Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/143/01. The contractual start date was in March 2013. The draft report began editorial review in September 2017 and was accepted for publication in July 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
David Price reports board membership fees paid to the Observational and Pragmatic Research Institute from Aerocrine AB, Amgen Inc., AstraZeneca plc, C.H. Boehringer Sohn AG & Ko. KG, Chiesi Farmaceutici S.p.A., Mylan N.V., Mundipharma GmbH, Napp Pharmaceutical Group Ltd, Novartis Pharmaceutical Company and Teva Pharmaceutical Industries Ltd; consultancy agreement fees paid to the Observational and Pragmatic Research Institute from Almirall S.A., Amgen Inc., AstraZeneca plc, C.H. Boehringer Sohn AG & Ko. KG, Chiesi Farmaceutici S.p.A., GlaxoSmithKline plc, Mylan N.V., Mundipharma GmbH, Napp Pharmaceutical Group Ltd, Novartis Pharmaceutical Company, Pfizer Inc., Teva Pharmaceutical Industries and Theravance Biopharma; grants from Aerocrine AB, AKL Research and Development Ltd, AstraZeneca plc, C.H. Boehringer Sohn AG & Ko. KG, the British Lung Foundation, Chiesi Farmaceutici S.p.A., Mylan N.V., Mundipharma GmbH, Napp Pharmaceutical Group Ltd, Novartis Pharmaceutical Company, Pfizer Inc., the Respiratory Effectiveness Group, Teva Pharmaceutical Industries, Theravance Biopharma, the UK National Health Service and Zentiva N.V.; lecture/speaking engagement fees paid to the Observational and Pragmatic Research Institute from Almirall S.A., AstraZeneca plc, C.H. Boehringer Sohn AG & Ko. KG, Chiesi Farmaceutici S.p.A., Cipla Ltd, GlaxoSmithKline plc, KYORIN Pharmaceutical Co., Ltd, Mylan N.V., Merck & Company, Inc., Mundipharma GmbH, Novartis Pharmaceutical Company, Pfizer Inc., Skyepharma and Teva Pharmaceutical Industries; manuscript preparation fees paid to the Observational and Pragmatic Research Institute from Mundipharma GmbH and Teva Pharmaceutical Industries; travel expenses fees paid to the Observational and Pragmatic Research Institute from Aerocrine AB, AstraZeneca plc, C.H. Boehringer Sohn AG & Ko. KG, Mundipharma GmbH, Napp Pharmaceutical Group Ltd, Novartis Pharmaceutical Company and Teva Pharmaceutical Industries; funding for patient enrolment or completion of research fees paid to the Observational and Pragmatic Research Institute from Chiesi Farmaceutici S.p.A., Novartis Pharmaceutical Company, Teva Pharmaceutical Industries and Zentiva N.V.; and payment for developing educational materials fees paid to the Observational and Pragmatic Research Institute from Mundipharma GmbH and Novartis Pharmaceutical Company. David Price is a peer reviewer for grant committees for the Efficacy and Mechanism Evaluation and Health Technology Assessment (HTA) programmes. He has stock/stock options from AKL Research and Development Ltd that produces phytopharmaceuticals, and owns 74% of the social enterprise Optimum Patient Care Ltd (in Australia, Singapore and the UK) and 74% of the Observational and Pragmatic Research Institute Pte Ltd (Singapore). Ian Pavord reports grants from GlaxoSmithKline during the conduct of the study; has received speaker’s honoraria from AstraZeneca plc, C.H. Boehringer Sohn AG & Ko. KG, Aerocrine AB, Almirall S.A., Novartis Pharmaceutical Company and GlaxoSmithKline; has received honoraria for attending advisory board panels from Almirall S.A., AstraZeneca plc, C.H. Boehringer Sohn AG & Ko. KG, Dey Pharma, L.P., GlaxoSmithKline, Merck Sharp & Dohme, Schering-Plough, Novartis Pharmaceutical Company, Napp Pharmaceutical Group Ltd and RespiVert Ltd; and has received sponsorship for attending international scientific meetings from AstraZeneca plc, C.H. Boehringer Sohn AG & Ko. KG, GlaxoSmithKline and Napp Pharmaceutical Group Ltd. Mike Thomas received speaker’s honoraria for speaking at sponsored meetings or satellite symposia at conferences from Aerocrine AB, GlaxoSmithKline and Novartis Pharmaceutical Company. He has received honoraria for attending advisory panels with Aerocrine AB, Boehringer Ingelheim, GlaxoSmithKline, Merck Sharp & Dohme, Novartis Pharmaceutical Company and Pfizer Inc. during the conduct of the study. He reports grants from the National Institute for Health Research during the conduct of the study; being a member of the HTA Primary Care Community and Preventative Interventions Panel during the conduct of the study; and personal fees from GlaxoSmithKline, Novartis Pharmaceutical Company, Boehringer Ingelheim and Aerocrine AB outside the submitted work. He is a member of the British Thoracic Society/Scottish Intercollegiate Guidelines Network’s Asthma Guideline Steering Group and the National Institute for Health and Care Excellence’s Asthma Diagnosis and Monitoring Guideline Development Group. Christopher Brightling received payment via his institution of grants and personal fees from AstraZeneca plc/MedImmune, LLC, GlaxoSmithKline plc, F. Hoffmann-La Roche AG/Genentech, Inc., Novartis Pharmaceutical Company, Chiesi Farmaceutici S.p.A., Pfizer Inc., Teva Pharmaceutical Industries, Sanofi S.A./Regeneron Pharmaceuticals, Inc., Glenmark Pharmaceuticals, Mologic Ltd and Vectura Group plc.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by McKeever et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Extracts of text, figures and tables throughout this chapter have been published in Skeggs et al. 1 This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution and reproduction in any medium, provided the original work is properly cited.
Background
Asthma is one of the commonest chronic diseases in the world, affecting an estimated 300 million people. Acute exacerbations of asthma cause considerable morbidity and account for a large component of the direct and indirect costs of asthma.
Previous studies have shown that the widespread use of an asthma self-management plan can reduce exacerbations requiring oral corticosteroids and emergency health-care utilisation, as well as reduce time away from work or school because of poorly controlled asthma. However, although written self-management plans are recommended for all patients with asthma, many patients are not provided with one. Reasons for not being provided with a self-management plan include a lack of time and confusion about what to include in the plan when asthma control is deteriorating but before there is a need for oral corticosteroids.
Two large randomised, double-blind, placebo-controlled clinical trials2,3 found no benefit from doubling the dose of a patient’s usual inhaled corticosteroid2 or doubling the dose of inhaled budesonide3 when asthma control starts to deteriorate. However, other studies have suggested that higher doses (e.g. a fivefold increase4 or 1 mg of inhaled fluticasone propionate twice daily5) may be effective for the treatment of established exacerbations. A previous single-centre, randomised, double-blind, placebo-controlled clinical trial carried out by the chief investigator explored whether or not asthma exacerbations could be prevented with a self-management plan that recommended quadrupling the dose of inhaled corticosteroid at the time when asthma control starts to deteriorate (n = 403). 6 The results showed that for those participants who started on the study inhalers (n = 94) quadrupling the dose of inhaled corticosteroid led to a 36% reduction in asthma exacerbations (per-protocol analysis, p = 0.004). Unfortunately, the number of participants starting on the study inhaler varied between the two groups and the primary outcome in the intention-to-treat analysis was not significant. There is no evidence to suggest that a higher dose (i.e. a fivefold increase) is any more effective and would be associated with greater systemic activity.
In view of the limited evidence for quadrupling the dose of inhaled steroid at the point when asthma control starts to deteriorate, the UK National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme issued a funding call in February 2010 and, subsequently, commissioned the Fourfold Asthma Study (FAST) (reference number 10/143/01).
Objectives
Primary objective
-
Determine whether or not the proposed asthma self-management plan reduces asthma exacerbations requiring oral steroids or unscheduled health-care consultations for asthma, compared with the usual self-management plan.
Secondary objectives
-
Determine whether or not the proposed asthma self-management plan reduces the deterioration in asthma control, compared with the usual self-management plan.
-
Determine if the proposed asthma self-management plan is cost-effective to the NHS and society overall, compared with the usual self-management plan.
Role of the funder
The study was funded by the NIHR HTA programme. The NIHR had input into the trial design through peer review of the proposal, but did not have a role in data collection, data analysis, data interpretation or the writing of the final report. The corresponding author had access to all the data and was responsible for the decision to submit the final report.
Chapter 2 Methods
Extracts of text, figures and tables throughout this chapter have been published in Skeggs et al. 1 and in the International Standard Registered Clinical/soCial sTudy Number (ISRCTN) Registry as ISRCTN15441965. 7 These are open access articles distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution and reproduction in any medium, provided the original work is properly cited.
Trial design
The FAST was a multicentre, pragmatic, normal care-controlled, randomised controlled trial (RCT) of 12 months’ duration (Figure 1). Adult asthma patients were randomised (1 : 1) to one of two asthma self-management plans: usual care or modified. Both self-management plans were identical at zones 1, 3 and 4, but at zone 2 (worsening of asthma symptoms) the usual-care group was advised to follow the current guidelines of increasing bronchodilator medication when asthma control begins to deteriorate. The modified self-management plan advised participants to increase bronchodilator medication and quadruple their inhaled corticosteroid dose. Both self-management plans advised participants to increase their medication for a maximum of 14 days, or for a shorter duration if asthma symptoms started to improve, before returning to their normal treatment, which was actively promoted in both self-management plans.
FIGURE 1.
Trial visit flow chart.
Participants were expected to attend three scheduled visits: baseline, 6 months and 12 months. These visits were conducted at the participants’ local general practitioner (GP)’s clinic or hospital (or via the telephone if easier for the participant). During these visits the research nurse reviewed the participant’s diary card to assess their adherence to the self-management plan and use of inhaled steroids, reviewed the participant’s asthma control to determine if there had been any unreported activation of zones 2, 3 or 4 since the previous visit and asked participants to complete the EuroQol-5 Dimensions, three-level version (EQ-5D-3L), questionnaire and Service Use Questionnaire (see Appendix 1).
In addition to the scheduled visits, participants were expected to attend post-activation visits no less than 14 days after activating zone 2 of the asthma self-management plan; for some participants multiple visits took place in the 12-month participation period. Prior to the visit participants were expected to complete a diary card. For FAST two diary cards were used: one diary card for those participants who used corticosteroid inhalers and a second for those on combination inhalers. The participants used the diary cards to record peak flow, to record the asthma medication used to manage symptoms (including the number of puffs of usual preventer inhaler, of extra corticosteroid inhaler and of reliever inhaler and whether or not any systemic corticosteroids were taken) and to document whether they had an asthma-related GP or hospital appointment. The diary cards acted as an aide-memoire during the post-activation visit; the research nurse reviewed the daily peak flow measurements, any health-care consultations attended, the use of inhaled corticosteroids and systemic corticosteroids, ascertained if any adverse events (AEs) had occurred and assessed the participant’s adherence to the asthma self-management plan. Participants were also asked to complete the Juniper et al. Mini Asthma Quality of Life Questionnaire (Mini AQLQ). 8 The study assessments are outlined in Table 1.
| Study assessments | Named trial visit | ||||
|---|---|---|---|---|---|
| Visit 1/screening | Activation of zone 2 (days 0–14) | Post-activation visit (14 days)a | Visit 2 (6 months after visit 1) | Visit 3 (12 months after visit 1) | |
| Demographics/eligibility, consent | ✓ | ||||
| Randomisation | ✓ | ||||
| Asthma diary card completionb | ✓ | ||||
| Mini AQLQc | ✓ | ✓ | |||
| Issue asthma diary card | ✓ | ✓ | |||
| EQ-5D-3Ld | ✓ | ✓ | ✓ | ||
| Service Use Questionnairee | ✓ | ✓ | |||
| Adherence to the self-management planf | ✓ | ✓ | ✓ | ||
| Asthma diary card review | ✓ | ||||
| Asthma review | ✓ | ✓ | |||
In addition, it was planned that the first 200 participants recruited at the Nottingham and Liverpool sites would have the option (if they agreed) to have their inhaled corticosteroid inhaler fitted with a smart inhaler electronic dose counter for adherence purposes. The main purpose of this was to compare an electronic record of inhaler use with the participants’ self-reported inhaler use and, therefore, the overall adherence to their allocated self-management plan. The smart inhaler was planned to be used to independently validate the accuracy of the participants’ self-reported adherence.
The implementation of the smart inhaler was challenging and, unfortunately, some technical issues (the smart inhaler not working correctly and a short battery life) prevented the implementation of the initial batch of devices into the trial.
Following a period of uncertainty around delivery and the reliability of new devices to replace those that did not function accurately, it was the unanimous view of the Trial Steering Committee (TSC), at a meeting held in July 2014, that it was not practical to pursue further use of monitoring devices as the chance of any informative data being obtained from them prior to the end of the study was low. As a result, the planned interim analysis to determine whether or not self-reported adherence to adjustments to inhaled steroid dose was similar to that captured by the electronic devices was not conducted.
Recruiting centres
Recruitment took place in both primary and secondary care across England and Scotland. There were 10 secondary care sites: Nottingham City Hospital, Leicester Glenfield Hospital, Freeman Hospital Newcastle, Aintree University Hospital, Aberdeen Royal Infirmary, Royal Liverpool University Hospital, King’s Mill Hospital, Arrowe Park Hospital, Blackpool Victoria Hospital and Bradford Royal Infirmary. In addition, there were 171 primary care Research Initiative Sites (RISs) across 11 CRN regions: North East and North Cumbria, North West Coast, Greater Manchester, East Midlands, West Midlands, West of England, Thames Valley and South Midlands, Eastern, Kent, Surrey, Sussex, Wessex and South West Peninsula.
Participants were identified through secondary care and primary care. In secondary care, participants were identified from patients attending respiratory outpatient appointments at the individual recruiting centres and also through running database searches of participants who had previously participated in asthma studies and had given consent to be contacted again for future studies. In Scottish centres, the Scottish Primary Care Research Network identified potential participants in primary care who were subsequently recruited at local secondary care sites. In primary care, local CRNs liaised directly with GP practices that acted as RISs that performed a database search to identify potential participants. Potentially eligible participants were sent a participation invitation pack that included an invitation letter flyer about the trial and, in some practices, a copy of the Participant Information Sheet.
A local press release was issued at the start of the trial. Posters and flyers were displayed in recruiting centres and, where possible, a digital flyer was displayed in recruiting centre waiting areas. Posters were also displayed in pharmacies in the Nottinghamshire area and information flyers were placed in the bags of patients who were collecting prescriptions for asthma medication. The trial was also promoted online by Asthma UK.
General practitioner surgeries local to the recruiting hospitals were used as Participant Information Centres (PICs) by displaying posters and flyers.
Participants
Patients were considered eligible for entry into the trial if the following inclusion criteria were met:
-
men or women aged ≥ 16 years
-
clinician-diagnosed asthma treated with a licensed dose of inhaled corticosteroid [i.e. steps 2–4 of the British Thoracic Society/Scottish Intercollegiate Guidelines Network (BTS/SIGN) guidelines]2
-
one or more exacerbations in the last 12 months requiring treatment with systemic corticosteroids
-
current smokers could be included provided that the recruiting centres had good evidence of underlying asthma (i.e. a life-long history of asthma, a > 12% forced expiratory volume in 1 second (FEV1) reversibility, or sputum or blood eosinophilia).
In addition, patients were not entered into the trial if any of the following exclusions applied:
-
a history more in keeping with smoking-related chronic obstructive pulmonary disease (COPD) (i.e. smoked > 20 pack-years, without evidence of significant reversibility or eosinophilia)
-
on maintenance systemic corticosteroids (i.e. step 5 of the BTS/SIGN guidelines2)
-
using a combination inhaler for both maintenance and relief treatment
-
experienced an exacerbation within 4 weeks of randomisation
-
women who were pregnant, breastfeeding or who were planning to become pregnant.
Interventions
Asthma self-management plans
The asthma self-management plans used in the trial were based on the plan that was available from Asthma UK9 and were widely used at the time of the trial’s design and protocol development. All participants were randomised to either the usual-care or modified self-management plans, which differed only in instruction at zone 2, which in the modified plan recommended a quadrupling of inhaled corticosteroid dose.
Zone 1 described the participant with well-controlled asthma and simply recommended that they continue their usual treatment. Zone 3 described the development of an exacerbation and when to start systemic corticosteroids and seek medical intervention and zone 4 described what to do with life-threatening exacerbations. Both plans had the same wording so that participants in each group should, on average, have started systemic corticosteroids at the same threshold.
Zone 2 included the current area of uncertainty and the research question under investigation.
Usual self-management plan
Participants in the usual-care group who reached zone 2 were instructed to use additional bronchodilator medication to relieve asthma symptoms, as outlined in their individual asthma self-management plan.
Modified self-management plan
Participants in the modified self-management group who reached zone 2 were instructed to use additional bronchodilator medication to relieve asthma symptoms and to increase their inhaled corticosteroid treatment fourfold, either by increasing the number of puffs of their current inhaler, if they used a corticosteroid inhaler (refer to Table 2), or by adding a corticosteroid inhaler to their treatment if they used a combination inhaler (Table 3), as outlined in their individual asthma self-management plan. Those participants on combination inhalers were not asked to simply increase the number of puffs because this would have led to an increase in long-acting beta-agonist dose as well as the corticosteroid dose.
| Current number of puffs per dose | Number of puffs per dose to achieve quadrupled dose |
|---|---|
| 1 o.d. | 4 o.d. |
| 2 o.d. | 8 o.d. |
| 1 b.i.d. | 4 b.i.d. |
| 2 b.i.d. | 8 b.i.d. |
| And so on | And so on |
| Current treatment | Additional treatment options | |
|---|---|---|
| Option 1 | Option 2 | |
| Seretide® MDI 50/25 (GlaxoSmithKline, Uxbridge, UK), 2 puffs b.i.d. | FP 50, 6 puffs b.i.d. | FP 125, 3 puffs b.i.d. |
| Seretide MDI 125/25, 2 puffs b.i.d. | FP 125, 6 puffs b.i.d. | FP 250, 3 puffs b.i.d. |
| Seretide MDI 250/25, 2 puffs b.i.d. | FP 250, 6 puffs b.i.d. | N/A |
| Seretide Accuhaler® 100/50 (GlaxoSmithKline, Uxbridge, UK), 1 puff b.i.d. | FP Disk 100, 3 puffs b.i.d. | N/A |
| Seretide Accuhaler 250/50, 1 puff b.i.d. | FP Disk 250, 3 puffs b.i.d. | N/A |
| Seretide Accuhaler 500/50, 1 puff b.i.d. | FP Diskhaler 500, 3 puffs b.i.d. | N/A |
| Symbicort® Turbo® 100/6 (AstraZeneca UK Ltd, Luton, UK), 1 puff b.i.d. | Bud Turbo 100, 3 puffs b.i.d. | N/A |
| Symbicort Turbo 100/6, 2 puffs b.i.d. | Bud Turbo 100, 6 puffs b.i.d. | Bud Turbo 200, 3 puffs b.i.d. |
| Symbicort Turbo 200/6, 1 puff b.i.d. | Bud Turbo 200, 3 puffs b.i.d. | N/A |
| Symbicort Turbo 200/6, 2 puffs b.i.d. | Bud Turbo 200, 6 puffs b.i.d. | Bud Turbo 400, 3 puffs b.i.d. |
| Symbicort Turbo 200/6, 4 puffs b.i.d. | Bud Turbo 400, 6 puffs b.i.d. | N/A |
| Symbicort Turbo 400/12, 1 puff b.i.d. | Bud Turbo 400, 3 puffs b.i.d. | N/A |
| Symbicort Turbo 400/12, 2 puffs b.i.d. | Bud Turbo 400, 6 puffs b.i.d. | N/A |
| Fostair MDI 100/6 (Chiesi Ltd, Manchester, UK), 1 puff b.i.d. | Qvar MDI 100, 3 puffs b.i.d. | N/A |
| Fostair MDI 100/6, 2 puffs b.i.d. | Qvar MDI 100, 6 puffs b.i.d. | N/A |
| Flutiform® MDI 50/5 (Napp Pharmaceuticals Ltd, Cambridge, UK), 2 puffs b.i.d. | FP MDI 50, 6 puffs b.i.d. | N/A |
| Flutiform MDI 125/5, 2 puffs b.i.d. | FP MDI 125, 6 puffs b.i.d. | FP MDI 250, 3 puffs b.i.d. |
| Flutiform MDI 250/10, 2 puffs b.i.d. | FP MDI 250, 6 puffs b.i.d. | N/A |
It was perceived at the outset of the trial that all participants enrolled in the trial would benefit, as their self-management plan would be explained to them in detail and monthly texts would be sent to prompt them to adhere to it (if participants consented to this). It was believed that this would increase the participant’s awareness of their asthma symptoms and allow them to implement their self-management plan more reliably.
During the baseline visit, a member of the research team randomised each participant to their self-management plan and talked through the allocated plan with the participant to ensure that they fully understood the guidance at each zone. Those participants who were randomised to usual self-management were instructed to ‘use your reliever inhaler to relieve your symptoms and continue your preventer medication at your normal dose’. Those participants randomised to the modified self-management plan were instructed to ‘use your reliever inhaler to relieve your symptoms and increase your preventer medication as described below’ and then implement the zone 2 dose instructions in the self-management plan according to either:
-
option 1: how to achieve a quadrupling dose for participants on an inhaled corticosteroid-only inhaler
-
option 2: how to achieve a quadrupling dose for participants on a combination inhaler.
Outcome measures
Primary outcome
The primary outcome of ‘time to first asthma exacerbation’ was defined as the need for systemic corticosteroids (for at least 3 consecutive days) and/or unscheduled health-care consultations for asthma (i.e. reaching zone 3 or 4 of the Asthma UK self-management plan).
Secondary outcomes
-
Number of participants who had an acute exacerbation of asthma.
-
Total number of exacerbations.
-
Number of participants using systemic corticosteroids for an acute exacerbation of asthma.
-
Number of participants requiring unscheduled health-care consultations for asthma.
-
Total number of courses of systemic corticosteroids for an acute exacerbation of asthma.
-
Total number of unscheduled health-care consultations for asthma.
-
Time to participants requiring systemic corticosteroids for an acute exacerbation of asthma.
-
Time to unscheduled health-care consultations for asthma.
-
Area under the morning peak flow curve over 2 weeks from the point of activating zone 2 of the asthma plan.
-
Change in Mini AQLQ score 2 weeks after activating zone 2 of the self-management plan.
-
Cumulative dose of inhaled and systemic corticosteroids used in the 12 months after randomisation.
-
Cost and resource audits of both trial arms, reported as incremental cost per asthma exacerbation prevented and cost per quality-adjusted life-year (QALY) gained.
Safety outcomes
Known side effects of inhaled corticosteroids were collected because of the quadrupling of the dose of inhaled corticosteroid in the modified self-management group.
Data collection
Trial data generated by all centres were entered by site staff directly into a web-based bespoke database, designed and maintained by the Nottingham Clinical Trials Unit (NCTU). Access to the trial database was controlled by user logins, and users could enter or edit data only for their regional centre. Participant questionnaires, completed at clinic visits, were entered into the trial database by site staff. If participants had not activated zone 2 of their self-management plan and were unable to attend the 6- and/or 12-month visit in person, site staff contacted participants via telephone and completed the trial database directly with the information provided to them over the telephone. Any missing and/or ambiguous data were queried with site staff, by the NCTU team, and resolved wherever possible.
Participants were asked to complete their diary cards prior to attending their post-activation visit. This diary card is where the participants recorded peak flow and the asthma medication used to manage symptoms (including the number of puffs of usual preventer inhaler, extra corticosteroid inhaler, reliever inhaler and whether or not any systemic corticosteroids were taken) and documented whether they had an asthma-related GP or hospital appointment. The diary was reviewed by the site staff at each clinic appointment and this information was used to complete the relevant sections of the trial database.
Site staff were also required to assess the participant’s adherence to the asthma self-management plan by reviewing the diary card and discussing, during the visit, whether or not, and how, participants changed their inhaled treatment since activating zone 2 of their self-management plan. Site staff completed the adherence assessment directly into the web-based bespoke system. Initially, there was the option for the first 200 participants recruited to measure their actuation adherence with the smart inhaler. The participant’s corticosteroid inhaler would be fitted with the electronic dose counter to record the date and time of each actuation. The data would then be directly downloaded from the device during the participant’s visit and uploaded to a separate database. The smart inhaler devices were not implemented because of the challenges described in Trial design and all adherence data were assessed by the site staff.
The web-based bespoke system generated automated notification e-mails for sites to remind them of upcoming and overdue 6- and 12-month visits. These notification e-mails were sent to sites on a monthly basis on the first day of every month during the recruitment and follow-up phase of the trial.
Those participants who provided additional consent, were sent monthly text reminders to remind them to adhere to their asthma self-management plan. The text message service was automated, with text messages automatically initiated on the first day of every month at approximately noon. To maintain confidentiality the participant’s mobile phone number was entered on to the database by site staff at the point of consent. These data were then encrypted and stored confidentially. Only research staff at their own specific site had access to their participants’ mobile phone numbers. If the participant withdrew, was lost to follow-up or had completed the trial, then the text messages automatically stopped being sent to the participant.
Informed consent
Written informed consent was obtained for all participants prior to any trial procedures being undertaken. Consent to receive a summary of the results of the study and for the research team to send monthly reminder text messages to the participant was included as optional.
Sample size
A reduction of one-third in the number of people requiring treatment with oral corticosteroids was considered an important treatment effect by a group of local GPs, asthma nurses and asthma experts.
With 1000 participants per group, a log-rank test (at the two-sided 5% significance level) provided at least 90% power to detect a difference of 30% (relative effect), assuming an exacerbation rate of 13% in the control group. A 13% exacerbation rate requiring systemic corticosteroids was the lowest level seen in the control group of previous studies of this type6,10 and so provided a conservative estimate. The study initially proposed to recruit 2300 participants to allow for participants lost to follow-up (i.e. approximately 15% lost to follow-up). The study was not powered for the subgroup analysis performed on smoking status or dose of maintenance inhaled steroid dose at baseline.
The power calculation was revised in March 2015 in consultation with the NIHR HTA programme. The overall event rate in the first 226 participants recruited was higher (around 50%) in those reaching the 12-month follow-up time point. The exacerbation rate at this time was thought to be high because of more participants being recruited from secondary care and, therefore, having more severe asthma; hence, a lower exacerbation rate was used to revise the sample size calculation. Assuming an exacerbation rate in the control group of 17% and 90% power, and still estimating a one-third reduction in the fourfold increase group, then a sample size of 1542 participants was needed for analyses. Allowing for 20% of participants being lost to follow-up, the study aimed to recruit between 1774 and 1850 participants before the close of recruitment on 31 January 2016.
For those participants who were lost to follow-up, where possible, site staff reviewed participant computerised medical records to document if they had had an asthma-related GP appointment, if their asthma had exacerbated or if they had been prescribed systemic steroids.
Stopping rules and discontinuation
Ongoing adherence to the self-management plans was assessed by the Data Monitoring Committee in accordance with the criteria outlined in Table 4.
| Level of adherence in both groups | Proposed action |
|---|---|
| ≥ 50% of participants with moderate or good adherence with self-management compliance | Continue with trial as planned |
| ≤ 49% – ≥ 30% of participants with moderate or good adherence with self-management compliance | Implement pragmatic strategies for improvement |
| ≤ 29% of participants with moderate or good adherence with self-management compliance | Stop trial unless rectifiable solution can be readily implemented |
Randomisation
Randomisation was stratified by regional centre (Box 1), smoking status (yes/no) and maintenance inhaled corticosteroid dose (high/low dose; Table 5). Participants were allocated with equal probability to the two trial treatment groups. Recruiting sites were grouped into regional centres, which grouped practices to the appropriate CRN regions or secondary hospital care sites (Box 1). The treatment group to which a participant was assigned was determined by a computer-generated pseudo-random code, with random permuted blocks of randomly varying size, that was created by the NCTU in accordance with its standard operating procedure. The data were held on a secure University of Nottingham server.
-
Aberdeen Royal Infirmary.
-
Aintree University Hospital.
-
Arrowe Park Hospital.
-
Blackpool Victoria Hospital.
-
Bradford Royal Infirmary.
-
East of England.
-
Freeman Hospital, Newcastle.
-
Hetton Group Practice.
-
Kent.
-
King’s Mill Hospital.
-
Leicester Glenfield Hospital.
-
Nottingham City Hospital.
-
Royal Liverpool and Broadgreen University Hospitals.
-
Southampton.
-
South West Peninsula.
-
Surrey and Sussex.
-
Thames Valley and South Midlands.
-
West Midlands (South).
-
West of England.
-
Wythenshawe Hospital, Manchester.
| Steroid | Device and formulation | Dose (mcg/day) | |
|---|---|---|---|
| Low | High | ||
| BDP | Non-proprietary | 100–1000 | > 1000–2000 |
| BDP | Clenil® (Chiesi Ltd, Manchester, UK) MDI | 100–1000 | > 1000–2000 |
| BDP | Qvar MDI | 50–500 | > 500–800 |
| Budesonide | MDI | 100–1000 | > 1000–1600 |
| Budesonide | Turbuhaler | 100–800 | > 800–1600 |
| Fluticasone propionate | MDI/Accuhaler | 50–500 | > 500–2000 |
| Ciclesonide | MDI | 80–320 | |
| Seretide | MDI/Accuhaler | 50–500 | > 500–1000 |
| Symbicort | Turbuhaler | 100–800 | > 800–1600 |
| Fostair | MDI | 400 | |
| Flutiform | MDI | 50–500 | > 500–1000 |
Research nurses accessed the randomisation website by means of a remote, internet-based randomisation system developed, and maintained, by NCTU. Access was controlled by unique user logins. The sequence of treatment allocations was concealed until interventions had all been assigned and recruitment and data collection were complete. The chief investigator, trial team and trial statisticians were blinded to treatment allocations until the database was locked.
Blinding
Owing to the nature of the self-management plans allocated in the trial, it was not possible to blind site staff or participants to their treatment allocation. Efforts were made to minimise the expectation bias by detailing in the trial documents that the evidence supporting the quadrupling of the inhaled dose of corticosteroid at the time of worsening asthma symptoms was limited, and it was not yet known whether or not the intervention offered any benefit over usual care. Both groups were also provided with tailored self-management plans that were explained to them in detail, ensuring that both groups received similar instruction on how to best use their asthma self-management plan.
Throughout the trial, prior to database lock, the blinding allocation was preserved for the chief Investigator, trial statisticians, the trial team and TSC members.
Full details of blinding arrangements are summarised in Table 6.
| Role within trial | Blinding status | Comments |
|---|---|---|
| Participants | Not blinded | Not possible to blind participants, efforts made to minimise expectation bias |
| Research nurses and principal investigators | Not blinded | Acted as the main point of contact for participants. Not possible to blind research staff, efforts made to minimise bias |
| Trial staff at NCTU | Blinded | Acted as the main point of contact for recruiting centres. All trial documentation finalised prior to revealing treatment codes |
| Statisticians | Blinded | Statisticians finalised the statistical analysis plan prior to revealing the treatment codes |
| Chief investigator | Blinded | Finalised all documentation prior to revealing treatment codes |
Statistical analysis
Analyses are detailed in the statistical analysis plan (www.nottingham.ac.uk/nctu/trials/respiratory.aspx#FAST), which was finalised prior to database lock and release of the treatment allocation codes for analysis.
All analyses were based on the intention-to-treat principle, for example analysed as randomised regardless of adherence to self-management plan. All analyses were carried out using Stata®/SE 13.1 (StataCorp LP, College Station, TX, USA).
Preliminary analyses
Descriptive statistics of demographic and clinical measures were used to examine balance between the randomised groups at baseline.
Descriptive analyses
The number of participants activating zone 2 or above of the self-management plan was derived from the following information on the electronic Case Report Form (eCRF): summary pages for diary cards, post-activation visit pages, summary pages for oral corticosteroid use for asthma, summary pages for health-care consultations for asthma and a question about unreported activations at the scheduled visits. Therefore, the source of the date of the first activation, diary card completion and post-activation visit attendance for the first activation to zone 2 is tabulated by allocated group. The research nurse rating of adherence is described with frequencies and percentages for the first activation to zone 2. Adherence information is unknown for participants who did not report their activation to zone 2 or complete their diary card.
Asthma exacerbation outcomes
An asthma exacerbation was defined as the need for a course of systemic corticosteroids and/or an unscheduled health-care consultation for asthma. A course of systemic corticosteroids was defined as taking 3 consecutive days or more of corticosteroids. Health-care consultations and courses of systemic corticosteroids were counted as part of the same exacerbation if they were within 14 days of the previous health-care consultation or course of systemic corticosteroids for asthma.
Similarly, for the derivation of the total number of courses of systemic corticosteroids, corticosteroids started within 14 days of the last date of the previous course of corticosteroids were counted as within the same course. For the derivation of the total number of unscheduled health-care consultations, GP/hospital visits were classed as one unscheduled health-care visit if they were within 14 days of the previous visit.
The analysis population for the asthma exacerbation outcomes (including systemic corticosteroids and unscheduled health visits) was all participants apart from those with whom there was no further contact after randomisation and, therefore, information was unavailable about oral steroid use or unscheduled health-care consultations for asthma (i.e. questions on eCRF answered as unknown or not answered). Attendance at a scheduled or post-activation visit or a completed diary card was considered as contact after randomisation.
The questions about oral corticosteroid use or unscheduled health-care consultations for asthma on the eCRF were not expected to be answered as unknown, as the protocol specified that health-care records could be checked for participants who did not complete the 12-month follow-up visit but who did not withdraw consent. Some sites, however, did not have access to health-care records if the participant had moved surgery or if the participant was recruited from a secondary care site. In these circumstances, the questions could be answered as unknown.
Primary outcome: time to first asthma exacerbation
For the analysis of time to first asthma exacerbation, the start time was the date of randomisation and the end time was either:
-
the date of first starting to take systemic corticosteroids (provided these were taken for at least 3 consecutive days) or the date of the first unscheduled health-care consultation for asthma (if within 365 nights after randomisation) (whichever happened first)
-
censored for participants who did not take systemic corticosteroids for more than 3 consecutive days or have an unscheduled health-care consultation (or if this occurred more than 365 days after randomisation) at the:
-
12-month follow-up date for participants who completed the trial (or 365 days if the 12-month follow-up date was after this)
-
date of withdrawal for participants who withdrew consent
-
date of death for participants who died
-
date of last contact in the case of participants who moved to another GP practice during the trial
-
scheduled 12-month follow-up date (i.e. 365 days after randomisation) for all other participants who did not complete the trial as a result of being lost to follow-up or other reasons (sites were asked to check GP records for these participants to ascertain the primary outcome over the trial period).
-
-
censored for participants where it was unknown if they took any systemic corticosteroids or had an unscheduled health-care consultations at the:
-
last date the participant was known to be in the trial, that is, whichever was last of the latest dates from the diary [provided peak expiratory flow (PEF) data or some information on the number of puffs on inhalers was recorded], post-activation visit date or 6-month visit date
-
randomisation date for all other scenarios.
-
The number of participants with an asthma exacerbation, the total number of person-years to first exacerbation and the rate for first asthma exacerbation are summarised by allocated group. The time to first asthma exacerbation is presented in Kaplan–Meier plots, with a table showing the number at risk.
The hazard ratio for an asthma exacerbation in the modified self-management group compared with the usual-care group was calculated using Cox proportional hazards regression (using the Breslow method for tied failure times), including the randomisation stratification variables dose of inhaled corticosteroid (high/low) and smoking status (never, former, current) as covariates and using a shared frailty model to account for stratification by regional centre. 11 In addition, the unadjusted hazard ratio is reported.
The proportional hazards assumption was tested by using a log–log plot of survival and using Schoenfeld’s residuals.
Sensitivity analyses for the primary outcome
The hazard ratio for time to asthma exacerbation was further adjusted for age, sex and peak flow at screening. These were chosen based on previous literature as being strong predictors of asthma exacerbation.
Prespecified subgroup analyses for the time to first asthma exacerbation for smoking status at trial entry (never, former, current) and dose of inhaled corticosteroid at trial entry (high/low) were conducted by including an interaction term with allocated group in the Cox proportional hazards regression model, adjusting for randomisation stratification variables.
The main analysis of the primary outcome specified above was repeated for the per-protocol population. The per-protocol population was defined as participants who activated zone 2 and had good adherence to their self-management plan during their first activation, as assessed by the research nurse, and participants who completed the study as planned (i.e. attended the 12-month follow-up) and did not activate zone 2. Note that this is a non-randomised comparison and, therefore, should be interpreted with caution.
Exploratory analyses were also performed to examine the robustness of the conclusions from the main analysis to the date of censoring for the participants who did not complete the 12-month visit.
Secondary outcomes
For secondary outcomes, the same approach for analyses was taken as for the primary outcome. The results are summarised by allocated group and the analysis models (used to estimate the intervention effect) included the randomisation stratification variables of corticosteroid dose, smoking status and regional centre as covariates.
Secondary exacerbation outcomes
The time to participants requiring a course of systemic corticosteroids and time to participants requiring an unscheduled health-care consultation were analysed using the methods described above for the primary outcome.
The difference in the percentage of participants with an asthma exacerbation requiring a course of corticosteroids and requiring an unscheduled health-care consultation was compared between the two allocated groups using generalised estimating equations with the binomial family and:
-
an identity link to estimate the risk differences
-
a log-link to estimate the risk ratios.
An exchangeable correlation matrix was used to account for randomisation being stratified by regional centre.
The total number of asthma exacerbations per participant, the total number of courses of systemic corticosteroids and the total number of unscheduled health-care consultations per participant were summarised in the two allocated groups and compared with a negative binomial model using generalised estimating equations to account for randomisation being stratified by regional centre. The number of days in the trial was used as time at risk in the negative binomial model. Incidence rate ratios with 95% confidence intervals (CIs) are presented.
Area under the morning peak flow curve over 2 weeks from the point of activating zone 2 of the asthma plan
The area under the peak flow curve was specified as a secondary outcome to explore whether or not the severity of the deterioration in asthma control differed between the allocated groups. After zone 2 activation, peak flow data were to be collected on the diary cards for 14 days.
The percentage baseline peak flow was used for analysis and was calculated as actual PEF × 100/(screening visit PEF). The area under the curve for each participant was calculated in Stata using the cubic spline method. 12
Participants with a diary card completed for the first activation to zone 2 and with a PEF measurement on day 1, at least one PEF measurement on or after day 10 and at least one PEF measurement in between day 1 and 10 were included in the analysis. The area under the curve was calculated for the two analysis populations:
-
participants with a PEF measurement on day 1, at least one PEF measurement on or after day 10 and at least one PEF measurement in between days 1 and 10
-
participants with a PEF value recorded on day 1 and day 14 and at least six PEF values between days 1 and 14.
For both of the analyses above on days when PEF was not recorded in the diary, the PEF value was imputed using the last PEF value recorded.
Linear regression with a random effect for regional centre was used to compare the area under the percentage baseline morning peak flow curve. Baseline PEF was also included as a covariate (along with the randomisation stratification variables), as this was felt likely to be prognostic for PEF values during activation to zone 2.
Change in the Mini Asthma Quality of Life Questionnaire score 2 weeks after activating zone 2 of the self-management plan
The Mini AQLQ measures the functional problems (symptoms, activities, emotions and environment) that are most troublesome to adults with asthma. It has 15 items each with seven response options (where 1 indicates severely impaired and 7 indicates not at all impaired) and a recall period of 2 weeks. The Mini AQLQ score was calculated from the mean of all 15 responses. The mean score for participants with missing items was calculated if no more than two items were missed. If more than two items were missed, the Mini AQLQ score was not calculated.
Participants with a Mini AQLQ for the first activation to zone 2 completed within 28 days of the start of the activation (because of the time window for the post-activation visit in relation to activation of zone 2) were included in the analysis of change in the Mini AQLQ. Mini AQLQ questionnaires completed more than 28 days after first activating zone 2 were not included.
The overall Mini AQLQ score is summarised at baseline and 2 weeks after activating zone 2 or above. In addition, the score was compared between allocated groups using a linear regression model adjusting for the Mini AQLQ score at baseline [i.e. analysis of covariance (ANCOVA)] and randomisation stratification variables with a random effect for regional centre.
If the Mini AQLQ score was missing at baseline, for inclusion in the regression analysis the score was imputed using the mean baseline score at the participant’s regional centre. 13
Cumulative dose of inhaled and systemic steroids used in the 12 months after randomisation
Participants attending and completing the 12-month follow-up visit were included in the analysis of cumulative dose of inhaled and systemic steroids.
The cumulative dose of inhaled corticosteroids was derived from the information collected at visits (scheduled and post activation) about permanent asthma medication, including changes in medication, and from the information entered from the diary cards about inhaler use during activation to zone 2 or above. Participants were assumed to have taken their normal number of puffs on their preventer inhaler for days when no information was recorded on the diary card.
The cumulative doses of inhaled corticosteroids and systemic corticosteroids taken per participant over the 365 days from randomisation are summarised descriptively by allocated group. The cumulative dose of systemic corticosteroids taken is summarised for all participants and includes only participants who took systemic corticosteroids.
Safety
Adverse events were reported during the 14 days following activation of zone 2 of the self-management plan; therefore, safety data are summarised for participants activating zone 2 or above of their self-management plan on at least one occasion.
The number and percentage of participants experiencing a serious adverse event (SAE), seriousness criteria, total number of SAEs, SAE description [using the Medical Dictionary for Regulatory Activities (MedDRA)14 terminology-preferred term] and classification (not related to trial treatment, related to trial treatment – not unexpected) are summarised by allocated group.
The number and percentage of participants experiencing a non-serious AE, total number of non-serious AEs, AE description (using the MedDRA-preferred term) and relationship to inhaled steroids are summarised by allocated group. Non-serious AEs reported that were not considered to be known side effects of inhaled corticosteroids (e.g. displaced fractures) are not included in the summaries.
Summary of changes to the protocol
The full protocol and statistical analysis plan are available on the NCTU’s website (www.nottingham.ac.uk/nctu/trials/respiratory.aspx#FAST). A summary of changes made to the protocol after the start of recruitment is listed in Appendix 2.
Chapter 3 Results
Recruitment
Recruitment to the trial took place between 17 May 2013 and 29 January 2016 (Figure 2).
FIGURE 2.
Cumulative recruitment.
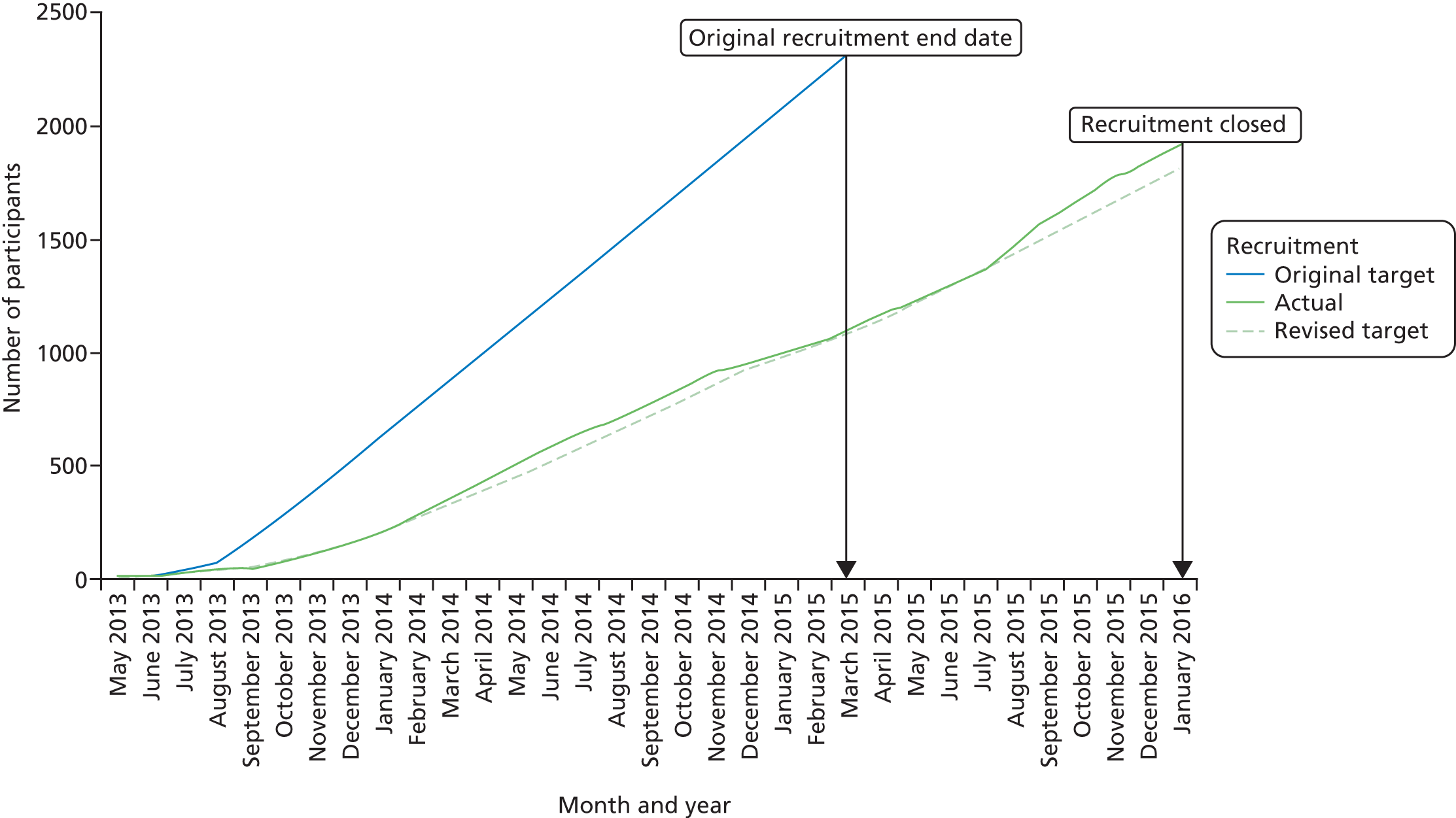
During this time 20,695 asthma patients were contacted and invited to take part in the study (patient contact data were not provided by 77 of the initiated sites). Of these, 4811 were assessed for eligibility and 1922 were subsequently randomised (Figure 3). Of the 2889 participants who were screened but not randomised, 860 (30%) declined to participate and 2029 (70%) failed to meet the eligibility criteria (Table 7).
FIGURE 3.
The Consolidated Standards of Reporting Trials (CONSORT) participant flow diagram. a, Invited figure reflective of the data provided by 132 initiated sites at the time of reporting.
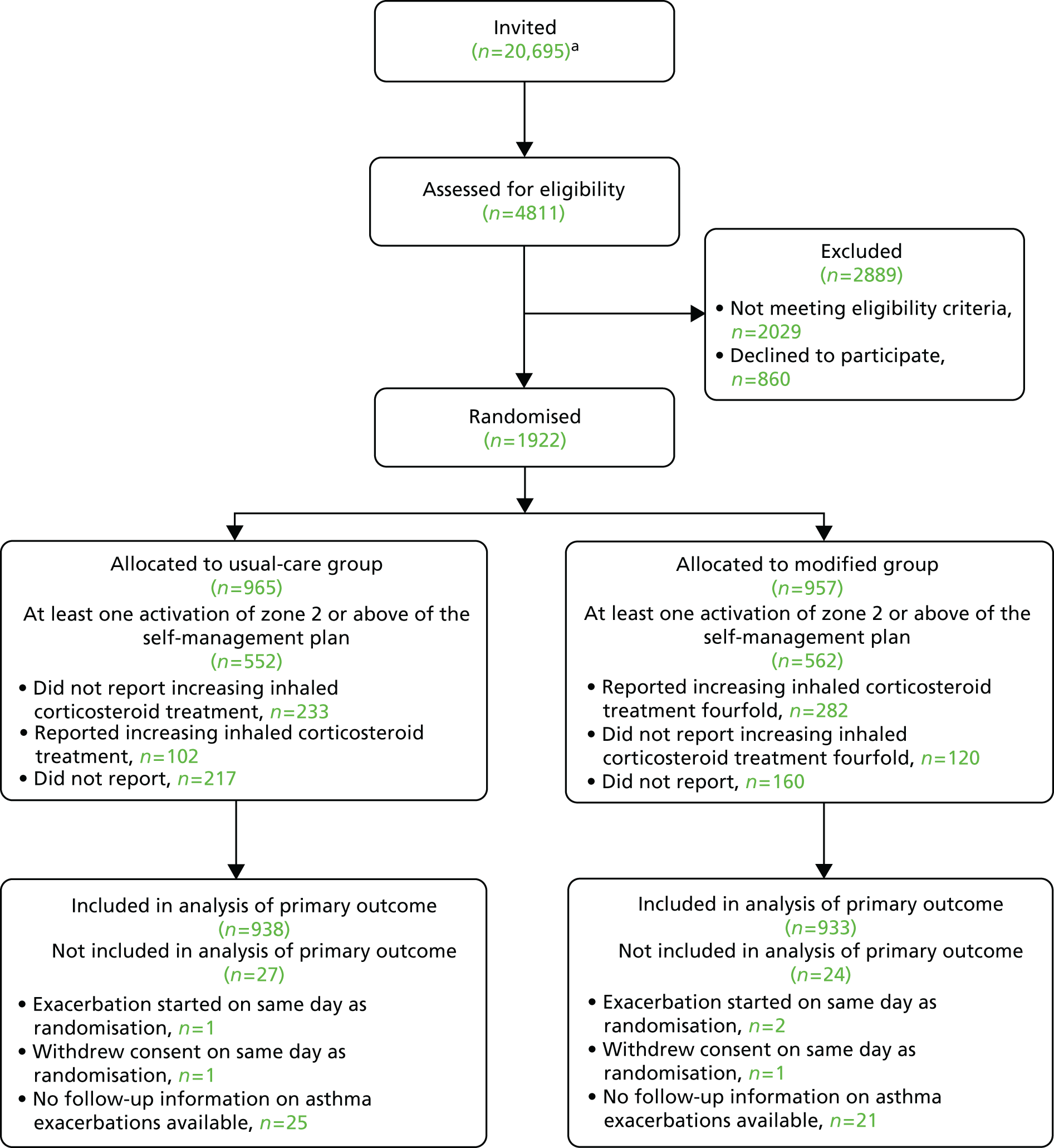
| Reason for not meeting eligibility criteria | Number of participants |
|---|---|
| No systemic corticosteroids in the past year | 594 |
| On maintenance corticosteroids | 340 |
| Exacerbated in the last 4 weeks | 47 |
| SMART regimen | 365 |
| COPD | 577 |
| Other | 106 |
| Pregnant/breastfeeding | 20 |
| Mental/learning difficulties | 39 |
| Unlicensed dose of inhaled steroid | 11 |
| On Relvar® Ellipta (GlaxoSmithKline UK Ltd, Brentford, UK) | 5 |
| No reason given | 31 |
Initially, recruitment was slow, and after 6 months was only 25% of the target as a result of a combination of delays with contracting and a higher than expected rate of non-eligibility. Following review of site recruitment and recruitment trends in primary and secondary care, focus was shifted to concentrate on primary care RISs. Although the RISs were initially achieving target after around 6 months, the pool of potential participants was exhausted and recruitment dropped. In February 2014, FAST’s Trial Management Group met the trial funders to agree on a strategy that new RISs should be opened to replace sites as they became inactive. Following the meeting, an ambitious initiation plan was undertaken, with 54 sites opening between March and December 2014 and a further 107 sites opening in 2015.
In order to meet recruitment targets the NIHR HTA programme agreed to an 11-month recruitment extension (January 2015). The trial completed recruitment on 31 January 2016 with 1922 randomised participants and 196 RISs and 11 secondary care sites initiated throughout the recruitment period. At the outset of the study it was envisaged that approximately 80% of participants would be recruited from primary care (i.e. PICs). In total, 81% of participants were recruited from primary care (63% RISs and 18% PICs) and 19% from secondary care sites. The number of participants randomised to each group was well balanced across regional randomisation centres (Table 8).
| Region | Intervention arm, n (% of total) | Total (N = 1922), n (% of total) | |
|---|---|---|---|
| Usual care (N = 965) | Modified (N = 957) | ||
| 1 | 133 (14) | 132 (14) | 265 (14) |
| 2 | 45 (5) | 45 (5) | 90 (5) |
| 3 | 31 (3) | 31 (3) | 62 (3) |
| 4 | 9 (1) | 9 (1) | 18 (1) |
| 5 | 77 (8) | 76 (8) | 153 (8) |
| 6 | 92 (10) | 93 (10) | 185 (10) |
| 7 | 99 (10) | 100 (10) | 199 (10) |
| 8 | 179 (19) | 176 (18) | 355 (18) |
| 9 | 22 (2) | 23 (2) | 45 (2) |
| 10 | 8 (1) | 8 (1) | 16 (1) |
| 11 | 5 (1) | 6 (1) | 11 (1) |
| 12a | 5 (1) | 5 (1) | 10 (1) |
| 13 | 38 (4) | 35 (4) | 73 (4) |
| 14 | 25 (3) | 25 (3) | 50 (3) |
| 15 | 103 (11) | 104 (11) | 207 (11) |
| 16 | 13 (1) | 13 (1) | 26 (1) |
| 17 and 18 | 12 (1) | 10 (1) | 22 (1) |
| 19 | 37 (4) | 35 (4) | 72 (4) |
| 20 | 29 (3) | 28 (3) | 57 (3) |
| 21 | 3 (< 0.5) | 3 (< 0.5) | 6 (< 0.5) |
Baseline data
Participants
The characteristics of the participants at baseline were well balanced between the usual-care and modified self-management groups (Table 9).
| Characteristic | Intervention arm | Total (N = 1922) | |
|---|---|---|---|
| Usual care (N = 965) | Modified (N = 957) | ||
| Age (years) | |||
| Mean (SD) | 56.7 (15.2) | 56.2 (15.5) | 56.5 (15.3) |
| Min., max. | 19, 94 | 16, 91 | 16, 94 |
| Sex, n (% of total) | |||
| Male | 316 (33) | 301 (31) | 617 (32) |
| Female | 649 (67) | 656 (69) | 1305 (68) |
| Recruited from, n (% of total) | |||
| Primary care | 774 (80) | 785 (82) | 1559 (81) |
| Secondary care | 191 (20) | 172 (18) | 363 (19) |
| PEF (l/minute) at screening | |||
| Mean (SD) | 381.1 (112.2) | 386.9 (110.8) | 384 (111.5) |
| Type of inhaler, n (% of total) | |||
| Corticosteroid | 303 (31) | 275 (29) | 578 (30) |
| Combination | 662 (69) | 682 (71) | 1344 (70) |
| Maintenance dose of inhaled corticosteroids (mcg/day of BDP) | |||
| Median (25th, 75th centiles) | 800 (400, 1000) | 800 (400, 1000) | 800 (400, 1000) |
| Min., max. | 100, 4000 | 80, 4000 | 80, 4000 |
| Maintenance dose of inhaled corticosteroids (used in randomisation stratification), n (% of total) | |||
| Low (≤ 1000 mcg/day of BDP) | 752 (78) | 743 (78) | 1495 (78) |
| High (> 1000 mcg/day of BDP) | 213 (22) | 214 (22) | 427 (22) |
| BTS step of asthma treatment, n (% of total) | |||
| Step 2 – regular preventer therapy | 259 (27) | 221 (23) | 480 (25) |
| Step 3 – initial add-on therapy | 363 (38) | 345 (36) | 708 (37) |
| Step 4 – persistent poor control | 330 (34) | 374 (39) | 704 (37) |
| Step 5 – omalizumab | 3 (0.3) | 2 (0.2) | 5 (0.3) |
| Not knowna | 10 (1) | 15 (2) | 25 (1) |
| Smoking status, n (% of total) | |||
| Never | 552 (57) | 564 (59%) | 1116 (58) |
| Current | 66 (7) | 59 (6) | 125 (7) |
| Former | 347 (36) | 334 (35) | 681 (35) |
| Pack-years for current or former smokers | |||
| n | 413 | 393 | 806 |
| Mean (SD) | 13.9 (16.1) | 12.3 (14.5) | 13.1 (15.4) |
| Mini AQLQ overall scoreb | |||
| n | 959 | 944 | 1903 |
| Mean (SD) | 5 (1.2) | 5.1 (1.2) | 5.1 (1.2) |
The mean age of participants was 57 years [standard deviation (SD) 15 years] and 1305 (68%) were female. Of those enrolled, 1344 (70%) were prescribed combination inhalers, and 1495 (78%), the majority of participants, were on a low dose of maintenance steroid [≤ 1000 mcg/day of beclometasone dipropionate (BDP)]. Overall, 1125 participants (59%) reported not taking any other respiratory medication at randomisation (Table 10).
| Medication | Intervention arm, n (% of total) | Total (N = 1922), n (% of total) | |
|---|---|---|---|
| Usual care (N = 965) | Modified (N = 957) | ||
| None | 572 (59) | 553 (58) | 1125 (59) |
| At least one medication reported | 360 (37) | 363 (38) | 723 (38) |
| Unknown | 33 (3) | 41 (4) | 74 (4) |
| Preventer medication | |||
| Theophylline/aminophylline | 16 (2) | 21 (2) | 37 (2) |
| Sodium cromoglycate | – | 1 (< 0.5) | 1 (< 0.5) |
| Omalizumab | 3 (< 0.5) | 2 (< 0.5) | 5 (< 0.5) |
| Reliever medication | |||
| Long-acting beta agonist | 12 (1) | 14 (1) | 26 (1) |
| Long-acting muscarinic antagonist | 38 (4) | 39 (4) | 77 (4) |
| Leukotriene antagonist | 81 (8) | 94 (10) | 175 (9) |
| Nebulised beta agonist | 3 (< 0.5) | 1 (< 0.5) | 4 (< 0.5) |
| Nebulised anticholinergic | 7 (1) | 8 (1) | 15 (1) |
| Short-acting beta agonist (not nebulised) | 311 (32) | 312 (33) | 623 (32) |
| Other respiratory medication | |||
| Antibiotics | 6 (1) | 5 (1) | 11 (1) |
| Oral/inhaled corticosteroids | 99 (10) | 112 (12) | 211 (11) |
| Other | 42 (4) | 47 (5) | 89 (5) |
Follow-up
Scheduled follow-up visits
Attendance by participants at the scheduled visits was good. In the usual-care group, 772 (80%) participants attended the 6-month visit, decreasing to 700 (73%) at the 12-month visit. Attendance was similar in the modified self-management group, with 773 (81%) participants attending the 6-month visit and 679 (71%) attending the 12-month visit.
A total of 67 (7%) participants in the usual-care group and 80 (8%) in the modified self-management group withdrew consent from the trial, 15% of participants were lost to follow-up and 5% of participants in each group were marked as not completing the trial for other reasons (Table 11). The other reasons reported for participants not attending the 12-month visit included moving area or GP surgery, being advised to switch to a Single inhaler Maintenance And Reliever Therapy (SMART) regimen, and a variation or stopping in inhaled steroid dose.
| Attendance | Intervention arm, n (% of total) | |
|---|---|---|
| Usual care (N = 965) | Modified (N = 957) | |
| Attended | 700 (73) | 679 (71) |
| Did not attend | 260 (27) | 274 (29) |
| No information | 5 (0.5) | 4 (0.4) |
| Reason if did not attend 12-month visit | ||
| Lost to follow-up | 147 (15) | 145 (15) |
| Withdrawal of consent | 67 (7) | 80 (8) |
| Other | 44 (5) | 47 (5) |
| Deatha | 1 (0.1) | 1 (0.1) |
| No information | 1 (0.1) | 1 (0.1) |
Activation to zone 2 or above of the self-management plan
A total of 1114 participants (58%) activated zone 2 or above of the self-management plan in the year after randomisation, with similar numbers in the two groups (552 in the usual-care group and 562 in the modified self-management group). The baseline characteristics of the participants who activated zone 2 or above were similar in the two groups (Appendix 6). However, a greater percentage of participants completed a diary card and attended the post-activation visit for the first activation of zone 2 in the modified self-management group than in the usual-care group (Table 12).
| Activation | Intervention arm, n (% of total) | |
|---|---|---|
| Usual care (N = 552) | Modified (N = 562) | |
| Source of date of first activation | ||
| Diary card | 328 (59) | 400 (71) |
| Post-activation visit | 2 (< 0.5) | 3 (1) |
| Health-care consultation or oral corticosteroid use for asthma | 203 (37) | 137 (24) |
| Date not known (unreported activationa) | 19 (3) | 22 (4) |
| Post-activation visit attended for first activation to zone 2 or above | 263 (48) | 341 (61) |
| Diary card completed for first activation to zone 2 or aboveb | 334 (61) | 403 (72) |
Nurse-assessed adherence to the allocated intervention
Adherence to the allocated self-management plan was rated by research nurses using either information entered in diaries or, if the diary was not completed, participant recall of this information at post-activation visits. The ratings were based on the criteria specified in the protocol and are shown in Table 13.
| Rating | Intervention arm | |
|---|---|---|
| Usual care | Modified | |
| Poor | Fourfold increase in maintenance dose | No or minimal change in medication |
| Moderate | Increase in maintenance dose, but less than fourfold | Change, but as fourfold or as instructed |
| Good | No change in inhaled corticosteroid dose | Fourfold change and followed instructions |
Adherence to the self-management plan was assessed as good (i.e. fourfold increase in corticosteroid dose as per instructions) for the first activation of zone 2 or above for 282 (50%) of the participants in the modified self-management group (Table 14). In the usual-care group, adherence for 15 participants (3%) was assessed as poor (i.e. used a fourfold increase in maintenance corticosteroid dose during first activation to zone 2) (Table 14).
| Adherence | Intervention arm, n (% of total) | |
|---|---|---|
| Usual care (N = 552) | Modified (N = 562) | |
| Poor | 15 (3) | 31 (6) |
| Moderate | 87 (16) | 89 (16) |
| Good | 233 (42) | 282 (50) |
| Not known | 217 (39) | 160 (28) |
Adherence information was unknown for 377 participants: 331 participants, first activation to zone 2 or above was an asthma exacerbation (health-care consultation or oral corticosteroid use for asthma; Table 12), 41 participants had an unreported activation and five participants had diary data but no nurse assessment of adherence entered on to the database. Adherence information is unknown for a greater percentage of participants who activated zone 2 or above in the usual-care group than in the modified self-management group (39% and 28%, respectively) (Table 14), as the percentage of participants completing diary cards was lower in the usual-care group.
Inclusion in the analysis of the primary outcome
There were 938 participants (97%) in the usual-care group and 933 participants (97%) in the modified self-management group included in the primary analysis of the primary outcome (Table 15). Of these, 134 and 158 participants in the usual-care and modified self-management group, respectively, were censored for the primary outcome as they did not have an exacerbation of asthma or complete the 12-month visit (Table 15).
| Primary outcome status | Intervention arm, n (% of total) | |
|---|---|---|
| Usual care (N = 965) | Modified (N = 957) | |
| Unknown information about oral corticosteroid use or unscheduled health-care consultations for asthma after randomisation – not included in analysis of primary outcome | 26 (3) | 22 (2) |
| Participant had asthma exacerbation and/or completed the 12-month follow-up visit | 805 (83) | 777 (81) |
| Exacerbation started on day of randomisation – not included in analysis of primary outcomea | 1 | 2 |
| Participant did not have asthma exacerbation and did not complete the 12-month follow-up visit – censored for primary outcome | 134 (14) | 158 (17) |
| Censored at | ||
| Date of death | 1 | 0 |
| Date withdrew consent | 32 | 34 |
| Date left surgery | 3 | 5 |
| Scheduled 12-month visit date | 91 | 107 |
| 6-month visit date | 6 | 10 |
| Post-activation visit date | 1 | 2 |
No information was collected after randomisation for 26 participants (3%) in the usual-care group and 22 (2%) in the modified self-management group (Table 15). Therefore, these participants could not be included in the analysis of the primary outcome.
Three participants had asthma exacerbations that started on the same day as randomisation. These participants are not included in the analysis of the primary outcomes, but are included in other analyses relating to exacerbations of asthma.
Primary and secondary outcomes
Primary outcome: time to first asthma exacerbation
Primary analysis
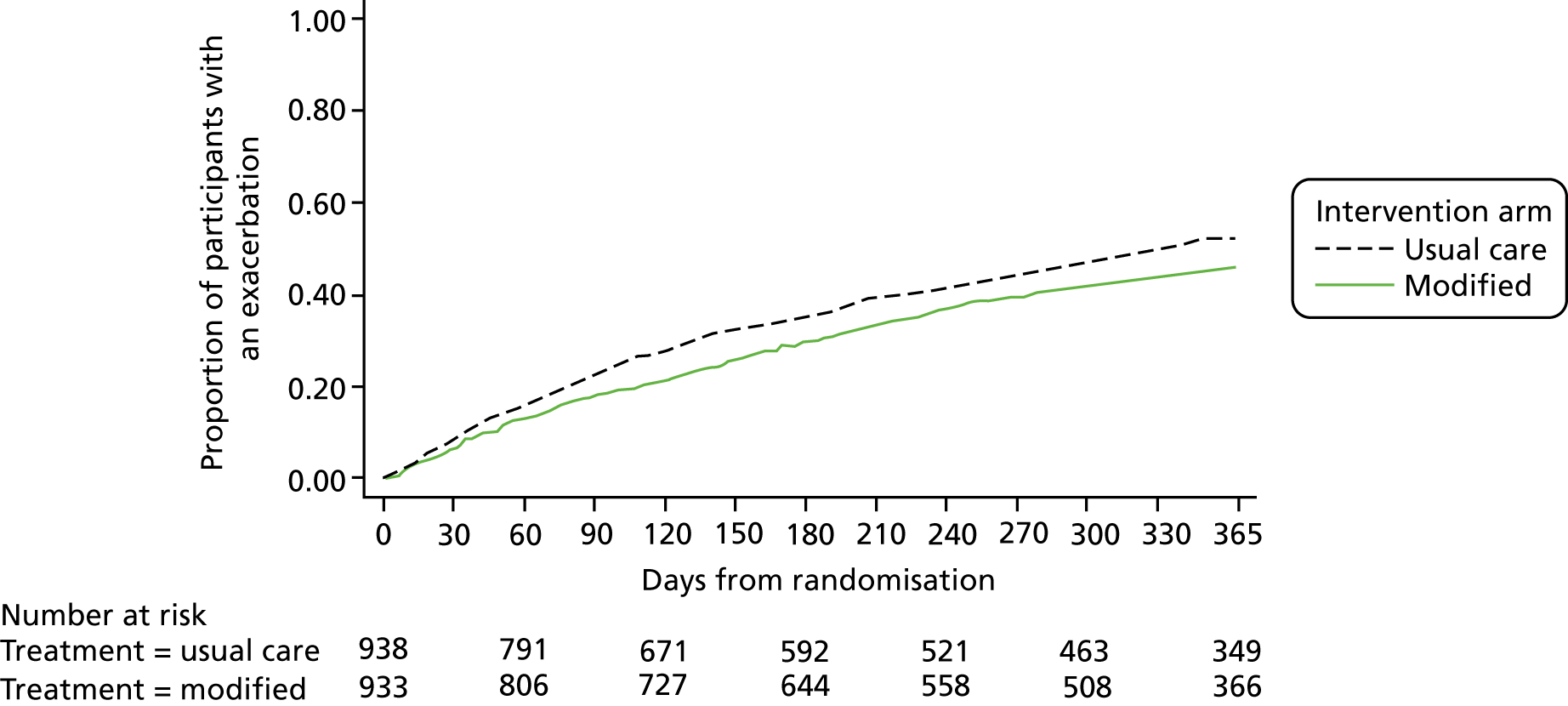
The number of participants having an exacerbation of asthma in the year after randomisation was 484 (51.6%) in the usual-care group and 420 (45.0%) in the modified self-management group (Figure 4). Kaplan–Meier curves for the time to first asthma exacerbation are shown in Figure 4. The adjusted hazard ratio for the time to first asthma exacerbation in the modified self-management group compared with the usual-care group was 0.81 (95% CI 0.71 to 0.92; p = 0.002; Table 16).
FIGURE 4.
Kaplan–Meier curves for the time to first asthma exacerbation by allocated group.
| Primary outcome | Intervention arm | Adjusted hazard ratioa (95% CI); p-value | |
|---|---|---|---|
| Usual care (N = 938) | Modified (N = 933) | ||
| Total number (% of total) with exacerbation | 484 (51.6%) | 420 (45.0%) | |
| Total follow-up time (person-years) | 610.3 | 649.8 | |
| Rate (per person-years) | 0.79 | 0.65 | 0.81 (0.71 to 0.92); p = 0.002 |
Secondary analysis for the primary outcome
Additional adjustment
The hazard ratio for the time to first asthma exacerbation in the modified self-management group compared with the usual-care group with additional adjustment for age, sex and PEF at screening was 0.80 (95% CI 0.71 to 0.92; p = 0.001).
Varying censoring time
If participants in both groups who did not complete the 12-month visit and did not exacerbate were censored at their date of last contact (either the 6-month visit date, last post-activation visit date or last completed diary card date), instead of as described in Table 15, the adjusted hazard ratio was 0.81 (95% CI 0.71 to 0.95; usual care, n = 860, and modified, n = 850).
If participants in the usual-care group were censored, as described in Table 15, and participants in the modified self-management group were censored at their date of last contact (i.e. favouring the usual-care group), the adjusted hazard ratio was 0.93 (95% CI 0.82 to 1.06; usual care, n = 938, and modified, n = 850).
Per-protocol analysis
Just over 50% of participants were included in the per-protocol population: 491 (51%) participants in the usual-care group and 524 (55%) participants in the modified self-management group (Table 17). A greater number of participants included in the per-protocol population in the modified self-management group had an activation to zone 2 or above.
| Inclusion | Intervention arm, n (% of total) | |
|---|---|---|
| Usual care (N = 965) | Modified (N = 957) | |
| Included in per-protocol population | 491 (51%) | 524 (55%) |
| Good adherence during the first activation to zone 2 or abovea | 233 | 281 |
| Did not activate zone 2 or aboveb | 258 | 243 |
For the participants included in the per-protocol population, 197 in the usual-care group and 189 in the modified self-management group had an asthma exacerbation, with an adjusted hazard ratio for the time to first asthma exacerbation of 0.83 (95% CI 0.68 to 1.01; p = 0.06).
Subgroup analysis for the primary outcome
There was no evidence of a difference in the hazard ratio for time to asthma exacerbation in the modified self-management group compared with the usual-care group according to smoking status or dose of maintenance inhaled steroid dose at baseline (Table 18).
| Subgroup | Adjusted subgroup specific hazard ratio (95% CI) | Adjusted interaction effect (95% CI) | p-value for interaction effect |
|---|---|---|---|
| Smoking status | |||
| Never smoked | 0.78 (0.66 to 0.93) | 0.80 | |
| Current | 0.92 (0.55 to 1.54) | 1.18 (0.68 to 2.03) | |
| Former | 0.83 (0.67 to 1.04) | 1.07 (0.80 to 1.41) | |
| Dose of maintenance inhaled corticosteroids | |||
| Low | 0.84 (0.72 to 0.98) | 0.37 | |
| High | 0.73 (0.57 to 0.94) | 0.87 (0.65 to 1.17) | |
Secondary outcomes
Unscheduled health-care consultations and the use of systemic corticosteroids for asthma
Time to first use of systemic corticosteroids for asthma and time to first unscheduled health consultation for asthma
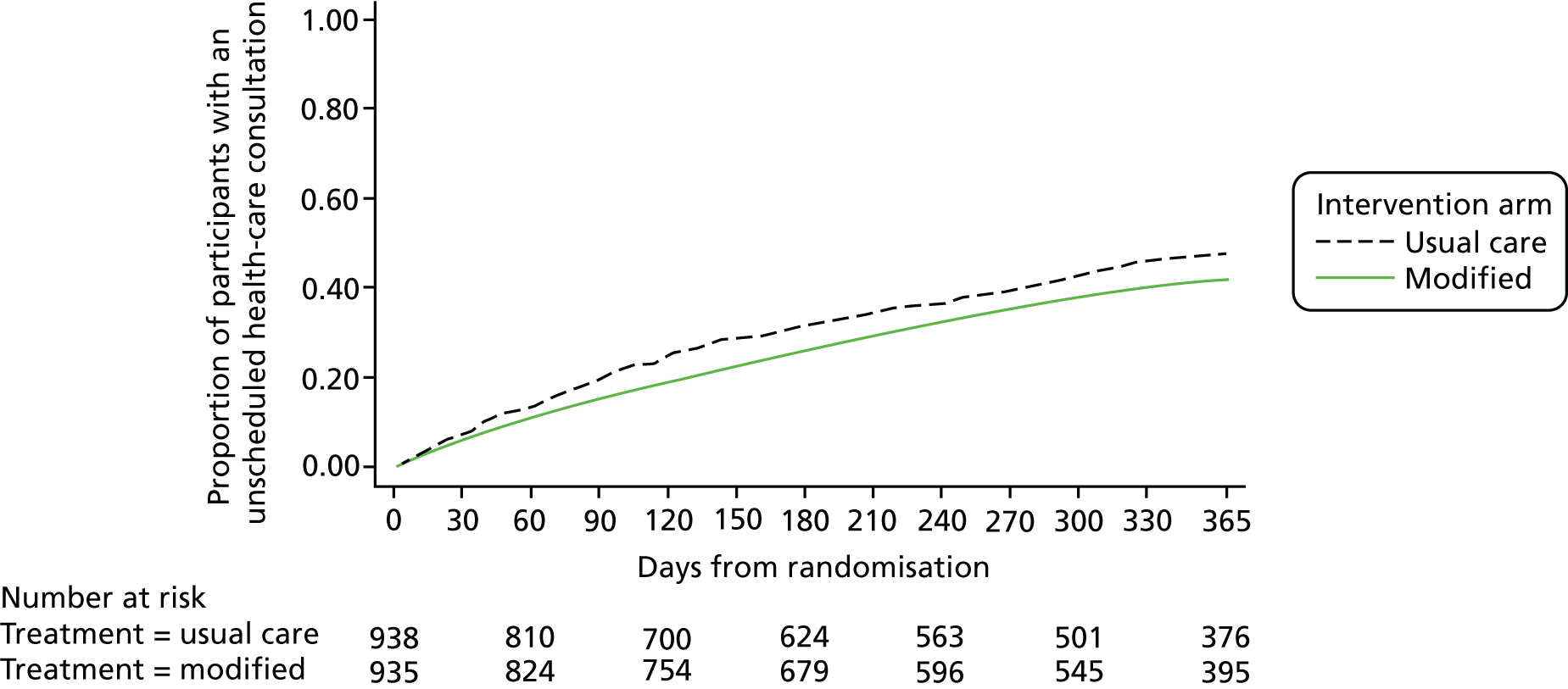
Figure 5 shows the Kaplan–Meier curves for the time to first use of systemic corticosteroids and the time to first unscheduled health-care consultation is shown in Figure 6. The adjusted hazards ratios are 0.76 (95% CI 0.65 to 0.88; p < 0.001) for the use of systemic corticosteroids and 0.82 (95% CI 0.70 to 0.92; p = 0.002) for unscheduled health-care consultations.
FIGURE 5.
Kaplan–Meier curves for the time to first requiring systemic corticosteroids for asthma by allocated group.
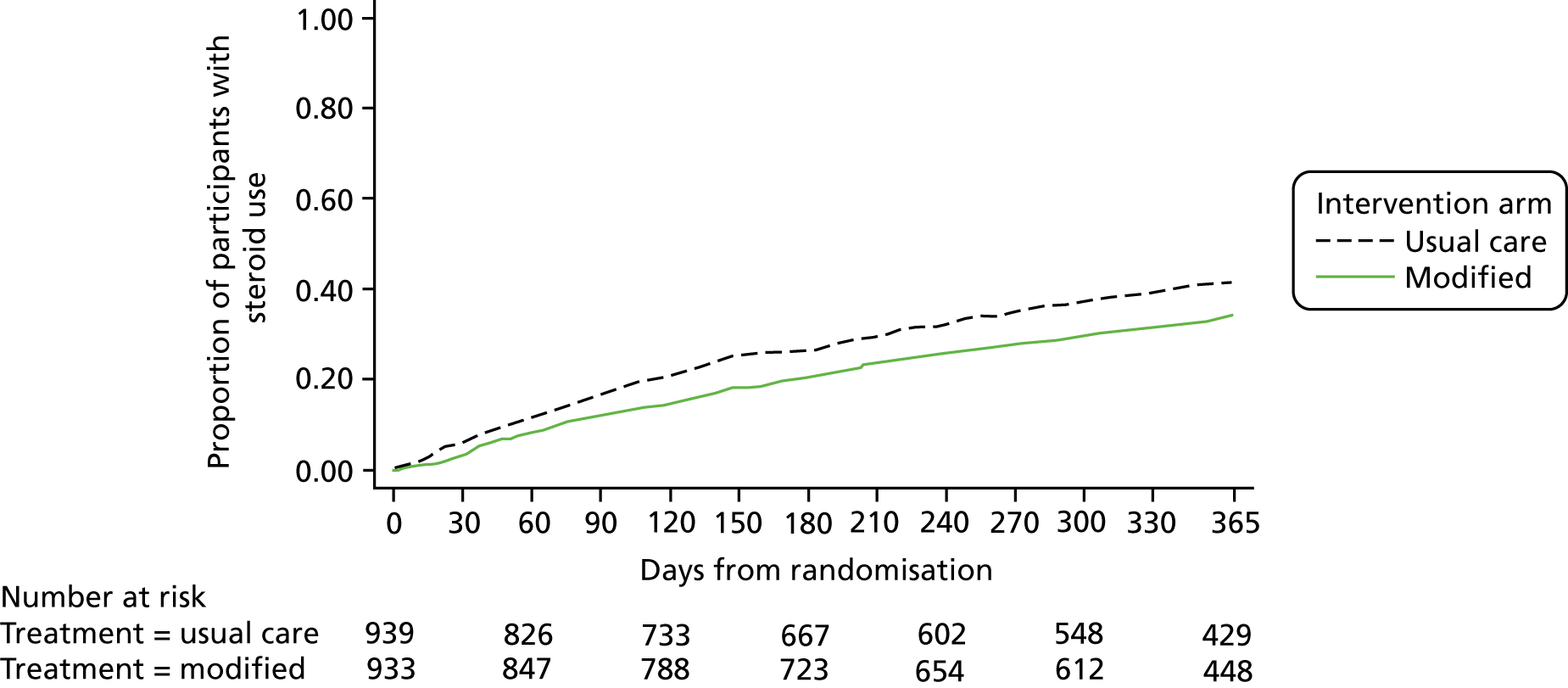
FIGURE 6.
Kaplan–Meier curves for the time to first unscheduled health-care consultation for asthma by allocated group.
Total number of courses of systemic corticosteroids for asthma, unscheduled health consultations and exacerbations for asthma
Table 19 shows that the number of participants using systemic corticosteroids, having an unscheduled health-care consultation and an exacerbation (systemic corticosteroids or unscheduled health-care consultation), was lower in the modified self-management group than in the usual-care group.
| Secondary outcome | Intervention arm | Adjusted intervention effecta (95% CI) | ||
|---|---|---|---|---|
| Usual care (N = 939) | Modified (N = 935) | |||
| Use of systemic corticosteroids | ||||
| Any courses, n (% of total) | ||||
| No | 552 (59) | 614 (66) | Risk difference –7.0% (–11.3% to –2.7%) | Risk ratio 0.83 (0.74 to 0.93) |
| Yes | 377 (40) | 311 (33) | ||
| Not knownb | 10 (1) | 10 (1) | ||
| Total number of courses | n = 929 | n = 925 | Incidence rate ratio 0.82 (0.70 to 0.96) | |
| Mean (SD) | 0.61 (0.93) | 0.5 (0.86) | ||
| 1, n (% of total) | 254 (27) | 212 (23) | ||
| 2, n (% of total) | 82 (9) | 68 (7) | ||
| 3 or more, n (% of total) | 41 (4) | 31 (3) | ||
| Any unscheduled health-care consultations | ||||
| Any, n (% of total) | ||||
| No | 490 (52) | 543 (58) | Risk difference –6.8% (–11.1% to –2.4%) | Risk ratio 0.86 (0.78 to 0.95) |
| Yes | 442 (47) | 379 (41) | ||
| Not knownb | 7 (1) | 13 (1) | ||
| Total number of unscheduled health-care consultations | n = 932 | n = 922 | Incidence rate ratio 0.86 (0.75 to 0.99) | |
| Mean (SD) | 0.84 (1.23) | 0.73 (1.19) | ||
| 1, n (% of total) | 261 (28) | 224 (24) | ||
| 2, n (% of total) | 96 (10) | 83 (9) | ||
| 3 or more, n (% of total) | 85 (9) | 72 (8) | ||
| Exacerbation: use of systemic corticosteroids and/or unscheduled health-care consultation for asthma | ||||
| Any exacerbations, n (% of total) | ||||
| No | 445 (47) | 499 (53) | Risk difference –6.7% (–11.2% to –2.3%) | Risk ratio 0.87 (0.80 to 0.95) |
| Yes | 485 (52) | 422 (45) | ||
| Not knownb | 9 (1) | 14 (1) | ||
| Total number of exacerbations | n = 930 | n = 921 | Incidence rate ratio 0.88 (0.77 to 1.01) | |
| Mean (SD) | 0.95 (1.29) | 0.84 (1.26) | ||
| 1, n (% of total) | 270 (29) | 235 (25) | ||
| 2, n (% of total) | 119 (13) | 97 (10) | ||
| 3 or more, n (% of total) | 96 (10) | 90 (10) | ||
Similarly, the total number of courses of systemic corticosteroids, unscheduled health-care consultations and exacerbations per participant was lower in the modified self-management group than in the usual-care group (Table 19).
Area under the percentage baseline morning peak flow curve over 2 weeks from the point of activating zone 2 (or above) of the asthma self-management plan
A higher percentage of participants who activated zone 2 or above of the self-management plan in the modified self-management group were able to be included in the analysis of the area under the PEF curve analysis (i.e. 54% compared with 41% in the usual-care group) as a result of a higher percentage of participants completing a diary card in the modified self-management group (Table 20). In both groups, however, there are high numbers of missing data for this analysis (Table 20). This is mainly due to participants not completing diaries for the first activation to zone 2 or not recording PEF values on or after day 10.
| Inclusion | Intervention arm, n (% of total) | |
|---|---|---|
| Usual care (N = 552); at least one activation to zone 2 or above | Modified (N = 562); at least one activation to zone 2 or above | |
| Included in analysis | 226 (41) | 303 (54) |
| Not included in analysis | 326 (59) | 259 (46) |
| Reason not included | ||
| No diary for first activation to zone 2 or above | 224 | 162 |
| No PEF values recorded in diary | 24 | 8 |
| PEF not recorded on day 1 | 17 | 17 |
| No PEF recorded on or after day 10 | 60 | 72 |
| PEF recorded on day 1 and 10, but no days in-between | 1 | – |
For the participants who did record sufficient PEF information on their diary cards, the area under the percentage baseline PEF curve in the 2 weeks from the point of first activating zone 2 or above of the self-management plan was slightly higher in the modified self-management group than in the usual-care group (Table 21).
| Analysis | Intervention arm | Adjusted difference in meansa (95% CI) | |
|---|---|---|---|
| Usual care (N = 552); at least one activation to zone 2 or above | Modified (N = 562); at least one activation to zone 2 or above | ||
| Analysis 1 | n = 226 | n = 303 | |
| Mean (SD) | 1130 (155) | 1166 (142) | 38 (13 to 62) |
| Median (25th, 75th centiles) | 1146 (1025, 1238) | 1165 (1073, 1258) | |
| Min., max. | 687, 1669 | 558, 1781 | |
| Analysis 2 | n = 197 | n = 269 | |
| Mean (SD) | 1133 (152) | 1164 (136) | 32 (7 to 59) |
| Median (25th, 75th centiles) | 1151 (1030, 1242) | 1158 (1069, 1249) | |
| Min., max. | 687, 1669 | 805, 1781 | |
Change in Mini Asthma Quality of Life Questionnaire 2 weeks after activating zone 2 (or above) of the self-management plan
The percentage of participants who activated zone 2 (or above) of the self-management plan was higher in the modified self-management group than in the usual-care group and who could be included in the analysis of change in the Mini AQLQ score (i.e. 51% compared with 39% in the usual-care group) because of a higher percentage of participants in the former attended the post-activation visit in the modified self-management group (Table 22). In both groups, however, there are substantial numbers of missing data for this analysis (Table 22).
| Mini AQLQ status | Intervention arm, n (% of total) | |
|---|---|---|
| Usual care (N = 552); at least one activation to zone 2 or above | Modified (N = 562); at least one activation to zone 2 or above | |
| Mini AQLQ within 28 days | 216 (39) | 284 (51) |
| More than two items missed, score not calculated | 0 | 1 |
| Mini AQLQ completed after 28 days | 36 (7) | 40 (7) |
| Mini AQLQ not done | 29 (5) | 38 (7) |
| Post-activation visit not attended | 60 (11) | 42 (7) |
| No post-activation record | 211 (38) | 158 (28) |
For the participants who did complete the Mini AQLQ within 28 days of the first activation to zone 2, the Mini AQLQ scores were slightly higher in the modified self-management group than in the usual-care group (Table 23).
| Intervention arm | Baseline | At post-activation visit following first activation to zone 2 (or above) | Adjusted difference in meansa (95% CI) |
|---|---|---|---|
| Usual care | |||
| n | 216 | 216 | |
| Mean (SD) | 5.0 (1.1) | 3.9 (1.3) | |
| Modified | |||
| n | 282 | 283 | |
| Mean (SD) | 5.1 (1.2) | 4.2 (1.2) | 0.2 (0.03 to 0.46) |
Cumulative dose of inhaled and systemic steroids used in the 12 months after randomisation
Among participants completing the 12-month follow-up visit, the mean total dose of inhaled corticosteroids used in the 12 months after randomisation was slightly higher in the modified self-management group than in the usual-care group (Table 24). The mean total dose of systemic corticosteroids taken in this time was slightly lower in the modified self-management group than in the usual-care group (Table 24) among participants completing the 12-month follow-up visit. The mean total dose of systemic corticosteroids was similar in the two groups among participants who took systemic corticosteroids in the 12 months after randomisation (Table 24).
| Cumulative dose of corticosteroid | Intervention arm, 12-month visit completed | |
|---|---|---|
| Usual care (N = 700) | Modified (N = 679) | |
| Total dose of inhaled corticosteroids (mg) | ||
| Mean (SD) | 328.5 (211.8) | 385.2 (265.5) |
| Median (25th, 75th centiles) | 292 (146, 365) | 304 (178.4, 444.5) |
| Min., max. | 36.5, 1414 | 29.2, 1592 |
| Total dose of systemic corticosteroids (mg) | ||
| Mean (SD) | 151.3 (256.9) | 120.9 (220.8) |
| Median (25th, 75th centiles) | 0 (0, 210) | 0 (0, 200) |
| Min., max. | 0, 2120 | 0, 1770 |
| Total dose of systemic corticosteroids (mg) in participants who took systemic corticosteroids | ||
| n | 306 | 247 |
| Mean (SD) | 346.1 (289.3) | 332.3 (252.6) |
| Median (25th, 75th centiles) | 240 (150, 400) | 210 (180, 400) |
| Min., max. | 25, 2120 | 15, 1770 |
Serious adverse events
Serious adverse events were reported during the 14-day period following activation of zone 2 (or above) of the self-management plan. In addition, diagnoses of pneumonia up to 1 month after the 14-day activation period of zone 2 or above were also considered to be SAEs.
A total of 22 (4%) participants in the usual-care group and 11 (2%) participants in the modified self-management group who activated zone 2 or above had at least one SAE (Table 25).
| SAE summary | Intervention arm | |
|---|---|---|
| Usual care (N = 552); at least one activation to zone 2 or above | Modified (N = 562); at least one activation to zone 2 or above | |
| Number of participants with at least one SAE, n (% of total) | 22 (4%) | 11 (2%) |
| Number of SAEs per participant, median (min., max.) | 1 (1, 4) | 1 (1, 1) |
| Total number of SAEs | 32 | 11 |
| Serious criterion (not mutually exclusive), n | ||
| Fatal | 0 | 1 |
| Life-threatening | 0 | 1 |
| Hospitalisation or prolongation | 32 | 10 |
| Persistent or significant disability/incapacity | – | – |
| Congenital anomaly or birth defect | – | – |
| Other (ongoing symptoms) | 0 | 1 |
| Classification, n | ||
| Serious, not related to trial treatment | 31 | 10 |
| Serious, possibly related to trial treatment | 0 | 1 |
| Not reportable per protocol | 1 | 0 |
| SAE description (MedDRA-preferred term), n | ||
| Asthma | 18 | 3 |
| Pneumonia | 1 | 5 |
| Lower respiratory tract infection | 3 | – |
| Influenza | 1 | 1 |
| Lobar pneumonia | 2 | – |
| Oesophageal candidiasis | 2 | – |
| Acute myocardial infarction | – | 1 |
| Allergy to animal | 1 | – |
| Atelectasis | 1 | – |
| Cardiac failure congestive | – | 1 |
| Gastroenteritis viral | 1 | – |
| Pneumonia bacterial | 1 | – |
| Renal impairment | 1 | – |
Eighteen of the 32 SAEs in the usual-care group were attributable to hospitalisations for asthma, compared with 3 of the 11 SAEs in the modified self-management group.
There were eight events in the usual-care group and six events in the modified self-management group relating to pneumonia, lower respiratory tract infections and influenza (Table 25).
One participant in the modified self-management group died after severe pneumonia. This event was not classified as related to trial treatment.
One of the 11 SAEs in the modified self-management group was classified as possibly related to trial treatment. This participant had pneumonia (outside the 14-day period following activation to zone 2) and had fourfolded their usual medication (4000 µg) on two occasions (once for 7 days and one for 6 days) prior to the event.
Non-serious adverse events
Non-serious AEs were reported during the 14 days following activation of zone 2 of the self-management plan. Only adverse events that are known side-effects of inhaled corticosteroids, such as oral candidiasis (i.e. thrush) and dysphonia (i.e. hoarseness), were intended to be collected from discussion with the participant and information from the diary card.
Ten (2%) participants in the usual-care group and 41 (7%) participants in the modified self-management group who activated zone 2 or above had at least one non-serious AE (Table 26). Of the 56 non-serious AEs in the modified self-management group, 44 were classified as definitely or probably related to inhaled corticosteroids, compared with 6 of the 13 non-serious adverse events in the usual-care group. The breakdown of the type of events is shown in Table 26.
| Non-serious AE summary | Intervention arm | |
|---|---|---|
| Usual care (N = 552); at least one activation to zone 2 or above | Modified (N = 562); at least one activation to zone 2 or above | |
| Number of participants with at least one non-serious AE, n (% of total) | 10 (2%) | 41 (7%) |
| Number of non-serious AEs per participant, median (min., max.) | 1 (1, 2) | 1 (1, 4) |
| Total number of non-serious AEs | 13 | 56 |
| Relationship to inhaled steroids, n | ||
| Definitely | 3 | 17 |
| Probably | 3 | 27 |
| Possibly | 2 | 8 |
| Not related | 5 | 4 |
| Severity, n | – | – |
| Mild | 7 | 31 |
| Moderate | 6 | 24 |
| Severe | 0 | 1 |
| AE description (MedDRA-preferred term), n | ||
| Oral candidiasis | 7 | 19 |
| Dysphonia | 2 | 17 |
| Asthma | 2 | 2 |
| Candidiasis | – | 4 |
| Oral pain | – | 4 |
| Oropharyngeal pain | – | 4 |
| Lower respiratory tract infection | 1 | 1 |
| Adverse drug reaction | – | 1 |
| Dry throat | – | 1 |
| Laryngitis | 1 | – |
| Mouth ulceration | – | 1 |
| Oral herpes | – | 1 |
| Pharyngitis | – | 1 |
Chapter 4 Health economics analysis
Introduction
The cost-effectiveness analysis (CEA) was conducted alongside the FAST to establish the value for money of temporarily quadrupling the dose of inhaled corticosteroid compared with usual care.
The objectives of the CEA were to:
-
identify the related costs associated with delivering the treatments
-
measure the participants’ use of respiratory-related health and social care services
-
compare the estimated mean cost per participant between the two intervention groups
-
estimate the health benefits of the trial interventions using QALYs calculated from the EQ-5D-3L questionnaire and the number of exacerbations prevented
-
compare the cost difference between the two intervention groups with difference in effectiveness and generate incremental cost-effectiveness ratios (ICERs)
-
test the uncertainty of the calculated ICERs, using the bootstrapping method, and generate cost-effectiveness acceptability curves (CEACs) to demonstrate the probability of the modified treatment being cost-effective over and above usual care.
Methods
Following the National Institute for Health and Care Excellence (NICE)’s Guide to the Methods of Technology Appraisal 2013,15 the analysis was conducted from the NHS/Personal Social Services perspective, with costs expressed in Great British pounds (£) for the financial year 2014–15. 15 A total of 1922 participants were randomised; however, 51 of these participants were excluded as they had no information from randomisation. The follow-up for the analysis was 6 months and 12 months from randomisation. All costs were inflated to 2014–15 price levels, where necessary, using the Hospital and Community Health Services pay and price inflation index. 16,17 No discount rate was applied as the follow-up was 12 months.
Treatment costs
A micro-costing exercise was conducted following the methods of technology appraisal recommended by NICE. 15 Treatment costs consisted of the total inhaled corticosteroid use during the 14-day activation period. This was calculated from the self-reported diary card and the number of extra corticosteroid inhalers provided. The number of puffs on each day was recorded on the diary card and these doses were rounded up to the nearest inhaler, depending on the number of doses for each particular inhaler, and costed using prescription cost analysis (PCA). 18 Where the information was missing from the diary card, the adherence, as assessed by the nurse, was used to estimate the total amount of inhaled corticosteroid use in the 14-day period. All participants in the modified self-management group who used a combination inhaler were given at least one extra corticosteroid inhaler, these were costed accordingly using the PCA. Any trial-related costs were not included in the analysis.
Unit costs of respiratory-related resource use
Respiratory-related health-care utilisation was collected for each participant alongside the trial. This was recorded using a comprehensive service-use questionnaire at 6 months and 12 months. Some questionnaires were not delivered at the correct follow-up time points, so a leeway of ± 2 months was used to decide those to be included and those to be considered missing. Wider societal costs were also collected, including travel time and productivity loss. However, as a result of the poor report rate, because of the burden of the questionnaire, only a tentative exploration of the potential impact of these costs could be made with the data available.
National unit costs were applied to the recorded resource use from a range of published sources. Table 27 shows the unit costs employed to generate a total respiratory-related resource cost per participant. The majority of the unit costs were from the Personal Social Services Research Unit’s Unit Costs of Health and Social Care 201517 and the Department of Health and Social Care (DHSC)’s NHS Reference Costs 2014/15. 19 Prescriptions were costed using the PCA and a weighted average unit cost for each drug was applied. 18
| Item | Unit cost (£) | Source |
|---|---|---|
| GP visit | 37.00 | Curtis17 |
| PN visit | 12.00 | Curtis17 |
| GP visit (at home) | 75.00 | Curtis17 |
| PN visit (at home) | 21.00 | Curtis17 |
| Other primary care costs incurred | 61.00 | Curtis17 |
| Walk-in centre | 54.00 | Curtis17 |
| Inpatient (visits) | 526.00 | DHSC’s NHS Reference Costs 2014–1519 |
| Outpatient (visits) | 169.00 | DHSC’s NHS Reference Costs 2014–1519 |
| A&E (visits) | 132.00 | DHSC’s NHS Reference Costs 2014–1519 |
| Emergency ambulance (journeys) | 231.00 | Curtis17 |
| Patient transport services (journeys) | 35.00 | DHSC’s NHS Reference Costs 2009–1020 |
| 111 call | 3.55 | Curtis;17 NHS employers’ Agenda for Change Pay Bands and Points from 1 April 2014;21 and NHS England’s NHS 111 Statistics – March 201522 |
Health outcome measures
In addition to the clinical outcomes collected in the statistical analyses, health benefits were measured in QALYs for the economic evaluation. QALYs are a generic measure of health that can be used to compare across all interventions, and are not constrained to just asthma-related treatment. QALYs were derived by calculating the area under the curve, using utility scores measured by the EQ-5D-3L questionnaire at baseline, and at the 6- and 12-month follow-ups. 23 As well as QALYs, the economic evaluation also presents cost-effectiveness results based on the total number of exacerbations per participant in the 12-month period.
Cost-effectiveness analysis
Incremental cost-effectiveness analysis was performed to combine the costs of the interventions with the outcomes. To generate an ICER, the mean difference in costs between the two intervention groups is divided by the mean difference in effect. The formula below is for the ICER, where Δ represents difference, E represents effects and C represents the cost of the intervention, and subscripts ‘I’ and ‘UC’ refer to intervention and usual care, respectively:24
An ICER is not needed if the treatment is both more clinically effective and less costly; in this instance the treatment is said to be dominant.
Handling uncertainty
The non-parametric bootstrap re-sampling technique was employed to explore the sensitivity of calculated ICERs. 25–28 Cost and outcome data were bootstrapped to account for skewness, sampling with replacement observations 5000 times to generate a new population of sample means with an approximate normal distribution. These bootstrap results were then displayed graphically using a cost-effectiveness plane (CEP) to show the uncertainty surrounding the mean estimates of incremental costs and effects, and a CEAC to show the probability of the treatment being cost-effective at different thresholds. To assess the uncertainty surrounding the ICER, bootstrapped 95% CIs were generated.
Handling missing data
In terms of missing data, 29% in the usual-care group and 32% in the modified self-management group were missing the QALY outcome. In total, 1% in each group were missing the number of exacerbations outcome, 30% in the usual-care and 26% in the modified self-management group were missing the 6-month costs and 35% in the usual-care and 36% in the modified self-management group were missing the 12 month’ costs (including those lost to follow-up).
Missing data for outcomes and costs were handled by using Rubin’s multiple imputation (MI) method,24,29,30 assuming that any missing data were missing at random.
Sensitivity analysis
A sensitivity analysis was undertaken to repeat the CEA using complete cases, that is, only those participants who had both cost and outcome data at the same time were included. This was done separately for the two outcome measures. Owing to questionnaires being delivered outside the specified 6- and 12-month time points, a 2-month leeway was applied to the primary analysis. To test the robustness of this, a second sensitivity analysis was done with just a 1-month leeway, putting all those participants who exceeded this as missing. The third sensitivity analysis was to include reliever inhaler costs, as reported on the diary card, in the total costs.
Results
A total of 1922 participants were recruited to the trial, with 51 excluded, leaving 1871 participants analysed in the economic evaluation (935 in the modified self-management group and 939 in the usual-care group). The base-case CEA was based on a MI data set, in which all the missing values were imputed using the MI method.
Costs
The intervention costs reported in Table 28 reflect the value of pharmacological resources needed to deliver the intervention. The mean cost per participant was £42 [standard error (SE) £2] for the modified self-management group and £17 (SE £1) for the usual-care group. This resulted in an adjusted difference of £25 (p < 0.001).
| Cost (£) | Intervention arm | |
|---|---|---|
| Usual care (N = 939) | Modified (N = 935) | |
| Intervention, mean (SE) | 17 (1) | 42 (2) |
| Resource use at 6 months, mean (SE) | 215 (123) | 168 (16) |
| Resource use at 12 months, mean (SE) | 198 (28) | 203 (38) |
| Total resource use,a mean (SE) | 413 (42) | 372 (42) |
| Total cost, mean (SE) | 431 (43) | 415 (42) |
| Adjusted differenceb (bootstrapped 95% CI) | –24 (–122 to 71); p = 0.681 | |
Respiratory-related resource-use costs were similar between interventions, with the modified self-management group costing slightly less, at £415 (SE £42) compared with £431 (SE £43), driven by the lower cost of resource use at 6 months (£168, SE £16). After adjusting for baseline characteristics, this resulted in a difference of –£24 (bootstrapped 95% CI –£122 to £71). Total resource cost included the cost of the two deaths, one in each intervention group. The death in the modified self-management group was costed using the five nights spent in hospital for pneumonia and the unit cost per night from the reference costs (£373). 20 The death in the usual-care group was sudden and the participant died at home, so the cost of an ambulance call-out was used as a conservative estimate (£231). The costs of deaths added £0.25 (SE 0.25) per participant and £1.99 (SE £1.99) to the cost per participant in the usual-care and modified self-management groups, respectively.
The modified self-management group had a lower total reported cost than the usual-care group, mostly driven by the difference in health-care resource use. This resulted in the modified self-management plan being £24 (bootstrapped 95% CI –£71 to £122) less costly than usual care; however, this difference did not reach statistical significance (p = 0.681).
Outcomes: quality-adjusted life-years and number of exacerbations
The primary health economic outcome was QALY gains over 12 months, which were estimated using the EQ-5D-3L. Table 29 reports mean EQ-5D-3L scores at baseline, and at 6 and 12 months. There was little difference between the intervention arms in EQ-5D-3L scores at baseline and both intervention arms saw a decline in score over the study period. The resulting difference in QALYs was 0.02 (bootstrapped 95% CI –0.005 to 0.04) greater for the modified self-management group, after adjusting for baseline EQ-5D-3L scores and characteristics; however, this difference did not reach statistical significance (p = 0.207).
| Time point | Intervention arm | |
|---|---|---|
| Usual care (N = 939) | Modified (N = 935) | |
| EQ-5D-3L scores, mean (SE) | ||
| Baseline | 0.79 (0.01) | 0.80 (0.01) |
| 6-month follow-up | 0.72 (0.01) | 0.75 (0.01) |
| 12-month follow-up | 0.72 (0.01) | 0.74 (0.01) |
| QALYs, mean (SE) | 0.74 (0.01) | 0.76 (0.09) |
| Adjusted differencea (bootstrapped 95% CI) | 0.02 (–0.00 to 0.04); p = 0.207 | |
The second health outcome, also assessed in the statistical analysis, was the number of exacerbations over the 12-month study period. Table 30 shows that the usual-care group has a higher mean number of exacerbations (0.95, SE 0.04 exacerbations) than the modified self-management group (0.84, SE 0.04 exacerbations) with an adjusted difference of –0.10 exacerbations (bootstrapped 95% CI –0.21 to 0.01 exacerbations), although this finding did not reach statistical significance (p = 0.080).
| Exacerbations | Intervention arm | |
|---|---|---|
| Usual care (N = 939) | Modified (N = 935) | |
| Number of exacerbations, mean (SE) | 0.95 (0.04) | 0.84 (0.04) |
| Adjusted difference (bootstrapped 95% CI) | –0.10 (–0.21 to 0.01); p = 0.080 | |
Cost-effectiveness analysis and uncertainty
Two sets of cost-effectiveness analyses were conducted using QALYs and the number of exacerbations per participant. As the modified treatment was both less costly and more effective for both health outcomes, the modified treatment is said to be ‘dominant’. However, Table 31 reflects the uncertainty with this result, as shown by the 95% bootstrapped CIs.
| CEA results | Intervention arm | Difference in mean (bootstrapped 95% CI) | |
|---|---|---|---|
| Usual care (N = 939) | Modified (N = 935) | ||
| Total cost, mean (SE) | £431 (£43) | £415 (£42) | –£24 (–£122 to £71) |
| QALY, mean (SE) | 0.74 (0.01) | 0.76 (0.09) | 0.02 (–0.00 to 0.04) |
| Exacerbations, mean (SE) | 0.95 (0.04) | 0.84 (0.04) | –0.10 (–0.21 to 0.01) |
| ICER | |||
| QALY (bootstrapped 95% CI) | Dominant (–£21,699 to £16,268) | ||
| Exacerbations (bootstrapped 95% CI) | Dominant (–£1999 to £2492) | ||
However, the difference between costs, QALYs and exacerbations was not statistically significant. This suggested that there is significant uncertainty surrounding these estimates. To investigate this, a non-parametric bootstrapping technique was investigated. A cost-effectiveness scatterplot was produced from the bootstrapping results for difference in QALYs and difference in cost and then, again, for difference in exacerbations and difference in cost. The results of the 5000 re-samples for each outcome were plotted on a cost-effectiveness plane (Figure 7), visually displaying any uncertainty surrounding the mean differences in costs and benefits between the intervention and usual-care groups. The CEP in Figure 7a shows the majority of the plots falling in the south-east quadrant (65%), suggesting greater QALYs and lower costs for the modified self-management group. With 29% of the plots falling in the north-east quadrant, there is some uncertainty surrounding the costs; however, the majority of plots fall below both the £30,000- and £20,000-threshold line, implying a high probability of cost-effectiveness for the modified self-management group.
FIGURE 7.
Cost-effectiveness planes (base case).
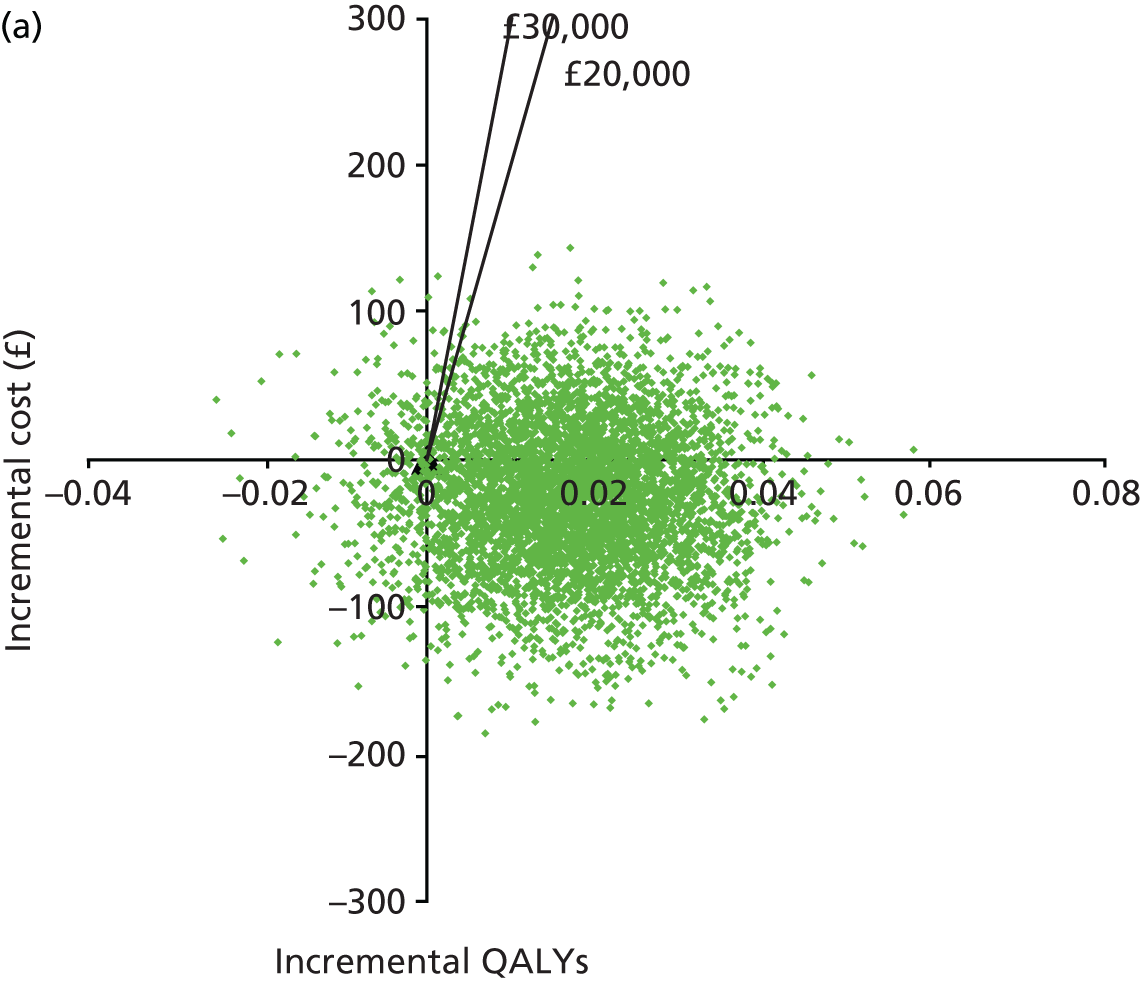
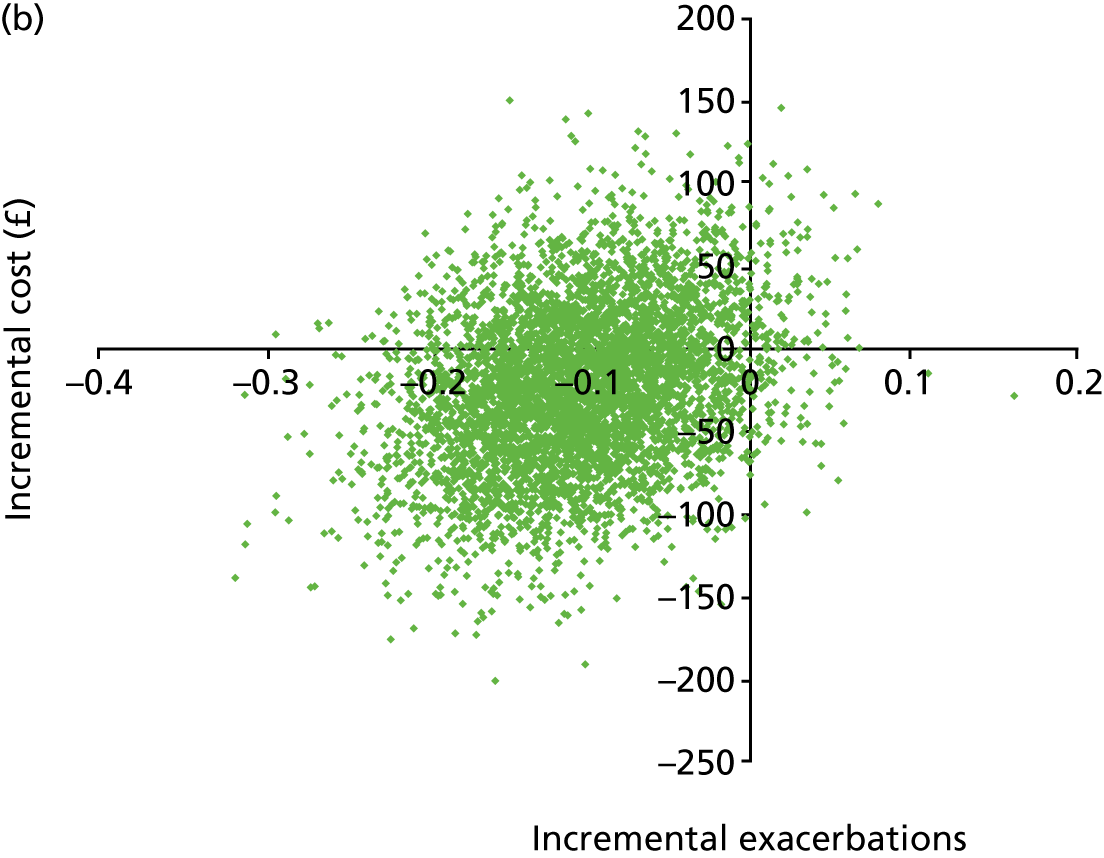
Figure 7b shows the majority of the plots falling in the west quadrants, suggesting a positive health gain in terms of exacerbations prevented, but also uncertainty in terms of costs with plots falling in both the north and south quadrants.
Using the bootstrapped replicates we also generated a CEAC (Figure 8), which provides a plot of probabilities that the intervention was cost-effective (y-axis) against all potential values of willingness-to-pay thresholds (x-axis). This can be generated only for the QALY outcome, as there is not a threshold for exacerbations prevented. The CEAC (Figure 8) shows that, with a willingness-to-pay threshold of £20,000, there is a 94% chance of the intervention being cost-effective.
FIGURE 8.
Cost-effectiveness acceptability curve.
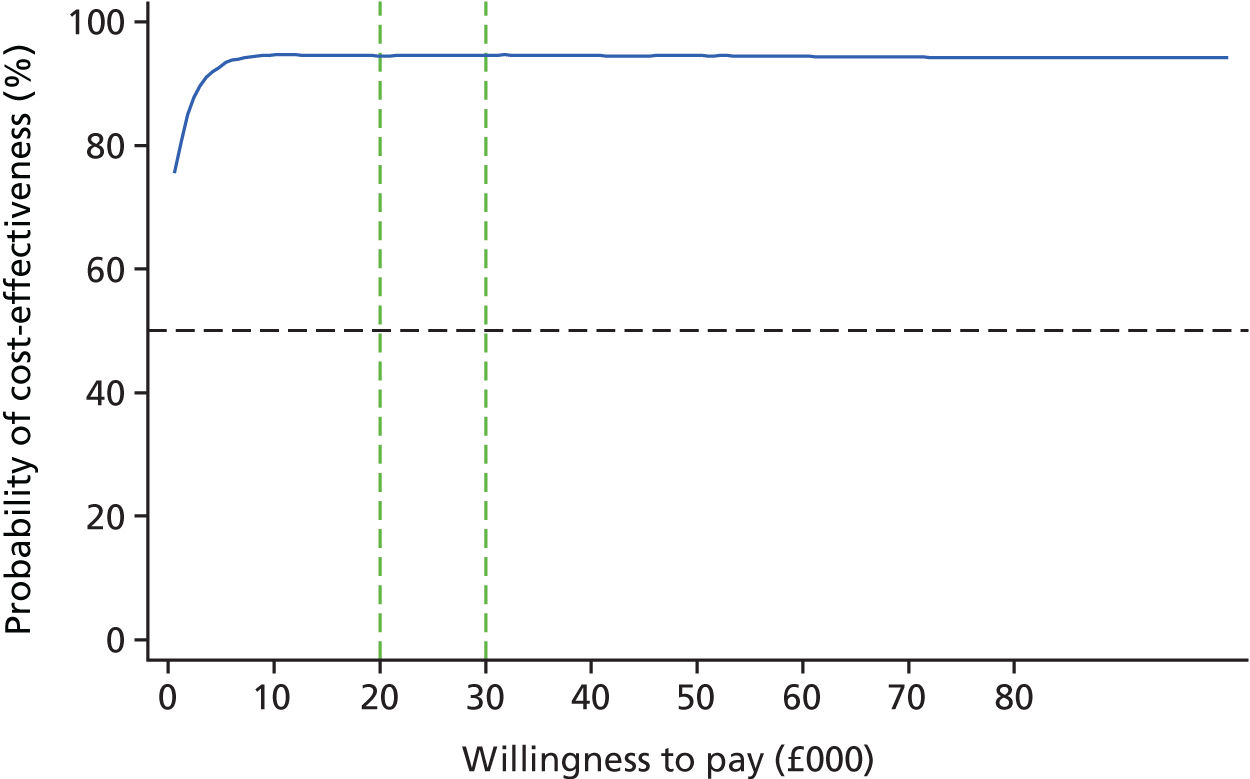
Complete-case analysis (sensitivity analysis)
In order to explore the potential impact of missing data on the results, a sensitivity analysis was conducted using complete cases. Complete costs and QALYs were available for 1041 participants and complete costs and the number of exacerbations were available for 1047 participants.
Table 32 shows the complete-case analysis results for complete QALYs and costs. As with the primary analysis, the results were cost-saving (–£109, bootstrapped 95% CI –£259 to £41; p = 0.148) and not statistically significant. The results of the QALYs showed a lower difference, of 0.01 (bootstrapped 95% CI –0.02 to 0.04; p = 0.517), compared with 0.02 in the primary analysis. However, the results did not reach statistical significance.
| Costs and QALYs | Intervention arm | |
|---|---|---|
| Usual care (N = 512) | Modified (N = 529) | |
| Intervention, mean (SD) | £22 (£34) | £51 (£74) |
| Resource use at 6 months, mean (SD) | £240 (£766) | £139 (£275) |
| Resource use at 12 months, mean (SD) | £220 (£920) | £191 (£853) |
| Total service use,a mean (SD) | £461 (£1459) | £333 (£930) |
| Total cost, mean (SD) | £483 (£1464) | £384 (£943) |
| Adjusted difference (bootstrapped 95% CI) | –£109 (–£259 to £41); p = 0.148 | |
| EQ-5D-3L scores, mean (SD) | ||
| Baseline | 0.80 (0.26) | 0.82 (0.23) |
| 6-month follow-up | 0.80 (0.26) | 0.82 (0.23) |
| 12-month follow-up | 0.81 (0.26) | 0.81 (0.24) |
| QALYs, mean (SD) | 0.80 (0.23) | 0.82 (0.21) |
| Adjusted difference (bootstrapped 95% CI) | 0.01 (–0.02 to 0.04); p = 0.517 | |
Table 33 shows the complete-case results for the number of exacerbations. As with the complete-case results for the QALYs, the costs for the complete exacerbations results were cost-saving, with an adjusted difference of –£110 (bootstrapped 95% CI –£265 to £39; p = 0.175). The complete-case results for the number of exacerbations showed a greater difference in the number of exacerbations, with an adjusted difference of –0.18 (bootstrapped 95% CI –0.34 to –0.02) and the results did reach statistical significance (p = 0.046).
| Costs and exacerbations | Intervention arm | |
|---|---|---|
| Usual care (N = 514) | Modified (N = 533) | |
| Intervention, mean (SD) | £23 (£34) | £50 (£74) |
| Resource use at 6 months, mean (SD) | £239 (£765) | £138 (£274) |
| Resource use at 12 months, mean (SD) | £219 (£918) | £191 (£850) |
| Total service use,a mean (SD) | £459 (£1457) | £333 (£927) |
| Total cost, mean (SD) | £482 (£1461) | £383 (£940) |
| Adjusted difference (bootstrapped 95% CI) | –£110 (–£265 to £39); p = 0.144 | |
| Exacerbations, mean (SD) | 1.07 (1.34) | 0.90 (1.30) |
| Adjusted difference (bootstrapped 95% CI) | –0.18 (–0.34 to –0.02); p = 0.046 | |
The modified self-management group had lower costs and better health outcomes; therefore, the modified treatment was said to be ‘dominant’. However, the cost and QALY difference were not statistically significant, so CEPs were used to explore this uncertainty. Figure 9 shows the CEP for QALYs and for exacerbations on the left-hand side and for exacerbations on the right-hand side. The majority of the plots in the CEP in Figure 9a fall in the south-east quadrant (71%), inferring lower costs and greater QALYs for the modified self-management group. However, compared with the base case, more plots fall in the south-west quadrant (21% vs. 4%), where both incremental costs and effects are negative. The majority of plots fall below both the £30,000- and £20,000-threshold line, suggesting a high probability of cost-effectiveness. The CEP in Figure 9b shows certainty in reducing exacerbations, with the majority of the plots falling in the west quadrants, which was also reflected in the significant p-values. The CEAC in Figure 10 shows an 86% probability of the intervention being cost-effective at a threshold of £20,000.
FIGURE 9.
Cost-effectiveness planes (complete case).
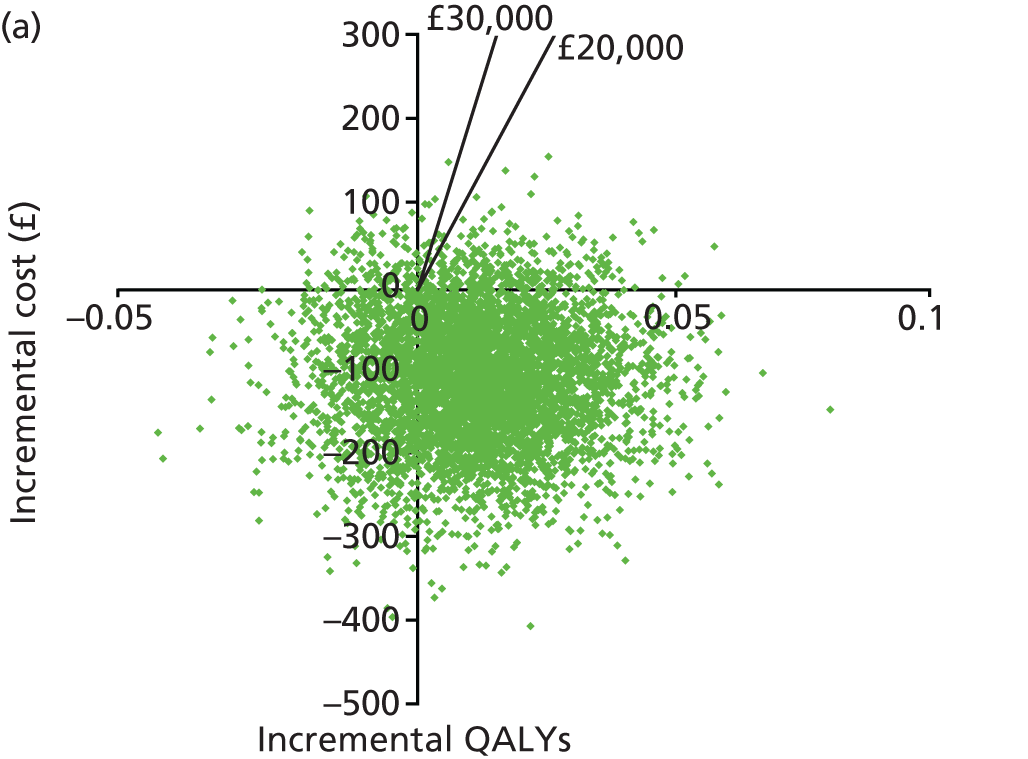
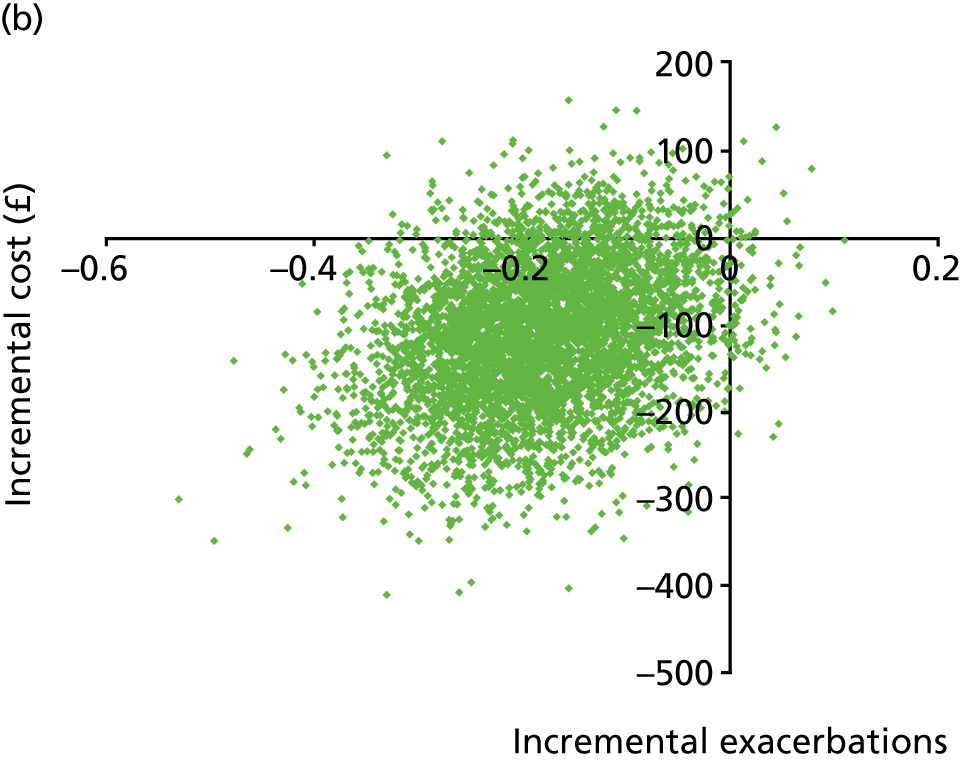
FIGURE 10.
Cost-effectiveness acceptability curve (complete case) for 12 months.
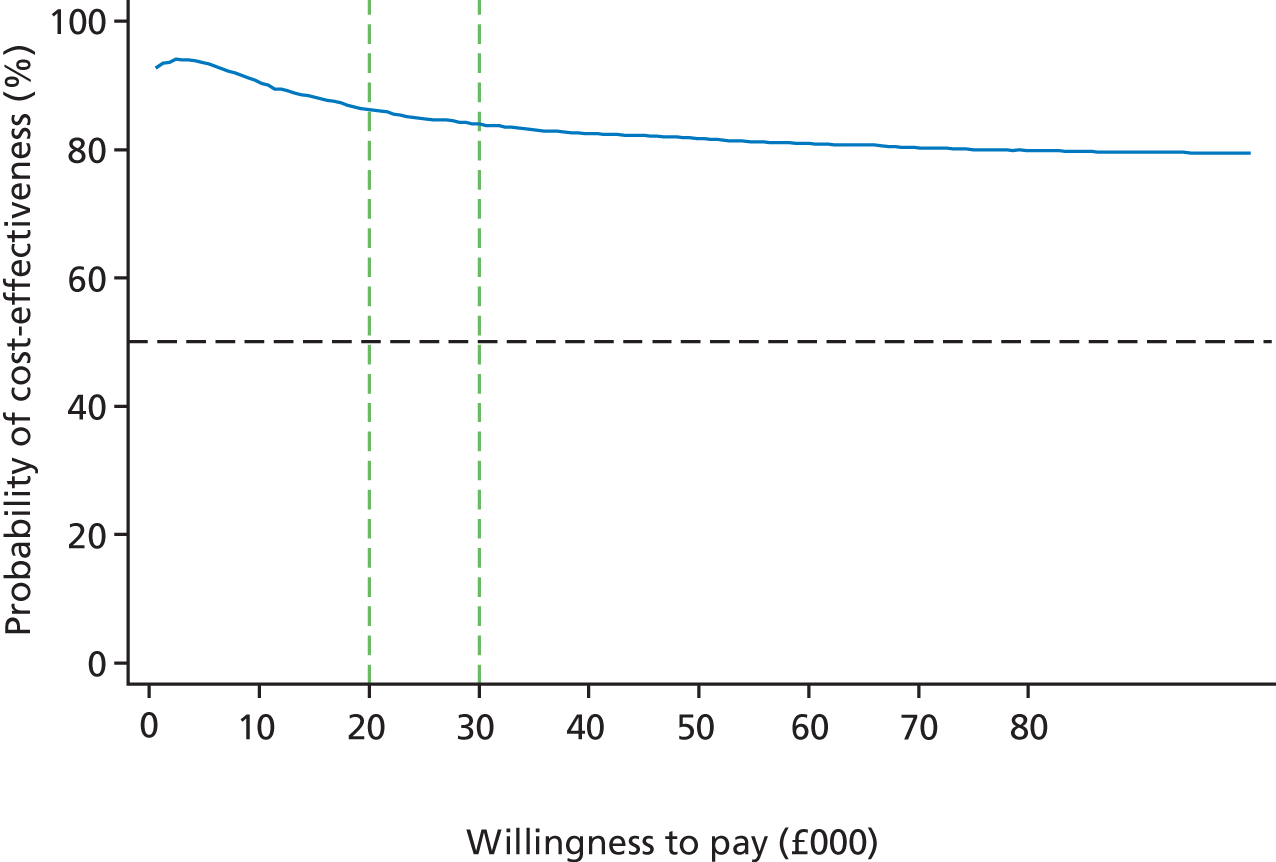
Sensitivity analysis
Table 34 presents the MI results of changing the questionnaire leeway to 1 month. For the usual-care group this resulted in 59% missing data and for the modified self-management group 56% missing data. The results showed a lower cost difference (–£10, bootstrapped 95% CI –£94 to £74) and a lower QALY difference (0.01, bootstrapped 95% CI –0.01 to 0.03). The difference in exacerbations remained the same.
| Summary | Intervention arm, mean (SE) | Adjusted difference (bootstrapped 95% CI) | |
|---|---|---|---|
| Usual care (N = 939) | Modified (N = 935) | ||
| Total cost | £404 (£41) | £402 (£39) | –£10 (–£94 to £74) |
| QALY | 0.79 (0.01) | 0.80 (0.01) | 0.01 (–0.01 to 0.03) |
| Exacerbations | 0.95 (0.04) | 0.84 (0.04) | –0.10 (–0.22 to 0.00) |
The reliever inhaler costs were very small (modified £0.86, SE £0.04; usual care £0.78, SE £0.04) per participant and, therefore, made very little difference to total costs.
Details of the wider societal costs analysed in the trial are presented in Appendix 7.
Summary/conclusion
This economic evaluation assessed the cost-effectiveness of temporarily quadrupling the dose of inhaled corticosteroid compared with usual care.
The mean intervention cost was £42 (SE £2) per participant in the modified self-management group and £17 (SE £1) per participant in the usual-care group. Taking into consideration the wider respiratory-related health-care resource use, participants who received the modified treatment had non-significantly lower total mean costs over the 12-month period after adjusting for covariates. The adjusted total cost difference was –£24 (bootstrapped 95% CI –£122 to £71).
It is recommended by NICE that cost-effectiveness be expressed in terms of cost per QALY. In this study, there was a non-significant trend towards higher QALYs associated with the modified treatment (adjusted difference of 0.02, bootstrapped 95% CI –0.005 to 0.04). The economic evaluation also used the number of exacerbations. There was, once again, a non-significant trend towards fewer exacerbations associated with the modified treatment (adjusted difference of –0.10, bootstrapped 95% CI –0.22 to 0.01).
The base-case results were based on a MI data set. As the modified treatment was both more effective and less costly, it was said to be ‘dominant’ in terms of both QALYs and the number of exacerbations prevented. Although the differences in QALYs and costs were not statistically significant, the CEAC demonstrated a 94% probability of it being cost-effective at a £20,000 threshold.
A sensitivity analysis was carried out using complete cases to explore the impact of missing data. The results showed a lower cost for the modified self-management group and higher cost for the usual-care group, resulting in the modified treatment still being cost-saving for both the exacerbation complete-case analysis (adjusted difference of –£110, bootstrapped 95% CI –£265 to £39) and the QALY complete-case analysis (–£109, bootstrapped 95% CI –£259 to £41). However, the difference in QALYs was reduced, resulting in the modified self-management group being less effective than in the base case (adjusted difference of 0.01, bootstrapped 95% CI –0.02 to 0.04). On the other hand, the difference in exacerbations was greater and statistically significant (adjusted difference of –0.18, bootstrapped 95% CI –0.34 to –0.02), unlike the base-case analysis. As both health outcomes saw greater improvement and lower costs than usual care, the modified treatment was said to be ‘dominant’. However, this should be interpreted with caution as the cost difference did not reach statistical significance and the CEPs demonstrate the uncertainty surrounding this result.
Including reliever inhaler costs within the intervention cost made little difference to the results, as these costs were so small. Changing the leeway of the questionnaires to 1 month, from 2 months, decreased the adjusted total cost difference to –£10 (bootstrapped 95% CI –£94 to £74) and reduced the QALY difference to 0.01 (bootstrapped 95% CI –0.01 to 0.03). However, changing the leeway of the questionnaires did not change the direction of the results and it still had an 87% chance of being cost-effective at a threshold of £20,000.
An exploration of wider societal costs was carried out with the available data. On the whole, there was little difference between the intervention groups, with slightly greater costs seen for the modified self-management group. However, some of this greater cost was due to one participant reporting very high travel costs. Although conclusions could not be drawn because of missing data, the exploration suggested that there may be costs incurred by participants as a result of hospital and GP visits.
In conclusion, the economic evaluation of the FAST has provided us with evidence showing that quadrupling the inhaled corticosteroid use during the activation zone results in better clinical outcomes. Moreover, the CEA shows that this intervention is likely to be a cost-effective intervention in comparison with usual care.
Chapter 5 Discussion
Summary/conclusion
Our results demonstrate that a self-management plan that recommends a temporary quadrupling of inhaled corticosteroids at the time of deteriorating asthma control can prevent asthma exacerbations, compared with the usual self-management plan. This supports the findings from a previous trial. 6
Overall, 51.6% of the usual-care self-management group had an asthma exacerbation (defined as the need for systemic corticosteroids and/or unscheduled health-care consultation) at least once in the 12-month study period, compared with 45% of the modified self-management group, with an adjusted hazard ratio for time to first exacerbation of 0.81 (95% CI 0.71 to 0.92; p = 0.02). This equates to 15 patients needing to be given the modified self-management plan for one additional patient to benefit (i.e. avoid an asthma exacerbation, 95% CI 9 to 43 patients). There was no evidence in the subgroup analysis that the intervention effect differed for the time to first exacerbation according to inhaled corticosteroid dose or smoking status.
Each of the 12 secondary outcomes favoured the modified treatment group. There were fewer participants who were prescribed systemic corticosteroids and attended unscheduled health-care consultations in the modified self-management group than in the usual-care self-management group. However, there was a large number of missing data for some secondary outcomes (i.e. peak flow and the Mini AQLQ) which limited confidence in the findings. However, the results were in keeping with the primary outcome and secondary outcomes for which data completion was greater, providing some reassurance around their reliability.
The safety data continued to support the clinical benefit of a temporary fourfold increase in of inhaled corticosteroids as participants in the modified treatment group reported fewer asthma-related hospitalisations (three in the modified self-management group compared with 18 in the usual-care group). The modified self-management group did experience a higher frequency of treatment-related side effects of inhaled corticosteroids, such as oral thrush (56 events reported by 41 participants in the modified self-management group and 13 events reported by 10 participants in the usual-care group), but this was expected because of the nature of the intervention. Local adverse effects such as these are not usually a major problem and are usually easily treated with local therapy. Of more concern are reports of pneumonia, especially in patients with COPD, and adverse effects related to systemic absorption, such as adrenal suppression, osteoporosis and cataract. The median dose of inhaled corticosteroid in our study was 800 mcg/day, so quadrupling this would equate to the equivalent of 3200 mcg/day of inhaled beclometasone. Unfortunately, the systemic effects of high-dose inhaled corticosteroids are not well described and the dose potency in terms of prednisolone equivalents appears to vary from tissue to tissue. 31 In terms of milligrams of prednisolone, adrenal suppression from 1.5 mg/day of fluticasone (approximately 3 mg of beclometasone) has been estimated to have approximately the same effects on morning cortisol suppression as between 10 and 20 mg of prednisolone. 31 As the study included patients on 2000 mcg of inhaled fluticasone, and if the dose potency ratio between fluticasone propionate and prednisone is linear, then the quadrupled dose could have the same systemic effects as a course of prednisolone used to treat asthma exacerbations.
Finally, although there have been reports of an increased risk of respiratory infections in patients on long-term high-dose inhaled steroids,32 the study found no evidence of an increased incidence of pneumonia.
Health economics
To date, FAST is the first large randomised controlled trial that has assessed the cost-effectiveness of temporarily quadrupling the dose of inhaled steroid to reduce asthma exacerbations.
When using QALYs as a standard health outcome measurement, the modified treatment was said to be ‘dominant’. When using the NICE decision threshold of £20,000–30,000, the modified treatment had a 94–95% probability of being cost-effective. The complete-case analysis also showed a 86% probability of the modified treatment being cost-effective at a threshold of £20,000; however, this probability decreased as the threshold increased. This is because the complete-case results had more plots falling into the south-west quadrant (i.e. less costly and less effective). The CEAC assumes that the amount saved in order to give up one QALY increases with the threshold. Therefore, more plots were excluded in the south-west quadrant as this threshold increased. This led to a lower probability of cost-effectiveness at higher thresholds, as many plots fell into the south-west quadrant.
When using the number of exacerbations as the health outcome, the modified treatment was once again ‘dominant’. However, as no decision-making threshold exists, the probability of cost-effectiveness could not be assessed.
Reducing the number of exacerbations per participant should lead to fewer hospitalisations, which account for the greatest cost of wider health care. A lower number of hospitalisations was reported at 6 months for the modified self-management group and was reflected in the much lower cost at this follow-up; however, this did not persist through to the 12-month follow-up and the overall difference in resource-use costs was not statistically significant. This may suggest that the overall reduction in the number of exacerbations was not great enough to make a significant impact on hospital admissions, but over a longer period of time this may accumulate a greater cost-saving. This may have also been impacted by an imbalance at baseline, but as the study did not collect baseline costs the analyses still need to be interpreted with caution.
The strength of the economic analysis has been impacted by a few limitations of the study. First, there was no baseline service-use questionnaire. Although the randomisation ensures that characteristics and confounders are balanced between the groups at baseline, it may not have been balanced for health-care service use. The questionnaires were also not always delivered at the right time, and so assumptions had to be made as to what was considered an appropriate time frame and data were lost because of this. For the intervention costing, only what was reported on the diary cards could be used. It is, therefore, possible that some participants may have activated but not recorded their inhaler use and the study would not have been able to cost this. Once more, nine participants in the usual-care group were reported being given extra steroid inhalers and self-reported using them on their diary cards, so there might have been some crossover between groups.
Relevance to existing literature
In June 2016, the Cochrane review relating to whether or not an increase in corticosteroid inhalers at the first sign of asthma exacerbation is better than, and as safe as, continuing with the usual prescribed inhaled corticosteroid dose was updated. 33 From the eight studies included (1669 participants with mild to moderate asthma) the authors concluded that it is unlikely that increasing the dose of inhaled corticosteroid reduces the need for courses of systemic corticosteroids, hospitalisations or recovery time. The Cochrane review made it clear that its results were rated as being moderate to low quality, as the findings were uncertain across the studies and the studies conducted included very few participants in whom it could definitively be shown that increasing the dose was beneficial.
The benefit of an increase in inhaled corticosteroid treatment at the time of asthma control worsening is also supported by the benefit seen from studies evaluating Symbicort for maintenance and relief medication. Studies have shown that a variable dose of Symbicort, for use only by patients whose asthma becomes severe or whose asthma symptoms start to worsen, results in fewer acute exacerbations than a constant dose of maintenance treatment.
Strengths and limitations
This was an adequately powered RCT, with high follow-up rates for the primary outcome and a moderate adherence to the trial intervention.
The trial was open-label to research site staff and participants so, although the possibility cannot be ruled out that the treatment effect in the modified treatment group was enhanced, the inclusion of any placebo effect makes the study more relevant to real-life clinical practice.
The diary card completion of participants with at least one activation to zone 2 or above was not balanced between the two groups, with poorer completion noted in the usual-care self-management group than in the modified self-management group. This could have contributed towards a null bias, which could strengthen the positivity of the result. Overall, diary cards were not completed for the first activation to zone 2 by 34% of participants; this meant that information was unknown about adherence to the allocated self-management plans for 39% of participants in the usual-care group and 28% of participants in the modified self-management group.
A large number of missing data were missing for the secondary outcomes of area under peak flow curve and change in the Mini AQLQ score 2 weeks after activating zone 2 or above of the self-management plan. In addition, there was an imbalance in the number of participants with these outcomes available in the two allocated groups. Reassuringly, the results were consistent with the findings where more complete data were available, but these analyses still need to be interpreted with caution because of the potential risk of bias attributable to the missing data.
Finally, additional information on the acceptability of carrying an additional inhaler for the 80% of patients on a combination inhaler would have been useful information to have collected. Although no evidence was found that this led to early withdrawal from the study, it would be interesting to know how acceptable this would be for patients in real life as it could represent a barrier to the widespread use of such a self-management plan.
Generalisability
The study has good external validity as it was a pragmatic design that reflected normal clinical practice across both primary and secondary care in the UK. Participants used their existing asthma medication, and those in the modified self-management group either increased the dose of their corticosteroid inhaler or added an extra corticosteroid inhaler, depending on whether their usual treatment comprised corticosteroid or a combination of corticosteroid/long-acting beta agonist inhaler. It is important to note that throughout the trial the site staff ensured that the self-management plans were well explained and supported, and if this was not carried through into routine care the results may not apply.
Participants were recruited from 207 UK centres, both hospitals and GP surgeries, covering a range of urban and rural settings. This trial included people aged ≥ 16 years with chronic asthma who had had at least one acute exacerbation in the previous 12 months. The trial was inclusive of patients who were prescribed to the upper limit of the licensed dose of their maintenance steroid, so participants on very high doses of maintenance steroids were included in the trial. The study identified a group of patients at risk of further exacerbation and, therefore, with most to gain from following a self-management plan.
It is important to note that the exacerbation rate in the usual-care group was higher than expected, which suggests that the study recruited a sample of patients whose asthma was more severe than initially anticipated. So despite the percentage of participants on the modified self-management plan having an exacerbation being lower than that in the usual-care group, the overall exacerbation was still high (45%). As children were not included in this study it is not known if this intervention would be beneficial to treat asthma symptoms in children.
Chapter 6 Conclusions
Main conclusions
This is the largest independent randomised controlled trial to assess the clinical effectiveness and cost-effectiveness of an asthma self-management plan that advises participants to increase fourfold their inhaled corticosteroid at the point at which asthma symptoms deteriorate. Both the clinical and economic analyses show this approach to asthma control to be effective for participants and health providers.
Implications for practice
The trial has shown that the use of an asthma self-management plan that advises patients to quadruple their dose of inhaled corticosteroid at the point of asthma deterioration is effective in reducing exacerbations that require unscheduled health-care consultations and the use of systemic corticosteroids in those patients who identify as having exacerbated within the last year, as well as proving cost-effective for health-care providers. Although quadrupling the corticosteroid dose appeared to be clinically effective and cost-effective across the licensed dose range, the systemic effects resulting from this advice in patients using high-dose inhaled corticosteroids need to be considered, and widespread adoption in these patients is not recommended. Clinical commissioners and national and international guideline developers can now be encouraged to make informed decisions regarding the use of self-management plans that advise a fourfold increase in inhaled corticosteroid dose on the basis of these robust findings. As only 15 patients need to be trained to use such a self-management plan to prevent one severe exacerbation, the study showed that all patients on low to medium doses of inhaled corticosteroids, especially if they have had an exacerbation in the last year, should be encouraged to follow such a plan.
Acknowledgements
We would like to thank those who took part in the trial, the clinical staff at the participating recruiting sites and PICs for their support.
The asthma self-management plans were designed in association with Asthma UK.
The trial was sponsored by the University of Nottingham, was co-ordinated from the Nottingham Clinical Trials Unit, and was supported by the NIHR CRN.
Contributors to the FAST
Independent Trial Steering Committee
Philip Ind (chairperson), Consultant Respiratory Physician, Imperial College Healthcare NHS Trust; Adel Mansur, Consultant Physician, Heartlands Hospital Birmingham; Jacqui Cooper, COPD/Respiratory Team Leader, Keyworth PCC; and Kim-Leng Hills, patient representative.
Independent Data Monitoring Committee
Steven Julious (chairperson), Professor of Medical Statistics, University of Sheffield; Ian Sabroe, Professor of Inflammation Biology, University of Sheffield; and Stephen Scott, Consultant Respiratory Physician, Countess of Chester Hospital.
Trial Management Group
Tim Harrison, chief investigator; Trisha McKeever, medical statistician; Lucy Bradshaw, medical statistician; Rebecca Haydock, trial manager (from December 2015); Lelia Duley, Director of NCTU and advisor on trial design; Richard Swinden, trial co-ordinator (from June 2015); Brian Barnes, data co-ordinator; Tessa Clarke, senior trial manager (until September 2013); Eleanor Mitchell, senior trial manager (from September 2013); Matthew Foster, data administrator; Daniel Simpkins, data manager; Keith Whitaker, IT programmer; Andrew Skeggs, trial manager (January 2013 to November 2015); Kate Frost, trial co-ordinator (March 2015 to December 2015); Desmond Dorairajoo, trial administrator (September 2014 to July 2015); and Trish Hepburn, research facilitator.
Secondary care sites
Nottingham University Hospitals NHS Trust: Janet Oborne (research fellow) and Lisa Williams (research nurse).
Leicester Royal Infirmary: Sarah Parker (research nurse).
Newcastle Freeman Hospital: Karen Martin (research nurse).
Aintree University Hospitals NHS Foundation Trust: Cathy Mordaunt (research nurse).
Aberdeen Royal Infirmary: Alison McKay (research nurse) and Vicki Fraser (research nurse).
Royal Liverpool and Broadgreen NHS Trust: Hassan Burhan (principal investigator), Sherald Barnes (research nurse) and Catherine Lowe (research nurse).
Kings Mill Hospitals: David Hodgkinson (principal investigator) and Sam Kemp (principal investigator).
Arrowe Park Hospitals: Nicola Stevenson (principal investigator) and Liz Bailey (research nurse).
Blackpool Victoria Hospitals: Tarek Saba (principal investigator), Melanie Caswell (research nurse) and Philomena Shooter (research nurse).
Bradford Royal Infirmary: Dinesh Saralaya (principal investigator) and Karen Walker (research nurse).
Primary care sites
City Medical Practice, Lincoln; Cripps Health Centre, Nottingham; Leen View Surgery, Nottingham; Lindum Medical, Lincoln; Nettleham Medical, Lincoln; Rivergreen Medical Centre, Clifton; Welton Family Health, Welton; The Surgery at Wheatbridge, Chesterfield; Danetre Medical Practice, Daventry; Hockley Farm Medical Practice, Leicester; Thurmaston Health Centre, Thurmaston; Beacon View Medical Centre, Gateshead; Belford Medical Practice, Belford; Branch End Surgery, Stocksfield; The Health Centre, Concord; Victoria Road Health Centre, Washington; Ellison View Surgery, Hebburn; Guide Post Medical, Choppington; Haydon Bridge, Northumberland; Hetton Medical Practice, Hetton-le-Hole; Humshaugh & Wark, Hexham; Marine Avenue Surgery, Whitley Bay; Marsden Road Health Centre, South Shields; Priory Medical Group, North Shields; Springwell Medical Group, Sunderland; The Village Surgery, Cramlington; Waterloo Medical Group (Blyth Health Centre), Northumberland; West Farm Surgery, Longbenton; Burnside Surgery (Waters Meeting Heath Centre), Bolton; Swan Lane Medical, Bolton; Wellfield Medical Centre, Manchester; Bollington Medical Centre, Bollington; Claughton Medical Centre, Birkenhead; Danebridge Medical Centre, Cheshire; Great Homer Street Medical Centre, Liverpool; Nantwich Health Centre, Nantwich; Vauxhall Medical Centre, Liverpool; Bermuda & Marlowe Practice, Basingstoke; The Bosmere Medical Practice, Hampshire; Brook Lane Surgery, Southampton; Chawton Park, Alton; Cowplain Family Practice, Waterlooville; Denmark Road Surgery, Bournemouth; Fleet Medical Centre, Fleet; Forest End Surgery, Waterlooville; Forest Surgery, Bordon; Friarsgate Surgery, Weeke; Gosport Medical Centre, Gosport; Highcliffe Medical Centre, Highcliffe; Homewell Surgery, Havant; Liphook & Liss Surgery, Liphook; Lordshill Health Centre, Southampton; Nicholstown Surgery, Southampton; Nightingale, Hamshire; Old Fire Station, Woolston; The Osbourne Practice, Southsea; Park and Francis Surgery, Eastleigh; Portsdown Group, Portsmouth; Rosemary Medical, Poole; The Bourne Valley Practice, Wiltshire; Swanage Health Centre, Swanage; The Adam Practice, Poole; Three Swans Surgery, Salisbury; Wareham Surgery, Wareham; Woolston Lodge Surgery, Woolston; Acorn Surgery, Huntingdon; Addison House Surgery, Harlow; Asplands Medical Centre, Woburn Sands; Beccles Medical Centre, Suffolk; Bridge Road Surgery, Suffolk; Buckden & Little Paxton, Huntingdon; Cornerstone Practice, March; Cottenham Surgery, Cottenham; Creffield Medical Centre, Creffield; Davenport House Surgery, Herts; Dolphin House Surgery, Herts; Elizabeth Courtauld Surgery, Halstead; Fakenham Medical Practice, Fakenham; Greensand Surgery, Ampthrill; Grove Surgery, Thetford; Harvey Group Practice, St Albans; Highlands Surgery, Leigh-on-Sea; Dr Hiscock and Partners, Benfleet; Ixworth Surgery, Ixworth; Kingfisher Practice, Luton; Leighton Road Surgery, Bedfordshire; Magdalen Medical Practice, Norwich; The Market Surgery, Aylsham; North Brink Practice, Wisbech; Oak Street Medical, Norwich; Papworth Surgery, Cambridge; Dr Patel and Partners, Benfleet; The Peninsula Practice, Alderton; Priory Fields Partnership, Huntingdon; Prospect Medical Practice, Norwich; The Riverside Practice, March; Rookery Medical Centre, Suffolk; Rosedale Surgery, Lowestoft; Roundwell Medical Centre, Norwich; Sheringham Medical Practice, Norfolk; St Stephens Gate, Norwich; Staploe Surgery, Cambridgeshire; Stow Health, Stowmarket; The Surgery, Thaxted; Trinity & Bowthorpe, Norwich; Watlington Medical Centre, Norfolk; West Street Surgery, Bedfordshire; King Street Surgery, Lancaster; Oakenhurst Medical Practice, Blackburn; Queen Square Medical Practice, Lancaster; Shifa Surgery, Blackburn; The Village Practice, Thorton-Cleveleys; Alcester Health Centre, Warwickshire; Broad Street Surgery, Coventry; Castle Medical, Warwickshire; Corbett Medical Practice, Worcestershire; Hazelwood Group Practice, Birmingham; Holbrooks Health Team, Coventry; Kensington Road Surgery, Coventry; Mortimer Medical Practice, Herefordshire; Much Birch Surgery, Herefordshire; The New Dispensary, Warwick; Phoenix Family Care, Coventry; Priory Gate Practice, Coventry; Spring Gardens, Worcester; Springfield Medical Practice, Coventry; St Stephen’s, Redditch; The Marches Surgery, Herefordshire; Westfield Surgery, Herefordshire; Winyates Surgery, Worcestershire; Backwell & Nailsea Medical Group, Bristol; Bradgate Surgery, Bristol; Chew Medical Practice, Bristol; Chipping Campden Surgery, Chipping Campden; Combe Down Surgery, Bath; Cotswold Medical Practice, Cheltenham; Elm Tree Medical Partnership, Shrivenham; Hathaway Medical Centre, Chippenham; Mann Cottage Surgery, Gloucestershire; Mythe Medical Practice, Gloucestershire; New Court Surgery, Swindon; Patford House Surgery, Calne; Rendcomb Surgery, Cirencester; Ridgeway View Family Practice, Swindon; Rowden Medical Partnership, Chippenham; St Augustine’s Surgery, Bristol; The Avenue Surgery, Warminster; The Lennard Surgery, Bristol; The Phoenix Surgery, Cirencester; The Tolsey Surgery, Malmesbury; Wellspring Surgery, Bristol; West Walk Surgery, Yate; Westbury on Trym Primary Care Centre, Bristol; Whiteladies Health Centre, Bristol; Winchcombe Medical Centre, Cheltenham; Yorkley Health Centre, Lydney; Arun Medical Group, West Sussex; Church View Practice, Kent; Cossington House Surgery, Kent; Downsway Medical Practice, Kent; Dr Elias & Partners, East Sussex; East Cliff Practice, Ramsgate; Fort House Surgery, Surrey; Henfield Medical Centre, West Sussex; Sackville Medical Centre, Hove; Southbourne Surgery, Hampshire; St James Medical Practice, Kent; The Butchery Surgery, Kent; Whitstable Health Centre, Kent; The Alverton Practice, Penzance; Axbridge & Wedmore Medical Practice, Axbridge; Brunel Medical Practice, Torquay; Claremont Medical Practice, Exmouth; East Quay Medical Centre, Bridgewater; Glastonbury Surgery, Glastonbury; Ide Lane Surgery, Exeter; Mendip Country Practice, Radstock; Oak Tree Surgery, Cornwall; Rame Group Practice, Torpoint; Richmond House Surgery, Devon; Rolle Medical Partnership, Exmouth; Vine Surgery, Somerset; The Abingdon Surgery, Abingdon; Broadshires Health Centre, Carterton; Church Street Practice, Wantage; Eynsham Medical Centre, Oxon; Montgomery House, Bicester; Summertown Health Centre, Oxford; The White Horse Medical Practice, Oxfordshire; and Wokingham Medical Centre, Wokingham.
Clinical Research Network regions
North East and North Cumbria hosted by Newcastle upon Tyne Hospitals NHS Foundation Trust.
North West Coast hosted by Royal Liverpool and Broadgreen University Hospitals NHS Trust.
Greater Manchester hosted by Central Manchester University Hospitals NHS Foundation Trust.
East Midlands hosted by University Hospitals of Leicester NHS Trust.
West Midlands hosted by The Royal Wolverhampton NHS Trust.
West of England hosted by University Hospitals Bristol NHS Foundation Trust.
Thames Valley and South Midlands hosted by Oxford University Hospitals NHS Foundation Trust.
Eastern hosted by Norfolk and Norwich University Hospitals NHS Foundation Trust.
Kent, Surrey and Sussex hosted by Royal Surrey County Hospital NHS Foundation Trust.
Wessex hosted by University Hospital Southampton NHS Foundation Trust.
South West Peninsula hosted by Royal Devon and Exeter NHS Foundation Trust.
Contributions of authors
Tricia McKeever (Medical Statistician) analysed the clinical effectiveness data and prepared the results for publication.
Kevin Mortimer (Senior Lecturer and Honorary Consultant in Respiratory Medicine) was a site principal investigator and contributed to the development of the grant application and trial protocol, and contributed important intellectual content to the report.
Lucy Bradshaw (Medical Statistician) analysed the clinical effectiveness data and prepared the results for publication.
Rebecca Haydock (Trial Manager) managed the trial from November 2015 and drafted the final report.
Ian Pavord (Professor of Respiratory Medicine) was a site principal investigator and contributed to the development of the grant application and trial protocol, and contributed important intellectual content to the report.
Bernard Higgins (Consultant Physician) was a site principal investigator and contributed to the development of the grant application and trial protocol, and contributed important intellectual content to the report.
Samantha Walker (Deputy Chief Executive and Director of Research and Policy) contributed to the development of the self-management plans, trial protocol and important intellectual content of the report.
Andrew Wilson (Clinical Professor) was a site principal investigator and contributed to the development of the grant application and trial protocol, and contributed important intellectual content to the report.
David Price (Chair of Primary Care Respiratory Medicine) was a site principal investigator and contributed to the development of the grant application and trial protocol, and contributed important intellectual content to the report.
Mike Thomas (Professor of Primary Care Research within Medicine) was a site principal investigator and contributed to the development of the grant application and trial protocol, and contributed important intellectual content to the report.
Graham Devereux (Professor and Honorary Consultant in Respiratory Medicine) was a site principal investigator and contributed to the development of the grant application and trial protocol, and contributed important intellectual content to the report.
Christopher Brightling (Senior Research Fellow and Clinical Professor in Respiratory Medicine) was a site principal investigator and contributed to the development of the grant application and trial protocol, and contributed important intellectual content to the report.
Charlotte Renwick (Health Economist) analysed the clinical cost-effectiveness data and prepared the results for publication.
Steve Parrott (Health Economist) contributed to the trial protocol, analysed the clinical cost-effectiveness data and prepared the results for publication.
Eleanor Mitchell (Senior Trial Manager) oversaw the trial management and contributed important intellectual content to the report.
Lelia Duley (Director Nottingham Clinical Trials Unit) contributed to the development of the grant application and trial protocol as well as important intellectual content to the report.
Tim Harrison (Professor and Honorary Consultant) was the chief investigator and co-authored the final report.
Publications
Skeggs A, McKeever T, Duley L, Mitchell E, Bradshaw L, Mortimer K, et al. Fourfold Asthma Study (FAST): a study protocol for a randomised controlled trial evaluating the clinical cost-effectiveness of temporarily quadrupling the dose of inhaled steroid to prevent asthma exacerbations. Trials 2016;17:499.
McKeever T, Mortimer K, Wilson A, Walker S, Brightling C, Skeggs A, et al. Quadrupling inhaled glucocorticoid dose to abort asthma exacerbations. NEJM 2018;378:902–10.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Skeggs A, McKeever T, Duley L, Mitchell E, Bradshaw L, Mortimer K, et al. Fourfold Asthma Study (FAST): a study protocol for a randomised controlled trial evaluating the clinical cost-effectiveness of temporarily quadrupling the dose of inhaled steroid to prevent asthma exacerbations. Trials 2016;17. https://doi.org/10.1186/s13063-016-1608-6.
- Harrison TW, Oborne J, Newton S, Tattersfield AE. Doubling the dose of inhaled corticosteroid to prevent asthma exacerbations: randomised controlled trial. Lancet 2004;363:271-5. https://doi.org/10.1016/S0140-6736(03)15384-6.
- FitzGerald JM, Becker A, Sears MR, Mink S, Chung K, Lee J, et al. Doubling the dose of budesonide versus maintenance treatment in asthma exacerbations. Thorax 2004;59:550-6. https://doi.org/10.1136/thx.2003.014936.
- Foresi A, Morelli MC, Catena E. Low-dose budesonide with the addition of an increased dose during exacerbations is effective in long-term asthma control. Chest 2000;117:440-6. https://doi.org/10.1378/chest.117.2.440.
- Levy ML, Stevenson C, Maslen T. Comparison of short courses of oral prednisolone and fluticasone propionate in the treatment of adults with acute exacerbations of asthma in primary care. Thorax 1996;51:1087-92. https://doi.org/10.1136/thx.51.11.1087.
- Oborne J, Mortimer K, Hubbard RB, Tattersfield AE, Harrison TW. Quadrupling the dose of inhaled corticosteroid to prevent asthma exacerbations: a randomized, double-blind, placebo-controlled, parallel-group clinical trial. Am J Respir Crit Care Med 2009;180:598-602. https://doi.org/10.1164/rccm.200904-0616OC.
- Nottingham Clinical Trials Unit . FAST – Four-Fold Asthma Study: The Clinical and Cost-Effectiveness of Temporarily Quadrupling the Dose of Inhaled Steroid to Prevent Asthma Exacerbations n.d. https://doi.org/10.1186/ISRCTN15441965 (accessed July 2017).
- Juniper EF, Guyatt GH, Cox FM, Ferrie PJ, King DR. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J 1999;14:32-8. https://doi.org/10.1034/j.1399-3003.1999.14a08.x.
- Asthma UK. Asthma Action Plan. London: Asthma UK; 2016.
- O’Byrne PM, Bisgaard H, Godard PP, Pistolesi M, Palmqvist M, Zhu Y, et al. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. Am J Respir Crit Care Med 2005;171:129-36. https://doi.org/10.1164/rccm.200407-884OC.
- Glidden DV, Vittinghoff E. Modelling clustered survival data from multicentre clinical trials. Stat Med 2004;23:369-88. https://doi.org/10.1002/sim.1599.
- pk — Pharmacokinetic (Biopharmaceutical) Data. Stata Manual 13. College Station, TX: Stata Press; 2013.
- White IR, Thompson SG. Adjusting for partially missing baseline measurements in randomized trials. Stat Med 2005;24:993-1007. https://doi.org/10.1002/sim.1981.
- Medical Dictorinary for Regulatory Activities n.d. www.meddra.org/ (accessed 14 July 2017).
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: Personal Social Services Research Unit, University of Kent; 2014.
- Curtis L. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
- Prescription Cost Analysis England 2015. Leeds: NHS Digital; 2016.
- NHS Reference Costs 2014–15. London: DHSC; 2015.
- NHS Reference Costs 2009–10. London: DHSC; 2011.
- NHS Employers . Agenda for Change Pay Bands and Points from 1 April 2014 2014. www.nhsemployers.org/∼/media/Employers/Documents/Pay%20and%20reward/AfC%20pay%20bands%20and%20points%20poster%202014.pdf (accessed 17 March 2016).
- NHS 111 Statistics – March 2015. London: NHS England; 2015.
- Richardson G, Manca A. Calculation of quality adjusted life years in the published literature: a review of methodology and transparency. Health Econ 2004;13:1203-10. https://doi.org/10.1002/hec.901.
- Drummond M, Schulper M, Claxton K, Stoddart GL, Geroge WT. Methods for the Economic Evaluation of Health Care Programmes, Fourth Edition. Oxford: Oxford University Press; 2015.
- Efron B, Tibshirani R. An Introduction to the Bootstrap. New York, NY: Chapman & Hall; 1993.
- Chaudhary MA, Stearns SC. Estimating confidence intervals for cost-effectiveness ratios: an example from a randomized trial. Stat Med 1996;15:1447-58. https://doi.org/10.1002/(SICI)1097-0258(19960715)15:13<1447::AID-SIM267>3.0.CO;2-V.
- Willan AR, O’Brien BJ. Confidence intervals for cost-effectiveness ratios: an application of Fieller’s theorem. Health Econ 1996;5:297-305. https://doi.org/10.1002/(SICI)1099-1050(199607)5:4<297::AID-HEC216>3.0.CO;2-T.
- Severens JL, De Boo TM, Konst EM. Uncertainty of incremental cost-effectiveness ratios. A comparison of Fieller and bootstrap confidence intervals. Int J Technol Assess Health Care 1999;15:608-14.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275-83. https://doi.org/10.1136/bmj.313.7052.275.
- Coyle D, Davies L, Drummond MF. Trials and tribulations. Emerging issues in designing economic evaluations alongside clinical trials. Int J Technol Assess Health Care 1998;14:135-44. https://doi.org/10.1017/S0266462300010588.
- McKeever T, Harrison TW, Hubbard R, Shaw D. Inhaled corticosteroids and the risk of pneumonia in people with asthma: a case-control study. Chest 2013;144:1788-94. https://doi.org/10.1378/chest.13-0871.
- Kew KM, Quinn M, Quon BS, Ducharme FM. Increased versus stable doses of inhaled corticosteroids for exacerbations of chronic asthma in adults and children. Cochrane Database Syst Rev 2016;6. https://doi.org/10.1002/14651858.CD007524.pub4.
- Distribution of Median and Mean Income and Tax by Age Range and Gender: 2014 to 2015. London: HM Revenue & Customs; 2017.
Appendix 1 Service Use Questionnaire
Appendix 2 Summary of changes to the protocol
Appendix 3 The Fourfold Asthma STudy’s asthma self-management plans
Appendix 4 Asthma UK’s self-management plan
Appendix 5 Asthma diary cards
Appendix 6 Baseline characteristics for participants’ activation in zone 2 or above by allocated intervention group
The baseline characteristics of the participants who activated zone 2 or above were well balanced between the usual-care and modified self-management groups (Table 35).
| Characteristic | Intervention arm | Total with at least one activation to zone 2 or above (N = 1114) | |
|---|---|---|---|
| Usual care (N = 552); at least one activation to zone 2 or above | Modified (N = 562); at least one activation to zone 2 or above | ||
| Age (years) | |||
| Mean (SD) | 57.0 (15.4) | 56.2 (15.5) | 56.6 (15.5) |
| Min., max. | 19, 94 | 16, 89 | 16, 94 |
| Sex, n (% of total) | |||
| Male | 154 (28) | 165 (29) | 319 (29) |
| Female | 398 (72) | 397 (71) | 795 (71) |
| Recruited from | |||
| Primary care | 426 (77) | 453 (81) | 879 (79) |
| Secondary care | 126 (23) | 109 (19) | 235 (21) |
| PEF (l/minute) at screening | |||
| Mean (SD) | 374.7 (111.2) | 386.9 (116.5) | 380.9 (114.0) |
| Type of inhaler, n (% of total) | |||
| Corticosteroid | 162 (29) | 141 (25) | 303 (27) |
| Combination | 390 (71) | 421 (75) | 811 (73) |
| Maintenance dose of inhaled corticosteroids (mcg/day of BDP) | |||
| Median (25th, 75th centiles) | 800 (400, 1350) | 800 (500, 1600) | 800 (400, 1600) |
| Min., max. | 100, 4000 | 80, 4000 | 80, 4000 |
| Maintenance dose of steroids (used in randomisation stratification), n (% of total) | |||
| Low (≤ 1000 mcg/day of BDP) | 413 (75) | 416 (74) | 829 (74) |
| High (> 1000 mcg/day of BDP) | 139 (25) | 146 (26) | 285 (26) |
| BTS step of asthma treatment, n (% of total) | |||
| Step 2 – regular preventer therapy | 136 (25) | 111 (20) | 247 (22) |
| Step 3 – initial add-on therapy | 213 (39) | 195 (35) | 408 (37) |
| Step 4 – persistent poor control | 195 (35) | 249 (44) | 444 (40) |
| Step 5 – omalizumab | 2 (0.4) | 1 (0.2) | 3 (0.3) |
| Not known | 6 (1) | 6 (1) | 12 (1) |
| Smoking status, n (% of total) | |||
| Never | 318 (58) | 338 (60) | 656 (59) |
| Current | 36 (7) | 29 (5) | 65 (6) |
| Former | 198 (36) | 195 (35) | 393 (35) |
| Pack-years for current or former smokers | |||
| n | 234 | 224 | 458 |
| Mean (SD) | 14.2 (15.8) | 12.9 (15.5) | 13.6 (15.7) |
| Mini AQLQ overall score | |||
| n | 551 | 557 | 1108 |
| Mean (SD) | 4.9 (1.2) | 5 (1.2) | 4.9 (1.2) |
The mean age of participants was 57 years (SD 15.5 years) and 795 (71%) were female.
Of those participants who activated zone 2 or above, 811 (73%) were prescribed combination inhalers and the majority of participants, 829 (74%), were on a low dose of maintenance steroid (≤ 1000 mcg/day of BDP).
Appendix 7 Health economics and wider societal costs
Wider societal costs
Only 41% of participants in both intervention groups reported their annual income and low numbers of participants reported costs of travel; therefore, an available case has been explored in this analysis to make the most of the available data. Table 36 shows the available cases for the number of days given up because of illness and the mean travel times to GP or hospital appointments as reported in the questionnaire. Using the participants’ number of GP visits and hospital outpatient visits, a mean total travel time per participant for each kind of appointment was calculated. There was little difference between intervention groups in the percentage of participants in paid employment (modified = 47% and usual care = 48% at 6 months). As for productivity loss, when looking at both time points together, there was little difference in the mean number of days given up as a result of respiratory illness between intervention groups.
| Productivity loss and travel time | Time point | |||||||
|---|---|---|---|---|---|---|---|---|
| 6 months | 12 months | |||||||
| Usual care | n | Modified | n | Usual care | n | Modified | n | |
| Productivity loss | ||||||||
| % in paid employment | 48 | 675 | 47 | 695 | 47 | 615 | 43 | 614 |
| % given up paid employment in last 6 monthsa | 4 | 321 | 12 | 329 | 3 | 286 | 4 | 266 |
| Number of days given up, mean (SD) | 1.5 (9.2) | 674 | 1.1 (7.7) | 702 | 1.3 (10.6) | 614 | 1.6 (11.9) | 614 |
| Number of hours given up by family members, mean (SD) | 0.12 (1.20) | 432 | 0.24 (2.21) | 427 | 0.32 (3.3) | 406 | 0.28 (2.3) | 401 |
| Travel time | ||||||||
| Mean travel time (minutes) to GP surgery | 22 (9.7) | 734 | 21 (9.4) | 742 | 21 (9.4) | 667 | 22 (10.2) | 652 |
| Mean total travel time (minutes) to GP surgery (calculated using number of visits) | 32 (78) | 729 | 26 (53) | 732 | 19 (38) | 664 | 16 (34) | 650 |
| Mean travel time (minutes) to hospital outpatient visit | 34 (17) | 734 | 36 (17) | 742 | 35 (16) | 665 | 37 (17) | 650 |
| Mean total travel time (minutes) to hospital outpatient visit (calculated using number of visits) | 7 (31) | 732 | 5 (28) | 737 | 6 (24) | 665 | 5 (21) | 648 |
Table 36 also shows how mean travel times to GP surgeries are mostly equal between groups at both time points; however, the modified self-management group has a slightly lower mean travel time per participant when the number of visits are taken into account, and both intervention groups have a lower travel time at 12 months. Once more, similar average travel times are seen for hospital outpatient visits and, when using the number of visits, there was little difference between the intervention groups and follow-ups.
The mode of travel to the GP surgery was mostly equal between intervention groups at both 6 months and 12 months, with car and walking the most popular methods. The majority of participants in both intervention groups and at both time points reported taking 0–15 minutes to reach the GP surgery [modified, 67% (6 months) and 64% (12 months); usual care, 61% (6 months) and 60% (12 months)]. Once again, the mode of transport to hospital outpatient visits was, on the whole, equally distributed between the two intervention groups, with both groups reporting more bus use than when travelling to the GP surgery. In both groups more participants reported having to travel for over 1 hour [modified, 15% (6 months) and 21% (12 months); usual care, 13% (6 months) and 17% (12 months)]. The percentages indicated the burden and time lost for travelling to hospital outpatient visits for both groups, as reflected in Table 36. Table 37 shows the mean travel costs per participant as reported and then how this translated into a total cost depending on their number of visits. The total travel costs are slightly greater for those in the modified self-management group when both time points are considered. This is because one participant in the modified self-management group reported very high costs for hospital outpatient visits.
| Travel cost (£) | Time point | |||||||
|---|---|---|---|---|---|---|---|---|
| 6 months | 12 months | |||||||
| Usual care | n | Modified | n | Usual care | n | Modified | n | |
| GP travel cost, mean (SD) | 1.33 (2.18) | 520 | 1.22 (1.79) | 531 | 1.24 (1.90) | 460 | 1.12 (1.72) | 450 |
| Hospital travel cost, mean (SD) | 4.00 (5.75) | 501 | 3.79 (4.79) | 515 | 4.14 (6.00) | 434 | 3.83 (5.00) | 437 |
| GP visits × cost, mean (SD) | 0.71 (2.49) | 630 | 0.60 (2.04) | 648 | 0.78 (4.10) | 560 | 0.39 (1.71) | 552 |
| Hospital visits × cost, mean (SD) | 0.53 (3.56) | 679 | 0.91 (9.68) | 708 | 0.28 (3.53) | 610 | 0.72 (11.90) | 608 |
| Total travel cost, mean (SD) | 1.23 (4.68) | 678 | 1.56 (10.26) | 682 | 1.12 (5.02) | 597 | 1.08 (12.20) | 583 |
A speculative estimate for the total cost at each follow-up time point has been calculated using productivity loss resulting from time off work and time spent travelling to appointments, as well as the travel costs reported in Table 37. However, the total numbers were very small because of the missing data. Table 38 shows the costs for the modified self-management group to be slightly higher than for the usual-care group at both time points. To make the most of the available data, the analysis was also repeated using the UK average wage rate (£31,800) to try and minimise the missing data caused by the poor report rate of income. 33 This resulted in a total of £220 (SD £1545, n = 487) for the usual-care group and £196 (SD £962, n = 504) for the modified self-management group.
| Societal cost (£) | Time point | |||
|---|---|---|---|---|
| 6 months | 12 months | |||
| Usual care (n = 256) | Modified (n = 318) | Usual care (n = 263) | Modified (n = 327) | |
| Total cost, mean (SD) | 20 (59) | 23 (66) | 17 (53) | 29 (137) |
List of abbreviations
- AE
- adverse event
- BDP
- beclometasone dipropionate
- BTS/SIGN
- British Thoracic Society/Scottish Intercollegiate Guidelines Network
- CEA
- cost-effectiveness analysis
- CEAC
- cost-effectiveness acceptability curve
- CEP
- cost-effectiveness plane
- CI
- confidence interval
- COPD
- chronic obstructive pulmonary disease
- CRN
- Clinical Research Network
- DHSC
- Department of Health and Social Care
- eCRF
- electronic Case Report Form
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- FAST
- Fourfold Asthma STudy
- GP
- general practitioner
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ISRCTN
- International Standard Registered Clinical/soCial sTudy Number
- MedDRA
- Medical Dictionary for Regulatory Activities
- MI
- multiple imputation
- Mini AQLQ
- Mini Asthma Quality of Life Questionnaire
- NCTU
- Nottingham Clinical Trials Unit
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- PCA
- prescription cost analysis
- PEF
- peak expiratory flow
- PIC
- Participant Information Centre
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RIS
- Research Initiative Site
- SAE
- serious adverse event
- SD
- standard deviation
- SE
- standard error
- TSC
- Trial Steering Committee
