Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 14/49/149. The contractual start date was in June 2016. The draft report began editorial review in January 2020 and was accepted for publication in July 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2021. This work was produced by Cockayne et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2021 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Material throughout this chapter has been reproduced from Cockayne et al. 1 © Author(s) (or their employer(s)) 2018. Re-use permitted under CC BY. Published by BMJ. This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Material throughout this chapter has been reproduced from Cockayne et al. 2 © 2021 Cockayne S et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Burden of falls and falling in the UK
Falls and fall-related fractures are highly prevalent among older people and are a major contributor to morbidity and cost to individuals and society. 3 Approximately one-third of people aged ≥ 65 years, and half of those aged > 80 years living in the community, will have a fall each year. 4,5 Although not all falls will have an impact on the individual, approximately one-fifth of all falls will require medical attention and 5% will result in a fracture,6 often a hip fracture. A significant number of falls (85%) occur within the home. 7 Older people who fall once are two to three times more likely to fall again within 1 year. Repeated falls tend to be experienced by frail older people aged ≥ 75 years. 4 These falls may lead to a loss of independence, resulting in the need for institutional care. It is likely that this burden will further increase, given the ageing population in the UK, with projections that the proportion of people aged ≥ 65 years is set to rise from 18% to 24% between 2016 and 2042. 8 The financial cost of treating injurious falls has been estimated at £2B per year, mainly as a result of the cost of treating hip fractures. 9
Risk factors for falling
Falls occur as a result of a complex interaction of risk factors. These risk factors can be separated into three broad categories: intrinsic, extrinsic and behavioural. Intrinsic risk factors are person-related and include factors such as having had a previous fall or fracture, impaired vision or impaired balance/gait. 10 Extrinsic risk factors are related to the environment, such as the presence of clutter, trip hazards or poor lighting. Behavioural risk factors include risk-taking activities, for example climbing on chairs, drinking alcohol, or having poor intake of nutrition or fluids.
Environmental hazards (extrinsic risk factors) are frequently attributed by older people as the primary causal factors in their fall and are also cited in the literature as a major contributor to falls. In a review by Rubenstein11 of 12 studies, environmental factors were identified as the primary cause of approximately one-third of falls (mean 31%, range 1–53%, n = 36,280). Similarly, in Talbot et al. ’s12 retrospective study, environmental factors were perceived by older people as the second most common cause of falls, with key contributors identified as objects on the floor, external forces and wet, uneven and icy surfaces. The latest Cochrane review in this area13 reported that home safety assessment and modification was effective in reducing the risk of falling [relative risk of falling 0.88, 95% confidence interval (CI) 0.80 to 0.96]. It also concluded that the intervention was more effective in people at higher risk of falling, including those with visual impairment, and if it was delivered by an occupational therapist (OT). 14
Environmental assessment and modification to reduce falls
The person–environment–occupation occupational therapy conceptual model of practice purports that the person, their environment and the activities in which they engage continually interact in ways that enhance or diminish the individual’s occupational performance. Environmental hazards constitute dynamic entities, which occur through the interaction between these three elements, and occupational therapy practice aims to restore a balance between these elements. Occupational therapy-led environmental interventions, therefore, comprise a comprehensive assessment of the older person, their environment and the tasks they perform, with intervention strategies focused on the person, their environment and their task performance.
At the time of applying for funding for this study, National Institute for Health and Care Excellence (NICE) guidance15 recommended the delivery of a home hazard assessment and safety intervention/modification for those receiving treatment in hospital as a result of a fall. It was recommended that the assessment should be undertaken by a ‘suitably trained professional’, in conjunction with follow-up and appropriate interventions. However, no such guidance existed for older people living in the community who had an elevated risk of falling but had not yet necessarily received hospital treatment as a result of falling. This was despite a pilot trial, undertaken by one of the authors,16 that assessed the effectiveness of a home hazard assessment and environmental modification in this population reporting a reduction in the number of falls as a secondary outcome in the study.
Consequently, the Occupational Therapist Intervention Study (OTIS) was undertaken to find out if these preliminary findings could be confirmed and to evaluate the cost-effectiveness of the intervention. If home hazard assessment and environmental modification were shown to be clinically effective and cost-effective, it would be likely that these would be implemented more widely, and could lead to important public health gains in preventing or delaying disability in the older population.
Research aims and objectives
OTIS was funded by the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme in response to a call for efficient study designs. The aim of OTIS was to establish the clinical effectiveness and cost-effectiveness of a home hazard assessment and environmental modification, delivered by OTs, on the number of falls among older, community-dwelling people at risk of falling.
The main objectives of OTIS were to:
-
investigate the clinical effectiveness of a home hazard assessment and environmental modification for falls prevention
-
investigate the cost-effectiveness of a home hazard assessment and environmental modification for falls prevention
-
explore the barriers to and facilitators of implementing the intervention among OTs and the wider community (e.g. commissioners of services).
Chapter 2 Methods
Material throughout this chapter has been reproduced from Cockayne et al. 1 © Author(s) (or their employer(s)) 2018. Re-use permitted under CC BY. Published by BMJ. This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Material throughout this chapter has been reproduced from Cockayne et al. 2 © 2021 Cockayne S et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Study design
OTIS was a modified cohort, pragmatic, two-armed, open randomised controlled trial (RCT), with an economic evaluation and nested qualitative study. In a cohort RCT (cRCT) design, participants are recruited to an observational cohort, and eligible participants are then randomised into an embedded RCT, following a period of outcome data collection. 17 Participants are unaware of when they have been randomised into the embedded RCT, and only those in the intervention group are informed that they have been offered the treatment. In our definition of a modified cRCT, participants were recruited into a cohort, but all were informed about the embedded RCT and that allocation to the intervention group and the usual-care group would be decided by chance. We implemented a run-in period for data collection before randomisation and a process for rescreening, after a period of time, the cohort participants who were not immediately eligible for the trial. We successfully used this design to improve recruitment rates and potentially minimise post-randomisation attrition in a previous NIHR-funded falls prevention RCT. 18 We therefore expected to observe similar benefits in this trial, as the previous trial not only was conducted in a comparable population, but also evaluated a falls prevention intervention.
The first benefit of using a modified cRCT design was the anticipated increase in recruitment rates in comparison with a traditional RCT design. Some participants would be immediately eligible for the trial and could be randomised straight after the initial ‘run-in’ period of data collection. Others who fulfilled all of the eligibility criteria apart from having had a fall within the previous 12 months were rescreened at a later date and could subsequently become eligible (e.g. if they had fallen in the meantime), in which case they could be randomised. These additional participants would have been lost to recruitment had a traditional RCT design been used. Second, we expected that this design would minimise post-randomisation attrition and the possibility of reporting bias. In a traditional RCT design, as participants can access usual care outside the trial, the only incentive to take part in the trial, apart from altruism, is the possibility of receiving the intervention. In this modified design, all participants were informed on enrolment to the cohort that they could at some point be offered a home assessment visit by an OT. The home assessment visit was offered to those participants subsequently randomised into the intervention group of the RCT; however, those in the usual-care group were not explicitly notified of their group allocation, as would have been the case in a traditional RCT. We expected that this would reduce attrition caused by ‘resentful demoralisation’ and minimise the risk of participants in the usual-care group biasing the trial, either knowingly or unknowingly, by reporting the number of falls they had experienced more or less conscientiously than those allocated to the intervention group. Finally, to ensure that participants were engaging with the study, and to reduce post-randomisation attrition and the risk of selection bias, we used a ‘run-in’ period to collect falls data prior to participants being randomised. Participants had to return their baseline questionnaire and at least one falls calendar before they could be randomised. As participants were required to provide a falls calendar each month for 1 year after being randomised, we hoped that only including those who had a proven track record of returning a calendar would reduce the chance that participants would not return calendars during the course of the trial.
Participants were randomised to either the usual-care group or the intervention group. The usual-care group continued to receive usual care from their general practitioner (GP) or other health-care professional and were sent a falls prevention leaflet. The intervention group received usual care and the falls prevention leaflet and were also offered a home assessment visit by an OT. Participants were allocated to a group using an unequal randomisation ratio (generally 2 : 1) in favour of the usual-care group in order to reduce costs and minimise the OT burden of delivering the intervention. The trial included an economic evaluation (see Chapter 4) and a nested qualitative study to explore treatment fidelity and the OTs’ experiences of delivering the intervention (see Chapter 5). The trial protocol has been published in full. 1
Public involvement
OTIS was informed throughout by the involvement of older people with a history of falls. Patient and public representatives were identified from the cohort of participants who had taken part in previous studies led by the study team. The group consisted of four older people, who met with the study team each year during the course of the study at face-to-face meetings held at the University of York. They also provided input over the telephone, if required. They helped develop the design and conduct of the study by providing feedback on the grant application submitted to the funder. They identified falls as an area of concern affecting many older people and agreed that reducing falls was an important issue. They further considered that strategies aimed at reducing falls was an area of research worth undertaking. During the trial, further advice was given on recruitment methods and on the phrasing and content of participant-facing documents, such as the participant information sheet, case report forms and newsletters. The group considered the burden of completing the trial documentation and whether or not it would be acceptable to trial participants. In addition, they reviewed the plain English summary in this report and advised on the dissemination of findings to a lay audience. They will provide input into the summary of results letter that will be sent to participants. One of the public involvement group members was also a member of the Trial Steering Committee (TSC)/Data Monitoring and Ethics Committee (DMEC). She attended meetings via teleconference and contributed from a non-medical perspective to ensure that the trial maintained its priorities of being patient focused and pragmatic.
Regulatory approvals and research governance
Ethics approval for the study was obtained from the West of Scotland Research Ethics Committee (REC) 3 (REC reference number 16/WS/0154) on 8 August 2016. The study was approved by the Health Research Authority on 8 September 2016. The University of York Department of Health Sciences Research Governance Committee approved the study on 20 May 2016. Approval and ‘Confirmation of Capacity and Capability’ was obtained for each participating NHS trust prior to the commencement of the trial at that site (see Appendix 1). Substantial amendments to approve changes to the protocol and study documentation were submitted to the REC, the Health Research Authority, the Department of Health Sciences Research Governance Committee and each site’s research and development office as required during the course of this study. The trial sponsor was the University of York, and responsibilities were delegated to York Trials Unit (YTU).
Trial registration
The trial was assigned the International Standard Randomised Controlled Trial Number (ISRCTN) ISRCTN22202133 on 21 June 2016.
Setting
Recruitment of participants took place across eight NHS trusts based in primary and secondary care in England. OTs employed in these trusts delivered the trial intervention. Fifteen GP surgeries within the geographical area covered by six of these NHS trusts mailed out invitation packs to their patients as part of the recruitment process.
Participant recruitment
Participants were first recruited to the OTIS cohort. Potential participants for the cohort were identified by one of the following methods:
-
A database search of existing trial cohorts held at YTU19–21 and the Yorkshire Health Study. 22 To be eligible for the mail-out, participants had to be aged ≥ 65 years and live in an OT catchment area. Participants known to live in residential or nursing homes were excluded from the mail-out.
-
A database search of the patient lists at GP surgeries within OT catchment areas. To be eligible for the mail-out, patients had to be aged ≥ 65 years. Patients known to have dementia or who lived in residential or nursing homes were excluded.
-
Advertising the study in GP surgeries, newspapers, faith magazines, posters, University of the Third Age (London, UK) and flyers.
-
Opportunistic screening undertaken by health-care professionals (GPs and podiatrists).
The recruitment methods used at each individual trust are reported in Appendix 2. As we planned to mail out a large number of invitation packs in order to recruit sufficient participants, we took the opportunity to evaluate the effectiveness of interventions designed to increase recruitment to studies. 23,24 As we were sending out large numbers of postal questionnaires to participants for follow-up data, we also took the opportunity to evaluate interventions aimed at improving response rates to postal questionnaires. 25,26
Any potential participant identified using one of the above strategies was sent a study recruitment pack consisting of an invitation letter (see Report Supplementary Material 1), a participant information sheet (see Report Supplementary Material 2), a consent form (see Report Supplementary Material 3), a screening questionnaire (see Report Supplementary Material 4) and a freepost envelope for returning the completed paperwork. For potential participants invited through a GP mail-out, the invitation letter was from their GP surgery, while those approached from the YTU cohorts and the Yorkshire Health Study were sent an invitation letter from YTU or the Yorkshire Health Study, respectively.
Potential participants who wished to take part in OTIS were requested to return a completed consent form and screening questionnaire by post to YTU. The research team assessed the forms to confirm the participant had given consent to take part in the study and for eligibility.
Consenting participants
Participation in OTIS was voluntary. Potential participants were given written information about the study and contact details for the research team if they, or a family member or friend, had any queries. The participants were asked to complete a consent form to indicate that they wanted to take part in the research and were willing to receive a home visit from an OT if this was offered. At the consent stage, participants were able to register their interest in helping the study team with other similar related studies. If willing, participants could opt in to being sent details about future research. On receipt of written consent for this study, researchers at YTU assessed participants’ responses to the screening questionnaire for eligibility in accordance with the criteria listed below.
Participant eligibility
Inclusion criteria for OTIS cohort
-
Aged ≥ 65 years.
-
Willing to receive a home assessment from an OT.
-
Community-dwelling.
-
Had had at least one fall in the past 12 months or reported a fear of falling on their screening questionnaire (for at least some of the time).
Exclusion criteria for OTIS cohort
-
Unable to walk 10 feet (3.05 m), even with the use of a walking aid.
-
Unable to give informed consent, for example due to dementia.
-
Lived in a residential or nursing home.
-
Unable to read or speak English and had no friend or relative to translate/interpret for them.
-
Had had an OT assessment for falls prevention in the previous 12 months or were on the waiting list for an OT assessment.
Participants who met any of the exclusion criteria for the study or did not meet the first three inclusion criteria were notified in writing that they were ineligible and no further correspondence was sent. Participants deemed eligible (i.e. those who met all of the inclusion criteria and none of the exclusion criteria) were sent a baseline questionnaire (see Report Supplementary Material 5) and a pack of monthly falls calendars (18 months’ worth; see Report Supplementary Material 6). Participants were eligible to be randomised once they had returned a completed baseline questionnaire and at least one falls calendar in the preceding 3 months. Participants who had neither had a fall in the past 12 months nor reported a fear of falling but were otherwise eligible for the study were deemed as ‘pending’ in terms of their eligibility and were followed up every 3 months for falls data. If individuals reported a fall, and were still willing to be in the study, they became eligible and were sent a pack of falls calendars and a baseline questionnaire to complete. If individuals did not report a fall by the end of the recruitment period, they were sent a letter to inform them that recruitment to the study had closed and that they were ineligible for the study.
Sample size
We proposed to recruit and randomise 1299 participants to OTIS in a 2 : 1 ratio (i.e. 866 to usual care and 433 to the intervention). This number allowed for 10% attrition and provided 90% power (using two-sided significance at the 5% level) to show a difference in the percentage of participants who experienced at least one fall in the 12 months following randomisation from 60% in the usual-care group to 50% in the intervention group, accounting for the unequal randomisation (Stata Statistical Software Release 13, StataCorp LP, College Station, TX, USA). In the REFORM (REducing Falls with ORthoses and a Multifaceted podiatry intervention) trial,19 which was conducted by some of the authors, an absolute difference of 5% was observed in the percentage of participants experiencing a fall (intervention group, 50%; usual-care group, 55%), with an upper CI limit of 13%; therefore, the decision was made to power OTIS for a 10% absolute difference.
The sample size is based on the proportion of participants experiencing at least one fall over 12 months, which is a key secondary outcome, rather than on the primary outcome, which is a count variable (number of falls). Powering a trial for count data is more complex and requires greater assumptions and so a binary approach to the sample size calculation was taken.
Randomisation
Participants were enrolled into the cohort study if they fulfilled the eligibility criteria and provided written consent to take part. They were then randomised to either the intervention group or the usual-care group once they had returned a valid baseline questionnaire and at least one falls calendar within 3 months prior to randomisation. Randomisation was carried out using YTU’s secure web-based randomisation service and was based on an allocation sequence generated by an independent data systems manager, who was not involved in the recruitment of participants. A ‘batch’ of participants from a particular centre was randomised at a time in a single block, most commonly in a 2 : 1 ratio in favour of the usual-care group (to reduce costs), according to when centres had the capacity to undertake intervention appointments and how many participants were available to be randomised. In some instances, the allocation ratio used per ‘batch’ varied (range 1 : 1 to 9.7 : 1) depending on the OTs’ capacity to carry out the assessments and the number of participants available to randomise at that centre (the overall ratio was, ultimately, 2.1 : 1). Unequal randomisation in favour of the usual-care group was used for two reasons. First, it reduced the cost of delivering the intervention arm of the study. Second, it reduced the OTs’ burden of having to deliver the home assessment visits. There was a total of 12 centres; some of the trial sites were split into two or three centres, according to the geographical areas covered by the OTs, for the purposes of randomisation. By nature of the procedure, the median time from completion of the eligibility form to randomisation was around 2.5 months. We did not repeat eligibility checks or baseline data collection before randomisation as we did not anticipate that many participants’ circumstances were likely to change in this short time frame such that they would become ineligible. This avoided further delays. Once intervention group participants had been randomised, they were sent a letter informing them of their group allocation and confirmation that an OT would be in contact to arrange a home assessment visit. Participants who were allocated to the usual-care group were not informed of their group allocation in order to minimise potential attrition and the possibility of resentful demoralisation. YTU wrote to all participants’ GPs (both the intervention and the usual-care groups) informing them of the participant’s study participation after they had been randomised and advised them of the possibility that the participant could be offered a home assessment.
Blinding
Owing to the nature of the intervention, participants in the intervention group were not blinded to group allocation. This was an open trial with an unequal allocation ratio; therefore, it was not possible to blind members of the research team who were actively involved in the administration of the study, the statistician or the health economist. Data entry staff were, however, blind to group allocation.
Group allocation
Usual-care group
Participants allocated to the usual-care group continued to receive usual care from their GP and other health-care professionals, which may have included referrals to falls clinics. They were sent a falls prevention leaflet produced by Age UK27 with their baseline questionnaire in the post.
Intervention group
In addition to the usual care and the falls prevention leaflet given to the usual-care group, the participants in the intervention group were offered home environmental assessment and modification to identify personal fall-related hazards. The assessment was undertaken by an OT registered with the Health and Care Professions Council (HCPC). The OT used the Westmead Home Safety Assessment (WeHSA) tool28 to structure their assessment visit. The WeHSA was developed in Australia in 1997 for older adults and is a validated tool. It comprises a 57-item functional assessment organised into 15 domains: internal/external traffic ways, general indoors, living area, seating, bedroom, toilet area, bathroom, kitchen, laundry, mobility aid, footwear, pets, medication management and safety call systems. The assessment consisted of an initial discussion about the participant’s history of falling, lifestyle, patterns of usage of areas in the home, risk-taking behaviour, strategies already adopted to reduce falls, environmental changes made and functional vision. The participant and the OT then moved through the home and identified potential falls hazards. The OT encouraged the participant to identify potential strategies to mitigate any hazards, but offered suggestions if needed. A list of recommendations was agreed and, if needed, the OT would either refer on to other agencies or liaise with family members regarding the provision of equipment and modifications or refer on to other health-care services as deemed clinically appropriate. The Timed Up and Go test29 was also conducted. The Timed Up and Go test is a standardised tool used as a falls risk indicator, with scores of > 14 seconds indicating a high risk of falling. These data were collected to allow secondary analysis to be undertaken once the main findings of the study are published. A second visit by the OT could have been undertaken if deemed clinically necessary, but, to our knowledge, none was. The OT contacted the participant 4–6 weeks after the home visit to collect data on whether or not the recommendations had been acted on. To document the activities undertaken at the visit, the OT completed an OT booklet (see Report Supplementary Material 7).
Occupational therapist training to deliver the intervention
The OTs delivering the intervention attended a 1-day face-to-face training session on how to conduct the assessment before conducting the home visits with participants. A standardised training package was developed by the co-applicant who had undertaken the pilot study16 (Alison Pighills) in collaboration with Professor Lindy Clemson, who originally developed the WeHSA. A standardised approach was used to ensure that the OTs who delivered the intervention did so consistently across all of the trial sites. The training materials included a presentation with detailed notes, a training manual and videos of older people undertaking activities of daily living, which the OTs assessed using the WeHSA. The training covered the following 10 domains: prevalence of falls; evidence underpinning environmental assessment and modification; falls risk factors; the person–environment–occupation conceptual model of practice and occupational performance;30 background on falls – categories, types and locations; environmental assessment; equipment and ideas for falls prevention; adherence to recommendations; action-planning; and scoring a video of an older person carrying out functional tasks at home using the WeHSA. Self-efficacy was addressed in the OT training in relation to its influence on participants’ confidence in their ability to engage in activities without falling, and the potential for low self-efficacy to lead to activity avoidance and deconditioning. OTs were encouraged to address both falls self-efficacy and fear of falling with participants as a component of the visit. The OT training addressed grading the demands that activities placed on the individual, particularly in the context of ‘environmental press’, which is the demand that the environment places on the individual, and reducing that demand for frail older people to enhance their self-efficacy. The initial training session was led by the co-applicant (Alison Pighills) who conducted the pilot trial. Two other HCPC-registered OTs and study co-applicants (Shelley Crossland and Avril Drummond) were also trained to facilitate the training at subsequent training sessions using a ‘train the trainer’ approach. 31,32 These face-to-face training sessions were audio-recorded for the purpose of evaluating training fidelity. The face-to-face training was supplemented by an online training module. 33 Those who could not attend the face-to-face training undertook the online training course in addition to attending ‘cascade’ training delivered by one of the OTs who had attended the face-to-face training sessions. These ‘cascade’ trainers were provided with the same training package used in the face-to-face sessions, which included extensive notes. All OTs trained to deliver the intervention were supported by Shelley Crossland, Avril Drummond and Alison Pighills, who addressed any queries or points for clarification raised during the training or throughout the trial.
Participant follow-up
All participants in OTIS were followed up with monthly falls calendars for 12 months post randomisation, and follow-up was completed by August 2019. If a participant did not return their falls calendar within 10 days of the due date, a member of the research team either contacted them by telephone (Monday to Friday between 9 a.m. and 5 p.m.) or sent a letter to collect falls data. Participants could also ring a freephone number to report a fall (participants could leave a message outside office hours or when a research team member was not available). Any participant who reported falling using their falls calendar was contacted by the research team in order to collect additional information about the fall(s) (see Report Supplementary Material 8). Participants occasionally gave permission for a relative or carer present to talk on the telephone to the researcher on their behalf. Permission for the study team to talk to a relative or carer was documented on the participant’s consent form. The relative or carer was asked to report on the participant’s own views. The relative or carer did not have to provide their own written consent.
Participants were also sent follow-up questionnaires in the post at 4, 8 and 12 months post randomisation along with a freepost return envelope (see Report Supplementary Material 9–11). Any participant who provided a mobile phone number and agreed to receive text messages was sent one Short Message Service text at the time they were expected to receive their questionnaire. Any participant who did not return their follow-up questionnaire within 21 days was sent a reminder letter together with an additional copy of the questionnaire. Participants were also sent a group-specific newsletter at 3 months post randomisation and 2 weeks before their final 12-month follow-up questionnaire. The newsletter informed participants of the study’s progress and aimed to minimise attrition and improve response rates to the postal questionnaires. The content of the newsletter was informed by issues raised by study participants and the public involvement group during the course of the trial. All participants were sent an unconditional £5 in cash with their 12-month questionnaire to cover any incidental expenses that they may have incurred when completing the questionnaires and in recognition of their participation in the study.
Outcomes
Primary outcome
The primary outcome of the trial was the number of falls per participant over the 12 months from randomisation. A fall was defined as ‘an unexpected event in which the participant comes to rest on the ground, floor or lower level’. 34 Data were collected from self-reported monthly falls calendars, on which participants were asked to mark the number of falls they had on each day or to indicate that they had not fallen that month. An explanation of what the researchers considered to be a fall was included in the participant information sheet and on the falls calendars. If a participant was uncertain whether an event would be classed as a fall, they were encouraged to ring the research team at YTU to discuss it. Participants who indicated on their falls calendar that they had a fall were contacted by the research team for further information. Information collected about the falls included the date and number of falls, the location of the fall, what the participant was doing when they fell (i.e. the cause/reason for the fall), injuries from the fall (e.g. superficial wounds, such as bruising, sprain, cuts, abrasions or fractures, including type of fracture) and any hospital admissions (see Report Supplementary Material 8).
Data collected on the 4-, 8- and 12-month post-randomisation follow-up participant questionnaires included the number of falls in the previous 4 months and were used to calculate the falls rates for participants who did not return any monthly falls calendars.
Secondary outcomes
All secondary outcomes were self-reported by the participant and collected using questionnaires at baseline and at 4, 8 and 12 months post randomisation, or on monthly falls calendars. The secondary outcomes were:
-
proportion of participants reporting at least one fall in the 12 months from randomisation
-
proportion of participants reporting multiple (two or more) falls in the 12 months from randomisation
-
time to first fall from date of randomisation and between subsequent falls
-
fracture rate
-
fear of falling
-
health-related quality of life, as measured using the EuroQol-5 Dimensions, five-level version (EQ-5D-5L)
-
health service utilisation.
Scoring of instruments
Fear of falling
Fear of falling was measured using the question ‘During the past 4 weeks have you worried about having a fall?’ Response categories were all of the time, most of the time, a good bit of the time, some of the time, a little of the time, and none of the time. These were scored from one to six, respectively, and treated as continuous data in the analysis. This measure has not been validated yet but was used by some of the authors in the earlier REFORM trial,18 where it correlated moderately well (r = 0.6) with the validated Short Falls Efficacy Scale (Short FES-I).
Other data collected
Items to identify participants with a history of falling or balance problems
We incorporated items into the baseline questionnaire that asked about history of falling or balance problems. These included selecting which of the following statements best applies: My balance is good and I want to keep it that way; My balance is quite good but I would like to improve it; or I have some problems with balance that I want to overcome. Additionally, we asked ‘Do you have any difficulties with your balance whilst walking or dressing?’, with the following response categories: yes, often or always; or no, or just occasionally. This item, in addition to asking about the number of falls sustained in the previous 12 months and the severity of problems doing usual activities (as part of the EQ-5D-5L), allowed us to construct a measure of risk of falling (adapted from a balance screening survey developed in the PreFIT study35). Participants reporting balance problems while walking or dressing or at least moderate problems doing usual activities, or those reporting one or more fall in the previous 12 months, were categorised as being at intermediate or high risk of falling. Conversely, those who reported no falls in the previous 12 months and no balance problems while walking or dressing and no or only slight problems doing usual activities were deemed to be at a low risk of falling.
Other important data
The following data were also collected during the study: date of birth, sex, ethnicity, height, weight, living arrangements, health problems, broken bones since the age of 18 years, number of medications prescribed by a doctor (≤ 4 vs. > 4), and difficulties with balance and, for intervention participants only, Timed Up and Go test scores and duration of home assessment.
Adverse events
With approval from the REC and the joint TSC and DMEC, it was agreed that only unexpected events that were related to taking part in the study had to be reported. Details of any adverse events reported directly to YTU by the participant, by a member of their family or by the OT who delivered the intervention were recorded on an OTIS adverse event form (see Report Supplementary Material 12). Participants reported adverse events by writing details of the event in the free-text comment section of a follow-up questionnaire, or they or a family member could report the event during a telephone call with a member of the study team. OTs were instructed to inform the study team by telephone if they found out that the participant had experienced an adverse event during their follow-up telephone call to elicit data on whether their recommendations had been actioned. Adverse events were categorised by two members of the study team, the trial manager and the chief investigator and reviewed by the Trial Management Group. Any serious adverse event judged to have been related and unexpected was required to be reported to the REC under the current terms of the standard operating procedures. The reporting period began as soon as the participant consented to being in the study and ended 12 months after they had been randomised.
In this study, a serious adverse event was defined as any untoward occurrence that:
-
resulted in death
-
was life-threatening
-
required hospitalisation or prolongation of existing hospitalisation
-
consisted of a congenital anomaly or birth defect
-
was otherwise considered medically significant by the investigator.
Owing to the age of the participants, expected events included incidences of hospitalisations, disabling/incapacitating/life-threatening conditions, ageing-associated diseases (e.g. cancer, cardiovascular disease, diabetes, arthritis, osteoporosis or dementia), other common illnesses such as depression, falls and deaths.
The occurrence of adverse events was monitored during the trial by an independent TSC/DMEC. Adverse event data are summarised descriptively by treatment group.
Non-consenting participants
Participants who did not wish to take part in the study were not required to return any forms to YTU; however, some chose to complete the screening questionnaire, and thus provided some demographic information about their age, sex, falls in the previous 12 months and fear of falling.
Participant withdrawal
Participants could withdraw from the trial at any point during the course of the study. If a participant indicated that they wanted to withdraw from the study, they were asked whether they wished to withdraw from the intervention only (i.e. withdrawal from treatment if allocated to the intervention group, only if withdrawal was requested before OT visit was received) or withdraw fully from the study. When withdrawal was only from the intervention, follow-up data continued to be collected. Data provided by participants who fully withdrew were retained for analysis, up to the point at which they withdrew. A member of the research team completed a change of circumstance form for any participant who changed status during the trial (see Report Supplementary Material 13).
Trial completion and exit
Participants completed the trial once they had completed the 12-month follow-up period post randomisation. Participants exited the trial if they had fully withdrawn (i.e. no further follow-up), were lost to follow-up or died.
Data analysis
All outcomes were analysed collectively after follow-up had ended. Analyses were conducted using Stata version 15 (StataCorp, College Station, TX, USA), following the principles of intention-to-treat (ITT), with outcomes analysed according to the participants’ original randomised group irrespective of deviations based on non-compliance. Statistical tests were two-sided at the 5% significance level, and 95% CIs are used.
Three Consolidated Standards of Reporting Trials (CONSORT) flow diagrams depict the flow of participants through the trial; one presents the recruitment of participants via GP and Yorkshire Health Study cohort mail-outs and another presents the recruitment of participants via the YTU cohort mail-outs, up to the point of randomisation; the third presents the flow of participants from randomisation onwards.
Recruitment graphs present the overall recruitment by month, and the actual compared with the target recruitment.
All participant baseline data are summarised descriptively by trial arm. No formal statistical comparisons were undertaken. Continuous measures are reported as means, standard deviations (SDs), median, minimum and maximum, and categorical data are reported as counts and percentages.
Follow-up response rates to the monthly falls calendars and the participant questionnaires (including time to response) are summarised overall and by treatment group. Details about the falls (e.g. cause, location) are also summarised.
The number of intervention participants receiving the OT home assessment and the time taken from randomisation to the home visit are also summarised.
Participant withdrawals (number, type and timing) are presented overall and by treatment group.
Primary analysis
The number of falls per person was analysed using mixed-effects negative binomial regression adjusting for sex (male/female), age at randomisation (continuous), history of falling and the allocation ratio used to randomise the participant as fixed effects, and centre as a random effect. Participants were classified into two groups for the history of falling covariate: (1) one or no falls in the 12 months prior to completion of the screening questionnaire; and (2) two or more falls reported in the 12 months prior to completion of the screening questionnaire. The model included an exposure variable for the number of months that the participant returned a monthly falls calendar (i.e. the number of months’ worth of falls they report).
The point estimate for the treatment effect in the form of an adjusted incidence rate ratio (IRR) is provided, along with its associated 95% CI and p-value.
This analysis primarily included falls data from the falls calendars, but, where no post-randomisation calendars were returned, data from the 4-, 8- and 12-month participant questionnaires were used for a participant, where available. When no falls data were provided at all, an assumption of zero falls over a negligible follow-up time of 0.1 months was made for the participant in the analysis.
Analysis of the primary outcome was checked and verified by a second statistician.
Sensitivity analyses
Non-compliance
The primary analysis follows the principles of ITT and thus estimates the effect of the offer of an OT visit; however, not all intervention participants received the home assessment. A complier-average causal effect (CACE) analysis to assess the impact of compliance on the primary treatment estimate was undertaken. 36–38 CACE analysis allows an unbiased treatment estimate of, in this case, receipt of an OT home assessment visit, in the presence of non-compliance. It is less prone to biased estimates than the more commonly used approaches of per-protocol or ‘on-treatment’ analysis, as it preserves the original randomisation and uses the randomisation status as an instrumental variable to account for the non-compliance. Compliance was defined as receiving the OT home assessment visit within 12 months of randomisation. A two-stage instrumental variable regression approach was used with negative binomial regression to reflect the primary analysis.
Post hoc adjustment for Parkinson’s disease
A chance imbalance in the proportion of participants in the two groups with Parkinson’s disease at baseline was observed. We therefore decided, as an unplanned, post hoc analysis, to consider the impact of this imbalance on the primary treatment estimate in a sensitivity analysis. We repeated the primary analysis including whether or not the participant had Parkinson’s disease as an additional fixed effect.
Missing data
A logistic regression model was used to predict non-response (no falls data received post randomisation either via monthly falls calendars or on the participant follow-up questionnaires), including all variables collected prior to randomisation. The primary analysis was then repeated, including as covariates all variables found to be statistically significantly predictive of non-response, to determine if these affected the parameter estimates and study conclusions.
Therapist effects
In some centres, more than one therapist delivered the intervention visits to the participants. We therefore have clustering by therapist in the intervention group. The value of the intervention may depend on the skill/experience of the therapist and their relationship with the participant. Therefore, to account for this potential variation between therapists, a sensitivity analysis was conducted using an artificial cluster method. With this method, every participant (whether allocated to the intervention group or to the usual-care group) was associated with a therapist. For intervention participants, their trial therapist was known; however, for usual-care participants, we assigned them a counterfactual therapist, that is, one they could have seen had they been randomised to the intervention group. For centres where only one therapist delivered the visits, all of the usual-care participants were assigned this therapist. For centres that had more than one trial therapist, the usual-care participants were randomly assigned one of these therapists in the proportion that the therapists saw intervention participants. Each therapist then had their own cluster of usual-care and intervention participants. Therapist (rather than centre) was then included as a random effect in the primary analysis model.
Subgroup analysis
The primary analysis was repeated including an interaction term between the treatment allocation and whether or not a participant received care in a hospital (outpatient appointment, day case, accident and emergency (A&E) presentation, or hospital admission) as a result of a fall in the 4 months prior to completion of the baseline questionnaire.
Secondary analyses
The following secondary outcomes were analysed by mixed-effects logistic regression adjusting for sex, age, history of falls and allocation ratio as in the primary analysis, and centre as a random effect:
-
proportion of participants who fell at least once over the 12 months from the date of randomisation*
-
proportion of multiple fallers (participants who had two or more falls in the 12 months from randomisation)*
-
proportion of participants who reported that they worried about falling at least some of the time at 12 months post randomisation
*using data from monthly falls calendars except where no post-randomisation calendars were returned, in which case data from the 4-, 8- and 12-month participant questionnaires were used, where available.
Adjusted odds ratios (OR) for the intervention effect and their associated 95% CIs and p-values are provided.
The primary and secondary analyses relating to falls (number of falls and proportion of single and multiple fallers) were also repeated using data only from participant follow-up questionnaires.
The proportion of participants having at least one fracture or multiple fractures resulting from a fall are reported but were not formally analysed owing to the rarity of these events.
Fear of falling was also analysed in its continuous form using a covariance pattern model incorporating all post-randomisation time points and adjusting for baseline fear of falling, sex, age, history of falling, allocation ratio, treatment group, time, and a treatment group-by-time interaction. The correlation of observations within participants over time was modelled using participant and centre as random effects. The Akaike information criterion was used to compare models specifying different correlation structures (smaller values were preferred). 39 An unstructured covariance structure was used in the final model. Model assumptions were checked visually. The normality of the standardised residuals was checked using a Q–Q plot, and homoscedasticity was assessed using a scatterplot of the standardised residuals against fitted values. There was no evidence to suggest a violation of the underlying assumptions, so data were not transformed. Adjusted mean differences in fear of falling between the two groups at 4, 8 and 12 months are provided, with their 95% CIs and p-values.
The time to the first fall was derived as the number of days from randomisation to the first fall reported on the monthly falls calendars. The time between any subsequent falls was also calculated. Participants who did not have a fall were treated as censored at their date of trial exit, or the date of their last available assessment, or 365 days or trial cessation, as appropriate. For months for which no calendar was returned, it is assumed, by default, that no falls were experienced in these months. The proportion of participants yet to experience a fall was summarised using a Kaplan–Meier survival curve for each group. Time to fall was analysed using the Andersen and Gill method40 for analysing time to event data when the event can be repeated. The analysis treats each time to event or censoring as a separate observation. The data were analysed by Cox proportional hazards regression, using robust standard errors to account for dependent observations by participant and adjusting for the same covariates as in the primary analysis model. Adjusted hazard ratios and their associated 95% CIs and p-values are provided.
Summary of changes to the protocol
Recruitment
The original funding application stated that we planned to randomise 1299 participants to OTIS over a 10-month period. Participants would be identified from either cohorts of participants held at the University of York and the University of Sheffield or direct mail-out to patients on GP lists. However, study commencement was delayed by approximately 4 months because of contractual issues and delays in obtaining regulatory approvals. As the trial progressed, recruitment fell below the expected level, due, in the main, to the delay in setting up sites. Approval was obtained from the funder to extend the study by 12 months to a total of 43 months (June 2016 to December 2019). This permitted the set-up of additional sites and mail-out of recruitment packs to potential participants from GP surgeries. Details of the recruiting sites and the dates that research and development departments confirmed their capacity and capability to undertake the study can be found in Appendix 1. To facilitate recruitment, approval to use additional recruitment strategies, namely opportunistic screening by OTs and other health-care professionals, media advertising for participants and rescreening participants, was received in January 2017, May 2017 and March 2017, respectively.
Treatment fidelity
In July 2017, additional strategies were included in the protocol to assess treatment fidelity. Discussions about fidelity strategies were guided by the TSC/DMEC and agreed by the funder. Additional strategies included some observational work of the OTs delivering the intervention and a review of the OT booklets to ensure that key elements of consultations had been included.
Intervention: undertaking the follow-up telephone call
In October 2017, a change to the protocol allowed members of the research team as well as OTs to undertake the follow-up telephone call to check participants’ adherence to the recommendations suggested in the home visit. This was intended to relieve some of the burden on the OTs; however, in the end the OTs had capacity to undertake all of the follow-up calls.
Chapter 3 Clinical effectiveness results
Material throughout this chapter has been reproduced from Cockayne et al. 2 © 2021 Cockayne S et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Participant flow
Participants were mainly enrolled into OTIS via mail-outs from GP surgeries or from previous trial cohorts. The flow of participants is illustrated in the CONSORT flow diagrams in Figures 1–3. Across the eight participatory sites (East Coast Community Healthcare, East Sussex Healthcare NHS Trust, Harrogate and District NHS Foundation Trust, Humber Teaching NHS Foundation Trust, Leeds Community Healthcare NHS Trust, Northern Lincolnshire and Goole NHS Foundation Trust, Sheffield Teaching Hospitals NHS Foundation Trust and York Teaching Hospital NHS Foundation Trust), 12 ‘centres’ were formed. These centres were formed for logistical reasons to stratify the randomisation and were based on the geographical areas covered by the OTs.
FIGURE 1.
The CONSORT flow diagram, up to randomisation, for GP and Yorkshire Health Study cohort mail-outs. a, More than one reason can apply.
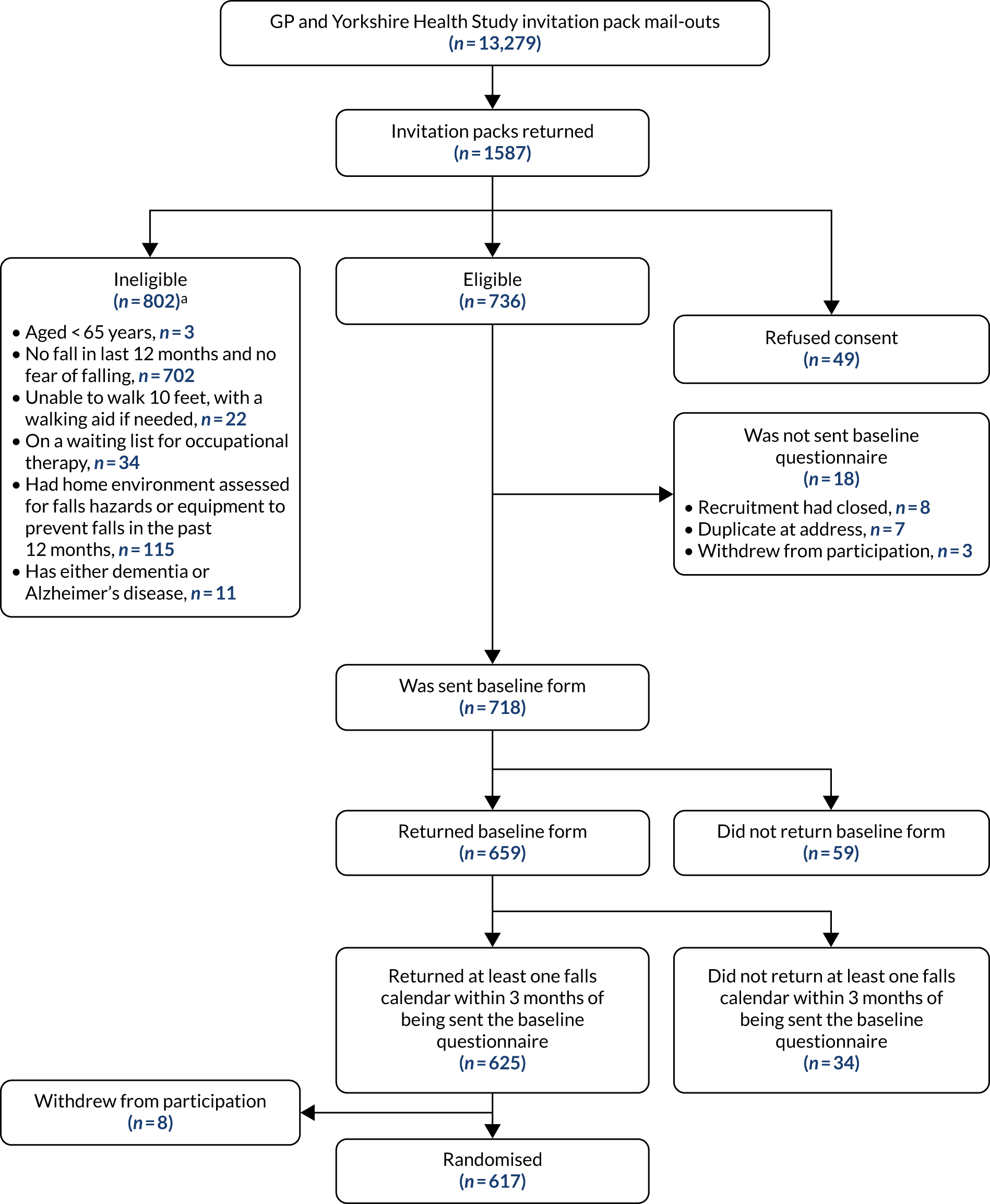
FIGURE 2.
The CONSORT flow diagram, up to randomisation, for YTU existing trial cohort mail-outs. a, More than one reason can apply. Reproduced with permission from Cockayne et al. 2 © 2021 Cockayne S et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
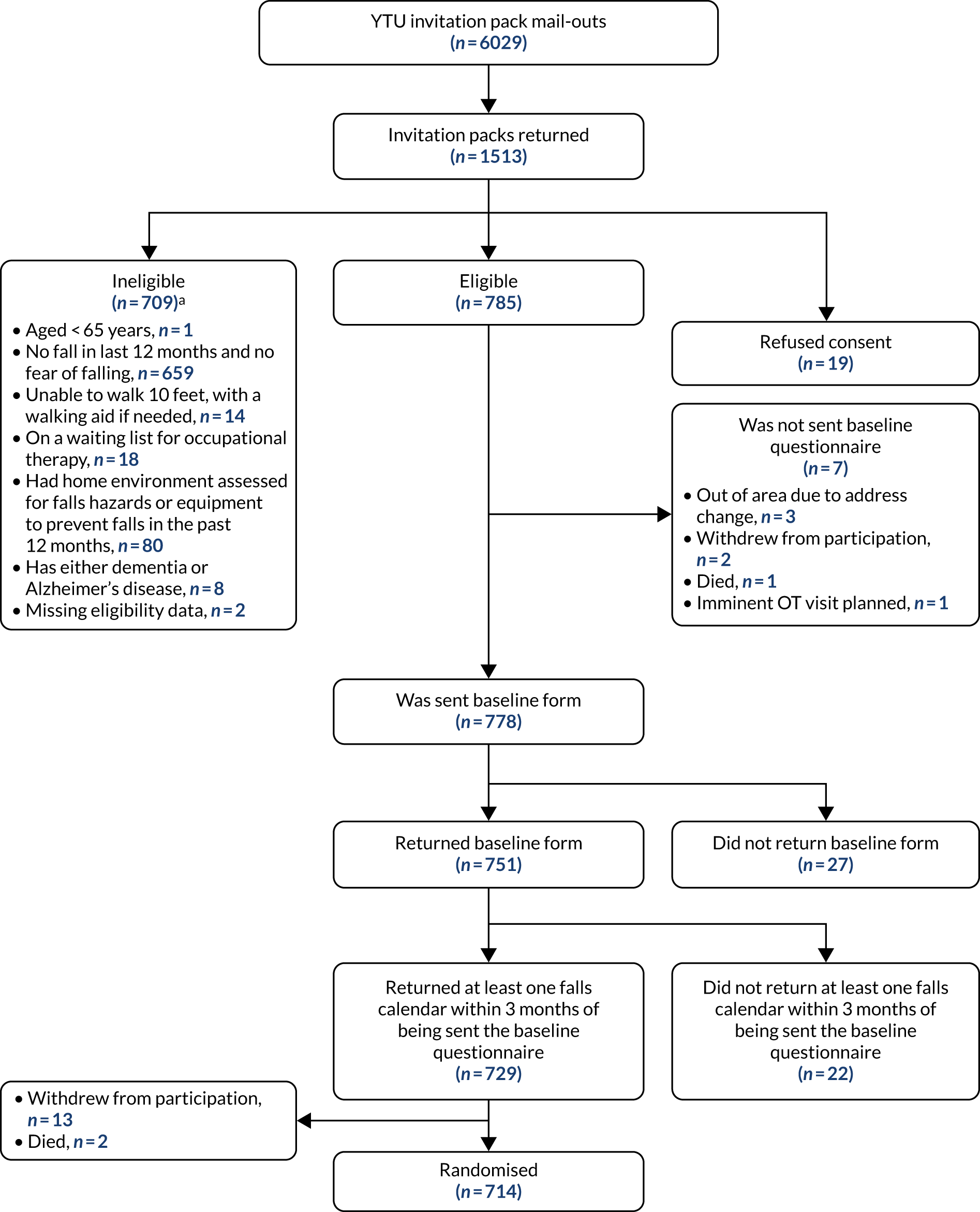
FIGURE 3.
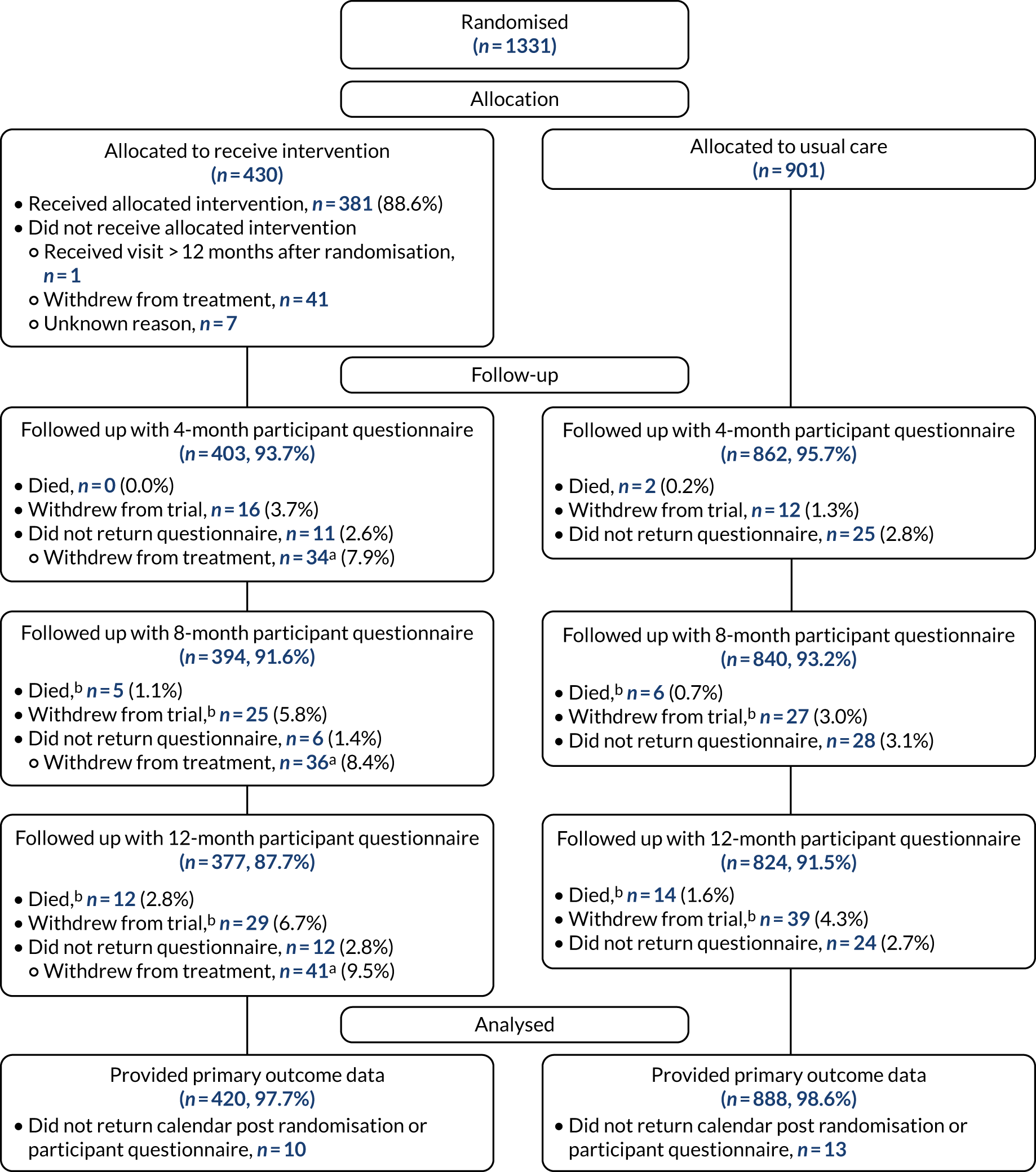
The CONSORT flow diagram depicting the flow of participants from randomisation. a, Includes withdrawals from trial/death where this was before the intervention was received; b, withdrawals and deaths over time are cumulative.
General practitioner mail-outs
A total of 11,965 recruitment packs were mailed or handed out to potential participants between March 2017 and April 2018 from GP surgeries, via opportunistic screening or through the University of the Third Age. The geographical locations covered included Harrogate, York and Elvington, Sheffield, Grimsby, East Coast Community (across Norfolk and Suffolk) and East Sussex.
Yorkshire Health Study
A recruitment pack was sent to 1314 participants from the Yorkshire Health Study cohort between April and July 2017.
Existing trial cohorts
A total of 6029 potentially eligible participants from previous trials conducted at YTU were mailed between October 2016 and March 2018: 3142 (52.1%) from CASPER (CollAborative care and active surveillance for Screen-Positive EldeRs),20 1741 (29%) from SCOOP (screening of older women for prevention of fracture)21 and 1146 (19.0%) from REFORM. 19
Recruitment
Recruitment commenced in October 2016 and ceased when the final participant was randomised in August 2018. Overall, 19,308 recruitment packs were distributed. Among these, no response was received to 15,491 (80.2%), 162 were returned as ‘addressee unknown’, 159 people had died, seven participants received more than one pack (duplicates), three packs were returned too late to be included in the trial, and two participants were out of area. A further 384 people returned incomplete documentation. In total, 3100 (16.1%) potential participants returned a screening questionnaire and a valid consent form and were assessed for eligibility; 68 (2.2% of 3100) declined to participate, 1468 (47.4%) were immediately eligible and 1564 (50.4%) were initially ineligible. The most predominant reason for ineligibility was not having had a fall in the previous 12 months or not having a fear of falling (n = 1361, 87.0%), although this was usually not the only reason (Table 1).
| Reason (not mutually exclusive) | n (% of 1564) |
|---|---|
| Aged < 65 years | 4 (0.3) |
| No fall in last 12 months and no fear of falling | 1361 (87.0) |
| Unable to walk 10 feet, with a walking aid if needed | 36 (2.3) |
| On a waiting list for occupational therapy | 52 (3.3) |
| Had home environment assessed for falls hazards or equipment to prevent falls in the past 12 months | 195 (12.5) |
| Had dementia | 19 (1.2) |
| Missing eligibility data | 2 (0.1) |
Based on their initial screening, 1289 participants were otherwise eligible except that they had not had a fall in the previous 12 months or did not have a fear of falling. These participants were eligible to be rescreened. A rescreening questionnaire was sent to 965 people (among the rest, they either declined to be rescreened or recruitment had closed before they were due to be rescreened). Of the 147 participants who returned a rescreening form, 53 (36.1%) subsequently became eligible (43 of whom went on to be randomised). Eligible and consenting participants were sent a baseline questionnaire and a pack of falls calendars (n = 1496). Twenty-five eligible, consenting participants were not sent a baseline pack because the trial had closed to recruitment (n = 8); the participant was at a duplicate address (n = 7); the participant withdrew consent (n = 5); the participant lived outside an area that an OT could visit (n = 3); an imminent OT visit was planned (n = 1); and the participant had died (n = 1). Of the 1410 participants (94.3% of 1496) who returned a baseline questionnaire, 1354 (96.0% of 1410) also returned at least one falls calendar. Of these 1354 participants, 1331 were randomised [the remaining 23 either withdrew (n = 21) or died (n = 2) before they were randomised].
The overall randomisation rate, from the total number of recruitment packs sent out, was 6.9%. The rate varied according to the mode of recruitment. From 6029 recruitment packs mailed out from YTU trial cohorts, 714 (11.8%) participants were randomised, relative to 59 out of 1314 (4.5%) from the Yorkshire Health Study and 558 out of 11,965 (4.7%) from GP surgeries.
Randomisation
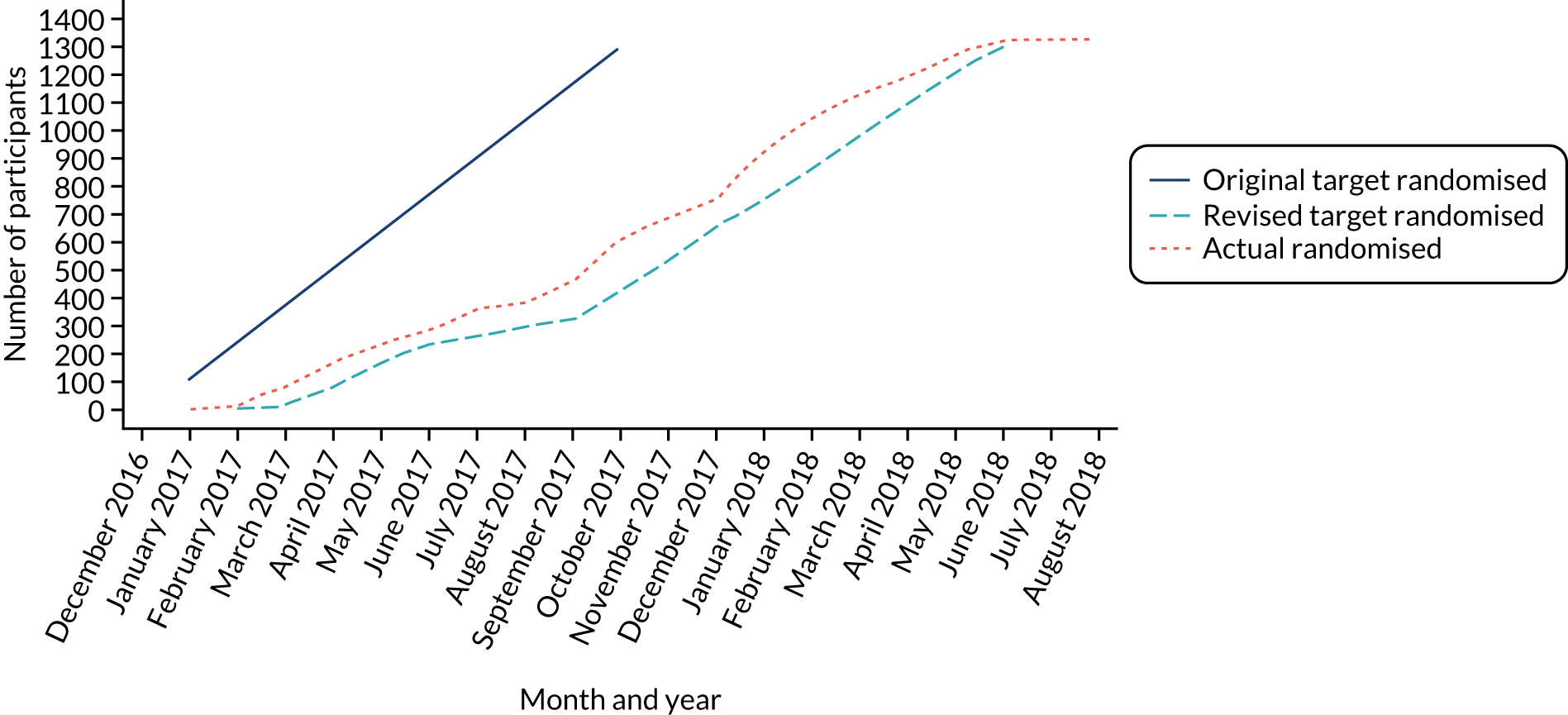
The first participant was randomised on 31 January 2017 and the last was randomised on 2 August 2018 (Figure 4), with follow-up ending in August 2019. Participants were randomised in 168 batches of between 2 and 32 patients. In total, 1331 participants were randomised into OTIS: 430 (32.3%) to the intervention group and 901 (67.7%) to the usual-care group (see Figure 3). We therefore exceeded our target of 1299 by 32 participants (Figure 5), albeit with the requirement of an extension to the recruitment period from October 2017, by which time we initially had hoped to complete recruitment, to August 2018. A median of 66 participants were recruited from each of the 12 centres (range 19–312 participants).
FIGURE 4.
Monthly recruitment to the OTIS trial.
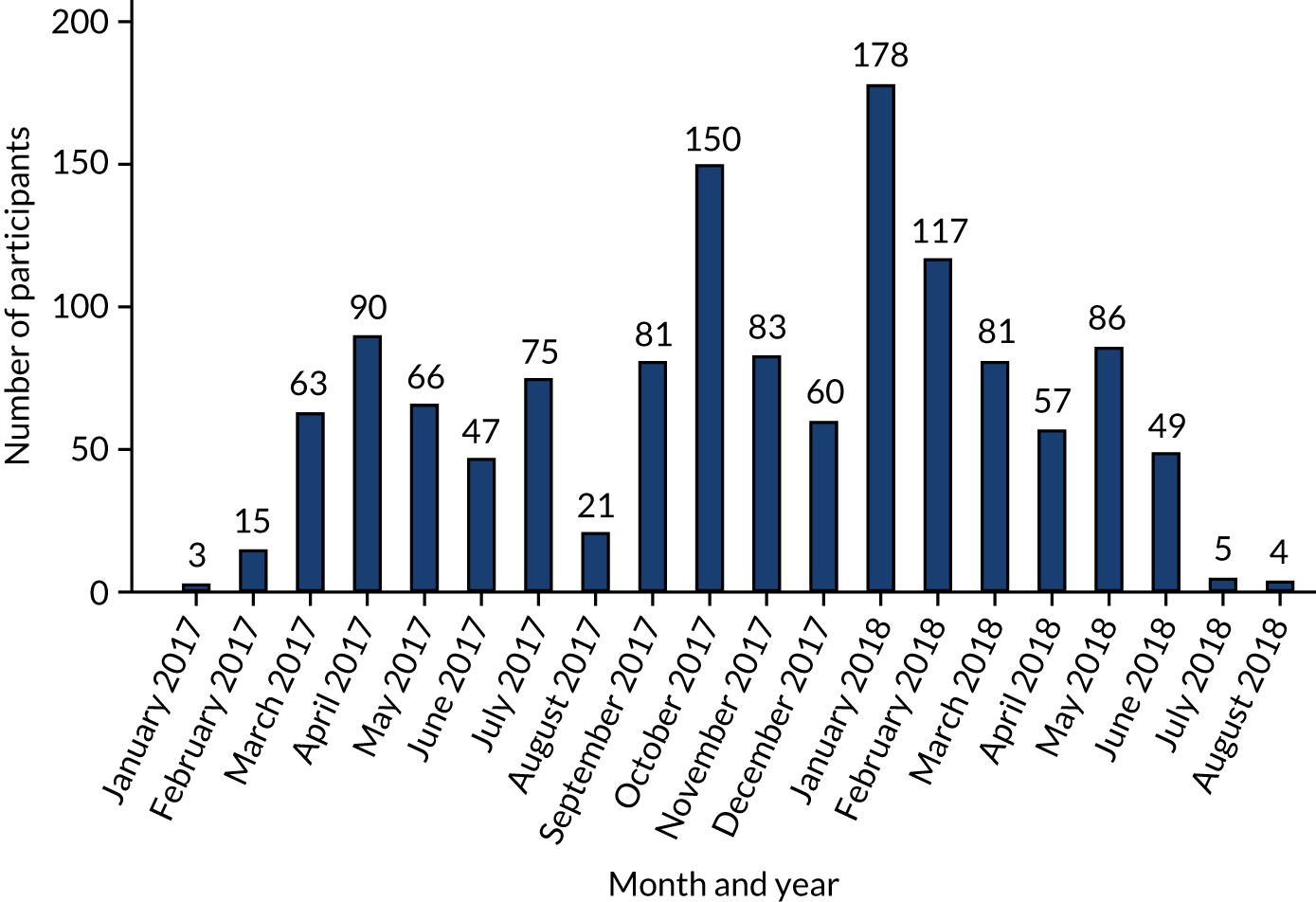
FIGURE 5.
Actual and target recruitment to the OTIS trial.
There was a median of 27 days (interquartile range 20 to 40 days) between completion of the screening questionnaire and completion of the baseline questionnaire. Participants were randomised a median of 44 days (interquartile range 25–73 days) after completing their baseline questionnaire. This allowed them time to return at least one falls calendar and for the OTs to confirm their capacity to deliver the intervention visits.
Baseline data
Baseline data for the 1331 randomised participants are presented in Tables 2 and 3. The mean age of participants was 80 years (range 65–98 years), and two-thirds (n = 872, 65.5%) were female. Three-quarters (n = 999) of the participants had sustained a fall in the 12 months prior to enrolment, among whom one in five (19.7%) had attended a hospital for treatment following their fall. The two groups were comparable on all baseline characteristics, except for a chance imbalance in the proportion of participants with Parkinson’s disease. Participants in the intervention group (n = 14, 3.3%) were more likely to have Parkinson’s disease than those in the usual-care group (n = 9, 1.0%).
| Characteristic | Intervention group (N = 430) | Usual-care group (N = 901) | Total (N = 1331) |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 79.9 (6.4) | 80.2 (6.3) | 80.1 (6.3) |
| Median (minimum, maximum) | 79.7 (67.3, 98.0) | 80.3 (65.5, 98.7) | 80.1 (65.5, 98.7) |
| Sex, n (%) | |||
| Male | 145 (33.7) | 314 (34.9) | 459 (34.5) |
| Female | 285 (66.3) | 587 (65.1) | 872 (65.5) |
| BMI (kg/m2) | |||
| Mean (SD) | 27.0 (5.4) | 26.8 (5.2) | 26.9 (5.3) |
| Median (minimum, maximum) | 26.3 (14.0, 59.7) | 26.0 (14.7, 63.8) | 26.1 (14.0, 63.8) |
| Taking > 4 medications prescribed by a doctor, n (%) | |||
| Yes | 212 (49.3) | 455 (50.5) | 667 (50.1) |
| No | 216 (50.2) | 437 (48.5) | 653 (49.1) |
| Missing | 2 (0.5) | 9 (1.0) | 11 (0.8) |
| Living arrangements, n (%)a | |||
| Alone | 202 (47.0) | 443 (49.2) | 645 (48.5) |
| With friend or relative | 20 (4.7) | 49 (5.4) | 69 (5.2) |
| With partner or spouse | 212 (49.3) | 417 (46.3) | 629 (47.3) |
| In sheltered accommodation | 8 (1.9) | 26 (2.9) | 34 (2.6) |
| Comorbidities, n (%)a | |||
| Osteoporosis | 67 (15.6) | 136 (15.1) | 203 (15.3) |
| High blood pressure | 192 (44.7) | 415 (46.1) | 607 (45.6) |
| Pain | 219 (50.9) | 452 (50.2) | 671 (50.4) |
| Angina or heart troubles | 94 (21.9) | 194 (21.5) | 288 (21.6) |
| Parkinson’s disease | 14 (3.3) | 9 (1.0) | 23 (1.7) |
| Arthritis (rheumatoid arthritis/osteoarthritis) | 226 (52.6) | 461 (51.2) | 687 (51.6) |
| Anxiety or depression | 55 (12.8) | 115 (12.8) | 170 (12.8) |
| Stroke | 25 (5.8) | 67 (7.4) | 92 (6.9) |
| Urinary incontinence | 89 (20.7) | 167 (18.5) | 256 (19.2) |
| Chronic lung disease | 34 (7.9) | 54 (6.0) | 88 (6.6) |
| Diabetes | 81 (18.8) | 153 (17.0) | 234 (17.6) |
| Ménière’s disease/conditions affecting balance/dizziness/vertigo | 32 (7.4) | 86 (9.5) | 118 (8.9) |
| Poor vision | 83 (19.3) | 178 (19.8) | 261 (19.6) |
| Cancer | 51 (11.9) | 65 (7.2) | 116 (8.7) |
| Other | 159 (37.0) | 341 (37.8) | 500 (37.6) |
| Characteristic | Intervention group (N = 430) | Usual-care group (N = 901) | Total (N = 1331) |
|---|---|---|---|
| Fall in last 12 months, n (%) | |||
| Yes | 323 (75.1) | 676 (75.0) | 999 (75.1) |
| If yes, number of falls | |||
| Median (minimum, maximum) | 1 (1, 40) | 1 (1, 24) | 1 (1, 40) |
| If yes, did you attend hospital for any of the falls?, n (%) | |||
| Yes | 60 (18.6) | 137 (20.3) | 197 (19.7) |
| History of falling in previous 12 months, n (%) | |||
| No or one fall | 283 (65.8) | 568 (63.0) | 851 (63.9) |
| Two or more falls | 147 (34.2) | 333 (37.0) | 480 (36.1) |
| Fear of falling, n (%) | |||
| All of the time | 13 (3.0) | 37 (4.1) | 50 (3.8) |
| Most of the time | 31 (7.2) | 75 (8.3) | 106 (8.0) |
| A good bit of the time | 67 (15.6) | 114 (12.7) | 181 (13.6) |
| Some of the time | 120 (27.9) | 279 (31.0) | 399 (30.0) |
| A little of the time | 117 (27.2) | 229 (25.4) | 346 (26.0) |
| None of the time | 82 (19.1) | 167 (18.5) | 249 (18.7) |
| Broken bone since age of 18 years, n (%) | |||
| Yes | 192 (44.7) | 378 (42.0) | 570 (42.8) |
| No | 235 (54.7) | 518 (57.5) | 753 (56.6) |
| Missing | 3 (0.7) | 5 (0.6) | 8 (0.6) |
| Judgement of balance, n (%) | |||
| Good and want to keep it that way | 116 (27.0) | 241 (26.7) | 357 (26.8) |
| Quite good but would like to improve it | 166 (38.6) | 327 (36.3) | 493 (37.0) |
| Some problems with balance that want to overcome | 144 (33.5) | 328 (36.4) | 472 (35.5) |
| Missing | 4 (0.9) | 5 (0.6) | 9 (0.7) |
| Difficulties with balance while walking or dressing, n (%) | |||
| Yes, often or always | 109 (25.3) | 274 (30.4) | 383 (28.8) |
| No, or just occasionally | 312 (72.6) | 611 (67.8) | 923 (69.3) |
| Missing | 9 (2.1) | 16 (1.8) | 25 (1.9) |
| Risk of falling, n (%) | |||
| High/intermediatea | 373 (86.7) | 775 (86.0) | 1148 (86.3) |
| Low | 52 (12.1) | 119 (13.2) | 171 (12.8) |
| Missing | 5 (1.2) | 7 (0.8) | 12 (0.9) |
Comparing randomised participants with ineligible or non-consenting participants, non-randomised participants tended to be very slightly younger (mean age 78.5 years) and less likely to be female (58.0%).
Number of falls calendars returned
The response rates for the monthly falls calendars, where month 0 is the month of randomisation, are presented in Table 4. Overall, the response rate per month was consistently > 90%; however, the response rate is lower in the intervention group each month than in the usual-care group. This difference increases from 1.2% (97.2% compared with 98.4%) at month 0 to 3.4% (87.9% compared with 91.3%) at month 12.
| Month post randomisation | Intervention group (N = 430), n (%) | Usual-care group (N = 901), n (%) | Overall (N = 1331), n (%) |
|---|---|---|---|
| 0 | 418 (97.2) | 887 (98.4) | 1305 (98.0) |
| 1 | 414 (96.3) | 879 (97.6) | 1293 (97.1) |
| 2 | 410 (95.3) | 877 (97.3) | 1287 (96.7) |
| 3 | 408 (94.9) | 870 (96.6) | 1278 (96.0) |
| 4 | 403 (93.7) | 862 (95.7) | 1265 (95.0) |
| 5 | 399 (92.8) | 855 (94.9) | 1254 (94.2) |
| 6 | 398 (92.6) | 851 (94.5) | 1249 (93.8) |
| 7 | 398 (92.6) | 852 (94.6) | 1250 (93.9) |
| 8 | 394 (91.6) | 846 (93.9) | 1240 (93.2) |
| 9 | 391 (90.9) | 843 (93.6) | 1234 (92.7) |
| 10 | 388 (90.2) | 834 (92.6) | 1222 (91.8) |
| 11 | 378 (87.9) | 828 (91.9) | 1206 (90.6) |
| 12 | 378 (87.9) | 823 (91.3) | 1201 (90.2) |
Participant questionnaire return rates
Within 4 months of randomisation, there were two reported deaths (both in the usual-care group) and 28 withdrawals from the trial [16 (3.7%) in the intervention group and 12 (1.3%) in the usual-care group]. These participants were therefore not sent a 4-month participant questionnaire [1301 (97.8%) were sent] (Table 5). Between 4 and 8 months post randomisation, a further nine deaths (intervention group, n = 5; usual-care group, n = 4) and 24 withdrawals (intervention group, n = 9; usual-care group, n = 15) were reported. Between 8 and 12 months post randomisation, 15 deaths (intervention group, n = 7; usual-care group, n = 8) and 16 withdrawals (intervention group, n = 4; usual-care group, n = 12) were reported. Therefore, at 12 months, 389 (90.5%) participants randomised to the intervention group and 848 (94.1%) in the usual-care group were sent a follow-up questionnaire. Overall, participant response rates to the follow-up questionnaires at 4, 8 and 12 months were consistently above 90%. The response rates, with the number sent as the denominator, were similar between the two groups across all three time points; however, when using the number randomised as the denominator, the response rates decrease over time and are lower in the intervention group than in the usual-care group. At 12 months, 87.7% of the intervention group returned a questionnaire compared with 91.5% of the usual-care group. This reflects that the intervention group had a higher withdrawal rate and so a higher proportion of participants in this group were not sent the questionnaire.
| Participant questionnaire | Intervention group (N = 430) | Usual-care group(N = 901) | Total (N = 1331) |
|---|---|---|---|
| 4 months | |||
| Sent (% randomised) | 414 (96.3) | 887 (98.5) | 1301 (97.8) |
| Received (% sent) | 403 (97.3) | 862 (97.2) | 1265 (97.2) |
| Received (% randomised) | 403 (93.7) | 862 (95.7) | 1265 (95.0) |
| Median time to response (interquartile range), days | 9 (8, 13) | 10 (8, 14) | 9 (8, 14) |
| 8 months | |||
| Sent (% randomised) | 400 (93.0) | 868 (96.3) | 1268 (95.3) |
| Received (% sent) | 394 (98.5) | 840 (96.8) | 1234 (97.3) |
| Received (% randomised) | 394 (91.6) | 840 (93.2) | 1234 (92.7) |
| Median time to response (interquartile range), days | 9 (8, 13) | 9 (8, 14) | 9 (8, 13) |
| 12 months | |||
| Sent (% randomised) | 389 (90.5) | 848 (94.1) | 1237 (92.9) |
| Received (% sent) | 377 (96.9) | 824 (97.2) | 1201 (97.1) |
| Received (% randomised) | 377 (87.7) | 824 (91.5) | 1201 (90.2) |
| Median time to response (interquartile range), days | 9 (8, 13) | 9 (7, 13) | 9 (7, 13) |
Occupational therapist-delivered environmental assessment and modification visits
A total of 382 participants allocated to the intervention group received an environmental assessment and modification visit from an OT. Of these participants, 362 (94.8%) completed the Timed Up and Go test, with a mean of 15.6 seconds (SD 8.4 seconds, range 5 to 70 seconds); 159 (43.9%) scored over 14 seconds, indicating a high risk of falling. The assessments were conducted by 23 OTs (median 16 visits per OT, range 1 to 54 visits per OT). Nineteen of the OTs attended a face-to-face training session, and four were ‘cascade’ trained by another OT who had attended face-to-face training. The visits took place between 1 and 411 days after randomisation (median 27 days), and lasted a median of 90 minutes (range 25 to 180 minutes). Nearly two-thirds of the intervention group (277/430, 64.4%) had received the visit within 6 weeks of being randomised, and 381/430 (88.6%) received it within 12 months. The delays in delivering the visits were due to availability of the participant and, despite agreeing to the number of participants to be randomised at a given time, OT capacity. One participant received the visit beyond 12 months after they were randomised as they lived on the border of two trusts and it could not be agreed which trust should undertake the visit. A total of 48 participants did not receive a visit.
Of the 48 participants randomised to the intervention group who did not receive a visit, 25 withdrew from the intervention and 16 withdrew fully from the trial before receiving a visit. Reasons for withdrawing from the intervention were as follows: the participant did not feel that they would benefit from an OT visit (often as they felt that they were fit and well), n = 12; an OT visit was not appropriate (house was well equipped/had ongoing renovations/was a rental property/had been already assessed and adapted for needs of spouse), n = 4; the OT was unable to arrange visit, n = 3; the participant lived outside the area that the OT would attend, n = 3; no reason given, n = 2; and the participant did not want a visit due to ill health, n = 1. Reasons for full withdrawal among these participants were broadly similar, as it was common for participants to inform the OT when they came to arrange the appointment that they did not want the visit (i.e. they were withdrawing from treatment). These participants were then asked if they would be willing to continue to provide outcome data, which some declined to do; hence, they were fully withdrawn from the trial.
Seven participants appeared to remain in full participation with the trial but did not receive an environmental assessment. It is possible that these participants received a home visit but, as no documentation relating to the visit was received for them, we have conservatively assumed these participants did not receive the intervention.
Primary outcome
Raw data
In total, 1303 (97.9%) trial participants returned at least one falls calendar following randomisation (intervention, n = 419, 97.4%; usual care, n = 884, 98.1%), with 1204 (90.5%) returning a complete 12 months’ worth of calendars post randomisation (intervention, n = 377, 87.7%; usual care, n = 827, 91.8%). Of the 28 participants who did not return any falls calendars, five (four usual care, one intervention) provided falls data on at least one of the 4-, 8- and 12-month participant questionnaires. These data were used in the analysis for these participants. In total, 2260 falls were reported: 826 in the intervention group (mean 1.9 falls, SD 5.5 falls; median 1 fall, range 0–94 falls) over an average of 338 days (median 365 days), and 1434 in the usual-care group (mean 1.6 falls, SD 3.0 falls; median 1 fall, range 0–41 falls) over a mean of 345 days (median 365 days).
At least some information, such as the location and perceived cause, was available for 2037 (90.1%) falls (intervention, n = 700, 84.7%; usual care, n = 1337, 93.2%; Table 6). Just over half of falls for which there was available location information occurred indoors (53.4%), with the majority (85.7%) of these occurring inside the participant’s own home rather than inside another premises. About half of the falls resulted in a superficial injury or worse, with 2.8% of the falls resulting in a broken bone (from 16 falls in the intervention group and 41 falls in the usual-care group), most frequently in the wrist or the hand (17/57, 29.8%).
| Details of fall | Intervention group (N = 700) | Usual-care group (N = 1337) | Total (N = 2037) |
|---|---|---|---|
| Where did you fall?, n (%) | |||
| Inside own home | 289 (41.3) | 590 (44.1) | 879 (43.2) |
| Inside, not in own home | 59 (8.4) | 88 (6.6) | 147 (7.2) |
| Outside own home | 136 (19.4) | 259 (19.4) | 395 (19.4) |
| Outside, beyond own home | 180 (25.7) | 322 (24.1) | 502 (24.6) |
| Missing | 36 (5.1) | 78 (5.8) | 114 (5.6) |
| What were you doing when you fell?, n (%)a | |||
| Getting in/out of bed, chair, bath, toilet, shower | 79 (11.3) | 150 (11.2) | 229 (11.2) |
| Turning | 41 (5.9) | 105 (7.9) | 146 (7.2) |
| Going up/down stairs or steps | 82 (11.7) | 142 (10.6) | 224 (11.0) |
| Walking | 323 (46.1) | 550 (41.1) | 873 (42.9) |
| Reaching/bending | 69 (9.9) | 161 (12.0) | 230 (11.3) |
| Rushing | 30 (4.3) | 38 (2.8) | 68 (3.3) |
| Unknown/cannot recall | 80 (11.4) | 160 (12.0) | 240 (11.8) |
| Other | 56 (8.0) | 138 (10.3) | 194 (9.5) |
| What caused you to fall?, n (%)a | |||
| Trip, did not pick up feet, fell over something | 205 (29.3) | 391 (29.2) | 596 (29.3) |
| Slip, skid | 69 (9.9) | 104 (7.8) | 173 (8.5) |
| Uneven surface | 59 (8.4) | 99 (7.4) | 158 (7.8) |
| Slippery surface | 50 (7.1) | 85 (6.4) | 135 (6.6) |
| Steps/gradient | 99 (14.1) | 179 (13.4) | 278 (13.6) |
| Access | 1 (0.1) | 2 (0.1) | 3 (0.1) |
| Legs gave away, just went over | 53 (7.6) | 154 (11.5) | 207 (10.2) |
| Dizzy, woozy, groggy, light-headed, passed out | 48 (6.9) | 115 (8.6) | 163 (8.0) |
| Lost balance | 221 (31.6) | 420 (31.4) | 641 (31.5) |
| Knocked, pulled or blown over | 12 (1.7) | 36 (2.7) | 48 (2.4) |
| Footwear issue | 19 (2.7) | 19 (1.4) | 38 (1.9) |
| Poor visibility/lighting | 24 (3.4) | 36 (2.7) | 60 (2.9) |
| Obstacle/obstruction/pet | 55 (7.9) | 110 (8.2) | 165 (8.1) |
| Unknown/cannot recall | 50 (7.1) | 121 (9.1) | 171 (8.4) |
| Other | 22 (3.1) | 51 (3.8) | 73 (3.6) |
| Injuries suffered, n (%)a | |||
| No injury | 343 (49.0) | 646 (48.3) | 989 (48.6) |
| Superficial wounds (e.g. bruising, sprain, cut, abrasion) | 287 (41.0) | 582 (43.5) | 869 (42.7) |
| Fractureb | 16 (2.3) | 41 (3.1) | 57 (2.8) |
| Other | 23 (3.3) | 28 (2.1) | 51 (2.5) |
| Unknown/cannot recall | 38 (5.4) | 53 (4.0) | 91 (4.5) |
| Overnight stay in hospital required due to fall, n (%) | |||
| Yes | 15 (2.1) | 47 (3.5) | 62 (3.0) |
| No | 673 (96.1) | 1266 (94.7) | 1939 (95.2) |
| Missing | 12 (1.7) | 24 (1.8) | 36 (1.8) |
| If yes, number of nights | |||
| Mean (SD) | 17.1 (17.1) | 13.2 (17.4) | 14.2 (17.3) |
| Median (minimum, maximum) | 14 (1, 56) | 6 (1, 70) | 9 (1, 70) |
Covariates
The primary analysis model controlled for sex, age at randomisation, history of falling and allocation ratio, and centre. Full covariate data were available for all randomised participants so the primary model was based on 1331 participants.
Primary analysis
The adjusted negative binomial model indicated weak evidence of a difference in falls, with an increase in the fall rate in the intervention group relative to usual care (IRR 1.17, 95% CI 0.99 to 1.38; p = 0.07). History of falling was seen to be a significant predictor in the model (IRR 3.15, 95% CI 2.69 to 3.69; p < 0.001).
Sensitivity analyses
Post hoc adjustment for Parkinson’s disease
One participant, in the intervention group, reported 94 falls over the 12 months from randomisation. This number of falls was significantly greater than the next largest of 41 (in the usual-care group). We observed that this participant had Parkinson’s disease, and also that there was a chance imbalance in the proportion of participants in the two groups who had Parkinson’s disease. We therefore decided, as a post hoc sensitivity analysis, to repeat the primary analysis, including whether or not the participant had Parkinson’s disease as an additional fixed effect. The resulting IRR was decreased to 1.11 (95% CI 0.94 to 1.31) and there was no evidence of a difference between the two groups (p = 0.23).
Non-compliance
When non-compliance with the intervention was accounted for using an instrumental variable CACE analysis approach, defining compliance as receipt of an OT-delivered environmental assessment within 12 months of randomisation, the CACE estimate of the intervention effect was very similar to that from the ITT analysis (IRR 1.18, 95% CI 0.98 to 1.43; p = 0.08).
Missing data
Ten (2.3%) participants in the intervention group and 13 (1.4%) participants in the usual-care group did not provide any falls data post randomisation. These participants were still included in the model as zero falls over a negligible time frame of 0.1 months was imputed for them. However, we conducted a logistic regression to assess whether or not any baseline factors were associated with missing outcome data and whether or not a participant reporting a fall in the 12 months prior to completing the screening questionnaire was the only statistically significant predictor. Participants who had had at least one previous fall were more likely to have missing outcome data (OR 5.84, 95% CI 1.13 to 30.21; p = 0.04). When this was added as a covariate in the primary analysis, the intervention effect estimate was virtually unchanged (IRR 0.17, 95% CI 0.99 to 1.38; p = 0.07).
Therapist effects
In three of the 12 centres, only one OT delivered the intervention visits. In the remaining eight centres, up to four OTs were involved. To account for potential therapist effects, we assigned each participant to an OT (i.e. the one they were seen by or a counterfactual therapist they could have seen had they been randomised to the intervention group). Therapist (rather than centre) was then included as a random effect in the primary analysis model. The intervention effect estimate was virtually unchanged (IRR 0.17, 95% CI 0.99 to 1.38; p = 0.07).
Subgroup analysis
About one-tenth (n = 124) of randomised participants (intervention group, 41/430, 9.5%; usual-care group, 83/901, 9.2%) reported that they had attended a hospital (outpatient appointment, day case, A&E presentation or overnight admission) for a fall in the 4 months prior to completing the baseline questionnaire.
We repeated the primary analysis in the subgroups of those who did and those who did not receive hospital treatment as a result of a fall in the 4 months prior to baseline and found qualitatively dissimilar treatment effects in each (received treatment: IRR 0.86, 95% CI 0.50 to 1.47; received no treatment: 1.21, 95% CI 1.01 to 1.44). However, when an interaction between this factor and treatment allocation was included in the primary model, the interaction was not observed to be statistically significant (p = 0.24).
Secondary analyses
Number of falls as reported for the previous 4 months on the 4-, 8- and 12-month participant questionnaires
In the intervention group, 404 participants (94.0%) provided a valid response to the number of falls they had experienced over the previous 4 months on at least one of the 4-, 8- and 12-month questionnaires. An average of 2.0 falls was reported (range 0–67 falls) over an average of 11.4 months. In the usual-care group, 870 participants (96.6%) provided a valid response to the number of falls they had experienced over the previous 4 months on at least one of the 4-, 8- and 12-month questionnaires. An average of 1.8 falls was reported (range 0–43 falls) over an average of 11.5 months. After imputing zero falls over a negligible time period of 0.1 months for those who did not provide any data, the adjusted IRR obtained from the negative regression model was 1.05 (95% CI 0.89 to 1.25; p = 0.56).
Comparison of the number of falls reported on falls calendars and in participant questionnaires
We compared the number of falls reported by participants in the questionnaires and on falls calendars. Of the 1331 randomised participants, 1132 (85.0%) returned 12 months’ worth of falls calendars and provided data on retrospective falls in all three participant questionnaires (4, 8 and 12 months). Among these, in the intervention group, the average number of falls reported on calendars was 1.9 (SD 5.7, median 1, range 0–94 falls) and in questionnaires was 2.0 (SD 5.0, median 1, range 0–67 falls). The correlation between the two counts was high, at 0.93 (95% CI 0.91 to 0.94). In the usual-care group, the average number of falls reported on calendars was 1.6 (SD 2.9, median 1, range 0–41 falls) and in questionnaires was 1.8 (SD 3.2, median 1, range 0–43), with correlation at 0.89 (95% CI 0.88 to 0.90). Figure 6 depicts a Bland–Altman plot of the agreement between falls reported on the calendars and falls reported in the questionnaires. There is strong agreement; the mean difference is –0.16 (participants tended to report marginally more falls in the questionnaires than on the calendars). The 95% limits of agreement are –3.5 to 3.2. There is a hint of a trend that the higher the average count of falls (between the two measurements), the larger the difference between the two tends to be.
FIGURE 6.
Bland–Altman plot of the agreement of falls reported in questionnaires and on calendars.
Proportion of fallers and multiple fallers
In total, 245 out of 430 (57.0%) intervention group participants and 506 out of 901 (56.2%) usual-care group participants reported at least one fall on their monthly falls calendars or in participant questionnaires (adjusted OR 1.06, 95% CI 0.83 to 1.34; p = 0.65).
Using only data from the participant questionnaires, 230 out of 430 (53.5%) randomised participants in the intervention group and 506 out of 901 (56.2%) participants in the usual-care group reported at least one fall. There was no evidence of a difference in the likelihood of reporting a fall between the two groups (adjusted OR 0.91, 95% CI 0.72 to 1.15; p = 0.42).
These analyses, by default, assume that (1) participants who did not return any falls data did not fall and (2) partial responders had no falls in the months for which no data were provided. In terms of point 1, 57 (4.3%) participants failed to provide any falls data in the participant questionnaires, and only 23 (1.7%) participants failed to provide any falls data either on the monthly calendars or in the participant questionnaires. At the extremes, we could assume that all 57 participants with missing questionnaire falls data in each group, in turn, did fall at least once in the 12 months following randomisation. Assuming that the 26 participants in the intervention group fell (but leaving the usual-care group unchanged) the adjusted OR favours usual care, but the result is not statistically significant (1.17, 95% CI 0.92 to 1.49; p = 0.19). Conversely, assuming that the 31 participants in the usual-care group fell (but leaving the intervention group unchanged), there is weak evidence of a reduction in the proportion of participants in the intervention group who experienced at least one fall (adjusted OR 0.79, 95% CI 0.63 to 1.01; p = 0.06). The conclusion that there is no difference in the proportion of fallers in the two groups is, therefore, reasonably robust.
Proportion of multiple fallers (those having two or more falls in the 12 months from randomisation)
The proportion of participants who reported two or more falls on their falls calendars (or participant questionnaires, where no falls calendars were returned) following randomisation was also slightly higher in the intervention group than in the usual-care group (148/430, 34.4%, vs. 298/901, 33.1%; adjusted OR 1.11, 95% CI 0.86 to 1.43; p = 0.42).
Using only data from the participant questionnaires, 149 out of 430 (34.7%) randomised participants in the intervention group, and 323 out of 901 (35.9%) randomised participants in the usual-care group reported at least two falls. There was no evidence of a difference in the likelihood of reporting multiple falls between the two groups (adjusted OR 0.96, 95% CI 0.75 to 1.24; p = 0.78).
Similarly, these analyses must be interpreted with the following in mind. By default, they assume that (1) participants who did not return any falls data actually fell no more than once in the 12 months from randomisation, (2) partial responders who reported one fall experienced no falls in all the months for which no data were provided; and (3) partial responders who reported no falls experienced no more than one fall in all the months for which no data were provided. No further analyses to test the robustness of these results to these assumptions were deemed warranted, as such a large proportion of participants (90%) returned 12 months’ worth of falls calendars.
Proportion of participants having at least one fracture over the 12 months’ follow-up
A total of 54 participants reported a fracture from a fall in the 12 months from randomisation (intervention group, 16/430, 3.7%; usual-care group, 38/901, 4.2%).
Proportion of participants obtaining multiple fractures
Only two participants, both in the usual-care group, reported more than one fracture resulting from a fall, from separate events, in the 12 months from randomisation. One reported three fractures (wrist, ribs and knee) and the other reported two fractures (wrist and knee).
Proportion of participants who reported that they were worried about falling at 12 months post randomisation (reported worrying about falling at least some of the time)
Fear of falling was measured at screening and in the 4-, 8- and 12-month questionnaires using the question ‘During the past 4 weeks how often have you worried about having a fall?’, with the following response categories:
-
1 – all of the time
-
2 – most of the time
-
3 – a good bit of the time
-
4 – some of the time
-
5 – a little of the time
-
6 – none of the time.
At 12 months, 197 out of 375 (52.5%) participants in the intervention group and 440 out of 818 (53.8%) participants in the usual-care group (where the denominator represents those providing a valid response to this question) reported that they worried about falling at least some of the time (some, a good bit, most or all of the time). There was no evidence of a difference in the likelihood of participants in the intervention group reporting a fear of falling relative to the usual-care group (adjusted OR 1.00, 95% CI 0.78 to 1.29; p = 1.00).
Fear of falling in its continuous form
Raw scores for fear of falling are summarised in Table 7 and were fairly consistent between groups and over time. At screening, the average score was 4.3 (SD 1.3) in the intervention group and 4.2 (SD 1.3) in the usual-care group. At 12 months, the average score was 4.2 (SD 1.3) in the intervention group and 4.1 (SD 1.3) in the usual-care group. There was no evidence of a difference in fear of falling between the two groups at any post-randomisation time point (see Table 7; Figure 7).
| Time point | Unadjusted | Adjusteda | ||||
|---|---|---|---|---|---|---|
| Intervention group (N = 430), n; mean (SD); median (min., max.) | Usual-care group (N = 901), n; mean (SD); median (min., max.) | Total (N = 1331), n; mean (SD); median (min., max.) | Intervention group, mean (SE); (95% CI) | Usual-care group, mean (SE); (95% CI) | Mean difference (95% CI); p-value | |
| Baseline | 430; | 901; | 1331; | – | – | – |
| 4.3 (1.3); | 4.2 (1.3); | 4.2 (1.3); | ||||
| 4 (1, 6) | 4 (1, 6) | 4 (1, 6) | ||||
| 4 months | 401; | 858; | 1259; | 4.3 (0.05); (4.2 to 4.4) | 4.3 (0.03); (4.2 to 4.3) | 0.04 (–0.07 to 0.16); 0.46 |
| 4.3 (1.3); | 4.2 (1.3); | 4.3 (1.3); | ||||
| 5 (1, 6) | 4 (1, 6) | 5 (1, 6) | ||||
| 8 months | 393; | 838; | 1231; | 4.3 (0.05); (4.2 to 4.4) | 4.2 (0.03); (4.2 to 4.3) | 0.07 (–0.05 to 0.19); 0.23 |
| 4.4 (1.2); | 4.2 (1.3); | 4.3 (1.3); | ||||
| 5 (1, 6) | 4 (1, 6) | 5 (1, 6) | ||||
| 12 months | 375; | 818; | 1193; | 4.2 (0.05); (4.0 to 4.3) | 4.2 (0.03); (4.1 to 4.2) | –0.01 (–0.13 to 0.11); 0.87 |
| 4.2 (1.3); | 4.1 (1.3); | 4.2 (1.3); | ||||
| 4 (1, 6) | 4 (1, 6) | 4 (1, 6) | ||||
FIGURE 7.
Adjusted means for fear of falling outcome by randomised group.
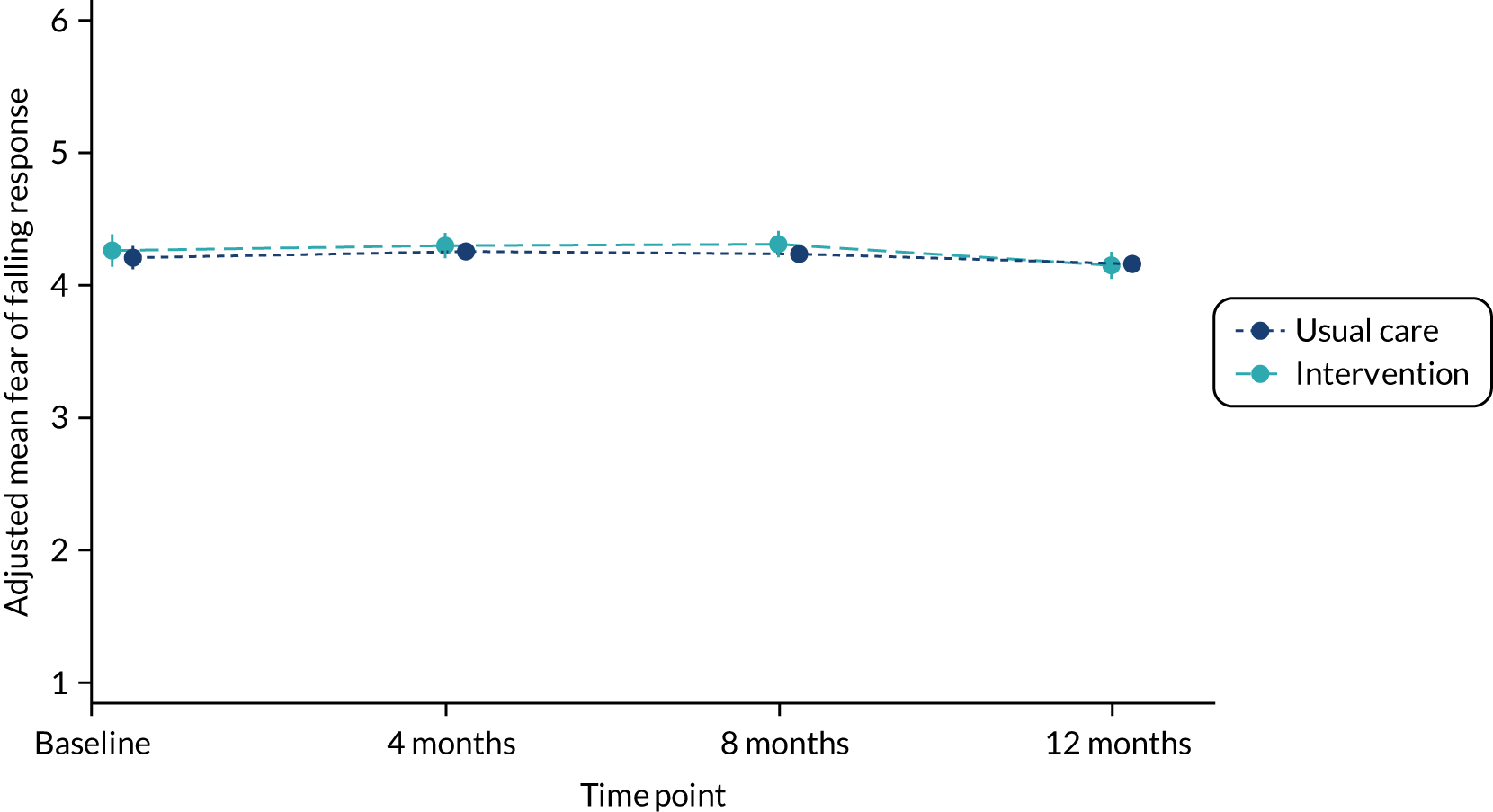
Time to fall
The median time to fall was 119 days in the intervention group (95% CI 105 to 133 days) and 144 days in the usual-care group (95% CI 132 to 155 days). Kaplan–Meier survival curves are presented for each group in Figure 8. The adjusted hazard ratio from the Cox proportional hazards model for the intervention effect was 1.24 (95% CI 0.94 to 1.63; p = 0.12), indicating that the hazard or chance of falling at any particular time was higher in the intervention group than in the usual-care group, but this ratio is not statistically significant. This analysis assumes that no falls were experienced in the months for which no falls calendar was returned; no further investigation or consideration of imputation of data was deemed necessary to evaluate the impact of this assumption, as such a high proportion of randomised participants (90%) returned a full 12 months’ worth of falls calendars.
FIGURE 8.
Kaplan–Meier curves by randomised group for time to fall.
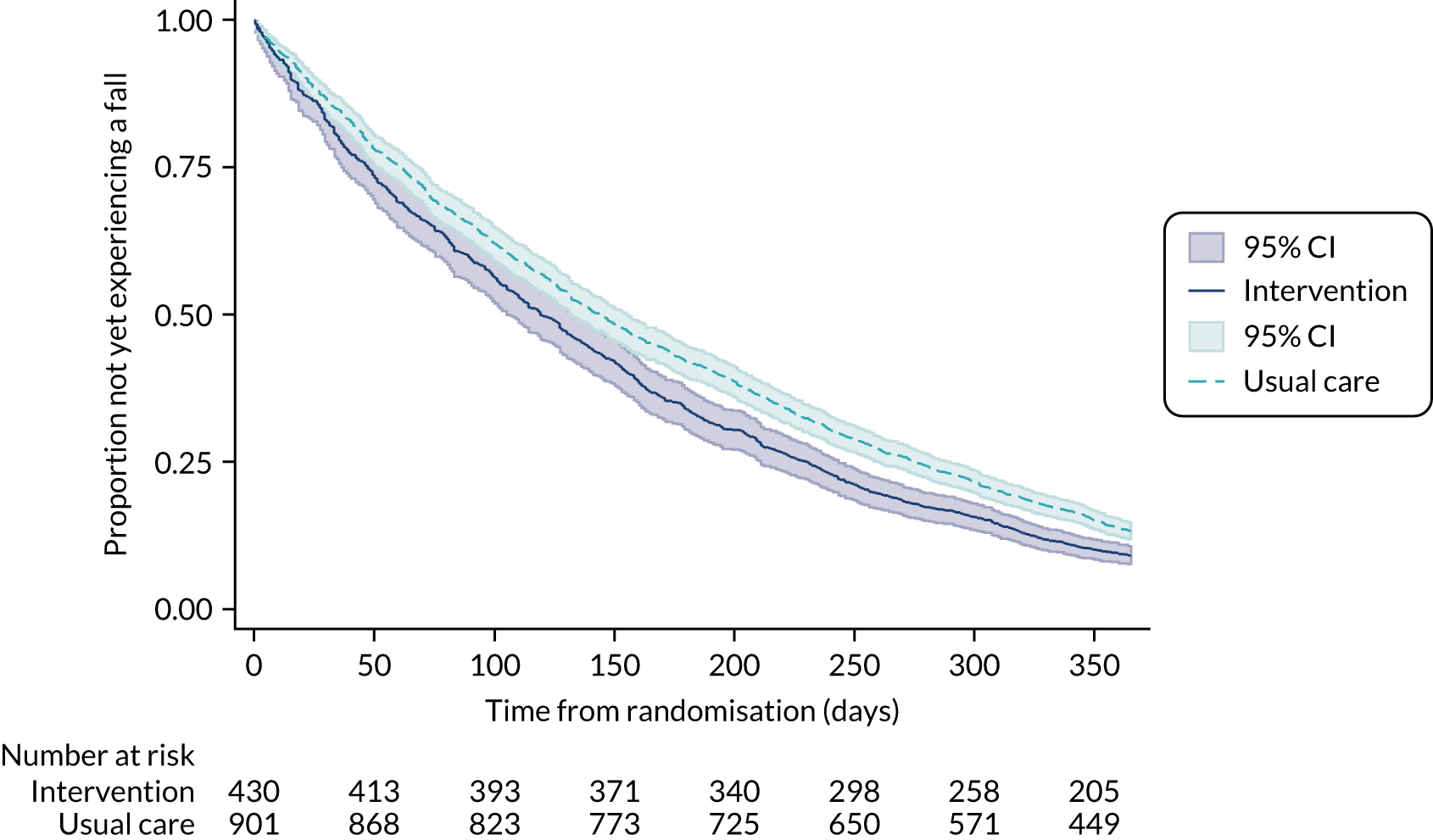
Adverse events
There were no serious or non-serious adverse events that were related to the intervention or trial procedures and that needed to be reported in accordance with the protocol.
Chapter 4 Economic evaluation
Introduction and aim
This chapter reports the methods and results of the economic evaluation that was undertaken as part of OTIS. The aim was to establish the cost-effectiveness of OT-delivered environmental assessment and modification (the intervention) compared with usual care, incorporating the impact of the intervention on both participants’ quality of life and falls prevention.
Methods
The within-trial economic evaluation involved:
-
a cost–utility analysis, which evaluated the intervention compared with usual care in terms of the incremental cost per quality-adjusted life-year (QALY)
-
a cost-effectiveness analysis, which assessed the intervention in terms of the cost per fall averted, thereby using the primary outcome measure of the trial.
The analyses included all costs related to health-care services used by the participant over the course of the trial, plus the cost of the intervention itself.
Within-trial economic evaluation
For both cost–utility and cost-effectiveness analyses, the base-case analysis was undertaken on an ITT basis from the perspective of the UK NHS and Personal Social Services (PSS), which included only health-care services and social services used by the participant during the trial that were related to falls. The base-case analysis used a multiply imputed data set, with the complete-case scenario also explored via sensitivity analysis. A secondary analysis was conducted from the societal perspective, which incorporated indirect costs in addition to the direct costs captured in the base-case analysis. A 12-month time horizon was used for the analyses, in line with the 12-month trial follow-up, and hence discounting of costs and health benefits was not required.
Economic data collection
Data regarding health-care resource use and health outcomes were collected during the 12-month follow-up period of the study. Health service utilisation and health-related quality of life data were collected using the questionnaires administered to participants at baseline and at 4, 8 and 12 months post randomisation. Falls data were collected via monthly falls calendars which were self-completed by participants. The equipment recommended by the OTs was documented using an equipment list that they completed at the home visit: confirmation that the equipment had been issued/installed was collected during the 4- to 6-week follow-up telephone call.
Health-related quality of life
The outcome for the cost–utility analysis was the QALY, with utilities used to represent health-related quality of life. QALYs provide a generic metric for the measurement of health that combines the impact on both longevity and quality of life, with one QALY equivalent to 1 year in full health. 41 Utility values for the different health states experienced by participants were estimated using the EQ-5D-5L, following NICE appraisal guidance. 42
The EQ-5D-5L43 was utilised to obtain utilities from participants at the four time points via participant questionnaires. The EQ-5D-5L comprises five domains (mobility, self-care, usual activities, pain/discomfort and anxiety/depression), each of which has five possible response levels: no problems, slight problems, moderate problems, severe problems and unable to/extreme problems. Based on the five responses across the five domains of the EQ-5D-5L classification system, a specific health state is defined. A utility score of one represents full health and a score of zero represents death, with it also being possible to have states worse than death as indicated by negative scores. Utility values were calculated using the mapping function developed by van Hout et al. ,44 following the advice provided by NICE in its most recent position statement regarding analyses that use data gathered using the EQ-5D-5L. 45
The raw EQ-5D-5L scores by domain are presented to examine the movements between levels for each domain according to study group. Descriptive statistics of the utilities for both groups are presented. The difference in utilities between the two groups for each time point have also been estimated. The overall difference in EQ-5D-5L index scores between the two groups were examined through regression methods, consistent with the model selected in the primary statistical analysis.
The cost–utility analysis was based on the difference in QALYs generated between both groups over the 12 months of the trial. For this, the utilities derived from the EQ-5D-5L were converted into QALYs for each participant using the area under the curve method, following the trapezium rule, which assumes linear interpolation between follow-up points. 46,47 The difference in QALYs gained between the two groups were adjusted for baseline utility weights48 to allow for any differences between the two groups at baseline.
Health benefits in terms of falls
The mean number of falls participants experienced during the trial was also incorporated into the economic evaluation, thereby corresponding to the trial’s primary outcome, the number of falls per participant over the 12 months post randomisation. Estimates of the mean number of falls for the two groups were used to estimate the number of falls averted as a result of the OT intervention. This was based on the negative binomial regression model that was used for the primary statistical analysis; this estimated the number of falls per participant per year, adjusting for fixed effects of sex, age at randomisation, history of falling and the allocation ratio used for randomising participants, plus centre as a random effect.
Health-care resource use
Data regarding the use of health-care services during the 12-month duration of the study were collected for each participant, specifically health-care resource use in primary care and the community (i.e. GP, nurse, physiotherapist, OT visits) and the hospital setting (i.e. outpatient attendances, day-case attendances, number of nights spent as an inpatient and A&E attendances). The resource use data were collected from self-reported participant questionnaires at baseline and at 4, 8 and 12 months. Participants were asked to record their resource use according to that which was falls-related and that which was not related to falls. In the base-case analysis, only resource use items relating to falls were utilised, with non-falls-related items incorporated as part of a sensitivity analysis.
Participants were contacted after a reported fall so that further information could be collected regarding the type, location and cause of the fall. This information was recorded on a falls data collection sheet. In addition, participants were asked whether they had been injured by the fall; if they answered ‘yes’, they were asked whether they had spent time in hospital overnight as an inpatient. This method of data collection provided an alternative way of gathering data regarding participants’ falls-related hospital stays to the participant follow-up questionnaires. The inpatient stay data collected via participants’ falls data collection sheets were used for a sensitivity analysis, to investigate how the findings differed from the base case (i.e. which used inpatient stay data obtained from participant questionnaires). Based on the falls data collection sheets, a total of 56 participants (intervention, n = 13; usual care, n = 43) stated that they stayed in hospital overnight because of a fall, with some participants recording multiple occasions for overnight hospital stays due to a fall. Where participants had reported no hospital stay and had not given an answer to the question regarding how many nights they had spent in hospital (i.e. the box was left blank), this was assumed to indicate no use of services and, thus, no nights spent in hospital. Where participants reported that they had stayed overnight in hospital due to a fall but left the question about the number of nights spent in hospital blank, it was assumed that these participants had stayed in hospital for one night.
In the 12-month questionnaire, participants were asked whether or not they had any attendances at a falls service/clinic and whether or not they had received a falls prevention visit that was not part of OTIS, in the last 12 months. As part of the falls prevention home visit question, participants were asked to select who had undertaken the visit from the following options: OT, home carer or helper, district nurse, social worker, physiotherapist or ‘other’.
The secondary analysis conducted from a societal perspective included additional costs relating to participants’ private/personal expenses; participants were asked to state how much they had paid for any equipment or modifications made to their house, and for travel costs for health-care attendances. A question was also included in the questionnaires regarding whether or not participants had received any visits from paid care workers. Individuals may need to pay for all, some or none of the cost of paid care workers, depending on their circumstances. 49 However, Age UK points out that it is rare for social care to be free. 49 Where individuals do not pay for their care, social services can fund the visits, and how much individuals pay depends on capital, income, benefits and/or expenses. 50 Paid care worker visits were assumed to be paid for by the participant/relatives, and therefore this fed into the societal perspective analysis rather than the base-case analysis, with this assumption explored via sensitivity analysis.
Resource use data are presented for both groups in terms of mean value, SD and mean difference (with 95% CIs) between the groups.
Costs
Costs of health-care services
Unit costs for health-care resource use items were obtained from established costing sources such as NHS Reference Costs 2017/1851 and the Unit Costs of Health and Social Care 2018 from the Personal Social Services Research Unit (PSSRU). 52 The unit cost items used in the analysis are summarised and presented in Table 8. The year of pricing was set as the mid-year of the trial (i.e. 2017–18), based on the trial period of June 2016–19. All costs were evaluated in Great British pounds for 2017–18. Unit costs were multiplied by the corresponding resource use data to give an estimation of the total cost per participant.
| Item | Unit cost (£) | Additional notes | Source |
|---|---|---|---|
| GP visit at general practice or home | 65.50 | Average of (1) £37.40 per GP visit at surgery of 9.22 minutes’ duration, and (2) £93.60 per home GP consultation, comprising 11.4-minute appointment and 12 minutes’ travel time | Unit Costs of Health and Social Care 2018 52 |
| Nurse visit at general practice or home | 24.65 | Average of (1) £10.85 per nurse visit at surgery lasting 15.5 minutes,52 and (2) £38.45 per consultation with District Nurse, Community Health Services sheet51 | Unit Costs of Health and Social Care 2018,52 NHS Reference Costs 2017/1851 |
| OT visit | 47.00 | Community OT (local authority), including training, assumed to be 1 hour in duration | Unit Costs of Health and Social Care 2018 52 |
| Physiotherapist visit | 57.26 | Community Health Services sheet: physiotherapist, one-to-one (adult) visit | NHS Reference Costs 2017/18 51 |
| Hospital outpatient visit | 125.01 | Total Outpatient Attendances sheet: total cost for all outpatient attendances divided by total activity | NHS Reference Costs 2017/18 51 |
| A&E attendance | 160.32 | Total HRGs sheet: total activity for all A&E attendances divided by total activity | NHS Reference Costs 2017/18 51 |
| Day-case hospital visit | 724.09 | Total HRGs sheet: sum of total cost divided by total activity for all day cases | NHS Reference Costs 2017/18 51 |
| Inpatient night in hospital | 345.76 | Total HRGs sheet: sum of total expenditure on excess bed-days (elective and non-elective) divided by total activity | NHS Reference Costs 2017/18 51 |
| Falls prevention visit by | |||
| OT | 47.00 | As above, for OT visit | Unit Costs of Health and Social Care 2018 52 |
| Home carer/helper | 28.00 | Page 143, PSSRU 2018. Cost per hour (see paid care worker cost below for further details) | NHS Reference Costs 2017/18,51 Unit Costs of Health and Social Care 201852 |
| District nurse | 38.45 | Community Health Services sheet | NHS Reference Costs 2017/18 51 |
| Social worker | 60.00 | Page 139, PSSRU 2018. Cost per hour | NHS Reference Costs 2017/18 51 |
| Physiotherapist | 57.26 | As above, for physiotherapist visit | NHS Reference Costs 2017/18 51 |
| Other | 104.26 | Responses to ‘other’ were categorised into the above individuals (i.e. OT, home carer, etc.). Where participants stated that more than one ‘other’ person visited them, the cost of an OT plus physiotherapist was included, as this was the most commonly occurring answer | NHS Reference Costs 2017/18,51 Unit Costs of Health and Social Care 201852 |
| Falls service/clinic attendance | 81.33 | Falls team visit | Unit Costs of Health and Social Care 2018 52 |
| Paid care worker | 14.00 | Page 143, PSSRU 2018. Cost based on price multipliers for independent sector home care provided for social services; £28 per hour for a face-to-face visit, which was the upper (and most commonly occurring) value of the different costs listed for the different times of the week over which visits take place. Duration of visit assumed to be 30 minutes, based on majority (63%) of home care visits lasting 16–30 minutes (also stated 10% last < 15 minutes and 16% last > 46 minutes) | Unit Costs of Health and Social Care 2018 52 |
The total cost for each participant comprised the following elements: health-care resource utilisation (i.e. primary, community and secondary care visits and attendances) and the cost of the OT intervention (for those in the intervention group only). Further details of the intervention cost are provided in Costing the occupational therapist intervention section. Participants in the usual-care group received usual care from their GP and health-care professionals, and hence the cost of usual care incorporates the cost of this resource use. As the falls prevention advice leaflet produced by Age UK27 was provided to participants in both groups, it has been excluded from the analysis.
Costing the occupational therapist intervention
The cost of implementing and delivering the intervention was estimated based on information obtained from the OT home assessment visit that participants in the intervention group received, plus information regarding the cost of training the OTs to undertake the home visits. The intervention cost therefore comprised (1) the cost of OTs’ time spent on the home visit, including travel for the visit, and follow-up telephone call(s); (2) the cost of training the OTs; and (3) the cost of the equipment provided to the participant following recommendations from the OTs at the home visit. The three elements of the intervention cost are described in detail in the sections below, and the corresponding unit costs are summarised in Table 9.
| Item | Unit cost (£) | Source |
|---|---|---|
| OT time (per hour) | ||
| Band 6 | 44.00 | Unit Costs of Health and Social Care 2018,52 page 119 |
| Band 7 | 53.00 | Unit Costs of Health and Social Care 2018,52 page 119 |
| Band 8a | 63.00 | Unit Costs of Health and Social Care 2018,52 page 119 |
| Training | ||
| Cost of trainers (per hour) | 53–63 | Unit Costs of Health and Social Care 2018,52 page 119 |
| Cost of trainees (per hour) | 44–63 | Unit Costs of Health and Social Care 2018,52 page 119 |
| Training pack | 8.22 | YTU cost information |
| Online training modules | 15.00 | YTU cost information |
| Equipmenta | ||
Time spent by occupational therapists on the home visit and follow-up calls
The duration of the intervention home visit was recorded and used to cost the visit. When visit duration was missing, an average visit duration was applied. The OTs who were trained to undertake the home visits ranged from band 6 to band 8a; the duration of the visit in minutes was multiplied by a cost per minute, using the unit costs of time, for the particular OT who visited. That is, a unit cost of £0.73 per minute of band 6 time was applied when a band 6 OT undertook the home visit, and, similarly, a cost of £0.88 per minute was applied for band 7 OTs. One visit occurred where the OT name was not recorded, and hence an average unit cost per minute was applied. No band 8a OTs undertook the visits, but they were involved in the intervention training, including the running of cascade training. A travel cost of £5.60 was applied for each visit, based on a cost of 56 pence for each mile52 for an assumed 10 miles of travel. The duration of the follow-up telephone call and associated administration time was not recorded for each participant, but instead it was assumed to be 15 minutes based on feedback from study OTs who undertook the follow-up calls. This call duration was then also multiplied by the OT unit cost per minute.
Training
The total costs associated with training comprised the time OTs spent attending the training workshop and the associated materials (£8 per training pack and £15 for access to online training modules), plus the cost of the trainers’ time. Information regarding the time spent on the training workshops/modules and the staff grade of the trainees/trainers was collected by the trial team. Capital costs such as room hire, catering and travel costs for the training were not costed separately in the analysis because the intervention was delivered in an NHS setting and these overheads were included already in the OT staff costs. The costs associated with developing the intervention (i.e. preparing the training materials) were also not incorporated as these costs would not be incurred when rolling out the intervention.
Costs were attached to the training that was undertaken to enable the study OTs to conduct the OTIS home visits, with the opportunity cost of each of the 27 trainees included. Training costs were estimated according to whether the training was provided on a face-to-face basis or via cascade training; both trainee and trainer costs differed according to the method used. For the face-to-face training, a cost for the OT attendees’ time spent attending the training (7 hours) was multiplied by their corresponding hourly unit cost, according to their band. The cascade training assumed a similar time spent by trainees (7 hours) plus the time spent on completing the online training modules (3 hours), with the cost of the online training of £15 applied for each trainee. For the trainers who undertook the training, a cost of their time was incorporated: the face-to-face training was conducted over 4 days, with either a grade 7 or a grade 8a trainer cost applied depending on who ran each training day. Similarly, trainer costs were applied for those involved in conducting the cascade training (two grade 7 and one grade 8a), along with the cost of spending an additional 3 hours on the online training modules as a refresher prior to running the cascade training. In addition, the cost of training packs for each of the 27 trained OTs was included.
Equipment
Information regarding the items of equipment that were installed following the home visits was incorporated in the intervention cost. This information was collected during the 4- to 6-week follow-up call.
In England, equipment and home adaptations may be provided and paid for by local councils/social services, or it may be the case that the individual will need to instead pay a proportion or the full amount of the cost. 53 The provision of equipment/adaptations depends on the type and cost of the item and the circumstances of the individual receiving them, that is, whether or not the individual meets the eligibility criteria for free provision. This takes into account the individual’s financial situation and whether they have an illness or a disability, with grants being available to apply for. 54
The base-case analysis includes equipment that was provided by the NHS and PSS, but not equipment that was provided by the participant/relative; this does, however, feed into the secondary analysis, which takes a wider societal perspective. A sensitivity analysis was undertaken to explore what would happen if all of the equipment that was provided were to be funded by the NHS and PSS.
The unit costs for the various types of equipment were sourced from PSSRU’s Unit Costs of Health and Social Care 201852 and via the Living Made Easy website55 using the AskSARA guide. 56 The guide provides information on daily living products and is governed by the NHS’s The Information Standard, a certification scheme for health and social care information. The cost of each equipment item is provided in Appendix 3.
Multiple imputation
Missing data are likely to arise in economic studies that involve the collection of patient-level data. 57 Reasons for missing data include participants failing to complete certain items in the questionnaire, and participants failing to return the questionnaire at all. The total mean cost generated as part of an economic analysis often involves the summation of the costs of several resource use items. If responses to any of the items are missing, however, the overall cost would be considered missing if an approach such as complete-case analysis was utilised. This form of analysis would lead to meaningful data being excluded;58 there might be only a small proportion of missing resource use items, but a high proportion of individuals with missing total costs. 57 Complete-case analysis is considered a useful starting point, although it is not recommended for use in the base case of a within-trial economic analysis. 59 Available case analysis uses data more efficiently, although it also has limitations, for instance relating to the use of different samples for the costs and outcomes. 59
Multiple imputation (MI) is an alternative approach to deal with missing data that relies on the assumption that data are missing at random; that is, the probability that data are missing is not dependent on the unobserved data, conditional on the observed data. 60 MI estimates a set of plausible values for the missing data using the distribution of the observed data,60 with multiple ‘complete’ data sets created. Owing to the creation of multiple predictions for each missing value, MI incorporates the uncertainty in the imputations and provides accurate standard errors. 61
Implementing MI with chained equations when data are not missing at random could result in biased estimates. 61 Descriptive analysis of missing economic data was undertaken to determine whether or not the missing at random assumption underpinning MI was plausible. Missing economic data at all follow-up points for each study group and missing data patterns were explored. Logistic regression was used to assess the association between missingness and baseline variables, and the association between missingness and observed costs and EQ-5D-5L scores.
The findings of our investigations into missing economic data indicated that the missing at random assumption is plausible, as presented in more detail in Results. Therefore, the base-case analysis was conducted using MI for the handling of missing data. The model used MI with chained equations, with predicted mean matching on utilities at 4, 8 and 12 months and the cost estimates, and hence ensured that only plausible values were imputed. The imputation model included age, sex, centre, history of falling at baseline, utilities (at baseline and at 4, 8 and 12 months) and total costs at the resource use level (e.g. total cost of GP visits over 12 months). Three imputations are suggested to be sufficient for a data set that has 20% of total data missing. 62 Owing to the extent of missing data in the present study, MI by chained equations was undertaken for a total of 10 imputations, with graphical plots used to compare the distributions of the observed data with the imputed data, thereby depicting whether or not the imputed data resemble the observed data. Mean cost and QALY estimates were generated using the combined imputed data sets following Rubin’s rules. 63 The multiply imputed data set was used for the base-case economic analysis, with incremental cost-effectiveness ratios (ICERs) generated as described in Incremental analysis.
For comparison purposes, we conducted a complete-case analysis as a separate sensitivity analysis, in which only participants with observed data for all costs and utilities were included. As complete-case analysis relies on the data being not missing at random, the sensitivity analysis allows us to explore the impact of this alternative assumption around missing data. Available case analysis has been used for initial exploration of the data.
For participants who died during the study, usual imputation methods were applied when these participants had missing data before their death. For resource use and QALY data that would have been obtained via questionnaires received at any follow-up time point after their death, zero resource use and zero QALYs were assumed.
Incremental analysis
Costs and health outcomes (i.e. QALYs and falls) are summarised for both groups in terms of mean value and SD, and mean difference (with 95% CIs) between the groups. The analyses used conventional decision rules when comparing the mean costs and outcomes for both groups, with the results presented in terms of ICERs (i.e. the additional cost per extra unit of benefit), where appropriate. The ICERs of (1) the cost per fall averted and (2) the cost per QALY gained were estimated based on Equation 1:
where incremental costs and incremental effects are shown by ΔC and ΔE, respectively.
The ICER is compared with the willingness-to-pay threshold, i.e. the amount that a decision-maker is willing to pay for an additional QALY. A willingness-to-pay threshold of £20,000–30,000 has been used for the analyses, in line with NICE recommendations;42 that is, if the estimated cost per QALY is below the threshold, the intervention will be considered cost-effective. Interventions with an ICER < £20,000 would generally be considered cost-effective.
The findings are also presented in terms of the net monetary benefit (NMB),64 which involves translating the health benefits associated with the intervention into monetary terms and use of the cost-effectiveness threshold λ. If the mean incremental NMB (i.e. the difference in NMB between the intervention and usual care) is greater than zero, then the intervention is considered to be cost-effective at the threshold under consideration:
The NMB was estimated at a range of willingness-to-pay thresholds, in addition to the uncertainty around the net benefit estimates that is presented graphically using cost-effectiveness acceptability curves (CEACs), as detailed in Analysis of uncertainty.
QALY data were adjusted for baseline EQ-5D-5L scores48 to allow for any differences at baseline between the two groups. Adjustment was made for covariates consistent with those used in the primary statistical analysis. Differences between the groups were found to be statistically significant if the p-value was < 0.05 and are presented alongside CIs around the differences in costs and outcomes.
The cost-effectiveness analysis produced estimates of the mean differences in costs and outcomes using seemingly unrelated regression equations, with 95% CIs estimated using bias-corrected and accelerated bootstrap methods. Bootstrapping was undertaken for 10,000 replications, thereby generating 10,000 estimates of incremental costs and incremental effects, and presented on the cost-effectiveness plane. Regression methods have been used to take into account differences in stratification or prognostic variables, and other sources of heterogeneity. Analyses were conducted using Stata release 16 (StataCorp LP, College Station, TX, USA).
Analysis of uncertainty
The decision uncertainty around the cost-effectiveness estimates has been explored by investigating the probability that the OT intervention is cost-effective relative to usual care at a range of values that decision-makers are willing to pay to gain one additional QALY. As mentioned above, CEACs have been used to illustrate this uncertainty. 65 Sensitivity analyses have been undertaken to explore the variability in estimating cost-effectiveness, as follows:
-
complete-case analysis as an alternative to the use of MI for dealing with missing data
-
inclusion of non-falls-related health-care resource use in addition to the falls-related resource use
-
inpatient stay data from falls data sheets, rather than from participant-completed questionnaires
-
exploration of the assumption that all equipment provided as part of the intervention is funded by the NHS and PSS (rather than in the base case, which attaches costs only to the items that were paid for by the NHS and PSS in the study and not to the items that were reported as funded by participants themselves, i.e. out-of-pocket expenditure)
-
paid care worker visits being paid for by the NHS and PSS (rather than by the participant/relative as in the base case).
Extrapolation to the longer term
A further analysis using an exploratory decision model was also due to be conducted to explore the possible long-term impact of the trial assuming that a falls reduction leads to a reduction in fractures. However, owing to the study findings regarding falls (i.e. that the intervention was associated with an increased falls rate), it was not possible to extrapolate to the future in terms of a fracture reduction, and hence a long-term model has not been included in the economic evaluation.
Results
Participant population and missing data
A total of 35 participants died during the study period: 13 out of 430 (3.0%) in the intervention group and 22 out of 901 (2.4%) in the usual-care group.
The complete-case analysis (sensitivity analysis 1) included participants who had complete data for all costs and utilities over the entire study duration. A total of 412 participants had complete economic data: 121 intervention participants and 291 usual-care participants. The proportion of participants who had complete economic data available remained at similar levels over the course of the study; 54.4% and 55.5% of participants had complete data for the intervention and usual-care groups at baseline, respectively, compared to 55.6% and 56.8% at 12-month follow-up. Further details on the remaining time points are available in Appendix 5. For both groups, there was a higher number of complete cases at 4 months than at any of the other time points. The missing data patterns displayed for EQ-5D-5L and cost data are not monotonic; there are participants with intermittent missing data. For example, some participants were lost to follow-up at 4 months (i.e. had missing data), but had observed data at 8 months and/or 12 months. The use of complete-case analysis would therefore be inefficient due to discarding data from these individuals altogether, on the basis of having one or more missing data items.
The findings of the logistic regressions of indicators of missing cost and QALY data on study group allocation, several baseline variables and observed outcomes are presented in Appendix 6. Age, sex and lower EQ-5D-5L scores at baseline were significantly associated with missing cost data at 12 months. Baseline EQ-5D-5L score, age and history of falling were found to be significant predictors of missing QALY data at 12 months. Observed values for QALYs at both 4 months and 8 months were significantly associated with missingness of QALY data at 12 months, and also with missingness of cost data at 12 months. Observed costs at 4 months and 8 months were not found to be significant predictors of missing QALY data at 12 months or of missing cost data at 8 months. Observed costs at 4 months were, however, significantly associated with missingness of QALY data at 12 months. These findings suggest that the data are unlikely to be missing completely at random and support our assumption that data are missing at random.
Economic data were, therefore, assumed to be missing at random, with MI by chained equations used to deal with the missing data for the health economic analysis. Missingness was assumed to depend on baseline covariates (sex, age, history of falling and EQ-5D-5L at baseline) and observed costs and QALYs. The distributions of the imputed data were compared with the distributions of the observed data, as a means of checking the fit of the imputation model, with the distributions found to be similar for both costs and utilities (see Appendix 6).
Health-related quality of life
The EQ-5D-5L is categorised as complete if each of its five dimensions contains a response. The completion rates for the EQ-5D-5L were 93% for both groups at baseline (see Appendix 7), and, although the completion rates decreased by 12 months, they remained high, at 91% and 86% for the usual-care and intervention groups, respectively. Full details of the missing dimensions for the incomplete EQ-5D-5L questionnaires are provided in Appendix 8.
A breakdown of the different EQ-5D-5L levels (1–5) reported by participants, according to dimension, time point and group, can be seen in Appendix 9, for all available cases. Over 80% of participants reported having problems in terms of pain/discomfort at all time points in both groups, and problems with mobility and usual activities were experienced by at least 62% of participants. Fewer participants reported problems with the dimensions of self-care and anxiety/depression, with over 25% and 37% experiencing these at the study follow-up points.
The mean EQ-5D-5L visual analogue scale scores were found to be similar at baseline: 73.3 in the intervention group and 73.7 in the usual-care group (see Appendix 10). Scores reduced slightly in both groups over time, with 12-month scores being 71.5 for the intervention group and 72.1 for the usual-care group. Median visual analogue scale scores remained at 75 throughout the study for both groups at all time points.
Participants’ mean utility scores at each time point are summarised in Table 10 for all available cases, alongside the mean difference between the groups, both unadjusted and adjusted for baseline utility. At baseline, utilities were higher, on average, for usual-care participants (0.69) than for participants in the intervention group (0.68). After adjusting the mean difference for baseline utility, a marginally greater health-related quality of life gain (of 0.0003) was seen in the intervention group than in the usual-care group at 12 months. However, the difference in mean utility score was not statistically significant at any time point, whether unadjusted or adjusted for baseline utility (see Table 10).
| Time point | Intervention (N = 430) | Usual care (N = 901) | Unadjusted mean difference (intervention – usual care) (95% CI); p-value | Adjusted mean differencea (intervention – usual care) (95% CI); p-value | ||
|---|---|---|---|---|---|---|
| n | Mean utility score (SD) | n | Mean utility score (SD) | |||
| Baseline | 398 | 0.684 (0.193) | 840 | 0.693 (0.197) | –0.0096 (–0.033 to 0.014); 0.422 | |
| 4 months | 397 | 0.676 (0.203) | 848 | 0.678 (0.197) | –0.0013 (–0.025 to 0.022); 0.912 | 0.0064 (–0.011 to 0.023); 0.459 |
| 8 months | 391 | 0.652 (0.226) | 830 | 0.673 (0.208) | –0.0205 (–0.046 to 0.005); 0.119 | –0.011 (–0.030 to 0.007); 0.233 |
| 12 months | 383 | 0.643 (0.228) | 834 | 0.653 (0.217) | –0.0099 (–0.037 to 0.017); 0.467 | 0.0003 (–0.020 to 0.021); 0.979 |
In terms of the total QALYs gained by participants over the trial duration for all available cases (Table 11), no statistically significant differences were found between the groups, when adjusting for baseline utility only or when adjusting for baseline utility and all covariates. QALYs were found to be marginally lower for the intervention group (reduction of 0.0015 QALYs; p = 0.814) when adjusting for baseline utility and all covariates.
| Treatment group | Total | Mean (SD) total QALYs | Difference (intervention – usual care) (95% CI);a p-value | Difference (intervention – usual care) (95% CI);b p-value |
|---|---|---|---|---|
| Intervention | 341 | 0.67 (0.18) | 0.0004 (–0.012 to 0.013); 0.951 | –0.0015 (–0.014 to 0.011); 0.814 |
| Usual care | 742 | 0.68 (0.18) |
Falls
At the primary end point of 12 months, participants in the intervention group experienced additional falls compared with those in the usual-care group (IRR 1.17, 95% CI 0.99 to 1.38; p = 0.07), as summarised in Chapter 3. As the intervention was associated with a higher falls rate (i.e. no falls were averted) and an additional cost, it was not clinically meaningful to calculate the ICER in terms of the cost per fall averted.
Health-care resource use and costs
The falls-related health-care services utilised by participants during the study period are summarised in Table 12. The resources participants used most commonly in relation to falls were visits to the GP, nurse and physiotherapist, in addition to hospital outpatient visits and inpatient overnight stays. The most notable difference between the groups was seen for inpatient hospital stays; participants in the intervention group stayed for an average of 0.05 nights over the past 4 months, when asked in the 12-month questionnaire, whereas participants in the usual-care group stayed for an average of 0.28 nights. Further resource use due to ‘other’ reasons is provided in Appendix 11. A greater proportion of missing data occurred for resource use related to falls than for ‘other’ resource use (i.e. non-falls-related). This applied for each resource use item, that is, for GP visits, nurse visits and all remaining resource use items, as shown in Table 12 and Appendix 11.
| Type of resource use | Intervention group | Usual-care group | ||
|---|---|---|---|---|
| Mean (SD) | Missing, n (%) | Mean (SD) | Missing, n (%) | |
| GP visit at general practice/home | ||||
| Baseline | 0.28 (1.88) | 139 (32.3) | 0.16 (0.54) | 297 (32.7) |
| 4 months | 0.20 (2.34) | 134 (31.2) | 0.09 (0.43) | 290 (32.2) |
| 8 months | 0.13 (0.63) | 132 (30.7) | 0.12 (0.52) | 296 (32.9) |
| 12 months | 0.10 (0.69) | 156 (36.3) | 0.16 (0.66) | 296 (32.9) |
| Nurse visit at general practice/home | ||||
| Baseline | 0.13 (0.80) | 143 (33.3) | 0.16 (1.04) | 314 (34.9) |
| 4 months | 0.09 (0.67) | 147 (34.2) | 0.70 (0.40) | 273 (30.3) |
| 8 months | 0.11 (0.81) | 141 (32.8) | 0.18 (1.22) | 314 (34.9) |
| 12 months | 0.06 (0.37) | 167 (38.8) | 0.18 (1.00) | 314 (34.9) |
| OT visit | ||||
| Baseline | 0.03 (0.23) | 129 (30.0) | 0.02 (0.18) | 295 (32.7) |
| 4 months | 0.17 (0.57) | 139 (32.3) | 0.06 (0.48) | 252 (28.0) |
| 8 months | 0.06 (0.36) | 131 (30.5) | 0.08 (0.74) | 287 (31.9) |
| 12 months | 0.06 (0.45) | 153 (35.6) | 0.07 (0.62) | 284 (31.5) |
| Physiotherapist visit | ||||
| Baseline | 0.18 (0.89) | 132 (30.7) | 0.11 (0.63) | 300 (33.3) |
| 4 months | 0.14 (0.74) | 136 (31.6) | 0.07 (0.56) | 258 (28.6) |
| 8 months | 0.13 (0.80) | 131 (30.5) | 0.14 (0.90) | 290 (32.2) |
| 12 months | 0.21 (1.09) | 154 (35.8) | 0.13 (0.86) | 299 (33.2) |
| Hospital outpatient visit | ||||
| Baseline | 0.11 (0.48) | 132 (30.7) | 0.17 (0.81) | 295 (32.7) |
| 4 months | 0.08 (0.47) | 131 (30.5) | 0.13 (1.03) | 255 (28.3) |
| 8 months | 0.08 (0.43) | 133 (30.9) | 0.10 (0.52) | 289 (32.1) |
| 12 months | 0.19 (1.18) | 151 (35.1) | 0.14 (0.74) | 292 (32.4) |
| A&E attendance | ||||
| Baseline | 0.08 (0.30) | 118 (27.4) | 0.12 (0.67) | 267 (29.6) |
| 4 months | 0.07 (0.37) | 122 (28.4) | 0.06 (0.30) | 234 (26.0) |
| 8 months | 0.08 (0.31) | 129 (30.0) | 0.08 (0.33) | 270 (30.0) |
| 12 months | 0.06 (0.26) | 143 (33.3) | 0.09 (0.37) | 272 (30.2) |
| Day-case hospital visit | ||||
| Baseline | 0.03 (0.18) | 127 (29.5) | 0.07 (0.71) | 287 (31.9) |
| 4 months | 0.06 (0.36) | 110 (25.6) | 0.06 (0.40) | 241 (26.8) |
| 8 months | 0.03 (0.20) | 126 (29.3) | 0.04 (0.26) | 262 (29.1) |
| 12 months | 0.05 (0.27) | 130 (30.2) | 0.07 (0.50) | 265 (29.4) |
| Inpatient hospital overnight stay | ||||
| Baseline | 0.11 (0.83) | 121 (28.1) | 0.18 (1.42) | 274 (30.4) |
| 4 months | 0.12 (1.02) | 112 (26.1) | 0.19 (1.38) | 227 (25.2) |
| 8 months | 0.14 (1.86) | 123 (28.6) | 0.38 (3.30) | 250 (27.8) |
| 12 months | 0.05 (0.61) | 132 (30.7) | 0.28 (3.26) | 254 (28.2) |
| Paid care worker visits | ||||
| Baseline | 1.92 (9.33) | 2 (0.5) | 2.70 (12.51) | 9 (1.0) |
| 4 months | 3.19 (13.60) | 27 (6.3) | 2.61 (12.22) | 42 (4.7) |
| 8 months | 3.54 (30.02) | 43 (10.0) | 3.67 (23.15) | 67 (7.4) |
| 12 months | 2.97 (14.02) | 46 (10.7) | 6.46 (44.91) | 64 (7.1) |
| Participants who received a falls prevention visit (non-OTIS) over the 12 months, n (%) | 83 (19.3) | 58 (13.5) | 83 (9.2) | 86 (9.5) |
| Participants who attended a falls service/clinic over the 12 months, n (%) | 24 (5.6) | 57 (13.3) | 41 (4.6) | 82 (9.1) |
The proportion who attended a falls service or clinic over the 12 months was slightly higher in the intervention group than in the usual-care group, at 6% and 5%, respectively. A higher proportion of participants in the intervention group (19%) received a (non-study) falls prevention visit over the 12 months of the study duration, than in the usual-care group (9%). A possible reason for this could be participants misunderstanding the question and instead recording their OTIS visit. Based on the possible confusion by participants, we explored the impact of excluding the non-study falls prevention visits from the analysis, as summarised in Cost–utility analysis and uncertainty.
Unit costs have been attached to the resource utilisation from Table 12 to indicate the cost differences for each resource. The largest differences can be seen in the costs of inpatient hospital stays and day-case hospital visits, with the mean costs being lower for both of these for participants in the intervention group. The cost of physiotherapist and OT visits was higher for the intervention group, with only small differences seen for the remaining items. However, it is worth pointing out the high proportion of missing data for the majority of these items. Table 13 indicates the total mean costs for each resource use item over the 12-month follow-up period for all available cases. The corresponding mean costs for the multiply imputed data set is provided in Table 14. Total out-of-pocket expenditure that features in the societal perspective analysis can be seen in Appendix 12.
| Cost item | Total mean (SD) cost (£) | Mean difference (intervention – usual care) (95% CI) | |
|---|---|---|---|
| Intervention (N = 430) | Usual care (N = 901) | ||
| GP visit at general practice/home | 17.79 (68.52); n = 173 | 20.18 (70.24); n = 409 | –2.38 (–14.81 to 10.04) |
| Nurse visit at general practice/home | 6.82 (29.73); n = 159 | 9.77 (45.58); n = 386 | –2.95 (–10.65 to 4.75) |
| OT visit | 15.85 (47.20); n = 172 | 8.11 (48.88); n = 423 | 7.74 (–0.86 to 16.33) |
| Physiotherapist visit | 31.23 (134.36); n = 176 | 14.60 (79.28); n = 404 | 16.63 (–0.97 to 34.24) |
| Hospital outpatient visit | 32.55 (131.73); n = 169 | 40.66 (140.94); n = 412 | –8.11 (–32.93 to 16.71) |
| A&E attendance | 28.75 (84.70); n = 184 | 31.47 (95.82); n = 433 | –2.72 (–18.73 to 13.29) |
| Day-case hospital visit | 67.46 (318.83); n = 198 | 93.19 (579.07); n = 430 | –25.73 (–112.02 to 60.56) |
| Inpatient hospital stay | 77.22 (804.14); n = 197 | 305.12 (2100.16); n = 451 | –227.90 (–531.09 to 75.29) |
| Paid care worker visitsa | 121.52 (627.36); n = 369 | 176.26 (934.45); n = 798 | –54.74 (–159.67 to –50.19) |
| Falls prevention visits (non-OTIS) | 10.80 (21.39); n = 373 | 5.77 (17.87); n = 816 | 5.04 (2.70 to 7.37) |
| Falls service/clinic attendances | 5.23 (19.98); n = 373 | 4.07 (17.7); n = 819 | 1.16 (–1.10 to 3.43) |
| Intervention cost | 136.53 (70.02);b n = 382 | N/A | N/A |
| Cost item | Total mean (SD) cost (£) | Mean difference (intervention – usual care) (95% CI) | |
|---|---|---|---|
| Intervention (N = 430) | Usual care (N = 901) | ||
| GP visit at general practice/home | 22.35 (75.44) | 23.30 (74.37) | –0.95 (–3.67 to 1.76) |
| Nurse visit at general practice/home | 9.61 (40.50) | 10.50 (45.98) | –0.89 (–2.50 to 0.72) |
| OT visit | 16.32 (52.27) | 10.06 (48.62) | 6.26 (4.45 to 8.07) |
| Physiotherapist visit | 38.88 (145.22) | 17.94 (89.16) | 20.94 (16.93 to 24.95) |
| Hospital outpatient visit | 45.64 (147.23) | 45.40 (147.18) | 0.25 (–5.10 to 5.59) |
| A&E attendance | 35.49 (91.98) | 33.04 (94.14) | 2.45 (–0.94 to 5.85) |
| Day-case hospital visit | 86.46 (374.80) | 94.22 (498.09) | –7.76 (–24.54 to 9.02) |
| Inpatient hospital stay | 186.31 (1380.17) | 315.98 (2090.87) | –129.67 (–198.36 to –60.98) |
| Falls prevention visits (non-OTIS) | 11.37 (21.98) | 6.23 (18.38) | 5.14 (4.43 to 5.85) |
| Falls service/clinic attendances | 5.67 (20.72) | 4.26 (18.12) | 1.41 (0.72 to 2.10) |
| Intervention cost | 121.29 (78.71) | N/A | N/A |
Costing the occupational therapist intervention
A total of 382 participants received the home visit as part of the intervention; the visit duration was recorded for 374 of these participants, with an average duration applied for the remaining eight participants for whom duration was not recorded. The mean intervention cost was found to be £137 for the 382 participants who received the home visit, which comprised the costs outlined in Table 15.
| Cost element | Total mean (SE) cost per participant (£) |
|---|---|
| Training of OTs to undertake the home visits | 35.30 (0.00) |
| OT time spent at home visit and on follow-up telephone call | 80.75 (1.02) |
| Equipment/adaptations installed following the home visit | 20.48 (3.20) |
| Total intervention cost | 136.53 (3.58) |
The training cost per participant was estimated by dividing the total cost of training the OTs involved in the study by the number of participants who received the intervention. Of the 27 trained OTs, 23 conducted the 382 visits, seeing 16 or 17 participants on average. However, if the intervention were to be rolled out in practice, an OT is likely to see more people than this, and hence the cost of their training will be spread over more people.
The average equipment cost was relatively low; a high proportion of 71% (273/382) of participants did not receive any equipment/adaptations. Participants had a mean of 0.48 (SD 0.94) items installed, with this ranging from 0 to a maximum of 6 items installed per participant (and the median being 0). A breakdown of the mean number of the 31 possible equipment items installed is provided in Chapter 5 (see Table 22). In addition to these items, OTs had space to enter five ‘other’ items. An average cost for ‘other’ equipment was applied based on the five most commonly occurring equipment items (full details are in Appendix 4).
Cost–utility analysis and uncertainty
The OT intervention was associated with an additional cost for the base-case analysis, shown in Table 16, of approximately £19 per participant, on average. In terms of the effect of the intervention on health-related quality of life, only marginal differences in QALYs were demonstrated. The base-case analysis found 0.004 fewer QALYs for participants in the intervention group than for those in the usual-care group, on average, over the 12 months. It was not appropriate to report the base-case results in terms of the cost per QALY gained owing to the finding that usual care was the dominant option; that is, it was less expensive than and generated additional QALYs to the intervention, although this QALY gain was marginal.
| SA | Incremental mean cost (95% CI)a | Incremental mean QALYs (95% CI)a | ICER (£): cost per QALY | Probability cost-effective at £30,000/QALY (%) |
|---|---|---|---|---|
| Base case (MI), NHS perspective | 18.78 (16.33 to 21.24) | –0.0042 (–0.0043 to –0.0041) | Dominatedb | 27 |
| Secondary analysis: societal perspective | 73.15 (68.41 to 77.89) | –0.0025 (–0.0026 to –0.0024) | Dominatedb | 34 |
| SA1: complete-case analysis | –68.60 (–315.92 to 178.73) | 0.0076 (–0.0107 to 0.0259) | Dominantc | 82 |
| SA2: non-falls-related resource use | –203.99 (–208.50 to –199.47) | –0.0038 (–0.0039 to –0.0037) | 53,900 per QALY lost | 62 |
| SA3: falls data inpatient stays | 119.84 (117.71 to 121.97) | –0.0037 (–0.0038 to –0.0035) | Dominatedb | 16 |
| SA4: all equipment funded by NHS and PSS | 39.68 (36.72 to 42.65) | –0.0026 (–0.0027 to –0.0025) | Dominatedb | 33 |
| SA5: paid care worker visits included | –54.70 (–57.46 to –51.95) | –0.0037 (–0.0038 to –0.0036) | 14,859 per QALY lost | 41 |
The NMB at £30,000 per QALY was –£145.31 (95% CI –£149.90 to –£140.72), which also indicates that the intervention is not a cost-effective option when compared with usual care. The NMB at £20,000 per QALY of –£103.13 (95% CI –£106.73 to –£99.53) similarly shows that the intervention is not cost-effective when this lower threshold is considered.
Figure 9 illustrates the 10,000 bootstrap sample estimates, which are spread across the four quadrants of the cost-effectiveness plane, although the north-west and south-west quadrants are more heavily populated than the remaining two quadrants. The probability of the intervention being cost-effective at different willingness-to-pay thresholds is shown in Figure 10. At the lower NICE threshold of £20,000 per QALY, the probability of the intervention being cost-effective is 29.1%, and is similar at 26.9% for a threshold of £30,000 per QALY (under the base-case scenario).
FIGURE 9.
Cost-effectiveness plane for the base-case analysis: NHS perspective (MI adjusted for all covariates).
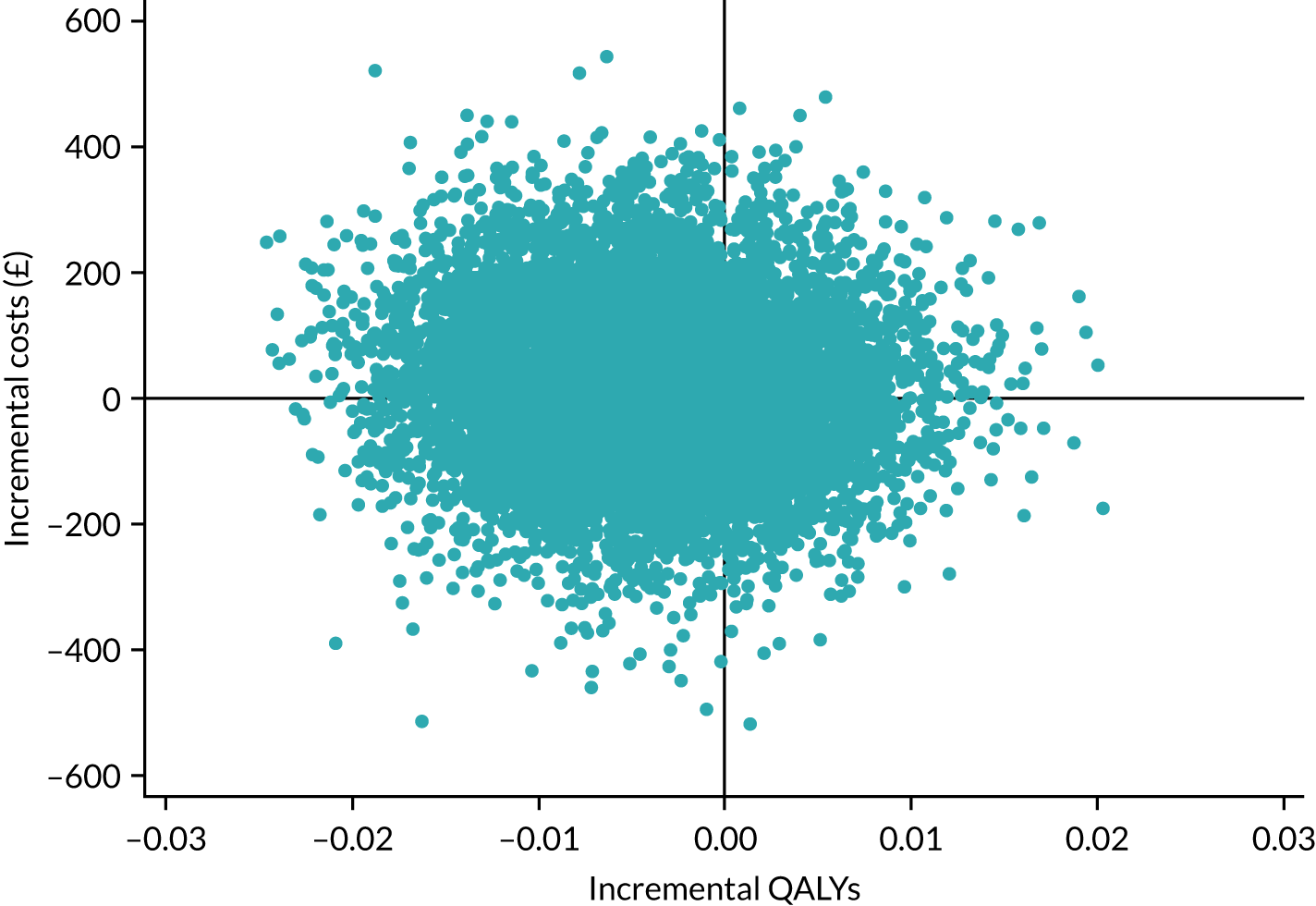
FIGURE 10.
Cost-effectiveness acceptability curve for the OT intervention relative to usual care, for the base-case analysis: NHS perspective (MI adjusted for all covariates).
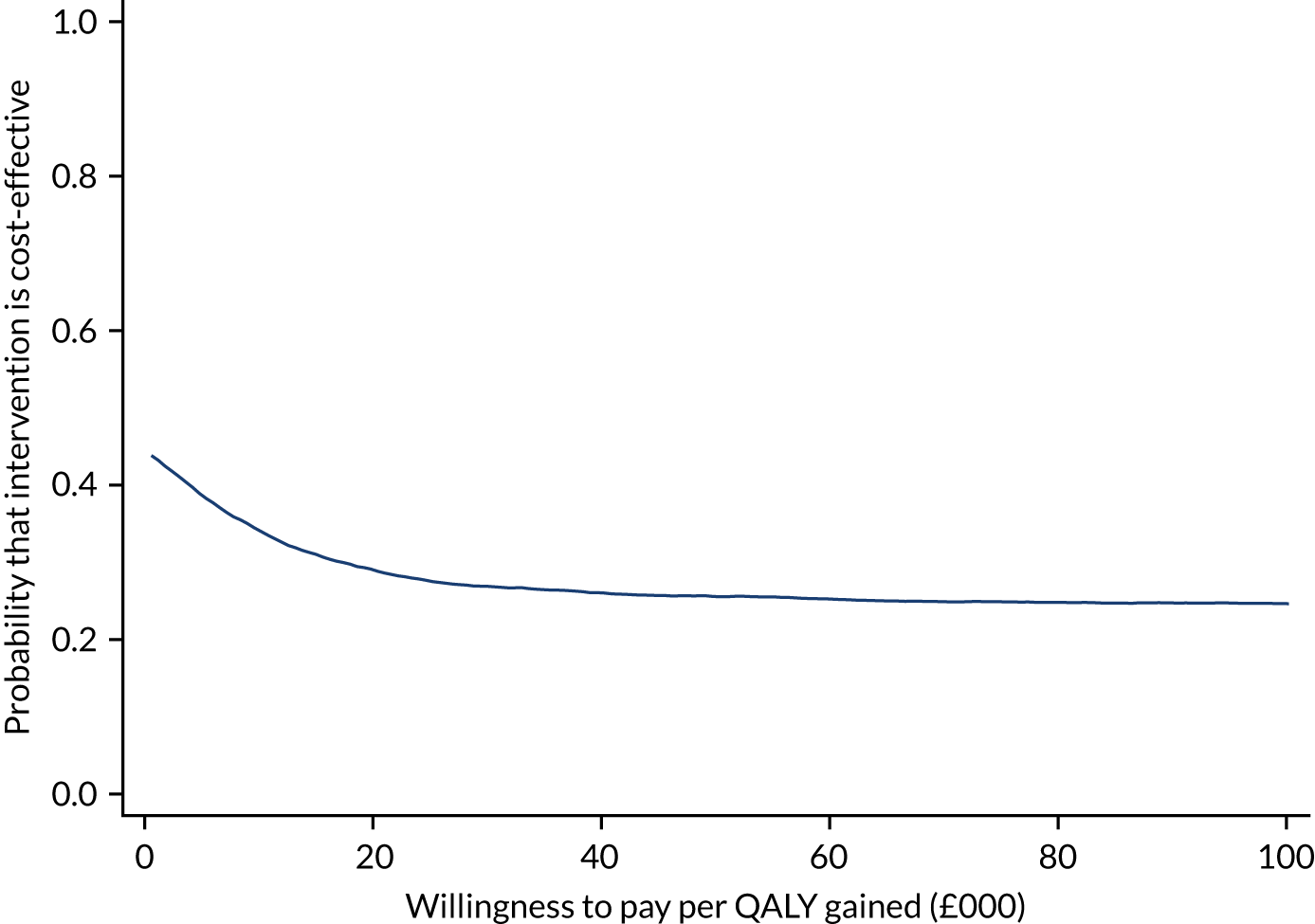
The secondary analysis undertaken from the societal perspective, which also included indirect costs rather than just considering the costs borne by the NHS and PSS under the base case, found an incremental mean cost per participant of £73.15 (95% CI £68.41 to £77.89). A reduction in QALYs (–0.0025, 95% CI –0.0026 to –0.0023) was again demonstrated for the intervention group versus the usual-care group. The cost-effectiveness plane displaying the bootstrap sample estimates for the secondary analysis is provided in Appendix 13. The corresponding CEAC (see Appendix 13) depicts a 34% chance of the intervention being cost-effective at the £30,000 per QALY threshold.
Sensitivity analysis
The findings for each of the sensitivity analyses are shown in Table 16 in terms of the incremental mean costs, the incremental mean QALYs, the ICER and the probability of the intervention being cost-effective. For all sensitivity analyses, the QALYs remained relatively similar, with the exception of sensitivity analysis 1, which comprised the complete-case analysis. We therefore focus on the cost findings here. When only complete cases were included (sensitivity analysis 1), the intervention was found to be the dominant option compared with usual care. Additional QALYs were experienced by the intervention group, on average, although this QALY difference was found to be small (0.0076) and not statistically significant. This sensitivity analysis found a cost saving of £69 for the intervention group, in contrast to the additional costs demonstrated in the base case. However, the complete-case analysis comprised only 412 participants. The corresponding cost-effectiveness plane (Figure 11) demonstrates the QALY gains, with more of the point estimates being situated in the north-east and south-east quadrants. The CEAC for the complete-case analysis can be seen in Figure 12. The cost-effectiveness planes and CEACs for sensitivity analyses 2–5 are provided in Appendices 14 and 15, respectively.
FIGURE 11.
Cost-effectiveness plane for the complete-case analysis (NHS perspective, adjusted for all covariates).
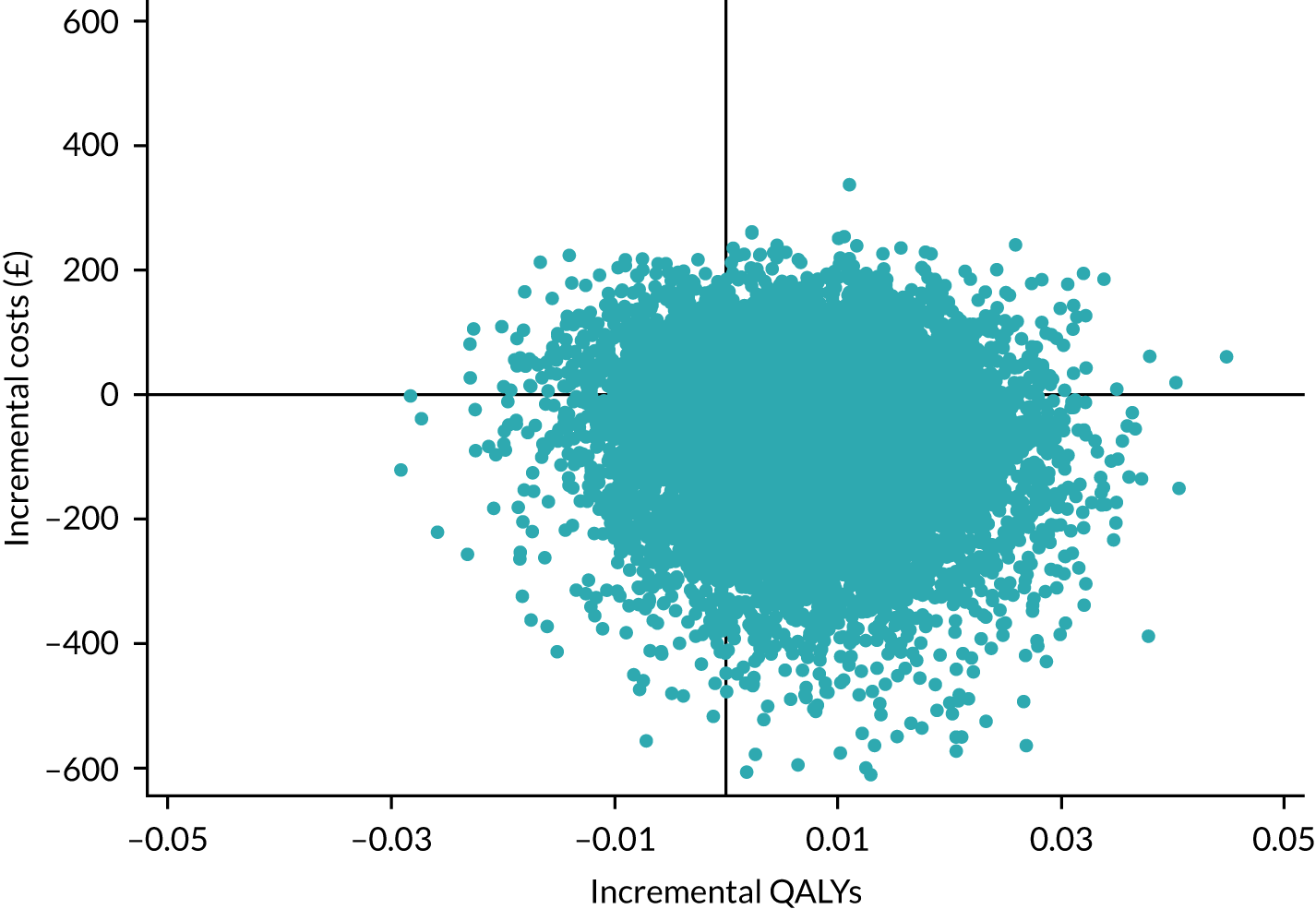
FIGURE 12.
Cost-effectiveness acceptability curve for the complete-case analysis (NHS perspective, adjusted for all covariates).
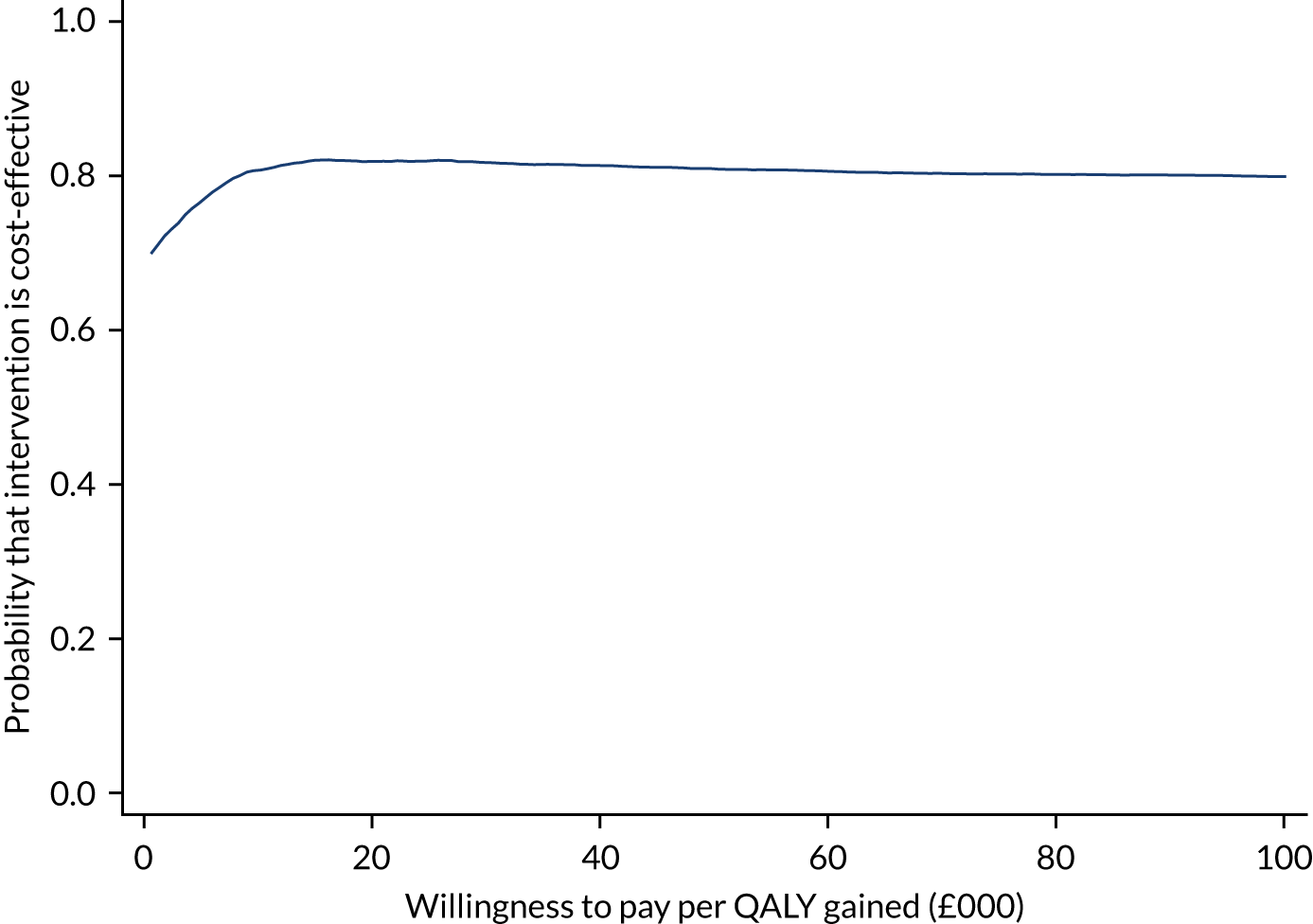
In the base-case analysis, the intervention was dominated by usual care, as the intervention generated additional costs and fewer QALYs. The intervention remained dominated for both sensitivity analysis 3 (alternative source of inpatient stay data) and sensitivity analysis 4 (all equipment funded by NHS and PSS). Sensitivity analysis 3 explored the use of an alternative source of data for the number of nights spent in hospital due to falls. When the falls data sheets were used instead of the follow-up questionnaires to obtain data on falls-related nights spent in hospital, additional costs were found for the intervention group, which in turn led to an additional mean cost of £120 per participant. Sensitivity analysis 4 investigated the findings when all the equipment installed following the OTIS home visit was funded by the NHS and PSS. As expected, because the equipment component of the intervention cost increased, an overall increase in the mean cost of £40 was found. For the analysis exploring the inclusion of non-falls-related resource use (sensitivity analysis 2) and the analysis of paid care worker costs being included (sensitivity analysis 5), the intervention group experienced an average cost saving of £204 and £55, respectively. The corresponding ICER results were presented in terms of the cost per QALY lost, whereby QALYs are being lost but costs are being saved. The ICERs here indicate how much would need to be saved to justify a loss of one QALY.
The effect of removing the non-study falls prevention visits from the analysis, owing to participants’ potential misunderstanding of these, was also explored; the overall cost saving was £68.28 per participant (as opposed to an additional cost of £18.78 in the base case).
Discussion
The within-trial cost–utility analysis findings indicate that the OT intervention was associated with slightly higher costs (£18.78, 95% CI £16.33 to £21.24) and generated marginally fewer QALYs (–0.0042 QALYs, 95% CI –0.0043 to –0.0041 QALYs) and additional falls over the 12 months’ study duration, when compared with usual care. However, the cost and QALY differences between the two groups were small. The cost-effectiveness plane indicated dispersion of the incremental costs and incremental QALYs, with the point estimates featuring in all four quadrants, further highlighting the uncertainty around the findings.
The base-case analysis, which took an NHS and PSS perspective and used MI to take account of the large proportion of missing economic data, found a 27% probability that the intervention was cost-effective, using a willingness-to-pay threshold of £30,000. The finding of additional costs and fewer QALYs for the intervention group indicates that the intervention was dominated by usual care and is, therefore, unlikely to provide a cost-effective use of NHS resources when compared with usual care.
For the secondary analysis conducted from a societal perspective, the intervention group again experienced higher costs and fewer QALYs than the usual-care group, and hence the intervention was dominated by usual care. It is worth noting that the societal perspective considered the key costs expected to be incurred by participants, and, owing to concerns about overburdening participants, the questionnaires did not provide an exhaustive list of items. We acknowledge that it would have been useful to collect information on whether the paid care worker visits participants reported were paid for by them or by social services.
The results remained robust under some of the sensitivity analyses undertaken to explore the use of different assumptions, although some of the analyses did demonstrate different findings. The complete-case analysis of 412 participants indicated the intervention to be a cost-effective option, as it dominated usual care in terms of finding a reduction in costs and an improvement in QALYs, although these differences were not statistically significant. However, for all other analyses, a mean loss in QALYs was found for the intervention group, although, again, these were not statistically significant. Cost savings arose when non-falls-related resource use was incorporated and when the cost of paid care worker visits was included. Higher costs were seen for the intervention group with the sensitivity analysis that used alternative data regarding hospital inpatient stay, and under the scenario in which all equipment provided as part of the OT intervention was funded by the NHS and PSS.
The breakdown of the EQ-5D-5L data collected for this population for the four time points was useful to investigate. Participants in both groups were found to have a marginal fall in utility over the course of the trial, perhaps indicating that health-related quality of life diminishes slightly over time for the elderly population included in the study.
In addition to potential recall issues for resource use questions in terms of participants possibly not remembering the details of a visit that happened a few months ago, participants may have struggled to decide whether their resource use related to a fall or an ‘other’ reason when completing their study questionnaires. The larger number of missing data for falls-related resource use could indicate that participants missed these questions out because they were not sure whether the visits were in relation to a fall, but instead found it easier to respond regarding visits that were due to ‘other’ reasons. It could, therefore, be considered a limitation to ask participants to assign their service use in this way because of the increased likelihood that data on the falls-related option will be missing.
The costs were heavily driven by the cost of hospital inpatient stays, hospital day-case visits and outpatient attendances, with A&E attendances, physiotherapist visits and GP visits also factoring to a lesser extent. The intervention cost of £137 inevitably was a prominent additional cost incurred by participants in the intervention group. The key cost drivers of the intervention were the OT time cost attached to the home visit and the training cost element. However, the training cost per person who receives a home visit will reduce with the number of people seen by a trained OT, as the initial training cost is then spread over more people. A point to note regarding the intervention cost relates to the possibility of OTs spending further time after the home visit finishing off their paperwork, which has not been included in the costs; the analysis assumes that all study forms were completed at the home visit as outlined to OTs in the initial study training. Another potential source of additional OT time is the time taken to make referrals. We did not collect information on the number of participants who received a referral following the home visit, and therefore this was not incorporated in the intervention cost, but we acknowledge that it would have been useful to capture such information.
The equipment costs were estimated based on the total cost of the equipment items under consideration. We recognise that such an approach will attach higher costs than if the mean annual cost for equipment had been applied. However, we took this approach because this represented the cost incurred by the NHS and PSS during the time horizon of the study. It also removed the need to make assumptions about the lifetimes of equipment and whether some equipment items may, in practice, be used in only a limited timeframe, less than their expected lifetime. A further point to raise around equipment costs relates to the sources used for these costs; we obtained costs for equipment/adaptations from PSSRU’s Unit Costs of Health and Social Care in the first instance. Where information was not available on particular items, we sourced this from AskSARA,56 which includes retail prices paid by individuals. Although we acknowledge that the use of different sources is not ideal, the aim of our approach was to obtain costs representative of those paid in practice, making the best use of the information available.
The original intention in developing a long-term model was to use the results of the trial, supplemented with data taken from the literature, where required, to extrapolate an improvement from the intervention in terms of falls reduction to a reduction in fractures in the longer term. However, given the finding of the trial, namely that intervention effects were not present, an extrapolation analysis using a long-term model was not considered to be meaningful.
In conclusion, the intervention was associated with additional costs, fewer QALYs and additional falls compared with usual care over the 12 months’ study duration. On this basis, the intervention would not be recommended from an economics perspective as it does not provide a cost-effective option when compared with usual care. However, the differences between groups were small, and findings changed when different assumptions were explored via sensitivity analyses.
Chapter 5 Intervention fidelity
Background
Material throughout this chapter has been reproduced from Scantlebury et al. 66 This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data. The text below includes minor additions and formatting changes to the original text.
OTIS evaluated a complex intervention, one that includes multiple interacting components. 67–69 Intervention fidelity, that is the extent to which interventions are implemented as intended, should be a priority when designing and evaluating RCTs. There are numerous examples of reviews and primary research70–72 that define key elements of fidelity. In 2007, Caroll et al. 73 undertook a critical review of existing research on conceptualising fidelity. The resulting theoretical framework considers fidelity to consist of seven elements: adherence to an intervention; exposure or dose; quality of delivery; participant responsiveness; programme differentiation; intervention complexity; and facilitation strategies. This framework has since been updated by Hasson74 to incorporate two additional moderating factors (context and recruitment; Table 17). We have adopted Hasson’s definition of fidelity in OTIS.
| Element of implementation fidelity | Description |
|---|---|
| Adherence | Whether an intervention is being delivered as intended |
| Exposure or dose | Whether the amount of an intervention received by participants (frequency and duration) is as intended |
| Quality of delivery | The way that those responsible for delivering the intervention deliver it |
| Participant responsiveness | How far participants respond to, or are engaged by, an intervention |
| Programme differentiation | Identifying unique features of different components of programmes and identifying which elements are essential |
| Intervention complexity | Complexity of an idea can act as a barrier to adoption – how complex is the intervention? |
| Facilitation strategies | When aiming to evaluate implementation fidelity, what are the specific strategies put in place to support implementation e.g. provision of manuals, training and incentives. How were these strategies perceived by those involved in delivery? |
| Recruitment | The recruitment strategies used to attract individuals to the intervention–what are the challenges to involvement? |
| Context | What factors at political, economic, organisational, and work group levels affected implementation? |
Methods and analysis
The strategies to assess fidelity to the trial protocol were overseen by a fidelity group, composed of Shelley Crossland, Alison Pighills, Avril Drummond, Sarah Cockayne and Sara Rodgers, which met quarterly. Both the independent TSC/DMEC and the funder approved the proposed strategies.
Training of providers
We used a standardised training package in order to maximise consistency of delivery of the intervention across all of the trial sites. The training materials included a presentation with detailed notes, a training manual and videos of older people undertaking activities of daily living, which the OTs assessed using the WeHSA. 28 The training was delivered by three modalities (face-to-face, ‘cascade’ and online training).
The initial training session for OTs was held face to face on 11 January 2016 (with eight attendees), led by Alison Pighills and observed by two other HCPC-registered OT co-applicants (Shelley Crossland and Avril Drummond). Three subsequent face-to-face training sessions were facilitated by Shelley Crossland and Avril Drummond (on 9 and 10 January 2017, with a total of 13 attendees) or Shelley Crossland alone (on 10 November 2017, with five attendees). Alison Pighills and Shelley Crossland each subsequently conducted a one-off training session for a single OT. All training sessions were audio-recorded. The audio-recordings of all but the one-off training delivered by Shelley Crossland were reviewed, and the training sessions were rated by Alison Pighills using the OTIS training workshop checklist (see Report Supplementary Material 14) for eight key elements that the trainer should have covered (prevalence of falls; evidence base underpinning environmental assessment and modification; falls risk factors; the person; environment; occupation conceptual model of practice and occupational performance; background on falls; environmental assessment; equipment and ideas for falls prevention; and adherence). These key elements were rated as N/A, not applicable; 0, omitted; 1, observed – some elements included; 2, observed – most elements included; or 3, observed – all elements included. Additionally, five sessions of cascade training were assessed by a single assessor (Alison Pighills) using the same checklist, as was the online training resource to ensure that it aligned with the face-to-face trial intervention training provided by the three facilitators. The scores for each of the key elements, and an overall mean score (calculated using the scores for each completed element, i.e. all for which N/A was not recorded), are summarised.
Delivery of treatment
To assess fidelity of treatment delivery, we used three strategies. First, one of the original training facilitators (SCr) directly observed the OTs delivering the intervention. Second, an audit of the WeHSA form and subsequent recommendations was conducted for all trial assessment visits to evaluate whether or not the delivery of the trial intervention was consistent with the training received. Finally, qualitative interviews were conducted with OTs who delivered the intervention and with the original training facilitators.
Structured occupational therapist observations
A purposive selection of nine intervention visits delivered by nine different OTs across seven centres were attended and observed by a HCPC-registered OT co-applicant (SCr) between 10 October 2017 and 3 May 2018. Selection was to ensure representation from the different geographical locations of the trial and the inclusion of each type of training modality in the sample (facilitator led, ‘cascade and online training’). The application of the WeHSA by the individual OT during the home assessment visit was assessed and graded using the OTIS observational checklist (see Report Supplementary Material 15). In addition, six OTs completed the OTIS observational checklist about a visit they had conducted. Twelve key elements (discussed history and risk of falls with participant if possible, otherwise with family/carer; assessed functional vision; assessed functional cognition; assessed functional balance; assessed functional mobility; WeHSA used to assess functional capacity while participant doing tasks within the context of their environment; all sections and items of WeHSA completed/clinical reasoning explained if unable to complete or item not applicable; participant engaged in identifying hazards; participant engaged in devising possible solutions to minimise identified hazards; list of mutually agreed recommendations prioritised by participant; action plan left with participant; and adequate follow-up planned and support identified for adaptations and modifications) were rated as N/A, not applicable; 0, omitted; 1, observed – some elements included; 2 – observed-most elements included; or 3 – observed-all elements included. The scores for each of these key elements, and an overall mean score (calculated using the scores for each completed element, i.e. all for which N/A was not recorded), were summarised.
Documentation audit
The completed WeHSA forms for all intervention visits were reviewed by a registered OT co-applicant (AP) and assessed using the OTIS documentation checklist (see Report Supplementary Material 16). As with the OTIS observational checklist, the same 12 key elements (e.g. discussed history and risk of falls with participant, if possible, otherwise with family/carer; assessed functional vision) were rated as N/A, not applicable; 0, omitted; 1, observed – some elements included; 2, observed – most elements included; or 3, observed – all elements included. The scores for each of these key elements, and an overall mean score (calculated using the scores for each completed element, i.e. all for which ‘N/A’ was not recorded), were summarised.
The OTIS documentation checklist was piloted by two of the training facilitators (AP and SCr), who independently assessed the same participant record. There was agreement on 11 of the 12 items; the item they disagreed about was discussed and a consensus was reached. The remaining 381 participant records were audited by one facilitator (AP) to enhance the consistency of the audit. In addition to the WeHSA documentation audit, YTU calculated the completion rates of the WeHSA among participants randomised to the intervention group.
The OTs telephoned the participants 4–6 weeks after their initial home assessment to ascertain whether or not advice given as part of the intervention had been followed and if the recommended equipment had been installed. This information was recorded in the OT booklet. We summarise each item of equipment that was recommended and whether or not it had been installed. OTs were also asked the general question ‘Taking into consideration all of the recommendations made and advice given, how many of these have been implemented?’, with the response categories of most, some and none.
Qualitative interviews
A qualitative study was undertaken to explore OTs’ views, experiences and acceptability of the OTIS intervention. A purposive sampling method was adopted to select service providers, from those delivering the trial intervention, to ensure a diverse interview sample (e.g. research sites, job roles, training method) and to maximise the potential variation of experiences reported. Sampling continued until a varied spread of participants from a number of sites and job roles had been achieved and thematic saturation had been reached. 75
All OTs listed in the trial’s database (n = 29) were sent an e-mail directly inviting them to participate in the interview. The recruitment e-mail included an information sheet (see Report Supplementary Material 17), which provided detailed information about this component of the trial, and a consent form (see Report Supplementary Material 18). This was followed up with an e-mail or a telephone call. OTs were requested to complete and return the hard-copy consent form to YTU via post or e-mail, and the researcher scheduled a suitable date and time for the interview.
Of the 29 OTs contacted, eight did not respond (up to two follow-up reminder e-mails were sent to all non-responders, before concluding contact), four declined to participate, stating that they were not involved in the delivery of the OTIS intervention itself, and 17 agreed to participate. Representation was achieved in the interview data from seven out of the eight sites. These 17 OTs had an average of 15 years (range 5–37 years) of occupational therapy experience; 15 had job roles that required them to be community based and have direct client contact and the remaining two were office-based team leads with limited or no client contact.
The semistructured interviews were carried out over the telephone at the end of the intervention period, over 2 months between October and December 2018, and on average lasted 40 minutes. An interview guide was used to ensure that all the necessary topics/questions were covered (see Report Supplementary Material 19). Areas covered broadly fell into the following categories: the participant’s job role, appraisal of the training received, feasibility of providing the intervention on a regular basis, identification of the barriers and facilitators to successfully providing the intervention, implications of workload and participating in the trial and their readiness to integrate the intervention into their routine practice.
All interviews were audio-recorded and transcribed anonymously prior to analysis to facilitate theme recognition. An initial thematic analysis was conducted as outlined by Braun and Clarke:76 familiarisation, generalisation, the creation and refinement of themes and reporting. Following this, secondary analysis was undertaken to explore the feasibility of mapping existing themes onto the nine elements of intervention fidelity adapted from Hasson et al. 74 The qualitative research team had no prior knowledge or experience of falls-related OT interventions; Arabella Scantlebury (Research Fellow) conducted the interviews, Lyn Robinson-Smith (Research Fellow) analysed the interviews and Joy Adamson oversaw the process.
Results
The results are described under the headings of the Hasson model.
Facilitation strategies
For four of the five training sessions, a perfect score of 3 was awarded for all eight elements (resulting in an overall mean score of 3). For one session (the one-off session delivered to a single OT by AP), a perfect score of 3 was awarded for seven elements, with ‘Equipment and ideas for falls prevention’ awarded one (resulting in an overall mean score of 2.75). This was because the training was done via videoconference and the trainee had only a set amount of time available, so the information on equipment and ideas was provided to the OT to review in their own time.
Additionally, five sessions of cascade training were assessed by a single assessor. Scores for this training are provided in Table 18.
| Key element | Audit of cascade training (n = 5) | |
|---|---|---|
| Prevalence of falls | Mean (SD) | 3.0 (0.0) |
| Median (minimum, maximum) | 3.0 (3.0, 3.0) | |
| Evidence base underpinning environmental assessment and modification | Mean (SD) | 3.0 (0.0) |
| Median (minimum, maximum) | 3.0 (3.0, 3.0) | |
| Falls risk factors | Mean (SD) | 3.0 (0.0) |
| Median (minimum, maximum) | 3.0 (3.0, 3.0) | |
| The person–environment–occupation conceptual model of practice and occupational performance | Mean (SD) | 2.4 (0.5) |
| Median (minimum, maximum) | 2.0 (2.0, 3.0) | |
| Background on falls | Mean (SD) | 3.0 (0.0) |
| Median (minimum, maximum) | 3.0 (3.0, 3.0) | |
| Environmental assessment | Mean (SD) | 3.0 (0.0) |
| Median (minimum, maximum) | 3.0 (3.0, 3.0) | |
| Equipment and ideas for falls prevention | Mean (SD) | 1.6 (0.5) |
| Median (minimum, maximum) | 2.0 (1.0, 2.0) | |
| Adherence | Mean (SD) | 2.4 (0.5) |
| Median (minimum, maximum) | 2.0 (2.0, 3.0) | |
| Total overall mean score | Mean (SD) | 2.7 (0.1) |
| Median (minimum, maximum) | 2.6 (2.6, 2.8) | |
Alison Pighills completed the OTIS WeHSA online training checklist, and all domains were awarded a perfect score of 3.
Overall, the training was well received by OTs. The content was considered to be balanced between evidence and practice and OTs liked how the online training built on the content of that received during the face-to-face workshop, particularly as it provided a ‘refresher’ between the face-to-face workshop and the first OTIS home visit. OTs reported that the training prepared them well for delivering the intervention in the context of the trial. Although the majority of OTs felt that the training did not provide them with any new information about falls prevention education, this was not viewed negatively. While some OTs implemented the Home Falls and Accidents Screening Tool (Home Fast) in current routine practice, the WeHSA was a tool that the OTs had not used previously:
I would say that the training was incredibly comprehensive. It was very relevant. It was well facilitated and delivered. I wouldn’t have any kind of suggestions in terms of improving it. The resources that they provided were excellent. Yeah it was very good.
OT 06, 267–270
The training was really very thorough and the presenters were extremely approachable and although they were working from an academic point of view, we felt that they were really quite in touch with the real world as well.
OT 01, 98–101
Adherence/exposure
Occupational therapists’ adherence to the intervention delivery and dose (i.e. whether or not all elements of the intervention were delivered as per protocol) was assessed as described in Box 1.
Participant was offered a home environmental assessment and modification to identify personal fall-related hazards by OT.
OT used the WeHSA tool to structure their visit and devise recommendation list.
The participant was contacted 4–6 weeks after the home visit for data to be collected on whether or not the recommendations had been acted on.
A total of 382 participants allocated to the intervention group received an environmental assessment and modification visit from an OT (Table 19). The assessments were conducted by 23 OTs (median 16 visits per OT, range 1–54 visits). Nineteen of the OTs attended a face-to-face training session, and four were ‘cascade’ trained by another OT who had attended face-to-face training. For all visits a WeHSA form was completed. The visits took place between 1 day and 411 days after randomisation (median 27 days) and lasted a median of 90 minutes (range 25–180 minutes). Nearly two-thirds of the intervention group (277/430, 64.4%) had received the visit within 6 weeks of being randomised, and 381 out of 430 (88.6%) had received it within 12 months. One participant received the visit more than 12 months after they had been randomised, and 48 did not receive a visit.
| n (N = 430) | % | |
|---|---|---|
| Number receiving home visit assessment | 382 | 88.8 |
| Number with completed WeHSA | 382 | 88.8 |
| Number receiving follow-up telephone call | 324 | 75.3 |
| Number with all three elements of the intervention | 324 | 75.3 |
It was not always possible to ascertain whether or not these 382 participants received a follow-up telephone call 4–6 weeks after their initial assessment. We know this call was conducted for 324 participants and definitely was not conducted for 28 participants (either because the participant could not be contacted even after several attempts or because it was agreed that no follow-up was necessary as no recommendations had been made at the initial visits). Therefore, we conservatively assume that 324 of the 430 (75.3%) participants randomised to the intervention group received all elements of the intervention.
The vast majority of OTs questioned the criteria used to recruit participants into the trial (having either one previous fall in the past 12 months or a fear of falling), as trial participants were often ‘poles apart’ (OT 11) in terms of functioning and demographics from patients/clients that the OTs would have on their ‘normal’ caseloads. OTs described such trial participants as active and ‘very able-bodied’ (OT 08, 62–63) with high standards of living in their home environments:
In my usual job I would probably see a little bit more of, you know, homes that were maybe not so well looked after or, you know, carpets that were a bit more worn and that sort of thing. I don’t know if it was just . . . the luck of the draw as it were but I did seem to get quite a lot of . . . well-kept houses, maybe more middle-class people.
OT 07, 249–254
Occupational therapists considered that some participants were participating in the trial only because they felt that they should ‘give something back to the community’ (OT 5) rather than because they felt that they would personally benefit from receiving a falls prevention service. A significant frustration for OTs was that some of their OTIS recipients had never experienced a fall or that the fall that had occurred was in an external environment and/or was an accidental ‘one-off’. This made it more difficult for OTs to deliver the intervention. Some participants questioned their own suitability for the trial as ‘quite a few of them felt that they were too good for the study and they were a bit embarrassed about having volunteered’ (OT 15, 277–280) and, as a result, some declined a home visit during the initial telephone contact with the OT:
A few people when I phoned them up and said, ‘is it OK if I come?’ They said, ‘oh no I think you’re wasting your time, I’ve never had a fall. I’m absolutely fine and don’t see the point of you coming to the house’. I would have thought that would have been made clear when they signed up to the trial that it’s all part of the process. Some people were quite defensive and didn’t want [me] going into their homes.
OT 14, 244–250
Some OTs chose to discontinue the home visit after learning the cause of the fall:
There was one lady I refused to do the assessment with . . . she had said on the phone ‘I don’t want you to waste your time, I’m not sure really I’m the sort of person you want to be speaking to’ and I said ‘well I’ve got the time I’ll come out and go through things’. She said it wasn’t a fall . . . She’d hit a pothole and caught her bike and had a fractured shoulder . . . So when I got there I thought this is totally inappropriate for me to do with somebody who’s fitter than most people . . . I actually thought I’m not going to do this because it’s not appropriate.
OT 5, 199–210
Active participants spent less time in the house, which made it more difficult for OTs to arrange the home visit and to contact them to complete the follow-up 4–6 weeks later:
I think I had a better response . . . from people that lived on their own, you know, maybe quite enjoy the company of like someone coming in and having a chat and taking a real interest in like their background and their sort of lifestyle and maybe a bit more vulnerable so quite keen to have help. The people that I found who weren’t so keen were some of the more sort of [more active], a couple of people I struggled to get appointment with because they play golf and yoga and gym, so they say ‘I’ve got an hour, you can come on this day’.
OT 14, 289–27
In addition, OTs participating in the trial found that delivering the OTIS intervention alongside their normal workload was sometimes problematic, with some OTs expressing the challenges they experienced in transitioning between their existing caseload and the OTIS trial. Despite having ‘allocated’ time to complete the OTIS intervention, some OTs were told by senior staff members to prioritise caseload work over OTIS. This was particularly the case for OTs whose casework regularly involved patients who required emergency/fast response or palliative care. Participation in the trial also presented tensions for some part-time OTs whose employers were explicit that they were to complete OTIS during non-working days only. As a result, there were instances where this tension affected the delivery of the OTIS intervention, as OTs were unable to deliver the follow-up telephone call to participants within the intended time scale of 4–6 weeks (post home visit):
In terms of follow-up, that was a big failing on my part I’ll be honest in that, particularly when I was transitioning between the two job roles and to be honest anyway when I was in palliative care service, because obviously we had to stick to the . . . the guidance was that we had to do a review in 6 weeks. I failed that quite significantly just through the pressure of my other caseload.
OT 12, 218–266
Quality of delivery
Findings from the observations of the nine OTs using the OTIS observational checklist are presented in Table 20, suggesting that OTs were delivering the intervention to a high standard. There was very little variation in scores across the domains, with a median score of 3 across all OTs observed for all 12 components.
| Key element | Unit | Visits audited by HCPC-registered co-applicant (n = 9) | Visits audited by OT who delivered it (n = 6) |
|---|---|---|---|
| Discussed history and risk of falls with participant, if possible, otherwise with family/carer | Mean (SD) | 3.0 (0.0) | 2.8 (0.4) |
| Median (minimum, maximum) | 3.0 (3.0, 3.0) | 3.0 (2.0, 3.0) | |
| Assessed functional vision | Mean (SD) | 3.0 (0.0) | 2.8 (0.4) |
| Median (minimum, maximum) | 3.0 (3.0, 3.0) | 3.0 (2.0, 3.0) | |
| Assessed functional cognition | Mean (SD) | 2.9 (0.3) | 2.8 (0.4) |
| Median (minimum, maximum) | 3.0 (2.0, 3.0) | 3.0 (2.0, 3.0) | |
| Assessed functional balance | Mean (SD) | 3.0 (0.0) | 2.7 (0.5) |
| Median (minimum, maximum) | 3.0 (3.0, 3.0) | 3.0 (2.0, 3.0) | |
| Assessed functional mobility (Timed Up and Go) | Mean (SD) | 3.0 (0.0) | 3.0 (0.0) |
| Median (minimum, maximum) | 3.0 (3.0, 3.0) | 3.0 (3.0, 3.0) | |
| WeHSA used to assess functional capacity while participant doing tasks within the context of their environment | Mean (SD) | 3.0 (0.0) | 2.7 (0.5) |
| Median (minimum, maximum) | 3.0 (3.0, 3.0) | 3.0 (2.0, 3.0) | |
| All sections and items of WeHSA completed/clinical reasoning explained if unable to complete or item not applicable | Mean (SD) | 3.0 (0.0) | 2.7 (0.5) |
| Median (minimum, maximum) | 3.0 (3.0, 3.0) | 3.0 (2.0, 3.0) | |
| Participant engaged in identifying hazards | Mean (SD) | 3.0 (0.0) | 2.8 (0.4) |
| Median (minimum, maximum) | 3.0 (3.0, 3.0) | 3.0 (2.0, 3.0) | |
| Participant engaged in devising possible solutions to minimise identified hazards | Mean (SD) | 2.9 (0.3) | 2.8 (0.4) |
| Median (minimum, maximum) | 3.0 (2.0, 3.0) | 3.0 (2.0, 3.0) | |
| List of mutually agreed recommendations prioritised by participant | Mean (SD) | 3.0 (0.0) | 3.0 (0.0) |
| Median (minimum, maximum) | 3.0 (3.0, 3.0) | 3.0 (3.0, 3.0) | |
| Action plan left with participant | Mean (SD) | 3.0 (0.0) | 2.6 (0.9) |
| Median (minimum, maximum) | 3.0 (3.0, 3.0) | 3.0 (1.0, 3.0) | |
| Adequate follow-up planned and support identified for adaptations and modifications | Mean (SD) | 3.0 (0.0) | 2.8 (0.4) |
| Median (minimum, maximum) | 3.0 (3.0, 3.0) | 3.0 (2.0, 3.0) | |
| Total overall mean score | Mean (SD) | 3.0 (0.0) | 2.8 (0.3) |
| Median (minimum, maximum) | 3.0 (2.9, 3.0) | 2.9 (2.3, 3.0) |
Additionally, six OTs across six centres completed the OTIS observational checklist about a visit they had conducted (see Table 20). The visits took place between 22 November 2017 and 3 May 2018.
The documentation audit of the WeHSA booklets were assessed for all 382 visits (Table 21). On average, the overall mean score for the 12 domains assessed was 2.6 out of 3. The three most poorly completed domains appear to be (1) WeHSA used to assess functional capacity while participant doing tasks within the context of their environment; (2) participant engaged in devising possible solutions to minimise identified hazards; and (3) action plan left with participant, as it was not always possible to tell from study documentation if this had taken place.
| Key element | Audit of completed WeHSA booklets (n = 382) | |
|---|---|---|
| Discussed history and risk of falls with participant, if possible, otherwise with family/carer | Mean (SD) | 2.8 (0.4) |
| Median (minimum, maximum) | 3.0 (2.0, 3.0) | |
| Assessed functional vision | Mean (SD) | 2.8 (0.4) |
| Median (minimum, maximum) | 3.0 (2.0, 3.0) | |
| Assessed functional cognition | Mean (SD) | 3.0 (0.1) |
| Median (minimum, maximum) | 3.0 (2.0, 3.0) | |
| Assessed functional balance | Mean (SD) | 2.3 (0.5) |
| Median (minimum, maximum) | 2.0 (1.0, 3.0) | |
| Assessed functional mobility (Timed Up and Go) | Mean (SD) | 3.0 (0.2) |
| Median (minimum, maximum) | 3.0 (0.0, 3.0) | |
| WeHSA used to assess functional capacity while participant doing tasks within the context of their environment | Mean (SD) | 2.0 (0.2) |
| Median (minimum, maximum) | 2.0 (2.0, 3.0) | |
| All sections and items of WeHSA completed/clinical reasoning explained if unable to complete or item not applicable | Mean (SD) | 2.7 (0.5) |
| Median (minimum, maximum) | 3.0 (1.0, 3.0) | |
| Participant engaged in identifying hazards | Mean (SD) | 3.0 (0.1) |
| Median (minimum, maximum) | 3.0 (2.0, 3.0) | |
| Participant engaged in devising possible solutions to minimise identified hazards | Mean (SD) | 2.0 (0.1) |
| Median (minimum, maximum) | 2.0 (2.0, 3.0) | |
| List of mutually agreed recommendations prioritised by participant | Mean (SD) | 2.3 (0.7) |
| Median (minimum, maximum) | 2.0 (1.0, 3.0) | |
| Action plan left with participant | Mean (SD) | 2.0 (0.2) |
| Median (minimum, maximum) | 2.0 (2.0, 3.0) | |
| Adequate follow-up planned and support identified for adaptations and modifications | Mean (SD) | 3.0 (0.1) |
| Median (minimum, maximum) | 3.0 (2.0, 3.0) | |
| Total overall mean score | Mean (SD) | 2.6 (0.1) |
| Median (minimum, maximum) | 2.6 (2.3, 3.0) | |
However, as described above, there was a general sense of disengagement among OTs towards completing the intervention with participants whom they considered unsuitable for the trial, and this influenced how they delivered it. For example, with participants who reported that falls were one-offs or had happened in external environments, such as while hiking, OTs reported ‘going through the motions’ (OT 12) of competing the trial documentation rather than identifying falls hazards, as they did not perceive the participant to be at any risk. This also meant that the OTIS intervention was modified by OTs, as they skipped questions they thought were irrelevant or had already made assumptions about the participant’s home environment:
Most of the participants, probably 90% of the participants we saw were very active and to go and ask them in detail about everything on the list was too much. Yeah so I wouldn’t ask them about every question.
OT 15, 125–129
Sometimes it didn’t seem appropriate, you know, sometimes I went to see people and they had no mobility problems at all and it wasn’t that they refused to do it but I got the feeling that they didn’t want to do it. So you know I used my judgement there as to whether or not it was appropriate to complete.
OT 02, 179–184
Although OTIS was designed to be delivered by OTs, qualitative data suggest that wider team members (e.g. physiotherapists) may have been involved in intervention delivery at one site. However, all intervention booklets indicated that home assessments were completed by a trained OT, so we are unable to provide characteristics of other team members or an estimate of how frequently this may have occurred:
We picked up some [participants] as our cases and carried out the intervention as a team, so it wasn’t necessarily that an OT that did it [delivered the OTIS intervention], you know, it could have been a physio or someone else.
OT 17, 53–55
Intervention complexity
The OTIS intervention was considered a very detailed tool that provided a very thorough assessment of a participant’s home environment. None of the OTs reported that the forms missed any key features of an environmental falls prevention assessment. For some participants, the OTIS intervention was too complex and identified areas for change over which they (the OT) felt they had no control, such as the participant’s external environment (e.g. path, driveway, gardens):
It’s almost too comprehensive . . . areas which I can’t do anything about . . . I can’t do anything with a garden if it’s got a slope on it . . . so then why are you kind of assessing and recording it?
OT 10, 251–257
The reporting form itself was not considered to be user-friendly and this may have had an impact on the quality of delivery. OTs raised issues with the format of the form (e.g. not providing enough space for free text), and the ordering and repetitiveness of questions. These issues meant that the form was complicated to complete, especially while also trying to establish and maintain a conversation with the participant. This resulted in some OTs completing the form after the home visit and relying on notes and memory to do so, or missing out parts of the form that they believed to be irrelevant or unnecessary:
A lot of it was repetitive and you’re having a conversation with somebody trying to work out where to write things or where things go, what you need to know, some of that I would write up when I got back to the office. In the end I just didn’t follow it . . . I wouldn’t ask them [the participant] about every single bit because I just look at what was relevant.
OT 15, 242–244, 267–271
I think probably generally speaking I filled the paperwork out once I got home. I usually came out and made come quick notes about things so that I didn’t forget things and then came back and sit and trawl through it all and if I was stuck on a point I would just ring the patient back. But I think I only did this one or two times.
OT 11, 384–387
Occupational therapists reported that, owing to the complexity of the form, it took on average 1.5 hours to deliver (ranging from 25 minutes to 3 hours). A number of factors had an impact on delivery time, including the size of the participant’s house, their current health condition(s) and the number of falls they had previously experienced. Some OTs felt that they took more time completing the form because it was for research, and if the form were used in routine practice they would be more inclined to skip over parts or leave some parts incomplete. The quality of delivery of the OTIS intervention improved the more frequently OTs completed the form. OTs reported that they became more familiar with the form the more often they used it, which enabled them to complete the form on-site during the home visits, rather than at a later time, and also led to shorter delivery times. For a number of OTs, the time it took to deliver the OTIS intervention made them uncertain about the feasibility of delivering it during routine practice:
In my everyday role . . . I would not have time to go through the whole of that.
Community OT 15, 369–370
I think the reason it took longer than perhaps one of our normal falls assessment is that you’re just trying . . . because it’s a research project, you are trying to really make sure that you have covered all the elements for the form as well. Whereas in normal practice, a little bit like I said before, that I might make certain assumptions and miss bits because I can see it’s not really relevant.
OT 01, 398–403
Participant responsiveness
The number of recommendations made to intervention participants following the OT home visit ranged from 0 to 11 [mean 2.46 (SD 2.08), median 2 recommendations]. Following the follow-up telephone call, OTs were asked to judge ‘Taking into consideration all of the recommendations made and advice given, how many of these have been implemented?’. Responses were as follows: most (49.5%), some (34.8%) and none (15.7%).
Table 22 presents data on items of equipment that were recommended following the home visit, whether or not these were installed and whether or not the installation was carried out by either health services or social services. We have calculated the proportion of participants who followed the recommendation for each item of equipment and also the proportion of participants who had the item installed by health or social care services.
| Equipment item | Number recommended | Number who had item installed | Number provided by NHS/PSS | Had item installed (%) | Installed by NHS/PSS (%) |
|---|---|---|---|---|---|
| Non-slip mat | 161 | 94 | 26 | 58.4 | 27.7 |
| Shower rails/bath safety bars | 76 | 32 | 26 | 42.1 | 81.3 |
| Trolley (e.g. for kitchen) | 75 | 45 | 9 | 54.5 | 20.0 |
| Grab rails/banister | 66 | 25 | 19 | 37.9 | 76.0 |
| Carpet glue/carpet tape | 62 | 10 | 0 | 16.1 | 0 |
| External rails by front/rear access | 60 | 34 | 27 | 56.7 | 79.4 |
| Alterations to the house | 49 | 15 | 5 | 30.6 | 33.3 |
| Emergency alarms (e.g. pendants) | 40 | 4 | 1 | 10.0 | 25.0 |
| Mobility aids (walking stick) | 40 | 21 | 16 | 52.5 | 76.2 |
| Perching stool | 35 | 17 | 4 | 48.6 | 23.5 |
| Safety aids | 27 | 15 | 7 | 55.6 | 46.7 |
| Lightweight step ladder | 26 | 10 | 1 | 38.5 | 10.0 |
| Steps/half-steps | 22 | 10 | 7 | 45.5 | 70.0 |
| Removable bath board | 22 | 10 | 9 | 45.5 | 90.0 |
| Furniture raisers (e.g. bed/chair) | 19 | 7 | 6 | 36.8 | 85.7 |
| Sensor-operated lights in the house | 16 | 7 | 1 | 43.8 | 14.3 |
| Toilet frame | 15 | 7 | 7 | 46.7 | 100 |
| Key safe provision | 13 | 3 | 3 | 23.1 | 100 |
| Bath lift | 12 | 5 | 5 | 41.7 | 100 |
| Combination toilet frame and seat | 12 | 4 | 3 | 33.3 | 75.0 |
| Handybar for car transfers | 12 | 6 | 0 | 50.0 | 0 |
| Half-step with/without a handle | 11 | 4 | 0 | 36.4 | 0 |
| Raised toilet seat | 10 | 4 | 4 | 40.0 | 100 |
| Easy reach/grabber | 10 | 7 | 1 | 70.0 | 14.3 |
| Ferrules | 9 | 8 | 2 | 88.9 | 25.0 |
| Outdoor lights | 9 | 2 | 0 | 22.2 | 0 |
| Bed hoist | 8 | 6 | 6 | 75.0 | 100 |
| Light bulbs | 7 | 2 | 0 | 28.6 | 0 |
| Bed rail/lever | 5 | 4 | 0 | 80.0 | 0 |
| New bed | 3 | 0 | 0 | 0 | |
| Remote control plugs/lights | 3 | 1 | 0 | 33.3 | 0 |
| Assistive technology devices | 2 | 0 | 0 | 0 | |
| Visual prompts | 2 | 1 | 0 | 50.0 | 0 |
| Ramp | 1 | 0 | 0 | 0 | 0 |
| Walking aid parking devices | 1 | 0 | 0 | 0 | 0 |
The mean (SD) number of items installed that were recommended per participant was 0.47 (0.92) (minimum 0, maximum 6; median 0). The most commonly recommended equipment was a non-slip mat (161/382, 42%); 58% of participants who were recommended this implemented it, 26 of whom were supplied it by formal services. Installation of recommended items varied from 0% to 89%; however, the numbers of some items recommended were very small. It was clear from the data that some items were more readily available from health and social care than others, for example the majority of grab rails were provided to participants. It is not clear from the data available the extent of local variation in the provision of equipment by formal services. However, even when equipment was available from formal services, uptake was not universal. It is also important to note that even in the absence of formal provision of recommended items/alterations, some participants were motivated to follow recommendations independently, indicating that support from services was not always essential for equipment to be purchased and fitted for those who had the resources to do so. Overall adherence to the recommendations was relatively low; on average, participants installed 17% of the items that they were recommended.
Occupational therapists reported that the majority of participants were accommodating during the OTIS visits and willingly participated in completing the activities that OTs requested they do around the home to enable the assessment to be completed. However, there were a couple of instances during home visits where participants considered the level of questioning to be intrusive, which prevented OTs from delivering the intervention as intended:
I phone up [the participant] and she was aware that I was coming. But when I got there she was quite defensive . . . she lived in the really, really big house and you could see that there was a lot of things everywhere. I think she might have been a bit of a hoarder. So when I explained for the purpose of my visit and, you know to ask about the environmental, she said I don’t mind you asking me the questions but I don’t want you to look around my house.
OT 14, 192–198
Completing the full environmental assessment enabled OTs to give participants informed recommendations about how to improve their home environments to minimise their risk of future falls, for example by installing of a piece of equipment. Participants who responded well to this advice completed the suggested modifications to their environment and/or purchased the equipment. OTs considered responsive participants to be older and less active and have lower functioning:
The ones who were really struggling with their mobility and things they were really grateful of the advice and so yeah they definitely took it and were happy to go ahead with any equipment and recommendations like referrals to social services and things.
OT 07, 161–166
Where recommendations had not been actioned, this was sometimes because the participants needed assistance to make the modifications or because they did not consider themselves at risk of falling and/or did not highly prioritise falls prevention. OTs reported the latter group of participants to be less receptive to their advice, and described them as being younger and more active and high-functioning (i.e. more able to undertake activities of daily living within their environment):
I would say probably the more high-functioning patients were the ones who weren’t quite ready to accept the sort of equipment side of things. So I was telling them maybe about the rails that they could get or bits of equipment maybe to help them around the house and they’d be like, ‘oh yes I’ll bear that in mind but I don’t think I’m quite ready for that yet’.
OT 07, 161–166
The well-functioning sort of very middle-class, yeah, reasonably well-off sort of groups of people that were definitely involved and they then struggled to make that transition into seeing themselves on being the receiving end of a treatment.
OT 5, 256–259
Although ‘high-functioning’ participants were less receptive to acting on the OTs’ recommendations, the home visit presented OTs with the opportunity to provide participants with advice and signpost them to useful services they may need in the future. OTs reported that this information was positively received by many high-functioning participants.
Occupational therapists felt that the functioning and the demographic characteristics of some trial participants prevented the uptake of advice when OTs identified areas of modification, as the participants did not deem themselves to be at risk of falls:
[Some] people just saw it for what it was, just an assessment of them and I think some of that is about the cohort of people I felt we were seeing . . . I think sometimes why people were reluctant to accept the advice because they didn’t see it as something relevant to them because they were doing this for the good of others and yet actually when the advice was being passed to them, they weren’t able to make that link . . . At that point they didn’t see themselves as . . . requiring the feedback.
OT 5, 228–236
For some participants (those with greatest need), support from health and social services in implementing recommendations will most likely have influenced their adherence to some recommendations. However, the qualitative data do suggest that for a large proportion of the participants the most likely reason for the relatively low uptake of many of the OT recommendations was that they did not feel that they would benefit from these suggested changes.
Programme differentiation
Occupational therapists confirmed that an environmental falls assessment was implemented as part of their routine practice when conducting falls-related home visits. Some reported using the Home Fast to carry out the assessment. Overall, there was no tool that was standardised between trusts. Many OTs spoke of how the tools they used had been developed ‘in house’ and how the delivery time was relatively short because environmental checks formed only a small part of a broad, multifactorial assessment [e.g. including checks on medication(s), osteoporosis, alcohol, footwear, memory]; this theme was particularly apparent among OTs whose roles were more community service based as opposed to those whose roles were falls-specific. The fact that the OTIS intervention was not a holistic assessment was considered a disadvantage by some OTs:
In our normal practice we would also be looking a little more at the psychological elements of patients, so how their mood is, perhaps their memory is, what sort of participation level they have in these activities . . . are they actually engaged in functional tasks? So that element isn’t really captured in OTIS.
OT 01, 326–333
Some OTs also felt that the key areas of the OTIS intervention were not too dissimilar to those of their routine environmental assessments. However, most agreed that the OTIS intervention was far more comprehensive, and this was positively received, particularly by OTs who had fewer years of experience and/or among those who did not specialise in falls prevention:
It [the OTIS intervention] was really comprehension . . . our assessment is nowhere near as in-depth as that, so it was really good. It was a great prompt for certain things . . . I liked that it made you really think about every little detail that maybe you might miss sometimes.
OT 14
The multifactorial assessment doesn’t have a particularly brilliant environmental assessment . . . so this was an added extra which they [OTs] liked.
OT 17, 115–117
Occupational therapists who were highly experienced in falls management did not believe that their practice would change as a result of using the OTIS intervention, as they felt that the tools they already used in their routine practice, coupled with their experience, were just as effective. Some experienced OTs suggested the WeHSA may be a useful tool to use in certain circumstances, but not during every falls-related home visit. However, they recognised that the comprehensive nature of the OTIS intervention would be of particular benefit to OTs who did not work specifically in falls, OTs who were newly qualified or those who were training to be OTs:
I would see it as a great tool for say newly qualified OTs who are not familiar with doing this particular, you know, type of assessment, so it gives them more structure . . . but [for more experienced OTs] I think a tool in a toolbox.
OT 17, 294–295
I can’t say there’s been anything else that it’s changed my practice as such. I guess from just so many years from doing similar or elements of it, you know, I think it helps to have, as an OT, to have the experience of doing falls assessments and environmental assessment . . . so for another OT it might actually give them other ‘oh I hadn’t really thought about that before’, you know, in the detail.
OT 01, 366–376
A number of OTs were willing to incorporate the OTIS intervention into routine practice should the trial prove it to be effective. However, the main concerns were in relation to (1) the time the intervention took to deliver, which was twice as long as routine appointments, and (2) the cost to the trust purchasing it:
Cost is always something that the trust considers and if it’s an expensive tool the trust will quite often look for a cheaper alternative.
OT 14, 165–167
A number of OTs described how they had begun incorporating some elements of the OTIS intervention into their routine practice, particularly where they had learned something new. The visual and sensory elements of the OTIS intervention were particularly praised:
From using the Westmead I’ve . . . started veering off . . . from my original form . . . and thinking more about the Westmead to ask more questions that are in that I wouldn’t normally ask because I’ve found it really useful for kind of identifying different information.
OT 14, 109–113
I’ve become a lot more aware of generally sensory alterations, obviously can come within ageing. So I think I’ve incorporated a lot more of that into my current practice . . . I’m a lot better at looking at some of the elements of people’s environments to do with lighting and flooring than I probably used to be.
OT 5, 347–354
Context
It is clear from the data collected that the participating OTs were committed to delivering the OTIS intervention thoroughly and to as high a standard as possible. All of the OTs who participated in the interviews had volunteered to take part in OTIS. OTs saw the trial as an opportunity for professional development and were keen to learn something new that they could apply to their current practice to improve patient experience. Many OTs were keen to be involved in research that was different from their daily job role, acknowledging both that research on the topic of falls prevention was very limited and the gap in evidence that OTIS was aiming to address:
I was interested just to see if there was anything really that I could learn that I could then apply to my practice. But also I was just really interested to get involved in some research and to try something new and different. Certainly when I spoke to [the developer of the OTIS intervention] . . . she was just so passionate about this falls intervention and she really kind of sparked my interest and really made me keen to be involved.
OT 07, 22–29
However, as the data suggest, despite this commitment, the most significant barrier to delivering the intervention was the relatively high well-being of the trial participants. In the context of sustained national government austerity measures across England and Wales, OTs observed that the participants they were being asked to provide the intervention to were ‘poles apart’ (OT 11) in terms of functioning and demographics from patients/clients they would see on their ‘normal’ caseloads. In current clinical practice, OTs see only the more severe cases who, as well as being in poorer health, are also less likely to have other resources (e.g. financial) to compensate for any lack in functional impairment. This difference in the sociodemographic profiles of people who are generally in receipt of OT services and those of the trial participants may have been exacerbated by the nature of recruitment strategy for the trial; responding to a mail-shot favours those with lesser morbidity and deprivation:77
I did seem to get quite a lot of . . . well-kept houses, maybe more middle-class people.
OT 07, 249–254
Discussion
The data suggested that the OTs received adequate training and delivered the intervention mainly as intended. However, in some cases both OTs and the participants themselves questioned their suitability for participation in the trial. As is the case in routine practice, the OTs chose to adapt the intervention in certain cases and did not deliver the whole intervention in the standardised way to participants who did not have a significant falls risk. Similarly, some participants felt that they were too mobile or active and that other people would benefit more from the OT home assessment.
This was a pragmatic trial testing the effectiveness and cost-effectiveness of OT home assessment with individualised recommendations. The OTs’ recommendations were followed by participants to varying degrees. Qualitative work from the OT perspective provides some insight into why participants may not have adhered to the recommendations based on a number of factors, including perceived need for change, provision from formal services and the personal resources available to enable a recommendation independently. While most participants in this sample did not feel that they were in need of environmental changes as a result of their low perceived risk of falls, recommendations were followed more closely by those with greater morbidity. It is important to note that the provision of environmental adaptations by health or social care services is likely to have played a significant role in whether or not those with greatest need were able to follow recommendations.
Although adherence to the OT recommendations was relatively low, this reflects the reality of a real-world implementation of the intervention in the context of current service provision. Therefore, low adherence to the individualised recommendations should not detract from the main finding that the intervention was not found to be effective in this population group.
We have observed that recommendations are followed to varying degrees by participants, which was significantly influenced by whether the recommendations were provided by health or social care services; a smaller proportion of individuals followed the equipment recommendations independently. This is likely to be due to a range of factors, including a lack of resources, a lack of desire to accept environmental changes and, as discussed above, feeling that they would not benefit from the recommendations. It has been noted previously that individuals make their own decisions about whether or not to follow through with environmental recommendations based on their knowledge of environmental risks, which is usually related to experience, perceptions of the degree of risk involved in the subject of the recommendation, perceived ability to mediate the risk through behaviour and the degree of freedom the individual has in decision-making. Of course, the person’s perceived risk and the therapist’s evaluation of risk may not be the same. There may be greater resistance to implement a modification if the objective of the modification is preventative as opposed to facilitative of an immediate functional outcome. 78
We have drawn on a range of data sources to assess the fidelity of the intervention training and delivery. Observations and documentary audits were conducted by OTs on the trial team, which may have led to some bias in the assessment of training and quality of delivery; as OTs, they may have felt a ‘vested interest’ in scoring individuals from a shared professional group more positively than was warranted. However, for the observations this is unlikely, as the OTs involved in the observations did not have an existing working relationship with the intervention OTs and every attempt was made to make the intervention OTs feel at ease with the process, Of course, it is impossible to rule out the Hawthorne effect, that the intervention OTs were conducting the home visit more thoroughly because they were being observed, which may have resulted in the scores being artificially high. For the documentation audit, it is unlikely that this assessment was biased in favour of a positive score as it was conducted remotely and anonymously. All documents were examined by one OT, ensuring consistency, and were conducted before the results of the trial were known. Qualitative interviews were conducted by two researchers who did not have a background in occupational therapy and were outside the main trial research team and, therefore, did not have any preconceived bias for or against the intervention or the professional group. It is clear from the data that OTs participating in the qualitative interview study were comfortable with being candid in their feelings about the trial and the intervention being tested.
Chapter 6 Discussion
In this chapter, we report the results of OTIS, a large, modified cRCT assessing the clinical effectiveness and cost-effectiveness of a home hazard assessment and environmental modification delivered by OTs for the prevention of falls. In this discussion, we summarise our key findings, compare these with findings from previous studies and discuss the strengths and limitation of OTIS.
Summary of key findings
Trial population
This large, multicentre modified cRCT comprised 1331 community-dwelling men and women aged ≥ 65 years: 430 were randomised to the intervention group and 901 were randomised to the usual-care group. We exceeded our original sample size target of 1299 by 32 participants. Participants were recruited primarily from mail-outs from general practice surgeries, from the Yorkshire Health Study, or from YTU cohorts of participants from previous trials in similar populations who had agreed to be contacted about future research projects. Recruitment was more successful (based on the conversion rate from mail-out to randomisation) from the YTU previous trial cohorts than from the general practice surgeries or Yorkshire Health Study, that is, it was more successful among people with prior experience of, and engagement in, similar research.
Two-thirds of OTIS participants were women, and participants’ average age was 80 years. Three-quarters of participants had reported a fall within the past 12 months at baseline, and one-fifth of these had attended hospital because of a fall. However, in the qualitative interviews, several of the OTs commented that they considered several of the intervention participants to be less frail and more active than the population of patients who would be referred in clinical practice.
Primary outcome
In total, 2260 falls were reported. The average number of falls per participant was slightly higher in the intervention group (1.9, range 0–94 falls) than in the usual-care group (1.6, range 0–41 falls). The two groups provided data for a similar amount of time on average (338 days in the intervention group and 345 days in the usual-care group). Details of each reported fall were sought over the telephone, and at least some information was obtained for about 90% of the falls. Just over half the falls occurred indoors (53%), with the majority (86%) of these occurring inside the participant’s own home rather than on another premises. Superficial injuries or worse were sustained in about half of the falls, with 3% of the falls resulting in a broken bone, most predominantly in the wrist or hand. There were nine reported hip fractures. This fracture rate is a lot lower than that reported in the SCOOP trial,21 where 16.0% of participants reported a clinical fracture and 3.5% reported a hip fracture. However, the population, although recruited from GP surgeries and of a similar age (70–85 years), comprised women who were not taking an antiosteoporotic drug.
We found that the environmental assessment did not lead to a reduction in falls over 12 months of follow-up. In fact, we found weak evidence of a difference in falls, with the intervention group reporting about 17% more falls than the usual-care group (p = 0.07). History of falling was seen to be significantly associated with a threefold increase in subsequent falls.
When compliance with the intervention was accounted for using an instrumental variable CACE analysis approach, the estimate of the intervention effect was very similar to the ITT analysis, likely to be because the vast majority of intervention participants (nearly 90%) received an assessment. The findings were robust to other sensitivity analyses exploring missing data and therapist effects.
There are some possible contributing factors to the increased reporting of falls in the intervention group, relative to the usual-care group. First, following their home assessment visits, participants in the intervention group may have been more likely to be mindful of falls, to consider an event as a fall and to recall and report a fall on their falls calendars. Second, there may be some risk compensation, whereby the intervention participants experienced a heightened sense of confidence and immunity from falls after the OT visit and so, perhaps counterintuitively, increased their risk-taking behaviour and were actually more likely to fall. These are reasonable theories and may have played a role; however, we believe it is likely that the weak evidence of a difference in falls rate is a chance finding and that the intervention neither significantly increases nor decreases the chance of falls. This is supported by the sensitivity analysis in which Parkinson’s disease is adjusted for, a known predictor of falls. We observed a chance imbalance in the proportion of participants with Parkinson’s disease in the two groups, with participants in the intervention group more likely to have Parkinson’s disease than those in the usual-care group. Although only 23 participants in the randomised population had Parkinson’s disease, adjusting for this reduced the difference in falls rate between the groups so there was little evidence of a difference (p = 0.23).
The intervention may be more effective in a ‘higher-risk’ population. In hypothesis-generating subgroup analyses, a qualitative difference was observed in the treatment effect estimate within those participants who reported having had hospital treatment due to a fall in the 4 months prior to enrolling in the trial compared with those who had not. In the former subgroup, considered at ‘higher risk’ of falls, the intervention participants reported 14% fewer falls than usual-care participants. However, the trial was not powered to detect subgroup effects and the interaction was not statistically significant.
Secondary outcomes
There were no differences in any of the secondary outcomes. Just over half (56%) of all participants reported at least one fall and one-third reported more than one fall. At 12 months, approximately half of the trial participants, in both groups, reported that they worried about falling at least some of the time.
Sample size
Our original sample size calculation was based on a binary outcome rather than a count (number of falls). We have considered, post hoc, a sample size calculation based on negative binomial regression. In REFORM, our earlier trial, we observed a mean predicted falls rate, over 12 months, of 1.66 in the usual-care group and 1.45 in the intervention group (IRR 0.88; p = 0.16). The dispersion parameter was estimated at 1.34. In OTIS, we observed a mean predicted falls rate, over 12 months, of 1.76 in the usual-care group and 2.05 in the intervention group (IRR 1.17; p = 0.07). The dispersion parameter was estimated at 1.29. We estimate that, assuming a falls rate in the usual-care group of 1.7 and a dispersion parameter of 1.3, with 1331 participants (2 : 1 allocation) we had 90% power to detect a 23% decrease in falls (two-sided 5% significance). 79 Ultimately, given the parameters observed in OTIS, we estimate that we were powered at about 70% to detect the 17% increase in falls we detected.
Cost-effectiveness
The economic evaluation found an additional cost of £18.78 (95% CI £16.33 to £21.24) per participant associated with the intervention when compared with usual care, and 0.0042 fewer (–0.0043 to –0.0041) QALYs per participant, in the base-case analysis. Therefore, the intervention was found to be dominated by usual care as a result of the intervention being more expensive and generating worse health outcomes, in terms of QALYs and falls. On this basis, the economic findings from the present study indicate that the intervention does not represent a cost-effective option when compared with usual care. The differences in costs and QALYs between the two groups were, however, found to be small, and sensitivity analyses indicated that the findings were not robust to changes in the explored assumptions.
Intervention fidelity
The data suggested that the OTs received adequate training and delivered the intervention mainly as intended. However, in some cases, both OTs and the participants themselves questioned their suitability for participation in the trial. As is the case in routine practice, OTs chose to adapt the intervention in certain cases and did not deliver the whole intervention in the standardised way for participants who did not have a significant falls risk. Similarly, some participants felt that they were too mobile or active and that other people would benefit more from the OT home assessment.
This was a pragmatic trial testing the effectiveness and cost-effectiveness of OT home assessment with individualised recommendations. The OTs’ recommendations were followed by participants to varying degrees. Qualitative work from the OT perspective provides some insight into why participants may not have adhered to the recommendations based on a number of factors, including perceived need for change, provision from formal services and the personal resources available to enable a recommendation independently. While most participants in this sample did not feel they were in need of environmental changes due to low perceived risk of falls, for those with greater morbidity recommendations were followed more closely. It is important to note that the provision of environmental adaptations by health or social care services is likely to have played a significant role in whether or not those with greatest need were able to follow recommendations. Although participants’ adherence to the OT recommendations was relatively low, this reflects a real-world implementation of the intervention in the context of current service provision for this population group.
Comparison with previous studies
The latest Cochrane review of interventions for preventing falls in older people living in the community identified six trials evaluating home safety assessment and modification to prevent falls, with rate of falls reported as an outcome. These six trials involved 4208 participants and concluded that home safety assessment and modification was an effective approach to reducing falls (relative risk of falling 0.81, 95% CI 0.68 to 0.97). It further concluded that these interventions were more effective in people at higher risk of falling, including those with severe visual impairment, and if they were delivered by an OT.
The results of this present study, therefore, do not support these previous findings. Of the six trials identified in this review, all but one showed a reduction in the rate of falls in favour of the intervention group. However, only the three smallest trials80–82 reached a statistically significant reduction. Campbell et al. ’s trial of 391 community-dwelling participants reported a 41% reduction in the rate of falls in the intervention group, who received a home safety programme, compared with no home safety intervention (rate ratio 0.59, 95% CI 0.42 to 0.82). Although the participants were of a similar age to those in the OTIS trial, they all had severe visual impairment and, therefore, constituted a different population. Linn et al.’s trial of 200 participants of residents in an agricultural area in Taiwan reported a 54% reduction (rate ratio 0.46, 95% CI 0.22 to 0.95) in the rate of falls in a trial evaluating home safety assessment, education and exercise training. Again, the population was different from that in OTIS. As well as being slightly younger (mean age 77 years), they had also sustained more injurious falls, as one of the inclusion criteria for the study was that participants had to have required medical attention for a fall in the previous 4 weeks. Although 75% of OTIS participants reported a fall in the 12 months prior to recruitment, the falls that they had were not as serious, as only approximately 20% had attended hospital as a result of a fall during that time. Finally, the participants (n = 360) in Nikolaus et al.’s trial were of a similar age to those in the OTIS study (mean age 82 years); however, they were recruited from a hospital setting and had to have at least two chronic conditions in order to be eligible for the trial. In addition, the intervention was different in that intervention participants received two home visits rather than one. Although the other three larger trials83–85 show a reduction in rate of falls, the results do not reach statistical significance, and the findings are more in keeping with those of OTIS.
Our trial’s design was heavily influenced by a three-group pilot RCT by Pighills et al. 16 This study of 238 participants, of which 87 were randomised to OT assessment and 78 to the control (the remaining 73 were randomised to have a home assessment from a non-professional), found a statistically significant reduction in falls associated with receipt of OT home assessment. Our population was similar to that in Pighills et al. ’s study, with a similar mean age (79 years) and a similar proportion of women (two-thirds). OTIS did have a lower proportion of participants having a fall (56% vs. 66%), suggesting that we recruited a lower-risk population; however, the intervention was very similar. The primary outcome of the pilot trial was fear of falling, which showed no between-group difference, therefore, the original pilot trial may have had a chance finding on the secondary outcome of falls and the OTIS result is more likely to be closer to the ‘true’ effects of implementing OT home assessments among this population.
Strengths and limitations of the study
To our knowledge, this is the largest pragmatic trial evaluating the effectiveness and cost-effectiveness of home hazard assessment and environmental modification by an OT for the prevention of falls within the UK to date, randomising a total of 1331 participants. The strength of the trial lies in its methodological quality and rigour. The trial was prospectively registered and the protocol was published. The design allowed the recruitment of participants who were initially ineligible because they had not fallen and were not particularly concerned about falling, but later became eligible because they had fallen while part of the observational cohort. This process of rescreening led to the randomisation of 43 additional participants who would otherwise have been lost to recruitment. Randomisation was conducted by a secure, remote, web-based system with concealed allocation. Those in the usual-care group were unaware of the exact time at which they were randomised, and, in theory, this should have limited resentful demoralisation. The initial engagement of participants with the intervention was high, with 89% of intervention participants receiving a home visit. The use of a run-in period with primary outcome data collection could have reduced the incidence of post-randomisation attrition. Response rates to the primary outcome were high. In total, 1303 (98%) trial participants returned at least one falls calendar following randomisation, with similar proportions in each group. The number of participants returning a complete set of 12 months’ worth of calendars was large (91%); however, there was a 4% difference in response rate in favour of the usual-care group (intervention group, 88%; usual-care group, 92%). The falls calendars were designed to be completed and returned at regular, frequent (monthly) intervals to minimise the risk of recall bias and to encourage high response rates while not causing excessive participant burden. The trial was reported in line with CONSORT and other relevant guidelines. An independent (joint) Trial Steering and Data Monitoring and Ethics Committee helped ensure that the trial was conducted as planned and that participant safety issues were considered.
One potential limitation of the study is that the outcome data were participant self-reported, which may have led to inaccuracies in the number of reported falls and the perceived cause of the fall. A definition of a fall was included on the participant information sheet, the monthly falls calendars, and the newsletter sent to participants at three months post-randomisation. However, it was the participants who had to interpret an event as a fall, recall it, report it on their falls calendar, and return the falls calendar to YTU. It is possible that minor falls were underreported as they were not considered significant enough to report or were forgotten about; and equally plausible that some major falls were missed if they resulted in significant injury which caused the participant to be unable to complete and/or return their falls calendar. The increase in the number of falls in the intervention group could be explained by the following reasons. First, it could just be a chance finding; the p-value was not particularly small and CIs were relatively large. Second, it could be that additional reporting bias may have been introduced, as participants were aware of their group allocation. Intervention participants may have been more mindful of the falls they experienced and more motivated to recall and report them, leading to a greater number of reported falls within this group. Finally it is also possible that, having received an intervention for falls, participants felt more confident about managing their falls risk, which resulted in them engaging in more risk-taking behaviours and activities. 86,87 Conversely, they could have felt less confident and this made them more likely to fall. However, we were unable to explore this further as trial participants were not interviewed as part of the study. Furthermore, the assessment visit was designed to identify falls risks in the home environment, but about one-quarter of reported falls occurred outside, beyond the home. It is feasible that the intervention could not address the cause of these falls. Although adherence to receipt of the home visits was high, the extent to which intervention participants followed the subsequent recommendations was generally low. Some of the recommended environmental adaptations were provided by formal services, however, this was variable and may reflect regional inequalities in service provision. However, the intervention being tested was not designed to incorporate mechanisms to enhance adherence to recommendations (e.g. behaviour change elements or free provision of all recommended environmental adaptations) and reflects real-world implementation. We were unable to explore reasons from the participant perspective; however, we did gain some insights into this from the qualitative interviews with the intervention OTs. In addition to this, there appeared to be a focus on equipment provision with little evidence of OTs addressing risk-taking behaviours.
Generalisability of the results
OTIS was a pragmatic modified cRCT across eight trusts in England, which included urban and rural areas. Participants were recruited from either cohorts of participants who previously participated in YTU research (who were initially identified from general practices or podiatry clinics), from direct GP mail-out, from the Yorkshire Health Study, or from direct advertising. The eligibility criteria were kept as broad as possible, and the findings are generalisable to community-dwelling, ambulatory, older adults in England. The exclusion criteria were minimal (unable to walk 10 feet, unable to give informed consent or understand English, received an OT assessment in the previous 12 months). However, all trials are susceptible to volunteer bias, that is, the notion that people who participate in research projects are likely to be different from those who do not, and this is likely to be based on unmeasured characteristics. This issue may have been exacerbated in OTIS because half of the participants had already taken part in one of three completed trials in a similar population in which YTU was involved. These participants may be different again, and plausibly more likely to engage in the research and data collection processes, as they have prior experience of it.
Implications for health care
As the study did not find an effect on falls among this population, it is unlikely that patients who have similar characteristics to those in the trial would benefit from a home assessment visit by an OT.
Recommendations for research
As OT resources are scarce, further research evaluating the effectiveness of the intervention in a similar population to OTIS would be wasteful. OTIS aimed to recruit a high-risk population by including participants who had a history of falls in the previous year or had a fear of falling. However, the OTIS qualitative study and the risk profile of recruited participants indicated that the population recruited had a low to moderate risk of falls. Previous, smaller studies have found environmental assessment and modification to be effective only in higher-risk populations16,80,82,85 when delivered by OTs, with studies focusing on moderate- to lower-risk populations showing no statistically significant effect. 81,83,84 Future research could focus on a higher-risk population that better reflects those seen in clinical practice, such as multiple fallers, those who also use mobility aids, those who report poor balance and those who require assistance with activities of daily living. 88 In addition, further qualitative research to develop a greater understanding of the impact of home hazard identification and the value of the recommendations from the service user’s perspective could be undertaken. Falls prevention advice given by other professionals should be evaluated to see if it is effective and provides value for money.
Conclusion
We did not find any effect on the rate of self-reported falls and falling among a population of older people who had an elevated falls risk. Consequently, we do not recommend OT-led home assessment for people who have similar characteristics to the participants described in our study. Scarce OT resources would be better employed elsewhere.
Acknowledgements
The authors wish to thank the participants for taking part in the trial. We would like to acknowledge the support of the NIHR Clinical Research Network. We would specifically like to thank David Goodge and Rachel Herdsman at YTU, University of York, for their contribution to data collection and data management and their assistance with the day-to-day running of the study. Additionally, we would like to thank the NIHR HTA programme for funding OTIS.
We would also like to thank independent members of the Trial Steering Committee and Data Monitoring and Ethics Committee for their support and guidance throughout the trial: Professor Roger Francis (Chairperson; Emeritus Professor of Geriatric Medicine, Newcastle University), Professor Lindy Clemson (Professor in Ageing and Occupational Therapy, The University of Sydney), Dr Claire Ballinger (Principal Research Fellow, University of Southampton, with research interests in falls prevention), Professor Ranjit Lall (Professor of Clinical Trials and Biostatistics, University of Warwick) and Mrs Margaret McCabe (Patient Involvement Group member).
Our thanks are also given to the following individuals for their input as members of the OTIS patient involvement group: Mrs Margaret McCabe, Mr Peter Minns and Mr Peter Grinyer.
Finally, we would like to thank the following principal investigators, OTs and other staff from participating sites for their involvement with either recruitment or delivering the intervention: David Sweeting (Principal Investigator), Jessica Lorraine, Jenny Harper and Regan Hood from East Coast Community Healthcare; Caroline Lees (Principal Investigator), Ann Barrett, Suzanne Dee, Rebecca Johnson, Mark Teehan and Maria Wantling from East Sussex Healthcare NHS Trust; Anne Mann (Principal Investigator), Karen Mepham, Julie Gosling, Anna Rowe and Cath Rudman from Harrogate and District NHS Foundation Trust; Derek Raitt (Principal Investigator), Andy Bonner, Katie Husband and SallyAnn Long from Humber Teaching NHS Foundation Trust; Clair Jones (Principal Investigator) and Joanne Wiles from Leeds Community Healthcare NHS Trust; Keith Littlewood (Principal Investigator) and David Bunn from Northern Lincolnshire and Goole NHS Foundation Trust; Ali Madden-Fitzgibbon (Principal Investigator), Kath Dugmore, Louise Patterson and Kay Powell from Sheffield Teaching Hospitals NHS Foundation Trust; and Sophie Boyes (Principal Investigator), Sally Hayler, Jennifer Hughes and Ruth Kay from York Teaching Hospital NHS Foundation Trust.
Contributions of authors
Sarah Cockayne (https://orcid.org/0000-0002-1288-5497) (Research Fellow) was a co-applicant and the OTIS trial manager. She contributed to the grant application and trial protocol and was the lead for study management. She was involved in writing the initial version of the report and was responsible for co-ordinating the compilation, formatting and proofreading of the final report.
Alison Pighills (https://orcid.org/0000-0002-4172-2056) (Research Fellow) was a co-applicant and wrote the original pilot protocol. She contributed to the grant application and main trial protocol, developed and delivered the training, undertook a review of treatment fidelity and contributed to writing the report.
Joy Adamson (https://orcid.org/0000-0002-9860-0850) (Professor, Applied Health Research) was a co-applicant and co-chief investigator. She contributed to the development of the grant application and trial protocol, supervised the conduct and analysis of the fidelity and qualitative research, and contributed to writing the report.
Caroline Fairhurst (https://orcid.org/0000-0003-0547-462X) (Research Fellow, Statistician) was a co-applicant, contributed to the grant application and trial protocol, wrote the statistical analysis plan, conducted the statistical analysis and was involved in writing the initial version of the report.
Shelley Crossland (https://orcid.org/0000-0003-0271-3488) (Occupational Therapist) was a co-applicant, contributed to the grant application and trial protocol, delivered the training, undertook the clinical observations and contributed to writing the report.
Avril Drummond (https://orcid.org/0000-0003-1220-8354) (Professor of Healthcare Research and Occupational Therapist) was a co-applicant, contributed to the grant application and trial protocol, delivered the training and contributed to writing the report.
Catherine E Hewitt (https://orcid.org/0000-0002-0415-3536) (Professor in Statistics) was a co-applicant, contributed to the overall study design and implementation, supervised the statistical analyses and reviewed the final report.
Sara Rodgers (https://orcid.org/0000-0003-1757-541X) (Research Fellow) was a co-applicant and trial co-ordinator, and she assisted with the day-to-day management of the study.
Sarah J Ronaldson (https://orcid.org/0000-0001-8321-786X) (Research Fellow, Health Economics), wrote the health economics analysis plan, conducted the health economics analysis and wrote the health economics section of the report.
Jennifer McCaffery (https://orcid.org/0000-0003-4420-290X) (Research Fellow) was a trial co-ordinator, assisted with data collection and the day-to-day management of the study, and contributed to writing the report.
Katie Whiteside (https://orcid.org/0000-0002-7610-5429) (Research Fellow) was a trial co-ordinator, assisted with data collection and the day-to-day management of the study, and contributed to writing the report.
Arabella Scantlebury (https://orcid.org/0000-0003-3518-2740) (Research Fellow) undertook the qualitative interviews.
Lyn Robinson-Smith (https://orcid.org/0000-0001-6826-4545) (Research Fellow) undertook the qualitative analysis and contributed to writing the final report.
Ann Cochrane (https://orcid.org/0000-0002-1502-6719) (Research Fellow) was a trial co-ordinator and contributed to the overall data collection.
Sarah E Lamb (https://orcid.org/0000-0003-4349-7195) (Professor of Rehabilitation and Physiotherapist) was a co-applicant, contributed to the overall study design, provided expertise in the field of falls and contributed to writing the report.
Sophie Boyes (https://orcid.org/0000-0002-1878-4494) (Occupational Therapist) was a co-applicant and local principal investigator in one centre.
Simon Gilbody (https://orcid.org/0000-0002-8236-6983) (Professor of Psychological Medicine and Health Services Research) was a co-applicant and contributed to the overall study design.
Clare Relton (https://orcid.org/0000-0001-8530-5011) (Senior Research Fellow) was a co-applicant and contributed to the study design of the studies within a trial.
David J Torgerson (https://orcid.org/0000-0002-1667-4275) (Professor, Director of YTU, University of York) was the chief investigator for OTIS. He had overall responsibility for the design and implementation of the study and writing of the report, with final approval of the report submission.
All authors read and gave final approval of the manuscript.
Publications
Cockayne S, Pighills A, Adamson J, Fairhurst C, Drummond A, Hewitt C, et al. Can occupational therapist-led home environmental assessment prevent falls in older people? A modified cohort randomised controlled trial protocol. BMJ Open 2018;8:e022488.
McCaffery J, Mitchell A, Fairhurst C, Cockayne S, Rodgers, Relton C, Torgerson D. Does handwriting the name of a potential trial participant on an invitation letter improve recruitment rates? A randomised controlled study within a trial. F1000Res 2019;8:659.
Whiteside K, Flett L, Mitchell A, Fairhurst C, Cockayne S, Rodgers S, Torgerson D. Using pens as an incentive for trial recruitment of older adults: an embedded randomised controlled trial. F1000Res 2019;8:315.
Cochrane A, Welch C, Fairhurst C, Cockayne S, Torgerson D, et al. An evaluation of a personalised text message reminder compared to a standard text message on postal questionnaire response rates: an embedded randomised controlled trial. F1000Res 2020;9:154.
James S, Parker A, Cockayne S, Rodgers S, Fairhurst C, Torgerson D, et al. Including a pen and/or cover letter, containing social incentive text, had no effect on questionnaire response rate: a factorial randomised controlled study within a trial. F1000Res 2020;9:623.
Cockayne S, Pighills A, Fairhurst C, Adamson J, Crossland S, Drummond A, et al. Home hazard assessment and environmental modification to prevent falls in older people: the OTIS trial [version 1; peer review: awaiting peer review]. F1000Res 2021;10:500.
Data-sharing statement
Requests to access OTIS data should be made to the corresponding author and will be considered on a case-by-case basis by the Trial Management Group. All data requests will be managed in accordance with YTU, University of York, processes and procedures.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Cockayne S, Pighills A, Adamson J, Fairhurst C, Drummond A, Hewitt C, et al. Can occupational therapist-led home environmental assessment prevent falls in older people? A modified cohort randomised controlled trial protocol. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2018-022488.
- Cockayne S, Pighills A, Fairhurst C, Adamson J, Crossland S, Drummond A, et al. Home hazard assessment and environmental modification to prevent falls in older people: the OTIS trial. F1000Res 2021;10. https://doi.org/10.12688/f1000research.52313.1.
- Iglesias CP, Manca A, Torgerson DJ. The health-related quality of life and cost implications of falls in elderly women. Osteoporos Int 2009;20:869-78. https://doi.org/10.1007/s00198-008-0753-5.
- Morris M, Osborne D, Hill K, Kendig H, Lundgren-Lindquist B, Browning C, et al. Predisposing factors for occasional and multiple falls in older Australians who live at home. Aust J Physiother 2004;50:153-9. https://doi.org/10.1016/S0004-9514(14)60153-7.
- Campbell AJ, Borrie MJ, Spears GF, Jackson SL, Brown JS, Fitzgerald JL. Circumstances and consequences of falls experienced by a community population 70 years and over during a prospective study. Age Ageing 1990;19:136-41. https://doi.org/10.1093/ageing/19.2.136.
- Vetter NJ, Lewis PA, Ford D. Can health visitors prevent fractures in elderly people?. BMJ 1992;304:888-90. https://doi.org/10.1136/bmj.304.6831.888.
- Tideiksaar R. Fall prevention in the home. Top Geriatr Rehab 1987;3:57-64. https://doi.org/10.1097/00013614-198710000-00010.
- Office for National Statistics . Overview of the UK Population: July 2017 2015. www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/articles/overviewoftheukpopulation/july2017 (accessed 6 March 2019).
- British Orthopaedic Association . The Care of Patients With Fragility Fracture 2007.
- Pighills A, Drummond A, Crossland S, Torgerson DJ. What type of environmental assessment and modification prevents falls in community dwelling older people?. BMJ 2019;364. https://doi.org/10.1136/bmj.l880.
- Rubenstein LZ. Falls in older people: epidemiology, risk factors and strategies for prevention. Age Ageing 2006;35:ii37-41. https://doi.org/10.1093/ageing/afl084.
- Talbot LA, Musiol RJ, Witham EK, Metter EJ. Falls in young, middle-aged and older community dwelling adults: perceived cause, environmental factors and injury. BMC Public Health 2005;5. https://doi.org/10.1186/1471-2458-5-86.
- Gillespie LD, Robertson MC, Gillespie WJ, Sherrington C, Gates S, Clemson LM, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev 2012;9. https://doi.org/10.1002/14651858.CD007146.pub3.
- Todd CJ, Ballinger C, Whitehead S. A Global Report on Falls Prevention: Reviews of Socio-Demographic Factors Related to Falls and Environmental Interventions to Prevent Falls Amongst Older People Living in the Community 2007.
- National Institute for Health and Care Excellence . Falls in Older People: Assessing Risk and Prevention 2013. www.nice.org.uk/guidance/cg161 (accessed 13 May 2015).
- Pighills AC, Torgerson DJ, Sheldon TA, Drummond AE, Bland JM. Environmental assessment and modification to prevent falls in older people. J Am Geriatr Soc 2011;59:26-33. https://doi.org/10.1111/j.1532-5415.2010.03221.x.
- Relton C, Torgerson D, O’Cathain A, Nicholl J. Rethinking pragmatic randomised controlled trials: introducing the ‘cohort multiple randomised controlled trial’ design. BMJ 2010;340. https://doi.org/10.1136/bmj.c1066.
- Cockayne S, Rodgers S, Green L, Fairhurst C, Adamson J, Scantlebury A, et al. Clinical effectiveness and cost-effectiveness of a multifaceted podiatry intervention for falls prevention in older people: a multicentre cohort randomised controlled trial (the REducing Falls with ORthoses and a Multifaceted podiatry intervention trial). Health Technol Assess 2017;21. https://doi.org/10.3310/hta21240.
- Cockayne S, Adamson J, Corbacho Martin B, Fairhurst C, Hewitt C, Hicks K, et al. The REFORM study protocol: a cohort randomised controlled trial of a multifaceted podiatry intervention for the prevention of falls in older people. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2014-006977.
- Mitchell N, Hewitt C, Adamson J, Parrott S, Torgerson D, Ekers D, et al. A randomised evaluation of CollAborative care and active surveillance for Screen-Positive EldeRs with sub-threshold depression (CASPER): study protocol for a randomized controlled trial. Trials 2011;12. https://doi.org/10.1186/1745-6215-12-225.
- Shepstone L, Fordham R, Lenaghan E, Harvey I, Cooper C, Gittoes N, et al. A pragmatic randomised controlled trial of the effectiveness and cost-effectiveness of screening older women for the prevention of fractures: rationale, design and methods for the SCOOP study. Osteoporos Int 2012;23:2507-15. https://doi.org/10.1007/s00198-011-1876-7.
- Relton C, Bissell P, Smith C, Blackburn J, Cooper CL, Nicholl J, et al. South Yorkshire Cohort: a ‘cohort trials facility’ study of health and weight – protocol for the recruitment phase. BMC Public Health 2011;11. https://doi.org/10.1186/1471-2458-11-640.
- Whiteside KE, Flett L, Mitchell AS, Fairhurst CM, Cockayne ES, Rodgers SA, et al. Using pens as an incentive for trial recruitment of older adults: an embedded randomised controlled trial. F1000 Res 2019;8. https://doi.org/10.12688/f1000research.18300.1.
- McCaffery J, Mitchell AS, Fairhurst C, Cockayne S, Rodgers S, Relton C, et al. OTIS Study Team . Does handwriting the name of a potential trial participant on an invitation letter improve recruitment rates? A randomised controlled study within a trial. F1000 Res 2019;8:1-11. https://doi.org/10.12688/f1000research.18939.1.
- Cochrane A, Welch C, Fairhurst CM, Cockayne S, Torgerson DJ. An evaluation of a personalised text message reminder compared to a standard text message on postal questionnaire response rates: an embedded randomised controlled trial [published online ahead of print February 26 2020]. F1000 Res 2020. https://doi.org/10.12688/f1000research.22361.1.
- James SJ, Parker A, Cockayne S, Rodgers SA, Fairhurst CM, Torgerson DJ, et al. Including a pen and/or cover letter, containing social incentive text, had no effect on questionnaire response rate: a factorial randomised controlled study within a trial [version 1; peer review: awaiting peer review]. F1000 Res 2020;9. https://doi.org/10.12688/f1000research.23767.1.
- Age UK . Staying Steady 2015.
- Clemson L, Fitzgerald MH, Heard R, Cumming RG. Inter-rater reliability of a home fall hazards assessment tool. Occup Ther J Res 1999;19:83-98. https://doi.org/10.1177/153944929901900201.
- Podsiadlo D, Richardson S. The timed ‘Up & Go’: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142-8. https://doi.org/10.1111/j.1532-5415.1991.tb01616.x.
- Law M, Cooper B, Strong S, Stewart D, Rigby P, Letts L. The person-environment-occupation model: a transactive approach to occupational performance. Can J Occup Ther 1996;63:9-23. https://doi.org/10.1177/000841749606300103.
- Martino S, Ball SA, Nich C, Canning-Ball M, Rounsaville BJ, Carroll KM. Teaching community program clinicians motivational interviewing using expert and train-the-trainer strategies. Addiction 2011;106:428-41. https://doi.org/10.1111/j.1360-0443.2010.03135.x.
- Gaskill D, Isenring EA, Black LJ, Hassall S, Bauer JD. Maintaining nutrition in aged care residents with a train-the-trainer intervention and Nutrition Coordinator. J Nutr Health Aging 2009;13:913-17. https://doi.org/10.1007/s12603-009-0251-2.
- Clemson LM. iSOLVE Project . Falls Prevention Online Workshops 2016. https://fallspreventiononlineworkshops.com.au/ (accessed 5 December 2019).
- Lamb SE, Jørstad-Stein EC, Hauer K, Becker C. Prevention of Falls Network Europe and Outcomes Consensus Group . Development of a common outcome data set for fall injury prevention trials: the Prevention of Falls Network Europe consensus. J Am Geriatr Soc 2005;53:1618-22. https://doi.org/10.1111/j.1532-5415.2005.53455.x.
- Bruce J, Lall R, Withers EJ, Finnegan S, Underwood M, Hulme C, et al. A cluster randomised controlled trial of advice, exercise or multifactorial assessment to prevent falls and fractures in community-dwelling older adults: protocol for the prevention of falls injury trial (PreFIT). BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2015-009362.
- Sussman JB, Hayward RA. An IV for the RCT: using instrumental variables to adjust for treatment contamination in randomised controlled trials. BMJ 2010;340. https://doi.org/10.1136/bmj.c2073.
- Dunn G. Instrumental Variables. Chichester: John Wiley & Sons; 2005.
- Hewitt CE, Torgerson DJ, Miles JNV. Is there another way to take account of noncompliance in randomized controlled trials?. CMAJ 2006;175:347-8. https://doi.org/10.1503/cmaj.051625.
- Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr 1974;19:716-23. https://doi.org/10.1109/TAC.1974.1100705.
- Andersen PK, Gill RD. Cox’s regression model for counting processes: a large sample study. Ann Stat 1982;10:1100-20. https://doi.org/10.1214/aos/1176345976.
- Brazier J, Ratcliffe J, Salomon JA, Tsuchiya A. Measuring and Valuing Health Benefits for Economic Evaluation. Oxford: Oxford University Press; 2007.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 2013.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- National Institute for Health and Care Excellence . Position Statement on Use of the EQ-5D-5L Valuation Set for England (Updated November 2018) 2018. www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l (accessed 6 January 2020).
- Billingham LJ, Abrams KR, Jones DR. Methods for the analysis of quality-of-life and survival data in health technology assessment. Health Technol Assess 1999;3. https://doi.org/10.3310/hta3100.
- Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ 1990;300:230-5. https://doi.org/10.1136/bmj.300.6719.230.
- Manca A, Palmer S. Handling missing data in patient-level cost-effectiveness analysis alongside randomised clinical trials. Appl Health Econ Health Policy 2005;4:65-7. https://doi.org/10.2165/00148365-200504020-00001.
- Age UK . Paying for Care 2020. www.ageuk.org.uk/information-advice/care/paying-for-care/ (accessed 18 May 2020).
- East Riding of Yorkshire Council . Paying for Care Services 2020. www.eastriding.gov.uk/living/care-and-support-for-adults/social-care-services/paying-for-care-services/ (accessed 18 May 2020).
- Department of Health and Social Care . NHS Reference Costs 2017 18 2018. https://improvement.nhs.uk/resources/reference-costs/ (accessed 6 January 2020).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: Personal Social Services Research Unit, University of Kent; 2018.
- NHS . Care and Support You Can Get for Free 2018. www.nhs.uk/conditions/social-care-and-support-guide/care-services-equipment-and-care-homes/care-and-support-you-can-get-for-free/ (accessed 21 May 2020).
- NHS . Household Gadgets and Equipment to Make Life Easier 2018. www.nhs.uk/conditions/social-care-and-support-guide/care-services-equipment-and-care-homes/household-gadgets-and-equipment-to-make-life-easier/ (accessed 21 May 2020).
- DLF Shaw Trust . Living Made Easy: Trusted Comparison Site for Independent Living Equipment 2020. www.livingmadeeasy.org.uk/ (accessed 6 January 2020).
- DLF Shaw Trust . AskSARA: Full List of Factsheets 2019. www.dlf.org.uk/content/full-list-factsheets (accessed 6 January 2020).
- Drummond M, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Briggs AH. Handling uncertainty in cost-effectiveness models. PharmacoEconomics 2000;17:479-500. https://doi.org/10.2165/00019053-200017050-00006.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work?. Int J Methods Psychiatr Res 2011;20:40-9. https://doi.org/10.1002/mpr.329.
- Rubin DB. Multiple imputation after 18+ years. J Am Stat Assoc 1996;91:473-89. https://doi.org/10.1080/01621459.1996.10476908.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. Hoboken, NJ: John Wiley; 1987.
- Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making 1998;18:S68-80. https://doi.org/10.1177/0272989X98018002S09.
- Fenwick E, O’Brien BJ, Briggs A. Cost-effectiveness acceptability curves – facts, fallacies and frequently asked questions. Health Econ 2004;13:405-15. https://doi.org/10.1002/hec.903.
- Scantlebury A, Cockayne S, Fairhurst C, Rodgers S, Torgerson D, Hewitt C, et al. Qualitative research to inform hypothesis testing for fidelity-based sub-group analysis in clinical trials: lessons learnt from the process evaluation of a multifaceted podiatry intervention for falls prevention. Trials 2020;21. https://doi.org/10.1186/s13063-020-04274-6.
- Mars T, Ellard D, Carnes D, Homer K, Underwood M, Taylor SJ. Fidelity in complex behaviour change interventions: a standardised approach to evaluate intervention integrity. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-003555.
- French SD, Green SE, Francis JJ, Buchbinder R, O’Connor DA, Grimshaw JM, et al. Evaluation of the fidelity of an interactive face-to-face educational intervention to improve general practitioner management of back pain. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-007886.
- Toomey E, Matthews J, Hurley DA. Using mixed methods to assess fidelity of delivery and its influencing factors in a complex self-management intervention for people with osteoarthritis and low back pain. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-015452.
- Dusenbury L, Brannigan R, Falco M, Hansen WB. A review of research on fidelity of implementation: implications for drug abuse prevention in school settings. Health Educ Res 2003;18:237-56. https://doi.org/10.1093/her/18.2.237.
- Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P. CONSORT Group . Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med 2008;148:295-309. https://doi.org/10.7326/0003-4819-148-4-200802190-00008.
- Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol 2004;23:443-51. https://doi.org/10.1037/0278-6133.23.5.443.
- Carroll C, Patterson M, Wood S, Booth A, Rick J, Balain S. A conceptual framework for implementation fidelity. Implement Sci 2007;2. https://doi.org/10.1186/1748-5908-2-40.
- Hasson H. Systematic evaluation of implementation fidelity of complex interventions in health and social care. Implement Sci 2010;5. https://doi.org/10.1186/1748-5908-5-67.
- Low J. A pragmatic definition of the concept of theoretical saturation. Sociol Focus 2019;52:131-9. https://doi.org/10.1080/00380237.2018.1544514.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Bonevski B, Randell M, Paul C, Chapman K, Twyman L, Bryant J, et al. Reaching the hard-to-reach: a systematic review of strategies for improving health and medical research with socially disadvantaged groups. BMC Med Res Methodol 2014;14. https://doi.org/10.1186/1471-2288-14-42.
- Clemson L, Cusick A, Fozzard C. Managing risk and exerting control: determining follow through with falls prevention. Disabil Rehabil 1999;21:531-41. https://doi.org/10.1080/096382899297189.
- Zhu H, Lakkis H. Sample size calculation for comparing two negative binomial rates. Stat Med 2014;33:376-87. https://doi.org/10.1002/sim.5947.
- Campbell AJ, Robertson MC, La Grow SJ, Kerse NM, Sanderson GF, Jacobs RJ, et al. Randomised controlled trial of prevention of falls in people aged > or = 75 with severe visual impairment: the VIP trial. BMJ 2005;331. https://doi.org/10.1136/bmj.38601.447731.55.
- Lin MR, Wolf SL, Hwang HF, Gong SY, Chen CY. A randomized, controlled trial of fall prevention programs and quality of life in older fallers. J Am Geriatr Soc 2007;55:499-506. https://doi.org/10.1111/j.1532-5415.2007.01146.x.
- Nikolaus T, Bach M. Preventing falls in community-dwelling frail older people using a home intervention team (HIT): results from the randomized Falls-HIT trial. J Am Geriatr Soc 2003;51:300-5. https://doi.org/10.1046/j.1532-5415.2003.51102.x.
- Stevens M, Holman CD, Bennett N. Preventing falls in older people: impact of an intervention to reduce environmental hazards in the home. J Am Geriatr Soc 2001;49:1442-7. https://doi.org/10.1046/j.1532-5415.2001.4911235.x.
- Day L, Fildes B, Gordon I, Fitzharris M, Flamer H, Lord S. Randomised factorial trial of falls prevention among older people living in their own homes. BMJ 2002;325. https://doi.org/10.1136/bmj.325.7356.128.
- Cumming RG, Thomas M, Szonyi G, Salkeld G, O’Neill E, Westbury C, et al. Home visits by an occupational therapist for assessment and modification of environmental hazards: a randomized trial of falls prevention. J Am Geriatr Soc 1999;47:1397-402. https://doi.org/10.1111/j.1532-5415.1999.tb01556.x.
- Haines TP, Lee DC, O’Connell B, McDermott F, Hoffmann T. Why do hospitalized older adults take risks that may lead to falls?. Health Expect 2015;18:233-49. https://doi.org/10.1111/hex.12026.
- Moreira NB, Rodacki ALF, Pereira G, Bento PCB. Does functional capacity, fall risk awareness and physical activity level predict falls in older adults in different age groups?. Arch Gerontol Geriatr 2018;77:57-63. https://doi.org/10.1016/j.archger.2018.04.002.
- Lusardi MM, Fritz S, Middleton A, Allison L, Wingood M, Phillips E, et al. Determining risk of falls in community dwelling older adults: a systematic review and meta-analysis using posttest probability. J Geriatr Phys Ther 2017;40:1-36. https://doi.org/10.1519/JPT.0000000000000099.
- B&Q . Light Bulb 2020. www.diy.com/departments/diall-e27-10w-806lm-gls-warm-white-led-light-bulb/3663602667216_BQ.prd (accessed 6 January 2020).
Appendix 1 Regulatory approvals
| Research site | Date R&D issued confirmation of capacity and capability |
|---|---|
| Humber Teaching NHS Foundation Trust | 31 October 2016 |
| Harrogate and District NHS Foundation Trust | 9 November 2016 |
| Northern Lincolnshire and Goole NHS Foundation Trust | 30 November 2016 |
| Sheffield Teaching Hospitals NHS Foundation Trust | 31 January 2017 |
| East Coast Community Healthcare | 3 March 2017 |
| York Teaching Hospital NHS Foundation Trust | 20 April 2017 |
| East Sussex Healthcare NHS Trust | 27 April 2017 |
| Leeds Community Healthcare NHS Trust | 28 December 2017 |
Appendix 2 Recruitment methods
| Trust | Recruitment method | |||||
|---|---|---|---|---|---|---|
| Mail-out from cohorts | Mail-out from general practices in the area | Advertising | Opportunistic screening | |||
| Faith magazine | Posters at libraries/general practices | U3A | ||||
| East Coast Community Healthcare | ✓ | ✓ | ||||
| East Sussex Healthcare NHS Trust | ✓ | ✓ | ✓ | |||
| Harrogate and District NHS Foundation Trust | ✓ | ✓ | ✓ | ✓ | ||
| Leeds Community Healthcare NHS Trust | ✓ | |||||
| Humber Teaching NHS Foundation Trust | ✓ | ✓ | ||||
| Northern Lincolnshire and Goole NHS Foundation Trust | ✓ | ✓ | ✓ | |||
| Sheffield Teaching Hospitals NHS Foundation Trust | ✓ | ✓ | ✓ | ✓ | ||
| York Teaching Hospital NHS Foundation Trust | ✓ | ✓ | ✓ | ✓ | ||
Appendix 3 Unit costs of equipment items recommended/installed by occupational therapists at OTIS home visits
| Equipment item | Unit cost (£) | Source |
|---|---|---|
| Steps/half-steps | 72 | Unit Costs of Health and Social Care 201852 (create step to front/back door) |
| Outdoor lights | 293 | Unit Costs of Health and Social Care 201852 (install lighting to outside steps/path) |
| External rails by front/rear accessa | 25 | Unit Costs of Health and Social Care 201852 (fit external handrail) |
| Rampa | 657 | Unit Costs of Health and Social Care 201852 (create ramp to front/back door) |
| Wheelchair | 797 | Unit Costs of Health and Social Care 201852 (active user wheelchair plus maintenance cost) |
| Grab rails/bannistera | 34 | Unit Costs of Health and Social Care 201852 (fit internal handrail) |
| Mobility aids (walking stick)b | 12 | Living Made Easy55 |
| Furniture raisers (e.g. bed and chair) | 25 | Living Made Easy55 |
| Shower rails/bath safety barsa | 23 | Unit Costs of Health and Social Care 201852 (handrail to bath) |
| Bath lift | 228 | Living Made Easy55 |
| Removable bath board | 31 | Living Made Easy55 |
| Raised toilet seat | 30 | Living Made Easy55 |
| Combination toilet frame and seat | 40 | Living Made Easy55 |
| Toilet frame | 36 | Living Made Easy55 |
| Half-step with/without a handle | 30 | Living Made Easy55 |
| Safety aids (rubber mat)c | 6 | Living Made Easy55 |
| Sensor-operated lights in the house | 293 | Assumed same cost as outdoor lights |
| Remote control plugs/lights | 25 | Living Made Easy55 |
| Emergency alarms (including pendant alarms and falls detectors) | 96 | Living Made Easy55 |
| Assistive technology devices (including motion sensors and motion-activated voice alerts) | 20 | Living Made Easy55 |
| Visual prompts | 30 | Living Made Easy55 |
| Light bulbs | 9 | B&Q89 (assumed two light bulbs) |
| Bed hoist | 144 | Living Made Easy55 |
| New bed | 714 | Living Made Easy55 |
| Easy reach/grabber | 12 | Living Made Easy55 |
| Key safe provision | 19 | Living Made Easy55 |
| Ferrules | 10 | Living Made Easy55 |
| Walking aid parking devices | 30 | Living Made Easy55 |
| Lightweight step ladder | 50 | Living Made Easy55 |
| Carpet glue/reflective anti-slip tape/adhesive carpet tape | 13 | Living Made Easy55 |
| Alterations to the housed | 235 | Living Made Easy,55 Unit Costs of Health and Social Care 201852 |
Appendix 4 Unit costs of ‘other’ items recommended/installed
| Equipment itema | Unit cost (£) | Source |
|---|---|---|
| Other 1: non-slip mat | 50.34 | Living Made Easy55 |
| Other 2: trolley (e.g. for kitchen) | 39.60 | Living Made Easy55 |
| Other 3: perching stool | 25.20 | Living Made Easy55 |
| Other 4: handybar for car transfers | 25.20 | Living Made Easy55 |
| Other 5: bed rail/lever | 30.00 | Living Made Easy55 |
| Average ‘other’ cost | 34.07 |
Appendix 5 Number and proportion of participants with complete-case data by group
| Time point | Intervention group (N = 430), n (%) | Usual-care group (N = 901), n (%) |
|---|---|---|
| Baseline | 234 (54.4) | 500 (55.5) |
| 4 months | 254 (59.1) | 554 (61.5) |
| 8 months | 252 (58.6) | 524 (58.2) |
| 12 months | 239 (55.6) | 512 (56.8) |
| Total trial duration | 121 (28.1) | 291 (32.3) |
Appendix 6 Additional missing data information
| Variable/value | OR in logistic regression for missing data (95% CI) | |
|---|---|---|
| Missing cost data at 12 months | Missing QALY data at 12 months | |
| Baseline variables | ||
| Sex (female) | 1.39 (1.08 to 1.78)a | 0.99 (0.69 to 1.42) |
| Age | 1.05 (1.03 to 1.07)a | 1.06 (1.03 to 1.09)a |
| History of falling | 1.01 (0.78 to 1.31) | 2.22 (1.56 to 3.15)a |
| EQ-5D-5L at baseline | 0.14 (0.07 to 0.28)a | 0.16 (0.07 to 0.36)a |
| Treatment allocation (intervention vs. usual care) | 1.18 (0.91 to 1.54) | 1.31 (0.91 to 1.88) |
| Observed values | ||
| QALYs at 4 months | 0.001 (0.0001 to 0.012)a | 0.01 (0.0003 to 0.36)a |
| QALYs at 8 months | 0.0015 (0.00019 to 0.013)a | 0.02 (0.0009 to 0.28)a |
| Costs at 4 months | 1.0002 (0.9998 to 1.0005) | 1.0005 (1.0002 to 1.0009)a |
| Costs at 8 months | 1.0001 (0.99992 to 1.0003) | 1.0002 (0.99996 to 1.0003) |
FIGURE 13.
Comparison of the distribution of imputed values (imputation number 1 to 10) with the observed data (imputation number 0) for costs.
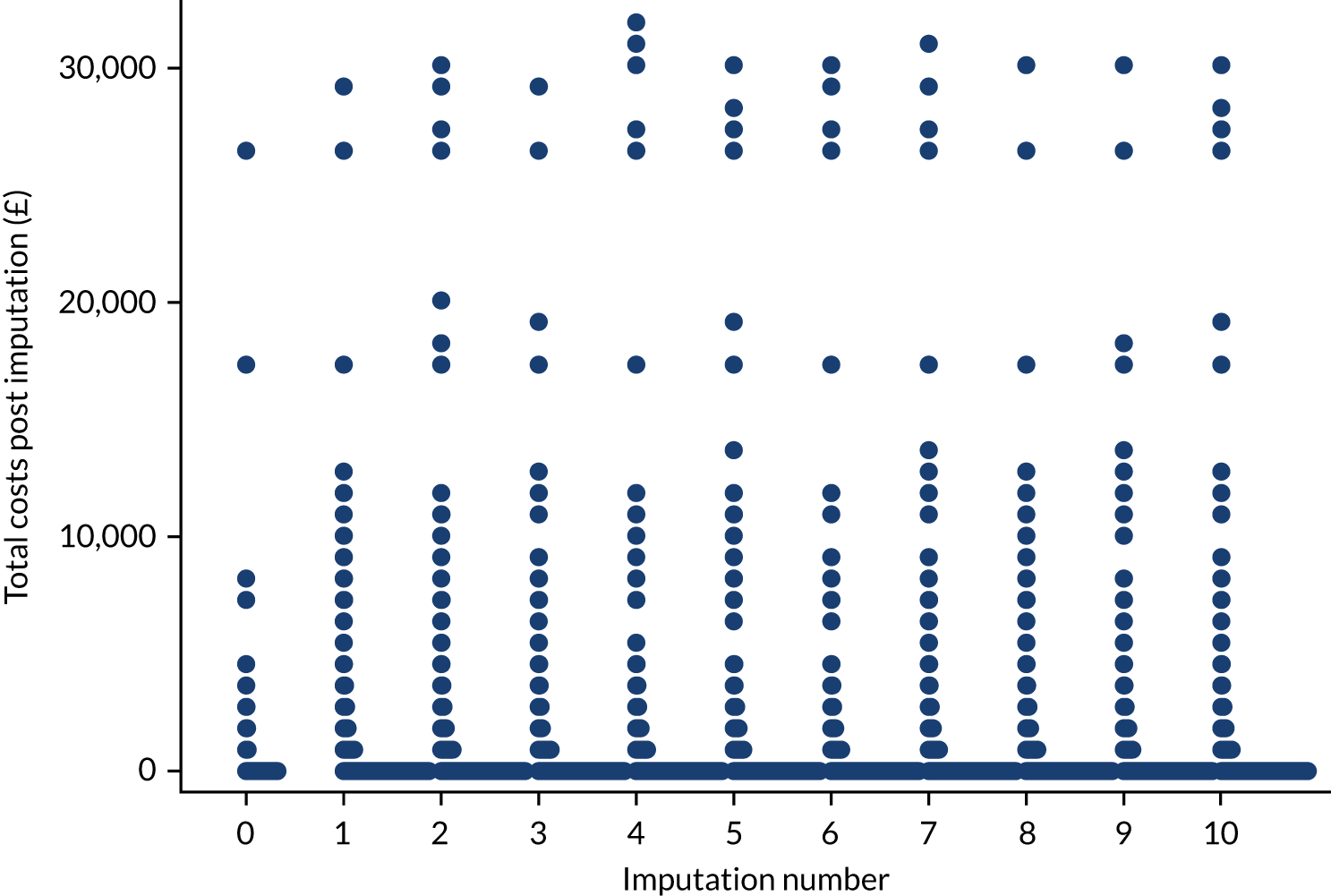
FIGURE 14.
Comparison of the distribution of imputed values (imputation number 1 to 10) with the observed data (imputation number 0) for QALYs.
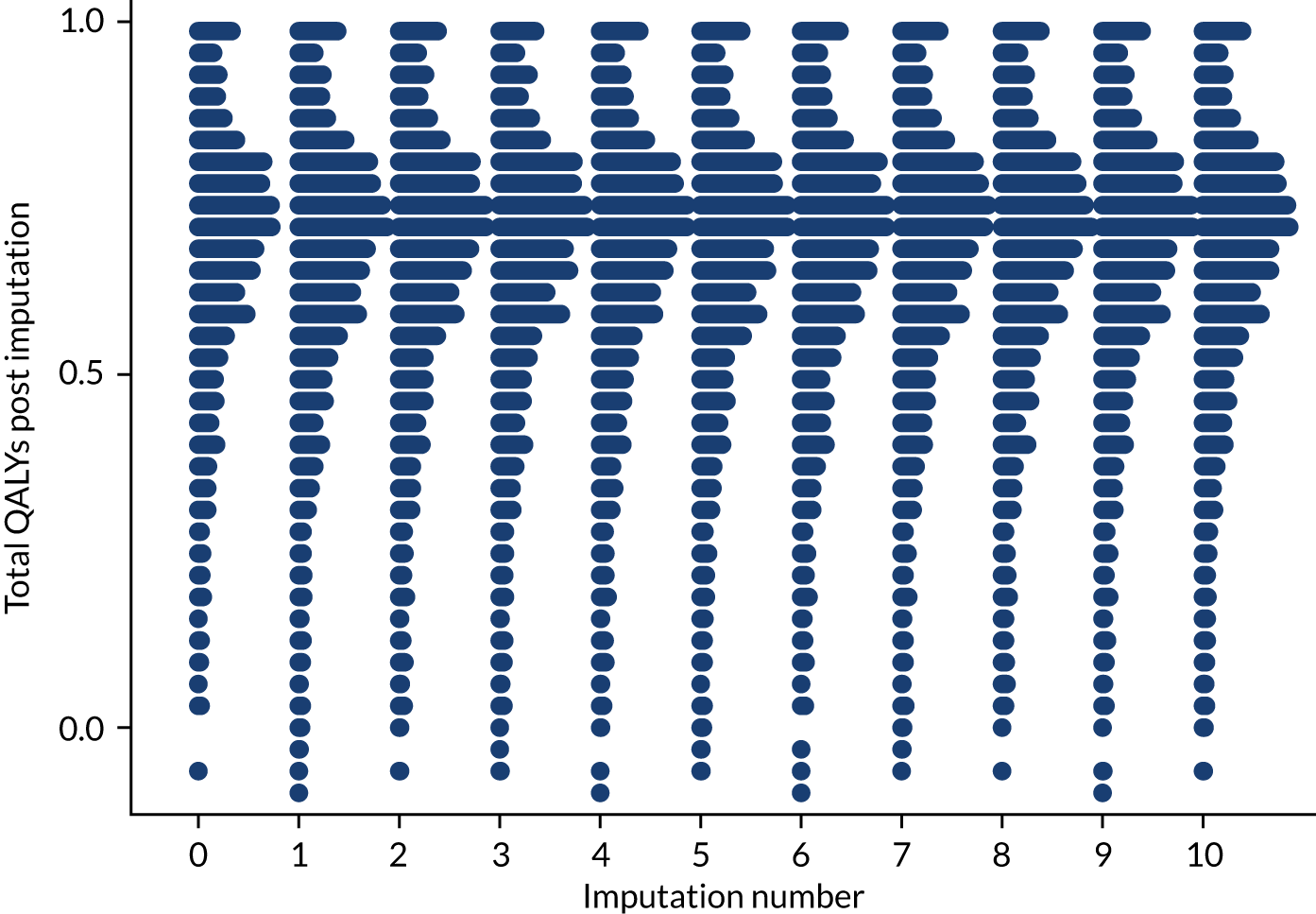
Appendix 7 Completion and missingness of EQ-5D-5L questionnaires
| Time point | Completed EQ-5D-5L, n (%) | Missing EQ-5D-5L (≥ 1 dimension missing), n (%) | ||
|---|---|---|---|---|
| Intervention group (N = 430) | Usual-care group (N = 901) | Intervention group (N = 430) | Usual-care group (N = 901) | |
| Baseline | 398 (92.6) | 840 (93.2) | 32 (7.4) | 61 (6.8) |
| 4 months | 394 (91.6) | 845 (93.8) | 36 (8.4) | 56 (6.2) |
| 8 months | 386 (89.8) | 823 (91.3) | 44 (10.2) | 78 (8.7) |
| 12 months | 371 (86.3) | 815 (90.5) | 59 (13.7) | 86 (9.5) |
Appendix 8 Number of missing dimensions in invalid EQ-5D-5L questionnaires
| Time point | Number of missing EQ-5D-5L dimensions | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention group | Usual-care group | |||||||||
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |
| Baseline | 21 | 9 | 0 | 0 | 2 | 48 | 10 | 2 | 1 | 0 |
| 4 months | 8 | 1 | 0 | 0 | 27 | 15 | 0 | 0 | 0 | 41 |
| 8 months | 6 | 1 | 1 | 0 | 36 | 14 | 2 | 1 | 0 | 61 |
| 12 months | 5 | 1 | 0 | 0 | 53 | 9 | 0 | 0 | 0 | 77 |
Appendix 9 Proportion reporting EQ-5D-5L levels 1 to 5 by dimension, group and time point
| EQ-5D-5L dimension | Health state severitya | Baseline, n (%) | 4 months, n (%) | 8 months, n (%) | 12 months, n (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention group | Usual-care group | Intervention group | Usual-care group | Intervention group | Usual-care group | Intervention group | Usual-care group | ||
| Mobility | Level 1 | 137 (31.9) | 328 (36.4) | 131 (30.5) | 278 (30.9) | 127 (29.5) | 267 (29.6) | 110 (25.6) | 250 (27.7) |
| Level 2 | 121 (28.1) | 229 (25.4) | 111 (25.8) | 255 (28.3) | 103 (24.0) | 237 (26.3) | 104 (24.2) | 233 (25.9) | |
| Level 3 | 125 (29.1) | 245 (27.2) | 119 (27.7) | 250 (27.7) | 115 (26.7) | 249 (27.6) | 119 (27.7) | 254 (28.2) | |
| Level 4 | 41 (9.5) | 91 (10.1) | 41 (9.5) | 69 (7.7) | 44 (10.2) | 79 (8.8) | 42 (9.8) | 79 (8.8) | |
| Level 5 | 0 (0.0) | 4 (0.4) | 0 (0.0) | 4 (0.4) | 1 (0.2) | 4 (0.4) | 0 (0.0) | 7 (0.8) | |
| Missing | 6 (1.4) | 4 (0.4) | 28 (6.5) | 45 (5.0) | 40 (9.3) | 65 (7.2) | 55 (12.8) | 78 (8.7) | |
| Number (%) reporting any problemsb | 287 (67.7) | 569 (63.4) | 271 (67.4) | 578 (67.5) | 263 (67.4) | 569 (68.1) | 265 (70.7) | 573 (69.6) | |
| Self-care | Level 1 | 316 (73.5) | 642 (71.3) | 283 (65.8) | 619 (68.7) | 267 (62.1) | 605 (67.1) | 261 (60.7) | 561 (62.3) |
| Level 2 | 72 (16.7) | 163 (18.1) | 74 (17.2) | 162 (18.0) | 79 (18.4) | 145 (16.1) | 68 (15.8) | 166 (18.4) | |
| Level 3 | 27 (6.3) | 65 (7.2) | 34 (7.9) | 65 (7.2) | 32 (7.4) | 76 (8.4) | 42 (9.8) | 81 (9.0) | |
| Level 4 | 8 (1.9) | 9 (1.0) | 7 (1.6) | 5 (0.6) | 8 (1.9) | 6 (0.7) | 6 (1.4) | 10 (1.1) | |
| Level 5 | 0 (0.0) | 2 (0.2) | 1 (0.2) | 4 (0.4) | 3 (0.7) | 2 (0.2) | 0 (0.0) | 3 (0.3) | |
| Missing | 7 (1.6) | 20 (2.2) | 31 (7.2) | 46 (5.1) | 41 (9.5) | 67 (7.4) | 53 (12.3) | 80 (8.9) | |
| Number (%) reporting any problemsb | 107 (25.3) | 239 (27.1) | 116 (29.1) | 236 (27.6) | 122 (31.4) | 229 (27.5) | 116 (30.8) | 260 (31.7) | |
| Usual activities | Level 1 | 154 (35.8) | 336 (37.3) | 140 (32.6) | 296 (32.9) | 131 (30.5) | 290 (32.2) | 123 (28.6) | 268 (29.7) |
| Level 2 | 146 (34.0) | 283 (31.4) | 133 (30.9) | 302 (33.5) | 141 (32.8) | 281 (31.2) | 116 (27.0) | 280 (31.1) | |
| Level 3 | 92 (21.4) | 200 (22.2) | 98 (22.8) | 193 (21.4) | 89 (20.7) | 202 (22.4) | 104 (24.2) | 204 (22.6) | |
| Level 4 | 28 (6.5) | 56 (6.2) | 26 (6.0) | 53 (5.9) | 21 (4.9) | 57 (6.3) | 28 (6.5) | 59 (6.5) | |
| Level 5 | 5 (1.2) | 12 (1.3) | 5 (1.2) | 15 (1.7) | 11 (2.6) | 7 (0.8) | 5 (1.2) | 11 (1.2) | |
| Missing | 5 (1.2) | 14 (1.6) | 28 (6.5) | 42 (4.7) | 37 (8.6) | 64 (7.1) | 54 (12.6) | 79 (8.8) | |
| Number (%) reporting any problemsb | 271 (63.8) | 551 (62.1) | 262 (65.2) | 563 (65.5) | 262 (66.7) | 547 (65.4) | 253 (67.3) | 554 (67.4) | |
| Pain/discomfort | Level 1 | 69 (16.0) | 166 (18.4) | 69 (16.0) | 144 (16.0) | 58 (13.5) | 145 (16.1) | 53 (12.3) | 119 (13.2) |
| Level 2 | 149 (34.7) | 376 (41.7) | 164 (38.1) | 378 (42.0) | 172 (40.0) | 343 (38.1) | 148 (34.4) | 360 (40.0) | |
| Level 3 | 148 (34.4) | 256 (28.4) | 124 (28.8) | 255 (28.3) | 112 (26.0) | 264 (29.3) | 130 (30.2) | 268 (29.7) | |
| Level 4 | 53 (12.3) | 87 (9.7) | 42 (9.8) | 74 (8.2) | 48 (11.2) | 76 (8.4) | 39 (9.1) | 69 (7.7) | |
| Level 5 | 3 (0.7) | 8 (0.9) | 2 (0.5) | 8 (0.9) | 4 (0.9) | 7 (0.8) | 4 (0.9) | 5 (0.6) | |
| Missing | 8 (1.9) | 8 (0.9) | 29 (6.7) | 42 (4.7) | 36 (8.4) | 66 (7.3) | 56 (13.0) | 80 (8.9) | |
| Number (%) reporting any problemsb | 353 (83.6) | 727 (81.4) | 332 (82.8) | 715 (83.2) | 336 (85.3) | 690 (82.6) | 321 (85.8) | 702 (85.5) | |
| Anxiety/depression | Level 1 | 257 (59.8) | 539 (59.8) | 225 (52.3) | 479 (53.2) | 214 (49.8) | 451 (50.1) | 192 (44.7) | 452 (50.2) |
| Level 2 | 113 (26.3) | 254 (28.2) | 124 (28.8) | 270 (30.0) | 129 (30.0) | 279 (31.0) | 137 (31.9) | 262 (29.1) | |
| Level 3 | 33 (7.7) | 63 (7.0) | 47 (10.9) | 96 (10.7) | 44 (10.2) | 95 (10.5) | 43 (10.0) | 98 (10.9) | |
| Level 4 | 3 (0.7) | 11 (1.2) | 4 (0.9) | 9 (1.0) | 6 (1.4) | 11 (1.2) | 3 (0.7) | 9 (1.0) | |
| Level 5 | 1 (0.2) | 2 (0.2) | 1 (0.2) | 2 (0.2) | 0 (0.0) | 1 (0.1) | 1 (0.2) | 3 (0.3) | |
| Missing | 23 (5.3) | 32 (3.6) | 29 (6.7) | 45 (5.0) | 37 (8.6) | 64 (7.1) | 54 (12.6) | 77 (8.5) | |
| Number (%) reporting any problemsb | 150 (36.9) | 330 (38.0) | 176 (43.9) | 377 (44.0) | 179 (45.5) | 386 (46.1) | 184 (48.9) | 372 (45.1) | |
Appendix 10 EQ-5D-5L visual analogue scale
| Baseline | 4 months | 8 months | 12 months | |||||
|---|---|---|---|---|---|---|---|---|
| Intervention group | Usual-care group | Intervention group | Usual-care group | Intervention group | Usual-care group | Intervention group | Usual-care group | |
| Mean EQ-5D-5L visual analogue scale score (SD) | 73.3 (17.3) | 73.7 (17.1) | 72.6 (18.5) | 73.2 (17.2) | 71.9 (18.2) | 72.3 (17.8) | 71.5 (18.7) | 72.1 (18.4) |
| Median EQ-5D-5L visual analogue scale score (interquartile range) | 75 (65–85) | 75 (65–85) | 75 (60–90) | 75 (60–85) | 75 (60–85) | 75 (60–85) | 75 (60–85) | 75 (60–85) |
Appendix 11 Mean resource use for ‘other’ reason only (i.e. non-falls related), based on all available cases
| Type of resource use | Intervention group | Usual-care group | ||
|---|---|---|---|---|
| Mean (SD) | Missing, n (%) | Mean (SD) | Missing, n (%) | |
| GP visit at general practice/home | ||||
| Baseline | 1.69 (1.96) | 39 (9.1) | 1.86 (3.58) | 106 (11.8) |
| 4 months | 1.48 (2.08) | 63 (14.7) | 1.52 (1.91) | 112 (12.4) |
| 8 months | 1.68 (2.23) | 71 (16.5) | 1.56 (2.10) | 132 (14.7) |
| 12 months | 1.67 (2.00) | 80 (18.6) | 1.55 (2.61) | 164 (18.2) |
| Nurse visit at general practice/home | ||||
| Baseline | 1.42 (2.56) | 45 (10.5) | 1.48 (3.06) | 121 (13.4) |
| 4 months | 1.25 (2.12) | 64 (14.9) | 1.47 (2.92) | 123 (13.7) |
| 8 months | 1.41 (2.97) | 70 (16.3) | 1.50 (3.63) | 139 (15.4) |
| 12 months | 1.57 (3.01) | 80 (18.6) | 1.43 (4.16) | 169 (18.8) |
| OT visit | ||||
| Baseline | 0.04 (0.30) | 60 (14.0) | 0.06 (0.53) | 154 (17.1) |
| 4 months | 0.40 (0.91) | 71 (16.5) | 0.10 (0.52) | 146 (16.2) |
| 8 months | 0.16 (0.96) | 78 (18.1) | 0.12 (0.79) | 159 (17.7) |
| 12 months | 0.17 (1.31) | 93 (21.6) | 0.12 (0.81) | 186 (20.6) |
| Physiotherapist visit | ||||
| Baseline | 0.36 (1.25) | 55 (12.8) | 0.35 (1.39) | 151 (16.8) |
| 4 months | 0.38 (1.15) | 72 (16.7) | 0.44 (1.61) | 146 (16.2) |
| 8 months | 0.50 (1.53) | 79 (18.4) | 0.44 (1.59) | 157 (17.4) |
| 12 months | 0.48 (1.69) | 92 (21.4) | 0.47 (2.08) | 187 (20.8) |
| Hospital outpatient visit | ||||
| Baseline | 1.19 (2.11) | 41 (9.5) | 1.42 (3.29) | 111 (12.3) |
| 4 months | 1.21 (1.98) | 55 (12.8) | 1.37 (2.58) | 103 (11.4) |
| 8 months | 1.19 (1.70) | 61 (14.2) | 1.34 (2.52) | 115 (12.8) |
| 12 months | 1.31 (2.45) | 76 (17.7) | 1.49 (3.17) | 151 (16.8) |
| A&E attendance | ||||
| Baseline | 0.10 (0.61) | 50 (11.6) | 0.11 (0.55) | 119 (13.2) |
| 4 months | 0.11 (0.39) | 64 (14.9) | 0.13 (0.44) | 118 (13.1) |
| 8 months | 0.15 (0.69) | 66 (15.4) | 0.11 (0.37) | 137 (15.2) |
| 12 months | 0.13 (0.47) | 89 (20.7) | 0.13 (0.53) | 175 (19.4) |
| Day-case hospital visit | ||||
| Baseline | 0.14 (0.46) | 48 (11.2) | 0.19 (0.69) | 120 (13.3) |
| 4 months | 0.15 (0.50) | 65 (15.1) | 0.19 (0.63) | 129 (14.3) |
| 8 months | 0.19 (0.79) | 78 (18.1) | 0.26 (1.49) | 142 (15.8) |
| 12 months | 0.22 (0.99) | 86 (20.0) | 0.22 (1.21) | 185 (20.5) |
| Inpatient hospital stay | ||||
| Baseline | 0.18 (1.05) | 42 (9.8) | 0.23 (1.81) | 111 (12.3) |
| 4 months | 0.38 (2.44) | 62 (14.4) | 0.44 (3.58) | 127 (14.1) |
| 8 months | 0.37 (2.07) | 75 (17.4) | 0.33 (2.25) | 138 (15.3) |
| 12 months | 0.30 (1.88) | 86 (20.0) | 0.53 (3.04) | 173 (19.2) |
Appendix 12 Out-of-pocket expenditure
| Out-of-pocket cost item | Intervention group | Usual-care group | ||
|---|---|---|---|---|
| Mean (SD) | Missing, n (%) | Mean (SD) | Missing, n (%) | |
| Equipment | ||||
| Baseline | 141.25 (718.94) | 2 (0.5) | 197.54 (1402.70) | 18 (2.0) |
| 4 months | 283.97 (1256.12) | 29 (6.7) | 124.57 (767.41) | 55 (6.1) |
| 8 months | 116.10 (778.72) | 35 (8.1) | 132.85 (898.24) | 69 (7.7) |
| 12 months | 86.58 (637.43) | 47 (10.9) | 134.29 (853.71) | 73 (8.1) |
| Travel to appointments | ||||
| Baseline | 10.52 (30.86) | 154 (35.8) | 11.31 (27.77) | 324 (36.0) |
| 4 months | 9.50 (43.61) | 125 (29.1) | 11.79 (35.42) | 244 (27.1) |
| 8 months | 10.74 (37.15) | 147 (34.2) | 10.78 (29.11) | 274 (30.4) |
| 12 months | 8.92 (24.66) | 141 (32.8) | 12.20 (42.34) | 292 (32.4) |
Appendix 13 Cost-effectiveness acceptability curve and cost-effectiveness plane: secondary analysis of the societal perspective
FIGURE 15.
Cost-effectiveness plane for secondary analysis.
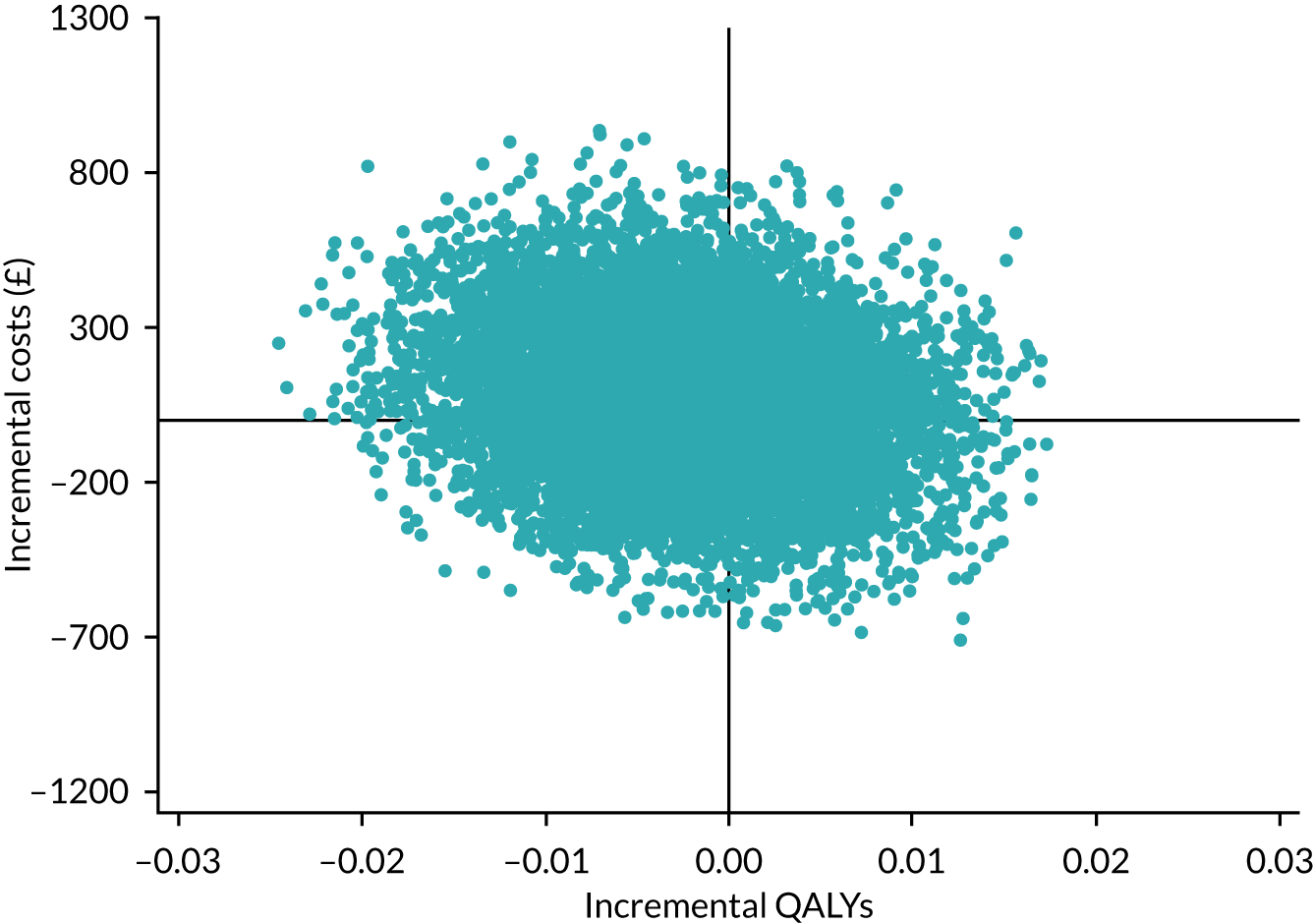
FIGURE 16.
Cost-effectiveness acceptability curve for secondary analysis.
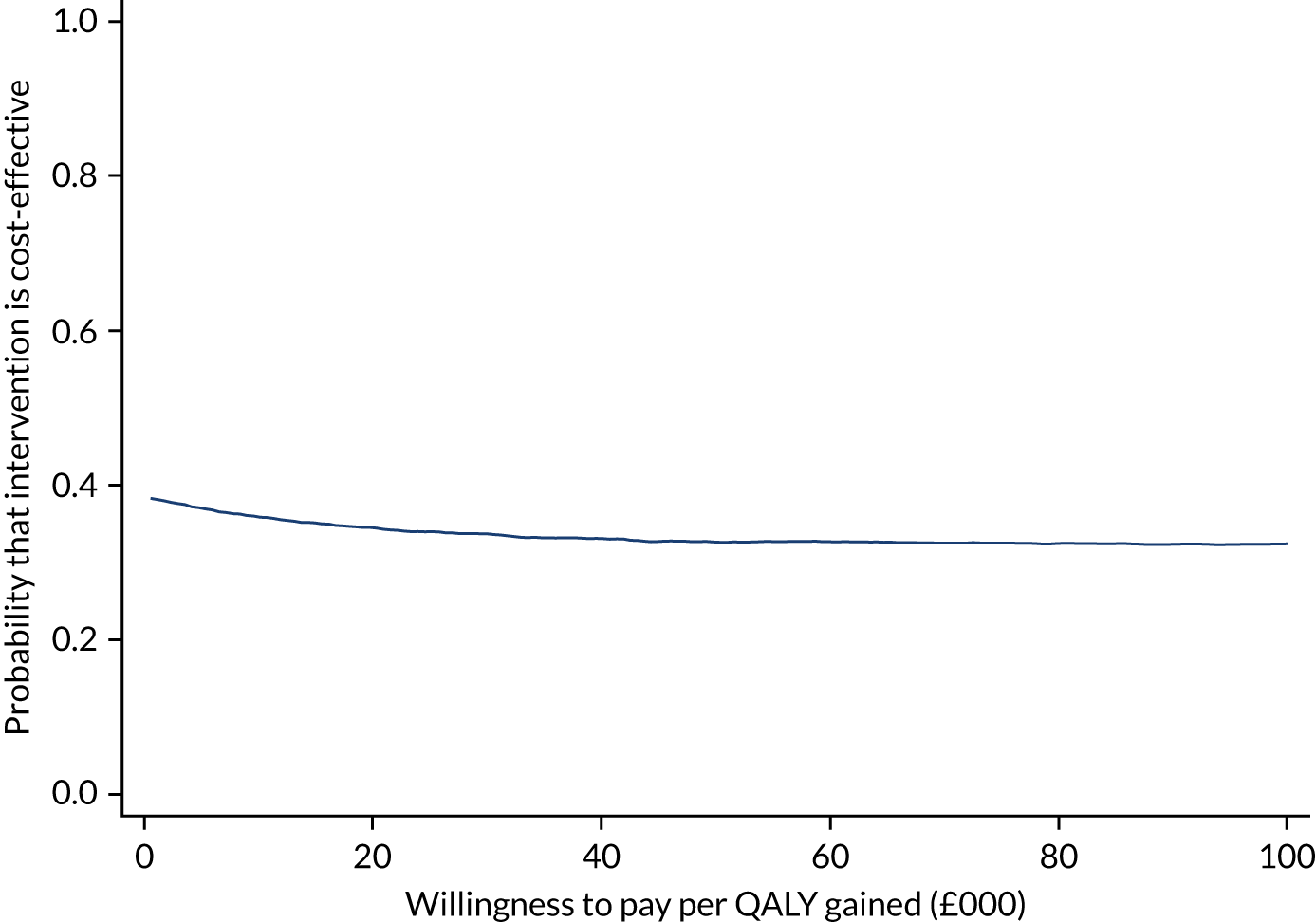
Appendix 14 Cost-effectiveness planes for sensitivity analyses 2–5
FIGURE 17.
Cost-effectiveness plane for sensitivity analysis 2: non-falls-related resource use.
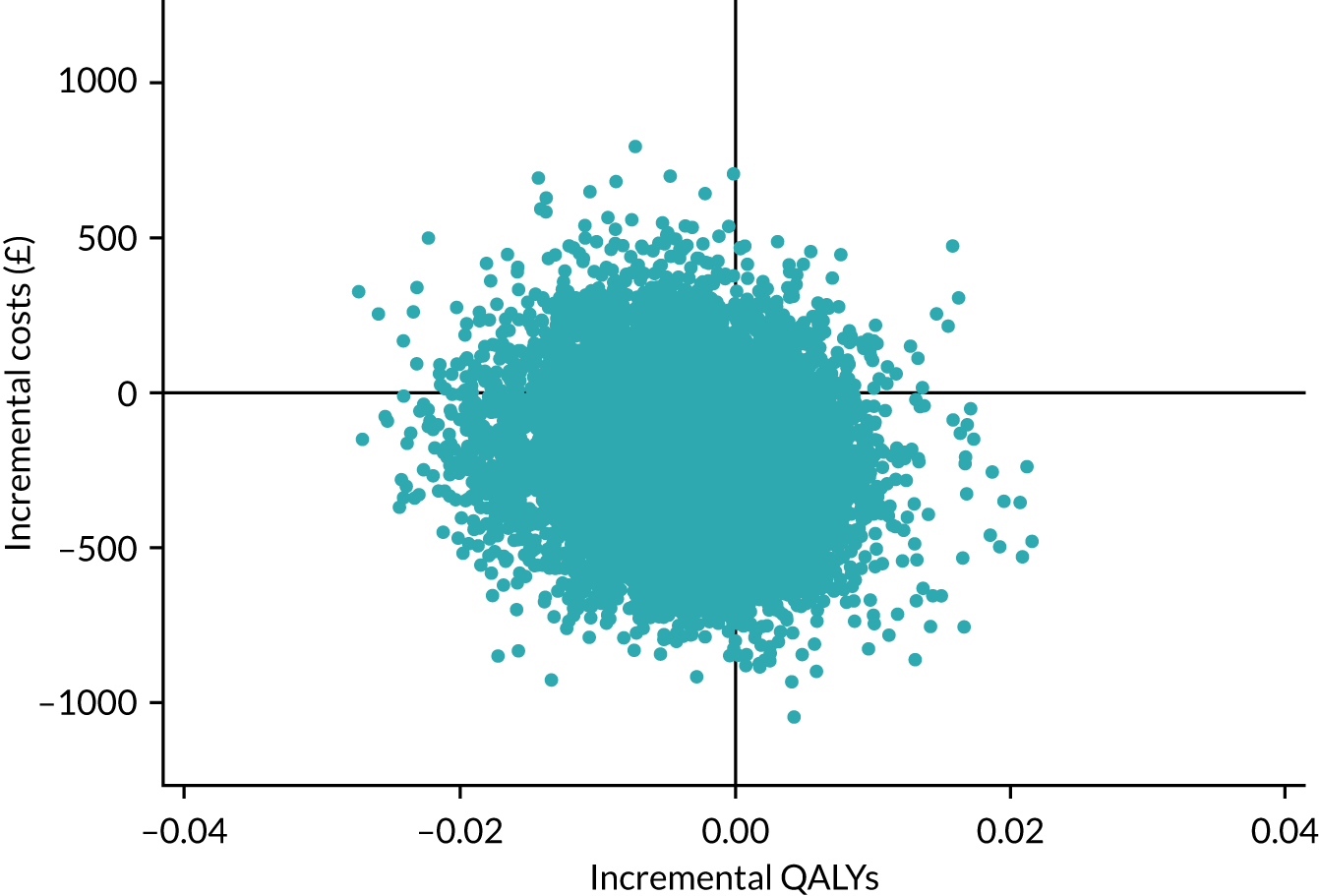
FIGURE 18.
Cost-effectiveness plane for sensitivity analysis 3: inpatient stay sourced from falls data sheets.
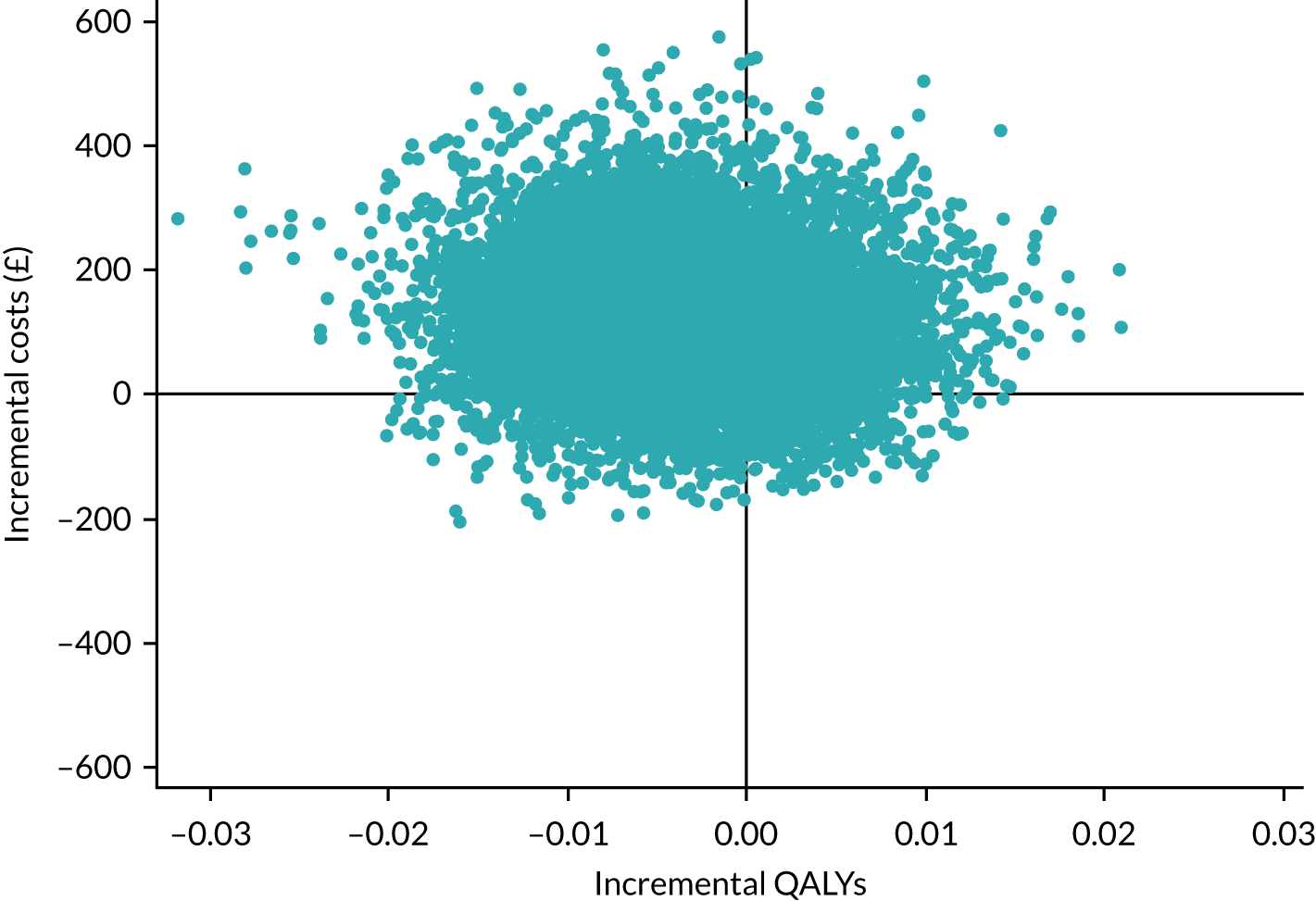
FIGURE 19.
Cost-effectiveness plane for sensitivity analysis 4: all equipment funded by NHS and PSS.
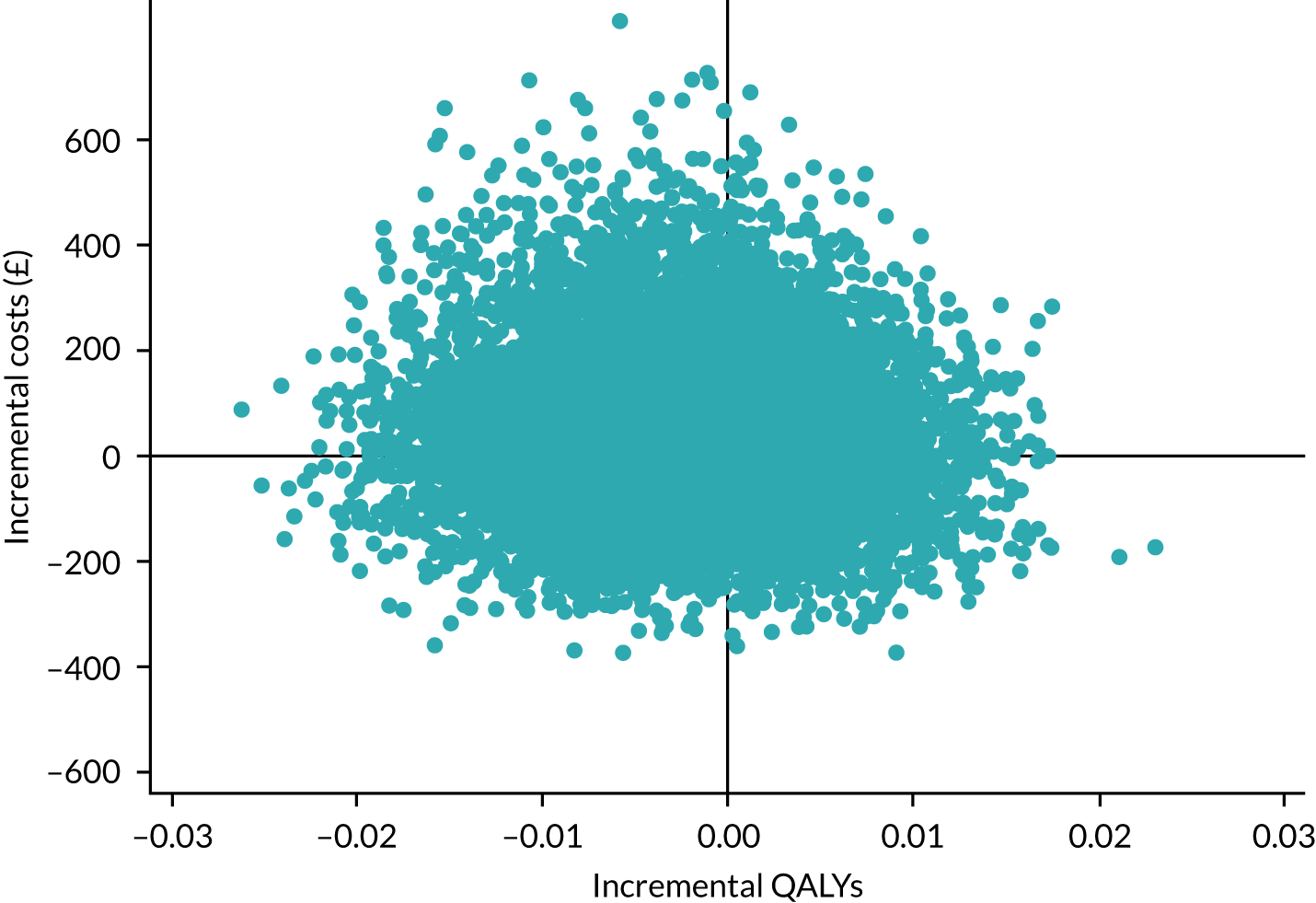
FIGURE 20.
Cost-effectiveness plane for sensitivity analysis 5: paid care worker visits paid for by NHS and PSS.
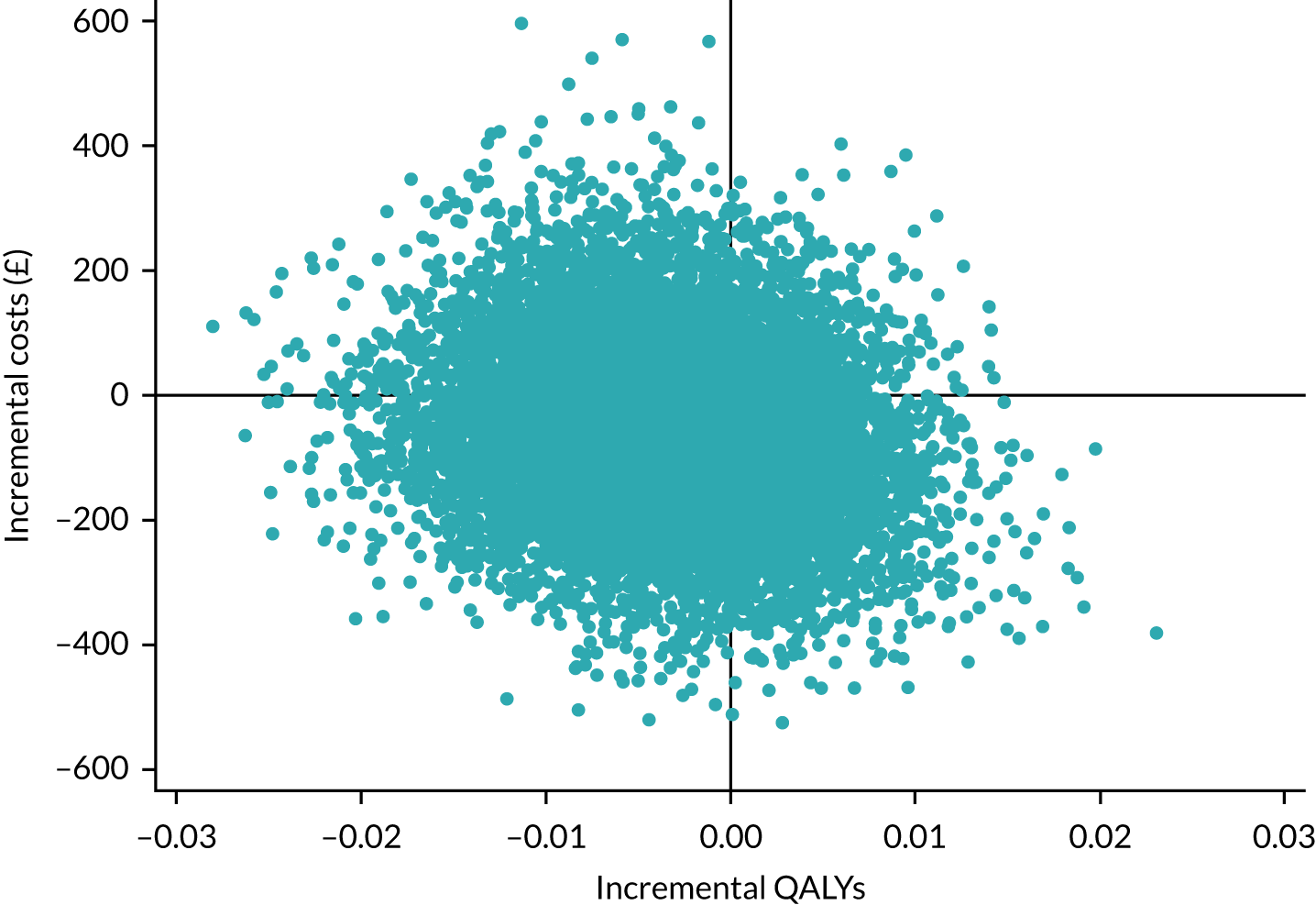
Appendix 15 Cost-effectiveness acceptability curves for sensitivity analyses 2–5
FIGURE 21.
Cost-effectiveness acceptability curve for sensitivity analysis 2: non-falls-related resource use.
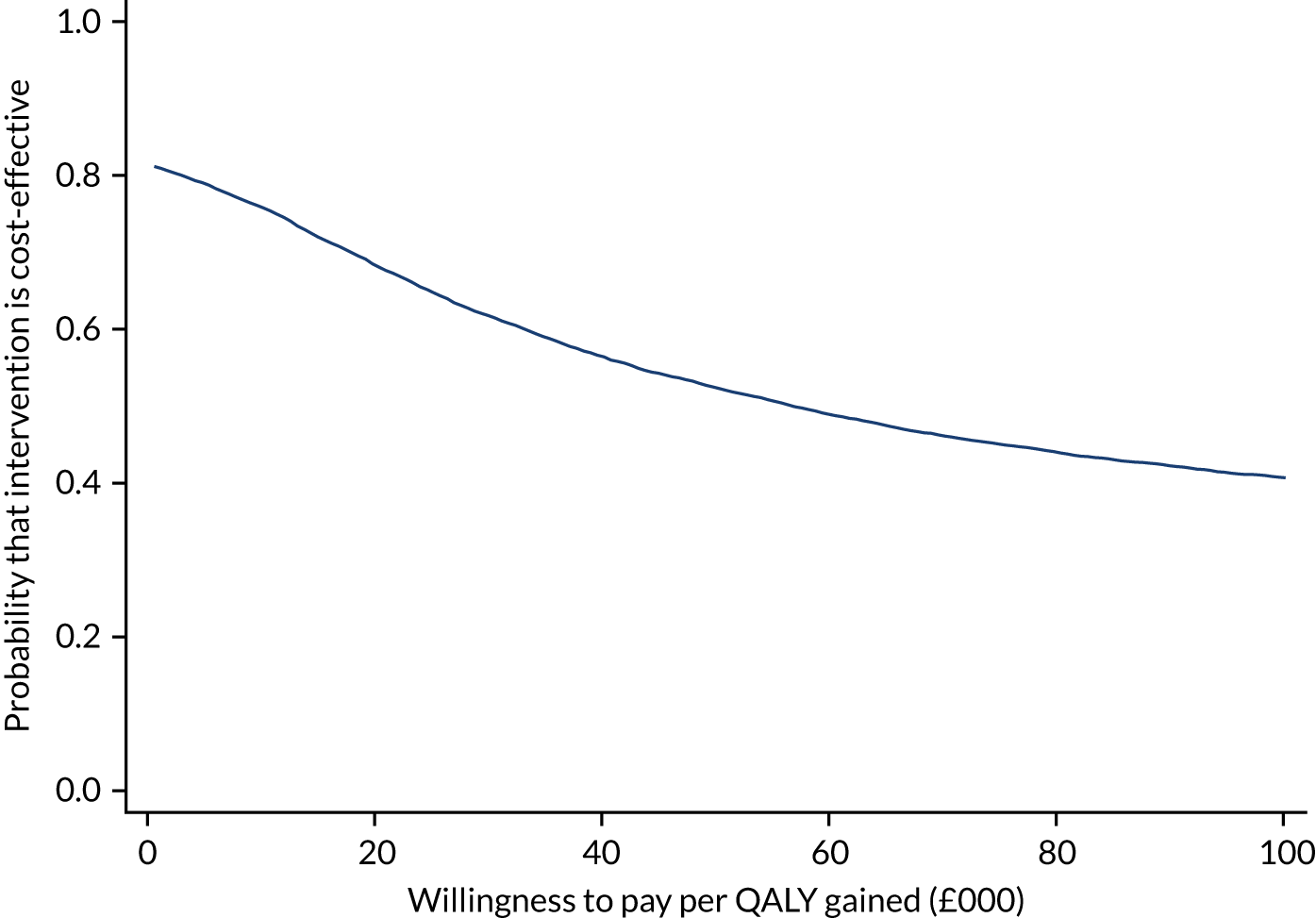
FIGURE 22.
Cost-effectiveness acceptability curve for sensitivity analysis 3: inpatient stay sourced from falls data sheets.
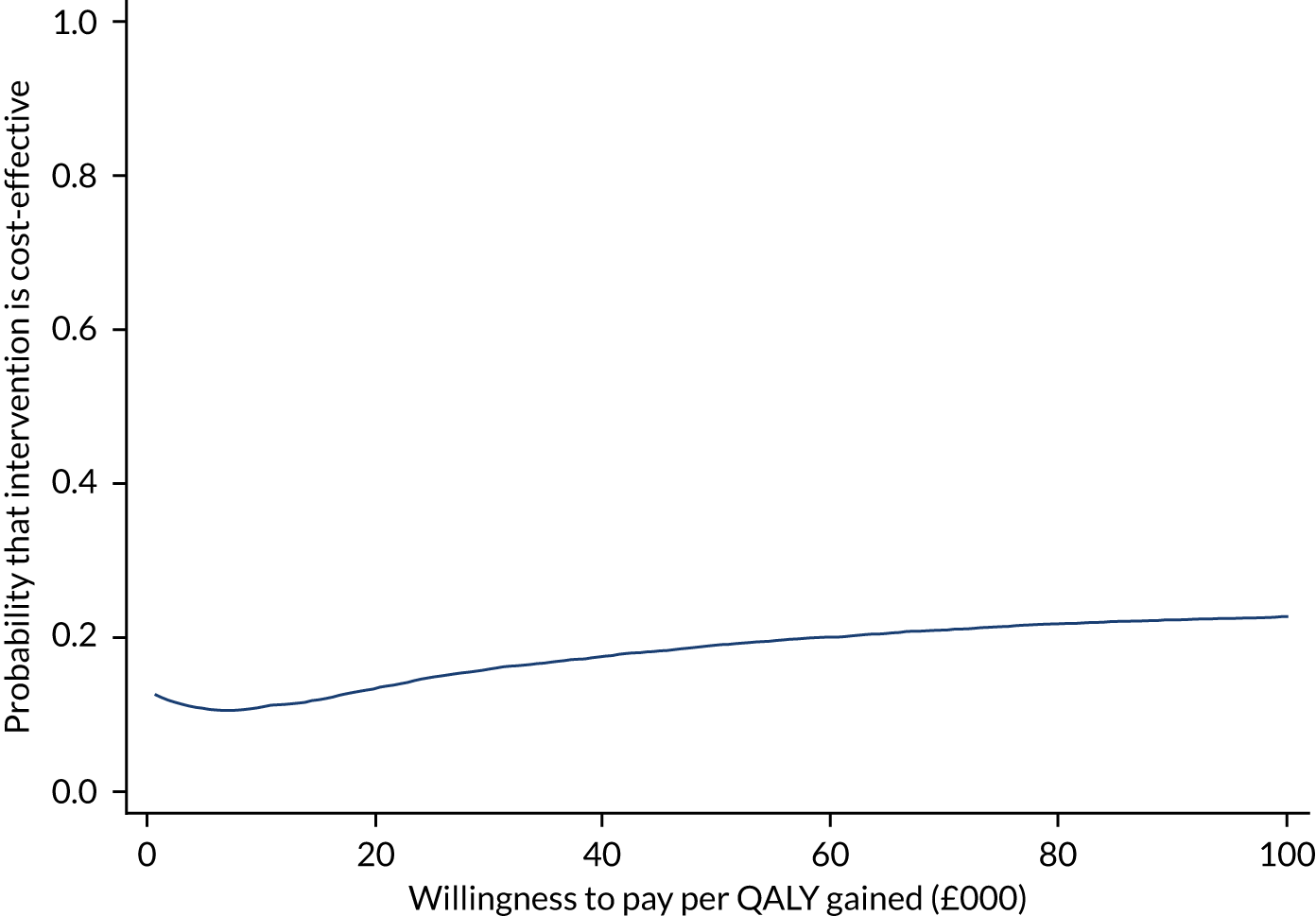
FIGURE 23.
Cost-effectiveness acceptability curve for sensitivity analysis 4: all equipment funded by NHS and PSS.
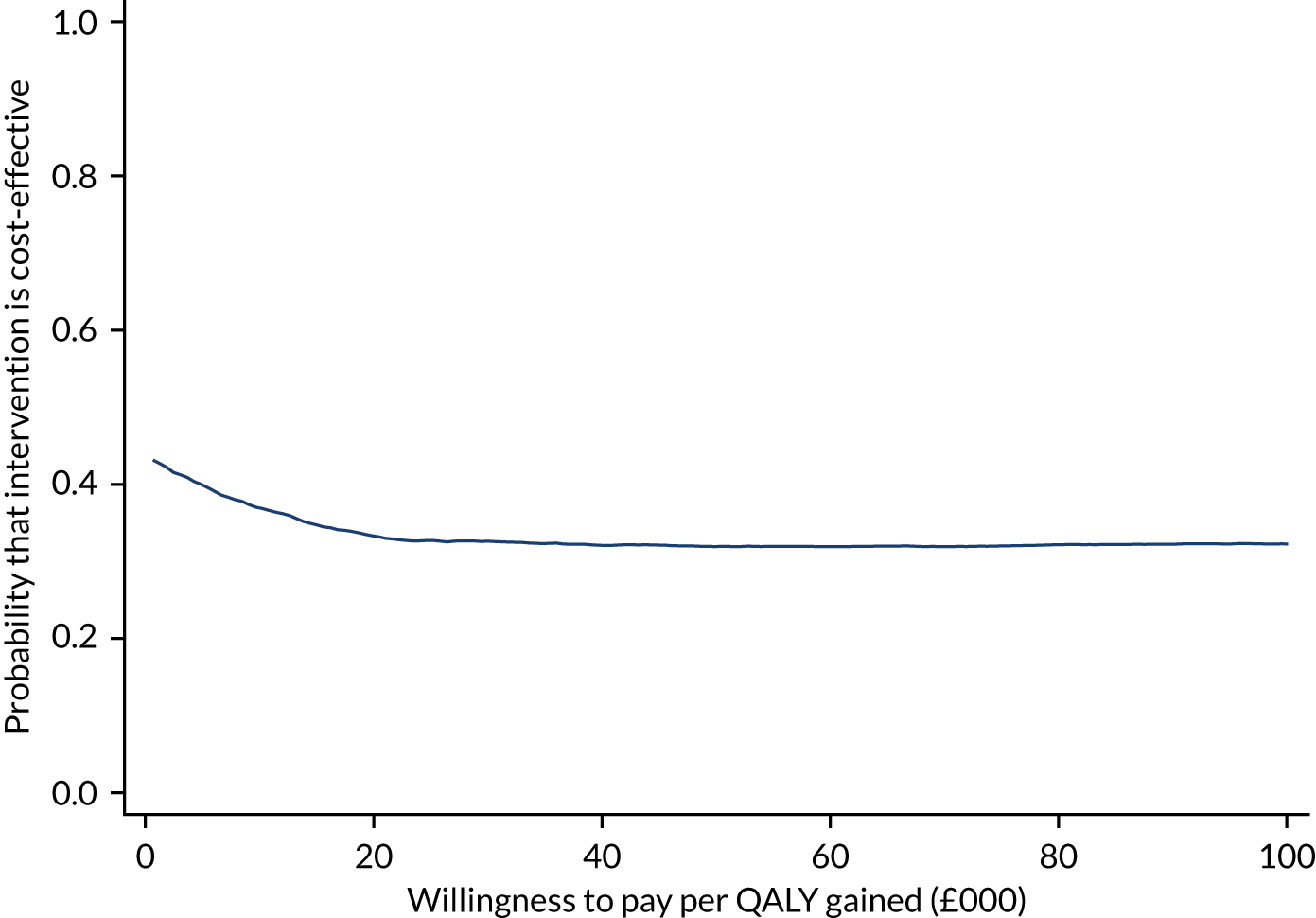
FIGURE 24.
Cost-effectiveness acceptability curve for sensitivity analysis 5: paid care worker visits paid for by NHS and PSS.
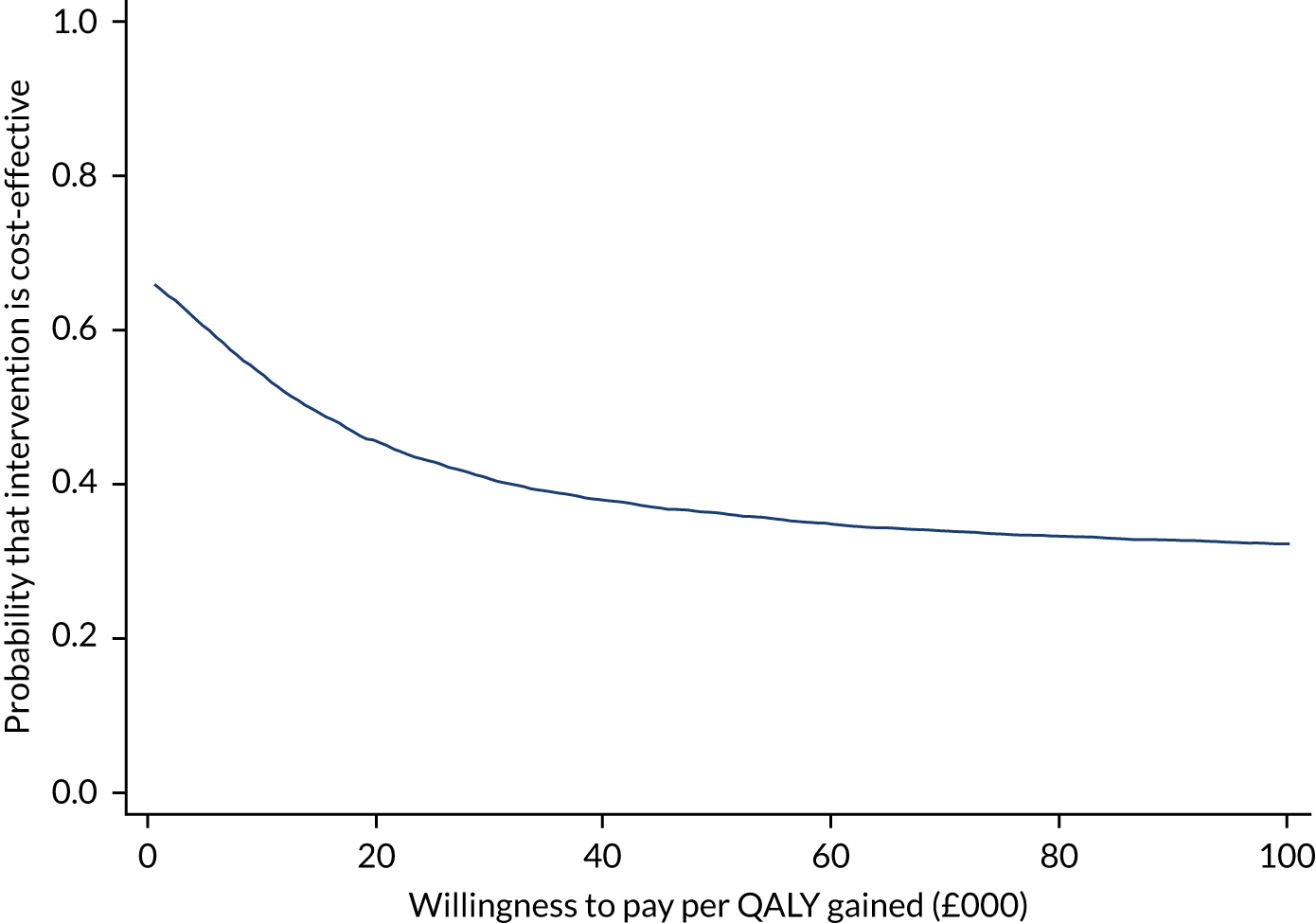
List of abbreviations
- A&E
- accident and emergency
- CACE
- complier-average causal effect
- CASPER
- CollAborative care and active surveillance for Screen-Positive EldeRs
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- cRCT
- cohort randomised controlled trial
- DMEC
- Data Monitoring and Ethics Committee
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GP
- general practitioner
- HCPC
- Health and Care Professionals Council
- Home Fast
- The Home Falls and Accidents Screening Tool
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IRR
- incidence rate ratio
- ITT
- intention to treat
- MI
- multiple imputation
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMB
- net monetary benefit
- OR
- odds ratio
- OT
- occupational therapist
- OTIS
- Occupational Therapist Intervention Study
- PSS
- Personal Social Services
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- REFORM
- REducing Falls with ORthoses and a Multifaceted podiatry intervention
- SCOOP
- screening of older women for prevention of fracture
- SD
- standard deviation
- TSC
- Trial Steering Committee
- WeHSA
- Westmead Home Safety Assessment
- YTU
- York Trials Unit
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/hta25460).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
