Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 15/57/160. The contractual start date was in January 2017. The draft report began editorial review in September 2020 and was accepted for publication in April 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
Copyright © 2022 Nugent et al. This work was produced by Nugent et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Nugent et al.
Chapter 1 Introduction
Scientific background
Description of the condition
Oral mucositis (OM) has the unfortunate distinctions of being both the most common and the most debilitating complication of head and neck cancer (HNC) irradiation. 1 It is characterised by inflammation of the mucous membranes with erythema and ulceration (Figure 1).
FIGURE 1.
Example of oral mucositis on the tongue.
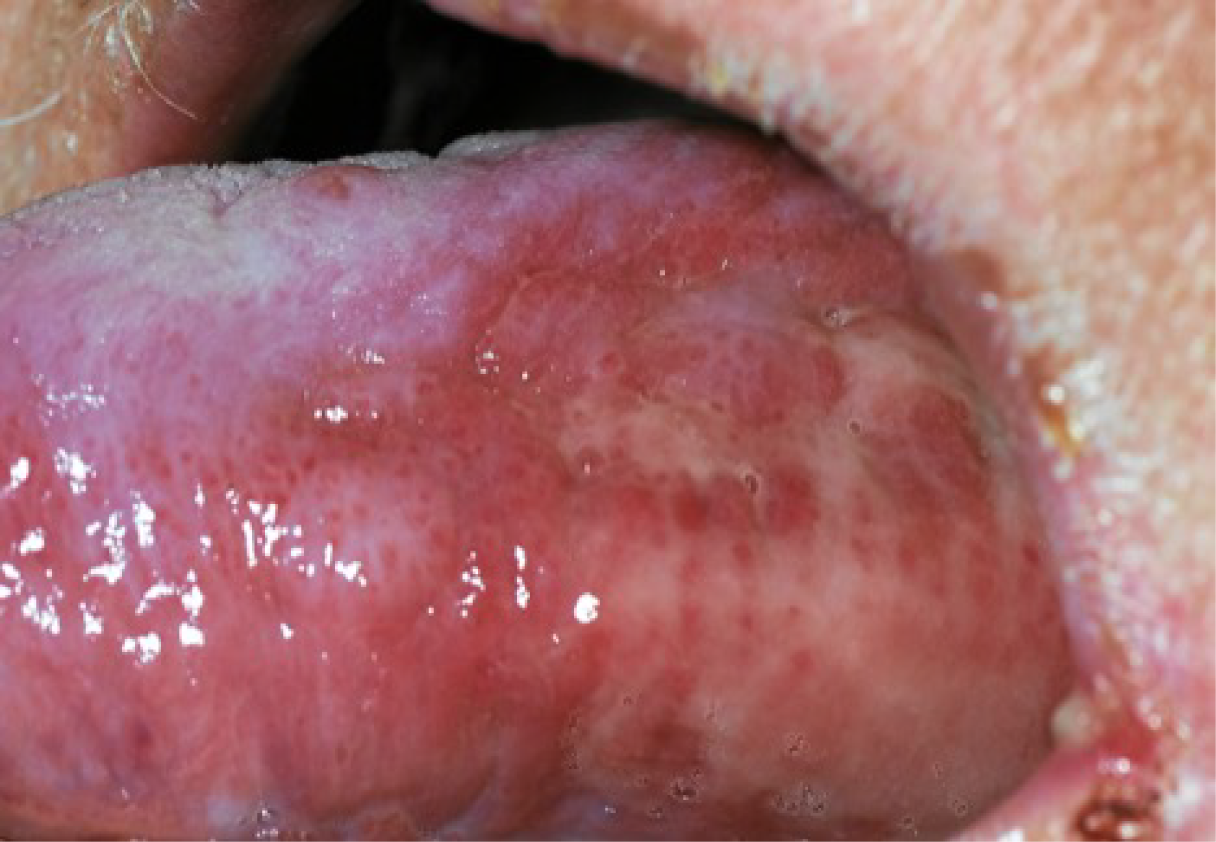
Many patients need opioid medications to control the pain, which affects their ability and willingness to eat and drink throughout radiotherapy and, for some, months thereafter. Consequently, around 90% of patients will require nutritional support and most will require tube feeding.
Feeding tubes can be inserted through the nose (Figure 2) or directly through the abdominal wall into the stomach. 2 The pain of OM for patients is only the beginning of the story. Patients’ general, psychosocial and financial well-being, and that of their carers, are also affected by OM. 3 Mucositis is an independent risk factor for pharyngo-oesophageal stricture. Stricture is a devastating complication, which can develop after HNC radiotherapy as a result of scarring partially or completely blocking the gullet and can result in a permanent inability to swallow, an aspiration of food and fluid into the lungs, and a long-term dependence on feeding tubes. 4 Strictures typically present within the first 6 months following the completion of irradiation, but can develop up to 5 years following completion. 5
FIGURE 2.
Example of a patient with a nasal feeding tube. Used with permission from the photo subject.
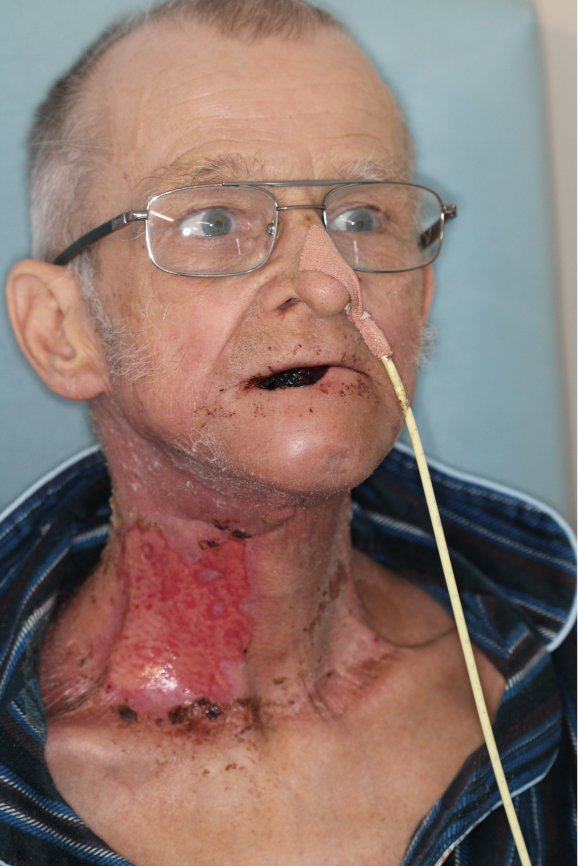
Size of the problem
Six thousand patients per year in England and Wales undergo (chemo)radiotherapy [(C)RT] for HNC. 6 Ninety-seven per cent of these patients will develop OM because of their (C)RT. Patients receiving (C)RT are at greater risk of more severe OM than those who are not because of potentiation of the effects of the radiotherapy by the chemotherapy. 7
How does oral mucositis develop?
The natural history of OM is gradually being unravelled. In 2004, Sonis et al. 3 described a five-stage mechanism by which the condition develops and then heals (Figure 3). These stages are initiation, the primary damage response, signalling and amplification, ulceration, and healing. In the first stage (initiation), the mucosal cells are injured by the cancer therapy. In the next two stages, reactive oxygen species and inflammatory cytokines (released by direct tissue injury) cause further damage to the submucosa, leading to ulceration (stage 4). Microbial toxins are also thought to stimulate further inflammation of the ulcerated lesion. In stage 5, which is the least well-understood phase, healing takes place. In the UK, cancer treatments are delivered daily from Monday to Friday, but not at weekends or bank holidays. Radiotherapy continues for 6–7 weeks, much longer than the 15–21 days in Sonis’s table. 3 Concurrent chemotherapy is given either weekly or every 3 weeks for the duration of the radiotherapy. 8 In effect, all five stages may be happening at the same time.
FIGURE 3.
Sonis et al. ’s3 five stages of mucositis. Reproduced with permission from Basile et al. 9 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
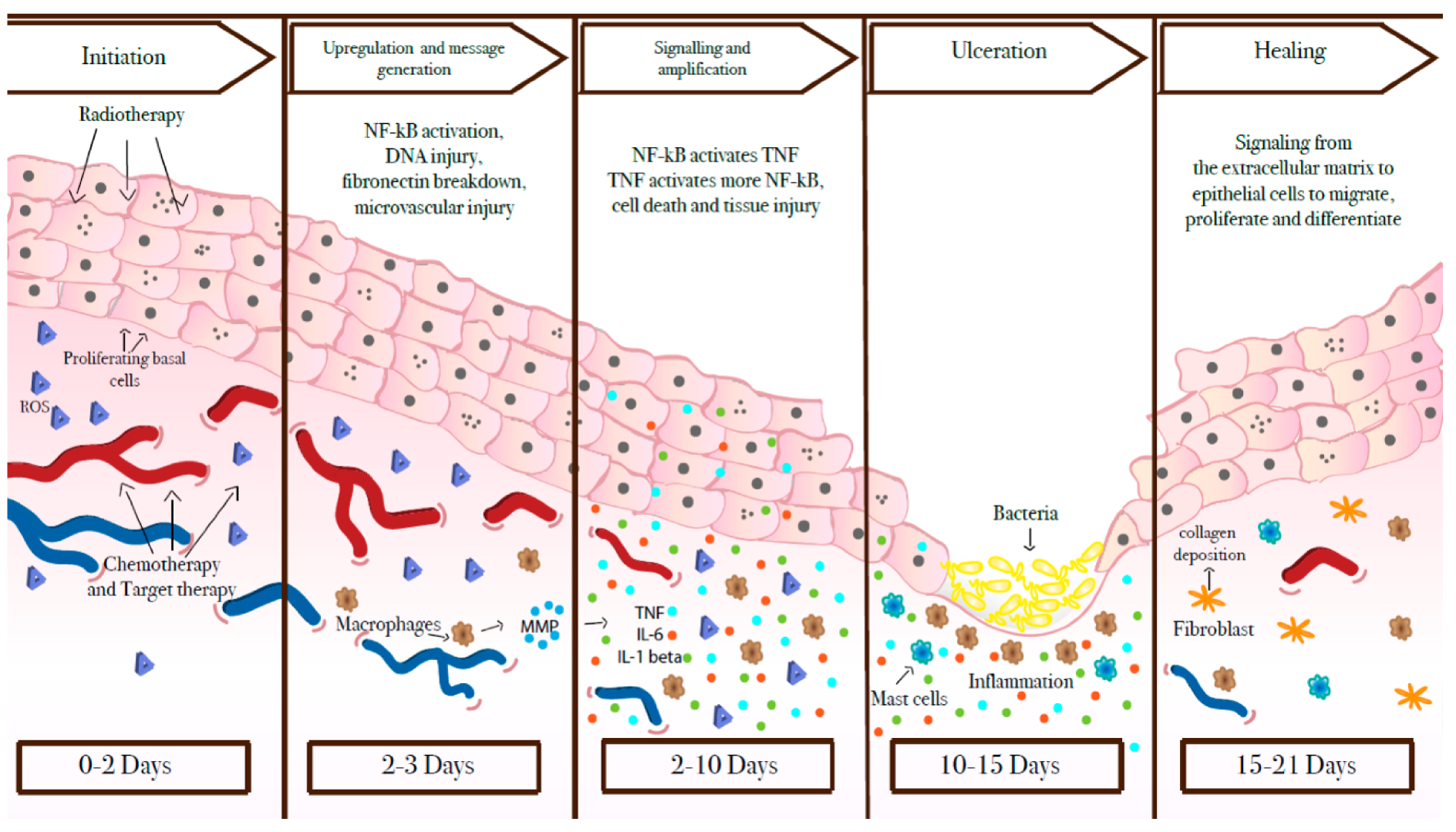
Current oral mucositis treatment strategies in the UK
In general, patients are encouraged to improve oral hygiene as a preventative measure. Mouthwashes are typically prescribed in an effort to protect the oral mucosa, by keeping the oral mucosa moist and clean during treatment. Mouthwashes, such as Biotène™ (GlaxoSmithKline plc, Brentford, UK), also help with the side effect of mouth dryness. A variety of mouthwashes are used, but there is no evidence of any of them being effective in relation to OM. It is suggested that chlorhexidine mouthwash is not used because its alcohol content makes it painful to use. 10
Nutrition is optimised by prescribing supplement drinks for patients who find chewing solid foods difficult and for those whose oral intake remains inadequate despite dietary changes to softer or liquid-textured foods. Pain is managed using combinations of topical and systemic analgesics, following the World Health Organization (WHO)’s analgesia ladder11 as symptoms progress. Anecdotal evidence suggests that there remains a variation in management strategies in the UK.
In terms of the process described by Sonis et al. 3, it is possible that stage 4 is suppressed by these measures. Mouthwash irrigation may reduce the bacterial load in the ulcers, and coating gels may cover mucosal breaches, reducing discomfort. However, none of the current approaches has the ability to affect the progression through the stages.
Rationale for the LiTEFORM trial
Low-level laser therapy (LLLT), or photobiomodulation, is a treatment that has the potential to reduce the severity of OM. LLLT involves the application of a low-powered laser to the affected tissue. The most familiar types of medical lasers are those that are used to cut or ablate tissues. LLLT works in dramatic contrast to this, modulating biochemical pathways within the cell to reduce inflammation and improve healing. The mechanism by which this happens is not fully understood. It is postulated that the light is absorbed into the mitochondria, which increases the activity of the cell, accelerates cell healing and inhibits pain. 12 It is plausible that LLLT is modulating all five stages of Sonis et al. ’s3 mucositis model. The effect of the laser depends on the wavelength and density of the light, as well as the duration and frequency of application. The timing of LLLT with respect to radiotherapy treatment sessions may also be significant.
Evidence for the use of low-level laser therapy
Low-level laser treatment for OM in HNC is gaining popularity outside the UK. 13 Results from trials have been encouraging. There has been a series of systematic reviews conducted over the last decade, including two by the Cochrane collaboration. 14 These reviews have pointed towards progressively stronger evidence supporting the use of low-level lasers for managing OM in HNC.
To our knowledge, the most up-to-date systematic review prior to starting the LiTEFORM trial was conducted by Oberoi et al. 15 This included 18 randomised controlled trials (RCTs) of LLLT for OM, 10 of which related to patients with conditions other than HNC who were treated with a mixture of chemotherapy and radiotherapy. This review concluded that prophylactic LLLT reduced severe OM in patients with cancer [risk ratio 0.37, 95% confidence interval (CI) 0.20 to 0.67; p = 0.001]. Many of these patients did not receive radiotherapy but experienced OM as a result of high-dose chemotherapy. The review suggested that future research should identify the optimal characteristics of LLLT and determine the feasibility of using LLLT in the clinical setting.
Limitations of current evidence
To our knowledge, much of the previous published evidence has methodological and other limitations.
Allocation concealment
Published LLLT trials have varied methods for delivering low-level laser treatment. The method of blinding both staff and patients to laser treatment tended to be poorly described, affecting the trial results owing to potential reporter bias. In Oberoi et al. ’s15 review, it was noted that only 21% of studies reported adequate allocation concealment. Furthermore, none of the studies included in the systematic reviews attempted to blind the clinicians delivering the LLLT. Some of these trials are badged as being double or triple blinded. There is a risk of unblinded clinicians transferring attitudes or providing differential treatment to active and sham arms. 16
Feasibility for use in routine practice
To our knowledge, no previous trial provided data on the human resource requirements to deliver this treatment. There is no guidance on required facilities or clinical governance, and there are no reported assessments on the impact of integrating this treatment into routine clinical care. Hence, there are still not many recommendations on how to set up and deliver this treatment.
Who should deliver low-level laser therapy?
The reported studies used physicians, dentists or physiotherapists to deliver LLLT. To our knowledge, there is no information on which health-care professionals should deliver this intervention to patients.
Lack of multicentre studies
Only one small study involving 30 participants attempted to use more than one site. 17 In this trial, 28 participants were recruited at one site and one each at the other two sites. The potential benefits of a multicentre RCT would be a larger number of participants and greater variety of locations, which may increase the generalisability of the findings. There is some evidence18 that single-site clinical trials with continuous outcomes show larger intervention effects than multicentre trials. This may reduce the generalisability of the results. 18,19
Acceptability to patients
There is little evidence on the acceptability to patients or on how they would perceive the LLLT treatment. In their systematic review, Oberoi et al. 15 recognised that this intervention requires patients to co-operate with an intervention delivered to an inflamed, painful oral cavity. Patients would undergo this repeatedly during a demanding, prolonged course of cancer treatment, with potential logistical and financial implications.
Acceptability to staff
Low-level laser therapy requires specialist equipment, a dedicated room and specially trained staff, as well as a degree of co-ordination for the appointments. There are no data on the acceptability of this treatment for clinicians or any occupational health hazards it entails.
Oncological safety
There are few published data on the safety of this treatment with regard to its effects on recurrence or persistence of disease.
Cost
Finally, there is little guidance on the cost of setting up and delivering LLLT, or its cost-effectiveness.
Summary with implications for trial design
We designed the LiTEFORM trial to address the issues raised in the prior, incomplete, research attempts.
Our aim was to investigate LLLT in a population attending for outpatient radiotherapy or chemoradiotherapy (CRT) in multiple regional cancer sites in the UK. The research aimed to show whether or not LLLT conferred a benefit over standard care, was acceptable to patients, was practical to deliver and was cost-effective.
Aims and objectives
The main aim of the trial was to estimate the magnitude of any benefit of LLLT delivered three times per week by staff trained in the management of OM in HNC irradiation when compared with sham LLLT. The trial aimed to measure this using a combination of patient- and clinician-reported outcome measures to allow the assessment of symptomatic responses to the treatment and its effect on quality of life (QoL) and function of HNC patients. We also intended to assess the financial impact for health-care providers. The qualitative arm of the trial aimed to assess the impact of setting up low-level laser services within various NHS trusts, all of which have an individual staff and service provision mix that has developed over time to specifically reflect that area’s population and resource availability.
Primary objective
-
To compare the clinical effectiveness of LLLT plus standard care with that of sham LLLT plus standard care, as measured by the Oral Mucositis Weekly Questionnaire-Head and Neck Cancer (OMWQ-HN), in adult HNC patients receiving (C)RT.
Secondary objectives
-
To determine the clinical effectiveness of LLLT in preventing severe OM during radiotherapy or CRT for HNC as shown by the clinician-measured WHO Oral Mucositis Grading Scale scores.
-
To apply evidence derived from the trial to inform NHS guidance in the use of LLLT for managing OM.
-
To investigate the short- and long-term benefits to patients in terms of dependence on feeding tubes, nutritional status, pain control, admission to hospital, treatment interruptions, swallowing function and QoL.
-
To investigate the long-term risks of LLLT (e.g. survival, recurrence and disease progression).
-
To identify barriers to and facilitators of implementing LLLT in routine clinical care through a qualitative process evaluation.
Economic evaluation
-
To compare the total costs of LLLT with those of sham LLLT, calculated by combining data collected from the electronic case report form (eCRF), Health Service Utilisation and Time and Travel Questionnaires (see Report Supplementary Material 1 and 2) with nationally available unit cost data.
-
To compare quality-adjusted life-years (QALYs) derived from the responses to the EuroQol-5 dimensions, five-level (EQ-5D-5L), questionnaire with those of the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire 30 (EORTC QLQ-C30) measured at baseline and throughout the trial.
-
To compare the cost-effectiveness measured in terms of the incremental cost per change (improvement) in OMWQ-HN score recorded between baseline and at 6 weeks of therapy (as detailed in the statistical primary end point).
-
To evaluate incremental cost per QALY of LLLT when compared with standard care (from the perspective of the NHS and Personal Social Services over 14 months).
Qualitative study
-
To identify barriers to and facilitators of recruitment by interviewing patients, interviewing health professionals, observing launch event and site initiation visits, and audio-recording recruitment consultations.
-
To feed back to sites barriers and facilitators that have been identified by developing a detailed action plan and preparing site-specific feedback.
-
To understand practitioners’ and sites’ experiences of training in and delivering LLLT and the ‘fit’ of LLLT within the treatment pathway.
-
To identify barriers to and facilitators of wider implementation of trial findings and LLLT.
Chapter 2 Methods
This chapter covers general trial methods, statistical analysis and governance. Details of the health economic and qualitative methods and analyses are provided in Chapter 4, Economic evaluation, and Chapter 5, Qualitative study, respectively.
Overview of the trial design
This was a multicentre, Phase III, individually randomised, double-blind, placebo-controlled superiority trial with an internal pilot and qualitative substudy set in secondary care. Patients with a treatment plan for HNC irradiation were identified and recruited from NHS head and neck multidisciplinary team (MDT) clinics. Participants were randomised in a 1 : 1 ratio to receive either standard care plus LLLT or standard care plus sham LLLT. This was a pragmatic trial embedded in current NHS clinical practice and attempts were not made to impose an external standard of care for OM or (C)RT regimes across sites. There was interest in any benefit added by LLLT in a real-world situation and the feasibility of setting up and running a new LLLT service within different NHS settings. The trial included an integrated internal pilot, economic evaluation (see Chapter 4, Economic evaluation) and parallel process evaluation (see Chapter 5, Qualitative study).
Trial registration and protocol availability
The LiTEFORM trial was registered with the International Standard Randomised Controlled Trial Number (ISRCTN) registry on 27 March 2017 (ISRCTN14224600). The protocol is available on the National Institute for Health and Care Research (NIHR) Health Technology Assessment (HTA) project web page (www.journalslibrary.nihr.ac.uk/programmes/hta/1557160/#/).
Ethics and governance
The Newcastle upon Tyne Hospitals NHS Foundation Trust was the sponsor for the trial (reference 08097). Favourable ethics opinion for the trial was obtained on 28 April 2017 from the NHS Research Ethics Service Committee West Midlands – Solihull Research Ethics Committee (REC) (REC reference: 17/WM/0096). Health Research Authority approval was received on 3 May 2017. Subsequent approval was sought and obtained for the three substantive protocol amendments (see Appendix 1, Table 28).
Setting
The trial was conducted in nine HNC treatment sites in England, Scotland and Wales, as follows:
-
City Hospital Sunderland NHS Foundation Trust (now South Tyneside and Sunderland NHS Foundation Trust).
-
University Hospital Southampton NHS Foundation Trust.
-
Velindre NHS Trust.
-
The Leeds Teaching Hospitals NHS Trust.
-
The Newcastle Upon Tyne Hospitals NHS Trust.
-
Taunton & Somerset NHS Foundation Trust.
-
Plymouth Hospitals NHS Trust.
-
Royal Cornwall Hospitals NHS Trust.
-
Royal United Hospitals Bath NHS Trust.
Participants
Participants were adults aged ≥ 18 years who had been diagnosed with HNC and were due to start treatment with irradiation at one of the participating sites.
Inclusion criteria
-
Adults aged ≥ 18 years diagnosed with HNC.
-
Patients who had the capacity to provide written informed consent.
-
Patients who had received a histological diagnosis of squamous cell carcinoma of the oral cavity, oropharynx, nasopharynx, larynx, hypopharynx or unknown squamous cell primary of head and neck origin histologically confirmed.
-
Patients who had been discussed in a head and neck MDT meeting and were deemed medically fit for an agreed treatment plan for primary or adjuvant radiotherapy ± concurrent or induction chemotherapy (cisplatin or cetuximab).
-
It had been planned for the patient to receive a minimum of 60 Gy to a defined clinical target volume in the oral cavity or oropharynx, or neck levels Ia/b, as defined by the current Radiation Therapy Oncology Group criteria. 20
Exclusion criteria
-
Patients who were known to be pregnant or planning to become pregnant within the trial treatment period.
-
Patients who had photosensitive epilepsy.
-
Patients who had parotid tumours.
-
Patients who had previous radiotherapy for HNC.
-
Patients who were experiencing current/ongoing OM and trismus, limiting laser access for treatment.
-
Patients who are experiencing active heavy tumour bleeding from their mouth (haemorrhage).
-
Patients for whom the MDT recommend short-course palliative radiotherapy.
-
Patients on immune suppressant drugs (except low-dose steroids).
-
Patients who were participating in other trials assessing different treatments for OM.
-
Patients who were unable to provide written informed consent.
Intervention
Low-level laser therapy
Participants were scheduled to receive three sessions of LLLT (or sham) per week for 6 weeks, with each session taking 20–30 minutes. The LLLT sessions took place prior to radiotherapy, ideally no longer than 2 hours before (in protocol version 2.0 this was 60 minutes and this was amended to 2 hours for protocol version 2.1) and a minimum of 24 hours after the last session. The LLLT was delivered via a non-contact method that involved shining a weak laser light on areas inside the oral cavity. Throughout the treatment, the participant had to be in a reclined position while keeping their mouth open.
At each session, 20–30 prespecified spots were treated with LLLT. Spots were located within the following anatomical sites of the oral cavity: hard and soft palate (four spots), ventral tongue and floor of the mouth (four spots), buccal mucosa (six spots), labial mucosa (four spots), dorsal tongue (six spots) and lateral border (six spots). The treatment of each spot required the laser to be shone on it for 60 seconds. The primary tumour site was avoided and a minimum of 20 spots could be expected to be treated at each session. Each participant had an individualised treatment exclusion diagram with any areas that must not be treated clearly marked by their treating clinician. These diagrams represented those patients who had not undergone surgery to remove the primary tumour prior to irradiation (approximately 60% of patients).
Low-level laser therapy equipment
The laser machine included a control unit (Figure 4) with an attached probe that was used to deliver the laser therapy. The probe was similar in size to a toothbrush (Figure 5) and was fitted with a new transparent sleeve at the start of each session for infection control purposes.
FIGURE 4.
The THOR® LX2.3 660-nm dental laser and control unit (reproduced with permission from THOR Photomedicine Ltd, Chesham, UK; 2021, personal communication).

FIGURE 5.
The THOR 660-nm, visible-red, single-laser dental probe (reproduced with permission from THOR Photomedicine Ltd, Chesham, UK; 2021, personal communication).

Low-level laser therapy was delivered using a red laser with the following specifications: wavelength 660 nm, power output 75 mW, beam area 1.5 cm2, irradiance 50 mW/cm2, exposure time 60 seconds and fluence 3 J/cm2. In addition to the laser system, the manufacturer supplied an accompanying sham adaptation switch box (see Laser device allocation concealment).
Training for staff delivering low-level laser therapy
Low-level laser therapy was delivered by a variety of clinical staff who had undergone the appropriate laser training, as documented on site delegation and training logs. A comprehensive training package was developed for staff to complete prior to delivering any LLLT. This included an online eLaser Training Course (provided by NHS Healthcare for Education England, www.e-lfh.org.uk/home/; accessed 16 February 2019) and practical training with the laser system. The practical training was initially provided by the machine manufacturer, but staff in the sites were able to provide this training in-house for new staff once they became more familiar with the equipment. The manufacturer was able to provide further face-to-face practical training on how to use the LLLT machine where requested.
Laser safety and maintenance
The low-level laser used in the trial is indicated for use for OM. This laser is classified as a 3b laser, which means that it does not cut or burn but may be hazardous for eye exposure. Laser operators, observers and participants were required to wear laser safety glasses while the laser was in operation, and these could be worn over prescription glasses when required. The use of laser safety glasses was documented at each session. LLLT was delivered in a locked room with reflective surfaces covered and a warning was placed outside the door.
All sites were required to appoint a laser protection advisor (LPA) if one was not already in place. The LPA role included approving the local laser rules and providing overarching advice on the general use of the laser. Sites were also required to appoint a laser protection supervisor (LPS), who had day-to-day knowledge of the laser therapy administered for the LiTEFORM trial. Their role included maintaining a list of laser operators and their training, and authorising them as competent to use the laser. Typically, this was a member of staff who was involved in the trial and named on the delegation log. The manufacturers serviced the equipment annually at no additional cost and provided a replacement laser machine at sites wherever possible during this time to allow for continued provision of service. All sites were also provided with an equipment decontamination guide for cleaning and disinfection in addition to following any local procedures.
Funding of the trial intervention
The THOR laser system [THOR LX2.3 laser and light-emitting diode (LED) therapy system (THOR Photomedicine Ltd, Chesham, UK)] was purchased by each site and was classed as an excess treatment cost (ETC). Each laser system included:
-
THOR LX2.3 laser and LED therapy system – control unit
-
THOR 660-nm, visible-red, single-laser dental probe
-
glass laser light guide(s) – spares provided free of charge
-
patient laser safety glasses
-
staff laser safety glasses
-
sham adaptation box
-
leads/cables/connectors.
The sham adaptation switch box was purchased upfront by sites in addition to the laser system package. All sites were reimbursed for the sham switch box as a research cost through the site agreement with the sponsor. All servicing for the trial performed by THOR was free of charge. Staff training and time to deliver the active LLLT were also classed as ETCs. Costs for delivering the sham LLLT therapy sessions were classed as research costs and were included in the per-participant payment.
Standard care for both arms
Aside from the randomised allocation to receive either LLLT or sham treatment, both arms received standard of care as per local policies. This varied between sites because of the lack of a standardised protocol for the treatment of OM in the UK (see Appendix 1, Table 29, for site-specific practices). However, this typically included patient education through reinforcing the importance of good oral hygiene and hydration, as well as providing nutritional advice and pain management using analgesics, mouthwashes and coating gels.
The variation in practice across the country regarding the prevention and treatment of OM was acknowledged during the design of the LiTEFORM trial, and no attempt to standardise care across sites was made. This was to ensure that sites were comfortable recruiting participants to the trial, knowing that they were going to be given all standard measures at that site in addition to LLLT/sham treatment. Given that the evidence for treatment of OM is, to our knowledge, very limited, this seemed to be reasonable.
Outcome measurements
Primary outcome measure
The primary outcome measure was the results of the OMWQ-HN. 21 The OMWQ-HN is a validated patient-reported outcome measure (PROM) with proven sensitivity and responsiveness in comparison with other patient-reported measures. 22 It has been recommended by the Head and Neck Steering Committee, part of the Coordinating Center for Clinical Trials, National Cancer Institute, Rockville, MD, USA. 23 There is good evidence of high completion rates, with patients returning over 90% of questionnaires, even during the last 2 weeks of radiotherapy when patients are at their lowest ebb. 22
The OMWQ-HN results were collected at baseline (after consent but before the first day of LLLT treatment), weekly during radiotherapy and at 4 and 14 months post radiotherapy. The OMWQ-HN is a nine-item patient-reported questionnaire specific to the HNC population, and measures symptoms of mucositis, including mouth and throat soreness, and their impact on patient well-being over the past 7 days. All questions use a Likert-type response format. The first question quantifies the mouth and throat symptoms that the patient is experiencing on a five-point scale, with 0 indicating no soreness and 4 indicating extreme soreness. There then follow five questions addressing the impact of soreness on patient function (sleeping, swallowing, drinking, eating and talking), which are rated on a five-point scale, with 0 indicating no limitations and 4 indicating unable to do. The remaining three questions assess the degree of mouth and throat pain and soreness using an 11-point scale, with 0 indicating no pain or soreness and 10 indicating the worst pain or soreness imaginable or possible. The responses to the OMWQ-HN are summed to give a total overall score between 0 and 54 points, with a higher score indicating poorer well-being and oral function. The decision to use a PROM for the primary outcome was guided by comments from the review board, as opposed to the original plan of a clinician-rated mucositis score. The patient and public involvement (PPI) group members were instrumental in deciding which PROM should be used; they were given a selection of PROMS that had been vetted for reliability, validity and responsiveness. The OMWQ-HN was selected by the PPI group because it was quick and tapped into meaningful things for patients at this time. The PPI group ruled out other PROMs for a variety of reasons. For example, one had a poor English translation and was felt to be ambiguous, onerous and more related to broader QoL issues that were not appropriate for people going for daily radiotherapy.
Secondary outcome measures
Economic and qualitative outcomes are detailed in Chapter 4, Economic evaluation, and Chapter 5, Qualitative study.
The WHO’s Oral Mucositis Grading Scale score24 is a clinician-rated score that measures objective, subjective and functional aspects of OM based on clinical observations, an oral examination of erythema and ulceration, and functional status. Data on the WHO scale were collected at baseline, weekly during the 6-week treatment period, and at the 4-month follow-up. The WHO scale is a single item that is scored on a five-point scale (Table 1). Owing to the subjective nature of this score, an intraoral photograph was taken at the time of the completion of the WHO mucositis score at the 4-month follow-up visit. Another member of the research team anonymised this for independent fully blinded evaluation.
| Grade | Description |
|---|---|
| 0 (none) | None |
| I (mild) | Oral soreness, erythema |
| II (moderate) | Erythema, ulcers, solid diet tolerated |
| III (severe) | Oral ulcers, liquid diet only |
| IV (life-threatening) | Oral feeding is impossible, requires parental nutrition |
The MD Anderson Dysphagia Inventory (MDADI) is a patient-reported swallowing outcome measure specifically designed for the HNC population. 25 The MDADI contains 20 items that constitute four subscales: global, emotional, functional and physical. Each item is scored on a five-point Likert scale ranging from 1 (strongly agree) to 5 (strongly disagree). Scores for one emotional item and one functional item were reverse scored in accordance with the scoring guidelines. Each subscale was calculated as the average of its items and rescaled to range from 20 (worst impairment) to 100 (no impairment). A subscale score was computed using ‘participant subscale mean’ imputation if at least half of its items were non-missing. A composite score was computed to summarise overall impairment on the emotional, functional and physical domains. The composite score was computed as the weighted average of the untransformed (before rescaling) emotional, functional and physical domain scores and was then scaled to range from 20 to 100. The composite score was computed if all three subscale scores were available. The MDADI was collected at baseline, 6 weeks and at the 4- and 14-month follow-ups.
The European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire 30 (EORTC QLQ-C30) (version 3.0) and EORTC Quality of Life Questionnaire Module for Head and Neck Cancer (EORTC QLQ-H&N35) were collected at baseline, 6 weeks and at the 4-month and 14-month follow-ups. The EORTC Quality of Life Questionnaire (QLQ), an integrated system for assessing the health-related QoL of cancer patients, comprises the following: the QLQ-C30 module, which contains 30 items that constitute 15 subscale scores – a global health status/QoL subscale, five functional subscales and nine symptom subscales (six of which are made up of single items). 26 All items are scored on a Likert scale from 1 (not at all) to 4 (very much), except for those related to global health status/QoL, which are scored from 1 (very poor) to 7 (excellent). The 15 subscales were each rescaled to range from 0 to 100. Higher scores on the global health status/QoL represent better QoL. Higher scores on the functional subscales represent higher levels of functioning. Higher scores on the symptom subscales represent greater symptomology/problems. We summarised 13 of the 15 subscales (i.e. excluding ‘global health status/QoL’ and ‘financial difficulties’) with a QLQ-C30 summary score; computation of the summary score was performed only when at least half of the subscale scores were non-missing. 27 The symptom subscales that formed part of the summary score were reverse scored to ensure that higher scores on the summary score reflected better functioning and lower symptomology.
The EORTC QLQ-H&N35 module is a diagnosis-specific module designed to be used in conjunction with the EORTC QLQ-C30. 27–29 It is intended for use among a wide range of HNC patients with disease of varying stage and being treated with various modalities. The EORTC QLQ-H&N35 contains 35 items that constitute 18 symptom subscales (11 of which are represented by single items). Higher scores on the subscales represent greater symptomology/problems.
Performance Status Scale for Head and Neck Cancer Patients (PSS-HN) is a three-item scale designed to evaluate functional performance of HNC patients on the domains of normalcy of diet, eating in public and understandability of speech. 30 The PSS-HN responses were collected at baseline, weekly during the 6-week treatment period and at the 4- and 14-month follow-ups. Each item is scored on an ordinal scale ranging from 0 to 100, with higher scores representing better functional performance. Responses on each item were dichotomised according to whether the participant had scored ≤ 50 or > 50. 30,31 For the eating in public item, a separate category was used to code participants who were inpatients.
The timed water swallow test (WST)32 was used to measure changes in swallow function. The WST provides an indication of overall swallowing performance. Participants were asked to drink 100 ml of water as they were timed and the number of swallows taken was recorded. Three measures of swallowing performance were computed: capacity (i.e. total volume swallowed divided by total time taken in seconds), volume (i.e. total volume swallowed divided by total number of swallows) and speed (i.e. total time taken divided by total number of swallows). If the participant displayed overt signs of significant aspiration or became distressed, the test was halted and the remaining amount of water in the cup was recorded. Participants scored 0 on all three WST outcomes if they had severe dysphagia or odynophagia and were nil by mouth. The WST was collected at baseline, 6 weeks and at the 4- and 14-month follow-ups.
Pain outcomes included the use of analgesics and topical treatments in mouthwashing assistance visits to an oral hygienist, as well as scores on the pain domains of the EQ-5D-5L and the OMWQ-HN.
The EQ-5D-5L ‘pain/discomfort’ item is a measure of patients’ self-reported pain/discomfort and is rated along an ordinal scale from 1 (no problems) to 5 (extreme pain or discomfort). 33
Data on the use of analgesics were categorised as no analgesia, anti-inflammatory analgesic/paracetamol (e.g. ibuprofen), opioids (e.g. morphine) and others. Data on the use of mouthwash were categorised as no mouthwash, simple mouthwash (e.g. saline), analgesic mouthwash [e.g. benzydamine hydrochloride (Difflam; Mylan UK Healthcare Ltd)], antiseptic mouthwash (e.g. chlorhexidine), mucosa-protecting mouthwash [e.g. oral mucoadhesive (Mugard, Norgine UK Ltd)] and others. Data were collected at baseline and weekly during the 6-week treatment period.
Weight and body mass index (BMI) changes from baseline were recorded on a weekly basis during treatment and at the 4- and 14-month follow-ups.
Participants’ oral intake as a proportion of normal (pre illness) and dependence on a feeding tube was recorded weekly during the 6-week treatment period and at the 4-month and 14-month follow-up visits.
Adverse events (AEs) attributed to LLLT and clinical complications included the number of days as an inpatient, the number of hospital admissions and the number of interruptions in CRT treatment.
Data on disease recurrence and persistence of disease were recorded at 14 months.
Safety
Adverse effects were recorded from day 1 of LLLT to the 12-week follow-up visit, serious adverse events (SAEs) were reported up to the last trial visit (at either 4 or 14 months) and any serious adverse reactions (SARs) were reported until trial closure. All events were graded according to severity (i.e. mild, moderate, severe or life-threatening) and their relationship to LLLT assessed (i.e. unrelated, unlikely, possible, probable or definite). Full guidance on AE and SAE reporting was provided in the protocol (see www.journalslibrary.nihr.ac.uk/programmes/hta/1557160/#/).
It was expected that most of the AEs that occurred during the trial would be related to the CRT that participants were receiving rather than the LLLT. However, it was anticipated that participants may experience the following AEs after receiving LLLT:
-
nausea
-
dizziness
-
increase in OM symptoms within 24 hours of receiving laser therapy
-
decrease in OM symptoms within 24 hours of receiving laser therapy
-
tingling sensation in their mouth
-
feeling of warmth in their mouth.
In the unlikely event that a participant experienced persistent or severe reaction to LLLT, staff were instructed to discontinue the intervention immediately.
Participant timeline
Screening and recruitment
Each site held weekly HNC MDT meetings at which the MDT decided whether or not to recommend (C)RT treatment to a patient. Research team members were embedded in the MDT and identified participants who would potentially be eligible following treatment recommendations. Staff at each site screened these potential participants against the eligibility criteria. Potentially eligible participants were then approached at one of their routine appointments prior to starting (C)RT and given a participant information sheet (PIS) (see Report Supplementary Material 3) to read and consider in their own time. In addition, a video-recording of a role-play of the consent discussion between a clinician and a patient was accessible for patients at sites, as well as on the trial website (www.liteform.org.uk; accessed 23 May 2019). The PIS was also available on this website.
Sites kept logs of screening activity, including the number of patients who were screened for eligibility and given PISs.
Consent procedure
Patients were reapproached about the trial at one of their subsequent standard hospital visits prior to attending for their planned (C)RT. A minimum of 48 hours was required to have elapsed since receipt of the PISs.
Informed consent discussions were undertaken by an appropriate member of site staff (as named on the delegation log) and patients were asked for verbal consent to audio-record these discussions. Patients were encouraged to discuss the PIS with an appropriate member of site staff and were given the opportunity to ask any questions.
Those indicating that they wished to participate gave written informed consent by signing and dating the trial consent form (see Report Supplementary Material 4), which was witnessed and dated by a delegated member of the local research team. Completed consent forms and eligibility checklists were sent securely to the Newcastle Clinical Trials Unit (NCTU) to be checked for accuracy and completeness prior to randomisation. Participants who declined to take part in the trial were given the option to providing a reason and this was recorded.
Schedule of events
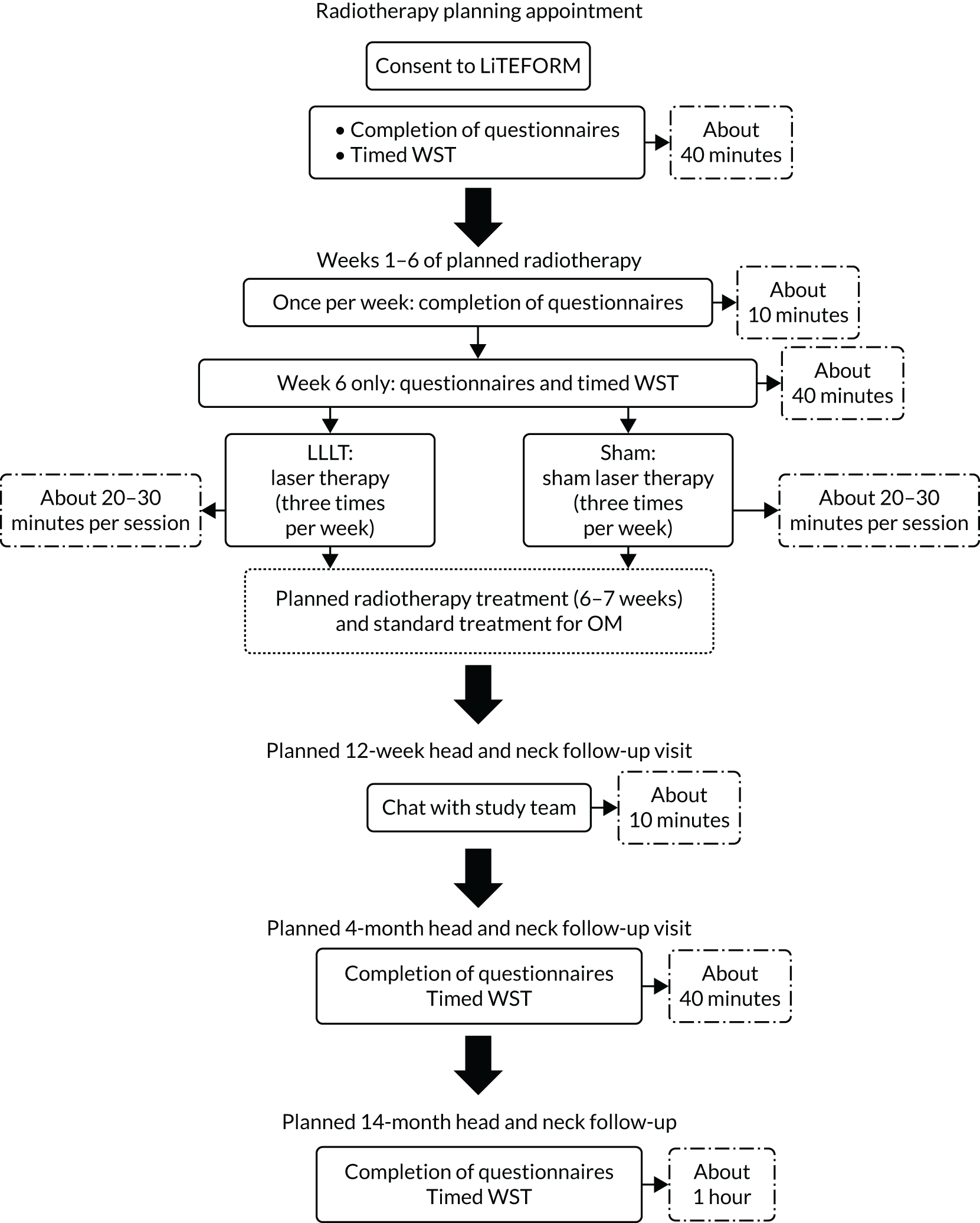
Figure 6 and Table 2 detail the participant flow and schedule of events, respectively. Baseline assessments were performed after consent at a standard care (C)RT planning appointment (and could be split across several appointments if needed), but always before the first day of LLLT treatment. During weeks 1–6 of the participant’s scheduled (C)RT, assessments were conducted weekly. These assessments typically took place on the same weekday as the first day of LLLT and sites were instructed to perform these assessments before LLLT was given that day. At 6 weeks, when the primary outcome was collected, further questionnaires and assessments took place.
FIGURE 6.
Trial flow chart showing the planned progress of participants.
| Event | Time point | ||||||
|---|---|---|---|---|---|---|---|
| Pre screening | Screening and planning (baseline) | 1–5 weeks | 6 weeks | 12 weeks | 4 months | 14 monthsa | |
| Patient given PIS | ✓ | ||||||
| Informed consent obtained | ✓ | ||||||
| Eligibility confirmed | ✓ | ||||||
| Demographic information/medical history recorded | ✓ | ||||||
| Randomisation | ✓ | ||||||
| MDADI administered | ✓ | ✓ | ✓ | ✓ | |||
| EORTC QLQ-C30 administered | ✓ | ✓ | ✓ | ✓ | |||
| EORTC QLQ-H&N35 administered | ✓ | ✓ | ✓ | ✓ | |||
| EQ-5D-5L administered | ✓ | ✓ | ✓ | ✓ | |||
| OMWQ-HN administered | ✓ | ✓ | ✓ | ✓ | |||
| PSS-HN administered | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Weight/BMI recorded | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Use of analgesics/topical treatments recorded | ✓ | ✓ | ✓ | ||||
| WHO Oral Mucositis Grading Scale administered | ✓ | ✓ | ✓ | ✓ | |||
| Intraoral photograph taken | ✓ | ||||||
| Hospitalisation details recorded | ✓ | ✓ | |||||
| Timed WST carried out | ✓ | ✓ | ✓ | ✓ | |||
| (C)RT administered | ✓ | ✓ | |||||
| LLLT/LLLT sham administered | ✓ | ✓ | |||||
| Clinical outcomes recorded | ✓ | ✓ | ✓ | ||||
| Health Care Utilisation Questionnaire administered | ✓ | ✓ | |||||
| Time and Travel Questionnaire administered | ✓ | ||||||
| AEs assessed and concomitant medications recorded | ✓ | ✓ | ✓ | ||||
Subsequent follow-up visits were designed to align with participants’ standard care visits. No assessments were performed at the week 12 visit, but AEs were recorded and concomitant medications were checked and recorded. At the 4- and 14-month follow-up visits, questionnaires and other assessments were repeated. Clinical outcomes regarding recurrence and disease progression were also recorded at the 14-month visit. In protocol V4.0 (amendment 3) (see Appendix 1, Table 28, and the project web page www.journalslibrary.nihr.ac.uk/programmes/hta/1557160/#/), the planned follow-up schedule was modified to reflect 14-month data being collected only for participants who commenced laser therapy prior to 6 July 2018 (see Appendix 1, Table 28).
Withdrawal of participants
Participants had the right to withdraw from the trial at any time without having to give a reason. The principal investigator could also discontinue an individual’s participation in the trial if this was considered to be in the patient’s best interest. Participants who withdrew consent for further follow-up were included in the analysis up to the date of withdrawal. Reasons for withdrawal were recorded, where available.
Randomisation
Randomisation was performed by computer allocation via the NCTU secure web-based randomisation service. Participants were randomised to receive standard care plus LLLT or standard care plus sham LLLT on a 1 : 1 basis. The method of random permuted blocks was used with block sizes of two, four and six. Randomisation was stratified by planned treatment [radiotherapy alone or (C)RT] and radiotherapy field (unilateral or bilateral). Randomisation was not stratified by site.
Laser device allocation concealment
A sham adaptation switch box was used to conceal participants’ allocation to the LLLT or sham arm. This was a small box situated between the main laser unit and the attached probe (Figure 7). The two dials outside the switch box (labelled ‘tens’ and ‘units’) were used to select a participant-specific machine number (in the range 1–99). This controlled the delivery of either sham therapy or LLLT as required. For example, for machine number 14, the operator would turn the tens dial to 1 and the units dial to 4.
FIGURE 7.
Sham adaption for laser machine (reproduced with permission from THOR Photomedicine Ltd, Chesham, UK; 2021, personal communication).
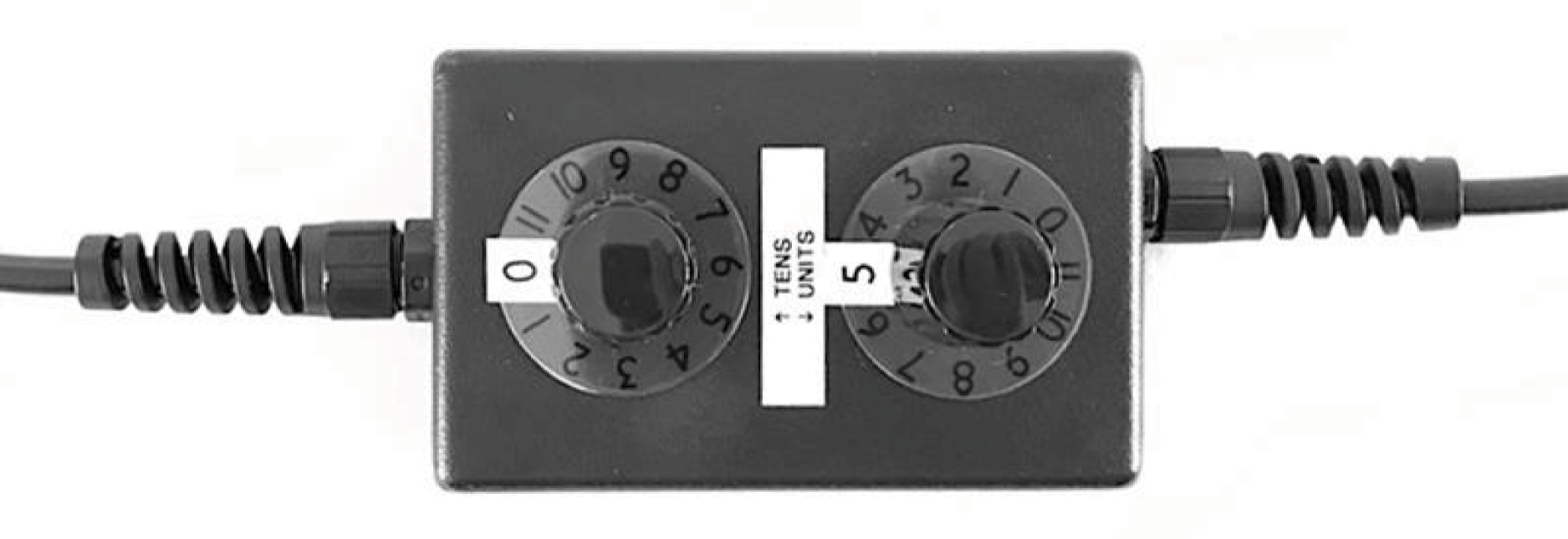
Internally, the switch box contained a circuit board laid out in a grid pattern. The rows in the grid correspond to the tens dial on the switch box and the columns to the units dial. There were two possible grids that could be used within the switch box (grid 1 or 2), with each grid containing a different set of randomly assigned on/off positions (see Appendix 1, Figure 17). The switch boxes were preset to an agreed grid by the manufacturer before delivery and the sites did not have access to the internal workings of the switch box.
Following randomisation, the secure web-based system generated an e-mail to staff at the site, notifying them of the participant’s allocated machine number. Within the randomisation system, the machine number was generated in an analogous way to a kit number or bottle number used in double-blind drug trials. Machine numbers were allocated uniquely within each site. This system ensured that, in the unlikely event of a participant being unblinded, staff would not become aware of the allocation of any other participants at their site.
Serious breach affecting treatment delivered
Following routine laser testing, it became apparent that the laser machine was not giving the expected output (i.e. LLLT or sham) for certain machine numbers (see Chapter 2, Laser device allocation concealment). This was further investigated by unblinded trial personnel, including a NCTU monitor who was not part of the trial team. It was found that laser machines at all sites had been consistently set up to use the incorrect grid [grid 2 was being used rather than grid 1 as pre-agreed at the time of randomisation set-up (see Appendix 1, Figure 17)]. At this point, 20 participants had commenced laser therapy (involving five sites in total). Of these 20 participants, seven had received treatment that was not what they should have received according to the trial randomisation schedule. It was agreed by the Trial Management Group (TMG) that the trial randomisation schedule should be updated to match the output grid that the laser machines had been set up with by the manufacturer (i.e. grid 2). The randomly assigned allocations for the first 20 participants were left unchanged in the randomisation log and the seven affected participants, three of whom had received LLLT and four of whom had received sham therapy, continued with the treatment that they had started receiving. 34 Although the incorrect grid had been used to programme the laser machine for 20 participants, their unique machine number was generated randomly from the randomisation schedule and, therefore, the allocation procedure was still random and unbiased.
Blinding
The laser safety glasses supplied to staff delivering LLLT were designed by the manufacturer to block the red light coming from the probe. This prevented staff from seeing if the machine was delivering the sham output or the active LLLT. Staff were instructed to wear the laser safety glasses before switching the laser on and not to remove them until the laser was switched off. This was primarily to keep staff safe, but also to reduce the risk of any accidental unblinding.
Participants were also instructed to wear laser safety glasses that emitted a pulsing red light inside the rims of laser safety glasses (Figure 8). The pulses emitted red light in time with the pulsing light from the laser machine. If a red light is shone on the roof of the mouth, it is possible to see/experience a faint red light travelling through the hard palate when the eyes are closed. This pulsing red light was designed to help maintain the trial blinding and stop the participant from knowing if they were receiving the sham output or the active LLLT.
FIGURE 8.
Laser safety glasses (reproduced with permission from THOR Photomedicine Ltd, Chesham, UK; 2021, personal communication).

Additional measures were taken to protect trial blinding, including the incorporation of additional resistors in the head of the sham LLLT probe to create warmth as if it was delivering the LLLT.
Trial management and oversight
Trial Management Group
The NCTU managed the trial on behalf of the trial sponsor. NCTU responsibilities included trial set-up, obtaining regulatory approvals, facilitating and performing site training, monitoring (on and off site), amendments, regular contact with site teams, maintenance of the central trial master file and trial close down.
The TMG was responsible for overseeing the day-to-day management of the trial and comprised the chief investigator, co-investigators, statisticians, health economists, qualitative researchers, a patient representative and the NCTU trial management team [i.e. trial manager, senior trial manager, clinical trial administrator and data(base) manager]. The TMG met approximately every month throughout the trial to ensure adherence to the trial protocol and monitor the conduct and progress of the trial.
Oversight committees
A Trial Steering Committee (TSC) was established to provide oversight of the trial on behalf of the funder. The TSC consisted of an independent clinical chairperson, an independent clinician, an independent statistician and a layperson. The TSC met four times throughout the trial and members were in regular contact through e-mail and teleconference when required.
An independent Data Monitoring Committee (DMC) was formed with the purpose of monitoring efficacy and safety end points. The DMC consisted of an independent clinical chairperson, two further independent clinicians and an independent statistician. DMC meetings were scheduled to take place prior to TSC meetings and the DMC made recommendations to the TSC regarding the continuation of the trial.
Statistical methods
Sample size
The sample size calculation assumed a group mean difference of four points in the OMWQ-HN, reflecting a meaningful treatment effect, and at 6 weeks a standard deviation (SD) of the OMWQ-HN of 10.7 points. 20 The trial was powered with a 5% alpha and 90% power. The original sample size calculation required 152 participants with primary outcome data in each treatment arm. This was inflated to 190 patients recruited in each arm (380 in total) to account for a maximum of 20% loss to follow-up or missing data.
Internal nine-month pilot phase
The LiTEFORM trial included a 9-month pilot phase with up to seven sites planned to open to recruitment during this period. A further three sites had been planned to open during the full RCT (taking the total number of sites to 10). Meetings of the DMC and TSC were held at the end of the pilot phase to review recruitment, any barriers, participant safety and data collection to date. These committees made recommendations as to whether or not the trial should continue and were guided by the following progression criteria specified in the protocol:
-
Site set-up to be complete for four pilot sites by 4 months post funding contractual start date (month 1), including the training of a minimum of two nurses or delegated staff to deliver LLLT in each site to a competent level to ensure that there are no gaps as a result of, for example, annual leave. The second phase of an additional three sites to be set up by month 6.
-
The first four pilot sites recruiting, on average, 1.5 participants per month for the first 4 months post funding.
-
The first four sites recruiting at full rate, on average, two participants per month from months 5–9 post funding.
-
The additional three sites recruiting, on average, 1.5 participants per month during months 3 and 4.
-
The additional three sites recruiting, on average, two participants per month during months 5–9.
-
Completion of the OMWQ-HN at 6 weeks in at least 80% of randomised participants.
-
A minimum of 100 participants recruited and randomised by the end of the 9-month pilot phase.
Data handling
Data were entered by sites on to the MACRO (Elsevier BV, Amsterdam, The Netherlands) database and were checked throughout the recruitment period to ensure that the eCRFs were as complete and accurate as possible. There were two types of validation to ensure data integrity: manual and electronic. The following types of checks were performed: range checks, consistency checks, protocol checks and accuracy checks. All issues arising from the checks were queried with site staff. All changes to the data were documented in the audit trail, including details of who made the change, when the change was made and why the change was made, to prove data integrity.
Essential data will be retained for a period of at least 5 years following close of the trial, in line with sponsor policy and the latest European Directive on good clinical practice (GCP) (2005/28/EC). 35 Data were handled, digitalised and stored in accordance with the Data Protection Act 199836 and the Data Protection Act 2018. 37 This was in accordance with General Data Protection Regulations (GDPR). For such purposes, the sponsor will act as the data controller for this study and NCTU as the data processor.
Statistical analysis plan
A complete statistical analysis plan (SAP), which provides full details of all statistical analyses, variables and outcomes, was finalised and signed before the final database lock and analysis (see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/1557160) (see Report Supplementary Material 5).
Owing to the trial under recruiting, the statistical analyses performed were descriptive in nature and no formal statistical testing between arms was carried out. Analyses followed the intention-to-treat (ITT) principle, including all participants randomised into the trial, regardless of their adherence to the entry criteria, subsequent discontinuation of laser therapy or deviation from the protocol. The ITT analysis set was modified (mITT) to allow the seven participants who randomly received treatment that was not what they should have received, according to the original randomisation schedule, to be included in the treatment arm corresponding to the treatment they received (see Serious breach affecting treatment delivered).
Statistical analyses were conducted on complete cases from the mITT analysis set, that is participants were included in the analyses if they attended the visit of interest and had evaluable data for the outcome measure of interest. Evaluable data are non-missing for the outcome measures listed in Outcome measurements, except for the three questionnaires MDADI, EORTC QLQ-C30 and EORTC QLQ-H&N35, for which simple imputation for missing questionnaire items in accordance with the questionnaire’s scoring manual was used (see Secondary outcome measures).
A Consolidated Standards of Reporting Trials (CONSORT) flow diagram was drafted to describe participant flow and retention throughout the trial (see Figure 10). Participants who discontinued with LLLT and participants who withdrew from the trial were presented in a line listing. Descriptive statistics were used to summarise participant follow-up, compliance with the LLLT schedule and baseline characteristics.
Primary outcome
The primary outcome was the OMWQ-HN total score at 6 weeks post start of LLLT. The OMWQ-HN total score was computed for all participants except those with missing data on more than one item, with the exception of participants who scored 0 (no soreness) on question 1 (who would not then proceed to the remaining questions and would be given a total score of 0). 21
Primary analysis of the primary outcome
Summary statistics for the OMWQ-HN total score at 6 weeks were reported by arm, along with the mean difference between arms and associated 95% confidence interval (CI). This analysis did not adjust for a participant’s OMWQ-HN total score at baseline, as the baseline score was collected before the start of (C)RT treatment and mucositis is a side effect of (C)RT treatment.
Additional summary statistics were reported for the OMWQ-HN at baseline, during weeks 1–6 of CRT and at the 4-month follow-up visit. Participants’ individual scores are plotted over the course of treatment. Changes in the OMWQ-HN total score from baseline to 6 weeks were also shown graphically (see Figure 14).
Planned subgroup analyses
The OMWQ-HN total score at 6 weeks was summarised descriptively within each level of trial stratification subgroup: (1) planned treatment (radiotherapy alone or CRT) and (2) unilateral or bilateral radiotherapy fields for those participants included in the primary analysis.
Secondary outcomes
The descriptive summaries for secondary outcomes, as presented in Chapter 3, Results, primarily focused on assessments undertaken at baseline, 6 weeks and 4 months, with further descriptive summaries included in Appendix 2.
For the WHO Oral Mucositis Grading Scale score, the number and percentage of participants with each grade were presented and the difference in proportion of participants reporting grades III or IV, indicating severe or life-threatening mucositis, was reported with associated 95% CIs.
Health-related QoL questionnaires (i.e. MDADI, EORTC QLQ-C30 and EORTC QLQ-H&N35) were scored according to their manuals and any missing data were handled as recommended. Outcomes were summarised descriptively as frequencies (and percentages) or means/medians [and SDs/interquartile ranges (IQRs)]. The difference between treatment arm means at 6 weeks and treatment arm means at 4 months for these measures were reported and presented graphically (see Figure 12).
Adverse events were coded using the Medical Dictionary for Regulatory Activities terminology (MedDRA®) and the preferred term was used for reporting. The number of AEs per participant and worst grade per participant were summarised descriptively by allocated treatment arm.
The number of participants reporting each AE was tabulated (for AEs occurring in at least 5% of participants in either treatment arm). SAEs were presented as a line listing that included the allocated treatment arm. All non-serious adverse reactions (AEs that were possibly, probably or definitely related to LLLT) that occurred were tabulated according to the allocated treatment arm.
Patient and public involvement
The patient perspective was central to the trial design and implementation, and will be important for the dissemination. A patient representative (Mrs Valerie Bryant) was actively involved in the LiTEFORM trial since the project idea was developed; she was a valued member and regular attendee of TMG meetings. As an author of this report, Mrs Valerie Bryant reviewed its content and continues to provide guidance on the dissemination of findings to lay audiences.
A PPI group, which was led by Mrs Valerie Bryant, was established at the outset. The group met throughout the trial and their views have been represented at the TMG meetings. The group members changed throughout the trial and included individuals with experience of HNC treatment at various post-treatment time points and some with previous experience of LLLT.
In planning this research, the PPI group identified OM as the worst part of receiving (C)RT, and anything that might ease this and prevent the eating and drinking problems was deemed a top priority. Subsequently, group members provided input, opinion and guidance for the trial. Examples of this include the selection of the PROM (primary outcome); trial launch event presentation; and revisions of the content and language used in the PIS, health economic questionnaires and the end-of-study information sheets (see Report Supplementary Material 6 and 7). The PPI group has advised on trial processes, including initial patient approach and informing participants about which treatment arm they had been allocated to after the trial had ended.
As part of dissemination, Mrs Valerie Bryant and a LiTEFORM trial participant planned and produced a video that captured the patient experience of the trial, once recruitment had closed. This video will be used for multiple purposes and audiences, with around 20 minutes of footage available to tailor use for each purpose.
Definition of the end of trial
The last participant trial visit was either the 4-month follow-up visit or the 14-month follow-up visit. The end of the study was defined as the database lock. Recruitment ended at the time planned; however, the study did not reach the recruitment target. Fourteen-month data were collected only for participants who commenced their laser therapy prior to 6 July 2018. Those participants affected by this shorter follow-up were provided information through an end-of-study information sheet (see Report Supplementary Material 6 and 7).
Chapter 3 Results
Introduction
Following the decision by the funder that the LiTEFORM trial would close before reaching its original recruitment target, it was agreed with the funder that recruitment would cease and follow-up would end when the last participant to enter the trial was scheduled to have reached their 4-month visit (the revised ‘last patient trial visit’). Fourteen-month visit data were collected only for participants who had been in the trial long enough at the time of the revised ‘last patient trial visit’. With only 87 participants randomised, not all of the statistical analyses proposed in the protocol were appropriate. In particular, formal comparative analyses of effectiveness were not performed and analyses were descriptive (see Chapter 2, Statistical analysis plan). Summaries of baseline, 6-week and 4-month visit data are presented in this chapter. The 14-month visit data are presented in Appendix 2 owing to the small numbers of participants followed up at that time point.
Recruitment
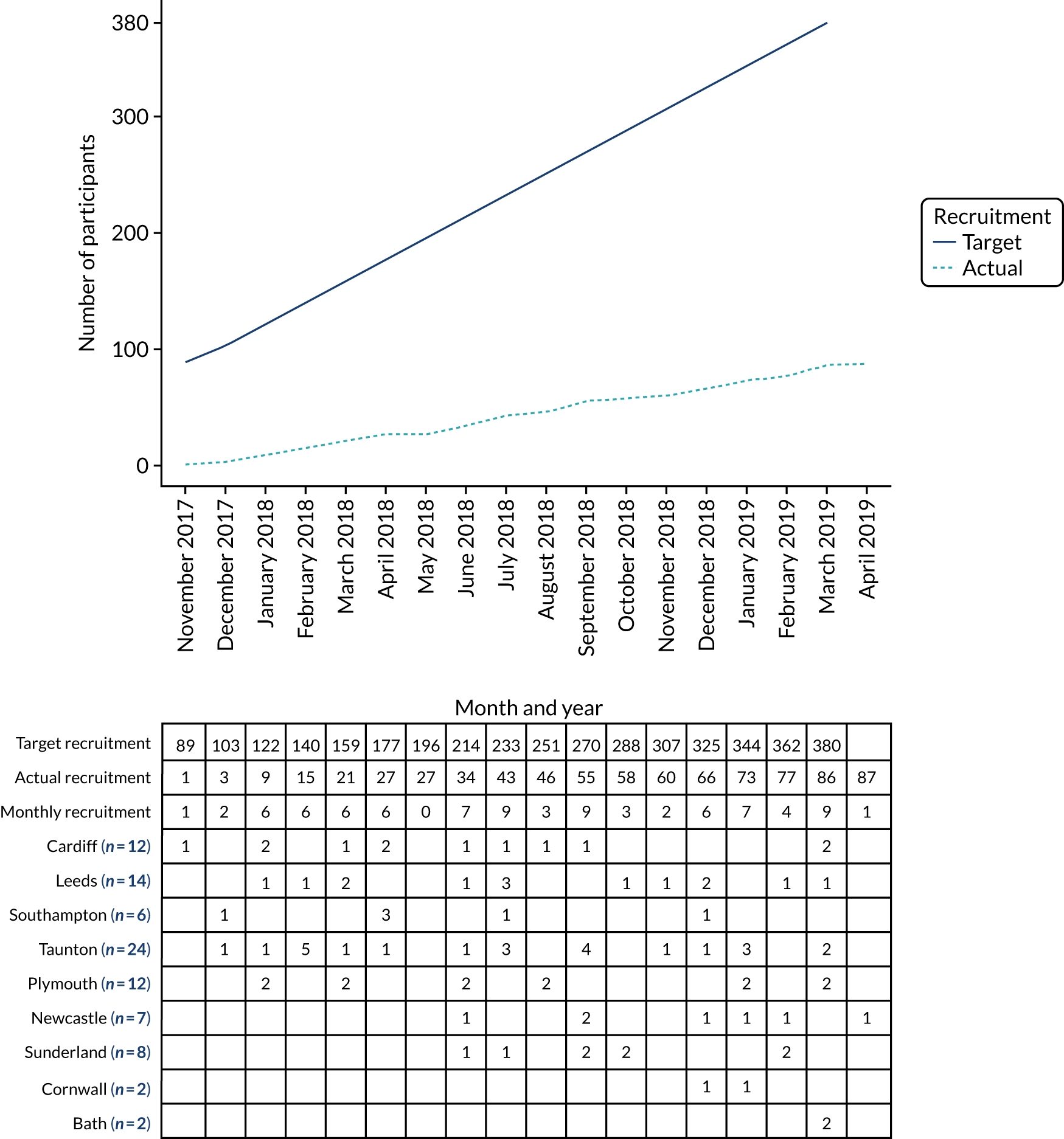
The trial was conducted in nine regional HNC sites in England, Scotland and Wales (see Chapter 2, Setting). Recruitment was expected to take place over 24 months, starting in April 2017. Recruitment opened on 27 October 2017; the first participant was randomised on 29 November 2017 and the last on 1 April 2019. Eighty-seven participants (23% of the original target of 380) were randomised over this 18-month period. The number of randomised participants per site ranged from two to 24, with a median of eight participants. Figure 9 shows the original recruitment target, the actual recruitment and the recruitment by site. Sites are listed from top to bottom in order of their opening dates. Figures in parentheses represent the total number of participants recruited for that site.
FIGURE 9.
Cumulative number of participants randomised by month.
Internal nine-month pilot phase
The LiTEFORM trial included a 9-month internal pilot phase, with up to seven sites planned to open to recruitment during this period and 100 participants randomised. A further three sites were planned to open during the full RCT with an additional 280 participants randomised. At the end of the 9-month pilot phase, the trial was reviewed by the DMC, TSC and NIHR HTA programme. Although, at that stage, the trial had not met the required progression criteria (see Chapter 2, Statistical methods, Internal nine-month pilot phase), we were advised by the funder, following recommendations and support from the oversight committees, to continue with site set-up and recruitment at the open sites. Owing to the length of time required to set up new sites, site set-up needed to continue while the NIHR HTA programme considered a recovery proposal extending the duration of the trial. However, because of the slower than expected opening of sites and participant recruitment, the trial was closed before reaching the original recruitment target of 10 sites and 380 participants.
Participant journey
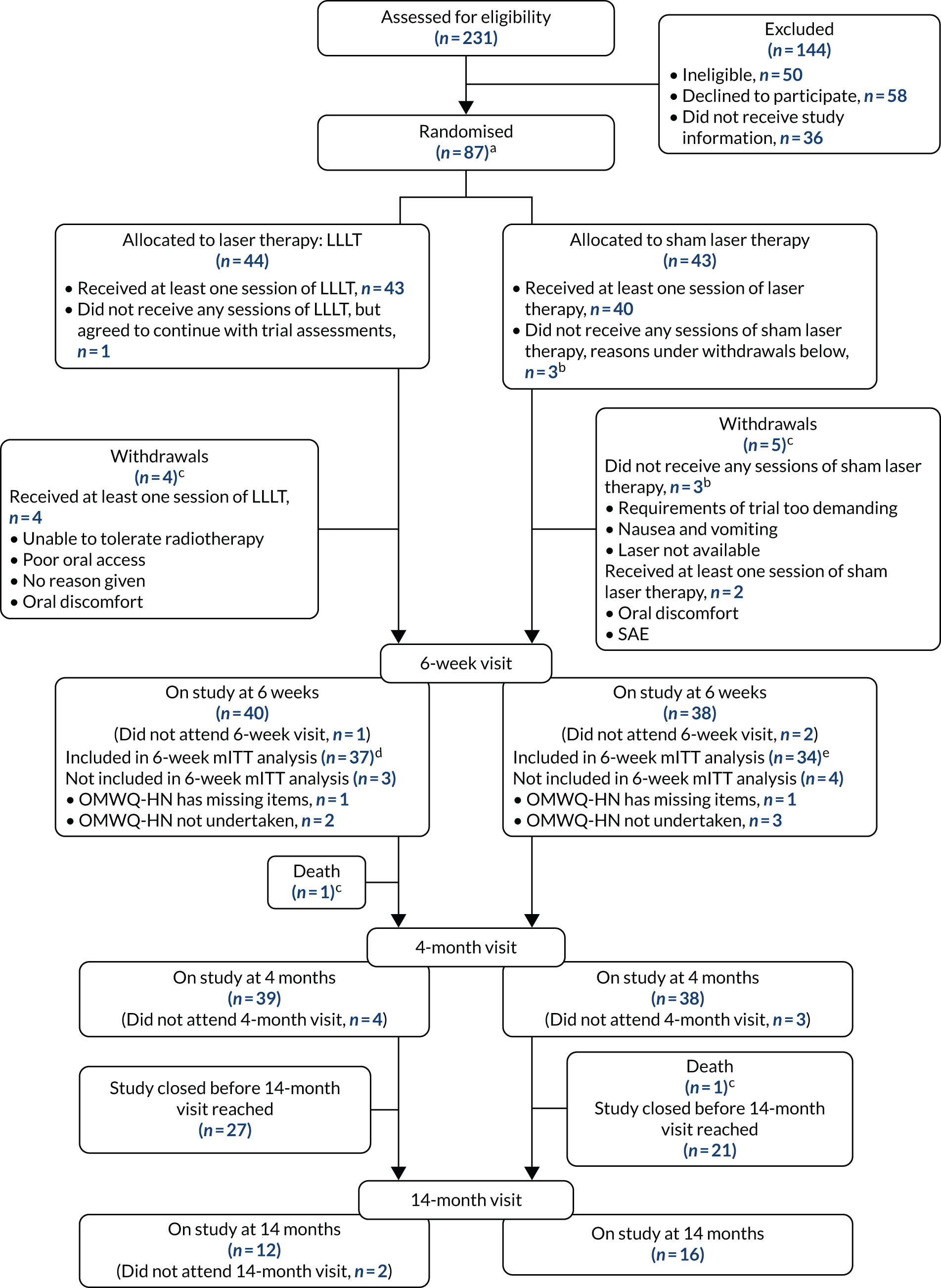
Patient/participant progress from the assessment for eligibility to the end of their LiTEFORM trial journey is shown in the CONSORT flow diagram in Figure 10.
FIGURE 10.
The CONSORT flow diagram of participant journey through the trial. a, Target recruitment was 380 participants; b, these are the same three participants (there were five withdrawals in the sham arm in total); c, see Table 4 for further details; d, OMWQ-HN primary analysis (n = 37) included six participants who had discontinued LLLT (see Table 8); and e, n = 34 OMWQ-HN primary analysis (n = 37) included nine participants who had discontinued sham laser therapy (see Table 8).
Screening and eligibility
A total of 231 patients were identified by trial sites and screened for eligibility. Of these participants, 50 out of 231 (22%) were deemed ineligible to take part by local research staff. When available, the main reasons given for the ineligibility of screened patients were as follows:
-
planned radiotherapy dose of < 60 Gy (n = 11)
-
non-NHS patient (n = 10)
-
OM and trismus prior to radiotherapy treatment (n = 7)
-
non-squamous cell carcinoma (n = 5)
-
participation in a competing trial (n = 5).
Of the 181 patients deemed eligible, 145 (80%) were given information about the trial and (20%) were not. When available, reasons for not having been given information about the trial were as follows:
-
patient was missed (n = 9)
-
there was no capacity at the site to take on new participants (n = 3)
-
patient did not want to commit to the additional time/burden (n = 2)
-
patient was participating in a competing trial (n = 2).
Of the 145 patients given information about the trial, 58 (40%) declined the offer to participate. The reasons given for non-participation (which were largely reported in free text) were as follows:
-
patient did not want to commit to the additional time/burden for the trial (n = 22)
-
patient felt that the trial was too much to consider after their cancer diagnosis (n = 6)
-
patient did not want to be randomised to receive sham (n = 3)
-
patient could not organise travel to accommodate the trial (n = 2)
-
staff were unable to contact the patient (n = 2).
Randomisation by arm
Following consent, 87 participants were randomised: 44 were allocated to the LLLT arm and 43 were allocated to the sham arm.
Numbers analysed
Statistical analyses were conducted on complete cases from the mITT analysis set (Table 3) (see Chapter 2, Statistical analysis plan).
| Analysis set | Treatment arm (n) | |
|---|---|---|
| LLLT | Sham | |
| ITT | 45 | 42 |
| mITT | 44 (including 41 from the LLLT ITT population) | 43 (including 39 from the sham ITT population) |
Withdrawals and deaths
All participants provided data at their baseline visit. Three participants in the sham arm withdrew from the trial before starting laser therapy (see Figure 10). During the 6-week treatment period, six further participants withdrew from the trial (LLLT arm, n = 4; sham arm, n = 2). The reasons and timings of withdrawals are given in Table 4. Two further participants died during the trial: one of metastatic squamous cell carcinoma (LLLT arm) and one of bilateral pulmonary thromboembolism (sham arm).
| Treatment arm | Reason | Last LLLT session received |
|---|---|---|
| LLLT | Unable to tolerate radiotherapy | 1 week (session 1) |
| LLLT | Poor oral access | 1 week (session 1) |
| LLLT | No reason given | 1 week (session 3) |
| LLLT | Oral discomfort | 3 weeks (session 2) |
| LLLT | Death (metastatic squamous cell carcinoma of the retromolar region) | 4 weeks (session 1) |
| Sham | Requirements of trial too demanding | No laser therapy received |
| Sham | Nausea and vomiting | No laser therapy received |
| Sham | Laser not available | No laser therapy received |
| Sham | Oral discomfort | 1 week (session 3) |
| Sham | SAE | 2 weeks (session 2) |
| Sham | Death (bilateral pulmonary thromboembolism) | 6 weeks (session 3) |
Participant follow-up visits
Participants were scheduled to return for their trial assessments at the following time points:
-
baseline (after consent but before day 1 of radiotherapy)
-
weeks 1–5
-
6 weeks
-
4 months after the final 6-week radiotherapy session (± 2 weeks)
-
14 months after the final 6-week radiotherapy session (± 2 weeks).
Only 28 participants (LLLT arm, n = 12; sham arm, n = 16) who started radiotherapy prior to 6 July 2018 had been in the study long enough to attend their 14-month trial visit.
Attendance at trial visits and compliance with trial visit windows
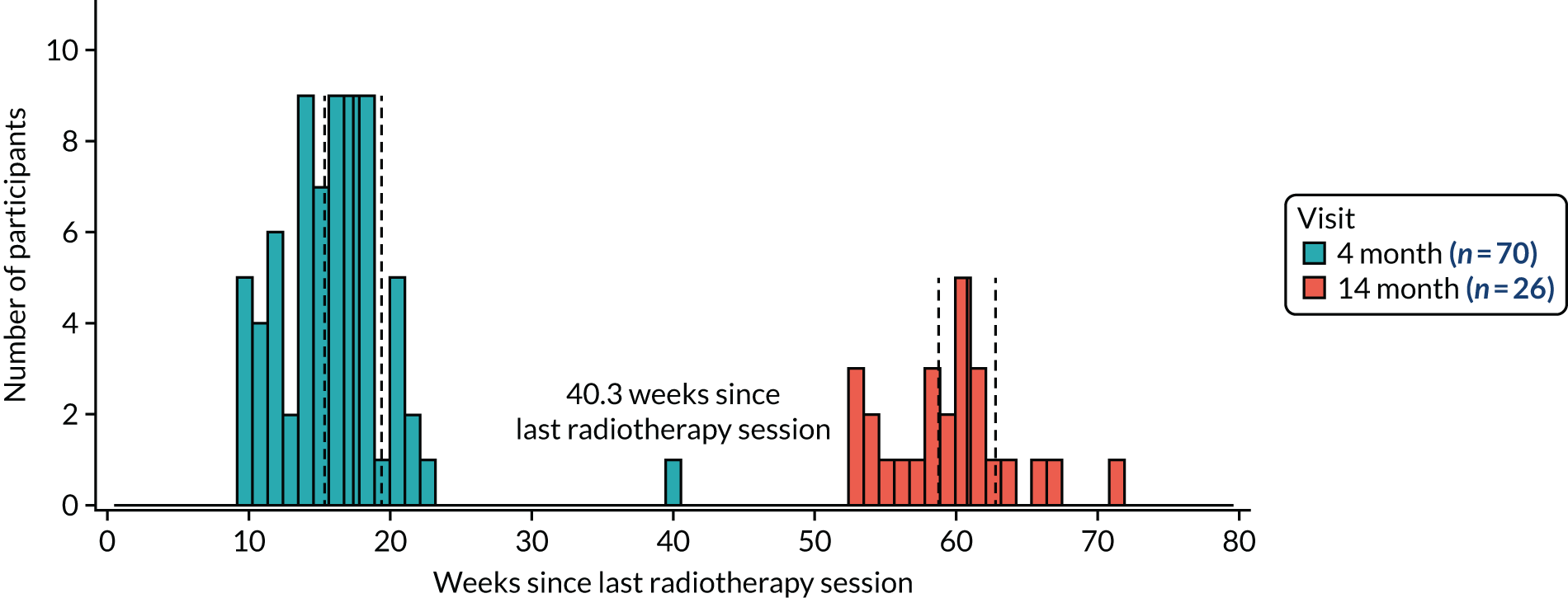
The attendance at trial visits for those participants still being followed up was high: 75 out of 78 (96%) at the 6-week visit and 70 out of 77 (91%) at the 4-month visit (Table 5). Nearly all participants (26/28, 83%) who had been in the trial long enough to have a 14-month visit scheduled attended. However, compliance with the ± 2-week trial visit windows at 4 and 14 months was poor: 28 out of 70 (40%) at 4 months and 14 out of 26 (54%) at 14 months (Figure 11). We suspect that the compliance with these trial visit windows reflects local follow-up protocols for cancer.
| Time point | Number attended/number on trial (% of randomised) | ||
|---|---|---|---|
| Treatment arm | Total (N = 87) | ||
| LLLT arm (N = 44) | Sham arm (N = 43) | ||
| 6 weeks | 39/40 (88.6) | 36/38 (83.7) | 75/78 (86.2) |
| 4 months | 35/39 (79.5) | 35/38 (81.4) | 70/77 (80.5) |
| 14 monthsa | 10/12 (22.7) | 16/16 (37.2) | 26/28 (29.9) |
FIGURE 11.
Compliance with the 4- and 14-month trial visit windows. The solid vertical lines are at 4 and 14 months since the last radiotherapy session; the dashed vertical lines are ± 2 weeks around these visit times. At 4 months, 28 out of 70 (40%) participants were within the 2-week visit window and at 14 months there were 14 out of 26 (54%) participants.
Baseline data
Baseline characteristics of the treatment arms were reported descriptively (Table 6), including the trial stratification factors, type of planned treatment (radiotherapy alone/CRT) and type of radiotherapy field (unilateral/bilateral). For categorical outcomes, it should be noted that with only 44 participants in the LLLT arm and 43 in the sham arm, one participant (in either arm) is equivalent to approximately 2.3%; therefore, an apparently ‘large’ difference of 9% between arms, for example, represents, on average, four participants more in one arm than the other.
| Characteristic | Treatment arm | Overall (N = 87) | |
|---|---|---|---|
| LLLT (N = 44) | Sham (N = 43) | ||
| Stratification factors (at randomisation), n (%) | |||
| Planned treatment | |||
| Radiotherapy alone | 12 (27.3) | 8 (18.6) | 20 (23.0) |
| Chemoradiotherapy | 32 (72.7) | 35 (81.4) | 67 (77.0) |
| Type of radiotherapy field | |||
| Unilateral neck | 9 (20.5) | 10 (23.3) | 19 (21.8) |
| Bilateral neck | 35 (79.5) | 33 (76.7) | 68 (78.2) |
| Clinical measurements | |||
| Age (years) | |||
| Mean (SD) | 59.1 (9.4) | 59.7 (8.2) | 59.4 (8.8) |
| Sex, n (%) | |||
| Male | 38 (86.4) | 31 (72.1) | 69 (79.3) |
| BMI (kg/m2)a | |||
| Mean (SD) | 26.7 (4.4) | 26.9 (5.6) | 26.8 (5) |
| Weight (kg) | |||
| Mean (SD) | 80.1 (14.8) | 80.4 (18.1) | 80.2 (16.4) |
| HNC information | |||
| Site of disease, n (%) | |||
| Nasopharynx | 1 (2.3) | 2 (4.7) | 3 (3.4) |
| Oropharynx: HPV positive | 24 (54.5) | 31 (72.1) | 55 (63.2) |
| Oropharynx: HPV negative | 8 (18.2) | 2 (4.7) | 10 (11.5) |
| Oropharynx: HPV undetermined | 1 (2.3) | 1 (2.3) | 2 (2.3) |
| Larynx | 3 (6.8) | 0 (0.0) | 3 (3.4) |
| Oral cavity | 6 (13.6) | 5 (11.6) | 11 (12.6) |
| Unknown primary | 1 (2.3) | 2 (4.7) | 3 (3.4) |
| TNM classification, n (%) | |||
| Primary tumour | |||
| T0 | 1 (2.3) | 2 (4.7) | 3 (3.4) |
| T1 | 9 (20.5) | 11 (25.6) | 20 (23.0) |
| T2 | 21 (47.7) | 14 (32.6) | 35 (40.2) |
| T3 | 5 (11.4) | 6 (14.0) | 11 (12.6) |
| T4 | 8 (18.2) | 10 (23.3) | 18 (20.7) |
| Regional lymph nodes | |||
| N0 | 7 (15.9) | 3 (7.0) | 10 (11.5) |
| N1 | 14 (31.8) | 11 (25.6) | 25 (28.7) |
| N2 | 23 (52.3) | 26 (60.5) | 49 (56.3) |
| N3 | 0 (0.0) | 3 (7.0) | 3 (3.4) |
| Distant metastasis | |||
| MX | 0 (0.0) | 2 (4.7) | 2 (2.30) |
| M0 | 44 (100.0) | 40 (93.0) | 84 (96.6) |
| M1 | 0 (0.0) | 1 (2.3) | 1 (1.1) |
| Radiotherapy and surgery details | |||
| Treatment received,b n (%) | |||
| Radiotherapy only | 10 (22.7) | 8 (18.6) | 18 (20.7) |
| Chemoradiotherapy | 33 (75.0) | 31 (72.1) | 64 (73.6) |
| Missing | 1 (2.3) | 4 (9.3) | 5 (5.7) |
| Surgery to primary tumour, n (%) | 15 (34.1) | 17 (39.5) | 32 (36.8) |
| Debulking | 1 | 0 | 1 |
| Transoral laser microsurgery | 2 | 1 | 3 |
| Transoral robotic surgery | 4 | 2 | 6 |
| Open surgery, no reconstruction | 1 | 3 | 4 |
| Open surgery with distant flap reconstruction | 2 | 2 | 4 |
| Neck dissection, unilateral/bilateral | 3 | 6 | 9 |
| Other | 2 | 3 | 5 |
| Comorbidity, n (%) | |||
| ACE-27 | |||
| Grade 0 (none) | 15 (34.1) | 20 (46.5) | 35 (40.2) |
| Grade 1 (mild) | 4 (9.1) | 4 (9.3) | 8 (9.2) |
| Grade 2 (moderate) | 6 (13.6) | 3 (7.0) | 9 (10.3) |
| Grade 3 (severe) | 1 (2.3) | 0 (0.0) | 1 (1.1) |
| Unknown | 7 (15.9) | 3 (7.0) | 10 (11.5) |
| Missing | 11 (25.0) | 13 (30.2) | 24 (27.6) |
| Health-related quality of life | |||
| MDADI, mean (SD) (higher score indicates better overall QoL) | |||
| Global score | 78.2 (26.2) | 81.4 (24.1) | 79.8 (25.1) |
| Composite score | 81.7 (16.7) | 83.9 (13.5) | 82.8 (15.1) |
| EORTC QLQ-C30 summary score38 (higher score indicates better functioning/lower symptoms) | |||
| Mean (SD) | 80.1 (15.8) | 81.3 (14.6) | 80.7 (15.2) |
| Eating and communication performance, n (%) | |||
| PSS-HN (lower scores reflect poorer performance status) | |||
| Normalcy of diet (score of ≤ 50) | 10 (22.7) | 6 (14.0) | 16 (18.4) |
| Public eating (score of ≤ 50) | 8 (18.2) | 2 (4.7) | 10 (11.5) |
| Understandability of speech (score of ≤ 50) | 2 (4.5) | 0 | 2 (2.3) |
| Swallowing | |||
| WST (volume, ml per swallow)c | |||
| Median (IQR) | 20 (14.3–25) | 20 (14.3–25) | 20 (14.3–25) |
| WST (capacity, ml per second)c | |||
| Median (IQR) | 12.5 (8.3–20) | 16.7 (9.1–20) | 14.3 (8.3–20) |
Baseline characteristics were approximately balanced across the arms with the exception of sex, for which descriptively (i.e. not in relation to the numerical values) there was a higher proportion of males in the LLLT arm (86%, 38/44), than in the sham arm (72%, 31/43). Although disease sites were balanced overall across the arms, for oropharynx there was some imbalance in the distribution of oropharynx-human papillomavirus (HPV)-positive and -negative cases.
Participants ranged in age from 40 to 76 years (mean 59.4 years, SD 8.8 years); this age distribution was similar to that in previously reported HNC LLLT studies. 39–42
Health-related quality-of-life scores were, descriptively, well balanced across arms. MDADI scores were in the region of those reported pre treatment from other HNC series (these scores, and EORTC QLQ-C30 scores, would typically be expected to decline during radiotherapy treatment). 43,44
Baseline characteristics are also reported for the subset of 71 participants who were included in the analysis of the primary outcome (see Appendix 2, Table 30).
Treatment received
Participants were scheduled to receive three sessions of LLLT or sham therapy per week during their 6 weeks of radiotherapy, giving a possible maximum of 18 sessions.
Laser therapy sessions
Table 7 presents a summary and a breakdown of the number of laser therapy sessions received by treatment arms for (1) all 87 randomised participants and (2) the 71 participants included in the analysis of the primary outcome. The lowest reported effective frequency of LLLT sessions for OM in HNC is two per week. 45 We, therefore, considered receiving at least two sessions of laser therapy per week for 6 weeks to be sufficient to deliver a therapeutic dose. Only 54% (47/87) of participants received at least two sessions of laser therapy per week for 6 weeks [with a slightly higher percentage, 63% (45/71), for the participants included in the analysis of the primary outcome].
| Events | All randomised participants | Participants in the primary analysis | ||||
|---|---|---|---|---|---|---|
| Treatment arm | Total (N = 87) | Treatment arm | Total (N = 71) | |||
| LLLT (N = 44) | Sham(N = 43) | LLLT (N = 37) | Sham (N = 34) | |||
| Median (IQR) number of sessions received | 17 (14.5–18) | 16 (7–17) | 16 (12–18) | 17 (16–18) | 17 (14–18) | 17 (15–18) |
| Received at least two sessions per week for 6 weeks, n (%) | 26 (59.1) | 21 (48.8) | 47 (54.0) | 25 (67.6) | 20 (58.8) | 45 (63.4) |
| Breakdown of number of sessions received, n (%) | ||||||
| 16–18 | 29 (65.9) | 22 (51.2) | 51 (58.6) | 28 (75.7) | 21 (61.8) | 49 (69.0) |
| 13–15 | 5 (11.4) | 8 (18.6) | 13 (14.9) | 4 (10.8) | 8 (23.5) | 12 (16.9) |
| 10–12 | 1 (2.3) | 2 (4.7) | 3 (3.4) | 1 (2.7) | 0 (0.0) | 1 (1.4) |
| 7–9 | 2 (4.5) | 1 (2.3) | 3 (3.4) | 1 (2.7) | 1 (2.9) | 2 (2.8) |
| 4–6 | 3 (6.8) | 3 (7.0) | 6 (6.9) | 3 (8.1) | 2 (5.9) | 5 (7.0) |
| 1–3 | 3 (6.8) | 4 (9.3) | 7 (8.0) | 0 (0.0) | 2 (5.9) | 2 (2.8) |
| 0 | 1 (2.3) | 3 (7.0) | 4 (4.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Discontinuation of treatment
Of the 87 randomised participants, 83 received at least one session of LLLT or sham laser therapy (see Table 7). Eighteen participants (LLLT arm, n = 6; sham arm, n = 12) discontinued treatment before completing their scheduled 18 sessions, but remained in the trial and continued with trial assessments (Table 8). The most frequently recorded reason for discontinuation was the participant becoming too unwell to tolerate the laser therapy sessions. Radiotherapy for HNC, as stated previously, is a very intense treatment with significant associated side effects. The reasons for withdrawal reflect a pattern of the side effects gradually building up. People who struggled with the laser treatment withdrew from the trial early, soon after starting laser treatment. As treatment continued, more participants then dropped out because of the side effects of their (C)RT.
| Treatment arm | Reason | Last session received | Participant 6-week OMWQ-HN data included in the primary analysis |
|---|---|---|---|
| LLLT | Unable to tolerate laser treatment | Week 2 (session 2) | Yes |
| LLLT | Oral discomfort | Week 2 (session 3) | Yes |
| LLLT | Lethargy | Week 3 (session 3) | Yes |
| LLLT | Radiotherapy treatment side effects | Week 4 (session 1) | Yes |
| LLLT | Radiotherapy treatment side effects | Week 5 (session 1) | Yes |
| LLLT | Laser fault | Week 5 (session 2) | Yes |
| Sham | Unable to tolerate flashing light | Week 1 (session 3) | Yes |
| Sham | Nausea and vomiting | Week 1 (session 3) | No |
| Sham | Radiotherapy treatment side effects | Week 2 (session 1) | Yes |
| Sham | Oral discomfort | Week 2 (session 1) | Yes |
| Sham | Radiotherapy treatment side effects | Week 2 (session 2) | Yes |
| Sham | Radiotherapy treatment side effects | Week 3 (session 3) | Yes |
| Sham | Patient choice | Week 4 (session 3) | No |
| Sham | Radiotherapy treatment side effects | Week 5 (session 1) | No |
| Sham | Radiotherapy treatment side effects | Week 5 (session 1) | Yes |
| Sham | Oral discomfort | Week 5 (session 1) | Yes |
| Sham | Nausea and vomiting | Week 5 (session 2) | Yes |
| Sham | Radiotherapy treatment side effects | Week 5 (session 3) | Yes |
Radiotherapy and chemotherapy received
Details of the therapy regimes for participants receiving radiotherapy and CRT are described in Tables 9 and 10. All participants receiving radiotherapy received intensity-modulated radiation therapy (IMRT).
| Events | Treatment arm, n (%) | |
|---|---|---|
| LLLT (N = 44) | Sham (N = 43) | |
| Aim of radiotherapy | ||
| Primary radical radiotherapy | 24 (55) | 31 (72) |
| Adjuvant radiotherapy | 18 (41) | 9 (21) |
| Missing | 2 (5) | 3 (7) |
| Radiotherapy received | ||
| Participants received all planned fractions | 40 (91) | 37 (86) |
| Participants missed at least one fraction | 1 (2) | 3 (7) |
| Reasons for missed fractions | ||
| Adverse event | 1 (2) | |
| Patient choice | 1 (2) | |
| Extreme weather conditions | 1 (2) | |
| Reason missing | 1 (2) | |
| Missing | 3 (7) | 3 (7) |
| Systemic treatment | Treatment arm, n (%) | |
|---|---|---|
| LLLT (N = 33) | Sham (N = 35) | |
| Cetuximab | 2 (6) | 1 (3) |
| Cisplatin | 27 (82) | 28 (80) |
| Carboplatin | 4 (12) | 2 (6) |
| Missing | 0 (0) | 4 (11) |
Primary outcome
The primary outcome was the OMWQ-HN score at 6 weeks post start of LLLT (see Chapter 2, Primary outcome). Responses to the OMWQ-HN were summed to give a total overall score between 0 and 54 points, with a higher score indicating poorer well-being and oral function.
Participants evaluable for the primary outcome
A total of 78 participants (LLLT arm, n = 40; sham arm, n = 38) remained in the trial at 6 weeks when the primary outcome was collected (see Figure 10). Of these participants, seven did not (fully) complete the OMWQ-HN and could not be included in the primary analysis (LLLT arm, n = 3; sham arm, n = 4). The primary analysis included 71 participants (LLLT arm, n = 37; sham arm, n = 34), that is 81% of the 87 randomised participants. The completion rate of the OMWQ-HN at each trial visit is presented in Appendix 2, Table 31.
Primary analysis
At 6 weeks, the OMWQ-HN total score in the LLLT arm ranged from 8 to 50 points (mean 33.2 points, SD 10.0 points) and in the sham arm ranged from 4 to 53 points (mean 27.4 points, SD 13.8 points) (Table 11 and Figure 12). The OMWQ-HN total score was, on average, 5.8 points higher (95% CI 0.1 to 11.5 points) in the LLLT arm than in the sham arm, with a higher score indicating poorer well-being and oral function. At the design stage of the trial, a minimal clinically important difference (MCID) between arm means was considered to be 4 points. The 95% CI provides the range of possible ‘true’ mean differences in the population of interest that are compatible with the trial data; it includes the prespecified MCID, but its lower limit is very close to a zero mean difference, so does not preclude the possibility of a very small mean difference between the arms. This analysis should be interpreted cautiously given the number of participants included and the role of chance variation.
| Treatment arm, OMWQ-HN score (points) at 6 weeksa | Mean score (points) differenceb (LLLT arm score minus sham arm score) | |
|---|---|---|
| LLLT (n = 37) | Sham (n = 34) | |
| 33.2 (10.0) | 27.4 (13.8) | 5.8 (0.1 to 11.5) |
FIGURE 12.
Distribution of OMWQ-HN total scores at 6 weeks by treatment arm (n = 71). a, Box plot of OMWQ-HN total scores at week 6 by treatment arm. The solid line represents the median and the dashed line represents the mean. Each black marker represents an individual participant. b, Histograms showing frequency of OMWQ-HN total scores at week 6 by treatment arm for LLLT (left) and sham (right).
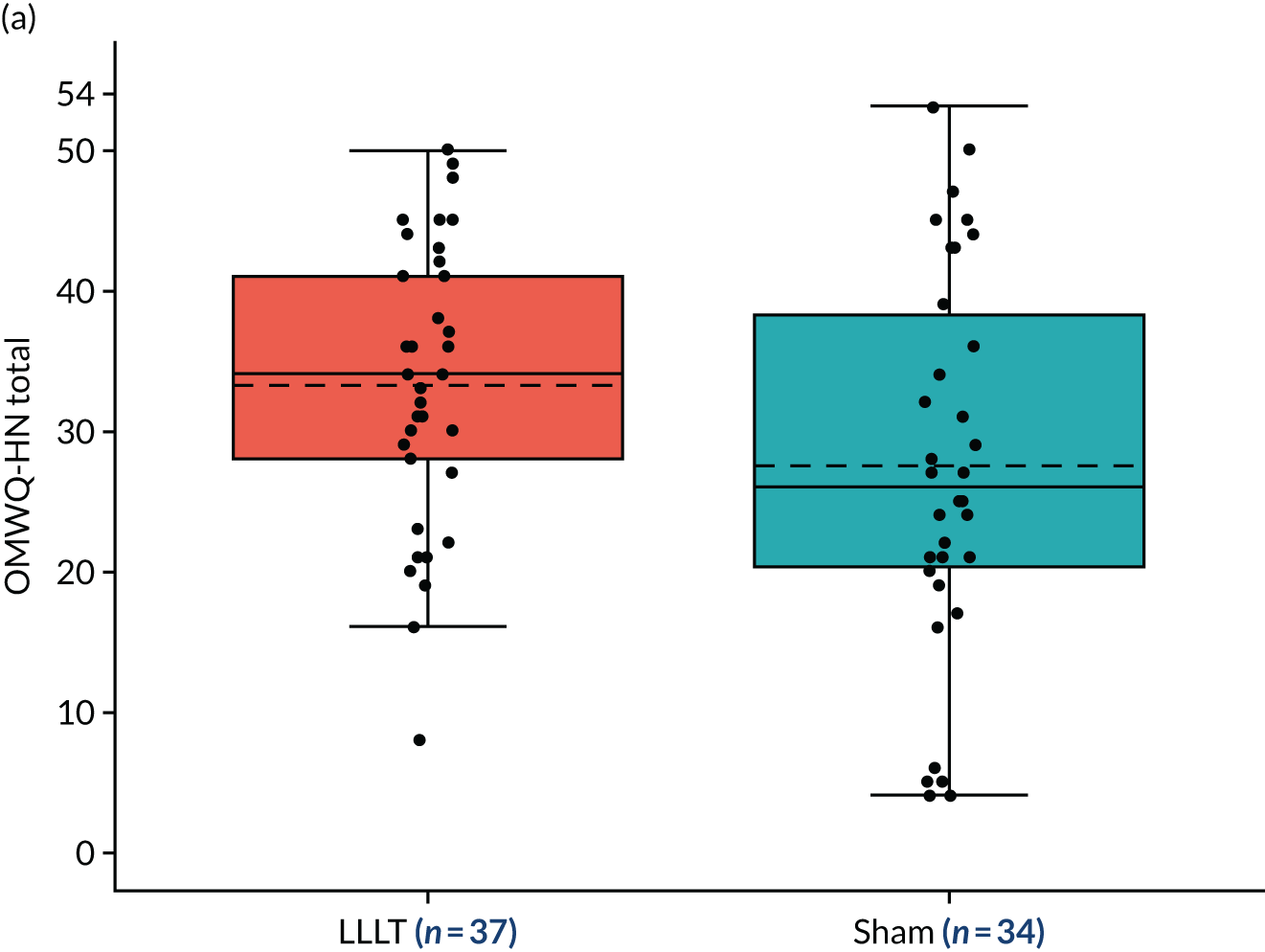
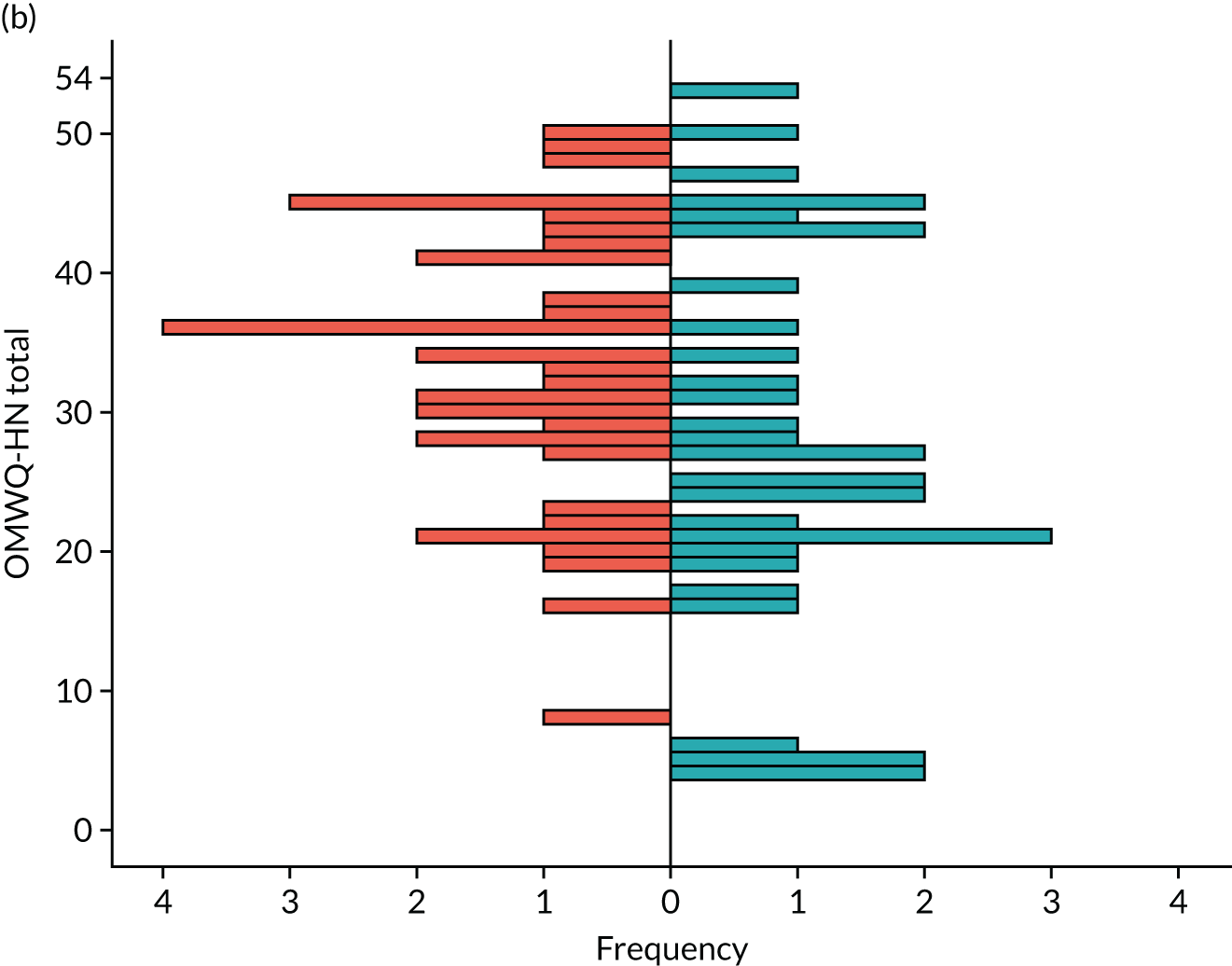
The summary statistics for the OMWQ-HN total score for each week are given in Table 12 and participants’ individual scores are plotted over the course of treatment in Figure 13. Changes in the OMWQ-HN total from baseline to 6 weeks are shown in Figure 14.
| Treatment arm | Time point | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 1 week | 2 weeks | 3 weeks | 4 weeks | 5 weeks | 6 weeks | 4 months | |
| LLLT | ||||||||
| Total, n | 44 | 44 | 41 | 41 | 40 | 40 | 40 | 39 |
| Available, n | 43 | 40 | 38 | 36 | 37 | 36 | 37 | 23 |
| OMWQ-HN total score (points) | ||||||||
| Minimum | 0 | 0 | 0 | 0 | 0 | 6 | 8 | 0 |
| Median (IQR) | 4 (0–13) | 0 (0–5) | 7 (0–14) | 16 (11–28.5) | 25 (19–36) | 27.5 (18.5–34.5) | 34 (28–41) | 18 (0–25) |
| Mean (SD) | 8.1 (10.1) | 4.7 (7.8) | 9.1 (10.1) | 19.2 (11.1) | 26.2 (11.2) | 27.2 (10.8) | 33.2 (10) | 14.6 (12.7) |
| Maximum | 35 | 30 | 39 | 47 | 46 | 51 | 50 | 36 |
| Sham | ||||||||
| Total, n | 43 | 40 | 39 | 38 | 38 | 38 | 38 | 38 |
| Available, n | 43 | 39 | 37 | 36 | 38 | 36 | 34 | 19 |
| OMWQ-HN total score (points) | ||||||||
| Minimum | 0 | 0 | 0 | 0 | 3 | 4 | 4 | 0 |
| Median (IQR) | 3 (0–7) | 0 (0–7) | 3 (0–7) | 15 (7.5–26) | 21 (14–32) | 28 (13.5–34) | 26 (20–39) | 7 (0–19) |
| Mean (SD) | 6.8 (10.6) | 5 (9.1) | 5.9 (8.3) | 17.8 (13.6) | 22.4 (13) | 25.6 (12.9) | 27.4 (13.8) | 10.7 (11.9) |
| Maximum | 38 | 40 | 35 | 54 | 54 | 54 | 53 | 38 |
FIGURE 13.
Individual change in OMWQ-HN total score across treatment arms. Participants in light blue were excluded from the primary analysis because of missing OMWQ-HN data at 6 weeks. Higher scores on the OMWQ-HN indicate poorer well-being and oral function.
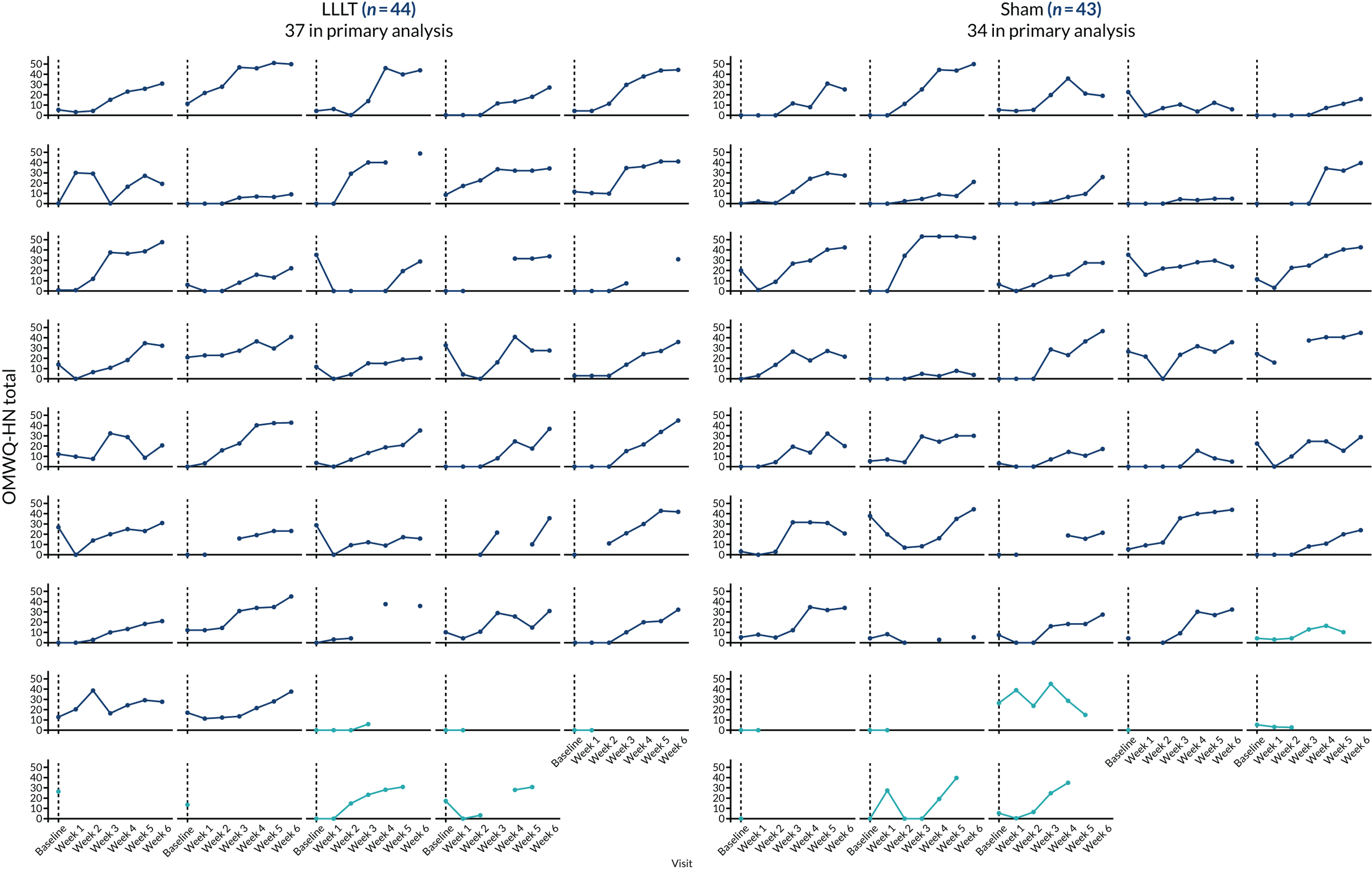
FIGURE 14.
The OMWQ-HN total score at baseline and 6 weeks. Higher scores on the OMWQ-HN indicate poorer well-being and oral function. One participant in the LLLT arm is not included because of missing data at baseline.
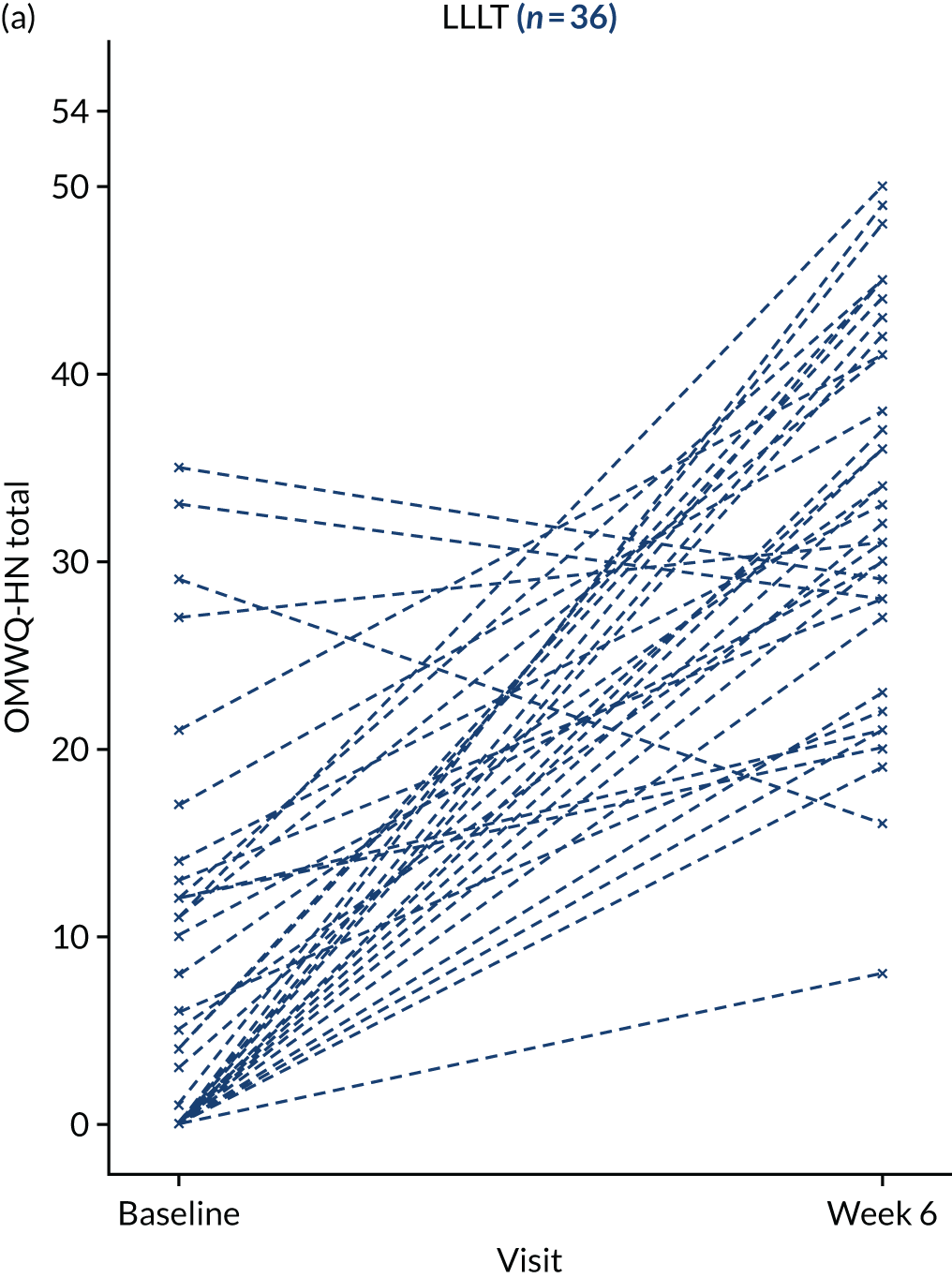
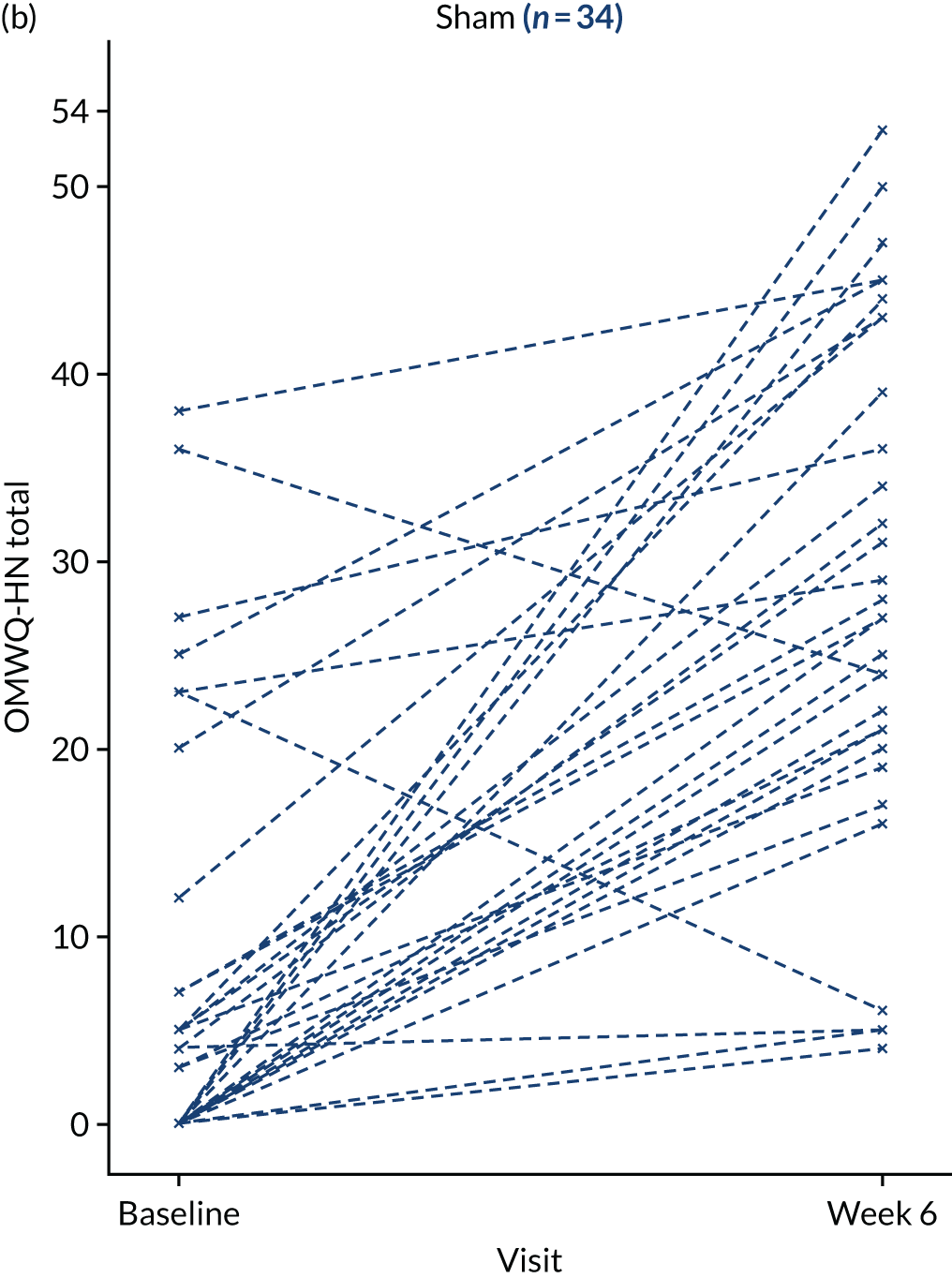
Planned subgroup analyses
To inform future studies, summary statistics for the OMWQ-HN total score at 6 weeks by level of trial stratification factors, for the 71 participants in the primary analysis, are given in Table 13.
| Stratification factors | Treatment arm | |||
|---|---|---|---|---|
| LLLT (N = 37) | Sham (N = 34) | |||
| n | OMWQ-HN score at 6 weeks, mean (SD) | n | OMWQ-HN score at 6 weeks, mean (SD) | |
| Treatment receiveda | ||||
| Radiotherapy | 9 | 27.2 (11.7) | 8 | 16.4 (9.9) |
| Chemoradiotherapy | 28 | 35.1 (8.8) | 26 | 30.8 (13.2) |
| Type of radiotherapy field | ||||
| Unilateral | 7 | 30.6 (14.8) | 9 | 29.6 (13.0) |
| Bilateral | 30 | 33.8 (8.8) | 25 | 26.6 (14.3) |
Secondary outcomes
Descriptive summaries for secondary outcomes are presented for assessments performed at baseline, 6 weeks and 4 months. Further descriptive summaries of additional assessments can be found in Appendix 2, including those collected during weeks 1–5 of radiotherapy and at the 14-month follow-up. Comprehensive summaries of the completeness of individual questions/domains for secondary outcomes are also provided in Appendix 2.
World Health Organization Oral Mucositis Grading Scale score
The number and percentage of participants with each WHO grade at baseline, 6 weeks and 4 months are given in Table 14. At baseline, no participants were assigned the highest grades III/IV, which indicate severe or life-threatening mucositis. At 6 weeks, data were available for 73 out of 78 participants (93.6%; two missing from the LLLT arm and three missing from the sham arm). In the LLLT arm, 19 out of 38 (50%) participants had grade III/IV mucositis, compared with 21 out of 35 (60%) participants in the sham arm. The proportion of participants with grade III/IV OM at 6 weeks was, on average, 10% (95% CI –32.7% to 12.7%) lower in the LLLT arm than in the sham arm. This 95% CI is wide and indicates that these trial data are compatible with a difference between treatment arms in the percentage of participants deemed to have grade III/IV OM at 6 weeks, in the population of interest, that ranges from 33% fewer participants in the LLLT arm than in the sham arm to 13% more.
| Outcome | Visit | |||||
|---|---|---|---|---|---|---|
| Baseline | 6 weeks | 4 months | ||||
| LLLT arm (N = 44) | Sham arm (N = 43) | LLLT arm (N = 40) | Sham arm (N = 38) | LLLT arm (N = 39) | Sham arm (N = 38) | |
| WHO mucositis score, n/N (%) | ||||||
| Grades 0–II | 43/43 (100) | 43/43 (100) | 19/38 (50) | 14/35 (40) | 30/31 (96.8) | 34/35 (97.1) |
| None | 39 | 41 | 3 | 4 | 18 | 19 |
| Mild | 4 | 2 | 6 | 3 | 8 | 13 |
| Moderate | 0 | 0 | 10 | 7 | 4 | 2 |
| Grade III or IV | 0/43 | 0/43 | 19/38 (50) | 21/35 (60) | 1/31 (3.2) | 1/35 (2.9) |
| Severe | 0 | 0 | 19 | 20 | 1 | 1 |
| Life-threatening | 0 | 0 | 0 | 1 | 0 | 0 |
| WST, median (IQR), n | ||||||
| Volume (ml per swallow) | 20.0 (14.3–25.0), 42 | 20.0 (14.3–25.0), 41 | 11.1 (0–16.7), 31 | 12.5 (6.7–16.7), 29 | 14.3 (11.1–20.0), 25 | 16.7 (11.8–25.0), 32 |
| Capacity (ml per second) | 12.5 (8.3–20.0), 43 | 16.7 (9.1–20.0), 41 | 2.4 (0–11.3), 32 | 4.7 (1.8–10), 29 | 10.0 (6.7–14.3), 25 | 12.5 (5.1–20.0), 32 |
| PSS-HN | ||||||
| Normalcy of diet | ||||||
| Scored ≤ 50,a n/N (%) | 10/44 (22.7) | 6/43 (14.0) | 33/39 (84.6) | 32/35 (91.4) | 23/35 (65.7) | 19/35 (54.3) |
| Median (range) | 100 (0, 100) | 100 (0, 100) | 10 (0, 100) | 20 (0, 100) | 50 (10, 100) | 50 (0, 100) |
| Eating in public | ||||||
| Inpatient, n/N | 0/44 | 0/43 | 2/38 (5.3)b | 3/34 (8.8)b | 0/35 | 0/35 |
| Scored ≤ 50,c n/N (%) | 8/44 (18.2) | 2/43 (4.7) | 28/36 (77.8) | 26/31 (83.9) | 13/35 (37.1) | 11/35 (31.4) |
| Median (range) | 100 (0, 100) | 100 (25, 100) | 25 (0, 100) | 25 (0, 100) | 75 (0, 100) | 75 (0, 100) |
| Understandability of speech | ||||||
| Scored ≤ 50,d n/N (%) | 2/44 (4.5) | 0/43 (0) | 4/39 (10.3) | 5/35 (14.3) | 1/35 (2.9) | 0/35 (0) |
| Median (range) | 100 (25, 100) | 100 (75, 100) | 100 (25, 100) | 100 (25, 100) | 100 (25, 100) | 100 (75, 100) |
| Weight (kg) | ||||||
| Mean (SD), n | 80.1 (14.8), 44 | 80.4 (18.1), 43 | 74.9 (12.7), 39 | 75.9 (15.8), 35 | 71.2 (11.5), 31 | 71.3 (15.1), 33 |
| BMI (kg/m2) | ||||||
| Mean (SD), n | 26.7 (4.4), 43 | 26.9 (5.6), 43 | 25 (4), 38 | 25.3 (4.9), 35 | 24.2 (3), 30 | 23.8 (4.6), 33 |
Health-related quality-of-life measures
Figures 15 and 16 present the 95% CIs for the difference between treatment arm means at 6 weeks and 4 months for health-related quality-of-life (HRQoL) measures. In addition, these figures include the means and SDs used to calculate the mean differences, along with, for context and to facilitate interpretation, the baseline means and SDs for the participants who were included in those calculations at each time point. The 95% CIs at 6 weeks and 4 months are wide, reflecting the small number of participants with evaluable data and the large variation between participants at each time point. The 95% CIs indicate the range of possible treatment effects that are compatible with the trial data and include effects in both directions (in favour of LLLT and in favour of sham); all include the null effect and, therefore (and given that there are 22 outcomes at each of two time points), these results should be interpreted cautiously.
FIGURE 15.
The MDADI and EORTC scores: mean difference between treatment arms at 6 weeks and 4 months. All MDADI and EORTC items ask participants to use ‘the past week’ as a reference. Higher scores on the MDADI and EORTC QLQ-C30 reflect relatively ‘positive’ outcomes (e.g. better functioning or QoL). Higher scores on the EORTC QLQ-H&N35 (pain to felt ill) reflect relatively ‘negative’ outcomes (e.g. greater symptoms or problems). Higher percentages on the EORTC QLQ-H&N35 (painkillers to weight gain) reflect a greater frequency of occurrence. n indicates, for each treatment arm, the number of participants included in the calculation of the mean and SD at 6 weeks or 4 months (and the corresponding mean and SD for the same participants at baseline is reported too). Difference estimates are unadjusted for baseline.
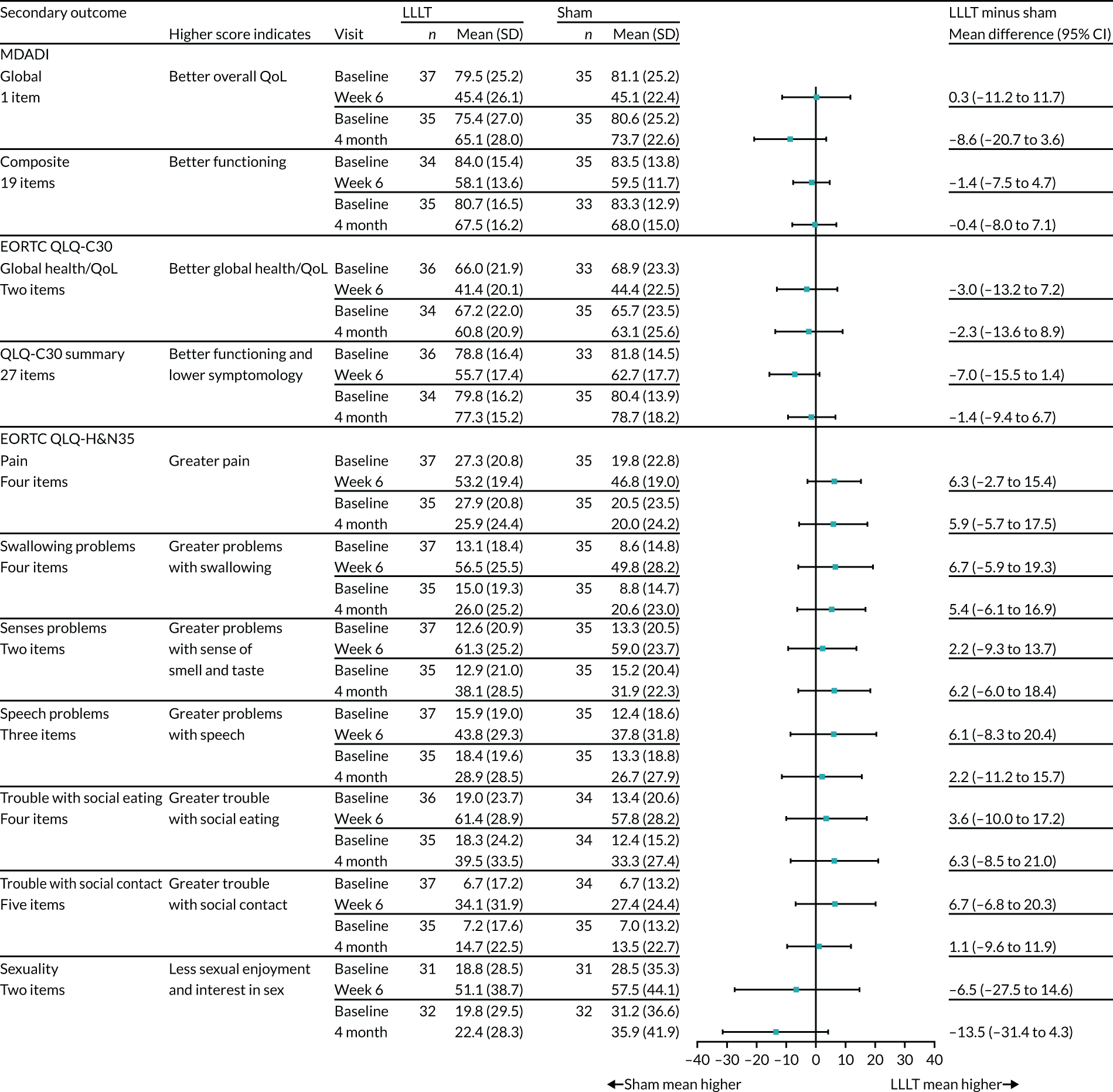
FIGURE 16.
The EORTC scores: mean and percentage difference between treatment arms at 6 weeks and 4 months. All EORTC QLQ items ask participants to use ‘the past week’ as a reference. Higher scores on the EORTC QLQ-H&N35 (pain to felt ill) reflect relatively ‘negative’ outcomes (e.g. greater symptoms or problems). Higher percentages on the EORTC QLQ-H&N35 (painkillers to weight gain) reflect a greater frequency of occurrence. n indicates, for each treatment arm, the number of participants included in the calculation of the mean and SD at 6 weeks or 4 months (and the corresponding mean and SD for the same participants at baseline is reported too). Difference estimates are unadjusted for baseline.
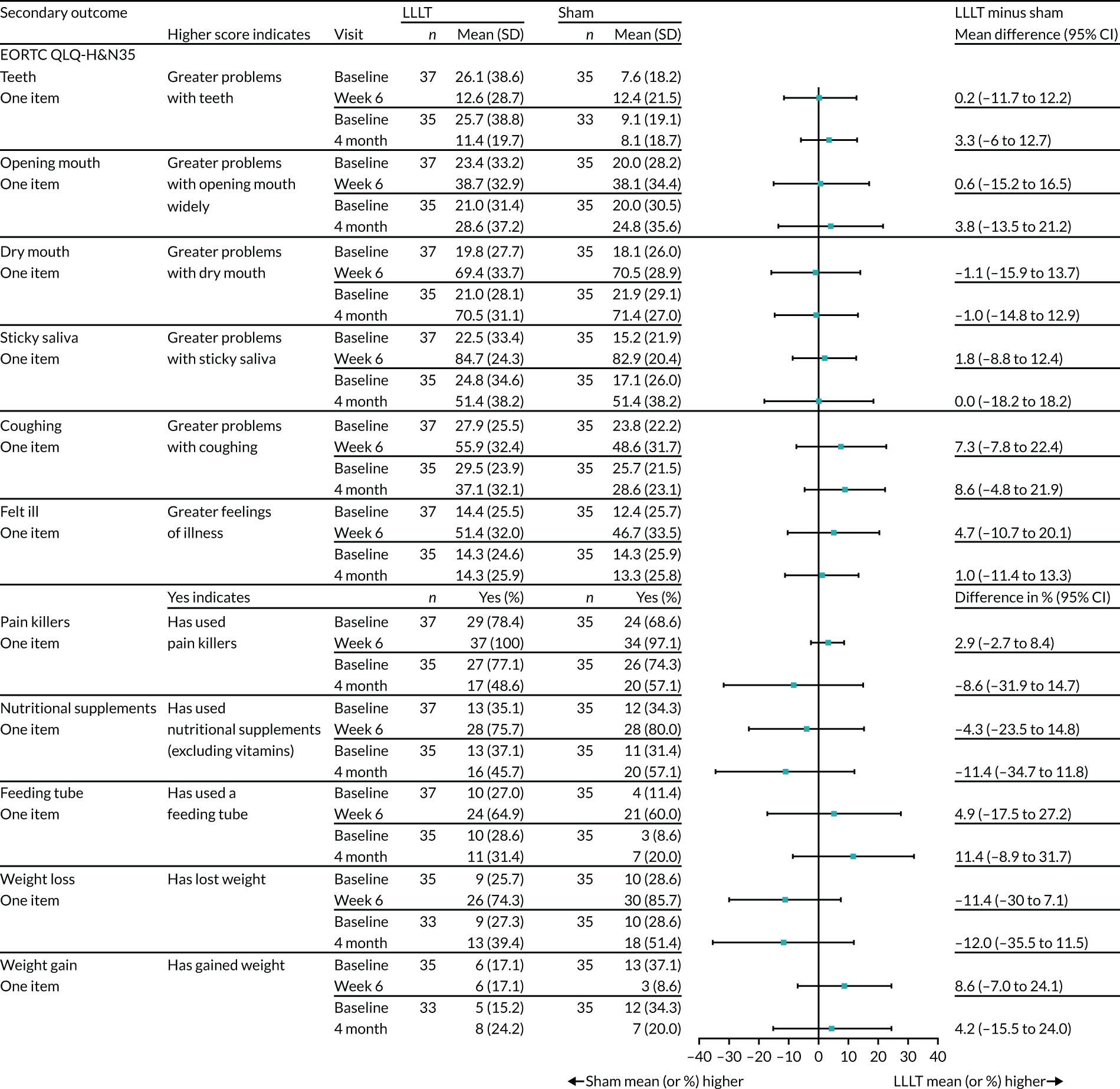
MD Anderson Dysphagia Inventory
The MDADI was administered at the baseline, 6-week, 4-month and 14-month visits. The global, physical, functional, emotional and composite score on the MDADI ranges from 20 to 100, with lower scores indicating greater impairment. The MDADI was well completed by participants at all visits for both treatment arms (see Appendix 2, Table 37). The global score (one item) was completed by at least 90% of participants at each visit, with the highest percentage at baseline (100%) and the lowest at the 4-month visit (70/77; 90.9%). The composite score was calculated as the weighted average of the emotional, functional and physical domain scores (see Chapter 2, Secondary outcome measures). The composite score completion rate was highest at baseline (100%) and lowest at the 4-month visit (68/77; 88.3%). At each visit, missing composite score data were almost always a result of all three domain scores being missing simultaneously.
Summary statistics at each visit (baseline, 6 weeks, 4 months and 14 months) for the MDADI global, emotional, functional and physical domains, and composite scores are presented in Appendix 2, Tables 32–36. Differences between treatment arms in the MDADI global and composite score means (with associated 95% CIs) at 6 weeks and 4 months are presented in Figure 15. MDADI global and composite scores were lower at 6 weeks than at baseline (pre treatment) and by 4 months had increased but were not back at the level at baseline. This drop during treatment would be expected. 43
European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire 30 and the European Organisation for Research and Treatment of Cancer Quality of Life Module for Head and Neck Cancer
The EORTC QLQ-C30 was well completed by participants at all visits for both treatment arms, with scores available for the global health/QoL and QLQ-C30 summary for at least 88% of participants throughout (see Appendix 2, Table 59). At each visit, the primary reason for the absence of QLQ-C30 summary score data was that all 13 scale scores were missing; partial availability of scale scores (i.e. less than half non-missing) occurred in only one case at 6 weeks.
The completion of the EORTC QLQ-H&N35 scales was > 80% at all visits (see Appendix 2, Table 59). Lower completion rates for this scale than for other parts of the EORTC QLQ-H&N35 have been reported in other studies. 46
Summary statistics for the EORTC QLQ-C30 global health/QoL, financial difficulties and summary scores and the 18 EORTC QLQ-H&N35 scores at each visit are presented in Appendix 2, Tables 38–58. Differences between treatment arms in the EORTC QLQ-C30 global health/QoL and summary score, and differences in score on all 18 EORTC QLQ-H&N35 subscales (with associated 95% CIs) at 6 weeks and 4 months, are presented in Figures 15 and 16.
The global health score at baseline in both treatment arms was consistent with pre-treatment values reported in other large HNC UK series. 38 Both the global health and the summary score showed a notable drop in score (consistent with worse quality of life/poorer functioning/more symptoms) between baseline and 6 weeks, with a rise at 4 months to a level slightly below that at baseline. As for the MDADI, this pattern would be anticipated. 46
Performance status scale for head and neck cancer patients
Table 14 shows the summary statistics for the PSS-HN subscales: normalcy of diet, eating in public and understandability of speech. Note that the summaries presented are for the available data at each time point and, therefore, the number of participants varies across time points.
Among the three subscales, participants were most burdened by a relatively reduced capacity to consume a normal diet. By 6 weeks, the majority of participants were, at best, able to manage only very soft food textures (87.8%; 65/74 with available data) and able to eat only with selected people, in selected places (80.6%; 54/67 with available data). On the other hand, participants had good speech intelligibility. The majority of participants were at least ‘understandable most of the time: occasional repetition necessary’ throughout the trial. A small percentage of participants had significant speech difficulties at 6 weeks (LLLT arm, 10.3%; sham arm, 14.3%), at which time face-to-face communication was necessary to make themselves understood. Descriptive statistics for the PSS-HN at each visit are presented in Appendix 2, Table 59.
Timed water swallow test
Descriptive statistics for the WST outcomes are displayed in Table 14 for both swallow volume and swallow capacity. Data for each visit are presented in Appendix 2, Tables 61 and 62. Participants scored zero on all WST outcomes if they had severe dysphagia or odynophagia and were nil by mouth. One participant at baseline, 12 participants at 6 weeks, two participants at 4 months and one participant at 14 months scored zero on all measures.
Data on the WST outcomes were available for 61 out of 78 participants (78.2%; eight missing from the LLLT arm and nine missing from the sham arm) at the 6-week visit and for 57 out of 77 participants (74%; 14 missing from the LLLT arm and six missing from the sham arm) at the 4-month visit. Reasons for missing WST data are presented in Appendix 2, Table 63. At the 6-week visit, the principal reasons for missing data were participants declining to take the WST (7/17; 41.2%), staff unavailability (3/17; 17.6%) and administrative/clerical errors (3/17; 17.6%). At the 4-month visit, most omissions were because of staff availability (8/20; 40%) and participants not attending the 4-month visit or the clinic session (8/20; 40%).
Weight and body mass index
Summary statistics for weight and BMI are reported in Table 14. Descriptively, there was a gradual decline in weight and BMI over time from baseline to the 4-month visit. Nutritional support is indicated for > 10% weight loss from baseline. By the 6-week visit, 8 out of 74 participants (10.8%) had lost > 10% of their baseline weight (LLLT arm, 6/39; sham arm, 2/35). By the 4-month visit, 29 out of 64 participants (45.3%) had lost > 10% of their baseline weight (LLLT arm, 12/31; sham arm, 17/33). Summary statistics for weight and BMI at each visit are presented in Appendix 2, Tables 64 and 65.
Oral intake and tube dependence
Data on feeding tube use were available for 73 out of 78 participants (93.6%; two missing from the LLLT arm and three missing from the sham arm) at the 6-week visit and for 67 out of 77 participants (87%; six missing from the LLLT arm and four missing from the sham arm) at the 4-month visit. Summary statistics for oral intake levels and feeding tube status at each visit are presented in Appendix 2, Tables 66 and 67.
There was a high prevalence of feeding tube dependence during radiotherapy for all participants. At the 6-week visit, there were equal proportions of participants on a feeding tube in both treatment arms [25/38 (66%) in the laser arm and 23/35 (66%) in the sham arm]. However, among those using feeding tubes at 6 weeks, the percentage of participants who were totally dependent on their feeding tube was greater in the LLLT arm (15/25, 60%) than in the sham arm (9/23, 39%) (Table 15). At the 4-month visit, 25% (17/67) of participants retained a feeding tube, with, descriptively, a higher percentage in the LLLT arm (11/33, 33%) than in the sham arm (6/34, 18%).
| Feeding tube use | Time point, n/N (%) | |||
|---|---|---|---|---|
| 6 weeks | 4 months | |||
| LLLT (N = 40) | Sham (N = 38) | LLLT (N = 39) | Sham (N = 38) | |
| Oral intake level | ||||
| 100% | 2/37 (5.4) | 1/33 (3.0) | 6/32 (18.8) | 8/34 (23.5) |
| 75% | 5/37 (13.5) | 2/33 (6.1) | 7/32 (21.9) | 14/34 (41.2) |
| 50% | 2/37 (5.4) | 3/33 (9.1) | 6/32 (18.8) | 4/34 (11.8) |
| 25% | 3/37 (8.1) | 4/33 (12.1) | 3/32 (9.4) | 3/34 (8.8) |
| < 25% | 25/37 (67.6) | 23/33 (69.7) | 10/32 (31.3) | 5/34 (14.7) |
| Use of feeding tube | 25/38 (65.8) | 23/35 (65.7) | 11/33 (33.3) | 6/34 (17.6) |
| Level of dependence on tube | ||||
| Total dependence on tube, nil by mouth | 15 | 9 | 0 | 1 |
| Tube dependence with minimal oral intake | 6 | 13 | 10 | 2 |
| Feeding tube supplements oral intake | 4 | 1 | 1 | 3 |
| Feeding tube type | ||||
| Gastronomy | 15 | 15 | 10 | 6 |
| Nasogastric | 6 | 7 | 1 | 0 |
| Nasojejunal | 3 | 0 | 0 | 0 |
| Missing | 1 | 1 | 0 | 0 |
Pain outcomes
Analgesics use at baseline and 6 weeks is summarised in Table 16. At baseline, 67.4% of participants (58/86 with data available) reported using analgesics in the past week. At 6 weeks, 97.4% of participants (72/74 with data available) reported using analgesics in the past week. Overall, anti-inflammatory analgesics/paracetamol were the most common analgesics used, followed by opioids. Further descriptive statistics on weekly analgesics use are presented in Appendix 2, Table 68.
| Oral care | Time point | |||
|---|---|---|---|---|
| Baseline | 6 weeks | |||
| LLLT (N = 44) | Sham (N = 43) | LLLT (N = 40) | Sham (N = 38) | |
| Used analgesics in past week, n/N (%) | 29/43 (67.4) | 29/43 (67.4) | 37/38 (94.9) | 35/36 (97.2) |
| Type of analgesic(s), n | ||||
| Anti-inflammatory analgesic/paracetamol | 26 | 27 | 34 | 32 |
| Opioid | 12 | 10 | 33 | 28 |
| Other | 1 | 3 | 0 | 2 |
| Used mouthwash in past week, n/N (%) | 14/43 (32.6) | 18/43 (41.9) | 34/39 (87.2) | 33/36 (91.7) |
| Type of mouthwash,a n | ||||
| Simple mouthwash | 3 | 6 | 10 | 10 |
| Analgesic mouthwash | 4 | 5 | 19 | 17 |
| Antiseptic mouthwash | 8 | 9 | 0 | 3 |
| Mucosa-protecting mouthwash | 0 | 1 | 21 | 22 |
| Visited an oral hygienist in past week, n/N (%) | 5/43 (11.6) | 2/43 (4.7) | 4/39 (10.3) | 1/36 (2.8) |
| Number of visits (range) | 0–1 | 0–1 | 0–3 | 0–1 |
Oral care
Mouthwash use at baseline and 6 weeks is summarised in Table 16. At baseline, 37.2% of participants (32/86 with data available) reported using mouthwash in the past week, and at 6 weeks 89.3% of participants (67/75 with data available) reported using mouthwash in the past week. Antiseptic mouthwash was the most frequently reported form of mouthwash used at baseline. Over the course of treatment, mucosa-protecting mouthwash became the most frequently reported type of mouthwash used, followed by analgesic mouthwash, simple mouthwash and antiseptic mouthwash. Further descriptive statistics on weekly mouthwash use are presented in Appendix 2, Table 69.
Visits to an oral hygienist are summarised in Table 16. At baseline, 8.1% of participants (7/86 with available data) reported visiting an oral hygienist in the past week: all made a single visit. At 6 weeks, 6.7% of participants (5/75) reported visiting an oral hygienist in the past week: four participants made a single visit and one participant made three visits. Further descriptive statistics on visits to oral hygienist during the treatment period are presented in Appendix 2, Table 70.
Hospital admissions and outpatient visits
Hospital admissions and outpatient visits were recorded from 2 weeks to 6 weeks of the treatment period. The frequency of hospital admissions was approximately balanced between arms over this period and ranged from 5% at week 3 (4/77 participants with available data) to 19% at week 5 (14/75 with available data). Outpatient appointments were frequent and ranged from 38% of participants at week 4 (having between 1 and 10 appointments) to 49% of participants at week 2 (having between 1 and 12 appointments). Few participants reported attending the head and neck ward as an outpatient, and no participant reported any outpatient visit to an accident and emergency (A&E) department during the treatment period. Further descriptive data on hospital admissions and outpatient visits are presented in Appendix 2, Table 71.
Alcohol use and smoking
Alcohol use (e.g. wine, beer, cider and spirits) and smoking (e.g. cigarettes, e-cigarettes and roll-ups) were defined as any use over the past 7 days and were recorded from 2 weeks to 6 weeks of the treatment period. Alcohol use appeared to decline over the treatment period, with 10 out of 78 (12.8%) participants reporting use at 2 weeks, 12 out of 77 (15.6%) at 3 weeks, 6 out of 77 (7.8%) at 4 weeks, 4 out of 74 (5.4%) at 5 weeks and 4 out of 72 (5.5%) at 6 weeks. Likewise, smoking appeared to decline over the treatment period, with 9 out of 78 (11.5%) participants reporting use at 2 weeks, 7 out of 77 (9.1%) at 3 weeks, 8 out of 77 (10.4%) at 4 weeks, 6 out of 74 (8.1%) at 5 weeks and 4 out of 72 (5.5%) at 6 weeks. Further descriptions of alcohol use and smoking are presented in Appendix 2, Table 72.
Disease outcomes
At the time the trial closed, 28 participants had been in the trial for at least 14 months and were still being followed up. At the 14-month visit, data on disease outcome (disease progression, recurrence or death) were available for 26 out of 28 participants (LLLT arm, 10/12; sham arm, 16/16). For all participants, there was no evidence that disease had progressed or recurred and no participants had died.
Adverse events
In total, 28 SAEs (LLLT arm, n = 17; sham arm, n = 11) were reported across 22 participants (LLLT arm, n = 13; sham arm, n = 9) (see Appendix 2, Table 75). None of these SAEs was classed as being related to the trial intervention.
The number of AEs reported was found to be similar across allocated treatment arms (Table 17). Commonly reported AEs are tabulated by treatment arm in Table 18 and a comprehensive list is provided in Appendix 2, Table 73. The most frequently reported events were gastrointestinal disorders [including stomatitis (OM), nausea, saliva altered, dry mouth and constipation], fatigue and oral pain.
| Number and severity of AEs | Treatment arm | Total (N = 87) | |
|---|---|---|---|
| LLLT (N = 44) | Sham (N = 43) | ||
| Number of events per participant | |||
| Mean (SD) | 7.3 (6.5) | 7.1 (6.3) | 7.2 (6.4) |
| Median (IQR) | 7.5 (1–11) | 6 (1–12) | 7 (1–12) |
| Minimum, maximum | 0, 25 | 0, 22 | 0, 25 |
| Worst grade reported per participant, n (%) | |||
| None | 8 (18.2) | 10 (23.3) | 18 (20.7) |
| Mild | 3 (6.8) | 0 (0) | 3 (3.4) |
| Moderate | 11 (25) | 11 (25.6) | 22 (25.3) |
| Severe | 21 (47.7) | 18 (41.9) | 39 (44.8) |
| Life-threatening | 1 (2.3) | 4 (9.3) | 5 (5.7) |
| AE | Number of participants, n (%) | ||
|---|---|---|---|
| Treatment arm | Total (N = 87) | ||
| LLLT (N = 44) | Sham (N = 43) | ||
| Stomatitis (OM) | 19 (43.2) | 16 (37.2) | 35 (40.2) |
| Fatigue | 18 (40.9) | 16 (37.2) | 34 (39.1) |
| Nausea | 12 (27.3) | 20 (46.5) | 32 (36.8) |
| Saliva altered | 17 (38.6) | 14 (32.6) | 31 (35.6) |
| Dry mouth | 15 (34.1) | 16 (37.2) | 31 (35.6) |
| Constipation | 15 (34.1) | 14 (32.6) | 29 (33.3) |
| Taste disorder | 10 (22.7) | 12 (27.9) | 22 (25.3) |
| Decreased appetite | 8 (18.2) | 12 (27.9) | 20 (23.0) |
| Dysphagia | 10 (22.7) | 9 (20.9) | 19 (21.8) |
| Mouth ulceration | 7 (15.9) | 12 (27.9) | 19 (21.8) |
| Oropharyngeal pain | 9 (20.5) | 9 (20.9) | 18 (20.7) |
| Oral candidiasis | 8 (18.2) | 10 (23.3) | 18 (20.7) |
| Vomiting | 8 (18.2) | 9 (20.9) | 17 (19.5) |
| Oral Pain | 6 (13.6) | 9 (20.9) | 15 (17.2) |
| Dysphonia | 8 (18.2) | 3 (7.0) | 11 (12.6) |
| Anaemia | 6 (13.6) | 5 (11.6) | 11 (12.6) |
| Tinnitus | 5 (11.4) | 6 (14.0) | 11 (12.6) |
| Dysgeusia | 7 (15.9) | 3 (7.0) | 10 (11.5) |
| Radiation skin injury | 7 (15.9) | 3 (7.0) | 10 (11.5) |
| Infection | 5 (11.4) | 4 (9.3) | 9 (10.3) |
| Diarrhoea | 3 (6.8) | 5 (11.6) | 8 (9.2) |
| Pain | 5 (11.4) | 2 (4.7) | 7 (8.0) |
| Weight loss | 4 (9.1) | 3 (7.0) | 7 (8.0) |
| Lymphocyte count decreased | 3 (6.8) | 4 (9.3) | 7 (8.0) |
| Skin reaction | 3 (6.8) | 4 (9.3) | 7 (8.0) |
| Tongue coated | 3 (6.8) | 4 (9.3) | 7 (8.0) |
| Dehydration | 4 (9.1) | 2 (4.7) | 6 (6.9) |
| Dyspepsia | 3 (6.8) | 3 (7.0) | 6 (6.9) |
| Depressed mood | 4 (9.1) | 1 (2.3) | 5 (5.7) |
| Pharyngeal inflammation | 4 (9.1) | 1 (2.3) | 5 (5.7) |
| Dry skin | 2 (4.5) | 3 (7.0) | 5 (5.7) |
| Rash | 1 (2.3) | 4 (9.3) | 5 (5.7) |
| Hypoalbuminaemia | 3 (6.8) | 1 (2.3) | 4 (4.6) |
| Pruritus | 3 (6.8) | 1 (2.3) | 4 (4.6) |
| Raised levels of alanine aminotransferase | 1 (2.3) | 3 (7.0) | 4 (4.6) |
| Deafness | 1 (2.3) | 3 (7.0) | 4 (4.6) |
| Salivary duct inflammation | 3 (6.8) | 0 (0) | 3 (3.4) |
| Pyrexia | 0 (0) | 3 (7.0) | 3 (3.4) |
Non-serious AEs that were deemed to be possibly, probably or definitely related to LLLT are reported in Appendix 2, Table 74. We note that many of these events recorded by sites as being related to LLLT could reasonably be expected to occur in patients undergoing CRT for HNC rather than those that would be expected after receiving LLLT.
Chapter 4 Economic evaluation
Introduction
Given that the LiTEFORM trial was closed prior to reaching the recruitment target, the proposed economic analysis, as described in the protocol, was no longer considered appropriate given the sample size of the trial at its closure. Therefore, no formal analysis was conducted to test for statistically significant differences in resource use, cost and HRQoL between the two randomised arms.
The economic evaluation presented in this chapter has three primary components:
-
a microcosting analysis of the LLLT intervention
-
presentation of health service utilisation data in the form of summary statistics.
-
presentation of HRQoL data in the form of summary statistics.
All data, with the exception of the cost of the intervention itself, are, therefore, presented as summary statistics. This includes the responses to the EQ-5D-5L and EuroQol visual analogue scale (EQ-5D-VAS) that were administered at baseline, 6 weeks post intervention, 4 months post intervention and 14 months post intervention, and the use of health services at 4 months post intervention. Detailed data on the use of health services at 14 months post intervention and data generated from the Time and Travel Questionnaire (completed at 14 months) (see Report Supplementary Material 2) are not presented because of the small number of participants providing data at this time point.
Methods
The design and conduct of the health economic evaluation followed guidelines for best practice throughout, conforming to Consolidated Health Economic Evaluation Reporting Standards. 47
A complete health economics analysis plan (HEAP), which provides full details of all analyses, variables and outcomes, was finalised and signed before the final database lock and analysis (see the project web page: www.journalslibrary.nihr.ac.uk/hta/15/57/160) (see Report Supplementary Material 8).
Intervention costs
Participants recruited to the trial could be allocated to one of two arms: the LLLT treatment arm or the sham treatment arm. As per protocol, both the LLLT intervention and the sham intervention had the same duration and staff component. The cost of providing the LLLT intervention was microcosted, using additional information provided by THOR48 and NHS employers ‘Agenda for Change’ pay scales (2018/19). 49 The base-case analysis assumed that the patient attended all scheduled laser sessions. Further analysis used information on session attendance recorded on the eCRFs to estimate the intervention cost for each trial participant and, therefore, to estimate the cost of delivering the intervention in the LiTEFORM trial.
Intervention costs for those randomised to receive the laser therapy session included:
-
equipment required for each laser therapy session
-
maintenance fee for the laser therapy equipment
-
estate/facilities costs for use of a treatment room
-
staff cost (per minute) for set-up and preparation for each therapy session
-
staff costs (per minute) of the staff members(s) who delivered the session
-
staff costs (per minute) of the staff member(s) who provided administrative support.
Calculation of the intervention costs assumed the following:
-
The usual lifespan of the laser is 5 years.
-
Patients receive LLLT three times per week for a period of 6 weeks.
-
Each session lasts 30 minutes, with 15 minutes of set-up time.
-
The equipment is serviced annually.
-
LLLT is delivered to the patient by a trained radiographer (band 6 mid-point).
In addition to staff time, capital costs were calculated for the LLLT using the ‘equivalent annual cost’ methodology. 50 This method converts the initial capital cost into an annual sum that equals the resources and investment plus their opportunity cost.
The equivalent annual cost of the LLLT was calculated under the following assumptions:
-
The laser is used for an average of 40 sessions per week (eight sessions per day, Monday to Friday).
-
The laser is in use 52 weeks per year.
-
The useful lifespan of the laser is 5 years.
-
The capital costs of the laser were spread over its lifespan (5 years).
A discount factor of 3.5% was applied to account for the individual’s time preference for costs to be incurred later rather than sooner. This follows guidance for best practice. 51
Hospitalisation during the intervention period
Data on the trial participant’s use of hospital services during the intervention period (a period of 2–6 weeks), including data on both the type and the frequency of the hospital service, were collected using an eCRF.
Specifically, data were collected on the:
-
number of inpatient nights
-
number of outpatient visits
-
number of A&E visits
-
number of visits to the head and neck ward
-
other hospital visits (e.g. hospital dentist visits).
Health service utilisation
A health service utilisation questionnaire was developed to capture the health-care resource use of the trial participants after the intervention period (see Report Supplementary Material 1). Participants were asked about the care that they received, retrospectively, at 4 and 14 months post randomisation. To avoid double-counting with the data on the use of hospital services during the intervention period, at the 4-month data collection point the questions specifically regarding hospital services asked the trial participants to consider hospital resource use since their last laser therapy session only. The 14-month questionnaire asked about health service utilisation in the previous 10 months only.
This questionnaire collected data on:
-
inpatient and day-case admissions and length of admission
-
number of outpatient visits
-
number of A&E visits
-
primary and community-based visits
-
use of private health care/personal care
-
work affected by illness.
The data collected through both the hospitalisation and the health service use questionnaires were supplemented with data collected through the eCRF.
Individual patient-level data on resource use of NHS and Personal Social Services collected via the Hospitalisation eCRF Health Service Utilisation Questionnaire (see Report Supplementary Material 1) were combined with unit costs obtained from routine data sources, such as the data collected by the Personal Social Services Research Unit (PSSRU)52 and the NHS Reference Costs 2018–19. 53 The unit costs are presented in Appendix 3, Tables 76–80. The price year for all analyses was 2019.
Medication costs
The cost of prescribed medication was also included in the trial. At each visit, participants were asked to record the name of the medication, as well as the dosage, frequency, format prescribed and start and end date (if applicable) of their prescription. Medications that were insufficiently specific to obtain costs for (e.g. ‘Vaseline’ or ‘Oxygen Therapy’) were excluded from the analysis because costs vary depending on the precise medication prescribed.
Units costs were taken from the British National Formulary and multiplied by the total units prescribed for the stated period. When there were missing or inconclusive data on any one of dosage, frequency, format prescribed, start date or end date of the medication, it was assumed that the participant had been issued one prescription for the most commonly prescribed format for the recommended time period. Information regarding prescription costs was gathered from NHS Digital’s Prescription Cost Analysis – England resources. 54 To ensure comparability with the other cost analyses, medications that were prescribed after the 4-month health services utilisation data collection point were excluded from the analysis.
Assessment of effects
EuroQol-5 Dimensions, five-level version and EuroQol-5 Dimensions, visual analogue scale
The EQ-5D-5L measure divides health status into five dimensions (i.e. mobility, self-care, usual activities, pain/discomfort and anxiety/depression). Each of these dimensions has five levels, resulting in 3125 possible health states existing. Completion rates and domain scores for the EQ-5D-5L and EQ-5D-VAS were initially reported for the two treatment arms. Following current National Institute for Health and Care Excellence (NICE) guidelines,55 a mapping/cross-walk algorithm was used to map the responses onto the EQ-5D-3L, with these values then converted into health state utility values at each time point for each patient based on a representative sample of the UK population. 56 These utility values have a possible range of –0.594 (worse than death) to 1 (perfect health).
Estimation of quality-adjusted life-years
The health state utilities calculated from the responses to EQ-5D-5L were used to estimate mean QALYs for both treatment arms at 4 months. This was carried out using the ‘area under the curve’ method, which allowed us to take into account differences in the rate of recovery following the interventions. 50 Owing to the limited sample size at 14 months, we did not estimate mean QALYs at this time point.
Results
Response rates
The response rates relating to participant-completed health economics questionnaires are outlined in Appendix 3, Tables 81–86. Although a progressive loss to follow-up occurred over the duration of the trial, the pattern of non-response was similar across the treatment arms.
Hospitalisation questionnaire
As shown in Appendix 3, Table 81, the completion rates of the hospitalisation questionnaires (issued between 2 weeks and 6 weeks) were very high. Aside from one trial participant at 2 weeks and one trial participant at 3 weeks, the questionnaire was fully completed by the trial participants.
Health Service Utilisation Questionnaire
As shown in Appendix 3, Table 82, the completion rates for the Health Service Utilisation Questionnaire issued at the 4-month and 14-month data collection points were 94% (67/71 participants) and 96% (25/26 participants) for each time point, respectively.
Time and Travel Questionnaire
As shown in Appendix 3, Table 83, the completion rate for the Time and Travel Questionnaire (issued at the 14-month data collection point) (see Report Supplementary Material 2) was 96% (25/26 participants).
Medications data
As shown in Appendix 3, Table 84, the completion rate for the concurrent medications data (completed via the eCRF) at the 4-month time period was 70% (54/77).
EuroQol-5 Dimensions, five-level version, and EuroQol-5 Dimensions, visual analogue scale
As shown in Appendix 3, Table 85, completion rates of the EQ-5D-5L were high across all data collection points. At baseline and 6 weeks, 84 out of 87 (97%) and 70 out of 71 (99%) trial participants fully completed the questionnaire, respectively. This high response rate was also replicated at the later data collection points, with 68 out of 71 (96%) and 26 out of 26 (100%) fully completing the questionnaire at 4 and 14 months, respectively.
As shown in Appendix 3, Table 86, the completion rates of the EQ-5D-VAS were similar to those for the EQ-5D-5L. At baseline and 6 weeks, the completion rates were 92% (80/87 participants) and 96% (68/71 participants), respectively. At 4 and 14 months, the completion rates were 93% (66/71 participants) and 92% (24/26 participants), respectively.
Estimation of costs
Microcosting of the intervention
Appendix 3, Tables 87 and 88, and Table 19, illustrate the microcosting of the intervention. Using information provided by THOR, it was assumed that the initial purchase cost of the laser was £6420, together with annual maintenance costs of £400 and annual training costs of £1200. For the staff costs, it was assumed that there would be 30 minutes of administrative time (band 3 mid-point) per therapy session to co-ordinate the sessions around other appointments and staff availability, and to check the patient in before the therapy session. The extensive level of co-ordination needed to deliver the intervention is discussed in detail in Chapter 5, Qualitative study. We also assumed that there would be 45 minutes of radiographer time (band 6 mid-point) for each therapy session, made up of 15 minutes set-up time and 30 minutes per laser session. Costs associated with the institution’s estate and facilities were included within the salary costs of the staff delivering the therapy.
| Cost of LLLT per session (capital) | Cost (£) |
|---|---|
| Opportunity cost of the capital (£1421.91 × 5) | 7109.55 |
| Annual cost of the laser | 1421.91 |
| Cost of laser per week (assume 52 weeks) | 27.34 |
| Cost of laser per session (assume 40 sessions per week) | 0.68 |
| Annual maintenance costs | 399.88 |
| Maintenance costs per week (assume 52 weeks) | 7.69 |
| Maintenance costs per session (assume 40 sessions per week) | 0.19 |
| Annual training costs | 1200 |
| Training costs per week (assume 52 weeks) | 23.08 |
| Training costs per session (assume 40 weeks) | 0.58 |
| Administrator staff costs (assume band 3 mid-point, 30 minutes) | 5.41 |
| Radiographera (assume band 6 mid-point, 45 minutes) | 37.70 |
| Total cost per session (laser cost + maintenance costs + training costs + staff costs) | 44.56 |
| Total cost per patient per week (total cost per session for three sessions) | 133.68 |
| Total intervention cost per patient (total cost per patient per week for six sessions) | 802.08 |
In the base-case analysis, the total cost of delivering the intervention was estimated to be £802 per patient, on average. This analysis assumed that the patient attended all 18 sessions (three sessions per week for 6 weeks). However, it is worth noting that only 28 out the 87 participants across both treatment arms attended all 18 sessions and, therefore, this figure is likely to be an overestimate of the true cost of delivering the LLLT intervention in the LiTEFORM trial. For those trial participants in the LLLT arm who were still on the trial at the end of the 6-week intervention period (n = 37), the mean number of sessions attended was approximately 16. Allowing for this, the average cost of delivering the intervention in the LiTEFORM trial was estimated to be £713 per patient.
To complement the base-case analysis, we also conducted a one-way sensitivity analysis, in which we assumed that each laser session would require a total of 60 minutes of radiographer time rather than the 45 minutes assumed in the base-case analysis. Owing to the increased time required for each session, it was also assumed that the number of sessions conducted per week would fall from 40 to 30, therefore increasing the capital costs of the laser per session. In this sensitivity analysis (see Appendix 3, Table 89), the total cost of delivering the intervention was estimated to be £1037 per patient on average. This sensitivity analysis assumed that the patient attended all 18 laser sessions.
Hospitalisation during the intervention period
Table 20 summarises the level of hospital services incurred in the two treatment arms during the intervention period (a period of 2–6 weeks). In the 6-week mITT sample, which included the 71 trial participants who had complete data on the primary outcome measure (OMWQ-HN) 6 weeks post intervention, the average total hospitalisation costs were £1615 in the LLLT arm and £1613 in the sham arm.
| Resource use | Treatment arm, mean cost (£) per patient (95% CI); n | |
|---|---|---|
| LLLT | Sham | |
| Intervention costs (assuming participants attend 18 laser sessions) | 802; 37 | N/A |
| Intervention costs (assuming participants attend 16 laser sessions) | 713; 37 | N/A |
| Hospitalisation costs (2–6 weeks) | 1615 (706 to 2523); 37 | 1613 (929 to 2298); 34 |
| Inpatient costs (6 weeks to 4 months) | 881 (1300 to 1631); 33 | 1417 (368 to 2467); 31 |
| Outpatient costs (6 weeks to 4 months) | 528 (308 to 748); 33 | 625 (329 to 920); 31 |
| Primary- and community-based NHS costs (4 months) | 107 (54 to 159); 33 | 150 (54 to 246); 31 |
| Concurrent medication costs (eCRF) | 284 (185 to 384); 24 | 217 (99 to 334); 24 |
Health service utilisation
Table 20 also summarises the level of health service utilisation in the two treatment arms at the 4-month post-intervention follow-up. The average per-patient inpatient costs were £881 in the LLLT arm and £1417 in the sham arm, and the average per-patient outpatient costs were £528 in the LLLT arm and £625 in the sham arm. The average per-patient primary care costs were £107 in the LLLT arm and £150 in the sham arm.
Summaries of the responses to the questions related to consultations with health-care professionals from charitable organisations, the use of private and/or personal health care and the number of workdays missed because of health problems are shown in Appendix 3, Tables 90–92. As shown in Appendix 3, Table 90, in both treatment arms combined there were four consultations with health-care professionals from charitable organisations reported at 4 months and no consultations reported at 14 months. As shown in Appendix 3, Table 91, in both treatment arms combined there was one incident of private and/or personal health-care use reported at 4 months and three consultations reported at 14 months. As shown in Appendix 3, Table 92, at the 4-month follow-up the average number of workdays missed because of health problems in the previous 4 months was 3 days in both treatment arms, and at the 14-month follow-up the average number of workdays missed because of health problems was approximately 2.5 days in both treatment arms.
Medications
Table 20 also summarises the cost of the medications prescribed before the 4-month post-intervention follow-up. For those individuals who reported prescribed medication use in sufficient detail, the average cost of the medications prescribed before the 4-month post-intervention data collection point was £284 in the LLLT arm and £217 in the sham arm.
Time and travel
Appendix 3, Tables 93 and 95, summarise the data collected from the Time and Travel Questionnaire, which was completed by the trial participants at 14 months and asked the trial participant about their most recent hospital admission, hospital outpatient appointment and GP or practice nurse consultation. As shown in Table 93, for their most recent hospital admission, the trial participants travelled 18 miles each way on average, taking 37 minutes for each journey and paying £3 in parking charges. A total of 69% of trial participants were accompanied by a relative or carer for this admission. As shown in Appendix 3, Table 94, for their most recent outpatient appointment the trial participants travelled 16 miles each way on average, taking 36 minutes for each journey and paying £3 in parking charges. A total of 36% of trial participants were accompanied by a relative or carer for this appointment. As shown in Appendix 3, Table 95, for their most recent GP or practice nurse consultation the trial participants travelled 8 miles each way on average, taking 20 minutes for each journey and paying £1 in parking charges. A total of 29% of trial participants were accompanied by a relative or carer for this consultation.
Health-related quality of life
EuroQol-5 Dimensions, five-level version
The responses to the EQ-5D-5L questionnaire at baseline, 6 weeks, 4 months and 14 months are shown in Appendix 3, Tables 96–99, and the utility scores derived from the responses to the EQ-5D-5L questionnaires are shown in Table 21. As shown in Table 21, at baseline the mean level of utility was 0.729 in the LLLT arm and 0.772 in the sham arm. At 6 weeks, the mean level of utility was 0.559 in the LLLT arm and 0.626 in the sham arm. At the 4-month follow-up point, the mean level of utility was 0.736 in the LLLT arm and 0.768 in the sham arm.
| Time point | Treatment arm, utility score | |
|---|---|---|
| LLLT | Sham | |
| Baseline | ||
| Mean (95% CI) EQ-5D-5L score, n | 0.729 (0.670 to 0.788), 42 | 0.772 (0.709 to 0.834), 42 |
| Median (IQR) EQ-5D-5L score, n | 0.759 (0.664–0.837), 42 | 0.816 (0.711–0.883), 42 |
| 6 weeks | ||
| Mean (95% CI) EQ-5D-5L score, n | 0.559 (0.485 to 0.633), 36 | 0.626 (0.553 to 0.699), 34 |
| Median (IQR) EQ-5D-5L score, n | 0.594 (0.411–0.743), 36 | 0.707 (0.458 to 0.796), 34 |
| 4 months | ||
| Mean (95% CI) EQ-5D-5L score, n | 0.736 (0.683 to 0.789), 31 | 0.768 (0.707 to 0.828), 31 |
| Median (IQR) EQ-5D-5L score, n | 0.740 (0.632–0.837), 31 | 0.837 (0.705–0.877), 31 |
| Mean (95% CI) QALYs, n | 0.218 (0.199 to 0.238), 29 | 0.231 (0.212 to 0.249), 30 |
| Median (IQR) QALYs, n | 0.220 (0.186–0.252), 29 | 0.244 (0.185–0.268), 30 |
Quality-adjusted life-years
As shown in Table 21, mean accumulated QALYs at 4 months were 0.218 in the LLLT arm and 0.231 in the sham arm. Given the limited sample size, no QALYs were reported at 14 months.
EuroQol-5 Dimensions, visual analogue scale
As shown in Table 22, at baseline the mean EQ-5D-VAS score was 72 in the LLLT arm and 71 in the sham arm. At 6 weeks, the mean EQ-5D-VAS score was 54 in the LLLT arm and 57 in the sham arm. At 4 months, the mean EQ-5D-VAS score was 72 in the LLLT arm and 71 in the sham arm.
| Time point | Treatment arm, EQ-5D VAS score, n | |
|---|---|---|
| LLLT | Sham | |
| Baseline | ||
| Mean (95% CI), n | 71 (65 to 77), 40 | 70 (64 to 77), 40 |
| Median (IQR), n | 73 (60–90), 40 | 73 (58–90), 40 |
| 6 weeks | ||
| Mean (95% CI), n | 54 (47 to 601), 34 | 57 (50 to 65), 34 |
| Median (IQR), n | 50 (40–70), 34 | 60 (45–75), 34 |
| 4 months | ||
| Mean (95% CI), n | 72 (66 to 78), 33 | 71 (64 to 78), 33 |
| Median (IQR), n | 75 (60–80), 33 | 75 (65–80), 33 |
| 14 months | ||
| Mean (95% CI), n | 80 (72 to 89), 9 | 83 (77 to 89), 15 |
| Median (IQR), n | 80 (75–88), 9 | 80 (75–95), 15 |
Discussion
Because the LiTEFORM trial closed before the original recruitment target was reached, no firm conclusions can be drawn from the data presented in this chapter. However, there are several aspects worth noting. First, the microcosting analysis indicates that the LLLT intervention is relatively inexpensive to implement. This low cost is mainly driven by the low yearly cost of the laser equipment, maintenance and training, with staff costs constituting the majority of the total cost of the intervention. Furthermore, if staff on lower salary bands can be trained to use the LLLT equipment, it may be possible to reduce the costs of delivering the intervention further.
Second, although the trial is not of sufficient size to detect statistically significant differences between the treatment arms, there seems to be a consistent pattern of results from the responses to the EQ-5D-5L and EQ-5D-VAS. In both arms of the trial, the EQ-5D-5L utility scores and EQ-5D-VAS scores are lower at 6 weeks (i.e. the end of the intervention period) than at baseline and at 4 months. This pattern of results is in line with the results from the cancer-specific HRQoL measures presented in Chapter 3, Results.
Finally, given the time-intensive nature of the LLLT intervention for the patient (as discussed in detail in Chapter 5 in relation to the difficulties co-ordinating the LLLT sessions around other hospital appointments), it is important that future trials in this clinical area (e.g. the trial currently being conducted in Brazil57) fully and accurately take into account the potentially substantial time and travel costs associated with an intervention of this nature. Using the limited data collected from the Time and Travel questionnaire as an illustrative example, we can see that some trial participants could spend > 30 minutes per journey getting to and from a hospital outpatient appointment, on top of the time spent waiting for their appointment and the time of the treatment itself. Furthermore, there may be car parking charges or other travel costs, as well as costs for relatives or carers who may accompany them to these appointments. Considering the intense treatment schedule of the intervention (i.e. three LLLT sessions per week for 6 weeks), the total patient costs of receiving the intervention may potentially be considerable.
There are some strengths of the analyses presented in this chapter. First, the completion rates for the measures of HRQoL (i.e. EQ-5D-5L and EQ-5D-VAS) were very high. Furthermore, the completion rates for the various data collection forms were also high, implying that these forms were well designed for the purposes of this trial.
However, there are also some associated weaknesses. Aside from the limited sample size, there were data collection issues with the concurrent medications at several trial sites (i.e. Newcastle and Plymouth), meaning that there was a relatively high level of missing data for this aspect of the cost data. Given that several medications prescribed to trial participants during the trial period were very high in cost, these missing data are likely to have increased the level of uncertainty surrounding the estimates of the medication cost data.
Conclusion
The results from the limited health economic analysis suggest that the data collection tools were fit for purpose for this trial. Owing to the limited data, all analyses of costs and effects should be interpreted with caution, and no firm conclusions can be drawn from these data. A fully powered future study in this area would provide evidence of the cost-effectiveness of LLLT.
Chapter 5 Qualitative study
Introduction
Qualitative research, in the form of embedded process evaluations, is now relatively common in trials of behavioural interventions,58 in which it can improve recruitment and conduct, contextualise findings, and contribute to effective scale-up and impact. 59 It also offers additional opportunities to add value beyond the specific trial questions through enabling broader questions about health and health care, such as the experience of health conditions, to be addressed. 59 However, qualitative research has been more rarely used in drug (5%), surgical (4%) or device (5%) trials. 58 This is despite increasing recognition that very few health interventions are truly ‘simple’,60 particularly in their implementation. A failure to recognise complexity may result in inadequate attention to the diverse factors that may affect the effectiveness of an intervention, for example concomitant interventions, the skill of the person delivering the intervention or the context in which it is delivered. 61
There is a rich body of qualitative work that considers factors influencing trial recruitment. Patient preference has often been perceived to be a key limiting factor; however, qualitative research has demonstrated that preferences may be based on misconceptions and that having an opportunity to explore preferences may reveal a more nuanced view and ultimately willingness to participate. 62 Trust in the trial and the associated health professionals is also an important factor, and patients are also strongly motivated by altruism63 – seeing trial participation as a way to achieve something positive from an otherwise difficult situation. 64,65 However, altruism has limits, and patients carefully weigh up the pros and cons of participation. 64,65 Clinicians also play an important part in recruitment, either deliberately or inadvertently revealing their own equivocality regarding equipoise, and sometimes struggling to reconcile their research and clinical roles. 64,66
The LiTEFORM trial was conceived without the inclusion of a placebo or sham treatment, and the potential impacts of the inclusion of the sham arm on recruitment and conduct were of particular interest to the team. Although health professionals widely accept that the benefits of placebo are real and significant,67–69 patients may see these effects as illusory or unreal70 and associate a placebo response with negative connotations of being susceptible and easy to fool. 69,70 It may be more difficult to recruit patients to trials with a placebo arm,71 leading recruiting clinicians to actively select participants whom they believe to be more likely to accept and comply with randomisation. 72 There have also been conflicting accounts regarding the adequacy of the consent process in placebo-controlled studies,72,73 with some patients motivated to participate because of a lack of alternative treatment options and having a preference for real rather than sham surgery. 74 The outcome of the placebo may vary according to personal characteristics of the patient and the symptoms being treated,72 and the patient–clinician relationship also plays a significant role in the placebo response. 69,70,72,75
The aims of the LiTEFORM trial qualitative process evaluation were to identify, describe, understand and address:
-
factors influencing trial participation, including willingness to randomise and be randomised
-
experiences of trial interventions and process including the ‘fit’ of LLLT within the treatment pathway
-
factors likely to influence wider implementation of LLLT beyond the LiTEFORM trial.
Exclusion of qualitative methodologists during trial design may lead to conflict between qualitative and quantitative components, and to poor integration and reporting. 58 In the LiTEFORM trial, qualitative methodologists were involved in trial design from the earliest stages of conception and were active members of the TMG throughout trial set-up and conduct.
In this chapter, we demonstrate how an apparently ‘simple’ intervention was deeply complex to integrate within the existing organisational, clinical and professional contexts. The LiTEFORM trial did not struggle because of an unwillingness on the part of staff to recruit or patients to be recruited to the trial, nor did it struggle because of a lack of engagement with the LLLT treatment: both staff and patients demonstrated commitment to the trial and worked hard to make it a success. Instead, we demonstrate how the LiTEFORM trial struggled because of the scale of the task of introducing, embedding and sustaining a new service into a complex care pathway. We show especially how patient flow was a central issue for all of the sites and how best to co-ordinate this, given that they only had a certain limited capacity – in terms of staff, suitable treatment rooms and time slots to see patients – to deliver the laser treatment to a specific number of people at any given time.
Methods
The qualitative process evaluation involved interviews and observations with a diverse sample of patients and hospital staff at all LiTEFORM trial sites. Our analysis of the introduction, embedding and sustaining of the LiTEFORM trial was informed by normalisation process theory (NPT). 76 NPT considers factors that affect implementation in four key areas: how people make sense of a new practice (coherence), the willingness of people to sign up and commit to the new practice (cognitive participation), their ability to take on the work required of the practice (collective action), and activity undertaken to monitor and review the practice (reflexive monitoring). The approach has been widely used in studies of the implementation of interventions in health care (www.normalizationprocess.org; accessed 21 April 2019). In the LiTEFORM trial, NPT helped us to understand how trial processes and interventions were introduced and embedded at each site for both patient and professional groups.
Sampling strategy
Our sampling strategy was informed by our current and prior experience in this area,64 our theoretical framework (i.e. NPT) and what was already known about the trial context. We aimed to achieve a balance between the spread of data (to avoid missing key events or issues) and the depth of data (a manageable data set that allows for in-depth analysis). We were also responsive to the trial context, with additional data collection in response to our emerging analysis or trial events.
Our sample of patients and staff recruited to interview was purposive, using the following criteria:
-
staff – a range of those professionals involved in the LiTEFORM trial at each site, including the site principal investigator, staff involved in recruitment and those involved in delivery of the intervention, and a range of professionals, including medical, nursing, allied health professional (AHP), research nurse and other staff (Table 23).
-
patients – a range of male and female patients from each site (Table 24).
| Characteristic | Number of participants (n) |
|---|---|
| Sitea | |
| 1 | 4 |
| 2 | 6 |
| 3 | 5 |
| 4 | 5 |
| 5 | 3 |
| 6 | 3 |
| 7 | 4 |
| 8 | 2 |
| 9 | 4 |
| Sex | |
| Male | 13 |
| Female | 23 |
| Profession | |
| Dental consultant/maxillofacial surgeon | 2 |
| Dental hygienist/dental nurse | 3 |
| Doctor: oncology | 7 |
| Doctor: ENT | 2 |
| Radiographer/radiotherapist | 7 |
| Nurse | 3 |
| Research nurse/research officer/research manager | 10 |
| Other (e.g. laser safety and SLT) | 2 |
| Role in studyb | |
| Site PI | 9 |
| Research support | 7 |
| Laser delivery | 15 |
| Wider clinical team | 4 |
| Other (laser safety) | 1 |
| Number of interviews | |
| 1 | 31 |
| 2 | 5 |
| Characteristic | Number of participants (n) |
|---|---|
| Sitea | |
| 1 | 3 |
| 2 | 5 |
| 3 | 4 |
| 4 | 5 |
| 5 | 3 |
| 6 | 4 |
| 7 | 3 |
| 8 | 2 |
| 9 | 1 |
| Randomisation | |
| Allocated to the LLLT arm | 17 |
| Allocated to the sham arm | 13 |
| Sex | |
| Male | 23 |
| Female | 7 |
| Age (years) | |
| 40–49 | 6 |
| 50–59 | 9 |
| 60–69 | 10 |
| 70–79 | 5 |
| Number of interviews | |
| Recruitment interview only | 10 |
| Post-treatment interview only | 7 |
| Recruitment and post-treatment interviews | 13 |
The timing of both staff and patient interviews varied depending on the activity at each site; for example, a change in recruitment frequency prompted a new phase of interviews. Our sample of audio-recorded recruitment conversations was partly a convenience one because sites varied in terms of their engagement with this part of the qualitative research (Table 25). Sampling was monitored and discussed regularly at each team meeting.
Data collection: recruitment and consent
Verbal consent was obtained to audio-record the recruitment conversation with patients at the start of the discussion. All of those present gave verbal consent, including any friends and family. If anyone declined, the discussion continued without being recorded. Consent could be withdrawn at any point during the discussion. Written consent was subsequently provided within the main trial consent form and on a separate consent form for any friends and family present; if those present chose not to give their written consent, the audio file was immediately deleted (see Report Supplementary Material 4 and 9). A follow-up information sheet was provided to explain how consenting individuals could contact the research team or qualitative researcher should they change their mind about the recording (see Report Supplementary Material 10).
Patient interviews
During the trial consent discussion, patients were asked if they could be contacted about a telephone interview and were given an information sheet (see Report Supplementary Material 11). All patients were invited to consent to being contacted, including those who declined taking part in the randomised trial. Patients were advised that not all of those who consented to be contacted would be invited to participate in an interview.
A purposive sample of patients who had given consent to be contacted were approached 1–2 weeks after the recruitment discussion and/or at approximately 4 months or 14 months (maximum of two interviews per patient). The qualitative researcher contacted the patient and, if the patient agreed, arranged a convenient time and date to conduct the interview. Verbal consent was taken and recorded at the start of the call, using an approved checklist (see Report Supplementary Material 12).
Staff interviews and observations
Staff were given an information sheet and completed a written consent form giving their consent (1) for an observer to be present and make notes at site initiation visits, training events and other LiTEFORM trial meetings; (2) to have their trial recruitment conversations audio-recorded; and (3) to be approached regarding participation in a LiTEFORM trial interview (see Report Supplementary Material 13 and 14). For all telephone interviews, consent was affirmed verbally at the start of the interview. Written informed consent was obtained prior to the start of the interview for all face-to-face staff interviews.
Interviews with health professionals took place throughout the trial, from set-up onwards. Most interviews were conducted via telephone, although some were carried out face to face (e.g. to coincide with a site initiation visit observation). No more than two interviews were conducted with any one staff member.
Topic guides (see Report Supplementary Material 15 and 16) were developed, agreed within the team and included the following:
-
Patient interviews –
-
trial processes (e.g. initial approach, information given, recruitment encounter, ideas and/or concerns about randomisation and consent), the intervention (e.g. willingness to undergo LLLT treatment and/or concerns about impact on health and acceptability), experiences of the delivery of the intervention (including the timing and location) and experiences of mucositis
-
-
Staff interviews –
-
views and experiences of LLLT, trial processes, and patient and recruitment pathways.
-
The content of the interview was flexible to accommodate additional unanticipated areas and to reflect the stage of the patient in the treatment process, the developing analysis and, in the case of a follow-up interview, what was known from the prior interview. Interviews and observations were conducted by an experienced qualitative researcher (the majority by LL with some by JM) who had experience in conducting interviews on sensitive topics. Interviewers were blind to patient allocation.
Qualitative data management and analysis
Interviews and recruitment discussions were, with consent, audio-recorded, transcribed verbatim and edited to ensure anonymity of the respondent. Contemporaneous field notes from non-participant observation in clinical settings were edited to ensure anonymity of the participants. Data were managed using NVivo software (QSR International, Warrington, UK). The analysis was theoretically informed by NPT and was conducted in accordance with the standard procedures of rigorous qualitative analysis,77 including open and focused coding, constant comparison, memoing,78 deviant case analysis79 and mapping. 80 A sample of data was independently coded and cross-checked: the purpose of this exercise was to identify and reflect on differences. A proportion of the data was analysed collectively in ‘data clinics’, where the research team shared and exchanged interpretations of key issues emerging from the data. The analysis was conducted by Lyndsay Lindley/Tim Rapley/Nikki Rousseau (all with a range of social science backgrounds) who were joined for some data clinics by the PPI lead Valerie Bryant (who has training in qualitative analysis).
Relationship between the process evaluation and the trial
The developing analysis was regularly discussed at TMG meetings and, when appropriate, it informed changes to trial processes. Examples include a recommendation that sites should show patients a picture of the LLLT device during the recruitment discussion (giving an indication of size and appearance) and the production of a checklist of areas that needed to be addressed during site set-up, which was provided to the post pilot phase sites.
Results
Forty-one interviews were conducted with 36 staff members across the nine participating sites, with the number of members of staff interviewed at each site ranging from two to six (see Table 23). The site PI was interviewed at least once at each site, and at least one person who was involved in the delivery of the laser was also interviewed. Interviews took place throughout the period of study data collection, with early interviews concentrating on activities around study set-up and late interviews focused on plans for the laser treatment post trial closure. Interviews lasted between 8 and 51 minutes, with most lasting approximately 30 minutes. Forty-three interviews were conducted with 30 patients, with between one and five patients interviewed at each site (see Table 24). Both the patient and the interviewer were blind to allocation, but we checked during data collection that our sample included both those receiving active laser therapy and those receiving sham treatment. Patient interviews typically lasted 20–25 minutes. Six sites contributed audio-recorded recruitment conversations, with between 1 and 10 conversations recorded at each site (see Table 25). Sites were encouraged to record recruitment conversations and were given equipment and support to do so, but sites and individual health professionals were free to choose whether or not to participate in audio-recording. When it emerged that the limiting factor on recruitment was the site capacity to treat patients rather than patient willingness to participate, the research team focused data collection on additional interviews rather than on encouraging sites to record additional recruitment conversations.
Findings
Of central importance to this trial was a small box with a wire leading to a small hand-held probe that delivered low-level light treatment. One member of staff who, after a short period of training, delivered LiTEFORM treatment to patients noted that ‘the actual laser treatment is quite easy to do’. Patients also commented that the actual treatment is ‘very simple to do’. The delivery may have been ‘quite easy’ and ‘simple’ to do, but introducing, embedding and sustaining a new (trial) treatment pathway to provide LiTEFORM treatment was deeply complex:
It was a very complex trial to do. It’s not just like any chemotherapy drug where everything is set up and you’re just trying a different drug but the process of giving chemotherapy is in place. This is a completely new treatment. It was just much trickier than anyone involved with the trial really envisaged.
OncCon 8
Centrally, the delivery required ‘setting . . . up a new service’. Staff at the sites had to work hard to align the trial pathway with the overall organisation of head and neck clinical pathways and with each trial participant’s specific clinical pathway. There was, for all sites, a large ‘hidden workload’, often seen as much more than that of other trials that they had been involved in:
It’s kind of like hidden workload but there is nothing to show for it at the end of it, if that makes sense. There is quite a bit of time making sure everybody knows where they are going and is going to get there at the time they expect. At the end that doesn’t come up on workload calendars for example. There is a lot of that kind of what you might call hidden workload.
ResNurse 4
This hidden workload not only was centred on co-ordinating the conversations, schedules and actions of patients and staff, but also included work such as enrolling organisations and teams; (re)finding and (re)adapting rooms; (re)finding and (re)training staff; financing, finding and adapting equipment; and developing optimal delivery approaches. Prior to the trial set-up starting, the mundane, seen but unnoticed co-ordination work around introducing, embedding and sustaining a new service was (relatively) black-boxed for everyone involved (e.g. trial team, sites, funder) given that this was a trial about ‘just introducing’ a new device. However, the trial was ‘much harder than we’d all anticipated’ because the delivery of the LiTEFORM treatment could not integrate easily and rapidly within a range of organisational, clinical and professional contexts.
Introducing: setting up the trial – integrating with bureaucratic and organisational orders
The time that it took from the initial contact with the trial team to the set-up of the trial at each of the sites ranged from just under 5 months to over 14 months. A range of factors affected this timeline. One participant outlined part of the process of setting up the new LiTEFORM trial service:
So, there’s lots of hoops to jump through. You have to get funding for the laser, buy the laser, then you have to get approved by the cleaning safety people. . . . You have to get medical physicists to say that it’s OK to use, that your room that you’re going to use it is OK and the people who are using it have been trained to use it. So, all those hoops you’ve got to go through.
MedCon 7
As we will see below, these ‘hoops’ also included the co-ordination work around enrolling teams and getting trust sign-off, as well as the work of finding an appropriate room and finding staff to deliver the LiTEFORM treatment.
‘We got the right people quite early on’: seeing value and enrolling people and organisations
Some sites had already expressed an interest in taking part in the trial during the grant application process. However, over time, five sites had decided for a range of reasons, including the key contact who was leading at a site leaving, the range of competing trials at a site, and formal and informal capacity and capability reviews, that they no longer had the local or broader organisational resources or support to take part. The main reason for wanting to take part routinely focused around an individual or group interest in the possibilities for LLLT for treating OM and exploring that through a rigorous design:
It’s like, we really like this study, we felt it was a really good study. There’s lots of studies that you do where you kind of go, ‘It’s this versus placebo’. And you kind of, it feels like well, let it go, whereas this felt like a really useful, directed, novel, clever kind of way of doing things.
OncCon 7
Taking part was ‘a no brainer’ for these core individuals or groups of people at these sites, with two sites already delivering LLLT. A core group of people at a site needed to see the potential (scientific) value of the clinical question to ‘think it’s worthwhile’. They then needed to take leadership, that is they needed to do ‘a lot of the organising’. Getting members of the broader teams together to both discuss the idea of the trial and think about how they could actually make this work at their site was central to driving forward the introduction and the embedding of the trial. At many sites, this work of enrolment went relatively smoothly, with teams being ‘really on board’ and engaged, with no ‘reservations . . . not even a negative’.
Overall, professionals were broadly in support of the design of the trial including a sham arm. However, at a few sites, notably those with experience of delivering LLLT, some people had concerns about the sham arm; in particular, they were worried that it would become apparent to them whether a patient was receiving active or sham treatment. They expected that those receiving LLLT would have less severe effects and the concern for them was around how they would manage conversations with participants whom they believed to be receiving placebo. However, as can be seen below, they felt that this potential issue was managed effectively through the development of technical solutions to blind operators and patients.
However, within some sites there were elements of resistance, which at times created issues and some delays around set-up. The degree of resistance of the broader team and the focus of that resistance varied between sites. For example, at one site questions were raised about the trial design:
There was also some open hostility to the trial from some of the people in the department. Their objection was that the benefit of the laser has already been proven so you don’t need to do this trial. I think also the design of the trial and the very open inclusion criteria . . . I also think there was an element of it being more of a commercial exercise for THOR than a robust scientific trial. That was another objection.
ResNurse 4
In this context, the focus was on the purity of the science, in terms of issues around equipoise, inclusion criteria and explicit links to an industrial partner. Overcoming such resistance took time and was an element that delayed a more rapid progress of the set-up at this site at which questions had been raised.
Alongside this, there were elements of milder resistance tied to a lack of priority for some team members. People at sites often felt that the trial may be ‘a nice thing to do’, but they lacked collective commitment and, at times, lacked key (senior) people driving the trial agenda. Where there was more collective buy-in and commitment to the trial across the department, the initial progress was more rapid. As teams were working to engage and enrol staff, they also worked to generate local organisational approval, going through what one described as ‘hurdle after hurdle’. Sites were aware of the potential issues:
[We] started the set-up period some months before, probably at least 6 months before, as is often the case there were issues with R&D [research and development] at our trust, which slowed things down.
MedCon 8
In part, this was tied to the nature of this trial: this was seen as a ‘new intervention’ or ‘novel treatment’, so a range of forms, committees and signatures had to be aligned. By contrast, at one site we were told that it was relatively easy, with ‘no major barriers from inside the hospital to it’.
‘Well actually the room’s not suitable’: co-ordinating a (safe) room to deliver the LiTEFORM trial
Another central task was finding a ‘suitable room’ in which to provide the LiTEFORM treatment. One person described part of the process that led to a delay in starting the trial:
I think there wasn’t somewhere to deliver the laser. There wasn’t a suitable room . . . There was almost like . . . whispers that, ‘there is a room, there is a room’ but when it came to the crunch it was clearly not suitable.
ResNurse 4
A suitable room had to fulfil specific dimensions in terms of spatiotemporal affordances, alongside specific physical and safety aspects to enable it to be ‘laser suitable’. These included the following:
-
The room’s relationship to the local geography of cancer treatment within each site. The location of the room in relation to the spatial organisation of patient care was key. It was especially important in relation to radiotherapy treatment suites because the LiTEFORM treatment would routinely be co-ordinated with patients’ outpatient appointments. Therefore, each site worked to find a room that would be convenient for patients to visit within a reasonable time scale within their overall appointment time.
-
The room’s relationship to the temporal organisation of care within each site. Whether or not access to the room was restricted to specific days and/or times was also central. Rooms had to be as accessible as possible, especially in terms of times available for the LiTEFORM treatment to be delivered. In part, staff became aware that flexibility in times of access could then accommodate any changes in specific patients’ LiTEFORM treatment slots. Sites had to work to find a room with limited calls for other type of use from their own or other clinical services.
-
The room’s affordances to support the (safe) delivery of care. The room also had to be able to be adjusted to enable the appropriate delivery of the LiTEFORM treatment. Given that LLLT is classified as a Class 3B laser, each room had to be prepared to meet specific laser protection standards. For example, the room should have no reflective surfaces and, if the room did have a window, full blackout blinds needed to be in place or be capable of being fitted. As some teams learnt over time, the room also had to have space to enable a chair, dental chair or treatment couch to be positioned to allow operators freedom of movement around the patient. Therefore, sites had to work to co-ordinate the adjustments to the local material geography of the room.
In practice, a suitable room was found relatively rapidly for a few sites, including at a site that was already providing therapeutic LLLT. However, for many sites, rooms ‘are always at a premium’ within their organisations.
Once the room was found, sites had to work to adjust the room to make it suitable. In one case, the LiTEFORM treatment room was moved three times. The initial room, located in a different department and different part of the hospital, ‘was obviously ideal’ because it had ‘a proper dental chair and everything’. However, the LiTEFORM participants and staff had to move to another room because the host department needed additional rooms as they expanded their own clinics. Albeit a more extreme case, it demonstrates the very practical organisational work that sites faced, which took time. Some suitable rooms were found within the radiotherapy department or reasonably close by, with others in different floors or areas of the organisation, one being around 5–10 minutes’ walk away.
‘. . . there wasn’t anybody to deliver it’: co-ordinating suitable staff (training) to deliver the LiTEFORM treatment safely
Sites also had to work to find a suitable number of staff who were adequately experienced and/or suited to deliver treatment and with the capacity to be released from other duties. Centrally, set-up at sites was affected by trying to find and co-ordinate these ‘suitable’ staff in a range of ways, including prospectively finding enough staff with potential capacity and managing their timetables to align with initial ideas about treatment delivery schedules, alongside specifying funding streams, contracts and goodwill between different parts of an organisation, as well as within a specific department. In part, this was further confounded because active LLLT was defined as an ETC; therefore, only half of the time of the site staff was funded through the trial. In some cases, this took quite some time and effort.
Alongside this, the co-ordination of training created problems for many sites. The practicalities of scheduling training to specially selected, often large, groups that could all attend at specific times was difficult to co-ordinate and, therefore, could create delays. Some sites conducted training sessions alongside NCTU set-up meetings. Training included laser safety training: internal and/or online (e-learning) training that covered a mandatory range of topics that, in this context, went beyond the specificities of the LiTEFORM trial device. Many found the training to be onerous and some described elements of it as ‘arduous’, ‘gruelling’ and ‘challenging’:
It was very, very tough and most of the online training wasn’t relevant to the laser that we’re doing and, actually, really frustrating, making people do mandatory training for something that’s not relevant.
OncCon 8
Staff outlined that despite being ‘very well trained’ and gaining continuing professional development credits, they were frustrated about the elements that had to be included in the trial because of specific national and local regulatory regimes, but were not viewed as pertinent to the trial.
The trial laser training that was delivered by the industry partner, the supplier THOR, also had a mixed reception. Some felt that it went into too much detail and others felt that they would have liked more detail. Some people regarded the training that THOR provided as inappropriate, a ‘waste of time’, ‘not great’ and of poor or inconsistent quality, or too heavy on background and embedded in a broader THOR ‘marketing’ agenda:
All we really needed to know was the safety and how to use it but I would say the THOR training really was a waste of time because we envisaged we’d [be] practicing with a sham laser, on each other, getting used to it, familiarising ourselves and it wasn’t, it was just going through a booklet reading.
Nurse 3
In addition, site staff agreed that the THOR training, which lasted around 2 hours, did not offer enough detail around the pragmatic ‘hands on skills’ that were required for working with the device and around the actual delivery of the LiTEFORM treatment to patients. These comments were fed back to THOR and they amended the content of the training. A couple of sites requested and then received a second training session from THOR around the pragmatics of working with the device, albeit this mainly focused on assembling and switching on the device, and through that the team members became more ‘comfortable’ using the laser. After these formal training sessions, some sites also arranged local sessions during which they undertook ‘practice runs’ or ‘walk-throughs’ in setting up and operating the equipment, albeit without turning the laser on. Finally, training could also include specific training around infection control, led by an infection control team or a specific industry representative. Some sites also undertook WST training. This also proved to be difficult to arrange because all those needing training had to be available at the same time as the sites.
Overall, the co-ordination, focus and content of the training at each site created practical issues as well as issues around trust in operators and workability of the device, especially in terms of developing adequate skillsets. In addition, some sites worked to cascade core elements of the training to other members of their teams [i.e. dieticians, speech and language therapists (SLTs) and specialist nurses] so that if patients asked a question ‘there was awareness of it throughout the department’.
‘The NHS won’t pay for a bit of kit for a trial’: co-ordinating access to (modifications to) devices
The majority of sites also needed to acquire the LiTEFORM trial devices themselves because the device was not funded through the trial. Each site had to organise and agree a specific funding stream to purchase the device. Nearly all sites requested funds from local charities affiliated to their site. This sourcing of funding (i.e. the process of applying, agreement and actual sign-off) was another ‘obstacle’ that created delays at most of the sites:
It just introduced a delay, again in terms of getting funding for the equipment. . . . So it wasn’t the amount of money, just the process that one has to go through, to get local charitable funding to agree to fund something, that is within a national trial, if that makes sense.
OncCon 4
Given that there was a sham arm in the trial, the devices themselves had to be adapted to maintain the blinding of staff delivering the treatment (and the patients). Therefore, all sites needed to acquire trial-compliant devices. At one stage, the system proposed by THOR to enable the randomisation was an ‘A/B switch’ that would deliver either active or sham ‘laser’ treatment. However, during the site set-up visits this led to concerns from staff, especially the proposed operators, who felt that they would come to know, by the condition of the patients, which treatment that they were receiving when they pressed a specific switch. The introduction of the new randomisation controls (the 0–99 randomisation grid) and laser safety glasses for staff and patients helped to increase staff confidence in the trial, with some feeling ‘more ethically comfortable’ providing sham treatment than they did previously.
Finally, a specific ‘cover’ or ‘sleeve’ had to be used over the probe used to deliver the laser. This added a 1-cm ‘cap’ on the end the standard probe used with the device to standardise the distance from the specific area, or spot, to be treated. Some sites had difficulty sourcing the new sleeves for covering the probe. At one site, they found technical problems with the laser equipment as they were undertaking mandatory tests:
So, we had various phone conversations and video conferences and I was being told to move it very carefully, which was causing me huge concern because I thought, ‘If we’ve got to do this to get the reading, how are we going to know we’re actually delivering treatment?’ . . . Well, eventually they came to us, erm, and it turned out they’d sent the wrong probes. So, they spent 5 weeks basically, telling me that I was an idiot and I didn’t know what I was doing; they could have been right because I’ve never used a laser before.
Radio 11
Staff at this site began to lose confidence that the laser would be working properly when they used it on patients and considered withdrawing from the trial. However, once this was resolved, they went on to open the site and recruit to the trial.
Embedding: running the trial – integrating organisational, clinical, lay and professional orders
During the set-up phases, staff at the sites had some concerns about whether or not patients would be willing to participate, given option of the sham treatment arm:
Well I think that first of all it was an assumption on my behalf that we might struggle with the sham. Because it is such a horrible treatment, the whole idea of signing up for something where it may be a sham treatment, I wondered whether people would be willing to sign up for something. But obviously that was completely unfounded.
Other 1
As we will outline, staff across all sites found that patients who were approached about participation were generally keen to take part. Staff learnt over time to optimise the actual delivery of the LiTEFORM treatment to each patient and patients, and staff were (mostly) very positive about the treatment and its impact. Despite this, the trial did not recruit to target. Capacity to deliver the therapy was a significant challenge:
It was easy for us to recruit patients, almost all patients that we approached wanted to go into the study but we couldn’t deliver it within a very busy NHS service, in the way that we had hoped that we would able to, so it limited our recruitment.
OncCon 4
Patient flow was a central issue for all of the sites: how best to co-ordinate this, given that they had only a certain capacity to deliver the laser to a specific number of people at any given time. This issue of capacity at sites is described and explored later and was the key factor limiting recruitment to the trial. However, although capacity to deliver the LiTEFORM treatment limited recruitment, other factors made recruitment challenging.
Approaching patients: integrating organisational and lay orders
Across the sites, staff reported that patients, when approached, often took up the offer of joining the trial. They described ‘people kind of falling over themselves to be involved’ and that ‘patients, they were just dying to go into it’:
They do seem quite willing, especially with LiTEFORM because it’s something that is felt it’s not going to make them any worse but it has got the potential to make them better, improve their symptoms, if you like.
ResNurse 3
Patient reports echoed those of the staff, with patients who agreed to randomisation describing participation as a ‘no-brainer as far as I was concerned’ and reporting that, after it was offered, they said ‘Yes, I’ll be delighted to’:
Nobody knows which you’re getting. It’s a 50/50 chance. In my view, if it’s something that can help me during the treatment, yes, I’ll go for it. It’s a no-brainer.
Patient 25
Therefore, despite some staff and the trial team’s potential concerns, the patients approached were comfortable with the sham element of the trial overall, with the majority willing to take part.
‘When we first started, we thought it would be straightforward’: identifying potential trial patients
Before approaching patients, the sites had to co-ordinate the work of identifying potential patients and understanding when was best to approach them. Some sites had competing trials and reported that they encountered patients who could have been suitable for the LiTEFORM trial who had already been recruited for another trial. The complexity of the patient pathway also made recruitment challenging, in part because of the inherent uncertainty about each patient’s treatment pathway. The trial could be offered only to those patients who would receive radiotherapy. For those patients who received surgery, staff would know if they were eligible only once they had a scan post surgery because this would inform whether or not radiotherapy would be offered. Site staff needed to find the optimum time to first approach patients: too early and the treatment might not be decided, too late and they might have been recruited onto another trial, or their treatment schedule might already have been set up:
It’s a lot of doing patient tracking, I am forever looking at my log if there is any that I have got a question mark over and haven’t quite got a conclusion yet on. Then I am regularly checking the appointment systems to just make sure that I am not missing anyone, and I am sifting through letters and notes to read exactly what has been discussed with the oncologist. And what is the plan? You know, when are they likely to have any treatment, if any . . . because everything is happening on a fast track.
Den 1
Treatment plans could change based on tests or new information. Multiple staff and specialties were involved and a large number of preparatory elements for treatment had to be completed in a short space of time. There was also the constant awareness of the need to start treatment urgently in line with government guidance81 (i.e. 31 days between the date of decision to treat and the start of treatment):
You just don’t really know. And then some take longer because they’ve got teeth extractions and what-have-you to deal with. And yet others, they can be seen on a Wednesday and they have their planning appointment on the Friday. Which from a consenting point of view makes life difficult. . . . And then actually get them scheduled. It’s a nightmare.
Other 7
In this context, there might be too little time (i.e. in terms of initial discussion, providing patient information materials and allowing at least 48 hours for decision-making, any follow-up discussions, consent discussions and baseline assessment) to actually recruit a patient prior to the first radiotherapy treatment appointment.
Sites worked hard to co-ordinate recruitment conversations with patients and to communicate effectively with the patients who they were approaching. They refined the process, discussing potential ways to optimise recruitment and developing specific resources, such as flow diagrams. After an initial round of treating a patient, some sites felt that some patients would be ‘better candidates’ than others. The excess of willing patients compared with capacity enabled some to try to approach specific types of patients:
I think that as we got on, we got better at selecting patients who might be more able to tolerate it . . . At the beginning, we would get them in because they fitted the inclusion criteria but as time went on, we looked for the younger, the fitter . . . who would be able to do it three times a week and keep their mouths open. But even for them, by the last weeks of treatment, it wasn’t easy but they did go through it.
OncCon 4
Some sites found that other aspects of treatment for HNC (e.g. nausea relating to chemotherapy and impacts of surgery) could make it harder for patients to tolerate the LiTEFORM treatment. Therefore, some tried to ‘opt for’ only patients who were having adjuvant radiotherapy as opposed to chemoradiation, as otherwise patients were ‘finding it too much’. However, irrespective of staff being ‘very enthusiastic’ about the trial, with some focusing on approaching specific types of patients, the flow of potentially eligible patients was often erratic. It was difficult to match to the availability of ‘free’ LiTEFORM treatment slots:
It’s all dried up a bit at the moment . . . we haven’t had anybody suitable for quite a couple of months now. . . . We had about four or five who were all suitable at the same time. So, a couple of weeks ago, we put two in and so the others had to go through treatment without it being offered to them and then nothing for a while. It’s just a waiting game sometimes.
OncCon 8
At times, there were several eligible patients and yet no capacity to deliver LiTEFORM treatment. At other times, there was capacity yet no eligible patients.
‘Just hopefully to lessen my symptoms and to help other people coming behind me’: discussing and deciding whether to (not) take part in the trial
Recruitment happened at a time when patients had a lot to deal with: they were meeting many different professionals and receiving unwelcome information about their diagnosis and treatment. They were also undergoing unpleasant procedures, such as tooth removal and fitting for radiotherapy masks, and many were recovering from surgery. However, most patients appeared to engage actively in the recruitment process.
In the recruitment consultations, several patients had some initial safety concerns about the trial that they discussed with the recruitment clinicians. These concerns were often associated with perceptions of lasers, which were seen as potentially hazardous. For example, in one recorded consultation the participant asked, ‘My daughter is pregnant and me having lasers isn’t going to affect that?’. In an interview, a patient outlined another pre-randomisation discussion around lasers:
I just asked the hygienist, when I met her before, starting to find out the information, ‘Would I feel it?’. You think of a laser, it gets hot, something that’s Star Wars [© Disney, Burbank, CA, USA] like being aimed in your mouth. But she explained everything fully and that it would just be really a light and that I don’t feel anything.
Patient 12
Some reported finding it reassuring that this was a relatively late phase trial, that they would not have ‘gone for something in its early stages’, and that the technology had been used in other contexts previously.
Staff were also ‘surprised’ at how ‘positive the patient feedback’ was towards the sham arm. In their interviews, the patients discussed the process of randomisation: that the trial team would ‘put the code into the computer’ and the specific arm selected, ‘it’s just random’, that ‘it’s a gamble really’. They also articulated their understanding of the purpose of a sham arm and what the implications were for the patients:
I mean that’s the only fair way you can test the thing to see if it works, to be honest. There has to be a winner and loser in every single one so it’s the only fairest way you can do it.
Patient 20
They were aware in some contexts, ‘that’s the way all these studies go, you’ve got to do it anyhow’. They also perceived there might be potential benefits from participation, often in relation to the ‘placebo thing’:
I suppose my reaction was, if I get the real thing, then the worst I can do is I’m wasting an hour every day, but it could help me. If I’m getting the sham thing, the placebo effect is not something to be ignored and it still helps people down the line anyway.
Patient 16
They also outlined how, irrespective of the arm and the efficacy of the treatment, ‘somebody is actually looking into my mouth. If there’s any problems there they can spot it’ (Patient 4). Overall, the patients who accepted randomisation were informed and comfortable with their choice: ‘double-blind testing, I’m absolutely happy with that’.
Patients also reported reading the provided information and discussing the trial with their families before making their decision. A small number of people had been actively looking for information about the best possible treatments available for their cancer. The majority of those who agreed to take part described their motives as tied to (conditional) altruism. 63 A sense of giving back for the care that they had received and a feeling of wanting something positive to come from a difficult situation ‘to help others as well’ were consistently mentioned by participants:
I have benefited from the trials that people have done with other things in the past and I was happy to continue in that vein, that if I can be of any assistance and it was of basically no risk to me, I was quite happy to do that.
Patient 3
The intervention seemed to appeal to many participants, as there was a sense that there was the potential for benefit and a relatively low risk of negative implications. The potential impact on those transporting them to and from the hospital was a key factor in questioning participation; many had considerable distances to travel. Those declining participation did not want to create any additional burden on themselves as well as their family. Given the broad range and number of appointments they would be facing, they ‘just don’t want to be doing any more’.
Scheduling the trial treatment: integrating with organisational and clinical orders
Staff had to co-ordinate the flow of trial patients. For all sites, this ‘steep learning curve’ created more work than they had anticipated:
It’s been quite difficult because the pathway is so intense and there are so many things that go on within it. It always just seems to be a difficult thing to organise.
Other 1
This was for them a question of ‘quite a lot of logistics’: co-ordinating the timing of LiTEFORM treatment appointments to align with patient, operator and room availability and integrate with all the other elements of HNC care. As a patient enters the care pathway, a whole range of appointments (e.g. one site quoted 18) are scheduled, including those tied to treatment (e.g. surgical, radiotherapy and chemotherapy), review and support (with a range of MDT actors, including, for example, specialist nurses and SLTs). Those managing the trial at the sites worked to:
Make sure that you don’t mess their system up too much. It’s massive . . . with anything to do with head and neck. But erm, you know, we could end up being a huge spanner in the works to put something else out of kilter.
Other 6
They did this, in the background, so that for patients this integration work became (relatively) invisible. The LiTEFORM treatment appointments needed to be delivered prior to radiotherapy treatment session, within 2 hours of the radiotherapy dose:
There wasn’t a sort of waiting time between, which was nice. There might have been a few minutes, but generally it wasn’t long. Logistically I don’t know how they did it.
Patient 15
Patients talked about how the co-ordination meant that the two sessions often ‘brushed up against each other’ and that they are not given ‘enough time in between’. Moving from LiTEFORM treatment to radiotherapy was usually relatively rapid, albeit in a few cases in which they might have to ‘wait a little bit’. All sites worked to minimise any such additional burden on the patients.
‘The timing perspective is it has to fit around the radiotherapy’: co-ordinating the delivery of LiTEFORM treatment
Staff worked to minimise patients’ overall appointment times through asking patients to arrive around 40 minutes before their radiotherapy appointment. They soon learnt to avoid days with other types of appointment, for example ‘to avoid . . . chemotherapy days, and the days where they see the multiprofession team’ (Radiol 5), as this could cause patients to have very long days with multiple appointments and gaps. Co-ordinating the two appointments (i.e. LiTEFORM trial and radiotherapy) could mean that patients were asked to leave home very early in the morning for a specific morning appointment or that they were leaving the site during rush hour, as radiotherapy was at the end of the working day.
Making sure that staff were available for a specific treatment slot was central and ‘a lot of the time, you had to jiggle’. They had to (re)check staff availability and manage the LiTEFORM trial rotas to match the radiotherapy schedule. As outlined above, the funding model for non-sham treatment delivery staff made this a little more complex because they needed to find some staff with ‘spare time on a regular basis’. Initially, sites expected that staff would need around half an hour per patient, yet all found that they ‘exceeded that’, especially at the start:
It took quite a bit longer than we were led to believe it would from the training. And also, the number of spots treated for each patient and obviously each of those takes a minute. So, with getting the machine ready, getting the patient ready, and then doing the treatment and then clearing away afterwards, it was meaning that staff were away from their other job for up to an hour at a time.
Other 2
Most sites outlined that the actual treatment part took between 20 and 40 minutes, partly because patients had to sometimes be given breaks, especially towards the end of their 6-week treatment cycle. However, they also had to include initial set-up and cleaning of equipment, completion of paperwork, and cleaning and storing equipment. At two of the sites, the session was delivered through the co-ordination of two members of staff. Therefore, one site used one LiTEFORM trial operator as well a student nurse or health-care assistant to manage paperwork and other tasks, such as getting the patient a drink. At the other site, a research nurse accompanied the operator. At a few sites, at the start of the trial people worked in pairs, learning from and ‘double checking’ with each other and over time gained confidence and began to work more autonomously. Over time, all sites reported that they had managed to reduce the time needed for a single session to around 45 minutes per patient.
The timing of each LiTEFORM treatment also had to align with the availability of the room in which the treatment was to be delivered, which could become another ‘limiting factor’. Some rooms were available only on specific morning or afternoon slots. Such constraints on access to rooms could affect potential recruitment flow. As can be seen below, close co-ordination with schedulers became central work for those organising LiTEFORM treatment sessions for new trial patients. In addition, in some sites, the delivery of the LiTEFORM trial was in a different location within the specific hospital (in some cases, in a different building). This meant that patients and staff had to co-ordinate travelling between different treatments:
I think it’s about a 10 minutes’ walk. I’m not sure . . . Yes, I think it’s about a good 5 minutes’ walk for the patient . . . I think a different floor, a different level . . . One of them [a patient], well, he did walk and then we got him a chair, because he wasn’t very well, but the other guy was much fitter than that guy.
Den 4
A few patients did find this movement between different areas, at times, a slight concern; they worried that they would not get to the radiotherapy suite on time. However, for most ‘it was fine’. In a few cases, staff delivering the treatment had to travel to a different site that was part of the same trust or different trust: these staff had to allow additional time. Other sites had a set-up that meant that the patients and staff had very little distance to travel.
‘It’s that certain amount of back and forwards to fit them in’: aligning treatment and trial treatment schedules
As outlined above, a whole range of patients’ appointments are scheduled ahead of time, once they enter the care of the head and neck team. For this trial, a central issue was the allocated timing of each radiotherapy appointment:
It was just really time consuming trying to work out where we can see the patients, without it disrupting too much of the radiotherapy appointments, having to readjust them. Because I think once the radiotherapy appointments are made, they’re set, they don’t like changing them too many times.
Den 2
Some radiotherapy schedules were ‘brilliant’ in that they easily aligned with the availability of LiTEFORM trial staff (and rooms). However, some ‘machines’ did not produce such ‘good schedules’ and, therefore, required a certain degree of negotiation, often on a weekly basis.
At the start, staff at some sites felt that they had little control over the specific timings of the LiTEFORM treatment. Others felt that they had more control and that it was ‘run quite smoothly’ when they were provided with 1 month’s worth of appointments in advance. The central line of communication was between the local trial co-ordinators and the schedulers:
I sit down with the schedulers to try and facilitate getting the patients’ appointments all sorted out because the radiotherapy schedulers do actually change, so I’ve been training up new people so I’m confidant . . . so it’s having sort of two eyes on the ball because . . . the schedulers know what they can do with patients and moving appointments around.
Other 7
For the trial team, getting to know and liaising with the team of schedulers ahead of time, either face to face or via e-mail, was essential. Trial co-ordinators needed to work with the team of schedulers to find ways to adapt the schedules to identify potential ways to try to adjust appointments. Even when working ahead of time, this was ‘not always that easy’.
However, there were some problems that were emergent in nature. Radiotherapy times did change on quite a frequent basis:
Radiotherapy . . . machines are so busy, often their times wouldn’t be the same time of the day or they would get changed because of machine breakdowns. Unfortunately, the communication between the change of appointments wasn’t getting relayed to the LiTEFORM staff. So the people who were prepared to do LiTEFORM say at 11 a.m. all of a sudden on that day the patient is not coming until 3 p.m. and you’re not available then.
Radiol 11
Such changes as a result of issues, such as breakdowns or people are running late, would affect the trial patient schedule and the operator’s (and room) availability. Constant monitoring, adjustment and accommodation work was needed by trial staff.
Many staff were taking on elements of the LiTEFORM trial work in addition to their ‘normal role’; therefore, at times, this could create ‘a strain’ on the local department(s). However, seeing the positive impact on patients, staff saw the value and, therefore, were willing to undertake the necessary accommodation work: ‘It did impact on the workload . . . when you see the patients benefiting so much it’s worthwhile to do that’ (ResNurse 8). All sites highlighted that all staff went ‘over and above’ normal expectations to integrate the trial into their routine work. The schedulers and the broader radiotherapy teams often worked to review, change and update the radiotherapy schedules to align with the availability of trial patients (staff and rooms). The ‘collaboration and the willingness to change things for the benefit of the trial’ (ResNurse 4) was central. When there had to be revision in schedule, people worked to accommodate this. Over time, the radiotherapy teams learnt to liaise with operators in a more timely manner: ‘they made sure that if any times were being changed, they would check with the LiTEFORM team’ (Radiol 11). Site staff were informed and engaged as many people as feasible, within large and shifting departments, about the trial and worked to engage any new starters. Good communication between different elements was central.
‘If you look at the numbers that you can physically do at one point, it is limited’: managing capacity to deliver LiTEFORM treatment over time
At the start of the recruitment work, site staff worked to understand the maximum capacity of trial patients that they felt that they could achieve at any one time point:
I think we’ve already determined that we’ll be able to have three to four having treatment at any one time in terms of capacity in our unit . . . just because we only have the machine, the room availability for half a morning.
Nurse 2
At another site, in an internal meeting, staff were trying to work out if they could ‘manage to have four patients at the same time’. However, staff at both of these sites learnt that this ideal of three or four at a single time point was not feasible. Some sites took a different approach and initially recruited single patients over 6-week periods to get more accustomed to the overall process. They did have ambitions to increase these numbers. One staff member outlined their thinking as their site moved up to having two patients going at the same time:
I think we will try to go up to three, but as I say, I really want to see how two goes because I’ve got the rota up in front of me now and I haven’t got someone down for – I’ve got someone down for every day this week, but there are a couple of gaps . . . there’s nobody down for delivering next Monday, and that’s the day we’ve got two, so that, I think it is going to be difficult.
Other 2
Quite early in this transition, they discovered the potential issues of managing more than a single patient at a time, and continued to have ‘concerns’ going forward. Over time, for nearly all sites, two trial patients seemed to be the maximum that could be managed at any single time. It was ‘a bit untenable to deliver too much per month’ (OncCon 2). However, some sites never got to the stage of recruiting two patients per 6-week cycle. The more successful (recruitment) sites tried to work with more patients at one time, but found that this was not sustainable.
Managing patient flow was a delicate balance at each site (especially in terms of numbers of trained staff) because if elements were breached, further issues could emerge. Most sites could manage issues such as covering staff while on holiday and occasional sickness, but anything more than temporary changes were hard to sustain. Staff availability could cause problems and, for this reason, affected numbers that could be recruited at any one time point:
I think probably the most important thing to say is that we’ve had to stop recruiting to the study because of shortages of staff . . . obviously we’re asking people who’ve already got busy full-time jobs to give up some time to do this. And, you know, ultimately that got the better of us, even with people’s goodwill and everything else and wanting to make it work.
Other 2
Several sites lost trial-trained staff because of people being promoted and moving on to other work. They also had to manage staff on long-term leave. Site staff did not imagine that they would need to cover people ‘off for such a long period of time’. This occurred in contexts where organisations were already at capacity.
Sites tried to build a team of people to focus on having spare capacity through training additional staff, yet it was, at times, ‘still . . . surprisingly difficult’. A staff member at one site outlined that, in retrospect, ‘perhaps it would have been better to have trained more people earlier’ (ResNurse 8). The number of trained staff who were available to deliver the LiTEFORM trial was central to managing the potential throughput. All sites worked over time to increase the capacity of trained staff. Those staff who initially received the training were radiotherapists, nursing staff (specialists and research) and dental hygienists. Sites also trained some medical staff, with a view to covering gaps in the rota. Over time, additional staff were identified, engaged and trained to increase the potential ‘pool’ and, therefore, to ‘spread the load’. The number of trained laser operators at the start of the trial at each site varied from two to five, with an average around three, and by the end of the trial varied between 3 and 12, with an average of six per site.
Despite the work to control patient throughput and increase the potential pool of operators, at times, sites had to restrict recruitment further. At one site, because of organisational changes, staff had to cap recruitment firmly to a maximum of two patients per month. They noted that, despite that ‘people kind of falling over themselves to be involved’, even if the trial remained funded for more time, they would still have been able to put through a maximum of only one patient per month, given changes in treatment scheduling. Notably, one site stopped recruiting altogether for around 6 months because of reduced staff capacity; other sites stopped recruitment for briefer periods. As some site staff highlighted, a shift in the protocol could have also maximised whatever level of patient throughput, as ‘having it unblinded would have allowed us to see and recruit and deliver laser to double the amount of patients’ (ResNurse 4).
Receiving treatment: integrating lay, material and embodied orders
Understanding trial patients’ experiences requires the patients to be placed within the broader context of the trajectory of their overall treatment. Patients developed a complex set of symptoms during and following their cancer treatment, which varied in part depending on the exact nature of the cancer that they had and the treatment(s) that they had undergone.
Patients typically felt more ill as treatment progressed, with symptoms at their worst at the end of radiotherapy and for a period after radiotherapy was completed. All patients had some symptoms, with severe symptoms and significant weight loss common:
I’d been losing weight through the treatment, but sometimes I was losing nearly a stone a week. Mainly just because of bringing food back up again having eating it with a mucositis. It meant I was getting very little calories quite often. I spent nearly 3 and a half weeks at the end of treatment, and after, purely on rig tube feeding.
Patient 16
Various problems were common (Table 26), compounded for many by the removal of teeth in preparation for treatment:
Before I had the cancer treatment I had a further six teeth out. All I was left with was incisors. That debilitated my eating more than the sore tongue and more than any of the treatment . . . As much as anything, having no teeth was more of a handicap to me than the discomfort with the throat and the tongue.
Patient 3
| Symptoms | Example from patient |
|---|---|
| Sores and ulcers in mouth and throat | It was hideous. It was just the most hideous thing I’ve ever [laughs] experienced in my life. No, I just had a mouth full of ulcersPatient 9 |
| Dry mouth | I’m unable to eat anything that isn’t moist or hasn’t a liquid element to it, basically. So any bread or anything like that is virtually impossible, I haven’t tried for a long timePatient 6 |
| Soreness and pain in mouth and throat | I think it was, going into the fourth week, I then couldn’t swallow – it was too, my throat was just so sore that I couldn’t bear to swallow my own saliva, it was that uncomfortablePatient 4 |
| Increased mucus or thicker saliva in their mouth | I just had to spit it out, it just was awful. My breath stunk, it was there constantly, and that as well didn’t help with drinking, all of the mucus that I had. It was the horriblest thingPatient 12 |
| Loss of taste | My taste is weird. Some things are absolutely fine. Some things really aren’t. Sweet things really aren’t OKPatient 15 |
| Vomiting | My husband took me to hospital because he thought that I was really dehydrated and maybe needed to stay because all I was doing was being sick as well. So, I couldn’t keep my feed down, I couldn’t even keep water down reallyPatient 12 |
| Fatigue | All I wanted to do was sleepPatient 11 |
| Weight loss | I became so unwell with the radiotherapy that I was unable to eat. And I then suffered all the usual problems – I lost 3 and a half stone in weight. I went from 15 stone 8 down to 12 stonePatient 1 |
Patients’ symptoms could have an effect on the experience of, and decisions about whether or not to continue on, the LiTEFORM treatment. They also affected patients’ appraisal of the impact of the LiTEFORM treatment, with difficulty in knowing which symptoms might be helped by the LiTEFORM treatment.
Who knew a minute was so long? Embodied experiences of receiving the LiTEFORM treatment
The LiTEFORM treatment appointment, a 45-minute to a 1-hour slot, would always be delivered prior to their radiotherapy treatment. All of the patients were aware that they would have to spend additional time at the hospital. As we saw above, staff at the sites worked hard to minimise this disruption. For many patients, this meant that the treatment took ‘an extra couple of hours every day’, especially if they were travelling some distance to the hospital, so this could create ‘a long day’. As one patient outlined:
It’s 50 miles to [hospital] and 50 miles back, for 6 weeks . . . whether the effects of radio[therapy] were starting to have an accumulative debilitating effect but it was increasing tiredness every afternoon when I got back, I have no reason to believe that the LiTEFORM in itself was tiring. All it did was to add 45 minutes each day . . . which meant that I was in the hospital confines longer each day before I could escape to sanctuary at home.
Patient 20
Any additional time at the hospital created another layer of disruption to their daily activities for patients and anyone accompanying them. The patients outlined that they routinely just wanted to ‘go home’. In this way, the trial ‘adds another complication’ when patients are already not feeling ‘100 per cent’.
The time that they spent in the LiTEFORM treatment appointment, positioned on a chair or a treatment couch, was very much an embodied experience. Patients could receive 20–30 1-minute periods of the probe focusing on a specific spot. They were very aware of the need to position their mouth and tongue to enable the operator to access the different parts of their mouth for each 1-minute period. Patients experienced some physical discomfort from keeping their mouth open for a period of time:
But it was more like your mouth was tiring but also it was that with me, it was the fact it was sitting still for that sort of length of time. Yes, it was sort of like, ‘God.’ As comfortable as the chairs are, you were starting to get a little bit uncomfortable. And I think it’s just timescale, for me, it was just the timescale, and by the end of the treatment, you was sort of thinking, ‘Right, yes, I’m three-quarters of the way now,’ or just sort of, ’I’ve got four more to do here . . .’
Patient 8
Their sense of time was often measured in minutes or the number of spots that needed to be treated, and embodied through the positions they ‘have to hold’.
Some people found the LiTEFORM treatment to be difficult: ‘I’m not going to lie, it was uncomfortable, it was painful at times’ (Patient 5). Being asked to ‘stick your tongue out and pull it over here’ and hold specific movements was described as ‘unpleasant’, ‘awkward’ and ‘tricky’. These difficulties appeared to be compounded by side effects that patients were already experiencing. It could affect their feelings of nausea and their pain (or anticipation of pain) when sores and ulcers were already present. Thick secretions, feeling a gagging reflex or sensation, or a fear of ‘any smell emanating’ while they held their mouths open could be made worse. However, for others, this was ‘easy’. One patient outlined: ‘for me, it was no trouble at all. Just lie back and open your mouth’ (Patient 3). In the broader context of their cancer treatment and its side effects, the LiTEFORM treatment was seen, by some, as a relatively benign interlude. All of the patients valued the efforts the operators made to make the process easier for them, including giving them rests and water, talking them through the process and sometimes playing music in the background.
‘So I had to stop the trial’: evaluations of taking part in the LiTEFORM trial
Patients described the experience of the LiTEFORM treatment as ‘intense’ and ‘intimate’. They were committed to the trial and tried hard to complete treatment. As they continued through treatment, those who started to feel more unwell (and this was the overwhelming majority) had to reflect on their participation in the trial. Being unaware of treatment allocation was key for continued engagement:
If it was suggested during the treatment that maybe I wasn’t getting the laser treatment I may well have knocked it on the head . . . I would have probably said ‘I’d rather not go ahead with this trial’. Particularly as the treatment went on and I was struggling a bit.
Patient 6
Continued engagement was, in part, contingent on an evaluation that the trial would still potentially benefit them: that they did not discover they were receiving the sham treatment. The broader context of helping others was also a central factor: that this was ‘an important thing’, that taking part was ‘wasn’t just about me’, that the trial had the potential to help other people if it demonstrated that LLLT was effective.
To carry on taking part, they described that they had to be ‘strong willed’ and, although they could ‘cope with it’, they were aware that they may need to ‘re-evaluate’ their position. They felt reassured that they had the option to withdraw. However, many found it ‘challenging’ and a few patients were unable to continue because they were feeling ‘generally quite unwell’:
The process of putting the laser in my mouth and laying with my mouth open, the nausea, I had such a high level of nausea that I just couldn’t actually lay still long enough for them to be effective with the laser.
Patient 1
This patient managed 4 weeks of treatment before they felt that they had to withdraw from the trial, as in the final stages of radiotherapy treatment they ‘became really unwell’. One patient withdrew because they could not tolerate the light emitting through the laser safety glasses.
Patients also valued some contingent elements. For example, some mentioned how being asked to keep their mouth open for a period of time, and moving the tongue around their mouth, mimicked exercises they had been given to do to maintain their swallowing musculature. Another outlined how this made them more focused on managing the ongoing care of their mouth, motivating them to undertake ‘mouth washing and cleaning thoroughly and doing everything I could’ (Patient 10). More commonly, patients referred to the positive experiences of interacting with the staff delivering the LiTEFORM treatment. Each visit meant that someone was focused on them and it enabled them to discuss how they were doing and their ongoing experiences. For this reason, they felt that they had an additional layer of support.
Delivering treatment: integrating professional, material and embodied orders
A central feature of the trial was the practical enactment of the LiTEFORM treatment, that is how teams learnt over time to make it more workable for them and their patients. For those operators without prior experience of working in the mouth, key aspects that facilitated the workability of the LiTEFORM treatment were not as present at the start of the trial. In retrospect, they felt that they needed more guidance on the delivery.
These staff learnt over time how to manage the work. They moved from periods of closely monitoring their own and others’ actions to periods of generating new habits and routines and becoming ‘more confident’ over time. As sites started delivering the LiTEFORM treatment, many operators felt physical discomfort, what one participant described as ‘some ergonomic issues’. For example, in one case, the staff member outlined that they were ‘quite tense, I did have a problem with my shoulder’ (ResNurse8), with another team member stopping delivery. At this site, the staff member worked with a dental hygienist and senior nurse to adjust the bodily position. They helped the operators to be ‘relaxed a bit more’ and to become more aware of how they are positioning and moving themselves. Operators across the sites had to learn to focus on how they positioned their bodies, for example to focus on not ‘stooping too much’. They had to learn to reappraise their approach to each element of the process:
I just make sure that I’m not holding my arm too far away because that’s when it gets tired, so making sure it’s quite close to my body and just making sure the patient is comfortable with you being so close to them as well. It’s just things like that that you get used to then.
Radiol 11
This new knowledge focused not only on their own posture, but also on how to work with patients, how to interact with them, how to get the patient to move in specific ways and how to move themselves to optimise their delivery.
The room that they were allocated to provide treatment in could have an impact on how best to work with patients in terms of what equipment was available. Staff learnt to change elements of the room, including the furniture, to enable the most effective and comfortable environment for both parties. Those with prior experience with working in the mouth had already developed a stock of knowledge:
See, I sit on, what you call, a saddle chair. So, it’s like a saddle seat, which dentists use. I sit on that and I use my stool to the height and I sit and ask the patient to turn their head towards me or different positions. I find it’s alright that way. I think if you’re standing, it does put pressure on your back. You’re better off sitting, but you’re better off [with] a saddle chair, which you can tilt it as well and it’s better for your back.
Den 4
Working with a specific arrangement of equipment (e.g. a suitable chair for patients, a specific chair for the operator that enables more ergonomically efficient movement and appropriate lighting, as well as water) transformed the delivery for both the operator and the patient. Some sites already had, or moved to a room that had, dental chairs for patients. Notably, staff at nearly all sites outlined that, going forward, they felt that they should be using dental chairs.
Others already used (or worked to obtain) couches that they could adjust so the patient was not flat and was positioned away from walls so they could move around the patient. The one site that started with patients in a more standard chair soon moved away from using these, as staff saw how uncomfortable these chairs were for patients as well as for themselves. Different sites found different practical solutions; in one site the patient should not be ‘too flat’, as this created the problem of secretions, in another, ‘almost flat’ was the best solution. This was a process of gaining knowledge and support from those trained in mouth work alongside experimentation of ongoing adjustment (‘a little bit trial and error in the beginning’) of their bodily actions and their tools, rooms and resources.
These operators also developed specific interactional skills in how to work with patients. This included how to put patients at ease, how to introduce and discuss the laser and how to create a good interactional environment (with some, playing music), as well as how best to co-ordinate breaks in the treatment. Making sense of how to manage breaks was key: learning when to remove the probe from the mouth so patients can manage the discomfort and/or excessive secretions and take a drink, rest and ‘reset themselves’. The process of blinding the operator with laser safety glasses took a few operators time to adjust. A couple found ‘it was quite hard to see’. Operators with prior experience of delivering LLLT found them ‘weird’ and ‘strange’ because they could no longer see the red beam so their routines were breached. Others found them ‘fine’. At times, with excessive heat (and lack of air conditioning) the laser safety glasses could ‘steam up’. High temperatures within rooms, especially during summer, also caused problems. Some operators felt ‘a bit lightheaded’ in the high temperatures, others ‘faint’ and some felt ‘tired’. They learnt to take regular breaks and have water to hand for themselves (as well as for the patient) and used fans when possible.
Some new operators commented that, over time, they found the work ‘really fulfilling’. Staff, especially those from radiotherapy, and patients outlined that they had enjoyed the opportunity to build new relationships with each other. The radiotherapy process is very swift and can feel impersonal. Note that there was no suggestion by patients that radiotherapy staff are not very supportive to patients, but rather that this is a different way to spend time with patients. Through providing the LiTEFORM treatment, the operators were able to interact with and come to know the patients in different ways.
Reflecting on impact: integrating scientific, lay and professional orders
Staff and patients understood that there was a range of possibilities including (but not limited to) the following:
-
patients had received sham treatment and had not received any benefit
-
patients had received sham treatment but had benefited from ‘the placebo effect’
-
patients had received active treatment and had benefited (but to an extent unknown because they may be someone who would not have had ‘bad symptoms’ anyway)
-
patients had received active treatment and it had had an adverse effect.
Overwhelmingly, the staff interviewed felt that through providing the LiTEFORM treatment they could see a positive impact for some of their patients on their OM. Echoing the sentiments of many others, a staff member outlined ‘I think my feeling is that it definitely makes a difference’ (Other 2). A number of patients also reported a positive impact from the LiTEFORM treatment:
To be honest with you I mean I was convinced I was having the treatment. Obviously I still don’t know if I did or not, but I had it in my head that I was having the treatment.
Patient 7
Others who felt that they did not benefit thought that they were taking part in the sham arm: ‘I’m starting to think that I’m in that group as well because my mouth is really sore’ (Patient 23).
Patients were, overall, very positive about the experience of taking part in the LiTEFORM trial, given the additional time, interest and care that they had received from staff. They were very engaged with the trial, curious about whether or not they had received active treatment and whether or not it would be effective. They tried to understand to which arm of the trial they were randomised:
I’ve tried guessing . . . I’m one of these that because I’ve got half an hour to sit in the chair, I’m thinking, ‘Wouldn’t that normal light do that?’. I just really, really cannot, sometimes I think I’m getting it and then other times I think, ’No, that’s not getting it.’ . . . the way that you’re doing it is very, very good because I’m one of these people who would go to the end of the earth to try and find out whether I was or not and honestly, I cannot.
Patient 8
However, despite this inability to know for certain which arm they were in, the above patient felt that they were getting some benefit: that this was probably due to having active treatment, but that this could also have been a product of placebo thinking. Patients described a number of ways that they tried to discover, or notice, which treatment (active or sham) that they were actually receiving. They did this by trying to look for specific signs, be that aural and optical signs from the device and/or its immediate reaction in their mouth, and trying to make sense of any sensations that they were feeling in their mouth. They asked the operator whether they felt that they were receiving active or sham, or tried to judge the operator’s reactions. They compared their experience with those going through the treatment at the same time, either noticing that they were the ‘only one’ who was really suffering or noticing that others were in a ‘worse state’ so they ‘must be getting it’. They also tried to interpret their own physical response, noticing the specific presence or absence of specific symptoms, such as ulcers in specific places of their mouth. Several patients had taken photos of their mouths to help to see any differences over time. However, some patients were unable to make sense of their situation, for some they had ‘no idea’. Symptom complexity could make it hard for the patient to separate out what elements they were experiencing as a result of OM and what was caused by side effects of other treatments. When asked if they felt that they had undergone active or sham treatment, a patient outlined that:
I suppose my ulcers were worse on the side that I was having the radiotherapy on, and they were alright on the other side I guess or not as bad. So I don’t know, I’ve no comparison have I really.
Patient 19
Unlike patients, staff had their prior experience seeing and managing OM and their experience of treating a range of patients with the LiTEFORM treatment to draw on when evaluating the potential impact.
Overall, staff were very positive about the trial because they felt that the technology made a difference to a significant problem for patients. They felt that they could, with some specific patients, see a difference, and had a reasonable degree of confidence in saying which specific people had received active treatment:
Some patients definitely looked to be, much improved over what we would normally expect to see. Especially, you know, how far they were in at times as well. We just kind of thought to ourselves, quietly, that maybe they were on the treatment and it was working. But obviously, you know, we don’t know.
Radiol 2
The impact of seeing some patients get through treatment substantially better than usual drove a sense of commitment to the trial. In a more extreme case, a member of staff was initially sceptical then became more engaged because of the ‘feeling amongst the trial staff that they could see real outcomes in some patients over others’ (Other 10). The enthusiasm for the trial, in many sites, was embedded in a broader sense that LLLT has a real potential to make an impact and that the trial itself had been ‘really good’ for the patients. The staff outlined how, even if it’s ‘just from psychological feel’, taking part has been a real benefit, and that some patients ‘feel that it’s a really, really positive treatment’.
Although the large majority of staff felt that LLLT was having a positive impact on patients, a few were not as convinced. When asked if they could tell whether patients had received active or sham treatment, one person noted that:
No, I don’t think I could.
No, you don’t think it had any effect on people?
Well it might have but I cannot honestly say that I’ve noticed a difference.
Others outlined that a range of mediating factors could have had an additional impact. Notably, someone outlined their ‘theory’:
This is not scientifically proven [laughter], but I found that those who had good oral hygiene, or good oral care, the laser therapy tended to actually work better. Those patients that weren’t keeping up with regular salt water rinses that would come in with a thick layer of gunk or saliva, a sticky tenacious saliva, it didn’t work so well.
Nurse 2
Given the complexity of factors influencing who does well in treatment (some ‘whizz through’ and ‘other end up with everything’), some staff felt that it would always be difficult to judge the specific impact of any active or sham treatment.
Sustaining: post-trial futures – integrating with bureaucratic, organisational and evidential orders
For staff at the sites involved, the trial closure was ‘a real shame’, ‘sad’ and for some even ‘unfair’:
We’re very, we’re kind of, disappointed . . . We put a lot of work and effort into opening the study. It was quite, it’s an involved process. So, our charity has funded us to buy the machine. Our staff has done extensive training. We’ve rearranged how we have the departments so that we’re able to deliver it and secured rooms and carried out quite a lot in terms of infrastructure.
OncCon 7
Site staff felt that they had all worked to integrate the trial and got over the ‘big hurdle’ of setting up a new service. There was reflection on what elements could have been carried out differently, but, as noted above, given the design of the LiTEFORM trial schedule, the rate-limiting factor was seen as a lack of capacity and capability to treat any more patients simultaneously. Some noted that a separate pilot trial, rather than an internal pilot, might have managed expectations and timelines. Other than waiting to see if any potentially meaningful results could emerge, they also had a question: ‘Now the trial’s finishing, I suppose the question then is, do we just offer it as a service, full stop?’ (Den 6).
Despite the practical integration problems that they had faced, staff at many sites could see a role for LLLT going forward. Staff at most sites talked about wishing to continue to deliver LLLT after the trial as standard care. For example, one person outlined that they had ‘got a meeting arranged with our manager next week or the week after to discuss how to set up a new service’ (OncCon 8). Another staff member was going to discuss it with the team at their upcoming service development meeting and another outlined that the team was ‘very pro’. The desire to try to enable this to become standard care is as a result of staff and patient enthusiasm.
The problem that many staff faced was around how to fund this new service: how to ‘make a case’ to their trust. Some highlighted that there was, currently, ‘no tariff for this’, so implementing it was going to be difficult. However, staff at some sites hoped they could fund it, one using an ‘approximate code’. Other staff felt that, given the recent NICE statement on LLLT (which many felt was deficient),82 the recommendation could be used to support the charge of an ‘interventional tariff’. Indeed, at one site while the trial was under way, a team member had been approached by their trust, which after reviewing the guidance, asked them ‘are you providing this low-level laser and if not should we consider doing it?’ (Den6). Embedded in such a new service would be the need to regularly audit laser safety, update the training and continue a formal process for authorisation of operators, alongside developing an overarching trust policy around such optical radiation devices. However, staff at a few sites were less optimistic about the potential, noting that ‘if we’re to drive this forward we need the evidence to say that it works’ (Nurse2). Without clear evidence of benefit, especially in terms of cost-effectiveness, these staff felt that the treatment could continue to be used only within a research setting, as commissioners would not support a new service.
Whether they were optimistic or pessimistic about the potential to fund such a service, staff at all sites were aware that to sustain LLLT over time elements would have to be adapted as ‘the logistics and feasibility . . . is just quite difficult’ (Radiol11). Providing LLLT is not something that people can fit in around their daily work; instead, dedicated staff were seen as needed to deliver it. Who these staff were varied between sites, but staff at most sites felt that you could not sustain LLLT using the higher band practitioners, such as radiographers or nurses. Some staff felt that, with adequate training and support, dental hygienists or dental nurses would be ideal, given that they are ‘used to dealing with mouths’, whereas others felt that it should be carried out by health-care assistants.
Other elements would also have to be adapted, including potentially increasing the number of lasers and dedicated operators. Service staff would have to be realistic about the number of patients that they could treat at any one time, as sites found that in its current form ‘it was not sustainable to treat that many patients’ (MedCon8). In part, the issue of sustainability is tied to the amount of time it takes to treat each patient. Patients themselves asked:
If there was something that could be either made, invented or looked into to speed the whole process up, say for instance inside the mouth . . . to administer the treatment, if the laser beam could be directed to two or three places at a time it would cut down the time that you were actually in the chair.
Patient 8
Staff also discussed the idea of whether or not a more efficient way of delivering LLLT will be available in future by adapting the probe, otherwise it would not be ‘able to work in practice’ due to the limited resources with the NHS. Future work could also focus on number of sites and optimum dosage per site.
Site staff were also keen that the evidence base for LLLT still be established. They were aware that the data collected by the end of the trial would not be sufficient to make any definitive claims. They wanted to continue (given the momentum, LLLT resources available and newly gained staff expertise) some form of data collection. For example, staff at one site outlined that they would be very interested in continuing to look at the role of laser ‘even if it were in the form of a non-randomised phase two study’ (Radiol5). Given that the trial was ‘very clunky’ to deliver, staff at one site argued that the focus should shift to a multicentre cohort study comparing patients who had LLLT with those who did not. Exploring the potential of delivering such collaborative evaluations would need resources in terms of time and money. Several people asked whether or not the manufacturer, THOR, should play a role, supporting some ‘sort of phase four work’. One person outlined:
Given the amount of investment there had already been . . . it’s a pity that this bit of research isn’t going a bit further. But you could argue that the people who are manufacturing the machine and stand to make money from it, should be investing more substantially in it . . . we have disseminated this equipment into quite a number of centres who didn’t have it before we started and are not going to throw it out. I would imagine they’ll be doing something with it in a certain number of patients.
MedCon 5
Within the broader context of the NICE recommendation,82 through the trial, LLLT devices are now introduced in a number of sites across the NHS. People have seen the potential value, are engaged and are motivated to support and provide LLLT. Service provision models have been introduced and integrated in their local contexts, so LLLT is workable, albeit with adaptations in expectations of patient throughput and who delivers the treatment. For sites, there is still a clear potential need and, most importantly, willingness and opportunity to collect data to generate ‘useful information’.
Discussion
Our qualitative process evaluation sought to identify, characterise and explain the factors that promote or inhibit the introduction, embedding and sustaining of the LiTEFORM trial. It focused primarily on issues of trial conduct and trial processes to understand issues of trial set-up and running the trial (in relation to recruitment and the practical organisation and experiences of delivery of the LiTEFORM treatment), as well as reflections on the current and future impact of LiTEFORM treatment.
Unlike many trials, be they those with a sham treatment83 or those without,66,84 recruitment was not the central problem that inhibited the successful conduct of the trial. The failure to recruit enough participants was not tied to ‘underlying issues among recruiters in terms of knowledge and views about evidence, equipoise, RCT design, role conflicts, specialty interests, and particular personal preferences’. 66 Instead, the pressures around the practical enactment of the scheduling, staffing and physical location of the LiTEFORM treatment and, importantly, a trial of LiTEFORM treatment, could not introduce or sustain the expected throughput of trial patients per month. Centrally, core elements of the (trial) pathway around the delivery of the LiTEFORM treatment could not integrate easily, at pace or at scale, within a wide range of organisational, clinical and professional contexts.
In terms of the core constructs of NPT, staff and patients could clearly understand the purpose of the trial: they could see the potential value and worth of the LiTEFORM trial. During the introduction of the trial, there were some initial elements of resistance in terms of the trial design at some sites. Questions were raised about issues of equipoise, inclusion criteria and explicit links to an industrial partner, as well as questions of how effectively a sham treatment could be provided. However, these were resolved, albeit they created some delays in the set-up phase at a few sites. Over time, we saw very high coherence,76 that is the LiTEFORM treatment, and a trial of LiTEFORM treatment that included a sham treatment, made sense to a large number of both staff and patients.
We also saw very high cognitive participation,76 in that staff and patients were very willing to be involved and commit to the implementation of the trial. Key people were driving the trial forward at each site, be they a few people championing the trial when the idea of the trial was first introduced at sites or a whole range of actors over time: those recruiting and those co-ordinating scheduling as well as those delivering, receiving or monitoring the treatment. We saw people ‘buy into’ the trial. Patients felt that taking part was acceptable and appropriate, with sites reporting that the number of those eligible and willing to take part exceeded the capacity to deliver treatment. Those patients who did decline did so in the context of the broader impact of HNC treatment in that they did not want to create any additional burden on themselves or their family. Notably, even when in the later stages of radiotherapy treatment, when patients’ symptom burden increased they were still keen to continue taking part in the LiTEFORM trial. Staff also clearly felt that delivering the trial was a legitimate part of their role. We repeatedly heard how staff (be they working directly on the trial or in the broader team) went over and above normal expectations to support the trial.
Staff and patients’ commitment to the delivery of the trial over time was a product of the potential value that they saw in the impact of the trial in supporting the experience of HNC patients going forward. Staff, especially trial staff, reported that they could see a positive impact of the LiTEFORM treatment for the OM of some of their patients. Patients also experienced a positive impact, for some in relation to their OM and for others in the additional time, interest and care that they had received from staff. As a result, both groups were very willing to undertake the necessary accommodation work, making adjustments (personal and practical) to support the work of the trial. The staff were clearly very disappointed that the trial ended without offering a definitive answer to the research question. Given the overall positivity that was generated through the process of reflexive monitoring,76 both by individuals and at sites, as well as the awareness that the hard major work of setting up a new service had been carried out, staff reported that they were working to find ways to continue to deliver the LLLT treatment. Future delivery was either as some element of standard care ( albeit with adaptations in the delivery, especially around who operates the laser) and/or with some form of ongoing data collection to support the generation of a suitable evidence base.
However, notwithstanding the very high levels of coherence, cognitive participation and reflexive monitoring (i.e. that this trial made sense, that people wanted to and were able to get involved and stayed committed, and the people evaluated the trial as worthwhile) the LiTEFORM trial recruitment was not operationalisable at the pace and scale that was expected and needed. This was a problem of workability and collective action (May 2009), not in terms of recruitment work or the one-to-one delivery of the LiTEFORM treatment, but rather in terms of the overall organisation of the delivery of the LiTEFORM as a new type of service. The pragmatics of the 20–40 minutes of the delivery of the one-to-one LiTEFORM treatment was (after initial periods of each operator developing embodied knowledge around LITEFORM tool use, mouth work and room set-up) relatively ‘simple’ and ‘easy’ for most. However, enabling the practical delivery required setting up a new type of service within already very busy departments. The initial work of set-up, which involved finding suitable rooms and suitable staff and then adequately adjusting the room and training the staff, as well as receiving appropriate organisational approvals, all took considerable time.
Capacity to deliver the therapy was the significant challenge over the life of the trial. Staff at the sites had to work hard to align the trial pathway with the overall organisation of head and neck clinical pathways and with each trial participant’s specific clinical pathway. How best to co-ordinate patient flow, given that sites had only a certain capacity in terms of the numbers of trained staff to deliver the laser at any given time, was a central question site staff all sought to manage on a day-by-day basis. They also often faced restrictions around when they could access the rooms that had been set-up and approved to deliver the LiTEFORM treatment in. Finally, despite establishing a good network of communication between the trial team and wider teams, staff needed to undertake constant monitoring, adjustment and accommodation work to (re)schedule patients’ LiTEFORM treatments alongside other treatments. Over time, for nearly all sites, two trial patients seemed the maximum that could be managed at any single time; some sites did not get to the stage of recruiting two patients at one time. In this way, managing patient flow was a delicate balance at each site, especially in terms of numbers of trained staff, if elements were breached, then recruitment to this new service would slow or even have to be stopped, albeit temporarily.
Prior to the trial set-up, the everyday, seen-but-unnoticed, co-ordination work around introduction, embedding and sustaining a new service was (relatively) black-boxed for everyone involved (i.e. the trial team, sites and funder) because this was a trial about only introducing a new device. The work of setting up a new service is often invisible work85 that becomes lost in the collective memory as the service is normalised. The two sites that already offered LLLT focused on treatment over prevention, and had more limited and ad hoc patient throughput. Even using a device such as the Nonadoption, Abandonment, Scale-up, Spread, and Sustainability (NASSS) framework86 would be unlikely to have focused attention to the range and depth of co-ordination work needed. The only item on the NASSS-CAT (Long Version)87 that would be core to questioning the potential to introduce the new service appears to be that ‘[o]rganisational routines and processes will need to change very considerably to accommodate the technology’. In a similar way, if a NPT analysis was carried out prior to the trial, a focus on questions of workability would also require people still being in post and remembering that invisible work. As some staff noted, a separate pilot trial over an internal pilot may have managed expectations around the potential capacity for a site to recruit as well as timelines for service set-up, embedding and patient throughput.
Strengths and limitations
A strength of our data was the spread of data across all sites. In a trial with a reasonable number of participating sites, we obtained a comprehensive account of the difficulties of trial implementation across the sites. A further strength was the multiple staff respondents from each site (including those that delivered the LiTEFORM treatment alongside those who undertook other trial-related tasks) enabling us to document the shared perspectives on problems and tensions around delivery within each site. Patient accounts were helpful in clarifying processes at sites as well as providing insight into their perspectives of experience of the LiTEFORM treatment, and were essential to the comprehensive picture of the trial obtained. We were less successful in obtaining recordings of recruitment consultations from all sites. However, the audio-recordings obtained tended to corroborate the information from both patient and professional interviews (which suggested that in the LiTEFORM trial, formal recruitment discussions were not problematic and that patients were keen to take part in the trial). Finally, we were generally successful at obtaining follow-up interviews with both staff and patients, which enabled us to understand changes over time.
Chapter 6 Discussion
Statement and interpretation of results
To our knowledge, this is the first multicentre RCT to test the effectiveness of LLLT in the prevention of OM in HNC irradiation using a pragmatic design in a routine NHS setting. Centrally, core elements of the trial pathway around the delivery of the LiTEFORM treatment could not integrate easily at a pace or scale within a wide range of organisational, clinical and professional contexts. For these reasons, the trial failed to recruit the planned number of participants (87 vs. 380) in the given time frame. Therefore, the results should be interpreted with caution. The primary outcome, mean OMWQ-HN total score at week 6 of radiotherapy, was 5.8 (95% CI 0.1 to 11.5) points higher (worse) in the arm that received LLLT three times per week than in those receiving sham LLLT. Although this exceeds the MCID for OMWQ-HN, which is 4 points, the wide CI means that this result (from an analysis including only 71 participants) should not be interpreted as the intervention being less effective than the sham. The only other trial to use OMWQ-HN found that at week 7 (final week of CRT) the mean OMWQ-HN score was 6.38 points lower (better) in the laser arm than in the sham arm. 22 That trial had greater power, having recruited 239 participants.
Secondary outcome measures
We compared the number of participants developing severe mucositis (WHO mucositis grades 3 and 4) in the LLLT and sham arms. The percentage of patients with a severe mucositis score was 10% (95% CI –32.7% to 12.7%) lower in the LLLT arm than in the sham arm. The CIs are too wide for us to conclude that LLLT reduced the risk of severe mucositis when compared with sham LLLT. Previous trials have shown that prophylactic LLLT significantly reduces the risk of severe OM. 22,39,40 The Laser Mucite trial41 in France recently published its results. This multicentre RCT was also too underpowered to show a difference between the sham and LLLT arms. 41
Feeding tubes
The intention had been to assess feeding tube use both during the 6-week period of HNC irradiation and at 14 months post treatment. At the 6-week visit, the proportion of participants on a feeding tube was the same in both treatment arms [25/38 (66%) in the laser arm; 23/35 (66%) in the sham arm]. Total dependence on a feeding tube was more common in the LLLT arm than in the sham arm (15/25 vs. 9/23, respectively). The trial ended early and, therefore, data on feeding tubes at the 14-month time point are available for only 26 participants. In addition, data are unavailable on whether tubes were used prophylactically or reactively. If it is assumed that nasogastric tubes are more commonly inserted reactively, then the data suggest equal numbers of gastrostomy and nasogastric tubes used across both arms.
Nutritional status
Approximately 45% of participants whose weight was measured at both baseline and 4 months had lost > 10% of their baseline weight. The results for both arms will have been affected by the use of feeding tubes and the time of their insertion (either prophylactically or reactively) as participants started to lose weight.
Pain control
The proportion of participants who used painkillers during the treatment period was similar in the two arms. Anti-inflammatory analgesics/paracetamol were the most common painkillers used, followed by opioids. This reflects the ongoing usual practice with regard to pain management for OM.
Admission to hospital
The rate of hospital admissions ranged from 5% to 19% per week during the treatment period and was approximately the same in both treatment arms.
Treatment interruptions
The number of participants who missed at least one fraction of radiotherapy was similar in both arms.
Swallowing function
This was assessed using the WST and the PSS-HN Normalcy of Diet. The pattern of diminishing swallowing function over the course of irradiation appeared similar in the sham and LLLT arms with respect to the PSS-HN. The available data indicated that objective swallowing performance based on the WST declined in both arms over the treatment period. At 6 weeks and 4 months, the main reasons for missing data were participants declining to take the WST, staff unavailability, administrative/clerical errors and participants not attending the 4-month visit.
Cancer-specific quality of life
We saw the pattern of cancer-specific QoL and symptoms that would have been anticipated, that is a drop in QoL (and worse symptoms) at 6 weeks compared with baseline, then a rise again at 4 months but not back to baseline levels. We observed wide CIs for the estimated differences in the MDADI, EORTC QLQ-C30 and EORTC QLQ-HN35 scores between the treatment arms. Although all CIs included zero, reliable conclusions regarding the differences in the QoL between treatment arms could not be made because of the small sample size. Other studies have shown improved QoL in the LLLT arm with respect to the physical, functional and emotional well-being scores. 22,40
Long-term risks of low-level laser therapy (survival, recurrence and disease progression)
Fourteen-month follow-up data were available for only 26 participants (LLLT; n = 10; sham, n = 16). None had recurrence and none showed evidence of disease progression. This small number of participants precludes any meaningful assessment of the long-term risks of LLLT. Other studies have now been published that show no increased risk of poor cancer treatment outcome associated with LLLT. 88,89
Economic evaluation
As with the statistical analysis, the economic evaluation was compromised by the small sample and lack of statistical power. However, there are aspects of the LiTEFORM trial economic component that may be useful for hospital trusts considering introducing a LLLT service for the treatment of the side effects of HNC.
The potential cost of delivering LLLT in an NHS setting has not previously been established. In the base-case analysis, the cost of delivering the intervention was estimated to be £802 per patient, based on the assumption that patients would have three 30-minute LLLT sessions per week for 6 weeks. This relatively low estimated cost of delivering the intervention was mainly because of the low yearly cost of the laser equipment (purchase price £6420), maintenance and training, with the bulk of the costs related to staff time.
The three times per week protocol chosen for the LiTEFORM trial was based on pilot work in Southampton,90 and was within Bensadoun’s guidelines13 for designing a treatment protocol. The recently published Multinational Association of Supportive Care in Cancer (MASCC) guidelines include a list of suitable protocols. 91 Although these are five times per week protocols,91 some are less time intensive than the protocol that we have chosen.
Of particular note, the Antunes et al. 39 and Oton-Leite et al. 42 treatment protocols can be completed in 11–12 minutes, rather than 30 minutes, as required for the LLLT sessions used in the LiTEFORM trial. These other protocols involve LLLT sessions on every day of irradiation rather than three times per week and, therefore, involve having to give 30 treatment sessions over 6 weeks rather than 18 treatment sessions. Although implementing a protocol such as this would mean that the number of patients who could be treated per week would increase, it is likely that the total cost of delivering the intervention would be similar to or potentially higher than the cost of delivering the protocol used in the LiTEFORM trial. This is because, although the total treatment time (810 minutes) would be approximately the same for either protocol (given an estimated 15-minute turnaround time between sessions), it is likely that more administrative time would be needed to co-ordinate the 12 extra LLLT sessions per patient around other hospital appointments and staff availability.
Qualitative study
This component of the LiTEFORM trial did recruit enough participants to fulfil its aims.
There was no lack of engagement from research teams at sites: this trial made sense to them, they wanted to be involved, and they stayed committed to it and evaluated it as worthwhile. Patients were enthusiastic about the trial and willing to participate. The qualitative work demonstrates the lengths to which sites were expected to go to to open the trial. Many sites had to apply for charitable or other funding to purchase a laser machine and then jump through many hoops to find a suitable room that was free for them to use at a range of times that conformed with laser regulations, and have it modified as necessary and install appropriate safety signage and get it approved by the local LPA. An appropriate dental chair or treatment couch had to be available. Staff who were willing to be trained in laser usage then had to be found and they had to have available time to deliver the treatment. Therefore, the trial required setting up a new type of service within already very busy departments with no additional funding.
The barriers to recruitment to this trial were logistical. In the words of one of the oncologists interviewed, ‘this was a very complex trial to do’. Capacity to deliver the therapy was the significant challenge over the life of the trial. How best to co-ordinate patient flow, given that sites only had a certain capacity in terms of the numbers of trained staff to deliver LLLT at any given time, was a central problem site staff had to manage on a day-to-day basis. They also often faced restrictions around when they could access the rooms that had been set up and approved to deliver the LLLT in. Even with a good network of communication between the trial team and the wider teams, they needed to undertake constant monitoring, adjustment and accommodation work to (re)schedule patients’ LLLT sessions alongside other treatments. Given their strong commitment to the trial, how best to manage the hidden logistical workload of this new type of service was the key issue all site teams faced.
Synthesising the results of the clinical, health economic and qualitative data
The trial had set out to recruit 380 participants in 18 months. In the end, 87 participants were recruited in that time frame. There were multiple factors contributing to this. The quantitative data show that setting up a new low-level laser service and opening the trial at each of the nine sites took between 5 and 15 months. This correlates well with the qualitative study, which clearly showed that the trial was very complex to set up and deliver, with a large hidden workload, including finding funding for the laser, obtaining approval for setting up a service and finding a suitable room and available staff. This meant that the mean time in recruitment for sites was 12 months. The mean recruitment rate was 0.9 participants per month. Although the University Hospital Southampton NHS Foundation Trust had had a functioning LLLT service before the LiTEFORM trial, its capacity was limited. Sunderland NHS Foundation Trust was also providing LLLT for those patients able to travel to Sunderland from Newcastle (where radiotherapy is delivered for Sunderland patients). This was not something that was practicable for most patients. Ideally, LLLT needed to be delivered at the site at which radiotherapy was delivered, so Sunderland and Newcastle had to be set up as two sites.
The pick-up rate for the trial of 60% (87/145) is broadly in line with other LLLT trials. 40 There was, therefore, no lack of willingness on the part of patients to engage in this trial. The qualitative study also showed that patients were willing to take part in the trial and that staff were engaged and committed. If the difficulties setting up the LiTEFORM trial were not enough, capacity constraints then limited recruitment for what time the sites were able to recruit. Several sites stopped recruiting when there were staffing shortages (i.e. Cardiff, Southampton and Leeds). The trial also had to stop recruiting when the major protocol breach occurred. Sunderland and Newcastle, although recruiting patients from two separate MDT settings, had to share access to a single laser device, with a shared staff rota.
It is useful at this point to compare the LiTEFORM trial’s recruitment with that of other published trials (Table 27). To our knowledge, no other LLLT trial has engaged so many sites (nine in total). The timescale in which these sites became involved also eclipses all other studies. Of particular note, the only trials to date with a multicentre design did not attempt to set up new services as part of their trial design. The Laser Mucite41 and the Bensadoun et al. 17 RCTs used sites that were already offering a LLLT service. These trials were open for recruitment for much longer (94 and 40 months respectively vs. 18 months for the LiTEFORM trial).
| Trial | Total number of participants | Time in recruitment (months) | Recruitment rate (participants per month) | Number of sites |
|---|---|---|---|---|
| Gautam et al.40 | 239 | 30 | 7.9 | 1 |
| LiTEFORM | 87 | 18 | 4.9 | 9 |
| Oton-Leite et al.42 | 60 | 14 | 4.2 | 1 |
| de Lima92 | 75 | 22 | 3.4 | 1 |
| Carvalho93 | 70 | 23 | 3.0 | 1 |
| Antunes et al.91 | 94 | 43 | 2.2 | 1 |
| Laser Mucite41 | 99 | 94 | 1 | 7 |
| Bensadoun et al.17 | 30 | 40 | 0.75 | 3 |
Determinants to wider implementation of trial findings and low-level laser therapy
Only 71 participants out of the 87 recruited were included in the final analysis. Eighteen participants discontinued LLLT during the 6-week period of treatment delivery. In five cases, this appeared to be specifically related to the LLLT treatment, whereas in the other 13 cases it was because of other symptoms. Only 28 out of the 87 participants attended all 18 LLLT sessions and only 47 out of the 87 attended at least two LLLT sessions per week for the 6-week treatment period. When the themes from the qualitative study are factored in, it is clear that LLLT is not completely benign from the patient perspective. Staff talked about how they observed and reflected on patients’ ability to complete LLLT treatment once the side effects of (C)RT had become significant. Given the limited capacity to deliver LLLT, staff at some sites reported a bias to select patients for the LiTEFORM trial who they felt might be less likely to suffer severe (C)RT side effects (because they felt that they would then be more likely to complete the LiTEFORM trial protocol). If this approach was mirrored in routine NHS care, it risks limiting access to LLLT to ‘lower-risk’ patients and excluding those with greatest potential to benefit from the treatment.
Our chosen protocol for LLLT was based around published guidelines13 and pilot work at Southampton, and was similar to other protocols in the literature. 90,91 There is a paucity of data on how well tolerated LLLT is, with few data on missed LLLT sessions, so the LiTEFORM trial contributes important data in this regard. In particular, it demonstrates that LLLT sessions can themselves be challenging for patients and, therefore, LLLT protocols with quicker treatment sessions would be preferable.
The aim of developing the LLLT protocol was to provide the regimen that would place the lowest burden on patients but still be likely to have a meaningful effect. Bensadoun and Nair13 recommended that LLLT be delivered at least three times per week, with between 6 and 20 spots in the oral cavity being treated per session. MASCC updated its guidelines while the LiTEFORM trial was still open for recruitment. 91 NICE also produced guidelines during this time frame. 82 Both of these guidelines agree that there is now sufficient evidence to recommend for LLLT to be used routinely. Neither guideline goes so far as to say which particular laser characteristics should be chosen. In this regard, the MASCC guidelines91 simply suggest copying a treatment protocol from one of the trials that they list in their systematic review. In the light of the fact that this trial is non-definitive, it would be prudent to suggest using a laser therapy protocol from the list in the MASCC guidelines.
Strengths and limitations
The strongest feature of the LiTEFORM trial was its teamwork-based multidisciplinary comprehensive approach. This statement is meant in the broadest sense, meaning that the trial included patient-reported and clinician-reported outcome measures combined with novel qualitative data from clinicians and patients. This extended team incorporated multiple sites. This approach was necessary if the trial was to succeed in comprehensively assessing LLLT being embedded as part of NHS HNC care. On reading reports from other trials, it appears that LLLT is a straightforward, easily delivered and well-tolerated intervention. 22,40,41 The LiTEFORM trial challenges this view with its account of the difficulties for sites, patients and clinicians. The LiTEFORM trial’s method for allocation concealment was another strength. To our knowledge, no other trial looking at LLLT in HNC irradiation has blinded the staff delivering the therapy, along with the patients and those assessing the clinical outcome. The LiTEFORM trial benefited greatly from the PPI group, which was highly influential. The high priority for treating mucositis identified by the PPI group was consistent with results that show a high level of engagement and commitment. The group was instrumental in selecting the primary outcome measure for the LiTEFORM trial, writing the PIS and promoting the trial through various media.
Serious breach
As noted, the trial benefited from close team working between researchers, clinicians and industry professionals. The allocation concealment procedures were borne out of those partnerships, and provided the methodological advantage of reduced risk of staff and participants becoming unblinded and knowledge of trial arm having an impact on outcome assessment. However, this came at the cost of an added layer of complexity (machine numbers allocated to determine treatment) and subsequent risk. In most RCTs, the unblinded members of the statistics team would have complete control over the procedures that protect allocation concealment. In this case, the coding device attached to the laser machines had been developed by the industry partner. The disparity in the matching of codes to treatment allocation meant that there was an interruption in recruitment at all sites as machines were all tested. It also dealt a blow to the confidence of the extended LiTEFORM trial team in sites. The early detection of this breach does highlight the diligence in the oversight of the trial.
Trial setting versus real-world setting
It is worth considering the extent to which the LiTEFORM trial was truly pragmatic. What is evident in this trial is the layer of additional work that the new service brings, in which clinicians are required to incorporate additional duties into their pre-existing job plan. In a routine NHS setting, this would be unlikely. It is more likely that new posts would be created specifically to provide this therapy. This artificial situation seen in the LiTEFORM trial is a product of the model of research in which excess treatment costs94 must be met by the trusts involved and are not provided by the funder so that they can be met from the trial budget. Trusts were not funded to recruit clinicians to provide LLLT, pay for laser devices, set aside a specific room or equip them for the treatment sessions. Trusts typically used charitable funds to buy the equipment and asked existing staff to find time to deliver the treatment. Room availability was also an issue. This observation is consistent with previous reports of ETCs being a barrier to site set-up and recruitment. 95,96
These significant constraints led to the most significant weakness of the LiTEFORM trial: its recruitment. It is clear from the data reported here, and those of other trials, that the LiTEFORM trial had set itself an unprecedented recruitment rate target. For comparison, the Laser Mucite trial41 took seven years to recruit 99 participants at seven sites in France, which were already providing LLLT treatment. In a little over 2 years, the LiTEFORM trial set up seven new LLLT services and recruited 87 participants.
Generalisability
Given the multicentre design, the LiTEFORM trial’s qualitative and economic data are generalisable across the NHS for those trusts considering setting up LLLT services for treating OM in HNC irradiation. It may also inform those wishing to set up LLLT services for OM caused by chemotherapy for other cancers and bone marrow transplantation.
Chapter 7 Conclusions
Given that it recruited only 87 participants, the LiTEFORM trial failed to meet all of its aims and objectives. However, the qualitative arm provides new evidence of the potential impact of LLLT on both patients and clinicians, and such methodology breaks new ground in LLLT research. Although the statistical and health economic analyses, with their broad CIs, confirm that it is not possible to draw any meaningful conclusions about the clinical effectiveness and cost-effectiveness of LLLT, the qualitative data give a vivid account of the human factors that must be considered when LLLT is introduced and embedded into a cancer treatment pathway. LLLT, although tolerated by most patients, is not entirely benign; it also raises occupational hazards and logistical obstacles for the delivering site. From a methodological point of view, the trial has demonstrated, for the first time (to our knowledge), that it is possible to maintain allocation concealment for clinicians delivering LLLT and patients receiving LLLT. In addition, the work has established (again for the first time) the cost to the NHS of LLLT in the treatment of OM.
Considerations for those wishing to set up low-level laser therapy services in the UK
In the context of newly published national and international guidelines82,91 recommending the use of LLLT in HNC treatment-related mucositis, NHS trusts may wish to consider the following:
-
LLLT is relatively inexpensive. Sites would require a laser device, a dedicated laser-safe room and one full-time equivalent band 7 AHP to treat up to eight patients at any given time. Additional human resources would be needed to cover leave.
-
Clinicians may wish to select protocols in which LLLT is delivered over shorter time frames to reduce the potential for increased discomfort from holding a sore mouth open. Shorter treatment protocols may also bring benefits in terms of patient throughput.
-
A treatment map/laser prescription will be required for each patient.
-
Dental hygienists adapt most readily to delivering intraoral LLLT. Other AHPs not experienced in mouth work benefit from training for both safety and comfort standpoints.
Considerations for researchers
There is still a need for well-designed pragmatic trials investigating the clinical effectiveness and cost-effectiveness of LLLT in HNC irradiation. In particular, research into less intrusive, more rapid LLLT delivery methods is a priority.
The set-up process for LLLT in NHS settings was complex and protracted, largely because of the bureaucratic, real estate, human resources and logistical demands. Once sites were open, recruitment was largely inhibited by capacity to deliver LLLT. These issues also prevented the LiTEFORM trial from being entirely pragmatic. Future trials would need to be designed to address these.
Working with an industry partner has benefits and risks. In the LiTEFORM trial, this produced a novel and effective sham adaptation. It also added a layer of complexity that resulted in a serious protocol breach.
Allocation concealment for the clinicians delivering LLLT and patients receiving it has been shown to be feasible in the LiTEFORM trial. This has important implications for future LLLT trials and not just those pertaining to HNC related mucositis. Future researchers may wish to consider our sham design and allocation concealment tactics.
Acknowledgements
The LiTEFORM trial was possible only because of the generous support and enthusiasm of a large number of individuals.
We would like to thank THOR Photomedicine Ltd for their technical support in creating the sham adaptation for this trial with supporting equipment, as well as providing free annual servicing. THOR have delivered face-to-face training on the laser machine to all sites, supported sites with technical and equipment queries, and have contributed to producing laser procedure documentation.
The investigators would also like to acknowledge the NIHR clinical research networks who provided NHS research support to the study.
Clinicians and research staff from across the UK contributed to the study at individual sites and we express our gratitude to all staff at sites in the UK for their support of study recruitment and data collection. We would like to thank staff at the following sites:
-
Sunderland Royal Hospital, with a special thanks to Pauline Oates
-
Southampton General Hospital and the radiotherapy research team
-
Velindre Cancer Centre, Dr Mererid Evans and the radiotherapy research team
-
The Leeds Dental Institute, Dr Shashwat Bhakta and Dr Peter Nixon and their team, with special thanks to Ashna Chavda
-
Freeman Hospital, with special thanks to Peter Wilson
-
Musgrove Park Hospital, Dr Petra Jankowska and her team, with special thanks to Susan Mahoney
-
Derriford Hospital and the cancer research team
-
Royal Cornwall Hospital, Dr Grant Stewart and the cancer research team
-
Royal United Hospital, Dr Emma de Winton and the cancer research team.
We would also like to thank all the site staff who have taken part in the qualitative interviews.
We thank the Newcastle Clinical Trials Unit staff, including previous team members: trial managers Shriya Sharma and Denise Clark, administrator Julia Phillipson, database manager Jon Pritchard and senior trial manager Jared Thornton. Furthermore, we would like to thank Joan Mackintosh, Qualitative Research Associate, from the Newcastle University’s Population Health Sciences Institute, who contributed to site training and conducted qualitative interviews for the trial. Likewise, we would like to thank Dr Colin Muirhead for his input in designing the trial.
We would like to thank all the members of the PPI group who have provided helpful advice and guidance when reviewing the trial and patient-facing documents.
We would also like to thank Mr David Stanley for his design of the LiTEFORM trial logo.
Finally, but most importantly, we would like to thank all our participants for taking part in the LiTEFORM trial.
Trial Steering Committee members
We would like to thank the members of the TSC: Professor Hisham Mehanna (chairperson), Dr Justin Roe, Dr Chris Foy, Professor Mary Wells and Mr John Telford.
Data Monitoring Committee members
We thank the members of the DMC for all their valuable guidance: Professor Richard Welbury (chairperson), Mr Chris Hurt, Professor Steve Thomas and Mr Jarrod Homer. For all his hard work on the trial we would also like to thank Mr James Morden, who was tragically killed while the study was ongoing.
Contributions of authors
Michael Nugent (https://orcid.org/0000-0002-2685-2426) (Oral Maxillofacial Surgeon) was the study chief investigator, designed the study, was principal applicant for funding, wrote the study protocol, supervised the overall conduct of the study, interpreted study data and co-wrote the final report.
Valerie Bryant (https://orcid.org/0000-0002-1413-9367) (Patient Representative) represented the views of patients in the design of the study, reviewed and edited all participant documentation, shared her experiences in a patient video, was a co-applicant for funding and co-wrote part of the final report.
Chrissie Butcher (https://orcid.org/0000-0002-1696-1506) (Trial Manager) was trial manager for the study, provided day-to-day management of trial conduct, performed monitoring to ensure that the trial was conducted to GCP requirements, and edited and contributed to the final report.
Holly Fisher (https://orcid.org/0000-0002-8389-9140) (Trial Statistician) designed the statistical plan in the study protocol, conducted the main study analysis and co-wrote the final report.
Sean Gill (https://orcid.org/0000-0001-9787-983X) (Data Manager) managed the data entered by sites as well as central data processes, enabled data cleaning and edited the final report.
Rebecca Goranova (https://orcid.org/0000-0002-1901-6075) (Consultant Clinical Oncologist) contributed to the design of the protocol, was a co-applicant for funding, interpreted study data, and co-wrote the final report.
Shaun Hiu (https://orcid.org/0000-0003-1699-4348) (Statistical Research Assistant) conducted the main study analysis and contributed to the final report.
Lyndsay Lindley (https://orcid.org/0000-0002-5982-9679) (Qualitative Research Associate) contributed to site training, conducted the qualitative interviews and analysed the interview data for the qualitative study.
James O’Hara (https://orcid.org/0000-0002-4096-3296) (Consultant Otolaryngologist and Head and Neck Surgeon) contributed to the design of the protocol and was a co-applicant for funding.
Yemi Oluboyede (https://orcid.org/0000-0002-9891-8279) (Senior Lecturer in Health Economics) contributed to the design of the HEAP in the protocol and reviewed and contributed to the final report.
Joanne Patterson (https://orcid.org/0000-0002-4990-302X) (Professor of Speech and Language Therapy) contributed to the design of the protocol, was a co-applicant for funding, interpreted study data, and reviewed and contributed to the final report.
Tim Rapley (https://orcid.org/0000-0003-4836-4279) (Professor of Applied Health Care Research) contributed to the design of the protocol, was a co-applicant for funding, interpreted study data and co-wrote the final report.
Tomos Robinson (https://orcid.org/0000-0001-8695-9738) (Health Economics Research Associate) conducted the health economic analysis, reviewed study results and wrote part of the final report.
Nikki Rousseau (https://orcid.org/0000-0001-8826-3515) (University Academic Fellow in Healthcare Technology Evaluation) contributed to the design of the protocol, was a co-applicant for funding, interpreted study data and co-wrote the final report.
Vicky Ryan (https://orcid.org/0000-0002-7008-3193) (Senior Statistician) wrote the SAP, led the final study statistical analysis, and co-wrote and edited the final report.
Ramkumar Shanmugasundaram (https://orcid.org/0000-0003-1491-7189) (Consultant Clinical Oncologist) contributed to the design of the protocol, was a co-applicant for funding, and reviewed and contributed to the final report.
Linda Sharp (https://orcid.org/0000-0001-9515-1722) (Senior Statistician) contributed to the design of the protocol, interpreted study data, and reviewed and contributed to the final report.
Ruby Smith Whelan (https://orcid.org/0000-0003-4389-8685) (Clinical Trials Administrator) was the trial administrator, providing high-level administrative support for the study; was involved in the management of the study; and contributed to, reviewed and edited the final report.
Deborah D Stocken (https://orcid.org/0000-0001-8031-1738) (Statistical Co-applicant) contributed to the design of the protocol and was a co-applicant for funding.
Laura Ternent (https://orcid.org/0000-0001-7056-298X) (Senior Lecturer in Health Economics) contributed to the design of the protocol, was a co-applicant for funding, wrote the HEAP and led the analysis of the health economics evaluation.
Janet Wilson (https://orcid.org/0000-0002-6416-5870) (Professor of Otolaryngology) contributed to the design of the protocol, was a co-applicant for funding, interpreted study data and reviewed and contributed to the final report.
Jenn Walker (https://orcid.org/0000-0002-7442-3060) (previous Trial Manager, Current Senior Trial Manager) contributed to the design, set-up and management of the trial; provided day-to-day management of trial conduct; performed monitoring to ensure that the trial was conducted to GCP requirements; interpreted study data; and co-wrote the report.
All authors provided critical comments on drafts of the final report.
Data-sharing statement
Anonymised data from this trial may be available to the scientific community subject to regulatory and ethics approval. Requests for data should be directed to the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Trotti A, Bellm LA, Epstein JB, Frame D, Fuchs HJ, Gwede CK, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol 2003;66:253-62. https://doi.org/10.1016/S0167-8140(02)00404-8.
- Nugent B, Lewis S, O’Sullivan JM. Enteral feeding methods for nutritional management in patients with head and neck cancers being treated with radiotherapy and/or chemotherapy. Cochrane Database Syst Rev 2013;1. https://doi.org/10.1002/14651858.CD007904.pub3.
- Sonis ST, Elting LS, Keefe D, Peterson DE, Schubert M, Hauer-Jensen M, et al. Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 2004;100:1995-202. https://doi.org/10.1002/cncr.20162.
- Best SR, Ha PK, Blanco RG, Saunders JR, Zinreich ES, Levine MA, et al. Factors associated with pharyngoesophageal stricture in patients treated with concurrent chemotherapy and radiation therapy for oropharyngeal squamous cell carcinoma. Head Neck 2011;33:1727-34. https://doi.org/10.1002/hed.21657.
- Laurell G, Kraepelien T, Mavroidis P, Lind BK, Fernberg JO, Beckman M, et al. Stricture of the proximal esophagus in head and neck carcinoma patients after radiotherapy. Cancer 2003;97:1693-700. https://doi.org/10.1002/cncr.11236.
- National Cancer Intelligence Network . Chemotherapy, Radiotherapy and Surgical Tumour Resections in England 2020.
- Luo DH, Hong MH, Guo L, Cao KJ, Deng MQ, Mo HY. Analysis of oral mucositis risk factors during radiotherapy for nasopharyngeal carcinoma patients and establishment of a discriminant model. Ai Zheng 2005;24:850-4.
- Kelly CG. Chemotherapy: United Kingdom national multidisciplinary guidelines. J Laryngol Otol 2016;130:S71-4. https://doi.org/10.1017/S0022215116000840.
- Basile D, Di Nardo P, Corvaja C, Garattini SK, Pelizzari G, Lisanti C, et al. Mucosal injury during anti-cancer treatment: from pathobiology to bedside. Cancers 2019;11. https://doi.org/10.3390/cancers11060857.
- Lalla RV, Bowen J, Barasch A, Elting L, Epstein J, Keefe DM, et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2014;120:1453-61. https://doi.org/10.1002/cncr.28592.
- World Health Organisation . WHO’s Cancer Pain Ladder for Adults n.d. www.who.int/cancer/palliative/painladder/en/ (accessed 23 July 2020).
- Jadaud E, Bensadoun RJ. Low-level laser therapy: a standard of supportive care for cancer therapy-induced oral mucositis in head and neck cancer patients?. Laser Ther 2012;21:297-303. https://doi.org/10.5978/islsm.12-RE-01.
- Bensadoun RJ, Nair RG. Low-level laser therapy in the prevention and treatment of cancer therapy-induced mucositis: 2012 state of the art based on literature review and meta-analysis. Curr Opin Oncol 2012;24:363-70. https://doi.org/10.1097/CCO.0b013e328352eaa3.
- Worthington HV, Clarkson JE, Bryan G, Furness S, Glenny AM, Littlewood A, et al. Interventions for preventing oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev 2010;12. https://doi.org/10.1002/14651858.CD000978.pub4.
- Oberoi S, Zamperlini-Netto G, Beyene J, Treister NS, Sung L. Effect of prophylactic low level laser therapy on oral mucositis: a systematic review and meta-analysis. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0107418.
- Grimes D, Shultz K. Use and abuses of screening tests. Lancet 2002;359:881-4. https://doi.org/10.1016/S0140-6736(02)07948-5.
- Bensadoun R, Franquin J, Ciais G, Darcourt V, Schubert MM, Viot M, et al. Low-energy He/Ne laser in the prevention of radiation-induced mucositis. A multicenter phase III randomized study in patients with head and neck cancer. Support Care Cancer 1999;7:244-52. https://doi.org/10.1007/s005200050256.
- Unverzagt S, Prondzinsky R, Peinemann F. Single-center trials tend to provide larger treatment effects than multicenter trials: a systematic review. J Clin Epidemiol 2013;66:1271-80. https://doi.org/10.1016/j.jclinepi.2013.05.016.
- Bafeta A, Dechartres A, Trinquart L, Yavchitz A, Boutron I, Ravaud P. Impact of single centre status on estimates of intervention effects in trials with continuous outcomes: meta-epidemiological study. BMJ 2012;344. https://doi.org/10.1136/bmj.e813.
- Grégoire V, Ang K, Budach W, Grau C, Hamoir M, Langendijk JA, et al. Delineation of the neck node levels for head and neck tumors: a 2013 update. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother Oncol 2014;110:172-81. https://doi.org/10.1016/j.radonc.2013.10.010.
- Epstein JB, Beaumont JL, Gwede CK, Murphy B, Garden AS, Meredith R, et al. Longitudinal evaluation of the oral mucositis weekly questionnaire-head and neck cancer, a patient-reported outcomes questionnaire. Cancer 2007;109:1914-22. https://doi.org/10.1002/cncr.22620.
- Gautam AP, Fernandes DJ, Vidyasagar MS, Maiya AG, Nigudgi S. Effect of low-level laser therapy on patient reported measures of oral mucositis and quality of life in head and neck cancer patients receiving chemoradiotherapy – a randomized controlled trial. Support Care Cancer 2013;21:1421-8. https://doi.org/10.1007/s00520-012-1684-4.
- Ringash J, Bernstein LJ, Cella D, Logemann J, Movsas B, Murphy B, et al. Outcomes toolbox for head and neck cancer research. Head Neck 2015;37:425-39. https://doi.org/10.1002/hed.23561.
- World Health Organization . WHO Handbook for Reporting Results of Cancer Treatment 1979.
- Chen AY, Frankowski R, Bishop-Leone J, Hebert T, Leyk S, Lewin J, et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg 2001;127:870-6.
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365-76. https://doi.org/10.1093/jnci/85.5.365.
- Fayers P, Aaronson N, Bjordal K, Groenvold M, Curran D, Bottomley A. EORTC QLQ-C30 Scoring Manual. Brussels: EORTC; 2001.
- Bjordal K, Ahlner-Elmqvist M, Tollesson E, Jensen AB, Razavi D, Maher EJ, et al. Development of a European Organization for Research and Treatment of Cancer (EORTC) questionnaire module to be used in quality of life assessments in head and neck cancer patients. EORTC Quality of Life Study Group. Acta Oncol 1994;33:879-85. https://doi.org/10.3109/02841869409098450.
- Bjordal K, Hammerlid E, Ahlner-Elmqvist M, de Graeff A, Boysen M, Evensen JF, et al. Quality of life in head and neck cancer patients: validation of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-H.N35. J Clin Oncol 1999;17:1008-19. https://doi.org/10.1200/JCO.1999.17.3.1008.
- List MA, D’Antonio LL, Cella DF, Siston A, Mumby P, Haraf D, et al. The performance status scale for head and neck cancer patients and the functional assessment of cancer therapy-head and neck scale. A study of utility and validity. Cancer 1996;77:2294-301. https://doi.org/10.1002/(SICI)1097-0142(19960601)77:11<2294::AID-CNCR17>3.0.CO;2-S.
- List MA, Siston A, Haraf D, Schumm P, Kies M, Stenson K, et al. Quality of life and performance in advanced head and neck cancer patients on concomitant chemoradiotherapy: a prospective examination. J Clin Oncol 1999;17:1020-8. https://doi.org/10.1200/JCO.1999.17.3.1020.
- Patterson JM, McColl E, Carding PN, Kelly C, Wilson JA. Swallowing performance in patients with head and neck cancer: a simple clinical test. Oral Oncol 2009;45:904-7. https://doi.org/10.1016/j.oraloncology.2009.03.012.
- Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ 5D 5L value set for England. Health Econ 2018;27:7-22. https://doi.org/10.1002/hec.3564.
- Yelland LN, Sullivan TR, Voysey M, Lee KJ, Cook JA, Forbes AB. Applying the intention-to-treat principle in practice: guidance on handling randomisation errors. Clin Trials 2015;12:418-23. https://doi.org/10.1177/1740774515588097.
- Barth I, Krafft H, Weber G, Keller-Stanislawski B, Cichutek K. Good clinical practice in the European Union. Hum Gene Ther 2008;19:441-2. https://doi.org/10.1089/hum.2008.0409.
- Great Britain . Data Protection Act 1998 1998.
- Great Britain . Data Protection Act 2018 2018.
- Rogers SN, Waylen AE, Thomas S, Penfold C, Pring M, Waterboer T, et al. Quality of life, cognitive, physical and emotional function at diagnosis predicts head and neck cancer survival: analysis of cases from the Head and Neck 5000 study. Eur Arch Otorhinolaryngol 2020;277:1515-23. https://doi.org/10.1007/s00405-020-05850-x.
- Antunes HS, Herchenhorn D, Small IA, Araújo CM, Viégas CM, Cabral E, et al. Phase III trial of low-level laser therapy to prevent oral mucositis in head and neck cancer patients treated with concurrent chemoradiation. Radiother Oncol 2013;109:297-302. https://doi.org/10.1016/j.radonc.2013.08.010.
- Gautam AP, Fernandes DJ, Vidyasagar MS, Maiya AG, Vadhiraja BM. Low level laser therapy for concurrent chemoradiotherapy induced oral mucositis in head and neck cancer patients – a triple blinded randomized controlled trial. Radiother Oncol 2012;104:349-54. https://doi.org/10.1016/j.radonc.2012.06.011.
- Legouté F, Bensadoun RJ, Seegers V, Pointreau Y, Caron D, Lang P, et al. Low-level laser therapy in treatment of chemoradiotherapy-induced mucositis in head and neck cancer: results of a randomised, triple blind, multicentre phase III trial. Radiat Oncol 2019;14. https://doi.org/10.1186/s13014-019-1292-2.
- Oton-Leite AF, Silva GB, Morais MO, Silva TA, Leles CR, Valadares MC, et al. Effect of low-level laser therapy on chemoradiotherapy-induced oral mucositis and salivary inflammatory mediators in head and neck cancer patients. Lasers Surg Med 2015;47:296-305. https://doi.org/10.1002/lsm.22349.
- Goepfert RP, Lewin JS, Barrow MP, Fuller CD, Lai SY, Song J, et al. Predicting two-year longitudinal MD Anderson Dysphagia Inventory outcomes after intensity modulated radiotherapy for locoregionally advanced oropharyngeal carcinoma. Laryngoscope 2017;127:842-8. https://doi.org/10.1002/lary.26153.
- Karimi AM, Gairola M, Ahlawat P, Tandon S, Pal M, Sachdeva N, et al. Health-related quality of life assessment for head-and-neck cancer patients during and at 3 months after radiotherapy – a prospective, analytical questionnaire-based study. Natl J Maxillofac Surg 2019;10:134-40. https://doi.org/10.4103/njms.NJMS_92_18.
- Zanin T, Zanin F, Carvalhosa AA, Castro PH, Pacheco MT, Zanin IC, et al. Use of 660-nm diode laser in the prevention and treatment of human oral mucositis induced by radiotherapy and chemotherapy. Photomed Laser Surg 2010;28:233-7. https://doi.org/10.1089/pho.2008.2242.
- Singer S, Arraras JI, Chie WC, Fisher SE, Galalae R, Hammerlid E, et al. Performance of the EORTC questionnaire for the assessment of quality of life in head and neck cancer patients EORTC QLQ-H.N35: a methodological review. Qual Life Res 2013;22:1927-41. https://doi.org/10.1007/s11136-012-0325-1.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Cost Eff Resour Alloc 2013;11. https://doi.org/10.1186/1478-7547-11-6.
- THOR . Photobiomodulation (PBM Therapy) 2020. www.thorlaser.com/ (accessed 23 March 2020).
- NHS . Annual Pay Scales 2020 21 n.d. www.nhsemployers.org/pay-pensions-and-reward/agenda-for-change/pay-scales/annual (accessed 23 March 2020).
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2019. Canterbury: PSSRU, University of Kent; 2019.
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2018–2019 2019.
- Prescribing & Medicines Team, NHS Digital . Prescription Cost Analysis England 2018 2019.
- National Institute for Health and Care Excellence (NICE) . Position Statement on Use of the EQ-5D-5L Value Set for England n.d. www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l (accessed 10 March 2020).
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Martins AFL, Nogueira TE, Morais MO, Oton-Leite AF, Valadares MC, Batista AC, et al. Effect of photobiomodulation on the severity of oral mucositis and molecular changes in head and neck cancer patients undergoing radiotherapy: a study protocol for a cost-effectiveness randomized clinical trial. Trials 2019;20. https://doi.org/10.1186/s13063-019-3196-8.
- Clement C, Edwards SL, Rapport F, Russell IT, Hutchings HA. Exploring qualitative methods reported in registered trials and their yields (EQUITY): systematic review. Trials 2018;19. https://doi.org/10.1186/s13063-018-2983-y.
- O’Cathain A, Thomas KJ, Drabble SJ, Rudolph A, Hewison J. What can qualitative research do for randomised controlled trials? A systematic mapping review. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-002889.
- Petticrew M. When are complex interventions ‘complex’? When are simple interventions ‘simple’?. Eur J Public Health 2011;21:397-8. https://doi.org/10.1093/eurpub/ckr084.
- Blencowe NS, Brown JM, Cook JA, Metcalfe C, Morton DG, Nicholl J, et al. Interventions in randomised controlled trials in surgery: issues to consider during trial design. Trials 2015;16. https://doi.org/10.1186/s13063-015-0918-4.
- Mills N, Donovan JL, Wade J, Hamdy FC, Neal DE, Lane JA. Exploring treatment preferences facilitated recruitment to randomized controlled trials. J Clin Epidemiol 2011;64:1127-36. https://doi.org/10.1016/j.jclinepi.2010.12.017.
- McCann SK, Campbell MK, Entwistle VA. Reasons for participating in randomised controlled trials: conditional altruism and considerations for self. Trials 2010;11. https://doi.org/10.1186/1745-6215-11-31.
- Paleri V, Patterson J, Rousseau N, Moloney E, Craig D, Tzelis D, et al. Gastrostomy versus nasogastric tube feeding for chemoradiation patients with head and neck cancer: the TUBE pilot RCT. Health Technol Assess 2018;22. https://doi.org/10.3310/hta22160.
- Phelps EE, Tutton E, Griffin X, Baird J. A mixed-methods systematic review of patients’ experience of being invited to participate in surgical randomised controlled trials. Soc Sci Med 2020;253. https://doi.org/10.1016/j.socscimed.2020.112961.
- Donovan JL, Paramasivan S, de Salis I, Toerien M. Clear obstacles and hidden challenges: understanding recruiter perspectives in six pragmatic randomised controlled trials. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-5.
- Sherman R, Hickner J. Academic physicians use placebos in clinical practice and believe in the mind-body connection. J Gen Intern Med 2008;23:7-10. https://doi.org/10.1007/s11606-007-0332-z.
- Tilburt JC, Emanuel EJ, Kaptchuk TJ, Curlin FA, Miller FG. Prescribing ‘placebo treatments’: results of national survey of US internists and rheumatologists. BMJ 2008;337. https://doi.org/10.1136/bmj.a1938.
- Kaptchuk TJ, Shaw J, Kerr CE, Conboy LA, Kelley JM, Csordas TJ, et al. ‘Maybe I made up the whole thing’: placebos and patients’ experiences in a randomized controlled trial. Cult Med Psychiatry 2009;33:382-411. https://doi.org/10.1007/s11013-009-9141-7.
- Bishop FL, Jacobson EE, Shaw JR, Kaptchuk TJ. Scientific tools, fake treatments, or triggers for psychological healing: how clinical trial participants conceptualise placebos. Soc Sci Med 2012;74:767-74. https://doi.org/10.1016/j.socscimed.2011.11.020.
- Welton AJ, Vickers MR, Cooper JA, Meade TW, Marteau TM. Is recruitment more difficult with a placebo arm in randomised controlled trials? A quasirandomised, interview based study. BMJ 1999;318:1114-17. https://doi.org/10.1136/bmj.318.7191.1114.
- Keller PH, Grondin O, Tison F, Gonon F. How health professionals conceptualize and represent placebo treatment in clinical trials and how their patients understand it: impact on validity of informed consent. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0155940.
- Kim SY, De Vries R, Holloway RG, Wilson R, Parnami S, Kim HM, et al. Sham surgery controls in Parkinson’s disease clinical trials: views of participants. Mov Disord 2012;27:1461-5. https://doi.org/10.1002/mds.25155.
- Swift TL. Sham surgery trial controls: perspectives of patients and their relatives. J Empir Res Hum Res Ethics 2012;7:15-28. https://doi.org/10.1525/jer.2012.7.3.15.
- Jakšić N, Aukst-Margetić B, Jakovljević M. Does personality play a relevant role in the placebo effect?. Psychiatr Danub 2013;25:17-23.
- May C, Finch T. Implementing, embedding, and integrating practices: an outline of normalization process theory. Sociol 2009;43:535-54. https://doi.org/10.1177/0038038509103208.
- Rapley T, Silverman D. Qualitative Research: Theory, Method & Practice. London: Sage Publications Ltd; 2011.
- Glaser BG. The constant comparative method of qualitative analysis. Social Problems 1965;12:436-45. https://doi.org/10.2307/798843.
- Seale C. The Quality of Qualitative Research. London: SAGE Publications Ltd; 1999.
- Charmaz K. Constructing Grounded Theory: A Practical Guide Through Qualitative Analysis. London: Sage Publications Ltd; 2006.
- Cancer Waiting Times Team . National Cancer Waiting Times Monitoring Dataset Guidance 2015.
- National Institute for Health and Care Excellence (NICE) . Low-Level Laser Therapy for Preventing or Treating Oral Mucositis Caused by Radiotherapy or Chemotherapy. Intervention Procedures Guidance (IPG615) 2018. www.nice.org.uk/guidance/ipg615 (accessed 2 August 2020).
- Hare KB, Lohmander LS, Roos EM. The challenge of recruiting patients into a placebo-controlled surgical trial. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-167.
- Sully BG, Julious SA, Nicholl J. A reinvestigation of recruitment to randomised, controlled, multicenter trials: a review of trials funded by two UK funding agencies. Trials 2013;14. https://doi.org/10.1186/1745-6215-14-166.
- Star SL, Strauss A. Layers of silence, arenas of voice: the ecology of visible and invisible work. Comp Supp Coop Work J 1999;8:9-30. https://doi.org/10.1023/A:1008651105359.
- Greenhalgh T, Wherton J, Papoutsi C, Lynch J, Hughes G, A’Court C, et al. Beyond adoption: a new framework for theorizing and evaluating nonadoption, abandonment, and challenges to the scale-up, spread, and sustainability of health and care technologies. J Med Internet Res 2017;19. https://doi.org/10.2196/jmir.8775.
- Greenhalgh T, Maylor H, Shaw S, Wherton J, Papoutsi C, Betton V, et al. The NASSS-CAT tools for understanding, guiding, monitoring, and researching technology implementation projects in health and social care: protocol for an evaluation study in real-world settings. JMIR Res Protoc 2020;9. https://doi.org/10.2196/16861.
- Fischlechner R, Kofler B, Schartinger VH, Dudas J, Riechelmann H. Does low-level laser therapy affect the survival of patients with head and neck cancer?. Lasers Med Sci 2021;36:599-604. https://doi.org/10.1007/s10103-020-03073-4.
- Antunes HS, Herchenhorn D, Small IA, Araújo CMM, Viégas CMP, de Assis Ramos G, et al. Long-term survival of a randomized phase III trial of head and neck cancer patients receiving concurrent chemoradiation therapy with or without low-level laser therapy (LLLT) to prevent oral mucositis. Oral Oncol 2017;71:11-5. https://doi.org/10.1016/j.oraloncology.2017.05.018.
- Ramkumar S N-JV. Implementation of low level laser therapy in head and neck cancer patients undergoing radical radiotherapy 2016.
- Zadik Y, Arany PR, Fregnani ER, Bossi P, Antunes HS, Bensadoun RJ, et al. Systematic review of photobiomodulation for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer 2019;27:3969-83. https://doi.org/10.1007/s00520-019-04890-2.
- de Lima AG, Villar RC, de Castro G, Antequera R, Gil E, Rosalmeida MC, et al. Oral mucositis prevention by low-level laser therapy in head-and-neck cancer patients undergoing concurrent chemoradiotherapy: a phase III randomized study. Int J Radiat Oncol Biol Phys 2012;82:270-5. https://doi.org/10.1016/j.ijrobp.2010.10.012.
- Carvalho PA, Jaguar GC, Pellizzon AC, Prado JD, Lopes RN, Alves FA. Evaluation of low-level laser therapy in the prevention and treatment of radiation-induced mucositis: a double-blind randomized study in head and neck cancer patients. Oral Oncol 2011;47:1176-81. https://doi.org/10.1016/j.oraloncology.2011.08.021.
- Department of Health Research and Development Directorate . Attributing the Costs of Health and Social Care Research &Amp; Development (AcoRD) 2012.
- Keetharuth AD, Galvan C, Ara R. Excess treatment costs (ETC) estimating the magnitude and distribution of ETC in England to inform policy formulation 2017.
- Palmer R, Harrison M, Cross E, Enderby P. Negotiating excess treatment costs in a clinical research trial: the good, the bad and the innovative. Trials 2016;17. https://doi.org/10.1186/s13063-016-1208-5.
- UK Oral Management in Cancer Care Group (UKOMiC) . Oral Care Guidance and Support in Cancer and Palliative Care: Third Edition 2019. www.ukomic.co.uk/guidance.html (accessed 21 November 2021).
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2017–2018 2018.
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
- Chesterton LS, Blagojevic-Bucknall M, Burton C, Dziedzic KS, Davenport G, Jowett SM, et al. The clinical and cost-effectiveness of corticosteroid injection versus night splints for carpal tunnel syndrome (INSTINCTS trial): an open-label, parallel group, randomised controlled trial. Lancet 2018;392:1423-33. https://doi.org/10.1016/S0140-6736(18)31572-1.
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: PSSRU, University of Kent; 2018.
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: PSSRU, University of Kent; 2017.
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2013. Canterbury: PSSRU, University of Kent; 2013.
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2014. Canterbury: PSSRU, University of Kent; 2014.
Appendix 1 Methods supplementary information
| Amendment number | Protocol version and date | Description |
|---|---|---|
| Substantial amendment 1 | 2.1, 31 May 2017 |
OMWH-HN questionnaire added to the 4-month visit at the request of the DMC Delivering laser therapy within 60 minutes prior to radiotherapy may not be practical at all sites. The protocol was updated to state that laser therapy will ideally be given within 2 hours of radiotherapy A withdrawal form for participant completion was introduced Data on patient smoking and drinking habits will be recorded from 2 weeks to 6 weeks of laser therapy |
| Substantial amendment 2 | 3.0, 15 November 2017 |
Following the site initiation visits it was clear that each hospital has different laser use policies. Therefore, the protocol was amended to state that sites will follow their local laser rules as written/finalised by their local LPS Patients may be approached about the trial at any of their standard care visits after diagnosis but prior to receiving their planned (C)RT The PIS was revised to state that the 20- to 30-minute time slot to deliver the laser therapy is an approximation |
| Substantial amendment 3 | 4.0, 3 September 2019 |
The trial under recruited; therefore, the analysis of the 14-month data was changed to be descriptive. The follow-up schedule was changed for all patients who started laser therapy after 6 July 2018. Their last visit was changed from 14 months to 4 months. These patients were informed that they would no longer be required to complete the 14-month study visit but would continue to attend the 4-month study visit (if it had not occurred already) To reflect the changes to the follow-up schedule, the following data will be recorded when the site find out about an event (as opposed to at 14 months): recurrence, disease progression, death, feeding tube use (stopping) SAE and SAR reporting timelines updated to reflect changes to follow-up schedule A change was added to optionally unblind patients at the end of study, after central database lock. This was added at the request of participants during their qualitative interviews |
| Site | Standard of care |
|---|---|
| Bath |
Caphasol and Difflam mouthwashes Gelclair or glycerin topical (Oralieve; Europharma Concepts Limited, Clara, Ireland) Analgesia |
| Cardiff |
Caphasol starting day 1 and continuing for 2 weeks after (C)RT At onset of OM: Difflam with or without Gelclair Analgesia as per WHO ladder |
| Cornwall | Supportive measures including mouthwash and opiate-based analgesia |
| Leeds |
Aspirin mouthwash Salty water Gelclair Pain relief as required |
| Newcastle | UK Oral Mucositis in Cancer Group ‘Mouth care guidance and support’97 |
| Plymouth |
Difflam and Biotène (GlaxoSmithKline Consumer Healthcare UK, Brentford, UK) mouthwashes Biotène gel Analgesia as per WHO ladder |
| Southampton | Current standard of care for OM is LLLT if possible, otherwise managed conservatively with mouthwash and analgesics |
| Sunderland |
Caphasol Gelclair Oral hygiene Difflam |
| Taunton | Caphasol: prophylactic |
FIGURE 17.
The LLLT/placebo combinations used in sham adaptation switch box. Two possible grid patterns were incorporated into the design of the placebo switch box for the delivery of sham/LLLT. Within each placebo switch box, internal switches (inaccessible to users at site) controlled which grid was active, either grid 1 (a) or grid 2 (b). Upon discovery of the serious breach, all devices were switched from grid 1 (a) to grid 2 (b).
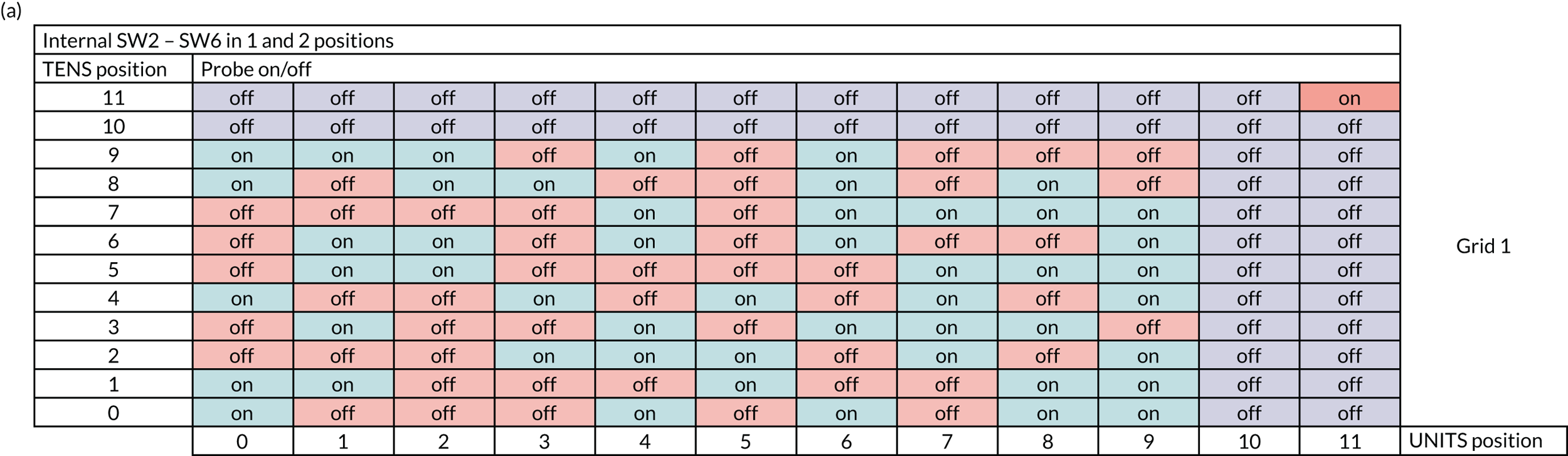
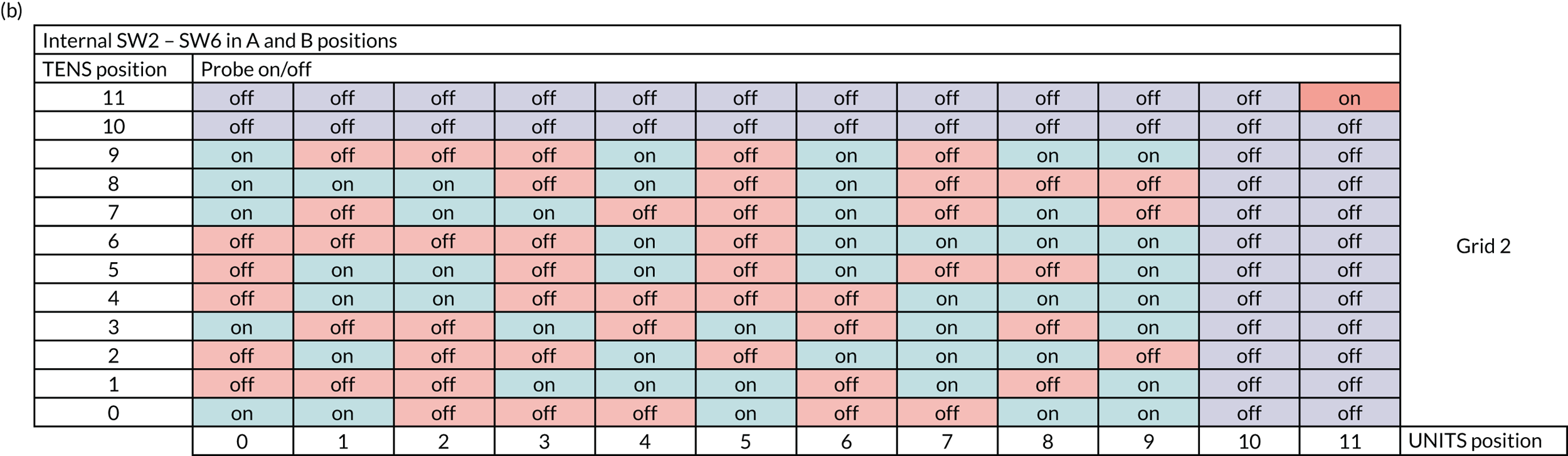
Appendix 2 Results supplementary information
| Characteristic | Treatment arm | Overall (N = 71) | |
|---|---|---|---|
| LLLT (N = 37) | Sham (N = 34) | ||
| Stratification factors (at randomisation), n (%) | |||
| Planned treatment | |||
| Radiotherapy alone | 10 (27) | 8 (23.5) | 18 (25.4) |
| Chemoradiotherapy | 27 (73) | 26 (76.5) | 53 (74.6) |
| Type of radiotherapy field | |||
| Unilateral | 7 (18.9) | 9 (26.5) | 16 (22.5) |
| Bilateral | 30 (81.1) | 25 (73.5) | 55 (77.5) |
| Clinical measures | |||
| Age (years) | |||
| Minimum | 40.3 | 44.1 | 40.3 |
| Median (IQR) | 58.6 (51.7–67.4) | 59.3 (54.4–64) | 59.1 (52.3–65.5) |
| Mean (SD) | 58.9 (10) | 59.7 (7.7) | 59.3 (8.9) |
| Maximum | 75.5 | 76.1 | 76.1 |
| BMIa (kg/m2) | |||
| Minimum | 14.6 | 17.8 | 14.6 |
| Median (IQR) | 26.3 (23.9–29.2) | 25.9 (23.5–31.1) | 25.9 (23.5–29.4) |
| Mean (SD) | 26.2 (4.2) | 27 (5.8) | 26.6 (5) |
| Maximum | 34.8 | 42.9 | 42.9 |
| Weight (kg) | |||
| Minimum | 46.8 | 50 | 46.8 |
| Median (IQR) | 78.8 (68.5–90.2) | 81.4 (67.8–91.2) | 79 (67.8–90.4) |
| Mean (SD) | 78.5 (14.1) | 80.9 (18.9) | 79.7 (16.5) |
| Maximum | 109.8 | 135 | 135 |
| Sex, n (%) | |||
| Female | 5 (13.5) | 10 (29.4) | 15 (21.1) |
| Male | 32 (86.5) | 24 (70.6) | 56 (78.9) |
| HNC information, n (%) | |||
| Site of disease | |||
| Nasopharynx | 0 | 0 | 0 |
| Oropharynx | |||
| HPV positive | 20 (54.1) | 24 (70.6) | 44 (62) |
| HPV negative | 6 (16.2) | 2 (5.9) | 8 (11.3) |
| HPV undetermined | 1 (2.7) | 1 (2.9) | 2 (2.8) |
| Larynx | 3 (8.1) | 0 (0) | 3 (4.2) |
| Oral cavity | 6 (16.2) | 5 (14.7) | 11 (15.5) |
| Unknown primary | 1 (2.7) | 2 (5.9) | 3 (4.2) |
| TNM classification, n (%) | |||
| Primary tumour | |||
| T0 | 1 (2.7) | 2 (5.9) | 3 (4.2) |
| T1 | 8 (21.6) | 7 (20.6) | 15 (21.1) |
| T2 | 19 (51.4) | 12 (35.3) | 31 (43.7) |
| T3 | 3 (8.1) | 6 (17.6) | 9 (12.7) |
| T4 | 6 (16.2) | 7 (20.6) | 13 (18.3) |
| Regional lymph nodes | |||
| N0 | 6 (16.2) | 3 (8.8) | 9 (12.7) |
| N1 | 12 (32.4) | 7 (20.6) | 19 (26.8) |
| N2 | 19 (51.4) | 21 (61.8) | 40 (56.3) |
| N3 | 0 | 3 (8.8) | 3 (4.2) |
| Distant metastasis | |||
| MX | 0 | 1 (2.9) | 1 (1.4) |
| M0 | 37 (100) | 32 (94.1) | 69 (97.2) |
| M1 | 0 | 1 (2.9) | 1 (1.4) |
| Planned treatment, n (%) | |||
| Type of treatment received | |||
| Radiotherapy only | 9 (24.3) | 8 (23.5) | 17 (23.9) |
| Chemoradiotherapy | 28 (75.7) | 26 (76.5) | 54 (76.1) |
| Radiotherapy and surgery details, n (%) | |||
| Patient to have IMRT | |||
| Yes | 37 (100) | 34 (100) | 71 (100) |
| Patient has had surgery to primary tumour | |||
| No | 24 (64.9) | 19 (55.9) | 43 (60.6) |
| Yes | 13 (35.1) | 15 (44.1) | 28 (39.4) |
| Debulking | 0 | 0 | 0 |
| Laser | 2 | 1 | 3 |
| Transoral robotic surgery | 4 | 2 | 6 |
| Open surgery, no reconstruction | 1 | 2 | 3 |
| Open surgery with distant flap reconstruction | 1 | 2 | 3 |
| Neck dissection, unilateral/bilateral | 3 | 5 | 8 |
| Other | 2 | 3 | 5 |
| Comorbidity details, n (%) | |||
| Adult comorbidity evaluation | |||
| None | 13 (35.1) | 17 (50) | 30 (42.3) |
| Grade 1 | 3 (8.1) | 2 (5.9) | 5 (7) |
| Grade 2 | 5 (13.5) | 2 (5.9) | 7 (9.9) |
| Grade 3 | 1 (2.7) | 0 | 1 (1.4) |
| Unknown | 7 (18.9) | 3 (8.8) | 10 (14.1) |
| Missing | 8 (21.6) | 10 (29.4) | 18 (25.4) |
| HRQoL | |||
| MDADI global score | |||
| Minimum | 20 | 20 | 20 |
| Median (IQR) | 80 (80–100) | 100 (60–100) | 80 (60–100) |
| Mean (SD) | 80 (24.5) | 80.6 (25.3) | 80.3 (24.7) |
| Maximum | 100 | 100 | 100 |
| MDADI composite score | |||
| Minimum | 52.6 | 51.6 | 51.6 |
| Median (IQR) | 88.4 (67.4–97.4) | 88.4 (73.7–95.8) | 88.4 (67.4–97.9) |
| Mean (SD) | 82.7 (15.6) | 83.8 (13.9) | 83.2 (14.7) |
| Maximum | 100 | 100 | 100 |
| EORTC QLQ-C30 summary scoreb | |||
| Minimum | 34.7 | 49.5 | 34.7 |
| Median (IQR) | 84.2 (72–90.8) | 88.1 (71.3–93.5) | 85.7 (71.3–92.3) |
| Mean (SD) | 79.4 (16.1) | 82.3 (14.3) | 80.8 (15.2) |
| Maximum | 99.4 | 99.4 | 99.4 |
| Nutritional parameters, n (%) | |||
| PSS-HN score of ≤ 50 | |||
| Normalcy of diet | 8 (21.6) | 6 (17.6) | 14 (19.7) |
| Public eating | 5 (13.5) | 2 (5.9) | 7 (9.9) |
| Understandability of speech | 0 (0) | 0 (0) | 0 (0) |
| Swallowing | |||
| WST median (IQR) | |||
| Volumec | 20 (14.3–25) | 20 (12.5–25) | 20 (14.3–25) |
| Capacitya | 12.5 (8.3–20) | 15.5 (8.3–20) | 13.4 (8.3–20) |
| Time point | Treatment arm | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||||||
| n | Fully missing | Partial missing | Complete | n | Fully missing | Partial missing | Complete | n | Fully missing | Partial missing | Complete | |
| Baseline | 44 | 1 | 0 | 43 | 43 | 0 | 0 | 43 | 87 | 1 | 0 | 86 |
| 1 week | 44 | 4 | 0 | 40 | 40 | 0 | 1 | 39 | 84 | 4 | 1 | 78 |
| 2 weeks | 41 | 1 | 2 | 38 | 39 | 2 | 0 | 37 | 80 | 3 | 2 | 75 |
| 3 weeks | 41 | 2 | 3 | 36 | 38 | 1 | 1 | 36 | 79 | 3 | 4 | 72 |
| 4 weeks | 40 | 1 | 2 | 37 | 38 | 0 | 0 | 38 | 78 | 1 | 2 | 75 |
| 5 weeks | 40 | 3 | 1 | 36 | 38 | 2 | 0 | 36 | 78 | 5 | 1 | 72 |
| 6 weeks | 40 | 2 | 1 | 37 | 38 | 3 | 1 | 34 | 78 | 5 | 2 | 71 |
| 4 months | 39 | 16 | 0 | 23 | 38 | 17 | 2 | 19 | 77 | 33 | 2 | 42 |
| Score | Treatment arm | |||||||
|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||
| Baseline (N = 44) | 6 weeks (N = 40) | 4 months (N = 39) | 14 months (N = 12) | Baseline (N = 43) | 6 weeks (N = 38) | 4 months (N = 38) | 14 months (N = 16) | |
| Available (patients), n | 44 | 37 | 35 | 10 | 43 | 35 | 35 | 16 |
| MDADI global domain score | ||||||||
| Minimum | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 40 |
| Median (IQR) | 80 (60–100) | 40 (20–80) | 80 (40–80) | 80 (40–100) | 100 (80–100) | 40 (40–40) | 80 (60–80) | 80 (40–100) |
| Mean (SD) | 78.2 (26.2) | 45.4 (26.1) | 65.1 (28) | 72 (30.1) | 81.4 (24.1) | 45.1 (22.4) | 73.7 (22.6) | 76.3 (26.6) |
| Maximum | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Score | Treatment arm | |||||||
|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||
| Baseline (N = 44) | 6 weeks (N = 40) | 4 months (N = 39) | 14 months (N = 12) | Baseline (N = 43) | 6 weeks (N = 38) | 4 months (N = 38) | 14 months (N = 16) | |
| Available (patients), n | 44 | 36 | 35 | 10 | 43 | 35 | 34 | 16 |
| MDADI emotional domain score | ||||||||
| Minimum | 43.3 | 30 | 43.3 | 43.3 | 50 | 43.3 | 33.3 | 43.3 |
| Median (IQR) | 85 (71.7–100) | 61.7 (51.7–75) | 70 (60–86.7) | 78.3 (66.7–90) | 86.7 (73.3–96.7) | 66.7 (53.3–76.7) | 76.7 (60–86.7) | 83.3 (69.3–93.3) |
| Mean (SD) | 83.1 (16.4) | 63.5 (15.9) | 72.5 (17.7) | 75.7 (18.1) | 83.4 (13.8) | 66.8 (13.6) | 72.2 (18) | 80.3 (16.6) |
| Maximum | 100 | 96.7 | 100 | 100 | 100 | 90 | 100 | 100 |
| Score | Treatment arm | |||||||
|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||
| Baseline (N = 44) | 6 weeks (N = 40) | 4 months (N = 39) | 14 months (N = 12) | Baseline (N = 43) | 6 weeks (N = 38) | 4 months (N = 38) | 14 months (N = 16) | |
| Available (patients), n | 44 | 35 | 35 | 10 | 43 | 35 | 33 | 16 |
| MDADI functional domain score | ||||||||
| Minimum | 52 | 32 | 36 | 30 | 52 | 36 | 28 | 40 |
| Median (IQR) | 84 (72–100) | 56 (44–72) | 68 (60–84) | 82 (60–84) | 84 (72–100) | 60 (52–68) | 72 (60–84) | 84 (70–98) |
| Mean (SD) | 83.4 (15.6) | 58.3 (17.9) | 70.3 (17.9) | 74.2 (20.2) | 84.5 (13.6) | 59.1 (13.6) | 67.7 (18.7) | 80 (19.4) |
| Maximum | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Score | Treatment arm | |||||||
|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||
| Baseline (N = 44) | 6 weeks (N = 40) | 4 months (N = 39) | 14 months (N = 12) | Baseline (N = 43) | 6 weeks (N = 38) | 4 months (N = 38) | 14 months (N = 16) | |
| Available (patients), n | 44 | 34 | 35 | 10 | 43 | 35 | 33 | 16 |
| Physical domain score | ||||||||
| Minimum | 47.5 | 32.5 | 32.5 | 52.5 | 52.5 | 32.5 | 40 | 52.5 |
| Median (IQR) | 83.8 (61.3–100) | 50 (47.5–55) | 62.5 (50–72.5) | 70 (57.5–72.5) | 92.5 (72.5–100) | 52.5 (45–60) | 62.5 (55–72.5) | 80 (65–90) |
| Mean (SD) | 79.7 (19.3) | 52.8 (12.9) | 62.1 (17.6) | 70.3 (15) | 83.8 (16.9) | 54.4 (14) | 64.7 (14.8) | 76.6 (16.1) |
| Maximum | 100 | 85 | 100 | 100 | 100 | 97.5 | 100 | 100 |
| Score | Treatment arm | |||||||
|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||
| Baseline (N = 44) | 6 weeks (N = 40) | 4 months (N = 39) | 14 months (N = 12) | Baseline (N = 43) | 6 weeks (N = 38) | 4 months (N = 38) | 14 months (N = 16) | |
| Available (patients), n | 44 | 34 | 35 | 10 | 43 | 35 | 33 | 16 |
| Composite score | ||||||||
| Minimum | 47.4 | 36.8 | 38.9 | 45.8 | 51.6 | 40 | 35.8 | 46.3 |
| Median (IQR) | 83.7 (66.8–98.4) | 53.2 (50.5–65.3) | 65.3 (55.8–78.9) | 74.2 (63.2–82.1) | 88.4 (73.7–96.8) | 58.9 (50.5–68.4) | 68.4 (56.8–76.8) | 80.3 (69.5–91.1) |
| Mean (SD) | 81.7 (16.7) | 58.1 (13.6) | 67.5 (16.2) | 73 (16.1) | 83.9 (13.5) | 59.5 (11.7) | 68 (15) | 78.7 (15.9) |
| Maximum | 100 | 88.4 | 100 | 100 | 100 | 94.7 | 100 | 100 |
| Time point | Treatment arm (n) | Total (n) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | ||||||||||||||
| N | All items missing | More than half items missing | Half or fewer items missing | Complete | N | All items missing | More than half items missing | Half or fewer items missing | Complete | N | All items missing | More than half items missing | Half or fewer items missing | Complete | |
| Global | |||||||||||||||
| Baseline | 44 | 0 | 0 | 0 | 44 | 43 | 0 | 0 | 0 | 43 | 87 | 0 | 0 | 0 | 87 |
| 6 weeks | 40 | 3 | 0 | 0 | 37 | 38 | 3 | 0 | 0 | 35 | 78 | 6 | 0 | 0 | 72 |
| 4 months | 39 | 4 | 0 | 0 | 35 | 38 | 3 | 0 | 0 | 35 | 77 | 7 | 0 | 0 | 70 |
| 14 months | 12 | 2 | 0 | 0 | 10 | 16 | 0 | 0 | 0 | 16 | 28 | 2 | 0 | 0 | 26 |
| Emotional | |||||||||||||||
| Baseline | 44 | 0 | 0 | 0 | 44 | 43 | 0 | 0 | 0 | 43 | 87 | 0 | 0 | 0 | 87 |
| 6 weeks | 40 | 3 | 1 | 3 | 33 | 38 | 3 | 0 | 0 | 35 | 78 | 6 | 1 | 3 | 68 |
| 4 months | 39 | 4 | 0 | 0 | 35 | 38 | 4 | 0 | 1 | 33 | 77 | 8 | 0 | 1 | 68 |
| 14 months | 12 | 2 | 0 | 0 | 10 | 16 | 0 | 0 | 1 | 15 | 28 | 2 | 0 | 1 | 25 |
| Functional | |||||||||||||||
| Baseline | 44 | 0 | 0 | 0 | 44 | 43 | 0 | 0 | 0 | 43 | 87 | 0 | 0 | 0 | 87 |
| 6 weeks | 40 | 3 | 2 | 1 | 34 | 38 | 3 | 0 | 0 | 35 | 78 | 6 | 2 | 1 | 69 |
| 4 months | 39 | 4 | 0 | 0 | 35 | 38 | 4 | 1 | 0 | 33 | 77 | 8 | 1 | 0 | 68 |
| 14 months | 12 | 2 | 0 | 1 | 9 | 16 | 0 | 0 | 0 | 16 | 28 | 2 | 0 | 1 | 25 |
| Physical | |||||||||||||||
| Baseline | 44 | 0 | 0 | 0 | 44 | 43 | 0 | 0 | 0 | 43 | 87 | 0 | 0 | 0 | 87 |
| 6 weeks | 40 | 3 | 3 | 1 | 33 | 38 | 3 | 0 | 0 | 35 | 78 | 6 | 3 | 1 | 68 |
| 4 months | 39 | 4 | 0 | 0 | 35 | 38 | 4 | 1 | 0 | 33 | 77 | 8 | 1 | 0 | 68 |
| 14 months | 12 | 2 | 0 | 0 | 10 | 16 | 0 | 0 | 0 | 16 | 28 | 2 | 0 | 0 | 26 |
| Composite | N | No domain scores | One domain score only | Two domain scores only | All domain scores available | N | No domain scores | One domain score only | Two domain scores only | All domain scores available | N | No domain scores | One domain score only | Two domain scores only | All domain scores available |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 44 | 0 | 0 | 0 | 44 | 43 | 0 | 0 | 0 | 43 | 87 | 0 | 0 | 0 | 87 |
| 6 weeks | 40 | 4 | 1 | 1 | 34 | 38 | 3 | 0 | 0 | 35 | 78 | 7 | 1 | 1 | 69 |
| 4 months | 39 | 4 | 0 | 0 | 35 | 38 | 4 | 1 | 0 | 33 | 77 | 8 | 1 | 0 | 68 |
| 14 months | 12 | 2 | 0 | 0 | 10 | 16 | 0 | 0 | 0 | 16 | 28 | 2 | 0 | 0 | 26 |
| Score | Treatment arm | |||||||
|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||
| Baseline (N = 44) | 6 weeks (N = 40) | 4 months (N = 39) | 14 months (N = 12) | Baseline (N = 43) | 6 weeks (N = 38) | 4 months (N = 38) | 14 months (N = 16) | |
| Available (patients), n | 44 | 36 | 34 | 10 | 42 | 34 | 35 | 16 |
| EORTC QLQ-C30 global health/QoL score | ||||||||
| Minimum | 8.3 | 0 | 16.7 | 50 | 16.7 | 16.7 | 8.3 | 50 |
| Median (IQR) | 66.7 (50–83.3) | 41.7 (25–50) | 66.7 (50–75) | 70.8 (66.7–83.3) | 66.7 (50–83.3) | 37.5 (25–58.3) | 66.7 (41.7–83.3) | 83.3 (66.7–95.8) |
| Mean (SD) | 64.8 (24.4) | 41.4 (20.1) | 60.8 (20.9) | 74.2 (13.9) | 67.9 (23.8) | 43.9 (22.4) | 63.1 (25.6) | 81.3 (15.1) |
| Maximum | 100 | 83.3 | 100 | 100 | 100 | 83.3 | 100 | 100 |
| Score | Treatment arm | |||||||
|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||
| Baseline (N = 44) | 6 weeks (N = 40) | 4 months (N = 39) | 14 months (N = 12) | Baseline (N = 43) | 6 weeks (N = 38) | 4 months (N = 38) | 14 months (N = 16) | |
| Available (patients), n | 43 | 36 | 34 | 10 | 42 | 34 | 35 | 16 |
| EORTC QLQ-C30 financial difficulties score | ||||||||
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Median (IQR) | 0 (0–33.3) | 0 (0–66.7) | 0 (0–33.3) | 0 (0–0) | 0 (0–33.3) | 0 (0–33.3) | 0 (0–33.3) | 0 (0–0) |
| Mean (SD) | 18.6 (29.4) | 28.7 (40.7) | 22.5 (29.3) | 0 (0) | 16.7 (32.3) | 20.6 (30.7) | 13.3 (27.1) | 6.2 (18.1) |
| Maximum | 100 | 100 | 100 | 0 | 100 | 100 | 100 | 66.7 |
| Score | Treatment arm | |||||||
|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||
| Baseline (N = 44) | 6 weeks (N = 40) | 4 months (N = 39) | 14 months (N = 12) | Baseline (N = 43) | 6 weeks (N = 38) | 4 months (N = 38) | 14 months (N = 16) | |
| Available (patients), n | 44 | 36 | 34 | 10 | 42 | 34 | 35 | 16 |
| EORTC QLQ-C30 summary score | ||||||||
| Minimum | 34.7 | 23.2 | 43.8 | 60.9 | 49.5 | 13.2 | 28.1 | 58.5 |
| Median (IQR) | 84.3 (70.9–91) | 54.3 (43.4–71.4) | 80.2 (66.3–88.1) | 83.2 (79.5–91) | 83.8 (70.8–94) | 66.5 (50.1–72.4) | 87.5 (65–92.7) | 92.9 (83.7–96.9) |
| Mean (SD) | 80.1 (15.8) | 55.7 (17.4) | 77.3 (15.2) | 83.5 (11) | 81.3 (14.6) | 62 (17.9) | 78.7 (18.2) | 89.7 (10.9) |
| Maximum | 100 | 88.3 | 100 | 100 | 99.4 | 91.9 | 99.5 | 100 |
| Score | Treatment arm | |||||||
|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||
| Baseline (N = 44) | 6 weeks (N = 40) | 4 months (N = 39) | 14 months (N = 12) | Baseline (N = 43) | 6 weeks (N = 38) | 4 months (N = 38) | 14 months (N = 16) | |
| Available (patients), n | 43 | 37 | 35 | 10 | 43 | 35 | 35 | 16 |
| EORTC QLQ-H&N35 pain score | ||||||||
| Minimum | 0 | 25 | 0 | 0 | 0 | 16.7 | 0 | 0 |
| Median (IQR) | 25 (8.3–41.7) | 50 (41.7–66.7) | 25 (8.3–33.3) | 8.3 (0–33.3) | 16.7 (0–33.3) | 50 (33.3–58.3) | 16.7 (0–25) | 8.3 (0–16.7) |
| Mean (SD) | 26.2 (20.5) | 53.2 (19.4) | 25.9 (24.4) | 15 (15.6) | 19.8 (22.3) | 46.8 (19) | 20 (24.2) | 12.5 (15.8) |
| Maximum | 83.3 | 100 | 91.7 | 41.7 | 83.3 | 83.3 | 83.3 | 41.7 |
| Score | Treatment arm | |||||||
|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||
| Baseline (N = 44) | 6 weeks (N = 40) | 4 months (N = 39) | 14 months (N = 12) | Baseline (N = 43) | 6 weeks (N = 38) | 4 months (N = 38) | 14 months (N = 16) | |
| Available (patients), n | 43 | 37 | 35 | 10 | 43 | 35 | 35 | 16 |
| EORTC QLQ-H&N35 swallowing score | ||||||||
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Median (IQR) | 8.3 (0–16.7) | 58.3 (41.7–83.3) | 16.7 (0–50) | 16.7 (8.3–33.3) | 0 (0–8.3) | 58.3 (25–66.7) | 8.3 (8.3–25) | 4.2 (0–20.8) |
| Mean (SD) | 15.3 (22.6) | 56.5 (25.5) | 26 (25.2) | 18.3 (13.5) | 7.6 (13.7) | 49.8 (28.2) | 20.6 (23) | 9.9 (11.9) |
| Maximum | 100 | 91.7 | 83.3 | 41.7 | 66.7 | 100 | 83.3 | 33.3 |
| Score | Treatment arm | |||||||
|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||
| Baseline (N = 44) | 6 weeks (N = 40) | 4 months (N = 39) | 14 months (N = 12) | Baseline (N = 43) | 6 weeks (N = 38) | 4 months (N = 38) | 14 months (N = 16) | |
| Available (patients), n | 43 | 37 | 35 | 10 | 43 | 35 | 35 | 16 |
| EORTC QLQ-H&N35 senses problems score | ||||||||
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Median (IQR) | 0 (0–16.7) | 50 (50–83.3) | 33.3 (16.7–50) | 16.7 (0–33.3) | 0 (0–16.7) | 50 (50–66.7) | 33.3 (16.7–50) | 33.3 (0–41.7) |
| Mean (SD) | 11.6 (19.8) | 61.3 (25.2) | 38.1 (28.5) | 21.7 (19.3) | 13.2 (19.4) | 59 (23.7) | 31.9 (22.3) | 25 (25.8) |
| Maximum | 83.3 | 100 | 100 | 50 | 66.7 | 100 | 83.3 | 83.3 |
| Score | Treatment arm | |||||||
|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||
| Baseline (N = 44) | 6 weeks (N = 40) | 4 months (N = 39) | 14 months (N = 12) | Baseline (N = 43) | 6 weeks (N = 38) | 4 months (N = 38) | 14 months (N = 16) | |
| Available (patients), n | 43 | 37 | 35 | 9 | 43 | 35 | 35 | 16 |
| EORTC QLQ-H&N35 speech problems score | ||||||||
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Median (IQR) | 11.1 (0–22.2) | 44.4 (22.2–66.7) | 22.2 (11.1–55.6) | 22.2 (11.1–44.4) | 0 (0–11.1) | 33.3 (11.1–55.6) | 11.1 (11.1–33.3) | 5.6 (0–16.7) |
| Mean (SD) | 17.3 (21) | 43.8 (29.3) | 28.9 (28.5) | 23.5 (20.4) | 11.4 (17.6) | 37.8 (31.8) | 26.7 (27.9) | 9 (10.9) |
| Maximum | 77.8 | 100 | 100 | 55.6 | 77.8 | 100 | 100 | 33.3 |
| Score | Treatment arm | |||||||
|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||
| Baseline (N = 44) | 6 weeks (N = 40) | 4 months (N = 39) | 14 months (N = 12) | Baseline (N = 43) | 6 weeks (N = 38) | 4 months (N = 38) | 14 months (N = 16) | |
| Available (patients), n | 43 | 36 | 35 | 10 | 43 | 34 | 34 | 16 |
| EORTC QLQ-H&N35 trouble with social eating score | ||||||||
| Minimum | 0 | 16.7 | 0 | 0 | 0 | 0 | 0 | 0 |
| Median (IQR) | 8.3 (0–33.3) | 58.3 (41.7–95.8) | 33.3 (8.3–50) | 8.3 (0–58.3) | 8.3 (0–22.2) | 50 (41.7–75) | 25 (16.7–50) | 12.5 (0–29.2) |
| Mean (SD) | 19.6 (25.9) | 61.4 (28.9) | 39.5 (33.5) | 26.7 (36) | 13.3 (19.8) | 57.8 (28.2) | 33.3 (27.4) | 18.7 (23.5) |
| Maximum | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 75 |
| Score | Treatment arm | |||||||
|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||
| Baseline (N = 44) | 6 weeks (N = 40) | 4 months (N = 39) | 14 months (N = 12) | Baseline (N = 43) | 6 weeks (N = 38) | 4 months (N = 38) | 14 months (N = 16) | |
| Available (patients), n | 43 | 37 | 35 | 10 | 43 | 34 | 35 | 16 |
| EORTC QLQ-H&N35 trouble with social contact score | ||||||||
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Median (IQR) | 0 (0–6.7) | 20 (6.7–66.7) | 0 (0–20) | 3.3 (0–13.3) | 0 (0–6.7) | 25.8 (6.7–46.7) | 0 (0–13.3) | 0 (0–3.3) |
| Mean (SD) | 8.2 (20.8) | 34.1 (31.9) | 14.7 (22.5) | 7.3 (10.6) | 5.9 (12.2) | 27.4 (24.4) | 13.5 (22.7) | 3.3 (7.3) |
| Maximum | 93.3 | 100 | 73.3 | 33.3 | 53.3 | 80 | 86.7 | 26.7 |
| Score | Treatment arm | |||||||
|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||
| Baseline (N = 44) | 6 weeks (N = 40) | 4 months (N = 39) | 14 months (N = 12) | Baseline (N = 43) | 6 weeks (N = 38) | 4 months (N = 38) | 14 months (N = 16) | |
| Available (patients), n | 41 | 32 | 33 | 8 | 40 | 32 | 34 | 13 |
| EORTC QLQ-H&N35 less sexuality score | ||||||||
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Median (IQR) | 0 (0–33.3) | 50 (16.7–100) | 0 (0–33.3) | 8.3 (0–50) | 8.3 (0–50) | 58.3 (0–100) | 25 (0–66.7) | 0 (0–33.3) |
| Mean (SD) | 19.5 (30.3) | 50.5 (38.2) | 21.7 (28.1) | 22.9 (29.5) | 29.2 (36.9) | 57.3 (43.4) | 35.8 (41.5) | 17.9 (32.2) |
| Maximum | 100 | 100 | 100 | 66.7 | 100 | 100 | 100 | 100 |
| Score | Treatment arm | |||||||
|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||
| Baseline (N = 44) | 6 weeks (N = 40) | 4 months (N = 39) | 14 months (N = 12) | Baseline (N = 43) | 6 weeks (N = 38) | 4 months (N = 38) | 14 months (N = 16) | |
| Available (patients), n | 43 | 37 | 35 | 10 | 43 | 35 | 33 | 16 |
| EORTC QLQ-H&N35 teeth score | ||||||||
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Median (IQR) | 0 (0–33.3) | 0 (0–0) | 0 (0–33.3) | 33.3 (33.3–66.7) | 0 (0–0) | 0 (0–33.3) | 0 (0–0) | 16.7 (0–33.3) |
| Mean (SD) | 24 (36.6) | 12.6 (28.7) | 11.4 (19.7) | 36.7 (24.6) | 9.3 (22.2) | 12.4 (21.5) | 8.1 (18.7) | 16.7 (17.2) |
| Maximum | 100 | 100 | 66.7 | 66.7 | 100 | 66.7 | 66.7 | 33.3 |
| Score | Treatment arm | |||||||
|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||
| Baseline (N = 44) | 6 weeks (N = 40) | 4 months (N = 39) | 14 months (N = 12) | Baseline (N = 43) | 6 weeks (N = 38) | 4 months (N = 38) | 14 months (N = 16) | |
| Available (patients), n | 43 | 37 | 35 | 10 | 43 | 35 | 35 | 16 |
| EORTC-QLQ-H&N35 opening mouth score | ||||||||
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Median (IQR) | 0 (0–33.3) | 33.3 (0–66.7) | 0 (0–33.3) | 0 (0–33.3) | 0 (0–33.3) | 33.3 (0–66.7) | 0 (0–33.3) | 0 (0–33.3) |
| Mean (SD) | 22.5 (33.9) | 38.7 (32.9) | 28.6 (37.2) | 16.7 (32.4) | 20.2 (30.1) | 38.1 (34.4) | 24.8 (35.6) | 22.9 (31.5) |
| Maximum | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Score | Treatment arm | |||||||
|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||
| Baseline (N = 44) | 6 weeks (N = 40) | 4 months (N = 39) | 14 months (N = 12) | Baseline (N = 43) | 6 weeks (N = 38) | 4 months (N = 38) | 14 months (N = 16) | |
| Available (patients), n | 43 | 37 | 35 | 10 | 43 | 35 | 35 | 16 |
| EORTC-QLQ-H&N35 dry mouth score | ||||||||
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 | 33.3 | 0 |
| Median (IQR) | 0 (0–33.3) | 66.7 (66.7–100) | 66.7 (33.3–100) | 66.7 (33.3–100) | 0 (0–33.3) | 66.7 (66.7–100) | 66.7 (33.3–100) | 66.7 (33.3–66.7) |
| Mean (SD) | 17.8 (26.6) | 69.4 (33.7) | 70.5 (31.1) | 60 (43.9) | 20.9 (28.2) | 70.5 (28.9) | 71.4 (27) | 56.2 (31.5) |
| Maximum | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Score | Treatment arm | |||||||
|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||
| Baseline (N = 44) | 6 weeks (N = 40) | 4 months (N = 39) | 14 months (N = 12) | Baseline (N = 43) | 6 weeks (N = 38) | 4 months (N = 38) | 14 months (N = 16) | |
| Available (patients), n | 43 | 37 | 35 | 10 | 43 | 35 | 35 | 16 |
| EORTC-QLQ-H&N35 sticky saliva score | ||||||||
| Minimum | 0 | 0 | 0 | 0 | 0 | 33.3 | 0 | 0 |
| Median (IQR) | 0 (0–33.3) | 100 (66.7–100) | 33.3 (33.3–100) | 66.7 (33.3–66.7) | 0 (0–33.3) | 100 (66.7–100) | 33.3 (33.3–100) | 33.3 (0–66.7) |
| Mean (SD) | 23.3 (34.5) | 84.7 (24.3) | 51.4 (38.2) | 56.7 (31.6) | 16.3 (24.5) | 82.9 (20.4) | 51.4 (38.2) | 39.6 (37) |
| Maximum | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Score | Treatment arm | |||||||
|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||
| Baseline (N = 44) | 6 weeks (N = 40) | 4 months (N = 39) | 14 months (N = 12) | Baseline (N = 43) | 6 weeks (N = 38) | 4 months (N = 38) | 14 months (N = 16) | |
| Available (patients), n | 43 | 37 | 35 | 10 | 43 | 35 | 35 | 16 |
| EORTC-QLQ-H&N35 coughing score | ||||||||
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Median (IQR) | 33.3 (0–33.3) | 33.3 (33.3–100) | 33.3 (0–66.7) | 33.3 (33.3–66.7) | 33.3 (0–33.3) | 33.3 (33.3–66.7) | 33.3 (0–33.3) | 0 (0–33.3) |
| Mean (SD) | 29.5 (26.4) | 55.9 (32.4) | 37.1 (32.1) | 40 (30.6) | 21.7 (21.7) | 48.6 (31.7) | 28.6 (23.1) | 18.7 (24.2) |
| Maximum | 100 | 100 | 100 | 100 | 66.7 | 100 | 100 | 66.7 |
| Score | Treatment arm | |||||||
|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||
| Baseline (N = 44) | 6 weeks (N = 40) | 4 months (N = 39) | 14 months (N = 12) | Baseline (N = 43) | 6 weeks (N = 38) | 4 months (N = 38) | 14 months (N = 16) | |
| Available (patients), n | 43 | 37 | 35 | 10 | 43 | 35 | 35 | 16 |
| EORTC-QLQ-H&N35 felt ill score | ||||||||
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Median (IQR) | 0 (0–33.3) | 33.3 (33.3–66.7) | 0 (0–33.3) | 0 (0–0) | 0 (0–33.3) | 33.3 (33.3–66.7) | 0 (0–33.3) | 0 (0–0) |
| Mean (SD) | 14.7 (24.5) | 51.4 (32) | 14.3 (25.9) | 6.7 (14.1) | 13.2 (24.3) | 46.7 (33.5) | 13.3 (25.8) | 8.3 (19.2) |
| Maximum | 100 | 100 | 100 | 33.3 | 100 | 100 | 100 | 66.7 |
| Score | Treatment arm | |||||||
|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||
| Baseline (N = 44) | 6 weeks (N = 40) | 4 months (N = 39) | 14 months (N = 12) | Baseline (N = 43) | 6 weeks (N = 38) | 4 months (N = 38) | 14 months (N = 16) | |
| Available, n | 43 | 37 | 35 | 10 | 43 | 35 | 35 | 16 |
| EORTC QLQ-H&N35 painkillers | ||||||||
| Yes, n (%) | 33 (76.7) | 37 (100) | 17 (48.6) | 4 (40) | 31 (72.1) | 34 (97.1) | 20 (57.1) | 4 (25) |
| Score | Treatment arm | |||||||
|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||
| Baseline (N = 44) | 6 weeks (N = 40) | 4 months (N = 39) | 14 months (N = 12) | Baseline (N = 43) | 6 weeks (N = 38) | 4 months (N = 38) | 14 months (N = 16) | |
| Available, n | 43 | 37 | 35 | 10 | 43 | 35 | 35 | 16 |
| EORTC QLQ-H&N35 nutritional supplements | ||||||||
| Yes, n (%) | 15 (34.9) | 28 (75.7) | 16 (45.7) | 3 (30) | 13 (30.2) | 28 (80) | 20 (57.1) | 5 (31.3) |
| Score | Treatment arm | |||||||
|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||
| Baseline (N = 44) | 6 weeks (N = 40) | 4 months (N = 39) | 14 months (N = 12) | Baseline (N = 43) | 6 weeks (N = 38) | 4 months (N = 38) | 14 months (N = 16) | |
| Available, n | 43 | 37 | 35 | 10 | 43 | 35 | 35 | 16 |
| EORTC QLQ-H&N35 feeding tube | ||||||||
| Yes, n (%) | 12 (27.9) | 24 (64.9) | 11 (31.4) | 2 (20) | 4 (9.3) | 21 (60) | 7 (20) | 0 (0) |
| Score | Treatment arm | |||||||
|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||
| Baseline (N = 44) | 6 weeks (N = 40) | 4 months (N = 39) | 14 months (N = 12) | Baseline (N = 43) | 6 weeks (N = 38) | 4 months (N = 38) | 14 months (N = 16) | |
| Available, n | 41 | 37 | 35 | 10 | 43 | 35 | 35 | 16 |
| EORTC QLQ-H&N35 weight loss | ||||||||
| Yes, n (%) | 10 (24.4) | 27 (73) | 13 (37.1) | 2 (20) | 12 (27.9) | 30 (85.7) | 18 (51.4) | 4 (25) |
| Score | Treatment arm | |||||||
|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||
| Baseline (N = 44) | 6 weeks (N = 40) | 4 months (N = 39) | 14 months (N = 12) | Baseline (N = 43) | 6 weeks (N = 38) | 4 months (N = 38) | 14 months (N = 16) | |
| Available, n | 41 | 37 | 35 | 9 | 43 | 35 | 35 | 16 |
| EORTC QLQ-H&N35 weight gain | ||||||||
| Yes, n (%) | 7 (17.1) | 6 (16.2) | 9 (25.7) | 6 (66.7) | 17 (39.5) | 3 (8.6) | 7 (20) | 6 (37.5) |
| Time point | Treatment arm (n) | Total (n) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | ||||||||||||||
| N | All items missing | More than half of subscale scores missing | Half or fewer of subscale scores missing | Complete | N | All items missing | More than half of subscale scores missing | Half or fewer of subscale scores missing | Complete | N | All items missing | More than half of subscale scores missing | Half or fewer of subscale scores missing | Complete | |
| QLQ-C30 summary | |||||||||||||||
| Baseline | 44 | 0 | 0 | 0 | 44 | 43 | 1 | 0 | 0 | 42 | 87 | 1 | 0 | 0 | 86 |
| 6 weeks | 40 | 4 | 0 | 0 | 36 | 38 | 4 | 0 | 1 | 33 | 78 | 8 | 0 | 1 | 69 |
| 4 months | 39 | 5 | 0 | 0 | 34 | 38 | 3 | 0 | 0 | 35 | 77 | 8 | 0 | 0 | 69 |
| 14 months | 12 | 2 | 0 | 0 | 10 | 16 | 0 | 0 | 0 | 16 | 28 | 2 | 0 | 0 | 26 |
| Global health status | |||||||||||||||
| Baseline | 44 | 0 | 0 | 0 | 44 | 43 | 1 | 0 | 0 | 42 | 87 | 1 | 0 | 0 | 86 |
| 6 weeks | 40 | 4 | 0 | 0 | 36 | 38 | 4 | 0 | 0 | 34 | 78 | 8 | 0 | 0 | 70 |
| 4 months | 39 | 5 | 0 | 0 | 34 | 38 | 3 | 0 | 0 | 35 | 77 | 8 | 0 | 0 | 69 |
| 14 months | 12 | 2 | 0 | 0 | 10 | 16 | 0 | 0 | 0 | 16 | 28 | 2 | 0 | 0 | 26 |
| Financial difficulties | |||||||||||||||
| Baseline | 44 | 1 | 0 | 0 | 43 | 43 | 1 | 0 | 0 | 42 | 87 | 2 | 0 | 0 | 85 |
| 6 weeks | 40 | 4 | 0 | 0 | 36 | 38 | 4 | 0 | 0 | 34 | 78 | 8 | 0 | 0 | 70 |
| 4 months | 39 | 5 | 0 | 0 | 34 | 38 | 3 | 0 | 0 | 35 | 77 | 8 | 0 | 0 | 69 |
| 14 months | 12 | 2 | 0 | 0 | 10 | 16 | 0 | 0 | 0 | 16 | 28 | 2 | 0 | 0 | 26 |
| HN pain | |||||||||||||||
| Baseline | 44 | 1 | 0 | 0 | 43 | 43 | 0 | 0 | 0 | 43 | 87 | 1 | 0 | 0 | 86 |
| 6 weeks | 40 | 3 | 0 | 0 | 37 | 38 | 3 | 0 | 1 | 34 | 78 | 6 | 0 | 1 | 71 |
| 4 months | 39 | 4 | 0 | 1 | 34 | 38 | 3 | 0 | 0 | 35 | 77 | 7 | 0 | 1 | 69 |
| 14 months | 12 | 2 | 0 | 0 | 10 | 16 | 0 | 0 | 0 | 16 | 28 | 2 | 0 | 0 | 26 |
| HN swallowing | |||||||||||||||
| Baseline | 44 | 1 | 0 | 0 | 43 | 43 | 0 | 0 | 0 | 43 | 87 | 1 | 0 | 0 | 86 |
| 6 weeks | 40 | 3 | 0 | 1 | 36 | 38 | 3 | 0 | 2 | 33 | 78 | 6 | 0 | 3 | 69 |
| 4 months | 39 | 4 | 0 | 1 | 34 | 38 | 3 | 0 | 2 | 33 | 77 | 7 | 0 | 3 | 67 |
| 14 months | 12 | 2 | 0 | 1 | 9 | 16 | 0 | 0 | 0 | 16 | 28 | 2 | 0 | 1 | 25 |
| HN senses problems | |||||||||||||||
| Baseline | 44 | 1 | 0 | 0 | 43 | 43 | 0 | 0 | 0 | 43 | 87 | 1 | 0 | 0 | 86 |
| 6 weeks | 40 | 3 | 0 | 0 | 37 | 38 | 3 | 0 | 0 | 35 | 78 | 6 | 0 | 0 | 72 |
| 4 months | 39 | 4 | 0 | 0 | 35 | 38 | 3 | 0 | 0 | 35 | 77 | 7 | 0 | 0 | 70 |
| 14 months | 12 | 2 | 0 | 0 | 10 | 16 | 0 | 0 | 0 | 16 | 28 | 2 | 0 | 0 | 26 |
| HN speech problems | |||||||||||||||
| Baseline | 44 | 1 | 0 | 0 | 43 | 43 | 0 | 0 | 0 | 43 | 87 | 1 | 0 | 0 | 86 |
| 6 weeks | 40 | 3 | 0 | 1 | 36 | 38 | 3 | 0 | 0 | 35 | 78 | 6 | 0 | 1 | 71 |
| 4 months | 39 | 4 | 0 | 0 | 35 | 38 | 3 | 0 | 0 | 35 | 77 | 7 | 0 | 0 | 70 |
| 14 months | 12 | 3 | 0 | 0 | 9 | 16 | 0 | 0 | 0 | 16 | 28 | 3 | 0 | 0 | 25 |
| HN trouble with social eating | |||||||||||||||
| Baseline | 44 | 1 | 0 | 0 | 43 | 43 | 0 | 0 | 1 | 42 | 87 | 1 | 0 | 1 | 85 |
| 6 weeks | 40 | 3 | 1 | 2 | 34 | 38 | 3 | 1 | 1 | 33 | 78 | 6 | 2 | 3 | 67 |
| 4 months | 39 | 4 | 0 | 0 | 35 | 38 | 4 | 0 | 1 | 33 | 77 | 8 | 0 | 1 | 68 |
| 14 months | 12 | 2 | 0 | 1 | 9 | 16 | 0 | 0 | 0 | 16 | 28 | 2 | 0 | 1 | 25 |
| HN trouble with social contact | |||||||||||||||
| Baseline | 44 | 1 | 0 | 0 | 43 | 43 | 0 | 0 | 1 | 42 | 87 | 1 | 0 | 1 | 85 |
| 6 weeks | 40 | 3 | 0 | 1 | 36 | 38 | 3 | 1 | 1 | 33 | 78 | 6 | 1 | 2 | 69 |
| 4 months | 39 | 4 | 0 | 0 | 35 | 38 | 3 | 0 | 0 | 35 | 77 | 7 | 0 | 0 | 70 |
| 14 months | 12 | 2 | 0 | 0 | 10 | 16 | 0 | 0 | 0 | 16 | 28 | 2 | 0 | 0 | 26 |
| HN less sexuality | |||||||||||||||
| Baseline | 44 | 3 | 0 | 1 | 40 | 43 | 3 | 0 | 2 | 38 | 87 | 6 | 0 | 3 | 78 |
| 6 weeks | 40 | 8 | 0 | 0 | 32 | 38 | 6 | 0 | 1 | 31 | 78 | 14 | 0 | 1 | 63 |
| 4 months | 39 | 6 | 0 | 1 | 32 | 38 | 4 | 0 | 1 | 33 | 77 | 10 | 0 | 2 | 65 |
| 14 months | 12 | 4 | 0 | 0 | 8 | 16 | 3 | 0 | 0 | 13 | 28 | 7 | 0 | 0 | 21 |
| HN teeth | |||||||||||||||
| Baseline | 44 | 1 | 0 | 0 | 43 | 43 | 0 | 0 | 0 | 43 | 87 | 1 | 0 | 0 | 86 |
| 6 weeks | 40 | 3 | 0 | 0 | 37 | 38 | 3 | 0 | 0 | 35 | 78 | 6 | 0 | 0 | 72 |
| 4 months | 39 | 4 | 0 | 0 | 35 | 38 | 5 | 0 | 0 | 33 | 77 | 9 | 0 | 0 | 68 |
| 14 months | 12 | 2 | 0 | 0 | 10 | 16 | 0 | 0 | 0 | 16 | 28 | 2 | 0 | 0 | 26 |
| HN opening mouth | |||||||||||||||
| Baseline | 44 | 1 | 0 | 0 | 43 | 43 | 0 | 0 | 0 | 43 | 87 | 1 | 0 | 0 | 86 |
| 6 weeks | 40 | 3 | 0 | 0 | 37 | 38 | 3 | 0 | 0 | 35 | 78 | 6 | 0 | 0 | 72 |
| 4 months | 39 | 4 | 0 | 0 | 35 | 38 | 3 | 0 | 0 | 35 | 77 | 7 | 0 | 0 | 70 |
| 14 months | 12 | 2 | 0 | 0 | 10 | 16 | 0 | 0 | 0 | 16 | 28 | 2 | 0 | 0 | 26 |
| HN dry mouth | |||||||||||||||
| Baseline | 44 | 1 | 0 | 0 | 43 | 43 | 0 | 0 | 0 | 43 | 87 | 1 | 0 | 0 | 86 |
| 6 weeks | 40 | 3 | 0 | 0 | 37 | 38 | 3 | 0 | 0 | 35 | 78 | 6 | 0 | 0 | 72 |
| 4 months | 39 | 4 | 0 | 0 | 35 | 38 | 3 | 0 | 0 | 35 | 77 | 7 | 0 | 0 | 70 |
| 14 months | 12 | 2 | 0 | 0 | 10 | 16 | 0 | 0 | 0 | 16 | 28 | 2 | 0 | 0 | 26 |
| HN sticky saliva | |||||||||||||||
| Baseline | 44 | 1 | 0 | 0 | 43 | 43 | 0 | 0 | 0 | 43 | 87 | 1 | 0 | 0 | 86 |
| 6 weeks | 40 | 3 | 0 | 0 | 37 | 38 | 3 | 0 | 0 | 35 | 78 | 6 | 0 | 0 | 72 |
| 4 months | 39 | 4 | 0 | 0 | 35 | 38 | 3 | 0 | 0 | 35 | 77 | 7 | 0 | 0 | 70 |
| 14 months | 12 | 2 | 0 | 0 | 10 | 16 | 0 | 0 | 0 | 16 | 28 | 2 | 0 | 0 | 26 |
| HN coughing | |||||||||||||||
| Baseline | 44 | 1 | 0 | 0 | 43 | 43 | 0 | 0 | 0 | 43 | 87 | 1 | 0 | 0 | 86 |
| 6 weeks | 40 | 3 | 0 | 0 | 37 | 38 | 3 | 0 | 0 | 35 | 78 | 6 | 0 | 0 | 72 |
| 4 months | 39 | 4 | 0 | 0 | 35 | 38 | 3 | 0 | 0 | 35 | 77 | 7 | 0 | 0 | 70 |
| 14 months | 12 | 2 | 0 | 0 | 10 | 16 | 0 | 0 | 0 | 16 | 28 | 2 | 0 | 0 | 26 |
| HN felt ill | |||||||||||||||
| Baseline | 44 | 1 | 0 | 0 | 43 | 43 | 0 | 0 | 0 | 43 | 87 | 1 | 0 | 0 | 86 |
| 6 weeks | 40 | 3 | 0 | 0 | 37 | 38 | 3 | 0 | 0 | 35 | 78 | 6 | 0 | 0 | 72 |
| 4 months | 39 | 4 | 0 | 0 | 35 | 38 | 3 | 0 | 0 | 35 | 77 | 7 | 0 | 0 | 70 |
| 14 months | 12 | 2 | 0 | 0 | 10 | 16 | 0 | 0 | 0 | 16 | 28 | 2 | 0 | 0 | 26 |
| HN painkillers | |||||||||||||||
| Baseline | 44 | 1 | 0 | 0 | 43 | 43 | 0 | 0 | 0 | 43 | 87 | 1 | 0 | 0 | 86 |
| 6 weeks | 40 | 3 | 0 | 0 | 37 | 38 | 3 | 0 | 0 | 35 | 78 | 6 | 0 | 0 | 72 |
| 4 months | 39 | 4 | 0 | 0 | 35 | 38 | 3 | 0 | 0 | 35 | 77 | 7 | 0 | 0 | 70 |
| 14 months | 12 | 2 | 0 | 0 | 10 | 16 | 0 | 0 | 0 | 16 | 28 | 2 | 0 | 0 | 26 |
| HN nutritional supplements | |||||||||||||||
| Baseline | 44 | 1 | 0 | 0 | 43 | 43 | 0 | 0 | 0 | 43 | 87 | 1 | 0 | 0 | 86 |
| 6 weeks | 40 | 3 | 0 | 0 | 37 | 38 | 3 | 0 | 0 | 35 | 78 | 6 | 0 | 0 | 72 |
| 4 months | 39 | 4 | 0 | 0 | 35 | 38 | 3 | 0 | 0 | 35 | 77 | 7 | 0 | 0 | 70 |
| 14 months | 12 | 2 | 0 | 0 | 10 | 16 | 0 | 0 | 0 | 16 | 28 | 2 | 0 | 0 | 26 |
| HN feeding tube | |||||||||||||||
| Baseline | 44 | 1 | 0 | 0 | 43 | 43 | 0 | 0 | 0 | 43 | 87 | 1 | 0 | 0 | 86 |
| 6 weeks | 40 | 3 | 0 | 0 | 37 | 38 | 3 | 0 | 0 | 35 | 78 | 6 | 0 | 0 | 72 |
| 4 months | 39 | 4 | 0 | 0 | 35 | 38 | 3 | 0 | 0 | 35 | 77 | 7 | 0 | 0 | 70 |
| 14 months | 12 | 2 | 0 | 0 | 10 | 16 | 0 | 0 | 0 | 16 | 28 | 2 | 0 | 0 | 26 |
| HN weight loss | |||||||||||||||
| Baseline | 44 | 3 | 0 | 0 | 41 | 43 | 0 | 0 | 0 | 43 | 87 | 3 | 0 | 0 | 84 |
| 6 weeks | 40 | 3 | 0 | 0 | 37 | 38 | 3 | 0 | 0 | 35 | 78 | 6 | 0 | 0 | 72 |
| 4 months | 39 | 4 | 0 | 0 | 35 | 38 | 3 | 0 | 0 | 35 | 77 | 7 | 0 | 0 | 70 |
| 14 months | 12 | 2 | 0 | 0 | 10 | 16 | 0 | 0 | 0 | 16 | 28 | 2 | 0 | 0 | 26 |
| HN weight gain | |||||||||||||||
| Baseline | 44 | 3 | 0 | 0 | 41 | 43 | 0 | 0 | 0 | 43 | 87 | 3 | 0 | 0 | 84 |
| 6 weeks | 40 | 3 | 0 | 0 | 37 | 38 | 3 | 0 | 0 | 35 | 78 | 6 | 0 | 0 | 72 |
| 4 months | 39 | 4 | 0 | 0 | 35 | 38 | 3 | 0 | 0 | 35 | 77 | 7 | 0 | 0 | 70 |
| 14 months | 12 | 2 | 0 | 0 | 10 | 16 | 0 | 0 | 0 | 16 | 28 | 2 | 0 | 0 | 26 |
| Score | Treatment arm | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||||||||||||
| Baseline (N = 44) | 1 week (N = 44) | 2 weeks(N = 41) | 3 weeks (N = 41) | 4 weeks(N = 41) | 5 weeks(N = 41) | 6 weeks (N = 40) | 4 months (N = 39) | 14 months (N = 12) | Baseline (N = 43) | 1 week (N = 40) | 2 weeks (N = 39) | 3 weeks (N = 38) | 4 weeks (N = 38) | 5 weeks(N = 38) | 6 weeks (N = 38) | 4 months (N = 38) | 14 months (N = 16) | |
| Normalcy of diet, available (patients), n | 44 | 40 | 40 | 39 | 39 | 38 | 39 | 35 | 10 | 43 | 39 | 38 | 37 | 37 | 37 | 35 | 35 | 16 |
| PSS-HN score | ||||||||||||||||||
| Scored ≤ 50, n (%) | 10 (22.7) | 9 (22.5) | 13 (32.5) | 25 (64.1) | 32 (82.1) | 31 (81.6) | 33 (84.6) | 23 (65.7) | 3 (30) | 6 (14) | 6 (15.4) | 8 (21.1) | 21 (56.8) | 29 (78.4) | 32 (86.5) | 32 (91.4) | 19 (54.3) | 5 (31.3) |
| Median (range) | 100 (0–100) | 100 (30–100) | 100 (20–100) | 50 (0–100) | 40 (0–100) | 35 (0–100) | 10 (0–100) | 50 (10–100) | 90 (0–100) | 100 (0–100) | 100 (30–100) | 100 (30–100) | 50 (0–100) | 50 (0–100) | 30 (0–100) | 20 (0–100) | 50 (0–100) | 90 (50–100) |
| Public eating, available (patients), n | 44 | 40 | 40 | 39 | 39 | 38 | 38 | 35 | 10 | 43 | 40 | 38 | 37 | 37 | 36 | 34 | 35 | 16 |
| PSS-HN score | ||||||||||||||||||
| Scored ≤ 50, n (%) | 8 (18.2) | 4 (10) | 9 (22.5) | 17 (43.6) | 23 (59) | 25 (65.8) | 28 (73.7) | 13 (37.1) | 2 (20) | 2 (4.7) | 3 (7.5) | 6 (15.8) | 12 (32.4) | 22 (59.5) | 23 (63.9) | 26 (76.5) | 11 (31.4) | 2 (12.5) |
| Inpatient | 0 | 0 | 0 | 1 (2.6) | 2 (5.1) | 3 (7.9) | 2 (5.3) | 0 | 0 | 0 | 0 | 1 (2.6) | 3 (8.1) | 1 (2.7) | 3 (8.3) | 3 (8.8) | 0 | 0 |
| Median (range)a | 100 (0–100) | 100 (25–100) | 100 (0–100) | 75 (0–100) | 50 (0–100) | 25 (0–100) | 25 (0–100) | 75 (0–100) | 100 (0–100) | 100 (25–100) | 100 (25–100) | 100 (25–100) | 75 (0–100) | 50 (0–100) | 25 (0–100) | 25 (0–100) | 75 (0–100) | 100 (25–100) |
| Understandability of speech, available (patients), n | 44 | 40 | 40 | 39 | 39 | 38 | 39 | 35 | 10 | 43 | 40 | 38 | 37 | 37 | 37 | 35 | 35 | 16 |
| PSS-HN score | ||||||||||||||||||
| Scored ≤ 50, n (%) | 2 (4.5) | 1 (2.5) | 3 (7.5) | 3 (7.7) | 3 (7.7) | 2 (5.3) | 4 (10.3) | 1 (2.9) | 1 (10) | 0 | 1 (2.5) | 1 (2.6) | 0 | 1 (2.7) | 1 (2.7) | 5 (14.3) | 0 | 0 |
| Median (range) | 100 (25–100) | 100 (25–100) | 100 (25–100) | 100 (25–100) | 100 (25–100) | 100 (25–100) | 100 (25–100) | 100 (25–100) | 100 (0–100) | 100 (75–100) | 100 (50–100) | 100 (50–100) | 100 (75–100) | 100 (50–100) | 100 (25–100) | 100 (25–100) | 100 (75–100) | 100 (75–100) |
| Score | Treatment arm | |||||||
|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||
| Baseline (N = 44) | 6 weeks (N = 40) | 4 months (N = 39) | 14 months (N = 12) | Baseline (N = 43) | 6 weeks (N = 38) | 4 months (N = 38) | 14 months (N = 16) | |
| Available (patients), n | 42 | 31 | 25 | 9 | 41 | 29 | 32 | 13 |
| Timed WST: volume (ml) | ||||||||
| Minimum | 2.8 | 0 | 0 | 0 | 0 | 0 | 0 | 7.1 |
| Median (IQR) | 20 (14.3–25) | 11.1 (0–16.7) | 14.3 (11.1–20) | 20 (16.7–33.3) | 20 (14.3–25) | 12.5 (6.7–16.7) | 16.7 (11.8–25) | 20 (14.3–25) |
| Mean (SD) | 20 (9.3) | 12.1 (11.3) | 15.6 (8.2) | 21 (11.1) | 21.1 (10.5) | 12.6 (8) | 18.6 (10.6) | 20.3 (8.5) |
| Maximum | 50 | 33.3 | 33.3 | 33.3 | 50 | 33.3 | 50 | 33.3 |
| Score | Treatment arm | |||||||
|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||
| Baseline (N = 44) | 6 weeks (N = 40) | 4 months (N = 39) | 14 months (N = 12) | Baseline (N = 43) | 6 weeks (N = 38) | 4 months (N = 38) | 14 months (N = 16) | |
| Available (patients), n | 43 | 32 | 25 | 9 | 41 | 29 | 32 | 13 |
| Timed WST: capacity (ml) | ||||||||
| Minimum | 0.4 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Median (IQR) | 12.5 (8.3–20) | 2.4 (0–11.3) | 10 (6.7–14.3) | 16.7 (11.1–20) | 16.7 (9.1–20) | 4.7 (1.8–10) | 12.5 (5.1–20) | 16.7 (12.5–20) |
| Mean (SD) | 15.2 (10.3) | 6.3 (7.7) | 11 (7.9) | 16.1 (9.5) | 15.5 (8.2) | 6.8 (6) | 14.1 (10.9) | 15.1 (6) |
| Maximum | 50 | 33.3 | 33.3 | 33.3 | 33.3 | 20 | 50 | 25 |
| ID | Treatment arm | Reason for unavailable WST data | Visit |
|---|---|---|---|
| 50002 | LLLT | Attempted 11 April 2019. Not completed successfully owing to coughing; note pain due to RIG fitting previous day | Baseline |
| 44006 | Sham | Patient not receiving LLLT, therefore withdrawn from study | Baseline |
| 46005 | Sham | Patient withdrew from study before this could be completed: no treatment given | Baseline |
| 40002 | LLLT | Declined | 6 weeks |
| 40006 | LLLT | Declined | 6 weeks |
| 41003 | LLLT | Did not attend 6-week visit | 6 weeks |
| 41005 | LLLT | Declined | 6 weeks |
| 46003 | LLLT | Patient unable to tolerate the taste of water | 6 weeks |
| 46006 | LLLT | Missed by speech and language team owing to staffing pressures | 6 weeks |
| 46016 | LLLT | No trained staff available | 6 weeks |
| 48006 | LLLT | Site error | 6 weeks |
| 14002 | Sham | Administrative error | 6 weeks |
| 18001 | Sham | Declined | 6 weeks |
| 40001 | Sham | Declined | 6 weeks |
| 40004 | Sham | Declined | 6 weeks |
| 44002 | Sham | Declined | 6 weeks |
| 44003 | Sham | Clerical error | 6 weeks |
| 45009 | Sham | Patient did not attend any 6-week laser sessions | 6 weeks |
| 46011 | Sham | Availability of SLT staff | 6 weeks |
| 48008 | Sham | Did not attend 6-week visit | 6 weeks |
| 12001 | LLLT | Lack of staff availability: decided that it was not in patient’s best interest to keep waiting much longer | 4 months |
| 40002 | LLLT | Declined | 4 months |
| 40003 | LLLT | Did not attend clinic | 4 months |
| 40005 | LLLT | Did not attend clinic | 4 months |
| 44001 | LLLT | No SLT available/unwilling to do as no report from local SLT | 4 months |
| 44004 | LLLT | No SLT available | 4 months |
| 44005 | LLLT | No SLT available to perform assessment | 4 months |
| 45007 | LLLT | Not carried out, no review took place. Patient’s choice not to attend this follow-up appointment | 4 months |
| 46003 | LLLT | Patient receiving bad news, so follow-up not carried out | 4 months |
| 46013 | LLLT | No staff available | 4 months |
| 46016 | LLLT | Staff availability | 4 months |
| 48001 | LLLT | Did not attend 4-month visit | 4 months |
| 48002 | LLLT | Did not attend 4-month visit | 4 months |
| 48006 | LLLT | Did not attend 4-month visit | 4 months |
| 15002 | Sham | Did not attend 4-month visit | 4 months |
| 45003 | Sham | Declined | 4 months |
| 45004 | Sham | Did not attend 4-month visit | 4 months |
| 46011 | Sham | Trained staff unavailable | 4 months |
| 46015 | Sham | No SLT staff available to complete the test | 4 months |
| 48005 | Sham | Did not attend 4-month visit | 4 months |
| 46001 | LLLT | Staff availability | 14 months |
| 48002 | LLLT | Did not attend 14-month visit | 14 months |
| 18001 | Sham | Patient has just had dental treatment | 14 months |
| 44003 | Sham | No SLT available | 14 months |
| 46002 | Sham | Staff availability | 14 months |
| Weight | Treatment arm | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||||||||||||
| Baseline (N = 44) | 1 week (N = 44) | 2 weeks (N = 41) | 3 weeks (N = 41) | 4 weeks (N = 41) | 5 weeks (N = 41) | 6 weeks (N = 40) | 4 months (N = 39) | 14 months (N = 12) | Baseline (N = 43) | 1 week (N = 40) | 2 weeks (N = 39) | 3 weeks (N = 38) | 4 weeks (N = 38) | 5 weeks (N = 38) | 6 weeks(N = 38) | 4 months (N = 38) | 14 months (N = 16) | |
| Available (patients), n | 44 | 37 | 37 | 40 | 39 | 37 | 39 | 31 | 9 | 43 | 38 | 38 | 36 | 38 | 35 | 35 | 33 | 16 |
| Weight (kg) | ||||||||||||||||||
| Minimum | 46.8 | 45.7 | 46.4 | 47.1 | 46.3 | 46.5 | 45.8 | 44.6 | 55.4 | 50 | 50.6 | 50.2 | 50.3 | 48.8 | 48.2 | 49.1 | 44.8 | 46.8 |
| Median (IQR) | 78.9 (70.5–90.5) | 79.4 (70.4–90.6) | 78.8 (68–91.8) | 78.4 (68.4–88.9) | 76.7 (67.6–88.4) | 74.4 (66.6–87.7) | 74.8 (65.8–84.8) | 72.1 (64.4–77.6) | 80 (64.2–81.2) | 80.2 (65–92.6) | 79.3 (66.9–91.9) | 80.3 (68.2–93) | 78.4 (67.1–90) | 76.8 (66.2–88) | 75.8 (65.6–87.1) | 75.3 (64.9–85) | 69.6 (61–80.6) | 74.1 (66.8–82.8) |
| Mean (SD) | 80.1 (14.8) | 79.8 (14.3) | 79.2 (14.9) | 78.6 (14.4) | 76.9 (13.7) | 75.4 (13.6) | 74.9 (12.7) | 71.2 (11.5) | 74.7 (11.5) | 80.4 (18.1) | 81 (18.3) | 80.7 (18) | 79.1 (18.2) | 77.5 (17.2) | 77 (16.6) | 75.9 (15.8) | 71.3 (15.1) | 77 (18.3) |
| Maximum | 110.9 | 108.8 | 106.9 | 108 | 104.2 | 102.7 | 100.4 | 98.5 | 89.4 | 135 | 136.4 | 132.4 | 133.4 | 131.5 | 127.2 | 124.3 | 119.2 | 126.2 |
| Weight | Treatment arm | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||||||||||||
| Baseline (N = 44) | 1 week (N = 44) | 2 weeks (N = 41) | 3 weeks (N = 41) | 4 weeks (N = 41) | 5 weeks (N = 41) | 6 weeks (N = 40) | 4 months (N = 39) | 14 months (N = 12) | Baseline (N = 43) | 1 week (N = 40) | 2 weeks (N = 39) | 3 weeks (N = 38) | 4 weeks (N = 38) | 5 weeks (N = 38) | 6 weeks(N = 38) | 4 months (N = 38) | 14 months (N = 16) | |
| Available (patients), n | 43 | 36 | 36 | 39 | 38 | 36 | 38 | 30 | 9 | 43 | 38 | 38 | 36 | 38 | 35 | 35 | 33 | 16 |
| BMI (kg/m2) | ||||||||||||||||||
| Minimum | 14.6 | 14.4 | 14.4 | 14.2 | 14 | 14.6 | 14.9 | 17.4 | 21.7 | 17.8 | 17.5 | 17.6 | 17.4 | 17.3 | 17.2 | 17.3 | 16.9 | 17.7 |
| Median (IQR) | 26.8 (24.5–29.8) | 26.4 (23.9–29.8) | 26.4 (23.7–29) | 26.1 (23.3–28.8) | 26 (23.1–28.2) | 24.9 (23–27.6) | 25 (22.8–27.2) | 24.2 (22.5–25.7) | 25.6 (23.6–26.3) | 25.8 (23.3–31.3) | 25.8 (23.7–31.2) | 25.9 (23–31.4) | 25 (22.8–29) | 24.7 (22.3–29.2) | 24.5 (22.3–29.7) | 24.1 (22.2–28.6) | 23.1 (21.1–25.5) | 23.4 (22.3–26.6) |
| Mean (SD) | 26.7 (4.4) | 26.7 (4.5) | 26.3 (4.4) | 26.2 (4.3) | 25.7 (4.2) | 25.2 (4.2) | 25 (4) | 24.2 (3) | 25.8 (3.1) | 26.9 (5.6) | 27.1 (5.8) | 26.9 (5.7) | 26.2 (5.6) | 25.9 (5.4) | 25.8 (5.2) | 25.3 (4.9) | 23.8 (4.6) | 25 (5.4) |
| Maximum | 37.9 | 37.2 | 36.5 | 35.2 | 35 | 34.7 | 33.8 | 30.1 | 32 | 42.9 | 42.9 | 41.9 | 42.3 | 40.7 | 39.3 | 38.4 | 36.8 | 39 |
| Oral intake | Treatment arm | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||||||||||
| 1 week (N = 44) | 2 weeks (N = 41) | 3 weeks (N = 41) | 4 weeks (N = 41) | 5 weeks (N = 41) | 6 weeks (N = 40) | 4 months (N = 39) | 14 months (N = 12) | 1 week (N = 40) | 2 weeks (N = 39) | 3 weeks (N = 38) | 4 weeks (N = 38) | 5 weeks (N = 38) | 6 weeks (N = 38) | 4 months (N = 38) | 14 months (N = 16) | |
| Available, n | 36 | 37 | 36 | 38 | 36 | 37 | 32 | 10 | 38 | 33 | 33 | 34 | 36 | 33 | 34 | 15 |
| Oral intake (% of normal), n (%) | ||||||||||||||||
| 100 | 31 (86.1) | 21 (56.8) | 15 (41.7) | 6 (15.8) | 4 (11.1) | 2 (5.4) | 6 (18.8) | 7 (70) | 31 (81.6) | 17 (51.5) | 13 (39.4) | 8 (23.5) | 6 (16.7) | 1 (3) | 8 (23.5) | 6 (40) |
| 75 | 2 (5.6) | 5 (13.5) | 6 (16.7) | 8 (21.1) | 6 (16.7) | 5 (13.5) | 7 (21.9) | 0 | 4 (10.5) | 7 (21.2) | 7 (21.2) | 2 (5.9) | 3 (8.3) | 2 (6.1) | 14 (41.2) | 5 (33.3) |
| 50 | 1 (2.8) | 7 (18.9) | 4 (11.1) | 3 (7.9) | 2 (5.6) | 2 (5.4) | 6 (18.8) | 2 (20) | 2 (5.3) | 6 (18.2) | 7 (21.2) | 9 (26.5) | 5 (13.9) | 3 (9.1) | 4 (11.8) | 3 (20) |
| 25 | 1 (2.8) | 1 (2.7) | 4 (11.1) | 5 (13.2) | 6 (16.7) | 3 (8.1) | 3 (9.4) | 0 | 0 | 1 (3) | 2 (6.1) | 5 (14.7) | 5 (13.9) | 4 (12.1) | 3 (8.8) | 1 (6.7) |
| < 25 | 1 (2.8) | 3 (8.1) | 7 (19.4) | 16 (42.1) | 18 (50) | 25 (67.6) | 10 (31.3) | 1 (10) | 1 (2.6) | 2 (6.1) | 4 (12.1) | 10 (29.4) | 17 (47.2) | 23 (69.7) | 5 (14.7) | 0 |
| Feeding tube use | Treatment arm | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||||||||||
| 1 week (N = 44) | 2 weeks (N = 41) | 3 weeks (N = 41) | 4 weeks (N = 41) | 5 weeks (N = 41) | 6 weeks (N = 40) | 4 months (N = 39) | 14 months (N = 12) | 1 week (N = 40) | 2 weeks (N = 39) | 3 weeks (N = 38) | 4 weeks (N = 38) | 5 weeks (N = 38) | 6 weeks (N = 38) | 4 months (N = 38) | 14 months (N = 16) | |
| Available (patients), n | 41 | 40 | 40 | 39 | 38 | 38 | 33 | 10 | 40 | 38 | 37 | 38 | 37 | 35 | 34 | 16 |
| Use of feeding tube, n (%) | 2 (4.9) | 4 (10) | 12 (30) | 21 (53.8) | 25 (65.8) | 25 (65.8) | 11 (33.3) | 1 (10) | 0 | 1 (2.6) | 7 (18.9) | 15 (39.5) | 23 (62.2) | 23 (65.7) | 6 (17.6) | 0 |
| Used feeding tube for the first time, n | 2 | 3 | 9 | 16 | 22 | 23 | 11 | 0 | 0 | 1 | 5 | 14 | 22 | 23 | 6 | 0 |
| Level of dependence on tube, n | ||||||||||||||||
| Total dependence on tube, nil by mouth | 0 | 0 | 2 | 4 | 9 | 15 | 0 | 1 | 0 | 0 | 0 | 6 | 5 | 9 | 1 | 0 |
| Tube dependence with minimal oral intake | 1 | 1 | 4 | 12 | 10 | 6 | 10 | 0 | 0 | 1 | 3 | 5 | 13 | 13 | 2 | 0 |
| Feeding tube supplements oral intake | 1 | 3 | 6 | 5 | 6 | 4 | 1 | 0 | 0 | 0 | 4 | 4 | 5 | 1 | 3 | 0 |
| Feeding tube type, n | ||||||||||||||||
| Gastronomy | 2 | 4 | 9 | 15 | 16 | 15 | 10 | 1 | 0 | 1 | 5 | 10 | 14 | 15 | 6 | 0 |
| Nasogastric | 0 | 0 | 1 | 5 | 6 | 6 | 1 | 0 | 0 | 0 | 2 | 4 | 8 | 7 | 0 | 0 |
| Nasojejunal | 0 | 0 | 0 | 0 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Missing feeding tube type | 0 | 0 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 |
| Painkiller use | Treatment arm | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||||||||
| Baseline (N = 44) | 1 week (N = 44) | 2 weeks (N = 41) | 3 weeks (N = 41) | 4 weeks (N = 40) | 5 weeks (N = 40) | 6 weeks (N = 40) | Baseline (N = 43) | 1 week (N = 40) | 2 weeks (N = 39) | 3 weeks (N = 38) | 4 weeks (N = 38) | 5 weeks (N = 38) | 6 weeks (N = 38) | |
| Available (patients), n | 43 | 41 | 39 | 40 | 39 | 38 | 38 | 43 | 39 | 38 | 37 | 37 | 36 | 36 |
| No painkiller use in past week, n (%) | 14 (32.6) | 19 (46.3) | 16 (41.0) | 6 (15.0) | 5 (12.8) | 6 (15.8) | 2 (5.1) | 14 (32.6) | 20 (51.3) | 21 (55.3) | 10 (27.0) | 5 (13.5) | 4 (11.1) | 1 (2.8) |
| Used painkiller(s) in past week, n | 29 (67.4) | 22 (53.7) | 23 (59.0) | 34 (85.0) | 34 (87.2) | 32 (84.2) | 36 (94.9) | 29 (67.4) | 19 (48.7) | 17 (44.7) | 27 (73.0) | 32 (86.5) | 32 (88.9) | 35 (97.2) |
| Type of painkiller(s), n | ||||||||||||||
| Anti-inflammatory analgesic/paracetamol | 26 | 20 | 22 | 33 | 34 | 31 | 34 | 27 | 19 | 13 | 24 | 28 | 29 | 32 |
| Opioid | 12 | 10 | 8 | 16 | 24 | 25 | 33 | 10 | 8 | 9 | 14 | 19 | 22 | 28 |
| Other | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 3 | 0 | 3 | 2 | 2 | 3 | 2 |
| Mouthwash use | Treatment arm | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||||||||
| Baseline (N = 44) | 1 week (N = 44) | 2 weeks (N = 41) | 3 weeks (N = 41) | 4 weeks (N = 40) | 5 weeks (N = 40) | 6 weeks (N = 40) | Baseline (N = 43) | 1 week (N = 40) | 2 weeks (N = 39) | 3 weeks (N = 38) | 4 weeks (N = 38) | 5 weeks (N = 38) | 6 weeks (N = 38) | |
| Available (patients), n | 43 | 40 | 40 | 40 | 39 | 38 | 39 | 43 | 40 | 37 | 37 | 38 | 36 | 36 |
| No mouthwash use in past week, n | 29 (67.4) | 17 (42.5) | 6 (15) | 5 (12.5) | 4 (10.3) | 3 (7.9) | 5 (12.8) | 25 (58.1) | 19 (47.5) | 14 (37.8) | 6 (16.2) | 4 (10.5) | 4 (11.1) | 3 (8.3) |
| Used mouthwash in past week, n (%) | 14 (32.6) | 23 (57.5) | 34 (85) | 35 (87.5) | 35 (89.7) | 35 (92.1) | 34 (87.2) | 18 (41.9) | 21 (52.5) | 23 (62.2) | 31 (83.8) | 34 (89.5) | 32 (88.9) | 33 (91.7) |
| Type of mouthwash, n | ||||||||||||||
| Simple | 3 | 6 | 11 | 11 | 11 | 11 | 10 | 6 | 7 | 7 | 12 | 13 | 10 | 10 |
| Analgesic | 4 | 7 | 7 | 14 | 18 | 19 | 19 | 5 | 3 | 3 | 7 | 14 | 15 | 17 |
| Antiseptic | 8 | 2 | 1 | 1 | 0 | 0 | 0 | 9 | 2 | 1 | 2 | 2 | 2 | 3 |
| Mucosa protecting | 0 | 11 | 22 | 22 | 24 | 22 | 21 | 1 | 11 | 15 | 21 | 22 | 23 | 22 |
| Oral hygienist visit | Treatment arm | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||||||||
| Baseline (N = 44) | 1 week (N = 44) | 2 weeks (N = 41) | 3 weeks (N = 41) | 4 weeks (N = 40) | 5 weeks (N = 40) | 6 weeks (N = 40) | Baseline (N = 43) | 1 week (N = 40) | 2 weeks (N = 39) | 3 weeks (N = 38) | 4 weeks (N = 38) | 5 weeks (N = 38) | 6 weeks (N = 38) | |
| Available (patients), n | 43 | 41 | 40 | 40 | 39 | 38 | 39 | 43 | 40 | 38 | 37 | 37 | 36 | 36 |
| Did not visit oral hygienist in past week, n (%) | 38 (88.4) | 39 (95.1) | 37 (92.5) | 37 (92.5) | 32 (82.1) | 36 (94.7) | 35 (89.7) | 41 (95.3) | 40 (100) | 37 (97.4) | 34 (91.9) | 36 (97.3) | 34 (94.4) | 35 (97.2) |
| Visited an oral hygienist in past week, n (%) | 5 (11.6) | 2 (4.9) | 3 (7.5) | 3 (7.5) | 7 (17.9) | 2 (5.3) | 4 (10.3) | 2 (4.7) | 0 | 1 (2.6) | 3 (8.1) | 1 (2.7) | 2 (5.6) | 1 (2.8) |
| Number of visits, n | ||||||||||||||
| 1 | 4 | 2 | 2 | 3 | 6 | 2 | 3 | 2 | 0 | 1 | 2 | 1 | 2 | 1 |
| 2 | 1 | 1 | ||||||||||||
| 3 | 1 | 1 | ||||||||||||
| Missing number of visits | 1 | |||||||||||||
| Hospital admissions and outpatient visits | Treatment arm | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||||
| 2 weeks (N = 41) | 3 weeks (N = 41) | 4 weeks (N = 40) | 5 weeks (N = 40) | 6 weeks (N = 40) | 2 weeks (N = 39) | 3 weeks (N = 38) | 4 weeks (N = 38) | 5 weeks (N = 38) | 6 weeks (N = 38) | |
| Available (patients), n | 40 | 40 | 39 | 38 | 39 | 38 | 37 | 38 | 37 | 36 |
| Stayed in hospital overnight or longer as an inpatient, n/N (%) | 2/40 (5) | 1/40 (2.5) | 5/39 (12.8) | 6/38 (15.8) | 6/39 (15.4) | 3/38 (7.9) | 3/37 (8.1) | 6/38 (15.8) | 8/37 (21.6) | 5/36 (13.9) |
| Had outpatient appointments, n/N (%) | 16/39 (41) | 15/39 (38.5) | 14/39 (35.9) | 11/38 (28.9) | 14/37 (37.8) | 22/38 (57.9) | 18/37 (48.6) | 15/38 (39.5) | 18/36 (50) | 19/36 (52.8) |
| Number of outpatient appointments | ||||||||||
| Median (IQR) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 1 (0–1) | 0 (0–2) | 0 (0–2) | 0.5 (0–2) | 1 (0–2) |
| Range | 0–12 | 0–8 | 0–10 | 0–9 | 0–5 | 0–6 | 0–5 | 0–8 | 0–9 | 0–10 |
| Attended the head and neck ward (but not admitted overnight), n/N (%) | 0/40 (0) | 1/40 (2.5) | 1/39 (2.6) | 2/38 (5.3) | 0/37 (0) | 2/38 (5.3) | 0/37 (0) | 0/38 (0) | 2/36 (5.6) | 0/36 (0) |
| Number of times, median (range) | – | 0 (0–2) | 0 (0–1) | 0 (0–1) | 0 (0–0) | 0 (0–1) | – | – | 0 (0, 1) | – |
| Had other hospital visits, n | 2 | 0 | 3 | 4 | 1 | 2 | 1 | 0 | 1 | 0 |
| Number of other hospital visits, range | 2–5 | – | 1–1 | 1–3 | 1–1 | 2–5 | 2–2 | – | 1–1 | – |
| Alcohol use and smoking | Treatment arm | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||||
| 2 weeks (N = 41) | 3 weeks (N = 41) | 4 weeks (N = 40) | 5 weeks (N = 40) | 6 weeks (N = 40) | 2 weeks (N = 39) | 3 weeks (N = 38) | 4 weeks (N = 38) | 5 weeks (N = 38) | 6 weeks (N = 38) | |
| Available (patients), n | 40 | 40 | 39 | 38 | 38 | 38 | 37 | 38 | 36 | 34 |
| Alcohol use | ||||||||||
| Consumed alcohol in the past 7 days, n (%) | 6 (15) | 9 (22.5) | 5 (12.8) | 2 (5.3) | 3 (7.9) | 4 (10.5) | 3 (8.1) | 1 (2.6) | 2 (5.6) | 1 (2.9) |
| Type, n | ||||||||||
| Wine | 4 | 3 | 1 | 1 | 1 | 3 | 2 | |||
| Beer | 1 | 5 | 2 | 1 | 1 | 1 | 1 | 1 | ||
| Cider | 1 | 1 | ||||||||
| Spirits | 1 | 1 | ||||||||
| Other | 1 | 1 | 1 | 1 | ||||||
| Frequency, n | ||||||||||
| < 2 times per week | 4 | 4 | 3 | 2 | 2 | 4 | 1 | 2 | ||
| 2–3 times per week | 3 | 1 | 1 | 1 | ||||||
| > 3 times per week | 1 | |||||||||
| Daily or almost daily | 2 | 2 | 1 | 1 | 1 | |||||
| Smoking | ||||||||||
| Smoked in the last 7 days, n (%) | 7 (17.5) | 5 (12.5) | 6 (15.4) | 6 (15.8) | 3 (7.9) | 2 (5.3) | 2 (5.4) | 2 (5.3) | 0 (0) | 1 (2.9) |
| Type, n | ||||||||||
| Cigarettes | 3 | 2 | 4 | 3 | 2 | 1 | 1 | |||
| E-cigarettes | 1 | 1 | 1 | 1 | ||||||
| Roll-ups | 2 | 1 | 1 | 2 | ||||||
| Other | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Frequency, n | ||||||||||
| Less than twice per week | 2 | 1 | 2 | 1 | 1 | 1 | ||||
| Two or three times per week | 1 | 1 | ||||||||
| More than three times per week | 1 | 1 | 2 | |||||||
| Daily or almost daily | 5 | 3 | 3 | 2 | 2 | 2 | 1 | 1 | 1 | |
| AE | Treatment arm (n) | |
|---|---|---|
| LLLT | Sham | |
| Number of participants with an AE | 36 | 33 |
| Blood and lymphatic system disorders | 7 | 5 |
| Anaemia | 6 | 5 |
| Mild | 4 | 3 |
| Moderate | 2 | 2 |
| Neutropenic sepsis | 2 | 0 |
| Severe | 2 | 0 |
| Ear and labyrinth disorders | 6 | 9 |
| Deafness | 1 | 3 |
| Mild | 0 | 1 |
| Moderate | 1 | 1 |
| Severe | 0 | 1 |
| Ear pain | 1 | 1 |
| Moderate | 1 | 1 |
| Tinnitus | 5 | 6 |
| Mild | 4 | 5 |
| Moderate | 0 | 1 |
| Severe | 1 | 0 |
| Eye disorders | 0 | 1 |
| Epiphora | 0 | 1 |
| Mild | 0 | 1 |
| Gastrointestinal disorders | 33 | 31 |
| Ageusia | 1 | 1 |
| Mild | 1 | 0 |
| Moderate | 0 | 1 |
| Chapped lips | 1 | 1 |
| Moderate | 1 | 1 |
| Constipation | 15 | 14 |
| Mild | 4 | 9 |
| Moderate | 11 | 5 |
| Diarrhoea | 3 | 5 |
| Mild | 3 | 5 |
| Dry mouth | 15 | 16 |
| Mild | 2 | 5 |
| Moderate | 12 | 8 |
| Severe | 1 | 3 |
| Dysgeusia | 7 | 3 |
| Mild | 2 | 0 |
| Moderate | 5 | 3 |
| Dyspepsia | 3 | 3 |
| Mild | 2 | 0 |
| Moderate | 1 | 3 |
| Dysphagia | 10 | 9 |
| Mild | 1 | 1 |
| Moderate | 6 | 5 |
| Severe | 3 | 3 |
| Erythema | 1 | 0 |
| Moderate | 1 | 0 |
| Faeces discoloured | 1 | 1 |
| Moderate | 1 | 1 |
| Gastro-oesophageal reflux disease | 2 | 1 |
| Mild | 0 | 1 |
| Moderate | 2 | 0 |
| Gingival bleeding | 0 | 1 |
| Mild | 0 | 1 |
| Gingival pain | 0 | 1 |
| Mild | 0 | 1 |
| Glossodynia | 2 | 0 |
| Moderate | 2 | 0 |
| Lip pain | 1 | 0 |
| Mild | 1 | 0 |
| Lip swelling | 0 | 1 |
| Mild | 0 | 1 |
| Malaise | 0 | 1 |
| Moderate | 0 | 1 |
| Mouth haemorrhage | 1 | 1 |
| Moderate | 1 | 0 |
| Severe | 0 | 1 |
| Mouth ulceration | 7 | 12 |
| Mild | 3 | 2 |
| Moderate | 4 | 9 |
| Severe | 0 | 1 |
| Nausea | 12 | 20 |
| Mild | 4 | 2 |
| Moderate | 5 | 16 |
| Severe | 3 | 2 |
| Non-infective sialoadenitis | 0 | 1 |
| Moderate | 0 | 1 |
| Odynophagia | 2 | 0 |
| Mild | 2 | 0 |
| Oral pain | 6 | 9 |
| Mild | 2 | 1 |
| Moderate | 3 | 6 |
| Severe | 1 | 2 |
| Oropharyngitis | 2 | 0 |
| Moderate | 2 | 0 |
| Pain | 5 | 2 |
| Mild | 4 | 1 |
| Moderate | 0 | 1 |
| Severe | 1 | 0 |
| Pain in jaw | 2 | 0 |
| Mild | 1 | 0 |
| Moderate | 1 | 0 |
| Paraesthesia oral | 1 | 0 |
| Mild | 1 | 0 |
| Regurgitation | 0 | 1 |
| Mild | 0 | 1 |
| Saliva altered | 17 | 14 |
| Mild | 5 | 3 |
| Moderate | 12 | 10 |
| Severe | 0 | 1 |
| Salivary duct inflammation | 3 | 0 |
| Mild | 1 | 0 |
| Moderate | 1 | 0 |
| Severe | 1 | 0 |
| Salivary hypersecretion | 2 | 0 |
| Mild | 1 | 0 |
| Moderate | 1 | 0 |
| Stomatitis | 19 | 16 |
| Mild | 4 | 4 |
| Moderate | 9 | 10 |
| Severe | 6 | 2 |
| Taste disorder | 10 | 12 |
| Mild | 3 | 5 |
| Moderate | 7 | 7 |
| Thirst | 0 | 1 |
| Mild | 0 | 1 |
| Tongue coated | 3 | 4 |
| Mild | 3 | 4 |
| Vomiting | 8 | 9 |
| Mild | 3 | 3 |
| Moderate | 3 | 3 |
| Severe | 2 | 3 |
| General disorders and administration site conditions | 19 | 20 |
| Body temperature decreased | 0 | 1 |
| Mild | 0 | 1 |
| Fatigue | 18 | 16 |
| Mild | 5 | 6 |
| Moderate | 10 | 10 |
| Severe | 3 | 0 |
| Lethargy | 2 | 0 |
| Moderate | 2 | 0 |
| Pain | 2 | 1 |
| Mild | 1 | 0 |
| Moderate | 1 | 0 |
| Severe | 0 | 1 |
| Pyrexia | 0 | 3 |
| Mild | 0 | 1 |
| Moderate | 0 | 1 |
| Severe | 0 | 1 |
| Swelling | 1 | 0 |
| Severe | 1 | 0 |
| Immune system disorders | 0 | 1 |
| Anaphylaxis | 0 | 1 |
| Life-threatening consequences | 0 | 1 |
| Infections and infestations | 12 | 14 |
| Candida infection | 1 | 0 |
| Moderate | 1 | 0 |
| Ear infection (fungal) | 1 | 0 |
| Mild | 1 | 0 |
| Herpes virus infection | 1 | 0 |
| Moderate | 1 | 0 |
| Infection | 5 | 4 |
| Mild | 1 | 0 |
| Moderate | 3 | 3 |
| Severe | 1 | 0 |
| Life-threatening consequences | 0 | 1 |
| Oral candidiasis | 8 | 10 |
| Mild | 2 | 4 |
| Moderate | 6 | 6 |
| Oral herpes | 1 | 0 |
| Moderate | 1 | 0 |
| Pharyngitis | 0 | 1 |
| Moderate | 0 | 1 |
| Pneumonia | 0 | 1 |
| Severe | 0 | 1 |
| Septic shock | 0 | 1 |
| Life-threatening consequences | 0 | 1 |
| Stoma site infection | 0 | 1 |
| Moderate | 0 | 1 |
| Upper respiratory tract infection | 1 | 0 |
| Moderate | 1 | 0 |
| Urinary tract infection | 1 | 1 |
| Moderate | 1 | 1 |
| Injury, poisoning and procedural complications | 12 | 11 |
| Erythema | 2 | 2 |
| Mild | 2 | 1 |
| Moderate | 0 | 1 |
| Neck pain | 0 | 1 |
| Moderate | 0 | 1 |
| Radiation skin injury | 7 | 3 |
| Mild | 4 | 2 |
| Moderate | 3 | 1 |
| Rash | 1 | 1 |
| Mild | 1 | 0 |
| Moderate | 0 | 1 |
| Skin reaction | 3 | 4 |
| Mild | 1 | 2 |
| Moderate | 2 | 2 |
| Investigations | 7 | 6 |
| Alanine aminotransferase levels increased | 1 | 3 |
| Mild | 1 | 1 |
| Moderate | 0 | 1 |
| Missing severity | 0 | 1 |
| Blood creatinine levels increased | 0 | 2 |
| Mild | 0 | 2 |
| Lymphocyte count decreased | 3 | 4 |
| Mild | 0 | 2 |
| Moderate | 1 | 0 |
| Severe | 2 | 2 |
| Neutrophil count decreased | 2 | 1 |
| Moderate | 2 | 1 |
| Platelet count decreased | 1 | 0 |
| Mild | 1 | 0 |
| Weight decreased | 4 | 3 |
| Mild | 1 | 2 |
| Moderate | 1 | 0 |
| Severe | 2 | 1 |
| White blood cell count decreased | 2 | 1 |
| Mild | 1 | 0 |
| Moderate | 1 | 1 |
| Metabolism and nutrition disorders | 15 | 13 |
| Decreased appetite | 8 | 12 |
| Mild | 1 | 2 |
| Moderate | 3 | 5 |
| Severe | 4 | 5 |
| Dehydration | 4 | 2 |
| Mild | 1 | 1 |
| Moderate | 1 | 1 |
| Severe | 2 | 0 |
| Gastrointestinal tube insertion | 1 | 1 |
| Moderate | 1 | 1 |
| Hypoalbuminaemia | 3 | 1 |
| Mild | 2 | 1 |
| Moderate | 1 | 0 |
| Hypokalaemia | 1 | 1 |
| Mild | 1 | 0 |
| Severe | 0 | 1 |
| Hyponatraemia | 0 | 1 |
| Life-threatening consequences | 0 | 1 |
| Hypophosphataemia | 2 | 1 |
| Mild | 1 | 0 |
| Moderate | 1 | 0 |
| Severe | 0 | 1 |
| Musculoskeletal and connective tissue disorders | 2 | 1 |
| Muscle spasms | 1 | 0 |
| Moderate | 1 | 0 |
| Pain in jaw | 0 | 1 |
| Mild | 0 | 1 |
| Trismus | 1 | 0 |
| Moderate | 1 | 0 |
| Nervous system disorders | 4 | 3 |
| Dizziness | 1 | 1 |
| Mild | 0 | 1 |
| Severe | 1 | 0 |
| Headache | 2 | 1 |
| Mild | 1 | 0 |
| Moderate | 0 | 1 |
| Severe | 1 | 0 |
| Neuropathy peripheral | 1 | 0 |
| Moderate | 1 | 0 |
| Presyncope | 1 | 0 |
| Severe | 1 | 0 |
| Syncope | 1 | 2 |
| Mild | 0 | 2 |
| Moderate | 1 | 0 |
| Tremor | 0 | 1 |
| Mild | 0 | 1 |
| Psychiatric disorders | 7 | 2 |
| Anxiety | 1 | 1 |
| Mild | 1 | 0 |
| Moderate | 0 | 1 |
| Depressed mood | 4 | 1 |
| Mild | 3 | 1 |
| Moderate | 1 | 0 |
| Depression | 1 | 0 |
| Moderate | 1 | 0 |
| Insomnia | 1 | 0 |
| Mild | 1 | 0 |
| Renal and urinary disorders | 0 | 2 |
| Acute kidney injury | 0 | 1 |
| Severe | 0 | 1 |
| Urinary retention | 0 | 1 |
| Moderate | 0 | 1 |
| Respiratory, thoracic and mediastinal disorders | 18 | 16 |
| Cough | 2 | 1 |
| Mild | 1 | 1 |
| Moderate | 1 | 0 |
| Dysphonia | 8 | 3 |
| Mild | 7 | 3 |
| Moderate | 1 | 0 |
| Dyspnoea | 1 | 0 |
| Severe | 1 | 0 |
| Epistaxis | 0 | 1 |
| Mild | 0 | 1 |
| Haemoptysis | 1 | 0 |
| Moderate | 1 | 0 |
| Hiccups | 1 | 0 |
| Mild | 1 | 0 |
| Laryngitis | 0 | 1 |
| Mild | 0 | 1 |
| Oropharyngeal pain | 9 | 9 |
| Mild | 3 | 3 |
| Moderate | 6 | 5 |
| Severe | 0 | 1 |
| Pharyngeal inflammation | 4 | 1 |
| Mild | 1 | 0 |
| Moderate | 3 | 1 |
| Pharyngitis | 1 | 0 |
| Moderate | 1 | 0 |
| Pneumonia | 0 | 1 |
| Life-threatening consequences | 0 | 1 |
| Productive cough | 0 | 1 |
| Mild | 0 | 1 |
| Stridor | 1 | 0 |
| Life-threatening consequences | 1 | 0 |
| Skin and subcutaneous tissue disorders | 6 | 9 |
| Alopecia | 1 | 2 |
| Mild | 1 | 2 |
| Dry skin | 2 | 3 |
| Moderate | 2 | 3 |
| Lymphoedema | 0 | 1 |
| Mild | 0 | 1 |
| Pain | 1 | 1 |
| Mild | 1 | 1 |
| Pruritus | 3 | 1 |
| Mild | 1 | 0 |
| Moderate | 2 | 1 |
| Rash | 1 | 4 |
| Mild | 1 | 2 |
| Moderate | 0 | 2 |
| Surgical and medical procedures | 3 | 1 |
| Fistula repair | 1 | 0 |
| Severe | 1 | 0 |
| Neck dissection | 2 | 0 |
| Severe | 2 | 0 |
| Tonsillectomy | 0 | 1 |
| Severe | 0 | 1 |
| Vascular disorders | 3 | 3 |
| Deep-vein thrombosis | 1 | 1 |
| Moderate | 0 | 1 |
| Severe | 1 | 0 |
| Hot flush | 2 | 0 |
| Mild | 2 | 0 |
| Hypertension | 0 | 1 |
| Mild | 0 | 1 |
| Pulmonary embolism | 0 | 1 |
| Severe | 0 | 1 |
| System organ class not specified | 1 | 0 |
| Elective admission for pharyngoscopy and biopsies | 1 | 0 |
| Mild | 1 | 0 |
| Non-SAEs | Treatment arm (n) | |
|---|---|---|
| LLLT | Sham | |
| Number of participants with an AE | 10 | 9 |
| Ear and labyrinth disorders | 0 | 2 |
| Deafness | 0 | 1 |
| Severe | 0 | 1 |
| Tinnitus | 0 | 1 |
| Mild | 0 | 1 |
| Gastrointestinal disorders | 9 | 9 |
| Constipation | 2 | 3 |
| Mild | 1 | 3 |
| Moderate | 1 | 0 |
| Dry mouth | 3 | 3 |
| Mild | 1 | 0 |
| Moderate | 2 | 2 |
| Severe | 0 | 1 |
| Dyspepsia | 0 | 2 |
| Moderate | 0 | 2 |
| Dysphagia | 0 | 1 |
| Moderate | 0 | 1 |
| Erythema | 1 | 0 |
| Moderate | 1 | 0 |
| Mouth ulceration | 0 | 2 |
| Mild | 0 | 1 |
| Moderate | 0 | 0 |
| Severe | 0 | 1 |
| Nausea | 0 | 2 |
| Moderate | 0 | 2 |
| Odynophagia | 1 | 0 |
| Mild | 1 | 0 |
| Oral pain | 1 | 1 |
| Mild | 1 | 0 |
| Moderate | 0 | 1 |
| Oropharyngitis | 1 | 0 |
| Moderate | 1 | 0 |
| Pain | 2 | 1 |
| Mild | 2 | 0 |
| Moderate | 0 | 1 |
| Paraesthesia oral | 1 | 0 |
| Mild | 1 | 0 |
| Saliva altered | 1 | 1 |
| Moderate | 1 | 0 |
| Severe | 0 | 1 |
| Salivary hypersecretion | 1 | 0 |
| Moderate | 1 | 0 |
| Stomatitis | 2 | 1 |
| Mild | 1 | 0 |
| Moderate | 1 | 0 |
| Severe | 0 | 1 |
| Taste disorder | 0 | 1 |
| Moderate | 0 | 1 |
| Tongue coated | 1 | 0 |
| Mild | 1 | 0 |
| General disorders and administration site conditions | 2 | 2 |
| Fatigue | 2 | 2 |
| Moderate | 2 | 2 |
| Pain | 1 | 0 |
| Moderate | 1 | 0 |
| Infections and infestations | 3 | 4 |
| Infection | 0 | 1 |
| Moderate | 0 | 1 |
| Oral candidiasis | 3 | 2 |
| Mild | 0 | 2 |
| Moderate | 3 | 0 |
| Pharyngitis | 0 | 1 |
| Moderate | 0 | 1 |
| Injury, poisoning and procedural complications | 0 | 2 |
| Neck pain | 0 | 1 |
| Moderate | 0 | 1 |
| Rash | 0 | 1 |
| Moderate | 0 | 1 |
| Musculoskeletal and connective tissue disorders | 0 | 1 |
| Pain in jaw | 0 | 1 |
| Mild | 0 | 1 |
| Psychiatric disorders | 1 | 1 |
| Anxiety | 0 | 1 |
| Moderate | 0 | 1 |
| Insomnia | 1 | 0 |
| Mild | 1 | 0 |
| Respiratory, thoracic and mediastinal disorders | 2 | 2 |
| Dysphonia | 0 | 1 |
| Mild | 0 | 1 |
| Haemoptysis | 1 | 0 |
| Moderate | 1 | 0 |
| Oropharyngeal pain | 1 | 1 |
| Mild | 0 | 1 |
| Moderate | 1 | 0 |
| Skin and subcutaneous tissue disorders | 0 | 2 |
| Rash | 0 | 2 |
| Mild | 0 | 1 |
| Moderate | 0 | 1 |
| SAE ID | Description | Severity | Treatment arm |
|---|---|---|---|
| SAE0001 | Vomiting | Moderate | LLLT |
| SAE0002 | Nausea, dizziness, fatigue, breathlessness, episode of pre-syncope, anorexia | Severe | LLLT |
| SAE0003 | Severe mucositis/odynophagia | Severe | LLLT |
| SAE0004 | General feeling unwell (suspected cisplatin reaction) nausea and dysphagia, oral mucositis, vomiting, lower respiratory tract infection | Severe | LLLT |
| SAE0005 | Septic shock: possibly due to Hickman line infection | Life-threatening | Sham |
| SAE0006 | Nausea and vomiting, dehydration, pain and oral mucositis | Severe | LLLT |
| SAE0007 | Deep-vein thrombosis (right leg) | Severe | LLLT |
| SAE0008 | Hyponatraemia | Life-threatening | Sham |
| SAE0009 | Hyponatraemia | Life-threatening | Sham |
| SAE0010 | Urinary retention | Moderate | Sham |
| SAE0011 | Left neck swelling | Severe | LLLT |
| SAE0012 | Vasovagal episodes (two: second due to dehydration) | Moderate | LLLT |
| SAE0013 | Sepsis of unknown origin | Life-threatening | Sham |
| SAE0014 | Pulmonary embolism | Severe | Sham |
| SAE0015 | Tonsillectomy (bilateral) | Severe | Sham |
| SAE0016 | Anorexia, nausea | Severe | Sham |
| SAE0017 | Dehydration secondary to nausea and vomiting | Moderate | Sham |
| SAE0018 | Closure and excision of otocutaneous fistula | Severe | LLLT |
| SAE0019 | Surgical and medical procedures: contralateral neck dissection | Severe | LLLT |
| SAE0020 | Neutropenic sepsis | Severe | LLLT |
| SAE0021 | Hospitalisation due to weight loss and need for nasogastric placement | Moderate | LLLT |
| SAE0022 | Hospital-acquired pneumonia | Severe | Sham |
| SAE0023 | Elective admission for pharyngoscopy and biopsies: no confirmed residual malignancy | Mild | LLLT |
| SAE0024 | Stridor with increasing respiratory effort | Life-threatening | LLLT |
| SAE0025 | Pain and vomiting | Severe | LLLT |
| SAE0026 | Surgical and medical procedures: neck dissection | Severe | LLLT |
| SAE0027 | Hospitalisation due to bleeding | Severe | Sham |
| SAE0028 | Total glossolaryngectomy with neck dissection (bilateral) | Severe | LLLT |
Appendix 3 Health economics supplementary information
| Item | Unit | Cost (£) | Reference | Code/page | Notes |
|---|---|---|---|---|---|
| A&E visit | Per visit | 142 | NHS Reference Costs 2017/1898 | Code T01NA – T04NA | Weighted average of all A&E visits (non-stay) |
| Outpatient appointment | Per visit | 136 | Unit Costs of Health and Social Care 2019 52 | Page 82 | Weighted average of all hospital outpatients attendances |
| Hospital overnight stay (non-A&E admission) | Per night | 346 | NHS Reference Costs 2017/1898 | Code AA35 A – AA35F (Elective: excess bed-days) | Elective: Excess bed-days (EL_XS) |
| Head and neck ward | Per visit | 166 | NHS Reference Costs 2017/1898 | Service Code 370 | Average of consultant-led and non-consultant-led medical oncology outpatient attendances |
| Item | Unit | Cost (£) | Reference | Page | Notes |
|---|---|---|---|---|---|
| GP practice consultation | Per appointment (9.22 minutes) | 40 |
Unit Costs of Health and Social Care 2019 52 Including direct care staff costs (with qualification costs) |
120 | – |
| Nurse practice consultation | Per appointment (15.5 minutes) | 11 | Unit Costs of Health and Social Care 201952 used for hourly rate (with qualifications). Unit Costs of Health and Social Care 201599 used for average direct patient contact | 118 (2020); 176 (2015) | – |
| Consultant practice consultation | Per appointment (9.22 minutes) | 40 |
Unit Costs of Health and Social Care 2019 52 Including direct care staff costs (with qualification costs) |
120 | Assumed same as GP practice consultation |
| Surgeon practice consultation | Per appointment (9.22 minutes) | 40 |
Unit Costs of Health and Social Care 2019 52 Including direct care staff costs (with qualification costs) |
120 | Assumed same as GP practice consultation |
| Carpal tunnel syndrome steroid injection | Per appointment | 213 | Chesterton et al.100 | Appendices page 5 | – |
| SLT appointment at general practice surgery | Per appointment (1 hour) | 35 | Unit Costs of Health and Social Care 2019 101 | 153 | Based on a band 5 scientific and professional salary cost per working hour |
| Dietitian practice consultation | Per appointment (1 hour) | 35 | Unit Costs of Health and Social Care 2019 101 | 153 | Based on a band 5 scientific and professional salary cost per working hour |
| Phlebotomist | Per appointment | 3 | Unit Costs of Health and Social Care 2017 102 | 18 | – |
| Item | Unit | Cost (£) | Reference | Code page | Notes |
|---|---|---|---|---|---|
| GP home consultation | Per appointment (23.4 minutes) | 100 | Unit Costs of Health and Social Care 2017102 for GP visit time, unit cost and reimbursement of travel costs. Unit Costs of Health and Social Care 201599 for average travel time and reimbursement of travel costs | 164 (2017); 176 (2015) | – |
| GP home visit travel time | Per visit (6 miles) | 4 | Unit Costs of Health and Social Care 2015 99 | 176 | – |
| Nurse home consultation | Per visit (25 minutes) | 19 | Unit Costs of Health and Social Care 2017102 used for hourly rate (with qualifications). Unit Costs of Health and Social Care 2013103 used for average direct patient contact and time for specialist nurse home visit | 160 (2017); 189 (2013) | – |
| Nurse home visit travel time | Per visit (6 miles) | 4 | Unit Costs of Health and Social Care 201599 Assumed same travel costs as for GP visits. Travel costs are reimbursed at 56p per mile as per assuming a 30mph speed limit, 6 miles will be travelled in 12 minutes totalling £3.36 per visit on average | 176 | – |
| Dietitian home consultation | Per appointment (1 hour) | 35 | Unit Costs of Health and Social Care 2018 101 | 153 | Assumed same as speech therapist (salaries seem comparable) |
| Dietitian home visit travel time | Per visit (6 miles) | 4 | Unit Costs of Health and Social Care 2015.99 Assumed same travel costs as for GP visits | 176 | – |
| Removal of percutaneous endoscopic gastrostomy tube | 1 hour (estimate) | 44 | Unit Costs of Health and Social Care 2017102 used for hourly rate (with qualifications), £42.00 per hour costs | Assumed that this was carried out by nurse | |
| Removal of percutaneous endoscopic gastrostomy tube travel time | Per visit (6 miles) | 4 | Unit Costs of Health and Social Care 2015.99 Assumed same travel costs as for GP visits | 176 | – |
| SLT home consultation | Per appointment (1 hour) | 38 | Unit Costs of Health and Social Care 2014 104 | 181 | – |
| SLT home visit travel time | 6 miles | 4 | Unit Costs of Health and Social Care 201599 Assumed same travel costs as for GP visits | 176 | – |
| Item | Unit | Cost (£) | Reference | Code page | Notes |
|---|---|---|---|---|---|
| GP telephone consultation | Per call (4 minutes) | 15 | Unit Costs of Health and Social Care 2017 102 | 164 | – |
| Nurse telephone consultation | Per call (6.56 minutes) | 8 | Unit Costs of Health and Social Care 2017.102 Cost per intervention (including other costs) | 164 | – |
| Diet nurse telephone consultation | Per call (6.56 minutes) | 8 | Unit Costs of Health and Social Care 2017.102 Cost per intervention (including other costs) | 164 | Assumed same as nurse (salaries comparable) |
| Dietitian telephone consultation | Per call (6.56 minutes) | 8 | Unit Costs of Health and Social Care 2017.102 Cost per intervention (including other costs) | 164 | Assumed same as nurse (salaries comparable) |
| Item | Unit | Cost (£) | Reference | Code page | Notes |
|---|---|---|---|---|---|
| GP out-of-hours consultation | Per consultation | 114 | Unit Costs of Health and Social Care 2018 101 | 18 | – |
| Nurse out-of-hours consultation | Per consultation | 69 | Unit Costs of Health and Social Care 2018 101 | 18 | – |
| Hospital doctor out-of-hours consultation | Per consultation | 114 | Unit Costs of Health and Social Care 2018 101 | 18 | Assumed same as out-of-hours GP consultation |
| Registrar out-of-hours consultation | Per consultation | 114 | Unit Costs of Health and Social Care 2018 101 | 18 | Assumed same as out-of-hours GP consultation |
| Hygienist out-of-hours consultation | Per consultation | 69 | Unit Costs of Health and Social Care 2018 101 | 18 | Assumed same as out-of-hours nurse consultation |
| Dentist out-of-hours consultation | Per consultation | 114 | Unit Costs of Health and Social Care 2018 101 | 18 | Assumed same as out-of-hours GP consultation |
| Dietitian out-of-hours consultation | Per consultation | 69 | Unit Costs of Health and Social Care 2018 101 | 18 | Assumed same as out-of-hours nurse consultation |
| SLT out-of-hours consultation | Per consultation | 69 | Unit Costs of Health and Social Care 2018 101 | 18 | Assumed same as out-of-hours nurse consultation |
| Time point | Treatment arm (n) | |||
|---|---|---|---|---|
| LLLT (N = 40) | Sham (N = 40) | |||
| Missing | Complete | Missing | Complete | |
| 2 weeks | 0 | 40 | 1 | 39 |
| 3 weeks | 0 | 40 | 1 | 39 |
| 4 weeks | 0 | 40 | 0 | 40 |
| 5 weeks | 0 | 40 | 0 | 40 |
| 6 weeks | 0 | 40 | 0 | 40 |
| Time point | Treatment arm (n/N) | |||||
|---|---|---|---|---|---|---|
| LLLT | Sham | |||||
| Did not attend visit | Missing | Complete | Did not attend visit | Missing | Complete | |
| 4 months | 3/39 | 4/39 | 32/39 | 3/38 | 1/38 | 35/38 |
| 14 months | 1/11 | 0/11 | 10/11 | 0/16 | 1/16 | 15/16 |
| Time point | Treatment arm (n) | |||||
|---|---|---|---|---|---|---|
| LLLT (N = 11) | Sham (N = 16) | |||||
| Did not attend visit | Missing | Complete | Did not attend visit | Missing | Complete | |
| 14 months | 1 | 0 | 10 | 0 | 1 | 15 |
| Time point | Treatment arm (n) | |||
|---|---|---|---|---|
| LLLT (N = 39) | Sham (N = 38) | |||
| Missing | Complete | Missing | Complete | |
| 4 months | 13 | 26 | 10 | 28 |
| Time point | Treatment arm (n/N) | |||||||
|---|---|---|---|---|---|---|---|---|
| LLLT | Sham | |||||||
| Did not attend visit | Missing | Partial | Complete | Did not attend visit | Missing | Partial | Complete | |
| Baseline | 0/44 | 1/44 | 1/44 | 42/44 | 0/43 | 0/43 | 1/43 | 42/43 |
| 6 weeks | 3/40 | 1/40 | 0/40 | 36/40 | 4/38 | 0/38 | 0/38 | 34/38 |
| 4 months | 3/39 | 2/39 | 1/39 | 33/39 | 3/38 | 0/38 | 0/38 | 35/38 |
| 14 months | 1/22 | 0/11 | 0/11 | 10/11 | 0/16 | 0/16 | 0/16 | 16/16 |
| Time point | Treatment arm (n/N) | |||||
|---|---|---|---|---|---|---|
| LLLT | Sham | |||||
| Did not attend visit | Missing | Complete | Did not attend visit | Missing | Complete | |
| Baseline | 0/44 | 4/44 | 40/44 | 0/44 | 3/43 | 40/43 |
| 6 weeks | 3/40 | 3/40 | 3/40 | 4/38 | 0/38 | 34/38 |
| 4 months | 3/39 | 3/39 | 33/39 | 3/38 | 2/38 | 33/38 |
| 14 months | 1/11 | 1/11 | 9/11 | 0/16 | 1/16 | 15/16 |
| Year | Discount factor at 3.5%a | Equivalent annual cost (£) of £6420b |
|---|---|---|
| 1 | 0.9662 | 6644.70 |
| 2 | 0.9335 | 3379.49 |
| 3 | 0.9019 | 2291.52 |
| 4 | 0.8714 | 1747.85 |
| 5 | 0.8420 | 1421.91 |
| Item | Unit | Cost (£) | Reference | Code/page | Notes |
|---|---|---|---|---|---|
| Administrator: band 3 midpoint | 30 minutes | 5.41 | NHS Employers AFC Pay Scales 49 | – | Hourly rate = £10.84 |
| Radiographer: band 6 midpoint | 1 hour | 50.26 | Unit Costs of Health and Social Care 2018 101 | – | – |
| Resource use (capital) | Cost (£) |
|---|---|
| Opportunity cost of the capital (1421.91 × 5) | 7109.55 |
| Annual cost of the laser | 1421.91 |
| Cost of laser per week (assume 52 weeks) | 27.34 |
| Cost of laser per session (assume 30 sessions per week) | 0.91 |
| Annual maintenance costs | 400 |
| Maintenance costs per week (assume 52 weeks) | 7.69 |
| Maintenance costs per session (assume 30 sessions per week) | 0.26 |
| Annual training costs | 1200 |
| Training costs per week (assume 52 weeks) | 23.08 |
| Training costs per session (assume 30 sessions per week) | 0.77 |
| Administrator staff costs (assume band 3 midpoint, 30 minutes) | 5.41 |
| Radiographera (assume band 6 midpoint, 60 minutes) | 50.26 |
| Total cost per session (laser cost + maintenance costs + training costs + staff costs) | 57.61 |
| Total cost per patient per week (total cost per session × 3) | 172.83 |
| Total intervention cost per patient (total cost per patient per week × 6) | 1036.98 |
| Time point | Treatment arm (n/N) | |||||
|---|---|---|---|---|---|---|
| LLLT | Sham | |||||
| Yes | No | Missing | Yes | No | Missing | |
| 4 months | 0/36 | 35/36 | 1/36 | 4/35 | 31/35 | 0/35 |
| 14 months | 0/10 | 10/10 | 0/10 | 0/16 | 16/16 | 0/16 |
| Time point | Treatment arm (n/N) | |||||
|---|---|---|---|---|---|---|
| LLLT | Sham | |||||
| Yes | No | Missing | Yes | No | Missing | |
| 4 months | 1/36 | 34/36 | 1/36 | 0/35 | 35/35 | 0/35 |
| 14 months | 0/10 | 9/10 | 1/10 | 3/16 | 12/16 | 1/16 |
| Days lost from work | Time point | |||
|---|---|---|---|---|
| 4 months | 14 months | |||
| LLLT arm (N = 30) | Sham arm (N = 30) | LLLT arm (N = 8) | Sharm arm (N = 13) | |
| Mean | 3.1 | 3.0 | 2.6 | 2.5 |
| IQR | 2.0–3.9 | 2.2–3.8 | 1.0–4.3 | 1.4–3.5 |
| Time and travel information | Values |
|---|---|
| Distance travelled to hospital (one way) (miles), mean (95% CI) | 18 (3 to 35) |
| Time taken (one way) (minutes), mean (95% CI) | 37 (10 to 60) |
| Parking costs (per hospital admission) (GBP), mean (95% CI) | 3 (0 to 7) |
| Accompanied by relative/carer, n (%) | 11 (69) |
| Time and travel information | Values |
|---|---|
| Distance travelled to hospital (one way) (miles), mean (95% CI) | 16 (3 to 50) |
| Time taken (one way) (minutes), mean (95% CI) | 36 (15 to 60) |
| Parking costs (per hospital admission) (GBP), mean (95% CI) | 3 (0 to 6) |
| Accompanied by relative/carer, n (%) | 4 (36) |
| Time and travel information | Values |
|---|---|
| Distance travelled to hospital (one way) (miles), mean (95% CI) | 8 (0 to 50) |
| Time taken (one way) (minutes), mean (95% CI) | 20 (5 to 60) |
| Parking costs (per GP or practice nurse visit) (GBP), mean (95% CI) | 1 (0 to 3) |
| Accompanied by relative/carer, n (%) | 5 (29) |
| Domain | Treatment arm (n) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LLLT (N = 44) | Sham (N = 43) | |||||||||||||
| Not included | Missing | No | Slight | Moderate | Severe | Unable to do | Not included | Missing | No | Slight | Moderate | Severe | Unable to do | |
| Mobility | 0 | 1 | 33 | 4 | 5 | 1 | 0 | 0 | 0 | 34 | 5 | 3 | 1 | 0 |
| Self-care | 0 | 1 | 38 | 3 | 1 | 1 | 0 | 0 | 0 | 37 | 4 | 2 | 0 | 0 |
| Usual activities | 0 | 1 | 24 | 9 | 5 | 2 | 3 | 0 | 0 | 23 | 8 | 10 | 1 | 1 |
| Pain and discomfort | 0 | 1 | 7 | 23 | 12 | 1 | 0 | 0 | 0 | 18 | 14 | 8 | 2 | 1 |
| Anxiety and depression | 0 | 2 | 20 | 13 | 8 | 1 | 0 | 0 | 1 | 20 | 17 | 5 | 0 | 0 |
| Domain | Treatment arm (n) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LLLT (N = 40) | Sham (N = 38) | |||||||||||||
| Not included | Missing | No | Slight | Moderate | Severe | Unable to do | Not included | Missing | No | Slight | Moderate | Severe | Unable to do | |
| Mobility | 3 | 1 | 23 | 8 | 4 | 1 | 0 | 4 | 0 | 26 | 6 | 2 | 0 | 0 |
| Self-care | 3 | 1 | 28 | 4 | 3 | 1 | 0 | 4 | 0 | 31 | 3 | 0 | 0 | 0 |
| Usual activities | 3 | 1 | 7 | 9 | 7 | 6 | 7 | 4 | 0 | 12 | 11 | 4 | 1 | 6 |
| Pain and discomfort | 3 | 1 | 2 | 9 | 16 | 8 | 1 | 4 | 0 | 2 | 10 | 16 | 4 | 2 |
| Anxiety and depression | 3 | 1 | 15 | 12 | 9 | 0 | 0 | 4 | 0 | 16 | 15 | 3 | 0 | 0 |
| Domain | Treatment arm (n) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LLLT (N = 39) | Sham (N = 38) | |||||||||||||
| Not included | Missing | No | Slight | Moderate | Severe | Unable to do | Not included | Missing | No | Slight | Moderate | Severe | Unable to do | |
| Mobility | 3 | 2 | 20 | 7 | 5 | 2 | 0 | 3 | 0 | 25 | 5 | 3 | 2 | 0 |
| Self-care | 3 | 2 | 28 | 4 | 1 | 1 | 0 | 3 | 0 | 32 | 2 | 1 | 0 | 0 |
| Usual activities | 3 | 2 | 12 | 13 | 7 | 2 | 0 | 3 | 0 | 18 | 6 | 5 | 3 | 3 |
| Pain and discomfort | 3 | 2 | 8 | 15 | 10 | 1 | 0 | 3 | 0 | 12 | 15 | 7 | 1 | 0 |
| Anxiety and depression | 3 | 3 | 19 | 10 | 3 | 1 | 0 | 3 | 0 | 20 | 7 | 8 | 0 | 0 |
| Domain | Treatment arm (n) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LLLT (N = 11) | Sham (N = 16) | |||||||||||||
| Not included | Missing | No | Slight | Moderate | Severe | Unable to do | Not included | Missing | No | Slight | Moderate | Severe | Unable to do | |
| Mobility | 1 | 0 | 8 | 1 | 1 | 0 | 0 | 0 | 0 | 12 | 4 | 0 | 0 | 0 |
| Self-care | 1 | 0 | 8 | 2 | 0 | 0 | 0 | 0 | 0 | 16 | 0 | 0 | 0 | 0 |
| Usual activities | 1 | 0 | 5 | 4 | 1 | 0 | 0 | 0 | 0 | 13 | 2 | 1 | 0 | 0 |
| Pain and discomfort | 1 | 0 | 4 | 6 | 0 | 0 | 0 | 0 | 0 | 8 | 7 | 1 | 0 | 0 |
| Anxiety and depression | 1 | 0 | 6 | 4 | 0 | 0 | 0 | 0 | 0 | 11 | 4 | 1 | 0 | 0 |
List of abbreviations
- A&E
- accident and emergency
- AE
- adverse event
- AHP
- allied health professional
- BMI
- body mass index
- (C)RT
- (chemo)radiotherapy
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRT
- chemoradiotherapy
- DMC
- Data Monitoring Committee
- eCRF
- electronic case report form
- EORTC
- European Organisation for Research and Treatment of Cancer
- EORTC QLQ-C30
- European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire 30
- EORTC QLQ-H&N35
- European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Module for Head and Neck Cancer
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- EQ-5D-VAS
- EuroQol-5 Dimensions visual analogue scale
- ETC
- excess treatment cost
- GCP
- good clinical practice
- HEAP
- health economics analysis plan
- HNC
- head and neck cancer
- HPV
- human papillomavirus
- HRA
- health research authority
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- IMRT
- intensity-modulated radiation therapy
- IQR
- interquartile range
- ITT
- intention to treat
- LED
- light-emitting diode
- LLLT
- low-level laser therapy
- LPA
- laser protection advisor
- LPS
- laser protection supervisor
- MASCC
- Multinational Association of Supportive Care in Cancer
- MCID
- minimal clinically important difference
- MDADI
- MD Anderson Dysphagia Inventory
- MDT
- multidisciplinary team
- MedDRA
- Medical Dictionary for Regulatory Activities
- mITT
- modified intention to treat
- NASSS
- Nonadoption, Abandonment, Scale-up, Spread, and Sustainability
- NASSS-CAT
- Nonadoption, Abandonment, Scale-up, Spread, and Sustainability complexity assessment tool
- NCTU
- Newcastle Clinical Trials Unit
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- NPT
- normalisation process theory
- OM
- oral mucositis
- OMWQ-HN
- Oral Mucositis Weekly Questionnaire-Head and Neck Cancer
- PIS
- participant information sheet
- PPI
- patient and public involvement
- PROM
- patient-reported outcome measure
- PSS-HN
- Performance Status Scale for Head and Neck Cancer Patients
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QLQ
- Quality of Life Questionnaire
- QoL
- quality of life
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SAR
- serious adverse reaction
- SD
- standard deviation
- SLT
- speech and language therapist
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- WHO
- World Health Organization
- WST
- water swallow test
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/UWNB3375).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
