Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 14/192/90. The contractual start date was in July 2016. The draft report began editorial review in January 2019 and was accepted for publication in December 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2021 Hall et al. This work was produced by Hall et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 The authors
Chapter 1 Introduction
Acute appendicitis is the most common surgical emergency in children. 1 The lifetime risk of developing appendicitis is 7–8% and the most common age point for developing appendicitis is the early teens. Appendicectomy is considered by most surgeons to be the gold-standard treatment for acute appendicitis. As a result, in 2017–18, there were 8105 emergency appendicectomies in England in children aged < 16 years.
Although appendicectomy is usually a simple procedure, it requires a general anaesthetic and an abdominal operation with inherent risks. Many parents find the proposal that their child needs emergency surgery frightening, and one that they are keen to avoid if an alternative is available. Work we undertook with patients and their families before the current study confirmed this. Families frequently ask ‘Does my child really need an operation?’.
An additional burden of paediatric appendicitis is the financial aspect. Treatment of children with appendicitis in England costs in excess of £21M per year. Appendicectomy also requires significant resource use, including need for out-of-hours surgery (45% of all paediatric appendicectomies are performed between 18.00 and 08.00).
An alternative approach to the treatment of children with acute appendicitis is treatment with antibiotics, without an appendicectomy. Although there is growing scientific interest in the use of non-operative treatment with antibiotics, we do not yet know whether or not this approach is safe and effective. However, there are several potential benefits of a non-operative approach over surgery, including:
-
avoiding the trauma, physiological stress, psychological distress and physical scarring of an operation
-
avoiding complications as a result of surgery or general anaesthesia
-
reduced NHS resource use with the potential for significant savings if non-operative treatment is effective.
However, such an approach would be acceptable only if antibiotic treatment is safe, if it is successful in the majority of cases and if the risk of recurrent appendicitis is low.
Although it has been known for some time that acute appendicitis can been treated successfully with antibiotics alone, in the context of remote environments without surgical service capability,2 the role of non-operative treatment as primary therapy within an established health-care system has only recently come under consideration. This was initially in adults,3–10 and only more recently in children. 11–13 Although a number of randomised controlled trials (RCTs) have been conducted among adults with acute appendicitis, extrapolating the findings of research in adults to children is problematic as there are key differences in appendicitis occurring in adults compared with children. Paediatric-specific research is necessary because appendicitis presents differently in children and adults. The intra-abdominal inflammatory response of adults is different to that of children;14,15 the inflammatory response in children may be more amenable to antibiotic treatment alone, and the psychosocial and economic impacts of appendicitis in children affect the whole family, rather than just the individual.
A systematic review and meta-analysis of the existing literature relating to non-operative treatment of appendicitis in children was undertaken prior to this study. 16 This identified 10 articles reporting just 413 children who received non-operative treatment. There was just one RCT, which was a pilot RCT, and therefore was not powered to compare the efficacy of non-operative treatment with that of surgery, but was conducted to inform the design of a large multicentre RCT, including North America, that is currently recruiting.
The systematic review concluded that further research into the clinical outcomes and cost-effectiveness of non-operative treatment compared with appendicectomy in the form of RCTs was needed to inform future decision-making for this group of patients. Importantly, none of the existing studies of non-operative treatment of acute uncomplicated appendicitis in children has identified any safety concerns regarding the intervention. 11,13,17–20
Given the current clinical interest, the evidence of the success of non-operative treatment and clear demand from patients, we believe that the time is right for a well-designed trial comparing non-operative treatment, namely antibiotics, with appendicectomy for acute uncomplicated appendicitis in children. However, prior to this, we identified a need for a feasibility study to inform the design and delivery of such a trial. A number of factors mean that a feasibility study is prudent prior to committing the resources to a full effectiveness trial. Such factors include a lack of experience of non-operative treatment of acute uncomplicated appendicitis in the UK, meaning that surgeons may not be willing to recruit; a challenging recruitment profile (children with appendicitis present to hospital as an emergency, often out of routine working hours and at the weekend), meaning that specific arrangements would have to be put in place to enable recruitment; a complex pattern of outcomes of interest to different stakeholder groups, meaning that identification of a primary outcome for an effectiveness trial is not clear; and a need to engage with relevant stakeholders to optimise the design and delivery of a trial.
We therefore designed a feasibility study, the aim of which was to answer the research question: is it feasible and acceptable to conduct a multicentre RCT testing the clinical effectiveness and cost-effectiveness of a non-operative treatment pathway for the treatment of acute uncomplicated appendicitis in children?
The specific objectives of this initial feasibility study were to:
-
assess the willingness of parents and children to be enrolled in, and surgeons to recruit to, a randomised trial comparing operative with non-operative treatment and to identify anticipated recruitment rate
-
identify strategies to optimise surgeon–family communication to inform the future RCT
-
enhance the design of a future RCT from the perspectives of stakeholders at participating sites (children, parents, surgeons and nurses)
-
identify what core outcomes family members and surgeons regard as important to measure in a future RCT and to develop a core outcome set (COS)
-
assess the equipoise and willingness of UK paediatric surgeons to participate in a future RCT
-
generate data to allow for the design of a definitive RCT, including sample size calculation and identification of key cost drivers and other parameters necessary to perform a full economic analysis
-
examine clinical outcomes of children with acute appendicitis treated without an operation, including an initial assessment of efficacy and safety of this treatment pathway in our centres
-
ensure that the whole of the research programme is well informed by a group of children and parents [the study-specific advisory group (SSAG)].
The CONservative TReatment of Appendicitis in Children a randomised controlled Trial (CONTRACT) feasibility study comprised a number of inter-related elements, carefully designed to fulfil the following objectives:
-
A randomised controlled feasibility trial of children with appendicitis comparing a non-operative treatment pathway with appendicectomy.
-
A detailed programme of embedded qualitative and quantitative research to optimise recruitment to the feasibility RCT. This was designed to inform the design and conduct of any future RCT of non-operative treatment versus appendicectomy in the treatment of acute uncomplicated appendicitis in children.
-
A health economics feasibility study to allow the identification of key cost drivers and other parameters necessary to perform a full economic evaluation in a future RCT. This included the design and piloting of data collection tools and the adoption of a microcosting approach.
-
The development of a COS for the treatment of children with uncomplicated acute appendicitis for use in the future RCT as well as the wider research community.
-
A patient and public involvement (PPI) workstream that reciprocally fed into elements 1, 2 and 4 (above). A SSAG was formed, made up of children who have had acute uncomplicated appendicitis, children who have not and parents.
Chapter 2 Methods of the feasibility randomised controlled trial
This chapter is focused on the methodology of the clinical component of the feasibility RCT. The methodology of the other elements of the wider study is described in the relevant later chapters.
Trial design
We performed a prospective feasibility RCT comparing appendicectomy with non-operative treatment in children with acute uncomplicated appendicitis. Recruitment lasted for 12 months and was open in three specialist paediatric surgery centres in England.
Participants
Participants were children aged 4–15 years inclusive with a clinical diagnosis of acute appendicitis, who would normally be treated with an appendicectomy as part of their standard care.
Inclusion criteria
-
Children aged 4–15 years.
-
Clinical diagnosis, either with or without radiological assessment, of acute appendicitis that, prior to study commencement, would be treated with appendicectomy.
-
Written informed parental consent, with child assent if appropriate.
Exclusion criteria
-
Clinical signs or radiological findings to suggest perforated appendicitis.
-
Presentation with appendix mass.
-
Previous episode of appendicitis or appendix mass treated non-operatively.
-
Major anaesthetic risk precluding allocation to the appendicectomy arm.
-
Known antibiotic allergy preventing allocation to the non-operative treatment arm.
-
Antibiotic treatment started at referring institution (defined as two or more doses administered).
-
Cystic fibrosis.
-
A positive pregnancy test.
-
Current treatment for malignancy.
Interventions
Non-operative treatment arm
Children randomised to non-operative treatment were treated according to a clinical pathway designed specifically for this trial. This treatment pathway comprised fluid resuscitation, a minimum of 24 hours of broad-spectrum intravenous (i.v.) antibiotics (per local antimicrobial policy), a minimum of 12 hours of nil by mouth (NBM) and regular clinical review to detect signs and symptoms of significant clinical deterioration, including, but not limited to, increasing fever, increasing tachycardia and increasing tenderness. After the initial 12-hour period of NBM, oral intake was advanced as tolerated. Children successfully treated without an operation were converted to oral antibiotics (per local policy) once they were afebrile for 24 hours and tolerating oral intake.
Clinical reviews were completed at approximately 24 and 48 hours post randomisation. Any children who showed signs of significant clinical deterioration by 24 hours, or at any point during the trial, were treated with appendicectomy. Children who were considered stable or improving continued with non-operative treatment. At 48 hours, any child who had not shown clinical improvement underwent an appendicectomy. The decision to continue non-operative treatment at these time points, or to recommend discontinuation of non-operative treatment and to recommend appendicectomy instead, was made by the treating consultant and based on clinical judgement rather than any specific features that are not evidence based. All reasons for a change in treatment were recorded in detail to guide a clinical pathway in a future trial.
Any child who received an appendicectomy for an incomplete response to non-operative treatment followed a standardised postoperative treatment regime already in use at each institution and identical to that used in the appendicectomy arm. The reason for having an appendicectomy was recorded.
Children treated non-operatively received a total of 10 days of antibiotics following randomisation, unless decided otherwise by the treating clinician. Children who received non-operative treatment were not routinely offered interval appendicectomy, but were counselled about the risk of recurrence.
Appendicectomy treatment arm
Children randomised to the appendicectomy arm underwent either open or laparoscopic appendicectomy at the surgeon’s discretion, performed by a suitably experienced trainee (as per routine current practice) or a consultant.
Participants received i.v. antibiotics from the time of diagnosis and were treated postoperatively with i.v. antibiotics according to existing institutional protocols; however, the following recommended regime was used to guide practice: children with acute uncomplicated appendicitis or a macroscopically normal appendix received no further antibiotics. Children with a perforated appendix (defined as a faecolith or faecal matter in the peritoneal cavity, or visualisation of a hole in the appendix) continued to receive i.v. antibiotics for a minimum of 3 days, and received a minimum total course of antibiotics of 5 days (i.v. and oral). The duration of antibiotic therapy was not standardised beyond this owing to anticipated variation in intraoperative findings and in response to treatment. The type of antibiotics used was identical to those used in the non-operative treatment arm in each centre. Any child failing to respond to first-line antibiotics was treated as was clinically appropriate with a longer course of antibiotics or a change in antibiotic therapy, with the choice of antibiotic determined by intraoperative swab or fluid culture.
Postoperatively, children with uncomplicated acute appendicitis or a normal appendix did not routinely have a nasogastric tube or a urinary catheter. They received oral intake as tolerated after surgery.
The participant flow through the two treatment arms is shown in Figure 1.
FIGURE 1.
Overview of the trial pathway. a, Non-operative treatment: NBM/sips for the initial 12 hours, minimum, then advance diet as tolerated; i.v. antibiotics for 24 hours, minimum, change to oral once afebrile for 24 hours, total course 10 days; and analgesia; b, appendicectomy group: no routine use of nasogastric tube or urinary catheter, advance diet as tolerates; c, defined as either seeing a hole in the appendix or seeing faecal matter/faecolith in the peritoneal cavity; d, continue i.v. antibiotics until afebrile for 24 hours, then change to oral; minimum 5 days total on antibiotics; e, criteria for discharge include vital signs within normal limits, tolerating light diet, adequate oral analgesia and mobile.
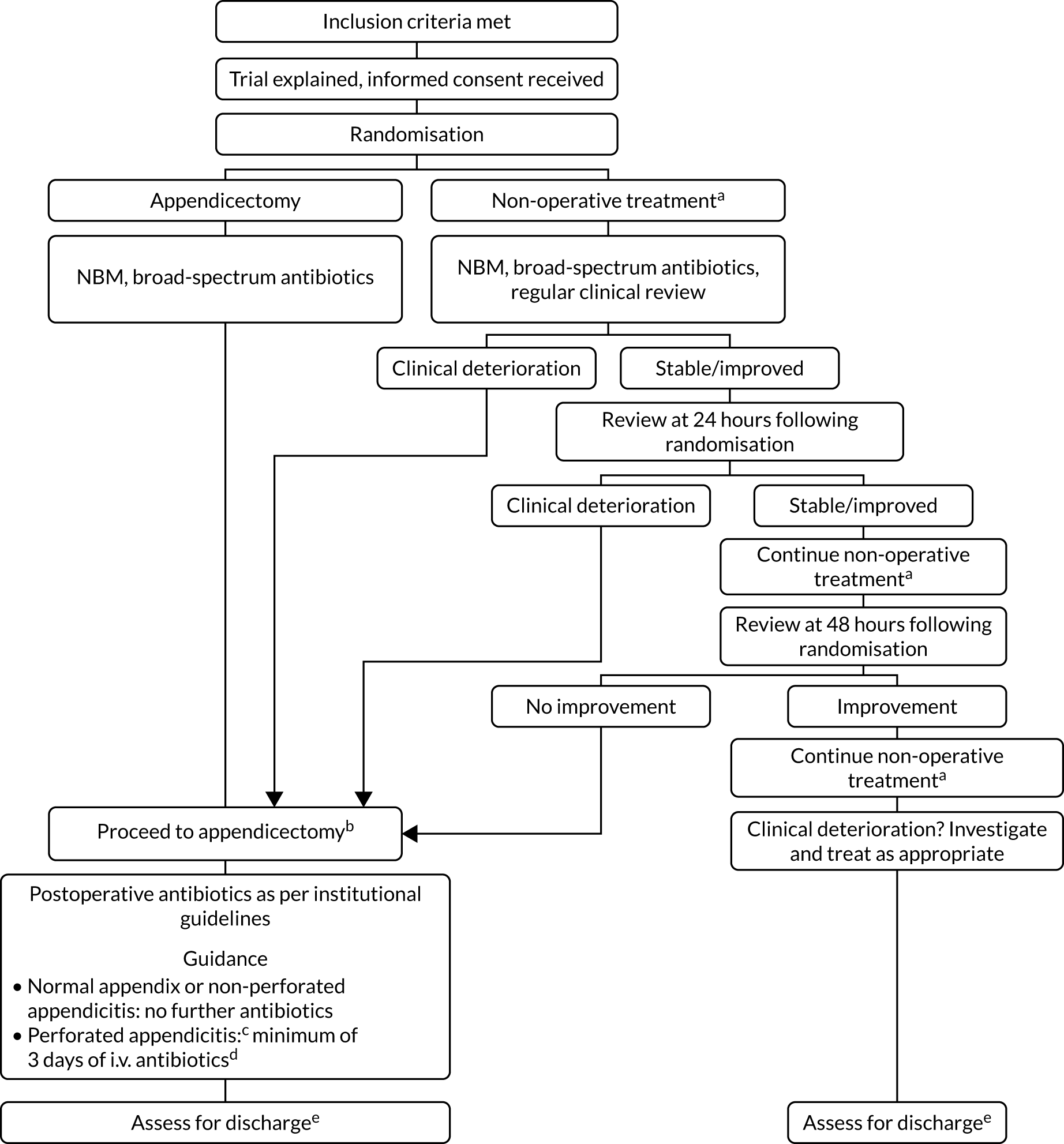
Discharge assessment
Criteria for discharge home were identical in both treatment arms and were as follows: vital signs within normal limits for age, afebrile for ≥ 24 hours, tolerating light diet orally, adequate oral pain relief and able to mobilise. We aimed to determine the feasibility of a blinded discharge assessment in a future RCT by attempting to complete a blinded discharge assessment for each participant as follows. Once a decision to discharge the child had been made, a member of the clinical team who had not been involved directly in the child’s treatment was asked to complete a discharge assessment. This assessor did not have prior knowledge of the randomisation or treatment received by the child. On completion of the discharge assessment, the assessor ‘guessed’ which treatment the child received. If the assessor became unblinded during the assessment, this was recorded.
Follow-up
All participants were given a diary card [see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/1419290/#/ (accessed February 2020)] to complete for the 14 days immediately following discharge from hospital and provided with a stamped addressed envelope to return this to the clinical trials unit on completion. This assessed whether or not the child had taken antibiotics and analgesia medication on each day following discharge, as well as an assessment of their recovery based on their ability to complete normal or full daily activities and attend school (if applicable). Finally, it also assessed whether or not parents had had to miss work as a result of their child’s illness. Follow-up appointments for all participants took place at 6 weeks and at 3 and 6 months following discharge, either in the outpatient clinic or in the clinical research facility at each centre. If a face-to-face appointment was not possible, the 3- and 6-month follow-ups were completed by telephone. Following an analysis of follow-up rates during the trial, we introduced an incentive in an attempt to improve follow-up rates. This was introduced in March 2018. All participants who attended all remaining follow-up visits from that point onwards were offered a £10 voucher.
Randomised controlled trial processes
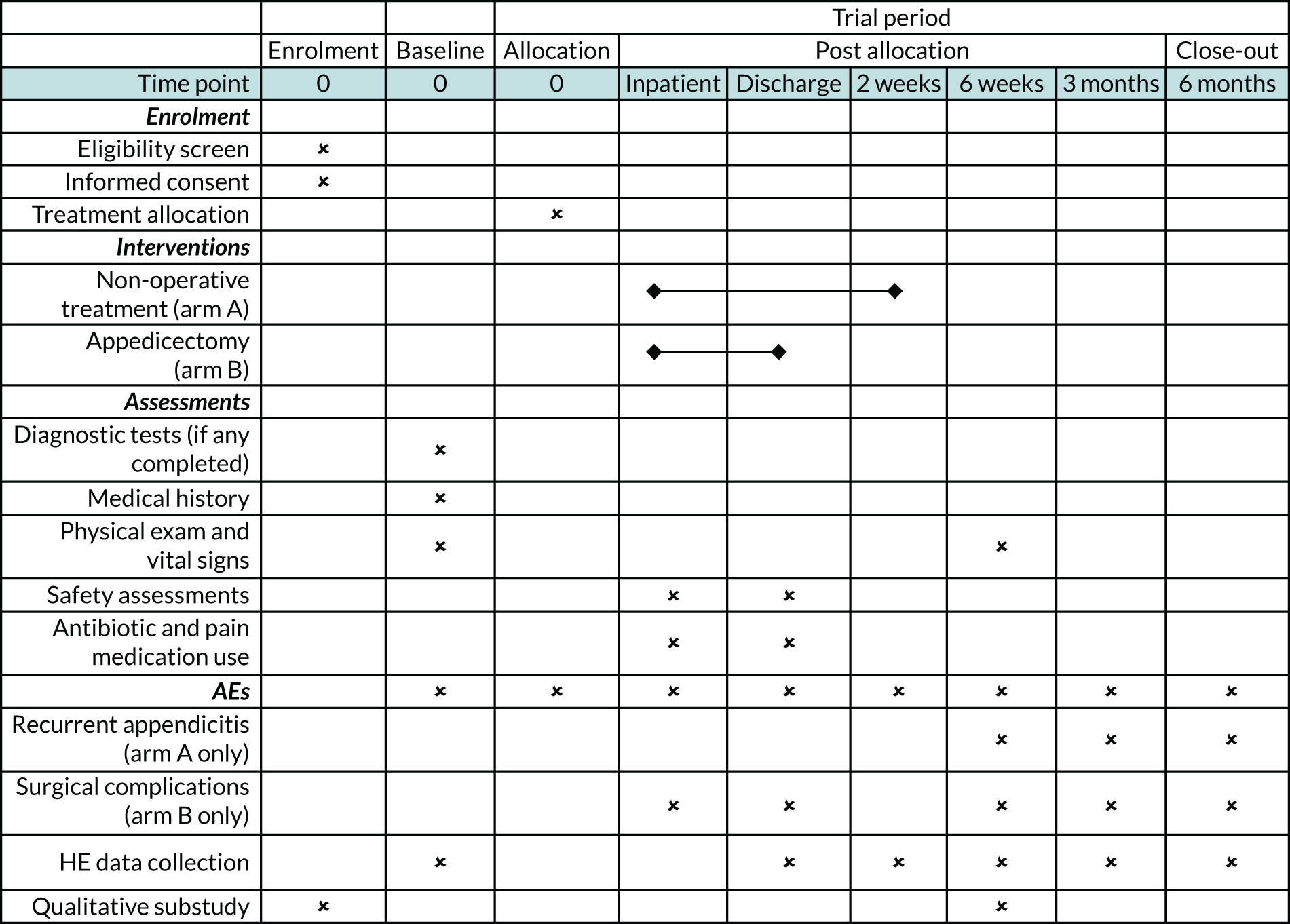
The schedule of enrolment, interventions and follow-up is shown in Figure 2.
FIGURE 2.
Overview of trial activity. AE, adverse event; HE, health economics.
Participant identification and recruitment
Participants were identified by the clinical team at the time of diagnosis and their eligibility was confirmed by the research team as soon as possible. Eligible patients were approached by the treating clinical teams with support from dedicated research nurses. Potential participants were provided with written information about the trial [see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/1419290/#/ (accessed February 2020) and shown a short video describing the study (see http://tinyurl.com/contract-f]. From the time of first discussing the trial with potential participants and their families, a maximum of 4 hours was permitted before a decision could be made regarding participation. This was to ensure that there was no delay in providing treatment as a result of considering trial participation. After receiving written informed consent (and assent from children aged ≥ 12 years who wished to give it), a member of the trial team randomised the participant to one of two treatment groups in a 1 : 1 ratio via an independent web-based system [TENALEA (Alea Clinical, Abcoube, the Netherlands)]. This online system allowed complete pre-randomisation concealment of treatment allocation and provided instant assignment to either the appendicectomy group or the non-operative treatment group. Minimisation was used to account for recruiting centre and to ensure balance between the groups in factors that may affect diagnostic accuracy and outcome of treatment. The factors taken into account were (1) sex, (2) age (4–8 years or 9–15 years), (3) duration of symptoms (onset of pain to recruitment to trial: < 48 hours or ≥ 48 hours) and (4) recruiting centre. In addition to the data required to complete randomisation, limited additional data were collected at baseline, including the use of any diagnostic imaging, and an Alvarado score21 (a scoring system used to help predict the severity of appendicitis) was calculated for each participant. This was used to provide an overview of the severity of illness of each child as a descriptive term; it was not used as a minimisation variable nor was a minimum Alvarado score used in the eligibility criteria.
Data collection and analysis
Data were recorded by dedicated research nurses at each site directly into an electronic, secure, web-based case report form (Medidata Rave® database; Medidata Solutions, Inc., New York, NY, USA). Data analysis was performed by the study statistician, who was blinded to treatment allocation by the use of coded data. As this is a feasibility study, all analyses were treated as preliminary and exploratory and data are reported descriptively. Feasibility outcomes (number of eligible patients, recruitment/retention rates, reasons for non-participation, success of blinding of the discharge assessor), treatment outcomes and complications are presented as simple summary statistics with 95% confidence intervals (CIs). Clinical outcomes were compared between treatment groups in an exploratory analysis.
Trial oversight
A Study Management Group (SMG) was responsible for overseeing the day-to-day management of the trial. An independent Trial Steering Committee and Data Monitoring and Safety Committee were convened to provide oversight of the trial. Their roles and responsibilities, which included adverse event (AE) monitoring, were agreed at the beginning of the trial and documented in specific charters. Specific processes to report AEs in a timely manner to the relevant committee were agreed.
Protocol, registration and ethics approval
The trial was carried out in accordance with a published protocol22 that was developed in accordance with the Standard Protocol Items: Recommendations for Interventional Trials – Children (SPIRIT-C) guidance,23 and was registered prior to recruitment of the first participant (International Standard Randomised Controlled Trial Number: ISRCTN15830435). The overall study was given ethics approval by the Hampshire A Research Ethics Committee (reference number 16/SC/0596). The study is reported in accordance with the Consolidated Standards of Reporting Trials 2010 statement. 24
Outcomes
Primary outcome
The primary outcome was to assess the feasibility of conducting a multicentre RCT testing the clinical effectiveness and cost-effectiveness of a non-operative treatment pathway for the treatment of acute uncomplicated appendicitis in children. This was evaluated as the proportion of eligible patients who were approached and recruited to the study over 12 months.
Secondary outcomes
The secondary outcomes were predominantly centred on the qualitative and COS substudies, contributing to the development of a future RCT. Those specifically relating to the RCT are marked with an ‘*’:
-
*Determine the willingness of parents, children and surgeons to take part in a randomised trial comparing operative treatment with non-operative treatment, and identify the anticipated recruitment rate. This was assessed from audio-recorded family–surgeon recruitment consultations; interviews with patients, parents, surgeons and nurses; surgeon surveys; and focus groups.
-
Identify strategies to optimise surgeon–family communication using the consultation and interview data.
-
Design a future RCT from the perspectives of stakeholders at participating sites (children, parents, surgeons, nurses, etc.), informed by the consultation and interview data, surgeon surveys and focus groups.
-
Assess the equipoise and willingness of UK paediatric surgeons to participate in a future RCT through surgeon surveys and focus groups.
-
*Assess the clinical outcomes of trial treatment pathways, including (1) overall success of initial non-operative treatment (measured as the number of participants who were randomised to non-operative treatment and discharged from hospital without appendicectomy), (2) complications of disease and treatment (measured during hospital stay and during the 6-month follow-up period) and (3) the rate of recurrent appendicitis during the 6-month follow-up period.
-
*Assess the performance of the study procedures, including retention of participants for the duration of the trial and the feasibility of outcome-recording and data collection systems.
Sample size
Participants were recruited from three centres for 12 months. It was expected that each centre would treat 80–100 children with acute appendicitis per year, with an estimate that at least 130 would be eligible out of the 240–300 potential patients. As this was a feasibility study, we did not specify a specific sample size, but aimed to define our recruitment rate within an approximate 10% margin of error. Based on an anticipated study population available for recruitment of approximately 130 participants, we would be able to estimate a true 40% recruitment rate with a 95% CI of 31% to 49% and a true 50% recruitment rate with a 95% CI of 41% to 59%. These numbers of participants in the feasibility RCT would be adequate to test treatment pathway procedures, data collection methods and loss to follow-up.
Changes to the original protocol
Version 2, 10 April 2017
-
Minor clarification of serious adverse event (SAE) exceptions in section 6.2.1.
-
Addition of ISRCTN reference on front page.
Version 3, 4 July 2017
-
Change to co-investigator at St George’s Hospital.
-
Reference to online patient video access.
-
Consent process oversight by Southampton Clinical Trials Unit (SCTU).
-
Telephone consent process for qualitative substudy.
-
Specification of office hours for randomisation backup.
-
Timeline for questionnaire completion.
-
Time frame for AE reporting.
-
Update to COS protocol.
Version 4, 8 March 2018
-
Addition of an incentive during the follow-up stage of the trial.
Chapter 3 Feasibility randomised controlled trial results
Trial timelines and recruitment
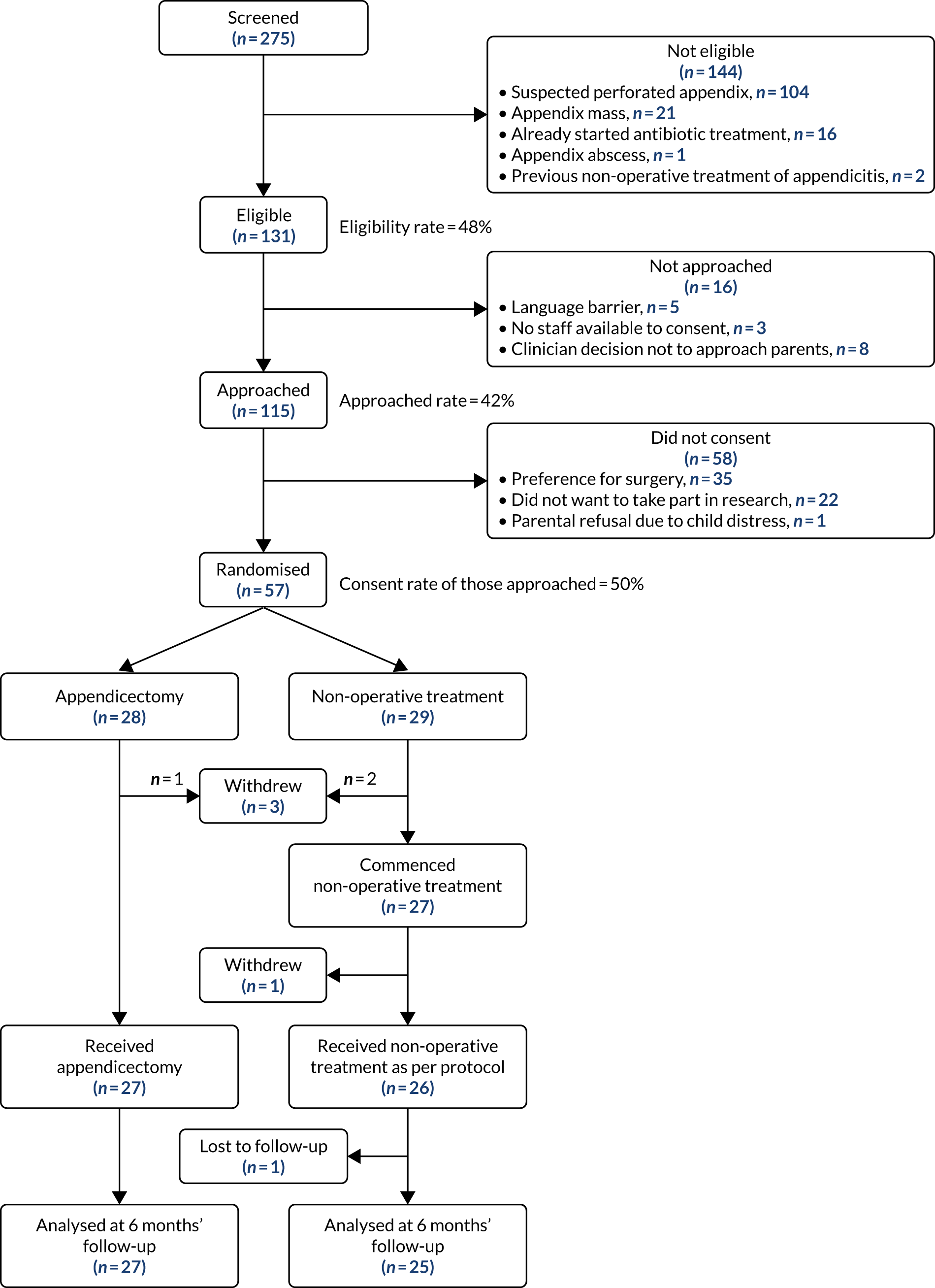
Pre-trial education and training visits, as well as site initiation visits, took place between December 2017 and February 2018. All three centres opened to recruitment simultaneously on 1 March 2017 at midnight. All three centres were open to recruitment for 12 months until midnight on 28 February 2018. During this time, a total of 275 children with acute appendicitis between the ages of 4 and 15 years, inclusive, presented to the three participating centres. Of these, 144 were ineligible for inclusion for the reasons shown in Figure 3. The remaining 131 children met the eligibility criteria for the CONTRACT feasibility RCT (48%, 95% CI 40% to 59%). Of these, 16 were not approached because they did not speak adequate English, no recruiting staff were available to approach the parents for consent or there was an active clinical decision not to approach the parents regarding the trial (typically in the presence of an additional medical comorbidity in the potential participant). A total of 16 children (12%) were therefore not approached despite meeting the eligibility criteria. The remaining 115 children (88% of those eligible) were all approached for participation in the trial: 57 agreed to participate and were successfully randomised. The remaining 58 did not consent to the trial because of a preference for surgical treatment (n = 35), because they did not want to take part in research (n = 22) or, in one case, because the parents were unable to consider the trial because they felt that their child was too distressed. Overall, 44% (57/131) of all eligible patients were recruited and the overall recruitment rate of those approached over the 12 months of the trial was 50% (95% CI 40% to 59%).
FIGURE 3.
The Consolidated Standards of Reporting Trials flow diagram of the CONTRACT feasibility RCT.
Feasibility of trial recruitment
The first participant was recruited to the trial on 2 March 2017, and recruitment continued for the 12-month duration of the study, as shown in Figure 4. Although no formal recruitment target was set for this trial, based on the anticipated number of eligible participants and anticipated recruitment rate, we aimed to recruit 52 participants. Overall, the number of participants recruited to this feasibility trial exceeded this ‘target’, and the recruitment rate of 50% (95% CI 40% to 59%) was at the upper end of the pre-trial target recruitment range of 40–50%.
FIGURE 4.
Trial recruitment by month.
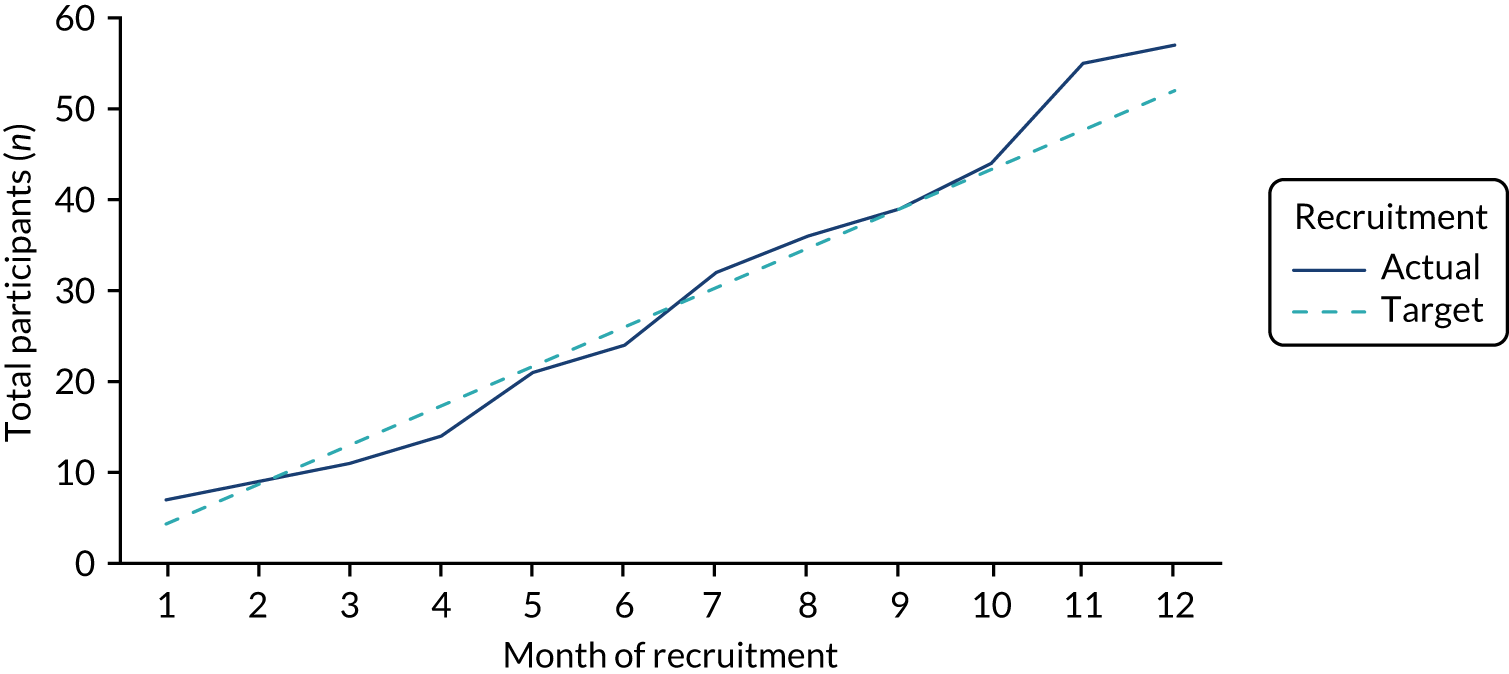
Following the initial recruitment training prior to the start of recruitment, further training was completed at all three centres in early July 2017 (month 5) and November 2017 (month 9). The relationship between retraining and recruitment rate is explored fully in Chapter 4. Recruitment rate during the initial 4 months of the trial was 38%, rose to 47% in months 5–9 and rose further to 72% in months 10–12.
Of note, all three centres were actively involved in screening and recruiting patients for the duration of the study (Table 1). The overall recruitment rate exceeded 40% at all three participating centres.
| Details | Total | Trial centre | ||
|---|---|---|---|---|
| Alder Hey | Southampton | St George’s | ||
| Total patients screened (n) | 275 | 145 | 78 | 52 |
| Eligible patients who entered trial (n) | 57 | 25 | 21 | 11 |
| Eligible patients who were approached but did not enter trial (n) | 58 | 27 | 16 | 15 |
| Recruitment rate (%) | 50 | 48 | 57 | 42 |
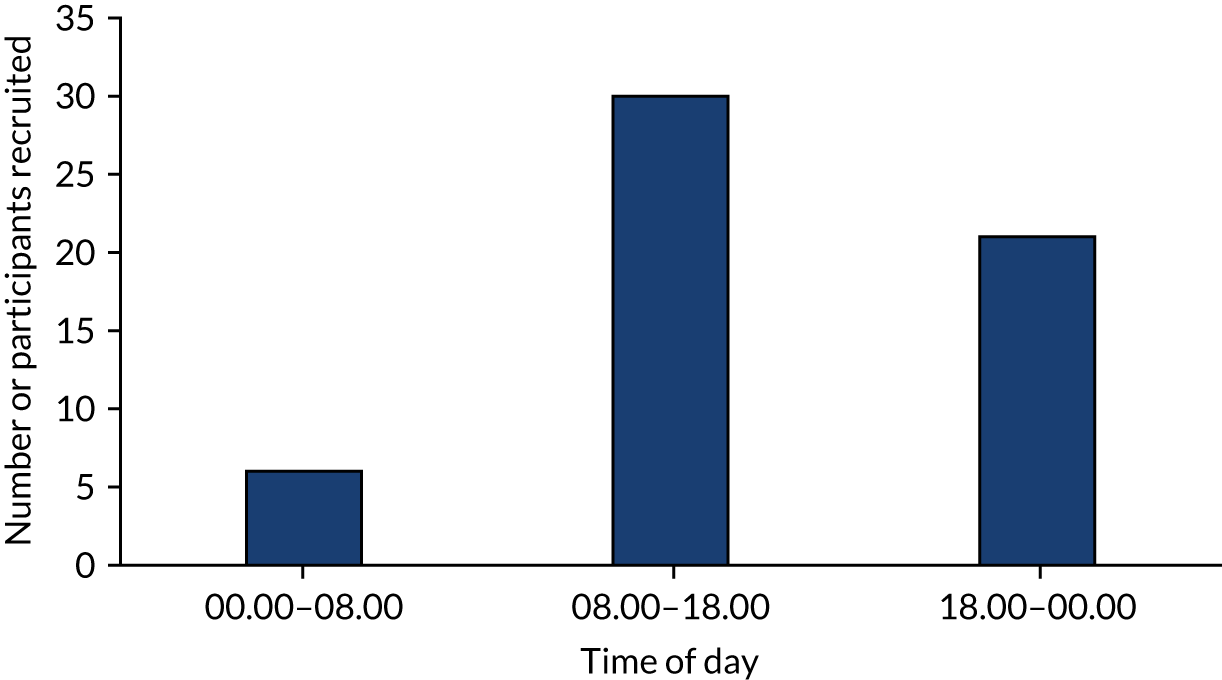
Of note, particularly in relation to the feasibility of a future trial, recruitment was successfully completed by over 21 different surgeons across the three centres. Furthermore, participants were successfully recruited to the trial outside normal working hours. Three-fifths of participants were recruited during working hours, and the remaining two-fifths were recruited either between the hours of 18.00 and 00.00 or between 00.00 and 08.00 (Figure 5).
FIGURE 5.
Number of participants recruited at different times of day.
Adherence to treatment allocation and protocol, trial retention and other feasibility outcomes
Figure 3 shows participant flow through the clinical trial. Three participants withdrew consent soon after randomisation and formally withdrew from the trial. Reasons given for this were dissatisfaction with the treatment allocated (n = 2) and being too overwhelmed with the diagnosis to continue in the trial (n = 1). The remaining 27 participants in the appendicectomy arm and 27 participants in the non-operative treatment arm commenced the assigned intervention. All 27 participants in the appendicectomy arm received the treatment as allocated; there was one protocol deviation in the non-operative treatment arm: the parents of one child withdrew consent for the study and requested appendicectomy 8 hours after randomisation, but did not withdraw consent for continued data collection.
A total of 34 out of 54 eligible children (63%) underwent a blinded discharge assessment. For the remaining 20 children, this was not possible owing to non-availability of appropriate members of staff. Data relating to the blinded discharge assessment are shown in Table 2. In one case, the assessor was unblinded during the assessment. In the remaining cases for which an assumed treatment was provided, the assessment was correct in 61% (n = 20) of cases and incorrect in 39% (n = 13) of cases. This is not statistically significantly different from the 50% accuracy that one would anticipate achieving by chance alone (p = 0.28; chi-squared test).
| Actual treatment allocation | Assumed treatment by blinded assessor (n) | Unblinded (n) | Total (n) | |
|---|---|---|---|---|
| Appendicectomy | Non-operative treatment | |||
| Non-operative treatment | 6 | 13 | 0 | 19 |
| Appendicectomy | 7 | 7 | 1 | 15 |
| Total | 13 | 20 | 1 | 34 |
Following discharge, 26 out of 54 (48%) participants returned a diary card: 15 in the appendicectomy arm and 11 in the non-operative treatment arm. Of the 11 in the non-operative arm who returned data, one completed the diary card until only day 4 following discharge. Thus, fewer than half of all participants in the study at the time of discharge from hospital (26/54 = 48%) returned diary card data for analysis. This equates to a total of 380 days of reporting.
One child was completely lost to follow-up: they did not attend any follow-up appointment and could not be contacted by telephone. This child was withdrawn from the study after the 3-month follow-up time point as they were known to have moved overseas. The remaining participants attended follow-up appointments or were contacted by telephone at 6 weeks (48/54, 89% of those remaining in the study), 3 months (46/54, 85%) and 6 months (n = 45/53, 85%). All other participants either did not attend or did not respond to repeated requests for contact by telephone. During the study, in an attempt to increase the follow-up rate, we added an incentive for completion of all remaining follow-up attendances by an individual participant in the form of a £10 shopping voucher. None of the 6-week follow-up appointments was incentivised owing to the time when the incentive was introduced. Of the 3-month follow-up appointments, 47 were not incentivised and were completed by 39 participants (83%, 95% CI 72% to 93%), whereas seven were incentivised and all seven were completed (100%, 95% CI 59% to 100%). Of the 6-month follow-up appointments, 34 were not incentivised and were completed by 28 participants (83%, 95% CI 65% to 93%), and 19 were incentivised and were completed by 17 participants (89%, 95% CI 67% to 99%).
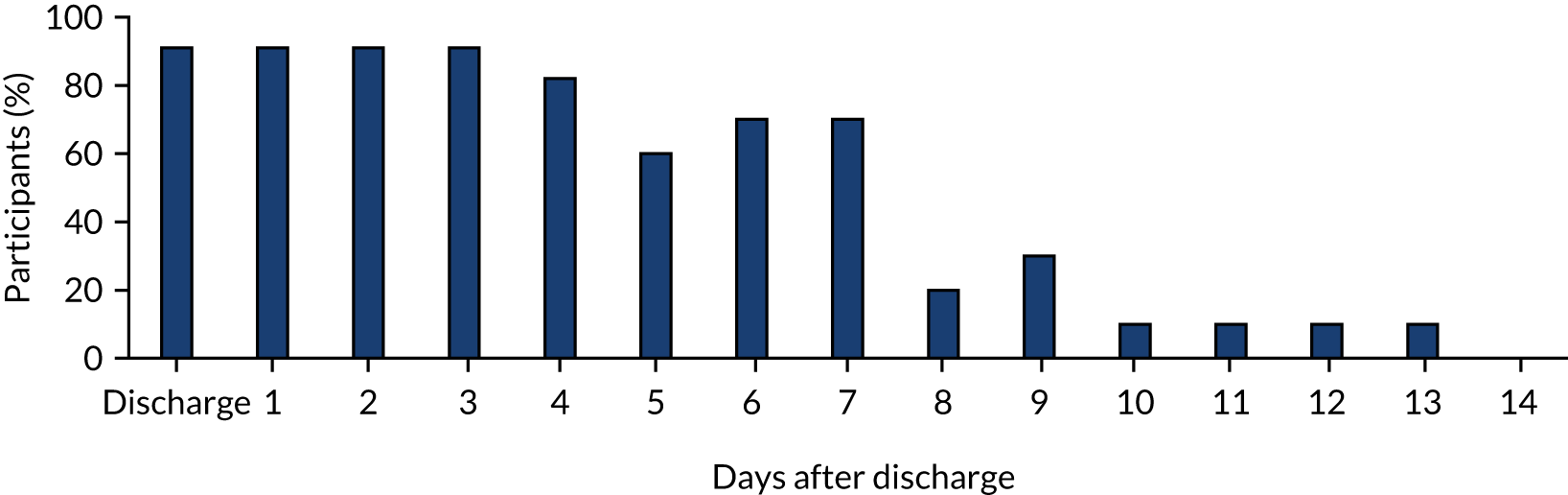
Compliance with outpatient antibiotic treatment
We had initially planned to assess compliance with outpatient antibiotic treatment in the non-operative treatment arm through the use of diary cards. However, owing to the low diary card completion rate and higher than anticipated crossover to appendicectomy during initial hospital admission, these data should be interpreted with some caution. They are included here for completeness (Figure 6).
FIGURE 6.
Compliance with oral antibiotics after discharge in the non-operative treatment arm (n = 11 until day 3, n = 10 thereafter). Proportion of participants in the non-operative treatment arm who reported taking antibiotics at or following discharge.
Clinical aspects of the feasibility trial
Baseline characteristics
Baseline characteristics for the participants overall and described by treatment allocation are shown in Table 3.
| Characteristic | Treatment arm | Total (N = 57) | |
|---|---|---|---|
| Appendicectomy (N = 28) | Non-operative treatment (N = 29) | ||
| Age, median (range) | 10 years and 7 months (6 years and 4 months–13 years and 6 months) | 10 years and 3 months (5 years and 0 months–15 years and 11 months) | 10 years and 5 months (5 years and 0 months–15 years and 11 months) |
| Sex, male : female (n) | 18 : 10 | 18 : 10a | 36 : 20a |
| Duration of symptoms (hours),b median (range) | 32 (12–63) | 34 (12–79) | 33 (12–79) |
| Ultrasonography during diagnostic workup,c n (%) | 8 (29) | 8 (28)a | 16 (28) |
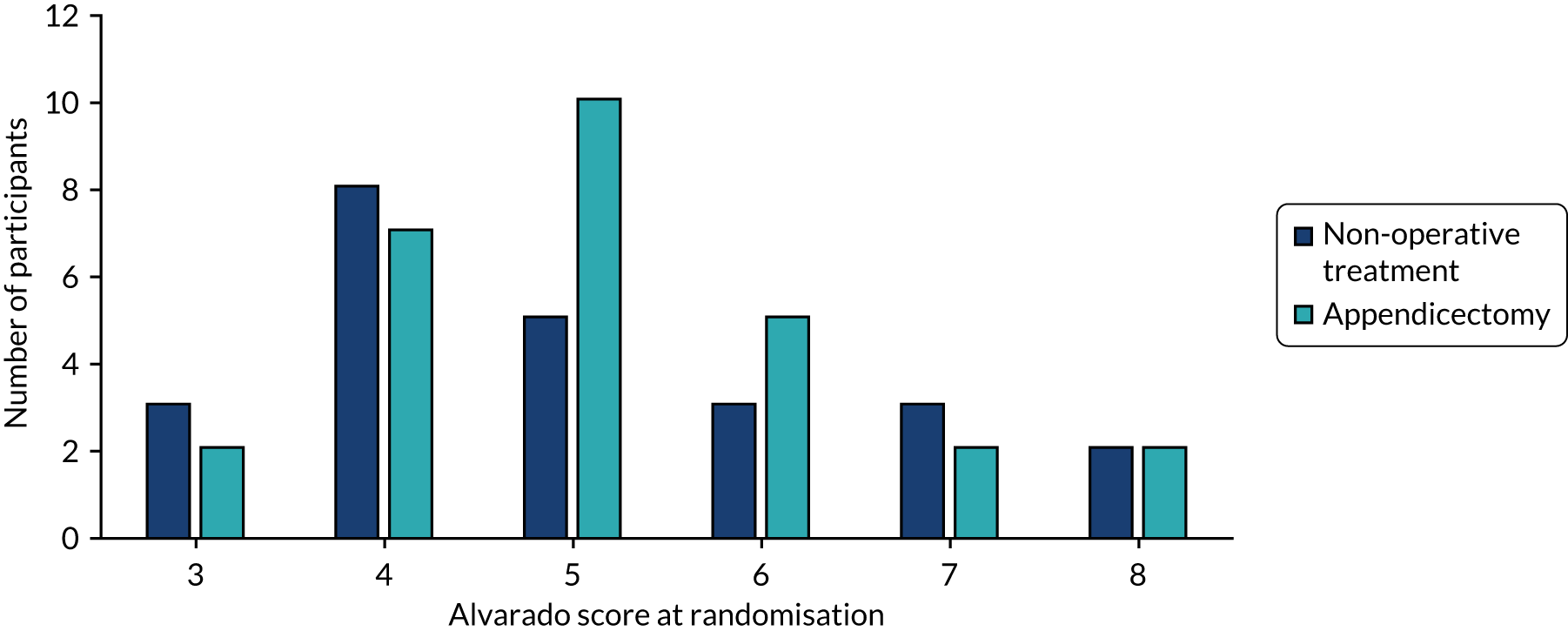
| Alvarado score,d median (range) | 5 (3–8) | 5 (3–8) | 5 (3–8) |
The distribution of Alvarado scores between groups is shown in Figure 7.
FIGURE 7.
Distribution of Alvarado scores across treatment arms.
Treatment received and outcome
In the appendicectomy arm, all 27 children received the allocated intervention and were treated according to the clinical pathway. Histological diagnosis of the resected appendix revealed simple acute appendicitis in 17 participants (63%), perforated appendicitis in eight participants (30%) and a normal appendix in two participants (7%).
In the non-operative treatment arm, 19 of the 27 children (70%) responded to non-operative treatment successfully, followed the clinical pathway and were successfully discharged from hospital. The remaining eight children underwent appendicectomy during their initial hospital admission. Reasons for appendicectomy were parental choice (withdrawal from treatment arm, as discussed in Adherence to treatment allocation and protocol, trial retention and other feasibility outcomes) at 8 hours following randomisation (n = 1), deterioration in clinical condition (according to protocol) at range 12.3–44.4 hours following randomisation (n = 6) and no improvement after 48 hours of non-operative treatment (in accordance with protocol) (n = 1). For the children whose clinical condition deteriorated (n = 6), reasons for deterioration included persistent fever and pain, worsening peritonism and worsening pain. For the one child who did not have any symptomatic improvement after 48 hours, the primary persisting complaint was that of ongoing severe abdominal pain. All eight patients underwent successful appendicectomy during the initial hospital admission. Histological findings were simple acute appendicitis in four and perforated appendicitis in four.
Clinical outcomes related to hospital stay are reported on an intention-to-treat basis and are shown in Table 4. Both decision to discharge and actual time of discharge were recorded as there may be non-medical factors (e.g. availability of transport or other social factors) that delay actual discharge.
| Time from randomisation to | Total time (hours), median (range) | Time by treatment arm (hours), median (range) | |
|---|---|---|---|
| Non-operative treatment | Appendicectomy | ||
| Decision to discharge | 67 (20–196) | 57 (21a–188) | 65 (20–196) |
| Actual discharge | 69 (21–196) | 76 (34–194) | 63 (21–196) |
For participants in the non-operative treatment group, the time from randomisation to discharge from hospital for children who responded to non-operative treatment and for those who underwent appendicectomy during the initial hospital admission are shown in Table 5.
| Time measure | Time (hours), median (range) | ||
|---|---|---|---|
| Total (n = 27) | Successful non-operative treatment (n = 19) | Appendicectomy during admission (n = 8) | |
| Randomisation to actual discharge | 76 (34–194) | 61 (34–125) | 116 (40–194) |
Of the 35 appendicectomies performed on participants in the trial during the initial hospital phase, 33 were performed laparoscopically, one was open surgery and one was laparoscopic converted to open surgery.
Early post-discharge outcomes
Data relating to early post-discharge recovery obtained from diary cards are summarised in Figures 8–10. A total of 26 diary cards were returned: 15 from the appendicectomy arm and 11 from the non-operative treatment arm. For one of the 11 participants in the non-operative treatment arm, data were reported up to and including day 4 only. For the remaining participants who returned diary cards, data were complete.
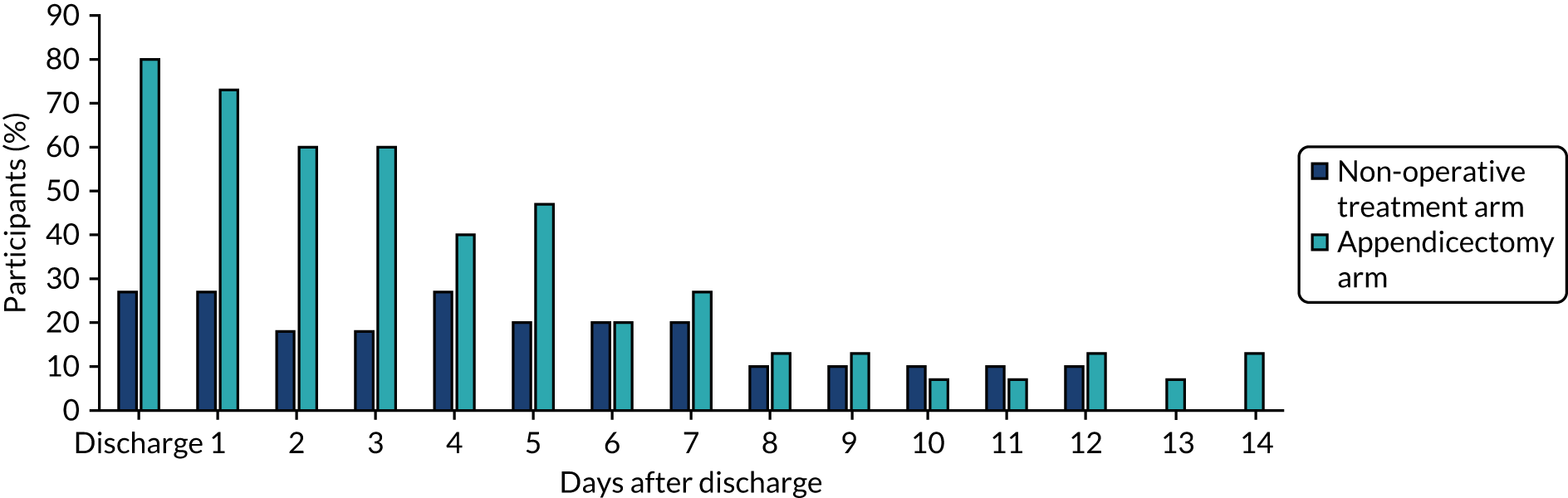
Pain medication after discharge home
The proportion of participants in each treatment arm who reported taking pain medication at and on the days following discharge home is shown in Figure 8. These data clearly suggest that analgesia use following discharge was lower following non-operative treatment than following appendicectomy. In accordance with the protocol, we have not performed a formal comparative analysis of these data.
FIGURE 8.
Analgesia use following discharge from hospital as reported in diary cards.
Return to normal activities
The proportion of participants in each treatment arm who reported being able to return to normal daily activities at and on the days following discharge home is shown in Figure 9. These data suggest that trial participants were able to return to normal activities faster following non-operative treatment than following appendicectomy.
FIGURE 9.
Return to normal activities as reported in diary cards.
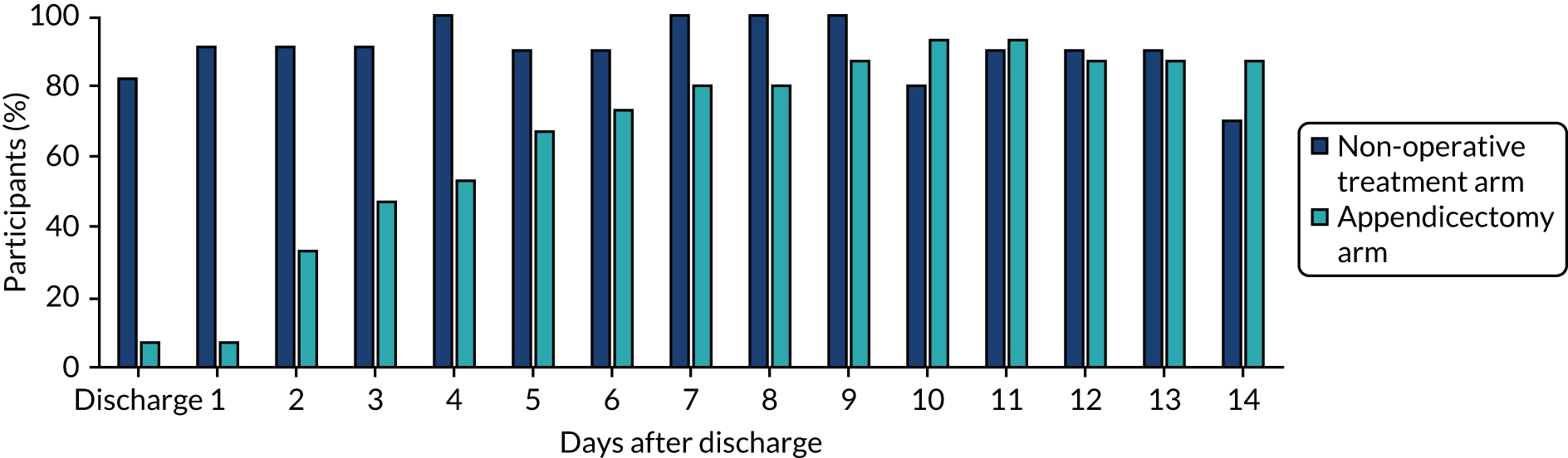
Return to full activities
The proportion of participants in each treatment arm who reported being able to return to full activities at and on the days following discharge home is shown in Figure 10. Like the data on return to normal activities, these data suggest that trial participants were able to return to full activities faster following non-operative treatment than following appendicectomy.
FIGURE 10.
Return to full activities as reported in diary cards.
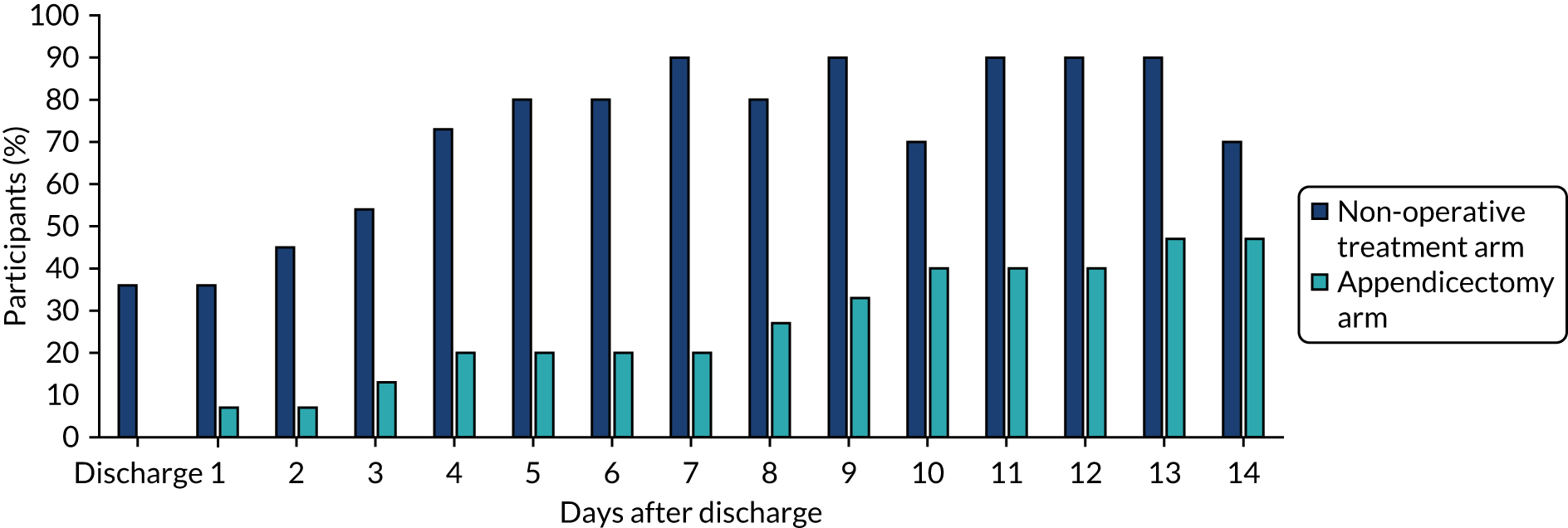
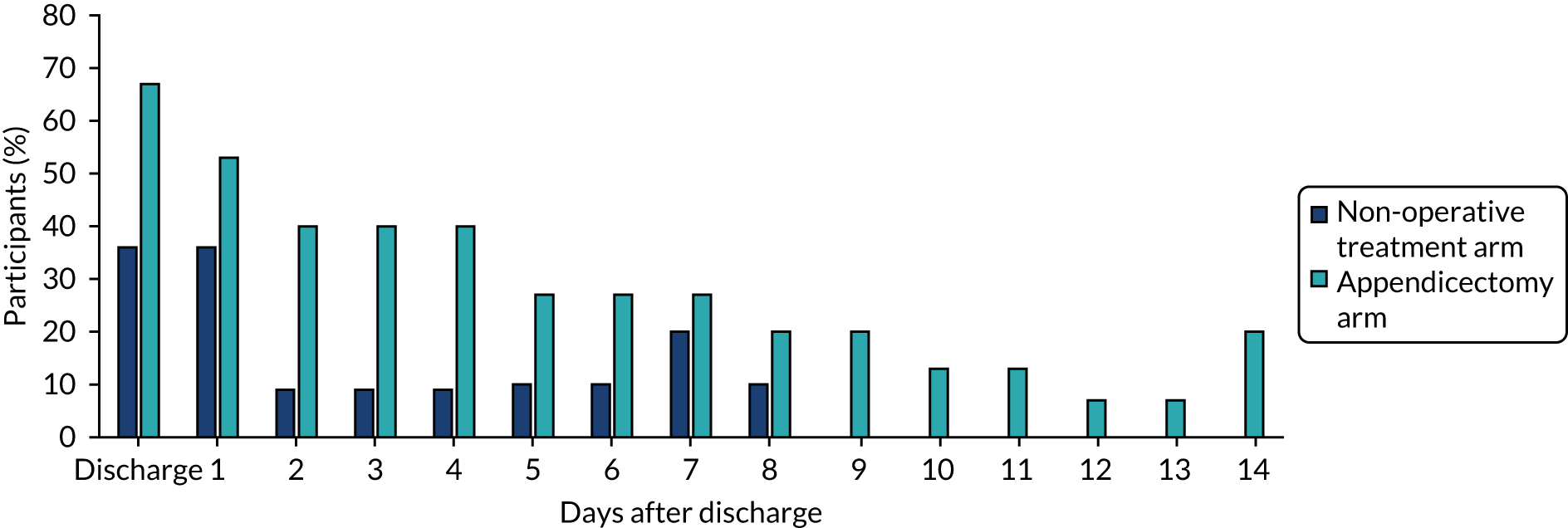
Parental absence from work
The proportion of participants in each treatment arm whose parents reported missing work as a result of their child’s illness or recovery is shown in Figure 11. These data suggest that parents were able to return to work more quickly if their child was allocated to non-operative treatment than if their child was allocated to appendicectomy. All parents of children allocated to non-operative treatment and who completed diary cards had returned to work by 9 days following discharge, whereas the parents of some children allocated to appendicectomy had not returned to work 2 weeks after discharge.
FIGURE 11.
Parental absence from work as reported in diary cards.
The 6-month follow-up
During the 6-month follow-up period, a total of seven children, all of whom were randomised to non-operative treatment, developed recurrent appendicitis. This equates to 24% of the total number randomised to non-operative treatment and 37% of the 19 participants who initially responded to non-operative treatment and were discharged from hospital without having had an appendicectomy. Of these seven children, six underwent appendicectomy (laparoscopic or open); histological findings were simple acute appendicitis in four of these children and perforated appendicitis in the remaining two. The remaining participant presented with an appendix mass at the time of recurrence, which was successfully treated non-operatively, and subsequently underwent interval laparoscopic appendicectomy. At the final follow-up of the trial, 11 children initially randomised to receive non-operative treatment (41%) had not undergone appendicectomy.
In the appendicectomy arm, three children (11%) were re-admitted to hospital following initial discharge for investigation and/or treatment of potential complications related to appendicectomy.
Adverse events
Adverse events during the 6-month follow-up period were captured and are summarised in Appendix 1, Tables 15 and 16. In the appendicectomy arm, 22 AEs were reported in eight participants; in the non-operative treatment arm, 24 AEs were reported in 15 participants. A small number of these AEs were assigned as being ‘possibly’ or ‘definitely’ attributable to the trial intervention.
In the non-operative treatment arm, the AEs included a rash at the time of receiving antibiotics in two participants. In the appendicectomy arm, three children developed wound complications (one dehiscence, one infection and one suture complications); one child developed a postoperative intra-abdominal abscess requiring percutaneous drainage and a peripherally inserted central catheter (PICC) for prolonged antibiotics; and one child had intra-abdominal fluid collection, which was treated with intravenous antibiotics. Other AEs, including sickness, pain, visits to the general practitioner (GP), visits to the emergency department and investigations through blood tests and ultrasonography, occurred in both treatment groups. Overall, three children in the appendicectomy arm and eight children in the non-operative treatment arm were re-admitted to hospital. All hospital re-admissions in the non-operative treatment arm were for treatment of recurrent appendicitis, which resulted in appendicectomy for seven participants and a PICC for one participant.
Discussion
This feasibility RCT was undertaken to address a number of specific objectives of the overall CONTRACT feasibility study. The feasibility of a future trial will be dependent on a number of factors, including the willingness of parents and children to be enrolled in a RCT in the context of uncomplicated acute appendicitis; the willingness of surgeons to recruit to such a trial (having been appropriately trained); an acceptable overall recruitment rate; a satisfactory and acceptable trial design, particularly with reference to a non-operative treatment pathway that is safe and usable; and continued justification for the underlying research question. In conducting this feasibility RCT, we have been able to address the majority of these objectives, but acknowledge that some remain outstanding.
We have clearly demonstrated that it is possible to train surgeons to approach children and families at the time of an emergency admission, discuss the trial with them and recruit to a RCT. This has been achieved equally successfully at several centres, suggesting that the procedures put in place to facilitate this are generalisable and not centre specific. We have also demonstrated that it is possible to recruit children with acute uncomplicated appendicitis to this trial outside normal working hours, an important consideration as children with appendicitis frequently present outside routine working hours, including at night and at weekends. It is also evident that there is adequate interest from parents and children to be involved in a trial, as half of all those approached with information about the study agreed to participate. Findings from the parallel communication study (see Chapter 4) confirmed continued interest from families.
Overall, the recruitment rate achieved across the 12 months of this feasibility RCT was 50% of those approached (95% CI 40% to 59%). This is at the upper end of our predicted recruitment target range and is in excess of the recruitment rate seen in other clinical trials involving either an emergency presentation or a complex intervention. We have no doubt that our embedded qualitative work (see Chapter 4) to understand the barriers to and facilitators of recruitment enhanced recruitment to this feasibility RCT. The sequential data on recruitment rate across the lifespan of this trial (rising from 38% to 47% to 72%) would support a role for iterative learning and retraining in improving trial recruitment; although, clearly, a causative relationship between retraining and an increased recruitment rate cannot be proven. Nonetheless, these findings suggest a continued role for qualitative methods in a future effectiveness trial, albeit at a reduced level. It is most likely that the qualitative methods would be particularly advantageous in new recruiting centres.
Following recruitment to the trial, a small number of participants withdrew, most likely because of dissatisfaction with the treatment to which they had been allocated. Although efforts were made to explore treatment preference during recruitment consultations, it is possible that, in some cases, a residual family preference for one treatment remained, and that this contributed towards the immediate withdrawal of three participants. To minimise this, it would be important that future recruitment consultations include adequate discussion and exploration of treatment preference to attempt to recruit only those families that are genuinely accepting of either treatment intervention, and therefore less likely to withdraw from the trial following randomisation. A further aspect that came to light during the running of this feasibility RCT was what children undergoing non-operative treatment were likely to experience ongoing pain despite not having surgery. Pain was cited as a reason for undergoing appendicectomy during the initial hospital admission in a number of cases in the non-operative treatment arm. Following identification of pain as an issue in the embedded qualitative research, we suggested that recruiters specifically discuss pain during recruitment consultations. In particular, we aimed to ensure that parents should anticipate that a certain amount of ongoing abdominal pain would continue with non-operative treatment, as it appeared that the discussion of ongoing pain was absent from recruitment consultations prior to that point. Data obtained from recruiting centres for participants randomised to non-operative treatment and whose clinical condition did not improve by 48 hours after randomisation showed that ongoing abdominal pain was a contributing factor in at least one case. Therefore, it is possible that some (but probably not all) appendicectomies during the initial hospital admission in the non-operative treatment arm may have been avoided with improved explanation about ongoing pain, and perhaps improved pharmacological pain management.
The data on blinded discharge assessment were collected to determine if it would be possible to collect outcome data in a blinded fashion in a future effectiveness trial, if desired. Clearly, in a trial such as this, it is impossible to blind those providing the intervention, those receiving the intervention and those caring for the patients from treatment allocation. Therefore, blinded outcome assessment remains a possible means of collecting outcome data in a blinded way. There were limitations to availability to do this in this feasibility RCT, mainly the fact that we required a member of nursing staff who did not have any familiarity with the trial participant to be available at the time of discharge and to remain blinded before, during and after the discharge assessment. In many cases, owing to staff non-availability, it was not possible to obtain a blinded discharge assessment. However it was possible in 34 out of 54 eligible cases; in just one of these 34 cases, the assessor became unblinded. Of the remaining cases, the assessor ‘guessed’ the treatment correctly 61% of the time, which is not statistically significantly different from what would be anticipated by chance (50%). It is therefore likely that, if a future trial were to require a blinded discharge assessment to minimise bias, this would be possible.
Overall adherence to inpatient treatment pathways by clinical teams and patient (family) compliance with home antibiotic consumption were good. Having agreed to recruit participants into the feasibility RCT, surgeons were accepting of the non-operative treatment pathway and generally compliant with it. In just one case, a decision was made (by a clinician) that was not in keeping with the clinical pathway: to perform an appendicectomy on a child whose clinical symptoms had not improved at 44 hours rather than at 48 hours, as defined in the treatment pathway. However, on closer review of the timelines, this appears to be a pragmatic decision related to the time of the day at which randomisation took place. Therefore, we have not recorded this as a protocol deviation. All participants allocated to the appendicectomy arm received the allocated intervention.
Rates of adherence to follow-up following trial discharge were variable across the different time points. We provided participants with a diary card to enter data on a daily basis following discharge from hospital in an attempt to record outcomes related to ongoing pain and time taken to return to normal and full daily activities, as well as an assessment of parental caring. Compliance with outpatient antibiotic use was also assessed by diary cards. Only 48% of diary cards were returned after a 2-week period, suggesting that this is not a useful method for recording this type of data. Interestingly, however, the majority of diary cards that were returned were fully completed, suggesting that the minority of parents who completed a diary card were engaged in the process. It is likely that a future effectiveness trial will require an alternative means of recording outcomes during the early discharge period, if desirable. The use of a mobile electronic data-capture platform, such as a smartphone-based application, may be a solution to this, although a previous paediatric study25 using such an approach suggested that data accuracy would need to be ascertained.
In this feasibility RCT, we aimed to follow up patients at 6 weeks and at 3 and 6 months following randomisation. Current routine clinical practice varies between sites and surgeons, but, at a maximum, would comprise a single follow-up visit. A key reason for multiple follow-up assessments in this feasibility RCT was for the purpose of optimising the design of a cost-effectiveness analysis in a future effectiveness trial. For the purposes of collecting relevant clinical outcomes, it is likely that an initial follow-up assessment followed by a longer-term assessment (most likely at approximately 1 year after randomisation) would be adequate. The optimal design of a potential cost-effectiveness study alongside a future effectiveness trial is further discussed in Chapter 6. Of note, completion of follow-up visits at 6 weeks, 3 months and 6 months was relatively high (> 80% at all time points), but was below what would be needed to achieve an acceptable ‘lost to follow-up’ rate in a future effectiveness trial. Interestingly, the offer of an incentive to those who completed all outstanding follow-up appointments seems to have increased compliance with follow-up (92% vs. 83%) and would probably be worth considering for a future effectiveness trial. Although we did not formally study the effect of this incentive in a randomised way, the findings are in keeping with the previous literature on questionnaire return rates and retention in trials. 26–28
A specific objective of this study was to assess the safety of the treatment interventions, and of the non-operative treatment pathway in particular, because non-operative treatment is not standard practice for uncomplicated acute appendicitis in the majority of UK centres. Overall, a similar number of AEs were reported in each treatment group, although more participants in the non-operative treatment arm than in the appendicectomy arm experienced AEs. AEs specifically related to treatment intervention were as anticipated and relatively infrequent. Other, less treatment-specific, AEs occurred in both treatment groups; there were no specific concerns about the safety of either treatment intervention. Of note, no unexpected SAEs were reported. During this feasibility trial, we encountered some challenges with the reporting and assignment of severity of AEs; as a result, the reporting pathways were altered. This experience will be valuable when proceeding to a future effectiveness trial.
Regarding the clinical features and outcomes of participants in this feasibility RCT, the baseline characteristics of trial participants were in keeping with those anticipated from the existing literature of children with acute appendicitis. Children allocated to receive appendicectomy were treated in accordance with the guidance of the clinical pathway provided for this trial. Of note, 30% of children were found to have perforated appendicitis and 7% were found not to have appendicitis at all (negative appendicectomy). Overall, postoperative outcomes in this group were as expected, given the operative findings. Of note, three participants had AEs related to their surgical wound, four had assessment with an abdominal ultrasonography and two had localised intra-abdominal fluid collection, of whom one was treated with percutaneous drainage.
In participants allocated to receive non-operative treatment, a higher proportion (30%) received appendicectomy during their initial hospital admission than had been anticipated based on the existing data on efficacy of non-operative treatment. In one case, this was due to parental withdrawal from the treatment allocation, but, in the remaining seven, it was due to non-response to non-operative treatment in accordance with the trial protocol. At surgery and on histology, the findings were simple acute appendicitis in four of these cases and perforated appendicitis in the remaining four. Despite this higher than anticipated non-response rate, overall clinical outcomes related to initial hospital admission were similar between treatment groups. Unsurprisingly, children allocated to receive non-operative treatment who subsequently underwent appendicectomy had a longer initial hospital stay than participants for whom non-operative treatment was successful.
Although data obtained from diary cards are limited by the low return rate, the data available suggest that there may be differences in recovery between the treatment arms (see Figures 8–11). Participants in the non-operative treatment arm appeared to stop taking analgesia sooner and were able to return to normal and, subsequently, full daily activities sooner than participants in the appendicectomy arm. Furthermore, their parents reported being able to return to work earlier. It will be important to capture these post-discharge outcomes in a future effectiveness trial. The potential for such differences in outcomes between treatment arms provides additional justification for pursuing an assessment of the relative efficacy of these two treatments. It also acts as a reminder that differences between treatment arms may exist beyond the initial hospital admission and therefore not immediately apparent to treating clinicans. Importantly, these outcomes may be of great interest and relevance to children and their families. Of note, few previous studies of non-operative treatment of children with acute uncomplicated appendicitis have reported outcomes relating to return to function or burden on the family. Only the study by Minneci et al. 29 reported a measure of ‘disability days’, which they defined as the sum of the length of stay (in days); the number of days of normal activity missed for the child; the number of days of normal activity missed for the parent or guardian; and clinic, emergency department and inpatient visits. Interestingly, they also reported fewer disability days for patients receiving non-operative treatment than for patients undergoing appendicectomy, but their study was not randomised. 29 Although Hartwich et al. 30 used a measure of quality-adjusted life-months in a cost–utility analysis of non-operative treatment, this was based on a measure of overall quality of life (QoL), rather than the specific detail relating to recovery time.
Although we have demonstrated that recruitment to a RCT is feasible from a number of perspectives, it is important to consider whether or not the clinical outcomes achieved in this feasibility RCT are compatible with a future effectiveness study of this population of children using the current clinical pathway. First, there was no evidence of an adverse safety profile of the non-operative treatment pathway. However, when considering clinical effectiveness, there are a number of differences between the clinical outcomes achieved in participants in both treatment arms and the existing comparative literature. Prior to undertaking this feasibility RCT, we performed a systematic review of the existing literature related to successful non-operative treatment of children with acute appendicitis and identified an initial success rate (defined as being discharged from hospital following initial hospital admission) of 97%. 16 However, in the current feasibility RCT, just 70% of children randomised to non-operative treatment were successfully discharged from hospital without receiving appendicectomy. Similarly in our feasibility RCT, the rate of recurrent appendicitis was higher, at 37%, than that reported in the previous literature. Of note, the difference in clinical outcomes between this feasibility RCT and previous studies is not confined to the non-operative treatment pathway, for instance the overall hospital length of stay is longer than is typically reported following appendicectomy for uncomplicated acute appendicitis. The probable explanation for this is that we have inadvertently recruited to our study more children with perforated appendicitis than we had intended to. Of the 57 children recruited to the study, 12 were known to have had perforated appendicitis at the time when they underwent appendicectomy. It is well recognised that children with perforated appendicitis have a more prolonged and complicated clinical course than children with acute uncomplicated appendicitis. It is interesting that, despite this, the overall hospital length of stay between groups was similar; however, there is no doubt that non-operative treatment in this feasibility RCT was not as clinically effective (however defined) as the previous literature suggests. 16
The likely explanation for this discrepancy is that our inclusion and exclusion criteria were too loose to allow the accurate identification of children who had acute uncomplicated appendicitis as opposed to more advanced disease. We deliberately designed this trial to be pragmatic to optimise its acceptability to surgeons, in particular. No alteration to current diagnostic pathways was made when designing this trial. In the UK, it is recognised that only a minority of children undergo diagnostic ultrasonography as part of their work-up when presenting with abdominal pain. 31 This is important as one of the pieces of information to be gained from ultrasonography may be a more reliable distinction between children with acute uncomplicated appendicitis and those with perforated appendicitis than can be achieved with clinical judgement alone. In our feasibility RCT, the exclusion of children suspected to have perforated appendicitis was based on clinical judgement alone, rather than on any specific physical signs, laboratory parameters or ultrasonograph findings. We continue to justify the absence of radiological parameters in our exclusion criteria because, even in this feasibility RCT, just 30% of enrolled participants underwent diagnostic ultrasonography. However, for a future effectiveness trial to be acceptable to all stakeholders, it will be necessary to determine, with a greater precision, children who have acute uncomplicated appendicitis as opposed to more advanced disease. Importantly, this should be done without the use of diagnostic imaging, as any such change would be a significant departure from current routine UK practice, would have logistical and cost implications, may decrease the acceptability of the trial to UK clinicians and would limit the generalisability of any findings. Therefore, we are in the process of using the data obtained during the screening process of this feasibility RCT to perform an analysis intended to identify a set of criteria that can be used in the setting of a child with a confirmed diagnosis of appendicitis (based on tests used in routine care). This set of criteria aims to assist with the judgement of whether the child is likely to have uncomplicated acute appendicitis or more advanced disease. We anticipate that, after adequate testing, the use of such criteria may facilitate the accurate identification of children with acute uncomplicated appendicitis so that a population in whom non-operative treatment can be anticipated to be of greater efficacy can be identified and enrolled in a future trial.
As a result of the alteration in trial design that will be necessary before proceeding to a future effectiveness trial, it is not valid to use outcome data recorded in this feasibility trial for the purposes of informing a sample size calculation for a future effectiveness trial. Such a RCT will therefore require an internal pilot phase as a minimum to test assumptions on which any provisional sample size calculation is based, as a result of these enhancements in trial design.
Chapter 4 Communication substudy: a qualitative study to optimise trial recruitment
Background
Recruitment of patients to RCTs is often challenging. Poor recruitment has been suggested as the most common reason for premature trial discontinuation,32 and almost half of the National Institute for Health Research (NIHR) Health Technology Assessment programme and Medical Research Council trials require additional funding, time extensions or both. 33
The CONTRACT feasibility RCT included several factors that can complicate or impede recruitment. The study compared surgical treatment with non-surgical treatment. Recruitment to trials that include such markedly different treatments is known to be particularly difficult because of treatment preference issues. 34,35 This is especially pertinent as the surgical treatment arm in CONTRACT (appendicectomy) has been a mainstay of treatment for acute appendicitis for more than 100 years. 36 Recruiting children (as opposed to adults) to trials is also challenging, because it is necessary to consider the needs of both child and parent(s),37 and children’s capacities to contribute to the decision-making process can vary markedly. 38 Recruitment to trials during an unscheduled hospital admission, as in CONTRACT, can also be complex owing to uncertainties regarding a patient’s clinical condition, the demanding clinical working environment and time limitations associated with the urgent need to deliver the treatments under investigation. 39 Furthermore, potential CONTRACT participants often present to hospital outside normal working hours, when the availability of recruiting staff is limited.
Trial recruitment can be optimised by embedding qualitative studies in the trials to identify barriers to recruitment and strategies to address these difficulties over the course of the trial. 40–42 Some strategies that have previously been identified via qualitative studies as important in optimising recruitment focus on communication are changes to the order of presenting treatments, avoiding misinterpreted terms, exploring patients’ treatment preferences42–45 and identifying the ‘hidden challenges’ to recruitment, such as a lack of clinical equipoise among health professionals. 46 Patients’ treatment preferences are complex, dynamic and may not always be well founded. Treatment preference exploration offers an opportunity to emphasise equipoise and address treatment preferences founded on misconceptions, thus making trial treatment arms more acceptable42 and optimising informed consent and recruitment. 43,44
Previous research in this field has focused on optimising recruitment to adult trials by embedding qualitative studies. The current embedded qualitative study (communication study) was needed as CONTRACT was a paediatric trial being conducted in an urgent care setting. It was anticipated that strategies to optimise recruitment and consent would need to be adapted to this specific setting, and, similarly, that lessons for future pilot and definitive trials would need to take account of the particularities of this paediatric setting.
This chapter describes the key findings of the communication study: how it informed recruitment to CONTRACT, how it informed the training delivered to health professionals and an evaluation of the training. The chapter documents the impact of the communication study on CONTRACT and outlines key messages for optimising recruitment to any future definitive trial comparing surgical with non-surgical treatment for uncomplicated acute appendicitis in children. More broadly, the findings can be used to inform the development and design of trials comparing surgical with non-surgical treatment, paediatric trials and research conducted in an acute setting.
Aims
The communication study aimed to:
-
systematically examine how families and health professionals communicated about and experienced CONTRACT
-
identify strategies to optimise trial recruitment to CONTRACT and to a future definitive trial.
Methods
Overview
We drew on an integrative qualitative communication approach, involving analysis of audio-recordings of CONTRACT consultations and in-depth interviews with parents, patients (i.e. children and young people) and health professionals. 47 This integrative qualitative communication approach is particularly suited to explore clinical communication in trials. The consultation recordings allowed us to explore how health professionals explained CONTRACT during consultations with families, and the interviews allowed us to explore children’s, parents’ and health professionals’ perspectives on communication about CONTRACT. 42 The communication study was included in the ethics approval given for CONTRACT. Patients and parents were provided with a summary of the results on completion of the study and we received no subsequent feedback from participants regarding the findings.
Recruitment training and phases
CONTRACT recruited for 12 months from March 2017 and the communication study ran concurrently. Before recruitment began, we delivered generic recruitment training in December 2016. We analysed the qualitative data iteratively throughout the CONTRACT recruitment period (and afterwards), and these ongoing analyses informed the development of bespoke recruitment training. While the trial was ongoing, we delivered two bespoke recruitment training sessions at all three CONTRACT sites in July 2017 and November 2017. We therefore classified ‘phase 1’ of recruitment as March–June 2017, ‘phase 2’ as July–October 2017, and ‘phase 3’ as November 2017–February 2018. We refer to these phases in Results. All training sessions involved Microsoft PowerPoint® (Microsoft Corporation, Redmond, WA, USA) presentations and discussion between the communication study team and health professionals at each individual site. The recruitment training that was delivered at the end of phases 1 and 2 was specifically informed by the ongoing analyses of the qualitative data. Health professionals were asked to complete training evaluation forms (available at www.journalslibrary.nihr.ac.uk/programmes/hta/1419290/#/) pre training, and after training sessions 1 (generic recruitment training), 2 and 3 (bespoke recruitment training sessions). Finally, we provided health professionals with written ‘hints and tips’ on ways to optimise CONTRACT consultation communication. We updated this information periodically in the light of the ongoing qualitative analysis.
Participants
Health professionals approached families of children who were eligible for CONTRACT at one of three UK hospitals. The families of all children who were eligible for CONTRACT were eligible for the communication study. During this period, we also approached and interviewed health professionals who had been involved in CONTRACT at one of the three sites.
Procedure
Sampling
Health professionals were invited for interview if they had either approached families about CONTRACT or been involved in other aspects of recruitment or patient care. Parents were invited for interview if they had been approached about CONTRACT. Children aged 7–15 years who had been approached about CONTRACT were also invited for interview. The sampling strategy for both consultation recordings and interviews aimed for diversity in CONTRACT participation status [including those who had consented or declined, child age, family socioeconomic status, health professional’s role (i.e. surgeon or nurse) and NHS site].
Consultations
Health professionals sought verbal permission to audio-record trial consultations at each of the study sites. If permission was granted, the audio-recorder was activated (the health professional subsequently sought written consent from parents and assent from children for the audio-recording to be included in the study). Consultations typically entailed health professionals describing the various elements of CONTRACT and the communication study, providing the relevant information sheet(s) and showing families a video about CONTRACT. Families then had time to decide before consenting to CONTRACT and/or the communication study; in the CONTRACT protocol, the proposed time given to decide was a maximum of 4 hours from the initial discussion until consent was obtained. Families were eligible for the communication study whether they declined or consented to CONTRACT. Consultation recordings and communication study consent forms were uploaded directly to a secure server. The recordings were subsequently transcribed by a professional agency and then anonymised and checked by the communication study team prior to analysis.
Interviews
Families who expressed an interest in being interviewed and provided written consent for their contact details to be forwarded were telephoned by a member of the communication study team. The team member attempted to contact the family up to three times by telephone to invite them to participate. The team member explained this part of the study to the family and forwarded them the relevant information sheet(s). If the family was interested in participating, an interview was provisionally scheduled. Family interviews were typically completed 1–4 weeks following discharge from hospital.
Health professionals were eligible for interview if they were involved in CONTRACT, although we were particularly interested in interviewing health professionals who had experience of inviting families to participate in CONTRACT. The communication study team typically obtained health professionals’ contact details via the local principal investigator and then contacted the health professional to provide the health professional information sheet and offer more information about the study, before obtaining consent and conducting the interview.
Before telephone interviews, informed consent and assent was audio-recorded; before face-to-face interviews, informed consent and assent was obtained in writing. Two experienced qualitative researchers (LB and FCS) with health research backgrounds conducted the semistructured interviews with children, parents and health professionals. Interviews were topic guided to ensure exploration of key topics (see Appendix 2), yet conversational to allow participants to raise issues of importance to them. As some of the concepts explored were rather complex, only children aged 7–15 years were eligible for interview. We devised separate topic guides for parents, health professionals and children, and used materials to facilitate the children’s interviews, such as art pads, pens and stickers. Topic guides were initially developed through consultations with the SSAG and adapted throughout the course of the study in response to developing analyses.
All interviews were audio-recorded, then transcribed by a professional agency. Transcripts were pseudo-anonymised (replacing names and places with codes) and checked by the study team prior to analysis. Sampling for interviews ceased when data saturation was reached. 48
Qualitative analysis
Salmon et al. 47 advocate an analytical approach that borrows from several methodological traditions when analysing data involving consultations and interviews. This involves two interlinked strands of analysis: a cross-case strand and a within-case integrative strand.
In cross-case strand analysis, consultation analysis focused on the interaction between recruiter and potential participants and information provision, communication techniques, intervention preferences and trial participation decisions. We analysed interview data for evidence of the needs, priorities and goals of families in relation to recruitment, randomisation, treatment preferences, their experiences and acceptability of the intervention and views regarding which outcomes are important. The analysis of interviews with health professionals at sites focused on their perceptions and experiences of recruitment, as well as their perceptions of the interventions and which outcomes they viewed as important (for information on outcomes of importance, see Chapter 5).
Within-case integrative strand analysis allowed us to explore associated consultations and interviews for each individual case [i.e. consultation and interviews with the parent(s), child and health professional present in the aforementioned consultation]. We produced narratives for each case by drawing on the codes and themes that arose from the cross-case analysis, allowing us to test and develop those codes and themes, and integrate the strands of analysis.
We analysed the recruitment consultations by listening to these as well as by reading the transcripts. If analyses of the audio-recordings suggested that recruitment difficulties were potentially linked to communication during the recruitment consultation, the communication study research group discussed the issue and integrated it into the health professional training sessions.
The analysis of recruitment consultations used content analytic methods to describe what was said by whom and how often in the audio-recordings of recruitment sessions. 49 More flexible constant comparison methods were used to identify common or divergent themes, particularly focusing on the impact of statements by the recruiter on parent responses and views. Thematic analysis was used to focus in great detail on certain sections of the transcripts, for example in the interactions during which randomisation was offered. 50 Families that declined randomisation or that did not accept their randomisation allocation were noted.
Again, analysis of all interview data drew on the principles of the constant comparative method and thematic analysis. 50
Members of the qualitative research team (FCS and LB) initially read and double-coded approximately 10% of consultations, health professional and family interview transcripts, and led a process of ‘cycling’ between the developing analysis and new data. Other members of the qualitative study team (BY) read a selection of transcripts and helped to develop and test the analysis by periodic discussion (LB, FCS, EC, NJH and BY) of detailed reports of the developing analysis.
Initially, each transcript was read several times (by LB/FCS) before developing open codes to describe each relevant unit of meaning, although coding occurred at multiple levels, from detailed descriptions of communication and experiences of the trial, to the general orientation of participants towards clinical research. Through comparison within and across the transcripts, the open codes were developed into categories to reflect and test the developing analysis.
The categories were organised into frameworks for each data type (consultations, and patient, parent and health professional interviews) to code and index the transcripts using NVivo 11 (QSR International, Warrington, UK). These frameworks are available in Report Supplementary Material 2. The framework categories were continually checked and modified to ensure an adequate ‘fit’ with the data, while also accounting for variation in the data and ‘deviant’ cases. A second coder (LB or FCS) checked categories and the assignment of data to them. Our analytic approach was informed by writings on quality in qualitative research. 51 Our approach was interpretive and considered both latent and manifest aspects of the data (e.g. what we can learn from the way that participants talk as well as the explicit content).
Quotations are labelled with data type (Cons = Consultation, Int = Interview). Family member or health professionals’ roles are described (e.g. surgeon, nurse, mother, father, child). Families are labelled by a number. CONTRACT treatment allocation and/or participation is indicated (NOT = non-operative treatment, App = appendicectomy, Declined = declined, Withdrew = withdrew). We have allocated a consistent number for each health professional for whom we have consultation and interview data, to aid the reader in linking consultations with interviews. Children’s ages are listed next to their quotations.
Results
In total, 115 families were approached about CONTRACT, but it is not possible to establish the exact number of families that were approached about the communication study. However, we asked health professionals at all three sites to routinely approach families about the communication study when inviting families to participate in CONTRACT. Health professionals audio-recorded 58 families’ CONTRACT consultations and obtained written consent for researcher contact from 62 families. Figure 12 provides an overview of recruitment of families with recorded CONTRACT consultations and recruitment of families without recorded CONTRACT consultations, showing families’ trajectories through CONTRACT and the communication study.
FIGURE 12.
Recruitment of families to the communication study. (a) Families with recorded CONTRACT consultations, recruited via recording of recruitment conversations; and (b) families without CONTRACT consultations, recruited via contact by researcher following discharge. a, Either they did not consent to researcher contact (n = 1) or the study had reached data saturation (n = 3). b, Because the study had reached data saturation. Uncontactable families included those with invalid contact numbers, those who did not respond after three telephone attempts and those who arranged interviews but cancelled and then did not respond to attempts to rearrange.
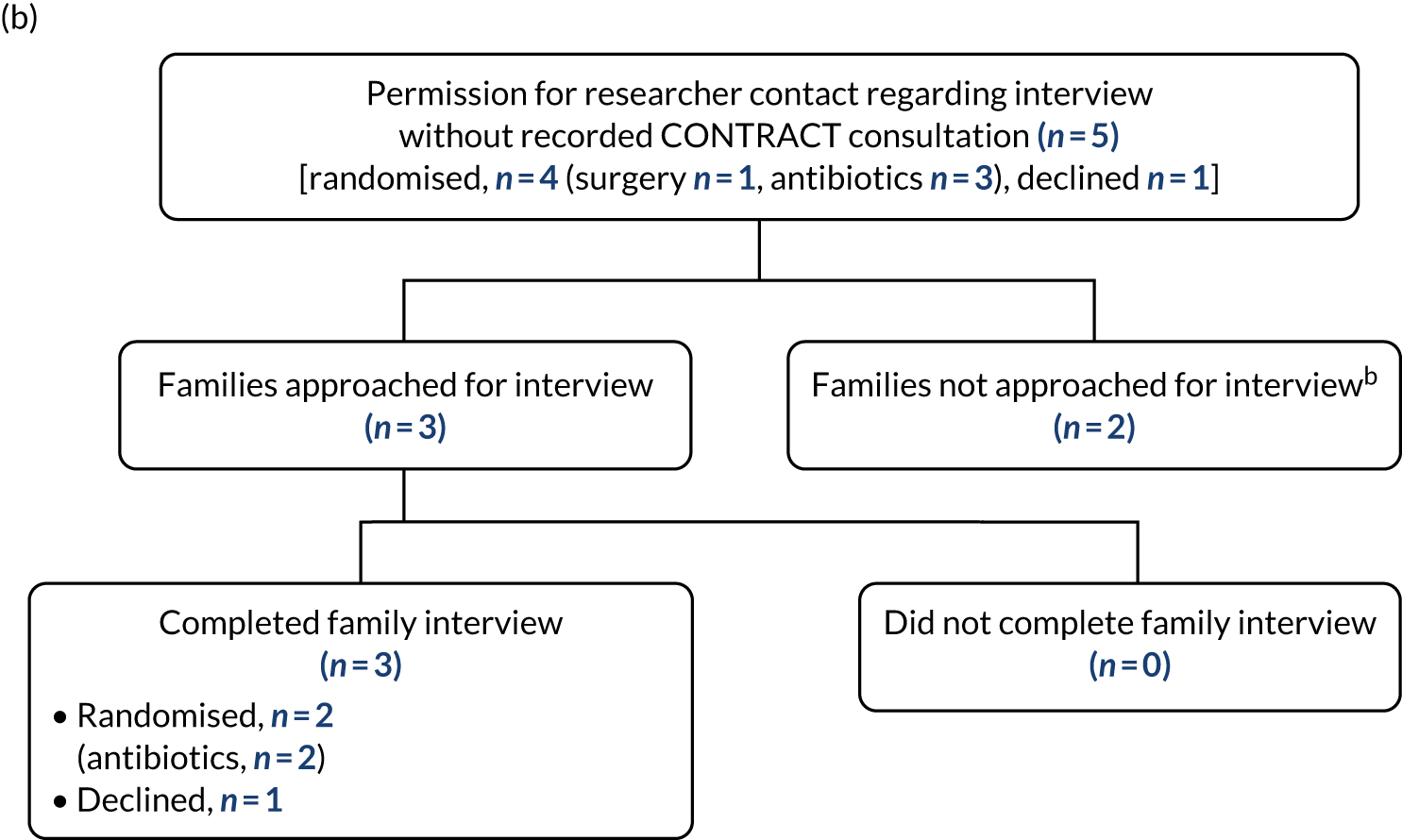
Consultations
We obtained consultation recordings for half of families that were approached about CONTRACT (58/115, 50%). Most of the families that we obtained consultation recordings from participated in CONTRACT (38/58, 66%). Of these 38 families that participated in CONTRACT, 19 were randomised to surgery and 19 to non-operative treatment (antibiotics). In recruitment phases 1, 2 and 3 (see Recruitment training and phases for definition of phases), consultations were recorded for 17, 23 and 18 families, respectively. Of the 58 families with consultation recordings, 23 were from site 1, 19 were from site 2 and 16 were from site 3. The majority of children were male (39/58, 67%) and children ranged from 4 to 15 years of age [median 10 years, interquartile range (IQR) 8–12 years]. We also obtained families’ postcodes when possible (52/58, 90%) and used the 2015 English Indices of Multiple Deprivation (IMDs)52 to indicate socioeconomic status: 15 out of 52 (29%) families lived in areas of high deprivation (IMD deciles 1–3), 18 out of 52 (35%) families lived in areas of moderate deprivation (IMD deciles 4–7) and 19 out of 52 (37%) families lived in the least deprived (IMD deciles 8–10) areas of England. 52
Often, health professionals had multiple brief consultations with families; we asked them to audio-record all of these if possible. Thirty-eight families had one consultation audio-recorded, 14 families had two audio-recorded consultations, five had three audio-recordings and one family had four audio-recordings. The duration of initial CONTRACT consultations ranged from 38 seconds to 24 minutes [median 10 minutes (IQR 5–12 minutes)], although initial consultations usually lasted longer than subsequent ones.
Family interviews
In total, the research team attempted to contact 57 families to arrange an interview. Common reasons for non-participation in the family interview included the family not responding to telephone calls and the family declining to participate because they was too busy. Twenty-eight families completed an interview (see Figure 12). Most families that completed an interview were randomised to CONTRACT (19/28, 68%) and, of those, just over half were randomised to the non-operative treatment arm (10/19, 53%).
Of the 10 families interviewed that were randomised to the non-operative treatment arm, four eventually had an appendicectomy owing to non-operative treatment failure. Of these four, one child was later re-admitted because of surgical complications, and three suffered recurrent appendicitis and were re-admitted. Of the three who were re-admitted with recurrent appendicitis, two were treated again with non-operative treatment (as opposed to appendicectomy), and one family withdrew from CONTRACT owing to concerns that the child was deteriorating; the child was subsequently treated with an appendicectomy. Of the nine families interviewed that were randomised to appendicectomy, one child was re-admitted to hospital because of surgical complications.
Eight, 12 and eight families from sites 1, 2 and 3, respectively, completed an interview. Of the 28 families interviewed, eight (29%) lived in areas of high deprivation (IMD deciles 1–3), seven (25%) lived in areas of moderate deprivation (IMD deciles 4–7) and 13 (46%) lived in the least deprived (IMD deciles 8–10) areas of England. 52 Twelve families were interviewed face to face in their homes, whereas the remaining interviews were completed by telephone. Overall, interviews lasted from 22 minutes to 89 minutes [median 59 minutes (IQR 46–66 minutes)]. In recruitment phases 1, 2 and, 3, seven, 14 and seven families were interviewed, respectively.
Most parents (19/28, 68%) completed an interview without their child being present. In total, 15 interviews were completed with mothers only, seven were completed with fathers only and six were completed with both parents present. Of the children eligible for interview (n = 25), 14 completed an interview. Children did not participate in interviews for several reasons, including parents feeling that their child was too young to be interviewed, parents stating that their child did not want to be interviewed and parents not talking to their child about doing an interview. Most children completed an interview with a parent present (11/14). Children who completed an interview were aged 8–14 years [median 11 years (IQR 9–13 years)], and most were male (11/14).
Health professional interviews
Fifty-one health professionals were invited to participate in an interview and 35 were interviewed on one or two occasions (constituting 40 interviews in total). Of the 35 professionals, 25 were surgeons, seven were research nurses and three were ward nurses. Fifteen, 11 and nine health professionals from sites 1, 2 and 3, respectively, completed an interview. Interviews were face to face in the place of work (n = 23) or by telephone (n = 17). First interviews lasted from 20 to 79 minutes [median 48 minutes (IQR 38–56 minutes)] and repeat interviews (n = 5) lasted from 39 to 69 minutes [median 51 minutes (IQR 42–67 minutes)].
Qualitative analysis
In the following sections, we describe how health professionals communicated about CONTRACT during consultations and family and health professional experiences of CONTRACT throughout the three recruitment phases (i.e. after each recruitment training session). We outline how the interim qualitative findings informed the recruitment training sessions, and examine the impact of training on recruitment practice and communication about CONTRACT.
Recruitment: phase 1
Training sessions (pre recruitment)
In December 2016, members of the CONTRACT and the communication study teams attended all three CONTRACT sites to provide generic pre-recruitment communication training to 29 surgeons and research nurses who were to be involved in approaching families about CONTRACT. The training focused on the rationale for the communication study and techniques to explore families’ treatment preferences, informed by previous research. 44
Families’ positive experiences of CONTRACT recruitment consultations
CONTRACT rationale
In phase 1, health professionals typically provided a clear rationale for the study, in which they explained the uncertainty regarding treatment for children with acute appendicitis and described how CONTRACT involved randomisation to either non-operative treatment or surgery:
What we are doing is looking at whether treating appendicitis with, um, an operation, or if you can avoid an operation and treat it with just antibiotics.
Cons_Surgeon29_Family25_Declined
Families that we interviewed during phase 1 recalled study processes, despite the interview taking place several weeks after they had been approached about CONTRACT:
She said you can either, the antibiotic drip and we’ll keep monitoring, or else [child] can just get, I think that it was already decided that she’d have to have surgery if she didn’t have that [antibiotics].
Int_Mother45_NOT
Generally, parents reported that health professionals had communicated clearly and compassionately:
He was really nice, very clear in what he, in the way that he explained everything and the kind of description of the study.
Int_Mother41_App
Emphasising voluntariness of participation
Our analysis of the consultations indicated that health professionals were clear that participation was optional, that families were free to withdraw from the study and that their decision would not affect patient care:
If you don’t like the sound of the study, you don’t have to be in it. Actually, if you decide you do want to be in the study but then you don’t want to be in it later on, you can withdraw. So, I always like to say those things upfront, so that you feel no pressure.
Cons_Surgeon8_Family45_NOT
You don’t have to be in it and if you don’t want to be in it, it doesn’t change our treatment of you in any way.
Cons_Surgeon7_Family6_Declined
When interviewed, some parents described how they had been concerned that their decisions about participating in CONTRACT may have jeopardised the quality of care their child would receive. However, parents commented that health professionals’ explanations had reassured them on these issues:
The only thing that I wanted to be reassured of is that [child’s] care was going to come above any research and that was made very clear by [surgeon] and was well explained by himself.
Int_Mother48_NOT
Audio-visual methods of providing information
A short video was produced to help explain CONTRACT to families (http://tinyurl.com/contract-f) and health professionals routinely provided families the opportunity to view the video (tablet computers were provided for this purpose), as well as time to read the CONTRACT information sheets. Parents and some children reported watching the video. Parents commented that the video was ‘basic, good’ adding that it was pitched at an appropriate level:
I don’t think you need to know too much really.
Int_Mother15_Declined
Indeed, both parents and children indicated that the video was easy to understand and that they found it helpful to receive the study information in a visual format and watch it together:
We watched a little video . . . and [child] watched it and he understood. He’s a bright boy. He was very able to understand exactly what was going on.
Int_Mother48_NOT
Nevertheless, the discussion with health professionals was the primary source of information for most families:
I’d say yeah, [the video] was good but I kind of understand it all from what they said.
Int_Child57_Age 12_NOT
Not all children watched the video, perhaps because of lack of interest or feeling acutely unwell:
Do you remember watching a video?
Mum and dad did.
Did they?
Yeah, I was playing on my tablet.
Were you sort of feeling quite poorly at that point?
Yeah.
Appropriate time to decide
The CONTRACT protocol proposed providing families with 4 hours from health professionals initially discussing CONTRACT with families to obtaining written consent. After being approached about CONTRACT, families reported that they were routinely provided with time alone to discuss participation, typically for ≥ 1 hour, before a health professional returned to allow them to voice their decision. Despite the urgent care setting of CONTRACT, all parents from phase 1 who commented on this during interviews said that they felt that they were given an appropriate amount of time to decide:
[Sh]e was very polite . . . I didn’t feel she was gonna, um, rush us or anything.
Int_Mother45_NOT
Yeah, I had plenty of time. I mean, I’d actually gone to sleep and got woken up a couple of hours later, before she’d even come back to find out everything anyway. So that was alright, I had more than enough time.
Int_Father60_NOT
Children interviewed during phase 1 tended not to discuss this, although one described how he had decided straight away that he wanted to participate, so he felt that the additional time he was given to decide was unnecessary:
Oh yeah, yeah, I think it was, yeah, a few hours or so [we were given to decide] . . . I had decided straight away. It was too long.
So you would have liked to just be able to voice your opinion straight away?
Yeah.
Opportunity for questions
Interviewed parents also described having the opportunity to ask questions about CONTRACT, and this was also regularly evidenced during the consultations. During consultations, if the child was present, they were often also offered the opportunity to ask questions. Few children had any questions, although some asked:
Which [treatment] do I get to go home earliest on?
Cons_Surgeon26_Child48_Age14_NOT
So what am I doing, antibiotics or the operation?
Cons_Surgeon33_Child15_Age8_Declined
Opportunities to optimise the CONTRACT recruitment consultations
Although families described positive aspects of communication about CONTRACT, through analysis of the consultation data and by drawing on findings from previous qualitative studies embedded in adult trials,42,44,53 we identified several opportunities for health professionals to enhance the way in which they communicated with families and to provide more balanced explanations of the treatments under investigation. In particular, we identified health professionals’ use of terminology that could inadvertently convey a lack of equipoise to families. We also identified a lack of family treatment preference exploration, and questions that families frequently had about CONTRACT.
Use of terminology
We found that health professionals often referred to the surgical treatment using terms that implied that it was superior to non-operative treatment, such as ‘gold standard’, ‘normal pathway’, ‘appropriate way’ or ‘normal’, ‘standard’ or ‘traditional’ treatment. In contrast, they sometimes referred to non-operative treatment as ‘experimental’ or ‘just antibiotics’:
Half the people will go on and have surgery, which is the . . . normal treatment, and half the people will be in the experimental side.
Cons_Surgeon29_Family25_Declined
Therefore, we encouraged health professionals to adopt neutral, non-evaluative terms for surgery, such as ‘operation’ or ‘surgery treatment’, and, similarly, for non-operative treatment, to simply refer to ‘antibiotic treatment’ or ‘medicine’. We also found that health professionals also often referred to CONTRACT using the term ‘trial’ during consultations. We encouraged them instead to simply refer to ‘CONTRACT’, ‘research project’ or ‘research study’, so as to avoid negative connotations with the term ‘trial’. 54
Health professionals also often inadvertently suggested that trial participation could be burdensome for either the family or the clinical team, which may have deterred some families from participating:
[If] you decide ‘oh no, I don’t want to have all of this done, I don’t want to go to all this trouble’ . . . our appropriate, our, our standard way would be at the moment is to go for an operation.
Cons_Surgeon33_Family15_Declined
If you agree to go ahead with the study, um, we have lots of paperwork, etc., [laughs] to fill in.
Cons_Surgeon36_Family21_Declined
We therefore encouraged health professionals to avoid framing CONTRACT as burdensome.
Exploring families’ treatment preferences
In phase 1, we found that health professionals rarely asked questions to elicit or explore families’ treatment preferences. Although some families spontaneously voiced their preferences, others did not and, in some cases, health professionals assumed this to mean that the family preferred surgery. Health professionals tended to provide some information to balance families’ views about treatments, but they did not explore the underlying reasons for families’ treatment preferences. The following excerpts, which show discussions that took place soon after the start of each of the consultations, indicate an unexplored assumption among families that surgery provided a more rapid treatment and resolution of symptoms than non-operative treatment, hence the preference for surgery:
Do you want to know a bit more about it [CONTRACT]?
Um, I don’t think . . . no, I’d just rather get . . .
You’d just rather get on?
Yeah, the normal way.
OK, that’s absolutely fine. Um, so in that case, what we’ll try to do is take his appendix out, OK.
. . . and if you need an operation [child], you recover very quickly regardless.
Best get it sorted love [directed to child].
Uh-huh.
But, by the same boat, if it is the fact that potentially you could try and avoid [surgery] with the antibiotics, it sounds as though that might be something you would be interested in.
Yeah?
Alright? Shall we give you a couple of minutes to have a read through that.
Family members with previous experience of appendicitis in themselves or a friend or relative tended to have a strong preference for surgery and often believed that prompt surgery was needed to prevent deterioration. However, the cases of appendicitis these families described had often occurred many years or decades previously and involved perforated, complicated appendicitis or other conditions, rather than the current treatment of acute or uncomplicated appendicitis that was the focus of CONTRACT:
I’d just rather get [child] . . . the normal way.
OK, that’s absolutely fine. Um, so in that case, what we’ll try to do is take his appendix out, OK . . . we tend to do it as a keyhole procedure.
Yeah, that’s how mine was, yeah.
You said you had your appendix out and it was burst, didn’t you? OK.
I think . . . without an appendix it can’t come back, so that’s a good [laughter] . . .
Oh absolutely.
It’s a good thing, especially with what we went through with my stepdaughter and her appendix bursting. We nearly lost her.
Mhm.
Health professionals did not usually address the belief underlying such preferences, which equated complicated/perforated appendicitis with simple appendicitis. In the second training session (see Recruitment: phase 2), we described the steps involved in exploring families’ treatment preferences, including identifying preferences, and particularly exploring the reasons for preferences, and gently challenging and balancing families’ preferences. 44 We highlighted cases where families’ preferences for surgery were based on their personal experiences of perforated or complicated appendicitis. We encouraged health professionals to explore these further, and where appropriate, gently challenge families’ views by explaining the differences between perforated and complicated appendicitis, and so highlight equipoise regarding the treatment of acute uncomplicated appendicitis.
Frequent questions that families had about CONTRACT
Most families asked questions in consultations about non-operative treatment and recurrence rates:
My concern is, if he has antibiotics, what’s the chance of the appendicitis recurring at a later date? . . . and, from evidence that you have, how many children who’ve had antibiotics have then gone on to need surgery because the antibiotics haven’t been effective?
Cons_Surgeon40_Mother7_App
The possibility of recurrence dissuaded many families from participating in CONTRACT.
In consultations, health professionals provided numerical estimates of antibiotic recurrence rates based on previous research, informed by the CONTRACT parent information sheet. However, the estimates and how these were explained often varied between health professionals:
. . . about 10% [have recurrent appendicitis after antibiotics].
Cons_Surgeon11_Family13_NOT
. . . of the children who do go home after the [antibiotics], about one in seven will come back in the first year with appendicitis and will need to have their appendix out. But the other six in the seven will not and they’ll be fine.
Cons_Surgeon10_Family57_NOT
About 85% will be successfully treated in their first, the first presentation . . . and of the ones that are successfully treated, about a quarter of them will need to have an appendicectomy . . . in the future.
Cons_Surgeon26_Family48_NOT
Families understood that the effect of non-operative treatment was uncertain:
I wasn’t sure about him having long-term antibiotics that potentially might not work.
Int_Mother15_Declined
Those with holiday plans were especially concerned about recurrence:
[Child’s] going [on holiday] in a few weeks and what if something happens when she’s there . . . that’s quite a big factor for me.
Cons_Surgeon29_Mother17_App
Families sometimes misinterpreted or incorrectly recalled numerical recurrence estimates.
As indicated earlier, families also had concerns about recovery and both parents and children commonly asked how long recovery would take or how long they would spend in hospital with each treatment arm:
Say he had the operation, what’s the recovery time, off school time, that sort of stuff?
Cons_Surgeon33_Mother15_Declined
So recovery periods and all those kinds of things, I need to know a bit more about.
Cons_Surgeon29_Mother17_App
In the second training session, we highlighted these common questions to health professionals, to help them feel better prepared.
Recruitment: phase 2
Training sessions
At the end of phase 1 of recruitment, further bespoke recruitment communication training, informed by the initial analyses of consultation and interview data, was provided at each site. Approximately 31 surgeons and research nurses (approximated based on the number of training evaluation forms received at the end of the session) who had been or were expected to become involved in approaching families about CONTRACT attended the training. The training focused on the issues identified from the analyses of phase 1 data, as described in Recruitment: phase 1. Sessions included discussion of strategies to address these issues, with the aim of optimising recruitment consultations in phase 2. The findings from phase 1 informed changes to the ‘hints and tips’ document, and the updated version, as well as study recruitment flow chart posters and handouts (available at www.journalslibrary.nihr.ac.uk/programmes/hta/1419290/#/) that we produced, were circulated to health professionals at the start of phase 2.
Assessing the impact of recruitment training on communication in phase 2 consultations: what changed and what did not change?
Use of terminology
We found a difference between phase 1 and phase 2 in the language that health professionals used to describe treatment arms. In phase 1, some health professionals had used highly unbalanced terms, such as ‘gold standard’, to refer to the surgical arm and ‘experimental’ to refer to the non-operative treatment arm in consultations; in phase 2, they completely avoided these terms.
We also found that use of terms like ‘normal’ and ‘standard’ to refer to the surgical arm became less frequent in phase 2 consultations. Nevertheless, some health professionals who attended the training continued to use such subtly imbalanced language or descriptions. For example, in phase 2, the use of the word ‘traditional(ly)’ to refer to surgery in consultations actually increased. Other examples included referring to surgery as the treatment ‘that’s been used for years and years and years’ and referring to non-operative treatment as ‘the alternative way of doing it’ (Cons_Surgeon8_Family42_Declined). In some instances, the imbalances were still overt. For example, one clinician referred to surgery as:
very effective. It gets rid of the appendicitis. It takes your appendix away
with a brief acknowledgement of the risks:
it’s a general anaesthetic and there are risks and complications of an operation
while adding that non-operative treatment has:
been used to successful[ly] treat appendicitis but we don’t know how effective it is and it can, um, have the risk of recurrence because we’ve left the appendix where it is.
Cons_Surgeon63_Family11_Declined
We also found that, although some health professionals continued to use the word ‘trial’ throughout phase 2 consultations, others started to describe CONTRACT as a ‘research study’. In phase 1, no health professional had described CONTRACT in this way.
Interestingly, our analysis of phase 2 consultations also identified instances when health professionals hesitated or corrected themselves, suggesting that they were consciously trying to avoid imbalanced language that might imply that surgery is superior:
So traditionally or, [hesitation] most people have always had an operation and we don’t know whether that’s the best treatment.
Cons_Surgeon32_Family20_NOT
In phase 2, health professionals less frequently indicated that CONTRACT could be burdensome and we found more examples of them framing CONTRACT positively:
Exciting opportunity to be involved in a part of a study looking at how is best to manage appendicitis in children.
Cons_Surgeon62_Family46_Declined
. . . an opportunity to help with future, um, treatments and also to get a better understanding of the treatment, best treatment for simple appendicitis.
Cons_Surgeon57_Family24_App
Exploring families’ treatment preferences
Following the discussion in the second training session emphasising the importance of treatment preference exploration, we found some changes to consultations. In phase 1, health professionals almost always asked families very generally if they had any questions. Most health professionals continued to ask families such general questions, but we observed some health professionals asking specific questions to elicit treatment preferences:
You’ve read the leaflet so, I can see where you’re coming from. So, are you saying that if you went into the study and the computer said ‘appendicectomy’, you would immediately drop out? Is that right?
Cons_Surgeon10_Family10_ App_Withdrew
Is there anything you think about that is sort of the idea of being involved in research, something that appeals to, that sort of worries you?
Cons_Surgeon29_Family17_App
Moreover, none of the health professionals asked questions in phase 1 to explore the reasons underlying families’ treatment preferences, whereas in phase 2 we found a small number of examples of this:
You would be randomised to either antibiotics or surgery but . . . what is it about, about the potential for, say antibiotics, that would make you think, ‘oh, no, I want surgery’? What’s, what’s the issue there?
Cons_Surgeon12_Family28_Declined
In phase 1, health professionals tended to accept families’ treatment preferences with little or no exploration, whereas in phase 2 we found more examples of health professionals gently exploring preferences and providing balanced information about treatment arms. Nevertheless, health professionals provided information on the risks and benefits of both treatments only in response to families whose treatment preference was for surgery. In such cases, either health professionals would provide additional information about the risks of surgery or, more often, they would provide reassurance or further information about the monitoring and care that would be provided to children allocated to non-operative treatment:
. . . some kids love to take antibiotics and medicine but my child is just not one of those.
. . . now, even if we take a child to theatre and have an operation, then potentially they may still need antibiotics after that time because the antibiotic duration is generally made on what the clinical findings are, sort of, when you do the surgery. So sometimes that can be up to a week . . . also . . . there is a risk that they can come back in with infections . . .
. . . My idea is just the . . . risk of it being an uncomplicated case at the moment and then, with antibiotics, if it’s not getting better and we get to a complicated case.
So, we’ve got, um, a sort of treatment plan and protocol for regular assessment and management of the patient . . . if he was enrolled into this study, then he would be, um, selected, you know, either to receive surgery or to receive the antibiotics and it would be a 50/50 chance. Um, with the antibiotic arm of it, we would then be regularly assessing him, clinically, um, all of his observations and if we have signs that he’s deteriorating despite the antibiotics, then at that point we would intervene, um, and look to perform an appendicectomy. So it’s not a kind of, you know, route to not being able to have an appendicectomy.
In phases 1 and 2, no health professionals tried to balance families’ treatment preferences for non-operative treatment, which, like balancing preferences for surgery, is important to ensure that families are in equipoise. Rather, when families expressed a preference for non-operative treatment, health professionals tended to reiterate that the family could be randomised to either treatment arm or further emphasised eligibility:
I was just going to say from, in the 12 hours we’ve been here, he’s had antibiotics and is considerably better.
Yes.
Now whether that’s antibiotics . . . fluids, whether that’s, you know, everything combined together.
It could be a number of those things, yeah . . . maybe being a little bit less dehydrated and having painkillers and everything else, erm, he’s had the antibiotics . . . Having one dose of antibiotics doesn’t affect you being, going into the study because if you’ve just had one dose, that’s a standard treatment for anybody we think’s got appendicitis and then, before the second dose, that’s when we make the decision about, you know, whether you’re happy to be included and, and which way we’re going.
Health professionals occasionally missed opportunities to record CONTRACT consultations; therefore, we may have missed some examples of health professionals exploring treatment preferences with families. However, the above results, alongside the results discussed in Health professionals’ experiences of CONTRACT, suggest that health professionals avoided balancing families’ treatment preferences for non-operative treatment, as such a preference was consistent with willingness to participate in CONTRACT.
Responding to families’ questions about CONTRACT
The updated ‘hints and tips’ sheet (available at www.journalslibrary.nihr.ac.uk/programmes/hta/1419290/#/) that we distributed to health professionals at the start of phase 2 presented current evidence, including numerical estimates of appendicitis recurrence rates following successful non-operative treatment. Families continued to ask about recurrence rate and health professionals often tended to frame the treatment failure and recurrence rate in various ways. As sites gained an impression of local non-operative treatment failure or recurrence rates over the course of phase 1, we found that some health professionals started to provide local estimates, albeit in response to direct questions from families and with caution:
You’d asked about in our experience of this trial here . . . and how many children have ended up, needed an appendicectomy that started off on the antibiotic arm . . . I haven’t got the exact figures . . . but so far, it’s, it is looking like almost a third . . . may have ended up with an appendicectomy . . . But it’s really important to stress that the numbers are very small. So those, those anomalies tend to skew things.
Cons_Surgeon8_Family40_App
However, as the above health professional acknowledged, the issue with providing local non-operative treatment failure or recurrence rates is that estimates are based on such small, site-specific figures in a pilot trial, which are an unreliable representation.
In phase 2, families also continued to ask about differences in recovery time between surgery and non-operative treatment. In contrast to phase 1, in phase 2, health professionals provided balanced responses to this question, by indicating that recovery was potentially similar (although acknowledging that it depended on the individual child’s response to surgery or non-operative treatment), or explaining that differences in recovery times between the two treatments were uncertain and that was partly the purpose of the trial:
. . . if she goes to the antibiotics arm, how long would she be off school for?
. . . [referring to both treatments] there’s no fixed . . . time. It’s when she’s ready. So when she’s, her pain’s OK, when she’s walking around, when she’s eating and drinking . . .
I don’t think a very great difference in the hospital time but obviously one of the things we’ll test is ‘does it take longer?’. That’s what we test again in these things.
Cons_Surgeon12_Family28_Declined
Further opportunities to optimise CONTRACT recruitment consultations
For the third training session, we revisited examples of the non-optimal communication covered in the first (pre-recruitment) and second training sessions, especially when we saw continuing examples of such communication during phase 2 consultations, including imbalanced descriptions of the treatments, and the absence of treatment preference exploration, or partial treatment exploration.
As we describe in the following sections, our analyses of the phase 2 data also identified several additional ways to optimise communication, which we included in the third training session. These included enhancing explanations of randomisation, managing families’ expectations about scheduling of surgery if non-operative treatment failed, balancing explanations of treatment risks, and responding to questions from families about the diagnosis of uncomplicated acute appendicitis.
Enhancing explanations of randomisation
Several health professionals specified that information about the child would be entered into a computer, which would then ‘pick’ a treatment:
Then we will actually go and put in a little bit of information about [child] into the computer and it will pick a treatment arm.
Cons_Surgeon8_Family45_NOT
Not surprisingly, the family interviews indicated that such explanations led families to think that the computer selected the treatment that was most appropriate for their individual child:
Once all the information had been gathered by the medics, it was being put into the computer to generate a random, profile to see whether or not he was eligible for, if he had to go down the, medical, the antibiotics route or the surgery route.
Int_Mother48_NOT
During the training, therefore, we advised health professionals to avoid explanations that might imply that CONTRACT treatments were selected based on specific information regarding which treatment might be suitable for an individual and, more generally, to be careful in referring to the use of computers in the randomisation process.
Managing families’ expectations about scheduling of surgery if non-operative treatment failed
In phases 1 and 2, in an effort to balance explanations of the treatments and reassure families about non-operative treatment, health professionals often emphasised that children randomised to non-operative treatment would be closely monitored and, if the child became increasingly unwell within 24 hours or showed no signs of improvement at 48 hours (i.e. treatment failure), they would be scheduled for surgery:
If we’ve got any doubt that he needs an operation at any time, he can have an operation at any time.
Cons_Surgeon10_Family47_NOT
However, health professionals rarely mentioned that, in the NHS setting, it is not possible to guarantee timing of unscheduled surgery and clinical cases are prioritised based on clinical need.
Interviews indicated that some families interpreted such comments to mean that surgery would be undertaken immediately following an assessment that non-operative treatment had failed. For example, when interviewed, a parent of a child randomised to non-operative treatment that had subsequently failed felt that communication about this aspect had been misleading:
We did expect it to be 24 hours, it’s worked, 24 hours, it hasn’t, she will get priority operation now . . . That’s what I would have expected.
Int_Father31_NOT
This finding informed an amendment to the participant information sheet (PIS) and was also included in the third training sessions, in an effort to enhance the management of families’ expectations regarding non-operative treatment failure and time to surgery.
Balancing explanations of treatment risks
By the end of phase 2, analyses showed that health professionals were routinely describing the key risks of non-operative treatment, and providing estimates of non-operative treatment failure or recurrence. However, some were giving minimal details of the risks of surgery, or were tending to emphasise how small or rare these risks were:
Equally, you’re exposed to the risks of an operation, so the, the, whilst general anaesthetics are very, very safe these days, there is a small risk associated with them. There’s a small risk, of doing things like injuring a little bit of bowel underneath. Again, that’s very rare or having scars on your tummy, and having problems with bleeding or infection.
Cons_Surgeon7_Family14__Declined
There are risks, during those operations that we, um, can cause damage doing that, that you can get infections and, as result of the operation itself, you can have complications that need other operations. That’s extremely rare, but we know that it definitely does treat appendicitis ’cause we take it away.
Cons_Surgeon26_Family48_NOT
As discussed earlier, health professionals routinely provided numerical estimates of the risks associated with non-operative treatment; however, they rarely provided numerical estimates of the risks of surgery unless prompted by families. In the third training sessions, we discussed how health professionals could advise families about the frequency of surgical complications while avoiding unduly alarming families. As part of this discussion, we showed a quotation from phase 2 to indicate one way in which this information could be presented:
We see complications in appendicitis in up to 25% of patients who have an appendicectomy. But appendicitis is a spectrum of disease. You have some that have quite advanced and you have some patients who are operated on very early . . .
Cons_Surgeon57_Family1_App
Responding to families’ questions about the diagnosis of uncomplicated acute appendicitis
Over the course of the interviews with health professionals in phases 1 and 2, it became clear that one of the key challenges in CONTRACT was diagnostic: distinguishing between children who had uncomplicated acute appendicitis and those who were ineligible for the trial as they had complicated or perforated appendicitis:
One of the other things I find difficult for some patients is picking the ones who are simple and not perforated, and there are at least one or two where we’ve not judged it quite right.
Int_Surgeon8
Reflecting this diagnostic challenge, during consultations, health professionals often indicated that, although they were confident that a child had acute appendicitis, they used phrases such as ‘we think’ to convey that the possibility of perforation or complications could not be excluded:
[Child’s] been diagnosed with having appendicitis which we think is simple appendicitis. And what we mean by that is that we don’t think it’s burst or it’s, it’s not gangrenous and sort of becoming like it’s going to burst.
Cons_Surgeon16_Family40_App
In consultations, some families asked a range of questions about health professionals’ confidence in the diagnosis, including the role of ultrasonography in verifying the diagnosis:
What about doing an ultrasound just to see if . . . we can further diagnose?
Cons_Surgeon12_Mother28_Declined
My only concern is we don’t know how advanced he is at the moment with appendicitis, and that’s why I asked you earlier is there not like a ultrasound you can do?
Cons_Surgeon57_Father1_App
In the third training sessions, therefore, we explained that parents were concerned about the certainty with which health professionals could diagnose uncomplicated acute appendicitis by examination alone and that they were asking about the need for ultrasonography. Our aim in doing this was to prepare health professionals for questions around diagnosis and eligibility, in order to improve their confidence in addressing such concerns when approaching families about CONTRACT.
Recruitment: phase 3
Training sessions
At the end of phase 2 of CONTRACT recruitment, a final round of communication training was provided at all three sites. Again, approximately 31 surgeons and research nurses (approximated based on the number of training evaluation forms we received back at the end of the session), who had been approaching or were likely to approach families about CONTRACT, attended. The training highlighted aspects of the CONTRACT consultations for which we had observed positive changes (e.g. referring to treatment arms in a more balanced way) and revisited examples of non-optimal communication (e.g. lack of family treatment preference exploration). The training also focused on the aforementioned issues and further opportunities we identified to optimise the CONTRACT recruitment consultations in phase 3, including enhancing explanations of randomisation, managing families’ expectations about the scheduling of surgery if non-operative treatment failed, balancing explanations of treatment risks and responding to families’ questions about the diagnosis of uncomplicated acute appendicitis. The sessions followed a format similar to the previous sessions.
Assessing the impact of recruitment training on communication in phase 3 consultations: what changed and what did not change?
Use of terminology
We continued to find that the language health professionals used throughout phase 3 was balanced. In particular, there were far fewer instances of terms that implied imbalance between the two treatments. The only exception was a few occasions when some health professionals continued to use ‘traditional’ to refer to surgery.
Exploring families’ treatment preferences
We found that health professionals were increasingly making statements to explore the underlying reasons for treatment preferences:
I mean obviously people have got individual reasons why they like the sound of it or don’t like the sound of it. We’re always keen to explore your thoughts about the studies really because we can’t run studies unless patients are happy to be in them.
Cons_Surgeon8_Family33_Declined
Nevertheless, as the below example demonstrates, although health professionals increasingly explored the underlying reasons for treatment preferences, they typically accepted these rather than gently challenged them:
I think the worry is, is that [patient’s] really adamant he wants the surgery.
OK . . . do you know what that’s based on, from him?
I think it’s just his old-school, gut feel thing . . . I think, erm, his brother had a really bad, his brother’s appendix, so I think he’s just got this, like emotional . . .
Yeah, no, no, absolutely.
In this phase, there was one example of a health professional attempting to balance a family’s treatment preference for non-operative treatment:
So, are you saying that if you went into the study and the computer said, appendicectomy, you would immediately drop out? Is that right?
Yes.
If we treat him with antibiotics now, there is still the possibility it could come back again. So, you know, we know that doing an operation definitively treats it. So, yeah, it does have to be either or. But you can change your mind.
Explanations of randomisation
Some health professionals in phase 3 elaborated on their previous explanations of randomisation to emphasise that treatment allocation was random and to avoid implying that treatment allocation was based on what might suit an individual child:
A computer is going to pick at random half the children to have an operation and half the children to have antibiotics, and it’s only by doing that that we can have two fairly distributed groups.
Cons_Surgeon10_Family44_App
Managing families’ expectations about scheduling of surgery if non-operative treatment failed
By the start of phase 3, the CONTRACT parent PIS had been amended to better manage parents’ expectations regarding non-operative treatment failure and time to surgery. In phase 3 consultations, health professionals also typically aimed to manage families’ expectations about the timing of surgery if non-operative treatment failed. Thus, they described how children were monitored and clarified the timescale of surgery:
We will monitor him, OK. And in the next 24 to 48 hours, we’ll see how he does. If things do not get better, OK, or if he becomes worse in any way but while he stays in the hospital, OK, we will proceed with an operation . . . it will not happen immediately when we make the decision but it may take a few hours, you know, until this happens.
Cons_Surgeon41_Family26_App
We’ll take her to theatre as when it’s appropriate to do so . . . It may not be this evening, even if she is randomised to the operation group, that may be tomorrow morning.
Cons_Surgeon63_Family23_Declined
Although rarer, we found that some health professionals were also managing families’ expectations of the time it would take for non-operative treatment to have an effect too:
We’ve learned from the first year of this study, just to explain, make sure we’re clear to you, that if we end up being in the study and initially having no operation, it still takes a couple of days to get better . . . we wouldn’t expect that within, you know, a couple of hours, he’s completely well . . . and no discomfort.
Cons_Surgeon31_Family5_NOT
Balancing explanations of treatment risks
In phase 3, some health professionals drew on the study rationale to balance treatment risks and benefits. Often health professionals would refer back to the study rationale later in discussions to emphasise clinical equipoise:
Like I said when I first started the conversation is, that if we knew for sure that one way is definitely better than the other, we wouldn’t have offered the other.
Cons_Surgeon59_Family37_Declined
Just to sort of counterbalance . . . so an operation gives certainty to some degree, in that we’ve taken the appendix out, so she won’t get appendicitis again, but there are risks with an operation, and they include having infections in the tummy.
Cons_Surgeon63_Family23_Declined
The third training session also covered how health professionals tended to focus on the risks associated with non-operative treatment, whereas mention of the risks associated with surgery were often minimal or vague. In training, we suggested that providing numerical estimates of the risks associated with surgery could help balance the explanation of the treatment arms. In phase 3 consultations, with a few exceptions, the following was stated:
20% of the people having an operation may have complications; not all of them are major.
Cons_Surgeon28_Family53_App
We did not identify any widespread changes in how health professionals described the risks of surgery. That is, most health professionals briefly explained that surgery entailed some risk, but numerical estimates of the risks and the specific risks involved were rarely mentioned. In Providing too much information on risk, we explore health professionals’ reservations about detailing surgical risks during CONTRACT consultations.
Responding to families’ questions about the diagnosis of uncomplicated acute appendicitis
In the final phase of recruitment, a few families continued to ask questions about health professionals’ confidence in the diagnosis and the role of ultrasonography in verifying the diagnosis. In the third training sessions, we explained that parents were concerned about the certainty with which health professionals could diagnose uncomplicated acute appendicitis alone. In the few example responses, health professionals provided justification for this:
. . . how do you know that that . . . it is definitely appendicitis?
Yeah, well I, I can’t say definitely . . .
Right, OK.
. . . and I can’t put my hand on my heart and say I know 100% . . . about 90% or so, 85% chance we, we’re right but, you know, looking at [patient], he’s tender, he’s got a temperature, he’s got his white blood cells, which are signs of infection, are high, there’s another protein in the blood that we look at that’s pretty high that’s showing us that, you know, it’s quite likely, based on everything and based on how his tummy feels and how tender he is over the right side, you know, I’m confident enough to say he has appendicitis.
Families’ experiences of CONTRACT
In the family interviews, we also explored families’ broader experiences of CONTRACT. In this section, we describe key topics of importance to families that should be considered in designing and implementing future trials in this context. Although some of the points are specific to CONTRACT and a future definitive trial of the treatment of simple appendicitis in children and young people, many of the issues we raise have implications for future trials in paediatric urgent care more broadly. In particular, families discussed their views on optimising children’s and young people’s involvement in research discussions in an acute setting, the optimal length of time for families to decide whether or not to participate, fear of perforation and post-surgical discussions with surgeons.
Optimising children’s and young people’s involvement in research discussions in an acute setting
Child’s capacity
Overall, children tended to have little involvement in CONTRACT discussions. Some older children did recall aspects of CONTRACT consultations:
They gave me an option of either taking part in the study or not having to. And then they said that your name and your symptoms and information will be put into a computer and then you get chosen whether you get the antibiotics or your appendix taken out.
Int_Child48_Age14_NOT
I think [the video was], simple to explain . . . I read the information sheet as well and that was, like, quite simple to read.
Int_Child14_Age13_Declined
In contrast, younger children often struggled when interviewed to recall discussions and often indicated that they were uninterested:
Um, I remember mummy and daddy watching it.
So did you, you didn’t see any of that yourself?
I might have done, but I can’t really remember.
So was there anything in particular that stood out for you, that you remember them saying and that really stood out?
Thinking about it I don’t really . . . I was more or less just thinking about, like, my pets and stuff.
However, it was clear that children of all ages were often in too much pain to engage in the discussions and decision-making, as evidenced by consultations and child and parent interviews:
It was hard for me to concentrate . . .
The lady was asking him questions, wasn’t she? And you were just going, ‘oh I just want it, I just want to stop it’.
Did the video make any sense to you [child] or are you feeling a bit too sore?
[Crying] . . . too sore.
Although most children, when asked, were able to recall that CONTRACT examined non-operative treatment of appendicitis, the above quotations suggest that maximising children’s and young people’s involvement in decision-making in this context could be challenging.
Managing conflicting treatment preferences within families
We often found that parents’ and children’s treatment preferences differed; therefore, their willingness to participate in CONTRACT also differed. Children tended to prefer non-operative treatment, whereas parents preferred surgery. Interviews with children and parents indicated that many children were frightened by the prospect of surgery, which probably explains their preference for non-operative treatment:
Just the idea of not really having to have an operation I thought would be good.
Int_Child48_Age14_NOT
Some also believed that being treated with non-operative treatment might allow them to go home sooner:
I just wanted the antibiotics so I could go home.
Int_Child44_Age9_App
Despite conflicting preferences, many parents were keen for their child to be actively involved in the discussion and in decision-making. In some cases, families participated in CONTRACT despite differences in the preferences of parent and child, with the preference of the child to participate taking precedence. One mother explained that she would have preferred for her child to have surgery and not to participate, but as her child had a preference for non-operative treatment and was keen to participate, the family jointly decided to participate in CONTRACT:
And did you feel as if the doctor, when you spoke to them, that they were interested in what you thought and that you got to kind of have a say about it?
Yeah. Yeah, ’cause as my mum said, they didn’t actually need my initials but they took them anyway so.
Yeah, but we’d decided, hadn’t we? I, I was respecting what you’d decided to do. You wanted to do the study.
Another child valued the opportunity to be involved in CONTRACT discussions and in decision-making. Furthermore, the child’s mother supported her daughter’s involvement, reasoning that children should have an opportunity to contribute to decision-making, as it is the child’s body that will be treated, not the parent’s:
I was glad that we discussed and I could have a say in whether I could also choose as well . . .
It’s her body.
Parents’ concerns about discussing treatment risks with children
Although several parents were keen for their child to be actively involved in research discussions and decision-making, some parents of younger children were concerned that discussing CONTRACT in front of children would provoke or aggravate, or had provoked or aggravated, anxiety in their children, when they were already unwell and often distressed. Some parents described how they discussed CONTRACT between themselves away from the child or that they would have preferred the health professional to discuss it without their child present. One parent of a 7-year-old child explained:
We wanted obviously to be a part in the study and then we . . . it was only for the fact we went downstairs to talk about it, ’cause we couldn’t talk about it in front of him because he was getting so upset.
Int_Mother49_ NOT_Withdrew
Parents were particularly concerned about their child hearing descriptions of the risks and benefits of CONTRACT treatments. One parent of a 9-year-old child commented that such details had undermined their efforts to allay their child’s anxieties:
When [the surgeon] went through all the complications . . . I even said to the doctor at that point, I said, ‘does he need to, does he really need to know this?’ . . . that’s when I could see him getting really scared and, like, I was quite annoyed over that because I, I’d been saying to him, ‘it’s fine, it’s just like getting your tooth out’, you know, and then [the surgeon] said, this [treatment risks] could happen and it, I could just see he was getting really frightened and I just think, when they’re in that much pain, and frightened anyway, I don’t think they need to know all of that. So I do think perhaps those conversations should be made outside the room, you know, away from the, from the child really.
Int_Mother44_App
Another parent of an 11-year-old also commented that providing children with information on the risks and benefits of CONTRACT treatments amounted to ‘scaring a child unnecessarily’ and . . . undermining the confidence they felt in . . . the doctor ‘. . . that they were making the decision’ before adding that ‘under 9 [years old] . . . I don’t really think . . . they should be involved in the study but not know that they’re involved in it’ (Int_Mother57_NOT).
She added that she chose not to consult with her 11-year-old child about CONTRACT because ‘I just don’t think he has the maturity’, which suggests that age should not be the only consideration in discussing CONTRACT treatment risks with children.
Optimal length of time for families to decide whether or not to participate
Families were often provided with several hours to deliberate about whether or not to participate, following the main consultation between families and health professionals about CONTRACT. Families reported intervals ranging from approximately 15 minutes to 7 hours from being informed about CONTRACT to having an opportunity to voice their decision, although most families reported waiting 1–2 hours.
This period of deliberation, although consistent with ethics guidance, meant that families typically experienced a time period when they were uncertain which treatment they were to be allocated to if they did participate, or a time period when they were uncertain as to when treatment would commence if they did not participate. One parent who had decided soon after the consultation that they wished to participate described how being provided with more time than they needed was particularly stressful for their child:
It was hard for [child]. [Child]’s 12 [years of age], you know, it was very, very difficult ’cause he kept asking ‘what are they doing next?’. You know, ‘are they coming back?’ . . . and he’d say to me ‘what do you think I’ll be on?’. I don’t know.
Int_Mother57_NOT
Some families reported that health professionals had delayed or withheld antibiotic treatment or pain relief until the family were able to voice their decision about CONTRACT participation. In these cases, families often reflected on whether or not the study had adversely affected their child’s care:
While you’re waiting for a decision to be made [on participation], whether, it’s going to be solely antibiotics, there might be a medical reason why you can’t do this but I think children should be started on antibiotics anyway because you’ve got hours there where they’re having to, you know . . . [Child] might not have ruptured if he’d have been on antibiotics over the night before he went into [surgery].
Int_Mother57_NOT
I was thinking, ‘did, did they delay the antibiotics until the other research surgeon had been to see us?’ . . . Because it, it seemed strange that the surgeon had told me earlier on in the day that they were gonna to start him on the i.v. antibiotics. But then he never started it until after we’d seen the, the lady surgeon from the research.
Int_Father33_Declined
The consultation corresponding to the preceding quotation corroborates the parent’s account that antibiotic delivery was delayed until around the time that the health professional returned to find out the family’s decision about participation. It also indicates that delays in antibiotic delivery could influence CONTRACT participation or retention:
I was thinking, doctor, to just go with the surgery . . . my only worry is seeing him in that way like we did before . . . It, it might have been different if he was starting the antibiotics earlier.
Yeah, maybe.
And that could have been working and we’ve seen a little bit of improvement or a dip, then I might have been tempted to go ‘well let’s carry on with the antibiotics and hopefully that will’ but . . .
Yeah, yes.
. . . because he hasn’t been on that and we haven’t see it.
You haven’t had the benefit of seeing him look a bit better, yep.
Parents’ reasons for surgery preference
As discussed, parents often preferred surgery to non-operative treatment, and therefore declined participation in CONTRACT. The most common underlying reasons for such preferences included the perception that surgery would avoid perforation and the belief that surgery would result in immediate pain relief. Furthermore, concerns regarding perforation and pain were compounded by treatment delays, which further drove families to opt for surgery.
Surgery to avoid perforation
Parents’ (and children’s) preferences for surgery tended to arise from concerns that the appendix could perforate:
I know that most people wouldn’t put their kids through an operation but that wasn’t really on my mind. It was sort of getting it dealt with and not letting it get too far and getting it done while he was well with it.
Int_Mother15_Declined
Many parents perceived perforation as severe and immediately life-threatening:
You know, going into an operation, well, is very different to going into an operation with a burst appendix. So it was just to get it sorted and done with rather than let it drag on really.
Int_Mother15_Declined
As noted earlier in this chapter, this was often influenced by narratives of family members or friends:
I did know that it could be life-threatening, so that was my major concern really. I think I wanted him to have the operation rather than try antibiotics that might not work because my friend had the peritonitis you see, where it burst and it, it was really, really serious.
Int_Mother44_App
Some parents sought reassurance that participation would not jeopardise the risk of perforation and some health professionals explained to parents that they were confident that the child had acute appendicitis and that perforation was unlikely. This seemed to reassure some parents and encouraged them to participate in CONTRACT:
As long that we’re fairly confident her appendix isn’t gonna pop anytime now . . .
Mm-hm.
. . . then there’s really no harm in trying the antibiotics first.
Surgery as immediate relief from pain
Parents described the pain and discomfort their child had experienced and the emotional upheaval they experienced as a parent, seeing their child become increasingly unwell and in pain:
My daughter was screaming, and I mean screaming, in pain. I said ‘what? This is so abnormal.’
Int_Mother32_App
Surgery was often preferred by parents as it was viewed as providing immediate pain relief:
He’s got quite a high pain threshold anyway, so to see him like that, I just think it just made the decision for us to just take it out, wasn’t it, straightaway?
Int_Mother33_Declined
One child also commented, with retrospect, that surgery offered him a faster pain-relief trajectory:
[By having surgery] it was over and done with, like I wouldn’t be in a lot of agony and it slowly . . . it would just be taken out and it would be over, you know, a slight pain from surgery.
Int_Child_Age12_NOT
Concerns about perforation and pain compounded by treatment delays
Parents’ concerns about perforation were compounded by delays in presenting and receiving treatment. Families who had experienced such delays were more likely to prefer surgery and less willing to participate in CONTRACT. Families described delays in presenting to hospital, as parents initially attributed their child’s symptoms to gastroenteritis, a sports injury or anxiety. It was often only after symptoms had persisted for several days that families sought medical care:
I got a phone call from the school saying I needed to pick her up ’cause she’d been sick, and I just thought . . . just a sickness bug . . . the next day she, she kept saying that her stomach hurt. Didn’t really think anything of it . . . then the pain got worse and worse and worse, and she was on the sofa literally screaming in pain . . . so obviously I had a feeling it might have been appendicitis, so obviously I took her to the doctor’s.
Int_Mother60_NOT
Some families consulted their GP before presenting at hospital; most GPs suspected appendicitis and advised them to attend accident and emergency (A&E). Some GPs did not, which added to delays in presenting to the hospital: ‘I rang our GP and they just said, it just sounded like a vomiting bug’; in this case, the GP advised waiting ‘24 hours’ and contacting the general practice again if the symptoms had not resolved (Int_Mother47_NOT). Finally, several families attended their local general hospital and were subsequently transferred to a children’s hospital, which occasionally contributed to additional delays.
Responses to treatment allocation
Families did not always respond well to receiving news of which treatment they had been allocated to. In particular, some children became upset on hearing that they had not been allocated to their favoured treatment:
[Child] broke down [when he heard which treatment he was allocated to].
Oh.
And I had a secret high five . . . he was really adamant he wanted the antibiotics and I think he was really gutted that it came up he needed surgery.
However, some families that initially had a preference for non-operative treatment but were allocated to surgery and were told later that the child’s appendicitis was more advanced retrospectively appreciated being randomised to surgery, as they believed that non-operative treatment would not have been effective:
I wanted antibiotics but I kind of got lucky, ’cause if I did [get antibiotics] it would have been worse for me anyway.
Int_Child57_Age 12_NOT
Although families did not always discuss their experience of treatment allocation in detail, one mother described her disappointment at not being allocated to her preferred treatment and being informed in a brief and unfavourable manner:
I was talking to a nurse . . . the consultant came round and said ‘no, sorry, she’s not got it’, I was like, ‘what? Not got what? What?’. So that was a bit of a blow. I think I’d rather have been told away from her about that . . . that felt like it was thrown at me . . . that seemed to happen quite quickly.
Int_Mother32_App
Post-surgical discussions and the impact of CONTRACT on families’ views in hindsight
In interviews, several parents who participated in CONTRACT commented that non-operative treatment would not have been effective in treating their child’s appendicitis; such views seemed to be informed by post-surgical discussions:
I don’t know if you’ve got his notes there, but actually . . . antibiotics would not have worked with him.
Int_Mother7_App
One family that participated in CONTRACT were informed by their surgeon that their child had ‘one of the worst [appendixes]’ that the surgeon had ever seen (Int_Mother57_NOT). Notably, a family at the same site who did not participate in CONTRACT were also informed that their child’s appendix was:
[O]ne of the worst [the surgeon had] ever seen.
Int_Mother61_Declined
Other families recalled surgeons’ vivid descriptions of the removed appendix post surgery:
He said it was this big, like five times the size. He said it was black, it stank. He said it was encrusted in infection, it had an abscess attached to it. She was in theatre for 3 hours. It pushed right underneath her bowel, all her bowel was infected.
Int_Mother61_Declined
Among families that had participated in CONTRACT and were randomised to non-operative treatment that had failed, some parents described feeling guilt or regret after surgeons had given details of the surgery and a feeling that consenting for their child to join CONTRACT was the ‘wrong’ choice and jeopardised their child’s condition:
So she’d had all the delay with the drip, it didn’t work . . . and I wondered if that’s, that’s why her appendix had perforated . . . [The health professional told me it had perforated] when they operated . . . So I have felt a bit guilty that maybe if I’d have gone with my initial instinct, which was to just get the operation over and done with . . . that she might not have had it perforate.
Int_Mother45_NOT
It is important to note that not all families reported that surgeons provided a description of the removed appendix. There were no reports of being informed that their child’s appendicitis was less severe or negative, although one parent questioned, during the interview, whether or not her child had appendicitis at all:
I’ve been like dying to know whether it was, whether it was that that caused it, you know, in the first place or not? . . . He might not have actually had to have the appendix out . . . if it was [the condition she suspected] . . . then it probably would have been treated with the antibiotics.
Int_Mother44_App
The preceding quotations indicate that post-surgical discussions can leave families retrospectively questioning whether or not their child should have been eligible for CONTRACT. In addition to inducing feelings of guilt among some families, such experiences could affect family–health professional trust, as families were confidently informed by health professionals that non-operative treatment is a viable treatment option and were later informed that non-operative treatment would not have been appropriate. Underlying these issues are the challenges that health professionals encounter in assessing patient eligibility for CONTRACT. This is discussed in greater detail in Concerns regarding patient eligibility.
Health professionals’ experiences of CONTRACT
In the interviews with health professionals, we explored their broad experiences of CONTRACT and the topics they raised regarding the design and conduct of a future trial. Some of the points are specific to CONTRACT and a future definitive trial of the treatment of acute uncomplicated appendicitis in children, but many have implications for future trials in paediatric urgent care more broadly.
Health professionals described influences on their clinical equipoise, their concerns regarding patient eligibility for CONTRACT, their experiences and reservations about exploring families’ treatment preferences, their experiences of dealing with conflicting treatment preferences within families, issues with conducting blinded discharge assessments and the challenges of explaining the concept of feasibility in the urgent care setting.
Clinical equipoise
Most health professionals felt that CONTRACT addressed an important research question:
I felt that this is a really important thing to be doing, because it’s in everybody’s interests to know if . . . we can treat appendicitis with antibiotics in the future.
Int_Surgeon40
and spoke of the value of evidence that might mean that children could avoid surgery and the risks that it entailed:
It’s a really important trial to be done because, for individuals, if you can avoid an operation and potentially avoid any other problems with appendicitis for the majority of patients in the future as well, then that’s really positive.
Int_Surgeon7
Some argued that if non-operative treatment is currently used to treat similar conditions, then its use in acute uncomplicated appendicitis should also be explored:
If you can treat masses with antibiotics, then theoretically you can treat all of them with antibiotics.
Int_Surgeon39
However, health professionals were aware of ‘one or two’ colleagues in their clinical teams who were not in equipoise or did not:
. . . fundamentally believe in the, in the underlying reasons for the study . . . don’t think we need to be doing it.
Int_Surgeon8
I perhaps know one other person who is perhaps not in equipoise.
Int_Surgeon35
Typically, such beliefs reflected a preference for surgery. The same surgeon explained that, although they saw value in CONTRACT, they hoped that surgery would be found to be superior to non-operative treatment in a larger trial:
Actually it would be really good to know this. I don’t really care which outcome it is. Personally, I’d quite like it if it was taking out the appendix, but actually it would be really nice to settle it ’cause then we could stop worrying about it.
Int_Surgeon35
In the following section, we elaborate on the reasons why health professionals preferred surgery.
Surgery as standard treatment
Surgeons in particular pointed to their long-standing experience of appendicectomy as ‘the treatment’ for acute uncomplicated appendicitis and of the challenges posed by treating children in a non-standardised way:
I’ve been doing surgery now for 15 years, so appendicitis equals an operation and it’s quite difficult to change your mindset.
Int_Surgeon54
[Some surgeons] see surgery as the answer and not antibiotics . . . culturally, that’s what was always done.
Int_Surgeon17
Some spoke of surgery as an ‘easier’ and ‘safer’ option than non-operative treatment:
We do generally want the best for the patients. It would be nice to avoid surgery but personally . . . it’s perhaps easier to just take the appendix out . . . I regard the process of taking out the appendix as generally safer than not taking it out.
Int_Surgeon35
Some health professionals also believed that parents were reluctant to accept non-operative treatment when surgery was the traditional treatment, and that parents’ and surgeons’ perceptions created a barrier to parents’ acceptability of non-operative treatment:
I was a little bit sceptical about [CONTRACT] . . . if someone came to me and said, ‘OK, well, let’s give your kid antibiotics’, I would say, ‘well, I don’t think so, I want the appendix out’. So I think it’s an issue of addressing your [as a surgeon] fixed ideas in addition to the fixed ideas of . . . the parents.
Int_Surgeon41
Conversely, one surgeon suggested that parents were more open to the concept of non-operative treatment than clinicians, and spoke of surgeons’ direct experiences of non-operative treatment failure over the course of CONTRACT as challenging their equipoise and attitude to CONTRACT:
As you go through the trial, you can start seeing the clinician towards, ‘oh we’re failing, we’re failing, we’re failing’ . . . It’s not the real impression, just the feeling gets more intense as it goes along after you have seen one or two [failures]. I find it’s actually the clinicians who are being more sceptical; the parents are being much more open than I had expected.
Int_Surgeon51
Concerns about antibiotic resistance and limiting opportunities for junior surgeons
A few surgeons had more specific concerns that increasingly treating children who were suspected to have acute uncomplicated appendicitis with antibiotics could contribute towards antibiotic resistance in the future:
I do worry a little bit from an antimicrobial resistance that, if we move to treating lots of early appendicitis with antibiotics, what that would mean in the long term from that microbiology point of view? Especially because I think it will become very easy to say ‘this patient has got abdominal pain, it’s appendicitis unless we’re proven otherwise, so we’ll just give antibiotics for it.’
Int_Surgeon7
You could argue that more [families] than not will go towards the antibiotics rather than surgery. Unless, of course, you have more scare stories about how antibiotic resistance is coming in . . . that may well influence how people decide in the longer term.
Int_Surgeon12
One surgeon also commented that increased use of non-operative treatment for acute uncomplicated appendicitis would deprive junior surgeons of training opportunities to improve their basic surgical skills:
Slightly facetious, but . . . you take away these straightforward . . . training operations which can become useful, you know, useful . . . for people building basic skills . . . In the longer term, you . . . have to become more inventive or find different ways . . . for people to gain their surgical experience, and that could be a counter-risk going forward.
Int_Surgeon12
Finally, some surgeons discussed how surgery is their passion and successfully performing surgery is satisfying, so, naturally, they felt reluctant to treat children in alternative ways:
I still quite like the certainty of, firstly, seeing it, so I know it’s there, and, secondly, taking it out and knowing what I’ve done . . . I’m not trying to make a case against the antibiotic arm at all, but I am confessing my personal bias.
Int_Surgeon35
We’re surgeons and we like doing operations.
Int_Surgeon10
It’s not as exciting to give people antibiotics as to operate.
Int_Surgeon12
Preference for non-operative treatment
As discussed, although most surgeons thought that CONTRACT addressed a valuable research question and could see the benefits of treating children with non-operative treatment, most tended to prefer surgery. However, one surgeon described how their experience of successfully treating a number of children recruited to CONTRACT non-operatively had ‘tempted’ them to consider treating children outside CONTRACT in a similar way:
You watch some patients get better with antibiotics and it’s really, really tempting to just not sort of bother with the trial and just offer patients antibiotics occasionally, which I haven’t done. But, you know, it’s quite hard to sort of, you know, keep your own personal views under control as you see it unfold.
Int_Surgeon17
Another surgeon explained:
I don’t know about anybody else, but my perspective is always to try and avoid an operation as much as possible.
Int_Surgeon10
This surgeon later approached a family who described a preference for non-operative treatment. The family was randomised to appendicectomy but withdrew from CONTRACT and subsequently received non-operative treatment outside the trial. It is unclear whether or not the health professional’s views about non-operative treatment influenced the family’s decision-making in this case.
Concerns regarding patient eligibility
Health professionals often perceived some children to be more or less suitable for either of the treatment arms, and this appeared to influence their view of clinical equipoise in the trial. Health professionals believed that children who were particularly poorly were more suitable for surgery, whereas those who were less poorly were felt to be more suitable for non-operative treatment, despite all children being eligible for CONTRACT according to the trial protocol:
How they look and if they obviously look pretty sick, then I think you’ll be more reluctant to do something that doesn’t feel standard . . . He was definitely eligible, for sure. But . . . he looked like he had appendicitis which, which is not entirely well.
Int_Surgeon37
We do agree that, for the selected group of patients, [antibiotics] would work . . . The irony is that sometimes we have selected certain people that we think ‘oh, they definitely, it’s more the early appendicitis type and not the complicated appendicitis and would definitely do well’, but . . . sometimes you feel sad that someone that looked really well and would do really well with antibiotics alone, is then randomised to having an operation.
Int_Surgeon11
Health professionals’ concerns regarding patient eligibility were usually born out of their worries about diagnosing children with acute uncomplicated appendicitis. A key inclusion criterion for CONTRACT was for children to have a clinical diagnosis, either with or without radiological assessment, of acute appendicitis that, prior to study commencement, would be treated with appendicectomy (see Chapter 2). CONTRACT thus brought a new challenge for surgeons: distinguishing between children with acute uncomplicated appendicitis and those with perforated appendicitis. Children who were very sick and suspected to have a perforated appendix were seen as relatively straightforward:
The really sick ones, they’re easy [to differentiate], you wouldn’t even consider them for the study, you know they need an operation.
Int_Surgeon8
However, as the same surgeon explained, other children were more challenging to differentiate, and surgeons described the difficulties that arose in a few cases when children whom they had originally suspected of having acute uncomplicated appendicitis were subsequently found to have a perforated appendix:
I find difficult for some patients is picking the ones who are simple and not perforated, and there are at least one or two where we’ve not judged it quite right. In retrospect, at the time it felt like the right decision, but in retrospect we haven’t.
Int_Surgeon8
They elaborated on the repercussions of such cases:
[Child] did OK for the first day [on antibiotics], but then by day 2 wasn’t well, had perforated appendicitis, got an operation and then had a, not a terrible time recovering, but more stormy than they would have done if they’d . . . had their appendix taken out. Mum was really angry, really angry.
Int_Surgeon8
Difficulties also arose in determining whether children had very early acute uncomplicated appendicitis and should be treated, or whether their symptoms were self-limiting and should not be treated:
A girl I saw the other day who needed an ultrasound showing signs of appendicitis to persuade yourself that this is really what was happening, rather than [a child] who you’re, you know, absolutely sure and you know they’ve got appendicitis . . . It’s those sort of ones who come very early, their blood tests are only minimally deranged, they’re minimally tender, they’re quite well in themselves, they may not even have had a temperature yet . . . and you’ve got enough in the history to persuade yourself . . . You’re swaying towards the fact that some children in that group might actually not have appendicitis and might never have needed anything . . . it’s a really fine line and it’s really difficult I think.
Int_Surgeon10
Outside CONTRACT, appendicectomy was the standard treatment for both acute uncomplicated appendicitis and perforated appendicitis, and as noted above, before CONTRACT, surgeons had not typically needed to preoperatively distinguish whether a child had acute uncomplicated or perforated appendicitis. Several suggested that further work to define acute uncomplicated appendicitis would be needed for a future trial to be feasible:
I think if we did a bigger study we might try and define a bit better, which was mainly about differentiating simple and perforated appendicitis clinically, preoperatively, which is not normally something you need to do.
Int_Surgeon31
That’s one of the key things underlying the whole reason for the study: are we good at picking out the patients for who antibiotic treatment is most likely to work? And it’s not something we’ve ever had to do before.
Int_Surgeon8
Health professionals offered suggestions to refine the inclusion criteria so they would be more confident in identifying only those children with acute uncomplicated appendicitis. Some indicated that blood test results [e.g. C-reactive protein (CRP)] could help to better determine appropriate children for the trial:
If that CRP was 20, 30 or 40 [mg/l], 4 is normal, less than 4 [mg/l]. She had 20 [mg/l], yeah, that’s OK, 30, 40 [mg/l]. If she had 200 [mg/l], that’s definitely not early appendicitis, that’s huge.
Int_Surgeon57
We might even look back and re-analyse blood tests and everything else so that we can try and say that these are exactly the [patients] who we think you can assume are not perforated.
Int_Surgeon31
More generally, another surgeon wanted the inclusion criteria to be refined to create:
[A]n extra safety netting thing, which would give other people confidence in the study.
Int_Surgeon8
A few health professionals also favoured restricting eligibility to older children. Older children were viewed as being more suitable for non-operative treatment than younger children:
I don’t know if like it’s an age thing and anyone older, more, you know the parents and the child would be more likely to go for the antibiotics side rather than a small child.
Int_Nurse2
This was particularly the case because younger children were reported to be more prone to present with complicated appendicitis:
I think [CONTRACT is] probably more suitable for the ones after 8 [years of age], like older than 8, probably, because, um, the, um, the younger ones tend to have more like, um, complicated appendicitis.
Int_Surgeon11
Nevertheless, other health professionals argued that restricting the eligibility could bring recruitment difficulties and limit the generalisability of the results of a future main trial:
For the study to mean anything, it sort of has to have that full [age] range, doesn’t it, otherwise it’s meaningless at the end of it . . . It’s important, as clinicians, to feel that there’s a point to what you’re doing . . . If it’s just sort of the over 12s, I’d be thinking ‘what’s the difference between this and, and the adult study?’.
Int_Surgeon17
One surgeon initially suggested that the eligibility criteria could be refined to include only children who had been symptomatic for < 24 hours, but continued to explain that children rarely present to hospital within this time frame:
The only way to refine the criteria would be to really bring it right back and say . . . ‘the history less than 24 hours’ . . . to make it so soft that there would be so few children that would be eligible to be included.
Int_Surgeon10
Exploring and balancing families’ treatment preferences
We provided health professionals with recruitment training, which partly focused on encouraging them to explore families’ treatment preferences with the aim of optimising informed consent and recruitment. 44 Health professionals varied in their views of treatment preference exploration. Several reported exploring treatment preferences with families and described the benefits of doing so. For example, one health professional explained that it can be useful when parents and their children had differing treatment preferences, particularly when the parents’ preferences were based on their personal experience of perforated appendicitis:
Usually the parents that have had experience with appendicitis, you know, they’ve had the old-style operation with a cut, OK? . . . So if they say, ‘I’ve had my appendix out’, you sort of get an idea about their experience and you utilise it accordingly . . . So many of them may have perforated appendicitis or have stayed in hospital for 7 days. OK, and you start there because they have an experience of what can actually go wrong with surgery.
Int_Surgeon41
For another health professional, it was essential to explore families’ treatment preferences, as the use of non-operative treatment for acute uncomplicated appendicitis in children was viewed as novel, and families needed support to understand it:
I don’t have a problem with [exploring treatment preference], with doing that because people just don’t know, do they? If you always assume that having appendicitis means that you get your appendix out and you’ve not heard that there’s another treatment, well you can’t be expected to, to sort of figure your way through that without information.
Int_Surgeon17
However, as we explain in the sections that follow, others expressed concerns about exploring treatment preferences with families.
Health professionals’ reservations about exploring treatment preferences
Health professionals explained that some families had strong treatment preferences and were not open to exploring the possibility of joining CONTRACT. For such families, health professionals were cautious not to ‘push’ (Int_Surgeon39) the trial or ‘persuade them’ (Int_Surgeon35), indicating that such health professionals equated preference exploration with persuasion. Reflecting on discussions with families more broadly, it was apparent that several surgeons viewed exploring treatment preferences as coercive. Referring to training advice to balance families’ treatment preferences by indicating the drawbacks of that treatment, one surgeon commented:
I mean children, young people, all the time would rather go for the less unpleasant option and I suppose one thing you could point out is that you may take longer to get better . . . if you go for the antibiotics, you may well be in hospital longer, there may be more tests, but I think you don’t want to coerce people.
Int_Surgeon35
One surgeon suggested that, if families did view them to be coercive by balancing treatment preferences, they might make a complaint, and this appeared to underline the surgeon’s concerns:
People come with their preconceptions and baggage, and though they understand it logically, they can’t, they still want to hang their hat on the, on a sort of operation and it’s quite hard to change it. And what I didn’t want to do was to be the person who pushes it too much and they complain.
Int_Surgeon18
As described earlier, in CONTRACT recruitment discussions, health professionals tended to focus more on the risks of non-operative treatment than on the risks of surgery. Moreover, some parents were concerned about health professionals discussing treatment risks in front of their children and some surgeons suggested that they provided a ‘distilled’ description of surgical risks, to avoid unduly worrying families:
I said to them . . . ‘we do, now and again, see children who’ve had . . . an operation to have their appendix out, coming back with bowel obstruction’ . . . And I don’t say it in such frank, scary terms, but I say, you know, ‘if you have an operation, you might come back at some point in the next year or 2 with a complication from the surgery’.
Int_Surgeon10
Surgeons talked of the importance of a balanced approach when describing surgical risks to families to maintain families’ trust:
It’s a balanced thing to say . . . ‘there are risks, including risk of us operating on the child’, which I suppose, to some degree, sounds like we’re making ourselves sound bad . . . And then you might worry if you’re making loose of anything you do, ‘oh we can do an operation, but maybe it won’t go so well’.
Int_Surgeon37
The same surgeon suggested that they would discuss surgical risks in greater detail, but only with parents who wanted to discuss them:
If in the parents who want to talk about it at length, which I’ve had a few of, then I would explain that to them.
Int_Surgeon37
As we also noted earlier, health professionals were less likely to explore treatment preferences with families with a preference for non-operative treatment than with families with a preference for surgery. One health professional suggested that they would explore a family’s preference for surgery, but if the family had a preference for non-operative treatment, exploring their preference could dissuade them from participating.
It’s difficult when you’re just trying to get people into the study . . . if you had . . . a family who was really keen for this patient just to have surgery and then you can explore their background and why they have such a strong opinion for one versus the other and try and unpick that a little bit more . . . If the situation arose again and there was some situation where they were . . . very pro . . . the non-operative arm, then that would have been an opportunity to, to go through that. But at that point, you know, it’s a success, it’s a tick in the success column, we just take it and run.
Int_Surgeon57
Dealing with conflicting family treatment preferences
In a context of parents and children within families having conflicting treatment preferences, some surgeons spoke of randomisation to CONTRACT as offering a way of resolving the conflict:
Sometimes you’ve got one parent who’s keen on antibiotics and the other one who’s keen on surgery. It might not just be the child. I use that as fuel to try and recruit them into the study because I was saying . . . ‘there’s a disagreement here within the family, let’s take it out of your hands as a family and, let the computer decide’, sort of thing.
Int_Surgeon10
Furthermore, as noted in Exploring and balancing families’ treatment preferences, health professionals suggested that discussions with family members to explore and balance treatment preferences helped to alleviate their reservations about participating in CONTRACT:
The kid didn’t want surgery . . . mum did want it, and I carefully balanced their expectations, and then they were all happy to go into it. And by the time the nurse came down then and recruited them properly, and it was all fine.
Int_Surgeon55
Issues with conducting blinded discharge assessments
Once a decision to discharge a CONTRACT participant had been made, a member of the clinical team who had not been directly involved in the child’s treatment and care completed a discharge assessment, in which they had to ‘guess’ which treatment the child had been allocated to (see Chapter 2); these data were subsequently captured. One nurse we interviewed was involved in these assessments and explained that, in working on the same ward as CONTRACT participants, it was often possible to deduce whether a child had been treated with non-operative treatment or surgery:
[Nurse] who said ‘oh can you come and do this patient’s discharge thing for the study?’. And I said, ‘oh that’s a bit difficult really, ’cause I’ve been clinical all week; therefore I’ve seen everybody who’s been in on a morphine infusion’. So if I’ve seen them, then I know that they’re on morphine infusion; therefore, they’ve had their appendix out.
Int_Nurse4
She advised that staff from a different clinical team in the hospital should conduct these assessments so that they are truly blinded to treatment arm:
[W]hether it needs to be someone completely, from a completely different team, I don’t know, diabetes nurse specialist or something that’s got nothing to do with anybody having any surgery.
Int_Nurse4
The CONTRACT protocol (see Chapter 2) advises that, if the assessor becomes unblinded during the assessment, this should be recorded. The same nurse commented that the rationale for this component of the study had not been explained to her, but she would have liked to have known why it was done:
I don’t know what . . . this sounds awful, but I don’t know what the point of it is . . . I suppose [I would have liked to have known] why they need me to do this part.
Int_Nurse4
Research nurse support
As anticipated, some health professionals suggested that it was more challenging to approach and recruit families to CONTRACT outside normal hours, including weekends, evenings and nights. This resulted in some eligible families not being approached about CONTRACT, although surgeons suggested that this was rare:
There’ve only been one or two situations where we’ve had patients who would’ve been just right for the study who weren’t approached . . . [that] tends to be the situation when it’s after hours. Registrars are busy with lots of patients to see, and it doesn’t come to . . . the forefront of their minds, CONTRACT.
Int_Surgeon57
Surgeons explained that having research nurses available to support them during normal working hours was highly beneficial:
It will take more time [approaching families about CONTRACT] . . . which is why sometimes it really depends, like if it’s during the daytime, then having the research nurses is great.
Int_Surgeon11
Research nurses also explained that, outside normal hours, occasionally, aspects of CONTRACT were missed:
Find that we have, it has been missed giving them the [CONTRACT] information sometimes . . . [the child has] gone home at the weekend and the nurses or people on the ward haven’t given them or it’s gone missing.
Int_Nurse2
Discussing ‘feasibility’ when approaching families about urgent care feasibility trials
Although CONTRACT was a feasibility trial, and this was explained in the PIS, in only three consultations did health professionals overtly describe CONTRACT as a feasibility trial:
This is the first part or the feasibility part of the, the UK trial, it’s three centres involved . . . we’re looking at seeing if, in local UK conditions, are we able to get enough patients on board to have a decent volume of patients.
Cons_Surgeon57_Family24_App
More typically, health professionals described CONTRACT’s aim as being to compare the efficacy of two treatments, namely surgery and non-operative treatment:
The aim of the study, is really to see which one is superior, because at the moment we have both feasibly there, but we want to really sort of get hard evidence to . . . be able to see which one is the best in comparison.
Cons_Surgeon39_Family50_Declined
When interviewed, health professionals typically reported that it was unnecessary to discuss feasibility:
I didn’t think I had to.
Int_Surgeon18
Others suggested that families would struggle to understand what a feasibility study is among the other concepts discussed during the consultation:
That’s quite a hard concept for people to understand. I think that, that would be even less attractive for a parent to understand. Um, ’cause I think it is a lot to take in, in there.
Int_Surgeon30
The acute setting could also exacerbate this issue:
At that stage, when they’re in the acute situation, it would be very difficult to grasp to them what that, what that actually, truly means.
Int_Surgeon12
Furthermore, some health professionals suggested that the term ‘feasibility’ could imply to families that the study was rudimentary and, therefore, it could make it challenging to gain their confidence in the study:
It makes it sound a little bit even more uncertain about the study, rather than, you know and about equipoise and that discussion.
Int_Surgeon30
[If we tell parents it’s a feasibility study] they might feel that their children are just guinea pigs and that we’re just messing about and playing with new kinds of treatment . . . it you tell them that it’s only a feasibility study, everyone will just say no.
Int_Surgeon11
The preceding quotations show that some health professionals worried that describing the study overtly as a feasibility study could deter some families from participating.
We asked health professionals if feasibility studies, like CONTRACT, are viewed as less valuable than larger trials, and whether or not this would influence how invested health professionals are in a trial. Some felt that the trial would be valued the same whether it is a feasibility or larger trial:
The feeling in [our site] about the study . . . the way that [site principal investigator] has really led on things . . . it’s made it feel like a, a privilege that we’re one of the involved centres. I think everyone see it as a really nice thing.
Int_Surgeon17
Others suggested that it could be valued less than a larger trial because:
[A] larger trial’s . . . got, erm, greater power.
Int_Surgeon18
Quantitative analysis of the impact of recruitment training on recruitment and surgeon confidence in approaching families about CONTRACT
In addition to assessing the impact of training qualitatively (by how CONTRACT was discussed with families in recruitment conversations), we have examined the effect of retraining quantitatively. Across the three phases of CONTRACT, recruitment rates rose from 38% at the end of phase 1 of recruitment to 50% in phase 2 and to 62% in phase 3, a modest rise throughout the duration of the study. Table 6 provides a breakdown of recruitment rates by phase and site; recruitment rate by month is shown in Figure 13.
| Phase | Site 1 | Site 2 | Site 3 | Overall, by phase | ||||
|---|---|---|---|---|---|---|---|---|
| Approached (n) | Randomised, n (%) | Approached (n) | Randomised, n (%) | Approached (n) | Randomised, n (%) | Approached (n) | Randomised, n (%) | |
| 1 | 13 | 4 (31) | 14 | 8 (57) | 10 | 2 (20) | 37 | 14 (38) |
| 2 | 9 | 6 (67) | 24 | 9 (38) | 11 | 7 (64) | 44 | 22 (50) |
| 3 | 15 | 11 (73) | 14 | 8 (57) | 5 | 2 (40) | 34 | 21 (62) |
FIGURE 13.
Recruitment to CONTRACT, by communication substudy phases.
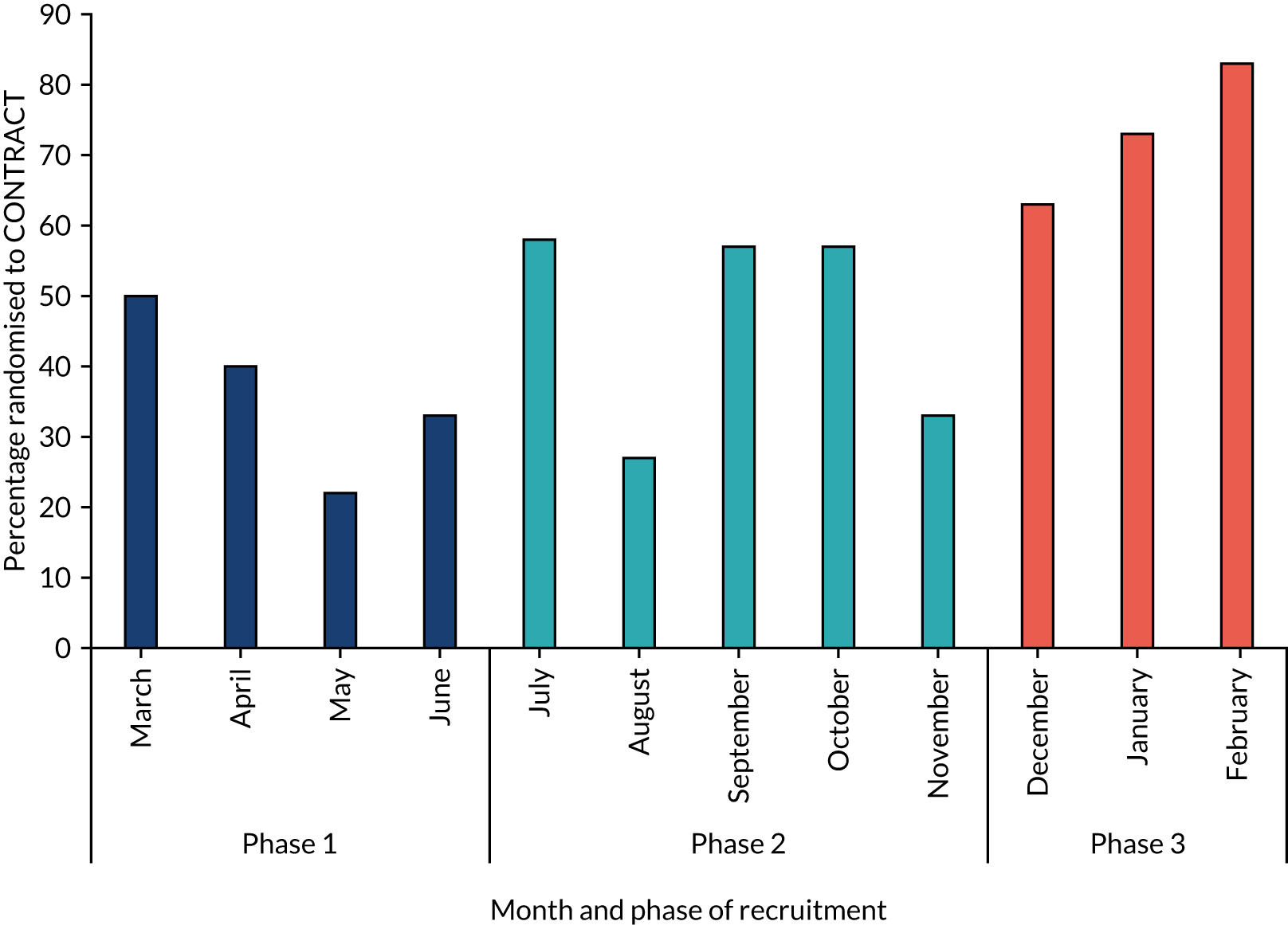
We used a chi-squared test for trend to investigate the relationship between recruitment rate and phase of training, with the assumption that each training episode (between phases 1 and 2, and then between phases 2 and 3) was identical. The increase in recruitment rate from phase 1 to phase 3 was statistically significant [χ2(1) = 4.06; p = 0.04].
Although we cannot solely attribute this rise in recruitment rates to a causal effect of the training, the change in how CONTRACT was discussed with families across the phases supports a beneficial effect of retraining. Furthermore, through evaluations of the training episodes provided by attendees, a trend of increased confidence in discussing various aspects of CONTRACT with increasing retraining is shown (see Appendix 2, Table 17).
Discussion
The CONTRACT communication study is one of a relatively small number of qualitative substudies to have been embedded within a feasibility trial with the aim of optimising recruitment and informing a future definitive trial. 55–58 Uniquely, our study drew on trial recruitment consultation audio-recordings, interviews with health professionals, children (patients) and parents. Although studies of this sort have been advocated,38 embedded qualitative studies rarely include the perspectives of both children and their parents. 56 This multiperspective approach offers a comprehensive approach to identifying barriers to and facilitators of recruitment, to enhancing families’ experience of the process and to informing future trial design. 59
In this section, we examine the effect of recruitment training (informed by the communication study findings) on health professionals’ CONTRACT consultations with families and discuss the strengths and weaknesses of the communication study. We offer recommendations (see Appendix 2) to inform a future definitive trial to compare non-operative treatment with appendicectomy, and discuss the broader implications of the findings for the design and conduct of surgical versus non-surgical trials, paediatric trials and research conducted in an acute setting.
Impact of communication training on CONTRACT recruitment
Informed by previous qualitative embedded studies, we identified key areas of non-optimal communication that can impede recruitment success, including use of imbalanced terminology42,54 and a lack of treatment preference exploration. 43,44 Following bespoke recruitment training, the use of imbalanced or potentially misinterpreted terminology decreased, and some health professionals began to elicit and balance families’ treatment preferences. However, health professionals did not alter all aspects of non-optimal communication: many continued to omit surgical risks because of concerns about unduly worrying families, and they rarely challenged families’ preferences for non-operative treatment owing to worries about dissuading families from participating in CONTRACT.
Similar to previous research,46,60 the current findings suggest that, although most health professionals saw the value in the research question CONTRACT was aiming to address, many had a preference for surgery. Health professionals spoke about their personal biases about treatments when interviewed, but these biases were also apparent in their communication with families when they used terms that were loaded in favour of one of the treatments, usually surgery. We identified frequently asked questions from parents and children; in the training, we highlighted and discussed these, which seemed to help health professionals feel more confident in addressing families’ questions. Treatment preference exploration has previously been found to optimise informed consent and recruitment,43,44 but some health professionals were reluctant to gently challenge or balance families’ treatment preferences owing to concerns that it might be coercive. It is currently unclear how parents and children experience treatment preference exploration and whether or not they ‘feel’ coerced by such approaches. Previous research has found that some health professionals can find approaching families about trials to be aversive, yet families were, at worst, neutral about being approached, and some were highly positive about it. 61 Further research with parents and children would help to establish whether their views about treatment preference exploration are similar to, or diverge from, those of health professionals.
We explored the effect of recruitment training on trial communication and recruitment both qualitatively and quantitatively, throughout the course of CONTRACT. The recruitment rate gradually increased throughout the recruitment phases, but it increased more markedly after the second session of tailored training. Future work is needed to robustly evaluate the impact of such recruitment training on trial recruitment and to examine sources of variation in response to training, such as site, staffing and the effect of being audio-recorded, as this was beyond the scope of the current study. Further evaluation might include a RCT of the training intervention. Our qualitative analysis indicated changes in health professionals’ communication that may explain the increases in recruitment rates that we observed. For example, many stopped using terms that could be misinterpreted. We know from health communication literature that tailored messages can be more persuasive and have a greater impact on behavioural outcomes;62 perhaps this is why we saw a cumulative, desirable effect on communication following the tailored recruitment training (sessions 2 and 3). Frequent, tailored recruitment training could have a more powerful effect on health professional communication and trial recruitment than the initial, generic training alone.
The statistical analyses of the relationship between recruitment training and recruitment rates from phase 1 to phase 3 using bivariate analyses indicated that the training was associated with an increase in the recruitment rate. However, a nested RCT of recruitment training would be needed to infer causality. Nevertheless, qualitatively, we identified changes in communication behaviour across all three sites and all three recruitment phases. We could not determine the impact of training on the communication of individual health professionals as we did not have a formal record of training attendees, nor were we able to identify recruiters from audio-recordings.
Strengths and limitations
We obtained audio-recorded CONTRACT consultations, interviewed health professionals involved in various aspects of CONTRACT and interviewed both children and parents. We recruited families and health professionals from all three CONTRACT sites. The captured audio-recorded CONTRACT consultations and family interviews were from a diverse range of families, including those who declined to participate in CONTRACT as well as those who consented to participate. Interviewed families also included those who were randomised to non-operative treatment and those randomised to surgery, and we explored the experiences of families of children reporting AEs in each of the treatment arms. In total, 115 families were approached about CONTRACT and we obtained qualitative data (consultation recordings, interviews or both) from 63 families. However, it is unclear how many families were approached about the communication study, as this information was not recorded on a screening log at CONTRACT sites. It is possible that the consultations and views of families who did not take part in the communication study differed from those who did take part. We note that families that agreed to participate in CONTRACT were more likely to also participate in the communication study than those that declined. Nevertheless, the consultations that we accessed showed a range of approaches to communicating about CONTRACT, and families that we interviewed had a range of views of CONTRACT, including critical views about aspects of their experiences.
Conclusion
This qualitative study, embedded in the CONTRACT feasibility trial, demonstrated how delivering bespoke recruitment training to health professionals during the course of the trial resulted in improvements in trial communication and was associated with increased overall recruitment rates. The results allowed us to generate a comprehensive list of recommendations (see Appendix 2) that should be considered in developing and conducting a future definitive trial to compare non-operative treatment with appendicectomy in children and young people with uncomplicated acute appendicitis. The findings can also be used to optimise recruitment to trials comparing surgical with non-surgical treatments and to paediatric trials in acute settings.
Chapter 5 Core outcome set development
This chapter discusses the development of a core outcome set to determine the overall treatment success of acute uncomplicated appendicitis in children.
Introduction
Background
A lack of knowledge and understanding regarding which outcomes are important to patients and clinicians may result in important outcomes being omitted from clinical trials. Differences in outcome selection and reporting between studies and how outcomes are defined and measured also make it difficult, sometimes impossible, to synthesise the results of studies (e.g. in meta-analysis) and apply them in a meaningful way. To address these problems, COSs have been proposed as a means of standardising outcome selection, measurement and reporting in health-care research, and in clinical trials in particular. 63,64 The development of a COS and its adoption by researchers is intended to help avoid inconsistencies in outcome selection, measurement and reporting that may otherwise exist. If an established COS is not adopted for a trial, there is a risk that suboptimal outcomes will be selected, and it is unlikely that such a trial will produce usable information. 65 Reporting outcomes in a consistent way between studies also facilitates data synthesis when combining resulting from more than one trial.
Prior to performing further efficacy studies of the treatment of appendicitis in children, it is imperative to identify the most relevant outcomes for inclusion in the design of comparative studies. This is of particular importance when evaluating a novel treatment approach, as the outcomes of importance may differ from those commonly reported with standard-of-care therapies. In particular, the pathway, complications and outcomes experienced by children and young people with acute uncomplicated appendicitis are potentially very different between those receiving non-operative treatment and those undergoing appendicectomy; therefore, an understanding of the outcomes that are important to different stakeholder groups is important.
A review of the relevant literature and electronic resources failed to identify a COS for children with appendicitis. 66 Furthermore, a wide range of outcomes were reported and a range of different primary outcomes were used across studies. 66 To advance our understanding of which outcomes are important and to fulfil an unmet need in our future research programme, we aimed to develop a COS for the measurement of effectiveness of treatment interventions in children and young people (aged < 18 years) with acute uncomplicated appendicitis.
Objectives
-
To determine which outcomes have previously been reported in studies comparing treatments for acute uncomplicated appendicitis in children.
-
To qualitatively explore outcomes of value to patients and parents of children who have had acute uncomplicated appendicitis, to inform the initial list of core outcomes.
-
To prioritise the treatment outcomes of children with acute uncomplicated appendicitis from key stakeholder groups’ perspectives [including paediatric surgeons, general surgeons, patients (aged 12–18 years) and parents of children who have had acute uncomplicated appendicitis].
-
To compare and contrast which outcomes of paediatric acute uncomplicated appendicitis treatment are prioritised by key stakeholder groups (detailed above).
-
To achieve consensus between key stakeholder groups on a COS to evaluate the overall success of treatment for acute uncomplicated appendicitis in children.
Scope of the core outcome set
The COS is intended to be used to evaluate the overall success of any treatment intervention in children who are assigned a clinical and/or radiological diagnosis of acute uncomplicated appendicitis. The finalised COS includes outcome measures identified as important within 12 months of treatment initiation and longer-term outcomes, if applicable. The COS focuses specifically on treatment of acute uncomplicated appendicitis (i.e. thought to be uncomplicated at the time of treatment initiation); the treatment of appendicitis thought to be perforated (with or without abscess) and appendix mass is outside the scope of this COS.
Design overview
The development of the COS entailed three key stages:
-
developing an initial list of outcomes
-
a three-phase online Delphi consensus process
-
a consensus meeting.
Protocol/registry entry
Development of the COS was in accordance with a published protocol. 67 The COS development study was registered with the Core Outcome Measures in Effectiveness Trials (COMET) Initiative in May 2017. 68
Ethics and consent
The COS study received ethics approval as part of the CONTRACT study. Two amendments relevant to the COS study were submitted and approved: the first for approval of the finalised COS study documentation (approved 18 August 2017) and the second to offer parents and patients £75 for participation in the consensus meeting (approved 30 August 2018). Potential participants were provided with PISs and informed consent was implied by completion of the online Delphi phases.
Study-specific advisory group
To inform and support the CONTRACT study, a PPI group specific to the CONTRACT study and called the SSAG was assembled, comprising 15 young people and parents (see Chapter 8). Three parents and two children and young people from the SSAG provided additional support to the COS study team and to inform study materials and processes. The SSAG informed the development of patient and parent information leaflets and invitation letters, addition and wording of outcomes and the development of the study video, and they helped to ensure that study materials were appropriately presented for parent and patient stakeholder panels. Some members (two parents and two young people) also attended the parent and patient consensus meeting to help facilitate discussion.
Developing an initial list of outcomes
An initial list of outcomes was developed from two main sources: first, from a systematic review of the existing literature to identify previously reported outcomes in trials of children with acute uncomplicated appendicitis, and, second, through interviews with children and parents as part of the CONTRACT communication study (a qualitative study, described in full in Chapter 4). From these interviews, we aimed to establish outcomes of importance that were not already identified in the systematic review. Outcomes identified from these two sources were combined to form an initial list of outcomes.
Systematic review
The COMET Initiative recommends the use of systematic reviews in informing the first phase of the Delphi process. 69 Two recent systematic reviews,16,66 both led by a member of the study team, were used to inform the initial list of potential outcomes to be considered for the COS. The first review identified 115 outcomes reported in RCTs and meta-analyses of appendicitis treatments administered to children. 66 Of these 115 outcomes, 106 were combined on the basis of their similarity, to give 37 outcomes, which were mapped to Outcome Measures in Rheumatology (OMERACT) domains (Figure 14).
FIGURE 14.
List of outcomes identified from a previous review of the literature, assigned to core areas. PROM, patient-reported outcome measure; WCC, white cell count. Reproduced from Hall et al. 66 © The Authors, 2015. This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (https://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
The second review aimed to determine the safety and efficacy of non-operative treatment for acute appendicitis, and identified 10 articles reporting on 413 children who had received non-operative treatment. 16 We updated this review, using the same search strategy, to include further articles published between 2016 and April 2017. This additional search revealed 28 articles, but only six of these that met the inclusion/exclusion criteria were not already in the review. 16 Appendix 3, Table 18, shows the characteristics of the 10 articles identified from the initial review of non-operative treatment16 and of the six further articles identified from the updated review.
We extracted outcomes from these papers as described in the previous review of outcomes. 66 In brief, two researchers (SE and FCS) extracted outcomes from the articles; disagreements were resolved by a third reviewer (NJH). Outcomes were mapped onto the condensed list of 37 outcomes identified in the previous review. 66 We also sought to list potentially novel outcomes that could not be mapped to the previously defined list; however, no completely new outcomes were identified. The outcomes that were identified from these 16 additional papers, and how they were mapped to those previously described, are shown in Appendix 3, Table 19.
Qualitative interviews
Full methods and results of the CONTRACT communication study are detailed in Chapter 4. Although the main aims of the communication study were to optimise recruitment and to inform a future trial, we also asked patients and parents about their views and experiences of acute appendicitis to ascertain the outcomes that were of particular value to them. We drew on the preliminary qualitative results to inform the initial list of outcomes for the Delphi process. Key outcomes identified from these interviews included pain, loss of appetite, re-admission to hospital/GP visits following treatment, wound healing, child’s psychological well-being, time away from school or physical activities, and fever. Raw data underlying the way in which these outcomes were identified from interview transcripts are shown in Appendix 3.
These outcomes identified in interviews were mapped to the list generated from the systematic reviews (see Appendix 3, Table 20). All outcomes identified could be mapped to outcomes already listed, meaning that no new outcomes were defined.
Finalising the initial list of outcomes
After generating an initial list of outcomes from the systematic reviews and interviews, the outcomes list was refined with the SSAG. The descriptors for each outcome were discussed with the SSAG to improve their clarity and comprehensibility to young people and parents. Other outcomes suggested by the SSAG were also considered. From all these sources, an initial list of 40 outcomes was generated, along with descriptions (see Appendix 3, Table 21).
Delphi consensus process and consensus meeting
Methods
Participant identification
For the COS to be meaningful and relevant to those involved in the treatment of acute appendicitis, the COS needed to reflect the views of patients who have been treated for acute appendicitis, patients’ parents and surgeons. As these groups may have different priorities that could obstruct reaching consensus, the stakeholders were separated into three panels, which we intended to be equally weighted: (1) patients, (2) parents and (3) paediatric surgeons and general surgeons. Potential members of each stakeholder group were selected and approached (see Appendix 3, Table 22). Patients and parents were identified from departmental databases at a total of seven specialist children’s hospitals in the UK, including the three CONTRACT feasibility RCT sites.
Initially, we discussed whether or not participants from outside the UK should be invited to participate. This would have had the potential advantage that patients from other countries had already been treated non-operatively, whereas at the time of the design of the COS, very few UK patients had been treated non-operatively, as CONTRACT recruitment had not yet started. However, there were several reasons against recruitment outside the UK: the treatment pathway for appendicitis in the UK differs to that in other countries, and organising recruitment of patients, parents and surgeons from outside the UK would have presented major logistic difficulties, not least for organisation of the consensus meeting. Thus, the COS study team decided that participants should be recruited from the UK only, but that COS recruitment should be delayed until a larger number of patients who had been treated non-operatively (as a result of participating in CONTRACT) were available to participate in the COS development and provide input via the communication study, as described above.
Participant registration
Potential participants were sent a letter/e-mail describing the study and why they had been identified as a potential participant. As NHS sites do not routinely collect patient e-mail addresses, patients and parents were invited by mail and surgeons via e-mail. The invitation included a one-page information sheet in plain language, with content tailored to each of the three stakeholder panels, describing the study aims and procedures and emphasising the importance of commitment to all three phases of the Delphi process. The invitations contained a link to a website, where they could read more about the study, view a video about COS development (available at https://tinyurl.com/contracthta), register their interest in participating and provide further information on their experience of the treatment of acute appendicitis. The importance of committing to three rounds of questionnaires was again emphasised.
The process of invitation and registration continued for 10 weeks, until the desired number of participants had registered (with at least 10 in each panel). There is no consensus on the optimal sample size for a Delphi process; therefore recruitment was based on previous Delphi studies. 70 We aimed to achieve 75–100 participants in the first round of the Delphi, with at least as many parents and patients as surgeons. We aimed to invite a diverse range of participants to each stakeholder panel.
Delphi consensus process
An online three-phase Delphi process was conducted across the three stakeholder panels in parallel. Participants were presented with the initial list of 40 outcomes, grouped into themes. We also provided participants with an opportunity to propose other outcomes at the end of Delphi process phase 1.
Outcome scoring and consensus definition
In all three phases of the Delphi consensus process, participants were asked to score each outcome using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) scale. 71 The scale was presented in the format 1–9, with 1–3 labelled ‘not important’, 4–6 labelled ‘important but not critical’ and 7–9 labelled ‘critical’.
When scoring each outcome, surgeon participants were asked the key question ‘How important do you consider the following outcomes to be when considering which treatment to offer children with uncomplicated acute appendicitis?’. A similar question was posed to parents and patients, with the wording altered as informed by our SSAG. Figure 15 displays a screenshot of the Delphi process phase 1 survey and shows how the outcomes were presented to participants.
FIGURE 15.
A screenshot of the Delphi process phase 1 survey.
‘Consensus in’ was defined as ≥ 70% of participants rating the outcome as 7–9, and < 15% rating it as 1–3. Outcomes were defined as ‘consensus out’ if ≥ 70% of participants rated it 1–3 and < 15% rated it 7–9. Outcomes not meeting these definitions were classified as ‘no consensus’.
Delphi process: data collection
Following registration, participants were sent a personalised link to access and complete the first phase of the Delphi process. Software for a COS Delphi process hosted on a secure server, developed by the National Perinatal Epidemiology Unit (University of Oxford, UK) and used successfully to develop two previous paediatric surgical COSs,72,73 was used to administer all three phases. Procedures were implemented throughout the Delphi process to attempt to limit attrition,70,74 such as sending reminder e-mails and newsletter summaries of the study progress. We aimed to send the link to the first phase to all participants on the same day that they registered,75 but this was not feasible owing to an administrative problem. The link to the first phase was sent to all participants concurrently.
Participants were asked to complete each phase of the Delphi process within 3 weeks, and two reminder e-mails were sent to non-responders during that time. Participants who did not complete the questionnaire within 4 weeks of being requested to do so were deemed not to have completed that phase.
Delphi process: phase 1 data analysis
When possible, we recorded the number of participants who were invited to register and, of those who registered, the number from each stakeholder panel who completed phase 1. The scores for each outcome were analysed for each stakeholder panel, and descriptive statistics were generated. All outcomes were carried forward to phase 2. Two members of the COS study team reviewed additional outcomes that participants had proposed at the end of phase 1 to consider if they represented new outcomes. Those that were deemed to be new were included in phase 2 if they were proposed by at least two participants. Participants were sent a study ‘newsletter’ prior to phase 2, which provided feedback from the previous phase and instructions on how to complete the forthcoming phase. Separate newsletters were tailored to the different stakeholder panels and e-mailed to participants prior to phase 2 [see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/1419290/#/ (accessed February 2020)].
Delphi process: phase 2
All participants who completed phase 1 were invited to participate in phase 2. Participants were individually presented with their own score for each outcome from phase 1, alongside the distribution of scores for each outcome from their stakeholder panel in phase 1. They were asked to rescore each outcome, taking into account the views of the other participants in their stakeholder panel. Participants were asked to score any new outcomes identified in phase 1. Figure 16 displays a screenshot of the phase 2 survey and shows how the scores from phase 1 were presented to participants.
FIGURE 16.
A screenshot of the Delphi process phase 2 survey. The gold circle around the radio button indicates the score given by the participant in phase 1. The blue bars display the distribution of scores for the given stakeholder panel, and the blue dot represents the panel’s mean score.
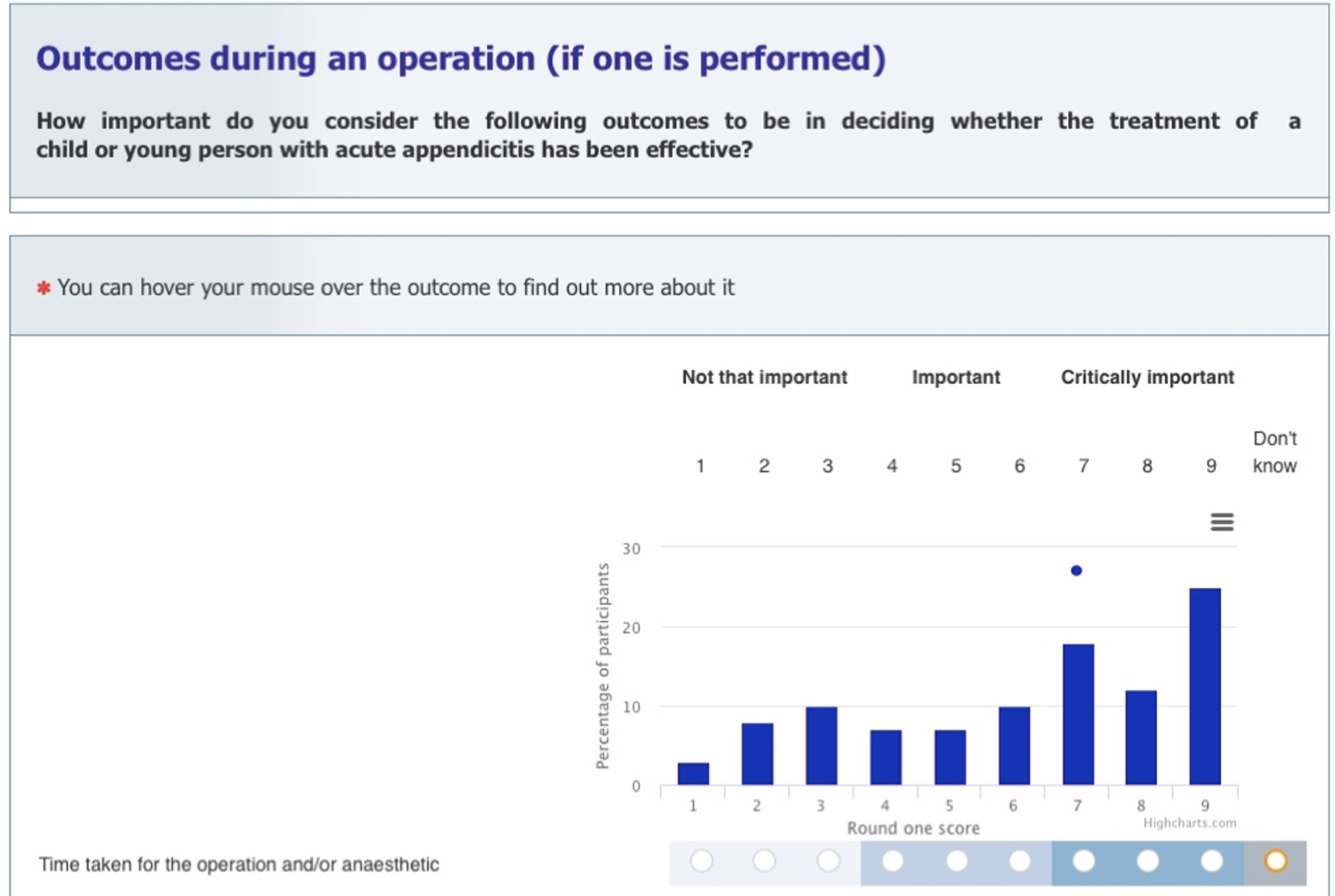
Delphi process: phase 2 data analysis
The data analysis process described for phase 1 (see Delphi process: phase 1 data analysis) was repeated. Any outcomes that met the criteria of ‘consensus out’ were removed from the outcomes list prior to phase 3. All other outcomes were carried forward to phase 3. Again, participants were sent a study ‘newsletter’ prior to phase 3, which provided feedback from the previous phase and instructions on how to complete the forthcoming phase. As with phase 1, newsletters were tailored to the different stakeholder panels and e-mailed to participants.
Delphi process: phase 3
Participants who completed phases 1 and 2 were invited to participate in phase 3. Owing to attrition among patients and a desire to ensure that the final COS represented the views of children and young people as much as possible, all patients who completed phase 1 were invited to complete phase 3 regardless of whether they had completed phase 2. The data collection process described for phase 2 (see Delphi process: phase 2) was repeated; however, as well as being shown scores for their own stakeholder panel, participants were shown scores for every other stakeholder panel separately. This allowed participants to consider other stakeholder panels’ views before rescoring the outcomes. 76 Figure 17 displays a screenshot of the phase 3 survey, showing how outcomes were presented to participants. We provided participants with instructions on how to complete the survey, highlighting that, on each outcome chart, the bars represented the distribution of scores among surgeons (blue bars), patients (green bars) and parents (orange bars). We also explained that mean scores were shown for surgeons (blue dot), patients (green diamond) and parents (orange square).
FIGURE 17.
A screenshot of the Delphi process phase 3 survey.
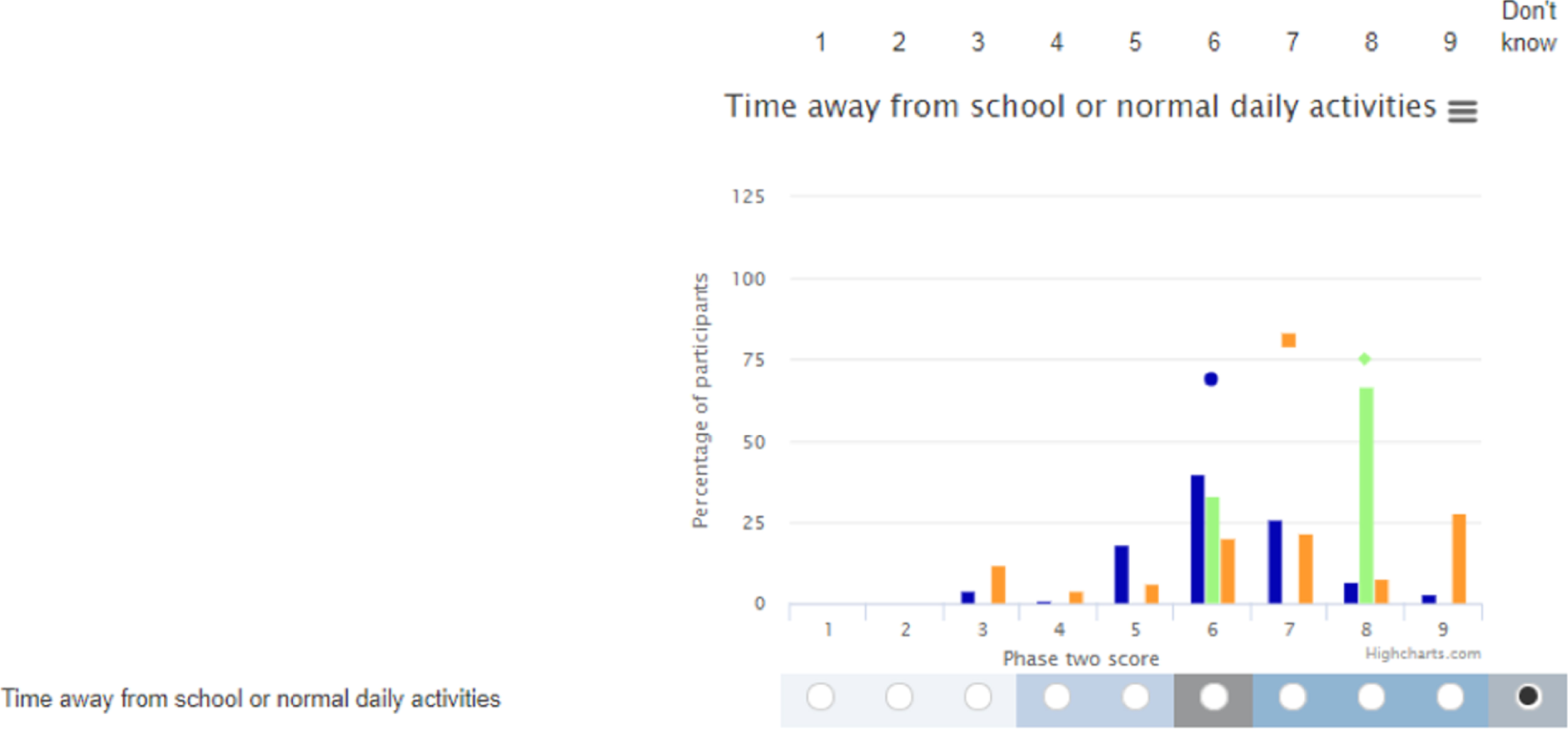
Delphi process: phase 3 data analysis
The data analysis process described for phase 2 (see Delphi process: phase 2 data analysis) was repeated. All outcomes from phase 3 were carried forward to the consensus meeting.
Attrition analysis
To address the potential for bias due to between-phase attrition, we compared (1) phase 1 outcome scores of participants who completed phases 1 and 2 with the scores of those who completed phase 1 only and (2) phase 1 outcome scores of participants who completed all three phases with the scores of those who completed phase 1 only. This was done using Mann–Whitney U-tests for each individual outcome. 72 The effect of attrition was also analysed across all outcomes using a multilevel modelling approach (level 1: outcome, level 2: participant and level 3: stakeholder panel) using MLwiN version 3.01 (Centre for Multilevel Modelling, University of Bristol, Bristol, UK).
Face-to-face consensus meetings
The aim of the consensus meeting was to ratify outcomes for which consensus (‘in’ or ‘out’) had been achieved, to discuss outcomes for which consensus could not be achieved and to finalise the COS. We invited all participants who completed all three rounds of the Delphi process to the consensus meeting and, owing to attrition during the Delphi phases, all children and young people who registered, to ensure that their views were represented. We aimed to have a minimum of 40 stakeholders confirm their attendance, with equally weighted participation across the three panels. Representatives from each stakeholder panel were required for the consensus meeting to be quorate.
Initial meeting and consultation process
At the end of the third phase of the Delphi process, Nigel J Hall e-mailed participants [parents (n = 32) and surgeons (n = 55) who had completed all three phases, and all patients who had registered (n = 15)] to invited them to participate in a consensus meeting on Saturday 30 June 2018 at a conference centre in Birmingham, UK. Tailored e-mails were sent to each of the three stakeholder groups and a reminder e-mail was sent to participants who had not responded after 1 week. Far fewer stakeholders confirmed their attendance than had previously registered their interest in attending [n = 4 (three surgeons and one parent)]. Therefore, the consensus meeting was postponed.
As a result, we consulted, via e-mail, with COS participants to identify barriers to and facilitators of attendance. Twelve parents and 25 surgeons responded, providing their views on opportunities to encourage participants to attend a future consensus meeting. Typically, surgeons suggested that they would prefer that a meeting takes place Monday–Friday, in London. Parents tended to suggest holding the meeting on a weekend, during term time, and in the afternoon. As expected, parents preferred the venue to be local to them and some felt that they would not be able to attend a central England venue without accommodation. Most parents agreed that families should receive some financial incentive for attending the meeting and that the offer of lunch, refreshments and childcare would also encourage some families to attend. We also consulted the SSAG regarding other ideas to improve parent and patient attendance at the forthcoming consensus meeting, but no further ideas were identified.
Final consensus meetings
As the consultation process indicated that surgeons’ and parents’ preferences for a consensus meeting differed, we arranged two separate consensus meetings: one for surgeons and one for parents and patients. Although this deviated from the study protocol, several other COS studies have held separate meetings for patients and health professionals to ensure that meetings are not dominated by health professionals’ views. 77–79
Invitations and registration
Surgeons (n = 55) who completed all three phases of the Delphi process were invited by e-mail to attend the surgeons’ consensus meeting on a weekday in London. Parents who completed all phases of the Delphi process (n = 32) and all patients who had registered for the COS development (n = 15) were invited by e-mail to attend the patients’ and parents’ (or families’) consensus meeting on a Saturday in Birmingham. E-mails were tailored to each stakeholder group and emphasised how we had incorporated feedback from the consultation process in devising the consensus meeting plans. Families were offered accommodation for the Friday or Saturday night, had travel arranged or travel expenses reimbursed and had childcare, lunch and refreshments provided at the venue, and each participant was offered a £75 voucher (or charity donation) for participating in the meeting, informed by guidance on PPI in research80 and approved by a second ethics amendment.
Pre-meeting information
All participants who confirmed their attendance were sent details of the event and a consensus meeting booklet [see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/1419290/#/ (accessed February 2020)]. The booklet described the aims of the project; the work completed so far; the plans and aims of the meeting; and an overview of the phase 3 results, including the consensus status of each outcome.
Surgeons’ meeting
The surgeons’ meeting was chaired by Dr Jamie Kirkham, a Senior Lecturer from the University of Liverpool with experience and knowledge of COS development. Participants were asked not to sit next to someone they knew in the meeting room. Nigel J Hall detailed participation rates across the Delphi phases, the rationale for a COS, the study video, the GRADE scoring scale and a description of how the outcomes would be grouped for discussion during the consensus meeting.
We had initially proposed that, following moderated discussion, each outcome would be anonymously rescored using the same scoring system as used in the Delphi process. Furthermore, we proposed that, for outcomes for which ‘no consensus’ was achieved across all stakeholder panels at the end of the Delphi process, and for which consensus was achieved in at least one but not all stakeholder groups, further discussion would take place, following which attendees would be asked to score each outcome anonymously.
Owing to attrition (particularly among young people, such that three young people started scoring phase 3 and only two completed it) and potential response bias, we prioritised outcomes to discuss and score in the meetings. For outcomes for which ≥ 70% of participants across all stakeholder panels rated the outcome 7–9, brief discussion took place. Following discussion, the chairperson asked participants whether or not they felt that any of those outcomes should be excluded from the final COS, and those outcomes were rescored only if participants voiced a preference to revote. For outcomes for which < 50% of participants across all stakeholder panels rated the outcome 7–9 at the end of the third Delphi phase, again, brief discussion took place. Following discussion, the chairperson asked participants whether or not they felt that any of those outcomes should be included in the final COS, and outcomes were rescored only if participants voiced a preference to revote. All other outcomes were presented, discussed and rescored, unless participants felt strongly that, following discussion, rescoring was unnecessary. Following discussion and rescoring, outcomes reaching ‘consensus in’ were included in the finalised COS. All other outcomes were excluded.
Voting was conducted using electronic voting devices, enabling participants to score outcomes anonymously from 1 to 9. Once all outcomes had been discussed and voted on (if applicable), the results were compiled and a list of outcomes classified as ‘consensus in’ was presented to participants before the meeting closed.
Families’ meeting
Unfortunately, Dr Jamie Kirkham was unavailable to also chair the families’ meeting; therefore, Nigel J Hall chaired the meeting and was supported by Erin Walker, who was the CONTRACT PPI Lead. The methods for the families’ meeting were similar to those of the surgeons’ meeting. Two parents and two young people from the SSAG who consulted with the COS study team at additional time points during the COS study attended the meeting to contribute to and facilitate discussion among the families, but did not vote.
Results
Delphi phases’ results
Invitations and registration
Between October and December 2017, 818 parents and patients, from seven NHS sites in England, were invited to participate in the study (see Appendix 3, Table 23). It was not possible to precisely measure the number of paediatric and general surgeons who were invited to participate, as they were invited via mailing lists from several organisations, including the British Association for Paediatric Surgeons and the Association of Surgeons of Great Britain and Ireland.
Participants
Appendix 3, Table 24, provides an overview of participant characteristics for those who registered to participate and characteristics by completion. Overall, 195 participants registered (15 patients, 67 parents, 57 paediatric surgeons and 56 general surgeons). Patients who registered ranged in age from 11 to 18 years, and the age of patients whose parents registered ranged from 3 to 18 years. All surgeons were consultants
Delphi phase 1
Of those who registered, 147 participants (75%) completed phase 1 of the Delphi survey: 11 patients (73%), 57 parents (85%), 45 paediatric surgeons (79%) and 34 general surgeons (61%). Appendix 3 shows participant characteristics for phase 1.
Consensus status of outcomes
Scores from phase 1 were analysed by a stakeholder panel. Outcomes for which ‘consensus in’ was reached are shown in Appendix 3, Table 25. Consensus varied across the stakeholder panels; ‘consensus in’ was reached for seven outcomes among patients, 12 outcomes among parents, and seven outcomes among surgeons. Two outcomes reached ‘consensus in’ in all three stakeholder panels in phase 1. No outcomes were scored ‘consensus out’ in phase 1.
Proposed new outcomes
Twenty-six participants proposed additional outcomes in phase 1 (one patient, 12 parents and 13 surgeons). Three outcomes met the predefined criteria for new outcomes and were included in the second phase of the Delphi process. These were ‘psychological distress’, ‘negative appendicectomy’ and ‘time to normal diet’.
Delphi phase 2
Forty-three outcomes were scored in phase 2. Of those who completed phase 1, 122 participants (63%) completed phase 2 of the Delphi survey (see Appendix 3).
Attrition bias in phase 2
The phase 1 scores of participants who completed phases 1 and 2 were compared with the scores of those who completed phase 1 only. The median scores were similar for each outcome, and there were no significant differences in any individual scores (see Appendix 3, Table 27). Multilevel modelling showed that, on average, phase 1 scores were 6.1 ± 0.2 for those who completed phase 1 only, and increased by 0.1 ± 0.2 for those who also completed phase 2 (p = 0.61).
Consensus status of outcomes
Scores from phase 2 were analysed and compared with the definitions of ‘consensus in’ and ‘consensus out’. The number of outcomes that reached ‘consensus in’ increased markedly for all stakeholder panels. Consensus varied across the stakeholder panels; ‘consensus in’ was reached for 20 outcomes among patients, 19 among parents and nine among surgeons (see Appendix 3, Table 26). None of the three newly introduced outcomes was voted ‘consensus in’. No outcomes were scored ‘consensus out’ in phase 2.
Delphi phase 3
All 43 outcomes were carried forward to phase 3 and rescored. Owing to a high attrition rate among patients, in particular between phases 1 and 2, we decided to invite all patient participants who had completed phase 1 to participate in phase 3, with the aim of increasing the response rate in this panel in phase 3. Of those who completed phase 2, 90 participants (74%) completed phase 3 of the Delphi survey, comprising three patients (one of whom had completed phase 2), 32 parents (63%), 34 paediatrics surgeons (87%) and 21 general surgeons (72%). Appendix 3 shows participant characteristics for phase 3.
Attrition bias in phase 3
We compared the phase 1 scores of those who completed phase 3 with the phase 1 scores of those who completed phase 1 only. The median scores were similar for each outcome, and there were no significant differences in any individual outcomes scored (see Appendix 3, Table 28). Multilevel modelling showed that, on average, phase 1 scores were 6.1 ± 0.2 for those who completed phase 1 only, and increased by 0.1 ± 0.3 for those who also completed phase 3 (p = 0.60).
Consensus status of outcomes
Scores from phase 3 were analysed and compared with the definitions of ‘consensus in’ and ‘consensus out’. Appendix 3 shows for which outcomes ‘consensus in’ was reached across stakeholder panels at the end of phase 3. Again, the number of outcomes that reached ‘consensus in’ increased markedly for all stakeholder panels. Consensus varied across the stakeholder panels; ‘consensus in’ was reached for 20 outcomes among patients, 15 outcomes among parents and 12 outcomes among surgeons. At the end of phase 3, five outcomes achieved ‘consensus in’ across all three stakeholder panels (see the shaded rows in Appendix 3, Tables 29–31). Once again, no outcomes were scored ‘consensus out’.
Variability in outcomes achieving consensus between rounds
There was variability in the number of outcomes classified as ‘consensus in’ between the phases by stakeholder groups. Outcomes were rated as increasingly important through the phases across all stakeholder panels. Table 7 shows the variability in the number of outcomes achieving consensus between the three phases. Appendix 3, Tables 29–31, show the outcomes achieving ‘consensus in’ between the three phases for each of the stakeholder panels.
| Stakeholder panel | Number of outcomes achieving consensus | |||
|---|---|---|---|---|
| In phase 1 only | In phases 1 and 2 | In phases 1–3 | In phase 3 only | |
| Patients | 7 | 7 | 4 | 6 |
| Parents | 12 | 11 | 11 | 0 |
| Surgeons | 7 | 7 | 7 | 2 |
| Totala | 17 | 16 | 15 | 8 |
Consensus meetings’ results
Participants
Overall, 28 participants attended the consensus meetings: 17 surgeons, nine parents and two patients. Attendees at the surgeons’ meeting comprised 10 paediatric surgeons, seven general surgeons, the chairperson, three members of the COS study team and one observer. All surgeons who attended voted during the meeting. Attendee characteristics for the patients’ and parents’ meeting are detailed in Appendix 3, Table 32.
One of the young people who participated in the consensus meeting did not complete the Delphi phases, but requested to participate as they met other aspects of the inclusion criteria (i.e. aged 12–18 years, with experience of acute uncomplicated appendicitis); because of the small numbers of young people confirming their attendance at the consensus meeting, the COS study team felt that it would be beneficial to allow for this protocol deviation to optimise patient representation. Two couples reported completing all three phases of the Delphi survey together and requested to vote separately in the consensus meeting. Again, the COS study team discussed this and allowed the couples to vote separately as, potentially, they may not have attended the meeting at all if one of them was not allowed to partake in the meeting, which would probably have had a greater impact on parent representation.
Results of voting and review of outcomes
Surgeons’ consensus meeting
As previously described, we first presented the outcomes rated ‘consensus in’ across all stakeholder panels in phase 3, then the outcomes for which < 50% of participants across all stakeholder panels rated the outcome 7–9 and, finally, the remaining outcomes were discussed. For each outcome, participants were provided with an opportunity to discuss it and to revote. Appendix 3, Table 33, provides a summary of all of the outcomes presented and participants’ decision regarding discussion and voting, alongside relevant notes. Participants’ decision categories comprised:
-
discussed but not voted on, as no participants opposed including the outcome in the COS
-
discussed but not voted on, as no participants opposed excluding the outcome from the COS
-
discussed and voted on
-
discussed and voted on, but later discussion to exclude, redefine or combine it with another outcome
-
discussed and not voted on, but later discussion to exclude, redefine or combine it with another outcome.
Proposals to redefine and combine outcomes
Redefining outcomes
The outcome ‘blood loss’ was not voted ‘consensus in’; however, participants suggested that the outcome name and description was quite vague and proposed redefining the outcome as ‘blood loss requiring transfusion’. The outcome was redefined and a new vote was held, but the outcome did not achieve ‘consensus in’ (see Appendix 3, Table 34).
Combining outcomes
The outcome ‘interventional radiology procedure’ was not voted ‘consensus in’; however, participants suggested that the outcome could be combined with another outcome: ‘reoperation’. Furthermore, ‘other infectious complication’ was initially discussed and not voted on. Later in the meeting, this outcome was discussed in greater detail: participants proposed that the important aspect of this outcome was the implication for treatment; in particular, an infectious complication would be deemed important if it resulted in reoperation, but not so important if it did not. Participants agreed to drop the outcome ‘other infectious complication’ on the basis that it had already been agreed to include ‘reoperation’ (see Appendix 3, Table 35).
Patients’ and parents’ consensus meeting
Again, for each outcome, participants were provided with the opportunity to discuss it and revote. Appendix 3, Table 36, provides a summary of all of the outcomes presented and participants’ decision regarding discussion and voting, alongside relevant notes. Participants’ decision categories comprised:
-
discussed but not voted on, as no participants opposed including the outcome in the COS
-
discussed but not voted on, as no participants opposed excluding the outcome from the COS
-
discussed and voted on
-
discussed and voted on, but later discussion to exclude, redefine or combine it with another outcome
-
discussed and not voted on, but later discussion to exclude, redefine or combine it with another outcome.
Finalising the core outcome set
Following both consensus meetings, the COS study team verified the results and finalised the COS. Figure 18 shows the study flow from the list of initial outcomes to the final COS. Overall, we aimed to achieve a manageable COS with a maximum of approximately 10 outcomes. For the final COS, surgeons and families mutually agreed on 10 outcomes to include in the COS. Additional outcomes voted ‘consensus in’ by only surgeons included ‘hospital length of stay’ and ‘time away from full activity’, whereas additional outcomes voted ‘consensus in’ by only families included ‘wound complication’ and ‘patient stress’ (which parents proposed should include a measure that examines psychological impact also). Overall, the COS includes 14 outcomes.
FIGURE 18.
Study flow and finalised COS. a, Antibiotic failure only reported in studies of non-operative treatment; b, outcomes that achieved consensus among parents and patients only; c, outcomes that achieved consensus among surgeons only.
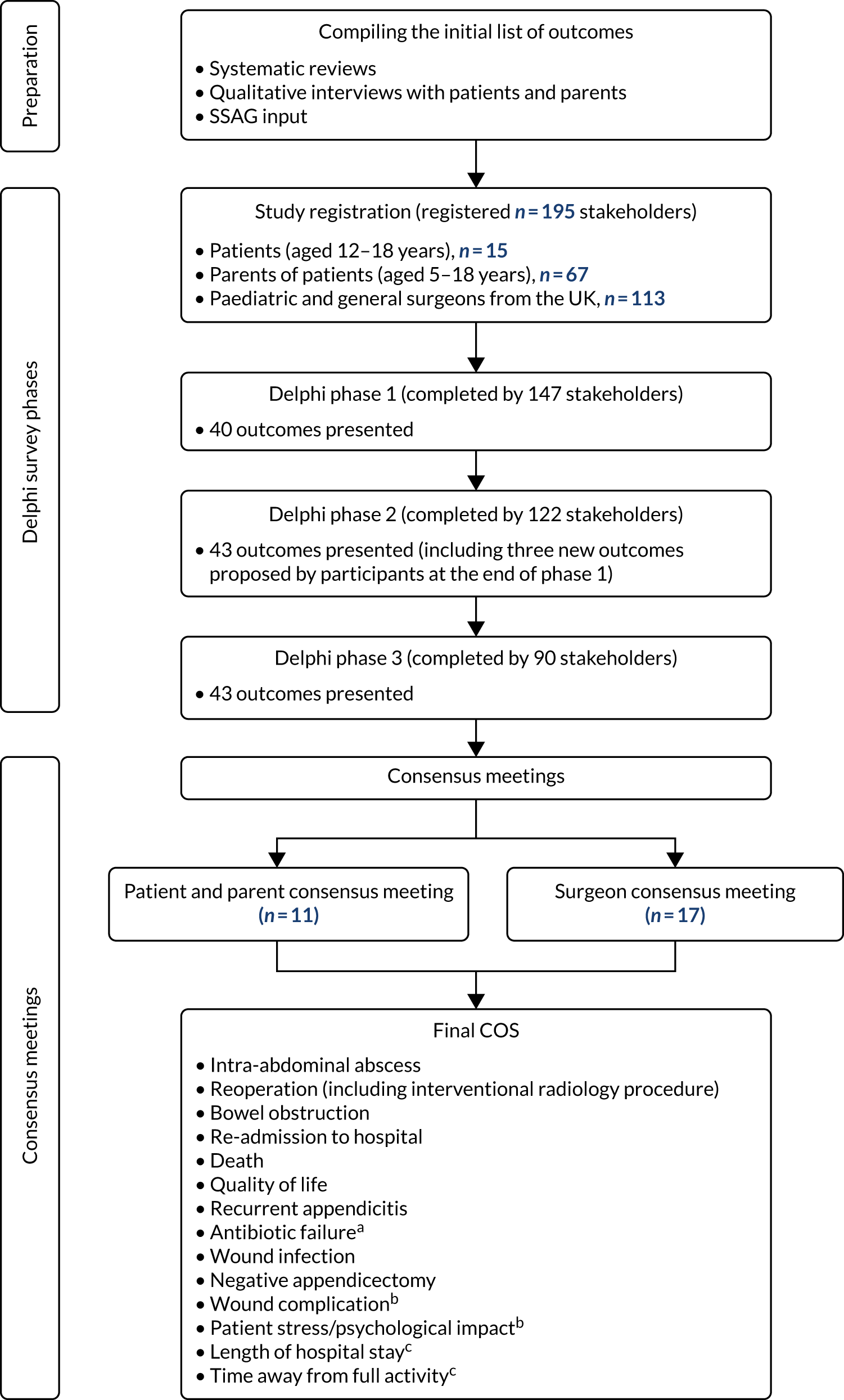
The OMERACT initiative provides a COS development framework that is useful across various health-care domains. 81 In developing the current COS, we also drew on this framework, which comprises three core domains (death, life impact and pathophysiological manifestations) and one strongly recommended domain (resource use). The framework recommends inclusion of at least one applicable measurement instrument for each core domain. It also recommends inclusion of AEs. Two researchers (FCS and SE) categorised the core outcomes by domain, and disagreements were resolved by a third researcher (NJH) (see Appendix 3).
Discussion
Prior to this study, there was no COS to determine the overall success of a treatment for uncomplicated acute appendicitis in children and young people. As previously reported, the outcomes used varied widely. 16,66 The ultimate aim of developing a COS in this field of research is to avoid inconsistencies in outcome selection, measurement and reporting in future studies, to reduce outcome reporting bias and to improve data synthesis. The development of a COS in this clinical area is particularly pertinent and timely given the increasing number of studies being conducted and published that evaluate novel treatment approaches to acute uncomplicated appendicitis in children and young people, as the outcomes reported can differ substantially from those reported with traditional treatments.
The finalised COS includes 10 outcomes agreed by patients, parents and surgeons, two additional outcomes agreed by patients and parents, and two additional outcomes agreed by surgeons (see Figure 18), totalling 14 outcomes. However, it was agreed in both consensus meetings that the outcome ‘antibiotic failure’ is applicable only to studies examining non-operative treatment. These 14 outcomes form the COS for treatments of acute uncomplicated appendicitis in children and young people that we recommend to all future researchers in this field.
‘Wound complication’ and ‘patient stress’ were the two outcomes voted ‘consensus in’ by patients and parents only. Surgeons suggested that ‘wound complication’ could be influenced by surgeons’ individual approaches and it was viewed as less important. At both meetings, it was suggested to combine ‘wound complication’ with ‘wound infection’; however, discussions indicated that the two outcomes were too distinct to be meaningfully combined. Surgeons suggested that ‘patient stress’ was to be expected for families, regardless of the intervention; some felt that it was very similar to QoL (which they had already voted ‘consensus in’); and some suggested that it could be challenging to effectively measure. Parents felt strongly that patient stress should be included in the COS and described how patient stress had a lasting impact on their everyday lives.
‘Length of hospital stay’ and ‘time away from full activity’ were the two outcomes voted ‘consensus in’ by surgeons only. Surgeons suggested that ‘length of stay’ was a good marker for treatment success and would also be easy to measure. However, patients and parents did not provide any arguments to include the outcome. Surgeons also felt that ‘time away from full activity’ was another indicator of overall treatment success. Although surgeons viewed it to be of importance, some surgeons suggested that the outcome might need redefining, as the concept of ‘full activity’ could be subjectively interpreted (and might be influenced by school holidays, for example). Patients and parents viewed the outcome to be less important, partly because it was viewed as a secondary outcome.
Further work (and consensus) is now needed to inform how each of the core outcomes should be defined and measured. 82 In order to define and measure core outcomes, we will seek guidance on the optimal processes,83 including the COnsensus-based Standards for the selection of health Measurement Instruments (COSMIN) Initative,84 which offers resources to help researchers to select the most suitable outcome measurement instruments. Key steps for us to consider include:
-
conceptual considerations (including construct of interest and target population)
-
finding existing outcome measurement instruments, by means of a systematic review and/or a literature search
-
quality assessment of outcome measurement instruments, by evaluating the measurement properties and feasibility aspects of outcome measurement instruments
-
generic recommendations on the selection of outcome measurement instruments for outcomes included in a COS.
Previous studies investigating treatments for acute uncomplicated appendicitis in children and young people have been conducted in a range of regions. The current COS has been developed with input from patients, parents and surgeons based in the UK. However, international consensus is needed to optimise the uptake of a COS internationally. A protocol for the development of a global COS for treatment of uncomplicated appendicitis in children has recently been registered on the COMET Initiative website85 and the present study’s chief investigator will be contributing to this future piece of work.
In undertaking this work, we encountered a number of challenges, primarily related to ensuring adequate engagement of participants. First, the proportion of parents and young people invited who registered was disappointingly low. Of the total 818 invited, just 10% registered. Owing to the acute nature of acute appendicitis, we invited participants who had recent experience of appendicitis (in the preceding 2 years). On discussion with some families, it became clear that, having recovered from acute appendicitis, life had very much moved on, such that appendicitis was a past memory and, in some cases, something that was long ago forgotten. Although this is positive for patients, we speculate that the absence of ongoing problems related to appendicitis may mean that interest in research is more limited than for chronic conditions. Another factor that may contribute to the limited registration rate is that potential participants were approached ‘cold’ by letter. It is possible that a more personal approach (e.g. telephone call or face-to-face discussion) would have resulted in a higher registration rate, but would have logistic and cost implications.
Attrition across the three phases of the Delphi survey was anticipated, but was particularly high in the patients’ panel. We adjusted our methods to maximise patient participation in particular, as we were keen for patients’ voices to be included in the entire consensus process, including the consensus meeting. Providing children and young people with the opportunity to develop COSs for paediatric conditions is important to ensure that important outcomes that might otherwise be overlooked by other stakeholder panels are included. 74 Compared with parents, few patients registered to participate in the project and only 20% of patients who registered completed phase 2 of the Delphi survey. Although the Delphi survey benefited from SSAG input, the online system we used was originally designed for health professionals and adult patients; therefore, further work to make consensus methods more appealing for children and young people could improve participation in this stakeholder panel.
When organising our consensus meeting, we aimed to hold one consensus meeting, bringing together patients, parents and surgeons. However, despite participants provisionally agreeing to attend, very few committed to this and it was necessary to postpone the meeting and consult with patients, parents and surgeons on their preferences for a future meeting. Previous studies have held two separate consensus meetings for patients and health professionals to ensure that meetings are not dominated by health professionals’ views. 77–79 Parents’ and surgeons’ preferences regarding timing (weekday vs. weekend) and location of a future consensus meeting differed, thus informing the decision to hold two separate meetings. Overall, parents preferred the prospect of a local consensus meeting. Future research should explore opportunities to design and implement alternative consensus meeting formats, such as online or remote meetings for geographically dispersed stakeholders. Overall, the consultation findings highlight important considerations that COS developers will need to consider in planning consensus meetings involving children and young people, parents and health professionals.
Although an increasing number of COSs have included patients as key stakeholders,82 few COS studies have included children and young people as participants, and those that have vary substantially regarding the degree of involvement. 76,86–88 There is currently no guidance on optimal methods to include children and young people and parents in the development of COSs that focus on paediatric conditions. Overall, further work is essential to effectively include those important stakeholders in COS development as it seems likely that the techniques currently employed do not engage or retain children and young people effectively.
In the current study, further work is also needed to effectively implement the COS. In doing so, we will consult with current guidance on COS implementation. 82 We have ensured that the final COS has been published in an Open Access format (British Journal of Surgery)89 and that the COMET entry is linked to this paper, and have presented the COS to the British Association of Paediatric Surgeons meeting (2019). We will continue to contact those responsible for planned and ongoing research identified through clinical trial registries, and contact journals in the field of paediatric surgery and gastroenterology to propose an editorial or commentary to promote use of the COS. This comprehensive approach will help to optimise the uptake of the COS in future research.
Identification of a candidate primary outcome and effect size for a future randomised controlled trial
Background
Central to the design of any RCT is the identification of an appropriate primary outcome. Historically in research, investigators have used a primary outcome that is most relevant to them in determining a specific aspect of their investigation in which they are most interested. More recently, greater importance has been placed on selecting a primary outcome that has relevance to a wide range of stakeholder groups that have an interest in the condition and treatments being investigated. Design of an appropriately powered RCT of appendicectomy versus non-operative management is especially problematic as the two arms may have markedly different outcomes. The outcomes from appendicectomy are likely to be superior to those from non-operative management if any of the usual measures are chosen as a single primary outcome. In the context of the current study, therefore, we propose that the primary outcome for a future RCT should be relevant not just to surgeons who care for children with acute uncomplicated appendicitis, but to a wider range of interested parties including, most importantly, the patients themselves and their families, and those delivering and funding health care. Consistent with our intention to deliver a future RCT that is pragmatic, we aim to ensure that the primary outcome selected is meaningful to patients and parents in particular, but that it also provides relevant information to surgeons.
Methods
We took the opportunity of having contact with a range of stakeholders in the COS development process to survey preferences for a candidate primary outcome in a future trial. We then held a discussion related to the survey responses at both COS consensus meetings to try to reach agreement among those present on a primary outcome that met the criteria of being important, relevant and acceptable. At the surgeon consensus meeting, we also discussed what an acceptable effect size for this putative primary outcome might be, on the assumption that a future trial would be designed on a non-inferiority basis.
Survey
At the end of phase 3 of the Delphi survey, participants from all stakeholder groups were also asked to identify the one outcome that they believed to be the most important for informing their treatment, their child’s treatment or their treatment choice, for patients, parents and surgeons, respectively, in a future trial comparing non-operative treatment with appendicectomy in children with acute uncomplicated appendicitis. They were provided with a list of the 15 highest-scoring outcomes across their stakeholder panel from round 2 of the Delphi consensus process and, from this list, were asked to select one single outcome or, if they felt that several factors were all of equal importance to measure, they were given the option of selecting more than one. These data were summarised by stakeholder panel.
Consensus meeting
Informed by these results, Nigel J Hall facilitated a discussion at the end of each COS consensus meeting to explore patients’, parents’ and surgeons’ preferences for the primary outcome in a future definitive trial of non-operative treatment for acute uncomplicated appendicitis in children and young people. This discussion was preceded by a brief verbal explanation of the importance of selecting a primary outcome and the relevance of selecting the correct primary outcome on trial design (e.g. sample size calculation, interpretation of trial results). Groups were informed that the primary outcome did not necessarily need to be one of the outcomes from the COS. The challenge of identifying a single primary outcome when the treatments being compared are very different was highlighted. The concepts of single and composite primary outcomes were explained to both groups, along with the potential benefits and challenges of each approach. Discussions were open and led to agreement by discussion, although no formal voting took place at either meeting.
At the surgeons’ meeting only (time did not allow for this at the parents’/young people’s meeting), a subsequent discussion was facilitated by Nigel J Hall regarding the size of an acceptable non-inferiority margin for a future trial. The concept of a non-inferiority margin was explained, along with how this type of trial design differs from a superiority trial. Again, an open discussion was held and views canvassed from participants. Participants were asked to consider what margin of inferiority they would be willing to accept in clinical practice when comparing non-operative treatment with appendicectomy for children with acute uncomplicated appendicitis. The assumption that non-operative treatment is likely to be inferior to appendicectomy, regardless of which specific primary outcome is eventually selected, was made and agreed. The potential benefits of non-operative treatment that would be the ‘trade-off’ of a marginally ‘inferior’ treatment were discussed in order to frame the discussion. These were agreed to include avoidance of surgery and general anaesthesia, avoidance of exposure to surgical complications, and allowing patient choice, with some participants suggesting that cost of treatment could be a potential benefit.
Results
Overall, 88 out of 90 (98%) phase 3 Delphi survey participants voted on their preferred primary outcome(s). Of these, 20 (22%) participants proposed a single outcome to be used as a primary outcome, whereas the remainder proposed that multiple outcomes should be used. Appendix 3, Table 37, shows how each stakeholder group voted and the cumulative total votes for each outcome are ranked in order of popularity.
Appendix 3, Figure 23, shows a matrix of the most frequently proposed outcomes across all 88 participants and allows determination of the most frequently proposed composite outcomes. These were as follows:
-
antibiotic failure AND recurrent appendicitis – 31 (36%)
-
antibiotic failure AND re-admission – 22 (26%)
-
antibiotic failure AND recurrent appendicitis AND re-admission – 14 (16%)
-
antibiotic failure AND recurrent appendicitis OR re-admission – 39 (45%)
-
antibiotic failure OR recurrent appendicitis OR re-admission – 74 (84%).
Three young people and 31 parents responded, of whom six suggested a single primary outcome:
-
re-admission, n = 1
-
recurrent appendicitis, n = 1
-
QoL, n = 1
-
other infectious complication, n = 1
-
wound infection, n = 2.
Fifty-four surgeons responded, of whom 14 suggested a single primary outcome:
-
antibiotic failure, n = 6
-
re-admission, n = 3
-
QoL, n = 2
-
time to ambulation, n = 2
-
death, n = 1.
The remainder proposed that more than one outcome be selected as a primary outcome. Responses from surgeons were compared with those from young people and parents to identify major differences between these stakeholder groups. Appendix 3, Figure 24, shows how surgeons and the group as a whole rated different outcomes.
Consensus meeting discussion: primary outcome selection
Initial discussion in the surgeons’ meeting was that they would want all data relating to important clinical outcomes specific to each treatment approach to be reported as a composite primary outcome. In the appendicectomy arm, this would include complications related to surgery, and in the non-operative treatment arm, this would include antibiotic failure and recurrence rate. Of note, negative appendicectomy was not considered to be important by the surgeons in this context. They also felt that it would be beneficial if any such composite primary outcome was defined in terms of defining treatment ‘success’ as opposed to treatment ‘failure’. Overall, however, the surgeons were attracted to the idea of trying to identify a single outcome that could be measured meaningfully in both treatment groups, and preferred this to a composite primary outcome as long as treatment-specific outcomes were also reported (as secondary outcomes). Some suggested that QoL would make a good secondary outcome if non-operative treatment was found to be non-inferior to surgery in terms of treatment success. Hospital length of stay and re-admission to hospital were also proposed as potential primary outcomes that could effectively assess the impact of both non-operative and operative treatment. The most popular single outcome was time to return to normal (premorbid) activity. Of note, this is not an outcome that was considered in the COS development process. It was noted that this would be difficult to measure as the premorbid state would not have been measured. It was also highlighted that this may be analysed on a superiority basis rather than on a non-inferiority basis.
In the young people and parents’ meeting, the initial preference was for a single primary outcome: QoL was identified as the forerunner. The group clearly understood the challenge in selecting a primary outcome that was meaningful to both groups but that was prevalent enough in this population to be distinguishable between patients treated with either non-operative treatment or appendicectomy. However, the group identified that QoL is difficult to measure and had concerns about the appropriate time to measure QoL, and hence, on balance, considered it less appropriate. The investigators mentioned that, in the CONTRACT study, QoL measures had been taken at various time points, meaning that data on the appropriate time to measure QoL might be available. The group discussed length of stay in hospital as a suitable primary outcome, as it reflects treatment success for both non-operative and operative treatment. However, on balance, this was rejected as the group was less concerned about the time it took to recover (so long as it was within a reasonable timeframe) than about recovery being complete. They were also concerned about not using the treatment-specific outcomes as the main determinant of treatment success and discussed this in some detail. On balance, they agreed that a composite primary outcome that included treatment-specific outcomes, including complications and negative appendicectomy (appendicectomy arm) and antibiotic failure rate and recurrence rate (non-operative treatment arm), was preferable.
Consensus meeting discussion: non-inferiority margin
The surgeon group considered the non-inferiority margin of a trial that was hypothetically designed as a non-inferiority trial and used a treatment-specific composite primary outcome. Although the surgeons felt that a 10% non-inferiority margin was too narrow as any such trial would almost certainly report in favour of appendicectomy for design reasons, they felt that a non-inferiority margin should not exceed 20%.
Discussion on primary outcome selection
In this work, we aimed to identify a primary outcome for use in a future effectiveness trial comparing non-operative treatment with appendicectomy in children with uncomplicated acute appendicitis. The identification of a primary outcome is an important step for any RCT. Along with the anticipated effect size, it is a key determinant of the sample size required. Perhaps more importantly, however, the primary outcome is the outcome that will be used to arrive at a decision on the overall result of the trial by those interested in its findings. In the past, this ‘headline’ outcome was typically the outcome that the investigators were most interested in. This approach risks using an outcome that is not interpretable by anyone other than the investigators and their stakeholder group; therefore, guidance90 suggests that greater emphasis should be given to selecting an outcome of greater interest to a broader range of stakeholders, including patients and, in the case of children, their families. The PRagmatic Explanatory Continuum Indicator Summary-2 (PRECIS-2)91 framework, which provides guidance on the design of pragmatic trials, recommends the selection of an outcome that is of obvious importance from the perspectives of all stakeholders. In designing a future effectiveness trial, therefore, we seek a primary outcome that is relevant and meaningful to patients, parents and surgeons, that is all the groups that we anticipate will be interested in the results of the trial.
To our knowledge, no precise guidance exists on how to identify a primary outcome with these characteristics. We therefore sought the opinions of multiple stakeholder groups that we were already in contact with through our COS development process. Through a survey and subsequent discussion, we have understood the views of important stakeholder groups. In the quantitative survey, it was evident that the majority of respondents identified a condition-specific measure as being the most important. Antibiotic failure was the outcome included by the most people as at least part of a primary outcome. A combination of at least one of antibiotic failure, recurrent appendicitis or re-admission appears to satisfy 84% of respondents.
Interestingly, in discussions, other less disease-specific outcomes were considered. The appeal appeared to come at least partly from the fact that a single outcome could be satisfactorily applied to both treatment arms. Eventually, one of these non-specific outcomes was preferred by surgeons over the disease-specific parameters. There was agreement that time to return to normal (premorbid) activities was a good marker of recovery from illness. Surgeons identified this as being important in discussion with one another, whereas previously, in a survey setting, disease-specific outcomes had been preferred. Parents and young people, on the other hand, having considered generic outcomes in discussion, felt that the disease-specific outcomes were more important to them in this context.
How to identify the target effect size in a clinical trial has recently been the topic of the Difference ELicitation in TriAls (DELTA2) guidance on how to determine this. 92 The authors acknowledge that deciding on a non-inferiority margin in a non-inferiority trial is a controversial topic. One proposal is that the non-inferiority margin be set as the largest difference that is clinically acceptable, so that a difference bigger than this would matter in practice. 93 Implicit in this is the concept that different stakeholders may have very different views on the biggest difference that would matter in practice.
This is perhaps particularly true when the treatments being compared are very different, as in the trial we propose. This is because the potential adverse effects (or benefits) of the treatments may be very different, giving rise to a potential ‘trade-off’ of one treatment against the other. For instance, those who are keen to avoid appendicectomy owing to the risk of perioperative complications may be willing to accept a reduction in efficacy of treatment (with non-operative treatment) in the interest of realising the potential benefit they seek. What margin of reduction in treatment efficacy they would be willing to accept may vary from person to person and will almost certainly vary between patients, parents and surgeons. Seeking a non-inferiority margin that is truly acceptable to all stakeholders may be challenging.
Chapter 6 Health economic analysis
Background
This chapter presents the economic analysis conducted alongside the CONTRACT feasibility study. The aims of this feasibility economic substudy were (1) to explore whether or not reliable health service use data can be obtained from hospital clinical records and to assess whether or not integration of clinical and research data could be reliably used to inform future trial-based economic evaluations, and (2) to assess two health-related quality-of-life (HRQoL) measures that are widely used in clinical studies for children in the context of comparing non-operative treatment with appendicectomy. By incorporating economic evidence into an early stage of the study, the research questions we aimed to address were as follows:
-
What are the resource use and cost implications of treating childhood appendicitis non-operatively, compared with appendicectomy, and how do the costs of both treatment options compare with widely used NHS reference costs?94
-
What could be the implications of differing costing methods and data collection tools?
-
How do two different HRQoL instruments compare, and could the timing of collecting HRQoL data affect utility values and cost-effectiveness analysis results?
Overall, the economic substudy aimed to provide evidence and guidance, with an emphasis on determining data collection tools to measure cost and benefit outcomes for a future RCT in which the cost-effectiveness of the non-operative treatment of appendicitis compared with appendicectomy will be assessed. The health economic work in this chapter follows the methods described in our publication of the health economic protocol for this study. 95
Methods
Identifying what resource utilisation and costs are to be included in an economic evaluation is an important part of the cost-effectiveness analysis, as the results of the economic evaluation will be greatly affected by the quality and accuracy of the measurements. 96–98 The feasibility stage gives the opportunity to define and refine resource utilisation, and to test data collection tools by piloting and assessing data collection methods. 96
The economic exploratory analysis was carried out from the perspective of the health system (NHS), which greatly determined the treatment pathways used and underpinned the classification and domains used in the analysis. The full sample (n = 57) was used for the overall economic analysis and a subsample (n = 28) was used for the microcosting exercise. This empirical investigation used data collected by the research team, clinicians accessing hospital records and questionnaires completed by parents/carers. Data management was performed by the SCTU and anonymised data were provided to the health economic team for analysis. The economic substudy has been conceptually divided into two parts: (1) resource use and costs – the assessment of developing tools and methods measuring resource utilisation and for conducting microcosting, and (2) HRQoL – measuring QoL using two different paediatric HRQoL instruments and assessing the impact of each of these on utility values and quality-adjusted life-years (QALYs).
Resource use, valuation and costs
In this feasibility study, health-care service use was measured for each participant using clinical records, case report forms completed by research nurses and the Client Service Receipt Inventory (CSRI)99 completed by parents/carers. In our empirical investigation, we sought to estimate and assess (1) the level of agreement between data sources, (2) the quality of the data and (3) the level of precision and impact in terms of the future cost-effectiveness analysis. Figure 19 presents the different costing methods and data sources used.
FIGURE 19.
Data sources and methods used.
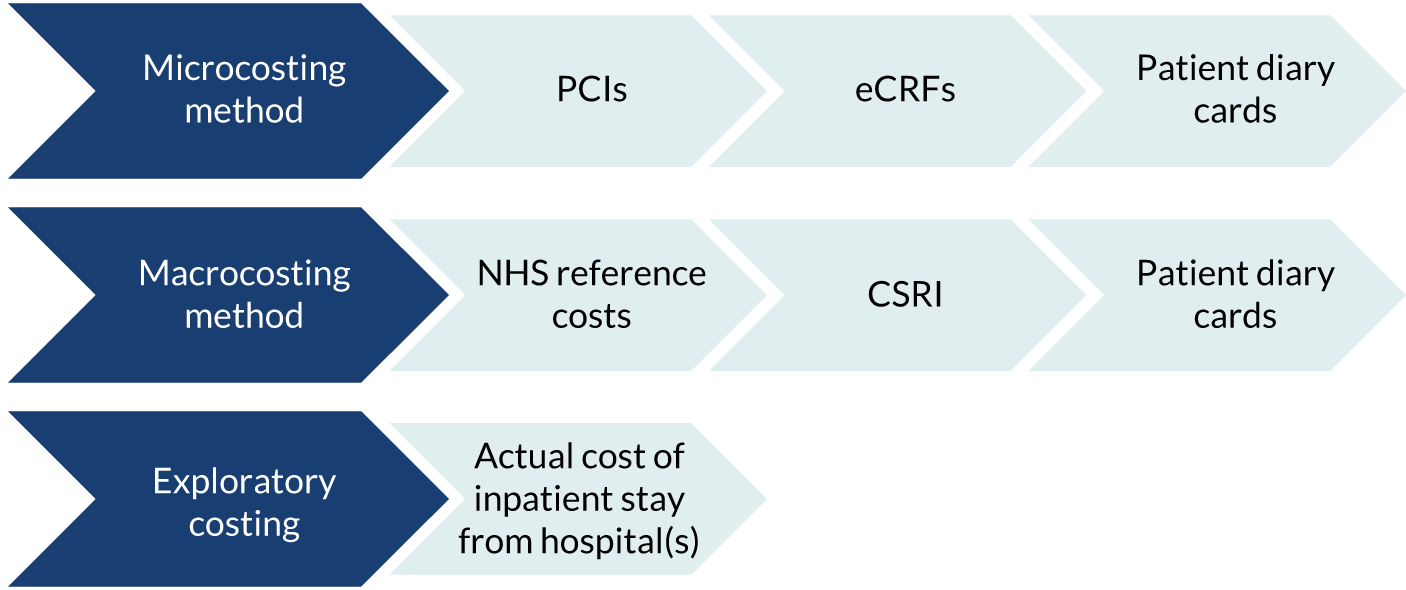
We adopted a comprehensive approach of collecting data during the inpatient phase of treatment from hospital, and from the wider health-care system following hospital discharge. The following data were recorded at the following time points.
-
During hospitalisation, discharge assessment, follow-up appointments and re-admissions:
-
patient clinical inventories (PCIs) that were designed to capture the full duration of resource use during hospitalisation and were informed from hospital records including in-hospital and outpatient clinical records, laboratory and pharmacy records, diaries, radiology department records and relevant correspondence. These allowed the capture of all resource use data relating to hospitalisation, discharge assessment, follow-up appointments and re-admissions (see Appendix 4, Figure 25, for an example of a PCI). In-hospital medication use was recorded from electronic case report forms (eCRFs) completed during hospitalisation by research nurses. These data were used not only to conduct microcosting, but also to assess the integration of routinely collected clinical data into research.
-
-
Post-discharge primary care, outpatients and emergency services:
-
eCRFs completed by research nurses interviewing parents/carers following discharge at 6 weeks and at 3 and 6 months (see Appendix 4, Figure 26, for a screenshot of an eCRF). These data were used as the ‘gold standard’ against which the patient-completed questionnaires (CSRI) were evaluated.
-
Patient diary cards were used to record resource use during the 14 days immediately after discharge from hospital [see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/1419290/#/ (accessed February 2020)]. These provided data on oral antibiotics, pain medications and anti-inflammatory medications (outpatient use). A modified version of the CSRI completed by parents/carers of participants was used to collect data on other health-care appointments and additional family-borne costs at 6 weeks and 6 months post discharge. The data collection also included reporting of days lost from work for parents and absence from school for children participating in our study [for the CSRI, see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/1419290/#/ (accessed February 2020)].
-
Data
The most commonly used approach incorporating the cost of hospitalisation in an economic evaluation is to use NHS reference costs94 for the relevant Healthcare Resource Group (HRG) code. These are national average unit costs for predefined services. Microcosting is the direct enumeration and costing of every input consumed in the treatment of a particular patient. 100–102 Our intention was not to identify every possible individual cost and its values, but rather to quantify resource use by treatment pathway and define domains within the treatment pathways by recording resource use and main cost drivers, hence providing valuable information and guidance on data collection requirements for a definitive RCT, that is identifying what costs need to be collected and how these resource use and cost data can be collected. 103
Although, primarily, our interest was to define the average cost for the ‘average patient’ (a macrocosting approach), there was also interest in following a microcosting approach and extending costing by including and defining an individual patient’s resource use. This allowed us to compare the total per-case cost against the widely used NHS reference costs. 94 We also compared our microcosting-derived cost with the actual cost of treatment provided by participating hospitals. This was especially important because non-operative treatment of appendicitis is a relatively new proposition and we needed to define the treatment pathway in terms of overall resource use and costs.
Data from PCIs were used to conduct microcosting of both treatment pathways and to explore what the determinants of variation in costs between the two treatment arms are and the potential economic implications for the NHS. For the valuation of resource use, we used the unit cost for each individual resource included in the microcosting exercise; these were obtained from participating hospitals. The method adopted includes identification of services, how the service works and which components of cost are incurred during the delivery of each service. In collaboration with the clinical team, we designed and mapped processes involved in service delivery and identified relevant resource use. During the data collection forms (PCIs) design stage, we created patients’ treatment pathways following the first 10 patients randomised to the study. This allowed us to identify resource use items in each domain within different stages of the treatment pathway. The PCIs were checked and discussed with the clinical team and, following modifications, the final PCIs were piloted for all patients recruited and randomised during the second 6 months of the study across all three participating sites. This process provided us with not only the design of a detailed individual PCI, but also the reporting of a comprehensive health resource use profile. Unit cost data used in the valuation of resource use were obtained from the finance department of participating hospitals.
Following discharge, the items selected for comparison were primary care resource use, outpatient and emergency appointments from secondary care and resource use due to hospital re-admission. Data were collected using two methods: first, by interviewing parents/carers (eCRFs) and, second, parents/carers completed questionnaires on their own time (CSRI). Unit cost data used in the valuation process of the eCRFs and CSRI data were obtained from NHS reference costs94 and Personal Social Services Research Unit data. 104
The process we followed intended not only to define the need for data collection in a future RCT, but also to assess the quality of data in terms of missing values and accuracy of data. During the feasibility stage, it is not appropriate to directly compare the trial treatment arms; therefore, our work is mainly reporting in the form of descriptive statistics. This allowed us to assess data quality, identifying the most appropriate method and tools to use in a future definitive RCT. All costs are presented in 2017/18 prices; when necessary, the unit costs were adjusted for inflation using the Hospital and Community Health Service (HCHS) index. 104
Effectiveness outcomes: health-related quality of life and estimating using utility values and quality-adjusted life-years
There is a strong debate among methodologists regarding the measurement and valuation of QoL in paediatric research studies. Rich literature105–109 highlights the problems associated with quantifying QoL for children; these problems range from who conducts these assessments (self vs. parent/proxy assessment) to what are the most appropriate value sets (tariffs) to use. In the UK, we are motivated to use the EuroQoL-5 Dimensions (EQ-5D)110,111 instrument in adult populations, following recommendations by the National Institute for Health and Care Excellence. 112 This creates a bias towards using the EuroQoL-5 Dimensions-Youth version (EQ-5D-Y)113 for children as most appropriate option for this population. However, the suitability of this approach has been questioned because the EQ-5D-Y applies the same tariff (at the time of the design of this study) as the adult version of the questionnaire; the only difference is the wording of the questions to make it more accessible for self-assessment by children. In this feasibility economics substudy, we decided to collect and assess two different instruments that have been used in paediatric research. The order of the two instruments was random when completed by parents/carers, making sure that the quality of data and missing values are not influenced or biased by the order of completion. The instruments used were the EuroQoL-5 Dimensions, five-level version (EQ-5D-5L),114 which comprises five levels of response assessing five dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression), and the Child Health Utility-9 Dimensions (CHU-9D)115–120 paediatric questionnaire developed at the University of Sheffield, which comprises five levels of response assessing nine dimensions (worried, sad, pain, tired, annoyed, school work/homework, sleep, daily routine and able to join activities). The CHU-9D is the only health utility questionnaire that has been developed with children and the value set was obtained by a UK-based general population sample.
Our aim was to detect the effects of the interventions on HRQoL by collecting and assessing the performance of the most commonly used (in the UK) HRQoL instruments. We collected both instruments at baseline, at discharge and at 2 weeks to determine any short-term difference in HRQoL that may not be apparent in later follow-up, and then at the 6-week and at the 3- and 6-month follow-ups. Responses from the two questionnaires were used to estimate utility values and to assess the short- and long-term implications of the two treatments for HRQoL. As we stated in our health economics protocol,95 our aim was not only to assess which instrument shows superior performance in terms of sensitivity to change and quality of data in this patient group, but also to assess how the different data collection points (timing) could affect the results of a cost-effectiveness analysis.
Results
Analysis followed a prespecified health economic analysis plan. A total of 57 participants were enrolled in the feasibility RCT over 12 months from March 2017. Data for all participants, with the exception of those who withdrew consent for data collection, were available for the economic analysis. Microcosting was performed on 28 of the 30 patients enrolled in the second 6 months of the trial recruitment period (two participants withdrew consent for ongoing data collection and were therefore excluded). These were 15 participants in the appendicectomy arm and 13 participants in the non-operative treatment arm. Three different data collection and costing methods were performed; results, quality of data and missing values are reported separately for each method. Baseline characteristics of the sample are detailed in Table 3. The following sections present the resource use and costs by source of data, and, for the effectiveness outcomes, side-by-side comparison for both HRQoL instruments.
Resource use and costs
Microcosting, source: patient clinical inventory from hospital records
Presentation of results in this section and the data collection method refers to event pathways for activity costing so that context and information are not lost in the final outcomes reported. We have therefore calculated, and report here, the estimates for each treatment profile and pathway, but we also define variation within each domain by reporting the mean and standard deviation (SD). The two treatment pathways presented are defined by the composition of events relevant to each arm. Figure 20 shows the treatment pathways as defined for this study and the classification of domains that describe the different stages of the treatment pathway. The process presented in this figure is not a clinical representation but, instead, follows the classification of resource use and costs following the same format as the tables with results. Crossover from the non-operative arm to the appendicectomy arm was possible at any point of this process if clinically necessary; in these cases, the process is identical to the one in the appendicectomy arm. Classification of service systems involved allows an itemised reporting and implies focusing on variation at individual and aggregate level by treatment arm.
FIGURE 20.
Treatment pathways for each treatment arm separated into domains. Please note that these are classification of cost domains, not clinical stages. Crossover from the non-operative arm to the appendicectomy arm could happen at any point of the process.
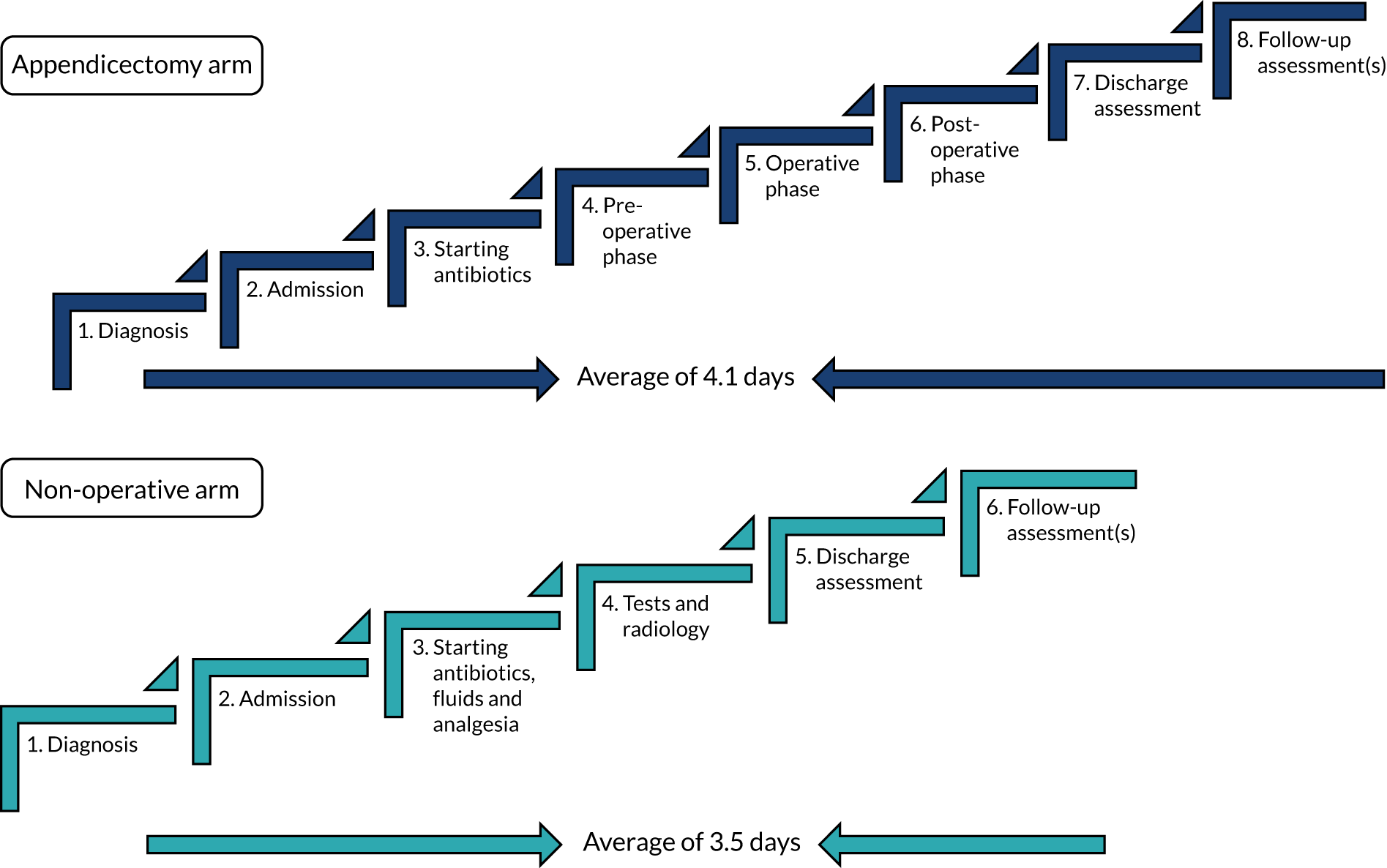
Treatment pathways and resource use
For the sample of 28 patients, detailed data were collected from randomisation up to and including discharge assessment and follow-up appointments. Data were available for all 28 patients. One participant randomised to the non-operative treatment arm had a re-admission for recurrent appendicitis resulting in appendicectomy. All treatment costs are included for this patient. Resource use, when possible, was measured in natural physical units, for example the number of blood tests performed. However, in some cases, it is necessary to report data for ‘bulk service’, for example reporting disposables and consumables; these were estimated in an itemised form, attaching unit cost for each item, but we report them here in a combined/summarised format.
Although we had originally intended to obtain the unit cost for each and every resource use item in the PCI from all three participating hospitals, this proved possible for only one participating hospital. The other two hospitals were unable to release full unit costs for reasons of commercial sensitivity. We therefore used the costs from the single hospital in the microcosting analysis for all 28 participants. Similarly, we were able to obtain the actual cost of treatment from only one participating hospital.
Table 8 shows the costs for the non-operative treatment arm and Table 9 shows the costs for the appendicectomy arm. Owing to the significant number of items included in each domain, we have presented the cost data as the mean (SD) cost for that domain following each treatment pathway.
| Classification | Domain | n | Cost (£), mean (SD) |
|---|---|---|---|
| Antibiotics phase | Antibiotics | See below for inpatient medications in details | |
| Other medications | 13 | 10.44 (14.4) | |
| Consumables and disposables | 13 | 23.9 (23.5) | |
| Tests and diagnostics | 13 | 3.90 (8.7) | |
| Other teams | 13 | 2.64 (9.5) | |
| Cost of antibiotics phase | 13 | 40.89 (44.5) | |
| In case of treatment failure, treated with appendicectomy | Medications before surgery | 13 | 0.16 (0.6) |
| Medications during surgery | 13 | 18.70 (47.2) | |
| Consumables and disposables | 13 | 19.32 (47.3) | |
| Clinical staff | 13 | 89.49 (239.5) | |
| Equipment and facilities | 13 | 334.38 (860.4) | |
| Laboratory tests | 13 | 14.00 (34.2) | |
| Cost of operation phase | 13 | 475.89 (1224.8) | |
| POC medications | 13 | 1.28 (3.6) | |
| POC consumables and disposables | 13 | 0.29 (0.7) | |
| Cost of POC phase | 13 | 1.58 (4.1) | |
| Total inpatient stay | Antibiotics | 13 | 93.53 (223.1) |
| Analgesics | 13 | 51.81 (60.0) | |
| Ward stay (days) | 13 | 3.54 (1.9) | |
| Ward stay cost | 13 | 1450.77 (760.0) | |
| Discharge assessment phase | Medications | 13 | 2.49 (7.8) |
| Consumables and disposables | 13 | 0.14 (0.5) | |
| Clinical review | 13 | 2.45 (8.8) | |
| Clinical staff | 13 | 49.45 (15.9) | |
| Laboratory tests | 13 | 8.42 (29.9) | |
| Cost of discharge assessment phase | 13 | 62.95 (32.2) | |
| Outpatient antibiotics | 13 | 1.83 (2.8) | |
| Follow-up appointments | 13 | 11.15 (40.2) | |
| Total cost of non-operative treatment arm | 13 | 2190.39 (1332.3) | |
| Classification | Domain | n | Cost (£), mean (SD) |
|---|---|---|---|
| Antibiotics phase | Medications | See below for inpatient medications in details | |
| Consumables and disposables | 15 | 44.39 (34.4) | |
| Preoperative phase | Medications | 15 | 2.29 (2.0) |
| Consumables and disposables | 15 | 2.89 (2.9) | |
| Cost of preoperative phase | 15 | 5.18 (3.5) | |
| Operation phase | Medications | 15 | 102.89 (17.0) |
| Consumables and disposables | 15 | 52.43 (26.9) | |
| Clinical staff | 15 | 348.82 (209.5) | |
| Equipment and facilities | 15 | 1827.91 (577.1) | |
| Laboratory tests | 15 | 21.85 (6.8) | |
| Cost of operation phase | 15 | 2353.90 (714.2) | |
| POC phase | Medications | 15 | 14.80 (18.6) |
| Consumables and disposables | 15 | 14.81 (12.3) | |
| Laboratory tests | 15 | 1.33 (2.9) | |
| Radiology | 15 | 3.87 (10.2) | |
| Other teams | 15 | 11.74 (21.8) | |
| Cost of POC phase | 15 | 46.55 (43.2) | |
| Total inpatient stay | Antibiotics | 15 | 53.23 (72.3) |
| Analgesics | 15 | 112.86 (182.3) | |
| Ward stay (days) | 15 | 4.13 (1.9) | |
| Ward stay cost | 15 | 1694.67 (788.1) | |
| Discharge assessment phase | Medications | 15 | 0.41 (0.9) |
| Consumables and disposables | 15 | 0.20 (0.7) | |
| Clinical review | 15 | 15.33 (36.1) | |
| Clinical staff | 15 | 57.42 (4.0) | |
| Laboratory tests | 15 | 0.68 (2.6) | |
| Cost of discharge assessment phase | 15 | 74.06 (40.0) | |
| Outpatient antibiotics | 5 | 0.90 (2.3) | |
| Follow-up appointments phase | 15 | 25.46 (52.7) | |
| Total cost of appendicectomy arm | 15 | 4411.20 (1270.6) | |
For the non-operative treatment arm, the antibiotics phase includes all domains relevant to this treatment pathway and discharge assessment and follow-up appointments. In case of treatment failure (one patient out of 13), the costs are included in the appendicectomy domain. Total inpatient stay refers to all patients, and it shows that the ward stay is the most significant cost driver for this treatment pathway, with a mean of 3.4 days (SD 1.9 days) of hospitalisation.
The mean total cost estimates for the non-operative treatment arm and the appendicectomy arm were £2190 (SD £1332.30) and £4463.63 (SD £1267.55), respectively. For the appendicectomy arm, the costs of the operation phase, including facilities and equipment costs, and the ward stay are the most significant cost drivers. The mean number of days of hospitalisation for this treatment arm was 4.13 days (SD 1.9 days).
The difference in costs between the two arms was £2220.81 (95% CI £1208.67 to £3232.95; p < 0.001), showing a cost reduction for the non-operative treatment arm. Our results from the microcosting approach are very similar to the actual per-patient cost incurred (as reported by hospital finance department): a mean cost of £2597 for the non-operative treatment arm and £4957 for the appendicectomy arm. Table 10 shows the total costs as estimated from the microcosting exercise, the actual costs as reported by one participating hospital and NHS reference costs94 for this condition, which are probably the figures that would be used in a macrocosting approach. The unit cost range of the NHS reference cost94 data refers to the different HRG codes for this condition.
| Treatment arm | Cost (£) |
|---|---|
| Total costs as per microcosting | |
| Non-operative treatment | 2190.39 |
| Appendicectomy | 4411.20 |
| Total actual costs incurred provided by hospital(s) | |
| Non-operative treatment | 2597.00 |
| Appendicectomy | 4957.00 |
| NHS reference costs, 2017/18 | |
| Paediatric other gastrointestinal disorders | 553.00–1918.00 |
| Appendicectomy procedures, aged ≤ 18 years | 2415.00–5055.00 |
Resource use and costs
Source: electronic case report forms completed by research nurses (Medidata Rave database)
The data were recorded on eCRFs by research nurses; the questions on the eCRF were regarding primary health-care service use and outpatient appointment and laboratory tests from secondary care. As expected, the rate of completion and the quality of the data reflect the quality of a ‘gold standard’, that of an interview-based completion method. Appendix 4, Table 38, summarises the mean (SD) resource use figures for each resource category as recorded in the eCRFs, presented by study group. Appendix 4, Table 39, presents the mean (SD) cost values for each cost category and total costs from baseline to 6 weeks, from 6 weeks to 3 months and from 3 months to 6 months.
Table 11 presents the total costs for each category for the trial duration (baseline to 6 months). Overall, the non-operative treatment arm incurred higher costs than the appendicectomy arm for the 6-month period.
| Classification | Treatment arm | Participants, n | Cost (£), mean (SD) |
|---|---|---|---|
| Costs: baseline to 6 months | |||
| A&E visits | Non-operative treatment | 18 | 41.53 (85.9) |
| Appendicectomy | 20 | 14.95 (46.0) | |
| GP visits | Non-operative treatment | 18 | 11.89 (19.7) |
| Appendicectomy | 20 | 10.70 (19.0) | |
| Practice nurse | Non-operative treatment | 18 | 0.75 (3.2) |
| Appendicectomy | 20 | 0.68 (3.0) | |
| Hospital outpatient | Non-operative treatment | 18 | 5.71 (17.6) |
| Appendicectomy | 20 | 11.99 (27.9) | |
| Laboratory tests | Non-operative treatment | 18 | 0.79 (1.6) |
| Appendicectomy | 20 | 0.88 (2.2) | |
| Walk-in centre and other health-related care | Non-operative treatment | 18 | 7.57 (17.4) |
| Appendicectomy | 20 | – (0.0) | |
| Total costs: baseline to 6 months | |||
| Summation of all services | Non-operative treatment | 18 | 67.54 (94.7) |
| Appendicectomy | 20 | 39.20 (75.8) | |
Source: parent-/carer-completed Client Service Receipt Inventory
The CSRI was completed when patients attended for follow-up consultation; if they did not attend in person, attempts were made to contact them by telephone to complete the CSRI. Appendix 4, Table 40, presents primary and secondary care use reported by parents/carers completing the CSRI questionnaire at 6 weeks. The 6-month data were extremely limited and are therefore not included in this report. The quality of data was relatively poor, even at 6 weeks, with more observations missing from the non-operative treatment arm than from the appendicectomy arm. Overall, patients in both arms had limited use of primary care services. Appendix 4, Table 41, presents the total costs following valuation of the resources used by cost category for each group. Comparing the 6-week data from both data sources, although the two data collection methods differ slightly as the CSRI includes more categories than the eCRFs, the common categories show a noticeable difference in costs reported. For example, the numbers of A&E visits reported for 6 weeks in the CSRI were 0.17 (SD 0.5) and 0.06(SD 0.3) for the non-operative treatment and appendicectomy arms, respectively, whereas the values for the same category as recorded by research nurses through the interview with parents/carers were a mean of 0.09 for both arms. It is difficult to know which one is more accurate without having an actual reference to A&E records, but, in general terms, the research nurse-recorded data by interview is considered the gold standard for research.
Family-borne costs, source: Client Service Receipt Inventory
The CSRI also asks parents/carers to report any additional costs (out of pocket) due to their child’s hospitalisation. The majority of parents reported only travel and parking costs. Appendix 4, Table 42, presents family-borne costs as reported at 6 weeks after discharge. Table 42 also shows days lost from school and days lost from work for parents/carers during the 6 weeks following discharge from hospital.
Health-related quality of life, health profiles, utility scores and quality-adjusted life-years
The EQ-5D-5L and the CHU-9D questionnaires were used to collect data at baseline, at discharge, at 2 and 6 weeks and at 3 and 6 months. Responses obtained using the EQ-5D-5L and the CHU-9D questionnaires were used to estimate utility values and QALYs using the health profiles reported for each participant. Figure 21 shows the health profiles from the EQ-5D-5L questionnaire for each dimension across time points and Table 12 states the estimated utility values from the EQ-5D-5L and the CHU-9D questionnaires, also showing that the completion rate for EQ-5D-5L was higher. Overall, a noticeable aspect from Figure 21, but mainly from Table 12 following transformation of health profiles into utility values, is that normalisation in terms of HRQoL and especially utility values was already achieved by 3 months for both groups, raising questions regarding the necessity to collect data beyond that timeframe. Regarding the EQ-5D-5L health profiles presented in Figure 21, it is clear that, although at baseline (dark blue bars), both groups reported low health values (higher values indicate more problems), at 3 months, both groups reported very similar health profiles (orange bars). The figure also shows that, at discharge (light blue bars), the appendicectomy arm participants reported lower health profiles than participants in the non-operative arm. Of note, this difference between treatment arms at hospital discharge was apparent for all dimensions assessed (mobility, self-care, usual activity, pain/discomfort and anxiety/depression). This is not surprising (following operation), but highlights the need to collect short-term data and to carefully consider the duration for which QALYs (area under the curve) will be estimated to assess cost-effectiveness.
FIGURE 21.
Quality-of-life scores (from the EQ-5D-5L) across time points.
| Timing of assessment | Treatment arm | EQ-5D-5L | CHU-9D | ||
|---|---|---|---|---|---|
| Participants, n | Mean (SD) | Participants, n | Mean (SD) | ||
| Baseline | Non-operative treatment | 28 | 0.53 (0.3) | 25 | 0.60 (0.2) |
| Appendicectomy | 28 | 0.56 (0.3) | 23 | 0.57 (0.1) | |
| Discharge | Non-operative treatment | 26 | 0.92 (0.1) | 21 | 0.90 (0.1) |
| Appendicectomy | 27 | 0.72 (0.3) | 22 | 0.69 (0.1) | |
| 2 weeks | Non-operative treatment | 13 | 0.99 (0.0) | 9 | 0.97 (0.0) |
| Appendicectomy | 15 | 0.89 (0.3) | 12 | 0.86 (0.2) | |
| 6 weeks | Non-operative treatment | 27 | 0.96 (0.1) | 24 | 0.95 (0.1) |
| Appendicectomy | 26 | 0.98 (0.0) | 23 | 0.97 (0.0) | |
| 3 months | Non-operative treatment | 27 | 0.98 (0.1) | 20 | 0.95 (0.1) |
| Appendicectomy | 27 | 0.99 (0.0) | 23 | 0.97 (0.0) | |
| 6 months | Non-operative treatment | 25 | 0.99 (0.0) | 20 | 0.97 (0.0) |
| Appendicectomy | 27 | 0.98 (0.1) | 23 | 0.97 (0.1) | |
The two instruments have produced relatively similar utility values. However, Appendix 4, Tables 43 and 44, shows the utility values at each time point for both instruments alongside the incremental (Δ) utility values between the two arms. It is worth noticing that even small differences in utility values can have a detrimental effect on estimating QALYs. This is due to the method used to calculate QALYs, taking the area under the curve approach, incorporating both duration and quality of life. As a result, even a small change in utility values over a long period of time may result in a significant effect in QALY terms. This is apparent in Table 13, in which the differences between the two arms is reported in QALY terms for both instruments; reporting 95% CIs shows that differences detected between the two arms are not statistically significant (including zero), and this is the same for both instruments. However, it is worth noticing that these are QALYs for the full duration of the study (6 months) where more than half of the time both groups are normalised and the true difference detected at discharge accounts for only a limited fraction of the time. An interesting aspect of these results is the need to consider very carefully the short- versus long-term implications in terms of QALYs.
| QoL instrument | Appendicectomy arm | Non-operative arm | Difference, mean (95% CI) | ||
|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | ||
| EQ-5D-5L | |||||
| Baseline to 6 months | 15 | 0.965 (0.04) | 13 | 0.973 (0.03) | 0.008 (–0.02 to 0.03) |
| CHU-9D | |||||
| Baseline to 6 months | 10 | 0.962 (0.02) | 8 | 0.943 (0.04) | –0.02 (–0.05 to 0.01) |
Discussion
Resource use, costs and effectiveness outcomes are an important part of any economic evaluation to determine whether or not a particular intervention is cost-effective and, therefore, better placed in terms of outcomes it generates, in comparison with standard care. In this economic substudy alongside the CONTRACT feasibility RCT, we collected and assessed alternative data collection tools for both resource use and HRQoL.
Assessment of resource use and costs data collection tools and methods
The first aim of this health economic substudy was to identify what type of costs need to be collected to assess the economic implications of the proposed intervention, to assess data collection tools and to identify sources for estimating these costs. We used treatment pathways during hospitalisation and the subsequent follow-up period for each trial treatment arm to ensure that all relevant costs were included and to define the most important cost drivers to consider in future research.
Assessing the tools used for data collection, our study shows that data of superior quality were collected by research nurses during interviews with parents/carers than those collected by CSRIs completed by parents in their own time. Our study also shows that hospital records provide a valid and extremely reliable data source when used in clinical research. Although hospital records are extremely reliable, the time requirement to obtain accurate data from them remains a restrictive factor to a great extent. However, it is thought that this approach will soon be easier as the adoption of electronic health records progresses in the majority of hospitals.
Reassuringly, the cost estimates produced using microcosting were in relative agreement with the actual costs incurred as reported at hospital level. However, they were less in agreement with the NHS reference cost data. 94 One important observation from this work is that the NHS reference cost for appendicectomy varies depending on the level of complications observed – an important factor to consider if macrocosting (NHS reference costs) is to be used. Similarly, the cost of non-operative treatment is difficult to identify using NHS reference costs (as there is no specific HRG tariff for this) and this is complicated even further when non-operative treatment is unsuccessful and is followed by appendicectomy during the same hospital admission.
Our results indicate that two costs are the main drivers of overall cost: the ward stay cost and the cost of the operation. The difference in overall cost between treatment arms confirms that a health economic analysis is an important component of any future effectiveness trial. The magnitude of difference in the total cost between the treatment arms suggests that non-operative treatment may have significant positive economic implications for the NHS. However, this is first dependent on ascertaining the clinical effectiveness of non-operative treatment compared with appendicectomy.
More generally, we have demonstrated that it is possible to use clinical data as valuable resources for health economic research. We have developed and tested the functionality of using clinical data to support research outcomes. We reviewed and assessed the quality of clinical data for this purpose. A further step forwards would be to amalgamate data derived from these sources with other patient-reported outcomes to enable economic evaluations alongside health technology assessments.
Assessment of health-related quality-of-life data collection tools and methods
Our assessment indicates that both HRQoL instruments provided similar results overall, yet the instruments produce different utility values and, potentially, the QALYs produced are not comparable. In terms of the quality of data, the EQ-5D-5L presented fewer missing values, indicating that it may be more acceptable to those completing it. The key implication from our assessment relates to the most appropriate timing of the collection of QoL data in a future trial. Although differences in QoL were observed between treatment arms in the short term (at discharge and 2 weeks), these differences had largely resolved by 6 weeks, and beyond, following randomisation. A failure to collect short-term data could result in missing differences in QoL between treatment arms that could have a significant effect in assessing utility. Similarly, there may be no added benefit of collecting QoL data beyond 6 weeks, as we would not (based on our data) anticipate any change over time, and reducing the number of time points when QoL is recorded would reduce burden to participants and cost. Therefore, our assessment highlights the need to carefully consider the timing of data collection, taking into account the short- and long-term implications of the intervention under scrutiny.
Strength and weaknesses
We consider a specific strength of this study to be the detailed identification and quantification of costs incurred in each arm in our microcosting process. Our ability to measure these costs is even more important owing to the lack of unit cost data (HRG tariff) for the non-operative treatment of acute appendicitis. Another strength of this study is the use of multiple time points for the collection of QoL data. This highlighted the crucial role that the timing of QoL data collection could play in estimating QALYs when assessing the cost-effectiveness of two treatment alternatives.
A potential weakness of the study is that the data informing our microcosting analysis used unit costs from a single participating hospital. It is known that there are differences in the costs of providing similar services between different NHS trusts because of, among other reasons, differences in contractual arrangements and estates costs. Therefore, the microcosting results may not be representative nationally for all UK hospitals. Unfortunately, we were unable to obtain financial information from all participating hospitals because of non-disclosure policies relating to commercially sensitive data (e.g. contractual cost of medications). However, our results strongly suggest that the main cost drivers identified through this study are likely to be the same for other hospitals, thereby supporting our conclusions.
Conclusions
Overall, this work provides evidence in support of an economic evaluation within a future effectiveness trial. Furthermore, we have been able to identify the most relevant and important cost data to collect and methods for their collection.
Our results show that detailed data collection from hospital records is extremely reliable when assessing secondary care interventions and confirms that research nurse-led interviews with parents are the most consistent data collection method. We have conducted a comprehensive assessment of costs of both treatment pathways and identified the main cost drivers for both treatment arms. We have also shown that the NHS reference cost data94 might not be completely accurate in cases when a new intervention is proposed, such as the non-operative treatment of appendicitis in children. This might be true in any case when no established unit cost data are available. However, for any future design of a study, these findings ought to be carefully considered against the time requirement and, hence, costs of adopting a detailed microcosting approach.
Assessing two frequently used HRQoL instruments, our results showed that the timing and duration of data collection could influence the result in terms of ‘cost per QALY’. Our findings emphasise the need to aid decision-making by reporting the short-term cost per QALY, in addition to reporting cost-effectiveness following the duration of the primary outcome. This issue might even be more important than the question of which of these two QoL instruments should be used. Our results support the use of the EQ-5D-5L, which performed slightly better than the CHU-9D. However, this should be judged against the issue of the tariff used; currently, there is no paediatric valuation available for this instrument – a subject that is beyond the scope of this report.
Therefore, we can conclude that our results highlight the need for analysts to use their judgement following appropriate justification dealing with these issues, in terms of both costs and QoL data collection methods, in the design stage of a definitive RCT as this could affect reporting the intervention as being cost-effective or not. All these findings will be integral parts of the design for the future definitive RCT, but are also extremely important in aiding decisions regarding the design of other RCTs and adding to the wider discussions in terms of methodological considerations when designing and conducting economic evaluation (assessing cost-effectiveness) alongside RCTs.
We anticipate that this will result in a more efficient (and cost-effective) data collection process as part of future clinical studies assessing clinical effectiveness and cost-effectiveness. Our results also indicate the need to remain extremely conscious of the most appropriate time point at which to collect QoL data, as this may affect the difference between considering an intervention cost-effective or not. This is an important outcome from this study. We recommend that all researchers undertaking a cost-effectiveness analysis alongside a health technology assessment consider carefully the appropriate time point at which to collect QoL data. The result of a cost-effectiveness analysis may depend crucially on this.
Chapter 7 A survey of UK-based paediatric surgeons
Introduction
A key factor that will determine the feasibility of a future effectiveness trial will be adequate interest, engagement and buy-in from specialist paediatric surgeons at centres in the UK beyond the three centres that participated in our feasibility trial. As it is likely that few UK paediatric surgeons routinely offer non-operative treatment for children with acute uncomplicated appendicitis, an understanding of their current views on non-operative treatment is likely to provide valuable information when designing a future main trial. Furthermore, an understanding of the value of our underlying research question, the position of equipoise of other surgeons and their current engagement with our proposed trial design are all important in order to design a main trial that is appealing, of interest and to which surgeons will actively recruit participants. We aimed to obtain data on these issues from UK-based paediatric surgeons in an online survey.
Methods
Questions for the online survey were drawn up following discussion among the research team and refined with particular input from a psychologist with experience in survey research (BY) in order to optimise the phrasing of questions to provide the most valuable data and to provide unambiguous responses. An online survey was created, and data were collected and managed in the REDCap electronic data capture tools121 hosted at University College London. Following design, the survey was tested by several members of the study team. The questions asked are shown in Appendix 5.
An invitation to participate in the survey was sent in early 2018 to all members of the British Association of Paediatric Surgeons (BAPS) and to consultant paediatric surgeons in the UK who are personal contacts of the study team. Trainees are not only potentially involved in the recruitment process, but are also the consultants of the future and therefore potential investigators in a main trial; therefore, we decided to include trainees in the survey. The survey remained open for completion for a 2-month period and a reminder e-mail was sent after 1 month to encourage participation.
Results were collated at the end of this period and analysed using descriptive statistics. Responses to questions are shown in the order in which they were asked. Percentages were calculated based on the number of respondents to each individual question. Free-text comments were grouped into themes for ease of reporting.
Results
Respondent characteristics
A total of 137 responses were received, of which 109 were complete (i.e. the participant answered every question). A total of 106 respondents (77%) were male; 121 respondents were UK consultant paediatric surgeons (88%) and the remaining 16 were UK-based trainees.
Responses to individual questions
Data for responses to all questions are shown graphically in Appendix 5, Figures 27–34.
Views and experiences of non-operative treatment of uncomplicated acute appendicitis
-
Question: Please indicate your level of clinical experience regarding non-operative treatment of acute uncomplicated appendicitis in children (single answer).
In their individual clinical experience of non-operative treatment for acute uncomplicated appendicitis in children, 70 (51%) respondents had never offered non-operative treatment, 55 (40%) had offered it in selective circumstances, 10 (7%) had offered non-operative treatment only within a research study and two (1%) had routinely offered non-operative treatment to children with uncomplicated acute appendicitis. Total respondents = 137.
-
Question: Please indicate your views of non-operative treatment of acute uncomplicated appendicitis in children and young people. This may be based on your reading of the literature, discussion with colleagues, clinical experience, etc. (single answer).
The most frequent response (53 respondents, 39%) was that they did not believe that non-operative treatment for acute uncomplicated appendicitis should be routinely discussed as a treatment option, but should be reserved for use only in a prospective research study. However, there was clear evidence of surgeons being willing to consider non-operative treatment: 29% were willing to consider it at parental request and 22% believed that it should be routinely discussed with parents. Only 9% responded that it should never be used. Total respondents = 137.
-
Question: Please rate how you view the strength of research evidence for the use of non-operative treatment as an alternative to appendicectomy for acute uncomplicated appendicitis in children and young people (single answer).
A total of 39% of respondents stated that they thought that the research evidence was weak or very weak, with the most popular response being ‘neither’ (39%). Only 13% felt that there was strong evidence. Total respondents = 137.
-
Question: In your opinion, how does the efficacy of non-operative treatment for acute uncomplicated appendicitis in children and young people compare to operative treatment? (single answer).
There was a clear view that appendicectomy was more effective (44%) or much more effective (15%) than non-operative treatment. Fourteen per cent responded that they were equally effective (24% were not sure). Total respondents = 137.
The following two questions were preceded by this stem: ‘Please indicate to what extent you agree or disagree with the following statements:’
-
Question: There is uncertainty as to whether non-operative treatment is as effective as operative treatment in treating children and young people with acute uncomplicated appendicitis (single answer).
A total of 58% of respondents either agreed (41%) or strongly agreed (17%) with this statement, whereas 22% either disagreed (15%) or strongly disagreed (7%). (Neither, 17%; not sure, 3%.) Total respondents = 132.
-
Question: There is currently enough evidence regarding non-operative treatment and enough uncertainty to justify a trial being performed comparing operative with non-operative treatment in children and young people (single answer).
A total of 47% of respondents either agreed (34%) or strongly agreed (13%) with this statement, whereas 26% either disagreed (20%) or strongly disagreed (6%). (Neither, 23%; not sure, 5%.) Total respondents = 134.
-
Question: Regardless of your current actual clinical practice, please indicate, by moving the slider, your preferred treatment strategy for children and young people with acute uncomplicated appendicitis.
Responses to this question were provided on a visual analogue scale with the statement ‘children should always be treated with an appendicectomy’ at one end (score 0) and the statement ‘children should always have an initial trial of antibiotics’ (score 100) at the other end, and ‘undecided’ indicated in the centre. The median score given was 17 (IQR 3–39; range 0–98).
Attitudes to, and design of, a randomised controlled trial to compare operative with non-operative treatment
-
Question: How important do you feel this research question is – is non-operative treatment of acute uncomplicated appendicitis in children and young people non-inferior to appendicectomy? (A non-inferiority trial aims to demonstrate that non-operative treatment is not worse than appendicectomy by more than a small predefined margin.)
A majority (83%) felt that it was either somewhat important (41%), very important (17%) or extremely important (15%), with only 17% stating that it was not important. Total respondents = 128.
Survey participants were then presented with a design of a hypothetical clinical trial and asked whether or not they would be willing to enrol participants in such a trial. The trial was identical to that used in the feasibility RCT reported in Chapters 2 and 3 and was described as follows:
-
Participants – children (aged 4–15 years) with a clinical ± radiological diagnosis of acute uncomplicated appendicitis.
-
Intervention – a non-operative treatment pathway composed of a minimum of 24 hours’ broad-spectrum i.v. antibiotics with clearly defined time points for clinical review and either:
-
discharge once responding with oral antibiotics to complete a 10-day course (i.v. + oral)
or
-
appendicectomy for those not responding within 48 hours.
-
-
Comparator – appendicectomy as currently practised.
-
Question: Please indicate your willingness to enrol participants in such a trial.
Fifty-one per cent of respondents stated that they would be willing to recruit; the remainder was split between undecided (25%) and unwilling (25%). Total respondents = 128.
-
Outcomes – relevant clinical and patient-centred outcomes (to be defined by ongoing work), as well as cost-effectiveness, with a minimum follow-up duration of 1 year.
The following two questions were conditional on the answer to the question above being either ‘undecided’ or ‘unwilling’.
-
Question: If you are undecided or unwilling to enrol, it would be helpful for us to understand the reasons behind this. Which one of the following best describes your reasoning for being undecided or unwilling to enrol?
Of 60 respondents who were undecided or unwilling, 24 (40%) stated that the study was not justified, 11 (18%) disagreed with the study design and 25 (42%) selected ‘other’.
-
Question: If you do not agree with the study design, please indicate how the trial may be modified in order that you would be willing to enrol. For example, this may involve modifying the patient population, the nature of the intervention or the comparator, the proposed outcomes or other reasons. Please give as much detail as possible.
For respondents who did not feel that the study was justified, comments were mainly related to a belief that non-operative treatment was not justified as a treatment of children with appendicitis. Underlying rationales included risk of missing a carcinoid tumour, risk of future cancer in the appendix, a belief that non-operative treatment either is unsafe or may harm patients, unwillingness to accept a recurrence rate of 15% and concern over antimicrobial stewardship. There was also a concern about therapeutic creep, namely the potential for clinicians to begin to use antibiotics for cases of abdominal pain without a clinical diagnosis of acute appendicitis, when in fact no treatment at all was needed.
Proposed modifications to the trial design made by respondents included the following:
-
population – increasing minimum age of participants to 10 years, to ensure a certain diagnosis of uncomplicated (as opposed to more advanced) appendicitis
-
intervention – ‘total antibiotic course is too long’, ‘intravenous antibiotic course is too short and should be 48 hours’ minimum’, ‘need a specific treatment pathway’
-
comparator – only permit laparoscopic appendicectomy
-
outcomes – should include morbidity, should include AEs, should be assessed over at least 1 year and perhaps 5 years
-
other – concern that it would not be possible to identify a population to actually recruit, lack of equipoise may hinder recruitment, consent process will be burdensome.
Finally, participants who had indicated a willingness to enrol were asked to give further detail to improve the design of the research if they felt it necessary. Although many comments were provided, there were no new comments that sought to improve the design of the study that are not included above.
Discussion
The overall aim of the survey of UK paediatric surgeons was to determine the attitudes towards non-operative treatment of acute appendicitis. This is important as the aim of the CONTRACT study was to test trial feasibility in only three centres. If non-operative treatment is to be evaluated in a future UK-wide definitive trial, then the willingness of paediatric surgeons to participate is essential. Thus, data from this survey provide important information on the feasibility of a future trial.
The first section of the survey sought information on the current experience and view of non-operative treatment of acute appendicitis. The first question related to current clinical experience. As the current standard treatment for acute appendicitis is appendicectomy, it is perhaps a little surprising that only 51% of respondents never offered non-operative treatment, and as many of 40% of respondents offered non-operative treatment in select circumstances. A total of 10% offered non-operative treatment in a research study only; presumably, these respondents were from one of the three centres taking part in the main CONTRACT trial. Whether the 40% of respondents who offered non-operative treatment in select circumstances occasionally care for children who appear to have some resolution of symptoms while waiting for their operation to take place, or whether these respondents intended to treat non-operatively, is not clear. These data are reflected in the current views of non-operative treatment, with 10% thinking that it should never be used, whereas 50% would discuss it with parents, either routinely or on parental request. A total of 40% of respondents thought that non-operative treatment should be used only in a research study.
The next section of the survey addressed the current perception of the evidence for use of non-operative treatment. Overall, the results from these questions indicated that, although respondents thought that there was some evidence to support the use of non-operative treatment, there was uncertainty regarding the efficacy. Approximately half of the respondents agreed that there was currently enough evidence to justify a trial being performed, with one-quarter disagreeing and one-quarter uncertain. Perhaps unsurprisingly, and in keeping with the responses regarding evidence base, the median score on the 0–100 visual analogue scale of ‘children should always have an appendicectomy’ versus ‘children should always have an initial trial of antibiotics’ was 17, favouring appendicectomy, although one surgeon respondent thought that all children should have an initial trial of antibiotics.
Only 17% of respondents thought that it was unimportant to answer the question ‘is non-operative treatment non-inferior to appendicectomy?’, with the rest responding that the question is at least somewhat important. Surgeons were then asked whether or not they would be potentially willing to recruit to a scenario trial of non-operative treatment compared with appendicectomy, with inclusion/exclusion criteria and clinical pathways similar to the CONTRACT feasibility trial. Of note, the primary outcome of this scenario was described as ‘relevant clinical and patient-centred outcomes (to be defined by ongoing work)’. Just over half of respondents stated that they would be willing to enrol patients to such a trial, with only 25% unwilling and the rest undecided. Of those who stated that they were unwilling to recruit to a trial, 40% did not think that such a study was justified, and a further 18% disagreed with the study design.
There are several potential biases inherent in a survey methodology that may make the results less generalisable. Usually, a key metric of a survey is the response rate, as this can indicate a potential bias: a very low response rate might be associated with only a particular subgroup responding. The link to the survey was sent out to all members of the BAPS, which, as well as active UK consultant and trainee paediatric surgeons, also includes international and retired surgeons. In addition, membership of the BAPS is not mandatory for UK consultant or trainee paediatric surgeons. It is therefore difficult to either estimate a response rate of eligible (UK consultant or trainee) BAPS members, or to accurately know the proportion of the UK paediatric surgical workforce that responded. In England, in December 2017, there were 189 consultant and 158 specialist registrars (NHS HCHS monthly workforce statistics, NHS Digital, 2018122); therefore, the 121 consultant responses represent about half of the UK consultants, whereas the 16 trainees represent a much smaller proportion of the UK trainee workforce. Thus, the responses from the study are probably reasonably representative of the UK consultant body, although the possibility cannot be excluded that those who did not respond might be those who are so against non-operative treatment that they did not want to even complete a survey on the topic.
Overall, there seems to be broad support for a definitive RCT by UK paediatric surgeons. Importantly, surgeons believe that, although there is some evidence for the use of non-operative treatment, the evidence in support of this is not strong enough to justify the routine treatment of children non-operatively. If the UK paediatric surgical community had been polarised into two groups, one group supportive of routine non-operative treatment and the other opposed to any treatment other than appendicectomy, this would have been problematic for equipoise and the conduct of a RCT. However, the data from this survey seem to suggest that there is equipoise, an important prerequisite for conducting a RCT.
The intention had been to follow this survey with some focus groups to discuss a future RCT, selecting both those surgeons who were in favour and those who were against such a trial. Although the comments on the free-text questions were useful, the study team decided, at least in part based on the discussion about primary outcomes at the COS consensus group meetings, that it would be better to postpone such a focus group until plans for a future trial were further developed, as that was a more appropriate time to engage with paediatric surgeons about the details of the trial design.
In conclusion, the survey of UK paediatric surgeons indicated that there is broad support for a future randomised trial, and the responses suggested that there is equipoise so that recruitment to such a trial could be feasible.
Chapter 8 Patient and public involvement in the CONTRACT study
Introduction
This chapter presents and discusses the patient and public involvement and engagement activity in the CONTRACT study. Chapters 2 and 3 address patient participation in the main RCT of the project, but it is important to distinguish between participation, involvement and engagement.
The CONTRACT study investigators strongly support all the arguments for PPI in research and are strong advocates for it. As a result, we considered very carefully the PPI activity before submitting the grant proposal, before it was subsequently funded. In fact, the research topic – can children be treated non-operatively for acute uncomplicated appendicitis? – had been asked of the chief investigator many times in routine clinical practice. It can therefore be argued that the research topic itself originated from patients and their families.
It was with this ethos that we planned, and carried out, an extensive programme of PPI throughout the study.
Methods
The PPI in this study took a flexible, blended approach. A parent co-investigator contributed to drafting the grant proposal, and was subsequently involved at several points throughout the research programme. We formed a SSAG of children, young people and parents. Although the involvement of that group occurred mostly in face-to-face meetings, involvement was also conducted virtually via e-mail, or in casual (e.g. in coffee shops) one-to-one meetings with individuals who were unable to attend some whole-group SSAG meetings. This enabled involvement of all those who were interested, in a way that was comfortable and acceptable to them, so that all members could contribute to the CONTRACT study. The SSAG was considered to be paramount to the successful conduct of the study by the research team, and, in fact, conceptually sat at the top of the study governance (Figure 22).
FIGURE 22.
The CONTRACT organisational chart.
Our approach incorporated all the values and principles advocated by INVOLVE, of respect, support, transparency, responsiveness, fairness of opportunity, and accountability. 123
Involved people: our study-specific advisory group
We involved 10 children and young people who, at the beginning of the study, were aged 9–18 years. Some had a history of appendicitis, and some did not. Five parents were involved as well, which represented three parent–child dyads. All parents had children who had had appendicitis. This group was the SSAG.
Children and young people were identified through the clinical connections of the chief investigator, the London Generation R Young Persons’ Advisory Group (YPAG), and a YPAG associated with the Southampton Children’s Hospital and Wellcome Trust Clinical Research Facility. For all but one child (who was aged 18 years, and with whom the PPI co-investigator had had a prior involvement relationship via the London Generation R YPAG), the parent was approached first, usually by the chief investigator. Then, following approval to share their contact details, the PPI co-investigator contacted them to explain the study, the PPI activity that was planned and to offer the parent and their child(ren) the opportunity to ask questions about the proposed involvement. All except two children subsequently joined the SSAG. The parents of those two children expressed a strong interest in their child’s involvement, but, as discussions developed, it transpired that the parent and/or child were not sufficiently available to join, or to facilitate their child joining, the SSAG.
Meetings
We planned to meet with our SSAG nine times throughout the course of the study, to correspond with times when the project most required the perspective of children, young people and parents. This meant that we met with the SSAG more frequently at the beginning and end of the study, when we most wanted the SSAG to contribute to the recruitment materials for the trial, and the dissemination materials. Actual meeting timings, topics covered and outputs are presented in Report Supplementary Material 1. We held nine meetings with our SSAG, as we had proposed at the very beginning, with our application for funding. All SSAG meetings were attended by the PPI co-investigator and the chief investigator. Other members of the study team attended meetings as required by the needs of the overall study.
Further involvement and engagement activities
One parent joined the SMG, as a representative of the SSAG. Then, following the 19 April 2017 meeting, two young people became more actively involved in the COS development work, or substudy, taking part in monthly meetings with the COS study team. The aim of this targeted PPI was to ensure that any decisions made by the SMG or COS team about the running of the study or the design of the COS work would be as acceptable as possible to children, young people and their families, in an attempt to optimise their engagement and participation. Finally, a parent–child dyad attended the study’s final results meeting on 5 October 2018, which involved presentations from all workstreams of the project, as a small engagement exercise.
Remuneration
We recognise the value that SSAG members added to the CONTRACT study, and remunerated them following guidelines (Mental Health Research Network and INVOLVE124) and experience of the PPI co-investigator (EW). Every member – regardless of whether they were a parent or child – was given a £25 Love2shop voucher (Love2shop Business Services, Liverpool, UK) following each SSAG meeting. These are vouchers that can be used in main high-street retailers. We provided catering at all meetings of the SSAG, reimbursed travel costs and offered to pay for the cost of childcare to enable parents to attend.
Impact
On the study
As demonstrated, the PPI work in this project was embedded throughout the research cycle and, we believe, made a great impact in numerous ways. The most substantial was that the SSAG helped keep the study grounded in the interests and priorities of children and young people, and parents of children and young people who have had appendicitis or whose child(ren) may develop appendicitis in the future. Although there were never any instances when the views of the study team differed wildly from those of the SSAG members, it was especially validating when views were congruent. It was revealing that the children and young people struggled to understand the concept of a COS, giving the study team an added appreciation for the complexity in communicating such a concept, and a warning that the Delphi process might not be as straightforward as anticipated. The videos used in the COS registration process, and for the COS substudy, were significantly improved by involving the SSAG in their production.
Although we did not assess this, it is possible that participants better understood the nature of the RCT and communication substudy as a result of the recruitment video and information sheets (which were improved as a result of the SSAG), and that their consent was truly and properly well informed. It is also possible that the same is true of the surgeons, parents and children and young people who participated in COS substudy.
It is the hope of the study team that the dissemination products we co-produce with members of the SSAG will be more accessible, interesting and relevant to children, young people and parents, as a direct result of their involvement.
On the researchers
The children and young people, in particular, made a profound impression on the study team, although the parent members also had an impact. The SSAG was highly enjoyable to work with and always embraced every involvement task. It was motivating and heart-warming to see the young people develop in the group – the two youngest girls (aged 11 years when they started) spoke little in the beginning and required more drawing-out to get their opinions, yet, over time, they became the first to offer their views confidently. The group worked well together, despite the members all being from different schools and of different ages. As young people often only mix with peers their own age, and as it can be intimidating for younger children to work with older children, or frustrating for older children to work with younger children, observing the children and young people of the SSAG treating each other with respect and compromising made all the hard work of organising and planning involvement activities rewarding and validating. Despite all the children, young people and parents being busy in their lives outside the study, the fact that they made time and committed to being involved was particularly rewarding to the study team, driving further motivation to enable the PPI activity to flourish. This commitment was particularly true of one parent member who returned following maternity leave, and one of the young people who returned to the group during her break from university – possibly because they found the work important and meaningful. Both the PPI co-investigator and the chief investigator further developed their skills in facilitating PPI and engagement activities; in facilitating, in particular, a mixed group of children, young people and parents, as this was a novel experience; and in how to involve children and young people in COS development.
The study team also found the experience of working with the SSAG an immensely enjoyable one from a personal perspective. Although the SSAG activity contributed in a material way to improving the study itself and optimising its acceptability to its target participants, the process of working with the SSAG was incredibly rewarding to the research team. We believe that this additional emotional motivation cannot be underestimated when considering the value that it brings to the research team.
On the children, young people and parents
In our final meeting in December 2019, we asked SSAG members to reflect on their experiences of being involved in the study, from July 2016. We asked, ‘In a couple of years from now, what I will remember is . . .’, ‘What I would have said to a younger me, back when I was joining the group . . .’ and ‘Final comments I want to share in a publication about this project . . .’. Although not all SSAG members attended, those six who did had been the most active children, young people and parents in the PPI work of the project. Anecdotal evidence, however, from e-mails and conversations in SSAG meetings, suggests that children and young people have gained confidence in themselves and in speaking in front of a group, the ability to balance and hold several viewpoints, a honed interest in medical research, improved abilities to work in a group of dissimilar individuals and knowledge about involvement in research that has been of great interest to them.
Discussion
This chapter reports on the PPI approach and activities that occurred over the course of the CONTRACT study, and those activities that are still to take place.
Our approach to involvement was framed by many compelling reasons, but mainly we believed that it is the right thing to do, based on knowledge and experience in the area. We were delighted to assemble a group of children, young people and parents who all believed passionately in the topic, and were able to make meaningful contributions to the study. As far as we are aware, our approach of assembling a large PPI group specifically for a study is novel. From the outset, we appreciated that there would be challenges in optimising engagement in the study; our approach reflects the importance we placed on understanding the challenges as fully as possible and our commitment to ‘getting the study right’. Although PPI in research studies is becoming more prevalent, frequently it involves a relatively small number of individuals. From the outset, we were committed to embedding PPI at the heart of all aspects of the study and believed that the best way to do this was to assemble a large group to bring a wide range of views over the entire course of the study. Furthermore, we are not aware of previous PPI groups that have included children and young people and parents. We identified that the overall study would benefit greatly from being informed by PPI across a range of ages. Although we have not formally measured it, we believe that assembling this large and multigenerational group has enabled us to maximise the impact of the SSAG, certainly to a much greater extent than if we had either a smaller group or a group of only adults or only children and young people.
Lessons learned
Although we had originally planned to involve children and young people from Southampton, Liverpool and London, the SSAG members were mostly based in or around Southampton. When forming the SSAG, principal investigators at all three CONTRACT clinical sites sought volunteers. However, the only volunteers who came forward were from the Southampton area. Although the representativeness and diversity of the group could be questioned as a result, the SSAG members were enthusiastic and engaged throughout. It could be argued that, in fact, we were able to develop a more intimate group dynamic, possibly due to group members’ regional proximity to each other and to the lead site, Southampton Children’s Hospital, where we held most of the SSAG meetings. It was also probably much easier to manage and co-ordinate activities this way. For our future work in this field, we will continue to attempt to achieve greater geographic diversity among PPI group members.
Keeping a group of children and young people together over 2.5 years, in a highly unique and cerebral extracurricular activity that happens at irregular time points, as young people continue to develop physically and psychosocially, can be very difficult. It requires semiregular contact, and a great deal of work on developing and maintaining interpersonal relationships. We believe that a named person who will lead on PPI activities is essential for any study such as this, to be able to develop and maintain these relationships over time. Another specific challenge of communicating with our SSAG was the need to engage in a meaningful way at an appropriate level with children, young people and adults, all of whom have different levels of understanding. During SSAG meetings, we endeavoured to use age-appropriate language for the attendees present, but were conscious of not ‘speaking down’ to adults because of a need to use language that was understandable to children and young people.
Many of the involvement tasks, for example reviewing PISs, helping to put together a recruitment video and advising on the COS Delphi process video, were fairly straightforward and easily comprehended by the children and young people. However, other aspects of the study were more difficult. For example, discussing the COS development substudy was particularly challenging. It took repeated efforts, by a few members of the study team (NJH, EW and SE), and variation in explanation of what a COS constitutes, and it is possible that a couple of the children and young people in the SSAG still did not acquire the concept.
Things we learned that worked well with the PPI in CONTRACT were maintaining regular contact with SSAG members, putting together a Doodle (Zurich, Switzerland) poll to pick future meeting dates, meeting at weekends (and once during the week, when it was the school summer holiday) and providing feedback to the SSAG members about what effect the involvement had on the study. Patients and the public are frequently dissatisfied with how little feedback and acknowledgement they are provided with, although feedback is an important component in learning and development, and in enjoyment of involvement. 125 As a result, at each meeting, we went over what had happened at the previous meeting and what suggestions the study team were able to action, and explained why suggestions were not actioned. We also repeatedly acknowledged that the group was making important contributions to the study and that members were viewed as experts on the project, and we expressed heartfelt gratitude for their involvement. We also contacted young members and parents at the same time via e-mail, so there was open and transparent communication.
Why do children and young people get involved in patient and public involvement? Is there a ‘right’ reason for involvement?
A matter of continuous reflection during the CONTRACT study was why the children, young people and parents became, and continued to be, involved. Although we cannot know for certain as we have yet to undertake our reflective exercise with the group, Johannesen126 can provide some compelling clues. These are gratitude, flattery, access, quest for answers, community, altruism/charity and agents for change. It is possible that reasons for involvement can change over the course of a project.
With so much concern over safeguarding and protecting children and young people, it can become easy to worry about children and young people having the ‘right’ reason(s) for involvement. In the past, with facilitating one child and young person involvement group for 2.5 years, it started to feel to the PPI co-investigator that some of the members attended meetings only for the vouchers received as remuneration. Even though it was not a large amount of money, £25 can be a significant amount to a child or young person. A couple of members of that group were also the children of members of staff whom the group was associated with, which called into question whether the children and young people themselves had a genuine interest in the group or whether it was an activity that the parents wanted their children to do. Although we do not have the sense that these reasons are the reasons members of our SSAG became involved or continued their involvement, we plan to explore the reflective experiences of the SSAG at a final meeting, with the aim of enhancing our future PPI activity.
It is possible that some members of the SSAG joined the group out of a sense of gratitude or duty to the chief investigator – a surgeon who treated some of the children and young people – and the PPI co-investigator – who had a previous PPI relationship with one member – as these were the individuals who recruited members to the group. However, this is an approach advocated for by the NIHR Research Design Service London. 127 The fact that all members continued their involvement over a period of 2.5 years may indicate that they joined for appropriate reasons of genuinely wanting to contribute in an involvement capacity. Moreover, the chief investigator and PPI co-investigator consciously worked to reduce any perceived power imbalances during meetings and to ensure that all members of the SSAG were not only valued, but each valued equally.
Finally, it could also be argued that, no matter what the reason for joining a study-specific involvement group of this nature, as long as people do join, continue to contribute and are meaningfully involved, then the reason does not matter. The involvement is what matters, as this very involvement enhances the research.
Conclusion
We took a multimethod, flexible approach to involving children and young people who had, and some who had not had, appendicitis, plus four parents, through a SSAG. The full breadth of the CONTRACT study benefited from this extensive programme of PPI. The group continued to be involved throughout the study, which enabled it to have the greatest impact. There remain future plans for co-producing child-, young person- and parent-friendly and accessible dissemination outputs regarding the research undertaken in CONTRACT, as well as the PPI workstream itself.
Chapter 9 Discussion
The overall aim of the CONTRACT feasibility study was to determine whether or not it is feasible and acceptable to conduct a future multicentre RCT in the UK to test the effectiveness of a non-operative treatment pathway for the treatment of acute uncomplicated appendicitis in children. In this overall aim, there were several specific objectives, each of which was designed to determine an aspect of feasibility of a future trial and to inform trial design. In addition, each objective would engage as far as possible with patients and parents, and also lead to outputs that would be informative to clinicians, researchers and health-care providers.
Although there are many important findings arising from this study that will need to be taken in consideration when designing a future multicentre study, outcomes from each of the objectives support the feasibility of a UK-based effectiveness trial.
Feasibility of recruitment to a future multicentre trial
Our successful recruitment to the feasibility RCT across three separate centres from geographically distinct areas suggests, first, that paediatric surgeons are willing to consider non-operative treatment and to discuss a RCT with parents and children and, second, that parents and children are willing to be enrolled in a RCT comparing appendicectomy with non-operative treatment. Our overall recruitment rate of those approached was 50%, which, of note, was at the upper end of our target range, a range that we believe was already an ambitious one. We therefore believe that, from the patient and parents’ perspective, there is adequate willingness to proceed to a full effectiveness trial. Our high consent rate also suggests that surgeons are willing to recruit patients to such a RCT. At the outset of our study, we were particularly concerned that this might not be the case, given that the majority of surgeons involved typically treat children with appendicitis with appendicectomy. However, we have clearly demonstrated that, with adequate education and training, surgeons will approach potential participants and subsequently successfully recruit them. Importantly, we have demonstrated that recruitment can take place out of hours and at weekends, an important consideration given the timing of presentation of children with acute appendicitis. We recognise that recruitment at three selected centres does not necessarily translate into successful recruitment across the large number of centres, which would be required in a future effectiveness trial. It is true that the three participating centres in our feasibility RCT were selected on the basis of their interest in the research; surgeons at other centres may not consider the research question to be of such importance. Nevertheless, at least 20 surgeons across the three centres successfully enrolled patients and data from our survey of UK paediatric surgeons suggest that there is adequate interest in the research question to proceed with a larger effectiveness trial. Furthermore, our detailed learning around how to train surgeons to recruit to this challenging trial reassures us that, so long as there is adequate interest in the research question at a centre, we will be able to train local surgical teams to recruit successfully.
In this study we have successfully identified strategies to optimise communication between recruiting surgeons and patients and their families. These strategies will be invaluable in the design and delivery of a future effectiveness trial. Having started with communication strategies based on experience and the best-available evidence from other contexts, we are now armed with much more detailed and relevant information that we can provide to recruiting teams to guide their practice with confidence. This can be delivered in a number of forms, including face-to-face training, written training information, role-play-type scenarios and the provision of a recruitment ‘hints and tips’ document. The ‘hints and tips’ document can be kept locally and made accessible to recruiting teams 24 hours a day so that they can remind themselves of the optimal recruitment strategy immediately prior to discussion with potential participants and their families. With all of these strategies and resources, we believe that we are well placed to proceed with a multicentre effectiveness trial, even among centres with limited recruiting experience.
An additional source of valuable information has been the interviews held with a wide range of stakeholders, including participants and non-participants and their families, surgeons and nursing staff at all participating sites. We have learned valuable lessons regarding the design of a future trial: in particular, how to make it as acceptable as possible to potential participants, as well as maximising compliance with treatment pathways and follow-up.
Our work to develop a COS for treatments of uncomplicated acute appendicitis in children and young people, which involved paediatric and general surgeons, young people and parents as stakeholder groups, successfully identified 14 outcomes that we put forward for use by all researchers in this field. Although further work is required to clarify how and when some of these outcomes should be measured, we intend to measure and report all of these in a future RCT. Contact with surgeons, young people and parents as part of the COS study provided us with opportunities to gather important information from different stakeholder groups about the choice of primary outcome for a future trial. Clearly, the identification of a primary outcome is extremely important, as it will determine the sample size requirement for a future trial and, thus, whether or not a future trial is indeed feasible as far as number of available participants is concerned.
As discussed earlier, a key determinant of future trial success is the expertise and willingness of an adequate number of UK paediatric surgeons to participate. Although there was not unanimous support from surgeons surveyed for performing an effectiveness trial, we believe that there is adequate support to proceed. Importantly, nearly 60% of the surgeons who responded to our survey strongly agreed that there was uncertainty as to whether or not non-operative treatment is as effective as operative treatment for children and young people with uncomplicated acute appendicitis, and nearly 50% agreed or strongly agreed that there was enough evidence to justify a trial being performed. When presented with a trial vignette similar to this feasibility trial, just over 50% indicated willingness to involve participants in such a trial. Of the remainder, half were unwilling and half were undecided. Taking these survey findings together with our detailed understanding of how to educate and train recruiting surgeons, we believe that a future effectiveness trial in the UK is deliverable.
Remaining areas of uncertainty
Despite the work we have undertaken, there remain several areas of uncertainty regarding the design of a future trial. Key areas of uncertainty are the primary outcome and effect size. Although we have consulted with a variety of stakeholders regarding these areas, it is clear that further consideration is necessary to finalise the nature of the primary outcome and, as a result, the effect size to be investigated. In our discussions with patients and families, and surgeons thus far, there appears to be a divergence of views between these groups regarding a preferred primary outcome. Although this is not necessarily unexpected, we are surprised that it is the patients and families who preferred disease-specific measures such as antibiotic failure, recurrent appendicitis and surgical complications, whereas the surgeons were attracted to a more generic patient-reported outcome: time to return to normal activities. Given the differing views of these key stakeholders regarding the optimal primary outcome for a future effectiveness trial, it is possible that further work with the stakeholders may not, in itself, be fruitful. It is therefore important that the future research team (including adequate PPI representation) considers the findings to date when reaching a decision on this. Although a single primary outcome would be highly desirable, a number of alternatives exist that may be achievable. These include the concept of a co-primary outcome (for which there is precedent in research funded by the Health Technology Assessment programme), particularly two outcomes, of particular interest to two different stakeholder groups, that can be measured side by side, or a composite primary outcome that is carefully designed and defined such that it is clinically unambiguous and statistically valid. Alongside a decision relating to the primary outcome is a decision relating to the effect size. It is probably not helpful to have a full discussion about how large an effect size should be until a primary outcome is determined. This is at least in part because the nature of the primary outcome will determine whether a future trial should be designed as a superiority trial or a non-inferiority trial. The work we have done with surgeons suggests that if a non-inferiority design is utilised, then a margin of between 10% and 20% non-inferiority would probably be acceptable.
We believe that the most essential aspect that requires optimisation before we can proceed to a full effectiveness trial is improved identification of a target population of interest, namely children with uncomplicated, as opposed to more advanced, acute appendicitis. Despite our best intentions and efforts in our feasibility trial, our pathway of using surgeons’ clinical skill to successfully identify children with uncomplicated acute appendicitis, as opposed to those with more advanced disease, was not as successful as we had anticipated. As a result, a higher proportion of children with more advanced acute appendicitis than had been intended were recruited to this trial. Although this has not been to the detriment of our overall feasibility findings or of the safety of trial participants, we believe that this is closely linked to the fact that the outcomes of non-operative treatment that we have observed in trial participants are not as good as those reported in the existing literature. 16 Therefore, it is essential that a pathway that enables better identification of children who definitely have uncomplicated (as opposed to more advanced) acute appendicitis can be identified. 128 There is no doubt that surgeons’ willingness to recruit to a future RCT depends on this, as surgeons from all three centres that participated in the feasibility trial indicated that they would require a greater degree of certainty that participants did have uncomplicated (as opposed to more advanced) acute appendicitis before they would be willing to recruit to an effectiveness trial.
A further consideration that follows from this is that we may not be able to rely solely on the overall clinical outcomes achieved in this study to provide data from which we can determine the magnitude of difference in important outcomes between treatment arms. This is because the target population for our future effectiveness trial will be different from the population that we have actually recruited to this feasibility trial (i.e. a future effectiveness trial will comprise a lower proportion of children with complicated appendicitis). We are likely, therefore, to include other existing literature comparing these treatment arms or anticipated meaningful effect sizes when determining future trial sample size in the first instance.
Patient and public involvement
One aspect of this feasibility study that has been extremely successful and of which we are extremely proud has been involvement of patient and public representatives through our SSAG. The full extent and breadth of this work is described in Chapter 8. There is no doubt that this group contributed enormously to the successful delivery of this complete feasibility study. We are extremely grateful to all members of the SSAG for their time, courage and enthusiasm, and we recommend this approach to other researchers in the future. Parents and young people were also key contributors to other aspects of the feasibility study, namely the COS and the qualitative research study. In our view, the dedicated involvement of a group of people who have experience of the condition of interest has provided us with the information and confidence to successfully deliver this feasibility study.
Other ongoing work in this field
As we consider developing our research programme into a multicentre effectiveness trial, it is important to consider other ongoing work in this field, particularly any work that has been completed since initiation of our feasibility study that may also be seeking to answer an identical or similar research question. Although there have been some recent reports of non-operative treatment in children with uncomplicated acute appendicitis,19,129–135 no trials have reported data beyond the pilot trial13 described previously. However, we are aware of a number of ongoing randomised trials comparing appendicectomy with non-operative treatment for children with uncomplicated acute appendicitis, none of which has yet reported its outcomes. 36,136 Therefore, uncertainty remains as to the relative efficacy of these two treatment options for this group of children. It is likely, however, that these trials will report their findings before we would be able to complete a multicentre effectiveness trial in the UK. Therefore, it is prudent to ask the question of whether or not a UK-wide effectiveness trial remains justified. To answer this, we must consider the setting of the trials currently recruiting, as well as their designs in relation to our proposed effectiveness trial. A summary of currently recruiting trials is presented in Table 14.
| Lead investigatora | Registration number | Setting | Protocol published | Sample size (n) | Summary of diagnostic criteria | Age of participants (years) | Estimated completion date | Primary outcome |
|---|---|---|---|---|---|---|---|---|
| St Peter | NCT02687464137 | International | Yes36 | 978 | Clinical and/or radiological diagnosis | 5–16 | January 2020 | Treatment failureb |
| Adams | NCT02795793138 | Australia | Yes136 | 226 | Clinical and/or radiological diagnosis | 5–16 | December 2019 | Unplanned or unnecessary operation or complication |
| Gorter | NCT02848820139 | The Netherlands | Yes140 | 334 | Radiological | 7–17 | December 2020 | Complications |
| Fisher | NCT02991937141 | The USA | No | 190 | Radiological | 6–17 | May 2020 | PedsQL |
Although all these trials are comparing non-operative treatment with appendicectomy for children with uncomplicated acute appendicitis, the precise diagnostic and inclusion criteria for each are all slightly different. Two trials139,141 require a radiological diagnosis of uncomplicated acute appendicitis for inclusion, and also exclude cases with inflammatory markers above a certain threshold. In contrast, the other two trials137,138 do not necessarily require a radiological diagnosis, nor do they specify any specific laboratory-based exclusion criteria. However, the St Peter trial137 has selected centres with a low (≈2%) negative appendicectomy rate only. The relevance of these differences is that the population each trial will recruit is likely to be different from the population that could be recruited in any future trial in the UK. In particular, the lack of routine imaging in the UK probably means that the negative appendicectomy rate in a population recruited to a trial in which diagnostic imaging is routine is lower than could be achieved in a UK-based trial. This will inevitably have implications for the efficacy of non-operative treatment. Furthermore, it is not clear whether or not the outcomes selected as primary outcomes are relevant to the UK population and NHS setting. Our work regarding outcomes to date suggests that outcomes other than those reported in these trials are important to UK stakeholders. Although some trials intend to report limited measures of the cost of health-care delivery, it is inevitable that these will not be relevant to the NHS. As a result of our feasibility work around a cost-effectiveness analysis, we are now well placed to design such an analysis alongside an assessment of clinical effectiveness. A UK-based trial is the only way to meaningfully undertake these tasks, in parallel generating data that have the ability to inform practice in the NHS and the UK health-care system. As a result, a UK-based trial remains highly relevant. Although trials that are currently recruiting will undoubtedly provide some data regarding the relative efficacy of non-operative treatment and appendicectomy in this population of children, their findings are likely not to be directly relevant to the UK setting.
Future work
We are currently performing an analysis of all children who were screened for entry into the CONTRACT feasibility RCT at the three participating sites and using these data to develop a method for identifying children with uncomplicated acute appendicitis that is more reliable than previous methods. 128 We anticipate this to be in the form of a scoring system that, crucially, will be applied not to determine whether or not a child has appendicitis (as is the case with some existing scoring systems, such as the Alvarado score or paediatric appendicitis score), but after a clinical decision has been made that the child has appendicitis. Its application at this point will be to determine whether appendicitis in this particular child is likely to be uncomplicated or more advanced. We anticipate, based on our initial analysis of data, that a useful scoring system can be developed: one that has adequate test sensitivity and specificity for the purposes of recruitment to a future effectiveness trial. This work is outside this CONTRACT feasibility study and will be reported separately. 128 Having established a more robust mechanism, we believe that we will then be in a position to proceed to an effectiveness trial.
Regarding the identification of an appropriate primary outcome, we intend to consult further with recruiting surgeons and patient representatives as we move forward to design a multicentre effectiveness trial. We will use the data collected in our work thus far to arrive at a relevant and meaningful primary outcome. We are dedicated to ensuring that a future trial is relevant to the full range of stakeholder groups, so that trial results are optimally placed to inform future decision-making and clinical practice. It is likely that this work will be undertaken during future trial design stage in focus group settings in order to reach agreement in an efficient way.
As a result, we intend to design our effectiveness trial with an internal pilot phase including clear ‘stop/go’ criteria. Criteria determining progression beyond the initial pilot phase will be based on adequate centre participation and adequate recruitment rate, and our ability to record primary and important secondary outcomes effectively. This will also allow us the opportunity to perform an interim analysis of the outcome data after the internal pilot phase for the purposes of confirming assumptions about effect size in relation to the primary outcome, with subsequent sample size adjustment should it be necessary.
Chapter 10 Conclusions
We believe that, in the CONTRACT feasibility study, we have demonstrated that it is feasible and acceptable to recruit to a RCT of non-operative treatment versus appendicectomy for the treatment of uncomplicated acute appendicitis in children. We have generated key data to support the design and implementation of such a trial.
Acknowledgements
The authors would like to acknowledge the key role played by the following:
-
all patients volunteering for the study
-
all those who contributed to our PPI group – the SSAG
-
all NHS trusts hosting the study
-
all surgeon and lay participants who attended the COS consensus meetings
-
Sam Tobin and Olivia Diggle (Psychology Interns at the University of Liverpool) for their help in drafting the dissemination material for the COS and communication study workstreams
-
Mr Ben Allin at the National Perinatal Epidemiology Unit, University of Oxford, for his assistance with designing and running the software for the COS development
-
Julia Andrews and Rose Watanabe (University of Bristol) for their support with the administration of the communication study
-
Gail Moors (University of Liverpool) for her help with the administration of the COS consensus meetings.
The authors received support from the NIHR Clinical Research Network.
Sponsor
University Hospital Southampton NHS Foundation Trust.
Data Monitoring and Safety Committee members
Professor John Gregory (chairperson), Mr Iain Yardley, Dr Nick Brown and Mr Stewart Cleeve.
Trial Steering Committee members
Mr Thomas Pinkney (chairperson), Mr David Gillespie, Dr Jonathan Grigg, Mr Jonathan Sutcliffe and Mrs Sam Lansdowne (lay representative).
Contributions of authors
Nigel J Hall (https://orcid.org/0000-0001-8570-9374) was involved in the design of the project, contributed to the communication substudy and health economic analysis, conducted the PPI aspects of the project and was responsible for study management, recruiting participants and writing the final report.
Frances C Sherratt (https://orcid.org/0000-0003-4147-9305) carried out the communication substudy and was responsible for the COS development, study management, recruiting participants and writing the final report.
Simon Eaton (https://orcid.org/0000-0003-0892-9204) was involved in the design of the project and was responsible for the COS development, study management and writing the final report.
Isabel Reading (https://orcid.org/0000-0002-1457-6532) was involved in the design of the project and was responsible for study management and writing the final report.
Erin Walker (https://orcid.org/0000-0003-2871-2547) was involved in the design of the project, conducted the PPI aspects of the project and was responsible for the COS development, study management and writing the final report.
Maria Chorozoglou (https://orcid.org/0000-0001-5070-4653) was involved in the design of the project, carried out the health economic analysis and was responsible for writing the final report.
Lucy Beasant (https://orcid.org/0000-0002-4279-5644) carried out the communication substudy and was responsible for recruiting participants and writing the final report.
Wendy Wood (https://orcid.org/0000-0001-9062-4455) was responsible for study management.
Michael Stanton (https://orcid.org/0000-0003-1130-9778), Harriet J Corbett (https://orcid.org/0000-0002-5470-1457) and Dean Rex (https://orcid.org/0000-0002-9040-1662) were responsible for recruiting participants.
Natalie Hutchings (https://orcid.org/0000-0001-9381-2750) and Elizabeth Dixon (https://orcid.org/0000-0002-9876-9533) were responsible for study management.
Simon Grist (https://orcid.org/0000-0001-7529-5665) was involved in the design of the project, conducted the PPI aspects of the project and was responsible for study management.
William van’t Hoff (https://orcid.org/0000-0003-4487-5530) was involved in the design of the project.
Esther Crawley (https://orcid.org/0000-0002-2521-0747) carried out the communication substudy and was responsible for writing the final report.
Jane Blazeby (https://orcid.org/0000-0002-3354-3330) was involved in the design of the project and COS work, she also provided advice and support for the chief investigator during the study.
Bridget Young (https://orcid.org/0000-0001-6041-9901) was involved in the design of the project, carried out the communication substudy and was responsible for the COS development, study management and writing the final report.
All authors reviewed the final report.
Publications
Sherratt FC, Eaton S, Walker E, Beasant L, Blazeby JM, Young B, et al. Development of a core outcome set to determine the overall treatment success of acute uncomplicated appendicitis in children: a study protocol. BMJ Paediatr Open 2017;1:e000151.
Chorozoglou M, Reading I, Eaton S, Hutchings N, Hall NJ. Health economics and quality of life in a feasibility RCT of paediatric acute appendicitis: a protocol study. BMJ Paediatr Open 2018;2:e000347.
Hutchings N, Wood W, Reading I, Walker E, Blazeby JM, van’t Hoff W, et al. CONTRACT Study – CONservative TReatment of Appendicitis in Children (feasibility): study protocol for a randomised controlled Trial. Trials 2018;19:153.
Sherratt FC, Allin BSR, Kirkham JJ, Walker E, Young B, Wood W, et al. Core outcome set for uncomplicated acute appendicitis in children and young people. Br J Surg 2020;107:1013–22.
Sherratt FC, Beasant L, Crawley EM, Hall NJ, Young B. Enhancing communication, informed consent and recruitment in a paediatric urgent care surgical trial: a qualitative study. BMC Pediatr 2020;20:140.
Data-sharing statement
Data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- St Peter SD, Tsao K, Spilde TL, Holcomb GW, Sharp SW, Murphy JP, et al. Single daily dosing ceftriaxone and metronidazole vs standard triple antibiotic regimen for perforated appendicitis in children: a prospective randomized trial. J Pediatr Surg 2008;43:981-5. https://doi.org/10.1016/j.jpedsurg.2008.02.018.
- Bowers WF, Hughes CW, Bonilla KB. The treatment of acute appendicitis under suboptimal conditions. U S Armed Forces Med J 1958;9:1545-57.
- Varadhan KK, Neal KR, Lobo DN. Safety and efficacy of antibiotics compared with appendicectomy for treatment of uncomplicated acute appendicitis: meta-analysis of randomised controlled trials. BMJ 2012;344. https://doi.org/10.1136/bmj.e2156.
- Vons C, Barry C, Maitre S, Pautrat K, Leconte M, Costaglioli B, et al. Amoxicillin plus clavulanic acid versus appendicectomy for treatment of acute uncomplicated appendicitis: an open-label, non-inferiority, randomised controlled trial. Lancet 2011;377:1573-9. https://doi.org/10.1016/S0140-6736(11)60410-8.
- Styrud J, Eriksson S, Nilsson I, Ahlberg G, Haapaniemi S, Neovius G, et al. Appendectomy versus antibiotic treatment in acute appendicitis. a prospective multicenter randomized controlled trial. World J Surg 2006;30:1033-7. https://doi.org/10.1007/s00268-005-0304-6.
- Hansson J, Körner U, Khorram-Manesh A, Solberg A, Lundholm K. Randomized clinical trial of antibiotic therapy versus appendicectomy as primary treatment of acute appendicitis in unselected patients. Br J Surg 2009;96:473-81. https://doi.org/10.1002/bjs.6482.
- Eriksson S, Granström L. Randomized controlled trial of appendicectomy versus antibiotic therapy for acute appendicitis. Br J Surg 1995;82:166-9. https://doi.org/10.1002/bjs.1800820207.
- Mason RJ. Surgery for appendicitis: is it necessary?. Surg Infect 2008;9:481-8. https://doi.org/10.1089/sur.2007.079.
- Ansaloni L, Catena F, Coccolini F, Ercolani G, Gazzotti F, Pasqualini E, et al. Surgery versus conservative antibiotic treatment in acute appendicitis: a systematic review and meta-analysis of randomized controlled trials. Dig Surg 2011;28:210-21. https://doi.org/10.1159/000324595.
- Liu K, Fogg L. Use of antibiotics alone for treatment of uncomplicated acute appendicitis: a systematic review and meta-analysis. Surgery 2011;150:673-83. https://doi.org/10.1016/j.surg.2011.08.018.
- Minneci PC, Sulkowski JP, Nacion KM, Mahida JB, Cooper JN, Moss RL, et al. Feasibility of a nonoperative management strategy for uncomplicated acute appendicitis in children. J Am Coll Surg 2014;219:272-9. https://doi.org/10.1016/j.jamcollsurg.2014.02.031.
- Gorter RR, van der Lee JH, Cense HA, Kneepkens CM, Wijnen MH, In ‘t Hof KH, et al. Initial antibiotic treatment for acute simple appendicitis in children is safe: short-term results from a multicenter, prospective cohort study. Surgery 2015;157:916-23. https://doi.org/10.1016/j.surg.2015.01.008.
- Svensson JF, Patkova B, Almström M, Naji H, Hall NJ, Eaton S, et al. Nonoperative treatment with antibiotics versus surgery for acute nonperforated appendicitis in children: a pilot randomized controlled trial. Ann Surg 2015;261:67-71. https://doi.org/10.1097/SLA.0000000000000835.
- Barsness KA, Bensard DD, Partrick DA, Calkins CM, Hendrickson RJ, Banerjee A, et al. IL-1beta induces an exaggerated pro- and anti-inflammatory response in peritoneal macrophages of children compared with adults. Pediatr Surg Int 2004;20:238-42. https://doi.org/10.1007/s00383-003-1118-y.
- Barsness KA, Bensard DD, Partrick DA, Calkins CM, Hendrickson RJ, McIntyre RC. Endotoxin induces an exaggerated interleukin-10 response in peritoneal macrophages of children compared with adults. J Pediatr Surg 2004;39:912-15. https://doi.org/10.1016/j.jpedsurg.2004.02.009.
- Georgiou R, Eaton S, Stanton MP, Pierro A, Hall NJ. Efficacy and safety of nonoperative treatment for acute appendicitis: a meta-analysis. Pediatrics 2017;139. https://doi.org/10.1542/peds.2016-3003.
- Abeş M, Petik B, Kazil S. Nonoperative treatment of acute appendicitis in children. J Pediatr Surg 2007;42:1439-42. https://doi.org/10.1016/j.jpedsurg.2007.03.049.
- Armstrong J, Merritt N, Jones S, Scott L, Butter A. Non-operative management of early, acute appendicitis in children: is it safe and effective?. J Pediatr Surg 2014;49:782-5. https://doi.org/https://doi.org/10.1016/j.jpedsurg.2014.02.071.
- Gorter RR, van der Lee JH, Heijsters FACJ, Cense HA, Bakx R, Kneepkens CMF, et al. Outcome of initially nonoperative treatment for acute simple appendicitis in children. J Pediatr Surg 2018;53:1849-54. https://doi.org/10.1016/j.jpedsurg.2017.12.012.
- Steiner Z, Buklan G, Stackievicz R, Gutermacher M, Erez I. A role for conservative antibiotic treatment in early appendicitis in children. J Pediatr Surg 2015;50:1566-8. https://doi.org/10.1016/j.jpedsurg.2015.04.008.
- Alvarado A. A practical score for the early diagnosis of acute appendicitis. Ann Emerg Med 1986;15:557-64. https://doi.org/10.1016/S0196-0644(86)80993-3.
- Hutchings N, Wood W, Reading I, Walker E, Blazeby JM, van’t Hoff W, et al. CONTRACT Study – CONservative TReatment of Appendicitis in Children (feasibility): study protocol for a randomised controlled Trial. Trials 2018;19. https://doi.org/10.1186/s13063-018-2520-z.
- Equator Network . SPIRIT Extension for Trials in Child Health: SPIRIT-C. n.d. www.equator-network.org/library/reporting-guidelines-under-development/reporting-guidelines-under-development-for-clinical-trials-protocols/#35 (accessed 14 February 2020).
- Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ Open 2010;340. https://doi.org/10.1136/bmj.c332.
- von Niederhäusern B, Saccilotto R, Schädelin S, Ziesenitz V, Benkert P, Decker ML, et al. Validity of mobile electronic data capture in clinical studies: a pilot study in a pediatric population. BMC Med Res Methodol 2017;17. https://doi.org/10.1186/s12874-017-0438-x.
- Singer E, Ye C. The use and effects of incentives in surveys. Ann Am Acad Political Soc Sci 2013;645:112-41. https://doi.org/10.1177/0002716212458082.
- Edwards PJ, Roberts I, Clarke MJ, Diguiseppi C, Wentz R, Kwan I, et al. Methods to increase response to postal and electronic questionnaires. Cochrane Database Syst Rev 2009;3. https://doi.org/10.1002/14651858.MR000008.pub4.
- Brueton VC, Tierney J, Stenning S, Harding S, Meredith S, Nazareth I, et al. Strategies to improve retention in randomised trials. Cochrane Database Syst Rev 2013;12. https://doi.org/10.1002/14651858.MR000032.pub2.
- Minneci PC, Mahida JB, Lodwick DL, Sulkowski JP, Nacion KM, Cooper JN, et al. Effectiveness of patient choice in nonoperative vs surgical management of pediatric uncomplicated acute appendicitis. JAMA Surg 2016;151:408-15. https://doi.org/10.1001/jamasurg.2015.4534.
- Hartwich J, Luks FI, Watson-Smith D, Kurkchubasche AG, Muratore CS, Wills HE, et al. Nonoperative treatment of acute appendicitis in children: a feasibility study. J Pediatr Surg 2016;51:111-16. https://doi.org/10.1016/j.jpedsurg.2015.10.024.
- Tiboni S, Bhangu A, Hall NJ. Paediatric Surgery Trainees Research Network and the National Surgical Research Collaborative . Outcome of appendicectomy in children performed in paediatric surgery units compared with general surgery units. Br J Surg 2014;101:707-14. https://doi.org/10.1002/bjs.9455.
- Chapman SJ, Shelton B, Mahmood H, Fitzgerald JE, Harrison EM, Bhangu A. Discontinuation and non-publication of surgical randomised controlled trials: observational study. BMJ 2014;349. https://doi.org/10.1136/bmj.g6870.
- Sully BG, Julious SA, Nicholl J. A reinvestigation of recruitment to randomised, controlled, multicenter trials: a review of trials funded by two UK funding agencies. Trials 2013;14. https://doi.org/10.1186/1745-6215-14-166.
- Cook JA. The challenges faced in the design, conduct and analysis of surgical randomised controlled trials. Trials 2009;10. https://doi.org/10.1186/1745-6215-10-9.
- Rosenthal R, Kasenda B, Dell-Kuster S, von Elm E, You J, Blümle A, et al. Completion and publication rates of randomized controlled trials in surgery: an empirical study. Ann Surg 2015;262:68-73. https://doi.org/10.1097/SLA.0000000000000810.
- Hall NJ, Eaton S, Abbo O, Arnaud AP, Beaudin M, Brindle M, et al. Appendectomy versus non-operative treatment for acute uncomplicated appendicitis in children: study protocol for a multicentre, open-label, non-inferiority, randomised controlled trial. BMJ Paediatr Open 2017;1. https://doi.org/10.1136/bmjpo-2017-000028.
- Caldwell PHY, Murphy SB, Butow PN, Craig JC. Clinical trials in children. Lancet 2004;364:803-11. https://doi.org/10.1016/S0140-6736(04)16942-0.
- Nuffield Council on Bioethics . Children and Clinical Research: Ethical Issues 2015.
- Rowlands C, Rooshenas L, Fairhurst K, Rees J, Gamble C, Blazeby JM. Detailed systematic analysis of recruitment strategies in randomised controlled trials in patients with an unscheduled admission to hospital. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2017-018581.
- Fletcher B, Gheorghe A, Moore D, Wilson S, Damery S. Improving the recruitment activity of clinicians in randomised controlled trials: a systematic review. BMJ Open 2012;2. https://doi.org/10.1136/bmjopen-2011-000496.
- Blazeby JM. Recruiting patients into randomized clinical trials in surgery. Br J Surg 2012;99:307-8. https://doi.org/10.1002/bjs.7818.
- Donovan J, Mills N, Smith M, Brindle L, Jacoby A, Peters T, et al. Quality improvement report: improving design and conduct of randomised trials by embedding them in qualitative research: ProtecT (prostate testing for cancer and treatment) study. Commentary: presenting unbiased information to patients can be difficult. BMJ 2002;325:766-70. https://doi.org/10.1136/bmj.325.7367.766.
- Mills N, Blazeby JM, Hamdy FC, Neal DE, Campbell B, Wilson C, et al. Training recruiters to randomized trials to facilitate recruitment and informed consent by exploring patients’ treatment preferences. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-323.
- Mills N, Donovan JL, Wade J, Hamdy FC, Neal DE, Lane JA. Exploring treatment preferences facilitated recruitment to randomized controlled trials. J Clin Epidemiol 2011;64:1127-36. https://doi.org/10.1016/j.jclinepi.2010.12.017.
- Wade J, Donovan JL, Lane JA, Neal DE, Hamdy FC. It’s not just what you say, it’s also how you say it: opening the ‘black box’ of informed consent appointments in randomised controlled trials. Soc Sci Med 2009;68:2018-28. https://doi.org/10.1016/j.socscimed.2009.02.023.
- Donovan JL, Paramasivan S, de Salis I, Toerien M. Clear obstacles and hidden challenges: understanding recruiter perspectives in six pragmatic randomised controlled trials. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-5.
- Salmon P, Mendick N, Young B. Integrative qualitative communication analysis of consultation and patient and practitioner perspectives: towards a theory of authentic caring in clinical relationships. Patient Educ Couns 2011;82:448-54. https://doi.org/10.1016/j.pec.2010.10.017.
- Fusch PI, Ness LR. Are we there yet? Data saturation in qualitative research. Qualitative Report 2015;20:1408-16.
- Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res 2005;15:1277-88. https://doi.org/10.1177/1049732305276687.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Mays N, Pope C. Qualitative research in health care. Assessing quality in qualitative research. BMJ 2000;320:50-2. https://doi.org/10.1136/bmj.320.7226.50.
- Ministry of Housing, Communities and Local Government . English Indices of Deprivation 2015 2015. www.gov.uk/government/statistics/english-indices-of-deprivation-2015 (accessed 16 August 2018).
- Donovan JL, Rooshenas L, Jepson M, Elliott D, Wade J, Avery K, et al. Optimising recruitment and informed consent in randomised controlled trials: the development and implementation of the Quintet Recruitment Intervention (QRI). Trials 2016;17. https://doi.org/10.1186/s13063-016-1391-4.
- Elliott D, Husbands S, Hamdy FC, Holmberg L, Donovan JL. Understanding and improving recruitment to randomised controlled trials: qualitative research approaches. Eur Urol 2017;72:789-98. https://doi.org/10.1016/j.eururo.2017.04.036.
- Rooshenas L. Bluebelle Study Group . Bluebelle study (phase A): a mixed-methods feasibility study to inform an RCT of surgical wound dressing strategies. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-012635.
- Crawley E, Mills N, Beasant L, Johnson D, Collin SM, Deans Z, et al. The feasibility and acceptability of conducting a trial of specialist medical care and the Lightning Process in children with chronic fatigue syndrome: feasibility randomized controlled trial (SMILE study). Trials 2013;14. https://doi.org/10.1186/1745-6215-14-415.
- Stein RC, Dunn JA, Bartlett JM, Campbell AF, Marshall A, Hall P, et al. OPTIMA prelim: a randomised feasibility study of personalised care in the treatment of women with early breast cancer. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20100.
- Blackshaw H, Carding P, Jepson M, Mat Baki M, Ambler G, Schilder A, et al. Does iaryngeal reinnervation or type I thyroplasty give better voice results for patients with unilateral vocal fold paralysis (VOCALIST): study protocol for a feasibility randomised controlled trial. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2017-016871.
- Sherratt FC, Roper L, Stones SR, McErlane F, Peak M, Beresford MW, et al. Protective parents and permissive children: what qualitative interviews with parents and children can tell us about the feasibility juvenile idiopathic arthritis trials. Pediatr Rheumatol Online J 2018;16. https://doi.org/10.1186/s12969-018-0293-2.
- Donovan JL, de Salis I, Toerien M, Paramasivan S, Hamdy FC, Blazeby JM. The intellectual challenges and emotional consequences of equipoise contributed to the fragility of recruitment in six randomized controlled trials. J Clin Epidemiol 2014;67:912-20. https://doi.org/10.1016/j.jclinepi.2014.03.010.
- Shilling V, Williamson PR, Hickey H, Sowden E, Smyth RL, Young B. Processes in recruitment to randomised controlled trials of medicines for children (RECRUIT): a qualitative study. Health Technol Assess 2011;15. https://doi.org/10.3310/hta15150.
- Jensen JD, King AJ, Carcioppolo N, Davis L. Why are tailored messages more effective? A multiple mediation analysis of a breast cancer screening intervention. J Commun 2012;62:851-68. https://doi.org/10.1111/j.1460-2466.2012.01668.x.
- Sinha IP, Altman DG, Beresford MW, Boers M, Clarke M, Craig J, et al. Standard 5: selection, measurement, and reporting of outcomes in clinical trials in children. Pediatrics 2012;129:146-52. https://doi.org/10.1542/peds.2012-0055H.
- Williamson P, Altman D, Blazeby J, Clarke M, Gargon E. Driving up the quality and relevance of research through the use of agreed core outcomes. J Health Serv Res Policy 2012;17:1-2. https://doi.org/10.1258/jhsrp.2011.011131.
- Gargon E, Gurung B, Medley N, Altman DG, Blazeby JM, Clarke M, et al. Choosing important health outcomes for comparative effectiveness research: a systematic review. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0099111.
- Hall NJ, Kapadia MZ, Eaton S, Chan WW, Nickel C, Pierro A, et al. Outcome reporting in randomised controlled trials and meta-analyses of appendicitis treatments in children: a systematic review. Trials 2015;16. https://doi.org/10.1186/s13063-015-0783-1.
- Sherratt FC, Eaton S, Walker E, Beasant L, Blazeby JM, Young B, et al. Development of a core outcome set to determine the overall treatment success of acute uncomplicated appendicitis in children: a study protocol. BMJ Paediatr Open 2017;1. https://doi.org/10.1136/bmjpo-2017-000151.
- COMET Initiative . A Core Outcome Set for Use in Determining the Overall Success of Acute Uncomplicated Appendicitis Treatment in Children n.d. www.comet-initiative.org/studies/details/987 (accessed 25 February 2020).
- Williamson PR, Altman DG, Blazeby JM, Clarke M, Devane D, Gargon E, et al. Developing core outcome sets for clinical trials: issues to consider. Trials 2012;13. https://doi.org/10.1186/1745-6215-13-132.
- Sinha IP, Smyth RL, Williamson PR. Using the Delphi technique to determine which outcomes to measure in clinical trials: recommendations for the future based on a systematic review of existing studies. PLOS Med 2011;8. https://doi.org/10.1371/journal.pmed.1000393.
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol 2011;64:395-400. https://doi.org/10.1016/j.jclinepi.2010.09.012.
- Allin BSR, Hall NJ, Ross AR, Marven SS, Kurinczuk JJ, Knight M. Development of a gastroschisis core outcome set. Arch Dis Child Fetal Neonatal Ed 2019;104:F76-82. https://doi.org/10.1136/archdischild-2017-314560.
- Allin BSR, Bradnock T, Kenny S, Kurinczuk JJ, Walker G, Knight M. NETS1HD Collaboration . NETS1HD study: development of a Hirschsprung’s disease core outcome set. Arch Dis Child 2017;102:1143-51. https://doi.org/10.1136/archdischild-2017-312901.
- Young B, Bagley H. Including patients in core outcome set development: issues to consider based on three workshops with around 100 international delegates. Res Involv Engagem 2016;2. https://doi.org/10.1186/s40900-016-0039-6.
- Delbecq AL, Van de Ven AH, Gustafson DH. Group Techniques for Program Planning: A Guide to Nominal Group and Delphi Processes. Glenview, IL: Scott, Foresman and Company; 1975.
- Broderick TL, Cifuentes J, Green D, Paulson DJ. Short-term carnitine deficiency does not alter aerobic rat heart function but depresses reperfusion recovery after ischemia. Can J Physiol Pharm 2001;79:892-7. https://doi.org/10.1139/y01-051.
- Coulman KD, Hopkins J, Brookes ST, Chalmers K, Main B, Owen-Smith A, et al. A core outcome set for the benefits and adverse events of bariatric and metabolic surgery: the BARIACT project. PLOS Med 2016;13. https://doi.org/10.1371/journal.pmed.1002187.
- McNair AG, Whistance RN, Forsythe RO, Macefield R, Rees J, Pullyblank AM, et al. Core outcomes for colorectal cancer surgery: a consensus study. PLOS Med 2016;13. https://doi.org/10.1371/journal.pmed.1002071.
- Potter S, Holcombe C, Ward JA, Blazeby JM. BRAVO Steering Group . Development of a core outcome set for research and audit studies in reconstructive breast surgery. Br J Surg 2015;102:1360-71. https://doi.org/10.1002/bjs.9883.
- INVOLVE . What Is Public Involvement in Research? 2017. www.invo.org.uk/find-out-more/what-is-public-involvement-in-research-2/ (accessed 21 November 2017).
- Boers M, Kirwan JR, Wells G, Beaton D, Gossec L, . Developing core outcome measurement sets for clinical trials: OMERACT filter 2.0. J Clin Epidemiol 2014;67:745-53. https://doi.org/10.1016/j.jclinepi.2013.11.013.
- Williamson PR, Altman DG, Bagley H, Barnes KL, Blazeby JM, Brookes ST, et al. The COMET Handbook: version 1.0. Trials 2017;18. https://doi.org/10.1186/s13063-017-1978-4.
- Prinsen CA, Vohra S, Rose MR, Boers M, Tugwell P, Clarke M, et al. How to select outcome measurement instruments for outcomes included in a ‘Core Outcome Set’ – a practical guideline. Trials 2016;17. https://doi.org/10.1186/s13063-016-1555-2.
- COSMIN Initiative . COSMIN Helps You Select the Most Suitable Outcome Measurement Instruments n.d. www.cosmin.nl/ (accessed 27 February 2020).
- COMET Initiative . Protocol for the Development of a Global Core Outcome Set for Treatment of Uncomplicated Appendicitis in Children n.d. www.comet-initiative.org/studies/details/1119 (accessed 27 February 2020).
- Morris C, Janssens A, Shilling V, Allard A, Fellowes A, Tomlinson R, et al. Meaningful health outcomes for paediatric neurodisability: stakeholder prioritisation and appropriateness of patient reported outcome measures. Health Qual Life Outcomes 2015;13. https://doi.org/10.1186/s12955-015-0284-7.
- Sinha IP, Gallagher R, Williamson PR, Smyth RL. Development of a core outcome set for clinical trials in childhood asthma: a survey of clinicians, parents, and young people. Trials 2012;13. https://doi.org/10.1186/1745-6215-13-103.
- Fair C, Cuttance J, Sharma N, Maslow G, Wiener L, Betz C, et al. International and interdisciplinary identification of health care transition outcomes. JAMA Pediatr 2016;170:205-11. https://doi.org/10.1001/jamapediatrics.2015.3168.
- Sherratt FC, Allin BSR, Kirkham JJ, Walker E, Young B, Wood W, et al. Core outcome set for uncomplicated acute appendicitis in children and young people. Br J Surg 2020;107:1013-22. https://doi.org/10.1002/bjs.11508.
- Kirkham JJ, Davis K, Altman DG, Blazeby JM, Clarke M, . Core Outcome Set-STAndards for Development: The COS-STAD recommendations. PLOS Medicine 2017;14. https://doi.org/10.1371/journal.pmed.1002447.
- Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ 2015;350. https://doi.org/10.1136/bmj.h2147.
- Cook JA, Julious SA, Sones W, Hampson LV, Hewitt C, Berlin JA, et al. DELTA2 guidance on choosing the target difference and undertaking and reporting the sample size calculation for a randomised controlled trial. Br Med J 2018;363. https://doi.org/10.1136/bmj.k3750.
- Committee for Medicinal Products for Human Use, Efficacy Working Party, Committee for Release for Consultation . Committee for Medicinal Products for Human Use (CHMP) guideline on the choice of the non-inferiority margin. Statist Med 2006;25:1628-38. https://doi.org/10.1002/sim.2584.
- NHS Improvement . Archived Reference Costs n.d. https://improvement.nhs.uk/resources/reference-costs/ (accessed 30 January 2019).
- Chorozoglou M, Reading I, Eaton S, Hutchings N, Hall NJ. Health economics and quality of life in a feasibility RCT of paediatric acute appendicitis: a protocol study. BMJ Paediatr Open 2018;2. https://doi.org/10.1136/bmjpo-2018-000347.
- Drummond M, Sculpher M, Claxton K, Stoddart G, Torrance G. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Drummond M, McGuire A. Economic Evaluation in Health Care: Merging Theory with Practice. New York, NY: Oxford University Press; 2004.
- Riewpaiboon A, Malaroje S, Kongsawatt S. Effect of costing methods on unit cost of hospital medical services. Trop Med Int Health 2007;12:554-63. https://doi.org/10.1111/j.1365-3156.2007.01815.x.
- Beecham J, Knapp M, Thornicroft G, Brewin C, Wing J. Measuring Mental Health Needs. London: Gaskell/Royal College of Psychiatrists; 2001.
- Smith MW, Barnett PG. Direct measurement of health care costs. Med Care Res Rev 2003;60:74-91. https://doi.org/10.1177/1077558703257001.
- Byford S, McDaid D, Sefton T. Because It’s Worth It: A Practical Guide to Conducting Economic Evaluations in the Social Welfare Field. York: Joseph Rowntree Foundation; 2003.
- Byford S, Leese M, Knapp M, Seivewright H, Cameron S, Jones V, et al. Comparison of alternative methods of collection of service use data for the economic evaluation of health care interventions. Health Econ 2007;16:531-6. https://doi.org/10.1002/hec.1175.
- Knapp M, Beecham J. Reduced list costings: examination of an informed short cut in mental health research. Health Econ 1993;2:313-22. https://doi.org/10.1002/hec.4730020404.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: Personal Social Services Research Unit, University of Kent; 2017.
- Petrou S. Methodological issues raised by preference-based approaches to measuring the health status of children. Health Econ 2003;12:697-702. https://doi.org/10.1002/hec.775.
- White H, Sabarwal S. Developing and Selecting Measures of Child Well-Being: Methodological Briefs – Impact Evaluation No. 11. Florence: UNICEF Office of Research; 2014.
- Khadka J, Kwon J, Petrou S, Lancsar E, Ratcliffe J. Mind the (inter-rater) gap. An investigation of self-reported versus proxy-reported assessments in the derivation of childhood utility values for economic evaluation: a systematic review. Soc Sci Med 2019;240. https://doi.org/10.1016/j.socscimed.2019.112543.
- Methodological challenges posed by economic evaluations of early childhood intervention programmes. Appl Health Econ Health Policy 2005;4:175-81. https://doi.org/10.2165/00148365-200504030-00006.
- Ungar WJ. Challenges in health state valuation in paediatric economic evaluation: are QALYs contraindicated?. PharmacoEconomics 2011;29:641-52. https://doi.org/10.2165/11591570-000000000-00000.
- Dolan P. EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Dolan P, Gudex C, Kind P, Williams A. A Social Tariff for EuroQol: Results from a UK General Population Study. York: Centre for Health Economics, University of York; 1995.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 2013.
- Wille N, Badia X, Bonsel G, Burström K, Cavrini G, Devlin N, et al. Development of the EQ-5D-Y: a child-friendly version of the EQ-5D. Qual Life Res 2010;19:875-86. https://doi.org/10.1007/s11136-010-9648-y.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Stevens K. Developing a descriptive system for a new preference-based measure of health-related quality of life for children. Qual Life Res 2009;18:1105-13. https://doi.org/10.1007/s11136-009-9524-9.
- Stevens KJ. Working with children to develop dimensions for a preference-based, generic, pediatric, health-related quality-of-life measure. Qual Health Res 2010;20:340-51. https://doi.org/10.1177/1049732309358328.
- Stevens K. Assessing the performance of a new generic measure of health-related quality of life for children and refining it for use in health state valuation. Appl Health Econ Health Policy 2011;9:157-69. https://doi.org/10.2165/11587350-000000000-00000.
- Stevens KJ, Freeman JV. An assessment of the psychometric performance of the Health Utilities Index 2 and 3 in children following discharge from a U.K. pediatric intensive care unit. Pediatr Crit Care Med 2012;13:387-92. https://doi.org/10.1097/PCC.0b013e318238969a.
- Stevens K, Ratcliffe J. Measuring and valuing health benefits for economic evaluation in adolescence: an assessment of the practicality and validity of the child health utility 9D in the Australian adolescent population. Value Health 2012;15:1092-9. https://doi.org/10.1016/j.jval.2012.07.011.
- Stevens K. Valuation of the Child Health Utility 9D Index. PharmacoEconomics 2012;30:729-47. https://doi.org/10.2165/11599120-000000000-00000.
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377-81. https://doi.org/10.1016/j.jbi.2008.08.010.
- NHS Digital . NHS Workforce Statistics, December 2017 2018. https://digital.nhs.uk/data-and-information/publications/statistical/nhs-workforce-statistics/nhs-workforce-statistics-december-2017 (accessed July 2020).
- INVOLVE . Public Involvement in Research: Values and Principles Framework 2015.
- Mental Health Research Network, INVOLVE . Budgeting for Involvement: Practical Advice on Budgeting for Actively Involving the Public in Research Studies 2013.
- Mathie E, Wythe H, Munday D, Millac P, Rhodes G, Roberts N, et al. Reciprocal relationships and the importance of feedback in patient and public involvement: a mixed methods study. Health Expect 2018;21:899-908. https://doi.org/10.1111/hex.12684.
- Johannesen J. Exploring the Purpose and Meaning of Patient Engagement in Pediatric Neurodisability Research 2017. https://johannesen.ca/2017/11/exploring-purpose-meaning-patient-engagement-pediatric-neurodisability-research (accessed 30 January 2019).
- National Institute for Health Research . Patient and Public Involvement in Health and Social Care Research 2018.
- Loukogeorgakis SP, Major C, Jones CE, Corbett HJ, Folaranmi SE, Stanton MP, et al. Derivation and validation of a novel clinical decision aid to distinguish between uncomplicated and complicated appendicitis in children. Gastrointestinal Surgery 2020. https://doi.org/10.21203/rs.3.rs-23218/v1.
- Knaapen M, van der Lee JH, Heij HA, van Heurn ELW, Bakx R, Gorter RR. Clinical recovery in children with uncomplicated appendicitis undergoing non-operative treatment: secondary analysis of a prospective cohort study. Eur J Pediatr 2019;178:235-42. https://doi.org/10.1007/s00431-018-3277-9.
- Miyano G, Ochi T, Seo S, Nakamura H, Okawada M, Doi T, et al. Factors affecting non-operative management of uncomplicated appendicitis in children: should laparoscopic appendectomy be immediate, interval, or emergency?. Asian J Endosc Surg 2019;12:434-8. https://doi.org/10.1111/ases.12677.
- Bachur RG, Lipsett SC, Monuteaux MC. Outcomes of nonoperative management of uncomplicated appendicitis. Pediatrics 2017;140. https://doi.org/10.1542/peds.2017-0048.
- Lee SL, Spence L, Mock K, Wu JX, Yan H, DeUgarte DA. Expanding the inclusion criteria for nonoperative management of uncomplicated appendicitis: outcomes and cost [published online ahead of print October 9 2017]. J Pediatr Surg 2017.
- Talan DA, Saltzman DJ, Mower WR, Krishnadasan A, Jude CM, Amii R, et al. Antibiotics-first versus surgery for appendicitis: a US pilot randomized controlled trial allowing outpatient antibiotic management. Ann Emerg Med 2017;70:1-11.
- Abbo O, Trabanino C, Pinnagoda K, Ait Kaci A, Carfagna L, Mouttalib S, et al. Non-operative management for uncomplicated appendicitis: an option to consider. Eur J Pediatr Surg 2018;28:18-21. https://doi.org/10.1055/s-0037-1607292.
- Caruso AM, Pane A, Garau R, Atzori P, Podda M, Casuccio A, et al. Acute appendicitis in children: not only surgical treatment. J Pediatr Surg 2017;52:444-8. https://doi.org/10.1016/j.jpedsurg.2016.08.007.
- Xu J, Liu YC, Adams S, Karpelowsky J. Acute uncomplicated appendicitis study: rationale and protocol for a multicentre, prospective randomised controlled non-inferiority study to evaluate the safety and effectiveness of non-operative management in children with acute uncomplicated appendicitis. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-013299.
- ClinicalTrials.gov . Appendectomy Versus Non-Operative Treatment For Acute Non-Perforated Appendicitis in Children (APPY) n.d. https://clinicaltrials.gov/ct2/show/NCT02687464 (accessed 2 March 2020).
- ClinicalTrials.gov . Non-Operative Management for Appendicitis in Children (APRES) n.d. https://clinicaltrials.gov/ct2/show/NCT02795793 (accessed 2 March 2020).
- ClinicalTrials.gov . Initial Non-Operative Treatment Strategy Versus Appendectomy Treatment Strategy for Simple Appendicitis in Children (APAC) n.d. https://clinicaltrials.gov/ct2/show/NCT02848820 (accessed 2 March 2020).
- Knaapen M, van der Lee JH, Bakx R, The SL, van Heurn EWE, Heij HA, et al. APAC collaborative study group . Initial non-operative management of uncomplicated appendicitis in children: a protocol for a multicentre randomised controlled trial (APAC trial). BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2017-018145.
- ClinicalTrials.gov . Comparison of Medical and Surgical Treatment of Uncomplicated Acute Appendicitis in Children n.d. https://clinicaltrials.gov/ct2/show/NCT02991937 (accessed 2 March 2020).
- Narsule CK, Kahle EJ, Kim DS, Anderson AC, Luks FI. Effect of delay in presentation on rate of perforation in children with appendicitis. Am J Emerg Med 2011;29:890-3. https://doi.org/10.1016/j.ajem.2010.04.005.
- Serres SK, Cameron DB, Glass CC, Graham DA, Zurakowski D, Karki M, et al. Time to appendectomy and risk of complicated appendicitis and adverse outcomes in children. JAMA Pediatr 2017;171:740-6. https://doi.org/10.1001/jamapediatrics.2017.0885.
- Byrne A, Morton J, Salmon P. Defending against patients’ pain: a qualitative analysis of nurses’ responses to children’s postoperative pain. J Psychosom Res 2001;50:69-76. https://doi.org/10.1016/S0022-3999(00)00207-5.
- Karlson CW, Rapoff MA. Attrition in randomized controlled trials for pediatric chronic conditions. J Pediatr Psychol 2009;34:782-93. https://doi.org/10.1093/jpepsy/jsn122.
- European Parliament, Council of the European Union . Directive 2001 20 EC of the European Parliament and of the Council of 4 April 2001 on the Approximation of the Laws, Regulations and Administrative Provisions of the Member States Relating to the Implementation of Good Clinical Practice in the Conduct of Clinical Trials on Medicinal Products for Human Use 2001. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32001L0020 (accessed 25 February 2020).
- Coats TJ, Shakur H. Consent in emergency research: new regulations. Emerg Med J 2005;22:683-5. https://doi.org/10.1136/emj.2005.024588.
- Briel M, Olu KK, von Elm E, Kasenda B, Alturki R, Agarwal A, et al. A systematic review of discontinued trials suggested that most reasons for recruitment failure were preventable. J Clin Epidemiol 2016;80:8-15. https://doi.org/10.1016/j.jclinepi.2016.07.016.
- World Medical Association (WMA) . WMA Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects 2013.
- Griffin D, Wall P, Realpe A, Adams A, Parsons N, Hobson R, et al. UK FASHIoN: feasibility study of a randomised controlled trial of arthroscopic surgery for hip impingement compared with best conservative care. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20320.
- Callahan MJ, Rodriguez DP, Taylor GA. CT of appendicitis in children. Radiology 2002;224:325-32. https://doi.org/10.1148/radiol.2242010998.
- Rothrock SG, Pagane J. Acute appendicitis in children: emergency department diagnosis and management. Ann Emerg Med 2000;36:39-51. https://doi.org/10.1067/mem.2000.105658.
- Chen CL, Chao HC, Kong MS, Chen SY. Risk factors for prolonged hospitalization in pediatric appendicitis patients with medical treatment. Pediatr Neonatol 2017;58:223-8. https://doi.org/10.1016/j.pedneo.2016.02.011.
- Jimbo K, Takeda M, Miyata E, Murakami H, Kyodo R, Orikasa H, et al. Is a pediatrician performed gray scale ultrasonography with power Doppler study safe and effective for triaging acute non-perforated appendicitis for conservative management?. J Pediatr Surg 2016;51:1952-6. https://doi.org/10.1016/j.jpedsurg.2016.09.018.
- Kaneko K, Tsuda M. Ultrasound-based decision making in the treatment of acute appendicitis in children. J Pediatr Surg 2004;39:1316-20. https://doi.org/10.1016/j.jpedsurg.2004.05.011.
- Koike Y, Uchida K, Matsushita K, Otake K, Nakazawa M, Inoue M, et al. Intraluminal appendiceal fluid is a predictive factor for recurrent appendicitis after initial successful non-operative management of uncomplicated appendicitis in pediatric patients. J Pediatr Surg 2014;49:1116-21. https://doi.org/10.1016/j.jpedsurg.2014.01.003.
- Mahida JB, Lodwick DL, Nacion KM, Sulkowski JP, Leonhart KL, Cooper JN, et al. High failure rate of nonoperative management of acute appendicitis with an appendicolith in children. J Pediatr Surg 2016;51:908-11. https://doi.org/10.1016/j.jpedsurg.2016.02.056.
- Mudri M, Coriolano K, Bütter A. Cost analysis of nonoperative management of acute appendicitis in children. J Pediatr Surg 2017;52:791-4. https://doi.org/10.1016/j.jpedsurg.2017.01.050.
- Steiner Z, Buklan G, Stackievicz R, Gutermacher M, Litmanovitz I, Golani G, et al. Conservative treatment in uncomplicated acute appendicitis: reassessment of practice safety. Eur J Pediatr 2017;176:521-7. https://doi.org/10.1007/s00431-017-2867-2.
- Tanaka Y, Uchida H, Kawashima H, Fujiogi M, Takazawa S, Deie K, et al. Long-term outcomes of operative versus nonoperative treatment for uncomplicated appendicitis. J Pediatr Surg 2015;50:1893-7. https://doi.org/10.1016/j.jpedsurg.2015.07.008.
Appendix 1 Adverse event profile of each treatment group
| Patient | AE | |||||
|---|---|---|---|---|---|---|
| Description | Actions | Severity | Seriousa | Related to treatment arm | Outcome | |
| 009 | Fevers – re-admission | Further course of antibiotics given | Moderate | Yesa | Definitely | Resolved with sequelae |
| PICC line insertion | Weekly follow-up appointments in clinic | Moderate | No | Unrelated | Resolved | |
| 025 | Abdominal pain | – | Moderate | Yesa | Unrelated | Resolved |
| Sore throat | Ibuprofen given | Mild | No | Unrelated | Resolved | |
| 103 | Abdominal pain | Antibiotics | Moderate | Yesa | Unlikely | Resolved |
| Recurrent appendicitis | Appendicectomy | Severe | Yesa | Unlikely | Resolved | |
| 162 | Abdominal pain | Appendicectomy and hospital admission | Moderate | No | Definitely | Ongoing |
| Fluid collection | Continued antibiotics, already in hospital | Moderate | No | Definitely | Ongoing | |
| 179 | Abdominal pain | A&E attendance, bloods taken | Mild | No | Unrelated | Resolved |
| 233 | Abdominal pain | Appendicectomy and hospital admission | Moderate | No | Definitely | Resolved |
| Recurrence of appendicitis | Appendicectomy | Moderate | No | Definitely | Resolved | |
| 266 | Recurrent appendicitis | Appendicectomy | Moderate | No | Definitely | Resolved |
| 002 | Abdominal pain | Admission to hospital | Mild | No | Probably | Resolved |
| 157 | Abdominal pain | – | Moderate | No | Possibly | Resolved |
| 049 | Abdominal pain | Advice given in A&E | Mild | No | Unrelated | Resolved |
| 089 | Patient visited GP surgery complaining of being sleepy for 2 weeks | None | Mild | No | Possibly | Ongoing |
| Patient visited GP complaining of lethargy for 2 weeks | None | Mild | No | Possibly | Ongoing | |
| Abdomen pain | Patient had appendicectomy | Moderate | No | Probably | Resolved | |
| 184 | Abdominal pain | Patient to be sent a clinic appointment | Mild | No | Unlikely | Ongoing |
| 185 | Rash over thighs | Patient given three doses of antihistamine | Mild | No | Unrelated | Resolved |
| 276 | Lethargic | Attended A&E. Discharged. Seen in clinic | Mild | No | Probably | Resolved |
| 289 | Generalised rash | Commenced antihistamines | Mild | No | Possibly | Resolved |
| Patient | AE | |||||
|---|---|---|---|---|---|---|
| Description | Actions | Severity | Seriousa | Related to treatment arm | Outcome | |
| 014 | Abdominal pain | i.v. antibiotics | Moderate | Yesa | Possibly | Resolved |
| Postoperative fever on re-admission | N/A | Mild | No | Possibly | Resolved | |
| 017 | Vomiting | Ultrasonography | Moderate | Yes | Unlikely | Resolved |
| 123 | Headache | Paracetamol given | Mild | No | Unrelated | Resolved |
| Abdominal pain | Blood test | Moderate | No | Possibly | Resolved | |
| Intermittent vomiting | Blood test taken on 6 November 2017 was fine | Moderate | No | Possibly | Resolved | |
| Headache | Ibuprofen, time off school | Mild | No | Possibly | Resolved | |
| Sickness | Rest, off sick from school | – | No | Possibly | Resolved | |
| Sickness | Surgical review | Mild | No | Unrelated | Resolved | |
| Sickness | Off school | Mild | No | Unrelated | Resolved | |
| 264 | Fluid collection | Drain insertion and hospitalisation | Moderate | No | Unrelated | Resolved |
| PICC line insertion | Hospitalisation | Moderate | No | Unrelated | Resolved | |
| Drain insertion | Hospitalisation | Moderate | No | Unrelated | Resolved | |
| 040 | Localised intra-abdominal fluid collection | Treatment with i.v. antibiotics | Moderate | No | Possibly | Resolved |
| 167 | Inflamed wound site | Patient started oral flucloxacillin | Moderate | No | Definitely | Resolved with sequelae |
| Wound dehiscence | Attended A&E | Moderate | No | Definitely | Resolved | |
| Diarrhoea | Telephone consultation with GP | Mild | No | Unlikely | Resolved with sequelae | |
| Vomiting | Call to GP – stomach bug diagnosed | Mild | No | Unrelated | Resolved | |
| Diarrhoea | Call to GP – stool sample | Mild | No | Unrelated | Resolved | |
| Pharyngitis | Commenced oral amoxicillin | Mild | No | Unrelated | Resolved | |
| 245 | Wound infection | Commenced antibiotics | Moderate | No | Definitely | Resolved |
| 247 | Suture-related complication | Ultrasonography and clinic | Moderate | No | Probably | Resolved |
Appendix 2 Communication substudy
-
Experience of illness.
-
Initial thoughts about CONTRACT.
-
Experience of being approached about CONTRACT.
-
Thoughts on how it was explained.
-
How the health professional explained the treatment options and family preferences.
-
Messages that resonated about CONTRACT.
-
-
Reasons for consent/decline.
-
Views and understanding of randomisation.
-
Experience of treatment.
-
Experience of recovery.
-
Reflections on CONTRACT since being approached.
-
Initial thoughts about CONTRACT.
-
Knowledge and awareness of CONTRACT.
-
Recruitment pathways.
-
Experiences of approaching families about CONTRACT.
-
Health professional treatment preferences.
-
Experience of delivering the treatments.
-
Anticipated CONTRACT results.
Health professionals’ recruiting confidence
| Training domains (by training session) | Health professionals (n) | Median respondent score (IQR)a |
|---|---|---|
| 1. I feel confident about explaining the CONTRACT study to families | ||
| Pre-training session (1) | 29 | 3 (1.5–4) |
| Post-training session (1) | 26 | 4.5 (4–5) |
| Post-training session (2) | 31 | 5 (4–5) |
| Post-training session (3) | 31 | 5 (4–5) |
| 2. I feel confident about dealing with families’ questions about CONTRACT | ||
| Pre-training session (1) | 28 | 3 (2–4) |
| Post-training session (1) | 26 | 4 (4–5) |
| Post-training session (2) | 31 | 4 (4–5) |
| Post-training session (3) | 31 | 5 (4–5) |
| 3. I feel confident about explaining randomisation to families | ||
| Pre-training session (1) | 28 | 4 (3–4) |
| Post-training session (1) | 26 | 5 (4–5) |
| Post-training session (2) | 31 | 4 (4–5) |
| Post-training session (3) | 31 | 5 (4–5) |
| 4. I feel confident about exploring families’ views about CONTRACT | ||
| Pre-training session (1) | 28 | 3 (2–4) |
| Post-training session (1) | 26 | 4.5 (4–5) |
| Post-training session (2) | 31 | 4 (4–5) |
| Post-training session (3) | 31 | 4 (4–5) |
| 5. I feel confident about balancing families’ treatment preferencesb | ||
| Post-training session (1) | 26 | 4 (4–5) |
| Post-training session (2) | 31 | 5 (4–5) |
| Post-training session (3) | 31 | 4 (4–5) |
Recommendations arising from the communication study regarding future trial design and delivery
Optimising recruitment in trials comparing surgical treatment with non-surgical treatment
CONTRACT compared a surgical with a non-surgical treatment. Trials that include such markedly different treatments are known to be particularly difficult to conduct as a result of treatment preference issues. 34,35 In CONTRACT, this was particularly pertinent because both parents and surgeons perceived surgery as a ‘trusted’ treatment, relative to non-operative treatment. 36 Parents’ preferences for surgery were often born out of concerns about the risk of perforation. Parents viewed perforation as a dangerous event that could severely compromise their child’s condition and worried that it could occur at any time. Such concerns influenced families’ willingness to participate, as some viewed surgery as a more immediate treatment and, in their view, as reducing the risk of perforation. Longer duration of symptoms has been reported as a risk factor for perforated appendicitis,142 but no association has been found between perforation and in-hospital time prior to surgery for children with acute appendicitis. 143 This suggests that parents’ concerns about the impact of in-hospital delays on perforation were not well founded. Moreover, parents often expressed these concerns in the qualitative interviews, but not in the recorded consultations, which further underscores the importance of exploring treatment preferences with families. Exploring parents’ treatment preferences, in a future definitive trial and for other trials comparing surgical treatment with non-surgical treatment, would be beneficial in alleviating families’ anxieties and could help address families’ concerns about participation. 44
Following surgery, the details surgeons provided to parents who participated in CONTRACT of the removed appendix were often vivid. For parents of children who had complicated or perforated appendicitis, these details seemed to add to their sense of guilt or regret for allowing their child to participate in the trial. This finding has implications for other trials comparing surgical treatment with non-surgical treatment. For a future definitive trial, health professionals, including those who are involved in the child’s treatment and care but not directly involved in the trial, should be aware of the possibility of inducing or adding to such feelings of regret when speaking with parents post surgery.
-
Recommendation 1: explore families’ treatment preferences in pre-consent trial discussions. In CONTRACT, this includes exploring families’ understanding of perforation and allaying their concerns.
-
Recommendation 2: be aware of family sensitivities when explaining post-surgery findings in the context of a trial.
Optimising recruitment in paediatric trials in acute settings
In the communication study interviews, many families reported making a shared decision as to whether or not to participate in CONTRACT. In consultation recordings, we found that few of the younger children engaged in CONTRACT discussions and decision-making. Regardless of age, children were often unwell and so they relied on their parents to make the decision for them. These findings align with current guidance on how best to involve children and young people in research. 38
Parents described how the pain their child experienced impeded their child’s engagement in CONTRACT discussions and decision-making. Parents who declined to participate in CONTRACT also often explained that they viewed surgery as a more immediate way of relieving their child’s pain than non-operative treatment. Health professionals have been found to adopt behavioural and cognitive strategies to emotionally cope with children’s pain, which can compromise clinical communication about pain. 144 Providing families with advance information about how a child’s pain is managed in both treatment arms could help to reduce their anxiety about this.
In CONTRACT, children and parents often described conflicting treatment preferences. Some surgeons suggested that randomisation in CONTRACT offered a means of resolving this conflict. In interviews, parents and children who had conflicting preferences and who were randomised to the parent’s preferred treatment spoke of this as upsetting for the child. Families suggested that allocation discussions were generally brief and parents were often left to comfort their child. Again, exploring treatment preferences pre allocation could help to avoid such difficulties. 42 Exploring treatment preferences pre allocation could also help to avoid families withdrawing from the trial because they do not want to continue with the allocated treatment, which is one of the most common reasons for attrition in paediatric trials. 145 If a child is upset at being allocated to a non-preferred treatment, it could help to further explore the reasons underlying their preferences. If the child remains upset about the prospect of continuing in the trial, the option of withdrawal should be discussed with them. 38
As discussed, previous research suggests that exploring treatment preferences can improve informed consent and trial recruitment. 43,44 In children mature enough to understand, this exploration should probably be done for both the child and the parents. This involves recruiters communicating in ways that help to balance views about treatments and so ensure that families have the necessary information to make an informed decision. 43 It can also include providing information about treatment risks. However, some parents of younger children expressed concerns about health professionals providing such information to their children and felt that such discussions compromised their child’s trust in health professionals. When exploring treatment preferences to improve informed consent in paediatric trials, health professionals therefore have the additional complexity of needing to do so while being sensitive to parents’ anxieties about what their children hear.
Informed consent should allow sufficient time for health professionals to explain the study thoroughly and for potential participants to decide whether or not to participate. 146 However, in time-critical settings, such as urgent care, it is rarely feasible to offer the traditionally advocated 24-hour timeframe to decide. 147 Some families in CONTRACT described making an instantaneous decision to participate, whereas others felt that the time they had was not sufficient. Families typically had 1 or 2 hours to decide, but a few families were left for up to 7 hours before a health professional returned to find out their decision. Parents appreciated that such delays were often unavoidable in the busy clinical setting, but extensive delays left them anxious about the appendicitis progressing. It also compromised their sense of being cared for, which ultimately influenced willingness to participate in CONTRACT. A process that allows families to signal when they have made a decision could help to allay parents’ concerns and optimise recruitment. Further work should explore how best to implement such a process in an urgent care setting.
Finally, CONTRACT recruited children in an acute hospital setting, often outside normal hours, when research nurse support was not available. A common reason for trial recruitment failure is a lack of recruiting staff or insufficient funds to reimburse recruiting staff. 148 Although families often presented at hospital outside normal working hours and surgeons described how having research nurse support was advantageous, few health professionals suggested that recruiting outside normal working hours was detrimental to recruitment. Nevertheless, staffing strategies to support health professionals to recruit families to a future definitive trial should be considered.
-
Recommendation 3: involve children and young people in research discussions and decision-making when possible, as per current guidance,38 while being sensitive to parents’ anxieties about what their children hear.
-
Recommendation 4: provide families with advance information about how a child’s pain will be managed in both treatment arms.
-
Recommendation 5: when child and parent treatment preferences conflict, randomisation may offer a means to resolve this conflict. Sensitivity is needed in communicating to families which treatment arm they have been allocated to; if a child is upset with treatment allocation, further exploring their treatment preference following randomisation may help to allay their concerns.
-
Recommendation 6: time to decide – develop a strategy to allow families to indicate when they have made a decision regarding participation, thereby minimising delays from the perspective of families.
-
Recommendation 7: consider staffing strategies to support health professionals in recruiting families outside normal working hours.
Optimising discussions about the purpose of feasibility trials
Informed consent is central to the ethical conducting of research, and conveying the ‘purpose’ or aim of a study is a necessary element of informed consent. 149 As found in a previous qualitative study embedded in a feasibility trial,150 health professionals in CONTRACT rarely described its feasibility aims. Rather, they described CONTRACT as aiming to find out how best to treat appendicitis. When interviewed, health professionals typically suggested that it was unnecessary to describe the feasibility aim of CONTRACT, adding that families might find the concept of feasibility confusing or overwhelming (especially in the urgent care context). Some health professionals thought that describing it could dissuade families from participating. In interviews, we did not ask families about their understanding of the purpose of feasibility trials and how best to discuss it because the topic was rarely mentioned in CONTRACT consultations. However, there is a need for work to explore children’s and parents’ understanding of feasibility, the acceptability of feasibility trials and optimal ways for health professionals to explain the aims of a feasibility trial, to optimise informed consent.
-
Recommendation 8: further research is needed to explore how best to discuss the purpose of feasibility trials with families in acute settings.
Optimising recruitment to a future definitive trial
This section discusses findings of the communication study and final recommendations, specifically in the context of developing a future definitive trial comparing non-operative treatment with appendicectomy for the treatment of acute uncomplicated appendicitis in children and young people.
In the UK, patient history and physical examination are routinely used to determine acute uncomplicated appendicitis, but 28–57% of children aged ≤ 12 years are misdiagnosed. 151 In CONTRACT, health professionals used the same diagnostic techniques to establish whether or not children had acute uncomplicated appendicitis, as opposed to perforated appendicitis. In their interviews, they explained the difficulties of accurately establishing patient eligibility. Some proposed introducing additional diagnostic measures to more confidently diagnose children with acute uncomplicated appendicitis, for instance level of CRP, which can indicate perforation, but not as a sole indicator. 152 Diagnostic uncertainty was a core concern for health professionals. Additional strategies to improve the accuracy of diagnosis should be considered to improve families’ and health professionals’ confidence in the trial.
As described earlier, some families who experienced non-operative treatment failure had expected that surgery would be performed almost immediately after such failure had been established clinically. These findings from the communication study informed changes to the PIS. Following discussion of this issue in the recruitment training, health professionals started providing families with information on the timing of surgery if non-operative treatment were to fail and the estimated timeframe in which families could expect to see an improvement in their child’s condition after starting non-operative treatment. The study investigators may wish to emphasise this finding in training for a future trial.
Some parents suggested that there had been delays in their child receiving the initial course of antibiotics. They questioned whether or not the delay was because of the time taken to voice their decision as to whether or not they wanted to participate in CONTRACT, and indicated that the experience discouraged them from participating in CONTRACT. Health professionals should avoid delay in commencing treatment prior to CONTRACT participation whenever possible.
Finally, one of the nurses who completed blinded discharge assessments highlighted that assessors working on the same wards as CONTRACT participants may become unblinded to their treatment allocation. Ideally, assessors should be from different wards to those on which CONTRACT participants are treated. Furthermore, assessors may appreciate brief training about the study and the opportunity to participate in study events.
-
Recommendation 9: consider additional strategies to improve the effectiveness of distinguishing children with acute uncomplicated appendicitis from those with perforated appendicitis in assessing patient eligibility.
-
Recommendation 10: provide families with balanced information regarding both treatment arms. This should include information on the timing of surgery if non-operative treatment fails and the estimated timeframe in which families allocated to the non-operative treatment arm should expect to see an improvement in their child’s condition. This could be included in training for a future trial.
-
Recommendation 11: some parents reported delays in their child starting the initial course of antibiotics, which they linked to the additional procedures required for CONTRACT, and so this discouraged them from participation. Whenever possible, health professionals should avoid delays in delivering the initial antibiotics.
-
Recommendation 12: ensure that blinded discharge assessments are completed by a health professional who does not work on the same wards as those where CONTRACT participants are treated, and offer the assessor brief training on the study.
Appendix 3 Core outcome set
Additional studies reviewed
| Study | Publication year | Treatments examined | Study type | Region | Sample size (n) (intention to treat non-operatively) |
|---|---|---|---|---|---|
| Abeş et al.17 | 2007 | Antibiotics | Retrospective | Middle East | 16 |
| Armstrong et al.18 | 2014 | Antibiotics vs. appendicectomy | Retrospective | North America | 12 |
| aCaruso et al.135 | 2017 | Antibiotics vs. appendicectomy | Retrospective | Europe | 197 |
| aChen et al.153 | 2016 | Antibiotics | Retrospective | Asia | 125 |
| Gorter et al.12 | 2015 | Antibiotics | Prospective | Europe | 25 |
| Hartwich et al.30 | 2016 | Antibiotics | Prospective | North America | 24 |
| aJimbo et al.154 | 2016 | Antibiotics | Retrospective | Asia | 71 |
| Kaneko and Tsuda155 | 2004 | Antibiotics | Prospective | Asia | 91 |
| Koike et al.156 | 2014 | Antibiotics | Retrospective | Asia | 134 |
| aMahida et al.157 | 2016 | Antibiotics | Prospective | North America | 5 |
| Minecci et al.29 | 2016 | Antibiotics | Prospective | North America | 37 |
| aMudri et al.158 | 2017 | Antibiotics and appendicectomy | Retrospective | North America | 26 |
| Steiner et al.20 | 2015 | Antibiotics | Retrospective | Middle East | 45 |
| aSteiner et al.159 | 2017 | Antibiotics | Retrospective | Middle East | 197 |
| Svensson et al.13 | 2015 | Antibiotics and appendicectomy | Prospective RCT | Europe | 24 |
| Tanaka et al.160 | 2015 | Antibiotics and appendicectomy | Retrospective | Japan | 78 |
Outcomes extracted from additional studies
| Outcome | Study | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abeş et al.17 | Armstrong et al.18 | Caruso et al.135 | Chen et al.153 | Gorter et al.12 | Hartwich et al.30 | Jimbo et al.154 | Kaneko and Tsuda155 | Koike et al.156 | Mahida et al.157 | Minecci et al.29 | Mudri et al.158 | Steiner et al.20 | Steiner et al.159 | Svensson et al.13 | Tanaka et al.160 | |
| Wound infection | ✓ | ✓ | ✓ | |||||||||||||
| Intra-abdominal abscess | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| (Adhesive) obstruction | ✓ | |||||||||||||||
| Complication of antibiotics or treatment intervention | ✓ | |||||||||||||||
| Major/minor complications | ✓ | |||||||||||||||
| Non-infectious wound complications | ||||||||||||||||
| Re-admission to hospital | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Other infectious complications | ✓ | ✓ | ✓ | |||||||||||||
| Interventional radiology procedure | ||||||||||||||||
| Need for operation/reoperation | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Conversion laparoscopic to open surgery | ||||||||||||||||
| Recurrent appendicitis | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Other complications | ✓ | ✓ | ||||||||||||||
| Bacterial isolates | ||||||||||||||||
| Post-treatment fever | ✓ | ✓ | ||||||||||||||
| Measure of recovery of gastrointestinal function | ✓ | |||||||||||||||
| Blood markers (WCC/CRP) | ✓ | ✓ | ✓ | |||||||||||||
| Duration of abdominal drainage | ||||||||||||||||
| Validated pain score | ||||||||||||||||
| Other pain assessment | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Time away from normal activities or school | ✓ | |||||||||||||||
| Recovery to full activity/sport | ||||||||||||||||
| Cosmesis | ||||||||||||||||
| Time to ambulation | ||||||||||||||||
| Other PROMs | ✓ | |||||||||||||||
| Duration of home health care | ✓ | |||||||||||||||
| Paediatric quality-of-life assessment | ✓ | ✓ | ||||||||||||||
| Parental quality-of-life assessment | ✓ | ✓ | ||||||||||||||
| Hospital length of stay | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Duration of surgery/analgesia | ✓ | |||||||||||||||
| Total charges | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| Duration of antibiotics | ✓ | |||||||||||||||
| Narcotic/analgesia doses | ||||||||||||||||
| Health-care visits | ✓ | |||||||||||||||
| Post-treatment imaging | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Cost-effectiveness | ✓ | ✓ | ✓ | |||||||||||||
| Death | ||||||||||||||||
Interview transcripts informing outcome selection
Families’ quotations are presented in this section to illustrate themes. Participant type is detailed (Patient, Mother, Father, Family), with patient age (e.g. 11y = 11 years) and initial treatment (NOT = non-operative treatment; App = appendicectomy). When patients allocated to non-operative treatment ultimately were treated with surgery owing to treatment failure, recurrence or withdrawal from CONTRACT, this is indicated in square brackets (e.g. [app]). Families’ interviews included both those who consented to CONTRACT and those who declined CONTRACT.
Pain was one of the most common outcomes described by patients and parents. Patients differed in the levels of pain they reported, but both parents and patients viewed a reduction in pain as an indicator for treatment success:
[The pain was] a bit worse after the operation, maybe because I lie so much that my tummy got used to it so actually it didn’t hurt as much, but when I moved, it actually hurted.
Patient52_13y_App
He went down for the surgery . . . it was obviously a few hours. He came round . . . he was fine, he was obviously in pain.
Father7_11y_App
Parents and patients also described how appendicitis had affected appetite, but some families described how appetite had not improved, or had not fully improved, since treatment:
My appetite didn’t come back until maybe 4 days after I was discharged the second time.
Patient48_14y_NOT
Definitely not eating the same foods . . . he’s not the same . . . I took him to McDonald’s [Chicago, IL, USA] and he normally demolishes the whole meal . . . and he’d leave stuff, and that’s not normal.
Father57_12y_NOT
Regardless of whether the patient had undergone conservative or surgical treatment, some patients had been re-admitted to hospital following treatment owing to appendicitis recurrence or surgical complications, and some had visited a GP owing to concerns following treatment:
He ended up back in hospital for near on 10 days afterwards.
Mother57_12y_NOT
He ended up getting the infection afterwards, and we ended up going back in, didn’t we?
Mother44_9y_App
Patients who had had surgery and their parents described how they regularly checked the wound’s appearance, looking for signs of infection or reassurance that the scar was healing as expected:
My belly button is a bit weird, like full of, um, full of scabs and stuff. Yeah, I had to clean that out a few times. Well, when I say I cleaned it out, I mean my mum cleaned it out for me.
Patient57_12y_NOT
Yeah, looking at the wounds, making sure they weren’t red, that they were healing but we didn’t need to, you know, we didn’t have any follow-up with the nurse or anything like that.
Mother15_8y_App
Their accounts indicate that they used the appearance of the wound as an indicator of the success of treatment.
Some parents talked about how the experience of appendicitis and its treatment had affected their child’s psychological well-being, describing that their child was more moody, subdued or anxious since their hospital episode:
After a few days it set in, and I think about a week, she was . . . very, very hypersensitive about everything and very . . . quick to lose her temper or quick to get upset . . . She still is a bit.
Mother45_10y_NOT[App]
I think the whole experience has just kind of left [patient] a little bit . . . I don’t know, what would you say? Yeah, a bit traumatised.
Just a bit, yeah, a bit guarded and a bit more subdued than normal.
Family45_10y_NOT[App]
Parents and patients spoke about how their treatment had resulted in time away from school and time away from physical activities:
We were hoping that, obviously, the antibiotics were going to work the first time, then she could have gone back to school.
Mother60_9y_NOT[App]
Lots of patients described how having surgery had prevented them from engaging in social and physical activities they enjoyed, such as sports:
I had PE [physical education] today and I had to sit there . . . I literally have a football tournament this week on Friday and I can’t go but I’m going to ask the teacher if I can, like, stay there and watch.
Patient61_11y_App
Finally, some parents described how fever and pale skin could also indicate decline in their child’s condition, especially while in hospital:
The nurses were [saying], ‘Her temperature’s still up a bit’ . . . I’d have thought your temperature should go back to normal if [the antibiotics are] doing what they’re meant to do, but it wasn’t.
Mother45_10y_NOT[App]
Outcome mapping between qualitative work and systematic reviews
| Outcome from qualitative work | Mapped outcome from systematic reviews |
|---|---|
| Pain | Pain score or analgesia |
| Loss of appetite | Recovery of bowel function |
| Re-admission to hospital | Re-admission to hospital |
| Wound healing | Wound infection, wound healing time or wound complication |
| Child’s psychological well-being | Child’s quality of life |
| Time away from school | Time away from school |
| Time away from physical activity | Time away from full activity |
| Post-treatment fever | Post-treatment fever |
List of initial outcomes and descriptions presented in phase 1 of the Delphi survey
| Outcome | Description |
|---|---|
| Intra-abdominal abscess | A pocket of infected fluid or pus deep inside the tummy that may occur after appendicectomy of treatment with antibiotics and may require another procedure or more treatment with antibiotics |
| Reoperation (wording changed from ‘Need for operation/reoperation’) | Having another operation that was not planned |
| Bowel obstruction (wording changed from ‘Adhesive obstruction’) | A blockage of the intestine that would require treatment in hospital or may require an operation to treat it |
| Major or minor complication | Any type of complication classified as minor or major (excluding re-admission to hospital, bowel obstruction and recurrent appendicitis) |
| Re-admission to hospital | Needing to be re-admitted to hospital with a stay at least one night |
| Total health-care visits (wording changed from ‘Health-care visits’) | How many times the child visits a health-care professional after they go home following their initial hospital treatment |
| Any unplanned imaging (wording changed from ‘Post-treatment imaging’) | Having any type of X-ray or ultrasonographic test (other than CT) after treatment |
| Total cost of treatment (wording changed from ‘Total charges’) | The total cost of treatment for the health service |
| Duration of antibiotics | How long a child is treated with antibiotics for |
| Duration of home health care | How long additional health care is needed at home after the child’s initial hospital treatment |
| Death | Dying (please bear in mind that the risk of dying from appendicitis is very low, but it may still be important to measure this as an outcome in studies) |
| Quality of life (wording changed from ‘Paediatric quality of life’) | The child’s quality of life that is measured using a specifically designed questionnaire |
| Recurrent appendicitis | Getting appendicitis again |
| Antibiotic failure (wording simplified from ‘Complication of antibiotics or treatment intervention’, as other complications can be included in ‘major/minor complications’) | Operation to remove the appendix, because of antibiotic failure |
| Blood loss (from additional nine unmapped outcomes) | Blood loss during the operation – losing lots of blood during an operation is unusual but you might think it is important to measure this |
| Unplanned central venous catheter (from additional nine unmapped outcomes) | Having a central venous catheter or not. A catheter is a fine tube inserted into a large vein to give medicines, and is usually used when medicines into a vein are likely to be needed for > 1 week |
| Wound infection | An infection at the site of the operation that requires treatment with antibiotics |
| Wound complication (wording changed from ‘non-infectious wound complication’) | A complication with the surgical wound, such as the wound opening up before healing, or bleeding. This may need treatment with medicine or another procedure |
| Interventional radiology procedure | Whether or not a child needs an interventional radiology procedure. An interventional radiology procedure is where a radiologist puts a tube inside the tummy to drain pus, using an X-ray for guidance |
| Patient stress (proposed by the SSAG) | A measure of stress in the child that would be measured using a specifically designed questionnaire |
| Hospital length of stay | How long a child has to spend in hospital |
| Pain score | How severe a child reports that their pain is, using a pain score |
| Fever after treatment | A high temperature after treatment has started |
| Duration of drainage | If a small tube (called a drain) is used after an operation or to drain pus from the tummy, the length of time this is needed for. This is unusual after treatment of uncomplicated appendicitis, but can sometimes be needed |
| Time away from school | How long a child has to spend away from school or their normal activities |
| Other infectious complication | Other infection during or after treatment that is not related to the appendix or surgical wound. For example, a urine infection or chest infection |
| Blood markers of inflammation | Results of blood tests that indicate how well the child is responding to treatment |
| Analgesia (pain relief) | Number of doses and types of painkiller medicine (analgesia) that are needed |
| Bacterial peritoneal cultures | Which bacteria are grown from inside a patient’s tummy when fluid is tested |
| Wound healing time (from additional nine unmapped outcomes) | How long the wound takes to heal after an operation |
| Cosmesis | Cosmesis is the neatness of a wound and whether the child or parent is happy with how it looks |
| Time away from full activity | How long a child spends away from full activity, such as sport |
| Cost-effectiveness | Cost-effectiveness involved working out how much a treatment costs, while also considering whether or not the treatment worked |
| Conversion to open operation | If an operation that started out as keyhole (a few small holes) needs to be converted to an open operation (a larger cut) |
| Unplanned CT (from additional nine unmapped outcomes; the SSAG suggested it was distinct enough from ‘Any unplanned imaging’ to be a separate outcome) | Whether or not CT is needed after treatment. CT makes more detailed pictures of parts of your body than an X-ray but uses higher doses of X-rays |
| Time to ambulation (or get out of bed) | How long before the child can walk around or move normally |
| Operation time | Time taken for the operation, including time that the child is asleep for (under general anaesthetic) |
| Recovery of bowel function | How long it takes to be able to eat or pass a stool normally |
| Parental stress (from additional nine unmapped outcomes) | A measure of stress in the parent/guardian that would be measured using a specifically designed questionnaire |
| Parent time off work (added as separate outcome following discussion of updated review, as distinct from parental stress, from ‘parent disability days’29) | How long a parent/guardian spends away from work |
Core outcome set stakeholder selection criteria and methods of approaching potential participants
| Participants | Selection criteria | Method of approach |
|---|---|---|
| Patients and parents |
|
|
| Paediatric surgeons | All practising consultant paediatric surgeons in the UK who treat children with acute uncomplicated appendicitis were considered potential participants | Invited to participate via the mailing list of the BAPS, and through personal contacts of the investigators |
| General surgeons | Adult general surgeons in the UK who regularly treat children for acute uncomplicated appendicitis were considered potential participants. This included those identified as having an interest in the treatment of children | Invited to participate via the Association of Surgeons of Great Britain and Ireland, existing personal contacts and through regional paediatric surgical networks in the UK |
Invitations to participate in core outcome set development
| NHS site | Parents | Young people (aged 12–15 years) | Young adults (aged 16–19 years)a | Total |
|---|---|---|---|---|
| St George’sb | – | – | – | 80 |
| Birmingham | 28 | 26 | 7 | 61 |
| Southampton | 128 | 44 | 13 | 185 |
| Manchester | 64 | 33 | 14 | 111 |
| Sheffield | 43 | 42 | 6 | 91 |
| Alder Heyb | – | – | – | 199 |
| Leeds | 40 | 39 | 12 | 91 |
| Total | – | – | – | 818 |
Participant characteristics by registration and Delphi phase
| Participant characteristics | Registration (N = 195, 100%) | Delphi phase | ||
|---|---|---|---|---|
| 1 (N = 147, 75%) | 2 (N = 122, 63%) | 3 (N = 90, 46%) | ||
| Patients, n (%) | 15 (100) | 11 (73) | 3 (20) | 2 (20) |
| Patient age (years), median (range) | 12.5 (11–18)a | 13.5 (11–18)a | 12 (12–14)a | 14a |
| Parents, n (%) | 67 (100) | 57 (85) | 51 (76) | 32 (48) |
| Patient age (years), median (range) | 10 (3–18) | 10 (3–18) | 10 (3–18) | 10 (3–18) |
| Paediatric surgeons, n (%) | 57 (100) | 45 (79) | 39 (68) | 34 (60) |
| General surgeons, n (%) | 56 (100) | 34 (61) | 29 (52) | 21 (38) |
| Total, n (%) | 195 (100) | 147 (75) | 122 (63) | 89 (46) |
Consensus status of outcomes by Delphi phase
| Outcome | Patients | Parents | Surgeons |
|---|---|---|---|
| Operation time | |||
| Conversion to open operation | ✓ | ||
| Blood loss | ✓ | ||
| Wound infection | ✓ | ||
| Intra-abdominal abscess | ✓ | ✓ | ✓ |
| Wound complication | ✓ | ||
| Fever after treatment | ✓ | ||
| Blood markers of inflammation | ✓ | ||
| Other infectious complication | |||
| Duration of antibiotics | |||
| Recovery of bowel function | |||
| Time to ambulation | |||
| Hospital length of stay | |||
| Duration of drainage | ✓ | ||
| Unplanned CT | |||
| Any unplanned imaging | |||
| Interventional radiology procedure | |||
| Unplanned central venous catheter | |||
| Re-operation | ✓ | ✓ | |
| Antibiotic failure | ✓ | ✓ | |
| Analgesia | |||
| Pain score | |||
| Re-admission to hospital | ✓ | ✓ | |
| Bowel obstruction | ✓ | ||
| Recurrent appendicitis | ✓ | ✓ | |
| Major or minor complication | ✓ | ||
| Death | ✓ | ✓ | ✓ |
| Time away from school | |||
| Time away from full activity | |||
| Parent time off work | |||
| Wound healing time | |||
| Child’s quality of life | ✓ | ✓ | |
| Cosmesis | |||
| Parental stress | |||
| Patient stress | ✓ | ||
| Total cost of treatment | |||
| Cost-effectiveness | |||
| Total health-care visits | |||
| Duration of home health care | |||
| Bacterial peritoneal cultures |
| Outcome | Patients | Parents | Surgeons |
|---|---|---|---|
| Operation time | |||
| Conversion to open operation | ✓ | ✓ | |
| Blood loss | ✓ | ✓ | ✓ |
| Wound infection | ✓ | ||
| Intra-abdominal abscess | ✓ | ✓ | ✓ |
| Wound complication | ✓ | ✓ | |
| Fever after treatment | ✓ | ✓ | |
| Blood markers of inflammation | ✓ | ✓ | |
| Other infectious complication | ✓ | ✓ | |
| Duration of antibiotics | |||
| Recovery of bowel function | |||
| Time to ambulation | |||
| Hospital length of stay | |||
| Duration of drainage | ✓ | ✓ | |
| Unplanned CT | |||
| Any unplanned imaging | |||
| Interventional radiology procedure | ✓ | ||
| Unplanned central venous catheter | ✓ | ||
| Reoperation | ✓ | ✓ | ✓ |
| Antibiotic failure | ✓ | ✓ | |
| Analgesia | |||
| Pain score | ✓ | ✓ | |
| Re-admission to hospital | ✓ | ✓ | |
| Bowel obstruction | ✓ | ✓ | ✓ |
| Recurrent appendicitis | ✓ | ✓ | |
| Major or minor complication | ✓ | ✓ | ✓ |
| Death | ✓ | ✓ | ✓ |
| Time away from school | |||
| Time away from full activity | |||
| Parent time off work | |||
| Wound healing time | |||
| Child’s quality of life | ✓ | ✓ | ✓ |
| Cosmesis | ✓ | ||
| Parental stress | |||
| Patient stress | ✓ | ✓ | |
| Total cost of treatment | |||
| Cost-effectiveness | ✓ | ||
| Total health-care visits | |||
| Duration of home health care | |||
| Bacterial peritoneal cultures | ✓ | ||
| Psychological distress | |||
| Negative appendicectomy | |||
| Time to normal diet |
Attrition bias analysis between Delphi phases 1 and 2
| Outcome | Phase 1 scores, median (range) | p-value | |
|---|---|---|---|
| Completed phases 1 and 2 | Completed phase 1 only | ||
| Operation time | 6 (1–9) | 5 (1–9) | 0.835 |
| Conversion to open operation | 6 (1–9) | 5 (2–9) | 0.488 |
| Blood loss | 7.5 (1–9) | 7 (1–9) | 0.606 |
| Wound infection | 7 (1–9) | 6 (1–9) | 0.099 |
| Intra-abdominal abscess | 8 (3–9) | 8 (2–9) | 0.285 |
| Wound complication | 7 (3–9) | 7 (1–9) | 0.452 |
| Fever after treatment | 6 (1–9) | 7 (1–9) | 0.754 |
| Blood markers of inflammation | 6 (1–9) | 7 (1–9) | 0.315 |
| Other infectious complication | 6 (1–9) | 6 (1–9) | 0.445 |
| Duration of antibiotics | 6 (1–9) | 6 (1–9) | 0.839 |
| Recovery of bowel function | 6 (1–9) | 5 (1–9) | 0.026 |
| Time to ambulation | 6 (1–9) | 6 (3–9) | 0.818 |
| Hospital length of stay | 6 (1–9) | 7 (3–9) | 0.583 |
| Duration of drainage | 6 (1–9) | 6 (1–9) | 0.507 |
| Unplanned CT | 5 (1–9) | 5.5 (1–9) | 0.888 |
| Any unplanned imaging | 5 (1–9) | 5 (1–9) | 0.823 |
| Interventional radiology procedure | 6 (1–9) | 5 (1–9) | 0.685 |
| Unplanned central venous catheter | 7 (1–9) | 5 (1–9) | 0.164 |
| Reoperation | 8 (1–9) | 8 (1–9) | 0.442 |
| Antibiotic failure | 7 (1–9) | 8 (3–9) | 0.211 |
| Analgesia | 6 (1–9) | 5 (1–9) | 0.046 |
| Pain score | 6 (2–9) | 6 (1–9) | 0.169 |
| Re-admission to hospital | 7 (4–9) | 7 (3–9) | 0.300 |
| Bowel obstruction | 8 (3–9) | 7 (3–9) | 0.099 |
| Recurrent appendicitis | 8 (3–9) | 8 (1–9) | 0.961 |
| Major or minor complication | 8 (3–9) | 8 (3–9) | 0.980 |
| Death | 9 (1–9) | 9 (3–9) | 0.890 |
| Time away from school | 6 (1–9) | 6 (1–9) | 0.785 |
| Time away from full activity | 6 (1–9) | 6 (2–9) | 0.749 |
| Parent time off work | 5 (1–9) | 6 (1–9) | 0.498 |
| Wound healing time | 6 (1–9) | 6 (3–9) | 0.864 |
| Child’s quality of life | 7 (1–9) | 7 (3–9) | 0.806 |
| Cosmesis | 5 (1–9) | 5 (1–9) | 0.724 |
| Parental stress | 5 (1–9) | 5 (1–9) | 0.846 |
| Patient stress | 7 (1–9) | 6 (1–9) | 0.277 |
| Total cost of treatment | 5 (1–9) | 5 (1–9) | 0.278 |
| Cost-effectiveness | 6 (1–9) | 6 (1–9) | 0.792 |
| Total health-care visits | 5 (1–9) | 5 (1–9) | 0.626 |
| Duration of home health care | 5 (1–9) | 6 (1–9) | 0.366 |
| Bacterial peritoneal cultures | 5 (1–9) | 5 (1–9) | 0.673 |
Attrition bias analysis between Delphi phases 1 and 3
| Outcome | Phase 1 scores, median (range) | p-value | |
|---|---|---|---|
| Completed phases 1–3 | Completed phase 1 only | ||
| Operation time | 6 (2–9) | 6 (1–9) | 0.872 |
| Conversion to open operation | 6 (1–9) | 6 (1–9) | 0.574 |
| Blood loss | 7 (1–9) | 7 (1–9) | 0.326 |
| Wound infection | 7 (1–9) | 7 (1–9) | 0.350 |
| Intra-abdominal abscess | 8 (3–9) | 8 (2–9) | 0.327 |
| Wound complication | 7 (3–9) | 7 (1–9) | 0.971 |
| Fever after treatment | 6 (1–9) | 7 (1–9) | 0.312 |
| Blood markers of inflammation | 5 (1–9) | 7 (1–9) | 0.038 |
| Other infectious complication | 6 (1–9) | 6 (1–9) | 0.887 |
| Duration of antibiotics | 6 (1–9) | 6 (1–9) | 0.495 |
| Recovery of bowel function | 6 (1–9) | 6 (1–9) | 0.388 |
| Time to ambulation | 6 (1–9) | 6 (3–9) | 0.660 |
| Hospital length of stay | 6 (1–9) | 7 (2–9) | 0.115 |
| Duration of drainage | 6 (1–9) | 6 (1–9) | 0.236 |
| Unplanned CT | 5.5 (1–9) | 5 (1–9) | 0.772 |
| Any unplanned imaging | 5 (1–9) | 5 (1–9) | 0.821 |
| Interventional radiology procedure | 6 (1–9) | 6 (1–9) | 0.754 |
| Unplanned central venous catheter | 6 (1–9) | 6 (1–9) | 0.512 |
| Reoperation | 8 (1–9) | 8 (1–9) | 0.246 |
| Antibiotic failure | 7 (1–9) | 7 (1–9) | 0.257 |
| Analgesia | 6 (2–9) | 6 (1–9) | 0.891 |
| Pain score | 6 (2–9) | 6 (1–9) | 0.894 |
| Re-admission to hospital | 7 (4–9) | 7 (3–9) | 0.514 |
| Bowel obstruction | 8 (3–9) | 7 (3–9) | 0.168 |
| Recurrent appendicitis | 8 (3–9) | 8 (1–9) | 0.755 |
| Major or minor complication | 7.5 (3–9) | 8 (3–9) | 0.695 |
| Death | 9 (1–9) | 9 (3–9) | 0.521 |
| Time away from school | 6 (3–9) | 7 (1–9) | 0.422 |
| Time away from full activity | 6 (1–9) | 6 (1–9) | 0.787 |
| Parent time off work | 5 (1–9) | 6 (1–9) | 0.934 |
| Wound healing time | 6 (2–9) | 6 (1–9) | 0.594 |
| Child’s quality of life | 7 (3–9) | 7 (1–9) | 0.500 |
| Cosmesis | 5 (2–9) | 5 (1–9) | 0.564 |
| Parental stress | 6 (1–9) | 5 (1–9) | 0.167 |
| Patient stress | 7 (3–9) | 7 (1–9) | 0.731 |
| Total cost of treatment | 5 (1–9) | 5 (1–9) | 0.257 |
| Cost-effectiveness | 6 (1–9) | 6 (1–9) | 0.268 |
| Total health-care visits | 5 (1–9) | 5 (1–9) | 0.960 |
| Duration of home health care | 5 (1–9) | 5 (1–9) | 0.341 |
| Bacterial peritoneal cultures | 5 (1–9) | 5 (1–9) | 0.525 |
Consensus status of outcomes by stakeholder group
| Outcome | Delphi phase | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Conversion to open operation | ✓ | ✓ | |
| Blood loss | ✓ | ||
| Wound infection | ✓ | ✓ | |
| Intra-abdominal abscess | ✓ | ✓ | ✓ |
| Wound complication | ✓ | ||
| Fever after treatment | ✓ | ✓ | |
| Blood markers of inflammation | ✓ | ✓ | ✓ |
| Other infectious complication | ✓ | ✓ | |
| Duration of drainage | ✓ | ✓ | ✓ |
| Unplanned central venous catheter | ✓ | ✓ | |
| Reoperation | ✓ | ✓ | |
| Antibiotic failure | ✓ | ✓ | ✓ |
| Analgesia | ✓ | ||
| Pain score | ✓ | ✓ | |
| Re-admission to hospital | ✓ | ||
| Bowel obstruction | ✓ | ✓ | |
| Major or minor complication | ✓ | ✓ | |
| Death | ✓ | ✓ | |
| Time away from school | ✓ | ||
| Time away from full activity | ✓ | ||
| Wound healing time | ✓ | ||
| Child’s quality of life | ✓ | ✓ | |
| Cosmesis | ✓ | ✓ | |
| Patient stress | ✓ | ||
| Cost-effectiveness | ✓ | ||
| Bacterial peritoneal cultures | ✓ | ✓ | |
| Negative appendicectomy | ✓ | ||
| Outcome | Delphi phase | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Conversion to open operation | ✓ | ||
| Blood loss | ✓ | ✓ | ✓ |
| Wound infection | ✓ | ✓ | |
| Intra-abdominal abscess | ✓ | ✓ | ✓ |
| Wound complication | ✓ | ✓ | ✓ |
| Fever after treatment | ✓ | ✓ | ✓ |
| Blood markers of inflammation | ✓ | ||
| Other infectious complication | ✓ | ||
| Duration of drainage | ✓ | ||
| Re-operation | ✓ | ✓ | ✓ |
| Antibiotic failure | ✓ | ✓ | ✓ |
| Pain score | ✓ | ✓ | |
| Re-admission to hospital | ✓ | ✓ | ✓ |
| Bowel obstruction | ✓ | ✓ | |
| Recurrent appendicitis | ✓ | ✓ | ✓ |
| Major or minor complication | ✓ | ✓ | |
| Death | ✓ | ✓ | ✓ |
| Child’s quality of life | ✓ | ✓ | ✓ |
| Patient stress | ✓ | ✓ | ✓ |
| Outcome | Delphi phase | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Blood loss | ✓ | ✓ | |
| Intra-abdominal abscess | ✓ | ✓ | ✓ |
| Interventional radiology procedure | ✓ | ✓ | |
| Unplanned central venous catheter | ✓ | ||
| Reoperation | ✓ | ✓ | ✓ |
| Re-admission to hospital | ✓ | ✓ | ✓ |
| Bowel obstruction | ✓ | ✓ | ✓ |
| Recurrent appendicitis | ✓ | ✓ | ✓ |
| Major or minor complication | ✓ | ✓ | ✓ |
| Death | ✓ | ✓ | ✓ |
| Child’s quality of life | ✓ | ✓ | |
Consensus meeting attendees
| Participant | Role | Stakeholder panel |
|---|---|---|
| 1a | Participant | Parent (mother) |
| 2a | Participant | Parent (mother) |
| 3a | Participant | Parent (mother) |
| 4a | Participant | Parent (mother) |
| 5a | Participant | Parent (mother) |
| 6a,b | Participant | Parent (mother) |
| 7a,b | Participant | Parent (father) |
| 8a,b | Participant | Parent (mother) |
| 9a,b | Participant | Parent (father) |
| 10a | Participant | Patient (female) |
| 11a,c | Participant | Patient (male) |
| 12 | PPI representative (CYP) | N/A |
| 13 | PPI representative (CYP) | N/A |
| 14 | PPI representative (parent) | N/A |
| 15 | PPI representative (parent) | N/A |
| 16 | PPI lead for CONTRACT | N/A |
| 17 | Chairperson | N/A |
| 18 | Study researcher | N/A |
| 19 | Study researcher | N/A |
| 20 | Study administrator | N/A |
| 21 | PhD student (observer) | N/A |
Summary of outcomes discussed during the surgeons’ meeting
| Outcome | Number of stakeholder panels that voted ‘consensus in’ during phase 3 | Meeting voters scoring 7–9 (%) | Meeting voters scoring 1–3 (%) | Decision category | Consensus in | Notes |
|---|---|---|---|---|---|---|
| Intra-abdominal abscess | 3/3 | N/A | N/A | 1 | ✓ | Agreed vote was unnecessary, as no participants opposed including the outcome |
| Re-operation | 3/3 | N/A | N/A | 1 | ✓ | Agreed vote was unnecessary, as no participants opposed including the outcome |
| Bowel obstruction | 3/3 | N/A | N/A | 1 | ✓ | Agreed vote was unnecessary, as no participants opposed including the outcome |
| Major or minor complication | 3/3 | N/A | N/A | 5 | ✗ | Discussed and not voted, left for later discussion, at which excluded. Participants felt the outcome was too broad to be meaningful and all relevant major complications included in other single outcomes |
| Re-admission to hospital | 3/3 | N/A | N/A | 1 | ✓ | Agreed vote was unnecessary, as no participants opposed including the outcome |
| Total health-care visits | 0/3 | N/A | N/A | 2 | ✗ | Agreed vote was unnecessary, as no participants opposed excluding the outcome |
| Any unplanned imaging | 0/3 | N/A | N/A | 2 | ✗ | Agreed vote was unnecessary, as no participants opposed excluding the outcome |
| Total cost of treatment | 0/3 | 18 | 35 | 3 | ✗ | Discussed and voted |
| Duration of antibiotics | 0/3 | 12 | 59 | 3 | ✗ | Discussed and voted |
| Duration of home health care | 0/3 | N/A | N/A | 3 | ✗ | Agreed vote was unnecessary, as no participants opposed excluding the outcome |
| Death | 2/3 | N/A | N/A | 1 | ✓ | Agreed vote was unnecessary, as no participants opposed including the outcome |
| Quality of life | 2/3 | 82 | 6 | 3 | ✓ | Discussed and voted |
| Recurrent appendicitis | 2/3 | N/A | N/A | 1 | ✓ | Agreed vote was unnecessary, as no participants opposed including the outcome |
| Antibiotic failurea | 2/3 | 82 | 0 | 3 | ✓ | Discussed and voted |
| Blood loss | 2/3 | 24 | 53 | 4 | ✗ | Discussed and voted, but subsequent discussion to redefine outcome and revote (see Outcomes that surgeons re-voted on once redefined) |
| Unplanned central venous catheter | 2/3 | 24 | 41 | 3 | ✗ | Discussed and voted |
| Wound infection | 2/3 | 88 | 6 | 3 | ✓ | Discussed and voted |
| Wound complication | 1/3 | 56 | 19 | 3 | ✗ | Discussed and voted |
| Interventional radiology procedure | 1/3 | 63 | 25 | 3 | ✗ | Discussed and voted, but subsequent discussion to combine with other outcome (reoperation). See Outcomes that surgeons re-voted on once combined with another outcome |
| Patient stress | 1/3 | 18 | 59 | 3 | ✗ | Discussed and voted |
| Hospital length of stay | 0/3 | 94 | 6 | 3 | ✓ | Discussed and voted |
| Pain score | 2/3 | 6 | 47 | 3 | ✗ | Discussed and voted |
| Fever after treatment | 2/3 | 0 | 71 | 3 | ✗ | Discussed and voted |
| Duration of drainage | 1/3 | N/A | N/A | 2 | ✗ | Agreed vote was unnecessary, as no participants opposed excluding the outcome |
| Time away from school | 1/3 | 12 | 59 | 3 | ✗ | Discussed and voted |
| Negative appendicectomy | 1/3 | 82 | 6 | 3 | ✓ | Discussed and voted |
| Other infectious complication | 1/3 | N/A | N/A | 5 | ✗ | Discussed and not voted, but subsequent discussion to exclude (see Outcomes that surgeons re-voted on once combined with another outcome) |
| Blood markers of inflammation | 1/3 | N/A | N/A | 2 | ✗ | Agreed vote was unnecessary, as no participants opposed excluding the outcome |
| Analgesia | 1/3 | N/A | N/A | 2 | ✗ | Agreed vote was unnecessary, as no participants opposed excluding the outcome |
| Bacterial peritoneal cultures | 1/3 | N/A | N/A | 2 | ✗ | Agreed vote was unnecessary, as no participants opposed excluding the outcome |
| Wound healing time | 1/3 | N/A | N/A | 2 | ✗ | Agreed vote was unnecessary, as no participants opposed excluding the outcome |
| Cosmesis | 1/3 | N/A | N/A | 2 | ✗ | Agreed vote was unnecessary, as no participants opposed excluding the outcome |
| Time away from full activity | 1/3 | 76 | 6 | 3 | ✓ | Discussed and voted |
| Psychological effects | 0/3 | N/A | N/A | 2 | ✗ | Agreed vote was unnecessary, as no participants opposed excluding the outcome |
| Cost-effectiveness | 0/3 | 53 | 6 | 3 | ✗ | Discussed and voted |
| Conversion to open operation | 0/3 | 18 | 65 | 3 | ✗ | Discussed and voted |
| Unplanned CT | 0/3 | N/A | N/A | 2 | ✗ | Agreed vote was unnecessary, as no participants opposed excluding the outcome |
| Time to ambulation | 0/3 | N/A | N/A | 2 | ✗ | Agreed vote was unnecessary, as no participants opposed excluding the outcome |
| Operation time | 0/3 | N/A | N/A | 2 | ✗ | Agreed vote was unnecessary, as no participants opposed excluding the outcome |
| Recovery of bowel function | 0/3 | N/A | N/A | 2 | ✗ | Agreed vote was unnecessary, as no participants opposed excluding the outcome |
| Parental stress | 0/3 | N/A | N/A | 2 | ✗ | Agreed vote was unnecessary, as no participants opposed excluding the outcome |
| Parent time off work | 0/3 | N/A | N/A | 2 | ✗ | Agreed vote was unnecessary, as no participants opposed excluding the outcome |
| Time to normal diet | 0/3 | N/A | N/A | 2 | ✗ | Agreed vote was unnecessary, as no participants opposed excluding the outcome |
Outcomes that surgeons re-voted on once redefined
| Outcome | Number of stakeholder panels that voted ‘consensus in’ during phase 3 | Meeting voters scoring 7–9 (%) | Meeting voters scoring 1–3 (%) | Decision category | Consensus in | Notes |
|---|---|---|---|---|---|---|
| Blood loss requiring transfusion | N/A | 76 | 18 | 3 | ✗ | Discussed and voted |
Outcomes that surgeons re-voted on once combined with another outcome
| Outcome | Number of stakeholder panels that voted ‘consensus in’ during phase 3 | Meeting voters scoring 7–9 (%) | Meeting voters scoring 1–3 (%) | Decision category | Consensus in | Notes |
|---|---|---|---|---|---|---|
| Interventional radiology procedure (as a subset of reoperation) | N/A | 88 | 6 | 3 | ✓ | Discussed and voted |
| Other infectious complication | 1/3 | N/A | N/A | 2 | ✗ | Discussed and agreed to exclude |
Summary of outcomes discussed during the parents’ and patients’ meeting
| Outcome | Number of stakeholder panels that voted ‘consensus in’ during phase 3 | Meeting voters scoring 7–9 (%) | Meeting voters scoring 1–3 (%) | Decision category | Consensus in | Notes |
|---|---|---|---|---|---|---|
| Intra-abdominal abscess | 3/3 | N/A | N/A | 1 | ✓ | Agreed vote was unnecessary, as no participants opposed including the outcome |
| Re-operation | 3/3 | N/A | N/A | 1 | ✓ | Agreed vote was unnecessary, as no participants opposed including the outcome |
| Bowel obstruction | 3/3 | N/A | N/A | 1 | ✓ | Agreed vote was unnecessary, as no participants opposed including the outcome |
| Major or minor complication | 3/3 | N/A | N/A | 5 | ✗ | Discussed and not voted, but later discussion to exclude. Participants felt that the outcomes already included (e.g. intra-abdominal abscess, bowel obstruction) accounted for the key major complications, and that ‘minor complications’ was too broad and less important |
| Re-admission to hospital | 3/3 | 91 | 0 | 3 | ✓ | Discussed and voted |
| Total health-care visits | 0/3 | N/A | N/A | 2 | ✗ | Agreed vote was unnecessary, as no participants opposed excluding the outcome |
| Any unplanned imaging | 0/3 | 27 | 27 | 3 | ✗ | Discussed and voted |
| Total cost of treatment | 0/3 | N/A | N/A | 2 | ✗ | Agreed vote was unnecessary, as no participants opposed excluding the outcome |
| Duration of antibiotics | 0/3 | 9 | 37 | 3 | ✗ | Discussed and voted |
| Duration of home health care | 0/3 | N/A | N/A | 2 | ✗ | Agreed vote was unnecessary, as no participants opposed excluding the outcome |
| Death | 2/3 | N/A | N/A | 1 | ✓ | Agreed vote was unnecessary, as no participants opposed including the outcome |
| Quality of life | 2/3 | N/A | N/A | 1 | ✓ | Agreed vote was unnecessary, as no participants opposed including the outcome |
| Recurrent appendicitis | 2/3 | N/A | N/A | 1 | ✓ | Agreed vote was unnecessary, as no participants opposed including the outcome |
| Antibiotic failurea | 2/3 | N/A | N/A | 1 | ✓ | Agreed vote was unnecessary, as no participants opposed including the outcome |
| Blood loss | 2/3 | 18 | 45 | 3 | ✗ | Discussed and voted |
| Unplanned central venous catheter | 2/3 | 45 | 9 | 3 | ✗ | Discussed and voted |
| Wound infection | 2/3 | 82 | 0 | 3 | ✓ | Discussed and voted |
| Wound complication | 1/3 | 90 | 0 | 3 | ✓ | Discussed and voted |
| Interventional radiology procedure | 1/3 | 90 | 0 | 3 | ✓ | Discussed and voted |
| Patient stress | 1/3 | 90 | 10 | 3 | ✓ | Discussed and voted |
| Hospital length of stay | 0/3 | 10 | 50 | 3 | ✗ | Discussed and voted |
| Pain score | 2/3 | 27 | 45 | 3 | ✗ | Discussed and voted |
| Fever after treatment | 2/3 | N/A | N/A | 2 | ✗ | Agreed vote was unnecessary, as no participants opposed excluding the outcome |
| Duration of drainage | 1/3 | N/A | N/A | 2 | ✗ | Agreed vote was unnecessary, as no participants opposed excluding the outcome |
| Time away from school | 1/3 | 9 | 54 | 3 | ✗ | Discussed and voted |
| Negative appendicectomy | 1/3 | 91 | 0 | 3 | ✓ | Discussed and voted |
| Other infectious complication | 1/3 | 30 | 50 | 3 | ✗ | Discussed and voted |
| Blood markers of inflammation | 1/3 | N/A | N/A | 2 | ✗ | Agreed vote was unnecessary, as no participants opposed excluding the outcome |
| Analgesia | 1/3 | N/A | N/A | 2 | ✗ | Agreed vote was unnecessary, as no participants opposed excluding the outcome |
| Bacterial peritoneal cultures | 1/3 | N/A | N/A | 2 | ✗ | Agreed vote was unnecessary, as no participants opposed excluding the outcome |
| Wound healing time | 1/3 | N/A | N/A | 2 | ✗ | Agreed vote was unnecessary, as no participants opposed excluding the outcome |
| Cosmesis | 1/3 | 0 | 72 | 3 | ✗ | Discussed and voted |
| Time away from full activity | 1/3 | N/A | N/A | 2 | ✗ | Agreed vote was unnecessary, as no participants opposed excluding the outcome |
| Psychological effects | 0/3 | 64 | 9 | 3 | ✗ | Discussed and voted |
| Cost-effectiveness | 0/3 | 18 | 45 | 3 | ✗ | Discussed and voted |
| Conversion to open operation | 0/3 | N/A | N/A | 2 | ✗ | Agreed vote was unnecessary, as no participants opposed excluding the outcome |
| Unplanned CT | 0/3 | N/A | N/A | 2 | ✗ | Agreed vote was unnecessary, as no participants opposed excluding the outcome |
| Time to ambulation | 0/3 | N/A | N/A | 2 | ✗ | Agreed vote was unnecessary, as no participants opposed excluding the outcome |
| Operation time | 0/3 | N/A | N/A | 2 | ✗ | Agreed vote was unnecessary, as no participants opposed excluding the outcome |
| Recovery of bowel function | 0/3 | N/A | N/A | 2 | ✗ | Agreed vote was unnecessary, as no participants opposed excluding the outcome |
| Parental stress | 0/3 | N/A | N/A | 2 | ✗ | Agreed vote was unnecessary, as no participants opposed excluding the outcome |
| Parent time off work | 0/3 | N/A | N/A | 2 | ✗ | Agreed vote was unnecessary, as no participants opposed excluding the outcome |
| Time to normal diet | 0/3 | N/A | N/A | 2 | ✗ | Agreed vote was unnecessary, as no participants opposed excluding the outcome |
Final core outcomes, grouped by Outome Measures in Rheumatology domains
Participants’ ranked votes for a primary outcome in a future trial
| Outcome | Stakeholder, n (%) | |||
|---|---|---|---|---|
| Young people (N = 3) | Parents (N = 31) | Surgeons (N = 54) | Total (N = 88) | |
| Antibiotic failure | 0 (0) | 18 (58) | 35 (65) | 53 (60) |
| Recurrent appendicitis | 2 (67) | 18 (58) | 24 (44) | 44 (50) |
| Re-admission | 1 (33) | 11 (36) | 25 (46) | 37 (42) |
| Quality of life | 2 (67) | 15 (48) | 14 (26) | 31 (35) |
| Wound infection | 3 (100) | 14 (45) | 11 (20) | 28 (32) |
| Death | 1 (33) | 12 (39) | 12 (22) | 25 (28) |
| Reoperation | 2 (67) | 12 (39) | 10 (19) | 24 (27) |
| Bowel obstruction | 1 (33) | 9 (29) | 9 (17) | 19 (22) |
| Other infectious complication | 1 (33) | 9 (29) | 8 (15) | 18 (20) |
| Negative appendicectomy | 0 (0) | 3 (10) | 11 (20) | 14 (16) |
| Cost-effectiveness | 0 (0) | 1 (3) | 12 (22) | 13 (15) |
| Patient stress | 1 (33) | 5 (16) | 6 (11) | 12 (14) |
| Blood loss | 1 (33) | 8 (26) | 3 (6) | 12 (14) |
| Central venous catheter | 0 (0) | 4 (13) | 9 (17) | 11 (13) |
| Time to ambulation | 1 (33) | 4 (13) | 5 (9) | 10 (11) |
Proposed primary outcome
FIGURE 23.
Combination matrix for the proposed primary outcome. Each column represents the response of a single participant. CVC, central venous catheter; QoL, quality of life.
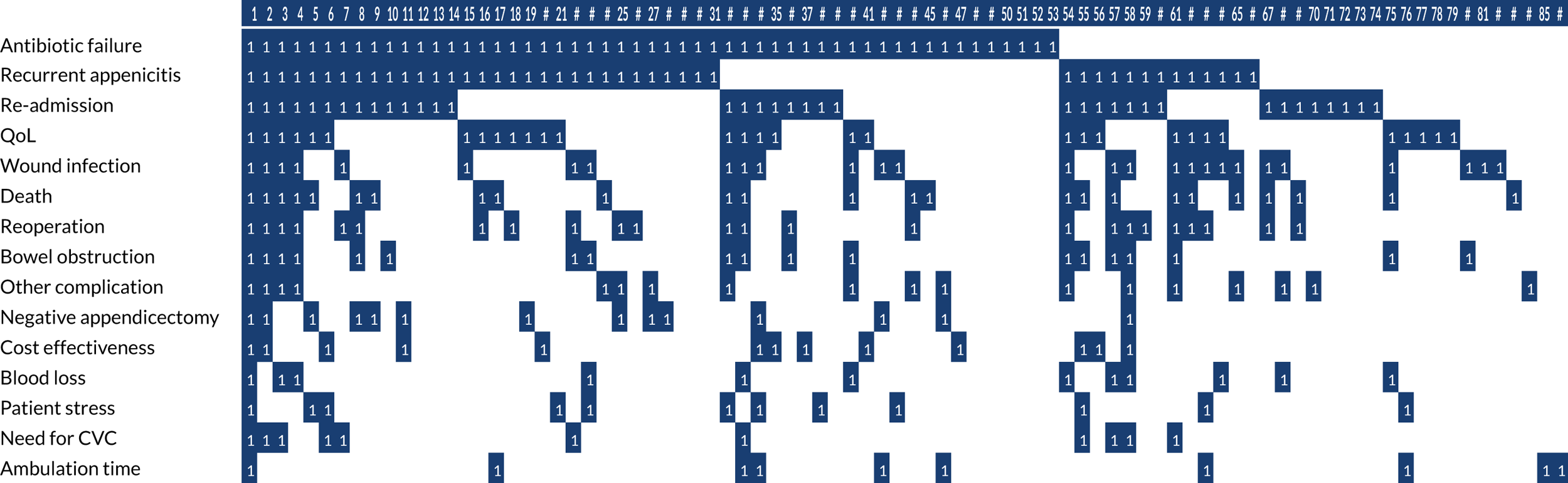
Surgeon and total respondents’ primary outcome preferences
FIGURE 24.
Percentage of surgeon and total respondents proposing each outcome be considered as a single outcome or as part of a composite primary outcome.
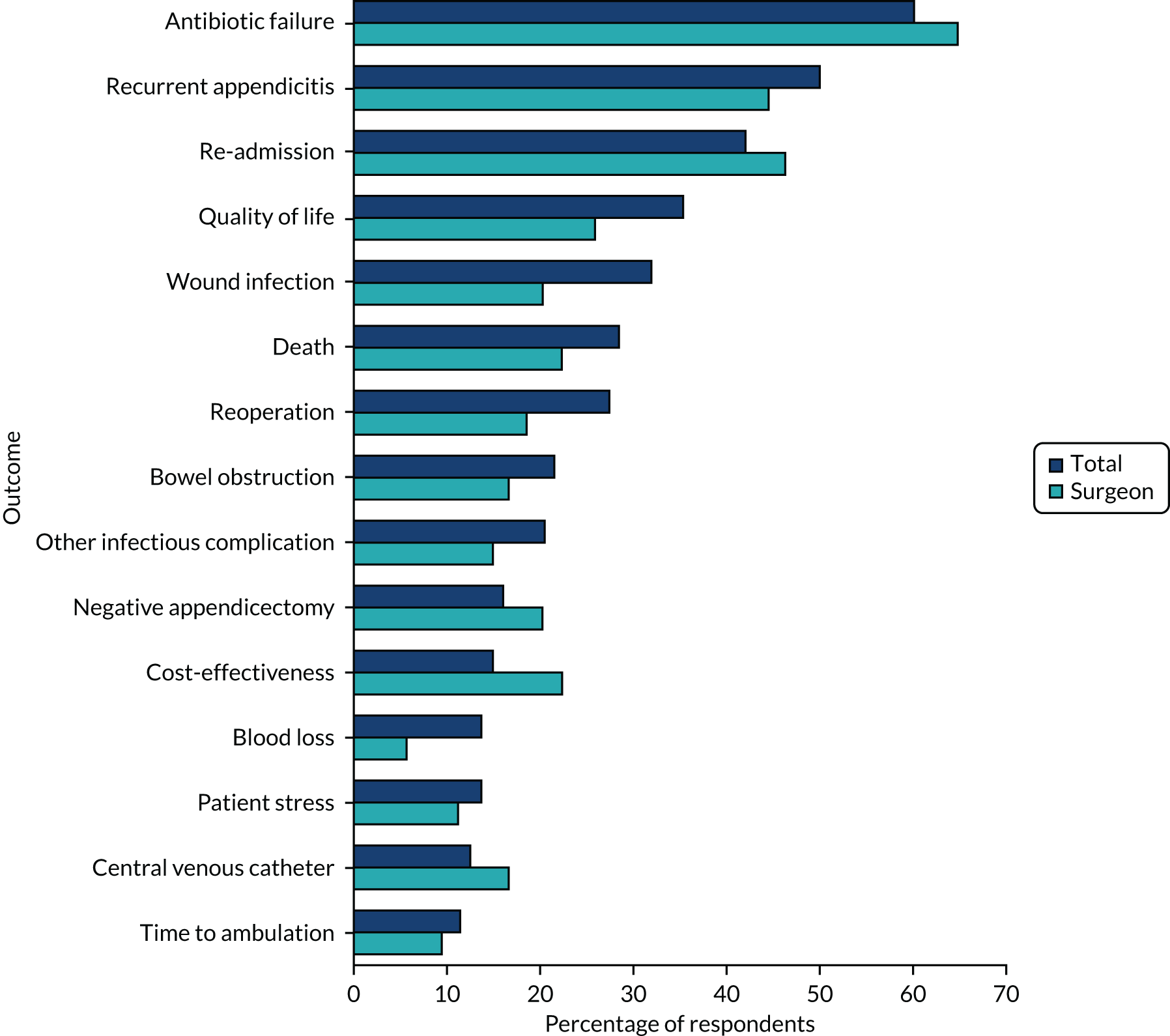
Appendix 4 Economic analysis
Example of a patient clinical inventory
FIGURE 25.
Patient clinical inventory for appendicectomy arm.
Electronic case report form
FIGURE 26.
Screenshot of the eCRF.
Resource use, source: electronic case report form (Medidata Rave database)
| Classification | Treatment arm | Trial participants, n | Resource use, mean (SD) |
|---|---|---|---|
| Resource use at 6 weeks | |||
| A&E visits | Non-operative treatment | 23 | 0.09 (0.4) |
| Appendicectomy | 23 | 0.09 (0.3) | |
| GP visits | Non-operative treatment | 23 | 0.13 (0.3) |
| Appendicectomy | 23 | 0.39 (0.9) | |
| Practice nurse visits | Non-operative treatment | 23 | 0.04 (0.2) |
| Appendicectomy | 23 | 0.04 (0.2) | |
| Hospital outpatient | Non-operative treatment | 23 | 0.09 (0.3) |
| Appendicectomy | 23 | 0.17 (0.7) | |
| Laboratory tests | Non-operative treatment | 23 | 0.52 (2.1) |
| Appendicectomy | 23 | 0.96 (2.5) | |
| Walk-in centres | Non-operative treatment | 23 | 0.13 (0.3) |
| Appendicectomy | 23 | – (0.0) | |
| Resource use at 3 months | |||
| A&E visits | Non-operative treatment | 21 | 0.24 (0.4) |
| Appendicectomy | 24 | 0.08 (0.3) | |
| GP visits | Non-operative treatment | 21 | 0.14 (0.4) |
| Appendicectomy | 24 | 0.04 (0.2) | |
| Practice nurse visits | Non-operative treatment | 21 | – (0.0) |
| Appendicectomy | 24 | – (0.0) | |
| Hospital outpatient | Non-operative treatment | 21 | 0.10 (0.3) |
| Appendicectomy | 24 | 0.08 (0.3) | |
| Laboratory tests | Non-operative treatment | 21 | 0.76 (1.8) |
| Appendicectomy | 24 | 0.13 (0.6) | |
| Walk-in centres | Non-operative treatment | 21 | 0.05 (0.2) |
| Appendicectomy | 24 | – (0.0) | |
| Resource use at 6 months | |||
| A&E visits | Non-operative treatment | 21 | – (0.0) |
| Appendicectomy | 23 | – (0.0) | |
| GP visits | Non-operative treatment | 21 | 0.10 (0.3) |
| Appendicectomy | 23 | – (0.0) | |
| Practice nurse visits | Non-operative treatment | 21 | – (0.0) |
| Appendicectomy | 23 | – (0.0) | |
| Hospital outpatient | Non-operative treatment | 21 | – (0.0) |
| Appendicectomy | 23 | 0.09 (0.3) | |
| Laboratory tests | Non-operative treatment | 21 | – (0.0) |
| Appendicectomy | 23 | – (0.0) | |
| Walk-in centres | Non-operative treatment | 21 | – (0.0) |
| Appendicectomy | 23 | – (0.0) | |
Costs (£), source: electronic case report form (Medidata Rave database)
| Classification | Treatment arm | Trial participants, n | Cost (£), mean (SD) |
|---|---|---|---|
| Costs from baseline to 6 weeks | |||
| A&E visits | Non-operative treatment | 23 | 13.00 (62.3) |
| Appendicectomy | 23 | 13.00 (43.1) | |
| GP visits | Non-operative treatment | 23 | 5.58 (14.7) |
| Appendicectomy | 23 | 16.75 (38.1) | |
| Practice nurse | Non-operative treatment | 23 | 0.59 (2.8) |
| Appendicectomy | 23 | 0.59 (2.8) | |
| Hospital outpatient | Non-operative treatment | 23 | 2.98 (9.9) |
| Appendicectomy | 23 | 5.96 (22.3) | |
| Laboratory tests | Non-operative treatment | 23 | 0.44 (1.8) |
| Appendicectomy | 23 | 0.80 (2.1) | |
| Walk-in centre and other health-related care | Non-operative treatment | 23 | 5.92 (15.6) |
| Appendicectomy | 23 | – (0.0) | |
| Total costs | Non-operative treatment | 23 | 28.51 (66.0) |
| Appendicectomy | 23 | 37.10 (83.0) | |
| Costs from 6 weeks to 3 months | |||
| A&E visits | Non-operative treatment | 21 | 35.60 (65.2) |
| Appendicectomy | 24 | 12.46 (42.2) | |
| GP visits | Non-operative treatment | 21 | 6.11 (15.3) |
| Appendicectomy | 24 | 1.78 (8.7) | |
| Practice nurse | Non-operative treatment | 21 | – (0.0) |
| Appendicectomy | 24 | – (0.0) | |
| Hospital outpatient | Non-operative treatment | 21 | 3.26 (10.3) |
| Appendicectomy | 24 | 2.86 (9.7) | |
| Laboratory tests | Non-operative treatment | 21 | 2.16 (9.9) |
| Appendicectomy | 24 | – (0.0) | |
| Walk-in centre and other health-related care | Non-operative treatment | 21 | 0.64 (1.5) |
| Appendicectomy | 24 | 0.11 (0.5) | |
| Total costs | Non-operative treatment | 21 | 47.14 (78.6) |
| Appendicectomy | 24 | 17.10 (48.8) | |
| Costs from 3 months to 6 months | |||
| A&E visits | Non-operative treatment | 21 | – (0.0) |
| Appendicectomy | 23 | – (0.0) | |
| GP visits | Non-operative treatment | 21 | 4.08 (12.9) |
| Appendicectomy | 23 | – (0.0) | |
| Practice nurse | Non-operative treatment | 21 | – (0.0) |
| Appendicectomy | 23 | – (0.0) | |
| Hospital outpatient | Non-operative treatment | 21 | – (0.0) |
| Appendicectomy | 23 | 2.98 (9.9) | |
| Laboratory tests | Non-operative treatment | 21 | – (0.0) |
| Appendicectomy | 23 | – (0.0) | |
| Walk-in centre and other health-related care | Non-operative treatment | 21 | – (0.0) |
| Appendicectomy | 23 | – (0.0) | |
| Total costs | Non-operative treatment | 21 | 4.08 (12.9) |
| Appendicectomy | 23 | 2.98 (9.9) | |
Resource use, source: Client Service Receipt Inventory
| Classification | Treatment arm | Trial participants, n | Resource use, mean (SD) |
|---|---|---|---|
| Resource use at 6 weeks | |||
| A&E visits | Non-operative treatment | 16 | 0.06 (0.3) |
| Appendicectomy | 24 | 0.17 (0.5) | |
| GP visits | Non-operative treatment | 16 | 0.19 (0.4) |
| Appendicectomy | 24 | 0.38 (0.9) | |
| GP home visits | Non-operative treatment | 16 | 0.06 (0.3) |
| Appendicectomy | 24 | 0.04 (0.2) | |
| GP telephone | Non-operative treatment | 16 | 0.06 (0.3) |
| Appendicectomy | 24 | – (0.0) | |
| Practice nurse visits | Non-operative treatment | 16 | 0.75 (3.0) |
| Appendicectomy | 24 | 0.08 (0.4) | |
| Health visitor | Non-operative treatment | 16 | – (0.0) |
| Appendicectomy | 24 | – (0.0) | |
| Community paediatrician | Non-operative treatment | 16 | 0.06 (0.3) |
| Appendicectomy | 24 | – (0.0) | |
| Hospital outpatient | Non-operative treatment | 16 | 0.25 (0.8) |
| Appendicectomy | 24 | 0.08 (0.3) | |
| Other health visits | Non-operative treatment | 16 | 0.19 (0.4) |
| Appendicectomy | 24 | – (0.0) | |
| Walk-in centres | Non-operative treatment | 16 | 0.06 (0.3) |
| Appendicectomy | 24 | 0.04 (0.2) | |
Costs (£), source: Client Service Receipt Inventory
| Classification | Treatment arm | Trial participants, n | Cost (£), mean (SD) |
|---|---|---|---|
| A&E visits | Non-operative treatment | 16 | 9.34 (37.4) |
| Appendicectomy | 24 | 24.92 (72.0) | |
| GP practice visits | Non-operative treatment | 16 | 8.03 (17.3) |
| Appendicectomy | 24 | 16.05 (37.5) | |
| GP home visits | Non-operative treatment | 16 | 13.63 (54.5) |
| Appendicectomy | 24 | 9.08 (44.5) | |
| GP telephone | Non-operative treatment | 16 | 1.74 (7.0) |
| Appendicectomy | 24 | – (0.0) | |
| Practice nurse visits | Non-operative treatment | 16 | 10.13 (40.5) |
| Appendicectomy | 24 | 1.13 (5.5) | |
| Health visitor | Non-operative treatment | 16 | – (0.0) |
| Appendicectomy | 24 | – (0.0) | |
| Community paediatrician | Non-operative treatment | 16 | 17.65 (70.6) |
| Appendicectomy | 24 | – (0.0) | |
| Hospital outpatient | Non-operative treatment | 16 | 8.57 (26.5) |
| Appendicectomy | 24 | 2.86 (9.7) | |
| Other health visits | Non-operative treatment | 16 | 8.51 (18.3) |
| Appendicectomy | 24 | – (0.0) | |
| Walk-in centres | Non-operative treatment | 16 | 2.84 (11.4) |
| Appendicectomy | 24 | 1.89 (9.3) | |
| Total costs, 6 weeks | Non-operative treatment | 16 | 80.43 (169.7) |
| Appendicectomy | 24 | 55.92 (88.0) |
Family-borne costs (£)
| CSRI, 6 weeks | Treatment arm | Trial participants, n | Cost (£), mean (SD) |
|---|---|---|---|
| Travel cost | Non-operative treatment | 16 | 5.44 (9.3) |
| Appendicectomy | 23 | 4.87 (10.4) | |
| Parking cost | Non-operative treatment | 16 | 3.00 (6.4) |
| Appendicectomy | 23 | 3.74 (9.1) | |
| Child care cost | Non-operative treatment | 16 | – (0.0) |
| Appendicectomy | 23 | – (0.0) | |
| Other household costs | Non-operative treatment | 16 | – (0.0) |
| Appendicectomy | 23 | – (0.0) | |
| Food cost | Non-operative treatment | 0 | – (0.0) |
| Appendicectomy | 0 | – (0.0) | |
| Other | Non-operative treatment | 0 | – (0.0) |
| Appendicectomy | 0 | – (0.0) | |
| School days lost (days) | Non-operative treatment | 10 | 8.65 (9.2) |
| Appendicectomy | 23 | 7.78 (5.2) | |
| Employment days lost (days) | Non-operative treatment | 16 | 2.44 (4.8) |
| Appendicectomy | 24 | 3.42 (4.5) | |
| CSRI time taken to complete (minutes) | Non-operative treatment | 11 | 7.27 (4.7) |
| Appendicectomy | 20 | 6.05 (3.0) |
Health-related quality of life: utility values
| Timing of assessment | Non-operative treatment | Appendicectomy | Difference, mean (95% CI) | ||
|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | ||
| Baseline | 28 | 0.532 (0.34) | 28 | 0.564 (0.33) | –0.032 (–0.21 to 0.15) |
| Discharge | 26 | 0.920 (0.10) | 27 | 0.721 (0.26) | 0.199 (0.09 to 0.31) |
| 2 weeks | 13 | 0.988 (0.03) | 15 | 0.894 (0.31) | 0.094 (–0.08 to 0.27) |
| 6 weeks | 27 | 0.962 (0.07) | 26 | 0.976 (0.05) | –0.014 (–0.05 to 0.02) |
| 3 months | 27 | 0.976 (0.05) | 27 | 0.993 (0.02) | –0.017 (–0.04 to 0.00) |
| 6 months | 25 | 0.995 (0.02) | 27 | 0.984 (0.06) | 0.011 (–0.01 to 0.03) |
| Timing of assessment | Non-operative treatment | Appendicectomy | Difference, mean (95% CI) | ||
|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | ||
| Baseline | 25 | 0.605 (0.18) | 23 | 0.571 (0.13) | 0.033 (–0.06 to 0.13) |
| Discharge | 21 | 0.895 (0.08) | 22 | 0.687 (0.14) | 0.208 (0.14 to 0.28) |
| 2 weeks | 9 | 0.972 (0.05) | 12 | 0.862 (0.17) | 0.110 (–0.01 to 0.23) |
| 6 weeks | 24 | 0.945 (0.06) | 23 | 0.970 (0.04) | –0.025 (–0.06 to 0.01) |
| 3 months | 20 | 0.949 (0.09) | 23 | 0.974 (0.04) | –0.025 (–0.06 to 0.01) |
| 6 months | 20 | 0.974 (0.05) | 23 | 0.967 (0.09) | 0.008 (–0.04 to 0.05) |
Appendix 5 Surgeon survey
Detail of questions and format of survey of UK-based paediatric surgeons
Detail of responses to survey of UK-based paediatric surgeons
Question: Please indicate your level of clinical experience regarding non-operative treatment of acute uncomplicated appendicitis in children (single answer).
-
51%: never offered non-operative treatment
-
40%: offered it in select circumstances
-
10%: offered it in a research study only.
Question: Please indicate your views of non-operative treatment of acute uncomplicated appendicitis in children and young people. This may be based on your reading of the literature, discussion with colleagues, clinical experience, etc. (single answer).
-
40%: should only be used in a research study.
-
30%: would be willing to consider it at parental request.
-
20%: should be routinely discussed with parents.
-
10%: should never be used.
FIGURE 27.
Question: Please rate how you view the strength of research evidence for the use of non-operative treatment as an alternative to appendicectomy for acute uncomplicated appendicitis in children and young people (single answer).
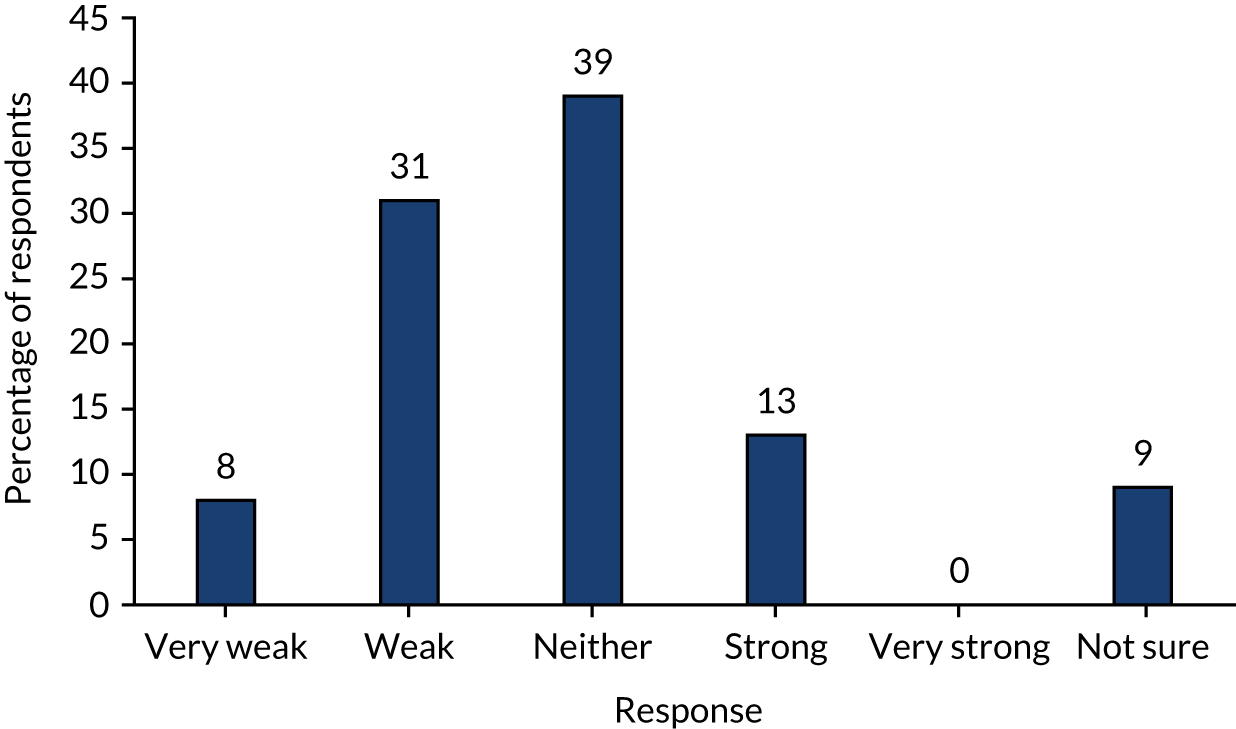
FIGURE 28.
Question: In your opinion, how does the efficacy of non-operative treatment for acute uncomplicated appendicitis in children and young people compare with operative treatment? (single answer.) App, appendicectomy; non-op, non-operative treatment.
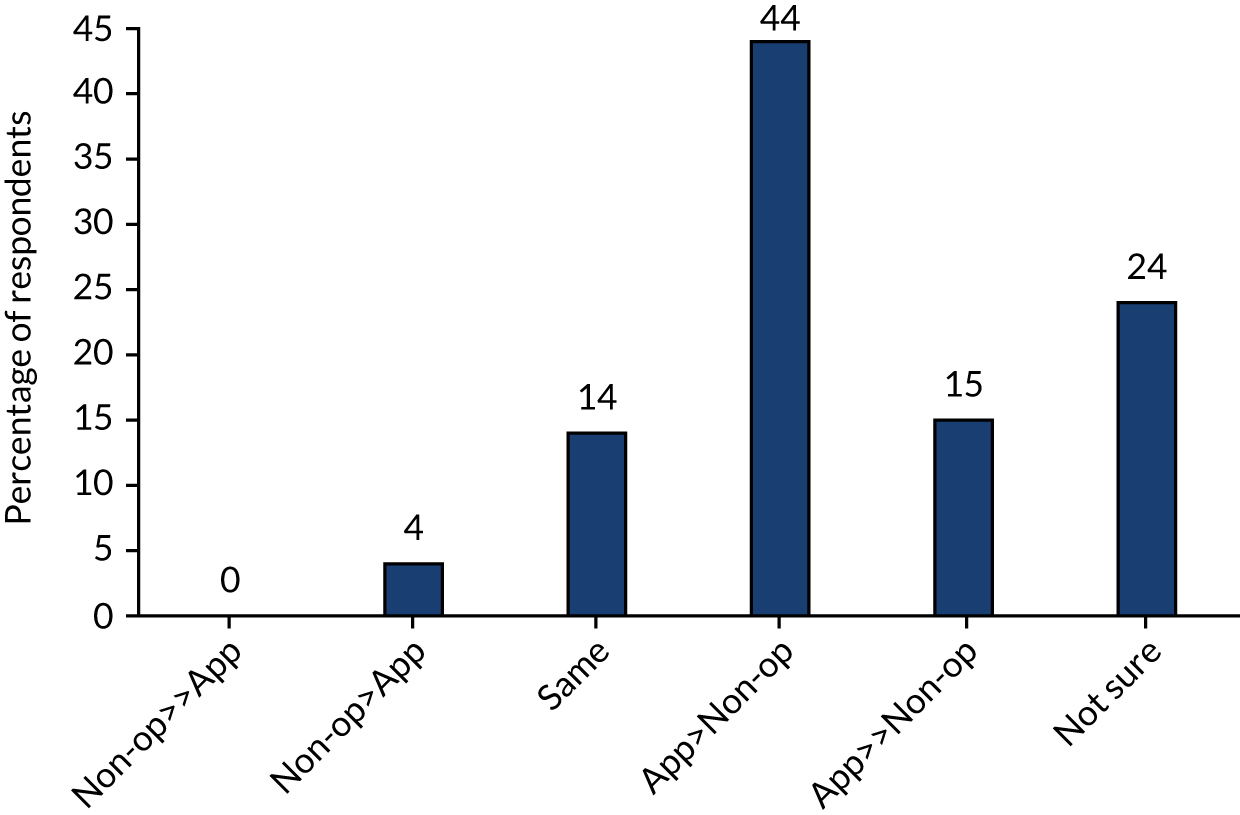
FIGURE 29.
There is uncertainty as to whether non-operative treatment is as effective as operative treatment in treating children and young people with acute uncomplicated appendicitis (single answer).
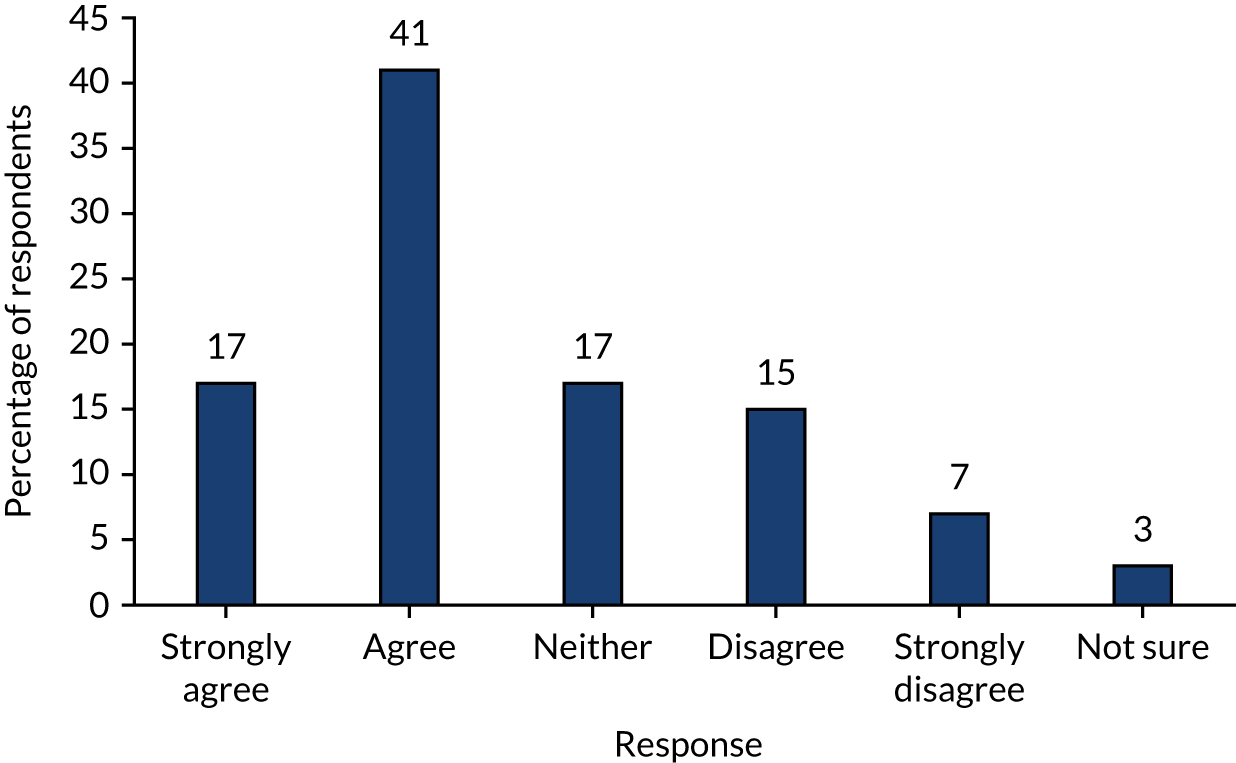
FIGURE 30.
There is currently enough evidence regarding non-operative treatment and enough uncertainty to justify a trial being performed comparing operative with non-operative treatment in children and young people (single answer).
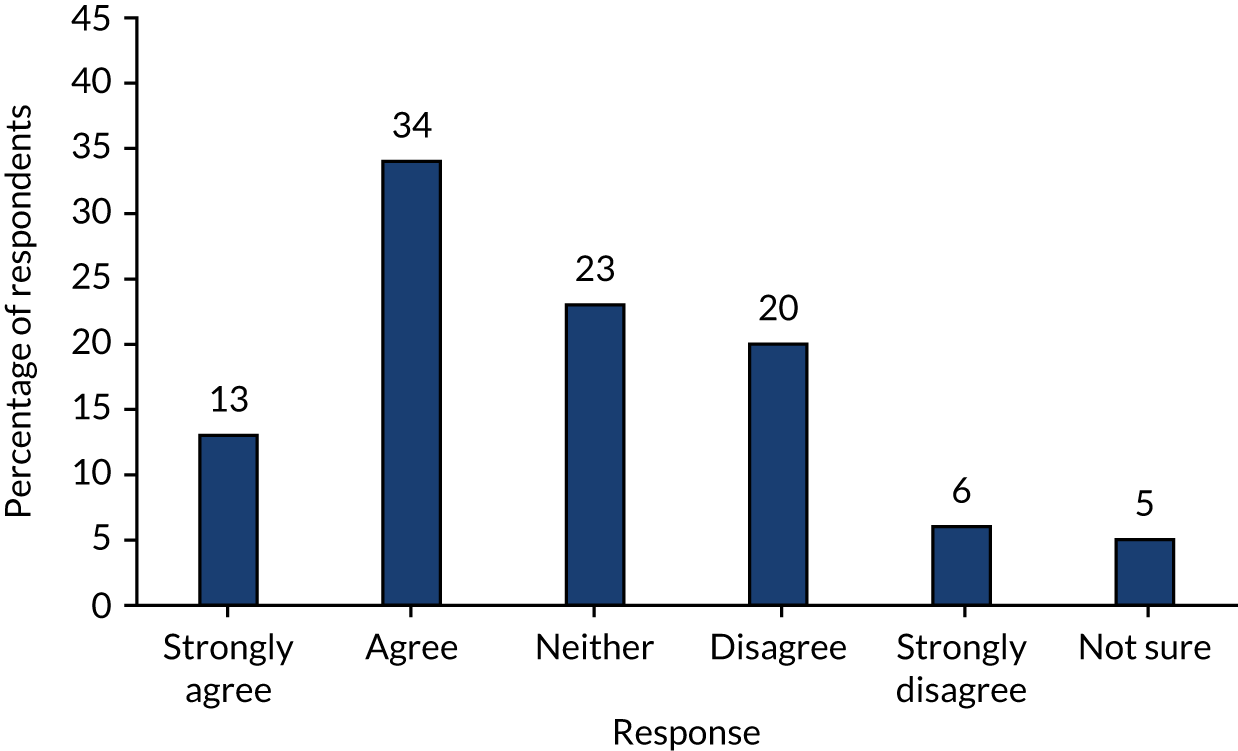
Responses to this question (see Figure 31) were provided on a visual analogue scale with the words ‘children should always be treated with an appendicectomy’ at one end (score 0) and the words ‘children should always have an initial trial of antibiotics’ (score 100) at the other end, and ‘undecided’ indicated in the centre. The median score given was 17 (IQR 3–39; range 0–98).
FIGURE 31.
Question: Regardless of your current actual clinical practice, please indicate, by moving the slider, your preferred treatment strategy for children and young people with acute uncomplicated appendicitis.
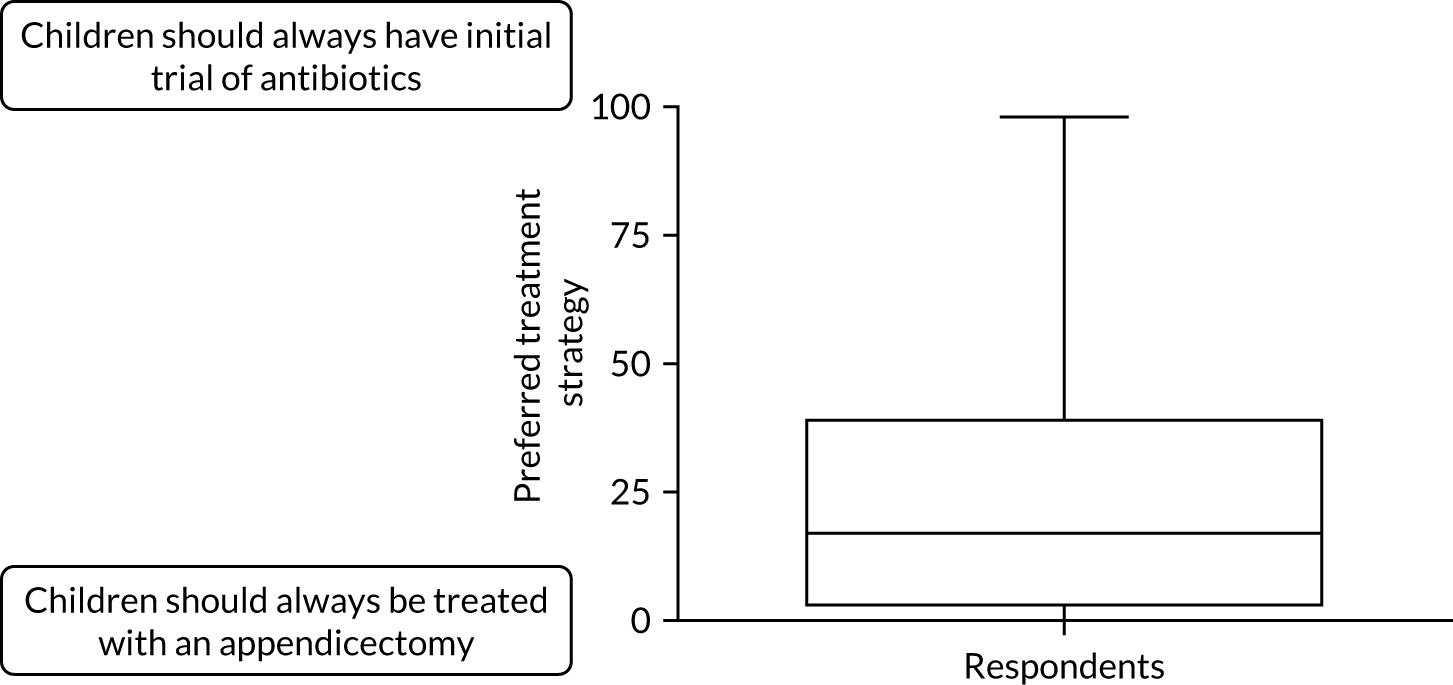
Section 3: attitudes to, and design of, a randomised controlled trial to compare operative with non-operative treatment
FIGURE 32.
Question: How important do you feel this research question is – is non-operative treatment of acute uncomplicated appendicitis in children and young people non-inferior to appendicectomy? (A non-inferiority trial aims to demonstrate that non-operative treatment is not worse than appendicectomy by more than a small predefined margin.)
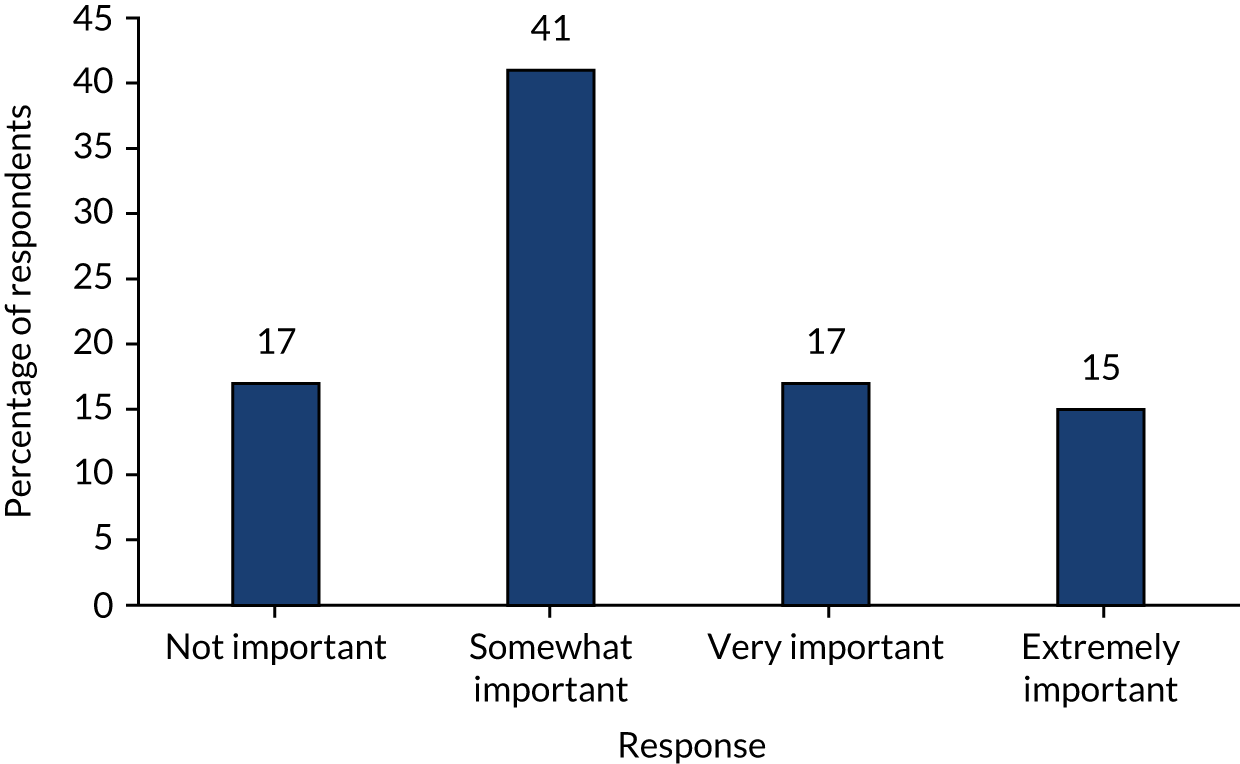
Survey participants were then presented with a design of a hypothetical clinical trial and asked whether or not they would be willing to enrol participants in such a trial. The trial was described as follows:
-
Participants – children (aged 4–15 years) with a clinical ± radiological diagnosis of acute uncomplicated appendicitis.
-
Intervention – a non-operative treatment pathway composed of a minimum of 24 hours’ broad-spectrum i.v. antibiotics with clearly defined time points for clinical review and either:
-
discharge once responding with oral antibiotics to complete a 10-day course (i.v. + oral)
or
-
appendicectomy for those not responding within 48 hours.
-
-
Comparator – appendicectomy as currently practised.
-
Outcomes – relevant clinical and patient-centred outcomes (to be defined by ongoing work), as well as cost-effectiveness, with a minimum follow-up duration of 1 year.
FIGURE 33.
Question: Please indicate your willingness to enrol participants in such a trial.
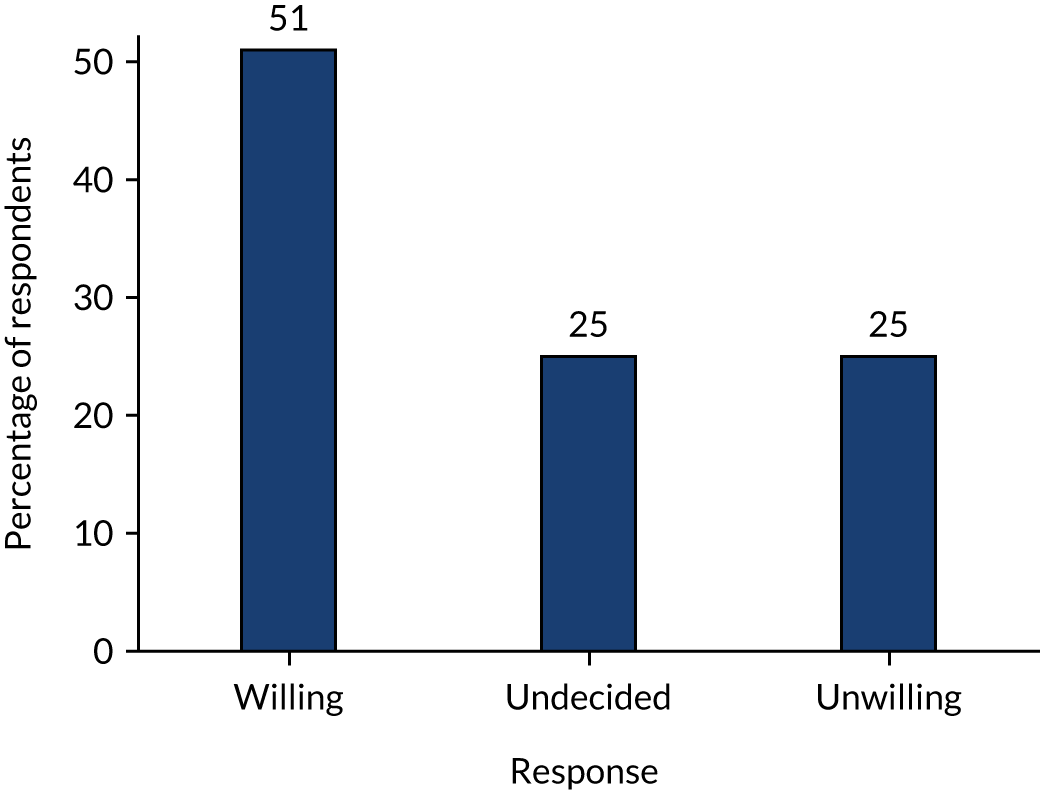
Responses to this question are shown in Figure 33. The final question (Figure 34) was conditional on the answer to the question above being either ‘undecided’ or ‘unwilling’.
FIGURE 34.
Question: If you are undecided or unwilling to enrol, it would be helpful for us to understand the reasons behind this. Which one of the following best describes your reasoning for being undecided or unwilling to enrol?
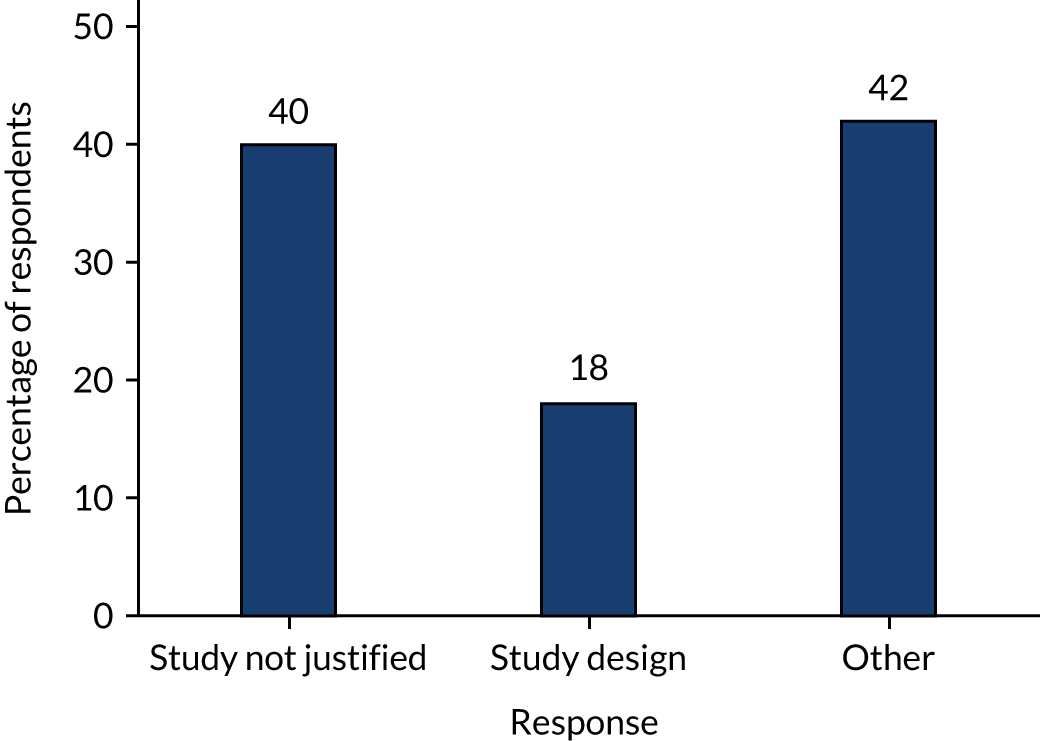
List of abbreviations
- A&E
- accident and emergency
- AE
- adverse event
- BAPS
- British Association of Paediatric Surgeons
- CHU-9D
- Child Health Utility-9 Dimensions
- CI
- confidence interval
- COMET
- Core Outcome Measures in Effectiveness Trials
- CONTRACT
- CONservative TReatment of Appendicitis in Children a randomised controlled Trial
- COS
- core outcome set
- CRP
- C-reactive protein
- CSRI
- Client Service Receipt Inventory
- eCRF
- electronic case report form
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-5L
- EuroQoL-5 Dimensions, five-level version
- EQ-5D-Y
- EuroQoL-5 Dimensions-Youth version
- GP
- general practitioner
- GRADE
- Grading of Recommendations Assessment, Development and Evaluation
- HCHS
- Hospital and Community Health Service
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- IQR
- interquartile range
- ISRCTN
- International Standard Randomised Controlled Trial Number
- i.v.
- intravenous
- NBM
- nil by mouth
- NIHR
- National Institute for Health Research
- OMERACT
- Outcome Measures in Rheumatology
- PCI
- patient clinical inventory
- PICC
- peripherally inserted central catheter
- PIS
- participant information sheet
- PPI
- patient and public involvement
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SCTU
- Southampton Clinical Trials Unit
- SD
- standard deviation
- SMG
- Study Management Group
- SSAG
- study-specific advisory group
- YPAG
- young persons’ advisory group
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/hta25100).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
