Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 15/156/02. The contractual start date was in July 2017. The draft report began editorial review in November 2019 and was accepted for publication in March 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2021. This work was produced by Parr et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2021 Queen’s Printer and Controller of HMSO
Chapter 1 Background and aims
Context
Long-term conditions affecting the brain, nerves and muscles are often grouped under the term ‘neurodisability’. ‘Neurodisability describes a group of congenital or acquired long-term conditions that are attributed to impairment of the brain and/or neuromuscular system and create functional limitations. A specific diagnosis may not be identified. Conditions may vary over time, occur alone or in combination, and include a broad range of severity and complexity. The impact may include difficulties with movement, cognition, hearing and vision, communication, emotion and behaviour’. (Reproduced with permission from Morris et al. ). 1
Eating, drinking and swallowing difficulties (EDSD) are common in young children with neurodisability. EDSD may lead to inadequate calorie intake, which affects a child’s nutrition, growth and general physical health. 2 There are three broad causes of EDSD: physical causes, which may affect control of the muscles of the lips, tongue, mouth and throat, and/or control of posture and movement, and/or impair the efficiency and safety of sucking, chewing and swallowing (e.g. children with cerebral palsy); non-physical causes, including sensory sensitivity that may lead to aversion, potential refusal of certain foods and rigidity or rituals associated with food or mealtimes [e.g. children with autism spectrum disorder (ASD), some of whom have avoidant/restrictive food intake disorder (ARFID)3]; and mixed, owing to both physical and non-physical causes. These three types of EDSD are used throughout the report to refer to these groups for ease of reference. Eating and drinking ability in children with EDSD of any cause is affected by their cognitive ability and their developmental age equivalent (rather than chronological age). Physical and non-physical EDSD frequently co-exist (e.g. children with cerebral palsy or Down syndrome). Both types of difficulties make mealtimes stressful for children and their families, and have negative impacts on quality of life and social participation.
The interventions that are available to address physical and non-physical EDSD are different;4–6 however, in practice, many children require judicious deployment of multiple interventions. 7 Descriptions of children with different types of EDSD and the interventions that they might receive are shown in Box 1.
Interventions are shown in italics. Further information on individual interventions can be found in the Glossary.
Physical EDSD‘A’ is 8 years old. He has a diagnosis of cerebral palsy, which affects his whole body. He is unable to walk, stand or sit unsupported, requires help to control his head position and has difficulties with eating and drinking. Specialist seating ensures that A is in the optimum position when eating and drinking. Working with the multidisciplinary team, A’s parents have modified the consistency of his food so that he is able to eat without choking and they have been helped to find a cup that reduces the amount of his drink that A loses from his lips. The feeding team and A’s parents were concerned that he was not gaining weight and so energy supplements were prescribed. A’s parents were also concerned that school and respite care staff needed help to understand how to feed him. The feeding team are helping those staff by, for example, showing them how to recognise when A is ready for another spoon of food.
A’s feeding difficulties and nutritional requirements may change as he gets older. The feeding team are sharing information with A’s parents to help them recognise when he may need reassessment, e.g. if he is not growing well or if there are concerns about the safety of his swallow or if he is not enjoying eating and drinking.
Mixed EDSD‘C’ has significant developmental disability; extensive medical investigations have not identified a cause. At the age of 3 years her parents are managing to feed her enough that her weight and growth are satisfactory. Some changes are needed to ensure that her nutritional intake is age appropriate and her dietitian has recommended a new milk.
C’s parents have modified her food consistencies so that she does not have foods that are a choking risk. C does not eat ‘mixed consistencies’ such as lumps in sauce: she sieves the lumps out and spits them out. She refused some other food textures. The feeding team have provided information to C’s parents about how her immature mouth movements are related to her overall level of development.
When C is fed she takes food passively. The feeding team and her parents are developing a programme to enhance mealtime communication. This, along with structured mealtime routines, will help C to learn to recognise when it is a mealtime. C’s parents are starting with using her highchair, putting her bowl where she can see it, letting her hold a spoon and reducing environmental distractions to help her focus on the meal more easily.
Non-physical EDSD‘D’ has a diagnosis of ARFID and, at the age of 4 years, has recently been diagnosed with ASD. He accepts a limited amount of food each day; he has a few preferred foods and refuses all others. To ensure that his nutritional intake is adequate, drink supplements have been prescribed. To minimise other stresses for D at school mealtimes, education staff have modified his social eating environment so that he always eats in a quiet area with the same small group of other pupils.
Working together with the multidisciplinary team and his school staff, D has made good progress. He now accepts some new flavours of his preferred foods and is happy to eat some foods that look different, e.g. lighter or darker toast and a broken biscuit. His parents and the feeding team have identified the texture and appearance of new foods that D is most likely to accept next, e.g. foods that are not sticky and do not have crumbs on them. A programme for a gradual graded exposure to these new foods has been started; D will now touch, smell and lick some of the new foods. The next step is that he will make bite marks in these foods.
Parents and primary carers of children with neurodisability and EDSD (referred to as parents for the remainder of the report) are usually supported in the NHS by multidisciplinary teams (MDTs) of health professionals (HPs);8 for example, paediatricians, speech and language therapists, occupational therapists, dietitians and, less frequently, clinical psychologists. HPs identify the cause(s) of a child’s EDSD by a combination of review of the child’s previous and current EDSD, understanding the origins of their condition, clinical observation and instrumental evaluation (e.g. videofluoroscopy). In the NHS, services to assess and support children with physical or mixed EDSD are more common than those to support children with non-physical EDSD.
Professionals take account of a range of parent and child factors, including parents’ views and their capacity to understand and implement interventions, and of the child’s cognitive ability. Individualised advice is then given on how and what to feed their child to improve the safety and efficiency of eating and drinking, improve the volume of oral solids and liquids if children can eat and drink safely, and how to manage behaviour so mealtimes are a positive experience. 7 This advice takes the form of individual interventions that are often delivered alongside each other; the actual intervention content received within each ‘intervention type’ varies with the child’s needs and neurodevelopmental profile. For children with either physical or non-physical EDSD, it has been unclear what advice is usually given, which interventions are commonly used, what constitutes ‘best clinical practice’ and whether or not there is robust evidence for such practice. 4–6 For instance, the National Institute for Health and Care Excellence (NICE) guidance for the treatment of both children with cerebral palsy and children with ASD recommends assessment and intervention as considered appropriate by clinicians, but does not specify how children should be assessed or which interventions should be provided (Box 2). 6,9
-
Guidelines on cerebral palsy in under-25s: assessment and management 6
-
Develop strategies and goals in partnership with the child or young person with cerebral palsy and their parents, carers and other family members for interventions to improve eating, drinking and swallowing.
-
Create an individualised plan for managing EDSD in children and young people with cerebral palsy, taking into account the understanding, knowledge and skills of parents, carers and any other people involved in feeding the child or young person. Assess the role of the following:
-
postural management and positioning when eating
-
modifying fluid and food textures and flavours
-
feeding techniques, such as pacing and spoon placement
-
equipment, such as specialised feeding utensils
-
optimising the mealtime environment
-
strategies for managing behavioural difficulties associated with eating and drinking
-
strategies for developing oral motor skills
-
communication strategies
-
modifications to accommodate visual or other sensory impairments that affect eating, drinking and swallowing
-
training needs of the people who care for the child or young person, particularly outside the home.
-
-
Advise parents or carers that intraoral devices have not been shown to improve eating, drinking and swallowing in children and young people with cerebral palsy.
-
Use outcome measures important to the child or young person and their parents or carers to review:
-
whether or not individualised goals have been achieved
-
the clinical and functional impact of interventions to improve eating, drinking and swallowing.
-
-
-
Autism in under-19s: support and management 9
Take into account the physical environment in which children and young people with autism are supported and cared for. Minimise any negative impact by:
-
providing visual supports, for example words, pictures and symbols that are meaningful for the child or young person
-
making reasonable adjustments or adaptations to the amount of personal space given
-
considering individual sensory sensitivities to lighting, noise levels and the colour of walls and furnishings.
Assess factors that may increase the risk of behaviour that challenges in routine assessment and care planning in children and young people with autism, including:
-
impairments in communication that may result in difficulty understanding situations or in expressing needs and wishes
-
co-existing physical disorders, such as pain or gastrointestinal disorders
-
co-existing mental health problems, such as anxiety or depression, and other neurodevelopmental conditions, such as attention deficit hyperactivity disorder
-
the physical environment, such as lighting and noise levels
-
the social environment, including home, school and leisure activities.
-
Challenging behaviour and learning disabilities: prevention and interventions for people with learning disabilities whose behaviour challenges 10
Develop a written behaviour-support plan for children, young people and adults with a learning disability and behaviour that challenges, which is based on a shared understanding about the function of behaviour. This should:
-
Identify proactive strategies designed to improve the person’s quality of life and remove the conditions likely to promote behavior that challenges, including:
-
changing the environment (e.g. reducing noise and increasing predictability)
-
promoting active engagement through structured and personalised daily activities, including adjusting the school curriculum for children and young people.
-
-
Identify adaptions to a person’s environment and routine and strategies to help them develop an alternative behaviour to achieve the function of the behavior that challenges, by developing a new skill (e.g. improved communication, emotional regulation or social interaction).
-
Identify preventative strategies to calm the person when they begin to show early signs of distress, including:
-
individual relaxation techniques
-
distraction and diversion onto activities that the person find senjoyable and rewarding.
-
Reproduced from NICE. © NICE [2017] Cerebral Palsy in Under 25s: Assessment and Management. NICE Guideline. Available from www.nice.org.uk/guidance/ng62. All rights reserved. Subject to Notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication.
Reproduced from NICE. © NICE [2013] Autism in Under 19s: Support and Management. Available from www.nice.org.uk/guidance/cg170. All rights reserved. Subject to Notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication.
Reproduced from NICE. © NICE [2015] Challenging Behaviour and Learning Disabilities: Prevention and Interventions for People with Learning Disabilities Whose Behaviour Challenges. Available from www.nice.org.uk/guidance/ng11. All rights reserved. Subject to Notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication.
The interventions that HPs may advise families to adopt can be time-consuming to administer, may involve considerable changes to parents’ usual feeding plans and may sometimes be in contrast to parents’ beliefs about how their child should be fed. 7 To date, it has not been known which interventions are viewed as acceptable and feasible to be delivered by parents at home, and how intervention success is or should be measured by parents and/or HPs.
Why this research is needed?
Most children with neurodisability and EDSD are identified by the age of 6 years, and many from infancy. The need for evidence about the timing, duration, dosage and effectiveness of individual EDSD therapies was ranked as the number-one priority by parents of children with neurodisability and professionals in a James Lind Alliance research priority-setting exercise. 11 HPs believe that effective interventions should benefit children’s health, growth, nutrition, development and learning; to some extent these are inter-related, as adequate nutrition is required for brain growth and for optimal development. 7 In addition to improving a child’s physical health, interventions targeting EDSD may also have positive psychosocial and education outcomes; mealtimes may be shorter and/or more enjoyable to children and their families, which increases the social participation and quality of life of both the child and their family at home, and for the child at school. However, interventions may have unintended adverse outcomes, such as increased parental or family stress, if the interventions conflict with parents’ beliefs and wishes about how to feed their child. 7
Thus, studies are needed to establish the effectiveness of intervention(s) that parents can deliver at home. However, before such studies can be undertaken, information is needed on which groups of children to include; the range of interventions available; what parents and HPs think are the most relevant outcomes (e.g. medical outcomes, such as nutrition, weight and health, and outcomes related to the International Classification of Functioning,12 such as social participation); what outcome measurement tools are valid, reliable, responsive and acceptable; and what types of study design would be acceptable to children, parents and HPs, and feasible to deliver. In this context, the National Institute for Health Research (NIHR) commissioned research in 2016 to address the following research question and objectives.
Research aims
The overall purpose of this study was to answer the question: what interventions, which could be delivered at home by parents, are available to improve eating in young children with neurodisability and are suitable for investigation in pragmatic trials? The aims were to:
-
review the clinical practice and research evidence for interventions, outcomes assessed and the tools used to measure these outcomes
-
determine which parent-delivered interventions are currently recommended by NHS professionals, which interventions parents use at home and how parents and professionals evaluate whether or not an intervention is successful
-
construct one or more trial frameworks acceptable to children, young people, parents and professionals; or to specify the additional evidence about interventions, outcomes and tools that would be needed to support a future trial.
Chapter 2 Summary of how the methods relate to the three aims of the study
This chapter outlines the aims, objectives and the main methodological approaches used in the study. More detail is provided in subsequent chapters.
Scope of the study
The NIHR commissioning brief requested a focus on ‘young children with neurodisability’. Following Morris et al. ,1 we defined neurodisability as any condition that is attributed to impairment of the brain and/or the neuromuscular system and creates functional limitations. We included any non-progressive neurodisability condition that gives rise to physical, non-physical or mixed EDSD. Throughout the research, we gathered information relevant to children with physical and non-physical EDSD separately as we considered that interventions to address these specific types of difficulties may differ. However, as many children with primarily physical EDSD also have non-physical EDSD, we present information relating to children with physical and mixed EDSD together throughout this report.
As there was no prior definition of ‘young children’ specifically relating to EDSD and neurodisability, in our study we defined the age range covered by ‘young children’ during the project. The co-investigators agreed to start the project with a conservative working definition of ‘children as aged up to and including 8 years’. Participants at a consultation group prior to the start of the research agreed that this was an acceptable initial working definition. Broader age ranges were used in some elements of the research to ensure that we captured all of the evidence relating to children aged 0–8 years and enabled all parents and professionals with recent experience to contribute their views. Individual sections of this report state the age range of children with EDSD included in specific elements of the study, and describe why any extensions that were applied were deemed necessary.
The commissioning brief focused on interventions used by parents at home. However, children aged up to 8 years are also fed in education and other care settings using techniques similar to those used at home. Therefore, we included research that focused on children’s eating and drinking at home or at school or nursery. Studies of feeding interventions that were delivered solely on paediatric or neonatal units in a hospital were not included. Enteral feeding, which involves direct feeding by a tube into the stomach, was not included in this study because this intervention is used when oral feeding is not enabling a child to receive sufficient fluid or calories.
Design
To address the study’s aims we used an iterative mixed-methods design. The individual methods comprised systematic reviews (updating three published reviews of interventions and undertaking one review to assess measurement properties of published outcome measures), a mapping review, surveys, focus groups, a Delphi survey and stakeholder consultation workshops (Figure 1).
FIGURE 1.
Methods used to address each aim of the study. TAU, treatment as usual.
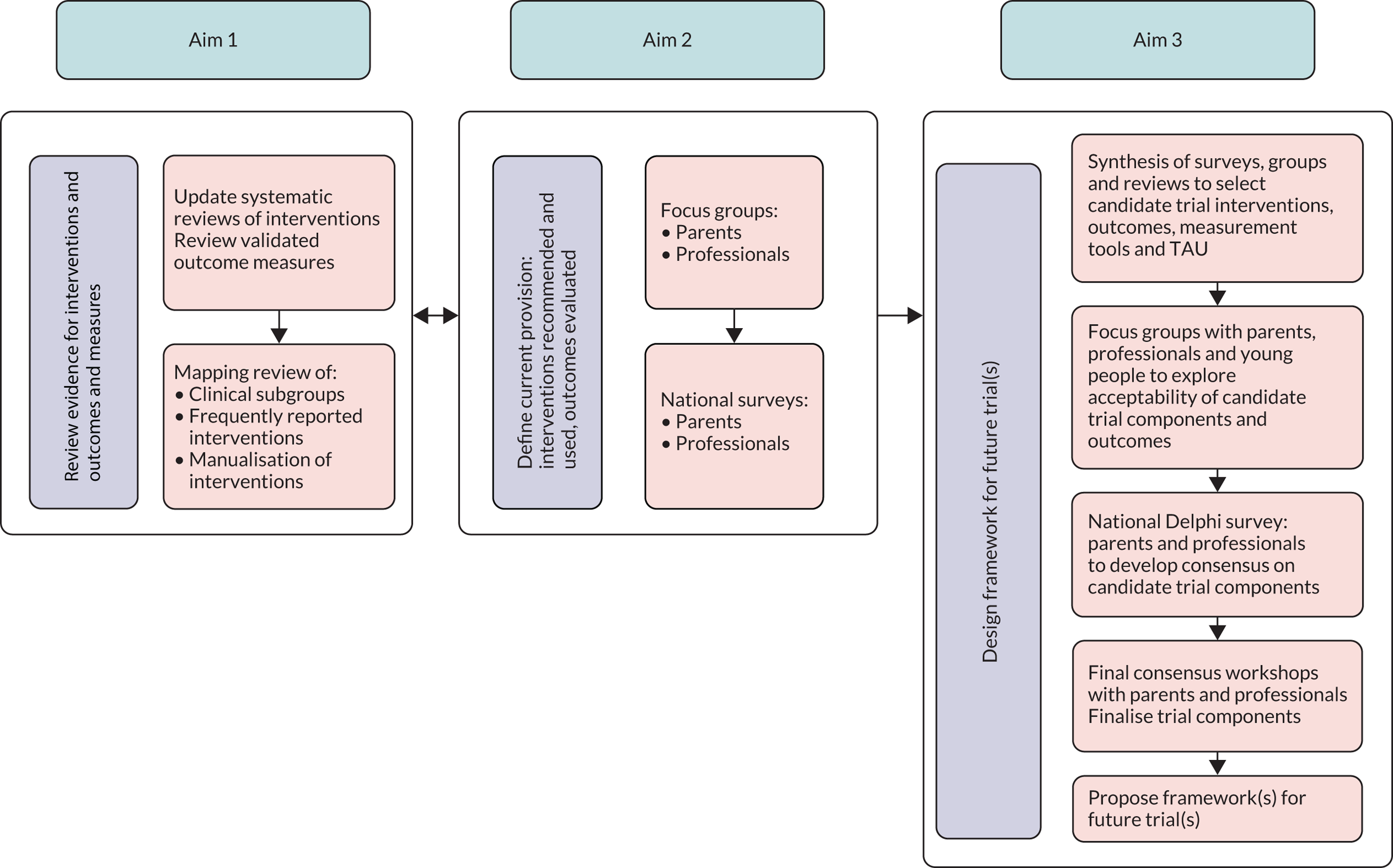
Aim 1: review of research evidence for interventions, outcomes and measures
To address the first aim of the research, we identified the range of interventions that parents could deliver at home to children with EDSD and the outcomes of the interventions that have been evaluated in previous research. We also assessed the extent of the research evidence for the effectiveness of identified interventions. Three relevant systematic reviews4–6 of interventions were published prior to the start of our research. These reviews appraised randomised controlled trials (RCTs) and quasi-experimental designs involving children with cerebral palsy,6 controlled group studies and single-case experimental designs involving children with ASD,5 and RCTs and quasi-experimental designs involving children who have oropharyngeal dysphagia of any neurological origin, including progressive conditions such as Friedreich’s ataxia. 4 We updated the first two reviews to include material published from 1 October 2013 and 1 October 2014, respectively. 5,6 The lead author of the review on oropharyngeal dysphagia4 updated her review during our study to include research findings from 1 October 2011 and provided us with pre-publication data.
As the three published systematic reviews of interventions appraised only a portion of the evidence on interventions for children with neurodisability and EDSD and their outcomes (i.e. those pertaining to children with the conditions listed above), we conducted a mapping review to find other published studies evaluating any EDSD intervention involving children with any non-progressive neurodisability condition and using any quantitative research design. The mapping review revealed the extent of the evidence for a greater range of types of intervention, in terms of the number of children who participated with physical and mixed EDSD and non-physical EDSD, the research designs used, the outcomes that were evaluated and the measures used to investigate progress in outcome areas.
In a further systematic review, we examined the measurement properties of the most relevant and promising candidate outcome measurement tools for EDSD identified in the systematic or mapping reviews.
Aim 2: defining current provision – interventions, outcomes and measures used in the UK
The second aim of the study was to find out which EDSD interventions HPs in the NHS currently provide or recommend, which interventions parents use at home and how parents and professionals judge whether or not these interventions work. We convened a consultation workshop to present the interventions and outcomes identified from the updates of the three published systematic reviews of interventions to HPs based in the north-east of England. Three speech and language therapists and one dietitian took part. They confirmed the co-investigators’ views that NHS HPs do currently provide or recommend the interventions appraised in the updates of the three published systematic reviews of interventions, sometimes in combination, and that the outcomes measured in the reviews are relevant when working with children with physical, non-physical and mixed EDSD. As all of the interventions and outcomes in the updates of the three published systematic reviews of interventions were deemed by the consultation group to be used with some children with neurodisability, none was removed from consideration in subsequent stages of the research following this consultation. The identified interventions and outcomes informed the development of a topic guide for the first round of focus groups with parents of children with EDSD and HPs working in the NHS. The focus groups, held in the north-east of England, explored whether or not the research-based interventions and outcomes are delivered to specific groups of children and if any interventions or outcomes were missing from our lists.
The interventions and outcomes identified in the updates of the three published systematic reviews of interventions, and discussed in the consultation workshop and focus groups, subsequently informed a UK-wide survey to quantify how frequently each of the parent-delivered interventions were recommended by NHS professionals; which interventions are used by parents; and how parents and professionals evaluate whether or not interventions are successful. We also included education professionals in the survey, to investigate which EDSD interventions are used by staff who feed children with neurodisability at school.
There was a considerable degree of overlap between the two streams of work to meet aims 1 and 2. The interventions and outcomes identified in the updates of the three published systematic reviews of interventions to meet aim 1 informed the topic guides for the focus groups, and were listed in the national survey to meet aim 2. Furthermore, the discussion in the focus groups and the answers to the survey (aim 2) informed searching and data extraction in the mapping review and systematic review of measurement properties (aim 1).
Aim 3: constructing trial frameworks to evaluate eating, drinking and swallowing difficulty interventions for children with neurodisability in the NHS
The third aim of the study was to construct one or more trial frameworks acceptable to children, young people, parents and professionals, or to specify the additional evidence about interventions, outcomes and tools that would be needed to support a future trial. We synthesised evidence gathered throughout the study: linking, building and merging findings to develop full lists of interventions and outcomes that are commonly used by families and/or are supported by research. 13,14 Groups of parents and HPs sense checked the summaries in a second round of focus groups held in the north-east, south-west and south-east of England. Following the focus groups, parents and HPs from across the UK rated the importance of each of the identified interventions and outcomes for future research in two rounds of a Delphi survey, which revealed the extent of established consensus on the interventions and outcomes that are essential. The last element of the study involved stakeholder consultation workshops with parents and HPs and separate focus groups with young people with EDSD to inform a framework for future research into EDSD management of children with neurodisability in the NHS.
Delivery of the research
The research team was multidisciplinary and comprised clinical academics, clinicians, health services research methodologists and parents. The team members were from the north-east, south-east and south-west of England. The parent co-investigators (DG and JS) had experience of mixed and non-physical EDSD, were part of UK and international networks of parents of children with neurodisability and had previous experience of working in applied health research. The clinicians and clinical academics provided services to families of children with neurodisability who had EDSD: clinical psychology (HM and HT), community paediatrics (AC), gastroenterology (JT), neurodisability paediatrics (JP, JC and MA) and speech and language therapy (CB, LP and DS). The health services research methodologists had particular interests in childhood disability research (CM), clinical trials and health-related surveys (EM and CM) and evidence synthesis (DC).
The full research team met regularly to monitor the conduct and progress of the study and to consider the findings from each research activity. We discussed decisions on whether or not any interventions, outcomes or measurement tools lacked evidence of use in clinical practice in the UK or supporting research and, therefore, should not be taken forward. In the final months of the study, the research team met three times to discuss the main study findings and to formulate the recommendations for the design of future evaluation studies of interventions.
The research was directly informed by a parent advisory group (PAG) (see Patient and public involvement) that consisted of parents of children with neurodisability and physical, mixed and non-physical EDSD. An external study steering group that included members with clinical and research expertise and links to parent groups provided oversight and advice on the conduct and reporting of the research.
Patient and public involvement
The PAG advised on the methods, procedures, analysis and dissemination of each element of the research design. We recruited parents from the north-east of England to the PAG via social media and local networks known to parent co-investigators Deborah Garland and Johanna Smith. Deborah Garland is a local National Autistic Society (London, UK) representative. Johanna Smith is a parent of a child with mixed EDSD and additional sensory impairment. Five parents expressed an interest in taking part and three or four parents took part in each meeting, with one of those parents contributing their views via e-mail. Equal representation was achieved from parents of children with physical and mixed EDSD (n = 2) and parents of children with non-physical EDSD (n = 2). Their children ranged in age from 6 to 16 years. Meetings took place on four occasions over 10 months in Newcastle. PAG members agreed the dates and venues in advance to ensure convenience and maximise attendance. Any documents that required attention in the meetings were circulated in advance via e-mail to allow PAG members sufficient time to read them. The sessions were led by a minimum of two facilitators (HT, DG and JS) with at least one of the parent co-investigators present. The sessions lasted 2 hours and refreshments were provided. Parents received a £75 shopping voucher to thank them for their contribution.
The first meeting outlined the purpose of the group and agreed how parents would like to receive communication. Members agreed that face-to-face meetings were preferable and that any additional work would be undertaken via e-mail as necessary. The members did not wish to join a closed Facebook group (Facebook, Inc., Menlo Park, CA, USA; www.facebook.com) or use other social media in relation to group tasks. The group members agreed terms of reference for the PAG (see Appendix 1). In the first meeting, the PAG also considered a summary of the findings of the national survey of interventions recommended to and used by parents, and the important outcomes. Members discussed whether or not the findings were consistent with their experiences of supporting their child with EDSD and of service provision.
In the second meeting, the PAG received a summary of all the evidence gathered to date, which included findings from the systematic and mapping reviews, the first round of focus groups and the national survey. The discussion focused on how best to share this information with parents in the second round of focus groups. The PAG also reviewed the wording and layout of the Delphi survey and the associated information sheets. The discussions covered the use of appropriate and accessible language in documents, the presentation of visual information (colours, layout and clarity) and how best to present the information simply, without repetition or unnecessary jargon. The PAG were also presented with three parent-reported tools for outcome measurement that had relatively stronger evidence for supporting robust measurement properties. They commented on their wording, layout and ease of use.
In the third meeting, the PAG reviewed the amended Delphi survey and information sheets that had been modified in response to the discussion in the second round of focus groups. Discussions again focused on keeping information clear and accessible. The PAG also discussed how to order the statements relating to the interventions and outcomes in the Delphi survey and suggested providing examples of each intervention for clarity.
In the fourth meeting, the PAG discussed the findings of the Delphi survey and provided advice on how to present this information to parents at the stakeholder consultation workshops. The PAG reviewed the individual tasks for parents attending the workshops in terms of content, structure and timings. They also advised on how best to present information about the study and key findings in a short presentation, including simplifying language and presenting the study data in a pictorial format. They also suggested creating a document to send to all attendees prior to the workshops to provide a background to the study and clarity on what would happen on the day. The PAG were also asked to give their views via e-mail on the proposed dissemination plan during the final months of the study.
In each chapter of the report, we describe the patient and public involvement (PPI) in the planning and conduct of the individual stages of the study and the interpretation of their findings, along with the strengths and limitations of the individual research methods.
Ethics
The West Midlands and the Black Country Research Ethics Committee approved the study procedures (17/WM/0439). There were four amendments: one non-substantial amendment (to add new sites as participant identification centres), two planned substantial amendments to seek approval for documents developed for the Delphi survey, informed by the findings from earlier study stages, and one further substantial amendment (to change the recruitment target). Newcastle upon Tyne Hospitals NHS Foundation Trust was the research sponsor.
Complete and transparent reporting
The following chapters of the report describe the objectives, methods, results, strengths and limitations of each element of the research, using EQUATOR (Enhancing the Quality and Transparency of Health Research) reporting guidelines (www.equator-network.org/; accessed 11 November 2019), GRIPP2,15 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)16 and Strengthening the Reporting of Observational studies in Epidemiology (STROBE). 17
Chapter 3 Aim 1: systematic reviews
Objectives
The objective was to update three published systematic reviews of interventions to improve the EDSD of children with neurodisability that were published before our research began.
This chapter outlines the methods and findings of the update of two of these reviews. 5,6 The third review4 was updated by the original review team during the period of our study. The update review followed the original review protocol. The authors of that review provided pre-publication data and these have been included at the end of this chapter for completeness.
Background
Three recently published systematic reviews relevant to interventions for young children with neurodisability and EDSD were identified at the proposal development stage. 4–6
Marshall et al. 5 reviewed evidence for interventions to improve feeding difficulties for children aged < 6 years with ASD that were published between January 2000 and October 2013. The review included 23 studies, which were a mix of single-case experimental design (n = 12) and small-group studies (n = 11), all with five or fewer participants who were aged 2–5 years. A total of 46 children participated, 37 of whom had ASD. The interventions were based on operant conditioning to ‘increase desirable eating behaviours or decrease undesirable eating behaviours’. 5 The risk of bias of each study was assessed using a tool developed for single-case experimental design by Horner et al. 18 The primary outcomes of interest were increased volume of food and variety of intake, which were considered to increase desirable mealtime behaviour. The secondary outcome of interest was a reduction of inappropriate mealtime behaviours. The authors concluded that there was a low level of evidence to support these types of interventions for children with ASD and that ‘favourable intervention outcomes were observed in terms of increasing volume, but not necessarily variety of foods consumed in young children with ASD and feeding difficulties’. 5
The NICE cerebral palsy guidance6 reviewed evidence generated by RCTs and observational studies of interventions for ‘management of eating, drinking and swallowing difficulties’ of children and young people with cerebral palsy aged < 25 years published before October 2014. The outcomes of interest in the review were:
-
physiological function of the oropharyngeal mechanism (as determined by clinical evaluation, videofluoroscopic swallow studies or fibreoptic endoscopic evaluation of swallowing)
-
changes in diet consistency that a child is able to consume (developmentally appropriate oral diet; texture and/or consistency of foods and fluids must be modified; supplementary feeding required)
-
respiratory health – presence of a history of confirmed aspiration pneumonia or recurrent chest infection (with or without pneumonia with suspected prandial aspiration aetiology)
-
nutritional status and/or changes in growth (weight and height percentiles)
-
child and young person’s level of participation in mealtime routine/length of mealtimes (time taken to feed)
-
psychological well-being of parents and/or carers
-
acceptability of the programme
-
survival.
The included publications comprised four RCTs and four cohort studies, which included a total of 235 participants who were aged 12 months to 21 years. The four RCTs all compared Oral sensorimotor therapy with routine therapy; each cohort evaluated a different intervention. One cohort considered the Innsbruck Sensorimotor Actuator and Regulator (ISMAR) compared with no ISMAR; one an Oral sensorimotor treatment; one a training programme delivered to children and caregivers; and one evaluated individual interventions delivered in combination (a multicomponent intervention), including carer training, behavioural interventions and Beckman Oral Motor Exercises. 19 The risk of bias was assessed using the Cochrane risk-of-bias tool. 20 Conclusions were not drawn from this review alone, but as part of the total guideline development process. This took account of stakeholder input to the evidence and consideration of the evidence by a committee made up of practitioners, professionals, care providers, commissioners, those who use services and family members or carers. Based solely on the published studies (n = 8) the Grading of Recommendations and Assessment, Development and Evaluation (GRADE) assessment rated the evidence to support these types of interventions in this population as being of very low to low quality. 6
Morgan et al. 4 considered interventions for children with any ‘neurologically based oropharyngeal dysphagia’. 4 The outcomes of interest were amount/variety of food and eating behaviours. Their review included two papers assessing sensorimotor treatment in 55 cerebral palsy patients aged 4–21 years; the studies were small and were rated as being at high risk of bias, as assessed by the Cochrane risk-of-bias tool. 20 Both of the papers were also included in the NICE6 review. Morgan et al. 4 concluded that there was ‘insufficient high-quality evidence from RCTs or quasi-RCTs to provide conclusive results about the effectiveness of any particular type of oral motor therapy for children with neurological impairment’. 4
Methods
We updated the review by Marshall et al. 5 and the NICE cerebral palsy review6 of EDSD interventions using the methods of the original reviews. Marshall et al. 5 provided the search strategy for the ASD review and the NICE review searches were based on the published search strategies. Updated searches were limited to 1 year before the date of the last searches undertaken for the original review, which allowed for delays in database updates. Protocols for the systematic reviews were registered on PROSPERO (www.crd.york.ac.uk/prospero/; accessed 11 November 2019). Full details of the search strategies are presented in Appendix 2.
Inclusion criteria
Studies were included in the update of Marshall et al. 5 if they met the following criteria:
-
Population – children aged 0–8 years with a diagnosis of ASD. (The original review included children aged 0–6 years, but we extended the age range to fit with our definition of young children with neurodisability.)
-
Intervention – non-pharmaceutical behavioural or environmental interventions.
-
Outcomes – amount of food and/or variety of foods consumed and/or desired or undesired eating behaviours.
-
Study design – an experimental design was used to investigate treatment outcomes, including the use of a control group, within-group designs or single-case-based experimental designs replicated across at least four participants (to give some indication of repeated effects of interventions). 18
-
Language – studies were published in English in peer-reviewed journals.
The update of the NICE review6 included studies that met the following criteria:
-
Population – children and young people aged 0–8 years with a diagnosis of cerebral palsy. (The original review considered young people aged < 25 years.)
-
Intervention – interventions that aimed to improve sucking/biting/chewing/swallowing of food, intake of food (amount and/or variety of food) and/or eating behaviours.
-
Outcomes – Co-ordination of chewing and swallowing, Increased amount and variety of intake, Duration of mealtimes, Nutritional status, Growth and Physical health (e.g. chest infections and mortality).
-
Study design – group experimental design.
-
Language – studies were published in English in peer-reviewed journals.
Screening/data extraction/quality assessment
For both review updates, two researchers (HT and LP) independently screened titles and abstracts to identify studies meeting the inclusion criteria. The full texts of potentially eligible articles were retrieved and assessed independently against inclusion criteria by two researchers (HT and LP or HM). Data extraction and quality assessment were conducted by one researcher (HT) and checked by a second researcher (LP).
We used the Cochrane risk-of-bias tool for randomised trials (Risk of Bias toolkit, version 2) to assess the quality of RCTs or quasi-RCTs that were included in either review update. 20 Critical judgements were made of the following domains: randomisation process, deviation from the intended intervention, missing outcome data, measurement of the outcome and selection of the reported results. Each included study was judged to be at ‘low risk of bias’, ‘high risk of bias’ or to give rise to ‘some concern of bias’ in each of these domains. An overall study risk of bias was established using the following criteria:
-
low risk of bias – the study was judged to be at low risk of bias for all domains
-
some concern of risk of bias – the study was judged to give rise to some concerns in at least one domain, but not judged to be at high risk of bias on any domain
-
high risk of bias – the study was judged to be at high risk of bias in at least one domain, or the study was judged to give rise to some concerns for multiple domains in a way that substantially lowered confidence.
As in Marshall et al. ,5 we used the quality assessment tool developed by Horner et al. 18 to assess studies using single-case experimental design. This tool grades the presence of absence of 21 quality indicators within single-case experimental designs relating to (1) the description of participants and settings (three criteria), (2) dependent variable(s) (five criteria), (3) independent variable(s) (three criteria), (4) the establishment of a robust baseline measure and replicable description of the intervention (two criteria), (5) experimental control/internal validity (three criteria), (6) external validity (one criterion) and (7) social validity (four criteria). Each criterion is rated as met or unmet, giving a possible total of 21 per study.
Results
The numbers of references included and excluded at each stage of the study selection process for both review updates are shown in the PRISMA flow diagram (Figure 2).
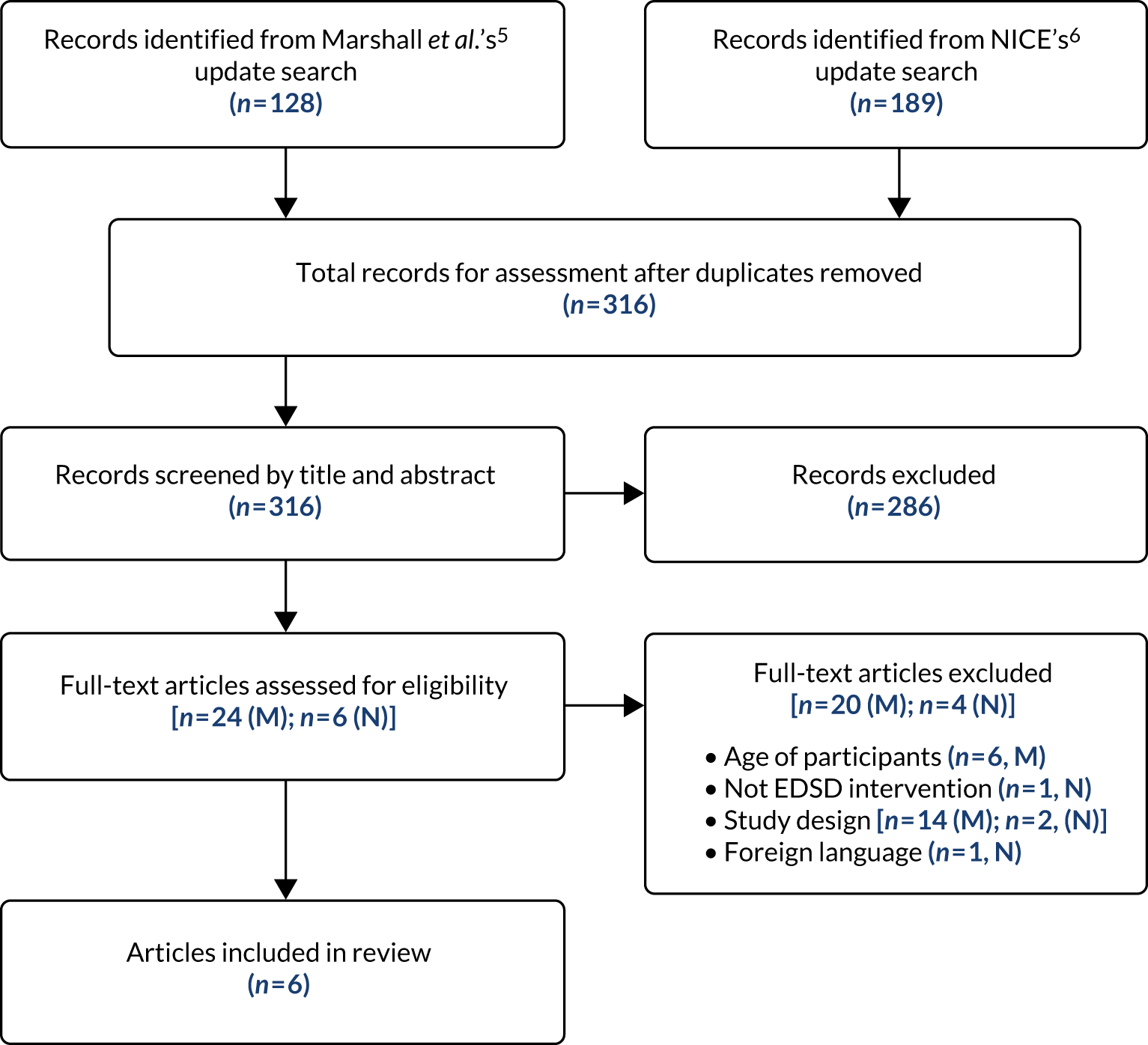
Following de-duplication, 316 references remained for assessment against the two sets of review inclusion criteria. Of these, 286 references were excluded based on the information in the title and abstract. The remaining 30 references were then assessed based on the full-text publication. Of these, a further 24 were excluded from the update review. A total of six references, reporting six studies, met the inclusion criteria adapted from Marshall et al. 5 and NICE6 and were included: four references and two references, respectively (see Figure 2).
Update of the Marshall et al.5 review focusing on children with autism spectrum disorder
Four studies were included in our update of the Marshall et al. 5 review (Box 3): two RCTs (one with a waiting list control21 and one with an active control22), one single-case experimental design23 (also referred to as a ‘n of 1’ study) replicated across participants and one pre–post pilot trial. 24 Nine single-case experimental design studies were excluded because they included fewer than five participants. These nine studies were included in the mapping review (see Chapter 4). Full descriptive details of the included studies are provided in the data extraction tables (see Appendix 3); however, a brief summary is provided here.
Johnson CR, Foldes E, DeMand A, Brooks MM. Behavioural parent training to address feeding problems in children with Autism spectrum disorder: a pilot trial. J Dev Phys Disabil 2015;27:591–607. 24
Marshall J, Hill RJ, Ware RS, Ziviani J, Dodrill P. Multidisciplinary intervention for childhood feeding difficulties. J Pediatr Gastroenterol Nutr 2015;60:680–7. 22
Peterson KM, Piazza CC, Volkert VM. A comparison of a modified sequential oral sensory approach to an applied behaviour-analytic approach in the treatment of food selectivity in children with autism spectrum disorder. J Appl Behav Anal 2016;49:485–511. 23
Sharp WG, Burrell TL, Jaquess DL. The Autism MEAL Plan: a parent-training curriculum to manage eating aversions and low intake among children with autism. Autism 2014;18:712–22. 21
The total population across all four studies was 107 children aged 3–8 years with a diagnosis of ASD. Three of the four studies included training interventions delivered to parents to address their children’s food aversions, restricted diets or mealtime behaviour. 21,22,24 Peterson et al. 23 delivered caregiver training after the study, whereas Johnson et al. ,24 Marshall et al. 22 and Sharp et al. 21 incorporated training of the caregiver/parent by the therapist in a clinic setting, either from the beginning of the study or incrementally as the child/parents progressed. All of the studies included a behavioural intervention, using prompts (verbal and visual) and reinforcement of the child’s behaviour. Both Marshall et al. 22 and Peterson et al. 23 included a comparison intervention, with Graded exposure to food through modelling and play. In both studies the comparison intervention was delivered by the therapist; however, Marshall et al. 22 incorporated training of the caregiver/parent, whose involvement incrementally increased as the sessions progressed. The number and duration of sessions varied across the studies for both interventions and comparators, with one22 offering the choice of sessions delivered weekly for 10 weeks, or sessions delivered intensively within 1 week. The duration of follow-up ranged from 8 to 16 weeks. The most common target outcome was mealtime behaviours, both positive and disruptive. Other outcomes measured across the four studies comprised Dietary intake, Dietary variety, Food acceptance, Mouth clean, Grams consumed, Weight, Height, Body mass index (BMI), Behaviour outside mealtimes, Parent stress, Caregiver satisfaction and Feasibility.
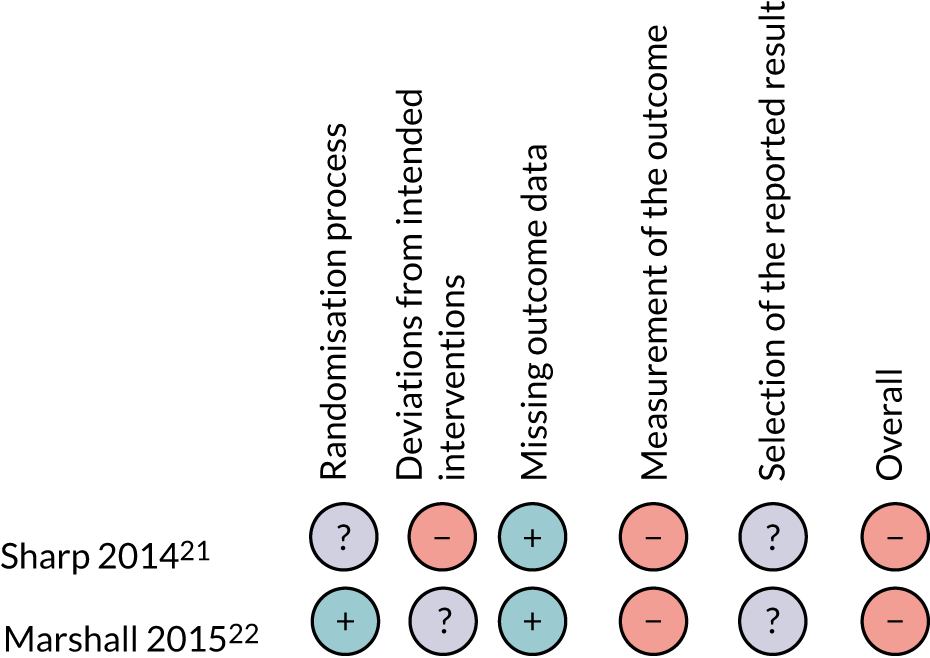
The overall quality of the studies that were included in the update was rated as low. The two studies that used a single-case experimental design both scored highly on baseline and external validity criteria and poorly on social validity; both failed to meet two of the four elements of this criteria [(1) magnitude of change of dependent variable is socially important and (2) independent variable is practical and cost-effective]. In addition, Johnson et al. 24 scored poorly on the criteria description of participants/setting. The two RCTs21,22 were both considered to be at high risk of bias, with the main concerns being the measurement of outcomes and deviation from the intended interventions. Summaries of the quality assessments are presented in Table 1 and Figure 3 (see also Table 2).
| Study (first author and year of publication) | Category | Total (21) | ||||||
|---|---|---|---|---|---|---|---|---|
| Description of participants/setting (3) | Dependent variable (5) | Independent variable (3) | Baseline (2) | Internal validity (3) | External validity (1) | Social validity (4) | ||
| Johnson 201524 | 1 | 4 | 3 | 2 | 2 | 1 | 2 | 15 |
| Peterson 201623 | 3 | 5 | 2 | 2 | 3 | 1 | 2 | 18 |
FIGURE 3.
Quality assessment of RCTs using the Cochrane risk-of-bias tool, version 2. –, high risk of bias; +, low risk of bias; ?, unclear risk of bias.
Summary of the overall evidence (from Marshall et al.5 and our update)
Twenty-three studies were included in the report by Marshall et al. ;5 adding the four studies included in the update, the total number of studies was 27, two of which were RCTs. In Marshall et al. ,5 only 5 out of the 23 (22%) studies reported an increase in the number of foods eaten, and only two studies used a formal outcome measure to capture this information. The four studies that were included in our update found a similar pattern, with only one study reporting outcomes relating to improvements in Total number of foods consumed and Total number of unprocessed fruits and vegetables.
In Marshall et al. ,5 all studies were appraised using the quality rating tool for single-case experimental designs:18 scores ranged from 7 to 18 (out of a target total of 21). The two studies21,22 identified in the update that were assessed using this tool were generally consistent with Marshall et al. ,5 but scored in a higher range (range 15–18). In their original review, Marshall et al. 5 had called for prospective randomised trials to further demonstrate experimental effect, and one RCT22 and one pilot RCT21 were included in the update. Sharp et al. 21 purported to represent the first RCT of a feeding intervention in ASD; however, this was a pilot RCT and, therefore, inadequately powered. Furthermore, this study21 was not sufficiently robust to allow strong conclusions to be made on the effects of EDSD interventions to improve the mealtime behaviour or foods eaten by children with ASD, or to inform decision-making. Overall, although the number of children with ASD who have taken part in research on interventions for EDSD has increased (from 37 children in 2014 to 144 children in 2018), the evidence base remains very limited. Although one RCT and one pilot RCT have now been included in the review, these studies contain methodological limitations. There continues to be a lack of rigorous studies and no high-quality prospective randomised trials to guide practice.
Update of the National Institute for Health and Care Excellence cerebral palsy in under-25s review6
Two new studies were included in our update of the NICE review6 to inform the management of EDSD in children with cerebral palsy. 25,26 One was a RCT25 and the other was a small pilot study26 in preparation for a RCT. Full descriptive details of the included studies are provided in the data extraction tables (see Appendix 3); however, a brief summary is provided in Box 4.
Serel Arslan S, Demir N, Karaduman AA. Effect of a new treatment protocol called Functional Chewing Training on chewing function in children with cerebral palsy: a double-blind randomised controlled trial. J Oral Rehabil 2017;44:43–50. 25
Song WJ, Park JH, Lee JH, Kim MY. Effects of neuromuscular electrical stimulation on swallowing functions in children with cerebral palsy: a pilot randomised controlled trial. Hong Kong J Occup Th 2015;25:1–6. 26
The population of the two studies (combined, n = 100) was children who were aged 1.1–8 years with cerebral palsy. Serel Arslan et al. 25 evaluated a parent training intervention that was delivered by speech and language therapists, whereas Song et al. 26 piloted a therapist-delivered intervention. Both studies included interventions with multiple components that were carried out simultaneously and both included Positioning and Oral/sensory desensitisation.
Serel Arslan et al. 25 (n = 80 children) evaluated an intervention that comprised Functional chewing training, which was made up of five steps: positioning the child, positioning food, sensory stimulation, chewing exercises and adjustments to food consistency. This was carried out alongside Oral motor exercises: five sets per day, five days per week, for 12 weeks. In addition, parents were given a brochure on exercises.
Song et al. 26 compared Oral sensorimotor treatment (10 minutes) and neuromuscular electrical stimulation (20 minutes) twice weekly for 8 weeks with Oral sensorimotor treatment plus sham neuromuscular electrical stimulation in a pilot study. Oral sensorimotor treatment included various sensory stimuli that were applied to the cheeks, chin, lips, tongue and oral palate using human fingers, a vibrator and an ice stick.
Both studies measured Feeding behaviour as a primary outcome, but used different tools to evaluate progress. Serel Arslan et al. 25 also measured Chewing function, whereas Song et al. 26 measured Severity of dysphagia. The study by Song et al. 26 was a pilot study and as a consequence it was judged to be at high risk of bias, whereas the full study by Serel Arslan et al. 25 was considered to be at lower risk of bias. A summary of the quality assessments are presented in Table 2.
| Study (first author and year) | Selection bias | Performance bias | Attrition bias | Detection bias |
|---|---|---|---|---|
| Serel Arslan 201725 | Low | Low | Low | Unclear |
| Song 201526 | High | High | Medium | High |
Summary of totality of the combined evidence (National Institute for Health and Care Excellence’s review6 plus our update)
Eight studies were included in the original NICE6 review of primary management of EDSD, and two more were included in our update (total number of included studies, n = 10; total number of participants, n = 335). The review included six RCTs and four cohort studies of various interventions and the studies were conducted in a number of countries: four in the USA, two in Turkey, and one each in the Republic of Korea, Canada, Bangladesh and the Islamic Republic of Iran, potentially limiting generalisability to the NHS setting. Based on its original review, NICE made a number of guideline recommendations (see Box 2). The combined evidence, including our updated review, does not support any changes to those guidelines.
Update of the review of oropharyngeal dysphagia by Morgan et al.4
Searches and data extraction for an update of the review by Morgan et al. 4 were conducted by the original review team in 2018. An updated review is due to be published in 2020. Inclusion criteria for this review are:
-
Population – children and young people aged < 18 years with oropharyngeal dysphagia (i.e. difficulties in chewing or preparing food, moving food posteriorly with the tongue and swallowing food) diagnosed by a medical officer. Studies involving children with oesophageal dysphagia, including lower oesophageal sphincter dysfunction and gastro-oesophageal reflux, were excluded.
-
Intervention – any intervention that aimed to improve body functions underpinning eating/drinking, eating/drinking or participation in mealtimes.
-
Outcomes – Physiological Function, Aspiration and chest health, Diet consumed, Growth, Participation at mealtimes and Parental stress.
-
Study design – RCTs and quasi-RCTs.
-
Language – studies were published in English in peer-reviewed journals.
The review by Morgan et al. 4 included three studies (two involving children with cerebral palsy, both of which were included in the NICE review,6 and one additional study that focused on children with myotonic dystrophy). In their recent searches, Morgan et al. 4 identified one additional study: Sığan et al. 27 This study was a RCT and included 81 children with cerebral palsy who were aged 12–42 months. At the time of this report, Morgan et al. 4 had not extracted data from this paper or reviewed its quality. However, it was included in the original review by NICE6 and, therefore, has already been systematically appraised; it was rated as being low quality in the NICE review. 6
Summary of systematic review findings
The original published systematic reviews of interventions and our updates have demonstrated that the evidence to address the questions around effective management of EDSD in children with cerebral palsy or ASD is of low quality and is accumulating slowly. A number of pilot RCTs were identified and included, some of which drew inferences around effectiveness (albeit with a lack of power); therefore, despite their primary aim being around feasibility, we have included them for completeness. Drawing the evidence together in one report has allowed us to identify the overlap in studies reviewed and some of the interventions that are being developed to improve children’s outcomes. Most RCTs involving children with cerebral palsy have focused on Sensorimotor treatments, with Behavioural techniques and parent training in Positioning, Modification of equipment, Food, and Environment and mealtime management. Interventions for children with ASD have used Behavioural techniques to improve mealtime behaviour and food aversion. A wide variety of outcomes have been targeted, using many different outcome measures. Meta-analysis has not yet been possible in any of the three reviews; no attempt was made to update the original, novel, meta-analysis by Marshall et al. 5 There remains a lack of high-quality studies and there are no high-quality prospective clinical trials that demonstrate the effectiveness on EDSD interventions for young children with neurodisability. We conclude that there is inadequate research evidence to demonstrate whether interventions to improve EDSD in children with cerebral palsy or ASD are effective.
Strengths and limitations of systematic reviews
To make best use of these published systematic reviews of interventions we updated each using the methodology of the original review, with the exception of the age criterion. To align with our project aims we extended the age criterion to 8 years for all of the review updates. By following each of the review methods different risk-of-bias and assessment tools were used across the updates to appraise the specific quality markers of RCTs and single-case experimental designs. Although the tools for assessment differ, resulting in different presentations, the criteria against which risk of bias and quality are being assessed are generally the same. We have assumed that the original searches and processes were robust enough to identify all relevant studies, as each of the reviews followed established systematic review processes. Each review includes and represented the best available evidence (with the inclusion of experimental designs) around effective management of EDSD in children with cerebral palsy or ASD. Our updates have ensured that the findings and recommendations of these reviews remain up to date.
Patient and public involvement in systematic reviews
The PAG considered the summaries of the findings from the updated systematic reviews of interventions alongside findings from the mapping review and national survey. The PAG advised on creating a pictorial summary of the identified interventions and outcomes to aid discussion in the second focus groups and the stakeholder consultation workshops (see Figures 9 and 10).
How did the systematic review findings inform the next step?
The interventions and outcomes identified in the updates of the published systematic reviews of interventions were considered by parents and professionals in the first focus groups with regard to their use in the UK (see Chapter 5). They also directly informed the design of the national survey of current practice (see Chapter 7) and the outcome measures used in studies included in the updates of the three published systematic reviews of interventions were listed for inclusion in the systematic review of measurement properties of tools (see Chapter 6).
Chapter 4 Aim 1: mapping review
Objectives
-
To review the clinical practice and research evidence for the interventions, outcomes measured and tools used to measure these outcomes for EDSD in young children with neurodisability.
-
To identify the subgroups of children for whom there is the most robust evidence on intervention success/failure.
-
To investigate the extent to which interventions have been defined and manualised to facilitate replication.
Methods
We searched for literature pertaining to any intervention that aimed to improve EDSD for children with neurodisability. This was a mapping review rather than a systematic review to establish an estimate of the effectiveness or assess the quality of the evidence. Nonetheless, the approach taken to searching and screening was rigorous and consistent with that used in a systematic review.
Inclusion criteria
Literature was included in the mapping review if it met the following criteria:
-
Population – children (aged 0–8 years) with any type of non-progressive neurodisability who had EDSD. The following conditions were excluded: cystic fibrosis, gastro-oesophageal reflux and structural abnormalities [e.g. cleft lip and palate, and CHARGE (coloboma, heart defects, choanal atresia, growth retardation, genital abnormalities and ear abnormalities) syndrome]. Children who had rumination (i.e. persistent regurgitation, re-chewing, re-swallowing or vomiting of previously eaten foods), eating disorders (unless specifically about food avoidance/restrictions not related to a desire for thinness) or problem behaviour at mealtimes that was not related to eating were also excluded. Studies were included if any of the participants were aged 0–8 years.
-
Intervention – any intervention to improve eating, drinking and swallowing that can be delivered by parents to their children aged 0–8 years. The following interventions were excluded: Pharmacological, Dietary or Nutritional interventions, Gastrostomy and Oral appliances. Interventions that focused on speech development or improvement and the swallowing of tablets were also excluded.
-
Comparator – any other intervention for eating, drinking and swallowing or mealtime behaviour, any intervention described as ‘treatment as usual’ or no intervention.
-
Outcome – any outcome pertaining to food intake, behaviour, health, well-being or acceptability.
-
Study design – systematic reviews of interventions and any controlled or non-controlled study of intervention effects or acceptability. Editorial/commentary/opinion articles were excluded.
-
Limitations – manuscripts written in English and published from January 1985 to October 2017.
Searches were designed by an information specialist in collaboration with the project team. The search strategy was designed on MEDLINE [via OvidⓇ (Wolters Kluwer, Alphen aan den Rijn, the Netherlands)] using thesaurus headings and title and abstract keywords, and translated as appropriate to the following databases: Cumulative Index to Nursing and Allied Health Literature (CINAHL) database [via EBSCOhost (EBSCO Information Services, Ipswich, MA, USA)], PsycINFO (via Ovid), Web of Science™ (WoS; Clarivate Analytics, Philadelphia, PA, USA), EMBASE™ (Elsevier, Amsterdam, the Netherlands) (via Ovid), Education Resources Information Center (ERIC) (via EBSCOhost), Cochrane Database of Systematic Reviews [via Wiley Online Library (John Wiley & Sons, Inc., Hoboken, NJ, USA)], Cochrane Central Register of Controlled Trials (CENTRAL) (via Wiley Online Library), The Speech Pathology Database for Best Interventions and Treatment Efficacy (speechBITE; The University of Sydney Lidcombe, NSW, Australia) (www.speechbite.com) and Occupational Therapy Systematic Evaluation of Evidence (OTseeker; www.otseeker.com). This search was run between 5 October and 17 October 2017. Full details of the search strategies are presented in Appendix 4. Two researchers (HT and LP) independently screened titles and abstracts to identify studies meeting the inclusion criteria. The full texts of potentially eligible articles were retrieved and assessed independently against inclusion criteria by two researchers (HT and LP or HM). Where there were discrepancies in these processes, a third person from the review team was consulted and a consensus was reached. One researcher (HT) extracted the data and classified each study; LP checked the data extraction and coding.
Results
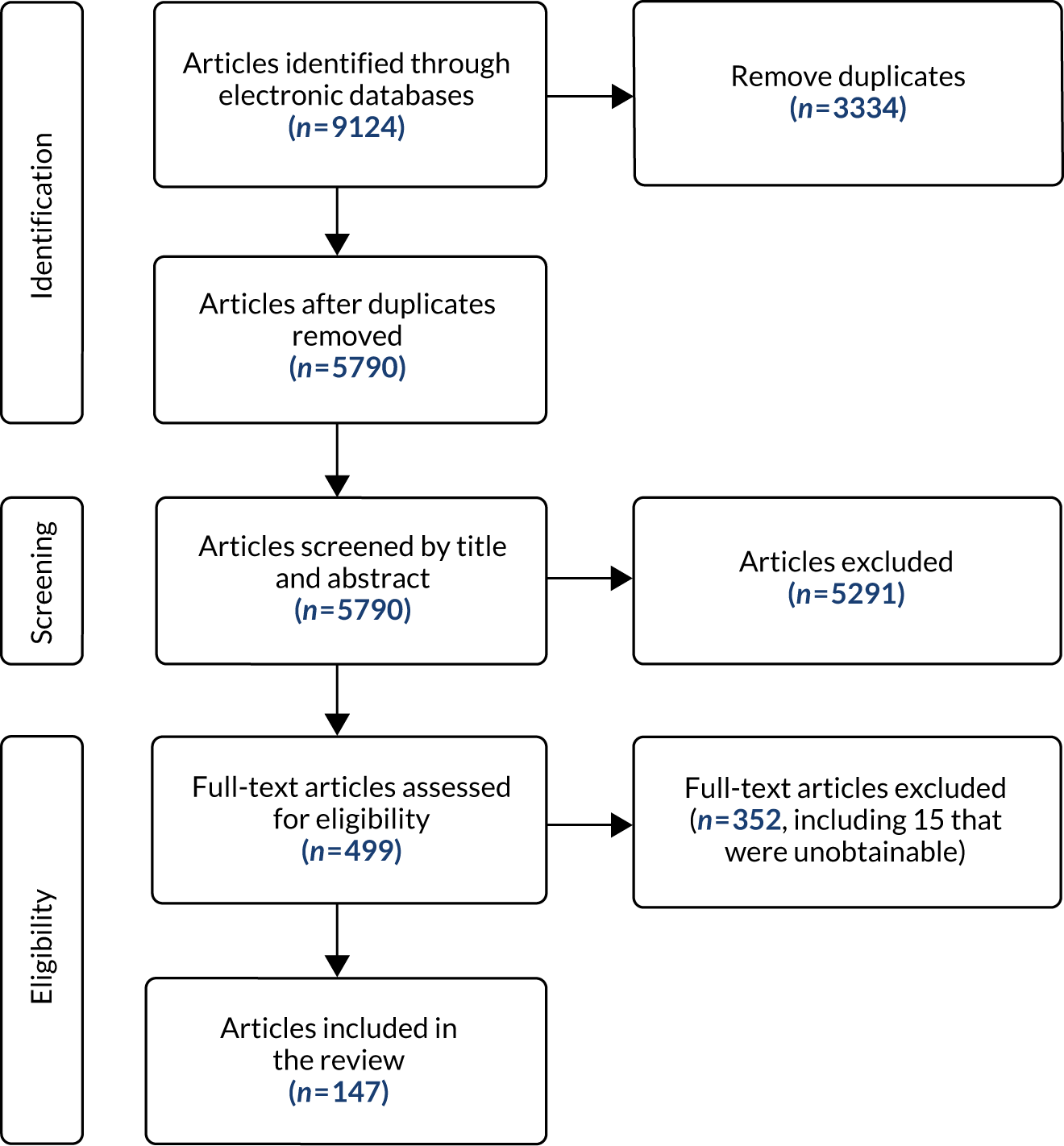
Our searches identified 5790 references; following sifting on title and abstract, we retrieved 492 full texts, of which 147 fitted the inclusion criteria (Figure 4). Fifteen of the papers identified through the updates of the published systematic reviews of interventions (those from the Marshall et al. 5 and NICE6 updates) were also found in the mapping review, including nine single-case experimental design studies replicated across fewer than four participants.
FIGURE 4.
The PRISMA flow chart of mapping review.
Study participants ranged in age from < 1 year to 31 years, with many of the studies including participants outside the age range that we defined as ‘young children’ (i.e. ≥ 9 years). In most cases, the results for our target group of young children (aged 0–8 years) could not be disaggregated. The interventions reported across the studies were grouped as addressing physical, mixed or non-physical factors affecting eating, drinking and swallowing: 27 studies addressed physical EDSD, 53 non-physical EDSD and 66 mixed EDSD. Most interventions directly targeted EDSD, such as Modifications (Environment, Equipment, Food or drink, Placement of food and Positioning), improved mealtime communication (Enhancing communication strategies, Responding to the child’s cues for feeding and Pace of feeding) and desensitisation strategies (Graded exposure to foods or textures, and Oral and sensory desensitisation). Other interventions that did not directly target EDSD included Psychological support for child and parent and Self-feeding. Teaching techniques (Prompting and Reinforcement) were referred to frequently in the teaching of any of these interventions. There was a range of outcomes measured across these studies including Swallowing function, Chest health, Amount of food eaten, Eating efficiency, Oral motor function, Number (percentage) of bites, Variety of food consumed, Mealtime behaviour, Self-feeding, Food acceptance and Amount of liquid consumed. Further details of the included studies are presented in Table 3.
| Clinical group | Research design | Total number of participants | Age of participants (years) | Outcomes measured | Published protocols/measures used |
|---|---|---|---|---|---|
| Physical and mixed EDSD | Systematic review including RCT, n = 4 | – | – | Improved nutrition; Better general health; Weight gain; Increased growth; Child enjoys mealtimes more; Child less frustrated or distressed at mealtimes; Better quality of life for child; Parent enjoys mealtimes more; Parent less frustrated or distressed at mealtimes; Better co-ordination of swallowing and breathing; Better sitting; Better oral motor function; Less drooling; Shorter mealtimes; Better self-feeding or independence skills; Wider range of foods eaten; Less aversion of avoidance of particular foods; More food or drink consumed; Better mealtime interaction one to one with child; More involvement in family’s activities; Better understanding of child’s difficulties and strategies to support them; and Mealtime behaviour | American Speech–Language–Hearing Association’s National Outcomes Measurement System Swallowing Scale (1);28 Battery for Oral-Motor Behavior in Children (1);29 Beckman Oral Motor Assessment (1);30 Behavioural Assessment of Oral Functions in Feeding (2);31 Behavioural Paediatric Feeding Assessment Scale (2);32,33 Canadian Occupational Performance Measures (1);34 Children’s Eating Behaviour Inventory (1);35 Classification system for complex feeding disorders (1);36 Drooling Rating Scale (1);37 Functional Feeding Assessment subtest of the Multidisciplinary Feeding Profileb (5);38 Functional Oral Intake Scale39 (1); Gisel Video Assessment (3);40 Karaduman Chewing Performance Scale (1);41 Morris Pre-speech Assessment Scale (2);42 Oral Motor Assessment Scale (1);43 Oral Motor Dysfunction Scale (1);43 Paediatric Feeding Evaluation Checklistc (1);44 Schedule of Oral Motor Assessment (1);45 Sitting Assessment Scale (1);46 Vulpe Assessment Battery (1);47 and the World Health Organization Quality of Life-BREF (1)48 |
| Systematic review, n = 4 | – | – | |||
| RCT, n = 6 | 257 | 1–13 | |||
| Quasi experimental design, n = 2 | 43 | 1–31 | |||
| Feasibility study, n = 0 | – | – | |||
| Single-case experimental design, n = 18 | 40 | 2–17 | |||
| Before-and-after study, n = 16 | 479 | < 1–18 | |||
| Case study, n = 14 | 27 | 0–6a | |||
| Literature review, n = 1 | – | – | |||
| Non-physical EDSD | Systematic review including RCT, n = 1 | – | – | Weight gain; Increased growth; Parent enjoys mealtimes more; Parent less frustrated or distressed at mealtimes; Better sitting; Shorter mealtimes; Better self-feeding or independence skills; Wider range of foods eaten; Less aversion of avoidance of particular foods; More food or drink consumed; and Mealtime behaviour | Aberrant Behaviour Checklist (1);49 Brief Autism Mealtime Behaviour Inventory (5);50,51 Behaviour Intervention Rating Scale (1);52 Behavioural Paediatric Feeding Assessment Scale (2);32,33 Client Satisfaction Questionnaire – Parent and Child (2);53 Eyberg Child Behaviour Inventory (1);54 Family Quality of Life Scale (2);55 Food Preference Inventory 1);56,57 Parenting Stress Index – Short Form (3);58 Screening Tool of feeding Problems (1);59 Social Responsiveness Scale (1);60 The Food Frequency Questionnaire (1);61 and 3 Day Food Records (1)62 |
| Systematic review, n = 5 | – | – | |||
| RCT, n = 4 | 113 | 1–8 | |||
| Quasi experimental design, n = 0 | – | – | |||
| Feasibility study, n = 1 | 11 | 8–11 | |||
| Single-case experimental design, n = 52 | 89 | 1–18 | |||
| Before-and-after study, n = 4 | 32 | 2–18 | |||
| Case study, n = 15 | 18 | 2–16 | |||
| Literature review, n = 0 | – | – |
The totals for the number of studies and included participants do not include the systematic or literature reviews to prevent double counting and due to the reviews including a large number of studies that did not meet the criteria for inclusion.
The majority of studies described multicomponent interventions; for example, an intervention might ensure that the children were in a safe position to eat and drink (Positioning), were fed textures that they could swallow easily (Modifying food or drink) and received praise for swallowing (Reinforcement). The frequency of individual interventions studies included in the mapping review is shown in Figure 5. The mapping process enabled us to disaggregate multicomponent interventions to explore a number of questions, including (1) which individual interventions were more frequently provided together as a multicomponent intervention; (2) the difference in the frequency of interventions between participants with physical and mixed EDSD and participants with non-physical EDSD; and (3) the number of participants in whom each intervention had been assessed.
FIGURE 5.
Number of studies and included participants by intervention and type of EDSD.
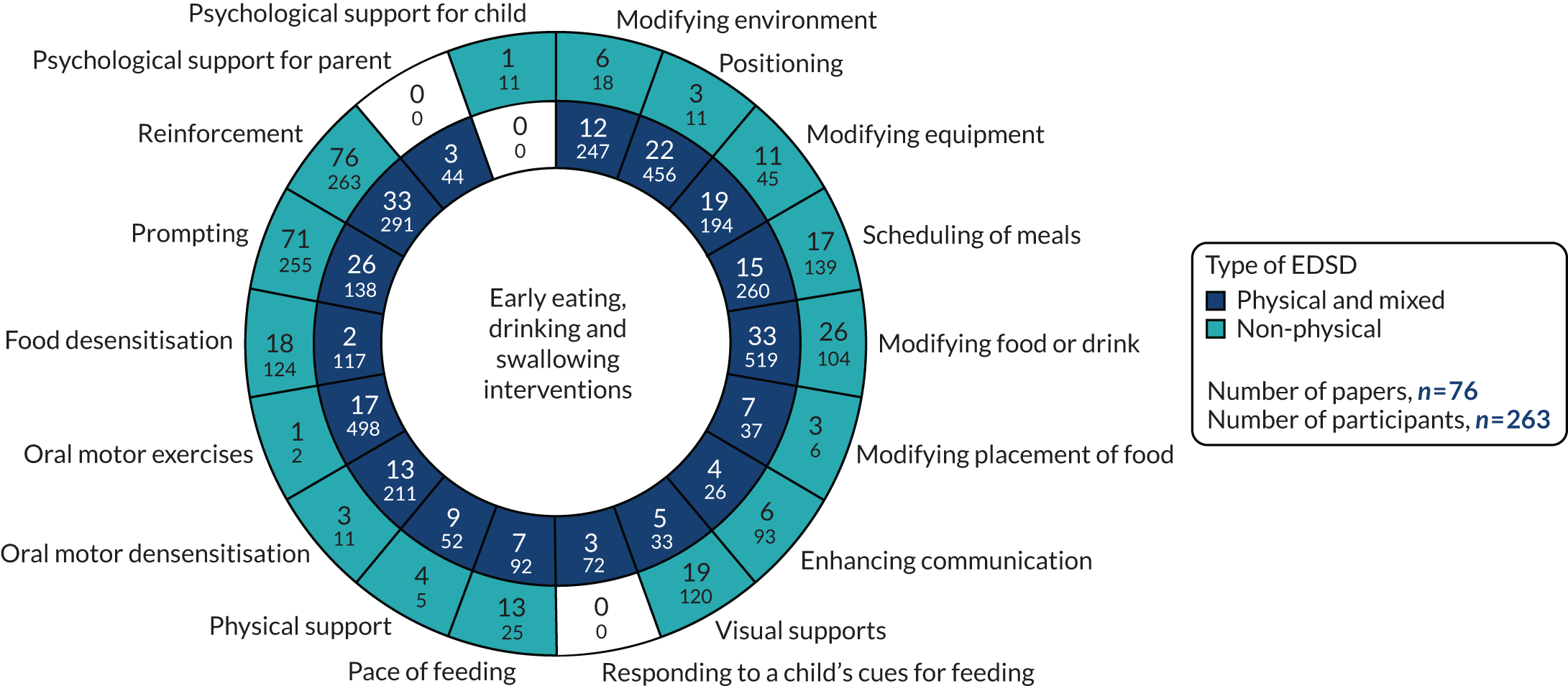
We identified 18 individual interventions, most of which had been assessed within a multicomponent intervention that included participants with physical, mixed and non-physical EDSD (see Figure 5). In Figure 5, we have presented the frequency of assessment of each intervention, based on the number of studies, alongside the total number of participants in those studies. The frequency count is based on the number of studies reporting the primary outcome. Based on the number of studies, the most common individual interventions considered across the populations were Reinforcement (109 studies, 554 participants) and Prompting (97 studies, 393 participants). However, these individual interventions are teaching techniques to support the delivery of specific EDSD interventions. Beyond the teaching interventions/techniques, the most commonly assessed interventions for children with physical or mixed EDSD were Modification of food or drink (33 studies, 519 participants), Positioning (22 studies, 456 participants), Modifying equipment (19 studies, 194 participants) and Oral motor exercises (17 studies, 498 participants). The most commonly assessed interventions for children with non-physical EDSD were Modification of food or drink (26 studies, 104 participants), followed by Visual supports (19 studies, 120 participants), Food desensitisation (18 studies, 124 participants) and Scheduling of meals (17 studies, 139 participants). Psychological support for parents (three studies, 44 participants) and Responding to a child’s cues for feeding (three studies, 72 participants) were assessed only for participants with physical or mixed EDSD; however, Psychological support for the child (one study, 11 participants) was found in an intervention assessing only children with non-physical EDSD. Although Psychological support for parents and the child and Responding to a child’s cues for feeding were included in studies infrequently (i.e. evaluated in fewer than five studies), a large number of individual interventions were seen in more than 10 studies of both children with physical or mixed EDSD and children with non-physical EDSD. Figure 5 illustrates the significant overlap in the individual interventions being considered for children with physical or mixed and non-physical EDSD. Only three interventions were considered in only one of the populations: Responding to a child’s cues for feedings (three studies) and Psychological support for the parents (three studies) were considered in only a non-physical population, and Psychological support for the child (one study) was considered in only children with physical and mixed EDSD. These three individual interventions were also the least frequently considered.
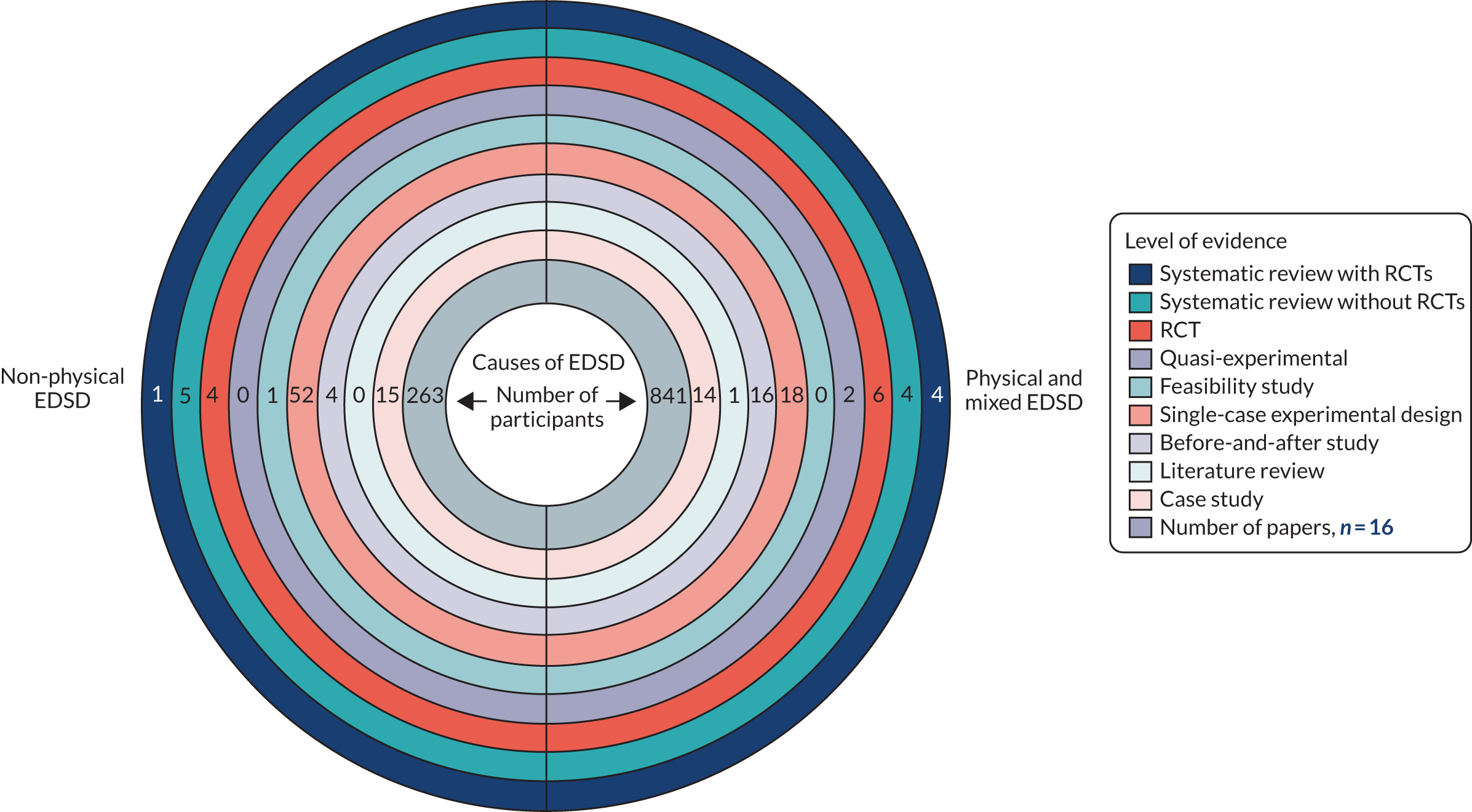
A range of study designs, from those providing the highest level of evidence (systematic reviews of RCTs) to those providing the lowest (case studies), were used to evaluate the interventions (Figure 6 and Table 3). Appendix 5 shows the study designs used to evaluate each intervention. Figure 6 shows that a large number of studies had designs that are widely considered to be less robust and, therefore, more prone to bias, such as case studies and before-and-after studies. In total we identified 147 studies, 121 of which were before-and-after studies, case studies, literature reviews or single-case experimental designs. We also identified 12 RCT/quasi-experimental design studies and 14 systematic reviews. The amount of evidence included in these reviews was variable and they provided no robust conclusions regarding the optimal multicomponent intervention.
FIGURE 6.
Types of study design and numbers of participants by causes of EDSD.
Outcomes
The studies in the review measured 24 different outcomes (as shown in Table 3), with most studies measuring multiple outcomes.
Measures
The studies used 33 published protocols/measures to assess change in the outcomes, as shown in Table 3. A total of 25 studies used published protocols/measures, with the remaining studies using bespoke measures that the authors had developed specifically for use in their study. These bespoke measures lacked evidence of reliability, validity or responsiveness to change.
Summary of mapping review
The mapping review collated a wide range of research evidence. The scope and purpose of the mapping review was not to assess the quality of the individual studies but rather to explore and understand the frequency and level of evidence for each of the individual interventions. The aim was to present a full picture of the interventions that have already been developed and/or evaluated, to ensure that all of the potential interventions were considered in the later stages of this work. The level of evidence found suggests that there are several studies evaluating some of the individual interventions identified; however, owing to the design of the studies it is likely to be low-level evidence, given that there is a lack of RCTs. The mapping review also highlighted the significant overlaps in the interventions delivered to children with physical or mixed and non-physical EDSD in these studies.
Strengths and limitations of the mapping review
The mapping review has elicited the number of published studies evaluating each individual intervention type, the study designs used and the number of participants with physical or mixed and non-physical EDSD included. Considering the number of studies identified, it was necessary to take a pragmatic approach to data extraction, ensuring that only the necessary information was retrieved. Furthermore, we have not explored the data beyond the scope of the question that we set out to address. There are many other questions that this literature base might support answering; however, these were out of scope of this research.
Patient and public involvement in the mapping review
Parent co-investigators checked the intervention and condition terms that were included in the searches for the mapping review. They also discussed the search findings with members of the research team, to help ensure that all relevant studies were being retrieved. The PAG considered the summaries of the findings from the mapping review alongside findings from the updates of the three published systematic reviews of interventions and national survey. The PAG commented on a pictorial summary of the identified interventions and outcomes to aid discussion in the second round of focus groups (see Chapter 9) and the stakeholder consultation workshops (see Chapter 11) (see Figures 9 and 10).
How did the mapping review inform the next step?
The mapping review provided information regarding the evidence base for interventions aimed at improving EDSD in children with neurodisability, the outcomes measured and the tools used to measure those outcomes. This information informed the list of interventions and outcomes included in the national survey (see Chapter 7), the discussions within the second round of focus groups (see Chapter 9) and the searches for papers examining relevant outcome measurement tools for the measurement properties review (see Chapter 6).
Chapter 5 Aim 2: first round of focus groups
Objective
The objective was to gain an understanding of the interventions offered through NHS services to parents of children with neurodisability who experience EDSD by consulting with parents and HPs to inform the development of a national survey of current practice.
Methods
Participant recruitment and selection
We aimed to recruit parents of young children with physical or mixed EDSD, parents of young children with non-physical EDSD and HPs working with young children with physical, mixed and non-physical EDSD in the north-east of England. Parents were recruited via invitations sent through social media, newsletters of local parent organisations, local specialist schools and charities. We recruited HPs working with children with neurodisability and EDSD via e-mails sent to regional professional networks in the north-east of England, including the Speech and Language Paediatric Dysphagia Clinical Excellence Network and the regional British Academy of Childhood Disability regional clinical network.
Procedure
Invitations about the focus groups contained e-mail contact details for the study team and a link to an information sheet. Parents who contacted the research team and expressed an interest in taking part provided information on the age of their child and the nature of their EDSD (i.e. whether these were physical, non-physical or mixed difficulties) and the geographical area in which they lived (e.g. Newcastle, County Durham) to ensure some variation in family experiences of EDSD and service provision. HPs provided information about the geographical area in which they worked, their professional group and the type of service they provided (e.g. community-based service or assessment service).
We held four stakeholder focus groups: two with parents and two with HPs. Parents of children with physical EDSD and parents of children with non-physical EDSD attended different groups. The HP groups included professionals working with children with any EDSD and were held outside working hours. All groups took place in February 2018 in the north-east of England. Participants provided written consent. Parents and HPs attending the sessions received a £50 shopping voucher to thank them for their time and to cover any travel costs.
All focus groups followed a similar format. Three of the research team attended each group: one or both of the parent investigators (DG and JS), Helen Taylor and Jeremy Parr, Julian Thomas or Lindsay Pennington. Helen Taylor led all four group discussions; other members of the research team asked supplementary questions or provided clarification. We asked each group about their experience of interventions for EDSD: who introduced them, where they were used, their perceived effectiveness and acceptability; how the success of interventions is or could be evaluated; and which measurement tools (if any) were used (see Appendix 6 for the focus group topic guide).
All recordings of focus group discussions were transcribed verbatim. Two investigators (LP and HT) read the transcripts repeatedly to identify all of the interventions, outcomes and measures discussed. Definitions of interventions and outcomes were taken from the systematic and mapping reviews. Any new interventions and outcomes identified by Helen Taylor and Lindsay Pennington were discussed by the research team to ensure mutual exclusivity (Tables 17 and 18). Following content analysis principles, we extracted excerpts of coded text from each focus group transcript into a matrix to chart the interventions and measures and who prescribed and used them. 63 We also charted participants’ reports of acceptability/effects of interventions and the importance of individual outcomes in the matrix. As the aim was to identify the range of interventions, outcomes and measures used, rather than the frequency of their use or strength of feeling about the features of EDSD management, we did not count the number of times each intervention/measure appeared in the transcripts.
Results
Participants
The characteristics of those who participated in the first round of focus groups are shown in Table 4. Seven parents participated, two of whom had a child with physical EDSD and five of whom had a child with non-physical EDSD; the age of the children ranged from 6 to 18 years. A parent of children with physical EDSD agreed to take part but was unable to attend on the day of the focus group. Six HPs took part (five speech and language therapists and one dietitian). Another speech and language therapist agreed to take part in the first focus group but was unable to attend on the day of the group, nor could they attend the second focus group. Parents resided in several areas across the north-east of England and received services from a number of NHS trusts. HPs worked in a range of NHS trusts in north-east England.
| Group | Location | Participants |
|---|---|---|
| Parent group 1 | Newcastle | Two parents of a child with physical EDSD |
| Parent group 2 | Newcastle | Five parents of a child with non-physical EDSD |
| HP group 1 | Newcastle | Two SLTs |
| HP group 2 | Newcastle | Three SLTs and one dietitian |
Interventions, outcomes and measures used by parents and health professionals
Parents reported that they currently used or had used a wide range of interventions, often in combination. Similarly, HPs reported that they or their colleagues recommended use of a range of interventions, sometimes simultaneously or in an additive approach (Box 5). Parents and HPs discussed the potentially stressful experience of mealtimes, and the importance of understanding the nature of both the children’s difficulties and their communication skills to implement any changes at mealtimes. Parents also stressed the variability in children’s eating and drinking from day to day. Both parents and HPs reported that children’s physical health and developmental progress were important outcomes of EDSD interventions, but also highlighted the social nature of mealtimes and outcomes related to enjoyment of food and eating with others. Although participants identified a substantial list of individual areas in which to measure progress or outcome, few participants reported a formal measurement that was used to evaluate progress. Tools that were mentioned were food diaries, weight, number of spoonfuls eaten in a meal and Therapy Outcome Measures (TOMs),64 which includes a Dysphagia scale.
-
Environment modification: adding or removing distractions and making eating into a game.
-
Positioning of the child, feeder or equipment.
-
Modification of equipment: plates, cutlery and cups.
-
Food modification: textures, temperature, colour and amount presented.
-
Modifying placement of food in mouth.
-
Enhancing communication between feeder and child.
-
Visual supports: mealtime plan.
-
Following child’s cues.
-
Manoeuvres.
-
Oral desensitisation.
-
Oral motor exercises: chewing practice of food in muslin bag and chewy tubes.
-
Desensitisation for food avoidance: preparation of foods, messy play, graded exposure to new foods and repeated exposure to new foods.
-
Behaviour change: prompting, rewards and explaining consequences.
-
Hand-over-hand prompting.
-
Parent support: counselling and parent to parent.
-
Energy supplements: calorie drinks, vitamins and special diets.
-
Sharing information on underlying causes of EDSD: HP to parent, and parent to parent.
-
Modifying social eating and drinking opportunities.
-
Nutrition: calorific intake and eating enough.
-
Health: chest health, constipation, vomiting, sleep and pain.
-
Weight.
-
Growth: rate of weight gain.
-
Child enjoyment of meals.
-
Reduced preparation time and reduced cost of preparing separate meals for child who eats a wider range of foods.
-
Parent mental health: feeling reassured, less stressed and less anxious.
-
Safety: choking, coughing and aspiration.
-
Oral motor function: chewing and drooling.
-
Mealtime duration.
-
Child independence in eating/drinking: no need for assistance, choosing what to eat and using cutlery.
-
Greater range of foods eaten: textures and trying new foods.
-
Parent understanding of child’s difficulties.
-
Parent–child interaction at mealtime.
-
Participation: eating with family, friends and outside the home.
-
Better able to read child’s cues around feeding.
-
Child comfort during meals.
-
Social acceptance.
Summary of findings
Parents and HPs reported using a wide range of interventions to enable children to use their current skills to eat and drink safely, or to teach new skills. They often used interventions in combination. They viewed children’s physical health and developmental progress, children’s enjoyment of meals and participation in meals as social activities as outcomes to target through intervention. HPs seldom used formal outcome measures to evaluate intervention success.
Strengths and limitations of the first round of focus groups
The first round of focus groups comprised a small sample of parents and HPs from one region of England served by numerous secondary-level services and one tertiary-level service. In the small sample, most of the parents had children with non-physical EDSD. The sample and the service organisations may not be representative of families receiving EDSD services or service providers across the UK. The children of some parents included in the groups were older than the target age range of the study. This meant that parents were recalling previous interventions and outcomes. However, their children had ongoing EDSD and parents were able to recall differences in interventions by school age, for example preschool and primary school, which was important for our study. The small size of the groups meant that there was time for all participants to relate their experiences.
Patient and public involvement in the first focus groups
The parent co-investigators recommended that separate groups be held for parents of children with physical or mixed EDSD and for parents of children with non-physical EDSD, as similar shared experiences may help the groups to gel and encourage discussion. The parent co-investigators reviewed the topic guide and agreed that the questions should initially be open, without examples of interventions and outcomes. The PAG reviewed the summary lists of the interventions and outcomes reported in the focus groups. They agreed that each intervention and outcome identified should be included in the survey.
How did the first focus groups inform the next step?
Parent and HPs in the focus groups endorsed all of the interventions and outcomes identified in the reviews; therefore, all were included in the national survey of UK parents’ and HPs’ use of EDSD interventions and evaluation of their outcomes (see Chapter 7).
Chapter 6 Aim 1: measurement properties review
Objective
To examine the psychometric robustness of tools used to measure change in EDSD in young children with EDSD.
Methods
Outcome measurement tools were primarily identified through the updates of the three published systematic reviews of interventions and mapping review, as well as the first round of focus groups and the survey (see Chapters 3–5 and 7). The list of 43 named tools focused on the evaluation of EDSD-related outcomes of interventions (see Appendix 7). Tools mentioned in the literature, or by participants in the focus groups and survey, that measured other important outcomes (e.g. Parent stress and Child quality of life) were not included in the listing and review of measurement properties.
Searches were conducted to specifically identify papers that examined the measurement properties of the named tools. The search strategy included terms for neurodisability or feeding disorders and children, and included a COnsensus-based Standards for the Selection of health status Measurement INstruments (COSMIN) filter (www.cosmin.nl; accessed 11 November 2019) (see Appendix 8). The COSMIN system was developed by an international group of experts to standardise assessment of the methodological quality of measurement studies. Papers identified from searching are examined in terms of the quality of the research study examining a tool’s measurement properties (e.g. whether the study had sufficient numbers of participants, clear hypotheses stated and a robust approach to the conduct of factor analysis); the paper is rated as ‘inadequate’, ‘doubtful’, ‘adequate’ or ‘very good’ on each property. Next, the data presented in each paper on reliability, validity and responsiveness to change are extracted and judged on COSMIN criteria (e.g. a cut-off point for a good reported level of inter-rater reliability) as being of a ‘sufficient’, ‘insufficient’ or ‘indeterminate’ level. Finally, the evidence is synthesised, following standard COSMIN criteria, to determine the strength of the evidence on each measurement property across all papers that examine any one particular measurement tool.
The following databases were searched: MEDLINE, CINAHL, PsycINFO and WoS. Following the initial searches of MEDLINE and PsycINFO on the 43 listed tools, further named tools of potential relevance were identified in the texts of the measurement properties papers, for example as criterion reference tools or revised versions (n = 21) [e.g. a paper on the Parent Mealtime Action Scale (PMAS) concerned development of a revised scale, the Parent Mealtime Action Scale – Revised (PMAS–R)]. The searches were updated to include the new tool names; thus, in total, we searched for papers on the measurement properties of 64 named tools.
Two reviewers (HM, a clinical psychologist who is experienced in systematic reviewing including use of the COSMIN approach, and CU, systematic reviewer in health research) sifted search results by title and abstract for likely relevance, using definitions and criteria agreed with the research team (see Appendix 9); uncertainty was resolved by requesting the full text. Papers were examined for inclusion at the full-text stage separately by both reviewers, with any disagreement settled by referral to a third reviewer (LP).
At the full-text stage, and before data extraction, a strategy was developed to focus on the most promising outcome measurement tools for application in a future trial. The criteria for exclusion comprised the age range covered (e.g. excluded the Neonatal Oral Motor Assessment Scale); not being about EDSD once further information was available from papers (e.g. Motivation Assessment Scale); cost of training and/or poor availability of training (e.g. Dysphagia Disorders Survey); precision of measurement [e.g. excluding the TOMs and the Eating and Drinking Ability Classification System for individuals with cerebral palsy (EDACS) and other similar classifications of function on 4- to 7-point scales]; or having been poorly rated on measurement properties in a published systematic review of measures of oropharyngeal dysphagia for preschool children with cerebral palsy and neurodevelopmental disabilities by Benfer et al. 65 Based on these criteria, 20 tools were not considered further.
Data extraction followed the COSMIN risk-of-bias checklist on reliability and validity of the evidence,66 with additional information noted on acceptability, feasibility, precision and interpretability from Fitzpatrick et al. 67 The COSMIN criteria for judgement of good measurement properties were taken from Prinsen. 66 The two reviewers trained together on papers and established reliability, before proceeding with data extraction (in addition, HM checked all data extracted by CU). Data were not extracted from papers with a sample of < 10 participants, with a non-relevant sample (e.g. a feeding clinic sample not further described) or when the paper was not about a measurement property.
Results
Results of searches
Papers from the first searches of PsycINFO and MEDLINE were sifted by title and abstract; 51 out of 560 papers and 18 out of 196 papers, respectively, were taken forward to sifting at full text. The reviewers checked 11% of articles (i.e. 88 articles) with 92% agreement on ‘get full text’/exclusion.
In the second search, CINAHL and WoS were added, as well as searching for the 21 additional named tools identified in the previous search. The searches of all four databases yielded 888 references, from which a total of 111 went forward to be sifted at full text. The two reviewers double checked 17.3% of articles, with 94% agreement on ‘get full text’/exclusion.
After de-duplication, a total of 127 papers were sifted as full text from the first and second searches. Of these, 86 papers were excluded and, therefore, 41 papers were included for data extraction (Figure 7).
FIGURE 7.
The PRISMA flow chart.
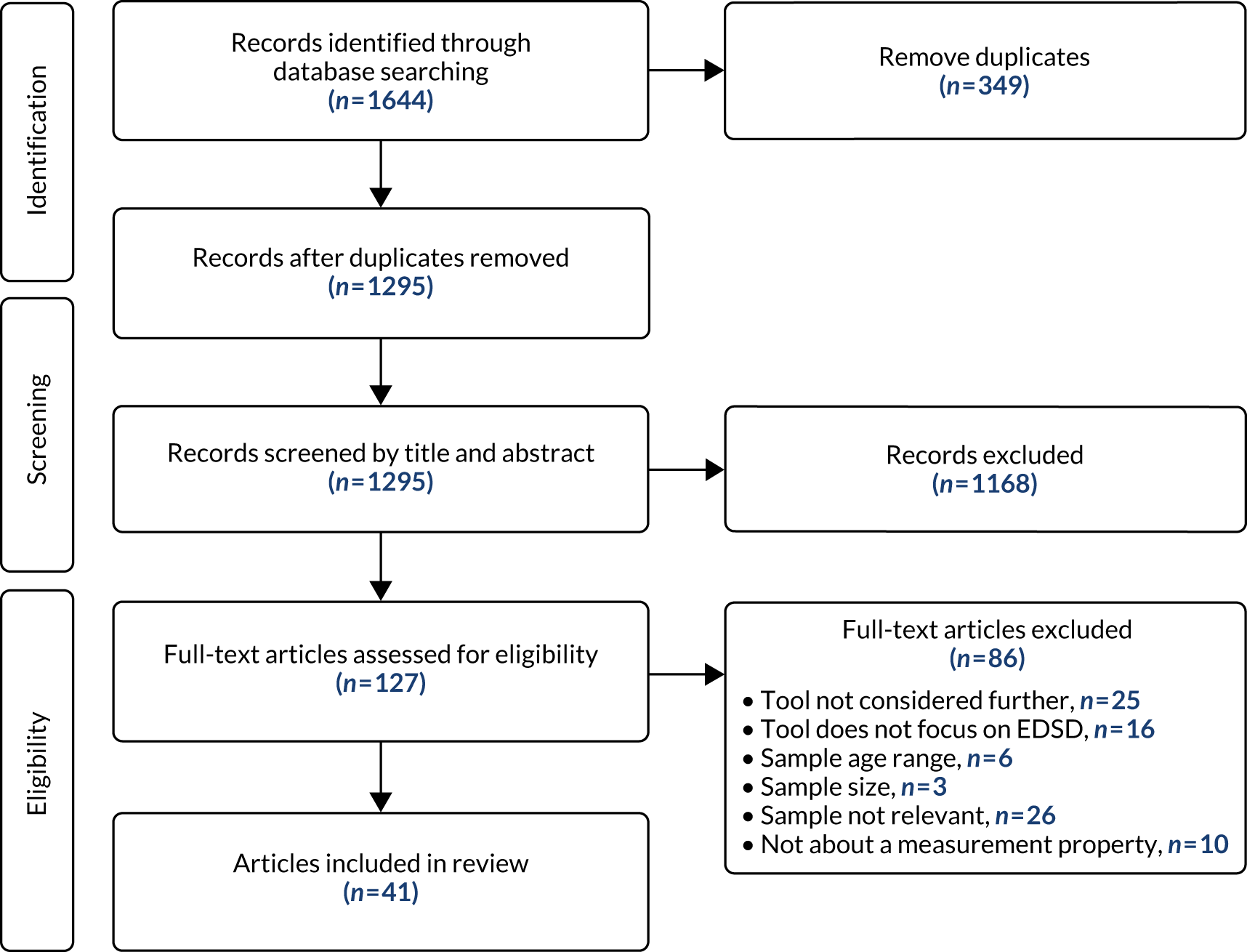
Results of data extraction
The 41 papers provided evidence on measurement properties relating to 22 tools used for children with neurodisability: 12 tools measuring child behaviours (three with parent domains also), five tools measuring parent strategies and five tools measuring oral motor skills. For the remaining 22 tools of the 44 considered in this review, no papers were found studying measurement properties with this population.
The information extracted for each paper in terms of the quality of the evidence about measurement properties is presented in Appendix 10, and the evidence for robust measurement is presented in Appendix 11. (Note that some papers provided evidence on more than one tool.)
The evidence is synthesised for each tool in Tables 5–7 and a description of each tool is presented in Tables 8–10 . The types of tools are divided into three categories: mainly assessing child behaviours in eating and drinking and at mealtimes, parent strategies for feeding their child and reported difficulties in managing mealtime situations or the child’s observable oral motor skills.
| Measurement tool | Summary ratings | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | PROM validity | Content validity | Structural validity | Internal consistency | Inter-rater reliability | Test–retest reliability | Criterion validity | Hypotheses testing: convergence/divergence | Hypotheses testing: known groups | Responsiveness | |
| Behavioural Paediatric Feeding Assessment Scale (child and parent domains)32,33 | 5 | – | + | + | + | – | |||||
| Brief Autism Mealtime Behaviour Inventory50,51 | 9 | – | – | ? | ? | + | + | + | |||
| Brief Assessment of Mealtime Behaviour in Children68 | 3 | – | +/– | ? | +/– | + | |||||
| Children’s Eating Behaviour Inventory (child and parent domains)35 | 1 | ? | + | + | |||||||
| Children’s Eating Behaviour Questionnaire69 | 3 | + | + | +/– | |||||||
| Food Frequency Questionnaire61 | 3 | + | + | ||||||||
| Food Preference Inventory56,57 | 2 | +/– | |||||||||
| Meals in Our Household (child and parent domains)70 | 1 | – | + | + | |||||||
| Paediatric Eating Assessment Tool71,72 | 2 | Adequate | Very good | – | + | + | + | + | |||
| Paediatric version of the Eating Assessment Tool73 | 1 | Inadequate | Doubtful | ? | ? | + | + | ||||
| Screening Tool of Feeding Problems59 | 1 | – | – | + | |||||||
| Child version of the Screening Tool of Feeding Problems59,74 | 1 | ? | – | + | ? | ||||||
| Measurement tool | Summary ratings | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | PROM validity | Content validity | Structural validity | Internal consistency | Inter-rater reliability | Test–retest reliability | Criterion validity | Hypotheses testing: convergence/divergence | Hypotheses testing: known groups | Responsiveness | |
| Child Feeding Questionnaire75 | 1 | – | |||||||||
| Feeding Strategies Questionnaire76 | 1 | – | – | ||||||||
| Parent Mealtime Action Scale77 | 3 | + | – | + | – | ||||||
| Parent Mealtime Action Scale – Revised78 | 1 | – | – | + | |||||||
| Parental Feeding Style Questionnaire79 | 1 | – | |||||||||
| Measurement tool | Summary ratings | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | PROM validity | Content validity | Structural validity | Internal consistency | Inter-rater reliability | Test–retest reliability | Criterion validity | Hypotheses testing: convergence/divergence | Hypotheses testing: known groups | Responsiveness | |
| Feeding Interaction Report Scale and Treatment80 | 1 | Inadequate | ? | – | |||||||
| Multidisciplinary Feeding Profile: subtests | |||||||||||
| Functional Feeding | 1 | + | |||||||||
| Oral-Facial Motor Function38 | 1 | + | |||||||||
| Mastication Observation and Evaluation81,82 | 2 | Very good | + | + | ? | ||||||
| Oral Motor Assessment Scale43 | 2 | + | – | + | |||||||
| Schedule of Oral Motor Assessment45 | 4 | + | ? | + | + | + | |||||
| Measurement tool | Study (first author and year of publication); revisions | Designed for | Number of items; time taken | Subscales | Scaling | Age range | Norms; clinical cut-off points |
|---|---|---|---|---|---|---|---|
| BPFAS (child and parent domains) | Crist 1994;32 Crist 200133 | Feeding problems | 35 (25 children, 10 parents) | Total, child and parent frequency and problem scores; restriction score and poor strategies | 5-point scale; problem score yes/no | 2–12 years | Crist and Napier-Phillips33 Canadian norms; Dovey et al.83 2013 UK norms and cut-off points |
| BAMBI | Lukens 200850 | Autism | 18 | Three subscales: limited variety, food refusal and features of autism | 5-point scale | 3–11 years | Cut-off point for ASD with/without feeding problems |
| DeMand 201551 | 15 | Four subscales: food selectivity, disruptive mealtime behaviour, food refusal and mealtime rigidity | 2–11 years | ||||
| BAMBIC | Hendy 201368 | Feeding problems | 10 | Three subscales: limited variety, food refusal and disruptive behaviour | 5-point scale | 18 months to 17 years | Norms for general population of children in Seiverling et al.84 2016 |
| Children’s Eating Behaviour Inventory (child and parent domains) | Archer 199135 | Feeding problems | 40 (28 children, 12 parents); 15 minutes | Total problems; per cent of items perceived as problem | 5-point scale | 2–12 years | Norms in Archer et al.;35 clinical cut-off point, but discrimination poor |
| Children’s Eating Behaviour Questionnaire | Wardle 200169 | General population | 35 | Eight subscales: food responsiveness, enjoyment of food, desire to drink, satiety responsiveness, slowness in eating, food fussiness, emotional overeating, emotional undereating | 5-point scale | 1–11 years | Norms in Wardle et al.69 |
| Food Frequency Questionnaire | Bandini 201061 | General population | 131 | Food refusal number and per cent of foods offered; foods eaten at high frequency | 11 frequency categories | 3–11 years | Variable |
| Food Preference Inventory | Schreck 2004;56 Paul 200757 | General population | Varies (e.g. 84, 154) | Food subgroups: proteins, starches, dairy, fruits and vegetables | 4-point scale | 3–8 years | Variable |
| Meals in Our Household (child and parent domains) | Anderson 201270 | General population | 50 | Six subscales: structure of family meals, problematic child mealtime behaviour, use of food as reward, parental concerns about child diet, spousal stress related to child’s mealtime behaviour and influence of child food preferences on what other family members eat | 4-, 5- or 6-point scales | 3–11 years | Not known |
| PediEAT | Thoyre 2014, 201871,72 | Feeding problems | 78 | Four subscales: physiological symptoms, problematic mealtime behaviours, selective/restricted eating and oral processing | 6-point scale | 6 months to 7 years | Norms in Pados 201885 |
| Paediatric version of the Eating Assessment Tool (PEDI-EAT 10) | Belafsky 200873 (adaptation of PEDI-EAT 10 for children) | Swallowing problems | 10 | 5-point scale | 18 months to 18 years | Not known | |
| Screening Tool of Feeding Problems | Matson 200159 (Screening Tool of Feeding Problems originally for people with intellectual disability) | Feeding problems | 23 | Five subscales: risk of aspiration, food selectivity, feeding skills deficits, food refusal and associated behaviour problems, and nutrition-related behaviour problems | 3-point scale | Not known | Not known |
| Child version of the Screening Tool of Feeding Problems | Matson 200159 (Screening Tool of Feeding Problems originally for people with intellectual disability); Seiverling 201174 for children | Feeding problems | 15 | Six subscales: chewing problem, rapid eating, food refusal, food selectivity, vomiting and stealing food | 3-point scale | Not known | Not known |
| Measurement tool | Study (first author and year of publication); revisions | Designed for | Number of items | Subscales | Scaling | Age range | Norms; clinical cut-off points |
|---|---|---|---|---|---|---|---|
| Child Feeding Questionnaire | Birch 200175 | General population | 31 | Five subscales: perceived responsibility for child feeding, concern about child weight, restriction, pressure for child to eat more food and monitoring | 5-point scale | 1–7 years | Not known |
| Feeding Strategies Questionnaire | Berlin 201176 | Feeding problems | 40 | Six subscales: mealtime structure, consistent mealtime routine, child control of intake, parent control of intake, between-meal grazing and encourages to clean plate | 5-point scale | 2–6 years | Not known |
| Parent Mealtime Action Scale | Hendy 200977 | General population | 31 | Nine subscales: snack limits, positive persuasion, daily fv availability, use of rewards, insistence on eating, snack modelling, special meals, fat reduction and many food choices | 3-point scale | 2–17 years | Norms in Hendry 201678 |
| Hendy 201678 (Parent Mealtime Action Scale – Revised) | 5-point scale | ||||||
| Parental Feeding Style Questionnaire | Wardle 200279 | General population | 27 | Four subscales: instrumental feeding, prompting and encouragement, emotional feeding and control over child eating | 5-point scale | 3–5 years | Not known |
| Measurement tool | Study (first author and year of publication); revisions | Designed for | Number of items, time taken | Subscales | Scaling | Age range | Norms; clinical cut-off points |
|---|---|---|---|---|---|---|---|
| Feeding Interaction Report Scale and Treatment (child and parent domains) | Sparling 198580 | Feeding problems | 12 | Three behavioural categories: readiness, process interaction, and specific oral motor behaviours (the first two are rated for parent and for child) | 3-point scale | 0–3 years | Not known |
| Multidisciplinary Feeding Profile: subtests Functional Feeding | Kenny 198938 | Oral motor skills | 4–9 behaviour per domain; 30–45 minutes | Eight domains of ingestion: spoon feeding, biting, chewing, cup drinking, straw drinking, swallowing, clearing and drooling | 5-point scale, scores transformed to per cent competence | Not known | Not known |
| Oral-Facial Motor Function | 3- to 5-point scale | ||||||
| Mastication Observation and Evaluation | Remijn 201381 | Chewing skills | 8 | Bread and biscuits | 4-point scale | 6–48 months (general population) | Not known |
| Remijn 201482 | 2–6 years (cp) | ||||||
| Oral Motor Assessment Scale | Ortega 200943 | Oral motor skills | 7 | Soft food, solid food and liquid food | 4-point scale | 3–13 years (cp) | Not known |
| Schedule of Oral Motor Assessment | Reilly 199545 | Oral motor skills | 8–22 per category; average 20 minutes | Seven challenge categories: purée, semisolid, solid, cracker, bottle, trainer cup and cup | Yes/no | 8–24 months (general population) | Not known |
There was patchy evidence of variable quality on measurement properties for most tools, with only one study providing any evidence of responsiveness to change (see Tables 5–7). Much of the evidence found related to hypothesis-testing of convergent/divergent validity or differences between known groups. The available evidence showed that hardly any tools met the COSMIN criteria for a sufficient quality of structural validity.
Conclusions
The strongest evidence for robust measurement properties of a tool measuring child behaviours was for the Paediatric Eating Assessment Tool (PediEAT),71,72 a 78-item parent questionnaire that is used with children aged 6 months to 7 years (see Tables 5–7). Evidence for the measurement properties of the Brief Autism Mealtime Behaviour Inventory (BAMBI),50 an 18-item parent questionnaire (or 15 items51) for children aged 2–11 years, is more mixed. The BAMBI was subsequently reworked for all children with feeding problems as the Brief Assessment of Mealtime Behaviour in Children (BAMBIC),68 a 10-item parent-reported scale relating to children aged 18 months to 17 years, which also had mixed evidence of robustness of its measurement properties. The evidence relating to seven further tools measuring child behaviours was limited and poor; therefore, these tools will not be considered further.
In addition, there were two types of tool used to measure children’s intake in this category (i.e. food frequency and food preferences) (see Tables 5–7). The Food Frequency Questionnaire (FFQ)61 evaluated was based on the youth/adolescent version of a list developed at Harvard University. 86,87 It has 131 items, and parents indicate foods refused and the number of times per day foods are eaten. Evidence was found only in relation to hypothesis testing of convergent validity and discrimination between groups.
In regard to parent strategies tools, the evidence of measurement property robustness is sparse and poor (see Tables 5–7). Among the child behaviours tools, the Behavioural Paediatric Feeding Assessment Scale (BPFAS),33 Child’s Eating Behaviour Inventory35 and Meals in Our Household70 also have parent domains. The BPFAS has a little more evidence of robustness for use with this population. The BPFAS has 25 items on child behaviours and 10 items on parent attitudes and strategies, relevant for those children aged 2–6 years.
The systematic reviews of parent-reported measures of feeding difficulties by Sanchez et al. 88 and Jaafar et al. 89 supported the BPFAS as the most robust measurement tool for children with feeding problems aged 2–5 years. In studies identified by our measurement properties review, it was used with children with a range of conditions, including ASD. 90,91
Regarding oral motor skills, the strongest evidence is for the Schedule of Oral Motor Assessment (SOMA). 45,92 The SOMA involves a structured assessment of children’s ability with a range of food textures and trained observers to rate the video-taped session. The strength of this tool was also concluded by the systematic review by Benfer et al. ,65 although more recent reviews by Barton et al. 93 and Speyer et al. 94 more cautiously reported the difficulty of reaching any conclusion on the basis of the patchy evidence.
Consultation
As the evidence from the review became available, three of the scales (PediEAT, BAMBIC and BPFAS) with the more robust properties were presented to the PAG in September 2018. For child behaviours, parents commented that the PediEAT was long. The BAMBIC was thought to be relatively easy to fill in, but short. The parents in the advisory group considered that some child behaviour items of the BPFAS were difficult to answer for particular situations, for example ‘eats junky snack foods but will not eat at mealtime’ as a child may eat junky snack foods as a meal at mealtime. The parent co-investigators also commented on the FFQ, with reservations expressed about the affordability of some of the range of foods included.
Summary of findings
The review of the papers on measurement properties of tools relevant to EDSD revealed the patchiness of the available evidence. A similar conclusion was reported by other recent reviews examining parts of this topic. 95,96 The review enabled the recommendation of candidate tools to be included in the design of outcome measurement in any future trial of interventions for EDSD in children with neurodisability. The PediEAT is a recently developed parent-report tool that measures child feeding difficulties and mealtime behaviours, and had the most evidence of robust measurement properties. For child intake, a FFQ might be acceptable. The BPFAS combines child and parent subscales, and had marginally stronger measurement properties than other parent strategy scales. The SOMA is the strongest measure of oral motor skills, and requires training of the assessors and observers.
Strengths and limitations of the measurement properties review
The identification of tools to be included in the review was comprehensive as it drew on a range of sources, including the mapping review. The inclusion of further tools identified within papers of measurement properties increased the breadth of the review. The pragmatic decision to focus data extraction on tools that were most likely to be candidates for use in evaluation in any future trial of interventions for EDSD was a limitation, but also an expedient best use of resources. The two reviewers worked closely together, to a detailed set of definitions, with access to another experienced reviewer to resolve discrepancies. Conclusions about the robustness of tools were checked against similar systematic reviews. The COSMIN approach to evaluate measurement properties is not applicable to the way in which some of the most valued outcomes are measured (e.g. Growth and Nutrition), which implies that a further review of approaches to their measurement will be required.
Patient and public involvement in the measurement properties review
Members of the PAG were presented with three parent-reported tools with the strongest evidence of their measurement properties, and commented on wording, layout and ease of use.
How did the measurement properties review inform the next step?
The three parent-reported tools that captured child behaviours, child intake of food and parent strategies were presented to groups at the stakeholder consultation workshops for further discussion (see Chapter 11). The process of the review also informed the thinking of the research team around how to conceptualise categories of outcomes valued by parents and professionals as elicited in the focus groups (see Chapter 9) and in the surveys (see Chapters 7 and 10). The tools reviewed for their measurement properties belong mostly to the intermediate category of child behaviours and parent strategies relating to EDSD, with child oral motor skills being grouped with proximal outcomes, such as Nutrition and Growth (see Chapter 9).
Chapter 7 Aim 2: national survey
Objectives
To establish current UK clinical practice in relation to the interventions recommended and used for EDSD in young children with neurodisability and the outcomes measured, from the perspective of parents and health and education professionals.
Methods
Population
Three UK populations were sampled:
-
Parents of children with neurodisability aged up to 12 years who experience EDSD. Although our focus was on young children aged up to 8 years, broadening the age limit to 12 years aimed to include parents with adequate recall in relation to the interventions that they had used historically.
-
Health professionals (e.g. speech and language therapists, occupational therapists, dietitians and paediatricians) working with children and young people (aged 0–18 years) with neurodisability who experience EDSD. Although the focus of the study was on young children (up to the age of 8 years), HPs often work with a wider range of ages which makes it difficult to separate those working exclusively with the younger age range.
-
Education professionals (e.g. teaching assistants, class teachers, head teachers and special education needs co-ordinators) working with children and young people (aged 0–18 years) with neurodisability who experience EDSD. As with HPs, education professionals often work with children from this wider age range, which makes it difficult to separate out those working exclusively with the younger age range.
Questionnaire development: UK clinical practice in managing eating, drinking and swallowing difficulties in young children with neurodisability
A questionnaire was developed to ascertain the interventions recommended and used for EDSD in young children with neurodisability and the outcomes measured, from the perspective of parents, HPs and education professionals. Lists of interventions and outcomes to be included within the questionnaire were generated from the updates of the three published systematic reviews of interventions (see Chapter 3), the mapping review (see Chapter 4) and the first focus groups (see Chapter 5). An additional four interventions were added by the research team based on their knowledge and expertise: Sensory stimulation, Medication, Sensory aids and Modifying social eating and drinking opportunities. An additional four outcomes were added by the research team based on their knowledge and expertise: Fewer breathing changes, More involvement in family activities, Fewer abnormal/unusual movements and More opportunity to talk to others about feelings regarding the child’s EDSD. The research team developed the questionnaires, drawing on clinical expertise, experience of survey design and best practice in the design and conduct of survey questionnaires. 97 The final online surveys and paper versions were piloted by a small number of parents, HPs, education professionals and researchers.
The final questionnaire had three sections: (1) demographic characteristics; (2) items about interventions, including usage, effectiveness, acceptability (i.e. was it acceptable to deliver at home or school), timescales for change and training; and (3) important outcomes. A total of 25 interventions and 32 outcomes were listed. The interventions were compiled from those identified through the updates of the three published systematic reviews of interventions (n = 13; see Chapter 3), the mapping review (n = 19; see Chapter 4) and the first round of focus groups (n = 21; see Chapter 5). The outcomes were compiled from those identified through the updates of the three published systematic reviews of interventions (n = 10; see Chapter 3), the mapping review (n = 24; see Chapter 4) and the first round of focus groups (n = 25; see Chapter 5). A number of the interventions and outcomes appeared in more than one research activity so duplicates were taken out. Respondents had the option to add additional interventions or outcomes. HPs were also asked about the tools that they used to measure outcomes. Most of the questions offered fixed-choice responses, with some opportunities for free-text responses. A small proportion of the questions were compulsory, such as those relating demographic information and whether or not participants had used a particular intervention. Questions asking for further detail about each intervention (e.g. whether or not it was acceptable to deliver) were answered only if respondents had experience of using that intervention. Respondents could use a ‘back’ button to review or change their answers as required, and the survey could be saved and completed at a later time or date. A ‘completion bar’ showed the respondents progress through the survey (see Report Supplementary Material 1 for the national survey questionnaire).
Procedure
Convenience samples of parents, HPs and education professionals were recruited. Recruitment was UK-wide, and took place between March and September 2018.
Parents were recruited via national and regional parent networks, parent support organisations and charities. These comprised:
-
parent carer forums
-
special needs networks
-
the Council for Disabled Children (London, UK)
-
Cerebra (Carmarthen, UK)
-
Contact (London, UK)
-
the National Autistic Society (London, UK)
-
the Down’s Syndrome Association (London, UK)
-
Down’s Syndrome North East (Newton Aycliffe, UK)
-
Cerebral Palsy UK
-
Action Cerebral Palsy (Bicester, UK)
-
SCOPE (London, UK)
-
Skills for People (Newcastle upon Tyne, UK)
-
the National Network of Parent Carer Forums (London, UK)
-
the Toby Henderson Trust (Bedlington, UK).
Parents were also recruited through two research databases: Autism Spectrum Database – UK (ASD-UK) and the Database of Children with Autism Spectrum Disorder Living in the North East (Daslne). Further sources of parent recruitment were 24 NHS trusts across England that joined as participant identification centres (PICs), mainstream and specialist schools across the UK, participants in the first round of focus groups and social media linked to relevant organisations, charities and parent forums.
Health professionals were approached through relevant professional bodies. These comprised:
-
the British Association of Community Child Health (London, UK)
-
the Royal College of Speech and Language Therapists (London, UK)
-
the British Dietetic Association (London, UK)
-
the British Academy of Childhood Disability (through the UK Child Development Team database) (London, UK)
-
the British Society of Paediatric Gastroenterology, Hepatology and Nutrition (London, UK)
-
the College of Occupational Therapy (London, UK)
-
the Chartered Society of Physiotherapists (London, UK).
Health professionals were also recruited through neurodisability and community paediatric networks, such as regional dysphagia clinical excellence networks, special interest dietetic groups, and local and national nursing and health visitor networks. In addition, some HPs were recruited through clinical services in the north-east and south-east of England (areas in which members of the research team were based) and the 24 PICs. Finally, HPs were also recruited through social media linked to relevant organisations and professional forums, and from participants who took part in the first round of focus groups (see Chapter 5).
Education professionals were recruited through independent and local authority schools across the UK, which were identified from websites such as Special Needs UK (URL: www.specialneedsuk.org, accessed 17 December 2020) and local authority websites. Education professionals were also recruited through local special education needs co-ordinator and Sure Start networks in the north-east of England. Further sources of education professional recruitment were via PICs and through relevant professional bodies, networks and voluntary organisations via social media, including:
-
the Department for Education
-
SEN magazine (Clitheroe, UK)
-
National Association of Head Teachers (Haywards Heath, UK)
-
Autism Education Trust (London, UK),
-
National Day Nurseries Association (Huddersfield, UK),
-
Early Education: The British Association for Early Childhood Education (St Albans, UK)
-
Teach Early Years magazine (London, UK)
-
Nursery World News (London, UK)
-
Percy Hedley Foundation (Newcastle upon Tyne, UK)
-
Childcare News (Milton Keynes, UK).
Separate, yet similar, versions of the questionnaire were developed for each of the three study groups (parents, HPs and education professionals). Electronic, web-accessible versions were hosted by Newcastle University, Newcastle, using Qualtrics (Provo, UT, USA), with paper versions available on request. E-mail and web-based flyers were sent to potential participants with a link to the appropriate version of the questionnaire alongside information on how to request a paper copy if this was preferred. Paper versions of the questionnaire were also distributed to PICs on request. At the end of the questionnaire, respondents were asked to provide their contact details if they would like to enter a prize draw to win one of five £100 vouchers for each of the three study groups, to be contacted with information about the Delphi survey (see Chapter 10) later in the study or to receive a summary newsletter about the results of the study. Reminders were sent out to parents, HPs and education professionals through the same sources to encourage non-respondents to participate.
Results
Participants
Figure 8 shows participant recruitment and flow through the study.
FIGURE 8.
Participant recruitment and flow through study.
Parents
Table 11 shows the characteristics of the 359 respondents who completed the parent version of the survey. Most of the respondents were mothers (95%) and most were from England (92%). The majority of respondents were aged between 31 and 50 years (85%). Most of the respondents were white British (89%). The children reported on ranged in age from 2 months to 12 years 11 months [mean 7 years 5 months, standard deviation (SD) 3 years 3 months]. Half of the respondents reported that their child had non-physical EDSD (51%) and half reported their child’s main diagnosis as ASD (51%). The majority of children attended specialist (40%) or mainstream (39%) schools. Around one-third of parents reported that they had heard about the study through the research databases of children with ASD (32%). 98,99 The remaining respondents heard about the survey through voluntary organisations or charities (22%), PICs (19%), schools (13%), social media (10%) and other sources (36%).
| Parent characterisics (N = 359) | Number (%) of respondents |
|---|---|
| Mother | 332 (93) |
| Father | 19 (5) |
| Carer of looked-after child | 6 (2) |
| Other | 2 (1) |
| Age (years) | |
| ≤ 20 | 6 (2) |
| 21–30 | 28 (8) |
| 31–40 | 165 (46) |
| 41–50 | 140 (39) |
| 51–60 | 18 (5) |
| ≥ 61 | 2 (1) |
| Gender | |
| Female | 339 (94) |
| Male | 20 (6) |
| Location | |
| England | |
| North-east | 64 (18) |
| North-west | 29 (8) |
| Yorkshire and Humber | 38 (11) |
| Midlands | 79 (22) |
| South-east, including London | 81 (23) |
| South-west | 39 (11) |
| Scotland | 15 (4) |
| Northern Ireland | 6 (2) |
| Wales | 8 (2) |
| Ethnicity | |
| White | 318 (89) |
| Black/African/Caribbean/black British | 5 (1) |
| Asian/Asian British | 24 (7) |
| Mixed/multiple ethnic group | 9 (3) |
| Other ethnic group | 0 (0) |
| Prefer not to say | 3 (1) |
| How they heard about the survey | |
| NHS trust | 67 (19) |
| School | 45 (13) |
| Voluntary organisation/charity | 80 (22) |
| ASD-UK/Daslnea | 114 (32) |
| Social media | 36 (10) |
| Other | 17 (5) |
| Type of EDSD of child | |
| Physical | 74 (21) |
| Non-physical | 183 (51) |
| Mixed | 91 (25) |
| Missing | 11 (3) |
| Primary diagnosis of child | |
| ASD | 183 (51) |
| Down syndrome | 69 (19) |
| Cerebral palsy | 30 (8) |
| Developmental delay | 26 (7) |
| Genetic condition | 23 (6) |
| Learning/intellectual disabilities | 4 (1) |
| Structural brain disorder | 4 (1) |
| Other | 19 (5) |
| Missing | 1 (< 1) |
| Age of child at time of survey completion | |
| Mean (SD) | 7 years 5 months (3 years 3 months) |
| Age of child at start of EDSD | |
| Mean (SD) | 1 year 4 months (1 year 9 months) |
| Missing | 6 (2) |
| Continuing difficulties experienced by child | |
| Yes | 316 (88) |
| No | 43 (12) |
| Educational setting of childb | |
| Specialist school | 143 (40) |
| Mainstream school | 141 (39) |
| Preschool | 53 (15) |
| Specialist unit in mainstream school | 8 (2) |
| Home schooled | 7 (2) |
| None | 24 (7) |
| Professionals involved with child and familyb | |
| Speech and language therapist | 215 (60) |
| Paediatrician | 205 (57) |
| Dietitian | 176 (49) |
| Occupational therapist | 121 (34) |
| Health visitor | 99 (28) |
| Nurse | 58 (16) |
| Gastroenterologist | 55 (15) |
| Physiotherapist | 50 (14) |
| Clinical psychologist | 28 (8) |
| Other | 39 (11) |
| None reported | 39 (11) |
Table 12 shows the characteristics of respondents who completed the HP and education professional versions of the survey.
| Characteristics, n (%) | |||
|---|---|---|---|
| HP (N = 421) | Education professionals (N = 62) | ||
| Role | |||
| Speech and language therapist | 131 (31) | Teaching assistant/learning support assistant | 25 (40) |
| Occupational therapist | 63 (15) | Class teacher | 18 (29) |
| Physiotherapist | 57 (14) | Higher-level teaching assistant | 6 (10) |
| Paediatrician | 50 (12) | Head teacher/deputy head teacher | 4 (7) |
| Dietitian | 40 (10) | Special educational needs co-ordinator | 2 (3) |
| Nurse | 32 (8) | Lunchtime assistant/midday meals supervisor | 1 (2) |
| Health visitor | 14 (3) | Nursery worker | 1 (2) |
| Clinical psychologist | 9 (2) | Childminder | 1 (2) |
| Gastroenterologist | 1 (< 1) | Other | 4 (7) |
| Other | 24 (6) | ||
| Location | |||
| England | |||
| North-east | 36 (9) | 14 (23) | |
| North-west | 25 (6) | 2 (3) | |
| Yorkshire and Humber | 63 (15) | 2 (3) | |
| Midlands | 57 (14) | 17 (24) | |
| South-east, including London | 172 (41) | 15 (24) | |
| South-west | 21 (5) | 11 (18) | |
| Scotland | 19 (5) | 0 (0) | |
| Northern Ireland | 11 (3) | 0 (0) | |
| Wales | 17 (4) | 1 (2) | |
| Years worked | |||
| Range | 0–38 years | 0–33 years | |
| Mean (SD) | 12 years 2 months (8 years 8 months) | 10 years 2 months (8 years 6 months) | |
| Missing | 2 (< 1) | 0 (0) | |
| Type of EDSD worked witha | |||
| Physical | 74 (18) | 11 (18) | |
| Non-physical | 32 (8) | 10 (16) | |
| Mixed | 314 (75) | 40 (65) | |
| Missing | 1 (< 1) | 1 (2) | |
| Age of children worked witha | |||
| 0–6 months | 281 (67) | 1 (2) | |
| 7–11 months | 298 (71) | 4 (7) | |
| 1–3 years | 360 (86) | 14 (23) | |
| 4–8 years | 377 (90) | 40 (65) | |
| ≥ 9 years | 353 (84) | 44 (71) | |
| Employed by | How heard about the study | ||
| NHS trust | 366 (87) | School | 34 (55) |
| Education | 21 (5) | Professional network | 12 (20) |
| Voluntary sector | 13 (3) | NHS trust | 10 (16) |
| Independent practitioner | 15 (4) | Voluntary organisation/charity | 4 (7) |
| Other | 5 (1) | Other | 2 (3) |
| Missing | 1 (< 1) | Missing | 0 (0) |
| Settings in which they worka | |||
| Community services | 301 (72) | Specialist school | 46 (74) |
| Hospital (secondary and tertiary) | 151 (36) | Mainstream school | 6 (10) |
| Education | 171 (41) | Preschool | 5 (8) |
| Other | 13 (3) | Child’s home | 3 (5) |
| Mainstream school with specialist unit | 0 (0) | ||
| Other | 3 (5) | ||
Health professionals
The majority of HPs were from England (89%) and the largest group of HPs was speech and language therapists (31%). The majority of HPs worked with children with mixed EDSD (i.e. physical and non-physical causes to their EDSD) (75%). Respondents reported working across a range of age groups. Respondents’ years of experience working with children with neurodisability ranged from 1 year to 38 years (mean 12 years 2 months, SD 8 years 8 months). The majority of respondents were employed by the NHS (87%) and most worked in community-based services (72%). The majority of HPs worked with parents (88%) and education professionals (69%) to deliver interventions. We did not ask HPs where they heard about the survey.
Education professionals
Education professionals were predominantly from England (98%) and the largest professional group was teaching assistants/learning support assistants (31%). The majority of education professionals worked with children with mixed EDSD (65%). Respondents reported working across a range of age groups, with the majority working with children aged ≥ 4 years. Respondents’ years of experience working with children with neurodisability ranged from 1 year to 33 years (mean 10 years 2 months, SD 8 years 6 months). The majority of respondents worked within specialist schools (74%) and heard about the study through schools (55%).
Use of interventions
Table 13 shows that parents reported using a wide range of interventions. The most commonly reported interventions used by parents were Food or drink modification (57%), Desensitisation programme for food avoidance (47%), Modification of utensils (41%), Enhancing parent–child communication strategies at mealtimes (41%) and Positioning (40%). Food or drink modification was the most frequently reported intervention used with children with physical and mixed EDSD (69%) and with children with non-physical EDSD (48%). Parents of children with physical and mixed EDSD also reported frequently using Positioning (62%), whereas parents of children with non-physical EDSD frequently reported the use of strategies to Enhance parent–child communication at mealtimes (46%). Few parents reported using Manoeuvres (11%), Modelling (10%), having Counselling (5%) or using Sensorimotor therapy (3%).
| Intervention | Parents | HPs | Education professionals | ||||||
|---|---|---|---|---|---|---|---|---|---|
| All (N = 359), n (%)a | Physical and mixed EDSD (N = 165), n (%)b | Non-physical EDSD (N = 183), n (%)c | All (N = 421), n (%)d | Physical and mixed EDSD (N = 388), n (%)e | Non-physical EDSD (N = 32), n (%)f | All (N = 62), n (%)g | Physical and mixed EDSD (N = 51), n (%)h | Non-physical EDSD (N = 10), n (%)i | |
| Modification of environment | 130 (36) | 46 (28) | 84 (46) | 218 (52) | 195 (50) | 22 (69) | 39 (63) | 33 (65) | 5 (50) |
| Positioning | 145 (40) | 102 (62) | 41 (22) | 325 (77) | 307 (79) | 17 (53) | 41 (66) | 39 (77) | 1 (10) |
| Modification of utensils | 146 (41) | 79 (48) | 65 (36) | 193 (46) | 178 (46) | 15 (47) | 42 (68) | 36 (71) | 5 (50) |
| Schedule of meals | 121 (34) | 48 (29) | 71 (39) | 157 (37) | 143 (37) | 14 (44) | 33 (53) | 28 (55) | 5 (50) |
| Food or drink modification | 203 (57) | 113 (69) | 88 (48) | 237 (56) | 221 (57) | 15 (47) | 42 (68) | 36 (71) | 5 (50) |
| Enhancing parent/child communication strategies at mealtimes | 146 (41) | 61 (37) | 85 (46) | 161 (38) | 143 (37) | 17 (53) | 44 (71) | 37 (73) | 6 (60) |
| Visual supports | 122 (34) | 41 (25) | 81 (44) | 133 (32) | 115 (30) | 18 (56) | 39 (63) | 33 (65) | 5 (50) |
| Training to wait for child’s cues for feeding | 53 (15) | 34 (21) | 18 (10) | 136 (32) | 130 (34) | 6 (19) | 23 (37) | 21 (41) | 1 (10) |
| Pacing of food at mealtimes | 78 (22) | 45 (27) | 31 (17) | 179 (43) | 168 (43) | 10 (31) | 30 (48) | 30 (59) | 0 (0) |
| Manoeuvres | 38 (11) | 35 (21) | 3 (2) | 152 (36) | 149 (38) | 2 (6) | 15 (24) | 14 (28) | 1 (10) |
| Desensitisation programme for oral sensations | 59 (16) | 38 (23) | 20 (11) | 109 (26) | 100 (26) | 9 (28) | 22 (36) | 17 (33) | 4 (40) |
| Sensory stimulation | 60 (17) | 37 (22) | 21 (12) | 60 (14) | 56 (14) | 4 (13) | 22 (36) | 18 (35) | 4 (40) |
| Oral motor exercises | 74 (21) | 54 (33) | 19 (10) | 102 (24) | 93 (24) | 9 (28) | 27 (44) | 24 (47) | 2 (20) |
| Sensorimotor therapy | 11 (3) | 9 (6) | 2 (1) | 12 (3) | 10 (3) | 2 (6) | 8 (13) | 8 (16) | 0 (0) |
| Desensitisation programme for food avoidance | 167 (47) | 83 (50) | 83 (45) | 204 (49) | 185 (48) | 18 (56) | 36 (58) | 30 (59) | 5 (50) |
| Strategies/programmes aimed at changing behaviour at mealtimes | 109 (30) | 29 (18) | 80 (44) | 146 (35) | 129 (33) | 17 (53) | 35 (57) | 29 (57) | 5 (50) |
| Modelling | 37 (10) | 22 (13) | 15 (8) | 52 (12) | 43 (11) | 9 (28) | 18 (29) | 15 (29) | 2 (20) |
| Hand-over-hand prompting | 123 (34) | 81 (49) | 41 (22) | 129 (31) | 118 (30) | 11 (34) | 44 (71) | 38 (75) | 5 (50) |
| Counselling | 17 (5) | 4 (2) | 12 (7) | 120 (29) | 109 (28) | 11 (34) | 7 (11) | 6 (12) | 1 (10) |
| Medicationj | 121 (34) | 89 (54) | 30 (16) | 167 (40) | 162 (42) | 5 (16) | 35 (57) | 33 (65) | 1 (10) |
| Energy supplementsj | 68 (19) | 40 (24) | 28 (15) | 88 (21) | 87 (22) | 1 (3) | 18 (29) | 18 (35) | 0 (0) |
| Sensory aidsj | 62 (17) | 34 (21) | 28 (15) | 58 (14) | 55 (14) | 3 (9) | 29 (47) | 26 (51) | 2 (20) |
| Information on impact of sensory difficulties on eating and drinkingj | 140 (39) | 56 (34) | 83 (45) | 213 (51) | 195 (50) | 17 (53) | 38 (61) | 34 (67) | 3 (30) |
| Information on impact of movement difficulties on eating and drinkingj | 50 (14) | 37 (22) | 12 (7) | 207 (49) | 198 (51) | 8 (25) | 28 (45) | 27 (53) | 0 (0) |
| Modifying social eating and drinking opportunitiesj | 83 (23) | 37 (22) | 45 (25) | 103 (25) | 89 (23) | 14 (44) | 36 (58) | 30 (59) | 5 (50) |
Health professionals also reported using a wide range of interventions, with the majority using multiple interventions (median 11). The most frequently used interventions by HPs were Positioning (77%); Food or drink modification, such as texture or consistency (56%); Modification of environment (52%); Information on the impact of sensory (51%) or movement (49%) difficulties on eating and drinking, and Desensitisation programme for food avoidance (49%). Some interventions were used by a minority of HPs, with over half reporting that they did not use them: Sensory stimulation (60%), Modelling (60%), Sensory aids (64%) and Sensorimotor therapy (71%).
The majority of education professionals had been involved in delivering strategies aimed at improving EDSD (82%). The most commonly delivered interventions were Enhancing parent–child communication strategies at mealtimes (71%), Hand-over-hand prompting (71%), Food or drink modification (68%) and Modification of utensils (68%). Education professionals working with children with physical and mixed EDSD reported Positioning as their most frequently used intervention (77%), alongside those listed above. Education professionals working with children with non-physical EDSD also frequently reported using Modification of environment (50%), Desensitisation programme for food avoidance (50%), Modification of utensils (50%), Visual supports (50%), Schedule of meals (50%), Strategies/programmes aimed at changing behaviour at mealtimes (50%) and Modifying social eating and drinking opportunities (50%). However, as only a small number of education professionals (n = 10) completing this survey worked solely with children with non-physical EDSD (16%), these findings should be interpreted with caution. Some interventions were rarely delivered by education professionals, with over half of the respondents reporting that they had not been involved with Sensorimotor therapy (60%) or Counselling (65%). There was considerable overlap in professionals’ use of interventions. Both HPs and education professionals frequently used a range of interventions, including Positioning, Modifications (to Food and drink, Utensils and the Environment), Information about the impact of the child’s sensory difficulties on EDSD and Food desensitisation.
Delivery of interventions
Health professionals delivered interventions across a range of settings: families’ homes; school and preschool settings; NHS settings including hospitals and community provision; respite services including short break services, residential care and hospices; and other settings, such as independent services. The majority of HPs reported offering ongoing support with individual interventions, although a proportion offered these only as part of a time-limited programme and a small minority provided advice around each intervention on a single occasion only. All interventions, with the exception of Sensorimotor therapy, were recommended for both children with physical and mixed EDSD and children with non-physical EDSD, indicating a common approach to working with children with neurodisability and EDSD regardless of the cause of their difficulty. All interventions were used across the age range that was included in the survey (0–18 years). The majority of HPs worked with parents (87.9%) and education professionals (68.6%) to deliver interventions. HPs reported offering training on delivering interventions to parents (mean across interventions 91.1%, range 73.7–97.0%) and education professionals (mean across interventions 71.6%, range 25.9–84.4%). Similar levels of training were reported as having been received from HPs by parents (mean 91.1%, range 74.1–97.0%) and education professionals (mean 68.6%, range 38.9–93.3%).
Acceptability of interventions
Table 14 shows that all of the interventions in use were acceptable to parents to deliver at home (mean 94%, range 80–100%) and to education professionals to deliver in school (mean 95.1%, range 75–100%).
| Intervention | Acceptable to deliver, n/N (%)a | |
|---|---|---|
| Parents | Education professionals | |
| Modification of environment | 123/129 (95) | 32/34 (94) |
| Positioning | 135/141 (96) | 34/35 (97) |
| Modification of utensils | 137/144 (95) | 35/35 (100) |
| Schedule of meals | 111/121 (92) | 28/29 (97) |
| Food or drink modification | 192/200 (96) | 37/38 (97) |
| Enhancing parent–child communication strategies at mealtimes | 135/144 (94) | 38/40 (95) |
| Visual supports | 116/122 (95) | 34/35 (97) |
| Training to wait for child’s cues for feeding | 47/51 (92) | 20/20 (100) |
| Pacing of food at mealtimes | 75/78 (96) | 26/27 (96) |
| Manoeuvres | 34/37 (92) | 11/12 (92) |
| Desensitisation programme for oral sensations | 57/59 (97) | 18/19 (95) |
| Sensory stimulation | 57/60 (95) | 17/18 (94) |
| Oral motor exercises | 71/73 (97) | 19/23 (83) |
| Sensorimotor therapy | 8/10 (80) | 6/6 (100) |
| Desensitisation programme for food avoidance | 156/167 (93) | 29/31 (94) |
| Strategies/programmes aimed at changing behaviour at mealtimes | 102/107 (95) | 31/32 (97) |
| Modelling | 33/37 (89) | 16/16 (100) |
| Hand-over-hand prompting | 119/123 (97) | 37/40 (93) |
| Counselling | 15/15 (100) | 3/4 (75) |
| Medication | 115/121 (95) | 30/30 (100) |
| Energy supplements | 65/68 (96) | 15/15 (100) |
| Sensory aids | 60/62 (97) | 25/25 (100) |
| Information on impact of sensory difficulties on eating and drinking | 135/140 (96) | 33/34 (97) |
| Information of impact of movement difficulties on eating and drinking | 44/50 (88) | 22/23 (96) |
| Modifying social eating and drinking opportunities | 77/83 (93) | 28/31 (90) |
Effectiveness of interventions
Table 15 shows the numbers and percentages of parents, HPs and education professionals who reported interventions as effective (based on the respondents who had used them). The interventions most frequently rated as effective by parents of children with physical and mixed EDSD were Energy supplements (64%), Hand-over-hand prompting (64%), Modification of utensils (63%), Modification of environment (63%), Food or drink modification (63%), Medication (62%) and Pace of feeding at mealtimes (60%). For parents of children with non-physical EDSD, Sensorimotor therapy (100%), Sensory aids (64%), Energy supplements (61%) and Hand-over-hand prompting (59%) were the most frequently rated effective by those who had used them. The interventions that were least frequently rated as effective by parents who had used them were Sensory stimulation (35%), Schedule of meals (35%), Oral motor exercises (34%) and Strategies/programmes aimed at changing behaviour at mealtimes (30%). The majority of HPs reported that the interventions they used were effective (mean 99%, range 91–100%). Parents’ views showed greater variability and differed in accordance with the nature of the child’s difficulties (i.e. whether the child’s EDSD were because of physical and mixed difficulties or non-physical difficulties). Education professionals’ views also showed considerable variation. Overall, the interventions most frequently rated as effective by the education professionals who had used them were Modification of environment (90%), Strategies/programmes aimed at changing behaviour at mealtimes (89%), Food or drink modification (86%), Visual supports (85%), Pacing of food at mealtimes (83%), Positioning (83%), Training to wait for child’s cues for feeding (83%), Enhancing parent/child communication strategies at mealtimes (82%) and Hand-over-hand prompting (82%).
| Interventions | Parents, n/N (%) | HPs, n/N (%) | Education professionals, n/N (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| All | Physical and mixed EDSD | Non-physical EDSD | All | Physical and mixed EDSD | Non-physical EDSD | All | Physical and mixed EDSD | Non-physical EDSD | |
| Modification of environment | 73/129 (57) | 29/46 (63) | 44/83 (53) | 216/218 (99) | 193/195 (99) | 22/22 (100) | 35/39 (90) | 29/33 (88) | 5/5 (100) |
| Positioning | 76/144 (54) | 58/100 (58) | 16/39 (41) | 298/299 (99) | 281/282 (99) | 16/16 (100) | 34/41 (83) | 32/39 (82) | 1/1 (100) |
| Modification of utensils | 82/145 (57) | 50/79 (63) | 32/64 (50) | 192/193 (99) | 177/178 (99) | 15/15 (100) | 32/42 (76) | 26/36 (72) | 5/5 (100) |
| Schedule of meals | 42/121 (35) | 21/48 (44) | 19/71 (27) | 153/155 (99) | 141/142 (99) | 12/13 (92) | 25/33 (76) | 21/28 (75) | 4/5 (80) |
| Food or drink modification | 108/200 (54) | 71/113 (63) | 36/85 (42) | 234/234 (100) | 219/219 (100) | 14/14 (100) | 36/42 (86) | 31/36 (86) | 4/5 (80) |
| Enhancing parent–child communication strategies at mealtimes | 74/144 (51) | 33/61 (54) | 41/83 (49) | 159/161 (99) | 141/143 (99) | 17/17 (100) | 36/44 (82) | 30/37 (81) | 5/6 (83) |
| Visual supports | 56/122 (46) | 20/41 (49) | 36/81 (44) | 132/133 (99) | 114/115 (99) | 18/18 (100) | 33/39 (85) | 27/33 (82) | 5/5 (100) |
| Training to wait for child’s cues for feeding | 28/51 (55) | 18/32 (56) | 10/18 (56) | 135/135 (100) | 130/130 (100) | 5/5 (100) | 19/23 (83) | 17/21 (81) | 1/1 (100) |
| Pacing of food at mealtimes | 45/78 (58) | 27/45 (60) | 16/31 (52) | 178/179 (99) | 167/168 (99) | 10/10 (100) | 25/30 (83) | 25/30 (83) | 0/0 (0) |
| Manoeuvres | 19/37 (51) | 18/34 (53) | 1/3 (33) | 149/149 (100) | 146/146 (100) | 2/2 (100) | 11/15 (73) | 10/14 (71) | 1/1 (100) |
| Desensitisation programme for oral sensations | 22/59 (37) | 14/38 (37) | 8/20 (40) | 107/109 (98) | 98/100 (98) | 9/9 (100) | 12/22 (55) | 10/17 (59) | 1/4 (25) |
| Sensory stimulation | 21/60 (35) | 14/37 (38) | 6/21 (29) | 57/60 (95) | 53/56 (95) | 4/4 (100) | 12/22 (55) | 10/18 (56) | 2/4 (50) |
| Oral motor exercises | 25/73 (34) | 19/54 (35) | 6/18 (33) | 90/96 (94) | 83/89 (93) | 7/7 (100) | 15/27 (56) | 12/24 (50) | 2/2 (100) |
| Sensorimotor therapy | 5/10 (50) | 3/8 (38) | 2/2 (100) | 10/11 (91) | 8/9 (89) | 2/2 (100) | 5/8 (63) | 5/8 (64) | 0/0 (0) |
| Desensitisation programme for food avoidance | 66/166 (40) | 36/82 (44) | 30/83 (36) | 202/204 (99) | 183/185 (99) | 18/18 (100) | 27/36 (75) | 23/30 (77) | 3/5 (60) |
| Strategies/programmes aimed at changing behaviour at mealtimes | 32/107 (30) | 10/29 (35) | 22/78 (28) | 142/143 (99) | 125/126 (99) | 17 (100) | 31/35 (89) | 27/29 (93) | 3/5 (60) |
| Modelling | 15/37 (41) | 11/22 (50) | 4/15 (27) | 52/52 (100) | 43/43 (100) | 9/9 (100) | 13/18 (72) | 11/15 (73) | 1/2 (50) |
| Hand-over-hand prompting | 77/123 (63) | 52/81 (64) | 24/41 (59) | 127/129 (98) | 116/118 (98) | 11/11 (100) | 36/44 (82) | 32/38 (84) | 3/5 (60) |
| Counselling | 9/16 (56) | 2/4 (50) | 6/11 (55) | 119/120 (99) | 108/109 (99) | 11/11 (100) | 4/6 (67) | 4/6 (67) | 0/0 (0) |
| Medicationa | 69/121 (57) | 55/89 (62) | 12/30 (40) | 161/163 (99) | 157/159 (99) | 4/4 (100) | 23/34 (68) | 21/32 (66) | 1/1 (100) |
| Energy supplementsa | 44/68 (65) | 27/40 (68) | 17/28 (61) | 85/87 (98) | 84/86 (98) | 1/1 (100) | 13/18 (72) | 13/18 (72) | 0/0 (0) |
| Sensory aidsa | 35/62 (57) | 17/34 (50) | 18/28 (64) | 58/58 (100) | 55/55 (100) | 3/3 (100) | 22/29 (76) | 19/26 (73) | 2/2 (100) |
| Information about impact of sensory difficulties on eating and drinkinga | 68/140 (49) | 26/56 (46) | 42/83 (51) | 210/213 (99) | 192/195 (99) | 17/17 (100) | 28/38 (74) | 24/34 (71) | 3/3 (100) |
| Information of impact of movement difficulties on eating and drinkinga | 21/50 (42) | 15/37 (41) | 6/12 (50) | 203/206 (99) | 194/197 (99) | 8/8 (100) | 18/27 (67) | 18/27 (67) | 0/0 (0) |
| Modifying social eating and drinking opportunitiesa | 41/83 (49) | 19/37 (51) | 21/45 (47) | 102/102 (100) | 88/88 (100) | 14/14 (100) | 27/36 (75) | 22/30 (73) | 4/5 (80) |
Time taken to produce change
Parents were asked about the duration over which they used each intervention. Parents reported using the majority of interventions for over 1 year. These interventions included using Information given about the child’s sensory (88%) and movement difficulties (78%), Modification of the environment (88%), Modifying social eating and drinking opportunities (86%), Sensory aids (84%) and Strategies to enhance communication between the child and the feeder at mealtimes (80%). In contrast, < 60% of parents reported using Scheduling of meals (58%), Oral motor exercises (56%), Sensory stimulation (48%) and Manoeuvres (44%) for over 1 year. Parents were not asked about the time taken for interventions to produce change, as it seemed likely that they would abandon interventions that they felt were not/no longer working.
Health professionals reported their perception that a large number of the interventions produced change quickly (0–3 months); these interventions included Manoeuvres (71%), Pacing of food at mealtimes (70%), Medication (66%), Modification of utensils (65%), Positioning (63%), Training to wait for a child’s cues for feeding (61%), Sensory aids (60%), Modification of environment (60%) and Food and drink modification (58%). For some of the interventions listed, such as Desensitisation programme for oral sensations, Oral motor exercises and Desensitisation programme for food avoidance, there was a relatively equal spread of responses from HPs across the different categories (0–3 months, 4–6 months, 7–9 months, 10–12 months and > 1 year), indicating that there was less consensus on how long these interventions took to produce change.
Education professionals agreed with HPs on most of the interventions that were thought to produce change quickly, including Modification of environment (76%), Modification of utensils (74%), Food and drink modification (67%), Medication (64%), Pacing of food at mealtimes (62%) and Training to wait for a child’s cues for feeding (59%). Education professionals also reported that Modifying social eating and drinking opportunities (62%), Energy supplements (60%) and Visual supports (60%) were quick to produce change (0–3 months). They also showed a spread of responses across the different categories (0–3 months, 4–6 months, 7–9 months, 10–12 months and > 1 year) for some of the interventions listed. These included Desensitisation programmes for oral sensations and food avoidance, Oral motor exercises, Sensory stimulation and Information about the impact of sensory or movement difficulties on a child’s EDSD, indicating there was less consensus among education professionals on how long these interventions took to produce change.
Potential benefits of interventions for eating, drinking and swallowing difficulties
Table 16 shows the numbers and percentages of parents, HPs and education professionals who viewed each potential benefit (referred to for remainder of report as ‘outcome’) as ‘important’. HPs, parents and education professionals all reported that the most important outcomes of interventions were Improved nutrition (parents, 40%; HPs, 31%; education professionals, 39%) and Better general health (parents, 31%; HPs, 32%; educational professionals, 48%). Parents also rated Weight gain (21%) and Increased growth (18%) as important, whereas HPs rated Fewer or shorter hospital admissions as important (17%). Outcomes related to the child’s and family’s overall well-being were also highly valued by professionals, including Better quality of life for the child (HPs, 26%; educational professionals, 36%), Less parental/carer stress (HPs, 17%), Child enjoying mealtimes more (education professionals, 23%) and Child being better able to communicate (education professionals, 19%).
| Outcomes | Parents, n/N (%) | HPs, n/N (%) | Education professionals, n/N (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| All (N = 359) | Physical and mixed EDSD (N = 165) | Non-physical EDSD (N = 183) | All (N = 421) | Physical and mixed EDSD (N = 388) | Non-physical EDSD (N = 32) | All (N = 62) | Physical and mixed EDSD (N = 51) | Non-physical EDSD (N = 10) | |
| Improved nutrition | 143 (40) | 62 (38) | 81 (44) | 130 (31) | 124 (32) | 6 (19) | 24 (39) | 18 (35) | 5 (50) |
| Better general health | 111 (31) | 62 (38) | 48 (26) | 133 (32) | 128 (33) | 4 (13) | 30 (48) | 27 (54) | 3 (30) |
| Weight gain | 76 (21) | 47 (29) | 28 (15) | 53 (13) | 52 (13) | 0 (0) | 10 (16) | 9 (18) | 0 (0) |
| Increased growth | 65 (18) | 45 (27) | 20 (11) | 46 (12) | 49 (13) | 0 (0) | 6 (10) | 6 (12) | 0 (0) |
| Child enjoys mealtimes more | 33 (9) | 12 (7) | 21 (12) | 59 (14) | 53 (14) | 6 (19) | 14 (23) | 12 (24) | 2 (20) |
| Parent/carer enjoys mealtimes more | 15 (4) | 6 (4) | 9 (5) | 47 (11) | 39 (10) | 8 (25) | 2 (3) | 2 (4) | 0 (0) |
| Better quality of life for child | 60 (17) | 25 (15) | 35 (19) | 110 (26) | 101 (26) | 8 (25) | 22 (36) | 18 (35) | 4 (40) |
| Child less frustrated or distressed at mealtimes | 47 (13) | 1 (10) | 31 (17) | 62 (15) | 55 (14) | 6 (19) | 7 (11) | 6 (12) | 1 (10) |
| Parent/carer less frustrated or distressed at mealtimes | 19 (5) | 5 (3) | 14 (8) | 57 (14) | 52 (13) | 5 (16) | 0 (0) | 0 (0) | 0 (0) |
| Less parental/carer/staff stress | 30 (8) | 8 (5) | 22 (12) | 73 (17) | 61 (16) | 12 (38) | 0 (0) | 0 (0) | 0 (0) |
| Better co-ordination of swallowing and breathing | 35 (10) | 31 (19) | 3 (2) | 50 (12) | 50 (13) | 0 (0) | 11 (18) | 10 (20) | 1 (10) |
| Better oral motor function | 36 (10) | 28 (17) | 8 (4) | 28 (7) | 26 (7) | 1 (3) | 10 (16) | 8 (16) | 2 (20) |
| Shorter mealtimes | 12 (3) | 8 (5) | 4 (2) | 10 (2) | 10 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Less food or drink spilled from lips | 7 (2) | 5 (3) | 2 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Better self-feeding or independence skills | 42 (12) | 25 (15) | 17 (9) | 33 (8) | 29 (8) | 4 (13) | 10 (16) | 7 (14) | 3 (30) |
| Wider range of foods eaten | 65 (18) | 11 (7) | 53 (29) | 20 (5) | 16 (4) | 4 (13) | 6 (10) | 2 (4) | 4 (40) |
| Less aversion or avoidance of particular foods | 39 (11) | 12 (7) | 27 (15) | 33 (8) | 28 (7) | 5 (16) | 6 (10) | 4 (8) | 2 (20) |
| More food or drink consumed | 30 (8) | 13 (8) | 17 (9) | 16 (4) | 16 (4) | 0 (0) | 1 (2) | 0 (0) | 1 (10) |
| Better mealtime one-to-one interaction with child | 11 (3) | 6 (4) | 5 (3) | 16 (4) | 15 (4) | 1 (3) | 10 (16) | 10 (20) | 0 (0) |
| More involvement in family’s activities | 32 (9) | 12 (7) | 19 (10) | 27 (6) | 23 (6) | 4 (13) | 5 (8) | 3 (6) | 1 (10) |
| Being able to eat a meal somewhere outside the home | 32 (9) | 14 (9) | 18 (10) | 7 (2) | 6 (2) | 1 (3) | –c | –c | –c |
| Better understanding of child’s EDSD and support strategies | 38 (11) | 15 (9) | 23 (13) | 52 (12) | 47 (12) | 5 (16) | 7 (11) | 7 (14) | 0 (0) |
| Fewer or shorter hospital admissions | 29 (8) | 25 (15) | 3 (2) | 73 (17) | 73 (19) | 0 (0) | 6 (10) | 5 (10) | 1 (10) |
| Fewer breathing changes | 19 (5) | 14 (9) | 5 (3) | 52 (12) | 51 (13) | 1 (3) | 5 (8) | 5 (10) | 0 (0) |
| Less pain | 14 (4) | 9 (6) | 5 (3) | 21 (5) | 21 (5) | 0 (0) | 9 (15) | 6 (12) | 3 (30) |
| Less drooling | 7 (2) | 6 (4) | 1 (1) | 3 (1) | 3 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Fewer abnormal or unusual movements | 11 (3) | 4 (2) | 7 (4) | 2 (1) | 2 (1) | 0 (0) | 1 (2) | 0 (0) | 1 (10) |
| Better sitting | 22 (6) | 13 (8) | 9 (5) | 7 (2) | 7 (2) | 0 (0) | 5 (8) | 4 (8) | 0 (0) |
| Child able to communicate better | 16 (5) | 7 (4) | 9 (5) | 16 (4) | 16 (4) | 0 (0) | 12 (19) | 9 (18) | 2 (20) |
| Not having to prepare separate meals for the child | 28 (8) | 8 (5) | 20 (11) | 3 (1) | 1 (< 1) | 2 (6) | –a | –a | –a |
| Less food waste or reduced cost of food | 13 (4) | 2 (1) | 11 (6) | 1 (< 1) | 1 (< 1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| More opportunity to talk to others about feelings about child’s EDSD | 3 (1) | 2 (1) | 1 (1) | 8 (29) | 4 (1) | 4 (13) | 1 (2) | 1 (2) | 0 (0) |
Use of tools to measure outcomes
Health professionals showed great variation when asked about whether or not they formally measured outcomes, with 18% reporting that they ‘usually’ formally measured progress following intervention, 29% reporting they ‘sometimes’ did and 25% reporting that they ‘never’ did (28% respondents did not answer this question). The most commonly used outcome measurement tools were published measures that focused on the child’s body structure, functioning and activity, such as TOMs (13%), the Oral Motor Assessment Scale (3%) and the SOMA (3%). HPs also reported using goal-based outcome measures (8%); published functional classification systems to describe functional ability (5%), such as the EDACS and the Gross Motor Function Classification System (GMFCS); adapted or self-developed non-standardised measures (4%); anthropometric measures (3%), such as Weight, Height, BMI, Proportion of food and liquid taken orally versus by tube feeding and whether the child was meeting their nutritional requirements; and parent-related measures (2%), such as measures of Depression, Anxiety, Stress and parent feedback questionnaires.
Summary of national survey
The survey provided insights into current UK-based clinical practice to treat children with EDSD based on the experiences of parents of children with neurodisability who experience EDSD, HPs and education professionals. Questions that were addressed included which interventions are used, how acceptable interventions are to deliver, the perceived effectiveness of interventions, how and where interventions are delivered or implemented, and which outcomes are important. The survey found that a wide range of interventions were used by parents, HPs and education professionals in the management of EDSD in children with neurodisability, despite limited research evidence to demonstrate clinical effectiveness. The majority of the interventions recommended by HPs were used with children with physical, non-physical and mixed EDSD, although the way these are implemented may differ based on the individual needs of the child and family. All interventions were viewed as acceptable to deliver in home and in school. The frequency of use did not reflect views on effectiveness; some less frequently used interventions were viewed as effective by parents and professionals in managing EDSD, such as the use of Sensorimotor therapy with children with non-physical EDSD. The most highly valued outcomes included those relating to the child’s physical health alongside those relating to the child’s and family’s overall well-being. The survey also highlighted low levels of formal outcome measurement by HPs as part of their clinical practice.
A small number of the outcomes listed in the survey were not taken forward into the second round of focus groups because < 5% of respondents rated them as important. These were Less drooling, Fewer abnormal or unusual movements, the Child being able to communicate better, Less food waste or reduced cost of food and More opportunity for parents to talk to others about their feelings about their child’s EDSD.
Strengths and limitations of national survey
To maximise recruitment to the survey, a large number of relevant parent and professional networks were contacted via e-mail and social media. As a result, we do not know how many eligible people received an invitation and the opportunity to take part in the survey; therefore, we cannot calculate a denominator for the response or examine the extent of any potential response bias. Only a small sample of education professionals responded to the survey and within this sample only 10 worked specifically with children with non-physical EDSD, which makes it difficult to draw conclusions about the use of interventions within schools and the outcomes valued by education professionals. We also have limited information about the frame of reference used by professionals responding to the study, especially in relation to the child’s age, with a large number of respondents reporting that they worked across the age range 0–18 years. Professionals were required to share only the nature of the EDSD of the child with whom they worked (i.e. whether the children had physical, non-physical or mixed EDSD) and, therefore, we cannot further explore any differences there might be in how professionals work with children within these groups. Although the same interventions were used with children with physical, non-physical and mixed EDSD, the delivery and specific details/content of the individual intervention will be guided by the needs of the child and family. Therefore, the delivery and details of each intervention are likely to vary across individual children and groups. For example, the intervention ‘Modifying equipment’ could, depending on the individual child’s needs, mean using a spoon with a bigger handle to facilitate a better grip for self-feeding or using a plastic spoon because of a heightened bite reflex. Although the number of respondents to the survey was sufficiently large to address the aim of identifying the interventions used, the survey was not sufficiently powered to allow statistical analysis of subgroup data, such as how different types of professionals deliver the interventions. The time taken to produce change from the interventions was unclear for a number of the interventions, making it difficult to draw conclusions regarding the duration of interventions and outcome measurement points for future studies.
Patient and public involvement in national survey
Parents and HPs were consulted on the list of interventions and outcomes to be included within the questionnaire to ensure that it was as inclusive as possible, through a discussion group and the first round of focus groups. Their views were also sought on supplementary questions, including the acceptability and effectiveness of interventions and the time taken to achieve change. The parent co-investigators were also involved in designing the questionnaires and advised on the use of simple language and clear examples of interventions to maximise participants understanding. The final online questionnaire and paper versions were piloted by a small number of parents, HPs, education professionals and researchers. The PAG considered the summaries of the findings of the national survey alongside the findings from the updates of the three published systematic reviews of interventions (see Chapter 3) and the mapping review (see Chapter 4). They suggested some amendments to the name of the interventions to improve clarity and accuracy, which are outlined in Chapter 8.
How did the national survey inform the next steps?
Following our iterative process, the national survey findings were used to inform the topic guide for the second round of focus groups (see Chapter 9), the Delphi survey questions on interventions and outcomes (see Chapter 10) and the stakeholder consultation workshops (see Chapter 11). For example, the survey showed that there was great variability in the interventions recommended by HPs; therefore, it was important to gather further information at the stakeholder consultation workshops about what comprises treatment as usual and how services are configured to facilitate the design of future trials. The survey, alongside the mapping review (see Chapter 4) and the first round of focus groups (see Chapter 5), showed that multiple interventions were being used by parents and recommended by HPs for both children with physical and mixed EDSD and children with non-physical EDSD. This information was considered as part of the evidence synthesis (see Chapter 8) and informed the discussions about interventions at the second round of focus groups (see Chapter 9). A large number of outcomes were valued by parents, HPs and education professionals and, therefore, further information was sought through the second round of focus groups (see Chapter 9), Delphi survey (see Chapter 10) and stakeholder consultation workshops (see Chapter 11) building towards agreement on the key areas to measure within future studies/clinical trials. Respondents to the survey had provided contact details if they wished to take part in the Delphi survey (see Chapter 10) and, therefore, the national survey acted as a vital part of sampling frame construction and recruitment for later stages of the study.
Chapter 8 Aim 3: evidence synthesis
Objectives
The objectives were to draw together information from preceding research activities on the support for individual EDSD interventions and their outcomes, and to inform a Delphi survey on the most important interventions and outcomes for future research evaluation.
Methods
Across the study, we collated evidence on the effects of EDSD interventions from the updates of three published systematic review of interventions (see Chapter 3), identified further interventions in a broader mapping review (see Chapter 4) and reviewed the measurement properties of published outcome measures (see Chapter 6). We also obtained the opinions of parents, HPs and education professionals on the current use and potential effectiveness of the EDSD interventions, and how they are evaluated in the UK, during the first round of focus groups (see Chapter 5) and from a national survey (see Chapter 7).
The previous chapters of this report have described the methods that we used in the individual activities and their results. Given our overall aim to identify interventions that could be delivered at home by parents to improve eating, drinking and swallowing in young children with neurodisability, we took an iterative, additive approach to synthesise/collate data regarding interventions and outcomes. To create full lists of all interventions and outcomes that have support from either research and/or current practice, we charted the data from each of the individual activities. We added the information obtained from each data set to the next data set, and iteratively created a full picture of potential interventions and outcomes. Each individual activity/data set was given equal weighting, as our aim was to develop a full picture rather than assess effectiveness.
Results of the evidence synthesis
Tables 17 and 18 show the interventions and outcomes that were identified and supported in the individual research activities.
| Intervention | Study task | |||
|---|---|---|---|---|
| Updated systematic reviews | Mapping review | First round of focus groups | National survey | |
| Modification of environment | ✓ | ✓ | ✓ | ✓ |
| Positioning | ✓ | ✓ | ✓ | ✓ |
| Modification of utensils | ✓ | ✓ | ✓ | ✓ |
| Schedule of meals | ✓ | ✓ | – | ✓ |
| Food or drink modification | ✓ | ✓ | ✓ | ✓ |
| Modifying placement of food in mouth | ✓ | ✓ | ✓ | – |
| Enhancing parent/child communication strategies at mealtimes | – | ✓ | ✓ | ✓ |
| Visual supports | – | ✓ | ✓ | ✓ |
| Training to wait for child’s cues for feeding | – | ✓ | ✓ | ✓ |
| Pace of food at mealtimes | – | ✓ | – | ✓ |
| Manoeuvres | ✓ | ✓ | ✓ | ✓ |
| Desensitisation programme for oral sensations | ✓ | ✓ | ✓ | ✓ |
| Sensory stimulation | – | – | – | ✓ |
| Sensorimotor therapy | ✓ | ✓ | – | ✓ |
| Oral motor exercises | ✓ | ✓ | ✓ | ✓ |
| Desensitisation programme for food avoidance | ✓ | ✓ | ✓ | ✓ |
| Strategies/programmes aimed at changing behaviour at mealtimes | ✓ | ✓ | ✓ | ✓ |
| Modelling | ✓ | ✓ | ✓ | ✓ |
| Hand-over-hand prompting | – | ✓ | ✓ | ✓ |
| Support for parents including counselling | – | ✓ | ✓ | ✓ |
| Psychological support for child | – | ✓ | ✓ | – |
| Medication | – | – | – | ✓ |
| Energy supplements | – | – | ✓ | ✓ |
| Vitamin or nutritional supplements | – | – | ✓ | – |
| Information on impact of movement difficulties on eating and drinking | – | – | ✓ | ✓ |
| Information on impact of sensory difficulties on eating and drinking | – | – | ✓ | ✓ |
| Sensory aids | – | – | – | ✓ |
| Modifying social eating and drinking opportunities | – | – | – | ✓ |
| Outcomes | Study task | ||||
|---|---|---|---|---|---|
| Updated Systematic reviews | Mapping review | First round of focus groups | National survey | Measurement properties review | |
| Improved nutrition | – | ✓ | ✓ | ✓ | ✓ |
| Better general health | ✓ | ✓ | ✓ | ✓ | – |
| Fewer or shorter hospital admissions | – | – | ✓ | ✓ | – |
| Less pain | – | – | ✓ | ✗ | – |
| Weight gain | ✓ | ✓ | ✓ | ✓ | – |
| Increased growth | ✓ | ✓ | ✓ | ✓ | – |
| Child enjoys mealtimes more | – | ✓ | ✓ | ✓ | ✓ |
| Child less frustrated or distressed at mealtimes | – | ✓ | ✓ | ✓ | ✓ |
| Better quality of life for child | – | ✓ | – | ✓ | – |
| Parent/carer enjoys mealtimes more | – | ✓ | – | ✓ | – |
| Parent/carer less frustrated or distressed at mealtimes | – | ✓ | ✓ | ✓ | ✓ |
| Less parental/carer/staff stress | – | ✓ | ✓ | ✓ | – |
| Not having to prepare separate meals for the child | – | – | ✓ | ✓ | ✓ |
| Better co-ordination of swallowing and breathing | ✓ | ✓ | ✓ | ✓ | ✓ |
| Fewer breathing changes | – | – | – | ✓ | – |
| Better sitting | – | ✓ | – | ✗ | – |
| Better oral motor function | ✓ | ✓ | ✓ | ✓ | ✓ |
| Less food or drink spilled from lips | ✓ | ✓ | ✓ | ✓ | ✓ |
| Less drooling | – | ✓ | ✓ | ✗ | ✓ |
| Shorter mealtimes | ✓ | ✓ | ✓ | ✓ | ✓ |
| Better self-feeding or independence skills | – | ✓ | ✓ | ✓ | ✓ |
| Wider range of foods eaten | ✓ | ✓ | ✓ | ✓ | ✓ |
| Less aversion or avoidance of particular foods | – | – | ✓ | ✓ | ✓ |
| More food or drink consumed | ✓ | ✓ | ✓ | ✓ | ✓ |
| Better mealtime one-to-one interaction with child | – | ✓ | ✓ | ✓ | – |
| More involvement in family’s activities | – | – | – | ✓ | – |
| Being able to eat a meal somewhere outside the home | – | – | ✓ | ✓ | – |
| Better understanding of child’s EDSD and support strategies | – | ✓ | ✓ | ✓ | – |
| Fewer abnormal or unusual movements | – | – | – | ✗ | – |
| Child able to communicate better | ✓ | ✓ | ✗ | – | |
| Less food waste or reduced cost of food | – | – | ✓ | ✗ | – |
| More opportunity to talk to others about feelings about child’s EDSD | – | – | – | ✗ | – |
| Appetite | – | ✓ | – | – | ✓ |
| Mealtime behaviour | ✓ | ✓ | – | – | ✓ |
| Child’s understanding of mealtime routines | – | – | – | – | – |
We reviewed the interventions and outcomes identified through the updates of the three published systematic reviews of interventions (see Chapter 3), the mapping review (see Chapter 4), the first round of focus groups (see Chapter 5) and the national survey (see Chapter 7). Given that individual interventions were rarely used on their own, the research team decided that the individual interventions were usually delivered in series or in combination, depending on the needs of the child and parent. We conceptualised this as a ‘toolkit of interventions’, and compiled a separate version for children with physical or mixed EDSD and children with non-physical EDSD (Figures 9 and 10), including the associated outcomes.
FIGURE 9.
Visual summary of the interventions and outcomes for children with physical or mixed EDSD from the evidence review and national survey.
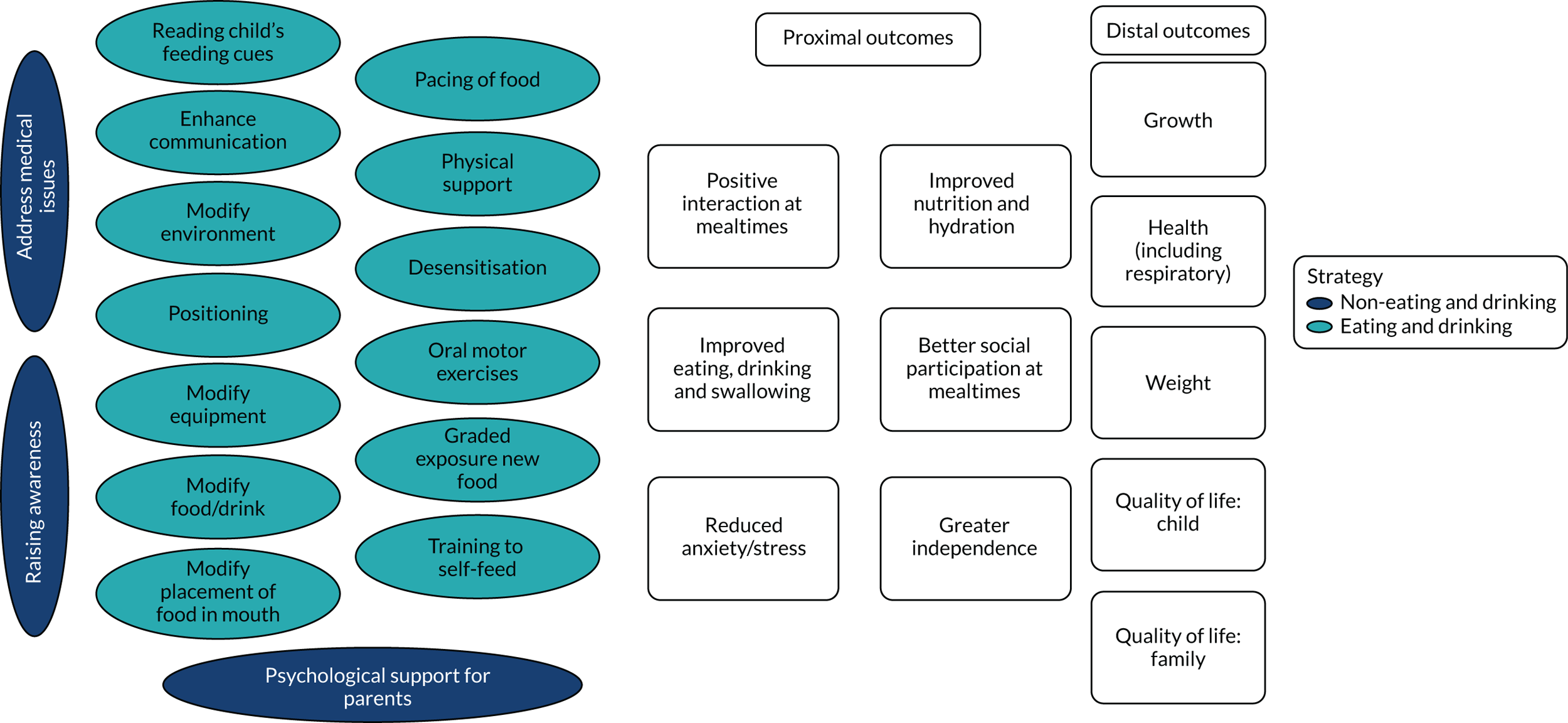
FIGURE 10.
Visual summary of the interventions and outcomes for children with non-physical EDSD from the evidence review and national survey. CBT, cognitive–behavioural therapy.
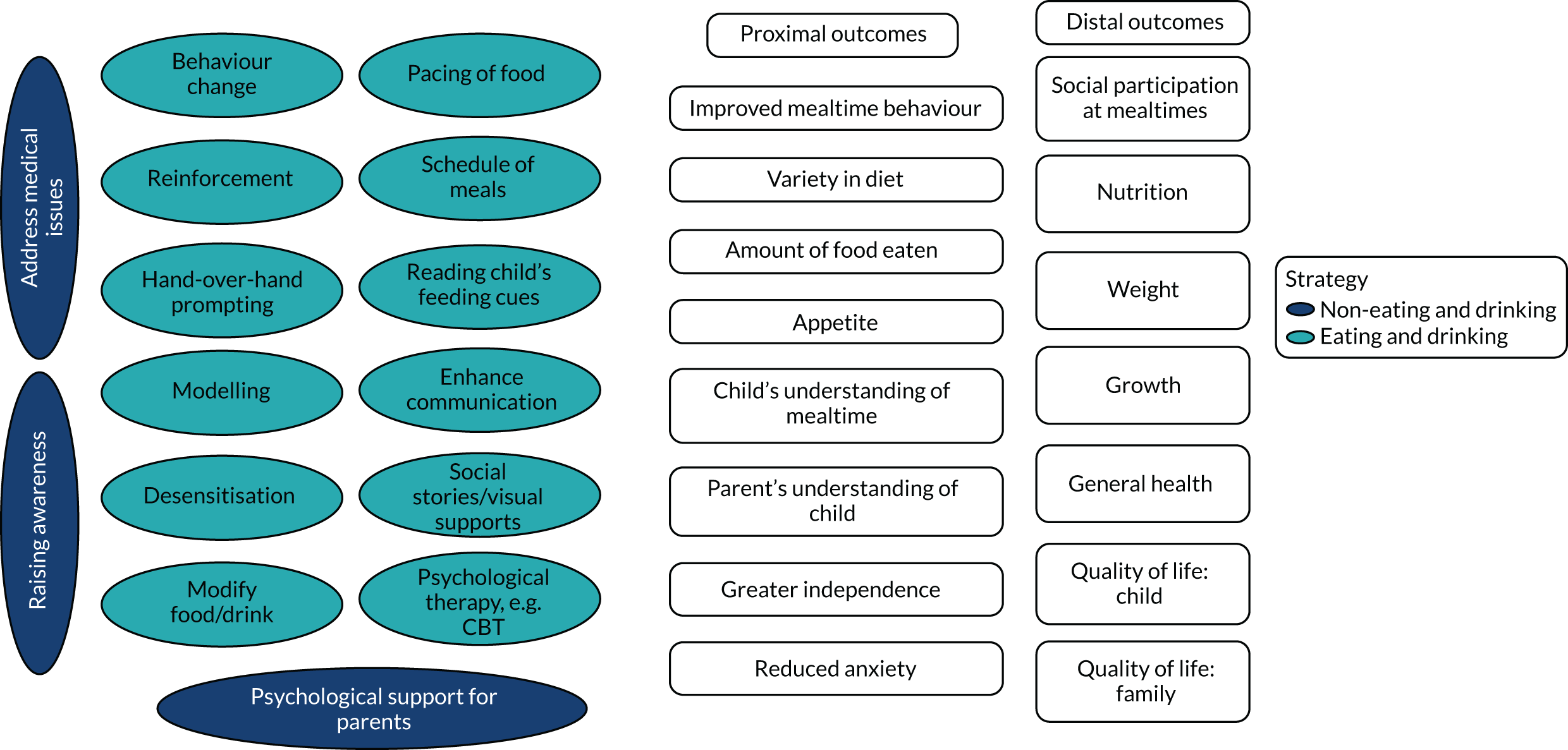
When compiling the first versions of the toolkit, the following decisions were taken by the research team on the basis of the evidence synthesis and feedback from the PAG.
Changes to interventions
For ease of understanding, we changed the names of the following interventions based on feedback from the PAG and the views of the research team:
-
Hand-over-hand prompting was changed to Training to self-feed.
-
Counselling was changed to Psychological support for parents.
-
Information on the impact of movement difficulties on EDSD and Information on the impact of sensory difficulties on EDSD were changed to Raising awareness.
-
Modifying social eating and drinking opportunities was included in Modifying environment.
We decided that the intervention Sensory aids should not be taken forward as this was not an intervention that related to improving EDSD.
Changes to outcomes
We renamed the following outcome based on feedback from the PAG and the views of the research team:
-
More food and drink consumed was changed to Amount.
In response to feedback, we grouped the following outcomes together:
-
Better general health, Fewer or shorter hospital admissions and Less pain as General health.
-
Child enjoys mealtimes more, Child less frustrated or distressed at mealtimes, Parent enjoys mealtimes more and Parent less frustrated or distressed at mealtimes as Reduced anxiety/stress.
-
Better co-ordination of swallowing and breathing and Fewer breathing changes as Improved eating, drinking and swallowing.
-
More involvement in family’s activities and Being able to eat a meal somewhere outside the home as Social participation.
Following feedback, the following outcomes were not taken forward owing to < 5% of respondents of the national survey rating them as important:
-
Less drooling
-
Fewer abnormal or unusual movements
-
Child able to communicate better
-
Less food waste or reduced cost of food
-
More opportunity for parents to talk to others about their feelings about their child’s EDSD.
Strengths and limitations of the evidence synthesis
The sequential approach to gathering data on interventions and outcomes that are supported by research and/or current practice in the UK enabled us to build on a robust foundation underpinned by the relevant evidence. We have been pragmatic and forward focused; therefore, we have not revisited or updated each stage of the work beyond the point at which it was used. For example, we did not revisit and update the search for the mapping review; although it is feasible that we would have identified new studies, findings could not have been incorporated into our survey of current practice or consensus seeking in the Delphi survey. Owing to the pragmatic nature of the work, we searched for and included only papers that were published in the English language.
How did the evidence synthesis inform the next step?
The toolkit of interventions and associated outcomes was shown to parents and HPs in the second round of focus groups (see Chapter 9) to capture their views on the idea of a toolkit and to identify any additional interventions or outcomes that were not found through the research activities to date.
Chapter 9 Aim 3: second round of focus groups
Objectives
We aimed to gain an understanding of parents’ and HPs’ views on which interventions identified in the previous study stages should be tested in research, on which outcomes identified in previous stages are important to measure and on which measurement tools could be used.
We also wanted to explore parents’ and HPs’ views on the future research priorities for interventions and choosing appropriate outcome measures for the interventions researched.
Methods
Participant selection and recruitment
We aimed to recruit parents of young children with physical or mixed EDSD, parents of young children with non-physical EDSD, HPs working with young children with physical or mixed EDSD and HPs working with young children with non-physical EDSD. The research team notified parents and professionals in their regional networks about the focus groups. People who expressed an interest in taking part were informed of the venues and dates of the focus groups. As the groups could include a maximum of eight participants, interested participants were asked to provide their geographical location, information on their child/client group, including age and type of difficulty (i.e. physical, non-physical or mixed EDSD), and for professionals their current role. Finally, participants were purposively selected to maximise variation in the difficulties their children or client group experienced and, hence, variation in participants’ knowledge of a wide range of interventions and outcomes.
Procedure
Each group was facilitated by Helen Taylor and two or three members of the research team (JC, DG, CM, JP, LP, DS or JS).
To address the first objective, the first part of the discussion focused on the interventions and outcomes identified in the previous stages of the research. Participants were provided with a brief verbal summary of the evidence reviews and survey. This was supported by visual representations of the proposed toolkits of interventions, and associated outcomes for children with physical or mixed EDSD and non-physical EDSD shown separately (see Figures 9 and 10) so that participants could discuss how these could be used at different times depending on parent and professional priorities. Participants were asked about their experience of these interventions, including their perceived effectiveness for children with physical, mixed and non-physical EDSD, the order of their use in clinical practice and the importance of individual outcomes. Information from the national survey (see Chapter 7) regarding what ‘treatment as usual’ may comprise was also discussed.
To address the second objective, the second part of the discussion focused on the potential feasibility of future research using a participant, intervention, comparator, outcome, time, setting (PICOTS) structure: which population of children could be included; whether one intervention or a combination of interventions could be evaluated; what the comparator or control condition could and should be; whether and how randomisation could be achieved; important outcomes and measurement tools; and the period over which outcomes should be evaluated. See Appendix 12 for the topic guide of the second round of focus groups.
Interviews were audio-recorded and transcribed verbatim, with participants identified by a research code. Analysis was undertaken by two of the research team (LP and HT) and was based on the framework method. 100 Transcripts were read repeatedly for familiarisation with their content. Text was coded using predefined codes relating to interventions and outcomes identified in the evidence reviews and survey, diagnostic groups and age categories (infant, preschool and school aged) on hard copies of the transcripts and in transcripts. Summaries of data coded under each category for each focus group/interview were then ‘charted’ by entering them into a matrix in a Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) spreadsheet in which rows referred to individual focus groups/interviews and columns referred to codes. The matrix was scrutinised to determine patterns across participant groups and interventions and outcomes. Any contrasting views of individual participants within a focus group or in individual interviews were highlighted within the matrix as ‘deviant cases’ and examined to determine possible reasons for their difference to the experience/views of other participants.
Results
Nine focus groups were held in September and October 2018: four in Newcastle (two for HPs and two for parents), two in London (for HPs), one in Sussex (for HPs) and two in Exeter (for parents). Three parents due to take part in a focus group in Exeter were unable to do so because of family circumstances. Subsequently, these parents continued to express a desire to participate and were interviewed individually by telephone.
Participants
Participants’ characteristics are summarised in Table 19. In total, 19 parents were recruited, of whom seven had a child with physical EDSD, nine had a child (or children) with non-physical EDSD, two had a child with mixed EDSD and one had a child with physical EDSD and a child with non-physical EDSD.
Twenty-nine HPs took part, comprising 11 speech and language therapists, six dietitians, nine paediatricians, two clinical nurse specialists and one physiotherapist.
| Group | Location | Participants |
|---|---|---|
| HPs | ||
| 1 | London | Three SLTs (one community, one tertiary and one specialist education) and two dietitians (one community/secondary level and one community and tertiary) |
| 2 | Sussex | Three paediatricians (community), two SLTs (one community and one specialist education) and one dietitian (community) |
| 3 | London | Four paediatricians (tertiary, one also community), two SLTs (tertiary), one dietitian (tertiary) and two nurse specialists (tertiary) |
| 4 | Newcastle | Two dietitians (community, mostly non-physical EDSD), two SLTs (both community), one paediatrician (tertiary) |
| 5 | Newcastle | One SLT (tertiary), two paediatricians (one secondary and one community and secondary care level) and one physiotherapist (community) |
| Parents | ||
| 1 | Newcastle | Two parents of a child with physical EDSD, two parents of a child with non-physical EDSD and one parent with two children with non-physical EDSD |
| 2 | Newcastle | Three parents of a child with physical EDSD, two parents of a child with non-physical EDSD and one parent with two children (one with physical EDSD and one with non-physical EDSD) |
| 3 | Exeter | Two parents of a child with non-physical EDSD, one parent of a child with physical EDSD and one parent of a child with mixed EDSD |
| 4 | Exeter | Parent of one child with mixed EDSD. Three parents who could not attend on the day were interviewed by telephone: parent of a child with physical EDSD, parent of a child with non-physical EDSD and parent of three children with non-physical EDSD |
Summary of themes identified from the discussion
Rich qualitative data were obtained from the extensive discussions. In addition to the planned topic guide to include interventions, outcomes, outcome measures and research design, there was a significant discussion regarding provision and organisation of services and their impact on intervention choice and delivery.
Interventions for eating, drinking and swallowing for young children with neurodisability
There was broad agreement among participants about the intervention headings presented (see Figures 9 and 10):
I think in reality you’d use a number of strategies in conjunction wouldn’t you, to try and improve your child’s eating. It’s not just one that you’d draw upon at any one time, you’d use a combination to reduce stress and anxiety and optimise their feeding.
Parent interview
Participants discussed details of what each intervention may comprise and how it may be delivered. Participants also confirmed that it is usual practice to use multiple interventions concurrently or sequentially for individual children. They also thought that most interventions could be relevant for children with physical, non-physical or mixed EDSD and suggested that a single toolkit was needed.
Participants considered some of the interventions as crucial to optimise safe eating and drinking. These formed first-line treatments and included Positioning, Food and drink modification, Position of food in the mouth, Modification of utensils and Modification of environment. Participants also designated Scheduling of meals, Enhancing the diet through supplementation or alteration, Cues for feeding, Pacing mealtimes appropriately, Strategies to enhance communication between child and mealtime helper, Increasing awareness of and understanding of child’s difficulties and Behaviour change programmes as first-line interventions. Parents identified Psychological support for parents and children as a further important first-line intervention because of the high levels of stress and anxiety associated with EDSD. However, they acknowledged that this type of intervention is rarely available from professionals, with the exception of support from some health visitors in early years. Parents also reported that informal support from peers via social media and support groups can be a means of reducing isolation, sharing ideas and recognising progress. Psychological support for children with non-physical EDSD was identified by participants as important because of children’s experience of food aversion and their high anxiety linked to mealtimes.
Participants thought that the following interventions were not relevant for all children, were less widely used by HPs or may be best tried after first-line interventions: Oral motor exercises; Hand-over-hand prompting; Food desensitisation; Sensory desensitisation; Visual support, especially when communication is limited; Informal modelling by peers; and Training to self-feed.
Multiple factors appeared to influence the choice of intervention(s) used and when to use them; these are outlined below.
Individual context of the child and family
Participants stressed the importance of individual context to determine the goals of an intervention and drive the selection of individual interventions. They felt strongly that determining priorities for an individual child required discussion between parents and professionals. Important considerations included the:
-
family’s capacity to understand and implement potential interventions
-
family’s culture and attitudes around food, eating habits and mealtimes
-
child and family’s motivation to change
-
child’s developmental profile and developmental (rather than chronological) age (cognitive ability, hearing, vision and communication abilities)
-
child and family’s psychological well-being related to personal factors, such as anxiety and stress, or additional stressors on the child/family
-
child’s health – impact of sleep, health and physical activity on food/fluid intake.
Underlying medical issues
Participants agreed that addressing medical concerns before and alongside EDSD-focused interventions was critical because their effect on health, well-being and quality of life could be significant. Specific EDSD interventions may by prevented from working because of a failure to address key underlying health issues, such as muscle tone, pain, constipation, reflux and other digestive system issues, seizures and allergies. There was acknowledgement in discussions that medical input may be variable across the country and that some general practitioners (GPs)/primary care providers may be reluctant to provide input when a paediatricians’ specialist knowledge may not be available.
Differences between professionals and between professionals and parents
Participants identified differences in knowledge, awareness and experience between different professionals of the same and different disciplines. Indeed, some HPs in the focus groups were not aware that all of the interventions listed, such as cognitive–behavioural therapy and social stories, were used to help EDSD. Participants described involvement in training education professionals, health visitors and GPs around EDSD as time well spent. They thought that there were particular gaps in HPs’ knowledge, skills and awareness of EDSD when a child had an intellectual disability. It was also considered important that the person providing mealtime assistance in any location understood the child’s needs at home, in school and in other contexts (e.g. that some children attend short breaks). Participants expressed that HPs do not always share their view of the long-term prognosis for a child’s EDSD with parents, which can lead to unrealistic and misinformed expectations around eating:
There’s pressure – it’s really, really tough . . . thinking about meeting what some professional wants you to do, against what you think is right, or even knowing what timescale they’re talking about, that can be quite unclear.
Parent group 1
Differences in services offered across the UK and the multidisciplinary team
Participants discussed the differences between services across the UK, including access to the wider MDT in some services.
Multidisciplinary team working
Participants agreed that MDT working is recommended as no one profession holds all of the answers of how to support children with EDSD, and that a typical MDT supporting children with EDSD comprises a speech and language therapist, dietitian, occupational therapist and paediatrician. Some professionals thought that all services for children with EDSD required routine input from dietitians. Support from nurses and psychologists was considered optimal, but rarely available, especially in secondary care. Participants thought that the MDT should also include members of school staff as education professionals provide mealtime assistance for children at nursery or school.
Parents commented that consistent messages from different HPs were important to support them with EDSD interventions and to help them manage their expectations of interventions. Consistency may be facilitated by professionals sharing the same geographical base and spending time talking together:
The problem with this kind of issues is that no one person holds the answer, and trying to get everyone together can be massively challenging.
HP group 1
Variation in services was thought to be due in part to funding reductions, service redesign and staff vacancies. However, participants acknowledged the variations in staff knowledge and expertise even within the same profession. Parents and HPs reported particular concerns about the lack of recognition and availability of formal support and interventions for children with non-physical EDSD.
Parents described that when there was no professional input for their child’s EDSD, or when a child’s needs were unrecognised by HPs, they would not seek help from HPs. Parents would also identify their own strategies, for example by using the internet, contacting charity groups and sharing their new information with education professionals. Participants further reported that limited HP resources promoted alternative ways of working with children and their families to address unmet needs. Examples included a parent forum in the local community setting, a MDT that was established to provide advice and information and some schools providing interventions separate from HP input, such as ‘Fun with Food’ groups or lunch clubs run by learning support staff.
Health professionals also referred to ‘transdisciplinary working’ in the context of limited professional resource. In contrast to MDT working, which involves different HPs working together and each drawing on their disciplinary knowledge in a MDT, transdisciplinary working involves exceeding usual disciplinary boundaries to share knowledge, skills and decision-making to address problems; children, parents and their families are included as stakeholders. 101 HPs had variable opinions about transdisciplinary working, particularly where no specific formal training takes place. They reported that transdisciplinary working was usually implemented when there was potential harm linked to lack of resources from, for example, dietetics and psychology. HPs considered it less risky for HPs other than psychologists to offer psychological support to parents; there was more risk associated with HPs offering nutritional advice in the absence of a dietitian. Some HPs expressed a reluctance to work therapeutically with parents as they had been specifically trained to work with children.
Service delivery
Participants identified that there are different service models for children with physical and non-physical EDSD, with variation across community and tertiary services and between NHS trusts. Some children with non-physical EDSD may receive limited support from local health services, with a small proportion of children seen in tertiary services. Children with physical and mixed EDSD are seen in local community services and are referred to tertiary services only when required. Participants found the lack of services for children with non-physical EDSD especially frustrating, with no input in many community services and schools. Participants reported that scarce resources were prioritised to children who were most at risk of harm from EDSD, such as those who are at risk of aspiration or choking.
Formal guidelines around service provision, role boundaries and care pathways were identified by parents as sometimes unhelpful and frustrating. Some HPs described using technology to facilitate input, for example using telephone calls, e-mails, teleconferencing and videos to communicate with families who did not live close to specialist provision.
Decisions about when to discharge children were described as varying between services and as being influenced by the alternative support services available and individual decisions by professionals. Some services (particularly tertiary services) are known to provide only assessment and advice; they discharge children after contact and require further referral by the local team to review the child and family again. This model focuses on intermittent support rather than ongoing support from the MDT and is particularly challenging for parents when there are communication or process barriers to re-referral, such as long waiting lists or lack of a single point of contact within a service.
Health professionals reported that lack of resource could lead to unmanageable caseloads, and that high staff turnover within teams resulted in decreased knowledge of local networks and individual cases.
Health professionals working in feeding services generally reported that they practise holistically and see a child as part of the family. However, parents reported that HPs often failed to assess the capacity of parents to implement recommended interventions.
Home–school link
Communication about EDSD between the home and the school was considered important, but many parents reported little contact with school-based HPs. Differences in approaches to EDSD between the home and the school could potentially be problematic; consistency of approach was considered crucial. Participants agreed that education professionals have a role in delivering interventions and effecting change, which supports the discussion above regarding educational professionals being included in the MDT.
Important outcomes for EDSD interventions
From the models presented, participants viewed broad outcomes linked to Health, Nutrition, Growth, Quality of life and participation, Increased awareness and understanding, and Reduced anxiety/stress levels as the most important outcomes for children with neurodisability and EDSD.
Health
General health was viewed as important for all children with EDSD. For children with physical or mixed EDSD, health was specifically linked to swallow safety, with the view that if swallowing is safe this should lead to a reduction in aspiration of food or fluid into the lungs and respiratory health issues (e.g. chest infections and lung damage). Parents reported anxiety and a sense of responsibility around the risk of aspiration and the impact on children’s respiratory health. In the case of children with non-physical EDSD, who often remain in good health even with a restrictive diet and are unlikely to suffer sudden acute medical emergencies, health was viewed in terms of chronic nutritional deficiencies. For all children with EDSD, energy levels were seen as a marker of General health. Some participants thought that potential benefits from interventions to address the safety of swallowing could be seen within weeks, whereas others thought that routine monitoring was required over time.
Growth
Growth was considered to be an important outcome for both children with physical EDSD and children with non-physical EDSD. HPs were reported to measure Growth as part of routine care and its measurement may or may not be related to an EDSD intervention. Participants discussed the challenges in measuring Growth. They agreed that measures should include changes in weight and height. HPs reported that proxy measures for height may be needed for children with physical disability who are unable to stand straight. Changes to weight were seen as easier to measure and might be expected in a matter of weeks, particularly for children with physical EDSD. However, HPs emphasised that Weight should be understood in relation to the amount of food eaten, previous weights and trajectories. Discussions indicated that sometimes Weight could be a contentious issue. Parents thought that HPs place greater value on Weight than they do and that a focus on Weight gain can increase parents’ anxiety. This was discussed in terms of Weight being difficult to gain, for example if children require a large number of calories owing to their movement disorder; others were concerned about excessive Weight gain among non-ambulant children, or that Weight gain would make it more difficult to lift and handle children with motor disorders. Some parents thought that focus on weight gain could have a detrimental affect on engagement with an intervention if they felt judged or criticised with respect to their abilities to care for their child if the child does not gain/maintain Weight as expected. HPs thought that some parents have an unrealistic view about their child’s weight, with limited recognition or acceptance if the child was underweight or overweight:
I think a good outcome would definitely be Growth because you can then compare it to lots of other studies.
HP group 1
There was significant variation of opinion regarding the timescale for expecting change in Growth from participants. One HP group thought that monthly monitoring was necessary to measure changes in Growth linked to interventions. Parents reported that changes might be apparent within 2 weeks, and another parent and group of HPs thought that longer-term monitoring of outcomes over 1–3 years may be needed to ensure an adequate Growth rate.
Nutrition
Adequate Nutrition and Hydration was thought to be an important outcome for children with physical and non-physical EDSD, with consequences for outcomes such as Growth, Health, Concentration, Energy levels and Well-being. Nutrition was linked to not only the amount of food but also variety, which was seen as an issue for children with physical and non-physical EDSD. Participants were concerned about the impact of unhealthy dietary strategies on children’s future health and parents expressed concern around limited future choices when children were more independent from them. Participants also reported that adequate food and fluid may be given to the child, but it might not be tolerated. Participants identified that parents could experience considerable stress when children’s mealtimes involved vomiting, reflux and food/fluid loss.
Professionals thought that the amount of food eaten was less relevant to children with EDSD than an assessment of nutritional intake, and that it was possible to pick up issues at the nutritional level even if a child’s weight was reasonable. However, participants did raise challenges to measuring food intake. HPs thought that parents did not always share a full picture of child mealtimes because of fear of being judged, but anxieties could be reduced by using something like a ‘Well plate’ (a pictorial representation of the recommended daily amount of major food groups). Other measures of Nutrition discussed included blood tests, which can indicate deficiencies that are not evident from the diet, but do not always reflect paucity of diet. However, participants identified a lack of resources to take blood in the community other than through the GP. Furthermore, they felt that children with non-physical EDSD could find the procedure of a blood test too difficult to undergo. Indictors of positive change in Nutrition could include reduction or discontinued use of nutritional supplements or enteral feeding.
Participants thought that changes associated with improved Nutrition could be seen within 6 months, although for some children a subjective improvement in a child’s energy levels may be seen as a proxy marker for Nutrition in a few weeks. Some participants thought that other changes were unlikely to be observed within 6 months; for example, changes to restricted dietary practices can be very slow to achieve, particularly for children with ASD:
I think you do get immediate effects from improving Nutrition and Hydration, like Energy levels, Better ability to concentrate and things like that. So I think it is both a short-term and a long-term goal.
HP group 4
Quality of life and participation
Although Quality of life was thought to be a key outcome for all children with EDSD, some parents thought it was secondary to outcomes linked to Growth, Health and Nutrition. All participants thought that children’s enjoyment of food and mealtimes was important and influenced their quality of life. Children’s enjoyment was especially important to parents. Some HPs thought that small changes, for example the ability to tolerate a different food or texture following an oral desensitisation intervention, could greatly affect a child’s quality of life.
Participants thought that Quality of life should be considered for the whole family, including siblings. Factors arising from children’s EDSD that could negatively affect the family’s quality of life included the stress and fatigue of a child’s lengthy, messy mealtimes in isolation; the demands of making separate meals and the time and skill required to prepare meals that required modifications (e.g. to texture, temperature and presentation); and parents being the sole provider of assistance to their children, which reduced their ability to work and act independently of their children.
Regarding Social participation, some families experienced considerable stress from not being able to participate in usual social situations, such as going out for meals. Parents also thought that it was important for their children to experience different tastes and textures of food and to be able to manage to eat in different environments, including sharing the same food and drink experiences as their family. There was also an understanding that there may need to be a balance between Safety of mealtimes and Experiencing different foods and mealtime environments. Similarly, Independent eating may be desirable, but it may have adverse effects on Length of mealtime and Nutritional intake. Participants thought that Enjoyment of mealtimes was more important than Independent eating/drinking, and that Self-esteem and Control around mealtimes was more important than Social participation. Some thought that supporting a child to self-feed and make choices around what they wanted to eat was important:
I’ve definitely lost count of the amount of times that I’ve approached a professional about issues around eating and then they’ve said to me ‘well its OK, because he’s tracking his height and weight chart’ and I’m saying but it’s not OK. This eating environment for all of us and for him is not OK and its reassuring that he’s still growing. It would feel much better if I was able to get him to enjoy food and be wanting to eat and be involved. So yes I do think that there is a tendency to just focus on those measurements and not necessarily on the actual social and emotional involvement of your child in food.
Parent group 3
The parent–child relationship was considered to be very important, with an acknowledgement that children’s EDSD could have adverse effects on this. Parents of children with ARFID and/or ASD experienced high levels of stress because of their children’s restricted eating, with resulting effects on their relationships with their children.
Quality of life for both children and their families was thought to be an outcome that needed long-term monitoring.
Psychological well-being
Participants thought that Reductions in stress and anxiety that were experienced by parents of children with EDSD were important outcomes, but that Psychological well-being was frequently overlooked. Participants discussed that anxiety and stress of the child and parents are interlinked, and they often used the terms ‘anxiety’ and ‘stress’ interchangeably in the discussions. When prompted for clarification, participants said that ‘stress’ may be more amenable to change because it is linked to external factors, that is ‘stressors’ that can be modified, whereas relief of anxiety may require psychological support because anxiety is closely linked to belief system and personal experiences. Stress was seen to arise from a number of different factors, including:
-
the limited availability of professional support and resources, especially linked to the specific group of children with ARFID
-
variability from day to day in children with non-physical EDSD
-
fear of asking for help because of not wanting to be judged
-
the desire to retain control of eating/drinking/feeding in the face of little control over other aspects of a child’s care
-
anxiety around weight gain, as this would make it more difficult to lift the child.
Parents thought that a reduction in anxiety or stress levels experienced by parents should be a secondary outcome compared with the child’s Growth, Nutrition and Health, and this in turn could facilitate parental engagement with other interventions linked to EDSD. Parents also expressed the need to select the aims and strategies used to address EDSD to feel more in control. They felt motivated to work on interventions when they worked with HPs using small shared goals with shared decision-making, particularly for children with non-physical EDSD.
The Child’s experience of stress and anxiety was a further important distinct outcome, especially for children with ASD. Participants reflected that children experienced frustration at their own limitations and hunger, as well as anxiety/fear linked to particular foods. Pressures to eat could increase children’s experience of anxiety/stress; this was particularly linked to potential demands to eat aversive foods in non-physical EDSD. Children were also described as becoming hypervigilant around food preparation. Some intervention strategies were thought to increase children’s distress, for example a child with ASD becoming overwhelmed by the experience of being offered a choice. Participants thought that anxiety and stress could be reduced quickly with appropriate HP involvement and interventions:
I think it would be quite good for the parents to get some support because it can be very frustrating and an anxious time when you’re trying to feed a child which is just going to choke and vomit the whole time, and the child reads off you. So it doesn’t help if the parent is stressed about giving the child food and wondering if they’re going to be choking and aspirating and everything else.
Parent group 3
Other outcomes
Participants suggested some additional outcomes that they did not consider to be included in the visual summaries presented to them (see Figures 9 and 10):
-
Duration of mealtimes – increased mealtime efficiency by a child will reduce the time and effort taken by parents
-
Prevention of new or further difficulties
-
Absence of vomiting once food/drink has been ingested, which could be included in oral motor control or health.
Outcome measures
Participants discussed measures of individual outcomes, but acknowledged that there was a lack of consistently used and validated outcome measurement tools in current practice. Furthermore, there is no eating, drinking and swallowing measure that includes nutritional intake alongside other aspects, such as safety. Participants considered some specific measures that they had come across in clinical practice and they thought may be suitable for use in future research, but none of these correlated with the outcome measures in the mapping review.
Research and potential trial design
There was unanimous agreement that further research is very important to all stakeholders. Elements of potential trial designs were discussed and thought to be challenging:
-
Population. This could be any child with neurodisability and EDSD, but, as some children with non-physical EDSD are not currently receiving services, some participants thought that there should be a focus for research on children with non-physical EDSD only.
-
Intervention. Parents and HPs use multiple interventions, often in parallel and at different times. Interventions may or may not be clearly defined and described. For example, parents or HPs may not be conscious that they are adjusting the position or environment, and that this may constitute an intervention. A research intervention would, therefore, need to be clearly defined and information about other interventions that may be in place should be systematically gathered. If multiple interventions were provided, it would be essential to ensure treatment fidelity, which could be difficult for parent-delivered interventions.
-
Comparator. This may be difficult to select and define as ‘treatment as usual’ varies so much between regions and type of HP and/or provider, and many children with EDSD have other comorbidities that would need treatment.
-
Outcomes. Again, these may be difficult to select; all groups discussed that outcome selection required further consideration. Appropriate outcomes for individual children and interventions can depend on the timescales over which change can be expected.
-
Timing. This would depend on the population, the intervention and the outcome chosen. Measurement of long-term outcomes will be challenging with respect to the heterogeneity of the population and confounding interventions.
-
Setting. Lack of resources and service provision across different settings may affect trial feasibility. HP participants discussed that the unit of randomisation should be at the NHS trust level and be dependent on existing service delivery models. This may be more acceptable to families; however, there may be contamination of interventions by parents offering support to one another (e.g. if they are in touch through schools or social media). There may be issues with generalisation of treatment effects across home and education settings; participants thought that this could be particularly challenging with children with ASD. Participants thought that schools could provide suitable trial settings to implement strategies first, which could later be translated into the home environment.
Summary of the second focus groups
The focus groups highlighted that all interventions and outcomes identified in previous stages of the research should be taken forward into the Delphi survey (see Chapter 10). Multiple interventions are often provided concurrently or sequentially, but there are several issues affecting their implementation. There is a need to build trust between parents and HPs for shared decision-making. There is significant variation in service organisation and available personnel, and the skills and experience available affect what is offered to children and their families. ‘Treatment as usual’ varies widely across the UK. A consistent feature that was highlighted by all groups was the general lack of psychology input for children with non-physical EDSD.
Participants universally agreed that further research is required, but the complexities of measuring outcomes, both clinically and for research, were acknowledged.
Strengths and limitations of the second round of focus groups
The focus groups were held in multiple UK locations, which reduced the chance of bias from regional practice opinion. However, as participants volunteered to take part, they may have had particularly strong views about certain issues discussed that were not representative of other parents and HPs. Where parents were unable to attend, their views were included through interviews. Terms such as ‘stress’ and ‘anxiety’ were not well defined, and there was not an opportunity to explore this in detail during groups in which a range of topics were discussed. Analysis was challenging owing to the wide range of topics discussed, as well as variation in opinion, practice and expertise. Themes contained useful views of relevance to future research.
Patient and public involvement in the second round of focus groups
The PAG advised on how best to share information about the interventions and outcomes that were identified through the updates of the three published systematic reviews of interventions (see Chapter 3), mapping review (see Chapter 4) and national survey (see Chapter 7). They advised on creating a pictorial summary of the interventions and outcomes to aid discussion in the groups, and using the term ‘strategies’ to describe the interventions (see Figures 9 and 10). Given the overlap between interventions identified for physical and mixed EDSD and for non-physical EDSD, they advised that it might be beneficial to run groups including parents of children with physical, mixed or non-physical EDSD together to capture the commonalities and differences within and between these groups.
How did the second round of focus groups inform the next steps?
The focus groups highlighted that all interventions and outcomes identified in previous stages of the research should be taken forward into the Delphi survey (see Chapter 10).
Presentation of the interventions in the visual format (see Figures 9 and 10) validated the research team’s concept of a single toolkit for any child with neurodisability and EDSD, which was made up of individual interventions to be selected in tandem or sequentially by parents and HPs. This concept evolved further at co-investigator meetings and was discussed at the stakeholder workshops (see Chapter 11), in which the themes about future research were also discussed.
The research team further reviewed the interventions and outcomes included in the proposed toolkit following the second round of focus groups in developing the Delphi survey. The PAG also offered further advice regarding the terms used within the toolkit, which resulted in a number of changes.
Changes to interventions
For ease of understanding, we renamed the following interventions based on feedback from the second round of focus groups and the PAG:
-
Modification of utensils changed to Modifying equipment.
-
Reading child’s feeding cues changed to Responding to a child’s cues for feeding.
-
Raising awareness changed to Sharing information.
Following feedback, for accuracy and clarity we renamed the following interventions to ensure that they were more clearly relevant to parents of children with physical or mixed EDSD and non-physical EDSD:
-
Food and drink modification into Modifying the consistency of food or drink and Modifying other aspects of the food.
-
Desensitisation programme for food avoidance into Graded exposure to new textures and Graded exposure to new food.
In response to feedback, we added the following intervention:
-
Vitamin or nutritional supplements.
Changes to outcomes
We renamed the following outcome following feedback from the PAG and the views of the second focus groups and research team:
-
Less parental/carer stress changed to Mental health of parent/caregiver.
In response to feedback, the following outcome was added:
-
Efficiency (to incorporate Duration of mealtimes).
One outcome was not taken forward, as it was not considered specific or measurable:
-
Prevention of further difficulties.
One further outcome was not taken forward, as we considered it had been covered by an outcome already included:
-
Vomiting was covered by General health.
A table describing all of the original intervention and outcome terms used in the national survey and the revised terms used in the Delphi survey is included in Appendix 13.
Chapter 10 Aim 3: Delphi survey
Objectives
The objectives were to seek agreement between parents of children with neurodisability and HPs on which interventions and which outcomes are considered to be the most appropriate for young people with neurodisability and EDSD.
Methods
Population
Two UK stakeholder groups were sampled:
-
Parents of children aged up to 12 years with neurodisability and EDSD. Although our focus was on young children aged up to 8 years, broadening the age limit up to 12 years aimed to include parents with adequate recall in relation to the interventions that they had used historically.
-
Health professionals, such as speech and language therapists, occupational therapists, dietitians and paediatricians, working with children and young people (aged 0–18 years) with neurodisability and EDSD. Although the focus of the study was on young children, HPs often work with a wide range of ages and so any HP working within the age span of 0–18 years was included.
Study design
As the aim was to establish consensus, an iterative online Delphi survey was employed. A questionnaire was sent to parents and HPs in two rounds; parents and HPs received the same questionnaire in each round. Both survey rounds were open for 3 weeks, with a week between the two rounds to allow for data analysis. In the first round, respondents were asked to rate the importance of individual intervention categories and outcomes for children with neurodisability who have EDSD. In the second round, respondents were shown the results of the first round in the form of bar charts of both parent and HP ratings of the importance of each intervention to be included as part of a package for children with EDSD, and the importance of each outcome. Respondents were then asked to re-rate the importance of each intervention and outcome in the light of the results from round 1. No items were removed from the survey between round 1 and round 2.
As an incentive, respondents were informed that at the end of the second round of the Delphi survey they would be entered into a prize draw to win one of five £100 vouchers available for each stakeholder group (parents and HPs).
Questionnaire development
The round 1 questionnaire was developed by listing the interventions and outcomes identified from the synthesis of evidence gathered through the systematic and mapping reviews (see Chapters 3 and 4, respectively), the national survey (see Chapter 7) and the second round of focus groups (see Chapter 9). The research team developed the structure and format of the questionnaire, drawing on previous experience of Delphi surveys and with reference to methodological recommendations. 102
The questionnaire comprised (1) demographic characteristics, (2) questions about outcomes that could be used to measure progress in eating, drinking and swallowing and whether or not interventions were effective, and (3) questions about the inclusion of specific interventions that could be delivered at home by parents of young children with neurodisability and EDSD. The majority of questions used fixed-choice rating options, although participants could suggest any additional outcomes or interventions not listed and/or comment through free-text boxes. Respondents were invited to rate how important they thought it was to include each of the 25 discrete interventions as part of a package of treatment for EDSD and their perception of how important they thought each of the 22 outcomes were. Ratings were made on a 9-point scale in which categories were labelled 0–3, ‘not important’; 4–6, ‘important but not essential’; and 7–9, ‘essential’. Respondents were able to tick ‘unable to score’ if they were not able to comment based on their knowledge or experience, or if the item was not relevant or applicable to them. Electronic, web-accessible versions were hosted by Newcastle University using Qualtrics (Provo, UT, USA).
The PAG reviewed the draft survey documents and offered advice about wording and layout. The final online questionnaire was piloted by a small number of parents, HPs and researchers. Respondents were able to use a ‘back’ button to review or change their answers as required and a progress bar told them how far through the questionnaire they were (see Report Supplementary Material 2 for the first round and Report Supplementary Material 3 for the second round).
Procedure
Parents and HPs who took part in the national survey (see Chapter 7) and who had expressed an interest in completing another survey were contacted via e-mail with an invitation to take part in the Delphi survey, and a hyperlink to the online questionnaire. To maximise participation, both respondents and non-respondents from round 1 were invited to take part in round 2.
Analysis
Consensus was conservatively defined as ≥ 67% of both stakeholder groups separately rating that it was essential (rated 7–9 at round 2) to assess an outcome domain or to incorporate intervention in the treatment package. 102
Results
Response
Invitations to participate were sent to 196 parents and 175 HPs (Figure 11). Of these responses, in each round there were:
-
parents –
-
round 1: n = 81/196 (41%)
-
round 2: n = 61/196 (31%)
-
with 52 parents taking part in both rounds.
-
-
HPs –
-
round 1: n = 76/175 (43%)
-
round 2: n = 61/175 (35%)
-
with 51 HPs taking part in both rounds.
-
FIGURE 11.
Flow diagram of Delphi survey recruitment.
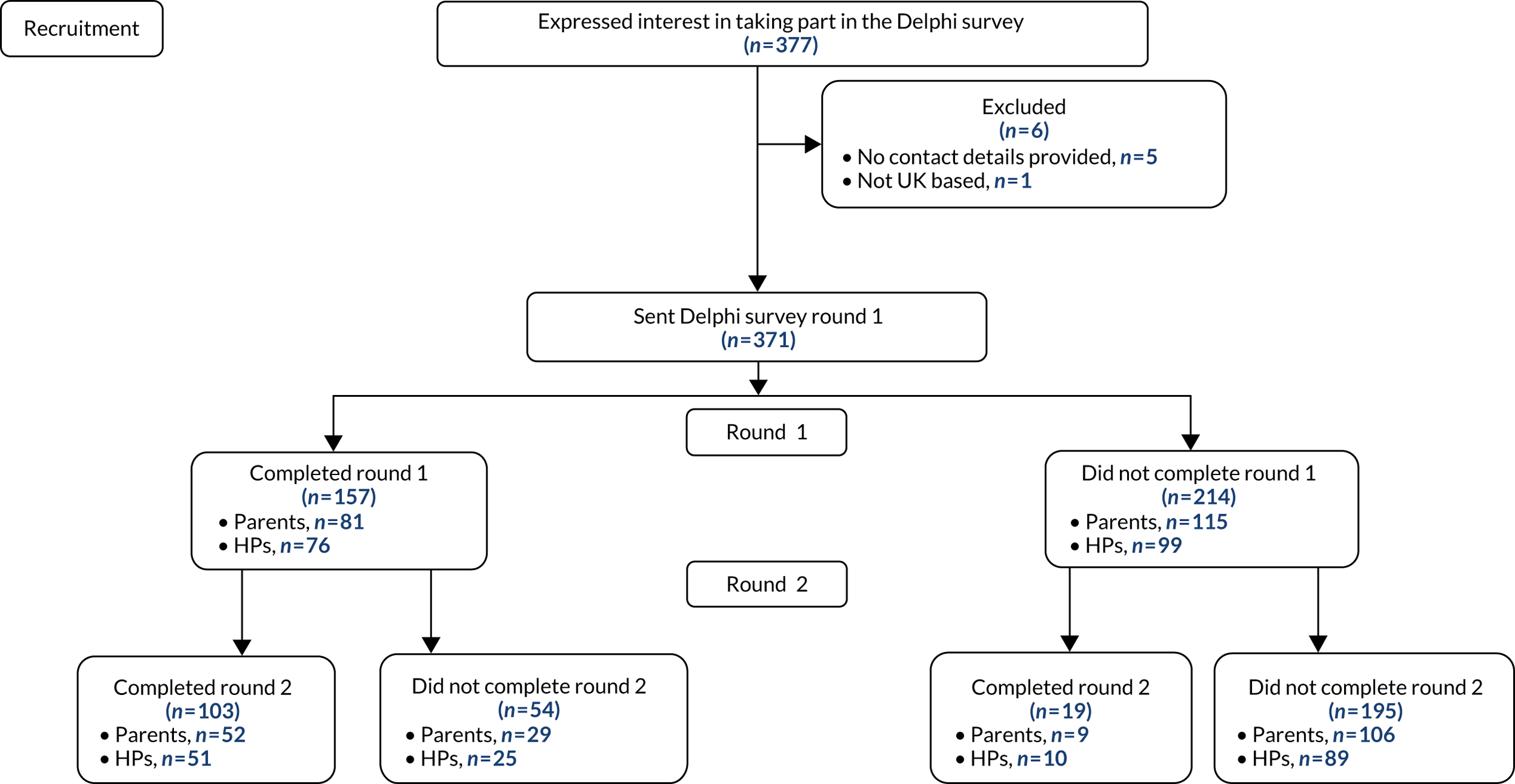
The round 1 HPs comprised:
-
speech and language therapists (n = 29)
-
dietitians (n = 5)
-
occupational therapists (n = 20)
-
physiotherapists (n = 5)
-
clinical psychologist (n = 1)
-
paediatricians (n = 12)
-
gastroenterologist (n = 1)
-
nurses (n = 2)
-
other (n = 1).
The round 2 HPs comprised:
-
speech and language therapists (n = 28)
-
dietitians (n = 3)
-
occupational therapists (n = 14)
-
physiotherapists (n = 2)
-
clinical psychologist (n = 1)
-
paediatricians (n = 10)
-
gastroenterologist (n = 1)
-
nurses (n = 2).
Sample characteristics
Table 20 shows the characteristics of the respondents who completed the first and second rounds of the survey, and the non-respondents. In the first round, the respondents comprised a similar proportion of parents and HPs (49% and 51%, respectively). Respondents were predominantly from England. The majority of respondents were aged 41–50 years (49% of parents; 33% of HPs) and most respondents were female (94% of parents; 92% of HPs). The majority of respondents identified as white British (96% of parents; 92% of HPs). Half of the parents had a child with non-physical EDSD (49%) and half had a child with mixed or physical EDSD (51%). The majority of HPs worked with children with mixed EDSD (75%).
| Characteristic | Round 1 (N = 157), n (%) | Round 2 (N = 122), n (%) | Non-respondents (N = 195), n (%) | |||
|---|---|---|---|---|---|---|
| Parents (N = 81) | HPs (N = 76) | Parents (N = 61) | HPs (N = 61) | Parents (N = 269) | HPs (N = 335) | |
| Age (years) (no missing data) | ||||||
| ≤ 20 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 5 (2) | –a |
| 21–30 | 2 (3) | 8 (11) | 2 (3) | 3 (5) | 23 (9) | –a |
| 31–40 | 32 (40) | 19 (25) | 23 (38) | 17 (28) | 130 (48) | –a |
| 41–50 | 40 (49) | 25 (33) | 32 (53) | 20 (33) | 95 (35) | –a |
| 51–60 | 7 (9) | 22 (29) | 4 (7) | 20 (33) | 14 (5) | –a |
| ≥ 61 | 0 (0) | 2 (3) | 0 (0) | 1 (2) | 2 (1) | –a |
| Gender (no missing data) | ||||||
| Female | 76 (94) | 71 (93) | 58 (95) | 58 (95) | 254 (94) | –a |
| Male | 5 (6) | 4 (5) | 3 (5) | 3 (5) | 15 (6) | –a |
| Prefer not to say | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | –a |
| Location | ||||||
| England | ||||||
| North-east | 14 (17) | 5 (7) | 11 (18) | 7 (12) | 48 (18) | 29 (9) |
| North-west | 8 (10) | 3 (4) | 6 (10) | 3 (5) | 20 (7) | 22 (7) |
| Yorkshire and Humber | 5 (6) | 10 (13) | 2 (3) | 9 (15) | 28 (10) | 49 (15) |
| Midlands | 11 (14) | 16 (21) | 9 (14) | 10 (16) | 66 (25) | 47 (14) |
| South-east, including London | 27 (33) | 26 (34) | 20 (33) | 21 (34) | 56 (21) | 136 (41) |
| South-west | 8 (10) | 8 (11) | 7 (12) | 4 (7) | 29 (11) | 14 (4) |
| Scotland | 3 (4) | 4 (5) | 2 (3) | 5 (8) | 11 (4) | 14 (4) |
| Northern Ireland | 2 (3) | 0 (0) | 2 (3) | 0 (0) | 4 (2) | 11 (3) |
| Wales | 1 (1) | 4 (5) | 1 (2) | 2 (3) | 7 (3) | 13 (4) |
| Missing | 2 (3) | 0 (0) | 1 (2) | 0 (0) | 0 (0) | 0 (0) |
| Ethnicity (no missing data) | ||||||
| White | 78 (96) | 70 (92) | 59 (97) | 55 (90) | 234 (87) | –a |
| Asian/Asian British | 2 (3) | 3 (4) | 0 (0) | 4 (7) | 22 (8) | –a |
| Black/African/Caribbean/black British | 0 (0) | 0 (0) | 1 (2) | 0 (0) | 4 (2) | –a |
| Mixed/multiple ethnic group | 1 (1) | 1 (1) | 1 (2) | 1 (2) | 7 (3) | –a |
| Other ethnic group | 0 (0) | 2 (3) | 0 (0) | 1 (2) | 0 (0) | –a |
| Prefer not to say | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (1) | –a |
| Nature of child’s EDSD | ||||||
| Physical | 14 (17) | 14 (18) | 9 (15) | 13 (21) | 58 (22) | 63 (19) |
| Non-physical | 40 (49) | 5 (7) | 32 (53) | 3 (5) | 141 (52) | 23 (7) |
| Mixed | 27 (33) | 57 (75) | 20 (33) | 45 (74) | 59 (22) | 248 (74) |
| Missing | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 11 (4) | 0 (0) |
In the second round, a similar proportion overall of parents and HPs responded (50% and 50%, respectively). Respondents were predominantly from England. The majority of parents were aged 41–50 years (53%) and the majority of HPs were aged 51–60 years (34%). The majority of respondents were female (95% of parents; 94% of HPs) and most identified as white British (97% of parents; 90% of HPs). Around half of the parents had a child with non-physical EDSD (52%) and half had a child with mixed or physical EDSD (48%). Most HPs worked with children with mixed EDSD (74%).
The characteristics of respondents who completed both rounds of the Delphi survey and those who completed the second round only were very similar apart from age, for which the majority of those completing both rounds were aged 41–50 years (46%), whereas the majority of those completing the second round only were aged 31–40 years (63%). See Appendix 14 for full details of the characteristics of respondents who completed both rounds of the Delphi survey and those who completed round 2 only.
Interventions
Table 21 shows the proportion of parents and HPs who rated interventions as essential in the first and second round of the Delphi survey. The percentage of respondents giving a rating of ‘essential’ (score of 7–9) increased from round 1 to round 2 for most interventions, for both parents and HPs. Consensus was achieved for 17 of the 25 interventions at round 1, increasing to 19 out of the 25 interventions at round 2. There was substantial agreement between the ratings of participants who completed both rounds of the survey and the ratings of participants who completed round 2 only (data not shown).
| Intervention | Round 1 (%) | Round 2 (%) | ||
|---|---|---|---|---|
| Parents (N = 81) | HPs (N = 76) | Parents (N = 61) | HPs (N = 61) | |
| Modifying environment | 67 | 87 | 77 | 95 |
| Positioning | 92 | 97 | 96 | 100 |
| Modifying equipment | 76 | 87 | 93 | 90 |
| Scheduling of meals | 53 | 82 | 50 | 83 |
| Modifying consistency of food or drink | 79 | 86 | 79 | 96 |
| Modifying other aspects of food or drink | 74 | 75 | 86 | 83 |
| Modifying placement of food | 68 | 79 | 75 | 90 |
| Enhancing communication | 76 | 82 | 86 | 90 |
| Visual supports | 52 | 63 | 52 | 72 |
| Responding to a child’s cues for feeding | 83 | 94 | 93 | 96 |
| Pace of feeding | 77 | 96 | 89 | 100 |
| Physical support | 72 | 69 | 82 | 81 |
| Oral and sensory desensitisation | 72 | 68 | 82 | 75 |
| Oral motor exercises | 73 | 40 | 70 | 35 |
| Graded exposure to new food | 66 | 85 | 70 | 84 |
| Graded exposure to new textures | 68 | 81 | 76 | 81 |
| Changing behaviour at mealtimes | 57 | 63 | 58 | 56 |
| Modelling | 80 | 82 | 77 | 83 |
| Training to self-feed | 68 | 47 | 55 | 46 |
| Support for parents | 81 | 84 | 95 | 96 |
| Psychological support for child | 72 | 63 | 77 | 59 |
| Medication | 78 | 86 | 87 | 91 |
| Energy supplements | 62 | 74 | 69 | 73 |
| Sharing information | 90 | 95 | 100 | 97 |
| Vitamin or nutritional supplementsa | 68 | 68 | 85 | 75 |
Figure 12 shows the interventions that were viewed as essential by ≥ 67% of parents and HPs in the second round of the Delphi survey (see Appendix 15 for the interventions viewed as essential by ≥ 67% of parents and HPs in the first round). In the second round, parents and HPs identified that a large number of interventions (n = 19) were an ‘essential’ part of an intervention package for young children with neurodisability and EDSD. These comprised interventions directly targeting EDSD, such as Modifications (Environment, Equipment, Food or drink, Placement of food and Positioning), improved mealtime communication (Enhancing communication strategies, Responding to the child’s cues for feeding and Pace of feeding) and desensitisation strategies (Graded exposure to foods or textures and Oral and sensory desensitisation). Other interventions not specifically targeting the child’s EDSD were also viewed as essential, such as Sharing information about the nature of the child’s difficulties, Medication and Parental support. None of the 25 interventions was viewed as ‘not important’ to include in an intervention package by either parents or HPs. See Appendices 16 and 17 for ratings of ‘not important’, ‘important but not essential’ and ‘essential’ for interventions by parents and HPs in the first and second rounds of the Delphi survey.
FIGURE 12.
Interventions viewed as essential by ≥ 67% of parents and HPs in round 2 of the Delphi survey.
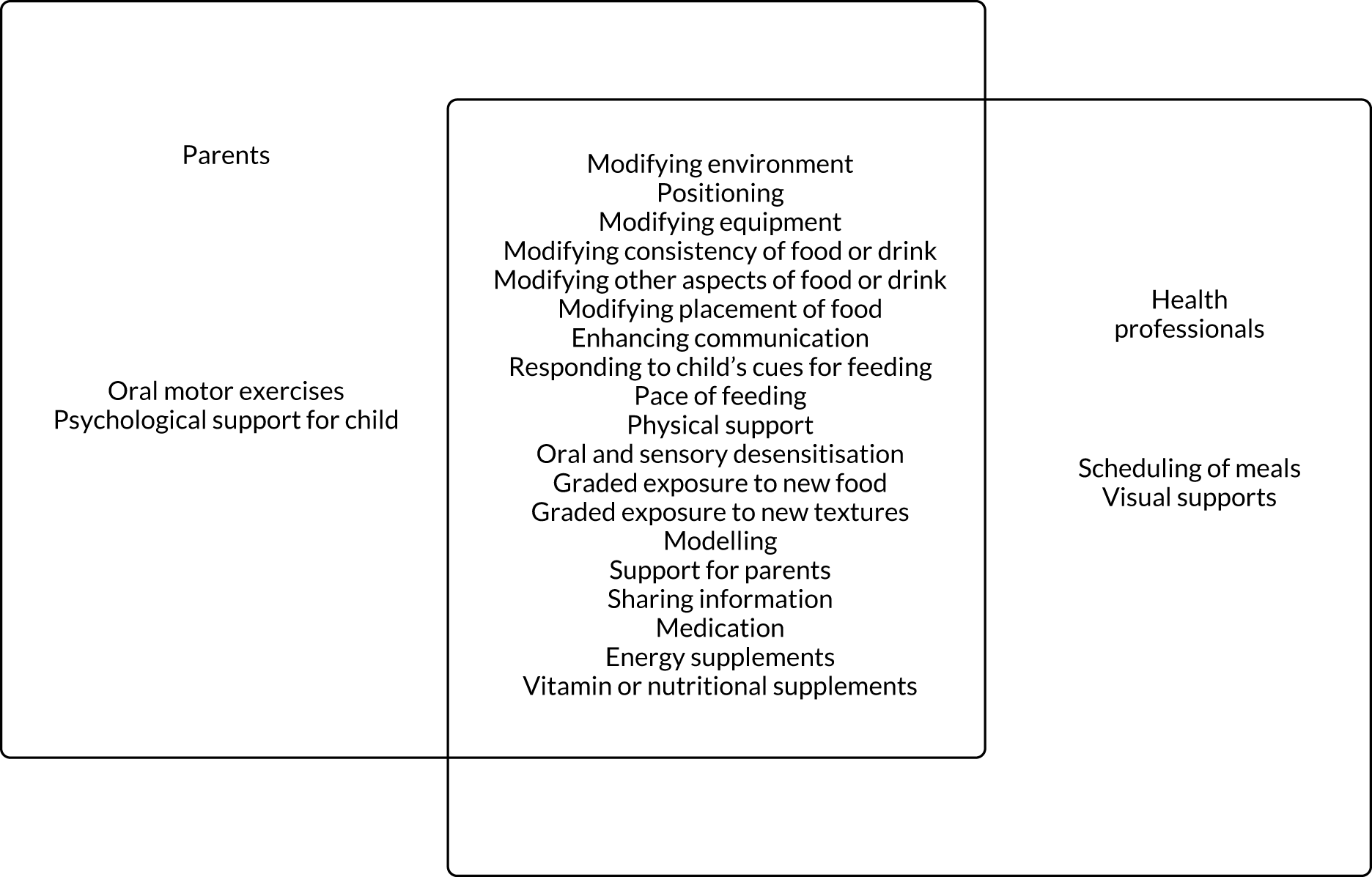
Outcomes
Table 22 shows parents’ and HPs’ agreement on outcomes rated as essential in the first and second rounds of the Delphi survey. In keeping with the findings about interventions, the percentage of respondents rating the outcome as ‘essential’ typically increased between the first and the second rounds. Figure 13 shows the outcomes viewed as essential by ≥ 67% of both parents and HPs in the first and second rounds of the Delphi survey. These are presented together given that the items for which there was consensus did not change between rounds. The 10 outcomes viewed as essential included child-focused outcomes, such as Physical health, Safety, Oral motor control and Quality of life. They also included family-focused outcomes, such as Quality of life of family, Parents’ understanding of the child’s difficulties and Parents’ mental health. None of the 22 outcomes was viewed as ‘not important’ by parents or HPs. See Appendices 18 and 19 for ratings of ‘not important’, ‘important but not essential’ and ‘essential’ on outcomes by parents and HPs in the first and second rounds of the Delphi survey.
| Outcome | Round 1 (%) | Round 2 (%) | ||
|---|---|---|---|---|
| Parents (N = 81) | HPs (N = 76) | Parents (N = 61) | HPs (N = 61) | |
| Nutrition | 89 | 97 | 95 | 98 |
| General health | 89 | 93 | 97 | 98 |
| Weight | 53 | 51 | 34 | 48 |
| Height | 31 | 32 | 12 | 12 |
| Growth | 75 | 76 | 82 | 89 |
| Child’s enjoyment of mealtimes | 83 | 91 | 90 | 98 |
| Parent’s enjoyment of mealtimes | 42 | 76 | 39 | 78 |
| Quality of life of child | 95 | 92 | 98 | 100 |
| Mental health of parent | 83 | 84 | 93 | 97 |
| Safety | 97 | 97 | 100 | 100 |
| Oral motor control | 87 | 74 | 86 | 72 |
| Efficiency | 44 | 60 | 17 | 46 |
| Independence | 60 | 31 | 43 | 28 |
| Variety | 51 | 23 | 26 | 12 |
| Amount | 62 | 40 | 53 | 25 |
| Mealtime Interaction | 61 | 81 | 65 | 79 |
| Social participation | 50 | 77 | 53 | 74 |
| Parent’s understanding of child’s EDSD | 89 | 89 | 95 | 93 |
| Quality of life of familya | 78 | 87 | 90 | 97 |
| Appetitea | 59 | 44 | 46 | 38 |
| Mealtime behavioura | 41 | 30 | 34 | 26 |
| Child’s understanding of mealtimesa | 51 | 51 | 58 | 40 |
FIGURE 13.
Outcomes viewed as essential by over 67% of parents and HPs in round 2 of the Delphi survey.
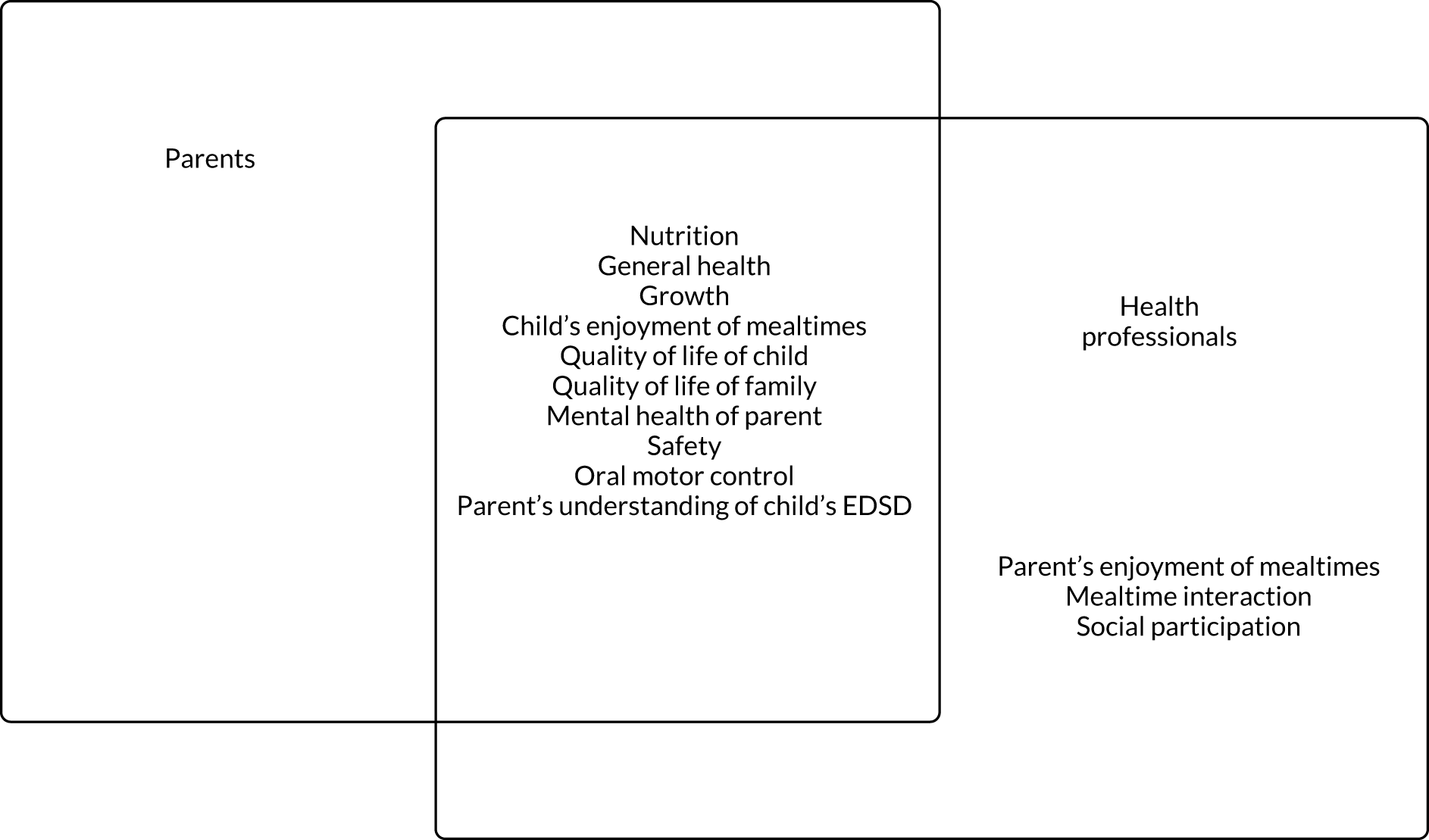
Summary of the Delphi survey findings
The Delphi survey established consensus between parents and HPs on the ‘essential’ interventions to include in an intervention package for young children with neurodisability and EDSD, and the important outcomes. The Delphi survey showed that for both parents and HPs 19 interventions were viewed as ‘essential’ to include in an intervention package and 10 outcomes were viewed as ‘essential’; these focused on both the child and the wider family. Schedule of meals and Visual supports were viewed as ‘essential’ interventions for inclusion in an intervention package by HPs only, and Oral motor exercises and Psychological support for children were viewed as ‘essential’ interventions to include by parents only. Training to self-feed and Changing behaviour at mealtimes were not viewed as ‘essential’ to include in an intervention package. None of the interventions or outcomes included in the Delphi survey was viewed as ‘not important’ to include in an intervention package, reflecting the complexity of neurodisability and highlighting the need to tailor interventions to meet the needs of individual children and their families.
Strengths and limitations of the Delphi survey
We used a wide range of recruitment sources that were also used in the national survey (see Chapter 7), and a reasonable number of respondents to that survey showed willingness to contribute further. This meant that we had information to enable a comparison of characteristics of those who participated and those who did not, and, therefore, the Delphi survey invitation was sent only to previous survey respondents. The overall response for the surveys was typical and acceptable (≈ 40%) for the Delphi approach, and there was little difference in the characteristics of respondents between round 1 and round 2 (see Appendix 14). The response ratings about ‘essential’ interventions and outcomes were very high; therefore, a higher response would have been unlikely to lead to different results. Our consensus definition of 67% was conservative; however, most interventions and outcomes were rated ‘essential’ by a much higher proportion than this. Nonetheless, if we had used a higher percentage to define consensus, our findings would have differed slightly. Contacting non-respondents from round 1 in round 2 increased the responses in round 2 and, therefore, improved precision; although this is not always carried out in Delphi surveys, there was substantial agreement between the ratings from participants who completed both rounds of the survey and those who completed round 2 only.
Patient and public involvement in the Delphi survey
The Delphi survey questionnaires and information sheets were developed by the research team, including the parent co-investigators. These draft documents were reviewed by the PAG who offered advice around content, wording and layout. These documents were further amended to take account of the discussions in the second round of focus groups and these amended versions were reviewed again by the PAG. The PAG advised on the ordering of statements relating to interventions and outcomes for the Delphi survey to ensure that these were presented in an order that made sense to the reader.
How did the Delphi survey findings inform the next study stage?
The Delphi survey findings informed decisions around which interventions and outcomes to take forward for further discussion at the stakeholder consultation workshops (see Chapter 11), and for consideration when designing future research and pragmatic trials.
The Delphi survey identified that a large number of interventions were viewed as essential to consider for improving EDSD in young children with neurodisability. The research team used this information and that gathered previously to make pragmatic decisions about which interventions to include in the toolkit so that they could be selected for use depending on the child and family’s individual needs.
The Delphi survey also showed that a large number of outcomes were valued by parents and HPs, focusing both on the child and the wider family. These essential outcomes were presented at the stakeholder consultation workshops (see Chapter 11) to facilitate further discussion regarding the important primary and secondary outcomes for use in future research and trials.
Chapter 11 Aim 3: stakeholder consultation workshops
Objectives
The objectives were to gauge consensus between HPs and parents of children with neurodisability on the interventions for EDSD, and their outcomes, that should be evaluated in future research, and to agree on frameworks for such research in the NHS.
Methods
Participant recruitment and selection
We sent invitations for the workshops to HPs and parents who took part in the national survey and/or the Delphi survey who had expressed an interest in taking part in subsequent stages of the research, and to members of HP professional networks (via e-mail). Parents who were interested in taking part in the workshops provided their location, the age of their child (preschool, primary school or secondary school) and the nature of their child’s EDSD (physical, non-physical or mixed). HPs stated their profession, the types of EDSD that they worked with (physical, non-physical or mixed) and the geographical location and types of services that they worked in (community, secondary care, tertiary service and education). We purposively selected participants to maximise variation in their experience of EDSD and service provision.
Procedure
We held two half-day workshops: one in Newcastle and one in London. Members of the research team designed and facilitated the workshops. Jeremy Parr, Lindsay Pennington and Helen Taylor led both workshops, with other members of the research team facilitating. The Newcastle workshop was facilitated by Charlotte Buswell, Allan Colver, Deborah Garland, Christopher Morris, Helen McConachie, Johanna Smith and Julian Thomas, and the London workshop was facilitated by Jill Cadwgan, Deborah Garland, Diane Sellers and Johanna Smith. We aimed for detailed discussion about the frameworks of future research, including the PICOTS and the elements of study design that related to feasibility of research in the NHS. At the start of the workshops, workshop leaders presented an overview of the study stages and the findings from the completed stages. Attendees then discussed individual topics in small groups. Most groups contained a mix of parents and HPs. One member of the research team facilitated each small group; notes from the discussions were written on flip charts.
The two workshops were iterative, with the results of the first workshop in Newcastle presented to participants at the second workshop in London. To do this, Helen Taylor summarised the notes from the Newcastle workshop. Jeremy Parr, Lindsay Pennington and Helen Taylor formed bullet points from the summaries to be presented in the small groups at the London workshop.
Topics discussed in the small groups included:
-
Interventions for EDSD. The concept of an intervention ‘toolkit’, which was first proposed and discussed at the second round of focus groups and formalised during subsequent co-applicant meetings, was presented (see Figure 15). The following aspects of a toolkit intervention were discussed –
-
How could the essential interventions identified in the Delphi survey be presented to parents as a list of treatment options?
-
What level of detail would parents need on each intervention?
-
How would a menu of treatment options be individualised?
-
What level of support would families need from HPs to use the toolkit?
-
Ways of measuring outcomes.
-
-
We presented the list of essential outcomes from the Delphi survey. Participants suggested the tools that they knew from professional and personal experience that could be used to measure each outcome, and discussed the pros and cons of each tool. In both workshops, one group of parents considered the three published parent-report measures of Children’s mealtime behaviour, Parent behaviour and Food intake that had been identified as the highest quality in the measurement properties review – BPFAS, PediEAT and a food frequency questionnaire.
-
Designing future research – defining treatment as usual; the concept of the toolkit and its implementation, including feasibility and barriers; unit of randomisation and acceptability of RCTs in EDSD; methods of recruitment and selection.
Parents received a £100 shopping voucher as a thank you for their time and to cover travel costs. Professionals received a £25 shopping voucher to cover their travel costs.
Data processing
Flip chart feedback compiled during the workshop discussions and the notes made at each group formed the data for analysis. Morag Andrew and Lindsay Pennington reviewed all data, identified key themes and presented findings to the full research team for discussion.
Results
A total of 15 parents and 19 HPs took part in the workshops. Nine of the parents had children with physical EDSD, two had children with non-physical EDSD, two had children with mixed EDSD and two had one child with physical EDSD and one child with non-physical EDSD. HP participants comprised speech and language therapists (n = 5), dietitians (n = 4), paediatricians (n = 4), occupational therapists (n = 3), clinical psychologists (n = 2) and a physiotherapist. One member of the NIHR-appointed external steering committee (speech and language therapist) participated in the Newcastle workshop and one (nurse) in the London workshop.
Use of eating, drinking and swallowing difficulty interventions in current clinical practice
Participants reported that multiple EDSD interventions are used in current clinical practice, but in an unstructured and unco-ordinated manner.
Toolkit of interventions
Participants agreed that no single intervention would be suitable for all children with EDSD; for many children several interventions may be delivered concurrently or sequentially. Participants liked the concept of an intervention ‘toolkit’ that parents and HPs could use together to identify the most appropriate interventions for individual children and their families.
Participants agreed that the toolkit should be represented visually and available in digital and hard copy, with interactive properties to aid communication with HPs. They thought that it should be flexible to allow families and teams to individualise intervention selection. Parents thought that some parents would want to have ownership of the toolkit and to be integral to decisions on which interventions were selected for their child. In that context, parents and HPs agreed that thorough information on each available intervention would allow families to share decision-making with HPs.
Support for families using the toolkit
Health professionals thought that toolkit use should be supported by a lead HP (e.g. a speech and language therapist) and MDT. Professional support would be required throughout toolkit use and may include psychological input; however, the nature of the support that was required would vary between families.
Participants raised several issues about the delivery of an intervention toolkit:
-
how to deliver the toolkit to meet the needs of a heterogeneous population with diverse EDSD
-
how to deliver the toolkit in geographical areas in which multidisciplinary EDSD teams are unavailable or under-resourced
-
how to avoid/overcome problems caused by delays in obtaining appropriate equipment (e.g. optimising positioning for feeding) to ensure efficient use of the toolkit
-
how best to deliver the toolkit for children with non-physical EDSD who are not currently linked to HP teams.
Ways to measure outcomes
The focus group and Delphi survey stages identified the important outcomes of any EDSD intervention (see Chapters 9 and 10). These outcomes were organised into three categories for presentation at the workshops:
-
category 1 – improvement in physical aspects of a child’s EDSD
-
Safety
-
General health
-
Nutrition
-
Growth
-
Oral motor control
-
-
category 2 – changes in behaviour of child or parent
-
Child’s behaviour at mealtimes
-
Parental understanding of child’s eating and drinking difficulties
-
-
category 3 – changes in child or family’s well-being
-
Quality of life of child
-
Quality of life of family
-
Parental mental health
-
Participation.
-
The groups discussed potential measures for each EDSD outcome and their strengths and weaknesses in clinical practice. For implementation in research, measures must be acceptable, valid and reliable. Not all of the measures that were suggested by participants fit all three of these criteria. A full list of measures generated in the stakeholder consultation workshop is provided in Appendix 20.
No acceptable measures that have evidence of reliability and validity were identified for Safety (measures discussed were videofluoroscopy, frequency of chest infections, frequency of choking episodes and observation of meals) or General health (use of a structured questionnaire capturing general health information, school attendance, bowel function including medications needed, skin pallor, hair thickness, dental health, hospital admissions or outpatient attendances, energy levels, concentration and sleep). For Nutrition, participants suggested 3-day food diaries; measuring blood micronutrient levels; food frequency questionnaires; body fat percentage using smart scales and the The Eatwell Guide [Public Health England, UK, URL: www.gov.uk/government/publications/the-eatwell-guide (accessed 23 December 2020)] (see Appendix 20); photographs of food at the beginning and end of a meal, including photographs of the floor to assess spillage; and the need for dietary supplements. Participants identified several measures of Growth including skinfold thickness, bioelectrical impedance, weight and height, and upper arm circumference (see Appendix 20 for further information). Several of the measures suggested for Growth and Nutrition have evidence of reliability and validity, but each would require further investigation in terms of their acceptability and validity in children with neurodisability.
As these outcomes had not been included in the measurement properties review, we undertook a brief review of their properties (Box 6).
Nutrition and Growth were considered as important outcomes by parents and HPs alike. As our measurement review did not focus on Nutrition and Growth, we undertook a brief literature review regarding measures for these outcomes. One challenge is that there is a lack of consensus on how best to measure nutritional status in children with neurodisability. Accurate height measurement can be challenging to obtain in many children with neurodisability owing to factors such as inability to stand, limb contractures and scoliosis. BMI (kg/m2) poorly reflects body composition in children with neurological impairment. 103,104 When height measurement cannot be achieved because of physical disability, segmental limb measurements provide an alternative measure of linear growth. The European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN), in its Guidelines for the Evaluation and Treatment of Gastrointestinal and Nutritional Complications in Children With Neurological Impairment,105 recommends that knee height or tibial length be routinely measured in children with neurological impairment when height cannot be obtained. The level and grade of evidence was assessed to be strong for this recommendation; however, there are different views about which measure should be used and whether raw measures or adjusted measures (using cerebral palsy-specific equations) are most valid. 106–109
Measurements of linear growth do not fully reflect body composition and nutritional status, and body composition is altered in some neurodisabling conditions. For example, children with cerebral palsy often have altered percentage fat mass and fat distribution, with higher central than peripheral fat stores. 104 Gold-standard body composition measures – doubly labelled water and dual-energy X-ray absorptiometry – are costly and time-consuming, making them unsuitable for routine clinical practice or as a pragmatic research outcome measure. Skinfold thickness combined with MUAC and BIA is more easily applied in these contexts. Raw measures from these methods are combined with height and weight to estimate body composition using predictive equations. Cerebral palsy-specific equations have been developed to predict body fat based on skinfold thickness. 110 These equations have better validity for children with GMFCS level I or II cerebral palsy, but perform less well in children with GMFCS level III or IV cerebral palsy. 111,112
The MUAC can be combined with triceps skinfold thickness to give a measure of fat mass and fat-free mass; when used in this way MUAC is subject to the same validity issues as skinfold thickness. MUAC does not take account of higher central fat stores in children with cerebral palsy. The ESPGHAN guideline currently recommends routine measurement of fat mass by skinfold thickness in children with neurological impairment, but acknowledges that the level of evidence for this recommendation is moderate and that the grade of evidence is weak. 105
A further technique is BIA, which estimates total body water through the application of a small current via electrodes placed on the feet and hands. As fat-free mass is a good conductor of current and fat mass is a non-conductor, total body water can be estimated from the measured resistance and can be used to predict the amount of fat mass and fat-free mass. Specific equations exist to convert resistance (ohms) to total body water or percentage body fat utilising height and weight data. Results obtained are sensitive to hydration status, which vary widely over time in children with neurodisability, as well as with age and sex. This variation is not accounted for in the hydration factor used in the equations, which is assumed to be static. A recent systematic review of the criterion validity of assessment methods to estimate body composition in children with cerebral palsy confirmed the reliability of the Gurka equation for comparison of percentage body fat between groups of children with GMFCS level I or II cerebral palsy; the equation was less accurate in children with GMFCS level III or V cerebral palsy. 111,112 The limits of agreement were too wide for use on an individual level, making skinfolds a less appropriate outcome measure for nutritional intervention studies assessing individual response over time. 109 For BIA, the Kushner113 and Fjeld114 equations were reliable for preschool children with cerebral palsy at the population level, but wide limits of agreement negated use on an individual level. The Pencharz equation115 was unreliable at the individual or population level. 109 Far less is known about the most appropriate measure of body composition in children with non-cerebral palsy diagnoses. Limited evidence shows wide variation in technical error of measurement in measures of body composition between children attending special school who are able or unable to stand. 116 Further research is needed to inform the choice of appropriate nutritional outcome measure(s) for evaluation of the toolkit, and the use in future studies or clinical trials across children with broad ranging neurodisability. Different outcome measures could be used depending on the nature of the child’s difficulties and what intervention aims to achieve.
BIA, bioelectrical impedance analysis; MUAC, mid-upper arm circumference.
For Oral motor control, participants identified the SOMA, which was included in the measurement properties review.
The measurement properties review showed that the PediEAT and the BPFAS had evidence of validity and reliability as measures of the Child’s enjoyment of meals and Parents’ understanding of child’s eating and drinking difficulties. In the consultation workshop, groups of parents considered these two measures in detail. Parents liked that the PediEAT has clear, specific questions, which feel quick to answer, and that the wording is factual and does not feel judgemental. The tool was deemed likely to identify the main issues and provide sufficient detail to help inform joint goal setting and identify comorbidities. The front page was considered off-putting owing to its length, as it included an outline of intended use, disclaimers and references relevant to the tool. However, the questionnaire was judged as lengthy for use during clinic appointments and better completed during a separate session to enable collection of the most detailed information. Although it is not a measure of participation, parents thought that some items were relevant to participation.
Parents liked the specificity of the BPFAS, but disliked its length. They felt that parents would need an explanation of why it was being used and why it was so long, so that they felt it would be worth the time taken to complete. However, they thought that it would be useful to consider results taken at different time points to review progress using this measure.
Participants did not identify measures of Participation, Quality of life of children or family members or Mental Health; however, generic measures have been validated, such as the CAPE117 and KIDSCREEN,118 that measure children’s Participation and Quality of life, respectively.
Designing future research (including randomisation and recruitment selection)
Usual care
Participants agreed that multiple interventions were recommended by HPs and delivered by parents. However, the way of introducing interventions to families, in terms of the methods of information sharing, the level of detail and the personnel providing the information, was not consistent within or across teams. It was acknowledged that interventions were not introduced in a systematic manner, resulting in inconsistency in treatment as usual.
Intervention delivery
Some participants questioned whether or not a trial of a toolkit of interventions would be necessary, as the toolkit provides a framework for existing practice and may feel onerous if tested within a trial. If a trial was conducted, there was uncertainty about which trial design would be most appropriate; some thought that a RCT may not be appropriate for evaluation of a toolkit containing multiple interventions, selected based on individual need. Case series studies were discussed as a potential alternative study design.
Randomisation
If a RCT design was chosen, participants agreed that randomisation would be acceptable to the majority of families. To avoid contamination between intervention and control arms, it was proposed that cluster randomisation by geographical area/region/service would be more appropriate than randomisation on an individual patient basis.
Population
Participants discussed that a future trial could include all children with EDSD (physical, mixed and non-physical EDSD), or focus on a specific condition or type of feeding difficulty (physical and mixed EDSD or non-physical EDSD). If the trial population included all children with EDSD, stratification by EDSD type or by diagnostic category was suggested. There was some support for focusing on a particular age group (e.g. preschool children), as this may reduce variability in service provision, for example access to therapy services for those children attending special school compared with those attending mainstream school.
Role of tertiary services in trial delivery
It was acknowledged that tertiary services cover large geographical areas with potentially important variability in resource. As tertiary services do not typically lead local delivery of therapy interventions, participants thought that tertiary teams should not lead the use of toolkit interventions directly.
Participant selection
A number of participant selection processes were discussed, including clear inclusion/exclusion criteria. Interviewing was discussed as a possible way of assessing family readiness for trial participation. Participants advised that careful consideration should be given to avoid selection bias.
Recruitment strategy
Participants thought that multiple recruitment sources would need to be considered, and may include:
-
health – health visitors, GPs, nursery nurses, allied health professionals, community nurses, paediatricians, feeding clinics/teams and dysphagia clinics
-
research registers (e.g. ASD-UK)
-
education (including home education communities) – special schools, additionally resourced schools, mainstream schools and independent schools
-
charities [e.g. National Autistic Society, Bliss (London, UK), SCOPE and Mencap (London, UK)]
-
local parent/carer groups – raising awareness and identification.
Potential barriers to recruitment
Identification of participants
Participants highlighted that a number of eligible children will not have been formally identified to have EDSD, and may not have recent input from HP teams (e.g. children with ASD prior to diagnosis or children with an ASD diagnosis whose EDSD have not been formally identified and are not under follow-up). In these circumstances, participants thought that staff in education settings may be well placed to identify eligible children.
Agreement of clinical services to participate
Participants thought that HPs may perceive trial participation as a burden on limited time and that the trial could generate an increase in referrals to feeding teams, particularly for children with non-physical EDSD. Current service capacity and treatment costs for any trial would need to be carefully considered.
Parent/carer and child factors
Parental stress, language and literacy, information technology skills and access, parents with additional needs and comorbidities affecting engagement in research were identified as additional potential recruitment barriers.
Challenges to intervention delivery
Participants thought that any trial must be deliverable within the current NHS infrastructure. The challenge of managing local resource variability within future intervention trials was highlighted. Equipment waiting times may also affect delivery of planned interventions. Further challenges may be faced where children attend school out of borough and have a lack of funded local therapy support for home-delivered interventions.
How would services manage the potential increase in referrals?
Participants thought that the actual increase in referrals to feeding teams would probably be small; it would be necessary to stress the potential benefits associated with trial participation to trusts to ensure engagement. It was suggested that trial-related (but clinically relevant) training and resources could be put in place to provide lasting benefit throughout participation.
Summary
The stakeholder consultation workshops showed consistent support from parents and HPs for the concept of a toolkit of interventions that could be worked through by HPs and parents. There was debate about how a toolkit might be best presented. The parents and HPs in the consultation workshops agreed with most of the outcomes that were deemed essential by the Delphi survey participants: Safety, General health, Nutrition, Oral motor control, Growth, Children’s enjoyment of mealtimes (incorporating reduced frustration and distress and associated behaviours), Parental understanding of child’s eating and drinking difficulties, Quality of life of child, Quality of life of family, Parental mental health and Children’s social participation. Ways of measuring most of these outcomes were proposed. Parents also endorsed the Pedi-EAT tool, which was found to have the strongest measurement properties (see Chapter 6). Parents and HPs felt that a trial of a toolkit could be achieved and supported a cluster design in which services were allocated to implement the toolkit or treatment as usual. However, treatment as usual would need defining in comparator group services, as this varies considerably. Participants also identified challenges to the identification and recruitment of participants for future NHS research, including increased referrals to services if the trial was advertised within trusts.
Patient and public involvement in the stakeholder consultation workshops
The parent co-investigators were involved in designing and delivering the stakeholder consultation workshops. The PAG reviewed the materials to be shown to parents and professionals, and commented on the structure of the workshops and the timings of the individual tasks. Changes to the layout and wording were subsequently made to the slide presentation summarising the study and key findings, and a document was created to send out to all attendees prior to the workshops to help provide a background to the study and clarity on what would happen on the day.
Strengths and limitations of the stakeholder consultation workshops
Consultation workshop strengths include participation of both parents and HPs across two diverse geographical areas. Parent/carers of young people with physical, non-physical and mixed EDSD participated, representing a broad range of EDSD experiences. Parent/carers had accessed secondary or secondary and tertiary-level EDSD services. Multidisciplinary professional representation was achieved during both workshops, with participation from HPs working in secondary and tertiary feeding services. The iterative nature of the workshops facilitated collection of detailed information on the topics discussed. We decided not to include young people with EDSD in the consultation workshops because of the abstract nature for older respondents of interventions for young children. This could be considered a limitation of the workshops; however, young people gave their opinion about outcomes during separate young people’s focus groups.
How did the stakeholder consultation workshops inform the next study stage?
The opinions of young people with EDSD on the outcomes identified as important to parents and HPs during the two stakeholder consultation workshops were sought during two young people’s focus groups (see Chapter 12).
Output from the stakeholder consultation workshops was used to generate recommendations, specifically those concerning the further development of an intervention toolkit, outcome measures and the design of future research to establish the effectiveness of an EDSD intervention toolkit.
Chapter 12 Aim 2: young people’s focus groups
Objective
The objective was to establish which of the outcomes of interventions for EDSD that were identified as important by parents and professionals were most meaningful to young people with neurodisability.
Methods
Two focus groups with young people with neurodisability who had EDSD, or had EDSD previously, were conducted. There was one focus group for young people with physical or mixed EDSD and one focus group for young people with non-physical EDSD.
Participant recruitment and selection
For focus group 1, seven young people with physical EDSD who were cognitively able to participate in the focus group were identified by a senior member of their local speech and language therapy team. Invitation letters were sent out to parents, followed by a telephone call to non-respondent parents to ascertain their willingness to participate. A familiar communication partner for each young person was also invited to attend the group.
For focus group 2, the focus group was advertised via a social group for young people with autism. Individuals who were aged 12–16 years with non-physical EDSD were invited to attend. The leader of the social group confirmed that participants were cognitively able to participate in the focus group. Each young person was accompanied by a parent.
Parental informed consent and participant assent was obtained for participants aged < 16 years. Informed consent was obtained from participants aged ≥ 16 years.
Procedure
The procedure for both focus groups was identical. One member of the research team (MA) led a 1-hour workshop in a room familiar to the participants. She presented images representing the outcomes identified as essential in the Delphi survey, printed on A4 paper. Figure 14 shows an example.
FIGURE 14.
Growth outcome image.

Morag Andrew presented two practice examples (friends and screen time) before the proposed outcome images; young people confirmed that they understood the process. Along with each of the outcome images, Morag Andrew provided an explanation of each of the nine outcomes (outcomes were described rather than named); Parent mental health was not considered appropriate to be discussed. One outcome at a time, Morag Andrew gave individual participants A5 images that matched each A4 outcome image. She placed three large A1 poster sheets headed ‘Very important’, ‘In the middle’ and ‘Less important’ on the wall in clear view of the participants. Morag Andrew repeated the description of the outcome, then asked the participants, in turn, to indicate whether they considered the outcome ‘Very important’, ‘In the middle’ or ‘Less important’. Morag Andrew presented the outcomes in the following order, using the given descriptor:
-
Growth – ‘a change in a child’s growth, including how much they weigh/how heavy they are and how tall they are’.
-
Health – ‘children are more healthy, feel ill less often’.
-
Safety – ‘children are able to eat and drink without choking or food going down the wrong way’.
-
Nutrition – ‘making sure that the foods children eat can give them enough energy and vitamins so that children can grow and stay healthy’.
-
Oral motor control – ‘children are able to control the movement of the mouth, jaw, tongue or lips, and swallow’.
-
Parental understanding of EDSD – ‘parents’ understanding of why eating and drinking may be hard for their child’.
-
Enjoyment of mealtimes – ‘child’s enjoyment of mealtimes at home and school’.
-
Quality of life of young person – ‘how happy a child is with their life’.
-
Quality of life of family – ‘how happy other members of the family are with their lives’.
Once each outcome had been assigned to an importance category, Morag Andrew took the poster sheets down and replaced them with new poster sheets entitled ‘Most important’ and ‘Very important’. Morag Andrew displayed all of the outcomes previously identified by participants as ‘Very important’ on the relevant poster sheet. She then asked participants to consider which of the ‘Very important’ outcomes were ‘Most important’. Morag Andrew moved the A5 images of the outcomes selected as ‘Most important’ to the ‘Most important’ poster sheet. She audio-recorded the focus groups and photographed the poster sheets so that the information collected could be checked or verified. Participants received a £50 shopping voucher as a thank you for their contribution.
Results
In focus group 1, four female participants aged 14, 15, 16 and 18 years agreed to participate. Three of the participants used Voice Output Communication Aid communication systems and one participant used verbal communication. All of the participants were supported by a familiar communication partner. Three of the participants regularly participated in small group work together. All participants had physical EDSD; none had mixed EDSD. All four young people had EDSD and fed orally; two participants had enteral feeding tubes to support intake but maintained partial oral feeding. All participants had current EDSD.
In focus group 2, six young people with autism and non-physical EDSD agreed to take part: four males and two females aged 12–15 years. Two participants also had selective mutism and gave their responses by writing on individual white boards. All young people had or previously had non-physical EDSD; none had mixed EDSD. All participants fed orally, without enteral feeding tube support. All participants were recruited on the criterion of recent or current EDSD, but we did not receive information on the current EDSD status.
Table 23 shows how young people from the focus groups rated outcome importance.
| Outcome | Physical EDSD (n) | Non-physical EDSD (n) | ||||
|---|---|---|---|---|---|---|
| Very important | In the middle | Less important | Very important | In the middle | Less important | |
| Growth | 4 | 0 | 0 | 1 | 4 | 1 |
| Health | 4 | 0 | 0 | 3 | 1 | 2 |
| Safety | 4 | 0 | 0 | 1 | 4 | 1 |
| Nutrition | 4 | 0 | 0 | 2 | 1 | 3 |
| Oral motor control | 4 | 0 | 0 | 3 | 1 | 2 |
| Parental understanding of EDSDa | 4 | 0 | 0 | 1 | 2 | 2 |
| Enjoyment of mealtimes | 2 | 2 | 0 | 1 | 2 | 3 |
| Quality of life of the young person | 4 | 0 | 0 | 3 | 2 | 1 |
| Quality of life of the familyb | 4 | 0 | 0 | 2 | 2 | 0 |
Young people with physical EDSD unanimously agreed that Safety was the ‘Most important’ outcome, closely followed by Nutrition. Participants also selected Oral motor control, Quality of life of young person and Health as being additional ‘Most important’ outcomes, in the order given. One young person with non-physical EDSD chose not to rate the most important outcome. The remaining five young people with non-physical EDSD selected Quality of life of young person (n = 2), Safety (n = 2) and Health (n = 1) as the ‘Most important’ outcomes.
Summary of the young people’s focus groups
For young people with physical EDSD, Safety and Nutrition were the most important outcomes. For young people with non-physical EDSD, Quality of life of the young person, Safety and Health were the most important outcomes.
Strengths and limitations of the young people’s focus groups
The inclusion of young people with physical and non-physical EDSD was a strength of the focus groups. Insufficient time to fully explore the rationale driving participant outcome selection was a limitation. The age range of the two focus groups differed slightly: participants in focus group 1 were slightly older than participants in focus group 2, which may have affected participant responses. Focus group 1 comprised females only; sex may have contributed to the responses obtained. The young people’s focus groups were all held in north-east England; additional data from focus groups in other regions of England would be valuable to establish any differences of opinion of young people living in socially and economically diverse areas of the country.
Patient and public involvement in young people’s focus groups
The parent co-investigators helped liaise with schools and local community support groups to recruit the young people.
How did the young people’s focus groups inform the next study stage?
The ratings of the importance of outcomes from the young people’s focus groups were considered by the research team alongside the information gathered in the stakeholder consultation workshops (see Chapter 11), and informed the recommendations of future research and trial design.
Chapter 13 Final outline of the FEEDS toolkit of interventions
Taking account of the results and conclusions from all of the FEEDS study stages, the final outline of the FEEDS toolkit of interventions was created. The FEEDS toolkit, as shown in Figure 15, comprises overarching principles of clinical care (shown in the dark blue box) that are relevant to all children and families and influence EDSD interventions (shown in light blue, orange and light orange).
FIGURE 15.
Final outline of FEEDS toolkit of interventions. © Newcastle University 2020.
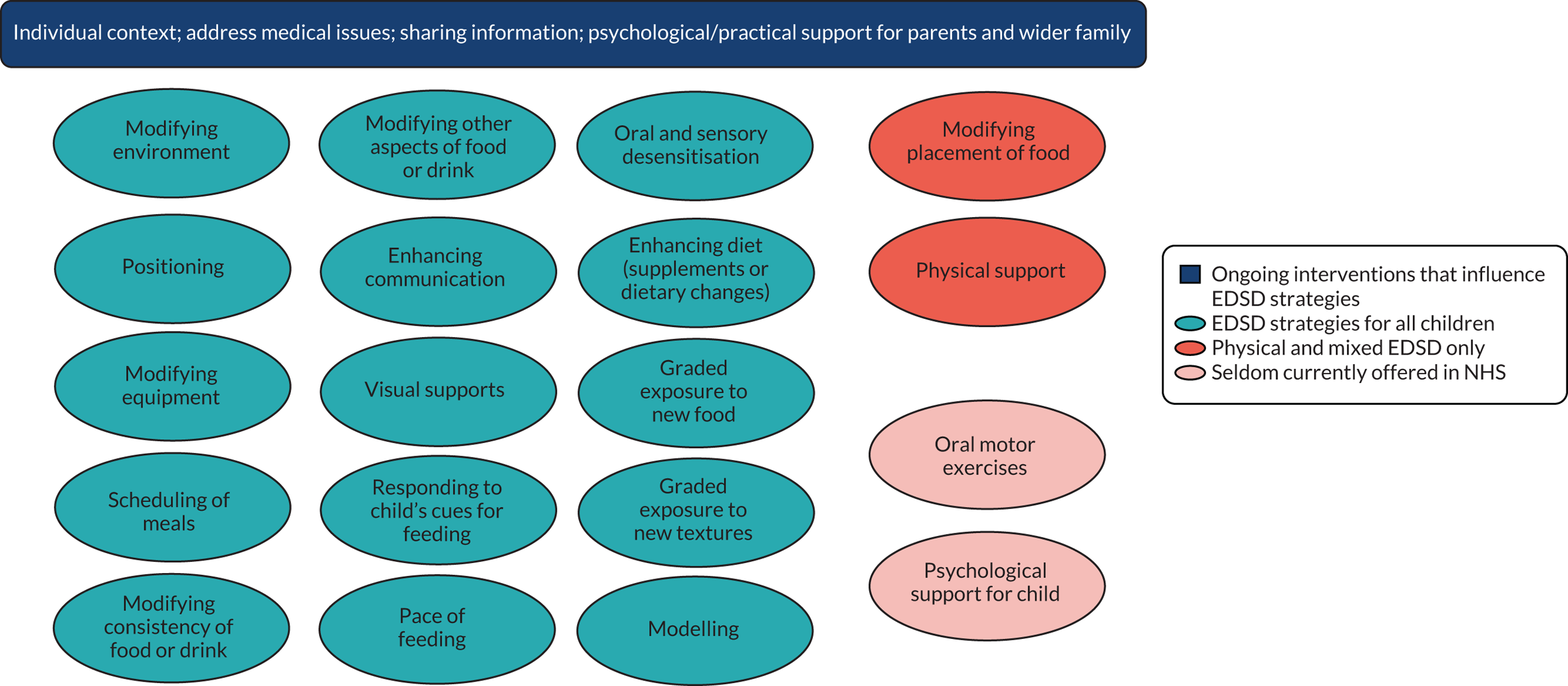
Chapter 14 Discussion
The FEEDS study is the first internationally to generate comprehensive information about how clinicians, researchers and parents might introduce and assess interventions delivered by parents at home to improve the EDSD of young children with neurodisability. The FEEDS study included systematic and mapping reviews of the literature and other evidence, and involved primary research with almost 1000 participants. The study incorporated the views of a wide range of stakeholders and, importantly, considered the needs of children with physical EDSD, non-physical EDSD and mixed EDSD. This means that the study findings are relevant to most children with neurodisability and EDSD. The research findings are relevant to an international audience of clinicians, researchers and parents, can be used to support service development for parents and children with neurodisability, and can be used by funders to consider whether or not further research should be commissioned, including trials of interventions.
The FEEDS study identified a range of interventions that have sufficient clinical and research evidence to warrant their use clinically and/or further evaluation. HPs and parents agreed that the proposed FEEDS toolkit of interventions would be a useful framework for HPs to use with parents to identify together the priority areas for them to work on to address a child’s EDSD. Not all of the toolkit interventions are applicable for all children. The order in which interventions are delivered, and their individual relevance, depends on the child and families personal circumstances, for example the child’s clinical condition, ability to follow instructions (cognitive ability), motivation to engage in intervention and motor disorder and function. Some interventions may need to be prioritised; for example, Positioning to maximise the safety of swallowing might be the first intervention for a child with cerebral palsy. Some interventions have been included because there is a clear clinical rationale to do so, despite limited research evidence. Some interventions were included in this version of the toolkit despite being rarely recommended or used in the NHS (e.g. Oral motor exercises and Psychological support for the child) as there is limited research evidence for their use but strong support from parents.
The aim of the FEEDS review was to identify whether one or more interventions should be evaluated in pragmatic evaluative trials. Additional development of the FEEDS toolkit (e.g. manualisation, optimisation of delivery strategies and piloting) is required prior to its use in clinical services or pragmatic trials. 119 Following piloting, initial evaluation through case series with individualised areas of outcome measurement may be an appropriate development step. At the FEEDS workshops, parents and HPs (end-user representatives) identified a number of ideas for the design and development of the toolkit, including creation of a web-based version that could become part of the clinical notes and have interactive elements to facilitate the recording process. Parents, HPs and the research team thought that the toolkit had interventions that were relevant for children with physical, non-physical and mixed EDSD, and that the order that these would be delivered in could be prioritised by parents and HPs through shared decision-making. As many parents reported that they have to learn about interventions from a range of sources (e.g. online or from other parents), information about what interventions are available may reduce parents’ feelings that access to interventions are ‘restricted’ and improve parents’ sense of control. Practical and psychosocial support for families would be required alongside or as part of toolkit delivery, perhaps through a key worker or lead professional model. Innovative technology-based solutions should be considered alongside HP support.
The delivery of all interventions for children with neurodisability needs to be seen in the context of the number of children requiring them and the current clinical capacity of the MDT. In parallel with toolkit development, it is necessary to evaluate whether or not there are sufficient numbers of therapists and psychologists with appropriate expertise available in local teams to deliver the toolkit, as well as various other interventions that aim to improve function in different developmental domains and social participation. Taking speech and language therapists as one example, an intervention may focus on speech, language and communication, in addition to eating and drinking; the same speech and language therapist may be responsible for intervention in both of these developmental areas and, therefore, the time they have available to support all of the interventions would be split. Current provision (treatment as usual) was very variable around the UK, depending on the availability of clinical staff. In some areas, there would be a substantial difference between the delivery of the FEEDS toolkit and what is provided in usual clinical care. We found that paediatricians and speech and language therapists were the staff group who were most available to support parents; there was less availability of dietitians, occupational therapists and clinical psychologists. This was particularly evident when considering the availability of these HPs to support parents of young children with non-physical EDSD: few parents reported receiving adequate support. In the focus groups and workshops, HPs suggested that, outside a small number of tertiary services for young children with non-physical EDSD, provision was very limited. Whether or not it is effective, intensive intervention for young children with non-physical EDSD may not be widely deliverable in some health services because of therapist/clinical psychologist capacity (number of children overall and number of professionals with the skills to deliver the intervention). In these contexts, research to evaluate how professionals would work together to deliver the toolkit in partnership with parents and the amount of time taken (in comparison with that usually available) should be a focus of future research. If there was evidence of feasibility and acceptability of delivery, economic evaluation would be required, ideally focused on toolkit use with young children following early recognition of their EDSD and considering parent, HP and education professional costs and savings compared with usual care.
Outcomes and measurement
We identified substantial agreement between parents and professionals in relation to the most important outcomes that should be measured in trials; young people also agreed with many of these. We do not think that there is a clear outcome area or measurement tool that could be currently recommended for use as a singular primary outcome. The ‘medical outcomes’, such as Safety and Growth, and those relating to the International Classification of Functioning Disability and Health, such as Social participation of the child, were widely endorsed and could be measured in future trials. 12
Families of children with neurodisability and the HPs who work with them seek interventions that are effective. They require evidence from research to inform decisions about which treatments to choose to invest in. As with most areas of health care, ‘evidence’ about effective interventions for children with neurodisability has emerged largely from clinical practice rather than from high-quality research. The James Lind Alliance Priority Setting Partnership conceptualised and funded by the British Academy of Childhood Disability11 identified priority areas that require higher-quality evidence; this influenced the commissioning of the FEEDS study. Our findings about provision, interventions and outcomes are directly relevant to current clinical care, and may influence current UK and international clinical practice and UK NICE guidelines. 6,9,10
Strengths of our study
The strengths of our component studies have been described in their individual chapters and are not repeated here. We consider that the main strengths were as follows.
The project used an iterative research process, with each phase of the work informing subsequent data collection and interpretation. We were able to use the findings from recently published systematic reviews of interventions to guide our research. 4–6 The published systematic reviews of interventions and the updates we undertook, and the mapping and measurement properties reviews, comprehensively identified the evidence base and found how limited the evidence is.
We recruited > 900 parents, young people and health and education professionals from across the UK. This meant that a wide range of views were identified from people with lived experience and expertise in physical and non-physical EDSD.
The focus groups, workshops and discussions with young people all gave face validity to the findings from the survey stages and overall results, and participants in those elements of the research expressed strikingly similar views about the direction for clinical development and future research. Thus, the results are likely to be widely applicable beyond the UK in countries with similar health service resources.
Limitations of our study
The limitations of our study components have been described in the individual chapters and are not repeated here. We consider that the main broader limitations were as follows.
Participants were not representative of the UK population as a whole. Evidence from children with disorders causing progressive neurodisability were not included in the study. Most study participants were female, white British and from England. The views of parents from an ethnic minority and the views of fathers were not well represented. We did not investigate the socioeconomic status of participants.
Despite the project being about young children, few parents of children aged < 5 years participated, with most being parents of children of primary school age. Recall bias may, therefore, have distorted some parents’ views, as expressed in the surveys and focus groups. On the other hand, the lived experience throughout their child’s early life lends validity to parental perspectives. Owing to the nature of the sampling frame for the national survey, it was not possible to investigate the extent or nature of non-response bias.
Chapter 15 Conclusions and recommendations
Conclusions
Parents and HPs reached consensus on 19 interventions and 10 outcomes as being ‘essential’. Across all strands of the research, we established that no single, standalone intervention is likely to be appropriate, effective or acceptable to parents and professionals in supporting children with neurodisability and EDSD. Therefore, evaluation of a specific intervention as a discrete entity in a RCT with a large sample of children with neurodisability is unlikely to be useful. Multiple interventions need to be used in combination, taking into account the underlying causes of the child’s EDSD, their individual needs and intervention goals. HPs and parents appear enthusiastic about the idea of the proposed FEEDS toolkit of interventions, which professionals could use in partnership with parents to identify and agree priority areas to address for a particular EDSD and to tailor the choice of interventions. Both stakeholder groups made useful suggestions for the development of the toolkit, including creation of a web-based version that could become part of the clinical notes and have interactive elements to facilitate recording. We believe that development and optimisation of the FEEDS toolkit is a prerequisite to any future deployment and evaluation thereof in, for example, pragmatic trials. This development work should be operationalised as a complex intervention, taking account of constituent content, delivery strategies considering fidelity of delivery and acceptability, sustainability of implementation and manualisation. Use of a toolkit approach in clinical practice needs to be informed by theories and models of behaviour change. A possible barrier to delivery of a novel intervention (and indeed standard care) is limited therapist and clinical psychologist capacity in terms of both the staff–child ratio and the skill base of professionals.
Our findings suggest that conducting a RCT at this stage may be challenging. We conclude that a development study should first be undertaken in which feasibility and acceptability of the toolkit, and primary and secondary outcomes and their measures, are investigated further. Subsequently, a clinical implementation study or RCT would be appropriate and achievable and lead to rigorous evaluation of the effectiveness of the toolkit.
Recommendations
-
Future research should evaluate whether or not a combination of interventions can be delivered effectively (sequentially or in parallel). Some of the interventions we identified are standard practices for many HPs. These already have clinical evidence of effectiveness (e.g. Positioning and Food modification). Therefore, if other discrete interventions were to be evaluated (e.g. Oral motor exercises and Psychological support for the child), they would have to be delivered in combination with standard practices.
-
Our proposed FEEDS toolkit requires further development in the context of guidance for complex interventions, optimisation and manualisation, including delivery strategies and media used (paper or electronic/web-based versions). The toolkit will include a menu of potential strategies and interventions, a brief description of what they mean to enable shared decision-making and signposting to more information or the appropriate local professional to contact.
-
Use of the FEEDS toolkit of interventions in clinical practice needs to be informed by theories and models of behaviour change, including identification of barriers to and facilitators of different care pathways and MDT provision. This would need to draw on expertise of health psychology colleagues and implementation scientists, who also draw on sociological and organisational theories of change.
-
Evaluation of the acceptability and feasibility of using the FEEDS toolkit in clinical practice for shared decision-making about selecting interventions collaboratively with professionals and parents is required. Acceptability and adherence in the home, school and other environments by parents and carers requires investigation.
-
Consideration should be given to a trial or other evaluative study of the FEEDS toolkit. Development work would be needed to operationalise the toolkit and investigate whether or not it can be delivered with fidelity and acceptability. If evaluation of intervention with a toolkit was considered possible in a trial, it would be challenging to determine and operationalise the control/comparator group, given the variability of what constitutes treatment as usual. Thus, in addition to a RCT, other evaluative research methods should be considered. A development study, followed by a clinical implementation or effectiveness/implementation hybrid design study, may be appropriate.
-
Further research is needed to identify the most appropriate tools to be used to measure the most valued outcomes. On the basis of work so far, we do not yet recommend a single primary outcome for any study: the outcomes would depend on individualised focus of change. The most appropriate and robust broad parent-reported outcome measurement tool to assess child feeding difficulties and mealtime behaviours for children with EDSD is the PediEAT questionnaire.
-
To underpin further evaluation research in this area, it would be desirable to have a consensus-agreed core outcomes set for young children with neurodisability and EDSD. Core Outcome Measures in Effectiveness Trials (COMET) methodology would provide the obvious methodology to follow for this process building incrementally on the work already achieved in the FEEDS study (URL: www.comet-initiative.org, accessed 17 December 2020).
Acknowledgements
We thank the following for their contributions to the study.
The young people, parents and professionals who participated in the consultation activities and research. The PAG members, Rachel Fay, Sally Walker, Rozita Mohamad and Mark Baston, for advising on the methods, procedures, analysis and dissemination of each element of the research design. Catherine Gibb for her contribution to project design and the funding application. Angela Morgan and Jeanne Marshall for sharing information about their systematic reviews. The Newcastle University staff: Fiona Beyer (Information Specialist), Chinyereugo Umemneku (systematic reviewer), Carla Black, Deborah Jones, Faye Wolstenhulme and Donna Armstrong (administrators). The research sponsor, Research and Development, Newcastle upon Tyne NHS Foundation Trust (in particular Aaron Jackson) and the staff at Percy Hedley School.
We would also like to thank the following NHS trusts for encouraging research participation: Airedale NHS Foundation Trust; Alder Hey Children’s NHS Foundation Trust; Birmingham Community Healthcare NHS Trust; Bradford Teaching Hospitals NHS Foundation Trust; Burton Hospitals NHS Foundation Trust; Calderdale and Huddersfield NHS Foundation Trust; Cambridgeshire and Peterborough NHS Foundation Trust; Cambridgeshire Community Services NHS Trust; Cumbria Partnership NHS Foundation Trust; Doncaster and Bassetlaw Teaching Hospitals NHS Foundation Trust; East Lancashire Hospitals NHS Trust; Leeds Community Healthcare NHS Trust; Leicestershire Partnership NHS Trust; The Mid Yorkshire Hospitals NHS Trust; Rotherham, Doncaster and South Humber NHS Foundation Trust; Sheffield Children’s NHS Foundation Trust; Midlands Partnership NHS Foundation Trust; The Royal Wolverhampton NHS Trust; University Hospital of North Midlands NHS Trust; University Hospitals of Morecambe Bay NHS Foundation Trust; Walsall Healthcare NHS Trust; Warrington and Halton Hospitals NHS Foundation Trust; Worcestershire Health and Care NHS Trust; and Wye Valley NHS Trust.
We would also like to thank the National Institute for Health Research Clinical Research Network.
We would like to thank the staff from the following organisations for distributing information about the study: Northeast Special Needs Network (Newcastle upon Tyne, UK); the Council for Disabled Children (London, UK); Cerebra (Carmarthen, UK); Contact (London, UK); the National Autistic Society (London, UK); the Down’s Syndrome Association (Teddington, UK); Down’s Syndrome North East (Newton Aycliffe, UK); Cerebral Palsy UK (London, UK); Action Cerebral Palsy (Bichester, UK); SCOPE (London, UK); Skills for People (Newcastle upon Tyne, UK); the Toby Henderson Trust (Bedlington, UK); National Network of Parent Carer Forums (London, UK); the British Association of Community Child Health (London, UK); the Royal College of Speech and Language Therapists (London, UK); the British Dietetic Association (Birmingham, UK); the British Society of Paediatric Gastroenterology, Hepatology and Nutrition; the College of Occupational Therapy (London, UK); the Chartered Society of Physiotherapists (London, UK); North East Dysphagia Clinical Excellence Network. In particular, Kelly Robinson and the British Academy of Childhood Disability (London, UK) CDT database review committee, and the Autism Spectrum Database-UK (ASD-UK) (Newcastle upon Tyne, UK) research committee members.
We would also like to thank the External Steering Committee members: Professor Bryony Beresford (Chairperson and Co-Director of Social Policy Research Unit, University of York), Amanda Allard (Assistant Director, National Children’s Bureau), Helen Cockerill (Senior Consultant in Speech and Language Therapy, Guy’s and St Thomas’ NHS Foundation Trust), Veronica Kelly (Consultant in Paediatric Neurodisability, Guy’s and St Thomas’ NHS Foundation Trust) and Sue Kirk (Professor of Family and Child Health, University of Manchester).
We also need to thank Roderick Delanougerede, Research Manager from NIHR, for his support.
Contributions of authors
Jeremy Parr (https://orcid.org/0000-0002-2507-7878) (Professor, Paediatric Neurodisability, Consultant, Paediatric Neurodisability and Study Lead Applicant) was chief investigator, co-led the design and delivery of the project; participated in all project stages; led on the survey and Delphi survey; supervised quantitative data analysis; wrote sections of the report; and finalised the report.
Lindsay Pennington (https://orcid.org/0000-0002-4540-2586) (Reader, Communication Disorders, Speech and Language Therapist and Co-Investigator) co-led the project with Jeremy Parr; participated in all project stages; led the systematic review; co-led the mapping review; co-led evidence synthesis and led on the young people’s focus groups; wrote sections of the report; and, with Jeremy Parr, finalised the report.
Helen Taylor (https://orcid.org/0000-0002-2847-9661) (Clinical Psychologist and Clinical Research Associate) developed the survey and Delphi survey materials; ran the survey and Delphi survey and analysed the data; organised and co-facilitated the first and second round of focus groups; analysed the data from the second round of focus groups; organised and co-facilitated stakeholder consultation workshops; produced materials for the PAG; and wrote sections of the report.
Dawn Craig (https://orcid.org/0000-0002-5808-0096) (Principal Scientist, Evidence Synthesis and Co-investigator) co-led on the mapping review, led on evidence synthesis for the study and wrote sections of the report.
Christopher Morris (https://orcid.org/0000-0002-9916-507X) (Associate Professor in Child Health Research and Co-Investigator) contributed to the design and delivery of the project, particularly the survey and Delphi survey, second round parent focus group and stakeholder consultation, and edited the final report.
Helen McConachie (https://orcid.org/0000-0002-0713-3987) (Professor, Clinical Psychology and Co-investigator) led on the measurement properties review and wrote sections of the report.
Jill Cadwgan (https://orcid.org/0000-0002-1198-8400) (Consultant, Paediatric Neurodisability, Associate Clinical Researcher and Co-Investigator) contributed to the design of the survey and Delphi survey, co-facilitated the second round of professional focus groups and wrote sections of the report.
Diane Sellers (https://orcid.org/0000-0002-3090-2107) (Clinical Specialist and Lead Speech and Language Therapist and Co-Investigator) co-facilitated the second round of professional focus groups, contributed to the design of the survey and Delphi survey, and wrote sections of the report.
Morag Andrew (https://orcid.org/0000-0003-2532-1655) (Consultant, Community Paediatrics) facilitated the young people’s focus groups, conducted the literature review on measures of nutrition and wrote sections of the report.
Johanna Smith (https://orcid.org/0000-0003-1549-0279) (Parent and Co-Investigator) led on the organisation of the PAG; recruited to and co-facilitated the first and second rounds of parent focus groups and stakeholder consultation workshops; contributed to the design of the survey and Delphi survey; and co-wrote sections of the report.
Deborah Garland (https://orcid.org/0000-0002-3708-4186) (Parent, Resource Centre Manager and Co-Investigator) led on the organisation of the PAG; recruited to and co-facilitated the first and second rounds of parent focus groups and stakeholder consultation workshops; contributed to the design of the survey and Delphi survey; and co-wrote sections of the report.
Elaine McColl (https://orcid.org/0000-0001-8300-3204) (Professor, Health Services Research and Co-Investigator) contributed to the design of the survey and Delphi survey, and wrote and reviewed sections of the report.
Charlotte Buswell (https://orcid.org/0000-0002-7530-7694) (Speech and Language Therapist and Co-Investigator) contributed to the design of the survey and Delphi survey, co-facilitated the first round of professional focus groups, co-facilitated the stakeholder consultation workshops and co-wrote Chapter 7 of the report.
Julian Thomas (https://orcid.org/0000-0002-6694-0762) (Consultant, Gastroenterology and Co-Investigator) contributed to the design of the survey and Delphi survey, co-facilitated the first round of professional focus groups and co-wrote Chapter 7 of the report.
Allan Colver (https://orcid.org/0000-0002-3623-8729) (Professor, Community Child Health and Co-Investigator) contributed to the design and delivery of the project and reviewed sections of the report.
Publications
Taylor H, Pennington L, Craig D, Morris C, McConachie H, Cadwgan J, et al. (in preparation). How do UK clinical teams work with families of children with neurodisability to alleviate, through parent-delivered interventions, eating, drinking and swallowing difficulties?
Taylor H, Pennington L, Craig D, Morris C, McConachie H, Cadwgan J, et al. (in preparation). Identifying key interventions and outcomes for children with neurodisability and eating, drinking and swallowing difficulties: a Delphi survey.
Data-sharing statement
Requests to share anonymised data will be considered after the research team have published research papers arising from the project.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Morris C, Janssens A, Tomlinson R, Williams J, Logan S. Towards a definition of neurodisability: a Delphi survey. Dev Med Child Neurol 2013;55:1103-8. https://doi.org/10.1111/dmcn.12218.
- Sullivan PB, Andersen GL, Andrew MJ. Nutrition in Neurodisability. London: Mac Keith Press; 2020.
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition 2013. https://doi.org/10.1176/appi.books.9780890425596.
- Morgan AT, Dodrill P, Ward EC. Interventions for oropharyngeal dysphagia in children with neurological impairment. Cochrane Database Syst Rev 2012;10. https://doi.org/10.1002/14651858.CD009456.pub2.
- Marshall J, Ware R, Ziviani J, Hill RJ, Dodrill P. Efficacy of interventions to improve feeding difficulties in children with autism spectrum disorders: a systematic review and meta-analysis. Child Care Health Dev 2015;41:278-302. https://doi.org/10.1111/cch.12157.
- National Institute for Health and Care Excellence (NICE) . Cerebral Palsy in Under 25s: Assessment and Management. NICE Guideline (NG62) 2017.
- Andrew MJ, Parr JR, Sullivan PB. Feeding difficulties in children with cerebral palsy. Arch Dis Child Educ Pract Ed 2012;97:222-9. https://doi.org/10.1136/archdischild-2011-300914.
- Parr JR, Jolleff N, Gray L, Gibbs J, Williams J, McConachie H. Twenty years of research shows UK child development team provision still varies widely for children with disability. Child Care Health Dev 2013;39:903-7. https://doi.org/10.1111/cch.12025.
- National Institute for Health and Care Excellence (NICE) . Autism in Under 19s: Support and Management 2013.
- National Institute for Health and Care Excellence (NICE) . Challenging Behaviour and Learning Disabilities: Prevention and Interventions for People With Learning Disabilities Whose Behaviour Challenges 2015.
- Morris C, Simkiss D, Busk M, Morris M, Allard A, Denness J, et al. Setting research priorities to improve the health of children and young people with neurodisability: a British Academy of Childhood Disability-James Lind Alliance Research Priority Setting Partnership. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2014-006233.
- World Health Organization (WHO) . International Classification of Functioning, Disability and Health 2001.
- Creswell JW, Plano Clark VL. Designing and Conducting Mixed Methods Research. Thousand Oaks, CA: SAGE Publications Ltd; 2011.
- Fetters MD, Curry LA, Creswell JW. Achieving integration in mixed methods designs – principles and practices. Health Serv Res 2013;48:2134-56. https://doi.org/10.1111/1475-6773.12117.
- Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. Res Involv Engagem 2017;3. https://doi.org/10.1186/s40900-017-0062-2.
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339. https://doi.org/10.1136/bmj.b2535.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. STROBE Initiative . Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806-8. https://doi.org/10.1136/bmj.39335.541782.AD.
- Horner RH, Carr EG, Halle J, McGee G, Odom S, Wolery M. The use of single-subject research to identify evidence-based practice in special education. Exceptional Child 2005;71. https://doi.org/10.1177/001440290507100203.
- Beckman D. Oral Motor Assessment and Intervention. Longwood: Beckman and Associates Inc.; 1995.
- Higgins JPT, Sterne JAC, Savovic J, Page MJ, Hrobjartsson A, Boutron I, et al. A revised tool for assessing risk of bias in randomized trials. Cochrane Database Syst Rev 2016;10:29-31.
- Sharp WG, Burrell TL, Jaquess DL. The Autism MEAL Plan: a parent-training curriculum to manage eating aversions and low intake among children with autism. Autism 2014;18:712-22. https://doi.org/10.1177/1362361313489190.
- Marshall J, Hill RJ, Ware RS, Ziviani J, Dodrill P. Multidisciplinary intervention for childhood feeding difficulties. J Pediatr Gastroenterol Nutr 2015;60:680-7. https://doi.org/10.1097/MPG.0000000000000669.
- Peterson KM, Piazza CC, Volkert VM. A comparison of a modified sequential oral sensory approach to an applied behavior-analytic approach in the treatment of food selectivity in children with autism spectrum disorder. J Appl Behav Anal 2016;49:485-511. https://doi.org/10.1002/jaba.332.
- Johnson CR, Foldes E, DeMand A, Brooks MM. Behavioral parent training to address feeding problems in children with autism spectrum disorder. A pilot trial. J Dev Phys Disabil 2015;25:591-607. https://doi.org/10.1007/s10882-015-9437-1.
- Serel Arslan S, Demir N, Karaduman AA. Effect of a new treatment protocol called functional chewing training on chewing function in children with cerebral palsy: a double-blind randomised controlled trial. J Oral Rehabil 2017;44:43-50. https://doi.org/10.1111/joor.12459.
- Song WJ, Park JH, Lee JH, Kim MY. Effects of neuromuscular electrical stimulation on swallowing functions in children with cerebral palsy: a pilot randomised controlled trial. Hong Kong J Occup Th 2015;25:1-6. https://doi.org/10.1016/j.hkjot.2015.05.001.
- Sığan SN, Uzunhan TA, Aydınlı N, Eraslan E, Ekici B, Calışkan M. Effects of oral motor therapy in children with cerebral palsy. Ann Indian Acad Neurol 2013;16:342-6. https://doi.org/10.4103/0972-2327.116923.
- Mullen R. Evidence for whom?: ASHA’s National Outcomes Measurement System. J Commun Disord 2004;37:413-17. https://doi.org/10.1016/j.jcomdis.2004.04.004.
- Long AD, Bahr DC, Kumin L. The Battery for Oral-Motor Behaviour in Children. Baltimore, MD: Loyola University Maryland; 1998.
- Beckman DA. Oral Motor Assessment and Treatment. Winter Park, FL: Beckman and Associates; 1986.
- Stratton M. Behavioural assessment scale of oral functions in feeding. Am J Occup Ther 1981;35:719-21. https://doi.org/10.5014/ajot.35.11.719.
- Crist W, McDonnell P, Beck M, Gillespie CT, Barrett P, Mathews J. Behavior at mealtimes and the young child with cystic fibrosis. J Dev Behav Pediatr 1994;15:157-61. https://doi.org/10.1097/00004703-199406000-00001.
- Crist W, Napier-Phillips A. Mealtime behaviors of young children: a comparison of normative and clinical data. J Dev Behav Pediatr 2001;22:279-86. https://doi.org/10.1097/00004703-200110000-00001.
- Law M, Baptiste S, McColl M, Opzoomer A, Polatajko H, Pollock N. The Canadian Occupational Performance Measure: an outcome measure for occupational therapy. Can J Occup Ther 1990;57:82-7. https://doi.org/10.1177/000841749005700207.
- Archer LA, Rosenbaum PL, Streiner DL. The children’s eating behavior inventory: reliability and validity results. J Pediatr 1991;16:629-42. https://doi.org/https://doi.org/10.1093/jpepsy/16.5.629.
- Burklow KA, Phelps AN, Schultz JR, McConnell K, Rudolph C. Classifying complex pediatric feeding disorders. J Pediatr Gastroenterol Nutr 1998;27:143-7. https://doi.org/10.1097/00005176-199808000-00003.
- Thomas-Stonell N, Greenberg J. Three treatment approaches and clinical factors in the reduction of drooling. Dysphagia 1998;3:73-8. https://doi.org/10.1007/BF02412423.
- Kenny DJ, Koheil RM, Greenberg J, Reid D, Milner M, Moran R, et al. Development of a multidisciplinary feeding profile for children who are dependent feeders. Dysphagia 1989;4:16-28. https://doi.org/10.1007/bf02407398.
- Crary MA, Mann GD, Groher ME. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehabil 2005;86:1516-20. https://doi.org/10.1016/j.apmr.2004.11.049.
- Gisel EG, Alphonce E, Ramsay M. Assessment of ingestive and oral praxis skills: children with cerebral palsy vs. controls. Dysphagia 2000;15:236-44. https://doi.org/10.1007/s004550000033.
- Serel Arslan S, Demir N, Barak Dolgun A, Karaduman AA. Development of a new instrument for determining the level of chewing function in children. J Oral Rehabil 2016;43:488-95. https://doi.org/10.1111/joor.12399.
- Morris SE. The Normal Acquisition of Oral Feeding Skills: Implications for Assessment and Treatment. New York, NY: Therapeutic Media Inc.; 1982.
- Ortega Ade O, Ciamponi AL, Mendes FM, Santos MT. Assessment scale of the oral motor performance of children and adolescents with neurological damages. J Oral Rehabil 2009;36:653-9. https://doi.org/10.1111/j.1365-2842.2009.01979.x.
- Abou Elsaad T, Abdel Latif G. Assessment of functional feeding and swallowing biomechanics in normal children. Banha Med J 2008;25:273-93.
- Reilly S, Skuse D, Mathisen B, Wolke D. The objective rating of oral-motor functions during feeding. Dysphagia 1995;10:177-91. https://doi.org/10.1007/bf00260975.
- Myhr U, Von Wendt L. Improvement of functional sitting position for children with cerebral palsy. Dev Med Child Neurol 1991;33:246-56. https://doi.org/10.1111/j.1469-8749.1991.tb05114.x.
- Vulpe SG. Vulpe Assessment Battery, 2nd Edition. Toronto, ON: Canadian Association for Retarded Citizens; 1977.
- WHOQOL Group . Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychol Med 1998;28:551-8. https://doi.org/10.1017/S0033291798006667.
- Aman MG, Singh NN, Stewart AW, Field CJ. Psychometric characteristics of the Aberrant behaviour checklist. Am J Ment Defic 1985;89:492-50. https://doi.org/10.1037/t10453-000.
- Lukens CT, Linscheid TR. Development and validation of an inventory to assess mealtime behavior problems in children with autism. J Autism Dev Disord 2008;38:342-52. https://doi.org/10.1007/s10803-007-0401-5.
- DeMand A, Johnson C, Foldes E. Psychometric Properties of the brief autism mealtime behaviors inventory. J Autism Dev Disord 2015;45:2667-73. https://doi.org/10.1007/s10803-015-2435-4.
- Elliott SN, Von Brock Treuting M. The Behaviour Intervention Rating Scale: development and validation of a pretreatment acceptability and effectiveness measure. J Sch Psychol 1991;29:43-51. https://doi.org/10.1016/0022-4405(91)90014-I.
- Attkisson CC, Zwick R. The client satisfaction questionnaire. Psychometric properties and correlations with service utilization and psychotherapy outcome. Eval Prog Plann 1982;5:233-7. https://doi.org/10.1016/0149-7189(82)90074-X.
- Eyberg S. ECBI: Eyberg Child Behaviour Inventory. Lutz, FL: Psychological Assessment Resources; 1999.
- Hoffman L, Marquis J, Poston D, Summers JA, Turnbull A. Assessing family outcomes: psychometric evaluation of the beach center quality of life scale. J Marriage Fam 2006;68:1069-83. https://doi.org/10.1111/j.1741-3737.2006.00314.x.
- Schreck KA, Williams K, Smith AF. A comparison of eating behaviors between children with and without autism. J Autism Dev Disord 2004;34:433-8. https://doi.org/10.1023/b:jadd.0000037419.78531.86.
- Paul C, Williams KE, Riegel K, Gibbons B. Combining repeated taste exposure and escape prevention: an intervention for the treatment of extreme food selectivity. Appetite 2007;49:708-11. https://doi.org/10.1016/j.appet.2007.07.012.
- Abidin R. Parenting Stress Index (PSI). Lutz, FL: Psychological Assessment Resources; 1995.
- Matson JL, Kuhn DE. Identifying feeding problems in mentally retarded persons: development and reliability of the screening tool of feeding problems (STEP). Res Dev Disabil 2001;22:165-72. https://doi.org/10.1016/S0891-4222(01)00065-8.
- Constantino JN. The Social Responsiveness Scale (SRS). Los Angeles, CA: Western Psychological Services; 2005.
- Bandini LG, Anderson SE, Curtin C, Cermak S, Evans EW, Scampini R, et al. Food selectivity in children with autism spectrum disorders and typically developing children. J Pediatr 2010;157:259-64. https://doi.org/10.1016/j.jpeds.2010.02.013.
- Center for Disease Control and Prevention . National Health and Nutrition Examination Survey 2013. www.cdc.gov/nchs/nhanes/index.htm (accessed 17 December 2020).
- Krippendorff K. Content Analysis: An Introduction to its Methodology. Thousand Oaks, CA: SAGE Publications Ltd; 2012.
- Enderby P, John A. Therapy Outcome Measures for Rehabilitation Professionals. Guildford: J&R Press Ltd; 2015.
- Benfer K, Weir K, Bell K, Davies P, Ware RS, Boyd R. Oral motor dysfunction on food and fluid textures, and its relationship with gross motor skills in children with cerebral palsy. Dev Med Child Neurol 2012;54:75-6.
- Prinsen CAC, Mokkink LB, Bouter LM, . COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual Life Res 2018;27:1147-57. https://doi.org/10.1007/s11136-018-1798-3.
- Fitzpatrick R, Davey C, Buxton MJ, Jones DR. Evaluating patient-based outcome measures for use in clinical trials. Health Technol Assess 1998;2. https://doi.org/10.3310/hta2140.
- Hendy HM, Seiverling L, Lukens CT, Williams KE. Brief assessment of mealtime behavior in children: psychometrics and association with child characteristics and parent responses. Children’s Health Care 2013;42:1-14. https://doi.org/10.1080/02739615.2013.753799.
- Wardle J, Guthrie CA, Sanderson S, Rapoport L. Development of the children’s eating behaviour questionnaire. J Child Psychol Psychiatry 2001;42:963-70. https://doi.org/10.1111/1469-7610.00792.
- Anderson SE, Must A, Curtin C, Bandini LG. Meals in our household: reliability and initial validation of a questionnaire to assess child mealtime behaviors and family mealtime environments. J Acad Nutr Diet 2012;112:276-84. https://doi.org/10.1016/j.jada.2011.08.035.
- Thoyre SM, Pados BF, Park J, Estrem H, Hodges EA, McComish C, et al. Development and content validation of the Pediatric Eating Assessment Tool (Pedi-EAT). Am J Speech Lang Pathol 2014;23:46-59. https://doi.org/10.1044/1058-0360(2013/12-0069).
- Thoyre SM, Pados BF, Park J, Estrem H, McComish C, Hodges EA. The Pediatric Eating Assessment Tool: factor structure and psychometric properties. J Pediatr Gastroenterol Nutr 2018;66:299-305. https://doi.org/10.1097/MPG.0000000000001765.
- Belafsky PC, Mouadeb DA, Rees CJ, Pryor JC, Postma GN, Allen J, et al. Validity and reliability of the Eating Assessment Tool (EAT-10). Ann Otol Rhinol Laryngol 2008;117:919-24. https://doi.org/10.1177/000348940811701210.
- Seiverling L, Hendy HM, Williams K. The Screening Tool of Feeding Problems applied to children (STEP-CHILD): psychometric characteristics and associations with child and parent variables. Res Dev Disabil 2011;32:1122-9. https://doi.org/10.1016/j.ridd.2011.01.012.
- Birch LL, Fisher JO, Grimm-Thomas K, Markey CN, Sawyer R, Johnson SL. Confirmatory factor analysis of the Child Feeding Questionnaire: a measure of parental attitudes, beliefs and practices about child feeding and obesity proneness. Appetite 2001;36:201-10. https://doi.org/10.1006/appe.2001.0398.
- Berlin KS, Davies WH, Silverman AH, Rudolph CD. Assessing family-based feeding strategies, strengths, and mealtime structure with the Feeding Strategies Questionnaire. J Pediatr Psychol 2011;36:586-95. https://doi.org/10.1093/jpepsy/jsp107.
- Hendy HM, Williams KE, Camise TS, Eckman N, Hedemann A. The Parent Mealtime Action Scale (PMAS). Development and association with children’s diet and weight. Appetite 2009;52:328-39. https://doi.org/10.1016/j.appet.2008.11.003.
- Hendy H, Harclerode W, Williams KE. The Parent Mealtime Action Scale revised (PMAS-R): psychometric characteristics and associations with variables of clinical interest. Appetite 2016;105:283-90. https://doi.org/10.1016/j.appet.2016.05.025.
- Wardle J, Sanderson S, Guthrie CA, Rapoport L, Plomin R. Parental feeding style and the inter-generational transmission of obesity risk. Obes Res 2002;10:453-62. https://doi.org/10.1038/oby.2002.63.
- Sparling JW, Rogers JC. Feeding assessment: development of a biopsychosocial instrument. Occup Ther J Res 1985;5:3-23. https://doi.org/10.1177/153944928500500101.
- Remijn L, Speyer R, Groen BE, Holtus PC, van Limbeek J, Nijhuis-van der Sanden MW. Assessment of mastication in healthy children and children with cerebral palsy: a validity and consistency study. J Oral Rehabil 2013;40:336-47. https://doi.org/10.1111/joor.12040.
- Remijn L, Speyer R, Groen BE, van Limbeek J, Nijhuis-van der Sanden MW. Validity and reliability of the Mastication Observation and Evaluation (MOE) instrument. Res Dev Disabil 2014;35:1551-61. https://doi.org/10.1016/j.ridd.2014.03.035.
- Dovey TM, Jordan C, Aldridge VK, Martin CI. Screening for feeding disorders. Creating critical values using the behavioural pediatrics feeding assessment scale. Appetite 2013;69:108-13. https://doi.org/10.1016/j.appet.2013.05.019.
- Seiverling LJ, Williams KE, Hendy HM, Adams K, Fernandez A, Alaimo C, et al. Validation of the Brief Assessment of Mealtime Behavior in Children (BAMBIC) for children in a non-clinical sample. Child Health Care 2016;45:165-76. https://doi.org/10.1080/02739615.2014.979925.
- Pados BF, Thoyre SM, Park J. Age-based norm-reference values for the Pediatric Eating Assessment Tool. Pediatr Res 2018;84:233-9. https://doi.org/10.1038/s41390-018-0067-z.
- Willett WC, Willett WC. Nutritional Epidemiology. New York, NY: Oxford University Press; 1998.
- Rockett HR, Breitenbach M, Frazier AL, Witschi J, Wolf AM, Field AE, et al. Validation of a youth/adolescent food frequency questionnaire. Prev Med 1997;26:808-16. https://doi.org/10.1006/pmed.1997.0200.
- Sanchez K, Spittle AJ, Allinson L, Morgan A. Parent questionnaires measuring feeding disorders in preschool children: a systematic review. Dev Med Child Neurol 2015;57:798-807. https://doi.org/10.1111/dmcn.12748.
- Jaafar NH, Othman A, Majid NA, Harith S, Zabidi-Hussin Z. Parent-report instruments for assessing feeding difficulties in children with neurological impairments: a systematic review. Dev Med Child Neurol 2019;61:135-44. https://doi.org/10.1111/dmcn.13986.
- Marshall J, Raatz M, Ward EC, Dodrill P. Use of parent report to screen for feeding difficulties in young children. J Paediatr Child Health 2015;51:307-13. https://doi.org/10.1111/jpc.12729.
- Allen SL, Smith IM, Duku E, Vaillancourt T, Szatmari P, Bryson S, et al. Behavioral pediatrics feeding assessment scale in young children with autism spectrum disorder: psychometrics and associations with child and parent variables. J Pediatr Psychol 2015;40:581-90. https://doi.org/10.1093/jpepsy/jsv006.
- Williams KE, Hendy HM, Seiverling LJ, Can SH. Validation of the parent mealtime action scale (PMAS) when applied to children referred to a hospital-based feeding clinic. Appetite 2011;56:553-7. https://doi.org/10.1016/j.appet.2011.01.021.
- Barton C, Bickell M, Fucile S. Pediatric oral motor feeding assessments: a systematic review. Phys Occup Ther Pediatr 2018;38:190-209. https://doi.org/10.1080/01942638.2017.1290734.
- Speyer R, Cordier R, Parsons L, Denman D, Kim JH. Psychometric characteristics of non-instrumental swallowing and feeding assessments in pediatrics: a systematic review using COSMIN. Dysphagia 2018;33:1-14. https://doi.org/10.1007/s00455-017-9835-x.
- Benfer KA, Weir KA, Bell KL, Ware RS, Davies PS, Boyd RN. Validity and reproducibility of measures of oropharyngeal dysphagia in preschool children with cerebral palsy. Dev Med Child Neurol 2015;57:358-65. https://doi.org/10.1111/dmcn.12616.
- Benfer KA, Weir KA, Boyd RN. Clinimetrics of measures of oropharyngeal dysphagia for preschool children with cerebral palsy and neurodevelopmental disabilities: a systematic review. Dev Med Child Neurol 2012;54:784-95. https://doi.org/10.1111/j.1469-8749.2012.04302.x.
- McColl E, Jacoby A, Thomas L, Soutter J, Bamford C, Steen N, et al. Design and use of questionnaires: a review of best practice applicable to surveys of health service staff and patients. Health Technol Assess 2001;5. https://doi.org/10.3310/hta5310.
- McConachie H, Barry R, Spencer A, Parker L, Le Couteur A, Colver A. Daslne: the challenge of developing a regional database for autism spectrum disorder. Arch Dis Child 2009;94:38-41. https://doi.org/10.1136/adc.2007.126326.
- Warnell F, George B, McConachie H, Johnson M, Hardy R, Parr JR. Designing and recruiting to UK autism spectrum disorder research databases: do they include representative children with valid ASD diagnoses?. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-008625.
- Spencer L, Ritchie J, O’Connor W, Ritchie J, Lewis J, McNaughton Nicholls C, et al. Qualitative Research Practice: A Guide for Social Science Students and Researchers. Thousand Oaks, CA: SAGE Publications Ltd; 2003.
- Van Bewer V. Transdisciplinarity in health care: a concept analysis. Nurs Forum 2017;52:339-47. https://doi.org/10.1111/nuf.12200.
- Sinha IP, Smyth RL, Williamson PR. Using the Delphi technique to determine which outcomes to measure in clinical trials: recommendations for the future based on a systematic review of existing studies. PLOS Med 2011;8. https://doi.org/10.1371/journal.pmed.1000393.
- Samson-Fang LJ, Stevenson RD. Identification of malnutrition in children with cerebral palsy: poor performance of weight-for-height centiles. Dev Med Child Neurol 2000;42:162-8. https://doi.org/10.1017/s0012162200000293.
- Kuperminc MN, Gurka MJ, Bennis JA, Busby MG, Grossberg RI, Henderson RC, et al. Anthropometric measures: poor predictors of body fat in children with moderate to severe cerebral palsy. Dev Med Child Neurol 2010;52:824-30. https://doi.org/10.1111/j.1469-8749.2010.03694.x.
- Romano C, van Wynckel M, Hulst J, Broekaert I, Bronsky J, Dall’Oglio L, et al. European Society for Paediatric Gastroenterology, Hepatology and Nutrition guidelines for the evaluation and treatment of gastrointestinal and nutritional complications in children with neurological impairment. J Pediatr Gastroenterol Nutr 2017;65:242-64. https://doi.org/10.1097/MPG.0000000000001646.
- Chumlea WC, Guo SS, Steinbaugh ML. Prediction of stature from knee height for black and white adults and children with application to mobility-impaired or handicapped persons. J Am Diet Assoc 1994;94. https://doi.org/10.1016/0002-8223(94)92540-2.
- Stevenson RD. Use of segmental measures to estimate stature in children with cerebral palsy. Arch Pediatr Adolesc Med 1995;149:658-62. https://doi.org/10.1001/archpedi.1995.02170190068012.
- Gauld LM, Kappers J, Carlin JB, Robertson CF. Height prediction from ulna length. Dev Med Child Neurol 2004;46:475-80. https://doi.org/10.1017/s0012162204000787.
- Snik DAC, de Roos NM. Criterion validity of assessment methods to estimate body composition in children with cerebral palsy: a systematic review [published online ahead of print May 31 2019]. Ann Phys Rehabil Med 2019. https://doi.org/10.1016/j.rehab.2019.05.003.
- Gurka MJ, Kuperminc MN, Busby MG, Bennis JA, Grossberg RI, Houlihan CM, et al. Assessment and correction of skinfold thickness equations in estimating body fat in children with cerebral palsy. Dev Med Child Neurol 2010;52:e35-41. https://doi.org/10.1111/j.1469-8749.2009.03474.x.
- Rieken R, van Goudoever JB, Schierbeek H, Willemsen SP, Calis EA, Tibboel D, et al. Measuring body composition and energy expenditure in children with severe neurologic impairment and intellectual disability. Am J Clin Nutr 2011;94:759-66. https://doi.org/10.3945/ajcn.110.003798.
- Finbråten AK, Martins C, Andersen GL, Skranes J, Brannsether B, Júlíusson PB, et al. Assessment of body composition in children with cerebral palsy: a cross-sectional study in Norway. Dev Med Child Neurol 2015;57:858-64. https://doi.org/10.1111/dmcn.12752.
- Kushner RF, Schoeller DA, Fjeld CR, Danford L. Is the impedance index (ht2/R) significant in predicting total body water?. Am J Clin Nutr 1992;56:835-9. https://doi.org/10.1093/ajcn/56.5.835.
- Fjeld CR, Freundt-Thurne J, Schoeller DA. Total body water measured by 18-O dilution and bioelectrical impedance in well and malnourished children. Pediatr Res 1990;27:98-102. https://doi.org/10.1203/00006450-199001000-00024.
- Pencharz PB, Azcue M. Use of bioelectrical impedance analysis measurements in the clinical management of malnutrition. Am J Clin Nutr 1996;64:485-8. https://doi.org/10.1093/ajcn/64.3.485S.
- Hardy J, Kuter H, Campbell M, Canoy D. Reliability of anthropometric measurements in children with special needs. Arch Dis Child 2018;103:757-62. https://doi.org/10.1136/archdischild-2017-314243.
- King G, Law M, King S, Hurley P, Hanna S, Kertoy M, et al. Children’s Assessment of Participation and Enjoyment (CAPE) and Preferences for Activities of Children (PAC). San Antonio, TX: Harcourt Assessment Inc.; 2004.
- Ravens-Sieberer U, Gosch A, Rajmil L, Erhart M, Bruil J, Duer W, et al. KIDSCREEN-52 quality-of-life measure for children and adolescents. Expert Rev Pharmacoecon Outcomes Res 2005;5:353-64. https://doi.org/10.1586/14737167.5.3.353.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and Evaluating Complex Interventions. London: Medical Research Council; 2019.
- Cockerill H, Kelly V, Dutton H, Jolleff N, Tiwana K. Developing a multidimensional outcome measure for us in the management of children with complex feeding difficulties. J Pediatr Gastroenterol Nutr 2011;52.
- Zhang R, Jia M, Zhang J, Xu X, Shou X, Zhang X, et al. Transcutaneous electrical acupoint stimulation in children with autism and its impact on plasma levels of arginine-vasopressin and oxytocin: a prospective single-blinded controlled study. Res Dev Disabil 2012;33:1136-46. https://doi.org/10.1016/j.ridd.2012.02.001.
- Twachtman-Reilly J, Amaral Sc, Zebrowski PP. Addressing feeding disorders in children on the autism spectrum in school-based settings: physiological and behavioural issues. Lang Speech Hear Serv Sch 2008;39:261-72. https://doi.org/10.1044/0161-1461(2008/025).
- Serel Arslan S, Demir N, Karaduman AA. Reliability and validity of a tool to measure the severity of tongue thrust in children: the Tongue Thrust Rating Scale. J Oral Rehabil 2017;44:119-24. https://doi.org/10.1111/joor.12471.
- Sonies BC, Cintas HL, Parks R, Miller J, Caggiano C, Gottshall SG, et al. Brief assessment of motor function: content validity and reliability of the oral motor scales. Am J Phys Med Rehabil 2009;88:464-72. https://doi.org/10.1097/PHM.0b013e3181a5abad.
- Santos MT, Manzano FS, Ferreira MC, Masiero D. Development of a novel orofacial motor function assessment scale for children with cerebral palsy. J Dent Child 2005;72:113-18.
- Serel Arslan S, Demir N, Karaduman AA, Belafsky PC. The Pediatric Version of the Eating Assessment Tool: a caregiver administered dysphagia-specific outcome instrument for children. Disabil Rehabil 2018;40:2088-92. https://doi.org/10.1080/09638288.2017.1323235.
- McConachie HM, Parr JR, Glod M, Hanratty J, Livingstone N, Oono IP, et al. Systematic review of tools to measure outcomes for young children with autism spectrum disorders. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19410.
- Mallick E, Sethy D, Bajpai P. Effect of oral sensorimotor stimulation on drooling and its relationship with feeding behavior in children with spastic cerebral palsy. Indian J Physiother Occup Ther 2017;11:105-11. https://doi.org/10.5958/0973-5674.2017.00129.0.
- Martins Y, Young RL, Robson DC. Feeding and eating behaviors in children with autism and typically developing children. J Autism Dev Disord 2008;38:1878-87. https://doi.org/10.1007/s10803-008-0583-5.
- Aponte CA, Romanczyk RG. Assessment of feeding problems in children with autism spectrum disorder. Research in Autism Spectrum Disorders 2016;21:61-72. https://doi.org/https://doi.org/10.1016/j.rasd.2015.09.007.
- Johnson CR, Turner K, Stewart PA, Schmidt B, Shui A, Macklin E, et al. Relationships between feeding problems, behavioral characteristics and nutritional quality in children with ASD. J Autism Dev Disord 2014;44:2175-84. https://doi.org/10.1007/s10803-014-2095-9.
- Meral BF, Fidan A, Laborda JC, Ozdamli F, Maasoglu Y. 5th World Conference on Educational Sciences. Amsterdam: Elsevier Science; 2014.
- Shmaya Y, Eilat-Adar S, Leitner Y, Reif S, Gabis LV. Meal time behavior difficulties but not nutritional deficiencies correlate with sensory processing in children with autism spectrum disorder. Res Dev Disabil 2017;66:27-33. https://doi.org/https://doi.org/10.1016/j.ridd.2017.05.004.
- Thullen M, Bonsall A. Co-parenting quality, parenting stress, and feeding challenges in families with a child diagnosed with autism spectrum disorder. J Autism Dev Disord 2017;47:878-86. https://doi.org/10.1007/s10803-016-2988-x.
- Tanner K, Case-Smith J, Nahikian-Nelms M, Ratliff-Schaub K, Spees C, Darragh AR. Behavioral and physiological factors associated with selective eating in children with autism spectrum disorder. Am J Occup Ther 2015;69:6906180030p1-8. https://doi.org/10.5014/ajot.2015.019273.
- Kral TV, Souders MC, Tompkins VH, Remiker AM, Eriksen WT, Pinto-Martin JA. Child eating behaviors and caregiver feeding practices in children with autism spectrum disorders. Public Health Nurs 2015;32:488-97. https://doi.org/10.1111/phn.12146.
- Williams KE, Hendy HM. Variables associated with the use of complete oral calorie supplements in children with feeding problems. J Nutr Educ Behav 2014;46:236-40. https://doi.org/10.1016/j.jneb.2014.01.003.
- Hubbard KL, Anderson SE, Curtin C, Must A, Bandini LG. A comparison of food refusal related to characteristics of food in children with autism spectrum disorder and typically developing children. J Acad Nutr Diet 2014;114:1981-7. https://doi.org/10.1016/j.jand.2014.04.017.
- Zimmer MH, Hart LC, Manning-Courtney P, Murray DS, Bing NM, Summer S. Food variety as a predictor of nutritional status among children with autism. J Autism Dev Disord 2012;42:549-56. https://doi.org/10.1007/s10803-011-1268-z.
- Serel Arslan S, Aydın G, Alemdaroğlu I, Tunca Yılmaz Ö, Karaduman AA. Reliability and validity of the Karaduman Chewing Performance Scale in paediatric neuromuscular diseases: a system for classification of chewing disorders. J Oral Rehabil 2018;45:526-31. https://doi.org/10.1111/joor.12642.
- Pinto VV, Alves LAC, Mendes FM, Ciamponi AL. The nutritional state of children and adolescents with cerebral palsy is associated with oral motor dysfunction and social conditions: a cross sectional study. BMC Neurol 2016;16. https://doi.org/10.1186/s12883-016-0573-8.
- Ko MJ, Kang MJ, Ko KJ, Ki YO, Chang HJ, Kwon JY. Clinical Usefulness of Schedule for Oral-Motor Assessment (SOMA) in children with dysphagia. Ann Rehabil Med 2011;35:477-84. https://doi.org/10.5535/arm.2011.35.4.477.
- Skuse D, Stevenson J, Reilly S, Mathisen B. Schedule for oral-motor assessment (SOMA): methods of validation. Dysphagia 1995;10:192-20. https://doi.org/10.1007/bf00260976.
- Meral BF. Parental feeding practices in Turkish children with autism spectrum disorder: factorial validation of the feeding strategies questionnaire. Child Health Care 2017;46:1-14. https://doi.org/10.1080/02739615.2015.1038679.
- Lewis SJ, Heaton KW. Stool form scale as useful guide to intestinal transit time. Stand J Gastroenterol 1997;32:920-4. https://doi.org/10.3109/00365529709011203.
- Burrows T, Goldman S, Rollo M. A systematic review of the validity of dietary assessment methods in children when compared with the method of doubly labelled water. Eur J Clin Nutr 2020;74:669-81. https://doi.org/10.1038/s41430-019-0480-3.
- Cichero JA, Lam P, Steele CM, Hanson B, Chen J, Dantas RO, et al. Development of international terminology and definitions for texture-modified foods and thickened fluids used in Dysphagia management: the IDDSI framework. Dysphagia 2017;32:293-314. https://doi.org/10.1007/s00455-016-9758-y.
Appendix 1 Parent advisory group terms of reference
Role of the group
The role of the PAG is to advise on the research activities conducted as part of the research project ‘FEEDS: Focus on Early Eating, Drinking and Swallowing’. This project aims to answer the question ‘What interventions, which could be delivered at home by parents, are available to improve eating in young children with neurodisability and are suitable for investigation in pragmatic trials?’.
Responsibilities
-
Provide advice on issues that affect parents who have a child with developmental difficulties who experience EDSD.
-
Provide a forum for discussion of study results.
-
Help to guide the development of future stages of the project (e.g. focus groups, Delphi survey and consensus workshops).
-
Provide feedback on the acceptability and feasibility of proposed trial designs, including:
-
reviewing appropriate documents
-
identifying, discussing and addressing issues of common concern
-
exchanging ideas, strengthening skills and sharing examples.
-
Membership
This group is for parents of children with a developmental difficulty who experience EDSD, or have in the past. There will be between six and eight members. Membership of this group is by invitation only and will last until July 2019.
Accountability
The group facilitators (Johanna Smith and Deborah Garland) will report on the activities of the group to members of the FEEDS research team.
Review
On an as-needed basis, the group will review the relevance and value of its work.
Working methods/ways of working
There will be a shared learning approach. Participation in this group will be primarily through face-to-face meetings and e-mails, in which information and resources will be shared. Therefore, group members must use their own e-mail account to access the content and participate alongside the face-to-face meetings. We ask that you respect other group members’ privacy and do not discuss or share the content of the group with non-members. Johanna Smith, Deborah Garland and Helen Taylor from Newcastle University will be the responsible for organising the face-to-face meetings and the administration of e-mails.
Appendix 2 Search strategies for systematic reviews
Marshall et al.’s5 2012 search update
PROSPERO registration number: CRD42017074408.
The following strategies were used to update the Marshall et al. 5 2012 review.
The search was conducted by Fiona Beyer on the 4–8 August 2017.
Database(s): Ovid MEDLINE® Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE®
Date range searched: 1946 to week 4 July 2017.
Date searched: 7 August 2017.
Search strategy
| # | Search terms | Results |
|---|---|---|
| 1 | autism spectrum disorder/or autistic disorder/ | 21,603 |
| 2 | Asperger Syndrome/ | 1735 |
| 3 | exp Child Development Disorders, Pervasive/ | 28,068 |
| 4 | (autis* or asperger* or pervasive development* disorder*).tw,kw. | 37,277 |
| 5 | or/1-4 | 40,422 |
| 6 | “Feeding and Eating Disorders of Childhood”/ | 452 |
| 7 | Feeding Behavior/ | 72,858 |
| 8 | exp Meals/ | 3521 |
| 9 | Food Preferences/ | 12,231 |
| 10 | ((feed* or food* or eat* or meal*) adj3 (difficult* or behavio* or disorder* or selectiv* or picky or habit*)).tw,kw. | 52,497 |
| 11 | or/6-10 | 121,072 |
| 12 | Behavior Therapy/ | 26,704 |
| 13 | Conditioning, Operant/ | 19,108 |
| 14 | Desensitization, Psychologic/ | 1620 |
| 15 | “reinforcement (psychology)”/or punishment/or reinforcement schedule/or reinforcement, social/ or reinforcement, verbal/ | 27,450 |
| 16 | exp Parents/ed [Education] | 11,875 |
| 17 | (behavio* adj3 (modif* or therap* or condition* or desensiti* or reinforc* or re-inforc* or punish* or interven* or nonremov* or non-remov*)).tw,kw. | 55,443 |
| 18 | (operant conditioning or systematic desensiti*).tw,kw. | 2314 |
| 19 | (parent* adj3 (educat* or train*)).tw,kw. | 12,466 |
| 20 | (interven* or reinforc* or re-inforc* or punish* or nonremov* or non-remov*).tw,kw. | 886,022 |
| 21 | or/12-20 | 965,604 |
| 22 | 5 and 11 and 21 | 137 |
| 23 | (201310* or 201311* or 201312* or 2014* or 2015* or 2016* or 2017*).ed. | 3,996,746 |
| 24 | 22 and 23 | 48 |
Database(s): Evidence-Based Medicine Reviews – Cochrane Database of Systematic Reviews
Date range searched: 2005 to 2 August 2017.
Date searched: 7 August 2017.
Search strategy
| # | Search terms | Results |
|---|---|---|
| 1 | (autis* or asperger* or pervasive development* disorder*).tw,kw. | 116 |
| 2 | ((feed* or food* or eat* or meal*) adj3 (difficult* or behavio* or disorder* or selectiv* or picky or habit*)).tw,kw. | 390 |
| 3 | (behavio* adj3 (modif* or therap* or condition* or desensiti* or reinforc* or re-inforc* or punish* or interven* or nonremov* or non-remov*)).tw,kw. | 1341 |
| 4 | (operant conditioning or systematic desensiti*).tw,kw. | 49 |
| 5 | (parent* adj3 (educat* or train*)).tw,kw. | 196 |
| 6 | (interven* or reinforc* or re-inforc* or punish* or interven* or nonremov* or non-remov*).tw,kw. | 9257 |
| 7 | or/3-6 | 9265 |
| 8 | 1 and 2 and 7 | 15 |
Database: PsycINFO
Date range searched: 1806 to week 5 July 2017.
Date searched: 7 August 2017.
Search strategy
| # | Search terms | Results |
|---|---|---|
| 1 | autism spectrum disorders/ | 35,797 |
| 2 | (autis* or asperger* or pervasive development* disorder*).ti,ab,id. | 44,307 |
| 3 | or/1-2 | 44,652 |
| 4 | eating behavior/ or food refusal/ | 10,455 |
| 5 | feeding disorders/ or eating disorders/ | 14,208 |
| 6 | eating attitudes/ or food preferences/ | 5542 |
| 7 | mealtimes/ | 638 |
| 8 | ((feed* or food* or eat* or meal*) adj3 (difficult* or behavio* or disorder* or selectiv* or picky or habit*)).ti,ab,id. | 41,721 |
| 9 | or/4-8 | 49,848 |
| 10 | behavior therapy/ or behavior modification/ or systematic desensitization therapy/ | 24,708 |
| 11 | exp operant conditioning/ | 34,283 |
| 12 | parent training/ | 6486 |
| 13 | intervention/ | 52,201 |
| 14 | exp reinforcement/ | 45,148 |
| 15 | (behavio* adj3 (modif* or therap* or condition* or desensiti* or reinforc* or re-inforc* or punish* or interven* or nonremov* or non-remov*)).ti,ab,id. | 74,358 |
| 16 | (operant conditioning or systematic desensiti*).ti,ab,id. | 5461 |
| 17 | (parent* adj3 (educat* or train*)).ti,ab,id. | 19,799 |
| 18 | (interven* or reinforc* or re-inforc* or punish* or nonremov* or non-remov*).ti,ab,id. | 408,099 |
| 19 | or/10-18 | 507,310 |
| 20 | 3 and 9 and 19 | 185 |
| 21 | (201310* or 201311* or 201312* or 2014* or 2015* or 2016* or 2017*).up. | 816,430 |
| 22 | 20 and 21 | 70 |
Cumulative Index to Nursing and Allied Health Literature (via EBSCOhost)
Date range searched: 10 October 2013 to 7 August 2017.
Date searched: 7 August 2017.
Search strategy
| # | Search term | Results |
|---|---|---|
| S20 | S3 AND S9 AND S18 (limit to 2013-date) | 25 |
| S19 | S3 AND S9 AND S18 | 52 |
| S18 | S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 | 229,721 |
| S17 | TI (interven* or reinforc* or re-inforc* or punish* or nonremov* or non-remov*) OR AB (interven* or reinforc* or re-inforc* or punish* or nonremov* or non-remov*) | 215,146 |
| S16 | TI (parent* N3 (educat* or train*)) OR AB (parent* N3 (educat* or train*)) | 4822 |
| S15 | TI (operant conditioning or systematic desensiti*) OR AB (operant conditioning or systematic desensiti*) | 127 |
| S14 | TI (behavio* N3 (modif* or therap* or condition* or desensiti* or reinforc* or re-inforc* or punish* or interven* or nonremov* or non-remov*)) OR AB (behavio* N3 (modif* or condition* or desensiti* or reinforc* or re-inforc* or punish* or interven* or nonremov* or non-remov*)) | 11,840 |
| S13 | (MH “Reinforcement (Psychology)”) OR (MH “Punishment”) | 2064 |
| S12 | (MH “Parents/ED”) OR (MH “Parents of Disabled Children/ED”) | 3610 |
| S11 | (MH “Conditioning (Psychology)”) | 550 |
| S10 | (MH “Behavior Modification”) OR (MH “Behavior Therapy”) OR (MH “Desensitization, Psychologic”) | 6943 |
| S9 | S4 OR S5 OR S6 OR S7 OR S8 | 25,748 |
| S8 | TI ((feed* or food* or eat* or meal*) N3 (difficult* or behavio* or disorder* or selectiv* or picky or habit*)) OR AB ((feed* or food* or eat* or meal*) N3 (difficult* or behavio* or disorder* or selectiv* or picky or habit*)) | 11,836 |
| S7 | (MH “Food Preferences”) OR (MH “Food Habits”) | 7983 |
| S6 | (MH “Meals+”) | 3390 |
| S5 | (MH “Eating Behavior”) | 6853 |
| S4 | (MH “Feeding and Eating Disorders of Childhood”) | 47 |
| S3 | S1 OR S2 | 15,119 |
| S2 | TI (autis* or asperger* or pervasive development* disorder*) OR AB (autis* or asperger* or pervasive development* disorder*) | 12,128 |
| S1 | (MH “Pervasive Developmental Disorder-Not Otherwise Specified”) OR (MH “Autistic Disorder”) OR (MH “Asperger Syndrome”) OR (MH “Child Development Disorders, Pervasive”) | 13,385 |
Education Resources Information Center (via EBSCOhost)
Date range searched: 10 October 2013 to 7 August 2017.
Date searched: 7 August 2017.
Search strategy
| # | Search term | Results |
|---|---|---|
| S15 | S3 AND S6 AND S13 (limit to 2013-date) | 15 |
| S14 | S3 AND S6 AND S13 | 79 |
| S13 | S7 OR S8 OR S9 OR S10 OR S11 OR S12 | 122,188 |
| S12 | TI (interven* or reinforc* or re-inforc* or punish* or nonremov* or non-remov*) OR AB (interven* or reinforc* or re-inforc* or punish* or nonremov* or non-remov*) | 83,329 |
| S11 | TI (parent* N3 (educat* or train*)) OR AB (parent* N3 (educat* or train*)) | 18,292 |
| S10 | TI (operant conditioning or systematic desensiti*) OR AB (operant conditioning or systematic desensiti*) | 592 |
| S9 | TI (behavio* N3 (modif* or therap* or condition* or desensiti* or reinforc* or re-inforc* or punish* or interven* or nonremov* or non-remov*)) OR AB (behavio* N3 (modif* or condition* or desensiti* or reinforc* or re-inforc* or punish* or interven* or nonremov* or non-remov*)) | 9390 |
| S8 | (DE “Parent Education”) OR (DE “Parent Workshops”) OR (DE “Intervention”) | 44,185 |
| S7 | DE “Behavior Modification” OR DE “Desensitization” OR DE “Operant Conditioning” OR DE “Verbal Operant Conditioning” OR DE “Reinforcement” OR DE “Negative Reinforcement” OR DE “Positive Reinforcement” OR DE “Punishment” OR DE “Rewards” OR DE “Social Reinforcement” OR DE “Timeout” OR DE “Token Economy” OR DE “Social Reinforcement” | 21,617 |
| S6 | S4 OR S5 | 5728 |
| S5 | TI ((feed* or food* or eat* or meal*) N3 (difficult* or behavio* or disorder* or selectiv* or picky or habit*)) OR AB ((feed* or food* or eat* or meal*) N3 (difficult* or behavio* or disorder* or selectiv* or picky or habit*)) | 3134 |
| S4 | DE “Eating Disorders” OR DE “Eating Habits” | 4263 |
| S3 | S1 OR S2 | 13,006 |
| S2 | TI (autis* or asperger* or pervasive development* disorder*) OR AB (autis* or asperger* or pervasive development* disorder*) | 12,387 |
| S1 | DE “Pervasive Developmental Disorders” OR DE “Asperger Syndrome” OR DE “Autism” | 12,398 |
National Institute for Health and Care Excellence’s Clinical Practice guideline6 search update
PROSPERO registration number: CRD42017074665.
The other searches are copied and pasted directly from the guideline appendix. However, they carried out the CINAHL search on Ovid and we have it through EBSCOhost; here is the EBSCOhost version. Note that a couple of the thesaurus headings listed in the appendix for CINAHL did not appear, so have been left out or substituted.
Cumulative Index to Nursing and Allied Health Literature (via EBSCOhost)
Date range searched: 10 October 2013 to 8 August 2017.
Date searched: 8 August 2017.
Search strategy
| # | Search term | Results |
|---|---|---|
| S60 | S6 AND S21 AND S58 | 11 |
| S59 | S6 AND S21 AND S58 | 68 |
| S58 | S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 OR S56 OR S57 | 161,492 |
| S57 | TI (feed* or eat* or masticat* or drink*) N3 (equipment or device* or technolog* or machine*) OR AB (feed* or eat* or masticat* or drink*) N3 (equipment or device* or technolog* or machine*) | 370 |
| S56 | TI (assistive or self help*) N3 (device* or technolog*) OR AB (assistive or self help*) N3 (device* or technolog*) | 2764 |
| S55 | TI ((equipment or device*) N3 design*) OR AB ((equipment or device*) N3 design*) | 1269 |
| S54 | (MH “Assistive Technology Devices”) | 4012 |
| S53 | (MH “Equipment Design”) | 17,365 |
| S52 | TI ((intra-oral* or intraoral* or orthodontic) N3 appliance*) OR AB ((intra-oral* or intraoral* or orthodontic) N3 appliance*) | 32 |
| S51 | (MH “Orthodontic Appliances+”) | 2199 |
| S50 | TI (occupational N3 therap*) OR AB (occupational N3 therap*) | 15,467 |
| S49 | (MH “Occupational Therapy”) | 14,195 |
| S48 | TI (speech N3 therap*) OR AB (speech N3 therap*) | 2142 |
| S47 | (MH “Speech Therapy”) | 1793 |
| S46 | TI ((speech or language) N3 patholog*) OR AB ((speech or language) N3 patholog*) | 3129 |
| S45 | (MH “Speech-Language Pathology”) | 3309 |
| S44 | TI (recover* N3 func*) OR AB (recover* N3 func*) | 4394 |
| S43 | TI oral screen* OR AB oral screen* | 496 |
| S42 | TI (lip* N3 (exercis* or strengthen*)) OR AB (lip* N3 (exercis* or strengthen*)) | 271 |
| S41 | (MH “Resistance Training”) | 1183 |
| S40 | TI (myofunctional N3 therap*) OR AB (myofunctional N3 therap*) | 22 |
| S39 | (MH “Muscle Strengthening”) | 9410 |
| S38 | TI (electric* N3 stimulat*) OR AB (electric* N3 stimulat*) | 4527 |
| S37 | (MH “Electric Stimulation+”) | 8893 |
| S36 | TI (oralmotor* or oral-motor*) N3 (therap* or treat* or train* or exercis*) OR AB (oralmotor* or oral-motor*) N3 (therap* or treat* or train* or exercis*) | 72 |
| S35 | TI (oromotor* or oro-motor*) N3 (therap* or treat* or train* or exercis*) OR AB (oromotor* or oro-motor*) N3 (therap* or treat* or train* or exercis*) | 10 |
| S34 | TI ISMAR OR AB ISMAR | 4 |
| S33 | TI (sensorimotor* or sensori-motor*) N3 (activator* or regulator*) OR AB (sensorimotor* or sensori-motor*) N3 (activator* or regulator*) | 4 |
| S32 | TI (Sens* N3 (therap* or treat* or train* or exercis*)) OR AB (Sens* N3 (therap* or treat* or train* or exercis*)) | 3387 |
| S31 | TI (speed* or slow* or fast* or pace* or pacing or efficien*) N3 (food* or feed* or fed or eat* or masticat* or meal* or drink*) OR AB (speed* or slow* or fast* or pace* or pacing or efficien*) N3 (food* or feed* or fed or eat* or masticat* or meal* or drink*) | 2183 |
| S30 | TI (food* or feed* or fed or eat* or masticat* or meal* or drink*) N3 (method* or technique* or practice* or experience*) OR AB (food* or feed* or fed or eat* or masticat* or meal* or drink*) N3 (method* or technique* or practice* or experience*) | 7493 |
| S29 | (MH “Feeding Methods”) | 690 |
| S28 | TI (seat* or sit*) N3 device*) OR AB (seat* or sit*) N3 device*) | 301 |
| S27 | TI (postur* or position*) OR AB (postur* or position*) | 53,290 |
| S26 | (MH “Patient Positioning+”) | 8087 |
| S25 | (MH “Posture+”) | 14,215 |
| S24 | TI (modif* N3 diet*) OR AB (modif* N3 diet*) | 1880 |
| S23 | TI (textur*) OR AB (textur*) | 1009 |
| S22 | TI (thick or thicken*) OR AB (thick or thicken*) | 3565 |
| S21 | S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 | 92,917 |
| S20 | TI (nutrition* or nourish*) N3 (disorder* or deficien* or poor or status) OR AB (nutrition* or nourish*) N3 (disorder* or deficien* or poor or status) | 5836 |
| S19 | TI (underweight* or under weight* or overweight* or over weight* or obes*) OR AB (underweight* or under weight* or overweight* or over weight* or obes*) | 45,536 |
| S18 | TI (malnutrition* or malnourish* or undernutrition* or under nutrition* or undernourish* or under nourish* or overnutrition* or over nutrition* or overnourish* or over nourish*) OR AB (malnutrition* or malnourish* or undernutrition* or under nutrition* or undernourish* or under nourish* or overnutrition* or over nutrition* or overnourish* or over nourish*) | 6975 |
| S17 | TI (oropharyn* or pharyng*) N3 (disorder* or dysfunc* or impair*) OR AB (oropharyn* or pharyng*) N3 (disorder* or dysfunc* or impair*) | 108 |
| S16 | TI ((oropharyn* or trachea* or lung* or pulmon*) N3 aspirat*) OR AB ((oropharyn* or trachea* or lung* or pulmon*) N3 aspirat*) | 512 |
| S15 | TI (dysphag*) OR AB (dysphag*) | 4068 |
| S14 | TI (eat* or fed or feed* or swallow* or deglut* or oral motor or oromotor or oro motor) N3 (disorder* or dysfunc* or function* or disabilit* or impair* or problem* or inabilit* or difficult* or abnormal*) OR AB (eat* or fed or feed* or swallow* or deglut* or oral motor or oromotor or oro motor) N3 (disorder* or dysfunc* or function* or disabilit* or impair* or problem* or inabilit* or difficult* or abnormal*) | 10,525 |
| S13 | (MH “Nutritional Status”) | 6254 |
| S12 | (MH “Child Nutritional Physiology+”) | 15,075 |
| S11 | (MH “Deglutition Disorders”) | 4250 |
| S10 | (MH “Eating Behavior”) | 6855 |
| S9 | (MH “Feeding and Eating Disorders of Childhood”) | 47 |
| S8 | (MH “Eating Disorders”) | 5597 |
| S7 | (MH “Nutrition Disorders+”) | 59,976 |
| S6 | S1 OR S2 OR S3 OR S4 OR S5 | 8430 |
| S5 | TI (“little* disease”) OR AB (“little* disease”) | 2 |
| S4 | TI (“pseudobulbar pals*” or “pseudo bulbar pals*”) OR AB (“pseudobulbar pals*”’ or “pseudo bulbar pals*”) | 18 |
| S3 | TI (“worster drought”) OR AB (“worster drought”) | 7 |
| S2 | TI (cerebral or brain or central) N2 (pals* or paralys* or pares*) OR AB (cerebral or brain or central) N2 (pals* or paralys* or pares*) | 6634 |
| S1 | (MH “Cerebral Palsy”) | 6852 |
Appendix 3 Data extraction for systematic reviews
Marshall et al.5 review update
| Study (first author and year of publication) | Study design | Intervention | Timescale of intervention | Outcomes | Outcome measures | N | Age range | ASD diagnosis |
|---|---|---|---|---|---|---|---|---|
| Johnson 201524 | Before and after | Manualised Parent Training – Feeding (group-based behavioural intervention) | 9 × 1–1.5 hours (over 16 weeks) | Mealtime behaviours, disruptive behaviours, dietary intake, parent stress and caregiver satisfaction | BAMBI, ABC, PSI-SF, 3-day food records, Caregiver satisfaction and effectiveness questionnaire (bespoke, non-validated) | 14 | 3 years 4 months to 6 years 2 months | Met diagnostic criteria according to DSM-IV and confirmed by ADOS in study |
| Marshall 201522 | RCT | Systematic desensitisation (SD) vs. operant conditioning (OC): both with parent training | 10 sessions (weekly or intensively) | Dietary intake and variety, mealtime behaviours, weight, height, BMI, behaviour outside mealtimes and parent stress | 3-day food records, food lists, BPFAS, ECBI and PSI-SF | 68 | 3 years 1 month to 5 years 2 months | Documented diagnosis by paediatrician, psychologist or psychiatrist: not formally assessed in study |
| Peterson 201623 | SCEDa | Applied behaviour analysis (ABA) vs. modified sequential oral sensory (MSOS) programme | 1.5 hours 3 times per week: ABA = 9–16 sessions; MSOS = 15–19 sessions | Food acceptance, mouth clean, inappropriate mealtime behaviours and grams consumed | N/A | 6 | 4–6 years | Diagnosis given by MDT using structured interview, ADOS and mental state exam: not formally assessed in study |
| Sharp 201421 | RCT | Group parent training – behavioural intervention vs. waiting list control | 8 × 1-hour sessions (over 6 weeks) | Mealtime behaviours, dietary variety, feasibility, parent stress and caregiver satisfaction | BAMBI, FPI, PSI-SF, caregiver satisfaction and effectiveness questionnaire | 19 | 3–8 years | DSM-IV and SRS used to confirm diagnosis in study |
National Institute for Health and Care Excellence guidelines review (2017)6 update
| Study (first author and year of publication | Study design | Intervention | Timescale of intervention | Outcomes | Outcome measures | N | Age range |
|---|---|---|---|---|---|---|---|
| Serel Arslan 201725 | RCT | FCTa vs. OMEb | FCTa = 12 weeks OMEb = 12 weeks | Chewing performance and feeding behaviours | KCPS and BPFAS | 80 | 1 year 1 month to 5 years 7 months |
| Song 201526 | RCT | OSTc + NMES vs. OSTc + NMES shamd | 8 weeks | Feeding behaviours and severity of dysphagia | BASOFF and NOMSe swallowing scale | 20 | 3 years 4 months to 8 years 9 months |
Appendix 4 Search strategies for mapping review
MEDLINE
Database(s): Ovid MEDLINE Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE
Date range searched: 1946 to 4 October 2017.
Date searched: 5 October 2017.
Search strategy
| # | Search term | Results |
|---|---|---|
| 1 | exp Brain Diseases/ | 1,193,723 |
| 2 | Pseudobulbar Palsy/ | 143 |
| 3 | (pseudobulbar pals$ or pseudo bulbar pals$).tw,kw. | 387 |
| 4 | Cerebral Palsy/ | 19,159 |
| 5 | ((cerebral or brain or central) adj2 (pals$ or paralys#s or pares#s)).tw,kw. | 20,996 |
| 6 | worster drought.tw,kw. | 34 |
| 7 | little? disease.tw,kw. | 93 |
| 8 | exp Neurodevelopmental Disorders/ | 173,103 |
| 9 | (neurodisabilit$ or neuro-disabilit$ or neurodisabl$ or neuro-disabl$).tw,kw. | 290 |
| 10 | ((neurodevelopment$ or development$) adj3 (disease$ or disabl$ or disabil$ or disorder$ or dysfunction$ or function$ or impair$ or abnormal$ or difficulty or difficulties)).tw,kw. | 142,205 |
| 11 | exp Nervous System Diseases/ | 2,691,703 |
| 12 | ((brain$ or nervous system$) adj2 (injur$ or damag$ or dysfunction$ or function$ or malform$ or disease$ or impair$ or abnormal$)).tw,kw. | 152,306 |
| 13 | ((neurologic$ or neuromuscular or motor) adj3 (disease$ or disabl$ or disabil$ or disorder$ or dysfunction$ or function$ or impair$ or abnormal$ or difficulty or difficulties)).tw,kw. | 160,841 |
| 14 | ((muscular or muscle$ or myopath$) adj3 (disorder$ or disease$ or dysfunction$ or function$ or dystroph$)).tw,kw. | 63,230 |
| 15 | (epilep$ or seizure$ or convuls$).tw,kw. | 197,466 |
| 16 | ((communicat$ or language or linguistic or speech or learning or intellectual$ or behaviour$ or behavior$) adj3 (disabl$ or disabil$ or disorder$ or dysfunction$ or function$ or impair$ or abnormal$ or difficulty or difficulties)).tw,kw. | 124,029 |
| 17 | (autis$ or asperger$ or kanner$ or pervasive development$ disorder$).tw,kw. | 41,371 |
| 18 | ((attention$ or behav$ or conduct) adj3 (defic$ or dysfunc$ or disorder$)).tw,kw. | 58,227 |
| 19 | ((disrupt$ adj3 disorder$) or (disrupt$ adj3 behav$) or (defian$ adj3 disorder$) or (defian$ adj3 behav$)).tw,kw. | 8220 |
| 20 | (impulsiv$ or inattentiv$ or inattention$).tw,kw. | 22,429 |
| 21 | (ADHD or ADDH or ADHS).tw,kw. | 22,515 |
| 22 | (down$ adj (syndrome$ or disease$)).tw,kw. | 21,757 |
| 23 | (trisomy adj “21”).tw,kw. | 5567 |
| 24 | (chromosome adj “21”).tw,kw. | 3737 |
| 25 | (mongol or mongols or mongoloid or mongolism).tw,kw. | 2669 |
| 26 | Cystic Fibrosis/ | 33,906 |
| 27 | cystic fibrosis.tw,kw. | 41,408 |
| 28 | or/1-27 | 3,189,618 |
| 29 | exp Deglutition Disorders/ | 50,791 |
| 30 | “Feeding and Eating Disorders of Childhood”/ | 471 |
| 31 | Food Preferences/ | 12,810 |
| 32 | dysphagi$.tw,kw. | 25,344 |
| 33 | ((swallow$ or deglut$ or oral motor or oromotor or oro motor or oropharyn$ or pharyng$) adj3 (disorder$ or dysfunction$ or function$ or disabl$ or disabilit$ or impair$ or abnormal$)).tw,kw. | 6776 |
| 34 | ((feed$ or food$ or eat$ or meal$) adj3 (problem$ or inabilit$ or difficult$ or behavio$ or selectiv$ or picky or habit$ or refus$)).tw,kw. | 45,753 |
| 35 | ((oropharynx$ or trachea$ or lung$ or pulmon$) adj3 aspirat$).tw,kw. | 4165 |
| 36 | nasal regurgit$.tw,kw. | 145 |
| 37 | or/29-36 | 126,373 |
| 38 | exp Child/ | 1,851,179 |
| 39 | Infant/ | 773,452 |
| 40 | (infant$ or toddler$ or child$ or preschool$ or pre-school$ or schoolchild$ or parent or parental or parents).tw,kw. | 1,761,763 |
| 41 | or/38-40 | 2,774,290 |
| 42 | (intervention$ or therap$ or counsel$ or psychol$ or treat$ or manag$ or rehab$ or educat$ or train$ or teach$ or taught or exercis$).tw,kw. | 8,179,590 |
| 43 | 28 and 37 and 41 and 42 | 3249 |
| 44 | limit 43 to (english language and humans and yr=“1985 -Current”) | 2493 |
PsycINFO
Database(s): PsycINFO
Date range searched: 1806 to week 4 September 2017.
Date searched: 5 October 2017.
Search strategy
| # | Search terms | Results |
|---|---|---|
| 1 | exp Brain Disorders/ | 190,680 |
| 2 | cerebral palsy/ | 4737 |
| 3 | ((cerebral or brain or central) adj2 (pals$ or paralys#s or pares#s)).ti,ab,id. | 6985 |
| 4 | worster drought.ti,ab,id. | 15 |
| 5 | little? disease.ti,ab,id. | 22 |
| 6 | (pseudobulbar pals$ or pseudo bulbar pals$).ti,ab,id. | 65 |
| 7 | Neurodevelopmental Disorders/ | 1778 |
| 8 | exp Developmental Disabilities/ | 13,284 |
| 9 | (neurodisabilit$ or neuro-disabilit$ or neurodisabl$ or neuro-disabl$).ti,ab,id. | 100 |
| 10 | ((neurodevelopment$ or development$) adj3 (disease$ or disabl$ or disabil$ or disorder$ or dysfunction$ or function$ or impair$ or abnormal$ or difficulty or difficulties)).ti,ab,id. | 47,070 |
| 11 | exp Nervous System Disorders/ | 269,833 |
| 12 | ((brain$ or nervous system$) adj2 (injur$ or damag$ or dysfunction$ or function$ or malform$ or disease$ or impair$ or abnormal$)).ti,ab,id. | 64,967 |
| 13 | ((neurologic$ or neuromuscular or motor) adj3 (disease$ or disabl$ or disabil$ or disorder$ or dysfunction$ or function$ or impair$ or abnormal$ or difficulty or difficulties)).ti,ab,id. | 42,505 |
| 14 | ((muscular or muscle$ or myopath$) adj3 (disorder$ or disease$ or dysfunction$ or function$ or dystroph$)).ti,ab,id. | 3671 |
| 15 | (epilep$ or seizure$ or convuls$).ti,ab,id. | 50,802 |
| 16 | exp Communication Disorders/ | 52,539 |
| 17 | exp Learning Disorders/ | 32,467 |
| 18 | ((communicat$ or language or linguistic or speech or learning or intellectual$ or behaviour$ or behavior$) adj3 (disabl$ or disabil$ or disorder$ or dysfunction$ or function$ or impair$ or abnormal$ or difficulty or difficulties)).ti,ab,id. | 135,880 |
| 19 | Autism Spectrum Disorders/ | 36,228 |
| 20 | (autis$ or asperger$ or kanner$ or pervasive development$ disorder$).ti,ab,id. | 45,029 |
| 21 | exp Attention Deficit Disorder/ | 23,915 |
| 22 | ((attention$ or behav$ or conduct) adj3 (defic$ or dysfunc$ or disorder$)).ti,ab,id. | 62,834 |
| 23 | ((disrupt$ adj3 disorder$) or (disrupt$ adj3 behav$) or (defian$ adj3 disorder$) or (defian$ adj3 behav$)).ti,ab,id. | 10,920 |
| 24 | (impulsiv$ or inattentiv$ or inattention$).ti,ab,id. | 26,847 |
| 25 | (ADHD or ADDH or ADHS).ti,ab,id. | 24,202 |
| 26 | (down$ adj (syndrome$ or disease$)).ti,ab,id. | 6955 |
| 27 | (trisomy adj “21”).ti,ab,id. | 360 |
| 28 | (chromosome adj “21”).ti,ab,id. | 322 |
| 29 | (mongol or mongols or mongoloid or mongolism).ti,ab,id. | 718 |
| 30 | Cystic Fibrosis/ | 819 |
| 31 | cystic fibrosis.ti,ab,id. | 1107 |
| 32 | or/1-31 | 576,626 |
| 33 | Feeding Disorders/ | 261 |
| 34 | Dysphagia/ | 752 |
| 35 | Eating Behavior/ | 10,543 |
| 36 | Food refusal/ | 98 |
| 37 | dysphagi$.ti,ab,id. | 1441 |
| 38 | ((swallow$ or deglut$ or oral motor or oromotor or oro motor or oropharyn$ or pharyng$) adj3 (disorder$ or dysfunction$ or function$ or disabl$ or disabilit$ or impair$ or abnormal$)).ti,ab,id. | 712 |
| 39 | ((feed$ or food$ or eat$ or meal$) adj3 (problem$ or inabilit$ or difficult$ or behavio$ or selectiv$ or picky or habit$ or refus$)).ti,ab,id. | 25,618 |
| 40 | ((oropharynx$ or trachea$ or lung$ or pulmon$) adj3 aspirat$).ti,ab,id. | 25 |
| 41 | nasal regurgit$.ti,ab,id. | 5 |
| 42 | or/33-41 | 32,228 |
| 43 | (infant$ or toddler$ or child$ or preschool$ or pre-school$ or schoolchild$ or parent or parental or parents).ti,ab,id. | 752,377 |
| 44 | (intervention$ or therap$ or counsel$ or psychol$ or treat$ or manag$ or rehab$ or educat$ or train$ or teach$ or taught or exercis$).ti,ab,id. | 2,146,104 |
| 45 | 32 and 42 and 43 and 44 | 1042 |
| 46 | limit 45 to (human and english language and yr=“1985 -Current”) | 875 |
Cochrane Library
Database(s): Cochrane Central Register of Controlled Trials (190), Cochrane Database of Systematic Reviews (29), Database of Abstracts of Reviews of Effects (3) via the Cochrane Library (via Wiley Online Library)
Search name: FEEDS mapping review October 2017.
Date range searched: 1985 to 5 October 2017.
Date searched: 5 October 2017.
Description: for Helen Taylor and Lindsay Pennington.
Search strategy
-
#1. MeSH descriptor: [Nervous System Diseases] explode all trees
-
#2. MeSH descriptor: [Pseudobulbar Palsy] this term only
-
#3. MeSH descriptor: [Cerebral Palsy] this term only
-
#4. (pseudobulbar pals* or pseudo bulbar pals*):ti,ab
-
#5. ((cerebral or brain or central) near/2 (pals* or paralys?s or pares?s)):ti,ab
-
#6. worster drought:ti,ab
-
#7. little? disease:ti,ab
-
#8. MeSH descriptor: [Neurodevelopmental Disorders] explode all trees
-
#9. (neurodisabilit* or neuro-disabilit* or neurodisabl* or neuro-disabl*):ti,ab
-
#10. ((neurodevelopment* or development*) near/3 (disease* or disabl* or disabil* or disorder* or dysfunction* or function* or impair* or abnormal* or difficulty or difficulties)):ti,ab
-
#11. MeSH descriptor: [Nervous System Diseases] explode all trees
-
#12. ((brain* or nervous system*) near/2 (injur* or damag* or dysfunction* or function* or malform* or disease* or impair* or abnormal*)):ti,ab
-
#13. ((neurologic* or neuromuscular or motor) near/3 (disease* or disabl* or disabil* or disorder* or dysfunction* or function* or impair* or abnormal* or difficulty or difficulties)):ti,ab
-
#14. ((muscular or muscle* or myopath*) near/3 (disorder* or disease* or dysfunction* or function* or dystroph*)):ti,ab
-
#15. (epilep* or seizure* or convuls*):ti,ab
-
#16. ((communicat* or language or linguistic or speech or learning or intellectual* or behaviour* or behavior*) near/3 (disabl* or disabil* or disorder* or dysfunction* or function* or impair* or abnormal* or difficulty or difficulties)):ti,ab
-
#17. (autis* or asperger* or kanner* or pervasive development* disorder*):ti,ab
-
#18. ((attention* or behav* or conduct) near/3 (defic* or dysfunc* or disorder*)):ti,ab
-
#19. ((disrupt* near/3 disorder*) or (disrupt* near/3 behav*) or (defian* near/3 disorder*) or (defian* near/3 behav*)):ti,ab
-
#20. (impulsiv* or inattentiv* or inattention*):ti,ab
-
#21. (ADHD or ADDH or ADHS):ti,ab
-
#22. (down* next (syndrome* or disease*)):ti,ab
-
#23. (trisomy next “21”):ti,ab
-
#24. (chromosome next “21”):ti,ab
-
#25. MeSH descriptor: [Cystic Fibrosis] this term only
-
#26. cystic fibrosis:ti,ab
-
#27. {or #1-#26}
-
#28. MeSH descriptor: [Deglutition Disorders] explode all trees
-
#29. MeSH descriptor: [Feeding and Eating Disorders] this term only
-
#30. MeSH descriptor: [Feeding Behavior] this term only
-
#31. MeSH descriptor: [Food Preferences] this term only
-
#32. dysphagi*:ti,ab
-
#33. ((feed* or food* or eat* or meal*) near/3 (problem* or inabilit* or difficult* or behavio* or selectiv* or picky or habit* or refus*)):ti,ab
-
#34. ((oropharynx* or trachea* or lung* or pulmon*) near/3 aspirat*):ti,ab
-
#35. nasal regurgit*:ti,ab
-
#36. {Ek, #28-#35}
-
#37. MeSH descriptor: [Child] explode all trees
-
#38. MeSH descriptor: [Infant] this term only
-
#39. (infant* or toddler* or child* or preschool* or pre-school* or schoolchild* or parent or parental or parents):ti,ab
-
#40. {or #37-#39}
-
#41. #27 and #36 and #40 Publication Year from 1985 to 2017.
Cumulative Index to Nursing and Allied Health Literature (via EBSCOhost)
Date range searched: 1980 to September 2017.
Date searched: 5 October 2017.
Search strategy
| # | Search terms | Results |
|---|---|---|
| S47 | S44 AND S45 (1985-date) | 637 |
| S46 | S44 AND S45 | 642 |
| S45 | S31 AND S40 AND S43 | 1208 |
| S44 | TI (intervention* or therap* or counsel* or psychol* or treat* or manag* or rehab* or educat* or train* or teach* or taught or exercis*) OR AB (intervention* or therap* or counsel* or psychol* or treat* or manag* or rehab* or educat* or train* or teach* or taught or exercis*) | 1,155,507 |
| S43 | S41 OR S42 | 442,678 |
| S42 | TI (infant* or toddler* or child* or preschool* or pre-school* or schoolchild* or parent or parental or parents) OR AB (infant* or toddler* or child* or preschool* or pre-school* or schoolchild* or parent or parental or parents) | 284,575 |
| S41 | (MH “Child+”) | 348,905 |
| S40 | S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 | 29,482 |
| S39 | TI dysphagi* OR AB dysphagi* | 4114 |
| S38 | TI nasal regurgit* OR AB nasal regurgit* | 21 |
| S37 | TI ((oropharynx* or trachea* or lung* or pulmon*) N3 aspirat*) OR AB ((oropharynx* or trachea* or lung* or pulmon*) N3 aspirat*) | 469 |
| S36 | TI ((feed* or food* or eat* or meal*) N3 (problem* or inabilit* or difficult* or behavio* or selectiv* or picky or habit* or refus*)) OR AB ((feed* or food* or eat* or meal*) N3 (problem* or inabilit* or difficult* or behavio* or selectiv* or picky or habit* or refus*)) | 7854 |
| S35 | TI ((swallow* or deglut* or oral motor or oromotor or oro motor or oropharyn* or pharyng*) N3 (disorder* or dysfunction* or function* or disabl* or disabilit* or impair* or abnormal*)) OR AB ((swallow* or deglut* or oral motor or oromotor or oro motor or oropharyn* or pharyng*) N3 (disorder* or dysfunction* or function* or disabl* or disabilit* or impair* or abnormal*)) | 1547 |
| S34 | (MH “Eating Behavior+”) | 17,322 |
| S33 | (MH “Feeding and Eating Disorders of Childhood+”) | 206 |
| S32 | (MH “Deglutition Disorders”) | 4306 |
| S31 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 | 455,296 |
| S30 | TI cystic fibrosis OR AB cystic fibrosis | 4072 |
| S29 | (MH “Cystic Fibrosis”) | 4144 |
| S28 | TI ((communicat* or language or linguistic or speech or learning or intellectual* or behaviour* or behavior*) N3 (disabl* or disabil* or disorder* or dysfunction* or function* or impair* or abnormal* or difficulty or difficulties)) OR AB ((communicat* or language or linguistic or speech or learning or intellectual* or behaviour* or behavior*) N3 (disabl* or disabil* or disorder* or dysfunction* or function* or impair* or abnormal* or difficulty or difficulties)) | 31,122 |
| S27 | (MH “Communicative Disorders+”) | 21,643 |
| S26 | TI (mongol or mongols or mongoloid or mongolism) OR AB (mongol or mongols or mongoloid or mongolism) | 17 |
| S25 | TI (chromosome N1 “21”) OR AB (chromosome N1 “21”) | 72 |
| S24 | TI (trisomy N1 “21”) OR AB (trisomy N1 “21”) | 324 |
| S23 | TI (down* N1 (syndrome* or disease*)) OR AB (down* N1 (syndrome* or disease*)) | 2992 |
| S22 | (MH “Down Syndrome”) | 3576 |
| S21 | (MH “Intellectual Disability+”) | 16,407 |
| S20 | (MH “Developmental Disabilities”) | 4936 |
| S19 | TI (ADHD or ADDH or ADHS) OR AB (ADHD or ADDH or ADHS) | 4604 |
| S18 | TI (impulsiv* or inattentiv* or inattention*) OR AB (impulsiv* or inattentiv* or inattention*) | 2864 |
| S17 | TI ((disrupt* N3 disorder*) or (disrupt* N3 behav*) or (defian* N3 disorder*) or (defian* N3 behav*)) OR AB ((disrupt* N3 disorder*) or (disrupt* N3 behav*) or (defian* N3 disorder*) or (defian* N3 behav*)) | 1550 |
| S16 | TI ((attention* or behav* or conduct) N3 (defic* or dysfunc* or disorder*)) OR AB ((attention* or behav* or conduct) N3 (defic* or dysfunc* or disorder*)) | 9336 |
| S15 | (MH “Attention Deficit Hyperactivity Disorder”) | 7457 |
| S14 | TI (autis* or asperger* or kanner* or pervasive development* disorder*) OR AB (autis* or asperger* or kanner* or pervasive development* disorder*) | 12,312 |
| S13 | TI ((neurodevelopment* or development*) N3 (disease* or disabl* or disabil* or disorder* or dysfunction* or function* or impair* or abnormal* or difficulty or difficulties)) OR AB ((neurodevelopment* or development*) N3 (disease* or disabl* or disabil* or disorder* or dysfunction* or function* or impair* or abnormal* or difficulty or difficulties)) | 14,219 |
| S12 | TI (neurodisabilit* or neuro-disabilit* or neurodisabl* or neuro-disabl*) OR AB (neurodisabilit* or neuro-disabilit* or neurodisabl* or neuro-disabl*) | 103 |
| S11 | (MH “Child Development Disorders, Pervasive+”) | 13,677 |
| S10 | TI (pseudobulbar pals* or pseudo bulbar pals*) OR AB (pseudobulbar pals* or pseudo bulbar pals*) | 18 |
| S9 | TI “little? Disease” OR AB “little? Disease” | 104 |
| S8 | TI “little? Disease” OR AB “little? Disease” | 104 |
| S7 | TI “worster drought” OR AB “worster drought” | 7 |
| S6 | TI ((cerebral or brain or central) N2 (pals* or paralys#s or pares#s)) OR AB ((cerebral or brain or central) N2 (pals* or paralys#s or pares#s)) | 6804 |
| S5 | TI (epilep* or seizure* or convuls*) OR AB (epilep* or seizure* or convuls*) | 12,862 |
| S4 | TI ((muscular or muscle* or myopath*) N3 (disorder* or disease* or dysfunction* or function* or dystroph*)) OR AB ((muscular or muscle* or myopath*) N3 (disorder* or disease* or dysfunction* or function* or dystroph*)) | 6616 |
| S3 | TI ((neurologic* or neuromuscular or motor) N3 (disease* or disabl* or disabil* or disorder* or dysfunction* or function* or impair* or abnormal* or difficulty or difficulties)) OR AB ((neurologic* or neuromuscular or motor) N3 (disease* or disabl* or disabil* or disorder* or dysfunction* or function* or impair* or abnormal* or difficulty or difficulties)) | 18,975 |
| S2 | TI ((brain* or nervous system*) N2 (injur* or damag* or dysfunction* or function* or malform* or disease* or impair* or abnormal*)) OR AB ((brain* or nervous system*) N2 (injur* or damag* or dysfunction* or function* or malform* or disease* or impair* or abnormal)) | 21,057 |
| S1 | (MH “Nervous System Diseases+”) | 390,878 |
Education Resources Information Center (via EBSCOhost)
Date range searched: 1985 to 12 October 2017.
Date searched: 12 October 2017.
Search strategy
| # | Search items | Results |
|---|---|---|
| S38 | S27 AND S34 AND S35 AND S36 (1985-2017) | 232 |
| S37 | S27 AND S34 AND S35 AND S36 | 273 |
| S36 | TI (intervention* or therap* or counsel* or psychol* or treat* or manag* or rehab* or educat* or train* or teach* or taught or exercis*) OR AB (intervention* or therap* or counsel* or psychol* or treat* or manag* or rehab* or educat* or train* or teach* or taught or exercis*) | 1,066,419 |
| S35 | TI (infant* or toddler* or child* or preschool* or pre-school* or schoolchild* or parent or parental or parents) OR AB (infant* or toddler* or child* or preschool* or pre-school* or schoolchild* or parent or parental or parents) | 329,708 |
| S34 | S28 OR S29 OR S30 OR S31 OR S32 OR S33 | 6163 |
| S33 | TI dysphagi* OR AB dysphagi* | 88 |
| S32 | TI nasal regurgit* OR AB nasal regurgit* | 0 |
| S31 | TI ((oropharynx* or trachea* or lung* or pulmon*) N3 aspirat*) OR AB ((oropharynx* or trachea* or lung* or pulmon*) N3 aspirat*) | 2 |
| S30 | TI ((feed* or food* or eat* or meal*) N3 (problem* or inabilit* or difficult* or behavio* or selectiv* or picky or habit* or refus*)) OR AB ((feed* or food* or eat* or meal*) N3 (problem* or inabilit* or difficult* or behavio* or selectiv* or picky or habit* or refus*)) | 2944 |
| S29 | TI ((swallow* or deglut* or oral motor or oromotor or oro motor or oropharyn* or pharyng*) N3 (disorder* or dysfunction* or function* or disabl* or disabilit* or impair* or abnormal*)) OR AB ((swallow* or deglut* or oral motor or oromotor or oro motor or oropharyn* or pharyng*) N3 (disorder* or dysfunction* or function* or disabl* or disabilit* or impair* or abnormal*)) | 81 |
| S28 | (DE “Eating Disorders”) OR (DE “Eating Habits”) | 4263 |
| S27 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 | 84,631 |
| S26 | TI cystic fibrosis OR AB cystic fibrosis | 95 |
| S25 | TI ((communicat* or language or linguistic or speech or learning or intellectual* or behaviour* or behavior*) N3 (disabl* or disabil* or disorder* or dysfunction* or function* or impair* or abnormal* or difficulty or difficulties)) OR AB ((communicat* or language or linguistic or speech or learning or intellectual* or behaviour* or behavior*) N3 (disabl* or disabil* or disorder* or dysfunction* or function* or impair* or abnormal* or difficulty or difficulties)) | 45,190 |
| S24 | TI (mongol or mongols or mongoloid or mongolism) OR AB (mongol or mongols or mongoloid or mongolism) | 112 |
| S23 | TI (chromosome N1 “21”) OR AB (chromosome N1 “21”) | 15 |
| S22 | TI (trisomy N1 “21”) OR AB (trisomy N1 “21”) | 30 |
| S21 | TI (down* N1 (syndrome* or disease*)) OR AB (down* N1 (syndrome* or disease*)) | 1944 |
| S20 | TI (ADHD or ADDH or ADHS) OR AB (ADHD or ADDH or ADHS) | 3280 |
| S19 | TI (impulsiv* or inattentiv* or inattention*) OR AB (impulsiv* or inattentiv* or inattention*) | 2592 |
| S18 | TI ((disrupt* N3 disorder*) or (disrupt* N3 behav*) or (defian* N3 disorder*) or (defian* N3 behav*)) OR AB ((disrupt* N3 disorder*) or (disrupt* N3 behav*) or (defian* N3 disorder*) or (defian* N3 behav*)) | 2494 |
| S17 | TI ((attention* or behav* or conduct) N3 (defic* or dysfunc* or disorder*)) OR AB ((attention* or behav* or conduct) N3 (defic* or dysfunc* or disorder*)) | 9247 |
| S16 | TI (autis* or asperger* or kanner* or pervasive development* disorder*) OR AB (autis* or asperger* or kanner* or pervasive development* disorder*) | 12,391 |
| S15 | TI ((neurodevelopment* or development*) N3 (disease* or disabl* or disabil* or disorder* or dysfunction* or function* or impair* or abnormal* or difficulty or difficulties)) OR AB ((neurodevelopment* or development*) N3 (disease* or disabl* or disabil* or disorder* or dysfunction* or function* or impair* or abnormal* or difficulty or difficulties)) | 10,466 |
| S14 | TI (neurodisabilit* or neuro-disabilit* or neurodisabl* or neuro-disabl*) OR AB (neurodisabilit* or neuro-disabilit* or neurodisabl* or neuro-disabl*) | 2 |
| S13 | TI (pseudobulbar pals* or pseudo bulbar pals*) OR AB (pseudobulbar pals* or pseudo bulbar pals*) | 1 |
| S12 | TI “little? Disease” OR AB “little? Disease” | 0 |
| S11 | TI “worster drought” OR AB “worster drought” | 2 |
| S10 | TI ((cerebral or brain or central) N2 (pals* or paralys#s or pares#s)) OR AB ((cerebral or brain or central) N2 (pals* or paralys#s or pares#s)) | 1272 |
| S9 | TI (epilep* or seizure* or convuls*) OR AB (epilep* or seizure* or convuls*) | 1313 |
| S8 | TI ((muscular or muscle* or myopath*) N3 (disorder* or disease* or dysfunction* or function* or dystroph*)) OR AB ((muscular or muscle* or myopath*) N3 (disorder* or disease* or dysfunction* or function* or dystroph*)) | 169 |
| S7 | TI ((neurologic* or neuromuscular or motor) N3 (disease* or disabl* or disabil* or disorder* or dysfunction* or function* or impair* or abnormal* or difficulty or difficulties)) OR AB ((neurologic* or neuromuscular or motor) N3 (disease* or disabl* or disabil* or disorder* or dysfunction* or function* or impair* or abnormal* or difficulty or difficulties)) | 2034 |
| S6 | TI ((brain* or nervous system*) N2 (injur* or damag* or dysfunction* or function* or malform* or disease* or impair* or abnormal*)) OR AB ((brain* or nervous system*) N2 (injur* or damag* or dysfunction* or function* or malform* or disease* or impair* or abnormal)) | 2576 |
| S5 | DE “Special Health Problems” | 1814 |
| S4 | DE “Learning Disabilities” OR DE “Pervasive Developmental Disorders” OR DE “Asperger Syndrome” OR DE “Autism” | 29,691 |
| S3 | ((DE “Head Injuries”) OR (DE “Intellectual Disability” OR DE “Down Syndrome” OR DE “Mild Intellectual Disability” OR DE “Moderate Intellectual Disability” OR DE “Severe Intellectual Disability”)) OR (DE “Language Impairments”) | 5521 |
| S2 | DE “Attention Deficit Disorders” OR DE “Attention Deficit Hyperactivity Disorder” OR DE “Behavior Disorders” OR DE “Addictive Behavior” OR DE “Communication Disorders” OR DE “Developmental Disabilities” | 16,384 |
| S1 | DE “Neurological Impairments” OR DE “Cerebral Palsy” OR DE “Epilepsy” | 5943 |
EMBASE
Database(s): EMBASE
Date range searched: 1974 to week 41 2017.
Date searched: 10 October 2017.
Search strategy
| # | Search terms | Results |
|---|---|---|
| 1 | exp *neurologic disease/ | 1,963,193 |
| 2 | (pseudobulbar pals$ or pseudo bulbar pals$).tw,kw. | 471 |
| 3 | ((cerebral or brain or central) adj2 (pals$ or paralys#s or pares#s)).tw,kw. | 28,470 |
| 4 | worster drought.tw,kw. | 39 |
| 5 | little? disease.tw,kw. | 81 |
| 6 | *mental disease/ or exp *autism/ or exp *behavior disorder/ or exp *emotional disorder/ or exp *learning disorder/ or exp *mental deficiency/ | 411,892 |
| 7 | *developmental disorder/ | 11,265 |
| 8 | (neurodisabilit$ or neuro-disabilit$ or neurodisabl$ or neuro-disabl$).tw,kw. | 472 |
| 9 | ((neurodevelopment$ or development$) adj3 (disease$ or disabl$ or disabil$ or disorder$ or dysfunction$ or function$ or impair$ or abnormal$ or difficulty or difficulties)).tw,kw. | 176,258 |
| 10 | ((brain$ or nervous system$) adj2 (injur$ or damag$ or dysfunction$ or function$ or malform$ or disease$ or impair$ or abnormal$)).tw,kw. | 203,264 |
| 11 | ((neurologic$ or neuromuscular or motor) adj3 (disease$ or disabl$ or disabil$ or disorder$ or dysfunction$ or function$ or impair$ or abnormal$ or difficulty or difficulties)).tw,kw. | 212,019 |
| 12 | ((muscular or muscle$ or myopath$) adj3 (disorder$ or disease$ or dysfunction$ or function$ or dystroph$)).tw,kw. | 78,516 |
| 13 | (epilep$ or seizure$ or convuls$).tw,kw. | 261,925 |
| 14 | ((communicat$ or language or linguistic or speech or learning or intellectual$ or behaviour$ or behavior$) adj3 (disabl$ or disabil$ or disorder$ or dysfunction$ or function$ or impair$ or abnormal$ or difficulty or difficulties)).tw,kw. | 155,797 |
| 15 | (autis$ or asperger$ or kanner$ or pervasive development$ disorder$).tw,kw. | 49,956 |
| 16 | ((attention$ or behav$ or conduct) adj3 (defic$ or dysfunc$ or disorder$)).tw,kw. | 75,099 |
| 17 | ((disrupt$ adj3 disorder$) or (disrupt$ adj3 behav$) or (defian$ adj3 disorder$) or (defian$ adj3 behav$)).tw,kw. | 9691 |
| 18 | (impulsiv$ or inattentiv$ or inattention$).tw,kw. | 28,167 |
| 19 | (ADHD or ADDH or ADHS).tw,kw. | 29,554 |
| 20 | (down$ adj (syndrome$ or disease$)).tw,kw. | 26,400 |
| 21 | (trisomy adj “21”).tw,kw. | 7614 |
| 22 | (chromosome adj “21”).tw,kw. | 4305 |
| 23 | (mongol or mongols or mongoloid or mongolism).tw,kw. | 1950 |
| 24 | *cystic fibrosis/ | 42,073 |
| 25 | cystic fibrosis.tw,kw. | 56,386 |
| 26 | or/1-25 | 2,922,784 |
| 27 | exp *dysphagia/ | 14,725 |
| 28 | *eating disorder/ or *food aversion/ | 10,534 |
| 29 | *feeding behavior/ or *eating habit/ or *food preference/ | 41,732 |
| 30 | dysphagi$.tw,kw. | 38,441 |
| 31 | ((swallow$ or deglut$ or oral motor or oromotor or oro motor or oropharyn$ or pharyng$) adj3 (disorder$ or dysfunction$ or function$ or disabl$ or disabilit$ or impair$ or abnormal$)).tw,kw. | 10,706 |
| 32 | ((feed$ or food$ or eat$ or meal$) adj3 (problem$ or inabilit$ or difficult$ or behavio$ or selectiv$ or picky or habit$ or refus$)).tw,kw. | 56,375 |
| 33 | ((oropharynx$ or trachea$ or lung$ or pulmon$) adj3 aspirat$).tw,kw. | 5496 |
| 34 | nasal regurgit$.tw,kw. | 186 |
| 35 | or/27-34 | 146,116 |
| 36 | exp *child/ | 166,529 |
| 37 | (infant$ or toddler$ or child$ or preschool$ or pre-school$ or schoolchild$ or parent or parental or parents).tw,kw. | 2,016,492 |
| 38 | or/36-37 | 2,059,013 |
| 39 | (intervention$ or therap$ or counsel$ or psychol$ or treat$ or manag$ or rehab$ or educat$ or train$ or teach$ or taught or exercis$).tw,kw. | 10,174,570 |
| 40 | 26 and 35 and 38 and 39 | 3970 |
| 41 | limit 40 to (human and english language and yr=“1985 -Current”) | 3167 |
Web of Science
Database(s): Science Citation Index – EXPANDED, Social Sciences Citation Index™ (Clarivate Analytics), Conference Proceedings Citation Index – Science, Conference Proceedings Citation Index – Social Science & Humanities, and Emerging Sources Citation Index (ESCI) (Clarivate Analytics, Philadelphia, PA, USA)
Date range searched: 1985 to 17 October 2017.
Date searched: 17 October 2017.
Search strategy
| # | Search items | Results |
|---|---|---|
| #33 | #31 NOT #32 | 1447 |
| #32 | TS=(bulimi* OR anorexi*) | 38,577 |
| #31 | #29 NOT #30 | 1616 |
| #30 | TS=(animal* or rat or rats or mouse or mice or murine or monkey* or pig or pigs or porcine) | 3,477,625 |
| #29 |
#27 AND #26 AND #25 AND #20 Refined by: LANGUAGES: ( ENGLISH ) |
1668 |
| #28 | #27 AND #26 AND #25 AND #20 | 1812 |
| #27 | TS=(intervention* or therap* or counsel* or psychol* or treat* or manag* or rehab* or educat* or train* or teach* or taught or exercis*) | 9,357,277 |
| #26 | TS=(infant* or toddler* or child* or preschool* or pre-school* or schoolchild* or parent or parental or parents) | 1,790,342 |
| #25 | #24 OR #23 OR #22 OR #21 | 108,968 |
| #24 | TS=“nasal regurgit*” | 91 |
| #23 | TS=((oropharynx* or trachea* or lung* or pulmon*) NEAR/3 aspirat*) | 3943 |
| #22 | TS=dysphagi* | 20,588 |
| #21 | TS=((feed* or food* or eat* or meal*) NEAR/3 (problem* or inabilit* or difficult* or behavio* or selectiv* or picky or habit* or refus*)) | 85,010 |
| #20 | #19 OR #18 OR #17 OR #16 OR #15 OR #14 OR #13 OR #12 OR #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 | 1,072,452 |
| #19 | TS=“cystic fibrosis” | 56,515 |
| #18 | TS=(trisomy NEAR/1 “21”) OR TS=(chromosome NEAR/1 “21”) | 9894 |
| #17 | TS=(down* NEAR/1 (syndrome* or disease*)) | 26,491 |
| #16 | TS=(ADHD or ADDH or ADHS) | 26,979 |
| #15 | TS=(impulsiv* or inattentiv* or inattention*) | 48,827 |
| # 14 | TS=(disrupt* NEAR/3 disorder*) or TS=(disrupt* NEAR/3 behav*) or TS=(defian* NEAR/3 disorder*) or TS=(defian* NEAR/3 behav*) | 11,266 |
| #13 | TS=((attention* or behav* or conduct) NEAR/3 (defic* or dysfunc* or disorder*)) | 77,070 |
| #12 | TI=(autis* or asperger* or kanner* or “pervasive development* disorder*”) | 34,923 |
| #11 | TS=((communicat* or language or linguistic or speech or learning or intellectual* or behaviour* or behavior*) NEAR/3 (disabl* or disabil* or disorder* or dysfunction* or function* or impair* or abnormal* or difficulty or difficulties)) | 191,165 |
| #10 | TS=(epilep* or seizure* or convuls*)Indexes=SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH, ESCI Timespan=1985-2017 | 188,368 |
| #9 | TS=((muscular or muscle* or myopath*) NEAR/3 (disorder* or disease* or dysfunction* or function* or dystroph*)) | 67,449 |
| #8 | TS=((neurologic* or neuromuscular or motor) NEAR/3 (disease* or disabl* or disabil* or disorder* or dysfunction* or function* or impair* or abnormal* or difficulty or difficulties)) | 150,256 |
| #7 | TS=((brain* or “nervous system*”) NEAR/2 (injur* or damag* or dysfunction* or function* or malform* or disease* or impair* or abnormal*)) | 196,035 |
| #6 | TS=((neurodevelopment* or development*) NEAR/3 (disease* or disabl* or disabil* or disorder* or dysfunction* or function* or impair* or abnormal* or difficulty or difficulties)) | 172,440 |
| #5 | TS=(neurodisabilit* or neuro-disabilit* or neurodisabl* or neuro-disabl*) | 234 |
| #4 | TS=“little* disease” | 65 |
| #3 | TS=worster drought | 51 |
| #2 | TS=(“pseudobulbar pals*” or “pseudo bulbar pals*”) | 236 |
| #1 | TS=((cerebral or brain or central) and (pals* or paralys*)) | 33,692 |
speechBITE
URL: http://speechbite.com/.
Date range searched: 1985–2017.
Date searched: 5 October 2017.
Number of results: 42.
Advanced search
Speech pathology practice area: dysphagia.
Age group: children.
OTseeker
URL: www.otseeker.com/Search/SearchBuilder.aspx.
Date searched: 5 October 2017.
Number of results: three.
Advanced search
Age group: paediatric/adolescent.
AND
Title/Abstract: meal* OR food* OR feeding OR eat* OR swallow*
AND
Title/Abstract: neuro* OR brain* OR nervous system OR disorder* OR autis* OR asperg* OR cystic* OR cerebral palsy OR Down* OR disabil* OR disabl*
Appendix 5 Study designs used to evaluate each intervention in the mapping review
| Intervention | Study design | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Systematic review including a RCT | Systematic review not including a RCT | RCT | Quasi-experimental design | Feasibility study | Single-case experimental design | Before-and-after study | Literature review | Case study | |
| Modifying environment | – | – | ✓ | – | – | ✓ | ✓ | – | ✓ |
| Positioning | ✓ | ✓ | ✓ | ✓ | – | ✓ | ✓ | ✓ | ✓ |
| Modifying equipment | – | – | ✓ | ✓ | – | ✓ | ✓ | – | ✓ |
| Scheduling of meals | – | – | ✓ | ✓ | – | ✓ | ✓ | – | ✓ |
| Modifying food or drink | ✓ | ✓ | ✓ | ✓ | – | ✓ | ✓ | ✓ | ✓ |
| Modifying placement of food | ✓ | ✓ | ✓ | ✓ | – | ✓ | – | – | – |
| Enhancing communication | – | – | ✓ | – | – | ✓ | ✓ | – | ✓ |
| Visual supports | – | ✓ | ✓ | – | ✓ | ✓ | ✓ | – | ✓ |
| Responding to a child’s cues for feeding | – | – | – | ✓ | – | – | ✓ | – | – |
| Pace of feeding | – | ✓ | ✓ | – | – | ✓ | ✓ | – | ✓ |
| Physical support | ✓ | – | – | ✓ | – | ✓ | – | – | ✓ |
| Oral or sensory desensitisation | ✓ | ✓ | ✓ | – | – | ✓ | ✓ | ✓ | ✓ |
| Oral motor exercises | ✓ | ✓ | ✓ | – | – | ✓ | ✓ | ✓ | ✓ |
| Food desensitisation | – | – | ✓ | – | ✓ | ✓ | ✓ | – | ✓ |
| Prompting | ✓ | ✓ | ✓ | – | ✓ | ✓ | ✓ | – | ✓ |
| Reinforcement | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Psychological support for parent | – | – | – | – | – | – | ✓ | – | ✓ |
| Psychological support for child | – | – | – | – | ✓ | – | – | – | – |
Appendix 6 First round of focus groups topic guide
Introduction
-
Thank you for attending.
-
Introductions of everyone involved in group: Research Associate (RA), other research team members and their roles.
-
Introductions of participants – acknowledge that some participants may know each other in a personal or professional role. If this is the case, reinforce confidentiality issues and clarify that they are comfortable with continued participation.
-
Explain purpose of project and this focus group:
-
We are looking for feedback from people involved in treatments for eating, drinking and swallowing for young children with neurodisability that can be delivered by parents at home: parents, professionals and young people.
-
We want to know about their experiences of treatments for EDSD for young children with neurodisability, the practicalities – good and bad.
-
No right or wrong answers – not a test of knowledge.
-
-
Ground rules of group – everyone’s contribution is valued; confidentiality.
-
Consent – written – because we want to be reviewing content and analysing data.
-
Explain group discussion audio-recorded to make sure that it is documented accurately, but details will be confidential.
-
If participants have any questions about care of a relative or client the RA the participant will be listened to, answered briefly and then offered the opportunity to discuss with the team afterward. We will try to signpost them to someone locally who can help.
-
Questions or concerns?
Ask participants to read and sign consent forms
Participants, experience of treatments for eating, drinking and swallowing for young children with neurodisability
-
Which interventions have you experience of?
-
Where and when – setting of use? School or home or other.
-
Who prescribes the treatments?
-
What are the good things about the treatments?
-
What are the challenges to the treatments? What do you not like?
-
What/who do the treatments work best for?
-
-
How has the success of the treatments been evaluated?
-
What are the important outcomes to measure regarding treatments for eating, drinking and swallowing?
-
How should these be measured? Are any specific tools known about?
Give participants debrief information sheet
Appendix 7 Initial list of outcome measurement tools compiled for searching
These were compiled from the systematic reviews and the mapping review, focus groups and survey, with additions from the measurement properties search from 20 June 2018.
| Tool | Reason for exclusion |
|---|---|
| American Speech–Language–Hearing Association’s National Outcomes Measurement System (studies use the swallowing scale) | |
| Battery for Oral-Motor Behavior in Children | |
| Beckman Oral Motor Assessment | |
| Behavioural Assessment of Oral Functions in Feeding | Poor rating in Benfer 201296 systematic review |
| Behavioural Paediatric Feeding Assessment Scale | |
| Brief Autism Mealtime Behaviour Inventory | |
| Children’s Eating Behaviour Inventory | |
| Dietary Intake for Children’s Eating | |
| Dyadic Interaction Nomenclature for Eating | |
| Dysphagia Disorders Survey (used as assessment tool) | Poor rating in Benfer 201296 systematic review and cost of training |
| Dysphagia Management Staging Scale (used as assessment tool) | Severity categories are derived from DDS (see above) |
| Exeter Dysphagia Assessment Technique | |
| Expanded Orofacial Myofunctional Evaluation with Scores | |
| Feeding Interaction Report Scale and Treatment | |
| Feeding Outcome Measure (cited in Cockerill et al.120 2011) | |
| Food choice questionnaire (cited in Zhang et al.121 2012) | |
| Food Preference Inventory | |
| Food Frequency Questionnaire | |
| Functional Analysis Interview Form | |
| Functional Assessment Scale | |
| Functional Oral Intake Scale | Not an outcome measure (7-point scale of function) |
| Gisel Video Assessment | Poor rating in Benfer 201296 systematic review |
| Karaduman Chewing Performance Scale | Lacks precision – 5-point scale of severity |
| Meals in Our Household (Anderson 201270) | |
| Morris Pre-speech Assessment Scale | |
| Motivation Assessment Scale | Not feeding |
| Multidisciplinary Feeding Profile (most use the Functional Feeding subtest of this measure) | |
| National Institutes of Health Toolbox for Assessment of Neurological and Behavioural Function | (Note that only the cognitive battery is reported) |
| Neonatal Oral Motor Assessment Scale | Exclude on restricted young age range |
| Oral Assessment Function Form | |
| Oral Motor Assessment Scale | |
| Oral Motor Dysfunction Scale | |
| Oral Sensory Motor Analysis (cited in Twatchman-Reilly et al.122 2008) | |
| Orofacial Myofunctional Evaluation with Scores | |
| Paediatric Feeding Evaluation Checklist – modified (cited from Abou ElSaad and Abdel Latif44 2008) | |
| Parent Mealtime Action Scale | |
| Pre-Speech Assessment Scale | Poor rating in Benfer 201296 systematic review |
| Schedule of Oral Motor Assessment | |
| Screening Tool of Feeding Problems | |
| Sensory Processing Measure | Not feeding |
| Therapy Outcome Measures | Lacks precision, 5-point scale for function |
| Vulpe Assessment Battery | |
| 3-day diet records | |
| New tools identified from the first searches of PsycINFO and MEDLINE | |
| Feeding Strategies Questionnaire | |
| About Your Child’s Eating | |
| Brief Assessment of Mealtime Behaviour in Children | |
| Mastication Observation and Evaluation | |
| Eating and Drinking ability classification system | Not an outcome measure – classification of function |
| Parent Mealtime Action Scale Revised | |
| Action MEAL Plan | Not an outcome measure (a curriculum) |
| Child Feeding Assessment Questionnaire | |
| Texture Problems (Seiverling et al.74 2011) | Lacks precision (4-item scale) |
| Feeding Interaction Report – Scale and Treatment | |
| Child Eating Behaviour Questionnaire (Wardle et al.69 2001) | |
| Novel Mealtime Duration Measure | Lacks precision |
| Cerebral Palsy Child Feeding Questionnaire | |
| Drooling Severity and Frequency Scale (Serel Arslan et al.123 2017) | Lacks precision (4-point scale) |
| Tongue Thrust Rating Scale (Serel Arslan et al.123 2017) | Lacks precision (single behaviour) |
| Brief Assessment of Motor Function Oral Motor Scales (in Sonies et al.124 2009) | Poor rating in Benfer 201296 systematic review |
| Orofacial Motor Function Assessment Scale (Santos et al.125 2005) | Not an outcome measure; tested in middle childhood |
| Paediatric Eating Assessment Tool (Thoyre71 2014) | |
| Functional Feeding Assessment Scale | |
| Feeding Behaviour Scale | Poor rating in Benfer 201296 systematic review |
| Paediatric version of the Eating Assessment Tool (PEDI-EAT-10) (Serel Arslan et al.126 2018) | |
Appendix 8 Search strategies for measurement properties review (example for MEDLINE)
The FEEDS measurement tools: ‘COSMIN’ review
Search 1
Database(s): Ovid MEDLINE In-Process & Other Non-Indexed Citations and Ovid MEDLINE
Date range searched: 1946 to week 2 May 2018.
Date searched: 12 May 2018.
This used the same strategy as for the mapping review in terms of the population, but combined differently so that it retrieves:
[neurodisability OR feeding disorders] AND children AND [any of the specified tools] AND COSMIN strategy (McConachie et al. 127)
PsycINFO search run first, and this search de-duplicated against it.
Search strategy
| # | Search terms | Results |
|---|---|---|
| 1 | exp Brain Diseases/ | 1,105,957 |
| 2 | Pseudobulbar Palsy/ | 133 |
| 3 | (pseudobulbar pals$ or pseudo bulbar pals$).tw,kw. | 364 |
| 4 | Cerebral Palsy/ | 18,709 |
| 5 | ((cerebral or brain or central) adj2 (pals$ or paralys#s or pares#s)).tw,kw. | 20,241 |
| 6 | worster drought.tw,kw. | 31 |
| 7 | little? disease.tw,kw. | 89 |
| 8 | exp Neurodevelopmental Disorders/ | 164,507 |
| 9 | (neurodisabilit$ or neuro-disabilit$ or neurodisabl$ or neuro-disabl$).tw,kw. | 275 |
| 10 | ((neurodevelopment$ or development$) adj3 (disease$ or disabl$ or disabil$ or disorder$ or dysfunction$ or function$ or impair$ or abnormal$ or difficulty or difficulties)).tw,kw. | 135,989 |
| 11 | exp Nervous System Diseases/ | 2,299,317 |
| 12 | ((brain$ or nervous system$) adj2 (injur$ or damag$ or dysfunction$ or function$ or malform$ or disease$ or impair$ or abnormal$)).tw,kw. | 146,151 |
| 13 | ((neurologic$ or neuromuscular or motor) adj3 (disease$ or disabl$ or disabil$ or disorder$ or dysfunction$ or function$ or impair$ or abnormal$ or difficulty or difficulties)).tw,kw. | 153,575 |
| 14 | ((muscular or muscle$ or myopath$) adj3 (disorder$ or disease$ or dysfunction$ or function$ or dystroph$)).tw,kw. | 59,337 |
| 15 | (epilep$ or seizure$ or convuls$).tw,kw. | 189,685 |
| 16 | ((communicat$ or language or linguistic or speech or learning or intellectual$ or behaviour$ or behavior$) adj3 (disabl$ or disabil$ or disorder$ or dysfunction$ or function$ or impair$ or abnormal$ or difficulty or difficulties)).tw,kw. | 117,682 |
| 17 | (autis$ or asperger$ or kanner$ or pervasive development$ disorder$).tw,kw. | 38,933 |
| 18 | ((attention$ or behav$ or conduct) adj3 (defic$ or dysfunc$ or disorder$)).tw,kw. | 55,066 |
| 19 | ((disrupt$ adj3 disorder$) or (disrupt$ adj3 behav$) or (defian$ adj3 disorder$) or (defian$ adj3 behav$)).tw,kw. | 7602 |
| 20 | (impulsiv$ or inattentiv$ or inattention$).tw,kw. | 21,069 |
| 21 | (ADHD or ADDH or ADHS).tw,kw. | 20,993 |
| 22 | (down$ adj (syndrome$ or disease$)).tw,kw. | 20,549 |
| 23 | (trisomy adj “21”).tw,kw. | 5248 |
| 24 | (chromosome adj “21”).tw,kw. | 3461 |
| 25 | (mongol or mongols or mongoloid or mongolism).tw,kw. | 2560 |
| 26 | Cystic Fibrosis/ | 32,659 |
| 27 | cystic fibrosis.tw,kw. | 39,524 |
| 28 | or/1-27 | 2,774,710 |
| 29 | exp Deglutition Disorders/ | 48,281 |
| 30 | “Feeding and Eating Disorders of Childhood”/ | 480 |
| 31 | Food Preferences/ | 12,780 |
| 32 | dysphagi$.tw,kw. | 23,841 |
| 33 | ((swallow$ or deglut$ or oral motor or oromotor or oro motor or oropharyn$ or pharyng$) adj3 (disorder$ or dysfunction$ or function$ or disabl$ or disabilit$ or impair$ or abnormal$)).tw,kw. | 6330 |
| 34 | ((feed$ or food$ or eat$ or meal$) adj3 (problem$ or inabilit$ or difficult$ or behavio$ or selectiv$ or picky or habit$ or refus$)).tw,kw. | 44,268 |
| 35 | ((oropharynx$ or trachea$ or lung$ or pulmon$) adj3 aspirat$).tw,kw. | 3949 |
| 36 | nasal regurgit$.tw,kw. | 142 |
| 37 | or/29-36 | 121,135 |
| 38 | 28 or 37 | 2,874,517 |
| 39 | exp Child/ | 1,770,134 |
| 40 | Infant/ | 738,151 |
| 41 | (infant$ or toddler$ or child$ or preschool$ or pre-school$ or schoolchild$ or parent or parental or parents).tw,kw. | 1,675,177 |
| 42 | or/39-41 | 2,638,929 |
| 43 | 38 and 42 | 580,527 |
| 44 | ((Speech adj1 Language adj1 Hearing) and (outcome* or NOMS)).ab,ti,id. | 55 |
| 45 | ((battery or behavior or behaviour or assessment) adj3 oral motor).ti,ab,id. | 95 |
| 46 | (((Assessment or Feeding) adj3 Oral Function*) or BASOFF).ti,ab,id. | 14 |
| 47 | (Behavio?ral P?ediatric Feeding Assessment Scale or BPFAS).ti,ab,id. | 19 |
| 48 | Autism Mealtime Behavio?r Inventory.ti,ab,id. | 4 |
| 49 | Eating Behavio?r Inventory.ti,ab,id. | 20 |
| 50 | Dietary Intake for Children* Eating.ti,ab,id. | 1 |
| 51 | Dyadic Interaction Nomenclature for Eating.ti,ab,id. | 4 |
| 52 | Dysphagia Disorder* Survey.ti,ab,id. | 20 |
| 53 | Dysphagia Manage* Staging Scale.ti,ab,id. | 1 |
| 54 | Exeter Dysphagia Assessment Technique.ti,ab,id. | 7 |
| 55 | (Expanded Orofacial Myofunctional Evaluation or OMES-E).ti,ab,id. | 4 |
| 56 | (Feeding Interaction Report Scale adj2 Treatment).ti,ab,id. | 0 |
| 57 | Feeding Outcome Measure*.ti,ab,id. | 4 |
| 58 | Food choice questionnaire.ti,ab,id. | 40 |
| 59 | Food Preference Inventory.ti,ab,id. | 1 |
| 60 | Food Frequency Questionnaire.ti,ab,id. | 8197 |
| 61 | Functional Analysis Interview Form.ti,ab,id. | 1 |
| 62 | Functional Assessment Scale.ti,ab,id. | 92 |
| 63 | Functional Oral Intake Scale.ti,ab,id. | 95 |
| 64 | Gisel Video Assessment.ti,ab,id. | 2 |
| 65 | Karaduman Chewing Performance Scale.ti,ab,id. | 4 |
| 66 | Meals in Our Household.ti,ab,id. | 2 |
| 67 | Morris Pre speech Assessment Scale.ti,ab,id. | 0 |
| 68 | Motivation Assessment Scale.ti,ab,id. | 19 |
| 69 | Multidisciplinary Feeding Profile.ti,ab,id. | 5 |
| 70 | ((National Institute or NIH) adj3 Toolbox).ti,ab,id. | 110 |
| 71 | (Neonatal Oral Motor Assessment Scale or Neo natal Oral Motor Assessment Scale or NOMAS).ti,ab,id. | 96 |
| 72 | Oral Assessment Function Form.ti,ab,id. | 0 |
| 73 | Oral Motor Assessment Scale.ti,ab,id. | 32 |
| 74 | Oral Motor Dysfunction Scale.ti,ab,id. | 0 |
| 75 | Oral Sensory Motor Analysis.ti,ab,id. | 0 |
| 76 | (Orofacial Myofunctional Evaluation or Oro facial Myofunctional Evaluation).ti,ab,id. | 24 |
| 77 | P?ediatric Feeding Evaluation Checklist.ti,ab,id. | 0 |
| 78 | (Parent Mealtime Action Scale or Parent Meal time Action Scale).ti,ab,id. | 10 |
| 79 | Pre Speech Assessment Scale.ti,ab,id. | 5 |
| 80 | (Schedule adj2 Oral Motor Assessment).ti,ab,id. | 13 |
| 81 | Sensory Processing Measure.ti,ab,id. | 25 |
| 82 | Vulpe Assessment Battery.ti,ab,id. | 4 |
| 83 | Day Diet Records.ti,ab,id. | 207 |
| 84 | or/44-83 | 9108 |
| 85 | 43 and 84 | 537 |
| 86 | (instrumentation or methods).sh. or Validation Studies.pt. or Comparative Study.pt. or psychometrics/ or psychometr*.ab,ti. or clinimetr*.tw. or clinometr*.tw. or “Outcome Assessment (Health Care)”/ or outcome assessment.ab,ti. or outcome measure*.tw. or observer variation/ or observer variation.ab,ti. or Health Status Indicators/ or reproducibility of results/ or reproducib*.ab,ti. or discriminant analysis/ or reliab*.ab,ti. or unreliab*.ab,ti. or valid*.ab,ti. or coefficient.ab,ti. or homogeneity.ab,ti. or homogeneous.ab,ti. or internal consistency.ab,ti. | 3,533,315 |
| 87 | ((cronbach* and (alpha or alphas)) or (item and (correlation* or selection* or reduction*))).ab,ti. | 31,105 |
| 88 | (agreement or precision or imprecision or precise values or test-retest or (test and retest) or (reliab* and (test or retest)) or stability).ab,ti. | 734,363 |
| 89 | (interrater or inter-rater or intrarater or intra-rater or intertester or inter-tester or intratester or intra-tester or interobserver or inter-observer or intraobserver or intraobserver or intertechnician or inter-technician or intratechnician or intra-technician or interexaminer or inter-examiner or intraexaminer or intra-examiner).ab,ti. | 39,903 |
| 90 | (interassay or inter-assay or intraassay or intra-assay).ab,ti. | 8476 |
| 91 | (interindividual or inter-individual or intraindividual or intra-individual).ab,ti. | 30,511 |
| 92 | (interparticipant or inter-articipant or intraparticipant or intra-participant).ab,ti. | 75 |
| 93 | (kappa or kappa? or kappas or repeatab*).ab,ti. | 173,798 |
| 94 | (((replicab* or repeated) and (measure or measures or findings or result or results or test or tests)) or generaliza* or generalisa* or concordance).ab,ti. | 241,147 |
| 95 | ((intraclass and correlation*) or discriminative or known group or factor analysis or factor analyses or dimension* or subscale* or (multitrait and scaling and (analysis or analyses)) or item discriminant or interscale correlation* or error or errors or individual variability or (variability and (analysis or values)) or (uncertainty and (measurement or measuring))).ab,ti. | 883,278 |
| 96 | (standard error of measurement or sensitiv* or responsive* or ((minimal or minimally or clinical or clinically) and (important or significant or detectable) and (change or difference)) or (small* and (real or detectable) and (change or difference)) or meaningful change).ab,ti. | 1,568,033 |
| 97 | (ceiling effect or floor effect or Item response model or IRT or Rasch or Differential item functioning or DIF or computer adaptive testing or item bank or cross-cultural equivalence).ab,ti. | 9728 |
| 98 | or/86-97 | 5,895,673 |
| 99 | 85 and 98 | 267 |
Search 2
Database(s): Ovid MEDLINE In-Process & Other Non-Indexed Citations and Ovid MEDLINE
Date range searched: 1946 to 6 July 2018.
Same as above but to capture some extra tools.
De-duplicated this strategy against the one above in EndNote: 123 further results.
Search strategy
| # | Search items | Results |
|---|---|---|
| 1 | exp Brain Diseases/ | 1,109,630 |
| 2 | Pseudobulbar Palsy/ | 134 |
| 3 | (pseudobulbar pals$ or pseudo bulbar pals$).tw,kw. | 365 |
| 4 | Cerebral Palsy/ | 18,798 |
| 5 | ((cerebral or brain or central) adj2 (pals$ or paralys#s or pares#s)).tw,kw. | 20,347 |
| 6 | worster drought.tw,kw. | 31 |
| 7 | little? disease.tw,kw. | 89 |
| 8 | exp Neurodevelopmental Disorders/ | 165,086 |
| 9 | (neurodisabilit$ or neuro-disabilit$ or neurodisabl$ or neuro-disabl$).tw,kw. | 278 |
| 10 | ((neurodevelopment$ or development$) adj3 (disease$ or disabl$ or disabil$ or disorder$ or dysfunction$ or function$ or impair$ or abnormal$ or difficulty or difficulties)).tw,kw. | 136,798 |
| 11 | exp Nervous System Diseases/ | 2,305,961 |
| 12 | ((brain$ or nervous system$) adj2 (injur$ or damag$ or dysfunction$ or function$ or malform$ or disease$ or impair$ or abnormal$)).tw,kw. | 146,976 |
| 13 | ((neurologic$ or neuromuscular or motor) adj3 (disease$ or disabl$ or disabil$ or disorder$ or dysfunction$ or function$ or impair$ or abnormal$ or difficulty or difficulties)).tw,kw. | 154,465 |
| 14 | ((muscular or muscle$ or myopath$) adj3 (disorder$ or disease$ or dysfunction$ or function$ or dystroph$)).tw,kw. | 59,599 |
| 15 | (epilep$ or seizure$ or convuls$).tw,kw. | 190,563 |
| 16 | ((communicat$ or language or linguistic or speech or learning or intellectual$ or behaviour$ or behavior$) adj3 (disabl$ or disabil$ or disorder$ or dysfunction$ or function$ or impair$ or abnormal$ or difficulty or difficulties)).tw,kw. | 118,242 |
| 17 | (autis$ or asperger$ or kanner$ or pervasive development$ disorder$).tw,kw. | 39,247 |
| 18 | ((attention$ or behav$ or conduct) adj3 (defic$ or dysfunc$ or disorder$)).tw,kw. | 55,267 |
| 19 | ((disrupt$ adj3 disorder$) or (disrupt$ adj3 behav$) or (defian$ adj3 disorder$) or (defian$ adj3 behav$)).tw,kw. | 7651 |
| 20 | (impulsiv$ or inattentiv$ or inattention$).tw,kw. | 21,145 |
| 21 | (ADHD or ADDH or ADHS).tw,kw. | 21,075 |
| 22 | (down$ adj (syndrome$ or disease$)).tw,kw. | 20,599 |
| 23 | (trisomy adj “21”).tw,kw. | 5269 |
| 24 | (chromosome adj “21”).tw,kw. | 3469 |
| 25 | (mongol or mongols or mongoloid or mongolism).tw,kw. | 2561 |
| 26 | Cystic Fibrosis/ | 32,718 |
| 27 | cystic fibrosis.tw,kw. | 39,692 |
| 28 | or/1-27 | 2,784,238 |
| 29 | exp Deglutition Disorders/ | 48,520 |
| 30 | “Feeding and Eating Disorders of Childhood”/ | 478 |
| 31 | Food Preferences/ | 12,782 |
| 32 | dysphagi$.tw,kw. | 24,034 |
| 33 | ((swallow$ or deglut$ or oral motor or oromotor or oro motor or oropharyn$ or pharyng$) adj3 (disorder$ or dysfunction$ or function$ or disabl$ or disabilit$ or impair$ or abnormal$)).tw,kw. | 6384 |
| 34 | ((feed$ or food$ or eat$ or meal$) adj3 (problem$ or inabilit$ or difficult$ or behavio$ or selectiv$ or picky or habit$ or refus$)).tw,kw. | 44,522 |
| 35 | ((oropharynx$ or trachea$ or lung$ or pulmon$) adj3 aspirat$).tw,kw. | 3969 |
| 36 | nasal regurgit$.tw,kw. | 141 |
| 37 | or/29-36 | 121,799 |
| 38 | 28 or 37 | 2,884,597 |
| 39 | exp Child/ | 1,774,420 |
| 40 | Infant/ | 739,609 |
| 41 | (infant$ or toddler$ or child$ or preschool$ or pre-school$ or schoolchild$ or parent or parental or parents).tw,kw. | 1,682,639 |
| 42 | or/39-41 | 2,648,333 |
| 43 | 38 and 42 | 582,488 |
| 44 | (instrumentation or methods).sh. or Validation Studies.pt. or Comparative Study.pt. or psychometrics/ or psychometr*.ab,ti. or clinimetr*.tw. or clinometr*.tw. or ‘Outcome Assessment (Health Care)’/ or outcome assessment.ab,ti. or outcome measure*.tw. or observer variation/ or observer variation.ab,ti. or Health Status Indicators/ or reproducibility of results/ or reproducib*.ab,ti. or discriminant analysis/ or reliab*.ab,ti. or unreliab*.ab,ti. or valid*.ab,ti. or coefficient.ab,ti. or homogeneity.ab,ti. or homogeneous.ab,ti. or internal consistency.ab,ti. | 3,546,312 |
| 45 | ((cronbach* and (alpha or alphas)) or (item and (correlation* or selection* or reduction*))).ab,ti. | 31,421 |
| 46 | (agreement or precision or imprecision or precise values or test-retest or (test and retest) or (reliab* and (test or retest)) or stability).ab,ti. | 740,344 |
| 47 | (interrater or inter-rater or intrarater or intra-rater or intertester or inter-tester or intratester or intra-tester or interobserver or inter-observer or intraobserver or intraobserver or intertechnician or inter-technician or intratechnician or intra-technician or interexaminer or inter-examiner or intraexaminer or intra-examiner).ab,ti. | 40,223 |
| 48 | (interassay or inter-assay or intraassay or intra-assay).ab,ti. | 8496 |
| 49 | (interindividual or inter-individual or intraindividual or intra-individual).ab,ti. | 30,666 |
| 50 | (interparticipant or inter-articipant or intraparticipant or intra-participant).ab,ti. | 75 |
| 51 | (kappa or kappa? or kappas or repeatab*).ab,ti. | 175,348 |
| 52 | (((replicab* or repeated) and (measure or measures or findings or result or results or test or tests)) or generaliza* or generalisa* or concordance).ab,ti. | 242,432 |
| 53 | ((intraclass and correlation*) or discriminative or known group or factor analysis or factor analyses or dimension* or subscale* or (multitrait and scaling and (analysis or analyses)) or item discriminant or interscale correlation* or error or errors or individual variability or (variability and (analysis or values)) or (uncertainty and (measurement or measuring))).ab,ti. | 889,813 |
| 54 | (standard error of measurement or sensitiv* or responsive* or ((minimal or minimally or clinical or clinically) and (important or significant or detectable) and (change or difference)) or (small* and (real or detectable) and (change or difference)) or meaningful change).ab,ti. | 1,576,641 |
| 55 | (ceiling effect or floor effect or Item response model or IRT or Rasch or Differential item functioning or DIF or computer adaptive testing or item bank or cross-cultural equivalence).ab,ti. | 9891 |
| 56 | or/44-55 | 5,924,916 |
| 57 | 43 and 56 | 126,434 |
| 58 | (Feeding Strateg* Questionnaire* or FSQ).ti,ab,kw. | 113 |
| 59 | “About Your Child”s Eating”.ti,ab. | 3 |
| 60 | ((Assessment adj2 Mealtime Behavio?r*) or BAMBIC).ti,ab,kw. | 1 |
| 61 | ((Mastication Observation adj2 Evaluation) or MOE).ti,ab,kw. | 1157 |
| 62 | ((Eating adj2 Drinking ability classification) or EDACS).ti,ab,kw. | 21 |
| 63 | (Parent Mealtime Action Scale* or PMAS or ‘PMAS–R’).ti,ab,kw. | 222 |
| 64 | (Child Feeding Assessment Questionnaire* or CFAQ).ti,ab,kw. | 5 |
| 65 | texture problem*.ti,ab,kw. | 9 |
| 66 | Feeding Interaction Report*.ti,ab,kw. | 0 |
| 67 | (Child Eating Behavio?r* Questionnaire* or CEBQ).ti,ab,kw. | 92 |
| 68 | child feeding questionnaire*.ti,ab,kw. | 124 |
| 69 | ((Drooling Severity adj2 Frequency Scale*) or DSFS).ti,ab,kw. | 49 |
| 70 | (Tongue Thrust Rating Scale or TTRS).ti,ab,kw. | 124 |
| 71 | (Brief Assessment adj2 Motor Function Oral Motor Scale*).ti,ab,kw. | 1 |
| 72 | (Orofacial Motor Function Assessment Scale* or OFMFAS).ti,ab,kw. | 3 |
| 73 | (P?ediatric Eating Assessment Tool* or “PEDI-EAT”).ti,ab,kw. | 5 |
| 74 | or/58-73 | 1916 |
| 75 | 57 and 74 | 138 |
Appendix 9 Sifting eligibility criteria for measurement properties review
Inclusion criteria
-
Study must be published as a ‘full-text original article’ (i.e. reviews are not eligible).
-
A relevant tool (i.e. used for monitoring and/or to measure outcome in a longitudinal or intervention study with children with neurodisability up to 8 years old) must be the focus of the study. However, if the paper is about the measurement properties of a ‘new’ tool not on the list, which seems very relevant, then please record it for listing in the report.
-
The study sample must at least overlap with the age range 0–8 years. For example, a sample with an age range 8–18 years would be eligible. A 100% adult sample (e.g. 18–60 years) would not be eligible.
-
A sample of participants with some kind of neurodisability is not absolutely essential (e.g. a paper monitoring feeding difficulties in a premature population could be eligible if exploring measurement properties of a tool used as an outcome). However, if the sample is drawn only from the general population of children, the paper is not included. If the sample is mixed, children with neurodisability should be at least 50%.
-
The sample size of children with neurodisability should be 10 at a minimum.
-
The aim of the study should be the development of a measurement tool or the evaluation of one or more of its measurement properties.
-
Studies that focus only on interpretability, for example the determination of minimal important change, can also be included.
-
If in doubt, include from the title and abstract sifting pending inspection of the full-text article; for example, if there appears to be hypothesis testing about features or subscales, include to clarify whether or not there is anything to extract.
-
-
Hypothesis testing applies in COSMIN to hypothesis testing within a paper about measurement properties of a tool (e.g. convergent/divergent validity against other tools; known-groups validity).
-
Almost all research tests hypotheses and trials may give evidence about sensitivity to change. So do not be over-inclusive.
-
Studies that test hypotheses (e.g. drug trials) re change over time or differences between groups as the result of an intervention but do not set out to test the measurement properties of the tool are to be excluded.
-
Studies that look at stability over time may be included, but in that case may better be considered an instance of test-retest reliability if interval is < 6 months.
-
Experimental studies can be included (e.g. exposure studies).
-
Exclusion criteria
-
Papers in which the measurement tool is being used only for diagnosis or screening and not for monitoring an outcome are to be excluded.
-
For each tool, we are not interested in its accuracy as a diagnostic tool, but only its usefulness as a monitoring/outcome tool.
-
-
Studies in which the focus of the paper is not the examination of psychometric properties are not eligible.
-
Example 1: RCTs may include data relevant to psychometric evaluation of outcome measurement tools within the paper, in particular responsiveness (to treatment). If responsiveness is the only property that could be rated, then exclude the paper.
-
Example 2: if the paper is focusing on creating a subtype of their population, or a subtype of a particular diagnosis, they can be excluded as the focus is not on measurement properties. Look for phrases such as ‘testing a model of autism’, etc.
-
Example 3: studies that look only to classify or group individuals by scores on the measure(s) can be excluded.
-
Example 4: studies with sample size of < 10 should be excluded.
-
Note
-
In regard to papers on translated tools, if the purpose of the papers is just to validate the translated version, then this is not eligible (as the ultimate purpose of the review is to develop a trial protocol for UK). If the purpose is to explore the tool’s validity in a different culture/country, include if the focus is on the properties of the tool, and the findings appear relevant for use in UK.
-
If there is any mention in the abstract of examination of ‘measurement properties’, this can be included.
-
Where this distinction is unclear regarding any of these issues, the team will discuss whether or not to include a paper at the full-text stage.
The measurement properties examined by COSMIN are as follows.
Appendix 10 The COSMIN ratings of measurement property papers
Outcome measurement: feeding, eating, drinking and swallowing
Parent/carer report on child
| Measurement tool | Study (first author and year of publication) | Summary rating | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PROM validity | Content validity | Structural validity | Internal consistency | Inter-rater reliability | Test–retest reliability | Criterion validity | Hypotheses testing – convergent/divergent | Hypotheses testing – known groups | Responsiveness | ||
| BPFAS (child and parent domains) | Allen 201591 | – | – | Adequate | Very good | – | – | – | Very good | – | – |
| Serel Arslan 201641 | – | – | – | – | – | – | – | – | Very good | – | |
| Mallick 2017128 | – | – | – | – | – | – | – | – | – | Doubtful | |
| Marshall 20155 | – | – | – | – | – | – | – | – | Very good | – | |
| Martins 2008129 | – | – | – | – | – | – | – | – | Doubtful | – | |
| BAMBI | Aponte 2016130 | – | – | – | – | – | – | – | Very good | – | – |
| DeMand 201551 | – | – | Very good | Very good | – | – | – | – | Adequate | – | |
| Hendy 201368 | – | – | Very good | Very good | – | – | – | – | – | – | |
| Johnson 2014131 | – | – | – | – | – | – | – | Very good | – | – | |
| Lukens 200850 | – | – | Adequate | Very good | Doubtful | Doubtful | Very good | Adequate | Very good | – | |
| Meral 2014132 | – | – | Very good | Doubtful | – | – | – | – | – | – | |
| Sharp 201421 | – | – | – | – | – | – | – | Very good | – | – | |
| Shmaya 2017133 | – | – | – | – | – | – | – | – | Very good | – | |
| Thullen 2017134 | – | – | – | Very good | – | – | – | Very good | – | – | |
| BAMBIC | Hendy 201368 | – | – | Very good | Very good | – | – | Doubtful | Adequate | Very good | – |
| Seiverling 201684 | – | – | Very good | Very good | – | – | – | Doubtful | Very good | – | |
| Tanner 2015135 | – | – | – | – | – | – | – | Very good | Very good | – | |
| Children’s Eating Behaviour Inventory | Archer 199135 | – | – | – | Doubtful | – | Adequate | – | – | Very good | – |
| Children’s Eating Behaviour Questionnaire | Kral 2015136 | – | – | – | – | – | – | – | – | Very good | – |
| Seiverling 201174 | – | – | – | – | – | – | – | Very good | – | – | |
| Williams 2014137 | – | – | – | Very good | – | – | – | – | Adequate | – | |
| FFQ | Hubbard 2014138 | – | – | – | – | – | – | – | – | Very good | – |
| Tanner 2015135 | – | – | – | – | – | – | – | Very good | Very good | – | |
| Zimmer 2012139 | – | – | – | – | – | – | – | – | Very good | – | |
| Food Preference Inventory | Seiverling 201174 | – | – | – | – | – | – | – | Very good | – | – |
| Sharp 201421 | – | – | – | – | – | – | – | Very good | – | – | |
| Meals in Our Household | Anderson 201270 | – | – | – | Very good | – | Adequate | – | – | Adequate | – |
| PediEAT | Thoyre 201471 | Adequate | Very good | – | – | – | – | – | – | – | – |
| Thoyre 201872 | – | – | Adequate | Very good | - | Adequate | Very good | – | Very good | – | |
| Paediatric version of the Eating Assessment Tool (PEDI-EAT-10) | Serel Arslan 2018140 | Inadequate | Doubtful | – | Doubtful | – | Doubtful | Very good | – | Very good | – |
| Screening Tool of Feeding Problems | Meral 2014132 | – | – | Very good | Very good | – | – | Very good | – | – | – |
| Child version of the Screening Tool of Feeding Problems | Seiverling 201174 | – | – | Adequate | Very good | – | – | Very good | Doubtful | – | – |
Parent/carer report on strategies
| Measurement tool | Study (first author and year of publication) | PROM validity | Content validity | Structural validity | Internal consistency | Inter-rater reliability | Test–retest reliability | Criterion validity | Hypotheses testing – convergent/divergent | Hypotheses testing – known groups | Responsiveness |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Child Feeding Questionnaire | Kral 2015136 | – | – | – | – | – | – | – | – | Very good | – |
| Feeding Strategies Questionnaire | Meral 2014132 | – | – | Very good | Very good | – | – | – | – | – | – |
| Parent Mealtime Action Scale | Seiverling 201174 | – | – | – | – | – | – | – | Very good | – | – |
| Williams 201192 | – | – | Very good | Very good | – | – | – | Very good | – | – | |
| Williams 2014137 | – | – | – | Very good | – | – | – | – | Adequate | – | |
| Parent Mealtime Action Scale – Revised | Hendy 201678 | – | – | – | Very good | – | Adequate | – | – | Very good | – |
| Parental Feeding Style Questionnaire | Kral 2015136 | – | – | – | – | – | – | – | – | Very good | – |
Professional observation/assessment
| Measurement tool | Study (first author and year of publication) | PROM validity | Content validity | Structural validity | Internal consistency | Inter-rater reliability | Test–retest reliability | Criterion validity | Hypotheses testing – convergent/divergent | Hypotheses testing – known groups | Responsiveness |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Feeding Interaction Report Scale and Treatment | Sparling 198580 | Inadequate | – | – | – | Inadequate | – | – | Inadequate | – | – |
|
Multidisciplinary Feeding Profile: subtests Functional Feeding Oral-Facial Motor Function |
Gisel 200040 | – | – | – | – | – | – | – | – | Very good | – |
| Gisel 200040 | – | – | – | – | – | – | – | – | Very good | – | |
| Mastication Observation and Evaluation | Remijn 201381 | – | Very good | – | – | Very good | – | – | – | – | – |
| Remijn 201482 | – | – | – | Very good | Very good | – | – | – | Doubtful | – | |
| Oral Motor Assessment Scale | Ortega 200943 | – | – | – | – | Adequate | – | – | – | Adequate | – |
| Pinto 2016141 | – | – | – | – | – | – | – | Adequate | Very good | – | |
| Schedule of Oral Motor Assessment | Benfer 201595 | – | – | – | – | Very good | – | Very good | Very good | Very good | – |
| Ko 2011142 | – | – | – | – | – | – | Very good | – | – | – | |
| Reilly 199545 | – | – | – | – | Very good | Doubtful | – | – | – | – | |
| Skuse 1995143 | – | – | – | – | – | – | Doubtful | – | – | – |
Appendix 11 The COSMIN quality assessment of measurement properties assessed in each paper
Outcome measurement: feeding, eating, drinking and swallowing
Parent/carer report on child
| Measurement tool | Study (first author and year of publication) | PROM validity | Content validity | Structural validity | Internal consistency | Inter-rater reliability | Test–retest reliability | Criterion validity | Hypotheses testing – convergent/divergent | Hypotheses testing – known groups | Responsiveness |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BPFAS (child and parent domains) | Allen 201591 | – | – | + | + | ||||||
| Serel Arslan 201641 | + | ||||||||||
| Mallick 2017128 | ? | ||||||||||
| Marshall 20155 | + | ||||||||||
| Martins 2008129 | + | ||||||||||
| BAMBI | Aponte 2016130 | + | |||||||||
| DeMand 201551 | – Borderline (15 items) |
– + for overall and 2/4 factors |
+ | ||||||||
| Hendy 201368 | – | – | |||||||||
| Johnson 2014131 | + | ||||||||||
| Lukens 200850 | – |
– + for overall and 2/3 factors |
– | + | + | + | + | ||||
| Meral 2014132 | – | – | – | + | – | – | – | – | – | – | |
| Sharp 201421 | – | – | – | – | – | – | – | + | – | – | |
| Shmaya 2017133 | – | – | – | – | – | – | – | – | + | – | |
| Thullen 2017134 | – | – | – |
– 15 items. + for 2/4 factors |
– | – | – | + | – | – | |
| BAMBIC | Hendy 201368 | – | – | – | + | – | – | ? | ? | – | – |
| Seiverling 201684 | – | – | – |
– + for 1/3 factors |
– | – | – | – | + | – | |
| Tanner 2015135 | – | – | – | – | – | – | – | + | + | – | |
| Children’s Eating Behaviour Inventory | Archer 199135 | – | – | – | + | – | + | – | – | + | – |
| Children’s Eating Behaviour Questionnaire | Kral 2015136 | – | – | – | – | – | – | – | – | – | – |
| Seiverling 201174 | – | – | – | – | – | – | – | + | – | – | |
| Williams 2014137 | – | – | – | + | – | – | – | – | + | – | |
| FFQ | Hubbard 2014138 | – | – | – | – | – | – | – | – | + | – |
| Tanner 2015135 | – | – | – | – | – | – | – | + | + | – | |
| Zimmer 2012139 | – | – | – | – | – | – | – | – | + | – | |
| Food Preference Inventory | Seiverling 201174 | – | – | – | – | – | – | – | – | – | – |
| Sharp 201421 | – | – | – | – | – | – | – | + | – | – | |
| Meals in Our Household (child and parent domains) | Anderson 201270 | – | – | – |
– + for 4/6 domains |
– | + | – | – | + | – |
| PediEAT | Thoyre 201471 | – | – | – | – | – | – | – | – | ||
| Thoyre 201872 | – | – | – | + | – | + | + | – | + | – | |
| Paediatric version of the Eating Assessment Tool (PEDI-EAT-10) | Serel Arslan 2018140 | – | + | – | + | + | – | + | – | ||
| Screening Tool of Feeding Problems | Meral 2014132 | – | – | – |
– + for overall, but 0/5 factors |
– | – | + | – | – | – |
| Child version of the Screening Tool of Feeding Problems | Seiverling 201174 | – | – | ? | – | – | – | + | + | – | – |
Parent/carer report on strategies
| Measurement tool | Study (first author and year of publication) | PROM validity | Content validity | Structural validity | Internal consistency | Inter-rater reliability | Test–retest reliability | Criterion validity | Hypotheses testing – convergent/divergent | Hypotheses testing – known groups | Responsiveness |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Child Feeding Questionnaire | Kral 2015136 | – | – | – | – | – | – | – | – | – | – |
| Feeding Strategies Questionnaire | Meral 2017144 | – | – | – |
– + for overall and 2/6 factors |
– | – | – | – | – | – |
| Parent Mealtime Action Scale | Seiverling 201174 | – | – | – | – | – | – | – | + | – | – |
| Williams 201192 | – | – | + |
– + for 2/9 factors |
– | – | – | + | – | – | |
| Williams 2014137 | – | – | – |
– + for 3/9 factors |
– | – | – | – | – | – | |
| Parent Mealtime Action Scale – Revised | Hendy 201678 | – | – | – |
– + for 4/9 factors |
– |
– + for 3/9 factors |
– | – | + | – |
| Parental Feeding Style Questionnaire | Kral 2015136 | – | – | – | – | – | – | – | – | – | – |
Professional observation/assessment of oral motor skills
| Measurement tool | Study (first author and year of publication) | PROM validity | Content validity | Structural validity | Internal consistency | Inter-rater reliability | Test–retest reliability | Criterion validity | Hypotheses testing – convergent/divergent | Hypotheses testing – known groups | Responsiveness |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Feeding Interaction Report Scale and Treatment | Sparling 198580 | – | – | – | ? | – | – | – | – | – | |
|
Multidisciplinary Feeding Profile: subtests Functional Feeding Oral-Facial Motor Function |
Gisel 200040 | – | – | – | – | – | – | – | – | + | – |
| Gisel 200040 | – | – | – | – | – | – | – | – | + | – | |
| Mastication Observation and Evaluation | Remijn 201381 | – | – | – | + | – | – | – | – | – | |
| Remijn 201482 | – | – | – | + | + | – | – | – | – | – | |
| Oral Motor Assessment Scale | Ortega 200943 | – | – | – | – | + | – | – | – | + | – |
| Pinto 2016141 | – | – | – | – | – | – | – | – | + | – | |
| Schedule of Oral Motor Assessment (SOMA) | Benfer 201595 | – | – | – | – | + | – | + | + | + | – |
| Ko 2011142 | – | – | – | – | – | – | + | – | – | – | |
| Reilly 199545 | – | – | – | – | + | + | – | – | – | – | |
| Skuse 1995143 | – | – | – | – | – | – | + | – | – | – |
Appendix 12 Second round of focus groups topic guide
Appendix 13 Original intervention and outcome terms used in the national survey and the revised terms for use in the Delphi survey
| Intervention | Delphi survey intervention term |
|---|---|
| Modification of environment | Modifying environment |
| Positioning | Positioning |
| Modification of utensils | Modifying equipment |
| Schedule of meals | Scheduling of meals |
| Food or drink modification | Modifying consistency of food or drink |
| Modifying other aspects of food or drink | |
| – | Modifying placement of food |
| Enhancing parent/child communication strategies at mealtimes | Enhancing communication |
| Visual supports | Visual supports |
| Training to wait for child’s cues for feeding | Responding to a child’s cues for feeding |
| Pacing of food at mealtimes | Pace of feeding |
| Manoeuvres | Physical support |
| Desensitisation programme for oral sensations | Oral and sensory desensitisation |
| Sensory stimulation | |
| Sensorimotor therapy | |
| Oral motor exercises | Oral motor exercises |
| Desensitisation programme for food avoidance | Graded exposure to new textures |
| Graded exposure to new food | |
| Strategies/programmes aimed at changing behaviour at mealtimes | Changing behaviour at mealtimes |
| Modelling | Modelling |
| Hand-over-hand prompting | Training to self-feed |
| Counselling | Support for parents |
| – | Psychological support for child |
| Sensory aids | – |
| Modifying social eating and drinking opportunities | – |
| Medication | Medication |
| Energy supplements | Energy supplements |
| – | Vitamin or nutritional supplements |
| Information on impact of movement difficulties on eating and drinking | Sharing Information |
| Information on impact of sensory difficulties on eating and drinking |
| Outcome | Delphi survey outcome term |
|---|---|
| Improved nutrition | Nutrition |
| Better general health | General health |
| Fewer/shorter hospital admissions | |
| Less pain | |
| Weight gain | Weight |
| – | Height |
| Increased growth | Growth |
| Child enjoys mealtimes more | Child’s enjoyment of mealtimes |
| Child less frustrated or distressed at mealtimes | |
| Better quality of life for child | Quality of life of a child |
| Parent/carer enjoys mealtimes more | Parent or caregivers enjoyment of mealtimes |
| Parent/carer less frustrated or distressed at mealtimes | |
| Less parental/carer stress | Mental health of parent/caregiver |
| Not having to prepare separate meals for the child | Quality of life of family |
| Better co-ordination of swallowing and breathing | Safety |
| Fewer breathing changes | |
| Better sitting | |
| Better oral motor function (e.g. chewing and biting) | Oral motor control |
| Less food/drink spilled from lips | |
| Shorter mealtimes | Efficiency |
| Better self-feeding/independence skills | Independence |
| Wider range of foods eaten | Variety |
| Less aversion/avoidance of particular foods | |
| More food/drink consumed | Amount |
| – | Appetite |
| – | Mealtime behaviour |
| Better mealtime one-to-one interaction with child | Mealtime interaction |
| More involvement in family’s activities | Social participation |
| Being able to eat a meal somewhere outside the home | |
| – | Child’s understanding |
| Better understanding of child’s difficulties and strategies to support them | Parent’s understanding |
| Less drooling | – |
| Fewer abnormal or unusual movements | – |
| Child able to communicate better | – |
| Less food waste/reduced cost of food | – |
| More opportunity to talk to others about feelings about child’s eating and drinking difficulties | – |
Appendix 14 Characteristics of respondents who completed both rounds of Delphi survey and those who only completed round 2
| Characteristic | Rounds 1 and 2 (N = 103), n (%) | Round 2 only (N = 19), n (%) |
|---|---|---|
| Role | ||
| Parent | 52 (51) | 9 (47) |
| HP | 51 (50) | 10 (53) |
| Age (years) | ||
| ≤ 20 | 0 (0) | 0 (0) |
| 21–30 | 5 (5) | 0 (0) |
| 31–40 | 28 (27) | 12 (63) |
| 41–50 | 47 (46) | 5 (26) |
| 51–60 | 22 (21) | 2 (11) |
| 61–70 | 0 (0) | 0 (0) |
| > 70 | 1 (1) | 0 (0) |
| Missing | 0 (0) | 0 (0) |
| Gender | ||
| Female | 98 (95) | 18 (95) |
| Male | 5 (5) | 1 (5) |
| Prefer not to say | 0 (0) | 0 (0) |
| Missing | 0 (0) | 0 (0) |
| Location | ||
| England | ||
| North-east | 15 (15) | 3 (16) |
| North-west | 8 (8) | 1 (5) |
| Yorkshire and Humber | 9 (9) | 2 (11) |
| Midlands | 15 (15) | 4 (21) |
| South-east, including London | 35 (34) | 6 (32) |
| South-west England | 10 (10) | 1 (5) |
| Scotland | 5 (5) | 2 (11) |
| Northern Ireland | 2 (2) | 0 (0) |
| Wales | 3 (3) | 0 (0) |
| Missing | 1 (1) | 0 (0) |
| Ethnicity | ||
| White | 98 (95) | 16 (84) |
| Black/African/Caribbean/black British | 0 (0) | 1 (5) |
| Asian/Asian British | 3 (3) | 1 (5) |
| Mixed/multiple ethnic group | 2 (2) | 0 (0) |
| Other ethnic group | 0 (0) | 1 (5) |
| Prefer not to say | 0 (0) | 0 (0) |
| Missing | 0 (0) | 0 (0) |
| Type of EDSD of child | ||
| Physical | 21 (20) | 1 (5) |
| Non-physical | 28 (27) | 7 (37) |
| Mixed | 54 (52) | 11 (58) |
| Missing | 0 (0) | 0 (0) |
Appendix 15 Interventions viewed as essential by over 67% of parents and health professionals in round 1 of the Delphi survey
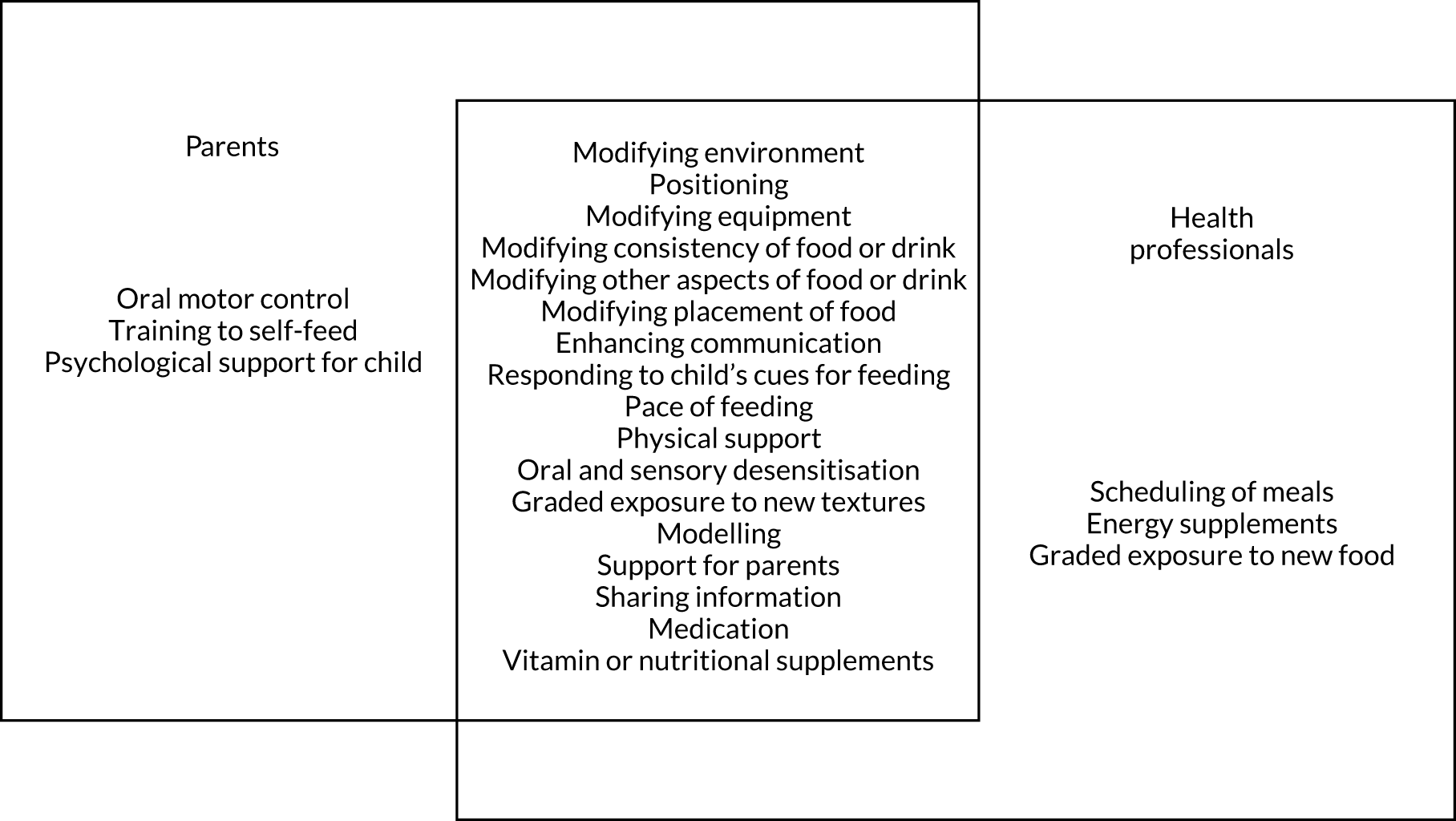
Appendix 16 Parents’ and health professionals’ ratings of interventions on round 1 of the Delphi survey
| Intervention | Parents (N = 81) | HPs (N = 76) | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Not important (score of 0–3) (%) | Important but not essential (score of 4–6) (%) | Essential (score of 7–9) (%) | n | Not important (score of 0–3) (%) | Important but not essential (score of 4–6) (%) | Essential (score of 7–9) (%) | |
| Modifying environment | 78 | 3 | 31 | 67 | 74 | 0 | 14 | 87 |
| Positioning | 72 | 1 | 7 | 92 | 74 | 0 | 3 | 97 |
| Modifying equipment | 75 | 7 | 17 | 76 | 74 | 0 | 14 | 87 |
| Scheduling of meals | 79 | 13 | 34 | 53 | 74 | 0 | 18 | 82 |
| Modifying consistency of food or drink | 70 | 9 | 13 | 79 | 72 | 1 | 13 | 86 |
| Modifying other aspects of food or drink | 76 | 5 | 21 | 74 | 73 | 3 | 22 | 75 |
| Modifying placement of food | 60 | 10 | 22 | 68 | 70 | 3 | 19 | 79 |
| Enhancing communication | 75 | 4 | 20 | 76 | 73 | 0 | 18 | 82 |
| Visual supports | 71 | 11 | 37 | 52 | 71 | 0 | 37 | 63 |
| Responding to a child’s cues | 64 | 5 | 13 | 83 | 71 | 1 | 4 | 94 |
| Pace of feeding | 70 | 1 | 21 | 77 | 71 | 0 | 4 | 96 |
| Physical supports | 54 | 13 | 15 | 72 | 67 | 3 | 28 | 69 |
| Oral and sensory desensitisation | 68 | 6 | 20 | 72 | 72 | 10 | 22 | 68 |
| Oral motor exercises | 59 | 7 | 20 | 73 | 68 | 27 | 34 | 40 |
| Graded exposure to new food | 73 | 6 | 29 | 66 | 72 | 0 | 15 | 85 |
| Graded exposure to new textures | 75 | 3 | 29 | 68 | 73 | 0 | 19 | 81 |
| Changing behaviour at mealtimes | 76 | 7 | 37 | 57 | 73 | 4 | 33 | 63 |
| Modelling | 79 | 3 | 18 | 80 | 73 | 0 | 18 | 82 |
| Support for parents | 74 | 3 | 16 | 81 | 73 | 0 | 16 | 84 |
| Psychological support for child | 65 | 9 | 19 | 72 | 70 | 3 | 34 | 63 |
| Training to self-feed | 69 | 6 | 26 | 68 | 72 | 4 | 49 | 47 |
| Sharing information | 76 | 0 | 11 | 90 | 73 | 0 | 6 | 95 |
| Medication | 49 | 8 | 14 | 78 | 70 | 0 | 14 | 86 |
| Energy supplements | 45 | 13 | 24 | 62 | 68 | 0 | 27 | 74 |
| Vitamin or nutritional supplements | 60 | 7 | 25 | 68 | 68 | 0 | 32 | 68 |
Appendix 17 Parents’ and health professionals’ ratings of interventions on round 2 of the Delphi survey
| Intervention | Parents (N = 61) | HPs (N = 61) | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Not important (score of 0–3) (%) | Important but not essential (score of 4–6) (%) | Essential (score of 7–9) (%) | n | Not important (score of 0–3) (%) | Important but not essential (score of 4–6) (%) | Essential (score of 7–9) (%) | |
| Modifying environment | 60 | 2 | 22 | 77 | 57 | 0 | 5 | 95 |
| Positioning | 54 | 2 | 2 | 96 | 57 | 0 | 0 | 100 |
| Modifying equipment | 54 | 4 | 4 | 93 | 57 | 0 | 11 | 90 |
| Scheduling of meals | 58 | 5 | 45 | 50 | 57 | 0 | 18 | 83 |
| Modifying consistency of food or drink | 56 | 2 | 20 | 79 | 54 | 0 | 4 | 96 |
| Modifying other aspects of food or drink | 59 | 3 | 10 | 86 | 57 | 2 | 16 | 83 |
| Modifying placement of food | 48 | 2 | 23 | 75 | 57 | 0 | 11 | 90 |
| Enhancing communication | 59 | 2 | 12 | 86 | 57 | 0 | 11 | 90 |
| Visual supports | 54 | 4 | 44 | 52 | 57 | 2 | 26 | 72 |
| Responding to a child’s cues | 55 | 0 | 7 | 93 | 56 | 0 | 4 | 96 |
| Pace of feeding | 56 | 0 | 11 | 89 | 56 | 0 | 0 | 100 |
| Physical supports | 44 | 5 | 14 | 82 | 57 | 4 | 16 | 81 |
| Oral and sensory desensitisation | 54 | 6 | 13 | 82 | 57 | 9 | 16 | 75 |
| Oral motor exercises | 50 | 4 | 26 | 70 | 57 | 35 | 30 | 35 |
| Graded exposure to new food | 60 | 3 | 27 | 70 | 57 | 4 | 12 | 84 |
| Graded exposure to new textures | 59 | 2 | 2 | 76 | 57 | 0 | 19 | 81 |
| Changing behaviour at mealtimes | 59 | 7 | 36 | 58 | 57 | 2 | 42 | 56 |
| Modelling | 60 | 2 | 22 | 77 | 57 | 0 | 18 | 83 |
| Support for parents | 60 | 2 | 3 | 95 | 56 | 0 | 4 | 96 |
| Psychological support for child | 52 | 4 | 19 | 77 | 56 | 4 | 38 | 59 |
| Training to self-feed | 56 | 5 | 39 | 55 | 56 | 4 | 50 | 46 |
| Sharing information | 60 | 0 | 0 | 100 | 57 | 0 | 4 | 97 |
| Medication | 47 | 4 | 9 | 87 | 57 | 2 | 7 | 91 |
| Energy supplements | 42 | 2 | 29 | 69 | 55 | 0 | 27 | 73 |
| Vitamin or nutritional supplements | 54 | 0 | 15 | 85 | 55 | 0 | 26 | 75 |
Appendix 18 Parents’ and health professionals’ ratings of outcomes on round 1 of the Delphi survey
| Outcome | Parents (N = 81) | HPs (N = 76) | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Not important (score of 0–3) (%) | Important but not essential (score of 4–6) (%) | Essential (score of 7–9) (%) | n | Not important (score of 0–3) (%) | Important but not essential (score of 4–6) (%) | Essential (score of 7–9) (%) | |
| Nutrition | 81 | 0 | 11 | 89 | 76 | 0 | 3 | 97 |
| General health | 80 | 1 | 10 | 89 | 76 | 0 | 7 | 93 |
| Weight | 80 | 6 | 41 | 53 | 76 | 4 | 45 | 51 |
| Height | 78 | 18 | 51 | 31 | 76 | 18 | 50 | 32 |
| Growth | 79 | 0 | 25 | 75 | 76 | 0 | 24 | 76 |
| Child’s enjoyment of mealtimes | 80 | 1 | 16 | 83 | 76 | 0 | 9 | 91 |
| Parent’s enjoyment of mealtimes | 81 | 7 | 51 | 42 | 76 | 0 | 24 | 76 |
| Quality of life of child | 81 | 1 | 4 | 95 | 75 | 0 | 8 | 92 |
| Quality of life of family | 81 | 1 | 21 | 78 | 75 | 0 | 13 | 87 |
| Mental health of parent | 81 | 0 | 17 | 83 | 76 | 0 | 16 | 84 |
| Safety | 78 | 0 | 3 | 97 | 75 | 0 | 3 | 97 |
| Oral motor control | 76 | 0 | 13 | 87 | 74 | 3 | 23 | 74 |
| Efficiency | 80 | 13 | 44 | 44 | 75 | 5 | 35 | 60 |
| Independence | 80 | 13 | 28 | 60 | 75 | 3 | 67 | 31 |
| Variety | 81 | 5 | 44 | 51 | 75 | 4 | 73 | 23 |
| Amount | 81 | 4 | 35 | 62 | 75 | 5 | 55 | 40 |
| Appetite | 81 | 3 | 38 | 59 | 75 | 3 | 53 | 44 |
| Mealtime behaviour | 80 | 14 | 45 | 41 | 74 | 10 | 61 | 30 |
| Mealtime Interaction | 79 | 4 | 35 | 61 | 74 | 1 | 18 | 81 |
| Social participation | 80 | 4 | 46 | 50 | 74 | 1 | 22 | 77 |
| Child’s understanding of mealtimes | 80 | 4 | 45 | 51 | 74 | 4 | 45 | 51 |
| Parent’s understanding of child’s EDSD | 80 | 1 | 10 | 89 | 72 | 1 | 10 | 89 |
Appendix 19 Parents’ and health professionals’ ratings of outcomes on round 2 of the Delphi survey
| Outcome | Parents (N = 61) | HPs (N = 61) | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Not important (score of 0–3) (%) | Important but not essential (score of 4–6) (%) | Essential (score of 7–9) (%) | n | Not important (score of 0–3) (%) | Important but not essential (score of 4–6) (%) | Essential (score of 7–9) (%) | |
| Nutrition | 61 | 0 | 5 | 95 | 60 | 0 | 2 | 98 |
| General health | 61 | 0 | 3 | 97 | 61 | 0 | 2 | 98 |
| Weight | 61 | 0 | 66 | 34 | 61 | 0 | 53 | 48 |
| Height | 61 | 13 | 75 | 12 | 61 | 13 | 75 | 12 |
| Growth | 61 | 0 | 18 | 82 | 61 | 0 | 12 | 89 |
| Child’s enjoyment of mealtimes | 61 | 0 | 10 | 90 | 60 | 0 | 2 | 98 |
| Parent’s enjoyment of mealtimes | 61 | 8 | 53 | 39 | 59 | 0 | 22 | 78 |
| Quality of life of child | 61 | 0 | 2 | 98 | 59 | 0 | 0 | 100 |
| Quality of life of family | 61 | 0 | 10 | 90 | 58 | 0 | 3 | 97 |
| Mental health of parent | 61 | 0 | 7 | 93 | 58 | 0 | 3 | 97 |
| Safety | 61 | 0 | 0 | 100 | 58 | 0 | 0 | 100 |
| Oral motor control | 56 | 0 | 14 | 86 | 58 | 0 | 28 | 72 |
| Efficiency | 60 | 13 | 70 | 17 | 57 | 5 | 49 | 46 |
| Independence | 61 | 10 | 48 | 43 | 58 | 3 | 69 | 28 |
| Variety | 61 | 3 | 71 | 26 | 57 | 4 | 84 | 12 |
| Amount | 61 | 0 | 48 | 53 | 56 | 2 | 73 | 25 |
| Appetite | 61 | 2 | 53 | 46 | 56 | 2 | 61 | 38 |
| Mealtime behaviour | 61 | 8 | 57 | 34 | 57 | 5 | 68 | 26 |
| Mealtime Interaction | 60 | 5 | 30 | 65 | 57 | 0 | 21 | 79 |
| Social participation | 60 | 10 | 37 | 53 | 57 | 2 | 25 | 74 |
| Child’s understanding of mealtimes | 60 | 3 | 38 | 58 | 57 | 2 | 58 | 40 |
| Parent’s understanding of child’s EDSD | 60 | 0 | 5 | 95 | 57 | 0 | 7 | 93 |
Appendix 20 List of outcome measures generated in the stakeholder consultation workshops
| Outcome | Participant views | Research team view on utility | |
|---|---|---|---|
| Strengths | Weaknesses | ||
| Safety | |||
| Videofluoroscopy | Objective measure of the swallowing mechanism, efficiency and safety (HP) |
Concerns about reliability (assessment findings may not be typical) (P/HP) High risks: children with compromised swallow safety may be advised to discontinue oral feeding post videofluoroscopy (P/HP) |
Not suitable for use as an outcome measure for all children |
| Frequency of choking episodes | Difficult to record accurately (HP). Stressful to record (P/HP). May have both physical and behavioural aetiology, which may be difficult to separate (P/HP) | Concerns regarding reliability as an outcome measure | |
| Episodes of chest infections | Lack of professional agreement on how to define an episode of chest infection (HP) | Would need clearer definition before being used as an outcome measure | |
| Daytime and/or nocturnal cough | Causes other than EDSD (e.g. gastro–oesophageal reflux) (HP) | Limited specificity | |
| Observation of meals |
Could provide information across different settings (P/HP). Could be captured using smartphone technology for sharing with HPs HPs could video link into a family meal (P/HP) |
Standardised scoring of mealtime observation could be challenging, potentially limiting its use as a trial outcome measure (HP) | Challenges with standardisation limit its use as an outcome measure |
| General health | |||
| Parent-reported child health questionnaire | May not capture relevant increments of improvement in general health (HP) | A standardised child health questionnaire with strong construct validity and reliability could be used as an outcome measure | |
| School attendance | Simple to measure (HP) | Factors other than health contribute (P/HP) | Not an appropriate measure of general health |
| Bowel function | Existing tools such as the Bristol Stool chart could be used (Lewis SJ145 1997) (HP) |
Stool consistency can vary day to day (HP) Dependent on carer report (HP) |
Inadequate measure of general health for use as an outcome measure Poor specificity |
| Hospital admissions/outpatient attendances | Identified as possible measure, no further discussion | ||
| Energy levels | Identified as possible measure, no further discussion | Inappropriate general health outcome measure | |
| Concentration | Identified as possible measure, no further discussion | Inappropriate general health outcome measure | |
| Sleep | Identified as possible measure, no further discussion | Inappropriate general health outcome measure | |
| Subjective measures of general health, e.g. skin pallor, hair thickness, dental health, nail condition | Identified as possible measure, no further discussion | Inappropriate general health outcome measure | |
| Nutrition (nutritional intake) | |||
| 3-day food diaries |
Short duration of information collection and scorability are advantageous (HP) Photographs of the mealtime plate could be used alongside (HP) |
Burdensome or stressful, especially if child not eating recommended healthy foods (P/HP) HPs disagreed on the utility of the diary for assessing micronutrient intake owing to uncertainties regarding the validity of dietary assessment methods as a measure of energy/micronutrient intake in children (HP) (Burrows T146 2019) |
Overly burdensome for families Validity concerns |
| Food frequency questionnaires | Could be used in conjunction with appropriate applications to facilitate ease of recording (P/HP) | Some families could find completion burdensome, some foods enquired about may not be within the budget of all families (P/HP) | Relies on parent/carer recall |
| The Eatwell Plate (a pictorial summary of the five food groups illustrating recommended proportions for a healthy balanced diet) | Easy to understand and is less demanding of parents/carers than a 3-day food diary (P/HP) | Poor specificity for use as a primary outcome measure (HP) | Poor specificity for use as an outcome measure, provides insufficient detail |
| Blood micronutrient levels | Objective and accurate. Some children would have samples collected through routine clinical care (Romano C105 2017) (HP) |
Trip to hospital required owing to sampling requirements off-putting for some families (HP) May be stressful for some children (P/HP) |
Not suitable as an outcome measure for all children |
| Body fat percentage using smart scales | Could be performed in clinic; experience may be needed for accurate measurement (HP) | Significant variability between different manufacturer systems (HP) | Further validity and reliability work required ahead of use as an outcome measure |
| Photographs of plate at the beginning and end of a meal, including photographs of the floor to assess spillage | Unsuitable for use as a primary outcome measure when used alone owing to insufficient detail on nutrient content of foods consumed (HP) | Poor specificity | |
| Need for dietary supplements | Identified as possible measure, no further discussion | Poor specificity | |
| Oral motor control | |||
| Assessment of food textures managed |
Standardised descriptions exist, e.g. International Dysphagia Diet Standardisation Initiative (Cichero JA147 2017) (HP) Sensory component to textures managed for some children (P/HP) |
May be more difficult for parents to assess (HP) | Poor specificity limits use as an outcome measure |
| Time to complete mealtimes | Could be measured by parents/carers at home (HP) | Parent/carer factors also contribute to total mealtime (P/HP) | Poor specificity limits use as an outcome measure |
|
Standardised measures: SOMA (Reilly S45 1995 and Skuse D143 1995) Drooling Impact Scale |
Could be incorporated into current NHS practice (HP) Drooling identified as important to families (P) |
Potential outcome measures | |
| Measures of breath control | Requires expert assessment, limiting utility as outcome measure (HP) | No available measure | |
| Growth (as a measure of nutritional status) | |||
| Growth trajectories | Identified as possible measure, no further discussion | ||
| Weight and height | Less reliable in children with severe physical disability owing to altered body composition and limb deformity contracture (HP) | Not suitable as outcome measure for children with severe physical disability | |
| Body summaries (e.g. mid-upper arm circumference and skinfold thicknesses) |
Reliable, quick and achievable within an NHS setting More acceptable to children |
Some children may find the use of callipers for skinfolds uncomfortable or off-putting | Potential outcome measure, further acceptability work required |
| Segmental measures (e.g. knee height) | Less well validated in children with neurodisability (Hardy J116 2018) | Validity concerns may limit current utility as an outcome measure (Snik DAC109 2019) | |
| Bioelectrical impedance monitoring | Reasonable reliability; test requirements and variation between systems may limit use as outcome measure (Hardy J116 2018) | Potential outcome measure | |
| Child’s enjoyment of mealtimes | |||
| Parental report of mealtime enjoyment, accompanied by a Likert scale | Parental report could be enhanced by school report where possible (P/HP) | Harder for parents to report if a child has limited communication ability (P/HP) | |
| PediEAT | Relates more to mechanical aspects of eating and drinking; inadequate measure of emotional response to food; some questions could be relevant (HP) | ||
| Parental understanding of child’s eating and drinking difficulties | |||
| PediEAT |
Could help parents to understand their child’s eating and drinking difficulties and identify appropriate interventions Potential outcome measure |
Potential outcome measure | |
| Parent subscale of the BPFAS | Questionnaire would need explanation regarding why it was being used to improve its acceptability (P/HP) | Unpopular with parents owing to length (P) | |
| Quality of life of child | |||
| No measures suggested by participants during the workshop | Robust measures are available | ||
| Quality of life of family | |||
| No measures suggested by participants during the workshop | Influenced by many factors, e.g. parental understanding of EDSD and a sense of normality (e.g. being able to visit friends around mealtimes, visiting a restaurant, and participation) | Robust measures are available for children and adults | |
| Parental mental health | |||
|
Mental health questionnaires Subjective questions accompanied by Likert scale (e.g. agreement with statements such as ‘I feel good today’) |
Anxiety provoking for some families; may be best completed with a psychologist during a protected session Lacks specificity |
Other life stressors may dominate, and so parental mental health may not be reflective of a child’s EDSD | Robust measures are available |
| Participation | |||
|
Particular standardised measure not identified by participants Attainment of child-specific participation goals |
Key outcome for many participants | Not equally important to all families | Robust measures are available |
Glossary
Throughout the text all interventions/strategies have the first letter capitalised to make them clear to the reader.
- Amount
- The quantity of food or volume of liquid a child eats or drinks.
- Appetite
- A child’s level of hunger and desire for food or drink.
- Changing behaviour at mealtimes
- Strategies to encourage a child to behave appropriately at mealtimes (e.g. a child sitting ready to eat, staying seated for the meal, not throwing food or not spitting food).
- Child’s understanding
- Child’s understanding of mealtime activities and routines.
- Efficiency
- A child’s ability to eat and drink at a reasonable pace and not lose food or liquid from the mouth while eating and drinking.
- Energy supplements
- Any energy or calorie supplement given orally or via a feeding tube.
- Enhancing communication
- Improving interaction between a child and the person feeding them during mealtimes (e.g. offering choices of food to a child and responding to a child’s use of non-verbal communication).
- Graded exposure to food or drink
- Activities aimed at gradually introducing a child to new or previously rejected foods and drinks (e.g. messy play activities involving a child touching new or disliked foods and using small steps towards a child accepting new or disliked foods, such as licking the food or putting it in their mouth with no expectation to swallow).
- Graded exposure to new textures
- Activities aimed at gradually introducing a child to more challenging food textures and fluid consistencies (e.g. messy play activities involving a child touching new or previously rejected textures and using small steps to introduce a child to lumpy food or foods that require chewing).
- Growth
- An increase over time in a child’s height and weight.
- Independence
- A child’s ability to feed themselves.
- Intellectual disability
- An IQ below the average range (i.e. < 70); also referred to as learning disability.
- Mealtime behaviour
- A child’s meal-related behaviour and other behaviour during mealtimes.
- Mealtime interaction
- The interaction between a child and the person feeding or sitting with them at mealtimes.
- Medication
- Any prescribed medicine that could affect eating and drinking (e.g. for epilepsy, pain, drooling, muscle tone and gastro-oesophageal reflux).
- Mental health of parent or caregiver
- A parent or caregiver’s mood and emotional well-being.
- Mixed eating, drinking and swallowing difficulties
- Eating, drinking and swallowing difficulties caused by physical difficulties (e.g. reduced control of the muscles of the lips, tongue, mouth and throat) and non-physical difficulties (e.g. sensory or behavioural issues leading to restricted or selective eating and rituals associated with food or mealtimes).
- Modelling
- Giving a child the opportunity to learn from others by eating and drinking with them (e.g. sitting a child with other children or family members at mealtimes).
- Modifying environment
- Changing the physical or social setting at mealtimes (e.g. reducing interference such as levels of noise; using distractions to reduce a child’s attention on their food).
- Modifying equipment
- Using different spoons, forks, plates, cups, bottles, etc. (e.g. adapted cup and plastic spoon).
- Modifying food or drink
- Changing aspects of the child’s food or drink, such as the consistency, temperature, taste, amount or presentation (e.g. puréeing food, thickening food or drink, presenting different foods so they do not touch each other and mixing liked foods with previously rejected foods).
- Multicomponent intervention
- Interventions delivered in combination.
- Nature of the child’s difficulties
- The physical, non-physical or mixed physical and non-physical causes of eating, drinking and swallowing difficulties, the extent of the difficulties and their impact on the child’s behaviour and/or participation at mealtimes.
- Non-physical EDSD
- Eating, drinking and swallowing difficulties caused by sensory or behavioural issues leading to restricted or selective eating and rituals associated with food or mealtimes.
- Nutrition
- A child’s intake of food nutrients, which should be sufficient to allow normal growth, health, activities and development.
- Oral and sensory desensitisation
- Activities that are aimed at reducing a child’s adverse reactions to different sensory experiences linked to eating and drinking (e.g. face massage and chewing non-food items, such as a chewy ‘toothbrush’).
- Oral motor control
- A child’s ability to co-ordinate the movements of their mouth, jaw, tongue or lips and swallow.
- Oral motor exercises
- Exercises carried out with a child with the aim of improving their control of their mouth, jaw, tongue or lips (e.g. a child moving a non-food item with their tongue and a child sucking through a straw).
- Outcome
- A possible effect of interventions for eating, drinking and swallowing difficulties.
- Outcome measurement tool
- A way to capture the possible effect of interventions for eating, drinking and swallowing difficulties.
- Pace of feeding
- The speed at which each mouthful of food or drink is taken by a child. The pace of feeding can be reduced by leaving more time between each spoonful. This helps to prevent overfilling of the mouth and readies the child for the next mouthful.
- Parent’s understanding
- A parent’s or caregiver’s insight into their child’s eating, drinking and swallowing difficulties.
- Physical eating, drinking and swallowing difficulties
- Eating, drinking and swallowing difficulties caused by reduced control/sensation of the muscles of the lips, tongue, mouth and throat, or by difficulties such as posture and physical control of movement.
- Physical support
- Direct physical support to a child given when eating or drinking to improve the movements that are needed to bite, chew and swallow (e.g. placing a thumb underneath the chin to help a child close their mouth).
- Positioning
- Placing the child in a position that affords the best posture to eat and drink food safely and efficiently (e.g. with child sitting upright and with support for head control).
- Psychological support for child
- Emotion-based approaches to help a child with their eating, drinking and swallowing difficulties (e.g. counselling or psychological therapy).
- Quality of life of the child
- How satisfied the child feels about their life.
- Quality of life of the family
- How satisfied family members feel about their own lives and the family unit.
- Responding to a child’s cues for feeding
- Helping parents/caregivers to recognise the signs that a child is ready to take another mouthful of food or drink (e.g. looking for breath alterations or repeated swallows from a child to indicate a lack of readiness).
- Restricted eating
- Picky eating, rituals and other behaviours that interfere with the child’s food intake.
- Safety
- A child’s ability to eat and drink without choking or aspirating food or fluid into the lungs.
- Scheduling of meals
- Setting the timing of mealtimes to encourage a child’s appetite and readiness to eat and drink, and establish a mealtime routine (e.g. spreading meals/snacks throughout the day and setting a 30-minute limit for mealtimes).
- Sensorimotor therapy
- Exercises to develop awareness of the lips, cheeks, tongue, and jaw and the strength, speed, consistency and endurance of their movements. Exercises include active and passive movement, stretching and sensory stimulation.
- Sensory stimulation
- Touch-based stimulation on and around the lips and mouth in an attempt to reduce sensory-based feeding difficulties.
- Shared information
- Any information shared between parents and professionals to help understand a child’s difficulties with eating and drinking and provide support (e.g. parents helping professionals understand what is important about mealtimes in their family and professionals teaching parents and education professionals about a child’s physical or sensory difficulties).
- Social participation at mealtimes
- A child’s overall involvement at mealtimes.
- Support for parents
- Help for parents around their child’s eating and drinking difficulties (e.g. professional support, counselling and parent support groups).
- Training to self-feed
- Teaching a child to feed themselves (e.g. placing a hand over a child’s hand to help guide the food into their mouth).
- Variety
- The range of foods or liquids that a child eats or drinks.
- Visual supports
- Pictures, a ‘countdown clock’ or social stories to increase a child’s understanding of what happens during mealtimes (e.g. showing a child pictures of what food will be on their plate and showing a child a story to explain what will happen during a mealtime).
- Vitamin or nutritional supplements
- Any supplements given or changes to a child’s diet to increase the vitamins or nutrients in their diet.
- Young children
- Children up to and including 8 years of age.
List of abbreviations
- ARFID
- avoidant/restrictive food intake disorder
- ASD
- autism spectrum disorder
- BAMBI
- Brief Autism Mealtime Behaviour Inventory
- BAMBIC
- Brief Autism Mealtime Behaviour Inventory in Children
- BMI
- body mass index
- BPFAS
- Behavioural Paediatric Feeding Assessment Scale
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- COSMIN
- COnsensus-based Standards for the Selection of health status Measurement INstruments
- EDACS
- Eating and Drinking Ability Classification System for individuals with cerebral palsy
- EDSD
- eating, drinking and swallowing difficulties
- ERIC
- Education Resources Information Center
- ESPGHAN
- European Society for Paediatric Gastroenterology, Hepatology and Nutrition
- FEEDS
- Focus on Early Eating, Drinking and Swallowing
- FFQ
- Food Frequency Questionnaire
- GMFCS
- Gross Motor Function Classification System
- GP
- general practitioner
- GRADE
- Grading of Recommendations and Assessment, Development and Evaluation
- HP
- health professional
- ISMAR
- Innsbruck Sensorimotor Actuator and Regulator
- MDT
- multidisciplinary team
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- PAG
- parent advisory group
- PediEAT
- Paediatric Eating Assessment Tool
- PIC
- participant identification centre
- PICOTS
- participant, intervention, comparator, outcome, time, setting
- PMAS
- Parent Mealtime Action Scale
- PMAS-R
- Parent Mealtime Action Scale – Revised
- PPI
- patient and public involvement
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RCT
- randomised controlled trial
- SD
- standard deviation
- SOMA
- Schedule of Oral Motor Assessment
- STROBE
- Strengthening the Reporting of Observational studies in Epidemiology
- TOM
- Therapy Outcome Measure
- WoS
- Web of Science
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/hta25220).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.