Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/22/169. The contractual start date was in October 2010. The draft report began editorial review in August 2012 and was accepted for publication in January 2013. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors:
none
Corrections
-
This article was corrected in September 2015. See Wardlaw J, Brazzelli M, Miranda H, Chappell F, McNamee P, Scotland G, et al. Erratum: An assessment of the cost-effectiveness of magnetic resonance, including diffusion-weighted imaging, in patients with transient ischaemic attack and minor stroke: a systematic review, meta-analysis and economic evaluation. Health Technol Assess 2015;18(27):369–370. http://dx/doi.org/10.3310/hta18270-c201509
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Wardlaw et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
In the UK, about 150,000 people have a stroke each year. About 30% die within 6 months and another 30% survive dependent on others for everyday activities, making stroke the commonest cause of dependency in adults and the second commonest cause of death in the world. 1,2 Stroke is estimated to cost the NHS between £4.6B3 and £7B per year. 4 Stroke is also one of the major causes of disability in adults. 5 Eighty per cent of strokes are ischaemic, and most (75%) ischaemic strokes are due to an artery in the brain becoming blocked by atherothromboembolism. Treatment of ischaemic stroke is limited to thrombolysis, which can be used only in the first few hours after the stroke,6 a minor effect of aspirin, and co-ordinated stroke unit care, so prevention is vital.
About 20–40% of people have a warning transient ischaemic attack (TIA) or minor non-disabling ischaemic stroke shortly before they have a major disabling stroke. 7,8 In the UK, there are estimated to be 80,000–90,000 TIAs per year. 9 If these people can be assessed quickly, potential stroke causes identified and treated appropriately to reduce risk, then many of these disabling strokes can be prevented. 10 Based on these figures, the average regional hospital serving a population of around 750,000 will see about 1000 suspected TIA/minor stroke cases per year, i.e. about 20 per week. Delivering effective stroke prevention to this number of people is challenging and requires highly organised stroke services that are able to respond rapidly, accurately and effectively for stroke prevention, while avoiding adverse affects on other services within finite resources. The personal, societal, public health and financial burden of stroke in the UK is such that every effort should be made to limit the damaging effects of having a major disabling stroke, and to determine how to make best use of our available resources. 5,11–13
Transient ischaemic attack is defined as ‘a sudden loss of focal cerebral or monocular function lasting less than 24 hours due to inadequate cerebral or ocular blood supply as a result of low blood flow, thrombosis or embolism associated with disease of the arteries, heart or blood’. 14 Although the definition of TIA purely on the basis of clinical grounds is the subject of debate,15,16 and a tissue-based definition has been proposed,17 for the present time we have used the clinical definition. Patients with minor stroke, which differs from TIA only by symptoms or signs lasting more than 24 hours, are also at high risk of early recurrent stroke and need the same assessment and treatment as for patients with TIA to prevent a further disabling stroke or death. A small proportion of TIA/minor stroke (< 5%)18–20 is actually due to a small haemorrhage in the brain but this can be distinguished from ischaemic stroke only by brain scanning.
The period of highest risk of disabling stroke is in the first few hours and days after a TIA/minor stroke, thus making suspected TIA/minor stroke a medical emergency:8,14,21,22 the Oxford Vascular Study (OXVASC) suggested that between 8.0% and 11.5% of patients will have a recurrent stroke by 1 week, and between 11.5% and 15.0% by 1 month after TIA/minor stroke unless effective secondary prevention is started. 9 In the USA, there are about 240,000 TIAs per annum, of whom 25% had experienced a further TIA, a stroke or died by 3 months. 23 Prevention of recurrent ischaemic stroke is by rapid identification of underlying risk factors [such as ipsilateral tight carotid artery stenosis, atrial fibrillation (AF), hypercholesterolaemia, hypertension] and implementation of optimal medical (antiplatelet agents, statins, antihypertensive drugs or anticoagulant drugs where necessary)10,14,24 and surgical treatment (endarterectomy for symptomatic moderate to severe carotid stenosis). 25
Hence, patients with definite acute ischaemic stroke are now given standard quadruple preventative therapy [antiplatelet agent, statin, angiotensin-converting enzyme (ACE) inhibitor and diuretic drug] and patients who present with suspected TIA/minor stroke are started on quadruple therapy pending specialist investigation and treatment. It is therefore important to ensure that the patients whose symptoms after due investigation are proven not to be caused by acute ischaemic cerebrovascular (CV) disease, particularly the small proportion (≈5%) whose symptoms are due to a small haemorrhage, then avoid inappropriate, ineffective, expensive, unnecessary or possibly hazardous26–30 drug treatment.
The conventional brain scanning technique for stroke and TIA is computed tomography (CT), now widely available in the NHS. Although CT excludes tumours and haemorrhage if performed acutely,31 it is insensitive to small ischaemic lesions, so does not ‘positively’ diagnose TIA/minor stroke. 32 CT demonstrates an infarct in a maximum of 50% of minor acute strokes33 and 43% of mixed minor stroke and TIA,34 although the sensitivity may be lower in older patients with brain tissue loss and leukoaraiosis. Nonetheless, the presence of an infarct on CT in patients presenting with TIA is associated with increased short-term risk of stroke,35 CT has high specificity for ischaemia,36 visible infarction on CT is an independent predictor of poor outcome (dependency or death) after ischaemic stroke of all severities,33,37 CT is very accurate for haemorrhage within the first 5 days of symptoms32,36 and for tumours and other non-vascular causes of sudden neurological symptoms, giving it many advantages to balance its two disadvantages: the low sensitivity for acute ischaemic stroke and for small haemorrhages that are > 5 days old. 31
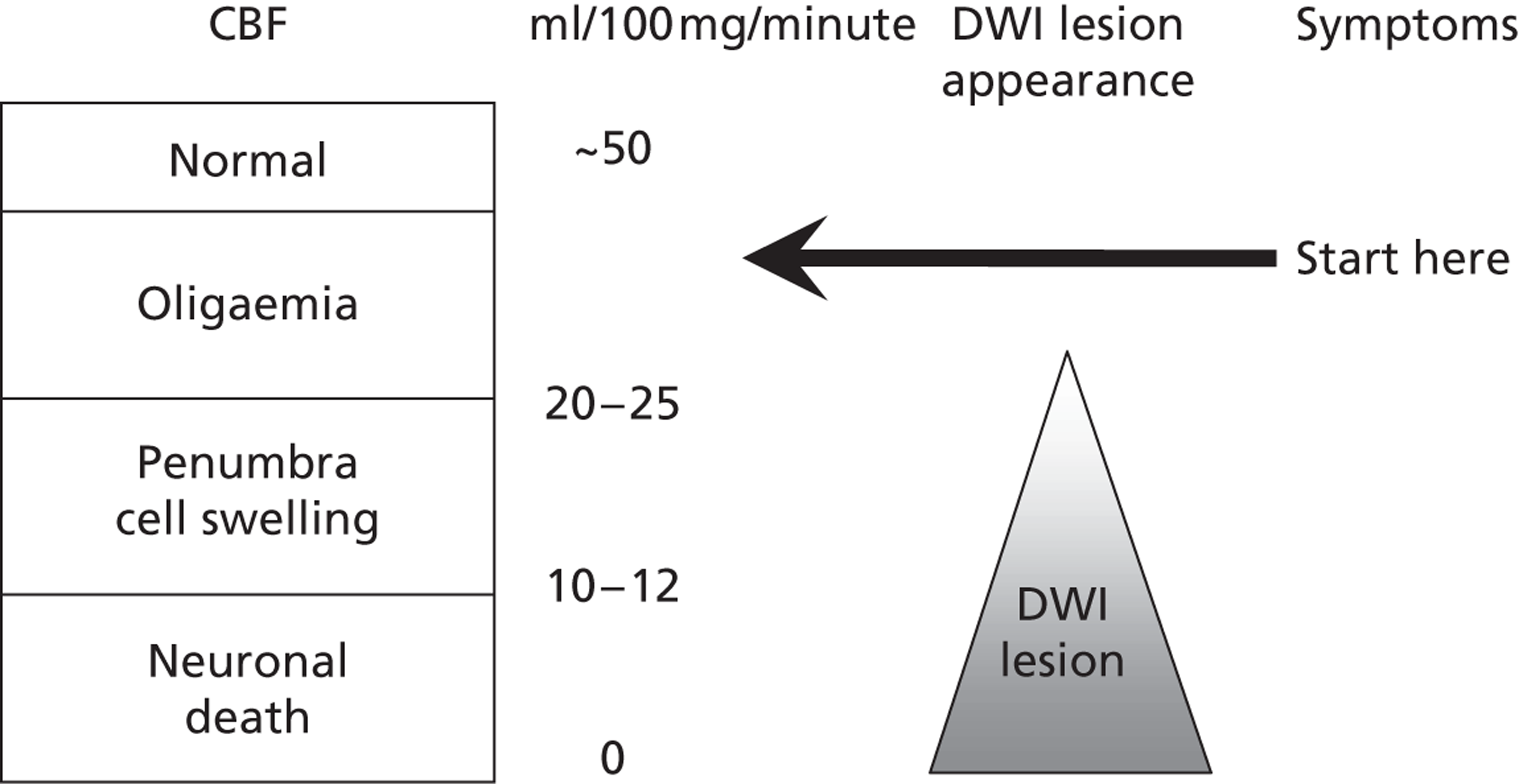
Magnetic resonance with diffusion-weighted imaging (MR DWI) is very sensitive to ischaemia. 38 Magnetic resonance imaging (MRI) is widely used to investigate many neurological disorders, musculoskeletal problems and in oncology. MRI is very versatile because different sequences can be used to highlight different types of pathology relevant to stroke. For example, DWI is very sensitive to changes in the mobility of water in the brain; one of the earliest changes in the brain at the onset of ischaemia is cell swelling, which reduces the extracellular space and hence restricts water movement. Thus, early ischaemia shows up well on DWI, even very early after the symptoms start and even in very small lesions causing mild symptoms38 if the effects of the ischaemic insult are sufficient to cause enough cell swelling to change the DWI signal. When considering how to use and interpret any brain imaging, it is important not to forget about the pathophysiological steps in ischaemia, knowledge that has been available since the early 1970s (Figure 1): the first step at the start of an ischaemic insult is that neurons cease their electrical activity when blood flow drops below about 35 ml/100 mg/minute (which is when the neurological symptoms start), well above the level required to precipitate loss of ion pump activity and cell swelling (about 20 ml/100 mg/minute) or cell death (10 ml/100 mg/minute). 39 It is therefore perfectly possible to have symptoms without any visible lesion on even the most sensitive scans, such as DWI, if the ischaemic insult is enough to drop the flow below the electrical shutdown level, but not so as to precipitate cell swelling or even on perfusion imaging if the amount of brain affected by the loss of flow is below the resolution of current perfusion imaging methods. If the flow recovers rapidly and never reached the level of causing cellular oedema then the symptoms will resolve and there will never be a lesion visible on the scan. If the flow recovers rapidly even after falling to a nadir below the cell oedema stage but not for long enough to result in cell death then the DWI-visible lesion will resolve rapidly as the cell swelling resolves and no lesion will be seen on the scan. 40 Only if the flow reduction persists for long enough and is low enough to have caused significant amounts of cell death will the scan show a positive lesion – the DWI lesion will last for variable times depending on the amount of tissue damage. 41 The chances of seeing an acute ischaemic lesion on DWI increase with increasing stroke severity,42,43 but even in patients with moderate to severe initial stroke symptoms the DWI hyperintensity/apparent diffusion coefficient reduction may disappear within a few days to a week and leave little evidence of permanent damage. 44 Permanent damage would appear on other sequences, notably T2-weighted, fluid-attenuated inversion recovery (FLAIR) or T1-weighted magnetic resonance (MR) images or CT, but after a week or so, when any acute swelling has disappeared, the recent lesion would be difficult to differentiate from an old prior lesion. 45 This physiological explanation is one of several valid and probably quite common reasons for having true ischaemic disease but a negative DWI even on acute scanning. Other reasons include delays to scanning, variation in sensitivity of sequences on individual MR machines and between MR machines of the same field strength and of different field strengths and generation of manufacture. 46
FIGURE 1.
Critical flow thresholds for development of symptoms and MR DWI imaging lesions.
Diffusion-weighted imaging primarily displays ischaemic areas as white on a dark background, so ischaemic lesions when present are much more obvious than they are on CT scanning, where ischaemic lesions appear as dark on a dark background. Thus even very tiny ischaemic lesions stand out on MR DWI (with the above caveat) soon after symptom onset in 16–67% of TIAs (mean 37%)47,48 and about 70–90% of minor strokes overall, even if scanned weeks after the event in some minor stroke patients. 36,38,45,48–50 However, in general, the chance of finding an ischaemic lesion on DWI falls rapidly with increasing time after symptoms,45,50 and probably most rapidly for small cortical ischaemic lesions,44 meaning that delays to scanning of even a few days will reduce the sensitivity of MR DWI to small ischaemic lesions. Currently, with CT scanning, if the scan provides no positive evidence of an ischaemic lesion, the diagnosis of ischaemia is often assumed if the scan has excluded haemorrhage and stroke mimics. By contrast, MR including DWI could help make a ‘positive’ diagnosis of brain ischaemia in TIA/minor stroke if it showed positive findings of ischaemia in a large proportion of patients, although a diagnosis of TIA/minor stroke would still have to be assumed if the DWI were negative and no alternative diagnosis could be made clinically.
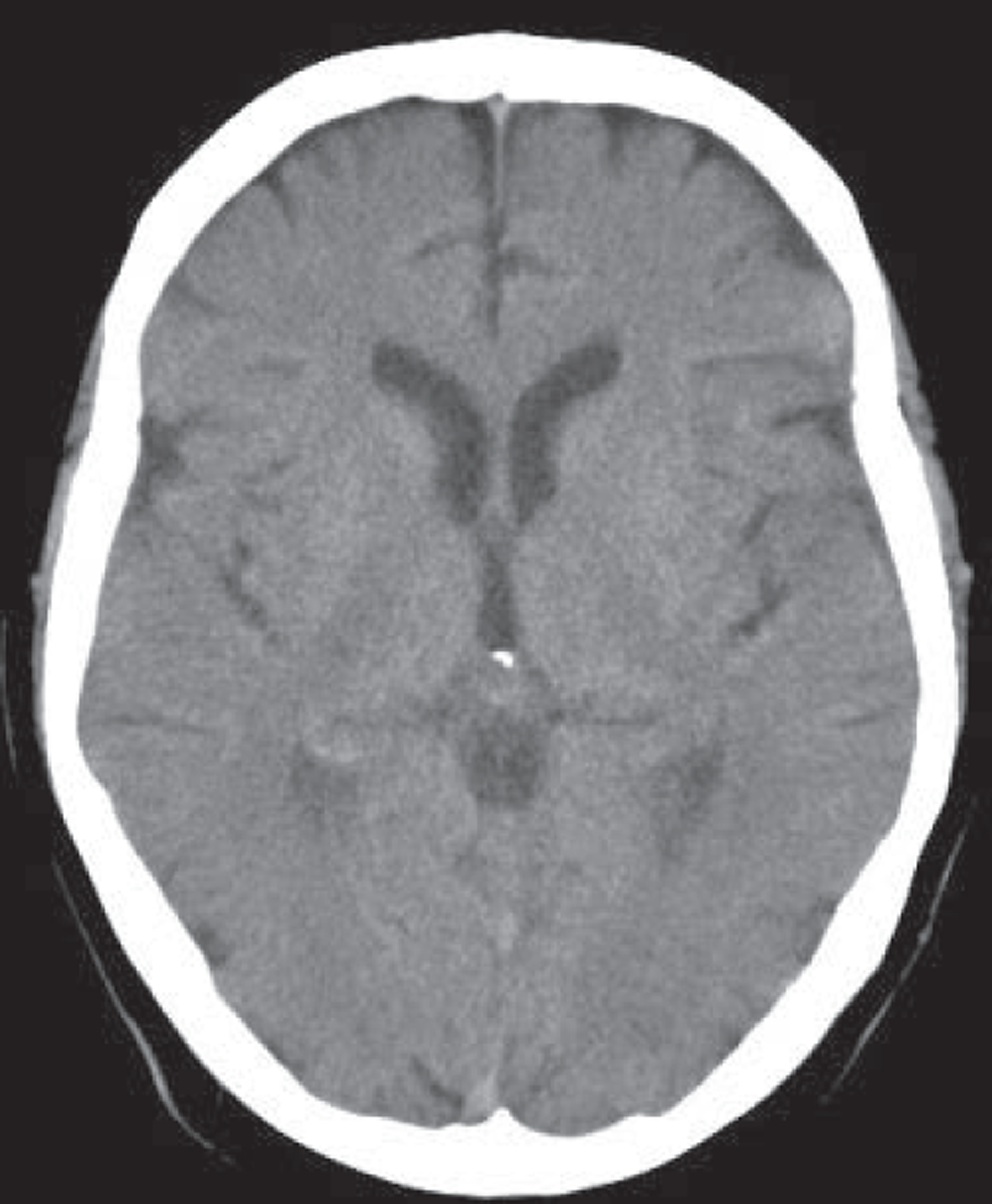
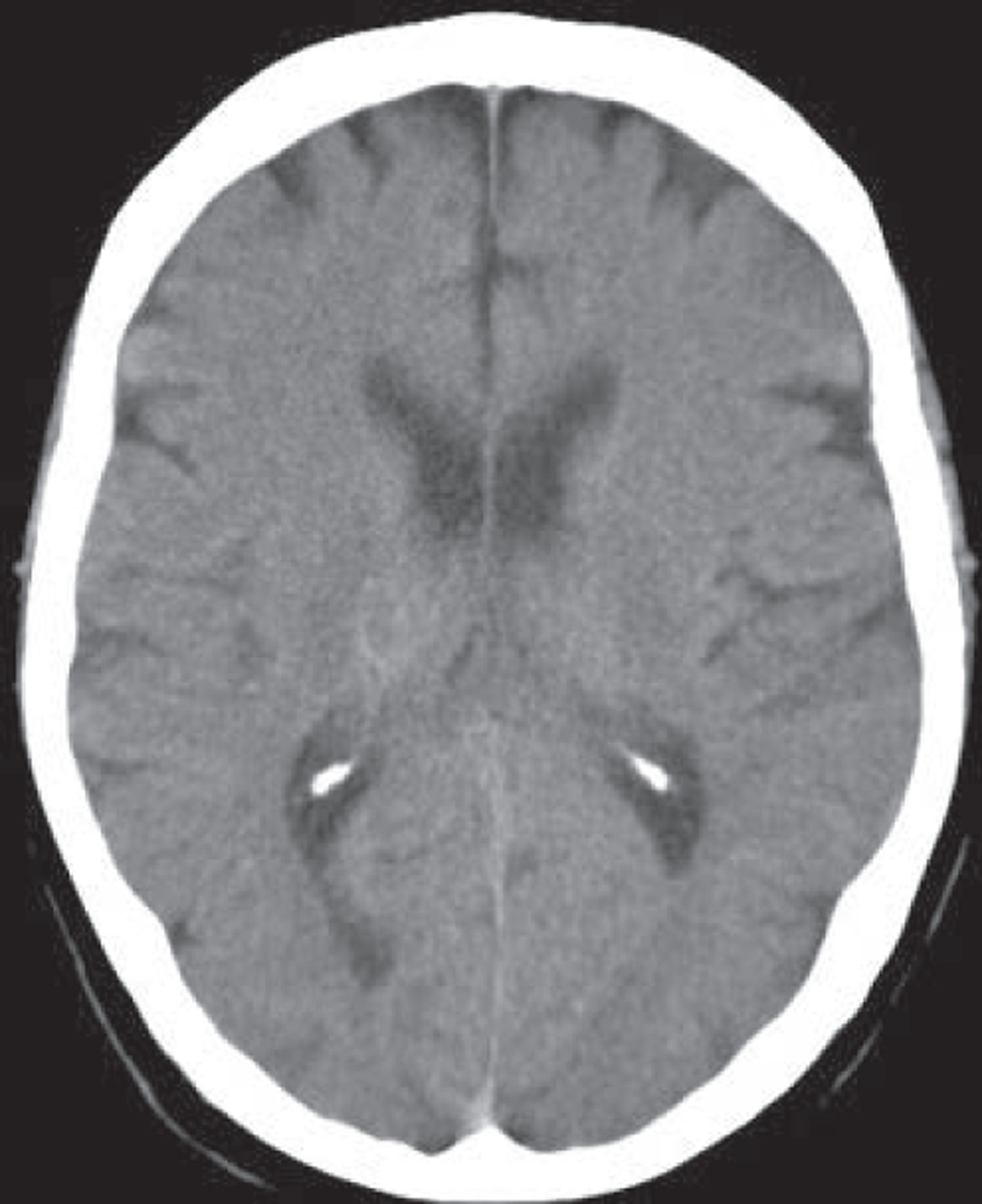
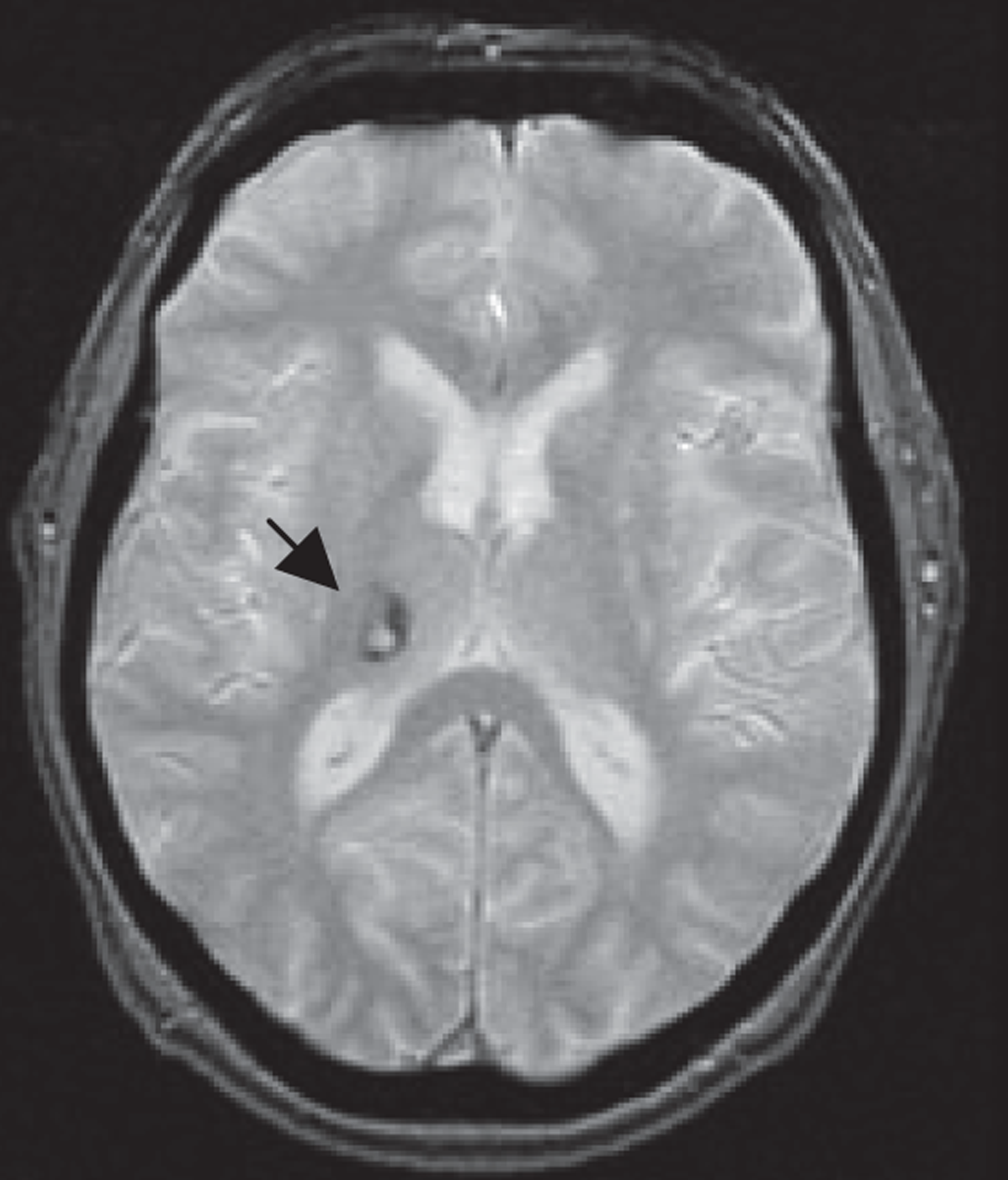
MR, if it includes T2*-weighted imaging or equivalent, is very sensitive to haemorrhage, even years after the event. 51 Other commonly used MR sequences (T2-weighted, T1-weighted and, particularly, FLAIR imaging) are much less sensitive. 51 Very acute haemorrhage (i.e. within the first few hours after symptom onset) is less easy to identify on MR and may be mistaken for ischaemia or a mass lesion until the characteristic paramagnetic features of blood breakdown products have had time to form. 52,53 In contrast, CT is very sensitive to acute haemorrhage54 but cannot reliably detect haemorrhage in patients who first present at > 5 days after a minor ischaemic stroke (Figure 2). 31 The high sensitivity of MR T2* sequences for haemorrhage is essential to diagnose haemorrhage correctly51,55,56 in patients who present late to medical services to avoid inappropriate use of antiplatelet agents, anticoagulants and carotid endarterectomy in patients with haemorrhagic stroke.
FIGURE 2.
Images from a patient presenting 8 days after developing mild left arm and leg numbness: top row, CT; bottom row, T2* MRI. Images from a patient presenting 8 days after developing mild left arm and leg numbness. CT is almost completely normal (a thin white circle is visible in the central image in the superior right thalamus, indicating a small resolving haemorrhage, arrow middle top image) but MR T2* clearly shows the low signal (black circle, arrow left bottom image) characteristic of a haemorrhage.
Magnetic resonance DWI could also help management in other ways. In some patients with carotid stenosis it is difficult to decide if the clinical event occurred in the territory of that artery or not. 57 DWI is helpful if it can confirm that a TIA/minor ischaemic stroke was in the territory of a tight carotid stenosis, so leading to endarterectomy. 57 The presence of lesions in several territories would indicate a need to search a more proximal source of embolism, for example in the heart. 58
The ability of MR to differentiate common stroke mimics from true ischaemic events is unclear. MR DWI is not specific to acute ischaemia: multiple sclerosis (MS), migraine, postictal changes, hypoglycaemia and even infection can produce high signal lesions on DWI (and reduced apparent diffusion coefficient). Many mimics do not produce any abnormality on either CT or MR brain imaging.
If MR is proven also to have high sensitivity and specificity for ischaemic events then by helping to exclude acute ischaemic CV disease as the cause of symptoms, and if in a large enough proportion of patients, it will potentially have a greater role in avoiding unnecessary or inappropriate treatment. However, if there is an over-reliance on the sensitivity of MR DWI to detect minor ischaemic change such that physicians start to think that patients presenting with suspected TIA/minor stroke without an acute ischaemic lesion on DWI do not have a TIA/minor stroke and therefore assign alternative diagnoses and do not implement secondary stroke prevention measures then greater use of MR could actually have a net damaging effect on stroke prevention. Similarly, if more MR is used – but without including all relevant sequences required to reliably identify haemorrhage,51,56 as well as ischaemia and any structural lesions acting as a mimic as the cause of symptoms – then it may also result in no benefit or net harm if patients with haemorrhage are misdiagnosed and given drugs that increase bleeding risk.
Several clinical scoring systems have been devised that aim to improve identification of patients at high risk of disabling recurrent stroke after TIA/minor stroke. 59–65 These scores could help to reduce delays to reaching medical attention and to triage patients,66 so that those needing most rapid treatment, such as carotid endarterectomy, would reach surgery more quickly. They might also improve the accuracy of clinical diagnosis of TIA by non-stroke physicians, thereby also making better use of resources. Not all of these have been externally validated. All except two60,64 use only simple clinical features so are rapid and easy to apply without needing complex technologies. 59,61,62,65 For example, the ABCD2 score uses age, blood pressure, clinical features, duration of symptoms and diabetes to derive a score from 0 to 7. The ABCD2 score has been adopted in National Institute for Health and Care Excellence (NICE) guidelines to aid patient triage in the UK:67 those with a score of ≥ 4 to be prioritised for assessment and treatment within 24 hours of reaching medical attention; those with a score of < 4 within 1 week.
Many of these scores performed well in the population from which they were derived and often also in initial testing in a different cohort68,69 but then wider use uncovered difficulties. For example, independent testing of the ABCD and ABCD2 scores62 has not been universally positive. 70,71 Although in OXVASC the ABCD2 score was highly predictive of recurrent stroke within 7 days of TIA (p < 0.01), it did not predict stroke at between 8 and 90 days, because in this group, patients with lower scores had a higher risk of stroke than those with high ABCD2 scores (p = 0.04). 71 The ABCD2 score also did not predict recurrent stroke in patients with minor stroke (as opposed to TIA). Thus TIA clinical risk prediction scores may not usefully predict stroke risk at > 7 days after TIA and may be of limited value in patients with minor stroke. Most scores were devised and tested in highly selected populations of patients with definite TIA/minor stroke such as randomised multicentre trials or observational studies after screening by a stroke expert where the patient had to have a definite TIA/minor stroke to get into the study. However, this is not the population of suspected TIA/minor stroke that typically presents to the ‘front door’ of the hospital. In this unselected population, up to 50% do not ultimately turn out to have had a TIA/minor stroke and therefore should have a low risk of recurrent stroke. 72 However, the net effect of applying a clinical risk score in this mixed population was that many true TIA/minor strokes were given a low ABCD2 score and therefore would have missed rapid access to stroke services. 72 In this sense, the use of a clinical risk score could actually lead to a net failure to prevent recurrent stroke. This reflects a general limitation of low specificity scoring or screening systems – namely that while they may detect the small proportion of patients with particularly high risk of recurrent stroke, many recurrent strokes actually occur in patients deemed to be at moderate or low risk only (because there are many more of them). Widespread use of clinical risk scores in this setting could therefore put at risk patients in a ‘slow stream’ who would then potentially not receive treatment quickly enough to prevent stroke and has not been tested.
Part of the problem may be that (1) the clinical diagnosis of TIA/minor stroke is difficult73–75 and (2) the clinical findings have low specificity for the likely underlying cause, but the future stroke risk is closely related to the cause. The clinical diagnosis of TIA and minor stroke is made difficult, especially for non-experts, by the high proportion of patients (up to 50%)5,72,76–78 who actually have common mimics of TIA/minor stroke. Common mimics include migraine, transient global amnesia, epilepsy, simple faints, tumours, functional disorders, etc. 72,77 It is not possible to determine the cerebral pathological cause of symptoms, i.e. whether infarct, haemorrhage or mimic (e.g. tumour), without a brain scan, although most mimics do not show specific positive findings on imaging, meaning that accurate diagnosis still rests heavily on clinical skills. 26,79
Some have questioned the usefulness of scores that do not incorporate significant carotid disease or other potential cardioembolic sources. 80,81 Two scoring systems that included carotid stenosis as part of the risk prediction60,64 found that adding tight carotid stenosis did improve risk prediction. Carotid imaging is an integral part of the assessment of TIA and minor stroke to identify those with ipsilateral tight carotid stenosis who will benefit from carotid endarterectomy. 82 The cost-effectiveness of carotid imaging was assessed in a previous Health Technology Assessment (HTA)-funded project by our group. 5 This work showed that rapid access to carotid imaging to identify and measure carotid stenosis was the most cost-effective way of using carotid imaging, and that the four non-invasive imaging methods functioned similarly in terms of accuracy83,84 and stroke prevention, although ultrasound was the most cost-effective of the four techniques if used early after TIA/minor stroke. 85 The focus of the present application is on how to improve stroke prevention through use of brain imaging techniques, so comparative carotid imaging will not be considered further.
The evidence on the contribution of MR to diagnosis, stroke prediction and hence potential for patient management and cost-effectiveness after TIA/minor stroke is uncertain – it could be very useful if used within its limitations in a targeted way, or it could be potentially harmful if used indiscriminately and without including all relevant sequences. No studies have considered these points or addressed the cost-effectiveness of using MR including DWI and T2* in TIA/minor stroke. Most studies of cost-effectiveness of imaging in stroke have either concentrated on hyperacute disabling stroke26,86 or on carotid imaging for carotid stenosis5 as part of carotid endarterectomy. 86 There are no clinical trials of diagnostic accuracy or effect on prediction of prognosis of MR including DWI, only observational studies using DWI in populations of patients with TIA/minor stroke. Many publications come from the same few research groups meaning there is likely to be data duplication: at the start of this project, all published studies were from single centres so lacked generalisability; some were retrospective; many only included a modest proportion of their TIA population (e.g. 53%)87 so were prone to various biases. 88–90 An acute lesion on DWI may be associated with increased risk of recurrent stroke and helps to confirm a positive diagnosis of stroke, but the additional prognostic value over clinical scoring, the role of carotid imaging, the effect of sample biases, etc., had not been evaluated thoroughly and was therefore unclear. 87,87,91–94 The accuracy of MR for common mimics of TIA/minor stroke was unclear, as most publications excluded patients who turned out after further testing not to have had a stroke.
There are barriers to the wider use of MR in stroke prevention. In addition to limitations owing to study methodological factors, listed above, MR is more expensive than CT scanning (about £400 for MR vs. £150 for CT brain scanning)5 and less available than CT in the UK, with long waiting lists even for patients with established indications for MR. There is limited capacity to provide rapid access to MR for people with TIA and stroke. MR delayed even by a few days would be of little value, as the patient would miss the period of highest recurrent stroke risk95 and the chance of finding an ischaemic lesion on DWI falls rapidly with increasing time after symptoms. 45,50 In the mid-2000s, although 75% of UK hospitals had MR on site, < 10% were able to scan patients early after stroke. 95 Similarly, across the EU, only 5% of hospitals met criteria for stroke care, which included availability of MR for stroke (and surprisingly the UK had the highest proportion of hospitals with MR for stroke). 96 Current availability is not known. Any increase in use of MR for stroke prevention could result in reduced availability for patients with MS, cancer and many other conditions for which MR is of established benefit. Some patients cannot have MR because of contraindications, for example having a pacemaker, claustrophobia or metal implant, so there would always be a need for CT. In one recent study, 90/904 patients (10%) considered for MR had contraindications and only 477/904 (53%) of all patients presenting with TIA actually underwent MR; of those having MR only 155/477 (33%, or 17% of the initial cohort) had a DWI-positive lesion. 87 Not all TIA patients have CT at present, so MR including DWI would partly replace, and partly be an additional investigation, if it proved to be cost-effective in stroke prevention.
What do guidelines say?
The 2008 UK NICE Guidelines advocate use of DWI in about 50%97 of TIA/minor stroke patients (www.dh.gov.uk/stroke). 67
Specifically, what is the guidance?
The objective for brain imaging for TIA is:4 ‘To confirm the diagnosis and/or vascular territory affected in those patients where either is unclear.’
Imaging for TIA (p. 8)
5. About 50 per cent of suspected TIAs require MRI of the brain. The diagnosis of TIA is difficult and doubt often remains, even after expert clinical assessment, whether or not the event was a TIA. Of patients presenting with symptoms suggesting a TIA, about half prove to have some other cause. For those who have had a TIA, the location of the lesion (damaged tissue) may not be known.
6. Computed Tomography (CT) is not sufficiently sensitive to demonstrate the small lesions found in TIA. MRI of the brain with diffusion-weighted imaging (DWI) is needed where the diagnosis or location of the lesion remains in doubt. The MRI may answer the question of whether the lesion is in vertebral (brain/brainstem) or carotid neck territory, prompting further carotid investigation where appropriate. If multiple lesions are found, this may suggest a cardiac source of the clot(s), and prompt investigation of the heart.
8. Carotid imaging should ideally be performed at initial assessment and should not be delayed for more than 24 hours after first clinical assessment of TIA for those at higher risk of stroke (i.e. ABCD2 score ≥ 4) or in those with non-cardioembolic carotid-territory minor stroke. Those people who are found to have carotid stenosis will require carotid intervention within 48 hours of presentation.
9. For those people at lower risk of subsequent stroke (i.e. ABCD2 score of < 4), carotid imaging should be performed within 7 days. If they are found to have carotid stenosis, they should have carotid intervention within two weeks of presentation.
10. Expert clinical assessment coupled with appropriate imaging provides the best diagnostic pathway for patients with TIA.
Furthermore, the Executive Summary states:
5. Imaging services managing TIA and stroke will need to be able to provide the following services:
5.1 TIA
MRI/MRA brain for those patients requiring it available 7 days a week with Contrast Enhanced MRA (CEMRA) for first-line carotid imaging. This requires MR software for diffusion weighted and gradient echo imaging and CEMRA, and a pump injector for CEMRA; and
Carotid imaging seven days a week, which will ideally include CEMRA and duplex ultrasound, and CT angiography.
5.2 Stroke
24-hour access to CT;
Rapidly accessible MRI, with the features described above, for those patients who require it; and
Ability to undertake more complex imaging examinations for stroke subtypes as required.
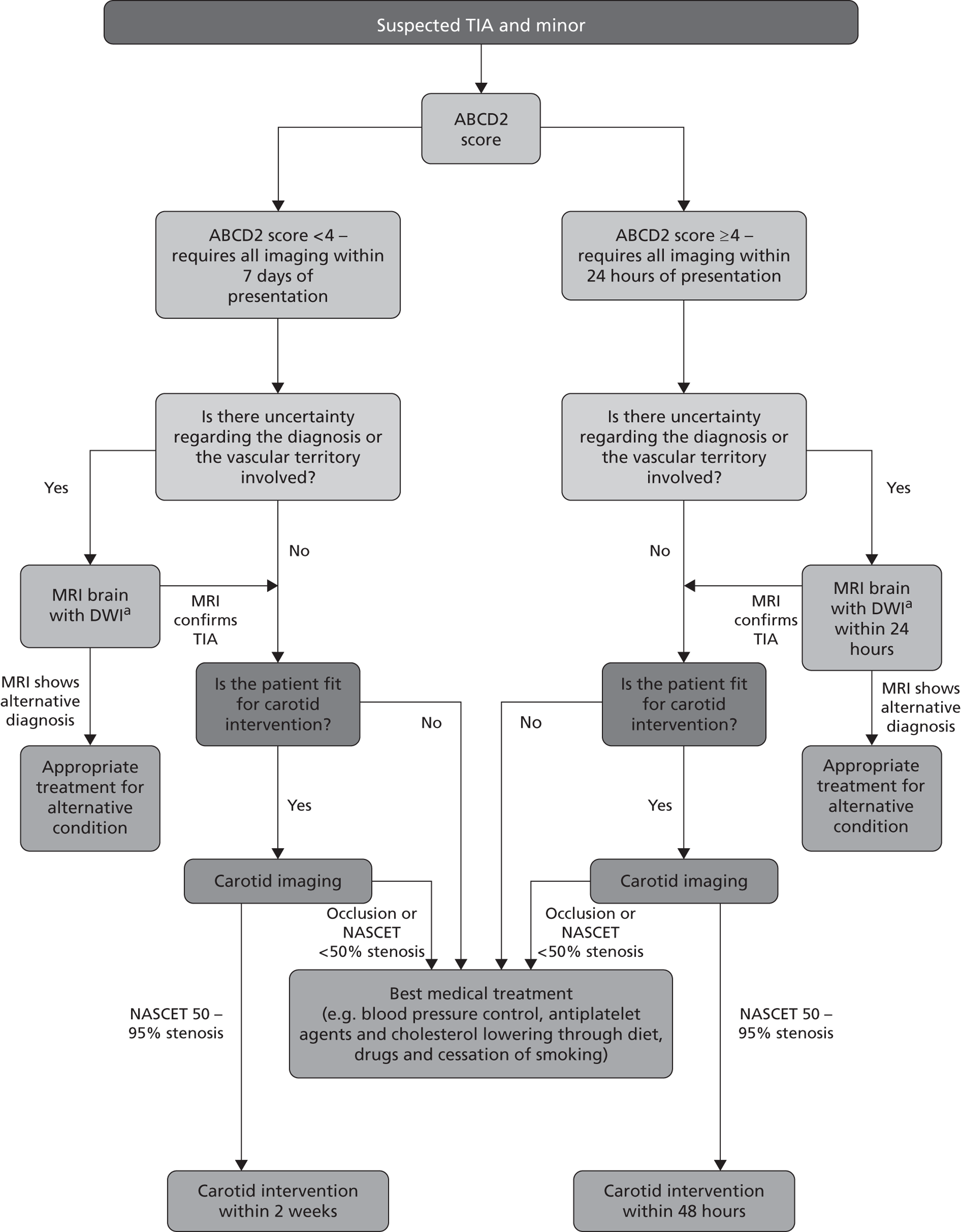
This policy is summarised in the NICE flow chart (Figure 3). 67 Whether or not this policy would reduce recurrent stroke and improve quality of life after TIA, or simply flood the system with large numbers of patients with negative MR scans while displacing others with diagnoses for which MR does have a clear indication, is uncertain. Since the start of this project, NHS Improvement in England have introduced tariffs to encourage the establishment of stroke prevention clinics that meet key performance criteria, and additional tariffs for rapid assessment and treatment of patients with high ABCD2 scores and for performing MRI in TIA patients (amount payable £450–634 per patient). 98,99
FIGURE 3.
Summary of current NICE guidance on investigation and management of patients with suspected TIA and minor stroke. Contains public sector information licensed under the Open Government License v1.0. NASCET, North American Symptomatic Carotid Endarterectomy Trial. a, Unless MRI contraindicated, in which case ct should be used.
Enthusiasm for a policy of more MR is expressed in many other guidelines. The American Heart Association (AHA)100 and related organisations101 stated that ‘TIA patients should undergo neuroimaging evaluation within 24 hours of symptom onset, preferably with magnetic resonance imaging including diffusion sequences’. 17 The revised National Clinical Guidelines for Stroke,102 Scottish Intercollegiate Guidelines Network (SIGN) Guidelines 2008,103 and European Guidelines104 are more cautious but still advocate immediate brain imaging and use of MR, including DWI in large proportions of patients especially those with mild stroke. Therefore, there is a ‘mismatch’ between national and international guidance on the one hand,17,97 and convincing evidence to support this approach,47,48 information to guide precise usage,105 details of cost-effectiveness and available technology to deliver it on the other,95,96 resulting in confusion about what to do in routine practice. This is mirrored in the NHS Purchasing and Supply Agency 2008 report, which stated only that ‘DWI shows significant potential in the study of TIA/minor stroke’, but also called for ‘more evidence’. 105 The limited evidence has also led some reviewers to call for more data on imaging to guide physicians treating TIA patients. 106,107
In summary, MR including DWI could make a substantial impact as a positive diagnostic test for TIA/minor stroke by improving diagnosis of the cause of stroke; efficient patient selection for medical secondary prevention in patients with a proven diagnosis; conversely, the avoidance of unnecessary treatment in patients in whom acute ischaemic CV disease was reliably excluded; and best use of carotid endarterectomy (especially where stroke expertise is limited). There are potential cost implications. TIA/minor stroke is so common that MR would be in daily use in every hospital if such a strategy were adopted wholeheartedly, but the direct costs to the NHS would be substantial – £16M per year to scan just the 50% of TIA patients suggested in recent UK guidelines, not including the minor strokes and all of the TIA mimics, assuming that MR were available. The opportunity cost to meet the demand by increasing MR scanning capacity, (without which there would also be substantial disadvantage to other MR users). It is not clear if the potential diagnostic and prognostic advantages of MR outweigh the disadvantages of the obvious expense, limited availability, failure to make a positive diagnosis of ischaemic lesion in up to 66% of TIA patients, unquantified accuracy for other stroke-related diagnoses, and possibility that the strokes that we are trying to prevent might occur during the wait for a scan if availability cannot be rapidly increased. Any strategy to increase MR usage would have to factor in the effect of varying delays introduced because of waiting for MR. We are not aware of any large or multicentre studies ongoing on this topic, although it is likely that single centre studies are ongoing.
This study aimed to resolve this controversy by summarising all available data on MR including DWI, diagnosis and stroke prediction, and modelling the cost-effectiveness of using MR including DWI in a range of stroke prevention strategies.
Our primary research objective is to determine whether MR DWI as well as other relevant structural and blood-sensitive sequences is cost-effective in the majority of patients with TIA or minor stroke to guide diagnosis and secondary stroke prevention, compared with the current alternative of CT brain scanning.
Second, to determine the cost and cost-effectiveness of increased use of MR including DWI and blood-sensitive sequences in patients presenting at > 5 days after TIA/minor stroke when CT will not be able to identify haemorrhage as the cause of stroke reliably.
Third, to estimate if ‘one-stop’ brain and carotid imaging is more cost-effective than individual separate brain and carotid examinations, in what proportion of patients a ‘one-stop’ approach could be used, and the practical and cost implications.
Fourth, to determine physicians and radiologists current attitudes to increased use of MR in TIA/minor stroke, the availability, barriers to greater use, costs of increasing availability, and net effect on other patient groups in whom MR is commonly used.
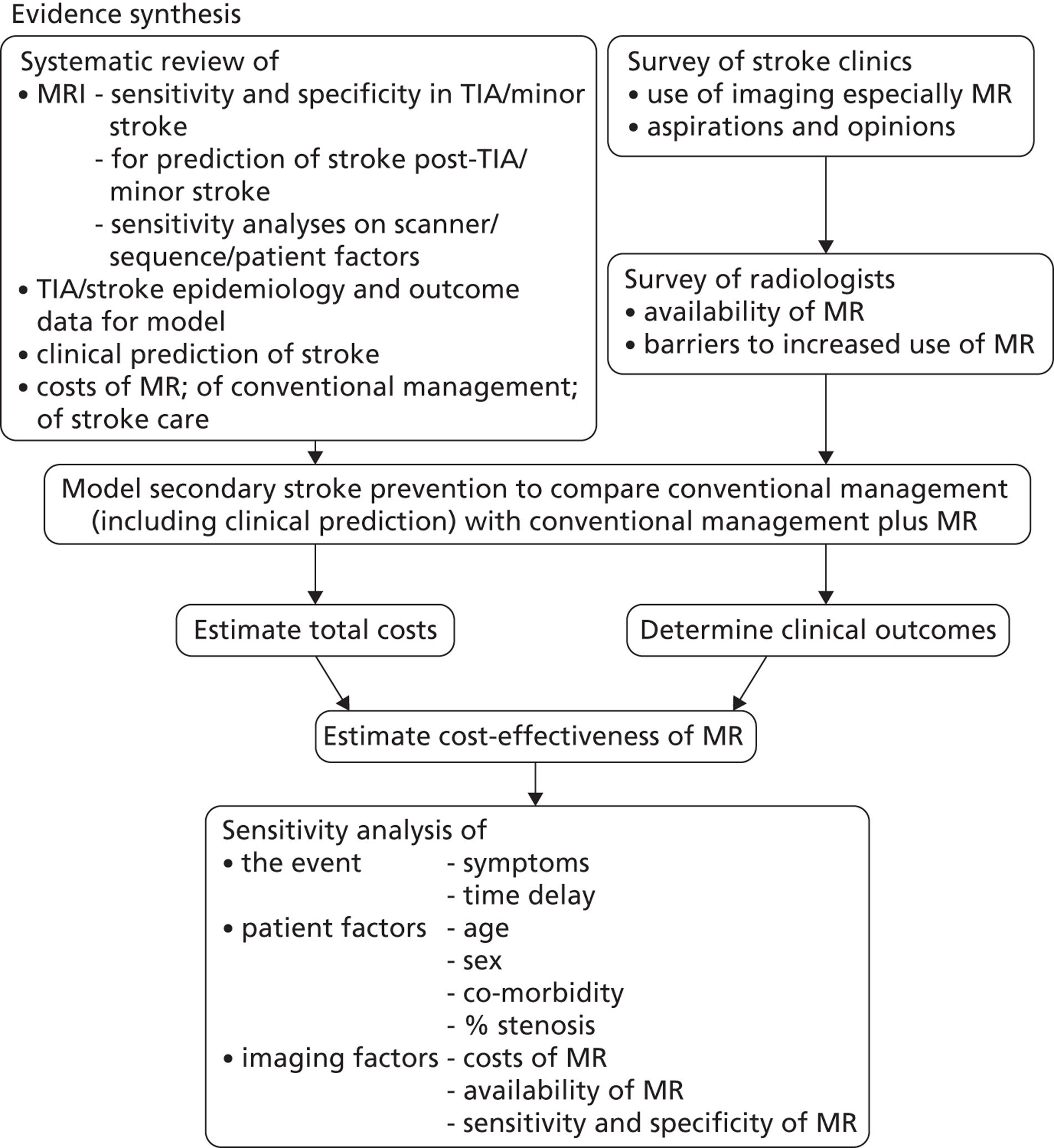
We planned to do this by using health economic decision-analytic modelling to assess the effectiveness, cost-effectiveness and practical implications of using MRI in different proportions of patients with TIA or minor stroke at different times after symptom onset, obtaining data from systematic reviews of the relevant literature, surveys of current practice and costs, existing stroke registry and cohort data. If MR cannot be justified in all patient then our aim was to determine in which subgroups of patients the use of MR is cost-effective. We aimed to provide a range of options reflecting the effect of using MR in different proportions of TIA/minor stroke patients at different times after TIA/minor stroke to guide decision-making by health service purchasers. We anticipated that the health economic modelling would be a hybrid of methods used in two previous HTA-funded cost-effectiveness analyses in stroke: a decision-analytic tree model to reflect the initial diagnosis of TIA/minor stroke compared with mimic, and ischaemic stroke compared with haemorrhagic stroke from work on the cost-effectiveness of CT scanning in acute stroke,26 followed by a probabilistic time-based model to reflect the varying risk of recurrent stroke and other vascular events following TIA/minor stroke, over a long time horizon from the work on cost-effectiveness of different carotid imaging methods in stroke prevention. 5
Chapter 2 General methods
Introduction
The research involved gathering information from many sources. This chapter provides a short résumé of the main objectives of the work and a brief general summary of the methods used. More detailed methods specific to the question being addressed are provided in each relevant chapter or section.
Objectives
Our primary research objective was to determine whether MR DWI and additional relevant structural and blood-sensitive sequences is cost-effective in the majority of patients with TIA or minor stroke to guide diagnosis and secondary stroke prevention, compared with the current alternative of CT brain scanning.
Our second objective was to determine the cost and cost-effectiveness of increased use of MR including DWI and blood-sensitive sequences in patients presenting at > 5 days after TIA/minor stroke when CT will not be able to identify haemorrhage as the cause of stroke reliably.
Our third objective was to estimate if ‘one-stop’ brain and carotid imaging was more cost-effective than individual separate brain and carotid examinations, in what proportion of patients a ‘one-stop’ approach could be used, and the practical and cost implications.
Our fourth objective was to determine physicians’ and radiologists’ current use of imaging in stroke prevention and attitudes to increased use of MRI in TIA/minor stroke, the availability, barriers to greater use, costs of increasing availability and net effect on other patient groups in whom MR is commonly used.
We undertook this work using a range of tools. We modelleding the effectiveness, cost-effectiveness and practical implications of using MRI in different proportions of patients with TIA or minor stroke at different times after symptom onset, using systematic reviews of the relevant literature, surveys of current practice and costs, and existing stroke registry and cohort data. If MRI could not be justified in all patients then we aimed to determine in which subgroups of patients the use of MR was cost-effective. We aimed to provide a range of options reflecting the effect of using MRI in different proportions of TIA/minor stroke patients so as to guide decision-making by health service purchasers. The extent to which these objectives would be fulfilled would depend on the amount of relevant evidence available in the literature on use of imaging in patients with TIA and minor stroke and mimics.
Design
The study was an evidence synthesis of data from the literature, new surveys of current UK practice and costs, and health economic modelling with sensitivity analyses of important variables (Figure 4). Further details on the following are given in relevant chapters.
-
Systematic reviews We systematically reviewed the literature to:
-
summarise the risk of recurrent stroke after TIA and minor stroke by time period (see Chapter 3)
-
summarise clinical risk scoring systems and in particular the ABCD2 score and its predictive accuracy (see Chapter 4)
-
estimate the sensitivity/specificity of CT and MR including DWI sequences in TIA/minor stroke, including the arterial territory; and
-
assess their role in prediction of stroke after TIA (see Chapters 5 and 6)
-
estimate the proportion of suspected TIA/minor stroke patients who are diagnosed as a mimic (see Chapter 7)
-
assess previous studies of cost-effectiveness of imaging in stroke prevention (see Chapter 9)
-
gather all of the data required to model stroke prevention after TIA, including estimating costs of CT and MR (summarised to 2003 in Wardlaw et al. 26 but requiring updating) (see Chapters 10 and 11).
-
-
All aspects of these systematic reviews, including literature searching, quality assessment, data extraction, and evidence synthesis were performed according to the Cochrane Collaboration and Stroke Group standards, including recommendations from the Screening and Diagnostic Tests Methods Group. 5,36 Occasionally we were not able to apply Cochrane methodology, and wherever this occurred, we give the reasons why and describe any alternative methodology used. The methods for evidence synthesis (meta-analyses performed according to the summary receiver operating characteristic (SROC) curve methodology or separate meta-analyses of sensitivity and specificity estimates) were determined by the data obtained and we used the most appropriate method, as described in each relevant chapter/section. 108 We explored heterogeneity by examination of forest plots and I2-statistics. 109 However, we did not use meta-regression, i.e. meta-analyses with covariates to explain heterogeneity, due to limited numbers of studies and a lack of information on relevant covariates. Where possible, we used a standardised quality assessment instrument [i.e. the Quality Assessment of Diagnostic Studies (QUADAS) tool],110 adapted to the topic in question as appropriate. 26,83 For chapters on prediction or prognosis, we created bespoke tools to suit the clinical context as there are no universally accepted instruments. Details are given as required in each chapter. As studies may be reported in multiple publications, we took care to include data from any given cohort only once in the reviews.
-
We obtained data on demographics, risk factors, medications, recurrent stroke and death in patients with TIA/minor stroke relevant to the UK, treatments and their effects, data on quality-adjusted life-years (QALYs) and utility weights and in order to construct the health economic model (see Chapters 10 and 11). We used existing data sets where possible, for example large stroke and TIA registries,111 individual patient data sets and routinely collected anonymised national audit data, as in previous work. 5,26
-
We surveyed UK stroke physicians through the Royal College of Physicians Stroke Audit and NHS Health Improvement,5 to ask about stroke prevention clinics including current access to and use of MR sequences, including DWI in patients with TIA/minor stroke in the NHS, aspirations and consensus on role of MR, and to gather additional unpublished data on MR including DWI in TIA/minor stroke (see Chapter 8).
-
We surveyed UK radiology departments across the UK to determine current use of imaging for stroke, types of imaging used, what capacity there is for increased throughput of TIA and minor stroke patients, and to identify the perceived major barriers to increased or faster throughput of TIA/minor stroke patients and what additional resources would be required to provide additional access for these patients (see Chapter 8).
-
We obtained UK data on imaging costs from the NHS Department of Health (DOH) NHS Reference Costs database, the literature and CT and MR from individual radiology departments,5,26 and the health-care costs associated with management of stroke patients by literature review of relevant electronic databases3 and by interrogation of specific sources, such as stroke registry data as in previous work (see below for specific details). 5,26 We also sought costs of stroke care by outcome and stroke prevention treatments (see Chapter 11).
-
We obtained data on quality of life after stroke for key subgroups from the literature, from previous work, unpublished sources and from our local stroke register data (see Chapter 11). 112
-
We built a model to reflect key stages and outcomes in secondary stroke prevention after TIA/minor stroke, including all data on assessments, medical and surgical interventions, outcomes, and timings, populated with representative data from TIA/stroke services in the UK. The timelines in the model were stratified by time after symptoms, key patient characteristics, use of prognostic scores (ABCD2),62 including conventional routinely available investigations (brain CT, carotid imaging), the comparator MRI and treatment decisions, and key outcomes of non-disabling and disabling stroke and death at various time intervals out to a lifetime time horizon (see Chapter 11).
-
We modelled the incremental cost-effectiveness of implementing MR including DWI with or without T2* instead of usual care in the diagnosis of TIA/minor stroke and a range of different strategies where proportions of patients would vary by key characteristics and timing of investigations, etc. in sensitivity analyses. We estimated the effect of using MR or CT also to diagnose carotid stenosis as a ‘one-stop’ investigation using magnetic resonance angiography (MRA) or CT angiography (CTA), respectively, instead of performing the MR or CT of the brain scan and separately performing carotid imaging with, for example, ultrasound. We used data from our previous HTA report (see Chapter 12). 5,84
-
We summarised the effect of different investigative approaches on recurrent stroke, costs, and QALYs, and hence the probability that the new diagnostic strategy is more cost-effective than usual care. The results are presented for a cohort of patients presenting to stroke prevention clinics with suspected TIA/minor stroke. Various analyses were conducted to investigate the worth of commissioning further research on the cost-effectiveness of MR DWI imaging and inform the design of any future research. All modelling work was performed according to recommended guidance (www.equator-network.org) (see Chapter 12). 113
FIGURE 4.
Study design.
Inclusion/exclusion criteria
We modelled a typical population of patients presenting with suspected TIA/minor stroke including mimics based on existing data reflecting current demographic, pre-stroke medications, and stroke treatments using information from the literature and a large national stroke audit.
These data sources included:
-
the North Edinburgh Stroke Study and Lothian Stroke Register (LSR, n = 2598, a hospital-based registry of all inpatients and outpatients with TIA and stroke presenting to the hospital between 1992 and 2000 with demographic information, imaging and laboratory investigations, past medical history, medications prior to and following the TIA/stroke, and quality-of-life data) and followed up to 4 years for recurrent stroke, cardiac events and death and used in two previous HTA reports5,26
-
the Edinburgh Stroke Study (ESS, n = 1367, a hospital-based registry of all inpatients and outpatients with TIA and stroke presenting to the hospital between 2002 and 2005 with the same information except quality of life) and so far followed up to 4 years after the stroke111
-
the Scottish Stroke Care Audit Study (SSCAS), a Scottish national routine collection of anonymised audit data from TIA and stroke services
-
data from other studies of stroke and TIA with more detailed imaging, including a study comparing CT and MR scans in minor stroke between 1998 and 2001 (n = 230) conducted as part of a previous HTA-funded project31
-
a study of patients with mild cortical and lacunar stroke between 2005 and 2007 (n = 250), all with MR DWI and T2* imaging114
-
audit data of TIA and stroke mimics from stroke prevention services in Leicester (Eveson; obtained through NHS Improvement)
-
data on quality of life at 6 months after stroke by functional outcome Oxford Handicap Score from the Clots in Legs Or sTockings after Stroke (CLOTS) trials. 115
Ethical arrangements
The use of existing anonymised data to populate the model and perform other analyses did not require additional ethics approvals. All data used in the proposed modelling were anonymised.
Proposed sample size
We modelled the effect of incremental change in proportions of TIA/minor stroke patients undergoing MRI for a typical UK population of 1000 patients. The existing data sets included several thousand patients with TIA, minor and major stroke, with initial clinical and imaging assessment, and data on functional status, stroke recurrence and death at 6 months, 1 year and later. We provide the results in the form of ‘events per a cohort of 1000 patients presenting to hospital with suspected TIA and minor stroke’.
Statistical analyses
Systematic review of diagnostic accuracy
Indices of diagnostic performance were extracted or derived from data presented in each primary study for each imaging test. Where data were available, we constructed 2 × 2 contingency tables of test results compared with reference standard to show the cross-classification of disease status and test outcome. We calculated sensitivity and specificity with 95% confidence intervals (CIs) for each imaging test for each study where possible. To describe and visualise the data, we used forest plots showing the pairs of sensitivity and specificity estimates for each study. We also used receiver operating characteristic (ROC) plots, random-effects SROC curves, survival curves, and various other meta-analytic techniques as appropriate. All reviews, analysis of observational data and other types of analysis including critical appraisal were conducted according to the established relevant recommendations (www.equator-network.org). 110,116
Outcome measures
The primary outcome was the incremental cost-effectiveness of MR scanning compared with CT for the whole population. Secondary outcome measures included the number of strokes prevented per strategy, costs and QALYs. We tested the effect of substituting MR for CT in key subgroups by time to imaging, ABCD2 score, assuming that DWI-negative TIA patients did not have a TIA/minor stroke, and combining carotid and brain imaging in one examination. We tested the effect of changing the proportion of patients with TIA/minor stroke with a visible ischaemic lesion on ‘MR including DWI’ and CT brain scanning and the association with key clinical variables; whether adding information from brain scanning to clinical risk scoring improves prediction of future risk of stroke or death; the association between a positive or negative brain scan and risk factors for stroke, such as carotid stenosis; delays to diagnosis, delays to starting medical or surgical treatment if MR including DWI were to replace CT brain scanning; the number of disabling strokes prevented at 6 months, 1 and 5 years after TIA/minor stroke if ‘MR including DWI’ were to be used in most patients for a cohort of 1000 patients. We tested the effect of QALYs stratified by key patient groups. We will provide a range of options reflecting effect of using MR in different proportions of TIA/minor stroke patients to guide decision-making by health service purchasers.
A brief discussion of the specific aspects of each chapter is provided at the end of each chapter and a discussion of the whole project and main findings in Chapter 13.
Chapter 3 A systematic review and meta-analysis of stroke risk after transient ischaemic attack
Introduction
The early risk of recurrent stroke after TIA has been underestimated for many years. Approximately 15–20% of ischaemic strokes are preceded by a TIA117 and the appropriate detection and urgent diagnostic work-up for patients with TIA can avoid a further disabling stroke if the correct treatment is indicated. A retrospective study of consecutive patients attending emergency departments (EDs) within 24 hours after TIA demonstrated that the stroke risk after the index event was higher than previously thought; the stroke rate was 10.5% at 90 days, with half of the events (5.3%) occurring within 2 days of symptoms onset. 8 A further analysis of a population-based TIA incidence study [Oxfordshire Community Stroke Project (OCSP)] also reported a high stroke rate after index TIA, with risks of 8.6% at 7 days and 12.0% at 30 days. 21 Several studies have been published after these publications. With respect to stroke immediately after TIA, in a prospective population-based incidence study of TIA and stroke (OXVASC), in 488 patients with a first TIA the risks of stroke at 6, 12 and 24 hours were 1.2% (95% CI 0.2% to 2.2%), 2.1% (0.8% to 3.2%) and 5.1% (3.1% to 7.1%), respectively. Furthermore, 42% of all stroke during the 30 days after a first TIA occurred within the first 24 hours. 118 Different studies have reported conflicting stroke rates after TIA, and cohorts from Oxford, UK and northern Portugal have published very high risks of stroke at 7 days (11% to 13%) and 90 days (17% to 21%), respectively. 9,119 A systematic review and meta-analysis of 11 observational studies reporting the early risk of stroke after TIA (total n = 7238), showed that risks of stroke ranged from 1.4–9.9% at 2 days, 3.2–17.7% at 30 days, and 3.9–17.3% at 90 days, with significant heterogeneity for all periods considered. Using a random-effects model, the pooled estimate of risk was 3.5% (95% CI 2.1% to 5.0%) at 2 days, 8.0% (5.7% to 10.2%) at 30 days, and 9.2% (6.8% to 11.5%) at 90 days; the risk was higher when the methodology of the studies involved active ascertainment of stroke outcome (9.9%, 13.4% and 17.3%, respectively). 120 Another systematic review and meta-analysis of studies reporting the risk of stroke exclusively within 7 days of TIA (total 18 cohorts, n = 10,126 patients) showed pooled risk of stroke of 3.1% (95% CI 2.0% to 4.1%) at 2 days and 5.2% (3.9% to 6.5%) at 7 days, with a substantial heterogeneity across studies for both pooled risk estimates. The risks of stroke after TIA observed in patients treated urgently by specialist stroke services were 0.6% (95% CI 0.0% to 1.6%) at 2 days, and 0.9% (95% CI 0.0% to 1.9%) at 7 days compared with 3.6% (95% CI 2.4% to 4.7%) at 2 days and 6.0% (95% CI 4.7% to 7.3%) at 7 days from other cohorts. 121
Recent studies24,122 included in the systematic review by Giles and Rothwell121 have reported very low risks of recurrent stroke for patients in whom a secondary prevention treatment [mainly antiplatelet drugs and blood pressure (BP)-lowering drugs] was started immediately after confirmed diagnosis of TIA or minor stroke. An early detection of medical conditions with a high risk of early stroke recurrence, such as severe carotid stenosis or a cardiac source of embolism requiring specific treatments (i.e. carotid endarterectomy or anticoagulant therapy) can also explain the significant reductions in stroke rates in those cohorts.
Methods
Objectives
For the purpose of this HTA project we have conducted a systematic review considering all primary studies reporting the overall rate of stroke in TIA patients. Recent published systematic reviews on stroke risk after TIA (e.g. Wu and colleagues120 and Giles and Rothwell121) were used only as a source of relevant references. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for systematic reviews of studies that evaluate health-care interventions to conduct this review. 123 We aimed to identify all published studies reporting the risk of recurrent stroke among patients with TIA and/or minor stroke irrespective of the clinical setting or type of study design.
Identification of studies
We searched indexed records that appeared in MEDLINE (Ovid) from January 1995 to November 2011. It is worth noting that for the purpose of this systematic review we combined the comprehensive literature searches we developed for Chapter 6 (DWI in patients with TIA and minor stroke) and for Chapter 4 (clinical prediction score and risk of stroke after TIA and minor stroke) of this assessment. The MEDLINE search strategy included both subject headings [medical subject headings (MeSH) terms] and text words for the target condition (e.g. stroke, TIA, minor stroke). We adapted the MEDLINE search to search EMBASE. In particular, we ‘translated’ the MEDLINE MeSH terms into the corresponding terms available in the Emtree vocabulary. We did not apply any language restrictions. The searches were initially run in November 2010 and updated in November 2011. Full details of the MEDLINE and the EMBASE search strategies are presented in Appendix 1. We imported all citations identified by the MEDLINE and EMBASE search strategies into the Reference Manager bibliographic database version 11 (ISI Research Software, San Francisco, CA, USA). We hand-searched all proceedings of the International Stroke Conference (2011) and the European Stroke Conference (2011, 2012). We also contacted experts in the field and perused the reference lists of all relevant articles to identify further published studies for possible inclusion in the review. Only full-text articles were deemed suitable for inclusion. 124
Inclusion/exclusion criteria
One review author (MB) examined the identified titles and abstracts and retrieved all potentially relevant citations in full. Full-text articles were assessed by two reviewer authors (MB, HM) and retained if they reported the number and proportion of patients with stroke recurrence at 7 days, 90 days, and/or > 90 days after index TIA. We analysed all studies regardless of whether they reported recurrent stroke at 7 days, 90 days or > 90 days. We excluded non-English articles for which a full-text translation was not available.
Quality assessment and data extraction
Two review authors (MB, HM) independently conducted data extraction and review the methodological quality of selected studies using the primary papers. Disagreements were resolved by discussion or referred to a third author (JMW) if necessary. We recorded data on study methods (e.g. setting, study design) characteristics of patients (first vs. recurrent TIA), and outcomes (stroke events at 7, 90 and > 90 days). We also collected information on the following methodological aspects, which we considered more likely to introduce potential biases: prospective compared with retrospective study design, data source for cohort identification (e.g. registries, databases, medical notes), patients’ selection criteria, definition of TIA and recurrent event, timing of clinical assessment, evaluating clinicians, and method of outcome ascertainment (active vs. passive).
Data synthesis
For each study we calculated the total number of patients with stroke recurrence after index event (first or recurrent TIA). The pooled proportion of TIA patients with stroke recurrence was calculated by means of a univariate random-effects meta-analysis with within-study variance modelled as binomial. Heterogeneity between studies was assessed visually by inspection of the forest plots and by calculating I2-statistics. 109 Data were analysed in R version 2.14.2 (The R Foundation for Statistical Computing, Vienna, Austria) (cran.r-project.org).
Results
Number of included/excluded studies
The literature searches identified 11,389 citations. After initial screening of titles and abstracts we selected 248 citations for full-text retrieval. Two additional studies published in 2005 were included after hand-searching of reference lists of relevant studies, yielding a total of 250 studies. After full-text examination, 197 studies were excluded, leaving 53 studies that fulfilled the inclusion criteria. The main reason for exclusion was that the number of recurrent events was not clearly reported (Figure 5). We translated one report published in Spanish; all other included reports were originally published in English. We observed, as have others,125 that some studies come from the same TIA cohorts (i.e. Oxford, north Dublin, California, Massachusetts, Lleida and Paris cohorts) and we experienced difficulty in avoiding double counting of the same patient data appearing in multiple publications. Many studies were published from the same research groups and the same cohorts of patients were described in multiple reports – with some reports assessing more than one patient cohort (i.e. Oxford, north Dublin, California, Massachusetts, Lleida and Paris cohorts). Even although we attempted to exclude multiple publications we found it challenging to identify which cohort was assessed in which study, as precise information were somehow lacking. When a single study included separate cohorts (e.g. community-based TIA patients and patients from specialist unit) for the purpose of the analyses we considered the separate cohorts as coming from separate studies.
FIGURE 5.
Identification and selection of studies.
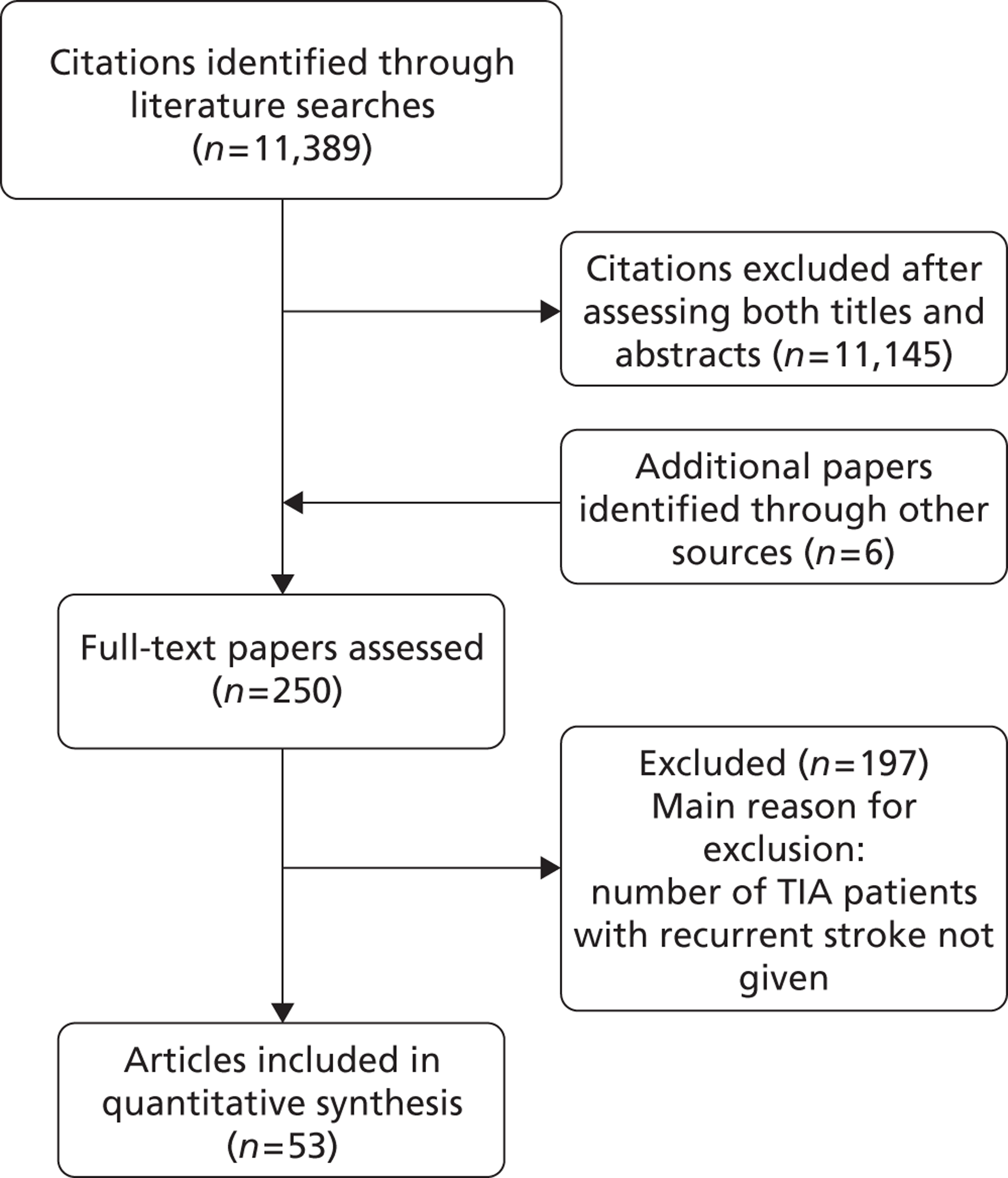
Characteristics of included studies
The methodological characteristics of selected studies are shown in Table 1.
| Study ID | Setting | Study design | TIA definition | Recurrent TIA included | Time from symptoms onset | Evaluating clinician | Consecutive patients | Follow-up | Recurrence definition (type of events) |
|---|---|---|---|---|---|---|---|---|---|
| Lovett and colleagues, 200321 | Population based | Prospective | Time based | No | Median 7 days | Neurologist | Yes | In person | Stroke |
| Lisabeth and colleagues, 2004126 | Population based (multiple EDs) | Prospective | Time based | Yes | NR | Neurologist (records) | No | Clinical records | Stroke (6.8% haemorrhagic) |
| Hill and colleagues, 2004127 | Population based (multiple EDs) | Retrospective | ICD 9 definition | Yes | NR | Emergency medicine physician | No | Clinical records (using ICD codes) | Stroke (1.2% haemorrhagic) |
| Coull and colleagues, 20049 | Population based | Prospective | Time based | Yes | Assessed ‘as soon as possible’ | Neurologist | Yes | In person | Stroke |
| Eliasziw and colleagues, 2004128 | NASCET | Prospective | Time based | Yes (first recorded event considered) | Within 180 days | Neurologist | No | In person | Stroke |
| Gladstone and colleagues, 2004129 | ED | Prospective | Time based | Yes | Median 120 minutes | Emergency medicine physician | Yes | Clinical records | Stroke |
| Kleindorfer and colleagues, 200523 | ED | Retrospective | Time based | Yes | NR | Emergency medicine physician, neurologist (records review) | Yes | Clinical records | Stroke |
| Rothwell and colleagues, 200561 | Population based | Prospective | Time based | Yes | Assessed ‘as soon as possible’ | Neurologist | Yes | In person | Stroke |
| Whitehead and colleagues, 2005130 | TIA clinics | Retrospective | NR | NR | NR | Stroke physician | Yes | Clinical records | Not clear |
| Van Wijk and colleagues, 200565 | Hospital | Prospective | Time based | NR | Within 3 months (near 23% within 7 days) | Neurologist | No | In person, letters, records | Stroke |
| Correia and colleagues, 2006119 | Population based | Prospective | Time based | No | 48% < 48 hours and 62% < 7 days | Neurologist | Yes | In person | Stroke |
| Bray and colleagues, 2007131 | ED | Retrospective | Time based | NR | Median 135 minutes | Emergency medicine physician | Yes | Clinical records (64%), telephone (36%) | Stroke |
| Johnston and colleagues, 200762 | ED | Retrospective | Time based | Yes | < 24 hours | Emergency medicine physician | Yes | Clinical records | Stroke (distinguishable from index TIA) |
| TIA clinic | Prospective | Time based | Yes | Assessed ‘as soon as possible’ | Neurologist | Yes | In person | Stroke | |
| Koton and colleagues, 2007132 | Population based | Prospective | Time based | Yes | Assessed ‘as soon as possible’ | Neurologist | Yes | In person | Stroke |
| Lavallée and colleagues, 2007122 | Specialist unit | Prospective | Time based | Not clear; patients with past medical history of CV disease included | 61% with < 48 hours and 75% with < 7 days of symptoms | Neurologist | Yes | In person, telephone | Stroke |
| Rothwell and colleagues, 200724 | TIA clinic | Prospective | Time based | Yes | 57% within 24 hours | Neurologist | Yes | In person | Stroke |
| Wu and colleagues, 2007120 | Hospital | Retrospective | NR | Yes | Immediately | NR | Yes | Clinical records | Probably stroke |
| Atanassova and colleagues, 2008133 | Hospital | Prospective | Time baseda | No | NR | Neurologist | Yes | In person | Stroke |
| bCoutts and colleagues, 2008134 | ED | Prospective | Time based | Yes | < 12 hours | Neurologist | Yes | In person | Stroke |
| bJosephson and colleagues, 2008135 | ED | Retrospective | Time based | Yes | < 24 hours | Emergency medicine physician | Yes | Clinical records | Stroke (distinguishable from index TIA) |
| Ois and colleagues, 200881 | Hospital | Prospective | Time based | Yes | Median 12 hours | Neurologist | Yes | In person, telephone | Stroke |
| Sciolla and Melis, 200869 | ED | Prospective | Time based | Not clear; patients with past medical history of CV disease were included | < 24 hours | Neurologist | Yes | In person, telephone | Stroke |
| Asimos and colleagues, 2009136 | ED | Prospective | Time based | Yes (first TIA included) | < 24 hours | Emergency medicine physician | No | Clinical records | Disabling stroke (mRS ≥3) |
| Ay and colleagues, 200987 | ED | Retrospective | Time based | NR | < 24 hours | Neurologist | Yes | Clinical records | Stroke |
| Calvet and colleagues, 2009137 | Specialist unit | Retrospective | Time based | Yes | < 48 hours | Neurologist | Yes | In person, telephone | Stroke |
| Chatzikonstantinou and colleagues, 2009138 | Specialist unit | Prospective | Time based | Yes | < 24 hours | NR | Yes | In person | Stroke |
| Cucchiara and colleagues, 2009139 | Specialist unit | Prospective | Time based | NR | < 48 hours | Neurologist | Yes | In person, telephone | Stroke |
| bFothergill and colleagues, 2009140 | Population based | Retrospective | Time based | No | < 72 hours | Neurologist (records) | Yes | Clinical records | Stroke |
| Mlynash and colleagues, 2009141 | Specialist unit | Prospective | Time based | Yes | < 48 hours | Neurologist | Yes | NR | Stroke |
| Tan and colleagues, 2009142 | Hospital | Prospective | Time based | NR | < 48 hours | Neurologist | Yes | NR | Stroke |
| Asimos and colleagues, 2010143 | ED | Prospective | Time based | Yes (first TIA included) | < 24 hours | Emergency medicine physician | No | Clinical records | Stroke |
| Chandratheva and colleagues, 2010144 | Population based | Prospective | Time based | Yes | Assessed ‘as soon as possible’ | Neurologist | Yes | In person | Stroke |
| Harrison and colleagues, 2010145 | TIA clinic | Retrospective | NR | NR | Median 15 days | Stroke physician | Yes | Clinical records | Stroke (ICD-9 definition) |
| Holzer and colleagues, 2010146 | Specialist unit | Retrospective | Time based | Yes | NR | Neurologist | Yes | Telephone, interview, medical records | Stroke |
| Merwick and colleagues, 2010147 | Population based | Retrospective | Time based | Yes | NR | Stroke physician | Yes | In person, telephone- clinical records | Stroke |
| Nakajima, and colleagues, 2010148 | Hospital | Prospective | Time based | Yes | NR | Neurologist | Yes | Clinical records, telephone interview | Stroke (TIA – asymptomatic brain infarction excluded) |
| Ong and colleagues, 2010149 | ED | Retrospective | Time based | NR | NR | Emergency medicine physician | Yes | In person | Stroke |
| Purroy and colleagues, 2010150 | Specialist unit | Prospective | Time based | Yes | < 48 hours | Neurologist | Yes | In person | Stroke |
| Sheehan and colleagues, 2010151 | Population based | Prospective | Time based | Yes | Median time 24 hours | Stroke physician | Yes | In person | Stroke |
| Tsivgoulis and colleagues, 2010152 | Hospital | Prospective | Time based | Yes | NR | Neurologist | Yes | In person | Stroke |
| Wasserman and colleagues, 2010153 | ED | Prospective | NR | NR | Within 7 days (68% < 24 hours) | Emergency medicine physician | No | Telephone questionnaire, records | Stroke |
| Weimar and colleagues, 2010154 | Specialist unit | Prospective | Time based | Yes | < 24 hours in 92%c | Neurologist | Yes | Telephone, paper questionnaire | Stroke |
| Yang and colleagues, 2010155 | Hospital | Retrospective | NR | Yes | NR | NR | Yes | In person, telephone | Stroke |
| Amort and colleagues, 2011156 | ED | Prospective | Time based | NR | NR | Neurologist | Yes | In person, telephone | Stroke |
| Arsava and colleagues, 2011157 | ED | Retrospective | Transient symptoms + DWI visible infarct | Yes | < 24 hours | Neurologistd | Yes | Clinical records | Stroke (new symptoms + visible DWI lesion) |
| Bonifati and colleagues, 2011158 | ED | Retrospective | Time based | Yes | NR | Neurologist and emergency medicine physician | Yes | Telephone interview, clinical records | Stroke (confirmed by brain imaging) |
| Kim and colleagues, 2011159 | ED | Retrospective | Time based | Yes | NR | Emergency medicine physician | No | Clinical records | Stroke (distinguishable from index TIA) |
| Meng and colleagues, 2011160 | Hospital | Prospective | Time based | Yes | Within 7 days | Neurologist | Yes | In person, clinical records | Stroke |
| Olivot and colleagues, 2011161 | ED | Prospective | Time based | NR | < 24 hours in 92% | Neurologist, stroke physician | Yes | In person, telephone interview | Stroke |
| Sanders and colleagues, 2011162 | Hospital | Retrospective | Time based | NR | NR | Emergency medicine and stroke physician | Yes | Clinical records, telephone | Stroke |
| Stead and colleagues, 2011163 | ED | Retrospective | Time based | Yes | NR | Mainly emergency medicine physician | Yes | Telephone interview, clinical records | Stroke |
| Amarenco and colleagues, 2012164 | Specialist unit | Prospective | Time based | Not clear; patients with past medical history of CV disease included | NR | Neurologist | Yes | In person, telephone interview | Stroke |
| Purroy and colleagues, 2012165 | Specialist unit | Prospective | Time based | Yes | < 48 hours | Neurologist | Yes | In person | Stroke |
The 53 studies varied in their primary objectives. Eleven were population-based studies including cohorts from the UK (Oxford), Ireland (Dublin), USA (Texas and Minnesota), Canada (Alberta) and northern Portugal. 9,21,61,119,126,127,132,140,144,147,151 Seventeen were ED-based studies,23,69,87,129,131,134–136,143,149,153,156–159,161,163 three were focused on TIA clinics (one included OXVASC participants),24,130,145 10 were hospital-based studies (the authors of which did not describe characteristics of the hospital unit),65,81,120,133,142,148,152,155,160,162 10 recruited patients admitted to a specialist unit [including stroke unit, neurological ward or highly-specialised neurovascular (NV) clinics],122,137–139,141,146,150,154,164,165 one focused on patients attending ED and TIA clinics (including OXVASC participants)62 and one128 recruited patients as part of a hospital-based clinical trial. The 53 studies included a total of 30,558 participants. The risk of recurrent stroke at 7 days was reported in 28 studies (30 cohorts) of 12,332 participants, 34 studies (35 cohorts) of 19,769 participants reported the stroke risk at 90 days and nine studies of 8699 participants reported the stroke risk > 90 days. For studies reporting the stroke risk > 90 days, the length of follow-up ranged from 6 months to 14 years.
The study design was prospective in 33 studies9,21,24,61,65,69,81,119,122,126,128,129,132–134,136,138,139,141–144,148,150–154,156,160,161,164,165 and retrospective in 19;23,87,120,127,130,135,137,140,145–147,149,155,157–159,162,163 one study62 included two cohorts recruited prospectively and retrospectively. Participants were included consecutively in 45 studies. The diagnosis of TIA was made by a neurologist or stroke physician in 36 studies,9,21,24,61,65,69,81,87,119,122,126,128,130,132–134,137,139–142,144–148,150–152,154,156,157,160,161,164,165 by an emergency medicine physician in 10 studies,23,127,129,131,135,136,143,149,153,159 and by both in four;62,158,162,163 in three studies120,138,155 this information was not reported.
With respect to the timing of patient assessment after the index event, in 10 studies TIA patients were assessed within 24 hours of symptoms onset,62,69,87,134–136,138,143,155,161 in six studies within 48 hours,137,139,141,142,150,165 in one study140 within 72 hours and in one study160 within 7 days. Another four studies9,61,132,144 reported that ‘patients were assessed as soon as possible after the event’ but did not give a time. One study62 (including two cohorts) assessed patients within 24 hours and ‘as soon as possible after the event’, another study120 assessed patients ‘immediately’ after the event, and in three studies65,128,145 the timing of assessment was beyond 7 days. The remaining 27 studies did not clearly provide this information (see Table 1). Four studies included patients with first-ever TIA, 34 included either first-ever or recurrent TIA, and in 15 studies the authors did not report this information.
The majority of studies (n = 46) used a time-based definition of TIA (see Table 1), one study145 recruited patients retrospectively from clinical records using the International Classification of Diseases (ICD), Ninth Edition, definition of TIA, one study157 included just patients with transient symptoms and DWI visible lesion, in five studies the information was not reported. One study128 included a highly selected sample of patients with TIA and carotid stenosis, and three studies65,141,155 excluded patients with specific conditions [i.e. cardioembolic events or blood coagulation disorders, AF and posterior circulation TIAs, respectively].
The ascertainment of stroke events after TIA was carried out by face-to-face patient assessment or telephone interviews in 25 studies,9,21,24,61,69,81,119,122,128,132–134,137–139,144,149–152,155,156,161,164,165 by medical records in 15 studies,23,62,87,120,126,127,129,130,130,135,136,140,143,145,157,159 by mixed methods involving face-to-face assessment plus another method (i.e. telephone interviews, medical records, letters) in 11 studies,65,131,146–148,153,154,158,160,162,163 and was not reported in two studies. 141,142
Main findings
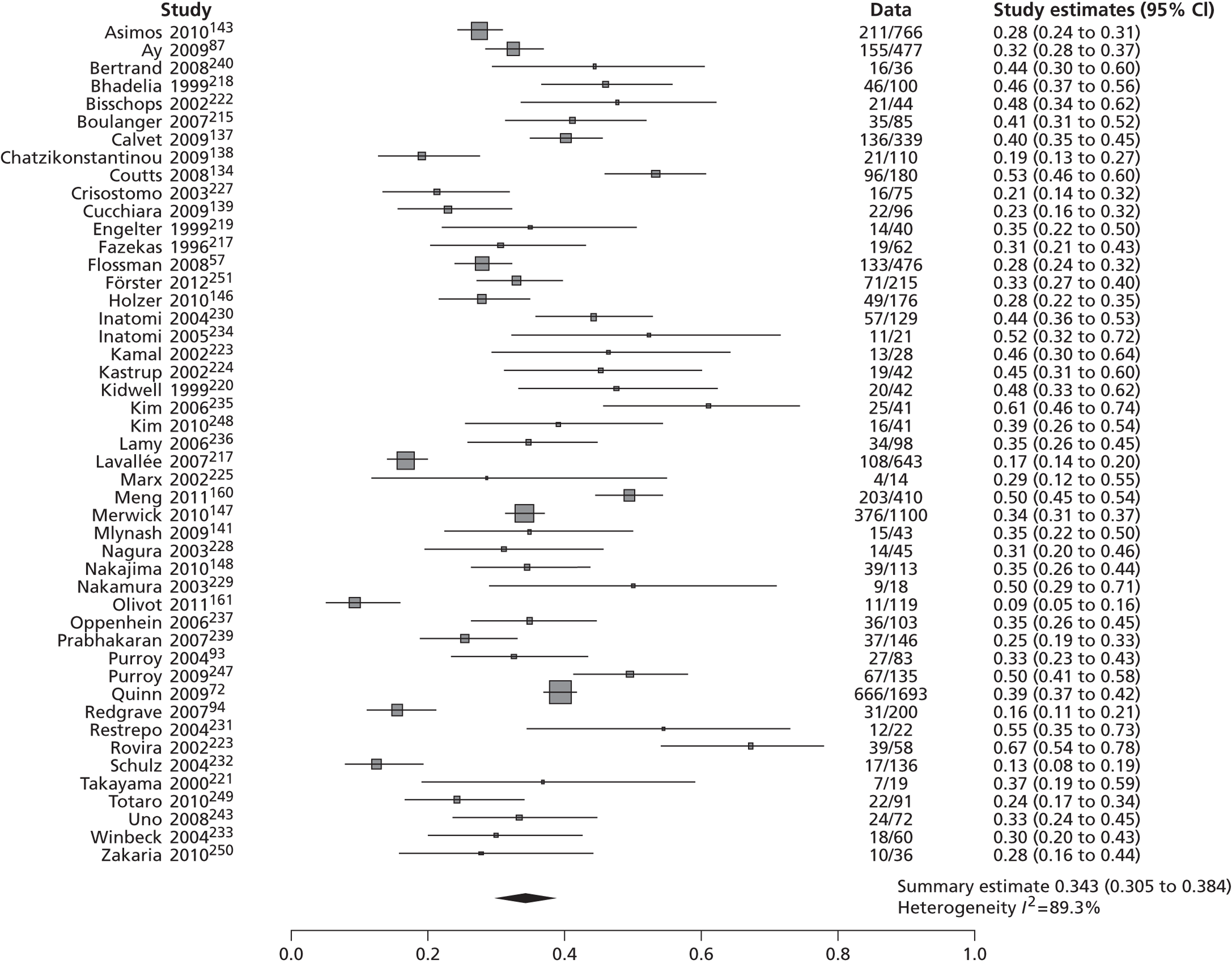
Table 2 shows the proportion of patients with stroke recurrence reported in all studies at different time points. The number of events was 974 out of 12,332 patients for studies reporting stroke risk at 7 days, 1567 out of 19,803 patients for risk at 90 days, and 927 out of 8699 patients for risk > 90 days. The risk of stroke reported across studies ranged from 0.0% to 22.4% at 7 days, 0.6% to 23.7% at 90 days, and 4.7% to 27% at > 90 days. There was evidence of significant heterogeneity between studies; the I2-statistics were 96.4%, 96.3%, and 97.8% for stroke risk at 7, 90 and > 90 days, respectively. Using random-effects meta-analysis, the pooled risk of stroke was 5.2% (95% CI 3.9% to 5.9%) at 7 days, and 6.7% (95% CI 5.2% to 8.7%) at 90 days. For stroke risk at > 90 days the pooled estimate was 11.3% (95% CI 7.5% to 16.6%). Figures 6–8 show the forest plots for studies reporting the proportion of patients with stroke recurrence at 7, 90 and > 90 days after index TIA.
| Study ID | Size | Setting | Study design | Percentage of stroke recurrence (n) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 7 days | 90 days | > 90 days | |||||||
| Lovett and colleagues, 200321 | 209 | Population based | Prospective | 12.0 | (25) | ||||
| Lisabeth and colleagues, 2004126 | 612 | Population based | Prospective | 1.9 | (12) | 4.0 | (25) | ||
| Hill and colleagues, 2004127 | 2285 | Population based | Retrospective | 9.5 | (217) | 15.1 | (346) | ||
| Coull and colleagues, 20049 | 87 | Population based | Prospective (OXVASC) | 17.2 | (15) | ||||
| Eliasziw and colleagues, 2004128 | 603 | NASCET | Prospective | 20.1 | (121) | ||||
| Gladstone and colleagues, 2004129 | 265 | ED | Prospective | 3.8 | (10) | 6.4 | (17) | ||
| Kleindorfer and colleagues, 200523 | 927 | ED | Retrospective | 14.6 | (135) | ||||
| Rothwell and colleagues, 200561 | 375 | Population based | Prospective (OXVASC) | 5.3 | (20) | ||||
| 206 | Prospective (TIA clinic) | 6.8 | (14) | ||||||
| Whitehead and colleagues, 2005130 | 121 | TIA clinics | Retrospective | 5.8 | (7) | ||||
| Van Wijk and colleagues, 200565 | 2447 | Hospital | Prospective | 4.7 | (115) | ||||
| Correia and colleagues, 2006119 | 141 | Population based | Prospective | 12.8 | (18) | ||||
| Bray and colleagues, 2007131 | 98 | ED | Retrospective | 4.0 | (4) | 7.1 | (7) | ||
| Johnston and colleagues, 200762 | 962 | ED | Retrospective (California) | 3.0 | (29) | 5.8 | (56) | ||
| 315 | TIA clinic | Prospective (Oxford) | 5.4 | (17) | 7.0 | (22) | |||
| Koton and colleagues, 2007132 | 69 | Population based | Prospective (OXVASC) | 8.7 | (6) | ||||
| Lavallée and colleagues, 2007122 | 770 | Specialist unit | Prospective | 1.7 | (13) | ||||
| Rothwell and colleagues, 200724 | 485 | TIA clinic | Prospective (OXVASC) | 8.2 | (40) | ||||
| Wu and colleagues, 2007120 | 639 | Hospital | Retrospective | 7.0 | (45) | ||||
| Atanassova and colleagues, 2008133 | 89 | Hospital | Prospective | 14.6 | (13) | ||||
| aCoutts and colleagues, 2008134 | 180 | ED | Prospective | 11.1 | (20) | ||||
| aJosephson and colleagues, 2008135 | 642 | ED | Retrospective (California) | 23.7 | (152) | ||||
| Ois and colleagues, 200881 | 221 | Hospital | Prospective | 19.0 | (42) | ||||
| Sciolla and Melis, 200869 | 274 | ED | Prospective | 3.6 | (10) | 5.5 | (15) | ||
| Asimos and colleagues, 2009136 | 944 | ED | Prospective (North Carolina) | 4.3 | (41) | ||||
| Ay and colleagues, 200987 | 479 | ED | Retrospective (Boston) | 5.2 | (25) | ||||
| Calvet and colleagues, 2009137 | 343 | Specialist unit | Retrospective | 1.5 | (5) | 3.0 | (10) | ||
| Chatzikonstantinou and colleagues, 2009138 | 122 | Specialist unit | Prospective | 9.0 | (11) | ||||
| Cucchiara and colleagues, 2009139 | 167 | Specialist unit | Prospective | 2.4 | (4) | 3.0 | (5) | ||
| aFothergill and colleagues, 2009140 | 284 | Population based | Retrospective | 12.7 | (36) | ||||
| Mlynash and colleagues, 2009141 | 97 | Specialist unit | Prospective | 0.0 | (0) | ||||
| Tan and colleagues, 2009142 | 136 | Hospital | Prospective | 11.8 | (16) | ||||
| Asimos and colleagues, 2010143 | 1667 | ED | Prospective (North Carolina) | 22.4 | (373) | ||||
| Chandratheva and colleagues, 2010144 | 500 | Population based | Prospective (OXVASC) | 10.0 | (50) | ||||
| Harrison and colleagues, 2010145 | 795 | TIA clinic | Retrospective | 17.4 | (138) | ||||
| Holzer and colleagues, 2010146 | 173 | Specialist unit | Retrospective | 5.2 | (9) | ||||
| Merwick and colleagues, 2010147 | 1232 | Population based | Retrospective (OXVASC and Dublin) | 7.5 | (92) | 12.0 | (139) | ||
| Nakajima and colleagues, 2010148 | 113 | Hospital | Prospective | 8.0 | (9) | 10.6 | (12) | ||
| Ong and colleagues, 2010149 | 470 | ED | Retrospective | 18.7 | (88) | 22.1 | (104) | ||
| Purroy and colleagues, 2010150 | 310 | Specialist unit | Prospective | 5.8 | (18) | 7.8 | (24) | ||
| Sheehan and colleagues, 2010151 | 443 | Population based | Prospective (Dublin) | 3.4 | (15) | 7.5 | (33) | ||
| Tsivgoulis and colleagues, 2010152 | 148 | Hospital | Prospective | 8.1 | (12) | 16.2 | (24) | ||
| Wasserman and colleagues, 2010153 | 982 | ED | Prospective | 3.2 | (31) | ||||
| Weimar and colleagues, 2010154 | 1897 | Specialist unit | Prospective | 5.6 | (107) | ||||
| Yang and colleagues, 2010155 | 490 | Hospital | Retrospective | 16.0 | (76) | ||||
| Amort and colleagues, 2011156 | 248 | ED | Prospective | 5.2 | (13) | ||||
| Arsava and colleagues, 2011157 | 257 | ED | Retrospective (Boston) | 6.2 | (16) | ||||
| Bonifati and colleagues, 2011158 | 502 | ED | Retrospective | 2.2 | (11) | 4.0 | (20) | ||
| Kim and colleagues, 2011159 | 475 | ED | Retrospective (California) | 5.3 | (25) | 9.9 | (47) | ||
| Meng and colleagues, 2011160 | 410 | Hospital | Prospective | 27.0 | (111) | ||||
| Olivot and colleagues, 2011161 | 157 | ED | Prospective | 0.6 | (1) | 0.6 | (1) | ||
| Sanders and colleagues, 2011162 | 289 | Hospital | Retrospective | 2.4 | (7) | ||||
| Stead and colleagues, 2011163 | 622 | ED | Retrospective | 2.4 | (15) | ||||
| bAmarenco and colleagues, 2012164 | 1679 | Specialist unit | Prospective (SOS-TIA) | 2.0 | (34) | ||||
| Purroy and colleagues, 2012165 | 1105 | Specialist unit | Prospective | 2.6 | (29) | 3.9 | (43) | ||
FIGURE 6.
Forest plot for studies reporting stroke recurrence at 7 days after index TIA. The Johnston paper62 reports results for two cohorts: (a) the California clinic and (b) the Oxford clinic. The Rothwell paper61 also reports results for two cohorts: (a) the OXVASC cohort and (b) the hospital clinic.
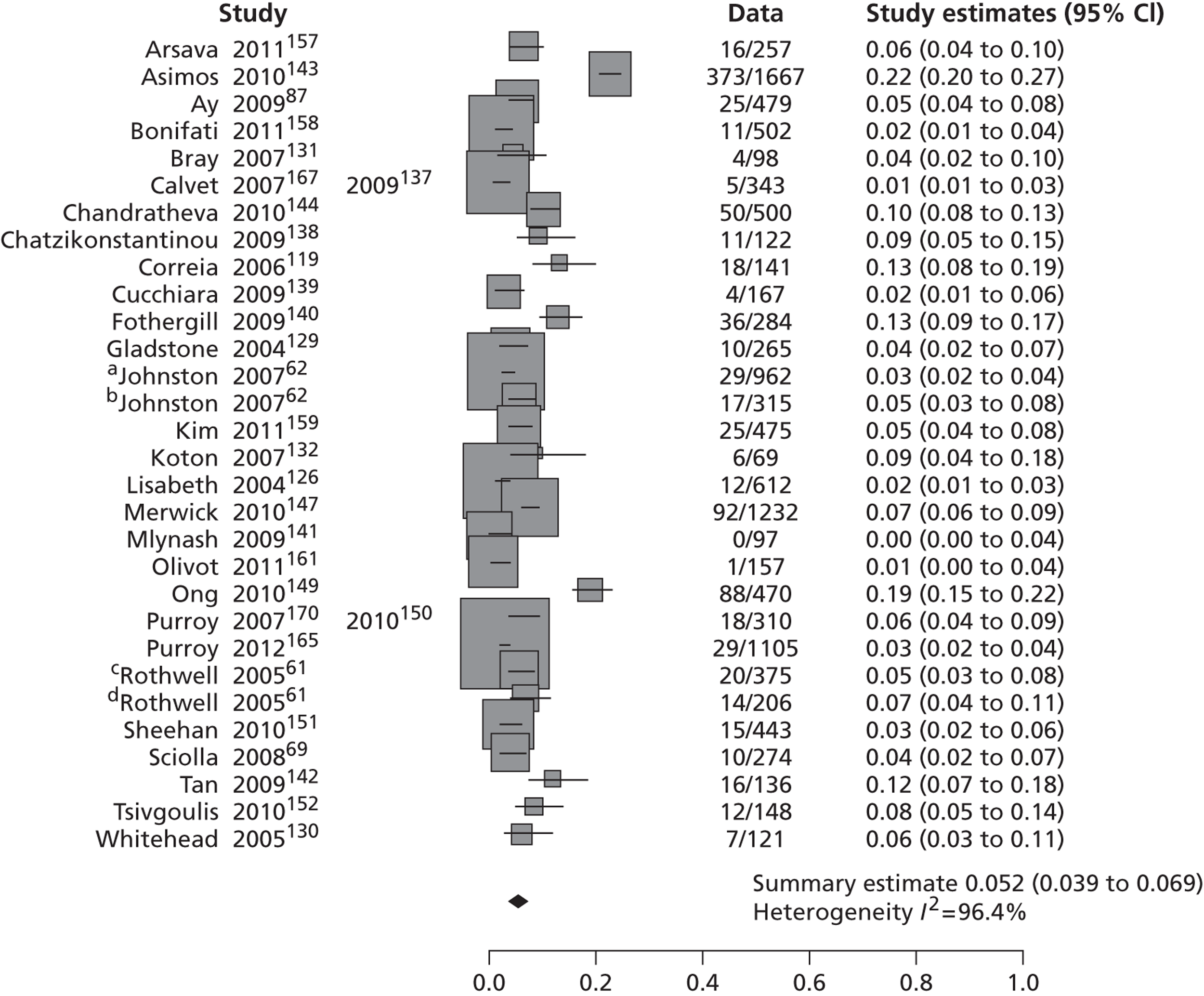
FIGURE 7.
Forest plot for studies reporting stroke recurrence at 90 days after index TIA. The Johnston paper62 reports results for two cohorts: (a) the California clinic and (b) the Oxford clinic.
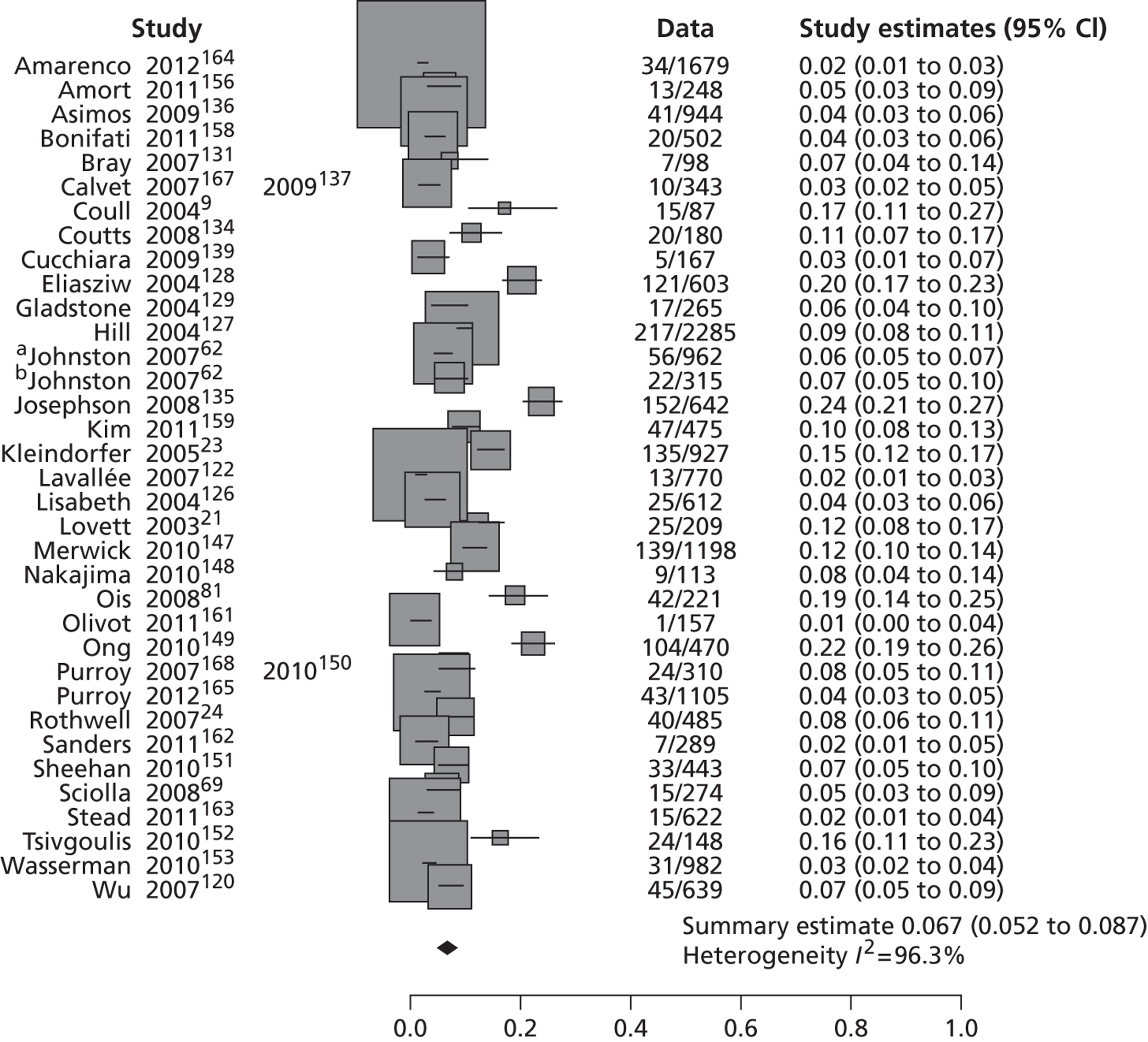
FIGURE 8.
Forest plot for studies reporting stroke recurrence beyond 90 days after index TIA.
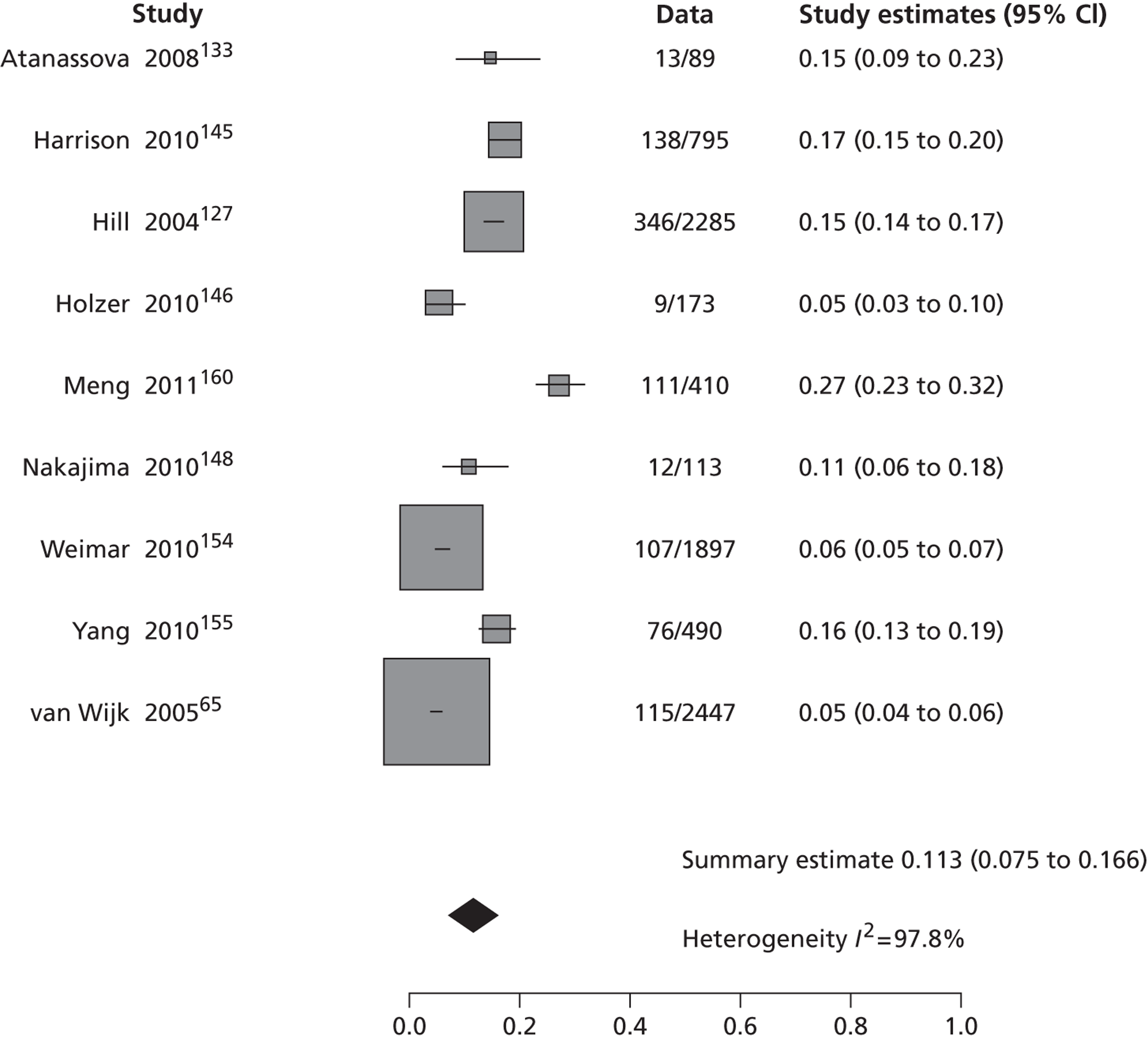
Discussion
We identified 53 studies with a total of 30,558 participants, published between 2003 and 2011, providing data on stroke risk at 7 days, 90 days or > 90 days after TIA. The rate of recurrent stroke at all time points assessed varied widely from 0% to 22.4% at 7 days, 0.6% to 23.7% at 90 days, and 4.7% to 27.0% at > 90 days. The random-effects meta-analysis showed the pooled risk of recurrent stroke at 7 days to be 5.2% (95% CI 3.9% to 5.9%), at 90 days to be 6.7% (95% CI 5.2% to 8.7%) and at > 90 days to be 11.3% (95% CI 7.5% to 16.6%). The heterogeneity between studies was huge. In some individual studies the rate was up to three times higher than the median rate and up to 4–4.5 times higher than the pooled risk rate. However, this summary of all available data indicates that the pooled risk of stroke after TIA is one-quarter of that stated in the current NICE guidance, which states in the Guide to Implementing the National Stroke Strategy Guide, Imaging for TIA, p. 8, that ‘Approximately 20% of TIAs will be followed by a stroke within four weeks’. 97 The upper 95% CI of our pooled risk barely passes one-quarter of that value. The few individual studies that reported such high rates were all very small.
Two systematic reviews of the risk of stroke after TIA120,121 and one systematic review of the risk of recurrent stroke after first-ever stroke169 have been published in 2007 and 2011, respectively. The review by Giles and Rothwell121 focused on stroke risk within 7 days of TIA and included 18 independent cohorts with 10,126 patients. The pooled stroke risk was 5.2% (95% CI 3.9% to 6.5%) at 7 days, ranging from 0.0% to 12.8%, and with substantial between study heterogeneity. The heterogeneity was largely explained by setting, with the lowest rates in emergency settings with urgent treatment and highest in population-based studies without urgent treatment. In the same year, Wu and colleagues120 published a systematic review focusing on stroke risk at 2, 30 and 90 days, and included 11 studies with 7238 patients. The pooled risk for all studies was 3.5%, 8.0% and 9.2% at 2, 30 and 90 days, respectively. The pooled risk for studies with active prospective case ascertainment was 9.9%, 13.4% and 17.3% at 2, 30 and 90 days, respectively. Despite both addressing the same topic, 11/18 studies included in Giles and Rothwell121 were not included in Wu and colleagues,120 and 5/11 studies in Wu and colleagues120 were not included in the Giles and Rothwell study,121 some because stroke rates were not reported at the precisely required time, but this did not account for all omissions. Mohan and colleagues169 published a review of 13 studies of 9115 patients published between 1961 and 2006 focusing on stroke recurrence after first-ever stroke (not TIA) showing a pooled risk of 3.1% (95% CI 1.7% to 4.4%) at 30 days and 11.1% (95% CI 9.0% to 13.3%) at 1 year, also with substantial heterogeneity between studies.
There has been considerable interest in the rate of stroke after TIA since the mid-2000s but this alone cannot account for the large difference between these earlier reviews of stroke risk after TIA and our study in the number of included studies or patients, our review including nearly treble the number of studies (53) and patients (30,558). Twenty-eight studies alone reported stroke risk at 7 days in 12,332 patients. We may inadvertently have included some of the same patients more than once as many of these studies come from the same few research groups and it was difficult to disentangle some of these studies. Substantial proportions of these studies were based in EDs (17, 32%) and, although details were a bit short in some studies, many of the rest could be considered to be recruiting in a rapid-access fast-response environment, and 33/53 were prospective, and 45/53 studies included consecutive patients. Our pooled risk rate is similar to that of Giles and Rothwell121 and midway between the estimates provided by Wu and colleagues. 120 Differences in the speed of assessment may account for differences in risk rates between studies, but many other factors are possible. For example, few studies reported on how patients were treated and how those with tight symptomatic carotid stenosis were managed. Presumably they were offered early endarterectomy, removing much of their stroke risk. Presumably many patients were offered immediate medical treatment, in many cases started by their family doctor prior to arriving at the specialist clinic or hospital ED. This practice might have increased since the publication of the Early use of eXisting PREventive Strategies for Stroke (EXPRESS) study in 200624 and might explain why some newer studies had lower stroke rates. With a few exceptions, these points are largely not mentioned. Regardless of that, lower rates were also observed in many older studies and therefore the real differences between studies are likely to be more complex than simply variation in rapidity of referral or early treatment.
Given the large variation in stroke risk between studies, and not having any good reasons to choose one group of studies over another, and, as the calculated pooled risk at each time point had pretty tight CIs indicating some reliability even if there is substantial heterogeneity, we will use the pooled risk rates at 7 days (28 studies, 12,332 patients), 90 days (34 studies; 19,769 patients) and > 90 days (nine studies; 8669 patients) as the most reliable base estimates of stroke risk in the present work.
Weaknesses of the review
Overall, our findings are limited by the great variation between included studies, especially in terms of study design, time of assessment from symptoms onset, type of TIA considered, and evaluating clinicians. As there is no a validated instrument for the quality assessment of observational studies on stroke risk, we pre-defined the methodological aspects that we considered more likely to represent potential sources of bias. The results of the quality assessment were used in only a descriptive way and not to inform further statistical analyses (e.g. meta-regression).
With regard to our literature searches, we focused mainly on two major electronic databases (MEDLINE and EMBASE). We did not search additional electronic databases, such as the Bioscience Information Service (BIOSIS), the Latin American and Caribbean Health Sciences Literature (LILACS) or the Science Citation Index (SCI), first because the number and relevance of indexed journals in these databases is limited compared with those indexed in MEDLINE and EMBASE, and, second, we are confident we have enhanced the overall sensitivity of our literature searches by hand-searching all recent conference proceedings of two major international stroke conferences, contacting experts in the field and perusing the reference lists of all relevant selected studies. Owing to time and resources constraints, we avoid searching grey literature sources, which are known to be time consuming and not particularly well developed.
Chapter 4 ABCD2 score and risk of stroke after transient ischaemic attack and minor stroke
Introduction
The incidence of TIA has been estimated as ranging from 20,000 to 90,000 in the UK,9,173 and from 200,000 to 500,000 in the USA. 169,170 The risk of recurrent stroke after TIA is high, especially during the first week after the index event (see Chapters 1 and 3). 121 The benefit of medical therapy to prevent recurrent stroke after TIA is greatest if given as early as possible after TIA. 24,122,153 Likewise, the benefit of endarterectomy for symptomatic carotid stenosis is highest when performed within 2 weeks after the index event and falls rapidly with increasing delay. 171 A deferred intervention would be futile, or at least of much reduced benefit, as the high-risk period without medical or surgical intervention will have passed. 10 Consequently, patients with TIA need a rapid comprehensive assessment to reduce the short- and long-term risks of recurrent stroke and other vascular conditions.
However, delays between the onset of symptoms and specialist assessment and treatment have previously been reported in the UK172,173 and are confirmed in a survey conducted for this report (see Chapter 8). These delays would decrease the proportion of patients started on appropriate secondary prevention treatment soon after the index event and before disabling stroke occurs. At this point, the use of a clinical score to predict the early risk of recurrent stroke after TIA emerges as a reasonable strategy for identifying patients with the highest stroke risk, in whom a more urgent clinical approach is needed. Assuming that a clinical score correctly predicts the early stroke recurrence after TIA, its use would allow efforts and resources to be concentrated on those with a higher risk, reducing the incidence of stroke in this population.
Numerous clinical risk prediction scores have been developed all aiming to do the same thing – identify patients at high risk of stroke to prioritise services. However, the ABCD2 and related scores have achieved particular prominence such that the ABCD2 score is recommended for use in UK stroke prevention services67 to triage the speed at which patients pass through clinics and access services. Hence, some detail is worthwhile here as to how it initially developed and has evolved.
A northern California study8 demonstrated that simple clinical variables were associated with the risk of stroke at 90 days in patients with a diagnosis of TIA who were initially admitted to an ED. Age, presence of diabetes mellitus, duration of episode > 10 minutes, weakness and speech impairment during episode were independently associated with the risk of stroke after TIA; the estimated risk of further stroke was 34% in patients presenting with all five predictors. In addition, a population-based study127 conducted in Canada showed that age, and diabetes mellitus together with hypertension, were associated with a higher risk of stroke 1 year after TIA. Subsequently, the ABCD score was created to predict the stroke risk during the first week after TIA using those clinical variables that have been independent predictors of stroke. 61 Derived from the OCSP population and validated in the OXVASC and local TIA clinic populations, the score showed a fair accuracy in the prediction of stroke risk at 7 days in these patients. A further validation of the ABCD and California scores showed that both had a fair accuracy in the prediction of early recurrent stroke at 2, 7 and 90 days. 62 By combining the components of these two scores, the authors generated a unified prediction tool, the ABCD2 score, in 2007 (Table 3). 62
| Age (years) | |
| > 60 | 1 |
| BP (mmHg) | |
| SBP > 140 or DBP > 90 | 1 |
| Clinical features | |
| Unilateral weakness | 2 |
| Speech disturbance without weakness | 1 |
| Duration of symptoms (minutes) | |
| ≥ 60 | 2 |
| 10–59 | 1 |
| Diabetes | 1 |
The ABCD2 score includes age, blood pressure elevation on first assessment after TIA, unilateral weakness, speech disturbance, duration of symptoms and diabetes mellitus as clinical variables, and predicted the early risk of stroke in the California and Oxford cohorts at 2, 7 and 90 days. The score classifies TIA or minor stroke patients at low, moderate or high risk using cut-off points of < 4, 4–5 and > 5. Recently, the ABCD2 risk prediction score has been adopted by many stroke prevention services in the UK as part of NICE guidance67 to prioritise patients who need urgent treatment. Stratification strategies to effectively prioritise TIA patients have become essential because (1) it is very challenging to assess every TIA/minor stroke patient within 24 hours of symptoms onset and (2) approximately half of the patients referred to stroke prevention services ultimately do not have a diagnosis of TIA or minor stroke (see Chapters 7 and 8). For these reasons, recent clinical guidelines have recommended the use of the ABCD2 score as a tool to select patients for whom an urgent management is required. 67 However, the ability of ABCD2 score to distinguish between TIA and non-TIA/mimics and to predict risk of stroke early after TIA has been questioned. Some independent validation studies of ABCD and ABCD2 scores have shown conflicting results in their discriminatory ability to predict stroke,69,70,151,152 and, in particular, a number of studies have suggested that high ABCD2 scores do not reliably identify patients with high stroke risk conditions, such as the presence of severe carotid stenosis. 132,170,174
The ABCD/ABCD2 scores have spawned numerous other variants (ABCD2-I, ABCD3, ABCD3-I),147,150 which add either more clinical or brain or carotid imaging variables, most of which have not been tested independently. Numerous variations may be causing confusion. For example, there is no consensus on the best ABCD2 cut-off for stratifying the risk of stroke and select patients for whom an urgent assessment is needed. Clinical guidelines issued by the AHA, for example, advocate admission to hospital and early assessment/treatment for patients with an ABCD2 score of ≥ 3,17 whereas national guidelines in the UK recommended a specialist assessment and investigation within 24 hours of symptoms for patients with an ABCD2 score of ≥ 4 and within 1 week for those patients with an ABCD2 score of < 4. 67
The aim of this systematic review was to evaluate all existing evidence on the performance of the ABCD2 prediction score to predict stroke recurrence in patients at high risk (score ≥ 4) and low risk (score of < 4) of stroke, i.e. at cut-off points used in current clinical guidelines. 67
Methods
Objectives
We followed the PRISMA guidelines for systematic reviews of observational studies to conduct this review. 175 We aimed to identify all published studies in which ABCD2 score was used to predict risk of stroke among patients with TIA and/or minor stroke irrespective of the clinical setting or type of study design and that reported on the actual rate of recurrent stroke.
Identification of studies
We searched indexed records which appeared in MEDLINE (Ovid) from January 2005 to November 2011. The choice of this time period reflected the introduction of the ABCD prediction score into clinical practice (even although the ABCD2 score was published for the first time in 2007, we adopted an over-inclusive approach and searched the literature from 2005 when the ABCD clinical prediction score was originally developed). The MEDLINE search strategy included both subject headings (MeSH terms) and text words for the target condition (e.g. stroke, TIA, minor stroke) and the prediction score under consideration (ABCD). We did not apply any language restrictions. We adapted the MEDLINE search to search EMBASE. In particular, we ‘translated’ the MEDLINE MeSH terms into the corresponding terms available in the Emtree vocabulary. The searches were initially run in November 2010, and updated in November 2011. Full details of both the MEDLINE and the EMBASE search strategies are presented in Appendix 1. We imported all citations identified by the MEDLINE and EMBASE search strategies into the Reference Manager bibliographic database. We hand-searched all proceedings of the International Stroke Conference (2011) and the European Stroke Conference (2011, 2012). We also contacted experts in the field and perused the reference lists of all relevant articles to identify further published studies for possible inclusion in the review. Only full-text articles were deemed suitable for inclusion.
Inclusion/exclusion criteria
One review author (MB) examined the identified titles and abstracts, and retrieved all potentially relevant citations in full. Full-text articles were retained if they studied the application of the ABCD2 score in cohorts of TIA and minor stroke patients and reported early risk of stroke assessed at 7, 90 and > 90 days. Studies that did not consider the incidence of further stroke in TIA/minor stroke patients; assess patients by means of the ABCD2 score; classify patients at high and low risk according to the cut-off score of 4; or report original data were excluded.
Quality assessment and data extraction
Two review authors (MB, HM) independently conducted data extraction and review the methodological quality of selected studies. Disagreements were resolved by discussion or referred to a third author (JMW) if necessary. We recorded data on study methods (e.g. setting, study design) characteristics of patients (first vs. recurrent TIA) and outcomes (stroke events at 7, 90 and > 90 days). We also collected information on the following methodological aspects, which we considered more likely to introduce potential biases: prospective compared with retrospective study design, data source for cohort identification (e.g. registries, databases, medical notes), patients’ selection criteria, spectrum of disease severity, definition of TIA, timing of clinical assessment, evaluating clinicians, and method of outcome ascertainment (active vs. passive).
Data synthesis
For each study we calculated the total number of patients with an ABCD2 score of > 4 and < 4, and the proportion of patients in each ABCD2 dichotomised category suffering a recurrent stroke at 7, 90 and > 90 days. The pooled risks of stroke for ABCD2 score of > 4 and < 4 at 7, 90 and > 90 days were calculated by means of univariate random-effects meta-analyses with within-study variance modelled as binomial. The discriminative ability of the ABCD2 cut-off score of 4 for stroke risk at 7, 90 and > 90 days was evaluated either by bivariate ROC curve random-effects meta-analyses or by random-effects univariate meta-analyses. We analysed all studies regardless of whether they reported recurrent stroke at only 7 or 90 days, and also restricted the analysis to just those studies that reported recurrent stroke at both 7 and 90 days, the latter to reduce the impact of some methodological differences between studies. We calculated estimates of sensitivity, specificity, positive and negative predictive values for a hypothetical cohort of 1000 TIA patients and expressed the proportion with recurrent stroke per 1000 patients assessed to improve the clinical relevance of the results. We used the statistical software R version 2.14.2 for our analyses.
Results
Number of included/excluded studies
The electronic searches identified 3406 citations. Of these, we considered 59 reports to be potentially relevant and retrieved the full-text articles for detailed assessment. Hand-searching of reference lists and of recent issues of Stroke journal (not yet indexed in MEDLINE) resulted in a further two reports to be included. We subsequently excluded 33 articles. The most common reason for exclusion was that the study did not provide data in sufficient detail to allow calculation of the ABCD2 score for above and below 4. Twenty-six studies published in 28 reports fulfilled our inclusions criteria (Figure 9). Thirteen were observational prospective cohort studies and 13 were observational retrospective cohort studies. The included studies ranged in size from 69 to 1679 patients, and the total number of TIA/minor stroke patients assessed was 12,586.
FIGURE 9.
Identification and selection of studies.
Characteristics of included studies
The methodological characteristics of the individual studies are shown in Table 4.
| Study ID | Setting | Study design | TIA definition | Time from symptoms onset | Evaluating clinician | Wide spectrum of patients | Method of ascertainment | ABCD2 score derivation | Secondary prevention drugs |
|---|---|---|---|---|---|---|---|---|---|
| Johnston and colleagues, 200762 | Specialist unit | Retrospective | Time-based definition | Assessed ‘as soon as possible after event’ | Neurologist | Yes | In person | Retrospective notes review | NR |
| Koton and Rothwell, 2007132 | Population based | Prospective | Time-based definition | Assessed ‘as soon as possible after event’ | Neurologist | No | In person | Patient assessment | NR |
| Coutts and colleagues, 2008134 | ED | Prospective | Time-based criteria | Within 24 hours | Neurologist | No | In person | Patient assessment | NR |
| Josephson and colleagues, 2008135 | ED | Retrospective | NR | NR | Emergency medicine physician | Yes | Medical records | Retrospective notes review | NR |
| Ay and colleagues, 200987 | ED | Retrospective | Time-based criteria | Within 24 hours | Neurologist | No | Medical records | Retrospective notes review | NR |
| Calvet and colleagues, 2009137 | Specialist unit | Retrospective | Time-based criteria | Within 48 hours | Neurologist | Yes | In person, telephone interviews | Patient assessment | NR |
| Chatzikonstantinou and colleagues, 2009138 | Specialist unit | Prospective | Time-based criteria | Within 24 hours | NR | Yes | In person | Patient assessment | NR |
| Cucchiara and colleagues, 2009139 | Specialist unit | Prospective | Time-based criteria | Within 48 hours | Neurologist | No | In person, telephone interviews | Patient assessment | NR |
| Fothergill and colleagues, 2009140 | Population based | Retrospective | Time-based criteria | Within 72 hours | Neurologist | No | Medical records | Retrospective notes review | NR |
| Purroy and colleagues, 2010150 | Specialist unit | Prospective | Time-based criteria | Within 48 hours | Neurologist | Yes | In person | Patient assessment | NR |
| Tan and colleagues, 2009142 | Hospital | Prospective | Time-based definition | Within 48 hours | Neurologist | NR | ‘Patients were followed up to document subsequent stroke’ | Patient assessment | NR |
| Asimos and colleagues, 2010143 Asimos and colleagues, 2009136 |
ED | Retrospective | Time-based criteria | Within 24 hours | Emergency medicine physician | No | Medical records | Retrospective notes review | Yes, 49% under antithrombotic therapy |
| Chandratheva and colleagues, 2010144 | Population based | Retrospective | Time-based criteria | Assessed ‘as soon as possible’ after event | Neurologist | No | In person | Patient assessment | NR |
| Holzer and colleagues, 2010146 | Specialist unit | Retrospective | Time-based criteria | NR | Neurologist | No | Telephone interviews, medical records | Retrospective notes review | NR |
| Harrison and colleagues, 2010145 | Specialist unit | Retrospective | Time-based criteria | Median 15 days | Stroke physician | Yes | Medical records | Retrospective notes review | NR |
| Ong and colleagues, 2010149 | ED | Retrospective | Time-based definition | Unclear | Emergency medicine physician | No | In person | Retrospective notes review | Yes, 15% under aspirin |
| Sheehan and colleagues, 2010151 | Population based | Prospective | Time-based definition | Within 24 hours | Stroke physician | No | In person | Patient assessment | NR |
| Tsivgoulis and colleagues, 2010152 | Specialist units | Prospective | Time-based definition | Within 24 hours | Neurologist | Yes | In person (no patients were lost at follow-up) | Patient assessment | Yes, 46% under antithrombotic therapy |
| Wasserman and colleagues, 2010153 | ED | Prospective | NR | Within 7 days | Emergency medicine physician | No | Standardised telephone questionnaire, medical records | Patient assessment | Yes, 45% under antithrombotic therapy |
| Yang and colleagues, 2010155 | Hospital | Retrospective | NR | NR | NR | Unclear | In person or telephone interviews | Retrospective notes review Prospectively calculated from hospital records and stroke registry | NR |
| Bonifati and colleagues, 2011158 | ED | Retrospective | Time-based criteria | NR | Neurologist + emergency medicine physician | Yes | Telephone interviews, medical records | Retrospective notes review | Yes, 49% under antithrombotic therapy |
| Meng and colleagues, 2011160 | Hospital | Prospective | Time-based definition | Within 7 days | Neurologist | No | In person or medical records | Patient assessment | NR |
| Sanders and colleagues, 2011162 | ED | Prospective | Time-based definition | Unclear | Emergency medicine physician | No | In person, telephone interviews (complete follow-up in 96% of patients) | Patient assessment | Yes, 56% under antithrombotic therapy |
| Stead and colleagues, 2011163 | ED | Retrospective | Time-based definition | NR | Emergency medicine physician plus neurologist | Unclear | Telephone interviews (complete follow-up in 95.4% of patients) | Retrospective assessment of medical notes | NR |
| Amarenco and colleagues, 2012164 | Specialist unit | Prospective | Time-based criteria | NR | Neurologist | Yes | In person, telephone interviews | Patient assessment | NR |
| Capone and colleagues, 2012177 | Specialist unit | Prospective | Time-based criteria | Within 24 hours | Neurologist | No | Unclear | Patient assessment | NR |
All studies used a time-based definition of TIA as opposed to the recently new proposed tissue-based definition. 176
Four studies assessed population-based cohorts,132,140,144,151 three studies hospital-based cohorts,142,155,160 nine studies patients from EDs,87,134,135,143,149,153,158,162,163 and 10 studies patients from specialist stroke or neurology units. 62,137–139,145,146,150,152,164,177
In 15 studies the ABCD2 score was derived directly by patient assessment, whereas in the remaining 11 studies it was retrospectively calculated from medical notes.
Timing of patient assessment after symptom onset varied across studies. In seven studies ,TIA patients were assessed within 24 hours of symptoms onset, in four studies within 48 hours, in one study within 72 hours, and in two studies within 7 days. Another three studies reported that ‘patients were assessed as soon as possible after the event’ but did not give a time, and another study stated that the median time from symptoms onset was 15 days. Eight studies did not clearly provide this information (see Table 4).
Most of the studies included only patients with a definite or confirmed TIA by a neurologist/stroke physician and excluded TIA mimics. TIA diagnosis was made by a neurologist in 14 studies, by an emergency medicine physician in six studies, initially by an emergency medicine physician and subsequently confirmed by a neurologist in two studies and by a stroke physician in another two studies; The status of the diagnosing physician was not reported in the remaining two studies.
Little information was available on patients with carotid stenosis or use of endarterectomy. Similarly, information on how many TIA patients received secondary prevention drugs was rarely reported in the included studies. Only five studies stated that approximately 45–50% of TIA patients were administered antithrombotic therapy,143,152,153,158,162 and another study that 15% of patients received aspirin. 149
Ascertainment of stroke events after TIA was carried out by face-to-face patient assessment or telephone interviews in 17 studies, by medical records in five studies, by mixed methods (i.e. both telephone interviews and medical records) in three studies, and was not clearly reported in one study (see Table 4). Six cohorts reported only stroke events at 7 days,87,132,138,140,142,144 seven studies at 90 days,134,135,145,153,162,164,177 10 studies reported stroke events both at 7 and 90 days,62,137,139,143,149–152,158,163 and three studies reported stroke events at > 90 days. 146,155,160
Main findings
Considering all 16 studies that reported stroke events at 7 days, the total number of TIA patients with an ABCD2 score of ≥ 4 was 4590/6920 (66%) and the total number of recurrent strokes at 7 days was 488. In contrast, the total number of TIA patients with an ABCD2 score of < 4 was 2330/6920 (34%) and the total number of recurrent strokes at 7 days was 76.
In the 19 studies reporting stroke events at 90 days, the total number of TIA patients with an ABCD2 score of ≥ 4 was 6294/9849 (64%), and the total number of recurrent strokes at 90 days was 544 (Table 5). In contrast, the total number of TIA patients with an ABCD2 score of < 4 was 3555/9849 (36%) and the total number of recurrent strokes at 90 days was 80.
| ABCD2 score of ≥ 4 | ABCD2 score of < 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study ID | No. of patients | No. of events | Stroke rate | No. of patients | No. of events | Stroke rate | ||||
| 7 days (%) | 90 days (%) | > 90 days (%) | 7 days | 90 days (%) | > 90 days (%) | |||||
| Amarenco and colleagues, 2012164 Same population as in Lavallée and colleagues, 2007122 |
701 | 24 | 3.4 | 978 | 11 | 1.1 | ||||
| Asimos and colleagues, 2010143 | 823 | 191 | 23.2 | 231 | 28 | 12.1 | ||||
| Asimos and colleagues, 2009136 Same population as in Asimos and colleagues, 2010143 |
695 | 39 | 5.6 | 208 | 2 | 1.0 | ||||
| Ay and colleagues, 200987 | 315 | 21 | 6.7 | 162 | 2 | 1.2 | ||||
| Bonifati and colleagues, 2011158 | 357 | 10 | 2.8 | 145 | 1 | 0.7 | ||||
| 19 | 5.3 | 1 | 0.7 | |||||||
| Calvet and colleagues, 2009,137 2007167 | 204 | 5 | 2.5 | 139 | 0 | 0.0 | ||||
| 9 | 4.4 | 1 | 0.7 | |||||||
| Capone and colleagues, 2012177 | 6 | 0 | 0.0 | 88 | 2 | 2.3 | ||||
| Chandratheva and colleagues, 2010144 | 308 | 40 | 13.0 | 192 | 10 | 5.2 | ||||
| Chatzikonstantinou and colleagues, 2009138 | 84 | 9 | 10.7 | 33 | 2 | 6.0 | ||||
| Coutts and colleagues, 2008134 | 166 | 20 | 12.0 | 14 | 0 | 0.0 | ||||
| Cucchiara and colleagues, 2009,139 | 106 | 3 | 2.8 | 61 | 1 | 1.6 | ||||
| Cucchiara and colleagues, 200670 | 4 | 3.8 | 0 | 0.0 | ||||||
| Fothergill and colleagues, 2009140 | 208 | 31 | 15.0 | 68 | 4 | 5.9 | ||||
| Harrison and colleagues, 2010145 | 644 | 23 | 3.6 | 145 | 1 | 0.7 | ||||
| Holzer and colleagues, 2010146 | 120 | 9 | 7.5a | 53 | 0 | 0.0 | ||||
| Johnston and colleagues, 200762 | 689 | 43 | 6.2 | 588 | 3 | 0.5 | ||||
| (California and Oxford data: clinical settings)62 | 65 | 9.4 | 13 | 2.2 | ||||||
| Josephson and colleagues, 2008135 | 494 | 141 | 28.5 | 148 | 11 | 7.4 | ||||
| Koton and Rothwell, 2007132 | 69 | 6 | 8.7 | |||||||
| Meng and colleagues, 2011160 | 250 | 76 | 30.4b | 160 | 35 | 21.9b | ||||
| Ong and colleagues, 2010149 | 325 | 76 | 23.4 | 145 | 12 | 8.3 | ||||
| 90 | 27.8 | 14 | 9.7 | |||||||
| Purroy and colleagues, 2010,150 2007168 | 243 | 15 | 6.2 | 67 | 3 | 4.5 | ||||
| 20 | 8.2 | 4 | 6 | |||||||
| Sanders and colleagues, 2011162 | 208 | 5 | 2.4 | 81 | 2 | 2.5 | ||||
| Sheehan and colleagues, 2010151 | 274 | 10 | 3.6 | 169 | 5 | 2.9 | ||||
| 25 | 9.1 | 8 | 4.7 | |||||||
| Stead and colleagues, 2011163 | 444 | 4 | 0.9 | 187 | 2 | 1.0 | ||||
| 11 | 2.5 | 4 | 2.1 | |||||||
| Tan and colleagues, 2009142 | 64 | 14 | 21.9 | 72 | 1 | 1.4 | ||||
| Tsivgoulis and colleagues, 2010152 | 77 | 10 | 12.9 | 71 | 2 | 2.8 | ||||
| 21 | 27.3 | 3 | 4.2 | |||||||
| Wasserman and colleagues, 2010153 | 661 | 28 | 4.2 | 321 | 3 | 0.9 | ||||
| Yang and colleagues, 2010155 | 366 | 67 | 18.3c | 124 | 9 | 7.3c | ||||
In the three studies146,155,160 reporting stroke events at > 90 days, 736/1073 (69%) patients with an ABCD2 score of ≥ 4 experienced a total of 152 recurrent strokes. In contrast, 337/1073 (31%) TIA patients with ABCD2 score of < 4 had a total of 44 recurrent strokes.
The corresponding pooled risks of stroke for patients with ABCD2 score of > 4 and < 4 at 7, 90 and > 90 days are shown in Table 6.
| ABCD2 score | Percentage | 95% CI |
|---|---|---|
| At 7 days | ||
| ≥ 4 | 7.5 | 4.7 to 11.7 |
| < 4 | 2.4 | 1.3 to 4.2 |
| At 90 days | ||
| ≥ 4 | 7.1 | 4.6 to 10.7 |
| < 4 | 2.1 | 1.3 to 3.3 |
| At > 90 days | ||
| ≥ 4 | 17.2 | 8.8 to 30.9 |
| < 4 | 5.2 | 1.0 to 28.9 |
The pooled risks of stroke for patients with an ABCD2 score of > 4 and < 4 at 7, 90 and > 90 days for all included studies are shown in Figures 10–12.
FIGURE 10.
ABCD2 score of ≥ 4 and risk prediction at 7 days: all included studies.
FIGURE 11.
ABCD2 score of ≥ 4 and risk prediction at 90 days: all included studies.
FIGURE 12.
ABCD2 score of ≥ 4 and risk prediction at > 90 days: all included studies.
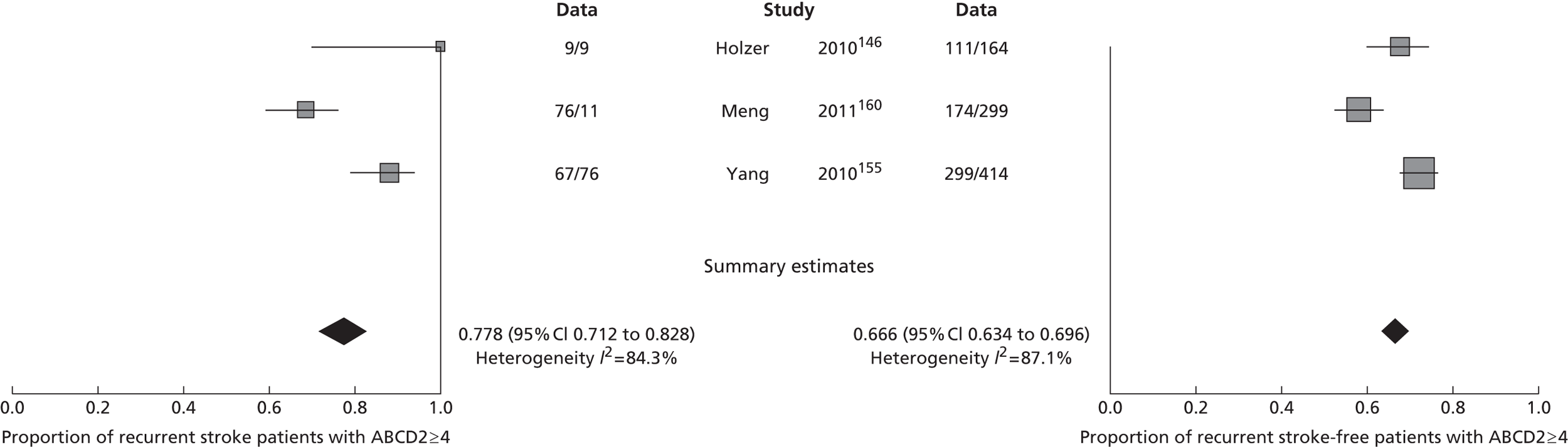
Many studies presented their results as accuracy of stroke risk prediction expressed as the area under receiver operating characteristic curve (AUROC) analyses. For completeness, we summarise the published area under the curve (AUC) values and 95% CI for stroke risk at 7 and 90 days in Table 7. We list these in order of publication year as it might be expected that patients would have been assessed more rapidly after TIA/minor stroke in the more recent studies (owing to greater awareness of the importance of seeking medical attention rapidly, better organised clinics, faster services, fewer recurrent strokes missed) and that this might have had some impact on the early stroke recurrence rate (higher rates in more recent studies) and in turn on the performance of risk prediction scores. However, there is no obvious trend in either the 7- or 90-day stroke risk or in the performance of the ABCD2 score in more recent years compared with earlier years, even allowing for the republication of some earlier data sets among later meta-analytic studies.
| Study | ABCD2 score | |||
|---|---|---|---|---|
| Stroke risk at 7 days (%) | AUC (95% CI) | Stroke risk at 90 days (%) | AUC (95% CI) | |
| OCSPa | 8.4 | 0.72 (0.60 to 0.84) | 14.3 | 0.69 (0.60 to 0.77) |
| OXVASCa | 10.6 | 0.87 (0.81 to 0.92) | 17.6 | 0.76 (0.68 to 0.85) |
| Oxford Clinica | 5.4 | 0.75 (0.68 to 0.83) | 7.0 | 0.75 (0.67 to 0.84) |
| aJohnston and colleagues, 20008 | 6.0 | 0.66 (0.62 to 0.71) | 10.5 | 0.67 (0.63 to 0.71) |
| Johnston and colleagues, 200762 | ||||
| California clinic | 3.0 | 0.75 (0.68 to 0.83) | 6.0 | 0.68 (0.61 to 0.75) |
| California ED | 7.0 | 0.63 (0.57 to 0.69) | 10 | 0.64 (0.58 to 0.69) |
| aMlynash, 2009141 | 1.0 | 0.62 (0.43 to 0.82) | 1.0 | 0.60 (0.38 to 0.81) |
| SOS-TIAa | 0.3 | 0.74 (0.57 to 0.90) | 1.2 | 0.71 (0.60 to 0.81) |
| Calvet and colleagues, 2009137 | 1.5 | 0.80a (0.62 to 0.98) | 2.9 | 0.75 (0.61 to 0.89) |
| aCucchiara and colleagues, 2009139 | 2.4 | 0.75 (0.45 to 1.00) | 3.0 | 0.71 (0.45 to 0.97) |
| Coutts and colleagues, 2008134 | 3.6a | 0.75a (0.56 to 0.94) | 11.1 | 0.78 (0.68 to 0.87) |
| Tan and colleagues, 2009142 | 11.8 | 0.76 (NR) | – | – |
| Fothergill and colleagues, 2009140 | 12.7 | 0.65 (NR) | – | – |
| aAy and colleagues, 2009136 | 4.8 | 0.66 (0.57 to 0.76) | – | – |
| Asimos and colleagues, 2010143 | 22.0 | 0.59 (0.56 to 0.62) | – | – |
| Sheehan and colleagues, 2010151 | 3.4 | 0.49 (0.35 to 0.63) | 7.5 | 0.55 (0.45 to 0.64) |
| Ong and colleagues, 2010149 | 18.7 | 0.66 (NR) | 22.1 | 0.66 (NR) |
| Arsava, 2011157 | 6.2 | 0.57 (0.45 to 0.69) | – | – |
| Tsivgoulis and colleagues, 2010152 | 8.0 | 0.72 (0.57 to 0.88) | 16.0 | 0.75 (0.65 to 0.86) |
| bSanders and colleagues, 2011162 | 1.4 | 0.80 (0.68 to 0.91) | 2.4 | 0.62 (0.40 to 0.83) |
| Purroy and colleagues, 2012165 | 2.6 | 0.56 (0.45 to 0.66) | 3.9 | 0.55 (0.46 to 0.64) |
Figures 10–12 show considerable heterogeneity between studies and wide CIs, which may reflect variation in study methods or different populations of patients contributing to estimates of recurrence at different times, as outlined in the results above, rather than true differences in stroke rates at 7 and 90 days or in times to assessment. A previous meta-analysis found that the predictive value for 7-day stroke risk was higher in studies using in-person or clinical records to calculate the score than in studies with retrospective calculations of the ABCD2 score from hospital records. 178
To reduce the impact of any between-study heterogeneity, we considered separately the 10 studies that provided data on stroke risk at both 7 and 90 days. 62,137,139,143,149–152,158,163 The study by Asimos and colleagues was published in two reports. 136,143 Even although the same cohort of patients was studied in both publications, data on risk of stroke at 7 days were reported in the 2010 publication, whereas data on risk of stroke at 90 days were reported in the 2009 publication. The number of recurrent events at 90 days was strangely low compared with that at 7 days (see Table 5). Therefore, we excluded this study from further analyses to avoid introducing conflicting or mismatched estimates and examined the remaining nine studies that considered risk of stroke at both 7 and 90 days in the exact same cohorts of patients. These nine studies included a total of 4291 patients. Five of the nine studies identified patients retrospectively from review of medical records or equivalent. These five studies included 2019 patients, 47% of the total data from studies providing information at both 7 and 90 days. In these nine studies, the total number of TIA patients with an ABCD2 score of ≥ 4 was 2719/4291 (63%), and the total number of recurrent strokes at 7 days and 90 days was 176 and 264, respectively. In contrast, the total number of TIA patients with an ABCD2 score of < 4 was 1572/4291 (37%), and the total number of recurrent strokes at 7 and 90 days was 29 and 44, respectively. The pooled stroke risk at 7 and 90 days for these nine studies is shown in Table 8 (these studies did not provide data on stroke risk after 90 days).
| ABCD2 score | Percentage | 95% CI |
|---|---|---|
| At 7 days | ||
| ≥ 4 | 4.7 | 2.4 to 8.7 |
| < 4 | 1.6 | 1.0 to 3.4 |
| At 90 days | ||
| ≥ 4 | 8.2 | 4.7 to 14.0 |
| < 4 | 2.7 | 1.5 to 4.7 |
The pooled sensitivity and specificity estimates for the ABCD2 score of ≥ 4 for predicting risk of stroke at 7 and 90 days are shown in Table 9.
| ABCD2 score of ≥ 4 | Accuracy (95% CI) | |
|---|---|---|
| Sensitivity | Specificity | |
| 7 days | 85.8 (80.4 to 90.0) | 36.1 (30.6 to 42.1) |
| 90 days | 84.6 (80.2 to 88.2) | 37.0 (40.0 to 43.4) |
Forest plots for the proportion of TIA/minor stroke patients with recurrent stroke at 7 days and ABCD2 score of > 4 and < 4 are shown in Figures 13 and 14 respectively, whereas forest plots for the proportion of patients with recurrent stroke at 90 days and ABCD2 score of > 4 and < 4 are shown in Figures 15 and 16, respectively. Results from the nine studies that provide stroke risk at both 7 and 90 days (see Figures 13 and 15: ABCD2 ≥ 4 and stroke recurrences), show reduced heterogeneity compared with those from all identified studies (see Figures 10 and 11). This suggests that as yet undefined methods differences between studies were the most likely sources of the considerable heterogeneity between studies rather than true differences in rates of recurrent stroke after TIA.
FIGURE 13.
Proportion of patients with an ABCD2 score of > 4 who have a stroke at 7 days from the subset of nine studies that provided data at both 7 days and 90 days.
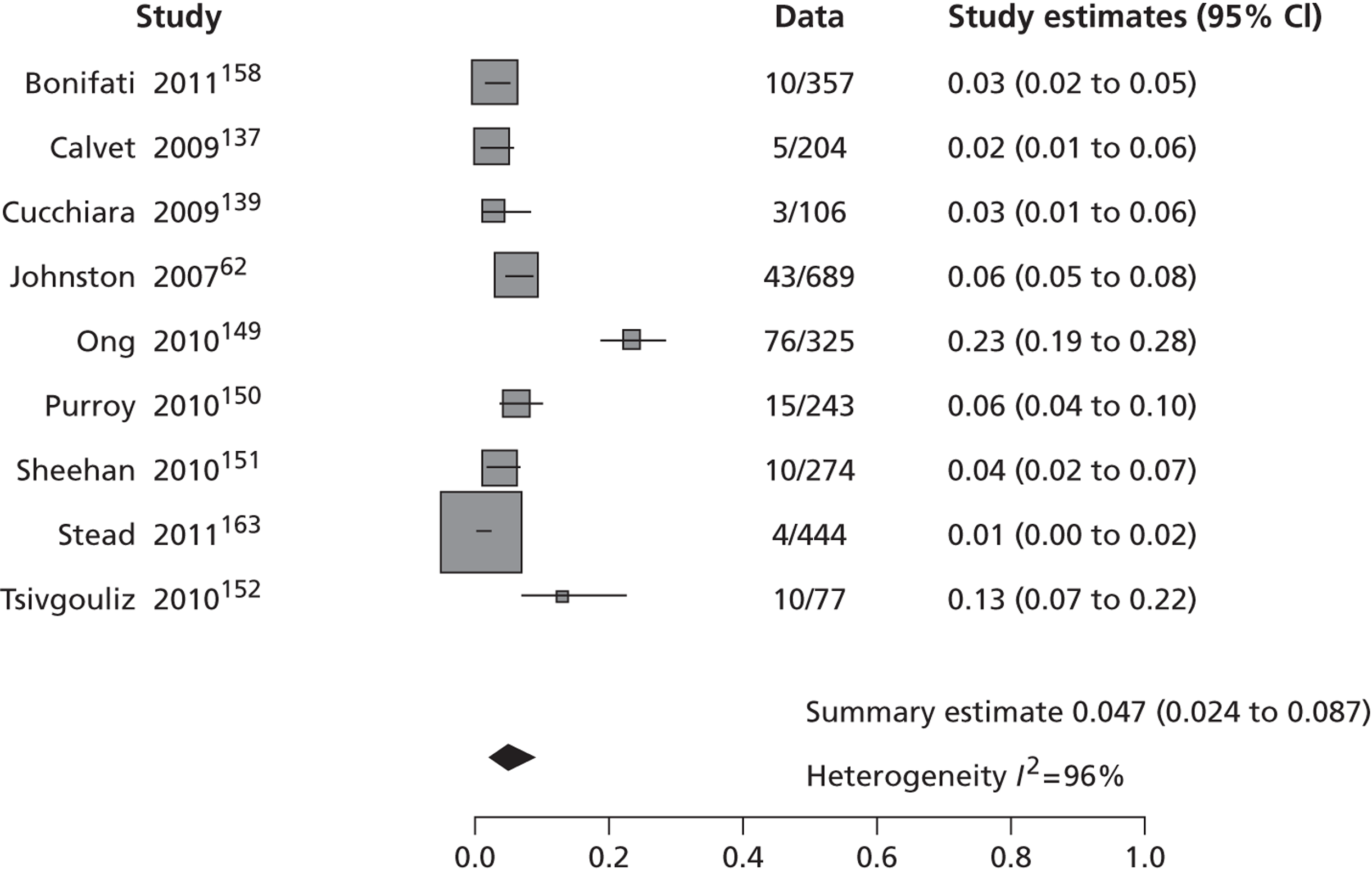
FIGURE 14.
Proportion of patients with an ABCD2 score of < 4 who have a stroke at 7 days from the subset of nine studies that provided data at both 7 days and 90 days.
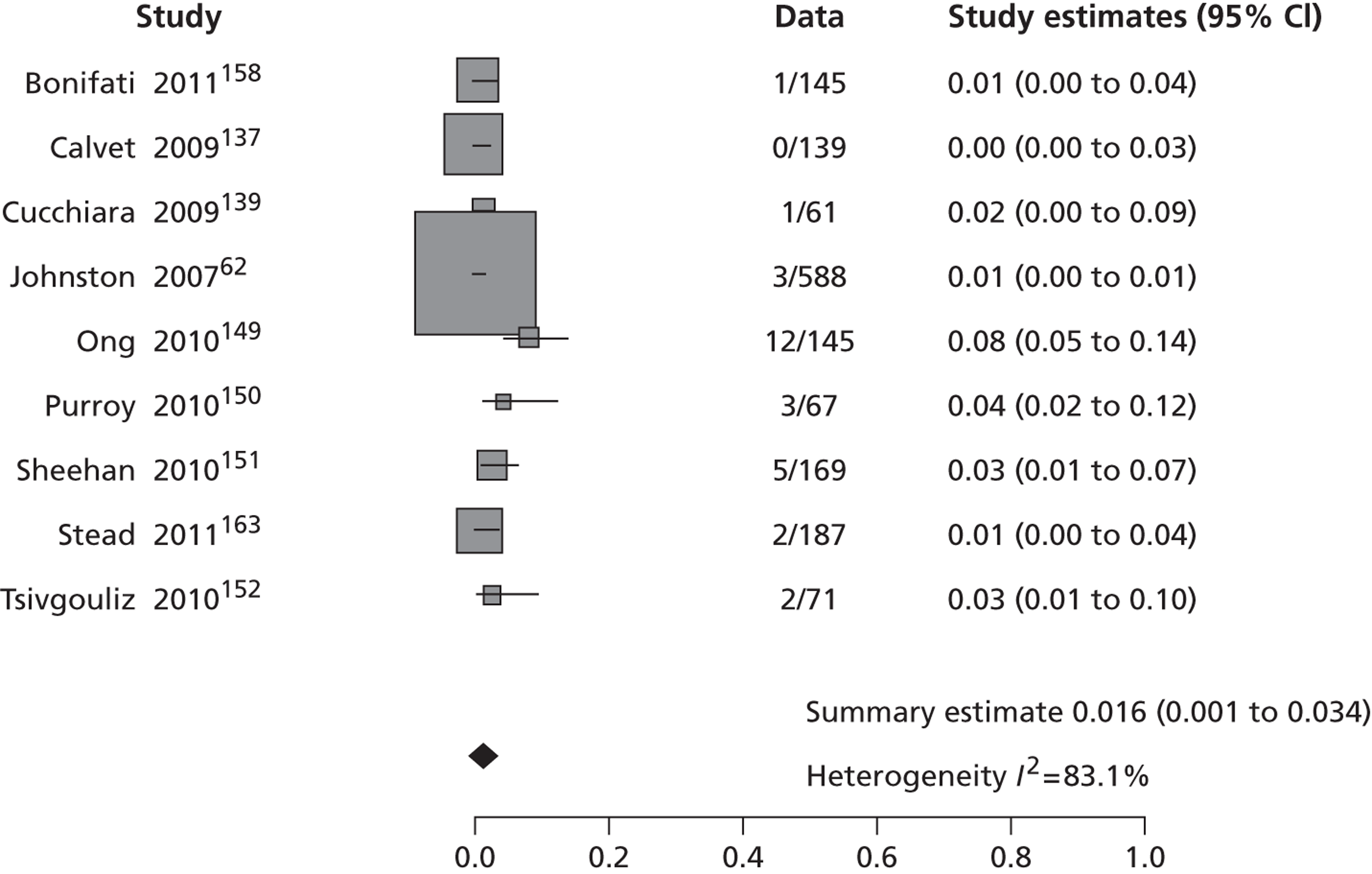
FIGURE 15.
Proportion of patients with an ABCD2 score of > 4 who have a stroke at 90 days from the subset of nine studies that provided data at both 7 days and 90 days.
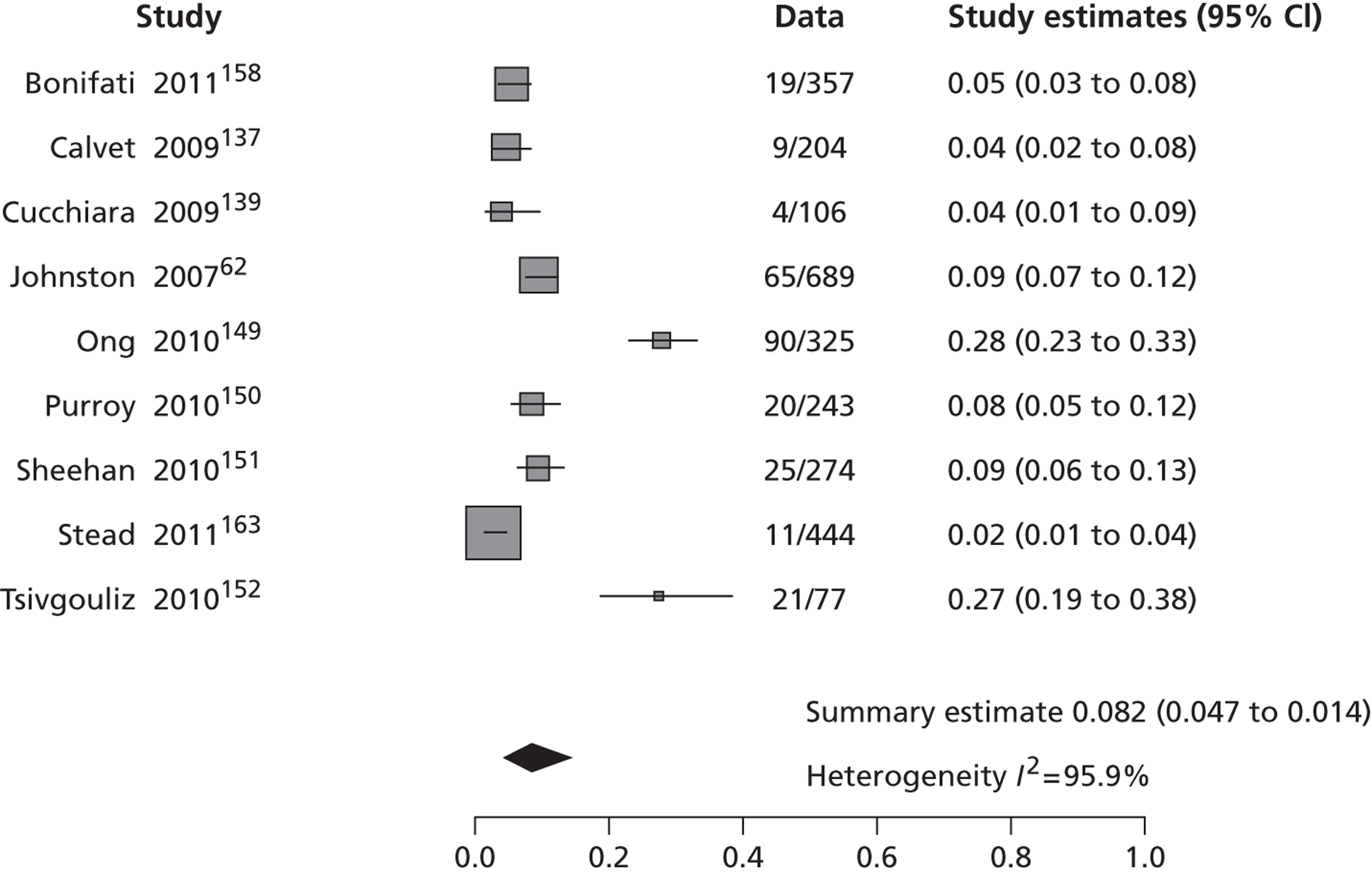
FIGURE 16.
Proportion of patients with an ABCD2 score of < 4 who have a stroke at 90 days from the subset of nine studies that provided data at both 7 days and 90 days.
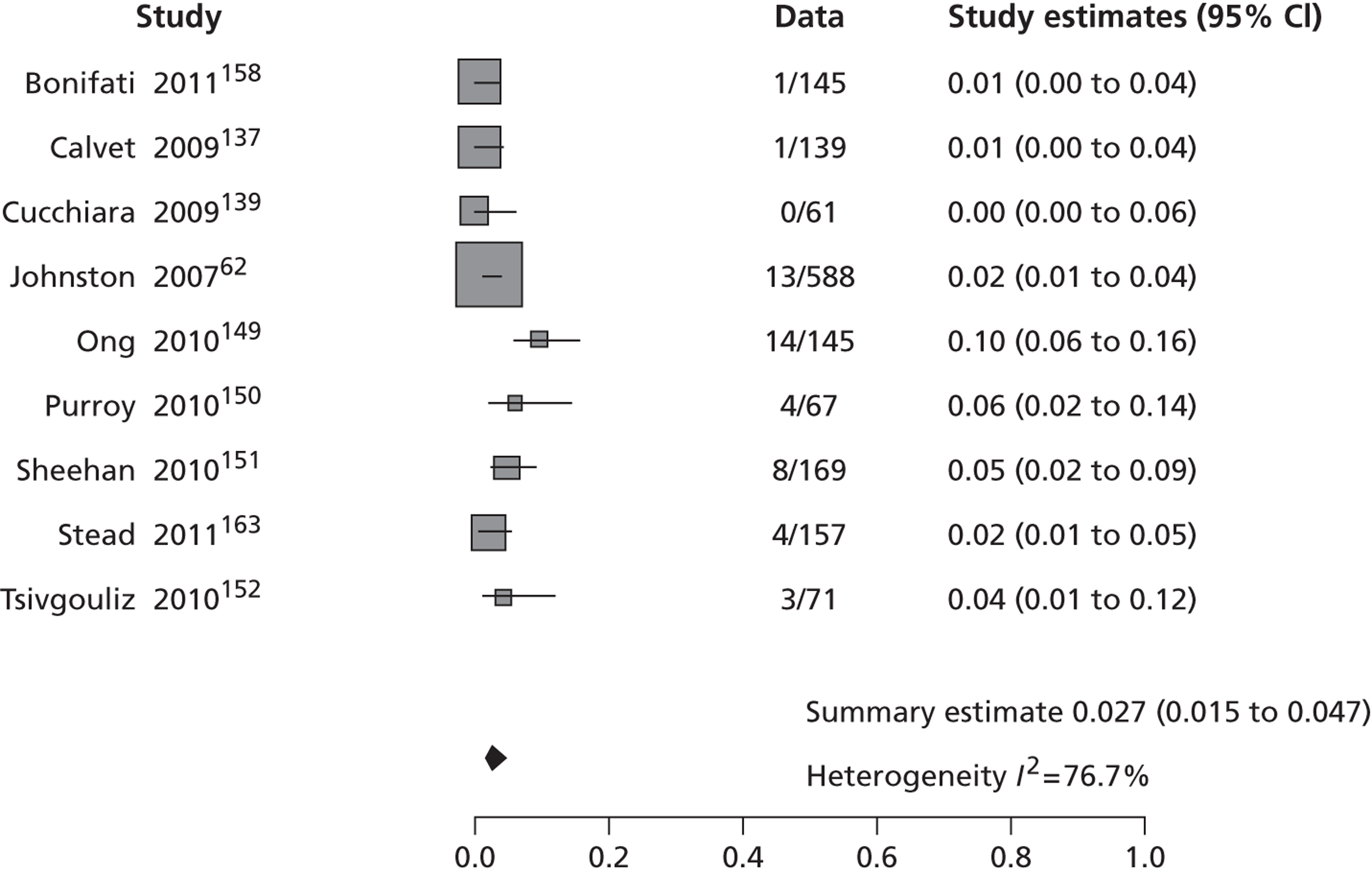
ABCD2 and proportions of patients with tight carotid stenosis
We examined all of the data on associations between the proportion of patients with high or low ABCD2 scores and an ipsilateral tight carotid stenosis, a key risk factor for recurrent stroke, to determine how many patients with tight carotid stenosis might have their investigations delayed and be at increased risk of stroke through having a low ABCD2 score. Four studies provided some data on carotid stenosis by ABCD2 score. 132,151,180,181 Ipsilateral carotid stenosis of > 50% was more frequent in patients with ABCD2 score of ≥ 4 but was not negligible in patients with ABCD2 score of < 4. In the SOS-TIA study (SOS Transient Ischaemic Attack), which enrolled a total of 1176 TIAs, among 679 of patients with ABCD2 scores of < 4, 9%, 6% and 8% of patients, respectively, had carotid stenosis of > 50%, AF or some other high-risk factor requiring immediate attention, compared with 14%, 11% and 10% of patients, respectively, among 497 with ABCD2 score of ≥ 4. 180 Basically, one-fifth of patients with an ABCD2 score of < 4 had a serious risk factor and one-third of patients with an ABCD2 score of ≥ 4. 180 In a further analysis of the SOS-TIA data, the 90-day stroke rate was 3.4% in patients with ABCD2 score of ≥ 4, 3.9% in patients with ABCD2 score of < 4 and carotid stenosis or other key risk factors, and 0.4% in patients with ABCD2 score of < 4 without key risk factors. 164 In another study assessing 220 TIAs, 11%, 22% and 22%, respectively, of 55 patients with ABCD2 scores of < 4 had carotid stenosis of > 50%, AF or both, compared with 14%, 18% and 18%, respectively, of 165 patients with an ABCD2 score of ≥ 4. 132 In a cohort of 443 specialist-confirmed TIAs, 23% of 169 patients with ABCD2 scores of < 4 had carotid stenosis of > 50%, compared with 25% of 274 patients with ABCD2 score of ≥ 4. 151 Another study that assessed TIA patients with an ABCD2 score of ≥ 5, showed that the recurrent stroke rate at 90 days increased with increasing degree of carotid stenosis. 182 Similarly, in a cohort of 40 patients with specialist-confirmed carotid territory TIA, 15% of 233 patients with ABCD2 scores of < 4 had carotid stenosis of > 50%, compared with 13% of 507 patients with an ABCD2 score of ≥ 4. 181 In short, the ABCD2 score does not predict carotid stenosis.
ABCD2 scores in transient ischaemic attack/minor stroke mimics
There was little information on ABCD and related scores in all patients presenting to stroke clinics with suspected TIA or minor stroke (i.e. including mimics), as most of the above studies specifically excluded non-TIA/minor stroke patients by specialist neurological examination and did not always differentiate TIA from minor stroke. In a retrospective cohort of 3646 patients presenting to an outpatient TIA service, of whom 1769 (48.5%) did not have TIA, a positive association was found between low ABCD2 scores and the diagnosis of non-CV events; after dichotomisation at 0–1 or 0–2 scores, the positive predictive values of ABCD2 score for a non-CV diagnosis were 0.81 and 0.74, respectively. 72 The analysis of the AUC for use of the score in the diagnosis of non-CV patients suggested a reasonable accuracy [0.74 (95% CI 0.73 to 0.76)]. 72 In a prospective cohort (north Dublin TIA study), the diagnostic usefulness of ABCD2 score to distinguish TIA or minor stroke from non-CV events was also assessed in 594 patients. 183 Mean ABCD2 score decreased across diagnostic groups [4.9, standard deviation (SD) 1.4 for minor ischaemic stroke; 3.9, SD 1.5 for TIA and 2.9, SD 1.5 for non-CV, p < 0.00001], as well the proportion of patients with ABCD2 scores of ≥ 4 and ≥ 6, respectively. An ABCD2 score of ≥ 4 increased the likelihood of a final diagnosis of confirmed TIA [odds ratio (OR) 2.8; 95% CI 2.0 to 3.9] and minor stroke (OR 8.4; 95% CI 3.8 to 18.9) compared with non-CV diagnosis. Scores of ≥ 4 had 60.3% sensitivity and 64.6% specificity for discriminating TIA from non-CV diagnosis and 82.2% sensitivity and 64.6% specificity for discriminating minor stroke from non-CV diagnosis. However, significant proportions of patients with a CV event (18% of minor stroke and 39% of TIA patients) had ABCD2 scores of < 4, and 35% of those with non-CV diagnoses had an intermediate or high ABCD2 score (≥ 4). 183 In a retrospective review (medical records) of 713 patients seen in 16 northern California EDs (from March 1997 to February 1998) who received an initial diagnosis of TIA, 79 patients (10%) had a final diagnosis of TIA mimic and 29 of them (41%) had an ABCD2 score of ≥ 4. 135 This indicates that, by placing over-reliance on the ABCD2 score, many true TIAs or minor strokes (with lower scores) would be missed while a substantial proportion of TIA mimics (35–41%) would be rapidly investigated.
ABCD2 scores and prediction of number per 1000 with recurrent stroke
Based on all available data for the ABCD2 score and predicted risk, we calculated the number of recurrent strokes by low or high ABCD2 scores in a hypothetical cohort of 1000 patients with probable or definite TIA attending a stroke prevention clinic. This requires that all patients initially referred to the TIA clinic as possible TIA/minor stroke have been filtered out by clinical assessment, which would have to be performed by a stroke physician or neurologist, as occurred in all published studies. We restricted the analysis to data from the nine studies that provided data on stroke at both 7 and 90 days. Among the 1000 selected probable or definite TIA/minor stroke patients there would be 634 with an ABCD2 score of ≥ 4 and 366 with ABCD2 score of < 4, of whom 30 and 6, respectively would have a recurrent stroke within 7 days, and 52 and 10, respectively, would have a recurrent stroke by 90 days. These numbers would shift substantially downwards if the starting population were the actual unfiltered population of patients that are referred to stroke prevention services with a suspected TIA/minor stroke, including the large proportion (45%) of patients with a stroke mimic as documented in the literature summarised in Chapter 7 and as found in the survey of UK stroke prevention services summarised in Chapter 8. Even a theoretical analysis of these data is substantially hampered by lack of information on the distribution of ABCD2 scores in all patients presenting to stroke prevention services. However, assuming that the neurologist was still filtering the patients into those with mimics and those with probable or definite TIA, then of the 1000 patients presenting to the TIA clinic, about 450, would have a mimic, and 550 would have TIA or minor stroke. Among patients with TIA/minor stroke, 349/550 would have ABCD2 score of ≥ 4 and 201/550 a core of < 4, of whom 16 and 5, respectively, would have a recurrent stroke by 7 days, and 27 and 5 by 90 days. Among the 450 with a mimic, 35–41%135,183 (say 38%) or 171 would have an ABCD2 score of ≥ 4 and 279 a score of < 4, making a total of 520/1000 (52%) clinic attendees with an ABCD2 score of ≥ 4, and 480/1000 48% with a score of < 4.
Discussion
Our systematic review combined data from 26 cohorts including 12,586 patients with TIA/minor stroke, representing all published studies to date on recurrent stroke after TIA/minor stroke in patients dichotomised at the ABCD2 cut-off point of ‘4’ as per UK National clinical guidelines. In terms of applying the ABCD2 score in routine practice, the review highlights several problems with the evidence that seems to have been overlooked previously and mean that the performance of the score will not be the same in routine practice as in these studies.
-
About half of the studies were retrospective, using patients identified through case note review and/or an ABCD2 score calculated also retrospectively from information derived from case notes or clinical databases, including five of the nine studies (47% of patients) that provided data on both 7- and 90-day recurrent stroke rate, rather than prospective collection of new patients presenting to stroke prevention services with the ABCD2 score assigned immediately in ‘real time’ after clinical assessment. Retrospective studies are known to be prone to numerous methodological biases that limit their reliability.
-
Most studies focused on patients with a definite (neurologist-determined) diagnosis of TIA/minor stroke, as opposed to patients with a possible diagnosis of TIA/minor stroke or even the large number of non-TIA/minor stroke patients who are referred to stroke prevention clinics. About 45% of patients attending TIA/minor stroke clinics do not have a TIA/minor stroke but have a mimic (see Chapters 7 and 8). Two studies that provided relevant data showed that about one-third of patients with non-CV diagnoses (35% and 41%, respectively) had an ABCD2 score of ≥ 4. This compares with one-fifth of minor stroke and 40% of true TIA patients who have a ABCD2 score of < 4. 72,183 The immediate and obvious implication of these findings is that the ABCD2 score should not be used in stroke prevention clinics except after the patient has been confirmed as a definite TIA/minor stroke by the examining stroke physician or neurologist, as it was not derived in, nor has its performance been ascertained in, the patients in whom it is being applied ‘at the front door’ by a non-specialist before a diagnosis of definite TIA/minor stroke has been made. It was not derived in patients with TIA/stroke mimics and therefore should not be used in mimics or even possible TIA/minor stroke patients. The equivalent for a drug would be testing the drug in an randomised controlled trial (RCT) in a population of patients with the disease that the drug was designed to treat (say thrombolysis for ischaemic stroke) but then using the drug in a different population (e.g. all stroke – including haemorrhagic and ischaemic stroke – and stroke mimics) coming to a hospital/clinic, many of whom did not actually have the disease that the drug was expected to treat and therefore might not only be precluded from gaining any benefit but could indeed be harmed by the intervention. This is an extreme example but serves to make the point.
-
Even if it were not obvious that possible TIAs and TIA mimics had been excluded from the studies, the high proportion of patients with ABCD2 scores of ≥ 4 (two-thirds or more across all studies and time points) should immediately raise alarm bells. Predicting risk when most of the patient population are regarded as high risk either indicates a fundamental flaw in the score (or in the choice of the cut-off point) or that the population is biased and not representative of routine clinical practice. We suspect that all of these three assumptions apply. These cohorts were highly filtered; the retrospective case ascertainment and assigning of the ABCD2 score will have inflated the number with risk points. Many people operating stroke prevention clinics would not regard the majority of patients as being at such high risk but rather most at low or moderate risk and only a few at high risk. What is the rationale of a score developed to identify and triage patients at high risk for rapid treatment if it suggests that most of them are at high risk and thereby swamps the system, the exact opposite of what it was supposed to achieve?
-
There is little evidence that the ABCD2 score picks out patients with established markers of high risk, such as carotid stenosis,164,181 supported by evidence that adding carotid stenosis to the ABCD2 or any other clinical prediction score (see Chapter 5) improves recurrent stroke prediction. 147,184 The high risk of early recurrent stroke after TIA or minor stroke in patients with significant carotid stenosis is well established. 66,128,182,185 Approximately 10–15% of patients with an ABCD2 score of < 4 have carotid stenosis of > 50% or even > 70% (very similar to the rate in patients with ABCD2 scores of ≥ 4) and should therefore be referred for endarterectomy. 132,162,164,181 Notably, in a French cohort, patients with TIA and ABCD2 score of < 4 had similar 90-day risk of recurrent stroke (3.9%) as those with a score of ≥ 4 (3.4%) in the presence of internal carotid or intracranial artery stenosis of ≥ 50% by North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria or major cardiac source of embolism. In this cohort, one TIA patient in five with a ABCD2 score of < 4 had a major finding that required immediate medical decision. 164 The presence of symptomatic carotid stenosis in patients with low ABCD score has also been shown in other cohorts, ranging from 9% to 23%. 132,151,164,181 Under present NICE guidance,67 patients with ABCD2 scores of < 4 would not be triaged for rapid assessment and would not reach endarterectomy in time for it to have maximum benefit. This risks undermining one of the strongest pieces of evidence that we have for stroke prevention, from the several 1000 patients in the carotid endarterectomy trials, which demonstrate that endarterectomy reduces the risk of ipsilateral ischaemic stroke in patients with a tight carotid stenosis by ≥ 50%. 25
-
Few studies indicated what secondary prevention measures were used in their patients (endarterectomy, medical therapy) yet these have been established in guidelines for at least 15 (endarterectomy) or more (aspirin, statins, BP-lowering drugs) years. It is unlikely that patients in these studies would have been denied such treatments and presumably they were not, but the application of endarterectomy and drugs may have reduced the risk of stroke that they were attempting to observe.
Other limitations with the literature are that there was significant heterogeneity between included studies, which could be explained by clinical and methodological factors. Clinical setting, time of assessment from symptom onset, diagnosis of TIAs, and evaluating clinicians varied considerably across studies. Our crude assessment of variation in recurrent stroke rates after TIA with year of study publication did not suggest that simple changes in referral rates would explain the heterogeneity. Many studies did not differentiate recurrent disabling stroke from any recurrent stroke and some studies may have counted a recurrent TIA as a stroke recurrence. Furthermore, in some studies the authors could not establish with certainty whether early strokes were due to progression of initial symptoms or a new event.
Various attempts have been made to improve the yield of clinical prediction scores in the stratification of stroke risk after TIA by including the use of brain and carotid imaging findings,69,147 and resulted in a confusing plethora of ABCD2 clones with limited validation or evidence of truly improved performance. The effect of adding brain imaging to the ABCD2 score is summarised in Chapters 5 and 6.
A recent systematic review and meta-analysis of ABCD2 scores and stroke risk125 in 33 studies, 16,070 patients, published shortly before submission of this report, is entirely consistent with our results. Differences between their work and ours are that they did not dichotomise on ABCD2 score of 4; they included information only published in abstract; and they did not identify the issues regarding case ascertainment, assignment of ABCD2 score, population distribution of scores, uncertainty over risk factor identification or treatment of these, or filtering by experts that we have highlighted. However, they did observe that many studies come from the same few research groups and, like us, experienced difficulty in avoiding double-counting of the same patient data appearing in multiple publications. Their pooled sensitivity and specificity for stroke risk at 7 days were nearly identical to ours. They concluded that ‘the ABCD2 score leads to only small revisions of baseline stroke risk particularly in settings of very low baseline risk and when used by non-specialists’, although most of the data in their review came from specialists. A systematic review and pooled analysis of validation studies of ABCD and ABCD2 scores showed pooled AUCs for the prediction of stroke at 7 days of 0.72 (95% CI 0.67 to 0.77) and 0.72 (95% CI 0.63 to 0.80) for studies using the ABCD and ABCD2 score, respectively; the pooled AUCs for studies using both scores were 0.72 (95% CI 0.66 to 0.78) and 0.72 (95% CI 0.63 to 0.82) respectively, with significant heterogeneity for all given estimates. 178 The pooled AUCs for the ABCD2 score assessed in the Oxford or California cohorts and independent studies were 0.77 (95% CI 0.63 to 0.91) and 0.69 (95% CI 0.64 to 0.74) respectively; the predictive value for 7-day stroke risk was higher for studies using in person or clinical records calculations of the score than studies with retrospective calculations from ED records.
Since the publication of this systematic review, a number of further studies evaluating the ABCD2 score have been published, with conflicting results. 19,20,37,39,46,67 A more recent meta-analysis of 16 ABCD2 score validation studies assessed the predictive value of the score in stroke risk at 7 and 90 days after TIA using three strata of risk: low, moderate and high (0–3, 4–5 and 6–7 points, respectively). The score correctly predicted the occurrence of stroke at 7 days after TIA in all strata of risk but tended to overpredict the occurrence of stroke at 90 days. 166 There is an indication that ABCD scores work differently in stroke and TIA patients, with some publications suggesting that ABCD scores may predict stroke recurrence with any accuracy only within the first 7 days of TIA. In the OXVASC population cohort the risk of recurrent stroke at 90 days in patients presenting with a first minor stroke was 11.7%; the ABCD2 score was poorly predictive of stroke recurrence within 7 and 90 days in this group of patients – AUCs 0.57 (95% CI 0.47 to 0.68) and 0.60 (95% CI 0.53 to 0.67), respectively. 186 Other scores have been used to predict the long-term risk of stroke. An external validation of several prediction models for long-term risk of major vascular events after TIA or minor stroke showed that the discrimination was poor for all prediction models for risk at 2 years. After adjustment for baseline risk and prevalence of risk factors, clinical usefulness may be best for the ABCD2 model, with a reasonable c-statistic (0.64). Notably, patients with AF, significant symptomatic carotid stenosis, mechanical heart valve and a proven dissection were excluded. 187 Another prospective assessment of prognostic scores in patients with TIA or non-disabling stroke [including Essen Stroke Risk Score (ESRS), Stroke Prognosis Instrument II (SPI-II), Life Long After Cerebral ischaemia (LiLAC) score and Hankey score, discussed in Chapter 5] showed that the discrimination for annual risk of stroke was similar across each score, with a marginal superiority of SPI-II score (AUC 0.65; 95% CI 0.6 to 0.7). 154
Current clinical guidelines67 recommend urgent treatment only for patients with high ABCD2 scores, whereas patients with low scores should be triaged for further clinical assessment within 1 week. 67,97 This would risk missing patients with carotid stenosis requiring endarterectomy. On the other hand, not everyone may be using these scores in real life, as indicated in the survey of stroke prevention services in the UK, which indicates that only 13% of the respondents described the use of ABCD2 score to stratify the promptitude of assessment of TIA patients (see Chapter 8).
Chapter 5 Clinical prediction models of stroke after transient ischaemic attack and the effect of adding information from plain computerised tomography or carotid imaging
Introduction
Patients who have recently experienced a TIA or minor stroke are at a higher risk of major disabling stroke and other vascular events. 188 Prediction of who will suffer recurrent disabling stroke may help triage those patients at highest risk to effective stroke prevention treatment faster. It may also help determine the intensity and type of treatment that is offered.
Numerous risk prediction scores have been developed over the last 20 years, which aim to improve identification of patients at high risk of disabling recurrent stroke after TIA/minor stroke: a score developed in 469 patients with TIA seen in the 1980s based on clinical variables;9 a score developed in the European Carotid Surgery Trial (ECST) (1980s to mid-1990s) data;11 the ABCD score developed using data from a population-based stroke and TIA registry conducted in the 1980s (OCSP) and tested in a repeat study in the same population in the early 2000s (OXVASC);61 an updated version of this derived in the OCSP TIA patients and patients attending a private health provider in the USA and tested in OXVASC and more USA patients – the ABCD2 score;62 the ESRS, which was derived from a subset of ischaemic stroke patients in the Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events (CAPRIE) trial and validated in two observational studies and in data from another stroke prevention trial;63 Stroke Prognosis Instrument I (SPI-I) and SPI-II were developed in medical stroke prevention trials and validated in three independent cohorts;64 and the LiLAC score, which was based on data from the Dutch TIA trial (1986–9) and has not been externally validated. 65
All except two60,64 of these scores use only simple clinical features and so they are rapid and easy to apply without needing complex technologies. 59,61,62,65 For example, the ABCD2 score uses age, blood pressure, clinical features, duration of symptoms and diabetes to derive a score from 0 to 7. These scores could help to reduce delays to reaching medical attention and to triage patients,66 so that those needing most rapid treatment, such as carotid endarterectomy, would reach surgery more quickly. They might also improve the accuracy of clinical diagnosis of TIA by non-stroke physicians, thereby also making better use of resources.
Development of risk prediction scores involves collecting a range of variables that are potentially associated with outcome (in this case recurrent stroke), performing statistical modelling to determine which of these are the most powerful predictors then turning these into a score and performing validation in external data sets that reflect the circumstances in which the score will be used in clinical practice. Proposed prediction scores include numerous ABCD and derivative scores, the Californian score, the ESRS score, SPI-II, and the clinical and imaging-based predictive (CIP) model. 61,63,64,189 These prediction scores aim to estimate the risk of recurrent stroke for a given patient in a specified timeframe, at the bedside. Although these scores are used in clinical practice, there are benefits from studying their accuracy and practicality when in wider use as validation is rarely extensive or adequate in the primary publication. 166,189,190
Stroke patients routinely undergo imaging of the brain and carotid arteries. It is reasonable to ask whether the addition of routinely available imaging of the brain and carotid arteries to models based on clinical variables alone improves prediction. To investigate this question, we systematically reviewed the literature. It was necessary to modify the methodology used for systematic reviews of RCTs to allow for the various study designs used by the original studies. We specifically examined the addition of CT brain imaging and carotid imaging by any modality to prediction rules or models of recurrent stroke using clinical predictors in patients with recent TIAs or minor strokes.
Methods
Identification of studies
Application of systematic review methodology to reviews of prognostic factors or prediction rules is not straightforward. Current methodology focuses on the retrieval and appraisal of particular kinds of study, particularly RCTs,191 yet most prediction rules are not developed in the context of RCTs. RCTs are carefully indexed as such in online medical databases. However, studies investigating the predictors of recurrent stroke are not restricted to any particular study design, especially not to RCTs, making it difficult to limit the number of hits found by a systematic literature search in medical databases, such as MEDLINE, by a study design filter. A simple, non-exhaustive, search strategy (see Appendix 1) in MEDLINE to find studies about recurrent stroke yielded 16,556 hits – an unworkably large number, given the resources available. Even for formal clinical prediction rules it is far more challenging to create an efficient yet exhaustive search strategy than for RCTs. 192
We therefore used a citation search method. Starting with a routinely collected reasonably comprehensive series of papers collected by one of the authors (JMW) over the last 20 years and several key papers,61,147,166,184 one reviewer (FMC) checked their references for relevant articles and also used Web of Knowledge ISI (http://wokinfo.com) to find articles that had cited the key papers. If a new study met our inclusion criteria, we also checked its references and found the studies that had cited it. This process was carried on iteratively until no new papers were found. This method has the advantages of transparency, repeatability and practicality. It can identify relevant literature without requiring the resources needed to administer thousands of abstracts and hundreds of full-text articles that would be identified by an exhaustive literature search but that would ultimately be irrelevant.
Inclusion/exclusion criteria
Each study had to contain data that could be used to ascertain recurrent stroke risk using either specified clinical prediction rules (ABCD, ABCD2, SP-II, the ESRS and the Californian rule; the CIP rule was not included as it is the ABCD rule plus DWI) or a described but un-named statistical model linking clinical predictors with recurrent stroke, with CT brain imaging or carotid imaging by any modality in adult patients who had had a recent TIA or minor stroke. Studies that did not use brain CT/carotid imaging, or used only imaging to predict stroke, were not included. Studies using a highly selected subset, for example young patients only, were noted but not included in the review. Studies were also excluded unless the predicted outcome was recurrent stroke. Studies published prior to 1990 were also excluded owing to developments in imaging technology since that time.
Quality assessment and data extraction
We derived a critical appraisal tool using a framework suggested for prognosis studies (see Appendix 2). 193 We particularly wished to examine patient recruitment into studies and patient selection for imaging as potential sources of bias. A brief inspection of the literature showed that patients may have had probable, possible or definite TIAs, imaging may have been reported for the entire or a subset of the sample, and recurrent stroke outcome events reported for 7, 30 or 90 days. Two other important issues were the definition of outcome and any stroke prevention treatment the patients may have received. Outcome was often simply defined as recurrent stroke without any indication of the severity. Also any treatment given will have an effect on stroke outcomes.
The data extracted from each article included the following:
-
number of patients
-
setting [e.g. hospital specialist clinic vs. community-based or accident and emergency (A+E) department]
-
percentage male
-
percentage TIA and minor stroke (possible, probable, definite)
-
recruitment procedure (e.g. consecutive recruitment)
-
age (mean/median age plus range)
-
length of follow-up
-
if patients at high risk of stroke were treated and the treatment given
-
timing of baseline imaging from symptom onset or presentation
-
clinical prediction rule or details of the statistical model
-
type of additional imaging
-
definition of recurrent event
-
definition of positive imaging
-
method of ascertaining effect of adding imaging to the clinical prediction rule/statistical model
-
the added value of imaging to the clinical prediction rule/statistical model (this last may be expressed in a number of ways, most simply as the changes in the numbers of patients correctly predicted to have or not have a recurrent stroke).
One reviewer (FMC) performed the critical appraisal and extracted the data; ambiguous papers were discussed with a second reviewer (JMW) to minimise appraisal or data extraction errors.
Data synthesis
We planned to conduct two meta-analyses of the CT and carotid stenosis data. Studies were grouped according to their outcome measure [AUC, hazard ratio (HR), relative risk (RR)] for separate analysis as different outcome measures generally cannot be combined. We used forest plots and calculated I2-statistics109 to assess heterogeneity. All analyses were done in R using the generic inverse variance method in the meta-package.
Additional individual patient data from Lothian cohort
We also had access to data from a large prospective observational study – the LSR – conducted between October 1991 and 1999 in the stroke service at the Western General Hospital (WGH), Edinburgh, the methods of which have been published previously. 37 For the stroke prediction analysis, we used data on all patients referred with TIA, amaurosis fugax (AFx), retinal artery occlusion (RAO) or stroke, outpatients and inpatients between October 1994 and December 1999. Patients were excluded if they had a primary intracerebral haemorrhage (PICH) on CT/MRI scanning or were diagnosed with major stroke (abnormal verbal score on Glasgow Coma Scale, could not walk, could not lift both arms at examination). All patients were seen by a stroke physician mostly as outpatients in a rapid access stroke prevention clinic that operated twice per week in addition to patients being seen in A+E and on the wards. The details of the current and past history, and examination (with particular emphasis on vascular risk factors), blood tests, chest radiography, electrocardiography (ECG), CT brain and carotid artery imaging were entered into a database, of the LSR. The diagnosis of definite, probable or possible stroke/TIA/retinal ischaemia and not stroke/TIA/retinal ischaemia were determined by a consultant stroke physician in discussion with a panel of expert stroke physicians, prospectively, held weekly and blind to all follow-up events. The stroke physician also assigned an OCSP stroke subtype total anterior circulation cortical stroke (TACS), partial anterior circulation cortical stroke (PACS), lacunar stroke (LACS) or posterior circulation stroke (POCS). 194 The CT scans were coded by an expert neuroradiologist for the presence of any visible, recent and ‘likely to be relevant to the recent symptoms’ infarct for any old stroke lesions and non-stroke lesions using a structured validated scoring method. 195 The rater was not blind to the clinical presenting symptoms and signs so as to determine if any visible lesion were likely to be relevant or not, but was blind to any recurrent stroke or other outcomes and to the presence of other risk factors including carotid stenosis. Carotid stenosis was measured by ultrasound, the imaging being performed by one of two trained operators (a radiographer or neuroradiologist) who recorded velocities in the internal, external and common carotid and vertebral arteries [internal carotid artery (ICA), external carotid artery, common carotid artery (CCA), vertebral artery, respectively] on both sides of the neck. 196 In addition, any visible stenosis was noted, including its location, the degree of luminal narrowing by calliper measurement of the residual and original lumen at the point of maximum stenosis, the CCA lumen, and the ICA lumen distal to the stenosis by calliper measurements on the ultrasound console. 197 The degree of stenosis was assigned according to the ECST measurement method,82 using the velocity readings and calliper measurements, a method that has been validated against intra-arterial angiography (IAA). 197 The appearance of the stenosing plaque (smooth surface and of uniform echoity indicating ‘quiescent plaque’ or irregular surface and mixed echoity indicating ‘active plaque’) was also recorded. 198 The ECST velocity and calliper measurements were also converted to stenosis, expressed using the NASCET method for comparison with other studies. 199 Patients with > 50% NASCET (> 70% ECST) stenosis in the symptomatic ICA underwent confirmatory imaging with IAA prior to 1998 and MRA or repeat Doppler ultrasound (DUS) by a blinded operator thereafter.
Patients were followed up at 6 months, 1 year and 2 years by a trained researcher using a postal/telephone questionnaire, blinded to initial presenting symptoms and imaging features, using validated methods, to identify whether they had had a further stroke, myocardial infarction (MI) or died (and if so from what), and to determine their functional status. Therefore, the data were representative of practice in a rapid-access, same-day investigation NV clinic that accepted unscreened referrals from general practitioners (GPs) and other hospitals in the geographical area of north Edinburgh.
In all patients with mild stroke [defined as non-disabling and usually equivalent to a PACS, LACS, or POCS syndrome according to the OCSP classification,194 or TIA, or eye ischaemic symptoms (AFx or RAO), including those who would be candidates for endarterectomy] we performed multivariate survival analyses (Cox proportional hazards). The final predictive model included:
-
residual neurological signs (yes/no)
-
carotid stenosis (deciles, ECST criteria)
-
age (years)
-
previous stroke (yes/no)
-
ocular not cerebral symptoms (yes/no)
-
coronary illness (yes/no)
-
visible infarct (yes/no).
Coronary illness was defined as the presence of one or more of the following: angina, previous MI, ECG showing acute or old MI. Predicted outcome was non-fatal disabling or fatal stroke. The model was rerun with and without carotid stenosis and CT visible infarct:
-
Model A included all of the above except carotid and brain imaging (clinical only).
-
Model B included all of the above except brain imaging (clinical plus carotid).
-
Model C included all of the above except carotid imaging (clinical plus CT).
-
Model D included all of the above (clinical plus carotid plus CT).
The models were used to generate an estimated risk of non-fatal disabling or fatal stroke for each patient, expressed as a probability from 0 to 1. A cut-off of 0.045 was applied to these risks to divide the patients into those predicted to have a stroke and those not predicted to have a stroke. The figure of 0.0045 was the cut-off chosen in the original LSR analyses for outcomes at 1 year.
The variables tested included age (years), residual neurological signs (yes/no), previous stroke, ocular as opposed to brain symptoms, coronary disease, the degree of ipsilateral carotid stenosis, atheroma plaque appearance or visible (likely to be relevant) infarct on CT. We tested the association between these variables and fatal or non-fatal disabling stroke during follow-up. Data were analysed with SAS version 9.1.3 (SAS Institute Inc., Cary, NC, USA; www.sas.com).
Results
Number of included/excluded studies
The citation search method produced 1116 papers after removing duplicates and papers published prior to 1990; among this were 117 potentially relevant studies that were considered for inclusion. Ninety-six studies were rejected for the following reasons: 36 did not use brain CT or carotid imaging to predict recurrent stroke, 15 did not have recurrent stroke as an outcome, the relevant data were not reported in 13 studies, and a further 32 were rejected for a variety of reasons. This left 21 studies. The study designs ranged from population-based epidemiological studies to RCTs.
One of these 21 studies was an individual patient data (IPD) meta-analysis, which provided estimates of accuracy of prediction of the ABCD2 and brain CT individually for seven of the included studies. 178 We extracted CT results for each of the seven studies, but did not use the meta-analysis further, for example it was not included in the critical appraisal process. One of these seven studies also provided CT results in the primary publication. 69 A further 11 studies provided CT data, i.e. there were 18 studies providing data on CT brain imaging in total. There were 10 studies with carotid imaging data, and another 12 studies that used large artery atherosclerosis (LAA) where carotid stenosis was a component as a predictor. We recorded the results of the LAA studies for interest, but, owing to the heterogeneous definitions of LAA, did not formally include them in the review. Six studies had data on both CT and carotid imaging (Table 10).
| Study | Data | ||
|---|---|---|---|
| CT | Carotid | LAA | |
| aAsimos and colleagues, 2010143 | Yes | No | No |
| Bonifati and colleagues, 2011158 | Yes | Yes | No |
| Calvet and colleagues, 2009137 | No | No | Yes |
| Cancelli and colleagues, 2011200 | No | Yes | No |
| Coutts and colleagues, 2008134 | Yes | No | Yes |
| Coutts and colleagues, 2011201 | No | Yes | No |
| aCucchiara and colleagues, 2009139 | Yes | No | No |
| aDouglas and colleagues, 200335 | Yes | No | No |
| The Dutch TIA Trial Study Group 1993202 | Yes | No | No |
| Eliasziw and colleagues, 1995203 | Yes | Yes | No |
| Giles and colleagues, 2010184 | Yes | No | No |
| Hankey and colleagues, 1992204 | Yes | Yes | No |
| Hier and colleagues, 1991205 | Yes | No | No |
| Holzer and colleagues, 2010146 | No | No | Yes |
| Kernan and colleagues, 1991206 | No | No | Yes |
| Kernan and colleagues, 200064 | Yes | Yes | No |
| Lavallée and colleagues, 2007122 | Yes | No | No |
| Lovett and colleagues, 2004207 | No | No | Yes |
| Marnane and colleagues, 2011208 | No | Yes | Yes |
| Merwick and colleagues, 2010147 | No | No | Yes |
| Ois and colleagues, 200881 | No | No | Yes |
| Purroy and colleagues, 2007182 | No | Yes | Yes |
| Purroy and colleagues, 2010150 | Yes | No | Yes |
| Purroy and colleagues, 2012165 | Yes | No | Yes |
| Purroy and colleagues, 2011209 | No | No | Yes |
| Rothwell and Warlow, 199960 | Yes | Yes | No |
| aRothwell and colleagues, 200561 | Yes | No | No |
| Sciolla and Melis, 200869 | Yes | No | No |
| aSheehan and colleagues, 2010151 | Yes | Yes | No |
We were careful to avoid duplicate publication of the same patient data, as some studies produced several publications. Only one reference per study was listed in the included studies, where that reference gave the required CT or carotid results. Secondary publications of included studies are given in Table 11, and were used for extra information in the critical appraisal and data extraction stages, with the exception of the studies used in the IPD meta-analysis,184 where the studies contributing to the IPD meta-analysis and which provided additional information on imaging findings, are listed as included studies, even if their own original publications did not provide CT brain or carotid imaging results.
| Primary publication listed in included studies | Secondary publications |
|---|---|
| Douglas VC, Johnston CM, Elkins J, Sidney S, Gress DR, Johnston SC. Head computed tomography findings predict short-term stroke risk after transient ischemic attack. Stroke 2003;34:2894–835 | Johnston SC, Gress DR, Bronwer WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA 2000;284:2901–68 |
| The Dutch TIA Trial Study Group. Predictors of major vascular events in patients with a transient ischemic attack or nondisabling stroke. Stroke 1993;24:527–31202 | Van Swieten JC, Kappelle LJ, Algra A, van Latum JC, Koudstaal PJ, van Gijn J, for the Dutch TIA Trial Study Group. Hypodensity of the cerebral white matter in patients with transient ischemic attack or minor stroke: influence on the rate of subsequent stroke. Ann Neurol 1992;32:177–83211 Van Wijk I, Kappelle LJ, van Gijn J, Koudstaal PJ, Franke CL, Vermeulen M, et al. A. Long-term survival and vascular event risk after transient ischaemic attack or minor ischaemic stroke: a cohort study. Lancet 2005;365:2098–10465 |
| Eliasziw M, Streifler JY, Spence JD, Fox AJ, Hachinski VC, Barnett HJM. Prognosis for patients following a transient ischaemic attack with and without a cerebral infarction on brain CT. Neurology 1995;45:428–31203 | Steifler JY, Eliasziw M, Benavente OR, Harbison JW, Hachinski VC, Barnett HJM, et al. The risk of stroke in patients with first-ever retinal vs. hemispheric transient ischemic attacks and high-grade carotid stenosis. Arch Neurol 1995;52:246–9212 North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991;325:445–53213 |
| Giles MF, Albers GW, Amarenco P, Arsava MM, Asimos A, Ay H, et al. Addition of brain infarction to the ABCD2 Score (ABCD2I): a collaborative analysis of unpublished data on 4574 patients. Stroke 2010;41:1907–13184 | Giles MF, Albers GW, Amarenco P, Arsava EM, Ay H, Calvet D, et al. Early stroke risk and ABCD2 score performance in tissue- vs. time-defined TIA: a multicenter study. Neurology 2011;77:1222–8210 Giles MF, Rothwell PM. Systematic review and pooled analysis of published and unpublished validations of the ABCD and ABCD2 transient ischemic attack risk scores. Stroke 2010;41:667–73178 Giles MF, Rothwell PM. Risk of stroke early after transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol 2007;6:1063–72121 |
| Hankey GJ, Slattery JM, Warlow CP. Transient ischaemic attacks: which patients are at high (and low) risk of serious vascular events? J Neurol Neurosurg Psychiatr 1992;55:640–52204 | Hankey GJ, Slattery JM, Warlow CP. The prognosis of hospital-referred transient ischemic attacks. J Neurol Neurosurg Psychiatr 1991;54:793–802214 |
| Purroy F, Montaner J, Molina CA, Delgado P, Ribo M, Álvarez-Sabín J. Patterns and predictors of early risk of recurrence after transient ischemic attack with respect to etiologic subtypes. Stroke 2007;38:3225–9168 | Purroy García F, Molina Cateriano CA, Montaner Villalonga J, Delgado Martínez P, Santmarina Pérez E, Toledo M, et al. Lack of usefulness of ABCD score in the early risk of recurrent stroke in transient ischemic attack patients. Med Clin 2007;128:201–3190 Purroy F, Montaner J, Rovira A, Delgado P, Quintana M, Álvarez-Sabín J. Higher risk of further vascular events among transient ischaemic attack patients with diffusion-weighted imaging acute ischemic lesions. Stroke 2004;35:2313–1993 |
| Rothwell PM, Warlow CP. Prediction of benefit from carotid endarterectomy in individual patients: a risk-modelling study. Lancet 1999;353:2105–1060 | European Carotid Surgery Trialists’ Collaborative Group. Randomised controlled trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998;351:1379–8782 |
Characteristics of included studies
The papers were broadly similar in their critical appraisal results (Figures 17 and 18). However, it was notable that the carotid stenosis papers mostly did define carotid stenosis adequately, whereas more than half of the CT papers did not give a description of a positive CT scan.
FIGURE 17.
Results of critical appraisal of studies contributing CT data. Each item on the checklist was coded ‘Yes’, ‘No’ or ‘Unclear’, with ‘Yes’ indicating better reporting or methodology (see Appendix 2). Note: item 5 is not included as it refers to carotid stenosis imaging.
FIGURE 18.
Results of critical appraisal of studies contributing carotid stenosis data. Each item on the checklist was coded ‘Yes’, ‘No’ or ‘Unclear’, with ‘Yes’ indicating better reporting or methodology (see Appendix 2). Note: item 4 is not included as it refers to CT imaging.
Many papers lacked details on exactly how the patients were recruited and, in particular, whether or not they were a consecutive sample, although the studies by Bonifati and colleagues,158 Cancelli and colleagues,200 and Sheehan and colleagues151 stressed the efforts made by the authors to identify all possible patients, and Sciolla and Melis69 and Purroy and colleagues150 explicitly stated that their sample was consecutively recruited. In addition, a few references had obtained data from RCTs (Table 12). 60,64,202,203
| Study | Recruitment process | Inclusion/exclusion criteria | TIAs vs. minor stroke |
|---|---|---|---|
| Asimos and colleagues, 2010143 | Non-consecutive sample | Exclusion criteria: history of stroke, unknown onset time, presentation at > 24 hours after onset | TIA only |
| Bonifati and colleagues, 2011158 | Retrospective analysis of hospital records | NR | TIA only |
| Cancelli and colleagues, 2011200 | Prospective review of patient records, including admission registers, and referrals to neurology and neuroradiology departments | Exclusion criteria: non-resident of the Udine area, non-consent, history of stroke, presenting at time of recurrent stroke rather than initial TIA, or non-definite TIA status | TIA only |
| Coutts and colleagues, 2011201 | Prospective cohort study | Inclusion criteria: examination by study neurologist within 12 hours of onset, aged ≥ 18 years, consent given, CT and MRI imaging completed within 24 hours of onset, no previous disability | TIA and ischaemic stroke |
| Cucchiara and colleagues, 2009139 | Prospective cohort study | Exclusion criteria: evaluation more than 48 hours after symptom onset, severe or terminal illness, on warfarin with an international normalised ratio of ≥ 1.5 Inclusion criteria: diagnostic testing for NV cause justified by patients’ symptoms |
TIA only |
| Douglas and colleagues, 200335 | Prospective cohort study | Exclusion criteria: patients with missing records or who were not members of the specified health plan, who had ED diagnoses other than TIA, or who had had a prior TIA | TIA only |
| The Dutch TIA Trial Study Group, 1993202 | Patients recruited to a RCT of aspirin | Exclusion criteria: final non-CV diagnosis, or ‘. . . cerebral ischemia from causes other than artery-to-artery embolism, such as haematological disorders, vasculitis, or a cardiac source of embolism’ | TIA and minor ischaemic stroke |
| Eliasziw and colleagues, 1995203 | Non-surgical arm of a RCT (NASCET) for carotid endarterectomy. All patients with 70–99% carotid stenosis with a TIA presenting event | Exclusion criteria: stroke or any history of stroke, ischaemic event attributed to a cardiac source, presenting event occurring at ≥ 120 days prior to randomisation, aged > 80 years, significant intracranial vascular disease, life-threatening or disabling conditions | TIA only |
| Giles and colleagues, 2010184 | Various methods used in the primary studies | Various inclusion/exclusion criteria used in the primary studies; the IPD meta-analysis did not use data from patients with stroke or non-neurovascular diagnosis | TIA only |
| Hankey and colleagues, 1992204 | Prospective cohort study | Inclusion criteria: ‘. . . presenting TIA was considered to be atheromatous thromboembolism, lipohyalinosis or cardiogenic embolism’ | TIA only |
| Hier and colleagues, 1991205 | Prospective cohort study | All patients presented with an ischaemic stroke. Exclusion criteria included cerebral oedema, hypoperfusion, metabolic disturbance, toxaemia, fever, epilepsy, stroke related to surgery, angiography or anticoagulants | Ischaemic stroke only |
| Kernan and colleagues, 200064 | Relevant results derived from patients recruited into a RCT (Women’s Oestrogen for Stroke Trial, WEST) | Inclusion criteria: postmenopausal women presenting with TIA or non-disabling stroke at < 90 days prior to randomisation | Ischaemic strokes (n = 403) and TIAs (n = 122) |
| Lavallée and colleagues, 2007122 | Prospective cohort of patients recruited to a TIA clinic | Patients thought to have non-neurological transient symptoms, or persistent ischaemic symptoms consistent with evolving stroke were excluded. Although this study did recruit and present results for patients with minor stroke, they were excluded from the IPD meta-analysis | TIA only |
| Marnane and colleagues, 2011208 | Population-based prospective study | Exclusion criteria: presenting with a TIA or haemorrhagic stroke, stroke associated with surgery, ipsilateral carotid or intracranial stenosis of > 50% | Ischaemic strokes only |
| Purroy and colleagues, 2007182 | Prospective study of patients presenting at A+E | Exclusion criteria: symptoms not attributed to brain ischaemia | TIA only |
| Purroy and colleagues, 2010150 | Prospective study of consecutive patients presenting at A+E | NR | TIA only |
| Purroy and colleagues, 2012165 | Prospective study of patients referred to stroke units | Exclusion criteria: non-TIA diagnosis or a mRS score of > 3 | TIA only |
| Rothwell and Warlow, 199960 | Non-surgical arm of a RCT (ECST) for carotid endarterectomy | Inclusion criteria: ‘. . . carotid distribution transient ischaemic attack, minor ischaemic stroke, non-disabling major stroke, or a retinal infarction in the previous 6 months, and had evidence of ipsilateral carotid stenosis on angiography’ | TIA and ischaemic stroke |
| Rothwell and colleagues, 200561 | Prospective population-based cohort study | Inclusion criteria: possible TIA | TIA only |
| Sciolla and Melis, 200869 | Consecutively recruited prospective sample | Inclusion criteria: TIA diagnosis confirmed by neurologist within 24 hours of onset Exclusion criteria: presentation more than 24 hours after onset, final diagnosis of stroke or non-ischaemic event |
TIA only |
| Sheehan and colleagues, 2010151 | Prospective population-based cohort study with ‘hot’ and ‘cold’ pursuit in the first year | Exclusion criteria: non-CV diagnosis | TIA only |
A more important point may be the various eligibility criteria used by the individual studies with regard to TIA status – some studies recruited patients if the emergency doctor suspected a TIA, whereas others recruited only patients with ischaemic stroke confirmed by a neurologist. The difference between these patient groups is likely to be a key factor in the rates of and prediction of recurrent stroke. However, the definitions of a TIA from study to study were generally very similar; a TIA was often defined as focal neurological symptoms lasting < 24 hours, thought to be of vascular origin (Table 13 and see Figures 17 and 18).
| Study | Definition of a TIA or stroke | How certain was TIA or stroke status? |
|---|---|---|
| Asimos and colleagues, 2010143 | Sudden focal loss of neurologic function involving the brain or retina, supplied by a specific vascular territory, with complete recovery in 24 hours | Emergency physicians or other admitting physicians identified . . . admitted presumptive patients with transient ischemic attack |
| Bonifati and colleagues, 2011158 | According to the World Health Organisation (WHO), TIA was defined as an episode of focal neurological symptoms with abrupt onset and rapid resolution lasting < 24 hours and thought to be due to altered circulation to a limited region of the brain | Retrospective analysis of ED discharge diagnosis The discharge diagnosis was not always coded as TIA but all patients discharged with neurological symptoms were evaluated. Clinical charts of all patients with a focal neurological deficit were further evaluated to identify TIAs |
| Cancelli and colleagues, 2011200 | . . . an acute loss of focal cerebral or ocular function lasting < 24 hours presumed, after adequate investigation, to be attributable to embolic or thrombotic vascular disease | Patients were assessed as soon as possible by a study neurologist in the hospital or at home . . . Hospital and outpatients records were reviewed to obtain a confirmation of the self-reported diagnosis . . . |
| Only definite TIA patients were included in the analysis – patients with ‘possible TIA’ were excluded | ||
| Coutts and colleagues, 2011201 | TIA patients needed to have motor or speech symptoms (or both) lasting at least five minutes . . . | . . . examined by a Stroke Neurologist within 12 hours of symptom onset . . . |
| Cucchiara and colleagues, 2009139 | . . . acute onset of focal cerebral or monocular symptoms lasting less than 24 hours and thought possibly to have a vascular cause in the opinion of the neurologist evaluating the patient | Presumed TIA on the basis of clinical symptoms |
| Douglas and colleagues, 200335 | . . . based on the World Health Organisation criteria as rapidly developed clinical signs of focal or global disturbance of cerebral function lasting fewer than 24 hours, with no apparent nonvascular cause | Presumed TIA based on diagnosis of emergency physician |
| The Dutch TIA Trial Study Group, 1993202 | A TIA was defined as a focal neurological deficit with abrupt or relatively rapid onset, persisting less than 24 hours; and a minor stroke, as a similarly focal neurological deficit persisting more than 24 hours | Patients were seen by a neurologist |
| Eliasziw and colleagues, 1995203 | . . . transient focal neurologic dysfunction of ischemic origin that resolved within 24 hours, leaving no deficit. Retinal events were defined as transient monocular visual loss | TIA diagnosis confirmed as part of recruitment procedure to the NASCET endarterectomy trial |
| Giles and colleagues, 2010184 | Patients were eligible if they had a diagnosis of TIA made according to the World Health Organisation time-based definition | Presumably various as the primary studies may have recruited patients with presumed TIA or definite TIA. The methods do not mention standardising diagnosis across the primary studies |
| Hankey and colleagues, 1992204 | . . . an acute loss of focal cerebral or monocular function with symptoms lasting less than 24 hours and which after adequate investigation was presumed to be due to embolic or thrombotic vascular disease | TIA diagnosis was confirmed by a neurologist |
| Hier and colleagues, 1991205 | Stroke was defined as a sudden, nonconvulsive, focal neurological deficit persisting for > 24 hours | Stroke diagnosis was confirmed by a neurologist |
| Kernan and colleagues, 200064 | Our case definitions for both a transient ischemic attack and a minor stroke in the carotid distribution included the sudden onset of dysphasia, unilateral motor weakness, or monocular blindness. For a transient ischemic attack, the case definition required the complete resolution of all neurologic signs and symptoms within 24 hours of symptom onset. The case definition for minor stroke required the persistence of neurologic signs beyond 24 hours . . . | NR |
| Lavallée and colleagues, 2007122 | TIA was defined as an acute loss of focal cerebral or ocular function with symptoms that lasted less than 24 hours and that were attributed to inadequate blood supply | Patients who had transient focal neurological features but whose clinical and radiological features were not sufficiently clear to affirm or exclude the diagnosis of TIA were judged to have a possible TIA. Patients with incomplete recovery were judged to have a minor stroke |
| Marnane and colleagues, 2011208 | Ischaemic stroke identified by an appropriate clinical syndrome (according to WHO definition) in whom brain imaging or pathologic confirmation was available | Definite ischaemic stroke |
| Purroy and colleagues, 2007182 | TIA was defined as a reversible episode of neurologic deficit of ischemic origin that resolved completely within 24 hours | Definite TIA ascertained by a neurologist |
| Purroy and colleagues, 2010150 | TIA was defined as a focal cerebral or ocular dysfunction with symptoms lasting less than 24 hours caused by vascular insufficiency or an arterial thromboembolism | TIA was diagnosed by a neurologist |
| Purroy and colleagues, 2012165 | A TIA was defined as a reversible episode of neurological deficit of ischemic origin that resolved completely with 24 hours | TIA was diagnosed by a neurologist |
| Rothwell and Warlow, 199960 | Not defined | TIA diagnosis confirmed as patients were assessed by neurologist and surgeon for suitability for carotid endarterectomy in the ECST endarterectomy trial |
| Rothwell and colleagues, 200561 | ‘. . . the standard definition.’ The reference gives, ‘rapidly developing clinical signs of focal (at times global) disturbance of cerebral function . . . with no apparent cause other than that of vascular origin’ | Results are given for suspected vs. probable and definite TIA diagnosis. However, the TIA status used by the IPD meta-analysis is not known |
| Sciolla and Melis, 200869 | WHO definition of a TIA | TIA diagnosis confirmed by neurologist |
| Sheehan and colleagues, 2010151 | TIA was defined clinically as an acute loss of focal cerebral or ocular function lasting < 24 hours, presumed, after investigation, to be due to embolic or thrombotic vascular disease . . . | TIA diagnosis was confirmed by a stroke specialist physician |
Most studies recruited patients from hospital settings, although three studies described themselves as population based. 61,151,208 The proportion of male patients ranged from 40.4% to 67.6%, but one study reported results for women only. 64 The reported average ages and age ranges were also broadly similar (Table 14).
| Study | No. of patients providing prediction data | Age (years), mean ± SD unless otherwise stated | Percentage male | Setting |
|---|---|---|---|---|
| Asimos and colleagues, 2010143 | 182 | 72.4 ± 14.4 | 40.4 | A+E departments |
| Bonifati and colleagues, 2011158 | 502 | 68.7 ± 17.1 | 46.4 | Primary care hospital |
| Cancelli and colleagues, 2011200 | 161 | 76.4 ± 11.7 | 51.0 | Various hospital departments |
| Coutts and colleagues, 2011201 | 334 | Median 70, range 18–95 | 58.1 | Hospital neurology department |
| Cucchiara and colleagues, 2009139 | 71 | 62.0 ± 14.0 | 44.9 | Hospital neurology department |
| Douglas and colleagues, 200335 | 322 | NR | 52.8 | 16 US A+E departments |
| The Dutch TIA Trial Study Group, 1993202 | 3127 | NR, but 1489 were > 65 years old | 60.2 | Hospital neurology department |
| Eliasziw and colleagues, 1995203 | 164 | 64.2 | 65.9 | Hospital neurology departments |
| Hankey and colleagues, 1992204 | 469 | Average not reported but 17.2% were < 60, 26.1% 60–70, and 36.4% > 70 | 67.6 | Hospital neurology department |
| Hier and colleagues, 1991205 | 1273 | Average not reported but 6.1% were < 44, 11.4% 45–54, 22.1% 55–64, 30.6% 65–74, 29.8% > 74 | 47.5 | Various hospital departments |
| Kernan and colleagues, 200064 | 525 | Average not reported, but 73.1% were > 65 | 0.0 | Hospital |
| Lavallée and colleagues, 2007122 | 204 | 64.7 ± 14.8 | 51.0 | Hospital-based TIA clinic |
| Marnane and colleagues, 2011208 | 314 | 70.3 | 51.6 | Population based |
| Purroy and colleagues, 2007182 | 388 | 70.8 ± 12.0 | 59.3 | Hospital A+E department (seen by neurologist) |
| Purroy and colleagues, 2010150 | 310 | 70.3 | 61.6 | Hospital neurology department |
| Purroy and colleagues, 2012165 | 1137 | 68.6 ± 13.1 | 59.3 | Hospital A+E department (seen by neurologist) |
| Rothwell and Warlow, 199960 | 1208 | 62.3 ± 8.0 | 71.8 | Hospital neurology departments |
| Rothwell and colleagues, 200561 | 227 | 73.7 ± 12.5 | 42.0 | Population based |
| Sciolla and Melis, 200869 | 274 | 71.5 ± 10.5 | 61.7 | Hospital A+E departments |
| Sheehan and colleagues, 2010151 | 88 | 68.0 ± 13.0 | 44.7 | Population based |
The reporting of secondary prevention therapy offered to patients generally listed the medical therapies without information on the proportion of patients receiving them, or speed of administration. Some studies gave the numbers of patients undergoing carotid surgery, but none indicated how the removal of what is known to be a major source of stroke risk was accounted for in the prediction modelling (Table 15).
| Study | TIA/minor stroke status | Secondary prevention | Proportion of patients with recurrent strokes |
|---|---|---|---|
| Asimos and colleagues, 2010143 | Probable TIA | NR | 0.042 |
| Bonifati and colleagues, 2011158 | Probable TIA | Antiplatelet therapy for nearly all patients, also anticoagulation and statins if indicated; nine patients underwent carotid endarterectomy | 0.060 |
| Cancelli and colleagues, 2011200 | Definite TIA | Patients received drug prescriptions | 0.111 |
| Coutts and colleagues, 2011201 | Definite TIA and ischaemic stroke | NR | 0.080 |
| Cucchiara and colleagues, 2009139 | Probable TIA | Heparin and warfarin; 11 patients had carotid revascularisation | 0.070 |
| Douglas and colleagues, 200335 | Probable TIA | NR | 0.109 |
| The Dutch TIA Trial Study Group, 1993202 | Definite TIA and minor ischaemic stroke | All patients were randomised to a low or high dose of aspirin | 0.183 |
| Eliasziw and colleagues, 1995203 | Definite TIA | Antiplatelet treatment (1300 mg of aspirin per day, antihypertensive, antilipid, and antidiabetic therapy, no carotid endarterectomy as this was the medical arm of the NASCET trial) | 0.136 |
| Hankey and colleagues, 1992204 | Definite TIA | Some patients received aspirin and carotid endarterectomy | 0.134 |
| Hier and colleagues, 1991205 | Definite stroke | NR | 0.101 |
| Kernan and colleagues, 200064 | Probable TIA and ischaemic stroke | Some patients had been randomised to received hormone replacement therapy (oestrogen) | 0.175 |
| Lavallée and colleagues, 2007122 | Probable TIA | Aspirin, blood pressure and lipid-lowering treatment, heparin, carotid revascularisation | 0.108 |
| Marnane and colleagues, 2011208 | Definite stroke | Five patients underwent carotid endarterectomy and three patients underwent carotid stenting. The majority of patients also received antiplatelet therapy, antihypertensive agents and statins | 0.083 |
| Purroy and colleagues, 2007182 | Definite TIA | Aspirin, clopidogrel, triflusal, anticoagulation, statins, renin–angiotensin system blockers, and carotid endarterectomy | 0.090 |
| Purroy and colleagues, 2010150 | Definite TIA | Nine patients received carotid endarterectomy, 80.9% of patients received antiplatelet therapy, 19.0% received anticoagulant therapy, 36.8% began using statins, and 50.6% angiotensin-converting enzyme (ACE) inhibitors | 0.078 |
| Purroy and colleagues, 2012165 | Definite TIA | Aspirin, clopidogrel, triflusal, anticoagulation, statins, renin–angiotensin system blockers, carotid endarterectomy, and carotid angioplasty | 0.038 |
| Rothwell and Warlow, 199960 | Definite TIA | Smoking advice, antihypertensive treatment, antiplatelet drugs, no carotid endarterectomy as this was the medical arm of ECST | 0.117 |
| Rothwell and colleagues, 200561 | Probable TIA | NR | 0.053 |
| Sciolla and Melis, 200869 | Definite TIA | Carotid endarterectomy (n = 7), intracranial stenting (n = 2), AF (n = 2), antiplatelet/anticoagulant (all patients) | 0.055 |
| Sheehan and colleagues, 2010151 | Definite TIA | Thirty-eight patients with endarterectomy, and carotid stenting in six | 0.114 |
Many studies used the same definition of stroke, which was often the World Health Organization (WHO) definition of stroke or very similar (Table 16, and see Figures 17 and 18). Only one study specified that the recurrent stroke had to have a certain severity,143 two studies defined recurrent stroke as worsening or new symptoms201,202 and one study collected information on whether or not the recurrent stroke was disabling, but did not use this information in the analyses. 204
| Study | Timing of baseline imaging | Definition of baseline positive imaging result | Definition of recurrent stroke |
|---|---|---|---|
| Asimos and colleagues, 2010143 | Within 7 days of symptom onset. | . . . brain imaging study . . . demonstrating infarction of brain tissue in a region consistent with the patient’s documented disturbance of neurological function | Recurrent stroke with a mRS score of ≥ 2 |
| Bonifati and colleagues, 2011158 | NR | NR | NR |
| Cancelli and colleagues, 201200 | NR | Carotid stenosis was defined according to TOAST criteria as narrowing of the ICA lumen of > 50% on carotid duplex ultrasound or angiography | WHO definition of stroke was used |
| Coutts and colleagues, 2011201 | Carotid imaging: NR | Carotid imaging: ‘An overall assessment of the presence or absence of symptomatic extracranial carotid artery disease (≥ 50% stenosis) was made by a stroke neurologist who combined all imaging and clinical data.’ Extracranial angiography was performed with a 3T MR scanner | If a patient deteriorated neurologically (defined as neurological worsening that affected function, persisting for at least 24 hours that was not caused by metabolic, infection or other such factors), the patient was either classified as recurrent stroke or a stroke progression. There was not a strict time threshold for the diagnosis of progression, but a temporal association with the index event was required . . . New symptoms in a new vascular territory were considered recurrent stroke |
| CT imaging: within 24 hours of symptom onset | CT imaging: NR | ||
| Cucchiara and colleagues, 2009139 | Within 48 hours of symptom onset | NR | NR |
| Douglas and colleagues, 200335 | Within 48 hours of presentation to A+E | Results are reported for new, old, and both new and old infarcts (brain CT); it is not clear what definition of positive CT scan was used in the IPD analysis | Stroke was defined as rapidly developing sings of focal or global disturbance or cerebral function, with no apparent nonvascular cause, lasting > 24 hours or resulting in death |
| The Dutch TIA Trial Study Group 1993202 | Within 1 month of symptom onset | NR | For the diagnosis of a nonfatal stroke, relevant clinical features persisting for more than 24 hours had to correspond with a new infarction or hemorrhage on a repeated CT scan. If sudden or focal neurologic deficits without CT changes (or in the absence of a CT scan) caused an increase in handicap of least one grade on the mRS, the event was also classified as a stroke |
| Eliasziw and colleagues, 1995203 | Carotid imaging: within 120 days of symptoms | Carotid imaging: ‘Biplane (anterior-posterior, lateral, and/or oblique) selective carotid angiography was used . . .’ The degree of stenosis was defined by the NASCET criteria | Outcome events included all strokes (defined as an acute ischemic event in which symptoms or signs persisted for more than 24 hours) ipsilateral to the entry TIA |
| CT imaging: median time between last TIA and CT scan was 10 days | CT imaging: ‘CTs were evaluated for the presence (or absence) of appropriate circulation of the brain and ipsilateral to the symptomatic carotid artery’ | ||
| Hankey and colleagues, 1992204 | NR | Carotid IAA | Minor stroke: patients with the clinical criteria of a stroke in whom symptoms lasted more than 24 hours and less than one week. Neurological signs of no functional significance . . . were acceptable thereafter |
| Major stroke: patients with the clinical criteria of a stroke in whom symptoms lasted for > 1 week or led to an earlier death. The severity of the stroke was assessed approximately 6 months after onset by a modification of the Rankin scale; scores of 0 to 2 were considered ‘non-disabling’, and scores of 3 to 5 were ‘disabling’. In the analyses, disabling strokes were not separated from other strokes | |||
| Hier and colleagues, 1991205 | Medians are provided for various stroke subtypes (infarct, embolic, atherosclerotic, lacunar and haemorrhagic); all medians are ≤ 26 hours | No explicit definition given. However, lesions on CT scans related to the stroke are discussed in the methods | Stroke was defined as a sudden, nonconvulsive, focal neurological deficit persisting for > 24 hours |
| Kernan and colleagues, 200064 | NR | Infarction | . . . stroke was defined as any new focal neurological deficit that persisted for more than 24 hours |
| Lavallée and colleagues, 2007122 | Unclear; IPD study reports that the delay to evaluation was 1 day (IQR 0 to 5 days); it is not stated whether the timing is made from symptom onset on admission to TIA clinic | If the brain CT or MRI scans revealed an acute infarction in an area that corresponded to the clinical symptoms, the patient was judged to have a TIA with a new lesion | A stroke endpoint was defined by focal neurological deficit of sudden onset that lasted for more than 24 hours, with or without confirmation from brain imaging results |
| Marnane and colleagues, 2011208 | NR | CS [carotid stenosis] was graded using a method similar to that in the NASCET study, with comparison of the lumen diameter at the maximum site of stenosis to that of the normal-appearing carotid artery distal to the stenosis.’ Imaging modalities were Doppler ultrasound, CT or MRA. Imaging was performed on the ipsilateral artery. Stenosis was dichotomised at 50% | Stroke recurrence was defined as a new, sudden-onset focal neurologic deficit in the same or other vascular territory lasting > 24 hours in a patient with examination findings which were initially stable for at least 24 hours |
| Purroy and colleagues, 2007182 | On day of admission | Baseline cervical ICA [internal carotid artery] atherosclerosis was categorized by echo Doppler as follows: absent; mild, if one or both ICAs had < 50% stenoses; moderate when any of the ICAs presented with 50% to 70% stenoses; and severe if any ICA had > 70% stenoses | Stroke was defined as rapidly developed clinical symptoms of focal disturbance of cerebral function lasting > 24 hours with an apparent vascular cause |
| Purroy and colleagues, 2010150 | Within 24 hours of symptom onset (median 6.5 hours, IQR 3.8 to 19.0 hours) | NR | NR |
| Purroy and colleagues, 2012165 | NR | . . . the presence of any infarction on CT imaging (given the unreliability of distinction between acute and old infarction) irrespective of whether it was appropriate to the infarction causing the presenting symptoms | NR |
| Rothwell and Warlow, 199960 | Within 120 days of symptom onset | Carotid imaging: stenosis defined by the ECST method [ref] measured on catheter angiograms | NR |
| Rothwell and colleagues, 200561 | Median 1 day (IQR 0 to 2 days); this figure was reported in the IPD meta-analysis; it is unclear whether it is time from event or admission | NR | The definition of stroke used in this and subsequent analyses was any stroke (by WHO criteria) occurring after full resolution of the initial TIA |
| Sciolla and Melis, 200869 | Within 24 hours; this figure was reported in the IPD meta-analysis. It is unclear whether it is time from event or admission | . . . from the CT scan performed in the ED (leukoaraiosis and/or old/new ischemic lesion = 1 point, normal = 0 point) | Stroke was defined as a cerebrovascular event of sudden onset lasting for > 24 hours, and clearly resulting in an increase of an existing or a new neurologic deficit . . . Brain imaging showing a new ischemic lesion in a vascular territory unaffected on the admission CT scan had to be present in all stroke cases |
| Sheehan and colleagues, 2010151 | Median 1 day; this figure was reported in the IPD meta-analysis; it is unclear whether it is time from event or admission | Carotid imaging: ‘Carotid stenosis was defined according to the TOAST method, as a narrowing . . . of 50% or greater . . . The degree of carotid stenosis of duplex ultrasonography was calculated with the use of NASCET-based criteria . . .’ From the analysis, it is clear they used 0–49%, 50–69% and 70–99% and occluded as categories CT imaging: NR | Recurrent strokes were defined as a new neurologic deficit fitting the standard definition of a stroke, which occurred after a period of neurologic stability or improvement lasting at least 24 hours |
The definition of carotid stenosis varied from study to study. Three studies stated that the stenosis was dichotomised at 50%, but did not say whether this meant 50% by the NASCET or ECST criteria,200,201,208 and others also categorised their data, for example into 0–49%, 50–69% and 70–99% by the NASCET criteria. 151 Carotid stenosis was most often measured by ultrasound.
The definition of a positive CT scan also varied from study to study. Some specified that the infarct had to be new or consistent with the patient’s symptoms. 122,143,203 Two studies said that, owing to the difficulty of distinguishing between old and new lesions, the appearance of any lesion meant that the scan was deemed positive. 69,165 All of the other studies either did not report or inadequately reported their definition of a positive CT scan (see Table 16). The time of imaging from either symptom onset or presentation was recorded because the ability of CT to detect stroke lesions is heavily time dependent, particularly so in the first few hours after symptom onset. The timing varied from within 24 hours to within 1 month of symptom onset.
Main findings
Prediction of recurrent stroke
Seven of the CT studies’ results were provided by the IPD meta-analysis,184 expressed as the AUC of logistic regression models for the ABCD2 and ABCD2I clinical prediction scores. The total number of patients contributing CT data was 1368. The increase in the AUC for both 7- and 90-day recurrent stroke outcome was often modest, with a large overlap of CI between the AUCs of the two scores. The IPD study reported a summary 7-day AUC increase from 0.64 (95% CI 0.49 to 0.79) to 0.71 (95% CI 0.57 to 0.84), and a summary 90-day AUC increase from 0.69 (95% CI 0.65 to 0.74) to 0.75 (95% CI 0.70 to 0.80). 184
Unfortunately, only three studies,60,202,203 with a total of 4499 patients, provided data suitable for a meta-analysis of HRs. Each of the three studies used a statistical model rather than a simple score, with predictors not found in the other two studies. There was also evidence of significant heterogeneity demonstrated by both the forest plot (Figure 19) and a high I2-statistic of 78.5%. The summary HR was 1.74 (95% CI 0.82 to 3.70, p = 0.15); however, this is difficult to interpret beyond there being a positive though non-significant relationship between having a lesion found by CT and recurrent stroke. The difficulties derive from different patients groups (probable TIA202 vs. definite TIA)60,203 different definitions of lesion positive and outcome, and timing of imaging (Table 17) and different statistical models (Table 18).
FIGURE 19.
Forest plot of HRs of having a lesion found by brain CT in a multivariate model in the prediction of recurrent stroke. Summary HR is 1.74 (95% CI 0.82 to 3.70) from a random-effects meta-analysis of data from 4499 patients.
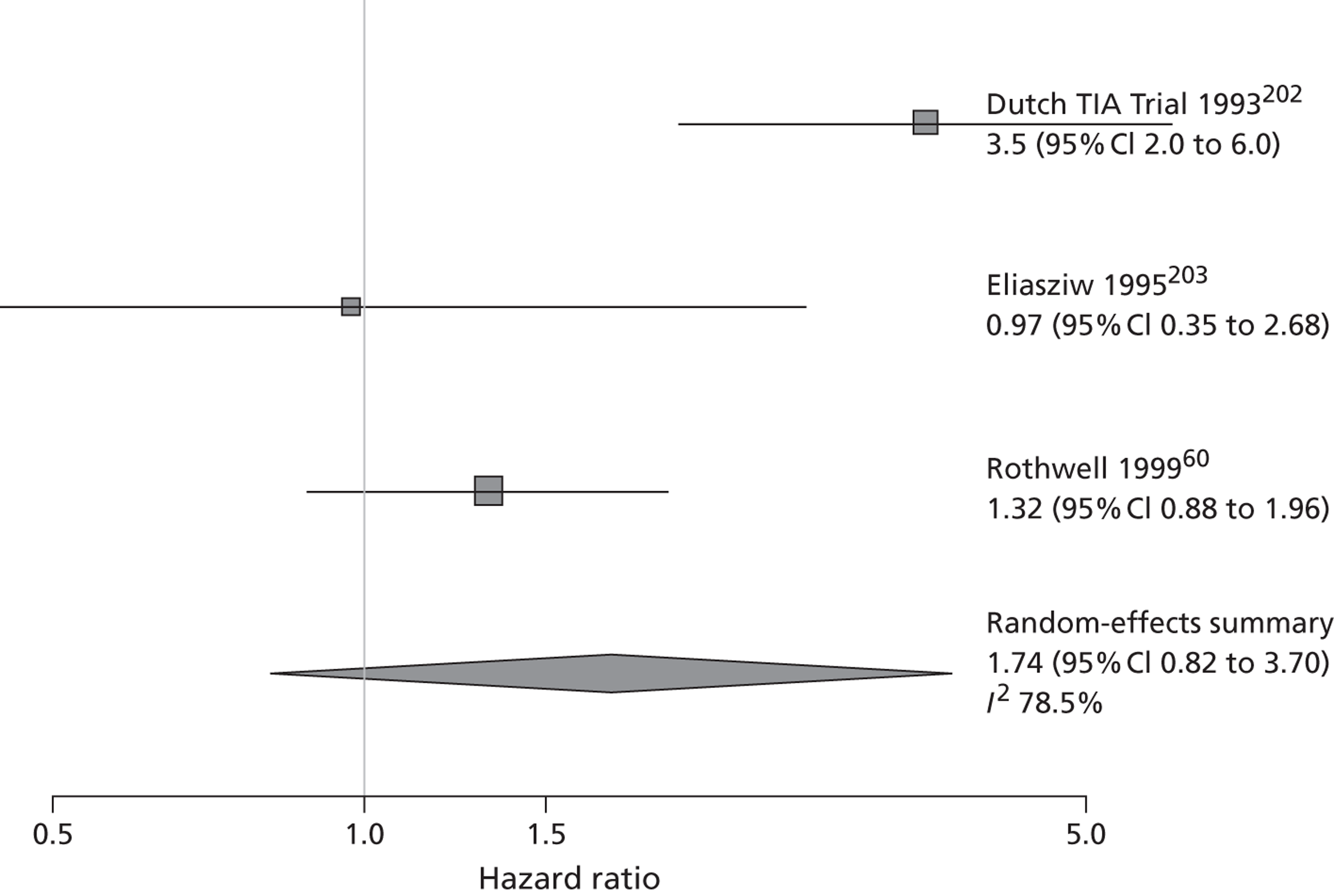
| Study | Model | Effect of addition of CT |
|---|---|---|
| Asimos and colleagues, 2010143 | ABCD2 vs. ABCD2I (patients given an additional point for a positive CT scan) | AUC for logistic regression model increased from 0.68 (95% CI 0.54 to 0.83) to 0.86 (95% CI 0.76 to 0.95) for recurrent stroke at 7 days; results were identical for recurrent stroke at 90 days |
| Bonifati and colleagues, 2011158 | ABCD2 with both CT and carotid stenosis added | Survival analysis (Cox proportional hazards) was used. CT (and carotid stenosis) remained significant (p = 0.02), whereas ABCD2 did not; only p-values reported |
| Coutts and colleagues, 2011201 | Results for CT are available from a univariate analysis only | Survival analysis (Cox proportional hazards); HR was 0.8 (95% 0.3 to 2.1) |
| Cucchiara and colleagues, 2009139 | ABCD2 vs. ABCD2I (patients given an additional point for a positive CT scan) | AUC for logistic regression model increased from 0.62 (95% CI 0.24 to 0.99) to 0.64 (0.23 to 1.00) for recurrent stroke at 7 days AUC for logistic regression model increased from 0.57 (95% 0.27 to 0.87) to 0.64 (0.32 to 0.95) for recurrent stroke at 90 days |
| Douglas and colleagues, 200335 | ABCD2 vs. ABCD2I (patients given an additional point for a positive CT scan) | AUC for logistic regression model increased from 0.64 (95% CI 0.55 to 0.73) to 0.67 (95% CI 0.57 to 0.77) for recurrent stroke at 7 days AUC for logistic regression model increased from 0.70 (95% CI 0.62 to 0.78) to 0.72 (0.64 to 0.80) for recurrent stroke at 90 days |
| The Dutch TIA Trial Study Group, 1993202 | Male sex, age > 65 years, dysarthria, multiple attacks, persisting > 6 weeks [minor strokes were also recruited], diabetes, intermittent claudication, haematocrit > 0.45 l/l, anteroseptal infarct, increased terminal P-wave | Survival analysis model (Cox proportional odds). Border zone infarct on CT, HR 3.5 (95% CI 2.0 to 6.0), any other infarct 1.6 (95% CI 1.2 to 2.0), white matter hypodensity 1.6 (95% CI 1.2 to 2.2) |
| Eliasziw and colleagues, 1995203 | Age, TIA duration, ipsilateral stenosis, most recent event and history of TIA, hypertension, coronary artery disease, ipsilateral ulcerated plaque, occluded contralateral artery | Survival analysis (Cox proportional hazards); presence of infarct on CT was significant in univariate analysis (HR 2.31; 95% CI 1.04 to 5.16) but not in a multivariate model (HR 0.97; 95% CI 0.35 to 2.68) |
| Hankey and colleagues, 1992204 | Sex, age, left ventricular hypertrophy, peripheral vascular disease, diabetes, carotid bruit, arcus senilis, cardiomegaly, ischaemic heart disease, AF, migraine, SBP, body mass index, number of cigarettes per day, alcohol units per day, carotid/vertebrobasilar presentation, AFx, residual neurological signs, low haematocrit, high haematocrit, infarct on CT on symptomatic side, infarct on CT for asymptomatic side, carotid stenosis on symptomatic side | Survival analysis (Cox proportional hazards). No numerical results reported. ‘. . . non-significant factors were . . . cranial CT evidence of infarction on the symptomatic and asymptomatic sides . . .’ |
| Hier and colleagues, 1991205 | Diabetes, diastolic hypertension (≥ 100 mmHg), prior stroke, infarct of unknown cause | Survival analysis (Kaplan–Meier); although an abnormal baseline CT scan was significant in univariate analysis (risk of stroke within 2 years, p = 0.03), it was not included in the multivariate model |
| Kernan and colleagues, 200064 | Age, physical functioning, cognitive skills, > 80% carotid stenosis, prior TIAs or strokes, hypertension, coronary artery disease, myocardial dysfunction, AF, left ventricular hypotrophy, diabetes peripheral vascular disease | RR for stroke or death within 2 years of infarct on CT imaging adjusted for all other variables was 1.0. No CI provided |
| Lavallée and colleagues, 2007122 | ABCD2 vs. ABCD2I (patients given an additional point for a positive CT scan) | AUC for logistic regression model increased from 0.77 (95% CI 0.58 to 0.95) to 0.85 (95% CI 0.72 to 0.98) for recurrent stroke at 7 days AUC for logistic regression model increased from 0.60 (95% CI 0.36 to 0.84) to 0.65 (95% CI 0.39 to 0.91) for recurrent stroke at 90 days |
| Purroy and colleagues, 2010150 | ABCD, ABCD2, ABCDI, ABCD2I (patients given an additional point for a positive CT scan) | None of the models was found to be statistically significant predictors of ischaemic stroke at either 7 or 90 days. AUCs are 0.55 (95% 0.43 to 0.67) for ABCD, 0.57 (95% CI 0.45 to 0.69) for ABCD2, 0.53 (95% CI 0.41 to 0.65) for ABCDI, and 0.55 (95% CI 0.43 to 0.67) for ABCD2I. It is not clear from the text whether these figures refer to outcome at 7 or 90 days. Other analyses by the authors (not reported here) clearly demonstrate no statistically significant relationship between the models and recurrent stroke at 7 or 90 days |
| Purroy and colleagues, 2012165 | ABCD, ABCD2, ABCDI, ABCD2I, ABCD3 (patients given an additional point for a positive CT scan, ABCD3 includes a point for prior TIA within 1 week of presenting event) | Method of analysis is described as ‘Cox regression analysis’, i.e. survival analysis; however, results are reported for specific time points, i.e. as if the method of analysis was logistic regression. Only ABCD3 was a significant predictor of recurrent stroke at either 7 or 90 days AUCs for stroke at 7 days: ABCD 0.57 (95% CI 0.46 to 0.68), ABCD2 0.56 (95% CI 0.45 to 0.66), ABCDI 0.56 (95% CI 0.44 to 0.67), ABCD2I 0.56 (95 % CI 0.45 to 0.67) and ABCD3 0.66 (95% CI 0.57 to 0.81) AUCs for stroke at 90 days: ABCD 0.55 (95% CI 0.46 to 0.64), ABCD2 0.55 (95% CI 0.46 to 0.64), ABCDI 0.56 (95% CI 0.45 to 0.67), ABCD2I 0.55 (95% CI 0.44 to 0.65) and ABCD3 0.61 (95% CI 0.52 to 0.70) |
| Rothwell and Warlow, 199960 | Cerebral vs. ocular events, residual neurological signs after 7 days, diabetes, any ischaemic event in previous 2 months, number of events in previous 3 years, previous MI, degree of carotid stenosis, plaque surface irregularity, poststenotic collapse of the ICA, age, male sex, SBP, DBP, peripheral vascular disease, angina, left ventricular hypertrophy, occlusion of contralateral carotid artery | Survival analysis (Cox proportional hazards). Cerebral infarction on symptomatic side by CT was not found to be significant in a multivariate model, HR 1.32 (95% CI 0.88 to 1.96) |
| Rothwell and colleagues, 200561 | ABCD2 vs. ABCD2I (patients given an additional point for a positive CT scan) | AUC for logistic regression model increased from 0.78 (95% CI 0.69 to 0.86) to 0.79 (95% CI 0.71 to 0.87) for recurrent stroke at 7 days AUC for logistic regression model increased from 0.67 (95% CI 0.58 to 0.76) to 0.71 (95% CI 0.63 to 0.8) for recurrent stroke at 90 days |
| Sciolla and Melis, 200869 | ABCD2 vs. ABCD2I (patients given an additional point for a positive CT scan) | AUC for logistic regression model increased from 0.75 (95% CI 0.63 to 0.88) to 0.78 (95% CI 0.64 to 0.92) for recurrent stroke at 7 days AUC for logistic regression model changed from 0.76 (95% CI 0.66 to 0.86) to 0.76 (95% CI 0.65 to 0.88) for recurrent stroke at 90 days |
| Sheehan and colleagues, 2010151 | ABCD2 vs. ABCD2I (patients given an additional point for a positive CT scan) | AUC for logistic regression model increased from 0.24 (95% CI 0.11 to 0.36) to 0.31 (95% CI 0.17 to 0.45) for recurrent stroke at 7 days AUC for logistic regression model increased from 0.61 (95% CI 0.38 to 0.85) to 0.70 (95% CI 0.50 to 0.90) for recurrent stroke at 90 days |
| Study | Model | |
|---|---|---|
| Bonifati and colleagues, 2011158 | ABCD2 with both CT and carotid stenosis added | Survival analysis (Cox proportional hazards) was used. Carotid stenosis (and CT) remained significant (p = 0.02), whereas ABCD2 did not; only p-values reported |
| Cancelli and colleagues, 2011200 | It is not clear whether or not carotid stenosis was included in a multivariate model | Survival analysis (Kaplan–Meier) was used. Symptomatic internal carotid stenosis > 50% was not statistically significant, 0.136 vs. 0.103, p = 0.63 |
| Coutts and colleagues, 2011201 | Final model for stroke recurrence included blood glucose > 8 mmol/l and symptomatic internal carotid stenosis ≥ 50% | Survival analysis (Cox proportional hazards) in patients with an NIHSS score of ≤ 5, HR for carotid stenosis 5.6 (95% CI 2.0 to 15.6) |
| Eliasziw and colleagues, 1995203 | Age, TIA duration, ipsilateral stenosis, most recent event and history of TIA, hypertension, coronary artery disease, ipsilateral ulcerated plaque, occluded contralateral artery | Survival analysis (Cox proportional hazards). Carotid stenosis was categorised to the nearest decile and was not found to be significant, HR 1.30 (95% CI 0.78 to 2.14), although this study excluded patients with < 70% stenosis |
| Hankey and colleagues, 1992204 | Sex, age, left ventricular hypertrophy, peripheral vascular disease, diabetes, carotid bruit, arcus senilis, cardiomegaly, ischaemic heart disease, AF, migraine, SBP, body mass index, number of cigarettes per day, alcohol units per day, carotid/vertebrobasilar presentation, AFx, residual neurological signs, low haematocrit, high haematocrit, infarct on CT on symptomatic side, infarct on CT for asymptomatic side, carotid stenosis on symptomatic side | Survival analysis (Cox proportional hazards). No numerical results reported, although results were non-significant at the 5% level |
| Kernan and colleagues, 200064 | Age, physical functioning, cognitive skills, > 80% carotid stenosis, prior TIAs or strokes, hypertension, coronary artery disease, myocardial dysfunction, AF, left ventricular hypotrophy, diabetes peripheral vascular disease | RR for stroke or death within 2 years for carotid stenosis > 80% in univariate analysis was 1.0; no CI or adjusted estimate provided |
| Marnane and colleagues, 2011208 | Age, sex, hypertension, diabetes, smoking, AF, acute antiplatelet and statin treatment | Survival analysis (Cox proportional hazards). Carotid stenosis was the only independent predictor of stroke at 72 hours (HR 36.1; 95% CI 1.6 to 837.5); results also available for 7 days (HR 9.1; 95% CI 1.1 to 79.2) and 14 days (HR 4.6; 95% CI 0.9 to 22.8) |
| Purroy and colleagues, 2007182 | Results available for a univariate analysis only | See Table 16 for definitions. Carotid stenosis absent, 5% risk of stroke, mild 25% risk of stroke, moderate, 11% risk of stroke, and severe 35% risk of stroke; p-value for trend < 0.0001 |
| Rothwell and Warlow, 199960 | Cerebral vs. ocular events, residual neurological signs after 7 days, diabetes, any ischaemic event in previous 2 months, number of events in previous 3 years, previous MI, degree of carotid stenosis, plaque surface irregularity, poststenotic collapse of the ICA, age, male sex, SBP, DBP, peripheral vascular disease, angina, left ventricular hypertrophy, occlusion of contralateral carotid artery | Survival analysis (Cox proportional hazards). Adjusted HR for carotid stenosis 1.25 (95% CI 1.18 to 1.40) in medically treated patients; carotid stenosis was no longer significant in the model for carotid endarterectomy patients |
| Sheehan and colleagues, 2010151 | Age and sex | Survival analysis (Cox proportional hazards); results available for ≥ 50% stenosis (HR 2.65; 95% CI 1.28 to 5.20) and ≥ 70% (HR 3.23; 95% CI 1.45 to 7.21) |
The possibility of bias must also be considered, given how small a subset of studies contributed to the meta-analysis.
Considering all CT studies, whether used in a meta-analysis or not, some found CT to be not significantly associated with recurrent stroke when adjusted for other predictors60,201,203–205 in survival analyses. Three studies also found CT to be non-significant with other analytic methods. 64,150,165 Only two studies found a significant relationship: one study that reported only p-values158 but another that gave fuller results202 (see Table 17).
The results for carotid stenosis provide stronger evidence of a relationship with recurrent stroke, with 6 out of 10 studies60,151,158,182,201,208 finding a significant relationship (see Table 18). One of the studies had not excluded patients with < 70% stenosis by the NASCET criteria,203 so could not provide an estimate of the risk of stroke between patients with lower degrees of stenosis compared with higher degrees.
Four of the studies provided data from 1944 patients suitable for a meta-analysis of HRs. 60,151,201,208 There was evidence of significant heterogeneity from both the forest plot (Figure 20) and the I2-statistic (81.9%), and the summary HR for carotid stenosis was 3.02 (95% CI 1.18 to 7.75, p = 0.02). Again, the interpretation of these summary statistics is challenging owing to different definitions of carotid stenosis and patient populations, and different statistical models used by the primary studies, but it does provide evidence of a link between carotid stenosis and recurrent stroke.
Perhaps the strongest evidence comes from an analysis of data from the ECST, in which carotid stenosis was a significant predictor of recurrent stroke in patients receiving medical treatment only, but not in those who underwent carotid endarterectomy. 60 When studies looked at the presence/absence of large artery atherosclerosis, which included carotid stenosis as a component, it was found to be a predictor of recurrent stroke at 90 days. 81,134,137,146,147,165,182,204–209
FIGURE 20.
Forest plot of HRs of carotid stenosis in a multivariate model in the prediction of recurrent stroke. Summary HR is 3.02 (95% CI 1.18 to 7.75) from a random-effects meta-analysis of data from 1944 patients.
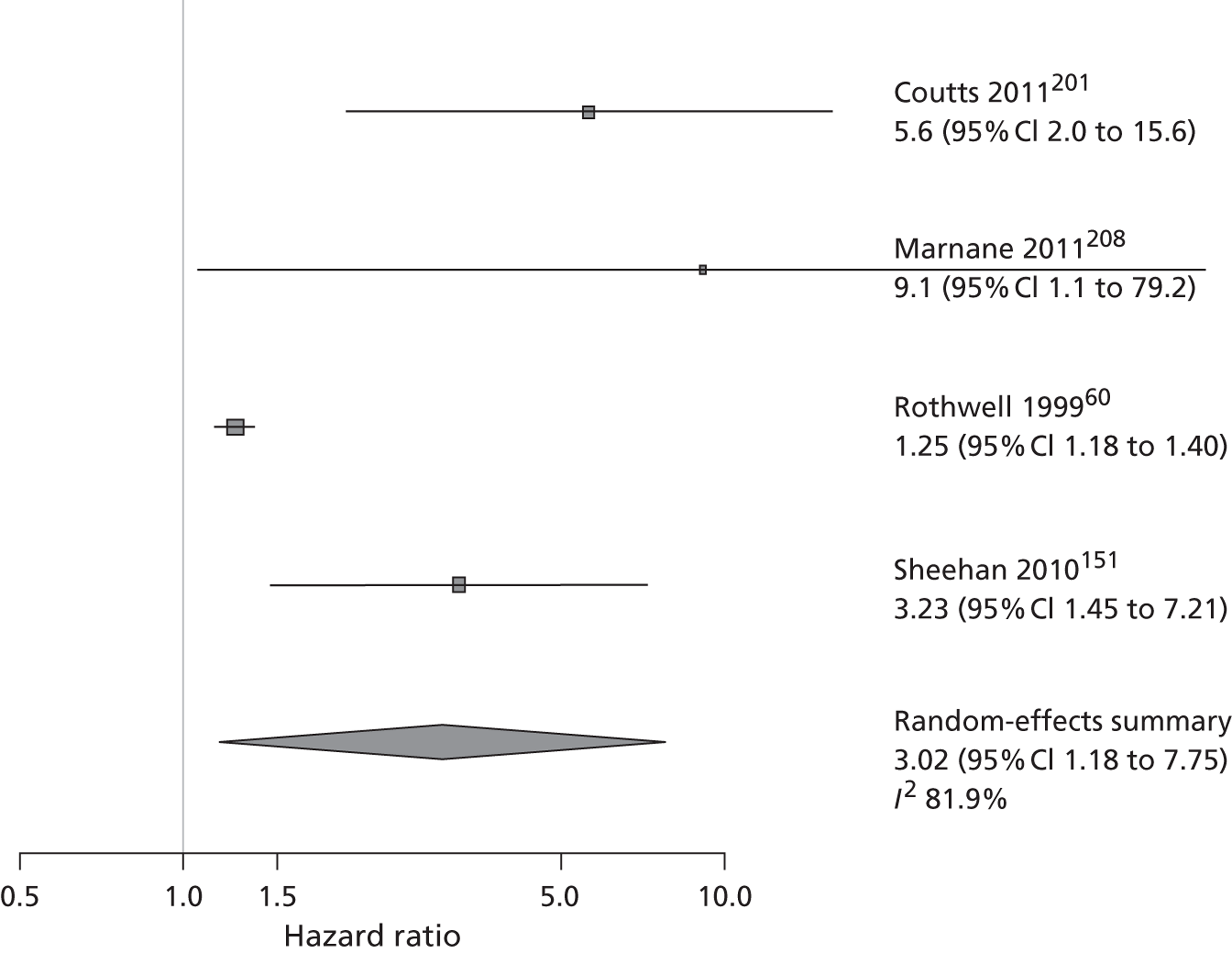
The LSR registered 2940 patients with definite, probable or possible stroke, TIA, AFx or RAO, but 929 were excluded from the present analysis because of severe stroke or only possible cerebral or ocular ischaemic symptoms, leaving 2011 patients for inclusion in the analysis [1093 (54%) minor stroke, 828 (41%) TIA and the remainder had RAO]. Ninety-seven per cent of patients were followed up to 6 months, 90% to 12 months, and 62% to 24 months. The numbers suffering a stroke were 65, 85 and 82 by 6, 12 and 24 months, respectively. Of the recurrent strokes, 93% were ischaemic, and 48% were small cortical (PACS), 28% large cortical (TACS) and 9% were lacunar (LACS). Overall, 189/2011 (9%) patients had a tight (70–99%) symptomatic carotid stenosis and 86 (4%) patients had a carotid endarterectomy at a mean of 3 months after the presenting event (range 6 days to 1.5 years).
The analysis of the data from the LSR found both carotid stenosis and visible infarct on CT to be significantly associated with recurrent disabling or fatal stroke (Table 19). Unadjusted HRs for carotid stenosis by the NASCET criteria and visible infarct on CT were 1.006 (95% CI 1.003 to 1.008) and 2.45 (95% CI 1.73 to 3.46), respectively. The HR for carotid stenosis is small because it represents the change in risk for an increase of just one degree of stenosis. If carotid stenosis is dichotomised at 70% then the HR for patients above compared with below this cut-off is 2.42 (95% CI 1.63 to 3.61). When adjusted for residual neurological signs, age, previous stroke, brain compared with ocular symptoms, and coronary disease, the HRs for carotid stenosis and visible infarct on CT are 1.009 (95% CI 1.004 to 1.013) and 1.47 (95% CI 1.01 to 2.14), respectively. If carotid stenosis is dichotomised at the 70% cut-off, its adjusted HR is 2.15 (95% CI 1.42 to 3.23). The carotid stenosis HRs are also adjusted for CT and vice versa.
| Model | Predictor | HR | 95% CI | p-value |
|---|---|---|---|---|
| Model A | Residual neurological signs | 1.818 | 1.238 to 2.668 | 0.0023 |
| Age | 1.031 | 1.014 to 1.048 | 0.0004 | |
| Previous stroke | 1.938 | 1.307 to 2.873 | 0.0010 | |
| Ocular symptoms | 0.449 | 0.195 to 1.036 | 0.0606 | |
| Coronary disease | 1.619 | 1.139 to 2.301 | 0.0072 | |
| Model B | Residual neurological signs | 1.834 | 1.249 to 2.693 | 0.0020 |
| Carotid stenosis | 1.009 | 1.005 to 1.013 | < 0.0001 | |
| Age | 1.029 | 1.011 to 1.047 | 0.0013 | |
| Previous stroke | 1.824 | 1.228 to 2.711 | 0.0029 | |
| Ocular symptoms | 0.379 | 0.164 to 0.879 | 0.0238 | |
| Coronary disease | 1.524 | 1.072 to 2.168 | 0.0190 | |
| Model C | Residual neurological signs | 1.577 | 1.050 to 2.368 | 0.0282 |
| Age | 1.030 | 1.013 to 1.048 | 0.0006 | |
| Previous stroke | 1.999 | 1.348 to 2.967 | 0.0006 | |
| Ocular symptoms | 0.509 | 0.218 to 1.188 | 0.1184 | |
| Coronary disease | 1.648 | 1.159 to 2.343 | 0.0054 | |
| Visible infarct | 1.518 | 1.043 to 2.210 | 0.0293 | |
| Model D | Residual neurological signs | 1.612 | 1.074 to 2.419 | 0.0211 |
| Carotid stenosis | 1.009 | 1.004 to 1.013 | < 0.0001 | |
| Age | 1.028 | 1.011 to 1.046 | 0.0016 | |
| Previous stroke | 1.898 | 1.276 to 2.823 | 0.0016 | |
| Ocular symptoms | 0.428 | 0.182 to 1.003 | 0.0509 | |
| Coronary disease | 1.552 | 1.091 to 2.209 | 0.0146 | |
| Visible infarct | 1.471 | 1.011 to 2.140 | 0.0437 |
None of the models A, B, C or D predicted that any patient would have a fatal or non-fatal but disabling stroke by 7 days. However, three patients did go on to have a non-fatal disabling stroke, another had a fatal stroke and there were also three other deaths within 7 days. All of these patients presented with minor stroke rather than TIA or RAO.
The results for 90 days and 2 years are given in Table 20. All of the models were better at correctly predicting a stroke outcome (fatal or non-fatal disabling stroke) at 2 years rather than 90 days. Conversely, all were better at predicting being alive and well at 90 days than 2 years.
| Outcome and time point | Model A | Model B | Model C | Model D |
|---|---|---|---|---|
| At 90 days | ||||
| No. of correctly predicted fatal or disabling strokes | 2 | 7 | 4 | 7 |
| No. of stroke outcomes that were not predicted | 26 | 21 | 24 | 21 |
| No. alive and well, and predicted no recurrent strokes | 1814 | 1798 | 1822 | 1800 |
| At 2 years | ||||
| No. of correctly predicted fatal or disabling strokes | 84 | 80 | 82 | 81 |
| No. of stroke outcomes that were not predicted | 10 | 14 | 12 | 13 |
| No. alive and well, and predicted no recurrent strokes | 647 | 724 | 687 | 764 |
At 90 days there were 27 fatal and non-fatal disabling strokes, 20 patients with another non-stroke event (MI, vascular or non-vascular death) and 1963 patients with no further events. At 2 years there were 94 fatal and non-fatal disabling strokes, 103 patients with another non-stroke event and 1814 patients with no further events.
For all models, 91–92% of patients had a correctly predicted outcome at 90 days. This fell to 41–47% of patients when considering outcomes at 2 years. Model D (clinical variables, plus carotid and brain CT imaging) had the highest proportion of correctly predicted outcomes at 2 years (47%). It is obvious that the time after presenting event at which the outcome is predicted is crucial in the prediction of stroke.
Discussion
In general, the performance of clinical prediction rules and statistical models are highly dependent on the characteristics of the patient sample used to test them. The studies found in this review used very different recruitment criteria, notably with regard to confirmation of TIA or ischaemic stroke diagnosis. A given model is likely to give different results depending on whether the patients are those with suspected TIA only, or confirmed ischaemic strokes only; this is particularly important given the high proportion of non-CV patients who present at TIA clinics (see Chapters 7 and 8). It must also be noted that the prognostic value of an infarct found by CT may be especially influenced by the patient sample, as the proportion of lesions is likely to increase with increasing severity of presenting symptoms. The studies were dissimilar in terms of the patients who they recruited.
The most common definition of outcome was simply recurrent stroke without any distinction made between disabling and non-disabling strokes. It may be that some factors are predictive of more severe outcomes, but any evidence of a relationship has been lost.
It is also difficult to draw any firm conclusions about any association that there may be between a positive CT scan and recurrent stroke. There are problems due to the timing of imaging – if a scan is done very soon after symptom onset, lesions are hard to detect. A further challenge to investigating the relationship is that some studies deliberately combined old and new lesions. The evidence found by this review on CT imaging is not robust enough to make a convincing case either way.
The case for carotid stenosis is stronger, with the majority of the studies and the multivariate survival analysis of the 2011 patients in the LSR finding a significant relationship with recurrent stroke. Perhaps the most convincing evidence comes from the endarterectomy trial data,60 where carotid stenosis was a predictor in medically treated patients, but not in those who had undergone carotid endarterectomy. It would be very hard to explain the efficacy of carotid endarterectomy if carotid stenosis were not a risk factor for recurrent stroke.
All of the studies contributing to the CT meta-analysis used data from RCTs. The studies contributing to the carotid stenosis meta-analysis used data from a RCT, two population-based studies and one prospective cohort study. Systematic review methodology is most developed in the area of interventions, where restriction to a particular study design, such as RCT, is sensible on both methodological (using the best available evidence) and pragmatic (limiting the number of studies to be assessed) grounds. Most systematic reviews use some kind of study design filter but our results suggest that it is important to consider using all study designs in a review of predictors so as to retrieve all of the available evidence. It is also worth noting that RCTs are often larger than observational studies – the three studies contributing to our CT meta-analysis had over three times as many patients as the seven observational studies contributing to the CT IPD meta-analysis.
Prediction of stroke is hard, and ascertainment of outcome is heavily dependent on the length of follow-up. The trade-off that must be made when using a clinical prediction score to designate patients into high- and low-risk categories, i.e. between not aggressively treating patients likely to have a recurrent stroke and asking patients to undergo stroke prevention treatment that is unlikely to be to their benefit, needs to consider the length of follow-up used when developing the original prediction score.
Weaknesses of the review
Owing to the nature of the underlying studies, our methodology had to be adapted from the ‘gold standard’ methodology used for RCTs. In particular, the citation search method and the critical appraisal checklist are unvalidated, although both were extremely thorough and produced in as methodologically sound way as possible.
The results of the meta-analyses are based on a small subset of the available studies due to factors beyond our control. However, we include a larger sample than were included in recent meta-analyses on stroke risk prediction, for example, 1368 patients with CT184,210 compared with our 4499. The results are difficult to interpret given the differences between the primary studies, and there is also a possibility of bias, not least because the studies with non-significant results could not be included owing to a lack of data.
Weaknesses of the Lothian Stroke Register analysis
The data were collected in the 1990s and so represent older CT technologies than those that are in use today (DUS with colour has changed relatively little in practical terms). For diagnosis of carotid stenosis, the same velocity criteria and image measurement have been in use since the 1990s so the stenosis measures are relevant and valid. The CT technology has moved to thinner scan sections, which may increase detection of small lesions, but the contrast has not improved. The presence of a visible infarct on 1990s technology is as relevant as on scanners today, although lesions may have been missed that would have been visible on current technology. On the other hand, many of the literature reports concern patients recruited in a similar time period on similar technology therefore the LSR data are still highly relevant. There would have been more delay between symptom onset and patient assessment in the 1990s owing to greater awareness now of the need to seek medical attention quickly after TIA/minor stroke. Although the LSR took place in a rapid access clinic that ran several times weekly with same day CT and US and direct access from GPs, patients may not have contacted their GP quickly, thereby incurring delays prior to reaching medical attention. Similarly, this problem will have affected all of the studies that use data from the 1990s but should not make a material difference in terms of identifying the factors which most strongly predict recurrent vascular events.
Conclusions
Carotid stenosis predicts recurrent stroke. However, investigating the link between carotid stenosis and disabling or fatal recurrent stroke compared with non-fatal non-disabling recurrent stroke would be of benefit. Carotid analyses that do not dichotomise the stenosis into severe and non-severe categories would increase the information available from the data. The evidence on brain CT imaging is inconclusive, as the studies were inconsistent in the timing of imaging and definition of a positive CT scan.
Chapter 6 Diffusion-weighted imaging in patients with transient ischaemic attack or minor stroke
Introduction
Patients with TIA are at high risk of early recurrent stroke. Accurate diagnosis and recognition of ischaemic causes of neurological symptoms are essential for effective management and stroke prevention. The observer agreement for diagnosis of TIA among physicians is poor, even with rating scales. 73,74 The territory affected by a TIA/minor stroke may be difficult to ascertain clinically. 57 A retrospective study75 used clinical records of 55 patients with suspected TIA to assess the diagnostic agreement between stroke neurologists. Using three scales with different rates of likelihood for the diagnosis of TIA, the study showed that the agreement for TIA was poor, regardless of the scale used. Scales with more points had fewer patients classed as unlikely TIA (suggesting that scale format influences diagnostic certainty); these results support that the interpretation of TIA likelihood is subjective and argue for use of other technologies to make diagnosis, as clinical diagnosis is so poor.
Magnetic resonance DWI is very sensitive to small ischaemic lesions. 38 Demonstration of an ischaemic lesion on MR DWI has been associated with an increased risk of stroke in TIA patients. The 29 studies of MR DWI in patients with TIA or TIA and minor stroke published between 1999 and the start of this project (in two systematic reviews and three additional primary studies) included 2881 patients imaged between a few hours of onset of symptoms and up to nearly 3 weeks after the event. DWI was positive for ischaemic change in 37% (mean ± 12% SD, range 16–67%). 47,48,87,137,215 Having an acute ischaemic lesion on DWI (compared with not having a lesion) may predict increased risk of subsequent stroke,87,92,93 but the effect of a DWI ischaemic lesion on stroke prediction independent of clinical scoring47,94 was unclear. 87,92–94 Clinical features and carotid stenosis may be a stronger predictor of recurrent stroke risk. 93,137 Given the uncertainty around the accuracy of the widely publicised ABCD and ABCD2 clinical scores62 in predicting stroke risk,71,144,168 DWI could add prognostic as well as diagnostic value. The amount of publication bias in this literature has not been assessed. Several other studies and meta-analyses of rates of positive DWI in patients with TIA/minor stroke and of DWI added to ABCD or related score in risk prediction have been published since the award of this project.
We aimed to assess the sensitivity and specificity of MR compared with CT in patients with minor stroke or TIA, but it became clear that there were no such comparisons. We had previously performed a systematic review and meta-analysis of studies comparing CT with MR in the diagnosis of acute ischaemic and haemorrhagic stroke. 36,216 These data were very limited. Most papers included few patients, included only hospital-admitted patients with moderate to severe stroke, so were not relevant to the present topic, suffered from lack of blinding and incorporation bias. One study that included patients with mild stroke and TIA as well as more severe stroke32 did not present data for these subgroups separately, had substantial incorporation bias (all TIAs with a DWI-positive lesion were reclassified as stroke), and was pseudo-retrospective because the diagnosis of TIA/stroke was made from the hospital records. Having initially attempted to update this review, but being unable to find any other more recent or more relevant literature, we therefore focused on assessing the frequency of DWI visible lesions in TIA or minor stroke patients. We also summarised the available data on the effect of adding MR DWI or CT to ABCD2 scores in predicting recurrent stroke.
Methods
Objectives
We performed this analysis according to the PRISMA guidelines for systematic reviews of observational studies. 175 Following an initial search for papers comparing MR with CT in the same TIA/minor stroke patients, we focused on identifying all published studies in which TIA and/or minor stroke patients were assessed by DWI irrespective of aims, type of study design, and clinical setting.
Identification of studies
We searched indexed records that appeared in MEDLINE (Ovid) from January 1995 to November 2011. The choice of this time period reflected the introduction in the mid-1990s of DW MR sequences into clinical practice. The MEDLINE search strategy included both subject headings (MeSH terms) and text words for the target condition (e.g. stroke, TIA, minor stroke) and the imaging modality under investigation (MRI, DWI). We did not apply any language restrictions. We adapted the MEDLINE search to search EMBASE. In particular, we ‘translated’ the MEDLINE MeSH terms into the corresponding terms available in the Emtree vocabulary. The searches were initially run in November 2010 and updated in November 2011. Full details of both the MEDLINE and the EMBASE search strategies are presented in Appendix 1. We imported all citations identified by the MEDLINE and EMBASE search strategies into the Reference Manager bibliographic database. We hand-searched all proceedings of the International Stroke Conference (2011) and the European Stroke Conference (2011, 2012). We also contacted experts in the field and perused the reference lists of all relevant articles, as well as the most recent issues of Stroke and Cerebrovascular Diseases (not yet indexed in MEDLINE or EMBASE) to identify further published studies for possible inclusion in the review.
Inclusion/exclusion criteria
One review author (MB) examined the titles and abstracts identified by the literature search and retrieved all potentially relevant citations in full. Full-text articles were retained if they focused on primary studies of TIA or minor stroke patients who had DWI as brain imaging in the acute and subacute phase. Studies that did not assess patients by means of DWI, did not report the proportion of patients with positive DWI lesions, or did not report original data, were excluded. In cases of multiple or subsequent publications from the same patient cohort, the most recent or more complete report was chosen.
Two review authors (MB, HM) independently conducted data extraction and reviewed the methodological quality of selected studies. Disagreements were resolved by discussion or referred to a third author (JW). We recorded data on study methods (e.g. setting, study design), characteristics of patients (TIA vs. minor stroke) and imaging findings (DWI-positive brain lesions) as the proportion of patients with an ischaemic lesion on DWI. We also recorded any analyses comparing the prediction of recurrent stroke using the ABCD2 score without and with DWI findings. We also collected information on the following methodological aspects: prospective compared with retrospective study design, definition of TIA, timing of imaging assessment and evaluating clinicians.
Data synthesis
For each study we calculated the total number of patients with a visible DWI lesion. The pooled proportion of TIA patients with a positive DWI lesion was calculated by means of a univariate random-effects meta-analysis with within-study variance modelled as binomial. Heterogeneity between studies was assessed visually by inspection of forest plots and by calculating I2-statistics. 109 We used the statistical software R for our analyses.
Results
Number of included/excluded studies
Diffusion-weighted imaging in patients with transient ischaemic attack/minor stroke
The electronic searches identified 7983 citations. Of these, we considered 185 reports potentially relevant and retrieved the full-text articles for detailed assessment. For two citations, full-text was not easily available and we examined the information contained in the abstracts. Hand-searching of reference lists and of recent issues of relevant medical journal (e.g. European Neurology), not yet indexed in MEDLINE or EMBASE, resulted in a further four reports to be included. We subsequently excluded 127 reports. The most common reason for exclusion was that the study did not provide the number of patients with positive DWI lesions. A total of 45 full-text studies published in 58 reports, and two abstracts, fulfilled our inclusions criteria (Figure 21).
FIGURE 21.
Study identification and selection.
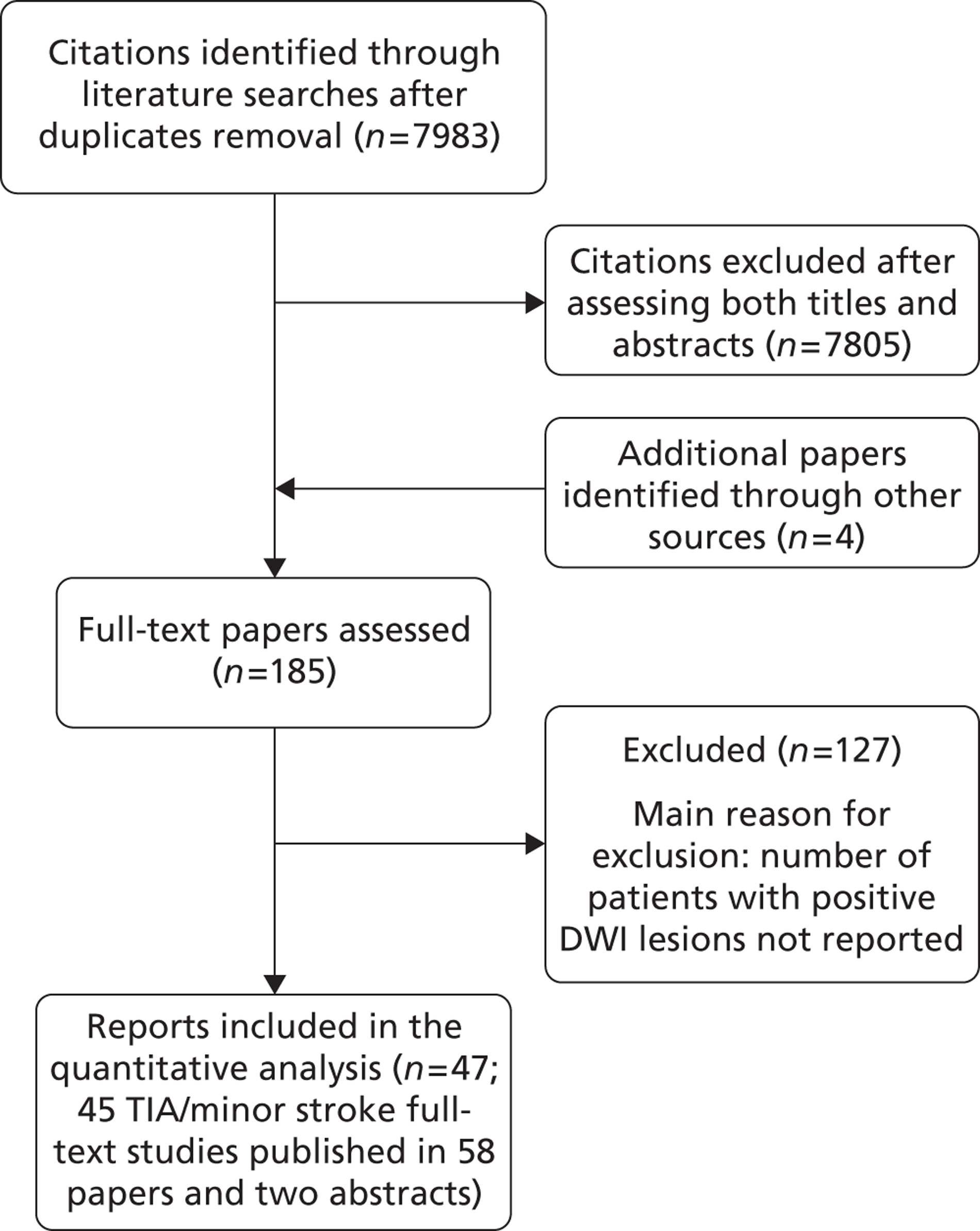
Characteristics of included studies
The methodological characteristics of the included studies are shown in Table 21. Eighteen were observational prospective cohort studies, 17 were observational retrospective cohort studies and 12 studies did not clearly state the type of study design (see Table 21).
| Study | Study design | Setting | TIA patients | TIA definition | Mean time from onset to DWI/MRI (range) | Evaluating clinician |
|---|---|---|---|---|---|---|
| Fazekas and colleagues, 1996217 | NR | Hospital | 62 | Time-based definition | 3 ± 3 days MRI+ 3.5 ± 3.0 days MRI+ |
NR |
| Bhadelia and colleagues, 1999218 | Prospective | Population based (Cardiovascular Health Study) | 100 | Time-based definition | NR | NR |
| Engelter and colleagues, 1999219 | NR | Hospital | 40 | Time-based definition | 36.5 hours (7–72 hours) | NR |
| Kidwell and colleagues, 1999220 | NR | Hospital | 42 | Time-based definition | 17 hours (1.25–73 hours) | Stroke specialist |
| Takayama and colleagues, 2000221 | NR | NR | 19 | NR | NR | NR |
| Bisschops and colleagues, 2002222 | Prospective | Hospital | 44 | Time-based definition | Within 3 days Within 24 hours in 22 (50%) patients |
NR |
| Kamal and colleagues, 2002223 | NR | Specialist unit | 28 | Time-based definition | Within 24 hours | Neurologist |
| Kastrup and colleagues, 2002224 | Prospective | Hospital | 42 | Time-based definition | 5.5 days | Stroke specialist |
| Marx and colleagues, 2002225 | Prospective | Hospital | 14 | Time-based definition | 9.4 hours (2 to 21 hours) | NR |
| Rovira and colleagues, 2002226 | Retrospective | Hospital | 58 | Time-based definition | 5 days (1 to 9 days) | NR |
| Crisostomo and colleagues, 2003227 | Retrospective | Specialist unit | 75 | Time-based definition | 23.0 ± 9.2 hours (0 to 76 hours) | Neurologist |
| Nagura and colleagues, 2003228 | NR | Specialist unit | 45 | Time-based definition | 17.3 hoursa (1.3 hours to 9 days) | NR |
| Nakamura and colleagues, 2003229 | NR | NR | 18 | NR | NR | NR |
| Inatomi and colleagues, 2004230 | Prospective | Specialist unit | 129 | Time-based definition | 4.7 ± 2.6 days (1 to 14 days) | NR |
| Purroy and colleagues, 200493 | Prospective | ED | 83 | Time-based definition | Within 7 days | Neurologist |
| Restrepo and colleagues, 2004231 | Retrospective | Specialist unit | 22 | Time-based definition | Within 24 hours | NR |
| Schulz and colleagues, 2004232 Schulz and colleagues, 200350 |
Prospective | Specialist unit | 136 | Time-based definition | 17 daysa (11 to 26 days) | Neurologist |
| Winbeck and colleagues, 2004233 | NR | Specialist unit | 60 | Time-based definition | 11 hours | NR |
| Inatomi and colleagues, 2005234 | Retrospective | Specialist unit | 21 | Time-based definition | 3.2 hours | Neurologist |
| Kim and colleagues, 2006235 | Retrospective | Specialist unit | 83 (41 with S-TIAs; 42 with R-TIAs) | Time-based definition | Within 3 days | NR |
| Lamy and colleagues, 2006236 | NR | Specialist unit | 98 | Time-based definition | 42.4 hours (3.3 hours to 11.7 days) | Neurologist |
| Oppenheim and colleagues, 2006237 | Retrospective | Specialist unit | 103 | Time-based definition | 24 hoursa (3.3 hours to 11.7 days) | Neurologist |
| Boulanger and colleagues, 2007215 | Prospective | ED | 85 | Time-based definition | 12.1 hours | Stroke specialist |
| Prabhakaran 2008238 | ||||||
| Lavallée and colleagues, 2007122 | Prospective | Specialist unit | 643 | Time-based definition | Within 15 days of symptoms onset | Neurologist |
| Prabhakaran and colleagues, 2007239 | Retrospective | Specialist unit | 146 | Time-based definition | Within 48 hours | NR |
| Redgrave and colleagues, 200794 | Prospective | Specialist unit | 200 | Time-based definition | 15 daysa | Neurologist |
| Bertrand and colleagues, 2008240 | Retrospective | Specialist unit | 36 | Time-based definition | 34 hoursa (3 hours to 8 days) | NR |
| Coutts and colleagues, 2008134 | Prospective | ED | 180 (TIA and minor stroke patients; 87/180 TIA) | Time-based definition | 8.5 hoursa (within 24 hours) | Neurologist |
| Coutts and colleagues, 2005241 | ||||||
| Coutts and colleagues, 200592 | ||||||
| Saver, 2006242 | ||||||
| Flossmann and colleagues, 200857 | NR | Specialist unit | 476 (TIA and minor stroke patients) | Time-based definition | NR | Neurologist |
| Uno and colleagues, 2008243 | Retrospective | Specialist unit | 72 | Time-based definition | 4.5 days in DWI+ 2.0 days in DWI– |
NR |
| Ay and colleagues, 200987 | Retrospective | ED | 477 | Time-based definition | 8.2 hours (IQR 5–12 hours)a | Neurologist |
| Ay and colleagues, 1999244 | ||||||
| Ay and colleagues, 2002245 | ||||||
| Ay and colleagues, 2005246 | ||||||
| Calvet and colleagues, 2009137 | Retrospective | Specialist unit | 339 | Time-based definition | 19.5 hoursa (8.0–28.3 hours) | Neurologist |
| Calvet and colleagues, 2007167 | ||||||
| Chatzikonstantinou and colleagues, 2009138 | NR | ED | 122 | Time-based definition | NR | Neurologist |
| Cucchiara and colleagues, 2009139 | Prospective | Specialist unit | 96 | Time-based definition | Within 48 hours | Neurologist |
| Cucchiara and colleagues, 200670 | ||||||
| Mlynash and colleagues, 2009141 | Prospective | Specialist unit | 43 | Time-based definition | 23.2 hours | Neurologist |
| Purroy and colleagues, 2009247 | Prospective | ED | 135 | Time-based definition | 3.8 ± 1.7 days from symptom onset | Neurologist |
| Quinn and colleagues, 200972 | Prospective | Specialist unit | 1693 (TIA and minor stroke patients) | Time-based definition | NR | Stroke specialist |
| Asimos and colleagues, 2010143 | Retrospective | ED | 766 | Time-based definition | Within 7 days | Emergency physician |
| Asimos and colleagues, 2009136 | ||||||
| Holzer and colleagues, 2010146 | Retrospective | Specialist unit | 176 | Time-based definition | Within 5 days | Neurologist |
| Kim and colleagues, 2010248 | Retrospective | Specialist unit | 41 (TIA and minor stroke patients) | Time-based definition | 19.6 days (3.0–40.0 days) | Neurologist |
| Merwick and colleagues, 2010147 | Retrospective | Population based | 1100 | Time-based definition | Within 24 hours | Stroke specialist |
| Nakajima and colleagues, 2010148 | Prospective | Specialist unit | 113 | Time-based definition | Within 14 days (median time 16.5 hours) | NR |
| Totaro and colleagues, 2010249 | Retrospective | Specialist unit | 91 | Time-based definition | Within 48 hours (mean 37.6 hours) | NR |
| Zakaria and Elghawabi, 2010250 | NR | NR | 36 | Time-based definition | Within 24 hours | NR |
| Meng and colleagues, 2011160 | Prospective | Specialist unit | 410 | Time-based definition | Within 7 days | Neurologist |
| Olivot and colleagues, 2011161 | Prospective | ED | 54 | Time-based definition | 1 daya | Emergency medicine physician + stroke specialist |
| Förster and colleagues, 2012251 | Retrospective | Specialist unit | 215 | Time-based definition | Within 24 hours | NR |
All studies used a time-based definition of TIA as opposed to the recently new proposed tissue-based definition. 176
Two studies assessed population-based cohorts, seven studies assessed hospital-based cohorts, eight studies assessed patients from EDs, 27 studies assessed patients from specialist stroke or neurology units and three studies did not clearly provide this information. TIA diagnosis was made by a neurologist in 20 studies, by a stroke specialist in five studies; by an emergency medicine physician in one study and initially by an emergency medicine physician and subsequently confirmed by a stroke specialist in another study; the status of the diagnosing physician was not reported in the remaining 20 studies. Timing of DWI assessment after symptom onset varied across studies (see Table 21). In 18 studies, TIA patients were assessed within 24 hours of symptom onset, in six studies within 48 hours, in 12 studies within 7 days, in three studies within 2 weeks and in the remaining two studies within 3 weeks. Six studies did not clearly provide this information.
Main findings
The total number of TIA/minor stroke patients assessed in the included studies was 9078 (range 18 to 1693 patients). All studies except eight57,72,134,224,225,232,233,248 restricted inclusion to TIA patients. The frequency of positive DWI findings ranged from 9% to 67% across studies (Table 22). Overall, 3048/9078 TIA patients had a visible lesion on DWI. Using a univariate random-effects meta-analysis, the pooled proportion of TIA patients with a positive DWI lesion was 34.3% (95.0% CI 30.5% to 38.4%), with heterogeneity between studies demonstrated by both the forest plot and a high I2-statistic of 89.3%. The forest plot of all included studies is shown in Figure 22.
| Study | TIA patients | Mean age (years) | Mean time from onset to DWI/MRI (range) | No. (%) with acute DWI lesions | Mean duration of symptoms | CT findings | Correlation: TIA duration and DWI+ | ||
|---|---|---|---|---|---|---|---|---|---|
| All TIA patients | DWI + patients | DWI – patients | |||||||
| Fazekas and colleagues, 1996217 | 62 | 61 | 3 ± 3 days MRI+ 3.5 ± 3.0 days MRI+ |
19 (31) | NR | 2 hours (median 30 minutes) | 3 hours (median 60 minutes) | NR | No |
| Bhadelia and colleagues, 1999218 | 100 | 74 | NR | 46 (46) | NR | NR | NR | NR | NR |
| Engelter and colleagues, 1999219 | 40 | 61 | 36.5 hours (7.0 to 72.0 hours) | 14 (35) | NR | 7.1 ± 9.2 hours | 3.2 ± 6.7 hours | NR | Yes |
| Kidwell and colleagues, 1999220 | 42 | 72 | 17 hours (1.25 to 73 hours) | 20 (48) | NR | 7.3 ± 6.0 hours | 3.2 ± 4.7 hours | NR | Yes |
| Takayama and colleagues, 2000221 | 19 | 71 | NR | 7 (37) | NR | NR | NR | NR | Yes |
| Bisschops and colleagues, 2002222 | 44 | 57 | Within 3 days Within 24 hours in 22 (50) patients |
21 (47) | NR | NR | NR | NR | NR |
| Kamal and colleagues, 2002223 | 28 | NR | Within 24 hours | 13 (46) | NR | NR | 2.3 hours | NR | NR |
| Kastrup and colleagues, 2002224 | 42 TIAs 36 minor stroke patients |
70 | 5.5 days | 19 (45) 36 (100) |
NR | 3.6 + 6.4 hours | 0.6 + 0.9 hours | NR | Yes |
| Marx and colleagues, 2002225 | 14 TIAs 19 minor stroke patients |
71 | 9.4 hours (2.0 to 21.0 hours) | 4 (29) 9 (47) |
NR | NR | NR | NR | NR |
| Rovira and colleagues, 2002226 | 58 | 60 | 5 days (1 to 9 days) | 39 (67) | 10.70 hours | 6.85 hours | 0.96 hours | Patients with CT non-ischaemic lesions were excluded | Yes |
| Crisostomo and colleagues, 2003227 | 75 | 67 | 23.0 ± 9.2 hours (0 to 76 hours) | 16 (21) | 3.3 ± 6.3 hours (40 seconds to 24 hours) | 5.2 ± 8.0 hours TIA ≤ 5 minutes: 10 (13) |
2.8 ± 5.7 hours | NR | Yes |
| Nagura and colleagues, 2003228 | 45 | 69 | 17.3 hoursa (1.3 hours to 9 days) | 14 (31) | 1.0 hoursa (3 minutes to 22.5 hours) | NR | NR | NR | No |
| Nakamura and colleagues, 2003229 | 18 | 71 | NR | 9 (50) | NR | NR | NR | NR | No |
| Inatomi and colleagues, 2004230 | 129 | 67 | 4.7 ± 2.6 days (1 to 14 days) | 57 (44) | NR | 3.1 ± 4.0 hours | 2.0 ± 3.0 hours | NR | Yes |
| Purroy and colleagues, 200493 | 83 | 66 | Within 7 days | 27 (32.5) | 30 minutesa | NR | NR | Chronic ischaemic infarct in 18 (21.7) of patients | NR |
| Restrepo and colleagues, 2004231 | 22 | 62 | Within 24 hours | 12 (55) | NR | 3.3 ± 5.0 hours | 0.6 ± 0.5 hours | NR | No |
| Schulz and colleagues, 2004232 | 136 TIAs | 70 | 17 daysa (11 to 26 days) | 17 (13) | NR | NR | NR | NR | No |
| Schulz and colleagues, 200350 | 164 minor stroke patients | 114 (70) | |||||||
| Winbeck and colleagues, 2004233 | 60 TIAs 37 minor stroke patients |
62 | 11 hours | 18 (30) 37 (100) |
NR | 5.3 hours (95% CI 1.8% to 8.7%) TIA < 1 hour: 8/25 (32%) TIA ≥≥ 1 hour: 10/35 (29%) |
5.2 hours (95% CI 2.9 hours to 7.5 hours) | NR | No |
| Inatomi and colleagues, 2005234 | 21 | 64 | 3.2 hours | 11 (52) | NR | 3.7 hours | 0.9 hours | NR | No |
| Kim and colleagues, 2006235 | 83 (41 with single TIA, S-TIA; 42 with recurrent TIAs, R-TIA) |
64 | Within 3 days | 25 (61) with S-TIAs 10 (24) with R-TIAs |
NR | NR | NR | NR | No |
| Lamy and colleagues, 2006236 | 98 | 61 | 42.4 hours (3.3 hours to 11.7 days) | 34 (35) | NR | 3.0 ± 4.0 hours | 1.4 ± 3.0 hours | NR | Yes |
| Oppenheim and colleagues, 2006237 | 103 | 60 | 24.0 hoursa (3.3 hours to 11.7 days) | 36 (35) | 1.8 ± 3.4 hours | 3.4 ± 4.5 hours | NR | NR | No |
| Boulanger and colleagues, 2007215 | 85 | NR | 12.1 hours | 35 (41) | NR | NR | NR | NR | NR |
| Prabhakaran, 2008238 | |||||||||
| Lavallée and colleagues, 2007122 | 643 | 66 | Within 15 days of symptoms onset | 108 (17) | 15 minutes (IQR 5–75 minutes)a | 15 minutes (IQR 5–60 minutes)a | 10 minutes (IQR 3–30 minutes)a | NR | NR |
| Prabhakaran and colleagues, 2007239 | 146 | 68.5 (median 70.0) | Within 48 hours | 37 (25) | NR | TIA > 1 hour: 22 (59.5) Four recurrent TIAs and six ischaemic strokes (combined events = 27) |
TIA > 1 hour: 29 (26.6) Three recurrent TIAs no ischaemic strokes |
NR | NR |
| Redgrave and colleagues, 200794 | 200 | 71 | 15 daysa | 31 (16) | NR | 5.3 ± 8.3 hours | 2.7 ± 5.0 hours | NR | Yes |
| Bertrand and colleagues, 2008240 | 36 | 66 | 34 hoursa (3 hours to 8 days) | 16 (44) | 2 hours and 50 minutesa | NR | NR | NR | NR |
| Coutts and colleagues, 2008134 | 180 (87 with TIA) | 66 | 8.5 hoursa (within 24 hours) | 96 (53) (from Coutts and colleagues 2005239) TIA and minor stroke patients |
NR | NR | NR | NR | NR |
| Coutts and colleagues, 2005241 | |||||||||
| Coutts and colleagues, 200592 | |||||||||
| Saver, 2006242 | |||||||||
| Flossmann and colleagues, 200857 | 476 | 73 | NR | 133 (28) TIA and minor stroke patients |
NR | NR | NR | NR | NR |
| Uno and colleagues, 2008243 | 72 | 69 | 4.5 days in DWI+ 2.0 days in DWI– |
24 (33) | NR | 4.0 ± 5.1 hours | 1.4 ± 2.5 hours | NR | Yes |
| Ay and colleagues, 200987 | 477 | 68 | 8.2 hours (IQR 5–12 hours)a | 155 (33) | NR | NR | NR | NR | NR |
| Ay and colleagues, 1999244 | |||||||||
| Ay and colleagues, 2002245 | |||||||||
| Ay and colleagues, 2005246 | |||||||||
| Calvet and colleagues, 2009137 | 339 | 62 | 19.5 hoursa (8.0–28.3) |
136 (40) | 30 hours a(10–80) |
TIA < 10 minutes: 15 (11) TIA ≥ 60 minutes: 79 (58) |
TIA < 10 minutes: 38 (19) TIA ≥ 60 minutes: 76 (37) |
NR | NR |
| Calvet and colleagues, 2007167 | |||||||||
| Chatzikonstantinou and colleagues, 2009138 | 122 | 67 | NR | 21 (17) | NR | NR | NR | 2 (1.6) | NR |
| Cucchiara and colleagues, 2009139 | 96 | 62 | Within 48 hours | 22 (23) | NR | NR | NR | NR | NR |
| Cucchiara and colleagues, 200670 | |||||||||
| Mlynash and colleagues, 2009141 | 43 | 71 | 23.2 hours | 15 (35) | 1–3 hours | NR | NR | NR | Yes |
| Purroy and colleagues, 2009247 | 135 | 69 | 3.8 ± 1.7 days | 67 (50) | NR | NR | NR | NR | NR |
| Quinn and colleagues, 200972 | 1693 | 67a | NR | 666 (39.3) TIA and minor stroke patients |
NR | NR | NR | NR | NR |
| Asimos and colleagues, 2010143 | 766 | 67 | Within 7 days | 211 (28) | NR | NR | NR | NR | NR |
| Asimos and colleagues, 2009136 | |||||||||
| Holzer and colleagues, 2010146 | 176 | 63 | Within 5 days | 49 (28) | NR | NR | NR | NR | NR |
| Kim and colleagues, 2010248 | 41 | 65 | 19.6 days (3 to 40 days) | 16 (39) TIA and minor stroke patients |
> 1 hour in 25 (61) patients | NR | NR | NR | No |
| Merwick and colleagues, 2010147 | 1100 | 65 | Within 24 hours | 376 (34) | NR | NR | NR | NR | NR |
| Nakajima and colleagues, 2010148 | 113 | 65 | Within 14 days (median time 16.5 hours) | 39 (34.5) | 1 houra | NR | NR | ||
| Totaro and colleagues, 2010249 | 91 | 68 | Within 48 hours (mean 37.6 hours) | 22 (24) | NR | NR | NR | NR | NR |
| Zakaria and Elghawabi, 2010250 | 36 | 65 | Within 24 hours | 10 (27.7) | 2.75 hoursa | 2.75 ± 3.5 hours | 2.0 ± 2.7 hours | NR | No |
| Meng and colleagues, 2011160 | 410 | 61 | Within 7 days | 203 (50) | NR | NR | NR | NR | NR |
| Olivot and colleagues, 2011161 | 119 | 71 | 1 daya | 11 (9) | NR | NR | NR | Non-contrast CT performed for all patients but findings not reported | NR |
| Förster and colleagues, 2012251 | 215 | 67 | Within 24 hours | 71 (33) | NR | NR | NR | 7/161 (4) | NR |
Two studies also reported findings on CT:138,251 Chatzikonstantinou and colleagues138 found that 2/122 patients had a relevant visible ischaemic lesion on CT (1.6%), compared with 21/122 (17.0%) on DWI; Förster and colleagues251 found that 7/161 (4%) of patients had a visible ischaemic lesion on CT, compared with 71/215 (33%) on DWI. A further study reported only old lesions on CT,93 and one study used CT to exclude non-stroke lesions. 226
Studies varied considerably in terms of sample size, time interval between symptom onset and DWI, symptom duration, aetiology, selection criteria, and setting. We explored potential reasons for heterogeneity of DWI-positive rates between studies including time and inclusion of minor stroke as well as TIA. Four studies provided data separately for TIA and minor stroke patients. 224,225,232,233 The proportion of minor stroke patients with visible ischaemic lesions on DWI was 47% in one study,227 70% in another study,232 and reached 100% in two studies. 224,233 It is worth noting that the two studies in which all minor stroke patients showed a positive DWI lesion were of small sample size and enrolled patients with a definite diagnosis of TIA or minor stroke as opposed to a possible diagnosis of TIA or minor stroke – for example Kastrup and colleagues224 included only patients with high-grade symptomatic carotid stenosis. Similarly, the study by Schulz and colleagues,232 in which 70% of minor stroke patients had a positive DWI lesion, focused on patients with a definite TIA or stroke and scanned patients 3 days after symptom onset or later. In another four studies that included patients with minor stroke as well as TIA but did not report the results separately, the proportion of patients with visible ischaemic lesions on DWI was not noticeably higher than in the studies reporting TIA alone: 53.0%,134 28.0%,57 39.3%72 and 39.0% respectively. 248
We examined the DWI-positive rate by time between symptom onset and scanning (Figure 23) using aggregate data from the primary studies, which showed no obvious time trend although most studies scanned patients within 8 days. Within those 8 days, the proportion with a DWI lesion ranged from 0.09 to 0.68.
FIGURE 23.
Scatterplot of time to imaging vs. proportion of patients with a positive DWI scan. Average time may indicate either a mean or median time.
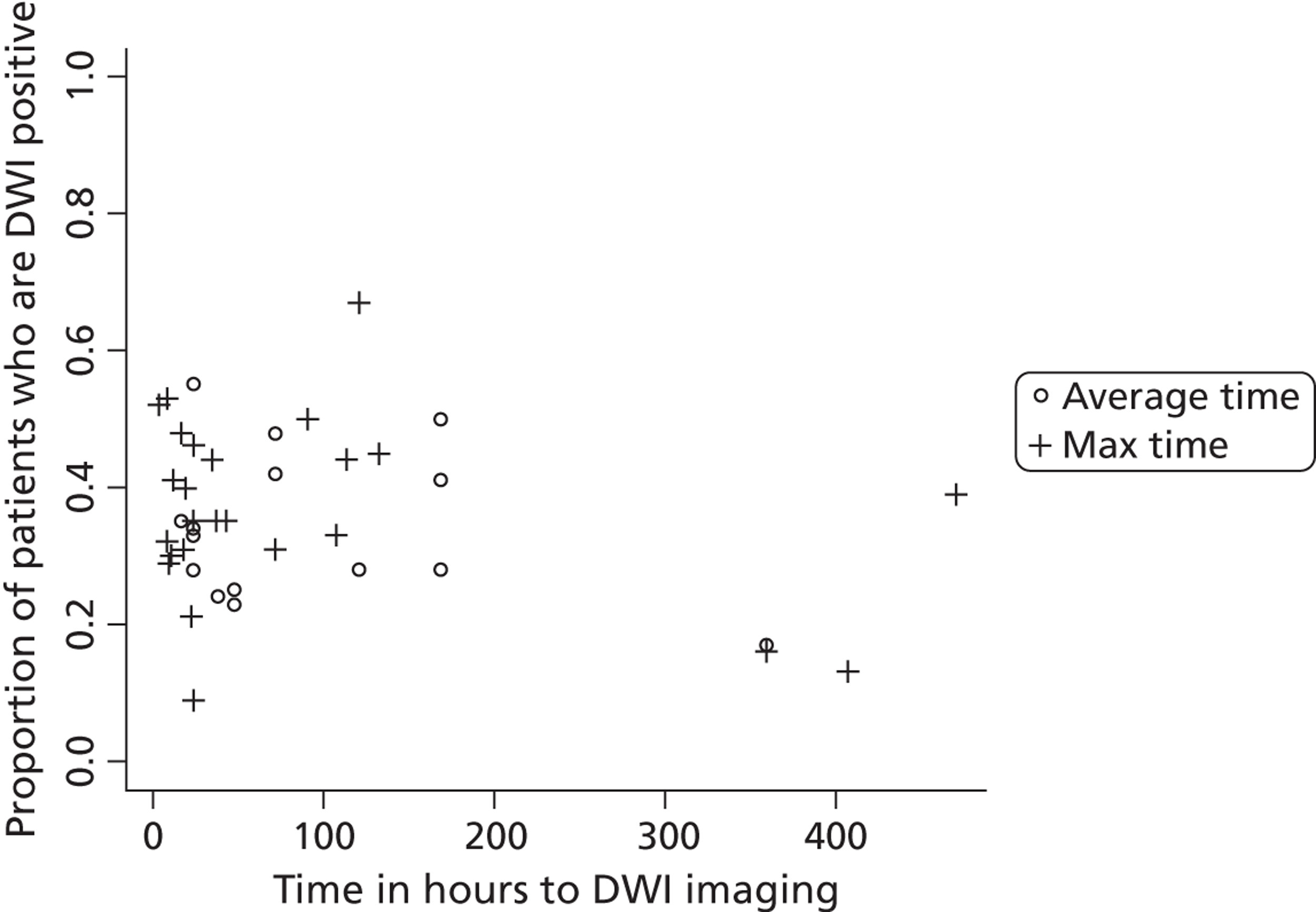
There is also no readily apparent difference between studies that reported average times and those that reported maximum times to imaging. Attempts to investigate the relationship between proportion DWI positive and time to imaging with statistical modelling did not manage to meet modelling assumptions.
Diffusion-weighted imaging in patients with minor stroke
From the results of our literature search we identified a further four studies that provided data on patients with minor stroke alone, i.e. not TIA. 45,252–254 Three of these studies were performed in the same centre in a regional NV clinic over a total of 12 years (1998–2011)252–254 and included a total of 627 patients (mean 209, range 153–246 patients). The patients were all assessed by expert stroke physicians and neurologists and were discussed at a weekly multidisciplinary meeting, and patients with non-vascular causes of minor stroke (i.e. mimics) were carefully excluded. The patients were scanned on two different MR scanners, about one-third on one scanner and the other two-thirds on the other scanner, but both used the same sequences. The time to scanning varied from within 24 hours to several weeks, but all were scanned on the day of presentation to the clinic. The average proportion of patients with a visible ischaemic lesion on DWI was 69%. Schulz and colleagues50 studied 51 patients with minor stroke and found that 29 patients had an ischaemic lesion on DWI at an average of 3 weeks after the stroke (57%), this lower proportion probably reflecting the greater delay to scanning. Chalela and colleagues32 included some patients with minor stroke [median National Institutes of Health Stroke Scale (NIHSS) score 3] in their prospective comparison of CT and MR DWI, but their assessment of sensitivity and specificity is confounded by switching the diagnosis from TIA to stroke in TIA patients with an ischaemic lesion on DWI.
ABCD2 score and diffusion-weighted imaging or computed tomography finding
Several publications assessed the use of MR DWI findings added to the ABCD2 score (Table 23). We have not re-meta-analysed these studies independently but present a summary of all studies providing information on ABCD2 and DWI findings here. In general, the addition of DWI findings to the ABCD2 score improved the risk prediction (higher AUC values in Table 23). A multicentre study184 assessed the early risk of recurrent stroke after TIA, and performance of ABCD2 score subcategorised as tissue positive or negative on either DWI or CT imaging. Among 3206 patients with DWI, 27.6% (884 patients) had an acute brain infarction. In 1368 patients imaged with CT, 23.9% (327 patients) had an acute or old infarction. For patients with DWI imaging, pooled rates of recurrent stroke at 7 days were 7.1% (95% CI 5.5% to 9.1%) in those with tissue-positive events compared with 0.4% (95% CI 0.2% to 0.7%) in those with tissue-negative events (p < 0.0001). For patients imaged with CT, rates were 12.8% (95% CI 9.3% to 17.4%) and 3.0% (95% CI 2.0% to 4.2%), respectively (p < 0.0001).
| Study | TIA patients | No. (%) with positive DWI | AUC (95% CI) | |||
|---|---|---|---|---|---|---|
| DWI | ABCD2 | ABCD2 + DWI | ABCD2I | |||
| Outcomes assessment at 7 days | ||||||
| Ay and colleagues, 200987 | 477 | 155 (33) | 0.76 (0.67 to 0.86) | 0.66 (0.57 to 0.76) | 0.81 (0.74 to 0.88) | |
| Arsava and colleagues, 2011157 | 257 | 257 (100) | 0.57 (0.45 to 0.69) | |||
| Asimos and colleagues, 2010143 | 1667 | 211 (28) | 0.59 (0.56 to 0.62) | 0.81a (0.75 to 0.87) | ||
| Calvet and colleagues, 2009137 | 339 | 136 (40) | 0.80a (0.62 to 0.98) | 0.87a (0.75 to 0.99) | ||
| Coutts and colleagues, 2008134 | 111 | 0.75a (0.56 to 0.94) | 0.85a (0.72 to 0.98) | |||
| Cucchiara and colleagues, 2009139 | 96 | 22 (23) | 0.99a (0.97 to 1.00) | 1.00a (1.00 to 1.00) | ||
| Lavallée and colleagues, 2007122 | 643 | 108 (17) | 0.49a (0.36 to 0.63) | 0.47a (0.33 to 0.59) | ||
| Mlynash and colleagues, 2009141 | 99 | 0.62a (0.43 to 0.82) | 0.81a (0.71 to 0.91) | |||
| Purroy and colleagues, 2007182/2009247 | 204 | 0.51a (0.21 to 0.80) | 0.62a (0.34 to 0.91) | |||
| Sheehan and colleagues, 2009183 | 443 379 (86) with brain imaging |
0.49 (0.35 to 0.63) | 0.44a (0.27 to 0.87) | |||
| Outcomes assessment at 90 days or beyond | ||||||
| Asimos and colleagues, 2010143 | 766 | 0.70a (0.62 to 0.78) | 0.81a (0.74 to 0.87) | |||
| Calvet and colleagues, 2009137 | 339 | 136 (40) | 0.75 (0.61 to 0.89) | 0.84 (0.70 to 0.97) | 0.81a (0.67 to 0.95) | |
| Coutts and colleagues, 2008134 | 180 | 96 (53) | 0.84 (0.72 to 0.92) | 0.78 (0.68 to 0.87) | 0.88 (0.79 to 0.94) | 0.82a (0.66 to 0.98) |
| Cucchiara and colleagues, 2009139 | 96 | 22 (23) | 0.76 | 0.63 | 0.83 | |
| Lavallée and colleagues, 2007122 | 643 | 108 (17) | 0.73a (0.63 to 0.84) | 0.73a (0.62 to 0.85) | ||
| bMeng and colleagues, 2011160 | 410 | 61 (15) | 0.59 (0.53 to 0.65) | 0.77 (0.72 to 0.82) | ||
| Mlynash and colleagues, 2009141 | 43 | 15 (35) | 0.60 (0.38 to 0.81) | 0.79 (0.69 to 0.90) | ||
| Purroy and colleagues, 2007,182 2009247 | 204 | 0.56a (0.38 to 0.73) | 0.61a (0.44 to 0.78) | |||
| Sheehan and colleagues, 2009183 | 443 379 (86) with brain imaging |
0.55 (0.45 to 0.64) | 0.38a (0.15 to 0.62) | |||
There was no information on DWI in TIA/minor stroke mimics, as these patients were excluded from all studies.
Patients with infarction on brain imaging had higher ABCD2 scores than those without any lesion. Tables 24–26 show the risk of recurrent stroke stratified by the presence of brain infarction on imaging and dichotomised ABCD2 score (above and below 4). Data were derived from the systematic review conducted by Giles and colleagues in 2011,210 who reported published and unpublished TIA data from 12 independent centres. Patients with ABCD2 score ≥ 4 and visible lesion on CT or DWI had a 7-day risk of stroke of 10.7%, compared with 1.9% in those without visible lesion; for patients with ABCD2 score of < 4 the risk of stroke was 2.3% and 0.2%, respectively. For patients with visible infarction on DWI and ABCD2 score ≥ 4, the 7-day risk of stroke was 8.9% compared with 0.6% in those without visible lesion; in patients with an ABCD2 score of < 4 these risks were 1.8% and 0.1%, respectively. For patients with visible infarction on CT and ABCD2 score ≥ 4, the 7-day risk of stroke was 15.5%, compared with 4.2% in those without visible lesion, and 3.9% and 0.9% in patients with ABCD2 score of < 4, respectively. The presence of infarction on DWI or CT imaging was the main determinant of early recurrent stroke; however, ABCD2 score had predictive value in the acute phase in both tissue-positive and tissue-negative patients, including both MRI and CT imaging, with an AUC for the prediction of stroke at 7 days of 0.68 (95% CI 0.63 to 0.73) and 0.73 (95% CI 0.67 to 0.80), respectively (p < 0.0001 for both results); AUCs for prediction of recurrent stroke at 90 days were 0.66 (95% CI 0.61 to 0.71) and 0.69 (95% CI 0.62 to 0.76), respectively. 210
| ABCD2 score | Visible lesion on CT-DWI | 7 days risk (%) | n/total patients | 90 days risk (%) | n/total patients |
|---|---|---|---|---|---|
| ≥ 4 | (+) | 10.7 | 98/912 | 14.6 | 106/728 |
| (–) | 1.9 | 37/1997 | 3.1 | 49/1602 | |
| < 4 | (+) | 2.3 | 7/299 | 3.9 | 9/230 |
| (–) | 0.2 | 3/1366 | 1.1 | 12/1140 | |
| Total | 3.2 | 145/4574 | 4.8 | 176/3700 |
| ABCD2 score | Visible lesion on DWI | 7 days risk (%) | n/total patients | 90 days risk (%) | n/total patients |
|---|---|---|---|---|---|
| ≥ 4 | (+) | 8.9 | 59/661 | 10.0 | 52/520 |
| (–) | 0.6 | 8/1299 | 1.2 | 13/1059 | |
| < 4 | (+) | 1.8 | 4/223 | 0.6 | 1/164 |
| (–) | 0.1 | 1/1023 | 0.7 | 6/863 | |
| Total | 2.2 | 72/3206 | 2.8 | 72/2606 |
| ABCD2 score | Visible lesion on CT | 7 days risk (%) | n/total patients |
|---|---|---|---|
| ≥ 4 | (+) | 15.5 | 39/251 |
| (–) | 4.2 | 29/698 | |
| < 4 | (+) | 3.9 | 3/76 |
| (–) | 0.9 | 3/343 | |
| Total | 5.4 | 74/1368 |
Discussion
This summary includes all available literature on DWI in patients with TIA and the effect of ABCD2 scores and DWI or CT in combination. In clinical practice, DWI shows a definite acute ischaemic lesion in one-third of TIA patients, but in most (two-thirds) patients with definite TIA DWI is negative. In 26 out of 47 studies, the diagnosis of definite TIA was made by a stroke physician or neurologist, and in most studies (25/47) the patients were scanned within 48 hours of their TIA. Therefore, the low proportion with a visible ischaemic lesion cannot be attributed either to dilution of the samples with non-TIA patients or to excessive delays to scanning. We conclude that absence of an ischaemic lesion on DWI is the commonest finding in patients with definite TIA and should not preclude effective secondary prevention, as these patients are at risk of recurrent stroke. 215 We also conclude that absence of an ischaemic lesion on DWI occurs in about one-third of patients with minor stroke alone who have been screened by a specialist neurologist or stroke physician and for whom no alternative cause of symptoms has been found. Therefore, absence of a lesion on DWI in patients with expert/physician-confirmed minor stroke should also not preclude use of effective secondary prevention treatment for stroke. It remains to be demonstrated whether an ischaemic DWI lesion is an independent risk factor for stroke in the population in whom it is currently being used in stroke prevention clinics and in what proportion the DWI is positive.
We found no apparent link between proportion of patients with a DWI lesion and time to imaging within the first 8 days in the studies. At least time to imaging did not explain the huge (sevenfold) variation in the proportion with a DWI lesion within the first 8 days. However, as we had to use summary data from the primary studies, our analysis does not provide strong evidence against there being an association. It may well be that the use of summary data results in a loss of information great enough to hide any relationship – an average time does not indicate the range of times to imaging – which would ideally be investigation with IPD, i.e. where the scan results and time to imaging for each patient are available.
We did not find any data from studies comparing DWI with CT directly in the same patients with TIA. The literature that does compare DWI with CT focuses on major disabling stroke requiring hospital admission, so is not relevant to the present analysis. 36,216 The paper by Chalela and colleagues32 comparing CT and DWI suffered from substantial incorporation bias by changing the diagnosis from TIA to stroke in patients with a DWI-positive lesion, among numerous other problems. In most studies it was difficult to disentangle the patients with minor stroke from those with TIA and the few studies that provided data separately included a small selected group of stroke patients, with, in some cases, a high proportion of other major risk factors. There were few studies on patients with minor stroke specifically. There is a larger literature on DWI in patients with mild stroke or specific subtypes such as lacunar stroke, but most of these focus on patients with a DWI lesion and some other specific characteristic so provide no information on proportions of patients with/without ischaemic lesions, i.e. the information needed for the present analysis. 38 The rate of ischaemic lesions in patients with minor stroke on DWI appears consistent however at around 69%, meaning that about one-third of physician-confirmed minor stroke patients do not have a DWI lesion and should not be denied secondary prevention treatment for stroke. 215
The studies assessing the addition of CT brain scan findings to ABCD2 suffer from lack of clarity whether the visible ischaemic lesion was thought to be recent, relevant to the presenting symptoms, or old and not relevant, or just areas of prominent leukoaraiosis. It was beyond the scope of this project to obtain additional data from the study investigators. It may be that having a visible infarct on CT simply indicates that patients who have had one infarct in the past are more likely to have another one, or that patients with visible lesions have more vulnerability to ischaemic brain damage. Thus, CT visible infarct may be acting differently to DWI visible infarct in terms of stroke prediction.
There is no information on which to calculate sensitivity or specificity of DWI or CT in TIA/minor stroke as all patients with mimics or haemorrhage were excluded and the reference standard for determining the diagnosis of stroke or TIA was varied and frequently uncertain. Many studies relied on retrospective case note identification of patients and for ascertainment of the ABCD2 score. These approaches will have biased the results. The proportion of TIA and minor stroke patients without a visible ischaemic lesion on DWI (66% and 31%, respectively) would be much higher if the denominator were all patients attending the clinic, including mimics, rather than just those with a definite TIA/minor stroke determined on expert neurology assessment. Assuming that the 45% of patients attending stroke prevention clinics with a final diagnosis of mimic (see Chapter 7) did not have DWI-visible lesions (which is incorrect as some with migraine, postictal states or hypoglycaemia would have DWI-positive lesions) and were included in the denominator then there would be much larger proportions of DWI-negative scans undertaken. As an approximation, DWI would be positive in only 19% of patients with suspected TIA and 38% of suspected minor stroke, leaving a large proportion of negative, non-contributory scans (see Chapter 4, Discussion, which provides an estimate of proportions with stroke, TIA or mimic by ABCD2 score).
What might happen if the DWI-negative patients in whom no alternative diagnosis apart from an ischaemic CV event can be found do not receive stroke secondary prevention? One-third of minor stroke and two-thirds of TIA patients might be denied effective stroke secondary prevention. Although these patients might be at lower risk by virtue of not having a DWI lesion, they still have some risk,215 particularly the 20% with a tight ipsilateral carotid stenosis (see Chapters 4 and 5). Failure to use routine and established investigations to identify established risk factors such as ipsilateral tight carotid stenosis through belief that a negative DWI excluded TIA/minor stroke would mean that patients with carotid stenosis would be denied an effective and established treatment (endarterectomy); others would be denied medical therapies, also evidence based and highly effective in combination. If patients with suspected TIA/minor stroke are to be given these therapies anyway then what is the point of placing so much emphasis on MR DWI? If effective stroke prevention can be achieved with a neurological assessment to differentiate definite or probable TIA/minor stroke from mimics, carotid ultrasound to identify those with tight ipsilateral carotid stenosis who require endarterectomy, cardiac investigation to identify AF or other key risk factors and a CT brain scan to exclude haemorrhage and structural mimics, it is unclear what the ABCD2 score or MR examinations are likely to add.
Chapter 7 Diagnosis of transient ischaemic attack, minor stroke and mimics: systematic review and meta-analysis of frequency, differential diagnosis, and prognosis of non-cardiovascular events
Introduction
The short-term risk of stroke after TIA or minor stroke is high, especially in the first few days after the event. In light of the early stroke risk, clinical guidelines advocate urgent diagnostic work-up and treatment of these patients. 15,103,255,256 However, after expert clinical assessment, an important number of patients referred with the diagnosis of suspected TIA have a final diagnosis of a non-CV event or mimic. 73,257
Accurate diagnosis of CV compared with non-CV causes of symptoms is essential to ensure appropriate management. However, the diagnosis is often difficult and disagreement on whether a patient has had a TIA is frequent in clinical practice. 258 Because of the lack of a universally accepted diagnostic criteria or objective confirmatory tests to identify TIA from other transient neurological symptoms, the final diagnosis relies mainly on clinical history alone and recollections of the patient who, by definition, was neurologically impaired. 168,259–261 Brain imaging is useful in identifying an acute ischaemic lesion or some non-vascular conditions (e.g. brain tumour, MS); however, a normal CT or MRI is still compatible with the diagnosis of both TIA (66% of TIAs do not have a visible lesion on imaging – see Chapter 5) and non-CV (many common non-vascular causes of TIA/minor stroke-like symptoms do not show any abnormality on brain imaging).
Less is known about the prognosis of patients with a non-CV event, although these are generally considered to be benign in most cases and given less attention. Several medical conditions can imitate a TIA or minor stroke, and require different therapeutic approaches from CV events. 26 Furthermore, previous publications reported a considerable proportion of patients with unclassifiable or miscellaneous events, probably including entities with different pathophysiology and unknown prognosis. 168,262,263
In order to determine the proportion of patients attending stroke prevention clinics with a final diagnosis of non-CV event, and the most frequent non-vascular diagnoses and their risk of recurrent neurological symptoms and functional outcome, we conducted a systematic review of studies reporting the frequency, main alternative diagnoses and outcomes in these patients.
Methods
Objectives
We performed this systematic review in accordance with Meta-analysis Of Observational Studies in Epidemiology (MOOSE)116 and PRISMA264 statements. We aimed to identify all studies reporting:
-
the proportion of patients with transient or mild non-disabling neurological symptoms with a final diagnosis of a non-vascular cause of their symptoms, irrespective of the study design or setting
-
the main causes (i.e. differential diagnosis) of non-CV events
-
the short and long-term prognosis of non-CV events.
Identification of studies
We identified potential relevant studies by searching Ovid MEDLINE and EMBASE from 1980 to December 2011 (see Appendix 1). We included the following search terms: transient ischaemic attack, TIA, atypical TIA, non-focal TIA, TIA mimics, transient neurological attack or non-CV. Considering that the number of studies assessing the prognosis of patients with non-CV events could be small, first we used a search strategy with broad definitions of TIA, minor stroke and non-CV events in order to retrieve the highest number of articles, and subsequently we included specific search terms for prognosis in these patients. We also screened the reference lists of all relevant studies and review articles. We excluded non-English articles for which a full-text translation was not available.
In the expectation that the literature might be biased towards highly specialist practice that was not relevant to routine clinics, we also identified several sources of unpublished data from operational clinics. These included a prospective data set of patients referred to a local stroke prevention clinic (WGH, Edinburgh, UK) from July 2009 to June 2011, who received a final diagnosis of TIA or minor stroke. 173 This data set is a subset of the SSCAS initiative that has collected anonymised information to monitor stroke care from all hospitals managing acute stroke in Scotland since 2002. We also identified data from an audit undertaken in Leicester.
Inclusion/exclusion criteria
One reviewer (HM) screened the identified titles and retrieved all potentially relevant citations in full-text. Articles were included in this systematic review if they provided any data on proportions of patients diagnosed as non-CV, TIA/minor stroke and also if those studies included a control group of healthy subjects or patients with an alternative condition as long as these subject categories could be clearly distinguished. Studies that did not include patients with non-CV events, did not report the proportion of patients with this condition, or published in abstract form were excluded. In cases when multiple publications were available for the same patient cohort, the most complete report was selected. Duplicates were removed using Reference Manager.
Quality assessment and data extraction
From the selected studies, one reviewer (HM) sought and extracted data on the following variables:
-
sample size of study
-
study design
-
setting of diagnosis
-
whether the final diagnosis had been made by a neurologist, geriatrician, stroke physician, GP or nurse
-
use of predefined diagnostic criteria for TIA or stroke and method of final diagnosis (i.e. clinical records, in person)
-
proportion of patients with a final diagnosis of a non-CV event
-
most frequent differential diagnoses in patients with non-CV events
-
prognosis of patients with non-CV events, in particular risk of further stroke, MI, and vascular death
-
method of follow-up (i.e. telephone, records, in person).
We contacted authors of one study for further information about the person making diagnosis of TIA or non-CV event. For the purpose of this review, patients with diagnosis of ‘possible TIA’ were included in the TIA group, as, in our experience, these patients are treated as TIA in clinical practice. Series including either TIA or minor stroke patients were considered for this review, in light of the frequent description of both conditions in studies reporting the risk of vascular events (i.e. recurrent stroke) after TIA, especially in the context of outpatient clinics.
Data synthesis
We aimed to investigate whether risk of outcome (stroke or vascular death at 7, 90 and > 90 days after presentation) varied between TIA, non-CV and asymptomatic/control patients. Specifically, we compared outcomes between TIA and non-CV patients, and also between non-CV and asymptomatic/control patients on which data on the above outcomes were available. We used simple logistic regression, as the outcomes were binary and the data were not amenable to more complex analyses, to allow for within-study clustering. We also assessed heterogeneity between studies owing to different settings (TIA clinics, hospital clinics and population-based studies) visually with forest plots and calculated I2-statistics. 109 Data were analysed in SAS and R.
Results
Number of included/excluded studies
The literature searches identified 1775 citations. After initial screening of titles and abstracts we selected 53 citations for full-text retrieval. An additional six studies were included after hand-searching of reference lists of relevant studies, yielding a total of 59 studies. After full-text examination, 43 studies were excluded, leaving 16 studies that fulfilled the inclusion criteria. The reasons for exclusion were: not relevant (n = 10), non-CV events excluded (n = 13), review articles (n = 5), conference abstracts (n = 13), risk of duplicated data (n = 1) and no description of the person making diagnosis of non-CV event (n = 1) (Figure 24). None was excluded because of language.
FIGURE 24.
Study selection and reasons for exclusion.
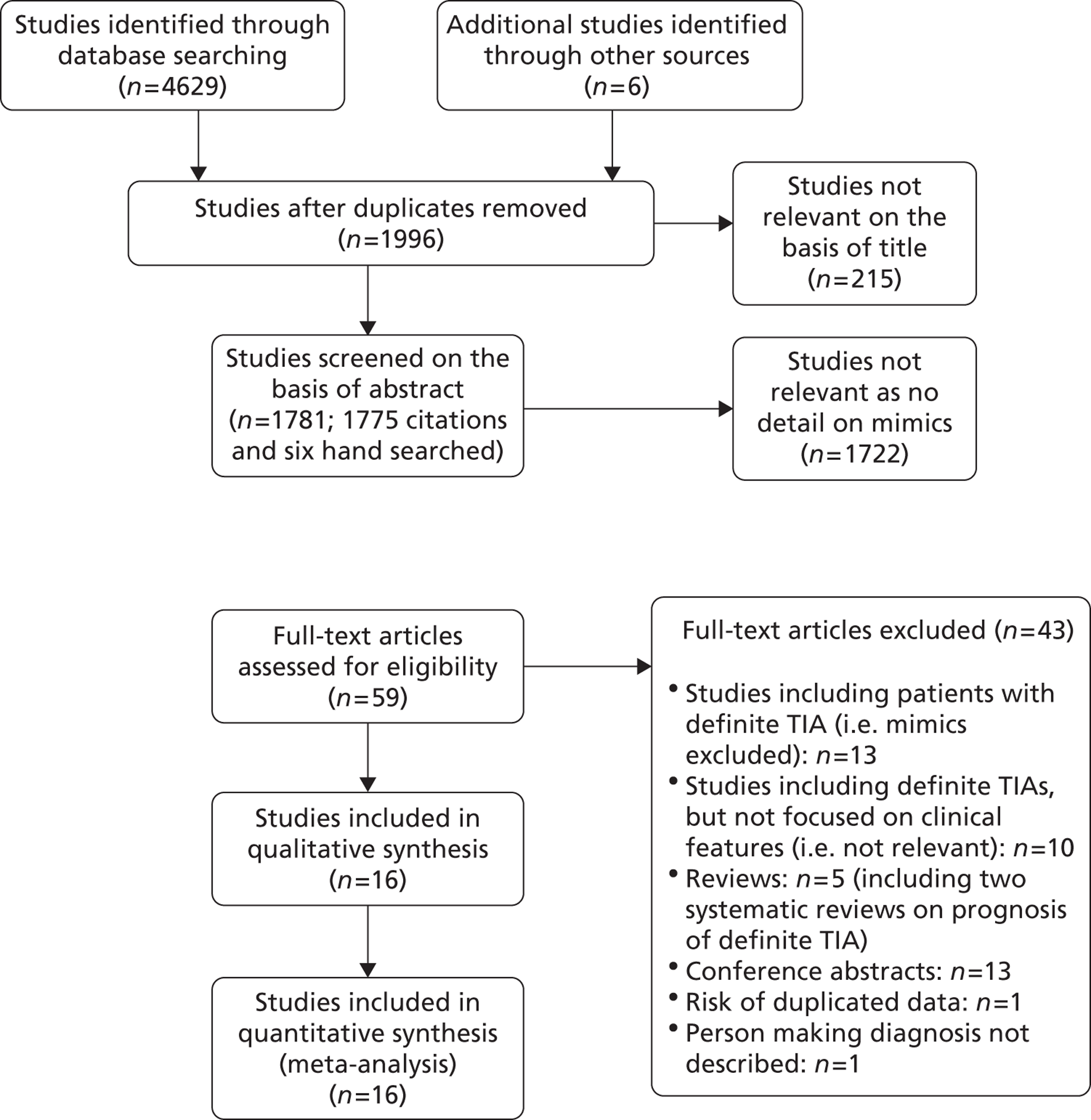
Characteristics of included studies
The 16 included studies7,73,122,156,161,173,257,263,265–271 varied in terms of their primary objectives. Three were population-based studies assessing the incidence of TIA in the general population in UK, the Netherlands and Spain (OCSP, Rotterdam Study and a community-based register in Segovia, respectively). 7,263,265 Eight studies focused on patients attending TIA clinics,122,173,257,266–270 and one study183 enrolled patients from multiple settings as part of a population-based study, even although the final diagnosis was made in a TIA clinic (North Dublin TIA Study). Two were ED-based studies,156,161 one included patients from ED and TIA clinics and reported the results for each setting separately and so contributed two independent data points,73 and one recruited patients as part of a hospital-based clinical trial. 271
The 16 studies included a total of 14,542 participants, and all provided data on frequency of mimics compared with true TIA/minor stroke;7,73,122,156,161,173,183,257,263,265–271 11 studies with a total of 3786 participants reported the main causes of non-CV events7,122,156,161,173,263,266–270 and five studies with a total of 8596 participants reported prognosis data. 122,156,265,270,271 One study deliberately excluded patients with some specific diagnoses other than TIA (i.e. migraine, epilepsy or vertigo). However, other non-specific transient neurological symptoms were incorporated (e.g. positive visual phenomena, dizziness or amnesia) and, consequently, the study was included in this review. 265 The same study used the term ‘mixed TIA’ for patients with focal and non-focal brain symptoms. For the statistical analysis, these patients were included in the group with focal brain symptoms (n = 38, 0.6% of the study sample). Forty-one per cent of the data related to frequency and 66% of the data related to differential diagnosis came from two studies,173,270 and 41% of the data for prognosis came from one study. 271 Follow-up was at least 1 year in all but one study assessing prognosis.
The study design was prospective in 13 studies and retrospective in three (Table 27). The final diagnosis of TIA or non-CV event was made by a neurologist, geriatrician or stroke physician in 15 studies, and one study reported the results of an anterior circulation TIA clinic led by a specialist NV nurse. 268 The final diagnosis of true compared with non-CV event was done in person in 14 studies (from consecutive referrals in eight studies); in two of them a predefined symptoms checklist was used [derived from the Face Arm Speech Test (FAST) in one study]. In two cohorts the final diagnosis was made using clinical records. Thirteen studies used time-based criteria of TIA, whereas three studies did not report which criteria were used. Subjects with minor stroke were included in 13 studies; 10 described the proportion of these events in all true CV events, ranging from 6% to 72%.
| Cohort details | Study quality | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Date | Size | Setting | Proportion of non-CV events (%) | Design | Person making final diagnosis of TIA vs. non-CV | Method of final diagnosis | Predefined diagnostic criteria for TIAa | Method of follow-up |
| Dennis and colleagues7 | 1989 | 512 | Population based | 53 | Prospective | Neurologist | In person | Yes | – |
| Koudstaal and colleagues271 | 1992 | 3127 | Hospital based | 18 | Prospective | Neurologist | In person (checklist) | Yes | In person |
| Sempere and colleagues263 | 1996 | 313 | Population based | 25 | Prospective | Neurologist | In person | Yes | – |
| Ferro and colleagues73 | 1996 | 52 | TIA clinic | 31 | Prospective | Neurologist | In person | Yes | – |
| Ferro and colleagues73 | 1996 | 31 | ED based | 55 | Prospective | Neurologist | In person | Yes | – |
| Martin and colleagues257 | 1997 | 508 | TIA clinic | 27 | Prospective | Neurologist | In person, consecutive referral | Yes | – |
| Karunaratne and colleagues266 | 1999 | 128 | TIA clinic | 50 | Prospective | Stroke physician | In person, consecutive referral | Yes | – |
| Murray and colleagues267 | 2007 | 811 | TIA clinic | 49 | Retrospective | Neurologist, stroke physician | In person, consecutive referral | Not described | – |
| Lavallée and colleagues122 | 2007 | 1085 | TIA clinic | 22 | Prospective | Neurologist | In person, consecutive referral | Yes | In person/telephone |
| Bos and colleagues265 | 2007 | 548 | Population based | 42 | Prospective | Neurologist | Note review | Yes | In person/records |
| Banerjee and colleagues267 | 2009 | 282 | TIA clinic | 14 | Prospective | Specialist nurse | In person,b consecutive referral | Yes | – |
| Sheehan and colleagues183 | 2009 | 594 | Population based | 43 | Prospective | Stroke physician | In person | Yes | Not described |
| WGH (unpublished data) | 2011 | 2368 | TIA clinic | 36 | Retrospective | Neurologist, stroke physician | In person, consecutive referral | Not described | – |
| Amort and colleagues156 | 2011 | 303 | ED-based | 18 | Prospective | Neurologist | Note review | Yes | In person/telephone |
| Olivot and colleagues161 | 2011 | 224 | ED-based | 48 | Prospective | Neurologist, stroke physician | In person | Yes | Records |
| Hörer and colleagues269 | 2011 | 123 | TIA clinic | 44 | Prospective | Neurologist | In person, consecutive referral | Yes | Telephone |
| Cameron and colleagues270 | 2011 | 3533 | TIA clinic | 47 | Retrospective | Stroke physician | In person, consecutive referral | Not described | Records |
Main findings
Frequency of non-cardiovascular events
Figure 25 shows the proportion of patients with a non-CV event in 17 cohorts reported in 16 studies (range 18% to 55%). The summary estimate for proportion of non-CV events was 34% (95% CI 28% to 42%); however, there was evidence of substantial heterogeneity across all selected studies – the I2-statistic was very high, 98.4%. The lowest proportion of non-CV events (14%) was described in a TIA clinic led by a specialist nurse and the highest (55%) in an ED-based study. The proportion of non-CV events was 40% (95% CI 23% to 60%) for TIA clinics, 40% (95% CI 23% to 60%) for population-based studies and 32% (95% CI 10% to 66%) for hospital-/ED-based studies, but with evidence of significant heterogeneity between studies in each setting (Figures 26–28 and Table 28) – the I2-statistics were 97.8%, 95.2%, and 97.9%, respectively, for hospital-/ED-based studies, population-based studies and TIA clinics.
FIGURE 25.
Proportion of patients with a non-CV diagnosis reported in the literature. NA, not applicable.
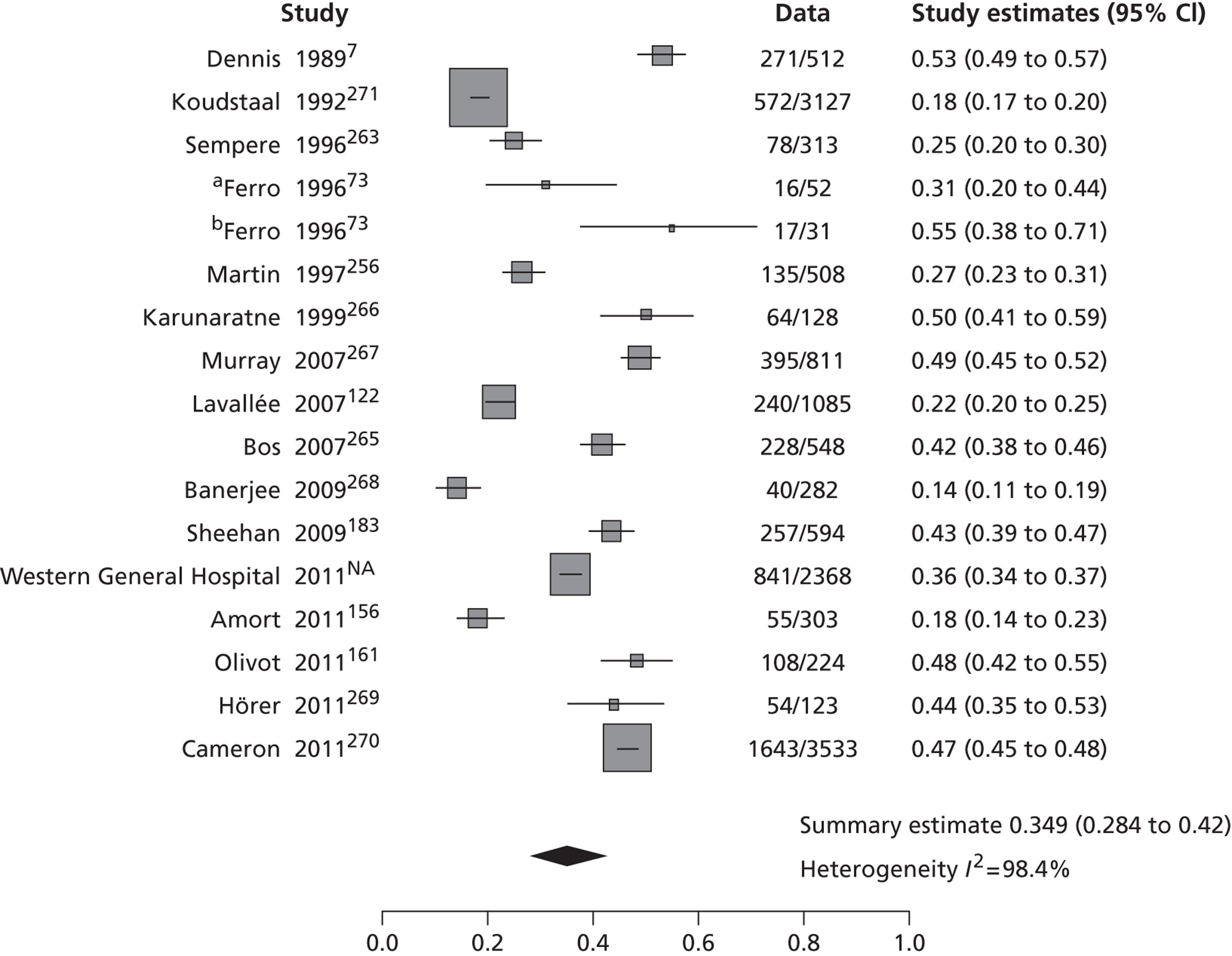
FIGURE 26.
Hospital/ED-based studies and proportion of non-CV events.
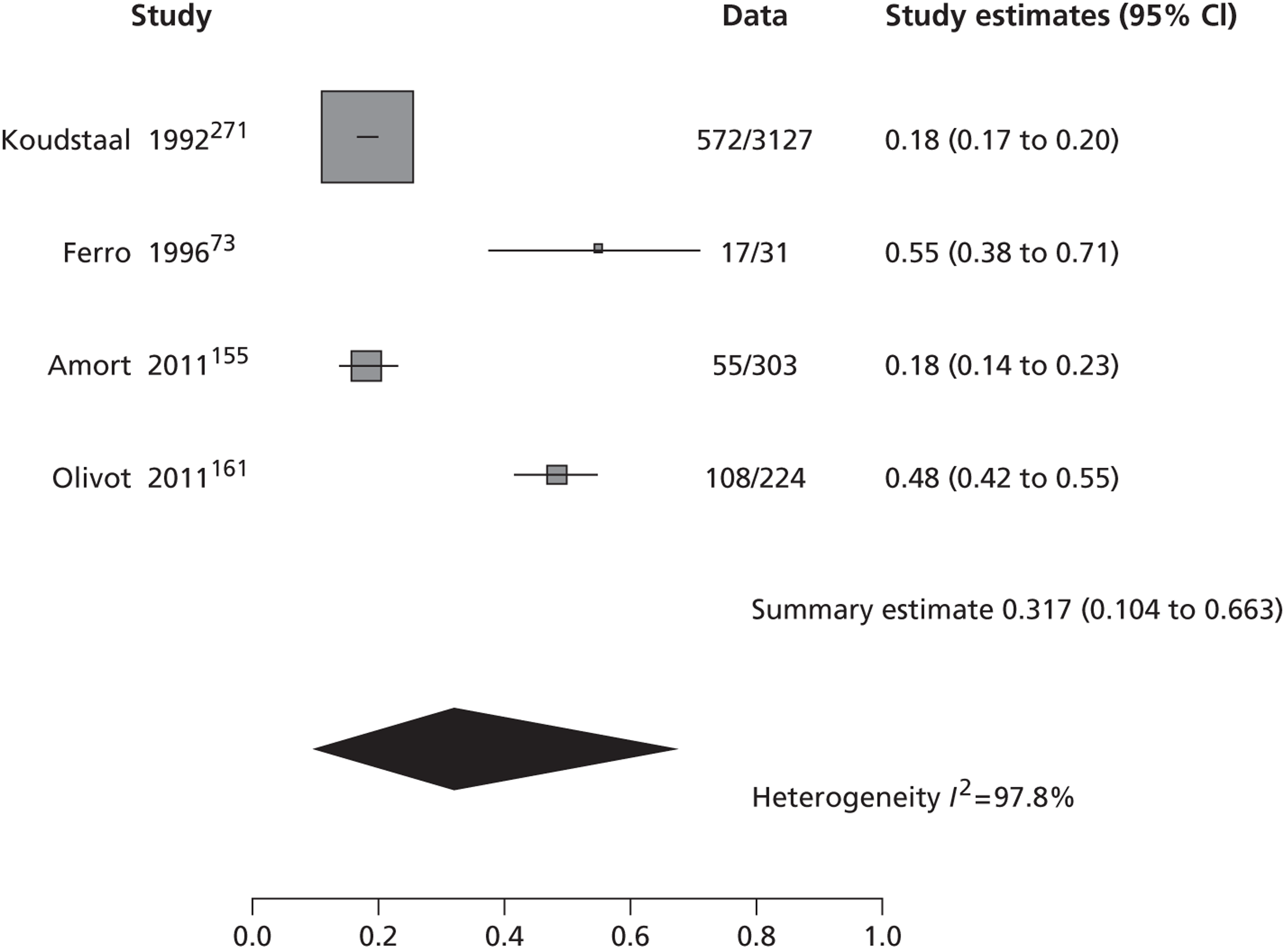
FIGURE 27.
Population-based studies and proportion of non-CV events.
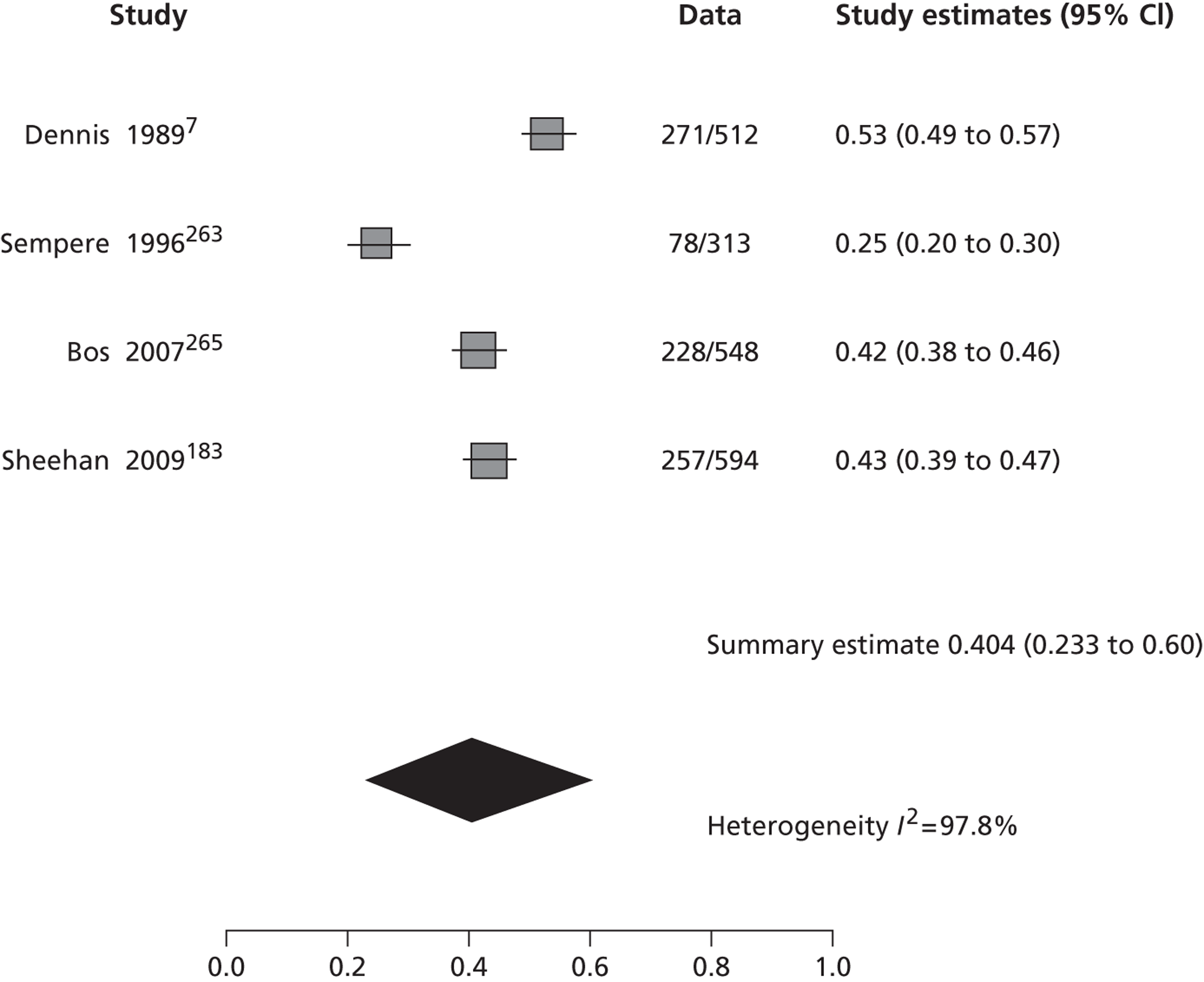
FIGURE 28.
Transient ischaemic attack clinic studies and proportion of non-CV events. NA, not applicable.
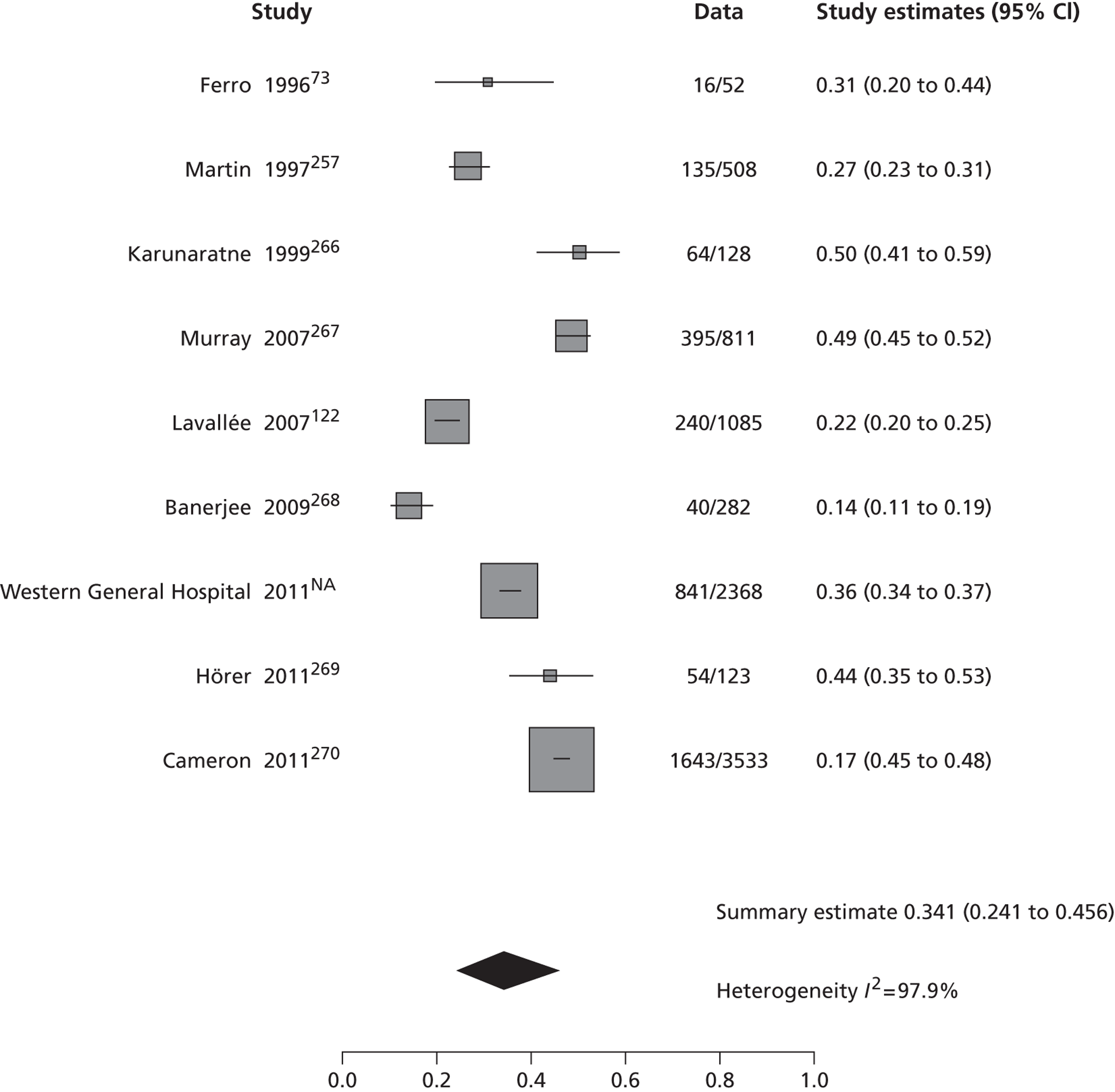
| Study location | Non-CV events (n) | Total patients (n) | Proportion of non-CVs |
|---|---|---|---|
| (95% CI) | |||
| Hospital – ED | |||
| Koudstaal and colleagues, 1992271 | 572 | 3127 | 0.18 (0.17 to 0.19) |
| Ferro and colleagues, 199673 | 17 | 31 | 0.55 (0.38 to 0.71) |
| Amort and colleagues, 2011156 | 55 | 303 | 0.18 (0.14 to 0.23) |
| Olivot and colleagues, 2011161 | 108 | 224 | 0.48 (0.42 to 0.55) |
| Summary | 0.32 (0.10 to 0.66) | ||
| Population based | |||
| Dennis and colleagues, 19897 | 271 | 512 | 0.53 (0.49 to 0.57) |
| Sempere and colleagues, 1996263 | 78 | 313 | 0.25 (0.21 to 0.30) |
| Bos and colleagues, 2007262 | 228 | 548 | 0.42 (0.38 to 0.46) |
| Sheehan and colleagues, 2009183 | 257 | 594 | 0.43 (0.39 to 0.47) |
| Summary | 0.40 (0.23 to 0.60) | ||
| TIA clinics | |||
| Ferro and colleagues, 199673 | 16 | 52 | 0.31 (0.20 to 0.45) |
| Martin and colleagues, 1997257 | 135 | 508 | 0.27 (0.23 to 0.31) |
| Karunaratne and colleagues, 1999266 | 64 | 128 | 0.50 (0.41 to 0.59) |
| Murray and colleagues, 2007267 | 395 | 811 | 0.49 (0.46 to 0.52) |
| Lavallée and colleagues, 2007122 | 240 | 1085 | 0.22 (0.20 to 0.25) |
| Banerjee and colleagues, 2009268 | 40 | 282 | 0.14 (0.10 to 0.19) |
| WGH 2011 | 841 | 2368 | 0.36 (0.34 to 0.38) |
| Hörer 2011269 | 54 | 123 | 0.44 (0.36 to 0.53) |
| Cameron and colleagues, 2011270 | 1643 | 3533 | 0.47 (0.45 to 0.49) |
| Summary | 0.40 (0.23 to 0.60) | ||
Main causes of non-CV events
The main causes of non-CV events were detailed in 11 studies. 7,122,156,161,173,263,266–270 The most frequent alternative diagnoses and the proportion of all non-CV diagnoses were migraine (10 studies, mean 14.4%7,122,156,161,173,266–270), syncope (seven studies, 6.6%7,161,173,262,267–269), seizure/epilepsy (11 studies, 5.0%7,122,156,161,173,266–268, 270), psychiatric (seven studies, 4.4%122,156,173,266–268, 270) and vertigo (nine studies, 4.0%7,122,156,161,173,263,266,267,269). Only one study reported a proportion of 8.8% of patients with cardiac disorders (144 out of 1643 participants). 266 Other less frequent diagnoses were peripheral nerve conditions, postural hypotension, transient global amnesia, toxic or metabolic causes (e.g. drugs, hypoglycaemia) and brain tumour (Table 29).
| Final diagnosis | Proportion of total non-CV events (%) | Mean | Study reference |
|---|---|---|---|
| Migraine | 4–33 | 14.4 | 7, 122, 156, 161, 173, 262–266 |
| Cardiac disorders | 8.8 | 8.8 | 266 |
| Syncope | 2–18 | 6.6 | 7, 161, 173, 257, 263–265 |
| Seizure/epilepsy | 3–44 | 5.0 | 7, 122, 156, 161, 173, 259, 262–266 |
| Psychiatric | 3–10 | 4.4 | 122, 156, 173, 262–264, 266 |
| Vertigo | 1–19 | 4.0 | 7, 122, 156, 161, 173, 259, 262, 263, 265 |
| Peripheral nerve | 1–15 | 3.1 | 156, 161, 173, 259, 262, 263–265 |
| Postural hypotension | 1–11 | 2.9 | 173, 262, 263 |
| Transient global amnesia | 2–7 | 2.7 | 7, 156, 161, 173, 259, 262, 263, 265, 266 |
| Toxic/metabolic | 0.3–8.0 | 2.3 | 156, 161, 263, 265 |
| Brain tumour | 0.7–6.0 | 2.0 | 7, 156, 161, 259, 262, 264, 265 |
| Subdural haemorrhage | 1.9 | 1.9 | 265 |
| Subarachnoid haemorrhage | 1.8–1.9 | 1.9 | 156, 265 |
Outcomes for transient ischaemic attack compared with non-cardiovascular events
The risks of stroke and other vascular events after TIA and non-CV event were described in five studies. 122,156,265,270,271 One retrospective study enrolled 3533 participants with TIA and non-CV events and 3915 control subjects without any neurological symptoms. A prospective population-based study enrolled 548 patients with transient neurological attacks and 5514 control subjects; however, the definite diagnosis of each event was derived retrospectively from medical records. Another three prospective studies included data for TIA and non-CV, but without asymptomatic control subjects.
The reported stroke risk up to 90 days was lower for non-CV events than for TIA (three studies,122,265,271 zero events vs. 1.5–5.2%, respectively). Owing to the absence of events in the first group, any further analysis proved unfeasible. With respect to stroke risk of > 90 days, two studies were considered. 265,271 We excluded one study because of the absence of events in the non-CV group. 122 The risk of stroke at > 90 days was significantly higher for TIA than for non-CV events (OR 1.63; 95% CI 1.21 to 2.20, p = 0.0013). However, some differences between the studies undermined the reliability of the data on stroke risk at > 90 days.
A combined outcome of stroke, MI or vascular death was specified in four studies. 122,156,270,271 The risk of this combined outcome was significantly higher for patients with TIA than for non-CV events (OR 1.597; 95% CI 1.400 to 1.810, p < 0.001); the OR remained unaffected after excluding from analysis a cohort without events for the non-CV group. 122 The risk of vascular death was described in three studies and tended to be lower in the group with non-CV events than in patients with TIA, without a significant difference (OR 0.86; 95% CI 0.71 to 1.05, p = 0.1614).
Outcomes for non-cardiovascular events compared with asymptomatic control subjects
There were limited data comparing prognosis in patients with non-CV events and asymptomatic control subjects. Only one study compared the risks of stroke at > 90 days in these groups, and found no significant difference between groups (OR 1.23; 95% CI 0.82 to 1.86, p = 0.3). 265 Two studies reported the risk of vascular death for these groups. 265,270 Again, there was no difference between asymptomatic control subjects and patients with non-CV events (OR 0.93; 95% CI 0.75 to 1.14, p = 0.46).
Non-vascular events in transient ischaemic attack and non- non-cardiovascular events
There is a lack of published data regarding fatal and non-fatal non-vascular events in patients with TIA and non-CV events. One study reported a HR of 1.59 (95% CI 1.11 to 2.26) for dementia in patients with non-CV compared with asymptomatic control subjects,265 and one recently published cohort reported that the risk of neurological events excluding vascular conditions was significantly higher in patients with non-CV events than in those with TIA (HR 1.37; 95% CI 1.10 to 1.69, p = 0.003). 270
Discussion
The complexity of the diagnosis of transient ischaemic attack
An accurate diagnosis of TIA is key to preventing a major CV event in patients with transient neurological symptoms of vascular origin and avoiding committing non-CV patients to taking antiplatelet drugs, statins and antihypertensive drugs for the rest of their lives and to stopping driving for a year, with its attendant inconveniences, which may include loss of employment. The correct diagnosis of TIA is frequently difficult and usually depends on the skill with which the clinical history is taken as objective findings on clinical examination as well as positive diagnostic findings are scarce. 180,272 Some atypical symptoms could mislead clinicians into diagnosing a non-CV event; this is particularly relevant in the diagnosis of posterior circulation TIA, considering they are often more difficult to identify with certainty. On the other hand, when the diagnosis of TIA is still not clear to different observers, they usually agree to diagnose TIA instead of a non-CV event. 74,260 Delays in time from onset of symptoms to assessment can cause patients to forget some important clinical features, making the diagnosis even more difficult. Patients with transient or mild neurological symptoms are evaluated in different settings, including emergency services, in-hospital consultations, ophthalmological clinics, primary health care centres and NV clinics. The diversity of settings could indicate differences in the clinical features of each event, making the diagnosis of TIA even more challenging. In view of the lack of universally accepted diagnostic criteria for TIA and non-CV events, the best standard is still the clinical experience or judgement of the physician. 259
Frequency of non-cardiovascular event in different settings
This systematic review shows a high frequency of non-CV events in TIA clinics (40%) higher than hospital/ED studies (32%); however, there was a substantial heterogeneity between studies in each setting, with both the forest plots and the I2-statistics confirming the impression given by reading the primary studies that there is significant variation between the primary studies, leading to difficulties with interpreting the summary estimates, the so-called ‘apples and oranges’ problem. 273 The differences between studies are attributable to several factors. The lowest proportion of non-CV events was described in a TIA clinic led by a specialist nurse. This clinical service was focused on anterior circulation TIAs, and the identification of anterior circulation events was done using a referral protocol based on the FAST instrument. 274 Patients identified by this method were eligible for further investigation in the nurse-led clinic, included in the study, and followed up in a weekly neurologist-led clinic, where the diagnosis was reviewed; referrals excluded by the protocol (e.g. possible posterior circulation events or other non-neurological diagnoses) were seen directly in a NV clinic led by neurologist. The use of predefined referral protocol and the exclusion of posterior circulation TIAs or other diagnoses could have improved the selection of true vascular events in this cohort. Likewise, different criteria for referral between the included studies could explain the observed heterogeneity in frequencies of non-CV events.
Differences could also be accounted for by the study design. A hospital-based RCT reported a proportion of 18% of ‘atypical TIA’. Subjects were included using a predefined checklist about symptoms and time course of the event; here the use of rigorous inclusion criteria may explain the lower frequency of those events.
Another cause can be found in the severity of the neurological symptoms and their probable true vascular origin; patients with a more severe condition are more likely to attend to emergency services and those with less severe or subtle symptoms (e.g. mild hypoaesthesia) are more likely to attend primary health care centres. Other plausible explanation could lie in who makes the diagnosis in each setting. Ferro and colleagues73 reported that, in patients referred for neurological evaluation by ED physicians with a suspected TIA, the final diagnosis was a non-CV event in 55%, with 32% having a definite stroke. However, other hospital/ED-based studies have reported a lower proportion of non-CV events, even when the final diagnosis was assessed by neurologists. 156,271
The availability of specialist assessment could be another important factor; services with more access to neurological consultation can have a higher proportion of patients referred with an uncertain diagnosis and consequently a higher probability of non-CV events. 73 Certainly, all of these proportions can be affected by the number of patients who decline to attend TIA clinics after the first assessment in primary health care centres or ED, patients with evident non-CV events who were identified in a first evaluation and the effect of multiple interviews on the ability to remember some symptoms. Considering that the final diagnosis of TIA or non-CV was made by neurologists or physicians with an interest in stroke in all but one included study, we could not estimate differences in proportions of each diagnosis between different observers (i.e. with less experience in stroke).
Causes of non-cardiovascular events
The most frequent conditions mimicking TIA were consistently reported across the included studies. It is well recognised that migraine and seizures can cause transient neurological symptoms as part of their manifestations. Migraine can present reversible focal neurological symptoms with gradual onset over a few minutes (i.e. aura), but typical aura can also occur without headache and last longer than a few minutes. 275 Generalised seizures can present with sudden loss of consciousness and involuntary jerking of limbs; postictal neurological findings (i.e. Todd paralysis) are frequent and can be confused with a CV event. Simple and complex partial seizures have less evident manifestations and also can cause a postictal deficit. 276 Psychiatric conditions (e.g. somatoform disorders) resemble transient or permanent neurological symptoms and the diagnosis can be demanding in some settings. Other diagnoses, such as vertigo, transient global amnesia or peripheral nerve diseases, can require an expert assessment or further exploration to exclude a CV event. Other rare causes of mimics include cerebral tumours (primary and secondary), subdural haematomas (SDHs), subarachnoid haemorrhage and MS. All of these are rare but require specific investigation, management and treatment and it is important to differentiate them from true TIA/minor ischaemic stroke. Tumours require sensitive investigation and treatment to make the best of remaining quality of life if aggressive malignant. SDHs probably do not benefit from antiplatelet agents and may require surgical evaluation. Similarly, subarachnoid haemorrhage and MS are important diagnoses not to overlook.
Whereas these conditions share clinical features with TIA, others are simply misdiagnosed as CV. Syncope and postural hypotension were present in 6.6% and 2.9% of patients with non-CV events, respectively; these entities usually have different clinical features from TIA, and diagnosis is usually straightforward. In a previous systematic review of patients with suspected stroke of all severities (not including TIA), the proportion of non-CV events ranged from 1% to 37% (mean 12.5%; 95% CI 6.7% to 18.3%) and the most common events were primary and secondary cerebral neoplasm, seizures, systemic infections and SDHs. 26 In a more recent systematic review of patients with suspected stroke (again generally excluding TIA), including 20 studies of 7221 patients, the proportion of patients with a final diagnosis of a true CV event was 73% (95% CI 62% to 84%); the most frequent non-CV diagnoses in order of frequency were seizures, syncope, sepsis, functional disorders, migraine and brain tumours, among others. 277 Compared with patients with stroke-like symptoms, our systematic review showed that in patients with transient neurological symptoms of non-vascular origin, migraine was the most frequent alternative diagnosis. Whereas seizures and syncope were also frequent in these patients, the presence of brain neoplasm or systemic infections were less important than its relative frequency as ‘stroke mimic’. 26 Differences could be partially explained by the presence of a permanent more than transient neurological deficit in patients with brain neoplasm or infections.
Awareness of the main differential diagnosis of transient neurological events can lead to improvements in the diagnosis of true compared with non-CV events; a more accurate identification and treatment of each condition can improve the prognosis of each condition and quality of life, especially in those with TIA.
Prognosis of non-cardiovascular events
There is a paucity of good-quality data comparing the prognosis of patients with non-CV events with TIA or general population. We identified five studies with data for prognosis of non-CV events; only two of them included a control group without any neurological symptom. Owing to the retrospective design, in two studies the event ascertainment relied on clinical records and would be less accurate than prospective studies. Despite these shortcomings, the findings deserve our attention. It is not surprising that patients with TIA can have a higher stroke risk compared with non-CV patients provided that the diagnosis of TIA was correct; the risk of a combined cardiovascular outcome was also higher for TIA patients. There was a trend towards a higher risk of stroke and vascular death for non-CV events than control subjects but without a significant difference.
Considering these results, the prognosis for any transient neurological event other than TIA has not yet been fully delineated. Diagnoses of atypical TIA, TIA mimic, non-focal transient neurological attack or non-CV event combine a wide array of conditions with distinct pathophysiologies and probably different cardiovascular risks;258 the assumed benign prognosis of these entities seems to be an oversimplification of their outcomes. Some conditions, such as syncope, can have different causes in elderly and young patients. In elderly persons, cardiac or orthostatic syncope can occur more often, and the prognosis is relatively poor in these patients. 278 Additionally, some TIA can be misdiagnosed as non-CV increasing the risk of stroke and other CV events in this group if the correct treatment is not indicated.
In view of the absence of a standard classification or clinical guidelines for the management of non-CV events, methodical research looking for a better classification and long-term follow-up of these patients is required. This large sector of TIA clinic patients have largely been ignored and further studies should determine the true risk of vascular or other non-vascular events in these patients, as well as identify conditions with a worse prognosis in which a more urgent approach is needed.
Chapter 8 A survey of clinical and imaging services for stroke prevention after suspected transient ischaemic attack or minor stroke in the UK
Introduction
Transient ischaemic attack and minor stroke are medical emergencies, with up to 90,000 cases every year in the UK. 9,170 Delivering effective stroke prevention requires highly organised services. We surveyed UK clinical and imaging secondary stroke prevention services to see if they met recent guidelines.
Methods
We devised two electronic survey questionnaires for clinical and imaging services for stroke prevention in the UK using the online survey software SurveyMonkey (www.surveymonkey.com). The survey questionnaires were initially developed by a neuroradiologist (JW) and a methodologist (MB). They were adapted from several previous questionnaires of imaging services for stroke in the UK which achieved high response rates. 26,95,279 They were then sent for comments and suggestions to three neurologists (MD, PS and Keith Muir), two health economists (PMcN and ZQ), a neuroradiologist (Donald Hadley) and a medical physicist (Janet De Wilde) within the project team. We phrased each question in a sensitive manner, avoiding ambiguous and complex terminology, and we made an effort to limit relevant questions to a manageable number. We used a ‘closed’, ‘structured’ response format for the majority of questions. Questions were numbered and organised in a series of linked pages, with a progress indicator on each page. The two first pages of both questionnaires provided information on the rationale and purpose of the study, including details of the research team, and addressed best research practice issues, such as confidentiality and informed consent.
When agreement was reached on the format and content of the two questionnaires, they were piloted among the members of the project team who were not involved in their original development. They were also sent for comment to the NHS Improvement team and the Royal College of Physicians of London Stroke Audit lead (see below). The purpose of the piloting phase was to evaluate both questionnaires in terms of their relevance, flow, acceptability and ease of use, and identification of redundant and/or poorly formulated questions. Following pilot testing, the agreed versions of the survey questionnaires (see Appendices 3 and 4) contained detailed questions regarding current provision of stroke prevention services and imaging services in the UK, with particular regard to volume of work, capacity, professionals involved, and type and timing of investigations, number and type of scanners, timing of both brain and carotid imaging, and endarterectomy rates. Specific questions were also included on the access of brain scanning for TIA and minor stroke patients during normal working hours and out of hours on weekdays and on weekends.
We considered eligible participants to be all clinical leads of UK stroke services and radiology departments. We used multiple overlapping methods to identify and contact leads of stroke services and imaging services in England, Wales and Scotland. Since the late 1990s and devolution, the organisation of these services has become increasingly regionalised to the respective devolved nations, necessitating different approaches in each country.
Clinical services
We contacted all leads of the 27 stroke networks’ clinical stroke services (with three to nine clinical sites within each network) in England through NHS Improvement (Ian Golton, Director, Stroke Improvement Programme, following contact with Dr Damian Jenkinson, Stroke Advisor, Department of Health) and to Stroke Leads in Trust hospitals through the Royal College of Physicians of London Sentinel Stroke Audit database of stroke services in England (Alex Hoffman, Professor Tony Rudd). The survey was also posted on the Royal College of Physicians of London Stroke Audit website and circulated in the NHS Improvement Stroke Bulletin (Barbara Zutshi). In Scotland, we contacted 15 stroke clinical leads through the SSCAS (Professor Martin Dennis).
Imaging services
In England, we contacted Radiology Directorate leads through the NHS Improvement database (Gail Powell) and information about the survey was also circulated in the bi-weekly Radiology Bulletin. We also contacted all known MRI services in England through the MAGNeT database (Dr Janet DeWilde). In Scotland, we contacted all 12 Directors of Imaging Services through the Diagnostic Imaging Clinical Network (Professor Ian Robertson, Mr James Cannon).
In Wales, we also contacted stroke services and imaging services through the Stroke Collaborative (Michelle Price).
Covering letters explaining the objectives of the two surveys and providing the links to access the questionnaires online were prepared for the clinical leads of both stroke prevention services and imaging services (see Appendices 3 and 4). Participants were assured that the information they provided would be treated in strictest confidence and with anonymity, and were informed about the amount of time required to complete the questionnaires. No incentives were offered to take part in the surveys. The covering letters were sent by e-mail or post to all UK stroke clinical leads and Radiology Directorates in April 2011 via multiple routes as above. In particular, they were sent to stroke leads of all 15 NHS Boards in Scotland, to all 40 network clinical leads covering the 27 stroke networks in the UK, to all 12 Scottish Clinical Directors of Radiology through the Diagnostic Imaging Clinical Network [Managed Diagnostic Imaging Clinical Network (MDICN)], to 171 Acute Trusts, 129 Foundation Trusts, and seven Welsh Trusts in the UK. Furthermore, details of the surveys, together with the associated online links, were disseminated through the Royal College of Physicians website and advertised through the NHS Improvement – Stroke newsletter and Radiology newsletter. Reminder messages were sent to non-respondents in June 2011.
The results of both internet surveys were reported according to the CHEcklist for Reporting Results of Internet E-Surveys (CHERRIES) statement. 280
Data management and analysis
Surveys results were generated using SurveyMonkey software and subsequently entered into a Microsoft Excel (version 11) spreadsheet (Microsoft Corporation, Redmond, WA, USA). We used descriptive statistics to examine clinical leads’ answers. Results were expressed as proportions, medians, or means where appropriate. Percentages reported were based on actual numbers of respondents. Not all respondents answered all of the questionnaires’ questions.
Results
A total of 114 stroke services surveys and 146 imaging facilities surveys were partially or fully completed. Response rate was 45% for both surveys. To calculate the response rate we divided the number of clinical leads to whom we initially sent the invitation to take part in the surveys by the number of participants who actually completed the online questionnaires. However, as both surveys were also disseminated and advertised through professional body websites and newsletters, it proved unfeasible to calculate the exact number of all of the potential respondents actually contacted.
As respondents were clinical leads in hospitals or specialist centres for the description of the surveys’ findings, we will use the terms ‘respondents’, ‘participants’ or ‘centres’ interchangeably.
Stroke services survey findings
In total, 97% of respondents (107 out of 110 who answered this question) indicated that a specialist stroke prevention service for TIA/minor stroke patients was available in their hospital/centre. In 99% of centres the TIA prevention service was run at least once per week; in 47% of centres every weekday; and in 31% of centres 7 days per week (Figure 29). The majority of centres (86%, 88 out of 102 respondents) indicated that one to five new patients attended the stroke service each time it was run, and only 13% of centres (13 out of 102 respondents) indicated that six to 10 new patients attended the stroke service each time it was run (Figure 30). In half of the centres (50%, 51 out of 102 respondents), the proportion of patients attending the stroke service who had a final diagnosis of TIA or minor stroke was between 41% and 60%, in 31% of centres between 21% and 40% and in 15% of centres between 61% and 80%. Only 3% of centres reported that the proportion of patients ultimately diagnosed as having TIA/minor stroke was ≤ 20%, and only 1% of centres reported that it was between 81% and 100% (Figure 31).
FIGURE 29.
Frequency of the specialist TIA/minor stroke service. Note: 109 out of 114 respondents answered this question.
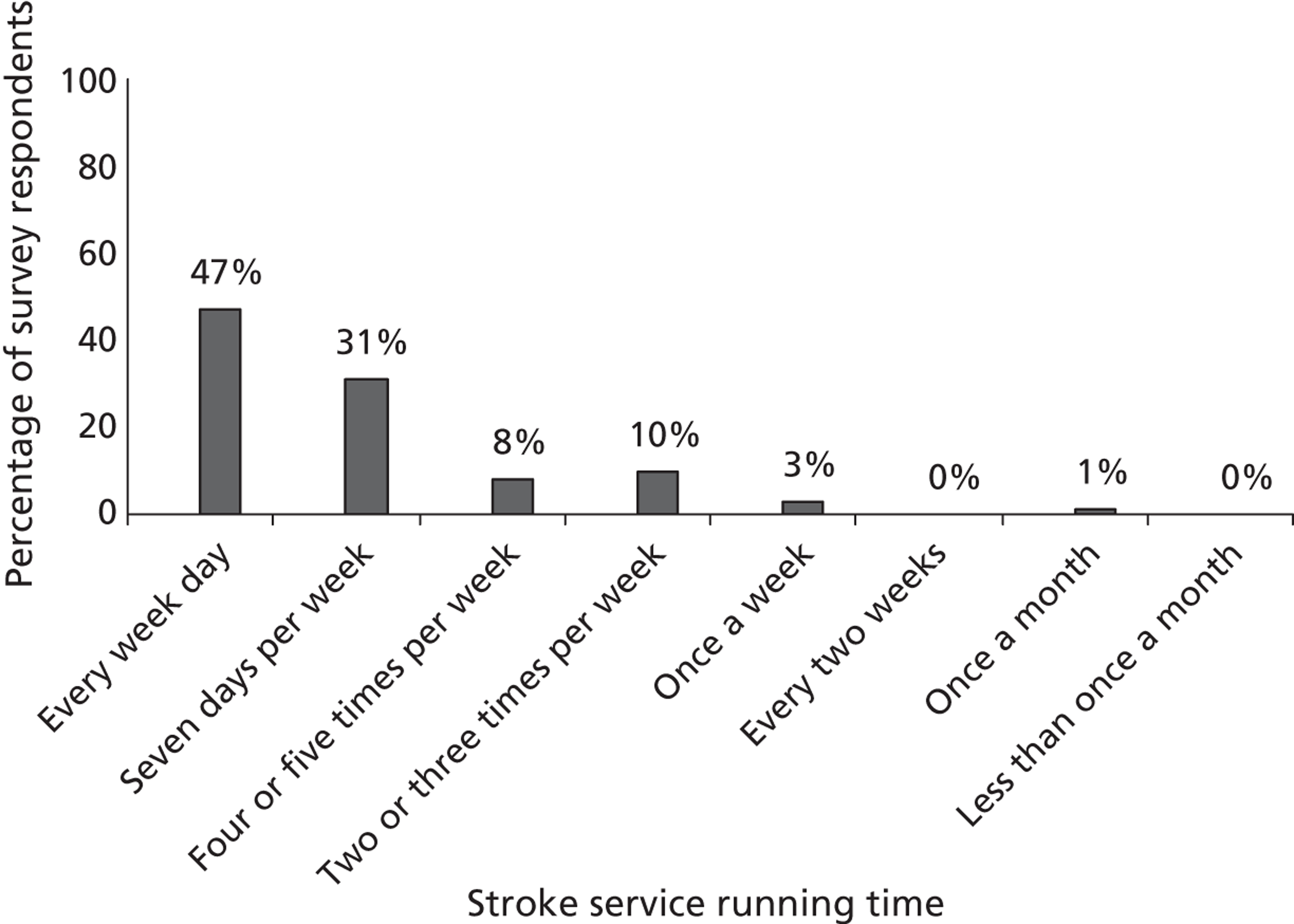
FIGURE 30.
Number of new patients suspected of TIA/minor stroke attending the specialist stroke service each time it runs. Note: 109 out of 114 respondents answered this question.
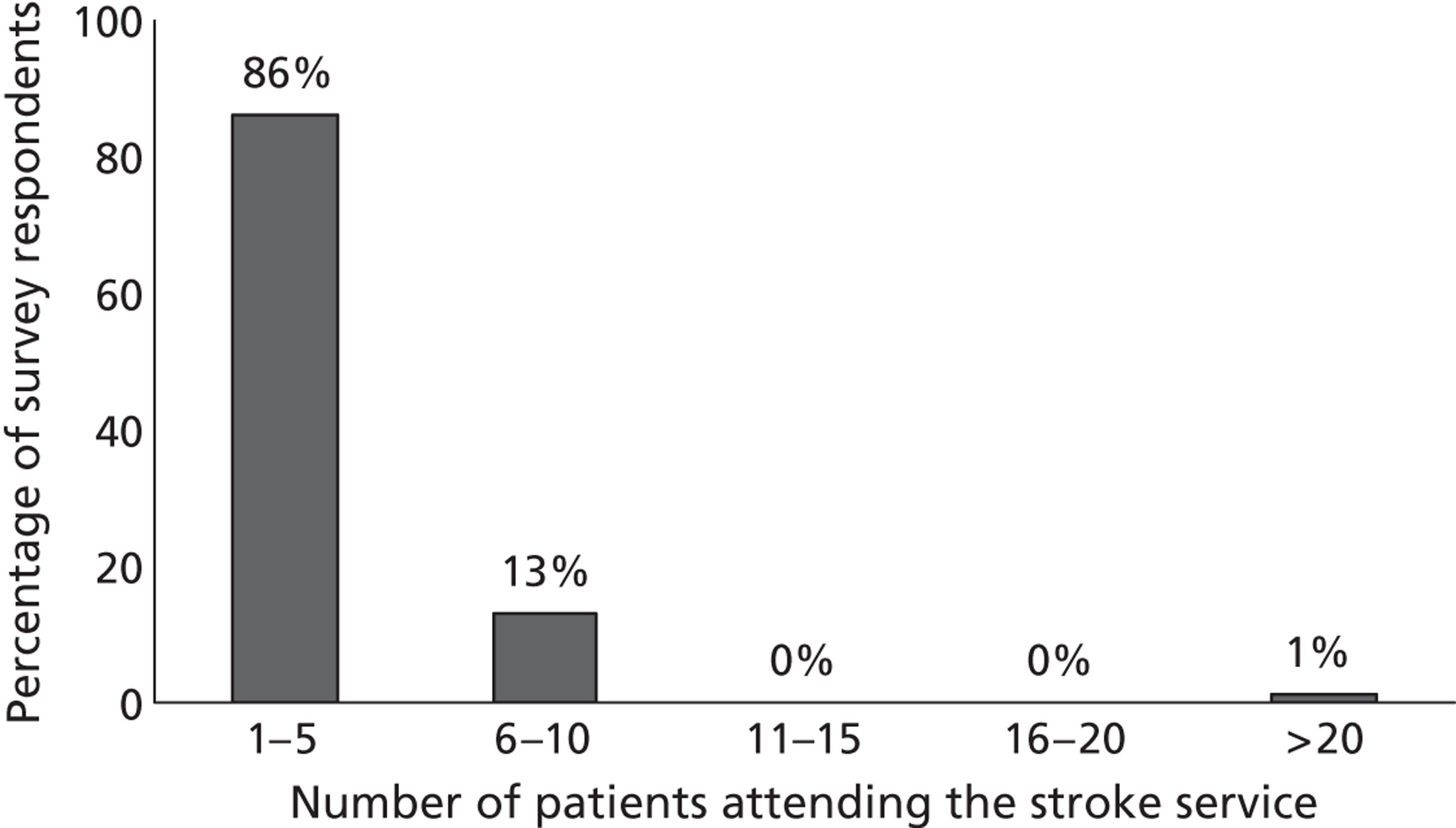
FIGURE 31.
Proportion of patients attending the stroke service who are ultimately diagnosed as having TIA or minor stroke. Note: 102 out of 114 respondents answered this question.
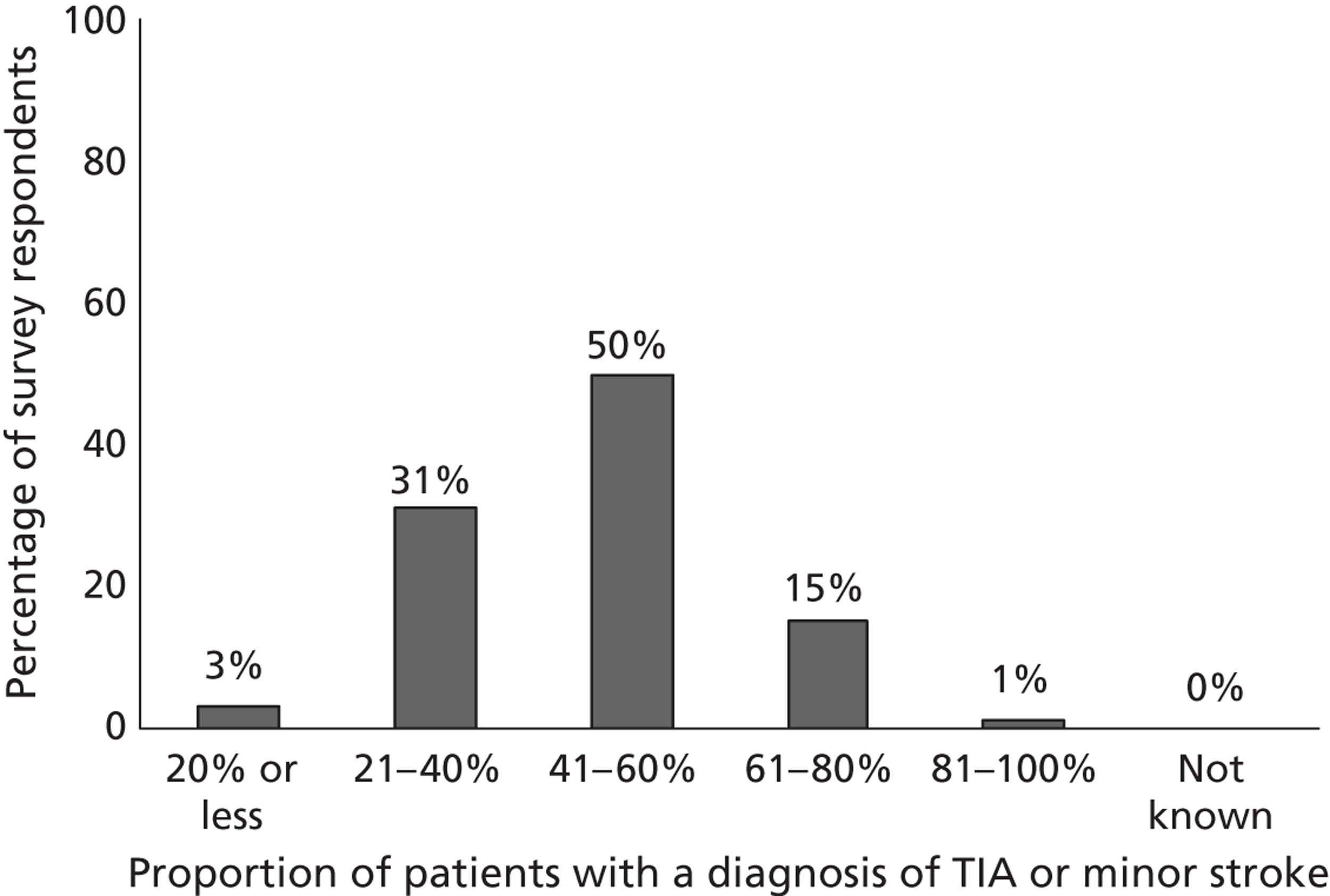
The proportion of patients who were already taking secondary prevention treatment, prescribed by their GP/referring doctor after the index event, when seen at the TIA clinic was between 41% and 60% in 20%, 61–80% in 24% and 81–100% in 29% of the responses. Thus, an overall average of 75% of patients had already been started on secondary prevention by the referring GP/ED before being seen at the secondary prevention clinic and before any investigations. In other words, in approximately 50% of the surveyed centres, the majority of patients (60–100%) seen at the specialist stroke prevention service had already been prescribed secondary prevention drugs (e.g. aspirin, statin) by their GP or referring doctor in response to their CV event.
The majority of stroke prevention services were run by consultants (stroke physicians, neurologists, geriatricians). However, 6% of the centres indicated that a nurse or stroke nurse specialist the service (Figure 32) and 19% of centres pointed out that a nurse was among the professionals who actually saw the patients in the stroke service (Figure 33). The main medical assessment or triaging of TIA/minor stroke patients was indeed undertaken by a nurse or nurse specialist in 28% of centres (Figure 34). The final diagnosis was established either with input from or by a consultant in 71% of centres (Figure 35). In most centres, the patient’s clinical assessment was performed by one or two doctors (one doctor in 57% of the centres and two doctors in 35% of the centres). Only in a few centres (Figure 36) did the patient’s assessment require three or more doctors (8%).
FIGURE 32.
Proportion of specialists running the stroke prevention service. Note: 101 out of 114 participants answered this question.
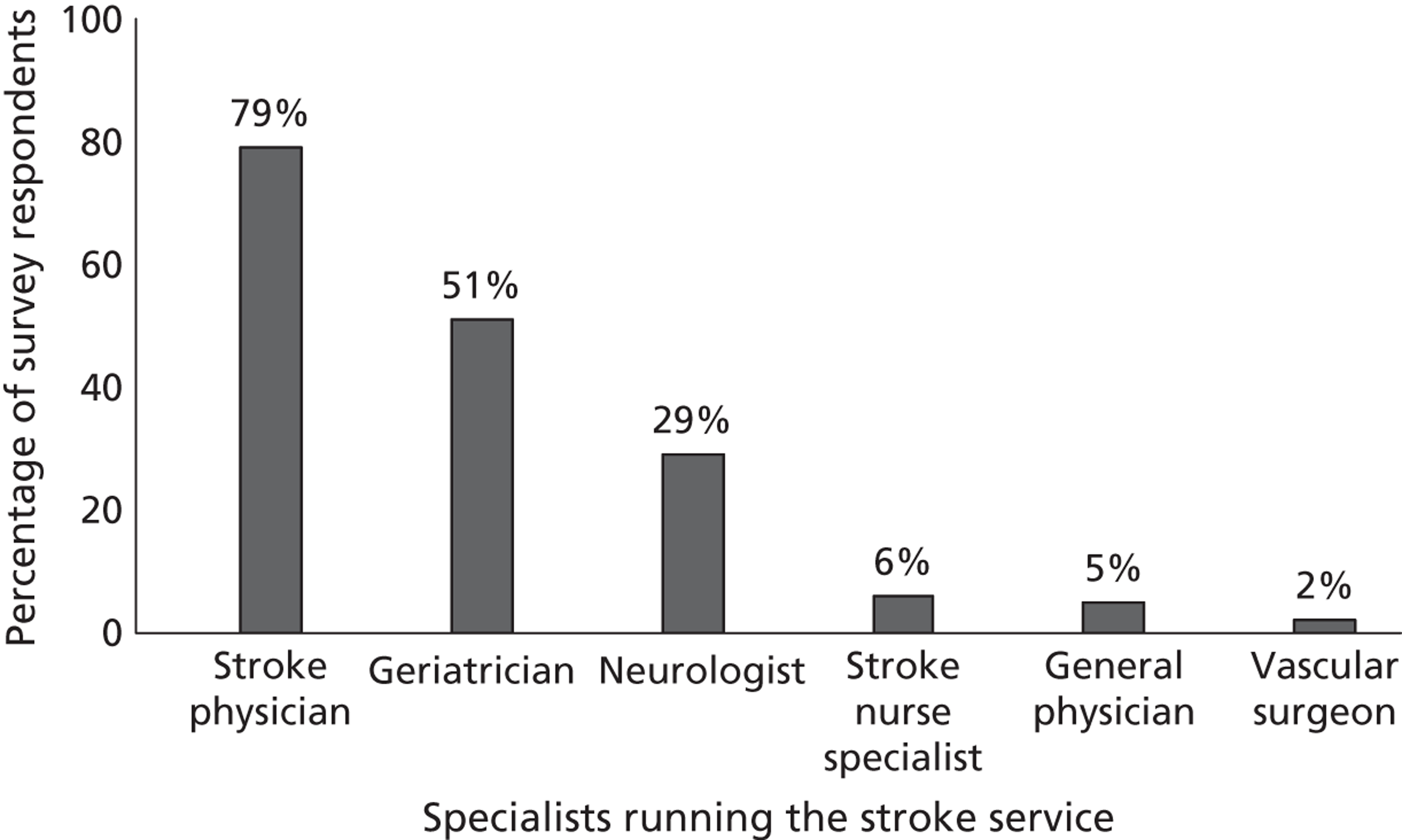
FIGURE 33.
Proportion of specialists who actually see the TIA/minor stroke patient in the prevention service. Note: 98 out of 114 participants answered this question.
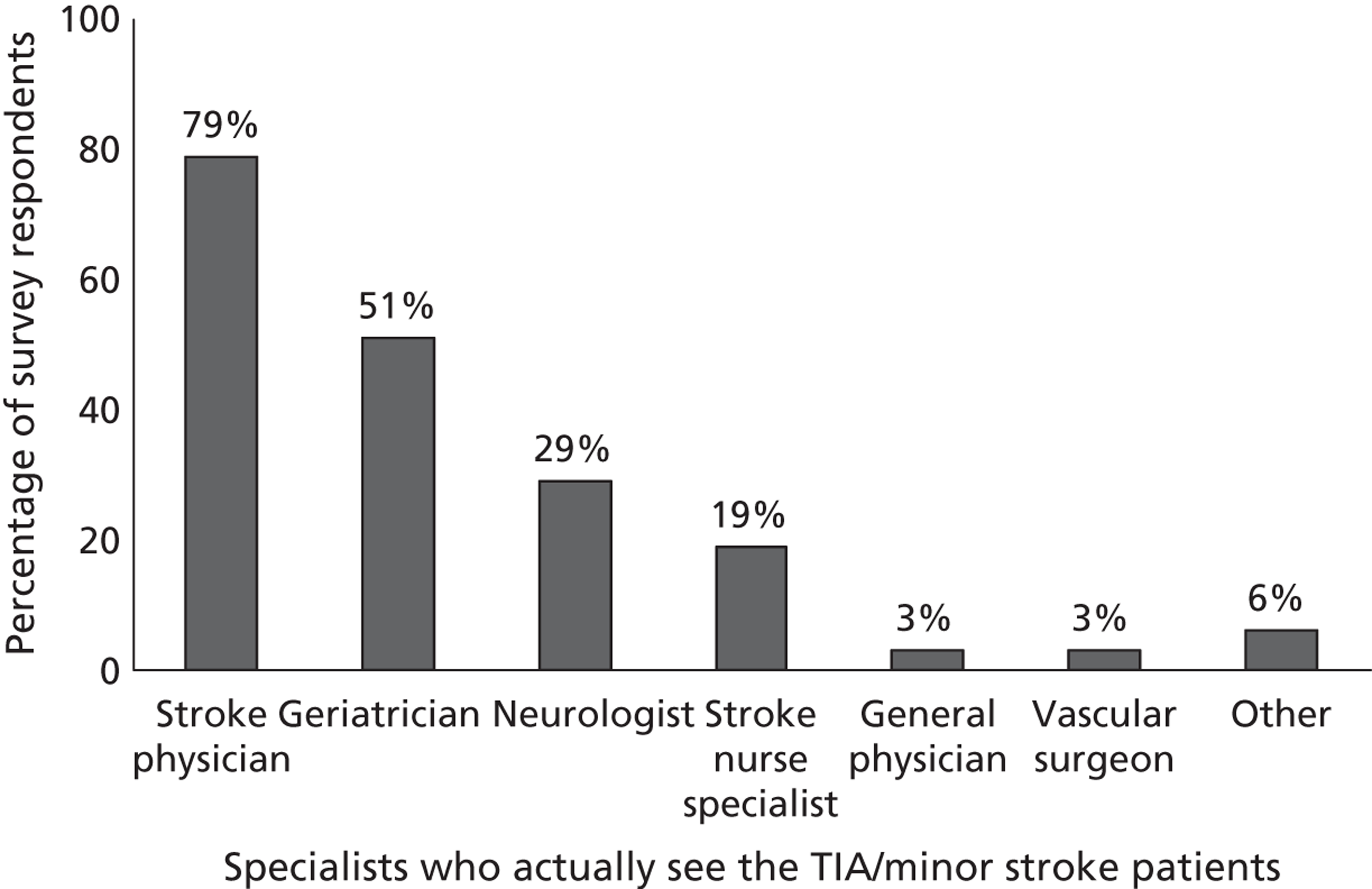
FIGURE 34.
Proportion of centres in which nurses or nurse specialists undertake the main medical assessment or triaging of TIA/minor stroke patients. Note: 99 out of 114 participants answered this question.
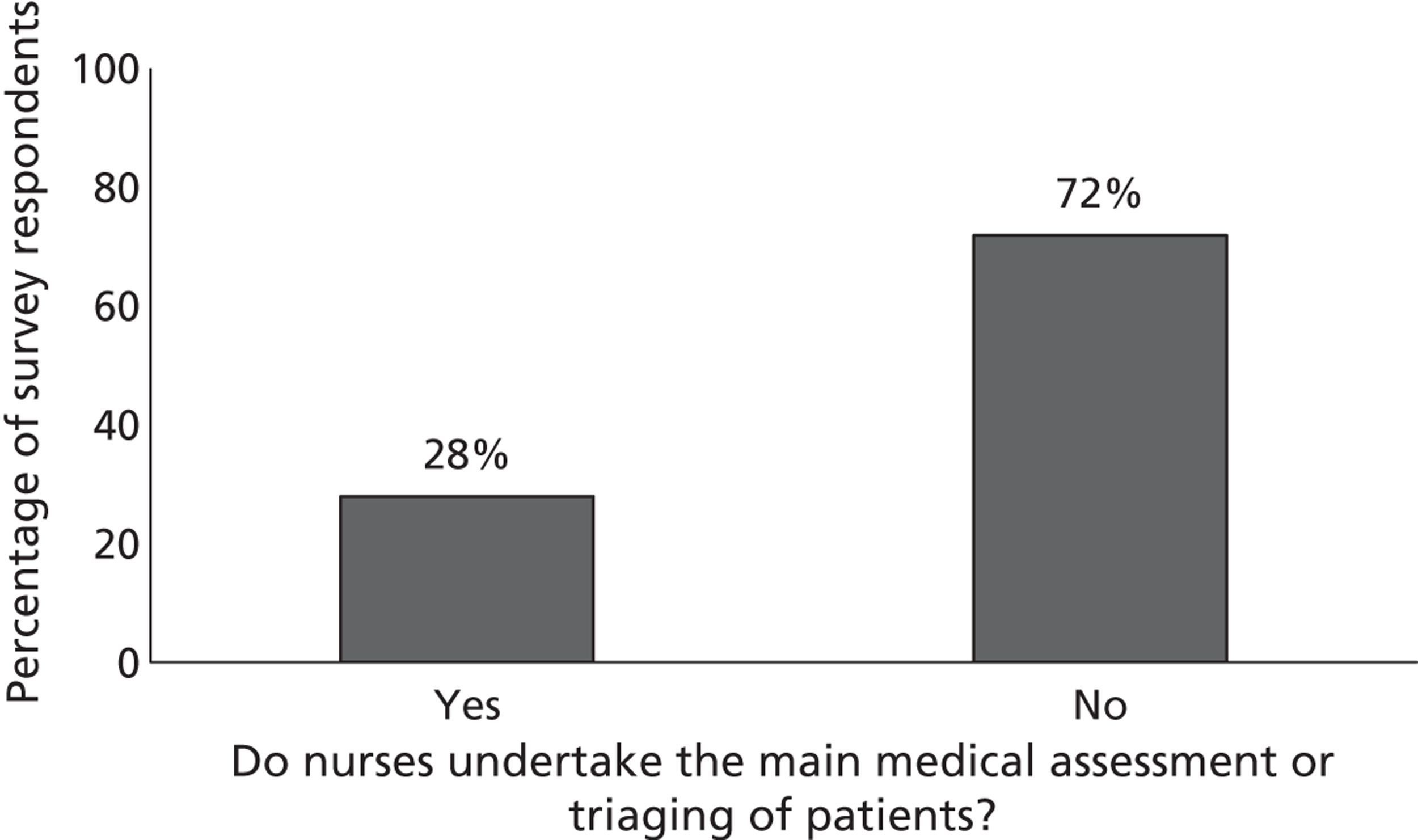
FIGURE 35.
Clinical diagnosis of TIA/minor stroke patients. Note: 99 out of 114 participants answered this question.
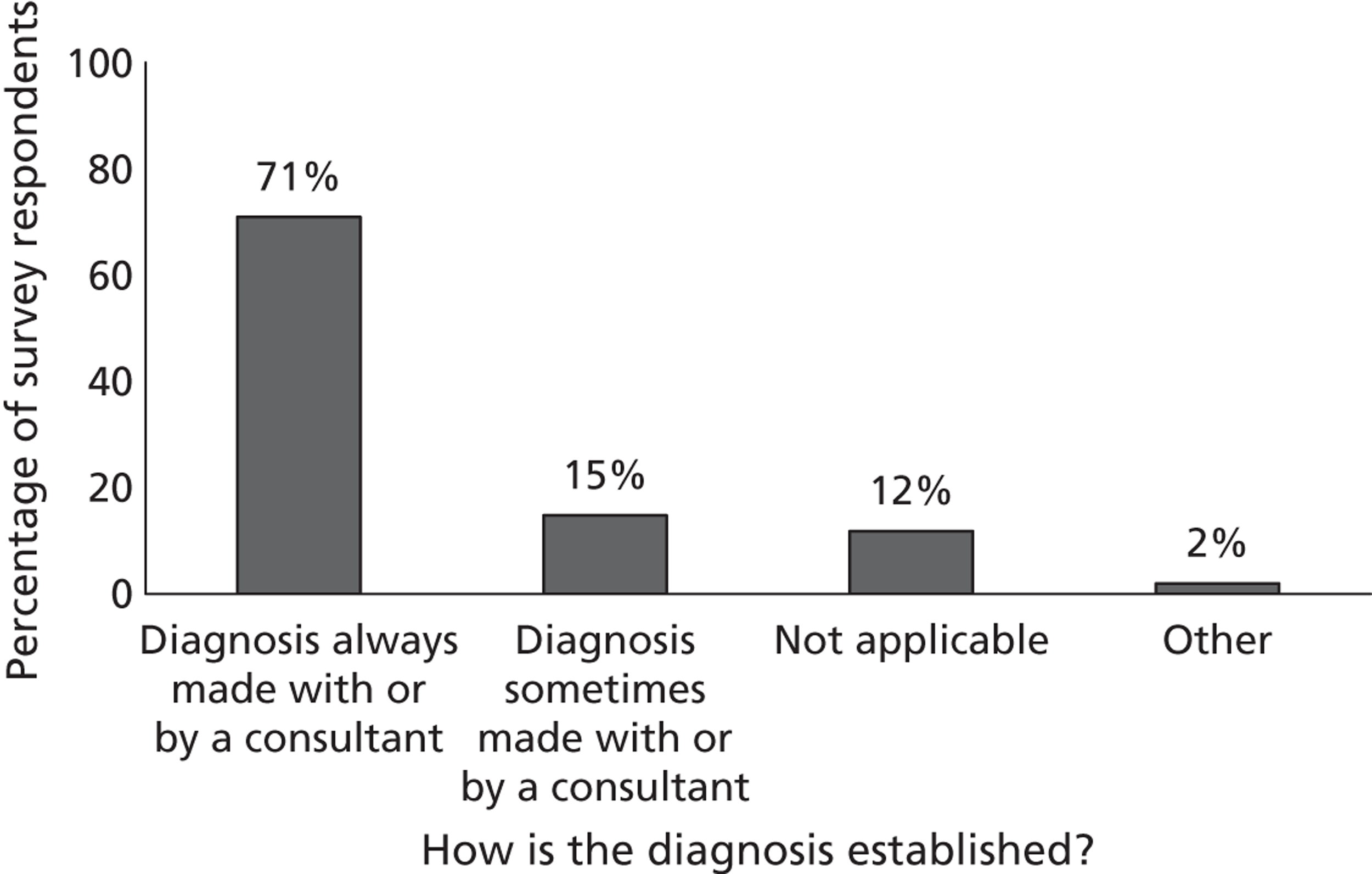
FIGURE 36.
Number of doctors involved in the patient’s clinical assessment in the prevention service. Note: 98 out of 114 participants answered this question.
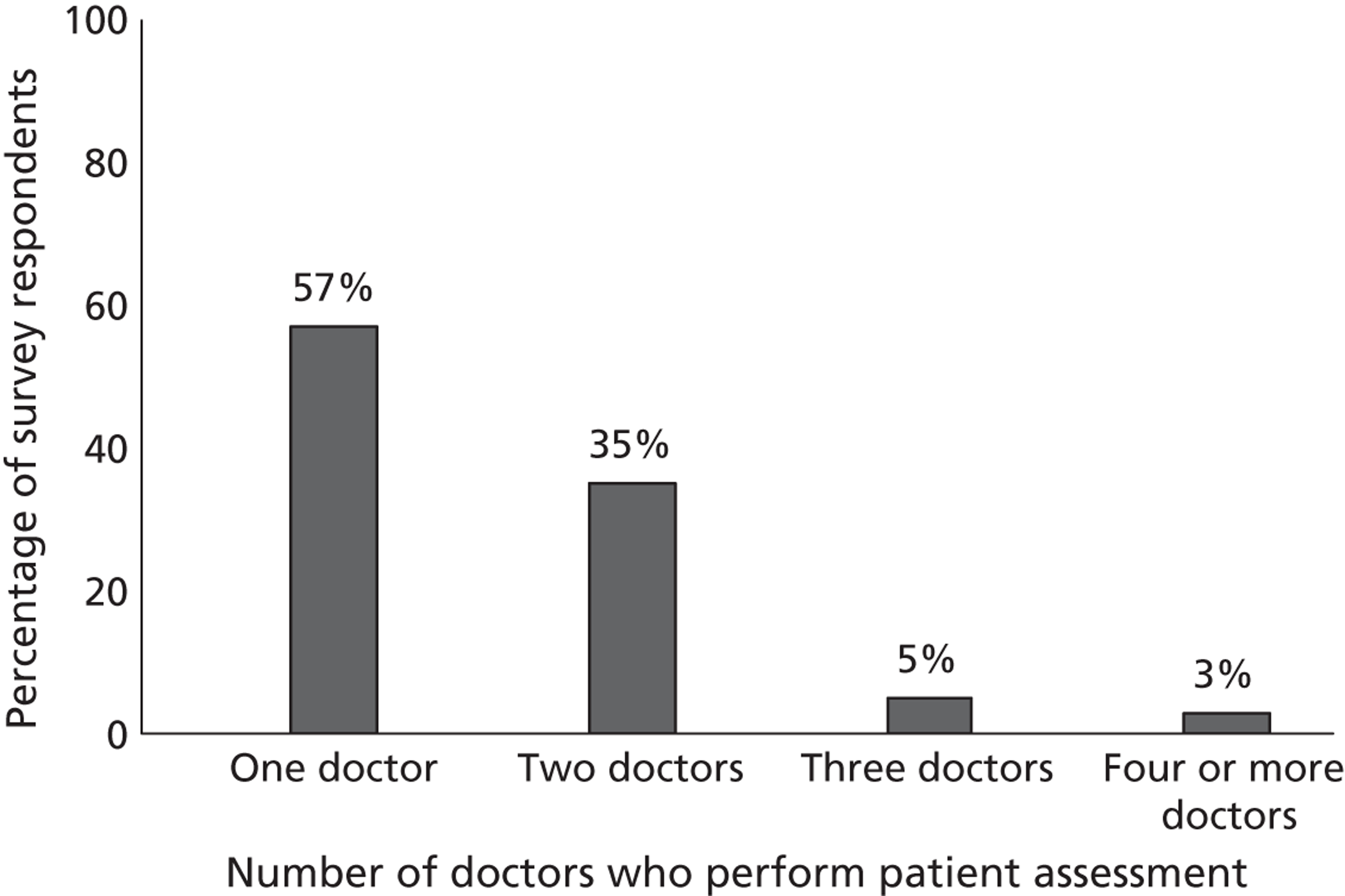
All centres responding indicated that TIA/minor stroke patients were referred primarily from GPs and other doctors within the same hospital/centre. Thirty per cent of centres also stated that patients were referred from other hospitals, 27% of centres accepted referrals from nurses and 17% of centres from other paramedics (e.g. paramedics in ambulance service). Self-referral was reported only by 4% of the centres (Figure 37). On average, about one-third of the TIA/minor stroke patients referred to the specialist stroke service were seen within 24 hours (35% same day or next day); one-quarter between 2 and 3 days (25%), and about 20% within 1 week. Thirteen per cent of the centres indicated also that patients considered at high risk according to the ABCD2 risk prediction score were seen within 24 hours from referral, whereas low-risk patients were seen within 7 days (Figure 38). It worth noting that we did not ask explicitly about assessment of patients according to the ABCD2 score but that respondents spontaneously provided this information.
FIGURE 37.
Referrals of patients suspected of TIA/minor stroke to the prevention service. Note: 101 out of 114 participants answered this question.
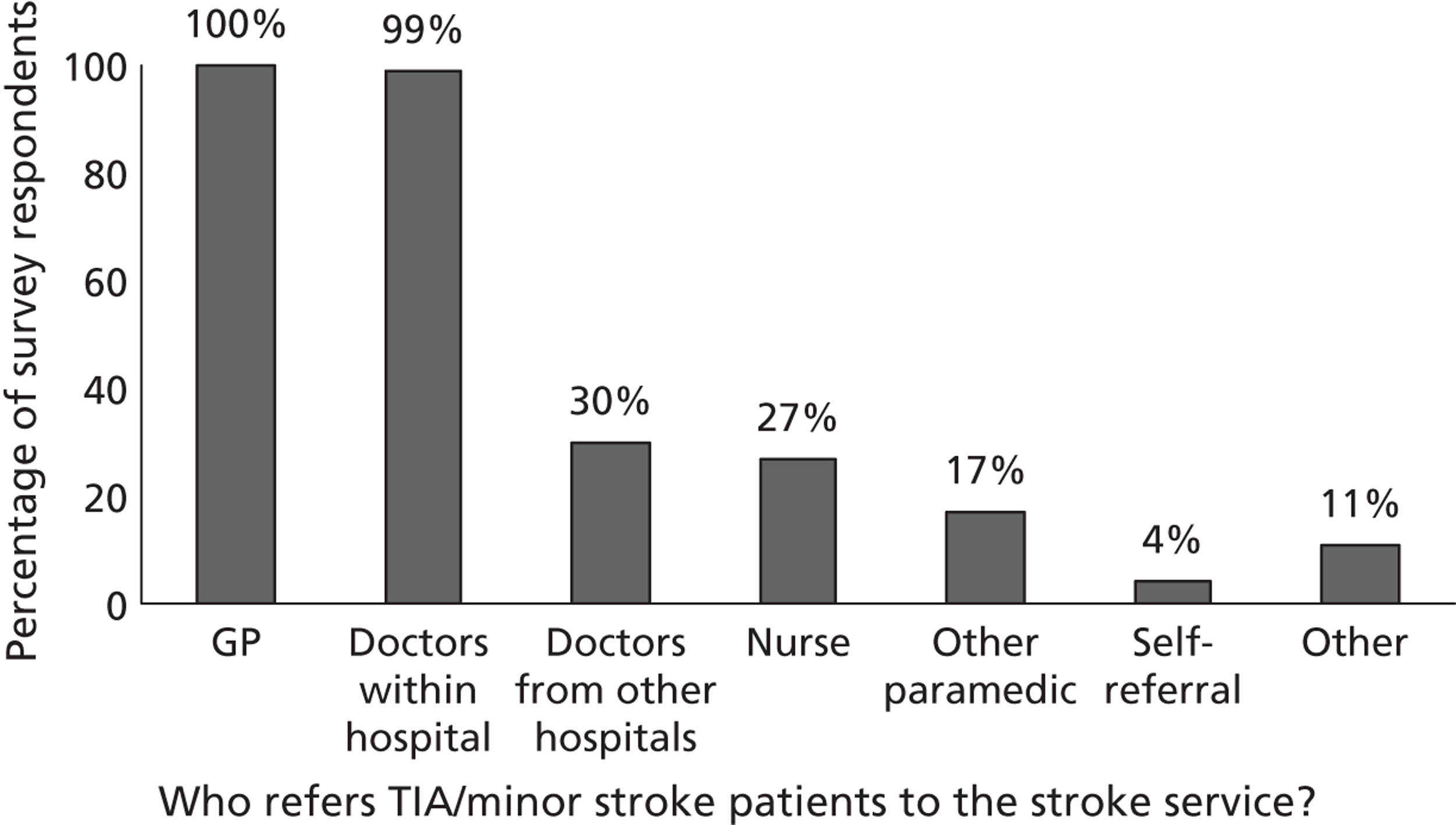
FIGURE 38.
Time to appointment after initial referral. Note: 102 out of 114 participants answered this question.
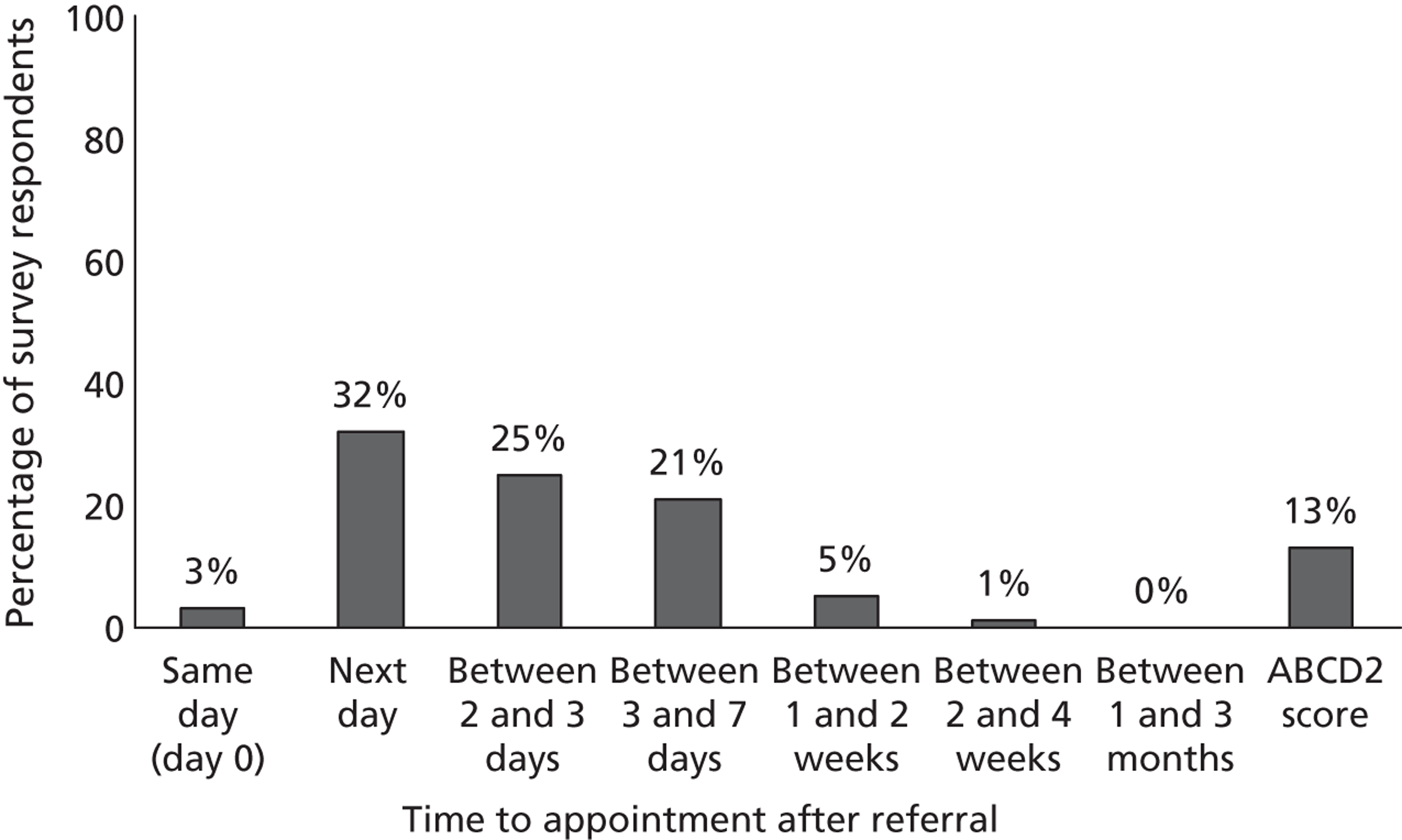
More frequently (in approximately 39% of the centres), imaging of the brain or neck arteries took place the same day of the patient’s appointment, at the same hospital visit, after clinical assessment (Figure 39). It took place the same day of the patient’s appointment but before clinical assessment in approximately 17% of centres and the next day in approximately 15% of centres. Seven per cent of centres indicated that patients did not undergo imaging of the brain or neck arteries.
FIGURE 39.
Time to brain or neck arteries imaging. Note: 100 out of 114 participants answered this question.
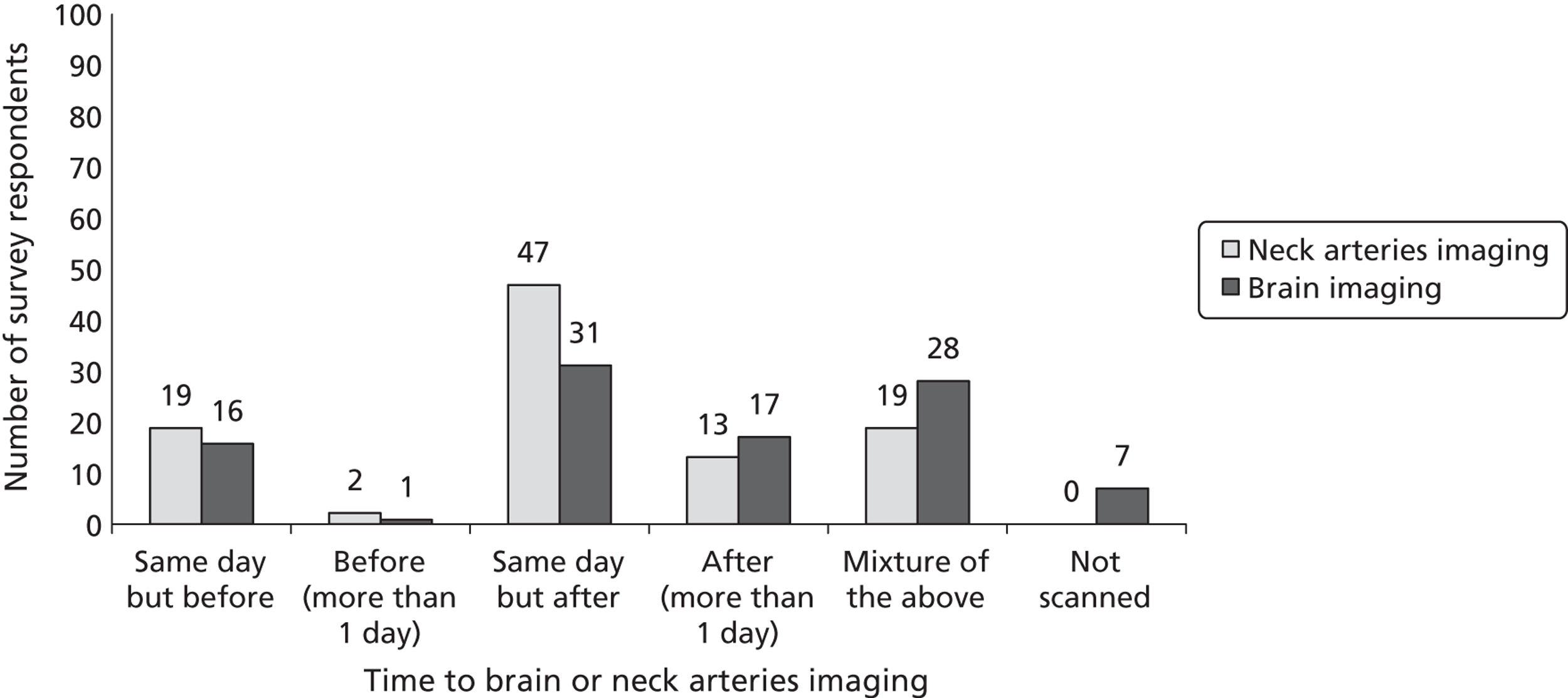
Computed tomography was routinely used as brain imaging modality in 84% of centres, whereas MRI in 51% (98/114 respondents answered this question). Moreover, CT was more often used as initial brain imaging investigation (Figure 40) for patients suspected of TIA/minor stroke. The majority of centres (66%) indicated that results of brain imaging were fed back to the stroke service the same day of the scanning; for 12% of the centres; however, it took 2–7 days to feed back the imaging results, and for 11% of centres it took > 7 days (Figure 41). In the majority of centres (76%), positive brain imaging results (e.g. haemorrhage) were fed back to the stroke prevention service immediately. The proportion of TIA/minor stroke patients for whom a subsequent MRI was requested after an initial CT varied considerably among centres (range 0% to 60%), with the majority of centres (33 centres), indicating that approximately 10% of patients were subsequently scanned with MRI (Figure 42). In approximately half of the centres (47%) the waiting time for MRI to be performed after the initial CT and the results sent back to the stroke service was 1 month; in 35% of the centres it was 1 week or less; in 7% of the centres MRI was performed one day after CT; and in 8% of the centres, the same day (Figure 43).
FIGURE 40.
Proportion of patients attending the stroke prevention service who receive CT or MRI as initial brain imaging investigation. Note: 101 out of 114 participants answered this question.
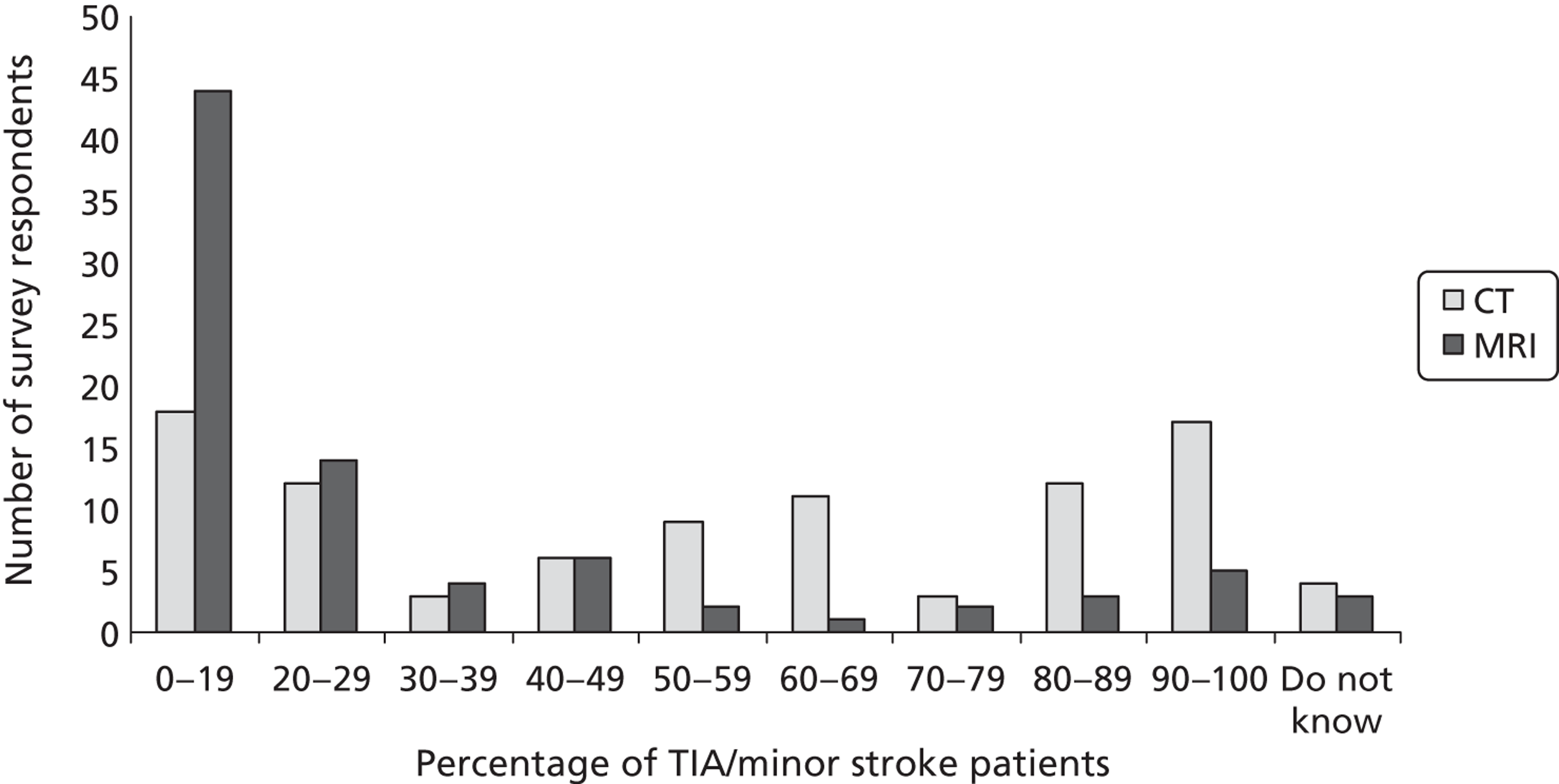
FIGURE 41.
Time for brain imaging results to reach the stroke prevention service. Note: 99 out of 114 participants answered this question.
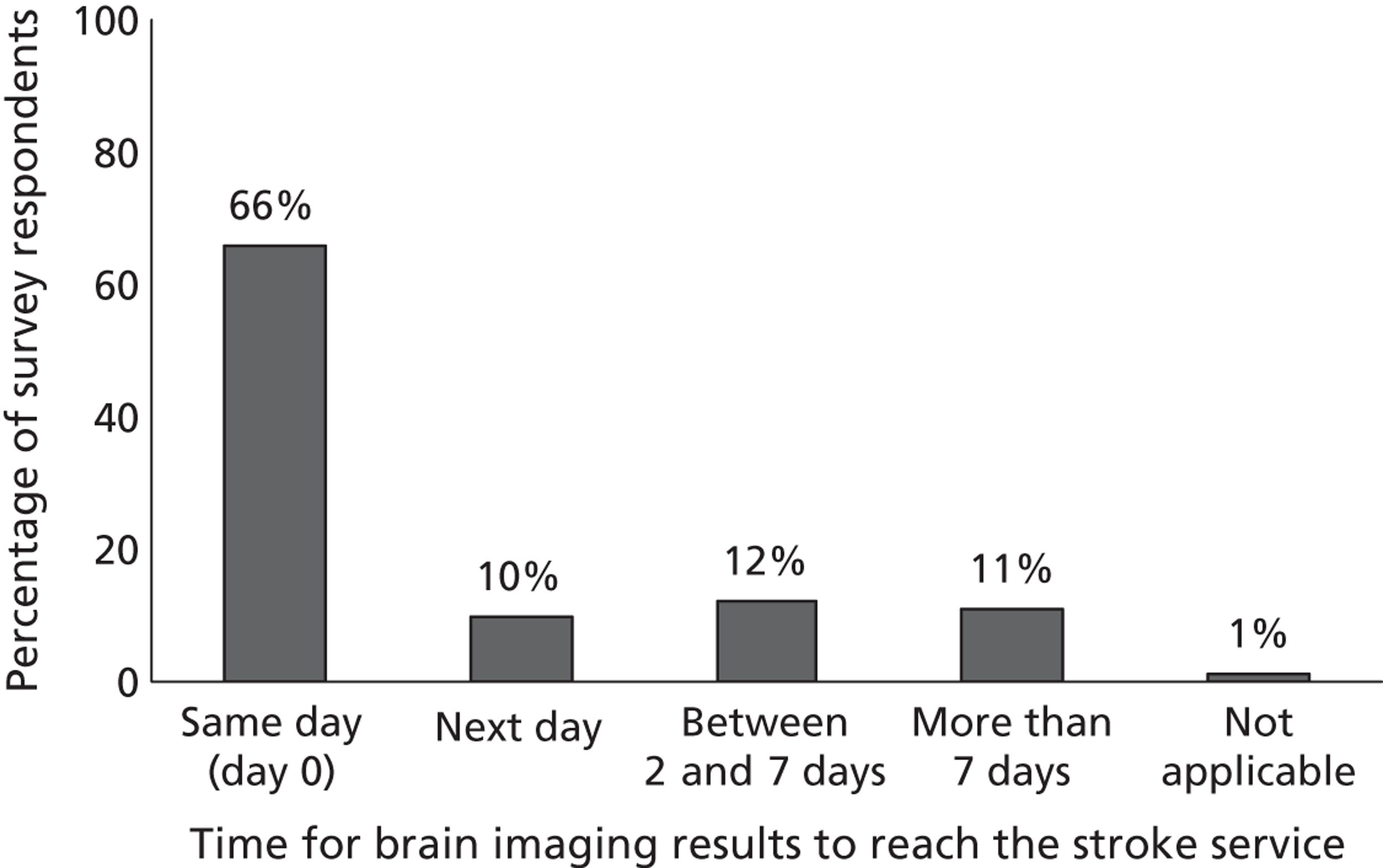
FIGURE 42.
Proportion of TIA/minor stroke patients who undergo MRI after an initial CT. Note: 86 out of 114 participants answered this question.
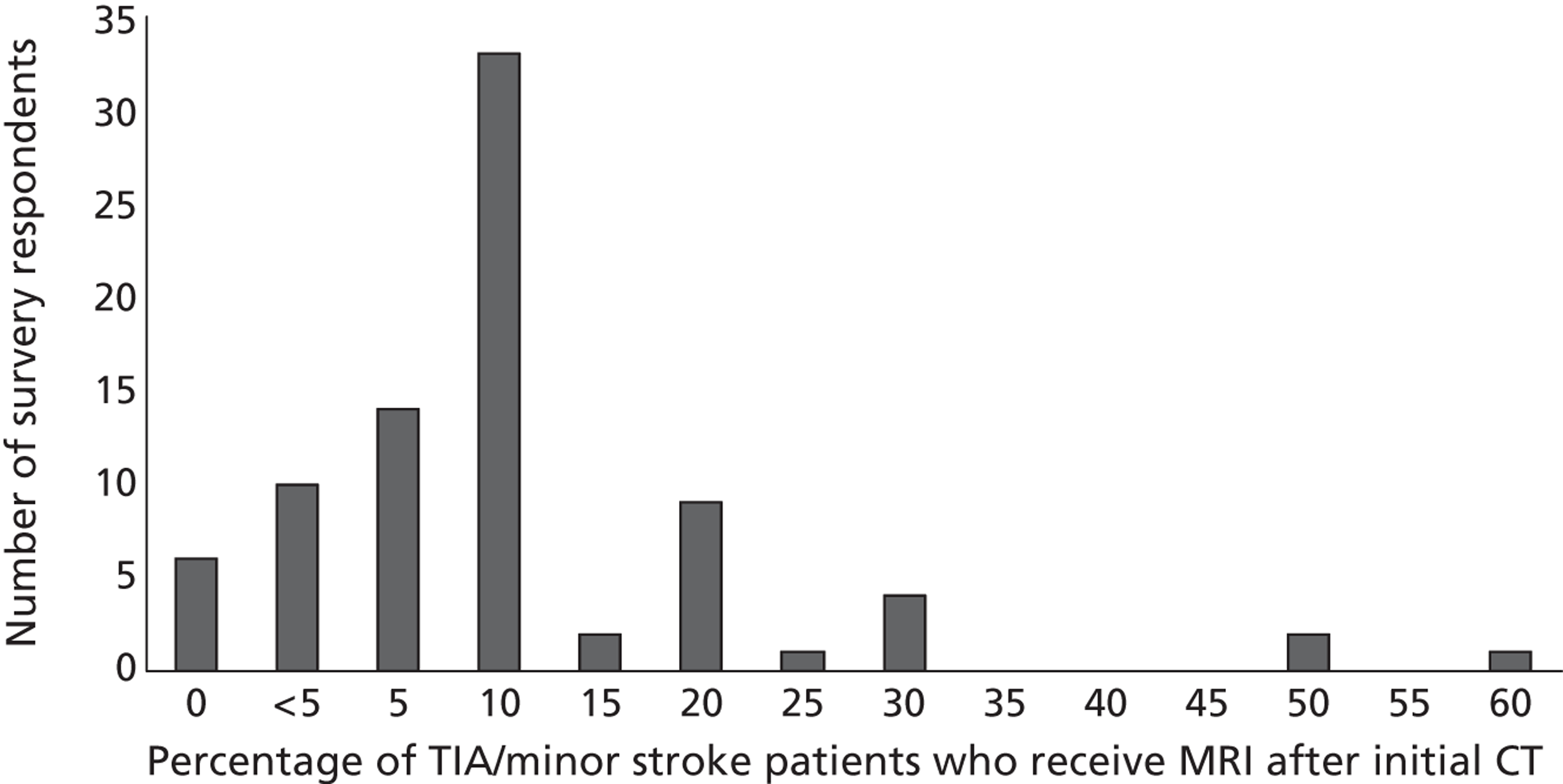
FIGURE 43.
Time to MRI after an initial CT. Note: 96 out of 114 participants answered this question.
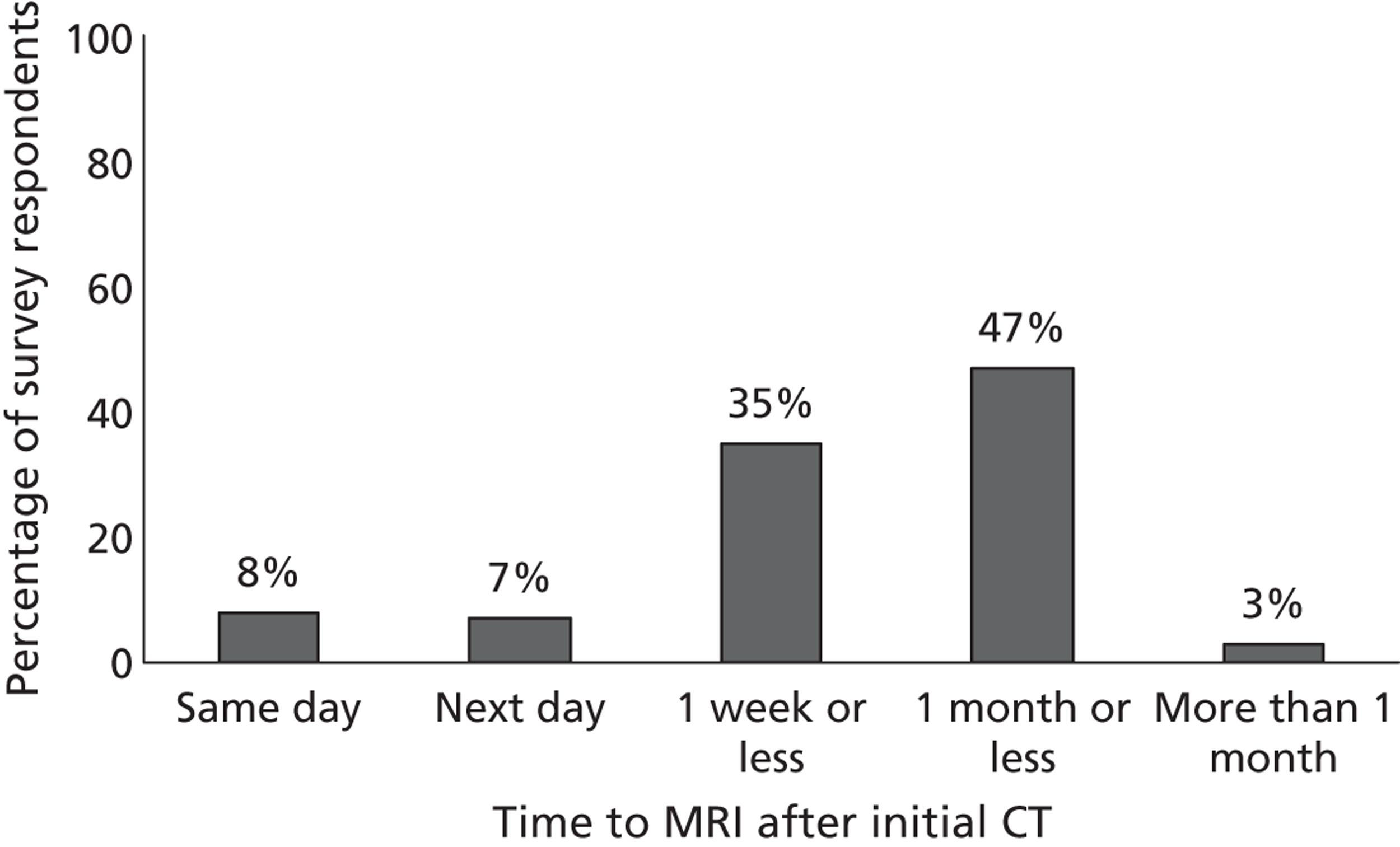
Doppler ultrasound was the first-line carotid/vertebral imaging in the majority of centres (95%). A few centres (14/100) provided information on other imaging tests used to scan the neck arteries. These included CTA (in 11 centres), MRA with contrast (in five centres), and MRA without contrast (in two centres). No centre indicated IAA as a first-line carotid/vertebral investigation (Figure 44).
FIGURE 44.
Tests used to scan the neck arteries (apart from DUS). Note: 14 out of 114 participants answered this question.
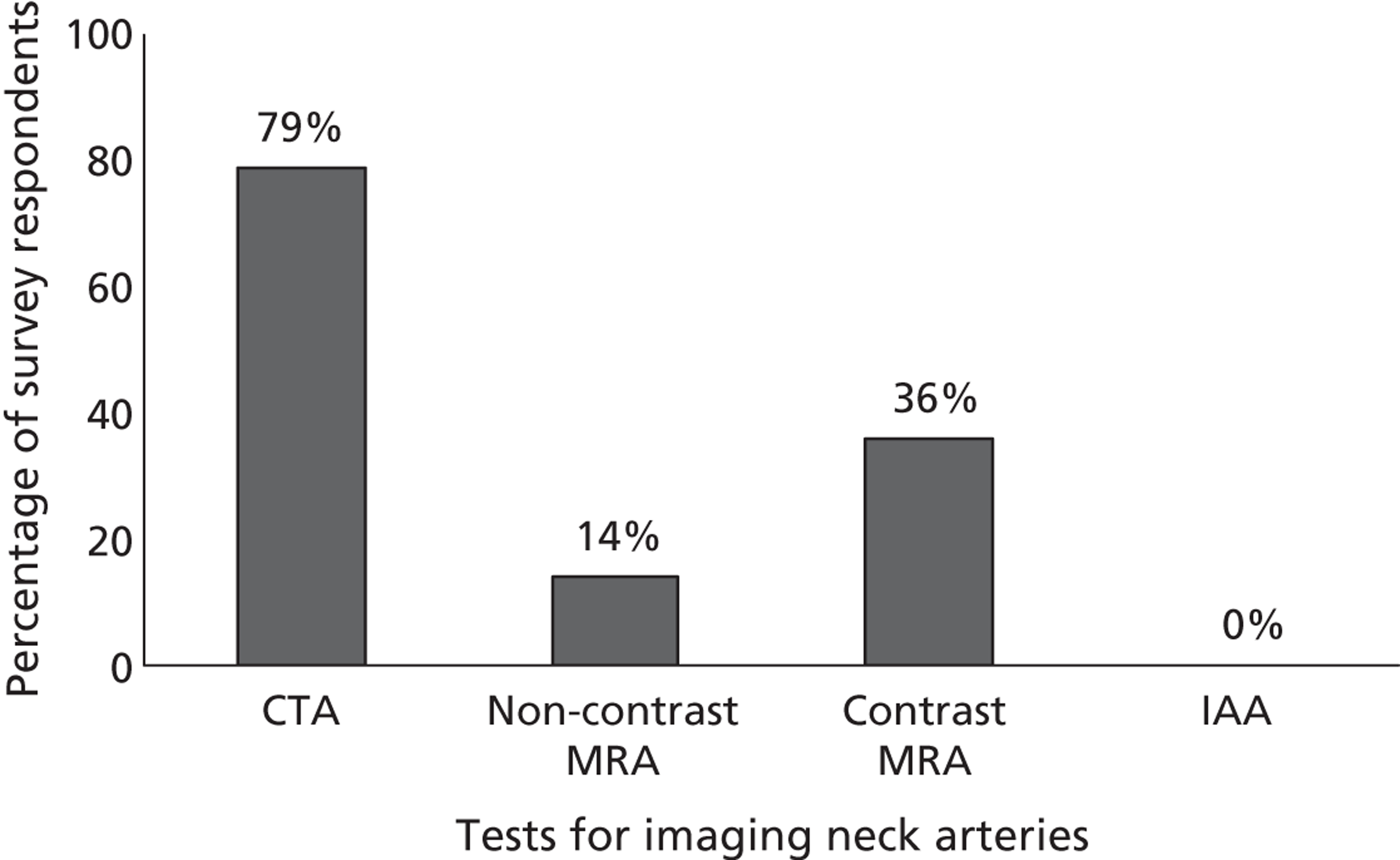
The majority of centres (80%) indicated that the results of neck imaging were fed back to the stroke service the same day (Figure 45). The majority of centres (86%; 85/99 centres that answered this question) indicated that important positive neck arteries results were fed back immediately to the stroke prevention service. With regard to further tests used to confirm the initial DUS or neck imaging results, prior to referral for endarterectomy, CTA and contrast MRA were among the choices more often indicated (Figure 46). However, in 19% of centres a second DUS was usually requested. The waiting time for further neck imaging tests to be performed and results fed back to the stroke service is shown in Figure 47. The DUS was the only test to be read and interpreted by a radiographer or vascular technician. All of the other neck arteries tests were interpreted by a radiologist or a neuroradiologist (Figure 48). The time between the decision to perform a carotid endarterectomy and the actual surgical procedure was reported to be < 1 week in 59% of centres and < 1 month in approximately 37% of the centres (Figure 49).
FIGURE 45.
Time for neck arteries findings to reach the stroke prevention service. Note: 99 out of 114 participants answered this question.
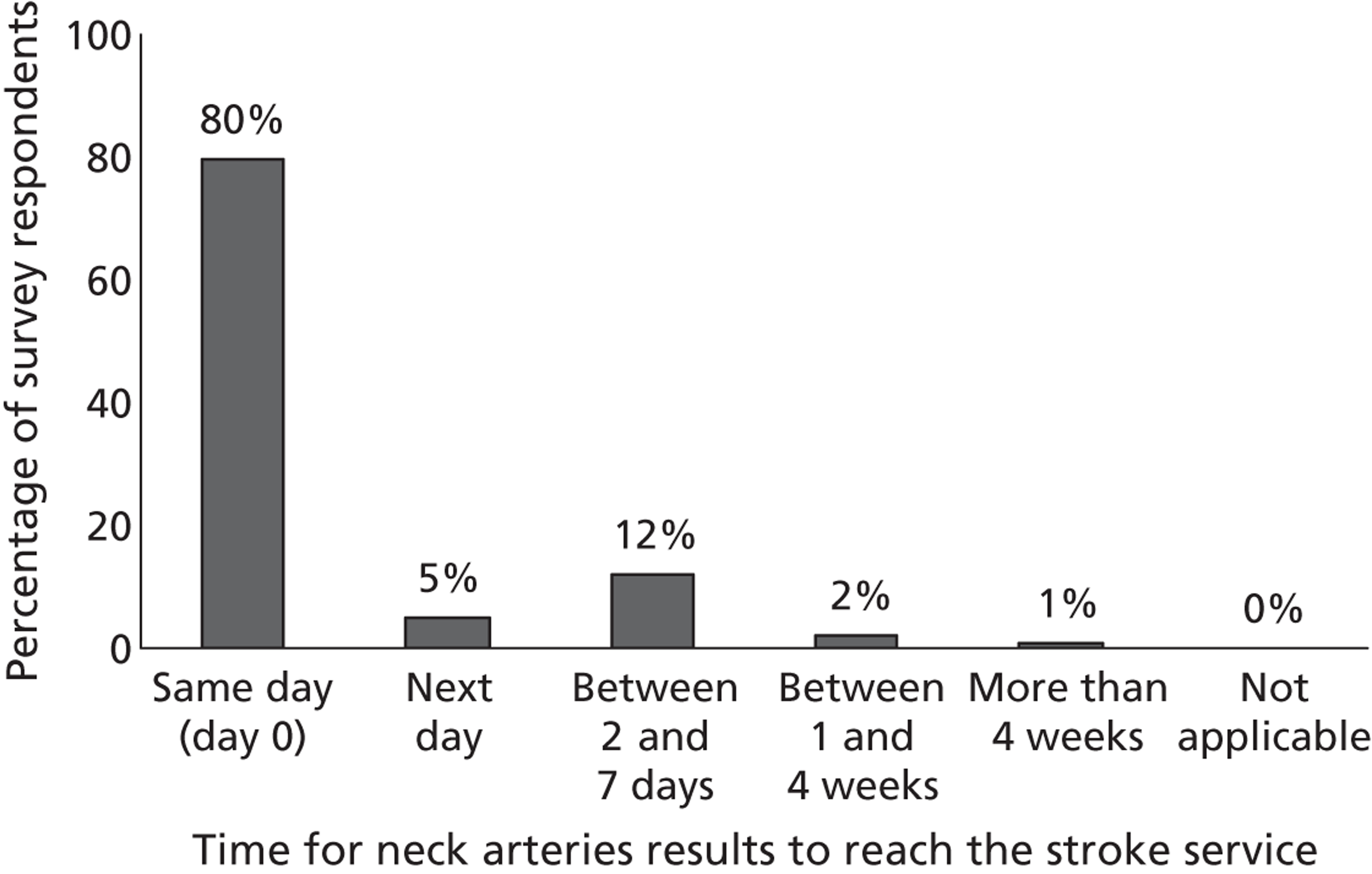
FIGURE 46.
Further tests used to confirm the initial DUS or neck imaging results prior to referral for endarterectomy. Note: 92 out of 114 participants answered this question.
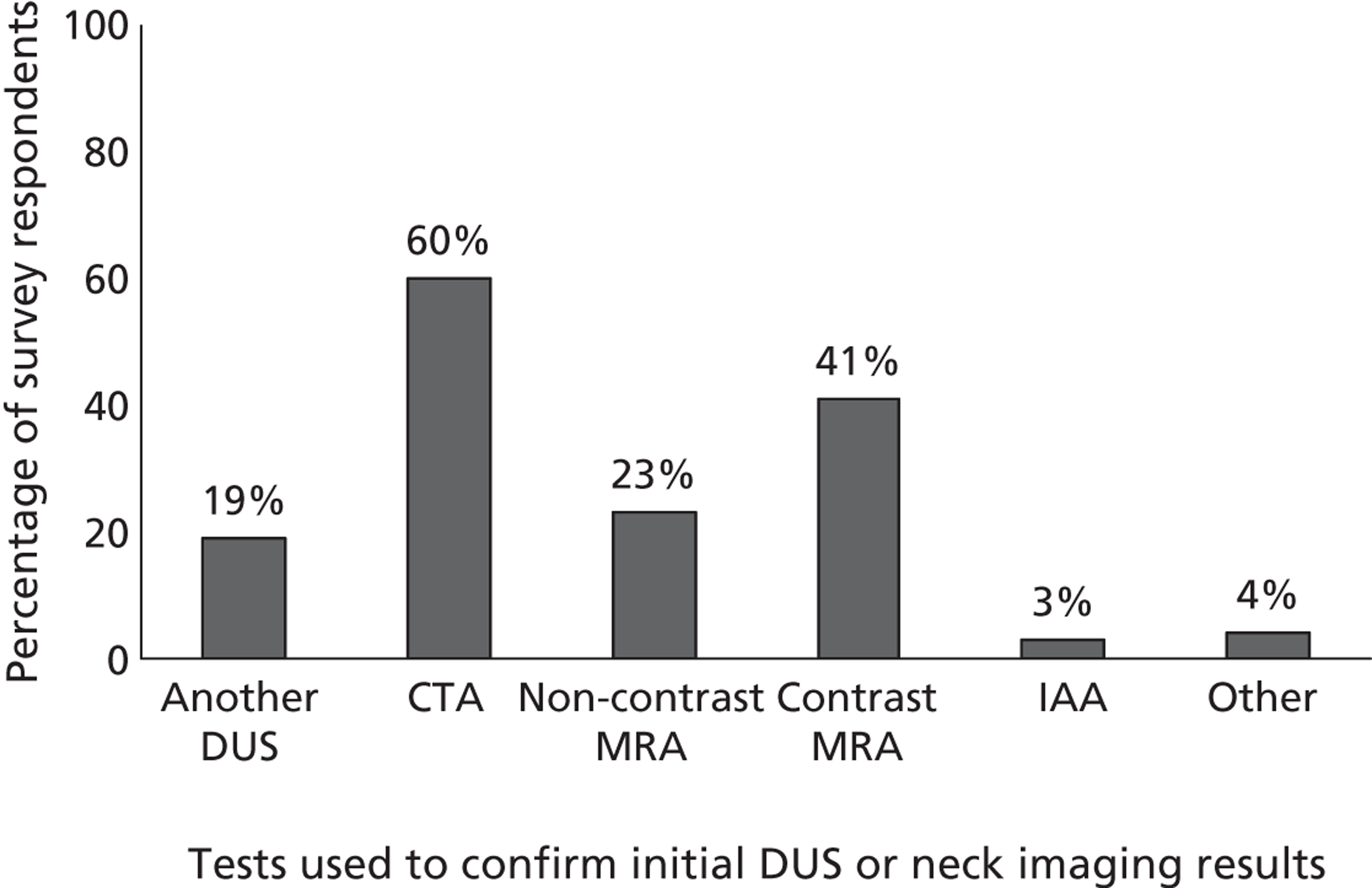
FIGURE 47.
Time for further neck arteries tests to be performed. Note: 92 out of 114 participants answered this question.
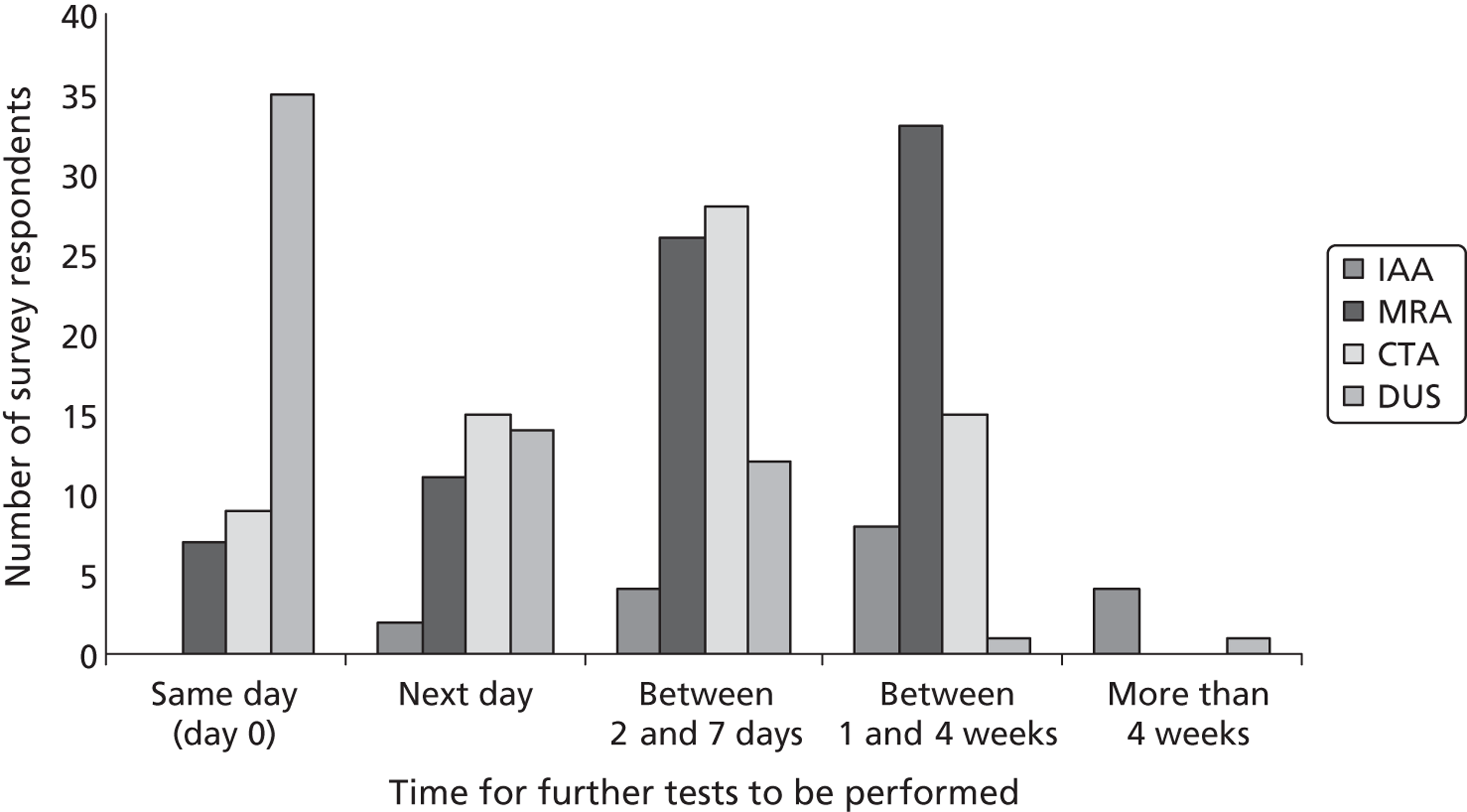
FIGURE 48.
Specialists who perform and interpret neck arteries tests. Note: 95 out of 114 participants answered this question.
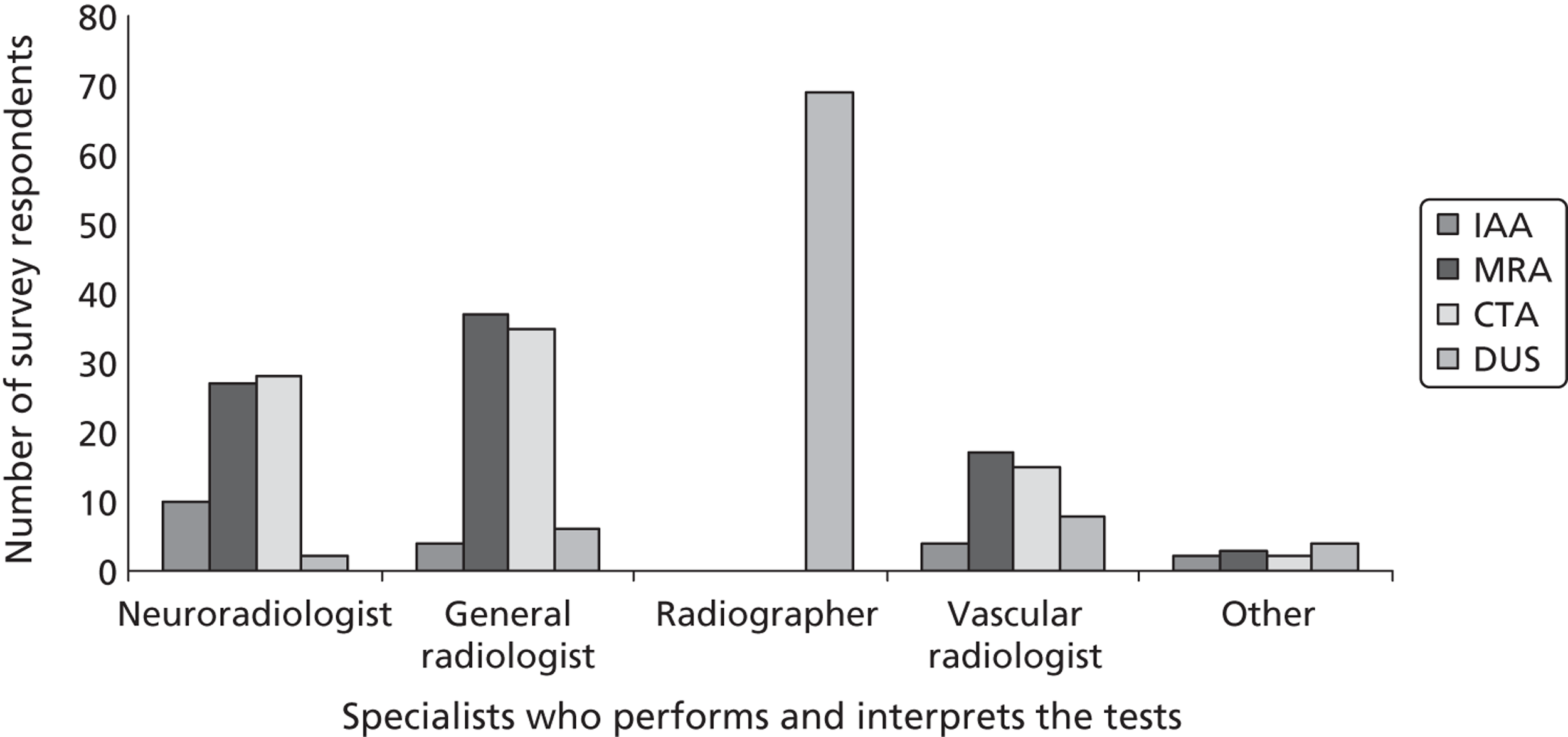
FIGURE 49.
Delay between the decision to perform endarterectomy and the actual surgical procedure. Note: 99 out of 114 participants answered this question.
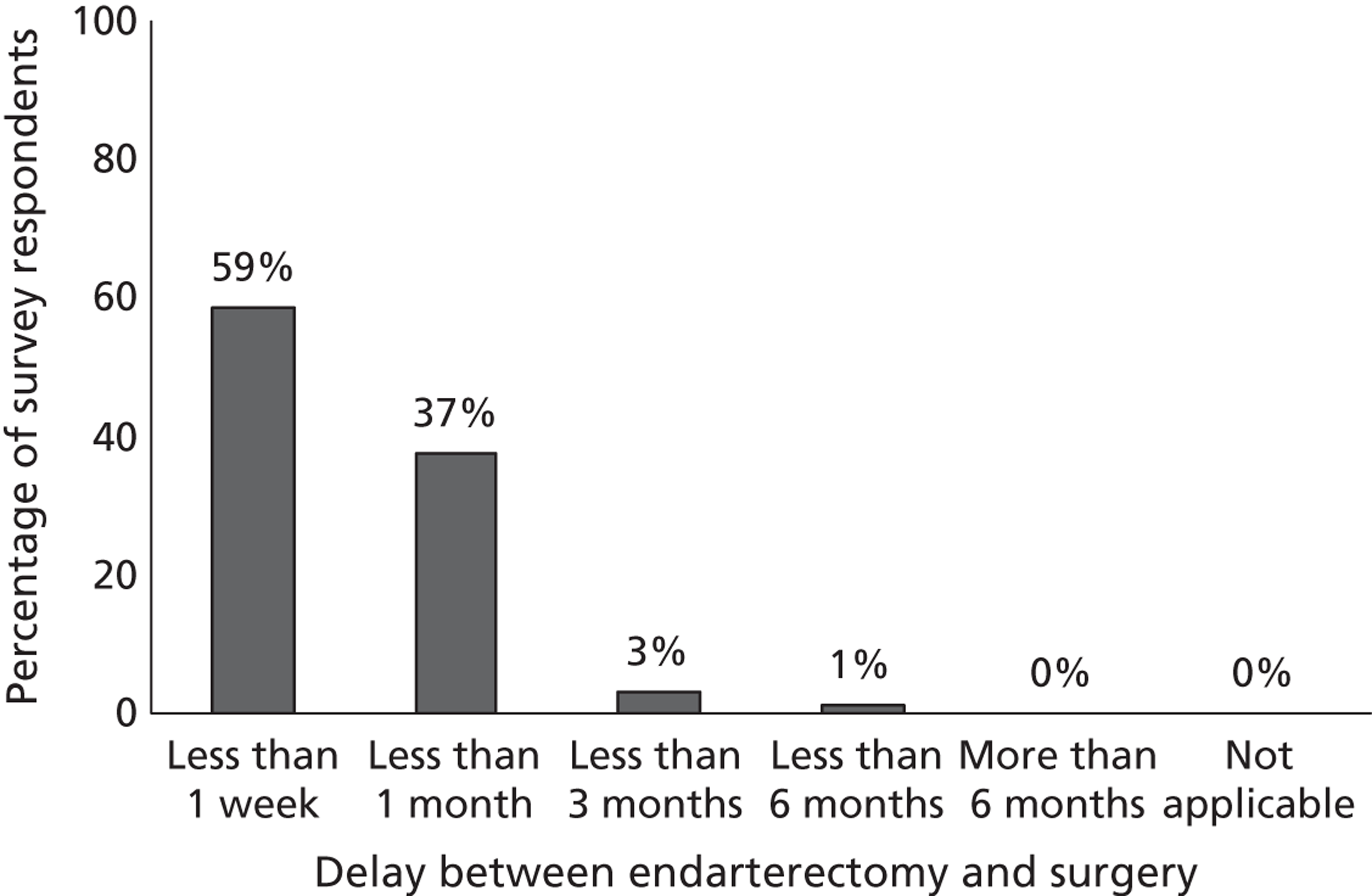
Forty-three per cent of the centres indicated that they had spare capacity to see more patients in the specialist stroke service, whereas 57% of centres did not. With regard to possible extra facilities to expand the current stroke service, different choices were indicated by different centres (Figure 50). When ‘more brain imaging’ was chosen as possible answer, all respondents opted for more MRI facilities (38/38 centres that answered this question) rather than CT (14/38). Similarly, when ‘more imaging of the neck arteries’ was chosen, the majority of centres indicated DUS as relevant neck imaging investigation (34/43 centres that answered this question), followed by MRA (16/43) and CTA (13/43 centres).
FIGURE 50.
Resources needed to expand capacity in fully saturated stroke prevention services. Note: 77 out of 114 participants answered this question.
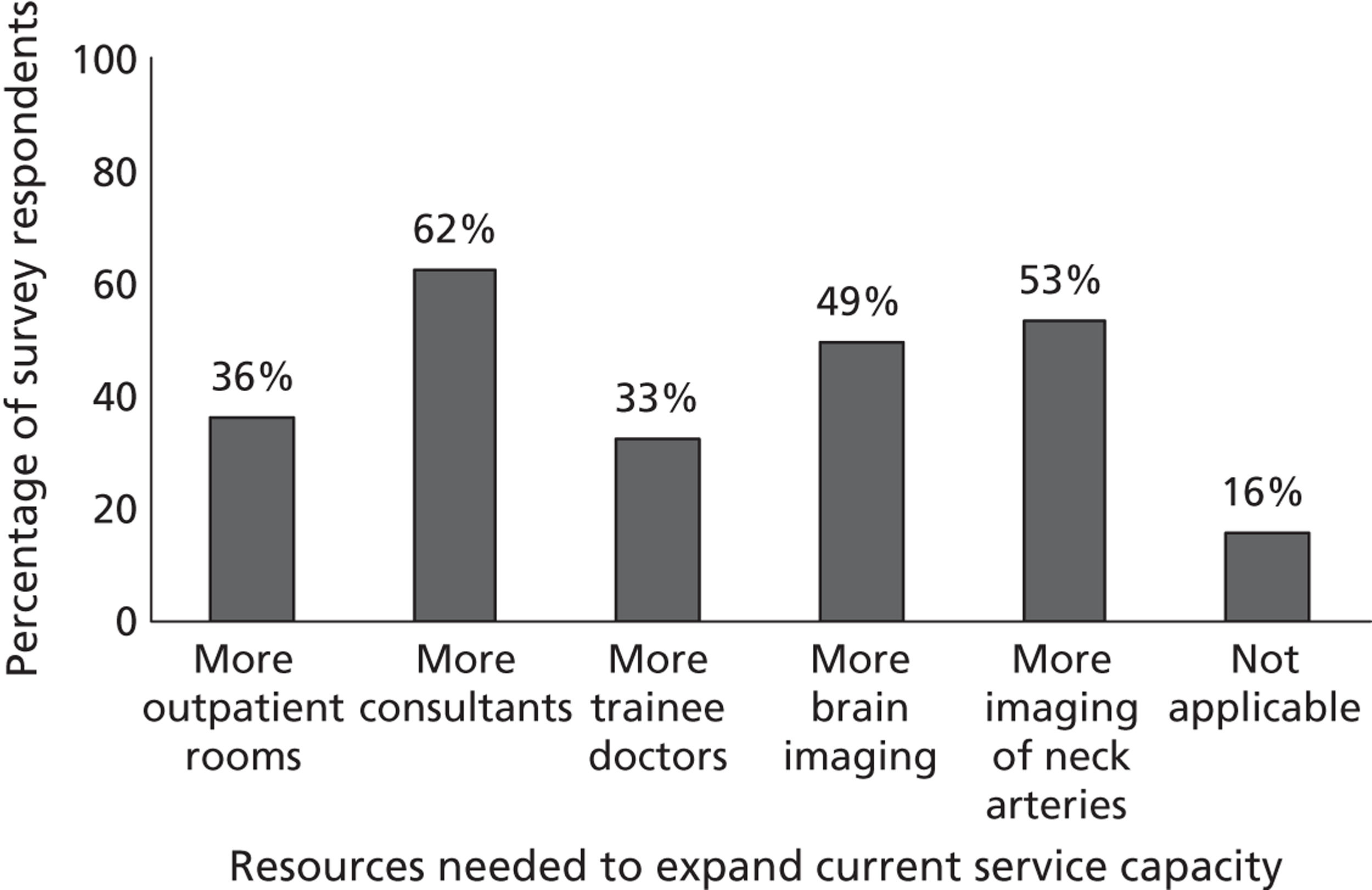
Among all respondent stroke services the mean catchment population was 400,000 people (median 300,000 people). Ninety-four clinical leads out of 114 respondents indicated the geographical location of their hospital/centre (Figure 51).
FIGURE 51.
Geographical location of 94 out of 114 stroke prevention services that provided this information.
Imaging services survey findings
Computed tomography and magnetic resonance imaging availability for transient ischaemic attack/minor stroke patients
Computed tomography and magnetic resonance imaging facilities were available in the majority of imaging departments. The number of CT scanners ranged from one to seven across departments, with most of the departments having one scanner (30% of departments) or two scanners (37%). Similarly, MRI scanners ranged from one to six across departments, with most of the departments having one scanner (44%) or two scanners (28%) (Figures 52 and 53).
FIGURE 52.
Number of CT scanners in radiology departments. Note: 135 out of 146 participants answered this question.
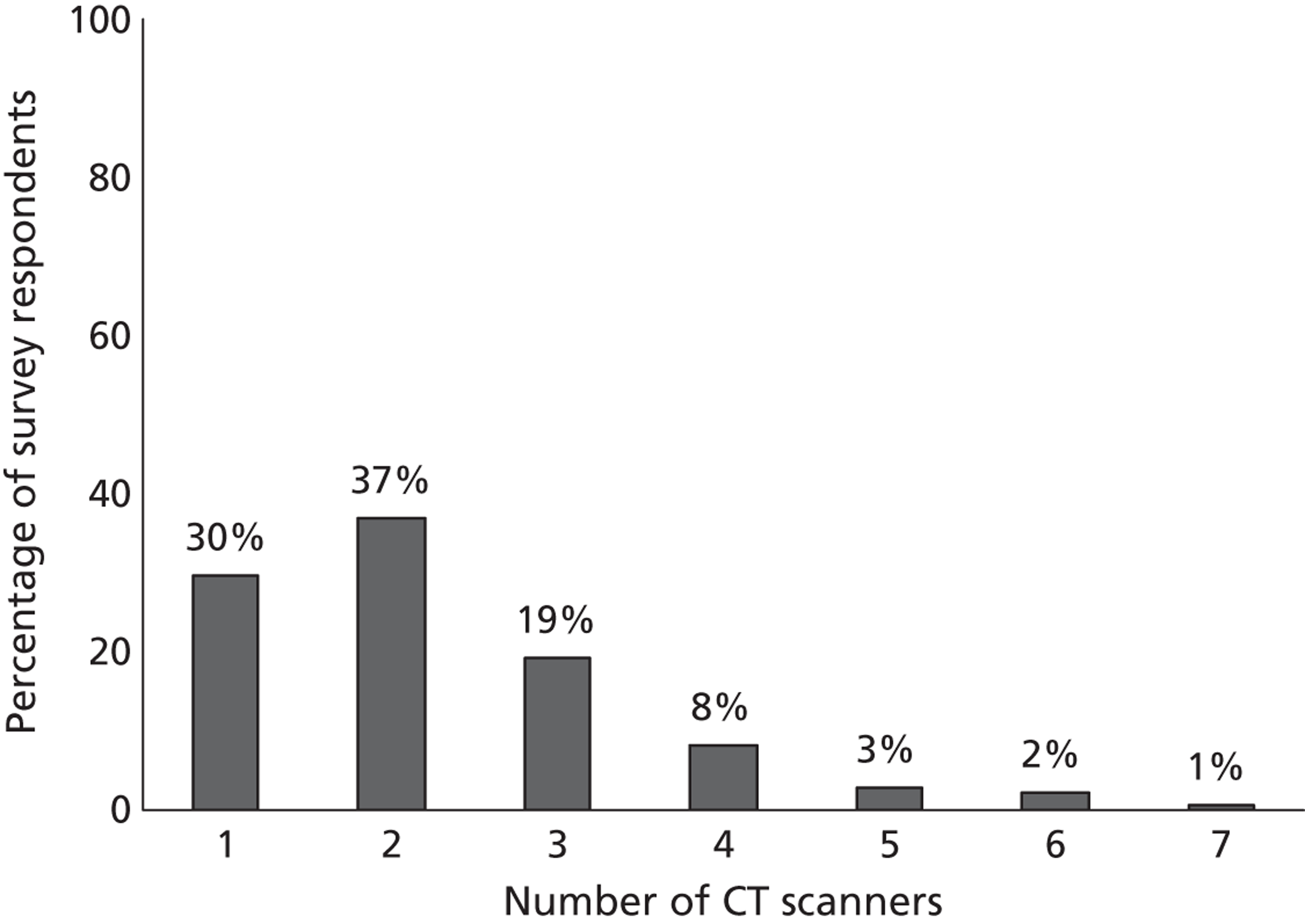
FIGURE 53.
Number of MR scanners in radiology departments. Note: 133 out of 146 participants answered this question.
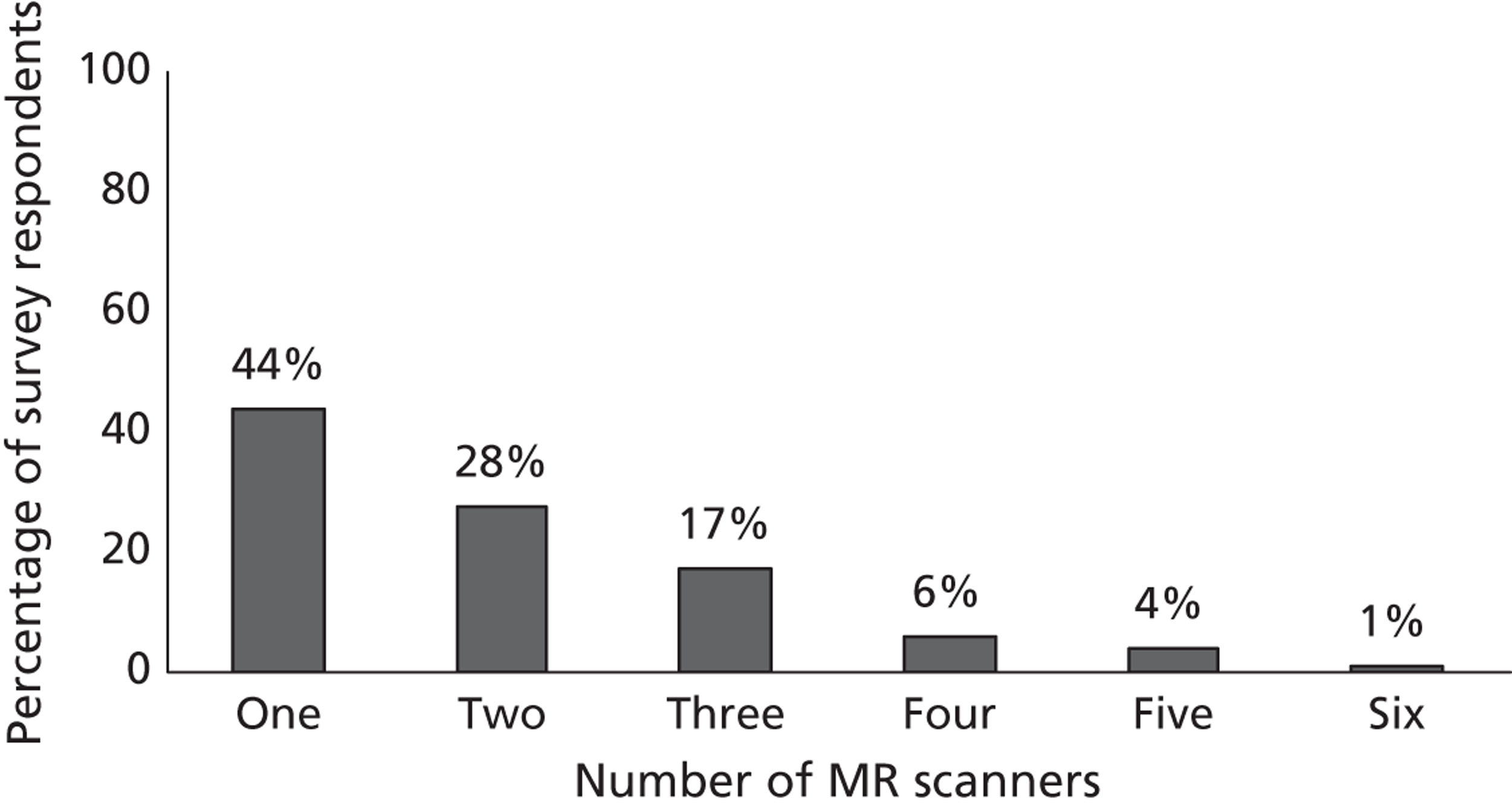
Ninety-four per cent of departments (136 out of 145 respondents who answered this question) indicated that CT was available to scan TIA and minor stroke patients. CT scanning was reported to be available out of hours in 82% of departments. In about 10% of imaging departments, TIA/minor stroke patients were referred to another hospital for CT scanning.
Magnetic resonance imaging facilities were reported to be available in 99% (133/134 respondents who answered this question), including MR diffusion imaging (96%), and were utilised to scan TIA/minor stroke patients in 88% of departments. Most departments routinely scanned patients using MR T1/T2, DWI and FLAIR sequences, but only half of the departments performed gradient echo (GRE) or GRE/T2* sequences (Figure 54). In 9% of imaging departments, TIA/minor stroke patients were referred to another hospital for MR scanning.
FIGURE 54.
Magnetic resonance sequences used to assess patients suspected of TIA/minor stroke in the surveyed radiology departments. Note: 128 out of 146 participants answered this question.
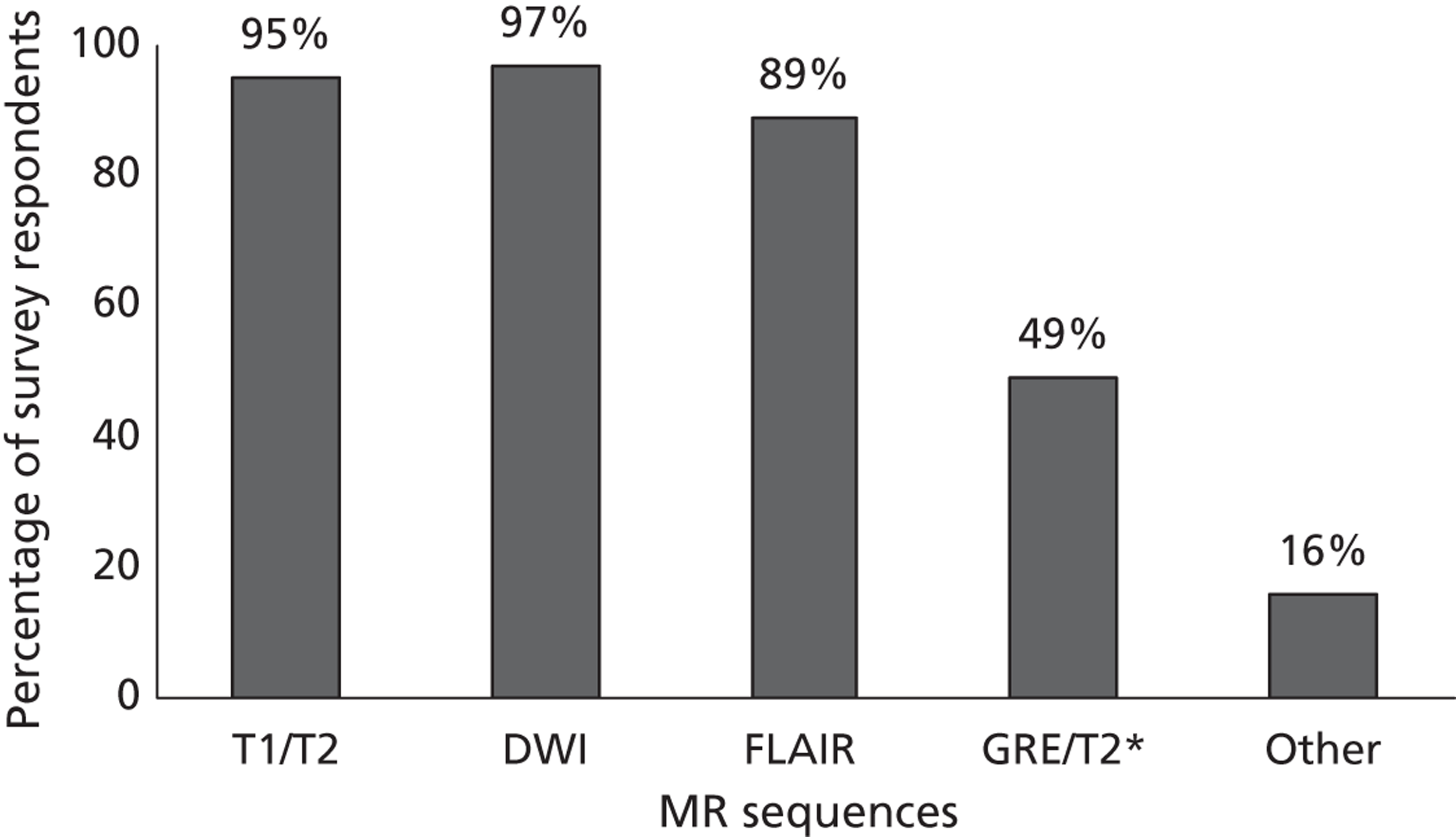
Thirty-nine per cent of surveyed radiology departments (57/146) provided information on which imaging modalities were commonly used to scan patients with TIA/minor stroke. Figure 55 shows that CT was the main modality in the majority of departments (49/57, 86%), followed by DWI/T1/T2/FLAIR sequences in 54% (31/57); DWI/T2/T1/FLAIR/GRE/T2* sequences in 46% (27/57), and DWI/T2 sequences in 18% (10/57).
FIGURE 55.
Imaging modalities commonly used to assess patients suspected of TIA/minor stroke in the surveyed radiology departments. Note: 57 out of 146 participants answered this question.
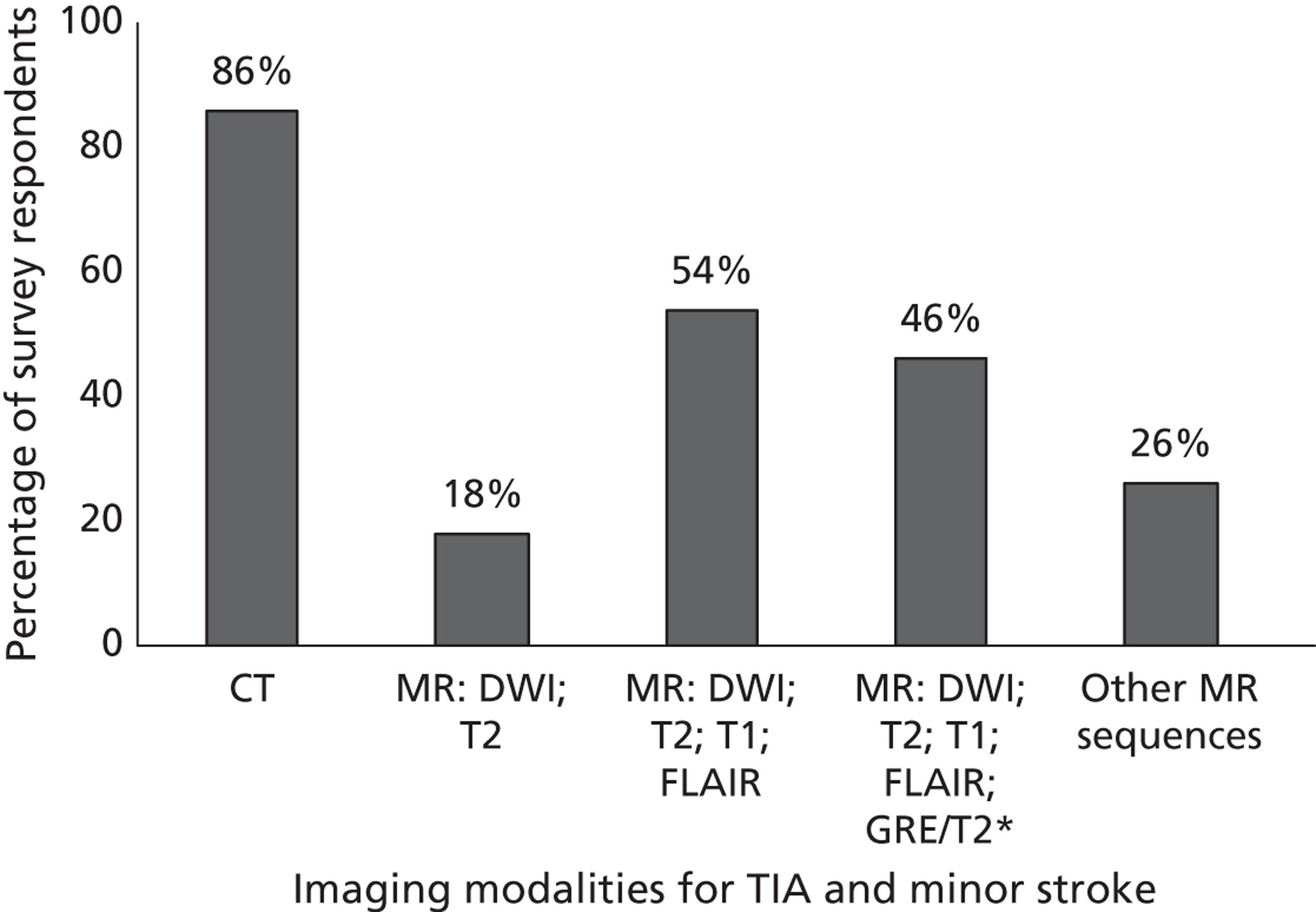
Thirty-eight per cent of departments (55/146) provided information on the proportion of TIA/minor stroke patients who received, at any time, CT or MRI as first-line brain imaging investigation. Most TIA/minor stroke patients were initially scanned with CT rather than MRI in many departments (Figure 56).
FIGURE 56.
Proportion of patients suspected of TIA/minor stroke, presenting at any time, who received CT or MRI as first-line brain imaging investigation.
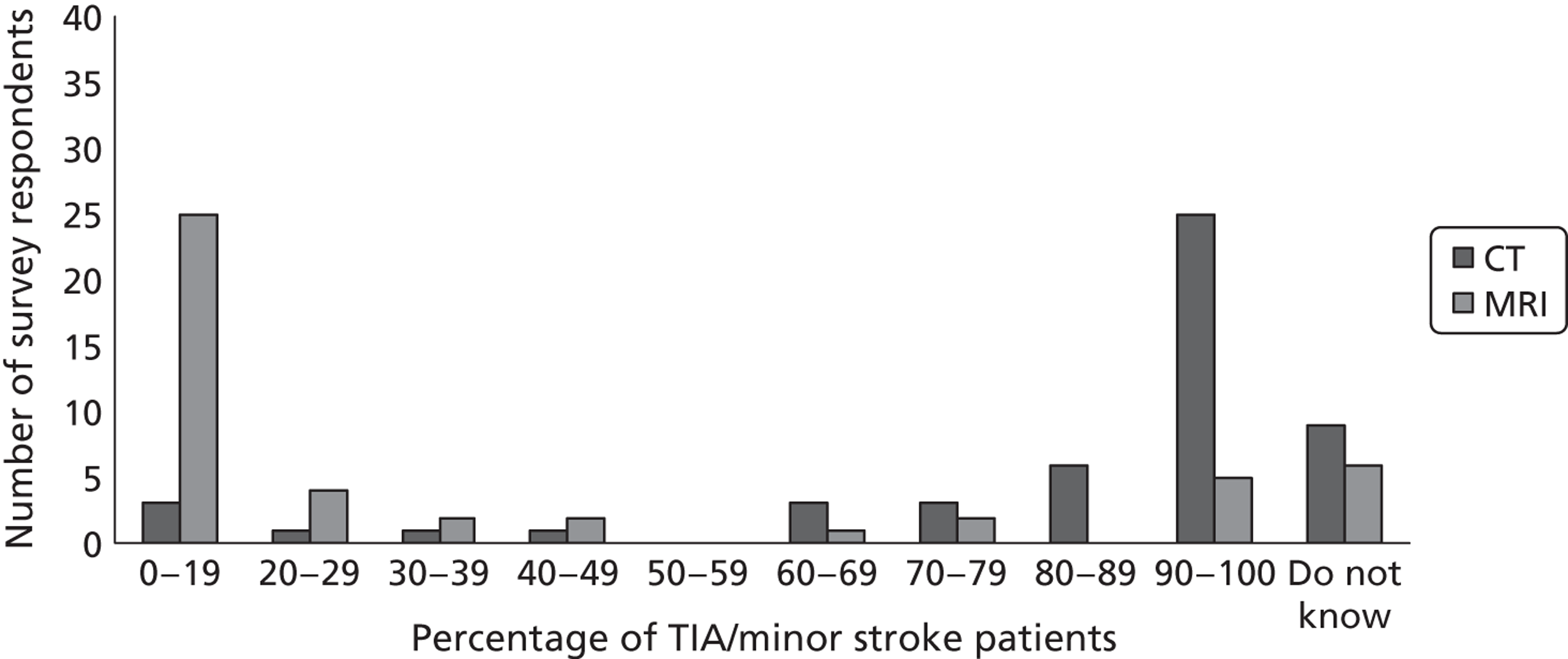
Volume of computed tomography and magnetic resonance brain scanning
Forty-two per cent of surveyed departments provided information on volume of CT brain scanning. During 2010, an average of 4905 CT brain scans were performed per radiology department (range 20–48, 346; median 2500). On average, 18% of brain scans were conducted out of hours. However, this varied greatly across departments, as some departments did not perform any CT brain scanning out of hours and other departments scanned up to 77% of patients (range 0% to 77%). Of the total CT brain scans, on average 686 were conducted on patients suspected of TIA/minor stroke (range 0–5000 scans; median 200 scans). On average, 9% of the TIA/minor stroke CT scans were performed out of hours (range 0–60% scans; median 2% scans).
Similarly, 38% of surveyed departments provided information on volume of MR brain scanning. During 2010, an average of 2888 brain MR images were performed per radiology department (range 20–24, 391 scans; median 1530 scans). On average, only 3% of MR scans were conducted out of hours and most of the departments did not perform any MR brain imaging out of hours (range 0–30%; median 0% scans). Of the total MR brain image scans, on average 258 were conducted on patients suspected of TIA/minor stroke (range 0–1200 scans; median 100 scans). On average, 3% of the TIA/minor stroke MR images were performed out of hours (range 0–50% scans; median 0% scans).
It is worth noting that data related to the number of brain scans were not routinely recorded in many surveyed departments, and therefore the estimates provided by the respondents should be interpreted with caution, as they are just a ballpark figure of the volume of brain scanning for TIA/minor stroke patients in clinical practice.
Access to computed tomography and magnetic resonance brain scanning
About one-third of centres provided information on access to CT scanning. In most radiology departments that provided this information (49/56, 87%), inpatients suspected of TIA/minor stroke presenting on weekdays, during normal working hours, were scanned with CT immediately or the same day and imaging results were fed back immediately or the same day in 22% and 72% of centres, respectively (Figures 57 and 58). Outpatients presenting on weekdays, during normal working hours, were scanned with CT immediately or the same day in 59% of departments that provided this information (29/51), and within 1–2 weeks in 32%. Imaging results were fed back the same day of the scanning (30/55, 54%) in the majority of centres (see Figures 57 and 58). CT scanning for TIA/minor stroke patients outside normal working hours or on weekends was available in 67% of centres that replied to this question (42/63); it could be performed but with difficulty in about 19% of centres (12/63) and was not available in 14% of centres (9/63). The proportion of inpatients suspected of TIA/minor stroke presenting out of hours or on weekends and scanned with CT is shown in Figure 59. The majority of centres indicated that scanning of inpatients presenting out of hours was performed with CT the same day of the patient assessment (48%, 25 out of 52 centres who answered this question) or the next day (33% of centres, 17/52), and results were fed back immediately (47% of centres, 25/43) or the same day (51% of centres, 27/53) (Figure 60). CT scanning for outpatients suspected of TIA/minor stroke presenting out of hours or on weekends was reported to be delayed for up to 2 weeks in some centres (see Figure 58). Outpatient imaging results were given back immediately after scanning or the same day in most centres (23% and 47%, respectively) (see Figure 60).
FIGURE 57.
Time of CT scanning for inpatients and outpatients suspected of TIA/minor stroke presenting on weekdays, during normal working hours.
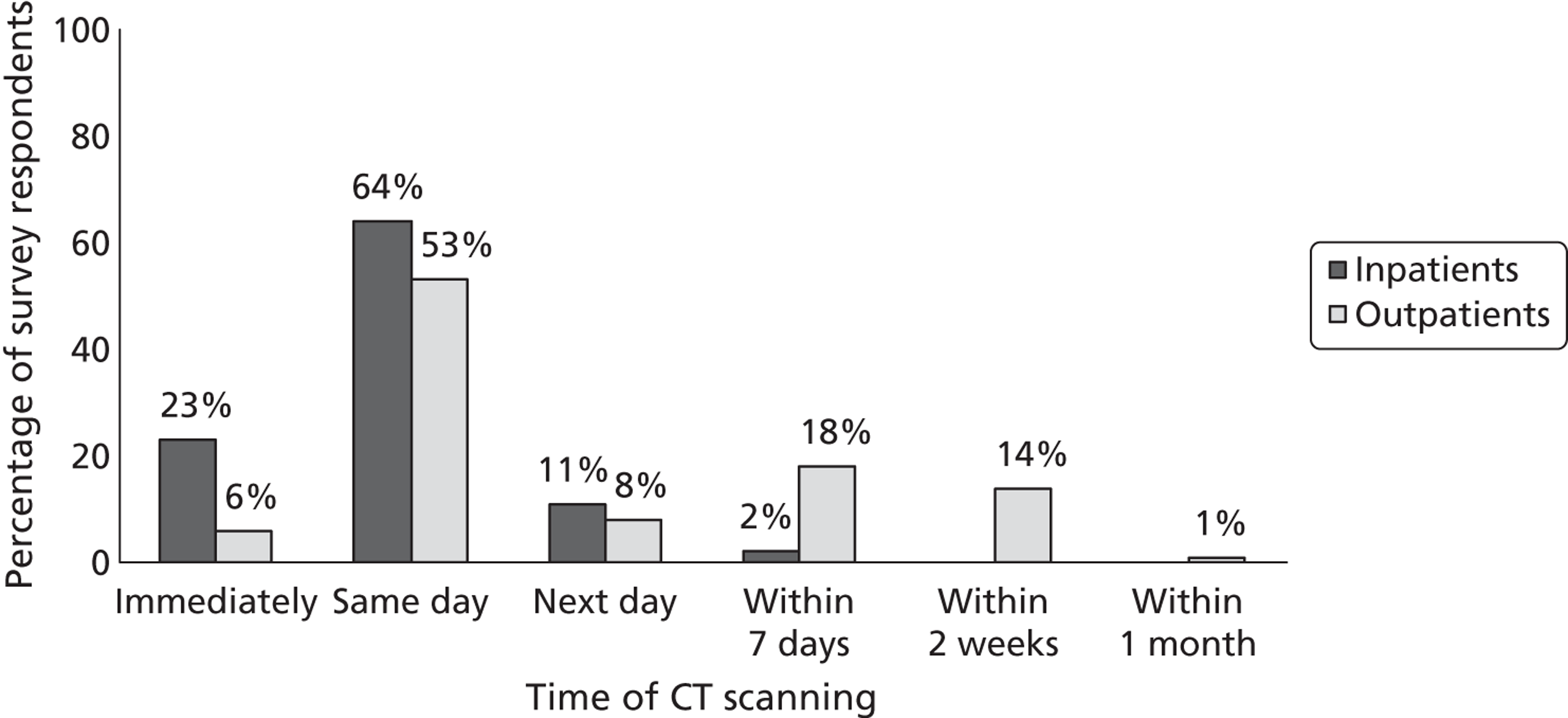
FIGURE 58.
Time for CT results to be received (when inpatients and outpatients are scanned on weekdays, during normal working hours).
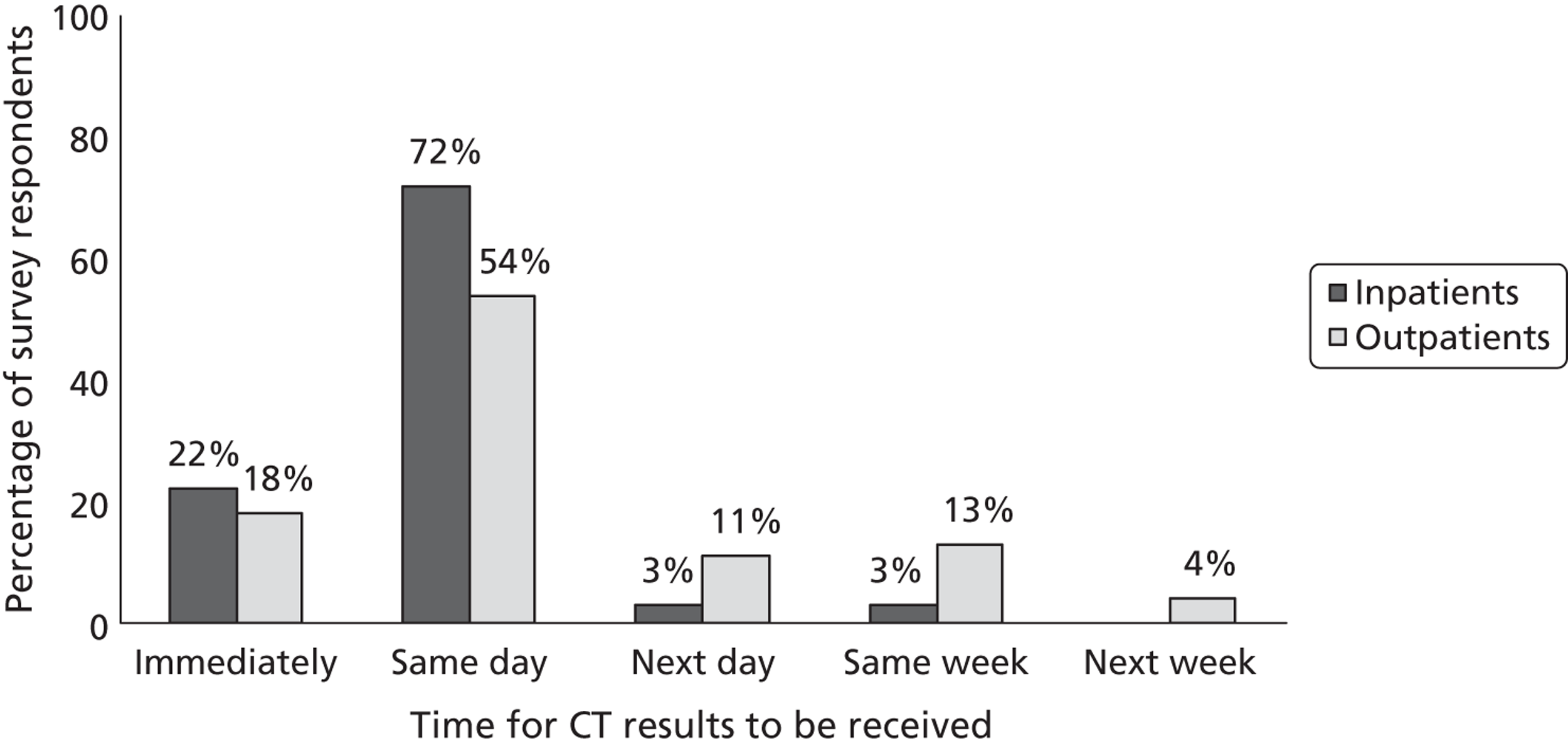
FIGURE 59.
Time of CT scanning for inpatients and outpatients suspected of TIA/minor stroke presenting on weekends or outside normal working hours.
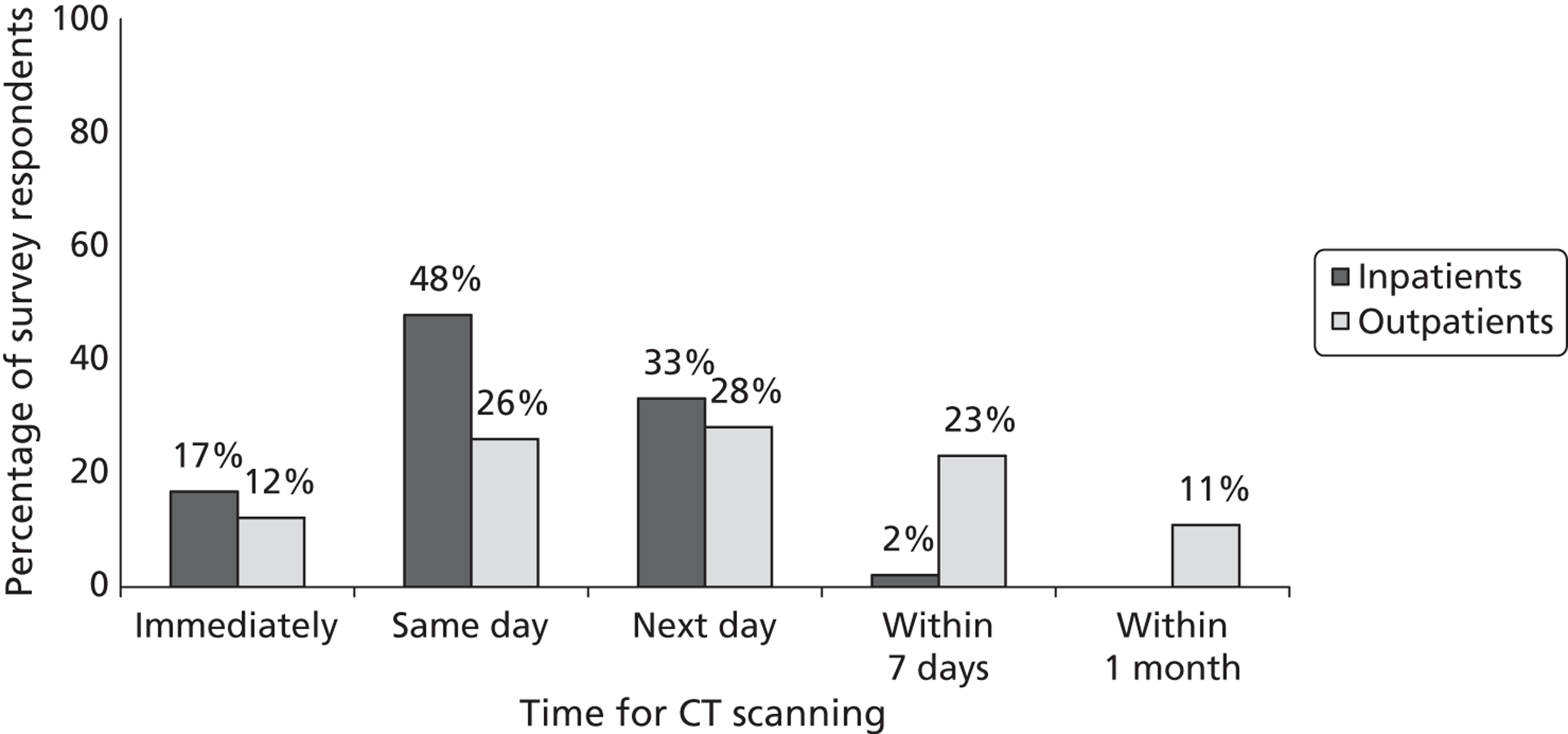
FIGURE 60.
Time for CT results to be received (when inpatients and outpatients are scanned on weekends or out of hours).
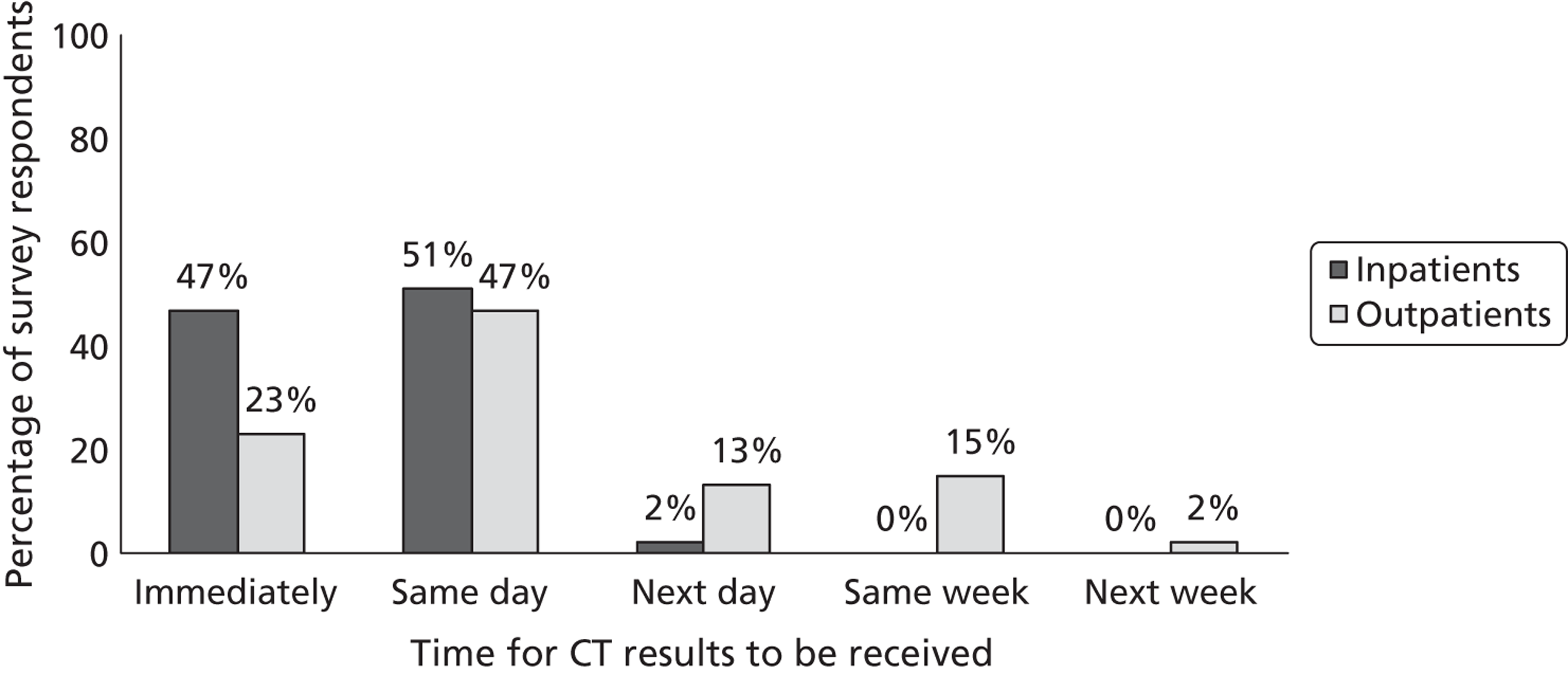
Sixty-one per cent of the centres that provided information on access to MRI (38/62) could provide scanning for TIA/minor stroke on weekdays, during normal working hours, without difficulty, whereas 31% of centres (19/62) could provide MR scanning but with some difficulty, and 8% of centres (5/62) indicated that they could not provide MR scanning. The proportion of both inpatients and outpatients scanned with MRI during weekdays, during normal working hours, is illustrated in Figure 61. For inpatients suspected of TIA/minor stroke presenting on weekdays, during normal working hours, MRI was performed on the same day in 35% of centres (in 19 out of 54 centres for which this information was supplied), the next day in 37% of centres (20/54) or within 7 days in 26% of centres (14/54). For outpatients suspected of TIA/minor stroke presenting on weekdays, during normal working hours, MRI was performed on the same day in 21% of centres (10 out of 47 centres who answered this question), the next day in 11% of centres (5/47), within 7 days in 30% of centres (14/47), within 2 weeks in 26% of centres (12/47) and within 1 month in 10% of centres (5/47). For inpatients, MR results were given back the same day of scanning in 83% of centres, whereas for outpatients it could take up to 7 days (27% of centres) to get results back (Figure 62). In the majority of centres, MR scanning was not available for TIA/minor stroke patients out of hours or on weekends (34/58, 59% of centres); could be performed with difficulty in about 24% of centres (14/58), and could be performed without any difficulty in only 17% of centres (10/58) (Figure 63). For inpatients suspected of TIA/minor stroke presenting on weekends or out of hours, MRI was performed on the same day in 14% of centres (6/42), the next day in 50% of centres (21/42), or within 7 days in 29% of centres (12/42). Outpatients suspected of TIA/minor stroke presenting on weekends or out of hours were scanned with MRI on the same day of clinical assessment in 9% of centres (3/35); the next day in 23% of centres (8/35); within 7 days in 40% of centres (14/35); within 2 weeks in 14% of centres (5/35) and within 1 month in the remaining centres (5/35, 14%; Figure 64). In 77% of centres (31/40), inpatient imaging results were returned the same day, whereas outpatient imaging results were given back on the same day in 43% of centres (15/35), returned within the same week in 43% centres (15/35), and delayed until the next week in 8% of centres (3/35) (Figure 65).
FIGURE 61.
Time of MR scanning for inpatients and outpatients suspected of TIA/minor stroke presenting on weekdays, during normal working hours.
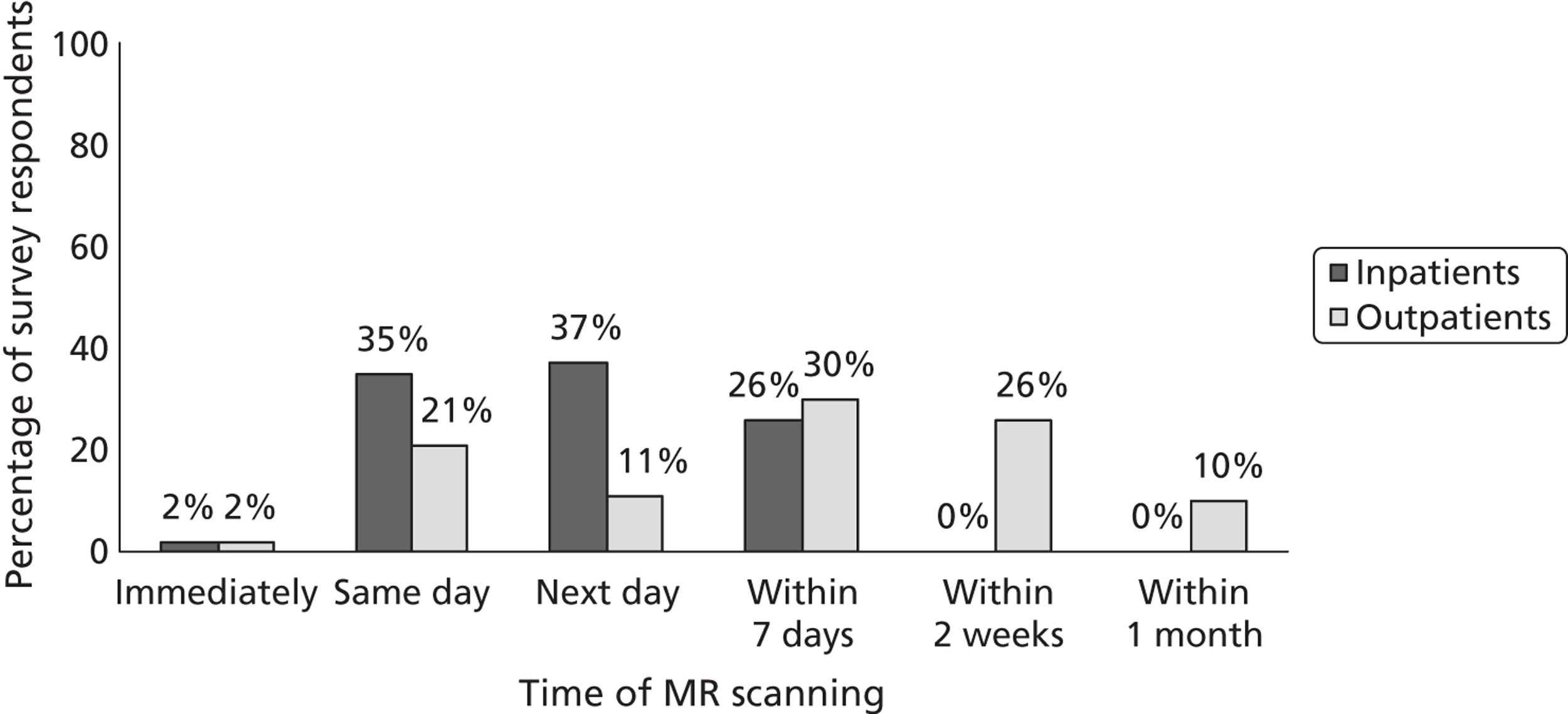
FIGURE 62.
Time for MR results to be received (when inpatients and outpatients are scanned on weekdays, during normal working hours).
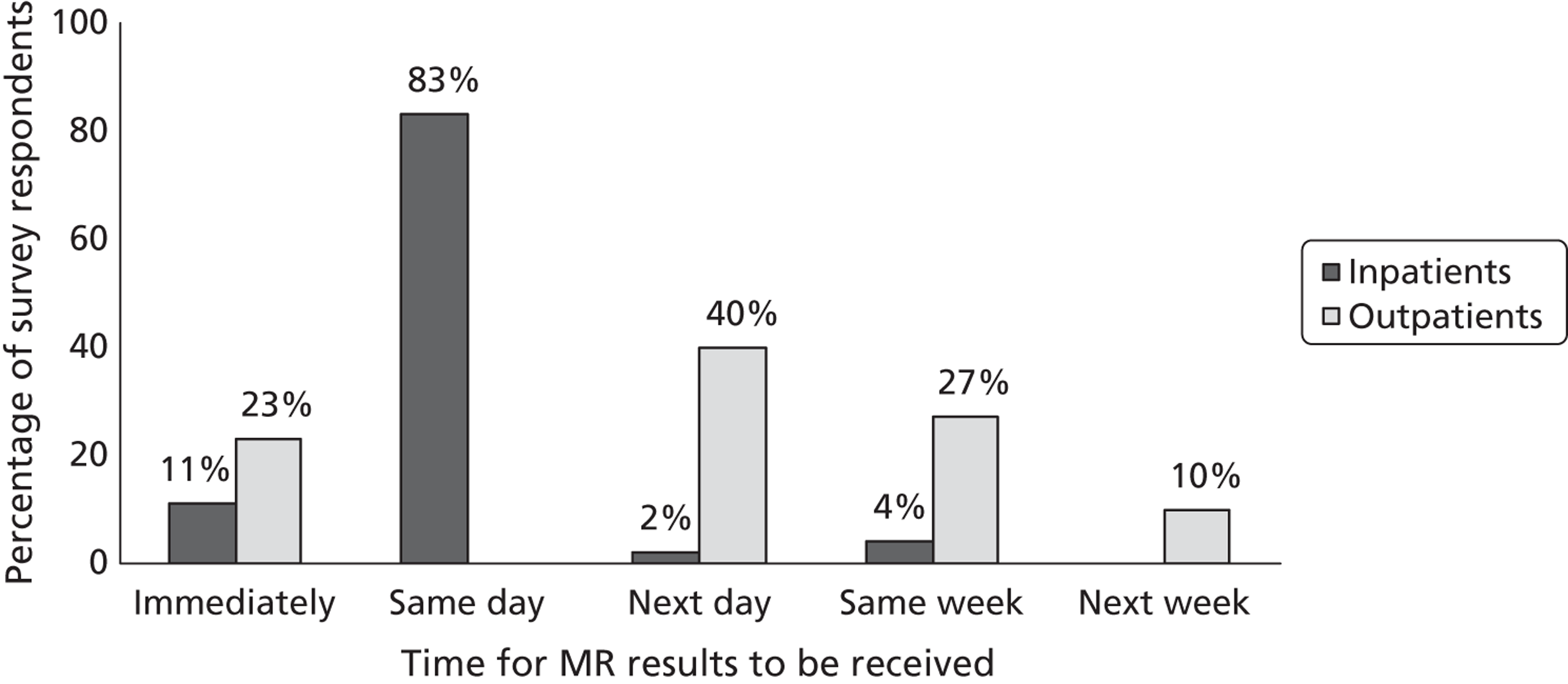
FIGURE 63.
Magnetic resonance scanning availability for patients suspected of TIA/minor stroke presenting on weekends or outside normal working hours. Note: 58 out of 146 participants answered this question.
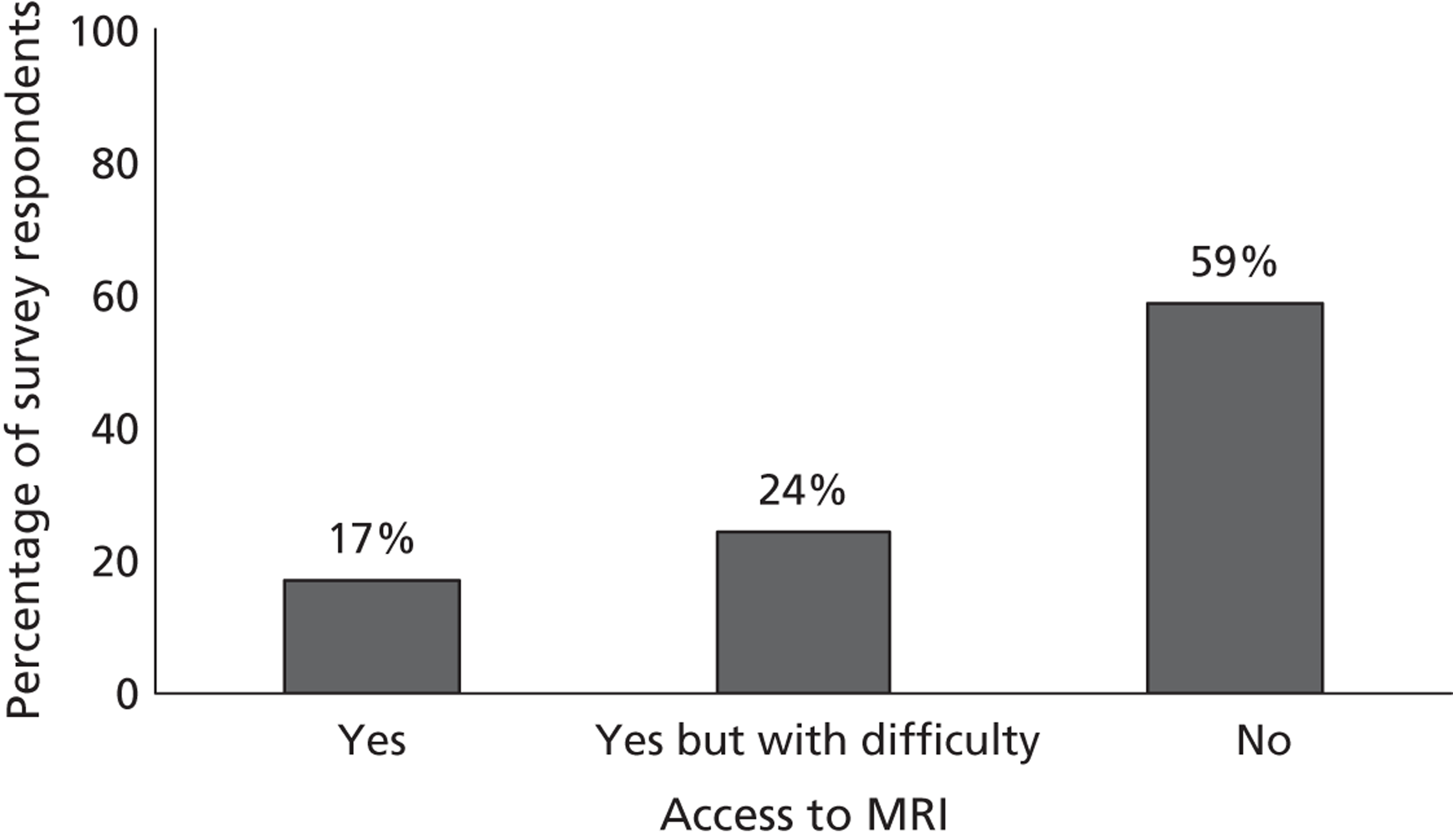
FIGURE 64.
Time of MR scanning for inpatients and outpatients suspected of TIA/minor stroke presenting on weekends or outside normal working hours.
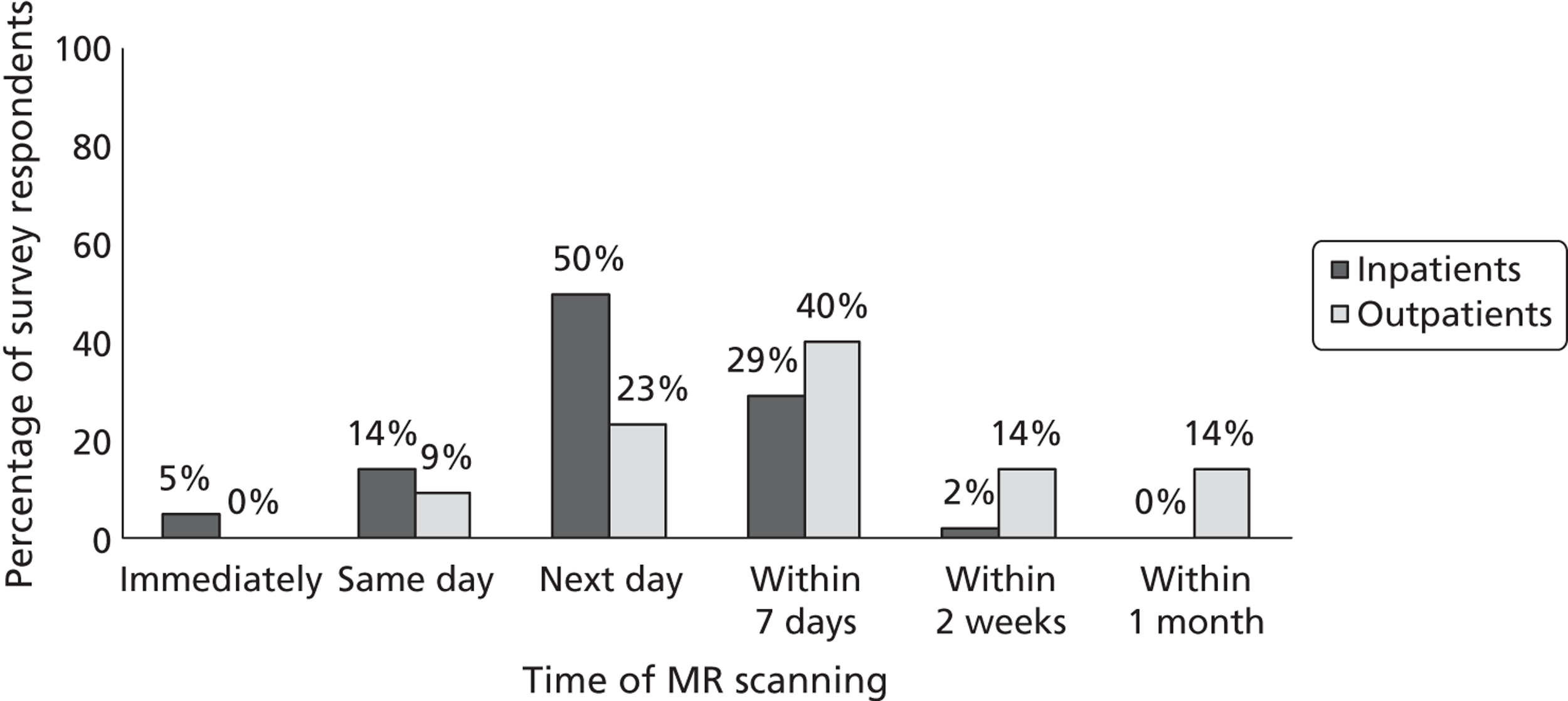
FIGURE 65.
Time for MR results to be received (when inpatients and outpatients are scanned on weekends or out of hours).
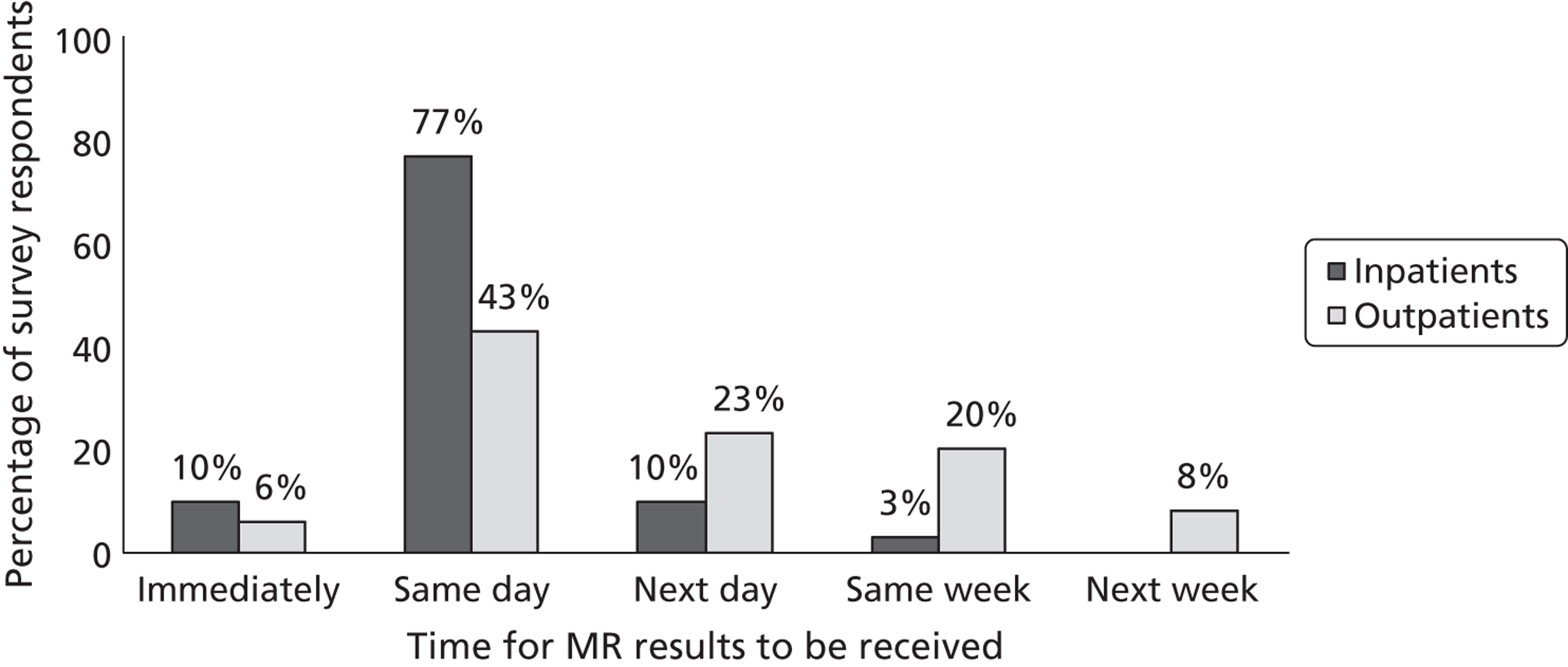
Discussion
The surveys were targeted to clinical leads of stroke prevention services and radiology departments in the UK. Response rate for both surveys was 45% which we considered overall satisfactory for surveys of clinical services.
The findings of the surveys indicate that prevention services for TIA/minor stroke are widely available in the UK, but their structure and organisation vary considerably across centres. In the majority of centres (78%) specialist stroke prevention services operate every day and on average 1–5 new patients attending the services each time. Even although the majority of specialist stroke services are run by consultants, nurses or nurse specialists undertake the main medical assessment or triaging of TIA/minor stroke patients in a considerable number of centres (28%). Approximately, 40–60% of patients attending the stroke services are ultimately diagnosed as having TIA or minor stroke. This means that approximately half of the presenting patients are non-TIA/minor stroke cases, consistent with the literature review in Chapter 7, with important implications in terms of workload and use of resources. CT and MRI facilities are available in the majority of centres for TIA and minor stroke patients. CT is the most common imaging investigation for inpatients and outpatients suspected of TIA/minor stroke. The consistency between the clinical services’ and imaging departments’ responses to the question about first-line imaging method was striking (see Figures 40 and 56, respectively). In most centres CT is performed immediately or the same day for patients presenting on weekdays during normal working hours. Access to CT during weekends or out of hours is available in many centres (approximately 70% of centres). In contast, MRI is available in many centres from 9 am to 5 pm on weekdays, but delays of many days or even weeks are usual, especially for outpatients. MR facilities for TIA/minor stroke during weekends or out of hours are available, without difficulty, in only a limited number of centres (17%). In approximately half of the centres, the important and only evidence-based MR sequence to diagnose or exclude haemorrhage (i.e. GRE/T2*) is omitted, reducing the potential benefit of MRI in stroke prevention. The surveys also confirmed the common use of DUS as the first-line carotid imaging for TIA patients (95%) rather than MRA or CTA. In just over half of the stroke services (57%) endarterectomy was performed within 1 week of the decision to operate and in 37% of centres within 1 month. No stroke service performed endarterectomy at > 6 months after the decision to operate.
Both questionnaires provided useful data to populate the cost-effectiveness model, especially with regard to the type and timing of clinical assessments, and imaging investigations and volume of non-CV diagnosis patients. Timing of imaging is a crucial factor in the model and the questionnaires provided up-to date information on the range of times taken to scan TIA/minor stroke patients in different UK stroke prevention services.
Although both internet surveys provided invaluable data for the economic model, we need to acknowledge some limitations. Some data were not routinely collected in all centres involved in the surveys, and respondents provided rough estimates to some of the survey questions. Furthermore, not all respondents answered all questions (especially for the survey of imaging services), limiting the generalisability of the surveys findings.
There are no other current data on activity in stroke prevention clinics or imaging departments in the UK or relevant data on service delivery in other countries. It is difficult to know what is happening across the UK as a whole or whether the non-responders to the present survey are undertaking more or less MR in TIA/minor stroke, seeing patients faster or slower, or making greater use of MRA or CTA for carotid imaging. The key points are that:
-
Nurses perform a primary role in patient diagnosis and assessment in nearly one-third of clinics.
-
In total, 34% of patients are seen the day after referral and 26% at between 2 and 3 days (not after TIA – these data are not available). In total 60% of patients are seen within 1 week of referral.
-
In total, 50% of patients attending these clinics do not have TIA/minor stroke but a mimic.
-
About 75% of patients have been started on secondary prevention treatment by their GP before attending the clinic.
-
CT is widely available and is the first-line test in the vast majority of centres, and is routinely used in 81% of centres.
-
MR is used in 51% of centres but in about half of those it is after an initial CT scan and there are greater delays to MRI.
-
The stroke clinical and imaging services provided remarkably consistent responses regarding the proportion of patients having CT or MR as the primary brain imaging investigation.
-
Imaging departments have more CT scanners than MR scanners.
-
The key MR sequence needed to diagnose haemorrhage is omitted in more than half (54%) of centres, making the diagnosis of haemorrhagic stroke unreliable with MRI.
-
Ultrasound is the primary carotid imaging test in 95% of centres.
-
60% of carotid endarterectomies are being performed within a week of taking the decision to operate
-
There is limited capacity to undertake more imaging, although about 40% of stroke services indicated that they had capacity to see more patients; however, the main barrier to seeing more patients was the limited availability of MRI.
The current Tariff for England and Wales98,99 incentivises services to deliver stroke prevention more rapidly by providing increased payment for patients who are correctly diagnosed, assessed and treated quickly through Integrated Performance Measures. A base tariff (£450) applies to patients who are treated in a dedicated stroke prevention clinic meeting minimum best practice criteria. Additional tariffs are paid for high-risk patients who are assessed and treated within 24 hours (£92) and for use of MRI (£92) in any suspected TIA patients. It uses the ABCD2 score to define high-risk patients (score ≥ 4) and the score is completed by the healthcare professional referring the patient – in most cases this would be the GP. In none of the studies in the literature as reviewed in Chapter 4 was the ABCD2 score assigned by GPs or nurses. There is little published information on agreement between ABCD2 scores assigned by GPs (or nurses) and by stroke specialists, but there are many examples of the large number of patients with a TIA/minor stroke mimic who are referred to stroke prevention clinics87,183 – in one population-based study set in north-eastern Italy, of 1150 patients referred by their GPs or presenting to hospital with suspected TIA, only 175 were confirmed as definite TIA by the stroke specialists (10%). 200 The investigation of high-risk patients, to be completed within 24 hours, includes brain imaging, preferably with DWI MRI, and carotid imaging. MR DWI is also encouraged in all other patients. Thus, a total of £634 could be paid for a high-risk patient (ABCD2 score of ≥ 4) investigated with MR DWI and started on treatment within 24 hours of first contact with health services.
Weaknesses of the clinical surveys
Even although we consider satisfactory a response rate of 45% for both surveys, we cannot be sure to have captured the full variance of current clinical practice. We were not able to compare characteristics of responders with those of non-responders, as only a very limited number of responders provided details and demographic characteristics of their hospitals/centres. Therefore, our findings may not be applicable to the entire stroke prevention community. However, considering the high consistency between the findings of the stroke services survey and those of the imaging services survey, it is likely that we have been successful in highlighting the most important clinical issues related to secondary stroke prevention and use of current stroke services.
Chapter 9 Systematic review of previous cost-effectiveness analyses of imaging in stroke treatment and prevention
Introduction
Stroke is a major illness in the UK and uses substantial amounts of health-care resources. About 150,000 people have a stroke in the UK each year, with about 30% dying within 6 months. A further 30% survive but become dependent on others for everyday activities. Treatment options for stroke are limited, so prevention is vital, particularly among those who have early warning TIA or minor non-disabling stroke, early assessment and management of whom can help to avoid future, more disabling strokes, thereby providing health gains to patients, cost savings to the health-care system and economic gains to society. The aim of this chapter is to review previous studies of the cost-effectiveness of MR including DWI compared with the current alternative of CT.
The specific objectives are to:
-
determine, by undertaking a systematic review of the literature, current knowledge regarding the cost-effectiveness of using MRI compared with CT, to identify and manage TIA and minor stroke
-
use this knowledge to inform development of an economic model to compare the cost-effectiveness of using MRI relative to CT for the purpose of identifying and managing cases of TIA and minor stroke.
Methods
Objectives
We assessed the literature that has compared the cost-effectiveness of CT and/or MRI strategies. We focused on UK studies and those from other countries in Europe; costs information from other countries is likely to be less relevant for NHS decision-making. The review also gathers information on costs of treatment for stroke patients in different health states. The review acts as an evidence base for the development of parameters for economic modelling.
Identification of studies
A comprehensive search was undertaken to identify studies that assessed the cost-effectiveness of CT and/or MRI in the management of stroke after TIA or minor stroke. The search strategy is in Appendix 1. For costs data, studies were relevant if they reported the costs of CT and/or MRI, or the costs of treatment averted (i.e. health-care resource use costs associated with management of TIA and minor stroke, as well as management of future stroke events after TIA or minor stroke, associated with provision of either imaging technique). For cost-effectiveness, studies were relevant if the costs and outcomes of two or more diagnostic strategies were compared. The databases that were searched were MEDLINE, MEDLINE in Process and Other Non-Indexed Citations, EMBASE Science Citation Index, NHS Economic Evaluations Database, the HTA database. Only studies that were published in English were considered. Also, only studies published in the year 2000 and beyond were included, as reviews of earlier studies published prior to 2000 were identified and appraised in previous HTA programme-commissioned research. 5,26 However, to inform the model development, the reference lists of all identified studies were checked for additional potentially relevant studies if it appeared from the title that they provided information on costs of imaging, costs of stroke care or health state outcomes/health state utilities. These include cost analyses or cost-effectiveness of different strategies for treating or managing stroke. The full texts of all potentially relevant reports were obtained and assessed in terms of their relevance to the stated objectives outlined earlier in the chapter.
Inclusion/exclusion criteria
In selecting studies for the cost-effectiveness review, studies had to be a cost-effectiveness analysis or a cost–utility analysis of alternative imaging techniques, specifically MRI and/or CT, to identify and manage TIA, minor stroke or stroke. Non-English language studies were excluded. Only studies published in the year 2000 and beyond were included.
Quality assessment and data extraction
Information and relevant data were extracted by an economist according to the guidelines produced by the Centre for Reviews and Dissemination for the critical appraisal of economic evaluations. 281 Where an economic evaluation had been based on a modelling exercise, additional data extraction criteria developed by Philips and colleagues282 and NICE283 were applied. A data extraction form was applied to the studies that met the inclusion criteria.
Results
Out of a total of 12 full cost-effectiveness studies that were identified as potentially relevant, four studies were deemed suitable for inclusion in the review: Wardlaw and colleagues,5,26 Buskens and colleagues,284 and Tholen and colleagues. 285 Among the eight not considered relevant, one study by the Centre for Evidence-based Purchasing published in 2008105 concluded that there was no information on which to base a cost-effectiveness assessment of DWI in TIA/minor stroke, and was not considered further. Four other studies,286–289 although relevant to stroke prevention in the UK, focused on organisation of stroke prevention clinics in general including factors such as whether TIA patients should be admitted to hospital or managed as outpatients,289 but did not address the cost-effectiveness of any contribution of imaging specifically, and one of them was > 10 years old. The three potentially relevant studies on imaging in stroke that were excluded (and the reasons) were (1) a study of cost-effectiveness of MR perfusion imaging for patient assessment prior to thrombolytic therapy for acute ischaemic stroke treatment290 (not relevant to stroke prevention); (2) a study of the cost-effectiveness of multimodal CT (i.e. plain CT, CTA, CT perfusion) for patient assessment prior to thrombolytic therapy for acute stroke treatment291 (not relevant to stroke prevention); and (3) a study of carotid imaging strategies in patients with carotid stenosis prior to carotid endarterectomy for stroke prevention292 (only considered patients with carotid stenosis).
All four included studies were model-based studies. The study by Wardlaw and colleagues26 determined the cost-effectiveness of different CT scan strategies for patients with suspected ischaemic or haemorrhagic stroke or stroke mimics. Using a decision-tree model, the aim was to compare standard care, defined as CT scanning for all within 48 hours of admission to hospital, with 12 alternative strategies:
-
Scan all patients immediately upon admission.
-
Scan patients on anticoagulants or in life-threatening conditions immediately and scan all remaining patients within 24 hours of admissions to hospital.
-
Scan patients on anticoagulants or in life-threatening conditions immediately and scan all remaining patients within 48 hours of admissions to hospital.
-
Scan patients on anticoagulants or in life-threatening conditions immediately and scan all remaining patients within 7 days of admissions to hospital.
-
Scan patients on anticoagulants or in life-threatening conditions immediately and scan all remaining patients within 14 days of admissions to hospital.
-
Scan patients on anticoagulants, those with life-threatening conditions or are candidates for hyperacute treatment immediately, and scan all remaining patients within 24 hours of admission to hospital.
-
Scan patients on anticoagulants, those with life-threatening conditions or are candidates for hyperacute treatment immediately, and scan all remaining patients within 48 hours of admission to hospital.
-
Scan patients on anticoagulants, those with life-threatening conditions or are candidates for hyperacute treatment immediately, and scan all remaining patients within 7 days of admission to hospital.
-
Scan patients on anticoagulants, those with life-threatening conditions or are candidates for hyperacute treatment immediately, and scan all remaining patients within 14 days of admission to hospital.
-
Scan only patients in arterial fibrillation, on anticoagulants or an antiplatelet drugs within 7 days of admission to hospital.
-
Scan only patients with life-threatening stroke or on anticoagulants within 7 days of admission to hospital.
-
Do not scan.
The study by Buskens and colleagues284 examined the cost-effectiveness of non-invasive strategies in patients with TIA or minor stroke suspected of having carotid artery stenosis (CAS). The non-invasive strategies in this study included DUS, MRA and the two examinations combined; all were compared against intra-arterial digital subtraction angiography (DSA), which was the reference standard. Both Buskens and colleagues284 and Tholen and colleagues285 used the ECST trial cohort to develop the model-based analysis. However, studies selected different cohorts for their analysis. Buskens and colleagues284 included only male patients, recruited during 1997 through 2000, and the average age was 55 years. Their economic evaluation was reported in 1998 US dollars.
The second study by Wardlaw and colleagues5 examined the cost-effectiveness of replacing a widely used method of diagnosis of carotid disease, IAA, with less invasive methods among patients with symptomatic carotid disease for patients who had suffered a TIA or minor stroke. IAA was compared against ultrasound, CTA, MRA and contrast-enhanced magnetic resonance angiography (CEMRA). The cost-effectiveness of a baseline strategy for which IAA was performed on all patients with an ultrasound reading of between 49% and 99% stenosis was compared with 21 alternative strategies, where test methods were varied and surgery was offered for different levels of stenosis. Expected costs and benefits were modelled over a 20-year time horizon for a cohort of 100 patients with TIA or minor stroke.
The study by Tholen and colleagues285 considered the cost-effectiveness of non-invasive diagnostic imaging strategies in patients with a TIA or minor stroke suspected of having carotid stenosis. The strategies that were compared included DUS, CTA, CEMRA and a combination of these strategies. In this study the main emphasis was on the time window between the first ischaemic symptoms and carotid endarterectomy, for different cut-off values as indications for surgery (i.e. 70–99% vs. 50–99% stenosis). Tholen and colleagues285 used data from 351 patients (both male and female) with TIA or minor stroke admitted between January 2002 and January 2005 to the Erasmus University Medical Centre, Rotterdam, The Netherlands. The age range of the population was 19–90 years, with an average age of 62 years. The economic evaluation was reported in 2007 euros. The alternatives that were compared with the base case included the following:
-
CTA for 70–99% stenosis
-
DUS and CTA for 50–99% stenosis
-
DUS for 50–90% stenosis and CTA for 70–90%
-
CTA for 50%–90% stenosis
-
DUS and CEMRA for 70–99%
-
DUS and CEMRA for 50–99%
-
DUS for 50–99% and CEMRA for 70–99%
-
CEMRA for 50–99% stenosis
-
CEMRA for 70–99% stenosis
-
DUS for 70–99% stenosis
-
DUS for 50–99% stenosis.
Table 30 summarises the economic evaluation of the strategies compared in the studies included in the review.
| Study | Country; trial; currency, price year | Perspectives (health services, third-party payers, societal perspective) | Patients | Comparisons | Age (years) | Outcomes | Modelling: type of model used | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TIA or minor stroke | Stenosis | Stroke | CT or CT immediate/delay | CTA | MRI | MRA | CEMRA | Ultrasound/DUS | IAA | Men and women | Survival/death | No. stroke | No. MI averted | Life-years gain | QALY | Cost-effectiveness ratio | ICER | Net benefit | Decision model | Markov | EVPI | Time period | ||||
| Years | Lifetime | |||||||||||||||||||||||||
| Wardlaw and colleagues, 20065 | UK; not trial based; £2004 | NHS UK | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 55+ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 20 | ||||||
| Wardlaw and colleagues, 200426 | UK; not trial based; £1999/00 prices | NHS UK | ✓ | ✓ | 45–89 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 5 | ||||||||||||||
| Tholen and colleagues, 2010285 | Netherlands; prospective cohort; €2007 | Netherlands teaching hospitals | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 60+ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 1 | |||||||||
| Buskens and colleagues, 2004284 | Netherlands, part of NASCET trial cohort, €1998 | Netherlands hospital perspective and societal perspective | ✓ | ✓ | ✓ | ✓ | 55+ | ✓ | ✓ | ✓ | ||||||||||||||||
Buskens and colleagues284 costed the imaging procedures using expenditures incurred for personnel, equipment, materials, maintenance, housing, cleaning, administration and overheads at one university hospital (University Medical Centre, Utrecht, The Netherlands) and one general hospital (Enschede Medical Centre, Enschede, The Netherlands). Estimates of costs associated with hospital stays and longer-term costs were obtained from individual patient-based data from previously published studies undertaken in the Netherlands. No information was reported on how health state utilities were calculated.
Tholen and colleagues285 costed the imaging procedures in a similar fashion to Buskens and colleagues,284 although figures were taken from a different local hospital (Erasmus Medical Centre, Rotterdam, The Netherlands). Estimates of costs associated with hospital stays and longer-term costs were obtained from Buskens and colleagues. 284 Disutilities for quality of life after TIA (0.0017 decrement), or after a minor stroke (0.0524 decrement), were estimated from Buskens and colleagues284 and Sullivan and colleagues. 293 A utility of 0.31 was estimated for major stroke, taken from Wardlaw and colleagues. 5
Data source on costs and outcomes of the economic evaluation studies reviewed
Further information relating to the data sources used in the above studies is shown in Tables 31 and 32. Wardlaw and colleagues26 conducted a detailed costing exercise based on three hospitals in Scotland and calculated the direct costs of equipment (CT scanner, laser printer and maintenance) in three different categories of hospitals (teaching, rural, and urban, during normal and out of hours) using a ‘bottom-up’ microcosting approach. These estimates were also used by Wardlaw and colleagues. 5 Further, Wardlaw and colleagues26 estimated the cost of treatment for stroke based on length of stay (LOS) data obtained from general acute inpatient and day-case discharge data sets from Information and Statistics Division (ISD), Scotland. These data were used to derive the mean duration of stay by type and severity of stroke. The data suggest that there are significant differences in LOS between patients who are alive and independent at 6 months and those who are dependent at 6 months. These data and the cost per in-patient bed-day were combined to derive average total acute care cost estimates. Similar approaches were undertaken in Wardlaw and colleagues. 5 Both studies by Wardlaw and colleagues5,26 used the EuroQol five-dimension (EQ-5D) instrument to derive quality-of-life weights, with weights taken from the study by Dorman and colleagues,112 who conducted a trial that included EQ-5D data from 867 UK patients.
| Study | Unit costs/cost of imaging | Cost of treatment | Effectiveness/outcomes | |||
|---|---|---|---|---|---|---|
| CT/CTA | MRI/MRA/CEMRA | Ultrasound/DUS/DSA | IAA | |||
| Wardlaw and colleagues, 20065 | CT: Wardlaw and colleagues, 200426 CTA: authors collected data from Edinburgh hospital – 2004 |
MRA, King-Im and colleagues, 2003;292 MRI, assumed to be same as MRA; CEMRA, King-Im and colleagues, 2003292 | Ultrasound: Benade and Warlow, 200286 | King-Im and colleagues, 2003292 | Cost of outpatient attendances – Benade and Warlow, 200286 (1997/98 price inflated) Cost of carotid endarterectomy – Berry and colleagues, 2002294 Cost of treatment, cost of stroke and for patient in different health states – Wardlaw and colleagues, 200426 |
QALYs calculated from EQ-5D responses published in Dorman and colleagues, 2000112 |
| Wardlaw and colleagues, 200426 | CT: detailed Micro-costing at different hospitals at different hours | Cost of treatment included LOS and cost per inpatient day. The LOS stay for each subtype of stroke (PICH) and cerebral infarction were obtained from ISD data of the NHS, Scotland Cost per inpatient day: NHS Scottish Health Services costs |
Quality-of-life weight associated with ‘independent survival after stroke’ and ‘dependent after stroke’, taken from UK data from the IST: based on EQ-5D responses, and published in Dorman and colleagues, 2000112 | |||
| Tholen and colleagues, 2010285 | Costs of CTA study by Buskens and colleagues284 and cost-analysis valuing resources, inflated at 2007 prices | Cost of MRA ($256) from Buskens and colleagues284 inflated at 2007 prices | Cost of DUS (US$53 (US$27–107) and DSA (US$1169) from Buskens and colleagues284 and cost-analysis valuing resources used, inflated at 2007 prices | Cost of minor and major stroke first year and subsequent year (Buskens and colleagues284) Cost of productivity loss using friction cost methods from Dutch Manual for costing in economic evaluation |
Disutility after TIA (Buskens and colleagues284). Quality-of-life weight after TIA taken from Wardlaw and colleagues, 2006;5 Sullivan and colleagues, 2006;293 and Hallan and colleagues, 1999.295 Utility value for quality of life after minor stroke, and after major stroke, was taken from Sullivan and colleagues, 2006,293 and Hallan and colleagues, 1999295 | |
| Buskens and colleagues, 2004284 | Cost at university and general hospital | Actual costs of treatment were from review of literature in Dutch context | Utility weights for the health states of well (1.0), minor stroke (0.8), and major stroke (0.2) and death obtained from Samsa and colleagues, 1998296 and Matchar and Pauker, 1987297 | |||
| Study | Comparators | Mean cost of strategies | QALYs | ICER | Net benefit for WTP1 | Net benefit for WTP2 |
|---|---|---|---|---|---|---|
| Wardlaw and colleagues, 20065 | Base case: ultrasound first, and IAA for 50–99% stenosis, and carotid endarterectomy for IAA reading of 70–99% stenosis | – | ||||
| Ultrasound, surgery for 50–99% | £1,335,994 (14 days to carotid endarterectomy) | 570.92 | NR | £86,977 for WTP of £30,000 per QALY | ||
| Wardlaw and colleagues, 200426 | Base case: scan all within 48 hours of admission | £10,279,728 for 1000 population | 1982.3 | – | ||
| Scan all immediately | £9,993,676 | 1982.4 | Superior to base case | |||
| Tholen and colleagues, 2010285 | Base case: DUS and CT for 70–99% stenosis (men) | €39,826 | 14.383 | _ | 13.587 for WTP of €50,000 per QALY | 13.885 for WTP of €80,000 per QALY |
| CTA for 70–99% (men) | €39,893 | 14.384 | €71,419 | 13.586 for WTP of €50,000 per QALY | 13.885 for WTP of €80,000 per QALY | |
| Base case: DUS and CT for 70–99% stenosis (women) | €45,911 | 16.460 | – | 15.542 for WTP of €50,000 per QALY | 15.887 for WTP of €80,000 per QALY | |
| DUS and CT angiography for 50–99% stenosis (women) | €46,546 | 16.456 | Dominated by base | 15.535 | ||
| Buskens and colleagues,284 2004 | Base case, DSA | US$33,500 | 11.23 | Dominated by DUS | ||
| DUS | US$20,400 | 11.30 | – | |||
| DUS and MRA | US$30,525 | 11.30 | Dominated by DUS |
Wardlaw and colleagues26 found that a strategy of immediate CT scans for all patients upon admission dominated all other strategies; it was not only the least costly strategy, but also it produced the greatest number of QALYs. The total expected QALYs for this strategy was estimated at 1982.2 for cohort of 1000 patients aged between 70 and 74 years, with total expected costs of £9994. Sensitivity analyses varied the cost of scanning, the cost-effectiveness of strategies for different age groups, the proportions of different types of strokes, the accuracy of CT, utility weights and lengths of hospital stays. The model was most sensitive to reductions in the costs of inpatient care; a cost per bed-day that differed by > 5% from the base-case assumption in a downwards direction led to the ‘scan all immediately’ strategy being less cost-effective (equally effective but more costly) than the main comparator strategy of scanning all within 48 hours.
Buskens and colleagues284 suggested that DUS was both less expensive and more effective than all other strategies included. The study suggested that, with the use of low stenosis-degree threshold as an indication for surgery, DUS as a single examination strategy would on average yield 11.4 QALYs at a cost of US$20,400. 284 The results were robust to a series of deterministic sensitivity analyses; changes to the likelihood of complications resulting from DSA or surgery, and the cost estimates, in general, revealed that the rank of the strategies in terms of incremental cost-effectiveness was largely unaffected.
In Wardlaw and colleagues,5 the net benefit of stroke prevention clinics was found to be very dependent on the speed with which patients could be investigated and treated, and also test accuracy. The study noted that the current stroke prevention approaches were too slow to prevent many strokes. 5 The clinical outcome in terms of time to endarterectomy did not differ much in those strategies that compared the first 24 hours of presentation of the patients. In strategies in which only one diagnostic test was used to determine CAS, the time to endarterectomy was on average 41 days. Two strategies (both using only one ultrasound) reduced it to 31 days, and eight strategies reduced it to 35 days.
The number of strokes suffered within the first year varied across the different strategies that were compared. The better strategies in terms of stroke prevention were those that enabled the endarterectomy to be undertaken expediently, i.e. those where the ultrasound show the stenosis level of 70–90% and the patient is subsequently provided with surgery. In this case 18.14 strokes occurred per 100 patients treated or investigated. If the patients were found to have stenosis level of > 49% and were offered endarterectomy, 17.94 strokes occurred per 100 patients investigated or treated. The number of QALYs gained from the different strategies examined in the analysis were related to the clinical results and ranged from 567.1 to 569.1 for 100 patients. The strategies where all patients with ultrasound reading of > 49% receive endarterectomy were found to be better in terms of QALY gains. Higher level of QALY gains were also observed where endarterectomy was offered to all patients with stenosis of > 69%.
The sensitivity of the model was examined by changes to assumptions regarding the assumed sensitivity of the tests, the time taken to reach surgery following the diagnostic tests, the costs of diagnostic tests, and the costs of endarterectomy. The results were moderately sensitive to changes in costs for endarterectomy and changes in accuracy of less invasive diagnostic tests. For example, the net monetary benefit changed from just over £10,000 to just over £50,000 by moving from a high to a low endarterectomy cost. Further, taking the extreme ends of the 95% CIs, for strategy 1 (surgery for stenosis of 70–99% following ultrasound), the low sensitivity showed a net monetary benefit of £13,000, whereas the high sensitivity showed a net monetary benefit of £35,000. However, the conclusion that less-invasive tests were preferred to DSA on cost-effectiveness grounds did not change, and the rank ordering of the less-invasive test strategies did not change.
Finally, Tholen and colleagues285 found that those strategies that used CTA or CEMRA, either as an independent strategy or in combination with an initial DUS, showed similar cost and effectiveness, where it is assumed that there is 2- to 4-week delay in surgery. For men, the combination strategy of DUS, followed by CTA with a cut off of 70–90% stenosis, was found to be the most cost-effective and dominated all other strategies except for the strategy of CTA with cut-off of 70–99% stenosis. The cost and QALY for the base case for men was estimated at €39,826, with QALY of 14.3832. The solo CTA strategy with a cut-off of 70–99% yielded slightly more QALYs (14.3841), giving a value of €71,419 per QALY gained. For the women-only cohort, likewise, the DUS–CTA combination strategy with a cut-off 70–90% dominated all other strategies. The costs for such a strategy were found to be €45,911 with QALYs of 16.4605. Probabilistic sensitivity analysis (PSA) showed that the combination strategy of DUS and CTA with a cut-off of 70–99% stenosis had the highest probability of cost-effectiveness (33–48%).
The main recommendation of the study was that the patient with a TIA or minor stroke should undergo DUS as initial diagnostic test, followed by CTA if the DUS results are positive, with surgery for 70–99% stenosis, whereas in patients with higher-risk profiles, with high prior probability of carotid stenosis, or who can undergo surgery without delay, immediate CTA and surgery for 50–99% stenosis are indicated. 285 Value-of-information analysis suggested an expected value of perfect information (EVPI) for further research of €318 per male patient, which implies a population EVPI of €1.5B for the European Union, and for females the EVPI was €40 per patient, implying a population EVPI of €193M for the European Union.
Discussion
This review found that no previous studies have assessed the cost-effectiveness of MRI compared with CT for minor stroke and TIA. One study attempted to perform a cost-effectiveness analysis of DWI in stroke prevention in 2008 but gave up through lack of data on accuracy of DWI or impact on management or other parameters required. 105 This is in contrast with the rapidly rising use of MR in stroke prevention, mostly performed in addition to CT rather than instead of, identified both in our UK survey of stroke services (see Chapter 8) and in a study performed using centralised health usage data in the USA. 298 In the USA between 1999 and 2008, the absolute utilisation of MRI for stroke increased by 38% but the relative use increased by 235%, from 28% of strokes in 1999 to 66% in 2008. 298 CT usage did not change over the same period.
The closest study on cost-effectiveness of imaging in stroke that we identified was that of Wardlaw and colleagues,26 who examined the role of CT. Three studies5,284,285 were identified that had addressed the question of diagnostic techniques for the management of carotid stenosis. Among these, Tholen and colleagues285 found very small differences in QALYs across the strategies, and larger differences in costs. These results were very different to those reported by Buskens and colleagues,284 which showed that Doppler ultrasound was the most effective and least costly strategy. Buskens and colleagues,284 however, compared Doppler ultrasound with non-enhanced rather than contrast-enhanced MR angiography, and did not consider CT angiography. Similarly, Wardlaw and colleagues5 concluded that Doppler ultrasound is the most cost-effective strategy.
As highlighted by Tholen and colleagues,285 a finding of large difference in costs but very small differences in QALYs is common in diagnostic test evaluation; model estimates may well be dominated by treatment costs and effects, which are common across different diagnostic strategies, with the only difference between strategies relating to any extra costs associated with capital and running expenditures of the imaging test itself. The importance of cost differences depends heavily on the local context. Where there is greater demand on MRI scanners for stroke as well as other conditions, revealed through waiting times that are unacceptable to clinicians and patients, it may be that the opportunity costs – i.e. the health benefits foregone as a result of delayed diagnosis and treatment – are so large that CT is indicated. For outcome differences, even where these are small on average on a per-patient basis, they are nevertheless important, as they apply to a large patient population, and therefore have a large effect once account is taken of total health benefit at a population level.
Chapter 10 Data required to populate the model of stroke prevention
Introduction
This chapter describes the data sources and summarises the data chosen for use in the model of stroke prevention clinics for components that have not been covered already in detail in the preceding chapters. It also presents some additional analyses of unpublished data where relevant. We have assumed that stroke prevention treatment (medical or surgical as appropriate) would be offered to patients with TIA or minor stroke due to ischaemic causes and not to patients with haemorrhagic stroke/TIA and non-vascular causes of their symptoms – the patients with haemorrhage would avoid ischaemic stroke prevention drugs/surgery and those with non-vascular diagnoses would be offered correct treatment for their true medical diagnosis. The effect of false-positive and negative diagnosis of stroke/TIA compared with not stroke/TIA and of ischaemic compared with haemorrhagic stroke/TIA and the use of imaging to differentiate these will be tested in the model. The supplementary table (at end of this chapter, see Table 61) lists full details of all values and ranges; ‘box’ refers to the relevant section identified by number in the left-hand column of this table.
Proportion of patients with non-vascular compared with vascular causes of symptoms (see Table 61, boxes 1–7)
Approximately half of the patients who attend NV clinics in the UK with transient or mild neurological symptoms do not have a final diagnosis of a CV event; less is know about the prognosis of these patients, although non-CV events are usually considered a benign condition. 266,267 These high proportions of TIA/minor stroke mimics among patients attending stroke prevention clinics in the UK are a common finding; in a recent survey of stroke prevention clinics in 2011, half of the centres indicated that the proportion of patients with a final diagnosis of TIA or minor stroke was between 11% and 60% (see Chapter 8). The final diagnosis of all patients (non-overlapping samples) referred to several different stroke prevention clinics in Scotland and Leicester, including the proportions of TIA, minor stroke and non-CV events, which are shown in Tables 33–35. In a large cohort of patients referred to a TIA clinic in Scotland with suspected TIA, 46% of the patients did not have a CV explanation for their symptoms (i.e. did not have a TIA or minor stroke). 271
| Variable | Before hotline | After hotline | p-value |
|---|---|---|---|
| No. of patients seen in clinic | 1625 | 2523 | |
| No. seen per month | 63 | 81 | |
| Demographics | |||
| Mean age, years (SD) | 68.5 (13) | 67.5 (13) | 0.02 |
| Male, n (%) | 802 (49) | 1259 (50) | 0.75 |
| Diagnoses, n (%) | |||
| Stroke definite | 458 (28) | 650 (26) | 0.09 |
| Stroke possible | 28 (2) | 91 (4) | < 0.01 |
| TIA definite | 427 (26) | 670 (27) | 0.87 |
| TIA possible | 57 (4) | 121 (5) | 0.05 |
| RAO | 50 (3) | 93 (4) | 0.34 |
| Transient monocular blindness | 95 (6) | 142 (6) | 0.82 |
| Definite non-CV | 399 (25) | 785 (31) | < 0.01 |
| Source of referral | |||
| Referred by GP, n (%) | 1298 (80) | 2048 (81) | 0.32 |
| Risk factors for those with CV disease | 1009 | 1547 | |
| History of diabetes, n (%) | 131 (13) | 199 (13) | 0.98 |
| Previous stroke, n (%) | 145 (14) | 240 (16) | 0.54 |
| Final diagnosis | Patients (n) | Percentage |
|---|---|---|
| TIA | 1137 | 29 |
| Minor ischaemic stroke | 650 | 17 |
| Retinal ischaemia | 155 | 4 |
| Primary haemorrhagic stroke | 17 | 0.4 |
| Subdural haemorrhage | 9 | 0.2 |
| Subarachnoid haemorrhage | 2 | 0.05 |
| Total | 1970 | 51 |
| Other diagnosis (non-CV) | ||
| Migraine | 337 | 9 |
| Syncope | 232 | 6 |
| Peripheral nerve | 89 | 2 |
| Vestibular | 86 | 2 |
| Transient global amnesia | 77 | 2 |
| Seizure | 54 | 1 |
| Tumour | 27 | 1 |
| Hypoglycaemia | 19 | 0 |
| Dementia | 11 | 0 |
| Parkinson’s disease | 5 | 0 |
| Others | 955 | 25 |
| Total non-CV | 1892 | 49 |
| Total patients | 3862 | 100 |
| Final diagnosis | Patients (n) | Percentage |
|---|---|---|
| TIA | 668 | 28 |
| Stroke | 631 | 27 |
| Transient monocular blindness | 139 | 6 |
| RAO | 89 | 4 |
| Total | 1527 | 64.4 |
| Other diagnosis (non-CV) | ||
| Migraine | 233 | 10 |
| Uncertain | 78 | 3 |
| Functional | 30 | 1 |
| Anxiety or panic | 24 | 1 |
| Seizure | 23 | 1 |
| Syncope | 18 | 1 |
| Transient global amnesia | 18 | 1 |
| Bell’s palsy | 14 | 1 |
| Vertigo | 12 | 1 |
| Postural hypotension | 11 | 0.5 |
| Other | 380 | 16 |
| Total non-CV | 841 | 35.5 |
| Total patients | 2368 | 100 |
The systematic review of studies of the frequency, main alternative diagnoses and outcomes in patients with a final non-CV diagnosis are reported in Chapter 7 and specific causes in the supplementary table (see Table 61, boxes 27–37). The summary estimate of the proportion for non-CV events reported across all selected studies was 34% (95% CI 28% to 42%), and ranged from 18% to 55%. However, there was evidence of substantial heterogeneity between studies; the summary estimates of proportions for non-CV events in TIA clinics, population-based and hospital/ED-based studies were 40% (95% CI 23% to 60%), 40% (95% CI 23% to 60%) and 32% (95% CI 10% to 66%), respectively, with evidence of significant heterogeneity between studies in each setting.
Prognosis among patients with suspected transient ischaemic attack and minor stroke
As regards prognosis of mimics, the HR for cardiovascular death in patients with both TIA and non-CV events was higher than in a control population without any neurological symptom (HR 3.61; 95% CI 3.06 to 4.25; HR 2.37/95% CI, 1.97 to 2.86, respectively) with a median follow-up of 87 months. 270 In a prospective population-based study of patients with transient neurological symptoms, the risks of stroke and dementia were also higher for patients with transient diffuse non-localising events than control subjects without any symptoms. 266 In the systematic review of all studies of mimics, a non-significant tendency for a higher risk of stroke and vascular death was found in patients with non-CV events compared with asymptomatic control subjects. Considering the few studies included with data for prognosis of non-CV compared with control subjects, the prognosis for any transient neurological event other than TIA is poorly documented. It would seem that from the perspective of stroke prevention clinics, that these non-CV patients are largely ignored.
Details on prognosis after definite TIA or minor stroke by underlying pathology and carotid stenosis are provided in Population of the model, below, and in the supplementary table (see Table 61, boxes 10–26 and 38–49).
Prognosis scoring systems in patients with transient ischaemic attack/minor stroke (see Table 61, box 54)
Several risk prediction scores based mainly on easy-to-elicit clinical features have been developed in the last 20 years to try to identify patients at higher stroke risk who could be fast tracked to secondary prevention. These are described in Chapters 4 and 6, and have largely coalesced into the ABCD2 score and its many variants in the last 5 years. 152,167,177,186,209
For the purpose of the present HTA project, we systematically reviewed studies that assessed ABCD score of ≥ 4 or < 4 in TIA patients (see Chapter 4). Among 26 cohorts, 15 cohorts reported the risk of recurrent stroke at 7 days; 18 cohorts at 90 days, and three cohorts at > 90 days, and nine studies63,139,141,151–154,160,165 provided stroke risk data at both 7 and 90 days. Among these nine studies63,139,141,151–154,160,165 the pooled estimates for stroke risk at 7 days were 4.76% (95% CI 2.40% to 8.70%) in patients with an ABCD2 score of ≥ 4 and 1.6% (95% CI 1.0% to 3.4%) in patients with score of < 4; at 90 days the risk was 8.2% (95% CI 4.7% to 14.0%) and 2.7% (95% CI 1.5% to 4.7%), respectively. The estimates for the risk of stroke at > 90 days were 17.2% (95% CI 8.8% to 30.9%) in patients with an ABCD2 score of ≥ 4 and 5.2% (95% CI 1 to 28.9%) in those with score of < 4.
The predictive value for CT visible ischaemic lesions and carotid stenosis for recurrent stroke is given in the supplementary table (see Table 61, boxes 55–58).
Proportion of ischaemic stroke/transient ischaemic attack with a relevant lesion on computed tomography, diffusion-weighted imaging (see Table 61, boxes 50–53)
Several studies have reported the proportion of patients with TIA and relevant visible lesion on CT; these values ranged from 0.0% to 11.5% (ESS data111). 35,69,81,122,138,150,158,249,251 A recent multicentre study showed that the proportion of patients with final diagnosis of TIA and visible lesion on CT was 23.9%; however, it is unclear if this was just the relevant lesion and in many cases it was an old and irrelevant lesion. 211 In patients with diagnosis of minor stroke, the proportion of patients with a visible relevant lesion on CT ranged from 42% to 52% (ESS data111). 33,252 With respect to minor stroke and visible lesion on DWI, few studies have shown that approximately two-thirds of patients have a relevant lesion during the first days after the event, ranging from 60% to 71%. 92,252,254
For the purpose of this HTA project we conducted a systematic review of DWI studies in TIA patients (see Chapter 6). We identified 47 studies with 9078 participants evaluating the proportion of TIA patients with positive DWI lesions. Sample size ranged from 14 to 1693 patients (mean 190 patients; median 85 patients). The proportion of patients with positive DWI lesions ranged from 9% to 67%. After random-effects meta-analysis, the proportion of TIA patients with a DWI abnormality was 34.3% (95% CI 31.0% to 38.4%). Studies varied in terms of time interval between symptoms onset and DWI, symptoms duration, aetiology, selection criteria and setting. The proportion of patients with minor stroke and a lesion on DWI was 69%.
Accuracy of computed tomography and magnetic resonance (diffusion-weighted imaging, gradient echo, T2/fluid-attenuated inversion recovery) for non-vascular compared with ischaemic stroke/transient ischaemic attack compared with haemorrhagic stroke/transient ischaemic attack by time after index event (see Table 61, boxes 59–64)
A recent systematic review aimed to estimate the accuracy of MR DWI compared with CT for the diagnosis of acute ischaemic stroke and to assess the accuracy of MRI (all feasible sequences) for the early detection of haemorrhagic stroke. 36 Most studies focused on acute hospital-admitted stroke patients who were being considered for acute primary treatment rather than for secondary prevention. Hence they are of limited relevance to the present topic. We found no studies that directly compared MR DWI and other appropriate sequences with CT in patients with TIA and minor stroke. For studies that assessed patients with ischaemic stroke (seven studies) in the systematic review of hospital-admitted patients,36 the pooled estimates for DWI sensitivity and specificity were 0.99 (95% CI 0.23 to 1.00) and 0.92 (95% CI 0.83 to 0.97) respectively, whereas the pooled estimates for CT sensitivity and specificity were 0.39 (95% CI 0.16 to 0.69) and 1.00 (95% CI 0.94 to 1.00), respectively. The one study that did include minor stroke suffered from incorporation bias because they changed the diagnosis of TIA to stroke in patients with a relevant lesion on DWI. They also excluded all mimics and haemorrhagic strokes. 32
Assessment of TIA/minor stroke patients may be delayed if the patient presents late or there are delays to investigations. We therefore estimated the likely sensitivity and specificity of MR DWI for ischaemic lesions, by time periods after symptom onset, for 100 patients presenting with suspected minor stroke for standard MR sequences required for diagnosis of ischaemic stroke (DWI, FLAIR, T2, T1) (Table 36). These estimates were based on the limited data available from observational studies of MR in patients with TIA/minor stroke. There are no reliable serial MRI studies of patients with minor stroke or TIA, or comparisons with CT. The estimates take account of the estimated loss of DWI hyperintensity with time after stroke and include the 45% with non-vascular diagnosis, assume that 66% of minor stroke would have DWI-positive lesion, and that without the DWI-positive lesion the other sequences T2, FLAIR and T1 are no better than CT, or actually worse because they show more white matter lesions, which are then mistaken for a recent infarct, thereby inflating the false-positive rate. DWI is also positive in migraine, epilepsy, MS, transient global amnesia, etc., hence the early false-positive rate. Between 7 and 21 days, fewer patients have a DWI-positive lesion (33%) and the rest may have a lesion visible on FLAIR/T2, etc., but this would be difficult to identify as the acute lesion – there would be other white matter lesions and old lesions that would be mistaken for the recent lesion, as they cannot be distinguished except with DWI, so the false-positive rate increases whereas the true-positive rate goes down. By 21 days or later, the DWI positives have almost vanished, the false positives have gone up a bit, etc. This estimation was necessary in the absence of other data. The effect of changing sensitivity and specificity would be the same for TIA patients except that the true-positive rate would be lower, as DWI shows a positive lesion in only one-third of TIA patients scanned within the first couple of days, and the lesion probably disappears even faster than for minor stroke and is less likely to leave any sign of permanent damage on T2, FLAIR or T1 than in minor stroke.
| MR DWI/FLAIR/T2/T1 ischaemic stroke | Total | |||
|---|---|---|---|---|
| Positive | Negative | |||
| < 7 days | ||||
| Ischaemic stroke | + | 35 | 20 | 55 |
| – | 5 | 40 | 45 | |
| Total | 40 | 60 | 100 | |
| Sensitivity = 63%, specificity = 88% | ||||
| 7–21 days | ||||
| Ischaemic stroke | + | 15 | 40 | 55 |
| – | 10 | 35 | 45 | |
| Total | 25 | 75 | 100 | |
| Sensitivity = 27%, specificity = 77% | ||||
| > 21 days | ||||
| Ischaemic stroke | + | 5 | 50 | 55 |
| – | 15 | 30 | 45 | |
| Total | 20 | 80 | 100 | |
| Sensitivity = 10%, specificity = 66% | ||||
For CT scanning, we calculated the proportion of patients with minor stroke with a visible relevant infarct by time of scanning within 7 days and after 7 days, using data on 892 relevant patients from the ESS (ESS data111). A visible infarct was seen within 7 days in 246/383 (64%) of patients and after 7 days in 277/507 (55%) of patients.
For sensitivity and specificity of MR in haemorrhagic stroke (two publications), one showed a sensitivity of 0.83 (95% CI 0.52 to 0.98) and a specificity of 1.00 (95% CI 0.95 to 1.00) for gradient-echo and diffusion-weighted MRI in patients assessed within 3 hours of stroke; compared with CT and clinical assessment, however, sensitivity was estimated from 13% of the 90 patients included in the study. The second study reported 1.00 (95% CI 0.91 to 1.00) for both sensitivity and specificity of diffusion-weighted MRI and 1.00 (95% CI 0.91 to 1.00) sensitivity and 0.98 (95% CI 0.87 to 1.00) specificity for GRE MRI performed within 6 hours of symptoms, but with no CT comparator in most of the patients. 36 Fifty per cent of patients in this study had haemorrhagic stroke compared with the 10–15% proportion of haemorrhagic stroke in the general population and about 5% among patients with minor stroke. This indicated the presence of highly selected patient sample that was likely to have increased sensitivity and influenced specificity, and is virtually irrelevant to the present work and the situation in daily practice. 36 MR detection of haemorrhage varies with sequence, T2* (GRE) or susceptibility-weighted sequences being the most sensitive, and T2 and FLAIR sequences being relatively insensitive. 51,299 We set the sensitivity and specificity of MR for haemorrhage as 0.83 and 1.00 if T2* was used and 0.50 if T2* was not used, respectively (see Table 61, boxes 62 and 64).
The signs of acute haemorrhage on CT have been well described and were discussed extensively in the HTA report on cost-effectiveness of CT scanning in acute stroke. 26 The characteristic CT features of acute haemorrhage (increased attenuation lesion – white – with respect to normal brain) appear virtually immediately on CT300 and last for between 5 days and perhaps 3 weeks,31 depending on the amount of initial blood. 301 Small amounts of blood are broken down more rapidly and can lose their characteristic whiteness by as early as 1 week after the stroke20,31 becoming isoattenuated and then hypoattenuated (dark) with respect to normal brain. 302 Eventually, a small slit-like cavity may remain, or the lesion may leave no trace, or it may leave a cavity with a white (haemosiderin-containing) rim. The latter is highly specific for old haemorrhage. There are almost no long-term systematic studies where patients with haemorrhage were scanned repeatedly to determine the rapidity with which haematomas resolve and what proportion remain visible or not as a cavity with or without a white rim for obvious reasons due to radiation dose. 302 However, for the purpose of the present analysis, the loss of characteristic whiteness in minor stroke and TIA patients with small haematomas is the important factor. We assumed that within the first week after stroke or TIA, the sensitivity and specificity of CT would be 0.99 and thereafter would fall to much lower levels (see Table 61, box 60).
There are few data on the sensitivity or specificity of CT or MR in the many potential mimics of TIA and minor stroke, with relevant few new studies since the publication of the HTA report on cost-effectiveness of CT scanning in acute stroke26 for which this topic was extensively reviewed. Unfortunately, most studies in which the data could have been collected excluded the mimics. Both CT and MR detect and misinterpret tumours and arteriovenous malformations, SDHs with equal sensitivity and specificity. MR may identify more patients with MS, but, on the other hand, MR also shows more incidental lesions, particularly white matter hyperintensities, which may be completely irrelevant to the presenting symptoms but be mistaken for vascular or non-vascular lesions. Neither technique will show much of relevance in migraine or epilepsy or other common mimics, although the former two pathologies may be associated with transient hyperintensities on DWI that would be mistaken for ischaemic events. Therefore, we used data from the HTA report on CT in acute stroke,26 and informed professional opinion and set the sensitivity and specificity of MR and CT for most mimics as equivalent (Table 37).
| Final diagnosis | Proportiona (see Table 61, boxes 27–37) | Scan finding | Action, consequence | Outcome | |
|---|---|---|---|---|---|
| CT | MR | ||||
| Migraine | 14 | –ve | –ve | Need antimigraine drugs | Unlikely to die from migraine but does need to be correctly identified and treated – death rate as for general population |
| Vertigo | 4 | –ve/NC | –ve/NC | Need symptom control – usually self-resolvingb | Unlikely to die from vertigo but does need to be correctly identified and treated – death rate as for general population |
| Psychiatric | 4 | –ve/NC | –ve/NC | Need relevant psychiatric consultation | Unlikely to die from psychiatric conditions but does need to be correctly identified and treated – death rate as for general population |
| Cardiac | 9 | NC | NC | Need relevant cardiac consultation | Depending on cardiac disorder, have increased risk of death from heart disease – heart attack (MI), arrhythmia and sudden death; suggest inflate death rate |
| Seizure | 5 | –ve | –ve | Need relevant antiepileptic treatment – failure may result in death or damage from further seizure | Increased risk of death from seizures and harm from damage during epileptic seizure if epilepsy untreated |
| Tumour | 2 | +ve | +ve | Needs proper assessment, including biopsy and other treatment | Increased risk of death/dependency from tumour – bad practice not to manage correctly even if tumour is ultimately not curable |
| SDH | 0.2 | +ve | +ve | Avoid aspirin/anticoagulation – may be self-resolving but may need surgery – otherwise increased risk of death or dependency | Disaster if give aspirin/warfarin/heparin to subdural – usually have warning signs, e.g. headache, history of bang on head, may be on warfarin already – set risk of death at 40%, risk of dependency at 40% if not correctly diagnosed |
| Subarachnoid haemorrhage | 0.05 | +veSensitivity: 0.95Specificity: 0.99 | –veSensitivity: 0.20Specificity: 0.20 | Needs angiogram to identify if has aneurysm and treatment of it – otherwise high risk of death/dependency from recurrent bleed | Disaster if not correctly diagnosed. Competent clinician should suspect on grounds of symptoms/signs and request scan. Common source of error in A+E departments by junior doctors. High risk of death (70%+) or dependency (30%) from recurrent bleed if not treated |
| Syncope | 7 | –ve/NC | –ve/NC | Identify cause (if any) of syncope on clinical/other tests (not scanning) and rectify to avoid falls, etc. and resulting damageb | Main problem ‘falls in elderly’ or over-treated hypertension resulting in drops in BP, falls, banged head, trauma, SDH, etc. So need to be correctly diagnosed and managed – risk of death/dependency 30%/30% (estimate) |
Population of the model
Epidemiological data: profile of the patients who have suffered a transient ischaemic attack
Only patients aged ≥ 55 years and who were non-disabled were considered in the model. Table 38 shows the distribution of age at first event against sex and type of TIA/stroke of all severities from the ESS. 111
| Frequency | < 55 years | 55–64 years | 65–74 years | 75–84 years | ≥ 85 years | Total |
|---|---|---|---|---|---|---|
| Female | 86 | 157 | 285 | 366 | 150 | 1044 |
| Male | 124 | 229 | 353 | 336 | 80 | 1122 |
| Haemorrhagic | 6 | 12 | 24 | 39 | 14 | 95 |
| Ischaemic | 180 | 329 | 541 | 611 | 200 | 1861 |
| Other | 22 | 44 | 59 | 43 | 10 | 178 |
| Uncertain | 2 | 1 | 14 | 9 | 6 | 32 |
| Total, n (%) | 210 (10) | 386 (18) | 638 (29) | 702 (32) | 230 (11) | 2166 |
The proportions of patients with haemorrhagic stroke in patients with a final diagnosis of TIA and minor stroke were taken from the ESS (ESS data111), previous population studies summarised in26 the SSCAS and other unpublished audit data (see Table 61, boxes 4 and 5).
The incidence of TIA per annum was taken from OXVASC,303 as were the data for minor stroke, assuming that one-third of ischaemic strokes would be minor. The rates of minor stroke and TIA per age and sex band were applied to a hypothetical population of 500,000 with an age and sex mixture equal to that of England and Wales,304 which resulted in an estimated 490 minor strokes and TIAs per annum. This resulted in estimated number of TIAs and minor strokes per annum as given in Table 39.
| Sex | Age (years) | ||||
|---|---|---|---|---|---|
| 55–64 | 65–74 | 75–84 | ≥ 85 | Total | |
| Male, n (%) | 22.5 (5) | 66.3 (14) | 63.4 (13) | 25.7 (5) | 177.9 (36) |
| Female, n (%) | 30.1 (6) | 65.3 (13) | 130.7 (27) | 86.0 (18) | 312.1 (64) |
| All, n (%) | 52.3 (11) | 131.7 (27) | 194.2 (40) | 111.6 (23) | 490.0 (100) |
Figures in parentheses indicate the percentage of the total number of TIAs or minor strokes. Figures may not tally exactly because of rounding.
The OXVASC study303 was a population-based incidence study of stroke and TIA in 2002–4. During the study period, 476 individuals had at least one TIA or stroke. Of these, 262 were first-ever incident strokes (223 ischaemic strokes), 76 were recurrent strokes and 138 individuals experienced at least one TIA (93 with incident TIA, 20 with previous TIA, 17 with previous stroke and eight with both). The crude incidence per 1000 population in OXVASC303 was 1.87 (95% CI 1.67 to 2.08) for any stroke, 1.45 (95% CI 1.28 to 1.63) for first-ever stroke and 0.42 (95% CI 0.33 to 0.53) for recurrent stroke. Table 40 shows the overall incidence of TIA adjusted to the 2001 Census population on England and Wales303 and Table 41 shows the age-specific incidence of TIA in men and woman in the OXVASC population. 305
| Incidence | Annual TIA incidence per 1000 (95% CI) |
|---|---|
| Crude incidence | |
| Men | 0.35 (0.24 to 0.49) |
| Women | 0.69 (0.53 to 0.89) |
| All | 0.51 (0.41 to 0.63) |
| Standardised incidence | |
| Men | 0.37 (0.24 to 0.50) |
| Women | 0.77 (0.57 to 0.96) |
| All | 0.58 (0.46 to 0.69) |
| Standardised incidence by age | |
| ≥ 85 years | 6.41 (3.45 to 9.38) |
| < 85 years | 0.46 (0.35 to 0.56) |
| < 75 years | 0.24 (0.17 to 0.32) |
| Sex | Age (years) | |||||||
|---|---|---|---|---|---|---|---|---|
| < 35 | 35–44 | 45–54 | 55–64 | 65–74 | 75–84 | ≥ 85 | Total | |
| Men | ||||||||
| Number | 2 | 3 | 3 | 11 | 15 | 19 | 10 | 63 |
| Rate (95% CI) | 0.03 (0.00 to 0.11) | 0.13 (0.03 to 0.39) | 0.16 (0.03 to 0.48) | 0.74 (0.37 to 1.32) | 1.45 (0.81 to 2.4) | 3.27 (1.97 to 5.11) | 7.94 (3.81 to 14.6) | 0.45 (0.34 to 0.57) |
| Women | ||||||||
| Number | 4 | 1 | 5 | 15 | 23 | 44 | 26 | 118 |
| Rate (95% CI) | 0.07 (0.02 to 0.17) | 0.05 (0.00 to 0.29) | 0.30 (0.10 to 0.70) | 1.05 (0.59 to 1.73) | 2.18 (1.38 to 3.26) | 5.61 (4.08 to 7.53) | 9.14 (5.97 to 13.4) | 0.89 (0.74 to 1.07) |
| All | ||||||||
| Number | 6 | 4 | 8 | 26 | 38 | 63 | 36 | 181 |
| Rate (95% CI) | 0.05 (0.02 to 0.10) | 0.10 (0.03 to 0.25) | 0.23 (0.10 to 0.45) | 0.89 (0.58 to 1.30) | 1.82 (1.29 to 2.50) | 4.61 (3.55 to 5.90) | 8.77 (6.14 to 12.14) | 0.66 (0.57 to 0.77) |
The Greater Cincinnati/Northern Kentucky Stroke Study23 was a large USA population-based metropolitan study (biracial population of 1.3 million) of incidence rates of TIA and short-term outcomes after TIA. During the study (1 year), a total of 1023 TIA events occurred among 927 patients (first ever or recurrent), with an overall annual age- and sex-adjusted incidence rate, for a single TIA, of 83 per 100,000 (95% CI 78 to 88) (Table 42). Black people and men had higher rates of TIA than white people and women. The risk of TIA and stroke after TIA was 8.6% and 14.6% at 3 months, respectively. 23 This indicates that the incidence of TIA is similar in western countries.
| Age (years) | Incidence rate (95% CI) | |||
|---|---|---|---|---|
| White | Black | |||
| Male | Female | Male | Female | |
| < 35 | 1.0 (0.0 to 2.2) | 1.8 (0.2 to 3.3) | 1.7 (0.0 to 5.2) | 3.2 (0.0 to 7.8) |
| 35–44 | 4.6 (0.1 to 9.0) | 12.3 (5.0 to 19.6) | 16.0 (0.0 to 38.3) | 12.7 (0.0 to 30.2) |
| 45–54 | 82.2 (59.2 to 105.2) | 73.3 (52.3 to 94.2) | 129.8 (49.4 to 210.3) | 30.2 (0.0 to 64.3) |
| 55–64 | 155.4 (118.0 to 192.1) | 96.3 (69.0 to 123.5) | 205.7 (89.3 to 322.1) | 249.0 (139.9 to 358.2) |
| 65–74 | 468.5 (395.7 to 541.3) | 246.6 (201.1 to 292.1) | 338.2 (172.5 to 503.9) | 330.2 (192.2 to 468.3) |
| 75–84 | 750.4 (620.9 to 879.9) | 521.1 (441.8 to 600.4) | 612.8 (279.7 to 945.9) | 647.9 (393.9 to 901.9) |
| ≥ 85 | 718.5 (457.0 to 980.0) | 589.3 (455.0 to 723.5) | 1,557.9 (478.3 to 2637.5) | 847.8 (346.8 to 1348.8) |
| Overall adjusted rates | 100.6 (91.1 to 110.1) | 67.6 (861.4 to 73.9) | 107.2 (79.8 to 134.7) | 92.6 (72.9 to 112.4) |
A systematic review of the incidence of all stroke in the UK showed a incidence among men of all ages ranging from 124 per 100,000 in south London to 185 per 100,000 in the Scottish Borders region, and from 88 to 146 per 100,000 among women in the same studies. 306
Carotid stenosis and risk of stroke
Owing to the significant correlation between stenosis level and the risk of stroke,307 the TIAs and minor strokes were subdivided into those occurring in patients with an ipsilateral internal carotid stenosis level of 70–99%, those occurring in patients with a stenosis level of 50–69% and those in patients where stenosis was < 50% or where the carotid artery had occluded. The percentages of patients suffering a TIA or minor stroke who fall into these stenoses bands are given in Tables 42–44 and the patients were assumed to be the same as those in the LSR5 and ESS111 data report. It was also assumed that the stenosis distribution in patients with a minor stroke was identical to that of patients with a TIA (see Tables 43 and 44). Details of key risk factors (carotid stenosis and AF) in patients with TIA and minor stroke were also obtained from multiple sources (see Table 61, boxes 8 and 9). 118,132,164,185
| Carotid stenosis band by NASCET | Minor stroke | TIA | ||
|---|---|---|---|---|
| % | n | % | n | |
| 0–49% and occluded | 90.3 | 901 | 90.9 | 421 |
| 50–69% | 3.5 | 35 | 2.8 | 13 |
| 70–99% | 6.2 | 62 | 6.3 | 29 |
| Stenosis level | Percentage of all TIAs |
|---|---|
| 70–99% | 6 |
| 50–69% | 4 |
| < 50% or occluded | 90 |
These groups were further subdivided into patients who were currently taking aspirin and those who were not, as this could affect the magnitude of benefit of initiating medical therapy at an earlier stage, when a diagnostic algorithm would result in treatment being initiated more rapidly. It was assumed that 51% of patients would be on aspirin at the time of experiencing TIA or minor stroke. 9,303 In the SOS-TIA and EXPRESS studies, 22% and 45% of patients respectively, with a diagnosis of TIA or minor stroke, were on antiplatelet drugs at baseline. In ED assessment of TIA, nearly 30% of patients were already on aspirin. 8,153 Audit data from Lothians 2010–11 show that 50–52% of patients were already on aspirin or other antithrombotic treatment at the time of their TIA/minor stroke (Table 45).
| Drugs at first assessment | 2010 | 2011 so far | ||||
|---|---|---|---|---|---|---|
| WGH | SJH | Lothian | WGH | SJH | Lothian | |
| Aspirin, n (%) | 453 | 153 | 606 (48) | 127 | 58 | 185 (38.7) |
| Other antiplatelet, n (%) | 38 | 14 | 52 (4.1) | 50 | 4 | 54 (11.3) |
| Anticoagulant, n (%) | 30 | 16 | 46 (3.6) | 8 | 4 | 12 (2.5) |
| Total patients | 1261 | 477 | ||||
We have assumed that these distributions are independent of age and sex, as there were no reliable population-based data on the age and sex distribution of patients with TIA or minor stroke who also gave information on the degree of carotid stenosis. The risks of stroke and MI for patients within the same carotid stenosis band were assumed to be homogeneous; however, death from all other causes would vary within stenosis bands dependent on the age and sex of the patient.
Risks of recurrent stroke after transient ischaemic attack and minor stroke
The proportions of patients experiencing a recurrent stroke by time after and severity of index stroke or TIA, including the risk of recurrent haemorrhage in patients with haemorrhagic stroke, were derived in the systematic reviews in Chapter 3 for ischaemic stroke and from multiple data sources for haemorrhagic stroke (see Table 61, boxes 38–41). 5,26,28,308
The systematic review of all published studies resulted in a pooled estimate of recurrent stroke after TIA of 5.2% (95% CI 3.9% to 5.9%) at 7 days, 6.7% (95% CI 5.2% to 8.7%) at 90 days and 11.3% (95% CI 7.5% to 16.6%) at > 90 days (see Chapter 3).
Data on recurrent haemorrhage in patients on antithrombotic drugs were taken from the HTA report on CT in acute stroke and a recent systematic review (see Table 61, box 49). 27,30
We obtained data on non-fatal and fatal stroke, MI and other causes of death at 7 and 90 days and 2 years after TIA and minor stroke from the LSR (Table 46).
| Outcomea | TIA | Minor stroke | ||||
|---|---|---|---|---|---|---|
| 7 days | 90 days | 2 years | 7 days | 90 days | 2 years | |
| Non-fatal stroke | 0 | 3 | 17 | 3 | 13 | 38 |
| Fatal stroke | 0 | 4 | 12 | 1 | 7 | 25 |
| Non-fatal MI | 0 | 0 | 7 | 0 | 1 | 10 |
| Fatal MI | 0 | 1 | 1 | 0 | 1 | 9 |
| Other vascular death | 0 | 4 | 16 | 1 | 4 | 22 |
| Non-vascular death | 0 | 3 | 18 | 2 | 5 | 19 |
| Alive, no events | 828 | 813 | 757 | 1086 | 1062 | 970 |
| Total ( n ) | 828 | 828 | 828 | 1093 | 1093 | 1093 |
Risks of stroke in relation to the degree of stenosis
The longer-term risks of stroke following a TIA have been well described. 9,21,22,127 Chapter 5 details the effect of adding carotid stenosis to clinical risk prediction scores. As determined in the carotid endarterectomy trials, carotid stenosis is a key risk factor for recurrent ipsilateral ischaemic stroke. However, although data were available on the longer-term risk stratified by stenosis level,25 the short-term data were not. 9 We have therefore assumed that the ratio of risks between stenosis bands observed in the initial year in the carotid surgery trials25 were also applicable to the initial 3-month period. The assumed risks associated with the patients within these trials are presented in Table 47. See Tables 48 and 49 for risks of stroke and MI by time and stenosis band from ESS data report (ESS data111). Table 48 shows the frequency of recurrent stroke by time and carotid stenosis bands, including six patients with endarterectomy. In these patients, there were five strokes, all at ≤ 6 months’ follow-up (one in a patient with 50–69% ipsilateral carotid stenosis and four in patients with 70–99% ipsilateral carotid stenosis) (ESS data111). However, this is under-reported, as ESS did not collect routinely the information on carotid endarterectomy. 111
| Time | Cumulative risk (%) of stroke in medically treated patients | ||
|---|---|---|---|
| Stenosis level | |||
| < 50% and occluded | 50–69% | 70–99% | |
| 1 week | 7 | 8 | 13 |
| 1 month | 9 | 12 | 19 |
| 3 months | 14 | 18 | 29 |
| 15 months | 20 | 25 | 39 |
| 27 months | 23 | 30 | 45 |
| 39 months | 26 | 33 | 48 |
| Time to recurrent stroke | Unknown | 0–49% and occluded | Stenosis band | ||
|---|---|---|---|---|---|
| 50–69% | 70–99% | All | |||
| ≤ 6 months | 20 (4.2) | 63 (4.2) | 1 (1.7) | 9 (8.0) | 93 |
| > 6 months to 1 year | 8 (1.7) | 30 (2) | 0 | 2 (1.7) | 40 |
| 13–24 months | 7 (1.4) | 29 (2) | 5 (8.7) | 2 (1.7) | 43 |
| 25–36 months | 3 (0.6) | 19 (1.3) | 0 | 3 (2.6) | 25 |
| 37 months to 5 years | 1 (0.2) | 2 (0.13) | 1 (1.7) | 0 | 4 |
| No second stroke | 440 (91) | 1,375 (91) | 50 (88.0) | 96 (86.0) | 1961 |
| All | 479 | 1518 | 57 | 112 | 2166 |
| Carotid stenosis group | Prior to first event | Time to first MI | No MI | All | ||||
|---|---|---|---|---|---|---|---|---|
| ≤ 6 months | > 6 months to 1 year | 13–24 months | 25–36 months | 37 months to 5 years | ||||
| Unknown | 2 | 5 | 4 | 1 | 3 | 1 | 463 | 479 |
| 0–49% and occluded | – | 11 | 7 | 7 | 5 | 1 | 1487 | 1518 |
| 50–69% | – | – | – | 1 | 1 | – | 55 | 57 |
| 70–99% | – | 1 | 1 | 1 | 1 | 1 | 107 | 112 |
| All | 2 | 17 | 12 | 10 | 10 | 3 | 2112 | 2166 |
| Cumulative risk, n (%) a | 2 (0) | 17 (0.8) | 2 (1.3) | 39 (1.8) | 49 (2.3) | 52 (2.4) | 2112 (97.5) | 2166 |
The early risk of ipsilateral ischaemic stroke after first TIA in medically treated patients with symptomatic ICA disease was obtained from multiple sources. In the medical arm of the NASCET trial, the 2- and 90-day risks of ipsilateral stroke were 5.5 and 20% for patients with hemispheric TIA and 0.0 and 2.3% respectively for hemispheric stroke. The risks were higher in the presence of infarct visible in brain imaging (adjusted HR 2.1; 95% CI 1.5 to 3.0) and intracranial major-artery disease (adjusted HR 1.9; 95% CI 1.3 to 2.7) but similar in patients with severe carotid stenosis (≥ 70%) compared with lower degrees of stenosis. 128
The consequences of the delays of carotid imaging and surgery for the effectiveness of stroke prevention have been assessed in the OXVASC study population. The median times from event to referral and event to imaging in this cohort were 7 and 28 days, respectively. The risk of recurrent stroke in patients with ≥ 50% stenosis censored before any surgery was 21% at 14 days and 32% at 12 weeks. 66 These values could reflect the delayed prescription of appropriate secondary prevention treatment soon after the index event and before surgery. More recent data from a prospective single-centre study described the recurrence rate at 2 weeks in 163 patients with symptomatic carotid stenosis of ≥ 50% and first-ever non-disabling stroke or TIA. Recurrence was defined as new neurological clinical event: TIA or stroke in patients with TIA, or worsening ≥ 4 points from the initial NIHSS or new symptoms suggesting a new neurological event in patients with mild stroke. During the first 2 weeks, 45 patients (27.6%) had recurrence, 26 (16.0%) patients in the first 24 hours after admission, 11 (6.7%) between 72 hours and 7 days, and 6 (3.7%) between 7 days and 2 weeks. When new TIAs were excluded, the risk of new stroke was 22.7% (37 patients) and increased with the degree of stenosis. 185 All of these rates seem a high estimate, perhaps because they admitted the patients and watched them very closely and maybe they were counting subtle neurological changes as recurrences during their strict follow-up. For the purpose of this project we applied the recurrence rates by per cent stenosis used in the 2006 HTA report (Wardlaw and colleagues5) derived from combined carotid endarterectomy trial data,25 because they are more consistent with local audit data and with the recurrence rates described in the literature reviews (see Table 47).
The risk of stroke in relation to medical treatment
The carotid surgery trials reported the risks of stroke following medical treatment. Antiplatelet treatment and BP-lowering drugs were prescribed in the NASCET, ECST and Veterans Administration carotid endarterectomy trials. 25 Lipid-lowering drugs are now in widespread use, and in the recent OXVASC epidemiology study 32% of patients with TIA and 24% of those with minor stroke were on lipid-lowering agents at the time of their stroke. 9
To calculate the risks of stroke in patients who remained untreated for a period after their TIA or minor stroke, we have assumed that the efficacy of medication seen in RCTs would apply (see Efficacy of medical treatment, below). 309–314 In fact, the survey of UK stroke prevention clinics shows that as in 2011, < 25% of patients were unmedicated for any length of time after their TIA/minor stroke. There are no other new data from clinical trials to change any assumptions regarding effects of medical treatment.
Long-term risks of stroke after transient ischaemic attack in the presence of carotid stenosis
We assumed that 39 months after a TIA or minor stroke (i.e. by just over 3 years) the risks of stroke were equal regardless of stenosis level. The risk of stroke in treated patients after 3 years has been set as 2.3% per annum and is the weighted average of data presented by Clark and colleagues,315 Cunningham and colleagues316 and Barnett and colleagues. 317 Therefore, immediately following successful endarterectomy in the model, we set the risk of stroke to 2.3% per annum, assuming it to be the same as in patients who had survived 3 years or more since a TIA or minor stroke.
Long-term outcomes after stroke
The proportion of patients dying as a result of index stroke or TIA by various time periods, by stroke type, by cause of death, were taken from multiple sources including data amassed in the two previous HTA reports of imaging in stroke5,26 the ESS (ESS data111), analysis of the LSR, and published data (see Table 61, boxes 10–26). 8,153,164,308,315,318–325
Data on the proportion of patients who would be alive and independent after stroke by severity and time after index stroke were obtained from multiple sources (see Table 61, boxes 42–49). 320,326 The proportion of patients dying, surviving in a dependent or independent state after stroke according to the Rankin score, by age of patient, was taken from the LSR327,328 and the ESS. 111 A Rankin score of ‘0–2’ indicates independent survival, ‘3–5’ indicates survival in a dependent state, and ‘6’ is dead. The LSR is a hospital-based register of all patients with TIA or stroke, attending the WGH in Edinburgh, collected between 1990 and 1999. The WGH has a catchment population of 500,000 and serves north Edinburgh. These data are not truly community based, as patients who die prior to hospital assessment are not included in the Registry, but there are only limited data on long-term outcome after stroke from other sources (see below). The outcome values derived from the LSR are provided in Table 50. These values are broadly similar to those presented in the study of Bond and colleagues,329 in which for all patients the fatality rate was 13%. The rates of disabling stroke and non-disabling stroke were 30% and 57%, respectively. Outcome at 6 months after stroke by age of the patient from ESS data report111 on a similar population of patients seen in Lothian with stroke between 2002 and 2005 are shown in Table 51. Long-term follow-up of 2447 patients recruited in the Dutch TIA Trial (excluding patients with cardiac source of embolism or a clotting disorder) over 10 years, showed that 1489 (60%) had died and 1336 (54%) had experienced at least one other vascular event. 65 Ten-year risk of death was 42% (95% CI 41% to 45%) and of a first vascular event was 44% (95% CI 42% to 46%) and similar factors predicted vascular event as vascular death (non-fatal stroke 480/1741 total events, non-fatal MI 185/1741, fatal stroke 139/1741, fatal MI 138/1741). 65 The Perth Community Stroke Study (PCSS)318,319 was a population-based study in Western Australia and also reported long-term survival after first-ever stroke. The 10-year cumulative risk of death was 79% (95% CI 74% to 84%). Case fatality was greatest in the first year after stroke (36%; 95% CI 30% to 41%), and particularly in the first 30 days after stroke 22% (95% CI 17% to 28%).
| Age (years) | Rankin score 0–2 | Rankin score 3–5 | Fatality | 30-day fatality in OXVASC300 |
|---|---|---|---|---|
| 55 to 64 | 76% | 22% | 2% | 12% |
| 65 to 74 | 79% | 17% | 4% | 10% |
| 75 to 84 | 73% | 21% | 6% | 15% |
| ≥ 85 | 52% | 39% | 10% | 31% |
| Age (years) | Unknown (n, %) | mRS | Dead (n, %) | All | |
|---|---|---|---|---|---|
| Rankin score 0–2 (n, %) | Rankin score 3–5 (n, %) | ||||
| < 55 | 37 (36) | 56 (54) | 10 (10) | 1 (1) | 104 |
| 55 to 64 | 53 (27) | 114 (59) | 18 (9) | 9 (5) | 194 |
| 65 to 74 | 106 (29) | 181 (49) | 52 (14) | 29 (8) | 368 |
| 75 to 84 | 128 (29) | 192 (43) | 60 (14) | 62 (14) | 442 |
| ≥ 85 | 55 (34) | 30 (19) | 22 (14) | 55 (34) | 162 |
| Total | 379 (30) | 573 (45) | 162 (13) | 156 (12) | 1270 |
Among 1-year survivors of stroke, the average annual case fatality over the next 9 years was 4.8%, 6.0% between years 1 and 5, and 4.0% between years 6 and 10. 319
The increased risk of all cause mortality following a stroke
Following stroke, the risk of death is increased 2.5-fold compared with patients of the same age and sex without stroke, as reported in the PCSS,330 with this effect persisting until death. The 10-year follow-up of this study showed that all patients with a first-ever stroke had an approximately three times greater risk of dying compared with individuals of the same age and sex in the general population of Western Australia, with a ninefold (95% CI 7.1 to 10.8) greater risk of death in the first year after stroke and then a twofold increase in risk in each of the subsequent years. 319
The longer-term outcome of patients alive at 6 months after stroke is related to Rankin status at 6 months: patients who were independent at 6 months had a median survival of 9.7 (95% CI 8.9 to 10.6) years, and in those who were dependent it was 6.0 (5.7 to 6.4) years; median survival of those with a modified Rankin Scale (mRS) score of 0–1 was 12.9 (10.0–15.9) years, reducing to 2.5 (1.4–3.5) years in those with a mRS of 5 (combined OCSP, LSR and [International Stroke Trial (IST) data] (Tables 52 and 53). 320 The patients who are dependent at 6 months after stroke onset had an overall RR of dying from a stroke-related cause of 1.68 (95% CI 1.49 to 1.91) (including OCSP, LSR and IST cohorts). The RR of dying from stroke-related causes increased with an increasing mRS score (OCSP and LSR cohorts) (Table 54). 321 Table 55 shows Kaplan–Meier estimates of the risk of death within defined time intervals after first-ever stroke. 319
| Functional status | Median survival, years (95% CI) |
|---|---|
| Rankin score in OCSP and Lothians cohorts (no. of patients) | |
| 0 (311) | > 15.0a |
| 1 (540) | 11.7 (8.4 to 14.9) |
| 2 (576) | 8.4 (7.6 to 9.3) |
| 3 (433) | 6.0 (5.2 to 6.8) |
| 4 (189) | 3.7 (2.9 to 4.6) |
| 5 (136) | 2.5 (1.4 to 3.5) |
| All three cohorts (no. of patients) | |
| Independent (2525) | 9.7 (8.9 to 10.6) |
| Dependent (3436) | 6.0 (5.7 to 6.4) |
| Timing of outcome | OCSP, n (%) | LSR, n (%) | IST,a n (%) | |||
|---|---|---|---|---|---|---|
| Independent (mRS Rankin score 0–2 at 6 months) | Dependent (mRS Rankin score 3–5 at 6 months) | Independent (mRS Rankin score 0–2 at 6 months) | Dependent (mRS Rankin score 3–5 at 6 months) | Independent (Oxford Handicap Scale, 0–2) | Dependent (Oxford Handicap Scale, 3–5) | |
| 6 months | 285 (100) | 154 (100) | 1142 (100) | 604 (100) | 1098 (100) | 2678 (100) |
| 1 year | 280 (98) | 138 (90) | 1101 (96) | 543 (90) | 1067 (97) | 2478 (93) |
| 5 years | 209 (73) | 69 (45) | 325 (28) | 147 (24) | 696 (63) | 1321 (49) |
| 9 years | 149 (52) | 42 (27) | 1 (0.08) | 0 | 28 (2.5) | 19 (0.7) |
| 10 years | 132 (46) | 39 (25) | – | – | – | – |
| 15 years | 68 (24) | 25 (16) | – | – | – | – |
| mRS | RR (95% CI) |
|---|---|
| 0–1 | 1.00 (baseline) |
| 2 | 1.12 (0.82 to 1.56) |
| 3 | 1.66 (1.24 to 2.23) |
| 4 | 1.92 (1.41 to 2.61) |
| 5 | 2.57 (1.92 to 3.43) |
| Data to calculate risk | < 30 days | 1 to < 6 months | 6 to < 12 months | 1 to < 2 years | 2 to < 3 years | 3 to < 4 years | 4 to < 5 years | 5 to < 6 years | 6 to < 7 years | 7 to < 8 years | 8 to < 9 years | 9 to 10 years |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| At risk, n | 251 | 195 | 172 | 162 | 144 | 128 | 117 | 100 | 84 | 71 | 63 | 59 |
| Deaths, n | 56 | 23 | 10 | 18 | 16 | 11 | 15 | 16 | 11 | 8 | 4 | 9 |
| Risk, % | 22 | 12 | 6 | 11 | 11 | 9 | 13 | 16 | 13 | 11 | 6 | 15 |
| Cumulative risk, % | 22 | 32 | 36 | 43 | 49 | 53 | 59 | 66 | 70 | 74 | 75 | 79 |
The risks of myocardial infarction
Data on MI after TIA or stroke were obtained from the LSR and a population study and a systematic review (see Table 61, box 44). 331,332 The risk of MI or cardiovascular death following a TIA or non-haemorrhagic minor stroke has been published by Clark and colleagues315 and was 27.8% at 10 years, which equates to 3.2% per annum. An updated systematic review and meta-analysis of risk of MI and vascular death after TIA and ischaemic stroke found an annual risk of 2.1% (95% CI 1.9% to 2.4%) for non-stroke vascular death, 2.2% (95% CI 1.7% to 2.7%) for total MI, 0.9% (95% CI 0.7% to 1.2%) for non-fatal MI, and 1.1% (95% CI 0.8% to 1.5%) for fatal MI; the time course of risk was linear. 332 In a cohort of patients of ≥ 65 years with first ischaemic stroke, the rates for recurrent stroke and coronary heart disease (CHD) were 105.4/1000 and 59.3/1000, respectively, during the first year of follow-up. After the first year, the stroke rate was 52/1000 and the CHD rate was 46.5/1000. 333 In the Rochester TIA study,331 the average annual incidence of MI after TIA was 1%, double that of the normal population and with higher rates in younger (< 60 years) than older patients. The risk remained constant over time and was an independent predictor of death after TIA (HR 3.11; 95% CI 2.11 to 4.57). Increasing age (HR 1.51 per 10 years; 95% CI 1.14 to 2.01), male sex (HR, 2.19; 95% CI 1.18 to 4.06) and the use of lipid-lowering therapy at the time of TIA (HR 3.10; 95%, 1.20 to 8.00) were independent risk factors for MI after TIA.
We assumed that these patients were taking antiplatelet therapy and BP-lowering medication. The risk for patients not on antiplatelet or antihypertensive treatment was calculated assuming the efficacy of treatment as seen in RCTs (see Efficacy of medical treatment, below). 309–314 We assumed that the fatality rates associated with MI were, for men, 35% for ages 55–64 years, and 48%, 69% and 82% for age bands 65–74 years, 75–84 years and ≥ 85 years, respectively; for women, the corresponding rates were 31%, 55%, 77% and 90%, respectively. These values were calculated by the authors from data reported by Volmink and colleagues. 334 Table 56 shows the time to MI from event by age, extracted from the ESS data report. 111
| Age (years) | Prior to first event, n (%) | Time to first MI, (n) (%) | No MI | All | ||||
|---|---|---|---|---|---|---|---|---|
| ≥ 6 months | < 6 months to 1 year | 13–24 months | 25–36 months | 37 months to 5 years | ||||
| < 55 | 1 | 1 | 208 | 210 | ||||
| 55–64 | 1 | 1 | 1 | 1 | 1 | 1 | 380 | 386 |
| 65–74 | 1 | 5 | 3 | 3 | 2 | 624 | 638 | |
| 75–84 | 6 | 6 | 3 | 4 | 1 | 682 | 702 | |
| ≥ 85 | 5 | 2 | 2 | 3 | 218 | 230 | ||
| All | 2 (0.09%) | 17 (0.78) | 12 (0.55) | 10 (0.46) | 10 (0.46) | 3 (0.14) | 2112 (97) | 2166 |
Effect of treatment
The beneficial effects of medical intervention were incorporated by reducing the risks of stroke and MI in accordance with efficacy values from RCTs and systematic reviews of these treatments (see below and Table 61, boxes 65–70). 309–314
The beneficial effects of surgery on reducing stroke risk in patients with symptomatic carotid stenosis and the possibility of surgical complications were incorporated using the values from RCTs (details given below). 25,329,335–339 For both medical therapy and surgery it was assumed that any effect would begin in the time period in which the intervention was initiated.
Carotid endarterectomy would be offered to most patients with 70–99% symptomatic carotid stenosis (on NASCET criteria)25 and some patients with 50–69% NASCET stenosis. Patients with stenoses of < 50% (NASCET) or patients in whom the carotid artery had occluded would be offered best medical therapy only.
The survey of services for patients with suspected TIA or minor stroke in the UK in 2011 showed that the proportion of patients under secondary prevention treatment, prescribed by their GP/referring doctor after the index event, was between 41% and 60% in 20% of the responses, between 61% and 80% in 24%, and between 81% and 100% in 29% (see Table 61, box 71). That is, an overall average of 75% of patients had already been started on secondary prevention by the referring GP/ED before being seen at the secondary prevention clinic and before any investigations. We tested the effect of initiating secondary prevention treatment by the GP prior to hospital referral compared with at stroke clinic assessment in the model.
The risks associated with carotid endarterectomy
The risks associated with carotid endarterectomy were taken from a systematic review and meta-analyses of symptomatic patients undergoing a carotid endarterectomy. 25 The estimated risk of death was 1.1% and the risk of non-fatal stroke was 6.0%. This review also showed that the risk of surgery was the same for patients with TIA and stroke. This work also showed no increase in mortality or complications with surgery performed early after stable stroke or TIA compared with surgery performed after a time lapse, although the benefit of surgery was greatest when surgery was performed quickly after TIA or stroke (in patients with evolving stroke symptoms the risk of surgery was higher). 171 A more recent meta-analysis showed that the pooled absolute risks of stroke and death after urgent surgery were high in patients with stroke-in-evolution (20.2%, 95% CI 12.0% to 28.4%) and in patients with crescendo TIA (11.4%; 95% CI 6.1% to 16.7%), but without significant difference between early and later surgery in neurologically stable patients with recent TIA or non-disabling stroke (< 1 week compared with ≥ 1 week, OR 1.2, 0.9 to 1.7, p = 0.17; < 2 weeks vs. ≥ 2 weeks, OR 1.2, 0.9 to 1.6, p = 0.13). 340 We assumed that the risk of a non-fatal MI following carotid endarterectomy was 0.6%, using data from Barnett and colleagues335 and Bond and colleagues. 329 The last UK carotid endarterectomy audit (June 2011) found a rate of complication at 30 days of 3% for stroke and death, 0.8% for MI, 4% for bleeding postoperatively and 4% for cranial nerve injury (CNI) (Table 57). 341
| Complication | Stage complication was experienced | National n (%) | |
|---|---|---|---|
| Round 2 | Round 3 | ||
| MI | Inpatient | 48/6983 (0.7) | 40/4971 (0.8) |
| Bleeding | Inpatient | 192/6983 (2.7) | 177/4971 (4) |
| CNI | Inpatient CNI | 136/6983 (1.9) | 96/4971 (2) |
| Overall CNI (inpatient/follow-up) | 135/5503 (2.5) | 184/4971 (4) | |
| TIA | Inpatient | 28/5274 (0.5) | 26/4971 (0.5) |
| Stroke | Inpatient stroke | 75/6983 (1.1) | 104/4971 (2) |
| Stroke at any point by follow-up | 109/6151 (1.8) | 134/4749 (3) | |
| Stroke within 30 days of operation | 83/6135 (1.4) | 124/4749 (3) | |
| Death | Inpatient death | 38/6983 (0.5) | 37/4954 (0.8) |
| Death within 30 days of operation | 50/6151 (0.8) | 39/4742 (0.8) | |
| Stroke/death | Death and/or stroke within 30 days | 112/6135 (1.8) | 139/4749 (3) |
| MI/stroke/death | Inpatient | 128/6983 (1.8) | 150/4954 (3) |
Risks of death through other causes
The risks of death through other causes were taken from UK life tables. 342 These values were adjusted to avoid double counting of deaths through stroke, MI or unsuccessful carotid endarterectomy.
Efficacy of medical treatment
We assumed that following a TIA a patient will be prescribed aspirin and dipyridamole, a BP-lowering drug and a lipid-lowering drug, respectively (see Table 61, boxes 65–70).
Assumed efficacy of aspirin
It was assumed that in the initial 3-month period following a TIA, aspirin produced a 23% RR reduction for stroke (95% CI 13% to 31%). 309 After this period it was assumed that efficacy fell to a 15% RR reduction (95% CI 6% to 33%). 310 Patients already taking aspirin at the time of the TIA were assumed to have a constant 15% risk reduction of stroke throughout the duration of the model. The impact of aspirin on MI was assumed to be constant at 27% (95% CI 12% to 39%) calculated using data for combined antiplatelet therapy from the Antithrombotic Trialists’ (ATT) collaboration. 311 A recent systematic review of antiplatelet therapy for acute ischaemic stroke showed a significant decrease in death or dependency in patients treated with aspirin 160–300 mg daily and started within 48 hours of onset with a maximum follow-up of 6 months (OR 0.95; 95% CI 0.91 to 0.99). For every 1000 patients treated, 13 patients would avoid death or dependency [number needed to treat to benefit (NNTB) 79]. The use of antiplatelet therapy (chiefly aspirin) was associated with a reduction in recurrent ischaemic strokes (OR 0.77; 95% CI 0.68 to 0.86, p < 0.00001, NNTB 140) and increased symptomatic intracranial haemorrhages [OR 1.33; 95% CI 1.1 to 1.62, number needed to treat to harm (NNTH) 574]. 343 In long-term secondary prevention trials, for patients with previous MI or TIA/stroke aspirin provided an absolute risk reduction of serious vascular events (stroke, MI or vascular death) of 1.5% per year (6.69% in aspirin vs. 8.19% in placebo, RR 0.81; 95% CI 0.75 to 0.87, p < 0.00001) and reductions of about one-fifth in total stroke (2.08% vs. 2.54% per year, p = 0.002). For patients with previous TIA/stroke, aspirin provided an absolute risk reduction (ARR) of serious vascular events of 1.28% per year (6.78% in aspirin vs. 8.06% in placebo, RR 0.83; 0.75 to 0.93, p = 0.001) and 0.78% per year in total stroke (3.9% vs. 4.68% per year, RR 0.83; 0.72–0.96, p = 0.01). 344
Assumed efficacy of dipyridamole
It was assumed that the additional use of dipyridamole added a further 10% RR reduction for stroke compared with aspirin alone. 312 A meta-analysis of dipyridamole plus aspirin versus aspirin alone showed a HR of 0.82 (95% CI 0.72 to 0.92) for the composite event of vascular death, non-fatal MI and non-fatal stroke. A proportion of 12.5% of patients in the combined aspirin-plus-dipyridamole group had a primary outcome event compared with 15.2% in the aspirin group, with a mean length of follow-up of 2.6 years. 345
In patients with non-cardioembolic ischaemic stroke or TIA, the use of antiplatelet agents is recommended by the European Stroke Organisation (ESO) and AHA/American Stroke Association (ASA) guidelines100 to reduce the risk of recurrent stroke and other CV events. Combined aspirin and dipyridamole or clopidogrel have been suggested by ESO and SIGN guidelines, as well as aspirin or triflusal (ESO255 and SIGN guidelines103).
The assumed efficacy of blood pressure-lowering drugs
Data on the efficacy of BP-lowering drugs were taken from the Perindopril pROtection aGainst Recurrent Stroke Study (PROGRESS; 2001). 313 The relative reduction in non-fatal stroke was 42% (95% CI 29% to 53%) and the relative reduction in non-fatal MI 42% (95% CI 11% to 62%). Analysing the graphs presented in the PROGRESS trial,313 we assumed that the effects of BP-lowering drugs would not be seen for 6 months (although clearly the drugs would be pharmacologically active as soon as started). A systematic review and meta-regression of RCTs showed that lowering BP or treating hypertension reduced stroke (OR 0.76; 95% CI 0.63 to 0.92), non-fatal stroke (OR 0.79; 0.65 to 0.95), MI (OR 0.79; 0.63 to 0.98) and total vascular events (OR 0.79; 0.66 to 0.95) in patients with prior ischaemic or haemorrhagic stroke or TIA. 346 BP reduction is recommended for both prevention of recurrent stroke and prevention of other vascular events in persons who have had an ischaemic stroke or TIA and are beyond the first 24 hours (AHA/ASA100 and ESO guidelines255), and this benefit extends to patients with or without hypertension. The use of ACE inhibitors and thiazides has been recommended by USA and local clinical guidelines. (AHA/ASA guidelines 2011,100 SIGN guidelines 2008103).
Adverse effects of giving aspirin, dipyridamole and clopidogrel to patients with haemorrhagic stroke were estimated from literature data. In a systematic review assessing the effect of antithrombotic or anticoagulant treatment given to patients with definite intracranial haemorrhage on outcome, for those with any acute intracranial haemorrhage (1997 patients) or just intraparenchymal cerebral haemorrhage (773 patients), the OR for death for those allocated to antiplatelet treatment compared with control was 0.85 (95% CI 0.63 to 1.15) and 0.96 (95% CI 0.62 to 1.50), respectively. For recurrent intracranial haemorrhage, in patients with any acute intracranial haemorrhage or just intraparenchymal cerebral haemorrhage, the OR was 1.00 (95% CI 0.73 to 1.37) and 1.02 (95% CI 0.58 to 1.80) respectively. 26,27 The effect of antiplatelet drugs on death and recurrent intracranial haemorrhage are neutral or favourable for patients with any intracranial haemorrhage, and less favourable for patients with intraparenchymal cerebral haemorrhage; however, the wide CIs cannot rule out modest harm or moderate benefit, and these drugs were not given for more than a few days. A systematic review of recurrent brain haemorrhage after intracranial haemorrhage showed an aggregate rate of recurrent haemorrhagic stroke of 2.4% and 8.8% of death per year. 28 If aspirin was given this would result in a 3.7% (95% CI 2.7% to 3.8%) increase in haemorrhagic stroke and a 12.3% (95% CI 7.3% to 15.4%) increase in death. 26 An observational study of 417 patients who survived to discharge for an intracerebral haemorrhage (ICH) described the level of antiplatelet drugs prescribing after haemorrhagic stroke and the risk of subsequent events. 29 There were 14 recurrent ICHs, two of those were on antiplatelet treatment and all in patients with an initial lobar haemorrhage. HRs associated with antiplatelet exposure were 1.07 (95% CI 0.24 to 4.84) for recurrent ICH and 0.23 (95% CI 0.03 to 1.68) for ischaemic stroke.
The assumed efficacy of lipid-lowering drugs
The efficacy of lipid-lowering drugs has been published by Kaste. 314 The relative reduction in stroke was assumed to be 25% (95% CI 15% to 34%), whereas the reduction in MI was 38% (95% CI 29% to 45%). Data from the Heart Protection Study347 imply that lipid-lowering drugs have a slow onset of effect. Accordingly we have assumed that the effects of lipid-lowering drugs were not realised for 12 months from the commencement of therapy. The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) study included patients who had had stroke or TIA (including 2% of haemorrhagic stroke) within 1–6 months before study entry, and had no known CHD; those patients were assigned to atorvastatin 80 mg (2365 patients) or placebo (2366 patients), with a 5-year absolute reduction in risk of fatal or non-fatal stroke of 2.2% in patients receiving atorvastatin (HR 0.84; 95% CI 0.71 to 0.99, p = 0.03, number needed to treat (NNT) 46) and a 5-year absolute reduction in risk of major cardiovascular events of 3.5% (HR 0.80; 95% CI 0.69 to 0.92, p = 0.002, NNT 29) compared with placebo. 348 There was a small increase in the incidence of haemorrhagic stroke in patients taking atorvastatin (HR 1.68; 95% CI 1.09 to 2.59, p = 0.02); multivariable regression showed that risk was higher in those patients with haemorrhagic stroke as the entry event (HR 5.65; 95% CI 2.82 to 11.30, p < 0.001), in men (HR 1.79; 95% CI 1.13 to 2.84, p = 0.01), with age (10-year increments, HR 1.42; 95% CI 1.16 to 1.74, p = 0.001) and Stage 2 [Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-7)] hypertension at the last study visit before a haemorrhagic stroke (HR 6.19; 95% CI 1.47 to 26.11, p = 0.01). 349 A systematic review (including SPARCL study349) showed that statin therapy had a marginal benefit in reducing subsequent CV events in patients with a previous history of stroke or TIA (OR 0.88; 95% CI 0.77 to 1.00), with a protective effect for ischaemic stroke (OR 0.78; 95% CI 0.67 to 0.92) and an increased risk of haemorrhagic stroke (OR 1.72; 95% CI 1.20 to 2.46). 350 Statin therapy with intensive lipid-lowering effects now is recommended to reduce the risk of stroke and other cardiovascular events after TIA or stroke by USA and local guidelines (AHA/ASA guidelines 2011,100 SIGN 2008103).
Estimating the combined efficacy of aspirin, dipyridamole, blood pressure-lowering drugs and lipid-lowering drugs
Few data are available on the combined efficacy of different classes of drugs. It has been hypothesised that the drugs work independently additively and that a reduction in stroke of 75% can be obtained (Professor R Collins, University of Oxford, 2005, personal communication). 351 Two studies (SOS-TIA122 and EXPRESS24) have reported very low risks of recurrent stroke for patients in whom a secondary prevention treatment (mainly antiplatelet and BP-lowering drugs) was started immediately after confirmed diagnosis of TIA or minor stroke. The EXPRESS study24 assessed the risk of stroke at 90 days before and after implementing a fast-access stroke prevention clinic at which secondary prevention treatment was started immediately after the diagnosis was confirmed. The before-and-after study found that, of 1278 patients presenting with TIA or minor stroke, the 90-day risk of recurrent stroke was 10.3% in phase I [slow: median time to tablets 20 days, interquartile range (IQR) 8–53 days] and 2.1% in phase II (fast: early medical intervention, median time to tablets 1 day). The adjusted HR was 0.20 (95% CI 0.08 to 0.49), independent of age and sex and without any increased hazard of early intervention. 24 The SOS-TIA clinic study assessed the effects of a NV clinic with 24-hour access for patients with symptoms of TIA, with a short standardised clinical assessment followed by initiation of a comprehensive stroke prevention programme. 122 The programme recommended starting therapy at the time of discharge from clinic or to family doctors with a loading dose of 300–500 mg aspirin, therapy to lower BP and blood lipids concentrations with predefined objectives. The recorded 90-day risk of stroke was 1.24% (95% CI 0.72% to 2.12%), whereas the expected risk from the ABCD2 scores was 5.96%. The risk of all stroke and the combined outcome of all stroke, MI and vascular death at 1 year as 1.95 (95% CI 1.26 to 3.00) and 2.54 (95% CI 1.74 to 3.72), respectively. 122 Thus, the 90-day risks of stroke were 1.24% and 2.10%, respectively, in these two before-and-after studies. 24,122
The cumulative benefit that could be obtained by combining multiple approaches for secondary prevention of TIA and stroke has been estimated using data from meta-analyses in secondary prevention trials. The calculated cumulative risk reduction for implementing diet, exercise, aspirin, statins and antihypertensive therapy was estimated in 80%. Using a 5-year major vascular event rate of 24.4% as seen in LiLAC (Dutch TIA trial cohort65), the ARR conferred by combination therapy was 20% (NNT 5) and the residual 5-year of major cardiovascular events after treatment was 5%. At 10 years the ARR, NNT and residual risk for treatment applied for a decade were 35%, 3 and 9%, respectively. 352 An exploratory analysis of the SPARCL study showed that the patients in whom the 1-month reduction in low-density lipoprotein (LDL) levels was higher the median (16%) experienced greater reductions in stroke and major cardiovascular events than those with smaller LDL reductions. 353 At each level of LDL reduction, patients with a 1-month high-density lipoprotein (HDL) above the median (47 mg/dl) had a reduction in the risk of stroke and major cardiovascular events, as did patients with a systolic blood pressure below the median (138 mmHg) compared with those above the median. An optimal control of LDL, triglycerides, BP and HDL had a cumulative effect on reducing stroke and major cardiovascular events, with HRs decreasing as optimal levels of one, two, three or four of these factors were achieved.
We have assumed that the different classes of drugs are independent, and we have used the mean efficacy estimate for aspirin and dipyridamole. In view of the controversy and lack of new direct data from clinical trials comparing effect of multiple treatments but those mentioned above, we have conservatively used the lower 95% CIs for BP- and lipid-lowering drugs. The reduction in stroke and MI assumed for a patient receiving all drugs are given in Table 58. Note that patients already taking aspirin at the time of their TIA or minor stroke are assumed to have less risk reduction from continuing to take aspirin than would a patient starting aspirin de novo – therefore we will apply the data on risk reduction between 3 and 6 months (see Table 58).
| Time since initiation of medical therapy | Reduction in stroke risk (%) | Reduction in MI risk (%) | Drugs assumed to be affecting risks |
|---|---|---|---|
| < 3 monthsa | 33 | 27 | Aspirin and dipyridamole |
| 3–6 months | 25 | 27 | Aspirin and dipyridamole |
| 6 months to 1 year | 47 | 35 | Aspirin, dipyridamole and BP-lowering drugs |
| ≥ 1 year | 55 | 54 | Aspirin, dipyridamole, BP- and lipid-lowering drugs |
As mentioned above, the use of antiplatelet therapy (chiefly aspirin) for acute ischaemic stroke was associated with increased symptomatic intracranial haemorrhages (OR 1.33; 95% CI 1.10 to 1.62, NNTH 574). 343 In long-term secondary prevention trials, the risk of haemorrhagic stroke was increased with aspirin use in patients with previous TIA/stroke (0.32% for aspirin vs. 0.14% for placebo per year: RR 1.90; 95% CI 1.06 to 3.44, p = 0.03). 344 The combination of aspirin plus clopidogrel for long-term secondary prevention of stroke or other vascular events for 18 months is associated with an increase in life-threatening bleeding with respect to aspirin alone (2.6% vs. 1.3%). 354 Recently, the data safety monitoring board of the Secondary Prevention of Small Subcortical Strokes (SPS3) study recommended terminating the dual antiplatelet arm of the study owing to an excess of overall and major non-central nervous system haemorrhagic side effects and total deaths in the patients receiving aspirin plus clopidogrel compared with aspirin. 355
Typical structure of neurovascular (stroke prevention) clinics in the UK
We considered that it was important to base the model on realistic times to first seeing patients after a TIA or minor stroke, investigation, obtaining results, making decisions about treatment and implementing those decisions. There was little reliable information on how long these stages took, or the typical structure of stroke prevention clinics in the UK172,173 and there have been many changes to speed up assessment of patients with TIA/minor stroke in the last 5 years. In a Scottish National Audit (SSCAS) the average delay from last TIA or stroke to assessment in the TIA clinic as 3 days, and 94% of the patients were seen by a specialist within 96 hours of the hotline call in the last Scottish Stroke Care Audit. 173 Therefore, we surveyed stroke prevention clinics and imaging services for stroke in the UK using multiple overlapping ascertainment methods (see Chapter 8).
Data from clinical services
Most (97%) centres in the UK had services for patients with suspected TIA or minor stroke, working at least once a week in 99% of the centres offering this service. On average, patients referred to clinics get an appointment on the same day in 3% of the responses, on the next day in 32%, after 2–3 days in 25%, and between 3 and 7 days later in about 20% of the responses. In about 29% of centres, the patient assessment was nurse led. In approximately 39% of the centres, imaging of the brain or neck arteries took place on the same day as the patient’s appointment, and at the same hospital, after clinical assessment; in 17% it took place on the same day but before clinical assessment, and in 15% imaging was performed the next day. CT was routinely used as brain imaging in 84% of centres, with MRI used in 51%; CT was more often used as initial brain imaging. The majority of centres indicated that results were fed back to the stroke service on the day of the scanning, and positive results (i.e. haemorrhages) were fed back immediately.
Data from imaging services
Patients with suspected TIA/minor stroke usually undergo imaging of the brain and neck arteries before a doctor’s assessment but on the same day in 16% and 19% of responses, on the same day but after assessment in 31% and 47%, after assessment but not more than 1 day later in 17% and 13%, and a mixture in 28% and 20% of responses, respectively. Brain CT was routinely used in 84% of response and brain MR in 51%. When important positive brain imaging results are found, these are fed back immediately to the clinic in 76% of the services. If MRI is required after initial CT, then in 8% of the services the test is performed the same day, in 7% the next day, in 34% within 1 week, in 47% within 1 month and in 3% in > 1 month. DUS is used as the first-line carotid and vertebral imaging investigation in 95% of the services; if DUS is not used as the first-line investigation, CTA is used in 79% of the services, MRA with contrast in 36%, and MRA without contrast in 14%. Important positive results of imaging neck arteries are fed back immediately to the clinic in 86% of the services. Ninety-four per cent of the centres have facilities for CT scanning in their department or directorate for patients with suspected TIA or minor stroke and 82% of them have CT scanning available out of hours. With respect to MRI, 88% of the services have facilities for MRI scanning for patients with suspected TIA/minor stroke and 19% have facilities for MRI scanning available out of hours. The MR sequences usually used are T1/T2 in 95% of the services, DWI in 97%, FLAIR in 89% and GRE susceptibility-weighted imaging in near 50%. For outpatients suspected of TIA/minor stroke during normal working hours, CT is usually performed immediately in 6% of the services, same day in 53%, next day in 8%, within 7 days in 18%, and within 2 weeks in 14%. For these presenting out of hours, CT is usually performed immediately in 12% of centres, 26% the same day, 28% the next day and 23% within 7 days. With respect to MRI, for outpatients with TIA/minor stroke during normal working hours, the test is usually performed immediately in 2% of the centres, same day in 21%, next day in 11%, within 7 days in 30%, within 2 weeks in 26% and within 1 month in 11%. For these patients during outside normal working hours, 9% usually perform the test the same day and 59% of the centres could not perform MRI.
Devising diagnostic algorithms
We considered that individual hospitals might have access to different types of brain imaging tests. In some hospitals only one modality might be available, whereas in others there might be a choice of several. Furthermore, there might be differences in the availability of some tests, so that the choice of which test to use might depend on the balance of time of day, day of the week, accuracy, availability and cost. There are also specific instructions given about timing and use of imaging in NICE and other national guidelines and incentive tariffs offered in parts of the UK which some centres have already adopted. For these reasons, we felt it important to model a number of different usages (algorithms) compared with the reference standard. This would allow individual hospitals to identify the algorithm which most closely resembled their availability of tests, and see how changing to another algorithm might affect their stroke prevention, costs, or QALYs. We therefore devised several algorithms to reflect the commonest patterns of practice and also the investigative pathway suggested in guidelines. These strategies are discussed further in Chapter 11.
Four key scanning strategies for testing
-
Base-case scenario. Scan all patients presenting to NV clinics with CT for brain imaging immediately; ultrasound for carotid imaging. (This includes mimics, but excludes retinal events.)
-
Scan all of those who are MR compatible with MR for brain imaging, immediately (CT for non-MR compatible patients); ultrasound for carotid. (This includes mimics, but excludes retinal events.)
-
Scan all those with ABCD2 score of ≥ 4 (and who are MR compatible) immediately with MR, and the rest within the first week of symptoms with MR (CT for non-MR compatible patients). (This is the DOH National Stroke Strategy Guidance; it does not distinguish and therefore includes mimics, but excludes retinal events.)
-
Exclude probable mimics on clinical grounds (with appropriate use of CT and MR for suspected diagnosis), scan those presenting at > 5 days after symptoms or on warfarin or with uncertain territory or with unusual symptoms (and who are MR compatible) with MR, and the rest with CT. [This is the pragmatic established clinic approach used in stroke prevention clinics led by experienced senior clinicians. ]
Sensitivity analyses
-
Assume that all patients without a visible ischaemic lesion on DWI do not have a TIA/minor stroke and withhold stroke prevention treatment.
-
Use MRA instead of ultrasound in patients undergoing MR.
-
Varying the proportion of mimics.
Effect of delays occurring before each diagnostic test
The availability of each diagnostic procedure was estimated from the results of the Stroke Clinic and Imaging Centres Questionnaires (see Chapter 8). We collated the responses and calculated the modal results. Where the distribution was bimodal we selected the earlier time period. We then translated the modal range into a time duration, assuming that the mid-point of the modal range was the true delay. For example, if the mean answer from the Questionnaire was the range between 1 day and 1 week, we have assumed that the delay would equate to an average of 4 days; if the modal range was between 1 week and 1 month, we have assumed that the delay would be 19 days. Given this methodology, the estimated times to each event are given in Table 59.
| Time to (from index TIA or minor stroke) | Estimated duration since the incident (days) |
|---|---|
| Appointment | 4 |
| Time to initial ultrasound | 4 |
| Time to receiving ultrasound results | 4 |
| Cumulative time to the results from an additional test where test is: | |
| Repeat ultrasound | 4 |
| CTA | 9 |
| MRA | 23 |
| CEMRA | 23 |
| Additional time to endarterectomy following results of the second test | 4 |
The last UK carotid endarterectomy audit (June 2011) showed the median number of days from symptom to operation was 21 (IQR 9–54), with a median number of days from symptom to referral and from referral to operation of six (IQR 2–20) and 12 (IQR 5–31), respectively. The reasons for delays of > 2 weeks between index symptom and surgery were delay in referral (41%), delay in patient presenting at GP or hospital (25%), operation cancellation as unfit or patient choice (18%) and delay in carotid imaging (13%). 341 The survey (see Chapter 8) showed that 60% of patients underwent carotid endarterectomy within a week of deciding to operate. Table 60 shows the delays between symptoms, referral to clinic and surgery in the Scottish Stroke Care Audit. 173
| Carotid surgery | Before hotline | After hotline | p-value |
|---|---|---|---|
| No. patients | 53 | 75 | |
| Median (range) delay from last TIA/stroke to clinic (days) | 19.0 (1–101) | 5.5 (1–54) | < 0.01 |
| Median (range) delay from clinic to surgery (days) | 33.0 (5–101) | 13.0 (2–50) | |
| Median (range) delay from last TIA/stroke to surgery (days) | 58.0 (6–135) | 21.5 (5–82) |
Further details of the model development and values used are provided in Chapter 11.
Table 61 shows parameters for the model.
| Parameters (model variable) | Values | Assumptions/source | ||
|---|---|---|---|---|
| Baseline % (n) | 95% CI | Range | ||
| Stroke and associated risk | ||||
| 1. Proportion of actual CV event in a population with stroke-like symptoms | 60 | Chapter 7 (systematic review of non-CV diagnosis; proportion for patients who attend TIA clinics) | ||
| 50 | 41–60 | Chapter 8. UK stroke services survey (50% of the services) | ||
| 55 | Suggested for the model | |||
| 2. Proportion of patients with final diagnostic of minor stroke among all patients presenting to NV clinics (including mimics) | 30 (1227/4066) | Data from diagnosis in stroke clinics, SSCAS | ||
| 17 (650/3862) | UHL TIA clinic database | |||
| 25 | Suggested for the model | |||
| 3. Proportion of patients with final diagnostic of TIA among all patients presenting to NV clinics (including mimics). | 31.3 (1275/4066) | Data from diagnosis in stroke clinics, SSCAS | ||
| 29 (1137/3862) | UHL TIA clinic database | |||
| 30 | Suggested for the model | |||
| 4. Proportion of haemorrhagic stroke in patients with final diagnosis of minor stroke | 3.7 (75/2018) | ESS data report111 | ||
| 5.0 | Expert opinion | |||
| 1.76 (11/622) | Data from diagnosis in stroke clinics (WGH-SJH) | |||
| 5. Proportion of haemorrhagic stroke in patients with final diagnosis of TIA | 0.86 (17/1970) | UHL TIA clinics (including TIA and minor stroke) | ||
| 0.5 | Suggested for the model | |||
| 6. Proportion of minor ischaemic stroke among all patients with final diagnosis of definite CV event (i.e. mimics excluded) | 42.5 (1227/2882) | Data from diagnosis in stroke clinics, SSCAS | ||
| 33.0 (650/1970) | UHL TIA clinic database | |||
| 37.8 | Suggested for the model | |||
| 7. Proportion of TIA among all patients with final diagnosis of definite CV event (i.e. mimics excluded) | 44.2 (1275/2882) | Data from diagnosis in stroke clinics, SSCAS | ||
| 57.7 (1137/1970) | UHL TIA clinic database | |||
| 51 | Suggested for the model | |||
| 8. Proportion of patients with first carotid territory TIA/minor stroke and 50–99% carotid stenosis | ||||
| TIA | 13.2 (29/220) | Koton and Rothwell, 2007132 (OXVASC) | ||
| 11.5 (41/357) | Marquardt and colleagues, 2009356 (OXVASC) | |||
| 9.2 (157/1713) | Amarenco and colleagues, 2012164 | |||
| 10.0 (42/421) | ESS data report111 (not clear if just carotid territory TIAs) | |||
| 14.1 (163/1151) | Ois and colleagues, 2009185 | |||
| TIA or minor stroke | 9.5 (139/1461) | ESS data report111 (not clear if just for carotid territory symptoms) | ||
| 10 | HTA report 2006 (LSR data) | |||
| 9. Proportions of patients with first TIA/minor stroke and AF: | ||||
| TIA | 14.7 (42/285) | Koton and Rothwell, 2007132 (OXVASC) | ||
| 14.9 (37/248) | Amort and colleagues, 2011156 | |||
| 25.7 (112/435) | Sheehan and colleagues, 2010151 | |||
| TIA or minor stroke | 17.6 (121/689) | Ois and colleagues, 200881 | ||
| 10.1 (72/711) | Selvarajah and colleagues, 2008322 | |||
| Death after stroke or TIA | ||||
| 10. Proportion of patients dying of any cause by 30 days after stroke of all severities | 22 (56/251) | 17 to 28 | Hardie and colleagues, 2005319 (PCSS) | |
| 17 (45/262) | Coull and colleagues, 20049 | |||
| 20 | Suggested for the model | |||
| 11. Proportion of patients dying of any cause by: | ||||
| 30 days after TIA | 3.2 (n = 927) | Kleindorfer 200523 | ||
| 1.34 (n = 612) | Lisabeth and colleagues, 2004126 (Texas, USA) | |||
| 2 | Suggested for the model | |||
| 90 days | 0.4 (3/709) | Selvarajah and colleagues, 2008322 | ||
| 0.9 (4/443) | Sheehan and colleagues, 2010151 | |||
| 1.9 (11/591) | Rothwell and colleagues, 200724 | |||
| 2.6 (45/1707) | Johnston and colleagues, 20008 | |||
| 3.9 (n = 612) | Lisabeth and colleagues, 2004126 | |||
| 5.2 (n = 927) | Kleindorfer and colleagues, 200523 | |||
| Summary estimate | 2.68 (134/4989) | |||
| 12. Proportion of patients dying of any cause by 6 months after stroke of all severities (pro_dth) | 12.2 (156/1270) | ESS data report111 | ||
| 22.6 (1749/7710) | OCSP-LSR-IST1 (Slot and colleagues, 2008 320 ) | |||
| 32 (79/251) | Hardie and colleagues, 2003319 (PCSS) | |||
| 19.6 (510/2603) | Bravata and colleagues, 2003357 | |||
| 13. Proportion of patients dying of any cause by 6 months after TIA (pro_dth) | 8.3 (n = 927) | Kleindorfer and colleagues, 200523 | ||
| 8.1 (87/1079) | Bravata and colleagues, 2003357 | |||
| 8.2 | Suggested for the model | |||
| 14. Proportion of patients dying of any cause by 1 year after stroke of all severities (pro_dth) | 17.6 (224/1270) | ESS data report111 | ||
| 36.0 (89/251) | 30 to 41 | Hardie and colleagues, 2003319 (PCSS) | ||
| 26.4 (688/2603) | Bravata and colleagues, 2003357 | |||
| 27.2 (2103/7710) | OCSP-LSR-IST1 (Slot and colleagues, 2008 320 ) | |||
| 15. Proportion of patients dying of any cause by 1 year after TIA (pro_dth) | 12.3 (n = 927) | Kleindorfer and colleagues, 200523 | ||
| 14.8 (160/1079) | Bravata and colleagues, 2003357 | |||
| 8.1 (15/184) | Dennis and colleagues, 1990358 (OCSP) | |||
| 9.77 (n = 612) | 7.5 to 12.7 | Lisabeth and colleagues, 2004126 (Texas, USA) | ||
| 3.4 (n = 2473) | 2.7 to 4.1 | Van Wijk and colleagues, 200565 (Dutch TIA trial) | ||
| 16. Proportion of patients dying of any cause by 5 years after stroke of all severities (pro_dth) | 27.8 (354/1270) | ESS data report111 | ||
| 59 (149/251) | 52 to 65 | Hardie and colleagues, 2005318 (PCSS) | ||
| 60 (1561/2603) | Bravata and colleagues, 2003357 | |||
| 64.1 (4943/7710) | OCSP-LSR-IST1 (Slot and colleagues, 2008 320 ) | |||
| 17. Proportion of patients dying of any cause by 5 years after TIA (pro_dth) | 19.4 (n = 2473) | 17.9 to 21.0 | Van Wijk and colleagues, 200565 (Dutch TIA trial) | |
| 31.3 (n = 184) | 23.3 to 39.3 | Dennis and colleagues, 1990358 (OCSP) | ||
| 49.6 (535/1079) | Bravata and colleagues, 2003357 (TIA) | |||
| 18. Proportion of patients dying of any cause by 10 years after stroke of all severities (pro_dth) | 79.0 (197/251) | Hardie and colleagues, 2005318 (PCSS) | ||
| 68.3 (368/539) | OCSP cohort (Slot and colleagues, 2008 320 ) | |||
| 19. Proportion of patients dying of any cause by 10 years after TIA (pro_dth) | 42.7 (n = 2473) | 41 to 45 | Van Wijk and colleagues, 200565 (Dutch TIA trial) | |
| 20. Proportion of patients dying of any cause after minor stroke: | ||||
| 7 days | 0.36 (4/1093) | LSR | ||
| 90 days | 1.55 (17/1093) | |||
| 2 years | 6.86 (75/1093) | |||
| 21. Proportion of vascular death in patients with stroke of all severities by: | ||||
| 30 days | 3.3 (72/2166) | ESS data report 111 | ||
| 6 months | 6.0 (131/2166) | |||
| 1 year | 7.8 (168/2166) | |||
| 5 years | 11.2 (244/2166) | |||
| 22. Proportion of vascular death in patients with minor stroke | ||||
| 7 days | 0.18 (2/1093) | LSR | ||
| 90 days | 1.1 (12/1093) | |||
| 2 years | 5.1 (56/1093) | |||
| 23. Proportion of vascular death in patients with TIA by: | ||||
| 7 days | 0.0 (0/828) | LSR | ||
| 3 months | 0.3 (2/711) | Selvarajah and colleagues, 2008322 | ||
| 1.08 (9/828) | LSR | |||
| 1.7 (29/1707) | Johnston and colleagues, 20008 | |||
| 2 years | 3.5 (29/828) | LSR | ||
| 3 years | 19.2 (15/78) | Falke and colleagues, 1989323 | ||
| 10 years | 30.0 (87/290) | Clark and colleagues, 2003315 | ||
| 24. Proportion of recurrent fatal stroke in patients with initial stroke of all severities by: | ||||
| 30 days | 0.3 (n = 655) | 0.0 to 0.7 | Dhamoon and colleagues, 2006324 | |
| 1 year | 1.1 (n = 655) | 0.3 to 2.0 | ||
| 5 years | 2.8 (385/13599) | Lee and colleagues, 2011325 (UK General Practice Research Database) | ||
| 3.7 (n = 655) | 2.1 to 5.4 | Dhamoon and colleagues, 2006324 | ||
| 25. Proportion of recurrent fatal stroke in patients with initial minor strokea | ||||
| 7 days | 0.1 (1/1093) | LSR | ||
| 90 days | 0.64 (7/1093) | |||
| 2 years | 2.3 (25/1093) | |||
| 26. Proportion of recurrent fatal stroke in patients with initial TIA by:a | ||||
| 7 days | 0.0 (0/828) | LSR | ||
| 30 days | 0.17 (3/1679) | Amarenco and colleagues, 2012164 | ||
| 3 months | 0.3 (3/982) | Wasserman and colleagues, 2010153 | ||
| 0.48 (4/828) | LSR | |||
| 2.2 38/1707) | Johnston and colleagues, 20008 | |||
| 2 years | 1.44 (12/808) | LSR | ||
| 10 years | 6.8 (20/290) | Clark and colleagues, 2003315 | ||
| Non-stroke | ||||
| 27. Proportion of non-stroke (all causes) in population with stroke-like symptoms (prevNst) | 40.0 | 23–60 | Chapter 7 (patients who attend TIA clinics) | |
| 50.0 | 41–60 | Chapter 8. UK stroke services survey (50% of the services) | ||
| 45.0 | Suggested for the model | |||
| 28. Proportion of migraine in population with non-stroke diagnosis | 14.4 | 4–33 | Chapter 7 | |
| 29. Proportion of vertigo in population with non-stroke diagnosis after stroke-like symptoms | 4.0 | 1–19 | Chapter 7 | |
| 30. Proportion of psychiatric symptoms in population with non-stroke diagnosis after stroke-like symptoms | 4.0 | 3–10 | Chapter 7 | |
| 31. Proportion of cardiac disorders in population with non-stroke diagnosis after stroke-like symptoms | 8.8 | Chapter 7 (West Glasgow Stroke Registry) | ||
| 32. Proportion of post seizure in population with non-stroke diagnosis after stroke-like symptoms | 5.0 | 3–44 | Chapter 7 | |
| 33. Proportion of primary brain tumours in population with non-stroke diagnosis after stroke-like symptoms | 2.0 | 1–6 | Chapter 7, data from UHL TIA clinics and diagnosis in WGH stroke clinics | |
| 34. Proportion of SDH in population with non-stroke diagnosis after stroke-like symptoms | 0.2 (9/3862) | UHL TIA clinics | ||
| 35. Proportion of subarachnoid haemorrhage in population with non-stroke diagnosis after stroke-like symptoms | 0.05 (2/3862) | UHL TIA clinics | ||
| 36. Proportion of syncope in population with non-stroke diagnosis after stroke-like symptoms | 6.6 | 2–18 | Chapter 7 | |
| 37. Proportion of all deaths in patients with non-stroke diagnosis after stroke-like symptoms (by 24 months) | 6.2 (103/1643) | Cameron and colleagues, 2011271 | ||
| Post scanning and treatment | ||||
| 38. Risk of recurrent stroke in patients with stroke of all severities by: | ||||
| 7 days | 1.8 (30/1709) | Lovett and colleagues, 2004207 | ||
| 30 days | 3.1 (n = 9115) | 1.7 to 4.4 | Mohan and colleagues, 2011168 | |
| 4.2 (72/1709) | Lovett and colleagues, 2004207 | |||
| 90 days | 6.6 (113/1709) | Lovett and colleagues, 2004207 | ||
| 1 year | 11.1 (n = 9115) | 9.0 to 13.3 | Mohan and colleagues, 2011168 | |
| 6.1 (133/2166) | ESS data report111 | |||
| 5 years | 26.4 (n = 9115) | 20.1 to 32.8 | Mohan and colleagues, 2011168 | |
| 9.46 (205/2166) | ESS data report111 | |||
| 10 years | 39.2 (n = 9115) | 27.2 to 51.2 | Mohan and colleagues, 2011168 | |
| 39A. Risk of recurrent stroke in patients with minor ischaemic stroke by: | ||||
| 7 days | 0.36 (4/1093) | LSR | ||
| 4.2 (22/514) | 2.4 to 6.0 | Chandratheva and colleagues, 2011186 | ||
| 90 days | 1.83 (20/1093) | LSR | ||
| 6.3 (50/793) | Rothwell and colleagues, 200724 (EXPRESS study) | |||
| 2 years | 5.76 (63/1093) | LSR | ||
| 39B. Risk of recurrent haemorrhage in patients with haemorrhagic stroke | 6.2% p.a. – community | Bailey and colleagues, 200128 (59% are recurrent haemorrhages, 15% uncertain and 26% ischaemic strokes) | ||
| 4% p.a. – hospital based | ||||
| 40. Risk of recurrent stroke in patients with TIA by: | ||||
| 2 days | 3.1 (290/9433) | Giles and Rothwell, 2007121 | ||
| 7 days | 5.2 (406/7830) | |||
| 30 days | 11.2 (n = 927) | Kleindorfer 200523 | ||
| 3.17 (n = 612) | Lisabeth and colleagues, 2004126 | |||
| 2.6 (26/982) | Wasserman and colleagues, 2010153 (Ottawa ED) | |||
| 4.4 (n = 184) | 1.5 to 7.3 | Dennis and colleagues, 1990358 (OCSP) | ||
| 6.7 (n = 2285) | 5.7 to 7.7 | Hill and colleagues, 2004127 (Alberta ED, Canada) | ||
| 3 months | 3.2 (31/982) | Wasserman and colleagues, 2010153 (Ottawa ED) | ||
| 1.24 (13/1052) | Lavallée and colleagues, 2007122 (SOS-TIA study) | |||
| 8.2 (40/485) | Rothwell and colleagues, 200724 (EXPRESS study) | |||
| 10.5 (180/1707) | Johnston and colleagues, 20008 | |||
| 4.05 (n = 612) | 2.7 to 5.9 | Lisabeth and colleagues, 2004126 | ||
| 9.5 (n = 2285) | 8.3 to 10.7 | Hill and colleagues, 2004127 (Alberta ED, Canada) | ||
| 1 year | 4.7 (n = 2473) | 3.8 to 5.5 | Van Wijk and colleagues, 200565 (Dutch TIA trial cohort) | |
| 11.6 (n = 184) | 6.9 to 16.3 | Dennis and colleagues, 1990358 (OCSP) | ||
| 14.5 (n = 2285) | 12.8 to 16.2 | Hill and colleagues, 2004127 (Alberta ED, Canada) | ||
| 6.62 (n = 612) | 4.8 to 9.1 | Lisabeth and colleagues, 2004126 | ||
| 5 years | 12.0 (n = 2473) | 10.7 to 14.7 | Van Wijk and colleagues, 200565 (Dutch TIA trial) | |
| 29.3 (n = 184) | 21.3 to 37.3 | Dennis and colleagues, 1990358 (OCSP) | ||
| 10 years | 18.4 (n = 2473) | 16.7 to 20.1 | Van Wijk and colleagues, 200565 (Dutch TIA trial) | |
| See Chapter 3 with all studies with risk of recurrent stroke | ||||
| 41. Risk of stroke after TIA (pooled estimate):a | ||||
| At 7 days | 5.2 (974/12332) | 3.9 to 5.9 | Chapter 3 (systematic review of risk of stroke after TIA, random-effects meta-analysis) | |
| At 90 days | 6.7 (1567/19769) | 5.2 to 8.7 | ||
| > 90 days | 11.3 (927/8699) | 7.5 to 16.6 | ||
| 42. Proportion of patients alive and dependent after stroke (pro_stdep) (mRS 3–5):a | ||||
| a. Minor stroke: | ||||
| At 6 months | 18.7 (141/754) | ESS data report111 (including PACS, POCS and LACS) | ||
| 1 year | 23.4 (241/1029) | |||
| 2 years | 21.3 (230/1049) | |||
| 5 years | 19.8 (207/1046) | |||
| b. Stroke of all severities | ||||
| At 6 months | 12.7 (162/1270) | ESS data report111 | ||
| 45.0 (3436/7710) | Slot and colleagues, 2008320 | |||
| 1 year | 22.0 (280/1270) | ESS data report111 | ||
| 2 years | 21.3 (271/1270) | |||
| 5 years | 19.4 (253/1270) | |||
| 43. Proportion of patients alive and independent after stroke (pro_stindep) (mRS 0–2):a | ||||
| a. Minor stroke | ||||
| At 6 months | 70.5 (532/754) | ESS data report (including PACS, POCS and LACS) | ||
| 1 year | 63.2 (651/1029) | |||
| 2 years | 59.6 (625/1049) | |||
| 5 years | 56.0 (591/1046) | |||
| b. Stroke of all severities | ||||
| At 6 months | 45.0 (573/1270) | ESS data report111 | ||
| 33.0 (2525/7710) | Slot and colleagues, 2008320 | |||
| 1 year | 55.8 (709/1270) | ESS data report111 | ||
| 2 years | 53.3 (678/1270) | |||
| 5 years | 50.4 (641/1270) | |||
| 44. Proportion of patients with stroke or TIA who suffered MI after the stroke or TIA (pro_MI)a | ||||
| Annual risk | 0.95 | Burns and colleagues, 2011331 (Rochester, just TIA patients) | ||
| Annual risk total MI | 2.2 | 1.7 to 2.7 | Touze and colleagues, 2005332 (including TIA and ischaemic stroke) | |
| Annual risk for fatal MI | 1.1 | 0.8 to 1.5 | ||
| Cumulative risk for fatal MI | ||||
| 7 days: | ||||
| TIA | 0.0 (0/828) | LSR | ||
| Minor stroke | 0.0 (0/1093) | |||
| 90 days: | ||||
| TIA | 0.12 (1/828) | LSR | ||
| Minor stroke | 0.1 (1/1093) | |||
| First year | 1.3 (29/2166) | ESS data report111 | ||
| 5 years | 2.5 (52/2166) | |||
| 45. Long-term survival after any stroke starting with independent status at 6 months: | Slot and colleagues, 2008320 | |||
| Independent at 6 months | 53 (285) | OCSP cohort (n = 539) | ||
| 56 (1142) | LSR cohort (n = 2054) | |||
| 21 (1098) | IST cohort (n = 5117) | |||
| Alive at: | ||||
| 1 year | 52 (280) | OCSP cohort (n = 539) | ||
| 54 (1101) | LSR cohort (n = 2054) | |||
| 21 (1067) | IST cohort (n = 5117) | |||
| 5 years | 39 (209) | OCSP cohort (n = 539) | ||
| 16 (325) | LSR cohort (n = 2054) | |||
| 14 (696) | IST cohort (n = 5117) | |||
| 9 years | 28 (149) | OCSP cohort (n = 539) | ||
| 0.04 (1) | LSR cohort (n = 2054) | |||
| 0.5 (28) | IST cohort (n = 5117) | |||
| 10 years | 24 (132) | OCSP cohort (n = 539) | ||
| 15 years | 13 (68) | OCSP cohort (n = 539) | ||
| 46. Long-term survival after any stroke starting with dependent status at 6 months: | ||||
| Dependent at 6 months | 29 (154) | OCSP cohort (n = 539) | ||
| 29 (604) | LSR cohort (n = 2054) | |||
| 52 (2678) | IST cohort (n = 5117) | |||
| Alive at: | ||||
| 1 year | 26 (138) | OCSP cohort (n = 539) | ||
| 26 (543) | LSR cohort (n = 2054) | |||
| 48 (2478) | IST cohort (n = 5117) | |||
| 5 years | 13 (69) | OCSP cohort (n = 539) | ||
| 7 (147) | LSR cohort (n = 2054) | |||
| 26 (1321) | IST cohort (n = 5117) | |||
| 9 years | 8 (42) | OCSP cohort (n = 539) | ||
| 0 (0) | LSR cohort (n = 2054) | |||
| 0.3 (19) | IST cohort (n = 5117) | |||
| 10 years | 7 (39) | OCSP cohort (n = 539) | ||
| 15 years | 5 (25) | OCSP cohort (n = 539) | ||
| 47. Functional status at 90 days after intraparenchymal haemorrhage | ||||
| Alive and independent | 26 (162/629) | Rost and colleagues, 2008327 (the FUNC score) | ||
| Alive and dependent | 29 (183/629) | |||
| Death | 45 (284/629) | |||
| 48. Proportion of patients with a stroke or TIA who died from all non-stroke vascular causes | ||||
| Annual risk | 2.1 | 1.9 to 2.4 | Touze and colleagues, 2005332 | |
| 5 years | 6.4 (n = 655) | 4.1 to 8.6 | Dhamoon and colleagues, 2006324 (just stroke, but > 50% were mild) | |
| 49. Treatment in the non-ischaemic stroke group: | ||||
| Patients with haemorrhagic stroke who received antiplatelet drugs | ||||
| Use of antiplatelet drugs in intraparenchymal cerebral haemorrhage: | ||||
| Death: | ||||
| Antiplatelet drugs | 11.3 (45/398) | Wardlaw and colleagues, 200426 HTA report; Keir and colleagues, 200227 | ||
| Control | 11.7 (44/375) | |||
| OR for death | 0.96 | 0.62 to 1.50 | ||
| Recurrent intracranial haemorrhage: | ||||
| Antiplatelet drugs | 6.8 (27/398) | Wardlaw and colleagues, 200426 HTA report; Keir and colleagues, 200227 | ||
| Control | 6.6 (25/375) | |||
| OR for recurrence just in patients with intraparenchymal ICH | 1.02 | 0.58 to 1.80 | Keir and colleagues, 200227 | |
| OR for any recurrent ICH after any spontaneous ICH | 1.0 | 0.73 to 1.37 | Keir and colleagues, 200227 | |
| OR for death or dependency | 0.68 | 0.46 to 1.02 | Keir and colleagues, 200227 | |
| Patients who have a haemorrhagic stroke and who were taking aspirin prior to the haemorrhagic stroke: | ||||
| Death (OR) | 1.27 | 1.10 to 1.47 | Thompson and colleagues, 201030 | |
| Poor functional outcome (death or dependent, mRS score of 4–6) multivariable adjusted | 1.10 | 0.93 to 1.29 | Thompson and colleagues, 201030 | |
| Sensitivity and Specificity for Imaging | ||||
| 50. Proportion of patients with final diagnosis of minor stroke and lesion on DWI | 67 (165/246) | Doubal and colleagues, 2011254 (median time from onset to MR scan: 12 days) | ||
| 71 (109/153) | Makin and colleagues, 2011253 (abstract UK stroke forum 2011) | |||
| 76 (39/51) | Coutts and colleagues, 200592 | |||
| 60 (137/228) | Keir and colleagues, 2004252 | |||
| 69 | Suggested for the model | |||
| 51. Proportion of patients with final diagnosis of TIA and lesion on DWI | 34.3 (3048/9078) | 31.0 to 38.4 | 9–67 | Chapter 6. Systematic review on DWI-positive lesions in TIAs (random-effects meta-analysis) |
| 52. Proportion of patients with final diagnosis of minor stroke and relevant ischaemic lesion on CT | 53.5 (122/228) | Keir and colleagues, 2004252 | ||
| 48.2 (527/1093) | Estimates from LSR data set | |||
| 53. Proportion of patients with final diagnosis of TIA and lesion on CT: | ||||
| Acute lesion | 0.0 (0/438) | Bonifati and colleagues, 2011158 | ||
| 0.0 (0/274) | Sciolla and Melis, 200869 | |||
| 1.2 (8/643) | Lavallée and colleagues, 2007122 | |||
| 1.8 (2/109) | Chatzikonstantinou and colleagues, 2009138 (lesion compatible with TIA symptoms) | |||
| 4.3 (7/161) | Förster and colleagues, 2012251 | |||
| 4.0 (13/322) | Douglas and colleagues, 200335 | |||
| 7.4 (23/310) | Purroy and colleagues, 2010150 | |||
| 8.0 (55/689) | Ois and colleagues, 200881 | |||
| 10.0 (16/160) | Totaro and colleagues, 2010249 | |||
| 11.5 (95/828) | LSR data | |||
| Suggested | 4 | Suggested for the model | ||
| Acute and old lesions | 27 | 8–42 | Giles and colleagues, 2011210 | |
| 54. ABCD2 score of < 4 versus ≥ 4 and risk of stroke in TIA patients: | ||||
| At 7 days: | Chapter 4. For nine studies that provided stroke risk data at both 7 and 90 days | |||
| ≥ 4 | 4.7 | 2.4 to 8.7 | ||
| < 4 | 1.6 | 1.0 to 3.4 | ||
| At 90 days: | ||||
| ≥ 4 | 8.2 | 4.7 to 14.0 | ||
| < 4 | 2.7 | 1.5 to 4.7 | ||
| > 90 days: | ||||
| ≥ 4 | 17.2 | 8.8 to 30.9 | ||
| < 4 | 5.2 | 1 to 28.9 | ||
| 55. Predictive value of ipsilateral lesion on CT for the prediction of ipsilateral major ischaemic stroke (HR + 95% CI) | 1.32 | 0.88 to 1.96 | Rothwell and Warlow, 199960 (patients in the medical arm of the ECST study with 0–99% carotid stenosis) | |
| 56. Visible lesion on brain imaging and risk of recurrent stroke: | ||||
| a. Predictive value of new lesion visible on CT for the prediction of ischaemic stroke (HR + 95% CI) | 2.45 | 1.73 to 3.46 | Chapter 5 . LSR data analysis | |
| b. Visible lesion on DWI and risk of recurrent stroke at 7 days after TIA | ||||
| DWI (+) | 7.1 | 5.5 to 9.1 | Giles and colleagues, 2011 212 | |
| DWI (–) | 0.4 | 0.2 to 0.7 | ||
| OR for recurrent stroke at 7 days in DWI (+) patients | 19.7 | 9.8 to 39.8 | ||
| 57. Predictive value of carotid stenosis in the prediction of ischaemic stroke (HR + 95% CI) | 2.423 | 1.63 to 3.61 | LSR (unadjusted HR, carotid stenosis dichotomised at 70%) | |
| 58. Predictive model for non-fatal disabling or fatal stroke up to 2 years (HR) | Chapter 5. Model derived from the LSR analysis | |||
| Model A: Clinical predictors: | ||||
| Residual neurological signs | 1.818 | 1.2 to 2.6 | p = 0.0023 | |
| Age | 1.031 | 1.0 to 1.04 | p = 0.0004 | |
| Previous stroke | 1.938 | 1.3 to 2.8 | p = 0.001 | |
| Ocular symptoms | 0.449 | 0.1 to 1.0 | p = 0.0606 | |
| Coronary disease | 1.619 | 1.1 to 2.3 | p = 0.0072 | |
| Model B: Clinical predictors plus carotid imaging: | ||||
| Residual neurological signs | 1.834 | 1.2 to 2.6 | p = 0.002 | |
| Carotid stenosis | 1.009 | 1.0 to 1.01 | p < 0.0001 | |
| Age | 1.029 | 1.0 to 1.04 | p = 0.001 | |
| Previous stroke | 1.824 | 1.2 to 2.7 | p = 0.002 | |
| Ocular symptoms | 0.379 | 0.1 to 0.8 | p = 0.023 | |
| Coronary disease | 1.524 | 1.0 to 2.1 | p = 0.019 | |
| Model C: Clinical predictors plus visible infarct: | ||||
| Residual neurological signs | 1.577 | 1.0 to 2.3 | p = 0.0282 | |
| Age | 1.030 | 1.0 to 1.04 | p = 0.0006 | |
| Previous stroke | 1.999 | 1.3 to 2.9 | p = 0.0006 | |
| Ocular symptoms | 0.509 | 0.2 to 1.1 | p = 0.1184 | |
| Coronary disease | 1.648 | 1.1 to 2.3 | p = 0.0054 | |
| Visible infarct | 1.518 | 1.0 to 2.2 | p = 0.0293 | |
| Model D: Clinical predictors plus carotid imaging plus visible infarct: | ||||
| Residual neurological signs | 1.612 | 1.0 to 2.4 | p = 0.0211 | |
| Carotid stenosis | 1.009 | 1.0 to 1.01 | p < 0.0001 | |
| Age | 1.028 | 1.0 to 1.04 | p = 0.0016 | |
| Previous stroke | 1.898 | 1.2 to 2.8 | p = 0.0016 | |
| Ocular symptoms | 0.428 | 0.1 to 1.0 | p = 0.0509 | |
| Coronary disease | 1.552 | 1.0 to 2.2 | p = 0.0146 | |
| Visible infarct | 1.471 | 1.0 to 2.1 | p = 0.0437 | |
| 59. Sensitivity and specificity CT in identifying the relevant symptomatic ischaemic lesion: | ||||
| a. Before 7 days | ||||
| Sensitivity | 0.39 | 0.16 to 0.69 | Brazzelli and colleagues, 200936 (Studies in acute stroke until 12 hours) but hospital admitted and not minor stroke so of limited relevance to our population of interest | |
| Specificity | 1.0 | 0.94 to 1.0 | ||
| 0.98 | Suggested for the model | |||
| b. After 7 days | ||||
| Sensitivity | 0.39 | |||
| Specificity | 0.30 | Suggested for the model | ||
| 60. Sensitivity and specificity CT in identifying haemorrhagic lesions: | ||||
| a. Haemorrhage within 7 days: | ||||
| Sensitivity | 0.99 | Suggested for the model | ||
| Specificity | 0.99 | |||
| b. Between 7 and 21 days: | ||||
| Proportion of patients with minor haemorrhagic stroke and visible haemorrhage on CT | 25 (2/8) | Wardlaw and colleagues, 200426 HTA report (compared with MRI) | ||
| Sensitivity | 0.40 | Suggested for the model | ||
| Specificity | 0.99 | |||
| c. After 21 days | ||||
| Sensitivity | 0.19 | Suggested for the model | ||
| Specificity | 0.55 | |||
| 61. Sensitivity and specificity of MRI scan in minor ischaemic stroke (sen_MRhm): | ||||
| ≤ 7 days: | Expert opinion: Assumes 67% minor stroke are DWI positive < 7 days, drops to 33% at 7–21 days and positive ID of infarct > 21 days of 20% – for further details see Chapter 10 | |||
| Sensitivity | 0.63 | |||
| Specificity | 0.88 | |||
| 7–21 days: | ||||
| Sensitivity | 0.27 | |||
| Specificity | 0.77 | |||
| > 21 days: | ||||
| Sensitivity | 0.10 | |||
| Specificity | 0.66 | |||
| 62. Sensitivity of MRI scan in haemorrhagic stroke of all severities: | ||||
| a. All sequences performed correctly | 0.83 | 0.52 to 0.98 | Chalela and colleagues, 2007:32 this information is of limited reliability – there are no good studies of MR detection of haemorrhage in minor stroke, in which there was a reference standard. Only if include a T2*/GRE sequence | |
| b. T2* missing | 0.50 | Suggested for the model | ||
| 63. Sensitivity and specificity of MR scan in ischaemic stroke of all severities: | ||||
| Sensitivity | 0.99 | 0.23 to 1.00 | Brazzelli and colleagues, 200936 there are no studies on accuracy of MR in stroke clinic population so this estimate is of limited reliability and it was not used in the model | |
| Specificity | 0.92 | 0.83 to 0.97 | ||
| 64. Specificity of MR scan in haemorrhagic stroke of all severities: | 1.00 | 0.95 to 1.00 | Chalela and colleagues, 200732 there are no studies on accuracy of MR in stroke clinic population so this estimate is of very limited reliability, particularly because the Chalela paper32 excluded all non-strokes. Only if include a T2*/GRE sequence and then there are some mimics – suggest reduce specificity slightly as no good data | |
| Suggested | 0.99 | Suggested for the model | ||
| Risk with or without treatment (medical-surgical) | ||||
| 65. Risk of cardiovascular or CV events after TIA/stroke: effect of combined therapies (antiplatelet drugs, statins, BP-lowering drugs): | ||||
| Aspirin and dipyridamole | Wardlaw and colleagues, 2006 HTA carotid imaging report5 | |||
| RR reduction of risk of stroke (%) | ||||
| < 3 months | 33 | |||
| 3–6 months | 25 | |||
| RR reduction in MI risk (%) | ||||
| < 3 months | 27 | |||
| 3–6 months | 27 | |||
| Aspirin, dipyridamole, and BP-lowering drugs | Wardlaw and colleagues, 2006 HTA carotid imaging report5 | |||
| 6–12 months: | ||||
| RR reduction in stroke risk (%) | 47 | |||
| RR reduction in MI risk (%) | 35 | |||
| Aspirin, dipyridamole, BP-lowering drugs and lipid-lowering drugs: 1 year and beyond | Wardlaw and colleagues, 2006 HTA carotid imaging report5 | |||
| RR reduction in stroke risk (%) | 55 | |||
| RR reduction in MI risk (%) | 54 | |||
| 66. Risk of death or dependency (mRS 3–6) after acute stroke (up to 6 months): | ||||
| Aspirin | 44.9 (9285/20647) | Sandercock and colleagues, 2008343 (Cochrane systematic review) | ||
| Control | 46.0 (9529/20644) | |||
| HR for patients treated with aspirin | 0.95 | 0.91 to 0.99 | ||
| 67. Risk of recurrent ischaemic/unknown stroke after acute stroke (up to 6 months): | ||||
| Aspirin | 2.4 (496/20427) | Sandercock and colleagues, 2008343 (Cochrane systematic review) | ||
| Control | 3.1 (636/20423) | |||
| HR for patients treated with aspirin | 0.77 | 0.69 to 0.87 | ||
| 68. Risk of cardiovascular or CV events after TIA/stroke – effect of aspirin: | ||||
| Events per year: | ||||
| Stroke, MI, vascular death | ||||
| Aspirin | 6.78 (698 events) | Baigent and colleagues, 2009344 ATT collaboration | ||
| Adjusted control | 8.06 (814 events) | |||
| Rate ratio | 0.83 | 0.75 to 0.93 | ||
| Any stroke | ||||
| Aspirin | 3.90 (408 events) | Baigent and colleagues, 2009344 ATT collaboration | ||
| Adjusted control | 4.68 (485 events) | |||
| Rate ratio | 0.83 | 0.72 to 0.96 | ||
| Definitely ischaemic stroke | ||||
| Aspirin | 1.32 (138 events) | Baigent and colleagues, 2009344 ATT collaboration | ||
| Adjusted control | 1.66 (172 events) | |||
| Rate ratio | 0.79 | 0.62 to 1.00 | ||
| Probably ischaemic stroke | ||||
| Aspirin | 3.58 (375 events) | Baigent and colleagues, 2009344 ATT collaboration | ||
| Adjusted control | 4.53 (470 events) | |||
| Rate ratio | 0.78 | 0.68 to 0.91 | ||
| 69. Risk of cardiovascular or CV events after TIA/stroke: effect of BP and lipid-lowering drugs | ||||
| Stroke recurrence | ||||
| BP-lowering drugs | 8.9 (689/7779) | Rashid and colleagues, 2003346 | ||
| Controls | 11.5 (888/7748) | |||
| OR | 0.76 | 0.63 to 0.92 | ||
| Statins | 12.1 (553/4579) | Manktelow and Potter, 2009350 | ||
| Controls | 10.8 (501/4645) | |||
| OR | 0.88 | 0.77 to 1.00 | ||
| MI | ||||
| BP-lowering drugs | 3.2 (244/7729) | Rashid and colleagues, 2003346 | ||
| Controls | 4.0 (311/7699) | |||
| OR | 0.79 | 0.63 to 0.98 | ||
| Major cardiovascular event (stroke, MI, vascular death) | ||||
| BP-lowering drugs | 12.8 (993/7729) | Rashid and colleagues, 2003346 | ||
| Controls | 16.0 (1232/7699) | |||
| OR | 0.79 | 0.66 to 0.95 | ||
| Statins | 22.7 (959/4209) | Manktelow and Potter, 2009350 | ||
| Controls | 28.4 (1192/4194) | |||
| OR | 0.74 | 0.67 to 0.82 | ||
| 70. Risk of ipsilateral stroke after TIA/minor stroke in presence of carotid stenosis, under medical treatment in patients who did not undergo carotid endarterectomy: | ||||
| 7-day risk, previous TIA/minor stroke: | Wardlaw and colleagues, 2006 HTA carotid imaging report5 | |||
| 50–69% stenosis | 8 | |||
| 70–99% stenosis | 13 | |||
| 30-day risk, previous TIA/minor stroke: | Wardlaw and colleagues, 2006 HTA carotid imaging report5 | |||
| 50–69% stenosis | 12 | |||
| 70–99% stenosis | 19 | |||
| 90-day risk, previous TIA/minor stroke: | Wardlaw and colleagues, 2006 HTA carotid imaging report5 | |||
| 50–69% stenosis | 18 | |||
| 70–99% stenosis | 29 | |||
| 15 months’ risk, previous TIA/minor stroke: | Wardlaw and colleagues, 2006 HTA carotid imaging report5 | |||
| 50–69% stenosis | 25 | |||
| 70–99% stenosis | 39 | |||
| 27 months’ risk, previous TIA/minor stroke: | Wardlaw and colleagues, 2006 HTA carotid imaging report5 | |||
| 50–69% stenosis | 30 | |||
| 70–99% stenosis | 45 | |||
| 39 months’ risk, previous TIA/minor stroke: | Wardlaw and colleagues, 2006 HTA carotid imaging report5 | |||
| 50–69% stenosis | 33 | |||
| 70–99% stenosis | 48 | |||
| 71. Proportion of patients already under medical treatment – summary estimates: | ||||
| Antiplatelet drugs | 37 (1981/5334) | Rothwell and colleagues, 200724 EXPRESS; Lavallée and colleagues, 2007122 SOS-TIA study; Wasserman and colleagues, 2010;153 Hannon and colleagues, 2010;359 Johnston and colleagues, 2000;8 Karunarante and colleagues, 1999266 | ||
| BP-lowering drugs | 45 (641/1440) | Rothwell and colleagues, 200724 EXPRESS; Hannon and colleagues, 2010;359 Wasserman and colleagues, 2010153 | ||
| Statins | 25 (674/2718) | Hannon and colleagues, 2010;359 Wasserman and colleagues, 2010153 | ||
| 72. Summary table with values for different outcomes after TIA/minor ischaemic strokeb | ||||
| Values for different outcomes after TIA-minor ischaemic stroke [%/(n)]: | ||||
| 7 days | 90 days | 1 year | ||
| Recurrence free | 94.65 | 92.35 | 81.80 | |
| Alive and independent | 3.35 | 4.00 | 5.90 | |
| Alive and dependent | 1.80 | 2.10 | 3.20 | |
| MI: alive | 0.00 | 0.05 | 0.90 | |
| Death: | ||||
| a. Recurrent stroke that is fatal | 0.05 (1/1921) | 0.57 (11/1921) | 2.3 | |
| b. Recurrent MI that is fatal | 0 (0/1921) | 0.1 (2/1921) | 1.0 | |
| c. Other fatal events | 0.15 (3/1921) | 0.83 (16/1921) | 4.9 | |
| All deaths (a + b + c) | 0.2 (4/1921) | 1.5 (29/1921) | 8.2 | |
| Total | 100 | 100 | 100.0 | |
Chapter 11 Development of the economic model
Introduction
Our review of cost-effectiveness studies (see Chapter 9) demonstrates that no previous research has been conducted to estimate the cost-effectiveness of MR including DWI and other relevant sequences in TIA/minor stroke. Most studies of cost-effectiveness of imaging in stroke have either concentrated on hyper acute disabling stroke or on carotid imaging for carotid stenosis as part of carotid endarterectomy. There are no clinical trials of diagnostic accuracy or effect on prediction of prognosis of MR including DWI and other relevant sequences.
Cost-effectiveness is an important criterion to address as TIA and minor stroke are so common that routine use of MR including DWI and other relevant sequences for diagnosis could be associated with additional resources and have a high impact on NHS budgets. Taking into account only the direct costs to the NHS associated with each scan, the budget impact could total £16M per year to scan only the 50% of TIA patients suggested in recent UK guidelines (i.e. not including the minor strokes and all of the TIA mimics). 360 However, if MR is more accurate in diagnosing TIA and minor stroke than CT, then it is possible that such levels of expenditure would lead to fewer strokes in future years, thereby generating some cost savings. An estimate at 2001–2 prices suggested that, for every patient who has a stroke, the cost to the NHS is £15,206 over a 5-year period (at 2009–10 prices, this would equate to £17,850). The estimates also suggest that, with informal care added, costs would total £29,405 (£34,292 at 2009–10 prices). 361
The current lack of a good evidence base means that there is considerable uncertainty over whether the potential diagnostic and prognostic advantages of MR outweigh any disadvantages. The potential benefits of MR, including DWI and other relevant sequences, include possible improvement in knowledge regarding the cause of stroke and therefore changes in treatment, more efficient patient selection for medical secondary prevention in patients with a proven diagnosis, the avoidance of unnecessary treatment in patients in whom acute ischaemic TIA/minor stroke was more reliably excluded, and better use of carotid endarterectomy.
The disadvantages include the additional expenditure, the current limited availability of the equipment and the resulting possibility that additional strokes might occur during the wait for a scan, the failure to make a positive diagnosis of an ischaemic lesion in up to 66% of TIA patients, and uncertain accuracy for other stroke-related diagnoses. Further, any strategy that recommends MR over CT would probably have to take into account subsequent increased MR usage, and such increased use would most likely lead to greater waiting time and delays to access MR, if no additional resources were allocated to hospitals.
It is clear then that there is a need to examine – using the best available evidence – the cost-effectiveness of MR including DWI and other relevant sequence usage, relative to existing stroke prevention strategies currently in use, for example CT. This is considered here through the development of an economic model that incorporates current knowledge regarding effectiveness and costs of the competing diagnostic strategies.
Methods
The research methods used are guided by the research questions that were previously outlined in the original study protocol, shown below:
-
Is MR including DWI, as well as other relevant structural and blood-sensitive sequences, cost-effective for the diagnosis and secondary prevention in the majority of patients with TIA or minor stroke compared with the current alternative of CT brain scanning?
-
What is the cost and cost-effectiveness of increased use of MR including DWI and blood-sensitive sequences in patients presenting at > 5 days after TIA/minor stroke when CT will not be able to identify haemorrhage as the cause of stroke reliably?
-
If ‘one-stop’ brain and carotid imaging is more cost-effective than individual separate brain and carotid examinations, in what proportion of patients could a ‘one-stop’ approach be used, and what are the practical and cost implications?
Economic modelling
A decision-analytic model was constructed to examine the cost-effectiveness of imaging using MRI compared with CT for the diagnosis and secondary prevention of stroke in patients with suspected TIA or minor stroke. The initial diagnostic pathways were modelled using a decision tree, whereas Markov models at the decision tree end nodes were used to simulate ongoing treatment costs and risks of recurrent ischaemic stroke, MI and haemorrhagic stroke or death over time. Risk of recurrent stroke was determined by a number of factors including type of initial event, ABCD score, degree of carotid stenosis, and appropriate diagnosis and treatment. The model incorporated costs associated with diagnosis and subsequent implementation of secondary prevention, as well as costs associated with recurrent stroke and MI. Health state utilities were assigned to the health states used to model recurrent stroke and MI, allowing both cumulative costs and QALYs to be estimated for a cohort of men and women aged 70 years (approximating the median age of patients presenting at stroke clinics). The model was initially run over a time horizon of 20 years (at which point ≈80% of the modelled cohort were dead) owing to limited availability of data to inform longer-term stroke risks and survival. However, we also carried out sensitivity analysis to assess the impact of running the model for 30 years in scenarios in which cost-effectiveness was potentially sensitive to the time horizon.
Important features of the economic model relate to the tree structure used to represent the care pathways for patients that follow from use of the alternative diagnostic and management strategies, the resulting health states that develop from these pathways, the probabilities associated with entering and exiting different states of health from these pathways, and the assumptions that govern patient transitions between different health states over time.
Diagnostic pathways
An outline schematic of the first part of the model is shown in Figure 66. The population cohort enters the model following arrival at the stroke clinic with a suspected minor stroke or TIA. Patients are initially triaged based on clinical assessment into those with a probable TIA, those with a probable minor stroke and probable mimics. At the next stage, using data from the evidence synthesis and expert opinion, the groups are further categorised according to the proportion of patients with a high ABCD score (ABCD > 4) and low ABCD score. Following this, the groups are subdivided again into true minor stroke, true TIA or true mimic, based on the combined findings of clinical assessment and subsequent diagnostic findings. The combined proportions to this point act as the reference standard for the model, and define the overall prevalence of different types of event by initial clinical assessment and ABCD score. These proportions do not vary between the diagnostic arms.
FIGURE 66.
Modelled diagnostic pathways for the management of minor stroke and TIA.
As shown in the model (see Figure 66), a small proportion (5%) of patients initially assessed as probable minor strokes turn out to be true mimics, and a small proportion of probable mimics (5%) turn out to be stroke or TIA. For the minor stroke and TIA groups, we also know from epidemiological prevalence data that a certain proportion of patients in fact turn out to have had a minor haemorrhagic stroke rather than an ischaemic stroke or TIA.
Following determination of the proportions of different types of stroke event, estimated diagnostic accuracy parameters of CT and MR are applied to determine the diagnostic outcome by type of event and time of presentation in the alternative arms of the model. For true mimics, the specificity of CT or MR is applied in the corresponding arms of the model to determine the proportion of patients correctly excluded as not having had a TIA or minor stroke. Based on consideration of the evidence and clinical opinion, it was felt reasonable to assume that these proportions would not differ significantly between the CT and MR arms. Thus a simplifying assumption was made that mimics would not affect the incremental comparison between MR and CT for diagnosing stroke, over and above their initial impact on scanning costs. As a result, downstream costs and subsequent events were not explicitly modelled for mimics. Instead, this group was subsequently modelled to cycle in a simple Markov model, exposed to the age-specific risk of all-cause mortality obtained from UK life tables. 361
For those patients with a true minor ischaemic stroke or TIA, a proportion of each of these groups were modelled to have an ischaemic lesion identified on MRI or CT, based on best available estimates for these parameters (see Table 61, boxes 59–62). 26,32,36 It was also assumed that ultrasound would be used in conjunction with both CT and MRI to pick up patients with moderate to high levels of carotid stenosis. For those patients with a haemorrhagic stroke, estimates of the sensitivity of CT or MR for haemorrhagic stroke were applied to determine the proportions of patients that would be correctly identified using the alternative imaging modalities.
In the CT arm, patients identified as having a visible ischaemic lesion (on CT) were confirmed as true-positive ischaemic strokes and treated appropriately. Those patients considered by the clinician to have a true TIA or minor ischaemic stroke, but no visible lesion detected by CT, were also modelled to receive secondary prevention for ischaemic stroke (antiplatelet drugs, statins, antihypertensive medication) based on the findings of clinical assessment and the assumption that CT findings would not rule out an ischaemic stroke in this group of patients. In the MR arm of the model, we also assumed that treatment decisions would follow the same pattern as described above for CT in the first instance. However, we also modelled scenarios in which treatment for ischaemic stroke in the MR arm was implemented only for those patients identified as having a visible ischaemic lesion on DWI.
Under the above initial specification, any patients with an undetected haemorrhagic stroke were also modelled to receive antiplatelet medication, putting them at a slightly elevated risk of suffering a recurrent haemorrhagic stroke (see Chapter 10).
In the initial base-case analysis, we compared a strategy of immediate CT plus ultrasound compared with immediate MR plus ultrasound in an immediate presenting cohort. In subsequent analyses we also assessed the impact of modelling the cost-effectiveness of immediate CT plus ultrasound compared with immediate MR plus ultrasound in later-presenting cohorts, and considered various delayed MR/ultrasound imaging strategies for selected groups of patients compared with immediate CT plus ultrasound for all. This was done to ascertain the potential impact of a MRI policy on waiting times to diagnosis and treatment. The probability parameters used in the diagnostic component of the model are presented in Table 62, along with their data sources.
| Variable/description | Value | 95% CI or estimate range high | Source |
|---|---|---|---|
| Proportion of patients classified as probable minor stroke, based on clinical assessment | 0.250 | 0.170 to 0.300 | Scottish stroke care audit; UHL TIA clinic database (see Table 61, box 2) |
| Proportion of patients classified as probable TIA, based on clinical assessment | 0.300 | 0.290 to 0.320 | Scottish stroke care audit; UHL TIA clinic database (see Table 61, box 3) |
| Proportion of patients classified as probable mimics, based on clinical assessment | 0.450 | 0.400 to 0.600 | Systematic review of non-CV diagnosis; UK stroke services survey (see Table 61, box 27) |
| Proportion of suspected minor stroke and TIA patients with low ABCD2 score | 0.580 | 0.550 to 0.650 | Amarenco and colleagues, 2009180 and other literature, Chapter 4 |
| Proportion of probable mimics with low ABCD score | 0.650 | 0.600 to 0.700 | Josephson and colleagues, 2008;135 Sheehan and colleagues, 2009,183 see Chapter 4 |
| Proportion of patients with probable minor stroke ultimately identified as true minor stroke | 0.950 | 0.400 to 0.980 | Clinic audit data, literature review – see Chapter 7; expert opinion |
| Proportion of patients with probable TIA ultimately identified as true minor TIA | 0.950 | 0.400 to 0.980 | As for minor stroke |
| Proportion of patients with a true minor stroke with final diagnosis of haemorrhagic stroke | 0.037 | 0.018 to 0.050 | ESS111 (see Table 61, box 4); previous HTA reports |
| Proportion of patients with final diagnosis of TIA with minor haemorrhagic stroke | 0.0050 | 0.0050 to 0.0086 | UHL TIA clinic database (see Table 61, box 5), literature |
| Specificity of CT (for excluding mimics) | 0.98 | Brazzelli and colleagues, 2009;36 Wardlaw and colleagues, 2004;26 Chapter 10, (see Table 61, boxes 59 and 60) | |
| Specificity of MR (for excluding mimics) | 0.99 | 0.95 to 1.00 | Chalela and colleagues, 2007;32 Chapter 10 (see Table 61, boxes 61 and 64) (assumed clinical assessment would prevent treatment of false-positive lesions) |
| Sensitivity of CT for ischaemic stroke | 0.39 | 0.16 to 0.69 | Brazzelli and colleagues, 2009;36 Chapter 10 (see Table 61, box 59) |
| Sensitivity of MR for ischaemic stroke: | Chapters 6 and 10, literature, expert opinion (see Table 61, box 61) | ||
| < 7 days | 0.63 | ||
| 7–21 days | 0.27 | ||
| > 21 days | 0.10 | ||
| Sensitivity of CT for haemorrhagic stroke: | Wardlaw and colleagues, 200426 (see Table 61, box 60) | ||
| < 7 days | 0.99 | ||
| 7–21 days | 0.40 | ||
| > 21 days | 0.19 | ||
| Sensitivity of MR for haemorrhagic stroke | 0.83 | 0.52 to 0.98 | Chalela and colleagues, 2007;32 Chapter 10 (see Table 61, box 62); assumes T2*/GRE sequences |
Modelling longer-term outcomes
After their movement through the diagnostic branches of the decision tree, the long-term outcomes for simulated patients were modelled using one of three Markov models: one for ischaemic stroke that accounted for level of carotid stenosis, one for haemorrhagic stroke and one for mimics. In all three it was assumed that all patients would start in a recurrence-free state. The models then cycled on a variable cycle length to simulate stroke recurrence over time, accounting for time since the initial event, ABCD score and level of carotid stenosis.
Group with no stenosis
For patients with a TIA or minor ischaemic stroke (without carotid stenosis), longer-term outcomes were modelled using four basic health states: ‘recurrence free’, ‘alive following a MI’, ‘alive and independent’ (following a recurrent stroke), ‘alive and dependent’ (following a recurrent stroke) and death (Figure 67). Within each cycle of the model, patients in the recurrence-free state could either have a recurrent stroke (with an associated risk of death), have a MI (again with an associated risk of death), or die from other causes (based on UK life table data). The risk of stroke was based on data derived from the systematic review meta-analysis and other available literature (see Table 61), and was estimated by ABCD score (< 4; ≥ 4) and time since initial TIA or minor stroke (see Chapter 4). Upon correct diagnosis, cycle risk estimates were adjusted downwards by converting them to average rates and then applying RR estimates associated with the implementation of appropriate secondary prevention5 before converting them back to risks. In estimating the cycle risks of stroke for patients with normal stenosis, the cumulative risks by ABCD score were adjusted downwards from those reported in Table 63, to account for the fact that proportions of the cohorts, upon which these estimates were based, had tight stenosis incurring higher risks (see below).
FIGURE 67.
Markov model for ischaemic stroke.
| Time from initial event | ABCD score | Stenosis level | 70–99% | |
|---|---|---|---|---|
| < 4 | ≥ 4 | 50–69% | ||
| 7 days | 0.016 | 0.047 | 0.08 | 0.13 |
| 1 month | – | – | 0.12 | 0.19 |
| 3 months | 0.027 | 0.082 | 0.18 | 0.29 |
| 1 year | 0.052 | 0.172 | – | – |
| 1.25 years | – | – | 0.25 | 0.39 |
| 2.25 years | – | – | 0.3 | 0.45 |
| 3.25 years | – | – | 0.33 | 0.48 |
A proportion of recurrent strokes were modelled to be fatal (within 30 days), whereas the remainder were modelled to progress to one of the survivor states (‘alive and independent’ or ‘alive and dependent’). The proportions moving to each of these states were determined from the estimated relative proportions of patients experiencing each of these outcomes at 1 year after an initial TIA or minor stroke, obtained from the systematic review (see Table 61, boxes 10 and 72). 9,318 Alternatively, patients in the recurrence-free state could experience a MI following their TIA or minor stroke (see Table 61, box 44),331,332 and again a proportion of these events were modelled to be fatal, with the remainder transiting to the ‘alive following a MI’ state for the next cycle of the model.
The Markov model was constructed to work on a variable cycle length: on a weekly basis for the first four cycles then on a monthly basis thereafter. This was done to enable modelling of the elevated risk of stroke immediately after TIA of MR, which then attenuates over time. A simplifying assumption of the model was that for those patients experiencing a stroke (fatal or otherwise) the expected costs associated with acute phase were all incurred in the model cycle in which the event occurred. Further, for fatal strokes, it was assumed that these patients would receive zero QALYs from the time of event until actual time of death.
Patients transiting to the ‘alive following a MI’ state, were modelled to remain at risk of stroke based on the same risk factors described above, and they were also exposed to an inflated risk of non-stroke related mortality, based on UK life tables362,363 with stroke deaths removed364 and a standardised mortality ratio of 2.68 associated with previous MI. 365 Patients in the ‘alive and independent’ and ‘alive and dependent’ states following a recurrent stroke were modelled to be at an inflated risk of death from all causes, based on UK life tables and the standardised mortality ratio of 2.72 associated with previous stroke. 366 Although further stroke deaths were not explicitly modelled for these patients, we applied the risk of recurrent stroke for patients in the ‘alive and independent’ state, based on risk factors and time since previous event, and used this to model the expected transitions form ‘alive and independent’ to ‘alive and dependent’ over time.
Group with tight stenosis
A proportion of the cohort presenting with a TIA or minor ischaemic stroke were also modelled to have moderate (50–69%) or high (70–99%) levels of carotid stenosis. Overall, 10% of patients with a low ABCD score and TIA or minor ischaemic stroke were modelled to fall into one of these categories, compared with 14% of patients with a TIA/minor stroke and high ABCD score. 132,180 These patients were assumed to be split between the high and moderate stenosis states in a ratio of 6 : 4. 5
The recurrent stroke risk for this group of patients was modelled to be significantly higher (pre-endarterectomy) as compared with patients with normal stenosis (see Table 63). 5 Separate model states (see Figure 67) mirroring the states for patients with normal levels of stenosis were used for this purpose. Within the model patients with tight stenosis were modelled to receive an endarterectomy upon stage of diagnosis, which, if successful, reduced their risk of subsequent stroke to 2.3% per year. 5 However, the procedure itself was modelled to bring with it 1.1% mortality risk and a 6.0% risk of non-fatal stroke. 5 The cumulative risks of recurrent stroke – by time, ABCD score and level of stenosis – that were used to derive transition probabilities to populate the Markov model for patients with a TIA or minor ischaemic stroke, are presented in Table 63. Note that the estimated risks for patients with low and high ABCD scores but normal stenosis were adjusted downwards to reflect the impact of removing the modelled 10% and 14% (respectively) of patients with moderate/high levels of stenosis within these groups.
Group with haemorrhagic stroke
Figure 68 shows the structure of the model for the group classified as having ‘true haemorrhagic stroke’. These patients again start the model in the recurrence-free state, and face the risk of a recurrent stroke (haemorrhagic or ischaemic) or death due to other causes. Those who experience a recurrent haemorrhagic stroke can move to one of three health states: ‘alive and independent’, ‘alive and dependent’ or dead as a result of fatal haemorrhagic stroke. The risks here were again based on data from the systematic review (see Chapter 10, Table 61, boxes 39 and 47). 28,21 Modelling here accounts for the possibility of MRI or CT missing some true cases of haemorrhagic stroke. In this situation patients are modelled to receive secondary prevention for ischaemic stroke, including with antiplatelet drugs, which slightly increases their risk of a recurrent haemorrhagic stroke. In the base-case analysis the RR was assumed, based on available data, to be 1.15, and this estimate was subjected to sensitivity analysis.
FIGURE 68.
Markov model for haemorrhagic stroke.
Group with a stroke mimic
Figure 69 describes the pathways for those individuals identified as having had a stroke mimic. As mentioned previously, the evidence review identified no clear evidence suggesting that the sensitivity and specificity of CT and MRI for detecting different types of mimic would vary significantly. Thus, no additional direct health benefit, associated with the use of one diagnostic technique over the other, was modelled in this subgroup of the patient population. Modelling for this group was restricted to the application of all-cause mortality, although the direct costs associated with imaging were included.
FIGURE 69.
Markov model for stroke mimics.
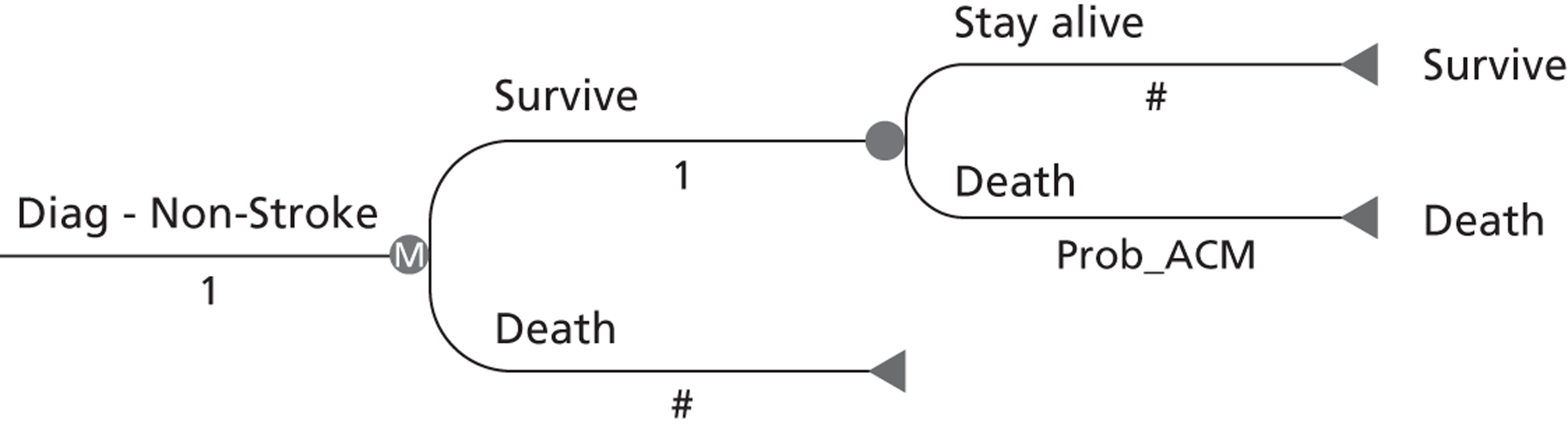
Strategies to be compared
Nine key scanning strategies were assessed in the modelling exercise. In the base-case analysis we compared a strategy of MRI plus ultrasound for all versus CT plus ultrasound for all in a cohort of patients presenting within a few hours of the initial event. Further scenarios assess were devised to assess the cost-effectiveness of various delayed MRI imaging strategies and immediate imaging strategies in later presenting cohorts:
-
Base-case scenario: Immediate MR for brain imaging plus immediate ultrasound for carotid imaging compared with immediate CT for brain imaging plus immediate ultrasound for carotid imaging in an immediate presenting cohort (within the first few hours of the event).
-
Delayed MRI and delayed ultrasound for those with ABCD score of < 4 (otherwise immediate MRI plus immediate ultrasound) compared with immediate CT plus immediate ultrasound for an immediate presenting cohort.
-
Immediate ultrasound for all, but delayed MRI for those with an ABCD score of < 4 and immediate MRI for those with an ABCD score of ≥ 4 compared with immediate CT plus immediate ultrasound for an immediate presenting cohort.
-
Immediate MR for brain imaging plus immediate ultrasound for carotid imaging compared with immediate MR for brain imaging plus immediate MRA for carotid imaging compared with immediate CT for brain imaging plus immediate ultrasound for carotid imaging compared with immediate CT for brain imaging plus CTA for carotid imaging (in immediate presenting cohort).
-
Repeat of scenario 1 but with secondary prevention for ischaemic stroke implemented in only the MRI arm for those with a visible lesion on MR brain imaging (immediate presenting cohort).
-
Secondary prevention implemented for all but MRI and ultrasound delayed for those with a low ABCD score (immediate presenting cohort).
-
Scenario 1 repeated for a cohort of patients presenting 7 days after the initial event.
-
Scenario 1 repeated for a cohort of patients presenting 21 days after the initial event.
-
Scenario 3 repeated for a cohort of patients presenting 7 days after the initial event.
Key assumptions of the modelling exercise
The following key assumptions apply to all component parts of the model:
-
The time horizon of the model is 20 years. All patients entering the model are 70 years of age. The total number of model cycles is 243; the first four cycles are weekly and the remainder are monthly, giving 15 cycles in the first year, and 228 over the remaining 19 years. The impact of adopting a 30-year time horizon is also explored through sensitivity analysis.
-
Patients remain recurrence free within the first 24 hours after imaging.
-
Baseline risks of stroke in the model are assumed to represent risks in patients not being treated with statins and antiplatelet drugs.
-
It is assumed that all patients who enter the model prior to imaging are not being treated with statins and antiplatelet drugs. After imaging the RR of recurrence is lower for those patients treated with these drugs.
-
The risk of stroke recurrence is initially high [particularly in the first 7 days but then the risk attenuates over time (see Table 63)]. In the longer term, we assume a constant risk of stroke as per the risk for the last observed interval.
-
The utility of the health outcomes after TIA, minor stroke, stroke and MI can be appropriately measured with six distinct health states: (1) good health with no further stroke recurrence after treatment (secondary prevention successful); (2) alive and independent after stroke recurrence; (3) alive and dependent after stroke recurrence, (4) alive after MI; (5) alive after being diagnosed as a non-stroke (no subsequent stroke events explicitly modelled); and (6) death, either due to fatal stroke or fatal MI or other causes.
-
In the base case it is assumed that patients present to the NV clinic for assessment within a few hours of symptom onset (cost-effectiveness in later presenting cohorts is also modelled).
-
The use of CT or MRI within the diagnostic pathway does not differentially influence the proportion of patients with stroke mimics that receive the correct diagnosis, and as such subsequent treatment and outcomes for these patients is assumed to be the same between the MRI and CT arms of the model.
-
Secondary prevention for ischaemic stroke is implemented after imaging unless a final diagnosis of haemorrhagic stroke or mimic is received.
Data requirements
The prevalence and epidemiological data that are used in the model were discussed earlier in the report and the sections above. This section presents studies that are relevant for the assumptions made regarding the costs of imaging, the length of hospital stay associated with recurrent stroke and MI, the costs per bed-day, the costs of treatment, and the utility of health states.
Cost-analysis studies
Luengo-Fernandez and colleagues3 conducted a review of the costs of stroke using patient-level data. They considered the cost of CV diseases within developed country settings. A total of 120 studies were reviewed; these included studies that estimated the costs for stroke and ischaemic stroke, TIA, subarachnoid haemorrhage and cardiovascular diseases (CVDs). Costs were subdivided into direct medical costs, direct non-medical costs and productivity costs. The direct medical costs included cost of diagnostics, inpatient care, outpatient care, day care, community health care and medication. The direct non-medical costs included social care, social benefits, travel costs and out-of-pocket care. The productivity costs included loss of productivity owing to illness, death and caregiving. Most of the studies provided information on inpatient costs (93%). However, only 40% of studies reported costs of diagnostic tests, 46% reported on costs of outpatient care, 34% on costs of medication, 40% on social care costs, and only 8% on travel costs. Only 14 studies reported estimates of productivity costs, with six studies reporting on productivity costs owing to illness, and a further eight studies reporting productivity cost due to informal caregiving. In terms of reporting of costs of different subgroups, some of the studies (39%) reported costs in relation to event severity and about 31% reported on event type. After having accounted for heterogeneity across studies, the authors compared costs in 71 studies, and converted the costs adjusting for purchasing power parity (PPP), expressing the costs in US dollars (US$) at 2006 prices. The mean cost of stroke was estimated at US$28,252 (median US$19,635) per patient for a follow-up period of 12 months. On average, the mean cost from all was estimated at US$19,018 per patient and the median cost was estimated at US$14,571 per patient. Not surprisingly, cost estimates were influenced by the length of the follow-up period; the mean cost for the groups with follow-up duration of between 3 and 6 months was estimated at US$10,216, whereas for the groups with follow-up duration of between 6 and 12 months it was US$16,973. The authors also noted that costs were significantly higher if charges were used to value resources rather than actual unit costs (US$27,835 vs. US$16,102). The types of stroke (overall stroke or ischaemic stroke) and study design (prospective, retrospective or RCT) did not influence the cost estimates. One of the important contributions of the study was comparison across countries and regions. There was a 10-fold difference in cost of stroke or ischaemic stroke between the studies conducted in Eastern Europe and those in the UK or the USA. The mean cost estimate for the studies in Eastern Europe was US$2822 (range from a minimum of US$468 to a maximum of US$11,523), US$9438 (US$1448–25,909) in countries in Western Europe other than the UK, US$22,377 (US$5206–107,860) in the UK, and US$28,253 (US$7309–146,149) in the USA. This study therefore reinforces the importance of only considering UK sources of costs data in stroke, as our cost-effectiveness modelling is conducted from the NHS perspective.
Grieve and colleagues367 reported significant differences in cost of acute stroke care across Europe. They conducted a study using patient-level data from 13 centres (hospitals) in 13 different countries across Europe, and analysed costs using multiple regression. The acute stroke costs were found to vary due to differences in clinical practices, such as the number of brain scans undertaken, the average staff time per contact and LOS. The study367 found that the mean number of scans ranged from 0.24 (at a centre in Lithuania) to 2.35 (at a centre in Austria). The average number of scans carried out at the participating UK centre was reported to be 0.98 and the mean LOS was 35.4 days (median 20 days), the highest among centres included in the study. There were no patients referred to the rehabilitation unit from this hospital. The shortest LOS was found to be for a centre in Spain (8.8 days), despite the fact that this centre was not referring patients to a rehabilitation unit. The study367 provided estimates of the predicted mean total cost (adjusting for purchasing power parity) of acute care for patients of > 74 years of age. For those who survived the stroke, the mean total cost, in 1995 prices, were estimated at $PPP5202 (95% CI $PPP 4423 to $PPP 6120) in Austria (highest), $PPP 3250 (95% CI $PPP698 to $PPP3916) in the UK, and $PPP240 (95% CI $PPP210 to $PPP274) in Latvia. This again highlights the importance of using UK data sources for costing the acute care received after stroke.
Cost data relevant for the economic modelling
In light of the above discussion, this section considers the cost data that are available in the literature for different brain imaging modalities, and acute and ongoing care for stroke. Owing to the large number of estimates that are available from different studies, we attempt here to provide justification for the sources that are used in the economic modelling.
Cost of imaging in stroke
Table 64 shows the range of UK estimates that are available for the costs of CT, MRI, ultrasound, MRA and CTA. The cost estimates of CT imaging provided by Wardlaw and colleagues26 used a bottom-up (microcosting) approach, i.e. measurement of actual resource use (staff time, expenditures and depreciation of capital equipment, consumables, apportionment of overheads were applied for stroke patients). The 2004 study26 is the most detailed microcosting study available on the costs of CT imaging; CT imaging costs are provided for teaching hospitals, as well as urban and rural general hospitals. In addition, estimates are available for normal working hours provision and out-of-hours provision. However, the estimate apply to only three Scottish hospitals, so they may not be generalisable to other settings within Scotland or other countries of the UK. An alternative set of estimates are available from the NHS reference costs;368 these are likely to include similar elements of resource use, but they are not specifically estimated for stroke patients. The Saka and colleagues369 estimates are in the middle of the range of those produced for CT by Wardlaw and colleagues. 26 However, their costs for MRI are much higher than other estimates and are based on data from one hospital trust in London; it is unclear why the estimate is much higher than others, as no detail is provided of the costing methods used. Owing to the availability of cost estimates for other techniques (MRA, CTA), for reasons of generalisability, and to enable comparisons with other interventions where NHS reference costs are widely used, the model uses the NHS reference costs for CT, MRI, MRA and CTA. 368 For patients considered to have suffered a minor ischaemic stroke we assume that they would receive appropriate blood pressure medication (£20.73) per annum), a generic statin (£24.31 per annum), aspirin (£11.05 per annum) and dipyridamole (£37.31 per annum) (British National Formulary, November 2012;370 www.medicinescomplete.com/mc/bnf/current). We assume that patients diagnosed as having suffered a minor haemorrhagic stroke do not receive antiplatelet drugs.
| Type of imaging | Description | Cost (£) | Source, price year | Inflated to 2009–10 (£) |
|---|---|---|---|---|
| CT | Bottom-up approach | 60.00 | Author estimates, 2010 | 60.00 |
| MRI | Bottom-up approach | 111.62 | Author estimates, 2010 | 111.62 |
| CT | One area – no contrast | 101.00 | DOH, 2011368 | 101.00 |
| MRI | One area – no contrast | 174.00 | DOH, 2011368 | 174.00 |
| Ultrasound < 20 minutes | Unknown | 55.00 | DOH, 2011368 | 55.00 |
| Ultrasound | Bottom-up approach | 54.08 | Wardlaw and colleagues, 20065 | 61.95 |
| DUS | Unknown | 120.00 | DOH, 2011368 | 120.00 |
| MRA | Bottom-up approach | 189.00 | Wardlaw and colleagues, 2006,5 2004 | 216.58 |
| CTA | Bottom-up approach | 122.94 | Wardlaw and colleagues, 2006,5 2004 | 140.85 |
| CT | Bottom-up approach | Wardlaw and colleagues, 2004,26 1999, 2000 | ||
| Teaching – normal hours | 42.90 | 50.03 | ||
| Teaching – out of hours | 79.35 | 92.54 | ||
| RDGH – normal | 81.02 | 94.48 | ||
| RDGH – out of hours | 126.30 | 147.29 | ||
| UDGH – normal | 69.47 | 81.02 | ||
| UDGH – out of hours | 72.57 | 84.63 | ||
| CT | 85.00 | Saka and colleagues, 2009,369 2005 | 96.00 | |
| MRI | 400.00 | Saka and colleagues, 2009,369 2005 | 450.00 |
Cost of stroke care
Acute phase
The costs of stroke can be divided into those incurred in the acute phase (initial hospitalisation) and also those incurred following discharge from hospital (chronic phase), including rehabilitation and ongoing care. The model uses cost estimates of stroke in the acute phase, based on LOS data reported by Wardlaw and colleagues26 combined with 2009–10 unit prices. The data reported by Wardlaw and colleagues26 were chosen as they provide LOS by stroke type (haemorrhagic and ischaemic) and severity (whether patients end up independent or dependent), which is consistent with the model structure. The LOS parameters used, along with other available estimates, are provided in Table 65. LOS in hospitals is important too, as it is the main cost driver in any estimates of costs associated with the acute phase of stroke. Table 65 shows the available estimates from UK and European studies. Given the wide variation in the estimated costs of stroke in the acute phase, we assessed the sensitivity of model findings to variation in this parameter.
| Type of stroke | Description | LOS (days) | Range | Source/comments |
|---|---|---|---|---|
| TIA | Days in stroke unit | 1.8 | ± 2.2 | Dodel and colleagues, 2004371 |
| Days in ICU | 0.3 | ± 2.2 | ||
| Days in regular ward | 7.2 | ± 5.6 | ||
| Ischaemic | Days in stroke unit | 2.5 | ± 2.9 | Dodel and colleagues, 2004371 |
| Days in ICU | 0.6 | ± 2.6 | ||
| Days in regular ward | 7.0 | ± 5.6 | ||
| Haemorrhagic | Days in stroke unit | 1.1 | ± 2.4 | Dodel and colleagues, 2004371 |
| Days in ICU | 5.4 | ± 4.8 | ||
| Days in regular ward | 5.4 | ± 6.8 | ||
| TIA | Days in stroke unit | 9.4 | ± 5.2 | Epifanov and colleagues, 2007,372 Germany – Hospital |
| Days in ICU | ||||
| Days in regular ward | 11.1 | ± 6.5 | ||
| Ischaemic | Days in stroke unit | 10.2 | ± 5.3 | Epifanov and colleagues, 2007,372 Germany – Hospital |
| Days in ICU | ||||
| Days in regular ward | 11.1 | ± 8.9 | ||
| Haemorrhagic | Days in stroke unit | 11.9 | ± 6.0 | Epifanov and colleagues, 2007,372 Germany – Hospital |
| Days in ICU | ||||
| Days in regular ward | 16.9 | ± 15.5 | ||
| Independent haemorrhagic | Average LOS, alive and independent – after haemorrhagic stroke | 11.6 | Wardlaw and colleagues, 200426 | |
| Independent ischaemic | Average LOS, alive and independent – after ischaemic stroke | 14.5 | Wardlaw and colleagues, 200426 | |
| Dependent haemorrhagic | Average LOS, alive and dependent – after haemorrhagic stroke | 16.3 | Wardlaw and colleagues, 200426 | |
| Dependent haemorrhagic | Average LOS, alive and dependent – after ischaemic stroke | 17.9 | Wardlaw and colleagues, 200426 | |
| Ischaemic | Average LOS in general medicine | 12.8 | Christensen and Munro, 2008373 | |
| Average LOS in geriatric ward | 41.1 | |||
| In readmission average | 13.2 | |||
| Haemorrhagic | Average LOS in general medicine | 12.2 | Christensen and Munro, 2008373 | |
| Average LOS in geriatric ward | 45.8 | |||
| 10.6 | ||||
| In re-admission average | 11.6 | |||
| Stroke (ICD10 – 160–66) | Average LOS SLSR register, South London, UK | 34.4 (SD 30.2) | Saka and colleagues, 2009,369 2005 | |
| LOS by quality of care | ||||
| Stroke (WHO criteria) | Median LOS | 13 | Svendsen and colleagues, 2009,374 Denmark | |
| Median LOS for 75–100% of the quality of care fulfilled | 9 | (IQR 5–20) | ||
| Median LOS for 0–24% of the quality of care fulfilled | 26 | (IQR 13–58) | ||
| Stroke | Mean LOS high non-compliance to guideline | 12.9 (± 6.5) | Quaglini and colleagues, 2004375 | |
| Mean LOS low non-compliance to guideline | 10.8 (± 6.5) | |||
| LOS by Rankin score | ||||
| TIA | ||||
| Rankin score 0–2 | LOS days | 9.5 | ± 5.1 | Dodel and colleagues, 2004371 |
| Rankin score 3–6 | LOS days | 8.1 | ± 6.2 | |
| Ischaemic | ||||
| Rankin score 0–2 | LOS days | 10.6 | ± 4.4 | Dodel and colleagues, 2004371 |
| Rankin score 3–6 | LOS days | 9.8 | ± 6.1 | |
| Haemorrhagic | ||||
| Rankin score 0–2 | LOS days | 14.0 | ± 2.9 | Dodel and colleagues, 2004371 |
| Rankin score 3–6 | LOS days | 11.6 | ± 6.3 | |
| Mean | Median | |||
| mRS 0 | LOS days | 9 | 17 | Dawson and colleagues, 2007376 |
| mRS 1 | LOS days | 17 | 25 | |
| mRS 2 | LOS days | 37 | 44 | |
| mRS 3 | LOS days | 64 | 58 | |
| mRS 4 | LOS days | 90 | 79 | |
| mRS 5 | 90 | 81 | ||
| LOS for patients who died after stroke | ||||
| Death from haemorrhagic | Average LOS for those who died after haemorrhagic stroke | 53 | Wardlaw and colleagues, 200426 | |
| Death from ischaemic | Average LOS for those who died after ischaemic stroke | 14 | Wardlaw and colleagues, 200426 | |
Chronic phase
Table 66 shows that there is a vast amount of information available on the health-care costs of stroke in the UK. In addition, there are now estimates of the social care costs and wider societal costs (productivity losses and informal care time). It can be difficult ,therefore, to gauge which estimates are the most suitable for estimating health service costs incurred beyond the initial acute phase. However, limited UK data are available regarding costs incurred beyond the initial hospitalisation. Among the alternative sources, the estimate of Lovibond and colleagues377 is chosen (£559 per 3 months) as the most suitable UK-based source, based on earlier individual patient data reported by Youman and colleagues. 361 As we were unable to obtain ongoing costs by level of dependence following stroke, this estimate was applied to patients in both the ‘alive and independent’ and the ‘alive and dependent’ states. However, an allowance was made for likely increased primary care costs for those alive and dependent.
| Type of stroke | Description | Cost (£) | Source, price year/comments | Inflated 2009/10 (£) |
|---|---|---|---|---|
| Initial stroke | 3-month costs | 9630 | Lovibond and colleagues, 2011377 used in NICE CG127, 2009–10 prices | 9630 |
| Post-stroke costs | 3-month costs | 559 | Lovibond and colleagues, 2011377 used in NICE CG127, 2009–10 prices | 559 |
| Stroke: pre-discharge, inpatient stay | HRG 1998–99, NHS DOH, 2001 | 1619 | Berry and colleagues, 2002,294 2001 prices | 2106 |
| Stroke: post-discharge inpatient stay | HRG 1998–99, NHS DOH, 2001 | 2009 | Berry and colleagues, 2002,294 2001 prices | 2613 |
| Stroke: disabled per year | Hospital episode statistics 1998/99 | 10,525 | Berry and colleagues, 2002,294 2001 prices | 13,690 |
| Stroke, dependent | Calculated on mean LOS 51 days, rehab cost £763 and average yearly cost of £11,292 | 22,255 | Wardlaw and colleagues, 200426 | 30,421 |
| Stroke, independent | Calculated on mean LOS 14 days, rehabilitation cost £40 and average yearly cost of £876 | 3716 | Wardlaw and colleagues, 200426 | 5079 |
| TIA | Cost per admission with LOS 9.4 (in stroke unit, ICU and regular wards) | €3020 (± 1790) | Epifanov and colleagues, 2007372 Germany – Hospital. Used Dodel and colleagues, 2004371 | 2421 |
| (Mean in £) | 1926 | 2002 price | ||
| Calculated cost/day | 258 | |||
| Ischaemic | Cost per admission with LOS 10.2 (in stroke unit, ICU and regular wards) | €3480 (± 1870) | Epifanov and colleagues, 2007372 Germany – Hospital. Used Dodel and colleagues 2004371 | 2790 |
| (Mean in £) | 2,220 | 2002 price | ||
| Calculated cost/day | 274 | |||
| Haemorrhagic | Cost per admission with LOS 11.9 (in stroke unit, ICU and regular wards) | €5080 (± 2900) | Epifanov and colleagues, 2007372 Germany – Hospital. Used Dodel and colleagues, 2004371 | 4072 |
| (Mean in £) | 3240 | 2002 prices | ||
| Calculated cost/day | 342 | |||
| TIA | Cost per admission with LOS 9.4 (in stroke unit, ICU and regular wards) | €3020 | Dodel and colleagues, 2004371 | 2421 |
| (Mean in £) | 1926 | 2002 prices | ||
| Calculated cost/day | 258 | |||
| Ischaemic | Cost per admission with LOS 10.2 (in stroke unit, ICU and regular wards) | €3480 | Dodel and colleagues, 2004371 | 2790 |
| (Mean in £) | 2220 | 2002 prices | ||
| Calculated cost/day | 274 | |||
| Haemorrhagic | Cost per admission with LOS 11.9 (in stroke unit, ICU and regular wards) | €5080 | Dodel and colleagues, 2004371 | 4072 |
| (Mean in £) | 3240 | 2002 price | ||
| Calculated cost/day | 342 | |||
| Post-TIA cost | Post TIA – 3 months’ description | 26 | Lovibond, and colleagues, 2011377 | 26 |
| Ischaemic | Average cost per initial hospitalisation | 10,924 (SD ± 15,202) | Christensen and Munro, 2008373 | 11,746 |
| Cost estimates based on ISD Scottish Health Services 2006 prices | ||||
| Haemorrhagic | Average cost per initial hospitalisation | 12,042 (SD ± 19,688) | Christensen and Munro, 2008373 | 12,948 |
| Cost estimates based on ISD Scottish Health Services 2006 price | ||||
| Ischaemic | Average cost per re-admission | 10,924 (SD ± 15,202) | Christensen and Munro, 2008373 | 11,746 |
| Cost estimates based on ISD Scottish Health Services 2006 prices | ||||
| Haemorrhagic | Average cost per re-admission | 3127 (SD ± 8101) | Christensen and Munro, 2008373 | 3362 |
| Cost estimates based on ISD Scottish Health Services 2006 prices | ||||
| Ischaemic | Average cost per 12 months’ survivor initial hospitalisation | 10,893 (SD ± 15,767) | Christensen and Munro, 2008373 | 11,713 |
| Total hospital cost for survivors – 12 months | 14,263 (SD ± 18,736) | Cost estimates based on ISD Scottish Health Services 2006 price | 15,336 | |
| Haemorrhagic | Average cost per 12 months’ survivor initial hospitalisation | 18,026 (SD ± 24,218) | Christensen and Munro, 2008373 | 19,383 |
| Total hospital cost – 12 months | 21,493 (SD ± 26,222) | Cost estimates based on ISD Scottish Health Services 2006 prices | 23,111 | |
| Stroke at Stroke Unit | Cost per patient per day | 165 | Saka and colleagues, 2009,369 2005 prices | 184 |
| Stroke at general medical ward | Cost per patient per day | 115 | Saka and colleagues, 2009,369 2005 prices | 128 |
| Acute stroke – mild | Cost of an acute event: mild stroke – 3 months | 5099 (95% CI 4558 to 5656) | Youman and colleagues, 2003,361 2001–2 prices | 6632 |
| Acute stroke – moderate | Cost of an acute event, moderate stroke – 3 months | 4816 (95% CI 4406 to 5225) | Youman and colleagues, 2003,361 2001–2 prices | 5754 |
| Acute stroke – severe | Cost of an acute event, severe stroke – 3 months | 10,555 (95% CI 9575 to 11,535) | Youman and colleagues, 2003,361 2001–2 prices | 12,612 |
| Acute stroke – fatal | Cost of an acute event, fatal stroke – 3 months | 6781 (95% CI 5444 to 9871) | Youman and colleagues, 2003,361 2001–2 prices | 8102 |
| Stroke patients | Cost per IP day, teaching hospital | 239 | Wardlaw and colleagues, 2004,26 2000 prices | 326.69 |
| Cost per IP day, large general hospital | 217 | 296.62 | ||
| Cost per IP day, long-stay hospital | 116 | 158.56 |
Costs associated with myocardial infarction
For patients experiencing a MI, the initial acute costs associated estimated based on a previous UK study by Palmer and colleagues,378 inflated to the 2010 prices. This estimate was chosen for its applicability to the NHS setting. Palmer and colleagues,378 reported hospital costs associated with MI, and so we added the cost of three GP visits to this estimate to allow for increased primary care costs in the period following MI. Beyond the first 3-month period, we applied the cost estimate of £141 per 3-month period. 377 For fatal MI we applied the cost of £2225 taken from NICE Technical Appraisal 90. 379 The costs applied to MI events are provided in Table 67.
| Event | Cost (£) | Source |
|---|---|---|
| Non-fatal MI (first-year hospital costs) | 5437 | Palmer and colleagues, 2004378 (hospital costs inflated to 2010 prices plus allowance made for increased primary care costs) (see Table 61, box 80) |
| Cost of fatal MI | 2225 | NICE TA 90 review, 2010379 |
| Post MI costs (3 monthly) | 141 | Lovibond and colleagues, 2011377 |
Utility values in transient ischaemic attack and stroke
Post and colleagues380 reviewed literature published between 1996 and 2000 to obtain estimates of health state utilities associated with stroke. They reviewed 23 studies where different methods were used to assess the utility of stroke outcomes, such as the visual analogue scale (VAS), Health Utilities Index (HUI) and the EQ-5D. They found the values/utilities elicited by the time trade-off (TTO) approach to be generally lower than standard gamble (SG) utilities. The utility scores derived from studies using the VAS or HUI instrument were found to be lower than those derived from studies using the TTO to directly elicit values for stroke outcomes. Utility weights for major stroke assigned by healthy participants were fairly similar to those assigned by patients at risk of stroke, however stroke survivors were found to have assigned higher utilities. The estimated mean utility for survivors of major stroke was found to be 0.32 when the EQ-5D was used, whereas it was 0.41 when the TTO was used to directly elicit values, and was 0.72 based on the SG method. For survivors of minor stroke, the mean utility score was 0.72 using the TTO method directly, and was 0.71 based on EQ-5D responses. These values were similar to those elicited from healthy persons. The study by Post and colleagues380 also suggests that variation in the description of minor stroke or major stroke influences the reported utilities. It needs to be noted here that estimates based on the SG method are generally less favoured than TTO values owing to the possibility of loss aversion and probability transformation causing upwards bias, whereas estimates derived using the VAS are less likely to be accepted for decision-making because the method does not incorporate the concepts of choice and opportunity cost. QALY weights derived from TTO scores are most frequently used to inform decision-making in the UK, with a particular emphasis placed on using weights based on EQ-5D responses scored using the UK TTO-based tariff. 381
There is an increasing interest in transforming scores from disease-specific instruments to utility-based scores to allow the estimation of QALYs. Mortimer and colleagues382 attempted to assess whether or not it would be possible to transform stroke-specific measures of health status to a preference-based measure suitable for generating QALY weights. Based on repeat responses of 859 patients suffering a stroke, they used a regression approach to derive an empirical transformation of three commonly used descriptive measures of health status in stroke – the NIHSS, the Barthel Index (BI), and the Short Form questionnaire-36 items (SF-36) – to the Assessment of Quality of Life (AQoL) (a generic preference-based measure). They showed that a transformation to the SF-36 could explain ≈72% of the observed variation in AQoL scores and concluded that the BI and SF-36 could be adequately transformed to preference-based values suitable for generating QALYs. Of course the SF-36 can also be used to generate utility weights in the UK setting through the Short Form questionnaire-6 Dimensions (SF-6D) (Brazier and colleagues383).
Rivero-Arais and colleagues384 studied the association between mRS scores and preference-based EQ-5D values using individual responses to both instruments collected as part of a large observational study (OXVASC). Their study sample included 1283 stroke and TIA patients recruited between April 2002 and March 2007 who were followed up at 1, 6, 12 and 24 months. The authors used ordinary least squares regression to predict the preference-based EQ-5D score (based on the UK TTO tariff) from mRS scores, and also tested a method whereby multinomial logistic regression (MLogit) was used to predict responses to each EQ-5D question. Although both methods predicted the mean EQ-5D utility estimate reasonably well, they also underestimated the uncertainty surrounding the observed mean EQ-5D score. They concluded that their derived algorithms should not replace prospective collection of utility data.
Van Exel and colleagues385 examined the direct relationship between a conventional clinical scale of functional status (BI) and the EQ-5D. Their findings were based on data collected in the Netherlands on 598 stroke patients who were followed over the period of 6 months. Using simple linear regression, they found evidence for a significant relationship between these measures suggesting an EQ-5D score of –0.25 for patients with a BI of 0, and a 0.05 increase in the EQ-5D score for each point increase on the BI. With this algorithm, independent patients (BI 20) received a utility weight of 0.75, which corresponded with the age-/sex-specific EQ-5D population reference score for their sample.
Although the above discussions highlight that a number of sources and approaches are available for estimating utility weights associated with stroke, more relevant estimates are available in the form of EQ-5D-based weights derived from the responses of individuals enrolled in the IST trial. 112 Dorman and colleagues’ study used the EuroQoL386 to measure the health status for 867 UK patients enrolled in the IST. The utility values were 0.31 (95% CI 0.29 to 0.34) for dependent health states, 0.71 (95% CI 0.68 to 0.74) for independent health states and 0.88 (95% CI 0.84 to 0.92) for fully recovered health states. Table 68 shows the mRS states and TTO weights based on EQ-5D updated with CLOTS trial data. These values were used in previous modelling studies by Wardlaw and colleagues5,26 and are importantly based on the responses of UK patients. We have now been able to update this with unpublished more recent EQ-5D data from UK stroke patients enrolled in the CLOTS trial115 (see Chapter 10, Table 61, boxes 99 and 100, and Table 68).
| mRS | CLOTS EQ-5D | Prior data from IST/LSR (EQ-5D) |
|---|---|---|
| Alive and independent | ||
| n | 1468 | 84 |
| Mean (SD) | 0.71 (0.24) | 0.78 |
| 95% CI | 0.70 to 0.72 | 0.73 to 0.82 |
| Median (IQR) | 0.73 (0.62 to 0.85) | 0.80 |
| Minimum | (–0.40) | 0.06 |
| Maximum | 1.00 | 1 |
| Alive and dependent | ||
| n | 2608 | 40 |
| Mean (SD) | 0.20 (0.34) | 0.34 |
| 95% CI | 0.19 to 0.21 | 0.23 to 0.45 |
| Median (IQR) | 0.19 (–0.07 to 0.52) | 0.33 |
| Minimum | (–0.59) | (–0.57) |
| Maximum | 1.00 | 1 |
| Total | 4076 | 124 |
Further, the CLOTS trial estimates are based on the responses of a larger sample of patients, and as such these values are used in the model. The values are very similar to those reported by Dorman and colleagues,112 with the exception of a lower value obtained for patients alive and dependent following a stroke. Table 69 below summarises available utility estimates for states of health associated with stroke and TIA.
| Stroke categories/health status | Utility values (95% CI) | Source | Comment |
|---|---|---|---|
| Recovered (mRS score 0) | 0.95 | Young and colleagues, 2010291 | Used Post and colleagues, 2001380 |
| Minor stroke (mRS score 1–2) | 0.71 | ||
| Major stroke (mRS score 3–5) | 0.22 | ||
| Recovered | 0.88 (0.80 to 0.96) | Dorman and colleagues, 2000112 using LSR data | Used LSR cohort |
| Independent | 0.74 (0.69 to 0.79) | ||
| Dependent | 0.38 (0.29 to 0.47) | ||
| Recovered | 0.88 (0.84 to 0.92) | Dorman and colleagues, 2000112 using IST data | Using IST data |
| Independent | 0.71 (0.68 to 0.74) | ||
| Dependent | 0.31 (0.29 to 0.34) | ||
| Fully recovered | 0.88 (0.84 to 0.92) | Wardlaw and colleagues, 20065 | Used Dorman and colleagues, 2000112 |
| Independent | 0.71 (0.68 to 0.74) | ||
| Dependent | 0.31 (0.29 to 0.34) | ||
| Alive and independent (mRS 0–2) | 0.78 (0.73 to 0.82) | Wardlaw and colleagues, 2004387 | Used Dorman and colleagues, 2000112 |
| Alive and dependent (mRS 3–5) | 0.34 (0.23 to 0.45) | ||
| Dead after stroke (mRS 6) | 0.00 | ||
| Minor stroke | 0.87 | Tseng and Lin, 2007,388 a review of literature | A review of literature used Tengs and Lin, 2003389 |
| Moderate stroke | 0.68 | ||
| Major stroke | 0.52 | ||
| Mild residua from stroke | 0.75 | Tseng and Lin, 2007,388 a review of literature | O’Brien and Gage, 2005,390 based on study published in 1996 |
| Moderate to severe | 0.39 | ||
| Residua from recurrent stroke | 0.12 | ||
| TIA | 0.90 | Tseng and Lin, 2007,388 a review of literature | Matchar and colleagues, 2005,391 based on large survey of patients |
| mRS 1 | 0.80 | ||
| mRS 2 | 0.65 | ||
| mRS 3 | 0.50 | ||
| mRS 4 | 0.35 | ||
| mRS 5 | 0.20 | ||
| mRS 0 | 0.80 (0.80 to 1.00) | Earnshaw and colleagues, 2009291 | Used study by Stahl and colleagues, 2003392 |
| mRS 1 | 0.80 (0.8 to 0.95) | ||
| mRS 2 | 0.65 (0.68 to 0.9) | ||
| mRS 3 | 0.50 (0.45 to 0.65) | ||
| mRS 4 | 0.35 (0.10 to 0.40) | ||
| mRS 5 | 0.20 (0.00 to 0.32) | ||
| mRS 6 | 0.00 | ||
| Ranking category | |||
| Mean and range | Stahl and colleagues, 2003392 | Used Tengs and Lin, 2003389 | |
| 0. No symptoms | 0.90 (0.85 to 0.95) | ||
| 1. No disability | 0.79 (0.70 to 0.90) | ||
| 2. Slight disability | 0.68 (0.35 to 0.90) | ||
| 3. Moderate disability | 0.65 (0.20 to 0.90) | ||
| 4. Moderate to severe disability | 0.40 (0.12 to 0.54) | ||
| 5. Severe disability | 0.32 (–0.20 to 0.54) | ||
| Death | 0 | ||
| Utility value in MI | |||
| Non-fatal MI | 0.76 (SE 0.018) | Goodacre and colleagues, 2004393 | |
| Post non-fatal MI | 0.76 (SE 0.018) | ||
Model parameters
In light of the above discussions, the final cost and utility values incorporated in the economic model are all provided in Table 70. The following chapter presents the results of the economic evaluation based on the model described here.
| Parameter | Value | Low | High | Data source and assumptions |
|---|---|---|---|---|
| Cost parameters | ||||
| Cost of drugs per month: | British National Formulary no. 62 (September 2011)370 | |||
| Pravastatin 40 mg | £26.01 | |||
| Aspirin 75 mg | £0.31 | |||
| Cost of CT (per scan) | £101 | £69 | £108 | DOH, 2011368 (CT one area, no contrast; average + lower and upper quartile) |
| Cost of MRI (per scan) | £174 | £117 | £199 | DOH, 2011368 (MRI one area, no contrast; average + lower and upper quartile) |
| Cost of ultrasound (< 20 minutes) | £55 | £40 | £65 | DOH, 2011368 (average + lower and upper quartile) |
| Cost of CTA for detecting stenosis | £116 | £88 | £126 | DOH, 2011368 (average + lower and upper quartile) |
| Cost of MRA for detecting stenosis | £213 | £180 | £249 | DOH, 2011368 (average + lower and upper quartile) |
| Cost per inpatient day | £297 | £180 | £372 | Wardlaw and colleagues, 2004;26 PSSRU, 2010394 (large general hospital inpatient cost, inflated to 2009–10 prices) |
| LOS – alive and dependent, haemorrhagic stroke | 16.3 days | 1.3 days | 31.3 days | Wardlaw and colleagues, 200426 |
| LOS – alive and dependent, ischaemic stroke | 17.9 days | 2.9 days | 32.9 days | Wardlaw and colleagues, 200426 |
| LOS – alive and independent haemorrhagic stroke | 11.6 days | 8 days | 19.6 days | Wardlaw and colleagues, 200426 |
| LOS – alive and independent, ischaemic stroke | 14.5 days | 10 days | 24.5 days | Wardlaw and colleagues, 200426 |
| LOS – death with haemorrhagic stroke | 53.0 days | Wardlaw and colleagues, 200426 | ||
| LOS – death ischaemic stroke | 33.3 days | Wardlaw and colleagues, 200426 | ||
| Cost of endarterectomy | £3697 | £2658 | £4466 | DOH, 2011368 (average plus lower and upper quartile for HRG QZ04Z) (see Table 61, box 92) |
| Annual health care costs following stroke (alive and dependent) | £2648 | Lovibond and colleagues, 2011;378 PSSRU, 2010394 (hospital costs £2236 + primary care costs of one GP visit per month assumed) | ||
| Annual health-care costs following stroke (alive and independent) | £2236 | Lovibond and colleagues, 2011377 | ||
| Initial MI costs (first 3 months) | £5437 | Palmer and colleagues, 2004378 (hospital costs inflated to 2010 prices plus allowance made for medication and primary care costs) (see Table 61, box 80) | ||
| Three-month health-care cost following a MI (after first 3 months) | £141 | Lovibond and colleagues, 2011377 | ||
| Cost of fatal MI | £2225 | NICE, 2010379 | ||
| Utility – alive and dependent following stroke | 0.20 | CLOTS data (see Table 61, box 99) | ||
| Utility – alive and Independent following stroke | 0.71 | CLOTS data (see Table 61, box 100) | ||
| Utility recurrence-free | 0.88 | Wardlaw and colleagues, 20065 (see Table 61, box 101) | ||
| Utility alive following MI | 0.76 | 0.712 | 0.939 | Goodacre and colleagues, 2004393 (see Table 61, boxes 93 and 94) |
| Utility for non-stroke | 0.88 | Assumed same as for recurrence-free state | ||
Chapter 12 Results of health economic modelling
This chapter presents the results of the health-economic modelling study, the methods and development of which were described in the previous chapter.
The nine key scenarios that were examined are again outlined below. Each scenario was analysed for a cohort of 70-year-old men and women over a 20-year time horizon:
-
Base-case scenario Immediate MRI for brain imaging plus immediate ultrasound for carotid imaging compared with immediate CT for brain imaging plus immediate ultrasound for carotid imaging in an immediate presenting cohort (within the first few hours of the event).
-
Delayed MRI and delayed ultrasound to within 7 days for those with ABCD2 score of < 4 (otherwise immediate MRI plus immediate ultrasound) compared with immediate CT plus immediate ultrasound for an immediate presenting cohort.
-
Immediate ultrasound for all, but delayed MRI to within 7 days, for those with an ABCD2 score of < 4 and immediate MRI for those with an ABCD2 score of ≥ 4, compared with immediate CT plus immediate ultrasound for an immediate presenting cohort.
-
Immediate MRI for brain imaging plus immediate ultrasound for carotid imaging compared with immediate MRI for brain imaging plus immediate MRA for carotid imaging, compared with immediate CT for brain imaging plus immediate ultrasound for carotid imaging, compared with immediate CT for brain imaging plus CTA for carotid imaging (in immediate presenting cohort).
-
Repeat of scenario 1 but with secondary prevention for ischaemic stroke implemented only in the MRI arm for those with a visible lesion on MRI brain imaging (immediate presenting cohort).
-
Secondary prevention implemented for all but MRI and ultrasound delayed to within 7 days for those with a low ABCD2 score (immediate presenting cohort).
-
Scenario 1 repeated for a cohort of patients presenting 7 days after the initial event.
-
Scenario 1 repeated for a cohort of patients presenting 21 days after the initial event.
-
Scenario 3 repeated for a cohort of patients presenting 7 days after the initial event.
Model analysis
The model was first of all run deterministically for the nine main scenarios identified above. Following this, distributions were placed on parameters for which sufficient sampling information was available, and PSA was conducted for scenarios 1, 7 and 8. The results of the PSA should be treated with caution, as data limitations and availability precluded the estimation of distributions for a number of key model inputs. Given the difficulty in assigning appropriate distributions to key diagnostic parameters, no formal value of information analysis was conducted. However, cost-effectiveness acceptability curves were derived based on the joint uncertainty surrounding those parameters for which distributions were assigned.
The parameters included in the PSA were the unit cost estimates for scanning, all of the utility estimates, the RR reductions associated with appropriate treatment, and the standardised mortality ratios associated with stroke and MI survival. Gamma distributions were fitted to cost parameters, beta distributions were assigned to utility weights, and log-normal distributions were assigned to the RRs and standardised mortality ratios. The results of the modelling study are presented in the following sections.
Cost-effectiveness findings
Table 71 shows the mean cost and QALY estimates associated with each strategy for each of the nine scenarios defined above. In each case, the more costly strategy is compared with the next less costly alternative to estimate the incremental costs and incremental QALYs. Where strategies are more costly and less effective an alternative strategy, they are said to be dominated. Where a strategy is more costly and more effective than its comparator, its incremental cost-effectiveness ratio (ICER) is reported. This represents the additional cost incurred per QALY gained over the next less costly alternative.
| Scenario/strategies | Cost (£) | Incremental costs (£) | QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| 1. Base case: immediate MRI and ultrasound vs. immediate CT and ultrasound | |||||
| CT + ultrasound | 3262 | – | 8.7313 | – | – |
| MR + ultrasound | 3340 | 77 | 8.7308 | –0.0005 | Dominated |
| 2. Delayed MRI and ultrasound for those with an ABCD2 score of < 4 vs. immediate CT and ultrasound | |||||
| CT + ultrasound | 3262 | – | 8.7313 | – | – |
| MR + ultrasound | 3388 | 126 | 8.7122 | –0.0191 | Dominated |
| 3. Immediate ultrasound but delayed MRI for those with ABCD2 score of < 4, vs. immediate CT and ultrasound | |||||
| CT + ultrasound | 3262 | – | 8.7313 | – | – |
| MR + ultrasound | 3340 | 78 | 8.7296 | –0.0017 | Dominated |
| 4. Immediate MRI vs. immediate CT vs. immediate MRA vs. immediate CTA | |||||
| CT + ultrasound | 3262 | – | 8.7313 | – | – |
| CT + CTA | 3323 | 61 | 8.7313 | 0.0000 | Dominated |
| MR + ultrasound | 3340 | 77 | 8.7308 | –0.0005 | Dominated |
| MR + MRA | 3498 | 235 | 8.7308 | –0.0005 | Dominated |
| 5. Only implement drug treatment for those with detected visible ischaemic lesion in MR arm | |||||
| CT + ultrasound | 3262 | – | 8.7313 | – | – |
| MR + ultrasound | 3669 | 407 | 8.5872 | –0.1440 | Dominated |
| 6. Immediate implementation of drug treatments but delayed MRI and ultrasound for those with a low ABCD2 score | |||||
| CT + ultrasound | 3262 | – | 8.7313 | – | – |
| MR + ultrasound | 3366 | 103 | 8.7214 | –0.0098 | Dominated |
| 7. Immediate MRI vs. immediate CT for cohort presenting 7 days after initial event | |||||
| CT + ultrasound | 3230 | – | 8.7425 | – | – |
| MR + ultrasound | 3296 | 66 | 8.7442 | 0.0017 | 39,509 |
| 8. Immediate MRI vs. immediate CT for cohort presenting > 21 days after initial event | |||||
| CT + ultrasound | 3233 | – | 8.7419 | – | – |
| MR + ultrasound | 3296 | 63 | 8.7442 | 0.0023 | 27,751 |
| 9. Immediate ultrasound but delayed MRI for those with an ABCD2 score of < 4, vs. immediate CT + ultrasound in the cohort presenting at 7 days after the initial event | |||||
| CT + ultrasound | 3230 | – | 8.742489 | – | – |
| MR + ultrasound | 3297 | 67 | 8.742934 | 0.000445 | 150,020 |
The base case considers the cost-effectiveness of immediate MRI plus ultrasound for patients presenting at stroke prevention clinics within a few hours of symptom onset compared with a policy of immediate CT imaging plus ultrasound. The results of this analysis indicate that a strategy of immediate MRI and ultrasound is very unlikely to be considered cost-effective in the immediate presenting cohort. It works out to be more expensive, primarily because the cost of MRI is higher than the cost of CT imaging and, if anything, slightly less effective. The small difference in effectiveness is caused by a slightly greater proportion of patients with haemorrhagic stroke being missed with MRI in the early presenting cohort, and therefore receiving inappropriate antiplatelet treatment. This is a consequence of the lower sensitivity of MRI for haemorrhagic stroke when used within a few hours of the event. At the same time, as individuals without any visible lesion detected on imaging are modelled to receive secondary prevention for ischaemic stroke, and ultrasound is used to detect carotid stenosis in both arms of the model, there is no benefit associated with the higher sensitivity of MRI for identifying ischaemic stroke. Hence the estimated increase in cost of £77 and the marginal QALY loss of 0.0005.
In scenario 2, MRI with ultrasound is again compared with immediate CT imaging with ultrasound. However, in this scenario selective imaging is used in the MRI arm, with MRI and ultrasound being delayed by 7 days for patients who have a low ABCD score; i.e. a score of < 4. This strategy also turns out to be less cost-effective than the CT option and also less cost-effective than when immediate MRI and ultrasound is carried out for all. The incremental costs are higher (£126) and the QALY losses greater (0.0191) with this selective delayed MRI strategy as a significant proportion of patients with a low ABCD2 score still have high or moderate levels of carotid stenosis, which without timely diagnosis and surgery puts them at high risk of suffering a recurrent stroke. The effect of delaying diagnosis and surgery for these patients translates into higher mean incremental costs and QALY losses.
In strategy 3, instead of delaying both ultrasound and MRI by 7 days, the strategy tests the effect of delaying only imaging with MRI for those with a low ABCD2 score (i.e. < 4). This strategy still costs more than the CT base strategy and there is still a loss of QALYs, although to a lesser extent than with strategy 2. However, the strategy is still dominated by CT plus ultrasound for all, i.e. it is more costly and no more effective, and it is therefore less cost-effective. This is a consequence of patients with a low ABCD2 score, but no stenosis, still having some degree risk for recurrent stroke that would benefit from immediate secondary preventative treatment.
A further option for the immediate presenting cohort is considered in scenario 4. Here we simultaneously compare four potential immediate imaging strategies: (1) CT for brain imaging plus ultrasound for carotid imaging; (2) CT plus CTA for carotid imaging; (3) MRI plus ultrasound for carotid imaging; and (4) MRI plus MRA for carotid imaging. The results for this scenario indicate that CT plus ultrasound dominates all of the other strategies. These results are based on the assumption that neither CTA nor MRA is any more sensitive for picking up carotid stenosis than ultrasound,83,84 whereas both are more costly.
Scenario 5 explores the impact of assuming secondary preventative drug treatment for ischaemic stroke is implemented only for patients with an identified ischaemic lesion on DWI in the MRI arm of the model (whereas treatment decisions remain unchanged from the base case in the CT arm). Under this scenario, the MRI strategy is less costly than it otherwise is owing to saving on the cost of treatment, but it is also significantly less effective owing to an increased incidence of recurrent stroke in the 66% of TIAs and 30% of minor strokes patients with no visible ischaemic lesion on DWI. It remains dominated by the CT strategy.
A final scenario explored for the immediate presenting cohort, is one where it is assumed that preventative drug treatment for ischaemic stroke is implemented for all patients upon presentation, but that brain and carotid imaging are delayed for the group with a low ABCD2 score in the MRI arm of the model (scenario 6). CT plus ultrasound remains the favoured strategy under this scenario.
Strategies 7–9 assess the cost-effectiveness of MRI strategies compared with immediate CT plus ultrasound in cohorts of patients presenting 7 days or 21 days after the initial event. Considering immediate MRI plus ultrasound compared with immediate CT plus ultrasound in the cohort presenting at 7 days (scenario 7), the MRI strategy turns out to be more costly but also slightly more effective than CT, with an ICER of £39,509 per QALY. The increase in effect observed for MRI under this scenario results from its superior sensitivity for haemorrhagic stroke (the sensitivity of CT for haemorrhages declines over time), whereas the sensitivity of MRI for ischaemic stroke also declines over time, this has no impact on outcomes as treatment for ischaemic stroke is still modelled to be implemented in this scenario unless an alternative cause of symptoms is identified. Similar results are obtained when the same strategies are compared in a cohort of patients presenting 21 days after the initial event. However, the QALY gains associated with the MRI strategy are higher in this case as a result of a further decline in the sensitivity of CT for haemorrhagic stroke and, as such, the ICER for MRI plus ultrasound is improved (£27,751 per QALY gained).
A final scenario explored for the cohort of patients presenting 7 days after the initial event was a repeat of scenario 3: delayed MRI for those with a low ABCD2 score but immediate ultrasound for all compared with immediate CT plus immediate ultrasound for all (scenario 9). Under this scenario the QALYs are again higher with MRI but less so than when immediate MRI is implemented for patients presenting at 7 days. This is, again, due to some patients with a low ABCD2 score and no carotid stenosis still being exposed to a risk of ischaemic stroke that would benefit from appropriate treatment. In this instance, however, the point estimate for the ICER is £159,325, which is well above the usually acceptable range of £20,000–30,000 per QALY gained.
The QALY differences shown in Table 71 are primarily driven by small changes in the relative incidence of recurrent stroke between the arms of the models. To illustrate this, Table 72 presents the modelled cumulative incidence of recurrent stroke in the MRI and CT arms of the model for scenarios 1, 2, 3, 5, 7 and 8 over the 20-year time horizon. In each instance, the strategy associated with the QALY gains is also associated with a lower cumulative recurrence of stroke. In the base case of immediate CT and ultrasound compared with immediate MRI and ultrasound (in the immediate presenting cohort), for example, the difference in recurrent stroke incidence between the strategies is very small, and would equate to an increase of one more haemorrhagic stroke for every 12,500 patients imaged using the MRI strategy (1/0.00007976).
| Scenario/strategies | Cumulative recurrence of stroke | Incremental cumulative recurrence of stroke | QALYs | Incremental QALYs |
|---|---|---|---|---|
| 1. Base case: immediate MRI vs. immediate CT; immediate DUS for both | ||||
| CT + ultrasound | 0.172593 | – | 8.7313 | – |
| MRI + ultrasound | 0.172673 | 0.00007976 | 8.7308 | –0.0005 |
| 2. Delayed MRI for those with an ABCD2 score of < 4 vs. immediate CT | ||||
| CT + ultrasound | 0.172593 | – | – | |
| MRI + ultrasound | 0.175366 | 0.002773 | 8.7122 | –0.0191 |
| 3. Immediate ultrasound but delayed MRI for those with an ABCD2 score of < 4, vs. immediate CT + ultrasound | ||||
| CT + ultrasound | 0.172593 | – | 8.7313 | – |
| MRI + ultrasound | 0.172657 | 0.00006453 | 8.7299 | –0.0014 |
| 5. Only implement drug treatment for those with detected visible lesion in MRI arm | ||||
| CT + ultrasound | 0.172593 | – | 8.7313 | – |
| MRI + ultrasound | 0.198549 | 0.0260 | 8.5872 | –0.1440 |
| 7. Immediate MRI vs. immediate CT for cohort presenting 7 days after initial event | ||||
| CT + ultrasound | 0.171517 | |||
| MRI + ultrasound | 0.171223 | –0.00029 | 8.7442 | 0.0017 |
| 8. Immediate MRI vs. immediate CT for cohort presenting > 21 days after initial event | ||||
| CT + ultrasound | 0.171621 | |||
| MRI + ultrasound | 0.171223 | –0.0004 | 8.7442 | 0.0023 |
Sensitivity analysis
Table 73 presents the results of one-way sensitivity analysis on several key variables for the base-case model specification: immediate MRI plus ultrasound compared with immediate CT plus ultrasound in an immediate presenting cohort.
| Scenario/strategies | Cost (£) | Incremental costs (£) | QALYs | Incremental QALYs | ICER |
|---|---|---|---|---|---|
| A. Immediate CT vs. immediate MRI with changes to the cost of the MRI scan | |||||
| a. Cost of MRI: base case – £174 | |||||
| CT + ultrasound | 3262 | – | 8.7313 | – | – |
| MRI + ultrasound | 3340 | 77 | 8.7308 | –0.0005 | Dominated |
| b. Cost of MRI – £90 | |||||
| MRI + ultrasound | 3255 | – | 8.7308 | – | – |
| CT + ultrasound | 3262 | 8 | 8.7313 | 0.0005 | 16,740 |
| c. Cost of MRI – £101 (making MRI the same cost as CT) | |||||
| CT + ultrasound | 3262 | – | 8.7313 | ||
| MR + ultrasound | 3265 | 2 | 8.7308 | –0.0005 | Dominated |
| B. Immediate CT vs. immediate MRI with changes to the sensitivity of CT for detecting haemorrhagic stroke | |||||
| Sensitivity of CT for detecting haemorrhagic stroke: Base case – 0.99 | |||||
| a. CT + ultrasound | 3262 | – | 8.7313 | – | – |
| MRI + ultrasound | 3340 | 77 | 8.7308 | –0.0005 | Dominated |
| b. Sensitivity of CT for detecting haemorrhagic stroke: 0.3925 (equivalent to the estimated value at 7 days) | |||||
| CT + ultrasound | 3271 | – | 8.7296 | – | – |
| MRI + ultrasound | 3340 | 68 | 8.7308 | 0.0012 | 55,123 |
| C. Immediate CT vs. immediate MRI with changes to the increased RR of recurrent haemorrhagic stroke in patients treated with anti–platelet medication following a minor haemorrhagic stroke | |||||
| a. Increased risk of haemorrhagic stroke with inappropriate antiplatelet drugs – Base value 1.15 | |||||
| CT + ultrasound | 3262 | – | 8.7313 | – | – |
| MRI + ultrasound | 3340 | 77 | 8.7308 | –0.0005 | Dominated |
| b. 1.05 | |||||
| CT + ultrasound | 3262 | – | 8.7313 | – | – |
| MRI+ ultrasound | 3339 | 77 | 8.7311 | –0.0002 | Dominated |
| c. 1.2 | |||||
| CT + ultrasound | 3262 | – | 8.7313 | – | – |
| MRI+ ultrasound | 3340 | 78 | 8.7307 | –0.0006 | Dominated |
Uncertainty around cost of imaging
First of all we varied the cost of MRI to examine the effect of changes in this parameter on the results. Immediate MRI and ultrasound remains dominated by immediate CT plus ultrasound unless we assume that the cost of MRI is less than that of CT (e.g. £90). Although the MRI strategy is less costly than the CT strategy at this price (holding the CT cost constant), it also remains marginally less effective. However, CT remains relatively cost-effective in comparison with MRI, with an ICER of approximately £20,000 per QALY gained.
Uncertainty around the sensitivity of computed tomography in detecting visible haemorrhagic lesions
Lowering the sensitivity of CT for identifying haemorrhages in the immediate presenting cohort, the MRI plus ultrasound option provides a greater number of QALYs compared with CT plus ultrasound. The declining sensitivity of CT for haemorrhage over time is responsible for the improved cost-effectiveness of MRI compared with CT in later presenting cohorts (see Table 71, scenarios 7 and 8). Here we demonstrate how the cost-effectiveness of MRI improves in relation to CT if the sensitivity of CT for haemorrhage drops in a more immediate presenting cohort (i.e. within a few hours of the initial event).
Uncertainty around the increased risk of recurrence of haemorrhagic stroke when incorrect treatment with antiplatelet therapy is given
With a reduced risk to 1.05 (the lower 95% CI on the risk of recurrent haemorrhage in patients with haemorrhagic stroke treated with antiplatelet agents), the option of immediate MRI and ultrasound is still dominated; however, there is a lower loss in QALYs than in the base case of immediate CT and ultrasound. The opposite effect occurs with an increase in the hazard rate; a higher QALY loss is observed.
Table 74 presents the results of one-way sensitivity analysis on several key variables for the comparison of immediate MRI plus ultrasound compared with immediate CT plus ultrasound in the later presenting (day 7) cohort of patients. The results indicate that the cost-effectiveness of the MRI strategy is quite sensitive to the cost of obtaining the MRI scan relative to the cost of CT, and also the relative increased risk of recurrent haemorrhagic stroke in patients with a minor haemorrhagic stroke treated with antiplatelet medication. When the cost of MRI is reduced or the RR associated with inappropriate antiplatelet medication is increased, the cost-effectiveness of MRI improves in the cohort of patients presenting at 7 days. The relative cost-effectiveness of alternative strategies is much less sensitive to the costs of recurrent stroke owing to only very small differences in the overall cumulative incidence of stroke between strategies. Cost-effectiveness also improves slightly when running the analysis over a period of 30 years.
| Scenario/strategies | Cost (£) | Incremental costs (£) | QALYs | Incremental QALYs | ICER |
|---|---|---|---|---|---|
| A. Immediate CT vs. immediate MRI with changes to the cost of the MRI scan | |||||
| a. Cost of MRI: base case – £174 | |||||
| CT + ultrasound | 3230 | – | 8.7425 | – | – |
| MR + ultrasound | 3296 | 66 | 8.7442 | 0.0017 | 39,509 |
| b. Cost of MRI £117 (lower quartile of NHS reference cost) | |||||
| MRI + ultrasound | 3230 | – | 8.7425 | – | – |
| CT + ultrasound | 3238 | 8 | 8.7442 | 0.0017 | 4870 |
| B. Immediate CT vs. immediate MRI with changes to the initial cost associated with recurrent haemorrhagic stroke | |||||
| a. Cost of haemorrhagic stroke: base case – (alive and independent £3445; alive and dependent £4841; fatal within 90 days £15,741) | |||||
| CT + ultrasound | 3230 | – | 8.7425 | – | – |
| MR + ultrasound | 3296 | 66 | 8.7442 | 0.0017 | 39,509 |
| b. Initial 3 month costs for non-fatal stroke increased to £371373 | |||||
| MRI + ultrasound | 3244 | – | 8.7425 | – | – |
| CT + ultrasound | 3310 | 65 | 8.7442 | 0.0017 | 39,069 |
| C. Immediate CT vs. immediate MRI with changes to the increased RR of recurrent haemorrhagic stroke in patients treated with antiplatelet medication following a minor haemorrhagic stroke | |||||
| a. Increased risk of haemorrhagic stroke with inappropriate antiplatelet drugs – base value 1.15 | |||||
| CT + ultrasound | 3230 | – | 8.7425 | – | – |
| MR + ultrasound | 3296 | 66 | 8.7442 | 0.0017 | 39,509 |
| b. Alternative value 1.05 | |||||
| CT + ultrasound | 3227 | – | 8.7436 | – | – |
| MR + ultrasound | 3296 | 69 | 8.7442 | 0.0006 | 120,140 |
| c. Alternative value 1.20 | |||||
| CT + ultrasound | 3231 | – | 8.7420 | – | – |
| MR + ultrasound | 3296 | 65 | 8.7442 | 0.0022 | 29,438 |
| D. Immediate CT vs. immediate MRI with changes to the modelled time horizon | |||||
| a. Base case – 20 years | |||||
| CT + ultrasound | 3230 | – | 8.7425 | – | – |
| MR + ultrasound | 3296 | 66 | 8.7442 | 0.0017 | 39,509 |
| b. 30 years | |||||
| CT + ultrasound | 3321 | – | 9.1987 | – | – |
| MR + ultrasound | 3387 | 66 | 9.2005 | 0.0018 | 36,156 |
Probabilistic sensitivity analysis
The results reported thus far are influenced by the mean values of the parameters. As each parameter estimate is associated with a degree of imprecision, it is important to gauge the effect of the level of imprecision on the reported values of cost-effective. To assess this, PSAs were conducted for the base-case model specification (scenario 1) and also the comparisons of immediate MRI plus ultrasound compared with immediate CT plus ultrasound in the later presenting cohorts (7 days and 21 days). In PSA the imprecision surrounding a number of variables is explicitly modelled; the estimated mean value of any random parameter has an associated measure of imprecision due to sampling variation; this is usually represented by the standard error of the mean estimate or other measure such as a 95% CI. Within PSA, this measure of imprecision is used to derive an empirical estimate of sampling distribution for each random variable (parameter) in the model, with assumptions made regarding the appropriate functional form distributions should take for different types of parameter. Monte Carlo simulation techniques are then used to randomly draw a value for each parameter from its assigned distribution, and the model is re-estimated using these values. This process is repeated a large number of times to build up a picture of the joint uncertainty surrounding the modelled costs and effects of alternative strategies, and also the ICER. By plotting the many estimates of incremental cost and effect pairs on the cost-effectiveness plane, cost-effectiveness acceptability curves can be derived which depict the probability of alternative options being preferred on grounds of cost-effectiveness by society’s per QALY gained.
The above process was followed to assign sampling distributions to the model parameters shown in Table 75. However, owing to limited availability of data illustrating the degree of precision surrounding several key inputs, several parameters were omitted from the PSA. These included the majority diagnostic performance parameters that were based to an extent on clinical opinion. Instead, we assessed the impact of variation in the key diagnostic performance parameters using deterministic sensitivity analysis (above). The other notable category of parameters omitted from PSA was to test variation in the baseline risks for recurrent stroke. With the approach used to estimate stroke risks by ABCD2 score, level of stenosis and time from event, it was not possible to assign distributions to these parameters. As such, the following analyses will likely underestimate the degree of uncertainty surrounding the cost-effectiveness of alternative options at different willingness-to-pay thresholds. Given the difficulty in assigning appropriate distributions to key model parameters, no formal value of information analysis was conducted.
| Description | Mean value | SE (estimated) | Distribution type |
|---|---|---|---|
| Sensitivity of CT for ischaemic stroke (< 7 days) | 0.39 | 0.135 | Beta |
| Cost of CT imaging | £101.00 | £29.10 | Gamma |
| Cost of MRI | £174.00 | £61.19 | Gamma |
| Cost per inpatient day | £297.00 | £104.48 | Gamma |
| Cost of treatment for MI | £4900.00 | £1623.00 | Gamma |
| Cost of endarterectomy | £3697.00 | £1349.00 | Gamma |
| Health state utility for recurrence free | 0.88 | 0.020 | Beta |
| Health state utility for alive and independent | 0.71 | 0.015 | Beta |
| Health state utility for alive and dependent | 0.20 | 0.005 | Beta |
| Health state utility for post MI | 0.76 | 0.058 | Beta |
| Health state utility non-stroke | 0.88 | 0.020 | Beta |
| LOS (haemorrhagic stroke moving to alive and dependent) | 16.3 days | 22.39 | Gamma |
| LOS (ischaemic stroke moving to alive and dependent) | 17.9 days | 22.39 | Gamma |
| LOS (haemorrhagic stroke moving to alive and independent) | 11.6 days | 11.94 | Gamma |
| LOS (ischaemic stroke moving to alive and dependent) | 14.5 days | 14.93 | Gamma |
| RR of ischaemic stroke with medical treatment | 0.53 | 0.148a | Log-normal |
| RR of MI with medical treatment | 0.65 | 0.120a | Log-normal |
| Increased RR of recurrent haemorrhagic stroke with antiplatelet drugs following a minor haemorrhagic stroke | 1.15 | 0.022a | Log-normal |
| Standardised mortality ratio for all cause mortality following a stroke | 2.72 | 0.02a | Log-normal |
| Standardised mortality ratio for all cause mortality following a TIA | 1.40 | 0.126a | Log-normal |
| Standardised mortality ratio for all cause mortality following a MI | 2.68 | 0.041a | Log-normal |
Figures 70–72 show the PSA results from scenarios 1, 7 and 8 in Table 71. Under scenario 1 (immediate CT compared with immediate MRI in an immediate presenting cohort), the CT strategy retains a high probability of being cost-effective in comparison with MRI (90–95%). This is primarily due to its significantly lower costs and very slightly improved effectiveness in comparison with the MRI strategy in the immediate presenting cohort.
FIGURE 70.
Scenario 1: immediate MRI plus ultrasound vs. immediate CT plus ultrasound.
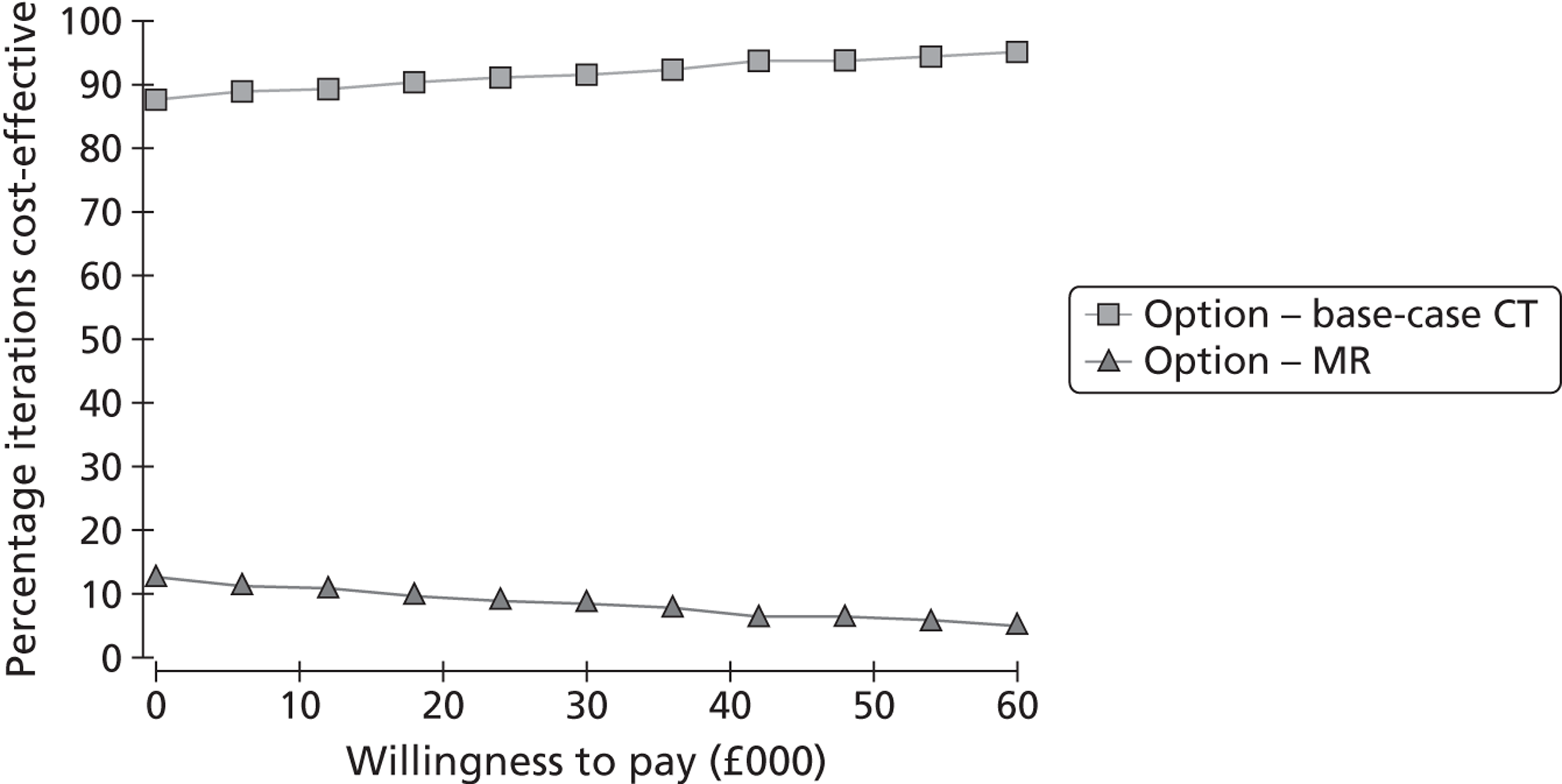
FIGURE 71.
Strategy 7: CT plus ultrasound vs. MRI plus ultrasound for cohort presenting 7 days of initial event.
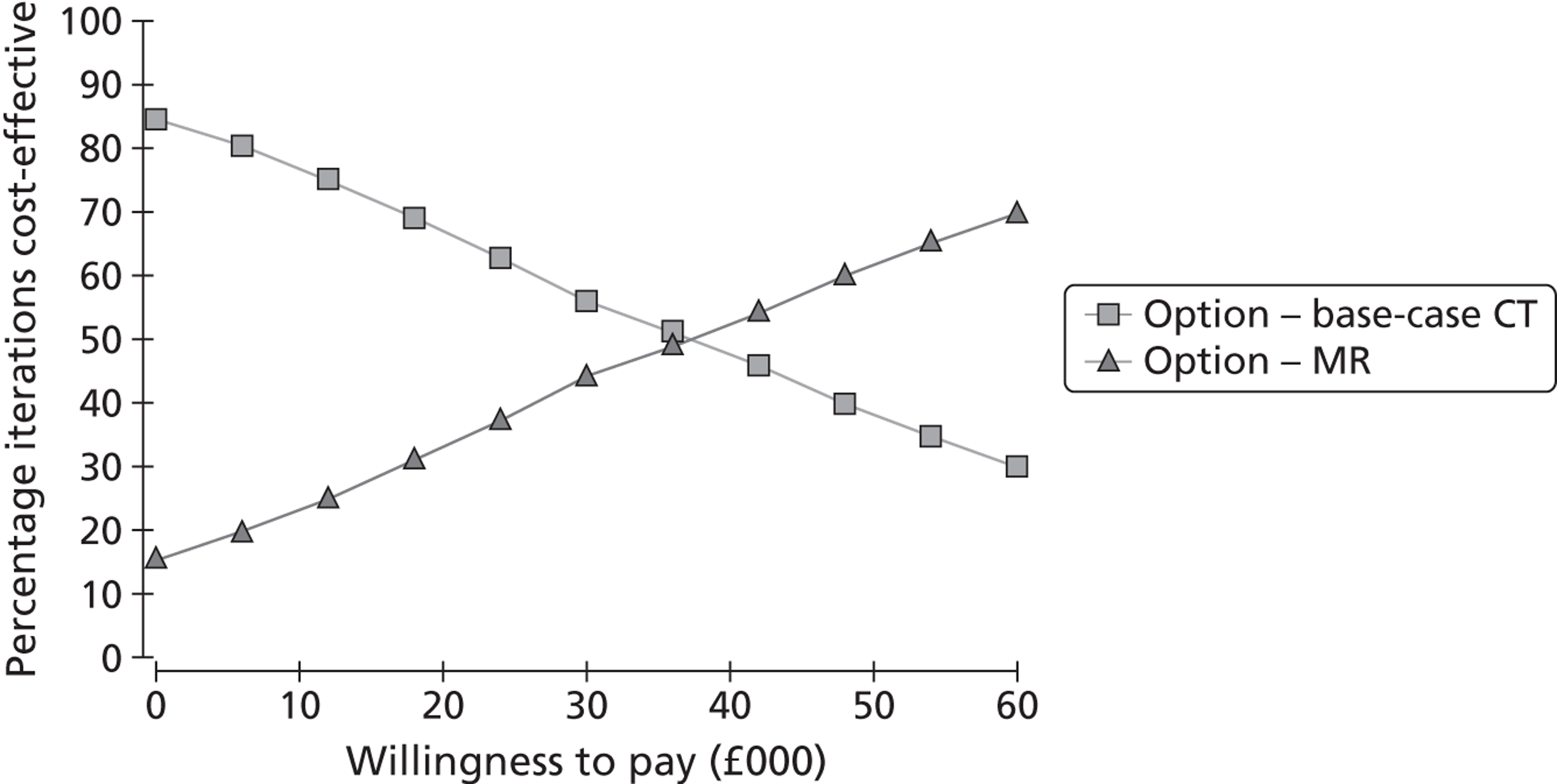
FIGURE 72.
Scenario 8: CT plus ultrasound vs. MRI plus ultrasound for cohort presenting 21 days after initial event.
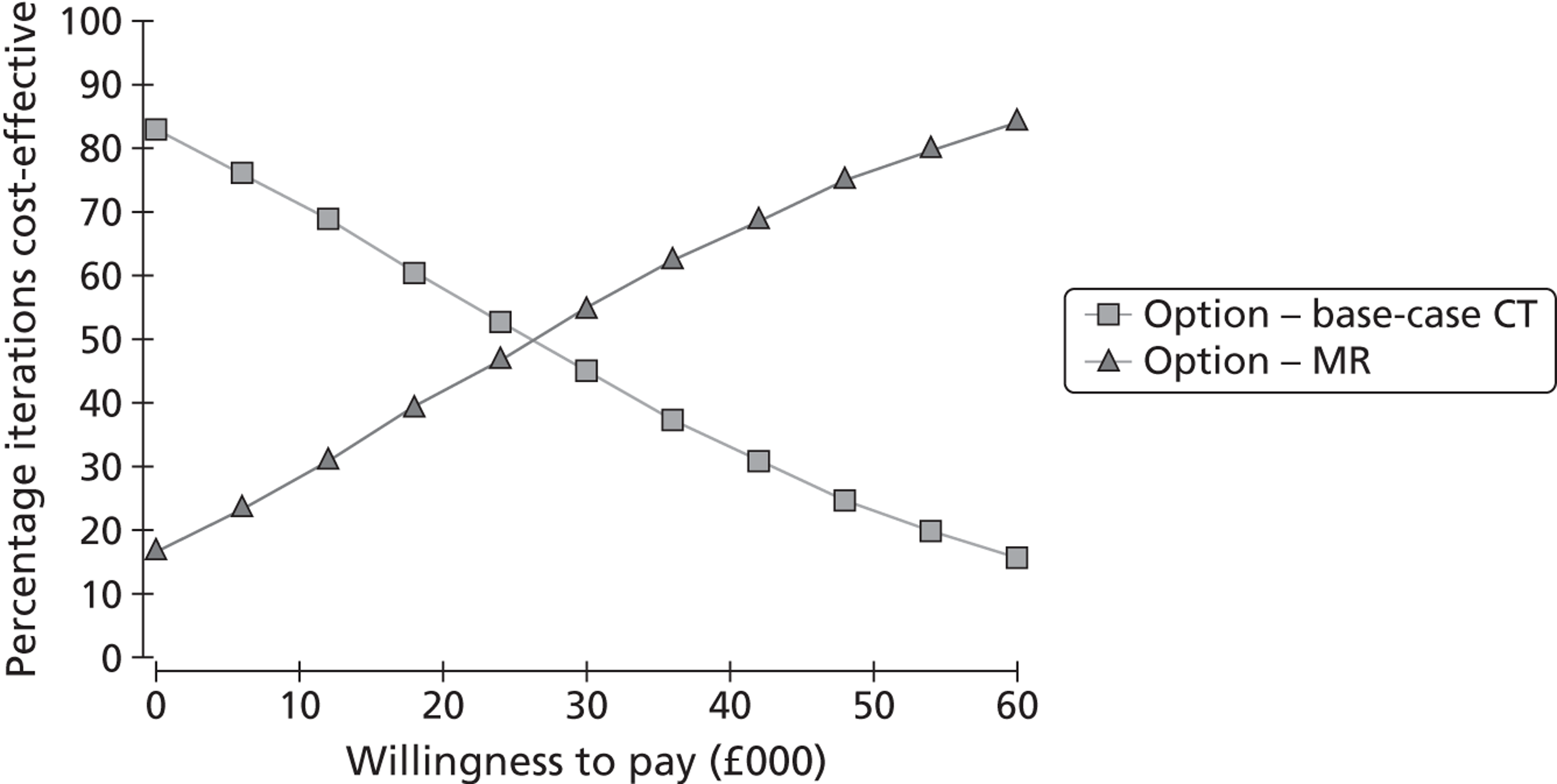
With scenario 7 [immediate CT vs. immediate MRI the later presenting cohort (7 days)], the probability of MRI being considered cost-effective increases as the threshold willingness to pay (WTP) per QALY increases. Above ≈£37,000, the MRI strategy has the higher probability of being cost-effective. In scenario 7 (immediate CT vs. immediate MRI for a cohort presenting at 21 days), the probability of MRI being cost-effective improves to ≈55% at the WTP threshold of £30,000 per QALY.
Discussion
Statement of principal findings
Magnetic resonance imaging strategies are generally found to be more expensive and no more effective than CT. The exceptions to this are for those strategies that involve later presenting cohorts. Here, MRI becomes more cost-effective, and its cost-effectiveness improves as its sensitivity for detecting haemorrhages improves over time, although its ICER falls only within, and does not fall below, conventionally accepted cost-effectiveness thresholds ranges (£20,000–30,000 per QALY) in the cohort presenting 21 day after the initial event. The cost-effectiveness of MRI in late-presenting cohorts (7 and 21 days) was found to be sensitive to fairly small changes in the cost of MRI relative to the cost of CT, and also the assumed relative increased risk of recurrent haemorrhagic stroke associated with inappropriate antiplatelet treatment of those patients who have suffered a minor haemorrhage. It should also be noted here that, although we present findings for the cost-effectiveness of MRI compared with CT at 7 days and 21 days, this was primarily due to available evidence suggesting that CT has high sensitivity for haemorrhagic stroke up to 7 days, and significantly lower sensitivity from day 7 onwards. However, if the sensitivity of CT for haemorrhage drops off more quickly, MRI may become the more effective strategy earlier than 7 days after the initial event.
Strengths and limitations of the economic model
A strength of the model is that it offers an explicit framework for the synthesis of both evidence and expert opinion. It uses structures developed in two previous HTA cost-effectiveness analyses that combine assessment of brain imaging (cross-sectional diagnostic decision tree approach) with risks of stroke (variable time-dependent approach), the former developed to assess the cost-effectiveness of brain imaging in acute stroke26 and the latter to assess the cost-effectiveness of carotid imaging in stroke prevention. 5 From this combined approach, gaps in the evidence base are revealed. There is good evidence from high-quality large RCTs on the effectiveness of different forms of medical and surgical management on the probability of stroke recurrence and by extrapolation on health-related quality of life. The main gaps appear to be in relation to the relative sensitivity and specificity of MRI and CT in TIA and minor stroke, and the impact these imaging modalities have on subsequent treatment and outcomes; the available evidence base is weighted towards sensitivity and specificity in moderate to severe stroke patients, and so, as discussed in earlier chapters, some assumptions have been made with respect to how these apply to TIA and minor stroke.
One potential limitation of the modelling strategy relates to the assumption regarding treatment of patients following information provided by imaging results. In the modelled base-case scenario, the sensitivity of MRI for detecting an ischaemic lesion is 0.63, whereas for CT it is 0.39. However, it is assumed that implementation of preventative treatment (for recurrent stroke) is based on clinical assessment as well as imaging, and not just the results from imaging in isolation, as is the case in clinical practice. Where either CT or MRI is unable to rule out a minor ischaemic stroke or TIA (i.e. by identifying a haemorrhage or mimic), we have assumed that implementation of secondary prevention for ischaemic stroke will prevail based on clinical assessment in patients without a visible ischaemic lesion. This is current practice. Further, we have assumed that CT and MRI, when combined with clinical assessment, are essentially equivalent in their ability to appropriately identify and rule out mimics, and that they do not result in different numbers of patients with true ischaemic stroke being misdiagnosed as mimics. The available evidence on the contribution of CT or MR to establishing a diagnosis in most of the mimics is not good but, on the other hand, there is information on the effect of MR in minor stroke on treatment decisions and on detection rates of positive findings that confirm the type of mimic in many types of mimics. There were no data on which to base other approaches, as mimics were excluded from all studies of DWI in TIA/minor stroke (see Chapter 6). It could be argued that the assumptions used may underplay the potential advantage that MRI offers through being better able to detect ischaemic lesions and therefore differentiate some true TIAs/minor strokes with a DWI lesion (one-third/two-thirds) from the two-thirds of TIAs/one-third of minor strokes without a DWI lesion and the mimics that make up 45% of clinic attendances in most of whom DWI would be negative. However, some mimics can produce a DWI-positive finding (migraine, hypoglycaemia, postictal, MS, etc.), so the impact on decision-making is not transparent. Differentiating these categories essentially boils down to clinical expertise with careful history and examination backed up by imaging. Thus, it is not clear whether that added advantage changes treatment decisions, and previous studies suggested that the differences conferred by MR DWI and T2* were small (< 10%),252 no treatment differences were modelled. It remains possible that in settings where clinical assessments and further tests are less expertly applied – and particularly where there was too much reliance on ABCD2 score-based filtering – the use of CT would result in more patients with a true ischaemic stroke or TIA (e.g. those with low ABCD2 scores) being misclassified as a mimic or haemorrhage, which could end up delaying appropriate preventative treatment for these patients. On the other hand, if MRI were to be relied on in place of expert opinion, it would only help sift out the one-third of TIAs and two-thirds of minor strokes with a DWI-positive lesion, leaving the two-thirds of TIAs/one-third of minor strokes without a DWI lesion, but still with a high risk of recurrent stroke, with no protection from stroke prevention therapy. Additionally, basing treatment decision more heavily on the findings of MRI, could result in more patients with a mimic being misclassified as an ischaemic stroke, or with an ischaemic event failing to receive treatment, which could lead to unnecessary or missed ischaemic stroke treatment and delayed diagnosis and appropriate treatment for the true underlying problem. These scenarios have been modelled by using ABCD2 score to triage the rapidity of imaging assessment and also by assuming that only patients with a DWI-positive lesion would receive stroke prevention treatment, neither of which was cost-effective.
Another issue that is problematic relates to estimation of the proportion of patients already on antiplatelet therapy and other medical treatment. HR data from randomised trials will probably overestimate potential benefits, as many patients who arrive with TIA or stroke may well already be on such therapy. The model may therefore overestimate the benefits of diagnosing and treating ischaemic stroke, although such an effect comes into play only in scenarios in which it is assumed that treatment is not implemented for those patients with no visible ischaemic lesion on MRI. In fact, as the UK survey and other sources298 indicate that many patients are started on stroke prevention treatment before they reach the clinic, and indeed this is the recommendation in the NICE guidelines,97 the benefit of MRI becomes even less and the relative cost even worse. Furthermore, the survey and other data indicate that MRI is often used in addition to CT, further increasing costs, burdening imaging services and reducing the incremental effectiveness of MRI.
A further potential limitation is that health-related quality of life has been aggregated into three broad categories: (1) alive and free from stroke; (2) alive and independent following a recurrent stroke; (3) and alive and dependent following a recurrent stroke. This makes the model insensitive to changes in health-related quality of life within particular categories; however, as the focus of the model relates to the contribution of imaging to diagnostic accuracy and subsequent prevention of recurrent stroke, these broad categories should be sufficient to capture the average expected impact of strategies on QALYs.
On the costs side, there is wide disparity in the available estimates for the costs of imaging. What is important for cost-effectiveness, however, is the size of the difference in costs between CT and MRI, rather than whether or not the exact values applied are too high or too low. In this case, there is a good deal of evidence available to suggest that MRI uses more health-care resources than CT; in addition to its greater acquisition price, the test takes longer to perform and it uses more consumables and materials. The important issue then is whether the cost increase is worth incurring in light of any expected health benefits or downstream cost savings. Taking the cost of a medical consultant as £146 per patient-hour,394 an extra 20 minutes of a consultant radiologist time costs approximately £60. It is possible then that the £70 difference is an underestimate. The effect of a larger and smaller difference, however, is tested in sensitivity analyses. In particular, the relative cost of MRI in comparison with CT is a key determinant of the cost-effectiveness of MRI in later-presenting cohorts.
Chapter 13 Discussion
Stroke is a devastating illness. Prevention of stroke is very important to minimise its disabling physical and cognitive consequences. Among those with a warning TIA or minor stroke, rapid assessment, identification of potential stroke causes and appropriate treatment can prevent many of these disabling strokes.
No one would question that the mild symptoms and signs of a TIA or minor stroke are difficult to separate from other causes of mild neurological or cardiac symptoms or that the diagnosis of TIA/minor stroke is difficult, particularly for non-experts. Or that identification of underlying risk factors, such as carotid stenosis or cardiac abnormalities, for which we have established treatments based on evidence from large multicentre randomised trials,395 should be a priority. Same-day clinics, where all priority investigations are obtained and treatment decisions implemented, save time and are cost-effective. 5,286 This much is straightforward.
Transient ischaemic attack/minor stroke is very common. So are mimics. Any system trying to deliver rapid assessment and treatment needs to be focused on priorities and streamlined to avoid becoming overwhelmed with the sheer numbers of patients. Stroke is a government priority with targets and incentives aiming to change practice to improve stroke prevention and treatment. 360 Methods to identify high-risk patients could quickly help streamline stroke services. Clinical risk prediction scores, particularly the ABCD2 score, have achieved prominence in high impact journals, becoming the subject of entire sessions at recent stroke conferences. Excitement about the sensitivity of MR DWI to detect ischaemic lesions in patients with TIA/minor stroke and clinical risk prediction scoring have changed guidelines,97,360 the DOH guidelines adopting the ABCD2 score with a cut-point of ≥ 4 against < 4 for triage, and suggesting that about 50% of TIA patients should have MRI to diagnose the TIA/minor stroke and triage speed of treatment.
Coordinated outpatient services with rapid evaluation, identification of key risk factors and treatment to prevent recurrent stroke are very important and, according to our survey, are now available in most parts of the UK. However, some aspects of current practice introduced in the last decade are not evidence based, may have been implemented rather hastily and may in fact not be as effective as was hoped, or have unintended consequences due to problems with interpretation and use of some information on which these practices are based. The rising use of MR in stroke in the last decade, often supplementing rather than replacing CT scanning, as shown in the survey but also happening outside the UK, makes neuroimaging the fastest growing component of hospital care costs for stroke in the last decade. 298 Furthermore, several organisations, including the AHA,17 have endorsed a change in the definition of TIA from time based to tissue based.
Many points relating to the gathering of evidence for this analysis have been discussed in detail in the relevant chapters and we do not propose to repeat those here. Rather we will highlight general and overarching points relevant to the use of imaging in stroke prevention in general and related issues that this work has thrown up. There are some large holes in the evidence on which some practices are currently based. That not so many clinics may actually be following these practices, as suggested in the survey of UK clinics, may be beneficial.
In general, in every topic that we meta-analysed, there was substantial heterogeneity (I2-statistic values typically in the 90s). We could not account for this heterogeneity by obvious causes such as retrospective compared with prospective recruitment, diagnosis, risk score, year of publication, time to assessment, etc. Second, despite this heterogeneity, the overall messages were clear.
The early risk of stroke after TIA may have been overestimated and certainly the pooled cumulative risk (see Chapter 3) was inconsistent with the 20% suggested in the 2008 NICE guidance on stroke. 97 Among 53 studies (30,558 patients) the pooled cumulative recurrent stroke rate was 5.2% (95% CI 3.9% to 5.9%) by 7 days, 6.7% (95% CI 5.2% to 8.7%) by 90 days and 11.3% (95% CI 7.5% to 16.6%) in the few studies that provided information for > 90 days. Of course, within this are subgroups for which recurrent stroke rate is much higher, notably those with symptomatic tight carotid stenosis. We applied subgroup-specific recurrent stroke rates where appropriate in the model. We cannot tell to what extent this lower stroke cumulative risk is a consequence of medical or surgical treatment of patients in these observational studies as secondary prevention was rarely mentioned. All we can say is that there was little evidence of any change in the rate over the last 20 years during which increasing awareness of stroke risk after TIA has probably increased speed of initiation of treatment.
The ABCD2 score does not identify all patients with key underlying risk factors which we know cause most recurrent stroke, particularly early recurrences, and for which there are well established evidence-based treatments from large RCTs and meta-analyses. 25 Symptomatic carotid stenosis and AF are the two common and obvious ones that together are responsible for a large proportion of recurrent stroke. When carotid stenosis is removed by successful endarterectomy, the stroke risk drops immediately to background rates. 169 Similarly, RCTs show that antithrombotic, cholesterol-lowering therapy and antihypertensive therapy reduce stroke risk, although the immediacy with which the risk is reduced is based on observational data24 rather than RCT evidence at present.
Use of ABCD2 scores, particularly by non-specialists, may in fact be failing , paradoxically, to prevent stroke. ABCD2 does not differentiate mimic from true TIA/minor stroke (see Chapter 4). 396 Mimics occupy a substantial part of the stroke prevention service – nearly half (see Chapters 7 and 8). This will slow down the access to services by true TIA/minor stroke. One-third of mimics have an ABCD2 score of ≥ 4 (see Chapter 4) and so would go down the fast-track route and undergo MR. Meanwhile, one-fifth of true TIA/minor stroke patients with low ABCD2 scores have carotid stenosis or cardiac embolic risk, and are at risk of having a recurrence while waiting for investigation if put in the slow stream. That this is a real problem has been amply demonstrated by some clinics in France164 and the UK,181 where patients with low ABCD2 scores have had recurrent stroke either by waiting too long in the slow stream or missing out on investigation altogether because of the creeping perception that patients with low ABCD2 scores do not have recurrent stroke. Furthermore, in the economic modelling, delaying investigation in patients with ABCD2 score of < 4 resulted in a fourfold reduction in QALYs over the QALY reduction that occurred with immediate MR and ultrasound for all.
It is unfortunate that the reliability of the data on the ABCD2 score and MR DWI was undermined by study methods, particularly factors such as nearly half of studies assigning the ABCD2 score or DWI findings retrospectively from case note review. This and related methodological problems are not uncommon in research on clinical risk prediction scores. 397 Greater awareness of these problems should improve this type of research. Many studies did not clearly differentiate TIA from minor stroke, yet the ABCD2 score may not work in minor stroke. 186 Few studies provided any information on treatment or identification of key risk factors like carotid stenosis or AF or how these patients were handled in the analysis of risk prediction. It would be unethical not to offer immediate treatment, but that would reduce by a large degree the recurrent strokes that these studies were trying to observe. Another point was that a large proportion of the data on several aspects of stroke prevention came from the same few research groups.
The ABCD2 score was not designed for, or tested for use by, non-specialists, yet DOH tariffs98,368 in England relate to ABCD2 scores assigned by GPs or other non-specialist referring doctors. Also, in about one-third of stroke prevention clinics, the assessments and ABCD2 score assigning are undertaken by nurses. A full work-up achieved quickly can earn between £450 and £634 per patient when the ABCD2 score and DWI have been used and the patient is started on treatment within 24 hours of referral. 98,99 Given that large proportions of patients arrive at the stroke prevention clinics having already been started on secondary prevention treatment by their GPs, it is even more unlikely that further fast-tracking with ABCD2 scores is going to add anything, although it could still detract by slowing endarterectomy in patients with tight stenosis who are put in the slow stream. One can only conclude that at present, as used in stroke prevention clinics, the ABCD2 score is unlikely to be contributing helpfully to prevention of stroke.
The ROC curve for any stroke prediction score improves by adding results of brain imaging with CT or DWI, but there are still patients with key risk factors who get low scores. In any case, it seems odd not to look for the risk factors directly with ultrasound and cardiac investigations. The survey (see Chapter 8) demonstrated that carotid imaging was widely available, mostly with ultrasound, on the same day as clinic assessment in most centres. Therefore, why use an indirect, proxy method that will inevitably be less accurate or effective than identifying the risk factor directly, particularly when the ultrasound or cardiac tests would still need to be performed in patients with high ABCD2 ± CT or DWI scores? It seems that all that the ABCD2 score ± brain imaging is achieving in this situation is delaying making the correct diagnosis, the one thing that stroke prevention clinics are supposed to avoid!
There is probably more information to be derived from plain CT findings as most studies (and we found about three times as many studies than in previous reviews, Chapter 5) did not specify if any infarct was relevant, recent, old and irrelevant, or just a sign of leukoaraiosis. That CT may help predict recurrent stroke is useful and not surprising, but getting the best information out of it may require knowing whether the lesion is likely to be relevant to the recent symptoms or not. Prior irrelevant infarct or leukoaraiosis may just indicate a vulnerability to infarction at some point in the future, for example, rather than a specific early risk of recurrence.
Most patients with TIA (two-thirds) and a large proportion of patents with minor stroke (one-third) do not have an acute ischaemic lesion on DWI, but equally they do not have any other cause of symptoms apart from the TIA/minor stroke. This questions the sense in moving towards a tissue-based definition of TIA or minor stroke. Reasons for substantial heterogeneity in rates of positive DWI (see Chapter 6) were not obviously explained by time to scanning or other factors that we were able to address, and may reflect variation in scanner technology, sequences used, expertise of the scan rater, etc. All of these are likely to persist meaning that it will be difficult to standardise the DWI results enough to remove this heterogeneity. However, it would probably be premature to change the definition of TIA if it leaves many patients potentially undiagnosed or completely changes the epidemiology of the condition for reasons that we cannot currently explain.
Ischaemic change on DWI disappears over a few days from lesions that produce mild symptoms such as TIA, even although we were not able to show an obvious change in the positive rate with time lapse after stroke in the published data (see Chapter 6). By extrapolation from ischaemic stroke where it is well known that the DWI high signal normalises over several days,398 and faster in milder than in more severe stroke, and faster in grey than white matter,44 the DWI-positive signal is likely to disappear in several days from the one-third of TIA patients with positive findings. Hence, it is worrying that the average time to undertaking MRI in TIA/minor stroke in the UK is 1 week or longer. Although late scans would detect haemorrhage, if a T2* sequence was used they would be less likely to detect recent ischaemic change, rendering the clinical value and cost-effectiveness even lower than with immediate scanning. If the definition of TIA changes to tissue based then patients who arrived late after their TIA, by which time the DWI acute lesion had disappeared, would be classed as not TIA or stroke. The public could use this information to their advantage, for example if they were concerned about loss of insurance or loss of a driving licence – simply delaying their attendance at clinic could avoid them being diagnosed as stroke or TIA and their unfortunate and inconvenient consequences. That they might also miss out on important secondary prevention measures, leaving themselves at high risk of recurrent stroke, would be very unfortunate.
More worryingly, until imaging departments can routinely use the correct and minimum set of sequences to diagnose stroke, whether haemorrhage or ischaemic and its mimics, wider use of MR should not be encouraged as it may be dangerous. Only 45% of departments included a T2* sequence to detect haemorrhage. This means that many departments are failing on the one established reason for performing a MR scan in TIA/minor stroke – to identify haemorrhage in patients presenting too late for CT to identify haemorrhage. 31
As an approximation, among 1000 suspected TIA/minor stroke patients referred to stroke prevention clinics, of whom about 45% would have a non-CV diagnosis, DWI would be positive in only 19% of patients with suspected TIA and 38% with suspected minor stroke, leaving a large proportion of negative, non-contributory scans and a large bill for the scanning.
In the UK, as elsewhere,298 MR seems to be used in addition to rather than instead of CT in many patients. This renders the cost-effectiveness of any imaging in stroke prevention even less than it would be if one or other were used alone. Reasons for this double use are unclear and may reflect ease of access. For example, if CT is perceived to be easy to get and ‘better to have than nothing’ while waiting for the MR then it is all too easy to request a CT immediately followed by a MRI. However, the point of this is unclear except in a small proportion of unusual patients. Moreover this practice inflates workloads for staff in imaging departments who have to perform and interpret the scans and perform receptionist and secretarial duties, the porters who have to make two trips to the imaging department for inpatients, and disrupts the recovery and lives of the patients themselves. Inpatients may have therapy sessions interrupted or miss visitors (important emotionally for recovery after stroke and for discharge planning); for outpatients, making two trips doubles the cost of transport and the time taken, and may disrupt relatives and friends as well if the patient (as is often the case) relies on someone else for transport, which, in turn, may disrupt other employment or child-care duties. This impact on inpatients and outpatients, and their carers may be far more costly or disruptive than the impact on staff, but we were unable to quantify these aspects of the effects of stroke prevention strategies in the modelling. It requires some thought on the part of those who are requesting these duplicate investigations as to what is really in the best interests of the patients and whether the additional information from a follow-up MR scan – performed, on average, when it is too late to see many ischaemic lesions and when most departments are not including blood-sensitive sequences and so will miss haemorrhage as well – is really worth the added cost and disruption to the patients and imaging departments.
Delays to carotid endarterectomy are still too long: median of 21 days in the 2011 report from the Royal College of Physicians of London,399 although this was a reduction from median 28 days in 2010400 or 30 days in Canada in 2009. 401 Delays were blamed previously, among other factors, on lack of awareness among the public and hence in presenting to medical attention. There is less reason for lack of awareness now following many public awareness campaigns but there will be little point in patients presenting early if they then encounter delays through being triaged inappropriately or waiting for imaging that is unlikely to be contributory. It is too easy to put off a decision for endarterectomy while ‘waiting for the scan’. If a CT scan can exclude a haemorrhage or a tumour/other important structural cause, the carotid imaging shows a tight stenosis and the clinician finds no other cause for symptoms apart from TIA/minor stroke then it is best to activate surgery (or other treatment) as fast as possible.
In the cost-effectiveness analysis, MRI strategies were generally more expensive and no more or even less effective than those using CT in some cases. The exceptions to this were for those strategies that involve later presenting patients. Here, MRI became more effective, and its cost-effectiveness improved as its sensitivity for detecting haemorrhages remains with time, although even in that scenario, its ICER did not fall below conventionally accepted cost-effectiveness thresholds (£20,000–30,000 per QALY). However, this result was sensitive to fairly small changes in the cost of MRI relative to the cost of CT, and also the assumed relative increased risk of recurrent haemorrhagic stroke associated with inappropriate antiplatelet treatment of those patients with minor haemorrhage.
The lack of relevant evidence on the effect of diagnostic imaging on outcomes, in contrast with the relatively large amount of data from randomised trials on the effects of medical or surgical interventions, is not unique, being a common problem afflicting diagnostic imaging and other technologies. A previous attempt in 2008 to calculate the cost-effectiveness of MR DWI in TIA was abandoned due to lack of relevant evidence. 105 We would argue that there is still a substantial gap in relevant evidence but not such that a reasonable attempt at estimating cost-effectiveness cannot be made. We would also emphasise the importance of not introducing new technologies, be they diagnostic tests or risk scores, without proper evaluation and an adequate evidence base on which to determine their risk and benefit and impact on clinically relevant outcomes. It would be more or less unthinkable to introduce a new drug into clinical practice without testing in large, generalisable RCTs in relevant patient populations. Diagnostic tests and risk scores should be no different. They work, if they do, by enabling a series of steps that leads to improvements in clinically relevant patient outcomes. Therefore, they should be tested in RCTs and there should be adequate data on their sensitivity and specificity and accuracy, instead of virtually none.
The effect on numbers of strokes prevented was small between different strategies, but, when multiplied across the population for such a common condition, should not be dismissed. In any case, no one should have a stroke if we have the knowledge and means to prevent it. We may have underestimated the number of recurrent strokes that would occur in some strategies because of the difficulty of introducing enough granularity in the various streams of patients with various risk factors passing through the model. Thus although we modelled an increased risk for patients with tight symptomatic carotid stenosis, we were not able to do this across a range of stenoses and nor did we model the effect of failing to treat cardiac sources of emboli, such as AF. However, regardless of the actual number of strokes not prevented, some strategies clearly would fail by a larger degree to prevent strokes, and failing to prevent stroke through failing to implement established evidence effectively and through the unintended consequences of changes in health policy or strategies is unsupportable and against all of the principles of medical practice.
Limitations of the work not mentioned above should be discussed. In general, only one reviewer performed the searches and initial checking of references. Having another reviewer to duplicate the searches and undertake initial paper triage was well outside the resources available for the work. However, we did other things to validate the searches and find all relevant papers, such as hand-searching of journals and cross-checking where relevant with other systematic reviews. Two researchers assessed potential papers and extracted data, so we are confident that these later steps have been as rigorous as possible. We did not exclude any non-English papers except when we were completely unable to obtain a translation. Our combined languages included Italian, Spanish, Portuguese, French, Japanese, Chinese and Russian, plus we have colleagues in Scandinavian and other countries who could have provided translations from other languages had we needed them. Thus in practice we excluded virtually no papers on the basis of being non-English. We did not check the extracted data with the original authors or contact the authors for unpublished data, as these steps were outside the staff resources and time limits available for the work. As it was, the work benefited from the contribution of a full-time visiting fellow from Chile (HM) and, towards the end of the project, a second health economist in Aberdeen (GS), without whom it would have been impossible to complete. We could have modelled other strategies but there seemed little point. It was already clear that MR was more costly than CT and (with one exception) no more effective, and the data were inadequate to support modelling of further strategies. For example, we did not repeat all of the strategies while assuming that all patients were already on stroke prevention treatment because use of MR in patients who were already on treatment would simply reduce the cost-effectiveness of MR even further.
Conclusions
The available data suggest that there is little justification for use of ABCD2 or other risk prediction scores to triage patients, particularly as currently implemented in many clinics. Rather, the resources that are already available across the UK according to the survey (see Chapter 8) should be focused on direct identification of underlying key risk factors, identifying the small proportion of patients for whom haemorrhage is the cause of the stroke, and differentiating mimics. This requires specialist input. Although we did not test specialists compared with non-specialists directly, the available data point to large numbers of mimics being referred as suspected TIA/minor stroke by non-specialists. The differentiation of many of the mimics from true TIA/minor stroke (see Chapter 7) require careful history and examination for subtle features that generally take many years of experience to develop. It is unlikely ever to be something that can be done by a non-specialist. The ABCD2 score and a MR scan do not replace the specialist. Patients with non-CV diagnoses, who make up a large proportion of these clinics, should be managed appropriately and sensitively. They need to be correctly distinguished from true TIA/minor stroke to avoid the consequences of being incorrectly diagnosed as TIA/minor stroke (driving cessation; ineffective and possibly risky drug treatment; insurance implications), as well as requiring correct treatment for the cause of their mimic, many of which are not benign.
The cost-effectiveness evidence would suggest that MRI does not appear to be a cost-effective use of resources for the routine diagnosis and management of TIA and minor stroke. However, in the subpopulation of patients who present at > 1 week after stroke and in whom CT will not be able reliably to identify haemorrhage, MR with T2* sequence is better than CT (thought to be still more expensive) and should be reserved for these patients. MR DWI might also help in the occasional patient in whom it is not clear if the symptoms are due to a tight carotid stenosis or are unrelated in the posterior circulation. Separately, patients with suspected unusual causes of TIA/minor stroke (dissection, rare genetic variants, etc., as are more common in young patients) are also more likely to benefit from MR. However, even the use of MR in these minority subpopulations is limited as the advantage in terms of added QALYs remains quite limited.
Detailed recommendations for current practice:
-
The evidence suggests that stroke prevention is more likely to achieve maximum effectiveness if services are staffed primarily by specialists with experience in diagnosis and differentiation of TIA, minor stroke and its mimics.
-
In all patients with probable TIA/minor stroke, evidence suggests that the focus should be on performing appropriate, rapid, risk factor-directed investigations to identify tight symptomatic carotid stenosis or AF/other cardiac embolic source and implementing appropriate treatment rapidly is likely to prevent most strokes.
-
Patients thought not to have a true TIA/minor stroke after review by an expert, i.e. the 45% with a mimic, many of which are cardiac or neurological, also require appropriate treatment including avoiding the costly and potentially harmful effects of unnecessary antiplatelet, statin and antihypertensive treatment.
-
Initial triage using the ABCD2 score alone risks relegating patients with key underlying causative lesions but with low ABCD2 scores to ‘slow stream’ investigation and treatment exposing them to high early recurrent stroke risk.
-
The benefits of the ABCD2 score on clinical practice and important outcomes have not yet been demonstrated in reliable, generalisable studies.
-
The justification for routine use of MR in most TIA or minor stroke is unclear. Exceptionally, MR is indicated in patients presenting at > 1 week after symptom onset when MR with T2* is better than CT for identifying haemorrhage, possibly where an unusual cause of symptoms is considered, or where there is doubt if a TIA/minor stroke is in the territory of a tight carotid stenosis. However, in most ‘typical’ TIA/minor stroke patients presenting rapidly, use of MR inflates costs substantially, confers no advantage in stroke prevention, may increase risk through increasing delays to treatment, and disadvantages patients with other conditions for which there is clear evidence of benefit.
-
The evidence indicates that patients diagnosed as TIA/minor stroke by a stroke specialist, but who do not have a visible ischaemic lesion on DWI, benefit from secondary stroke prevention treatment; not implementing stroke prevention treatment will leave two-thirds of patients with TIA, and one-third of patients with minor stroke who have no lesion on DWI, at high risk of recurrent stroke.
-
Failure to include T2* sequence as part of the MR examination for stroke or TIA, as currently happens in 55% of UK services, will fail to diagnose haemorrhage and expose patients with haemorrhage to the risk of recurrent haemorrhage if incorrectly diagnosed and treated with antiplatelet or anticoagulant drugs.
-
MR DWI alone will not identify underlying risk factors, nor will it correctly differentiate stroke/TIA from mimic, identify haemorrhage or even correctly identify all true TIAs or minor strokes.
-
The use of MR in addition to CT is not justified, at least not at its current frequency: using either CT or MR but not both would help streamline flow through TIA/minor stroke investigative pathways.
-
Ultrasound is cost-effective and its position as the most widely used investigation for carotid stenosis continues to be justified; CT or MR angiography are not cost-effective alternatives, even when combined into one examination session with brain CT or MR.
-
The justification for changing the definition of TIA from time based to tissue based is unclear, as two-thirds of specialist-diagnosed TIAs and one-third of minor stroke do not have a DWI-visible lesion.
-
There are still many delays during assessment of patients with suspected TIA/minor stroke, including to endarterectomy; efforts to identify and overcome the causes of those delays would increase speed of investigation and prevent more strokes.
Recommendations for research
As stated in two prior analyses for the HTA on cost-effectiveness of imaging in stroke, more information is needed on costs of stroke and of investigations – it seems increasingly bizarre that there is not better information on how much it costs to deliver health care for common disorders.
No technology, including scoring systems of which there are now many in different areas of medical practice, should be introduced into clinical practice without a thorough evaluation of the evidence demonstrating its impact on clinically relevant outcomes, including comparisons of the score with the best previous alternative,402,403 ideally from RCTs. Observational comparisons may be misleading or uninformative. 179 The principle of using the least biased evidence, which is most likely to come from randomised trials, applies even more to technologies like diagnostic imaging – diagnostic imaging affects treatment decisions and therefore clinical outcomes and should be regarded as no different to the testing of a new drug. The methods required for testing diagnostic imaging may be a bit different but the principle of identifying the benefit and hazard is the same as for drugs or surgical treatments.
More data are needed to determine which imaging strategy is best in routine stroke prevention, preferably from a randomised trial on the subsequent treatment, health-care costs, health-related quality of life and survival of patients with suspected TIA or minor stroke presenting at stroke clinics – randomised to initial diagnosis and management with MRI or CT. Such studies could control for other confounding variables at baseline, such as number of days delay between initial onset of TIA/minor stroke and time of imaging, and number of days between imaging and initiation of treatment. Further microcosting studies on CT and MRI, to gauge the size of the cost difference between the two techniques would also be beneficial. Information on the cost of many other aspects of stroke care is also limited and needs to be improved.
The definition of TIA is being changed from a time-based to a tissue-based definition, because of excitement around the ability of DWI to detect small ischaemic lesions in TIA and minor stroke. 17 This may be premature, given that two-thirds of TIA and one-third of minor stroke after assessment by a specialist and in whom no other diagnosis is available, do not have a DWI lesion. Does this mean that they will no longer be classed as TIA or minor stroke? What if the variation between stroke centres is as much as that seen in the DWI meta-analysis (see Chapter 6)? Does that mean that the proportions of TIA or minor stroke patients seen in different hospitals could vary so widely, and for as yet unaccounted for reasons? It also means that a patient could be diagnosed as stroke if arriving early at one hospital with rapid access to DWI but be diagnosed as TIA if admitted to another hospital that either had no MR scanner or limited access or the patient just arrived to late for their lesion to still be visible. Basing the diagnosis of a common condition, such as TIA or stroke on such a variable phenomenon as visible lesion on DWI, is likely to be problematic? Redefinition of TIA with infarction on DWI as stroke will, overnight, increase the annual incidence of stroke by about 15% and improve statistics on independent survival substantially, as these new strokes will be mild and raise the overall average of good outcomes after stroke. 404
Further recommendations are as follows:
-
Reliable, relevant and generalisable data on use of MR in TIA/minor stroke is needed, preferably from a RCT in which patients are randomly allocated to a policy of ‘early CT brain scan’ compared with ‘early MR DWI and T2* brain scan’ with a primary outcome based on recurrent stroke and vascular death.
-
Data on accuracy of MR DWI and T2* in TIA/minor stroke and its mimics, in comparison with CT scanning is required – there are no reliable data at present.
-
Use of the ABCD2 score by non-specialists including nurses and GPs should be tested against use by stroke experts to determine the reliability of the ABCD2 score.
-
The effect of changing TIA definition from time based to tissue based is required in large populations across multiple stroke centres and scanners in order to determine the effect on TIA/stroke incidence, prevalence and outcome, preferably before the definition is changed.
-
Research should examine why MR is frequently used after CT as reducing the rate of duplicate examinations and finding effective ways to triage patients to one examination would help to improve efficient use of the staff and equipment resources.
-
Reliable data on all costs related to stroke including all different imaging modalities and the effect of different operational settings, and their variation with different health-care scenarios are required.
-
Research into the reasons for substantial heterogeneity between studies on all topics related to TIA assessed here is required to improve consistency of diagnosis. The wide variation observed is unlikely to reflect true differences in disease frequency.
Acknowledgements
We thank Dr Peter Tothill, lay representative, who attended project meetings and commented on the relevance of the design and of the model to stroke prevention clinics, and of the outcomes to patients. We thank Professor Keith Muir and Professor Donald Hadley for assistance with obtaining costs on imaging in different hospitals and on the design of the questionnaires for the survey of stroke services in the UK. We thank Dr Janet De Wilde for assistance with the design of the questionnaire for the survey of UK imaging departments and for contacting imaging departments across the UK.
Contributions of authors
Joanna Wardlaw (Professor, Neuroradiology) designed the original concept, obtained funding, conducted project oversight, protocol writing, data identification, extraction, quality assessment, data analysis and interpretation, report writing, and had overall responsibility for co-ordinating the project and the final report.
Miriam Brazzelli (Research Fellow, Health Services Research) contributed to study selection, data extraction, quality assessment, data analysis and report writing.
Hector Miranda (Staff Neurologist, Neurology) contributed to study selection, data identification and extraction, quality assessment, data analysis and report writing.
Francesca Chappell (Medical Statistician, Medical Statistics) contributed to study selection, data extraction, quality assessment, data analysis and report writing.
Paul McNamee (Reader, Health Economics) contributed to obtaining funding, oversight of the health economic modelling, data identification, health-economic modelling, interpretation and report writing.
Graham Scotland (Senior Research Fellow, Health Technology Assessment) contributed to health-economic model construction and testing, data analysis, interpretation and report writing.
Zahid Quayyum (Research Fellow, Health Economics) contributed to data identification and extraction, model construction, data analysis and report writing.
Duncan Martin (Scientific Business Manager, Financial Management of Healthcare) contributed to data collection for costs data and report writing.
Kirsten Shuler (Project Administrator, Scientific Administration) co-ordinated references and publication databases, and assisted with project co-ordination and compilation of the final report.
Peter Sandercock (Professor, Neurology and Stroke) contributed to the obtaining of funding, provided clinical and/or methodological input and advice throughout the project and commented on ongoing analyses and drafts of the report.
Martin Dennis (Professor, Stroke) contributed to the obtaining of funding, provided clinical and/or methodological input and advice throughout the project and commented on ongoing analyses and drafts of the report.
Publications
Brazzelli M, Shuler K, Quayyum Z, Hadley D, Muir K, McNamee P, et al. Clinical and imaging services for TIA and minor stroke: results of two surveys of practice across the UK. BMJ Open 2013;3:e003359.
Brazzelli M, Chappell FM, Miranda H, Shuler K, Dennis MS, Sandercock PAG, et al. Diffusion-weighted imaging and diagnosis of transient ischaemic attack. Ann Neurol 2014;75:67–76.
Disclaimer
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Feigin VL, Lawes CMM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol 2003;2:43-5. http://dx.doi.org/10.1016%2FS1474-4422%2803%2900266-7.
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 2006;367:1747-57. http://dx.doi.org/10.1016%2FS0140-6736%2806%2968770-9.
- Luengo-Fernandez R, Gray AM, Rothwell PM. Costs of stroke using patient-level data: a critical review of the literature. Stroke 2009;40:e18-23. http://dx.doi.org/10.1161%2FSTROKEAHA.108.529776.
- Swain S, Turner C, Tyrrell P, Rudd A. Guideline Development Group . Diagnosis and initial management of acute stroke and transient ischaemic attack: summary of NICE guidance. BMJ 2008;337:291-3. http://dx.doi.org/10.1136%2Fbmj.a786.
- Wardlaw JM, Chappell FM, Stevenson M, De Nigris E, Thomas S, Gillard J, et al. Accurate, practical and cost-effective assessment of carotid stenosis in the UK. Health Technol Assess 2006;10.
- Wardlaw JM, Murray V, Berge E, del Zoppo G, Sandercock P, Lindley RL, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet 2012;379:2364-72. http://dx.doi.org/10.1016%2FS0140-6736%2812%2960738-7.
- Dennis MS, Bamford JM, Sandercock PA, Warlow CP. Incidence of transient ischemic attacks in Oxfordshire, England. Stroke 1989;20:333-9. http://dx.doi.org/10.1161%2F01.STR.20.3.333.
- Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA 2000;284:2901-6. http://dx.doi.org/10.1001%2Fjama.284.22.2901.
- Coull AJ, Lovett JK, Rothwell PM. Oxford Vascular Study . Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ 2004;328. http://dx.doi.org/10.1136%2Fbmj.37991.635266.44.
- Sandercock PAG. Should I start all my ischaemic stroke and TIA patients on a statin, an ACE inhibitor, a diuretic, and aspirin today?. J Neurol Neurosurg Psychiatry 2003;74:1461-4. http://dx.doi.org/10.1136%2Fjnnp.74.11.1461.
- Evers SMAA, Struijs JN, Ament AJHA, van Genugten MLL, Jager C, van der Bos GAM. International comparison of stroke cost studies. Stroke 2004;35:1209-15. http://dx.doi.org/10.1161%2F01.STR.0000125860.48180.48.
- Joining Forces to Deliver Improved Stroke Care. London: The Stationery Office; 2007.
- Emergency and comprehensive care for stroke needed . Lancet 2009;373.
- Hankey GJ. Redefining risks after TIA and minor ischaemic stroke. Lancet 2005;365:2065-6. http://dx.doi.org/10.1016%2FS0140-6736%2805%2966713-X.
- Easton JD, Albers GW, Caplan LR, Saver JL, Sherman DG. Discussion: reconsideration of TIA terminology and definitions. Neurology 2004;62:S29-34. http://dx.doi.org/10.1212%2FWNL.62.8_suppl_6.S29.
- Warach S, Kidwell CS. The redefinition of TIA: the uses and limitations of DWI in acute ischemic cerebrovascular syndromes. Neurology 2004;62:359-60. http://dx.doi.org/10.1212%2FWNL.62.3.359.
- Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke 2009;40:2276-93.
- Keir SL, Wardlaw JM, Warlow CP. Stroke epidemiology studies have underestimated the frequency of intracerebral haemorrhage. A systematic review of imaging in epidemiological studies. J Neurol 2002;249:1226-31.
- Sudlow CLM, Warlow CP. Comparable studies of the incidence of stroke and its pathological types. Results from an international collaboration. Stroke 1997;28:491-9. http://dx.doi.org/10.1161%2F01.STR.28.3.491.
- Gunatilake SB. Rapid resolution of symptoms and signs of intracerebral haemorrhage: case reports. BMJ 1998;316:1495-6. http://dx.doi.org/10.1136%2Fbmj.316.7143.1495.
- Lovett JK, Dennis MS, Sandercock PAG, Bamford J, Warlow CP, Rothwell PM. Very early risk of stroke after a first transient ischemic attack. Stroke 2003;34:e138-140. http://dx.doi.org/10.1161%2F01.STR.0000080935.01264.91.
- Coull AJ, Rothwell PM. Underestimation of the early risk of recurrent stroke. Evidence of the need for a standard definition. Stroke 2004;35:1925-9. http://dx.doi.org/10.1161%2F01.STR.0000133129.58126.67.
- Kleindorfer D, Panagos P, Pancioli A, Khoury J, Kissela B, Woo D, et al. Incidence and short-term prognosis of transient ischemic attack in a population-based study. Stroke 2005;36:720-3. http://dx.doi.org/10.1161%2F01.STR.0000158917.59233.b7.
- Rothwell PM, Giles MF, Chandratheva A, Marquardt L, Geraghty O, Redgrave JNE, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet 2007;370:1432-42. http://dx.doi.org/10.1016%2FS0140-6736%2807%2961448-2.
- Rothwell PM, Eliasziw M, Gutnikov SA, Fox AJ, Taylor DW, Mayberg MR, et al. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet 2003;361:107-16. http://dx.doi.org/10.1016%2FS0140-6736%2803%2912228-3.
- Wardlaw JM, Keir SL, Seymour J, Lewis S, Sandercock PA, Dennis MS, et al. What is the best imaging strategy for acute stroke?. Health Technol Assess 2004;8.
- Keir SL, Wardlaw JM, Sandercock PA, Chen Z. Antithrombotic therapy in patients with any form of intracranial haemorrhage: a systematic review of the available controlled studies. Cerebrovasc Dis 2002;14:197-206. http://dx.doi.org/10.1159%2F000065661.
- Bailey RD, Hart RG, Benavente O, Pearce LA. Recurrent brain hemorrhage is more frequent than ischemic stroke after intracranial hemorrhage. Neurology 2001;56:773-7. http://dx.doi.org/10.1212%2FWNL.56.6.773.
- Flynn RW, MacDonald TM, Murray GD, MacWalter RS, Doney AS. Prescribing antiplatelet medicine and subsequent events after intracerebral hemorrhage. Stroke 2010;41:2606-11. http://dx.doi.org/10.1161%2FSTROKEAHA.110.589143.
- Thompson BB, Bejot Y, Caso V, Castillo J, Christensen H, Flaherty ML, et al. Prior antiplatelet therapy and outcome following intracerebral hemorrhage: a systematic review. Neurology 2010;75:1333-42. http://dx.doi.org/10.1212%2FWNL.0b013e3181f735e5.
- Wardlaw JM, Keir SL, Dennis MS. The impact of delays in computed tomography of the brain on the accuracy of diagnosis and subsequent management in patients with minor stroke. J Neurol Neurosurg Psychiatry 2003;74:77-81. http://dx.doi.org/10.1136%2Fjnnp.74.1.77.
- Chalela JA, Kidwell CS, Nentwich LM, Luby M, Butman JA, Demchuk AM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet 2007;369:293-8. http://dx.doi.org/10.1016%2FS0140-6736%2807%2960151-2.
- Wardlaw JM, West TM, Sandercock PA, Lewis SC, Mielke O. Visible infarction on computed tomography is an independent predictor of poor functional outcome after stroke, and not of haemorrhagic transformation. J Neurol Neurosurg Psychiatry 2003;74:452-8. http://dx.doi.org/10.1136%2Fjnnp.74.4.452.
- Widjaja E, Manuel D, Hodgson TJ, Connolly DJ, Coley SC, Romanowski CA, et al. Imaging findings and referral outcomes of rapid assessment stroke clinics. Clin Radiol 2005;60:1076-82. http://dx.doi.org/10.1016%2Fj.crad.2005.04.010.
- Douglas VC, Johnston CM, Elkins J, Sidney S, Gress DR, Johnston SC. Head computed tomography findings predict short-term stroke risk after transient ischemic attack. Stroke 2003;34:2894-8. http://dx.doi.org/10.1161%2F01.STR.0000102900.74360.D9.
- Brazzelli M, Sandercock PAG, Chappell FM, Celani MG, Righetti E, Arestis N, et al. Magnetic resonance imaging versus computed tomography for detection of acute vascular lesions in patients presenting with stroke symptoms. Cochrane Database Syst Rev 2009;4. http://dx.doi.org/10.1002%2F14651858.CD007424.
- Wardlaw JM, Lewis SC, Dennis MS, Counsell C, McDowall M. Is visible infarction on computed tomography associated with an adverse prognosis in acute ischemic stroke?. Stroke 1998;29:1315-19. http://dx.doi.org/10.1161%2F01.STR.29.7.1315.
- Gass A, Ay H, Szabo K, Koroshetz WJ. Diffusion-weighted MRI for the ‘small stuff’: the details of acute cerebral ischaemia. Lancet Neurol 2004;3:39-45.
- Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia: the ischemic penumbra. Stroke 1981;12:723-5. http://dx.doi.org/10.1161%2F01.STR.12.6.723.
- Rivers CS, Wardlaw JM. What has diffusion imaging in animals told us about diffusion imaging in patients with ischaemic stroke?. Cerebrovasc Dis 2005;19:328-36. http://dx.doi.org/10.1159%2F000084691.
- Rivers CS, Wardlaw JM, Armitage PA, Bastin M, Carpenter T, Cvoro V, et al. Persistent infarct hyperintensity on diffusion-weighted imaging late after stroke indicates heterogeneous, delayed, infarct evolution. Stroke 2006;37:1418-23. http://dx.doi.org/10.1161%2F01.STR.0000221294.90068.c4.
- Wardlaw JM, Keir SL, Bastin ME, Armitage PA, Rana AK. Is diffusion imaging appearance an independent predictor of outcome after ischaemic stroke?. Neurology 2002;59:1381-7. http://dx.doi.org/10.1212%2F01.WNL.0000032495.71720.C3.
- Hand PJ, Wardlaw JM, Rivers CS, Armitage PA, Bastin ME, Lindley RI, et al. MR diffusion-weighted imaging and outcome prediction after ischemic stroke. Neurology 2006;66:1159-63. http://dx.doi.org/10.1212%2F01.wnl.0000202524.43850.81.
- Muñoz Maniega S, Bastin ME, Armitage PA, Farrall AJ, Carpenter TK, Hand PJ, et al. Temporal evolution of water diffusion parameters is different in grey and white matter in human ischaemic stroke. J Neurol Neurosurg Psychiatry 2004;75:1714-18.
- Keir S, Wardlaw JM, Bastin ME, Dennis MS. In which patients is diffusion-weighted magnetic resonance imaging most useful in routine stroke care?. J Neuroimaging 2004;14:118-22. http://dx.doi.org/10.1177%2F1051228404263277.
- Wardlaw JM, Brindle W, Casado AM, Shuler K, Henderson M, Thomas B, et al. A systematic review of the utility of 1.5 versus 3 Tesla magnetic resonance brain imaging in clinical practice and research. Eur Radiol 2012;22:2295-303.
- Redgrave JNE, Coutts SB, Schulz UG, Briley D, Rothwell PM. Systematic review of associations between the presence of acute ischemic lesions on diffusion-weighted imaging and clinical predictors of early stroke risk after transient ischemic attack. Stroke 2007;38:1482-8. http://dx.doi.org/10.1161%2FSTROKEAHA.106.477380.
- Engelter ST, Wetzel SG, Bonati LH, Fluri F, Lyrer PA. The clinical significance of diffusion-weighted MR imaging in stroke and TIA patients. Swiss Med Wkly 2008;138:729-40.
- Keir SL, Wardlaw JM. Systematic review of diffusion and perfusion imaging in acute ischaemic stroke. Stroke 2000;31:2723-31. http://dx.doi.org/10.1161%2F01.STR.31.11.2723.
- Schulz UG, Briley D, Meagher T, Molyneux A, Rothwell PM. Abnormalities on diffusion weighted magnetic resonance imaging performed several weeks after a minor stroke or transient ischaemic attack. J Neurol Neurosurg Psychiatry 2003;74:734-8. http://dx.doi.org/10.1136%2Fjnnp.74.6.734.
- Dimigen M, Keir S, Dennis M, Wardlaw J. Long-term visibility of primary intracerebral haemorrhage on MRI. J Stroke Cerebrovasc Dis 2004;13:104-8.
- Patel MR, Edelman RR, Warach S. Detection of hyperacute primary intraparenchymal hemorrhage by magnetic resonance imaging. Stroke 1996;27:2321-4. http://dx.doi.org/10.1161%2F01.STR.27.12.2321.
- Fiebach JB, Schellinger PD, Gass A, Kucinski T, Siebler M, Villringer A, et al. Stroke magnetic resonance imaging is accurate in hyperacute intracerebral hemorrhage. A multicenter study on the validity of stroke imaging. Stroke 2004;35:502-7. http://dx.doi.org/10.1161%2F01.STR.0000114203.75678.88.
- Franke FL, Ramos LMP, van Gijn J. Development of multifocal haemorrhage in a cerebral infarct during computed tomography. J Neurol Neurosurg Psychiatry 1990;53:531-2. http://dx.doi.org/10.1136%2Fjnnp.53.6.531.
- Wardlaw JM, Statham PF. How often is haemosiderin not visible on routine MRI following traumatic intracerebral haemorrhage?. Neuroradiology 2000;42:81-4. http://dx.doi.org/10.1007%2Fs002340050019.
- Alemany Ripoll M, Stenborg A, Sonninen P, Terent A, Raininko R. Detection and appearance of intraparenchymal haematomas of the brain at 1.5 T with spin-echo, FLAIR and GE sequences: poor relationship to the age of the haematoma. Neuroradiology 2004;46:435-43.
- Flossmann E, Redgrave JN, Briley D, Rothwell PM. Reliability of clinical diagnosis of the symptomatic vascular territory in patients with recent transient ischemic attack or minor stroke. Stroke 2008;39:2457-60. http://dx.doi.org/10.1161%2FSTROKEAHA.107.511428.
- Ay H, Oliveira-Filho J, Buonanno FS, Ezzeddine M, Schaefer PW, Rordorf G, et al. Diffusion-weighted imaging identifies a subset of lacunar infarction associated with embolic source. Stroke 1999;30:2644-50. http://dx.doi.org/10.1161%2F01.STR.30.12.2644.
- Hankey GJ, Slattery JM, Warlow CP. Can the long term outcome of individual patients with transient ischaemic attacks be predicted accurately?. J Neurol Neurosurg Psychiatry 1993;56:752-9. http://dx.doi.org/10.1136%2Fjnnp.56.7.752.
- Rothwell PM, Warlow CP. Prediction of benefit from carotid endarterectomy in individual patients: a risk-modelling study. European Carotid Surgery Trialists’ Collaborative Group. Lancet 1999;353:2105-10.
- Rothwell PM, Giles MF, Flossmann E, Lovelock CE, Redgrave JN, Warlow CP, et al. A simple score (ABCD) to identify individuals at high early risk of stroke after transient ischaemic attack. Lancet 2005;366:29-36. http://dx.doi.org/10.1016%2FS0140-6736%2805%2966702-5.
- Johnston SC, Rothwell PM, Nguyen-Huynh MN, Giles MF, Elkins JS, Bernstein AL, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 2007;369:283-92. http://dx.doi.org/10.1016%2FS0140-6736%2807%2960150-0.
- Weimar C, Diener H-C, Alberts MJ, Steg PG, Bhatt DL, Wilson PWF, et al. The Essen Stroke Risk Score predicts recurrent cardiovascular events: a validation within the REduction of Atherothrombosis for Continued Health (REACH) Registry. Stroke 2009;40:350-4. http://dx.doi.org/10.1161%2FSTROKEAHA.108.521419.
- Kernan WN, Viscoli CM, Brass LM, Makuch RW, Sarrel PM, Roberts RS, et al. The Stroke Prognosis Instrument II (SPI-II): A clinical prediction instrument for patients with transient ischemia and nondisabling ischemic stroke. Stroke 2000;31:456-62. http://dx.doi.org/10.1161%2F01.STR.31.2.456.
- Van Wijk I, Kappelle LJ, van Gijn J, Koudstaal PJ, Franke CL, Vermeulen M, et al. Long-term survival and vascular event risk after transient ischaemic attack or minor ischaemic stroke: a cohort study. Lancet 2005;365:2098-104.
- Fairhead JF, Mehta Z, Rothwell PM. Population-based study of delays in carotid imaging and surgery and the risk of recurrent stroke. Neurology 2005;65:371-5. http://dx.doi.org/10.1212%2F01.WNL.0000170368.82460.b4.
- Stroke. The Diagnosis and Acute Management of Stroke and Transient Ischaemic Attacks. London: NICE; 2008.
- Tsivgoulis G, Spengos K, Manta P, Karandreas N, Zambelis T, Zakopoulos N, et al. Validation of the ABCD score in identifying individuals at high early risk of stroke after a transient ischaemic attack. Stroke 2006;37:2892-7. http://dx.doi.org/10.1161%2F01.STR.0000249007.12256.4a.
- Sciolla R, Melis F. Rapid identification of high-risk transient ischemic attacks: prospective validation of the ABCD score. Stroke 2008;39:297-302. http://dx.doi.org/10.1161%2FSTROKEAHA.107.496612.
- Cucchiara BL, Messe SR, Taylor RA, Pacelli J, Maus D, Shah Q, et al. Is the ABCD score useful for risk stratification of patients with acute transient ischemic attack?. Stroke 2006;37:1710-14. http://dx.doi.org/10.1161%2F01.STR.0000227195.46336.93.
- Chandratheva A, Marquardt L, Geraghty OC, Rothwell NJ. Limits of risk scoring systems for the early risk of stroke after TIA. Int J Stroke 2009;3.
- Quinn TJ, Cameron AC, Dawson J, Lees KR, Walters MR. ABCD2 scores and prediction of noncerebrovascular diagnoses in an outpatient population. A case-control study. Stroke 2009;40:749-53. http://dx.doi.org/10.1161%2FSTROKEAHA.108.530444.
- Ferro JM, Falcao I, Rodrigues G, Canhao P, Melo TP, Oliveira V, et al. Diagnosis of transient ischemic attack by the nonneurologist. A validation study. Stroke 1996;27:2225-9. http://dx.doi.org/10.1161%2F01.STR.27.12.2225.
- Koudstaal PJ, van Gijn J, Staal A, Duivenvoorden HJ, Gerritsma JG, Kraaijeveld CL. Diagnosis of transient ischemic attacks: improvement of interobserver agreement by a check-list in ordinary language. Stroke 1986;17:723-8. http://dx.doi.org/10.1161%2F01.STR.17.4.723.
- Castle J, Mlynash M, Lee K, Caulfield AF, Wolford C, Kemp S, et al. Agreement regarding diagnosis of transient ischemic attack fairly low among stroke-trained neurologists. Stroke 2010;41:1367-70. http://dx.doi.org/10.1161%2FSTROKEAHA.109.577650.
- Goldstein LB, Simel DL. Is this patient having a stroke?. JAMA 2005;293:2391-402. http://dx.doi.org/10.1001%2Fjama.293.19.2391.
- Hand PJ, Kwan J, Lindley RI, Dennis MS, Wardlaw JM. Distinguishing between stroke and mimic at the bedside: the brain attack study. Stroke 2006;37:769-75. http://dx.doi.org/10.1161%2F01.STR.0000204041.13466.4c.
- Warlow C, van Gijn J, Dennis M, Wardlaw J, Bamford J, Hankey G, et al. Stroke: Practical Management. Malden, MA: Blackwell Publishing; 2008.
- Saver JL, Kidwell C. Neuroimaging in TIAs. Neurology 2004;62:S22-5. http://dx.doi.org/10.1212%2FWNL.62.8_suppl_6.S22.
- Hill MD, Weir NU. Is the ABCD score truly useful?. Stroke 2006;37. http://dx.doi.org/10.1161%2F01.STR.0000230125.04355.e5.
- Ois A, Gomis M, Rodriguez-Campello A, Cuadrado-Godia E, Jimenez-Conde J, Pont-Sunyer C, et al. Factors associated with a high risk of recurrence in patients with transient ischemic attack or minor stroke. Stroke 2008;39:1717-21. http://dx.doi.org/10.1161%2FSTROKEAHA.107.505438.
- European Carotid Surgery Trial Collaborative Group . Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998;351:1379-87.
- Wardlaw JM, Chappell FM, Best JJK, Wartolowska K, Berry E. NHS Research & Development Health Technology Assessment Carotid Stenosis Imaging Group . Non-invasive imaging compared with intra-arterial angiography in the diagnosis of symptomatic carotid stenosis: a meta-analysis. Lancet 2006;367:1503-12. http://dx.doi.org/10.1016%2FS0140-6736%2806%2968650-9.
- Chappell FM, Wardlaw JM, Young GR, Gillard JH, Roditi GH, Yip B, et al. Carotid artery stenosis: accuracy of noninvasive tests: individual patient data meta-analysis. Radiology 2009;251:493-502.
- Wardlaw JM, Stevenson MD, Chappell F, Rothwell PM, Gillard J, Young G, et al. Carotid artery imaging for secondary stroke prevention. Both imaging modality and rapid access to imaging are important. Stroke 2009;40:3511-17. http://dx.doi.org/10.1161%2FSTROKEAHA.109.557017.
- Benade MM, Warlow CP. Costs and benefits of carotid endarterectomy and associated preoperative arterial imaging: a systematic review of health economic literature. Stroke 2002;33:629-38. http://dx.doi.org/10.1161%2Fhs0202.102880.
- Ay H, Arsava EM, Johnston SC, Vangel M, Schwamm LH, Furie KL, et al. Clinical- and Imaging-based Prediction of stroke risk after transient ischemic attack. The CIP model. Stroke 2009;40:181-6. http://dx.doi.org/10.1161%2FSTROKEAHA.108.521476.
- Whiting P, Rutjes AWS, Reitsma JB, Glas AS, Bossuyt PMM, Kleijnen J. Sources of variation and bias in studies of diagnostic accuracy. Ann Intern Med 2004;140:189-202. http://dx.doi.org/10.7326%2F0003-4819-140-3-200402030-00010.
- Ransohoff DF, Feinstein AR. Problems of spectrum and bias in evaluating the efficacy of diagnostic tests. N Engl J Med 1978;299:926-30. http://dx.doi.org/10.1056%2FNEJM197810262991705.
- Begg CB, McNeil BJ. Assessment of radiologic tests: control of bias and other design considerations. Radiology 1988;167:565-9.
- Calvet D, Lamy C, Oppenheim C, Domigo V, Touze E, Essabiri M, et al. Early risk of stroke after transient ischemic attack in stroke unit care. Cerebrovasc Dis 2005;19.
- Coutts SB, Simon JE, Eliasziw M, Sohn CH, Hill MD, Barber PA, et al. Triaging transient ischemic attack and minor stroke patients using acute magnetic resonance imaging. Ann Neurol 2005;57:848-54. http://dx.doi.org/10.1002%2Fana.20497.
- Purroy F, Montaner J, Rovira A, Delgado P, Quintana M, Alvarez-Sabin J. Higher risk of further vascular events among transient ischemic attack patients with diffusion-weighted imaging acute ischemic lesions. Stroke 2004;35:2313-19. http://dx.doi.org/10.1161%2F01.STR.0000141703.21173.91.
- Redgrave JN, Schulz UG, Briley D, Meagher T, Rothwell PM. Presence of acute ischaemic lesions on diffusion-weighted imaging is associated with clinical predictors of early risk of stroke after transient ischaemic attack. Cerebrovasc Dis 2007;24:86-90. http://dx.doi.org/10.1159%2F000103121.
- Kane I, Whiteley WN, Sandercock PA, Wardlaw JM. Availability of CT and MR for assessing patients with acute stroke. Cerebrovasc Dis 2008;25:375-7. http://dx.doi.org/10.1159%2F000120688.
- Leys D, Ringelstein EB, Kaste M, Hacke W. Facilities available in European hospitals treating stroke patients. Stroke 2007;38:2985-91. http://dx.doi.org/10.1161%2FSTROKEAHA.107.487967.
- Implementing the National Stroke Strategy: An Imaging Guide. London: Crown; 2008.
- Technical Guidance for the 2011/12 Operating Framework. London: Crown; 2011.
- Payment by Results Guidance for 2011–12. London: Crown; 2011.
- Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:227-76. http://dx.doi.org/10.1161%2FSTR.0b013e3181f7d043.
- Ackerman RH, Candia MR, May Z. Technical advances and clinical progress in carotid diagnosis. AJNR Am J Neuroradiol 1999;20:187-9.
- National Clinical Guideline for Stroke. London: Royal College of Physicians; 2008.
- Management of Patients with Stroke or TIA: Assessment, Investigation, Immediate Management and Secondary Prevention. A National Clinical Guideline. Edinburgh: SIGN; 2008.
- Ringleb PA, Bousser M-G, Ford G, Bath P, Brainin M, . The European Stroke Organization (ESO) Executive Committee and the ESO Writing Committee . Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis 2008;25:457-50.
- Delakis I, Price D, Renaud C, Dickinson R, Kitney R. Evidence Review: Diffusion-weighted Magnetic Resonance Imaging and Competing Imaging Technologies for the Diagnosis of Stroke and Transient Ischaemic Attack (TIA). London: Crown; 2008.
- Cucchiara B, Ross M. Transient ischemic attack: risk stratification and treatment. Ann Emerg Med 2008;52:S27-39. http://dx.doi.org/10.1016%2Fj.annemergmed.2008.05.019.
- White P, Wardlaw J. Brain attack imaging: making sense of multiple guidelines. Part one of two: transient ischaemic attack and minor stroke; diagnosis and secondary prevention. Stroke Matters 2008;1:6-7.
- Chappell FM, Raab GM, Wardlaw JM. When are summary ROC curves appropriate for diagnostic meta-analyses?. Stat Med 2009;28:2653-68. http://dx.doi.org/10.1002%2Fsim.3631.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58.
- Whiting P, Rutjes AWS, Reitsma JB, Bossuyt PMM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3.
- Jackson C, Dennis M, Wardlaw J, Lewis S, Hutchinson A, Sudlow C. Recurrent stroke rates and subtype patterns among lacunar versus non-lacunar ischaemic stroke patients in a hospital-based cohort: evidence for distinct vascular pathologies?. Cerebrovasc Dis 2008;25.
- Dorman P, Dennis M, Sandercock P. United Kingdom Collaborators in the International Stroke Trial (IST) . Are the modified ‘simple questions’ a valid and reliable measure of health related quality of life after stroke?. J Neurol Neurosurg Psychiatry 2000;69:487-93.
- Briggs A, Claxton K, Sculpher K. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Wardlaw JM, Doubal F, Armitage P, Chappell F, Carpenter T, Maniega SM, et al. Lacunar stroke is associated with diffuse blood–brain barrier dysfunction. Ann Neurol 2009;65:194-202.
- Dennis M, Sandercock PA, Reid J, Graham C, Murray G, . CLOTS Trials Collaboration . Effectiveness of thigh-length graduated compression stockings to reduce the risk of deep vein thrombosis after stroke (CLOTS trial 1): a multicentre, randomised controlled trial. Lancet 2009;373:1958-65.
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12.
- Rothwell PM. Incidence, risk factors and prognosis of stroke and TIA: the need for high-quality, large-scale epidemiological studies and meta-analyses. Cerebrovasc Dis 2003;16:2-10. http://dx.doi.org/10.1159%2F000070271.
- Chandratheva A, Mehta Z, Geraghty OC, Marquardt L, Rothwell PM. Population-based study of risk and predictors of stroke in the first few hours after a TIA. Neurology 2009;72:1941-7. http://dx.doi.org/10.1212%2FWNL.0b013e3181a826ad.
- Correia M, Silva MR, Magalhaes R, Guimaraes L, Silva MC. Transient ischemic attacks in rural and urban northern Portugal: incidence and short-term prognosis. Stroke 2006;37:50-5. http://dx.doi.org/10.1161%2F01.STR.0000195209.26543.8f.
- Wu CM, McLaughlin K, Lorenzetti DL, Hill MD, Manns BJ, Ghali WA. Early risk of stroke after transient ischemic attack: a systematic review and meta-analysis. Arch Intern Med 2007;167:2417-22. http://dx.doi.org/10.1001%2Farchinte.167.22.2417.
- Giles MF, Rothwell PM. Risk of stroke early after transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol 2007;6:1063-72. http://dx.doi.org/10.1016%2FS1474-4422%2807%2970274-0.
- Lavallée PC, Meseguer E, Abboud H, Cabrejo L, Olivot JM, Simon O, et al. A transient ischaemic attack clinic with round-the-clock access (SOS-TIA): feasibility and effects. Lancet Neurol 2007;6:953-60. http://dx.doi.org/10.1016%2FS1474-4422%2807%2970248-X.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009;151:W65-94. http://dx.doi.org/10.7326%2F0003-4819-151-4-200908180-00136.
- Dundar Y, Dodd S, Dickson R, Walley T, Haycox A, Williamson PR. Comparison of conference abstracts and presentations with full-text articles in the health technology assessments of rapidly evolving technologies. Health Technol Assess 2006;10.
- Sanders LM, Srikanth VK, Blacker DJ, Jolley DJ, Cooper KA, Phan TG. Performance of the ABCD2 score for stroke risk post TIA: meta-analysis and probability modelling. Neurology 2012;79:971-80. http://dx.doi.org/10.1212%2FWNL.0b013e31825f9d02.
- Lisabeth LD, Ireland JK, Risser JMH, Brown DL, Smith MA, Garcia NM, et al. Stroke risk after transient ischemic attack in a population-based setting. Stroke 2004;35:1842-6. http://dx.doi.org/10.1161%2F01.STR.0000134416.89389.9d.
- Hill MD, Yiannakoulias N, Jeerakathil T, Tu JV, Svenson LW, Schopflocher DP. The high risk of stroke immediately after transient ischemic attack: a population-based study. Neurology 2004;62:2015-20. http://dx.doi.org/10.1212%2F01.WNL.0000129482.70315.2F.
- Eliasziw M, Kennedy J, Hill MD, Buchan AM, Barnett HJM. North American Symptomatic Carotid Endarterectomy (NASCET) Group . Early risk of stroke after a transient ischemic attack in patients with internal carotid artery disease. CMAJ 2004;170:1105-9. http://dx.doi.org/10.1503%2Fcmaj.1030460.
- Gladstone DJ, Kapral MK, Fang J, Laupacis A, Tu JV. Management and outcomes of transient ischemic attacks in Ontario. CMAJ 2004;170:1099-104. http://dx.doi.org/10.1503%2Fcmaj.1031349.
- Whitehead MA, McManus J, McAlpine C, Langhorne P. Early recurrence of cerebrovascular events after transient ischaemic attack. Stroke 2005;36. http://dx.doi.org/10.1161%2F01.STR.0000149929.92356.f1.
- Bray JE, Coughlan K, Bladin C. Can the ABCD Score be dichotomised to identify high-risk patients with transient ischaemic attack in the emergency department?. Emerg Med J 2007;24:92-5. http://dx.doi.org/10.1136%2Femj.2006.041624.
- Koton S, Rothwell PM. Performance of the ABCD and ABCD2 scores in TIA patients with carotid stenosis and atrial fibrillation. Cerebrovasc Dis 2007;24:231-5. http://dx.doi.org/10.1159%2F000104483.
- Atanassova PA, Chalakova NT, Dimitrov BD. Major vascular events after transient ischaemic attack and minor ischaemic stroke: post hoc modelling of incidence dynamics. Cerebrovasc Dis 2008;25:225-33. http://dx.doi.org/10.1159%2F000113860.
- Coutts SB, Eliasziw M, Hill MD, Scott JN, Subramaniam S, Buchan AM, et al. An improved scoring system for identifying patients at high early risk of stroke and functional impairment after an acute transient ischemic attack or minor stroke. Int J Stroke 2008;3:3-10. http://dx.doi.org/10.1111%2Fj.1747-4949.2008.00182.x.
- Josephson SA, Sidney S, Pham TN, Bernstein AL, Johnston SC. Higher ABCD2 score predicts patients most likely to have true transient ischemic attack. Stroke 2008;39:3096-8. http://dx.doi.org/10.1161%2FSTROKEAHA.108.514562.
- Asimos AW, Rosamond WD, Johnson AM, Price MF, Rose KM, Murphy CV, et al. Early diffusion weighted MRI as a negative predictor for disabling stroke after ABCD2 score risk categorization in transient ischemic attack patients. Stroke 2009;40:3252-7. http://dx.doi.org/10.1161%2FSTROKEAHA.109.555425.
- Calvet D, Touze E, Oppenheim C, Turc G, Meder JF, Mas JL. DWI lesions and TIA etiology improve the prediction of stroke after TIA. Stroke 2009;40:187-92. http://dx.doi.org/10.1161%2FSTROKEAHA.108.515817.
- Chatzikonstantinou A, Willmann O, Jager T, Szabo K, Hennerici MG. Transient ischemic attack patients with fluctuations are at highest risk for early stroke. Cerebrovasc Dis 2009;27:594-8. http://dx.doi.org/10.1159%2F000214224.
- Cucchiara BL, Messe SR, Sansing L, MacKenzie L, Taylor RA, Pacelli J, et al. D-dimer, magnetic resonance imaging diffusion-weighted imaging, and ABCD2 score for transient ischemic attack risk stratification. J Stroke Cerebrovasc Dis 2009;18:367-73. http://dx.doi.org/10.1016%2Fj.jstrokecerebrovasdis.2009.01.006.
- Fothergill A, Christianson TJ, Brown RD, Rabinstein AA. Validation and refinement of the ABCD2 score: a population-based analysis. Stroke 2009;40:2669-73. http://dx.doi.org/10.1161%2FSTROKEAHA.109.553446.
- Mlynash M, Olivot JM, Tong DC, Lansberg MG, Eyngorn I, Kemp S, et al. Yield of combined perfusion and diffusion MR imaging in hemispheric TIA. Neurology 2009;72:1127-33. http://dx.doi.org/10.1212%2F01.wnl.0000340983.00152.69.
- Tan S, Zhao L, Song B, Li Z, Zhang R, Gao Y, et al. Clinical application of ABCD2 score system. Life Science Journal 2009;6:23-6.
- Asimos AW, Johnson AM, Rosamond WD, Price MF, Rose KM, Catellier D, et al. A multicenter evaluation of the ABCD2 score’s accuracy for predicting early ischemic stroke in admitted patients with transient ischemic attack. Ann Emerg Med 2010;55:201-10.
- Chandratheva A, Geraghty OC, Luengo-Fernandez R, Rothwell PM. Oxford Vascular Study . ABCD2 score predicts severity rather than risk of early recurrent events after transient ischemic attack. Stroke 2010;41:851-6. http://dx.doi.org/10.1161%2FSTROKEAHA.109.570010.
- Harrison JK, Sloan B, Dawson J, Lees KR, Morrison DS. The ABCD and ABCD2 as predictors of stroke in transient ischemic attack clinic outpatients: a retrospective cohort study over 14 years. QJM 2010;103:679-85. http://dx.doi.org/10.1093%2Fqjmed%2Fhcq108.
- Holzer K, Feurer R, Sadikovic S, Esposito L, Bockelbrink A, Sander D, et al. Prognostic value of the ABCD2 score beyond short-term follow-up after transient ischemic attack (TIA): a cohort study. BMC Neurol 2010;10. http://dx.doi.org/10.1186%2F1471-2377-10-50.
- Merwick A, Albers GW, Amarenco P, Arsava EM, Ay H, Calvet D, et al. Addition of brain and carotid imaging to the ABCD(2) score to identify patients at early risk of stroke after transient ischaemic attack: a multicentre observational study. Lancet Neurol 2010;9:1060-9.
- Nakajima M, Hirano T, Naritomi H, Minematsu K. Symptom progression or fluctuation in transient ischemic attack patients predicts subsequent stroke. Cerebrovasc Dis 2010;29:221-7. http://dx.doi.org/10.1159%2F000267844.
- Ong ME, Chan YH, Lin WP, Chung WL. Validating the ABCD(2) Score for predicting stroke risk after transient ischemic attack in the ED. Am J Emerg Med 2010;28:44-8.
- Purroy F, Pinol-Ripoll G, Quilez A, Sanahuja J, Brieva L, Suarez Luis I. Validation of the ABCDI and ABCD2I scales in the registry of patients with transient ischemic attacks from Lleida (REGITELL) Spain. Med Clin 2010;135:351-6.
- Sheehan OC, Kyne L, Kelly LA, Hannon N, Marnane M, Merwick A, et al. Population-based study of ABCD2 score, carotid stenosis, and atrial fibrillation for early stroke prediction after transient ischemic attack: the North Dublin TIA study. Stroke 2010;41:844-50. http://dx.doi.org/10.1161%2FSTROKEAHA.109.571844.
- Tsivgoulis G, Stamboulis E, Sharma VK, Heliopoulos I, Voumvourakis K, Teoh HL, et al. Multicenter external validation of the ABCD2 score in triaging TIA patients. Neurology 2010;74:1351-7. http://dx.doi.org/10.1212%2FWNL.0b013e3181dad63e.
- Wasserman J, Perry J, Dowlatshahi D, Stotts G, Stiell I, Sutherland J, et al. Stratified, urgent care for transient ischemic attack results in low stroke rates. Stroke 2010;41:2601-5. http://dx.doi.org/10.1161%2FSTROKEAHA.110.586842.
- Weimar C, Benemann J, Michalski MD, Muller M, Luckner K, Katsarava Z, et al. Prediction of recurrent stroke and vascular death in patients with transient ischemic attack or nondisabling stroke. A prospective comparison of validated prognostic scores. Stroke 2010;41:487-93. http://dx.doi.org/10.1161%2FSTROKEAHA.109.562157.
- Yang J, Fu JH, Chen XY, Chen YK, Leung TW, Mok V, et al. Validation of the ABCD2 score to identify the patients with high risk of late stroke after a transient ischemic attack or minor ischemic stroke. Stroke 2010;41:1298-300. http://dx.doi.org/10.1161%2FSTROKEAHA.110.578757.
- Amort M, Fluri F, Schafer J, Weisskopf F, Katan M, Burow A, et al. Transient ischemic attack versus transient ischemic attack mimics: frequency, clinical characteristics and outcome. Cerebrovasc Dis 2011;32:57-64. http://dx.doi.org/10.1159%2F000327034.
- Arsava EM, Furie KL, Schwamm LH, Sorensen AG, Ay H. Prediction of early stroke risk in transient symptoms with infarction: relevance to the new tissue-based definition. Stroke 2011;42. http://dx.doi.org/10.1161%2FSTROKEAHA.110.604280.
- Bonifati DM, Lorenzi A, Ermani M, Refatti F, Gremes E, Boninsegna C, et al. Carotid stenosis as predictor of stroke after transient ischemic attacks. J Neurol Sci 2011;303:85-9. http://dx.doi.org/10.1016%2Fj.jns.2011.01.005.
- Kim AS, Sidney S, Bernstein AL, Douglas VC, Johnston SC. Urgent neurology consultation from the ED for transient ischemic attack. Am J Emerg Med 2011;29:601-8. http://dx.doi.org/10.1016%2Fj.ajem.2009.12.025.
- Meng X, Wang Y, Liu L, Pu Y, Zhao X, Wang C, et al. Validation of the ABCD(2)-I score to predict stroke risk after transient ischemic attack. Neurol Res 2011;33:482-6. http://dx.doi.org/10.1179%2F016164111X13007856084043.
- Olivot JM, Wolford C, Castle J, Mlynash M, Schwartz NE, Lansberg MG, et al. Two aces: transient ischemic attack work-up as outpatient assessment of clinical evaluation and safety. Stroke 2011;42:1839-43. http://dx.doi.org/10.1161%2FSTROKEAHA.110.608380.
- Sanders LM, Srikanth VK, Psihogios H, Wong KK, Ramsay D, Phan TG. Clinical predictive value of the ABCD2 score for early risk of stroke in patients who have had transient ischaemic attack and who present to an Australian tertiary hospital. Med J Aust 2011;194:135-8.
- Stead LG, Suravaram S, Bellolio MF, Enduri S, Rabinstein A, Gilmore RM, et al. An assessment of the incremental value of the ABCD2 score in the emergency department evaluation of transient ischemic attack. Ann Emerg Med 2011;57:46-51. http://dx.doi.org/10.1016%2Fj.annemergmed.2010.07.001.
- Amarenco P, Labreuche J, Lavallée PC. Patients with transient ischemic attack with ABCD2 < 4 can have similar 90-day stroke risk as patients with transient ischemic attack with ABCD2 ≥ 4. Stroke 2012;43:863-5. http://dx.doi.org/10.1161%2FSTROKEAHA.111.636506.
- Purroy F, Jimenez Caballero PE, Gorospe A, Torres MJ, Alvarez-Sabin J, Santamarina E, et al. Prediction of early stroke recurrence in transient ischemic attack patients from the PROMAPA study: a comparison of prognostic risk scores. Cerebrovasc Dis 2012;33:182-9. http://dx.doi.org/10.1159%2F000334771.
- Galvin R, Geraghty C, Motterlini N, Dimitrov BD, Fahey T. Prognostic value of the ABCD(2) clinical prediction rule: a systematic review and meta-analysis. Fam Pract 2011;28:366-76.
- Calvet D, Lamy C, Touze E, Oppenheim C, Meder JF, Mas J-L. Management and outcome of patients with transient ischemic attack admitted to a stroke unit. Cerebrovasc Dis 2007;24:80-5. http://dx.doi.org/10.1159%2F000103120.
- Purroy F, Molina CA, Montaner J, Alvarez-Sabin J. Absence of usefulness of ABCD score in the early risk of stroke of transient ischemic attack patients. Stroke 2007;38:855-6. http://dx.doi.org/10.1161%2F01.STR.0000257306.00512.d3.
- Mohan KM, Wolfe CD, Rudd AG, Heuschmann PU, Kolominsky-Rabas PL, Grieve AP. Risk and cumulative risk of stroke recurrence: a systematic review and meta-analysis. Stroke 2011;42:1489-94. http://dx.doi.org/10.1161%2FSTROKEAHA.110.602615.
- Giles MF, Rothwell PM. Substantial underestimation of the need for outpatient services for TIA and minor stroke. Age Ageing 2007;36:676-80. http://dx.doi.org/10.1093%2Fageing%2Fafm088.
- Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJM. Carotid Endarterectomy Trialists’ Collaboration . Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroup and timing of surgery. Lancet 2004;363:915-24. http://dx.doi.org/10.1016%2FS0140-6736%2804%2915785-1.
- Briley D, Durkin C, Meagher T. An urgent access neurovascular clinic: audit of timeliness. Clin Med 2009;9:236-8. http://dx.doi.org/10.7861%2Fclinmedicine.9-3-236.
- Kerr E, Arulraj N, Scott M, McDowall M, van Dijke M, Keir S, et al. A telephone hotline for transient ischaemic attack and stroke: prospective audit of a model to improve rapid access to specialist stroke care. BMJ 2010;341. http://dx.doi.org/10.1136%2Fbmj.c3265.
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics: 2011 update: a report from the American Heart Association. Circulation 2011;123:e18-209. http://dx.doi.org/10.1161%2FCIR.0b013e3182009701.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b2700.
- Albers GW, Caplan LR, Easton JD, Fayad PB, Mohr JP, Saver JL, et al. Transient ischemic attack: proposal for a new definition. N Engl J Med 2002;347:1713-16. http://dx.doi.org/10.1056%2FNEJMsb020987.
- Capone FT, Cavallari M, Casolla B, Caselli G, Pieroni A, Di LV, et al. Stroke prediction after transient ischemic attacks in patients admitted to a stroke unit. Eur Neurol 2012;67:34-8. http://dx.doi.org/10.1159%2F000332999.
- Giles MF, Rothwell PM. Systematic review and pooled analysis of published and unpublished validations of the ABCD and ABCD2 transient ischemic attack risk scores. Stroke 2010;41:667-73. http://dx.doi.org/10.1161%2FSTROKEAHA.109.571174.
- Song F, Xiong T, Parekh-Bhurke S, Loke YK, Sutton AJ, Eastwood AJ, et al. Inconsistency between direct and indirect comparisons of competing interventions: meta-epidemiological study. BMJ 2011;343. http://dx.doi.org/10.1136%2Fbmj.d4909.
- Amarenco P, Labreuche J, Lavallée PC, Meseguer E, Cabrejo L, Slaoui T, et al. Does ABCD2 score below 4 allow more time to evaluate patients with a transient ischemic attack?. Stroke 2009;40:3091-5. http://dx.doi.org/10.1161%2FSTROKEAHA.109.552042.
- Walker J, Isherwood J, Eveson D, Naylor AR. Triaging TIA/minor stroke patients using the ABCD2 score does not predict those with significant carotid disease. Eur J Vasc Endovasc Surg 2012;43:495-8. http://dx.doi.org/10.1016%2Fj.ejvs.2012.02.002.
- Purroy F, Montaner J, Molina CA, Delgado P, Ribo M, Alvarez-Sabin J. Patterns and predictors of early risk of recurrence after transient ischemic attack with respect to etiologic subtypes. Stroke 2007;38:3225-9. http://dx.doi.org/10.1161%2FSTROKEAHA.107.488833.
- Sheehan OC, Merwick A, Kelly LA, Hannon N, Marnane M, Kyne L, et al. Diagnostic usefulness of the ABCD2 score to distinguish transient ischemic attack and minor ischemic stroke from noncerebrovascular events: the North Dublin TIA Study. Stroke 2009;40:3449-54. http://dx.doi.org/10.1161%2FSTROKEAHA.109.557074.
- Giles MF, Albers GW, Amarenco P, Arsava MM, Asimos A, Ay H, et al. Addition of brain infarction to the ABCD2 Score (ABCD2I): a collaborative analysis of unpublished data on 4574 patients. Stroke 2010;41:1907-13. http://dx.doi.org/10.1161%2FSTROKEAHA.110.578971.
- Ois A, Cuadrado-Godia E, Rodriguez-Campello A, Jimenez-Conde J, Roquer J. High risk of early neurological recurrence in symptomatic carotid stenosis. Stroke 2009;40:2727-31. http://dx.doi.org/10.1161%2FSTROKEAHA.109.548032.
- Chandratheva A, Geraghty OC, Rothwell PM. Poor performance of current prognostic scores for early risk of recurrence after minor stroke. Stroke 2011;42:632-7. http://dx.doi.org/10.1161%2FSTROKEAHA.110.593301.
- Wijnhoud AD, Maasland L, Lingsma HF, Steyerberg EW, Koudstaal PJ, Dippel DW. Prediction of major vascular events in patients with transient ischemic attack or ischemic stroke. A comparison of 7 models. Stroke 2010;41:2178-85. http://dx.doi.org/10.1161%2FSTROKEAHA.110.580985.
- Rothwell PM, Buchan A, Johnston SC. Recent advances in management of transient ischaemic attacks and minor ischaemic strokes. Lancet Neurol 2006;5:323-31. http://dx.doi.org/10.1016%2FS1474-4422%2806%2970408-2.
- Shah KH, Metz HA, Edlow JA. Clinical prediction rules to stratify short-term risk of stroke among patients diagnosed in the emergency department with a transient ischemic attack. Ann Emerg Med 2009;53:662-73. http://dx.doi.org/10.1016%2Fj.annemergmed.2008.08.004.
- Purroy García F, Molina Cateriano CA, Montaner Villalonga J, Delgado Martinez P, Santmarina Perez E, Toledo M, et al. Lack of usefulness of ABCD score in the early risk of recurrent stroke in transient ischemic attack patients. Med Clin 2007;128:201-3.
- Leeflang MG, Deeks JJ, Gatsonis C, Bossuyt PMM. Systematic reviews of diagnostic test accuracy. Ann Intern Med 2008;149:889-97. http://dx.doi.org/10.7326%2F0003-4819-149-12-200812160-00008.
- Keogh C, Wallace E, O’Brien KK, Murphy PJ, Teljeur C, McGrath B, et al. Optimized retrieval of primary care clinical prediction rules from MEDLINE to establish a web-based register. J Clin Epidemiol 2011;64:848-60. http://dx.doi.org/10.1016%2Fj.jclinepi.2010.11.011.
- Altman DG, Eggar M, Davey Smith G, Altman DG. Systematic Reviews in Health Care: Meta-analysis in Context. London: BMJ Books; 2001.
- Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 1991;337:1521-6. http://dx.doi.org/10.1016%2F0140-6736%2891%2993206-O.
- Wardlaw JM, Sellar RJ. A simple practical classification of cerebral infarcts on CT and its interobserver reliability. AJNR Am J Neuroradiol 1994;15:1933-9.
- Lewis SC, Wardlaw JM. Which Doppler velocity is best for assessing suitability for carotid endarterectomy?. Eur J Ultrasound 2002;15:9-20. http://dx.doi.org/10.1016%2FS0929-8266%2801%2900168-9.
- Wardlaw JM, Lewis S. Carotid stenosis measurement on colour Doppler ultrasound: agreement of ECST, NASCET and CCA methods applied to ultrasound with intra-arterial angiographic stenosis measurement. Eur J Radiol 2005;56:205-11. http://dx.doi.org/10.1016%2Fj.ejrad.2005.04.021.
- Lammie GA, Wardlaw J, Allan P, Ruckley CV, Peek R, Signorini DF. What pathological components indicate carotid atheroma activity and can these be identified reliably using ultrasound?. Eur J Ultrasound 2000;11:77-86. http://dx.doi.org/10.1016%2FS0929-8266%2899%2900076-2.
- Rothwell PM, Gibson RJ, Slattery J, Sellar RJ, Warlow CP. Equivalence of measurements of carotid stenosis: a comparison of three methods on 1001 angiograms. Stroke 1994;25:2435-9. http://dx.doi.org/10.1161%2F01.STR.25.12.2435.
- Cancelli I, Janes F, Gigli GL, Perelli A, Zanchettin B, Canal G, et al. Incidence of transient ischemic attack and early stroke risk: validation of the ABCD2 score in an Italian population-based study. Stroke 2011;42:2751-7. http://dx.doi.org/10.1161%2FSTROKEAHA.110.612705.
- Coutts SB, Hill MD, Eliasziw M, Fischer K, Demchuk AM. Final 2 year results of the vascular imaging of acute stroke for identifying predictors of clinical outcome and recurrent ischemic eveNts (VISION) study. BMC Cardiovasc Disord 2011;11. http://dx.doi.org/10.1186%2F1471-2261-11-18.
- The Dutch TIA Trial Study Group . Predictors of major vascular events in patients with a transient ischemic attack or nondisabling stroke. Stroke 1993;24:527-31.
- Eliasziw M, Streifler JY, Spence JD, Fox AJ, Hachinski VC, Barnett HJ. Prognosis for patients following a transient ischemic attack with and without a cerebral infarction on brain CT. North American Symptomatic Carotid Endarterectomy Trial (NASCET) Group. Neurology 1995;45:428-31.
- Hankey GJ, Slattery JM, Warlow CP. Transient ischaemic attacks: which patients are at high (and low) risk of serious vascular events?. J Neurol Neurosurg Psychiatry 1992;55:640-52. http://dx.doi.org/10.1136%2Fjnnp.55.8.640.
- Hier DB, Foulkes MA, Swiontoniowski M, Sacco RL, Gorelick PB, Mohr JP, et al. Stroke recurrence within 2 years after ischemic infarction. Stroke 1991;22:155-61. http://dx.doi.org/10.1161%2F01.STR.22.2.155.
- Kernan WN, Horwitz RI, Brass LM, Viscoli CM, Taylor KJ. A prognostic system for transient ischemia or minor stroke. Ann Intern Med 1991;114:552-7. http://dx.doi.org/10.7326%2F0003-4819-114-7-552.
- Lovett JK, Coull AJ, Rothwell PM. Early risk of recurrence by subtype of ischemic stroke in population-based incidence studies. Neurology 2004;62:569-73. http://dx.doi.org/10.1212%2F01.WNL.0000110311.09970.83.
- Marnane M, Ni Chroinin D, Callaly E, Sheehan OC, Merwick A, Hannon N, et al. Stroke recurrence within the time window recommended for carotid endarterectomy. Neurology 2011;77:738-43. http://dx.doi.org/10.1212%2FWNL.0b013e31822b00cf.
- Purroy F, Begue R, Gil MI, Quilez A, Sanahuja J, Brieva L, et al. Patterns of diffusion-weighted magnetic resonance imaging associated with etiology improve the accuracy of prognosis after transient ischaemic attack. Eur J Neurol 2011;18:121-8. http://dx.doi.org/10.1111%2Fj.1468-1331.2010.03080.x.
- Giles MF, Albers GW, Amarenco P, Arsava EM, Asimos AW, Ay H, et al. Early stroke risk and ABCD2 score performance in tissue- vs time-defined TIA: a multicenter study. Neurology 2011;77:1222-8. http://dx.doi.org/10.1212%2FWNL.0b013e3182309f91.
- Van Swieten JC, Kappelle LJ, Algra A, van Latum JC, Koudstaal PJ, van Gijn J. Dutch TIA Trial Study Group . Hypodensity of the cerebral white matter in patients with transient ischemic attack or minor stroke: influence on the rate of subsequent stroke. Ann Neurol 1992;32:177-83. http://dx.doi.org/10.1002/ana.410320209.
- Steifler JY, Eliasziw M, Benavente OR, Harbison JW, Hachinski VC, Barnett HJM, et al. The risk of stroke in patients with first-ever retinal vs. hemispheric transient ischemic attacks and high-grade carotid stenosis. Arch Neurol 1995;52:246-9. http://dx.doi.org/10.1001/archneur.1995.00540270034016.
- North American Symptomatic Carotid Endarterectomy Trial Collaborators . Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991;325:445-53. http://dx.doi.org/10.1056/NEJM199108153250701.
- Hankey GJ, Slattery JM, Warlow CP. The prognosis of hospital-referred transient ischemic attacks. J Neurol Neurosurg Psychiatr 1991;54:793-802. http://dx.doi.org/10.1136/jnnp.54.9.793.
- Boulanger JM, Coutts SB, Eliasziw M, Subramaniam S, Scott J, Demchuk AM. Diffusion-weighted imaging-negative patients with transient ischemic attack are at risk of recurrent transient events. Stroke 2007;38:2367-9. http://dx.doi.org/10.1161%2FSTROKEAHA.106.475541.
- Brazzelli M, Sandercock PAG, Grazia Celani M, Righetti E, Chappell FM, Arestis N, et al. MRI versus CT for detection of acute vascular lesions in patients presenting with stroke symptoms. Stroke 2010;41:e27-8. http://dx.doi.org/10.1161%2FSTROKEAHA.109.568667.
- Fazekas F, Fazekas G, Schmidt R, Kapeller P, Offenbacher H. Magnetic resonance imaging correlates of transient cerebral ischemic attacks. Stroke 1996;27:607-11. http://dx.doi.org/10.1161%2F01.STR.27.4.607.
- Bhadelia RA, Anderson M, Polak JF, Manolio TA, Beauchamp N, Knepper L, et al. Prevalence and associations of MRI-demonstrated brain infarcts in elderly subjects with a history of transient ischemic attack. The Cardiovascular Health Study. Stroke 1999;30:383-8. http://dx.doi.org/10.1161%2F01.STR.30.2.383.
- Engelter ST, Provenzale JM, Petrella JR, Alberts MJ. Diffusion MR imaging and transient ischaemic attacks. Stroke 1999;30:2759-68. http://dx.doi.org/10.1161%2F01.STR.30.12.2759-c.
- Kidwell CS, Alger JR, Di Salle F, Starkman S, Villablanca P, Bentson J, et al. Diffusion MRI in patients with transient ischemic attacks. Stroke 1999;30:1174-80. http://dx.doi.org/10.1161%2F01.STR.30.6.1174.
- Takayama H, Mihara B, Kobayashi M, Hozumi A, Sadanaga H, Gomi S. Usefulness of diffusion-weighted MRI in the diagnosis of transient ischemic attacks. Brain Nerve 2000;52:919-23.
- Bisschops RH, Kappelle LJ, Mali WP, van der Grond J. Hemodynamic and metabolic changes in transient ischemic attack patients: a magnetic resonance angiography and (1)H-magnetic resonance spectroscopy study performed within 3 days of onset of a transient ischemic attack. Stroke 2002;33:110-15.
- Kamal AK, Segal AZ, Ulug AM. Quantitative diffusion-weighted MR imaging in transient ischemic attacks. AJNR Am J Neuroradiol 2002;23:1533-18.
- Kastrup A, Schulz JB, Mader I, Dichgans J, Kuker W. Diffusion-weighted MRI in patients with symptomatic internal carotid artery disease. J Neurol 2002;249:1168-74. http://dx.doi.org/10.1007%2Fs00415-002-0793-2.
- Marx JJ, Mika-Gruettner A, Thoemke F, Fitzek S, Fitzek C, Vucurevic G, et al. Diffusion weighted magnetic resonance imaging in the diagnosis of reversible ischaemic deficits of the brainstem. J Neurol Neurosurg Psychiatry 2002;72:572-5. http://dx.doi.org/10.1136%2Fjnnp.72.5.572.
- Rovira A, Rovira-Gols A, Pedraza S, Grive E, Molina C, Alvarez-Sabin J. Diffusion-weighted MR imaging in the acute phase of transient ischemic attacks. AJNR Am J Neuroradiol 2002;23:77-83.
- Crisostomo RA, Garcia MM, Tong DC. Detection of diffusion-weighted MRI abnormalities in patients with transient ischemic attack: correlation with clinical characteristics. Stroke 2003;34:932-7. http://dx.doi.org/10.1161%2F01.STR.0000061496.00669.5E.
- Nagura J, Suzuki K, Johnston SC, Nagata K, Kuriyama N, Ozasa K, et al. Diffusion-weighted MRI in evaluation of transient ischemic attack. J Stroke Cerebrovasc Dis 2003;12:137-42. http://dx.doi.org/10.1016%2FS1052-3057%2803%2900040-5.
- Nakamura T, Uchiyama S, Shibagaki Y, Iwata M. Abnormalities on diffusion-weighted magnetic resonance imaging in patients with transient ischemic attack. Rinsho Shinkeigaku 2003;43:122-5.
- Inatomi Y, Kimura K, Yonehara T, Fujioka S, Uchino M. DWI abnormalities and clinical characteristics in TIA patients. Neurology 2004;62:376-80. http://dx.doi.org/10.1212%2F01.WNL.0000110303.57683.3A.
- Restrepo L, Jacobs MA, Barker PB, Wityk RJ. Assessment of transient ischemic attack with diffusion- and perfusion-weighted imaging. AJNR Am J Neuroradiol 2004;25:1645-52.
- Schulz UG, Briley D, Meagher T, Molyneux A, Rothwell PM. Diffusion-weighted MRI in 300 patients presenting late with subacute transient ischemic attack or minor stroke. Stroke 2004;35:2459-65. http://dx.doi.org/10.1161%2F01.STR.0000143455.55877.b9.
- Winbeck K, Bruckmaier K, Etgen T, Grafin von Einsiedel H, Rottinger M, Sander D. Transient ischemic attack and stroke can be differentiated by analyzing early diffusion-weighted imaging signal intensity changes. Stroke 2004;35:1095-9. http://dx.doi.org/10.1161%2F01.STR.0000125720.02983.fe.
- Inatomi Y, Kimura K, Yonehara T, Fujioka S, Uchino M. Hyperacute diffusion-weighted imaging abnormalities in transient ischemic attack patients signify irreversible ischemic infarction. Cerebrovasc Dis 2005;19:362-8. http://dx.doi.org/10.1159%2F000085203.
- Kim SH, Han SW, Heo JH. Predictive implications of recurrent transient ischemic attacks in large-artery atherosclerosis. Cerebrovasc Dis 2006;22:240-4. http://dx.doi.org/10.1159%2F000094010.
- Lamy C, Oppenheim C, Calvet D, Domigo V, Naggara O, Meder JL, et al. Diffusion-weighted MR imaging in transient ischaemic attacks. Eur Radiol 2006;16:1090-5. http://dx.doi.org/10.1007%2Fs00330-005-0049-5.
- Oppenheim C, Lamy C, Touze E, Calvet D, Hamon M, Mas JL, et al. Do transient ischemic attacks with diffusion-weighted imaging abnormalities correspond to brain infarctions?. AJNR Am J Neuroradiol 2006;27:1782-7.
- Prabhakaran S. Triaging patients with transient ischemic attack: what can we learn from diffusion-weighted imaging?. Nat Clin Pract Neurol 2008;4:14-5. http://dx.doi.org/10.1038%2Fncpneuro0656.
- Prabhakaran S, Chong JY, Sacco RL. Impact of abnormal diffusion-weighted imaging results on short-term outcome following transient ischemic attack. Arch Neurol 2007;64:1105-9. http://dx.doi.org/10.1001%2Farchneur.64.8.1105.
- Bertrand A, Oppenheim C, Lamy C, Rodrigo S, Naggara O, Mas JL, et al. Comparison of optimized and standard diffusion-weighted imaging at 1.5T for the detection of acute lesions in patients with transient ischemic attack. AJNR Am J Neuroradiol 2008;29:363-5. http://dx.doi.org/10.3174%2Fajnr.A0802.
- Coutts SB, Hill MD, Simon JE, Sohn CH, Scott JN, Demchuk AM. Silent ischemia in minor stroke and TIA patients identified on MR imaging. Neurology 2005;65:513-17. http://dx.doi.org/10.1212%2F01.WNL.0000169031.39264.ff.
- Saver JL. Can acute MRI predict outcome following transient ischemic attack and minor stroke?. Nat Clin Pract Neurol 2006;2:14-5. http://dx.doi.org/10.1038%2Fncpneuro0049.
- Uno H, Taguchi A, Oe H, Nagano K, Yamada N, Moriwaki H, et al. Relationship between detectability of ischemic lesions by diffusion-weighted imaging and embolic sources in transient ischemic attacks. Eur Neurol 2008;59:38-43. http://dx.doi.org/10.1159%2F000109259.
- Ay H, Buonanno FS, Rordorf G, Schaefer PW, Schwamm LH, Wu O, et al. Normal diffusion-weighted MRI during stroke-like deficits. Neurology 1999;52:1784-92. http://dx.doi.org/10.1212%2FWNL.52.9.1784.
- Ay H, Oliveira-Filho J, Buonanno FS, Schaefer PW, Furie KL, Chang YC, et al. ‘Footprints’ of transient ischemic attacks: a diffusion-weighted MRI study. Cerebrovasc Dis 2002;14:177-86. http://dx.doi.org/10.1159%2F000065682.
- Ay H, Koroshetz WJ, Benner T, Vangel MG, Wu O, Schwamm LH, et al. Transient ischemic attack with infarction: a unique syndrome?. Ann Neurol 2005;57:679-86. http://dx.doi.org/10.1002%2Fana.20465.
- Purroy F, Begue R, Quilez A, Pinol-Ripoll G, Sanahuja J, Brieva L, et al. The California, ABCD, and unified ABCD2 risk scores and the presence of acute ischemic lesions on diffusion-weighted imaging in TIA patients. Stroke 2009;40:2229-32. http://dx.doi.org/10.1161%2FSTROKEAHA.108.537969.
- Kim JT, Kim HJ, Yoo SH, Park MS, Kwon SU, Cho KH, et al. MRI findings may predict early neurologic deterioration in acute minor stroke or transient ischemic attack due to intracranial atherosclerosis. Eur Neurol 2010;64:95-100. http://dx.doi.org/10.1159%2F000315138.
- Totaro P, Toni D, Durastanti L, Bozzao L, Gualdi GF, Raz E, et al. Diffusion-weighted MRI in patients with non-diagnostic CT in the post-acute phase of cerebral ischemia. Eur Neurol 2010;63:94-100. http://dx.doi.org/10.1159%2F000276399.
- Zakaria Y, Elghawabi H. Neurological evaluation and diffusion-weighted MRI assessment of patients with transient ischemic attacks. Egypt J Neurol Psychiatr Neurosurg 2010;47:447-52.
- Förster A, Gass A, Kern R, Ay H, Chatzikonstantinou A, Hennerici MG, et al. Brain imaging in patients with transient ischemic attack: a comparison of computed tomography and magnetic resonance imaging. Eur Neurol 2012;67:136-41. http://dx.doi.org/10.1159%2F000333286.
- Keir SL, Wardlaw JM, Dennis MS. The impact of neuroimaging on clinical decisions in minor stroke. Age Ageing 2004;33:312-14. http://dx.doi.org/10.1093%2Fageing%2Fafh070.
- Makin S, Doubal FN, Dennis MS, Wardlaw JM. Factors associated with negative MRI scan in minor stroke. Int J Stroke 2011;6.
- Doubal FN, Dennis MS, Wardlaw JM. Characteristics of patients with minor ischaemic strokes and negative MRI: a cross sectional study. J Neurol Neurosurg Psychiatry 2011;82:540-2. http://dx.doi.org/10.1136%2Fjnnp.2009.190298.
- The European Stroke Organization (ESO) Executive Committee, ESO Writing Committee . Guidelines for Management of Ischaemic Stroke and Transient Ischaemic Attack 2008 (updated 2009) n.d. www.eso-strokeorg/pdf/ESO08_Guidelines_Original_english.pdf (accessed 9 July 2012).
- Stroke – National Clinical Guideline for Diagnosis and Initial Management of Acute Stroke and Transient Ischaemic Attack (TIA). London: Royal College of Physicians; 2008.
- Martin PJ, Young G, Enevoldson TP, Humphrey PR. Overdiagnosis of TIA and minor stroke: experience at a regional neurovascular clinic. Q J Med 1997;90:759-63. http://dx.doi.org/10.1093%2Fqjmed%2F90.12.759.
- Johnston SC. Transient neurological attack: a useful concept?. JAMA 2007;298:2912-3. http://dx.doi.org/10.1001%2Fjama.298.24.2912.
- Fonseca AC, Canhao P. Diagnostic difficulties in the classification of transient neurological attacks. Eur J Neurol 2011;18:644-8. http://dx.doi.org/10.1111%2Fj.1468-1331.2010.03241.x.
- Landi G. Clinical diagnosis of transient ischaemic attacks. Lancet 1992;339:402-5. http://dx.doi.org/10.1016%2F0140-6736%2892%2990086-I.
- Johnston SC. Clinical practice. Transient ischemic attack. N Engl J Med 2002;347:1687-92.
- Bos MJ, Koudstaal PJ, Breteler MMB. Prognosis of transient neurological attacks: reply. JAMA 2008;299:1772-3. http://dx.doi.org/10.1001%2Fjama.299.15.1772.
- Sempere AP, Duarte J, Cabezas C, Claveria LE. Incidence of transient ischemic attacks and minor ischemic strokes in Segovia, Spain. Stroke 1996;27:667-71. http://dx.doi.org/10.1161%2F01.STR.27.4.667.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339. http://dx.doi.org/10.1136%2Fbmj.b2535.
- Bos MJ, van Rijn MJ, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM. Incidence and prognosis of transient neurological attacks. JAMA 2007;298:2877-85. http://dx.doi.org/10.1001%2Fjama.298.24.2877.
- Karunaratne PM, Norris CA, Syme PD. Analysis of six months’ referrals to a ‘one-stop’ neurovascular clinic in a district general hospital: implications for purchasers of a stroke service. Health Bull 1999;57:17-28.
- Murray S, Bashir K, Lees KR, Muir K, MacAlpine C, Roberts M, et al. Epidemiological aspects of referral to TIA clinics in Glasgow. Scott Med J 2007;52:4-8. http://dx.doi.org/10.1258%2Frsmsmj.52.1.4.
- Banerjee S, Natarajan I, Biram R, Sutton K, Ekeng G, Ames D, et al. FAST-TIA: a prospective evaluation of a nurse-led anterior circulation TIA clinic. Postgrad Med J 2009;85:637-42. http://dx.doi.org/10.1136%2Fpgmj.2009.083162.
- Hörer S, Schulte-Altedorneburg G, Haberl RL. Management of patients with transient ischemic attack is safe in an outpatient clinic based on rapid diagnosis and risk stratification. Cerebrovasc Dis 2011;32:504-10. http://dx.doi.org/10.1159%2F000331919.
- Cameron AC, Dawson J, Quinn TJ, Walters MR, McInnes GT, Morrison D, et al. Long-term outcome following attendance at a transient ischemic attack clinic. Int J Stroke 2011;6:306-11. http://dx.doi.org/10.1111%2Fj.1747-4949.2011.00591.x.
- Koudstaal PJ, Algra A, Pop GAM, Kappelle LJ, van Latum JC, van Gijn JV. Risk of cardiac events in atypical transient ischaemic attack or minor stroke. Lancet 1992;340:630-3. http://dx.doi.org/10.1016%2F0140-6736%2892%2992170-K.
- National Institute of Neurological and Communicative Disorders and Stroke . A classification and outline of cerebrovascular diseases II. Stroke 1975;6:564-616.
- Sharpe D. Of apples and oranges, file drawers and garbage: why validity issues in meta-analysis will not go away. Clin Psychol Rev 1997;17:881-90. http://dx.doi.org/10.1016%2FS0272-7358%2897%2900056-1.
- Harbison J, Hossain O, Jenkinson D, Davis J, Louw SJ, Ford GA. Diagnostic accuracy of stroke referrals from primary care, emergency room physicians, and ambulance staff using the face arm speech test. Stroke 2003;34:71-6. http://dx.doi.org/10.1161%2F01.STR.0000044170.46643.5E.
- Headache Classification Subcommittee of the International Headache Society . The International Classification of Headache Disorders. Cephalalgia 2004;24:9-160.
- Werhahn KJ. Weakness and focal sensory deficits in the postictal state. Epilepsy Behav 2010;19:138-9. http://dx.doi.org/10.1016%2Fj.yebeh.2010.06.029.
- Gibson L, Whiteley WN. Stroke patients as a proportion of patients with suspected stroke: a systematic review and meta-analysis. Cerebrovasc Dis 2012;33.
- Van Dijk JG, Thijs RD, Wieling W. Prognosis of transient neurological attacks. JAMA 2008;299:1771-2.
- Lindley RI, Amayo EO, Marshall J, Sandercock PA, Dennis M, Warlow CP. Hospital services for patients with acute stroke in the United Kingdom: the Stroke Association survey of consultant opinion. Age Ageing 1995;24:525-32. http://dx.doi.org/10.1093%2Fageing%2F24.6.525.
- Eysenbach G. Improving the quality of web surveys: the CHEcklist for Reporting Results of Internet E-Surveys (CHERRIES). J Med Internet Res 2004;6. http://dx.doi.org/10.2196%2Fjmir.6.3.e34.
- Centre for Reviews and Dissemination . Systematic Reviews: CRD’s Guidance for Undertaking Systematic Reviews in Health Care n.d. www.york.ac.uk/inst/crd/SysRev/!SSL!/WebHelp/SysRev3.htm (accessed 1 February 2012).
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8.
- Guide to the Methods of Technology appraisal. London: NICE; 2008.
- Buskens E, Nederkoorn PJ, Bujis-van der Woude T, Mali WP, Kappelle LJ, Eikelboom BC, et al. Imaging of carotid arteries in symptomatic patients: cost-effectiveness of diagnostic strategies. Radiology 2004;233:101-12.
- Tholen AT, de Monye C, Genders TS, Buskens E, Dippel DW, van der Lugt A, et al. Suspected carotid artery stenosis: cost-effectiveness of CT angiography in work-up of patients with recent TIA or minor ischemic stroke. Radiology 2010;256:585-97. http://dx.doi.org/10.1148%2Fradiol.10091157.
- Blight A, Periera AC, Brown MM. A single consultation cerebrovascular disease clinic is cost effective in the management of transient ischaemic attack and minor stroke. J R Coll Physicians Lond 2000;34:452-5.
- Jackson D, Moshinsky J, Begg AJ. Addressing shortfalls in TIA care in the UK: an economic perspective. J Med Econ 2009;12:331-8. http://dx.doi.org/10.3111%2F13696990903365000.
- Luengo-Fernandez R, Gray AM, Rothwell PM. Effect of urgent treatment for transient ischaemic attack and minor stroke on disability and hospital costs (EXPRESS study): a prospective population-based sequential comparison. Lancet Neurol 2009;8:235-43. http://dx.doi.org/10.1016%2FS1474-4422%2809%2970019-5.
- Joshi JK, Ouyang B, Prabhakaran S. Should TIA patients be hospitalized or referred to a same-day clinic? A decision analysis. Neurology 2011;77:2082-8. http://dx.doi.org/10.1212%2FWNL.0b013e31823d763f.
- Earnshaw SR, Jackson D, Farkouh R, Schwamm L. Cost-effectiveness of patient selection using penumbral-based MRI for intravenous thrombolysis. Stroke 2009;40:1710-20. http://dx.doi.org/10.1161%2FSTROKEAHA.108.540138.
- Young KC, Benesch CG, Jahromi BS. Cost-effectiveness of multimodal CT for evaluating acute stroke. Neurology 2010;75:1678-85. http://dx.doi.org/10.1212%2FWNL.0b013e3181fc2838.
- King-Im JM, Hollingworth W, Trivedi RA, Cross JJ, Higgins NJ, Graves MJ, et al. Contrast-enhanced MR angiography vs intra-arterial digital subtraction angiography for carotid imaging: activity-based cost analysis. Eur Radiol 2004;14:730-5.
- Sullivan PW, Ghushchyan V. Mapping the EQ-5D index from the SF-12: US general population preferences in a nationally representative sample. Med Decis Making 2006;26:401-9. http://dx.doi.org/10.1177%2F0272989X06290496.
- Berry E, Kelly S, Westwood ME, Davies LM, Gough MJ, Bamford JM. The cost-effectiveness of magnetic resonance angiography for carotid artery stenosis and peripheral vascular disease: a systematic review. Health Technol Assess 2002;6.
- Hallan S, Asberg A, Indredavik B, Wideroe TE. Quality of life after cerebrovascular stroke: a systematic study of patients’ preferences for different functional outcomes. J Intern Med 1999;246:309-16.
- Samsa GP, Matchar DB, Goldstein L, Bonito A, Duncan PW, Lipscomb J, et al. Utilities for major stroke: results from a survey of preferences among persons at increased risk for stroke. Am Heart J 1998;136:703-13. http://dx.doi.org/10.1016%2FS0002-8703%2898%2970019-5.
- Matchar DB, Pauker SG. Endarterectomy in carotid artery disease. A decision analysis. JAMA 1987;258:793-8. http://dx.doi.org/10.1001%2Fjama.1987.03400060069032.
- Burke JF, Kerber KA, Iwashyna TJ, Morgenstern LB. Wide variation and rising utilization of stroke magnetic resonance imaging: data from 11 states. Ann Neurol 2012;71:179-85. http://dx.doi.org/10.1002%2Fana.22698.
- Liang L, Korogi Y, Sugahara T, Shigematsu Y, Okuda T, Ikushima I, et al. Detection of intracranial hemorrhage with susceptibility-weighted MR sequences. AJNR Am J Neuroradiol 1999;20:1527-34.
- Franke C, van Swieten J, van Gijn J, De Jonge J, Op de Coul AAW. Intracerebral hematomas during anticoagulant treatment. Stroke 1991;22.
- Wardlaw JM. Stroke: A Practical Guide to Management. Oxford: Blackwell Scientific Ltd; 2001.
- Sung CY, Chu NS. Late CT manifestations in spontaneous putaminal haemorrhage. Neuroradiology 1992;34:200-4. http://dx.doi.org/10.1007%2FBF00596335.
- Rothwell PM, Coull AJ, Giles MF, Howard SC, Silver LE, Bull LM, et al. Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study). Lancet 2004;363:1925-33. http://dx.doi.org/10.1016%2FS0140-6736%2804%2916405-2.
- Census 2001. Population Pyramids. UK.; n.d.
- Rothwell PM, Coull AJ, Silver LE, Fairhead JF, Giles MF, Lovelock CE, et al. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study). Lancet 2005;366:1773-83. http://dx.doi.org/10.1016%2FS0140-6736%2805%2967702-1.
- Bhatnagar P, Scarborough P, Smeeton NC, Allender S. The incidence of all stroke and stroke subtype in the United Kingdom, 1985 to 2008: a systematic review. BMC Public Health 2010;10. http://dx.doi.org/10.1186%2F1471-2458-10-539.
- Rothwell PM, Gutnikov SA, Warlow CP. Reanalysis of the final results of the European Carotid Surgery Trial. Stroke 2003;34:514-23. http://dx.doi.org/10.1161%2F01.STR.0000054671.71777.C7.
- Vermeer SE, Algra A, Franke CL, Koudstaal PJ, Rinkel GJE. Long-term prognosis after recovery from primary intracerebral hemorrhage. Neurology 2002;59:205-9. http://dx.doi.org/10.1212%2FWNL.59.2.205.
- Sandercock P, Gubitz G, Foley P, Counsell C. Antiplatelet therapy for acute ischaemic stroke. Cochrane Database Syst Rev 2003;2. http://dx.doi.org/10.1002%2F14651858.CD000029.
- Johnson ES, Lanes SF, Wentworth CEI, Satterfield MH, Abebe BL, Dicker LW. A metaregression analysis of the dose–response effect of aspirin on stroke. Arch Intern Med 1999;159:1248-53.
- Baigent C, Sudlow C, Collins R, Peto R. Antithrombotic Trialists’ Collaboration . Collaborative meta-analysis of randomised trials of antiplatelet therapy for the prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324:71-86.
- De Schryver EL, Algra A, van Gijn J. Dipyridamole for preventing stroke and other vascular events in patients with vascular disease. Cochrane Database Syst Rev 2003;1. http://dx.doi.org/10.1002%2F14651858.CD001820.pub2.
- PROGRESS Collaborative Group . Randomised trial of a peridopril-based blood-pressure-lowering regimen among 6105 individuals with previous stroke or transient ischaemic attack. Lancet 2001;358:1033-41.
- Kaste M. Statins in threatened stroke. Stroke 2003;34:351-3. http://dx.doi.org/10.1161%2F01.STR.0000054260.05136.7D.
- Clark TG, Murphy MF, Rothwell PM. Long term risks of stroke, myocardial infarction, and vascular death in ‘low risk’ patients with a non-recent transient ischaemic attack. J Neurol Neurosurg Psychiatry 2003;74:577-80.
- Cunningham EJ, Bond R, Mehta Z, Mayberg MR, Warlow CP, Rothwell PM. Long-term durability of carotid endarterectomy for symptomatic stenosis and risk factors for late postoperative stroke. Stroke 2002;33:2658-63. http://dx.doi.org/10.1161%2F01.STR.0000034397.72390.D3.
- Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1998;339:1415-25.
- Hardie K, Jamrozik K, Hankey GJ, Broadhurst RJ, Anderson C. Trends in five-year survival and risk of recurrent stroke after first-ever stroke in the Perth Community Stroke Study. Cerebrovasc Dis 2005;19:179-85. http://dx.doi.org/10.1159%2F000083253.
- Hardie K, Hankey GJ, Jamrozik K, Broadhurst RJ, Anderson C. Ten-year survival after first-ever stroke in the Perth Community Stroke Study. Stroke 2003;34:1842-6. http://dx.doi.org/10.1161%2F01.STR.0000082382.42061.EE.
- Slot KB, Berge E, Dorman P, Lewis S, Dennis M, Sandercock P. Impact of functional status at six months on long term survival in patients with ischaemic stroke: prospective cohort studies. BMJ 2008;336:376-9. http://dx.doi.org/10.1136%2Fbmj.39456.688333.BE.
- Slot KB, Berge E, Sandercock P, Lewis SC, Dorman P, Dennis M. Causes of death by level of dependency at 6 months after ischemic stroke in 3 large cohorts. Stroke 2009;40:1585-9. http://dx.doi.org/10.1161%2FSTROKEAHA.108.531533.
- Selvarajah JR, Smith CJ, Hulme S, Georgiou RF, Vail A, Tyrrell PJ. Prognosis in patients with transient ischaemic attack (TIA) and minor stroke attending TIA services in the North West of England: the NORTHSTAR Study. J Neurol Neurosurg Psychiatry 2008;79:38-43. http://dx.doi.org/10.1136%2Fjnnp.2007.129163.
- Falke P, Stavenow L, Young M, Lindgarde F. Differences in mortality and cardiovascular morbidity during a 3-year follow-up of transient ischemic attacks and minor strokes. Stroke 1989;20:340-4. http://dx.doi.org/10.1161%2F01.STR.20.3.340.
- Dhamoon MS, Sciacca RR, Rundek T, Sacco RL, Elkind MS. Recurrent stroke and cardiac risks after first ischemic stroke: the Northern Manhattan Study. Neurology 2006;66:641-6. http://dx.doi.org/10.1212%2F01.wnl.0000201253.93811.f6.
- Lee S, Shafe AC, Cowie MR. UK stroke incidence, mortality and cardiovascular risk management 1999–2008: time-trend analysis from the General Practice Research Database. BMJ Open 2011;1.
- Rost NS, Smith EE, Chang Y, Snider RW, Chanderraj R, Schwab K, et al. Prediction of functional outcome in patients with primary intracerebral hemorrhage: the FUNC score. Stroke 2008;39:2304-9. http://dx.doi.org/10.1161%2FSTROKEAHA.107.512202.
- Counsell C, Dennis M, McDowall M, Warlow C. Predicting outcome after acute and subacute stroke. Development and validation of new prognostic models. Stroke 2002;33:1041-7. http://dx.doi.org/10.1161%2Fhs0402.105909.
- Mead G, Lewis S, Wardlaw JM, Dennis MS. Comparison of risk factors in patients with transient and prolonged eye and brain ischemic syndromes. Stroke 2002;33:2383-90. http://dx.doi.org/10.1161%2F01.STR.0000029827.93497.97.
- Bond R, Narayan SK, Rothwell PM, Warlow CP. Clinical and radiographic risk factors for operative stroke and death in the European Carotid Surgery Trial. Eur J Vasc Endovasc Surg 2002;23:108-16. http://dx.doi.org/10.1053%2Fejvs.2001.1541.
- Hankey GJ, Jamrozik K, Broadhurst RJ, Forbes S, Burvill PW, Anderson CS, et al. Five-year survival after first-ever stroke and related prognostic factors in the Perth Community Stroke Study. Stroke 2000;31:2080-6. http://dx.doi.org/10.1161%2F01.STR.31.9.2080.
- Burns JD, Rabinstein AA, Roger VL, Stead LG, Christianson TJ, Killian JM, et al. Incidence and predictors of myocardial infarction after transient ischemic attack: a population-based study. Stroke 2011;42:935-40. http://dx.doi.org/10.1161%2FSTROKEAHA.110.593723.
- Touze E, Varenne O, Chatellier G, Peyrard S, Rothwell PM, Mas JL. Risk of myocardial infarction and vascular death after transient ischemic attack and ischemic stroke: a systematic review and meta-analysis. Stroke 2005;36:2748-55. http://dx.doi.org/10.1161%2F01.STR.0000190118.02275.33.
- Kaplan RC, Tirschwell DL, Longstreth WT, Manolio TA, Heckbert SR, Lefkowitz D, et al. Vascular events, mortality, and preventive therapy following ischemic stroke in the elderly. Neurology 2005;65:835-42. http://dx.doi.org/10.1212%2F01.wnl.0000176058.09848.bb.
- Volmink JA, Newton JN, Hicks NR, Sleight P, Fowler GH, Neil HA. Coronary event and case fatality rates in an English population: results of the Oxford myocardial infarction incidence study. The Oxford Myocardial Infarction Incidence Study Group. Heart 1998;80:40-4.
- Barnett HJ, Meldrum HE, Eliasziw M. The appropriate use of carotid endarterectomy. CMAJ 2002;166:1169-79.
- Johnston DC, Chapman KM, Goldstein LB. Low rate of complications of cerebral angiography in routine clinical practice. Neurology 2001;57:2012-14. http://dx.doi.org/10.1212%2FWNL.57.11.2012.
- Hankey GJ, Warlow CP, Molyneux AJ. Complications of cerebral angiography for patients with mild carotid territory ischaemia being considered for carotid endarterectomy. J Neurol Neurosurg Psychiatry 1990;53:542-8. http://dx.doi.org/10.1136%2Fjnnp.53.7.542.
- Cloft HJ, Joseph GJ, Dion JE. Risk of cerebral angiography in patients with subarachnoid hemorrhage, cerebral aneurysm, and arteriovenous malformation: a meta-analysis. Stroke 1999;30:317-20. http://dx.doi.org/10.1161%2F01.STR.30.2.317.
- Davies KN, Humphrey PRD. Complications of cerebral angiography in patients with symptomatic carotid territory ischaemia screened by carotid ultrasound. J Neurol Neurosurg Psychiatry 1993;56:967-72. http://dx.doi.org/10.1136%2Fjnnp.56.9.967.
- Rerkasem K, Rothwell PM. Systematic review of the operative risks of carotid endarterectomy for recently symptomatic stenosis in relation to the timing of surgery. Stroke 2009;40:e564-72. http://dx.doi.org/10.1161%2FSTROKEAHA.109.558528.
- UK Carotid Endarterectomy Audit. London: Royal College of Physicians; 2011.
- Expectation of Life, United Kingdom. London: Government Actuary Department; 1999.
- Sandercock PA, Counsell C, Gubitz GJ, Tseng MC. Antiplatelet therapy for acute ischaemic stroke. Cochrane Database Syst Rev 2008;3. http://dx.doi.org/10.1002%2F14651858.CD000029.
- Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373:1849-60.
- Halkes PH, Gray LJ, Bath PM, Diener HC, Guiraud-Chaumeil B, Yatsu FM, et al. Dipyridamole plus aspirin versus aspirin alone in secondary prevention after TIA or stroke: a meta-analysis by risk. J Neurol Neurosurg Psychiatry 2008;79:1218-23. http://dx.doi.org/10.1136%2Fjnnp.2008.143875.
- Rashid P, Leonardi-Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review. Stroke 2003;34:2741-8. http://dx.doi.org/10.1161%2F01.STR.0000092488.40085.15.
- Heart Protection Study Collaborative Group . MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:7-22.
- Amarenco P, Bogousslavsky J, Callahan A, Goldstein LB, Hennerici M, Rudolph AE, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006;355:549-59.
- Goldstein LB, Amarenco P, Szarek M, Callahan A, Hennerici M, Sillesen H, et al. Hemorrhagic stroke in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels study. Neurology 2008;70:2364-70. http://dx.doi.org/10.1212%2F01.wnl.0000296277.63350.77.
- Manktelow BN, Potter JF. Interventions in the management of serum lipids for preventing stroke recurrence. Cochrane Database Syst Rev 2009;3. http://dx.doi.org/10.1002%2F14651858.CD002091.
- Yusuf S. Two decades of progress in preventing vascular disease. Lancet 2002;360:2-3. http://dx.doi.org/10.1016%2FS0140-6736%2802%2909358-3.
- Hackam DG, Spence JD. Combining multiple approaches for the secondary prevention of vascular events after stroke: a quantitative modeling study. Stroke 2007;38:1881-5. http://dx.doi.org/10.1161%2FSTROKEAHA.106.475525.
- Amarenco P, Goldstein LB, Messig M, O’Neill BJ, Callahan A, Sillesen H, et al. Relative and cumulative effects of lipid and blood pressure control in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels trial. Stroke 2009;40:2486-92. http://dx.doi.org/10.1161%2FSTROKEAHA.108.546135.
- Yip S, Benavente O. Antiplatelet agents for stroke prevention. Neurotherapeutics 2011;8:475-87. http://dx.doi.org/10.1007%2Fs13311-011-0060-2.
- Benavente OR, Hart RG, McClure LA, Szychowski JM, Coffey CS, . SPS3 Investigators . Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med 2012;367:817-25. doi:10.1056/NEJMoa1204133.
- Marquardt L, Kuker W, Chandratheva A, Geraghty O, Rothwell PM. Incidence and prognosis of ≥ or = 50% symptomatic vertebral or basilar artery stenosis: prospective population-based study. Brain 2009;132:982-8. http://dx.doi.org/10.1093%2Fbrain%2Fawp026.
- Bravata DM, Ho SY, Brass LM, Concato J, Scinto J, Meehan TP. Long-term mortality in cerebrovascular disease. Stroke 2003;34:699-704. http://dx.doi.org/10.1161%2F01.STR.0000057578.26828.78.
- Dennis M, Bamford J, Sandercock P, Warlow C. Prognosis of transient ischemic attacks in the Oxfordshire Community Stroke Project. Stroke 1990;21:848-53. http://dx.doi.org/10.1161%2F01.STR.21.6.848.
- Hannon N, Sheehan O, Kelly L, Marnane M, Merwick A, Moore A, et al. Stroke associated with atrial fibrillation: incidence and early outcomes in the north Dublin population stroke study. Cerebrovasc Dis 2010;29:43-9. http://dx.doi.org/10.1159%2F000255973.
- Department of Health, Vascular Programme, Stroke . National Stroke Strategy 2007.
- Youman P, Wilson K, Harraf F, Kalra L. The economic burden of stroke in the United Kingdom. Pharmacoeconomics 2003;21:43-50. http://dx.doi.org/10.2165%2F00019053-200321001-00005.
- Office for National Statistics . Wales, Interim Life Tables, 1980–82 to 2008–10 n.d. www.ons gov uk/ons/taxonomy/index html?nscl = Interim+Life+Tables (accessed 1 August 2012).
- Office for National Statistics . England, Interim Life Tables, 1980–82 to 2008–10 n.d. www.ons gov uk/ons/taxonomy/index html?nscl = Interim+Life+Tables (accessed 1 August 2012).
- Mortality Statistics: Deaths Registered in England and Wales. Newport: Office for National Statistics; 2009.
- Bronnum-Hansen H, Jorgensen T, Davidsen M, Madsen M, Osler M, Gerdes LU, et al. Survival and cause of death after myocardial infarction: the Danish MONICA study. J Clin Epidemiol 2001;54:1244-50.
- Bronnum-Hansen H, Davidsen M, Thorvaldsen P. Long-term survival and causes of death after stroke. Stroke 2001;32:2131-6. http://dx.doi.org/10.1161%2Fhs0901.094253.
- Grieve R, Dundas R, Beech R, Wolfe C. The development and use of a method to compare the costs of acute stroke across Europe. Age Ageing 2001;30:67-72. http://dx.doi.org/10.1093%2Fageing%2F30.1.67.
- NHS Reference Costs 2009–10. London: Crown; 2011.
- Saka O, McGuire A, Wolfe C. Cost of stroke in the United Kingdom. Age Ageing 2009;38:27-32. http://dx.doi.org/10.1093%2Fageing%2Fafn281.
- British National Formulary. MedicinesComplete n.d. www.medicinescomplete com/mc/bnf/current (accessed 1 November 2012).
- Dodel RC, Haacke C, Zamzow K, Paweilik S, Spottke A, Rethfeldt M, et al. Resource utilization and costs of stroke unit care in Germany. Value Health 2004;7:144-52. http://dx.doi.org/10.1111%2Fj.1524-4733.2004.72314.x.
- Epifanov Y, Dodel R, Haacke C, Schaeg M, Schoffski O, Hennerici M, et al. Costs of acute stroke care on regular neurological wards: a comparison with stroke unit setting. Health Policy 2007;81:339-49. http://dx.doi.org/10.1016%2Fj.healthpol.2006.07.004.
- Christensen MC, Munro V. Ischemic stroke and intracerebral hemorrhage: the latest evidence on mortality, re-admissions and hospital costs from Scotland. Neuroepidemiology 2008;30:239-46.
- Svendsen ML, Ehlers LH, Andersen G, Johnsen SP. Quality of care and length of hospital stay among patients with stroke. Med Care 2009;47:575-82. http://dx.doi.org/10.1097%2FMLR.0b013e318195f852.
- Quaglini S, Cavallini A, Gerzeli S, Micieli G. Economic benefit from clinical practice guideline compliance in stroke patient management. Health Policy 2004;69:305-15. http://dx.doi.org/10.1016%2Fj.healthpol.2003.12.015.
- Dawson J, Lees JS, Chang TP, Walters MR, Ali M, Davis SM, et al. Association between disability measures and healthcare costs after initial treatment for acute stroke. Stroke 2007;38:1893-8. http://dx.doi.org/10.1161%2FSTROKEAHA.106.472381.
- Lovibond K, Jowett S, Barton P, Caulfield M, Heneghan C, Hobbs FD, et al. Cost-effectiveness of options for the diagnosis of high blood pressure in primary care: a modelling study. Lancet 2011;378:1219-30. http://dx.doi.org/10.1016%2FS0140-6736%2811%2961184-7.
- Palmer S, Sculpher M, Phillips Z. A Cost Effectiveness Model Comparing Alternative Management Strategies for the Use of Glycoprotein IIB/IIIA Antagonists in Non-ST-Elevation Acute Coronary Syndrome. York: York Centre for Health Economics; 2004.
- Review of Technology Appraisal Guidance 90. London: NICE; 2010.
- Post PN, Stiggelbout A, Wakker P. Utility of health states after stroke. A systematic review of the literature. Stroke 2001;32:1425-9. http://dx.doi.org/10.1161%2F01.STR.32.6.1425.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. http://dx.doi.org/10.1097%2F00005650-199711000-00002.
- Mortimer D, Segal L, Sturm J. Can we derive an ‘exchange rate’ between descriptive and preference-based outcome measures for stroke? Results from the transfer to utility (TTU) technique. Health Qual Life Outcomes 2009;7.
- Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002;21:271-92. http://dx.doi.org/10.1016%2FS0167-6296%2801%2900130-8.
- Rivero-Arias O, Ouellet M, Gray A, Wolstenholme J, Rothwell PM, Luengo-Fernandez R. Mapping the Modified Rankin Scale (mRS) measurement into the generic EuroQol (EQ-5D) health outcome. Med Decis Making 2010;30:341-54. http://dx.doi.org/10.1177%2F0272989X09349961.
- Van Exel NJ, Scholte Op Reimer WJ, Koopmanschap MA. Assessment of post-stroke quality of life in cost-effectiveness studies: the usefulness of the Barthel Index and the EuroQoL-5D. Qual Life Res 2004;13:427-33.
- Dorman PJ, Slattery J, Farrell B, Dennis MS, Sandercock PA. A randomised comparison of the EuroQol and Short Form-36 after stroke. United Kingdom Collaborators in the International Stroke Trial. BMJ 1997;315.
- Wardlaw JM, Seymour J, Cairns J, Keir S, Lewis S, Sandercock P. Immediate computed tomography scanning of acute stroke is cost-effective and improves quality of life. Stroke 2004;35:2477-83. http://dx.doi.org/10.1161%2F01.STR.0000143453.78005.44.
- Tseng MC, Lin HJ. Health-related quality of life after stroke: review of the literature and implications for future research. Acta Neurol Taiwan 2007;16:7-12.
- Tengs TO, Lin TH. A meta-analysis of quality-of-life estimates for stroke. Pharmacoeconomics 2003;21:191-200. http://dx.doi.org/10.2165%2F00019053-200321030-00004.
- O’Brien CL, Gage BF. Costs and effectiveness of ximelagatran for stroke prophylaxis in chronic atrial fibrillation. JAMA 2005;293:699-706.
- Matchar DB, Samsa GP, Liu S. Cost-effectiveness of antiplatelet agents in secondary stroke prevention: the limits of certainty. Value Health 2005;8:572-80. http://dx.doi.org/10.1111%2Fj.1524-4733.2005.00050.x.
- Stahl JE, Furie KL, Gleason S, Gazelle GS. Stroke: Effect of implementing an evaluation and treatment protocol compliant with NINDS recommendations. Radiology 2003;228:659-68.
- Goodacre S, Nicholl J, Dixon S, Cross E, Angelini K, Arnold J, et al. Randomised controlled trial and economic evaluation of a chest pain observation unit compared with routine care. BMJ 2004;328. http://dx.doi.org/10.1136%2Fbmj.37956.664236.EE.
- Unit Costs of Health and Social Care 2010. Canterbury: PSSRU; 2010.
- Rothwell PM, Mayberg MR, Barnett HJM, Warlow CP. Meta-analysis of individual patient data from randomised controlled trials of carotid endarterectomy for symptomatic stenosis: (2) Overall results by degree of stenosis. Cerebrovasc Dis 2000;10.
- Navi BB, Kamel H, Shah MP, Grossman AW, Wong C, Poisson SN, et al. Application of the ABCD2 score to identify cerebrovascular causes of dizziness in the emergency department. Stroke 2012;43:1484-9. http://dx.doi.org/10.1161%2FSTROKEAHA.111.646414.
- Bouwmeester W, Zuithoff NP, Mallett S, Geerlings MI, Vergouwe Y, Steyerberg EW, et al. Reporting and methods in clinical prediction research: a systematic review. PLoS Med 2012;9. http://dx.doi.org/10.1371%2Fjournal.pmed.1001221.
- Warach S, Mosley M, Sorensen AG, Koroshetz W. Time course of diffusion imaging abnormalities in human stroke. Stroke 1996;27:1254-6.
- UK Carotid Endarterectomy Audit. Clinical Audit Report. Round 3 Public Report. London: Royal College of Physicians of London; 2010.
- UK Audit of Vascular Surgical Services & Carotid Endarterectomy. London: Royal College of Physicians; 2010.
- Gladstone DJ, Oh J, Fang J, Lindsay P, Tu JV, Silver FL, et al. Urgency of carotid endarterectomy for secondary stroke prevention: results from the Registry of the Canadian Stroke Network. Stroke 2009;40:2776-82. http://dx.doi.org/10.1161%2FSTROKEAHA.109.547497.
- Siontis GC, Tzoulaki I, Siontis KC, Ioannidis JP. Comparisons of established risk prediction models for cardiovascular disease: systematic review. BMJ 2012;344. http://dx.doi.org/10.1136%2Fbmj.e3318.
- Collins GS, Moons KG. Comparing risk prediction models. BMJ 2012;344. http://dx.doi.org/10.1136%2Fbmj.e3186.
- Mullen MT, Cucchiara BL. Redefinition of transient ischemic attack improves prognosis of transient ischemic attack and ischemic stroke: an example of the Will Rogers phenomenon. Stroke 2011;42:3612-13. http://dx.doi.org/10.1161%2FSTROKEAHA.111.627877.
Appendix 1 Search strategies
Chapter 3: stroke risk after transient ischaemic attack
The literature searches developed for Chapters 4 and 6 (below) have been combined for the purpose of Chapter 3.
Chapter 4: ABCD2 score to predict risk of stroke after transient ischaemic attack/minor stroke
MEDLINE (Ovid) search strategy
Searched: January 2005 to November 2011.
-
cerebrovascular disorders/ or basal ganglia cerebrovascular disease/ or brain ischemia/ or exp brain infarction/ or carotid artery diseases/ or carotid artery thrombosis/ or intracranial arterial diseases/ or cerebral arterial diseases/ or exp “intracranial embolism and thrombosis”/ or stroke/ or Hypoxia-Ischemia, Brain/
-
((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw.
-
(isch?emi$ adj6 (stroke$ or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva)).tw.
-
1 or 2 or 3
-
(minor or mini or mild or warning).tw.
-
4 and 5
-
Ischemic Attack, Transient/
-
(transient adj3 (ischemi$ or ischaemi$)).tw.
-
(transient adj3 attack$).tw.
-
(TIA or TIAs).tw.
-
6 or 7 or 8 or 9 or 10
-
ABCD$.tw.
-
((clinical or predict$ or risk$ or prognos$) adj3 scor$).tw.
-
(Predict$ adj3 (stroke$ or risk)).tw.
-
(stratif$ adj2 risk$ adj2 stroke$).tw.
-
exp risk/
-
“Predictive Value of Tests”/
-
16 and 17
-
“Severity of Illness Index”/
-
age factors/ and blood pressure/ and time factors/
-
(age and blood pressure and clinical features and duration).tw.
-
12 or 13 or 14 or 15 or 18 or 19 or 20 or 21
-
11 and 22
-
limit 23 to human
Chapter 4: ABCD2 score to predict risk of stroke after transient ischaemic attack/minor stroke
EMBASE (Ovid) search strategy
Searched: January 2005 to November 2011.
-
cerebrovascular disease/ or brain infarction/ or brain stem infarction/ or cerebellum infarction/ or brain ischemia/ or exp carotid artery obstruction/ or cerebral artery disease/ or cerebrovascular accident/ or exp occlusive cerebrovascular disease/ or stroke/ or stroke patient/
-
((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw.
-
(isch?emi$ adj6 (stroke$ or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva)).tw.
-
1 or 2 or 3
-
(minor or mini or mild or warning).tw.
-
4 and 5
-
transient ischemic attack/
-
(transient adj3 (ischemi$ or ischaemi$)).tw.
-
(transient adj3 attack$).tw.
-
(TIA or TIAs).tw.
-
6 or 7 or 8 or 9 or 10
-
scoring system/ or ABCD$.tw.
-
((clinical or predict$ or risk$ or prognos$) adj3 scor$).tw.
-
(Predict$ adj3 (stroke$ or risk)).tw.
-
(stratif$ adj2 risk$ adj2 stroke$).tw.
-
risk/ or cardiovascular risk/
-
exp “prediction and forecasting”/ or stratification/
-
16 and 17
-
age/ and blood pressure/ and (disease duration/ or time/)
-
(age and blood pressure and clinical features and duration).tw.
-
12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20
-
11 and 21
-
limit 22 to human
Chapter 6: proportion of transient ischaemic attack minor stroke patients with an ischaemic lesion on diffusion-weighted imaging
MEDLINE (Ovid) search strategy
Searched: January 1995 to November 2011.
-
cerebrovascular disorders/ or basal ganglia cerebrovascular disease/ or brain ischemia/ or exp brain infarction/ or carotid artery diseases/ or carotid artery thrombosis/ or intracranial arterial diseases/ or cerebral arterial diseases/ or exp “intracranial embolism and thrombosis”/ or stroke/ or Hypoxia-Ischemia, Brain/
-
((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw.
-
(isch?emi$ adj6 (stroke$ or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva)).tw.
-
1 or 2 or 3
-
(minor or mini or mild or warning).tw.
-
4 and 5
-
Ischemic Attack, Transient/
-
(transient adj3 (ischemi$ or ischaemi$)).tw.
-
(transient adj3 attack$).tw.
-
TIA.tw.
-
6 or 7 or 8 or 9 or 10
-
exp Magnetic Resonance Imaging/
-
((magnetic resonance or MR or NMR or diffusion weighted or T2-weighted) adj2 imag$).tw.
-
((MR or NMR) adj2 tomograph$).tw.
-
(MRI or DWI).tw.
-
12 or 13 or 14 or 15
-
exp Tomography, X-Ray Computed/
-
(CT or CAT).tw.
-
(comput$ adj3 tomograph$).tw.
-
17 or 18 or 19
-
16 or 20
-
(“1995$” or “1996$” or “1997$” or “1998$” or “1999$” or “20$”).ed.
-
11 and 21 and 22
-
limit 23 to humans
Chapter 6: proportion of transient ischaemic attack minor stroke patients with an ischaemic lesion on diffusion-weighted imaging
EMBASE (Ovid) search strategy
Searched: January 1995 to November 2011.
-
cerebrovascular disease/ or brain infarction/ or brain stem infarction/ or cerebellum infarction/ or brain ischemia/ or exp carotid artery obstruction/ or cerebral artery disease/ or cerebrovascular accident/ or exp occlusive cerebrovascular disease/ or stroke/ or stroke patient/
-
((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw.
-
(isch?emi$ adj6 (stroke$ or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva)).tw.
-
1 or 2 or 3
-
(minor or mini or mild or warning).tw.
-
4 and 5
-
transient ischemic attack/
-
(transient adj3 (ischemi$ or ischaemi$)).tw.
-
(transient adj3 attack$).tw.
-
TIA.tw.
-
6 or 7 or 8 or 9 or 10
-
exp nuclear magnetic resonance imaging/
-
((magnetic resonance or MR or NMR or diffusion weighted or T2-weighted) adj2 imag$).tw.
-
((MR or NMR) adj2 tomograph$).tw.
-
(MRI or DWI).tw.
-
12 or 13 or 14 or 15
-
exp computer assisted tomography/
-
(CT or CAT).tw.
-
(comput$ adj3 tomograph$).tw.
-
17 or 18 or 19
-
16 or 20
-
(“1995$” or “1996$” or “1997$” or “1998$” or “1999$” or “20$”).em.
-
11 and 21 and 22
-
limit 23 to human
Chapter 5: the addition of computed tomography brain and carotid imaging to clinical risk prediction scores of recurrent stroke after transient ischaemic attack
Search strategy in MEDLINE
Search strategy resulted in 16,556 hits (strategy was last run May 2012).
-
brain ischemia/
-
brain infarction/
-
cerebral infarction/
-
stroke
-
1 or 2 or 3 or 4
-
recur$.tw
-
predict$.tw
-
second.tw
-
6 or 7 or 8
Restriction of the search with CT- or carotid-related search terms would have missed relevant references, as some imaging was not a major part of the study.
Chapter 7: transient ischaemic attack and minor stroke mimics
MEDLINE (Ovid) search strategy
-
cerebrovascular disorders/ or basal ganglia cerebrovascular disease/ or brain ischemia/ or exp brain infarction/ or carotid artery diseases/ or carotid artery thrombosis/ or intracranial arterial diseases/ or cerebral arterial diseases/ or exp “intracranial embolism and thrombosis”/ or stroke/ or Hypoxia-Ischemia, Brain/
-
((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw.
-
(isch?emi$ adj6 (stroke$ or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva)).tw.
-
1 or 2 or 3
-
(minor or mini or mild or warning).tw.
-
4 and 5
-
Ischemic Attack, Transient/
-
(transient adj3 (ischemi$ or ischaemi$)).tw.
-
(transient adj3 attack$).tw.
-
(transient adj3 neurologic$ attack$).tw.
-
TIA.tw.
-
TNA.tw.
-
or/7-12
-
6 or 13
-
(mimic$ or misdiagnosis).tw.
-
(focal or atypical).tw.
-
(stroke?like or true stroke or pseudostroke).tw.
-
15 or 16 or 17
-
14 and 18
-
exp Mortality/ or Fatal Outcome/
-
exp Risk Factor/ or Cardiovascular Disease/
-
exp Secondary Prevention/
-
exp Prognosis/
-
20 or 21 or 22 or 23
-
19 and 24
-
limit 25 to yr = “1980-Current”
Chapter 7: transient ischaemic attack and minor stroke mimics
EMBASE (Ovid) search strategy
-
cerebrovascular disease/ or exp cerebrovascular accident/ or carotid artery disease/ or carotid artery obstruction/ or carotid artery thrombosis/ or brain infarction/ or brain stem infarction/or cerebellum infarction/ or exp brain ischemia/ or exp occlusive cerebrovascular disease/
-
((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw.
-
(isch?emi$ adj6 (stroke$ or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva)).tw.
-
1 or 2 or 3
-
(minor or mini or mild or warning).tw.
-
4 and 5
-
transient ischemic attack/
-
(transient adj3 (ischemi$ or ischaemi$)).tw.
-
(transient adj3 attack$).tw.
-
(transient adj3 neurologic$ attack$).tw.
-
TIA.tw.
-
TNA.tw.
-
or/7-12
-
6 or 13
-
(mimic$ or misdiagnosis).tw.
-
(focal or atypical).tw.
-
(stroke?like or true stroke or pseudostroke).tw.
-
15 or 16 or 17
-
14 and 18
-
exp mortality/ or fatality/
-
exp risk/ or risk factor/ or cardiovascular disease/ or neurologic disease/
-
secondary prevention/ or prophylaxis/
-
prognosis/ or disease course/
-
20 or 21 or 22 or 23
-
19 and 24
-
limit 25 to yr = “1980-Current”
Chapter 9 and 11: costs and previous cost-effectiveness analyses
EMBASE (Ovid) search strategy
Searched: January 1996 to 2011 week 8.
-
exp cerebrovascular disorders/ (228,191)
-
stroke$.tw. (107,527)
-
cerebrovascular$.tw. (23,460)
-
(cerebral or cerebellar or brainstem or vertebrobasilar).tw. (188,738)
-
(infarct$ or isch?emi$ or thrombo$ or emboli$).tw. (430,400)
-
4 and 5 (51,042)
-
(cerebral or intracerebral or intracranial or parenchymal).tw. (199,114)
-
(brain or intraventricular or brainstem or cerebellar).tw. (429,046)
-
(infratentorial or supratentorial or subarachnoid).tw. (18,781)
-
or/7-9 (558,740)
-
(haemorrage or hemorrhage or haematoma or hematoma).tw. (70,777)
-
(bleeding or aneurysm).tw. (121,745)
-
11 (70,777)
-
10 and 13 (27,294)
-
carotid$.tw. (52,648)
-
thrombo$.tw. (163,020)
-
(intracranial or (venous adj5 sinus$) or (sagittal adj5 venous) or (sagittal adj5 vein)).tw. (44,254)
-
16 and 17 (4464)
-
transient isch?emic attack$.tw. (6048)
-
reversible isch?emic neurologic$ defecit.tw. (0)
-
venous malformation$.tw. (986)
-
arteriovenous malformation$.tw. (5925)
-
21 or 22 (6773)
-
10 and 23 (3405)
-
exp aphasia/ (8498)
-
hemianopsia/ (1453)
-
hemiplegia/ (5357)
-
(aphasi$ or dysphasi or hemianop$).tw. (7902)
-
(hemiplegi$ or hemipar$).tw. (11,021)
-
or/25-29 (23,098)
-
or/1-3,6,14-15,18-20,24,30 (306,678)
-
leukomalacia, periventricular/ (1509)
-
cerebral anoxia/ (5821)
-
exp dementia, vascular/ (5570)
-
exp Vascular Headaches/ (145)
-
migrain$.tw. (18,258)
-
or/32-36 (31,090)
-
31 not 37 (293,645)
-
exp Computer Assisted Tomography/ (328,357)
-
(computed tomograph$ or ct).tw. (206,560)
-
39 or 40 (383,395)
-
38 and 41 (36,930)
-
exp economics/ (103,662)
-
exp technology assessment, biomedical/ (8431)
-
exp health resources/ (39,605)
-
cost$.tw. (240,084)
-
charge$.tw. (81,504)
-
economic$.tw. (97,136)
-
finance$.tw. (4768)
-
economic evaluation.tw. (4046)
-
cost effective$.tw. (55,597)
-
economics model$.tw. (15)
-
markov$.tw. (8779)
-
monte carlo.tw. (16,251)
-
(decision$ adj2 (tree? or anly$ or model$)).tw. (6411)
-
quality of life/ (146,257)
-
quality adjusted life years/ (7026)
-
quality adjusted life.tw. (4490)
-
or/43-58 (655,474)
-
38 and 41 and 59 (862)
-
limit 60 to english language (749)
-
limit 61 to yr = “2000 -Current” (671)
-
Nuclear Magnetic Resonance Imaging/ (274,871)
-
mri.tw. (110,011)
-
63 or 64 (291,418)
-
38 and 59 and 65 (661)
-
limit 66 to english language (591)
-
limit 67 to yr = “2000 -Current” (548)
Chapters 9 and 11: costs and previous cost-effectiveness analyses
Ovid MEDLINE(R) without Revisions
Searched: January 1996 to February week 2 2011.
-
exp “cerebrovascular disorders”/ (126,102)
-
stroke$.tw. (76,361)
-
cerebrovascular$.tw. (17,086)
-
(cerebral or cerebellar or brainstem or vertebrobasilar).tw. (144,798)
-
(infarct$ or isch?emi$ or thrombo$ or emboli$).tw. (318,894)
-
4 and 5 (36,596)
-
(cerebral or intracerebral or intracranial or parenchymal).tw. (150,327)
-
(brain or intraventricular or brainstem or cerebellar).tw. (337,459)
-
(infratentoriral or supratentorial or subarachnoid).tw. (13,217)
-
or/7-9 (434,276)
-
(haemorrage or hemorrhage or haematoma or hematoma).tw. (52,406)
-
(bleeding or aneurysm).tw. (88,982)
-
11 or 12 (130,357)
-
10 and 13 (26,725)
-
carotid$.tw. (39,004)
-
thrombo$.tw. (119,079)
-
(intracranial or (venous adj5 sinus$) or (sagittal adj5 venous) or (sagittal adj5 vein)).tw. (32,586)
-
16 and 17 (2992)
-
transient isch?emic attack$.tw. (4465)
-
reversible isch?emic neurologic$ defecit.tw. (0)
-
venous malformation$.tw. (702)
-
arteriovenous malformation$.tw. (4383)
-
21 or 22 (4987)
-
10 and 23 (2478)
-
exp aphasia/ (3078)
-
hemianopsia/ (825)
-
hemiplegia/ (2715)
-
(aphasi$ or dysphasi or hemianop$).tw. (5041)
-
(hemiplegi$ or hemipar$).tw. (7941)
-
or/25-29 (14,176)
-
or/1-3,6,14-15,18-20,24,30 (199,900)
-
leukomalacia, periventricular/ (800)
-
cerebral anoxia/ (2574)
-
exp dementia, vascular/ (3548)
-
exp Vascular Headaches/ (110)
-
migrain$.tw. (12,180)
-
or/32-36 (19,027)
-
31 not 37 (192,528)
-
Tomography, X-Ray Computed/ (148,332)
-
(computed tomograph$ or ct).tw. (149,853)
-
39 or 40 (222,171)
-
38 and 41 (20,937)
-
exp economics/ (228,595)
-
exp technology assessment, biomedical/ (4949)
-
exp health resources/ (6488)
-
cost$.tw. (180,366)
-
charge$.tw. (60,699)
-
economic$.tw. (72,526)
-
finance$.tw. (3794)
-
economic evaluation.tw. (2990)
-
cost effective$.tw. (41,336)
-
economics model$.tw. (12)
-
markov$.tw. (6964)
-
monte carlo.tw. (12,959)
-
(decision$ adj2 (tree? or anly$ or model$)).tw. (4942)
-
quality of life/ (73,130)
-
quality adjusted life years/ (4632)
-
quality adjusted life.tw. (3430)
-
Tomography, X-Ray Computed/ec [Economics] (726)
-
or/43-58 (537,088)
-
42 and 60 (402)
-
limit 61 to english language (349)
-
Magnetic Resonance Imaging/ (177,270)
-
mri.tw. (78,522)
-
63 or 64 (197,809)
-
magnetic resonance Imaging/ec (560)
-
or/43-58,66 (537,174)
-
38 and 61 and 67 (402)
-
limit 68 to english language (349)
-
limit 69 to yr = “2000-current” (284)
Chapters 9 and 11: costs and previous cost-effectiveness analyses
Database: Evidence-based Medicine reviews – Health Technology Assessment first quarter 2011, EBM reviews – NHS Economic Evaluation Database first quarter 2011
Search strategy
-
cerebrovascular disorders/ or basal ganglia cerebrovascular disease/ or brain ischemia/ or exp brain infarction/ or carotid artery diseases/ or carotid artery thrombosis/ or intracranial arterial diseases/ or cerebal arterial diseases/ or exp “intracranial embolism and thrombosis”/ or stroke/ or Hypoxia-Ischemia, Brain/ (583)
-
((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw. (164)
-
(isch?emi$ adj6 (stroke or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva)).tw. (159)
-
1 or 2 or 3 (692)
-
(minor or mini or mild or warning).tw. (1233)
-
4 and 5 (53)
-
Ischemic Attack, Transient/ (31)
-
(transient adj3 (ischemi$ or ischaemi$)).tw. (78)
-
(transient adj3 attack$).tw. (77)
-
TIA.tw. (25)
-
6 or 7 or 8 or 9 or 10 (117)
-
exp Magnetic Resonance Imaging/ (386)
-
((magnetic resonance or MR or NMR or diffusion weighted or T2-weighted) adj2 image$).tw. (14)
-
((MR or NMR) adj2 tomograph$).tw. (0)
-
(MR or DWI).tw. (107)
-
12 or 13 or 14 or 15 (424)
-
exp Tomography, X-Ray Computed/ (509)
-
(CT or CAT).tw. (491)
-
17 or 18 or “19”.mp. [mp = ti, tx, hw] (13,868)
-
17 or 18 or 19 (13,868)
-
16 or 20 (14,085)
-
(“1995$” or “1996$” or “1997$” or “1998$” or “1999$” or “20$”).ed. (0)
-
11 and 21 and 22 (0)
-
limit 23 to humans (0)
-
(di or du).fs. (3995)
-
24 and 25 (0)
-
24 not 26 (0)
-
exp basal ganglia hemorrhage/ or exp intracranial hemorrhages/ (86)
-
((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or subarachnoid or putaminal or putamen or posterior fossa) adj15 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. (183)
-
((haemorrhag$ or hemorrhag$) adj6 (stroke$ or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva)).tw. (62)
-
28 or 29 or 30 (208)
-
(minor or mini or mild or warning).tw. (1233)
-
31 and 32 (43)
-
21 and 22 and 33 (0)
-
limit 34 to humans (0)
-
35 not 24 (0)
-
(stroke adj5 (mimic$ or misdiagnosis)).tw. (1)
-
(stroke?like or true stroke or pseudostroke).tw. (1)
-
37 or 38 (1)
-
21 and 22 and 39 (0)
-
40 not (24 or 36) (0)
-
exp “costs and cost analysis”/ (25,213)
-
economics/ (50)
-
economics, hospital/ (96)
-
economics, medical/ (122)
-
economics, pharmaceutical/ (635)
-
economic evaluation.mp. (23,416)
-
exp budgets/ (172)
-
exp decision theory/ (733)
-
monte carlo method/ (324)
-
markov chains/ (1161)
-
exp technology assessment, biomedical/ (528)
-
cost$.ti. (16,475)
-
(cost$ adj2 (effective$ or utilit$ or benefit$ or minimis$)).ab. (0)
-
economics model$.tw. (3)
-
(economic$ or pharmacoeconomic$ or pharmo-economic$).ti. (5381)
-
(price$ or pricing$).tw. (7874)
-
(financial or finance or finances or financed).tw. (1250)
-
(value adj2 (money or monetary)).tw. (231)
-
markov$.tw. (1792)
-
monte carlo.tw. (946)
-
(decision$ adj2 (tree? or anly$ or model$)).tw. (2706)
-
or/42-62 (30,875)
-
63 and 4 (568)
-
63 and 11 (109)
-
63 and 11 and 23 (0)
-
(4 or 6 or 7 or 8 or 9 or 10) and 47 (390)
-
quality of life/ (2132)
-
quality adjusted life years/ (2434)
-
“Value of Life”/ (271)
-
health status indicators/ (221)
-
health status/ (550)
-
sickness impact profile/ (101)
-
disability evaluation/ (198)
-
activities of daily living/ (299)
-
disability.mp. (796)
-
cost utility analysis.mp. (2106)
-
rating scale.mp. (171)
-
questionnaire/ (0)
-
(quality adj1 life).tw. (38)
-
quality adjusted life.tw. (3704)
-
disability adjusted life.tw. (107)
-
(qaly? or qald? or qale? or qtime? or daly?).tw. (2374)
-
(euroqol or euro qol or eq5d or eq 5d).tw. (539)
-
(hql or hqol or h qol or hrqol or hr qol).tw. (61)
-
(hye or hyes).tw. (2)
-
health$ year$ equivalent$.tw. (5)
-
(hui or hui1 or hui2 or hui3).tw. (50)
-
(health adj3 (utilit$ or disutili$)).tw. (474)
-
(health adj3 (state or status)).tw. (1870)
-
(sf36 or sf 36 or short form 36 or shortform 36).tw. (240)
-
(sf6 or sf 6 or short form 6 or shortform 6).tw. (3)
-
(sf12 or sf 12 or short form 12 or shortform 12).tw. (32)
-
(sf20 or sf 20 or short form 20 or shortform 20).tw. (1)
-
willingness to pay.tw. (942)
-
standard gamble.tw. (177)
-
trade off.tw. (358)
-
conjoint analys?s.tw. (360)
-
discrete choice.tw. (376)
-
or/68-99 (7282)
-
11 and 100 (53)
-
limit 101 to yr = “2000 -Current” (36)
Chapters 9 and 11: costs and previous cost-effectiveness analyses
Database: Evidence-based Medicine reviews – Cochrane Database of Systematic Reviews (2005 to February 2011), Evidence-based Medicine reviews – ACP Journal Club (1991 to February 2011), Evidence-based Medicine reviews – Database of Abstracts of Reviews of Effects (first quarter 2011), Evidence-based Medicine reviews – Cochrane Central Register of Controlled Trials (first quarter 2011), Evidence-based Medicine reviews – Cochrane Methodology Register (first quarter 2011), Evidence-based Medicine reviews – Health Technology Assessment (first quarter 2011), Evidence-based Medicine reviews – NHS Economic Evaluation Database (first quarter 2011)
Search strategy
-
(stroke or cerebrovascular or vascular or cerbral or subrachnoid)/All Fields OR dementia/Subject Headings) AND (tomography OR ct OR magnetic OR mri/All fields {Including Limited Related Terms} (281)
-
limit 1 to english language [Limit not valid in CDSR,ACP Journal Club,DARE,CCTR,CLCMR; records were retained] (281)
-
limit 2 to yr = “2000 -Current” [Limit not valid in DARE; records were retained] (225)
-
limit 3 to humans [Limit not valid in CDSR,ACP Journal Club,DARE,CCTR,CLCMR; records were retained] (222)
Evidence-based Medicine Reviews – Cochrane Database of Systematic Reviews
Searched: 2005 to January 2011.
Search strategy
-
[exp economics/] (0)
-
[exp Technology Assessment, Biomedical/] (0)
-
[exp health resources/] (0)
-
cost$.tw. (3434)
-
charge$.tw. (184)
-
economic$.tw. (1679)
-
finance$.tw. (60)
-
economic evaluation.tw. (206)
-
cost effective$.tw. (1221)
-
or/1-9 (3821)
-
[exp cerebrovascular disorders/] (0)
-
stroke$.tw. (842)
-
cerebrovascular$.tw. (414)
-
(cerebral or cerebellar or brainstem or vertebrobasilar).tw. (811)
-
(infarct$ or isch?emi$ or thrombo$ or emboli$).tw. (1738)
-
14 and 15 (429)
-
[exp Ischemic Attack, Transient/] (0)
-
or/11-17 (2350)
-
Magnetic Resonance Angiography/ ec {Including Related Terms} (71)
-
18 and 19 (20)
-
10 and 20 (16)
-
limit 21 to yr = “2000 -Current” (16)
-
from 22 keep 1-5 (5)
-
[exp economics/] (0)
-
[exp Technology Assessment, Biomedical/] (0)
-
[exp health resources/] (0)
-
cost$.tw. (3434)
-
charge$.tw. (184)
-
economic$.tw. (1679)
-
finance$.tw. (60)
-
economic evaluation.tw. (206)
-
cost effective$.tw. (1221)
-
or/24-32 (3821)
-
[exp cerebrovascular disorders/] (0)
-
stroke$.tw. (842)
-
cerebrovascular$.tw. (414)
-
(cerebral or cerebellar or brainstem or vertebrobasilar).tw. (811)
-
(infarct$ or isch?emi$ or thrombo$ or emboli$).tw. (1738)
-
37 and 38 (429)
-
[exp Ischemic Attack, Transient/] (0)
-
or/34-40 (2350)
-
Tomography, X-Ray Computed/ec[Economics] {Including Related Terms} (8)
-
41 and 42 (3)
-
10 and 43 (3)
-
limit 44 to english language [Limit not valid; records were retained] (3)
-
limit 45 to yr = “2000-Current” (3)
Appendix 2 Critical appraisal tools
Chapter 5: the addition of computed tomography brain and carotid imaging to clinical risk prediction scores of recurrent stroke after transient ischaemic attack
| Item | Description | Scoring |
|---|---|---|
| 1 | Patient recruitment Were the patients a consecutively recruited sample? |
Y – Patients were recruited consecutively. In prospective studies, this means that efforts were made to recruit all patients who met the eligibility criteria over the time frame on the study. In retrospective studies, this means that efforts were made to identify all patient records within the time frame of the study N – Patients formed, for example, a convenience sample U – Insufficient detail reported in the study |
| 2 | Definition of a TIA | Y – The definition of a TIA is given, for example the WHO definition of sudden neurological deficits lasting < 24 hours with full recovery |
| 3 | TIA status Was the TIA status of the patients defined? |
Y – The TIA status of the patients was described in the study. For example, patients are described as confirmed, probable, suspected TIA cases N – For example, patients described as TIA only, with no indication of whether this is suspected, confirmed, probable, possible U – Unclear |
| 4 | Positive CT scan | Y – The definition of a positive CT scan is described, for example a new lesion apparent on CT consistent with the clinical symptoms N – No definition given U – Unclear |
| 5 | Positive carotid stenosis scan | Y – The definition of a positive carotid stenosis diagnosis is given, for example > 70% stenosis by the NASCET method N – No definition is given U – Unclear |
| 6 | Definition of outcome | Y – The definition of recurrent disabling stroke is given. For example, new neurological symptoms lasting > 24 hours, confirmed by a neurologist, resulting in a mRS score of > 2 N – No definition of recurrent disabling stroke is given. This includes studies that state that the outcome was recurrent stroke but do not give an indication of whether it was disabling or not U – Unclear |
| 7 | Time interval between symptom onset and scanning | Y – The time interval between presenting event and CT brain scan/carotid imaging is given. This is coded as ‘Y’ if the maximum time is given (i.e. the greatest possible time interval is known for all patients) N – The time interval is not given U – The time interval is given as a mean or some way such that it is not possible to infer how many patients had late CT scans |
| 8 | Treatment | Y – Description of treatments given, with some indication of the number of patients undergoing treatment and relation to outcome N – No description given U – The treatments are described but with no indication of patient numbers or how they might have affected outcomes |
| 9 | All patients included in analysis | Y – The results refer to all or most (> 80%) of patients recruited into the study, or there is an explanation for any mismatch that does not indicate that the subsample is a biased subsample N – There is a mismatch between the number recruited and the number included in the analysis, and the subsample is biased U – There is a mismatch between the number recruited and the number included in the analysis, but it is not clear whether the subsample is biased or not |
Chapter 7: diagnosis of transient ischaemic attack, minor stroke and mimics: systematic review and meta-analysis of frequency, differential diagnosis and prognosis of non-cardiovascular events
| Item | Description | Data recorded |
|---|---|---|
| 1 | Sample size | The number of patients recruited into the study |
| 2 | Study design – was the study prospective or retrospective? | Recorded as prospective if data were collected from patients over time, at time of recruitment outcomes had not occurred, retrospective if data were taken from existing records or similar, and unknown if insufficient detail reported in the study |
| 3 | Specialism of person making final diagnosis of TIA | Recorded as neurologist, geriatrician, stroke physician, GP or nurse |
| 4 | Setting of study | Recorded as TIA clinics, hospital, ED and population based |
| 5 | Did the study use predefined criteria for a TIA | Recorded as having used predefined criteria if a description given, also noted if the description was time based |
| 6 | Method of final diagnosis | Recorded as having been made in person or by review of patient records |
| 7 | Method of follow-up | Recorded as having been made in person or by review of patient records |
| 8 | Numbers of patients with a final diagnosis of a non-CV event | Recorded as the number of patients with a non-CV event and the total number recruited, to enable the calculation of the proportion of non-CV patients with associated CI |
| 9 | Other diagnoses | The most frequent differential diagnoses for non-CV patients |
| 10 | Outcomes of non-CV patients | The number of non-CV patients with further strokes, MI or vascular causes of death |
Appendix 3 Services for patients with suspected transient ischaemic attack or minor stroke in the UK: cover letter and questionnaire
Appendix 4 Access to imaging facilities for the diagnosis of stroke/transient ischaemic attack in the UK: cover letter and questionnaire
Appendix 5 Project description
Project description
1. Title
An assessment of the cost-effectiveness of magnetic resonance including diffusion weighted brain imaging in patients with transient ischaemic attack and minor stroke
2. Funding body
NIHR HTA 09/22/169
Kate Fenton
Programme Manager
NETSCC, Health Technology Assessment
Alpha House
University of Southampton Science Park
Southampton
SO16 7NS
3. Project steering group
-
Professor Joanna Wardlaw, Professor of Applied Neuroimaging and an Honorary Consultant Neuroradiologist, at the University of Edinburgh and NHS Lothian
-
Dr Paul McNamee, a Senior Research Fellow in Health Economics at the Health Economics Research Unit (HERU), University of Aberdeen
-
Professor Martin Dennis, Professor of Stroke Medicine and an Honorary Consultant Stroke Physician at the University of Edinburgh and NHS Lothian
-
Professor Peter Sandercock, Professor of Neurology and Honorary Consultant Neurologist at the University of Edinburgh and NHS Lothian
-
Ms Miriam Brazzelli, Research Fellow at the Division of Clinical Neurosciences, University of Edinburgh
-
Dr Janet de Wilde, Medical Physicist and Project Co-ordinator of SINAPSE
-
Professor Keith Muir, SINAPSE Professor of Clinical Imaging and Honorary Consultant Neurologist with an interest in stroke, University of Glasgow and NHS Glasgow
-
Professor Donald Hadley, Professor and Honorary Consultant Neuroradiologist, University of Glasgow and NHS Glasgow
-
Dr Peter Tothill, Lay representative
-
Dr G Scotland, Health economics research fellow, University of Aberdeen
-
Dr Francesca Chappell, Statistician, University of Edinburgh.
4. Research objectives
Our primary research objective is to determine whether magnetic resonance (MR) including diffusion-weighted imaging (DWI) as well as other relevant structural and blood-sensitive sequences is cost-effective in the majority of patients with transient ischaemic attack (TIA) or minor stroke to guide diagnosis and secondary stroke prevention, compared with the current alternative of computerised tomography (CT) brain scanning.
Secondly to determine the cost and cost-effectiveness of increased use of MR including DWI and blood-sensitive sequences in patients presenting more than five days after TIA/minor stroke when CT will not be able to identify haemorrhage as the cause of stroke reliably.
Thirdly to estimate if ‘one-stop’ brain and carotid imaging is more cost-effective than individual separate brain and carotid examinations, in what proportion of patients a ‘one-stop’ approach could be used, and the practical and cost implications. Fourth, to determine physicians and radiologists current attitudes to increased use of MR in TIA/minor stroke, the availability, barriers to greater use, costs of increasing availability and net effect on other patient groups in whom MR is commonly used. We will do this by modelling the effectiveness, cost-effectiveness and practical implications of using MR imaging in different proportions of patients with TIA or minor stroke at different times after symptom onset, using systematic reviews of the relevant literature, surveys of current practice and costs, existing stroke registry and cohort data and data linkage. If MR cannot be justified in all patients, then we will also determine in which subgroups of patients the use of MR is cost-effective. We will provide a range of options reflecting effect of using MR in different proportions of TIA/minor stroke patients to guide decision-making by health service purchasers.
5. Background
Stroke remains a major public health burden: In the UK, about 150,000 people have a stroke each year. About 30% die within six months and another 30% survive dependent on others for everyday activities, making stroke the commonest cause of dependency in adults and the third commonest cause of death in the world. 1 Stroke costs the NHS in England around £7bn per year. 2 Eighty per cent of strokes are ischaemic and most ischaemic strokes are due to an artery in the brain becoming blocked by atherothromboembolism. Treatment of stroke is limited, so prevention is vital. About 20–40% of people have a warning TIA or minor non-disabling ischaemic stroke shortly before they have a major disabling stroke. 3,4 If these people can be assessed quickly, potential stroke causes identified and given the appropriate treatment, then many of these disabling strokes can be prevented. 5
TIA is defined as ‘a sudden loss of focal cerebral or monocular function lasting less than 24 hours and which is thought to be due to inadequate cerebral or ocular blood supply as a result of low blood flow, thrombosis or embolism associated with disease of the arteries, heart or blood’. 6 While the definition of TIA purely on the basis of clinical grounds is the subject of debate,7,8 for the present time we will use this clinical definition. Patients with minor stroke, which only differs from TIA by symptoms or signs lasting more than 24 hours, are also at high risk of early recurrent stroke and need the same assessment and treatment as for patients with TIA to prevent a further disabling stroke or death. A small proportion of TIA/minor stroke (less than 5%) are actually due to a small haemorrhage in the brain, but this can only be distinguished by brain scanning.
TIA and minor stroke are common: In the UK, there are estimated to be 80,000 to 90,000 TIAs and minor strokes per year. 9 Therefore, the average regional hospital serving a population of 750,000 will see about 1000 suspected cases per year, i.e. 20 per week. Delivering effective stroke prevention to this number of people is challenging and requires highly organised stroke services that are able to respond rapidly and precisely. However the personal, societal, public health and financial burden of stroke in the UK is such that every effort should be made to limit the damaging effects of having a major disabling stroke, and to determine how to make best use of our available resources. 10–13
TIA and minor stroke is a medical emergency: The period of highest risk of disabling stroke is in the first few hours and days after a TIA/minor stroke, thus making suspected TIA/minor stroke a medical emergency:4,6,14,15 between 8% and 11.5% of patients will have a recurrent stroke by one week and between 11.5% and 15% by one month after TIA/minor stroke unless effective secondary prevention is started. 9 In the USA, there are about 240,000 TIAs per annum, of whom 25% had had a further TIA, a stroke or died by three months. 16 Prevention of recurrent stroke is by rapid identification of underlying risk factors (such as ipsilateral tight carotid artery stenosis, atrial fibrillation, hypercholesterolaemia, hypertension) and implementation of optimal medical (antiplatelet agents, statins, antihypertensives or anticoagulants where necessary)5,6,17 and surgical treatment (endarterectomy for symptomatic moderate to severe carotid stenosis). 18
Appropriate treatment of those whose symptoms are not due to vascular disease: The diagnosis of TIA/minor stroke brings a threat of disabling stroke or death and causes much worry for patients. Patients with definite acute ischaemic cerebrovascular disease are now given standard quadruple preventative therapy (antiplatelet, statin, angiotensin converting enzyme inhibitor and diuretic). In the light of the EXPRESS study, many patients with suspected acute ischaemic cerebrovascular disease following presentation with TIA/minor stroke are started on quadruple therapy pending specialist investigation and treatment. It is therefore important to ensure that the patients, whose symptoms after due investigation are proven not to be due to acute ischaemic cerebrovascular disease, particularly the small proportion (< 5%) whose symptoms are due to a small haemorrhage, then avoid inappropriate or unnecessary and possibly hazardous drug treatment. So, if MR imaging can reliably help exclude acute ischaemic cerebrovascular disease as the cause of symptoms, then an additional benefit is that patients will avoid the inconvenience and risk (of adverse drug reactions) and the health service will avoid the cost of unnecessary drug treatment.
Clinical stroke risk prediction: Several scoring systems have been devised that aim to improve identification of patients at high risk of disabling recurrent stroke after TIA/minor stroke: a score developed in 469 patients with TIA seen in the 1980s based on clinical variables;19 a score developed in the European Carotid Surgery Trial (1980s to mid 1990s) data;20 the ABCD score developed using data from a population-based stroke and TIA registry conducted in the 1980s (Oxfordshire Community Stroke Project – OCSP) and tested in a repeat study in the same population in the early 2000s (Oxford Vascular Study – OxVasc);21 an updated version of this derived in the OCSP TIA patients and patients attending a private health provider in the USA and tested in OxVasc and more USA patients, the ABCD2 score;22 the Essen Stroke Risk Score which was derived from a subset of ischaemic stroke patients in the Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events (CAPRIE) trial and validated in two observational studies and in data from another stroke prevention trial;23 the Stroke Prognosis Instruments I and II (SPI-I and SPI-II) were developed in medical stroke prevention trials and validated in three independent cohorts;24 and the LIfe Long After Cerebral Ischaemia score (LiLAC), which was based on data from the Dutch TIA trial (1986–1989), has not been externally validated. 25 All except two20,24 of these scores use only simple clinical features so are rapid and easy to apply without needing complex technologies. 19,21,22,25 For example, the ABCD2 score uses age, blood pressure, clinical features, duration of symptoms and diabetes to derive a score from zero to seven. These scores could help to reduce delays to reaching medical attention and to triage patients,26 so that those needing most rapid treatment such as carotid endarterectomy would reach surgery more quickly. They might also improve the accuracy of clinical diagnosis of TIA by non-stroke physicians, thereby also making better use of resources.
Problems with clinical stroke risk prediction: Many of these scores performed well in the population from which they were derived and often also in initial testing in a different cohort,27,28 but then wider use uncovered difficulties. For example, independent testing of the ABCD and ABCD2 scores22 has not been universally positive. 29,30 While in the OxVasc Study, the ABCD2 score was highly predictive of recurrent stroke within 7 days of TIA (p < 0.01), it did not predict stroke between eight and 90 days, because in this group, patients with lower scores had a higher risk of stroke than those with high ABCD2 scores (p = 0.04). 30 The ABCD2 score also did not predict recurrent stroke in patients with minor stroke (as opposed to TIA). Thus TIA clinical risk prediction scores may not usefully predict stroke risk more than 7 days after TIA and may be of limited value in patients with minor stroke. Most scores were devised and tested in highly selected populations of patients with definite TIA/minor stroke such as randomised multicentre trials or observational studies where the patient had to have a definite TIA/minor stroke to get into the study. However this is not the population of suspected TIA/minor stroke that typically presents to the ‘front door’ of the hospital. In this unselected population, up to 50% do not ultimately turn out to have had a TIA/minor stroke and therefore should have a low risk of recurrent stroke. 31 However the net effect of applying a clinical risk score in this mixed population was that many true TIA/minor strokes were given a low ABCD2 score and therefore would have missed rapid access to stroke services. 31 In this sense, the use of a clinical risk score could have actually led to a net failure to prevent recurrent stroke. This reflects a general limitation of low specificity scoring or screening systems – namely that while they may detect the small proportion of patients with particularly high risk of recurrent stroke, many recurrent strokes actually occur in patients deemed to be at moderate or low risk only (because there are many more of them). Widespread use of clinical risk scores in this setting could therefore put at risk patients in a ‘slow stream’ who would then potentially not receive treatment quickly enough to prevent stroke.
Could stroke prediction scores be improved with imaging? Part of the problem may be that a) the clinical diagnosis of TIA/minor stroke is difficult and b) the clinical findings have low specificity for the likely underlying cause, but the future stroke risk is closely related to the cause. The clinical diagnosis of TIA and minor stroke is made difficult, especially for non-experts, by the high proportion of patients (up to 50%)11,31–34 that actually have common mimics of TIA/minor stroke. Common mimics include migraine, transient global amnesia, epilepsy, simple faints, tumours, functional disorders, etc. 31,33 It is not possible to determine the cerebral pathological cause of symptoms, i.e. whether infarct, haemorrhage or mimic (e.g. tumour), without a brain scan. 35,36 The conventional brain scanning technique for stroke and TIA is CT, now widely available in the NHS. While CT excludes tumours and haemorrhage if done acutely,37 it is insensitive to small infarcts, so does not ‘positively’ diagnose TIA/minor stroke. 38 CT demonstrates an infarct in a maximum of 50% of minor acute strokes39 and 43% of mixed minor stroke and TIA,40 although the sensitivity may be lower in older patients with brain tissue loss and leukoaraiosis. Nonetheless, the presence of an infarct on CT in patients presenting with TIA is associated with increased short term risk of stroke,41 CT has high specificity for ischaemia,42 visible infarction on CT is an independent predictor of poor outcome (dependency or death) after any ischaemic stroke,39,43 CT is very accurate for haemorrhage within the first five days of symptoms38,42 and for tumours and other non-vascular causes of sudden neurological symptoms, giving it many advantages to balance its two disadvantages – the low sensitivity for acute ischaemic stroke and for small haemorrhages that are more than five days old.
Some have questioned the usefulness of scores that do not incorporate significant carotid disease or other potential cardioembolic sources. 44 However, only two scoring systems included carotid stenosis as part of the risk prediction,20,24 and found, along with other studies,45 that adding tight carotid stenosis did improve risk prediction. Carotid imaging is an integral part of the assessment of TIA and minor stroke. Its cost-effectiveness was assessed in a previous HTA-funded project by our group. 11 This work showed that rapid access to carotid imaging to identify and measure carotid stenosis was the most cost-effective way of using carotid imaging, and that the four non-invasive imaging methods functioned similarly in terms of stroke prevention, although ultrasound was the most cost-effective of the four techniques if used early after TIA/minor stroke. 46 The focus of the present application is on how to improve stroke prevention through use of brain imaging techniques, so comparative carotid imaging will not be considered further.
The alternative brain imaging test – advantages of MR:
a) Higher sensitivity for ischaemic lesions. MR imaging, if it includes DWI, is very sensitive to ischaemia. MR imaging is widely used to investigate many neurological disorders, musculoskeletal problems and in oncology. MR imaging is very versatile because different sequences can be used to highlight different types of pathology relevant to stroke. For example, DWI is very sensitive to changes in the mobility of water in the brain; one of the earliest changes in the brain at the onset of ischaemia is cell swelling which reduces the extracellular space and hence restricts water movement. Thus, early ischaemia shows up well on DWI, even very early after the symptoms start and even in very small lesions causing mild symptoms. 47 DWI primarily displays ischaemic areas as white on a dark background, so ischaemic lesions are much more obvious than they are on CT scanning, where ischaemic lesions appear as dark on a dark background. MR DWI shows even very tiny ischaemic lesions soon after symptom onset in 16% to 67% of TIAs (mean 37%)48,49 and about 70% to 90% of minor strokes overall, even weeks after the event. 42,47,49–52 Currently, with CT scanning, if the scan provides no positive evidence of an ischaemic lesion, the diagnosis of ischaemia is often assumed if the scan has excluded haemorrhage and stroke mimics. By contrast, MR including DWI could help make a ‘positive’ diagnosis of brain ischaemia in TIA/minor stroke in a larger proportion of (but not all) patients.
b) Higher sensitivity for haemorrhage in patients presenting late. MR, if it includes T2*-weighted imaging (also known as Gradient Echo) or equivalent, is very sensitive to haemorrhage, even years after the event. CT is very sensitive to acute haemorrhage but cannot reliably detect haemorrhage in patients who first present more than five days after a minor ischaemic stroke. The high sensitivity of MR T2* sequences for haemorrhage is clinically very helpful in such patients – and can avoid inappropriate use of antiplatelet agents, anticoagulants and carotid endarterectomy in patients with haemorrhagic stroke.
c) Defining the arterial territory(ies) affected. MR can also help management in other ways. In some patients with carotid stenosis it is difficult to decide if the clinical event occurred in the territory of that artery if not. 53 DWI is helpful, if it can confirm that a TIA/minor ischaemic stroke was in the territory of a tight carotid stenosis, so leading to endarterectomy. 53 The presence of lesions in several territories would indicate a need to search a more proximal source of embolism, e.g. in the heart. 54
d) Excluding acute ischaemic cerebrovascular disease as the cause. If MR is proven also to have both high specificity, it will, by helping to exclude acute ischaemic cerebrovascular disease as the cause of symptoms and if in a large enough proportion of patients, potentially have a greater role in avoiding unnecessary or inappropriate treatment.
The evidence on the contribution of MR to diagnosis, stroke prediction and hence potential for patient management and cost-effectiveness after TIA/minor stroke is conflicting. No studies have addressed the cost-effectiveness of using MR including DWI and other relevant sequences in TIA/minor stroke. Most studies of cost-effectiveness of imaging in stroke have either concentrated on hyperacute disabling stroke35,55 or on carotid imaging for carotid stenosis11 as part of carotid endarterectomy. 56 There are no clinical trials of diagnostic accuracy or effect on prediction of prognosis of MR including DWI and other relevant sequences, only smallish observational studies, from which it is unclear whether DWI adds prognostic information to simple clinical scoring systems. The 29 studies of MR DWI in patients with TIA or TIA and minor stroke published since 1999 include 2881 patients imaged between a few hours of onset of symptoms and nearly three weeks after the event. DWI was positive for ischaemic change in 37% (mean, ± 12% SD, range 16–67%). 48,49,57–59 Note: some studies are by the same research groups (so there may be some data duplication): all are single centre studies (so lack generalisability); some were retrospective; and many only included a modest proportion of their TIA population (e.g. 53%)59 (so may be prone to selection and small study bias). Many studies suggested that having a lesion on DWI (versus not having a lesion) predicted increased risk of subsequent stroke,59–62 but others found that lesions on DWI matched the same clinical features that are predictive of stroke after TIA and are already predicted by clinical scoring. 48,63 Whilst having a lesion on DWI was an independent risk factor for stroke in some studies,59–62 several others failed to confirm this independent association63 and instead found that clinical features and carotid stenosis were stronger predictors. 58,62 However, the accuracy of the widely publicised ABCD and ABCD2 clinical scores22 in predicting stroke risk has recently been questioned,30,64 so DWI could add prognostic as well as diagnostic value after all. Furthermore, for the published literature of the diagnostic and prognostic utility of MR, we cannot exclude the possibility of publication bias. Finally, the focus on DWI has overlooked the contribution of other MR sequences to identifying recent and old haemorrhage and its accuracy in detecting stroke mimics (which may be no better than CT).
The problems with MR. The available data are limited by the study methodological factors, listed above. MR is also much more expensive than CT scanning (about £400 for MR versus £150 for CT brain scanning.)11 It is still in very short supply in the UK, with long waiting lists even for patients with established indications for MR. Thus, there is very limited capacity to provide rapid access to MR for people with TIA and stroke (MR delayed even by a few days is of little value since the patient will miss the window of opportunity to have preventative treatment during the early days of highest risk). 65 Although 75% of UK hospitals had MR on site, less than 10% were able to scan patients early after stroke. 65 The UK is not alone in this as across the EU, only 5% of hospitals met criteria for stroke care which included availability of MR for stroke (and surprisingly the UK had the highest proportion of hospitals with MR for stroke). 66 Any increase in use of MR for stroke prevention could result in reduced availability for – for example – patients with cancer, where MR is of established benefit. Some patients cannot have MR because of contraindications, e.g. having a pacemaker, claustrophobia or metal implant, so there would always be a need for CT. In one recent study, only 90/904 patients [12%] considered for MR had contraindications and only 477/904 (53%) of all patients presenting with TIA actually underwent MR; of those having MR only 155/477 (33%, or 17% of the initial cohort) had a DWI-positive lesion. The accuracy of MR for common mimics of TIA/minor stroke is unclear as most publications excluded patients who turned out after further testing not to have had a stroke. Not all TIA patients have CT at present, so MR including DWI would partly replace, and partly be an additional investigation, if it proved to be cost-effective in improving the management of TIA.
Are guideline statements supported by robust evidence? The limited evidence has led some reviewers to call for more data on imaging to guide physicians treating TIA patients. 67,68 The NHS Purchasing and Supply Agency 2008 report stated only that “DWI shows significant potential in the study of TIA/minor stroke”, but also called for “more evidence”. 69 Despite this – rather surprisingly – the 2008 UK Stroke Strategy guidelines and the National Institute for Health and Clinical Excellence (NICE) guidelines advocate use of DWI in either 50% of70 or most71 TIA/minor stroke patients (www.dh.gov.uk/stroke). The American Heart Association and related organisations stated that “TIA patients should undergo neuroimaging evaluation within 24 hours of symptom onset, preferably with magnetic resonance imaging including diffusion sequences”. 72 The revised National Clinical Guidelines for Stroke,73 SIGN Guidelines 2008,74 and European Guidelines75 are more cautious but still advocate immediate brain imaging and use of MR including DWI in large proportions of patients especially those with mild stroke. The guidance tends to overlook the need for other sequences to identify haemorrhage and stroke mimics, hence there is a need for further work to define the accuracy of MR for all aspects of stroke diagnosis and the incremental difference, if any, from the accuracy of CT. Therefore, there is a ‘mismatch’ between national and international guidance on the one hand,70,72 and convincing evidence to support this approach,48,49 information to guide precise usage,69 details of cost-effectiveness and available technology to deliver it on the other,65,66 resulting in confusion about what to do in routine practice.
Summary: Potential benefits. MR including DWI and other relevant sequences, could make a substantial impact as a positive diagnostic test for TIA/minor stroke by: improving diagnosis of the cause of stroke; efficient patient selection for medical secondary prevention in patients with a proven diagnosis; conversely, the avoidance of unnecessary treatment in patients in whom acute ischaemic cerebrovascular disease was reliably excluded; and best use of carotid endarterectomy (especially where stroke expertise is limited). Potential cost implications. TIA/minor stroke is so common that MR including DWI and other relevant sequences would be in daily use in every hospital if such a strategy were adopted, but the direct costs to the NHS would be substantial – £16 million per year to scan just the 50% of TIA patients suggested in recent UK guidelines, not including the minor strokes and all the TIA mimics, assuming that MR were available. Opportunity costs. The opportunity cost to meet the demand by increasing MR scanning capacity, (without which there would also be substantial disadvantage to other MR users). Overall cost-effectiveness unclear. It is not clear if the potential diagnostic and prognostic advantages of MR outweigh the disadvantages of the obvious expense, limited availability, failure to make a positive diagnosis of ischaemic lesion in up to 66% of TIA patients, unquantified accuracy for other stroke-related diagnoses, and possibility that the strokes that we are trying to prevent might occur during the wait for a scan if availability cannot be rapidly increased. Any strategy to increase MR usage would have to factor in the effect of varying delays introduced because of waiting for MR. We are not aware of any large or multicentre studies ongoing on this topic, although it is likely that single centre studies are ongoing. The proposed study aims to resolve this controversy by summarising all available data on MR including DWI and other relevant sequences, diagnosis and stroke prediction, and model the cost-effectiveness of increasing MR including DWI and other relevant sequences usage to existing stroke prevention strategies.
6. Plan of investigation
Design: The study is an evidence synthesis of literature data, surveys of practice and costs, and health economic modelling with sensitivity analyses of important variables (see Diagram).
1) Systematic review: We will systematically review the literature to: estimate the sensitivity/specificity of CT and MR including DWI and other relevant sequences in TIA/minor stroke, including the arterial territory; to assess their role in prediction of stroke after TIA; to estimate costs of CT and MR (summarised to 2003 in35 but requiring updating); and to gather all data required to model stroke prevention after TIA. All aspects of the systematic review, including literature searching, quality assessment, data extraction, and evidence synthesis will be performed according to the Cochrane Collaboration and Stroke Group standards, including recommendations from the Screening and Diagnostic Tests Methods Group, as in previous work. 11,42 The methods for evidence synthesis (meta-analyses performed according to the summary receiver operator characteristic (SROC) curve methodology or separate meta-analyses of sensitivity and specificity estimates) will be contingent on the data obtained and the most appropriate method will be used. 76 We will use a standardised quality assessment instrument (i.e. the Quality Assessment of Diagnostic Studies tool – QUADAS),77 with modifications as appropriate as in previous work. 35,78
2) We will obtain data on demographics, risk factors, medications, recurrent stroke and death in patients with TIA/minor stroke relevant to the UK in order to construct the health economic model. We will use existing datasets where possible, e.g. large stroke and TIA registries,79 individual patient datasets, and routinely collected anonymised national audit data, as in previous work. 11,35
3) We will systematically review the literature for data on costs of CT and MR in the UK and other countries with relevant healthcare systems (data available to us from previous HTA-funded projects will help this process but requires updating). We will obtain up to date imaging costs from the NHS Department of Health NHS Reference costs database and NHS Scotland Information and Statistics Division (ISD) Costs Book. We will also obtain specific up to date costs of diagnostic imaging for stroke with CT and MR from individual radiology departments in a range of hospitals in the Scottish Imaging Network – A Platform for Scientific Excellence (SINAPSE) Collaboration, which includes six University centres including four regional specialist neuroscience centres and networks with district general hospitals in different large and small cities. Together, these costs will provide a realistic range of current costs for the health economics model and information on which to base sensitivity analyses of the effects of higher or lower costs on the outcome of the health economics modelling as in previous work. 11,35
4) We will survey UK stroke physicians through the British Association of Stroke Physicians as in previous work11 to ask about stroke prevention clinics including current access to and use of MR sequences, including DWI and other relevant sequences in patients with TIA/minor stroke in the NHS, aspirations and consensus on role of MR, and to gather additional unpublished data on MR including DWI and other relevant sequences in TIA/minor stroke.
5) We will survey UK radiology departments through the SINAPSE Collaboration in Scotland, and the College of Radiologists and British Society of Neuroradiologists elsewhere in the UK to determine what capacity there is for increased throughput of TIA and minor stroke patients, and to identify the perceived major barriers to increased or faster throughput of TIA/minor stroke patients and what additional resources would be required to provide additional access for these patients.
6) We will obtain UK data on the health care costs associated with management of stroke patients by literature review of relevant electronic databases such as MEDLINE, EMBASE and SCOPUS,80 and interrogation of specific sources such as stroke registry data as in previous work (see below for specific details). 11,35
7) We will obtain data on quality of life after stroke for key subgroups from the literature and from our previous work and from our local stroke register data. 81
8) We will build a model that reflects key stages and outcomes in secondary stroke prevention after TIA/minor stroke, including all data on assessments, medical and surgical interventions, outcomes, and timings, populated with representative data from TIA/stroke services in the UK. The timelines in the model will be stratified by time after symptoms, key patient characteristics, use of prognostic scores (ABCD2)22 including conventional investigations (CT, carotid imaging) and treatment decisions, and key outcomes of non-disabling and disabling stroke and death at six months, one and five years.
9) We will model the incremental cost-effectiveness of implementing MR including DWI and other relevant sequences instead of usual care in the diagnosis of TIA/minor stroke. The clinical outcomes of non-disabling stroke, disabling stroke and death will be summarised through the calculation of Quality Adjusted Life Years (QALYs). QALYs will be calculated by the generation of quality of life weights for disabling and non-disabling stroke, and the assignment of probabilities to these outcomes. Literature review and statistical modelling of registry data will inform the calculation of probabilities of clinical outcomes with and without MR including DWI and other relevant sequences. Quality of life weights will be based on values from published literature and, where feasible, registry data. The definition of usual care will be developed following literature review of current UK studies and interrogation of stroke registry data; however, following previous work it is anticipated that it will be based on CT scanning varying proportions of cohorts of 1000 patients presenting to hospital with suspected TIA/minor stroke. Modelling will account for patient heterogeneity; e.g. in spite of increased efforts to raise awareness of the importance of acting quickly on noticing stroke symptoms, a proportion of patients with TIA/minor stroke may continue to present late after symptoms. In these patients, CT scanning will not identify the small proportion (5%) who have a small haemorrhage as the cause of their stroke and MR scanning is required. 37 We have already analysed the sensitivity and specificity of CT and MR for haemorrhage in patients presenting beyond five days after symptoms35 but did not perform a cost-effectiveness analysis of increased use of MR in this predominantly out patient population. Therefore it is anticipated that cost-effectiveness will be measured separately for both ‘early’ and ‘delayed’ presentation of symptoms, with ‘delayed’ defined as presenting over five days after stroke. To test the robustness of the point estimates generated and to quantify the degree of decision uncertainty, probabilistic sensitivity analysis (PSA) will be conducted. This will involve taking repeated random draws from specific distributions of all stochastic parameters (clinical outcome probabilities, relative risk reduction of associated events with MR DWI and other relevant sequences, usual care quality of life score, disabling and non-disabling stroke quality of life score, usual care health care costs, and imaging costs). The output from the PSA will then be used to calculate the probability that the new diagnostic strategy is more cost-effective than usual care. Value of information analysis will be conducted to investigate the worth of commissioning further research on the cost-effectiveness of MR DWI imaging and inform the design of any future research. Population Expected Value of Perfect Information (EVPI) will be computed to assess whether the benefits of future research in the form of reduced uncertainty exceed the likely costs of research, and to assess which parameter, or set of parameters, should be the focus for any future research; analysis of covariance techniques will be used to explore the proportionate contribution of each parameter to variation in incremental cost and QALYs. Further, the EVPI for parameters will be calculated, in which repeated model simulations will be run with each parameter held constant in turn, whilst allowing other stochastic parameters to vary, so as to provide clinically-relevant data on key age groups, stroke risk strata and MR availability. All modelling work will be performed according to recommended guidance (http://www.equator-network.org/). 82
10) Finally, using information on investigation costs, we will estimate the effect of using MR or CT also to diagnose carotid stenosis as a ‘one-stop’ investigation, instead of performing the MR or CT brain scan and separately performing carotid imaging with, for example, ultrasound. This will reflect the reality that different imaging modalities are used in different centres for reasons of equipment availability. We have already determined the accuracy and cost-effectiveness of different carotid imaging tests in the diagnosis and management of carotid stenosis in stroke prevention. 11,46 However, we have not assessed the cost and practicality of performing ‘one-stop’ brain and carotid imaging. Obtaining all the information required from imaging in one scan is attractive, but factors such as increased machine occupancy, radiation dose (CT), patient unacceptability (MR), and contraindications to contrast agents required for the carotid imaging (both CT and MR) will limit the number of patients who can actually have ‘one-stop’ brain and carotid imaging. We will use data from our previous HTA report11 combined with updated information on accuracy of carotid imaging,83 data on practicality from Glasgow Neuroradiology where CT with CTA (rather than CT plus ultrasound) is the routine investigation for suspected carotid stenosis and the cost information that we will obtain in the present work, to examine the effect of replacing traditional separate brain and carotid imaging tests with ‘one-stop’ CT or MR imaging. In this analysis, the base case scenario will be CT brain scan and carotid ultrasound.
Planned inclusion/exclusion criteria: We will model a typical population of patients presenting with suspected TIA/minor stroke based on existing data as in previous work, updated to reflect current demographic, pre-stroke medications, and stroke treatments using information from the literature and a large national stroke audit. These include: the North Edinburgh Stroke Study and Lothian Stroke Register (n = 2598, a hospital-based registry of all in and out patients with TIA and stroke presenting to our hospital between 1990 and 2000 with demographic information, imaging and laboratory investigations, past medical history, medications prior to and following the TIA/stroke, and quality of life data) and followed up to four years for recurrent stroke, cardiac events and death and used in two previous HTA reports;11,35 the Edinburgh Stroke Study (n = 1367, a hospital-based registry of all in and out patients with TIA and stroke presenting to our hospital between 2002 and 2005 with the same information except quality of life) and so far followed up to four years after the stroke;79 and the Incidence of Stroke in the Lanarkshire Area (ISLA) study (K Muir). We will obtain longer term follow-up for stroke, other major vascular events and death for both cohorts at the start of the project through data linkage with the ISD (http://www.isdscotland.org). We also have data from other studies of stroke and TIA with more detailed imaging, including a study comparing CT and MR scans in minor stroke between 1998 and 2001 (n = 230),37 a study of patients with mild cortical and lacunar stroke between 2005 and 2007 (n = 250),84 and a study of practicalities and workload implications of increased use of MR imaging in patients with TIA in Glasgow (K Muir ongoing). Other population-based studies of TIA/minor stroke which may provide additional details are available to us and additional new data may become available from other stroke prognosis studies. The Scottish Stroke Care Audit (SSCA) routinely collects data from hospitals across Scotland that provide stroke services (PI M Dennis). Data from SSCA will be routinely linked through ISD to provide anonymised data on the process of stroke care across the Scottish population in late 2009 (project currently under way jointly run by SSCA and ISD, with a research governance committee to guide access and usage). We will apply to use anonymised data from the linked audit to provide up to date population-based data that reflect current stroke care. We will stratify data in the model by age, underlying vascular risk factors, sex, prior use of medical treatments, and other factors which may affect stroke risk and therefore require data with this degree of granularity for the model.
Ethical arrangements: The use of existing anonymised data to populate the model and perform other analyses does not require additional ethics approvals. We will apply to the ISD Privacy Advisory Committee for permission to link the LSR and ESS data (this has been done previously and no current problems are anticipated with repeating this request). We will also apply to the new committee which is being formed to review requests for use of ISD-linked data from SSCAS (see above). All data used in the proposed modelling will be anonymised.
Proposed sample size: We will model the effect of incremental change in proportions of TIA/minor stroke patients undergoing MR imaging for a typical UK population of 1000 patients as performed previously in HTA-funded projects assessing the cost-effectiveness of CT scanning in acute stroke35 and of carotid imaging in stroke prevention. 11 We have several existing datasets including several 1000 patients with TIA, minor and major stroke, with initial clinical and imaging assessment, and data on functional status, stroke recurrence and death at six months, one year and four years, which we propose to data link with centralised health care statistics as details above. We will provide the results in the form of “events per a cohort of 1000 patients presenting to hospital with suspected TIA and minor stroke”.
Statistical analyses:
Systematic review of diagnostic accuracy: Indices of diagnostic performance will be extracted or derived from data presented in each primary study for each imaging test. Where data are available, we will construct 2 × 2 contingency tables of test results versus reference standard to show the cross-classification of disease status and test outcome. We will calculate sensitivity and specificity with 95% confidence intervals for each imaging test for each study. To describe and visualise the data, we will draw forest plots showing the pairs of sensitivity and specificity estimates for each study. For a descriptive analysis we will also plot the imaging test results on a receiver operating characteristic (ROC) plot of true positive rate (sensitivity) against false positive rate (1 – specificity). The choice of statistical methods to combine studies will depend upon the pattern of heterogeneity observed between study results. Where appropriate, we will pool data from included studies using SROC curve methods,84 which are based on a random effects approach and take into account the degree of heterogeneity between studies. Where possible, co-variates will be incorporated in the SROC model to examine the effect of potential sources of heterogeneity on sensitivity and specificity estimates. If the SROC model proves inappropriate we will summarise results using univariate meta-analyses of true and false positive rates. 76
Economic modelling: Data from included studies will be appraised, summarised, and interpreted alongside the results of the systematic review of diagnostic accuracy so that conclusions will be drawn on the effect of MR including DWI and other relevant sequences compared with the current alternative of CT brain scanning, as detailed in 9 above.
Proposed outcome measures: The primary outcome will be the incremental cost-effectiveness of MR scanning compared with the reference standard. Proposed secondary outcome measures include: the estimates of sensitivity/specificity of ‘MR including DWI and other relevant sequences’ versus ‘conventional clinical assessment plus CT’ to diagnose TIA/minor stroke; the proportion of patients with TIA/minor stroke with a visible ischaemic lesion on ‘MR including DWI and other relevant sequences’ and CT brain scanning and the association with key clinical variables; whether adding information from brain scanning to clinical risk scoring improves prediction of future risk of stroke or death; the association between a positive or negative brain scan and risk factors for stroke such as carotid stenosis; delays to diagnosis, delays to starting medical or surgical treatment if MR including DWI and other relevant sequences were to replace CT brain scanning; the number of disabling strokes prevented at six months, one and five years after TIA/minor stroke if ‘MR including DWI and other relevant sequences’ were to be used in most patients for a cohort of 1000 patients; quality adjusted life years stratified by key patient groups (e.g. elderly); the cost-effectiveness of substituting MR including DWI and other relevant sequences for CT brain scanning in patients with TIA/minor stroke in important demographic subgroups and by different times to presentation; and an estimate of the increase in availability of MR for TIA/minor stroke that would be required (if any) to accommodate TIA/minor stroke patients rapidly after the index event. We will provide a range of options reflecting effect of using MR in different proportions of TIA/minor stroke patients to guide decision-making by health service purchasers.
7. Project timetables and milestones
We anticipate that the project will take 18 months to be completed.
Month 1–2: Appoint staff, develop a protocol, develop literature searches, design data extraction forms, discuss structure for model;
Month 3–8: Diagnostic review, economic review and model development, design survey questionnaire, survey stroke prevention clinics;
Month 9–12: Test model, writing up diagnostic review;
Month 12–14: Modelling (run model and sensitivity analyses);
Month 14–18: Report writing and papers preparation.
8. References
- Feigin VL, Lawes CMM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2:43-5.
- Swain S, Turner C, Tyrrell P, Rudd A. Guideline Development Group . Diagnosis and initial management of acute stroke and transient ischaemic attack: summary of NICE guidance. BMJ 2008;337:291-3.
- Dennis MS, Bamford JM, Sandercock PA, Warlow CP. Incidence of transient ischemic attacks in Oxfordshire, England. Stroke 1989;20:333-9.
- Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA 2000;284:2901-6.
- Sandercock PAG. Should I start all my ischaemic stroke and TIA patients on a statin, an ACE inhibitor, a diuretic, and aspirin today?. J Neurol Neurosurg Psychiatry 2003;74:1461-4.
- Hankey GJ. Redefining risks after TIA and minor ischaemic stroke. Lancet 2005;365:2065-6.
- Easton JD, Albers GW, Caplan LR, Saver JL, Sherman DG. Discussion: reconsideration of TIA terminology and definitions. Neurology 2004;62:S29-S34.
- Warach S, Kidwell CS. The redefinition of TIA: the uses and limitations of DWI in acute ischemic cerebrovascular syndromes. Neurology 2004;62:359-60.
- Coull AJ, Lovett JK, Rothwell PM. Oxford Vascular Study . Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ 2004;328.
- Evers SMAA, Struijs JN, Ament AJHA, van Genugten MLL, Jager C, van der Bos GAM. International comparison of stroke cost studies. Stroke 2004;35:1209-15.
- Wardlaw JM, Chappell F, Stevenson M, DeNigris E, Thomas S, Gillard J, et al. Accurate, practical and cost-effective assessment of carotid stenosis in the UK. HTA 2006;10:1-200.
- London: TSO (The Stationery Office); 2007.
- The Lancet . Emergency and comprehensive care for stroke needed. Lancet 2009;373.
- Lovett JK, Dennis MS, Sandercock PAG, Bamford J, Warlow CP, Rothwell PM. Very early risk of stroke after a first transient ischemic attack. Stroke 2003;34:1-3.
- Coull AJ, Rothwell PM. Underestimation of the early risk of recurrent stroke. Evidence of the need for a standard definition. Stroke 2004;35:1925-9.
- Kleindorfer D, Panagos P, Pancioli A, Khoury J, Kissela B, Woo D, et al. Incidence and short-term prognosis of transient ischemic attack in a population-based study. Stroke 2005;36:720-3.
- Rothwell PM, Giles MF, Chandratheva A, Marquardt L, Geraghty O, Redgrave JNE, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet 2007;370:1432-42.
- Rothwell PM, Eliasziw M, Gutnikov SA, Fox AJ, Taylor DW, Mayberg MR, et al. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet 2003;361:107-16.
- Hankey GJ, Slattery JM, Warlow CP. Can the long term outcome of individual patients with transient ischaemic attacks be predicted accurately?. J Neurol Neurosurg Psychiatry 1993;56:752-9.
- Rothwell PM, Warlow CP. Prediction of benefit from carotid endarterectomy in individual patients: a risk-modelling study. European Carotid Surgery Trialists’ Collaborative Group. Lancet 1999;353:2105-10.
- Rothwell PM, Giles MF, Flossmann E, Lovelock CE, Redgrave JN, Warlow CP, et al. A simple score (ABCD) to identify individuals at high early risk of stroke after transient ischaemic attack. Lancet 2005;366:29-36.
- Johnston SC, Rothwell PM, Nguyen-Huynh MN, Giles MF, Elkins JS, Bernstein AL, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 2007;369:283-92.
- Weimar C, Diener H-C, Alberts MJ, Steg PG, Bhatt DL, Wilson PWF, et al. The Essen Stroke Risk Score predicts recurrent cardiovascular events: a validation within the REduction of Atherothrombosis for Continued Health (REACH) Registry. Stroke 2009;40:350-4.
- Kernan WN, Viscoli CM, Brass LM, Makuch RW, Sarrel PM, Roberts RS, et al. The Stroke Prognosis Instrument II (SPI-II): A clinical prediction instrument for patients with transient ischemia and nondisabling ischaemic stroke. Stroke 2000;31:456-62.
- van Wijk I, Kappelle LJ, van Gijn J, Koudstaal PJ, Franke CL, Vermeulen M, et al. Long-term survival and vascular event risk after transient ischaemic attack or minor ischaemic stroke: a cohort study. Lancet 2005;365:2098-104.
- Fairhead JF, Mehta Z, Rothwell PM. Population based study of delays in carotid imaging and surgery and the risk of recurrent stroke. Neurology 2005;65:371-5.
- Tsivgoulis G, Spengos K, Manta P, Karandreas N, Zambelis T, Zakopoulos N, et al. Validation of the ABCD score in identifying individuals at high early risk of stroke after a transient ischaemic attack. Stroke 2006.
- Sciolla R, Melis F. Rapid identification of high-risk transient ischemic attacks: prospective validation of the ABCD score. Stroke 2008;39:297-302.
- Cucchiara BL, Messe SR, Taylor RA, Pacelli J, Maus D, Shah Q, et al. Is the ABCD score useful for risk stratification of patients with acute transient ischemic attack?. Stroke 2006;37:1710-4.
- Chandratheva A, Marquardt L, Geraghty OC, Rothwell NJ. Limits of risk scoring systems for the early risk of stroke after TIA. Int J Stroke 2009;3.
- Quinn TJ, Cameron AC, Dawson J, Lees KR, Walters MR. ABCD2 scores and prediction of noncerebrovascular diagnoses in an outpatient population. A case–control study. Stroke 2009;40:749-53.
- Goldstein LB, Simel DL. Is this patient having a stroke?. JAMA 2005;293:2391-402.
- Hand PJ, Kwan J, Lindley RI, Dennis MS, Wardlaw JM. Distinguishing between stroke and mimic at the bedside: the brain attack study. Stroke 2006;37:769-75.
- Warlow C, van Gijn J, Dennis M, Wardlaw J, Bamford J, Hankey G, et al. Stroke: practical management. Malden, MA: Blackwell Publishing; 2008.
- Wardlaw JM, Keir SL, Seymour J, Lewis S, Sandercock PA, Dennis MS, et al. What is the best imaging strategy for acute stroke?. Health Technol Assess 2004;8:1-180.
- Saver JL, Kidwell C. Neuroimaging in TIAs. Neurology 2004;62:S22-S25.
- Wardlaw JM, Keir SL, Dennis MS. The impact of delays in CT brain imaging on the accuracy of diagnosis and subsequent management in patients with minor stroke. J Neurol Neurosurg Psychiatry 2003;74:77-81.
- Chalela JA, Kidwell CS, Nentwich LM, Luby M, Butman JA, Demchuk AM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet 2007;369:293-8.
- Wardlaw JM, West TM, Sandercock PA, Lewis SC, Mielke O. Visible infarction on computed tomography is an independent predictor of poor functional outcome after stroke, and not of haemorrhagic transformation. J Neurol Neurosurg Psychiatry 2003;74:452-8.
- Widjaja E, Manuel D, Hodgson TJ, Connolly DJ, Coley SC, Romanowski CA, et al. Imaging findings and referral outcomes of rapid assessment stroke clinics. Clin Radiol. 2005;60:1076-82.
- Douglas VC, Johnston CM, Elkins J, Sidney S, Gress DR, Johnston SC. Head computed tomography findings predict short-term stroke risk after transient ischemic attack. Stroke 2003;34:2894-8.
- Brazzelli M, Sandercock PAG, Chappell FM, Celani MG, Righetti E, Arestis N, et al. Magnetic resonance imaging versus computed tomography for detection of acute vascular lesions in patients presenting with stroke symptoms. Cochrane Database of Systematic Reviews 2008.
- Wardlaw JM, Lewis SC, Dennis MS, Counsell C, McDowall M. Is visible infarction on computed tomography associated with an adverse prognosis in acute ischemic stroke?. Stroke 1998;29:1315-9.
- Hill MD, Weir NU. Is the ABCD score truly useful?. Stroke 2006;37.
- Ois A, Gomis M, Rodriguez-Campello A, Cuadrado-Godia E, Jimenez-Conde J, Pont- Sunyer C, et al. Factors associated with a high risk of recurrence in patients with transient ischemic attack or minor stroke. Stroke 2008;39:1717-21.
- Wardlaw JM, Stevenson MD, Chappell F, Rothwell P, Gillard J, Young G, et al. Carotid artery imaging for secondary stroke prevention: both imaging modality and rapid access to imaging are important. Stroke 2009.
- Gass A, Ay H, Szabo K, Koroshetz WJ. Diffusion-weighted MRI for the “small stuff”: the details of acute cerebral ischaemia. Lancet Neurol. 2004;3:39-45.
- Redgrave JNE, Coutts SB, Schulz UG, Briley D, Rothwell PM. Systematic review of associations between the presence of acute ischemic lesions on diffusion-weighted imaging and clinical predictors of early stroke risk after transient ischemic attack. Stroke 2007;38:1482-8.
- Engelter ST, Wetzel SG, Bonati LH, Fluri F, Lyrer PA. The clinical significance of diffusion-weighted MR imaging in stroke and TIA patients. Swiss Med Wkly 2008;138:729-40.
- Keir S, Wardlaw JM, Bastin ME, Dennis MS. In which patients is diffusion-weighted magnetic resonance imaging most useful in routine stroke care?. J Neuroimaging 2004;14:118-22.
- Keir SL, Wardlaw JM. Systematic review of diffusion and perfusion imaging in acute ischaemic stroke. Stroke 2000;31:2723-31.
- Schulz UGR, Briley D, Meagher T, Molyneux A, Rothwell PM. Abnormalities on diffusion weighted magnetic resonance imaging performed several weeks after a minor stroke or transient ischaemic attack. J Neurol Neurosurg Psychiatry 2003;74:734-8.
- Flossmann E, Redgrave JN, Briley D, Rothwell PM. Reliability of clinical diagnosis of the symptomatic vascular territory in patients with recent transient ischemic attack or minor stroke. Stroke 2008;39:2457-60.
- Ay H, Oliveira-Filho J, Buonanno FS, Ezzeddine M, Schaefer PW, Rordorf G, et al. Diffusion-weighted imaging identifies a subset of lacunar infarction associated with embolic source. Stroke 1999;30:2644-50.
- Wardlaw JM, Seymour J, Cairns J, Keir S, Lewis S, Sandercock P. Immediate computed tomography scanning of acute stroke is cost-effective and improves quality of life. Stroke 2004;35:2477-83.
- Benade MM, Warlow CP. Costs and benefits of carotid endarterectomy and associated preoperative arterial imaging: a systematic review of health economic literature. Stroke 2002;33:629-38.
- Boulanger JM, Coutts SB, Eliasziw M, Subramaniam S, Scott J, Demchuk AM. Diffusion-weighted imaging-negative patients with transient ischemic attack are at risk of recurrent transient events. Stroke 2007;38:2367-9.
- Calvet D, Touze E, Oppenheim C, Turc G, Meder JF, Mas JL. DWI lesions and TIA etiology improve the prediction of stroke after TIA. Stroke 2009;40:187-92.
- Ay H, Arsava EM, Johnston SC, Vangel M, Schwamm LH, Furie KL, et al. Clinical- and Imaging-based Prediction of stroke risk after transient ischemic attack. The CIP model. Stroke 2009;40:181-6.
- Calvet D, Lamy C, Oppenheim C, Domigo V, Touze E, Essabiri M, et al. Early risk of stroke after transient ischemic attack in stroke unit care. Cerebrovasc Dis. 2005;19.
- Coutts SB, Simon JE, Eliasziw M, Sohn CH, Hill MD, Barber PA, et al. Triaging transient ischaemic attack and minor stroke patients using acute magnetic resonance imaging. Ann Neurol. 2005;57:848-54.
- Purroy F, Montaner J, Rovira A, Delgado P, Quintana M, Alvarez-Sabin J. Higher risk of further vascular events among transient ischemic attack patients with diffusion weighted imaging acute ischemic lesions. Stroke 2004;35:2313-19.
- Redgrave JN, Schulz UG, Briley D, Meagher T, Rothwell PM. Presence of acute ischaemic lesions on diffusion-weighted imaging is associated with clinical predictors of early risk of stroke after transient ischaemic attack. Cerebrovasc Dis. 2007;24:86-90.
- Purroy F, Molina CA, Montaner J, Varez-Sabin J. Absence of usefulness of ABCD score in the early risk of stroke of transient ischemic attack patients. Stroke 2007;38:855-6.
- Whiteley W, Kane IJ, Sandercock PAG, Wardlaw JM. Availability of CT and MR for assessing patients with acute stroke. Cerebrovasc Dis. 2008;25:375-7.
- Leys D, Ringelstein EB, Kaste M, Hacke W. Facilities available in European hospitals treating stroke patients. Stroke 2007;38:2985-91.
- Cucchiara B, Ross M. Transient ischemic attack: risk stratification and treatment. Ann Emerg Med. 2008;52:S27-S39.
- White P, Wardlaw J. Brain attack imaging: making sense of multiple guidelines. Part one of two: transient ischaemic attack and minor stroke; diagnosis and secondary prevention. Stroke Matters 2008;1:6-7.
- Delakis I, Price D, Renaud C, Dickinson R, Kitney R. London: Crown; 2008.
- London: Crown; 2008.
- Ackerman RH, Candia MR, May Z. Technical advances and clinical progress in carotid diagnosis. AJNR Am J Neuroradiol. 1999;20:187-9.
- Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke 2009;40:2276-93.
- London: Royal College of Physicians; 2008.
- Edinburgh: Scottish Intercollegiate Guidelines Network; 2008.
- Ringleb PA, Bousser M-G, Ford G, Bath P, Brainin M, . The European Stroke Organisation (ESO) Executive Committee and the ESO Writing Committee . Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008;25:457-50.
- Chappell FC, Raab GM, Wardlaw JM. When are summary ROC curves appropriate for diagnostic meta-analyses?. Stat Med. 2009;DOI. URL: 10.1002/sim.3631.
- Whiting P, Rutjes AWS, Reitsma JB, Bossuyt PMM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3.
- Wardlaw JM, Chappell FM, Best JJK, Wartolowska K, Berry E. NHS Research & Development Health Technology Assessment Carotid Stenosis Imaging Group . Non-invasive imaging compared with intra-arterial angiography in the diagnosis of symptomatic carotid stenosis: a meta-analysis. Lancet 2006;367:1503-12.
- Jackson C, Dennis M, Wardlaw J, Lewis S, Hutchinson A, Sudlow C. Recurrent stroke rates and subtype patterns among lacunar versus non-lacunar ischaemic stroke patients in a hospital-based cohort – evidence for distinct vascular pathologies?. Cerebrovasc Dis. 2008;25.
- Luengo-Fernandez R, Gray AM, Rothwell PM. Costs of stroke using patient-level data: a critical review of the literature. Stroke 2009;40:e18-e23.
- Dorman P, Dennis M, Sandercock P. United Kingdom Collaborators in the International Stroke Trial (IST) . Are the modified “simple questions” a valid and reliable measure of health related quality of life after stroke?. J Neurol Neurosurg Psychiatry 2000;69:487-93.
- Briggs A, Claxton K, Sculpher K. Oxford: Oxford University Press; 2006.
- Chappell FM, Wardlaw JM, Young GR, Gillard JH, Roditi GH, Yip B, et al. Accuracy of noninvasive tests for carotid stenosis – an individual patient data meta-analysis. Radiology 2009.
- Wardlaw JM, Doubal F, Armitage P, Chappell F, Carpenter T, Maniega SM, et al. Lacunar stroke is associated with diffuse blood–brain barrier dysfunction. Ann Neurol. 2009;65:194-202.
Appendix 6 Project management plan
HTA 09/22/169
Project timetables and milestones
Start date: 1 October 2010
We anticipate that the project will take 18 months to be completed (see chart below):
-
Months 1–2: Appoint staff, develop a protocol, develop literature searches, design data extraction forms, discuss structure for model.
-
Months 3–8: Diagnostic review, economic review and model development, design survey questionnaire, survey stroke prevention clinics.
-
Months 9–12: Test model, writing up diagnostic review.
-
Months 12–14: Modelling (run model and sensitivity analyses).
-
Months 14–18: Report writing and papers preparation.
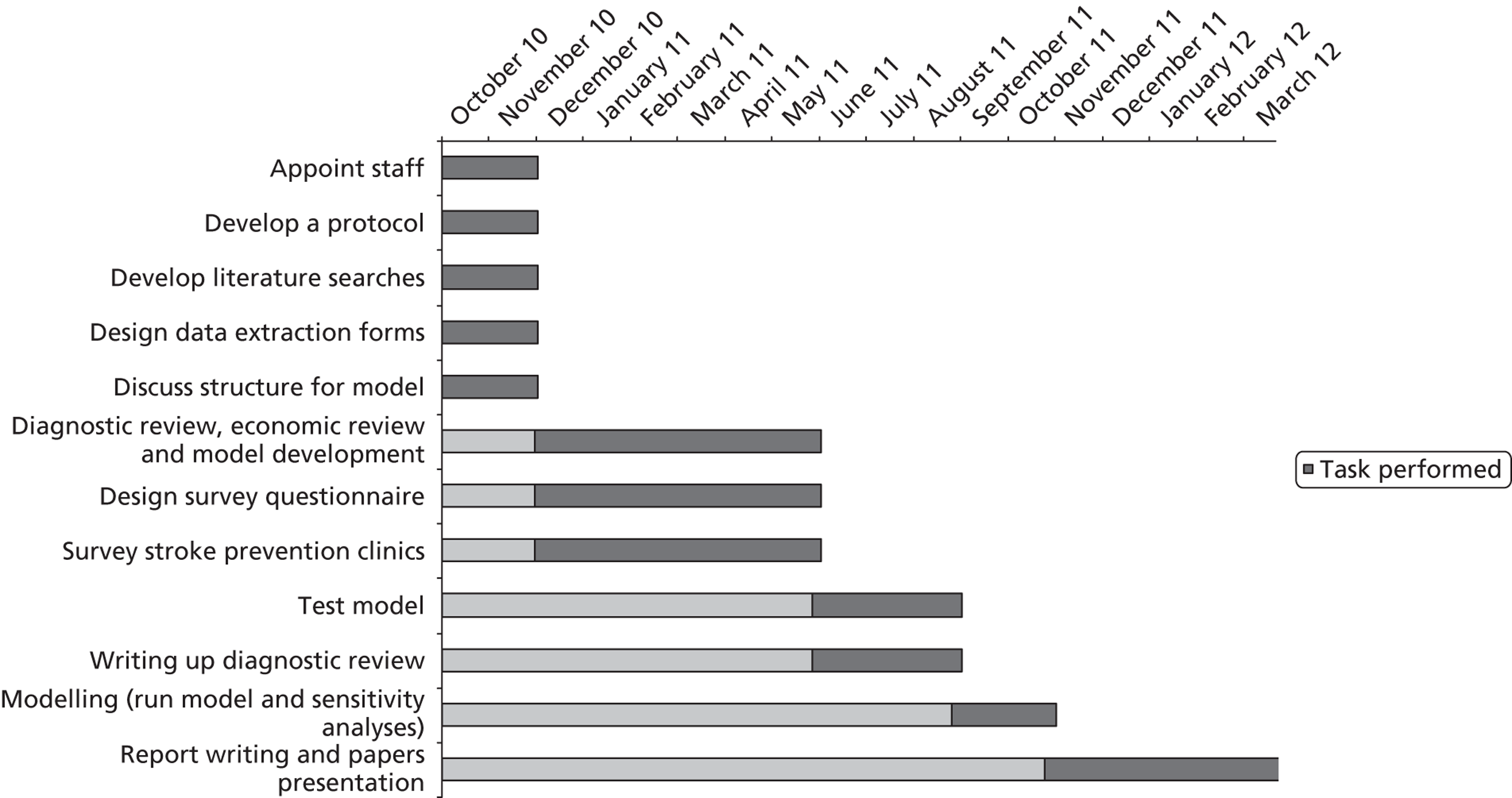
Project resources
Steering committee
Professor Joanna Wardlaw – Edinburgh, Neuroradiologist, PI
Dr Paul McNamee – Aberdeen, Health Economist
Professor Martin Dennis – Edinburgh, Stroke Physician
Professor Peter Sandercock – Edinburgh, Neurologist and Cochrane Stroke Group Lead
Ms Miriam Brazzelli – Edinburgh, Research fellow
Dr Janet de Wilde – SINAPSE co-ordinator
Professor Keith Muir – Glasgow, Neurologist
Professor Donald Hadley – Glasgow, Neuroradiologist
Dr Peter Tothill (Public representative)
Dr G Scotland – Aberdeen, Health Economics research fellow
Dr Francesca Chappell – Edinburgh, Statistician
The full Project Steering Committee will meet three monthly, starting in October 2010 until the end of the project. A core group made up of J Wardlaw, M Brazzelli, P McNamee, and G Scotland will meet at least monthly partly in person and partly by teleconference to reduce time wasted in travelling. Web teleconferences will be used where possible to reduce in person meeting frequency.
Expertise and resources available
Professor Joanna Wardlaw will lead and co-ordinate all aspects of the project. She is Professor of Applied Neuroimaging and an Honorary Consultant Neuroradiologist at the University of Edinburgh and NHS Lothian. She is an internationally recognised expert on imaging in the diagnosis and prevention of stroke, in the use of imaging to study pathophysiology of large artery and lacunar ischaemic stroke, systematic reviews of diagnostic test accuracy in stroke, and has conducted several cost-effectiveness analyses of imaging in stroke. She has performed two HTA-funded studies of effectiveness and cost-effectiveness of imaging in stroke (CT in acute stroke; carotid imaging for secondary prevention) which have changed UK, European, North American and Australasian stroke guidelines. She is a member of the Cochrane Diagnostic Tests Methods Group, helped to evaluate methods for meta-analysis of diagnostic tests,76 and helped to pilot two Cochrane Diagnostic Tests Reviews. She was a European Editor of the AHA journal STROKE, from 2005 to 2010. She is an executive member of the Scottish Stroke Research Network (SSRN) and was founding director of a large imaging collaboration between six Scottish universities, the Scottish Imaging Network – A Platform for Scientific Excellence (SINAPSE).
Dr Paul McNamee is a Senior Research Fellow in Health Economics at the Health Economics Research Unit (HERU), University of Aberdeen. He is Director of the Preference Elicitation and Assessment of Technologies (PEAT) Research Programme within HERU, and has over 15 years of research experience in health economics, mainly in studies investigating the cost-effectiveness of new forms of health-care delivery. Among current grants, he is a co-investigator on the NIHR HTA funded multicentre Surgical Trial In Traumatic intraCerebral Haemorrhage (STITCH).
Professor Martin Dennis is Professor of Stroke Medicine and an Honorary Consultant Stroke Physician at the University of Edinburgh and NHS Lothian. He is an expert on stroke services, models of stroke prevention and prediction. He was the founding President of the British Association of Stroke Physicians and has been the Chairman of the Advisory Committee on Stroke to the Scottish Government (i.e. the Scottish Stroke ‘Czar’) for nearly 10 years. He is an executive member of SSRN, the PI of the SSCA, and the PI of the FOOD (HTA funded) and CLOTS (MRC funded) multicentre trials.
Professor Peter Sandercock is Professor of Neurology and Honorary Consultant Neurologist at the University of Edinburgh and NHS Lothian. He was PI of the world’s largest ever acute stroke treatment trial, the MRC-funded International Stroke Trial, and is currently PI of the world’s largest trial of thrombolysis in acute stroke, the Third International Stroke Trial. He has been the Coordinating Editor of the Cochrane Stroke Group since 1998. He has worked with the Cochrane Diagnostic Tests Methods Group, tested the methods for systematic reviews of diagnostic tests, and helped pilot one of the first Cochrane reviews in the field. He was lead author of an HTA review of effectiveness and cost-effectiveness of thrombolytic and neuroprotective therapies for acute stroke, is currently joint Director of the Edinburgh MRC Trials Methodology Hub and Director of Edinburgh Neuroscience, and jointly with Professor Wardlaw, has previously surveyed availability of MR for patients admitted to UK hospitals with acute stroke.
Ms Miriam Brazzelli is a Research Fellow at the Division of Clinical Neurosciences, University of Edinburgh. She is experienced in systematic reviews of interventions and cost-effectiveness analyses of diagnostic imaging. She has conducted and published several Cochrane systematic reviews as well as Technology Assessment Reviews (TAR) and Interventional Procedures Reviews commissioned by NICE and worked on systematic reviews for the Royal College of Radiologists Guideline ‘Making the Best Use of a Radiology Department 5th Edition’. She has also been awarded a Chief Scientist Office (CSO), Scottish Executive Health Department, fellowship to study the methodology of systematic reviews of diagnostic accuracy studies and she is currently completing her PhD on this topic. She is a member of the Cochrane Screening and Diagnostic Tests Methods Group and of the Stroke Group and has recently completed, together with J Wardlaw and P Sandercock, a Cochrane review of diagnostic accuracy studies on CT and MR for acute stroke.
Dr Janet de Wilde, Medical Physicist, is the Project Coordinator of SINAPSE, a joint imaging network set up between six universities and funded by the Scottish Funding Council. She is also a very experienced MR physicist having authored several papers and books on MRI and is the former Director of MagNET (the UK’s central MR advisory body). She recently led a successful bid, on behalf of SINAPSE, to become a UK Centre for Evidence-Based Purchasing (CEP) now part of NICE, and now leads the first CEP-commissioned work.
Professor Keith Muir, SINAPSE Professor of Clinical Imaging and Honorary Consultant Neurologist with an interest in stroke, University of Glasgow and NHS Glasgow is an experienced clinical investigator in trials in acute and secondary prevention studies in stroke since 1992 (e.g. SAINT 1&2, ECASS 3, lubeluzole programme, MATCH, PRoFESS), UK chief investigator for phase 2 trials, and member of the steering committees for IMAGES, EPITHET, ENOS, and others. He is experienced in brain imaging analysis and interpretation for acute stroke trials and in research (e.g. SELESTIAL trial, CT perfusion studies). He runs an acute stroke treatment and prevention service for south Glasgow and adjacent regions and has been piloting practicalities in the use of MR DWI for TIA.
Professor Donald Hadley, Professor and Honorary Consultant Neuroradiologist, University of Glasgow and NHS Glasgow, is one of, if not the most, experienced neuroradiologists in the UK in MR, has co-ordinated the SW Scotland regional neuro-MR service since the early 1980s as well as research uses and has extensive experience in applications and practicalities of MR in general and in particular for stroke.
Dr Graham Scotland is a health economics research fellow in Aberdeen at HERU.
Dr Francesca Chappell is a statistician specialising in assessment diagnostic tests including meta-analyses and use of Summary receiver operator characteristic curves and other methods for data synthesis.
Dr Peter Tothill is a retired medical physicist who will act as public representative on the Steering Committee. As a medical physicist, he has very valuable insight into medical imaging as well as being able to bring experience of stroke investigations from a lay perspective to the project.
Infrastructure: The SINAPSE network of six Scottish university research imaging centres and NHS imaging facilities, offers an unparalleled ‘virtual clinical imaging laboratory’ facilitating many aspects of the project (www.sinapse.ac.uk). The network members have considerable experience of systematic reviews and health economic assessments of diagnostic imaging as evidenced by the recent award Centre for Evidence Based Purchasing status. The HERU, together with the Health Services Research Unit (HSRU) and the Department of Public Health at the University of Aberdeen, are one of only seven academic groups that hold contracts to conduct TARs and Single Technology Appraisals (STAs) for NICE, as well as other customers. Further, HERU provides an external quality assurance function to the work of the Scottish Medicines Consortium (SMC). The Cochrane Stroke Groupbased at the University of Edinburgh is one of the longest established Cochrane disease groups and has been proactive in piloting and implementing diagnostic tests systematic reviews into the Cochrane Library.
The key elements of the work and deliverables are covered in the diagram (below).

List of abbreviations
- A+E
- accident and emergency
- ACE
- angiotensin-converting enzyme
- AF
- atrial fibrillation
- Afx
- amaurosis fugax
- AHA
- American Heart Association
- AQoL
- Assessment of Quality of Life
- ARR
- absolute risk reduction
- ASA
- American Stroke Association
- ATT
- Antithrombotic Trialists
- AUC
- area under the curve
- AUROC
- area under receiver operating characteristic
- BI
- Barthel Index
- BP
- blood pressure
- CAPRIE
- Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events
- CAS
- carotid artery stenosis
- CCA
- common carotid artery
- CEMRA
- contrast-enhanced magnetic resonance angiography
- CHD
- coronary heart disease
- CHERRIES
- CHEcklist for Reporting Results of Internet E-Surveys
- CI
- confidence interval
- CIP
- clinical and imaging-based predictive model
- CLOTS
- Clots in Legs Or sTockings after Stroke trial
- CNI
- cranial nerve injury
- CT
- computed tomography
- CTA
- computed tomography angiography
- CV
- cerebrovascular
- CVD
- cardiovascular disease
- DOH
- Department of Health (NHS)
- DSA
- digital subtraction angiography
- DUS
- Doppler ultrasound
- DWI
- diffusion-weighted imaging
- ECG
- electrocardiography
- ECST
- European Carotid Surgery Trial
- ED
- emergency department
- EQ-5D
- European Quality of Life-5 Dimensions
- ESO
- European Stroke Organisation
- ESRS
- Essen Stroke Risk Score
- ESS
- Edinburgh Stroke Study
- EVPI
- expected value of perfect information
- EXPRESS
- Early use of eXisting PREventive Strategies for Stroke
- FAST
- Face Arm Speech Test
- FLAIR
- fluid-attenuated inversion recovery
- GP
- general practitioner
- GRE
- gradient echo
- HDL
- high-density lipoprotein
- HR
- hazard ratio
- HTA
- Health Technology Assessment
- HUI
- Health Utilities Index
- IAA
- intra-arterial angiography
- ICA
- internal carotid artery
- ICD
- International Classification of Diseases
- ICER
- incremental cost-effectiveness ratio
- ICH
- intracerebral haemorrhage
- IPD
- individual patient data
- IQR
- interquartile range
- ISD
- Information and Statistics Division
- IST
- International Stroke Trial
- LAA
- large artery atherosclerosis
- LACS
- lacunar stroke
- LDL
- low-density lipoprotein
- LiLAC
- Life Long After Cerebral ischaemia score
- LOS
- length of stay
- LSR
- Lothian Stroke Register
- MeSH
- medical subject heading
- MI
- myocardial infarction
- MR
- magnetic resonance
- MR DWI
- magnetic resonance diffusion-weighted imaging
- MRA
- magnetic resonance angiography
- MRI
- magnetic resonance imaging
- mRS
- modified Rankin Scale
- MS
- multiple sclerosis
- NASCET
- North American Symptomatic Carotid Endarterectomy Trial
- NICE
- National Institute for Health and Care Excellence
- NIHSS
- National Institutes of Health Stroke Scale
- NNT
- number needed to treat
- NNTB
- number needed to treat to benefit
- NNTH
- number needed to treat to harm
- NV
- neurovascular
- OCSP
- Oxfordshire Community Stroke Project
- OR
- odds ratio
- OXVASC
- OXford VASCular study
- PACS
- partial anterior circulation cortical stroke
- PCSS
- Perth Community Stroke Study
- PICH
- primary intracerebral haemorrhage
- POCS
- posterior circulation stroke
- PPP
- purchasing power parity
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROGRESS
- Perindopril pROtection aGainst REcurrent Stroke Study
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- RAO
- retinal artery occlusion
- RCT
- randomised controlled trial
- ROC
- receiver operating characteristic
- RR
- relative risk
- SD
- standard deviation
- SDH
- subdural haematoma
- SF-36
- Short Form questionnaire-36 items
- SF-6D
- Short Form questionnaire-6 Dimensions
- SG
- standard gamble
- SIGN
- Scottish Intercollegiate Guidelines Network
- SOS-TIA
- SOS Transient Ischaemic Attack study
- SPARCL
- Stroke Prevention by Aggressive Reduction in Cholesterol Levels
- SPI-I
- Stroke Prognosis Instrument I
- SPI-II
- Stroke Prognosis Instrument II
- SROC
- summary receiver operating characteristic
- SSCAS
- Scottish Stroke Care Audit Study
- TACS
- total anterior circulation cortical stroke
- TIA
- transient ischaemic attack
- TTO
- time trade-off
- VAS
- visual analogue scale
- WGH
- Western General Hospital
- WHO
- World Health Organization
- WTP
- willingness to pay