Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 03/13/06. The contractual start date was in March 2005. The draft report began editorial review in May 2009 and was accepted for publication in December 2009. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
None
Permissions
Copyright statement
© 2010 Queen’s Printer and Controller of HMSO. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2010 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Chronic obstructive pulmonary disease (COPD) is a slowly progressive, not fully reversible constriction of the airways causing breathlessness, cough and respiratory distress. The primary cause is repeated exposure to cigarette smoke, which inflames the lungs and reduces lung tissue elasticity. The prevalence of COPD is estimated at between 2% and 4%, representing approximately 1–2 million people in England. 1
COPD is a condition for which economic evaluation of therapeutic interventions is particularly relevant. The high prevalence, chronic nature of the disease, and range of therapeutic interventions make the management of COPD a considerable financial burden to health-care services. The National Institute for Health and Clinical Excellence (NICE) estimates that the direct cost of COPD to the UK NHS exceeds £491M per year (expenditure based on 2001–2 activity). 2 More than half of this cost relates to the provision of care in hospital, with more than 1 million inpatient ‘bed days’ per year attributable to the disease. 3 One in eight unplanned hospital stays concern COPD, making it the second largest cause of emergency admissions in the UK. 4
Scientific background
Key features of COPD are chronic cough and excessive sputum production. These symptoms occur as a result of mucus hypersecretion and ciliary dysfunction. Manual chest physiotherapy (MCP) involves external manipulation of the thorax using the techniques of percussion and vibration. The purpose of percussion (also referred to as cupping, clapping and tapotement) is to intermittently apply kinetic energy to the chest wall and lung. This is accomplished by using a cupped hand with rhythmical flexion and extension action of the wrist. Vibration involves the application of a tremorous action over the area being drained. This is performed by manually pressing with both hands in the direction of the normal movement of the ribs during expiration. Percussion and vibration are designed to dislodge bronchial secretions which the patient then clears through involuntary or assisted coughing. The assumption underlying the use of MCP is that removing sputum from the airway improves ventilation perfusion ratios (V/Qs), prevents further mucosal injury and thereby improves lung function.
In 2001, this study’s Chief Investigator (J Cross) led a comprehensive review of the literature regarding manual physiotherapy techniques. 5 The project was commissioned by the Association of Chartered Physiotherapists in Respiratory Care and its remit was to identify and critically review the literature on MCP in relation to mobilisation and clearance of secretions. The review focused on patients with compromised respiratory function and impaired mucociliary clearance who were not being mechanically ventilated. The intention of the review was to identify studies of acceptable quality designed to evaluate the use and mode of manual techniques with a view to compiling clear and concise clinical practice guidelines. This proved impossible to achieve owing to the lack of suitable evidence. However, certain key points emerged from this literature review and these are reported below.
Eight papers reported designs that evaluate a specific manual technique, using secretion clearance as the main outcome. 6–13 In these studies, comparisons were made against either a ‘control’ or ‘standard’ treatment, augmented by the addition of the manual technique in the experimental group. Four studies found no evidence that manual techniques conferred greater efficacy. 7,9,12,13 The remaining found that manual percussion was associated with sputum mobilisation,7 vibrations and percussion were associated with an increased wet weight of sputum,9 there was a significant increase in sputum clearance at 60 minutes post treatment with mechanical vibration but no difference over 24 hours,12 and fast manual percussion produced the greatest sputum volume 60 minutes after treatment. 13 Chest physiotherapy appeared to be inappropriate in acutely ill patients with little or no sputum. 14 On occasion it was associated with oxygen desaturation, V/Q mismatch, a decrease in forced expiratory volume in 1 second (FEV1) and bronchospasm. 5
De Boeck and Zinman15 performed a crossover trial of stable COPD patients receiving twice daily physiotherapy at home with randomisation of treatment order. Chest physiotherapy, including manual techniques, was compared with vigorous coughing. The results of this study showed no clear benefit of chest physiotherapy over cough alone. However, the small sample size means that, even with paired data analysis, only very large effect sizes are likely to be identified. In many studies, the effect of manual techniques independent of encouragement to cough was not separately determined. Rossman et al. 11 reported that cough alone appeared as effective as manual techniques
Lung volume measures
Manual techniques have sometimes been reported as producing falls in lung volumes. Campbell et al. 16 compared two groups of patients with chronic bronchitis and applied chest percussion in a postural drainage position. They reported an immediate reduction in FEV1 associated with the procedure, this effect being lessened by the administration of a bronchodilator. The reduction in FEV1 was negated within 20 minutes. It was concluded that this fall was due to bronchoconstriction brought about by the physiotherapy techniques of percussion and vibration. However, neither sputum volume at baseline was reported nor whether participants had been tested for airway reversibility prior to the study.
Newton and Stephenson17 considered the effect of chest physiotherapy (breathing exercises, chest vibration and percussion in different positions or postural drainage) on pulmonary function and, in a small number of subjects, arterial blood gases (ABGs). They found no change in FEV1, vital capacity, specific conductance or ABGs. However, functional residual capacity (FRC) and airway conductance and resistance were all seen to increase after these manoeuvres. While this study does support the view that MCP may not be appropriate in small sputum producers, the precise physiotherapy techniques used are inadequately described. May and Munt18 suggest that forced vital capacity (FVC) increases with both chest physiotherapy and cough alone, though neither technique shows an advantage over the other.
Feldman et al. 19 used a mixed group of patients with either chronic bronchitis or cystic fibrosis (CF) characterised by chronic copious sputum production. They found that chest physiotherapy produced a significant improvement in lung function, predominately at low lung volumes, and that the effect could persist for 45 minutes after treatment. However, the heterogeneous nature of their study group raised the possibility that these benefits might be confined to higher sputum-producing patients with CF.
Rivington-Law et al. 20 conducted a crossover study of 12 patients, all with chronic bronchitis. Deep breathing exercises were compared with deep breathing exercises and chest vibrations and with no intervention. They report statistically significant increases in expiratory reserve volume in association with deep breathing exercises alone.
Sputum clearance
In patients with copious secretions, movement of sputum appears more likely to relieve airway obstruction. However, the long-term benefit of increased sputum clearance is unclear as increase in volume does not appear to be maintained 24 hours post treatment. 12 Bateman et al. 21 produced a simple and clearly reported study measuring radioisotope clearance of sputum from the lungs of 10 patients with chronic airways obstruction (not in exacerbation). These patients were regular sputum producers with a mean volume of 100 ml per day. Clearance rates were measured twice: once after physiotherapy (comprising drainage, percussion and vibration for 20 minutes) and on the other occasion without physiotherapy. Clearance, both centrally and peripherally, increased by up to five times after physiotherapy as did sputum weight produced (up to 15 times).
Bateman et al. 22 also studied six patients with chronic obstructive airway disease (COAD) in a repeated measures design. Researchers compared control (no cough), with cough alone and with MCP and cough. They report significantly greater clearance of radioactive aerosol for both intervention modalities compared with control. However, only MCP produced a statistically significant difference in clearance from the peripheral areas of the lungs (p < 0.05) and increased sputum weight (p < 0.05).
Wollmer et al. 6 undertook a study in which inhalation of radiolabelled particles (aerosol scintigraphs) was employed to measure particle deposition and clearance during chest physiotherapy. Although there was no overall effect on the deposition and clearance of radiolabelled particles, two patients with the highest sputum production (100 ml and 130 ml) had a substantially higher clearance with chest percussion. This observation supports the suggestion that there may be differential effects of manual techniques in patients with differing levels of sputum production.
There is some evidence that contradicts this hypothesis. Van Der Schans et al. 10 investigated the effect of manual percussion as a single procedure, as well as in combination with postural drainage (PD), coughing and breathing exercises, on tracheobronchial clearance in patients with chronic airflow obstruction (CAO) and excessive tracheobronchial secretions. Again the study was small (only nine subjects) but PD and coughing, with or without manual percussion, did appear to improve mucociliary clearance more than manual percussion alone. In contrast, manual percussion did not appear to add to the efficiency of the combination of PD, coughing and breathing exercises.
Oxygenation levels
The study by Connors et al. 14 is often quoted to substantiate the claim that chest physiotherapy produces hypoxaemia. However, that study had significant methodological and analytical weaknesses. May and Munt18 reported no significant effect (clinical or statistical) of manual techniques on either oxygen or carbon dioxide levels. Buscaglia and St Marie23 presented a well-designed study of a homogenous group of patients, supporting the evidence that patients’ response to MCP in terms of oxygenation depends on the amount of sputum produced. Wollmer et al. 6 found no significant difference in arterial oxygen saturation (SaO2) between pre- and post-treatment values, either with or without percussion.
Update of 2001 review
The numbers of patients investigated in the studies described above are small and the focus was either on groups of patients that were very specific or heterogeneous in nature. An update of the studies presented above reveals that little has changed since 2001. 24 A systematic review conducted in 2004 to produce the American College of Chest Physicians (ACCP) guidelines found that, although some airway clearance techniques improve sputum expectoration, no high-quality evidence exists for long-term outcomes. 25 Moreover, while MCP was recommended for CF, there was some evidence that manually assisted cough might be detrimental in COPD. Thus, it was advised that this technique should not be used to treat acute exacerbations. In 2007, Garrod and Lasserson26 conducted an overview of systematic reviews of the role of physiotherapy in the management of chronic lung diseases. With respect to MCP they considered that randomised controlled trials (RCTs) were still required to evaluate effects on health-related quality of life, exacerbation frequency and hospital admission.
Thus, a clear state of clinical equipoise remains as to whether MCP confers any benefit to patients with COPD. As a consequence, current UK guidelines for the management of COPD do not propose a clear place for MCP techniques. A National Clinical Strategy for COPD is being developed by the Department of Health (previously known as a National Service Framework [NSF]). National Strategies are 10-year plans for the NHS which aim to raise the quality of care for all people living with specified conditions. Currently a draft strategy is out for consultation with the key stakeholders and one of its remits is to ensure that if someone is admitted to hospital, the time is used effectively to avoid recurrent hospitalisation. 27 Thus, this study is ideally placed to inform evidence-based recommendations concerning MCP.
Study rationale
Crossover designs permit only short-term outcomes to be studied and require either a high degree of stability in the underlying condition or repeated and similar episodes to manifest in the same patient. Acute exacerbations of COPD do not meet this criterion and there is a need for long-term as well as short-term outcomes to be studied. Therefore, this study adopts a pragmatic RCT design, powered for equivalence.
Choice of study outcome measures
The choice of outcome measure appropriate for a trial of this type is somewhat contentious owing to the changing nature of health-care evaluation. Traditionally the focus of effectiveness trials has been on the physiological outcomes of interventions. More recently there has been recognition that quality of life is an important indicator of efficacy that is often not addressed. The choice of outcome measures in this study was therefore predicated on the assumption that long-term effectiveness must be based largely on quality of life considerations. However, because physiological measures may provide useful short-term indicators of effectiveness, these were also included as secondary outcomes in this study.
Quality of life
Chronic obstructive pulmonary disease is a life-limiting condition with considerable effect on quality of life. A study of 141 patients with COPD admitted to hospital for exacerbations reported a considerable loss of health utility compared with individuals in a non-exacerbated state. The majority of hospitalised patients reported a state considered ‘worse than death’ (mean health utility –0.21). 28 Furthermore, the adverse impact on health utility appeared to be greater with increasing severity of COPD.
St George’s Respiratory Questionnaire
The St. George’s Respiratory Questionnaire (SGRQ) is a quality of life measure specifically designed for patients with COPD. It provides valid and reliable measures of respiratory symptoms and is sensitive to change in objective measures of respiratory function. It comprises a self-completed questionnaire containing 76 items divided into three domains. These are:
-
symptoms:frequency of cough, sputum production, wheeze, breathlessness and duration and frequency of attacks
-
activity:physical activities that either cause or are limited by breathlessness.
-
impact:employment, being in control of health, panic, stigma, need for medication and side effects, health expectations, disturbances in daily life.
The SGRQ is rated as easy to use by 90% of respondents29 and has been used extensively in RCTs of rehabilitation and early discharge of COPD patients. 30 It provides an effective measure of health-related quality of life during acute exacerbations31 and reliably predicts mortality for COPD. 32–34 For these reasons, the SGRQ was selected as the primary outcome measure for this study and used as the basis for the statistical power calculation to determine sample size.
Breathlessness Cough and Sputum Scale
The Breathlessness Cough and Sputum Scale (BCSS) is a self-completed symptom-severity scale. One of the advantages of the BCSS is the simultaneous inclusion of breathlessness, cough and sputum assessments. This relatively new scale has demonstrated strong correlation with cough-specific items from the SGRQ. 35 Validation studies of the BCSS indicate that it is able to demonstrate sensitivity to within-group change and between-group differences. 36
European Quality of Life-5 Dimensions questionnaire
The European Quality of Life-5 Dimensions (EQ-5D) questionnaire is a standardised instrument for measuring health outcomes. It provides a simple descriptive profile and a well-validated single-index value of health status. Designed for self-completion by the respondent, the EQ-5D takes only a few minutes to complete. It comprises five dimensions (mobility, self-care, usual activities, pain/discomfort, anxiety/depression) and is supplemented by a visual analogue scale (hereafter referred to as EQ-VAS) recording the respondent’s self-rated health status on a vertical graduated ‘thermometer’, which ranges between 0 (worst imaginable health state) and 100 (best imaginable health state). Responses to the five dimension questions are then converted into a single utility index (where 0 is equivalent to death and 1 is equivalent to full health) using equations relevant to the UK population. 37
While the SGRQ correlates well with the Short Form-36 items (SF-36) quality of life measure, the EQ-5D has been shown to be less responsive to change in the SGRQ. 38 That said, it has been shown that the EQ-5D can discriminate between COPD patients with different levels of known severity. 39 As a result, the EQ-5D was included within this study in order to complement other quality of life measures and provide a fuller description of changes in health-related quality of life.
Physiological impact of MCP
Oxygen saturation and sputum volume
With regard to the physiological impact of MCP, useful indicators suggested by the literature are its short-term impact on sputum volumes40,41 and oxygen saturation. 20
Medical Research Council-Dyspnoea scale
The Medical Research Council-Dyspnoea (MRC-D) scale is a five-item questionnaire in which patients categorise their own level of disability. 42 As some research suggests that lung function measures are useful predictors of morbidity but of little value in predicting quality of life,43,44 this outcome was included solely as a baseline indicator of severity of disease.
Six-minute Walk Test
With respect to evaluating longer term physiological impacts, the Six-minute Walk Test (6MWT)45 is easy to administer, well tolerated by patients and regarded as the most useful functional walk test for research purposes. 46 Therefore, in order to provide comparative functional outcome data, the 6MWT was selected for completion by a subsample of participants at 6 months post randomisation.
Health economics issues
As health-care resources are scarce, and the amount of funding available to the NHS is relatively fixed, there is a need to evaluate the cost-effectiveness of different health-care interventions. 47,48 Here we seek to evaluate whether the provision of MCP represents an efficient use of resources. Alternatively, it may be that a greater health benefit would be afforded by spending the same limited resources elsewhere.
Chapter 2 Methods
In this chapter, the development of the MCP treatment protocol and the methods used to conduct the intervention are described in detail. Prior to start-up, the study protocol and associated documents were reviewed and approved by the Norfolk Research Ethics Committee (REC – ref.06/Q0101/140) and relevant NHS research consortia. The study was conducted in accordance with good clinical practice (GCP) research guidelines.
Development of MCP treatment protocol
Manual chest physiotherapy is a time-consuming, labour intensive treatment requiring significant skill and strength on the part of the therapist and the mental and physical co-operation of the patient. While many physiotherapists perform MCP, the precise method, sequence and duration of its component parts can vary considerably. In order to provide a precise description of the study intervention and standardise delivery as far as possible, a treatment protocol for MCP was developed with physiotherapists involved in the trial. This comprised a series of meetings to reach consensus on the essential elements of MCP, identify potential areas of ambiguity and provide a set of clear parameters within which treatment would be based. The fundamental premise of these meetings was to arrive at a treatment protocol that clearly defined the MCP to be delivered, but allowed sufficient flexibility to preserve the profession’s ethos of providing treatment according to individual need. Thus, the content, number and duration of treatments could remain at the discretion of the physiotherapist as long as variation remained within the bounds set by the protocol.
This iterative approach resulted in a treatment protocol that combined current practice with the best research evidence available to date (see Appendix 1). To prevent ambiguity, definitions for the various elements of MCP were provided, along with pictures of ideal hand positions to adopt when performing percussion and vibration techniques (see Appendix 2). With respect to the positioning of patients during MCP, a photographic list of the six most common treatment positions was provided from which the two most appropriate could be selected according to clinical need (see Appendix 3). If deemed necessary, the physiotherapist could select additional positions, provided these were recorded at the time.
The experience of the research team at the University of East Anglia (UEA) in conducting large, complex, hospital-based RCTs has highlighted the importance of employing active recruiters at each trial site. The study protocol stipulates that research associates (RAs) would identify, recruit and randomise patients and collect all trial-associated data. However, an important issue to emerge from meetings with physiotherapists was their concern that involvement in the trial would impact on already heavy case loads. In order to reassure clinicians that their time commitment would be kept to a minimum, the treatment protocol made clear the division of responsibilities between RAs and physiotherapists delivering the intervention.
Study objectives
Primary objectives
-
To estimate the effect, if any, of MCP administered to patients hospitalised with COPD exacerbation on disease-specific quality of life at 6 months post randomisation.
-
To compare the costs to the NHS and personal social services (PSS) for those who either receive or do not receive MCP while in hospital.
Secondary objectives
-
To compare clinically relevant outcomes between treatment and control groups at 6 weeks and 6 months post randomisation. These included frequency of exacerbation, hospital readmission and sputum volume produced per 24 hours while in hospital.
-
To undertake a prespecified subgroup analysis comprising subjects producing ≥ 15 ml and < 15 ml of sputum per 24 hour period during hospitalisation.
-
To undertake a prespecified subsample analysis of participants undertaking 6MWTs.
-
To describe and quantify the component parts of the MCP given to patients hospitalised with a COPD exacerbation. These included position selection, duration and frequency of treatment and associated change in oxygen saturation.
As this study’s design was pragmatic in nature, the major objective for data collection was to obtain information on the primary outcome measure (SGRQ) at the primary end point (6 months post randomisation).
Screening and recruitment
MATREX was designed as a multisite trial with a phased start-up for each hospital depending on recruitment rates achieved. The clinical population from which study participants were drawn comprised all patients admitted to participating hospitals with an exacerbation of COPD.
Inclusion criteria
-
Diagnosis of COPD as defined by the British Thoracic Society,49 namely:
-
progressive, predominantly irreversible airflow obstruction in which
-
FEV1 is < 80% of the predicted value and FEV1/FVC is less than 0.7
-
symptoms may include worsening breathlessness, cough, increased sputum production and change in sputum colour.
-
-
A COPD exacerbation as set out by the British Thoracic Society,46 namely:
-
a sustained worsening of the patient’s symptoms from his or her usual stable state that is beyond normal day-to-day variations
-
the exacerbation is acute in onset.
-
Exclusion criteria
-
Contraindications to the use of MCP techniques,50 namely:
-
osteoporosis
-
frank haemoptysis
-
bronchial hyper-reactivity
-
known respiratory system malignancy
-
raised intracranial pressure
-
uncontrolled hypertension (diastolic > 110 mmHg)
-
pulmonary embolism
-
coagulopathy [platelets < 50,000mm3 and/or INR (international normalised ratio) ≥ 3]
-
bronchopleural fistula
-
subcutaneous emphysema
-
left ventricular failure as primary diagnosis.
-
-
No evidence of excess sputum production after examination (i.e. the patient does not report excess secretions and there are no signs of excess secretions on auscultation).
-
Cognitive impairment, rendering the patient unable to give fully informed consent.
Screening and recruitment procedure
Each day, RAs screened admission lists at participating hospitals to identify potential study participants. A checklist based on study inclusion and exclusion criteria was compiled for this purpose (see Appendix 4). When a potential participant was identified, the RA liaised with the physiotherapist who then made a clinical assessment of the patient’s suitability for MCP. A checklist comprising known contraindications for MCP and clinical risk factors associated with potential adverse events (AEs) was provided for this purpose (Appendix 5). Once eligibility had been confirmed, the RA went through the patient information sheet (see Appendix 6) with the potential participant and answered any queries they might have. Because rapid change in clinical condition is likely in this patient group, the RA needed to strike a balance between enabling the study intervention to occur during the most acute phase of the exacerbation and not rushing the patient in their decision. After due process, if the patient was willing to participate, the RA obtained informed consent (see Appendix 7).
Baseline data collection
On receipt of written consent, the RA assisted the participant to complete the following baseline questionnaires:
-
SGRQ (Appendix 8)
-
BCSS (Appendix 9)
-
MRC-D scale (Appendix 10)
-
EQ-5D (Appendix 11)
-
COPD cost questionnaire (see Health economics measures)
Additional baseline data collected by the RA included the date of the participant’s admission to hospital, the ward/area to which they had been admitted and the attending physician responsible for their care. Additional personal and demographic information obtained at this point included the participant’s name, sex, date of birth, address, post code and general practitioner (GP) details. A case report form (CRF) was compiled for this purpose (see Appendix 12).
Randomisation
Randomisation was conducted via a voice-activated, automatic telephone response system. This provided each participant with a unique study number, recorded the date of their randomisation and assigned them to receive, or not receive, MCP. The automated system also stratified randomisation by hospital, using a block size of six. The participant was provided with an information card detailing which study arm they had been allocated to (see Appendices 13 and 14). Hospital notes were marked with a removable label to inform RAs and physiotherapists in the event of readmission during the study’s follow-up period.
Blinding
Baseline questionnaire data was collected prior to randomisation. Given the nature of the study intervention, it was not possible to blind participants, clinicians or research staff to study arm allocation during the intervention. However, blinding to arm allocation was achievable for certain individuals at specific points in the study, namely RAs when collecting retrospective data on health service use (see Health economics measure) and the trial statistician and trial health economist during initial data analysis.
Intervention
MCP arm
For participants randomised to receive MCP, the physiotherapist administered treatment within the bounds set by the treatment protocol (Appendix 1). After auscultation, the physiotherapist selected the most appropriate positions to achieve optimal clearance of secretions. The patient’s chest was percussed while they performed thoracic expansion exercises and vibration was applied on expiration. Treatment was interspersed with periods of relaxed abdominal breathing, and the forced expiration technique (FET) in accordance with active cycle breathing techniques (ACBT) to enable chest clearance.
The precise nature of each intervention was recorded by the attending RA on a CRF compiled for this purpose (see Appendix 15). Oxygen saturation was monitored during treatment with a finger pulse oximeter (Konica Minolta Pulsox-300, Tokyo, Japan). Any sputum produced during treatment was collected in a pot which was dated and labelled accordingly.
Following MCP, the physiotherapist provided the patient with advice on positioning, with ACBT. This information was reinforced by providing the patient with an information sheet that summarised the advice (see Appendix 16). The content, number and duration of further MCP treatments during hospitalisation were at the discretion of the physiotherapist and varied according to clinical need. The patient was asked to continue to collect all further expectorant produced during the remainder of their hospital stay. Additional pots were provided for this purpose and collected by the RA as often as practical. The volume of sputum in each pot was recorded.
Control arm
The physiotherapist provided the patient with advice on positioning, cough and sputum mobilisation in accordance with ACBT. This information was reinforced by providing the patient with an information sheet that summarised this advice (Appendix 16). Oxygen saturation was obtained at this visit by means of a finger pulse oximeter. The patient was asked to collect any expectorant produced during their hospital stay. Sputum pots, dated and labelled accordingly, were provided for this purpose. These were collected by the RA as often as practical and the volume of sputum recorded. All information pertaining to participants in the control arm was recorded on a CRF compiled for this purpose (see Appendix 17).
Procedure for handling adverse events
According to the literature, possible AEs associated with MCP include: increased intracranial pressure; acute hypotension; pulmonary haemorrhage; dysrhythmia; vomiting; hypoxia; and bronchospasm. 47 Pain and/or injury to muscles, ribs, and spine can also occur as an immediate consequence of the percussion and vibration elements of this therapy. A list of potential AEs and associated symptoms was included in the treatment protocol along with recommended actions should any occur (see Appendix 1, Section 5).
In addition to individual NHS trust’s policies on AE/incident reporting, a procedure for trial-specific reporting was set in place. To this aim, an AE report form was compiled to record AEs and evidence their management (see Appendix 18). This reiterated the list of possible events and defined the reporting procedure for each one. The physiotherapist was required to provide a brief description of each AE and what action was taken, including details of any investigations and treatments. They were also asked to state whether, in their opinion, the event was related to the MCP being administered.
Movement between arms
The MCP treatment protocol (Appendix 1, Section 4) defines the circumstances under which participants would switch from the control arm to receive MCP. Essentially, these circumstances constitute a working definition for respiratory failure. If the physiotherapist or attending physician became concerned that a patient’s condition had deteriorated to the extent that MCP was warranted, all of the following criteria were required to switch arm:
-
clinical evidence of sputum retention (e.g. auscultation, chest radiograph)
-
ABGs: pH less than 7.26
-
ABGs: rising carbon dioxide
-
already receiving controlled oxygen therapy
-
already receiving other supportive treatment(s).
Outcome measures
MCP treatment measures
In order to describe and quantify the component parts of the MCP administered, the following measures were obtained for each treatment session:
-
treatment position(s) selected
-
oxygen saturation immediately before treatment
-
lowest oxygen saturation during treatment
-
time taken by physiotherapist to deliver treatment
-
deviation(s) from MCP treatment protocol
-
AE(s) experienced.
MCP treatment efficacy measures
In order to estimate the effect of MCP administered to patients hospitalised with COPD exacerbation on disease-specific quality of life, the following questionnaires were administered at baseline, 6 weeks and 6 months post randomisation:
-
SGRQ
-
BCSS
-
EQ-5D.
Follow-up questionnaires were posted to participants with a cover letter requesting that they complete and return them to the study office in the pre-paid, addressed envelope provided.
In order to compare clinically relevant outcomes between treatment and control groups, the following measures were obtained for each study participant:
-
sputum volume (ml per 24 hours) during hospitalisation (see Intervention)
-
number of hospital readmissions during study period
-
number of hospital ‘bed days’ during study period.
The last two were obtained retrospectively by scrutinising hospital databases at the end of follow-up.
In addition to the measures listed above, the 6MWT was completed by a subsample of participants at 6 months post randomisation. All participants at one site (see Six-minute Walk Test) were invited by letter to undertake a walk test at the hospital. In order to minimise the inconvenience to participants, tests were arranged as far as practicable to coincide with routine outpatient appointments. Participants were recompensed for any travel costs they incurred for this visit. Each test was supervised by the physiotherapist according to specified standards51 and undertaken in an area suitably marked with known distances. The distance (metres) achieved in 6 minutes was recorded.
Health economics measures
In order to examine the cost-effectiveness of MCP, the following data were collected:
-
SGRQ
-
EQ-5D
-
COPD cost questionnaire – baseline (Appendix 19)
-
COPD cost questionnaire – follow-up (Appendix 20)
-
secondary care health service use (see Measuring costs).
The baseline COPD cost questionnaire was a study-specific, non-validated instrument designed to capture the participant’s use of health services during the previous 3 months (e.g. visits to hospital, home visits from health professionals), their personal circumstances (e.g. how they travel to hospital, do they have dependents) and health-related financial costs incurred (e.g. purchase of specialised equipment, private health care). The follow-up COPD cost questionnaire was designed to complement the equivalent baseline instrument by capturing change in health service use and cost measures during follow-up.
Questionnaire response rate
Regular audits during the pilot and early part of the main trial alerted the research team to the importance of maximising returns, particularly with respect to the primary outcome at the primary end point. Therefore, an action plan was established to improve questionnaire return rates (see Appendix 24).
Data management and data quality
All paper records pertaining to study participants were collated and stored in the trial office at UEA. Study data were entered on to secure computer systems with limited access measures enforced via user names and passwords. For data files where personal information was not required (e.g. name, address, etc.), individual participants were identifiable only by the study-specific number generated at randomisation. Prior to analysis, the final data set was audited for completeness and accuracy.
Sample size
Sample size was based on the primary outcome measure, SGRQ. Treating this study as non-superiority, where an effect size of 0.3 (typically considered small) is taken as the threshold for superiority then, assuming a true zero difference in the population (90% power, 5% significance) a total of 233 subjects in each arm were required. To allow for a 15% dropout rate, we aimed to recruit 275 participants to each study arm, resulting in a total target sample size of 550 participants.
To conduct the analysis of participants undertaking 6MWTs, a randomly selected subsample of 114 participants per arm was required. This would confer 90% power (5% significance) to detect a clinically significant difference in mean distance of 54 metres assuming a standard deviation (SD) of 125 metres. 52
Statistical methods
All statistical analyses were undertaken using the stata (Version 9.1 SE) statistical software package (StataCorp LP, College Station, TX, USA). This section outlines the statistical analysis procedures that were performed.
Baseline analysis
Baseline comparability between the treatment arms was evaluated by summarising and comparing the following parameters. Continuous outcomes were summarised using the mean and SDs in each group separately, and for categorical outcome the number and percentage were reported:
-
demographic measures:age, gender, smoking status and site
-
measures of disease severity: SGRQ total score, SGRQ symptom score, SGRQ activity score, SGRQ impact score, BCSS score, oxygen saturation (%), sputum (ml), MRC-D score, EQ-5D health thermometer and EQ-5D score.
Efficacy analysis
Primary outcome measure
The primary efficacy analyses were based on the intention-to-treat (ITT) principle, including all randomised patients according to the treatment arm allocation using a full analysis set (i.e. those patients with valid outcome measurements). Additionally, we imputed data, using the method described below, and completed an imputed ITT analysis. An analysis of covariance was used, with treatment as a fixed effect and baseline scores and site as covariates. A 95% confidence interval (CI) was constructed for the mean difference in outcome between the treatment arms. Equality was regarded as a difference in effect size of 0.3 or less in absolute value, i.e. if the upper limit of the 95% CI was less than 0.3 and the lower limit was greater than –0.3. The effect size was defined as the mean difference divided by the pooled, over treatment arm, SD of the outcome. No adjustment for multiple testing was made.
Secondary outcome measures
Analyses of the secondary outcome measures, BCSS, EQ-5D score and EQ-VAS, were also based on the analysis of covariance with treatment as a fixed effect and baseline scores and site as covariates. The analysis of the secondary outcome measure 6MWT was based on the two-sample t-test since no baseline measurements were available and it was only recorded at one site (see Six-minute Walk Test). Analysis of the number of days in hospital was based on a negative binomial regression model with treatment as a fixed effect and site as a covariate.
Secondary analyses
In order to assess the sensitivity of the results to missing or incomplete data, both missing outcome and baseline data were imputed by means of iterative chain equations using all outcome measures (bar the 6MWT), and the number of hospital days, demographic details and treatment allocation. In total, 10 data sets were imputed using the ‘ICE’ command in stata. 53 Estimates were then combined using Rubin’s multiple imputation approach. 54 This is considered preferable to alternative approaches such as last value carried forward as it allows for uncertainty in the missing values themselves. 55 Multiple imputations were carried out using the stata software. This method assumes that the data are missing at random.
Previously published papers have reported that, in equivalence trials, per-protocol (PP) analyses can be preferable to ITT. 56 Hence PP analyses were also conducted using the same models as described in the section Secondary outcome measure.
Planned subgroup analyses of the primary end points by sputum levels (15 ml or less versus more than 15 ml) were undertaken by testing for an interaction between the subgroup and the treatment arm in an analysis of covariance model, with treatment as a fixed effect and baseline scores, site and subgroup as covariates.
Health economics analysis
Measuring health-specific quality of life
The economic evaluation component of this study used both the EQ-5D and the SGRQ quality of life scores to assess the cost-effectiveness of the intervention, in line with guidance from NICE. 57 Justification for using the SGRQ is provided above. Although there is some evidence that the EQ-5D may not be responsive in patients with COPD,37 given its wide usage in health service research and the fact that it is recommended for use in cost-effectiveness analyses,53 it was considered important to include this measure in the study. The SGRQ has the capacity to detect both physiological and functional changes which are essential for detecting any direct improvement resulting from the intervention. Thus, a number of effectiveness end points were compiled and analysed using both total and disaggregated scores.
Measuring costs
Overview
In line with guidance from NICE, published 2008,57 in the base-case analysis we adopted an NHS and PSS perspective and sought to estimate those costs that were considered to potentially relate to the intervention in question. A patient self-report baseline cost questionnaire, the COPD cost questionnaire (Appendix 19), was developed in order to assess whether there were any differences between the two groups at randomisation. Also, for each participant over the 6-month trial period, we sought to monitor the levels of resource associated with physiotherapy input (including MCP), inpatient admissions, outpatient visits, rehabilitation and early discharge, and any other NHS and PSS costs. This enabled the total NHS and PSS cost for those resources considered to potentially relate to the intervention in question (hereafter referred to as the overall health service cost) to be estimated.
Unit costs
All costs were estimated in UK sterling (£) at 2007/8 financial year levels. Unit costs associated with the time spent with various health-care professionals were taken from Curtis,58 where these costs were adjusted to reflect the appropriate pay scale for those who provided the care (see below for further details). NHS reference costs55 were used to estimate unit costs for hospital admissions.
Specific cost components
Baseline health service use
A patient self-report baseline cost questionnaire (Appendix 19) was developed, where information was requested for the last 3 months (prior to randomisation) and included the number of hospital attendances and the number of consultations with other community health and social services. All participants were asked to complete this questionnaire, except those who took part in the pilot phase of the study. The mean number of visits to hospital and consultations with various health-care professionals were reported in order to assess whether there were any differences between the two groups at baseline.
Physiotherapy input
Throughout the trial period the number of MCP sessions and associated ‘hands-on’ time was recorded for all participants (see Appendix 15). In order to estimate the actual level of physiotherapy input each participant received, the following assumptions were made. At baseline all participants received general respiratory physiotherapy advice from a hospital physiotherapist, which was estimated to last 10 minutes. This was added to any MCP hands-on time reported to have occurred at this session in order to estimate the patient contact time at baseline. In order to estimate the actual patient contact with a hospital physiotherapist in subsequent sessions, it was assumed that each follow-up session would last a further 5 minutes in addition to any MCP hands-on time reported. Hospital physiotherapy unit costs were extracted from Curtis58 and adjusted to reflect the different pay scales for those who provided the care (within this study MCP sessions were generally performed by a Band 6 hospital physiotherapist). This enabled the total cost of providing general respiratory physiotherapy advice and any subsequent MCP to be estimated for each participant. The mean cost was thereby calculated for both the MCP arm and the no MCP arm, with the mean incremental cost of MCP estimated by subtracting the latter from the former.
Hospital admissions
Throughout the 6-month trial period, details of all hospital admissions were recorded for each participant. For each admission the following data was extracted from medical records and hospital computer systems: time spent in hospital (days); whether the admission was respiratory or non-respiratory related; ward type (general, coronary care unit, intensive therapy unit/high-dependency unit); day care; and accident and emergency department (A&E). All admissions were assumed to be non-elective. This enabled the total number of days post randomisation (categorised by ward type) to be calculated for each participant. Unit costs in terms of average cost per bed day (for each ward type and respiratory/non-respiratory related) were estimated using NHS reference costs 2006/7. 59 As these costs were estimated at 2006/7 levels, all unit costs were inflated by 3.35% (the hospital and community health services pay and price inflation rate for 2007/858) in order to equate to 2007/8 levels. This enabled the 6-month hospital admission cost to be estimated for each participant and, in turn, the mean 6-month hospital admission cost was estimated for each trial group. By subtracting the mean hospital admission cost in the no MCP arm from that in the MCP arm, it was also possible to calculate the mean incremental hospital admission cost for MCP.
Outpatient visits
Throughout the 6-month trial period, details of all outpatient visits were recorded for each participant. For each outpatient visit the following data were extracted from hospital computer systems: type of visit (first or follow-up); and speciality (respiratory or non-respiratory related). Neither the NHS reference costs 2006/759 nor the Personal Social Services Research Unit58 provides cost per outpatient visit data for respiratory-related conditions. Consequently, both respiratory- and non-respiratory-related visits were assigned the appropriate weighted average cost per visit for either all first attendances or all follow-up attendances, as reported by Curtis. 58 This enabled the 6-month outpatient visit cost to be estimated for each participant and, in turn, the mean 6-month outpatient visit cost was estimated for both the MCP arm and the no MCP arm. Subsequently, the mean incremental cost of MCP was estimated by subtracting the latter from the former.
Pulmonary rehabilitation and early discharge service
At one of the hospitals (University Hospital Aintree, UHA) more intensive rehabilitation support was also available to patients with COPD (hereafter referred to as rehabilitation). For all participants at this site, throughout the 6-month trial period, details of all such contacts were thereby extracted from medical records. Each type of contact is now described. Pulmonary rehabilitation assessments were provided at hospital by a hospital physiotherapist (Band 6), where each assessment lasted an average of 1 hour. Pulmonary rehabilitation group sessions were provided in hospital by a physiotherapist (Band 6) and a physiotherapist assistant (Band 3). Each group session lasted an average of 1.25 hours and was attended by eight patients. The early discharge from hospital scheme, which ran at the same hospital, selected patients on the basis of their clinical severity and suitability to be monitored and treated by a home team. Assessments were made by one-to-one hospital visits with a hospital physiotherapist (Band 6) and lasted an average of 2 hours. Subsequent home visits were provided by a nurse (Band 6) or a hospital physiotherapist (Band 6) and lasted an average of 0.75 hours. Finally, telephone calls were undertaken by a nurse (Band 6) and lasted an average of 5 minutes. In line with aforementioned methods, unit costs for the staff time associated with each of these contacts was estimated from Curtis58 after making adjustments to reflect the different pay scale for those who provided the care. This enabled the 6-month rehabilitation cost to be estimated for each participant. Subsequently, the mean 6-month rehabilitation cost was estimated for each trial group, where this was calculated across all participants in each trial group, not just those at UHA. Finally, the mean incremental cost for the MCP arm was estimated by subtracting the mean rehabilitation cost in the no MCP arm from that in the MCP arm.
Other NHS and PSS costs
These were monitored by a patient self-report measure at 6 weeks and 6 months post randomisation, the COPD cost questionnaire (see Appendix 20), where respondents were asked to report the level of health service use since randomisation and being sent the previous 6-week cost questionnaire respectively. Variables which were monitored included visits to A&E, GP services, consultations with other health professionals and contact with social services.
After considering the response rates to each of the aforementioned component costs, we estimated the overall health service cost for each participant. Subsequently, the mean overall health service cost was estimated for both the MCP arm and the no MCP arm. By subtracting the latter from the former the mean incremental overall health service cost of MCP was also estimated.
Measuring effects
In order to enable the effectiveness of many interventions to be compared on a common scale, within cost-effectiveness analyses benefits are commonly assessed in terms of utility (where 0 is equivalent to death and 1 is equivalent to full health). 44 In this study, in line with recommendations by NICE,57 we used the EQ-5D to estimate utility values and compare the benefits of MCP with no MCP. The EQ-5D asks about the level of problems (none, some/moderate or severe/extreme) with regard to mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. 60 Responses to the EQ-5D were sought at baseline, 6 weeks and 6 months post randomisation. However, in the pilot phase of this study we did not collect baseline data on the EQ-5D. Utility scores were subsequently assigned to each of the elicited health-state descriptions using the York A1 tariff37 on which utility scores range between –0.594 and 1.00. Additionally, in line with a previous analysis,61 those participants who died within the study period were assigned a score of zero. Multiple imputation62 was used to estimate missing EQ-5D scores, as described below.
Multiple imputations were performed using the method of chained equations and 10 sets of imputations as implemented in the stata ‘ICE’ command add-on. 53 This routine uses iterative chain equations based on regression models to impute plausible values for the missing data based upon the relationships observed in the non-missing data. The variables included in the regression models are listed in Table 1.
| Variable | Time point | ||
|---|---|---|---|
| Baseline | 6 weeks | 6 months | |
| Hospital site | ✗ | ||
| Original trial arm allocation | ✗ | ||
| Number of days in hospital | ✗ | ||
| BCSS score | ✗ | ✗ | ✗ |
| SGRQ total score | ✗ | ✗ | ✗ |
| SGRQ symptom score | ✗ | ✗ | ✗ |
| SGRQ activity score | ✗ | ✗ | ✗ |
| SGRQ impact score | ✗ | ✗ | ✗ |
| MRC-D score | ✗ | ||
| Age | ✗ | ||
| Gender | ✗ | ||
| Sputum (ml) | ✗ | ||
| Oxygen saturation (%) | ✗ | ||
| EQ-5D | ✗ | ✗ | ✗ |
| Smoking status (current vs non-current) | ✗ | ✗ | ✗ |
| Academic attainment – degree level (yes/no) | ✗ | ||
| Schooling past minimum leaving age (yes/no) | ✗ | ||
Ten imputed values were estimated for each missing EQ-5D score, where the mean value was used within the subsequent analysis. The exception to this was when the imputed EQ-5D score was outside the range of utility scores estimated by the EQ-5D York A1 tariff (range –0.594 to 1.00),37 where imputed scores were truncated at these values.
Mean EQ-5D scores are reported for both the MCP and no MCP arm at baseline, 6 weeks and 6 months post randomisation, along with the 6-month change scores. The EQ-5D data were also used to calculate the quality-adjusted life-year (QALY) gain/loss accrued over the 6-month trial period for each participant, where this was calculated using the area under the curve method (with adjustment for baseline differences). 63 The mean 6-month QALY gain/loss was subsequently calculated for both the MCP arm and the no MCP arm, along with the mean incremental QALY gain for the MCP arm.
In addition to the mean incremental QALY gain for the MCP arm we also estimated the incremental effect on the SGRQ (both for the total score and for each of the three domains). This was calculated by first using the aforementioned imputation methods to estimate the missing SGRQ scores. Second, the mean change on the SGRQ (both for the total score and for each of the three domains) was estimated for both the no MCP and the MCP group. Finally, the incremental change on the SGRQ for MCP was calculated (both for the total score and for each of the three domains) by subtracting the mean score for the no MCP group from that for the MCP group.
Cost-effectiveness analysis
In the base-case analysis, the level of cost-effectiveness was estimated from the viewpoint of the NHS using the aforementioned incremental overall health service cost of MCP and mean incremental QALY gain of MCP. When two options are compared one is said to ‘dominate’ the other, and thereby be considered to be the more cost-effective option if it is associated with a mean cost saving (a negative incremental cost) and positive mean incremental effect. Where one intervention does not dominate the other it is common to calculate the incremental cost-effectiveness ratio (ICER) associated with each intervention group, relative to the next best alternative. 47 The ICER is calculated by dividing the mean incremental cost (ΔC) by the mean incremental effect (ΔE) (ICER = ΔC/ΔE, where E is the QALY gain and C is the cost). Subsequently, in line with guidance by NICE,57 one might then deem options that have an ICER of less than the threshold (λ) of £20,000 per QALY to be cost-effective. However, certain ICER values are open to misinterpretation64 as, for example, the same ICER value can be reached from both (1) a costing saving and positive incremental effect and (2) an increase in cost and negative incremental effect, where both of these situations have quite different interpretations (in contrast to the latter, the former would be deemed favourable). As a result it is recommended that the net monetary benefit (NMB) is calculated (where NMB = λ × E – C), with a positive NMB denoting that the option was estimated to be cost-effective at the threshold in question. 65 Within this study, where dominance did not occur, we calculated the range of λ values over which the incremental net benefit (i.e. NMB for no MCP – NMB for MCP) was positive, where the point estimate of the ICER for MCP is given by the value of λ when the incremental net benefit is zero (assuming that neither a cost saving nor a negative effect occurs). Additionally, as NICE guidance suggests that options that have a positive incremental net benefit at λ values of £20,000 to £30,000 per QALY will be deemed cost-effective,57 we also calculated the incremental net benefit when λ was equivalent to £20,000 per QALY.
Additional cost-effectiveness analyses were undertaken using the aforementioned incremental overall health service cost of MCP and the incremental effect of MCP according to the SGRQ (both for the total score and each of the three domains).
Decision uncertainty
In order to estimate the level of uncertainty associated with the decision as to which option was most cost-effective, probabilistic methods were used to estimate the cost-effectiveness acceptability curve (CEAC) for each option, where the CEAC depicts the probability that an intervention is cost-effective at different levels of the cost-effectiveness threshold (λ). 65,66 The CEAC was constructed using the technique of non-parametric bootstrapping,67 whereby 10,000 simulations of the (per participant) cost and effect were drawn for each option (with replacement) from the original cost and effect data. The probability of being cost-effective was then equivalent to the proportion of the 10,000 simulations for which each option had the highest net benefit at different values of λ.
With regard to these calculations, it should be noted, as has been pointed out previously,65,66 that as the ICER and CEAC are calculated in different ways it is possible for the most cost-effective option (as determined by the ICER) to have the lowest probability of being cost-effective (according to the CEAC).
Subgroup analysis
Due to the potential for the costs and benefits of MCP compared with no MCP to vary according to the level of sputum, we undertook a prespecified subgroup analysis in which we estimated the costs and benefits for both participants who produced ≥ 15 ml of sputum and participants who produced < 15 ml of sputum (where sputum was the average level of production over a 24-hour period during initial hospitalisation). Our a priori hypothesis was that, if MCP were to be more cost-effective for a particular group, it would be for those who had produced ≥15 ml. 21,6, 22 Thus, for high and low sputum production, in addition to estimating the incremental cost and incremental QALY gain for MCP, the incremental net benefit of MCP was calculated when λ was equivalent to £20,000 per QALY.
Sensitivity analysis
Sensitivity analysis is often undertaken in order to assess how robust conclusions are to methodological assumptions that were made as part of the analysis. 47 Our aforementioned methods were considered to be those of the ‘base-case’ analysis. Therefore, we conducted the following sensitivity analyses in order to assess what impact different assumptions had on our results. First, due to the fact that missing EQ-5D scores were imputed, we conducted a complete case analysis,68 whereby results were analysed only for those participants who had complete cost and EQ-5D data. Second, we changed the assumptions about the unit costs to be applied to respiratory-related admissions. This was undertaken as it was unclear that all ‘respiratory-related’ conditions within the NHS reference costs 2006/759 would be representative for our population group. First, we assumed that the unit cost for respiratory-related conditions was equivalent to those denoted as COPD-related in the NHS reference costs [analysis (a)]. Second, we assumed that the unit cost for respiratory-related conditions was equivalent to the weighted average for all admissions as denoted in NHS reference costs 2006/7 [analysis (b)]. Third, we changed our assumption about what services might potentially relate to the intervention in question. In analysis (a) we assumed that only respiratory-related admissions could potentially relate to MCP, and in analysis (b) we assumed that only physiotherapy time at baseline and subsequent follow-up MCP sessions could potentially relate to MCP. Finally, we conducted a PP analysis. For each of the above analyses the following factors are reported, both in terms of the overall mean levels for both the MCP and no MCP groups and the incremental level for MCP: (1) hospital admission costs (this is the largest cost-driver); (2) overall health service costs; (3) 6-month QALY gain; and (4) net benefit at λ = £20,000 per QALY. Finally, the range of λ values for which MCP was estimated to be cost-effective was also reported.
Changes to study protocol
With respect to the primary outcome measure for the MATREX trial, length of stay in hospital was proposed in our original bid. However, the funder considered that this was not an appropriate outcome measure as it can be influenced by other, non-intervention factors and that alternative, patient-orientated outcomes should be used in power calculations. The primary outcome measure was therefore changed to a COPD-specific quality of life measure (SGRQ) and the power of the study recalculated accordingly.
In order to assess the adequacy of the MCP treatment protocol (see Development of MCP treatment protocol) and proposed outcome measures, the study commenced with a pilot phase for the first 6 months of recruitment. Close monitoring and review of preliminary data by the study’s management groups [Trial Management Group (TMG), see Appendix 21; Trial Steering Committee (TSC), see Appendix 22; Data Monitoring and Ethics Committee (DMEC), see Appendix 23] indicated the need for certain changes to the study protocol and the rationale for these are detailed below. Changes were approved by the lead REC, relevant NHS research consortia and the research commissioning body (NIHR HTA programme).
Testing and refining the MCP treatment protocol
The original treatment protocol stipulated that patients in the MCP arm should be encouraged to cough (Appendix 1, Section 2.5.1). However, this was not listed as an explicit instruction in the control arm. Thus, when assessing the effect of MCP, ‘deliberate’ coughing could act as a confounding variable. In order to ensure parity between trial arms, the treatment protocol was amended to include this instruction for control arm patients (Appendix 1, Section 3.1.1). In addition, an AE report form was compiled to make reporting procedures more explicit (Appendix 18). A number of CRFs were also compiled to ensure consistency in data collection (Appendices 12, 15 and 17).
Changes to inclusion and exclusion criteria
The original commissioning brief included the term ‘infective exacerbation’ in its call for research proposals. While COPD patients tend to be admitted under the rubric of an ‘infective exacerbation’, infectivity status is not routinely established. In clinical practice it is increased sputum volume (regardless of infectivity) that triggers the administration of MCP. Therefore, infective status was removed as a prerequisite for trial eligibility on the grounds that this most closely reflects clinical practice and clinical decision-making.
Six patients who appeared to meet the trial’s inclusion criteria were excluded by the physiotherapy team at one particular site because they were receiving anticoagulant therapy. These exclusions were in line with the physiotherapists’ clinical practice guidelines. As clotting risk factors were implicit in two of the study protocol’s exclusion criteria (haemoptysis and low platelet count), MCP was considered to pose an additional risk of internal bleeding for patients taking anticoagulant medication. After advice from the TSC it was agreed that conducting an additional screen for raised INR was sufficient to ensure that MCP would not be administered inappropriately. As INR is routinely checked on admission, this information would be readily available to trial recruiters. Therefore, the exclusion criterion (INR > 3) was adopted with immediate effect at all sites for the remainder of the recruitment period.
Finally, the definition of COPD given in the study protocol was updated to reflect current NICE guidelines. 49
Changes to recruitment and follow-up periods
The original study protocol stated that follow-up would take place at 6 weeks, 3 months, 6 months and 1 year post randomisation. However, issues that arose during the early stages of recruitment led to the following changes:
-
The pilot phase indicated relatively poor questionnaire response rates for the first two follow-ups. Therefore, in order to minimise the demands made on participants and maximise future questionnaire return rates, the 3-month follow-up was withdrawn.
-
As a result of slower than anticipated recruitment and in order to achieve an adequate sample size, the recruitment period was extended by 12 months. To compensate for slow recruitment during the first year and complete the study within a reasonable length of time, the 1-year follow-up was withdrawn. Thus, the 6-month follow-up became the study’s primary end point.
Health economics protocol changes
In order to calculate the QALY gain associated with the intervention, in the original protocol it was stated that the EQ-5D would be administered at 3 months and 1 year post randomisation. However, in 2005 it was recommended that baseline differences be adjusted for when estimating the QALY gain associated with an intervention. 63 Thus, from the start of the main trial (participant 100 onwards) the EQ-5D was also administered at baseline to provide a more complete picture of change in quality of life. Additionally, following the removal of 3-month and 1-year follow-up, the time points for administering subsequent EQ-5Ds were switched to 6 weeks and 6 months post randomisation.
In the original protocol it was stated that a societal perspective would be taken with regard to the health economic analysis. Guidance from NICE, which was issued after this trial started,47 does however recommend that an NHS and PSS perspective be taken within cost-effectiveness analyses. In accordance with this guidance we thereby changed the perspective of the cost-effectiveness analysis to be from an NHS and PSS viewpoint, in order to enable our results to be compared with those for other studies adopting a similar perspective.
In the original protocol it was stated that health service use declared by patients would be cross-checked with the relevant hospital/primary care records. This was not undertaken for the reasons stated in Chapter 3 in the section Measuring costs.
Six-minute Walk Test
The study pilot revealed logistic problems in setting up and conducting 6MWTs. In order to satisfy concerns regarding patient safety, NHS trusts require walk tests to be conducted by individuals with appropriate medical training and at a venue where cardiopulmonary resuscitation equipment and additional medical support are readily to hand. None of the RAs working at three of the hospital sites was suitably qualified and physiotherapists delivering the study intervention did not have time to conduct additional procedures. However, at the fourth site to join the study, a qualified physiotherapist was seconded full time to deliver the MCP and undertake all RA functions. This provided the opportunity to satisfy concerns regarding patient safety during 6MWTs. In order to capture this important measure of physical function, it was decided that all participants at the fourth site would be approached to undertake walk tests as opposed to the 50% sample across all sites as stipulated in the protocol. Shortening the follow-up period meant that walk tests, originally planned for completion at 1 year, were conducted at 6 months post randomisation.
Amendments to study title
Given that infective status was no longer a prerequisite for participation the word ‘infective’ was removed from the study title (see Changes to inclusion and exclusion criteria). Following review of the first draft of this report by the TMG two further changes have been made. First, the Consolidated Standards of Reporting Trials (CONSORT) statement for RCTs recommends that when trials are powered to test for equivalence, this should be stated in the study title. 56 Therefore, the word ‘equivalence’ has been inserted into the title of this report. Second, while blinding to arm allocation was conducted where possible (see Blinding) use of the term ‘single blind’ is considered to be misleading. 69 Therefore, the phrase ‘single blind’ has been removed from the title of this report. These last two changes to the study title do not appear in the latest approved version of the study protocol (version 7.1, 1 July 2007).
Chapter 3 Results
Recruitment
The study commenced recruitment on 21 November 2005 and closed to recruitment on 30 April 2008 (total recruitment period was 29 months, 9 days).
Recruitment sites
The original study timetable allowed for a 3-month pilot at one site to test the adequacy of the MCP treatment protocol, the suitability of the proposed questionnaires and the feasibility of the original recruitment target (three participants per week per site). Consequently, the trial opened to recruitment at the Norfolk and Norwich University Hospital (NNUH: Norfolk and Norwich University Hospital Trust, Norwich, UK) on 21 November 2005. At the end of 3 months only 24 participants had been recruited, yielding insufficient data to adequately assess the pilot’s aims. Therefore, the pilot was extended to the James Paget Hospital (JPH: James Paget Hospital NHS Trust, Great Yarmouth, UK) which opened to recruitment on 27 February 2006.
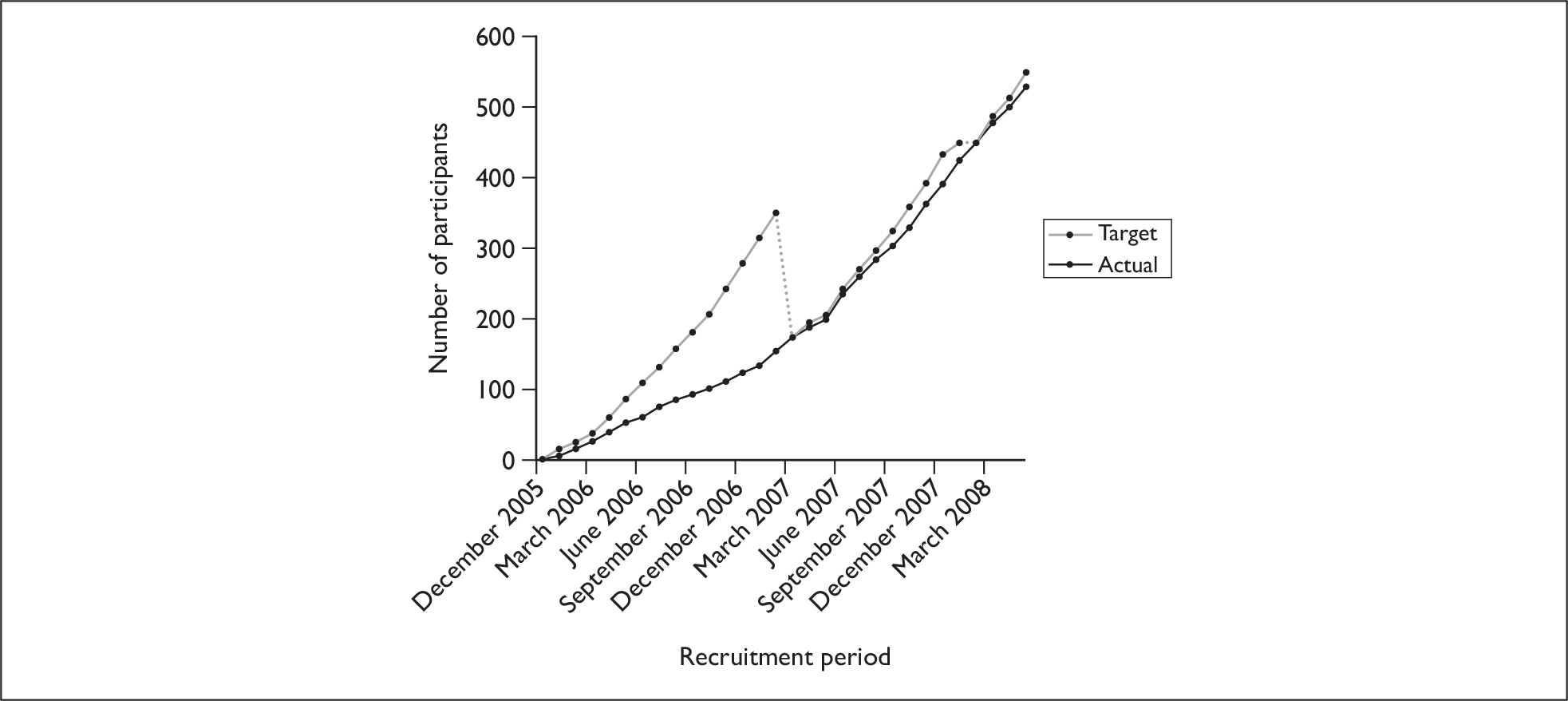
After obtaining REC approval for study amendments arising from the pilot (see Chapter 2, Changes to study protocol) the main trial commenced with the Queen Elizabeth Hospital (QEH: Queen Elizabeth Hospital NHS Trust, King’s Lynn, UK) opening to recruitment on 11 October 2006. Participants recruited during the pilot at NNUH (n = 65) and JPH (n = 36) were retained and incorporated into the main trial. Recruitment continued at all three sites for a further 6 months. During this time it became clear that a fourth site would be required to achieve an adequate sample size within a reasonable time frame. Therefore, University Hospital Aintree (UHA: University Hospital Aintree NHS Trust, Liverpool, UK) joined the trial on 30 April 2007 and recruitment continued at all four sites for a further 12 months. All sites closed to recruitment on 30 April 2008. Figure 1 shows cumulative monthly recruitment for the entire recruitment period. In total, 526 participants were consented and randomised. This figure was 96% of the original recruitment target (550).
FIGURE 1.
Cumulative monthly recruitment against target, 1 December 2005 to 30 April 2008.
CONSORT statement
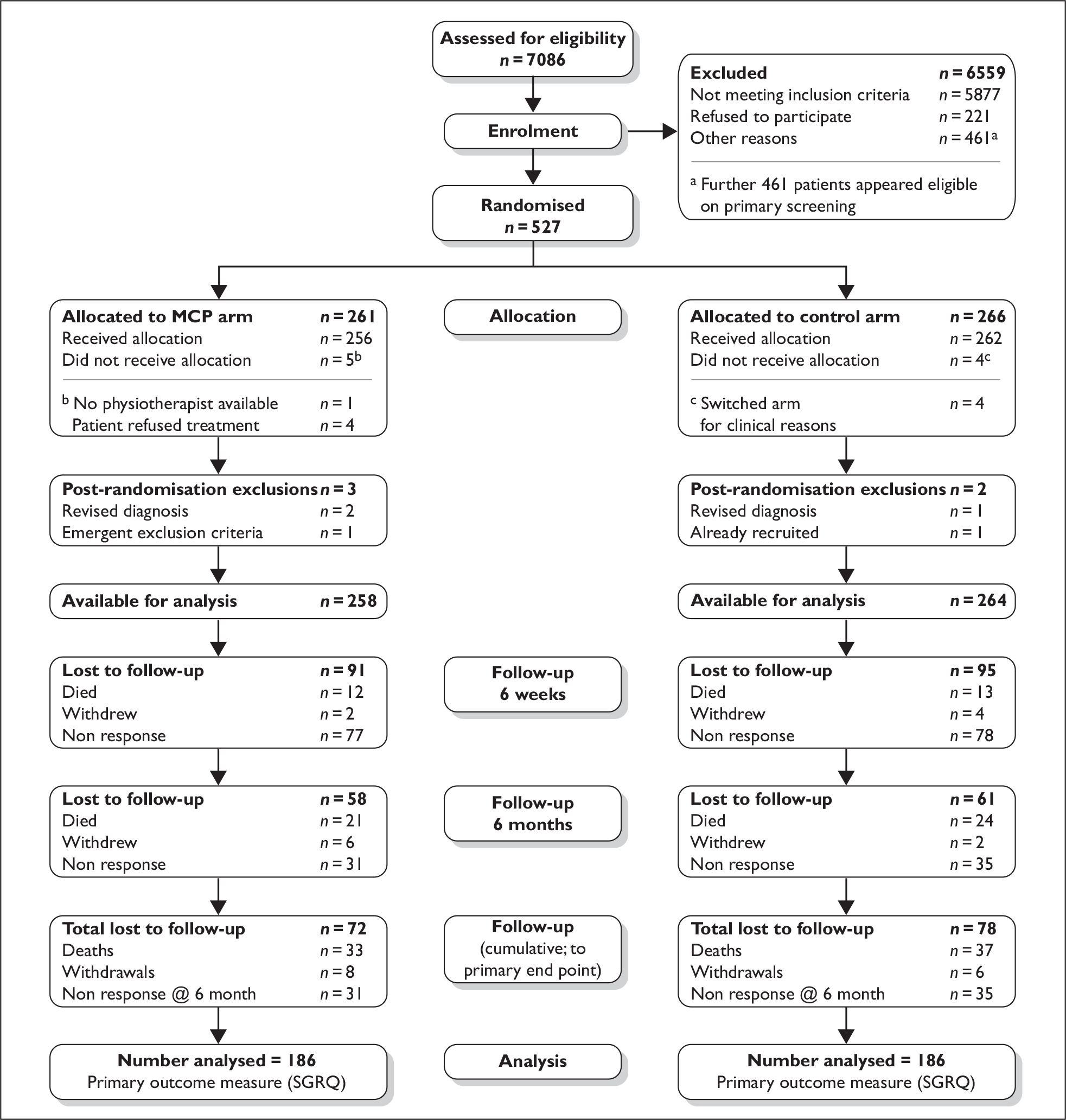
A summary of participant flow through each phase of the trial is provided in Figure 2. This CONSORT diagram provides a summary of recruitment and retention at all four sites combined. Non-responses reported for the two follow-up periods refer to the primary outcome measure only (SGRQ). Details of each phase are described below.
FIGURE 2.
CONSORT flow diagram.
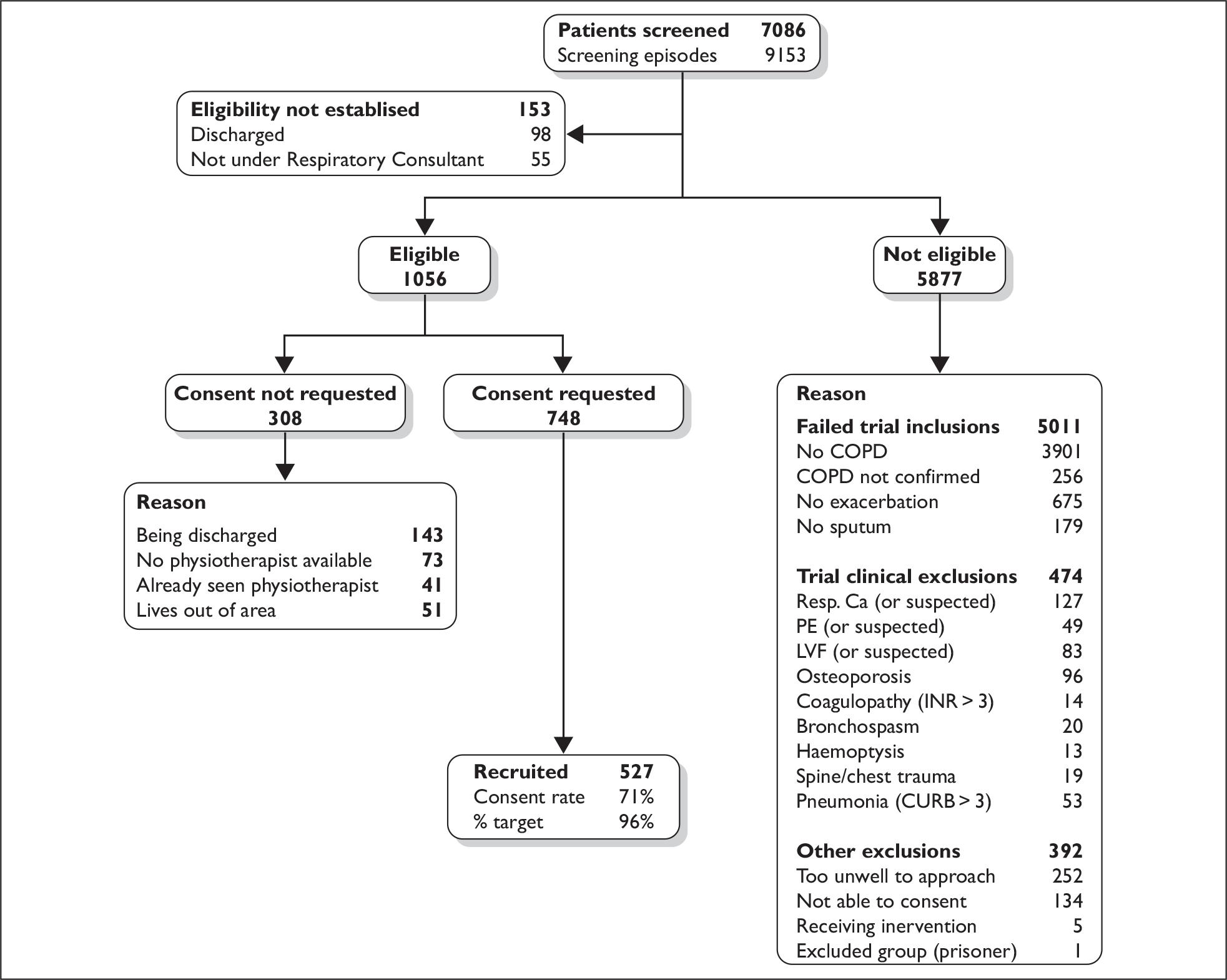
Screening for eligibility
During the 29-month recruitment period, 7086 patients admitted with respiratory symptoms were screened, of which 5877 (83%) did not meet the trial’s inclusion/exclusion criteria. Of the remaining 1209 patients, a further 461 patients appeared eligible on preliminary screening, but were not approached for logistical reasons. These comprised:
-
being discharged (n = 241)
-
no physiotherapist available (n = 73)
-
not under care of respiratory consultant (n = 55)
-
lives out of area (n = 51)
-
already seen by a physiotherapist (41).
Inclusion and exclusion criteria
Full details of trial inclusion and exclusion screenings are provided in Figure 3. It indicates that the bulk of respiratory admissions (83%) did not meet the trial’s eligibility criteria. Of these, the majority concerned patients who did not have COPD or the reason for their admission was not an exacerbation of their condition (85%). The remaining exclusions were either owing to clinical contraindications for MCP (8%) or where ability to give informed consent was compromised in some way (7%).
FIGURE 3.
Screening pathway from admission to consent, 21 November 2005 to 30 April 2008. CURB score, a composite score comprising 1 point for each of the following: confusion (defined as an AMT of 8 or less), urea > 7 mmol/l (blood urea nitrogen > 19), respiratory rate of 30 breaths per minute or greater and blood pressure < 90 mmHg systolic or diastolic blood pressure 60 mmHg or less; LVF, left ventricular failure; PE, pulmonary embolism; Resp. Ca., respiratory cancer.
Repeat screenings
More than 2000 screenings involved trial recruiters scrutinising records of people already excluded on a previous admission. Of these repeat screenings, 141 eventually yielded an additional 117 trial participants, which constituted 22% of the final sample size. Table 2 provides a breakdown of repeat screenings that led to recruitment and the reasons for initial exclusion.
| Rationale for initial exclusion | Number of screenings |
|---|---|
| Clinical reasons | |
| COPD diagnosis not established | 27 |
| Not admitted for COPD exacerbation | 22 |
| No sputum | 11 |
| Clinical exclusion suspected | 16 |
| Too unwell to consent | 14 |
| Other reasons | |
| Discharged | 23 |
| Consent declined | 15 |
| No physiotherapist available | 8 |
| Already seen physiotherapist | 5 |
| Total | 141 |
Consent
In total, 748 patients were approached to participate in the study, 526 of whom gave their consent. This equates to an overall consent rate of 71% for the trial. The consent rate during the first 3 months of recruitment was considerably lower (38%) leading to an audit of reasons for refusal and strategies to ameliorate them (Table 3).
| Patient profile | Number/detail | |
|---|---|---|
| Gender | 20 male, 18 female | |
| Age | Mean 69 (range 59–86) | |
| Number of hospitalisations during last year | Mean 2.2 (range 1–9) | |
| Number of days in hospital during last year | Mean 15 (range 2–104) | |
| Mortality | Two deaths during 12-week time frame | |
| Reasons given for non-consent | Number | Strategy to ameliorate |
| Feeling too unwell to think about study | 15 | Additional phrase added to introduction scripta |
| Unwilling to receive MCP | 7 | Stress that the physiotherapist tailors the treatment to each individual |
| Unwilling to be randomised to control arm | 4 | Reiterate importance of RCT principle |
| Need time to think about it | 4 | Repeat visits (next day and/or next admission) |
| Need to ask family member (leading to subsequent non-consent) | 4 |
Delay specific consent request for patients who appear unwilling Facilitate conversation with treating physician |
| Unwilling to collect sputum | 2 | Empathy and encouragement |
| Reason not given and/or unclear | 2 | Gentle enquiry |
Randomisation
In total 527 participants were randomised to receive either MCP plus advice on chest clearing or advice on chest clearing alone. Unfortunately, this included one person who was consented and randomised twice. This error was realised shortly after the participant’s second ‘recruitment’ and the corresponding randomisation number was abandoned for all follow-ups. Fortunately the participant was randomised to the same arm (control) on both occasions. The CONSORT diagram (Figure 2) describes this double allocation as a post-randomisation exclusion. Thus, the actual sample size achieved was 526 with 261 randomised to the MCP arm and 265 to the control arm. Results quoted in the remainder of this report are based on these figures using the format: total n – MCP n = control n (i.e. 526 – 261 = 265).
Post-randomisation exclusions
In total, there were 5 – 3 = 2 post-randomisation exclusions during the course of the trial. Retrospective changes in diagnosis (whereby inclusion criteria were no longer satisfied) led to 3 – 2 = 1 participants being withdrawn shortly after recruitment. These comprised two individuals who were rediagnosed with asthma and one who was rediagnosed with Kennedy syndrome. Additionally, one participant was withdrawn from the MCP arm owing to an emergent contraindication to treatment (i.e. pulmonary embolism). This patient had not received MCP prior to their exclusion from the trial. Finally, as mentioned previously, one person in the control arm was recruited twice. Follow-up data were not requested from these post-randomisation exclusions; consequently all subsequent analyses are based on the data provided by 522 – 258 = 264 participants (see Figure 2).
Movement between study arms
In total, 9 – 5 = 4 participants did not receive the intervention to which they had been allocated. Four patients randomised to receive advice on chest clearing alone were considered by the physiotherapist to be sufficiently ill to make MCP essential for clinical reasons. Conversely, four patients allocated to receive MCP declined the treatment offered by the physiotherapist. One patient allocated to receive MCP was discharged before the physiotherapist could give the treatment. This participant’s study allocation number was held open and they were readmitted with another COPD exacerbation within the 6-month follow-up period. However, on that occasion treatment with MCP was declined.
Follow-up
6-week follow-up
In total, 186 = 91 + 95 participants did not complete questionnaires at the 6-week time point. This equates to a 35% loss to follow-up, significantly higher than the 15% target set in the study protocol. Deaths (25 = 12 + 13) accounted for 5% of the total number recruited and withdrawals (6 = 2 + 4) for 1%, leaving the majority (29%) attributable to non-return of postal questionnaires (155 = 77 + 78).
6-month follow-up
In total, 119 = 58 + 61 losses occurred between 6 weeks and 6 months post randomisation. These comprised: 45 = 21 + 24 deaths, 8 = 6 + 2 withdrawals and 66 = 31 + 35 non-return of questionnaires. Thus, the total loss to follow-up from randomisation to the study end point comprised: 5 = 3 + 2 post-randomisation exclusions, 70 = 33 + 37 deaths, 14 = 8 + 6 withdrawals and 66 = 31 + 35 non-responses at 6 months post randomisation. These losses equate to 1%, 13%, 3% and 12% respectively of the starting sample size (n = 526). This equates to a final retention figure of 71% for the primary outcome measure at the study’s primary end point.
Site-specific recruitment, retention and follow-up
In line with recent recommendations for reporting complex RCTs of non-pharmacological treatment interventions70 an additional CONSORT diagram is provided in Figure 4.
FIGURE 4.
Site-specific CONSORT flow diagram (including follow-up details for secondary outcome measures). a, inter quartile range for number of participants. b, inter quartile range for number of treatments.
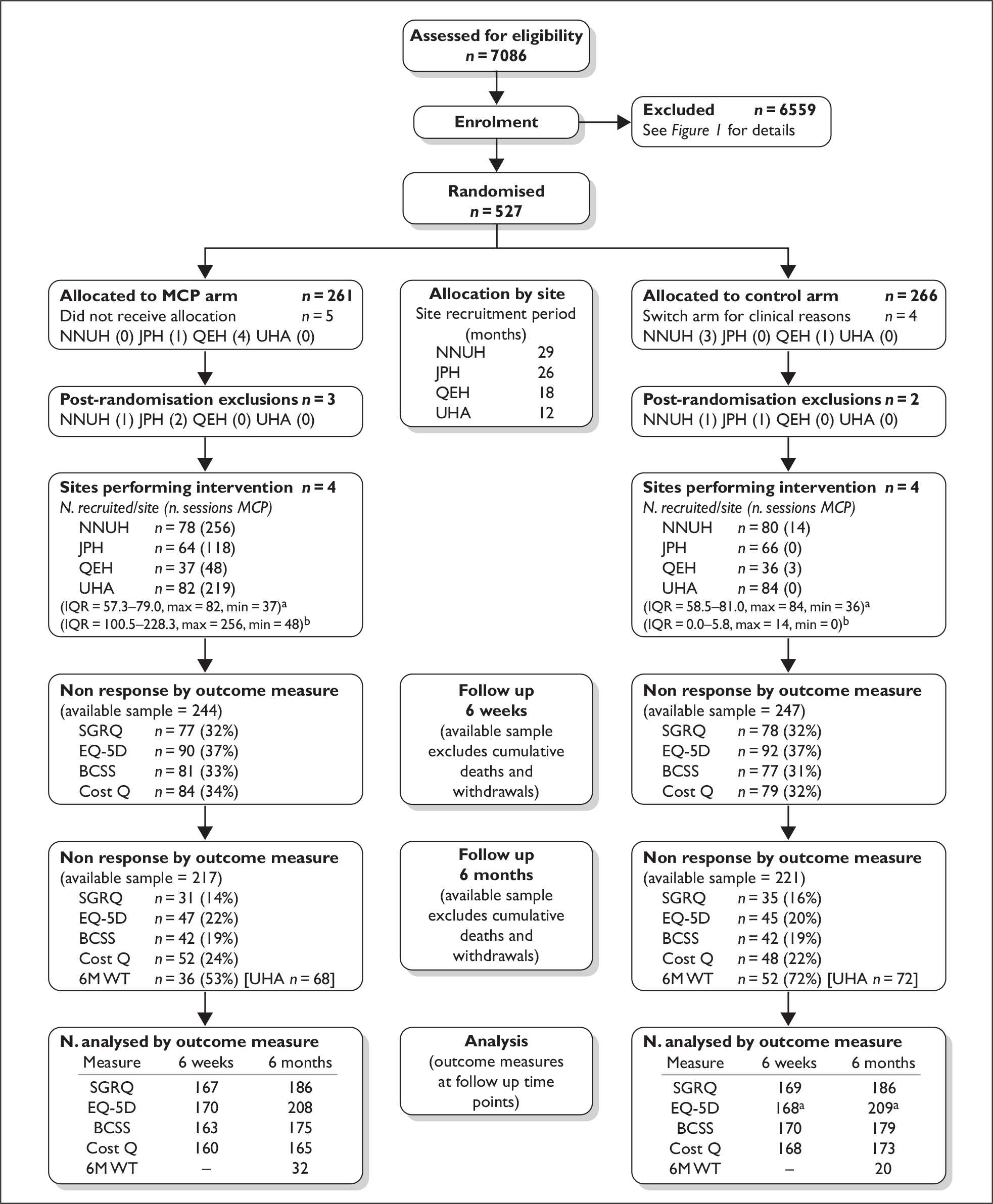
Extra boxes relating to care-providers have been added to show recruitment achieved and MCP treatment delivered at each hospital site. Follow-up phases have also been expanded to give information on non-response rates for secondary outcome measures. Details of particular note are summarised below.
Site-specific recruitment rates
Both the length of time open to recruitment and the consequent accrual achieved shows variation between sites. The site yielding the largest number of participants (166) in the shortest time period (12 months) was UHA, achieving an average monthly recruitment rate of 13.8 participants. Recruitment rates at NNUH (158 over 29 months) and JPH (130 over 26 months) were broadly similar, with an average recruitment per month of 5.4 and 5.0 respectively. Recruitment at QEH accrued 73 participants over an 18-month period, equating to an average of 4.1 participants per month.
Post-randomisation exclusions and study arm switching
Of the three post-randomisation exclusions owing to revised diagnoses, two occurred at JPH and one at NNUH. The exclusion concerning an emergent contraindication to MCP occurred at JPH and the participant randomised twice was recruited at NNUH. Of the four control arm participants switched to the MCP arm for clinical reasons, three occurred at NNUH and one at QEH. Of the five participants in the MCP arm who refused treatment, four occurred at QEH and one at JPH.
Follow-up response rates for secondary outcome measures
At 6 weeks post randomisation, the non-response rates for all questionnaire-based outcome measures were broadly similar (range 30–34%). With the exception of the COPD cost questionnaire, all 6-month non-response rates were lower (range 4–19%). Following amendments to the study protocol (see Chapter 2, Six-minute Walk Test) the 6MWT was conducted at one site only (UHA). This physiological outcome measure showed the highest non-response rate. More than half of those contacted to conduct walk tests either refused to participate or subsequently failed to attend the appointment that had been arranged with the physiotherapist.
MCP treatment
Information on the number, frequency and duration of MCP delivered during the study period is provided in Table 4. In total, 257 participants received 658 sessions of MCP over the 3-year recruitment/follow-up period. The number of MCP sessions administered to patients varied considerably (range 1–25) with the majority receiving two or three sessions between randomisation and the end of their follow-up period. In the majority of sessions (61%) the physiotherapist selected two different positions in which to place the patient when performing percussion and vibration techniques. However, in approximately one-third of sessions, only one treatment position was adopted.
| MCP treatment parameter | Min. | Max. | Mean/median | Breakdown of parameter: n (% total sessions) | |||
|---|---|---|---|---|---|---|---|
| Number of MCP sessions/patient | 1 | 21 | 2.53/2 | n sessions per patient | n patients (total = 257) | n sessionsa (total = 658) | % Total sessions |
| 1 | 97 | 97 | 14 | ||||
| 2 | 70 | 140 | 21 | ||||
| 3 | 47 | 141 | 22 | ||||
| 4 | 20 | 80 | 12 | ||||
| 5 | 6 | 30 | 5 | ||||
| 6 | 3 | 18 | 3 | ||||
| 7 | 5 | 35 | 5 | ||||
| 8 or more | 9 | 117 | 18 | ||||
| Number of positions/session | 1 | 3 | 1.91/2 | 1 position: 248 sessions (38%) | |||
| 2 positions: 404 sessions (61%) | |||||||
| 3 positions: 6 sessions 1%) | |||||||
| Time taken per session | 1 | 41 | 11.9/11 | < 5 minutes: 14 sessions (2%) | |||
| 5–10 minutes: 266 sessions (40%) | |||||||
| 11–19 minutes: 323 sessions (49%) | |||||||
| 20–25 minutes: 44 sessions (7%) | |||||||
| ≥ 26 minutes: 11 sessions (2%) | |||||||
| O2 saturation (%) – immediately prior to MCP | 74 | 100 | 92.0/93 | Less than 85%: 30 (4%) | |||
| 85% to 89%: 111 (17%) | |||||||
| 90% to 94%: 413 (63%) | |||||||
| 95% to 100%: 98 (15%) | |||||||
| O2 saturation (%) – lowest during MCP | 69 | 99 | 91.3/92 | < 85%: 44 (7%) | |||
| 85–89%: 130 (20%) | |||||||
| 90–94%: 385 (58%) | |||||||
| 95–100%: 93 (14%) | |||||||
| O2 saturation (%) – change during MCP | –18 | +13 | –0.7/0 | Drop in O2 saturation: 268 (41%) | |||
| No change in O2 saturation: 258 (39%) | |||||||
| Increase in O2 saturation: 126 (19%) | |||||||
| Deviations from MCP treatment protocol | n = 258 | One position only: 248 (38%) | |||||
| O2 saturation not recorded: 6 (< 1%) | |||||||
| Patient declined treatment: 4 (< 1%) | |||||||
| Alternative positions selected | n = 44 | Upright: 31 (5%) | |||||
| Leaning forward: 10 (2%) | |||||||
| Flat on back: 3 (<1%) | |||||||
While the length of time spent performing MCP varied considerably (range 1–41 minutes), half of all sessions lasted between 11 and 19 minutes (average session length 11.9 minutes).
On four occasions patients requested that the physiotherapist stopped treatment and on six occasions an AE truncated treatment. These scenarios made up the majority of sessions lasting less than 5 minutes (n = 14).
Immediately prior to each MCP session, the patient’s oxygen saturation was recorded with a finger pulse oximeter. While this reveals an average pretreatment reading of 92.0%, again wide variation is apparent across the total number of sessions (range 69–99%). Similarly, while the average lowest oxygen saturation during MCP appears little changed from baseline (91.3%) this figure was compiled from readings ranging from 69% to 99%. With respect to change in oxygen levels, nearly half of all MCP sessions were associated with a drop in oxygen saturation (41%). However, for a similar proportion (39%) no change was evident. The largest drop in oxygen saturation was from 92% prior to treatment to 74% during MCP (see Adverse events). Averaging all change values reveals a slight drop in oxygen saturation overall (–0.7%) but, again, this figure conceals wide variation across all treatment sessions (range –18% to + 15%).
Adverse events
Of the 658 MCP treatments performed by physiotherapists during the study, a total of 15 AEs were reported (Table 5). These comprised: increased shortness of breath (n = 5); pain (n = 5); arrhythmia (n = 3); bronchospasm (n = 1); and thoracic haematoma (n=1). The shortness of breath reported by patients was accompanied by varying degrees of reduced oxygen saturation (–18% to 0%). Four patients requested that MCP treatment be stopped. AEs were subject to periodic review by the study’s management groups. Given their nature (i.e. consistent with the literature) and frequency (i.e. 2% of total treatments), these AEs were not considered to present any significant issues with respect to patient safety and continuation of the trial.
| Site | Adverse event | Response | Outcome | Attributed to MCP (clinical review) |
|---|---|---|---|---|
| NNUH | Tachycardia (130 b.p.m.) | This treatment stopped | Symptoms resolved | Yes |
| NNUH | Atrial fibrillation | This treatment stopped | Symptoms resolved | No – exacerbation of pre-existing condition |
| NNUH | Thoracic haematoma 1 day post treatment | Treatment discontinued, no further MCT given | Further three admissions owing to cardiac events – patient died | No |
| NNUH | Patient reported chest wall pain | Treatment position changed | Pain alleviated | No – exacerbation of pre-existing condition |
| NNUH |
Tachycardia (125 b.p.m.) Starting 02 saturation: 79% Lowest 02 saturation: 71% |
This treatment stopped | Symptoms resolved | Yes |
| JPH |
Patient reported very SOB Starting 02 saturation: 88% Lowest 02 saturation: 80% Patient asked to stop MCP |
This treatment stopped | Symptoms resolved | Yes |
| NNUH | Patient reported worsening pleuritic pain | This treatment stopped | Symptoms resolved | Yes |
| JPH |
Patient reported very SOB in second position Starting 02 saturation: 95% Lowest 02 saturation: 94% |
This treatment stopped Patient auscultated Breathing exercises and coughing implemented |
Symptoms resolved | Yes |
| QEH | Patient reported cramp | Treatment position changed. | Pain alleviated | No – exacerbation of pre-existing condition |
| JPH |
Patient reported SOB and asked to stop MCP Starting 02 saturation: 95% Lowest 02 saturation: 84% |
Treatment suspended Nurse alerted Nebuliser given |
Symptoms stabilised | Yes |
| JPH |
Patient reported SOB and asked to stop MCP Starting 02 saturation: 88% Lowest 02 saturation: 85% |
This treatment stopped | Symptoms resolved | Yes |
| JPH |
Patient exhibited increased wheeze 02 saturation constant: 98% |
Treatment suspended Broncospasm confirmed on auscultation |
Symptoms stabilised | Yes |
| UHA | Patient reported back pain | Symptoms reported 1 day after treatment | No further treatment given | No |
| JPH |
02 saturation drop on turning (patient on 35% oxygen) Starting 02 saturation: 92% Lowest 02 saturation: 74% |
Treatment suspended Patient returned to sitting position. 02 saturation quickly recovered to 92% |
Symptoms stabilised MCP restarted |
Yesa |
| NNUH | Patient reported sharp pain in lower abdomen and asked to stop MCP | This treatment stopped | Symptoms resolved | No – exacerbation of pre-existing condition |
Data quality
Prior to analysis, the final data set was audited for completeness and accuracy. This comprised a cross-check of electronic database entries against original paper records for a randomly selected sample of participants (n = 26, 5% of full data set). In addition, a double data entry check of questionnaire returns entered on electronic databases was performed for participants recruited before 1 January 2007 (n = 125, 23% of total recruited). The results of this audit revealed no significant issues in terms of data quality (see Appendix 25).
Baseline data
Characteristics of randomised participants are shown in Table 6. No differences were identified between the treatment arms.
| MCP arm (n = 258) | No MCP arm (n = 264) | |||||
|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | |
| Age (years) | 258 | 69.08 | 9.85 | 264 | 69.58 | 9.51 |
| SGRQ symptom score | 249 | 79.23 | 14.42 | 255 | 79.61 | 14.18 |
| SGRQ activity score | 249 | 84.97 | 15.46 | 258 | 84.10 | 15.87 |
| SGRQ impact score | 249 | 56.58 | 19.13 | 258 | 57.57 | 18.85 |
| SGRQ total score | 249 | 68.94 | 14.66 | 255 | 69.13 | 14.76 |
| BCSS score | 249 | 6.23 | 2.11 | 256 | 6.44 | 2.18 |
| O2 saturation (%) | 254 | 92.33 | 3.67 | 252 | 92.77 | 5.03 |
| Sputum volume (ml) | 240 | 8.17 | 11.09 | 255 | 7.89 | 9.63 |
| EQ-VAS score | 196 | 44.95 | 21.03 | 202 | 46.64 | 21.42 |
| EQ-5D score | 199 | 0.45 | 0.32 | 202 | 0.43 | 0.36 |
| n/N | % | n/N | % | |||
| Female | 115/258 | 44.57 | 109/264 | 41.29 | ||
| Smoking status | ||||||
| Current | 43/221 | 19.46 | 49/224 | 21.88 | ||
| Ex-smoker | 175/221 | 79.19 | 172/224 | 76.79 | ||
| Never | 3/221 | 1.36 | 3/224 | 1.34 | ||
| Sputum > 15 ml | 38/240 | 15.83 | 42/255 | 16.47 | ||
| Site | ||||||
| JPH | 62/258 | 24.03 | 65/264 | 24.62 | ||
| NNUH | 77/258 | 29.84 | 79/264 | 29.92 | ||
| QEH | 37/258 | 14.34 | 36/264 | 13.64 | ||
| UHA | 82/258 | 31.78 | 84/264 | 31.82 | ||
| MRC-D score | ||||||
| 1 | 0/250 | 0.00 | 1/255 | 0.39 | ||
| 2 | 11/250 | 4.40 | 14/255 | 5.49 | ||
| 3 | 27/250 | 10.80 | 27/255 | 10.59 | ||
| 4 | 68/250 | 27.20 | 75/255 | 29.41 | ||
| 5 | 144/250 | 57.60 | 138/255 | 54.12 | ||
Numbers analysed
A total sample size of 522 was used for all analyses. The proportion of participants for which information was not available on the primary outcome measures at 6 weeks did not differ significantly between treatment arms (p = 0.96, chi-squared test) or at 6 months (p = 0.786, chi-squared test).
Primary analyses
The results for the primary ITT analyses are given in Table 7a and 7b.
| MCP arm | No MCP arm | Unadjusted analysis no MCP – MCP |
Adjusted analysisa no MCP – MCP |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | Mean difference | 95% CI | p-value | Mean difference | 95% CI | p-value | |
| 6 weeks | ||||||||||||
| SGRQ total score | 167 | 62.96 | 18.50 | 169 | 63.81 | 19.37 | 0.84 | –3.22 to 4.91 | 0.6833 | 1.61 | –1.33 to 4.55 | 0.282 |
| Effect size | 0.04 | –0.17 to 0.26 | 0.09 | –0.07 to 0.24 | ||||||||
| SGRQ symptom score | 168 | 67.29 | 22.08 | 169 | 70.02 | 22.29 | 2.73 | –2.02 to 7.48 | 0.2594 | 3.12 | –1.00 to 7.25 | 0.137 |
| Effect size | 0.12 | –0.09 to 0.34 | 0.14 | –0.04 to 0.33 | ||||||||
| SGRQ activity score | 170 | 81.30 | 19.01 | 173 | 79.37 | 20.93 | –1.93 | –6.18 to 2.32 | 0.3714 | –0.16 | –3.55 to 3.23 | 0.926 |
| Effect size | –0.10 | –0.31 to 0.12 | –0.01 | –0.18 to 0.16 | ||||||||
| SGRQ impact score | 170 | 50.32 | 21.55 | 173 | 52.04 | 21.85 | 1.72 | –2.89 to 6.33 | 0.4639 | 2.12 | –1.30 to 5.53 | 0.223 |
| Effect size | 0.08 | –0.13 to 0.29 | 0.10 | –0.06 to 0.25 | ||||||||
| 6 months | ||||||||||||
| SGRQ total score | 186 | 63.88 | 19.05 | 186 | 63.52 | 19.68 | –0.36 | –4.31 to 3.59 | 0.8573 | 0.51 | –2.67 to 3.69 | 0.753 |
| Effect size | –0.02 | –0.22 to 0.19 | 0.03 | –0.14 to 0.19 | ||||||||
| SGRQ symptom score | 186 | 68.38 | 23.13 | 186 | 68.40 | 23.01 | 0.02 | –4.68 to 4.73 | 0.9925 | 0.87 | –3.50 to 5.25 | 0.695 |
| Effect size | 0.00 | –0.20 to 0.21 | 0.04 | –0.15 to 0.23 | ||||||||
| SGRQ activity score | 188 | 82.49 | 18.81 | 187 | 80.91 | 19.74 | –1.58 | –5.50 to 2.34 | 0.4279 | –0.36 | –3.76 to 3.04 | 0.836 |
| Effect size | –0.08 | –0.29 to 0.12 | –0.02 | –0.20 to 0.16 | ||||||||
| SGRQ impact score | 188 | 51.53 | 22.58 | 187 | 51.60 | 22.50 | 0.07 | –4.51 to 4.65 | 0.9752 | 0.43 | –3.29 to 4.14 | 0.822 |
| Effect size | 0.00 | –0.20 to 0.2 | 0.02 | –0.15 to 0.18 | ||||||||
| MCP arm | No MCP arm | Unadjusted analysis no MCP – MCP |
Adjusted analysisa no MCP – MCP |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | Mean difference | 95% CI | p-value | Mean difference | 95% CI | p-value | |
| 6 weeks | ||||||||||||
| SGRQ total score | 258 | 62.89 | 26.45 | 264 | 63.56 | 23.71 | 0.67 | –3.79 to 5.13 | 0.764 | 0.60 | –3.65 to 4.84 | 0.776 |
| Effect size | 0.04 | –0.20 to 0.27 | 0.03 | –0.19 to 0.26 | ||||||||
| SGRQ symptom score | 258 | 66.71 | 31.64 | 264 | 68.66 | 34.13 | 1.95 | –3.76 to 7.65 | 0.496 | 1.97 | –3.57 to 7.52 | 0.479 |
| Effect size | 0.08 | –0.15 to 0.30 | 0.08 | –0.14 to 0.29 | ||||||||
| SGRQ activity score | 258 | 80.59 | 28.44 | 264 | 78.92 | 24.64 | –1.67 | –6.07 to 2.72 | 0.449 | –1.27 | –5.51 to 2.98 | 0.550 |
| Effect size | –0.08 | –0.31 to 0.14 | –0.06 | –0.28 to 0.15 | ||||||||
| SGRQ impact score | 258 | 50.44 | 31.36 | 264 | 52.03 | 27.75 | 1.59 | –3.76 to 6.95 | 0.551 | 1.05 | –4.01 to 6.11 | 0.674 |
| Effect size | 0.07 | –0.17 to 0.31 | 0.05 | –0.18 to 0.28 | ||||||||
| 6 months | ||||||||||||
| SGRQ total score | 258 | 63.496 | 23.47 | 264 | 63.30 | 21.64 | –0.15 | –3.77 to 3.46 | 0.935 | –0.23 | –3.56 to 3.10 | 0.891 |
| Effect size | –0.01 | –0.19 to 0.18 | –0.01 | –0.18 to 0.16 | ||||||||
| SGRQ symptom score | 258 | 67.79 | 39.50 | 264 | 67.72 | 40.75 | –0.07 | –4.72 to 4.58 | 0.976 | –0.01 | –4.51 to 4.49 | 0.996 |
| Effect size | 0.00 | –0.20 to 0.20 | 0.00 | –0.19 to 0.19 | ||||||||
| SGRQ activity score | 258 | 79.19 | 32.40 | 264 | 77.92 | 34.65 | –1.27 | –6.12 to 3.57 | 0.605 | –0.95 | –5.66 to 3.77 | 0.692 |
| Effect size | –0.05 | –0.24 to 0.14 | –0.04 | –0.22 to 0.15 | ||||||||
| SGRQ impact score | 258 | 51.44 | 24.41 | 264 | 51.55 | 23.56 | 0.11 | –4.22 to 4.44 | 0.961 | –0.43 | –4.47 to 3.61 | 0.832 |
| Effect size | 0.00 | –0.20 to 0.21 | –0.02 | –0.21 to 0.17 | ||||||||
Total SGRQ score
No statistically significant difference in mean total SGRQ score was found at the 6-week time point in either unadjusted or adjusted for baseline values and hospital site analyses. At 6 weeks, the mean difference (95% CI) in the unadjusted analysis was 0.84 (–3.22 to 4.91) and for the adjusted analysis was 1.61 (–1.33 to 4.55), with the advice for chest therapy arm having a, non-significantly, higher score. Converting these CIs to effect size CIs, the result of the unadjusted analysis was 0.04 (–0.17 to 0.26) and for the adjusted analysis was 0.09 (–0.07 to 0.24). Both unadjusted and adjusted CIs are within the predefined limits of equivalence, indicating equivalence.
No statistically significant difference in total SGRQ score was found at the 6-month time point in either unadjusted or adjusted for baseline values and hospital site analyses. At 6 months, the mean difference (95% CI) in the unadjusted analysis was –0.36 (–4.31 to 3.59) and for the adjusted analysis was 0.51 (–2.67 to 3.69), with the advice for chest therapy arm having a lower unadjusted score but a higher adjusted score. Converting these to effect sizes, the result of the unadjusted analysis was –0.02 (–0.22 to 0.19) and the result of the adjusted analysis 0.03 (–0.14 to 0.19). Both unadjusted and adjusted CIs are within the predefined limits of equivalence, indicating equivalence.
SGRQ symptom score
No statistically significant difference in mean SGRQ symptom score was found at the 6-week time point in either unadjusted or adjusted for baseline values and hospital site analyses. At 6 weeks, the mean difference (95% CI ) in the unadjusted analysis was 2.73 (–2.02 to 7.48) and for the adjusted analysis was 3.12 (–1.00 to 7.25). Converting these to effect sizes, the result of the unadjusted analysis was 0.12 (–0.09 to 0.34) and for the adjusted analysis 0.14 (–0.04 to 0.33). Both unadjusted and adjusted CIs are outwith the predefined limits of equivalence with the advice for chest therapy arm having a possibly higher symptom score than the MCP.
No statistically significant difference in mean SGRQ symptom score was found at the 6-month time point in either unadjusted or adjusted for baseline values and hospital site analyses. At 6 months, the mean difference (95% CI) in the unadjusted analysis was 0.02 (–4.68 to 4.73) and for the adjusted analysis was 0.87 (–3.50 to 5.25), with the advice for chest therapy arm having a, non-significant, higher score. Converting these to effect sizes, the results of the unadjusted analysis was 0.00 (–0.20 to 0.21) and for the adjusted analysis was 0.04 (–0.15 to 0.23; these are within the predefined limits of equivalence, indicating equivalence.
SGRQ activity score
No statistically significant difference in mean SGRQ activity score was found at the 6-week time point in either unadjusted or adjusted for baseline values and hospital site analyses. At 6 weeks, the mean difference (95% CI) in the unadjusted analysis was –1.93 (–6.18 to 2.32) and for the adjusted analysis was –0.16 (–3.55 to 3.23). Converting these to effect sizes, the result for the unadjusted analysis was –0.10 (–0.31 to 0.12) and for the adjusted analysis was –0.01 (–0.18 to 0.16). The unadjusted interval includes the possibility that the MCP arm is slightly superior to the advice for chest therapy arm; however, the adjusted analysis interval is within the predefined limits of equivalence.
No statistically significant difference in mean SGRQ activity score were found at the 6-month time point in either unadjusted or adjusted for baseline values and hospital site analyses. At 6 months, the mean difference (95% CI) in the unadjusted analysis was –1.58 (–5.50 to 2.34) and for the adjusted analysis was –0.36 (–3.76 to 3.04). Converting these to effect sizes, the result for the unadjusted analysis was –0.08 (–0.29 to 0.12) and for the adjusted analysis was –0.02 (–0.20 to 0.16); both of these intervals are within the predefined limits of equivalence.
SGRQ impact score
No statistically significant difference in mean SGRQ impact score was found at the 6-week time point in either unadjusted or adjusted for baseline values and hospital site analyses. At 6 weeks, the mean difference (95% CI) in the unadjusted analysis was 1.72 (–2.89 to 6.33) and for the adjusted analysis was 2.12 (–1.30 to 5.53). Converting these to effect sizes, the result for the unadjusted analysis was 0.08 (–0.13 to 0.29) and for the adjusted analysis 0.10 (–0.06 to 0.25), both intervals within the predefined limits of equivalence.
No statistically significant difference in mean SGRQ impact score was found at the 6-month time point in either unadjusted or adjusted for baseline values and hospital site analyses. At 6 months, the mean difference (95% CI) in the unadjusted analysis was 0.07 (–4.51 to 4.65) and for the adjusted analysis was 0.43 (–3.29 to 4.14). Converting these to effect sizes, the result for the unadjusted analysis was 0.00 (–0.20 to 0.21) and for the adjusted analysis was 0.02 (–0.15 to 0.18); both of these intervals are within the predefined limits of equivalence.
Secondary analyses
Secondary outcome measures
Results of the secondary outcome measures are given in Table 8a and 8b.
| MCP arm | No MCP arm | Unadjusted analysis no MCP – MCP |
Adjusted analysisa no MCP – MCP |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | Mean difference | 95% CI | p-value | Mean difference | 95% CI | p-value | |
| 6 weeks | ||||||||||||
| BCSS score | 163 | 5.41 | 2.70 | 170 | 5.87 | 2.62 | 0.45 | –0.12 to 1.03 | 0.1204 | 0.33 | –0.18 to 0.84 | 0.208 |
| EQ-VAS score | 148 | 52.07 | 20.04 | 152 | 51.95 | 21.87 | –0.11 | –4.88 to 4.66 | 0.9626 | –0.68 | –5.90 to 4.55 | 0.798 |
| EQ-5D score | 168 | 0.48 | 0.32 | 166 | 0.50 | 0.32 | 0.01 | –0.05 to 0.08 | 0.6891 | 0.03 | –0.04 to 0.10 | 0.442 |
| 6 months | ||||||||||||
| BCSS score | 175 | 5.60 | 2.96 | 179 | 5.66 | 2.84 | 0.06 | –0.55 to 0.66 | 0.8577 | 0.01 | –0.54 to 0.56 | 0.978 |
| 6MWT (metres) | 32 | 174.72 | 109.53 | 20 | 257.95 | 141.15 | 83.23 | 13.09 to 153.37 | 0.0210 | |||
| Number of days in hospitalb | 258 | 15.95 | 16.49 | 264 | 16.98 | 18.04 | 1.07c | 0.91 to 1.24 | 0.4209 | |||
| EQ-VAS score | 167 | 51.29 | 20.97 | 173 | 52.25 | 19.65 | 0.96 | –3.37 to 5.29 | 0.6630 | 2.65 | –2.35 to 7.65 | 0.297 |
| EQ-5D score | 209 | 0.48 | 0.33 | 207 | 0.45 | 0.35 | –0.03 | –0.10 to 0.04 | 0.3720 | –0.01 | –0.07 to 0.06 | 0.886 |
| MCP arm | No MCP arm | Unadjusted analysis no MCP – MCP |
Adjusted analysisa no MCP – MCP |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | Mean difference | 95% CI | p-value | Mean difference | 95% CI | p-value | |
| 6 weeks | ||||||||||||
| BCSS score | 258 | 5.56 | 4.21 | 264 | 6.01 | 3.24 | 0.45 | –0.10 to 0.99 | 0.107 | 0.35 | –0.17 to 0.86 | 0.188 |
| EQ-VAS score | 258 | 50.42 | 40.10 | 264 | 50.13 | 47.26 | –0.29 | –5.45 to 4.88 | 0.912 | –0.53 | –5.74 to 4.67 | 0.837 |
| EQ-5D score | 258 | 0.46 | 1.64 | 264 | 0.46 | 1.45 | 0.01 | –0.08 to 0.09 | 0.897 | 0.01 | –0.08 to 0.10 | 0.768 |
| 6 months | ||||||||||||
| BCSS score | 258 | 5.65 | 5.81 | 264 | 5.65 | 4.74 | 0.00 | –0.68 to 0.69 | 0.994 | –0.10 | –0.75 to 0.55 | 0.759 |
| EQ-VAS score | 258 | 49.76 | 26.39 | 264 | 51.74 | 27.15 | 1.97 | –2.16 to 6.11 | 0.347 | 1.78 | –2.34 to 5.91 | 0.395 |
| EQ-5D score | 258 | 0.46 | 0.74 | 264 | 0.42 | 0.92 | –0.04 | –0.11 to 0.03 | 0.246 | –0.03 | –0.10 to 0.04 | 0.344 |
EQ-VAS score
No statistically significant differences in mean EQ-VAS score were found at the 6-week time point in either the unadjusted (p = 0.963) or adjusted analyses (p = 0.798). At 6 weeks the mean difference (95% CI) in the unadjusted analysis was –0.11 (–4.88 to 4.66) and for the adjusted analysis was –0.68 (–5.90 to 4.55). Similarly, no statistically significant differences were found at the 6-month time point in either the unadjusted (p = 0.663) or adjusted analyses (p = 0.297). At 6 months the mean difference (95% CI) in the unadjusted analysis was 0.96 (–3.37 to 5.29) and for the adjusted analysis was 2.65 (–2.37 to 7.65).
EQ-5D score
No statistically significant difference in mean EQ-5D score was found at the 6-week time point in either the unadjusted (p = 0.689) or adjusted analyses (p = 0.442). At 6 weeks the mean difference (95% CI) in the unadjusted analysis was 0.01 (–0.05 to 0.08) and for the adjusted analysis was 0.03 (–0.04 to 0.10). Similarly, no statistically significant differences were found at the 6-month time point in either the unadjusted (p = 0.372) or adjusted analyses (p = 0.886). At 6 months the mean difference (95% CI) in the unadjusted analysis was –0.03 (–0.10 to 0.04) and for the adjusted analysis was –0.01 (–0.07 to 0.06).
BCSS score
No statistically significant difference in mean BCSS score was found at the 6-week time point in either the unadjusted (p = 0.120) or adjusted analyses (p = 0.208). At 6 weeks the mean difference (95% CI) for the unadjusted analysis was 0.45 (–0.12 to 1.03) and for the adjusted analysis was 0.33 (–0.18 to 0.84). Similarly, no statistically significant differences were found at the 6-month time point in either the unadjusted (p = 0.858) or adjusted analyses (p = 0.978). At 6 months the mean difference (95% CI) for the unadjusted analysis was 0.06 (–0.55 to 0.66) and for the adjusted analysis was 0.01 (–0.54 to 0.56).
Six-minute Walk Test
A statistically significant difference in mean total distance walked in 6 minutes was found between the treatment arms at the 6 months time point (p = 0.0210). The mean difference (95% CI) was 83.23 (13.09 to 153.37) with the no MCP arm walking further on average than the MCP arm.
Number of days in hospital
No significant difference was found in the total number of days spent in hospital (p = 0.4209). The 95% CI for the incidence rate ratio or the ratio of the means was 0.91 to 1.24), indicating that the advice only arm could result in a 24% higher mean number of days in hospital or that the MCP arm could result in a 9% lower mean number of days in hospital.
Subgroup analysis of SGRQ score by sputum volume
Subgroup analyses of the primary outcome measures by baseline sputum volume, split into 15 ml or less versus more than 15 ml, are given in Table 9 and Figures 5 and 6. Neither subgroup analysis was significant.
FIGURE 5.
6-week subgroup analysis of SGRQ by sputum.
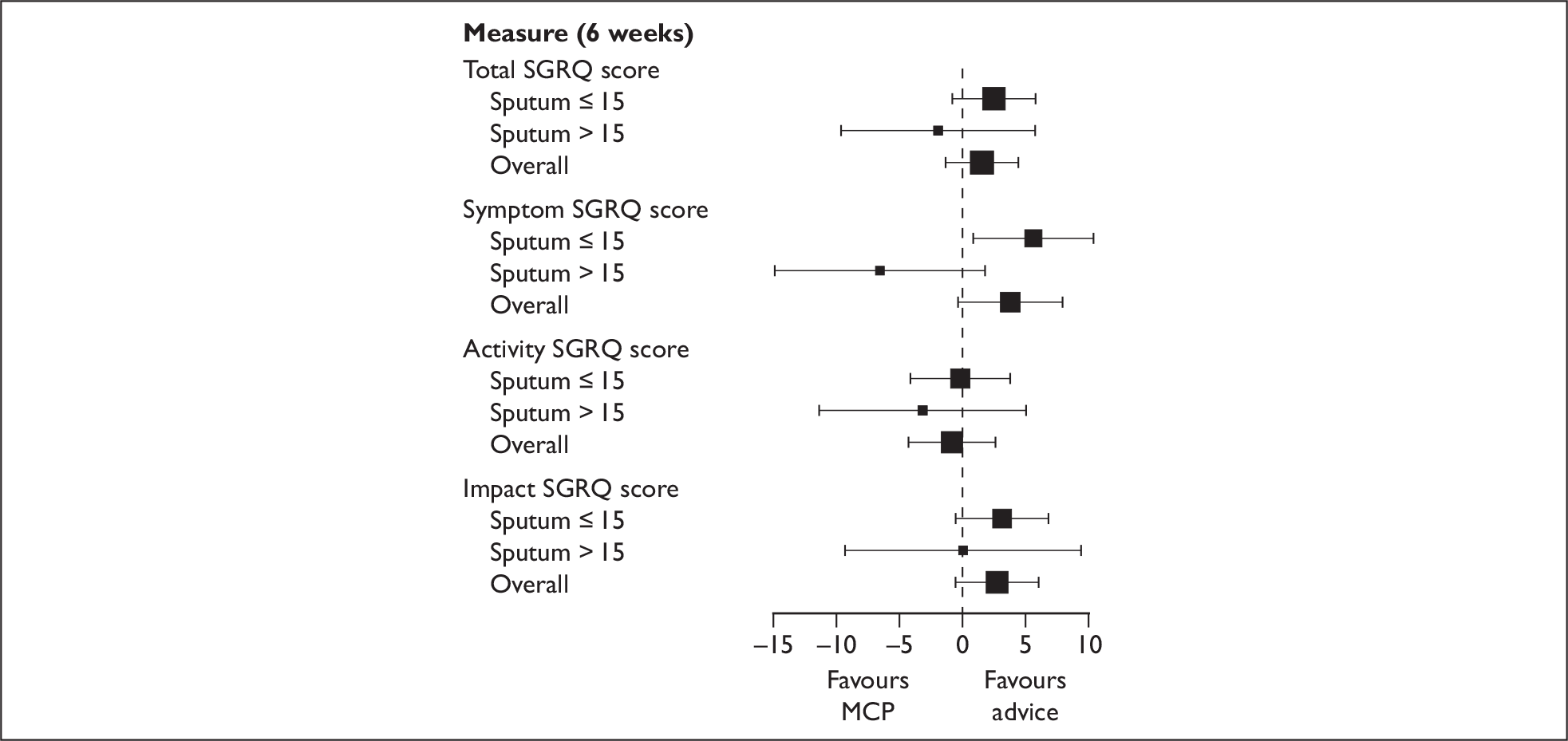
FIGURE 6.
6-month subgroup analysis of SGRQ by sputum.
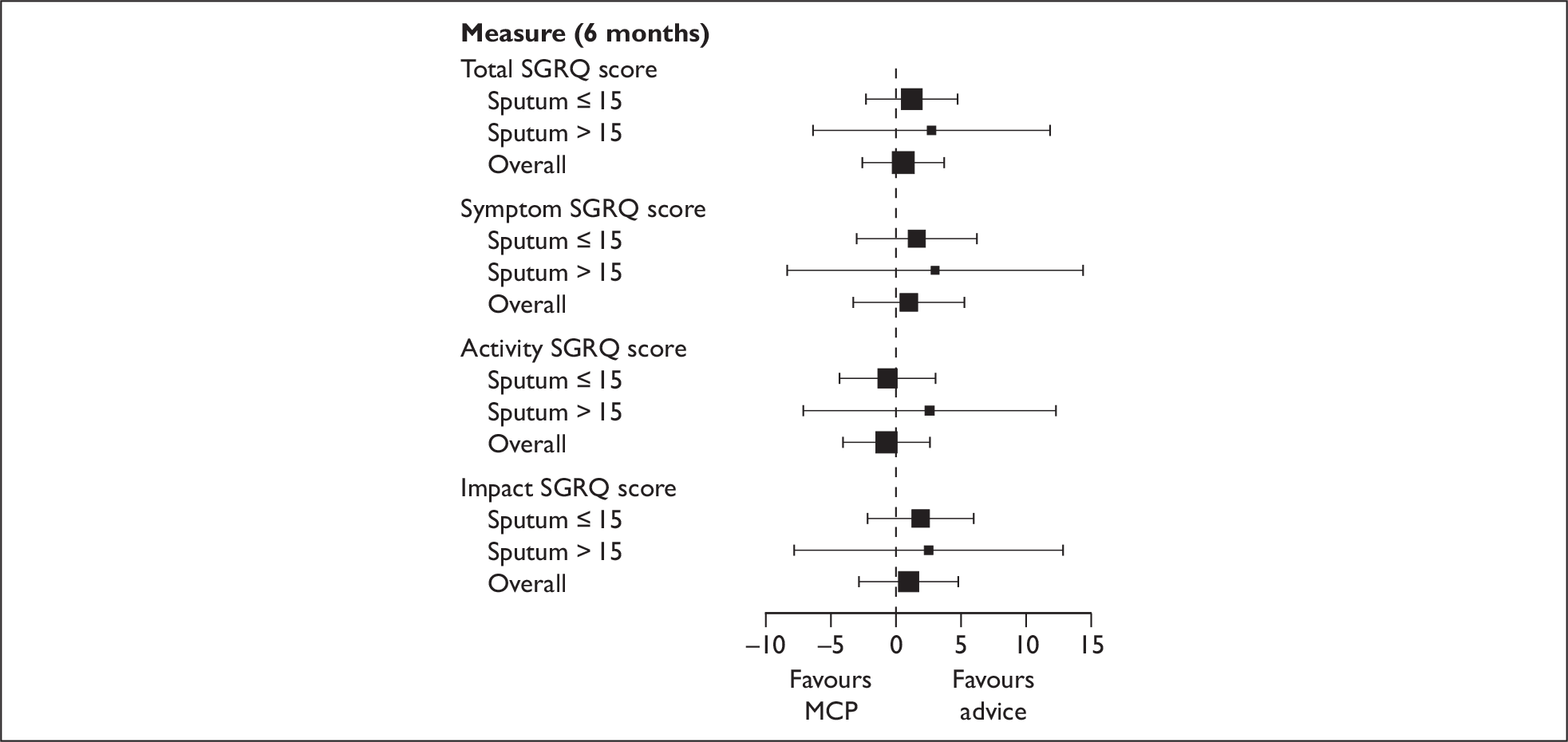
| Outcome | Sputum < 15 ml | Sputum ≥ 15 ml | Interaction p-value | ||
|---|---|---|---|---|---|
| Effect | 95% CI | Effect | 95% CI | ||
| 6 weeks | |||||
| SGRQ total score | 2.41 | –0.89 to 5.72 | –2.00 | –9.72 to 5.72 | 0.348 |
| SGRQ symptom score | 5.57 | 0.85 to 10.29 | –6.51 | –15.01 to 1.99 | 0.209 |
| SGRQ activity score | –0.12 | –4.13 to 3.89 | –3.16 | –11.24 to 4.93 | 0.870 |
| SGRQ impact score | 3.13 | –0.57 to 6.83 | 0.03 | –9.33 to 9.38 | 0.283 |
| 6 months | |||||
| SGRQ total score | 1.11 | –2.38 to 4.59 | 2.62 | –6.47 to 11.70 | 0.932 |
| SGRQ symptom score | 1.57 | –3.03 to 6.17 | 2.97 | –8.28 to 14.23 | 0.951 |
| SGRQ activity score | –0.69 | –4.45 to 3.07 | 2.51 | –7.18 to 12.19 | 0.495 |
| SGRQ impact score | 1.93 | –2.17 to 6.02 | 2.45 | –7.80 to 12.70 | 0.741 |
Per-protocol analyses
The results of the PP analyses are given in Tables 10 and 11.
| MCP arm | No MCP arm | Unadjusted analysis no MCP – MCP |
Adjusted analysisa No MCP – MCP |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | Mean difference | 95% CI | p-value | Mean difference | 95% CI | p-value | |
| 6 weeks | ||||||||||||
| SGRQ total score | 163 | 62.77 | 18.62 | 168 | 63.73 | 9.41 | 0.96 | –3.15 to 5.08 | 0.6448 | 1.60 | –1.37 to 4.58 | 0.290 |
| Effect size | 0.05 | –0.17 to 0.27 | 0.08 | –0.07 to 0.24 | ||||||||
| SGRQ symptom score | 163 | 67.48 | 22.06 | 168 | 69.86 | 22.26 | 2.38 | –2.42 to 7.17 | 0.3302 | 2.90 | –1.27 to 7.06 | 0.173 |
| Effect size | 0.11 | –0.11 to 0.32 | 0.13 | –0.06 to 0.32 | ||||||||
| SGRQ activity score | 166 | 80.90 | 19.05 | 172 | 79.25 | 20.94 | –1.65 | –5.94 to 2.64 | 0.4501 | 0.07 | –3.37 to 3.50 | 0.970 |
| Effect size | –0.08 | –0.30 to 0.13 | 0.00 | –0.17 to 0.17 | ||||||||
| SGRQ impact score | 166 | 50.16 | 21.69 | 172 | 52.02 | 21.91 | 1.86 | –2.80 to 6.53 | 0.4324 | 2.07 | –1.38 to 5.53 | 0.239 |
| Effect size | 0.09 | –0.13 to 0.30 | 0.10 | –0.06 to 0.25 | ||||||||
| 6 months | ||||||||||||
| SGRQ total score | 182 | 63.88 | 18.88 | 184 | 63.40 | 19.73 | –0.48 | –4.45 to 3.49 | 0.8121 | 0.34 | –2.84 to 3.53 | 0.832 |
| Effect size | 0.02 | –0.23 to 0.18 | 0.02 | –0.15 to 0.18 | ||||||||
| SGRQ symptom score | 182 | 68.45 | 23.07 | 184 | 68.36 | 23.10 | –0.09 | –4.83 to 4.66 | 0.9710 | 0.87 | –3.56 to 5.30 | 0.699 |
| Effect size | 0.00 | –0.21 to 0.20 | 0.04 | –0.15 to 0.23 | ||||||||
| SGRQ activity score | 184 | 4.28 | 0.98 | 185 | 4.19 | 1.03 | –1.68 | –5.64 to 2.28 | 0.4040 | –0.46 | –3.90 to 2.99 | 0.795 |
| Effect size | –0.09 | –0.29 to 0.12 | –0.02 | –0.20 to 0.15 | ||||||||
| SGRQ impact score | 184 | 51.52 | 22.31 | 185 | 51.47 | 22.55 | –0.05 | –4.64 to 4.54 | 0.9829 | 0.18 | –3.53 to 3.89 | 0.924 |
| Effect size | 0.00 | –0.21 to 0.20 | 0.01 | –0.16 to 0.17 | ||||||||
| MCP arm | No MCP arm | Unadjusted analysis no MCP – MCP | Adjusted analysisa No MCP – MCP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | Mean difference | 95% CI | p-value | Mean difference | 95% CI | p-value | |
| BCSS score | 158 | 5.43 | 2.70 | 169 | 5.87 | 2.63 | 0.44 | –0.14 to 1.02 | 0.1345 | 0.31 | –0.20 to 0.83 | 0.232 |
| EQ-VAS score | 145 | 52.17 | 20.09 | 151 | 51.83 | 21.89 | –0.33 | –5.15 to 4.47 | 0.8902 | –0.56 | –5.85 to 4.72 | 0.834 |
| EQ-5D score | 165 | 0.48 | 0.32 | 164 | 0.50 | 0.32 | 0.02 | –0.05 to 0.09 | 0.6116 | 0.03 | –0.04 to 0.11 | 0.403 |
| BCSS score | 171 | 5.64 | 2.95 | 178 | 5.63 | 2.83 | –0.01 | –0.62 to 0.60 | 0.9716 | –0.05 | –0.60 to 0.49 | 0.848 |
| 6MWT (metres) | 32 | 174.72 | 109.53 | 20 | 257.95 | 141.15 | 83.23 | 13.09 to 153.37 | 0.0210 | |||
| Number of days in hospitalb | 253 | 16.02 | 16.57 | 260 | 16.85 | 18.11 | 1.05 | 0.90 to 1.23 | 0.5208 | |||
| EQ-VAS | 163 | 51.53 | 20.90 | 171 | 51.93 | 19.54 | 0.40 | –3.95 to 4.75 | 0.8559 | 2.35 | –2.66 to 7.36 | 0.356 |
| EQ-5D score | 206 | 0.48 | 0.34 | 203 | 0.45 | 0.35 | –0.02 | –0.09 to 0.04 | 0.4869 | –0.00 | –0.07 to 0.07 | 0.933 |
Primary outcomes
The results of the PP analyses of primary outcomes were similar to those of the ITT analyses with equivalence being demonstrated for total SGRQ score, SGRQ activity score and SGRQ impact score at 6 weeks and 6 months. Equivalence was also demonstrated for SGRQ symptom score at 6 weeks for both unadjusted and adjusted for baseline and site analyses. However, equivalence was not demonstrated for SGRQ symptom score at 6 weeks in adjusted for baseline and site analysis as the 95% CI for the effect size (–0.07 to 0.32) extended beyond 0.30 SDs.
Secondary outcomes
The results of the PP analyses of secondary outcomes were similar to those of the ITT analyses with no significant differences in scores on EQ-VAS, EQ-5D or BCSS. The results of the 6MWT were identical as the PP and ITT groups did not differ for this outcome.
Health economics analysis
Measuring costs
Baseline health service use
In total, 367 participants completed one or more sections of the baseline cost questionnaire. In Table 12 the number of participants who responded to particular questions are detailed for both the no MCP group (overall n = 264) and the MCP group (overall n = 258), along with either the percentage who reported they had a hospital attendance or the corresponding mean number of visits for those who responded. In retrospect, particular questions within the baseline questionnaire were poorly designed. For example, with regard to question 14 (Appendix 19), if a box was not ticked it was not clear whether a patient did not have a contact or did not answer that particular question. That said, very few participants reported that they had seen any of the listed health professionals (two reported seeing a health visitor and 15 said they had seen a chiropodist or podiatrist). Overall, it can be seen that the use of particular health services in the 3 months prior to randomisation was comparable in both study arms. However, the percentage reporting hospital attendance for COPD or using oxygen at home and the mean number of GP surgery visits that were COPD related was higher in the no MCP group than in the MCP group.
| MCP arm | No MCP arm | |||
|---|---|---|---|---|
| n | % Yes | n | % Yes | |
| Hospital attendance (COPD related) | 181 | 38.7% | 186 | 44.1% |
| Hospital attendance (other reasons) | 168 | 15.5% | 178 | 15.2% |
| Use of oxygen at home | 185 | 22.2% | 185 | 23.8% |
| Mean | Mean | |||
| GP surgery visit (COPD related) | 174 | 2.23 | 178 | 2.35 |
| GP surgery visit (other reasons) | 158 | 0.43 | 158 | 0.37 |
| GP home visit (COPD related) | 170 | 0.64 | 174 | 0.76 |
| GP home visit (other reasons) | 157 | 0.06 | 157 | 0.00 |
| GP telephone consultation (COPD related) | 168 | 0.42 | 169 | 0.83 |
| GP telephone consultation (other reasons) | 157 | 0.08 | 157 | 0.01 |
| Nurse surgery visit (COPD related) | 168 | 0.65 | 164 | 0.51 |
| Nurse surgery visit (other reasons) | 157 | 0.27 | 162 | 0.20 |
| Nurse home visit (COPD related) | 167 | 0.26 | 161 | 0.61 |
| Nurse home visit (other reasons) | 155 | 0.27 | 162 | 0.10 |
| Nurse telephone consultation (COPD related) | 164 | 0.04 | 159 | 0.20 |
| Nurse telephone consultation (other reasons) | 152 | 0.06 | 160 | 0.01 |
Physiotherapy input
Complete data was obtained for all 522 participants who were followed up. At baseline 4 of the 264 in the control arm received MCP, compared with 251/258 in the intervention arm, where the mean hands-on time for those receiving such treatment was 12.25 minutes (range 7–16 minutes) and 12.37 minutes (range 1–41 minutes) respectively. Further MCP follow-up sessions were provided to the same four participants in the control arm (range 1–9 sessions), and to 155 participants in the intervention arm (range 1–16 sessions), where the average total MCP hands-on time associated with all follow-up treatments was equal to 32.75 minutes (range 3–88 minutes) and 28.37 minutes (range 2–260 minutes) respectively. Thus, the per-participant average total MCP hands-on time was equal to 0.68 minutes (range 0–99 minutes) in the control arm, compared with 29.08 minutes (range 0–272) in the intervention arm. After adding a further 10 minutes to each baseline contact and a further 5 minutes to each follow-up session, the mean physiotherapy contact time was estimated to be 10.93 minutes in the control arm, compared with 46.27 minutes in the intervention arm.
Physiotherapy advice and MCP was generally provided by a Band 6 hospital physiotherapist, the average salary for which was £27,120 in 2007/8. 58 Curtis58 estimated that the unit cost per hour of client contact was £40 for a Band 5 hospital physiotherapist. When this unit cost was adjusted to reflect band 6 costs, the unit cost per hour of client contact was estimated to be £44.91. When combined with the aforementioned average physiotherapy contact time the mean cost of the physiotherapy input was estimated to be £8.18 in the control arm, compared with £34.63 in the intervention arm. The mean incremental cost, for those allocated to receive MCP, was thereby estimated to be £26.45 per patient.
Hospital admissions
Complete data was obtained for all 522 participants who were followed up. The mean length of stay (post randomisation) at the initial inpatient admission was 5.31 days in the no MCP arm (range 1–27 days), compared with 5.84 in the MCP arm (range 1–51 days). The mean number of admissions (including the initial visit) in the 6-month trial period was 3.89 for participants in the control arm (range 1–23 admissions), compared with 3.47 in the intervention arm (range 1–28 admissions), where the mean length of stay in each of those admissions was estimated to be 5.04 in the no MCP arm, compared with 5.50 days in the MCP arm. The associated mean total number of days was estimated to be 16.98 in the control arm (range 0–118 days), compared with 15.95 in the intervention arm (range 0–102 days). The Healthcare Resource Group (HRG) codes from the NHS references costs 2006/0759 which were deemed to relate to general respiratory admissions are listed in Table 13 (respiratory neoplasms were considered not to be applicable to this population group). 57
| HRG code | HRG label |
|---|---|
| DZ19A | Other Respiratory Diagnoses with Major CC |
| DZ19B | Other Respiratory Diagnoses with CC |
| DZ19C | Other Respiratory Diagnoses without CC |
| DZ22A | Unspecified Acute Lower Respiratory Infection with Major CC |
| DZ22B | Unspecified Acute Lower Respiratory Infection with CC |
| DZ22C | Unspecified Acute Lower Respiratory Infection without CC |
| DZ21A | COPD or Bronchitis with length of stay 1 day or less, discharged home |
| DZ21B | COPD or Bronchitis with Intubation with Major CC |
| DZ21C | COPD or Bronchitis with Intubation with CC |
| DZ21D | COPD or Bronchitis with Intubation without CC |
| DZ21E | COPD or Bronchitis with NIV without Intubation with Major CC |
| DZ21F | COPD or Bronchitis with NIV without Intubation with CC |
| DZ21G | COPD or Bronchitis with NIV without Intubation without CC |
| DZ21H | COPD or Bronchitis without NIV without Intubation with Major CC |
| DZ21J | COPD or Bronchitis without NIV without Intubation with CC |
| DZ21K | COPD or Bronchitis without NIV without Intubation without CC |
| DZ22A | Unspecified Acute Lower Respiratory Infection with Major CC |
| DZ22B | Unspecified Acute Lower Respiratory Infection with CC |
| DZ22C | Unspecified Acute Lower Respiratory Infection without CC |
| DZ27A | Respiratory Failure with Intubation with Major CC |
| DZ27B | Respiratory Failure with Intubation with CC |
| DZ27D | Respiratory Failure without Intubation with Major CC |
| DZ27E | Respiratory Failure without Intubation with CC |
| DZ27F | Respiratory Failure without Intubation without CC |
| DZ49Z | Respiratory Nurse education/support |
| PA09A | Major Upper Respiratory Tract Disorders with CC |
| PA09B | Major Upper Respiratory Tract Disorders without CC |
| PA10A | Minor Upper Respiratory Tract Disorders with CC |
| PA10B | Minor Upper Respiratory Tract Disorders without CC |
| PA11Z | Acute Upper Respiratory Tract Infection and Common Cold |
| PA14A | Lower Respiratory Tract Disorders without Acute Bronchiolitis with CC |
| PA14B | Lower Respiratory Tract Disorders without Acute Bronchiolitis without CC |
| PA33A | Intermediate Upper Respiratory Tract Disorders with CC |
| PA33B | Intermediate Upper Respiratory Tract Disorders without CC |
| CC, complications; NIV non-invasive ventilation. | |
The estimated weighted average cost per bed-day for both these respiratory-related general admissions and non-respiratory-related admissions are reported in Table 14. Assessment costs were not reported in the NHS reference costs 2006/0759 and we consequently assumed that these were equivalent to the aforementioned average cost per bed-day on a general ward, where assessments were again categorised as either respiratory- or non-respiratory-related. NHS reference costs do not categorise coronary care unit, intensive therapy unit/high-dependency unit, day care, or A&E admissions as either respiratory or non-respiratory related; consequently the same unit cost was applied to both these types of admissions.
| Ward type | Specialty | Cost per bed day (£) |
|---|---|---|
| General | Respiratory | 332.47 |
| Non-respiratory | 422.21 | |
| Assessment | Respiratory | 332.47 |
| Non-respiratory | 422.21 | |
| Day case | Respiratory | 151.07 |
| Non-respiratory | 151.07 | |
| Intensive therapy unit | Respiratory | 1121.11 |
| Non-respiratory | 1121.11 | |
| Coronary care unit | Respiratory | 465.41 |
| Non-respiratory | 465.41 | |
| A&E | Respiratory | 160.95 |
| Non-respiratory | 160.95 |
When these unit costs were assigned with the corresponding length of stay data, it was possible to estimate per-participant hospital admission costs. In the control arm the mean 6-month hospital admission cost was £6075.95 (range £332.47–£40,055.06), compared with £5650.26 (range £332.47–£37,728.11) in the intervention arm, giving an incremental cost of –£425.68 for the MCP arm.
Outpatient visits
Complete data were obtained for all 522 participants who were followed up. Throughout the 6-month trial period 200 of the 264 participants in the control arm had one or more outpatient visits, compared with 201/258 in the intervention arm. Overall the mean number of visits was 2.11 for those in the control group (range 0–13 visits), compared with 2.10 visits in the intervention arm (range 0–17 visits). Curtis58 estimated that the weighted average cost per visit for a first attendance was £55, compared with £71 for a follow-up attendance. It was thereby estimated that the mean 6-month outpatient visit cost was £140.43 in the control arm, compared with £140.69 in the intervention arm, which is equivalent to an incremental cost of £0.25.
Rehabilitation and early discharge service
In total 166 participants were recruited at the hospital providing this service (UHA) and complete data were recorded for each of these participants. Five of the 84 participants in the control group received at least one pulmonary rehabilitation assessment, compared with 4/82 in the intervention group. The mean number of attendances was 0.07 per participant (range 0–2 assessments) in the no MCP arm compared with 0.07 per participant (range 0–2 assessments) in the MCP arm. Pulmonary rehabilitation group sessions were attended by 3/84 in the no MCP arm, compared with 3/82 in the MCP arm. The mean number of sessions was 0.25 per participant (range 0–16 sessions) in the no MCP arm compared with 0.40 per participant (range 0–16 sessions) in the MCP arm. Hospital visits to a hospital physiotherapist were made by 25 of the 84 participants in the no MCP arm compared with 19/82 in the MCP arm, in order to assess their suitability for the early discharge service. The corresponding mean number of hospital visits was 0.54 (range 0–7 visits), and 0.32 (range 0–3 visits) respectively. Each of these 25/84 participants in the no MCP arm and 19/82 in the MCP arm received a subsequent home visit from a Band 6 nurse. The corresponding figures for a hospital physiotherapist were 17/84 and 11/82 respectively. The mean number of home visits was 2.25 for a specialist nurse (range 0–32 visits) in the no MCP arm [hospital physiotherapist = 0.25 (range 0–2 visits)], compared with 1.87 (range 0–29 visits) in the MCP arm [hospital physiotherapist = 0.24 (range 0–5 visits)]. Of those in the no MCP arm 24/84 received at least one telephone contact, compared with 18/82 in the MCP arm. The corresponding mean number of telephone calls per participant was 2.23 (range 0–40) and 1.94 (range 0–35) respectively.
The unit cost of 1 hour patient contact time was estimated to be £44.91 for a physiotherapist (Band 6), £31.16 for a physiotherapist assistant Band 3: average salary £15,678 in 2007/0858), and £49.03 for a Band 6 nurse, where this increased to £57.50 for a home visit by a Band 6 hospital physiotherapist. The cost of a home visit by a nurse was not estimated by Curtis,58 consequently we assumed that the cost of a home visit by a Band 6 nurse was equivalent to that for a Band 6 hospital physiotherapist. The subsequently estimated per-participant cost for each type of contact is shown in Table 15, where this includes a travel cost of £2.60 for each home visit, as estimated by Curtis. 58
| Contact type | Cost per participant contact (£) |
|---|---|
| Pulmonary rehabilitation assessment | 44.91 |
| Pulmonary rehabilitation group sessions | 11.89 |
| Home visit – Band 6 nurse | 45.72 |
| Home visit – hospital physiotherapist | 45.72 |
| Hospital visit – hospital physiotherapist | 89.83 |
| Telephone contact | 4.09 |
In Table 16 the mean cost of each of the above types of rehabilitation are reported for each trial group. The costs are similar in both arms, though the mean cost was slightly higher in the control group in relation to home visits (from a Band 6 nurse) and hospital physiotherapy visits. Thus, the overall rehabilitation cost was £56.54 in the no MCP arm, compared with £44.72 in the MCP arm, giving an incremental cost of –£11.82.
| Contact type | Mean cost per participant (£) | |
|---|---|---|
| No MCP arm | MCP arm | |
| Pulmonary rehabilitation assessment | 1.02 | 1.04 |
| Pulmonary rehabilitation group sessions | 0.95 | 1.52 |
| Home visit – specialist nurse | 32.73 | 27.10 |
| Home visit – hospital physiotherapist | 3.64 | 3.44 |
| Hospital visit – hospital physiotherapist | 15.31 | 9.10 |
| Telephone contact | 2.89 | 2.52 |
Other NHS and PSS costs
In order to estimate the total number of visits over the 6-month trial period, with regard to the other resource use variables listed in the follow-up questionnaire (see Appendix 20), it was necessary for a participant to complete the particular follow-up questions at both the 6-week and 6-month follow-up time point. With regard to the questions concerning A&E visits, GP visits and nurse consultations at the GP practice, the number of participants who fulfilled this task is listed in Table 17. This indicates a high level of missing data with responses available for only approximately half of the participants in each trial arm. However, the unit costs associated with these visits [A&E visit (£161, see Table 14), GP visit (£3258) and nurse consultation (£1158] are relatively small compared with those previously reported for hospital admission costs for which complete data were obtained. Given that the mean number of visits were approximately equal in both arms, we did not attempt to estimate the level of other NHS and PSS costs for each participant. Further difficulties arose with regard to analysing results from this questionnaire in that certain questions returned ambiguous data with respect to zero responses and/or missing data (i.e. when asked to report any contact with social services, if the respondent left the box unticked it was not clear whether there had been no contacts or whether they had failed to answer this particular question). In light of the decision to exclude these costs from the analysis, we did not cross-check them against the relevant hospital/primary care records as specified in the original protocol.
| MCP arm | No MCP arm | |||||
|---|---|---|---|---|---|---|
| n | % | Mean | n | % | Mean | |
| A&E visit | 125 | 48.4 | 1.10 | 137 | 51.9 | 1.36 |
| GP visit | 124 | 48.1 | 4.40 | 140 | 53.0 | 4.98 |
| Nurse consultation | 122 | 47.3 | 2.43 | 136 | 51.5 | 2.74 |
Given that no costs were assigned to other NHS and PSS levels of resource use, the overall health service cost for each participant was estimated by summing the aforementioned specific component costs (i.e. physiotherapy cost, hospital admission cost, outpatient visit cost, rehabilitation cost), where complete data on each of these variables was available for all 522 participants (no imputation was undertaken). The estimated mean costs derived from these four components are given in Table 18. The mean value was estimated to be £6281.10 in the no MCP arm compared with £5870.31 in the MCP arm. Thus the mean incremental overall health service cost of MCP was estimated to be equivalent to a cost saving of £410.79.
| No MCP arm | MCP arm | Incremental cost of MCP | |
|---|---|---|---|
| Physiotherapy cost | £8.18 | £34.63 | 26.45 |
| Hospital admission cost | £6075.95 | £5650.26 | –425.68 |
| Outpatient visit cost | £140.43 | £140.69 | 0.25 |
| Rehabilitation cost | £56.54 | £44.72 | –11.82 |
| Overall health service cost | £6281.10 | £5870.31 | –410.79 |
Measuring effects
Responses to the EQ-5D were as follows. At baseline 401 (76.2%) of the 522 participants completed the EQ-5D (99 pilot phase participants were not asked to complete EQ-5D at baseline). By 6 weeks post-randomisation 25 participants had died and this number rose to 70 at 6 months post-randomisation. Over the 6-month trial period, for the 37 who died in the no MCP arm the date of death was on average 74.89 days post-randomisation (median = 37 days, range 7 to 179 days). The corresponding mean value for the 33 in the MCP arm was 68.30 days post-randomisation (median = 33 days, range 4 to 172 days). Each of these participants was assigned an EQ-5D score of 0.00 from their date of death. A further 309 participants completed the EQ-5D at 6 weeks post-randomisation, compared to 346 at 6 months. Hence, EQ-5D scores were available for 58.7% and 65.8% of participants at 6 weeks and 6 months, respectively (see Table 19).
| MCP arm | No MCP arm | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | n | % | Mean | SD | n | % | |
| Baseline | 0.447 | 0.323 | 199 | 77.1 | 0.428 | 0.356 | 202 | 76.5 |
| 6 weeks | 0.484a | 0.318 | 168 | 65.1 | 0.498d | 0.323 | 166 | 62.9 |
| 6 months | 0.479b | 0.335 | 209 | 81.0 | 0.449e | 0.346 | 207 | 78.4 |
| 6-month QALY gain | 0.003c | 0.149 | 116 | 43.8 | 0.010f | 0.148 | 121 | 45.7 |
After using multiple imputation to estimate missing EQ-5D scores, the mean score at baseline was estimated to be 0.418 in the no MCP arm compared with 0.438 in the MCP arm. At 6 weeks (6 months) these scores were 0.496 (0.439) and 0.507 (0.466) respectively (see Table 20). The mean 6-month QALY gain was estimated to be 0.020 for the no MCP arm compared with 0.018 in the MCP arm, giving an incremental QALY gain of –0.002 for MCP.
| MCP arm | No MCP arm | |
|---|---|---|
| Baseline | 0.438 | 0.418 |
| 6 weeks | 0.507c | 0.496a |
| 6 months | 0.466d | 0.439b |
| 6-month QALY gain | 0.018d | 0.020b |
Response rates for the SGRQ are listed in Figure 4. The mean scores for the SGRQ (both for the total score and each of the three domains) are presented in Tables 21–24, where these are estimated for the 264 participants in the no MCP and the 258 in the MCP arm as missing values were estimated via imputation. These mean values can be seen to be comparable to those based on available data (see Tables 6 and 7). When the 6-month change scores are calculated (see Tables 21–24) it can be seen that, on average, both groups improved post intervention according to both the SGRQ total score and each of the three domains (a negative change score denotes an improvement). However, the mean change was higher for the no MCP group, compared with the MCP group, on the SGRQ activity, impacts and total score. Thus, according to each of these measures, no MCP was estimated to be more effective than MCP, where the mean incremental effect of MCP was estimated to be 0.50 (activity), 0.91 (impact) and 0.89 (total). In contrast, the mean incremental effect on the SGRQ symptoms scale was –0.09 for MCP.
| MCP arm | No MCP arm | |
|---|---|---|
| Baseline | 79.07 | 78.91 |
| 6 weeks | 66.71 | 68.66 |
| 6 months | 67.79 | 67.72 |
| 6-month change | –11.28 | –11.19 |
| MCP arm | No MCP arm | |
|---|---|---|
| Baseline | 84.69 | 83.92 |
| 6 weeks | 80.59 | 78.92 |
| 6 months | 79.19 | 77.92 |
| 6-month change | –5.50 | –6.00 |
| MCP arm | No MCP arm | |
|---|---|---|
| Baseline | 57.61 | 56.59 |
| 6 weeks | 52.03 | 50.44 |
| 6 months | 51.55 | 51.44 |
| 6-month change | –6.06 | –5.15 |
| MCP arm | No MCP arm | |
|---|---|---|
| Baseline | 68.97 | 69.10 |
| 6 weeks | 62.89 | 63.56 |
| 6 months | 63.76 | 62.99 |
| 6-month change | –5.22 | –6.10 |
Cost-effectiveness analysis
As reported above, the incremental cost of MCP was estimated to be equivalent to a mean cost saving of £410.79, and the incremental effect was estimated to be equivalent to a mean QALY loss of 0.002. The resulting incremental net benefit was estimated to be positive for λ values ≤ £237,100.51, which implies that if society was willing to pay ≤ £237,100.51 per QALY gain, then MCP would represent an efficient use of NHS resources as it would enable resources to be freed up and spent elsewhere in a more efficient manner. Indeed the incremental net benefit of MCP was estimated to be £376.14 when λ was equivalent to £20,000 per QALY, suggesting that MCP was cost-effective.
Similar methods were used to estimate the cost-effectiveness of MCP according to the SGRQ total and domain scores. As the λ for each of these measures is unknown, we simply calculated the range of threshold values below which MCP would be deemed cost-effective. As MCP was associated with a cost saving and, compared with no MCP, an improvement in effect on the SGRQ symptoms domain, MCP was estimated to dominate no MCP on to this domain. Conversely, no MCP was more effective than MCP according to the SGRQ activity scores (Table 24), such that the cost saving associated with MCP would mean that MCP was cost-effective if the λ was below £817.62 (Table 25). The similarly calculated λ for the SGRQ impacts and total score are listed in Table 25. Again these can be interpreted such that the implementation of MCP may increase the level of resources that can be spent elsewhere, where the gain in SGRQ associated with these extra resources being spent elsewhere can more than offset any loss associated with implementing MCP.
| Incremental effect | Range of cost-effectiveness for MCP | |
|---|---|---|
| SGRQ symptoms | –0.09 | Dominates no MCP |
| SGRQ activity | 0.50 | λ ≤ £817.62 |
| SGRQ impacts | 0.91 | λ ≤ £450.99 |
| SGRQ total | 0.89 | λ ≤ £464.02 |
Decision uncertainty
The CEAC for each option are plotted in Figure 7. It can be seen that the probability of each option being cost-effective is very similar, and at λ = £20,000 per QALY the probability of MCP being cost-effective was estimated to be 52.6%. Equally, at this value of λ it is estimated that there was a 47.6% chance of making the wrong decision by choosing to implement MCP. This demonstrates that there is a high degree of uncertainty over which is the more cost-effective option.
FIGURE 7.
Decision uncertainty: cost-effectiveness acceptability curves: MCP and no MCP arms.
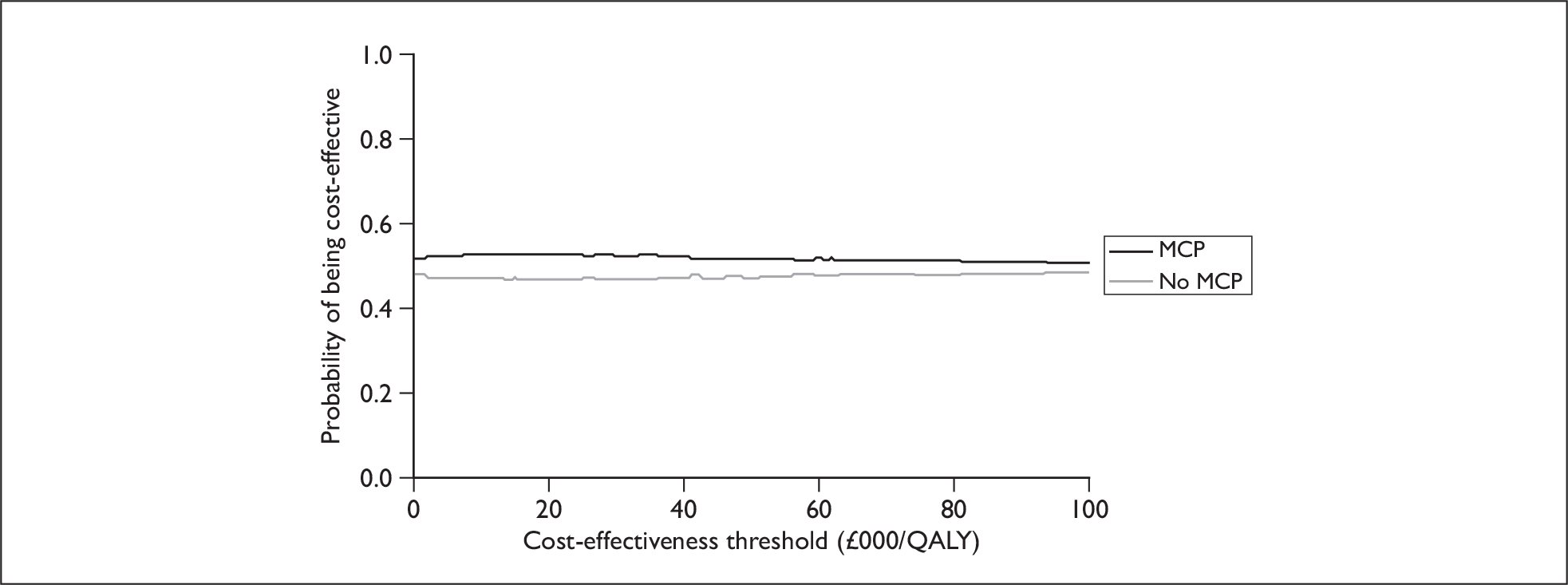
Subgroup analysis
At baseline, sputum levels were measured for 495 of the 522 participants, with rates for the no MCP and MCP arms of 255/264 and 240/258 respectively. Of these, 42 produced ≥ 15 ml of sputum per 24-hour period in the no MCP arm compared with 38 in the MCP arm. The mean overall health service cost for these participants was £5991.80 and £7602.49, respectively, giving an incremental cost of £1610.70 for MCP. The associated QALY gains were estimated to be 0.027 for no MCP and 0.040 for MCP, giving an incremental 6-month QALY gain of 0.013. At a value of = £20,000 per QALY this gave an incremental net benefit of –£1352.73 for MCP, suggesting that MCP was not cost-effective for those who had produced ≥ 15 ml of sputum. This is supported by the associated ICER estimate of £124,874.60 per QALY.
For those producing < 15 ml of sputum, the mean overall health service cost was £6424.00 in the no MCP arm compared with £5730.13 in the MCP arm, giving an incremental cost of –£693.87. Associated QALY gains were estimated to be 0.021 and 0.014, giving an incremental 6-month QALY gain of –0.007 for MCP. At a value of λ = £20,000 per QALY this gave an incremental net benefit of £551.91 for MCP, suggesting that MCP was cost-effective for this subgroup. Indeed the incremental net benefit was estimated to be positive for λ values ≤ £97,754.58, implying that if society was willing to pay this amount per QALY gain, then MCP would represent an efficient use of NHS resources as it would enable them to be freed up and spent elsewhere in a more efficient manner. These results are counter to our a priori expectations.
Sensitivity analysis
Complete cost data was available for all 522 participants who were followed up for analysis purposes. The response rate for the EQ-5D is shown in Table 19, where it can be seen that only 121 in the no MCP arm and 116 in the MCP arm completed the EQ-5D at each of the three follow-up points. The results of the complete case analysis were in line with the base case as compared to no MCP, MCP was estimated to be associated with lower hospital admission costs (Table 26), lower overall health service costs (Table 27), lower quality of life (Table 28) and to be cost-effective at a threshold of λ = £20,000 per QALY (Table 29). Similar results were also achieved when different unit costs were attached to respiratory related admissions, non respiratory-related admissions were excluded from the analysis, and a PP analysis was undertaken (see Tables 25–28). The exception to this was when it was assumed that only physiotherapy time could potentially relate to MCP (analysis 3b), where MCP was shown to be associated with higher health service costs and lower quality of life than no MCP (see Tables 27 and 28 respectively). Here MCP was dominated by no MCP as it was more expensive and less effective.
| MCP arm (£) | No MCP arm (£) | MCP incremental cost (£) | |
|---|---|---|---|
| Base case | 5650.26 | 6075.95 | –425.68 |
| 1. Complete case analysis | 5967.47 | 6334.09 | –366.62 |
| 2a. Unit cost per bed day (COPD specific) | 4890.58 | 5294.24 | –403.66 |
| 2b. Unit cost per bed day (average for NHS) | 6826.93 | 7286.72 | –459.78 |
| 3a. Exclusion of non-respiratory admissions | 4386.58 | 4614.91 | –228.34 |
| 3b. Physiotherapy time costs (only) | – | – | – |
| 4. Per protocol | 5673.89 | 6048.21 | –374.31 |
| MCP arm (£) | No MCP arm (£) | Incremental cost of MCP (£) | |
|---|---|---|---|
| Base case | 5870.31 | 6281.10 | –410.79 |
| 1. Complete case analysis | 6202.23 | 6586.04 | –383.81 |
| 2a. Unit cost per bed day (COPD specific) | 5110.62 | 5499.40 | –388.78 |
| 2b. Unit cost per bed day (average for NHS) | 7046.97 | 7491.87 | –444.90 |
| 3a. Exclusion of non-respiratory admissions | 4606.62 | 4820.07 | –213.45 |
| 3b. Physiotherapy time costs (only) | 34.63 | 8.18 | 26.45 |
| 4. Per protocol | 5893.06 | 6235.72 | –342.67 |
| MCP arm | No MCP arm | Incremental effect of MCP | |
|---|---|---|---|
| Base case | 0.018 | 0.020 | –0.002 |
| 1. Complete case analysis | 0.003 | 0.010 | –0.007 |
| 2a. Unit cost per bed day (COPD specific) | 0.018 | 0.020 | –0.002 |
| 2b. Unit cost per bed day (average for NHS) | 0.018 | 0.020 | –0.002 |
| 3a. Exclusion of non-respiratory admissions | 0.018 | 0.020 | –0.002 |
| 3b. Physiotherapy time costs (only) | 0.018 | 0.020 | –0.002 |
| 4. Per protocol | 0.017 | 0.018 | –0.001 |
| Net benefits at λ = £20,000 per QALY | Range of cost-effectiveness for MCP | |
|---|---|---|
| Base case | £376.14 | λ ≤ £237,100.51 |
| 1. Complete case analysis | £243.12 | λ ≤ £54,561.94 |
| 2a. Unit cost per bed day (COPD specific) | £354.13 | λ ≤ £224,392.74 |
| 2b. Unit cost per bed day (average for NHS) | £396.95 | λ ≤ £64,656.66 |
| 3a. Exclusion of non-respiratory admissions | £178.80 | λ ≤ £123,197.41 |
| 3b. Physiotherapy time costs (only) | –£61.11 | Dominated |
| 4. Per protocol | £410.24 | λ ≤ £256,783.35 |
Chapter 4 Discussion
In this chapter the interpretation, limitations and generalisability of this study will be considered.
Interpretation
Recruitment rate
From the start, recruitment to the study was slower than anticipated. The original project timetable was based on a phased start across three hospitals, each with an average recruitment target of three participants per week. This recruitment rate was derived from mean admission data for the target population at intended sites during 2001–2. However, after 10 months’ screening of all respiratory admissions at two hospitals, the number of COPD cases identified by trial recruiters was lower than predicted from hospital coding data. Study screening indicated that less than 30% of admissions were the result of COPD whereas hospital episode data from previous years suggested a figure nearer 60%. To some extent, this mismatch might be explained by the introduction of early discharge and admission prevention policies implemented since 2001. Adoption of COPD Guidelines49 published in 2004 (which place an emphasis on managing exacerbations in the community where possible) had a further impact upon the feasibility of achieving the original recruitment target.
These combined issues forced the trial management group to re-examine the recruitment strategy in order to complete the study within a reasonable time frame. This process identified UHA as a suitable additional site. Hospital episode statistics (HES) for UHA indicated between approximately 143 and 187 COPD admissions per month (based on 2006–7 figures). Assuming similar levels of exclusions and non-consents as seen at the Norfolk sites, this would yield an additional eight participants per week. However, learning the lessons from recruitment estimates based on HES data at Norfolk sites, an on-site feasibility study was also conducted. This yielded 2 months of admission/discharge data against trial eligibility and indicated that UHA did admit a sufficient number of patients with COPD to substantially boost recruitment. In the event, this site achieved an average recruitment rate of 3.5 participants per week (based on 48 weeks active recruitment during the 12-month recruitment period).
Consent rate
Consent was kept under review by the trial management group and strategies that were employed to maximise this proved successful. Overall a consent rate of 71% would appear excellent for this population group, which was overall elderly and with significant health impairment as judged by SGRQ and EQ-5D scores at baseline.
Movement between study arms
Movement between arms was minimal and was driven by clinical need; however, the strict ‘switch arm’ criteria developed for the protocol were not always strictly adhered to. These criteria were intentionally set at a very significant level of illness,49 and perhaps this was too high for the clinicians to adhere to. The impact of this is minimal on the study; however, it raises important issues with regard to developing satisfactory criteria in the protocol for switching arms and methods of protocol development to ensure that clinicians adhere to these.
Follow-up – losses and response rates
The death rate within this study of 13% is consistent with others reported in the literature. Miravitlles et al. 71 report a death rate of 10.3% during a 2-year follow-up of patients following acute exacerbation of COPD; however, their average SGRQ (total) scores were much lower (better) than those reported the current study. Fruchter72 reports an overall mortality of 22% at the 6-month time point in a study that considered long-term survival in elderly patients with COPD. However, that study population had a minimum age of 65 and a mean of 75.8 compared with 69.33 for this current study. The withdrawal rate from this study was low, but there was a loss to follow-up greater than the 15% predicted at inception. This was despite implementing an effective action plan (Appendix 24).
MCP treatment protocol
The MCP protocol was designed to be as representative as possible of current practice and followed the best available research evidence. Yohannes73 conducted a survey of physiotherapists working in UK acute admitting hospitals regarding their treatment of patients admitted with acute exacerbation of COPD. This asked which physiotherapy treatments they employed and with what frequency: 77% responded that they treated this patient group, with 88% of these reporting using ACBT. A statistically significantly smaller proportion used manual techniques in conjunction with the active cycle (26% vibration, 8% percussion, 11% shaking) always or often; however, 66% still used these techniques sometimes or rarely.
The protocol for this study included ACBT in both trial arms. This was a very useful standardisation as it reflects current practice. For participants randomised to the control arm, the physiotherapist delivered a short training session on the principles of ACBT and explained how this technique could be used to help clear their chest. Participants in the MCP arm were actively guided through at least one session of ACBT with the physiotherapist percussing their chest on expiration. Thus, it could be argued that what was being compared was ACBT plus or minus MCP. However there is a suggestion in the results that perhaps a short teaching session on ACBT is equally effective in terms of quality of life 6 month post intervention as several sessions of ACBT performed with support from the physiotherapist. Therapists could therefore be encouraged to provide ACBT training (or an equivalent airway clearance technique) sooner rather than later particularly in light of the perception that the sputum-rich phase of the disease is early in its course. Current initiatives that delay first admission as long as possible will compound this situation unless there is a change in service provision. Thus, it will be important to communicate any emergent ACBT training message to primary as well as secondary care.
Review of the literature suggests that there is little evidence of efficacy for these MCP techniques and that clinically their use is diminishing while the active cycle remains the treatment of choice. 73 This would appear to be substantiated by the acceptability of the protocol to physiotherapists, and their high level of adherence indicates that the main aim of defining and generalising the intervention was achieved. The one exception was in often selecting one treatment position when two were stipulated (248 sessions, 38%). However, it is important that treatments are tailored in response to findings on clinical assessment, and clinical expertise indicates that it is likely that treatment conducted with the patient seated is equally efficacious when using the ACBT in this group of patients. Additionally there may have been no clinical rationale for treating in more than one position, for example if there was clinical evidence of a unilateral lung problem.
Changes to protocol
Some changes to the protocol were made at the recommendation of the TSC; these included the recording of an INR value within prespecified limits as an inclusion criterion. This was to reassure clinicians that there was little chance that a pulmonary embolus was part of the presenting clinical picture. The TSC considered a similar scenario that had arisen with regard to patients on oral steroids being excluded from the study. There is no evidence that MCP is contraindicated for patients on prophylactic bone protection. Even where osteoporosis exists there is little research evidence to suggest a likelihood of percussion and vibration causing rib damage, although clinically this is considered a contraindication. Therefore, while it was considered reasonable to exclude patients with overt osteoporosis, it was not deemed necessary to extend this to those at risk of the disease.
Baseline characteristics
This study demonstrated higher than average SGRQ scores at baseline. It would appear that these were approximately 5–10 points higher than reported by other studies on similar populations. 71,74 This perhaps reflects recent improvements in treatment (i.e. bronchodilators and steroids) that keep people out of hospital for longer. Anecdotal evidence suggests that there is an increasing trend for admitted patients to be very sick with end-stage disease and multiple comorbidities. In addition, the average age for patients admitted with COPD has also increased. These factors are, however, balanced at baseline by the randomisation process, giving excellent comparability.
Oxygen saturation
Manual chest physiotherapy has been associated in the literature with clinically significant falls in oxygen saturation. 14 The results from this study indicate that almost half the sessions of MCP resulted in a fall in oxygen saturation (268; 41%). However, 258 (39%) sessions resulted in no change in oxygen saturation and a further 126 (19%) sessions resulted in an increase in oxygen saturation. This raises the possibility that de-saturation is happening more often than previously reported. This is possibly due to the heterogeneous patient groupings and/or small sample size in these studies. Interpretation of these results is difficult because MCP did not occur in isolation. Therapists were required to choose positions in which to administer the treatment, and hence these changes could result from position changes altering V/Q ratios as much as from the MCP itself. It is however interesting to note the high frequency of falling oxygen saturation and this might be an indication for the routine use of oxygen saturation monitoring during physiotherapy treatment. It should be noted that the SGRQ scores of this group indicate a significant level of impairment and these factors may be related. Hence this may be considered an important finding as people hospitalised with COPD are now increasingly likely to be in end-stage disease and there is little robust information to guide clinicians on the risk of significant de-saturation in this patient group. Importantly, clinically significant falls in oxygen saturation were recorded as AEs and the rate of these is very small. Details can be found in Table 5.
MCP treatment efficacy
The primary outcome of this study was to find equivalence in total SGRQ between the intervention and the control group 6 months after intervention. This result suggests that there is no gain in quality of life when including MCP in the physiotherapy management of acute exacerbation of COPD. The difference in total SGRQ at 6 months, after adjusting for baseline, was 0.51 (–2.67 to 3.69) which is within the prespecified limits of equivalence stated in the protocol. This also excludes the minimal clinically important difference (MCID) of 4, as suggested by Jones et al.,75,76 although the trial was not powered to demonstrate equivalence by the MCID. The differences in SGRQ subscores at 6 months were again within the prespecified limits of equivalence stated in the protocol: the difference in symptom score was 0.87 (–3.50 to 5.25), activity score –0.36 (–3.76 to 3.04) and impact score 0.43 (–3.29 to 4.14). Thus the MCID is not excluded from the symptom score or the impact score, but is excluded from the activity score.
In the short-term time point, 6 weeks after intervention, the difference in total SGRQ score was 1.61 (–1.33 to 4.55) which was within the prespecified limits of equivalence stated in the protocol. However, it does not exclude the MCID but does exclude an effect greater than 4.55 with 95% confidence. The difference in SGRQ subscores at 6 weeks was mixed, with equivalence not being demonstrated for symptom score with a difference of 3.12 (–1.00 to 7.25), which exceeds our definition of equivalence by 3% of a SD, but being demonstrated for activity score with a difference of –0.16 (–3.55 to 3.23) and impact score with a difference of 2.12 (–1.30 to 5.53). There were no major differences from the ITT analysis with either the imputed ITT analysis or the PP analysis.
The secondary outcome measures included BCSS, EQ-VAS, EQ-5D utility score, number of days in hospital and the 6MWT. The BCSS difference at 6 months was 0.01 (–0.54 to 0.56) demonstrating no significant difference and equivalent to with 0.56, i.e. almost half a point. At 6 weeks the difference was 0.33 (–0.18 to 0.84), suggesting equivalence to within 1 point on the scale. The EQ-VAS difference at 6 months was 2.65 (–2.35 to 7.65) on a scale of 0 to 100, suggesting no large difference, and at 6 weeks the difference was –0.68 (–5.90 to 4.55). The difference in EQ-5D utility score at 6 months was –0.01 (–0.07 to 0.06) on a scale of 0 to 1, implying no large differences; similarly at 6 weeks the differences was 0.03 (–0.04 to 0.10). The difference in the number of nights in hospital during the 6 months post intervention was not significant with the ratio of means (non-MCP/MCP) being 1.07 (0.91 to 1.24), suggesting that on average the non-MCP group spent 7% longer in hospital. Not providing MCP could increase the number of days in hospital by 24% or, alternatively, providing MCP could increase the number of days in hospital by 9%. A difference in the 6MWT was found (p = 0.0210) with the non-MCP arm walking further on average than the MCP arm. However, this was only available for 52 individuals in one centre and therefore results were statistically underpowered with limited ensuing generalisablity. There were no major differences from the ITT analysis with either the imputed ITT analysis or the PP analysis.
Subgroup analyses showed no evidence that the effectiveness of MCP differed by baseline sputum levels in terms of SGRQ or its subscores at either 6 months or 6 weeks. However, it should be noted that the study was not sufficiently statistically powered to detect a difference in effect by subgroup.
Cost-effectiveness
As NHS resources are relatively fixed one has to assess the impact that providing MCP or no MCP would have, both in terms of overall costs and benefits. Provision of MCP did not improve overall quality of life. However, it was associated with lower overall health service costs, compared with no MCP, as the cost of providing MCP was offset by lower hospital admissions costs. Although there is much uncertainty over which is the more cost-effective option (see Figure 7), economists would argue that decisions as to which option is most efficient have to be made on the basis of available evidence. 65,77 Moreover, in contrast to the classic statistical approach, it is generally accepted within health economics that it is the mean estimate that is of interest to policy makers78,79 where, assuming one seeks to maximise health subject to a budget constraint, this equates to choosing the option that has the most favourable cost-effectiveness ratio. 66 Our mean estimates suggest that provision of MCP would reduce overall costs, and thereby enable resources to be spent elsewhere. Moreover, as the health benefits provided by those extra resources are likely to more than offset any loss in quality of life that may be associated with provision of MCP, rather than no MCP, this would suggest that MCP represents a cost-effective use of resources. Additionally, a number of sensitivity analyses were conducted, which generally suggested that these results were robust to the assumptions we made within our analysis.
Limitations
Subgroup analysis of > 15ml sputum
The preplanned subgroup analysis for patients producing more than 15 ml of sputum per day demonstrated equivalence. It should however be noted that this finding is limited by the number of participants who met this criteria and the sample size was statistically underpowered. This small subset is probably consistent with patients at the end stage of their disease, substantiated by their very poor SGRQ scores. This patient group is more likely to have stopped smoking with a consequential reduction in inflammatory lung response. It is suggested that overproduction of sputum is most apparent in the prediagnosis phase of COPD and that sputum production per se is not a headline diagnostic feature of the disease. 2
Cost-effectiveness
The plausibility of the above result does depend upon whether MCP was truly associated with lower hospital admission costs, or whether this result occurred by chance. In terms of explanations, we did not find that MCP was associated with shorter hospital stays (mean = 5.50 days, compared with 5.04 with no MCP), but rather that the lower costs seemed to arise because of fewer hospital admissions (mean = 3.47 for MCP, compared with 3.89 for no MCP). Moreover, as MCP was actually associated with a (non-significant) loss in quality of life we cannot explain why hospital admission costs were lower in the MCP group. Indeed, although the baseline characteristics were similar in the two groups, we cannot rule out the possibility that hospital admission costs were lower in the MCP groups owing to the presence of fewer comorbidities (i.e. that hospital admission costs would have been lower for this group) even if MCP had not been provided. This argument is partially supported by the fact that the mean level of quality of life at baseline was estimated to be lower for those with no MCP (0.418), compared with those with MCP (0.438) (see Table 16). Moreover, at baseline, the percentage reporting a hospital attendance for COPD and the mean number of GP surgery visits that were COPD related was also higher in the no MCP group, compared with the MCP group. The uncertainty of our results is further supported by the fact that MCP was estimated to be more cost-effective for those with lower levels of sputum, which was counter to our a priori expectations. Further evidence of the difficulty in explaining variation in hospital admission costs is provided by Wong et al. 80 who suggest that, in addition to disease severity, the number of comorbidities, social factors such as marital status and the need for social work intervention are also linked to readmission rates and length of stay of patients with acute exacerbation of COPD.
With regard to the economic methods, it is acknowledged that there are no a priori guidelines about how much data is sufficient to collect within cost-effectiveness studies, and no data as to the incremental value of collecting specific cost items. 81 As a result, the general recommendation is to focus on: (1) high-cost services that are likely to make up a high proportion of the total cost; and (2) those services that are likely to account for a large proportion of the difference in costs between the two interventions in question. 47 Within this study we implemented these recommendations by monitoring the costs associated with the intervention (MCP), hospital admissions, outpatient visits and levels of rehabilitation. However, in line with other economic studies,82–88 we did not monitor medication costs. Thus this constitutes one potential limitation, along with the fact that oxygen use at home was not monitored for the duration of the study. Similarly, though we advanced upon certain economic studies that did not monitor baseline levels of resource use,76,77 there were limitations with regard to the baseline questionnaire in that it did not request sufficient information for one to assign a unit cost to each hospital attendance. Moreover, there were also deficiencies in that it was difficult to differentiate as to whether no response to certain questions meant that a participant had not used the service in question, or whether they had not completed the respective question (i.e. data was missing). That said, with regard to the resources that were monitored for the duration of the study we did manage to collect complete data for each participant, something which is rarely achieved in an economic analysis.
One further aspect to note is that the costs and benefits of both MCP and no MCP were only estimated over the 6-month trial period. It should therefore be noted that had, for example, a lifetime perspective been taken, the results might have been quite different. We chose not to extrapolate beyond the 6-month trial period as, for the reasons outlined above, we consider it to be unclear as to whether MCP was truly associated with lower hospital admission costs. Similarly, threshold analysis47 was not undertaken as we consider there to be uncertainty as to whether MCP was truly associated with a cost-saving or a loss in quality of life.
Generalisibility
Initially this study could have had a limited generalisability as the catchment characteristics could have led to a charge of its being representative of a rural, relatively affluent patient population. However the study’s generalisability was greatly broadened by the inclusion of UHA and the balanced recruitment that was achieved from the variety of sites.
The study’s pragmatic stance, adopted throughout the trial, means the results of the MATREX trial have a high degree of generalisibility.
Chapter 5 Conclusions
Implications for healthcare
-
Owing to a clear state of clinical equipoise as to whether MCP confers any benefit to patients with COPD, current UK guidelines for its management do not propose a clear place for MCP techniques. This study addressed the limitations of previous research by standardising the delivery of MCP and obtaining a sample of sufficient size to derive statistically robust results for a patient-orientated, clinically meaningful outcome.
-
This study found no gain in longer-term quality of life when MCP was included in the physiotherapy management of acute exacerbation of COPD. However, the findings do not mean that MCP is of no therapeutic value to patients with COPD in specific circumstances.
-
This study found that MCP was associated with lower overall health service costs, with the cost of providing therapy offset by savings associated with fewer hospital admissions among patients assigned to receive MCP. However, interpretation of this apparent saving should be examined in the light of the primary outcome, which demonstrated no evidence of efficacy above normal care. In light of this we consider that, as MCP was not found to be effective, it is difficult to justify providing MCP on the basis of the results of the cost-effectiveness analysis alone. Furthermore, there is no guarantee that any cost savings identified would be realised by employing MCP in routine care.
-
In order to standardise treatment given during the course of this study, an MCP treatment protocol was developed in collaboration with physiotherapists involved in the trial. This protocol reflects professional consensus on best practice with respect to the essential elements of MCP, clarifies potential areas of ambiguity and provides a set of clear parameters within which treatment can be given. The high level of adherence to the MCP treatment protocol used in this trial suggests that it would be acceptable among the profession as a generic tool for delivering therapy.
Recommendations for research
With respect to the primary aim of the MATREX trial, further research is not required to demonstrate equivalence between receiving and not receiving MCP. Further research on the level of cost-effectiveness is unlikely to yield gains as the benefits of both MCP and no MCP are similar, and thus the consequences of making the wrong decision are small. As such, the cost of further research is likely to outweigh the value of information that would be gained. However, the findings of this study do not mean that MCP is of no therapeutic value to COPD patients in specific circumstances. Research questions arising from this study are listed below in order of priority.
Is MCP effective for COPD patients producing high volumes of sputum?
While the subgroup analysis for patients producing more than 15 ml of sputum per day demonstrated equivalence, the significance of this finding is limited by the number of participants who met this criteria and is thus statistically underpowered. Given that overproduction of sputum is most apparent among patients with COPD early in the course of their disease history, staging the intervention in the primary care setting may overcome the difficulties this study experienced with sample size.
Can the risk of oxygen desaturation during MCP be predicted?
The results from this study indicate that almost half the sessions of MCP resulted in a fall in oxygen saturation from baseline. This raises the possibility that desaturation is happening more often than previously reported. Given that people hospitalised with COPD are increasingly likely to be in end-stage disease, there is little robust information to guide clinicians on the risk of significant desaturation in this patient group. Examining SGRQ BCSS and MRC-D scale as predictors of oxygen desaturation during therapy interventions may provide useful information for clinical decision-making.
Is ACBT effective in treating COPD exacerbation?
The protocol for this study included ACBT in both trial arms. Thus, to some extent what was being compared was ACBT plus or minus MCP. There is a need to formalise this emergent element of the MATREX study design and examine the effectiveness of ACBT in isolation. There is also an opportunity to examine the mode of delivery of ACBT. Results from this study suggest that a short teaching session on ACBT might be equally effective in terms of quality of life 6 months post intervention as several sessions of ACBT performed with support from the physiotherapist. Given recent trends in hospital admissions for COPD, future research regarding physiotherapy intervention with this patient population should focus on examining the effectiveness of ACBT taught in primary care settings.
What are the trends over time in admission and survival rates for COPD?
This study’s high attrition rate between screening and recruitment (over 7000 respiratory admissions screened to yield 526 participants) suggests caution against over-reliance on hospital coding to identify eligible patients. This is an important principle to pass on to future studies when calculating potential recruitment rates. In particular, the changing nature of COPD treatment pathways has meant that extrapolating historical admission rates is liable to overinflate the number of patients available. Extending the study of this cohort of COPD patients as a longitudinal design would produce important data regarding admission rates and survival. There is also the potential to map SGRQ and/or EQ-5D to other instruments as a predictor of outcome. In particular, the DOSE index is a simple valid tool for assessing the severity of COPD. The index is derived from the MRC-D score, airflow Obstruction, Smoking status and Exacerbations; it is related to a range of clinically important outcomes such as health-care consumption and has the capability to predict future events. 89
How can health-related resource use be more accurately identified?
There is a need to develop robust instruments to identify health-related resource use for specific patient groups. Within this study there were deficiencies in the COPD cost questionnaire relating to non-acute NHS and PSS levels of resource use. Specifically, it was difficult to conclude whether no response to certain questions meant that a participant had not used the service in question or whether they had not completed the respective question (i.e. data was missing). Future studies might overcome this by inserting a ‘Not used’ option for particular questions.
Acknowledgements
The Trial Steering Committee is thanked for their support throughout the project. This committee comprised David Price (Professor of Primary Care, University of Aberdeen), who chaired the committee, and study site lead investigators and physiotherapists, whose input was invaluable in achieving project aims. These included: Simon Watkin (Consultant Physician) and Rachel Ellis (Superintendent Physiotherapist) from the Norfolk and Norwich Hospital University Hospital; David Ellis (Consultant Physician) and Rachel Matthews (Senior Respiratory Physiotherapist) from the James Paget Hospital; Anna Pawlowicz (Consultant Physician) and Julia Kerrigan (Senior Physiotherapist) from the Queen Elizabeth Hospital; and, Robert Angus (Consultant Physician) and Verity Ford (Senior Respiratory Physiotherapist) from University Hospital Aintree. Thanks also go to committee members: Judy Close (Independent NHS Advisor for Allied Health Professions), Sandra Olive and Paula Browne (Respiratory Nurse Specialists) and Katherine Jones (Research and Development Manager) from the Norfolk and Norwich University Hospital.
We also wish to thank members of the Data Monitoring and Ethics Committee, namely Richard Lilford (DMEC Chairperson and Professor of Clinical Epidemiology, University of Birmingham), Jennifer Pryor (Senior Research Fellow in Physiotherapy, Royal Brompton and Harefield NHS Trust) and Michael Roughton (Medical Statistician, Imperial College London).
Our thanks to Helene Talbot, Charlotte Minter and Kim Clipsham (Research Associates, University of East Anglia) who recruited trial participants, to Ric Fordham (Senior Lecturer in Health Economics, University of East Anglia) who designed the non-validated health economics questionnaire, and to Tony Dyer (Research Fellow, University of East Anglia) for support with database design and management.
The MATREX Research Group
Dr Simon Watkin, Site Lead Investigator, Norfolk and Norwich University Hospital, Norwich, UK
Dr David Ellis, Site Lead Investigator, James Paget Hospital, Great Yarmouth, UK
Dr Anna Pawlowicz, Site Lead Investigator, Queen Elizabeth Hospital, King’s Lynn, UK
Dr Robert Angus, Site Lead Investigator, University Hospital Aintree, Liverpool, UK
Rachel Ellis, Site Physiotherapy Lead, Norfolk and Norwich University Hospital, Norwich, UK
Rachel Mathews, Site Physiotherapy Lead, James Paget Hospital, Great Yarmouth, UK
Julia Lodge (née Kerrigan) Site Physiotherapy Lead, Queen Elizabeth Hospital, King’s Lynn, UK
Verity Ford, Site Physiotherapy Lead, University Hospital Aintree, Liverpool, UK
Contribution of authors
The Project Management Group met throughout the project and comprised the authors contributing to this report. Jane Cross (Senior Lecturer in Respiratory Physiotherapy) was the Chief Investigator and grant holder and had overall responsibility for the integrity of the work as a whole. Frances Elender (Senior Research Associate) was responsible for trial management, compiling the treatment protocol and drafting the final report. Garry Barton (Lecturer in Health Economics) had overall responsibility for economic evaluation and its reporting. Allan Clark (Lecturer in Medical Statistics) carried out and reported on the efficacy analysis. Lee Shepstone (Professor of Medical Statistics) had overall responsibility for statistical elements of the study protocol and implementation of the efficacy analysis. Annie Blyth (Research Associate) designed and implemented recruitment strategies, drew up hospital resource use data collection instruments and drafted treatment elements of the final report. Max Bachmann (Professor of Health Care Interfaces) and Ian Harvey (Professor of Epidemiology and Public Health) contributed substantially to the study design, interpretation of results and revising the final report for important intellectual content. All authors read and approved the final manuscript.
Publications
Sauerzapf V, Jones AP, Cross J. Environmental factors and hospitalisation for chronic obstructive pulmonary disease in a rural county of England. J Epidemiol Community Health 2009;63:324–8.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- COPD prevalence. Bandolier 2003;113.
- The National Collaborating Centre for Chronic Conditions . Chronic Obstructive Pulmonary Disease. National clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care. Appendix D: Economic costs of COPD to the NHS. Thorax 2004;59:192-4.
- Chief Medical Officer . On the State of the Public Health: Annual Report of the Chief Medical Officer of the Department of Health 2004 2005.
- Dixon J, Damiani M. COPD medical admissions in the UK, 2000/01–2001/02. London: The King’s Fund; 2004.
- Tydeman DE, Cross JL. Respiratory physiotherapy manual techniques: Review of the literature. Physiotherapy 2001;87.
- Wollmer P, Ursing K, Midgren B, Eriksson L. Inefficiency of chest percussion in the physical therapy of chronic bronchitis. Eur J Respir Dis 1985;66:233-9.
- Mazzocco MC, Owens GR, Kirilloff RN, Rogers RM. Chest percussion and postural drainage in patients with bronchiectasis. Chest 1985;88:360-3.
- Webber BA, Parker RA, Hofmeyr J, Hodson M. Evaluation of self percussion during postural drainage using the forced expiration technique. Physiother Pract 1985;1:42-5.
- Sutton PP, Lopez-Vidriero MT, Pavia D, Newman SP, Clay MM, Webber B, et al. Assessment of percussion, vibratory-shaking and breathing exercises in chest physiotherapy. Eur J Respir Dis 1985;66:147-52.
- Van Der Schans CP, Piers DA, Posta DS. Effect of manual percussion on tracheobronchial clearance in patients with chronic airflow obstruction and excessive tracheobronchial secretion. Thorax 1986;41:448-52.
- Rossman CM, Waldes R, Sampson D, Newhouse MT. Effect of chest physiotherapy on the removal of mucus in patients with cystic fibrosis. Am Rev Respir Dis 1982;126:131-5.
- Brown PM, Manfreda J, McCarthy DS, MacDonald S. The effect of mechanical vibration in patients with acute exacerbations of chronic obstructive pulmonary disease. Physiother Can 1987;39:371-4.
- Gallon A. Evaluation of chest percussion in the treatment of patients with copious sputum production. Respir Med 1991;85:45-51.
- Connors A, Hammon W, Martin R, Rogers R. Chest physical therapy: the immediate effect on oxygenation in acutely ill patients. Chest 1980;78:559-64.
- De Boeck C, Zinman R. Cough versus chest physiotherapy. Am Rev Respir Dis 1984;129:182-4.
- Campbell A, O’Connell J, Wilson F. The effect of chest physiotherapy upon the FEV1 in chronic bronchitis. Med J Aust 1975;1:33-5.
- Newton D, Stephenson A. Effect of physiotherapy on pulmonary function. Lancet 1978;2:228-30.
- May D, Munt P. Physiologic effects of chest percussion and postural drainage in patients with stable chronic bronchitis. Chest 1979;75:29-32.
- Feldman J, Traver GA, Taussig LM. Maximal expiratory flows after postural drainage. Am Rev Respir Dis 1979;119:239-45.
- Rivington-Law BA, Epstein SW, Thompson GL, Pulm R, Corey PN. Effect of chest wall vibration on pulmonary function in chronic bronchitis. Chest 1984;85:378-81.
- Bateman J, Daunt K, Newman S, Pavia S, Clarke S. Regional lung clearance of excessive bronchial secretions during chest physiotherapy in patients with stable chronic airways obstruction. Lancet 1979;1:294-7.
- Bateman J, Newman S, Daunt K, Sheahan N, Pavia D, Clarke S. Is cough as effective as chest physiotherapy in the removal of excessive tracheobronchial secretions?. Thorax 1981;36:683-7.
- Buscaglia AJ, St. Marie MS. Oxygen saturation during chest physiotherapy for acute exacerbation of severe chronic obstructive pulmonary disease. Respir Care 1983;28:1009-13.
- Holland A, Button B. Is there a role for airway clearance techniques in chronic obstructive pulmonary disease?. Chron Respir Dis 2006;3:83-91.
- McCool FD, Rosen MJ. Nonpharmacologic airway clearance therapies: ACCP evidence-based clinical practice guidelines. Chest 2006;129:250S-259S.
- Garrod R, Lasserson T. Role of physiotherapy in the management of chronic lung diseases: an overview of systematic reviews. Respir Med 2007;101:2429-36.
- National Service Frameworks COPD n.d. http://www.dh.gov.uk/en/Healthcare/NationalServiceFrameworks/COPD/DH_085153 (accessed 25 April 2009).
- O’Reilly J, Williams AE, Ledger G, Rice L. Health Utility Burden of Exacerbation in COPD Requiring Admission to Hospital As Measured by EQ-5D n.d.
- Stahl E. Health-related quality of life, utility, and productivity outcomes instruments: ease of completion by subjects with COPD. Health Qual Life Outcomes 2003;1.
- Singh SJ, Sodergren SC, Hyland ME, Williams J, Morgan MD. A comparison of three disease-specific and two generic health-status measures to evaluate the outcome of pulmonary rehabilitation in COPD. Respir Med 2001;95:71-7.
- Doll H, Miravitlles M. Health-related QOL in acute exacerbations of chronic bronchitis and chronic obstructive pulmonary disease: a review of the literature. Pharmacoeconomics 2005;23:345-63.
- Gudmundsson G, Gislason T, Lindberg E, Hallin R, Ulrik CS, Brondum E, et al. Mortality in COPD patients discharged from hospital: the role of treatment and co-morbidity. Respir Res 2006;7.
- Jones P, Calverley P, Larsson T, Peterson S. St George’s Respiratory Questionnaire (SGRQ) Scores May Help Identify COPD Patients at Increased Risk of Death over 1 Year n.d.
- Yohannes AM, Baldwin RC, Connolly MJ. Predictors of 1-year mortality in patients discharged from hospital following acute exacerbation of chronic obstructive pulmonary disease. Age Ageing 2005;34:491-6.
- Bausewein C, Farquhara M, Booth S, Gysels M, Higginson IJ. Measurement of breathlessness in advanced disease: a systematic review. Respir Med 2007;101:3339-410.
- Leidy NK, Schmier JK, Jones MKJ, Lloyd J, Rocchicciou K. Evaluating symptoms in chronic obstructive pulmonary disease: validation of the Breathlessness, Cough and Sputum Scale. Respir Med 2003;97:59-70.
- Dolan P. Modelling valuations for EuroQol health states. Med Care 1997;35:1095-108.
- Harper R, Brazier JE, Waterhouse JC, Walters SJ, Jones NM, Howard P. Comparison of outcome measures for patients with chronic obstructive pulmonary disease (COPD) in an outpatient setting. Thorax 1997;52:879-87.
- Rutten-van Mölken PMH, Oostenbrink JB, Tashkin DP, Burkhart D, Monz BU. Does Quality of life of COPD patients as measured by the generic EuroQoL five-dimension questionnaire differentiate between COPD severity stages?. Chest 2006;130:1117-28.
- Cecins NM, Jenkins SC, Pengelley J, Ryan G. The active cycle of breathing techniques – to tip or not to tip?. Respir Med 1999;93:660-5.
- Sutton PP, Parker RA, Webber BA, Newman SP, Garland N, Lopez-Vidriero MT, et al. Assessment of the forced expiration technique, postural drainage and directed coughing in chest physiotherapy. Eur Respir J 1983;64:62-8.
- Medical Research Council, Committee on the Aetiology of Chronic Bronchitis . Standardised questionnaires on respiratory symptoms. Br Med J 1960;2.
- Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999;54:581-6.
- Wedzicha JC, Paul EA, Garrod R, Garnham R, Jones PW. Randomized controlled trial of pulmonary rehabilitation in severe chronic obstructive pulmonary disease patients, stratified with the MRC dyspnoea scale. Eur Respir J 1999;54:581-6.
- Butland RJA, Pang J, Gross ER, . Two, Six and Twelve Minute Walking Tests. Br Med J 1982;284:1607-8.
- Solway S, Brooks D, Lacasse Y, Thomas S. A qualitative systematic overview of the measurement properties of functional walk test used in the cardiorespiratory domain. Chest 2001;119:256-70.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brian BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. New York, NY: Oxford University Press; 2005.
- Barton GR, Sach T, Jenkinson C, Avery A, Doherty M, Muir KR. Do estimates of cost–utility based on the EQ-5D differ from those based on the mapping of utility scores?. Health Qual Life Outcomes 2008;6.
- National Institute for Clinical Excellence (NICE) . Chronic obstructive pulmonary disease: national clinical guideline for management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax 2004;59:1-232.
- American Association for Respiratory Care (AARC) . Clinical practice guideline, postural drainage therapy. Respir Care 1991;36:1418-26.
- Thoracic Society . ATS Statement: Guidelines for the Six-minute Walk Test. Am J Respir Crit Care Med 2002;166:111-17.
- White RJ, Rudkin ST, Harrison ST, Day KL, Harvey IM. Pulmonary rehabilitation compared with brief advice for severe COPD. J Cardiopulm Rehabil Prev 2002;22:338-44.
- Royston P. Multiple imputation of missing values: update of ice. Stata J 2005;5:527-36.
- Rubin DB. Multiple imputation for nonresponse in surveys. Hoboken, NJ: John Wiley & Sons; 1987.
- Molenberghs G, Kenward MG. Missing data in clinical studies. London: Wiley; 2007.
- Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SJW. Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT statement. JAMA 2006;295:1152-61.
- National Institute for Clinical Excellence (NICE) . Guide to the Methods of Technology Appraisal 2008.
- Curtis LA. Unit costs of health and social care. Canterbury: PSSRU, University of Kent; 2008.
- Department of Health . NHS Reference Costs 2006 07 n.d.
- Brooks R. Euroqol: the current state of play. Health Policy 1996;37:53-72.
- Longworth L, Bryan S. An empirical comparison of EQ-5D and SF-6D in liver transplant patients. Health Econ 2003;12:1061-7.
- Schafer JL. Multiple imputations: a primer. Stat Methods Med Res 1999;8.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96.
- Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making 1998;18:S68-S80.
- Barton GR, Briggs AH, Fenwick EA. Optimal cost-effective decisions: the role of the cost-effectiveness acceptability curve (CEAC), cost-effectiveness acceptability frontier (CEAF) and expected value of perfect information (EVPI). Value Health 2008;11:886-97.
- Fenwick E, Claxton K, Sculpher MJ. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87.
- Briggs AH, Wonderling DE, Mooney CZ. Pulling cost-effectiveness analysis up by its bootstraps: a non-parametric approach to confidence interval estimation. Health Econ 1997;6:327-40.
- Briggs AH, Clark T, Wolstenholme J, Clarke P. Missing . presumed at random: cost-analysis of incomplete data. Health Econ 2003;12:377-92.
- Day SJ, Altman DG. Statistics Notes: Blinding in clinical trials and other studies. BMJ 2000;321.
- British Thoracic Society . Guidelines for the management of chronic obstructive pulmonary disease. Thorax 2003;52.
- Miravitlles M, Ferrer M, Pont A, Zalacain R, Alvarez-Sala JL, Masa F, et al. Effect of exacerbations on quality of life in patients with chronic obstructive pulmonary disease: a 2 year follow up study. Thorax 2004;59.
- Fruchter O. Predictors of long-term survival in elderly patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. Respirology 2008;13:851-5.
- Yohannes AM, Connolly MJ. A national survey: percussions, vibration, shaking and active cycle breathing techniques in patients with acute exacerbations of chronic obstructive pulmonary disease. Physiotherapy 2007;93:110-13.
- Spencer S, Jones PW. Time course of recovery of health status following an infective exacerbation of chronic bronchitis. Thorax 2003;58:589-93.
- Jones PW, Quirk FH, Baveystock CM. The St. George’s Questionnaire. Respir Med 1991;85:25-31.
- Jones PW. Group NSQoLS . Quality of life, symptoms, and pulmonary function in asthma: long-term treatment with nedocromil sodium examined in a controlled multicentre trial. Eur J Respir Dis 1994;7.
- Claxton K. The irrelevance of inference: a decision-making approach to the stochastic evaluation of health care technologies. Health Econ 1999;18:341-64.
- Claxton K, Sculpher MJ, Drummond M. A national framework for decision making by the National Institute for Clinical Excellence (NICE). Lancet 2002:360-15.
- Richardson G, Bloor K, Williams J, Russell I, Durai D, Cheung WY, et al. Cost effectiveness of nurse delivered endoscopy: findings from randomised multi-institution nurse endoscopy trial (MINuET). BMJ n.d.
- Wong A, Gan W, Burns J, Sin D, van Eeden S. Acute exacerbation of chronic obstructive pulmonary disease: influence of social factors in determining length of hospital stay and readmission rates. Can Respir J 2008;15:361-4.
- Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic evaluation in clinical trials. Oxford: Oxford University Press; 2007.
- McCrone P, Knapp M, Proudfoot J, Ryden C, Cavanagh K, Shapiro DA, et al. Cost-effectiveness of computerised cognitive behavioural therapy for anxiety and depression in primary care: randomised controlled trial. Br J Psychiatry 2004;185:55-62.
- Byford S, Knapp M, Greenshields J, Ukoumunne OC, Jones V, Thompaon S, et al. Cost-effectiveness of brief cognitive behaviour therapy versus treatment as usual in recurrent deliberate self-harm: a decision-making approach. Psychol Med 2003;33:977-86.
- Beecham J, Sleed M, Knapp M, Chiesa M, Drahorad C. The costs and effectiveness of two psychosocial treatment programmes for personality disorder: a controlled study. Eur J Psychiatry 2006;21:102-9.
- Van Roijen LH, Van Straten A, Rutten F, Donker M. Cost-utility of brief psychological treatment for depression and anxiety. Br J Psychiatry 2006;188:323-9.
- McCrone P, Risedale L, Darbishare L, Seed P. Cost-effectiveness of cognitive behavioural therapy, graded exercise and usual care for patients with chronic fatigue in primary care. Psychol Med 2004;34:991-9.
- Severens JL, Prins JB, Van der Wilt GJ, Van der Meer JWM, Bleijenber G. Cost-effectiveness of cognitive behaviour therapy for patients with chronic fatigue syndrome. Q J Assoc Phys 2004;97:153-61.
- Torrance GW, Raynauld JP, Walker V, Goldsmith CH, Bellamy N, Band PA, et al. A prospective, randomized, pragmatic, health outcomes trial evaluating the incorporation of hylan G-F 20 into the treatment paradigm for patients with knee osteoarthritis: economic results. Osteoarthritis Cartilage 2002;10:518-27.
- Jones RC, Donaldson GC, Chavannes NH, Kida K, Dickson-Spillmann M, Harding S, et al. Derivation and validation of a composite index of severity in chronic obstructive pulmonary disease – the DOSE Index. Am J Respir Crit Care Med 2009;180:1189-95.
Appendix 1 Manual chest physiotherapy treatment protocol
Appendix 2 Manual chest physiotherapy – treatment definitions
Appendix 3 Manual chest physiotherapy – treatment positions
Appendix 4 Trial recruiter screening checklist
Appendix 5 Physiotherapist screening checklist
Appendix 6 Patient information sheet
Appendix 7 Study consent form
Appendix 8 St George’s Respiratory Questionnaire
Appendix 9 Breathlessness, Cough and Sputum Scale
Appendix 10 Medical Research Council-Dyspnoea scale
Appendix 11 EQ-5D questionnaire
Appendix 12 Case report form – randomisation
Appendix 13 Study arm allocation reminder card – MCP arm
Appendix 14 Study arm allocation reminder card – control arm
Appendix 15 Case report form – MCP arm
Appendix 16 Advice leaflet on chest clearing
Appendix 17 Case report form – control arm
Appendix 18 Adverse Event Report Form
Appendix 19 COPD cost questionnaire – baseline
Appendix 20 COPD cost questionnaire – follow-up
Appendix 21 MATREX Trial Management Group
The Chief Investigator (Dr Jane Cross) was responsible for the day-to-day management of the trial. A TMG was established to assist with this function.
Meetings were held monthly with minutes circulated to each member. TMG members included:
Dr Jane Cross – Chief Investigator
Senior Lecturer in Respiratory Physiotherapy
School of Allied Health Professions
University of East Anglia
Tel.: 01603 593315
E-mail: j.cross@uea.ac.uk
Professor Ian Harvey – study design, project management
Professor of Epidemiology and Public Health
School of Medicine, Health Policy and Practice
University of East Anglia
Tel.: 01603 593605
E-mail: ian.harvey@uea.ac.uk
Professor Max Bachmann – study design, project management
Professor of Health Care Interfaces
School of Medicine, Health Policy and Practice
University of East Anglia
Tel. 01603 591220
E-mail: m.bachmann@uea.ac.uk
Dr Garry Barton, health economics
Lecturer in Health Economics
School of Medicine, Health Policy and Practice
University of East Anglia
Tel.: 01603 591936
E-mail: g.barton@uea.ac.uk
Professor Lee Shepstone – study design, medical statistics
Senior Lecturer in Medical Statistics
School of Medicine, Health Policy and Practice
University of East Anglia
Tel.: 01603 592100
E-mail: l.shepstone@uea.ac.uk
Dr Allan Clark – medical statistics
Lecturer in Medical Statistics
School of Medicine, Health Policy and Practice
University of East Anglia
Tel.: 01603 593629
E-mail: allan.clark@uea.ac.uk
Dr Frances Elender – Project Manager
MATREX Trial Manager
School of Allied Health Professions
University of East Anglia
Tel.: 01603 591675
E-mail: frances.elender@uea.ac.uk
Appendix 22 Trial Steering Committee
TSC membership
| Name and address | Current position | Current member (Yes/No) |
|---|---|---|
|
Professor David Price, GPIAG Prof of Primary Care Respiratory Medicine, Department of General Practice and Primary Care, University of Aberdeen, Foresterhill Health Centre, Westburn Road, Aberdeen AB25 2AY E-mail: d.price@abdn.ac.uk. |
Chairperson | Yes |
|
Professor Max Bachmann, Professor Health Care Interfaces, Department of Health Policy and Practice, University of East Anglia, School of Medicine, University Plain, Norwich NR4 7TJ E-mail: m.bachmann@uea.ac.uk |
Ordinary | Yes |
|
Ms Judy Close, Independent NHS Advisor – Allied Health Professions, Norfolk and Norwich University Hospital NHS Trust, Colney Lane, Norwich, NR4 7UY E-mail: judy.close.nnuh.nhs.uk |
Ordinary | Yes |
|
Dr Jane Cross, Senior Lecturer in Respiratory Physiotherapy, School of Allied Health Professions, University of East Anglia, Queen’s Building, University Plain, Norwich NR4 7TJ E-mail: j.cross@uea.ac.uk |
Ordinary | Yes |
|
Dr Frances Elender, Trial Manager, MATREX trial, University of East Anglia, Queen’s Building, University Plain, Norwich NR4 7TJ E-mail: frances.elender@uea.ac.uk |
Ordinary | Yes |
|
Dr David Ellis, Consultant Physician, Department of Respiratory Medicine, James Paget Healthcare NHS Trust, Lowestoft Road, Gorleston, Great Yarmouth NR31 6LA E-mail: david.ellis@ukdoctor.org |
Ordinary | No |
|
Dr Venkat Mahadevan, Consultant Physician, Department of Respiratory Medicine, James Paget Healthcare NHS Trust, Lowestoft Road, Gorleston, Great Yarmouth NR31 6LA E-mail: venkat.mahadevan@jpaget.nhs.uk |
Ordinary | Yes |
|
Ms Rachel Ellis, Superintendent Physiotherapist, Department of Physiotherapy, Norfolk and Norwich University Hospital NHS Trust, Colney Lane, Norwich NR4 7UY E-mail: rachel.ellis@nnuh.nhs.uk |
Ordinary | Yes |
|
Dr Garry Barton, Lecturer in Health Economics, School of Medicine, Health Policy and Practice, University of East Anglia, University Plain, Norwich NR4 7TJ E-mail: g.barton@uea.ac.uk |
Ordinary | Yes |
|
Professor Ian Harvey, Professor of Epidemiology and Public Health, Health Policy and Practice, University of East Anglia, University Plain, Norwich NR4 7TJ E-mail: ian.harvey@uea.ac.uk |
Ordinary | Yes |
|
Ms Kathryn Andrews, R&D Manager, R&D Office, Norfolk and Norwich University Hospital NHS Trust, Colney Lane, Norwich NR4 7UY E-mail: kathryn.andrews@nmuh.nhs.uk |
Ordinary | Yes |
|
Ms Katherine Jones, R&D Manager, R&D Office, Norfolk and Norwich University Hospital NHS Trust, Colney Lane, Norwich NR4 7UY E-mail: kathryn.jones@nmuh.nhs.uk |
Ordinary | No |
|
Ms Julia Kerrigan, Respiratory Physiotherapist, Queen Elizabeth Hospital, Gayton Road, King’s Lynn, Norfolk PE30 4ET E-mail: julia.kerrigan@gehkl.nhs.uk |
Ordinary | No |
|
Ms Rachel Mathews, Senior Respiratory Physiotherapist, James Paget Healthcare Trust, Lowestoft Road, Gorleston, Great Yarmouth NR31 6LA E-mail: rachel.mathews@jpaget.nhs.uk |
Ordinary | No |
|
Ms Sandra Olive, Respiratory Nurse Specialist, Department of Respiratory Medicine, Norfolk and Norwich University Hospital NHS Trust, Colney Lane, Norwich, NR4 7UY E-mail: sandra.olive@nnuh.nhs.uk |
Ordinary | Yes |
|
Paula Brown, Respiratory Nurse Specialist, Department of Respiratory Medicine, Norfolk and Norwich University Hospital NHS Trust, Colney Lane, Norwich, NR4 7UY E-mail: paula.brown@nnuh.nhs.uk |
Ordinary | Yes |
|
Dr Anna Pawlowicz, Consultant Physician in Respiratory Medicine, Department of Respiratory Medicine, Queen Elizabeth Hospital, Gayton Road, King’s Lynn, Norfolk PE30 4ET E-mail: anna.pawlowicz@qehkl.nhs.uk |
Ordinary | Yes |
|
Professor Lee Shepstone, Senior Lecturer in Medical Statistics, School of Medicine, Health Policy and Practice, University of East Anglia, University Plain, Norwich NR4 7TJ E-mail: l.shepstone@uea.ac.uk |
Ordinary | Yes |
|
Dr Simon Watkin, Consultant Physician, Department of Respiratory Medicine, Norfolk and Norwich University Hospital NHS Trust, Colney Lane, Norwich NR4 7UY E-mail: simon.watkin@nnuh.nhs.uk |
Ordinary | No |
TSC terms of reference
-
1.0 Summary functions
-
1.1 To monitor and supervise trial progress towards its interim and overall objectives.
-
1.2 To review relevant information from other sources (e.g. other related trials).
-
1.3 To consider recommendations of the DMEC.
-
1.4 To inform NIHR Evaluation, Trials and Studies Coordinating Centre (NETSCC), HTA on the progress of the trial.
-
1.5 To advise NETSCC, HTA on publicity and the presentation of all aspects of the trial.
-
-
2.0 Membership composition
-
2.1 Independent Chairperson (no direct trial involvement other than as TSC member).
-
2.2 Two additional independent expert members.
-
2.3 Chief Investigator (CI).
-
2.4 MATREX trial HTA grant holders.
-
2.5 Lead Investigator at each participating site.
-
2.6 Patient/lay representative.
-
2.7 Trial Manager.
-
2.8 In attendance:
-
2.8.1 HTA representative.
-
2.8.2 Trial recruiters.
-
-
-
3.0 Meetings
-
3.1 The inaugural meeting will take place:
-
3.1.1 After Research Ethics Committee/NHS R&D approval.
-
3.1.2 Prior to recruitment of the first patient.
-
-
3.2 The TSC will meet at 6-month intervals for the duration of the trial (× 6).
-
3.3 Meetings will be organised by the Chief Investigator.
-
3.4 Papers for the meeting will be circulated in advance.
-
3.5 An accurate minute will be prepared by the Chief Investigator and:
-
3.5.1 Agreement sought by all the members.
-
3.5.2 A copy sent to NETSCC, HTA.
-
-
-
4.0 Trial steering and management
-
4.1 The role of the TSC is to provide supervision of the trial on behalf of NETSCC, HTA.
-
4.2 The TSC will concentrate on:
-
4.2.1 Trial progress.
-
4.2.2 Adherence to trial protocol.
-
4.2.3 Patient safety.
-
4.2.4 Consideration of new information.
-
-
4.3 Day-to-day management of the trial is the responsibility of the Chief Investigator:
-
4.3.1 A trial management group will assist with this function.
-
-
-
5.0 Good clinical practice
-
5.1 The TSC will endeavour to ensure that the trial is conducted at all times to the standards set out in the Guidelines for Good Clinical Practice (GCP).
-
-
6.0 Patient safety
-
6.1 In all the deliberations of the TSC, the rights, safety and well-being of trial participants are the most important considerations.
-
6.2 The Chief Investigator will provide the TSC with sufficient information to enable it to assess the quality of the patient consent process.
-
6.3 The TSC will advise the investigators on the continued completeness and suitability of the patient information provided.
-
-
7.0 Progress
-
7.1 It is the role of the TSC to monitor the progress of the trial and to maximise the chances of completing the trial within the agreed time scale.
-
7.2 At the first TSC meeting targets for recruitment, data collection, compliance, etc. will be agreed with the Chief Investigator.
-
7.3 Targets will be used to compile a template for presentations to all further meetings.
-
7.3.1 The Chief Investigator will submit biannual reports to NETSCC, HTA based on this template.
-
7.3.2 These reports will be endorsed by the TSC prior to submission.
-
-
-
8.0 Adherence to protocol
-
8.1 The full protocol will be presented and agreed at the first TSC meeting.
-
8.2 Subsequent changes to the protocol will require approval from the TSC.
-
8.2.1 The Chief Investigator will inform REC, NHS R&D and HTA of any changes.
-
-
-
9.0 Data Monitoring and Ethics Committee
-
9.1 The DMEC will meet regularly to review the data and results of any interim analyses.
-
9.2 Members of the DMEC will be independent of both the trial and the TSC.
-
9.3 The DMEC will produce summary reports after each meeting for consideration by the TSC.
-
-
10.0 Consideration of new information
-
10.1 The TSC will consider new information relevant to the trial (including DMEC).
-
10.2 It is the responsibility of the Chief Investigator, the Chairperson and other independent members to bring results from other studies that may have a direct bearing on the future conduct of the trial to the attention of the TSC.
-
10.3 On consideration of such information, the TSC will recommend appropriate action such as changes to the protocol, additional patient information, or stopping the trial.
-
10.4 The rights, safety and well-being of the trial participants will be the most important consideration in this regard.
-
10.5 It is the responsibility of the Chief Investigator to notify the TSC, DMEC and relevant regulatory authority immediately of any unexpected serious adverse events occurring during the course of the trial.
-
Suggested template for Trial Steering Committee agendas and reports
The list below outlines the information that will be provided by the Chief Investigator at each meeting. This list will be used as a basis for the agenda of TSC meetings and a template for biannual reports to NETSCC, HTA:
-
trial progress (with respect to targets)
-
recruitment to date (with respect to targets)
-
follow-up to date (with respect to targets)
-
AEs
-
DMEC report
-
issues/problems (specifically since last report)
-
new information
-
changes to protocol.
Appendix 23 Data Monitoring and Ethics Committee – membership and terms of reference
DMEC membership
Research expertise – Chairperson
Professor Richard Lilford
Department of Public Health and Epidemiology
University of Birmingham
Birmingham B15 2TT
Tel.: 0121 414 6772
Fax.: 0121 414 7878
E-mail: r.j.lilford@bham.ac.uk
Statistical expertise
Mike Roughton
Cancer Trials Unit
University College London
London WC1E
Tel.: 020 7679 2000
Mob.: 07966 086325
E-mail: m.roughton@ctc.ucl.ac.uk
Clinical expertise
Jennifer A Pryor
Research Fellow in Physiotherapy
Royal Brompton Hospital
London SW3 6NP
Tel.: 020 7352 8121, extension 4925 or bleep 7313
Fax.: 020 7351 8052
E-mail: j.pryor@rbh.nthames.nhs.uk
No longer a current member
Dr Fotios Siannis
MRC Biostatistics Unit
Institute of Public Health
University of Cambridge, Forvie Site
Cambridge CB2 2SR
E-mail: fotios.siannis@mrc-bsu.cam.ac.uk
DMEC role
The DMEC is the only body involved in the trial that has access to the unblinded comparative data. Its role is to monitor these data and make recommendations to the TSC and NETSCC, HTA on whether there are any ethical or safety reasons why the trial should not be continued.
All members of the DMEC are independent of the trial they are monitoring.
The DMEC Chairperson organises work related to the trial. The DMEC includes a statistician and a clinically qualified specialist in the field of physiotherapy.
The Chief Investigator and the Chairperson of the TSC will agree with the DMEC Chairperson a timely mechanism for reporting to the DMEC. With the help of the trial statistician, the Chief Investigator will provide blinded data, in strict confidence, to the DMEC as frequently as the members of the DMEC request. A template for reporting interim data will be used by the Chief Investigator (Appendix I).
The DMEC discusses the data on AEs and efficacy data, either in a meeting or by teleconference. If necessary, it may request further data from the Chief Investigator and trial statistician. In the light of interim data, and other evidence from relevant studies (including updated overviews of the relevant randomised controlled trials), the Chairperson of the DMEC informs the Chairperson of the TSC if, in its view, the trial should proceed or be terminated.
Unless cessation of the trial is recommended by the DMEC, the TSC, Chief Investigator and Trial Manager will remain in ignorance of the results of the interim analysis of efficacy.
The DMEC may also advise the TSC on modification of the protocol.
DMEC terms of reference
-
To meet at least once a year during the course of the trial (either at a meeting or by teleconference).
-
To set up and maintain direct communication with the Chief Investigator and Chairperson of the TSC. The Chairperson of the TSC will be made aware of all communication between the Chief Investigator and DMEC.
-
To receive a copy of the trial protocol and any plans for interim analysis as early as possible in the conduct of the trial.
-
To receive reports (as per the template in Appendix I) during the trial at intervals agreed with the TSC and Chief Investigator.
-
To consider data from interim analyses, unblinded if considered appropriate, plus any additional safety issues for the trial and relevant information from the template and other sources.
-
To report to the TSC and recommend on the continuation of the trial.
DMEC output and reporting
-
The first meeting of the DMEC is an open meeting with the Chief Investigator. The output of that meeting includes agreement on the relevant material that needs to be reported subsequently.
-
The report of the trial statistician to the DMEC is seen only by DMEC members. Each meeting is summarised in the form of brief minutes.
-
The Chairperson of the DMEC provides a brief summary of the recommendations of each meeting to the TSC, Chief Investigator and NETSCC, HTA.
Appendix I Template for Chief Investigator’s report to DMEC
Date of report:
-
1. Title of trial:
-
2. Trial progress
-
2.1 Trial recruitment
-
2.1.1 Plan of recruitment
Start date of recruitment
End date of recruitment
Recruitment period
Expected average monthly recruitment
Recruiting centres
-
2.1.2 Recruitment to date
Recruitment period to date
Total recruitment to date
Observed average monthly recruitment
Recruitment stratified by centre
Expected recruitment period (based on current recruitment rate)
End date of recruitment (based on current recruitment patterns)
Graph showing the planned and actual recruitment rates
-
2.1.3 Recruitment based on eligibility
Inclusion/exclusion
Number ineligible
Non-consent
Protocol violation
-
-
2.2 Internal validity
-
2.2.1 Comparability of selected baseline characteristics between the treatment groups
-
-
2.3 External validity
-
2.3.1 Selected baseline characteristics of subjects in high and low recruiting centres
-
-
2.4 Protocol compliance
-
2.4.1 Number of patients withdrawn from treatment but continued being followed up
-
2.4.2 Number of patients who have been lost to follow-up
-
2.4.3 Number of patients with missing follow-up data
-
2.4.4 Number of patients who have crossed over to alternative treatment
-
-
-
3. Safety data – to be presented overall and by blinded group
-
3.1 Serious adverse events*
-
3.2 Other adverse events
-
-
4. Details of new information since start of trial/last report
-
4.1 Publications
-
4.2 National or international guidelines on the treatment of the disease
-
*A serious adverse event (SAE) is defined as any untoward medical occurrence or effect that:
-
results in death
-
is life-threatening (i.e. with an immediate, not hypothetical, risk of death)
-
requires hospitalisation or prolongs existing hospitalisation (excluding hospitalisation for elective treatment of a pre-existing condition)
-
results in persistent or significant disability or incapacity
or any other important medical condition which may jeopardise the patient and may require medical or surgical intervention to prevent one of the outcomes listed above (e.g. bronchospasm requiring intensive emergency treatment).
Appendix 24 Action plan to improve questionnaire response rates
-
Trial recruiters to assess at baseline whether the patient may have particular difficulties that might lead to a non-response (e.g. literacy level, very poor eyesight, changing home circumstances). For these patients, alternative methods/routes for data collection are to be negotiated (e.g. identifying a relative/friend to help with completion, large-type questionnaires, telephone call, home visit).
-
Compile a follow-up database containing information on follow-up due dates and questionnaire returns. Include information on patients with special needs and routinely update database when new information becomes available. Scrutinise database before questionnaires are sent out.
-
Conduct a weekly audit to identify overdue questionnaires (i.e. 3 weeks after sending). For overdue returns, check hospital/GP records to establish patient status (i.e. still alive, still at the same address). If all is well, telephone the participant to enquire whether they received their questionnaires and/or whether they require help to complete them.
-
Where it is not possible to contact the patient by telephone, issue a reminder letter that offers support if required.
-
If/when a ‘non-responder’ is readmitted to hospital, trial recruiters are to offer help to complete duplicate questionnaires. (Note: this tactic is dependent on the admission being within 2 weeks of the planned follow-up date.)
-
When undertaking follow-up strategies, priority is to be given to completion of the primary outcome measure (SGRQ) at the primary end point.
A 3-month audit conducted in October 2007 indicated the following action plan activities:
-
49 reminder telephone calls conducted
-
six reminder letters issued
-
four patients identified as deceased
-
three follow-ups administered in hospital
-
two nursing home visits to help complete questionnaires
-
one patient identified as having moved.
Appendix 25 Data quality audit (conducted February 2009)
|
Total participant record set N = 526 |
Paper record checka n = 26 (5% sample) |
Double data entryb n = 125 (23% sample) |
||||
| Data | Cells checked | Errors found | % Error | Cells checked | Errors found | % Error |
| MRC-D score | 26 | 0 | 0 | 121 | 2 | 1.6 |
| SGRQ score | 3900 | 11 | 0.2 | 6032 | 45 | 0.7 |
| BCSS score | 1124 | 2 | 0.2 | 5775 | 121 | 2.0 |
| EQ-5D score | 671 | 3 | 0.4 | |||
| Other participant datac | 585 | 5 | 0.8 | |||
| Use of hospital services | 891 | 22 | 2.4 | |||
| Total | 7197 | 43 | 0.59 | 11,928 | 168 | 1.40 |
List of abbreviations
- λ
- cost-effectiveness threshold
- 6MWT
- six-minute walk test
- ABG
- arterial blood gas
- ACBTw
- active cycle of breathing techniques
- ACCP
- American College of Chest Physicians
- AE
- adverse event
- BCSS
- Breathlessness Cough and Sputum Scale
- CAO
- chronic airflow obstruction
- CEAC
- cost-effectiveness acceptability curve
- CF
- cystic fibrosis
- CI
- confidence interval
- COAD
- chronic obstructive airway disease
- CONSORT
- Consolidated Standards of Reporting Trials
- COPD
- chronic obstructive pulmonary disease
- CRF
- case report form
- DMEC
- Data Monitoring and Ethics Committee
- EQ-5D
- European Quality of Life-5 Dimensions questionnaire
- EQ-VAS
- EQ-5D visual analogue scale
- FET
- forced expiratory technique
- FEV1
- forced expiratory volume in 1 second
- FRC
- functional residual capacity
- FVC
- forced vital capacity
- GCP
- good clinical practice
- GP
- general practitioner
- HRG
- Healthcare Resource Group
- ICER
- incremental cost-effectiveness ratio
- INR
- international normalised ratio
- ITT
- intention to treat
- JPH
- James Paget Hospital
- MCID
- minimal clinically important difference
- MCP
- manual chest physiotherapy
- MRC-D
- Medical Research Council-Dyspnoea (scale)
- NETSCC
- NIHR Evaluation, Trials and Studies Coordinating Centre
- NICE
- National Institute for Health and Clinical Excellence
- NMB
- net monetary benefit
- NNUH
- Norfolk and Norwich University Hospital
- NSF
- National Service Framework
- PD
- postural drainage
- PP
- per protocol
- PSS
- personal social services
- QALY
- quality-adjusted life-year
- QEH
- Queen Elizabeth Hospital
- QoL
- quality of life
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RA
- research associate
- SaO2
- arterial oxygen saturation
- SGRQ
- St George’s Respiratory Questionnaire
- SD
- standard deviation
- SF-36
- Short Form–36 items
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- UEA
- University of East Anglia
- UHA
- University Hospital Aintree
- V/Q
- ventilation/perfusion ratio
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment reports published to date
-
Home parenteral nutrition: a systematic review.
By Richards DM, Deeks JJ, Sheldon TA, Shaffer JL.
-
Diagnosis, management and screening of early localised prostate cancer.
A review by Selley S, Donovan J, Faulkner A, Coast J, Gillatt D.
-
The diagnosis, management, treatment and costs of prostate cancer in England and Wales.
A review by Chamberlain J, Melia J, Moss S, Brown J.
-
Screening for fragile X syndrome.
A review by Murray J, Cuckle H, Taylor G, Hewison J.
-
A review of near patient testing in primary care.
By Hobbs FDR, Delaney BC, Fitzmaurice DA, Wilson S, Hyde CJ, Thorpe GH, et al.
-
Systematic review of outpatient services for chronic pain control.
By McQuay HJ, Moore RA, Eccleston C, Morley S, de C Williams AC.
-
Neonatal screening for inborn errors of metabolism: cost, yield and outcome.
A review by Pollitt RJ, Green A, McCabe CJ, Booth A, Cooper NJ, Leonard JV, et al.
-
Preschool vision screening.
A review by Snowdon SK, Stewart-Brown SL.
-
Implications of socio-cultural contexts for the ethics of clinical trials.
A review by Ashcroft RE, Chadwick DW, Clark SRL, Edwards RHT, Frith L, Hutton JL.
-
A critical review of the role of neonatal hearing screening in the detection of congenital hearing impairment.
By Davis A, Bamford J, Wilson I, Ramkalawan T, Forshaw M, Wright S.
-
Newborn screening for inborn errors of metabolism: a systematic review.
By Seymour CA, Thomason MJ, Chalmers RA, Addison GM, Bain MD, Cockburn F, et al.
-
Routine preoperative testing: a systematic review of the evidence.
By Munro J, Booth A, Nicholl J.
-
Systematic review of the effectiveness of laxatives in the elderly.
By Petticrew M, Watt I, Sheldon T.
-
When and how to assess fast-changing technologies: a comparative study of medical applications of four generic technologies.
A review by Mowatt G, Bower DJ, Brebner JA, Cairns JA, Grant AM, McKee L.
-
Antenatal screening for Down’s syndrome.
A review by Wald NJ, Kennard A, Hackshaw A, McGuire A.
-
Screening for ovarian cancer: a systematic review.
By Bell R, Petticrew M, Luengo S, Sheldon TA.
-
Consensus development methods, and their use in clinical guideline development.
A review by Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CFB, Askham J, et al.
-
A cost–utility analysis of interferon beta for multiple sclerosis.
By Parkin D, McNamee P, Jacoby A, Miller P, Thomas S, Bates D.
-
Effectiveness and efficiency of methods of dialysis therapy for end-stage renal disease: systematic reviews.
By MacLeod A, Grant A, Donaldson C, Khan I, Campbell M, Daly C, et al.
-
Effectiveness of hip prostheses in primary total hip replacement: a critical review of evidence and an economic model.
By Faulkner A, Kennedy LG, Baxter K, Donovan J, Wilkinson M, Bevan G.
-
Antimicrobial prophylaxis in colorectal surgery: a systematic review of randomised controlled trials.
By Song F, Glenny AM.
-
Bone marrow and peripheral blood stem cell transplantation for malignancy.
A review by Johnson PWM, Simnett SJ, Sweetenham JW, Morgan GJ, Stewart LA.
-
Screening for speech and language delay: a systematic review of the literature.
By Law J, Boyle J, Harris F, Harkness A, Nye C.
-
Resource allocation for chronic stable angina: a systematic review of effectiveness, costs and cost-effectiveness of alternative interventions.
By Sculpher MJ, Petticrew M, Kelland JL, Elliott RA, Holdright DR, Buxton MJ.
-
Detection, adherence and control of hypertension for the prevention of stroke: a systematic review.
By Ebrahim S.
-
Postoperative analgesia and vomiting, with special reference to day-case surgery: a systematic review.
By McQuay HJ, Moore RA.
-
Choosing between randomised and nonrandomised studies: a systematic review.
By Britton A, McKee M, Black N, McPherson K, Sanderson C, Bain C.
-
Evaluating patient-based outcome measures for use in clinical trials.
A review by Fitzpatrick R, Davey C, Buxton MJ, Jones DR.
-
Ethical issues in the design and conduct of randomised controlled trials.
A review by Edwards SJL, Lilford RJ, Braunholtz DA, Jackson JC, Hewison J, Thornton J.
-
Qualitative research methods in health technology assessment: a review of the literature.
By Murphy E, Dingwall R, Greatbatch D, Parker S, Watson P.
-
The costs and benefits of paramedic skills in pre-hospital trauma care.
By Nicholl J, Hughes S, Dixon S, Turner J, Yates D.
-
Systematic review of endoscopic ultrasound in gastro-oesophageal cancer.
By Harris KM, Kelly S, Berry E, Hutton J, Roderick P, Cullingworth J, et al.
-
Systematic reviews of trials and other studies.
By Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F.
-
Primary total hip replacement surgery: a systematic review of outcomes and modelling of cost-effectiveness associated with different prostheses.
A review by Fitzpatrick R, Shortall E, Sculpher M, Murray D, Morris R, Lodge M, et al.
-
Informed decision making: an annotated bibliography and systematic review.
By Bekker H, Thornton JG, Airey CM, Connelly JB, Hewison J, Robinson MB, et al.
-
Handling uncertainty when performing economic evaluation of healthcare interventions.
A review by Briggs AH, Gray AM.
-
The role of expectancies in the placebo effect and their use in the delivery of health care: a systematic review.
By Crow R, Gage H, Hampson S, Hart J, Kimber A, Thomas H.
-
A randomised controlled trial of different approaches to universal antenatal HIV testing: uptake and acceptability. Annex: Antenatal HIV testing – assessment of a routine voluntary approach.
By Simpson WM, Johnstone FD, Boyd FM, Goldberg DJ, Hart GJ, Gormley SM, et al.
-
Methods for evaluating area-wide and organisation-based interventions in health and health care: a systematic review.
By Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ.
-
Assessing the costs of healthcare technologies in clinical trials.
A review by Johnston K, Buxton MJ, Jones DR, Fitzpatrick R.
-
Cooperatives and their primary care emergency centres: organisation and impact.
By Hallam L, Henthorne K.
-
Screening for cystic fibrosis.
A review by Murray J, Cuckle H, Taylor G, Littlewood J, Hewison J.
-
A review of the use of health status measures in economic evaluation.
By Brazier J, Deverill M, Green C, Harper R, Booth A.
-
Methods for the analysis of quality-of-life and survival data in health technology assessment.
A review by Billingham LJ, Abrams KR, Jones DR.
-
Antenatal and neonatal haemoglobinopathy screening in the UK: review and economic analysis.
By Zeuner D, Ades AE, Karnon J, Brown J, Dezateux C, Anionwu EN.
-
Assessing the quality of reports of randomised trials: implications for the conduct of meta-analyses.
A review by Moher D, Cook DJ, Jadad AR, Tugwell P, Moher M, Jones A, et al.
-
‘Early warning systems’ for identifying new healthcare technologies.
By Robert G, Stevens A, Gabbay J.
-
A systematic review of the role of human papillomavirus testing within a cervical screening programme.
By Cuzick J, Sasieni P, Davies P, Adams J, Normand C, Frater A, et al.
-
Near patient testing in diabetes clinics: appraising the costs and outcomes.
By Grieve R, Beech R, Vincent J, Mazurkiewicz J.
-
Positron emission tomography: establishing priorities for health technology assessment.
A review by Robert G, Milne R.
-
The debridement of chronic wounds: a systematic review.
By Bradley M, Cullum N, Sheldon T.
-
Systematic reviews of wound care management: (2) Dressings and topical agents used in the healing of chronic wounds.
By Bradley M, Cullum N, Nelson EA, Petticrew M, Sheldon T, Torgerson D.
-
A systematic literature review of spiral and electron beam computed tomography: with particular reference to clinical applications in hepatic lesions, pulmonary embolus and coronary artery disease.
By Berry E, Kelly S, Hutton J, Harris KM, Roderick P, Boyce JC, et al.
-
What role for statins? A review and economic model.
By Ebrahim S, Davey Smith G, McCabe C, Payne N, Pickin M, Sheldon TA, et al.
-
Factors that limit the quality, number and progress of randomised controlled trials.
A review by Prescott RJ, Counsell CE, Gillespie WJ, Grant AM, Russell IT, Kiauka S, et al.
-
Antimicrobial prophylaxis in total hip replacement: a systematic review.
By Glenny AM, Song F.
-
Health promoting schools and health promotion in schools: two systematic reviews.
By Lister-Sharp D, Chapman S, Stewart-Brown S, Sowden A.
-
Economic evaluation of a primary care-based education programme for patients with osteoarthritis of the knee.
A review by Lord J, Victor C, Littlejohns P, Ross FM, Axford JS.
-
The estimation of marginal time preference in a UK-wide sample (TEMPUS) project.
A review by Cairns JA, van der Pol MM.
-
Geriatric rehabilitation following fractures in older people: a systematic review.
By Cameron I, Crotty M, Currie C, Finnegan T, Gillespie L, Gillespie W, et al.
-
Screening for sickle cell disease and thalassaemia: a systematic review with supplementary research.
By Davies SC, Cronin E, Gill M, Greengross P, Hickman M, Normand C.
-
Community provision of hearing aids and related audiology services.
A review by Reeves DJ, Alborz A, Hickson FS, Bamford JM.
-
False-negative results in screening programmes: systematic review of impact and implications.
By Petticrew MP, Sowden AJ, Lister-Sharp D, Wright K.
-
Costs and benefits of community postnatal support workers: a randomised controlled trial.
By Morrell CJ, Spiby H, Stewart P, Walters S, Morgan A.
-
Implantable contraceptives (subdermal implants and hormonally impregnated intrauterine systems) versus other forms of reversible contraceptives: two systematic reviews to assess relative effectiveness, acceptability, tolerability and cost-effectiveness.
By French RS, Cowan FM, Mansour DJA, Morris S, Procter T, Hughes D, et al.
-
An introduction to statistical methods for health technology assessment.
A review by White SJ, Ashby D, Brown PJ.
-
Disease-modifying drugs for multiple sclerosis: a rapid and systematic review.
By Clegg A, Bryant J, Milne R.
-
Publication and related biases.
A review by Song F, Eastwood AJ, Gilbody S, Duley L, Sutton AJ.
-
Cost and outcome implications of the organisation of vascular services.
By Michaels J, Brazier J, Palfreyman S, Shackley P, Slack R.
-
Monitoring blood glucose control in diabetes mellitus: a systematic review.
By Coster S, Gulliford MC, Seed PT, Powrie JK, Swaminathan R.
-
The effectiveness of domiciliary health visiting: a systematic review of international studies and a selective review of the British literature.
By Elkan R, Kendrick D, Hewitt M, Robinson JJA, Tolley K, Blair M, et al.
-
The determinants of screening uptake and interventions for increasing uptake: a systematic review.
By Jepson R, Clegg A, Forbes C, Lewis R, Sowden A, Kleijnen J.
-
The effectiveness and cost-effectiveness of prophylactic removal of wisdom teeth.
A rapid review by Song F, O’Meara S, Wilson P, Golder S, Kleijnen J.
-
Ultrasound screening in pregnancy: a systematic review of the clinical effectiveness, cost-effectiveness and women’s views.
By Bricker L, Garcia J, Henderson J, Mugford M, Neilson J, Roberts T, et al.
-
A rapid and systematic review of the effectiveness and cost-effectiveness of the taxanes used in the treatment of advanced breast and ovarian cancer.
By Lister-Sharp D, McDonagh MS, Khan KS, Kleijnen J.
-
Liquid-based cytology in cervical screening: a rapid and systematic review.
By Payne N, Chilcott J, McGoogan E.
-
Randomised controlled trial of non-directive counselling, cognitive–behaviour therapy and usual general practitioner care in the management of depression as well as mixed anxiety and depression in primary care.
By King M, Sibbald B, Ward E, Bower P, Lloyd M, Gabbay M, et al.
-
Routine referral for radiography of patients presenting with low back pain: is patients’ outcome influenced by GPs’ referral for plain radiography?
By Kerry S, Hilton S, Patel S, Dundas D, Rink E, Lord J.
-
Systematic reviews of wound care management: (3) antimicrobial agents for chronic wounds; (4) diabetic foot ulceration.
By O’Meara S, Cullum N, Majid M, Sheldon T.
-
Using routine data to complement and enhance the results of randomised controlled trials.
By Lewsey JD, Leyland AH, Murray GD, Boddy FA.
-
Coronary artery stents in the treatment of ischaemic heart disease: a rapid and systematic review.
By Meads C, Cummins C, Jolly K, Stevens A, Burls A, Hyde C.
-
Outcome measures for adult critical care: a systematic review.
By Hayes JA, Black NA, Jenkinson C, Young JD, Rowan KM, Daly K, et al.
-
A systematic review to evaluate the effectiveness of interventions to promote the initiation of breastfeeding.
By Fairbank L, O’Meara S, Renfrew MJ, Woolridge M, Sowden AJ, Lister-Sharp D.
-
Implantable cardioverter defibrillators: arrhythmias. A rapid and systematic review.
By Parkes J, Bryant J, Milne R.
-
Treatments for fatigue in multiple sclerosis: a rapid and systematic review.
By Brañas P, Jordan R, Fry-Smith A, Burls A, Hyde C.
-
Early asthma prophylaxis, natural history, skeletal development and economy (EASE): a pilot randomised controlled trial.
By Baxter-Jones ADG, Helms PJ, Russell G, Grant A, Ross S, Cairns JA, et al.
-
Screening for hypercholesterolaemia versus case finding for familial hypercholesterolaemia: a systematic review and cost-effectiveness analysis.
By Marks D, Wonderling D, Thorogood M, Lambert H, Humphries SE, Neil HAW.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of glycoprotein IIb/IIIa antagonists in the medical management of unstable angina.
By McDonagh MS, Bachmann LM, Golder S, Kleijnen J, ter Riet G.
-
A randomised controlled trial of prehospital intravenous fluid replacement therapy in serious trauma.
By Turner J, Nicholl J, Webber L, Cox H, Dixon S, Yates D.
-
Intrathecal pumps for giving opioids in chronic pain: a systematic review.
By Williams JE, Louw G, Towlerton G.
-
Combination therapy (interferon alfa and ribavirin) in the treatment of chronic hepatitis C: a rapid and systematic review.
By Shepherd J, Waugh N, Hewitson P.
-
A systematic review of comparisons of effect sizes derived from randomised and non-randomised studies.
By MacLehose RR, Reeves BC, Harvey IM, Sheldon TA, Russell IT, Black AMS.
-
Intravascular ultrasound-guided interventions in coronary artery disease: a systematic literature review, with decision-analytic modelling, of outcomes and cost-effectiveness.
By Berry E, Kelly S, Hutton J, Lindsay HSJ, Blaxill JM, Evans JA, et al.
-
A randomised controlled trial to evaluate the effectiveness and cost-effectiveness of counselling patients with chronic depression.
By Simpson S, Corney R, Fitzgerald P, Beecham J.
-
Systematic review of treatments for atopic eczema.
By Hoare C, Li Wan Po A, Williams H.
-
Bayesian methods in health technology assessment: a review.
By Spiegelhalter DJ, Myles JP, Jones DR, Abrams KR.
-
The management of dyspepsia: a systematic review.
By Delaney B, Moayyedi P, Deeks J, Innes M, Soo S, Barton P, et al.
-
A systematic review of treatments for severe psoriasis.
By Griffiths CEM, Clark CM, Chalmers RJG, Li Wan Po A, Williams HC.
-
Clinical and cost-effectiveness of donepezil, rivastigmine and galantamine for Alzheimer’s disease: a rapid and systematic review.
By Clegg A, Bryant J, Nicholson T, McIntyre L, De Broe S, Gerard K, et al.
-
The clinical effectiveness and cost-effectiveness of riluzole for motor neurone disease: a rapid and systematic review.
By Stewart A, Sandercock J, Bryan S, Hyde C, Barton PM, Fry-Smith A, et al.
-
Equity and the economic evaluation of healthcare.
By Sassi F, Archard L, Le Grand J.
-
Quality-of-life measures in chronic diseases of childhood.
By Eiser C, Morse R.
-
Eliciting public preferences for healthcare: a systematic review of techniques.
By Ryan M, Scott DA, Reeves C, Bate A, van Teijlingen ER, Russell EM, et al.
-
General health status measures for people with cognitive impairment: learning disability and acquired brain injury.
By Riemsma RP, Forbes CA, Glanville JM, Eastwood AJ, Kleijnen J.
-
An assessment of screening strategies for fragile X syndrome in the UK.
By Pembrey ME, Barnicoat AJ, Carmichael B, Bobrow M, Turner G.
-
Issues in methodological research: perspectives from researchers and commissioners.
By Lilford RJ, Richardson A, Stevens A, Fitzpatrick R, Edwards S, Rock F, et al.
-
Systematic reviews of wound care management: (5) beds; (6) compression; (7) laser therapy, therapeutic ultrasound, electrotherapy and electromagnetic therapy.
By Cullum N, Nelson EA, Flemming K, Sheldon T.
-
Effects of educational and psychosocial interventions for adolescents with diabetes mellitus: a systematic review.
By Hampson SE, Skinner TC, Hart J, Storey L, Gage H, Foxcroft D, et al.
-
Effectiveness of autologous chondrocyte transplantation for hyaline cartilage defects in knees: a rapid and systematic review.
By Jobanputra P, Parry D, Fry-Smith A, Burls A.
-
Statistical assessment of the learning curves of health technologies.
By Ramsay CR, Grant AM, Wallace SA, Garthwaite PH, Monk AF, Russell IT.
-
The effectiveness and cost-effectiveness of temozolomide for the treatment of recurrent malignant glioma: a rapid and systematic review.
By Dinnes J, Cave C, Huang S, Major K, Milne R.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of debriding agents in treating surgical wounds healing by secondary intention.
By Lewis R, Whiting P, ter Riet G, O’Meara S, Glanville J.
-
Home treatment for mental health problems: a systematic review.
By Burns T, Knapp M, Catty J, Healey A, Henderson J, Watt H, et al.
-
How to develop cost-conscious guidelines.
By Eccles M, Mason J.
-
The role of specialist nurses in multiple sclerosis: a rapid and systematic review.
By De Broe S, Christopher F, Waugh N.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of orlistat in the management of obesity.
By O’Meara S, Riemsma R, Shirran L, Mather L, ter Riet G.
-
The clinical effectiveness and cost-effectiveness of pioglitazone for type 2 diabetes mellitus: a rapid and systematic review.
By Chilcott J, Wight J, Lloyd Jones M, Tappenden P.
-
Extended scope of nursing practice: a multicentre randomised controlled trial of appropriately trained nurses and preregistration house officers in preoperative assessment in elective general surgery.
By Kinley H, Czoski-Murray C, George S, McCabe C, Primrose J, Reilly C, et al.
-
Systematic reviews of the effectiveness of day care for people with severe mental disorders: (1) Acute day hospital versus admission; (2) Vocational rehabilitation; (3) Day hospital versus outpatient care.
By Marshall M, Crowther R, Almaraz- Serrano A, Creed F, Sledge W, Kluiter H, et al.
-
The measurement and monitoring of surgical adverse events.
By Bruce J, Russell EM, Mollison J, Krukowski ZH.
-
Action research: a systematic review and guidance for assessment.
By Waterman H, Tillen D, Dickson R, de Koning K.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of gemcitabine for the treatment of pancreatic cancer.
By Ward S, Morris E, Bansback N, Calvert N, Crellin A, Forman D, et al.
-
A rapid and systematic review of the evidence for the clinical effectiveness and cost-effectiveness of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer.
By Lloyd Jones M, Hummel S, Bansback N, Orr B, Seymour M.
-
Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature.
By Brocklebank D, Ram F, Wright J, Barry P, Cates C, Davies L, et al.
-
The cost-effectiveness of magnetic resonance imaging for investigation of the knee joint.
By Bryan S, Weatherburn G, Bungay H, Hatrick C, Salas C, Parry D, et al.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.
By Forbes C, Shirran L, Bagnall A-M, Duffy S, ter Riet G.
-
Superseded by a report published in a later volume.
-
The role of radiography in primary care patients with low back pain of at least 6 weeks duration: a randomised (unblinded) controlled trial.
By Kendrick D, Fielding K, Bentley E, Miller P, Kerslake R, Pringle M.
-
Design and use of questionnaires: a review of best practice applicable to surveys of health service staff and patients.
By McColl E, Jacoby A, Thomas L, Soutter J, Bamford C, Steen N, et al.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.
By Clegg A, Scott DA, Sidhu M, Hewitson P, Waugh N.
-
Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives.
By Brookes ST, Whitley E, Peters TJ, Mulheran PA, Egger M, Davey Smith G.
-
Depot antipsychotic medication in the treatment of patients with schizophrenia: (1) Meta-review; (2) Patient and nurse attitudes.
By David AS, Adams C.
-
A systematic review of controlled trials of the effectiveness and cost-effectiveness of brief psychological treatments for depression.
By Churchill R, Hunot V, Corney R, Knapp M, McGuire H, Tylee A, et al.
-
Cost analysis of child health surveillance.
By Sanderson D, Wright D, Acton C, Duree D.
-
A study of the methods used to select review criteria for clinical audit.
By Hearnshaw H, Harker R, Cheater F, Baker R, Grimshaw G.
-
Fludarabine as second-line therapy for B cell chronic lymphocytic leukaemia: a technology assessment.
By Hyde C, Wake B, Bryan S, Barton P, Fry-Smith A, Davenport C, et al.
-
Rituximab as third-line treatment for refractory or recurrent Stage III or IV follicular non-Hodgkin’s lymphoma: a systematic review and economic evaluation.
By Wake B, Hyde C, Bryan S, Barton P, Song F, Fry-Smith A, et al.
-
A systematic review of discharge arrangements for older people.
By Parker SG, Peet SM, McPherson A, Cannaby AM, Baker R, Wilson A, et al.
-
The clinical effectiveness and cost-effectiveness of inhaler devices used in the routine management of chronic asthma in older children: a systematic review and economic evaluation.
By Peters J, Stevenson M, Beverley C, Lim J, Smith S.
-
The clinical effectiveness and cost-effectiveness of sibutramine in the management of obesity: a technology assessment.
By O’Meara S, Riemsma R, Shirran L, Mather L, ter Riet G.
-
The cost-effectiveness of magnetic resonance angiography for carotid artery stenosis and peripheral vascular disease: a systematic review.
By Berry E, Kelly S, Westwood ME, Davies LM, Gough MJ, Bamford JM, et al.
-
Promoting physical activity in South Asian Muslim women through ‘exercise on prescription’.
By Carroll B, Ali N, Azam N.
-
Zanamivir for the treatment of influenza in adults: a systematic review and economic evaluation.
By Burls A, Clark W, Stewart T, Preston C, Bryan S, Jefferson T, et al.
-
A review of the natural history and epidemiology of multiple sclerosis: implications for resource allocation and health economic models.
By Richards RG, Sampson FC, Beard SM, Tappenden P.
-
Screening for gestational diabetes: a systematic review and economic evaluation.
By Scott DA, Loveman E, McIntyre L, Waugh N.
-
The clinical effectiveness and cost-effectiveness of surgery for people with morbid obesity: a systematic review and economic evaluation.
By Clegg AJ, Colquitt J, Sidhu MK, Royle P, Loveman E, Walker A.
-
The clinical effectiveness of trastuzumab for breast cancer: a systematic review.
By Lewis R, Bagnall A-M, Forbes C, Shirran E, Duffy S, Kleijnen J, et al.
-
The clinical effectiveness and cost-effectiveness of vinorelbine for breast cancer: a systematic review and economic evaluation.
By Lewis R, Bagnall A-M, King S, Woolacott N, Forbes C, Shirran L, et al.
-
A systematic review of the effectiveness and cost-effectiveness of metal-on-metal hip resurfacing arthroplasty for treatment of hip disease.
By Vale L, Wyness L, McCormack K, McKenzie L, Brazzelli M, Stearns SC.
-
The clinical effectiveness and cost-effectiveness of bupropion and nicotine replacement therapy for smoking cessation: a systematic review and economic evaluation.
By Woolacott NF, Jones L, Forbes CA, Mather LC, Sowden AJ, Song FJ, et al.
-
A systematic review of effectiveness and economic evaluation of new drug treatments for juvenile idiopathic arthritis: etanercept.
By Cummins C, Connock M, Fry-Smith A, Burls A.
-
Clinical effectiveness and cost-effectiveness of growth hormone in children: a systematic review and economic evaluation.
By Bryant J, Cave C, Mihaylova B, Chase D, McIntyre L, Gerard K, et al.
-
Clinical effectiveness and cost-effectiveness of growth hormone in adults in relation to impact on quality of life: a systematic review and economic evaluation.
By Bryant J, Loveman E, Chase D, Mihaylova B, Cave C, Gerard K, et al.
-
Clinical medication review by a pharmacist of patients on repeat prescriptions in general practice: a randomised controlled trial.
By Zermansky AG, Petty DR, Raynor DK, Lowe CJ, Freementle N, Vail A.
-
The effectiveness of infliximab and etanercept for the treatment of rheumatoid arthritis: a systematic review and economic evaluation.
By Jobanputra P, Barton P, Bryan S, Burls A.
-
A systematic review and economic evaluation of computerised cognitive behaviour therapy for depression and anxiety.
By Kaltenthaler E, Shackley P, Stevens K, Beverley C, Parry G, Chilcott J.
-
A systematic review and economic evaluation of pegylated liposomal doxorubicin hydrochloride for ovarian cancer.
By Forbes C, Wilby J, Richardson G, Sculpher M, Mather L, Riemsma R.
-
A systematic review of the effectiveness of interventions based on a stages-of-change approach to promote individual behaviour change.
By Riemsma RP, Pattenden J, Bridle C, Sowden AJ, Mather L, Watt IS, et al.
-
A systematic review update of the clinical effectiveness and cost-effectiveness of glycoprotein IIb/IIIa antagonists.
By Robinson M, Ginnelly L, Sculpher M, Jones L, Riemsma R, Palmer S, et al.
-
A systematic review of the effectiveness, cost-effectiveness and barriers to implementation of thrombolytic and neuroprotective therapy for acute ischaemic stroke in the NHS.
By Sandercock P, Berge E, Dennis M, Forbes J, Hand P, Kwan J, et al.
-
A randomised controlled crossover trial of nurse practitioner versus doctor-led outpatient care in a bronchiectasis clinic.
By Caine N, Sharples LD, Hollingworth W, French J, Keogan M, Exley A, et al.
-
Clinical effectiveness and cost – consequences of selective serotonin reuptake inhibitors in the treatment of sex offenders.
By Adi Y, Ashcroft D, Browne K, Beech A, Fry-Smith A, Hyde C.
-
Treatment of established osteoporosis: a systematic review and cost–utility analysis.
By Kanis JA, Brazier JE, Stevenson M, Calvert NW, Lloyd Jones M.
-
Which anaesthetic agents are cost-effective in day surgery? Literature review, national survey of practice and randomised controlled trial.
By Elliott RA Payne K, Moore JK, Davies LM, Harper NJN, St Leger AS, et al.
-
Screening for hepatitis C among injecting drug users and in genitourinary medicine clinics: systematic reviews of effectiveness, modelling study and national survey of current practice.
By Stein K, Dalziel K, Walker A, McIntyre L, Jenkins B, Horne J, et al.
-
The measurement of satisfaction with healthcare: implications for practice from a systematic review of the literature.
By Crow R, Gage H, Hampson S, Hart J, Kimber A, Storey L, et al.
-
The effectiveness and cost-effectiveness of imatinib in chronic myeloid leukaemia: a systematic review.
By Garside R, Round A, Dalziel K, Stein K, Royle R.
-
A comparative study of hypertonic saline, daily and alternate-day rhDNase in children with cystic fibrosis.
By Suri R, Wallis C, Bush A, Thompson S, Normand C, Flather M, et al.
-
A systematic review of the costs and effectiveness of different models of paediatric home care.
By Parker G, Bhakta P, Lovett CA, Paisley S, Olsen R, Turner D, et al.
-
How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study.
By Egger M, Jüni P, Bartlett C, Holenstein F, Sterne J.
-
Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of home versus hospital or satellite unit haemodialysis for people with end-stage renal failure.
By Mowatt G, Vale L, Perez J, Wyness L, Fraser C, MacLeod A, et al.
-
Systematic review and economic evaluation of the effectiveness of infliximab for the treatment of Crohn’s disease.
By Clark W, Raftery J, Barton P, Song F, Fry-Smith A, Burls A.
-
A review of the clinical effectiveness and cost-effectiveness of routine anti-D prophylaxis for pregnant women who are rhesus negative.
By Chilcott J, Lloyd Jones M, Wight J, Forman K, Wray J, Beverley C, et al.
-
Systematic review and evaluation of the use of tumour markers in paediatric oncology: Ewing’s sarcoma and neuroblastoma.
By Riley RD, Burchill SA, Abrams KR, Heney D, Lambert PC, Jones DR, et al.
-
The cost-effectiveness of screening for Helicobacter pylori to reduce mortality and morbidity from gastric cancer and peptic ulcer disease: a discrete-event simulation model.
By Roderick P, Davies R, Raftery J, Crabbe D, Pearce R, Bhandari P, et al.
-
The clinical effectiveness and cost-effectiveness of routine dental checks: a systematic review and economic evaluation.
By Davenport C, Elley K, Salas C, Taylor-Weetman CL, Fry-Smith A, Bryan S, et al.
-
A multicentre randomised controlled trial assessing the costs and benefits of using structured information and analysis of women’s preferences in the management of menorrhagia.
By Kennedy ADM, Sculpher MJ, Coulter A, Dwyer N, Rees M, Horsley S, et al.
-
Clinical effectiveness and cost–utility of photodynamic therapy for wet age-related macular degeneration: a systematic review and economic evaluation.
By Meads C, Salas C, Roberts T, Moore D, Fry-Smith A, Hyde C.
-
Evaluation of molecular tests for prenatal diagnosis of chromosome abnormalities.
By Grimshaw GM, Szczepura A, Hultén M, MacDonald F, Nevin NC, Sutton F, et al.
-
First and second trimester antenatal screening for Down’s syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS).
By Wald NJ, Rodeck C, Hackshaw AK, Walters J, Chitty L, Mackinson AM.
-
The effectiveness and cost-effectiveness of ultrasound locating devices for central venous access: a systematic review and economic evaluation.
By Calvert N, Hind D, McWilliams RG, Thomas SM, Beverley C, Davidson A.
-
A systematic review of atypical antipsychotics in schizophrenia.
By Bagnall A-M, Jones L, Lewis R, Ginnelly L, Glanville J, Torgerson D, et al.
-
Prostate Testing for Cancer and Treatment (ProtecT) feasibility study.
By Donovan J, Hamdy F, Neal D, Peters T, Oliver S, Brindle L, et al.
-
Early thrombolysis for the treatment of acute myocardial infarction: a systematic review and economic evaluation.
By Boland A, Dundar Y, Bagust A, Haycox A, Hill R, Mujica Mota R, et al.
-
Screening for fragile X syndrome: a literature review and modelling.
By Song FJ, Barton P, Sleightholme V, Yao GL, Fry-Smith A.
-
Systematic review of endoscopic sinus surgery for nasal polyps.
By Dalziel K, Stein K, Round A, Garside R, Royle P.
-
Towards efficient guidelines: how to monitor guideline use in primary care.
By Hutchinson A, McIntosh A, Cox S, Gilbert C.
-
Effectiveness and cost-effectiveness of acute hospital-based spinal cord injuries services: systematic review.
By Bagnall A-M, Jones L, Richardson G, Duffy S, Riemsma R.
-
Prioritisation of health technology assessment. The PATHS model: methods and case studies.
By Townsend J, Buxton M, Harper G.
-
Systematic review of the clinical effectiveness and cost-effectiveness of tension-free vaginal tape for treatment of urinary stress incontinence.
By Cody J, Wyness L, Wallace S, Glazener C, Kilonzo M, Stearns S, et al.
-
The clinical and cost-effectiveness of patient education models for diabetes: a systematic review and economic evaluation.
By Loveman E, Cave C, Green C, Royle P, Dunn N, Waugh N.
-
The role of modelling in prioritising and planning clinical trials.
By Chilcott J, Brennan A, Booth A, Karnon J, Tappenden P.
-
Cost–benefit evaluation of routine influenza immunisation in people 65–74 years of age.
By Allsup S, Gosney M, Haycox A, Regan M.
-
The clinical and cost-effectiveness of pulsatile machine perfusion versus cold storage of kidneys for transplantation retrieved from heart-beating and non-heart-beating donors.
By Wight J, Chilcott J, Holmes M, Brewer N.
-
Can randomised trials rely on existing electronic data? A feasibility study to explore the value of routine data in health technology assessment.
By Williams JG, Cheung WY, Cohen DR, Hutchings HA, Longo MF, Russell IT.
-
Evaluating non-randomised intervention studies.
By Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, et al.
-
A randomised controlled trial to assess the impact of a package comprising a patient-orientated, evidence-based self- help guidebook and patient-centred consultations on disease management and satisfaction in inflammatory bowel disease.
By Kennedy A, Nelson E, Reeves D, Richardson G, Roberts C, Robinson A, et al.
-
The effectiveness of diagnostic tests for the assessment of shoulder pain due to soft tissue disorders: a systematic review.
By Dinnes J, Loveman E, McIntyre L, Waugh N.
-
The value of digital imaging in diabetic retinopathy.
By Sharp PF, Olson J, Strachan F, Hipwell J, Ludbrook A, O’Donnell M, et al.
-
Lowering blood pressure to prevent myocardial infarction and stroke: a new preventive strategy.
By Law M, Wald N, Morris J.
-
Clinical and cost-effectiveness of capecitabine and tegafur with uracil for the treatment of metastatic colorectal cancer: systematic review and economic evaluation.
By Ward S, Kaltenthaler E, Cowan J, Brewer N.
-
Clinical and cost-effectiveness of new and emerging technologies for early localised prostate cancer: a systematic review.
By Hummel S, Paisley S, Morgan A, Currie E, Brewer N.
-
Literature searching for clinical and cost-effectiveness studies used in health technology assessment reports carried out for the National Institute for Clinical Excellence appraisal system.
By Royle P, Waugh N.
-
Systematic review and economic decision modelling for the prevention and treatment of influenza A and B.
By Turner D, Wailoo A, Nicholson K, Cooper N, Sutton A, Abrams K.
-
A randomised controlled trial to evaluate the clinical and cost-effectiveness of Hickman line insertions in adult cancer patients by nurses.
By Boland A, Haycox A, Bagust A, Fitzsimmons L.
-
Redesigning postnatal care: a randomised controlled trial of protocol-based midwifery-led care focused on individual women’s physical and psychological health needs.
By MacArthur C, Winter HR, Bick DE, Lilford RJ, Lancashire RJ, Knowles H, et al.
-
Estimating implied rates of discount in healthcare decision-making.
By West RR, McNabb R, Thompson AGH, Sheldon TA, Grimley Evans J.
-
Systematic review of isolation policies in the hospital management of methicillin-resistant Staphylococcus aureus: a review of the literature with epidemiological and economic modelling.
By Cooper BS, Stone SP, Kibbler CC, Cookson BD, Roberts JA, Medley GF, et al.
-
Treatments for spasticity and pain in multiple sclerosis: a systematic review.
By Beard S, Hunn A, Wight J.
-
The inclusion of reports of randomised trials published in languages other than English in systematic reviews.
By Moher D, Pham B, Lawson ML, Klassen TP.
-
The impact of screening on future health-promoting behaviours and health beliefs: a systematic review.
By Bankhead CR, Brett J, Bukach C, Webster P, Stewart-Brown S, Munafo M, et al.
-
What is the best imaging strategy for acute stroke?
By Wardlaw JM, Keir SL, Seymour J, Lewis S, Sandercock PAG, Dennis MS, et al.
-
Systematic review and modelling of the investigation of acute and chronic chest pain presenting in primary care.
By Mant J, McManus RJ, Oakes RAL, Delaney BC, Barton PM, Deeks JJ, et al.
-
The effectiveness and cost-effectiveness of microwave and thermal balloon endometrial ablation for heavy menstrual bleeding: a systematic review and economic modelling.
By Garside R, Stein K, Wyatt K, Round A, Price A.
-
A systematic review of the role of bisphosphonates in metastatic disease.
By Ross JR, Saunders Y, Edmonds PM, Patel S, Wonderling D, Normand C, et al.
-
Systematic review of the clinical effectiveness and cost-effectiveness of capecitabine (Xeloda®) for locally advanced and/or metastatic breast cancer.
By Jones L, Hawkins N, Westwood M, Wright K, Richardson G, Riemsma R.
-
Effectiveness and efficiency of guideline dissemination and implementation strategies.
By Grimshaw JM, Thomas RE, MacLennan G, Fraser C, Ramsay CR, Vale L, et al.
-
Clinical effectiveness and costs of the Sugarbaker procedure for the treatment of pseudomyxoma peritonei.
By Bryant J, Clegg AJ, Sidhu MK, Brodin H, Royle P, Davidson P.
-
Psychological treatment for insomnia in the regulation of long-term hypnotic drug use.
By Morgan K, Dixon S, Mathers N, Thompson J, Tomeny M.
-
Improving the evaluation of therapeutic interventions in multiple sclerosis: development of a patient-based measure of outcome.
By Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ.
-
A systematic review and economic evaluation of magnetic resonance cholangiopancreatography compared with diagnostic endoscopic retrograde cholangiopancreatography.
By Kaltenthaler E, Bravo Vergel Y, Chilcott J, Thomas S, Blakeborough T, Walters SJ, et al.
-
The use of modelling to evaluate new drugs for patients with a chronic condition: the case of antibodies against tumour necrosis factor in rheumatoid arthritis.
By Barton P, Jobanputra P, Wilson J, Bryan S, Burls A.
-
Clinical effectiveness and cost-effectiveness of neonatal screening for inborn errors of metabolism using tandem mass spectrometry: a systematic review.
By Pandor A, Eastham J, Beverley C, Chilcott J, Paisley S.
-
Clinical effectiveness and cost-effectiveness of pioglitazone and rosiglitazone in the treatment of type 2 diabetes: a systematic review and economic evaluation.
By Czoski-Murray C, Warren E, Chilcott J, Beverley C, Psyllaki MA, Cowan J.
-
Routine examination of the newborn: the EMREN study. Evaluation of an extension of the midwife role including a randomised controlled trial of appropriately trained midwives and paediatric senior house officers.
By Townsend J, Wolke D, Hayes J, Davé S, Rogers C, Bloomfield L, et al.
-
Involving consumers in research and development agenda setting for the NHS: developing an evidence-based approach.
By Oliver S, Clarke-Jones L, Rees R, Milne R, Buchanan P, Gabbay J, et al.
-
A multi-centre randomised controlled trial of minimally invasive direct coronary bypass grafting versus percutaneous transluminal coronary angioplasty with stenting for proximal stenosis of the left anterior descending coronary artery.
By Reeves BC, Angelini GD, Bryan AJ, Taylor FC, Cripps T, Spyt TJ, et al.
-
Does early magnetic resonance imaging influence management or improve outcome in patients referred to secondary care with low back pain? A pragmatic randomised controlled trial.
By Gilbert FJ, Grant AM, Gillan MGC, Vale L, Scott NW, Campbell MK, et al.
-
The clinical and cost-effectiveness of anakinra for the treatment of rheumatoid arthritis in adults: a systematic review and economic analysis.
By Clark W, Jobanputra P, Barton P, Burls A.
-
A rapid and systematic review and economic evaluation of the clinical and cost-effectiveness of newer drugs for treatment of mania associated with bipolar affective disorder.
By Bridle C, Palmer S, Bagnall A-M, Darba J, Duffy S, Sculpher M, et al.
-
Liquid-based cytology in cervical screening: an updated rapid and systematic review and economic analysis.
By Karnon J, Peters J, Platt J, Chilcott J, McGoogan E, Brewer N.
-
Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement.
By Avenell A, Broom J, Brown TJ, Poobalan A, Aucott L, Stearns SC, et al.
-
Autoantibody testing in children with newly diagnosed type 1 diabetes mellitus.
By Dretzke J, Cummins C, Sandercock J, Fry-Smith A, Barrett T, Burls A.
-
Clinical effectiveness and cost-effectiveness of prehospital intravenous fluids in trauma patients.
By Dretzke J, Sandercock J, Bayliss S, Burls A.
-
Newer hypnotic drugs for the short-term management of insomnia: a systematic review and economic evaluation.
By Dündar Y, Boland A, Strobl J, Dodd S, Haycox A, Bagust A, et al.
-
Development and validation of methods for assessing the quality of diagnostic accuracy studies.
By Whiting P, Rutjes AWS, Dinnes J, Reitsma JB, Bossuyt PMM, Kleijnen J.
-
EVALUATE hysterectomy trial: a multicentre randomised trial comparing abdominal, vaginal and laparoscopic methods of hysterectomy.
By Garry R, Fountain J, Brown J, Manca A, Mason S, Sculpher M, et al.
-
Methods for expected value of information analysis in complex health economic models: developments on the health economics of interferon-β and glatiramer acetate for multiple sclerosis.
By Tappenden P, Chilcott JB, Eggington S, Oakley J, McCabe C.
-
Effectiveness and cost-effectiveness of imatinib for first-line treatment of chronic myeloid leukaemia in chronic phase: a systematic review and economic analysis.
By Dalziel K, Round A, Stein K, Garside R, Price A.
-
VenUS I: a randomised controlled trial of two types of bandage for treating venous leg ulcers.
By Iglesias C, Nelson EA, Cullum NA, Torgerson DJ, on behalf of the VenUS Team.
-
Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of myocardial perfusion scintigraphy for the diagnosis and management of angina and myocardial infarction.
By Mowatt G, Vale L, Brazzelli M, Hernandez R, Murray A, Scott N, et al.
-
A pilot study on the use of decision theory and value of information analysis as part of the NHS Health Technology Assessment programme.
By Claxton K, Ginnelly L, Sculpher M, Philips Z, Palmer S.
-
The Social Support and Family Health Study: a randomised controlled trial and economic evaluation of two alternative forms of postnatal support for mothers living in disadvantaged inner-city areas.
By Wiggins M, Oakley A, Roberts I, Turner H, Rajan L, Austerberry H, et al.
-
Psychosocial aspects of genetic screening of pregnant women and newborns: a systematic review.
By Green JM, Hewison J, Bekker HL, Bryant, Cuckle HS.
-
Evaluation of abnormal uterine bleeding: comparison of three outpatient procedures within cohorts defined by age and menopausal status.
By Critchley HOD, Warner P, Lee AJ, Brechin S, Guise J, Graham B.
-
Coronary artery stents: a rapid systematic review and economic evaluation.
By Hill R, Bagust A, Bakhai A, Dickson R, Dündar Y, Haycox A, et al.
-
Review of guidelines for good practice in decision-analytic modelling in health technology assessment.
By Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al.
-
Rituximab (MabThera®) for aggressive non-Hodgkin’s lymphoma: systematic review and economic evaluation.
By Knight C, Hind D, Brewer N, Abbott V.
-
Clinical effectiveness and cost-effectiveness of clopidogrel and modified-release dipyridamole in the secondary prevention of occlusive vascular events: a systematic review and economic evaluation.
By Jones L, Griffin S, Palmer S, Main C, Orton V, Sculpher M, et al.
-
Pegylated interferon α-2a and -2b in combination with ribavirin in the treatment of chronic hepatitis C: a systematic review and economic evaluation.
By Shepherd J, Brodin H, Cave C, Waugh N, Price A, Gabbay J.
-
Clopidogrel used in combination with aspirin compared with aspirin alone in the treatment of non-ST-segment- elevation acute coronary syndromes: a systematic review and economic evaluation.
By Main C, Palmer S, Griffin S, Jones L, Orton V, Sculpher M, et al.
-
Provision, uptake and cost of cardiac rehabilitation programmes: improving services to under-represented groups.
By Beswick AD, Rees K, Griebsch I, Taylor FC, Burke M, West RR, et al.
-
Involving South Asian patients in clinical trials.
By Hussain-Gambles M, Leese B, Atkin K, Brown J, Mason S, Tovey P.
-
Clinical and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes.
By Colquitt JL, Green C, Sidhu MK, Hartwell D, Waugh N.
-
Identification and assessment of ongoing trials in health technology assessment reviews.
By Song FJ, Fry-Smith A, Davenport C, Bayliss S, Adi Y, Wilson JS, et al.
-
Systematic review and economic evaluation of a long-acting insulin analogue, insulin glargine
By Warren E, Weatherley-Jones E, Chilcott J, Beverley C.
-
Supplementation of a home-based exercise programme with a class-based programme for people with osteoarthritis of the knees: a randomised controlled trial and health economic analysis.
By McCarthy CJ, Mills PM, Pullen R, Richardson G, Hawkins N, Roberts CR, et al.
-
Clinical and cost-effectiveness of once-daily versus more frequent use of same potency topical corticosteroids for atopic eczema: a systematic review and economic evaluation.
By Green C, Colquitt JL, Kirby J, Davidson P, Payne E.
-
Acupuncture of chronic headache disorders in primary care: randomised controlled trial and economic analysis.
By Vickers AJ, Rees RW, Zollman CE, McCarney R, Smith CM, Ellis N, et al.
-
Generalisability in economic evaluation studies in healthcare: a review and case studies.
By Sculpher MJ, Pang FS, Manca A, Drummond MF, Golder S, Urdahl H, et al.
-
Virtual outreach: a randomised controlled trial and economic evaluation of joint teleconferenced medical consultations.
By Wallace P, Barber J, Clayton W, Currell R, Fleming K, Garner P, et al.
-
Randomised controlled multiple treatment comparison to provide a cost-effectiveness rationale for the selection of antimicrobial therapy in acne.
By Ozolins M, Eady EA, Avery A, Cunliffe WJ, O’Neill C, Simpson NB, et al.
-
Do the findings of case series studies vary significantly according to methodological characteristics?
By Dalziel K, Round A, Stein K, Garside R, Castelnuovo E, Payne L.
-
Improving the referral process for familial breast cancer genetic counselling: findings of three randomised controlled trials of two interventions.
By Wilson BJ, Torrance N, Mollison J, Wordsworth S, Gray JR, Haites NE, et al.
-
Randomised evaluation of alternative electrosurgical modalities to treat bladder outflow obstruction in men with benign prostatic hyperplasia.
By Fowler C, McAllister W, Plail R, Karim O, Yang Q.
-
A pragmatic randomised controlled trial of the cost-effectiveness of palliative therapies for patients with inoperable oesophageal cancer.
By Shenfine J, McNamee P, Steen N, Bond J, Griffin SM.
-
Impact of computer-aided detection prompts on the sensitivity and specificity of screening mammography.
By Taylor P, Champness J, Given- Wilson R, Johnston K, Potts H.
-
Issues in data monitoring and interim analysis of trials.
By Grant AM, Altman DG, Babiker AB, Campbell MK, Clemens FJ, Darbyshire JH, et al.
-
Lay public’s understanding of equipoise and randomisation in randomised controlled trials.
By Robinson EJ, Kerr CEP, Stevens AJ, Lilford RJ, Braunholtz DA, Edwards SJ, et al.
-
Clinical and cost-effectiveness of electroconvulsive therapy for depressive illness, schizophrenia, catatonia and mania: systematic reviews and economic modelling studies.
By Greenhalgh J, Knight C, Hind D, Beverley C, Walters S.
-
Measurement of health-related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology.
By Smith SC, Lamping DL, Banerjee S, Harwood R, Foley B, Smith P, et al.
-
Clinical effectiveness and cost-effectiveness of drotrecogin alfa (activated) (Xigris®) for the treatment of severe sepsis in adults: a systematic review and economic evaluation.
By Green C, Dinnes J, Takeda A, Shepherd J, Hartwell D, Cave C, et al.
-
A methodological review of how heterogeneity has been examined in systematic reviews of diagnostic test accuracy.
By Dinnes J, Deeks J, Kirby J, Roderick P.
-
Cervical screening programmes: can automation help? Evidence from systematic reviews, an economic analysis and a simulation modelling exercise applied to the UK.
By Willis BH, Barton P, Pearmain P, Bryan S, Hyde C.
-
Laparoscopic surgery for inguinal hernia repair: systematic review of effectiveness and economic evaluation.
By McCormack K, Wake B, Perez J, Fraser C, Cook J, McIntosh E, et al.
-
Clinical effectiveness, tolerability and cost-effectiveness of newer drugs for epilepsy in adults: a systematic review and economic evaluation.
By Wilby J, Kainth A, Hawkins N, Epstein D, McIntosh H, McDaid C, et al.
-
A randomised controlled trial to compare the cost-effectiveness of tricyclic antidepressants, selective serotonin reuptake inhibitors and lofepramine.
By Peveler R, Kendrick T, Buxton M, Longworth L, Baldwin D, Moore M, et al.
-
Clinical effectiveness and cost-effectiveness of immediate angioplasty for acute myocardial infarction: systematic review and economic evaluation.
By Hartwell D, Colquitt J, Loveman E, Clegg AJ, Brodin H, Waugh N, et al.
-
A randomised controlled comparison of alternative strategies in stroke care.
By Kalra L, Evans A, Perez I, Knapp M, Swift C, Donaldson N.
-
The investigation and analysis of critical incidents and adverse events in healthcare.
By Woloshynowych M, Rogers S, Taylor-Adams S, Vincent C.
-
Potential use of routine databases in health technology assessment.
By Raftery J, Roderick P, Stevens A.
-
Clinical and cost-effectiveness of newer immunosuppressive regimens in renal transplantation: a systematic review and modelling study.
By Woodroffe R, Yao GL, Meads C, Bayliss S, Ready A, Raftery J, et al.
-
A systematic review and economic evaluation of alendronate, etidronate, risedronate, raloxifene and teriparatide for the prevention and treatment of postmenopausal osteoporosis.
By Stevenson M, Lloyd Jones M, De Nigris E, Brewer N, Davis S, Oakley J.
-
A systematic review to examine the impact of psycho-educational interventions on health outcomes and costs in adults and children with difficult asthma.
By Smith JR, Mugford M, Holland R, Candy B, Noble MJ, Harrison BDW, et al.
-
An evaluation of the costs, effectiveness and quality of renal replacement therapy provision in renal satellite units in England and Wales.
By Roderick P, Nicholson T, Armitage A, Mehta R, Mullee M, Gerard K, et al.
-
Imatinib for the treatment of patients with unresectable and/or metastatic gastrointestinal stromal tumours: systematic review and economic evaluation.
By Wilson J, Connock M, Song F, Yao G, Fry-Smith A, Raftery J, et al.
-
Indirect comparisons of competing interventions.
By Glenny AM, Altman DG, Song F, Sakarovitch C, Deeks JJ, D’Amico R, et al.
-
Cost-effectiveness of alternative strategies for the initial medical management of non-ST elevation acute coronary syndrome: systematic review and decision-analytical modelling.
By Robinson M, Palmer S, Sculpher M, Philips Z, Ginnelly L, Bowens A, et al.
-
Outcomes of electrically stimulated gracilis neosphincter surgery.
By Tillin T, Chambers M, Feldman R.
-
The effectiveness and cost-effectiveness of pimecrolimus and tacrolimus for atopic eczema: a systematic review and economic evaluation.
By Garside R, Stein K, Castelnuovo E, Pitt M, Ashcroft D, Dimmock P, et al.
-
Systematic review on urine albumin testing for early detection of diabetic complications.
By Newman DJ, Mattock MB, Dawnay ABS, Kerry S, McGuire A, Yaqoob M, et al.
-
Randomised controlled trial of the cost-effectiveness of water-based therapy for lower limb osteoarthritis.
By Cochrane T, Davey RC, Matthes Edwards SM.
-
Longer term clinical and economic benefits of offering acupuncture care to patients with chronic low back pain.
By Thomas KJ, MacPherson H, Ratcliffe J, Thorpe L, Brazier J, Campbell M, et al.
-
Cost-effectiveness and safety of epidural steroids in the management of sciatica.
By Price C, Arden N, Coglan L, Rogers P.
-
The British Rheumatoid Outcome Study Group (BROSG) randomised controlled trial to compare the effectiveness and cost-effectiveness of aggressive versus symptomatic therapy in established rheumatoid arthritis.
By Symmons D, Tricker K, Roberts C, Davies L, Dawes P, Scott DL.
-
Conceptual framework and systematic review of the effects of participants’ and professionals’ preferences in randomised controlled trials.
By King M, Nazareth I, Lampe F, Bower P, Chandler M, Morou M, et al.
-
The clinical and cost-effectiveness of implantable cardioverter defibrillators: a systematic review.
By Bryant J, Brodin H, Loveman E, Payne E, Clegg A.
-
A trial of problem-solving by community mental health nurses for anxiety, depression and life difficulties among general practice patients. The CPN-GP study.
By Kendrick T, Simons L, Mynors-Wallis L, Gray A, Lathlean J, Pickering R, et al.
-
The causes and effects of socio-demographic exclusions from clinical trials.
By Bartlett C, Doyal L, Ebrahim S, Davey P, Bachmann M, Egger M, et al.
-
Is hydrotherapy cost-effective? A randomised controlled trial of combined hydrotherapy programmes compared with physiotherapy land techniques in children with juvenile idiopathic arthritis.
By Epps H, Ginnelly L, Utley M, Southwood T, Gallivan S, Sculpher M, et al.
-
A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study.
By Hobbs FDR, Fitzmaurice DA, Mant J, Murray E, Jowett S, Bryan S, et al.
-
Displaced intracapsular hip fractures in fit, older people: a randomised comparison of reduction and fixation, bipolar hemiarthroplasty and total hip arthroplasty.
By Keating JF, Grant A, Masson M, Scott NW, Forbes JF.
-
Long-term outcome of cognitive behaviour therapy clinical trials in central Scotland.
By Durham RC, Chambers JA, Power KG, Sharp DM, Macdonald RR, Major KA, et al.
-
The effectiveness and cost-effectiveness of dual-chamber pacemakers compared with single-chamber pacemakers for bradycardia due to atrioventricular block or sick sinus syndrome: systematic review and economic evaluation.
By Castelnuovo E, Stein K, Pitt M, Garside R, Payne E.
-
Newborn screening for congenital heart defects: a systematic review and cost-effectiveness analysis.
By Knowles R, Griebsch I, Dezateux C, Brown J, Bull C, Wren C.
-
The clinical and cost-effectiveness of left ventricular assist devices for end-stage heart failure: a systematic review and economic evaluation.
By Clegg AJ, Scott DA, Loveman E, Colquitt J, Hutchinson J, Royle P, et al.
-
The effectiveness of the Heidelberg Retina Tomograph and laser diagnostic glaucoma scanning system (GDx) in detecting and monitoring glaucoma.
By Kwartz AJ, Henson DB, Harper RA, Spencer AF, McLeod D.
-
Clinical and cost-effectiveness of autologous chondrocyte implantation for cartilage defects in knee joints: systematic review and economic evaluation.
By Clar C, Cummins E, McIntyre L, Thomas S, Lamb J, Bain L, et al.
-
Systematic review of effectiveness of different treatments for childhood retinoblastoma.
By McDaid C, Hartley S, Bagnall A-M, Ritchie G, Light K, Riemsma R.
-
Towards evidence-based guidelines for the prevention of venous thromboembolism: systematic reviews of mechanical methods, oral anticoagulation, dextran and regional anaesthesia as thromboprophylaxis.
By Roderick P, Ferris G, Wilson K, Halls H, Jackson D, Collins R, et al.
-
The effectiveness and cost-effectiveness of parent training/education programmes for the treatment of conduct disorder, including oppositional defiant disorder, in children.
By Dretzke J, Frew E, Davenport C, Barlow J, Stewart-Brown S, Sandercock J, et al.
-
The clinical and cost-effectiveness of donepezil, rivastigmine, galantamine and memantine for Alzheimer’s disease.
By Loveman E, Green C, Kirby J, Takeda A, Picot J, Payne E, et al.
-
FOOD: a multicentre randomised trial evaluating feeding policies in patients admitted to hospital with a recent stroke.
By Dennis M, Lewis S, Cranswick G, Forbes J.
-
The clinical effectiveness and cost-effectiveness of computed tomography screening for lung cancer: systematic reviews.
By Black C, Bagust A, Boland A, Walker S, McLeod C, De Verteuil R, et al.
-
A systematic review of the effectiveness and cost-effectiveness of neuroimaging assessments used to visualise the seizure focus in people with refractory epilepsy being considered for surgery.
By Whiting P, Gupta R, Burch J, Mujica Mota RE, Wright K, Marson A, et al.
-
Comparison of conference abstracts and presentations with full-text articles in the health technology assessments of rapidly evolving technologies.
By Dundar Y, Dodd S, Dickson R, Walley T, Haycox A, Williamson PR.
-
Systematic review and evaluation of methods of assessing urinary incontinence.
By Martin JL, Williams KS, Abrams KR, Turner DA, Sutton AJ, Chapple C, et al.
-
The clinical effectiveness and cost-effectiveness of newer drugs for children with epilepsy. A systematic review.
By Connock M, Frew E, Evans B-W, Bryan S, Cummins C, Fry-Smith A, et al.
-
Surveillance of Barrett’s oesophagus: exploring the uncertainty through systematic review, expert workshop and economic modelling.
By Garside R, Pitt M, Somerville M, Stein K, Price A, Gilbert N.
-
Topotecan, pegylated liposomal doxorubicin hydrochloride and paclitaxel for second-line or subsequent treatment of advanced ovarian cancer: a systematic review and economic evaluation.
By Main C, Bojke L, Griffin S, Norman G, Barbieri M, Mather L, et al.
-
Evaluation of molecular techniques in prediction and diagnosis of cytomegalovirus disease in immunocompromised patients.
By Szczepura A, Westmoreland D, Vinogradova Y, Fox J, Clark M.
-
Screening for thrombophilia in high-risk situations: systematic review and cost-effectiveness analysis. The Thrombosis: Risk and Economic Assessment of Thrombophilia Screening (TREATS) study.
By Wu O, Robertson L, Twaddle S, Lowe GDO, Clark P, Greaves M, et al.
-
A series of systematic reviews to inform a decision analysis for sampling and treating infected diabetic foot ulcers.
By Nelson EA, O’Meara S, Craig D, Iglesias C, Golder S, Dalton J, et al.
-
Randomised clinical trial, observational study and assessment of cost-effectiveness of the treatment of varicose veins (REACTIV trial).
By Michaels JA, Campbell WB, Brazier JE, MacIntyre JB, Palfreyman SJ, Ratcliffe J, et al.
-
The cost-effectiveness of screening for oral cancer in primary care.
By Speight PM, Palmer S, Moles DR, Downer MC, Smith DH, Henriksson M, et al.
-
Measurement of the clinical and cost-effectiveness of non-invasive diagnostic testing strategies for deep vein thrombosis.
By Goodacre S, Sampson F, Stevenson M, Wailoo A, Sutton A, Thomas S, et al.
-
Systematic review of the effectiveness and cost-effectiveness of HealOzone® for the treatment of occlusal pit/fissure caries and root caries.
By Brazzelli M, McKenzie L, Fielding S, Fraser C, Clarkson J, Kilonzo M, et al.
-
Randomised controlled trials of conventional antipsychotic versus new atypical drugs, and new atypical drugs versus clozapine, in people with schizophrenia responding poorly to, or intolerant of, current drug treatment.
By Lewis SW, Davies L, Jones PB, Barnes TRE, Murray RM, Kerwin R, et al.
-
Diagnostic tests and algorithms used in the investigation of haematuria: systematic reviews and economic evaluation.
By Rodgers M, Nixon J, Hempel S, Aho T, Kelly J, Neal D, et al.
-
Cognitive behavioural therapy in addition to antispasmodic therapy for irritable bowel syndrome in primary care: randomised controlled trial.
By Kennedy TM, Chalder T, McCrone P, Darnley S, Knapp M, Jones RH, et al.
-
A systematic review of the clinical effectiveness and cost-effectiveness of enzyme replacement therapies for Fabry’s disease and mucopolysaccharidosis type 1.
By Connock M, Juarez-Garcia A, Frew E, Mans A, Dretzke J, Fry-Smith A, et al.
-
Health benefits of antiviral therapy for mild chronic hepatitis C: randomised controlled trial and economic evaluation.
By Wright M, Grieve R, Roberts J, Main J, Thomas HC, on behalf of the UK Mild Hepatitis C Trial Investigators.
-
Pressure relieving support surfaces: a randomised evaluation.
By Nixon J, Nelson EA, Cranny G, Iglesias CP, Hawkins K, Cullum NA, et al.
-
A systematic review and economic model of the effectiveness and cost-effectiveness of methylphenidate, dexamfetamine and atomoxetine for the treatment of attention deficit hyperactivity disorder in children and adolescents.
By King S, Griffin S, Hodges Z, Weatherly H, Asseburg C, Richardson G, et al.
-
The clinical effectiveness and cost-effectiveness of enzyme replacement therapy for Gaucher’s disease: a systematic review.
By Connock M, Burls A, Frew E, Fry-Smith A, Juarez-Garcia A, McCabe C, et al.
-
Effectiveness and cost-effectiveness of salicylic acid and cryotherapy for cutaneous warts. An economic decision model.
By Thomas KS, Keogh-Brown MR, Chalmers JR, Fordham RJ, Holland RC, Armstrong SJ, et al.
-
A systematic literature review of the effectiveness of non-pharmacological interventions to prevent wandering in dementia and evaluation of the ethical implications and acceptability of their use.
By Robinson L, Hutchings D, Corner L, Beyer F, Dickinson H, Vanoli A, et al.
-
A review of the evidence on the effects and costs of implantable cardioverter defibrillator therapy in different patient groups, and modelling of cost-effectiveness and cost–utility for these groups in a UK context.
By Buxton M, Caine N, Chase D, Connelly D, Grace A, Jackson C, et al.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.
By Shepherd J, Jones J, Takeda A, Davidson P, Price A.
-
An evaluation of the clinical and cost-effectiveness of pulmonary artery catheters in patient management in intensive care: a systematic review and a randomised controlled trial.
By Harvey S, Stevens K, Harrison D, Young D, Brampton W, McCabe C, et al.
-
Accurate, practical and cost-effective assessment of carotid stenosis in the UK.
By Wardlaw JM, Chappell FM, Stevenson M, De Nigris E, Thomas S, Gillard J, et al.
-
Etanercept and infliximab for the treatment of psoriatic arthritis: a systematic review and economic evaluation.
By Woolacott N, Bravo Vergel Y, Hawkins N, Kainth A, Khadjesari Z, Misso K, et al.
-
The cost-effectiveness of testing for hepatitis C in former injecting drug users.
By Castelnuovo E, Thompson-Coon J, Pitt M, Cramp M, Siebert U, Price A, et al.
-
Computerised cognitive behaviour therapy for depression and anxiety update: a systematic review and economic evaluation.
By Kaltenthaler E, Brazier J, De Nigris E, Tumur I, Ferriter M, Beverley C, et al.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.
By Williams C, Brunskill S, Altman D, Briggs A, Campbell H, Clarke M, et al.
-
Psychological therapies including dialectical behaviour therapy for borderline personality disorder: a systematic review and preliminary economic evaluation.
By Brazier J, Tumur I, Holmes M, Ferriter M, Parry G, Dent-Brown K, et al.
-
Clinical effectiveness and cost-effectiveness of tests for the diagnosis and investigation of urinary tract infection in children: a systematic review and economic model.
By Whiting P, Westwood M, Bojke L, Palmer S, Richardson G, Cooper J, et al.
-
Cognitive behavioural therapy in chronic fatigue syndrome: a randomised controlled trial of an outpatient group programme.
By O’Dowd H, Gladwell P, Rogers CA, Hollinghurst S, Gregory A.
-
A comparison of the cost-effectiveness of five strategies for the prevention of nonsteroidal anti-inflammatory drug-induced gastrointestinal toxicity: a systematic review with economic modelling.
By Brown TJ, Hooper L, Elliott RA, Payne K, Webb R, Roberts C, et al.
-
The effectiveness and cost-effectiveness of computed tomography screening for coronary artery disease: systematic review.
By Waugh N, Black C, Walker S, McIntyre L, Cummins E, Hillis G.
-
What are the clinical outcome and cost-effectiveness of endoscopy undertaken by nurses when compared with doctors? A Multi-Institution Nurse Endoscopy Trial (MINuET).
By Williams J, Russell I, Durai D, Cheung W-Y, Farrin A, Bloor K, et al.
-
The clinical and cost-effectiveness of oxaliplatin and capecitabine for the adjuvant treatment of colon cancer: systematic review and economic evaluation.
By Pandor A, Eggington S, Paisley S, Tappenden P, Sutcliffe P.
-
A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness.
By Chen Y-F, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, et al.
-
Telemedicine in dermatology: a randomised controlled trial.
By Bowns IR, Collins K, Walters SJ, McDonagh AJG.
-
Cost-effectiveness of cell salvage and alternative methods of minimising perioperative allogeneic blood transfusion: a systematic review and economic model.
By Davies L, Brown TJ, Haynes S, Payne K, Elliott RA, McCollum C.
-
Clinical effectiveness and cost-effectiveness of laparoscopic surgery for colorectal cancer: systematic reviews and economic evaluation.
By Murray A, Lourenco T, de Verteuil R, Hernandez R, Fraser C, McKinley A, et al.
-
Etanercept and efalizumab for the treatment of psoriasis: a systematic review.
By Woolacott N, Hawkins N, Mason A, Kainth A, Khadjesari Z, Bravo Vergel Y, et al.
-
Systematic reviews of clinical decision tools for acute abdominal pain.
By Liu JLY, Wyatt JC, Deeks JJ, Clamp S, Keen J, Verde P, et al.
-
Evaluation of the ventricular assist device programme in the UK.
By Sharples L, Buxton M, Caine N, Cafferty F, Demiris N, Dyer M, et al.
-
A systematic review and economic model of the clinical and cost-effectiveness of immunosuppressive therapy for renal transplantation in children.
By Yao G, Albon E, Adi Y, Milford D, Bayliss S, Ready A, et al.
-
Amniocentesis results: investigation of anxiety. The ARIA trial.
By Hewison J, Nixon J, Fountain J, Cocks K, Jones C, Mason G, et al.
-
Pemetrexed disodium for the treatment of malignant pleural mesothelioma: a systematic review and economic evaluation.
By Dundar Y, Bagust A, Dickson R, Dodd S, Green J, Haycox A, et al.
-
A systematic review and economic model of the clinical effectiveness and cost-effectiveness of docetaxel in combination with prednisone or prednisolone for the treatment of hormone-refractory metastatic prostate cancer.
By Collins R, Fenwick E, Trowman R, Perard R, Norman G, Light K, et al.
-
A systematic review of rapid diagnostic tests for the detection of tuberculosis infection.
By Dinnes J, Deeks J, Kunst H, Gibson A, Cummins E, Waugh N, et al.
-
The clinical effectiveness and cost-effectiveness of strontium ranelate for the prevention of osteoporotic fragility fractures in postmenopausal women.
By Stevenson M, Davis S, Lloyd-Jones M, Beverley C.
-
A systematic review of quantitative and qualitative research on the role and effectiveness of written information available to patients about individual medicines.
By Raynor DK, Blenkinsopp A, Knapp P, Grime J, Nicolson DJ, Pollock K, et al.
-
Oral naltrexone as a treatment for relapse prevention in formerly opioid-dependent drug users: a systematic review and economic evaluation.
By Adi Y, Juarez-Garcia A, Wang D, Jowett S, Frew E, Day E, et al.
-
Glucocorticoid-induced osteoporosis: a systematic review and cost–utility analysis.
By Kanis JA, Stevenson M, McCloskey EV, Davis S, Lloyd-Jones M.
-
Epidemiological, social, diagnostic and economic evaluation of population screening for genital chlamydial infection.
By Low N, McCarthy A, Macleod J, Salisbury C, Campbell R, Roberts TE, et al.
-
Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation.
By Connock M, Juarez-Garcia A, Jowett S, Frew E, Liu Z, Taylor RJ, et al.
-
Exercise Evaluation Randomised Trial (EXERT): a randomised trial comparing GP referral for leisure centre-based exercise, community-based walking and advice only.
By Isaacs AJ, Critchley JA, See Tai S, Buckingham K, Westley D, Harridge SDR, et al.
-
Interferon alfa (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation.
By Shepherd J, Jones J, Hartwell D, Davidson P, Price A, Waugh N.
-
Systematic review and economic evaluation of bevacizumab and cetuximab for the treatment of metastatic colorectal cancer.
By Tappenden P, Jones R, Paisley S, Carroll C.
-
A systematic review and economic evaluation of epoetin alfa, epoetin beta and darbepoetin alfa in anaemia associated with cancer, especially that attributable to cancer treatment.
By Wilson J, Yao GL, Raftery J, Bohlius J, Brunskill S, Sandercock J, et al.
-
A systematic review and economic evaluation of statins for the prevention of coronary events.
By Ward S, Lloyd Jones M, Pandor A, Holmes M, Ara R, Ryan A, et al.
-
A systematic review of the effectiveness and cost-effectiveness of different models of community-based respite care for frail older people and their carers.
By Mason A, Weatherly H, Spilsbury K, Arksey H, Golder S, Adamson J, et al.
-
Additional therapy for young children with spastic cerebral palsy: a randomised controlled trial.
By Weindling AM, Cunningham CC, Glenn SM, Edwards RT, Reeves DJ.
-
Screening for type 2 diabetes: literature review and economic modelling.
By Waugh N, Scotland G, McNamee P, Gillett M, Brennan A, Goyder E, et al.
-
The effectiveness and cost-effectiveness of cinacalcet for secondary hyperparathyroidism in end-stage renal disease patients on dialysis: a systematic review and economic evaluation.
By Garside R, Pitt M, Anderson R, Mealing S, Roome C, Snaith A, et al.
-
The clinical effectiveness and cost-effectiveness of gemcitabine for metastatic breast cancer: a systematic review and economic evaluation.
By Takeda AL, Jones J, Loveman E, Tan SC, Clegg AJ.
-
A systematic review of duplex ultrasound, magnetic resonance angiography and computed tomography angiography for the diagnosis and assessment of symptomatic, lower limb peripheral arterial disease.
By Collins R, Cranny G, Burch J, Aguiar-Ibáñez R, Craig D, Wright K, et al.
-
The clinical effectiveness and cost-effectiveness of treatments for children with idiopathic steroid-resistant nephrotic syndrome: a systematic review.
By Colquitt JL, Kirby J, Green C, Cooper K, Trompeter RS.
-
A systematic review of the routine monitoring of growth in children of primary school age to identify growth-related conditions.
By Fayter D, Nixon J, Hartley S, Rithalia A, Butler G, Rudolf M, et al.
-
Systematic review of the effectiveness of preventing and treating Staphylococcus aureus carriage in reducing peritoneal catheter-related infections.
By McCormack K, Rabindranath K, Kilonzo M, Vale L, Fraser C, McIntyre L, et al.
-
The clinical effectiveness and cost of repetitive transcranial magnetic stimulation versus electroconvulsive therapy in severe depression: a multicentre pragmatic randomised controlled trial and economic analysis.
By McLoughlin DM, Mogg A, Eranti S, Pluck G, Purvis R, Edwards D, et al.
-
A randomised controlled trial and economic evaluation of direct versus indirect and individual versus group modes of speech and language therapy for children with primary language impairment.
By Boyle J, McCartney E, Forbes J, O’Hare A.
-
Hormonal therapies for early breast cancer: systematic review and economic evaluation.
By Hind D, Ward S, De Nigris E, Simpson E, Carroll C, Wyld L.
-
Cardioprotection against the toxic effects of anthracyclines given to children with cancer: a systematic review.
By Bryant J, Picot J, Levitt G, Sullivan I, Baxter L, Clegg A.
-
Adalimumab, etanercept and infliximab for the treatment of ankylosing spondylitis: a systematic review and economic evaluation.
By McLeod C, Bagust A, Boland A, Dagenais P, Dickson R, Dundar Y, et al.
-
Prenatal screening and treatment strategies to prevent group B streptococcal and other bacterial infections in early infancy: cost-effectiveness and expected value of information analyses.
By Colbourn T, Asseburg C, Bojke L, Philips Z, Claxton K, Ades AE, et al.
-
Clinical effectiveness and cost-effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: a systematic review.
By Garrison KR, Donell S, Ryder J, Shemilt I, Mugford M, Harvey I, et al.
-
A randomised controlled trial of postoperative radiotherapy following breast-conserving surgery in a minimum-risk older population. The PRIME trial.
By Prescott RJ, Kunkler IH, Williams LJ, King CC, Jack W, van der Pol M, et al.
-
Current practice, accuracy, effectiveness and cost-effectiveness of the school entry hearing screen.
By Bamford J, Fortnum H, Bristow K, Smith J, Vamvakas G, Davies L, et al.
-
The clinical effectiveness and cost-effectiveness of inhaled insulin in diabetes mellitus: a systematic review and economic evaluation.
By Black C, Cummins E, Royle P, Philip S, Waugh N.
-
Surveillance of cirrhosis for hepatocellular carcinoma: systematic review and economic analysis.
By Thompson Coon J, Rogers G, Hewson P, Wright D, Anderson R, Cramp M, et al.
-
The Birmingham Rehabilitation Uptake Maximisation Study (BRUM). Homebased compared with hospital-based cardiac rehabilitation in a multi-ethnic population: cost-effectiveness and patient adherence.
By Jolly K, Taylor R, Lip GYH, Greenfield S, Raftery J, Mant J, et al.
-
A systematic review of the clinical, public health and cost-effectiveness of rapid diagnostic tests for the detection and identification of bacterial intestinal pathogens in faeces and food.
By Abubakar I, Irvine L, Aldus CF, Wyatt GM, Fordham R, Schelenz S, et al.
-
A randomised controlled trial examining the longer-term outcomes of standard versus new antiepileptic drugs. The SANAD trial.
By Marson AG, Appleton R, Baker GA, Chadwick DW, Doughty J, Eaton B, et al.
-
Clinical effectiveness and cost-effectiveness of different models of managing long-term oral anti-coagulation therapy: a systematic review and economic modelling.
By Connock M, Stevens C, Fry-Smith A, Jowett S, Fitzmaurice D, Moore D, et al.
-
A systematic review and economic model of the clinical effectiveness and cost-effectiveness of interventions for preventing relapse in people with bipolar disorder.
By Soares-Weiser K, Bravo Vergel Y, Beynon S, Dunn G, Barbieri M, Duffy S, et al.
-
Taxanes for the adjuvant treatment of early breast cancer: systematic review and economic evaluation.
By Ward S, Simpson E, Davis S, Hind D, Rees A, Wilkinson A.
-
The clinical effectiveness and cost-effectiveness of screening for open angle glaucoma: a systematic review and economic evaluation.
By Burr JM, Mowatt G, Hernández R, Siddiqui MAR, Cook J, Lourenco T, et al.
-
Acceptability, benefit and costs of early screening for hearing disability: a study of potential screening tests and models.
By Davis A, Smith P, Ferguson M, Stephens D, Gianopoulos I.
-
Contamination in trials of educational interventions.
By Keogh-Brown MR, Bachmann MO, Shepstone L, Hewitt C, Howe A, Ramsay CR, et al.
-
Overview of the clinical effectiveness of positron emission tomography imaging in selected cancers.
By Facey K, Bradbury I, Laking G, Payne E.
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.
By Garside R, Pitt M, Anderson R, Rogers G, Dyer M, Mealing S, et al.
-
Drug-eluting stents: a systematic review and economic evaluation.
By Hill RA, Boland A, Dickson R, Dündar Y, Haycox A, McLeod C, et al.
-
The clinical effectiveness and cost-effectiveness of cardiac resynchronisation (biventricular pacing) for heart failure: systematic review and economic model.
By Fox M, Mealing S, Anderson R, Dean J, Stein K, Price A, et al.
-
Recruitment to randomised trials: strategies for trial enrolment and participation study. The STEPS study.
By Campbell MK, Snowdon C, Francis D, Elbourne D, McDonald AM, Knight R, et al.
-
Cost-effectiveness of functional cardiac testing in the diagnosis and management of coronary artery disease: a randomised controlled trial. The CECaT trial.
By Sharples L, Hughes V, Crean A, Dyer M, Buxton M, Goldsmith K, et al.
-
Evaluation of diagnostic tests when there is no gold standard. A review of methods.
By Rutjes AWS, Reitsma JB, Coomarasamy A, Khan KS, Bossuyt PMM.
-
Systematic reviews of the clinical effectiveness and cost-effectiveness of proton pump inhibitors in acute upper gastrointestinal bleeding.
By Leontiadis GI, Sreedharan A, Dorward S, Barton P, Delaney B, Howden CW, et al.
-
A review and critique of modelling in prioritising and designing screening programmes.
By Karnon J, Goyder E, Tappenden P, McPhie S, Towers I, Brazier J, et al.
-
An assessment of the impact of the NHS Health Technology Assessment Programme.
By Hanney S, Buxton M, Green C, Coulson D, Raftery J.
-
A systematic review and economic model of switching from nonglycopeptide to glycopeptide antibiotic prophylaxis for surgery.
By Cranny G, Elliott R, Weatherly H, Chambers D, Hawkins N, Myers L, et al.
-
‘Cut down to quit’ with nicotine replacement therapies in smoking cessation: a systematic review of effectiveness and economic analysis.
By Wang D, Connock M, Barton P, Fry-Smith A, Aveyard P, Moore D.
-
A systematic review of the effectiveness of strategies for reducing fracture risk in children with juvenile idiopathic arthritis with additional data on long-term risk of fracture and cost of disease management.
By Thornton J, Ashcroft D, O’Neill T, Elliott R, Adams J, Roberts C, et al.
-
Does befriending by trained lay workers improve psychological well-being and quality of life for carers of people with dementia, and at what cost? A randomised controlled trial.
By Charlesworth G, Shepstone L, Wilson E, Thalanany M, Mugford M, Poland F.
-
A multi-centre retrospective cohort study comparing the efficacy, safety and cost-effectiveness of hysterectomy and uterine artery embolisation for the treatment of symptomatic uterine fibroids. The HOPEFUL study.
By Hirst A, Dutton S, Wu O, Briggs A, Edwards C, Waldenmaier L, et al.
-
Methods of prediction and prevention of pre-eclampsia: systematic reviews of accuracy and effectiveness literature with economic modelling.
By Meads CA, Cnossen JS, Meher S, Juarez-Garcia A, ter Riet G, Duley L, et al.
-
The use of economic evaluations in NHS decision-making: a review and empirical investigation.
By Williams I, McIver S, Moore D, Bryan S.
-
Stapled haemorrhoidectomy (haemorrhoidopexy) for the treatment of haemorrhoids: a systematic review and economic evaluation.
By Burch J, Epstein D, Baba-Akbari A, Weatherly H, Fox D, Golder S, et al.
-
The clinical effectiveness of diabetes education models for Type 2 diabetes: a systematic review.
By Loveman E, Frampton GK, Clegg AJ.
-
Payment to healthcare professionals for patient recruitment to trials: systematic review and qualitative study.
By Raftery J, Bryant J, Powell J, Kerr C, Hawker S.
-
Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation.
By Chen Y-F, Jobanputra P, Barton P, Bryan S, Fry-Smith A, Harris G, et al.
-
The clinical effectiveness and cost-effectiveness of central venous catheters treated with anti-infective agents in preventing bloodstream infections: a systematic review and economic evaluation.
By Hockenhull JC, Dwan K, Boland A, Smith G, Bagust A, Dundar Y, et al.
-
Stepped treatment of older adults on laxatives. The STOOL trial.
By Mihaylov S, Stark C, McColl E, Steen N, Vanoli A, Rubin G, et al.
-
A randomised controlled trial of cognitive behaviour therapy in adolescents with major depression treated by selective serotonin reuptake inhibitors. The ADAPT trial.
By Goodyer IM, Dubicka B, Wilkinson P, Kelvin R, Roberts C, Byford S, et al.
-
The use of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer: systematic review and economic evaluation.
By Hind D, Tappenden P, Tumur I, Eggington E, Sutcliffe P, Ryan A.
-
Ranibizumab and pegaptanib for the treatment of age-related macular degeneration: a systematic review and economic evaluation.
By Colquitt JL, Jones J, Tan SC, Takeda A, Clegg AJ, Price A.
-
Systematic review of the clinical effectiveness and cost-effectiveness of 64-slice or higher computed tomography angiography as an alternative to invasive coronary angiography in the investigation of coronary artery disease.
By Mowatt G, Cummins E, Waugh N, Walker S, Cook J, Jia X, et al.
-
Structural neuroimaging in psychosis: a systematic review and economic evaluation.
By Albon E, Tsourapas A, Frew E, Davenport C, Oyebode F, Bayliss S, et al.
-
Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in adults and children aged 12 years and over.
By Shepherd J, Rogers G, Anderson R, Main C, Thompson-Coon J, Hartwell D, et al.
-
Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in children under the age of 12 years.
By Main C, Shepherd J, Anderson R, Rogers G, Thompson-Coon J, Liu Z, et al.
-
Ezetimibe for the treatment of hypercholesterolaemia: a systematic review and economic evaluation.
By Ara R, Tumur I, Pandor A, Duenas A, Williams R, Wilkinson A, et al.
-
Topical or oral ibuprofen for chronic knee pain in older people. The TOIB study.
By Underwood M, Ashby D, Carnes D, Castelnuovo E, Cross P, Harding G, et al.
-
A prospective randomised comparison of minor surgery in primary and secondary care. The MiSTIC trial.
By George S, Pockney P, Primrose J, Smith H, Little P, Kinley H, et al.
-
A review and critical appraisal of measures of therapist–patient interactions in mental health settings.
By Cahill J, Barkham M, Hardy G, Gilbody S, Richards D, Bower P, et al.
-
The clinical effectiveness and cost-effectiveness of screening programmes for amblyopia and strabismus in children up to the age of 4–5 years: a systematic review and economic evaluation.
By Carlton J, Karnon J, Czoski-Murray C, Smith KJ, Marr J.
-
A systematic review of the clinical effectiveness and cost-effectiveness and economic modelling of minimal incision total hip replacement approaches in the management of arthritic disease of the hip.
By de Verteuil R, Imamura M, Zhu S, Glazener C, Fraser C, Munro N, et al.
-
A preliminary model-based assessment of the cost–utility of a screening programme for early age-related macular degeneration.
By Karnon J, Czoski-Murray C, Smith K, Brand C, Chakravarthy U, Davis S, et al.
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.
By Shepherd J, Jones J, Frampton GK, Tanajewski L, Turner D, Price A.
-
Absorbent products for urinary/faecal incontinence: a comparative evaluation of key product categories.
By Fader M, Cottenden A, Getliffe K, Gage H, Clarke-O’Neill S, Jamieson K, et al.
-
A systematic review of repetitive functional task practice with modelling of resource use, costs and effectiveness.
By French B, Leathley M, Sutton C, McAdam J, Thomas L, Forster A, et al.
-
The effectiveness and cost-effectivness of minimal access surgery amongst people with gastro-oesophageal reflux disease – a UK collaborative study. The reflux trial.
By Grant A, Wileman S, Ramsay C, Bojke L, Epstein D, Sculpher M, et al.
-
Time to full publication of studies of anti-cancer medicines for breast cancer and the potential for publication bias: a short systematic review.
By Takeda A, Loveman E, Harris P, Hartwell D, Welch K.
-
Performance of screening tests for child physical abuse in accident and emergency departments.
By Woodman J, Pitt M, Wentz R, Taylor B, Hodes D, Gilbert RE.
-
Curative catheter ablation in atrial fibrillation and typical atrial flutter: systematic review and economic evaluation.
By Rodgers M, McKenna C, Palmer S, Chambers D, Van Hout S, Golder S, et al.
-
Systematic review and economic modelling of effectiveness and cost utility of surgical treatments for men with benign prostatic enlargement.
By Lourenco T, Armstrong N, N’Dow J, Nabi G, Deverill M, Pickard R, et al.
-
Immunoprophylaxis against respiratory syncytial virus (RSV) with palivizumab in children: a systematic review and economic evaluation.
By Wang D, Cummins C, Bayliss S, Sandercock J, Burls A.
-
Deferasirox for the treatment of iron overload associated with regular blood transfusions (transfusional haemosiderosis) in patients suffering with chronic anaemia: a systematic review and economic evaluation.
By McLeod C, Fleeman N, Kirkham J, Bagust A, Boland A, Chu P, et al.
-
Thrombophilia testing in people with venous thromboembolism: systematic review and cost-effectiveness analysis.
By Simpson EL, Stevenson MD, Rawdin A, Papaioannou D.
-
Surgical procedures and non-surgical devices for the management of non-apnoeic snoring: a systematic review of clinical effects and associated treatment costs.
By Main C, Liu Z, Welch K, Weiner G, Quentin Jones S, Stein K.
-
Continuous positive airway pressure devices for the treatment of obstructive sleep apnoea–hypopnoea syndrome: a systematic review and economic analysis.
By McDaid C, Griffin S, Weatherly H, Durée K, van der Burgt M, van Hout S, Akers J, et al.
-
Use of classical and novel biomarkers as prognostic risk factors for localised prostate cancer: a systematic review.
By Sutcliffe P, Hummel S, Simpson E, Young T, Rees A, Wilkinson A, et al.
-
The harmful health effects of recreational ecstasy: a systematic review of observational evidence.
By Rogers G, Elston J, Garside R, Roome C, Taylor R, Younger P, et al.
-
Systematic review of the clinical effectiveness and cost-effectiveness of oesophageal Doppler monitoring in critically ill and high-risk surgical patients.
By Mowatt G, Houston G, Hernández R, de Verteuil R, Fraser C, Cuthbertson B, et al.
-
The use of surrogate outcomes in model-based cost-effectiveness analyses: a survey of UK Health Technology Assessment reports.
By Taylor RS, Elston J.
-
Controlling Hypertension and Hypotension Immediately Post Stroke (CHHIPS) – a randomised controlled trial.
By Potter J, Mistri A, Brodie F, Chernova J, Wilson E, Jagger C, et al.
-
Routine antenatal anti-D prophylaxis for RhD-negative women: a systematic review and economic evaluation.
By Pilgrim H, Lloyd-Jones M, Rees A.
-
Amantadine, oseltamivir and zanamivir for the prophylaxis of influenza (including a review of existing guidance no. 67): a systematic review and economic evaluation.
By Tappenden P, Jackson R, Cooper K, Rees A, Simpson E, Read R, et al.
-
Improving the evaluation of therapeutic interventions in multiple sclerosis: the role of new psychometric methods.
By Hobart J, Cano S.
-
Treatment of severe ankle sprain: a pragmatic randomised controlled trial comparing the clinical effectiveness and cost-effectiveness of three types of mechanical ankle support with tubular bandage. The CAST trial.
By Cooke MW, Marsh JL, Clark M, Nakash R, Jarvis RM, Hutton JL, et al. , on behalf of the CAST trial group.
-
Non-occupational postexposure prophylaxis for HIV: a systematic review.
By Bryant J, Baxter L, Hird S.
-
Blood glucose self-monitoring in type 2 diabetes: a randomised controlled trial.
By Farmer AJ, Wade AN, French DP, Simon J, Yudkin P, Gray A, et al.
-
How far does screening women for domestic (partner) violence in different health-care settings meet criteria for a screening programme? Systematic reviews of nine UK National Screening Committee criteria.
By Feder G, Ramsay J, Dunne D, Rose M, Arsene C, Norman R, et al.
-
Spinal cord stimulation for chronic pain of neuropathic or ischaemic origin: systematic review and economic evaluation.
By Simpson, EL, Duenas A, Holmes MW, Papaioannou D, Chilcott J.
-
The role of magnetic resonance imaging in the identification of suspected acoustic neuroma: a systematic review of clinical and cost-effectiveness and natural history.
By Fortnum H, O’Neill C, Taylor R, Lenthall R, Nikolopoulos T, Lightfoot G, et al.
-
Dipsticks and diagnostic algorithms in urinary tract infection: development and validation, randomised trial, economic analysis, observational cohort and qualitative study.
By Little P, Turner S, Rumsby K, Warner G, Moore M, Lowes JA, et al.
-
Systematic review of respite care in the frail elderly.
By Shaw C, McNamara R, Abrams K, Cannings-John R, Hood K, Longo M, et al.
-
Neuroleptics in the treatment of aggressive challenging behaviour for people with intellectual disabilities: a randomised controlled trial (NACHBID).
By Tyrer P, Oliver-Africano P, Romeo R, Knapp M, Dickens S, Bouras N, et al.
-
Randomised controlled trial to determine the clinical effectiveness and cost-effectiveness of selective serotonin reuptake inhibitors plus supportive care, versus supportive care alone, for mild to moderate depression with somatic symptoms in primary care: the THREAD (THREshold for AntiDepressant response) study.
By Kendrick T, Chatwin J, Dowrick C, Tylee A, Morriss R, Peveler R, et al.
-
Diagnostic strategies using DNA testing for hereditary haemochromatosis in at-risk populations: a systematic review and economic evaluation.
By Bryant J, Cooper K, Picot J, Clegg A, Roderick P, Rosenberg W, et al.
-
Enhanced external counterpulsation for the treatment of stable angina and heart failure: a systematic review and economic analysis.
By McKenna C, McDaid C, Suekarran S, Hawkins N, Claxton K, Light K, et al.
-
Development of a decision support tool for primary care management of patients with abnormal liver function tests without clinically apparent liver disease: a record-linkage population cohort study and decision analysis (ALFIE).
By Donnan PT, McLernon D, Dillon JF, Ryder S, Roderick P, Sullivan F, et al.
-
A systematic review of presumed consent systems for deceased organ donation.
By Rithalia A, McDaid C, Suekarran S, Norman G, Myers L, Sowden A.
-
Paracetamol and ibuprofen for the treatment of fever in children: the PITCH randomised controlled trial.
By Hay AD, Redmond NM, Costelloe C, Montgomery AA, Fletcher M, Hollinghurst S, et al.
-
A randomised controlled trial to compare minimally invasive glucose monitoring devices with conventional monitoring in the management of insulin-treated diabetes mellitus (MITRE).
By Newman SP, Cooke D, Casbard A, Walker S, Meredith S, Nunn A, et al.
-
Sensitivity analysis in economic evaluation: an audit of NICE current practice and a review of its use and value in decision-making.
By Andronis L, Barton P, Bryan S.
-
Trastuzumab for the treatment of primary breast cancer in HER2-positive women: a single technology appraisal.
By Ward S, Pilgrim H, Hind D.
-
Docetaxel for the adjuvant treatment of early node-positive breast cancer: a single technology appraisal.
By Chilcott J, Lloyd Jones M, Wilkinson A.
-
The use of paclitaxel in the management of early stage breast cancer.
By Griffin S, Dunn G, Palmer S, Macfarlane K, Brent S, Dyker A, et al.
-
Rituximab for the first-line treatment of stage III/IV follicular non-Hodgkin’s lymphoma.
By Dundar Y, Bagust A, Hounsome J, McLeod C, Boland A, Davis H, et al.
-
Bortezomib for the treatment of multiple myeloma patients.
By Green C, Bryant J, Takeda A, Cooper K, Clegg A, Smith A, et al.
-
Fludarabine phosphate for the firstline treatment of chronic lymphocytic leukaemia.
By Walker S, Palmer S, Erhorn S, Brent S, Dyker A, Ferrie L, et al.
-
Erlotinib for the treatment of relapsed non-small cell lung cancer.
By McLeod C, Bagust A, Boland A, Hockenhull J, Dundar Y, Proudlove C, et al.
-
Cetuximab plus radiotherapy for the treatment of locally advanced squamous cell carcinoma of the head and neck.
By Griffin S, Walker S, Sculpher M, White S, Erhorn S, Brent S, et al.
-
Infliximab for the treatment of adults with psoriasis.
By Loveman E, Turner D, Hartwell D, Cooper K, Clegg A
-
Psychological interventions for postnatal depression: cluster randomised trial and economic evaluation. The PoNDER trial.
By Morrell CJ, Warner R, Slade P, Dixon S, Walters S, Paley G, et al.
-
The effect of different treatment durations of clopidogrel in patients with non-ST-segment elevation acute coronary syndromes: a systematic review and value of information analysis.
By Rogowski R, Burch J, Palmer S, Craigs C, Golder S, Woolacott N.
-
Systematic review and individual patient data meta-analysis of diagnosis of heart failure, with modelling of implications of different diagnostic strategies in primary care.
By Mant J, Doust J, Roalfe A, Barton P, Cowie MR, Glasziou P, et al.
-
A multicentre randomised controlled trial of the use of continuous positive airway pressure and non-invasive positive pressure ventilation in the early treatment of patients presenting to the emergency department with severe acute cardiogenic pulmonary oedema: the 3CPO trial.
By Gray AJ, Goodacre S, Newby DE, Masson MA, Sampson F, Dixon S, et al. , on behalf of the 3CPO study investigators.
-
Early high-dose lipid-lowering therapy to avoid cardiac events: a systematic review and economic evaluation.
By Ara R, Pandor A, Stevens J, Rees A, Rafia R.
-
Adefovir dipivoxil and pegylated interferon alpha for the treatment of chronic hepatitis B: an updated systematic review and economic evaluation.
By Jones J, Shepherd J, Baxter L, Gospodarevskaya E, Hartwell D, Harris P, et al.
-
Methods to identify postnatal depression in primary care: an integrated evidence synthesis and value of information analysis.
By Hewitt CE, Gilbody SM, Brealey S, Paulden M, Palmer S, Mann R, et al.
-
A double-blind randomised placebo-controlled trial of topical intranasal corticosteroids in 4- to 11-year-old children with persistent bilateral otitis media with effusion in primary care.
By Williamson I, Benge S, Barton S, Petrou S, Letley L, Fasey N, et al.
-
The effectiveness and cost-effectiveness of methods of storing donated kidneys from deceased donors: a systematic review and economic model.
By Bond M, Pitt M, Akoh J, Moxham T, Hoyle M, Anderson R.
-
Rehabilitation of older patients: day hospital compared with rehabilitation at home. A randomised controlled trial.
By Parker SG, Oliver P, Pennington M, Bond J, Jagger C, Enderby PM, et al.
-
Breastfeeding promotion for infants in neonatal units: a systematic review and economic analysis.
By Renfrew MJ, Craig D, Dyson L, McCormick F, Rice S, King SE, et al.
-
The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation.
By Picot J, Jones J, Colquitt JL, Gospodarevskaya E, Loveman E, Baxter L, et al.
-
Rapid testing for group B streptococcus during labour: a test accuracy study with evaluation of acceptability and cost-effectiveness.
By Daniels J, Gray J, Pattison H, Roberts T, Edwards E, Milner P, et al.
-
Screening to prevent spontaneous preterm birth: systematic reviews of accuracy and effectiveness literature with economic modelling.
By Honest H, Forbes CA, Durée KH, Norman G, Duffy SB, Tsourapas A, et al.
-
The effectiveness and cost-effectiveness of cochlear implants for severe to profound deafness in children and adults: a systematic review and economic model.
By Bond M, Mealing S, Anderson R, Elston J, Weiner G, Taylor RS, et al.
-
Gemcitabine for the treatment of metastatic breast cancer.
By Jones J, Takeda A, Tan SC, Cooper K, Loveman E, Clegg A.
-
Varenicline in the management of smoking cessation: a single technology appraisal.
By Hind D, Tappenden P, Peters J, Kenjegalieva K.
-
Alteplase for the treatment of acute ischaemic stroke: a single technology appraisal.
By Lloyd Jones M, Holmes M.
-
Rituximab for the treatment of rheumatoid arthritis.
By Bagust A, Boland A, Hockenhull J, Fleeman N, Greenhalgh J, Dundar Y, et al.
-
Omalizumab for the treatment of severe persistent allergic asthma.
By Jones J, Shepherd J, Hartwell D, Harris P, Cooper K, Takeda A, et al.
-
Rituximab for the treatment of relapsed or refractory stage III or IV follicular non-Hodgkin’s lymphoma.
By Boland A, Bagust A, Hockenhull J, Davis H, Chu P, Dickson R.
-
Adalimumab for the treatment of psoriasis.
By Turner D, Picot J, Cooper K, Loveman E.
-
Dabigatran etexilate for the prevention of venous thromboembolism in patients undergoing elective hip and knee surgery: a single technology appraisal.
By Holmes M, C Carroll C, Papaioannou D.
-
Romiplostim for the treatment of chronic immune or idiopathic thrombocytopenic purpura: a single technology appraisal.
By Mowatt G, Boachie C, Crowther M, Fraser C, Hernández R, Jia X, et al.
-
Sunitinib for the treatment of gastrointestinal stromal tumours: a critique of the submission from Pfizer.
By Bond M, Hoyle M, Moxham T, Napier M, Anderson R.
-
Vitamin K to prevent fractures in older women: systematic review and economic evaluation.
By Stevenson M, Lloyd-Jones M, Papaioannou D.
-
The effects of biofeedback for the treatment of essential hypertension: a systematic review.
By Greenhalgh J, Dickson R, Dundar Y.
-
A randomised controlled trial of the use of aciclovir and/or prednisolone for the early treatment of Bell’s palsy: the BELLS study.
By Sullivan FM, Swan IRC, Donnan PT, Morrison JM, Smith BH, McKinstry B, et al.
-
Lapatinib for the treatment of HER2-overexpressing breast cancer.
By Jones J, Takeda A, Picot J, von Keyserlingk C, Clegg A.
-
Infliximab for the treatment of ulcerative colitis.
By Hyde C, Bryan S, Juarez-Garcia A, Andronis L, Fry-Smith A.
-
Rimonabant for the treatment of overweight and obese people.
By Burch J, McKenna C, Palmer S, Norman G, Glanville J, Sculpher M, et al.
-
Telbivudine for the treatment of chronic hepatitis B infection.
By Hartwell D, Jones J, Harris P, Cooper K.
-
Entecavir for the treatment of chronic hepatitis B infection.
By Shepherd J, Gospodarevskaya E, Frampton G, Cooper, K.
-
Febuxostat for the treatment of hyperuricaemia in people with gout: a single technology appraisal.
By Stevenson M, Pandor A.
-
Rivaroxaban for the prevention of venous thromboembolism: a single technology appraisal.
By Stevenson M, Scope A, Holmes M, Rees A, Kaltenthaler E.
-
Cetuximab for the treatment of recurrent and/or metastatic squamous cell carcinoma of the head and neck.
By Greenhalgh J, Bagust A, Boland A, Fleeman N, McLeod C, Dundar Y, et al.
-
Mifamurtide for the treatment of osteosarcoma: a single technology appraisal.
By Pandor A, Fitzgerald P, Stevenson M, Papaioannou D.
-
Ustekinumab for the treatment of moderate to severe psoriasis.
By Gospodarevskaya E, Picot J, Cooper K, Loveman E, Takeda A.
-
Endovascular stents for abdominal aortic aneurysms: a systematic review and economic model.
By Chambers D, Epstein D, Walker S, Fayter D, Paton F, Wright K, et al.
-
Clinical and cost-effectiveness of epoprostenol, iloprost, bosentan, sitaxentan and sildenafil for pulmonary arterial hypertension within their licensed indications: a systematic review and economic evaluation.
By Chen Y-F, Jowett S, Barton P, Malottki K, Hyde C, Gibbs JSR, et al.
-
Cessation of attention deficit hyperactivity disorder drugs in the young (CADDY) – a pharmacoepidemiological and qualitative study.
By Wong ICK, Asherson P, Bilbow A, Clifford S, Coghill D, R DeSoysa R, et al.
-
ARTISTIC: a randomised trial of human papillomavirus (HPV) testing in primary cervical screening.
By Kitchener HC, Almonte M, Gilham C, Dowie R, Stoykova B, Sargent A, et al.
-
The clinical effectiveness of glucosamine and chondroitin supplements in slowing or arresting progression of osteoarthritis of the knee: a systematic review and economic evaluation.
By Black C, Clar C, Henderson R, MacEachern C, McNamee P, Quayyum Z, et al.
-
Randomised preference trial of medical versus surgical termination of pregnancy less than 14 weeks’ gestation (TOPS).
By Robson SC, Kelly T, Howel D, Deverill M, Hewison J, Lie MLS, et al.
-
Randomised controlled trial of the use of three dressing preparations in the management of chronic ulceration of the foot in diabetes.
By Jeffcoate WJ, Price PE, Phillips CJ, Game FL, Mudge E, Davies S, et al.
-
VenUS II: a randomised controlled trial of larval therapy in the management of leg ulcers.
By Dumville JC, Worthy G, Soares MO, Bland JM, Cullum N, Dowson C, et al.
-
A prospective randomised controlled trial and economic modelling of antimicrobial silver dressings versus non-adherent control dressings for venous leg ulcers: the VULCAN trial
By Michaels JA, Campbell WB, King BM, MacIntyre J, Palfreyman SJ, Shackley P, et al.
-
Communication of carrier status information following universal newborn screening for sickle cell disorders and cystic fibrosis: qualitative study of experience and practice.
By Kai J, Ulph F, Cullinan T, Qureshi N.
-
Antiviral drugs for the treatment of influenza: a systematic review and economic evaluation.
By Burch J, Paulden M, Conti S, Stock C, Corbett M, Welton NJ, et al.
-
Development of a toolkit and glossary to aid in the adaptation of health technology assessment (HTA) reports for use in different contexts.
By Chase D, Rosten C, Turner S, Hicks N, Milne R.
-
Colour vision testing for diabetic retinopathy: a systematic review of diagnostic accuracy and economic evaluation.
By Rodgers M, Hodges R, Hawkins J, Hollingworth W, Duffy S, McKibbin M, et al.
-
Systematic review of the effectiveness and cost-effectiveness of weight management schemes for the under fives: a short report.
By Bond M, Wyatt K, Lloyd J, Welch K, Taylor R.
-
Are adverse effects incorporated in economic models? An initial review of current practice.
By Craig D, McDaid C, Fonseca T, Stock C, Duffy S, Woolacott N.
-
Multicentre randomised controlled trial examining the cost-effectiveness of contrast-enhanced high field magnetic resonance imaging in women with primary breast cancer scheduled for wide local excision (COMICE).
By Turnbull LW, Brown SR, Olivier C, Harvey I, Brown J, Drew P, et al.
-
Bevacizumab, sorafenib tosylate, sunitinib and temsirolimus for renal cell carcinoma: a systematic review and economic evaluation.
By Thompson Coon J, Hoyle M, Green C, Liu Z, Welch K, Moxham T, et al.
-
The clinical effectiveness and cost-effectiveness of testing for cytochrome P450 polymorphisms in patients with schizophrenia treated with antipsychotics: a systematic review and economic evaluation.
By Fleeman N, McLeod C, Bagust A, Beale S, Boland A, Dundar Y, et al.
-
Systematic review of the clinical effectiveness and cost-effectiveness of photodynamic diagnosis and urine biomarkers (FISH, ImmunoCyt, NMP22) and cytology for the detection and follow-up of bladder cancer.
By Mowatt G, Zhu S, Kilonzo M, Boachie C, Fraser C, Griffiths TRL, et al.
-
Effectiveness and cost-effectiveness of arthroscopic lavage in the treatment of osteoarthritis of the knee: a mixed methods study of the feasibility of conducting a surgical placebo-controlled trial (the KORAL study).
By Campbell MK, Skea ZC, Sutherland AG, Cuthbertson BH, Entwistle VA, McDonald AM, et al.
-
A randomised 2 × 2 trial of community versus hospital pulmonary rehabilitation for chronic obstructive pulmonary disease followed by telephone or conventional follow-up.
By Waterhouse JC, Walters SJ, Oluboyede Y, Lawson RA.
-
The effectiveness and cost-effectiveness of behavioural interventions for the prevention of sexually transmitted infections in young people aged 13–19: a systematic review and economic evaluation.
By Shepherd J, Kavanagh J, Picot J, Cooper K, Harden A, Barnett-Page E, et al.
-
Dissemination and publication of research findings: an updated review of related biases.
By Song F, Parekh S, Hooper L, Loke YK, Ryder J, Sutton AJ, et al.
-
The effectiveness and cost-effectiveness of biomarkers for the prioritisation of patients awaiting coronary revascularisation: a systematic review and decision model.
By Hemingway H, Henriksson M, Chen R, Damant J, Fitzpatrick N, Abrams K, et al.
-
Comparison of case note review methods for evaluating quality and safety in health care.
By Hutchinson A, Coster JE, Cooper KL, McIntosh A, Walters SJ, Bath PA, et al.
-
Clinical effectiveness and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes: systematic review and economic evaluation.
By Cummins E, Royle P, Snaith A, Greene A, Robertson L, McIntyre L, et al.
-
Self-monitoring of blood glucose in type 2 diabetes: systematic review.
By Clar C, Barnard K, Cummins E, Royle P, Waugh N.
-
North of England and Scotland Study of Tonsillectomy and Adeno-tonsillectomy in Children (NESSTAC): a pragmatic randomised controlled trial with a parallel non-randomised preference study.
By Lock C, Wilson J, Steen N, Eccles M, Mason H, Carrie S, et al.
-
Multicentre randomised controlled trial of the clinical and cost-effectiveness of a bypass-surgery-first versus a balloon-angioplasty-first revascularisation strategy for severe limb ischaemia due to infrainguinal disease. The Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial.
By Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FGR, Gillespie I, et al.
-
A randomised controlled multicentre trial of treatments for adolescent anorexia nervosa including assessment of cost-effectiveness and patient acceptability – the TOuCAN trial.
By Gowers SG, Clark AF, Roberts C, Byford S, Barrett B, Griffiths A, et al.
-
Randomised controlled trials for policy interventions: a review of reviews and meta-regression.
By Oliver S, Bagnall AM, Thomas J, Shepherd J, Sowden A, White I, et al.
-
Paracetamol and selective and non-selective non-steroidal anti-inflammatory drugs (NSAIDs) for the reduction of morphine-related side effects after major surgery: a systematic review.
By McDaid C, Maund E, Rice S, Wright K, Jenkins B, Woolacott N.
-
A systematic review of outcome measures used in forensic mental health research with consensus panel opinion.
By Fitzpatrick R, Chambers J, Burns T, Doll H, Fazel S, Jenkinson C, et al.
-
The clinical effectiveness and cost-effectiveness of topotecan for small cell lung cancer: a systematic review and economic evaluation.
By Loveman E, Jones J, Hartwell D, Bird A, Harris P, Welch K, et al.
-
Antenatal screening for haemoglobinopathies in primary care: a cohort study and cluster randomised trial to inform a simulation model. The Screening for Haemoglobinopathies in First Trimester (SHIFT) trial.
By Dormandy E, Bryan S, Gulliford MC, Roberts T, Ades T, Calnan M, et al.
-
Early referral strategies for management of people with markers of renal disease: a systematic review of the evidence of clinical effectiveness, cost-effectiveness and economic analysis.
By Black C, Sharma P, Scotland G, McCullough K, McGurn D, Robertson L, et al.
-
A randomised controlled trial of cognitive behaviour therapy and motivational interviewing for people with Type 1 diabetes mellitus with persistent sub-optimal glycaemic control: A Diabetes and Psychological Therapies (ADaPT) study.
By Ismail K, Maissi E, Thomas S, Chalder T, Schmidt U, Bartlett J, et al.
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
Prioritisation Strategy Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
-
Dr Bob Coates, Consultant Advisor, NETSCC, HTA
-
Dr Andrew Cook, Consultant Advisor, NETSCC, HTA
-
Dr Peter Davidson, Director of Science Support, NETSCC, HTA
-
Professor Robin E Ferner, Consultant Physician and Director, West Midlands Centre for Adverse Drug Reactions, City Hospital NHS Trust, Birmingham
-
Professor Paul Glasziou, Professor of Evidence-Based Medicine, University of Oxford
-
Dr Nick Hicks, Director of NHS Support, NETSCC, HTA
-
Dr Edmund Jessop, Medical Adviser, National Specialist, National Commissioning Group (NCG), Department of Health, London
-
Ms Lynn Kerridge, Chief Executive Officer, NETSCC and NETSCC, HTA
-
Dr Ruairidh Milne, Director of Strategy and Development, NETSCC
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Ms Pamela Young, Specialist Programme Manager, NETSCC, HTA
HTA Commissioning Board
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
-
Senior Lecturer in General Practice, Department of Primary Health Care, University of Oxford
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Deborah Ashby, Professor of Medical Statistics, Queen Mary, University of London
-
Professor John Cairns, Professor of Health Economics, London School of Hygiene and Tropical Medicine
-
Professor Peter Croft, Director of Primary Care Sciences Research Centre, Keele University
-
Professor Nicky Cullum, Director of Centre for Evidence-Based Nursing, University of York
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, University of Sheffield
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford?
-
Professor Stuart Logan, Director of Health & Social Care Research, The Peninsula Medical School, Universities of Exeter and Plymouth
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, Univeristy of Oxford
-
Professor Ian Roberts, Professor of Epidemiology & Public Health, London School of Hygiene and Tropical Medicine
-
Professor Mark Sculpher, Professor of Health Economics, University of York
-
Professor Helen Smith, Professor of Primary Care, University of Brighton
-
Professor Kate Thomas, Professor of Complementary & Alternative Medicine Research, University of Leeds
-
Professor David John Torgerson, Director of York Trials Unit, University of York
-
Professor Hywel Williams, Professor of Dermato-Epidemiology, University of Nottingham
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Professor of Evidence-Based Medicine, University of Oxford
-
Consultant Paediatrician and Honorary Senior Lecturer, Great Ormond Street Hospital, London
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, Imaging Science and Biomedical Engineering, Cancer & Imaging Sciences, University of Manchester
-
Mr A S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital
-
Dr Dianne Baralle, Consultant & Senior Lecturer in Clinical Genetics, Human Genetics Division & Wessex Clinical Genetics Service, Southampton, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Service User Representative
-
Professor Anthony Robert Kendrick, Professor of Primary Medical Care, University of Southampton
-
Dr Susanne M Ludgate, Director, Medical Devices Agency, London
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee
-
Dr David Mathew Service User Representative
-
Dr Michael Millar, Lead Consultant in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mr Stephen Pilling, Director, Centre for Outcomes, Research & Effectiveness, University College London
-
Mrs Una Rennard, Service User Representative
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Ultrasound Department, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr W Stuart A Smellie, Consultant, Bishop Auckland General Hospital
-
Professor Lindsay Wilson Turnbull, Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Dr Alan J Williams, Consultant in General Medicine, Department of Thoracic Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Catherine Moody, Programme Manager, Neuroscience and Mental Health Board
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Disease Prevention Panel
-
Medical Adviser, National Specialist Commissioning Advisory Group (NSCAG), Department of Health
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook Clinical Programmes Director, Bazian Ltd, London
-
Dr Elizabeth Fellow-Smith, Medical Director, West London Mental Health Trust, Middlesex
-
Dr Colin Greaves Senior Research Fellow, Peninsular Medical School (Primary Care)
-
Dr John Jackson, General Practitioner, Parkway Medical Centre, Newcastle upon Tyne
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Chris McCall, General Practitioner, The Hadleigh Practice, Corfe Mullen, Dorset
-
Miss Nicky Mullany, Service User Representative
-
Dr Julie Mytton, Locum Consultant in Public Health Medicine, Bristol Primary Care Trust
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Professor Carol Tannahill, Glasgow Centre for Population Health
-
Mrs Jean Thurston, Service User Representative
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Ms Kay Pattison NHS R&D Programme/DH, Leeds
-
Dr Caroline Stone, Programme Manager, Medical Research Council
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust, Bristol
-
Reader in Wound Healing and Director of Research, University of Leeds, Leeds
-
Professor Bipin Bhakta Charterhouse Professor in Rehabilitation Medicine, University of Leeds, Leeds
-
Mrs Penny Calder Service User Representative
-
Professor Paul Carding, Professor of Voice Pathology, Newcastle Hospital NHS Trust, Newcastle
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry, London
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol, Bristol
-
Mrs Anthea De Barton-Watson, Service User Representative
-
Professor Christopher Griffiths, Professor of Primary Care, Barts and the London School of Medicine and Dentistry, London
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester, Manchester
-
Dr Peter Martin, Consultant Neurologist, Addenbrooke’s Hospital, Cambridge
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham, Nottingham
-
Dr Kate Radford, Division of Rehabilitation and Ageing, School of Community Health Sciences. University of Nottingham, Nottingham
-
Mr Jim Reece, Service User Representative
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton, Southampton
-
Dr Pippa Tyrrell, Stroke Medicine, Senior Lecturer/Consultant Stroke Physician, Salford Royal Foundation Hospitals’ Trust, Salford
-
Dr Sarah Tyson, Senior Research Fellow & Associate Head of School, University of Salford, Salford
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University, Cardiff
-
Dr Phillip Leech, Principal Medical Officer for Primary Care, Department of Health , London
-
Ms Kay Pattison, Section Head R&D, DH, Leeds
-
Dr Morven Roberts, Clinical Trials Manager, MRC, London
-
Dr Ursula Wells PRP, DH, London
Interventional Procedures Panel
-
Consultant Surgeon & Honorary Clinical Lecturer, University of Sheffield
-
Mr David P Britt, Service User Representative, Cheshire
-
Mr Sankaran ChandraSekharan, Consultant Surgeon, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Mr Seamus Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor David Taggart, Consultant Cardiothoracic Surgeon, John Radcliffe Hospital
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Nadim Malik, Consultant Cardiologist/ Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Mr Michael Thomas, Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Mrs Isabel Boyer, Service User Representative, London
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Unit Manager, Pharmacoepidemiology Research Unit, VRMM, Medicines & Healthcare Products Regulatory Agency
-
Mrs Nicola Carey, Senior Research Fellow, School of Health and Social Care, The University of Reading
-
Mr John Chapman, Service User Representative
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Professor Robin Ferner, Consultant Physician and Director, West Midlands Centre for Adverse Drug Reactions, City Hospital NHS Trust, Birmingham
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr Bill Gutteridge, Medical Adviser, London Strategic Health Authority
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Yoon K Loke, Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Dr Martin Shelly, General Practitioner, Leeds, and Associate Director, NHS Clinical Governance Support Team, Leicester
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Mr David Symes, Service User Representative
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Service User Representative, Gloucestershire
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician & Cardiologist, County Durham & Darlington Foundation Trust
-
Mr John Needham, Service User, Buckingmashire
-
Ms Mary Nettle, Mental Health User Consultant, Gloucestershire
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Howard Ring, Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry & Warwickshire Partnership Trust
-
Dr Alastair Sutcliffe, Senior Lecturer, University College London
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Ms Kay Pattison, Section Head, R&D, DH, Leeds
-
Dr Morven Roberts, Clinical Trials Manager, MRC, London
-
Professor Tom Walley, HTA Programme Director, Liverpool
-
Dr Ursula Wells, Policy Research Programme, DH, London
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation for Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Dr Katherine Darton, Information Unit, MIND – The Mental Health Charity, London
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Mr George Levvy, Chief Executive, Motor Neurone Disease Association, Northampton
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Rajan Madhok, Medical Director and Director of Public Health, Directorate of Clinical Strategy & Public Health, North & East Yorkshire & Northern Lincolnshire Health Authority, York
-
Professor Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, National Co-ordinator, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Susan Schonfield, Consultant in Public Health, Hillingdon Primary Care Trust, Middlesex
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington