Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 15/160/02. The contractual start date was in February 2017. The draft report began editorial review in August 2020 and was accepted for publication in April 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
Copyright © 2021 Devall et al. This work was produced by Devall et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 Devall et al.
Chapter 1 Introduction
Parts of this chapter are reproduced or adapted with permission from Chu et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) license, which permits others to copy and redistribute this work, for non-commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Clinical background
Miscarriage is a common complication of pregnancy (15–25% of pregnancies; approximately 125,000 miscarriages per year in England). 2,3 Miscarriage is associated with not only physical harm, such as excessive bleeding, infection and uterine perforation during surgery, but also substantial psychological impact on patients; studies have shown that distress from miscarriage is equivalent to that from the stillbirth of a term-born baby,4,5 and miscarriage is estimated to cost the NHS £81M per year. 6
There are two main types of miscarriage that require medical intervention: missed miscarriage and incomplete miscarriage. 6 A missed miscarriage, also known as a delayed or silent miscarriage, is diagnosed when a non-viable pregnancy is identified on ultrasound scan during the first 14 weeks of gestation. Commonly, women who suffer missed miscarriage are asymptomatic or experience small amounts of vaginal bleeding or pain before the diagnosis is made. 6–8 All pregnancy tissue is retained in the uterus in a missed miscarriage. In contrast, an incomplete miscarriage is diagnosed when pregnancy tissue has already been partly expelled by the uterus. 6
Management of miscarriage can be expectant (i.e. waiting for natural miscarriage), medical (i.e. with drugs) or surgical. A UK survey conducted by this study team has shown that 25% of women opt for medical management. However, there is uncertainty about the optimal drug regimens for medical management. Misoprostol is a prostaglandin analogue used for the medical management of miscarriage to induce myometrial contractions to aid the expulsion of pregnancy tissue. 9 However, misoprostol alone is not always effective, and 15–40% of women require an additional dose of misoprostol, thus prolonging the duration of treatment. 10–14 This treatment failure can culminate in the need for surgical management, which can be particularly undesirable to women who have actively chosen to have medical management. 15,16 To strengthen the effect of misoprostol, a steroidal anti-progestogen called mifepristone (Mifegyne®, Exelgyn, Paris, France) is sometimes used in combination with misoprostol. Mifepristone is a competitive progesterone receptor antagonist that primes the myometrium before prostaglandin exposure. 9
The reported effectiveness of combination treatment with mifepristone and misoprostol for the medical management of missed miscarriage in previous clinical trials ranges from 64% to 84%. 17–19 However, given the lack of placebo-controlled studies, the usefulness of mifepristone in the management of missed miscarriage has remained unclear. Before National Institute for Health and Care Excellence (NICE) guideline CG154 was published in 2012,20 common practice was to use a combination of mifepristone and misoprostol (MifeMiso combination). The 2012 NICE guideline, however, recommended that misoprostol alone should be given to women undergoing medical management. 20 This recommendation was based on very limited evidence from one study of 115 women,17 which found no difference between MifeMiso combination and misoprostol alone. Recognising the limited available evidence, the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) issued a commissioned call for a trial investigating the clinical effectiveness and cost-effectiveness of mifepristone in combination with misoprostol compared with misoprostol alone in the management of first-trimester miscarriage.
Existing evidence
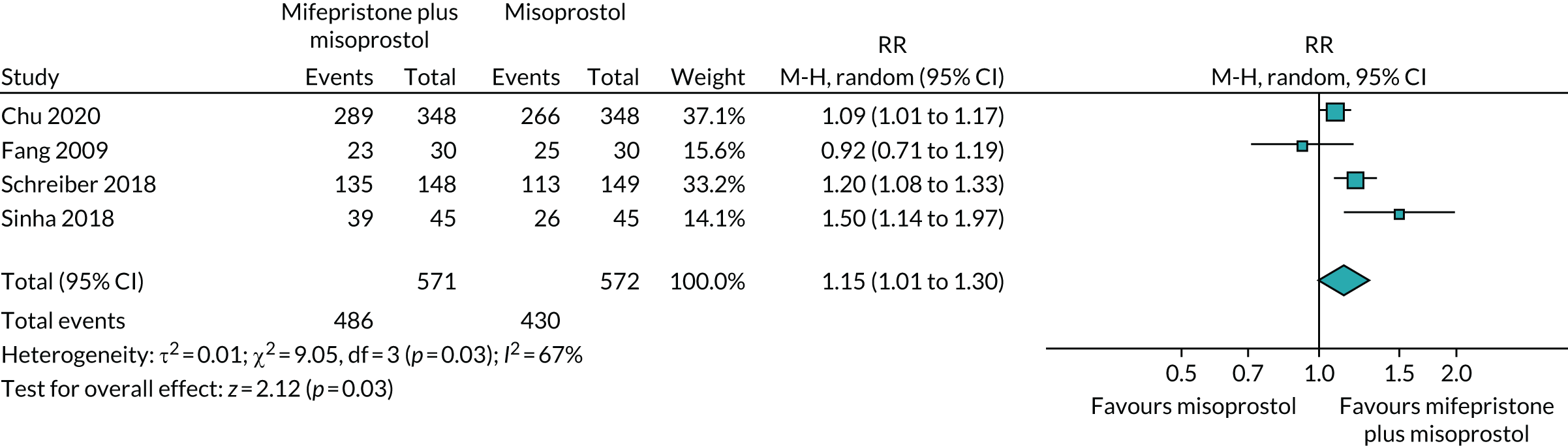
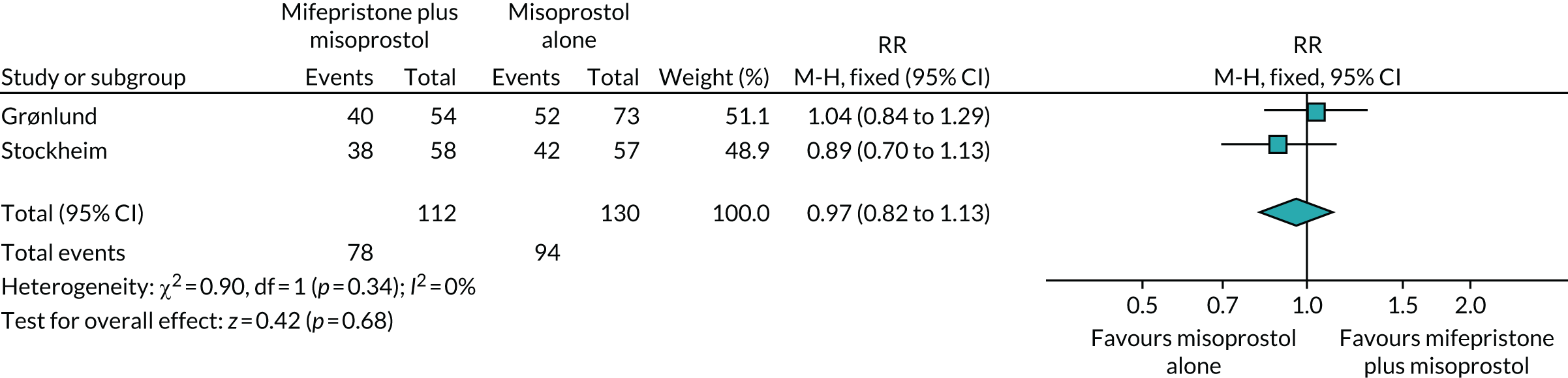
We conducted a systematic review of trials investigating the use of mifepristone and misoprostol in women with miscarriage to gain a better understanding of studied interventions, outcomes and resolution rates. These studies are listed in Table 1. In addition to the publication by Stockheim et al. ,17 one more trial was identified,18 which compared MifeMiso combination with misoprostol alone in the missed miscarriage population. Together, the two trials included a total of 242 patients treated with MifeMiso or misoprostol alone. Meta-analysis of the results showed no significant difference between the two trial groups for outcome of resolution of miscarriage [risk ratio (RR) 0.97, 95% confidence interval (CI) 0.82 to 1.13] (Figure 1). However, given the imprecision that inevitably accompanies such small sample sizes, it is not possible to draw any firm inferences.
| Study (first author and year of publication) | Intervention | Comparison | Risk of bias |
|---|---|---|---|
| Grønlund 200218 (n = 127) | 600 mg of oral mifepristone and 400 µg of misoprostol 48 hours later | 400 µg of vaginal misoprostol and 200 µg of misoprostol 2 hours later if no vaginal bleeding occurred | Centres randomised to treatment regimens with crossover every 4 months; allocation concealment inadequate; no blinding of patients and study personnel |
| Stockheim 200617 (n = 115) | 600 mg of oral mifepristone and 800 µg of oral misoprostol 48 hours later | 800 µg of oral misoprostol and 800 µg of oral misoprostol 48 hours later | Method of randomisation clear; no allocation concealment; no blinding of patients and study personnel |
FIGURE 1.
Forest plot of completed trials comparing MifeMiso combination with misoprostol alone for the medical management of missed miscarriage, for the outcome of resolution of miscarriage.
Rationale
A trial comparing MifeMiso to misoprostol alone for the medical management of missed miscarriage was required because:
-
NICE guideline CG154 called for a definitive trial to evaluate whether or not there is any benefit in using mifepristone in addition to misoprostol.
-
A patient survey supported the study. The survey (n = 188) showed that 66% of women would consider taking part in the study.
-
A UK clinician survey supported the study. In the survey of 152 practitioners, 79% believed that a clinical trial was needed to investigate whether or not the mifepristone and misoprostol combination is more effective than misoprostol alone in the medical management of missed miscarriage.
-
The Association of Early Pregnancy Units, the Early Pregnancy Clinical Studies Group, the Miscarriage Association and Tommy’s Charity supported the research.
-
If benefit was confirmed in the MifeMiso trial, women and the NHS stand to gain substantially. On the other hand, if mifepristone was found to be ineffective, treatment with mifepristone could be avoided.
-
Mifepristone treatment is cheap (£17.55 per 200-mg oral tablet), and if benefit was confirmed, rapid uptake of this intervention was expected.
Specific objectives
Primary objective
The primary objective of the MifeMiso trial is to test the hypothesis that treatment with mifepristone plus misoprostol is superior to misoprostol alone for the resolution of miscarriage within 7 days in women diagnosed with missed miscarriage by pelvic ultrasound scan in the first 14 weeks of pregnancy.
Key secondary objective
To test the hypothesis that the addition of mifepristone reduces the need for surgical intervention to resolve the miscarriage up to discharge from early pregnancy unit (EPU).
Other secondary objectives
-
To evaluate if the addition of mifepristone reduces the need for further doses of misoprostol.
-
To evaluate if the addition of mifepristone improves other clinical outcomes, including surgical intervention up to and including 7 days post randomisation and after 7 days post randomisation, duration of bleeding, infection, negative pregnancy test at 21 days post randomisation, time from randomisation to discharge from EPU care, side effects and complications.
-
To evaluate if the addition of mifepristone improves patient satisfaction.
-
To assess the cost-effectiveness of the combination of mifepristone and misoprostol in the medical management of missed miscarriage.
Chapter 2 Methods
Parts of this chapter are reproduced or adapted with permission from Coomarasamy et al. 21,22 Contains information licensed under the Non-Commercial Government Licence v2.0.
Our study registration is published on www.clinicaltrials.gov/ct2/show/NCT03065660. 23
Design
The MifeMiso trial was conducted as a randomised, double-blind, placebo-controlled, multicentre trial of mifepristone and misoprostol compared with misoprostol alone in the medical management of missed miscarriage. The trial had a favourable ethical opinion from National Research Ethics Service (NRES) Committee West Midlands – Edgbaston. The final protocol version was v5.0, 27 June 2019.
Participants
Potential participants were women attending EPUs in secondary or tertiary care NHS hospitals located across the UK. They were eligible for the MifeMiso trial if they fulfilled the following criteria (see Recruitment for more details on the recruitment process):
-
A missed miscarriage had been diagnosed by pelvic ultrasound scan in the first 14 weeks of pregnancy and they had opted for medical management of miscarriage.
-
They were aged ≥ 16 years.
-
They were willing and able to give informed consent.
Potential participants could not be included if any of the following criteria were applicable:
-
They had opted for alternative methods of miscarriage management (expectant or surgical).
-
They had a diagnosis of incomplete miscarriage.
-
They were suffering life-threatening bleeding.
-
They had contraindications to mifepristone or misoprostol use (e.g. chronic adrenal failure, known hypersensitivity to either drug, haemorrhagic disorders and anticoagulant therapy, prosthetic heart valve or history of endocarditis, existing cardiovascular disease, severe asthma uncontrolled by therapy or inherited porphyria).
-
They were currently participating in another blinded, placebo-controlled trial of investigational medicinal products (IMPs) in pregnancy.
-
They had previously participated in the MifeMiso trial.
-
They were not able to attend for a day 6–7 ultrasound scan.
Recruitment
Potential participants were identified from dedicated EPUs and approached by clinic doctors, research nurses and midwives, who had received appropriate training relating to the trial.
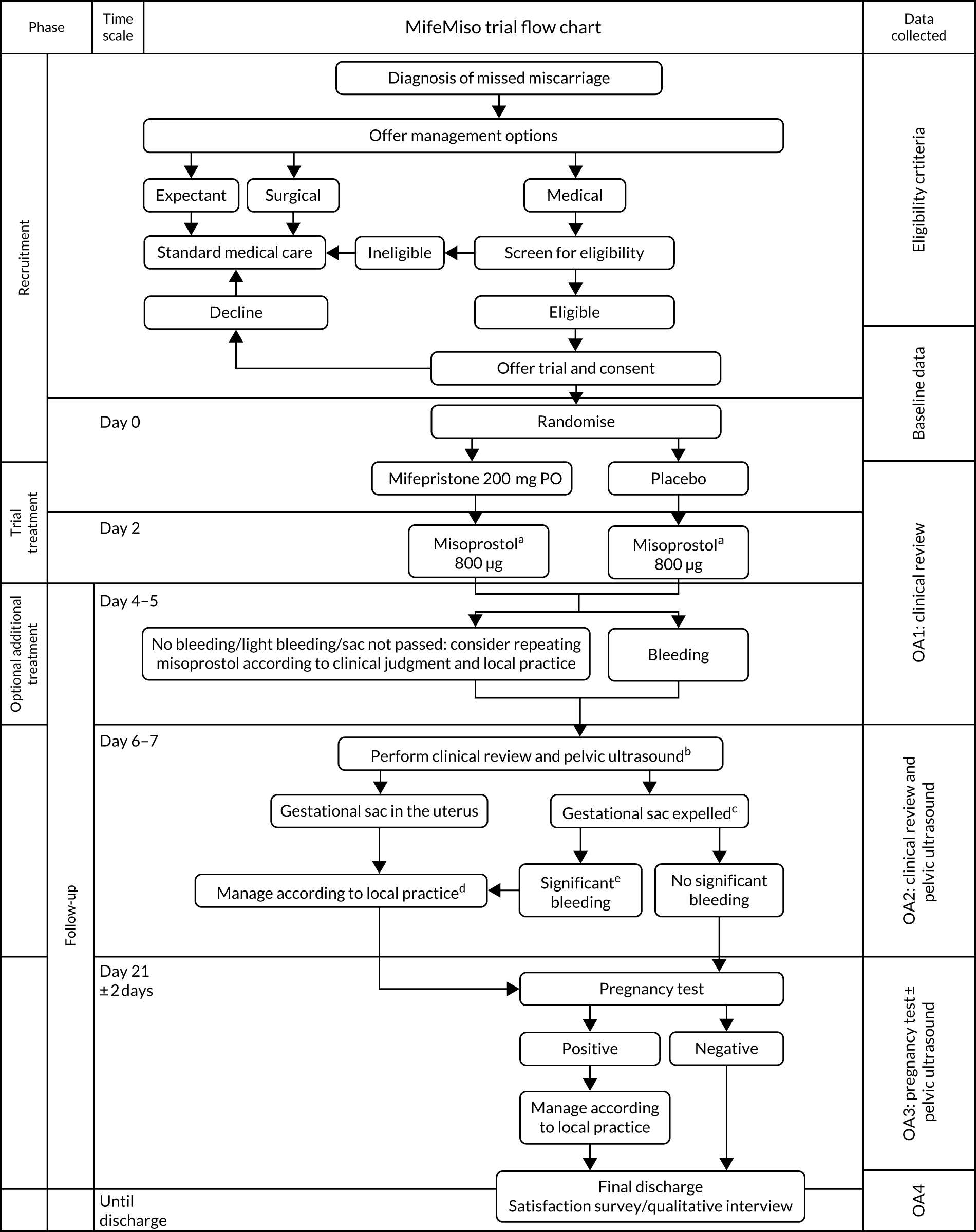
The participant eligibility pathway to recruitment and randomisation is illustrated in Figure 2. Eligible women were given verbal and written explanations about the trial. They were informed clearly that participation in the trial was entirely voluntary, with the option to withdraw at any stage, and that participation or non-participation would not affect their usual care. They were provided with a participant information sheet (PIS) (see Report Supplementary Material 1). Eligible women were then given the opportunity to decide if they wished to participate, if they needed more time to consider their decision or if they did not wish to participate. In all three scenarios, the decision of the woman was respected. If a woman needed more time to consider her potential involvement, the research nurse or midwife contacted her after the initial discussion to follow this up. If an initially undecided woman decided later to participate, the research nurse or midwife arranged a mutually convenient opportunity for the woman to be consented, providing she still met the eligibility criteria. A written consent form (see Report Supplementary Material 2) was provided to each woman who agreed to participate in the trial. The investigator and the participant both signed the consent form. The original copy was kept in the investigator site file, one copy was given to the participant, one copy was retained in the woman’s hospital records and one copy was returned to the MifeMiso trial office at Birmingham Clinical Trials Unit. Baseline demographic and medical data were collected, anonymised and stored in an electronic integrated trial management system (ITMS). Any identifying information was collected and stored in a password-protected local database on a secure computer with restricted access. We made provision for translation, if necessary, to communicate with non-English speakers and accommodate any special communications requirements of potential study participants.
FIGURE 2.
MifeMiso trial flow chart. a, If gestational sac has been passed before the scheduled time for misoprostol, misoprostol can be omitted; b, if scan performed earlier than day 6–7 and sac passed then repeat scan at day 6–7 not required; c, primary outcome; d, advice: avoid surgical evacuation unless clinically indicated; e, according to clinical judgement.
Randomisation
Confirmation of eligibility according to inclusion and exclusion criteria was provided by a medically trained doctor and all the necessary information was gathered before randomisation. Participants were randomised online to receive the trial intervention (either mifepristone or placebo) via a purpose-designed ITMS. Each authorised member of the research team was provided with a unique username and password to the ITMS for this purpose. Online randomisation was available 24 hours per day, 7 days per week, apart from short periods of scheduled maintenance.
Sequence generation and minimisation
Participants were randomised online via a secure internet facility. This third party independent ITMS was designed, developed and delivered by MedSciNet® (London, UK) according to standards of the International Organisation for Standardisation (ISO) 27000 and the requirements of the US Food and Drug Administration CFR21:11.
Participants were randomised to receive mifepristone or placebo in a 1 : 1 ratio. A ‘minimisation’ procedure using a computer-based algorithm was used to avoid chance imbalances in important stratification variables. The stratification variables used for minimisation are listed below:
-
Maternal age (< 30 or ≥ 30 years). The aim was to achieve a balance in the number of participants from younger and older age groups. However, there is no known biological reason why the intervention should lead to differing efficacy in women aged < 30 or ≥ 30 years.
-
Body mass index (BMI) (< 35 or ≥ 35 kg/m2). It was postulated that the pharmacokinetics and metabolism of mifepristone and misoprostol may be different in women with a BMI that is < 35 or ≥ 35 kg/m2.
-
Previous parity (nulliparous, parous women). It was postulated that parous women may be able to complete their missed miscarriage at a higher resolution rate than nulliparous women. The aim was, therefore, to achieve balance in the participants according to their previous parity.
-
Gestational age (< 70 or ≥ 70 days). It was postulated that women with a gestational age of < 70 days may have a higher resolution rate than women with a gestational age of ≥ 70 days.
-
Amount of bleeding [Pictorial Blood loss Assessment Chart (PBAC) score: ≤ 2 or ≥ 3]. It was proposed that women with a PBAC score of ≥ 3 may achieve a higher resolution rate than those with a score of ≤ 2, as an increased amount of bleeding would suggest that the miscarriage was more likely to resolve with the allocated intervention.
-
Randomising centre. It was hypothesised that different centres may respond to patients with different ultrasonography findings in different ways. For example, some centres may have a quicker recourse to miscarriage than others. Therefore, the aim was to achieve balance in the number of participants randomised to mifepristone and misoprostol and placebo and misoprostol at each recruiting centre.
Allocation
When all the eligibility criteria and baseline data items were entered online, the ITMS generated a trial number that took into account the minimisation variables recorded for the individual and was linked to a specific trial intervention pack. The pack number was revealed via e-mail to the local principal investigator (PI), the relevant trial pharmacist (see Blinding) and the research nurse or midwife performing the randomisation. The trial intervention pack was dispensed to the patient by the clinical trial pharmacist at the randomising hospital. Each trial intervention pack contained either mifepristone or an identical-looking placebo tablet.
Interventions
Each participant in the MifeMiso trial received either a mifepristone or placebo tablet, to be administered orally. Both products were supplied by MODEPHARMA Limited (Beckenham, Greater London, UK), a pharmaceutical wholesaler and project management company with a Medicines and Healthcare Products Regulatory Agency (MHRA) wholesale dealer’s licence, in compliance with good manufacturing practice (GMP) standards, good clinical practice (GCP) requirements and Medicines for Human Use (Clinical Trials) Regulations 2004. MODEPHARMA Limited also provided qualified person (QP) release of the trial drug under the requirements of the Medicines for Human Use (Clinical Trials) Regulations 2004.
Mifepristone tablets
The IMP was mifepristone at a single dose of 200 mg, taken following randomisation. Mifepristone was based on licensed and commercially available Mifegyne (Mifepristone) 200-mg tablets.
Placebo tablets
Placebo tablets were oral tablets, composed of Pearlitol 200SD Dev, Microcrystalline Cellulose NF, Ph. Eur.; DAB, Crospovidone, NF/Ph. Eur.; Magnesium Stearate, Ph. Eur.; and Quinoline Yellow produced in the same form as the IMP, and identical in colour, shape and weight, for use in the control group of the MifeMiso trial. The dose, route and timing of administration were also identical to those in the active mifepristone group of the study.
Dose
The dose of 200 mg of mifepristone was chosen as it is the most commonly used dose for the medical management of miscarriage when used with misoprostol and it is the most commonly studied dose in published trials investigating its efficacy in the medical management of miscarriage.
Concomitant non-trial treatments
Concomitant therapy was provided at the discretion of the care-providing clinicians, and all concomitant treatment and medications were documented via the ITMS. Other than identified contraindicated drugs (see Participants), the initiation of treatment for another indication did not necessitate withdrawal from the MifeMiso trial.
Blinding
Participants, investigators, research nurses, midwives and other attending clinicians remained blind to the trial drug allocation throughout the duration of the trial.
In the case of any serious adverse event (SAE), the recommendation was to initiate management and care of the participant as though the woman had taken mifepristone. The occurrence of any adverse event considered serious, unexpected and possibly, probably or definitely related to the trial intervention (for more information please see additional documentation: www.journalslibrary.nihr.ac.uk/programmes/hta/1516002/#/documentation; accessed September 2021) led to unblinding as appropriate. In any other circumstances, investigators and research nurses and midwives remained blind to drug allocation while the participant remained in the trial. However, if medical management required clinicians to know a participant’s drug allocation, then this could be requested from the central trial team 24 hours per day, 7 days per week.
Compliance assessment and treatment withdrawal
Compliance monitoring
The dispensing of the MifeMiso trial drug was recorded in the pharmacy drug accountability log. Ingestion of the drug was observed by a health-care professional (HCP) and documented in the patient’s notes. Compliance to trial treatment was defined as taking the allocated mifepristone or placebo tablet on day 0 (day of randomisation) and subsequently misoprostol on day 2 unless the gestational sac had passed before the scheduled time for misoprostol; in the latter case, the patient was deemed to be compliant to the trial medication as long as the allocated mifepristone or placebo tablet was taken on day 0.
Withdrawal from trial
Participants could voluntarily withdraw their consent to study participation at any time. If a participant did not return for a scheduled visit, attempts were made to contact them and (when possible) to review compliance and adverse events (AEs). We documented the reason(s) for self-withdrawal when possible. Each woman remained able to change her mind about withdrawal, and re-consent to participate in the trial, at any time. If a participant explicitly withdrew consent to any further data recording then this decision was respected and recorded via the ITMS. All communications surrounding the withdrawal were noted in the study records and no further data were collected for such participants.
Outcomes and assessment
Primary outcome
Failure to spontaneously pass the gestational sac within 7 days after randomisation.
Secondary outcomes
Key secondary outcome: surgical intervention to resolve the miscarriage (collected up to discharge from EPU care).
Additional secondary outcomes are as follows (as a proportion of those randomised unless stated):
-
Surgical intervention to resolve the miscarriage up to and including day 7 post randomisation.
-
Surgical intervention to resolve the miscarriage after day 7 post randomisation to discharge from EPU care.
-
Need for further doses of misoprostol up to day 7 post randomisation.
-
Need for further doses of misoprostol up to discharge from EPU care.
-
Overall patient satisfaction score [measured using the Client Satisfaction Questionnaire (CSQ-8) questionnaire and collected at discharge from EPU care].
-
Patient quality of life (index value and overall health status measured using the EuroQol-5 Dimensions, five-level version (EQ-5D-5L), questionnaire and collected on date of randomisation, day 6–7 post randomisation or day of follow-up ultrasound scan if different from day 6–7 and day 212 days post randomisation. If a woman obtains an initial positive pregnancy test result at day 21 ± 2 days post randomisation, then a further EQ-5D-5L questionnaire is collected (at discharge from EPU care).
-
Duration of bleeding reported by woman (days) (collected up to discharge from EPU care).
-
Diagnosis of infection associated with miscarriage requiring outpatient antibiotic treatment (collected up to discharge from EPU care).
-
Diagnosis of infection associated with miscarriage requiring inpatient antibiotic treatment (collected up to discharge from EPU care).
-
Negative pregnancy test result 21 days (± 2 days) after randomisation.
-
Time from randomisation to discharge from EPU care (described using summary statistics only).
Safety outcomes:
-
blood transfusion required (collected up to discharge from EPU care)
-
side effects (collected up to discharge from EPU care)
-
death (collected up to discharge from EPU care)
-
any serious complications (collected up to discharge from EPU care).
Mixed-methods evaluation resource use outcomes
These are detailed in Chapter 4.
Resource use outcomes
These are detailed in Chapter 5.
Future outcomes
Women’s consent was sought for future evaluation of themselves and any subsequent pregnancies using their health records. Although long-term follow-up was outside the scope of this trial, we plan to conduct further studies on outcomes such as subsequent successful pregnancies post-miscarriage resolution.
Outcome generation
Details of how outcome measures were generated are given in Table 2. The ITMS was utilised to capture baseline and outcome data, and to maintain an audit trail. Relevant trial data were transcribed directly into the ITMS. Source data comprised the research clinic notes, hospital notes, self-reports, the EQ-5D-5L questionnaire completed at 21 days post randomisation and subsequently at discharge (for women who obtained an initial positive pregnancy test result), the patient satisfaction survey completed at discharge, and the transcript of the in-depth qualitative interviews. All case report forms are provided in the additional documentation (see Report Supplementary Material 4).
| Outcome assessed | When? | How? | By whom? | PD or SP |
|---|---|---|---|---|
| Baseline data: EQ-5D-5L questionnaire | Day 0 | Face-to-face clinical appointment with participant | Self-administered questionnaire | PD |
| OA1: clinical review | Day 4–5 | Telephone or face-to-face clinical appointment with participant | Local research nurse/midwife or doctor | SP and PD |
| OA2: clinical review ± pelvic ultrasound, to determine whether or not the gestation sac has been expelled | Day 6–7 | Face-to-face clinical appointment with participant | Local research nurse/midwife or doctor | PD (and SP in some hospitals depending on trust policy) |
| OA2: EQ-5D-5L questionnaire | Day 6–7 | Face-to-face clinical appointment with participant | Self-administered questionnaire | PD |
| OA3: pregnancy test | Day 21 ± 2 days (point of discharge for women with negative pregnancy test result) | Clinical records and/or telephone interview or face-to-face clinical appointment with participant | Local research nurse/midwife or doctor | SP |
| OA3: EQ-5D-5L questionnaire | Day 21 ± 2 days (point of discharge for women with negative pregnancy test result) | Telephone or face-to-face clinical appointment with participant | Self-administered questionnaire | PD |
| OA4: final discharge | At discharge | From clinical records or interview with the participant | Local research nurse/midwife or doctor | SP |
| OA4: EQ-5D-5L questionnaire (if initial positive pregnancy test result) and patient satisfaction survey | At discharge | Telephone or face-to-face clinical appointment with participant | Self-administered questionnaires | PD |
| OA4: semistructured qualitative interview | Within 6 weeks of discharge | Face-to-face, via telephone or via video call with participant | Qualitative/mixed-methods researcher | PD |
First outcome assessment (day 6–7 post randomisation)
At the time of randomisation, arrangements for an ultrasound appointment through EPU were made for day 6–7 post randomisation. The research nurse or midwife assisted with booking an appointment if necessary, and was responsible for ensuring that the details of the scan were recorded in the ITMS. If the woman did not have a scan for any reason, this was recorded in the ITMS. If the woman did not attend for an ultrasound scan, or attended for a scan after day 7 post randomisation, then the research nurse or midwife asked the woman if she could provide the date of passing the gestational sac.
Blinded Endpoint Review Committee assessment of the primary outcome
For women who did not undergo an ultrasound scan on day 6 or 7 and had not already passed their gestational sac according to an earlier scan, a Blinded Endpoint Review Committee (BERC) was convened to assess whether or not the primary outcome had been met. The BERC consisted of four medical members of the central trial management group and an independent nurse specialist who works in early pregnancy. All members of the committee were blinded to treatment allocation and were required to sign the BERC charter before the meeting taking place (see Report Supplementary Material 5). The committee was convened at a face-to-face meeting following the end of trial recruitment to review participant data for women who had not undergone ultrasound scanning within 7 days of randomisation.
Before the committee meeting, a medical member of the central trial management group contacted these women to ask when they had experienced their vaginal bleeding and pelvic pain following the trial medications. The date when the participant experienced miscarriage symptoms and the date of any subsequent scan were used to determine whether the gestational sac was passed by day 7. The committee needed to agree unanimously that the participant could have primary outcome data collected. Please see below for some examples of participants who underwent BERC assessment.
Example 1
A participant reported vaginal bleeding and pain for 2 days starting from the day of misoprostol administration and then experienced no further bleeding and underwent ultrasound scan on day 8. The committee agreed that this meant that the participant would have passed the gestational sac by day 7.
Example 2
A participant reported vaginal bleeding on the day of misoprostol administration and no further bleeding thereafter. The ultrasound scan was performed on day 9, and the gestational sac was seen in situ. The committee agreed that this meant that the participant failed to pass the gestational sac by day 7.
The BERC adhered to the prespecified committee charter and convened to discuss the clinical details of each of these participants. The decision of whether or not the primary outcome was met needed to have been decided unanimously by the committee.
Second outcome assessment (day 21 ± 2 days post randomisation)
The second outcome assessment was conducted at 21 days (± 2 days) post randomisation. The research nurse or midwife at each study site contacted the participant to obtain their pregnancy test result. If the result was negative, this was used as the point of discharge from EPU care. If the pregnancy test result was positive, the participant was asked to repeat the pregnancy test until a negative test result was obtained. Several clinical outcomes were collected up to discharge from EPU care, including requirement for surgical intervention, duration of bleeding, requirement for blood transfusion and diagnosis of infection associated with miscarriage. The research nurse or midwife obtained the duration of bleeding by asking the participant and collected the remaining information from clinical notes.
Definition of the end of the trial
The interventional phase of the trial ended when the last woman recruited had taken her last dose of the trial treatment. The observational phase of the trial to assess clinical outcomes ceased and the primary analysis was scheduled to occur when the final outcome of the last woman recruited had been completed and data had been entered onto the database and validated as being ready for analysis.
Notes on adverse events and serious adverse events
All the trial participants were asked to report any hospitalisations, consultations with other medical practitioners, disability, incapacity or any other AEs to their local research team; if the local study nurse or midwife was unavailable for any reason, the participants were able to report the events to the trial manager via telephone at any time. Moreover, at the time of each outcome assessment, investigators and research nurses and midwives at each study centre proactively asked each participant about any AEs in the preceding weeks. AEs were assessed by clinical investigators, further reported as appropriate, and recorded on the ITMS.
Serious AEs and serious adverse reactions (SARs) were recorded on a purpose-designed SAE form. Local investigators notified the trial manager within 24 hours of the local investigators becoming aware of these events. In addition, local investigators were responsible for reporting SAEs to their host institutions, in accordance with local regulations, and instituting supplementary investigations as appropriate based on clinical judgement of the causative factors. SAEs and SARs were followed up until resolution even if this was beyond the participant’s discharge date from EPU care. The trial manager reported all SAEs to the Data Monitoring Committee (DMC) approximately once every 6 months. The DMC viewed data blinded to treatment, but was able to review unblinded data if requested.
Sample size
The MifeMiso trial investigators believed that it was important to ensure that the study was large enough to detect reliably moderate but clinically important treatment effects. The sample size was calculated using the Sample Size Tables for Clinical Studies Software application based on a chi-squared test for comparing two proportions. Our calculations indicated that 670 women would be required to detect a minimally important difference (MID) of a 10% reduction in the rate of failure to spontaneously pass the gestational sac within 7 days (i.e. from 25% to 15%), assuming 90% power and a type I error rate of 5%. However, assuming and adjusting for a worst case scenario of 5% attrition, the total number of participants required was 710 (355 participants in each group). The 25% (95% CI 23% to 27%) control group estimate was taken from our systematic review (unpublished data) and the 10% MID was the most popular selection from our HCP survey (41% of those surveyed). The estimate of the control group rate was monitored throughout the recruitment period by the independent DMC to ascertain if any deviations from this assumption had an impact on the sample size calculation.
Statistical methods
A comprehensive statistical analysis plan (SAP) was drawn up before any analysis and provided to the independent DMC for review. All analyses were prespecified in a SAP. Full details of the statistical analysis can be found in the SAP (see Report Supplementary Material 6).
All women randomised were included in the analysis and were analysed in the group into which they were randomly allocated, regardless of treatment received (an intention-to-treat analysis).
For all binary outcomes, a log-binomial regression model was used to calculate the adjusted RRs and 95% CIs. For continuous outcomes, a linear regression model was used to estimate adjusted mean differences and 95% CIs. All estimates of treatment effects between groups were adjusted for the minimisation variables where maternal age, BMI and gestational age were treated as continuous fixed effects, parity and bleeding score as categorical fixed effects, and randomising centre as a random effect. If covariate adjustment was not possible (i.e. due to lack of convergence when carrying out the modelling procedure), alternative models were explored. The clustering effect of randomising centres was accounted for by using robust standard errors at centre level. When required, p-values from the associated models were produced and used to assess statistical significance.
A hierarchical testing procedure was prespecified to allow for multiple comparisons: the null hypothesis for the primary outcome was tested first and, if it was statistically significant at the 5% level, the key secondary outcome would be tested. Otherwise, no further hypothesis testing would be performed. For all safety outcomes, to assess any signal within specific organ groups, p-values are presented unadjusted for multiple testing. The results from all other secondary outcomes are treated as exploratory rather than confirmatory. No adjustments for multiple testing were made for CIs.
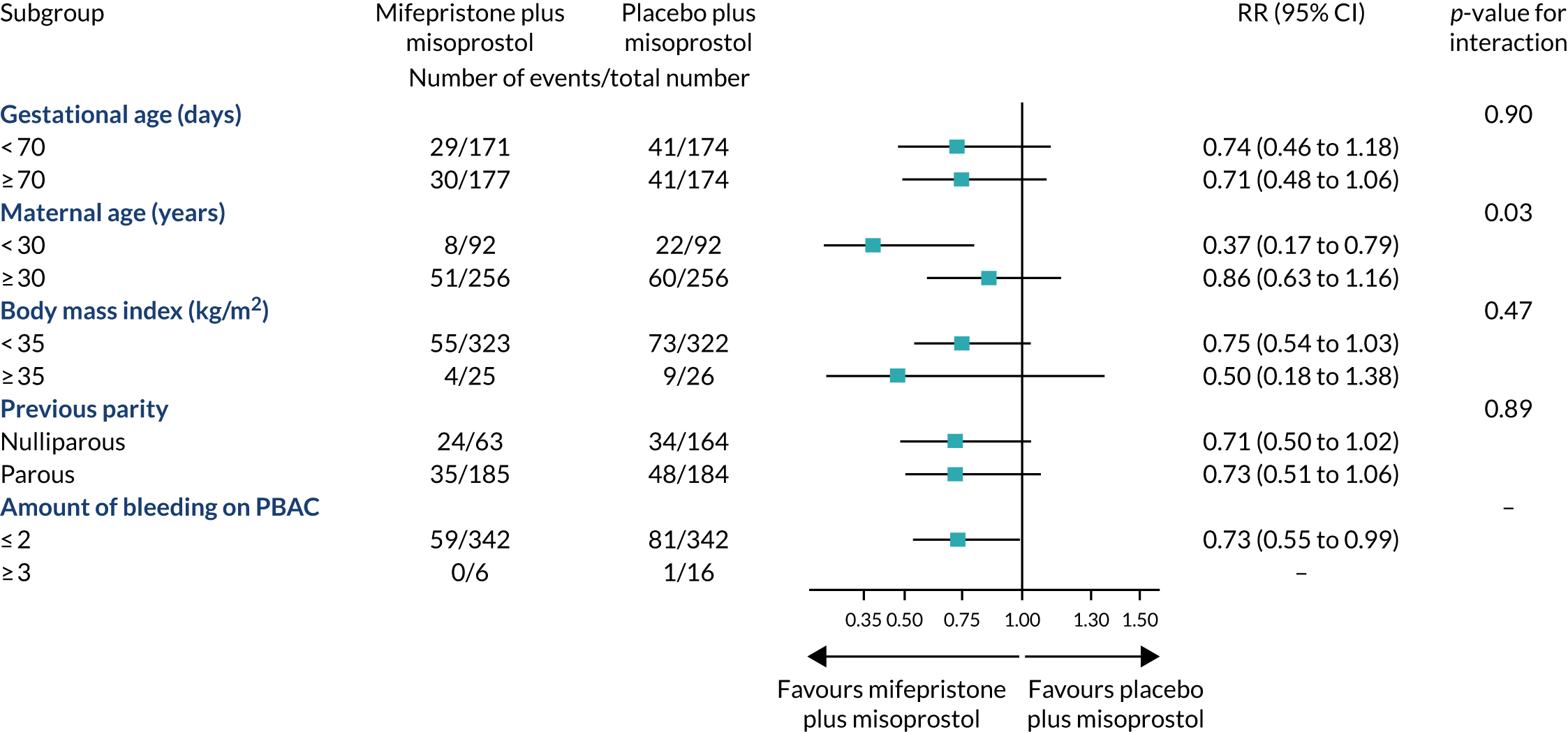
We analysed the treatment effect for the primary outcome in prespecified subgroups defined according to maternal age (< 30 or ≥ 30 years), BMI (< 35 or ≥ 35 kg/m2), previous parity (nulliparous or parous women), gestational age (< 70 or ≥ 70 days) and amount of bleeding (PBAC score,24 ≤ 2 or ≥ 3). The subgroup defined by gestational age was prespecified as of special interest; the results of other subgroup analyses are treated with caution and used for the purposes of hypothesis generation only. 25 The effects of these subgroups were examined by adding the variables for the interaction of subgroup with trial group to the regression model; an F-test was used to determine whether or not the effects of mifepristone and misoprostol differed to the effects of placebo and misoprostol in the various subgroups.
Analyses of principal safety and effectiveness outcomes were performed on behalf of the DMC by the trial statistician (who remained unaware of the treatment assignments) on two occasions during the recruitment period. Because these analyses were performed with the use of the Peto principle,26 no adjustment was made to the final p-values presented.
Trial oversight
Study oversight was provided by a Trial Steering Committee (TSC) (chaired by Mr Rajendra Rai, Imperial College London) and a DMC (chaired by Dr Abha Maheshwari, University of Aberdeen).
The TSC provided independent supervision for the trial, providing advice to the chief investigator, co-investigators and the sponsor on all aspects of the trial throughout the study. The DMC adopted the DAMOCLES27 charter to define its terms of reference and operation in relation to oversight of the MifeMiso trial. The DMC met on an approximately 6-monthly basis during the study.
Chapter 3 Results
Parts of this chapter are reproduced or adapted with permission from Chu et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) license, which permits others to copy and redistribute this work, for non-commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
This chapter reports the results of the MifeMiso trial. It starts with a description of the flow of participants through the trial and is followed by demographic information and results of the primary and secondary outcomes including safety outcomes. The clinical findings from this trial have been previously published in The Lancet. 1
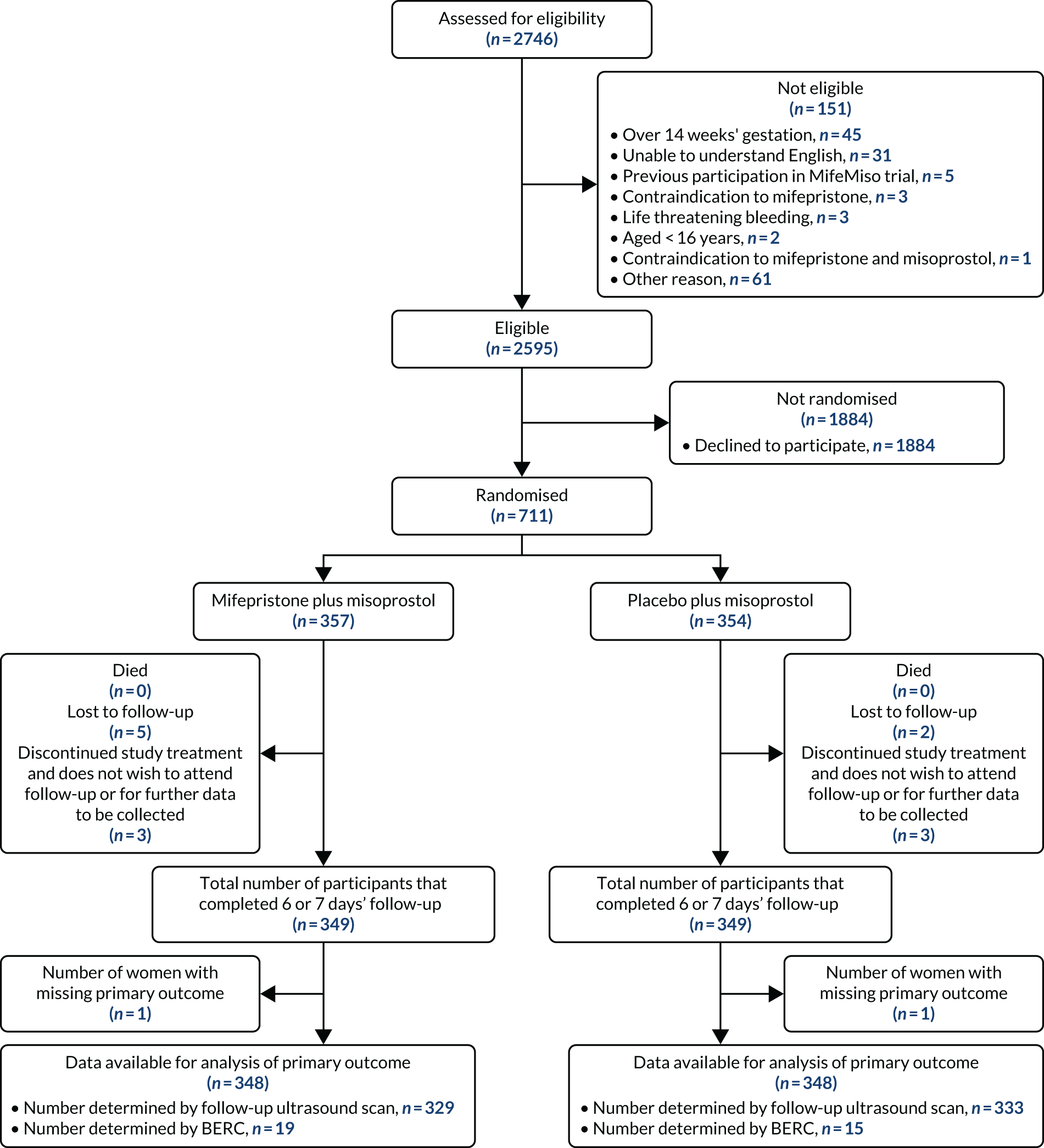
Participant flow
Participant flow is illustrated in Figure 3. A total of 2746 participants were screened for eligibility to take part in the MifeMiso trial, of whom 151 were not eligible for randomisation and a further 1884 declined to participate in the trial. A total of 711 women proceeded to randomisation, with 357 allocated to the mifepristone and misoprostol group and 354 allocated to the placebo and misoprostol group. Six participants were withdrawn from the study, a further seven were lost to follow-up and two had missing primary outcome data. This meant that 696 participants (98% of those randomised) were available for analysis of the primary outcome.
FIGURE 3.
The MifeMiso trial CONSORT flow diagram.
Recruitment
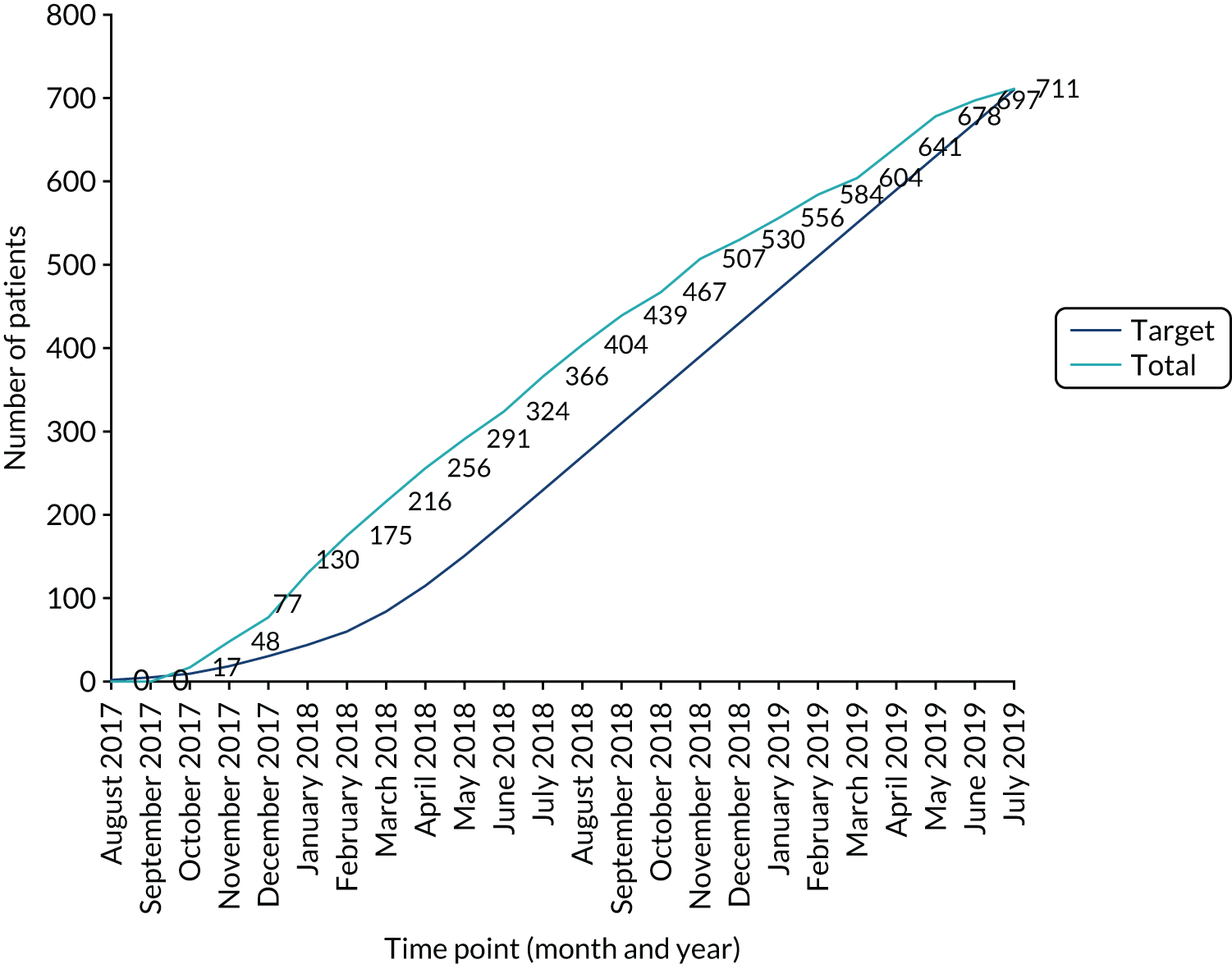
Recruitment and randomisation took place over 22 months in 28 UK NHS hospitals (Figure 4, Table 3) from October 2017 to July 2019 (Figure 5).
FIGURE 4.
Map of the MifeMiso trial recruiting sites. Map data © Google 2021.
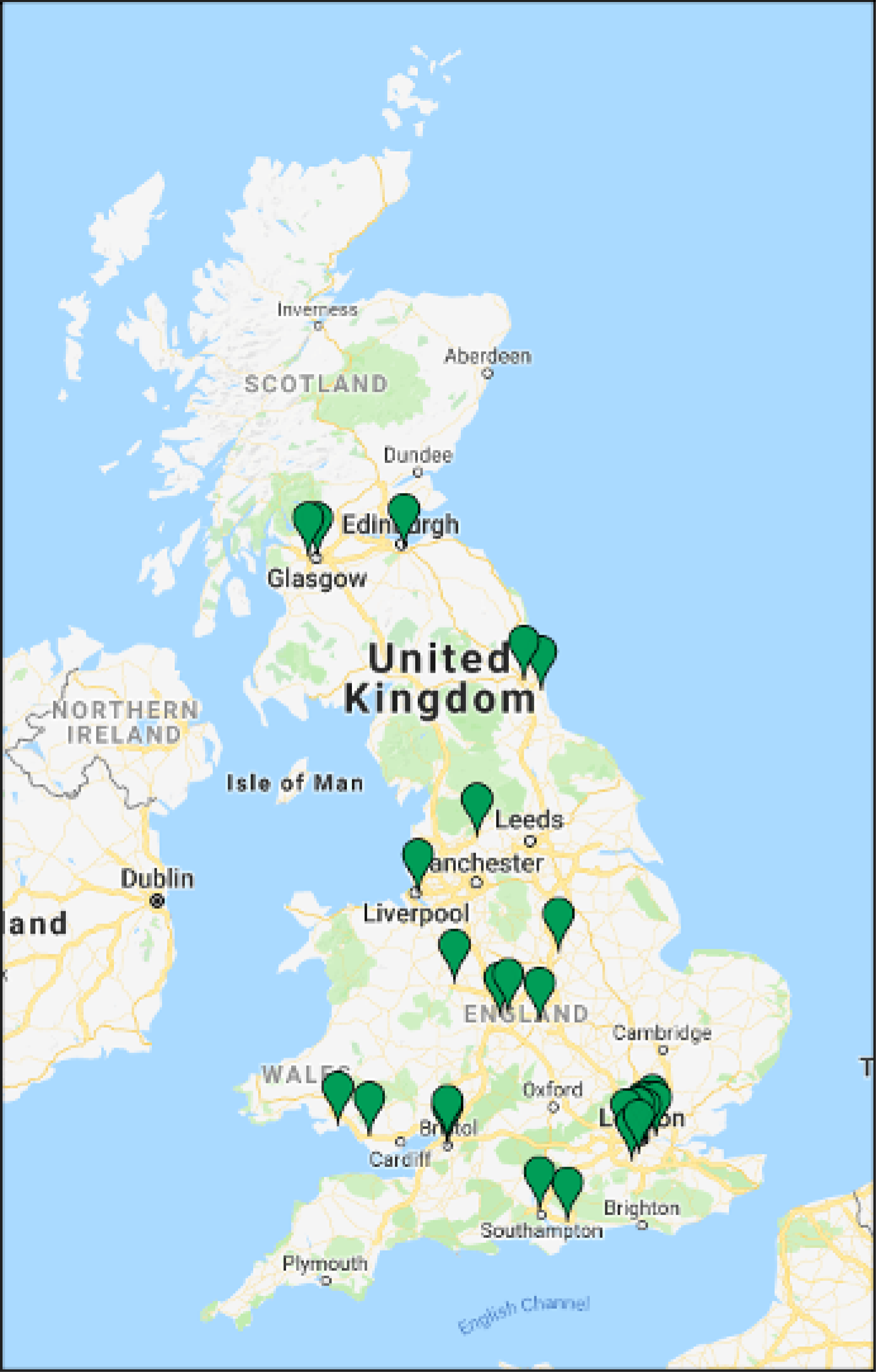
| Centre | PI | Activation date | Total recruitment |
|---|---|---|---|
| Sunderland Royal Hospital | Amna Ahmed | 25 September 2017 | 108 |
| University College London Hospital | Joel Naftalin | 27 September 2017 | 60 |
| West Middlesex University Hospital | Natalie Nunes | 29 November 2017 | 59 |
| St Michael’s University Hospital | Abigail Oliver | 25 September 2017 | 49 |
| Queen Alexandra Hospital | Nirmala Vaithilingam | 20 September 2017 | 47 |
| University Hospital Coventry | Feras Izzat | 25 September 2017 | 38 |
| Singleton Hospital | Frances Hodge | 11 January 2018 | 31 |
| Burnley General Hospital | Kalsang Bhatia | 5 April 2018 | 30 |
| St Thomas’ Hospital | Judith Hamilton | 2 October 2017 | 28 |
| Birmingham Women’s Hospital | Yadava Jeve (previously Ismail Hassan) | 17 November 2017 | 28 |
| Southmead Hospital | Jane Mears | 1 June 2018 | 25 |
| Queen’s Medical Centre, Nottingham | Shilpa Deb | 20 September 2017 | 20 |
| Liverpool Women’s Hospital | Linda Watkins (previously Penny Robshaw) | 9 October 2017 | 18 |
| Chelsea and Westminster Hospital | Natalie Nunes | 29 November 2017 | 18 |
| Princess Royal Hospital, Telford | Martyn Underwood | 6 December 2017 | 18 |
| King’s College Hospital | Jackie Ross | 1 February 2018 | 18 |
| Princess Anne Hospital, Southampton | Ying Cheong | 9 October 2017 | 17 |
| Glasgow Royal Infirmary | Chitra Kumar | 26 October 2017 | 16 |
| Queen Elizabeth University Hospital | Stewart Pringle | 25 October 2017 | 13 |
| Birmingham Heartlands Hospital | Pratima Gupta | 30 November 2017 | 13 |
| Royal Victoria Infirmary, Newcastle | Meenakshi Choudhary | 29 September 2017 | 12 |
| St Helier Hospital | Sangeetha Devarajan | 5 April 2018 | 12 |
| Whipps Cross University Hospital | Anupama Shahid | 2 October 2017 | 11 |
| Royal Infirmary of Edinburgh | Andrew Horne | 3 October 2017 | 11 |
| Royal London Hospital | Anupama Shahid | 11 October 2017 | 4 |
| Epsom Hospital | Sangeetha Devarajan | 8 May 2018 | 4 |
| Princess of Wales Hospital | Frances Hodge | 11 January 2018 | 2 |
| Newham University Hospital | Anupama Shahid | 2 November 2017 | 1 |
FIGURE 5.
Cumulative recruitment to the MifeMiso trial.
Baseline data
The baseline demographic characteristics of participants in the two groups were comparable, with the minimisation algorithm ensuring balance for the factors indicated in Table 4.
| Characteristic | Mifepristone plus misoprostol group (N = 357) | Placebo plus misoprostol group (N = 354) | Overall (N = 711) |
|---|---|---|---|
| Minimisation variables | |||
| Maternal age (years) | |||
| < 30, n (%) | 95 (27) | 95 (27) | 190 (27) |
| ≥ 30, n (%) | 262 (73) | 259 (73) | 521 (73) |
| Mean (SD) | 32.8 (5.6) | 32.7 (5.7) | 32.8 (5.6) |
| Median [IQR] | 33 [29–37] | 33 [29–36] | 33 [29–37] |
| BMI (kg/m2) | |||
| < 35, n (%) | 332 (93) | 328 (93) | 660 (93) |
| ≥ 35, n (%) | 25 (7) | 26 (7) | 51 (7) |
| Mean (SD) | 25.8 (5.6) | 26.5 (5.5) | 26.1 (5.6) |
| Median [IQR] | 24.4 [22.1–27.8] | 25.5 [22.3–29.7] | 24.8 [22.2–28.9] |
| Previous parity, n (%) | |||
| Nulliparous | 167 (47) | 168 (47) | 335 (47) |
| Parous | 190 (53) | 186 (53) | 376 (53) |
| Gestational age (days) | |||
| < 70, n (%) | 176 (49) | 175 (49) | 351 (49) |
| ≥ 70, n (%) | 181 (51) | 179 (51) | 360 (51) |
| Mean (SD) | 70.5 (13.1) | 70.7 (13.8) | 70.6 (13.4) |
| Median [IQR] | 70 [61–81] | 70 [63–82] | 70 [62–82] |
| Amount of bleeding, n (%) | |||
| PBACa score ≤ 2 | 351 (98) | 348 (98) | 699 (98) |
| PBACa score ≥ 3 | 6 (2) | 6 (2) | 12 (2) |
| Other demographic and clinical characteristics | |||
| Ethnicity, n (%) | |||
| White | 296 (83) | 280 (79) | 576 (81) |
| Black | 10 (3) | 17 (5) | 27 (4) |
| Asian | 38 (11) | 42 (12) | 80 (11) |
| Mixed | 5 (1) | 8 (2) | 13 (2) |
| Other ethnic group | 7 (2) | 7 (2) | 14 (2) |
| Missing | 1 (< 1) | 0 (–) | 1 (< 1) |
| Progesterone levels | |||
| Mean (SD) | 17.0 (4.2) | 22.8 (12.8) | 20.9 (10.6) |
| Median [IQR] | 17.0 [14.0–20.0] | 26.0 [12.7–33.0] | 19.5 [14.0–33.0] |
| Not measured, n (%) | 355 (99) | 350 (99) | 705 (99) |
| Missing, n (%) | 0 (–) | 0 (–) | 0 (–) |
| Pregnancy-related pain score at randomisationb | |||
| Mean (SD) | 1.0 (1.8) | 1.2 (2.0) | 1.1 (1.9) |
| Median [IQR] | 0 [0–1] | 0 [0–2] | 0 [0–2] |
| Missing, n (%) | 0 (–) | 0 (–) | 0 (–) |
| Number of gestational sacs, n (%) | |||
| 1 | 351 (98) | 348 (98) | 699 (98) |
| 2 | 6 (2) | 6 (2) | 12 (2) |
| ≥ 3 | 0 (–) | 0 (–) | 0 (–) |
| Missing | 0 (–) | 0 (–) | 0 (–) |
| Days from date of ultrasound scan diagnosing missed miscarriage to randomisation | |||
| Mean (SD) | 1.5 (3.3) | 1.9 (4.6) | 1.7 (4.0) |
| Median [IQR] | 0 [0–1] | 0 [0–1] | 0 [0–1] |
| Not measured | 0 (–) | 0 (–) | 0 (–) |
| Missing, n (%) | 0 (–) | 0 (–) | 0 (–) |
| Current concomitant medication, n (%) | |||
| Yes | 71 (20) | 80 (23) | 151 (21) |
| No | 285 (80) | 274 (77) | 559 (79) |
| Missing | 1 (< 1) | 0 (–) | 1 (< 1) |
| If yes, type of medication,c n (%) | |||
| Analgesic | 7 (10) | 6 (8) | 13 (9) |
| Antibacterial | 5 (7) | 5 (6) | 10 (7) |
| Anticonvulsant | 1 (1) | 2 (3) | 3 (2) |
| Antidepressant | 8 (11) | 7 (9) | 15 (10) |
| Antifungal | 0 (–) | 1 (1) | 1 (1) |
| Anti-inflammatory agents | 1 (1) | 0 (–) | 1 (1) |
| Blood glucose regulators | 4 (6) | 6 (8) | 10 (7) |
| Blood products/modifiers/volume expanders | 1 (1) | 0 (–) | 1 (1) |
| Cardiovascular agent | 2 (3) | 4 (5) | 6 (4) |
| Dermatological agent | 2 (3) | 1 (1) | 3 (2) |
| Gastrointestinal agent | 3 (4) | 4 (5) | 7 (5) |
| Hormonal agents, stimulant/replacement/modifying (sex hormones/modifiers) | 3 (4) | 9 (11) | 12 (8) |
| Hormonal agents, stimulant/replacement/modifying (thyroid) | 12 (17) | 10 (13) | 22 (15) |
| Immunological agent | 1 (1) | 2 (3) | 3 (2) |
| Migraine | 1 (1) | 0 (–) | 1 (1) |
| Respiratory tract agent | 12 (17) | 15 (19) | 27 (18) |
| Sedative/hypnotic | 0 (–) | 2 (3) | 2 (1) |
| Antiplatelet | 1 (1) | 2 (3) | 3 (2) |
| Medical history, n (%) | |||
| Diabetes | |||
| Yes | 4 (1) | 7 (2) | 11 (2) |
| No | 352 (99) | 347 (98) | 699 (98) |
| Missing | 1 (< 1) | 0 (–) | 1 (< 1) |
| Renal disease | |||
| Yes | 3 (1) | 1 (< 1) | 4 (1) |
| No | 353 (99) | 353 (99) | 706 (99) |
| Missing | 1 (< 1) | 0 (–) | 1 (< 1) |
| Cardiac disease | |||
| Yes | 1 (< 1) | 1 (< 1) | 2 (< 1) |
| No | 355 (99) | 353 (99) | 708 (99) |
| Missing | 1 (< 1) | 0 (–) | 1 (< 1) |
| Chronic hypertension | |||
| Yes | 2 (1) | 1 (< 1) | 3 (< 1) |
| No | 354 (99) | 353 (99) | 707 (99) |
| Missing | 1 (< 1) | 0 (–) | 1 (< 1) |
| Thyroid disease | |||
| Yes | 12 (3) | 13 (4) | 25 (4) |
| No | 344 (96) | 341 (96) | 685 (96) |
| Missing | 1 (< 1) | 0 (–) | 1 (< 1) |
| Cancer | |||
| Yes | 2 (1) | 0 (–) | 2 (< 1) |
| No | 354 (99) | 354 (100) | 708 (99) |
| Missing | 1 (< 1) | 0 (–) | 1 (< 1) |
| Other | |||
| Yes | 46 (13) | 44 (12) | 90 (13) |
| No | 310 (87) | 310 (88) | 620 (87) |
| Missing | 1 (< 1) | 0 (–) | 1 (< 1) |
| Number of previous pregnancies, n (%) | |||
| Live birth | |||
| 0 | 63 (18) | 70 (20) | 133 (19) |
| 1 | 111 (31) | 118 (33) | 229 (32) |
| 2 | 45 (13) | 39 (11) | 84 (12) |
| ≥ 3 | 25 (7) | 23 (7) | 48 (7) |
| No previous pregnancies | 112 (31) | 104 (29) | 216 (30) |
| Missing | 1 (< 1) | 0 (–) | 1 (< 1) |
| Stillbirth | |||
| 0 | 238 (67) | 246 (69) | 484 (68) |
| 1 | 6 (2) | 3 (1) | 9 (1) |
| 2 | 0 (–) | 1 (< 1) | 1 (< 1) |
| ≥ 3 | 0 (–) | 0 (–) | 0 (–) |
| No previous pregnancies | 112 (31) | 104 (29) | 216 (30) |
| Missing | 1 (< 1) | 0 (–) | 1 (< 1) |
| Miscarriage | |||
| 0 | 120 (34) | 121 (34) | 241 (34) |
| 1 | 62 (17) | 77 (22) | 139 (20) |
| 2 | 29 (8) | 24 (7) | 53 (7) |
| ≥ 3 | 33 (9) | 28 (8) | 61 (9) |
| No previous pregnancies | 112 (31) | 104 (29) | 216 (30) |
| Missing | 1 (< 1) | 0 (–) | 1 (< 1) |
| Ectopic pregnancy | |||
| 0 | 231 (65) | 241 (68) | 472 (66) |
| 1 | 13 (4) | 9 (3) | 22 (3) |
| 2 | 0 (–) | 0 (–) | 0 (–) |
| ≥ 3 | 0 (–) | 0 (–) | 0 (–) |
| No previous pregnancies | 112 (31) | 104 (29) | 216 (30) |
| Missing | 1 (< 1) | 0 (–) | 1 (< 1) |
| Molar pregnancy | |||
| 0 | 244 (68) | 249 (70) | 493 (69) |
| 1 | 0 (–) | 1 (< 1) | 1 (< 1) |
| 2 | 0 (–) | 0 (–) | 0 (–) |
| ≥ 3 | 0 (–) | 0 (–) | 0 (–) |
| No previous pregnancies | 112 (31) | 104 (29) | 216 (30) |
| Missing | 1 (< 1) | 0 (–) | 1 (< 1) |
| Termination | |||
| 0 | 191 (54) | 192 (54) | 383 (54) |
| 1 | 44 (12) | 45 (13) | 89 (13) |
| 2 | 7 (2) | 10 (3) | 17 (2) |
| ≥ 3 | 2 (1) | 3 (1) | 5 (1) |
| No previous pregnancies | 112 (31) | 104 (29) | 216 (30) |
| Missing | 1 (< 1) | 0 (–) | 1 (< 1) |
| Pregnancy of unknown location | |||
| 0 | 241 (68) | 250 (71) | 491 (69) |
| 1 | 3 (1) | 0 (–) | 3 (< 1) |
| 2 | 0 (–) | 0 (–) | 0 (–) |
| ≥ 3 | 0 (–) | 0 (–) | 0 (–) |
| No previous pregnancies | 112 (31) | 104 (29) | 216 (30) |
| Missing | 1 (< 1) | 0 (–) | 1 (< 1) |
Compliance with treatment
Adherence with the treatment regimen was high in both groups: 94% (337/357 women) in the mifepristone plus misoprostol group and 96% (341/354 women) in the placebo plus misoprostol group (Table 5).
| Outcome | Mifepristone plus misoprostol group (N = 357) | Placebo plus misoprostol group (N = 354) |
|---|---|---|
| Number adherent to treatment regimena | 337 (94) | 341 (96) |
| Number non-adherent | 20 (6) | 13 (4) |
The route of administration for the first dose of misoprostol was vaginal for the vast majority of women who needed to take this intervention [89% (286/321) in the mifepristone plus misoprostol group and 90% (299/334) in the placebo plus misoprostol group]. The number of women who received oral or sublingual misoprostol for their first dose was small, and the same in each group (Table 6).
| Description of interventions | Mifepristone plus misoprostol (N = 357) | Placebo plus misoprostol (N = 354) |
|---|---|---|
| Mifepristone or placebo taken, n (%) | ||
| Yes | 354 (99) | 350 (99) |
| No | 2 (1) | 4 (1) |
| If no, reason, n (%) | ||
| Woman changed her mind | 2 (100) | 3 (75) |
| Sac already passed | 0 (–) | 0 (–) |
| Othera | 0 (–) | 1 (25) |
| Missing | 1 (< 1) | 0 (–) |
| Misoprostol taken, n (%) | ||
| Yes | 321 (90) | 334 (94) |
| No | 35 (10) | 20 (6) |
| If no, reason, n (%) | ||
| Woman changed her mind | 0 (–) | 0 (–) |
| Woman did not attend hospital | 3 (9) | 0 (–) |
| Sac already passed | 27 (77) | 16 (80) |
| Other | 5 (14)b | 4 (20)c |
| Missing | 1 (< 1) | 0 (–) |
| If yes, route of administration of first dose of misoprostol, n (%) | ||
| PV | 286 (89) | 299 (90) |
| PO | 30 (9) | 28 (8) |
| Sublingual | 5 (2) | 7 (2) |
| Missing | 0 (–) | 0 (–) |
| Additional doses of misoprostol taken | ||
| Yes, n (%) | 50 (14) | 65 (18) |
| One additional dose | 40 (80) | 51 (78) |
| Two additional doses | 7 (14) | 12 (18) |
| Three or more additional doses | 3 (6) | 2 (3) |
| Mean number of additional doses (SD) | 1.3 (0.7) | 1.3 (0.6) |
| Median number of additional doses [IQR] | 1 [1–1] | 1 [1–1] |
| No, n (%) | 271 (76) | 269 (76) |
| First dose not taken | 35 (10) | 20 (6) |
| Missing | 1 (< 1) | 0 (–) |
| If yes, route of administration of first additional dose, n (%) | ||
| PV | 36 (72) | 46 (71) |
| PO | 7 (14) | 11 (17) |
| Sublingual | 7 (14) | 8 (12) |
| Missing | 0 (–) | 0 (–) |
| If yes, dose of first additional dose (µg) | ||
| Mean (SD, n) | 656.0 (185.3, 50) | 692.3 (169.8, 65) |
| Median [IQR] | 800 [400–800] | 800 [600–800] |
| Missing | 0 (–) | 0 (–) |
| If yes, days since mifepristone/placebo of first additional dose | ||
| Mean (SD, n) | 6.7 (6.6, 50) | 6.2 (6.8, 64) |
| Median [IQR] | 4 [3–7] | 4 [4–7] |
| Missing | 0 (–) | 1 (2) |
Primary outcome results
The proportion of participants who did not pass the gestational sac spontaneously within 7 days was 17% (59/348 women) in the mifepristone plus misoprostol group and 24% (82/348 women) in the placebo plus misoprostol group (RR 0.73, 95% CI 0.54 to 0.98; p = 0.04) (Table 7).
| Mifepristone plus misoprostol group (N = 357) | Placebo plus misoprostol group (N = 354) | RRa (95% CI) | p-value | |
|---|---|---|---|---|
| Primary outcome – failure to pass the gestational sac spontaneously within 7 days of randomisation | ||||
| Yesb | 59 (17.0) | 82 (23.6) | 0.73 (0.54 to 0.98) | 0.035 |
| No | 289 (83.0) | 266 (76.4) | ||
| Missing | 9 | 6 | ||
Secondary outcome results
For the prespecified key secondary outcome, the proportion of participants who required surgical intervention to resolve the miscarriage was 17% (62/355 women) in the mifepristone plus misoprostol group, as compared with 25% (87/353 women) in the placebo plus misoprostol group (RR 0.70, 95% CI 0.52 to 0.94; p = 0.02) (Table 8). Among the women who underwent surgery, the most common reason for surgery was pregnancy tissue remaining in the uterus: 89% (55/62 women) in the mifepristone plus misoprostol group, and 91% (79/87 women) in the placebo plus misoprostol group.
| Mifepristone plus misoprostol group (N = 357) | Placebo plus misoprostol group (N = 354) | RRa (95% CI) | p-value | |
|---|---|---|---|---|
| Key secondary outcome – surgical intervention to resolve the miscarriage up to discharge from EPU | ||||
| Yesb | 62 (17) | 87 (25) | 0.70 (0.52 to 0.94) | 0.019 |
| No | 293 (83) | 266 (75) | ||
| Missing | 2 | 1 | ||
| Reason for surgeryc | ||||
| Pregnancy tissue remaining | 55 (89) | 79 (91) | ||
| Significant bleeding | 19 (31) | 14 (16) | ||
| Other | 8 (13) | 6 (7) | ||
| Missing | 0 (–) | 0 (–) | ||
| Outcome of surgery | ||||
| Complicatedc | 4 (6) | 5 (6) | ||
| Bleeding at surgery | 4 (100) | 3 (60) | ||
| Uterine damage | 0 (–) | 0 (–) | ||
| Need for extensive surgical intervention | 2 (50) | 0 (–) | ||
| Other | 1 (25) | 4 (80) | ||
| Missing | 0 (–) | 0 (–) | ||
| Uncomplicated | 58 (94) | 82 (94) | ||
| Missing | 0 (–) | 0 (–) | ||
| Surgical intervention to resolve the miscarriage up to and including day 7 post randomisation | ||||
| Yesb | 23 (6) | 19 (5) | 1.23 (0.68 to 2.21)d | |
| No | 332 (94) | 334 (95) | ||
| Missing | 2 | 1 | ||
| Surgical intervention to resolve the miscarriage after day 7 and up to discharge from EPU | ||||
| Yesb | 39 (11) | 68 (19) | 0.56 (0.39 to 0.79) | |
| No | 316 (89) | 285 (81) | ||
| Missing | 2 | 1 | ||
| Need for further doses of misoprostol within 7 days after randomisation | ||||
| Yesb | 34 (10) | 48 (14) | 0.73 (0.45 to 1.18) | |
| No | 322 (90) | 306 (86) | ||
| Missing | 1 | 0 (–) | ||
| Need for further doses of misoprostol up to discharge from EPU care | ||||
| Yesb | 50 (14) | 65 (18) | 0.78 (0.54 to 1.12) | |
| No | 307 (86) | 289 (82) | ||
| Missing | 0 (–) | 0 (–) | ||
| Infection requiring outpatient antibiotic treatment | ||||
| Yesb | 8 (2) | 11 (3) | 0.72 (0.29 to 1.78)d | |
| No | 343 (98) | 340 (97) | ||
| Missing | 6 | 3 | ||
| Infection requiring inpatient antibiotic treatment | ||||
| Yesb | 5 (1) | 4 (1) | 1.10 (0.30 to 4.01)d | |
| No | 346 (99) | 347 (99) | ||
| Missing | 6 | 3 | ||
| Negative pregnancy test result 21 days (± 2 days) after randomisation | ||||
| Yesb | 237 (77) | 230 (76) | 1.02 (0.93 to 1.11)e | |
| No | 71 (23) | 72 (24) | ||
| Test not provided | 33 | 28 | ||
| Missing | 16 | 24 | ||
| Overall patient satisfaction score (CSQ-8)f | ||||
| Mean (SD, N) | 29.1 (3.8, 265) | 29.2 (3.8, 256) | –0.05 (–0.50 to 0.40) | |
| Median [IQR] | 31 [27–32] | 31 [28–32] | ||
| Duration of bleeding reported by woman (days) | ||||
| Mean (SD, N) | 16.0 (12.6, 326) | 16.3 (15.2, 330) | –0.44 (–2.73 to 1.84) | |
| Median [IQR] | 13 [8–19] | 12.5 [7–19] | ||
| Time from randomisation to discharge from EPU care (days) | ||||
| Mean (SD, N) | 27.0 (14.2, 340) | 27.3 (14.4, 337) | ||
| Median [IQR] | 21 [21–28] | 21 [21–27] | ||
The proportion of participants who required further doses of misoprostol up to discharge was 14% (50 out of 357 women) in the mifepristone plus misoprostol group, as compared with 18% (65 out of 354 women) in the placebo plus misoprostol group (RR 0.78, 95% CI 0.54 to 1.12). The mean time from randomisation to discharge was 27 days (SD 14.2) in the mifepristone plus misoprostol group, as compared with 27.3 days (SD 14.4) in the placebo plus misoprostol group. There was no evidence of a subgroup effect according to gestational age, which was prespecified as a subgroup of special interest (Figure 6).
FIGURE 6.
Subgroup analyses.
There was no evidence of a between-group difference in the proportions of participants experiencing a SAE: 5 out of 357 women (1%) in the mifepristone plus misoprostol group and 2 out of 354 women (1%) in the placebo plus misoprostol group. Similarly, there was no evidence of a difference in the proportions of participants experiencing an AE: 26 out of 357 women (7%) in the mifepristone plus misoprostol group and 24 out of 354 women (7%) in the placebo plus misoprostol group (Table 9). For further detailed information on AEs, see Report Supplementary Material 7.
| Mifepristone plus misoprostol group (N = 357) | Placebo plus misoprostol group (N = 354) | Adjusted RRa (95% CI) | p-value | |
|---|---|---|---|---|
| Blood transfusion requiredb | ||||
| Yes | 11 (3) | 5 (1) | 2.15 (0.76 to 6.12)c | 0.150 |
| No | 341 (97) | 346 (99) | ||
| Missing | 5 | 3 | ||
| Any side effects (AEs)b,d | ||||
| Yes | 26 (7) | 24 (7) | 1.11 (0.77 to 1.61) | 0.576 |
| No | 331 (93) | 330 (93%) | ||
| Deathd | ||||
| Yes | 0 (–) | 0 (–) | Unable to calculate | Unable to calculate |
| No | 357 (100) | 354 (100) | ||
| Any serious complications (SAEs)b,d | ||||
| Yes | 5 (1) | 2 (1) | 2.33 (0.45 to 11.95)c | 0.311 |
| No | 352 (99) | 352 (99) | ||
Sensitivity analysis
A sensitivity analysis was conducted to exclude the 34 cases that were included in the primary outcome analysis following review by the BERC. This analysis showed that the proportion of participants who did not pass the gestational sac spontaneously within 7 days was 18% (59 out of 348 women) in the mifepristone plus misoprostol group and 24% (81 out of 348 women) in the placebo plus misoprostol group (RR 0.75, 95% CI 0.56 to 1.00; p = 0.05) (Table 10).
| Mifepristone plus misoprostol group (N = 357) | Placebo plus misoprostol group (N = 354) | RRa (95% CI) | p-value | |
|---|---|---|---|---|
| Primary outcome – failure to pass the gestational sac spontaneously within 7 days of randomisation | ||||
| Sensitivity analysis – excluding women requiring primary outcome information provided by BERC | ||||
| Yesb | 59 (17.9%) | 81 (24.3%) | 0.75 (0.56 to 1.00) | 0.052 |
| No | 270 (82.1%) | 252 (75.7%) | ||
| Number excluded | 19 | 15 | ||
| Missing | 9 | 6 | ||
Interpretation of the clinical findings
Our large multicentre, randomised, double-blind, placebo-controlled trial showed that the combination treatment of mifepristone plus misoprostol resulted in an increase in the number of missed miscarriages that were completed within 7 days compared with misoprostol alone. There were also fewer incidences of surgical management to complete miscarriage in the mifepristone plus misoprostol group than in the placebo plus misoprostol group. These findings are consistent with other previously published trials. 19,28,29 The results from our trial show clearly the importance of optimising the medical management of missed miscarriage using the combined mifepristone and misoprostol treatment regimen, which improves outcomes and safety by increasing the proportion of women who have miscarriage resolution and by reducing the need for surgical management. Women choosing medical management of missed miscarriage often wish to have expedited treatment and resolution of their miscarriage while also avoiding the risks of surgery. 30 The risks of miscarriage surgery are dependent on the clinical context, the setting in which the procedure is performed, the surgeon and the available equipment. Complications of miscarriage surgery are rare, but can include bleeding, infection and uterine perforation requiring more extensive surgery, which carries significant morbidity. 30 Our trial findings demonstrate that the combination treatment of mifepristone and misoprostol reduces the need for surgery after medical management and this is likely to be of great importance to women wishing to undergo medical management of missed miscarriage.
It is also important to note that the women who received placebo and misoprostol can be considered to have had an extra 2 days of expectant management compared with women receiving mifepristone and misoprostol. If the mifepristone was not found to have a beneficial effect in achieving passage of the gestational sac, one would have expected either similar rates of miscarriage resolution by day 7 or higher miscarriage resolution rates in the placebo and misoprostol group.
The strengths of this study include its multicentre approach, increasing generalisability across a wide range of settings, and the placebo-controlled design with high adherence to treatment, enhancing the trial’s internal validity. A pragmatic trial design was used in our study, which also adds to the generalisability of our findings. In particular, the route of administration of misoprostol reflects standard UK clinical practice and NICE guidance for the medical management of missed miscarriage. 6 The majority of our participants received vaginal misoprostol and it is important to note that the route of misoprostol administration was similar in both trial groups. The primary outcome used in the MifeMiso trial was carefully selected through a consultation and survey of clinicians working with women diagnosed with miscarriage and the women themselves through patient and public involvement (PPI). We were able to collate near-complete data for primary outcome, which was aided by the use of a BERC. The committee convened using a strict charter and considered each individual participant’s clinical data in turn. The clinical data were collected on a standardised case report form and the decision as to whether or not the participant had met the primary outcome could only be made unanimously. The sensitivity analysis excluding the findings of the BERC does not alter the conclusions of our trial and is consistent with the primary analysis, showing a similar treatment effect and 95% CI.
Some limitations of our study should also be considered. We studied the effect of mifepristone and misoprostol in missed miscarriage; therefore, these results are not generalisable to patients diagnosed with incomplete miscarriage, who will already have passed some pregnancy tissue. The biological rationale for focusing exclusively on missed miscarriage in this trial is that the antiprogestogenic effect of mifepristone is less likely to be relevant in incomplete miscarriage, as in this case the expulsion of pregnancy tissue has already begun. 31
Chapter 4 Women’s perspectives on the acceptability of and their satisfaction with medical management of missed miscarriage: a nested qualitative study
Introduction
Medical management of miscarriage was introduced as an alternative to surgical or expectant management > 20 years ago. 32 Quantitative studies exploring the efficacy of medical management, with administration of either misoprostol or a combination of misoprostol and mifepristone, have also considered women’s satisfaction with the treatment and the factors influencing acceptability. 32–38
These studies have established that medical management is an effective alternative to surgical management or expectant management. 19,34,35,38,39 Furthermore, they have identified benefits to medical management, including lower health-care costs,35,40 and avoidance of risks associated with surgical management. 34 Medical management has also been found to offer an alternative to expectant management that is responsive to women’s increasing preference for a timely resolution to the miscarriage. 41
However, research has also highlighted that women’s satisfaction with medical management may be limited in certain contexts. In particular, it is suggested that satisfaction is dependent on the success of medical management in achieving a complete resolution to the miscarriage. 32,36,37,42 These studies have shown that women who experience treatment failure resulting in the need for surgical intervention experience lower quality of life36 and lower satisfaction with treatment37,42 and are less likely to say that they would choose medical management again in the event of further miscarriages. 32,37 This finding is further reinforced in a nested qualitative study within a trial of different types of miscarriage management that explored women’s experiences of management options. 34,43 Smith et al. 43 found that, although medical management is viewed as an acceptable alternative to surgery, women’s view of it becomes negative when it fails and surgery is then required. This finding contrasts directly with women’s perspectives after expectant management had failed, where they did not perceive the resulting need for surgery as a negative outcome. 43 It seems possible that lower satisfaction may at least in part reflect the need for two forms of active management as opposed to only one, in the case of women who initially opted for expectant management, particularly as women had expressed concerns about the lengthy duration of the process of medical management. 43
Qualitative and mixed-methods studies have considered aspects of the experience of miscarriage treatment, and some of these have addressed factors influencing the acceptability of medical management. 41,43–46 The results suggest that acceptability is influenced by a range of factors, including the wish to avoid the risks associated with surgical intervention, such as adhesions or scarring, and a fear of either anaesthesia or hospital intervention. 41,43,44 Women may also choose medical management because they perceive it to be more aligned with a natural experience of miscarriage, including being able to grieve and experience the loss. 41,43 In addition, it seems possible that medical management provides some women with a greater sense of control than can be achieved with expectant management, particularly given that women express the desire to bring an end to the miscarriage in a timely manner. 43
However, in both qualitative and quantitative studies, women report negative side effects associated with medical management, including significant levels of pain, cramping and bleeding as well as nausea and sickness. 43,47,48 It has also been reported that bleeding, pain and prolonged treatment reduce the acceptability of medical management to women, particularly if the treatment is subsequently unsuccessful. 35,36
It is also important to recognise that satisfaction and acceptability of medical management cannot be viewed in isolation of the care process within which it is delivered. Both UK and international qualitative studies exploring women’s perceptions of miscarriage care have found that choice about treatment options, adequate information-giving, control over where treatment takes place, the environment of care and follow-up support can also significantly influence the acceptability of and satisfaction with treatment for miscarriage. 43,44 Women and their partners have also reported the value of empathic, sensitive and responsive HCPs in assisting them to cope with the miscarriage and promote their recovery from the psychological and physical consequences. 49,50 However, many of these studies evaluate all forms of management; thus, it is difficult to determine those aspects of satisfaction that are specific to medical management.
It is evident that satisfaction with and acceptability of medical management of miscarriage is a complex area, with many possible factors influencing women’s choice of medical management, their experience of it and whether or not they would choose it again. However, limited literature exists that specifically explores in depth women’s perspectives on the process of medical management and considers which factors may be important to women in determining the acceptability of medical management and their satisfaction with it.
Furthermore, different forms of miscarriage occur in the first trimester of pregnancy, including missed or silent miscarriage. 51 Missed miscarriage is defined as the presence of a pregnancy either with a fetus but no evidence of fetal heart activity or with an empty gestational sac. 51,52 In missed miscarriage, women may not have experienced any signs of miscarriage until later in the first trimester and may not know the baby has died until the dating scan, even though fetal death may have occurred some weeks before. Some women will have had a feeling that something is wrong, but these concerns may have been dismissed by themselves or by HCPs. 52,53 In addition, some women continue to experience pregnancy symptoms even though their baby has died. 51–54
Although medical management is recommended for missed miscarriage,55 there is limited evidence of differences in satisfaction between women diagnosed with different types of miscarriage and how this might influence their choices and experience of treatment. One recent study54 concluded that the type of treatment was more important than the type of pregnancy loss in all miscarriages, with treatment effectiveness and timeliness strongly influencing recovery. However, other studies have suggested that the experience of missed miscarriage may be different from that of other types of miscarriage. 53,56 For example, Adolfsson et al. 56 found that women experiencing a missed miscarriage have prolonged and greater levels of grief and difficulty coping than women experiencing other forms of miscarriage. It seems plausible that the features of missed miscarriage, such as those mentioned above, may create particular challenges in emotional processing of the diagnosis. They may also influence decision-making and experience of treatment, given that unspoken emotional considerations have been found to influence women’s decision-making about miscarriage treatment. 44 However, as far as we are aware, an in-depth understanding of women’s experience of the process of medical management of missed miscarriage and their satisfaction with it has not been explored. Consequently, further in-depth research is required to develop our understanding.
Aim of the study
To explore women’s satisfaction with and acceptability of medical management of missed miscarriage for those who complete the MifeMiso trial protocol.
Research questions
-
Why do women choose medical management?
-
Why do women choose to participate in the trial?
-
What is their lived experience of the process of treatment and care?
-
What factors influence the acceptability of and satisfaction with the treatment they have received?
-
Does receipt of mifepristone with misoprostol influence women’s satisfaction with and acceptability of medical management?
-
What implications does this have for the care offered to women and their partners?
Methods
Study design and rationale
To address the identified aim and research questions, we undertook a qualitative study nested within the MifeMiso trial, which sought to develop an understanding of:
-
women’s experience of the trial treatment, considering the structures, resources and processes occurring during their participation as well as their perceptions concerning the treatment itself
-
the contextual factors influencing women’s experience of the trial treatment and their satisfaction with it. 57,58
In conducting this study, we therefore sought to contribute an in-depth understanding to the overall findings of the trial, by addressing aims that illuminate aspects of women’s experience that are currently less well understood but that may influence their satisfaction with medical management and its acceptability. 57 Results such as this can make substantial contributions to the implementation of findings of the trial in the future. 57,59
The primary source of data collection was qualitative, semistructured interviews. Semistructured interviews are a widely used method of data collection in studies that explore people’s personal experiences and which aim to develop a deep understanding of their attitudes, perceptions and beliefs. Interviews are particularly suited to discussing sensitive topics when an open-ended approach is needed. 60
Two additional sources of data collection were utilised. All participants taking part in the MifeMiso trial were asked to complete the CSQ-8. 61 This questionnaire was designed for use in health studies and has been used recently in treatment studies for miscarriage. 42 The CSQ-8 has eight questions, each of which offers responses ranging from quite dissatisfied (scoring 1 point) to very satisfied (scoring 4 points). A total score of 32 can, therefore, be achieved. The higher the total score, the more satisfied the woman is with the treatment she has received. In this study, the CSQ-8 was utilised as a sampling frame when selecting participants for interview. In addition, participants completing the CSQ-8 were invited to provide additional thoughts concerning their satisfaction in a free-text box at the end of the questionnaire. These free-text comments were used as an additional source of data.
Methodological approach
We have framed this qualitative study within an epistemological position of constructivism62 and an ontological position of critical realism. 63 Constructivist theory proposes that there is no single unassailable truth; rather, meaning is constructed by individuals to make sense of their world, and these meanings are constantly being revised and re-interpreted. 64,65 By using a constructivist epistemology, we seek to understand the multiple realities and meanings that individuals create, by asking questions that relate to complicated features of human activity. 57,65 In applying constructivism in this study, we seek to understand how medical management of missed miscarriage is perceived by women and why they experience the treatment as acceptable or unacceptable. Although constructivism is grounded in relativism, it is argued that qualitative health-care research is commonly based on assumptions about the objective existence of specific conditions, such as, in this research, miscarriage. 63 Willig63 therefore suggests that it is appropriate to adopt a critical realist ontology alongside a constructivist epistemology, in which it is accepted that miscarriage exists, but that what we are concerned with is how medical management of miscarriage is constructed and understood by the women who experience it.
Recruitment
Recruiting HCPs provided eligible women with a PIS and informed them about the opportunity to participate in a qualitative interview when they were approached to take part in the trial. A subsequent invitation was provided at day 21, when they were asked to complete the CSQ-8 satisfaction survey. At this point, women self-selected to be approached for participation in the interviews by providing their contact details and indicating their agreement to be contacted by the qualitative research team. Satisfaction survey questionnaires were returned directly to the trials office along with signed permission to be contacted about an interview. These contact details were then passed onto the qualitative research team (JLFP and LJ).
Satisfaction scores were used as an initial sampling frame. We purposively selected participants from those who were satisfied/found the treatment acceptable, and those who did not. We also sought to increase the diversity by purposively recruiting a maximum variation sample of participants according to trial site, age, BMI, gestational age, parity and ethnicity. Within this context we sought to achieve diversity in the sample to ensure that a broad range of views, characteristics and experiences of participation were obtained.
We recruited women for interview between January 2018 and March 2019. Of the 238 women agreeing to be approached, we purposively sampled 85 (35.71%) for follow-up. After completion of the trial protocol and discharge from the service, we contacted the women using their stated preferred method (telephone, e-mail or post) and provided them with another PIS. If no response had been received, we contacted women again between 1 and 2 weeks later. Over the 15-month period, 50 (58.82%) women initially responded positively to contact and agreed to participate, although eight (9.4%) of these women subsequently withdrew before an interview could take place. All interviews were conducted within 6 weeks of completion of the trial protocol. Figure 7 provides a summary of the recruitment process and identifies reasons for non-participation where available.
FIGURE 7.
Summary of recruitment January 2018 to March 2019.
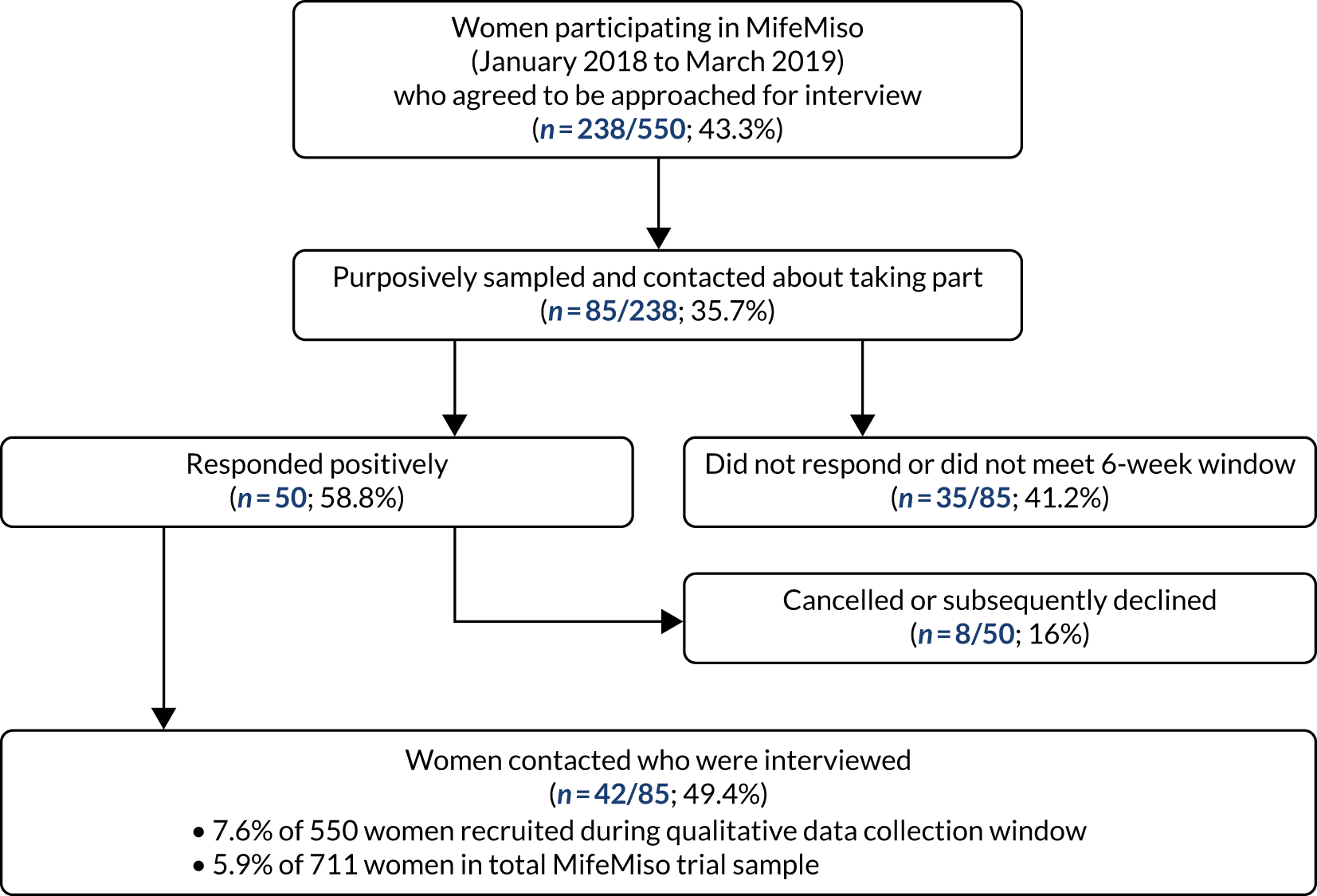
Consent
Women who responded positively to our initial approach were offered further information concerning the purpose of the interviews and their rights regarding participation and withdrawal. We gave them the opportunity to ask questions and time to consider whether or not they wanted to take part, although the majority indicated that they did not require this. If women agreed to participate, we set an interview date and consent forms were posted along with a prepaid reply envelope. Completed consent forms were returned to the trial office. Consent was discussed again at the agreed interview date for all women and any further questions were addressed before commencing the discussion.
Data collection
Semistructured interviews were the primary source of data. We developed the interview guide through a process of reflection and discussion, considering relevant literature and the aims of the research. During our discussions, we also explored any bias we might hold by exploring our preconceptions and personal experiences that could influence our thinking in this research. The interview guide explored the main areas outlined in Box 1.
-
The woman’s journey from discovering her pregnancy through to the diagnosis of missed miscarriage.
-
Previous experience of miscarriage, related treatment and management.
-
Previous experience of childbirth.
-
Reasons for choosing medical management and for participating in the trial.
-
Experience of the treatment and the care received.
-
Satisfaction with treatment and care.
-
Whether or not women would choose medical management again and recommend it to others.
-
What care and treatment women perceive should be offered for miscarriage in the future.
We used the interview guide flexibly to respond to the women’s pace and experience and encouraged each participant to tell her story in the way that best suited her. The guide was subject to development over the duration of the interviews as we moved iteratively between analysis and interviews to ensure that early themes achieved saturation and no new themes were interpreted. 66
Interviews lasted on average 59 minutes (range 28 to 89 minutes). Thirty-eight (90.5%) of the interviews were conducted by telephone, three (7.1%) face to face and one (2.38%) by Skype. Although it was possible to offer (where the researcher was able to travel) a face-to-face interview as an alternative to 10 women, seven chose telephone as the medium they preferred. There may be a number of reasons influencing the choice of telephone interviews; for example, some of the women participating had returned to work and/or were caring for their children. As a result, interviews commonly took place in the evening or during women’s days off from work. It is also possible that the anonymity offered by telephone interviews was valued, given the sensitivity of the research. 67 Where interviews were face to face, two took place in the participants’ own home at their request. One face-to-face interview occurred in a private space at the University of Birmingham.
Ethics
For many women, the experience of miscarriage represents an unanticipated loss that has significant implications for psychological and emotional well-being. 7,56,68–70 Consequently, research with women at such a vulnerable time required us to be reflexive and sensitive to the potential distress that revisiting the experience might cause, particularly given that the interviews were occurring very soon after their miscarriage. Furthermore, some of the women had identified difficult experiences during previous miscarriage or birth-related trauma. To address potential difficulties we implemented a number of strategies:
-
All women who were approached were provided with a list of support services (via the PIS) they could access to address their emotional and informational needs.
-
We employed ethical mindfulness during the recruitment and interview process,71,72 considering women’s experiences and addressing needs as these arose. This included explaining what would be involved in the interview during initial discussions, stating that they could pause or stop the interview as required and maintaining awareness of the woman’s emotional well-being during the interview.
-
Any questions regarding additional support needs were addressed at the end of the discussion following completion of the interview.
-
At the end of the interview, we sought feedback from women about the interview process and used their feedback to adapt our approach as appropriate.
-
Reflexive notes were maintained for each interview.
-
We engaged in regular reflexive discussions following interviews.
Reflexive statement
I [JLFP] am a research fellow and trained as a mental health nurse, although I worked in a different area of health during my career as a nurse. I have an interest in women’s health and particularly the psychological and emotional needs of women in health care. I am also a woman and have one child of my own, two step-children and five grandchildren. I have experienced miscarriage and have undergone fertility treatment, both of which contributed to my interest in this research study.
My experience as a mental health nurse and my work in research with vulnerable populations has sensitised me to the potential challenges and sensitivities that may be present when conducting research with women in this context. However, I also appreciate that this and my personal experience can lead to assumptions about how a woman might view her miscarriage. For example, there is a risk that I uncritically apply my own experience and values and, therefore, assume that miscarriage is only a negative experience, which could influence the way questions were phrased, how I responded to women during interviews and how I analysed the data.
Consequently it was very important that I engaged in reflexive practice involving ethical mindfulness71,72 throughout the period of planning the interviews, recruitment, interviewing and analysis. In addition, my work as a health-care professional sensitised me to the potential challenges both women and health-care services might experience but I needed to ensure that these experiences informed rather than dominated my thinking during the research and analysis. Research supervision and reflection with LJ was, therefore, an essential process to ‘check in’, explore and challenge perceptions, as well as reflect on my own emotional well-being.
Analysis
Following completion of the interviews, all recordings were transcribed to a clean verbatim standard by a GDPR compliant transcription company. JLFP checked transcripts for consistency with the recordings and anonymised them, removing identifying details including names, locations and other details as appropriate. Interviews were then uploaded to NVivo qualitative software version 12 (QSR International, Warrington, UK) to support data analysis.
We used qualitative thematic analysis (QTA),73 informed by our theoretical position, to analyse the 42 interview transcripts. QTA is described as a flexible approach to analysis that has theoretical freedom; thus, it is possible to apply a constructivist paradigm and an inductive approach to arrive at a detailed interpretive account of the data. 73 These authors describe the analytical approach as involving six steps.
Step 1 involves becoming familiar with the data. JLFP read transcripts repeatedly alongside listening to the associated recordings, identifying emotions and emphasis. JLFP took notes to identify areas of interest and potential recurring messages that were discussed with LJ. LJ read a sample of the transcripts and compared thoughts and ideas on an initial coding structure with JLFP. At this point in the analysis of early interviews, an interpreted analytic memo reflected the variation in women’s experience of the scan and the sonographer’s approach when the diagnosis of missed miscarriage was made. JLFP then carried out steps 2 and 3 in tandem, involving developing initial codes and beginning to group nodes into low level themes. In developing the analytical memo further as other interviews contributed to these initial interpretations, two nodes were identified, reflecting different dimensions of the interaction between the woman and the sonographer (Box 2).
Putting my needs at the centre ↔ focusing on the task.
Sensitively communicated ↔ lack of care in communication.
Given that part of the focus of the research was to explore women’s experience of the process of delivering the trial, these nodes were then collected together under the theme ‘the experience of the scan’. As interviews were conducted over a prolonged period of time, this step involved coding new interviews at nodes and grouping nodes into themes, which then informed subsequent interviews in an iterative process. These were regularly reviewed in analytical meetings between LJ and JLFP. Steps 1, 2 and 3 were completed following the end of data collection. Step 4 then involved exploring, reviewing and refining the content of the themes to determine if they belonged together or were more appropriately situated elsewhere or in new nodes, followed by a review of the whole set of themes. This process involved revisiting transcripts and holding analytical meetings to discuss progress and coding.
We determined that specific features of these and other nodes grouped under the theme ‘experience of the scan’ needed to remain within the context of a descriptive superordinate theme named ‘the pathway of care’ (not reported here). However, other aspects of the theme were more appropriately located in a separate theme that addressed the contextual factors influencing women’s satisfaction with medical management. This theme was labelled ‘empathic’, as women explicitly referred to the extent to which the HCPs involved in their care were empathic towards themselves and their partners. At step 5, JLFP further developed and refined the themes through discussion with LJ, revisiting data as appropriate to develop an overall thematic map. In this context, ‘empathic’ was determined as a subtheme of ‘alongside me’, reflecting the relative importance women placed on the extent to which HCPs were able to be present, empathic and responsive to their needs and enabled women to feel supported during their care. Analysis of the free-text comments was carried out using the themes derived from the interviews as a framework. No additional themes were identified from these comments.
Results
Sample
In total, 42 women participated in the qualitative interviews. Table 11 provides a summary of the demographic characteristics of the women as a total figure and by group allocation. Twenty-three (55%) women were randomly allocated to the placebo and misoprostol group and 19 were randomly allocated (45%) to the mifepristone and misoprostol group. Participants were geographically distributed across the UK regions. Thirty-one (73.8%) participants were aged > 30 years. Thirty participants (71.4%) identified themselves as white British, with the remaining women coming from a range of different ethnicities. It is notable that it was not possible to recruit women from South Asian communities into the qualitative interviews, despite making contact with a number of women from this ethnic group who had participated in the trial.
| Characteristic | Mifepristone plus misoprostol group (N = 19), n (%) | Placebo plus misoprostol group (N = 23), n (%) | Total interview sample (N = 42), n (%) |
|---|---|---|---|
| Geographical location | |||
| East Midlands | 2 (10.5) | 0 (0.0) | 2 (4.8) |
| North East | 2 (10.5) | 1 (4.3) | 3 (7.1) |
| North West | 0 (0.0) | 2 (8.7) | 2 (4.8) |
| Scotland | 3 (15.8) | 1 (4.3) | 4 (9.5) |
| South East | 2 (10.5) | 6 (26.0) | 8 (19.0) |
| South West | 6 (31.6) | 6 (26.0) | 12 (28.6) |
| Wales | 2 (10.5) | 1 (4.3) | 3 (7.1) |
| West Midlands | 2 (10.5) | 6 (26.0) | 8 (19.0) |
| Age (years) | |||
| 16–20 | 1 (5.3) | 1 (4.3) | 2 (4.8) |
| 21–25 | 1 (5.3) | 2 (8.7) | 3 (7.1) |
| 26–30 | 2 (10.5) | 4 (17.4) | 6 (14.3) |
| 31–35 | 7 (36.8) | 7 (30.4) | 14 (33.3) |
| 36–40 | 7 (36.8) | 6 (26.1) | 13 (30.9) |
| ≥ 41 | 1 (5.3) | 3 (13.0) | 4 (9.5) |
| Ethnicity | |||
| African | 1 (5.3) | 1 (4.3) | 2 (4.8) |
| Any other white background | 2 (10.5) | 3 (13.0) | 5 (11.9) |
| Black Caribbean | 0 (0.0) | 1 (4.3) | 1 (2.4) |
| Chinese | 1 (5.3) | 1 (4.3) | 2 (4.8) |
| White British | 15 (78.9) | 15 (65.2) | 30 (71.4) |
| White/black Caribbean | 0 (0.0) | 2 (8.7) | 2 (4.8) |
| BMI (kg/m2) | |||
| Underweight (< 18.5) | 1 (5.3) | 0 (0.0) | 1 (2.4) |
| Healthy (18.5–24.9) | 8 (42.1) | 11 (47.8) | 19 (45.2) |
| Overweight (25–29.9) | 5 (26.3) | 7 (30.4) | 12 (28.6) |
| Obese (30–39.9) | 5 (26.3) | 5 (21.7) | 10 (23.8) |
| Parity | |||
| Yes | 12 (63.2) | 12 (52.2) | 24 (57.1) |
| No | 7 (36.8) | 11 (47.8) | 18 (42.9) |
| Children | |||
| 0 | 12 (63.1) | 13 (56.5) | 25 (59.5) |
| 1 | 3 (15.8) | 9 (39.1) | 12 (28.6) |
| 2 | 4 (21) | 0 (0.0) | 4 (9.5) |
| 3 | 0 (0.0) | 1 (4.3) | 1 (2.4) |
| Previous miscarriage(s) | |||
| 0 | 11 (57.9) | 12 (52.2) | 24 (57.1) |
| 1 | 2 (10.5) | 5 (21.7) | 7 (16.7) |
| 2 | 3 (15.8) | 5 (21.7) | 8 (19) |
| 3 | 1 (5.3) | 0 (0.0) | 1 (2.4) |
| ≥ 4 | 0 (0.0) | 1 (4.3) | 1 (2.4) |
| Previous still birth | |||
| 1 | 1 (5.3) | 1 (4.3) | 2 (4.8) |
| Gestational age at current pregnancy loss | |||
| 4 weeks to 6+6 weeks | 7 (36.8) | 5 (21.7) | 12 (28.6) |
| 7 weeks to 8+6 weeks | 10 (52.6) | 12 (52.5) | 22 (52.4) |
| 9 weeks to 10+6 weeks | 2 (10.5) | 3 (13) | 5 (11.9) |
| 11 weeks to 12+6 weeks | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 13 weeks to 13+6 weeks | 1 (5.3) | 2 (8.7) | 3 (7.1) |
| Weeks of pregnancy at diagnosis | |||
| 7 weeks to 8+6 weeks | 5 (26.3) | 4 (17.4) | 9 (21.4) |
| 9 weeks to 10+6 weeks | 5 (26.3) | 9 (39.1) | 14 (33.3) |
| 11 weeks to 12+6 weeks | 6 (31.6) | 5 (21.7) | 11 (26.2) |
| 13 weeks to 13+6 weeks | 2 (10.5) | 2 (8.7) | 4 (9.5) |
| Not sure | 1 (5.3) | 3 (13) | 4 (9.5) |
| Surgery required | |||
| No | 15 (78.9) | 17 (73.9) | 32 (76.2) |
| Yes | 4 (21) | 6 (26.1) | 10 (23.8) |
Of the women participating, 24 (57.1%) were nulliparous (in the context of the trial, this was defined as women who had never carried a pregnancy beyond 24 weeks). Seventeen women (40.47%) had children (range 1–3), of whom one (2.4%) had previously experienced still birth. One other woman had experienced still birth and had no children. Sixteen (38.1%) of the women had experienced between one and three miscarriages in addition to the loss being treated within the trial. One further woman had experienced more than four miscarriages in addition to the loss treated within the trial.
Table 12 provides a summary of the satisfaction scores for the 42 women participating. As can be seen from the table, 37 (90%) of those who fully completed the CSQ-8 (n = 41) scored above 21, indicating satisfaction with the trial and service they had received.
| Satisfaction scorea | Mifepristone plus misoprostol group (N = 19), n (%) | Placebo plus misoprostol group (N = 23), n (%) | Total interview (N = 42), n (%) |
|---|---|---|---|
| < 16 | 2 (10.5) | 1 (4.3) | 3 (7.1) |
| 17–20 | 1 (5.3) | 0 (0.0) | 1 (2.4) |
| 21–24 | 1 (5.3) | 7 (30.4) | 8 (19.0) |
| 25–28 | 4 (21.0) | 3 (13.0) | 7 (16.7) |
| 29–32 | 10 (52.6) | 12 (52.5) | 22 (52.4) |
| Missing data | 1 (5.3) | 0 (0.0) | 1 (2.4) |
In addition, 233 comments were analysed from the free-text box of the CSQ-8. These comments included the perspectives of women who chose not to agree to an interview, some of whom were not satisfied with the care they received. The demographic data for these respondents is not shown and their quotations are not included in the presentation of the findings.
Findings
We begin the presentation of our findings by exploring women’s satisfaction, discussing the perspectives of women on a continuum ranging from those who would choose medical management again to those who would not. Five superordinate themes are discussed within this, representing the factors influencing satisfaction and acceptability of medical management: (1) alongside me, (2) feeling informed and in control, (3) timely resolution to the physical process, (4) treatment experience and (5) treatment success. Following this, we briefly describe views on participation in the trial.
Satisfaction with and acceptability of medical management for missed miscarriage
Having completed the pathway, women were asked to consider and reflect on their overall satisfaction with the treatment they had received. This is now explored, along with the contextual factors influencing their perceptions of the satisfaction and acceptability of medical management. Women’s experiences suggested that satisfaction with and acceptability of medical management need to be considered on a continuum, as represented in Figure 8.
FIGURE 8.
Satisfaction with and acceptability of medical management of missed miscarriage.
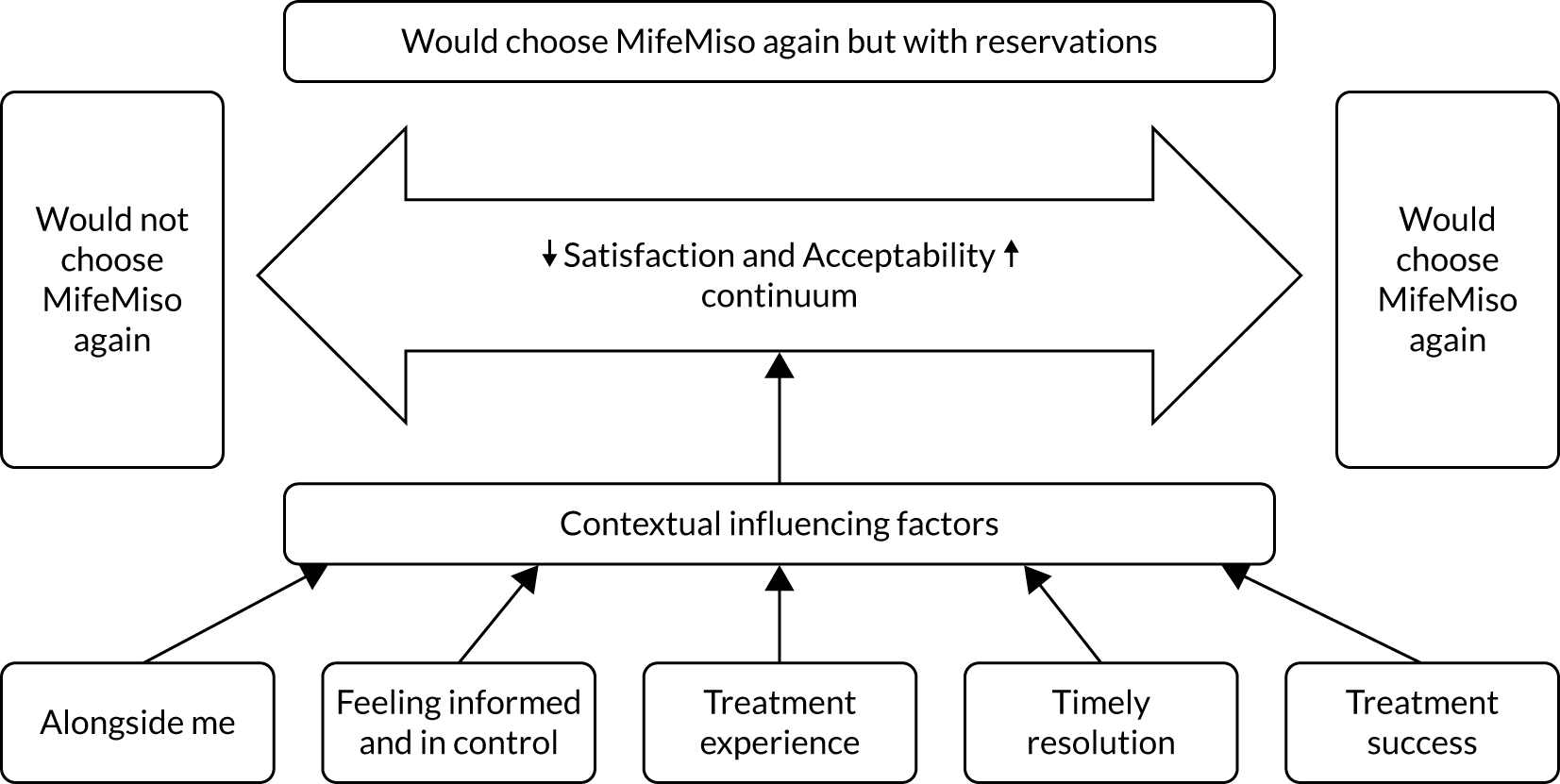
Figure 8 shows that some women reported being entirely satisfied and would choose medical management again if this was needed, or would recommend medical management to someone they knew who was experiencing a miscarriage (note that all names used are pseudonyms):
For me, if it’s something that you know is going to happen anyway then for me speeding up that process is a lot better. It helps you then be able to grieve a lot quicker and then get back to normality a lot easier I think, so I would definitely recommend it. . . . Oh I’m really happy with everything that’s happened.
Jacinta
Other women reported unequivocally that they would not choose medical management should they experience a further missed miscarriage:
So you go through . . . we went through a transition of 8 weeks thinking this is the weekend, it’s all going to be over and each time something else was thrown at us. . . . If I knew then what I know now I would have done surgery in the very first instance, there would have been absolutely no doubt in my mind I would have just went for the surgical option.
Gemma
In addition, some women, despite reporting that they were satisfied, appeared from our interpretation of their responses to have reservations about medical management. For example, Florrie reported that in the context of recurrent miscarriage, it was the most appropriate option, but not one she was completely comfortable with:
If like me it’s becoming quite a recurrent thing then I would suggest medical management, even though it’s quite a horrible experience I think I would still have to choose that if it happened again.
Florrie
All three women quoted above gave specific answers to questions about satisfaction. However, their responses also illustrated that there are contextual factors underpinning their satisfaction that had an impact on their experience of the treatment, both positively and negatively. Although these may not result in women refusing medical management, they nevertheless have a significant influence on women’s views on the acceptability of medical management. Five main subthemes were identified, as illustrated in Figure 8. These contextual factors reflect both the experience of the treatment itself and the care environment in which medical management took place.
Alongside me
Alongside me reflects the extent to which women appeared to feel that the care and the environment in which their treatment was offered supported them throughout their miscarriage. There are three subthemes within this: (1) being present and empathic, (2) feeling supported and (3) being responsive to my needs.
Being present and empathic
Women reported that it was important that the HCPs who supported them were present and able to show empathy and concern for their well-being. This began at the point of first contact with HCPs, when the diagnosis of missed miscarriage was made and during discussions about their diagnosis and treatment options. A range of HCPs were reported as being involved in these interactions, including sonographers, nurses, midwives and doctors. Some women reported the news being shared and the subsequent discussions as being conducted with sensitivity and empathy during this difficult time:
[The doctor said] ‘I’m sorry this is an awful situation, do you want to look at the screen?’ And was measuring things and just talking us through this is the yolk sac, this is the first fetus, this is the second one. He did a sound thing so you could hear what was going on, there was a 3D [three-dimensional] picture as well, . . . but he was just talking it through, talking through our options and just talking to us, and I just felt like there was some care in it and it made a difference.
Jan
Jan’s experience suggests that the doctor conducting the scan was able to ‘be present’ by focusing on her and being respectful at the same time as being thorough in his approach. She described how he gave her choices about the process and sensitively explained what was happening. HCPs’ ability to be present and empathic continued to be important to women throughout the pathway. Many women valued the care they received because they believed that the HCPs were thoughtful and treated them as an individual:
I think they were . . . just very caring, and very aware that it’s not the nicest thing for somebody to go through and everybody is different in how they deal with it, . . . and actually it was quite a painful experience, emotionally it was quite hard for me, and everyone was just very thoughtful and very caring and very individual. . . . I know they deal with it every day and it is part of their job, but I felt like they were just saying it to me, like it was personal to me that they were saying it, and it’s just very . . . it was a very caring environment to be in.
Olivia
Furthermore, women reported that HCPs showed concern and gave them time as well as facilitating the opportunity to talk about what might be worrying them. For example, Elise described the care she received between the day 7 scan and day 21:
I went to that ward in [city], . . . they were just so lovely, I rang them up and said I’m still bleeding, I’m really worried that something is wrong, and then they were like just come in, it’s absolutely fine, just come in if you’re worried, . . . So I came in and they . . . sat me down on the bed, they were just very gentle about it, and they were very helpful, and didn’t make me feel like I was over-reacting, that was my main concern.
Elise
In addition, women appeared to emphasise that the ability of HCPs to recognise their emotional journey and to tailor their approach accordingly was valuable. This was evident in Phoebe’s example, where the HCPs were empathic and attuned to the couple’s mood and personal ways of coping as they moved from initial distress at the news to finding ways to manage the impact of the miscarriage:
I don’t always come across as taking everything seriously, and both me and my husband, my husband comes out with the most inappropriate things at the wrong time, because we’ve had so many scans over the last year . . . But they were able to respond to us being like that, . . . they reflected our mood if you get what I mean? When we were upset to begin with they reflected that, they responded in a considerate way whereas when we were a bit . . . and we were just trying to get on and deal with it in the best way we could, they reflected that so they weren’t morbid about anything, . . . they were the right type of people of what you needed at that time.
Phoebe
Thus, it appeared that women valued the relationship that they developed with HCPs over the duration of the treatment. In contrast, some women reported that they felt that the care they received during the pathway was un-empathic, lacking compassion and lacking concern for their well-being. For example, Kyla described the approach of the two people conducting her scan:
It was very matter of fact and they told me ‘it’s not viable, it’s stopped growing’, . . . and then they came back in and they explained to me it’s nature way and that it was better now than I’m told at 20 weeks that there is some kind of deformity or anything like that which . . . I do feel like in that time it could have been a little nicer, and a little less about how much worse it could have been, because at that moment in time I didn’t think it could be any worse . . . One of the things she said to me was, ‘All you have done so far is pee on a stick and then you have changed your life because you have peed on a stick’.
Kyla
Kyla appears to feel that the HCPs involved in her scan lacked awareness of the impact of the news on her and their responses served to diminish the validity of her feelings. It seems possible that these negative experiences may challenge women’s ability to attend to discussions about treatment options following diagnosis. For example, Kyla went on to describe how emotional she felt and that she wanted to leave:
After that they then went into what the options would be, and I didn’t think that at that point I was even . . . I wanted to leave, I wanted out of there as soon as possible because I felt uncomfortable and I just wanted to go home and be in my own space. . . . because I was crying and completely and . . . totally not with it . . . So I found out in all honesty I got more comfort and more information from the two leaflets than I got from the actual midwife that told me the news.
Kyla
In this context, Kyla described gaining more comfort from the written information than from the HCP involved. Therefore, the extent to which HCPs are able to be empathic and focused on women during the scan and discussion of options would appear to be particularly important because they may influence women’s decision-making about treatment. They may also influence women’s psychological well-being going forward.
Some women reported that there continued to be a lack of communication, lack of time to be present with them and little concern for their well-being. These occasions were most commonly described in relation to contact with gynaecology and accident and emergency where women perceived that their miscarriage was not viewed as a priority. For example, Sarah received misoprostol in the gynaecology ward. During her appointment there, she described delays in receiving the medication that she felt were understandable but reported that the ward staff were cold in comparison with the EPU HCPs:
I felt [the ward was] very clinical, cold, compared to the early pregnancy unit. I just felt one of many . . . There was probably four women been and gone while I was still there, so you just felt you were just on a conveyer belt if I’m honest, and nobody came. I went in . . . I was told . . . that’s the room you need to go to, so I just took myself in there, and then nobody came near us for over an hour, which I found really . . . it was very matter of fact. I don’t know what I expected but a little bit of compassion. They weren’t horrible, they weren’t rude, but there was no . . . it was a task to them, and I felt very much it was very task orientated . . . Nobody there acknowledged what was happening.
Sarah
Similarly, some women reported differences in the extent to which HCPs were able to be present with them and give them time to talk during such an emotional time. Vicky commented on her interaction with a doctor at the follow-up scan, reporting that she was ‘matter of fact’ about her experience and appeared to be focused on getting the task done, rather than being concerned with Vicky’s well-being:
Actually [the doctor] . . . she wasn’t warm, she was really matter of fact about things, and I felt like my time in front of her was very much about her . . . the things that she needed rather than about me, and that’s quite damming but I think it’s true . . . I feel like everyone that goes into that horrible clinic deserves 10 minutes in a room, and a cup of tea.
Vicky
Her response highlighted the importance to her of having time and a gentle and personal approach from HCPs, something she did not feel she had from this interaction.
Feeling supported
Knowing that HCPs were alongside them also contributed to women feeling supported during their treatment. Women reported feeling supported when they knew who to contact and/or were in regular contact with a person or team who were responsible for their care during the treatment process:
Yeah, so I had all the information, I had the nurse who was managing the trial, I had her number . . . and I knew I could turn to get at any point, or call [the city hospital] and I had the direct dial number to the wards that I needed . . . But if I started to get to a point where I couldn’t manage it, I couldn’t handle it, I knew that I had options to call, get reassurance or just go in to the hospital.
Sophia
As the above quotation illustrates, some women reported that just knowing who to call was meaningful, even if contact was not ultimately needed. Others reported that they appreciated HCPs making regular contact with them, which offered them reassurance that their concerns would be listened to and that action would be taken:
She called on Sunday because they said they will check on me anyway . . . to make sure I take the pessaries and I was feeling okay. So I did wait for the nurse to call me . . . because I wasn’t really sure if I should take them or not, and then I described what happened and she was like okay I don’t know if you should take it, and I will contact the leading doctor and she will ask her what to do. So she did come back to me after 20 minutes, and she said proceed with the pessaries, because if I feel like it wasn’t all of it I should take them it will be alright . . . So as I said it was like amazing the way with everything, they really look after me, and over the phone or in the hospital it was really good experience.
Milena
For some women, particularly women who had experienced recurrent miscarriage and/or previous stillbirth, feeling supported also involved continuity of care, such as Rose’s experience during her scan:
Actually the doctor that scanned us with this one at my local hospital was the same one that scanned us when we went in because [our daughter] hadn’t been moving so there was a nice bit of continuity of care there. So I knew her and she remembered me, and she remembered [our daughter], and actually that was really important . . . helped a lot. It wasn’t going in and being seen by someone completely that I didn’t know.
Rose
Rose emphasised that the doctor’s knowledge of her previous history made a significant difference to her well-being at such a stressful time. Feeling supported continued to be important throughout the care pathway, with women valuing the opportunity to explore their feelings and concerns at the follow-up scan and pregnancy test.
Conversely, some women reported that they did not feel supported during their miscarriage treatment:
I only got contacted once, and that was it, and I thought I would have been contacted at least every other day just to see how the process is going, even if it’s just like a 2 minutes call, something like that. I just didn’t want to feel alone, I wanted to speak to someone so I can tell them my journey at each step of the way so I am understanding what my body is going through, I just wanted clarification.
Keisha
As Keisha’s experience illustrates, some women reported feeling alone and without opportunity to check that what was happening to them was normal. Furthermore, for some a lack of co-ordination in care within and between services compounded the sense of being alone. This resulted in feelings of abandonment for two women in particular, one of whom expressed her dissatisfaction with what had happened:
During that time there was a junior doctor came in and took me into a side room and just basically said these are your three options . . . [I said] ‘Can I just stop you there, have you actually read any of my notes?’ And she said no, . . . nobody in the hospital then knew why I was there . . . they didn’t know I was part of the trial. The doctors were basically thinking I had just had a miscarriage and I was just there to find out what to do next, and the research team thought I was just there as part of the trial to close off their files. From there the consultant wasn’t available because my notes had been lost, so I was sent away that day after waiting about 2 or 3 hours, just hanging around, various people coming in and out not really knowing what to say to me, not really knowing what to do next.
Gemma
In this quotation, Gemma highlighted the absence of a supportive relationship in which her needs were not recognised and thus felt forgotten about and just one of many.
Responsive to my needs
Finally, being alongside women also involved HCPs and the environment of care being responsive to their needs. Women reported HCPs as being responsive to their needs when they listened to them, took their concerns and needs seriously, and consequently were flexible with arrangements, where possible. Flexibility about the location of treatment was valued, with many women indicating that the option of treatment at home influenced their decision to take part in the trial. For example, Hailey reported that the fact that the treatment could take place at home was particularly relevant to her needs, allowing her to be with those she felt cared for her:
She said the second option was to go with the MifeMiso research project, and I could go home. So I liked the idea of that more because I didn’t really want to be in the hospital, I wanted to be at home with my loved ones . . . I just felt if I was at home I would be more comfortable being in an environment where I would be relaxed.
Hailey
Some women did not want home management and were grateful when the option of hospital treatment was offered. For example, Grace emphasised that she did not want to miscarry at home and appreciated that the HCPs had offered day patient attendance for the misoprostol. She was therefore unhappy that the miscarriage occurred at home, after taking the mifepristone/placebo:
The one thing I said to [my partner] is I don’t want to flush it down the toilet, and that’s literally what happened to it. So that was rubbish because I felt like the decision I was making I would have been in hospital, it wouldn’t have gone down the loo.
Grace
Flexibility was also valued by women when making decisions about treatment options, for example being able to go home after the diagnosis and make contact a day or so later:
It was quite hard to process everything, and I think bless her she was explaining this trial to me and I thought . . . I mean, I’m a nurse, she was explaining and I thought [I] don’t know what you’re going on about, because I think it was really hard to take all the information . . . For me I just wanted to get out of the hospital . . . Then she let me go home and then she called me the next day and explained.
Sarah
The environment of care was viewed as responsive to women’s needs when women were offered privacy following the diagnosis of missed miscarriage, for example:
[The doctor] . . . also offered me a quiet room that my husband and I can stay in that room and just release our emotion and think about what to do next.
Biyu
Minimising women’s exposure to other women who were pregnant or who had babies was viewed as a difficulty:
I think the hardest part is the scan because your scan is next to other pregnant women as well at that point, so you’re going through your own emotions, and then they are all excited to be getting the 28-week scan or whatever they are in for, so yeah it’s quite difficult.
Florrie
Women who reported concerns about this indicated that their difficulty related not only to their own feelings but also to how it might feel for the other women to be exposed to their distress.
Thus, when women felt that the HCPs were alongside them and supportive of them, they expressed satisfaction with medical management and would have the treatment again:
There was never a time when she wasn’t on the blower or at the end of the phone if I needed her or anything. The support was just fab, and I would definitely do it again. If I was put in that scenario again and I hope not, really hope not, but I would do the same thing again.
Christie
However, when women felt that they had been forgotten about or that their needs were not a priority, then they were more likely to have reservations or to say that they would not choose to have medical management again:
If I knew then what I know now I would have done surgery in the very first instance, there would have been absolutely no doubt in my mind I would have just went for the surgical option . . . from the experience that we’ve had and I think the lack of support, because as soon as I went in for the surgical option the surgical team were a totally different team to what we were used to dealing with in the medical management side. We knew exactly what was going to happen, we knew when it was happening, they were lovely, the anaesthetist came out and everything was just explained from start to finish.
Gemma
Feeling informed and in control
Women’s narratives suggested that the provision of tailored information about the miscarriage and treatment process had the potential to make a difference to the level of control they felt they had over what was happening. As women’s accounts already indicate, this theme and the previous one of ‘alongside me’ are interlinked, in that how information is shared is as important as the quality of the information provided so that women can make informed choices and feel in control.
Women’s narratives highlighted three significant points for information provision in the pathway: (1) at the point of diagnosis of missed miscarriage, (2) knowing what to expect regarding treatment and (3) knowing the next steps following the miscarriage.
Diagnosis
In Being present and empathic, women’s experiences of the sensitivity of the HCPs conducting the scan were discussed. In addition to a sensitive approach, women appreciated it when practitioners took time to explain, offered clear information and gave explanations during and after the scan, including being clear with women about what they were going to do. Some women also found it helpful to be offered the opportunity to see the screen and for details about measurements to be explained. Informational interventions at this point also afforded opportunities to challenge self-blame:
She explained to me as well what could happen, and she said to me that it’s not my fault at all, these things just happen and they happen to more women than I think unfortunately, and she was brilliant.
Milena
In contrast, some women experienced the professionals sharing the diagnosis as dismissive and the information sharing as very ‘matter of fact’. Others also described the difficulty they experienced when the sonographer was silent and uncommunicative; did not prepare them for what they were going to do; or where their non-verbal communication indicated there was something wrong, even though they were not expressing this verbally. For example, Gemma described the process as secretive even though non-verbally, she felt the sonographer was giving a very clear message:
I felt like it was a secret throughout all of the scan processes. I felt like you was trapped behind a curtain, and the look on the scan lady’s face just said everything. You could tell it was bad news every time without her even showing you or looking at you or anything.
Gemma
In addition, Aaliyah reported that she felt she had to ask for information:
I was told the baby died and I had to ask, I wasn’t even given that information, I had to ask how far did the fetus grow to, and that was because of past experience and having read so much I was able to ask that question for myself, because they don’t show you the screen, they don’t tell you anything, they just it’s dead and there’s no heartbeat and that’s it, and you have to get out for the next person to come and lie down.
Aaliyah
Indeed, she seemed to feel that, without her previous knowledge, information that she needed would not have been forthcoming. Some women also described the experience as confusing, with HCPs providing conflicting information or offering false hope. Ultimately, where information was withheld or communicated in a way that did not support women’s understanding of what had happened with sensitivity and honesty, then this was experienced as hurtful and influenced their ability to attend subsequent treatment discussions and therefore to make an informed choice. It also seemed that this negatively influenced women’s evaluation of their treatment and care.
Knowing what to expect
For those women who reported knowing what to expect, there were specific features of the information sharing in regard to the effects of the treatment that they identified as helpful. These were information about how the treatment would affect their body; the extent of the bleeding and pain associated with the treatment; pain management; and the plan of care, including when and who to contact in an emergency:
So the information provided to me by the midwife about what would happen and saying . . . I really appreciated how forthright she was, and she said, ‘Some women describe it as a really heavy period, you are going to be going into labour, it is going to hurt, I would strongly recommend that you use the drugs to help ease the pain. I have given you co-codamol or codeine or whichever one it was, because it is painful, and I don’t want [you] to be shocked when it starts to hurt, you can come to the hospital at any time.’ So she was very clear in her management of my expectations of that which I am so grateful for because there’s a solid chance again with drugs, I don’t do drugs, so when I do take strong painkillers it really affects me, and I probably would be like maybe I’ll take one, maybe I won’t take them, but because she advised that I did, and thank God because it hurt like hell. [She] provided me with a better understanding of what was going to happen to me physically, and that helped remove some of the fear, and obviously there’s still uncertainty because it’s gnarly, that whole process, my gosh, talk about getting kicked when you’re down. But I felt like the information they provided me with allowed me to prepare myself and make an informed decision about what was going to happen.
Sophia
As Sophia’s narrative highlights, she found the information that she was given to be clear and helpful, alleviating some of her fears about what would happen. It also meant that she felt informed enough to make appropriate decisions about pain management, something she may have ignored under other circumstances. Some women also described being given written and visual as well as verbal information about the bleeding and what to expect:
They gave a visual prompt that actually if your pads are looking like this then this is when we need to worry . . . But actually the visual prompts were really helpful, . . . because . . . saying it’s like a heavy period isn’t helpful if you only ever have really light periods, because then you just get a false perception of what a heavy period is, whereas . . . an image of if there is this much blood on your pad every half an hour then you need to come in, actually is a very clear way of describing it.
Rose
This visual information, as Rose indicates, was particularly helpful in enabling her to know when to ask for help. Some women also reported that they appreciated information about what the baby and placenta might look like and also that they should have someone with them if they were having home management. It appears that being informed in this way led some women to feel that they were aware of what was going to happen and what actions they could take to support themselves.
However, other women reported that they did not feel informed to the same degree about treatment and management. Their concerns were related to knowing what would happen overall, how much bleeding and pain to expect, how to manage their pain, where to get information from, the risks that it might fail, a lack of written information and the duration and timing of the treatment.
Where women felt they lacked information, some sought this from the internet. Elena described searching Google (Google Inc., Mountain View, CA, USA) for more information. She reported that this helped her to gain a better understanding of what might happen, as she did not feel that the hospital had given her enough information about the ‘nitty gritty’ of the treatment effects:
I think it was quite helpful because I feel like at the hospital they gave me some codeine phosphate and said it would be very painful, . . . and to go and get lots of pads and things. But I think they don’t really explain so much the actual nitty gritty of it . . . Google can be a friend and foe sometimes, because it can scare you a bit, but also I feel like in this situation it did prepare me a bit more. But I still wasn’t prepared I don’t think for the pain, but I think you don’t really know what that pain is like until you have had it. It’s really hard to explain it, unless you have given birth I guess, but then I don’t know, it’s maybe slightly different.
Elena
However, as Elena alludes to, the internet can be a foe as well as a friend. In particular, the variability, type and quality of the information women encountered could increase distress and uncertainty rather than informing them as described here:
So I looked online which is a terrible place for information, especially on this topic because everybody deals with it differently, and there’s no right and there’s no wrong, but for me I have a right and I have a wrong which I can handle, and graphical mum stories are not my bag, it’s just too much crazy . . . When you’re scared and you don’t know what’s coming up, you don’t know how much it’s going to hurt, it’s terrifying, it’s really terrifying. So I struggled to find factual medical knowledge about the process that your body goes through, and I don’t know, maybe my Google searching it wasn’t even the right words or whatever but I really I struggle with that and I found that a bit frustrating.
Sophia
Some women reported that it would have been helpful if they had been provided with written as well as verbal information to act as a reference point. For example, Jan felt that the information about pain management was difficult to take in when she was first told about the miscarriage:
But I think I was overwhelmed with what was going on I couldn’t really remember at that point anything that they had said and it’s only in hindsight that we talked it through and remembered what they had said. But when you’re experiencing it I wish I had a sheet saying . . . this is when you take your tablets, and this is up until this point you might feel this.
Jan
Women also reported difficulties with knowing when to take misoprostol. For example, Jacinta described self-administering the misoprostol early in the afternoon:
So then took the tablets around about two/half two time, and I actually thought even though no one actually told me in my head I thought it would take a couple of hours to kick into your system and then it would start, but it actually didn’t. It didn’t start until one o’clock in the morning, so it was quite a long period of time actually from when I took the tablets to when it actually started the process in the body.
Jacinta
Although this did not appear to affect her satisfaction with the outcome, she nevertheless indicated that it would have been helpful to know what to expect. In addition, information about the duration of the effects of the misoprostol would have been valued by some women who chose home management. Vicky described how she thought the miscarriage was over and had not arranged any further support from family. The following day, she experienced a further period of significant pain and bleeding when alone, which she described as being traumatic:
So that was a really horrible experience actually, that being alone, and it was very traumatic, and quite frightening.
Vicky
Consequently, detailed information about what to expect and what support is required would have been beneficial.
Knowing the next steps
Following the treatment, some women reported the value of support and information to address their needs going forward. They reported having follow-up appointments and knowing they could contact the service if they required any advice concerning their experiences post miscarriage. Women also reported that that they appreciated receiving information about what they might experience:
It was a nice close-out conversation . . . But I remember the midwife on the day being particularly nice and they spent a lot of time talking to us about how this doesn’t mean the end of it all, and preparing you for the fact that it is more common than you realise, you do need to find some friends and you will probably find there’s a lot more people out there have been through the same thing as you, and a lot of discussions around how everywhere you go there’s just going to be a sea of pregnant ladies everywhere all of the time, and it’s exactly what happened . . . She spent a bit more time talking about that which I am glad she did because I would have felt like it was just me, but it’s not.
Nita
They also reported that they appreciated receiving information about the reasons for the miscarriage:
We asked the question about our age, we asked the question about is there anything we could have done differently, and really no one can give you that answer because it’s the genetics or the process the body has to go through it’s so amazing that it only takes one chromosome or one link not to be right and it doesn’t happen, and that’s . . . and to be fair I wasn’t aware how common it was, that it’s one in four women that actually experience it.
Olivia
Women realising how common miscarriages are was identified as reassuring and, as both quotations indicate, informed women in a manner that supported their well-being and their adjustment. In addition, women experiencing recurrent miscarriage identified that they were given information and referred onto specialist clinics and/or were informed that they could attend the EPU for an early scan in their next pregnancy. However, others felt that post-miscarriage support was not available to them and, in particular, the opportunity to ask questions about their miscarriage and the future:
. . . and there’s no information on stuff like when can we start trying for a baby again, is there anything I can do from a health perspective to I don’t know look after my body in a better way potentially to help avoid this in the future, because we just seem to be told there’s no investigation until you’ve had three miscarriages, but I think people having had one or two are still confused as to why it happened, what can I do next, and any more face-to-face emotional support as opposed to just being directed to websites or being given leaflets.
Karen
As Karen points out, some women will have questions about a range of issues, and she felt that the lack of opportunity to talk these through with someone would leave them feeling alone with their concerns. Furthermore, as this quotation highlights, the lack of information for women who had not experienced three or more miscarriages and were therefore not able to access specialist recurrent miscarriage clinics was particularly difficult. Some women reported that they had not received information about counselling or a referral for support following the miscarriage:
When it happened I think they should have had someone to speak to me more to help me through that really . . . and I didn’t really get that.
Chantale
Treatment experience
Women’s reports of satisfaction with treatment experience appeared to be influenced by three factors: (1) administration of misoprostol, (2) location of miscarriage and (3) experience of bleeding and pain.
Administration of misoprostol
Administration of misoprostol varied according to local protocols and women’s choice of location for treatment. Some women reported self-administration at home whereas others had to return to the hospital. Some of those women who were required to go to hospital for the misoprostol found the number of visits acceptable and relatively quick. For example, Jan, although she had to have surgery as medical management failed, reported that the process itself was efficient and managed well:
Well, in terms of the drugs themselves I thought it was a very straightforward way of going about it . . . if I remove what my experience was like you say, I think that’s a very efficient way of dealing with it. It’s very good in terms of time management, you have to go in twice, not hugely invasive taking one orally, well you can do that one yourself, and the vaginal well it’s pretty quick in terms of what we have to go through as women anyway, it’s far quicker and less difficult to deal with physically than a smear test for example, and then just the waiting for an hour and having your blood pressure checked twice that’s incredibly efficient I think.
Jan
Other women, however, found the number of visits to be onerous:
I think the main thing is that having the two sets of treatment it does extend the period of time that you’re having to go back and forth and that sort of thing, and it doesn’t sound like a lot but when you’re in that where you’ve already been back and forth to the hospital and you’re trying to maintain your normal life as well, it just does add that extra level of something that you’re doing.
Sian
Clearly, Sian, given her other commitments, found it difficult to fit in the required number of visits. Consistent with other women in this study, Sian’s reasons for choosing medical management appeared to be influenced by competing life demands and the need to achieve a timely resolution while managing work and other commitments:
. . . with the other two children and work I just felt like I wanted to get back to normal as soon as possible really.
Sian
In this context, repeated visits to the hospital may have contributed to reservations about medical management.
Location of miscarriage
As indicated previously, women valued being able to choose the location of the treatment; indeed, this was an influencing factor in their choice of treatment and subsequently taking part in the trial. It seems evident that choice of location continues to be important to women when considering their satisfaction with treatment. For example, in common with other women, Rose reported that her satisfaction was influenced by being able to experience the miscarriage at home:
The treatment itself very satisfied with. I think that actually for me it was great to be at home.
Rose
Conversely, some women thought that being in hospital and able to manage the treatment there was particularly important:
I work full time, I have a 2-year-old, I didn’t want to be miscarrying on a random Wednesday in the middle of work, . . . I wanted to be able to be able and be here for my 2-year-old . . . Because like I said my husband works away so throughout the week I am a single mum kind of thing, . . . medical management . . . was managed and I knew when it was happening.
Nansi
Nansi appeared to value the control that being in hospital gave her, given that her husband worked away from home during the week. Indeed, she subsequently reported that she was satisfied with the process of medical management because it enabled her to feel in control and clear about what would happen:
. . . knew exactly where I was, I knew exactly what was happening, I wasn’t caught off guard on a random day, I was in control, I knew what I was doing. So if it was to happen again and I didn’t miscarry naturally, I would definitely do it again 100%.
Nansi
Experience of bleeding and pain
Following administration of the misoprostol, 13 women reported experiencing bleeding and/or pain that was perceived as manageable. Many of these women reported symptoms that they associated with a normal, albeit heavy or painful, period. For example, Jo said:
The pain, it’s hard to gauge really, the pain was unpleasant but it wasn’t in any way unbearable, it was just like a really bad period pain I think.
Jo
Some women reported that, although the bleeding or pain was significant initially, it quite quickly reduced to a manageable level. However, over half (n = 24) of the women reported experiencing either heavy bleeding or significant pain, or both. For example, one woman reported the intensity and duration of her pain as being similar to labour and the bleeding as very heavy, resulting in dizziness and light-headedness:
And then on the Saturday . . . about 4 o’clock I took the first lot, and then laid down for a couple of hours, and wasn’t until about 7/8 o’clock that the pain started, and then probably from then until about well we were up quite late really, about three o’clock in the morning I had just what I would say having not been through giving birth but it felt like contraction type pain, and a lot of heavy bleeding, and I think probably around midnight or 1 o’clock I had a really dizzy spell where I’d gone to change one of the pads and put a new pad on and then went to wash up and then immediately soaked through that pad, so then I sat on the loo trying to sort myself out and suddenly just felt very dizzy and very light-headed.
Sally
The majority of women reported receiving a prescription for pain-relieving medication, such as codeine and paracetamol, and some also received antiemetics. However, as described in Knowing what to expect, this did not always result in women reporting that they knew how to manage their pain effectively. In addition, some women reported that they did not receive any pain medication. Consistent with Sally’s experience, some women reporting heavy bleeding described having to spend considerable amounts of time in the bathroom:
Anyway so at about 12 o’clock I went to the toilet and had really super heavy bleeding, and I just got this instant terrible agonising abdominal pain, and I hadn’t taken any paracetamol, I hadn’t taken any ibuprofen, I hadn’t take any codeine, I hadn’t taken any antiemetics, and I was just stuck in the toilet in absolute agonising pain by myself . . . my phone was downstairs. I didn’t have any analgesia to hand and I just couldn’t move. So I spent about 2 hours on the toilet absolutely sweating profusely in terrible pain by myself which is a really horrible experience. I started feeling worried, the pain was so intense and I thought I could pass out, I need to let someone know this is happening, and so after . . . about an hour and a half or something I thought when it got to the point I need to let someone know, I crawled downstairs and got my phone and then rang mum, and said, ‘I’m in terrible pain do you mind coming over?’.
Vicky
As Vicky’s quotation suggests, this level of bleeding and pain was frightening for women, particularly if they were alone. Some women reported fearing that they would lose consciousness, with all the implications of this if they were at home and had children who might see them or where they were without support, even for brief periods:
I actually had to be home alone for a while because my sister had to go and pick up my daughter from nursery at that point, and I basically felt like I was going to faint. My ears started ringing, and I just was on the loo with my pants down and thinking if I faint now . . . naked basically on the floor with all this disgusting stuff that’s hanging outside, it’s disgusting, but this is what it was like.
Emma
For some women, the level of blood loss and pain resulted in emergency admission to hospital and a need for further intervention within the 7-day period:
I had . . . the four pessaries and then I would say about 3 hours into that the pain was starting really bad, and then the blood loss was unreal, I’ve never seen that much amount of blood, and basically the pregnancy tissue was stuck in my cervix, so that’s what was causing the blood loss. So they said it was like a mini labour, and I had gas and air and everything, and then they had to try and get the tissue out manually themselves. But I was in hospital for the night, and then I went home, and then about 2 days later I was still in pain, so I went back up and there was more pregnancy tissue . . . Looking back now I think if I could turn back time I would have probably had . . . [surgery] because they could have got it all out properly instead of it having . . . yeah definitely, I would have done that.
Chantale
In common with some women, Chantale reported that she did not feel she was informed about the potential severity of the bleeding and pain and indicated that she would choose surgery if she were to miscarry again. Thus, the level of the bleeding and pain appeared to negatively influence the acceptability of medical management, particularly if women did not feel they had been given adequate information and were unprepared for the extent of the symptoms.
Timely resolution
Women reported choosing medical management because they hoped that it would achieve a timely resolution to the physical process of miscarriage. In this context, the duration of the miscarriage appeared to be an important contextual factor in the acceptability of medical management. The majority of the women reported that they had passed the baby and placenta before the follow-up scan and seemed to experience this as beneficial:
So it was very quick for me which is what I needed.
Jacinta
However, for some women, the duration of bleeding post miscarriage appeared to add further delay to the end of the physical miscarriage. A number described experiencing bleeding and loss of clots for what they perceived to be a prolonged period of time:
I always think the surgery is a lot easier because . . . you don’t have to go through all that and you go to sleep and you wake up and it’s all gone, and it’s like you don’t have to deal with it. So emotionally I think it’s more difficult to deal with it medically than the surgical process . . . After the medical management you are not quite sure if it’s finished or not, you are not quite sure when or if there’s going to be more or if you’ve fully passed it, and I found that it was quite a lengthy process. So I had to go for a scan again and the sonographer said that everything seemed to be clear, but then a week later I passed big clots again, and then a week after that there was more, so it was like it was still happening . . . It takes a while for your cycle to get back to normal compared to when you have the surgery.
Florrie
Therefore, as Florrie indicates, even after the placenta and baby appear to have been passed, continued bleeding and the time taken until normal menstruation resumes prolongs the physical process and, hence, the time taken to achieve emotional resolution. As a result, Florrie felt that surgery was an easier option.
Treatment success: ‘being able to draw a line under it’
The care received after the miscarriage treatment, and in particular the follow-up scan, were valued as reasons for participating in the trial and to confirm that the miscarriage had completed:
So we just sat briefly and I can’t really remember, I suppose [the doctor] just asked briefly about what had happened the past few days or something, and then she did the internal scan, and told me that I’d passed the pregnancy sac, and then I got very upset and was just really crying. So it took me by surprise, I was so relieved, I was just so relieved that it was over, that I’d reached an end point.
Vicky
As a result, some women reported positively that they were able to reach an end point in the physical process and, as described by Vicky, this resulted in a sense of relief that the physical process of the miscarriage was over.
Other women found that the duration of the bleeding and/or presence of clots and tissue identified during the follow-up scan extended the time before they were able to draw a line under it, as described by Sarah:
So I had the scan but I hadn’t had a complete . . . it hadn’t all come away, so I was obviously upset about that because you expect that’s draw a line under it.
Sarah
Nevertheless, these women mainly evaluated medical management positively and indicated that they would choose it again, although others clearly had reservations. However, some women reported that the treatment was not a success and that further intervention was required. Of those women reporting the need for further intervention, two appeared not to feel negatively about it failing, for example:
It was quite bad luck [that I had] all these complications but otherwise I was quite happy with everything, and even I did this scan after because of the trial, and that something was quite happy to have that to make sure . . . She said that after I would have a scan to check that everything was okay. This is something you don’t have usually, and I think it was something very important for me because to know that the womb is clean now and that was something very important for me. So yeah that was . . . yeah otherwise I guess I am quite happy, and unlucky about what has happened I guess.
Francine
Francine chose medical management because she wanted to avoid the risks associated with surgery and it is clear that these remained important to her. Despite having prior experience of heavy blood loss with misoprostol in the past, she seemed to evaluate her experience as ‘bad luck’. She subsequently explained that she would choose medical management as a first option because of the risk that surgery would have an adverse effect on her fertility.
In contrast, the remaining women requiring further intervention evaluated medical management negatively. They reported that the failure of medical management, and therefore the inability to be able to move on from the miscarriage and ‘claim their body back’, was one reason for this negative evaluation, as described by Donna:
I think if I was to have to experience a miscarriage again I think next time I would just go straight for the surgical route, not least because when this one came to an end shall we say and I had the check-up at the hospital the following week I think it is, I was still within the bracket of needing more assistance with it, and I think that’s what got me as well is I suppose miscarriages are hard enough to deal as it is, but to go through it all and to get to what you think is the end to be told actually you’ve still got some left and to have to make a decision on how you want to get rid of that bit as well that was a hard pill to swallow . . . I just wanted it done really, I wanted my body back, I wanted to stop bleeding, I wanted to be able to move on from it all really.
Donna
This inability to move on was particularly an issue for those experiencing long periods of time before the miscarriage was complete, such as Kyla, who had a manual vacuum aspiration (MVA) a month after her scan revealed that the baby had died:
We [found] out that the baby had died on the [DATE] and I had the MVA on the [DATE], so there’s a long period of time, so if somebody was short of time I would go straight for the MVA because it was as dignified as it possibly . . . something like that could be, it was relatively over very quickly and the pain is short lived, and the recovery seemed to be pretty good.
Kyla
However, other factors also influenced the way in which women evaluated medical management, including the care and support that they received and the severity of the pain and blood loss, as described earlier. Common to all of the women who negatively evaluated medical management was the emotional distress associated with the failure of treatment, as indicated in Donna’s quotation.
These findings highlight that there are a range of contextual factors that influence women’s evaluation of medical management. These factors are not only concerned with the treatment itself, but incorporate the service context and care it is delivered within.
Women’s personal journeys, experiences and needs
Although not directly influencing women’s satisfaction with medical management, our interpretation of our participants’ experiences suggest that there are additional elements that are important context when understanding women’s experience of medical management of missed miscarriage. These elements are (1) women’s personal journey, (2) women’s desire for active management of missed miscarriage and (3) women’s experience of missed miscarriage and its influence on treatment choice.
My personal journey
The results indicated that the pathway for women in this study began with their lived experience of conception and pregnancy. These experiences and the choices they made provided the frame in which their perceptions of the miscarriage and the care and treatment they received for it were contextualised.
The majority of women reported that they had planned their pregnancy with their partners. Consequently, they had begun the process of envisaging a different life for themselves that, for many, would have commenced before conception or at the point of a positive pregnancy test:
Yes, it [pregnancy] was definitely something that we planned . . . I think it felt like this could be a really lovely nurturing way to, a good time to have a child while we’re in this set-up . . . So I think that it all fitted together as a bit of a life choice altogether really.
Jo
Although miscarriage is undeniably a common event, for many of these women it was nevertheless an unanticipated, significant and distressing experience, involving the loss of a baby, emerging identity as a mother and a planned-for future. Additional characteristics further influenced the context in which these women experienced the miscarriage and in which they received the trial treatment. First, 17 participants had experienced recurrent miscarriage. Thus, their perception of the treatment and care they received was influenced by a range of factors, including their previous treatment and care experiences, the cumulative grief and loss associated with repeated miscarriages and, on occasions, the lack of understanding from others concerning their loss:
I think that people think that you have a miscarriage and you have whatever procedure you need and then that’s it, but it isn’t. It’s longer lasting than that, . . . and even things like when it’s . . . when you know the baby would be due you can get upset then, . . . I think when it happens the people that I have around me have been supportive, but I think that’s probably short lived because for them it’s an instant shock and you just move on, but for us it has longer lasting impact than that.
Tasha
Furthermore, three of the women experienced missed miscarriage following in vitro fertilisation (IVF) treatment. This added a further dimension to their experience and perceptions of the current loss and its significance to them, particularly given the significant personal, financial and emotional costs that such treatment entails. For example, Jan described giving up her career, highlighting the considerable costs associated with the treatments:
I don’t really know what I say I do career wise, I [had a successful career], but we’ve been having fertility treatment for over three and a half years now, we’ve been trying to conceive for 5 years, so it’s been quite a journey for us . . . we had eight cycles of IVF and this was the first time that I had managed to get pregnant in 5 years.
Jan
Moreover, 13 of the women were aged between 36 and 39 years and a further four were aged ≥ 41 years. Some of these women’s experiences of miscarriage appeared to be framed within a fear that they may be ‘running out of time’ in which to realise their desire to have a child:
It’s just I just feel sad that I have to go through [the miscarriage] in part I just feel teary because it’s not my fault, and again I live in fear because what if it never happens again?
Aaliyah
Finally, five of the women had not purposefully planned the pregnancy that resulted in miscarriage, although none of these women indicated that the pregnancy was unwelcome. None of the participants taking part in the qualitative interviews indicated that the pregnancy was unwanted.
Active management of missed miscarriage
All but six women opted for medical management as their first choice. Expectant management appeared to be rejected by the majority of women because of the lack of control associated with not knowing when and where the miscarriage would happen:
I didn’t really want to do just do that because it was only 2 weeks before Christmas, I didn’t really want anything to happen at Christmas . . . but I just thought in my head I was going to be back at work as well. I went to work the next day and I was like but I can’t be at work, or shopping, or be with my daughter, I just thought that would be too traumatic.
Zoe
A small proportion of women initially chose expectant management but then subsequently returned for medical management. For most of these women the uncertainty of expectant management, in particular not knowing when the miscarriage would happen, appeared to engender a sense of being out of control. This lack of control seemed to be a motivating factor in their decision to change to medical management:
So I went home and over the weekend I was scared to go anywhere too far away from home just in case, because again I had read other people’s accounts of miscarrying naturally, and the blood loss was something that was worrying me, because if I was to be at work for example, I work 20 miles away from home so it would be very difficult for me to get . . . and also I am the only person here that can drive so it would be very difficult for me to get home . . . But even just little things like I didn’t really want to go to anybody’s house, or go anywhere just in case.
Kyla
Delays in confirming the missed miscarriage also seemed to reinforce the desire to bring closure to the experience:
. . . because I was already so devastated and then I had already waited a week I just felt that I couldn’t just wait really for something to happen.
Ruth
In addition, the need for control seems to relate in part to competing demands in women’s lives, including work, childcare commitments and family life, as described in the quotations above. Women’s reasons for rejecting expectant management, therefore, appear to illuminate why active management is preferable. Control is deemed important because it allows women the opportunity to manage the competing demands in their lives as well as bringing some closure.
Women’s experience of missed miscarriage and its influence on treatment choice
There also appears to be specific emotional considerations underpinning women’s desire for active management of a missed miscarriage. As indicated above, many of the women reported the need to have closure to bring the physical process of the miscarriage to an end, having discovered that their baby had died some weeks previously:
I just want to get it done, cleaned, get it over and done with and just close . . . I just felt like I had been a walking tomb for nearly 3 weeks and had no idea, and my body had totally tricked me and it was horrible.
Emma
I said no I just want this thing out. Psychologically once you are told, it’s like having cancer, when you have got this cancer but you don’t know you’ve got it, but the moment you are told that you have got cancer psychologically your body changes, your mind. So when I was told that there is no baby you’ve had a missed miscarriage they said, psychologically my body just changed, all of a sudden my bump shrunk, and I was really emotionally . . . and I broke down.
Oyinlola
There is a sense in both quotations that these women felt betrayed by their bodies. Thus, it seems that women struggle with the dissonance created for them in continuing to feel pregnant despite having discovered that their baby has died. Indeed, some women continued to experience pregnancy-related symptoms, such as nausea and breast tenderness, which further intensified this dissonance. Consequently, they reported a strong desire to bring the physical process to an end:
Because the thing is [I] would be worrying that . . . the sac would continue to grow and I would continue to have the symptoms of being pregnant, and I couldn’t deal . . . with that mentally and emotionally.
So even up to that point you still were having pregnancy symptoms?
Yeah, I felt sick, my breasts were tender.
Accordingly, it seemed that active management in the form of medical management afforded women the opportunity to bring the difficult feelings engendered by the specific circumstances of a missed miscarriage to an end and enable them to begin to grieve for the loss.
Reasons for choosing to participate in the trial
In addition to the need for active management, women reported that their reasons for choosing medical management were a desire to avoid surgery and/or because of advice they had received from friends and family. Women subsequently identified a range of reasons for agreeing to participate in the trial. One of the main reasons seemed to involve an altruistic notion of being able to help others in the same position as themselves:
I felt at that point that if I couldn’t help myself then I would try and help someone else maybe in the future to get the process over with quicker.
Elena
Nevertheless, other reasons given suggested that there were aspects of the trial that were also meaningful to women in regard to their own needs and so the altruism was on some level conditional. These reasons were (1) location of the treatment, (2) a follow-up scan and (3) achieving a successful conclusion to the process.
Women indicated that having control over where the treatment took place was important to them. For example, Hailey reported that the fact that the treatment could take place at home was particularly relevant to her needs, allowing her to be with those she felt cared for her:
She said the second option was to go with the MifeMiso research project, and I could go home. So I liked the idea of that more because I didn’t really want to be in the hospital, I wanted to be at home with my loved ones . . . I just felt if I was at home I would be more comfortable being in an environment where I would be relaxed.
Hailey
Not all women preferred home management though. For example, Grace emphasised that she did not want to miscarry at home. She appreciated that the staff had offered day patient attendance for the misoprostol and so was unhappy that the miscarriage occurred at home, after taking the mifepristone/placebo:
The one thing I said to [my partner] is I don’t want to flush it down the toilet, and that’s literally what happened to it. So that was rubbish because I felt like the decision I was making I would have been in hospital, it wouldn’t have gone down the loo.
Grace
Women also reported that having a scan following the treatment was a strong influencing factor as, for the majority, this was not an option that would have been offered according to local protocols:
She said, ‘Normally when you do the medically managed one you don’t get a scan afterwards, it’s only when you’re doing the research trial thing’ and I was like . . . obviously you would want to have the scan afterwards . . . especially because you’re doing it on your own, you just never know if it’s going to all come away, and with me it didn’t, in the end I had to go and have the ERPC [Evacuation of Retained Products of Conception]. So that was a major reason for me . . . why I wanted to do the trial, because of that extra scan at the end.
Emma
Finally, women reported that the potential to ensure a greater chance of success with the miscarriage if they received mifepristone was an influencing factor:
The other thing was if I did get the real one that they’re supposed to work well, better with taking that as well as the pessary, because I know the pessary by itself doesn’t always work. So that was another reason why I decided.
Ruth
Women’s perspectives on what should be offered in miscarriage care and treatment in the future
Women identified two main areas that they felt were needed in miscarriage care and treatment in the future: (1) the need for an accessible, woman- and partner-centred pathway of care and (2) personal support needs throughout the experience of pregnancy loss.
An accessible, woman- and partner-centred pathway of care
Women identified that miscarriage care and treatment in centres such as EPUs needed to be accessible and available to women via self-referral. Furthermore, consistent with the themes identified in our analysis, women reported that the care offered within EPUs should involve HCPs who are specialist in miscarriage and who are alongside them:
Compassionate care would be my first one, so the human being not automation . . . so having that care with someone who is supporting me through it is important.
Sophia
The care offered within EPUs should also involve providing continuity of care:
I think having a clear contact point is very helpful to the person . . . a clear person that you’re allocated to always feels very reassuring, with a clear number and route to call them.
Jo
Furthermore, women indicated that these specialist HCPs should be knowledgeable and able to appropriately address their information needs. This involved providing women with clear and evidence-based information about medical management while tailoring this to their specific needs and requirements:
Yeah, and I think some people . . . my partner he is a scientist by nature, and so he’s really interested in what happens in the process, so he asked questions because he wanted to understand it. Some people might not want to understand it and they are happy with the answer that you give and that’s it, but I think it’s being able to explore that or have that opportunity to explore it if you want to, to find out. Everyone is different aren’t they?
Olivia
In the context of recurrent miscarriage, some women, including Christie, identified that it was important to offer women relevant information, rather than what they perceived as platitudes:
. . . someone that’s specialised into miscarriage, because I can’t be . . . hearing oh one in four have a miscarriage again, I’m like shut up with that, because that’s one in three now with me on the trot, so one in four you can really stick that.
Christie
In addition, women indicated that they thought that follow-up services should be available, offering women support to understand the reasons for their miscarriage, what to do for their fertility and early support in future pregnancies. Many of these wishes reflected the emotional journey of miscarriage for these women, including the uncertainty and loss of control it engendered. Therefore, women’s perspectives on what should be offered reflected the need to feel supported and informed rather than alone with their fears and concerns.
Personal support needs
Alongside the need for follow-up support, women also reported that they thought that formal and informal opportunities for emotional and psychological support following the miscarriage should be available to women and their partners generally. The forms of support included peer support in a variety of different formats, including one to one, online or in groups:
Some people don’t necessarily want to talk one on one because they find it too intense, sometimes just going to a group and listening to people’s stories can be a great help. Sometimes just having someone to sit in silence with is a great help as well. So I think that support network, having a support network where you can direct people to if they do have things like this happen would just be amazing.
Ceri
Women reported that the value of peer support was the knowledge that they were not alone and that others understood their experience. Women also reported that they felt that counselling should be available to women during and after the miscarriage:
Absolutely counselling. I think once you’re going through something like this the only support you can really have is just know that there is someone there who will just listen to you. Just someone to listen to you because there’s no one can say anything that’s going to make you feel any better, no one can do anything that makes you feel better, or you just need a space or someone that you can go to just to spill those emotions out on. Not everyone has a good support unit at home I guess.
Elise
In common with other women, Elise recognised the value of having someone present and able to hear her distress and facilitate opportunities to explore those emotions. It was evident from women’s narratives that this counselling needed to be provided by a skilled and knowledgeable professional who would be able to help women and their partners explore and make sense of the miscarriage. This was particularly emphasised by women experiencing recurrent miscarriage.
Discussion
The purpose of this nested qualitative study was to explore women’s perspectives on the acceptability of and their satisfaction with medical management of missed miscarriage. In total, 42 women participated, the majority of whom were either satisfied or very satisfied according to the CSQ-8 score. 61 This study has uncovered a deeper and nuanced understanding of these women’s experiences, their perspectives on the acceptability of medical management and their satisfaction with this treatment.
We have found that satisfaction with medical management is best viewed as a continuum, from satisfied to dissatisfied. In exploring women’s experiences through the pathway, we have uncovered their perspectives on what aspects of the treatment and care did or did not work well for them. We have found that some women were satisfied and would choose medical management again or recommend it. However, we have also found that there were other women whose experience at key points in the care pathway lead them to report that they had reservations about it or would not choose medical management again. We have identified five contextual factors that appeared to influence women’s satisfaction positively or negatively and therefore, the extent to which they find the treatment acceptable. These are (1) alongside me, (2) feeling informed and in control, (3) timely resolution to the physical process, (4) treatment experience and (5) treatment success. A central feature of these contextual factors is the extent to which women felt that they were supported, informed and in control.
All of the women’s narratives emphasised the importance that they placed on the care they received from HCPs and the extent to which they felt supported. It appeared that this care, when positive, was delivered in the context of a therapeutic relationship involving listening, warmth, empathy, responsiveness to need and interest in the woman and her experience. Women clearly valued and found this relationship with HCPs to be beneficial to their well-being. Kornhaber et al. 74 described three core features of therapeutic, interpersonal relationships that are necessary in acute health care: therapeutic listening, responding to emotions and unmet needs, and patient centredness and therapeutic engagement. There is evident synchronicity between these concepts and the experiences that women report in this study. Research evidence further demonstrates the significance of such contact, in that positive therapeutic relationships are strongly associated with increased satisfaction with care, improved outcomes and clinical effectiveness. 74,75
In contrast, where women in this study experienced care that they perceived as negative, this was associated with practices that they found to be invalidating, diminishing of their experience and lacking concern for their needs. Care such as this can increase psychological distress and, furthermore, risks poor recovery. 45,74,76,77 In this study, these experiences more commonly reflected contact with HCPs in services outside EPUs, such as scanning departments, gynaecology or accident and emergency, although on occasions their experiences related to EPU or research staff as well. Although recent research has indicated that improvements may be needed in EPUs too,45 it is nevertheless clear that acute health-care environments, in particular, struggle with increasing workloads, patient acuity and technological demands that can result in a lack of person centeredness. 74
Nevertheless, our findings suggest that some HCPs in these areas were able to meet women’s psychological and emotional needs. It therefore seems possible that HCPs may benefit from developing the necessary skills and knowledge to respond appropriately to women’s psychological needs in the context of miscarriage. 49,78,79 Jensen et al. 78 suggest that this may be achieved through tailored education and training programmes, particularly when they are linked to bereavement care.
Our findings highlighted that feeling informed and in control was important to all of the women participating in our study. Existing research highlights that women are often dissatisfied with the amount and quality of information that they receive, as well as the sensitivity with which it is communicated. 45,76,80–82 Therefore, for information to be helpful, our findings have identified a number of features of information sharing that can assist women to feel informed and in control, thus enhancing their satisfaction.
First, information needs to be provided in a variety of formats (verbal, written and visual) so that women’s differing information needs, learning preferences, literacy and language needs are met. Pictorial and written information served as important reference points and reinforced verbal explanations when it was difficult to retain information during discussions about treatment options and the potential effects of medical management. Our participants’ experiences, therefore, suggest that these different forms of information need to be used in combination to ensure that women know what to expect and can make informed decisions.
Second, we have found that women have individual preferences and needs for the information that they require as well as differing levels of prior knowledge and experience. Some will want information about other women’s experiences of missed miscarriage and medical management, whereas others clearly prefer technical information or a combination of the two. Consequently, it seems that HCPs might benefit from training in advanced communication skills to be able to explore women’s needs and expectations and tailor their approach accordingly. 45,50,79,83 In addition, given women’s variable experiences of accessing the internet, the provision of written information detailing trusted websites and what sort of content these provide might assist women to access the additional information that they require. 84 This sort of information may also reduce potential anxiety related to stories that can be found on the internet.
Third, it seems that information concerning what to expect during and after treatment was essential for the women participating in our study. We found valuable examples of this that enabled women to make an informed choice, prepare for their personal care needs during the treatment and manage the aftermath, particularly when having home-based treatment. In this context, explicit information was provided on what to expect regarding the extent and duration of blood loss and pain, pain management, care needs and who to contact in an emergency. Where women were not provided with enough information, this appeared to negatively influence their satisfaction. In some cases, women appeared to feel that, as a result of the lack of information, they had not made an informed choice, something that is enshrined in current health-care policy and guidance. 85,86 Thus, it seems a necessary step forward in early pregnancy loss care to explicitly address women’s information needs for the likely effects of medical management and the management strategies that they will need going forward. 81–83,87
Finally, our findings indicated that information sharing and discussion following completion of the treatment was highly desirable. Women valued the follow-up scan and contact with HCPs at this point as an opportunity to bring the physical process to a close and address specific information needs, including concerns about fertility, available support services and counselling support. Given the evidence concerning the psychological and emotional impact of miscarriage, it seems likely that the amount of information required will vary and sensitivity is needed to establish what women need, are ready to hear and discuss. 77,88 Nevertheless, such support is a valuable opportunity to address women’s well-being and adjustment needs, as well as bring closure to the physical process of the miscarriage. 76,81
When considering women’s experience of the treatment, it seems that there are further implications for miscarriage care arising from our findings, including women’s preference for the location of the treatment, women’s experience of bleeding and pain and the needs of women who require further intervention.
Our participants reported that being able to choose their preferred location for undergoing the miscarriage was influential in their choice of taking part in the trial initially and then again when expressing satisfaction with the outcomes. Research evidence indicates that being able to undergo medical management at home is viewed as a safe, effective and acceptable alternative to hospital care for the majority of women. 41,89,90 However, given some women’s experiences with the level and intensity of the bleeding and pain, it would seem particularly important to ensure that women have comprehensive information and support to manage their needs appropriately during home management. 89 Furthermore, it is clear that some women valued the opportunity to undergo the miscarriage in hospital. It therefore appears that, where possible, the location of the miscarriage needs to be considered with due regard to the women’s specific needs, understanding and experiences. 41,89,90
Although a proportion of women in our study reported significant pain and/or heavy bleeding during medical management, some did not. Some research evidence indicates that increased blood loss is more common with medical management than with surgical management,48 although not all studies agree. 91 Davis et al. 48 suggest that severity of bleeding is more likely to be greater in younger and parous women and when the pregnancy is of longer gestation. However, it seems that limited research evidence exists that has explored the characteristics that are most likely to be associated with heavy blood loss and/or bleeding. Therefore, it would seem that further research exploring the factors influencing heavy bleeding and/or pain might provide information, which can aid in decision-making, treatment and management.
Our findings are consistent with existing research in finding that the failure of medical management, and thus the need for surgery, or the need for further intervention because of an incomplete resolution, increases the likelihood of dissatisfaction with treatment. 32,36,37,42,43 Although women were mainly satisfied with the intervention that they ultimately received to resolve the physical process of the miscarriage, they nevertheless appeared to experience emotional distress. This distress was particularly evident in the case of women who reported difficulties with the care provided and/or extended delays to resolution.
Research suggests that women experiencing missed miscarriage may have a greater need for post-miscarriage counselling and follow-up. 53,56 Furthermore, Kong et al. 42 suggest that women with high baseline scores of psychological distress following miscarriage may particularly benefit from follow-up counselling. We, therefore, suggest that women experiencing a delayed resolution to their miscarriage may have a significant need for follow-up support and counselling.
In addition to the findings on satisfaction and acceptability, we have also identified additional elements that we believe add important context to the experience of care and treatment for women experiencing miscarriage and in particular, missed miscarriage. These are (1) women’s personal journey, (2) active management and (3) women’s experience of missed miscarriage.
Despite being a commonplace occurrence, our findings demonstrate that miscarriage is a significant event in a woman’s life when the pregnancy is wanted. Consistent with the experience of the women in this study, research evidence suggests that the process of planning, conceiving and being pregnant involves fundamental changes to identity and selfhood as a woman prepares to become a mother. 46,92,93 As our findings illustrate, women re-evaluate their lives, make plans and appear to be developing an ‘internal sense of motherhood’. 93
In this context, miscarriage may be experienced as a traumatic event in which women grieve for the loss of their baby as well as the hoped-for future they had envisaged. 94,95 Furthermore, miscarriage may cause some women to feel caught in an ‘in-between state’, unable to return to their previous concept of self, but equally unable to continue their journey to becoming a mother. 94,95 This may have consequences for emotional well-being, particularly where the threat that motherhood will never be realised increases, such as with recurrent miscarriage, IVF or where women are trying to conceive later in their reproductive life. This emotional and personal journey frames women’s experience of miscarriage care and treatment and their recovery. Our participants’ experiences of the actions of HCPs in validating or minimising their changing identity and self-concept highlights the powerful impact that these professionals can have on a women’s well-being at this time. 53 This adds further weight to the need for training and education for HCPs. It also supports women’s view that follow-up counselling and support should be offered. 76,81,96
Furthermore, it seemed that there are features of a missed miscarriage that may be particularly influential in the shock and distress following diagnosis. We found that women varied in the extent to which they are aware that something was wrong and even where they had experienced bleeding and/or pain, this is most commonly late in the first trimester. Consequently, women had begun to develop strong attachments to their baby as well as the aforementioned changes to identity. In this context, our participants experienced powerful emotional dissonance when discovering that their baby had died some weeks ago, while they continued to believe themselves to be pregnant and in some instances had ongoing pregnancy symptoms. It appeared that women may feel a sense of betrayal, that their bodies have ‘tricked’ them into believing they were having a baby. Given that existing literature identifies that unspoken emotions influence women’s decision-making, it seems important that HCPs are aware of the potential impact that these feelings have and the implications that they have for their approach and the support that they offer. 41,44
One way in which this experience might influence decision-making is apparent in our findings. In common with another study,41 our participants’ experience suggests that, in the light of the diagnosis, active management of miscarriage is preferable to them. Being able to achieve closure and reclaim their body would appear to be strong influencing factors in women’s decisions to reject expectant management in favour of an approach that brings the physical experience of the miscarriage to a close in a timely way. Active management also enables these women to achieve a sense of control and manage the impact of the miscarriage within the context of the competing demands on their lives. So, although existing guidance recommends expectant management for missed miscarriage,6 it would appear that in the context of missed miscarriage, women’s preference in our study was for an active and managed solution that achieves a timely resolution.
Strengths and limitations
There are strengths and limitations to this aspect of the study. We have considered the perspectives of 42 women taking part in the MifeMiso trial. We were able to recruit a range of participants representing different characteristics and demographics and achieve saturation in the thematic structure that we developed. This is therefore a large qualitative sample that has resulted in new insights and a depth of understanding of women’s experiences of medical management of missed miscarriage. These women’s experiences are likely to be transferable to women in similar contexts and we believe we have provided sufficient detail from which transferability to different contexts can be assessed. Nevertheless, this study relates to women’s experience of medical management for missed miscarriage in the UK, where EPUs have been in place for more than 20 years. This needs to be considered when exploring transferability of the results in countries with health-care systems that differ from this provision.
Our study has focused on women’s perspectives of medical management of missed miscarriage. Although it is a strength of this research that it provides an in-depth exploration of women’s experiences, it would have been further strengthened by considering the perspectives of professionals and partners alongside the women. This would have provided a broader, systemic consideration of the experience of medical management of missed miscarriage and the contextual factors influencing satisfaction. Considering these different perspectives may have contributed towards the development of a model of best practice for women and their partners. However, given that patient experience is considered to be a central tenent of clinical safety and effectiveness,75,86 our findings offer an important insight into women’s experience of medical management of missed miscarriage. In particular, we have been able to highlight the factors that positively influence satisfaction, a finding that has been largely absent from existing research.
One limitation of the study is that it only compares two different regimens for the medical management of missed miscarriage, and therefore cannot compare this against expectant management. However, our research group are currently undertaking a network meta-analysis that will estimate the relative effectiveness and safety profiles for all methods of management of miscarriage and rank the available methods according to their effectiveness and safety profile. 97
Another potential limitation is that the observed effect size was smaller than that considered in the sample size calculation. The observed difference in primary outcome rates between the two treatment groups was 7.6%, which is lower than the 10% difference that was deemed important by the survey of clinicians prior to commencing the study.
One final limitation of the qualitative study is the absence of the perspective of South Asian women. Existing research findings98 emphasise that participation of South Asian people in health research is likely to be increased if opportunities are provided to develop trusting and personal relationships. It therefore seems possible that a reason for the non-participation of South Asian women in this study reflects constraints of time and frequency of contact, which limited opportunities to develop a relationship that might facilitate their participation. Solutions to this will need to be addressed in subsequent research to ensure that the perspectives of women from South Asian communities are heard.
Chapter 5 Health economics analysis
Parts of this chapter are reproduced or adapted with permission from Chu et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) license, which permits others to copy and redistribute this work, for non-commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Parts of this chapter are reproduced or adapted with permission from Okeke Ogwulu et al. 99 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to copy and redistribute this work, for non-commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Introduction
This chapter reports the economic evaluation conducted alongside the MifeMiso trial. The overall aim of the economic evaluation was to assess the resource use costs, clinical outcomes and health-related quality of life associated with the use of the combination of mifepristone and misoprostol (the MifeMiso intervention) compared with misoprostol alone in the medical management of a missed miscarriage.
The primary economic evaluation was a within-trial cost-effectiveness analysis (CEA) based on the outcome of additional cost per additional successfully managed miscarriage. We also conducted a cost–utility analysis (CUA), with the outcome measured in terms of quality-adjusted life-years (QALYs) gained at specified end points. In addition to the within-trial analysis, a decision-analytic model was developed to assess the cost-effectiveness of the medical management of missed miscarriage with mifepristone plus misoprostol (as explored in the trial), compared with alternative strategies beyond the trial comparisons, including surgical and expectant management and the current practice of medical management, based on available secondary sources.
The report for the trial analysis and the model-based analysis are presented separately – first the within-trial economic evaluation and then the model-based economic evaluation.
Health economic analysis objectives
To explore the relative cost-effectiveness of the combination of mifepristone and misoprostol (the MifeMiso intervention) compared with misoprostol alone in the medical management of a missed miscarriage.
Health economic analysis methods
All statistical analyses were performed using Stata® 15 (StataCorp LP, College Station, TX, USA). The economic evaluation adopted the perspective of the NHS; hence, only direct health-care costs were included. Costs from a Personal Social Services perspective were likely to be negligible and so were not collected. As part of the sensitivity analyses, we explored a range of costs. Given the duration of the trial, discounting was not applied to either the costs or outcomes. The reporting of the economic evaluation is in keeping with the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. 100
Data collection
Data on resource use and outcomes were collected during the MifeMiso trial using researcher-recorded trial collection forms. Data on health utility were collected using the EQ-5D-5L questionnaire. 101 As in standard practice, for the base-case analysis, only resource use incurred because of the trial intervention was considered. To calculate the total cost of the treatment, resource use data were multiplied by relevant unit costs.
Outcomes
The primary outcome of the economic evaluation, based on the main outcome of the trial (failure to spontaneously pass the gestational sac within 7 days post randomisation) was the number of successfully managed miscarriages. In keeping with the recommendation of NICE,101 an additional outcome of the economic evaluation was the QALYs gained. The QALY is a single index measure incorporating both quantity and quality of life using health utilities. 101
Some secondary clinical outcomes explored in the economic evaluation were the number of women who needed surgical intervention to resolve the miscarriage and the number of women who needed additional doses of misoprostol.
Resource use and costs
Resource use data within the NHS (secondary care costs) were collected to estimate the overall cost of drug administration, management of miscarriage and follow-up care (until discharge from EPU care) associated with the two groups of the trial. For each resource item, unit costs (see Table 13) were identified from established national sources such as the NHS Reference Costs 2018–19102 and the Personal Social Services Research Unit (PSSRU) costs. 103 In cases where there are different categories associated with resource use, weighted averages were used (see Table 13). All costs were reported in 2019–20 Great British pounds values and cost estimates from earlier years were inflated using the NHS Cost Inflation Index (NHSCII). 103
The main resource categories related to miscarriage that were collected included:
-
the quantity of medication administered.
-
the management of miscarriage in terms of the number of visits to secondary care facilities, such as outpatient visits, emergency visits and hospital admissions until final discharge, for example, if surgery is needed to resolve the miscarriage.
-
The frequency of AEs and complications, such as blood transfusion and infection.
Drug administration (medication cost)
The trial medication was a single dose of 200 mg of mifepristone, taken orally after confirmation of missed miscarriage by pelvic ultrasound scan. The cost of mifepristone (Mifegyne,® 200 mg) was identified from the British National Formulary (BNF)104 as £52.66 for a pack of three oral tablets, equivalent to £17.55 per 200-mg tablet (Table 13). As every woman in the trial received an initial dose of vaginal, oral or sublingual 800 μg of misoprostol, this cost was not included in the analysis.
| Resource use items | Unit cost (£)a | Sourceb |
|---|---|---|
| Medication | ||
| Mifepristone (Mifegyne®) 200 mg | 18 | BNF 104 |
| Misoprostol (Topogyne®) 800 µg | 16 | BNF 104 |
| Vaginal misoprostolc (Mysodelle®) 800 µg | 372 | BNF 104 |
| Secondary care costs | ||
| Hospital visit | 150 | PSSRU 2002 105 |
| Emergency visit | 98 | NHS Reference Costs 2018–19102 (VB09Z VB11Z) |
| Outpatient admission (specialised non-routine U/S) | 127 | NHS Reference Costs 2018–19102 (NZ22Z) |
| Inpatient admission (< 24 hours) | 325 | NHS Reference Costs 2018–19102 (MA54Z MA55A MA55B MA56 A MA56B) |
| Night of patient admission | 413 | NHS Reference Costs 2018–19102 (MA51Z MA52A MA52B MA54Z MA55B) |
| Surgical management (dilatation and evacuation) | 1462 | NHS Reference Costs 2017–18106 (MA17C MA17D) |
| Manual vacuum aspiration (MVA) | 1182 | NHS Reference Costs 2017–18106 (MA19A MA19B) |
However, for women who required additional doses of misoprostol, these were costed. The BNF provides the cost of 400 µg of oral misoprostol (Topogyne®, 400 µg) as £128.00 for a pack of 16 tablets; therefore, a 400-µg tablet costs £8, which is equivalent to a cost of £16 for two tablets (800 µg). The routine practice within the NHS is to use oral tablets for vaginal and sublingual administration. Hence, the same cost was applied for all misoprostol administration irrespective of the route. A cost was not available in the BNF for sublingual misoprostol; however, the cost of misoprostol for vaginal administration (Mysodelle®, 200 µg) was provided as £465 for a pack of five tablets, which is equivalent to £372 for 800 µg. The impact of the cost of vaginal misoprostol was explored in a sensitivity analysis.
Management of miscarriage (secondary care cost)
Resource use data were collected on the number of visits to the hospital and the emergency department as well as the number of outpatient admissions, inpatient admissions (a hospital stay of < 24 hours) and nights of admissions (a stay of > 24 hours). The cost of a regular hospital visit was obtained from the PSSRU. 105 For the cost of an emergency visit, the weighted average of emergency medicine for patients who require a category 0–2 treatment and a category 0–1 investigation were obtained from the NHS Reference Costs 2018–19. 102
Outpatient admission in the trial literature involved procedures such as an additional ultrasound and was, therefore, costed as an antenatal visit with a non-routine ultrasound. 102 Inpatient admission was described in the trial literature as the admission of a participant because of vaginal bleeding or any complication for observation. The trial literature further assumed that bleeding ceased and the participant was discharged on the day of admission and, hence, this situation was costed as a day-case management of medical/surgical, abortion or miscarriage care102 (Table 14). Nights in the hospital were recorded as inpatient night admissions costs within the trial. The weighted average cost per non-elective long-stay day was obtained102 to arrive at a mean cost per patient night of admission (see Table 14).
| HRG code | HRG label | Number of cases | Unit costs (£) |
|---|---|---|---|
| Inpatient admission (< 24 hours) | |||
| MA54Z | Medical, abortion or miscarriage care, from 14–20 weeks’ gestation | 1222 | 410 |
| MA55A | Medical, abortion or miscarriage care, from 9 to < 14 weeks’ gestation, with insertion of long-acting contraceptive | 660 | 390 |
| MA55B | Medical, abortion or miscarriage care, from 9 to < 14 weeks’ gestation, without insertion of long-acting contraceptive | 7124 | 317 |
| MA56A | Medical, abortion or miscarriage care, < 9 weeks’ gestation, with insertion of long-acting contraceptive | 1504 | 296 |
| MA56B | Medical, abortion or miscarriage care, < 9 weeks’ gestation, without insertion of long-acting contraceptive | 15,549 | 311 |
| Weighted average inflated to 2019–20 prices | 325 | ||
| Inpatient nights of admission | |||
| MA51Z | Surgical, abortion or miscarriage care, from 14–20 weeks’ gestation | 549 | 3943 |
| MA52A | Surgical, abortion or miscarriage care, < 14 weeks’ gestation, with insertion of long-acting contraceptive | 37 | 2549 |
| MA52B | Surgical, abortion or miscarriage care, < 14 weeks’ gestation, without insertion of long-acting contraceptive | 3045 | 2705 |
| MA54Z | Medical, abortion or miscarriage care, from 14–20 weeks’ gestation | 465 | 3444 |
| MA55A | Medical, abortion or miscarriage care, from 9 to < 14 weeks’ gestation, with insertion of long-acting contraceptive | 80 | 832 |
| MA55B | Medical, abortion or miscarriage care, from 9 to < 14 weeks’ gestation, without insertion of long-acting contraceptive | 367 | 1875 |
| Weighted average inflated to 2019–20 pricesa | 413 | ||
| Type of surgery | |||
| MA17C | Dilatation and evacuation, < 14 weeks’ gestation | 9878 | 1296 |
| MA17D | Dilatation and evacuation, 14–20 weeks’ gestation | 1642 | 2111 |
| Weighted average inflated to 2019–20 prices | 1462 | ||
| MA19A | Vacuum aspiration with cannula, < 14 weeks’ gestation | 39,593 | 1115 |
| MA19B | Vacuum aspiration with cannula, 14–20 weeks’ gestation | 1560 | 1816 |
| Weighted average inflated to 2019–20 prices | 1182 | ||
Further non-medical intervention for the miscarriage was categorised within the trial as manual vacuum aspiration (MVA) and surgical management (dilatation and evacuation) of miscarriage. These costs were obtained from the corresponding HRGs in the NHS cost schedule 2017–18. 106
Measuring health-related quality of life
To calculate QALYs for the CUA, health utility data were collected using the EQ-5D-5L questionnaire. 101 The EQ-5D-5L questionnaire is a self-reported preference-based measure of health-related quality-of-life index, which assigns a weight to different health states. It consists of five dimensions: mobility, self-care, usual activities, pain or discomfort, and anxiety or depression. Each dimension has five levels of functioning: no problem, slight problems, moderate problems, extreme problems and unable to. The EQ-5D-5L measure produces a possible 3125 distinct health states ranging from 11111 (full health) to 55555 (worst health).
Responses to the EQ-5D-5L were collected at baseline and at 6 or 7 days and 21 days post randomisation and at discharge from EPU care. For women in the trial that had a negative pregnancy test following the intervention, day 21 was the point of discharge. Baseline EQ-5D-5L data were collected immediately after randomisation. Follow-up EQ-5D-5L data were also collected at different clinical contact points.
Serious adverse events
Data on SAEs were collected using SAE forms. The trial’s literature defines a SAE as an untoward event resulting in maternal death, immediate threat to life, hospitalisation or persistent or significant disability. Only clinically specified SAEs deemed to have arisen from the trial intervention were considered to be relevant to the economic analysis. As there were no SAEs that were clearly related to the administration of mifepristone, we did not include such costs in the analysis.
Missing data
Multivariate regressions and t-tests were used to explore whether or not resource use and QALY values missingness could be predicted by other variables in the existing data. 107 If the associations between variables were not statistically significant at the 5% level, we assumed that data were missing completely at random. Based on this assumption, missing costs and QALY values were imputed using multiple imputations108 by applying chained equations with predictive mean matching across 25 imputations. 109
Primary cost-effectiveness analysis
A within-trial incremental CEA was conducted to estimate the costs and benefits of a combination of mifepristone and misoprostol (the MifeMiso intervention) compared with misoprostol alone. The CEA, which is the base-case primary analysis, was expressed in terms of the cost per successfully managed miscarriage. The analysis focused on the secondary care costs for participants incurred within the trial period.
The total cost during the trial period was estimated by multiplying the resource items used by the corresponding unit cost and summing these costs. We calculated the mean total cost and mean total resource use for participants across trial groups. Given the skewness inherent in most cost data and the concern of economic analyses with mean costs, using the bias-corrected and accelerated (BCa) non-parametric bootstrap method110 95% CI around mean differences were estimated by analysing 1000 resamples.
Multivariate cost analyses were conducted using seemingly unrelated regressions111,112 to assess heterogeneity in the trial population. Seemingly unrelated regression is an accepted method that factors in any congruity in the error terms between costs and outcomes113 and is robust to skewed data. Based on the minimisation variables for the trial, model covariates included baseline data on maternal age (< 30 or ≥ 30 years), BMI (< 35 or ≥ 35 kg/m2), gestational age (< 70 or ≥ 70 days) and the quantity of bleeding (PBAC score: ≤ 2 or ≥ 3). All results were presented as mean values with standard deviations (SDs) and, when applicable, as mean differences in costs and effects with 95% CIs.
Incremental cost-effectiveness ratios (ICERs) were calculated by dividing the difference in mean total cost by the difference in the number of successfully managed miscarriages between the trial groups. The ICER is a measure that depicts the additional cost ascribed to an additional effect. We used the formula below to calculate ICERs. Here C represents cost and E represents effects.
To quantify the uncertainty that is often attributable to sampling variations, we resampled the joint distribution in the mean cost and outcome difference using non-parametric bootstrapping. 114 The distributions were simulated 5000 times to generate paired estimates of incremental costs and outcomes, which were plotted in a cost-effectiveness plane. 115 A cost-effectiveness plane comprises four quadrants: north-east, north-west, south-east and south-west. The scatterplot that is produced on the plane represents the simulations. Depending on the location of the scatterplot dots on the quadrant, an intervention may be judged as more effective and more costly (north-east), more effective and less costly (south-east), less effective and less costly (south-west) or less effective and more costly (north-west) than the alternative intervention.
Cost-effectiveness acceptability curves (CEACs) were generated to depict the probabilities that the combination of mifepristone and misoprostol for the medical management of miscarriages is a cost-effective intervention compared with misoprostol alone across a range of values representing the decision-maker’s willingness to pay (WTP) for an additional benefit. 116
Cost-utility analysis
The EQ-5D-5L values were converted to utility scores using the interim crosswalk value set for the UK population. 117 This involved mapping the EQ-5D-5L scores back to the EQ-5D-3L valuation set, as recommended by NICE. 101 At each time point, participants’ responses to the EQ-5D-5L questionnaires were converted to utility score ranging from –0.594 (for worst health description) to 1.000 (for full health). Multiple imputations as described earlier were used to estimate missing EQ-5D-5L scores.
The number of QALYs gained by each participant was calculated with the area under the curve method using the trapezoidal rule. This method links the utility scores of each participant at different time points. To avoid bias, QALYs were estimated using multiple linear regressions with baseline EQ-5D-5L utility (in addition to other minimisation variables) as a covariate to adjust for any difference between the trial groups. 118
An incremental CUA was conducted to estimate the differences in costs and QALYs between the trial groups. The mean total QALYs across participants in the trial was calculated. We divided the cost difference between the two groups by the difference in QALYs gained to estimate the ICER and reported the outcome in terms of additional cost per additional QALYs gained. As the distribution of QALYs is typically skewed, 95% CIs around mean values were calculated based on 1000 replications using the bias-corrected and accelerated (BCa) bootstrap method. A CEAC was constructed to show the probability of the MifeMiso intervention being cost-effective across a range of possible values of WTP for an additional QALY.
Typically, ICERs are compared against the benchmark thresholds for cost-effectiveness in the NHS context of £20,000 to £30,000 per QALY gained, as recommended by NICE. 116 An ICER below £20,000 per QALY suggests that the intervention is a cost-effective alternative. An ICER above £30,000 per QALY suggests that the intervention is not cost-effective. For values between £20,000 and £30,000, the result is indeterminate.
Sensitivity analysis
One-way and/or multiway sensitivity analyses were performed, depending on the results of the deterministic analysis. These comprised an exploration of alternative unit costs applied to the different resources used or the variation of some parameters while leaving others at their baseline value. When appropriate, these analyses were combined with the stochastic sensitivity analysis. Sensitivity analysis was used to quantify the inherent uncertainty relating to assumptions and sampling variations in the methods used for the economic analysis and ultimately assess the generalisability of the results.
Sensitivity analyses for the CEA included:
-
Exploring an alternative cost for additional misoprostol via the vaginal route – for the base-case analysis, as per the practice within the NHS, the additional doses of misoprostol were costed as the same irrespective of the route of administration (oral, vaginal or sublingual). The cost of 800 µg of vaginal misoprostol is £372 whereas the equivalent dose in the form of oral tablets costs £16. We explored the impact of assigning different costs (according to the route) on the analysis.
-
Removing the cost of additional doses of misoprostol – in a situation where initial treatment was successful, there will be no need for additional doses of misoprostol. Hence, we explored the impact of removing these costs.
-
Removing the cost of surgery – again, given a successful initial treatment, there would be no need for surgery.
-
Imputation of missing secondary care costs – an analysis was carried out to assess the impact of missing outcome data on the analysis for all women with primary outcome data.
Sensitivity analyses for the CUA included:
-
In the trial protocol, the plan was to derive utilities from EQ-5D-5L data using the English values. 119 However, following a recent recommendation by NICE,101 we used the crosswalk value set for the base-case analysis. For the sensitivity analysis, therefore, we explored the impact of using the English values.
-
Some authors recommend adjusting for the baseline utility when calculating QALYs. In the sensitivity analyses, we assessed the effect of not adjusting for baseline utility values.
Within-trial economic evaluation results
A total of 711 women were recruited for the MifeMiso trial, of whom 357 were randomised to the mifepristone plus misoprostol group, and 354 were randomised to the placebo plus misoprostol group. Of the 711 women recruited, seven were lost to follow-up and six withdrew from the trial. In addition, there were two women with a missing primary outcome. Women were included in the economic analyses if they had data for the primary outcome measure; hence, a base-case analysis was conducted for 696 women (348 in each group).
Outcomes
Of the 696 women with primary outcome data (Table 15), 289 (83%) in the mifepristone plus misoprostol group and 266 (76%) women in the placebo plus misoprostol group had a complete resolution of the miscarriage by day 6–7 post randomisation. This translates to an effect difference of 6.6% (95% CI 0.7% to 12.5%).
| Outcomes | Mifepristone plus misoprostol group (N = 348), n (%) | Placebo plus misoprostol group (N = 348), n (%) | Bootstrap difference, adjusted mean (95% CI) |
|---|---|---|---|
| Primary outcome | |||
| Successfully managed miscarriage | 289 (83.05) | 266 (76.44) | 0.066 (0.007 to 0.125) |
| Secondary outcome | |||
| Need for surgery | 62 (17.82) | 87 (25.00) | –0.072 (–0.133 to –0.011) |
| Surgery complication | 4 (6.45) | 5 (5.75) | 0.003 (–0.019 to 0.014) |
| Need for additional misoprostol | 50 (14.37) | 65 (18.68) | –0.043 (–0.097 to 0.011) |
A breakdown of the resource use data by the trial group is presented in Table 16. For all resource use items with the exception of nights of admissions, women in the placebo group utilised, on average, more resources than women in the intervention group. These differences are also reflected in the costs, as shown in Table 17, which presents the mean secondary care costs per woman by trial group.
| Resource items | Mifepristone plus misoprostol group (N = 348) | Placebo plus misoprostol group (N = 348) | Bootstrap difference,a adjusted mean (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | n | Mean | SD | n | ||
| Secondary care costs | |||||||
| Hospital visit | 0.67 | 1.18 | 340 | 0.92 | 1.32 | 343 | –0.25 (–0.44 to –0.06) |
| Emergency visit | 0.19 | 0.43 | 340 | 0.29 | 0.65 | 343 | –0.10 (–0.18 to –0.02) |
| Outpatient admission (specialised non-routine ultrasound scan) | 0.40 | 0.99 | 338 | 0.51 | 1.01 | 343 | –0.11 (–0.25 to 0.04) |
| Inpatient admission (< 24 hours) | 0.21 | 0.46 | 340 | 0.28 | 0.53 | 343 | –0.08 (–0.15 to –0.002) |
| Nights of patient admission | 0.20 | 0.78 | 340 | 0.17 | 0.52 | 343 | 0.03 (–0.08 to 0.13) |
| Additional dose of misoprostol | 0.14 | 0.35 | 348 | 0.19 | 0.39 | 348 | –0.04 (–0.10 to 0.01) |
| Surgical management (dilatation and evacuation) | 0.14 | 0.34 | 348 | 0.19 | 0.40 | 348 | –0.06 (–0.11 to –0.005) |
| Manual vacuum aspiration | 0.04 | 0.20 | 348 | 0.06 | 0.23 | 348 | –0.01 (–0.05 to 0.02) |
| Resource item | Mifepristone plus misoprostol group (N = 348) | Placebo plus misoprostol group (N = 348) | Bootstrap mean cost difference,a adjusted mean (95% CI) | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Intervention | 18 | 0 | 0 | 0 | |
| Secondary care costs | |||||
| Hospital visit | 100 | 177 | 138 | 198 | –37 (–65 to –9) |
| Emergency visit | 18 | 42 | 28 | 64 | –10 (–18 to –2) |
| Outpatient admission (specialised non-routine ultrasound scan) | 50 | 125 | 64 | 128 | –14 (–33 to –6) |
| Inpatient admission (< 24 hours) | 68 | 148 | 92 | 172 | –24 (–48 to –0.79) |
| Nights of patient admission | 81 | 323 | 71 | 213 | 11 (–31 to 53) |
| Additional dose of misoprostol | 3 | 8 | 4 | 9 | –0.77 (–2 to 0.5) |
| Surgical management (dilatation and evacuation) | 197 | 500 | 281 | 577 | –85 (–165 to –4) |
| Manual vacuum aspiration | 50 | 240 | 68 | 276 | –17 (–55 to 22) |
Mean total costs
The mean total cost by trial group for different variables is presented in Table 18. The mean hospital costs per woman for the trial period was £621 in the intervention group and £803 in the placebo group, generating a mean cost difference of –£182 (95% CI –£338 to –£26).
| Cost | Mifepristone plus misoprostol group (N = 348) | Placebo plus misoprostol group (N = 348) | Bootstrap difference, adjusted mean (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| Intervention | 18 | 0 | 0 | 0 | |||
| Hospital visits/admissions | 328 | 629 | 388 | 556 | –58 (–148 to 32) | ||
| Need for surgery | 248 | 537 | 349 | 609 | –101 (–185 to –18) | ||
| Additional dose of misoprostol | 3 | 8 | 4 | 9 | –0.77 (–2 to 0.5) | ||
| Mean total cost | 580 | 1012 | 741 | 1028 | –161 (–309 to –12) | ||
Cost-effectiveness analysis
The primary cost-effectiveness outcome of the MifeMiso trial was the cost of a successfully managed miscarriage as evidenced by the passage of the fetal sac by day 6 or 7. The analysis showed that the combination of mifepristone and misoprostol was more effective than misoprostol only, with an additional gain in seven successfully managed miscarriages per 100 women (Table 19). The use of mifepristone plus misoprostol resulted in a cost saving of £182 (95% CI £26 to £338) per woman.
| Treatment | Mean cost (£) | Mean effect | Cost difference (£) (95% CI) | Effect difference (95% CI) | ICER (£) |
|---|---|---|---|---|---|
| Mifepristone plus misoprostol | 621 | 0.831 | –182 (–338 to –26) | 0.066 (0.007 to 0.125) | Dominant |
| Placebo plus misoprostol | 803 | 0.764 |
The results of 5000 bootstrap replications plotted on the cost-effectiveness plane for the primary analysis is presented in Figure 9. Each point on the plane depicts a pair of incremental cost and incremental effectiveness estimates for the comparison between the trial groups. As shown in the figure, the majority of the scatter plots are in the south-east quadrant. This suggests that the MifeMiso intervention is dominant (i.e. less costly and more effective).
FIGURE 9.
Cost-effectiveness plane for the primary outcome.
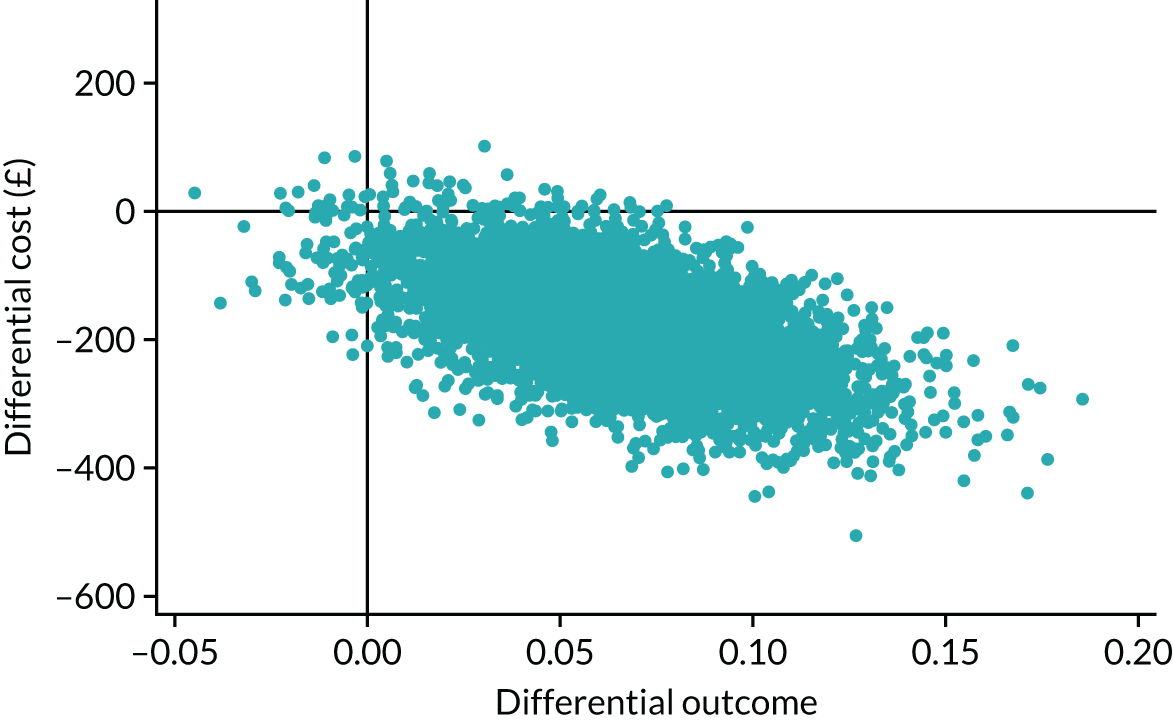
Figure 10 provides the CEAC for the primary analysis, which illustrates the probability of the intervention being cost-effective at various values of decision-makers’ WTP per additional successfully managed miscarriage. The figure shows that for thresholds of WTP > £3000, the probability of the MifeMiso intervention being cost-effective is over 90%.
FIGURE 10.
The CEAC for the primary outcome.
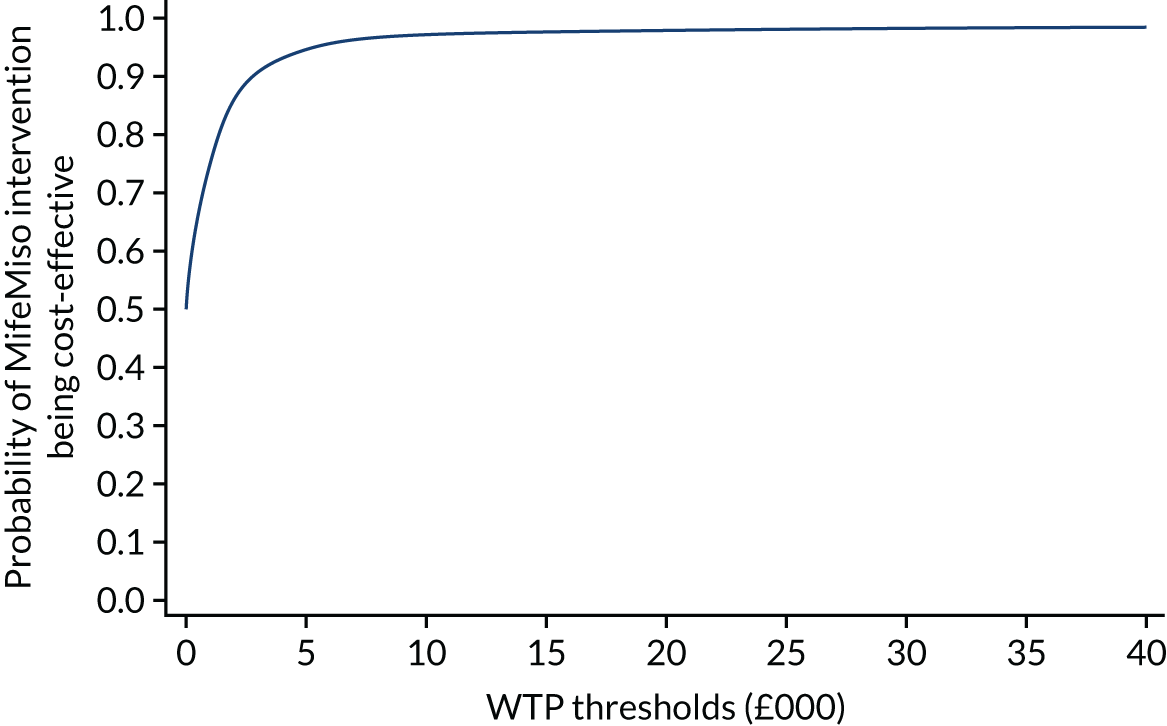
Cost-utility analysis
Utility and quality-adjusted life-years
The response rates for the health economics participant-completed EQ-5D-5L questionnaires are presented in Table 20. There was a progressive decrease in data over the trial. Complete economic data were available for 593 (85%) women (intervention group, n = 296; placebo group, n = 297).
| Time point | Mifepristone plus misoprostol group, n (%) | Placebo plus misoprostol group, n (%) | Total | Missing |
|---|---|---|---|---|
| Baseline | 348 | 348 | 696 | 0 |
| Day 6–7 | 337 | 336 | 673 | 23 |
| Day 21 | 300 | 303 | 603 | 93 |
| Discharge | 59 | 55 | 114 | 582 |
| Complete data baseline to day 21 | 296 | 297 | 593 | 103 |
Of particular note is the data collected on discharge from the EPU, which were available for < 17% of the participants. The low level of data available for this variable is mostly because the last contact for women who had a negative pregnancy test following the intervention was on day 21. Owing to the paucity of data at discharge from the EPU, these data were not included in the analysis. In addition, the time points for discharge from EPU varied for each woman and it would be inappropriate to include the time points for discharge from EPU in the total QALYs calculation.
The level of missingness of the EQ-5D-5L data shows that, at day 21, complete data were available for approximately 85% of women in both groups. Comparisons were made between baseline covariates and the missing utility scores and t-tests were used to assess the rate of missingness. Although no relationship was found between age, BMI, gestational age or quantity of bleeding, missingness was related to baseline utilities. EQ-5D-5L scores were lower for individuals with missing data.
The EQ-5D-5L responses were converted into utility scores using the Crosswalk tariff. EQ-5D-5L scores at different trial time-points are shown in Table 21. For all time points except for discharge from EPU, the utility scores were higher in the intervention group than in the placebo group.
| Measure | Mifepristone plus misoprostol group (N = 348) | Placebo plus misoprostol group (N = 348) | Bootstrap mean cost difference, adjusted mean (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||
| Baseline EQ-5D-5L score | 348 | 0.8776 | 0.1370 | 348 | 0.8716 | 0.1325 | 0.0058 (–0131 to 0.247) |
| Baseline EQ-VAS | 348 | 76.7 | 20.1 | 348 | 77.4 | 19.9 | –0.80 (–3.8 to 2.2) |
| Day 6/7 EQ-5D-5L score | 337 | 0.8558 | 0.13754 | 336 | 0.8291 | 0.1742 | 0.0269 (0.0023 to 0.0515) |
| Day 6/7 EQ-VAS | 337 | 77.4 | 18.7 | 336 | 77.0 | 18.8 | 0.39 (–2.4 to 3.2) |
| Day-21 EQ-5D-5L score | 300 | 0.9129 | 0.1307 | 303 | 0.9081 | 0.1345 | 0.0060 (–0.0150 to 0.0269) |
| Day-21 EQ-VAS | 300 | 85.1 | 15.6 | 303 | 84.2 | 16.3 | 0.88 (–1.7 to 3.5) |
| Discharge EQ-5D-5L score | 59 | 0.8809 | 0.1815 | 55 | 0.9182 | 0.1076 | –0.0353 (–0.0892 to 0.0185) |
| Discharge EQ-VAS | 59 | 81.9 | 17.1 | 56 | 85.2 | 13.0 | –3.46 (–8.9 to 1.96) |
| Total QALYs | 296 | 0.0324 | 0.0039 | 297 | 0.0319 | 0.0045 | 0.0004 (–0.0001 to 0.001) |
| Total imputed QALYs | 348 | 0.0324 | 0.0040 | 348 | 0.0317 | 0.0046 | 0.0005 (0.0000 to 0.001) |
The mean QALYs attained over the trial period were estimated using the area-under-the-curve method (Table 22). These estimates are based on data for participants with complete EQ-5D-5L data from baseline to day 21. The CUA showed a slight effect in the intervention group compared with the placebo group with a QALY difference of 0.0004 (95% CI –0.0001 to 0.001). The combination of mifepristone and misoprostol remained a cost-saving intervention.
| Treatment | Mean cost (£) | Mean effect | Cost difference (£) (95% CI) | Effect difference (95% CI) | ICER (£) |
|---|---|---|---|---|---|
| Mifepristone plus misoprostol | 621 | 0.0324 | –182 (–338 to –26) | 0.0004 (–0.0001 to 0.001) | Dominant |
| Placebo plus misoprostol | 803 | 0.0319 |
The cost-effectiveness plane for the CUA (complete-case analysis) is presented in Figure 11. As illustrated by the figure, the majority of the scatter plots are in the south-east quadrant, suggesting that the MifeMiso intervention is dominant (i.e. less costly and more effective). The CEAC (Figure 12) shows that for all WTP thresholds, the probability of MifeMiso being cost-effective is > 50%.
FIGURE 11.
Cost-effectiveness plane for the CUA.
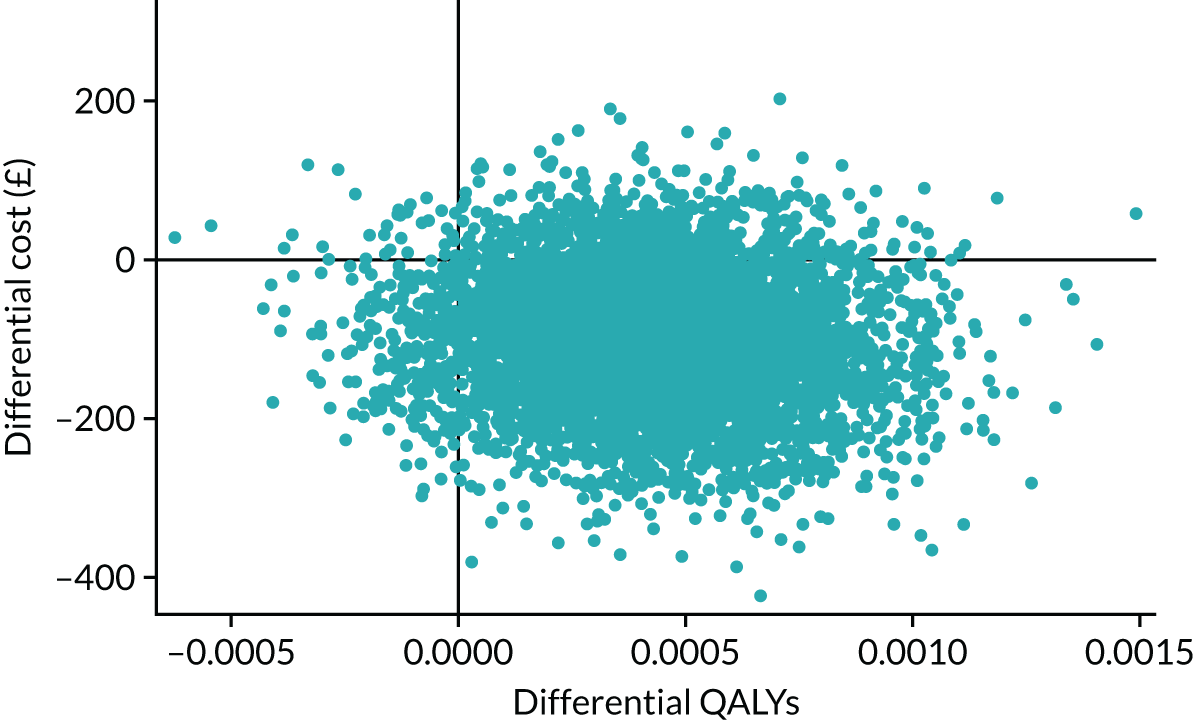
FIGURE 12.
The CEAC for the CUA.
Similar analyses were conducted on the QALYs following the application of multiple imputations (Table 23 and Figure 13).
| Treatment | Mean cost (£) | Mean effect | Cost difference (£) (95% CI) | Effect difference (95% CI) | ICER (£) |
|---|---|---|---|---|---|
| Mifepristone plus misoprostol | 621 | 0.0324 | –182 (–338 to –26) | 0.0005 (0.0003 to 0.001) | Dominant |
| Placebo plus misoprostol | 803 | 0.0317 |
FIGURE 13.
Imputed QALYs: (a) cost-effectiveness plane; and (b) CEAC.
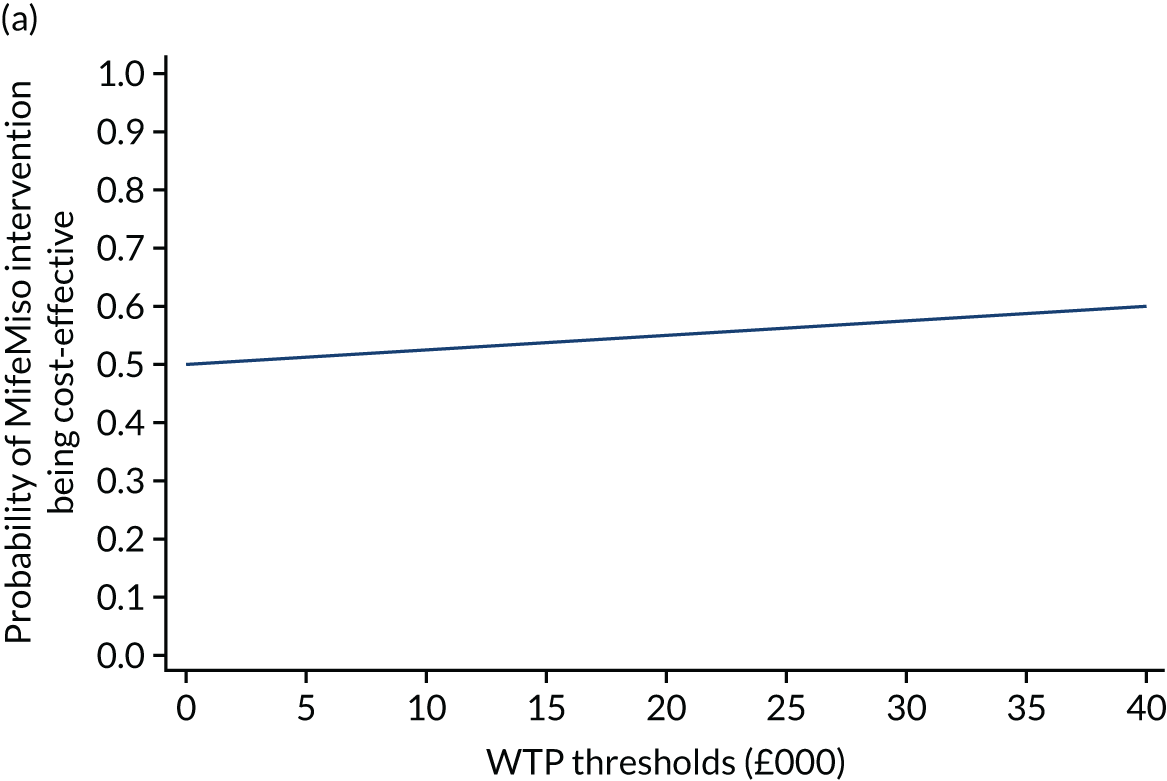
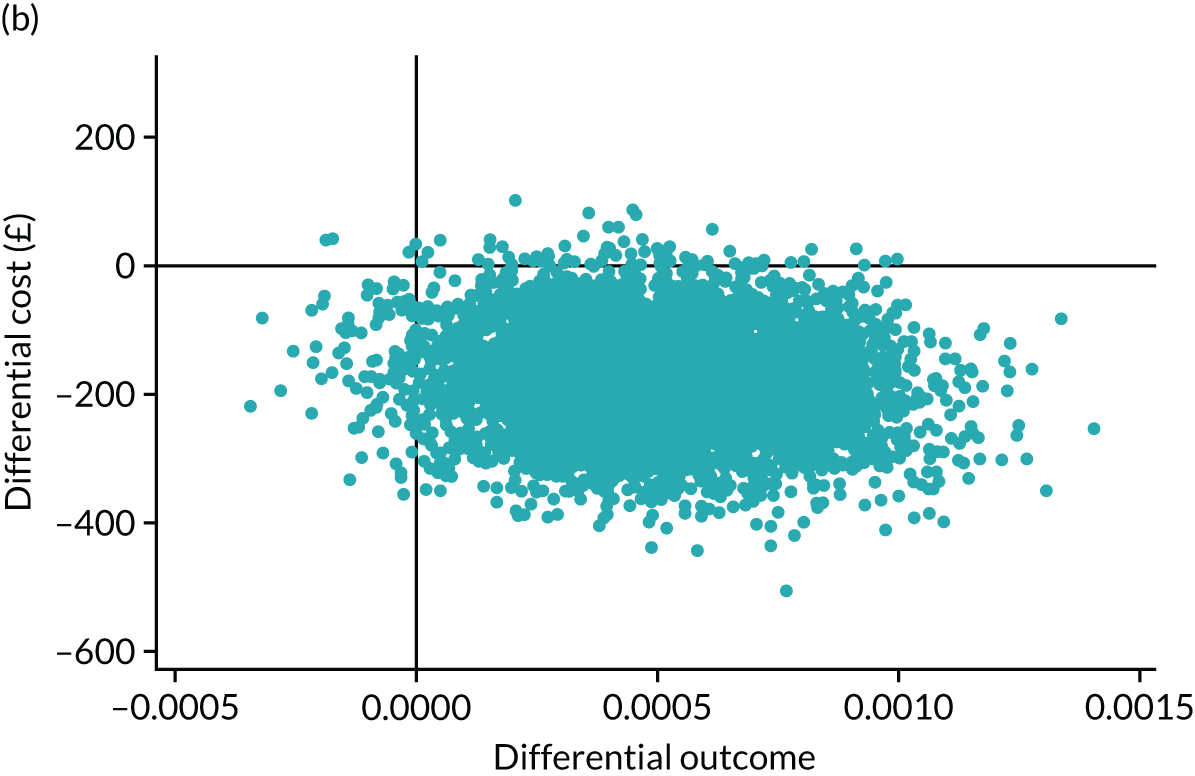
Sensitivity analysis
One-way deterministic sensitivity analyses were carried out using alternative costs for resource use items. For all scenarios (Table 24), the use of mifepristone and misoprostol remained less costly and more effective than misoprostol alone. Similarly, the sensitivity analyses conducted on the QALYs did not find any impactful differences (Table 25).
| Sensitivity analyses | Mean costs (£) | Cost difference (£), adjusted mean (95% CI) | ICER (£) | |
|---|---|---|---|---|
| Mifepristone plus misoprostol (SD) | Placebo plus misoprostol (SD) | |||
| Different cost for vaginal route of additional dose of misoprostol | 621 (1030) | 794 (1058) | –173 (–329 to –17) | Dominant |
| Removing costs for additional dose of misoprostol | 577 (1010) | 737 (1026) | –160 (–314 to –5) | Dominant |
| Removing costs for surgery | 331 (632) | 391 (558) | –59 (–148 to 30) | Dominant |
| Imputation of hospital care costs | 590 (1012) | 746 (1030) | –156 (–305 to 7) | Dominant |
| Sensitivity analyses | Mean QALYs | Mean difference, adjusted mean (95% CI) | |||
|---|---|---|---|---|---|
| Mifepristone plus misoprostol | n | Placebo plus misoprostol | n | ||
| QALYs as per protocol, derived using English values (adjusted for baseline utility) | 0.0345 | 296 | 0.0341 | 297 | 0.0003 (–0.0000 to 0.0007) |
| QALYs without adjustment for baseline utility | 0.0324 | 296 | 0.0319 | 297 | 0.0005 (–0.0002 to 0.001) |
Discussion of the within-trial economic evaluation
The main analysis was a CEA in terms of cost per successfully managed miscarriage. A CUA that was reported in terms of QALYs was also conducted. We collected utility values at specified timelines using the EQ-5D-5L questionnaire.
Principal findings
The results of the primary CEA suggest that the MifeMiso trial intervention (the combination of mifepristone and misoprostol) was less costly than the use of misoprostol only for the management of a missed miscarriage, with a cost saving of £182 (95% CI £26 to £338). The trial intervention is also more effective and led to an additional 6.6 (95% CI 0.7% to 12.5%) completely resolved miscarriages per 100 women. Hence, the combination of mifepristone and misoprostol is less costly and more effective, which suggests that ‘MifeMiso’ is a dominant intervention for the trial participants.
Similarly, the CUA showed that the intervention was slightly more effective for the management of a missed miscarriage with an additional QALY of 0.0004 (95% CI –0.0001 to 0.001). However, owing to limited data for the CUA, the results should be interpreted with caution. Sensitivity analyses were conducted to explore the robustness of primary analysis results to changes in the assumptions made. The conclusions drawn from the main analysis were shown to be robust to all sensitivity analyses.
Strengths and limitations of the trial-based economic analysis
A key strength of this analysis is that the economic evaluation was conducted in keeping with the recommended design and reporting guidelines. It was based on a multicentre randomised and controlled trial and provided the channel for prospective data collection. Data on outcomes and resource use were collected during the trial using CRFs and at specified points. Unit costs were drawn from established national sources and where variables were not clearly depicted by HRGs, we collaborated with the clinical teams to select the most suitable HRG. These are likely to enhance the generalisability of the study’s findings. The robustness of the main analyses as evidenced by the sensitivity analyses is a strength.
In addition, we carried out a CUA, thereby further measuring the effectiveness of the trial intervention in terms of QALYs as recommended by NICE. 116 The use of a preference-based measure of health outcome is more useful for comparative purposes. However, there were some missing EQ-5D-5L data in this trial, which we accounted for by imputing missing values. Although imputation is not ideal, the results are robust to these methods, as the complete-case analysis shows similar results. However, caution should be applied when making conclusions on the results of the CUA.
The trial protocol stated that we would collect data on primary care and from a societal perspective based on findings from preliminary data. There was no evidence in the trial or preliminary data to suggest that these data would have had an impact on the result. Therefore, to avoid unnecessary data collection and imposition on the women following a distressing event, this wider data collection was deemed inappropriate to pursue.
Comparison with the literature
To our knowledge, this is the first UK-based economic evaluation of the cost-effectiveness of mifepristone plus misoprostol versus misoprostol alone for the medical management of a missed miscarriage. A recent study in the USA120 assessed the relative cost-effectiveness of the two alternatives for the management of early pregnancy loss from the health-care sector and societal perspective and reported their results in terms of QALYs at 30 days post-intervention.
From the health-care sector perspective, the authors120 reported the trial intervention as minimally more costly, with a cost of US$2.87, and from the societal perspective they reported that the intervention was less costly with a cost saving of US$999.32. 120 Furthermore, the intervention group had higher QALYs with a difference of 0.0014 QALYs. From the health-care sector perspective, the use of mifepristone and misoprostol was cost-effective relative to misoprostol alone with an ICER of US$4225.43 (95% CI −US$195,053.30 to US$367,625.10) per QALY gained. Mifepristone and misoprostol also dominated misoprostol alone when the analysis was conducted from the societal perspective. When evaluating the cost-effectiveness by treatment success rates (complete expulsion of gestational sac after a single dose of misoprostol), the mifepristone and misoprostol combination was also found to be a cost-effective intervention in comparison with misoprostol alone.
Implications for policy
The within-trial economic evaluation found that the combination of mifepristone and misoprostol is likely to be considered by decision-makers for the medical management of women presenting with a missed miscarriage.
Model-based economic evaluation
Objectives
The objective was to explore the relative cost-effectiveness of the combination of mifepristone and misoprostol (the MifeMiso intervention) versus all relevant alternative options for miscarriage management for which there are available and suitable data.
Methods
A decision analytic model was constructed to facilitate the comparison of all relevant alternatives for miscarriage management to determine the most cost-effective way of managing a missed miscarriage. The model was parameterised using a combination of evidence gathered in a clinical systematic review conducted by co-investigators of the MifeMiso trial and a systematic review of economic evaluations on early miscarriage management, which were supplemented by other published literature and expert opinion from within the research study team. The analysis was performed from the NHS perspective primarily because of the reliance on secondary data, which was limited from a wider perspective.
The primary outcome was the additional cost per additional successfully managed miscarriage, defined by the expulsion of the fetal sac within 7 days following randomisation. Given the short-term nature of the decision problem, a decision tree model was deemed appropriate. 121 The model was constructed and analysed in TreeAge Pro (2020) (TreeAge Software, Inc., Williamstown, MA, USA).
Extrapolation beyond the end of the trial using decision analytical modelling was not undertaken because the within-trial analysis found no demonstrable difference between groups in factors impacting long-term health outcomes.
Model structure
The model was developed to represent the alternative strategies for management of missed miscarriage in early pregnancy. The MifeMiso trial results were incorporated into the model structure to facilitate the comparison. The pathways of the model were finalised using expert opinion from within the research study team and, as far as possible, represent the practices followed in the UK in the event of a missed miscarriage. The decision tree is presented in Figure 14.
FIGURE 14.
Economic evaluation model pathway.
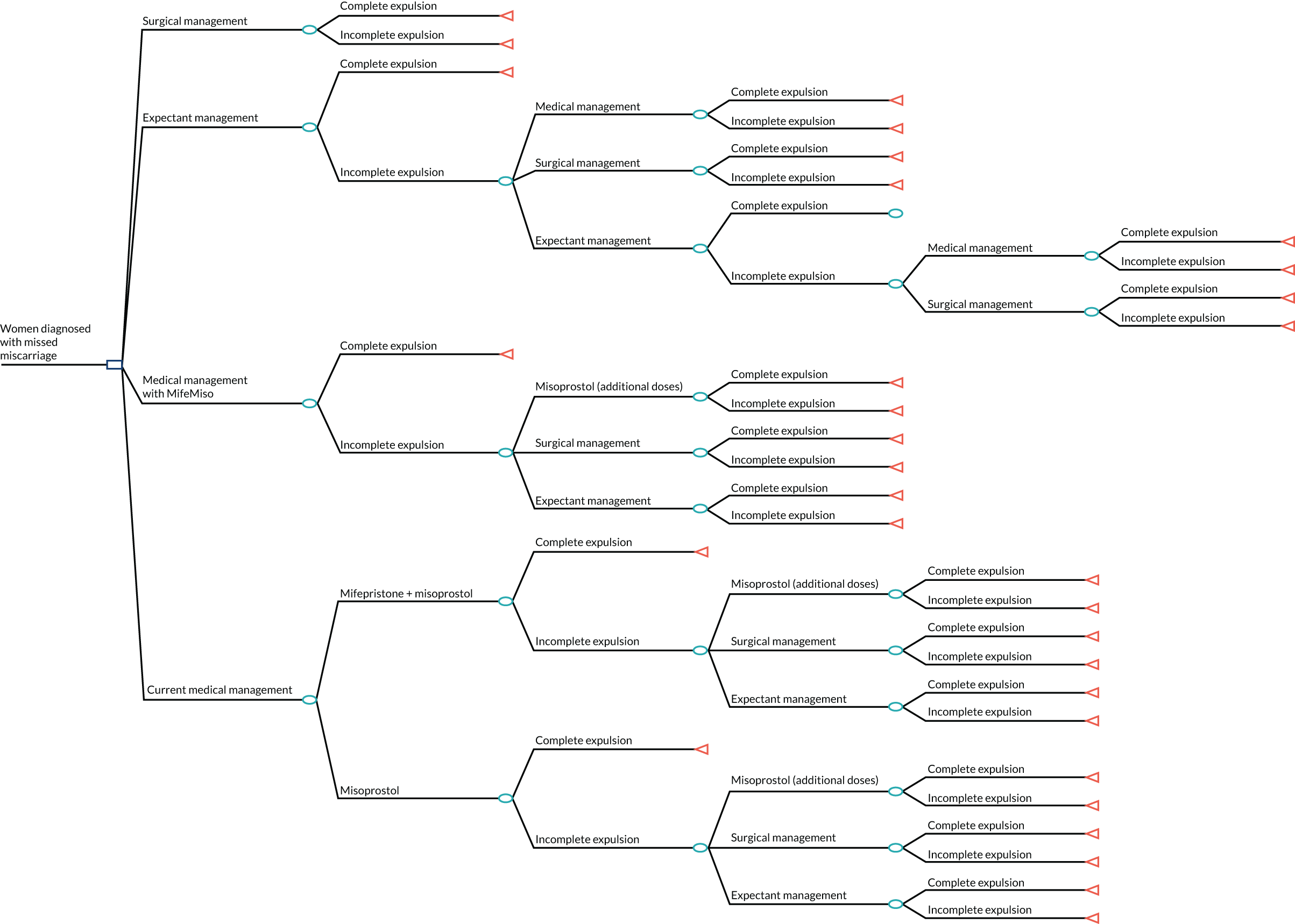
The model commences following the diagnosis of missed miscarriage in the first 14 weeks of pregnancy. Women can receive four alternative management strategies: surgical management, expectant management, current medical management or medical management with mifepristone plus misoprostol (medical management as proposed by the MifeMiso trial).
Surgical management refers to a procedure to remove the pregnancy tissue using a suction device. If, after the initial surgical intervention, the expulsion is incomplete, women will undergo a repeat surgical intervention.
Women receiving the current medical management could be given either mifepristone plus misoprostol, or misoprostol alone. It is assumed that women who were given mifepristone plus misoprostol and women given misoprostol alone follow the same pathway, which is equivalent to the pathway followed by participants of the MifeMiso trial. If, after the initial stage of medical management, the expulsion is incomplete, women undergo a second stage of medical management [additional dose(s) of misoprostol] or follow expectant or surgical management. If, after the second stage of medical management and surgical or expectant management, the expulsion is incomplete, women will undergo surgical intervention.
Expectant management refers to waiting for the pregnancy tissue to pass out naturally. If the initial stage of expectant management is unsuccessful, women will follow surgical management or medical management (with misoprostol) or undergo a second stage of expectant management. If, after subsequent surgical or medical management, the expulsion is incomplete, women will undergo surgical intervention. If, after the second stage of expectant management the expulsion is incomplete, women will undergo surgical or medical management. If the expulsion is still incomplete, women will undergo surgical intervention.
Model assumptions
Several pragmatic assumptions were required to develop a workable model. These are summarised and described below and divided into three categories: trial centres, model pathways and model inputs.
Trial centres
-
All centres (both UK and non-UK) were assumed to have similar expertise and protocols for management of women diagnosed with missed miscarriage.
Model pathways
-
Women were assumed to have received no management for missed miscarriage following their diagnosis.
Model inputs
-
It was assumed that the cost of misoprostol was the same regardless of the route of administration.
-
Administration costs (i.e. staff time) were assumed to be broadly captured by other costs within the model. Therefore, no extra staffing costs were added.
-
Both surgical interventions performed using MVA and dilatation and evacuation were assumed to be equally effective.
-
In all model branches, if expulsion is incomplete at the terminal node, the woman receives surgical intervention, which was assumed to be 100% effective. This means that after an unsuccessful second stage of medical management, and unsuccessful medical or surgical management in the expectant management branches, the effectiveness of the surgical intervention is assumed to be 100%.
-
In all model branches populated by secondary source data, it was assumed that 77% of surgical interventions were carried out using dilatation and evacuation and 23% were carried out using MVA. This assumption was derived from the results of both the placebo and mifepristone groups of the trial.
-
Clinical data from non-UK based studies were assumed to be transferable to an NHS setting.
-
Where necessary, clinical data from studies assessing the management of all early miscarriages were assumed to be transferable.
-
When data on resource use for specific management strategies were unavailable, transferability of resource use data from alternative strategies was assumed.
All assumptions were agreed with the clinical team before the model-based analysis was undertaken.
Model parameters
Effectiveness data
The effectiveness data for the base-case model were, as far as possible, based on the results of published clinical trials of management strategies for missed miscarriage. When necessary, data were supplemented by other published literature or sourced via expert opinion from within the research study team (Table 26).
| Item | Management strategy | Probability of success | Standard error | Source |
|---|---|---|---|---|
| Management stage 1 | Surgical management | 0.959 | 0.028 | Grønlund et al.18 |
| Management stage 1 | Expectant management | 0.289 | 0.074 | Bagratee et al.122 |
| Management stage 1 | Current medical management: misoprostol only | 0.712 | 0.053 | Grønlund et al.18 |
| Management stage 1 | Current medical management: mifepristone plus misoprostol | 0.740 | 0.072 | Grønlund et al.18 |
| Management stage 2 (medical) | Current medical management: misoprostol only | 0.400 | 0.126 | Schreiber et al.19 |
| Management stage 2 (expectant) | Current medical management: misoprostol only | 0.300 | 0.102 | Schreiber et al.19 |
| Management stage 2 (surgical) | Current medical management: misoprostol only | 0.966 | 0.033 | Lemmers et al.123 |
| Management stage 2 (medical) | Current medical management: mifepristone plus misoprostol | 0.500 | 0.250 | Schreiber et al.19 |
| Management stage 2 (expectant) | Current medical management: mifepristone plus misoprostol | 0.380 | 0.121 | Schreiber et al.19 |
| Management stage 2 (surgical) | Current medical management: mifepristone plus misoprostol | 0.966 | 0.033 | Lemmers et al.123 |
| Management stage 2 (medical) | Expectant management | 0.750 | 0.025 | Assumption based on expert opinion |
| Management stage 2 (expectant) | Expectant management | 0.412 | 0.050 | Derived from data presented in Luise et al.124 |
| Management stage 2 (surgical) | Expectant management | 0.960 | 0.023 | Graziosi et al.125 |
| Management stage 3 (medical) | Expectant management | 0.850 | 0.080 | Rafi and Khalil126 |
| Management stage 3 (surgical) | Expectant management | 0.960 | 0.023 | Graziosi et al.125 |
Table 27 presents data on the proportion of women undergoing particular management strategies. For stage 1 of current medical management, the proportion of women managed by using mifepristone plus misoprostol and misoprostol alone were sourced from the practice impact survey performed in conjunction with the MifeMiso trial. The proportion of women undergoing stage 2 surgical, medical and expectant management following an incomplete miscarriage in stage 1 of current medical management were drawn from Schreiber et al. 19
| Item | Management strategy | Probability of using strategy | Standard error | Source |
|---|---|---|---|---|
| Management stage 1 | Current medical management: misoprostol only | 0.740 | 0.048 | Practice impact survey, 2020 |
| Management stage 1 | Current medical management: mifepristone plus misoprostol | 0.260 | 0.048 | Practice impact survey, 2020 |
| Management stage 2 (medical) | Current medical management: misoprostol only | 0.306 | 0.066 | Schreiber et al.19 |
| Management stage 2 (expectant) | Current medical management: misoprostol only | 0.408 | 0.070 | Schreiber et al.19 |
| Management stage 2 (surgical) | Current medical management: misoprostol only | 0.286 | 0.065 | Schreiber et al.19 |
| Management stage 2 (medical) | Current medical management: mifepristone plus misoprostol | 0.167 | 0.076 | Schreiber et al.19 |
| Management stage 2 (expectant) | Current medical management: mifepristone plus misoprostol | 0.667 | 0.096 | Schreiber et al.19 |
| Management stage 2 (surgical) | Current medical management: mifepristone plus misoprostol | 0.167 | 0.076 | Schreiber et al.19 |
| Management stage 2 (medical) | Expectant management | 0.400 | 0.163 | Assumption based on expert opinion (based on discussions with the National Clinical Coordinator and the Chief Investigator of the MifeMiso trial, May 2020) |
| Management stage 2 (expectant) | Expectant management | 0.200 | 0.133 | Assumption based on expert opinion (based on discussions with the National Clinical Coordinator and the Chief Investigator of the MifeMiso trial, May 2020) |
| Management stage 2 (surgical) | Expectant management | 0.400 | 0.163 | Assumption based on expert opinion (based on discussions with the National Clinical Coordinator and the Chief Investigator of the MifeMiso trial, May 2020) |
| Management stage 3 (medical) | Expectant management | 0.426 | 0.072 | Rafi and Khalil126 |
| Management stage 3 (surgical) | Expectant management | 0.574 | 0.072 | Rafi and Khalil126 |
The proportions of women undergoing stage 2 surgical, medical and expectant management following an incomplete miscarriage in expectant stage 1 were estimated via expert opinion, the significance of which was explored in the sensitivity analysis. The proportion of women undergoing stage 3 surgical and medical management following an incomplete miscarriage in expectant stage 2 were drawn from Rafi and Khalil. 126
The data from the MifeMiso trial that were used to populate the medical management with mifepristone plus misoprostol branch of the model are presented in Table 28.
| Item | Probability (n/N) | Distribution |
|---|---|---|
| Stage 1 medical management – success | 0.830 (289/348) | Beta |
| Stage 1 medical management – failure | 0.170 (59/348) | Beta |
| Proportion of patients undergoing stage 2 medical management | 0.271 (16/59) | Dirichlet |
| Stage 2 medical management – success | 0.500 (8/16) | Beta |
| Stage 2 medical management – failure | 0.500 (8/16) | Beta |
| Proportion of patients undergoing stage 2 expectant management | 0.237 (14/59) | Dirichlet |
| Stage 2 expectant management – success | 0.643 (9/14) | Beta |
| Stage 2 expectant management – failure | 0.357 (5/14) | Beta |
| Proportion of patients undergoing stage 2 surgical management | 0.492 (29/59) | Dirichlet |
| Stage 2 surgical management – success | 0.965 (28/29) | Beta |
| Stage 2 surgical management – failure | 0.035 (1/29) | Beta |
Costs and resource use data
The cost inputs for the model were estimated from the perspective of the UK NHS. Standard practice dosages were identified for mifepristone and misoprostol. With the exception of surgical intervention, the unit costs applied in the model are equivalent to those utilised in the within-trial analysis. The unit cost of surgical intervention in the medical management with mifepristone plus misoprostol branch was derived from the results of mifepristone plus misoprostol group of the MifeMiso trial, in which 22% of surgical interventions were performed using MVA and 78% of surgical interventions were performed using dilatation and evacuation.
The unit costs for surgical intervention in the branches populated by secondary source data were based on an assumption that 77% of surgical interventions are performed using dilatation and evacuation and 23% are performed using MVA. This assumption was derived from the results of both groups of the MifeMiso trial and is explored in the sensitivity analysis.
For surgical interventions performed in stage 1 of management, a gestational age of ≤ 14 weeks is assumed. For surgical interventions performed in the second or third stages of management, it is assumed that gestational age may be < 14 weeks or 14–20 weeks. The corresponding costs were obtained from the NHS Reference Costs 2017–18. 102 All costs sourced are reported in 2019–20 Great British pounds, having been inflated using the NHS Cost Inflation Index (NHSCII),103 if necessary. Table 29 presents the key costs for the model.
| Item | Unit cost (£) | Other information | Source |
|---|---|---|---|
| Medical management | |||
| Mifepristone (Mifegyne) | 18 | Per 200-mg tablet | BNF 104 |
| Misoprostol (Topogyne) | 16 | Per 800 µg | BNF 104 |
| Misoprostol (Mysodelle) | 372 | Per 800 µg, vaginally | BNF 104 |
| Surgical management | |||
| Surgical intervention (stage 2, medical management with mifepristone plus misoprostol) | 1400 | Per procedure, figure is weighted cost based on 78% dilatation and evacuation/22% MVA | NHS Reference Costs 2017–18 106 |
| Surgical intervention (stage 1, surgical management) | 1254 | Per procedure, < 14 weeks’ gestation. The figure is weighted cost based on an assumption of 77% dilatation and evacuation/23% MVA | NHS Reference Costs 2017–18 106 |
| Surgical intervention (stages 2 and 3, expectant management and current medical management) | 1398 | Per procedure, < 14 weeks’ gestation. The figure is weighted cost based on an assumption of 77% dilatation and evacuation/23% MVA | NHS Reference Costs 2017–18 106 |
| Secondary care | |||
| Hospital visit | 150 | Scheduled visit | PSSRU 2002 105 |
| Emergency visit | 98 | The figure is a weighted average of costs from corresponding HRGs | NHS Reference Costs 2018–19 102 |
| Outpatient admission | 127 | Antenatal, specialised non-routine ultrasound | NHS Reference Costs 2018–19 102 |
| Inpatient admission (day) | 325 | The figure is a weighted average of costs from corresponding HRGs | NHS Reference Costs 2018–19 102 |
| Night of inpatient admission | 413 | The figure is a weighted average of costs from corresponding HRGs | NHS Reference Costs 2018–19 102 |
The assumed resource utilisation of the secondary care items for surgical management, expectant management and the current medical management were based on published literature identified in the systematic review of economic evaluations of early miscarriage management (Table 30). All items were agreed with the research study team before the model-based analysis was undertaken. Given a lack of studies assessing the cost-effectiveness of expectant management, where data were unavailable, the corresponding resource use for current medical management was applied. When data on medical management with mifepristone plus misoprostol or misoprostol alone were unavailable, the corresponding resource use for the alternative medical management regime was applied. In addition, data on nights of inpatient admission were not available; therefore, an assumption based on expert opinion from within the research study team was implemented. This inherent uncertainty is explored in the sensitivity analyses.
| Item | Surgical management | Current medical management (misoprostol) | Current medical management (mifepristone plus misoprostol) | Expectant management | Source(s) |
|---|---|---|---|---|---|
| Hospital visit | 2 | 2 | 2 | 2 | You and Chung127 |
| Emergency visit | 0.09 | 0.21 | 0.21 | 0.56 | Petrou et al.128 |
| Outpatient admission | 0.04 | 0.63 | 0.63a | 0.63b | Graziosi et al.125 |
| Inpatient admission (< 24 hours) | 0.122 | 0.200a | 0.200 | 0.200b | Niinimäki et al.35 |
| Night of inpatient admission | 0.122 | 0.200 | 0.200 | 0.200 | Assumption based on expert opinion (based on discussions with the National Clinical Coordinator and the Chief Investigator of the MifeMiso trial, May 2020) |
| Additional dose(s) of misoprostol within 7 days | – | 0.589 | 0.241 | – | Grønlund et al.18 |
The resource use data for the mifepristone plus misoprostol branch of the model, based on the MifeMiso trial, is presented in Table 31. An additional hospital visit was added to the figure detailed in Table 4 of the trial-based analysis to account for the initial visit attended by each woman at randomisation. As all women in the model attend an initial visit, we consider only uncertainty for subsequent visits.
| Item | Resource use |
|---|---|
| Hospital visit | 1.670 |
| Emergency visit | 0.190 |
| Outpatient admission | 0.400 |
| Inpatient admission (< 24 hours) | 0.210 |
| Night of inpatient admission | 0.200 |
| Additional dose(s) of misoprostol within 7 days | 0.144 |
| Surgical intervention after the passage of gestational sac (within 7 days) | 0.069 |
Outcome measure
The outcome in this model was measured as a successfully managed miscarriage. This is reported in terms of the ICER, which indicates the additional cost per additional successfully managed miscarriage.
Base-case analysis
Our base-case analysis was conducted from an NHS perspective in which direct health service costs are taken into account.
Deterministic sensitivity analyses
Additional scenarios were investigated following the base-case analysis, which tested the significance of the assumptions made in the model. Scenarios investigated included:
-
increasing the proportion of women undergoing a second stage of expectant management by 50%
-
reducing the proportion of women undergoing a second stage of expectant management by 50%
-
all surgical interventions performed by MVA
-
all surgical interventions performed by dilatation and evacuation
-
equal proportions of surgical interventions performed by MVA and dilatation and evacuation
-
increasing the effectiveness of medical management following an initial stage of expectant management by 10%
-
decreasing the effectiveness of medical management following an initial stage of expectant management by 10%
-
decreasing the nights of inpatient admission in all strategies populated by secondary source data by 50%.
Probabilistic sensitivity analysis
A probabilistic sensitivity analysis was conducted to explore the uncertainty of the model input data. For each uncertain model input parameter, distributions were assigned, from which a value was randomly drawn. In total, 10,000 Monte Carlo simulations were subsequently computed, which generated mean cost and effectiveness estimates by simultaneously varying all relevant parameters. These estimates were used jointly to form an empirical distribution of the differences in both the cost and effectiveness of interventions. Beta distributions were applied to probabilities where only two outcomes were possible. Dirichlet distributions, the multinomial extension of the beta distribution, were applied if three outcomes were possible. As there is no uncertainty around unit costs, no distribution was fitted. However, resource use is uncertain; therefore, gamma distributions were applied to costs including resource use. When resource use data were derived from alternative strategies or only point estimates were available, the widest possible uncertainty was applied.
Model-based cost-effectiveness analysis results
Base-case analysis
The results of the base-case analysis are shown in Table 32. Medical management with mifepristone plus misoprostol is the least costly strategy, with a mean cost of £761 per woman. The most effective strategy is surgical management, whereas medical management with mifepristone plus misoprostol is the second most effective strategy. Both the current medical management and expectant management strategies are dominated by medical management with mifepristone plus misoprostol, as they are more costly and less effective than medical management with mifepristone plus misoprostol.
| Management strategy | Mean cost (£) per woman | Effectiveness | ICER (£) |
|---|---|---|---|
| Medical management with mifepristone plus misoprostol | 761 | 0.830 | – |
| Current medical management | 876 | 0.717 | Dominated |
| Expectant management | 1177 | 0.289 | Dominated |
| Surgical management | 1658 | 0.959 | 6969 |
However, compared with medical management with mifepristone plus misoprostol, surgical management is more costly but more effective. The estimated ICER for surgical management compared with medical management with mifepristone plus misoprostol is £6969 per additional successfully managed miscarriage. This means that every additional successfully managed miscarriage achieved using surgical management over medical management with mifepristone plus misoprostol costs an additional £6969.
Deterministic sensitivity analyses
The results of the deterministic sensitivity analyses are shown in Report Supplementary Material 7. Each scenario investigated made no substantial difference to the base-case results.
Probabilistic sensitivity analysis
Probabilistic sensitivity analysis using 10,000 Monte Carlo simulations showed moderate uncertainty.
Medical management with mifepristone plus misoprostol compared with surgical management
The cost-effectiveness plane in Figure 15 shows the modelled uncertainty in the cost and effectiveness between medical management with mifepristone plus misoprostol and surgical management. The ICER of each simulation is plotted on the cost-effectiveness plane, providing information about the joint density of the differences in cost and effectiveness between the strategies. It is shown that medical management with mifepristone plus misoprostol is highly likely to be less effective than surgical management while being less costly.
FIGURE 15.
Cost-effectiveness plane for medical management with mifepristone plus misoprostol relative to surgical management.
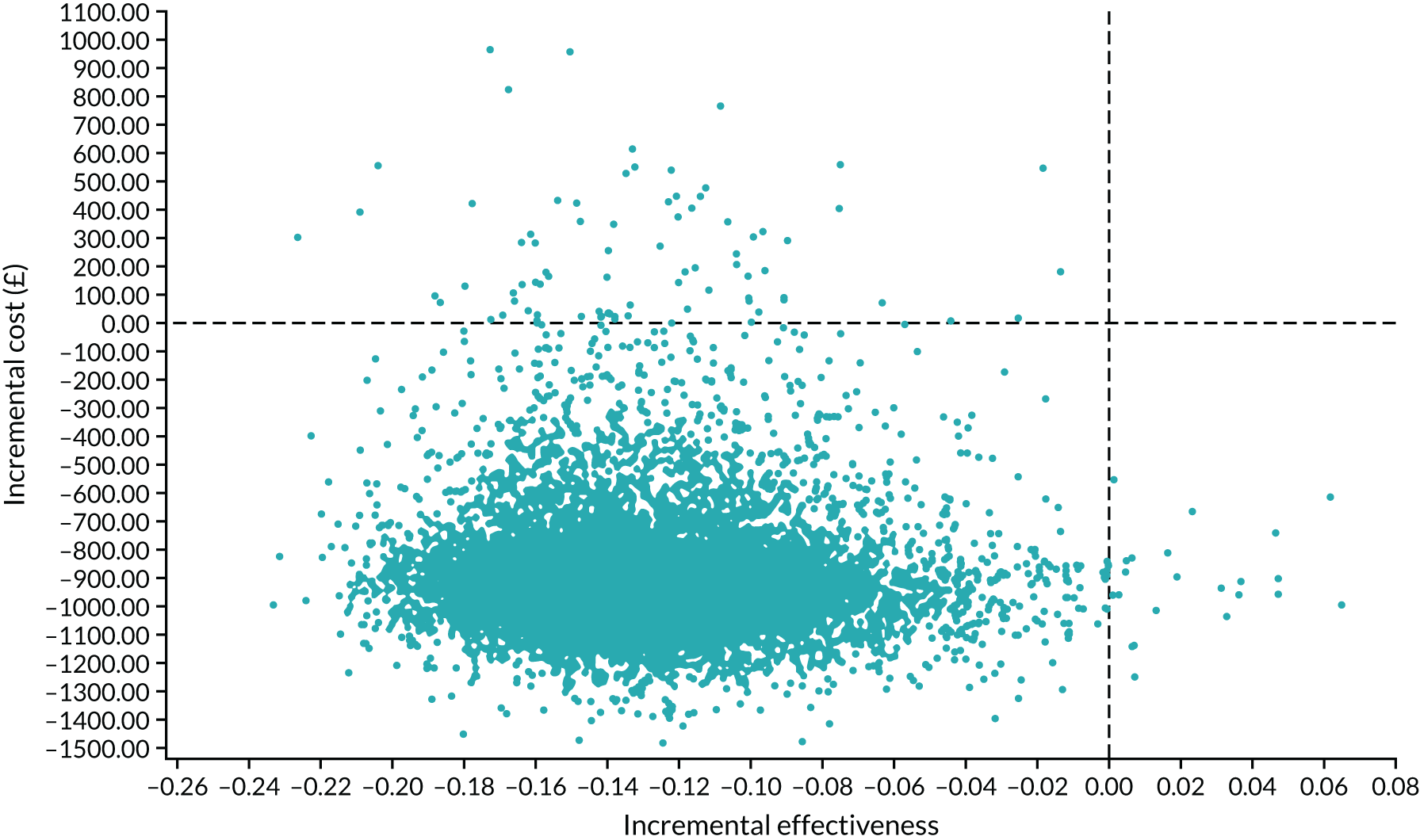
The CEAC for medical management with mifepristone plus misoprostol compared with surgical management is shown in Figure 16. Given an arbitrary WTP threshold of £5000 per additional successfully managed miscarriage, the probability that medical management with mifepristone plus misoprostol is cost-effective is 86% and the probability that surgical management is cost-effective is 14%. However, if the WTP threshold is higher at £10,000 per additional successfully managed miscarriage, the probability that medical management with mifepristone plus misoprostol is cost-effective is 15% whereas the probability that surgical management is cost-effective is 85%. As the WTP per additional successfully managed miscarriage tends to infinity, the probability that surgical management is cost-effective compared with medical management with mifepristone plus misoprostol tends to 99%. The difference in probabilities over WTP thresholds reflects uncertainty in the model.
FIGURE 16.
The CEAC for medical management with mifepristone plus misoprostol compared with surgical management.
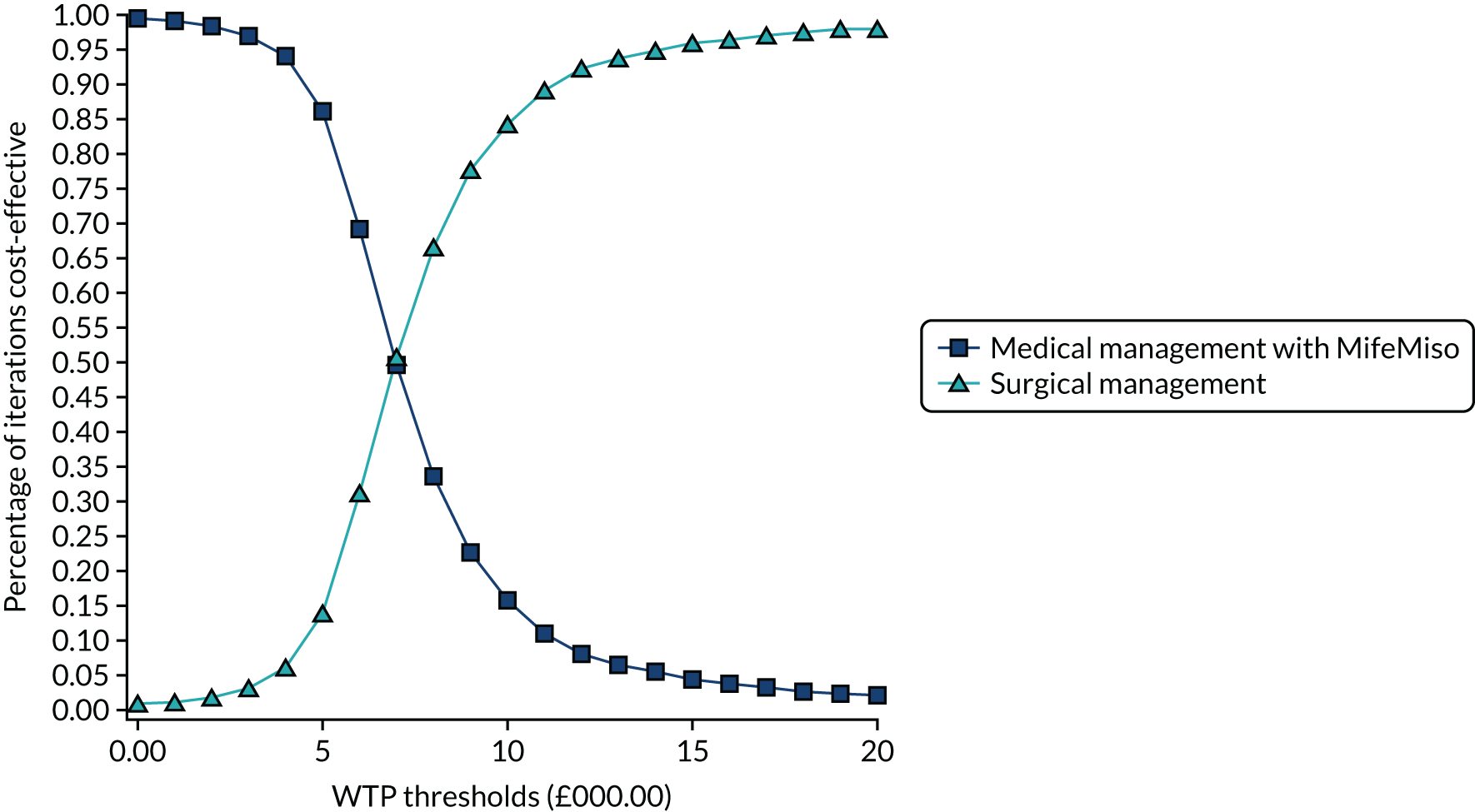
Medical management with mifepristone plus misoprostol compared with current medical management
The cost-effectiveness plane in Figure 17 shows the modelled uncertainty in the cost and effectiveness between medical management with mifepristone plus misoprostol compared with the current practice of medical management. It is shown that medical management with mifepristone plus misoprostol is highly likely to be more effective than the current practice of medical management; however, it is uncertain as to whether or not it is less costly.
FIGURE 17.
Cost-effectiveness plane for medical management with mifepristone plus misoprostol relative to current medical management.
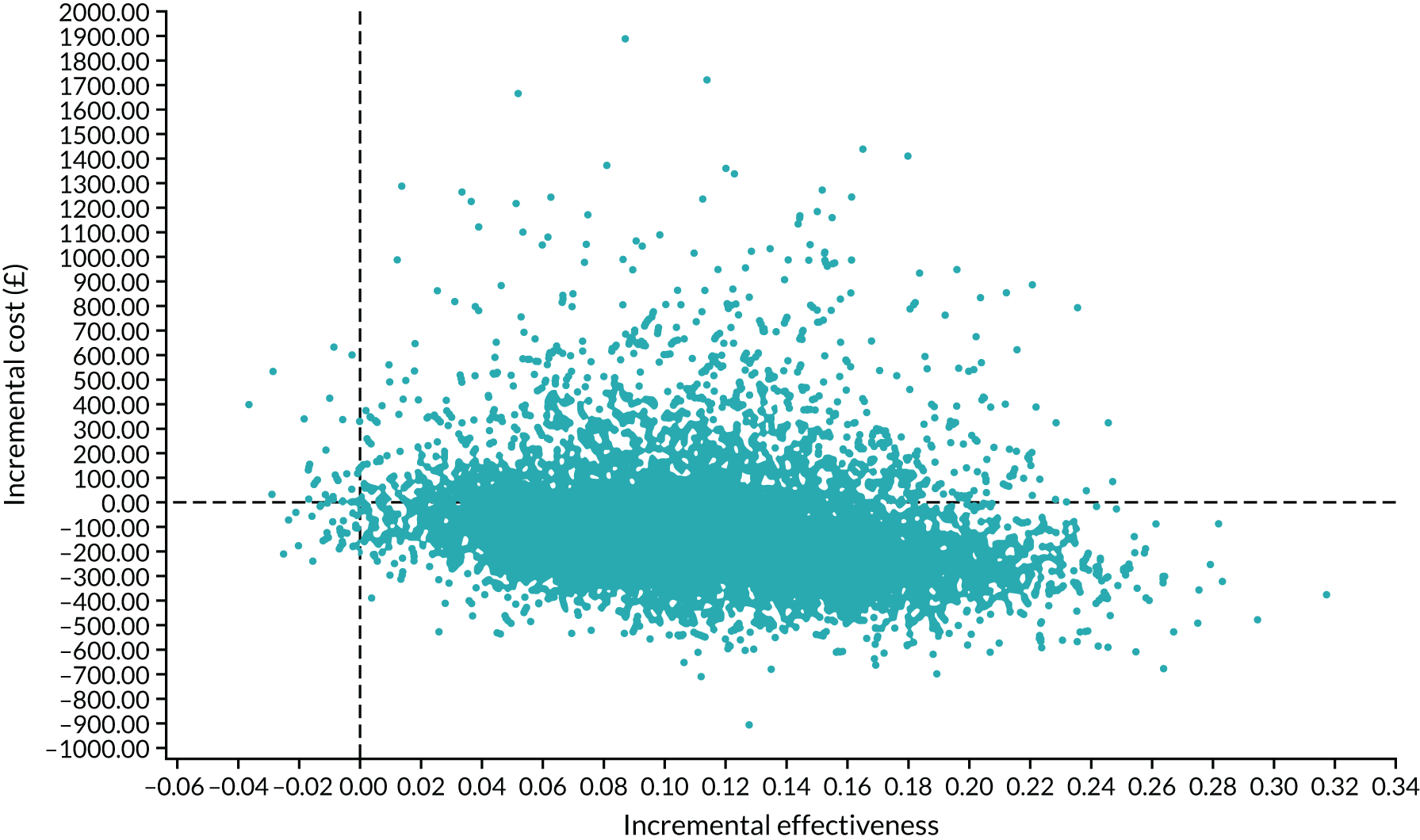
Figure 18 shows the CEAC for medical management with mifepristone plus misoprostol compared with current medical management. The CEAC shows that at any WTP threshold > 0, medical management with mifepristone plus misoprostol is the preferred strategy compared with the current medical management. As the WTP per additional successfully managed miscarriage tends to infinity, the probability that medical management with mifepristone plus misoprostol is cost-effective compared with the current practice of medical management tends to 99%.
FIGURE 18.
The CEAC for medical management with mifepristone plus misoprostol relative to current medical management.
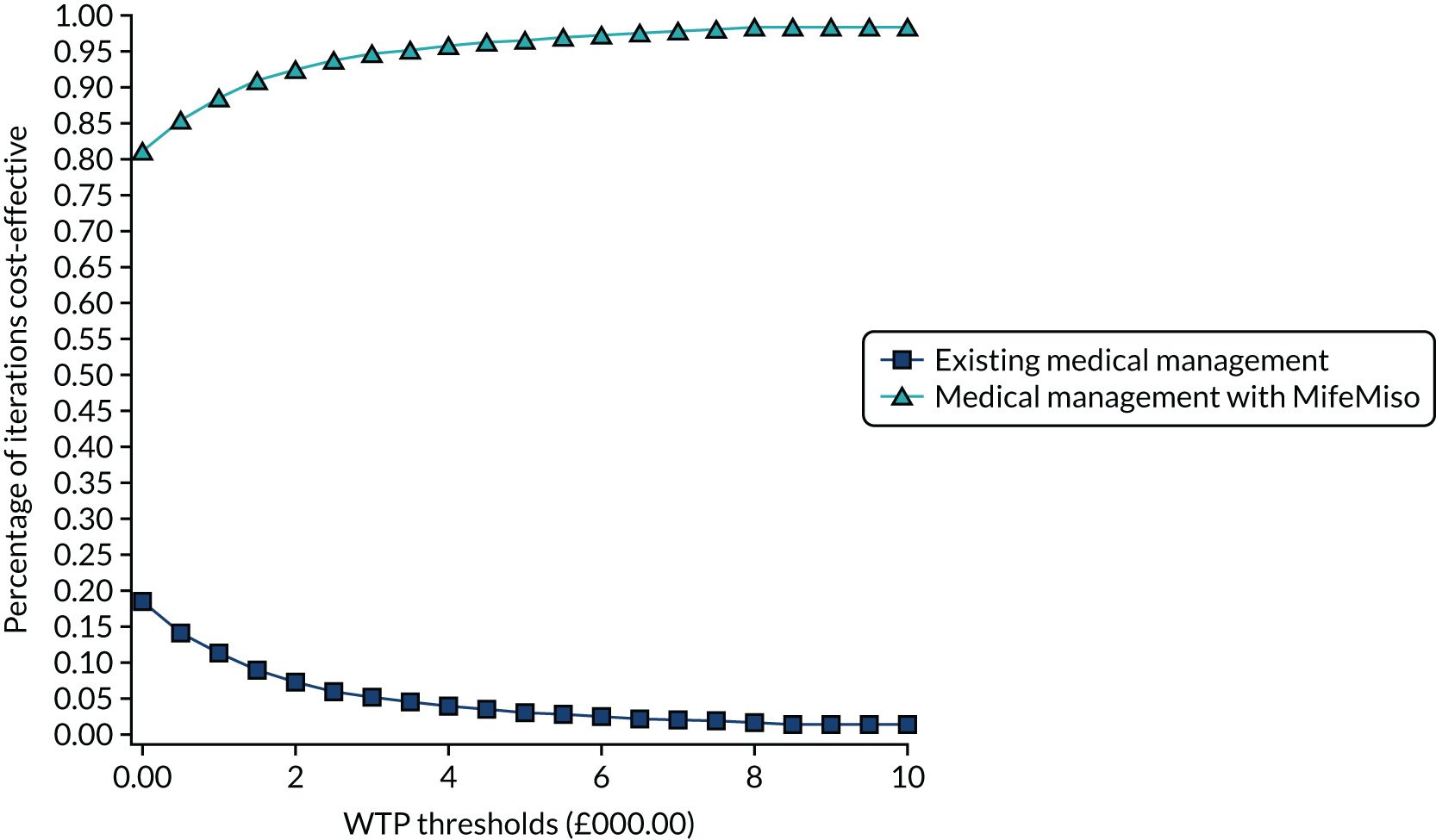
Medical management with mifepristone plus misoprostol compared with expectant management
The cost-effectiveness plane in Figure 19 shows the modelled uncertainty in the cost and effectiveness of medical management with mifepristone plus misoprostol compared with expectant management. It is shown that medical management with mifepristone plus misoprostol is consistently more effective than expectant management; however, it is uncertain as to whether or not it is less costly.
FIGURE 19.
Cost-effectiveness plane for medical management with mifepristone plus misoprostol relative to expectant management.
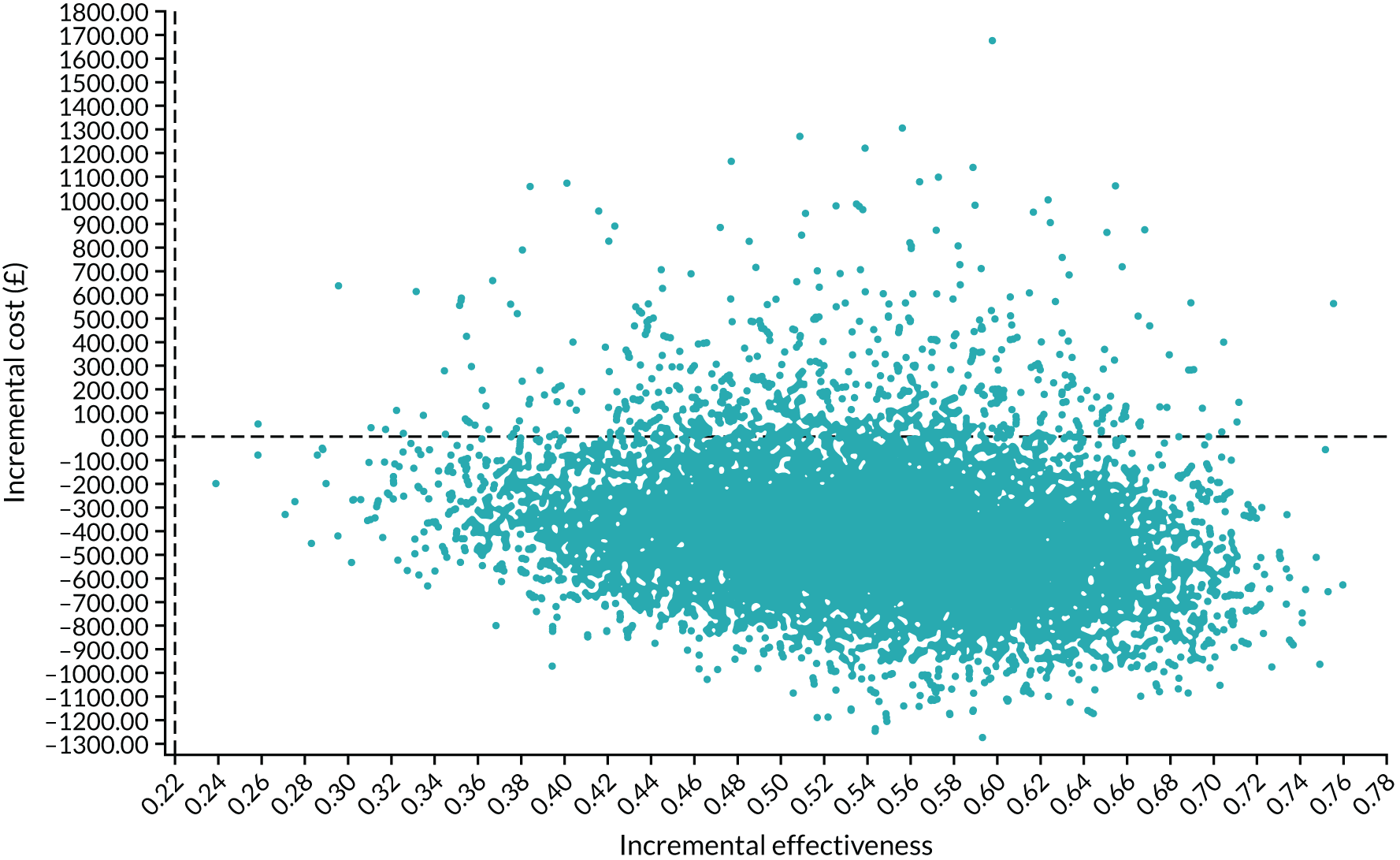
Figure 20 shows the CEAC for medical management with mifepristone plus misoprostol compared with expectant management. The CEAC shows that at any WTP threshold > 0, medical management with mifepristone plus misoprostol is the preferred strategy compared with expectant management. As the WTP per additional successfully managed miscarriage tends to infinity, the probability that medical management with mifepristone plus misoprostol is cost-effective compared with the current practice of medical management tend to 99%.
FIGURE 20.
The CEAC for medical management with mifepristone plus misoprostol relative to expectant management.
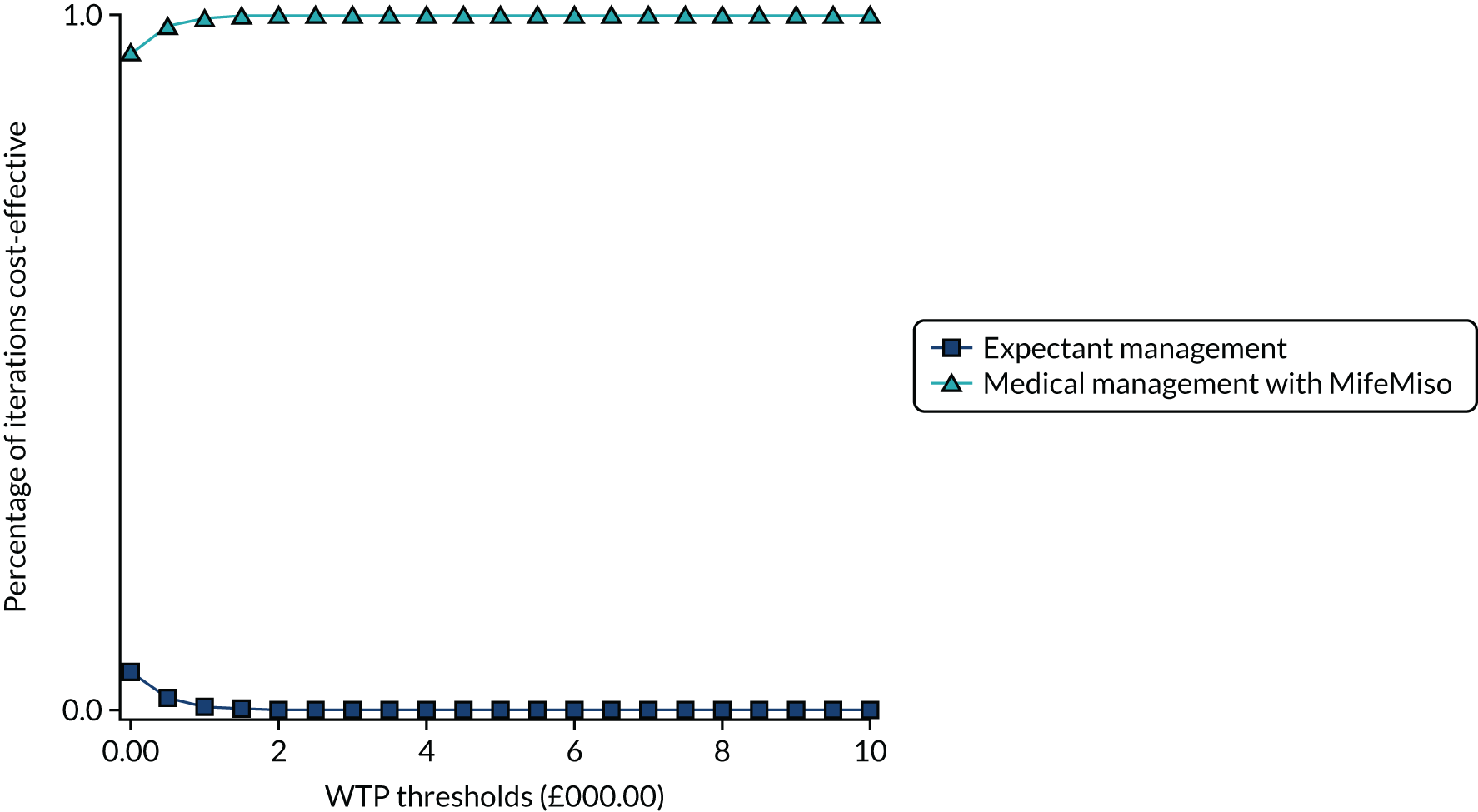
Discussion of the model-based analysis
We carried out a model-based CEA in which we compared the trial results with alternative miscarriage management strategies, surgical management, expectant management and the current practice of medical management, based on the best available evidence in the literature.
Principal findings
The results of the model-based analysis show that for the management of an early missed miscarriage the strategy proposed by the MifeMiso trial (medical management with mifepristone plus misoprostol) is dominant when compared with expectant management and the current medical management strategy, as it is more effective and less costly than both strategies. However, medical management with mifepristone plus misoprostol is a less effective strategy than surgical management, though it is less costly.
The ICER for surgical management compared with medical management with mifepristone plus misoprostol compared with surgical management is £6969 per additional successfully managed miscarriage. The PSA suggests that at a WTP threshold below approximately £7000, medical management with mifepristone plus misoprostol is the preferred strategy relative to surgical management. The results of this analysis were shown to be robust over the full range of deterministic sensitivity analyses.
Strengths and limitations of the model-based economic analysis
A key strength of the model-based economic evaluation is that it is the first model-based economic evaluation to compare the cost-effectiveness of the three broad alternative management strategies. Furthermore, the model-based economic evaluation considers the cost-effectiveness of a management strategy proposed by a clinical trial in the context of current practice for missed miscarriage management. Being able to directly compare alternative management strategies and to rank them in terms of cost and effectiveness is especially useful for policymakers.
The principal limitation of the model-based economic analysis is that in the absence of a network meta-analysis on the management strategies for missed miscarriage over the relevant intervention period, the effectiveness data were based on the results of published clinical trials. Although the quality and relevance of the trials were stringently assessed, several biases may be attached to the trials that could compromise the accuracy of the data.
In addition, data on resource use items were not available for all management strategies. This meant that assumptions had to be made about the resource use from alternative management strategies. Similarly, no data were available on nights of inpatient admission for the expectant, surgical and current medical management strategies; therefore, assumptions were made using expert opinion from within the research study team. Attempts were made to make missing resource use data as accurate as possible, and the significance of the assumptions made was tested in the deterministic sensitivity analysis in an attempt to rectify this limitation.
No published studies analysed the effect of medical management with misoprostol following an initial stage of expectant management over the relevant period. Similarly, no data were available on the proportion of women undergoing various management strategies following an unsuccessful initial stage of expectant management. Therefore, assumptions based on expert opinion from within the research study team were made. The significance of these assumptions was tested in the deterministic sensitivity analysis where the results showed they made no substantial difference to the base-case result.
Information on the impact on quality of life was not available for all management strategies included in this analysis; therefore, the outcome for the model-based analysis was expressed in terms of clinical effectiveness rather than in terms of the standard unit of benefit, the QALY, which means that the meaning of the results is not as easy to interpret. A model-based analysis based on an outcome of additional QALYs at discharge may be useful for policy-makers.
The analysis did not examine how patient preferences inform decisions, although a previous study on stated preference using a discrete choice experiment found that women undergoing management for first trimester miscarriage expressed a general preference for surgical over medical management, and would value being offered alternatives to expectant management. 129 Furthermore, the model-based economic evaluation makes no comparisons for different dosages of mifepristone and misoprostol or different routes of administration. Comparing the effects of different dosages and routes of administration in the medical management of miscarriage may be useful for future research.
Comparison with the literature
To the best of our knowledge, there is currently no published evidence on the cost-effectiveness of the medical management with mifepristone plus misoprostol compared with alternative management strategies including surgical and expectant for the management of missed miscarriage.
Implications for policy
The results of the model-based economic analysis suggest that medical management with mifepristone plus misoprostol is likely to be recommended by decision-makers for the medical management of missed miscarriage than the current practice, which consists of a combination of mifepristone plus misoprostol or misoprostol alone. When alternative methods of miscarriage management are considered, the results suggest that the best choice is between medical management with mifepristone plus misoprostol and surgical management, but that medical management with mifepristone plus misoprostol is likely to be recommended by decision-makers ahead of expectant management.
Chapter 6 Discussion
Parts of this chapter are reproduced or adapted with permission from Chu et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) license, which permits others to copy and redistribute this work, for non-commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Study strengths
To the best of our knowledge, this study is to date the largest randomised placebo-controlled trial comparing mifepristone and misoprostol with misoprostol alone for medical management of missed miscarriage. The multicentre approach that has been used increases the generalisability of the study findings across a wide range of settings. The robust study design including blinding to treatment allocation of both participants and investigators ensured internal validity, enabling the results to be interpreted with confidence. Randomisation via a computer-generated allocation sequence was effective in achieving balanced groups with respect to important prognostic factors. There was also high adherence to treatment allocation in both groups. This trial also included both qualitative and cost-effectiveness analysis, which enables further interpretation and understanding of the clinical findings.
The size of the study was driven by a MID, determined following consultations among HCPs, patients and representatives of patient bodies as well as through a clinician’s survey. The primary outcome used in the MifeMiso trial was carefully selected through a consultation and survey of clinicians working with women diagnosed with miscarriage and the women themselves through PPI. A consensus of a 10% reduction in the failure rate to spontaneously pass the gestational sac within 7 days evolved from this consultation, resulting in a target sample size of 710 participants with primary outcome data. A total of 711 women, from 28 hospitals in the UK, were randomised, receiving either mifepristone (357 women) or placebo (354 women). The follow-up rate for the primary outcome was 98% (696 of 711 women).
A pragmatic trial design was used in our study, which also adds to the generalisability of our findings. In particular, the route of administration of misoprostol reflects standard UK clinical practice and NICE guidance for the medical management of missed miscarriage. 6 The majority of our participants received vaginal misoprostol and it is important to note that the route of misoprostol administration was similar in both trial groups. We were able to collate near-complete data for the primary outcome, which was aided by the use of a BERC. The committee convened using a strict charter and considered each individual participant’s clinical data in turn. The clinical data were collected on a standardised case report form and the decision as to whether or not the participant had met the primary outcome could only be made unanimously. The sensitivity analysis excluding the findings of the BERC does not alter the findings of our trial and is consistent with the primary analysis.
Limitations and critique
We studied the effect in missed miscarriage; therefore, these results are not generalisable to women diagnosed with incomplete miscarriage, who will have already passed some pregnancy tissues. First, the biological rationale for focusing exclusively on missed miscarriage in this trial is that the antiprogestogenic effect of mifepristone is less likely to be relevant in incomplete miscarriage where the expulsion of pregnancy tissue has already begun. Second, we are unable to compare medical management of missed miscarriage against expectant management in this trial data.
Findings in the context of existing literature
Our large multicentre, randomised, double-blind, placebo-controlled trial showed that the combination treatment of mifepristone plus misoprostol resulted in an increase in the number of missed miscarriages that were completed within 7 days compared with misoprostol alone. There were also fewer incidences of surgical management to complete miscarriage in the mifepristone plus misoprostol group compared with the placebo plus misoprostol group. These findings are consistent with the findings of previously published trials. 18,19 A Cochrane review published in 201910 identified three previously published trials evaluating the effectiveness of mifepristone and misoprostol compared with misoprostol alone in the medical management of miscarriage. 19,28,29 These three trials included smaller numbers of participants (60 to 300 participants). Although they used the same dose of mifepristone as our trial (200 mg), the dose of misoprostol varied (400–800 μg). In addition, these three trials assessed outcome of miscarriage management by varying methods including clinical and ultrasound assessment, and at differing time points. We have updated the Cochrane meta-analysis10 that investigated the effectiveness of mifepristone and misoprostol with misoprostol alone for the medical management of miscarriage to incorporate the findings from our trial (Figure 21). This demonstrates a clear benefit for mifepristone plus misoprostol in comparison to misoprostol alone for the resolution of miscarriage (RR 1.15, 95% CI 1.01 to 1.30) (see Figure 20).
Interpretation of the clinical findings
These results clearly show the importance of optimising the medical management of missed miscarriage using the combined mifepristone and misoprostol treatment regimen, which improves outcomes and safety by increasing the proportion of women who have miscarriage resolution by day 7 and by reducing the need for surgical management. Women choosing medical management of missed miscarriage often wish to have expedited treatment and resolution of their miscarriage while also avoiding the risks of surgery. 30 The risks of surgery include bleeding, infection and uterine perforation requiring more extensive surgery, which carries significant morbidity. 30 Our trial findings demonstrate that the combination treatment of mifepristone and misoprostol reduces the need for surgery after medical management and this is likely to be of great importance to women wishing to undergo medical management of missed miscarriage. Equally, in the light of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, a key aim is to reduce the footfall of individuals in hospital, as well as the need for surgical interventions that will place a demand on already stretched health-care resources and personnel. Our trial shows that adding mifepristone to misoprostol can reduce the number of women who will have not passed their gestational sac by day 6 or 7 by 7% (95% CI 0.01 to 0.13) and reduce the need for surgery by 8% (95% CI 0.01 to 0.13). This evidence is therefore important to improve care and can help rationalise services during the SARS-CoV-2 pandemic.
Interpretation of the qualitative findings
Consistent with existing research, when considering the satisfaction scores of women participating in this study, their responses would suggest that women are largely satisfied with medical management of missed miscarriage. However, our study has achieved a greater depth of understanding concerning satisfaction with and acceptability of medical management. In particular, we have found that a range of factors, associated with both the treatment itself and the care that women experienced, frequently act in combination to influence women’s perspectives on whether or not they would choose medical management again. In contrast to existing qualitative studies, we have been able to elicit those experiences that positively contribute to satisfaction as well as confirming existing evidence concerning the factors associated with dissatisfaction.
Our findings suggest that when women experienced care that supported their psychological well-being throughout the care pathway, and information was delivered in a skilled and sensitive manner such that women felt informed and in control, they were more likely to express satisfaction with medical management. These findings emphasise the need for skilled, knowledgeable and empathic HCPs who are able to better understand and respond to women’s emotional, psychological and informational needs, as well as their physical needs, when they experience missed miscarriage. Ways in which HCPs may be supported to achieve this have been suggested in previous studies, such as bereavement-focused training and education as well as advanced communication skills. Our findings lend weight to the suggestion that training and education such as this would be beneficial.
As miscarriage may create considerable emotional and cognitive challenges for women in being able to process information and make informed decisions, our findings highlight that approaches to information giving necessarily require a flexible and tailored approach throughout the care pathway. Furthermore, women’s ability to attend to the information, their learning needs, preferences and personal factors that may influence their decision-making are all factors that need to be taken into account in supporting women and enabling them to feel informed and in control. Information provision needs to be available in a range of formats including visual, verbal and written and used in combination to support women’s understanding. This is particularly important in the light of the individual variability in the experience of bleeding and pain. As many of the women experienced significant levels of bleeding and pain and this appeared to influence the acceptability of and satisfaction with medical management, providing clear evidence-based information and a management plan may empower women to make appropriate informed decisions about their needs, treatment choices and preferred location of treatment, which can, therefore, assist them in preparing for the miscarriage.
In addition, our findings suggest that for many women (although not all) miscarriage is a highly significant life event, with profound implications for women’s psychological and emotional well-being. Furthermore, missed miscarriage would appear to have particular characteristics that increase the risk of prolonged psychological and emotional distress. In this context women appear to have a preference for active management of their miscarriage, to bring a timely conclusion to the physical process. It also seems that women experiencing missed miscarriage may have a greater need for follow-up support and counselling to address their well-being needs.
Interpretation of the cost-effectiveness findings
The within-trial economic evaluation found that the combination of mifepristone and misoprostol is likely to be recommended by decision-makers for the medical management of women presenting with a missed miscarriage based on cost-effectiveness grounds.
The results of the model-based analysis compared the strategy proposed by the MifeMiso trial (medical management with mifepristone plus misoprostol) with a comprehensive range of management options. The model-based analysis shows that MifeMiso is dominant when compared with expectant management and the current medical management strategy, as it is both more effective and less costly than both strategies.
However, medical management with mifepristone plus misoprostol is a less effective strategy than surgical management, although it is less costly. Thus, when alternative methods of miscarriage management are considered, the results suggest that there is a clear choice between MifeMiso (medical management with mifepristone plus misoprostol) and surgical management. But for medical management alone, medical management with MifeMiso (mifepristone plus misoprostol) is likely to be recommended by decision-makers ahead of expectant management and other medical options.
Patient and public involvement
In the MifeMiso trial, PPI was utilised at all stages of the study design, development and monitoring, which included engagement in the development of patient facing literature for participants. The Trial Steering Committee included a representative of the Miscarriage Association (Wakefield, UK) charity. We believe these roles were important to ensure appropriate communication with study participants and project oversight throughout the duration of the research. Dissemination of results will be supported by the Miscarriage Association and Tommy’s (London, UK) charities.
Generalisability
Centres participating in the study were geographically spread across the UK, improving the generalisability of the results for women with missed miscarriage. The exclusion criteria were kept to a minimum and the heterogeneity of the population was well reflected by trial participants.
Chapter 7 Conclusions
Implications for health care
Our trial findings are consistent with another high-quality clinical trial19 and, therefore, combination mifepristone and misoprostol treatment should be considered as first-line treatment for women who wish to have medical management of missed miscarriage. We recommend that this new evidence is reviewed by the UK clinical guideline bodies with a view to updating their recommended first-line medical treatment of missed miscarriage. Providing clear evidence-based information and a management plan may empower women to make appropriate, informed decisions about their needs, treatment choices and preferred location of treatment, which can therefore assist them in preparing for the miscarriage.
Recommendations for research
Large, robustly designed and conducted randomised controlled trials should compare different forms of the same management options for miscarriage. This could include the comparison of different durations for expectant management of miscarriage, or comparison of different surgical management regimen. These studies could not only examine the time to miscarriage resolution, but also investigate the impact that treatment has on future pregnancies and the incidence of future pregnancy success. In addition, there is a need for standardised woman-centred clinical outcomes through the development of a core outcome set.
Acknowledgements
The full trial protocol is available in Report Supplementary Material 3. We would like to acknowledge Mary Nulty and Hannah Noordali for their support in administering the trial; Mr Lee Middleton for his statistical support in the design of the trial; Mr Rajendra Rai for chairing the Trial Steering Committee; Dr Maya Al-Memar and Mrs Ruth Bender-Atik for participating in the Trial Steering Committee; Dr Abha Maheshwari for chairing the Data Monitoring Committee; Miss Neelam Potdar and Mr Mike Bradburn for participating in the Data Monitoring Committee; and all those not otherwise mentioned above who have contributed to the MifeMiso study. We thank all the women who participated in this study and all the MifeMiso research nurses and midwives who assisted in the collection of data.
Contributions of authors
Adam Devall (https://orcid.org/0000-0001-5632-079X) (Lecturer in Maternal Health Clinical Trials), Justin Chu (https://orcid.org/0000-0002-5355-9989) (Consultant in Obstetrics and Gynaecology), Pollyanna Hardy (https://orcid.org/0000-0003-2937-8368) (Senior statistician), Tracy Roberts (https://orcid.org/0000-0002-0624-0537) (Professor of Health Economics), Laura Jones (https://orcid.org/0000-0002-4018-3855) (Senior Lecturer in Qualitative and Mixed-Methods), Ruth Bender-Atik (https://orcid.org/0000-0002-6751-5901) (Director of the Miscarriage Association), Jane Brewin (https://orcid.org/0000-0002-8411-1802) (Chief Executive Officer of Tommy’s Charity), Kim Hinshaw (https://orcid.org/0000-0003-0468-4326) (Consultant in Obstetrics and Gynaecology), Meenakshi Choudhary (https://orcid.org/0000-0003-0483-3627) (Consultant in Obstetrics and Gynaecology), Andrew Horne (https://orcid.org/0000-0002-9656-493X) (Professor of Gynaecology and Reproductive Sciences), Siobhan Quenby (https://orcid.org/0000-0003-3221-5471) (Professor of Obstetrics) and Arri Coomarasamy (https://orcid.org/0000-0002-3261-9807) (Professor of Gynaecology) contributed to the study design and methodology.
Leanne Beeson (https://orcid.org/0000-0003-0980-9837) (Senior Trial Manager) was the trial manager and contributed to data collection.
Amna Ahmed (https://orcid.org/0000-0003-2960-0731) (Consultant in Obstetrics and Gynaecology), Meenakshi Choudhary, Joel Naftalin (https://orcid.org/0000-0002-9358-1291) (Consultant in Obstetrics and Gynaecology), Natalie Nunes (https://orcid.org/0000-0003-1801-6281) (Consultant in Obstetrics and Gynaecology), Abigail Oliver (https://orcid.org/0000-0003-0774-9279) (Consultant in Obstetrics and Gynaecology), Feras Izzat (https://orcid.org/0000-0001-9673-4276) (Consultant in Obstetrics and Gynaecology), Kalsang Bhatia (https://orcid.org/0000-0003-0892-4892) (Consultant in Obstetrics and Gynaecology), Ismail Hassan (https://orcid.org/0000-0003-3243-8341) (Consultant in Obstetrics and Gynaecology), Yadava Jeve (https://orcid.org/0000-0002-5389-9588) (Consultant in Obstetrics and Gynaecology), Judith Hamilton (https://orcid.org/0000-0001-6182-8360) (Consultant in Obstetrics and Gynaecology), Shilpa Deb (https://orcid.org/0000-0003-4456-8194) (Consultant in Obstetrics and Gynaecology), Cecilia Bottomley (https://orcid.org/0000-0003-1914-2482) (Consultant in Obstetrics and Gynaecology), Jackie Ross (https://orcid.org/0000-0003-4168-6910) (Consultant in Obstetrics and Gynaecology), Linda Watkins (https://orcid.org/0000-0002-2031-6577) (Consultant in Obstetrics and Gynaecology), Martyn Underwood (https://orcid.org/0000-0001-6891-7390) (Consultant in Obstetrics and Gynaecology), Ying Cheong (https://orcid.org/0000-0001-7687-4597) (Professor of Reproductive Medicine), Chitra Kumar (Consultant in Obstetrics and Gynaecology), Pratima Gupta (https://orcid.org/0000-0001-7006-9917) (Consultant in Obstetrics and Gynaecology), Rachel Small (https://orcid.org/0000-0002-4338-2198) (Specialist Research Midwife), Stewart Pringle (https://orcid.org/0000-0002-5883-5196) (Consultant in Obstetrics and Gynaecology), Frances Hodge (https://orcid.org/0000-0003-1162-0611) (Consultant in Obstetrics and Gynaecology), Anupama Shahid (https://orcid.org/0000-0001-6836-7333) (Consultant in Obstetrics and Gynaecology), Andrew Horne and Siobhan Quenby were responsible for the oversight of the study at their respective hospitals and contributed to the recruitment of participants.
Versha Cheed (https://orcid.org/0000-0002-6713-0913) (Trial Statistician), Yongzhong Sun (https://orcid.org/0000-0003-0659-9811) (Trial Statistician) and Pollyanna Hardy were responsible for data analysis.
Laura Jones and Jenny La Fontaine Papadopoulos (https://orcid.org/0000-0002-5485-0719) (Qualitative Researcher) were responsible for qualitative data collection, analysis and interpretation.
Ioannis Gallos (https://orcid.org/0000-0002-2766-358X) (Senior Lecturer in Global Women's Health)conducted the updated meta-analysis.
Laura Jones and Jenny La Fontaine Papadopoulos wrote the first draft of Chapter 4.
Tracy Roberts, Chidubem Okeke Ogwulu (https://orcid.org/0000-0002-8133-7021) (Health Economics Research Fellow) and Eleanor Williams (https://orcid.org/0000-0003-4641-2409) (Health Economics Research Fellow) wrote the first draft of Chapter 5.
Adam Devall, Justin Chu, Leanne Beeson and Arri Coomarasamy wrote the first draft of all other sections of the report.
All authors contributed to data interpretation, critical revision of the manuscript for important intellectual content and gave final approval.
Publications
Chu J, Devall AJ, Hardy P, Beeson L, Coomarasamy A. What is the best method for managing early miscarriage? BMJ 2020;368:l6438.
Chu JJ, Devall AJ, Beeson LE, Hardy P, Cheed V, Sun Y, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet 2020;396:770–8.
Okeke Ogwulu CB, Williams EV, Chu JJ, Devall AJ, Beeson LE, Hardy P, et al. Cost-effectiveness of mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage: an economic evaluation based on the MifeMiso trial. BJOG 2021;128:1534–45.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Chu JJ, Devall AJ, Beeson LE, Hardy P, Cheed V, Sun Y, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet 2020;396:770-8. https://doi.org/10.1016/S0140-6736(20)31788-8.
- NHS Digital . NHS Maternity Statistics – England, 2012–13 2013. https://digital.nhs.uk/data-and-information/publications/statistical/nhs-maternity-statistics/2012-13 (accessed 18 June 2020).
- Quenby S, Gallos ID, Dhillon-Smith RK, Podesek M, Stephenson MD, Fisher J, et al. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet 2021;397:1658-67. https://doi.org/10.1016/S0140-6736(21)00682-6.
- Murphy FA, Lipp A, Powles DL. Follow-up for improving psychological well being for women after a miscarriage. Cochrane Database Syst Rev 2012;3. https://doi.org/10.1002/14651858.CD008679.pub2.
- Farren J, Jalmbrant M, Falconieri N, Mitchell-Jones N, Bobdiwala S, . Posttraumatic stress, anxiety and depression following miscarriage and ectopic pregnancy: a multicenter, prospective, cohort study. Am J Obstet Gynecol 2020;222:367.e1-22. https://doi.org/10.1016/j.ajog.2019.10.102.
- National Institute for Health and Clinical Excellence (NICE) . Ectopic Pregnancy and Miscarriage: Diagnosis and Initial Management in Early Pregnancy of Ectopic Pregnancy and Miscarriage. National Guideline [NG126] 2019. www.nice.org.uk/guidance/ng126 (accessed 30 November 2020).
- Jurkovic D, Overton C, Bender-Atik R. Diagnosis and management of first trimester miscarriage. BMJ 2013;346. https://doi.org/10.1136/bmj.f3676.
- American College of Obstetricians and Gynecologists' Committee on Practice Bulletins—Gynecology . ACOG practice bulletin no. 200: early pregnancy loss. Obstet 2018;132:e197-207. https://doi.org/10.1097/AOG.0000000000002899.
- World Health Organization (WHO) . Medical Management of Abortion 2018.
- Lemmers M, Verschoor MA, Kim BV, Hickey M, Vazquez JC, Mol BWJ, et al. Medical treatment for early fetal death (less than 24 weeks). Cochrane Database Syst Rev 2019;6. https://doi.org/10.1002/14651858.CD002253.pub4.
- Chen BA, Creinin MD. Contemporary management of early pregnancy failure. Clin Obstet Gynecol 2007;50:67-88. https://doi.org/10.1097/GRF.0b013e31802f1233.
- Zhang J, Gilles JM, Barnhart K, Creinin MD, Westhoff C, Frederick MM. A comparison of medical management with misoprostol and surgical management for early pregnancy failure. N Engl J Med 2005;353:761-9. https://doi.org/10.1056/NEJMoa044064.
- Robledo C, Zhang J, Troendle J, Barnhart K, Creinin MD, Westhoff C, et al. Clinical indicators for success of misoprostol treatment after early pregnancy failure. Int J Gynaecol Obstet 2007;99:46-51. https://doi.org/10.1016/j.ijgo.2007.04.031.
- Creinin MD, Huang X, Westhoff C, Barnhart K, Gilles JM, Zhang J. Factors related to successful misoprostol treatment for early pregnancy failure. Obstet Gynecol 2006;107:901-7. https://doi.org/10.1097/01.AOG.0000206737.68709.3e.
- Major B, Cozzarelli C, Cooper ML, Zubek J, Richards C, Wilhite M, et al. Psychological responses of women after first-trimester abortion. Arch Gen Psychiatry 2000;57:777-84. https://doi.org/10.1001/archpsyc.57.8.777.
- Pud D, Amit A. Anxiety as a predictor of pain magnitude following termination of first-trimester pregnancy. Pain Med 2005;6:143-8. https://doi.org/10.1111/j.1526-4637.2005.05030.x.
- Stockheim D, Machtinger R, Wiser A, Dulitzky M, Soriano D, Goldenberg M, et al. A randomized prospective study of misoprostol or mifepristone followed by misoprostol when needed for the treatment of women with early pregnancy failure. Fertil Steril 2006;86:956-60. https://doi.org/10.1016/j.fertnstert.2006.03.032.
- Grønlund A, Grønlund L, Clevin L, Andersen B, Palmgren N. Lidegaard Ø. Management of missed abortion: comparison of medical treatment with either mifepristone + misoprostol or misoprostol alone with surgical evacuation. A multi-center trial in Copenhagen county, Denmark. Acta Obstet Gynecol Scand 2002;81:1060-5. https://doi.org/10.1080/j.1600-0412.2002.811111.x.
- Schreiber CA, Creinin MD, Atrio J, Sonalkar S, Ratcliffe SJ, Barnhart KT. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med 2018;378:2161-70. https://doi.org/10.1056/NEJMoa1715726.
- National Institute for Health and Care Excellence (NICE) . Ectopic Pregnancy and Miscarriage: Diagnosis and Initial Management in Early Pregnancy of Ectopic Pregnancy and Miscarriage. Clinical Guideline [CG154] 2012. www.nice.org.uk/CG154 (accessed 30 November 2020).
- Coomarasamy A, Harb HM, Devall AJ, Cheed V, Roberts ET, Goranitis I, et al. Progesterone to prevent miscarriage in women with early pregnancy bleeding: the PRISM RCT. Health Technol Assess 2020;24. https://doi.org/10.3310/hta24330.
- Coomarasamy A, Williams H, Truchanowicz E, Seed PT, Small R, Quenby S, et al. PROMISE: first-trimester progesterone therapy in women with a history of unexplained recurrent miscarriages – a randomised, double-blind, placebo-controlled, international multicentre trial and economic evaluation. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20410.
- U.S. National Library of Medicine . Mifepristone and Misoprostol Versus Misoprostol Alone in the Medical Management of Missed Miscarriage (MifeMiso) n.d. www.clinicaltrials.gov/ct2/show/NCT03065660 (accessed 4 October 2021).
- Bottomley C, Van Belle V, Pexsters A, Papageorghiou AT, Mukri F, Kirk E, et al. A model and scoring system to predict outcome of intrauterine pregnancies of uncertain viability. Ultrasound Obstet Gynecol 2011;37:588-95. https://doi.org/10.1002/uog.9007.
- Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine – reporting of subgroup analyses in clinical trials. N Engl J Med 2007;357:2189-94. https://doi.org/10.1056/NEJMsr077003.
- Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer 1976;34:585-612. https://doi.org/10.1038/bjc.1976.220.
- DAMOCLES Study Group, NHS Health Technology Assessment Programme . A proposed charter for clinical trial data monitoring committees: helping them to do their job well. Lancet 2005;365:711-22. https://doi.org/10.1016/S0140-6736(05)17965-3.
- Fang AH, Chen QF, Zheng W, Li YH, Chen RY. Termination of missed abortion in a combined procedure: a randomized controlled trial. J Reprod Contracep 2009;20:45-9. https://doi.org/10.1016/S1001-7844(09)60006-7.
- Sinha P, Suneja A, Guleria K, Aggarwal R, Vaid NB. Comparison of mifepristone followed by misoprostol with misoprostol alone for treatment of early pregnancy failure: a randomized double-blind placebo-controlled trial. J Obstet Gynaecol India 2018;68:39-44. https://doi.org/10.1007/s13224-017-0992-5.
- Chu J, Devall AJ, Hardy P, Beeson L, Coomarasamy A. What is the best method for managing early miscarriage?. BMJ 2020;368. https://doi.org/10.1136/bmj.l6438.
- van den Berg MM, Goddijn M, Ankum WM, van Woerden EE, van der Veen F, van Wely M, et al. Early pregnancy care over time: should we promote an early pregnancy assessment unit?. Reprod Biomed Online 2015;31:192-8. https://doi.org/10.1016/j.rbmo.2015.04.008.
- Lee DT, Cheung LP, Haines CJ, Chan KP, Chung TK. A comparison of the psychologic impact and client satisfaction of surgical treatment with medical treatment of spontaneous abortion: a randomized controlled trial. Am J Obstet Gynecol 2001;185:953-8. https://doi.org/10.1067/mob.2001.117661.
- Graziosi GC, Bruinse HW, Reuwer PJ, Mol BW. Women’s preferences for misoprostol in case of early pregnancy failure. Eur J Obstet Gynecol Reprod Biol 2006;124:184-6. https://doi.org/10.1016/j.ejogrb.2005.06.010.
- Trinder J, Brocklehurst P, Porter R, Read M, Vyas S, Smith L. Management of miscarriage: expectant, medical, or surgical? Results of randomised controlled trial (miscarriage treatment (MIST) trial). BMJ 2006;332:1235-40. https://doi.org/10.1136/bmj.38828.593125.55.
- Niinimäki M, Jouppila P, Martikainen H, Talvensaari-Mattila A. A randomized study comparing efficacy and patient satisfaction in medical or surgical treatment of miscarriage. Fertil Steril 2006;86:367-72. https://doi.org/10.1016/j.fertnstert.2005.12.072.
- Harwood B, Nansel T. Quality of life and acceptability of medical versus surgical management of early pregnancy failure. BJOG 2008;115:501-8. https://doi.org/10.1111/j.1471-0528.2007.01632.x.
- Tomasik P, Zwierzchowska A, Barcz E. Assessment of patient acceptability of medical treatment in case of non-viable first trimester pregnancy. Ginekol Pol 2015;86:383-7. https://doi.org/10.17772/gp/2427.
- Al Wattar BH, Murugesu N, Tobias A, Zamora J, Khan KS. Management of first-trimester miscarriage: a systematic review and network meta-analysis. Hum Reprod Update 2019;25:362-74. https://doi.org/10.1093/humupd/dmz002.
- Fernlund A, Jokubkiene L, Sladkevicius P, Valentin L. Misoprostol treatment vs. expectant management in women with early non-viable pregnancy and vaginal bleeding: a pragmatic randomized controlled trial. Ultrasound Obstet Gynecol 2018;51:24-32. https://doi.org/10.1002/uog.18940.
- Cubo AM, Soto ZM, Haro-Pérez A, Hernández ME, Doyague MJ, Sayagués JM. Medical versus surgical treatment of first trimester spontaneous abortion: A cost-minimization analysis. PLOS ONE 2019;14. https://doi.org/10.1371/journal.pone.0210449.
- Schreiber CA, Chavez V, Whittaker PG, Ratcliffe SJ, Easley E, Barg FK. Treatment decisions at the time of miscarriage diagnosis. Obstet Gynecol 2016;128:1347-56. https://doi.org/10.1097/AOG.0000000000001753.
- Kong GW, Lok IH, Yiu AK, Hui AS, Lai BP, Chung TK. Clinical and psychological impact after surgical, medical or expectant management of first-trimester miscarriage – a randomised controlled trial. Aust N Z J Obstet Gynaecol 2013;53:170-7. https://doi.org/10.1111/ajo.12064.
- Smith LF, Frost J, Levitas R, Bradley H, Garcia J. Women’s experiences of three early miscarriage management options: a qualitative study. Br J Gen Pract 2006;56:198-205.
- Olesen ML, Graungaard AH, Husted GR. Deciding treatment for miscarriage – experiences of women and healthcare professionals. Scand J Caring Sci 2015;29:386-94. https://doi.org/10.1111/scs.12175.
- Norton W, Furber L. An exploration of how women in the UK perceive the provision of care received in an early pregnancy assessment unit: an interpretive phenomenological analysis. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2018-023579.
- Adolfsson A. Applying Heidegger’s interpretive phenomenology to women’s miscarriage experience. Psychol Res Behav Manag 2010;3:75-9. https://doi.org/10.2147/PRBM.S4821.
- Nansel TR, Doyle F, Frederick MM, Zhang J. Quality of life in women undergoing medical treatment for early pregnancy failure. J Obstet Gynecol Neonatal Nurs 2005;34:473-81. https://doi.org/10.1177/0884217505278319.
- Davis AR, Hendlish SK, Westhoff C, Frederick MM, Zhang J, Gilles JM, et al. Bleeding patterns after misoprostol vs surgical treatment of early pregnancy failure: results from a randomized trial. Am J Obstet Gynecol 2007;196:31.e1-7. https://doi.org/10.1016/j.ajog.2006.07.053.
- Radford EJ, Hughes M. Women’s experiences of early miscarriage: implications for nursing care. J Clin Nurs 2015;24:1457-65. https://doi.org/10.1111/jocn.12781.
- Larivière-Bastien D, deMontigny F, Verdon C. Women’s experiences of miscarriage in the emergency department. J Emerg Nurs 2019;45:670-6. https://doi.org/10.1016/j.jen.2019.06.008.
- Bottomley C, Bourne T. Diagnosing miscarriage. Best Pract Res Clin Obstet Gynaecol 2009;23:463-77. https://doi.org/10.1016/j.bpobgyn.2009.02.004.
- Jansson C, Adolfsson A. A Swedish study of midwives’ and nurses’ experiences when women are diagnosed with a missed miscarriage during a routine ultrasound scan. Sex Reprod Healthc 2010;1:67-72. https://doi.org/10.1016/j.srhc.2010.01.002.
- Adolfsson A. Women’s well-being improves after missed miscarriage with more active support and application of Swanson’s Caring Theory. Psychol Res Behav Manag 2011;4:1-9. https://doi.org/10.2147/PRBM.S15431.
- Volgsten H, Jansson C, Darj E, Stavreus-Evers A. Women’s experiences of miscarriage related to diagnosis, duration, and type of treatment. Acta Obstet Gynecol Scand 2018;97:1491-8. https://doi.org/10.1111/aogs.13432.
- Kulier R, Kapp N, Gülmezoglu AM, Hofmeyr GJ, Cheng L, Campana A. Medical methods for first trimester abortion. Cochrane Database Syst Rev 2011;11. https://doi.org/10.1002/14651858.CD002855.pub4.
- Adolfsson A, Berterö C, Larsson PG. Effect of a structured follow-up visit to a midwife on women with early miscarriage: a randomized study. Acta Obstet Gynecol Scand 2006;85:330-5. https://doi.org/10.1080/00016340500539376.
- O’Cathain, A . Using Qualitative Research With Randomized Controlled Trials 2018. https://doi.org/10.1093/med/9780198802082.001.0001.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Plano Clark VL, Schumacher K, West C, Edrington J, Dunn LB, Harzstark A, et al. Practices for embedding an interpretive qualitative approach within a randomized clinical trial. J Mix Methods Res 2013;7:219-42. https://doi.org/10.1177/1558689812474372.
- DeJonckheere M, Vaughn LM. Semistructured interviewing in primary care research: a balance of relationship and rigour. Fam Med Community Health 2019;7. https://doi.org/10.1136/fmch-2018-000057.
- Larsen DL, Attkisson CC, Hargreaves WA, Nguyen TD. Assessment of client/patient satisfaction: development of a general scale. Eval Program Plann 1979;2:197-20. https://doi.org/10.1016/0149-7189(79)90094-6.
- Guba EG, Lincoln YS, Denzin NK, Lincoln YS. Handbook of Qualitative Research. London: SAGE Publications; 1994.
- Willig C. Constructivism and ‘the real world’: can they co-exist?. Qual Methods Psychol Bull 2016;21:33-7.
- Elliott J. Using Narrative in Social Research: Qualitative and Quantitative Approaches. London: SAGE Publications; 2005.
- Esin C, Frost N. Qualitative Research Methods in Psychology Combining Core Approaches. Berkshire: Open University Press; 2011.
- Saunders B, Sim J, Kingstone T, Baker S, Waterfield J, Bartlam B, et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant 2018;52:1893-907. https://doi.org/10.1007/s11135-017-0574-8.
- Oltmann S. Qualitative interviews: a methodological discussion of the interviewer and respondent contexts. Forum Qual Soc Res 2016;17.
- Volgsten H, Jansson C, Svanberg AS, Darj E, Stavreus-Evers A. Longitudinal study of emotional experiences, grief and depressive symptoms in women and men after miscarriage. Midwifery 2018;64:23-8. https://doi.org/10.1016/j.midw.2018.05.003.
- Krosch DJ, Shakespeare-Finch J. Grief, traumatic stress, and posttraumatic growth in women who have experienced pregnancy loss. Psychol Trauma 2017;9:425-33. https://doi.org/10.1037/tra0000183.
- Lee C, Rowlands IJ. When mixed methods produce mixed results: integrating disparate findings about miscarriage and women’s wellbeing. Br J Health Psychol 2015;20:36-44. https://doi.org/10.1111/bjhp.12121.
- Bowtell EC, Sawyer SM, Aroni RA, Green JB, Duncan RE. ‘Should I send a condolence card?’ Promoting emotional safety in qualitative health research through reflexivity and ethical mindfulness. Qual Inq 2013;19:652-63. https://doi.org/10.1177/1077800413500927.
- Warin J. Ethical mindfulness and reflexivity: managing a research relationship with children and young people in a 14-year qualitative longitudinal research (QLR) study. Qual Inq 2011;17:805-14. https://doi.org/10.1177/1077800411423196.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Kornhaber R, Walsh K, Duff J, Walker K. Enhancing adult therapeutic interpersonal relationships in the acute health care setting: an integrative review. J Multidiscip Healthc 2016;9:537-46. https://doi.org/10.2147/JMDH.S116957.
- Doyle C, Lennox L, Bell D. A systematic review of evidence on the links between patient experience and clinical safety and effectiveness. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2012-001570.
- Bellhouse C, Temple-Smith M, Watson S, Bilardi J. ‘The loss was traumatic. . . some healthcare providers added to that’: women’s experiences of miscarriage. Women Birth 2019;32:137-46. https://doi.org/10.1016/j.wombi.2018.06.006.
- deMontigny F, Verdon C, Meunier S, Dubeau D. Women’s persistent depressive and perinatal grief symptoms following a miscarriage: the role of childlessness and satisfaction with healthcare services. Arch Womens Ment Health 2017;20:655-62. https://doi.org/10.1007/s00737-017-0742-9.
- Jensen KLB, Temple-Smith MJ, Bilardi JE. Health professionals’ roles and practices in supporting women experiencing miscarriage: a qualitative study. Aust N Z J Obstet Gynaecol 2019;59:508-13. https://doi.org/10.1111/ajo.12910.
- Edwards S, Birks M, Chapman Y, Yates K. Bringing together the ‘threads of care’ in possible miscarriage for women, their partners and nurses in non-metropolitan EDs. Collegian 2018;25:293-301. https://doi.org/10.1016/j.colegn.2017.09.004.
- Emond T, de Montigny F, Guillaumie L. Exploring the needs of parents who experience miscarriage in the emergency department: a qualitative study with parents and nurses. J Clin Nurs 2019;28:1952-65. https://doi.org/10.1111/jocn.14780.
- National Bereavement Care Pathway for Pregnancy and Baby Loss . Miscarriage, Ectopic Pregnancy and Molar Pregnancy. Full Guidance Document 2019. https://nbcpathway.org.uk/sites/default/files/2019-05/NBCP%20Miscarriage%20Full%20Guidance%20-%20May%202019.pdf (accessed 14 July 2020).
- van den Berg MMJ, Dancet EAF, Erlikh T, van der Veen F, Goddijn M, Hajenius PJ. Patient-centered early pregnancy care: a systematic review of quantitative and qualitative studies on the perspectives of women and their partners. Hum Reprod Update 2018;24:106-18. https://doi.org/10.1093/humupd/dmx030.
- Wallace RR, Goodman S, Freedman LR, Dalton VK, Harris LH. Counseling women with early pregnancy failure: utilizing evidence, preserving preference. Patient Educ Couns 2010;81:454-61. https://doi.org/10.1016/j.pec.2010.10.031.
- Betts D, Dahlen HG, Smith CA. A search for hope and understanding: an analysis of threatened miscarriage internet forums. Midwifery 2014;30:650-6. https://doi.org/10.1016/j.midw.2013.12.011.
- Department of Health . Public Health White Paper. Liberating the NHS: No Decision About Me Without Me 2012.
- National Institute for Health and Care Excellence . Patient Experience in Adult NHS Services: Improving the Experience of Care for People Using Adult NHS Services. Clinical Guideline [CG138] 2012. www.nice.org.uk/guidance/CG138 (accessed 14 July 2020).
- Shorter JM, Atrio JM, Schreiber CA. Management of early pregnancy loss, with a focus on patient centered care. Semin Perinatol 2019;43:84-9. https://doi.org/10.1053/j.semperi.2018.12.005.
- Kolte AM, Bernardi LA, Christiansen OB, Quenby S, Farquharson RG, Goddijn M, et al. Terminology for pregnancy loss prior to viability: a consensus statement from the ESHRE early pregnancy special interest group. Hum Reprod 2015;30:495-8. https://doi.org/10.1093/humrep/deu299.
- Shankar M, Economides DL, Sabin CA, Tan B, Kadir RA. Outpatient medical management of missed miscarriage using misoprostol. J Obstet Gynaecol 2007;27:283-6. https://doi.org/10.1080/01443610701213927.
- Scott L. Medical management in the community as an option for first trimester miscarriage. Nurs Times 2010;106:17-9.
- Shelley JM, Healy D, Grover S. A randomised trial of surgical, medical and expectant management of first trimester spontaneous miscarriage. Aust N Z J Obstet Gynaecol 2005;45:122-7. https://doi.org/10.1111/j.1479-828X.2005.00357.x.
- Smith JA. Identity development during the transition to motherhood: an interpretative phenomenological analysis. J Reprod Infant Psychol 1999;17:281-99. https://doi.org/10.1080/02646839908404595.
- Laney EK, Hall MEL, Anderson TL, Willingham MM. Becoming a mother: the influence of motherhood on women’s identity development. Identity 2015;15:126-45. https://doi.org/10.1080/15283488.2015.1023440.
- Reiheld A. ‘The event that was nothing’: miscarriage as a liminal event. J Soc Phil 2015;46:9-26. https://doi.org/10.1111/josp.12084.
- Anderson ML, Goodman J, Schlossberg NK. Counseling Adults in Transition: Linking Schlossberg’s Theory with Practice in a Diverse World. New York, NY: Springer Publishing Company; 2011.
- Nikčević AV, Nicolaides KH. Search for meaning, finding meaning and adjustment in women following miscarriage: a longitudinal study. Psychol Health 2013;29:50-63. https://doi.org/10.1080/08870446.2013.823497.
- Gallos ID, Williams HM, Price MJ, Eapen A, Eyo MM, Tobias A, et al. Methods for managing miscarriage: a network meta analysis – protocol. Cochrane Database Syst Rev 2017;3. https://doi.org/10.1002/14651858.CD012602.
- Quay TA, Frimer L, Janssen PA, Lamers Y. Barriers and facilitators to recruitment of South Asians to health research: a scoping review. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-014889.
- Okeke Ogwulu CB, Williams EV, Chu JJ, Devall AJ, Beeson LE, Hardy P, et al. Cost-effectiveness of mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage: an economic evaluation based on the MifeMiso trial. BJOG 2021;128:1534-45. https://doi.org/10.1111/1471-0528.16737.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BJOG 2013;120:765-70. https://doi.org/10.1111/1471-0528.12241.
- National Institute for Health and Care Excellence . Position Statement on Use of the EQ-5D-5L Value Set for England 2019. www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l (accessed 14 July 2020).
- Reference Cost Collection . National Schedule of Reference Costs, 2018–19 – NHS Trusts and NHS Foundation Trusts n.d. https://digital.nhs.uk/binaries/content/assets/website-assets/data-collections/refcost-collection-guidance-2018_2019.pdf (accessed 20 January 2020).
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2019. Canterbury: PSSRU, University of Kent; 2019.
- Joint Formulary Committee . British National Formulary (online). 2019. www.medicinescomplete.com (accessed 14 July 2020).
- Curtis L, Netten A. Unit Costs of Health and Social Care 2002. Canterbury: PSSRU, University of Kent; 2002.
- UK Government . Health Trust Reference Costs 2017–18 n.d. https://data.gov.uk/dataset/71367291-5636-460f-a8fe-374780a16a8e/health-trust-reference-costs-2017-18 (accessed 4 October 2021).
- Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338. https://doi.org/10.1136/bmj.b2393.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. Hoboken, NJ: John Wiley & Sons; 2004.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Barber JA, Thompson SG. Analysis of cost data in randomized trials: an application of the non-parametric bootstrap. Stat Med 2000;19:3219-36. https://doi.org/10.1002/1097-0258(20001215)19:23<3219::AID-SIM623>3.0.CO;2-P.
- Zellner A, Huang DS. Further properties of efficient estimators for seemingly unrelated regression equations. Int Econ Rev 1962;3:300-13. https://doi.org/10.2307/2525396.
- Moon H, Perron B, Durlauf SN, Blume LE. The New Palgrave Dictionary of Economics. London: Palgrave Macmillan; 2008.
- Willan AR, Briggs AH, Hoch JS. Regression methods for covariate adjustment and subgroup analysis for non-censored cost-effectiveness data. Health Econ 2004;13:461-75. https://doi.org/10.1002/hec.843.
- Glick HA, Briggs AH, Polsky D. Quantifying stochastic uncertainty and presenting results of cost-effectiveness analyses. Expert Rev Pharmacoecon Outcomes Res 2001;1:25-36. https://doi.org/10.1586/14737167.1.1.25.
- Black WC. The CE plane: a graphic representation of cost-effectiveness. Med Decis Making 1990;10:212-14. https://doi.org/10.1177/0272989X9001000308.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013.
- van Hout B, Janssen MF, Feng Y-S, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2018;27:7-22. https://doi.org/10.1002/hec.3564.
- Nagendra D, Koelper N, Loza-Avalos SE, Sonalkar S, Chen M, Atrio J, et al. Cost-effectiveness of mifepristone pretreatment for the medical management of nonviable early pregnancy: secondary analysis of a randomized clinical trial. JAMA Netw Open 2020;3. https://doi.org/10.1001/jamanetworkopen.2020.1594.
- Brennan A, Chick SE, Davies R. A taxonomy of model structures for economic evaluation of health technologies. Health Econ 2006;15:1295-310. https://doi.org/10.1002/hec.1148.
- Bagratee JS, Khullar V, Regan L, Moodley J, Kagoro H. A randomized controlled trial comparing medical and expectant management of first trimester miscarriage. Hum Reprod 2004;19:266-71. https://doi.org/10.1093/humrep/deh049.
- Lemmers M, Verschoor MAC, Bossuyt PM, Huirne JAF, Spinder T, Nieboer TE, et al. Cost-effectiveness of curettage vs. expectant management in women with an incomplete evacuation after misoprostol treatment for first trimester miscarriage: a randomized controlled trial and cohort study. Acta Obstet Gynecol Scand 2018;97:294-300. https://doi.org/10.1111/aogs.13283.
- Luise C, Jermy K, May C, Costello G, Collins WP, Bourne TH, et al. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. BMJ 2002;324. https://doi.org/10.1136/bmj.324.7342.873.
- Graziosi GC, van der Steeg JW, Reuwer PH, Drogtrop AP, Bruinse HW, Mol BW. Economic evaluation of misoprostol in the treatment of early pregnancy failure compared to curettage after an expectant miscarriage. Hum Reprod 2005;20:1067-71. https://doi.org/10.1093/humrep/deh709.
- Rafi J, Khalil H. Expectant management of miscarriage in view of NICE Guideline 154. J Pregnancy 2014;2014. https://doi.org/10.1155/2014/824527.
- You JH, Chung TK. Expectant, medical or surgical treatment for spontaneous abortion in first trimester of pregnancy: a cost analysis. Hum Reprod 2005;20:2873-8. https://doi.org/10.1093/humrep/dei163.
- Petrou S, Trinder J, Brocklehurst P, Smith L. Economic evaluation of alternative management methods of first-trimester miscarriage based on results from the MIST trial. BJOG 2006;113:879-89. https://doi.org/10.1111/j.1471-0528.2006.00998.x.
- Petrou S, McIntosh E. Women’s preferences for attributes of first-trimester miscarriage management: a stated preference discrete-choice experiment. Value Health 2009;12:551-9. https://doi.org/10.1111/j.1524-4733.2008.00459.x.
List of abbreviations
- AE
- adverse event
- BERC
- Blinded Endpoint Review Committee
- BMI
- body mass index
- BNF
- British National Formulary
- CEA
- cost-effectiveness analysis
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CSQ-8
- Client Satisfaction Questionnaire 8
- CUA
- cost–utility analysis
- DMC
- Data Monitoring Committee
- EPU
- early pregnancy unit
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GCP
- good clinical practice
- GMP
- good manufacturing practice
- HCP
- health-care professional
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IMP
- investigational medicinal product
- ITMS
- integrated trial management system
- MHRA
- Medicines and Healthcare products Regulatory Agency
- MID
- minimally important difference
- MifeMiso
- mifepristone and misoprostol
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NRES
- National Research Ethics Service
- PBAC
- Pictorial Blood loss Assessment Chart
- PI
- principal investigator
- PIS
- participant information sheet
- PPI
- patient and public involvement
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QP
- qualified person
- QTA
- qualitative thematic analysis
- RR
- risk ratio
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SAR
- serious adverse reaction
- TSC
- Trial Steering Committee
- WTP
- willingness to pay
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/hta25680).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.