Notes
Article history
The research reported in this issue of the journal was commissioned by the National Coordinating Centre for Research Methodology (NCCRM), and was formally transferred to the HTA programme in April 2007 under the newly established NIHR Methodology Panel. The HTA programme project number is 06/92/03. The contractual start date was in October 2007. The draft report began editorial review in June 2010 and was accepted for publication in February 2011. The commissioning brief was devised by the NCCRM who specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2011. This work was produced by Avery et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2011 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
An adverse drug reaction (ADR) is a reaction to a drug and/or a combination of drugs that is harmful and unintended, and that occurs at a dose that is normally used for prophylaxis, diagnosis or treatment. 1 It is widely acknowledged that ADRs ‘significantly diminish quality of life, increase hospitalisations, prolong hospital stay and increase mortality’. 2 It has been estimated that 6.5% of acute hospital admissions are due to ADRs with an annual cost to the UK NHS (in 2004) of £466M,3 and that one in seven hospital inpatients experience an ADR. 4
Pharmacovigilance is the science and activities relating to the detection, assessment, understanding and prevention of adverse effects of drugs. The monitoring of ADRs through pharmacovigilance is vital to patient safety, as rare, serious and/or unexpected reactions often appear only when drugs are used in everyday practice by many people. Spontaneous reporting of ADRs is one method of pharmacovigilance. Other methods include postmarketing surveillance and interrogation of large electronic data sets.
In the UK, spontaneous reporting is undertaken through the YCS, which was established in 1964 as a consequence of the thalidomide disaster. 1 Since the 1960s, doctors have been able to use this system to report suspected ADRs to the Medicines and Healthcare products Regulatory Agency (MHRA). From 1997, reports were also accepted from pharmacists. In 2002, the Yellow Card Scheme (YCS) was extended so that nurses, midwives and health visitors could also report suspected ADRs. An example of a health-care professional (HCP) report form is shown in Appendix 1.
The history of patient reporting to the Yellow Card Scheme
Patient reporting to the UK YCS was first considered by a working party of the Committee on Safety of Medicines (CSM) in 1983 following the withdrawal of benoxaprofen,5 but it decided against this and advised patients with suspected ADRs to consult their doctors in the hope that they would report appropriately.
The potential benefits of patient reporting were summarised at the First International Conference on Consumer Reports on Medicines in 2000 and included the promotion of consumer rights and equity, acknowledging that consumers have unique perspectives and experiences, and that health-care organisations would benefit from consumer involvement. 6 It is widely recognised that there is substantial under-reporting of ADRs by HCPs; therefore, adding patients to the range of potential reporters may increase spontaneous reporting and earlier detection of important ADRs. 2,7–9 Additional support for direct patient reporting came from the Metters report in 2004;1 although, concerns were documented from some stakeholders.
In 2003, UK patients were able to report ADRs using the telephone helpline NHS Direct. There was a relatively low number of reports and there were criticisms that patients were not able to report to the MHRA directly. 1
In 2004, the CSM approved the formation and membership of the CSM working group on patient reporting of ADRs (ADRWG). The ADRWG had representatives from patients and patient organisations, academia, pharmacy, general practice and clinical pharmacology, and was chaired by Dr Patricia Wilkie, a lay member of the CSM. The first meeting was held in September 2004. The ADRWG held focus groups and recommended that a patient reporting system had to be:
-
accessible to patients and the public
-
easy to use
-
available in different reporting systems
-
able to provide information needed to effectively monitor medicines.
The first patient Yellow Card for use by the public was designed to include the important questions in the professionals’ Yellow Card and retained a similar format to the professional card. Consultations were held with patients and patient organisations and the first pilot of the patient Yellow Card was made available on the MHRA website in January 2005. A paper version was also distributed to 4000 general practices, representing some 30,000 general practitioners (GPs).
Between January and September 2005, over 650 patient reports were received. The reports contained detailed descriptions of suspected ADRs and were considered useful by the MHRA.
The ADRWG recommended that:
-
A range of methods, including paper, electronic and telephone, should be available to report suspected ADRs.
-
Yellow Cards should be available in a variety of outlets, including pharmacies, general practices and voluntary organisations.
-
The report could be made by any member of the public, who should provide name and contact details.
-
The MHRA would follow-up reports wherever necessary, but would not be able to give medical advice.
-
Reports could be made on any medicine on the market, including prescribed medicines, over-the-counter (OTC) products and complementary medicines including herbals.
Other recommendations included:
-
carrying out further evaluation into aspects of patient reporting
-
making Yellow Card information more widely available
-
making HCPs more aware of patient reporting
-
examining the possibility of one Yellow Card for all
-
informing patients when they are prescribed a drug that is undergoing intense monitoring by the MHRA.
Following the pilot, the forms were further adapted and a nationwide-direct patient reporting scheme was officially launched along with a media campaign in September 2005. An evaluation of 407 reports received in the first 6 months of the scheme suggested that patient reports were of a similar quality to HCP reports. 10 Following the redesign of the paper-based reporting form and web interface for reporting, the scheme was launched again in February 200811 with another media campaign. On this occasion, community pharmacies received promotional literature, including display posters and credit card-sized information for informing patients about the scheme.
Examples of the patient report forms used from September 2005 to February 2008 and from February 2008 onwards are shown in Appendices 2 and 3, respectively. The reports can be submitted to the MHRA by post or telephone or via the internet. 12,13 Patients are encouraged to report suspected side effects of any prescribed or non-prescribed medicine or herbal remedy,14 especially if:
-
it is not mentioned in the medicine patient information leaflet (PIL)
-
it causes problems bad enough to interfere with everyday activities
-
it happens when taking more than one medicine and could be caused by interactions.
The MHRA electronically records and reviews information submitted so that major safety issues can be detected using signal generation and additional methods. 14 The UK scheme was one of the earliest to introduce patient ADR reporting and, as such, has generated considerable number of data to enable comparison between patient and ADR reports; by 2009 the MHRA had received around 9000 patient reports. 11
In April 2006 there was a call for research to evaluate patient reporting to the YCS and our team was successful in bidding for funding. The aims, objectives and research questions of our evaluation are outlined below.
Aims
To evaluate patient reporting of suspected ADRs to the YCS in the UK by assessing the pharmacovigilance contribution of patient reports compared with those of health professionals; exploring the views of patient reporters and members of the public, and comparing study findings with those from existing schemes worldwide.
Objectives and research questions
The objectives and research questions relate to:
-
a review of the literature
-
studies based on the analysis of Yellow Card data for patients and health professionals
-
studies considering the views and experiences of patients and members of the public regarding patient reporting.
For ease of reference, we have numbered the separate studies as follows.
-
Literature review:
-
– Study 1 Review of the world literature describing and comparing patient and HCP reporting of ADRs.
-
-
Evaluating the pharmacovigilance impact of patient reporting of suspected ADRs – analysis of reports of suspected ADRs from the UK YCS:
-
– Study 2 Description of the characteristics of reports from patients and HCPs.
-
– Study 3 Assessment of the pharmacovigilance impact of reports from patients and HCPs using signal generation analysis and clinical assessment of reports.
-
– Study 4 Qualitative analysis of reports from patients and HCPs.
-
-
Considering the views and experiences of patients and members of the public regarding patient reporting:
-
– Study 5 Questionnaire survey to capture the views and experiences of patients who have made reports.
-
– Study 6 Telephone interviews to explore further the views and experiences of patients who have made reports.
-
– Study 7 Focus groups and usability testing with members of the public regarding patient reporting.
-
– Study 8 Omnibus survey to assess public awareness of the YCS.
-
The objectives and research questions relating to these studies are listed in Table 1. These are derived from the original application.
| Study | Objectives | Research questions |
|---|---|---|
| Study 1 Review of the world literature describing and comparing patient and HCP reporting of ADRs | To review the published literature on patient and HCP-reporting systems in different settings |
How do the study findings compare with the literature? Are there any recommendations that could be made to amend the UK system, derived from the literature? |
| Study 2 Description of the characteristics of reports from patients and HCPs | To identify the characteristics of patient reporting to the YCS | What are the characteristics of patients who report ADRs through the YCS? |
| To identify the types of drug, types of suspected adverse reactions and seriousness of suspected reactions reported by patients |
What classes of drugs are most commonly reported by patients? What categories of suspected adverse reactions are most commonly reported by patients? What are the outcomes of suspected side effects and do these differ across reporter groups? |
|
| To determine whether there are differences in the time lag between ADR occurrence and reporting for patients and HCPs | Are there important differences in the time taken to report an ADR between patients and HCPs? | |
| To investigate the factors associated with patient reports compared with those made by HCPs |
Are patients more or less likely than HCPs to report serious reactions? Are patients more likely than HCPs to report suspected adverse reactions to particular classes of drug? Are patients more likely than HCPs to report suspected adverse reactions to newer drugs? Are patients more likely than HCPs to report particular categories of suspected adverse reactions? For children, are the events reported directly by parents different from those reported by HCPs, acknowledging possible small numbers? |
|
| Study 3 Assessment of the pharmacovigilance impact of reports from patients and HCPs using signal generation analysis and clinical assessment of reports | To explore the relative contribution of patient reporting to signal generation |
Do patient reports add ‘weight’ to known signals or signals generated by reporting by HCPs? Do patient reports generate ‘new’ signals? If they do, are these signals for events which are expected to be of more concern to patients than HCPs? Do patient reports generate new signals sooner? |
| To estimate the extent to which duplicate reporting occurs | What is the proportion of patient reports that also seem to have been reported by a HCP? | |
| Study 4 Qualitative analysis of reports from patients and HCPs | To explore the richness of patients’ descriptions of their suspected adverse reactions compared with HCPs |
How do patients describe suspected side effects? How does the richness of patient reports compare with those of HCPs? Are there similarities or differences between patient and HCP reports and between the three methods of reporting? |
| Study 5 Questionnaire survey to capture the views and experiences of patients who have made reports | To describe the views and experiences of patients reporting to the YCS |
How did patients find out about the YCS? How many times have they used the scheme? What method of reporting did they use for their latest Yellow Card report (electronic, paper or telephone)? Who did the report (the patient or the patient’s representative)? How easy did they find it to make a report? Were there any difficulties encountered in making reports, including whether patients needed additional help in completing the electronic- or paper-based forms? Are there any suggestions for improvements in the reporting system? Did reporters inform a HCP about the suspected reaction? Were patients discouraged by HCPs from reporting? What are the characteristics of respondents (age, gender, ethnicity and educational attainment) and do these influence reporting methods? |
| Study 6 Telephone interviews to explore further the views and experiences of patients who have made reports | To explore in detail the experiences and views of patients who have made reports to the YCS |
Do reporters have difficulties in making Yellow Card reports, and suggestions for improving the reporting system? What are patients’ motivations for making a report, and anticipated contribution of their report? What are patients’ expectations about what would happen to their report? Are patients satisfied or dissatisfied with making a report? Are patients willing to report in future? |
| Study 7 Focus groups and usability testing with members of the public regarding patient reporting | To ascertain the views of members of the public on the YCS | What do members of the public think about the YCS? |
| To ascertain the views of members of the public on the user-friendliness, effectiveness and usability of different mechanisms of patient reporting | When given the opportunity to try out different methods of reporting in a simulated setting, what do members of the public think about these methods? | |
| To obtain suggestions for potential ways in which the reporting system could be improved | What suggestions do members of the public have for ways in which patient reporting to the YCS could be improved? | |
| Study 8 Omnibus survey to assess public awareness of the YCS | To estimate the percentage of the general public in Great Britain who have heard of the YCS for patient reporting of ADRs | What percentage of respondents have heard about the YCS? |
| To determine whether those respondents who believe they had experienced an ADR and who were aware of the YCS had made a report using the YCS |
Have respondents used any types of medicines regularly or taken any types of medicines in the last year? Do respondents believe that they have experienced a side effect from a medicine (or complementary therapy) in the past and, if so, have they told anyone about it? For those respondents who had experienced side effects from a medicine in the past and who had heard about the YCS for patient reporting: Did they make a report? If they did not make a report, why not? |
|
| To assess the views of members of the public on the convenience of the three different ways of reporting (online, telephone, obtaining a paper form from a GP/pharmacy to fill in and post) | Which of the following ways of reporting suspected side effects might be most convenient to respondents:
|
Structure of the report
Each of the above studies is described in a separate chapter containing objectives, methods, results and a summary. In addition, a chapter is devoted to an explanation of data used for the studies evaluating the pharmacovigilance impact of patient reporting (see Chapter 3). The final chapter contains the discussion for all of the studies, including strengths and limitations, recommendations for patient reporting and recommendations for research.
Research team
The research was undertaken by a multicentre, multidisciplinary team co-ordinated by the University of Nottingham, Nottingham, UK.
In terms of main areas of responsibility:
-
The Drug Safety Research Unit from Southampton carried out the initial processing of Yellow Card Report data from MHRA and also undertook the signal generation analyses.
-
The University of Aberdeen undertook all of the other quantitative analyses in the study and led the literature review.
-
The Liverpool John Moores University undertook aspects of the qualitative analyses.
-
The University of Nottingham led on survey design and execution, interviews, focus groups, usability testing and qualitative analyses.
Advisory group
A multidisciplinary advisory group was set up 1 year after the start of the project. It was led by Millie Kieve, founder of the charity APRIL (Adverse Psychiatric Reactions Information Link; www.april.org.uk). The group commented on the design and progress of the study, advised on the qualitative analysis of Yellow Card reports and commented on drafts of the report. Membership of the group is shown in Appendix 4.
Medicines and Healthcare Regulatory Authority
The MHRA contributed to this study by providing data, administering the questionnaire and answering numerous queries from the research team. The MHRA did not, however, have a role in the interpretation of the findings and did not comment on the report before it was submitted.
Ethical approval
Our evaluation received a favourable opinion from:
-
Warwickshire Research Ethics Committee (07/H1211/117)
-
The Independent Scientific Advisory Committee of MHRA.
Chapter 2 Study 1: literature review on the international experience of consumer reporting schemes
Objective
To review the published literature on patient- and HCP-reporting systems in different settings.
Methods
A range of methods was used to identify countries with patient reporting as part of their national pharmacovigilance activities, including a questionnaire, personal communication with key contacts and literature review. The questionnaire (Appendix 5) was e-mailed to pharmacovigilance staff in 47 countries to obtain information on their ADR reporting schemes. The contact details were identified from the World Health Organization (WHO) Uppsala Monitoring Centre website (www.WHO-UMC.org). The questionnaire explored whether the national scheme permitted patient reporting and details about these schemes. The questionnaire was designed for rapid completion and the content was informed by the authors’ knowledge of the characteristics of previously identified national spontaneous reporting schemes. The questionnaire was not piloted prior to dissemination. One e-mail reminder was sent to non-respondents. In addition, the authors liaised with key contacts in pharmacovigilance to identify relevant information.
A literature review was performed to identify comparative studies and reports of patient and HCP ADR reports. A search was conducted of the databases MEDLINE (Ovid), EMBASE (Ovid) and Pharm-line using both medical subject heading (MeSH) and text search terms ‘ADR reporting’, ‘side effect reporting’, ‘pharmacovigilance’, ‘patient report’, ‘patient report’ and ‘public report’. Details of the search terms used are shown in Appendix 6. The search dates were from 1996 to May 2009. The searches were last run in May 2009. Only studies in the English language were included owing to financial and time constraints. Studies that included only HCP or only consumer reports were excluded. The reference lists of each included study were checked to identify additional studies. Personal reference lists were also searched. Internet searches were performed using similar search terms. The search results were checked by one individual (SA-U) for relevant studies. Data from the included studies were extracted using a data abstraction form. These data were synthesised by classifying the different types of study, and comparing and contrasting the findings.
Results
Table 2 shows the countries that, on the basis of the survey or literature review, were identified as having consumer reporting schemes. Appendix 7 shows the countries that did not appear to have consumer reporting based on the literature review or that did not respond to the survey.
| Country | Pharmacovigilance system (year of introduction) | Methods of report submission | Comparison of patient and HCP data available? | Feedback provided to patient reporter | Feedback to survey from pharmacovigilance contact |
|---|---|---|---|---|---|
| Argentinaa | |||||
| Armeniaa | |||||
| Australia |
TGA (2003) AME Line |
Hard copy form ‘Blue card’, online, post, telephone | Yes17 |
Referred to TGA website for information requested No comparisons made |
|
| bBelgium15 | Test-Achats/Test-Aankoop (2006) |
Data from Herxheimer et al. 15 Form downloaded from internet |
No |
No response. Data from Herxheimer et al. 15 Scheme run by Test-Achats/Test-Aankoop, national consumer organisation |
|
| Botswanaa | |||||
| Brazila | |||||
| Burkina Fasoa | |||||
| Canada | Canada Vigilance Program (2003) | Post, fax, telephone, online | Yes18 |
Comparison made in 2008 HCPs 70% and patients 30% of reports submitted |
|
| Congoa, The Democratic Republic of The | |||||
| Côte d’Ivoirea | |||||
| Czech Republic | National Drug Information And Pharmacovigilance Centre | Online | Unclear/unlikely | Referred to website for information requested | |
| Denmark | Danish Medicines Agency (2003) | E-mail, post, online, fax | Yes19 | ||
| Ghanaa | |||||
| Guatemalaa | |||||
| Iceland |
Iceland Medicines and Control Agency (2007) |
Unclear – possibly only online Not by telephone |
No | Unclear | No comparisons made yet |
| Irana, Islamic Republic of | |||||
| bIreland15 |
Irish Medicines Board |
Online, freepost | No | Unclear | No response |
| bItaly15 | Italian Drug Regulatory Agency (2004) |
Data from Herxheimer et al. 15 Form downloaded from internet; electronic form also available |
No | ||
| Kenyaa | National Pharmacovigilance Systems of Kenya (2009) (www.pharmacyboardkenya.org/index.php?id=2) |
Hard copy forms, post, fax, e-mail, to local pharmacy Unclear whether online or telephone submission possible |
No | Unclear | Campaigns and programmes are being organised to create public awareness |
| Madagascara | |||||
| Mexicoa | |||||
| Moldovaa, Republic of | |||||
| Myanmara | |||||
| Namibia | The Safety Yellow Form (2008) (www.nmrc.com.na/PVSystem/tabid/1347/language/en-US/Default.aspx) |
Form can be downloaded and submitted by post, e-mail or fax Unclear whether online or telephone submission possible |
No | Unclear | No comparison has been made as patient reports are low |
| Netherlands |
The Netherlands Pharmacovigilance Centre Lab (Lareb): government run (www.lareb.nl) (2003) IVM (Institute for Responsible Medicines Use): consumer run (www.medicijngebruik.nl) (2004) |
Post, e-mail, fax, online, telephone | Yes20 | ||
| New Zealand | Medicines and Medical Devices Safety Authority (< 1970) |
Report forms available online or as hard copy For submission by freepost, fax or e-mail; telephone reports also accepted |
No |
Yes Acknowledgement and feedback letter sent to all reports Letters include causality assessment, no. of similar reports in NHS, additional relevant information |
No comparisons made yet |
| bNorway15 | Norwegian Medicines Agency (2010) (www.legemiddelverket.no/) | No | Unclear | No comparisons made | |
| Omana | |||||
| Philippinesa | |||||
| Saudi Arabiac | |||||
| Sierra Leonea | |||||
| Slovakia | State Institute for Drug Control (NK) (www.sukl.sk/en) | Online, post | No | Unclear | No comparisons made |
| South Africaa | |||||
| Sri Lankaa | |||||
| Saint Luciaa | |||||
| Sweden |
Swedish Drug Information system (www.kilen.org) (1978) |
Forms, e-mail, electronic method launched in 2008 | No | Yes | No comparisons made as number of reports received from patients is low |
| Switzerland | Swiss Agency for Therapeutic Products (NK) (www.swissmedic.ch/index.html?lang=en) |
Forms obtained online, or by telephone, post Possibly only by post (online commencing soon for HCPs only) |
No |
Yes Primary reported receives a ‘brief evaluation’ within 7 days |
Referred to the website |
| United Republic of Tanzaniaa | |||||
| Thailanda | |||||
| Togoa | |||||
| UK | The YCS (2003, pilot; 2005, full introduction) | Hard copy form ‘Yellow Card’, post, e-mail, fax, telephone, online | Yes10,21,22 | No | |
| Uruguaya | |||||
| USA | US Food and Drug Administration (~1960s) | Post, online, telephone | Yes23 | ||
| Zambiaa | |||||
| Zanzibara | |||||
| Zimbabwea |
Sixteen countries responded to the questionnaire (response rate 34.0%). The characteristics of the longer-established European schemes were summarised in a report by Health Action International Europe,15 authored by one of the key contacts (Dr A Herxheimer). Since completion of the questionnaire, the results of a survey of low- and middle-income countries regarding their pharmacovigilance activities have been published. 16 An additional 27 countries with consumer reporting were identified. Information was received that consumer reporting has been introduced in Saudi Arabia (Dr H Aljadhey, King Saud University, Riyadh, Saudi Arabia, 2010, personal communication). In total, 46 countries were identified as having consumer reporting schemes (see Table 2).
Findings from the survey and literature review
International experiences of patient reporting
Blenkinsopp et al. 24 completed a systematic review of patient reporting of suspected ADRs, which was first published online in 2006. Seven studies were included, although none involved spontaneous reporting by patients. Where comparisons were available with HCP reports, quality of reports appeared to be similar. There was some evidence that patients were more likely to report ADRs if they felt that their HCP had not acknowledged their concerns. We now report further on international experiences of patient reporting of ADRs drawing on literature from before and after this systematic review.
In Australia, patients have access to the ‘Adverse Medicine Events (AME) Line’,25 through which they can report ‘suspected AMEs, possible errors or “near misses” with their medicines’. Patients can also report to the Adverse Drug Reactions Unit (ADRU) of the Therapeutic Goods Administration (TGA). 26 The AME Line is operated by the Australian Council of Safety and Quality in Health Care and was launched in 2003. Suspected errors and ADRs are reported using two different forms; thus, reporting rates for each can be analysed separately. A recent case series of patient reports to AME relating to the use of zolpidem indicated that memory disturbances, hallucinations and dependence were more common than was previously thought. 27 An audit of the use of the AME Line showed that 43% of the 3415 calls received in a 2-year period were prompted by media publicity. 17 Females and older patients were more likely to call the line. One-fifth of ADRs reported by callers were previously unrecognised and 8% were related to complementary medicines. In total, 105 serious ADRs and drug-induced hospitalisations that callers reported had not been reported by health professionals. 17 The case series and audit were reported as conference abstracts and, as such, very limited information is available regarding their methods and conduct.
The Canadian Adverse Drug Reaction Monitoring Program (CADRMP) at Health Canada is responsible for the collection and assessment of adverse reaction reports made voluntarily by health professionals and patients. 28 In 2008, 20,360 adverse reaction reports were submitted, but these were not limited solely to medicines. 18 The majority (72%) of these reports were submitted by the market authorisation holder. Of the 16,272 reports for which a reporter was identifiable, 4851 (29.9%) were submitted by ‘consumers’ and ‘patients’, and 10,898 (67%) were submitted by health professionals. In total, 1719 (10.6%) reports were submitted solely by patients. The number of domestic reports for ADRs has nearly doubled between 2001 and 2008. 18 A quarterly newsletter is produced for HCPs and patients; the former receive mailed paper copies, whereas the latter need to access the publication online. These data are presented as annual reports, which do not include detailed methodological information.
In Denmark, patient reporting of ADRs commenced in 2004 and the reports received from 2004 to 2006 have been analysed. 19 The analysis comprised 6319 reports and 15,531 ADRs in total, with 544 (8.6%) reports submitted by patients. Highly significant differences were shown in the proportions of ADRs classified as ‘serious’ or ‘non-serious’ by different types of reporter (patient, physician, pharmacists, lawyers, other HCPs) (p < 0.0001). Patients and physicians reported similar proportions of serious reactions (46% and 45%, respectively). Compared with all other types of reporters, patients were significantly less likely to report ADRs relating to the following: blood and lymphatic system [odds ratio (OR) 0.22, 95% confidence interval (CI) 0.08 to 0.59)]; hepatobiliary disorders (OR 0.14, 95% CI 0.04 to 0.57); infections and infestations (OR 0.45, 95% CI 0.23 to 0.87); investigations (OR 0.71, 95% CI 0.50 to 0.99); and pregnancy, puerperium and perinatal conditions (OR 0.33, 95% CI 0.11 to 1.06). However, compared with all other types of reporters, patients were significantly more likely to report ADRs relating to the following: ear and labyrinth disorders (OR 2.09, 95% CI 1.01 to 4.30); nervous system disorders (OR 1.27, 95% CI 1.05 to 1.53); psychiatric disorders (OR 1.70, 95% CI 1.31 to 2.20); and reproductive system and breast disorders (OR 2.02, 95% CI 1.13 to 3.61). Differences were also shown between patient and other types of reporter regarding the types of medicines for which they submitted ADR reports. Although patients were more likely to submit reports for medicines used to treat the nervous system and sensory organs, along with antiparasitic products, they were less likely than other types of reporters to report products for blood and blood-forming organs, anti-infectives for systemic use, antineoplastics and immunomodulating agents. In addition, it was noted that patients reported nine ADRs that had not been identified by other reporters, including dysgraphia, parosmia, and thromboembolic stroke, all of which are regarded as serious. As this study focused solely on ADRs that were classified as serious, it does not reflect the full range of ADRs reported by consumers (and HCPs).
In the Netherlands, patients can report suspected ADRs directly to the Netherlands Pharmacovigilance Centre (Lareb),29 a government-run organisation. Of the 6305 reports received in 2005, 819 (13%) were submitted by patients. 29 A comparative study was conducted in the Netherlands of all suspected ADR reports from patients and HCPs, based on reports received between April 2004 and April 2007,30 i.e. the first 3 years of consumer reporting. A total of 2522 reports (5401 suspected ADRs) were received from patients and 10,635 reports (16,722 suspected ADRs) from HCPs. Patient reports were more likely to be submitted by females; the mean age of patient reporters was 48 years. The types of drugs most often reported by patients were statins, selective serotonin reuptake inhibitors, beta-adrenoceptor antagonists, anticoagulants and proton pump inhibitors. The drugs and ADRs most frequently reported by patients were similar to those reported by doctors, including the organ systems affected. No difference was found between patient and HCP reports in terms of overall seriousness, but some differences were reported between the type of reporter and different categories of seriousness, i.e. patients reported significantly more disability than HCPs (2.3% and 0.4%, respectively) and more life-threatening reactions (5.2% and 2.7%, respectively). The Lareb system accepts only electronic reports from consumers, whereas HCPs can submit online or using a paper form. This may limit the numbers of reports by consumers, especially those who have little or no access to the internet.
In addition to Lareb, a patient-run reporting scheme31 has operated since 2004. Higher numbers of reports are made to this scheme than to Lareb, but the amount and type of information recorded by the two schemes differs, and so the data are not entirely comparable. During the first 10 months of the scheme, 49% of reports related to side effects, of which 6% were severe and 30% were not mentioned on the PIL. A short report was published of a comparison of the reports associated with the use of paroxetine made with Lareb and those reported to a free telephone medicines information service, introduced in 1990, which enabled patients to consult a pharmacist about the correct use of medicines and problems related to their medicine use. 20 Out of 23,625 calls from patients, 120 suspected ADRs were reported for paroxetine compared with 89 of the 7665 suspected ADRs from HCPs. Proportionally fewer reports were made about paroxetine via the telephone service than to Lareb (0.5% vs 1.2%, respectively). However, suspected ADRs were reported sooner using the telephone service than using Lareb (mean 229 days; 95% CI 160 to 298 days). No difference was found between the two reporting systems in terms of new suspected reactions (i.e. those not included in the PIL). Nine new ADRs were identified by both systems. Each reaction was first reported using the telephone system for all nine reactions, with a mean time lag of 273 days (95% CI 89 to 458 days) between the telephone and Lareb reports. Owing to this study being published as a short report, limited methodological data were presented. More recently, a comparative study was conducted using patient reports submitted to the patient-run reporting scheme for antidepressants,32 with HCP reports submitted during the same data collection period as Lareb. This study was published as an abstract, therefore only limited methodological information was available and there was limited interpretation of the results. In total, 258 reports were submitted by patients, most (72%) of whom were female. Of these reports, 217 were associated with side effects. Significant differences were shown between the ADRs reported by patients and those reported by HCPs. For example, the patients reported more sexual problems and weight gain, whereas HCPs reported more skin, muscle and joint complaints.
In the USA, the Adverse Events Reporting System (AERS) is the national database used by the Food and Drug Administration23 to support postmarketing drug surveillance. The MedWatch33 programme is the reporting scheme for patients and HCPs to use to submit reports of serious problems associated with medicines or medical devices. All reports submitted to MedWatch are entered into AERS. The same reporting form is used for both types of reporters and reports can be submitted online or by post, fax or telephone. Reports submitted by patients are acknowledged. Patient reporters are also contacted by MedWatch if further information is needed. The scheme includes reports for serious adverse events, product problems and errors. Serious events are those that led to death, disability, congenital anomaly, hospitalisation or ‘other serious events’ and those that are life-threatening. In 2008, of the 226,647 reports received by MedWatch, 46% were submitted by patients. The proportion of total reports submitted by patients has increased since the mid-1990s. 34 Relatively detailed annual reports were available regarding patient and HCP reporting to this system in the 1990s,34 and these are now available from the AERS website. 23 These data are presented as annual reports, which do not include detailed methodological information.
Overall, considerable variation exists across national schemes in terms of the proportion of total ADR reports submitted by patients, and this is illustrated in Figure 1.
FIGURE 1.
Percentage of total reports submitted by patients by country.

Other comparative studies of patient- and health-care professional-suspected adverse drug reaction reporting
In addition to the comparative studies of spontaneous ADR reporting described above, several comparative research studies were identified which provide more information regarding the differences between these types of reporters, although they did not involve national spontaneous reporting. Only studies that have been published since the earlier review by Blenkinsopp et al. 24 are presented below. Studies were excluded if they were restricted to specific patient populations, for example children or the elderly.
A 6-month study conducted on medical wards in a university teaching hospital in north-east Thailand compared doctor and patient reporting of 13 new drugs newly introduced into the market in three medical wards. 35 Patients who were either receiving one of the target drugs on admission or had one of these drugs prescribed during admission were included. Doctors were asked to report ADRs on forms attached to the patients’ records during the patient’s admission, or post discharge, on forms in the patient’s outpatient record. Patients were interviewed daily during admission and all reports recorded in the forms were attached to their records. The forms were also attached to outpatient records. During the inpatient phase, patients were interviewed and reported 28 suspected ADRs, whereas doctors reported 13, and in the outpatient phase, patients reported 88 suspected ADRs, whereas doctors reported five. Patients reported more ADRs than doctors, but doctors reported more serious ADRs than patients. Two methods of patient reporting were used: one for inpatients and one for outpatients. The effect of the two different methods of data collection was not assessed. Causality was assessed for ADR reports from doctors using validated criteria, although different criteria were used to assess reports from patients following discharge from hospital. It is unclear whether the latter were validated criteria.
In 2009, a study was conducted with outpatients from one hospital in France, to determine whether they had experienced a suspected ADR and, if so, whether it had also been identified by their doctor(s). 36 In total, 66 patients reported 91 ADRs. The majority (77%) of patients with ADRs were women. Reports of ADRs were included in the medical notes of 44% of these patients; however, it is unclear whether these were the same ADRs reported by the patient. This study was reported as a conference abstract and minimal data were presented regarding the methods used.
A second study in France, by Nasrallah-Irles et al. 37 analysed patient reports of adverse events to health products submitted via patient associations. In the first year of the service, 200 reports were submitted by, or on behalf of, patients. Of these reports, only 130 (65%) were evaluable (others had missing information or related to medical devices, which were excluded by the authors). More reports were received for female patients and the average age of the affected person was 54 years (range 0.5 to 90 years). In the case of 93 reports, the adverse event was attributed with having a major effect on the patient’s well-being (physical, psychological and/or social). No direct comparison was made between reports received by this novel service and national pharmacovigilance statistics. Limited methodological information was presented. Validation of patient reports by their doctor was attempted, but achieved a response rate of only 40%. Overall, validation by HCPs of patient reports was achieved for 34.6% of all reports submitted. Although it is assumed that the 130 reports that formed the body of data for the analyses were associated with adverse events related to medicines, this is not described explicitly by the authors.
Summary
-
A range of methods was used to identify countries with patient reporting as part of their national pharmacovigilance activities, including a questionnaire to pharmacovigilance staff in 47 countries, personal communication with key contacts and a literature review.
-
A literature review was performed to identify comparative studies of patient and HCP ADR reports. A search was conducted of MEDLINE (Ovid), EMBASE (Ovid) and Pharm-line databases using both MeSH and text search terms. The search dates were from 1996 to May 2009.
-
Forty-six countries were identified as having consumer reporting schemes. A number of studies of patient reporting of suspected ADRs were identified, including a recent systematic review. Since the time of that review, one large-scale comparative study from the Netherlands has shown similarities in the classes of drug most commonly reported by patients and HCPs, and similar proportions of reactions were judged to be serious. Another large-scale comparative study from Denmark has shown that, compared with other sources, patients reported different types of medicines for categories of ADR. In this study consumers were as likely as physicians to report ADRs that were judged to be serious.
Chapter 3 Data used for the analysis of Yellow Card reports from patients and health-care professionals
Introduction
This chapter describes the methods used by the MHRA when processing Yellow Cards, the data provided by the MHRA for the purposes of the project and the methods used by the project team when processing the data received from the MHRA. The data were used for the following studies:
-
the descriptive study comparing the characteristics of patient reports and HCP reports (see Chapter 4)
-
the study comparing signals generated by patient reports and HCP reports (see Chapter 5)
-
the clinical evaluation of signals generated by patient reports and HCP reports (see Chapter 5)
-
the qualitative study exploring the similarities and differences between patient reports and HCP reports (see Chapter 6).
Processing/coding of reports by the Medicines and Healthcare Regulatory Authority
Data capture and data entry by the Medicines and Healthcare Regulatory Authority
Yellow Card reports can be made on paper, by telephone and online.
Paper reports are optically scanned and the information is then entered manually into the MHRA database using a variety of coded and free-text fields.
For reports received by telephone, the MHRA staff member taking the telephone call records the relevant case information stated by the patient on to a Yellow Card, which is transcribed to the database in the first person.
Online reports were originally transcribed manually into the Adverse Drug Reactions Online Information Tracking (ADROIT) database that was used by the MHRA, but on 31 May 2006 the MHRA changed to a custom database called ‘Sentinel’; this system allows for direct importing of data fields from reports completed electronically.
Data entry and quality checking is a four-step process involving different MHRA staff members:
-
Step 1 basic data entry (this requires the following minimum information: an identifiable reporter and patient, a suspected medicinal product and a reaction or event)
-
Step 2 full data entry
-
Step 3 checking the quality of the data before committing the report to the database
-
Step 4 a final check on the quality of data entry and initiation of follow-up, if appropriate.
The MHRA performs a monthly audit on a sample of data entered.
Once a report is committed to the database, updates can be made if new information becomes available, for example if:
-
follow-up information has been received, either at the request of the MHRA or because the reporter spontaneously provided additional information for the case
-
a duplicate report for the same case has been received from another reporter (whereby additional details will be merged with the original report)
-
the classification of the report has been changed (for instance, if an error is identified through the MHRA’s internal quality audit procedures).
Reports are flagged by the MHRA if they have been updated, but this does not distinguish between reports updated with follow-up information and those updated on receipt of duplicate reports for the same case.
Coding of data by the Medicines and Healthcare Regulatory Authority
Reporter category
More than one reporter category may be coded for in an individual report if:
-
the MHRA receives the same report from different reporters – these are merged into one case folder and contact details retained from both reporters
-
follow-up information is obtained from a different HCP – again, the contact details for the new informant are retained in the same case folder
-
the patient is also a HCP.
Reaction terms
The description of the reaction is entered both as a free-text narrative and as coded fields. The MHRA code the reaction using the most appropriate lowest level term (LLT) in the Medical Dictionary for Regulatory Affairs (MedDRA). This is a hierarchical grouping dictionary structured at the highest level by system organ class (SOC) then high-level group term (HLGT), high-level term (HLT), preferred term (PT) and LLT. MedDRA is used widely for data processing and analysis by regulatory authorities and the pharmaceutical industry, and is clinically validated. It is maintained and updated by the Maintenance and Support Services Organization (MSSO).
Preferred terms within MedDRA may be mapped to more than one SOC. For example, the PT ‘dizziness’ may be mapped (via the corresponding HLT and HLGT) to ‘cardiac disorders’, ‘vascular disorders’ or ‘nervous system disorders’ SOCs. This ‘multiaxiality’ allows for flexible analysis, depending on the research question or specific regulatory concern. However, within MedDRA a primary SOC is provided. For the example of ‘dizziness’ this would be ‘nervous system disorders’.
Seriousness
There are two ways in which seriousness can be coded: if ‘reporter considered serious’ has been flagged as ‘yes’ or the report contains at least one reaction term classified as ‘dictionary serious’. These terms are explained below.
‘Reporter considered serious’ flag
This relates to the report as a whole and is based on the response to the question regarding seriousness requested on the Yellow Card. The way in which this question is asked on the patient Yellow Card differs from that on the HCP Yellow Card. The HCP is asked ‘Do you consider the reaction to be serious?’ and is given a ‘yes’/‘no’ response option. If ‘yes’ is ticked then the HCP is requested to specify one or more of six reasons for considering the reaction to be serious. These are based on Council for International Organizations of Medical Sciences (CIOMS) criteria as:
-
patient died owing to reaction
-
life threatening
-
involved or prolonged inpatient hospitalisation
-
involved persistent or significant disability or incapacity
-
congenital abnormality
-
medically significant (reporter asked to provide details).
If the ‘reporter considered serious’ question is not ticked, but one or more of the six CIOMS subcategories are set to ‘yes’ then the ‘reporter considered serious’ flag would nevertheless be coded as ‘yes’ by MHRA.
Patient reporters are not asked directly whether they consider the reaction to be serious. At the time of our study, they were asked ‘How bad was the reaction?’ and asked to select one option from:
-
mild or slightly uncomfortable
-
uncomfortable, a nuisance or irritation, but able to carry on with everyday activities
-
bad enough to affect everyday activities
-
bad enough to be admitted to hospital
-
life-threatening
-
caused death.
If any of the last three of these options is ticked then the MHRA sets the ‘reporter considered serious’ flag to ‘yes’.
‘Dictionary serious’ flag
The second method for classifying the seriousness is at the reaction term level using the flag ‘dictionary serious’. Here, PTs within the MedDRA dictionary are assessed by medically-qualified personnel within the MHRA, and individually assigned a ‘dictionary serious’ status of either ‘yes’ or ‘no’. This is the MHRA’s own ‘in-house’ medical opinion and is independent of the CIOMS criteria. Where a reporter has not indicated any seriousness criteria, but at least one MedDRA reaction term is flagged as ‘dictionary serious’, the ‘medically significant’ CIOMS flag is set to ‘yes’ for that report.
Reaction outcome
Information on the outcome of the reaction is also requested in slightly different ways for patient and HCP reports. Table 3 shows the way in which the different options are coded by the MHRA.
| Patient report options | HCP report options | Coded as |
|---|---|---|
| Recovered completely | Recovered | Recovered/resolved |
| Recovered, but with some lasting effects (details requested) | Recovered, but free text indicates sequelae | Recovered/resolved with sequelae |
| Getting better | Recovering | Recovering/resolving |
| Still has the reaction | Continuing | Not recovered/not resolved |
| Other (details requested) | Other | Free-text field |
| Caused death | Caused death | Fatal |
Drugs
The MHRA codes the name of the medicines involved in a suspected ADR as they are reported on the Yellow Card (after correction of spelling errors). Thus, the medicine may be named using the generic drug substance, its brand or proprietary name, the combination name (e.g. co-codamol) or the individual constituents of a product (including, in some cases, its excipients).
An internal drug dictionary is used by the MHRA to select the most appropriate medicine or product name. The medicine/drug/product is then classified as either the ‘suspect drug’ or ‘other drug’ based on the information specified on the report. The MHRA does not code suspected drugs into hierarchical therapeutic classes within the report.
Black triangle status
Drugs with ‘black triangle’ status are those that require intensive monitoring by the MHRA to further assess their risk–benefit profile. This occurs, for instance, if the product contains a new active substance or a new formulation or combination of a previously licensed product or if a significant new prescribing indication has been added to the product’s licence. The time period for black triangle status varies. The status is usually reviewed after the product has been on the market for 2 years, although it may remain until the safety of the drug is more fully established. The MHRA allocates a black triangle flag to drugs specified on the Yellow Card report, according to the drug’s status at the time of the report. HCPs are made aware of black triangle drugs through publications such as the British National Formulary (BNF),38 but, currently, there is no system for informing patients.
Partial data entry
All data from online reports are imported into the database. For paper and telephone reports, all data are entered in to the database if the report is classified as ‘serious’ (as described above), but data from other reports are not always entered in full. The minimum information recorded for these other reports is the patient identifier, the suspect drug(s) involved and the coded MedDRA reaction terms. Hence, the data on the database may not always include, for example, the free-text description of the reaction. Such reports are flagged as ‘partially classified’ on the database. Where more complete information is required for assessment of reports that have been partially classified, full data capture is carried out and the partial classification flag is updated.
Narrative field
When free-text descriptions of the reaction are entered by the MHRA in to the database, they are transcribed in a narrative field, generally verbatim. Sometimes, however, if certain elements have been coded in other fields, the narrative may be abridged, for example to remove personal information, dates or information which can be recorded in structured fields elsewhere in the database. The narrative field is important in situations in which the MHRA wishes to undertake detailed assessment of the potential causality and/or impact of a suspected ADR.
Data provision by the Medicines and Healthcare Regulatory Authority
The MHRA provided the Drug Safety Research Unit (DSRU) with anonymised data on all patient and HCP reports received between 1 October 2005 and 30 September 2007. Reports from the pharmaceutical industry were excluded.
The data were provided electronically in the form of several encrypted Microsoft excel 2003 spreadsheets (Microsoft Corporation, Redmond, WA, USA). A case number was provided as a means of identifying unique patients and linking data between the different excel spreadsheets. The data fields available are shown in Box 1.
Gender
Age
Weight
Height
Report/reporter characteristicsReporter status:
-
For patient reports: patient, carer or parent
-
For HCP reports: qualification (e.g. doctor, pharmacist, nurse, other HCP, coroner)
Method of reporting (telephone, paper, internet)
Date report received by the MHRA
A flag to indicate, for example, that the report had been updated since first received
The opinion of the reporter (in the case of HCPs) as to the seriousness of the reaction
Various permissions requested by the MHRA from patient reporters:
-
Permission to send a copy of the report to the reporter’s GP
-
Permission to request follow-up information from the GP or reporter
Whether a patient reporter had informed a HCP of the ADR and whether the HCP had submitted a Yellow Card report
Reaction(s)Free-text description of reaction experienced
MedDRA-coded reaction terms: LLT and corresponding PT
Reaction start and end date
Onset of reaction from first and last dose of drug taken
Reaction duration
‘Dictionary seriousness’ (as noted above, this is a flag given by the MHRA to individual MedDRA terms and is independent from any opinion provided by the reporter as to the seriousness of the reaction)
Reaction outcome
Recovery time
Any specific treatment given
Reaction severity
Any sequelae
Suspect and concomitant drugsName of drug as reported
Prescribing indication(s)
Dose description as text (in various formats)
Dosage form and strength
Route of administration
Start and stop dates and/or duration of therapy
Status of whether drug was reported as the ‘suspect’ drug involved or an ‘other’ drug prescribed in the 3 months prior to the reaction
A flag to indicate whether the drug was a ‘black triangle’ product (the MHRA reported some technical difficulties in the use of the black triangle flag in their database at the time of our study and this may have affected the quality of the data extracted)
A flag indicating evidence of rechallenge
Patient medical and drug historyMedDRA LLT terms and free-text description of medical conditions
Start/end dates or duration of these conditions and a flag to indicate whether ongoing
Name of drugs taken in the past (with dates)
Details of any deaths reportedReported cause of death (MedDRA LLT term)
Certified cause(s) of death (MedDRA LLT term)
Flag to indicate if sudden death
Flag to indicate if postmortem on file at MHRA
Postmortem-reported cause of death (MedDRA LLT term)
Test resultsName of tests performed (MedDRA LLT term)
Dates and results (with reference range)
Flag to show further information held at MHRA
Information on the parent (this may be requested by the MHRA where a reaction is reported in a child) including:Details on the parent’s age, gender, weight, height, last menstrual period (details potentially relevant for medicines taken during pregnancy causing suspected ADRs to the fetus)
Parents’ medical and drug history including any dates and any previous parental ADRs
Data processing
All data received from the MHRA were stored on a secure server network at the DSRU. Data were uploaded for data processing on to a Microsoft SQL Server database. Data were reviewed for initial exploratory analysis and logical checks using stata (version 10, StataCorp LP, College Station, TX, USA).
Data cleaning
It was not the purpose of this study to investigate the quality of the data coded by the MHRA. Various logical checks were performed, however, and any apparent anomalies or inconsistencies checked with the MHRA (see Appendix 8). Where appropriate (e.g. in the case of a coding error) the data were corrected, both by the MHRA at source and by the DSRU, manually, in the data obtained from the MHRA.
The MHRA has an automated method of identifying potential duplicate reports at the time of data capture (e.g. if a patient and his/her GP complete separate Yellow Cards). In these circumstances the electronic data are merged into one case folder allocated according to the ‘primary’ reporter by the MHRA. Hence, if duplicate case numbers were identified in the patient and HCP data sets received from the MHRA then these were checked at source by the MHRA in order to identify which reporter was the ‘primary’ reporter. Using this information the data from both reports were then merged into one record stored in the database of the ‘primary’ reporter. It should be noted that this occurred for only six of the patient reports and so the merging of records should not have skewed our findings.
The research team had originally planned to search systematically for duplicate reports, but we decided not to do this when we learned of the system used by the MHRA. We felt that the approach taken by the MHRA was more sensitive than anything that we could have done, particularly as the MHRA has access to full details on the patients (whereas we had only anonymised data). Hence, only limited checking for duplicates was undertaken by the research team as outlined below and as part of the qualitative assessment of potential signals detected by the disproportionality analysis (Chapter 5).
Grouping of reaction terms
The coded reaction terms were provided by the MHRA as LLTs and the corresponding PTs. The DSRU mapped the LLTs provided by the MHRA to the corresponding PTs, HLTs, HLGTs and SOCs using the database files for MedDRA (version 12). The ‘default’ SOC provided in the MedDRA database files was used where more than option was available (see Reaction terms, above).
Grouping of suspect drugs reported
As noted above, the data provided by the MHRA contained the name of the suspect drug as reported. To group drugs into categories, the drug name (as reported) was first converted to a generic equivalent and then mapped to the most appropriate code within the Anatomical Therapeutic Chemical (ATC) drug classification system. 39 The ATC system is used worldwide to classify drugs into groups according to their therapeutic use.
A bespoke ‘matching tool’ was designed at the DSRU to enable each suspect drug to be mapped to the most appropriate ATC code. Data from the Dictionary of Medicines and Devices (‘dm+d’) were incorporated into this software (www.dmd.nhs.uk/). The dm+d database contains unique identifiers and descriptions for medicines and medical devices, and is used within the NHS for the purpose of procurement and reimbursement of medicines. Data from the dm+d were used to map brand names for proprietary products to generic equivalent drug names.
Having converted brand name to generic equivalent, the most appropriate ATC code was then selected by the DRSU researcher (LH, a pharmacist with experience of both community and hospital practice) for the generic equivalent. In some cases, there may be more than one ATC code for a drug, so the selection was based on assumptions taken from the contextual information provided on each report, such as prescribing indication, dose/strength, dosage form, administration route, other drugs prescribed and in some cases the free-text description of the reaction. In the absence of this contextual information, the ATC code was selected assuming the drug had been prescribed for systemic administration, unless this was unusual for the particular drug (e.g. salbutamol would be coded as an inhalation product). In addition, some drugs were mapped pragmatically, based on experience of the researcher involved in the mapping process, according to the most common prescription indication for the drug. For example, aspirin was coded as an antithrombotic agent rather than as an analgesic in the absence of other contextual information. Complementary therapies were identified as those containing herbal and/or homeopathic ingredients. These were not allocated an ATC code, but grouped as a separate category. An overview of the assumptions used in this mapping process is given in Appendix 9.
The database was screened for patients with the same age, gender, suspect drug name and reaction term (as PT). This produced a list of 462 reports. Of these, 246 were related to fairly common reactions to immunisations, for example in babies of a similar age; 66 were unlikely to be duplicates as the reaction date or date of starting the suspect drug were different and 135 potential duplicates had no information on dates to allow verification without viewing the source documentation held by the MHRA. The remaining 10 reports were reviewed more closely. Three appeared to be for different patients and seven were thought likely to include duplicate reports (relating to three patients). Hence, there appeared to be a few duplicate reports in the database.
Summary of differences between Yellow Card reports from health-care professionals and patients
A number of differences between the data obtained from HCPs and patients have been noted above and Table 4 gives a summary of these.
| Patient Yellow Card | HCP Yellow Card | |
|---|---|---|
| Current status of patient | Reporter asked ‘How is the person feeling now?’ and selects one of:
|
Reporter selects outcome of the reaction:
|
Please note that the MRHA recategorised these five options in the data set to make them comparable across reporter types. They were recorded as:
|
||
| Is the reaction serious? | Not asked | Reporter asked ‘Do you consider the reactions to be serious?’ and selects ‘yes’ or ‘no’ |
| How serious is the reaction? | Reporter asked ‘How bad was the suspected side effect? (tick one)’ and selects from:
|
After answering yes to the question above, the reporter asked to indicate why the reaction is considered serious, with the option of ticking any number of six boxes:
|
| Height of patient | Asked for | Not asked for |
Chapter 4 Study 2: descriptive study of Yellow Card reports
Objectives
This study was designed to provide important background information for evaluating the pharmacovigilance impact of patient reports of suspected ADRs. The objectives were to:
-
identify the characteristics of patients reporting to the YCS
-
identify the types of drug, types of suspected adverse reaction and seriousness of suspected reactions reported by patients
-
determine whether there are differences in the time lag between ADR occurrence and reporting for patients and health professionals
-
investigate the factors (see below) associated with patient reports compared with those made by health professionals.
Methods
After completing the data cleaning and processing, as described in Chapter 3, data analysis was undertaken at the University of Aberdeen.
Descriptive statistics were calculated for Yellow Card reports from patients and HCPs. Appropriate statistical tests compared the following factors across reporter type:
-
age and gender of patients
-
reported seriousness of the suspected ADRs (as coded by the MHRA)
-
types and number of suspected ADRs using MedDRA terms
-
word count used to describe the suspected reaction
-
number of suspect drugs per report and class of suspected drug using the ATC classification
-
time lag between suspected ADR and its reporting
-
reported outcome of the suspected ADR.
The time to report a suspected ADR for reactions occurring in the first and second year of the study period was also calculated by reaction outcome.
Reports with zero word counts were excluded when comparing word counts of reporter types because this provides the most valid comparison given that the MHRA does not always include the narratives in the database unless reports have been made online or are classified as serious.
Continuous variables were compared using an independent t-test if they were normally distributed, otherwise the non-parametric Mann–Whitney U-test was used. The median word count used to describe the suspected reaction for the different methods of reporting was compared using the Kruskal–Wallis test. Associations between two categorical variables were examined using Pearson’s chi-squared test. To minimise the chances of a type 1 error arising from multiple comparisons, a p-value of ≤ 0.01 was used to denote statistical significance throughout. Multiple logistic regression models were fitted to quantify the ORs for reporting specific reactions (based on the system organ class of the MedDRA classification system) between reporter type. The ORs were adjusted for age and gender of the patient affected by the ADR. All statistical analyses were performed using sas (version 9; SAS Institute, Cary, NC, USA).
Results
Characteristics of those experiencing an adverse drug reaction
In total, 26,129 ADR Yellow Card reports were received from the MHRA over the 2-year study period. Of these, 5180 (19.8%) were patient and 20,949 (80.2%) were HCP reports. Significantly more Yellow Card reports were made for female patients, whether reported by the patient or via HCPs (both p < 0.001) (Table 5). The median age of patients, as reported by either patients or HCPs, was similar (p = 0.06).
| Characteristic | Patient report | HCP report | |||||
|---|---|---|---|---|---|---|---|
| Male | Female | All | Male | Female | All | ||
| Age (years) | Median (IQR) | 57.0 (40.0 to 69.0) | 51.0 (35.0 to 64.0) | 54.0 (37.0 to 66.0) | 54.0 (32.0 to 68.0) | 53.0 (34.0 to 68.0) | 53.0 (33.0 to 68.0) |
| Missing, n (%) | 402 (22.4) | 723 (22.3) | 1230 (23.7) | 506 (6.2) | 737 (6.2) | 1371 (6.5) | |
| Total | N (%) | 1796 (34.7) | 3249 (62.7) | 5180 (100) | 8208 (39.2) | 11,935 (57.0) | 20,949 (100) |
Method of reporting
The reporting method used was not documented for 22.7% of reports from patients and 29.3% from HCPs. Excluding these reports, the most frequent method used to report an ADR was the paper Yellow Card form for both reporter groups (79.0% of patients and 87.7% of HCPs); the internet was the next most frequent method (17.6% of patients and 12.3% of HCPs), followed by the telephone (3.5% of patients and 0.03% of HCPs). There was a highly significant association between the method of reporting used and reporter type (p < 0.001).
Reactions
Patients reported 20,358 ADRs in total, whereas HCPs reported 44,429 ADRs. Patients reported a significantly higher number of suspected ADRs per Yellow Card report than HCPs {median [interquartile range (IQR)] of 3 (2 to 5) vs 2 (1 to 3), respectively; p < 0.001 (Table 6)}. Almost one-half (45.2%) of HCP Yellow Card reports contained only one ADR compared with 21.6% of patient Yellow Card reports; only 3.3% of HCPs reported over five reactions per report compared with 21.8% of patient reports (p < 0.001 overall).
| No. of reactions | No. of patient reports (%) | No. of HCP reports (%) | p-value |
|---|---|---|---|
| Median (IQR) | 3 (2 to 5) | 2 (1 to 3) | < 0.001a |
| 1 | 1120 (21.6) | 9475 (45.2) | < 0.001b |
| 2 | 1041 (20.1) | 5405 (25.8) | |
| 3 | 812 (15.7) | 3070 (14.7) | |
| 4 or 5 | 1076 (20.8) | 2316 (11.1) | |
| > 5 | 1131 (21.8) | 683 (3.3) | |
| Total (%) | 5180 (19.8) | 20,949 (80.2) |
The most frequent LLT reaction reported by patients was nausea (n = 458; 2.2% of all patient reactions reported), followed by headache (n = 440; 2.2%) and dizziness (n = 334; 1.6%) (Table 7). Nausea (n = 987; 2.2%) and headache (n = 758; 1.7%) were also the two most frequently reported LLTs on the HCP reports, followed by vomiting (n = 647; 1.5%).
| No. of patient-reported reactions (N = 20,358) | No. of HCP-reported reactions (N = 44,429) | |||
|---|---|---|---|---|
| LLT name | n (% of reactions) | LLT name | n (% of reactions) | Rank for patient reports |
| Nausea | 458 (2.2) | Nausea | 987 (2.2) | 1 |
| Headache | 440 (2.2) | Headache | 758 (1.7) | 2 |
| Dizziness | 334 (1.6) | Vomiting | 647 (1.5) | 6 |
| Depression | 300 (1.5) | Rash | 577 (1.3) | 10 |
| Diarrhoea | 280 (1.4) | Diarrhoea | 479 (1.1) | 5 |
| Vomiting | 242 (1.2) | Dizziness | 418 (0.9) | 3 |
| Tiredness | 230 (1.1) | Depression | 273 (0.6) | 4 |
| Anxiety | 196 (1.0) | Abdominal pain | 265 (0.6) | 44 |
| Itching | 178 (0.9) | Erythema | 262 (0.6) | 130 |
| Rash | 174 (0.9) | Shortness of breath | 256 (0.6) | 49 |
| Suicidal ideation | 156 (0.8) | Redness | 252 (0.6) | 108 |
| Appetite lost | 154 (0.8) | Chest pain | 249 (0.6) | 28 |
| Pain | 148 (0.7) | Pain | 222 (0.5) | 13 |
| Muscle pain | 145 (0.7) | Itching | 220 (0.5) | 9 |
| Joint pain | 133 (0.7) | Confusion | 214 (0.5) | 21 |
| Shaking | 133 (0.7) | Swelling | 213 (0.5) | 47 |
| Stomach pain | 131 (0.6) | Palpitations | 212 (0.5) | 22 |
| Constipation | 128 (0.6) | Anxiety | 211 (0.5) | 8 |
| Dry mouth | 127 (0.6) | Fever | 206 (0.5) | 55 |
| Sweating | 126 (0.6) | Sweating | 205 (0.5) | 20 |
Table 8 presents the number of patient and HCP reports that had at least one of each type of reaction grouped according to the System Organ Classification of MedDRA. More patient reports had mention of a nervous system disorder problem (41.5%) than those of another organ system. This was followed by problems categorised as ‘general disorders and administration site conditions’ (39.8%), problems that were also the second most common organ system affected according to the HCP reports (23.1%). The most common category in the HCP reports was skin and subcutaneous tissue disorders (23.2%).
| Reaction | No. of patient reports (%)a | No. of HCPb reports (%)a | Patient vs HCP: adjusted ORb (99% CI) |
|---|---|---|---|
| Nervous system disorders | 1626 (41.5) | 3912 (20.7) | 2.72 (2.47 to 2.99) |
| General disorders and administration site conditions | 1561 (39.8) | 4371 (23.1) | 2.20 (2.00 to 2.42) |
| Gastrointestinal disorders | 1266 (32.3) | 3722 (19.7) | 1.95 (1.76 to 2.15) |
| Psychiatric disorders | 1213 (30.9) | 2312 (12.2) | 3.22 (2.89 to 3.57) |
| Skin and subcutaneous tissue disorders | 997 (25.4) | 4391 (23.2) | 1.13 (1.02 to 1.25) |
| Musculoskeletal and connective tissue disorders | 766 (19.5) | 1865 (9.9) | 2.22 (1.97 to 2.50) |
| Respiratory, thoracic and mediastinal disorders | 498 (12.7) | 1598 (8.5) | 1.58 (1.37 to 1.81) |
| Investigations | 488 (12.5) | 1713 (9.1) | 1.43 (1.24 to 1.64) |
| Eye disorders | 353 (9.0) | 797 (4.2) | 2.25 (1.89 to 2.67) |
| Metabolism and nutrition disorders | 284 (7.2) | 695 (3.7) | 2.05 (1.70 to 2.47) |
| Vascular disorders | 182 (4.6) | 881 (4.7) | 1.00 (0.80 to 1.23) |
| Renal and urinary disorders | 176 (4.5) | 609 (3.2) | 1.41 (1.13 to 1.77) |
| Cardiac disorders | 163 (4.2) | 1025 (5.4) | 0.76 (0.61 to 0.95) |
| Infections and infestations | 141 (3.6) | 711 (3.8) | 0.96 (0.75 to 1.22) |
| Injury, poisoning and procedural complications | 128 (3.3) | 481 (2.5) | 1.29 (1.00 to 1.68) |
All of the age- and gender-adjusted ORs between patient and HCP reports by SOC were statistically significant except for vascular disorders, infections and infestations, and injury, poisoning and procedural complications (see Table 8). In general, patients tended to report more ADRs in each SOC, apart from cardiac disorders, in which case patients were significantly less likely than a HCP to report a relevant ADR.
The median (IQR) word count (excluding reports with zero word counts) used to describe the suspected reaction was significantly higher for patient reports [45.0 (22.0 to 74.0)] than HCP reports [15.0 (9.0 to 26.0); p < 0.001]. There was a significant difference between the median word count used to describe the reaction in each method of reporting (Table 9).
| Type of reporter and method of reporting | n | Median (IQR) | Kruskal–Wallis p-value |
|---|---|---|---|
| HCP Yellow Card reporter | |||
| Internet | 1231 | 17.0 (9.0 to 33.0) | < 0.001 |
| Paper | 9012 | 14.0 (8.0 to 24.0) | |
| Telephone | 4 | 21.5 (10.5 to 36.0) | |
| Not specified | 5533 | 18.0 (10.0 to 29.0) | |
| Patient Yellow Card reporter | |||
| Internet | 607 | 94.0 (43.0 to 174.0) | < 0.001 |
| Paper | 2718 | 42.0 (21.0 to 71.0) | |
| Telephone | 126 | 37.5 (19.0 to 64.0) | |
| Not specified | 1139 | 40.0 (19.0 to 64.0) | |
| All Yellow Card reporters | |||
| Internet | 1838 | 27.0 (12.0 to 69.0) | < 0.001 |
| Paper | 11,730 | 17.0 (9.0 to 32.0) | |
| Telephone | 130 | 37.0 (18.0 to 61.0) | |
| Not specified | 6672 | 20.0 (11.0 to 34.0) | |
Seriousness of reaction
Similar percentages of both patient and HCP reports contained at least one reaction term coded as ‘dictionary serious’ by the MHRA (patients 58.3% vs HCPs 58.8%; p = 0.58).
Over one-half (55.5%) of HCP reporters stated that they considered their patient’s reaction serious.
Of the three subtypes of serious ADRs that were comparable between the two types of reporter, HCPs reported a higher proportion of each event than patient reports (caused hospitalisation 18.8% vs 12.9%; life-threatening 11.1% vs 6.2%; caused death 2.6% vs 0.7%; p < 0.001 for each).
Nearly one-half (44.8%) of the patient Yellow Card reports said that the suspected ADR was bad enough to affect everyday activities, whereas 15.4% said it was uncomfortable or a nuisance and 2.6% said it was mild or slightly uncomfortable. Some patients appeared to have completed a HCP version of the Yellow Card or had a follow-up report by their HCP merged with theirs. Owing to the structure of the database these occurrences were difficult to quantify.
Drugs
A higher proportion of patient reports (16.1%) than of HCP reports (9%, p < 0.001) contained more than one suspect drug. The median (IQR) number of suspect drugs reported was 1 (1 to 1) for both reporter types, although they were statistically different, with the HCPs reporting fewer (p < 0.001). The 20 most frequent suspect drugs reported by patients and HCPs are presented in Figures 2 and 3, and substantial differences can be seen.
FIGURE 2.
The 20 most frequent suspect drugs reported by patients (by reporter type). Note: the rank of the drug for HCP reporters is shown to the right of the HCP bars.
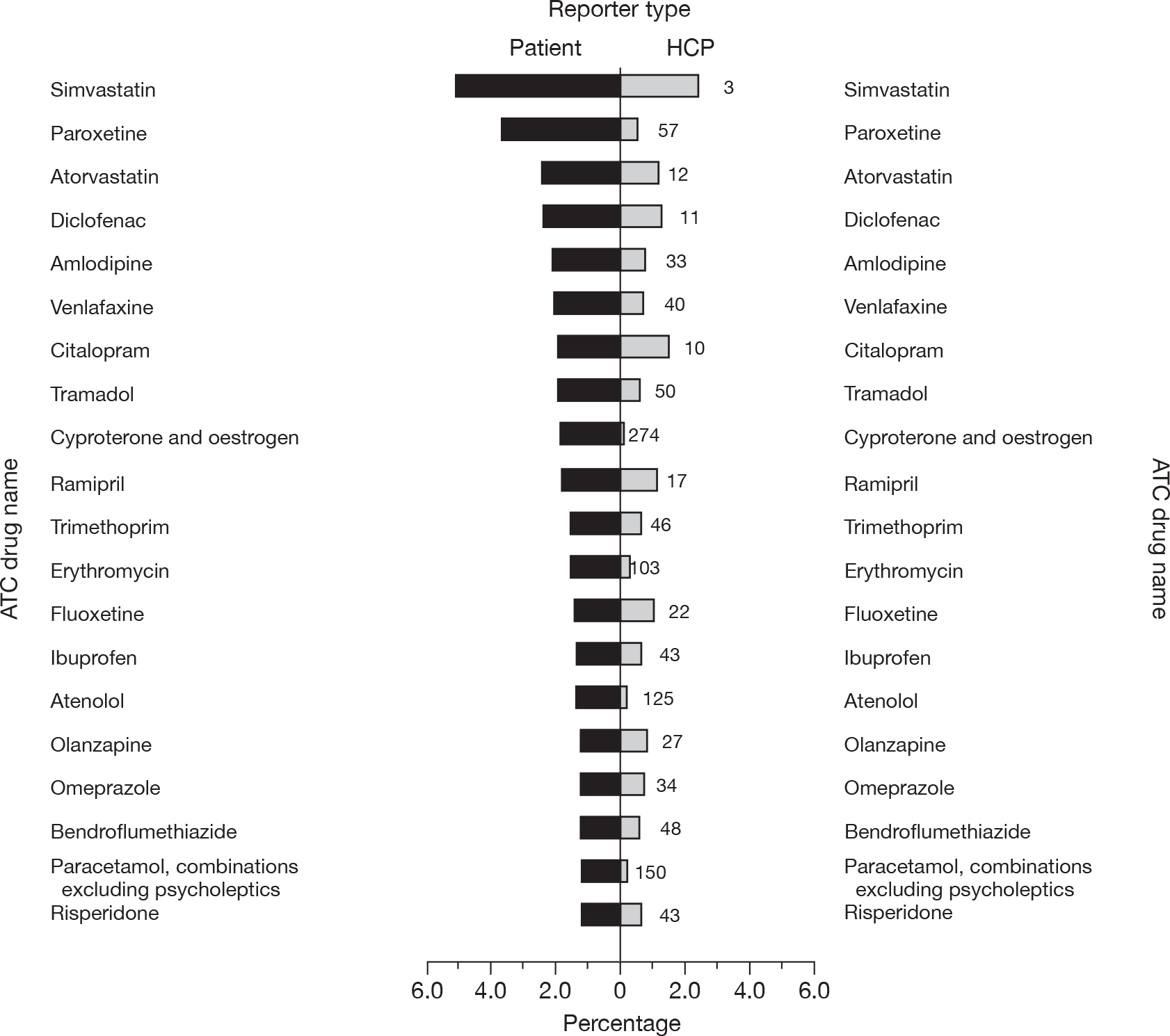
FIGURE 3.
The 20 most frequent suspect drugs reported by HCPs (by reporter type). Note: the ordered rank of the drug for patient reporters is shown to the right of the patient bars.
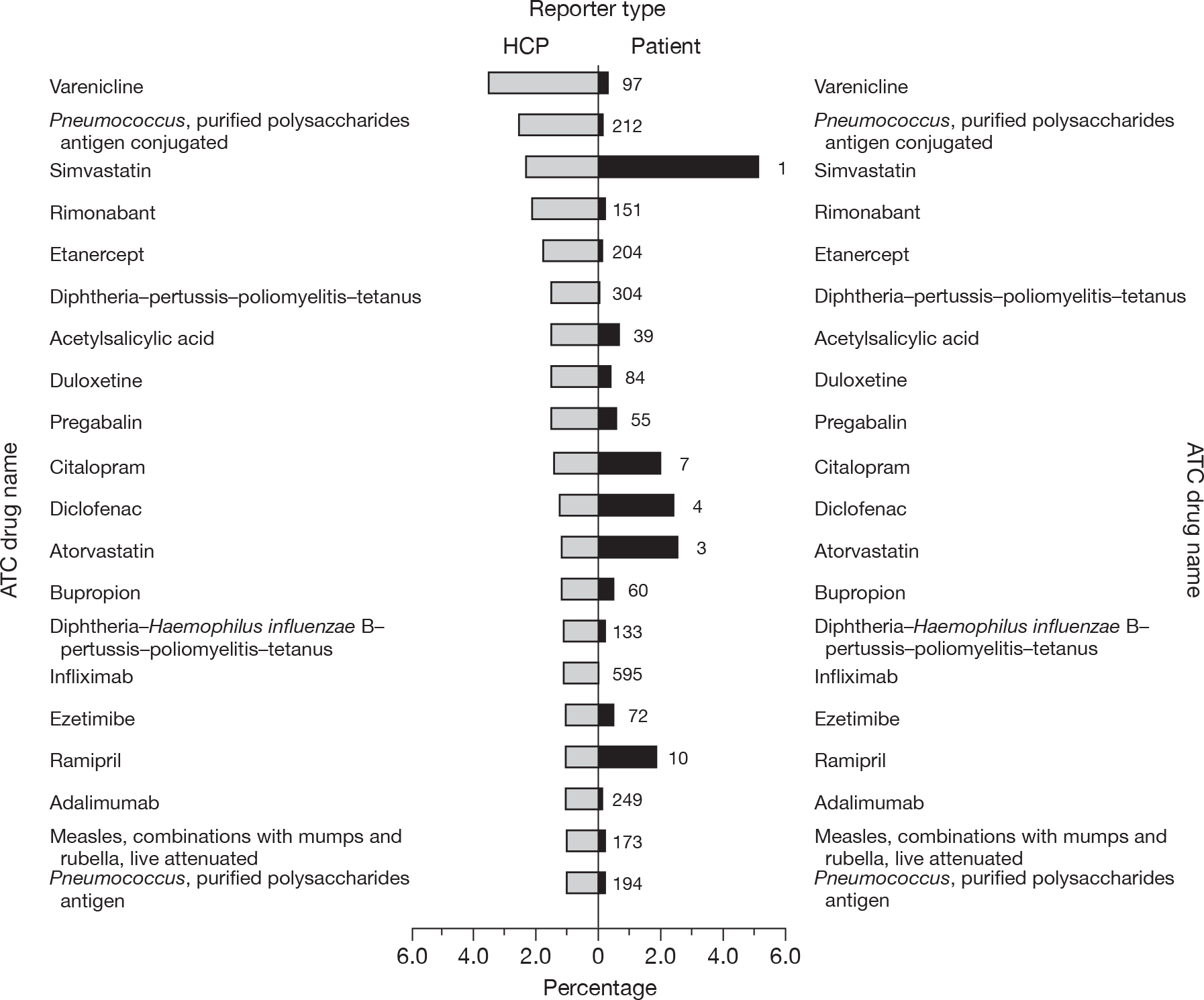
The ATC anatomical classification of suspect drugs on the patient and HCP reports is presented in Table 10. The most frequent category of drug suspected of being linked to an ADR was for the nervous system, for both patient (33.2%) and HCP (26.2%) reports. This was followed by cardiovascular system drugs from patient reports (21.8%) and anti-infectives for systemic use from HCP reports (19.4%). A statistically significant difference in the percentage of type of suspect drug between reporters was shown for drugs of the nervous system; cardiovascular system; systemic hormonal preparations, excluding sex hormones and insulin; antiparasitic products, insecticides and repellents; herbals/complementary medicine (which all had higher proportions in the patient than HCP reports); anti-infectives for systemic use; antineoplastic and immunomodulating agents; blood and blood-forming organs; and, ‘various’ (all of which had higher proportions in the HCP than the patient reports).
| Main group (first level) of the ATC classification system | Patient report (%) | HCP report (%) | Patient vs HCP reporta | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient (n = 4836) | Representative (n = 344) | All (N = 5180) | Doctor (n = 12,088) | Pharmacist (n = 3690) | Nurse (n = 2725) | Other (n = 2446) | All (N = 20,949) | p-value | |
| Nervous system | 1604 (33.2) | 116 (33.7) | 1720 (33.2) | 3304 (27.3) | 897 (24.3) | 493 (18.1) | 789 (32.3) | 5483 (26.2) | < 0.001 |
| Cardiovascular system | 1087 (22.5) | 44 (12.8) | 1131 (21.8) | 1936 (16.0) | 635 (17.2) | 101 (3.7) | 150 (6.1) | 2822 (13.5) | < 0.001 |
| Anti-infectives for systemic use | 617 (12.8) | 86 (25.0) | 703 (13.6) | 1770 (14.6) | 533 (14.4) | 1139 (41.8) | 614 (25.1) | 4056 (19.4) | < 0.001 |
| Alimentary tract and metabolism | 411 (8.5) | 21 (6.1) | 432 (8.3) | 1274 (10.5) | 300 (8.1) | 238 (8.7) | 145 (5.9) | 1957 (9.3) | 0.03 |
| Musculoskeletal system | 395 (8.2) | 16 (4.7) | 411 (7.9) | 889 (7.4) | 437 (11.8) | 41 (1.5) | 113 (4.6) | 1480 (7.1) | 0.03 |
| Genitourinary system and sex hormones | 267 (5.5) | 6 (1.7) | 273 (5.3) | 770 (6.4) | 97 (2.6) | 73 (2.7) | 100 (4.1) | 1040 (5.0) | 0.39 |
| Respiratory system | 181 (3.7) | 19 (5.5) | 200 (3.9) | 337 (2.8) | 119 (3.2) | 153 (5.6) | 53 (2.2) | 662 (3.2) | 0.01 |
| Blood and blood-forming organs | 120 (2.5) | 12 (3.5) | 132 (2.6) | 375 (3.1) | 398 (10.8) | 66 (2.4) | 72 (2.9) | 911 (4.4) | < 0.001 |
| Antineoplastic and immunomodulating agents | 118 (2.4) | 10 (2.9) | 128 (2.5) | 1022 (8.5) | 369 (10.0) | 320 (11.7) | 283 (11.6) | 1994 (9.5) | < 0.001 |
| Systemic hormonal preparations, excluding sex hormones and insulin | 118 (2.4) | 6 (1.7) | 124 (2.4) | 164 (1.4) | 79 (2.1) | 27 (1.0) | 33 (1.4) | 303 (1.5) | < 0.001 |
| Dermatologicals | 99 (2.1) | 13 (3.8) | 112 (2.2) | 298 (2.5) | 62 (1.7) | 29 (1.1) | 38 (1.6) | 427 (2.0) | 0.61 |
| Antiparasitic products, insecticides and repellents | 57 (1.2) | 7 (2.0) | 64 (1.2) | 85 (0.7) | 18 (0.5) | 25 (0.9) | 9 (0.4) | 137 (0.7) | < 0.001 |
| Herbalsb | 48 (1.0) | 1 (0.3) | 49 (1.0) | 35 (0.3) | 35 (1.0) | 4 (0.2) | 7 (0.3) | 81 (0.4) | < 0.001 |
| Sensory organs | 39 (0.8) | 5 (1.5) | 44 (0.9) | 86 (0.7) | 31 (0.8) | 17 (0.6) | 40 (1.6) | 174 (0.8) | 0.96 |
| Various | 36 (0.7) | 4 (1.2) | 40 (0.8) | 120 (1.0) | 39 (1.1) | 43 (1.6) | 90 (3.7) | 292 (1.4) | < 0.001 |
| Unmappedc | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.01) | 1 (0.03) | 0 (0.0) | 0 (0.0) | 2 (0.01) | – |
Patient Yellow Card reports were also split into those completed by patients themselves and those completed by a representative (parent, carer, etc.) and HCP Yellow Card reports into those reported by doctors, pharmacists, nurses or another health professional (e.g. dentists). Among the patient reports, a higher proportion of patients reported drugs of the cardiovascular (p < 0.001) and genitourinary and sex hormone system (p = 0.003) than representatives who completed the patient report. In contrast, representatives reported a higher proportion of anti-infectives for systemic use (p < 0.001). Among HCP reports, there were significant differences in the proportion of drugs in each ATC anatomical category reported by the four HCP groups, except for antiparasitic products, insecticides and repellents. A higher proportion of nurses reported a suspected ADR for anti-infectives for systemic use (41.8%) or respiratory system drugs (5.6%) than any other HCP. A higher proportion of pharmacists reported a suspected ADR for a drug for the cardiovascular system (17.2%), musculoskeletal system (11.8%), blood and blood-forming organs (10.8%), and systemic hormonal preparations (2.1%). Of all the HCP groups examined, doctors reported a greater proportion of suspected ADRs for drugs of the alimentary tract and metabolic drugs (10.5%) as well as genitourinary system and sex hormone drugs (6.4%).
Time lag between suspect adverse drug reaction and reporting
Patient reporters took a significantly longer median (IQR) time than HCPs to report their reaction to the MHRA [104 (27 to 463) vs 28 (13 to 75) days, respectively; p < 0.001]. However, there was a higher percentage of missing data for the variable ‘time from reaction to report’ among patient reports (61.0% of such reports) than among HCP reports (33.2%).
The median time to report a suspected ADR for reactions occurring in the first and second year of the study period is presented in Table 11, by reaction outcome. The median time to report an ADR among patients who were still recovering or whose reaction was resolving was significantly longer in year 2 than in year 1, regardless of who reported the ADR [patient (p = 0.01) and HCP (p = 0.02) reports]. However, these data should be interpreted with caution as there were large numbers of missing data related to this issue, in both groups of reporter.
| Reaction outcome | Time to report in first year (days) | No. of reports in first year | Time to report in second year (days) | No. of reports in second year | p-valuea | ||||
|---|---|---|---|---|---|---|---|---|---|
| Median (IQR) | Range | Missing (%) | Median (IQR) | Range | Missing (%) | ||||
| Not recovered/resolved | 195.0 (44.0 to 852.0) | 2–14,994 | 675 (54.7) | 1234 | 147.0 (35.0 to 644.0) | 2–5389 | 441 (67.6) | 652 | 0.08 |
| Recovering/resolving | 51.0 (13.0 to 212.0) | 1–10,700 | 277 (49.2) | 563 | 88.5 (27.0 to 331.0) | 2–4093 | 195 (63.9) | 305 | 0.01 |
| Recovered/resolved with sequelae | 247.5 (48.0 to 670.0) | 6–5986 | 52 (53.1) | 98 | 306.5 (77.0 to 925.0) | 9–2985 | 81 (61.8) | 131 | 0.91 |
| Recovered/resolved | 68.5 (21.0 to 305.0) | 1–14,717 | 760 (52.8) | 1440 | 88.0 (24.0 to 352.0) | 2–10,003 | 530 (71.3) | 743 | 0.54 |
| Reaction outcome | Time to report in first year (days) | No. of reports in first year | Time to report in second year (days) | No. of reports in second year | p-valuea | ||||
|---|---|---|---|---|---|---|---|---|---|
| Median (IQR) | Range | Missing (%) | Median (IQR) | Range | Missing (%) | ||||
| Not recovered/resolved | 35.0 (14.0 to 105.0) | 0 to 4829 | 652 (27.7) | 2354 | 34.0 (14.0 to 98.0) | 1 to 4328 | 1064 (46.4) | 2291 | 0.86 |
| Recovering/resolving | 20.0 (10.0 to 46.0) | 0 to 5614 | 637 (20.3) | 3135 | 21.0 (11.0 to 52.0) | 0 to 1642 | 960 (34.0) | 2822 | 0.02 |
| Recovered/resolved with sequelae | 99.0 (41.0 to 107.0) | 7 to 203 | 2 (28.6) | 7 | 90.5 (30.0 to 167.0) | 14 to 1655 | 7 (28.0) | 25 | 0.74 |
| Recovered/resolved | 33.0 (14.0 to 81.0) | 0 to 3971 | 987 (23.1) | 4282 | 31.0 (14.0 to 84.0) | 0 to 3092 | 1725 (39.7) | 4350 | 0.77 |
Reaction outcome
A significantly higher proportion of HCPs than patient representatives reported fatal outcomes to the reaction (2.6% vs 0.7%; p < 0.001). A significantly higher proportion of HCPs than patient representatives reported that the patient was recovering or that the ADR was resolving (28.4% vs 16.8%; p < 0.001). More patients than HCPs reported that they had not recovered or that the reaction had not resolved (36.4% vs 22.2%; p < 0.001).
Yellow Card reports involving children
One of our objectives was to specifically examine Yellow Card reports involving children. The findings are shown in Appendix 10. The median age and percentage of females was similar between patient and HCP reporters. Patient reports contained more reactions per report and there were differences in the types of reactions reported. For example, in keeping with all patient reports, patient reports for children were more common for reactions in the following SOC reaction groups: nervous system, psychiatric, gastrointestinal, metabolism and nutritional disorders.
Summary
-
Anonymised data were provided by the MHRA for all patient and HCP reports received by the YCS between 1 October 2005 and 30 September 2007. To compare the two reporter groups, suspected adverse reaction terms were grouped within the hierarchical structure of the industry standard ‘Medical Dictionary for Regulatory Affairs’ (MedDRA, version 12) and suspect drug names were mapped to the most appropriate code within the ATC drug classification system (2007 version).
-
For the 2-year study period, 26,129 Yellow Card reports were received from the MHRA [5180 (19.8%) patient reports and 20,949 (80.2%) HCP reports]. Patient reports contained a significantly higher number of suspected ADRs per report than did HCP reports [median (IQR) of 3 (2 to 5) vs 2 (1 to 3), respectively; p < 0.001]. A higher proportion of patient reports than of HCP reports contained more than one suspect drug (16.1% vs 9% respectively; p < 0.001). The median (IQR) word count (excluding reports with zero word counts) used to describe the suspected reaction was significantly higher for patient reports than for HCP reports [45.0 (22.0 to 74.0) vs 15.0 (9.0 to 26.0), respectively; p < 0.001].
-
Patients showed different patterns of reporting of drugs and ADRs compared with HCPs. Nevertheless, similar proportions of reports contained at least one reaction term that was classified as ‘serious’ by the MHRA (58.3% for patients vs 58.8% for HCPs; p = 0.58). The following were recorded more commonly in HCP reports than in patient reports: ‘caused hospitalisation’ (18.8% vs 12.9%, respectively); ‘life-threatening’ (11.1% vs 6.2%, respectively); and ‘caused death’ (2.6% vs 0.7% respectively) (p < 0.001 for each comparison). Of the patient Yellow Card reports, 44.8% stated that the suspected ADR was bad enough to affect everyday activities. Patient reporters took a significantly longer time to report suspected ADRs than HCPs [median (IQR) of 104 (27 to 463) days vs 28 (13 to 75) days, respectively; p < 0.001], although there were missing data on ‘time taken to report’ for over 60% of patient reports.
Chapter 5 Study 3: pharmacovigilance impact of patient reporting: signal generation analysis
A large part of this chapter has been reproduced with permission from Adis, a Wolters Kluwer Business (see McLernon et al. 40 for the final published version).
Introduction to signal generation analysis
An important objective in pharmacovigilance is the early detection of a potential signal of an ADR. Traditionally, this includes using observations by HCPs and patients, case reports in the literature, reports to spontaneous reporting systems and/or data from larger studies, such as clinical trials and observational studies. It is important to have robust systems that detect previously unsuspected problems, as well as those that are able to investigate emerging problems.
In order to further evaluate a signal of a previously unrecognised ADR, it is necessary to determine whether there is evidence of a causal relationship between the drug and the reaction. This will involve not only clinical evaluation of cases reported, but also a search for further information in the literature and an assessment of the pharmacological plausibility of a causal association. 41 Causality assessment on individual or clusters of reports depends on the completeness of the information provided. It may require follow-up information from reporters. Particularly relevant are details on the patient’s medical history, concomitant drug therapies and the time course of the reaction in relation to the suspect drug therapy.
It is not practical to perform such assessments on all events reported to pharmacovigilance organisations. Triage methods may be applied. 42,43 For example, the MHRA currently uses a system of filtering ADRs within its signal detection database so as to prioritise important ADRs for further clinical evaluation. Hence, ADR reports that have a fatal outcome, involve children or pregnant women, or include ‘alert’ terms (a list of reaction terms specified in-house by the MHRA) are regarded as high priority. In addition, reports relating to black triangle drugs that are regarded as serious by the reporter or that involve reactions coded as ‘dictionary serious’ by the MHRA are also prioritised for clinical assessment. For established drugs on the UK market, however, an additional filter is applied to serious reactions using quantitative signal detection methods. Essentially, these methods generate ‘statistical’ signals of ADRs using disproportionality analysis to identify drug–reaction pairs that are reported more frequently than expected, based on the background reporting rates for the drug and reaction of interest within the database. 44 The MHRA uses a method based on the Empirical Bayesian Geometric Mean (EGBM) method. 45 Vaccines are analysed in a similar way, but separately from other medicinal products.
Within the context of this project it was not feasible to analyse all reports meeting the high-priority criteria outlined above. Hence, we have used disproportionality analysis as the primary means of identifying potential signals of ADRs, regardless of the patient’s age, seriousness of reaction, time of marketing of the suspect drug or the reaction outcome. In addition, reports relating to vaccines have been analysed together with drugs to maximise the size of our data sets.
The method of disproportionality analysis used in our project was the proportional reporting ratio (PRR), in which the reporting rate for an ADR in association with a particular medicine is compared with that of other products in the database. 46 This method is used by the EudraVigilance (European Union Drug Regulating Authorities Pharmacovigilance) Data Analysis System, which supports pharmacovigilance activities within the European Community. The system uses the term ‘signal of disproportionate reporting (SDR)’ instead of the more ambiguous term ‘signal’, as the latter term might be interpreted as implying a causal relationship between suspect drug and ADR. 47
Unlike formal clinical trials or observational studies, disproportionality analysis within spontaneous reporting schemes does not provide an estimate of the frequency of ADRs (as it does not provide denominator information for drug exposure) and is subject to bias from under-reporting and selective reporting (e.g. when the media report a particular safety issue). The merits and limitations of different statistical methods for disproportionality analysis in the context of signal generation have been discussed elsewhere. 44 A key concept is that signals detected using any quantitative method indicate a higher proportionate reporting of a specific reaction for a specific drug than would be expected considering the whole ADR database; it does not necessarily prove causality. Further qualitative evaluation is essential when determining whether a real ADR is being signalled.
The SDRs identified by our analysis formed the sampling frame from which reports were selected for further evaluation. We did not plan to undertake an exhaustive signal assessment. Instead, we aimed to conduct an assessment of causality using information provided by patients and, where possible, compare this with similar assessments undertaken on information supplied by HCPs.
Aim
To investigate the relative contribution of patient reports to signal generation.
Objectives
The following objectives are more detailed than those in our original application and were:
-
Quantitatively:
-
– to compare the types of SDRs generated by patient reports with those generated by HCP reports, in terms of their importance within the context of pharmacovigilance including:
-
– the seriousness of the ADRs involved (using the MHRA’s ‘dictionary seriousness’ classification)
-
– the time of marketing of the suspect drugs involved (black triangle drugs)
-
– whether the ADR had been previously been documented on the summary of product characteristics (SPC) for the suspect drug involved
-
-
– to investigate how SDRs generated in a spontaneous reporting database of HCP reports may be affected (statistically) by the inclusion of patient reports
-
– to estimate the extent to which duplicate reporting occurs.
-
-
Qualitatively:
-
– to investigate how the information provided by patient reports may contribute to the assessment of a causal association for selected drug–ADR pairs.
-
– to identify problems incurred when assessing causality from patient reports.
-
Methods
Data collection and processing
As described in Chapter 3, data were received from the MHRA regarding details of patient and HCP reports submitted to the YCS during the 2-year period from October 2005 to September 2007. Reports from the pharmaceutical industry were excluded.
Data synthesis
Following the initial data processing described in Chapter 3, three data sets of drug–ADR pairs were compiled: one for patient reports (‘patient only’), one for HCP reports (‘HCP only’) and a combined data set for reports from both groups (‘combined’). For the purposes of the signal generation analysis the drug–ADR pair consisted of the drug name coded at the lowest hierarchical level within the ATC classification system for drug substances and the reaction term coded at the PT level within the hierarchical MedDRA dictionary.
Signals of disproportionate reporting were generated for each drug–ADR pair reported using a modification of the PPR, calculated by the formula:
where:
-
A = the number of patients who had drug X and reaction Y
-
B = the number of patients who had drug X and did not have reaction Y
-
C = the number of patients who did not have drug X, but had reaction Y
-
D = the number of patients who did not have drug X and did not have reaction Y.
That is:
| Drug | Reaction Y | All other reactions | Total |
|---|---|---|---|
| Drug X | A | B | A + B |
| All other drugs | C | D | C + D |
| Total | A + C | B + D | N = A + B + C + D |
For each drug–ADR pair, each patient contributes to only one of the four A, B, C or D cells, even if a report involved more than one suspect drug or reaction term. This maintains independence between the variables used to calculate the PRR, so that variance is not underestimated.
In order to improve statistical robustness of the PRR where few or no drug–ADR pairs were observed in the data set, a shrinkage method was adopted. Thus, the ‘PRRshrunk’ was calculated using a modification of the PRR formula:48
where ‘observed’ = A and ‘expected’ = [(A + B)C/C + D].
For each PRRshrunk, 95% CIs were calculated using the formula:
where:
Within each of the three data sets the PRRshrunk was generated for each drug–ADR pair reported over the 2-year study period. Based on previously published arbitrary thresholds, drug–ADR pairs were flagged as SDRs, if at the end of the 2-year study period all of the following three conditions were fulfilled:46
-
The PRRshrunk was > 2.
-
The number of reports for the drug–reaction pair (A) was ≥ 3.
-
The lower 95% CI for the PRRshrunk was ≥ 1.
All PRR calculations were computed within a SQL Server (2000) database.
Quantitative analysis of signals of disproportionate reporting
Comparison of signals of disproportionate reporting generated by the patient and health-care professional data sets
The following descriptive statistics were calculated for the patient and the HCP data sets:
-
Number and proportion of SDRs generated in each data set.
-
Number and proportion of SDRs generated in each data set where the reaction preferred term was classified as ‘dictionary serious’ by the MHRA at least once (regardless of who reported such an event).
-
Number and proportion of SDRs generated in each data set where the suspect drug may have been available as a recently marketed product or formulation. For the purposes of this analysis drug–ADR pairs were flagged as ‘black triangle’ if any report relating to that drug, regardless of the brand or formulation reported, had been classified by the MHRA as ‘black triangle’.
-
Number of SDRs that were documented, i.e. listed within section 4.8 (‘undesirable effects’) of the SPC for the suspect drug involved. It was not practical to evaluate whether all of the SDRs generated in this analysis had been previously documented on the SPC. Hence, a random sample of 300 SDRs from each data set was selected. This sample size would be sufficient to detect a 10% difference in proportion of SDRs that were not documented on the product’s SPC between the patient and HCP data sets, at the 5% significance level with 80% power. In addition, the SPC for a medicinal product changes over time, so it was not feasible within the context of the project to evaluate whether the SDRs generated had been documented on the SPC at the time of the individual report or reaction. Furthermore, the retrospective nature of our study meant that it was not always possible to identify when the SPC for a particular drug first recorded a particular ADR. In order to provide an estimate of whether new information about SDR/ADRs was being revealed by each data set, we referred to the version of SPC available at the beginning of the study period (2004) rather than estimate which version was available at the time of the report or reaction, or the first available SPC for drugs launched on the UK market during the course of the study.
-
The top five system organ classes for reactions and top five anatomical classes for drugs most commonly associated with the SDRs generated by each data set.
Univariate comparisons between reports from patients and HCPs were performed using Pearson’s chi-squared test for categorical variables, or the difference in proportions calculated with its 95% CI. These were computed using stata (version 10).
Analysis of signals of disproportionate reporting generated after combining data from patients and health-care professional data sets
The following descriptive statistics were calculated:
-
Number of SDRs generated in the ‘patient-only’ and ‘HCP-only’ data sets that remained SDRs in the ‘combined’ data set.
-
Number of SDRs generated in the ‘patient-only’ and ‘HCP-only’ data sets that were no longer SDRs in the ‘combined’ data set, along with the proportion of these SDRs that were potentially serious, involved suspect drugs flagged as ‘black triangle’ and/or were not documented on the SPC.
-
Number of new SDRs in the ‘combined’ data set compared with those generated in the ‘HCP-only’ data set, along with the proportion of these SDRs that were potentially serious, involved suspect drugs flagged as ‘black triangle’ and/or were not documented on the SPC.
-
Number of SDRs generated by the ‘HCP-only’ data set that were detected later or earlier after the combination with the patient data set.
Causality assessment in a sample of reports
Drug–ADR pairs identified as SDRs in the disproportionality analysis were first categorised according to whether the patient reports appeared to be adding, or taking away, information provided by the HCP reports (Table 12). This categorisation provided a sampling frame for the selection of reports for an assessment of causality.
| Category | Description |
|---|---|
| 1 | Potential new information provided by the patient report: drug–ADR pair identified as SDR in the ‘patient-only’ data set, but not identified as SDR in the ‘HCP-only’ data set, although drug–ADR pair in the HCP data set |
| 2 | Potential new information provided by the patient report: drug–ADR pair identified as SDR in the ‘patient-only’ data set, but drug–ADR pair not present in the ‘HCP-only’ data set (and, hence, could not be identified as an SDR in ‘HCP-only’ data set) |
| 3 | Potential new information from the addition of patient reports to HCP reports: new SDR generated in the ‘combined’ data set, which was not identified in the ‘patient-only’ or ‘HCP-only’ data sets alone, irrespective of whether the drug–ADR pair was in either data set |
| 4 | Potential loss of information from the addition of patient reports to HCP reports: SDR generated in the ‘HCP-only’ data set, which was no longer a SDR in the ‘combined’ data set |
Within each of the four categories, SDRs were subgrouped according to whether the ADR term was classified as ‘dictionary serious’ by the MHRA, whether the ADR (or similar term) was documented on the SPC and whether the suspect drug was available in a form or brand classified as a black triangle drug by the MHRA. Four SDRs were then randomly selected from the SDRs in each of the 12 subcategories. It is important to note that the categories in Table 12 were not mutually exclusive; if a randomly selected SDR had already been selected for one category it was replaced by another randomly selected suitable SDR.
The sampling frame was used to select SDRs that involved no more than 10 individual patient and/or HCP reports so that a variety of drugs (including OTC products and complementary therapies) and reactions could be assessed for causality. This number was chosen to give a range of reported problems while ensuring a manageable workload for the project.
An assessment of causality was made for each identified report, by two DSRU researchers (a pharmacist and a biomedical scientist). Conflicting assessments were discussed by the two researchers and, where agreement could not be reached, a third member of DSRU staff (a medically-qualified doctor) arbitrated. The assessment involved reviewing all of the data provided by the MHRA. Each ADR was classified into one of four categories (Table 13). The process also enabled:
-
the identification of likely duplicates
-
the identification of limitations when making the assessment of causality for patient reports
-
an assessment of whether important information provided in the narrative description was lost by coding using MedDRA terminologies.
| Causality | Criteria |
|---|---|
| Probable | An event that was clinically well defined, occurring within a reasonable time sequence to drug administration, more likely to be attributable to the drug than concurrent disease or medication and that followed a clinically reasonable response on drug withdrawal or dose reduction (dechallenge) or reintroduction of the drug or dose increase (rechallenge) |
| Possible | An event with a reasonable clinical definition occurring with a reasonable time sequence to drug administration, but which could also be explained by concurrent disease or medication; information on dechallenge and rechallenge may have been incomplete or inconclusive |
| Unlikely | An event with a temporal association to drug administration which makes causality improbable or in which an illness or another medication provided a more plausible explanation for the event |
| Not assessable | Incomplete or conflicting information, which prevented an assessment of causal association |
Results
Comparison of patient and health-care professional reports: overview
There were 5180 (19.8% of total) reports supplied by patients and 20,949 (80.2%) by HCPs. Table 14 shows the number of different reaction terms coded (at PT level), suspect drugs (at ATC code level) and drug–ADR pairs in the three data sets. The ‘patient-only’ data set contained less than one-half (approximately 40%) of all of the drug–ADR pairs reported in this study. Figure 4 shows that most drug–ADR pairs were present in either the ‘patient-only’ or the ‘HCP-only’ data set – only 10.6% drug–ADR pairs were common to both data sets. This indicates marked differences between the two data sets in the identification of drug–ADR pairs.
| Drug–ADR pairs, ATC codes and reaction terms | Exclusive to ‘patient-only’ data set | Exclusive to ‘HCP-only’ data set | Present in both the ‘patient-only’ and ‘HCP-only’ data sets | In ‘combined’ data set |
|---|---|---|---|---|
| Drug–ADR pairs (% of row total) | 12,226 (29.8) | 24,435 (59.6) | 4340 (10.6) | 41,001 |
| ATC codes (% of row total) | 163 (11.6) | 626 (44.4) | 622 (44.1) | 1411 |
| ADR PTs (% of row total) | 275 (9.6) | 1514 (52.9) | 1073 (37.5) | 2862 |
FIGURE 4.
Distribution of drug–reaction pairs across the patient and HCP data sets. aTotal: 41,001 pairs.
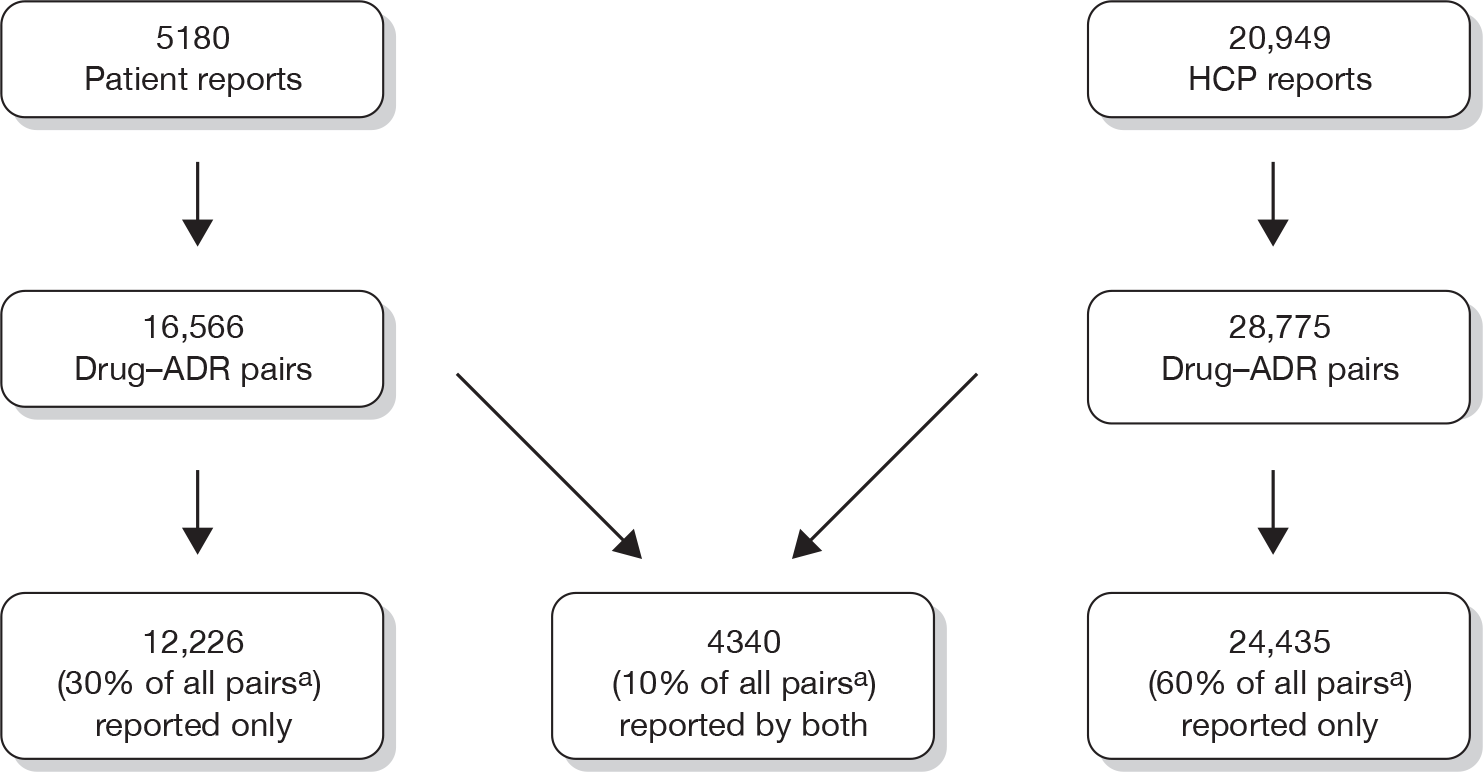
Comparison of signals of disproportionate reporting generated by patient and health-care professional data sets
Figure 5 shows the number of SDRs generated using the PRRshrunk method in the ‘patient-only’ and ‘HCP-only’ data sets. A significantly higher proportion of SDRs were generated from drug–ADR pairs contained in the ‘HCP-only’ data set than in the ‘patient-only’ data set (6.7% and 3.9% of pairs, respectively; difference 2.8%, 95% CI 2.4% to 3.2%). Only 136 drug–ADRs pairs (21.0% of those generated by the ‘patient-only’ data set; 7.0% of those from the ‘HCP-only’ data set) were generated as SDRs in both data sets. For these 136 SDRs, 56 (41.2%) were detectable earlier in the ‘patient-only’ data set, 71 (52.2%) were detectable later and for nine (6.6%) there was no difference in the time to reach SDR threshold.
FIGURE 5.
Signals of disproportionate reporting generated from drug–ADR pairs in the patient and HCP data sets.
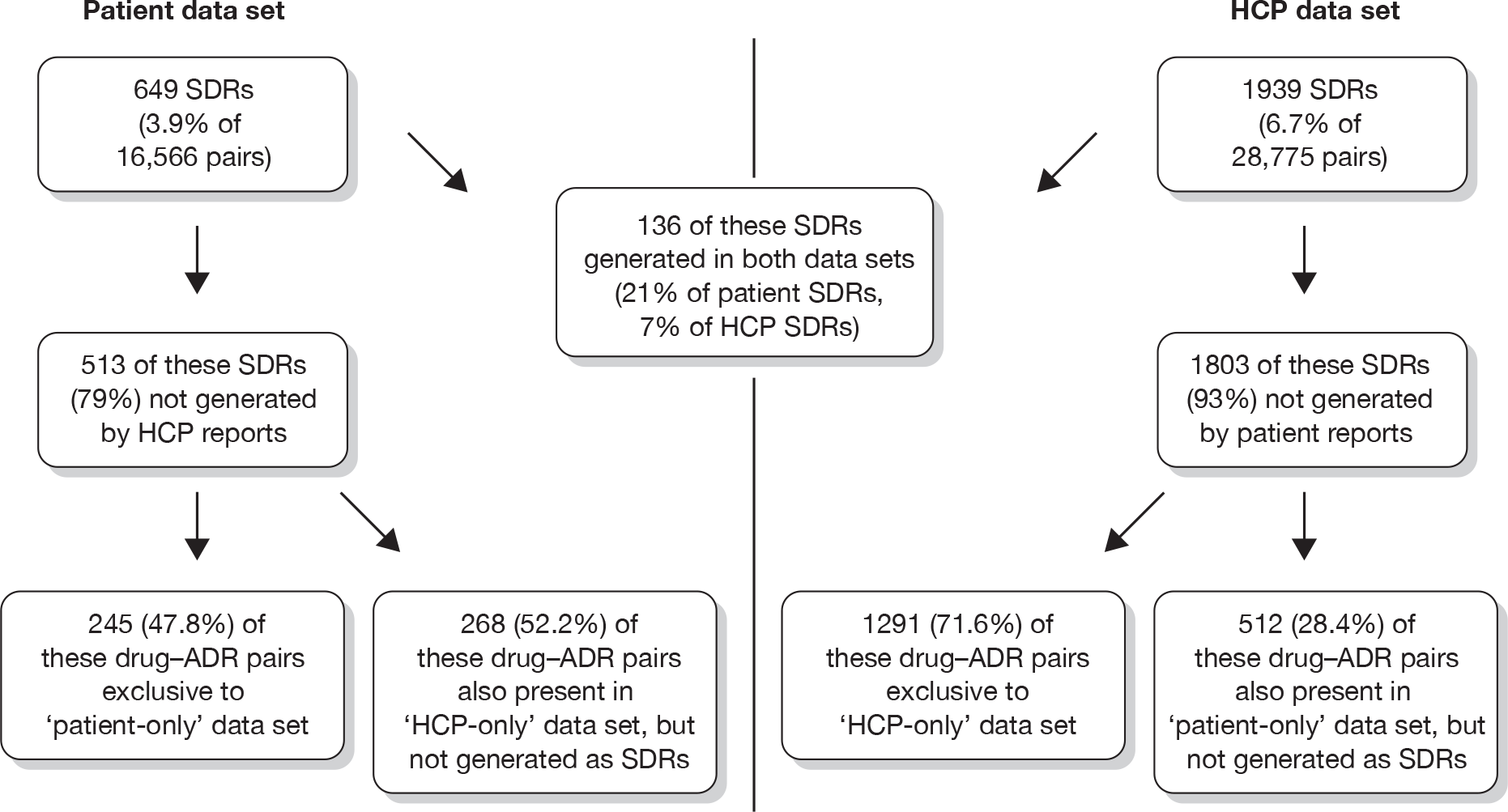
It would appear, therefore, that patient and HCP reports generate different SDRs, although this may partly be owing to differences in the way in which reactions are described and then subsequently coded at the MedDRA PT level. Further assessment of the 649 SDRs generated by the ‘patient-only’ data set revealed that, in addition to the 136 SDRs also generated by the ‘HCP-only’ data set, another 88 used a reaction term for the suspect drug similar or related to that of some SDRs generated using the ‘HCP-only’ data set. For example, the ‘patient-only’ data set identified amlodipine and ‘heart rate irregular’ as an SDR, whereas the ‘HCP-only’ identified amlodipine and ‘palpitations’ as an SDR. Nevertheless, 425 of the 649 (65.5%) SDRs generated using the ‘patient-only’ data set were quite distinct and so unlikely be generated using the ‘HCP-only’ data set.
Table 15 shows whether the SDRs generated by each data set involved reactions which were classified as ‘dictionary serious’, or involved a black triangle drug. Compared with those generated using the ‘patient-only’ data set, a significantly higher proportion of the SDRs generated using the ‘HCP-only’ data set was classified as involving serious reactions (difference in proportions 19.5%, 95% CI 15.4% to 23.6%) or a black triangle suspect drug (difference in proportions 19.8%, 95% CI 16.6% to 23.0%).
| Classification of SDRs | ‘Patient-only’ data set | ‘HCP-only’ data set |
|---|---|---|
| No. of SDRs generated | 649 | 1939 |
| No. classified as ‘dictionary serious’ reaction terms (% of SDRs generated) | 185 (28.5) | 931 (48.0) |
| No. involving a suspect drug flagged as ‘black triangle’ (% of SDRs generated) | 71 (10.9) | 596 (30.7) |
In both data sets the majority of SDRs generated were documented on the SPC, either specifically as described or using a similar or related clinical term for the suspect drug (Table 16). There was no significant difference between the two data sets in the proportion of SDRs which were regarded as not documented (difference in proportions 2.0%, 95% CI –3.7% to 7.7%).
| Classification of SDRs | ‘Patient-only’ data set | ‘HCP-only’ data set |
|---|---|---|
| No. of SDRs generated | 649 | 1939 |
| Sample assessed | 300 | 300 |
| No. documented on SPC (%) | 164 (54.7) | 132 (44.0) |
| No. with similar clinical term documented (%) | 73 (24.3) | 80 (26.7) |
| No. not documented (%) | 48 (16.0) | 42 (14.0) |
| No. that were documented during or after the period of study (%) | 11 (3.7) | 33 (11.0) |
| No. of SDRs that could not be unclassified, e.g. non-specific terms such as ‘feeling abnormal’ (%) | 4 (1.3) | 13 (4.3) |
Table 17 shows the top five most common system organ classes involved in SDRs generated by each data set and the top five anatomical drug classes involved. The same five system organ classes were involved when using each data set although their ranking differed (Table 17). Four of the five top anatomical drug classes were common to both data sets, although again the ranking differed (Table 18).
| ‘Patient-only’ data set | ‘HCP-only’ data set | ||
|---|---|---|---|
| SOC | No. of SDRs (%)a | SOC | No. of SDRs (%)a |
| Psychiatric disorders | 144 (22.2) | General disorders and administration site conditions | 319 (16.4) |
| Nervous system disorders | 95 (14.6) | Gastrointestinal disorders | 234 (12.1) |
| Gastrointestinal disorders | 94 (14.5) | Nervous system disorders | 228 (11.8) |
| Skin and subcutaneous tissue disorders | 67 (10.3) | Skin and subcutaneous tissue disorders | 204 (10.5) |
| General disorders and administration site conditions | 66 (10.2) | Psychiatric disorders | 194 (10.0) |
| ‘Patient-only’ data set | ‘HCP-only’ data set | ||
|---|---|---|---|
| Anatomical drug class | No. of SDRs (%)a | Anatomical drug class | No. of SDRs (%)a |
| Nervous system | 253 (39.0) | Nervous system | 482 (24.9) |
| Cardiovascular system | 124 (19.1) | Anti-infectives for systemic use | 452 (23.3) |
| Anti-infectives for systemic use | 76 (11.7) | Cardiovascular system | 207 (10.7) |
| Alimentary tract and metabolism | 38 (5.9) | Alimentary tract and metabolism | 167 (8.6) |
| Genitourinary system and sex hormones | 36 (5.6) | Antineoplastic and immunomodulating agents | 160 (8.2) |
Table 19 shows the top 10 individual suspect drugs involved in the SDRs generated by each data set and Table 20 the 10 most common preferred terms for the SDRs generated. The drugs and preferred terms for SDRs generated by the two data sets tended to be different, with none of the drugs and only four of the preferred terms being the same. The drugs involved in SDRs generated by the ‘HCP-only’ data set tended to be for recently introduced (black triangle) drugs, whereas the drugs involved in the SDRs generated by the ‘patient-only’ data set were for older, more established drugs.
| ‘Patient-only’ data set | ‘HCP-only’ data set | ||
|---|---|---|---|
| Suspect drug | No. of SDRs (%)a | Suspect drug | No. of SDRs (%)a |
| Paroxetine | 48 (7.4) | Pneumococcal vaccine | 46 (2.4) |
| Venlafaxine | 23 (3.5) | Rimonabant | 41 (2.1) |
| Cyproterone and oestrogen | 21 (3.2) | Pregabalin | 39 (2.0) |
| Citalopram | 17 (2.6) | Varenicline | 38 (2.0) |
| Trimethoprim | 16 (2.5) | Duloxetine | 34 (1.8) |
| Simvastatin | 14 (2.2) | Acetylsalicylic acid | 32 (1.6) |
| Tramadol | 14 (2.2) | Bupropion | 30 (1.6) |
| Atorvastatin | 13 (2.0) | Atomoxetine | 27 (1.4) |
| Diclofenac | 12 (1.8) | Diphtheria–HIB–pertussis–poliomyelitis–tetanus | 27 (1.4) |
| Zopiclone | 12 (1.8) | Etonogestrel | 26 (1.4) |
| ‘Patient-only’ data set | ‘HCP-only’ data set | ||
|---|---|---|---|
| Preferred term | No. of SDRs (%)a | Preferred term | No. of SDRs (%)a |
| Diarrhoea | 16 (2.5) | Anaphylactic reaction | 27 (1.4) |
| Anxiety | 15 (2.3) | Rash | 26 (1.3) |
| Arthralgia | 14 (2.2) | Pyrexia | 24 (1.2) |
| Weight increased | 14 (2.2) | Headache | 22 (1.1) |
| Nausea | 13 (2.0) | Vomiting | 22 (1.1) |
| Tremor | 13 (2.0) | Dizziness | 21 (1.1) |
| Vomiting | 13 (2.0) | Urticaria | 21 (1.1) |
| Dizziness | 12 (1.8) | Liver function test abnormal | 20 (1.0) |
| Rash | 12 (1.8) | Dyspnoea | 19 (1.0) |
| Dyspnoea | 11 (1.7) | Pruritus | 19 (1.0) |
Analysis of signals of disproportionate reporting generated after the combination of data from patients and health-care professional data sets
Table 21 presents the number of drug–ADR pairs generated as SDRs before and after combining the patient and HCP reports and the number of drug–ADRs pairs no longer generated as SDRs in the ‘combined’ data set.
The majority of SDRs generated in the ‘patient-only’ and ‘HCP-only’ data sets remained SDRs after combining both sets of reports. However, 278 of the drug–ADR pairs generated as SDRs (approximately 11% of all SDRs generated by the ‘patient-only and ‘HCP-only’ data sets independently) were no longer generated as SDRs in the ‘combined’ data set, including SDRs involving drugs flagged as ‘black triangle’, potentially serious reactions or reactions that were not documented on the SPC of the suspect drug. Three of the drug–ADR pairs generated as SDRs by the ‘HCP-only’ data set that were no longer generated as SDRs in the ‘combined’ data set were potentially serious, involved a drug flagged as ‘black triangle’ and were not documented as a problem on the SPC (levetiracetam/drug interaction, pregabalin/overdose and rimonabant/blood pressure increased).
| Classification of SDRs | ‘Patient-only’ data set | ‘HCP-only’ data set |
|---|---|---|
| SDRs generated before data combined | 649 | 1939 |
| SDRs remaining after data combined (% of SDRs generated in each data set before combination) | 557 (85.8) | 1753 (90.4) |
| SDRs no longer present after data combined: | ||
| All (% of SDRs generated before combination) | 92 (14.2) | 186 (9.6) |
| Serious (% of all) | 18 (19.6) | 47 (25.3) |
| Flagged as ‘black triangle’ | 10 (10.9) | 69 (37.1) |
| Not documented on SPC (% of all) | 14 (15.2) | 32 (17.2) |
| Serious, black triangle and not documented (% of all) | 0 (0.0) | 3 (1.6) |
| Serious, established drug and not documented (% of all) | 4 (4.3) | 5 (2.7) |
Of the 1753 SDRs generated in the ‘HCP-only’ data set that remained SDRs in the combined data set, 139 (7.9%) were detectable later in the combined data set, 247 (14.1%) were detectable earlier and in the remaining 1367 (77.0%) the month of detection was unchanged. This should be interpreted with caution, however, as this assumes that both patient and HCP reporting started at the same ‘day 0’, which is not true in practice (HCP reporting to the YCS commenced in 1964).
In practice, disproportionality analysis is not relied upon in signal detection for black triangle drugs. Instead, serious reactions to black triangle drugs are scrutinised on a case-by-case basis by the regulatory authorities. ‘Loss’ of SDRs, however, may be of concern for established drugs, for which disproportionality analysis is used to prioritise serious reactions for clinical assessment. Five of the SDRs generated when using the ‘HCP-only’ data set were ‘lost’ after combination of patient and HCP reports, involving established drugs, potentially serious reactions and reactions that were not documented on the product’s SPC (anastrazole/depression, citalopram/hypoglycaemia, simvastatin/drug interaction, trimethoprim/chest discomfort and trazadone/myalgia). Four of the SDRs generated by the ‘patient-only’ data set involved established drugs, potentially serious reactions and reactions that were not documented on the product’s SPC, which were ‘lost’ when the patient reports were combined with the HCP reports (citalopram/aggression, doxazosin/muscular weakness, perindopril/hypertension and prednisolone/face swelling).
The addition of patient reports to HCP reports generated an extra 929 SDRs for drug–ADR pairs that either had not been generated as SDRs when the ‘HCP-only’ data set was used (705 SDRs) or that were not present in ‘HCP-only’ data set (i.e. 224 SDRs that were only seen in the ‘patient-only’ data set) (Figure 6). Of these 929 additional SDRs, 299 (32.2%) were classified as being potentially serious, 139 (15.0%) involved black triangle drugs and 222 (23.9%) involved reactions that were not documented on the SPC.
FIGURE 6.
Additional SDRs generated after combining the patient and the HCP reports.
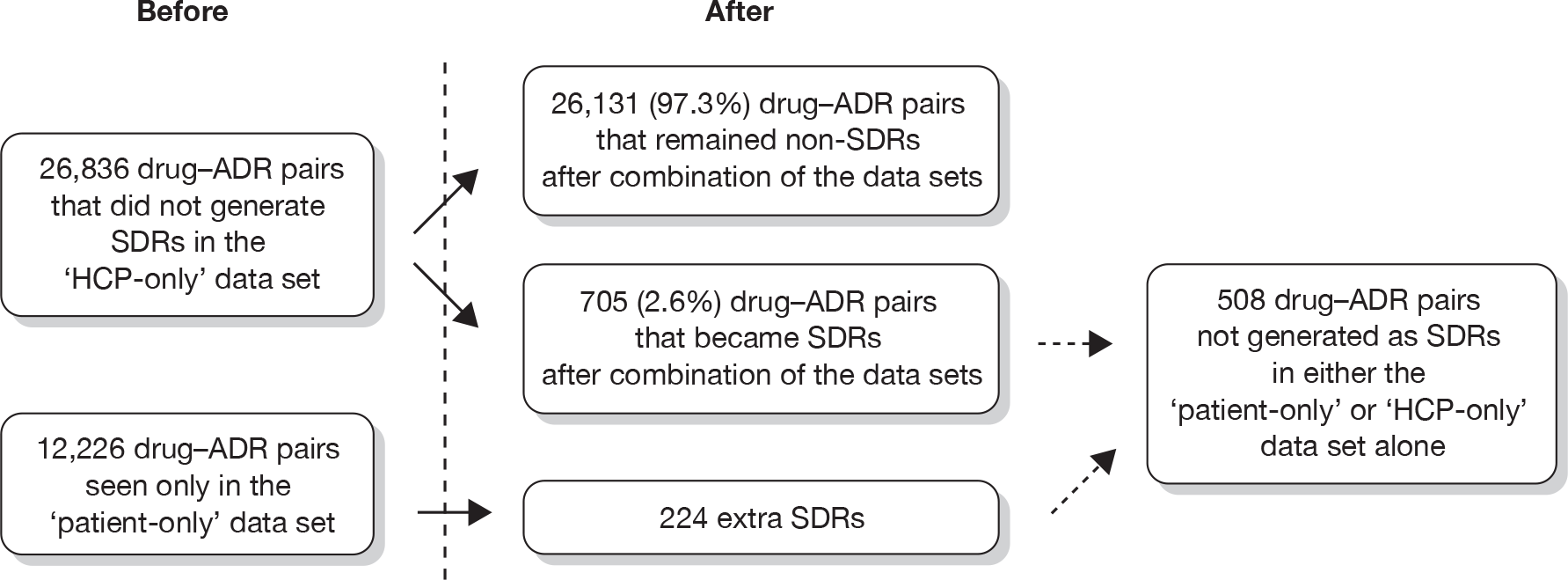
Of the 929 additional SDRs generated, 508 would not have been generated by disproportionality analysis in either the ‘patient-only’ or the ‘HCP-only’ data sets alone. Of these 508 extra SDRs, 185 (36.4%) were classified as being potentially serious, 95 (18.7%) involved drugs flagged as ‘black triangle’ and 137 (26.9%) involved reactions which were not documented as problems on the SPC. Ten of these additional SDRs were potentially serious, involved a drug flagged as ‘black triangle’ and were not documented on the product’s SPC (see Appendix 11), whereas 37 of the additional SDRs were potentially serious reactions involving established drugs that were not documented on the product’s SPC (see Appendix 12). Some of these SDRs were related to conditions that were prescribing indications for the suspect drug, for example diazepam/muscle spasms, chloramphenicol/eyelid oedema and donepezil/confusional state.
Causality assessment in a sample of reports
Overall, 41 drug–ADR pairs were randomly selected from the sampling frame for causality assessment, involving 199 patient reports and 184 HCP reports, to show the findings from the causality assessments performed within each category of drug–ADR pairs of interest. These have not been tested statistically owing to the small numbers within each category. The full breakdown of causality assessments is included in Appendix 13.
In Category 1, 11 drug–ADR pairs present in both the ‘patient-only’ and the ‘HCP-only’ data sets but identified as SDRs only in the ‘patient-only’ data set were selected from the sampling frame. The distribution of causality assessments performed on the 56 patient and the 43 HCP reports involving these drug–ADR pairs was similar for both reporter groups (Figure 7). Most reports were assessed as ‘possible’ with a relatively small number assessed as ‘probable’. Slightly more patient reports than HCP reports could not be assessed.
FIGURE 7.
Category 1: drug–ADR pairs present in both the ‘patient-only’ and the ‘HCP-only’ data sets; SDRs generated only by the ‘patient-only’ data set (11 pairs selected).
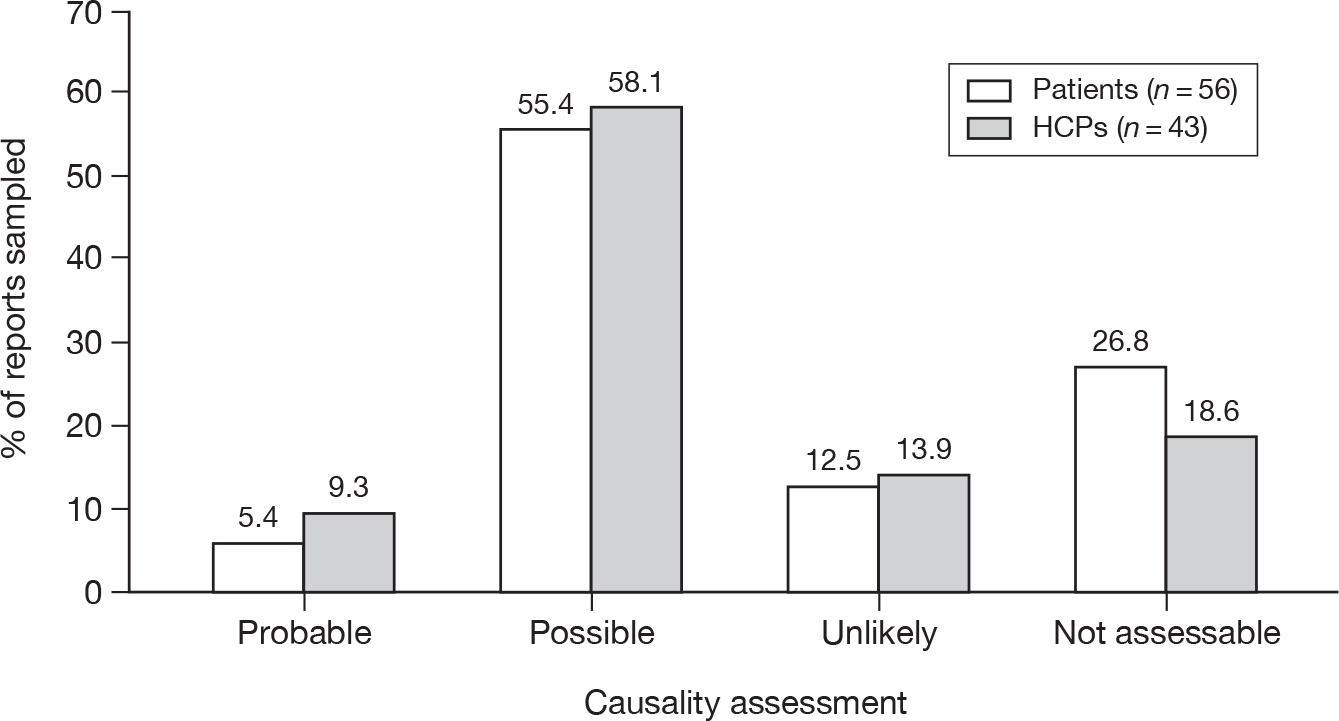
In Category 2, 12 drug–ADR pairs identified as SDRs in the ‘patient-only’ data set but not present in the ‘HCP-only’ data set were selected. Just under half of the 73 patient reports involved in these drug–ADR pairs were assessed as having a ‘possible’ causal relationship, with a minority assessed as ‘probable’. Compared with Category 1, however, a slightly smaller proportion was assessed as ‘probable’ or ‘possible’ and a higher proportion was assessed as ‘unlikely’ (Figure 8). In addition, almost a quarter of reports in Category 2 were regarded as ‘not assessable’.
FIGURE 8.
Category 2: drug–ADR pairs only present in the ‘patient-only’ data set; SDRs generated by patient, but not the HCP reports (12 pairs selected).
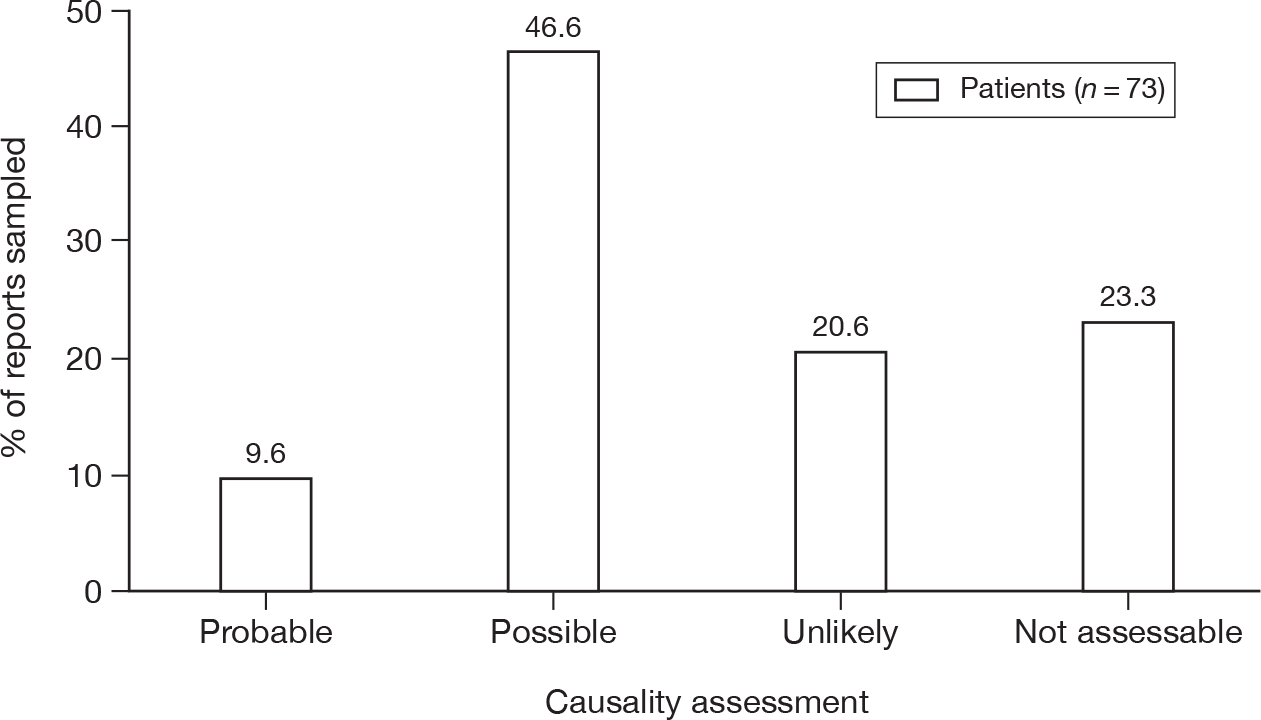
In Category 3, 12 drug–ADR pairs were selected that were identified as SDRs only after combining the patient and HCP reports. The distribution of causality assessments performed on the 52 patient and the 46 HCP reports involving these drug–ADR pairs was again similar for both groups (Figure 9). Compared with the patient reports, a higher proportion of the HCP reports was assessed as ‘probable’ or ‘unlikely’ and a lower proportion was assessed as ‘possible’.
FIGURE 9.
Category 3: drug–ADR pairs present in both the ‘patient-only’ and the ‘HCP-only’ data sets, but SDRs generated only after combining both sets of reports (12 pairs selected).
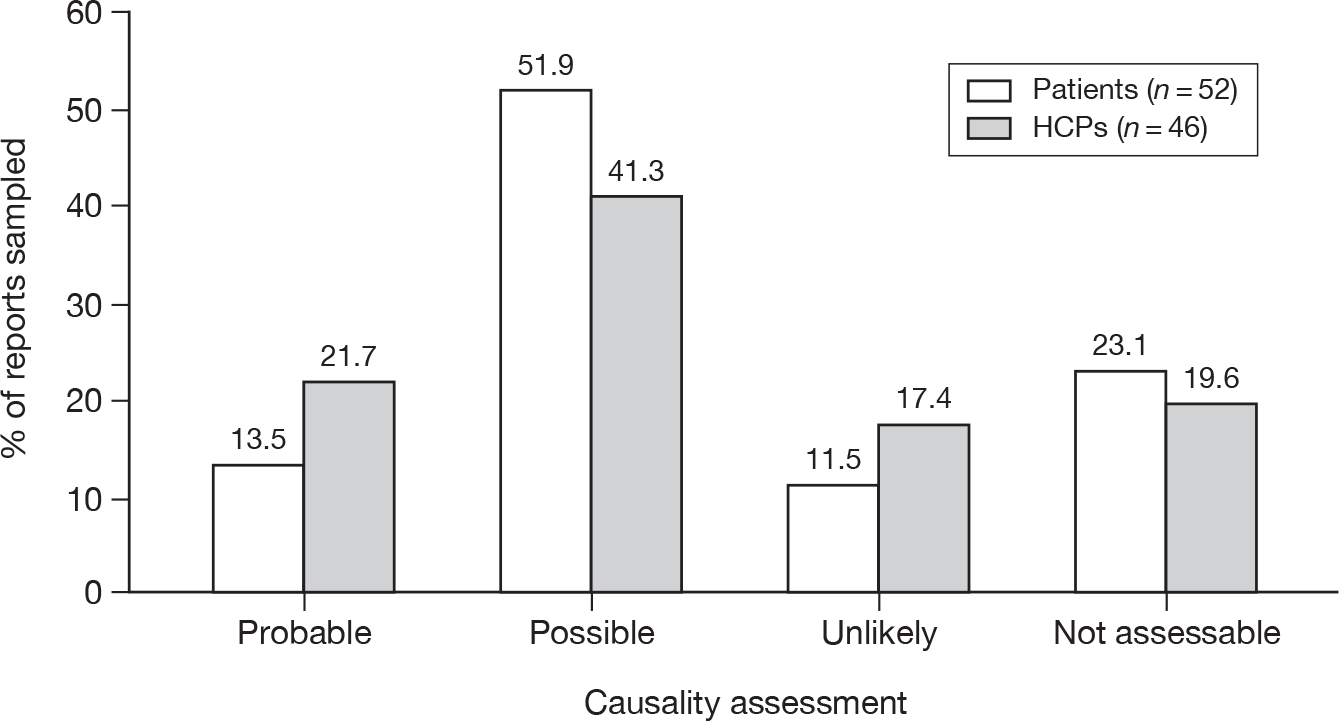
In Category 4, 12 drug–ADR pairs identified as SDRs by the ‘HCP-only’ data set were ‘lost’ when patient and HCP reports were combined and were selected for causality assessment. The assessment was performed on 84 HCP reports involving these drug–ADR pairs. Most reports were assessed as either ‘possible’ or ‘probable’, with few assessed as ‘unlikely’ (Figure 10).
A number of limitations to causality assessment were observed. These have not been systematically quantified and compared for each reporter group, but were observed when using both patient and HCP reports.
FIGURE 10.
Category 4: drug–ADR pairs generated as SDRs in the ‘HCP-only’ data set, but no longer regarded as SDRs when patient reports were combined with the HCP reports (12 pairs selected).
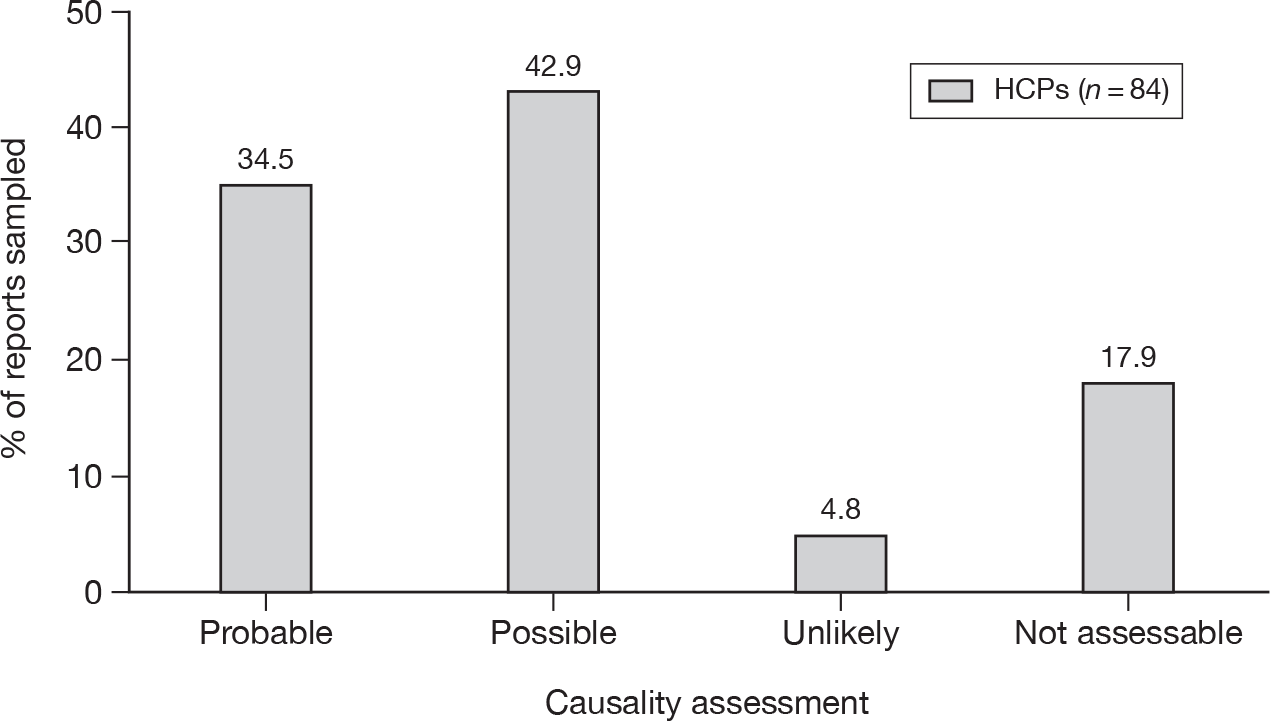
-
Missing information In some reports, no narrative or relevant dates were given, preventing an assessment of temporality. In some cases only a list of symptoms/events was reported. Information on past medical history was generally sparse, making it difficult to assess whether there were other reasons for the reported event(s).
-
Common or coincidental events Several events in the sample of pairs selected involved reaction terms that are common complaints in everyday life, making it difficult to determine whether these ‘reactions’ were in fact causal or coincidental, for example hair loss, cough, hypertension, weight gain, headache, diarrhoea or insomnia.
-
Possible confounding by indication This limited the ability to determine causality for a number of drug–ADR pairs, for example pregabalin/convulsion, fluoxetine/anxiety, iofendylate/arachnoiditis, olanzapine/agitation, tiotropium/dyspnoea, atomoxetine/depression and anti-malarial/vomiting or diarrhoea or pyrexia.
-
Long time to onset of reaction It is difficult to assess causality in events or symptoms that develop or are recognised by the patient many months or years after starting the medication. There may be many other unknown or unreported events/factors that may have contributed over this time period. This was observed in assessing reports of ‘loss of confidence’ with cyproterone/oestrogen, many of whom also experience depression.
-
The reporting of vague symptoms/less well defined terms These were difficult to assess, for example lack of confidence, anorexia, asthenia.
-
In some cases a reaction was reported which was specific to the drug’s formulation or brand, or the medication was suspected of being counterfeit These reactions were regarded as ‘not assessable’ for this project.
-
Multiple reaction terms/multiple suspect drugs In some reports a long list of reaction terms and/or suspect drugs was reported. It was not always possible to determine whether the information provided regarding a possible temporal relationship or outcome related to the specific reaction term or drug being assessed for causality. Patient reports tended to contain a larger number of coded reaction terms and were more likely to contain more than one suspect drug than HCP reports.
-
Ambiguity of coded terms For each of the 383 reports sampled, the drug–ADR pair of interest was cross-checked with the free-text description of the reaction provided by the reporter. In the majority of cases (314; 81.9%) the MedDRA term used to code the reaction appeared to be appropriate; in one of these cases an appropriate coding term had been used for the drug–ADR pair risperidone/musculoskeletal stiffness, but from the narrative the intended meaning of the reaction may have been an extrapyramidal side effect. In the remaining cases there was either no narrative (45; 11.7%) or the narrative did not contain any reference to the reaction of interest (24; 6.3%). Overall, however, there was little evidence to suggest that the intended meaning of the reporter’s description was lost in coding.
Finally, of the 383 forms evaluated, 18 were thought to be duplicate reports relating to the same nine patients: seven of these were detected twice in the ‘patient-only’ data set, one was detected twice in the ‘HCP-only’ data set and one was detected in both data sets. This suggests that the MHRA system to identify and merge reports about the same reaction in the same patient from different sources is not 100% effective.
Summary
-
Using the anonymised data provided by the MHRA for all patient and HCP reports received by the YCS between 1 October 2005 and 30 September 2007, signal generation analysis was undertaken on the whole database of patient and HCP reports.
-
We identified SDRs, which are ‘statistical signals’ when the reporting rate for a suspected ADR in association with a particular medicine is disproportionate to that of other products in the database. We then investigated the effects (on SDRs) of including and excluding patient reports from the HCP database. We also did clinical causality assessments on selected drug–ADR pairs from patients and HCPs.
-
For the signal generation analysis there were 16,566 drug–reaction pairs from patient reports and 28,775 from HCPs, with only 4340 (10.6%) pairs common to both groups. The HCP data set generated a significantly higher proportion of SDRs from the different drug–reaction pairs reported [1939 SDRs (6.7%) vs 649 (3.9%) respectively; difference in proportions 2.8%, 95% CI 2.4% to 3.2%]. Also, a higher proportion of HCP SDRs were for reactions classified as ‘serious’ by the MHRA compared with patient SDRs (48% vs 28.5% respectively; difference in proportions 19.5%, 95% CI 15.4% to 23.6%) or for drugs undergoing intensive surveillance (black triangle drugs) (30.7% vs 10.9% respectively; difference in proportions 19.8%, 95% CI 16.6% to 23.0%). A similar proportion of SDRs in both groups (15%) was assessed as not being listed on the product’s SPC and therefore potentially providing new information.
-
After combining the patient and the HCP data sets, an additional 508 SDRs were generated that were not produced by either data set alone, whereas 186 SDRs generated by the HCP data set alone were no longer present. The combined data set identified 47 SDRs for reactions classified as serious by MHRA which had not previously recorded on SPCs, whereas eight generated by the HCP data set alone were no longer present. Among the sample of individual reports assessed for causality, most were assessed as having a ‘possible’ causal association, regardless of reporter group.
-
Overall, patients appeared to have the potential to make a positive contribution to signal generation by:
-
– reporting different drug–ADR pairs and generating different SDRs from HCPs
-
– generating SDRs that may be considered important in the context of pharmacovigilance
-
– generating additional SDRs when combined with data from HCP reports
-
– providing information that may be valuable when assessing the likelihood of a causal association between a particular drug and reaction.
-
Chapter 6 Study 4: qualitative analysis of Yellow Card reports from patients and health-care professionals
Objective
To explore the richness of patients’ descriptions of their suspected adverse reactions compared with health professionals.
Methods
Case selection
Following extensive discussions with the advisory group, and within the project team, a purposive sample was taken from a range of different categories of Yellow Card reports. The advisory group suggested sampling by reaction type as well as by drug and suggested we cover a wide range of different drugs and different types of reaction. The details of the approach taken to sampling are provided in Appendix 14. The reports were divided as follows:
-
drugs most commonly reported by patients
-
black triangle drugs
-
drugs purchased OTC
-
complementary therapies.
The original plan was to examine around 300 patient reports and 300 HCP reports for similar types of drug–ADR pairs. There were, however, marked differences in reporting between patients and HCPs (as described in Chapter 5) and this meant that it was not possible to obtain the same number of reports for each group. In addition, there were no drugs reported by HCPs that were purchased OTC and virtually no complementary therapy reports; we examined only the patient reports for these two categories.
The details of the final sample of reports are shown in Appendix 14, and are summarised in Table 22. In total, we selected 270 patient reports and 179 HCP reports. This is fewer than originally planned, but following discussion with the advisory group we judged that there were sufficient numbers of cases for the qualitative analysis.
| Drug category | Drug | Nos. of reports selected | |
|---|---|---|---|
| Patients | HCPs | ||
| Most commonly reported drugs by patients | Citalopram | All 28 | All 23 |
| Co-cyprindiol (Dianette®, Bayer Schering) | Randoma 30 | All 3 | |
| Paroxetine | Random 30 | All 17 | |
| Simvastatin | Random 30 | Random 30 | |
| Venlafaxine | Random 30 | All 19 | |
| Subtotal | 148 | 92 | |
| Black triangle drugs | Insulin glargine | All 13 | All 9 |
| Levetiracetam | All 13 | All 8 | |
| Olanzapine | All 25 | All 13 | |
| Pregabalin | All 17 | All 38 | |
| Proguanil with atovaquone (Malarone®, GlaxoSmithKline) | All 14 | All 19 | |
| Subtotal | 82 | 87 | |
| Drugs purchased OTC | Subtotal | 25 | 0 |
| Complementary therapies | Subtotal | 15 | 0 |
| All drug categories | Grand total | 270 | 179 |
Analysis
Descriptive analysis
Data were exported to spss (SPSS Inc., Chicago, IL, USA) for descriptive analysis of the reports to compare age, gender, total number of drugs reported, total number of reactions reported and the word count used to describe the reaction between the two reporter types. Association between gender of reporter and reporter type was tested using the continuity-corrected chi-squared test. The independent samples t-test was used to compare the continuous variables that were normally distributed for both reporter types. If data were skewed then the non-parametric Mann–Whitney U-test was used. To minimise the chance of a type 1 error arising from multiple comparisons, a p-value of ≤ 0.01 was used to denote statistical significance throughout all analyses.
Content analysis
Following initial piloting using 10 patient reports and 10 HCP reports, a number of categories were identified from the descriptions given in the free-text field of the reports. These categories were identified inductively through iterative reading of the texts and discussion among researchers (CA and EM). Once the main data analysis began it was necessary to refine some of the categories in order to improve their precision or to widen the category definition. For example, in relation to text regarding clinical ‘signs’ we decided that it would be most appropriate to focus on ‘physical signs observed by a HCP’ as it was difficult otherwise to distinguish clearly between symptoms and signs. In relation to text indicating that an ADR improved when a drug had been discontinued, we found examples of improvement when a dose had been reduced, and so, widened the definition to include this possibility. The categories were stable at an early stage in the analysis to allow for quantification across the sample.
In broad terms, the categories covered the following:
-
description of the problem
-
impact of the adverse reaction on the patient
-
descriptions of the possible association between the drug and adverse effects
-
the patient's background medical history
-
actions taken by the patient
-
involvement of health professionals.
In addition, fields were created to allow for assessment of other features of the reports, such as whether there appeared to be ambiguities or possible recording errors. We made a qualitative judgement on how elaborate the accounts were and scored them as follows: 0, no narrative; 1, scant narrative; 2, moderately elaborate; and 3, very elaborate. We also recorded whether the presentation of symptoms stressed their extreme nature.
One researcher (VG) then read through each ADR report and noted the presence of information on a record sheet containing the categories of information shown in Appendix 15.
The researcher met regularly with the supervisor (AA) throughout this process to ensure accuracy of interpretation of information from the Yellow Card reports and accuracy of data extraction. Data from the record sheets were entered into excel by an administrator, who then double checked all of the data entry. One in 10 reports were checked visually for accuracy of data entry (by AA) and no problems were detected.
For the content analysis of Yellow Card reports, data were analysed in excel. The number and percentage of reports containing each of the information items was calculated for patient reports and HCP reports.
In-depth qualitative analysis
Having developed the categories detailed in the previous section, the researchers then carried out a detailed qualitative content analysis by iterative reading of the data relating to each category, with particular reference to those categories for which there were substantial differences between the frequency of use by patients and HCPs. The data were read by one researcher (CA), entered into the nvivo (QSR International Inc., Cambridge, MA, USA) software package and coded. The initial categories identified were checked and verified by two other researchers (AA and EM). Where there was not full agreement over the codes or the interpretation of the reports, these were discussed and reviewed. The analysis used the technique of constant comparison and the identification of outlying cases. The transcripts were repeatedly read and discussed by the researchers in relation to the key categories identified. As well as looking for differences between patient and HCP reports, we initially categorised those issues that had been highlighted in the content analysis of these data. Particular types of reactions that appeared to be reported at different rates by patients and health professionals were also categorised, for example electric shock sensations and suicidal ideation. In analysing and interpreting the data, we have tried to remain aware of the influence of the research process and the researchers’ backgrounds on the analyses produced. Factors such as the researchers’ backgrounds as a pharmacist, a GP and a medical sociologist are acknowledged.
Results
Description of the sample
In total, 449 reports were analysed, with 270 of these coming from patients and 179 from HCPs.
Descriptive statistics of the reports by patient (n = 230) and HCP (n = 179) reporters for the categories of ‘most commonly reported drugs by patients’ and black triangle drugs are presented in Table 23. Combining the data for these two categories, it can be seen from the table that the main differences between the patient and HCP reports were:
-
Mean age [standard deviation (SD)] in patient reports was slightly lower [44.2 (16.1) years vs 47.8 (18.5) years].
-
The number of drugs that the patient was reported as taking was lower for patient reports [median 2 (IQR 1 to 3) compared with 2 (IQR 1 to 4) for HCP reports].
-
Median number of distinct reactions reported was higher for patient reports [5 (IQR 3 to 8) compared with 3 (IQR 2 to 5) for HCP reports].
-
Median word count was greater in patient reports [58 words (IQR 29 to 121)] than in the reports from HCPs [16 words (IQR 9 to 31)].
-
Just over half (53.0%) of patient reports made using the paper form compared with 81.0% of HCP reports.
| Characteristics | Drugs most commonly reported by patients (n = 240) | Black triangle drugs (n = 169) | Combined categories of drug (n = 409) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient | HCP | p-value | Patient | HCP | p-value | Patient | HCP | p-value | |
| No. of reports | 148 | 92 | 82 | 87 | 230 | 179 | |||
| Mean (SD) age (years) at time of reaction | 42.8 (16.6) | 51.2 (19.5) | 0.001a | 46.8 (14.8) | 44.3 (16.7) | 0.31a | 44.2 (16.1) | 47.8 (18.5) | 0.04a |
| No. (%) of reports for women | 103 (70) | 57 (62) | 0.28b | 53 (65) | 54 (64) | 0.93b | 156 (68) | 111 (62) | 0.30b |
| No. of concurrent medications, median (IQR) | 1 (1 to 3) | 3 (1 to 5) | < 0.001c | 2 (1 to 3) | 2 (1 to 4) | 0.94c | 2 (1 to 3) | 2 (1 to 4) | 0.01c |
| No. distinct reactions, median (IQR) | 5 (3 to 8) | 3 (2 to 5) | < 0.001c | 5 (3 to 7) | 3 (2 to 4) | < 0.001c | 5 (3 to 8) | 3 (2 to 5) | < 0.001c |
| Word count, median (IQR) | 76.5 (32 to 138) | 16 (10 to 28) | < 0.001c | 46 (24 to 68) | 16.5 (9 to 36) | < 0.001c | 58 (29 to 121) | 16 (9 to 31) | < 0.001c |
| Methods of reporting n (%) | |||||||||
| Paper | 77 (52) | 76 (83) | 45 (55) | 69 (79) | 122 (53) | 145 (81) | < 0.001d | ||
| Internet | 68 (46) | 7 (7) | 16 (20) | 3 (3) | 84 (36) | 10 (6) | |||
| Telephone | 3 (2) | 0 (0) | 6 (7) | 0 (0) | 9 (4) | 0 (0) | |||
| Not specified | 0 (0) | 9 (10) | 15 (18) | 15 (17) | 15 (7) | 24 (13) | |||
There was no statistically significant difference between patient reports and HCP reports in the proportion relating to women.
The breakdown of HCP reporters was:
-
doctors (65%):
-
– GP 51%
-
– hospital doctor 8%
-
– physician 6%
-
-
pharmacists (13%):
-
– community pharmacist 6%
-
– hospital pharmacist 5%
-
– pharmacist – background not specified 2%
-
-
other health professionals (22%):
-
– nurses (11%):
-
– hospital nurse 8%
-
– nurse – background not specified 3%
-
-
– other health professional – background not specified 7%
-
– hospital health professional 3%
-
– dentist 1%.
-
Of the patient reports, 216 (94%) were made by the patient themselves, 10 (4%) came from carers and four (2%) were from a parent.
Most commonly reported drugs category
A total of 240 reports were extracted for qualitative analysis, with 148 of these coming from patients and 92 from HCPs. Descriptive analysis of these reports is shown in Table 23.
Black triangle drugs category
A total of 169 reports were extracted for qualitative analysis, with 82 of these coming from patients and 87 from HCPs. Descriptive analysis of these reports is shown in Table 23.
Drugs purchased over-the-counter by patients
Twenty-five reports were extracted for qualitative analysis of drugs purchased OTC by patients. This number was lower than originally expected because of the large number of duplicates. The mean age of patients at the time the reaction was reported was 42 years (SD 20 years). Fifteen (60%) of the patients were female.
Nineteen (76%) patients reported by paper, four (16%) by internet and one by telephone; the source of the report was not classified for another patient.
Twenty-one reports (84%) were from the patients themselves, two from parents and one from a carer.
Complementary therapies reported by patients
Fifteen reports were extracted for qualitative analysis. The median age was 39 years (IQR 36 to 56 years). Nine (60%) of the patients were female.
Eight of the patients reported by paper, four by internet, two by telephone and for one the method of reporting was not specified.
Qualitative analysis
Content analysis of Yellow Card reports
The contents of Yellow Card reports in relation to the combined categories of ‘drugs most commonly reported by patients’ and ‘black triangle drugs’ were compared. Table 24 shows the findings of the content analysis.
We decided, on the advice of the lead statistician on the study (AL), not to statistically compare the patient reports and HCP reports, as it was felt that this would give spurious precision to what was mainly a qualitative analysis. Nevertheless, it is apparent that patient reports were more likely to include:
-
information about symptoms
-
information about the impact on the patient (particularly the emotional and/or social impact)
-
comments on temporality (particularly in terms of text indicating that the ADR followed administration of the drug).
| Information categorya | Patient reports (total 230) | HCP reports (total 179) | |||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| 1 | Report no. | 230 | 100 | 179 | 100 |
| 2 | Suspect drug | 230 | 100 | 179 | 100 |
| 3 | Symptoms | 214 | 93 | 139 | 78 |
| 4 | Signs | 1 | 0 | 8 | 4 |
| 5 | Any impact | 108 | 47 | 22 | 12 |
| 6 | Emotional impact | 79 | 34 | 12 | 7 |
| 7 | Social Impact | 62 | 27 | 12 | 7 |
| 8 | Occupational impact | 15 | 7 | 1 | 1 |
| 9 | Involvement of health services | 87 | 38 | 144 | 80 |
| 10 | Investigations | 17 | 7 | 17 | 9 |
| 11 | Treatment | 57 | 25 | 17 | 9 |
| 12 | HCP advice | 35 | 15 | 3 | 2 |
| 13 | Past medical history | 10 | 4 | 2 | 1 |
| 14 | General health | 11 | 5 | 3 | 2 |
| 15 | Temporality 1: ADR followed administration of drug | 141 | 61 | 48 | 27 |
| 16 | Temporality 2: ADR improved when drug discontinued or dose was reduced | 60 | 26 | 22 | 12 |
| 17 | Temporality 3: ADR occurred on discontinuation of drug | 50 | 22 | 16 | 9 |
| 18 | Temporality 4: ADR recurred when drug started again | 15 | 7 | 1 | 1 |
| 19 | Dose relationship | 13 | 6 | 5 | 3 |
| 20 | Differential diagnosis | 16 | 7 | 4 | 2 |
| 21 | Past history | 12 | 5 | 4 | 2 |
| 22 | Causal theorising | 7 | 3 | 6 | 3 |
| 23 | Objective evidence | 8 | 3 | 15 | 8 |
| 24 | Endorsement by HCP of causal link | 7 | 3 | 4 | 2 |
| 25 | Self-management | 13 | 6 | 6 | 0 |
| 26 | Medicotechnical | 0 | 0 | 24 | 13 |
| 27 | Extreme nature of signs and symptoms | 107 | 47 | 31 | 17 |
| 28 | ‘Elaboration’ score | ||||
| 0 = no narrative | 14 | 6 | 32 | 18 | |
| 1 = scant narrative | 86 | 37 | 110 | 61 | |
| 2 = moderately elaborate | 90 | 39 | 30 | 17 | |
| 3 = very elaborate | 40 | 17 | 7 | 4 | |
| 29 | Ambiguity | 16 | 7 | 14 | 8 |
| 30 | Apparent recording error | 0 | 0 | 0 | 0 |
In addition, 56% of patient reports were judged to be at least moderately elaborate, whereas 61% of HCP reports were judged to have scant narrative. Furthermore, although we judged that 47% of patient reports highlighted the extreme nature of symptoms, this was the case for only 17% of HCP reports.
Health-care professionals were more likely to use medicotechnical language, although this was judged to be present in only 13% of their reports.
Ambiguities were uncommon in the reports examined, and there were no apparent recording errors, for example patient reports appearing to come from a health professional or vice versa, or where the coding of reaction appeared to be based on misinterpretation of the text.
In-depth qualitative analysis of Yellow Card reports
When describing patients’ and health professionals’ reports, extracts have been quoted verbatim and identified by reporter type (patient or professional group), gender of patient, age of patient (years), suspect drug name and reporting method. A range of extracts from Yellow Card Reports have been used to illustrate our findings, representing different patients, reactions and drugs. Spelling errors in extracts were corrected for ease of reading; any emphasis in bold has been added by the researchers. A number of reports are written in the third person as someone else reported on behalf of the patient.
A number of major categories arose from the content analysis and these informed the in-depth qualitative analysis. We note, however, that some of the categories were linked or interrelated so that many text fragments could be related to more than one category. For example, there was a strong link between the categories of ‘symptom description’ and ‘extreme nature of symptoms’ and so these categories are not mutually exclusive. We have dealt with this issue by providing extensive detailed quotes so that the reader can see how these cover different categories, even when they have been used to highlight specific themes.
The main part of the results compares patient and health professional reports and examines in detail the characteristics of patient and professional reports with particular reference to those categories of content that patients use more frequently than HCPs, including symptom reporting, with emphasis on the extreme nature of these, and the impact of suspected ADRs on relationships, social life, occupation and emotions. The final part describes and discusses patient reporting of involvement of health professionals.
Symptom description
Both patients (93%) and health professionals (78%) described symptoms of ADRs. However, patients’ reports tended to be much more detailed and extensive, as reflected in the mean elaboration score (patients 1.68, health professionals 1.07) and the word count. Comparing the following two reports, one by a health professional and the other by a patient, relating to the same suspect drug but involving different patients, it can be seen that the HCP described the symptoms and what the patient had been advised to do to: withdraw from the medicine. In contrast, the patient not only listed the symptoms experienced, but also described their intensity. She refers to ‘unbearable anxiety’, describing the current stage of dose reduction as ‘the worse stage so far’, and the medicine as ‘horrendous’. The symptom of headache is highlighted by repeating it and putting it in block capitals:
Dizziness, blurred vision, light headedness and electric shock sensations following withdrawal from Seroxat. These sensations are abolished by taking half a Seroxat tablet, but then recur after I switched him to Seroxat suspension 20 mg/10 ml and advised him to reduce dose in very small steps from 0.7 ml daily. Not seen since.
GP, male, 44 years, paroxetine, paper
Since beginning to reduce this medicine, I have had terrible withdrawal symptoms, these have included: sudden changes in emotion and mood, crying, insomnia, excessive anxiety and agitation, sweating and palpitations. There have also been bouts of stomach upsets, nausea, dizziness and headaches. Since reaching an amount of 5 mg I have had to use the liquid version with a syringe and make reductions of 1 mg per month, this has been the worst stage so far and I have been prescribed medication to alleviate the unbearable anxiety that this is causing! Still 2 mg to go before I am off this horrendous medicine! HEADACHE HEADACHE.
Patient, female, 46 years, paroxetine, paper
Similarly, comparing two different reports for levetiracetam, the health professional report was very short and the patient report more elaborate and descriptive:
Movements feel dull and heavy, tiredness, nausea and shaky.
Community pharmacist, female, 71 years, levetiracetam, reporting method not specified
I couldn’t stay awake, sick, staggering, loss of balance, eyesight got worse. When I increased the evening dose, my knuckles got swollen and sore, then I had 5 weeks of sleeplessness nights with the agony of carpal tunnel syndrome in my right hand. A steroid injection helped, but I still have a sore hand. Keppra – pain in my feet, ankles, shins, knee, hips, thighs, fingers, hands and arms, I could hardly walk, also couldn’t sleep at night which made me tired during the day. Before taking the medication for epilepsy, I had no pain and could move freely. Since medication I have been in constant pain and hobble about like a crippled old woman. I can’t even sit cross legged any more.
Patient, female, 58 years, levetiracetam, paper
The patient quoted below gives an elaborate description of the history and symptoms of her hypoglycaemic reaction to insulin glargine, emphasising the unpredictability, rapidity of onset and associated dangers of the reaction:
Reaction started almost immediately. The analogue insulin made me have hypoglycaemic reactions that came upon me with little or absolutely no warning signs at all. Not only did this happen, but the window of opportunity of recognising a symptom to being too stroppy to do or have something done for me was less than 5 minutes – on Porcine I have at least 30 to 40 minutes. On top of this a hypoglycaemic reaction at night did not wake me up. Very dangerous and could lead to severe damage or even death if no one found me. This happened on several occasions, but fortunately others were with me. It did involve me going to A&E twice and having a doctor called out to house twice. Other times husband has given me 2 or 3 glucagons! I have after considerable difficulty, persuaded my Diabetic Consultant to put me back on to some porcine insulin – for the present only the neutral short acting, but as it is going so well, I am hoping to revert to the porcine long acting as well. It took me 5 months to get this to happen and that should not be the case. Stopped as soon as I switched back to porcine neutral insulin.
Patient, female, 54 years, insulin glargine, internet
In contrast, a GP report of a different patient’s reaction to insulin glargine is brief and to the point, but gives no history:
Erratic diabetic control.
GP, male, 36 years, insulin glargine, paper
Other health professional reports were equally brief:
Raised CK 350.
GP, simvastatin, paper
Major withdrawal, panic attacks tremors, eye pain, neck pain, leg pain, found it difficult to walk. Eye problems, sees floaters like looking through net curtain. Life threatening because depressed and anxious to end life.
Community pharmacist, male, 44 years, paroxetine, paper
Very few patients made short, unelaborated reports that were similar to those from HCPs, for example:
Severe depression with suicidal thoughts.
Patient, female, 30 years, Dianette, internet
On the other hand, there were very occasional elaborated reports from health professionals, such as the following, which describes the effect on the patient in great detail:
The patient was complaining that on a bad day she had palpitations, dyspnoea, nightmares, difficulty speaking, disorientation, difficulty walking with poor balance, a feeling as though she is drunk and feeling of cold alternating with episodes of sweating. The current symptoms are of difficulty focusing which varies from one eye to another on a daily basis, electric shock type symptoms shooting down her limbs periodically, poor balance, poor memory and word finding and severe insomnia. In addition, she has recently complained of heartburn and of extreme frequency of micturition. The patient tried a course of St John’s wort. This was to try and control the insomnia, electric shock and tremor symptoms. Approximately 2 weeks after starting this she had disturbing and visual hallucinations. She was agitated and felt driven to throwing herself off a roof. She discontinued the St John’s wort and did not seek medical attention at the time. Her mother observed her closely and after approximately 48 hours the symptoms resolved. Currently the patient continues with complaints of hallucinations, slurred speech and difficulty in word finding, intermittent blurred vision and intermittent dyspnoea which sounds like chest muscle spasms. She had a severe episode of abdominal discomfort last month which resulted in an attendance at the acute medical unit.
GP, female, 37 years, paroxetine, paper
Some patients stated why they were originally prescribed the offending medicine. The health professionals tended not to do this in the free-text, comments, but past medical history, which may include these details, is also recorded in coded fields as a MedDRA term in response to relevant questions on patient and HCP Yellow Cards:
I was first prescribed Seroxat as I was feeling low in mood …
Patient, female, 37 years, paroxetine, paper
I was prescribed this medication for mild sleeplessness. I became addicted to it and after 18 months of severely debilitating symptoms, the principal symptom being persistent suicidal thoughts.
Patient, female, 31 years, paroxetine, paper
Patients also described what their relatives and health professionals said about the adverse effect as if to confirm that it was real:
My daughter, my wife and a sister in a hospital said I looked dreadful in the face …
Patient, male, 61 years, simvastatin, paper
In addition to providing the expected details about the ADR, some patients also gave reasons for making the report, including a diagnosis or to find other people with the same reaction:
I am desperate to discuss my condition with someone who is prepared to listen. I don’t know where else to turn and a friend gave me the enclosed Yellow Card and suggested that I might get someone to diagnose and tell me which of the many diagnosis [sic] I am actually suffering from. I’m told I do not have the right to get advice from another doctor but I am sinking into depression now and feel there must be someone somewhere who can help me and not just leave me to it.
Patient, male, 61 years, simvastatin, paper
Emphasis on extreme nature of symptoms
Patients were much more likely than health professionals to discuss the extreme nature of signs and symptoms. Forty-three per cent of patients did this compared with 17% of health professionals. The following examples clearly illustrate how out of the ordinary and frightening the reactions could be. This patient discusses the extreme reaction he/she suffered after taking the complementary medicine Angelica sinensis:
Soon after taking the first dose of Tiao He Cleanse I got a severe headache, I had to take paracetamol which I continued to do until the following week in order to get through each day. I continued to take dose. The headache was just the beginning of a serious problem. Every internal organ became intolerably painful including my liver, kidney, stomach, I could scarcely move, sit, lie. I became dehydrated and took copious amount of water. I would wake at least four times in the night both to go to the toilet and to drink considerable amount of water. It would take me several minutes to work out how I could even move from the bed. Extraordinary heat came from the lower part of my body as if it was burning. I became bloated. I became constipated. My mouth became ulcerated. My teeth felt so on edge that I found them difficult to brush and could not eat all foods normally. I had to force myself to eat but maintained their recommended diet. I rang a Chinese herbalist practitioner and was advised to cease taking the medication (which I had already done) and to go to my doctor and get a liver enzyme test and to drink lots of water.
Patient, female, 61 years, Angelica sinensis, paper
The report below emphasises the extreme nature of this patient’s reaction to two selective serotonin reuptake inhibitors (SSRIs), citalopram and sertraline:
Became increasingly confused, violent and abusive towards his partner. Disorientated, and in his words thoroughly pissed off with life in total. Did not reach the point of suicidal thoughts, but had completely collapsed inside himself and unable to function or look at himself. He was re-prescribed sertraline which stabilised some of the worst of the above but not sufficiently enough to become fully effective. Citalopram and sertraline each caused mild to moderate involuntary muscle spasms, extreme libido problems (impotency), extreme sleeping disorders (nightmares, early wakening, afternoon lethargy), extreme anxiety, moderate-to-severe loss of interest in basic personal hygiene.
Patient, male, 48 years, citalopram, paper
This patient discusses the extreme adverse effects suffered after taking levetiracetam and compares them with the single effect of weight gain caused by her previous antiepileptic medicine, sodium valproate (Epilim, Sanofi-Aventis):
The first Keppra pill I took, I took at night, I was phasing off Epilim Chrono and coming onto Keppra – I have night time seizures, it just sent me to sleep automatically. It was like a really, really strong sleeping tablet, I’m not exactly tiny, so I was very surprised, I had to wait until the Christmas holidays before starting to take it during the day, as I felt I would end up like a narcoleptic otherwise, falling asleep at my desk in the middle of the day. Not only that over a period of time, I also got extremely agitated, stressed out, nervous, paranoid, leading up to a night where I honestly thought I was losing my mind totally. I’ve never, ever felt like this, I’m normally an extremely moderate and chilled-out person, to feel like this was extremely worrying … I have been on Epilim for years previously and the only issue with Epilim is that it makes me put on weight when I start taking it! I’d rather be a few pounds heavier than risk losing my mental wellbeing!
Patient, female, 26 years, levetiracetam, internet
This report from a doctor describes a similar reaction to levetiracetam, but the report was more detailed and factual:
Levetiracetam introduced 500 mg twice daily. Sodium valproate reduced by 100 mg per week from 800 mg total/day. Patient very sleepy, slow eating, observed by staff to have tremors, head shaking no associated temperature, hands cold and slightly blue, very agitated, needing support from carers. Blood pressure, heart rate and respiratory rate normal. Vomited once. Levetiracetam stopped. Discussion with drug company recommend 250 mg twice daily introduction. Urine output and intake monitored throughout 5 days total. Gradual improvement over next 48 hours back to normal after 1 week. Liver enzymes normal these checked pre levetiracetam and after patient had recovered as very difficult to obtain blood when having side effects.
Hospital doctor, levetiracetam, paper
This patient describes the severity of a reaction having taken the OTC antihistamine cetirizine for hay fever:
Soon after starting the medicine I experienced difficulty breathing, weariness, especially at night. This was so severe it affected a trip to [place name]. Had to stop taking the tablets, but still had trouble breathing at night and persistent cough.
Patient, male, 34 years, cetirizine, paper
A number of patients described their reactions as terrifying. The reports below illustrate just how frightening the patients felt their reactions were:
I have suffered horrific adverse reactions to venlafaxine for over two and a half years. I stopped taking the drug and the withdrawal effect has been the most terrifying experience of my life. I could write a book – it has wrecked my life, my body, my mind. I have been unable to work for over eighteen months and started part time again, but have had another month of debilitating withdrawal. I shall attempt to describe the symptoms extreme drowsiness, exhaustion, severe heart palpitations, shakes, hypoglycaemia, diarrhoea up to three or four times a day, confusion – difficulty in thinking logically, orderly. Memory problems, electric shock sensations, and involuntary spasms. Severe dizziness, balance problems, co-ordination problems, weight/distance perception disturbed, feelings of waking drugged, insomnia, tinnitus, nausea, digestive problems – stomach ache, flatulence. Severe fogginess in head, horrendous concentration. Personality change (is the most unbearable effect – unable to think with my own mind), aggression, irritability, depersonalised feelings. This last week to 10 days I have literally felt like I have been physically hit over the head with a hammer – the symptoms are that severe and physical and tangible as well as mental. I almost feel a physical constricting sensation in my brain. At one point last year on the detox I even had trouble reading and interpreting English. I am staggered at how dangerous this drug is and how much it has the potential to destroy. It affects not only your mind, but brain function and your digestive system, central nervous system and much more. Still has not recovered – and is still suffering horrific side effects. The information on the PIL was not adequate enough.
Patient, female, 37 years, venlafaxine, paper
… The seizures with St John’s wort were happening totally unexpectedly, daily and were terrifying. I had to hang onto a lamp-post on my way into town to stop myself from falling. I could not read after the seizures, I could not count, I could not speak. My regular seizures do not have these effects. My doctor told me to come off the St John's wort straight away as there has been much speculation about the dangers. Other doctors I have spoken to said the same.
Patient, age and gender unknown, St John’s wort, internet
Took tablet at 14:00. Sleepy all evening … confusion, loss of memory, completely disorientated. Increased respiration, drowsy, unable to follow simple instructions, unable to recall recent events and past medical history … Able to read and write again … fully orientated. Nauseous, but able to tolerate clear fluids Monday. First time I was able to shower and dress since Thursday pm. Shaky, very scared by the reaction especially the confusion.
Patient, female, 41 years, sumatriptan, paper
Twelve HCPs out of the 21 who had reported withdrawal symptoms mentioned the distress that withdrawal symptoms caused to patients, but these descriptions were never as elaborate as those reports by patients discussed above:
Very severe withdrawal leading to severe distress.
Hospital doctor, female, 46 years, venlafaxine, not specified
Electric shock sensation
Twenty-three per cent of patient reports and 14% of HCP reports about citalopram, paroxetine and venlafaxine included descriptions of ‘electric shock sensations’ or similar reactions. Patient reports of this particular symptom exemplify the tendency among patients to stress the severity of the reaction, which was described above. Compared with the professional reports, patient reports were particularly vivid, comparing this reaction to a range of other extreme experiences and stressing the intensity of the symptoms. These provide an important example of how, if such a serious and unpleasant adverse effect were to occur from another drug in the future, patients’ reports could contribute to pharmacovigilance and to understanding of the effects of an ADR:
Shocks these are like dropping out of an aircraft as a diver for the first time or a speeding car hitting a hill at speed then flying through the air. Your breath is taken away …
Patient, male 51 years, paroxetine, internet
… electrical zaps …
Patient, female, 32 years, paroxetine, internet
… ‘fireworks’ exploding in my head …
Patient, female, 50 years, venlafaxine, paper
… electric shock-like sensations also called brain shivers.
Patient, male, 27 years, venlafaxine, internet
One patient went to great length in her description of the adverse reaction:
First symptoms constant headache, extreme agitation, pressure inside head followed by sudden explosion inside head. Sensation of chemical being forcibly released from a particular area in brain, which then felt like a cold trickle down neck, across chest down spine. Neck went rigid and painful, cold shivers then feverishness, chest felt heavy, heart rate increased [although felt very calm and was not hyperventilating (this was not a panic attack)], legs then began shaking and convulsing uncontrollably for half an hour, accompanied by regular release of chemical in head symptoms subsided after an hour, but then began to reoccur approximately every 6 days in the same pattern, but with increasing severity. Arms and legs would convulse uncontrollably, arms and legs would be numb and pins and needles and loss of feeling, neck would go rigid and jaw clench, pressure inside head would rise and fall with each explosion. Symptoms would last for up to 3 hours each time. Headache would return once symptoms had subsided each time. After 3 weeks of this, I reduced the dosage of citalopram back to 10 mg, concerned that it was causing these symptoms
Patient, female, 34 years, citalopram, paper
A number of health professionals listed electric shocks as a reaction, but only one HCP in our sample described it in any more depth, quoting the patient’s own description:
… reported that her brain was fizzing like being connected to the national grid.
GP, female, 35 years, venlafaxine, paper
Impact on patients’ lives
Forty-seven per cent of patient reports discussed the impact of the reaction on their lives compared with only 12% of health-professional reports. Three types of impact were discussed: impact on relationships and social life, occupational impact and emotional impact.
Impact on relationships and social life
Twenty-seven per cent of patients in the sample reported the social impact of their reaction, while only 7% of health professionals mentioned this. Not only were patients more likely to mention this effect, but also their accounts of the social impact of the reaction were much more elaborate and referred more to the feelings that this impact caused. These differences are illustrated by the extracts from professional and patient reports reproduced below. Professionals who did refer to the impact of the reaction tended to give brief, factual reports, for example:
Severe muscle pain restricting normal activities. Required analgesics four times a day.
GP, female, 56 years, simvastatin, paper
Somnolence. Odd visual effects. Difficult to drive.
GP, male, 61 years, pregabalin, paper
In contrast, a number of the patient reports vividly described the effect the ADR had on the patient’s normal functioning in society and the feelings which this generated. For example, they reported how they have been confined to their bed, how they could not go out at all and how the ADR was ruining their life. These extracts from reports by two patients who have suffered muscle pain as an ADR from simvastatin exemplify this:
… So many things have happened to me over the past year all originating from start of this medication. That is my opinion since I know how I feel and how my life has almost come to a standstill …
Patient, female, 71 years, simvastatin, paper
… but since starting simvastatin 10 mg, 1 at night, my life has been turned upside down. I can’t make any plans as I used to. Why do people force you to take cholesterol tablets because you are diabetic? If I did not get them I would not feel the way I feel now. I can’t eat my food like I used to, I look at food and am full up. I know I am 70 years old, but I only take the diabetic medication and am not even a problem to my family, I look after them and now this simvastatin ruins my life.
Patient, female, 72 years, simvastatin, paper
Other patients described how they could not function normally:
Two weeks into starting Dianette, started to feel depressed, anxious and low self esteem. After 3 weeks really depressed, felt suicidal, couldn’t function and couldn’t get out of bed. Was crying all the time and was very frightened. Stopped 3 days later and starting to feel better – though still feels wobbly.
Patient, female, 37 years, Dianette (Bayer plc), telephone
While the excerpts above refer to the direct impact of the drug, similar accounts were given in relation to the withdrawal of drugs:
After the dose drop to 10 mg, I had mood swings, palpitations and a weird head lagging behind sensation which I believe were withdrawal symptoms. Five weeks after the dose drop I got more symptoms – tiredness to the point of not being able to go to shops, headaches, aching joints and muscles like having the flu, anxiety, sweating, finding it hard to speak, unable to cope with any stress, and feeling like I had been punched in the eyes. This belated effect is the bit I am worried about …
Patient, female, 30 years, citalopram, paper
In addition to the effects on functioning described above, some patients described the profound effect that the reaction had on their relationships with family and friends, as well as the impact of the reaction on others:
I feel so down a lot of the time I can’t even phone a friend as I’m afraid I’ll just cry down the phone. I have everything going for me and yet find it so hard to look on the bright side it’s a disgrace. So, I’ve decided to take a break from the pill altogether for a few months.
Patient, female, 30 years, Dianette, internet
The last year has been hell for me and has been awful for my parents who are 62 and 68 respectively …
Patient, male, 31 years, paroxetine, internet
I don’t know why I was put on this medication either, as I have tonic–clonic grand mal seizures at night, which I don’t think they are for. I have never felt so odd on a medication and thank God I decided to come off it, I think my boyfriend and I would of split up otherwise, due to my change in mood.
Patient, female, 26 years, levetiracetam, internet
One patient reported harming herself, her pets and her husband:
Self harming myself, my pets and husband…
Patient, female, 54 years, pregabalin, paper
Occupational impact
Fifteen (7%) patient reports and one HCP in the sample commented on the effect of the suspected ADR the patient’s occupation. Only the health professional report mentioned how long the patient had been off work:
Reaction on withdrawal – headaches, nausea, vomiting, sweating, sleep disturbance, diarrhoea. Off work for 4 weeks.
GP, female, 49 years, venlafaxine, paper
Patients taking a number of different medicines portrayed how the ADR had affected their occupation – some had had to stop work, whereas others were able to work only part-time:
An increased heart rate, deep breathing and ‘rushes’ of sensation throughout body. Intense tingling in scalp, loss of spatial awareness and mild hallucinations. General mania, rambling of speech and difficulty stringing sentences together. Erratic behaviour, possibly a bit aggressive at times, although I don’t feel any animosity towards anyone. I am a lab-based research student and the above symptoms make it unsafe for me to work …
Patient, male, 27 years, citalopram, internet
I would say that it was a gradual decline in my mental state within months whilst starting Dianette. I became extremely low and very unhappy, struggling with work, socialising, becoming withdrawn, distressed, short tempered and finding difficulty in coping generally (crying a lot, etc.). I didn’t talk to anyone over how I was feeling and issues at work worsened. I dealt with complaints within a hospital and very much struggled with the sensitive issues I was dealing with – anger, grief, bereavement, etc. I continued for a number of months until I could function no more and shared with my family how I was feeling.
Patient, female, 30 years, Dianette, internet
I was prescribed this medication for mild sleeplessness. However, a period of 18 months of severe symptoms has had a huge effect on my professional and personal life. I am now working part time, having lost fundamental confidence.
Patient, female, 32 years, paroxetine, internet
I have suffered horrific adverse reactions to venlafaxine. I have been unable to work for over 18 months and started part time again, but have had another month of debilitating withdrawal.
Patient, female, 37 years, venlafaxine, paper
One patient described the effect of the ADR on her work, stating that she had lost her edge:
It is the single most distressing experience. Anxiety, tearfulness/shakiness/feelings of panic/feelings of hopelessness, loss of cognitive function – can’t remember specific words, inarticulate, lose ‘edge’ at work as not thinking as sharply, gastrointestinal problems.
Patient, female, 43 years, venlafaxine, paper
Emotional impact
Thirty-four per cent of patient reports and 12% of HCP reports discussed the emotional impact of the ADR on the patient.
These patient reports clearly show how much emotional distress particular reactions cause:
Became increasingly confused, violent and abusive towards his partner. Disorientated, and in his words thoroughly pissed off with life in total …
Patient, male, 48 years, citalopram, paper
Become very anxious, sweaty and shaky
Patient, female, 23 years, citalopram, paper
I also get very confused, forgetful, mood swings, highs and very lows.
Patient, female, 54 years, pregabalin, paper
Nearly all the patient reports about reactions to Dianette in the sample vividly described their mood swings and depression, for example:
I had been suffering from violent mood swings for years, but had not linked it to the pill I was taking as I had always been told that the pill should help stabilise my moods. I had read the PIL however still did not link my mood swings to the pill. I just put it down to bad PMT. Then over the past couple of years I was getting quite depressed – crying all the time for silly reasons, not being able to cope with much at all, not being able to concentrate. Feeling pretty useless …
Patient, female, 29 years, Dianette, internet
Twenty-seven patients described how taking the antidepressants citalopram, paroxetine and venlafaxine and the acne treatment Dianette, had led them have suicidal thoughts:
This medication made me feel suicidal. Although I felt pretty bad anyway from my depression which was acute at the time, I experienced the effect of thinking in detail about how I could best kill myself. I thought about different methods and researched this on the internet. I have suffered depression for a long time and have often felt I wanted not to be alive any more but have never ever thought about ways of killing myself, much less researching them.
Patient, female, 45 years, citalopram, paper
I have noticed that the longer I have been taking the Dianette pill the more severe my depression has become. I cry about three to four times during the week and have thought about suicide.
Patient, female, 17 years, Dianette, internet
These two patients had suicidal thoughts on withdrawing from paroxetine and venlafaxine:
Side effects include not feeling myself, dizzy, agitated, sick, vertigo, harming myself, suicide thoughts, unbearable pains, muscle spasms, mental clicking inside head, headaches lasting weeks. I can only imagine it’s like the withdrawal from heroin. My son was also born with withdrawal symptoms shakes.
Patient, female, 26 years, paroxetine, paper
After tapering down as per doctor's instructions then stopping experienced nausea, ongoing irritable bowel syndrome, dizziness, fatigue, sweating, nightmares, electric shock-like sensations also called brain shivers, akathisia, abnormal vision, nervousness, panic attacks, depressed feelings, suicidal thoughts and confusion.
Patient, male, 27 years, venlafaxine, paper
Some patients taking paroxetine, citalopram and venlafaxine described suicide attempts:
I tried suicide on several attempts and even attacked my father for no reason.
Patient, male, 31 years, paroxetine, paper
Two weeks after starting the medication, it caused me to have suicidal thoughts. On the first occasion I took 4 sleeping tablets on the second I took 6 sleeping tablets. The doctor then increased the dosage of citalopram and I had an uncontrollable desire to commit suicide. I took aspirin, Nurofen, Night Nurse and paracetamol. I was admitted to hospital and after 4 days of not taking citalopram all suicidal thoughts had disappeared.
Patient, female, 58 years, citalopram, paper
This patient made suicide attempts on withdrawal from citalopram:
Withdrawing from this drug caused me to feel suicidal. I made two suicide attempts during withdrawal. I am now on no medication at all.
Patient, female, 44 years, citalopram, internet
One patient complained of feeling suicidal following taking the OTC hay fever medicine cetirizine:
I took 2 cetirizine tablets in total over the space of approximately 1 week. On each occasion, within 24 hours of taking the tablet I was extremely depressed and felt suicidal. Have NEVER felt this way before. I woke in the night feeling that I might as well end it all. I have no previous history of depression. I stopped taking the cetirizine completely and preferred to suffer from the hay fever as I felt the tablets were responsible but was not sure.
Patient, female, 45 years, cetirizine, internet
Two HCP reports also mentioned patients’ suicidal ideation:
Suicidal thoughts and suicide attempt (potentially lethal paracetamol overdose). No recent suicidal thoughts prior to initiating citalopram, patient did not want to die.
Hospital pharmacist, female, 16 years, citalopram, paper
Brought out more thoughts of suicide especially in bed at night. She said they caused hallucinations as well. Stopped 2 days after stopping citalopram.
GP, female, 18 years, citalopram, paper
Causality
Patients commonly provided information that would be useful when assessing causal links between the suspect drug and the reaction. This will now be illustrated by discussing causality under a number of headings that have been derived from a series of questions designed for assessing the likelihood of an adverse reaction being related to the use of a medicine. 49
Adverse drug reaction following administration of the drug
Patients often described reactions occurring after first administration of a drug or a particular formulation of that drug:
Suspected side effects (from the start of treatment), gastrointestinal problems, dizziness/light-headedness on standing up, insomnia, weight gain, strange dreams, heightened awareness of colour.
Patient, female, 43 years, citalopram, paper
Started having leg problems shortly after start of simvastatin. I associate problem with medication because I had never had leg problems prior to starting medication. I pointed out to doctor that leaflet accompanying medication stated there could be muscle problems.
Patient female, 71 years, simvastatin, paper
Side effects immediate, chest pain worsened, couldn’t walk or talk, shaking, shock, ‘put myself in the recovery position and passed out’
Patient, female, 42 years, olanzepine, telephone
This patient’s reaction started on changing to a slow release formulation of tramadol:
I had two glasses of red wine per evening whilst on the tablets, contrary to what was recommended. This may have complicated the issue. However, having been on conventional tramadol, there had been no ill effects, and it was only when my prescription was changed to slow release tramadol that the seizures occurred. Two seizures within 3 hours of each other – the first at home the second in Accident and Emergency.
Patient, male, 51 years, tramadol, paper
This doctor related a reaction to insulin glargine to the administration of the drug:
Felt light headed after first dose also developed symptoms of the room spinning after subsequent doses.
GP, female, 70 years, insulin glargine, paper
Adverse drug reaction improved when drug discontinued
One-quarter of patient reports highlighted how a reaction improved on discontinuation of a drug. Patient reports about myalgia and arthralgia following simvastatin also give the history of the reaction as well as the effect of stopping the medicines:
My muscles became stiff in my neck, then in my arms a month later, then by 2 months later the muscles in my whole body were so painful I could not get out of bed and function. I thought it was from the simvastatin, but my doctor said no and told me to continue taking it as the symptoms got worse. I stopped taking simvastatin. Blood tests showed my sedimentation rates were very very high and I was diagnosed with polymyalgia rheumatica (PMR). I am 100% convinced it was caused by simvastatin. It would not have progressed to PMR if I had stopped earlier.
Patient, female, 58 years, simvastatin, paper
This patient clearly links the start and finish of her problems to taking Dianette:
Unfortunately I had no indication that the contraceptive was having such an effect until I had been taking it for three years and decided to have a break. Of course I would not like to spur severe accusations about Dianette; however, I strongly believe that the contraceptive was affecting my mood, giving me anxiety and panic attacks and quite bad depression on occasions, it seems too much of a coincidence that the anxiety and depression began after a few months of taking the pill and then evaporated not long after stopping. During the time I was on Dianette, in one burst of depression I took an overdose, it is for this reason that I would like to report what I think were side effects of what is generally thought of as a harmless drug.
Patient, female, 19 years, Dianette, internet
Adverse drug reaction occurred on discontinuation of the drug
Patients (22%) were more likely than health professionals (9%) to discuss how the reaction had occurred on discontinuation of the drug. One of the reactions most dramatically described by patients was drug withdrawal; this was experienced whilst reducing the dose or stopping taking a number of medicines including the antidepressants citalopram, venlafaxine and paroxetine:
As I started to come off the medicine I started to feel anxious all over again despite feeling perfectly well prior to deciding to stop. Each time I have tried to come off the drug it has resulted in returning to the medication as the side effect s have too much impact on my daily routine. I have been taking the medicine again and plan to start to gradually withdraw using a liquid replacement of the tablet in the immediate future. Hopefully, this will allow me to reduce the quantity very gradually and have less effect.
Patient, female, 28 years, paroxetine, internet
Dizziness, nausea, alternate sweats and chills, unable to stand properly, balance affected. Dislike of bright lights, slurred speech, no appetite not even wanted liquids, pains in abdomen. Re-started medication, and symptoms increased in severity, vomited after 36 hours, once after taking first capsule and soup, saw out of hours doctor as blood pressure was raised, heart rate fast and blood in urine – on test strip. Away from home for few days, forgot medication. After 48 hours from previous dose symptoms began. Continued to worsen. Family members called NHS direct helpline. Doctor contacted – advised withdrawal symptoms. Gave emergency prescription. GP said it was like ‘heroin cold turkey’. I thought SSRI’s were non-addictive, therefore I am very concerned about the severity of these symptoms and the duty doctor comparing it to a controlled drug withdrawal.
Patient, female, 58 years, venlafaxine, paper
This GP also related the patient’s reaction to withdrawal:
Patient who received Efexor (venlafaxine hydrochloride tablet) therapy at 150 mg for 3 years, reduced the dose to 37.5 mg for 1 month and then stopped therapy and after 24 hours of taking her last tablet the patient experienced withdrawal effects (drug withdrawal syndrome) and felt over stimulated (psychomotor hyperactivity), reported that her brain was fizzing like being connected to the national grid (paraesthesia) felt very sick (malaise) and heightened alertness (hypervigilance).
GP, female, 35 years, venlafaxine, paper
Adverse drug reaction recurred when drug started again
Seven per cent of patients and 1% of HCP reports discussed how the reaction started when the drug was restarted. This patient talks about how the reaction stopped within a few days of stopping their simvastatin but resumed on re-challenge:
Pain in right and left shoulder joint with lack of movement increasing, with constant pain in both upper arms and shoulders which becomes severe during night and early hours of morning. Diagnosed in November by GP as ‘Frozen Shoulders’. Further pain increase since December 2006 in neck and across top of both shoulders and down to upper arm as far as elbow joint. Link with Simvastatin identified when having course of physiotherapy and Simvastatin stopped in March after discussion with GP. Pain in neck disappeared within a 6 days of stopping Simvastatin, pain in arms no change or improvement with physiotherapy. Simvastatin resumed after 4 week break in dose and pains in neck re-appeared within 3 days, Simvastatin stopped after 7 days and pain in neck now subsided, Simvastatin dosage now ceased. Constant pain in both arms and ‘Frozen Shoulders’ with lack of movement not improving despite intensive physiotherapy regime problem now existed for 13 months.
Patient, male, 48 years, simvastatin, internet
Dose relationship
The following quotes show how patient reports linked suspected ADRs to a change in dose:
I am presently taking 40 mg daily of this medication. On increasing the dose of citalopram, first to 60 mg and then reducing to 50 mg daily – I experienced severe agitation and a recurrent thought of ending my life. On 60 mg, I felt seriously suicidal, and had constant morbid thoughts and fixation. Increasing citalopram from 40mg daily to 60 mg daily made my depression and anxiety much worse, therefore having the reverse effect on my mood – inducing suicidal feelings.
Patient, female, 27 years, citalopram, paper
I have been taking simvastatin for a number of years, on doubling the dose 2 years ago to 40mg I developed severe muscle pains acute in arms, hands and shoulders. This was diagnosed as carpel tunnel both wrists, trapped nerves at both elbows and both shoulders frozen. I have been in severe pain for a long time. I stopped the simvastatin and felt a hell of a lot better (much less pain) to check that the simvastatin was intensifying the pain I started to take it again. One 20 mg tablet left me again in pain and feeling dreadfully confused and muddle headed. I therefore stopped taking the simvastatin and again felt better.
Patient, male, 55 years, simvastatin, internet
These are examples of reported dose-related reactions from HCPs:
Patient had been on a small dose of Lyrica since last year for pain control. After increasing dose had an asthma attack followed by acute neurological symptoms – dysarthria, robotic speech, dizziness, drowsiness, ataxia, and difficulty controlling urine.
GP, female, 43 years, pregabalin, paper
This lady was in with a scalding injury and looked more depressed. Her paroxetine dose was increased to 20 mg daily from 10 mg and she was also prescribed tramadol for pain. She then started to hallucinate and developed myoclonic jerking and tremor with increased tone in all limbs. I think she had serotonin syndrome and as there were no other culprit drugs. Stopped her tramadol and paroxetine. All other tests normal and she improved rapidly.
Hospital doctor, female, 78 years, paroxetine, internet
Differential diagnoses
Patients sometimes provided information that showed they had considered different possible causes for their reactions. This patient tells of her reaction and how she decided it was due to Malarone and not due to food poisoning:
On day 10 of taking Malarone, one minute I was ok, next minute I was extremely dizzy and started to vomit, at 10 pm, within 3 minutes with severe diarrhoea. At the time I was sharing a meal with four others, none of whom were sick from the same food. I vomited several times in the night and had diarrhoea all night and all the following day. Next day I had severe flu-like pains in my whole body, particularly in my spine, and I was walking from my bed to the toilet like a woman of 100! I had to stay in bed the following day because I was very weak and slightly feverish. My arms and legs felt cold but my torso and neck felt hot to the touch. The joint pains moved into my wrists. My stomach and intestines felt tender to the touch. I could eat nothing for 60 hours, the thought of food was nauseating. My digestive system seemed to block up too when I started to eat again. I started to take Malarone again on day 12 but stomatitis started so I stopped taking Malarone altogether and will never use it again. Local people said Oh, you got Malarone malaria. One other detail: I noticed from the start of taking Malarone that my urine was extremely dark. It was clear that once I stopped taking the Malarone everything got back to normal.
Patient, female, 50 years, malarone, internet
This patient wondered if one or both of their drugs was causing their reaction:
Nose starts to tingle and the same effect moves down and around mouth and chin, it’s just like the feeling if you have injection in your gum at the dentist. Wonder if it’s a combination of both drugs – or one or the other – also it only happens when take medication late of forget.
Patient, female, 45 years, venlafaxine, paper
Involvement of health professionals
Many of the patient reports commented favourably on the advice and care they had received from HCPs, particularly that received from doctors. A small minority, however, reported that their doctor had not understood them, not known about the existence of the side effect, ignored them or told them that they were wasting their doctor’s time:
… After 10 or more years on these pills I can feel my stomach isn’t right but have had to put up with it. I feel I’ve been lied to over the years even by the doctor who did my endoscopy who didn’t want to do it, I had to persevere to try and get to the bottom of this. Still haven’t. These drugs are evil.
Patient, male, 40 years, paroxetine, internet
A few of the patients who suffered from depression following taking Dianette complained that their doctor did not know that Dianette caused depression:
… My doctor suggested I take anti depressives just until my moods had become more stable but I refused. He never mentioned that my depression and anxiety could be linked to Dianette…
Patient, female, 29 years, Dianette, internet
Other patients described how they felt ignored by their doctor:
… I pointed out to doctor that leaflet accompanying medication stated there could be muscle problems. If experienced patient should discuss with doctor. I pointed out problem to my doctor and pain I was having all of which he chose to ignore. I’m told I do not have the right to get advice from another doctor but I am sinking into depression now and feel there must be someone somewhere who can help me and not just leave me to it.
Patient, female, 71 years, simvastatin, paper
One patient described a particularly unfortunate experience with their doctor over the adverse effects they had experienced from pregabalin:
Side effects started straight away, was violently sick, fit and blacked out. Dizzy head, tendency to fall over, itching, blurry eyes, swelling of the stomach, food tastes awful, mouth dry on waking and I feel like there is a heavy weight on my chest and I have to sit up to breathe. I can’t go about doing things and my husband has Parkinson’s disease. Five other women who suffer as I do and they had the same, and stopped them. I tell you this in confidence, my doctor was very rude to me, I had a couple of drugs that didn’t work, he prescribed the pregabalin. I went back to see him and he assured me of wasting NHS money and told me if I didn’t take them he would strike me off his medical list and I wouldn’t be seen again. He also wouldn’t believe me that I couldn’t use my arm, he grabbed hold of my finger, tugged my arm up into the air, let it drop down and it slammed down on to his desk, no apology. I was told I should have reported him, but I couldn’t have coped with the outcome it may have caused. I don’t see him anymore, I go to another one who is very nice.
Patient, female, 78 years, pregabalin, paper
Influence of reporting method
It was thought that the free text in patient telephone reports might differ from those reported on paper or via the internet because the report was transcribed during the patients’ telephone call by a staff member at the MHRA, who may be selective in what they chose to record. Although a few telephone reports were short and more like health professional reports, a number of them were more elaborate, mentioning the impact of the reaction on the patients’ life and included what are assumed to be direct quotes from patients, as illustrated below:
Rigidity – not complete but a degree. Trembling feverishness – didn’t take temperature, loss of sleep, panic attacks, loss of concentration, depression worse, loss of sense of taste (partial), facial movements, blurred vision – more than usual, dizziness, agitation, feeling faint, weakness, confusion. Higher level of anxiety than usual – felt chemical in origin. Loss of initiative, being ‘full of words’ in minds ear, coupled with being ‘keyed up’ – not a good combination. Taken 2 months to die down since then.
Patient, male, 51 years, citalopram, telephone
One telephone report was written in the first person:
Twenty minutes in, as though I had been drinking – couldn’t stop laughing. I do not drink. Palpitations, very drowsy had to be prodded awake, slept till 11 pm. Arms ‘heavy’, nauseous, dreadful, slurry. Next day, violently sick. Went to GP next day, didn’t give anything as has had a mild antihistamine reaction. Tearful and aggressive/argumentative. Frightened. Feeling ill. Trying to disguise slurred speech. Headache.
Patient, female, 46 years, certrizine, telephone
Summary
-
We undertook a qualitative analysis of reports from patients and HCPs and purposively selected a wide range of different types of report. Focusing on the free text describing the ADRs we undertook a content analysis to describe the characteristics of 230 patient and 179 HCP reports, followed by a more detailed inductive qualitative analysis of the free text (which included 40 additional patient reports of drugs purchased OTC and complementary therapies).
-
The content analysis of text describing suspected reactions showed that patient reports were more likely than those from HCPs to include information about symptoms (93% vs 78%) and to stress the extreme nature of these (47% vs 17% of reports). They were also more likely to highlight the impact of the reaction on the patient (47% vs 12%), particularly the emotional impact (34% vs 7%) or social impact (27% vs 7%). Patients commonly reported on temporal associations, with 61% stating that the suspected ADR had followed the administration of the drug; 26% that it had improved on stopping the drug, 22% that it had occurred on withdrawal of the drug, and 7% that it had recurred on restarting the drug.
-
The in-depth qualitative analysis demonstrated the richness of accounts from patients and provided numerous detailed and elaborate descriptions of suspected reactions. Patient Yellow Card reports also contained information on reasons for drugs being prescribed, reasons for reporting, how patients identified the ADR, and responses from HCPs. Particularly striking were reports, often in relation to central nervous system drugs, which were extremely distressing, and sometimes frightening, describing confusion, agitation, panic symptoms, mood swings, suicidal thoughts and electric shock sensations. Patient reports vividly described the effects of suspected ADRs on patients’ lives, illustrating impact in terms of serious disruption to social and occupational functioning and marked emotional effects. By contrast, where HCPs did comment on the effects of suspected ADRs on patients’ lives, the accounts were usually brief and rarely illustrated the profound impact recorded in patient reports.
Chapter 7 Study 5: questionnaire survey of patients reporting to the Yellow Card Scheme
Objective
To describe the views and experiences of patients reporting to the YCS.
Methods
Study population
The study population was all patients reporting an ADR to the MHRA via the YCS between March 2008 and December 2008.
Questionnaire development
A postal questionnaire was developed to explore patient reporters’ views and experiences of making a Yellow Card report. Members of the project team worked on several iterations of the questionnaire to ensure that it covered all key areas regarding reporters’ views and experiences, and that it had face validity. The questionnaire (see Appendix 16) asked about how patient reporters learned about the YCS, how often they had made reports, details of their most recent Yellow Card report, reasons for submitting a report, their experience of reporting the ADR and their sociodemographic characteristics. Most questions used closed formats, including some scaled responses. However, open questions were included to obtain views on problems with reporting, suggested improvements to the YCS, expectations and reasons for future use of the scheme. In addition, the patient reporters were asked if they would be willing to talk to a researcher about their report in a more detailed telephone interview (see Chapter 8 for further details).
For piloting, the questionnaire was distributed to a convenience sample of adults of varying ages and educational levels to assess ease of completion and identify areas for amendment. We did not assess test–retest reliability. A final version of the questionnaire was produced after taking account of feedback.
Questionnaire administration
To maintain confidentiality, the MHRA distributed the questionnaires on behalf of the research team with a covering letter (see Appendix 17). To minimise recall bias, the questionnaires were sent out within a week of a patient’s report being received by the MHRA. Only MHRA staff knew the identity of the reporters, and they assigned a unique identifier to each questionnaire holding the code separately. Completed questionnaires were returned directly to the researchers, who sent MHRA staff regular updates of identifiers for returned questionnaires. This enabled the MHRA to identify non-responders and send reminders (see Appendix 18), while maintaining anonymity. A single reminder was sent 3 weeks after the original mailing.
Data management and analysis
Data from the questionnaires were entered into spss and validated by checking a 10% systematic sample against the original questionnaires. The medicine that the respondent suspected as causing their ADR was coded according to the ATC classification system. 39 Categorical responses were described using frequencies and percentages. The distribution of respondent age was skewed and therefore was described using the median and IQR, and compared between groups using the Kruskal–Wallis test. Pearson’s chi-squared test was used to examine the associations between the method of reporting (online, postal, telephone) and the following responses to the questionnaire: where they learned about the YCS, the ATC anatomical classification of the suspect drug, how soon they reported ADR, ease of reporting using the YCS, whose idea it was to report the ADR, and whether they expected (and would have liked) feedback on their report. Associations between two categorical variables were examined using Pearson’s chi-squared test. To minimise the chance of a type 1 error arising from multiple comparisons, a p-value of ≤ 0.01 was used to denote statistical significance throughout.
Each free-text question was analysed separately, using a thematic approach. All comments were read individually and sorted thematically using an iterative approach. For questions with large numbers of responses, two researchers initially independently read 100 responses to identify themes. Final themes were agreed and all responses were coded independently. Any discrepancies were discussed and agreed.
Results
The MHRA posted the questionnaire to a total of 2008 patient reporters and 1362 (68%) evaluable forms were returned and entered into the database. From a 10% systematic sample (n = 136), only seven minor errors were found relating to illegible handwriting on the questionnaire, misspelling, and data recording error, and these were rectified to reflect the original questionnaire.
Respondent characteristics
The respondents’ median (IQR) age was 56.5 years (43.0 to 67.0 years], 910 (66.8%) were female and 1274 (93.5%) were of white ethnicity (Table 25). Over two-thirds of respondents had been educated beyond 18 years. The Yellow Card report was submitted by post for 814 (59.8%), online for 447 (32.8%) and by telephone for 86 (6.3%) respondents. Respondents who made an online report were significantly younger (p < 0.001) and had a higher level of education than those using another method of reporting (p < 0.001). Only 105 (7.7%) respondents had sent in more than one Yellow Card report, and 133 respondents (9.8%) had submitted a Yellow Card report on someone else’s behalf.
| Characteristic | Method of reporting | All (n = 1362) | p-value | ||
|---|---|---|---|---|---|
| Post (n = 814) | Online (n = 447) | Telephone (n = 86) | |||
| Median age – years (IQR) | 61 (46 to 71) | 50 (36 to 58) | 63 (52 to 72) | 56.5 (43 to 67) | < 0.001 |
| Gender | |||||
| Female | 534 (65.6) | 310 (69.8) | 56 (66.7) | 910 (66.8) | 0.31 |
| Male | 280 (34.4) | 134 (30.2) | 28 (33.3) | 447 (32.8) | |
| Education | |||||
| I left school aged 16 years or younger and did no further education | 222 (28.4) | 58 (13.1) | 24 (29.6) | 309 (22.7) | < 0.001 |
| I left school or college aged 17 or 18 years and did no further education | 48 (6.1) | 34 (7.7) | 7 (8.6) | 90 (6.6) | |
| I did a further education qualification beyond the age of 18 | 297 (37.9) | 140 (31.5) | 24 (29.6) | 465 (34.1) | |
| I did an undergraduate degree | 131 (16.7) | 127 (28.6) | 17 (21.0) | 277 (20.3) | |
| I did a postgraduate degree | 85 (10.9) | 85 (19.1) | 9 (11.1) | 181 (13.3) | |
| Ethnicity | |||||
| White | 758 (95.3) | 427 (96.6) | 75 (93.8) | 1274 (93.5) | 0.72a |
| Asian or Asian British | 12 (1.5) | 7 (1.6) | 1 (1.3) | 20 (1.5) | |
| Black or Black British | 10 (1.3) | 2 (0.5) | 1 (1.3) | 13 (1.0) | |
| Mixed | 6 (0.8) | 4 (0.9) | 1 (1.3) | 11 (0.8) | |
| Chinese | 2 (0.3) | 0 (0.0) | 0 (0.0) | 2 (0.1) | |
| Other | 7 (0.9) | 2 (0.5) | 2 (2.5) | 11 (0.8) | |
How respondents learnt about the Yellow Card Scheme
Almost one-half of the respondents learnt about the YCS from a pharmacy (n = 667; 49.0%), followed by their GP (n = 220; 16.2%). Of those who learnt about the YCS from other sources not listed as options in the questionnaire (n = 252; 18.5%), the most frequent response was that they had a HCP background/professional knowledge (n = 88; 35.2%). A higher proportion of respondents reporting by post learnt about the YCS from a pharmacy or GP surgery than either of those using the other two methods (both p < 0.001; Table 26). One-hundred and thirteen (25.3%) patients reporting online found out about the scheme from an internet search, compared with only 24 (2.9%) and 7 (8.1%) of those reporting by post and telephone, respectively.
| Source | Method of reporting | All (n = 1362) | p-value | ||
|---|---|---|---|---|---|
| Post (n = 814) | Online (n = 447) | Telephone (n = 86) | |||
| Pharmacy | 484 (59.5) | 135 (30.2) | 41 (47.7) | 667 (49.0) | < 0.001 |
| GP surgery | 167 (20.5) | 45 (10.1) | 8 (9.3) | 220 (16.2) | < 0.001 |
| Other | 109 (13.4) | 114 (25.5) | 29 (33.7) | 252 (18.5) | < 0.001 |
| Internet search | 24 (2.9) | 113 (25.3) | 7 (8.1) | 145 (10.6) | < 0.001 |
| Family member or friend | 46 (5.7) | 34 (7.6) | 7 (8.1) | 89 (6.5) | 0.32 |
| Hospital | 48 (5.9) | 21 (4.7) | 3 (3.5) | 73 (5.4) | 0.49 |
| Magazine or newspaper | 21 (2.6) | 17 (3.8) | 1 (1.2) | 40 (2.9) | 0.28 |
| MHRA website | 5 (0.6) | 28 (6.3) | 4 (4.7) | 37 (2.7) | < 0.001 |
Information regarding respondents’ Yellow Card reports
Table 27 presents the ATC anatomical classifications of the suspect drugs reported by the method of reporting used. Overall, the biggest percentage of reports was for a drug for the nervous system and this did not differ by method of reporting. However, there were some differences. Over one-quarter (n = 213) of respondents reporting by post reported a drug related to the cardiovascular system compared with 22.1% (n = 19) of those reporting by telephone and 17.2% (n = 77) of those reporting online (p = 0.001). A higher proportion of online users (n = 34; 7.6%) reported adverse events from drugs for the genitourinary system and sex hormones than users of other reporting methods [post n = 31 (3.8%) and telephone n = 2 (2.3%)] (p = 0.006).
| ATC class | Method of reporting | All (n = 1362) | p-valuea | ||
|---|---|---|---|---|---|
| Post (n = 814) |
Online (n = 447) |
Telephone (n = 86) |
|||
| Nervous system | 229 (28.1) | 124 (27.7) | 26 (30.2) | 384 (28.2) | 0.90 |
| Cardiovascular system | 213 (26.2) | 77 (17.2) | 19 (22.1) | 311 (22.8) | 0.001 |
| Anti-infectives for systemic use | 84 (10.3) | 66 (14.8) | 13 (15.1) | 163 (12.0) | 0.046 |
| Alimentary tract and metabolism | 74 (9.1) | 33 (7.4) | 6 (7.0) | 114 (8.4) | 0.51 |
| Musculoskeletal system | 52 (6.4) | 22 (4.9) | 5 (5.8) | 79 (5.8) | 0.57 |
| Genitourinary system and sex hormones | 31 (3.8) | 34 (7.6) | 2 (2.3) | 68 (5.0) | 0.006 |
| Respiratory system | 28 (3.4) | 17 (3.8) | 4 (4.7) | 49 (3.6) | 0.83 |
| Blood and blood-forming organs | 24 (2.9) | 8 (1.8) | 3 (3.5) | 35 (2.6) | 0.40 |
| Antineoplastic and immunomodulating agents | 21 (2.6) | 10 (2.2) | 2 (2.3) | 33 (2.4) | 0.93 |
| Systemic hormonal preparations, excluding sex hormones and insulins | 23 (2.8) | 9 (2.0) | 1 (1.2) | 33 (2.4) | 0.49 |
| Dermatologicalsb | 8 (1.0) | 5 (1.1) | 1 (1.2) | 14 (1.0) | – |
| Sensory organsb | 10 (1.2) | 1 (0.2) | 1 (1.2) | 12 (0.9) | – |
| Antiparasitic products, insecticides and repellentsb | 3 (0.4) | 7 (1.6) | 0 (0.0) | 10 (0.7) | – |
| Variousb | 1 (0.1) | 0 (0.0) | 1 (1.2) | 2 (0.1) | – |
Figure 11 shows that those reporting by telephone did so relatively sooner than those reporting by either of the other two methods, whereas those who reported by post took the longest time to report the event (p = 0.01). In total, 1304 (95.7%) respondents were at least fairly sure that the side effect was due to the medicine, with no significant difference between methods of reporting (p = 0.22).
FIGURE 11.
Time the report was made after the side effect was first noticed by method of reporting. Note: Pearson’s chi-squared test showed a significant association between the time the report was made after the ADR and method of reporting used (p = 0.01).
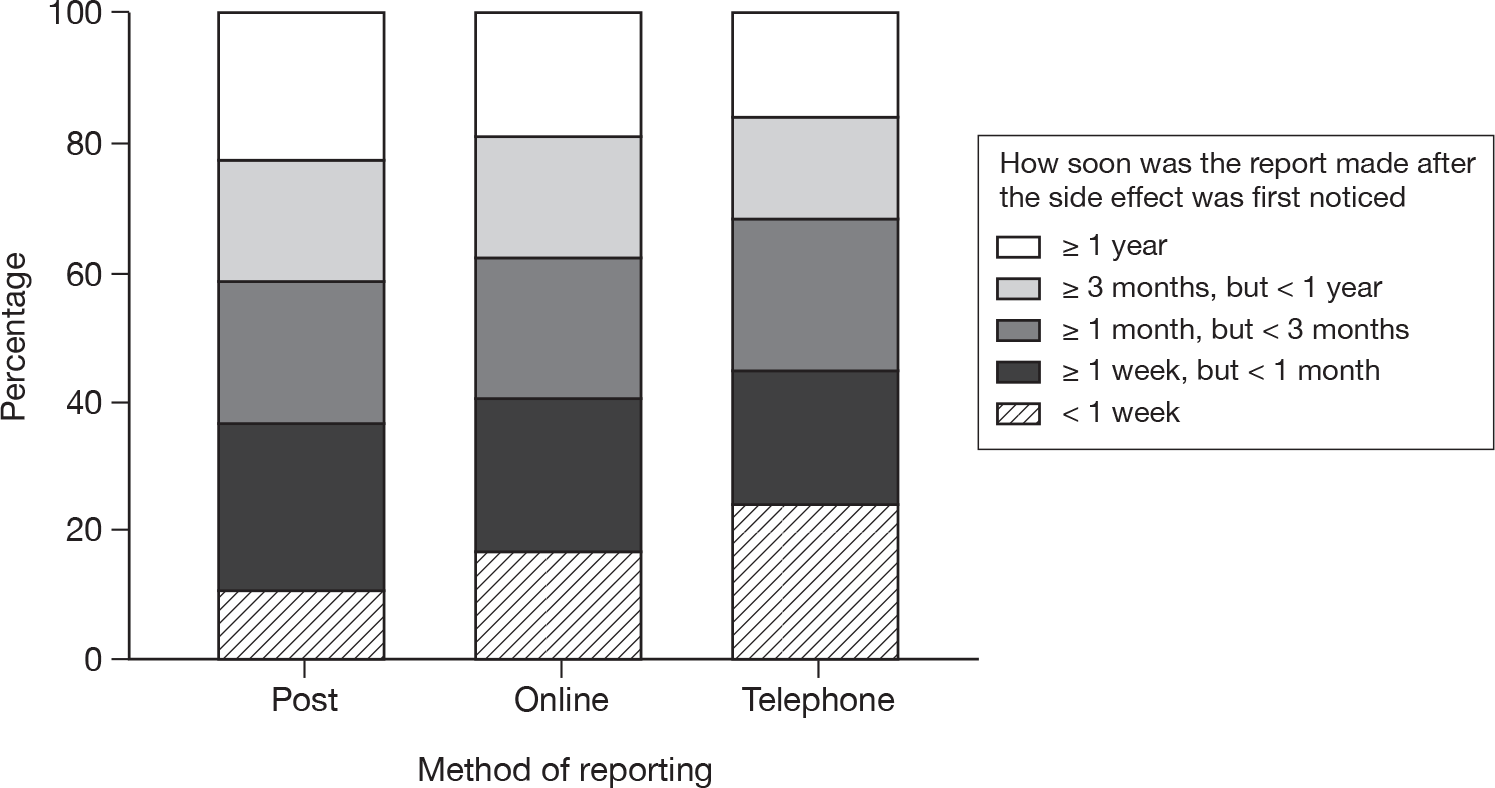
Respondents’ views on submitting Yellow Card reports
Most respondents – 1274 (93.6%) – thought that the report was fairly or very easy to complete (Table 28), but in free-text comments 216 (15.9%) noted difficulties that they had experienced. A higher proportion of telephone reporters (6.8%) felt that it was ‘not very easy’ to report their ADR compared with those using other methods of reporting [online (3.4%); post (2.5%)] (p < 0.001).
| Ease of completing the report | Method of reporting | All (n = 1362) | ||
|---|---|---|---|---|
| Post (n = 814) | Online (n = 447) | Telephone (n = 86) | ||
| Very easy | 577 (72.7) | 245 (54.9) | 44 (59.5) | 874 (64.2) |
| Fairly easy | 187 (23.6) | 184 (41.3) | 25 (33.8) | 400 (29.4) |
| Not very easy | 20 (2.5) | 15 (3.4) | 5 (6.8) | 40 (2.9) |
| Very difficult | 10 (1.3) | 2 (0.4) | 0 (0.0) | 12 (0.9) |
| Total | 794 (100) | 446 (100) | 74 (100) | 1326 (100) |
Suggestions for improvements to the form or processes were made by 307 (22.5%) respondents. These included more space (n = 31), simpler questions or language (n = 18) and larger print (n = 7). Respondents who reported difficulties, included 39 who had general difficulties owing to problems with writing/poor sight, anxiety or confusion, 15 who indicated uncertainties about what to report and 10 who felt that some questions were missing or lacked relevant options. Several mentioned the inability to use imperial units for height and weight online.
Not much room on the form, not very well set out. I found it difficult the second time to fill in the form because I was suffering from severe anxiety which was the problem.
Female, 60 years, further educational qualification
There were 39 respondents who raised specific issues about internet reporting, many of whom gave up and reported via telephone or paper:
The proforma asked for the major symptoms. When I wrote paralysis, a long list of types of paralysis appeared – it needed medical knowledge to complete. I could not move past it. I gave up and sent a written report direct to your enquiry desk.
Male, 54 years, graduate
Seven raised issues relating to telephone reporting:
I first telephoned 08081 003 352 to be told by recorded message calls could only be taken between 10–2 Mon–Fri. The hours need to be extended. The Yellow Card says you can report suspected side effects by filling in Yellow form or by calling which I did first, but the person only asked for my name and address in order to send the Yellow Card and did not ask would I like to give details over the phone.
Female, 71 years, graduate
A total of 143 respondents suggested that greater publicity of the scheme, wider availability and accessibility of the reporting forms and encouragement to report were required. Twenty-three respondents indicated that health professionals lacked awareness of the patient reporting scheme, but should promote patient reporting. A substantial number suggested posters and leaflets should be available in GP surgeries, hospitals and pharmacies. Other suggestions included forms in post offices, libraries and local government offices or their issue with medicines:
My GP and receptionist did not know how to access a Yellow form! The hospital told me that a GP had to do it.
Female, 65 years, further educational qualification
A description of the scheme with the MHRA website should be (by law if necessary) included in the manufacturers’ information contained in the medicine packages.
Male, 69 years, graduate
Why the respondent made a Yellow Card report
Most respondents (n = 1098; 80.6%) said that making the report had been their own idea, with a significantly higher proportion of the online completers (86.4%; n = 386) stating this than postal (78.1%; n = 636) or telephone (77.9%; n = 67) reporters (p = 0.001). Among those reporting by telephone, 19.8% (n = 17) said that it was their pharmacist’s idea to report the event, compared with 17.4% (n = 142) of postal reporters and 6.9% (n = 31) of online reporters (p < 0.001). Only 43 (3.2%) respondents were discouraged by someone from making a report. Of these, most were discouraged by their GP (n = 26; 60.5%). Fifty-six respondents (4.1%) stated that a HCP had refused to make a report on their behalf.
Respondent experiences and expectations of reporting
Some respondents (n = 104; 7.6%) had received help to make the report, with most assistance coming from a family member or friend (n = 56) or a pharmacist (n = 26). Almost one-third (n = 448; 32.9%) of respondents expected feedback from the MHRA and 828 (60.8%) said that they would have liked feedback. Of those reporting their suspected ADR by telephone, 42.7% (n = 35) expected feedback compared with 36.0% (n = 290) of postal reporters (p = 0.001) and 26.2% (n = 117) of online reporters. A higher proportion of telephone reporters (74.3%; n = 55) would have liked feedback than other reporters [postal 67.3% (n = 495) and online 63.0% (n = 269)], although this difference was not statistically significant (p = 0.05).
Responses to open questions indicated that 134 respondents expected an acknowledgement of their report and 112 expected to receive information about the reaction or the medicine about which they had reported. Many of those who desired feedback (323/707) wanted specific information about the frequency with which other similar reports had been received, how common the effect was or whether it was a well-known problem. However, 149 wanted to know whether any investigation or action would take place as a result of their report, including informing manufacturers or health professionals:
Would like to know how often this particular problem has been reported in the past and how well known the side effect is within the medical profession.
Male, 53 years, postgraduate
Tell me what you are doing with the information I give you and any action you plan to take to prevent others suffering as I did.
Male, 33 years, graduate
A minority (n = 24) had expected to be approached for further information or provided with advice on what action to take (n = 25); more (n = 84) wanted to receive such advice. There were 46 respondents who wanted confirmation that the medicine and symptom were causally associated and 27 expected to receive this.
Almost all respondents (n = 1302; 95.6%) would make a report again if they, or someone they knew, had a suspected ADR. A similar number would encourage other people to report through the YCS (n = 1300; 95.4%).
The most frequently cited reasons for reporting again related to the need for collection of data on medicines (97 respondents), to help others (n = 57) or to improve the quality of medicines, medicines information or care (n = 51). The high prevalence of ADRs, the distress caused and the need to record patients’ experiences were also mentioned:
I think it is important to have reports from patients themselves as part of the post-marketing surveillance. Patients’ experiences are very important.
Female, 32 years, graduate
Some comments (n = 25) explained reasons for respondents not reporting again, including the time taken to make the report, the lack of feedback received and the perception that their report has little impact:
But think some feedback should be given, otherwise what’s the point? How would I know if it was making any difference?
Female, 50 years, further educational qualification
Although 23 respondents stated that they definitely would or indeed already had encouraged others to report, 22 provided reasons for not encouraging others and a further 34 indicated conditional encouragement only:
I wouldn’t want people to report unless they feel strongly that the product caused the effect, because it could reduce the value of these reports.
Female, 26 years, graduate
General views on Yellow Card reporting
An open question ‘Why do you think it is important for people to report side effects from medicines using the YCS?’ elicited a total of 1802 specific comments from 1238 respondents, although 103 were judged to be unrelated to the question. The view most frequently expressed (by 355 respondents) illustrated an awareness of the purpose and importance of the scheme as a means of gathering data from a large population taking medicines. Respondents also cited the need to prevent others from suffering similar problems (n = 165) and to make the public or other patients aware of side effects from medicines (n = 152):
Might be small number of people scattered over country or something you think people are aware of but aren’t. Is good for someone to collate this info together to be able to see the whole picture and spot major problems with a particular medicine or any trends to stop others from suffering or make sure people are aware before they take them.
Female, 34 years, left school at 16 years
Others felt it was important to make MHRA or manufacturers aware of ADRs (n = 100), with many respondents expressing the hope that as a result medicines may be improved or PILs amended (n = 173) or products withdrawn from the market if necessary (n = 41):
This should ensure that drug companies are made aware of severe side effects so that they can issue appropriate warnings. They should also carry out further research to reduce the risks of side effects.
Female, 65 years, higher educational qualification
The view that health professionals need to be informed about ADRs and perhaps to change their practice was raised by 167 respondents. Others criticised government incentives to prescribe certain drugs and a number of respondents expressed distrust in the pharmaceutical industry, as well as in health care and government (n = 79):
Absolutely necessary to act as backup to clinical trials, plus acting as a brake on government instructions to issue pills on a one-size-fits-all basis, i.e. how many blood pressure and cholesterol tablets are issued to borderline cases in order to meet targets and gain bonuses?
Male, 72 years, left school at 16 years
For too long there does not seem to have been any method the general public can input side effects from their medication. It also means (hopefully) things can be collated, monitored, and fixed in shorter period of time.
Female, 38 years, higher educational qualification
The attitude of health professionals to suspected ADRs was raised by 106 respondents, who indicated both dismissive attitudes among HCPs and their failure to report ADRs. The need for a reporting mechanism which is independent of health professionals (n = 32) and for patients’ voices to be heard were also identified as issues (n = 49). A further 90 comments related to the importance of the patient’s perspective, indicating that side effects were, or could be, severe; may be perceived differently by patients or may be worse than the underlying medical problem:
Because I feel if I told my doctor about my side effect, he would belittle it and I would wrongly feel I was making too much of a fuss, whereas the reality of my experience of the side effect was not wrong or little. I feel scared of my GP’s response. Being able to report side effects effectively anonymously allows side effects to be known by pharmacologists, which would otherwise remain unreported or lost in a GP’s database somewhere.
Female, 43 years, graduate
So the MHRA have independent information about prescribed drugs. Sometimes GPs may not believe patients reporting side effects. It helps patients recover from side effects ‘getting it off your chest’. Patients hope that someone might take notice/make amends on patients’ suffering. People need to know they have a voice.
Female, 58 years, postgraduate
What doctors and nurses regard as a side effect worth reporting may differ from what the patient thinks. My mother has had a life changing experience that came out of the blue and is keen to ensure others don’t experience the same problem.
Female, 57 years, postgraduate
Some comments related to the need for research data on medicines, whereas others hoped that by drawing attention to problems, novel research would be initiated (n = 66). A wide range of other comments were received (n = 116), covering issues such as side effects arising from drug combinations or changes in brand, suggestions of ‘fake’ products, mistakes in production, or reactions to excipients. Criticisms of both health care and licensing were also raised:
Different (as far as I can tell) manufacturers may have different recipes. One may be okay and another not. I don’t know.
Female, 80 years, left school at 16 years
Because too many doctors prescribe inappropriately bowing to pressure from reps, patients and health authorities. In addition, a number of pharma companies tend not to look for AE data and so medicines are licensed on the basis of too small a safety database. Real life experience is the only way to build the database.
Male, 49 years, postgraduate
I think it is too easy for HCPs to go for the ‘quick fix’ if they know that a particular medication will ease a problem, but they don’t follow up often on repeat prescriptions and don’t really view subsequent problems holistically so can miss links to other reported problems. I see older people in relation to my work, they often have repeat prescriptions with many items on and some items are to deal with side effects of other meds. GPs don’t have time to follow through. I think we, as patients, should do more to raise awareness of these issues.
Female, 53 years, higher educational qualification
Attribution of symptoms to medicines
The questionnaire also explored respondents’ views about what made them think that the medicine caused the side effect. There were 1348 responses to this question, 99% of all respondents. Of these, 181 (13.3%) were not analysable as 78 listed only symptoms and 103 were comments on other issues or inadequate descriptions. Of the remaining 1167 respondents, most (689; 59.0%) provided only one piece of information to explain causal association. However, 382 (32.7%) respondents provided two pieces of information, whereas 87 (7.5%) provided three and nine respondents (0.8%) provided four.
Among these 1167 responses, 675 (57.2%) mentioned reasons relating to timing only. A further 238 (20.2%) indicated using information sources and 145 (12.3%) mentioned both. Explanations included: symptom not present before medicine started (n = 195, 16.7%), symptom began soon after starting medicine (n = 320: 27.4%), symptom reduced on stopping (n = 275, 23.6%) and symptom re-occurred on re-challenge (n = 89, 7.6%). A further 37 noted symptom changes with dose changes, 25 noted symptoms started after medicine withdrawal and 29 on changing brand. In addition, 22 indicated it was a new medicine and 78 cited other timing-related issues:
Never had it before. Didn’t have it after stopped. Was worse on higher doses. Was better on lower doses.
Male, 32 years, graduate
I had been taking Pfizer’s Istin (amlodipine) for over six months and when I suddenly could not find it anywhere I was forced to take a generic version. Within half an hour of taking this I could feel symptoms which I had never experienced with Pfizers Istin. I reported them immediately to the doctor.
Female, 63 years, higher educational qualification
The most common sources of information used to associate the symptom with the medicine were HCP (n = 186; 15.9%), and information from the internet, information leaflet or books (n = 182; 15.6%). A further 57 (4.9%) indicated other information sources:
Read about possible side effects on internet – commencement of taking medication coincided with my own medical problems. This was backed up by consultant.
Female, 65 years, postgraduate
The onset was a short time (few weeks) after commencing the drug. I looked it up in the literature and consulted several clinical friends.
Male, 77 years, postgraduate
In addition, 33 indicated previous reactions to the same or similar drugs, while 25 mentioned they or their doctor had seen similar effects in other people. A substantial number (n = 130) also felt that, for various reasons, no other explanation for their reaction was possible, including elimination of other possible causes. Other reasons/views were given by 27 respondents, including the severity of the event, interactions, specific excipients and confirmation by tests:
Only medication at the time. Several of my friends on this drug have had similar side effects.
Female, 71 years, higher educational qualification
I know that I’m allergic to E104 and discovered after suffering severe migraines that this colouring was in some of my medication.
Female, 47 years, graduate
Summary
-
A questionnaire was developed for distribution by the MHRA to all patients reporting through the YCS between March 2008 and January 2009. The questionnaire elicited information on how patients found out about the YCS, their experiences of, and views on, reporting and their demographic characteristics.
-
There were 1362 evaluable responses to the questionnaire sent to 2008 patient reporters (68%). The most frequent reporting method was postal (59.8%), followed by online (32.8%) and telephone (6.3%). Online reporters were younger (median age in years of reporters: online 50, postal 61, telephone 63; p < 0.001) with a higher education level than those using other reporting methods (e.g. proportion of reporters with a degree: online 48%, postal 28%, telephone 32%; p < 0.001). Almost one-half learned about the YCS from a pharmacy (n = 667; 49.0%). In response to a closed question, most respondents 1274 (93%) indicated that the report was ‘fairly’ or ‘very’ easy to complete, although in free-text comments 216 (15.9%) noted difficulties that they had experienced. Suggestions for enhancements were made by 307 (22.5%). One-third (n = 448; 32.9%) expected feedback from the MHRA on their report and 828 (60.8%) would have liked feedback. Almost all respondents (n = 1302; 95.6%) would report again. Respondents indicated a need for increasing health professionals’ awareness of patient reporting. Some stressed the importance of having a reporting mechanism that is independent of health professionals so that patients’ perspectives can be recognised.
Chapter 8 Study 6: telephone interview follow-up of patients reporting to the Yellow Card Scheme
Objective
To explore, in detail, the experiences and views of patients who have made reports to the YCS.
Methods
Semistructured telephone interviews were conducted with a theoretical sample of patients that was purposively selected from those who had completed questionnaires and had indicated a willingness to be approached for a telephone interview (Chapter 7). We aimed to interview around 30 people in order to gain a wide range of opinions and maximum variation sampling was used to do this in order to obtain a wide range of opinions. Factors that were taken into account in the sampling included: age, gender and educational attainment of patients, and the mode of reporting. In addition, we selected some patients based on issues raised in the questionnaire, such as the perceived ease of reporting. An iterative process was applied as we conducted the interviews to try to ensure that we interviewed people with different perspectives based upon the preliminary analysis and emergent themes.
Conduct and analysis of interviews
The semistructured telephone interviews were conducted by two researchers using an interview guide (Appendix 19) developed by the research team. The first three interviews were performed by one researcher (AG) and the remaining 24 by a second researcher (TP). The development of the guide was informed by a preliminary analysis of the questionnaire data and issues identified by the project team including:
-
exploration of any difficulties in making Yellow Card reports and suggestions for improvement in the reporting system
-
patients’ motivations for making the report and anticipated contribution of their report
-
patients’ expectations about what would happen to their report
-
patients’ satisfaction or dissatisfaction with the process of making a report
-
patients’ willingness to report in future.
The areas to be explored were reviewed and revised in the light of the data obtained from the initial interviews to ensure that the data obtained were relevant to the focus of the research. For example, in the early interviews respondents tended to focus on the story of their ADR rather than their experience of reporting it. So, in subsequent interviews we shifted the focus away from the reaction towards the reporting scheme. Interviews were audio-recorded digitally, with consent, and transcribed verbatim. The interview transcripts were analysed by an academic member of the research team (CA) and the analysis checked by another academic (EM). Data were analysed for both anticipated (based on the questions asked) and emergent themes using the constant comparison method. The researcher first read the interviews and noted the main themes. The data were then categorised into the major themes, which were finding out about reporting, recognition of adverse effects, reason for reporting, involvement of others in decision to report, how patient reports might differ from health professional reports, ease of reporting, what people expected to happen following making their report, advertising the scheme, and reporting again and encouraging others to report. The researcher then identified from the data a number of subcategories for each of these themes. For example, under the major theme of ‘reasons for reporting’ a number of subcategories emerged from the interview data, including altruism, solidarity and pharmacovigilance. Quotes are used to illustrate the themes, and for each quote the following information is provided: interview number, gender and age of reporter and mode of reporting, for example ‘interview 12, female 54 years, paper’.
Results
Twenty-seven telephone interviews were carried out; 23 interviewees had originally completed paper reports, two internet and two telephone reports. Two reports were done by parents on behalf of their children. A summary of the characteristics of the interviewees is shown in Table 29 and more detailed information on each interviewee is shown in Table 30. All of the interviewees were of white-British ethnic origin.
| Characteristics | n (%) or median (IQR) |
|---|---|
| Gender of patient, n (%) | |
| Female | 19 (70) |
| Male | 8 (30) |
| Educational attainment of patient, n (%) | |
| Finished education at 15 years | 2 (7) |
| Finished education at 16 years | 5 (19) |
| Finished education at 18 years | 1 (4) |
| Further education | 9 (33) |
| Graduate | 3 (11) |
| Postgraduate | 3 (11) |
| Not stated | 4 (15) |
| Age of intervieweea | |
| Age (years): median (IQR) | 60 (49 to 69) |
| Interview no. | Gender | Age (years) | Educational attainment |
|---|---|---|---|
| 1 | Female | 74 | Not stated |
| 2 | Female | 72 | Not stated |
| 3 | Female | 30 (report on behalf of daughter aged 5) | Not stated |
| 4 | Female | 69 | Graduate |
| 5 | Female | 52 | Finished education at 16 years |
| 6 | Female | 60 | Finished education at 16 years |
| 7 | Female | 48 | Postgraduate |
| 8 | Female | 66 | Finished education at 16 years |
| 9 | Female | 47 | Finished education at 16 years |
| 10 | Female | 75 | Further education |
| 11 | Female | 57 | Further education |
| 12 | Female | 54 | Further education |
| 13 | Female | 32 | Finished education at 16 years |
| 14 | Female | 52 | Graduate |
| 15 | Female | 43 | Further education |
| 16 | Female | 60 | Further education |
| 17 | Male | 49 | Not stated |
| 18 | Female | 50 | Graduate |
| 19 | Female | 71 | Finished education at 18 years |
| 20 | Female | 36 | Further education |
| 21 | Male | 72 | Further education |
| 22 | Male | 52 (on behalf of son) | Postgraduate |
| 23 | Male | 61 | Further education |
| 24 | Male | 66 | Finished education at 15 years |
| 25 | Male | 64 | Finished education at 15 years |
| 26 | Male | 73 | Further education |
| 27 | Male | 61 | Postgraduate |
Finding out about reporting
For the majority of people, awareness of the YCS was by chance either in the pharmacy, GP surgery or on the internet. One woman had found out about the YCS because her husband was a member of the Stroke Association and one from her diabetic nurse. Two interviewees already knew about the scheme because they were HCPs. Fifteen of the interviewees had found out about the scheme via the pharmacy because the cards were on display, they saw a poster or because pharmacy staff were proactive in encouraging people to report:
I think I was given that form in the chemist … No, well I’ve never been given cards before. She just said, ‘These forms now are given to people if certain tablets have not agreed with them. If you wish, fill it in’.
Interview 8, female, 66 years, paper
… I went and saw the doctor … and she referred me to a pharmacist. That’s when he said, ‘Well we can put you on a different variety and you can put in a Yellow Card Report’, which I’d never heard of at the time.
Interview 27, male, 61 years, internet
Others or their partners had seen the forms in the pharmacy:
And then I saw them, they were on the counter in [name and location of pharmacy] so I picked one up and thought well the reaction to this thing was so upsetting.
Interview 10, female, 75 years, paper
No, it was my wife that saw the card in the chemist and she brought one home for me.
Interview 22, male, 52 years, paper
Two interviewees had seen the scheme advertised on a poster in a community pharmacy. Others had found the forms while waiting at their GP surgery. However, in contrast with some of the interviewees’ experiences of a proactive approach in community pharmacies, there was no sense that GPs or their staff were playing an active part in facilitating reporting.
I, being the sort of person who, when I’ve been having difficulty sitting round in, down in surgery you know, I tend to stay on my feet and prowl, so I read everything on the wall that’s going er and go through the rack of whatever else is available and this is where I picked up the Yellow Card information on one occasion.
Interview 4, female, 69 years, paper
I had changed doctors and they have a load of those forms sitting there, I’ve never seen them before. So I just automatically picked a couple up and brought them home and read through them and just filled them in.
Interview 5, female, 52 years, paper
Others had found or ‘stumbled’ across the web-based form while looking on the internet:
It was actually, I think it was at the time when he was being poorly with it all, I think I just Googled the drug name and I don’t, I think there might have been some tag to your page but I’m sure that’s how I found out about the YCS anyhow … I kind of stumbled across it and I’ve never seen any leaflets in a surgery
Interview 13, female, 32 years, internet
One interviewee heard about the YCS on the Radio 4 Today programme and had then obtained a Yellow Card form from a pharmacy:
Well I didn’t know there was such a thing as a Yellow Card. But then I heard a little, er a little bit on Radio 4, er in the Today programme it mentioned this Yellow Card and said you could get it in a chemists. And that is the first I heard of it. Yes, I went and got it from the chemists …Well they were out on display but not very visible so I had to ask. And then it was produced.
Interview 2, female, 72 years, paper
Another interviewee found out about reporting to the MHRA via a television programme:
It was a programme on the television about patients in hospital and this sort of thing and they had discovered through you know this information, that 300,000 people died last year from prescribed drugs and that didn’t include people who suffered damaging side effects, which damaged their internal organs in some way or another. Well when I saw this, at the end of the programme it said that if you think you have had a problem with drugs why not get in touch with the MHRA and it gave an address. So this is how I found out about it.
Interview 1, female, 74 years, paper
Recognition of adverse effects
Most interviewees were clearly able to link the ADR with taking a particular medicine, including making temporal links:
I took this one capsule of the antibiotic and er I was talking to a friend on the phone and er within half an hour my tongue started to swell and I had to ring off because I couldn’t speak … and then I had mucous streaming from my nose and mouth. Oh it was awful, I felt sick and broke out into a rash all over my body which itched and felt hot.
Interview 2, female, 72 years, paper
… that was the Friday evening then on the Saturday morning I started to itch and noticed that I had a rash that was fairly extensive, pretty well covered in a very fine rash and didn’t feel very well at all and in the evening I just went to bed early. I didn’t take any more of the medication, I only had the one dose I think and it was because of the rash and feeling really sick and I think I felt very cold, I was very shivery.
Interview 11, female, 57 years, paper
For another reporter’s daughter, Stevens–Johnson syndrome started within 12 hours of starting penicillin.
Patients also noted a ‘dose response’. For someone who had taken pregabalin for pain, the reaction started the next morning after taking the first dose and became worse on the second dose. This patient recalled a previous similar experience:
Researcher: So how long had you been taking your medication before you realised that there was a problem?
Interviewee: Oh gosh erm, er a matter of days. Erm I sh’ it should be on the form.
Interviewee: Yes, it appeared following the morning after taking the dose the night before?
Interviewee: That’s right. Yeah.
Researcher: And then got worse after the second dose.
Interviewee: That’s right yeah because I’d had a similar reaction on, on meloxicam.
Researcher: Right so you phoned your GP who told you to …
Interviewee: Stop, stop!
Interviewee: within 24 hours I was feeling a lot better.
Interview 4, female, 69 years, paper
An interviewee who had been encouraged to fill in the report by a pharmacist at the store where she worked had been taking azathioprine for a flare-up of Crohn’s disease for 2 weeks when she started feeling sick. The doctor had asked her to take the azathioprine again as a re-challenge to ensure it was causing the ADR:
So I started taking that and about maybe two weeks into taking it and I started feeling sick. Reading on the instructions it just said that it can cause nausea but these are sort of like, when you read these instructions it’s maybe, you know, one in however many people will have these … Yes, so I started feeling sick as I say and I thought right, that will be fine, I’ll just sort of ride through it and it just got worse until in the end I was being sick, violently sick. So I went back to my GP rather than the hospital because it was just every so often that I went to the hospital and he said, ‘Right let’s stop taking it.’ So I stopped taking it on a particular Wednesday and then he said, ‘Leave it a full week and then start taking the drug again and then we’ll know whether it’s that that’s actually making you be sick’. So I started taking it on the Wednesday morning and I remember taking it at half past 8 just before going into work and by 9 o’clock I was being violently sick again and I was being sick about every 5 minutes.
Interview 9, female, 47 years, paper
Another man had been taking a prostate medication and had experienced recurrence of symptoms on re-challenge:
I think it was one day, two days and it was most pronounced, the symptom was pronounced and definite and I was very surprised. Stopped taking it for I think a few days, repeated it and got exactly the same results much to my amazement. It was more pronounced, most definite and repeatable.
Interview 23, male, 61 years, paper
One man was convinced that his tablets for hypertension were causing the problem as all his adverse effects stopped when he ran out of tablets while on holiday, only to come back when he started to take extra tablets:
Well I had no problems up to start of taking the tablets and when I went abroad and ran out of the tablets everything returned to normal … But it was only when I came back, started retaking the tablets that the symptoms started to reappear and when I started taking the extra tablets the symptoms, they got extremely bad.
Interview 24, male, 66 years, paper
Why interviewees had made the report
Altruistic reporting
A number of interviewees had made their report because they thought that it might prevent the adverse effect from happening to someone else in the future:
It might help and it might save somebody going through the same symptoms because they’re so horrible.
Interview 4, female, 69 years, paper
I just thought he’d been in so much pain and I felt so frustrated because no-one had listened or no-one seemed to consider that it could be that and I just thought well it’s got to be logged for future because no doubt someone else in the country or the world will probably experience the same thing and … But I just thought, no I don’t want to see, or don’t want anyone else to be suffering like he did when there’s no need because if someone’s made aware of it then I just think it’s better to make sure that everyone is aware so if it does happen.
Interview 13, female, 32 years, internet
There was a very strong sense of responsibility and duty that led some of the interviewees to report:
… it wasn’t making any difference to me one way or the other but I felt I had a duty.
Interview 13, female, 32 years, internet
Well I would really expect it to be sort of 50/50 as far as responsibility goes. Yes I do think that the GP or the pharmacist or whoever might just have a duty of care to report side effects like this in all instances. I also think that the people who, ie myself, who are swallowing all these nasty concoctions have got a duty to report back any untoward effects as well.
Interview 12, female, 54 years, paper
Others reported as they thought that as well as helping other people it would make the pharmaceutical manufacturer aware of the adverse effect:
And I did it because I thought well if this has happened to me there’s a good chance it’s going to happen to somebody else and I felt the company should be aware …
Interview 23, male, 61 years, paper
Solidarity and a desire for personal feedback
Some of the interviewees were keen to find out if other people had experienced the same thing and to find other people with the same problem. There was a strong sense that these interviewees had a different motive in reporting to that of others; they wanted something back. They also wanted to be reassured that they were not the only one who had suffered and wished to know how to alleviate their problem:
… thought well the reaction to this thing was so upsetting...that I felt perhaps it was worth sending it off just in case other people had experienced the same sort of thing … I had felt so unwell taking it that I just felt it justified giving a report. A degree of curiosity to know whether was I being neurotic or was it something that other people have experienced.
Interview 10, female, 75 years, paper
Well I just wanted, I felt as though it could be another outlet for easing this problem and also if other people had had the same problems they might have reported it and somebody somewhere might know what I can do to alleviate the problem.
Interview 24, male, 66 years, paper
Pharmacovigilance
Some of the interviewees showed some understanding of the pharmacovigilance process and had reported to help with this process:
Hopefully it can highlight more causes and erm more alerts for parents … Gathering information.
Interview 3, female, 30 years, telephone
Mainly I hoped that it would, as I say, when the doctors look in the symptoms book in the future that if somebody came to them with similar side effects that we were having that they could actually see it straightaway because I think sometimes if it’s something that’s not in the book they just completely dismiss what you’re saying.
Interview 13, female, 32 years, internet
The following woman was against being ‘force fed’ with drugs and felt that if a lot of reports were coming through then the drugs should be taken off the market:
Because I mean all these things are force fed us as panaceas for this and that and the other and yes some of them are life savers, I’m not disputing that. What I am saying is that with some people the side effects are far, far worse than the actual illness that they may be experiencing at the time and I don’t think these drugs, if you’re getting a lot of reports about a certain drug it should be removed.
Interview 12, female, 54 years, paper
Involvement of others in decisions to report adverse drug reactions
A number of interviewees had been encouraged to report by their pharmacist. One woman who had been taking statins for 6 months was discussing her thinning hair with her hairdresser who asked her if she was taking any medicines. She then went to her pharmacist who confirmed the hairdressers’ diagnosis. This is the only example in our sample of a layperson identifying an ADR:
She said, ‘Are you on any new medications?’ But of course that was something I hadn’t considered and I told her about the statins and she said, ‘That’s funny’, and this girl that does my hair, she said, ‘My mum stopped taking statins for the same reason because she thought her hair was falling out’. So I filled it in. It was my pharmacist when I went to get a repeat prescription and she said, ‘Oh simvastatin’, and I said, ‘It’s making my hair fall out’. And she said, ‘Oh you should report that’, she said, you know, ‘they’re keen to know of any contra-indications sort of thing.’
Interview 7, female, 48 years, paper
Others said that they made their report because they did not think that they could rely on their GP to make a report on their behalf or could not rely on it being accurate. These interviewees felt that their report would compensate for their GP’s lack of commitment to reporting:
Yes. Because I’ve used it before. I’d reported pregabalin and meloxicam both of them I’d reacted badly to. I thought well if people don’t report you don’t know erm and I couldn’t rely on GP reporting.
Interview 4, female, 69 years, paper
If it had just been going to the doctor and reporting it I wouldn’t have gone to the doctor and reported it but the fact that this card was available, I filled it in. I gather from what I’ve read that a lot of GPs don’t bother reporting side effects anyway. I mean that’s just hearsay, I mean that’s what I’ve read but I don’t know if it’s true or not. Maybe that was the case 5 years ago, maybe it isn’t now.
Interview 23, male, 61 years, paper
How patient reports might differ from health professional reports
Three interviewees commented about how a patient report might differ from a HCP report, compensating for the perceived shortcomings of health professional reporting:
I think by the time it’s filtered through, through various other people it doesn’t actually go in the form in which the patient actually reports it … to, to make mine fairly specific erm and it was fairly specific and quite, and fairly detailed but I’m sure that anybody else would simply, a lot of health professionals would simply specify some general adverse symptoms and that would be it.
Interview 4, female, 69 years, paper
I think it is important that people do get the opportunity to report their own understanding of a problem which may be different from a professional’s understanding of a problem. And that, you know, some people just prefer to do things themselves than to have to go through an official course, you know, to have to go to the doctors and ask them to report it or, I think if people knew the reporting system was there and available more then they would use it.
Interview 11, female, 57 years, paper
I think it’s far better than reporting it to your GP because, you know, you have more time to sit and think about it and it doesn’t get filtered in the process if you know what I mean, you know what actually happened because you get so little time with the GP, you wouldn’t have time to think it through and put all the exact details down.
Interview 23, male, 61 years, paper
Ease of reporting
Although some of those interviewed found it straightforward to report, a number of difficulties were mentioned including lack of space on the paper form. Further details are not given here, as the issues concerning ease of reporting are covered more fully in Chapters 7 and 9.
What interviewees expected to happen after making their report
Eight interviewees had not expected a response to their report, although were pleased when they received one:
Nothing. I expected absolutely nothing and I was very surprised when I was informed that they’d received it and it had been noted and I thought well this is progress, most of the time things that you send off to official bodies just sort of disappear into the ether and you never hear about them again. So I was quite chuffed to hear back, feedback was the word I was looking for and yeah that was fine.
Interview 12, female, 54 years, paper
Well I did get a letter thanking me for the card, which I didn’t expect and that was nice because it made me feel that actually someone is taking notice there.
Interview 15, female, 43 years, paper
Ten interviewees said that they had expected to receive an acknowledgement and or some action:
Well I don’t know, I suppose maybe an acknowledgement that somebody had received it but nothing particularly further than that other than it might be of some use to somebody who was monitoring different medications.
Interview 11, female, 57, paper
Others showed some understanding of the pharmacovigilance process, but were cynical as to whether anything would actually change as a result of their report:
Then I expected them to collect them and if there were more than a certain number of people report the side effect then it would get flagged up as a common one in the NHS or, I don’t know whether anything actually goes back to the manufacturers or if there are any studies that might come out of, you know, even if there might be something new that might put up a red flag, they might think well maybe we should look at this and find out what’s happening there and instigate some further research. That would be my hope but I tend to think that once something’s licensed and they’re making money then they’re not really interested in going back and doing further research
Interview 15, female, 43 years, paper
What was I expecting to happen? Very little, I suppose I expected, I hoped there’d be an acknowledgement because you’re never sure when you send something off it’s going to get there but I didn’t expect any benefit at all or anything to happen to me, I was just reporting what happened for some database somewhere really. I felt I wanted to be sure someone actually got it as an input.
Interview 23, male, 61 years, paper
One had not heard anything and felt ignored:
I take it from the fact that I’ve heard nothing is that I’m a maverick and they’ve ignored it, don’t worry about it, it’s not a significant problem so let’s not worry about it.
Interview 25, male, 64 years, paper
Advertising the Yellow Card Scheme
There was a general feeling among the interviewees that people ought to be aware of the scheme and not find out about it by chance, like they had. There was also a feeling that people should be encouraged to report as some people thought that they might not be believed:
… but one thing I do want to say is could the MHRA advertise more? The more they advertise it, the more people will be aware and the less people will suffer side effects.
Interview 1, female, 74 years, paper
Well I think the Yellow Card could be advertised better so people know what’s going on. I only heard about it by chance from the radio.
Interview 2, female, 72 years, paper
Well I’m not sure that the little Yellow Card is the best way of bringing it to people’s attention. I think we’ve got some in our surgery but I think on the whole people are reluctant to report any side effects just in case they’re not believed.
Interview 10, male, 75 years, paper
The interviewees had a number of ideas as to how the scheme might be better advertised to the public. Interviewees suggested putting information on or in the pharmacy bag or on the medicine label:
Another thing I think you could do, you know on your pharmacy bag, you get another bag to put your tablets in, it could even be printed on the paper bags they put your medication in … Or even put a Yellow Card in with your medication to just say if these tablets have unforeseen, upset you in any way after two or three days, get back in touch with your doctor and tell them that you’re going to fill this form in or go back into the chemist and say, ‘Right they’re not agreeing with me, I’ve been in touch with the doctor, I want to fill this form in.’
Interview 8, female, 66 years, paper
In fact I would go so far as to say that in every single box of medication … there should, or information about the Yellow Card.
Interview 12, female, 54 years, paper
She also suggested running a television advertising campaign:
I mean why don’t they even do a TV ad. I bet a sort of TV ad when people are sat there in front of their tellies watching Coronation Street or something, I don’t know, look at everybody out there that takes medication, ‘Do you know there is a scheme that’s called the Yellow Card de da, de da, de da’ and make people aware that it’s out there.
Interview 12, female, 54 years, paper
Reporting again and encouraging others to report
The majority of interviewees were very positive about reporting again or encouraging other people to report ADRs:
Yes I think it’s a really good thing, I still think most people aren’t aware of it or really understand, I think lots of people who are on medication don’t even know what the YCS is or were aware of it even when it was just the GPs could fill them in…There were people I suggested it to.
Interview 15, female, 43 years, paper
A number of interviews had already actively promoted the YCS to others as a matter of course:
I run a shop in a village and I’ve heard of people who’ve had side effects and I’ve explained what the Yellow Card System is and I don’t know if it’s sinking in or not but it helps, it’s helpful. It’s not guaranteed that it helps for everything or they even fill it in.
Interview 22, male, 52 years, paper
And I run a support group in [place name] for thyroid patients, when they’re having problems I’ll always mention the Yellow Card to them as well.
Interview 21, male, 72 years, paper
Because I can tell you, I have been in touch, with friends, colleagues, from the different organisations I belong to. I do a lot of charity work, you know, and people in the village here you know and it is amazing how many people are on prescribed drugs which are giving them dreadful side effects. I am trying to persuade them to get in touch you know on the YCS. So their information is recorded and some sort of investigation can be carried out, you know.
Interview 1, female, 74 years, paper
Some saw the importance of reporting for pharmacovigilance:
Well yes, I mean I suppose each individual is probably different aren’t they, maybe what one person suffers maybe the other person doesn’t … But, you know, occasionally there’s a pattern maybe there’s a lot of people have had the same thing …
Interview 17, male, 49 years, paper
Yes unless the public know about it people are going to be worried. I think it’s important to report as many side effects as possible so that they can list them then when people take the drug they know what to expect and they don’t think it’s their health.
Interview 18, female, 50 years, paper
Summary
-
Semistructured telephone interviews were conducted with a purposeful sample of patient reporters selected from those who had completed questionnaires and given consent to further contact by the research team. The interviews explored motivations for reporting, expectations of, and satisfaction with, the reporting system and suggestions for improvements.
-
Twenty-seven telephone interviews were conducted with patient reporters. Most became aware of the YCS by chance and many suggested that greater publicity was needed for patient reporting. Motivations for reporting included altruism and a desire to find out if others had experienced similar problems. Several suggestions were made for enhancements to reporting systems, including more space for writing free-text comments on the paper form.
Chapter 9 Study 7: focus groups and usability testing with members of the public
Objectives
To ascertain the views of members of the public on the YCS.
To ascertain the views of members of the public on the user-friendliness, effectiveness and usability of different mechanisms of patient reporting.
To obtain suggestions for potential ways in which the reporting system could be improved.
Methods
Recruitment
We planned to recruit six focus groups with up to eight subjects in each. For logistical purposes participants were recruited from only the Nottingham area and recruitment took place between June and November 2008. Although we recruited from only one area of the country, we aimed to recruit a broad demographic sample of people. Originally, we had planned to include patients who believed they may have experienced side effects from medications, but had not previously filled in a Yellow Card report. We decided, however, that this would make recruitment difficult and that there would be some advantages to widening our selection criteria to all adults. For example, this allowed us to recruit younger people, who might not have had side effects from drugs, but who might be more familiar with the use of online reporting systems. Three main approaches were used to recruit participants with a range of demographic characteristics.
Recruitment posters and leaflets were displayed in general practices, pharmacies, social centres and in the University of Nottingham. In addition, an article about the research study was placed in the local press. Finally, the pool of simulated patients utilised by the University of Nottingham Medical School for the purposes of supporting medical student training were invited by letter. Those interested in participating in the research were invited to contact the research team directly by telephone. Also, the simulated patients had the option of returning a consent form in a stamped addressed envelope provided.
All of those volunteering to participate in the research were offered a choice of dates for attendance at the focus group/usability sessions. The dates were confirmed by letter. Sessions were run on different days of the week and times of the day, to enable participants to attend at a time of their convenience. Volunteers were all offered a £25 inconvenience allowance. Sessions were conducted at the University of Nottingham where computer facilities were available. Refreshments were offered prior to the commencement of the focus groups.
Focus groups
The topic schedule for the focus group was developed by the research team, informed by the literature and project objectives. It included the participants’ thoughts about the YCS, whether they felt they might utilise the scheme and how they felt the scheme might be improved. Finally, each participant was asked which method of reporting they felt that they would prefer to use. After completing the usability tests, participants were asked whether their views had changed.
A pilot focus group with members of the research team was conducted to confirm clarity and appropriateness of wording. As a result, the schedule was revised. The final version is shown in Appendix 20.
Before the focus group/usability session began participants were asked to complete a short questionnaire asking for basic demographic data and experience of using computers. These data were used to inform which of the usability tests participants were allocated to.
At the start of the focus group/usability session there was a 15-minute presentation, by a member of the research team, explaining the YCS and the research project. We felt it necessary to give a presentation to provide context for the focus group discussions and for the subsequent usability testing. At the end of the presentation, there was an opportunity for participants to ask questions. They were then asked to complete and sign a consent form to document their agreement to participate in the research and to the audio-recording of the focus group/usability session. The signed consent form was returned to the research team. Participants retained a duplicate copy. Once consent had been obtained from each participant, the focus group was conducted. Recordings of the focus group/usability sessions were transcribed verbatim. The focus group data were analysed thematically (by CA and AG), identifying the major predicted and emerging themes and ideas from the data.
Usability tests
Simulating the Yellow Card reporting scheme
Online reporting
In order to ensure that the online reporting system did not receive a number of ‘fake’ reports, the MHRA provided the team with access to a dummy system, identical to the real one, but which did not add reports to the database used for pharmacovigilance. The MRHA web address for the dummy system was used to ensure that the computers were logged onto the correct site and then left at the front page of the reporting system for participants.
Paper forms
Paper forms for reporting to the YCS were provided by the MHRA. These were the same as those available for members of the public through community pharmacies and GP surgeries.
Telephone reporting
The telephone reporting system at the MHRA is staffed between 10 am and 2 pm, Monday to Friday. As not all of the focus group/usability sessions were conducted during these times, the decision was made to use a member of the research team to replicate the telephone reporting system during the usability testing. In order to ensure that the participants’ experience of reporting using the telephone method was as realistic as possible, a pharmacist was used to answer the call, as the MHRA use science graduates (some of whom are pharmacists) to answer the telephone-reporting line. The MHRA provided their training materials for answering the telephone reporting line and these were used by our pharmacist to structure the telephone conversation. The research team pharmacist answered the usability test call and recorded notes of the conversation before completing a YCS paper report.
Adverse drug reaction scenarios
Six scenarios describing a patient experiencing an adverse effect to a medicine were developed by the researchers (Appendix 21). Each participant was given a randomly selected set of four scenarios from which they chose two for use when completing two YCS reporting methods during the usability tests. The use of scenarios avoided the need for participants to disclose confidential medical information, allowed those who had not experienced a side effect to participate and gave consistency to data collection. Scenarios were provided in large print for participants who required them.
The participants were asked to read the scenarios then complete a report. Participants were given an opportunity to ask questions relating to the scenarios, to help clarify any issues arising. Each participant was assigned to two of the three possible reporting methods, informed by the demographic information collected prior to the focus group to ensure that only those familiar with the internet were given this option. In each group a maximum of two people were assigned to the telephone reporting system; numbers were limited by staff availability.
Participants were asked to think aloud as they completed the reporting process, highlighting any issues encountered. There was no time limit placed for completion of the report, but participants were told that if they felt that they would have given up completing the form at home, they should do the same during the usability tests. As the participants completed their report they were observed by a researcher to whom they had been previously introduced.
Observers made field notes and asked questions for clarification, and reminded the participants to keep talking if they stopped verbalising their thoughts. To aid analysis of the data, each participant and observer pair was digitally audio-recorded throughout the process. At the end of the session, the observer asked their participant which of the reporting methods they would choose to use if they were to report an ADR.
The observers were debriefed and their observation notes were transcribed, these were supplemented by further information and verbatim quotes from the audio recording.
The data from the field notes were analysed thematically by identifying the major themes and ideas emerging from the data by reporting method (telephone, internet and paper). A constant comparison comparative approach was taken and this was supplemented with relevant information from the audio recordings (these were not transcribed). For example, the major theme ‘ease of navigation of online form’ was sub categorised into ‘challenging’ and ‘easy’. The ‘challenging’ theme subcategory was then further subdivided into, ‘drop-down menus’, ‘lack of understanding of medical terms’ and ‘saving data’. ‘Saving data’ was then further subcategorised into ‘losing information’ and ‘stopping reporting’.
Results
Characteristics of participants
Overall 40 participants took part in seven focus groups/usability sessions (22 recruited via the posters, 11 from the simulated patients and seven from the newspaper article). Table 31 shows the characteristics of participants.
| Demographic data | Participant nos |
|---|---|
| Gender | |
| Male | 13 |
| Female | 27 |
| Age (years) | |
| 18–29 | 5 |
| 30–39 | 1 |
| 40–49 | 5 |
| 50–59 | 5 |
| 60–69 | 15 |
| 70–79 | 7 |
| 80+ | 2 |
| Ethnic origin | |
| White | 34 |
| Black | 2 |
| Asian | 4 |
| Other | 0 |
| Educational level achieved | |
| Left school at 16 years | 4 |
| Left school at 18 years | 2 |
| Further education | 17 |
| Higher education | 14 |
| Postgraduate degree | 2 |
| Not stated | 2 |
Over two-thirds of the participants were female and most (74%) were aged 50 years or over. Despite widely publicising the project there were few men or younger people.
Two of the participants were hearing impaired and one had to use a magnifying glass with the large print materials in order to overcome his visual impairment.
Thirty-seven people completed simulated online reports, 36 people completed paper reports and eight people completed telephone reports (81 reports in total).
Focus group findings
The focus groups lasted between 30 and 45 minutes. The main themes identified were awareness and value of the YCS, and direct patient reporting, identifying ADRs, issues regarding reporting and advertising the scheme, and these are discussed below.
Awareness and value of the Yellow Card Scheme and direct patient reporting
Of the 40 participants, two had previous knowledge of the YCS: one was a retired dentist, who knew of the scheme through his profession and another had heard of it on a radio programme.
After hearing the initial presentation introducing the YCS, all of the participants felt that it was a very worthwhile scheme and they could see the benefit of asking patients to report ADRs directly to the MHRA:
It’s very sensible, you don’t know that the general practitioners are going to complete the forms even if you go back to them, but this way they will get more information I suppose. Whether they can use the information or not I don’t know, or whether they get too much information or not, I don’t know. But at least they are getting information.
Female, 60–69 years
Several of the participants also commented that they had reported side effects from medications to their doctor previously and had felt that the doctor was disinterested or dismissive. They believed that direct patient reporting would avoid information being reported to the MHRA through a professional lens.
In two of the focus groups there was some discussion relating to the quality of data that would be reported by patients and the possibility that this could be influenced by varying levels of literacy and education.
Although most of the participants felt that it was a positive step to allow members of the public to make reports to the YCS, a few felt that the GP should still be informed about the potential adverse reaction. Three participants stated that it should remain their GP’s responsibility to report such ADRs to the YCS, as this would be the most appropriate way of screening out those problems that were minor and not worth reporting. One participant expressed concern that direct patient reporting could result in the patient not discussing the issue with the GP. Others saw direct patient reporting as an option in situations where the GP did not agree to report a suspected ADR. Nevertheless, several participants felt that it was a professional responsibility of HCPs to report ADRs. Some participants recognised that direct patient reporting provided a mechanism to report adverse events from medicines bought from pharmacies.
Identifying adverse drug reactions
One issue raised in all of the focus groups was the difficulty of determining whether the symptoms experienced were a side effect of the medicine, due to the patient’s illness, or unrelated to their health problems. In addition, several participants discussed the potential complexity of deciding which drug was causing an ADR if the patient was taking multiple drug therapies:
I think my problem would be on the complexity of it all. I wouldn’t be able to really say which drug it was coming from. I find it useless information to give as I have a mishmash. If you give them the wrong one it could be wrong.
Female, 70–79 years
Determining the severity of the adverse reaction was also discussed. There was great concern over what would constitute a side effect worth reporting; whereas some participants felt they would be keen to report any potential side effect, many feared that reports of minor or unimportant side effects would ‘waste’ the MHRA’s time. There was general agreement that participants would only report side effects that were serious enough to be debilitating:
I wouldn’t bother reporting it unless I thought it [the ADR] was serious.
Female, 18–29 years
If it was just a headache or something I wouldn’t make a report, but something life threatening I would.
Female, 40–49 years
Participants also raised the issue of potential inter-patient variability on determining of the severity of ADRs:
How do I know, that, you know, my headache which I think is 8 on a scale of 1 to 10 is the same as yours (nodding at another participant) or yours?
Male, 50–59 years
The participants saw potential issues relating to the consistency of reporting of the severity of an ADR from members of the public, with no effective ‘filtering’ by a prescriber with greater medical knowledge.
Issues regarding reporting
Participants were informed that they would only receive an acknowledgement of their report from the MHRA. Concern was raised about this. Several participants felt that the lack of detailed feedback would potentially discourage them from completing a report:
If you are still suffering from the side effect, are still ill, er then I am not sure I would think it was worth the effort required to complete the form if I wasn’t going to get any advice or something, perhaps some personal feedback.
Female, 40–49 years
The majority of the participants felt that there ought to be more feedback provided to the reporter than a simple acknowledgement. It was suggested that this feedback might simply to give the reporter an idea of the frequency of the side effect reported for the drug involved. The participants felt that reporters would want to know how their experience of a side effect related to that of other people reporting side effects from the same drug. It was felt important that the reporting forms and information provided about the YCS made clear that feedback on an individual’s specific case and medical condition would not be possible. This would avoid disappointment or anger when reporters did not receive a more detailed response.
Advertising of the Yellow Card Scheme
None of the participants had seen a poster or leaflet relating to the YCS, despite some of those involved in the focus groups being individuals who attended regularly both GP and outpatient clinics and received regular prescriptions from their local pharmacy. A high level of concern was expressed by participants about this apparent lack of promotion of the scheme.
Advertising the YCS was discussed in some detail, with participants mentioning that there needed to be much wider awareness in order to ensure that the maximum volume of reports were obtained from the patient reporting system. Without extensive advertising, there would be a limited response from the public:
If you don’t know about it you can’t do anything can you? … I think the surgeries or pharmacies or whoever being more proactive in displaying all of this with posters and what have you so that the general public really do know what it is for. I don’t think that there is any other way that the general public are going to learn about it.
Male, 20–29 years
Several suggestions were made on how to improve awareness of the scheme among those most likely to suffer from ADRs. Simple measures, such as ensuring that posters are displayed in all pharmacies, general practice surgeries and hospital outpatient departments were seen to be a good method for raising general awareness. To target those receiving prescribed medicines, it was suggested that there could be a leaflet included in the bag with repeat prescription items:
Mmm … would it not be better that any time you collect any medication or something from your chemist, then one of these little Yellow Cards are put in separately. Because then it’s, it’s in your face as soon as you open your bag …
Female, 50–59 years
However, some felt that the leaflet might be treated as ‘junk mail’, and so would be an expensive and inefficient method of raising awareness. Another suggestion was to include information about the YCS on the bottom of each PIL provided within the medicine packaging by the manufacturer. Participants suggested that even if people do not read these leaflets routinely, they were much more likely to go back and read them if they suspected that they were suffering from an adverse reaction. Therefore, this would be an appropriate place to site information on the YCS.
Another suggestion for raising awareness included advertising on radio stations, particularly those targeted at the older population, as this section of the population takes the largest proportion of prescribed medicines. Attempts could also be made to have the YCS discussed on popular television programmes that deal with viewers’ medically related issues.
Following on from the need to raise awareness of the system, participants felt there was also a need to ensure easy access to the reporting forms in surgeries and pharmacies, with forms being clearly displayed in both settings. The participants also felt that HCPs could play a more proactive role in encouraging reporting by patients, and suggested that GPs and pharmacists could hand out the reporting forms to all patients they saw.
Usability testing findings
The usability test data are presented below, with each reporting method discussed in turn.
Paper reporting
The form was generally well received, with many of the participants commenting positively:
This is an excellent form and with my own information I could er … fill it in easily.
Female, 60–69 years
Two participants with visual impairment found the font size too small. Several participants commented that they would not bother reading the information about the YCS provided within the integrated information leaflet and reporting form; they would just start filling in the form. It should be noted, however, that the scheme had been explained to participants in the focus group directly before they undertook the usability tests.
In some of the scenarios, details were provided about a number of drugs that were being taken by the fictional individual and some of the participants found it difficult to fit all the required information onto the form. Although the form suggests that further information can be provided on a separate sheet, participants were generally not keen on this:
Having to attach extra sheets to give all the information would annoy me, so I wouldn’t bother!
Male, 70–79 years
I’m frustrated to have to add additional sheets.
Female, 70–79 years
And some felt that this part of the form was tedious and long winded:
I have lost the will to live (comment made when entering all details of the drugs).
Female, 50–59 years
Some individuals commented that they would struggle to remember the date on which their various drugs had started and finished and they would need to look up this information.
The layout of the form was commented on by some participants. Details about the reporter are asked for at the end of the report after details of the ADR and the severity of the side effect. This did not feel natural to some of participants, who felt that detailed ADR information should be requested after more basic information has been collected.
Whereas all the scenarios included the height and weight of the fictional character experiencing the side effect, some participants commented that they would not necessarily be able to provide these details, particularly their weight.
When participants tried to insert their completed form in to the reply-paid envelope, which is also part of the leaflet, they were disappointed to discover that the form did not slide neatly into it; instead, the form had to be folded:
The envelope is the same width as the form, so it doesn’t go in without folding. And the adhesive is not very good.
Male, 18–29 years
One participant commented that the use of a first-class reply-paid envelope made her feel that the MHRA was taking the whole system very seriously.
It was suggested that access to the system would be improved by making the paper-based and online forms available in languages other than English:
It might be useful to er you know, provide the service in other languages, er not only English.
Male, 60–69 years
Telephone reporting
The telephone reporting system was popular with all of those who used it. The interactive nature of the conversation with the person staffing the telephone was highlighted as a factor that made the process much easier for the reporter. Participants expressed concern that such a convenient method of reporting was only available for a limited number of hours, thereby limiting access. They were pleased to be told that the reporting line has a direct-dial number which does not require reporters to enter a queuing system with an automated response menu:
I might be put off by having to pay for the phone call … the time restriction of the service might put me off too.
Female, 40–49 years
The only issue encountered with the telephone system occurred to one participant who had impaired hearing. He found that he was unable to hear the person taking the report because of background noise; the test had to be abandoned because of this. However, the participant stated that at home he would not have encountered this problem and he did not consider this to be an inherent problem.
Online reporting
The online reporting system received mixed comments from participants. There were a few who found the system easy to navigate, but many found it very challenging, even though they used the internet regularly and completed forms online.
When reporting the nature of the suspected ADR, there are a number of drop-down menus (dictionaries) that provide mixed-response options. However, the complexity of the terms used within these menus confused a number of participants. For example, entering an ADR term of ‘rash’ produced over 70 medical terms for rash (such as ‘rash desquamating’, ‘rash morbilliform’). Participants were often unable to understand these terms, and many participants felt forced to choose the first term on the list (which is always a general term) or a term at random.
A number of participants did not see the drop-down menus appear, as they were concentrating on the next part of the form that they had to complete. This resulted in lack of further detail being provided.
There appeared to be no facility to save data at each stage of completing the online form. At least two participants had problems with losing information after taking some considerable time completing sections of the form. Having wasted their time, these participants told us that they would not have finished completing the form in real life.
One comment made by a number of participants related to the different ordering of questions on the online and paper forms. A consistent approach would make it easier for users who may use the paper form to collate their information, but then choose to submit the data using the online system.
The online system also asks for extra information to that requested on the paper form. It is important to consider whether this additional information is required and, if so, whether it should also be collected on the paper form.
Summary
-
Members of the public in Nottingham, UK, were invited to seven focus groups at which views on patient reporting of ADRs were explored. Participants were then observed completing Yellow Card reports for simulated ADR scenarios and detailed information was recorded on their experiences and suggestions for improvements.
-
Forty participants took part in seven focus groups. After hearing an introductory presentation, a number of suggestions were made about improving publicity for the YCS. Usability testing with the 40 participants indicated that telephone reporting worked well, but identified specific suggestions for enhancing online and paper reports.
Chapter 10 Study 8: omnibus survey
Omnibus survey
The omnibus survey is a multipurpose survey that was developed in the 1990s by the Office of National Statistics in the UK. It is most commonly used to measure the efficacy of publicity campaigns, assess awareness of new policies, test questions and provide a sample of respondents for follow-up investigations. 50
The omnibus survey contains a set of classificatory questions, including socioeconomic status and ethnicity, and these can be supplemented to provide information on a range of health and health-related topics. 51
Objectives
-
To estimate the percentage of members of the general public in Great Britain who had heard of the YCS for patient reporting of ADRs.
-
To determine whether those respondents, who believe that they have experienced an ADR and who were aware of the YCS, have made a report using the YCS.
-
To assess the views of members of the public on the convenience of the three different ways of reporting (online, telephone, obtaining a paper form from a GP/pharmacy to fill in and post).
Methods
Eight questions, three of which were contingent on answers to the other questions, were added to the national omnibus survey carried out by the British Market Research Bureau (BMRB) (www.tns-ri.co.uk/what-we-do/6772.aspx) by telephone over two weekends in January 2009. Probability sampling was attained by using random digit dialling (RDD). This method ensures that numbers not listed in telephone directories are included in the sample. Each residential number has an equal chance of being dialled.
A UK database of residential numbers is the source of the master sample used by BMRB for its telephone omnibus service. New numbers are created by adding or subtracting up to 40 from each original number. Duplicate numbers are removed from the listing. Samples for each omnibus survey are selected from the master sample using a random start and constant sampling interval. Any adult aged 16 years or above living in the household is eligible for interview. To ensure that the final sample does not over-represent groups that spend more of their time at home, criteria known to relate to an individual’s likelihood of being at home are used to control selection from the list of eligible adults. If no reply is received at the first calling, or if the respondent requests an appointment at a different time, the household is recalled.
Questions were developed to assess the public’s knowledge of the YCS and preference for different methods of reporting ADRs. We asked about respondents’ previous experiences of side effects from medications and whether they had ever reported these. In addition, respondents were asked to rank the convenience of each reporting method on a scale from 1 (very inconvenient) to 10 (very convenient). The exact wording of the questions asked is shown in Appendix 22.
Demographic information was obtained for the sample about gender, age, geographical region, social grade, highest level of education, working status, ethnicity and internet use in the past month.
Analysis
Data were analysed using spss for Windows version 15.0. Data were described using frequencies and percentages. Original BMRB codes for ethnic background were combined into five major ethnic groups: (1) white Irish, white British and any other white background; (2) mixed African, mixed Caribbean, mixed Asian and any other mixed background; (3) Indian, Pakistani and any other Asian background; (4) African, Caribbean and any other black background; and (5) other ethnic background. The chi-squared test was used to compare the sociodemographic profile of our sample with UK general population figures of 2008. Similarly, the chi-squared test was used to examine sociodemographic variables across level of awareness of the patient reporting to the YCS. Multiple logistic regression was used to identify independent patient demographic predictors of awareness of the YCS. Mann–Whitney or Kruskal–Wallis tests were used to compare median convenience of reporting scores across sociodemographic factors. A p-value of ≤ 0.01 was used to denote statistical significance throughout.
Results
Over the two weekends, 62,018 telephone calls were made, resulting in 31,390 contacts (50.6%) and 2028 responses (6.5% of contacts). The analysis was based on the 2028 subjects who responded to the survey. Demographic details of the respondents are shown in Table 32 together with comparisons for the general population of England, Scotland and Wales in 2008 unless stated otherwise. Compared with the general population in 2008, respondents were more likely to be female, of older age, living in London/south-east/south-west, of lower middle class, and economically inactive. It should be noted, however, that although all differences reached statistical significance, which was partly owing to the general population sample size; overall percentage differences were fairly small.
| Factor | Sample, % (n) | General population (2008, England, Scotland and Wales): % (n) | p-value |
|---|---|---|---|
| Gender | |||
| Male | 44.9 (911) | 49.1 (29,280,500) | < 0.001 |
| Female | 55.1 (1117) | 50.9 (30,327,700) | |
| Age group (years) | |||
| 16–24 | 6.5 (130) | 16.2a (7,956,200) | < 0.001 |
| 25–34 | 9.8 (196) | 15.6 (7,665,100) | |
| 35–44 | 17.4 (347) | 18.1 (8,894,400) | |
| 45–54 | 20.2 (403) | 16.1 (7,929,200) | |
| 55–64 | 20.3 (404) | 14.4 (7,084,400) | |
| 65+ | 25.8 (514) | 19.5 (9,581,300) | |
| Missing (n) | 34 | – | |
| Geographical region | |||
| London/SE/SW | 41.2 (836) | 35.6 (21,209,100) | < 0.001 |
| Wales | 5.1 (104) | 5.0 (2,993,400) | |
| E Anglia/E and W Midlands | 22.1 (448) | 26.1 (15,572,800) | |
| Yorkshire/NW/NE/Scotland | 31.6 (640) | 33.3 (19,832,900) | |
| Social gradeb | |||
| AB (upper middle) | 22.0 (434) | 22.0 (8,934,482) | < 0.001 |
| C1 (lower middle) | 33.3 (657) | 29.7 (12,065,333) | |
| C2 (skilled working) | 18.4 (363) | 15.1 (6,149,928) | |
| DE (working class) | 26.3 (520) | 33.2 (13,516,803) | |
| Missing (n) | 54 | – | |
| Highest-level education | |||
| None/primary/few years secondary | 7.9 (153) | c | – |
| Secondary completed | 35.6 (688) | ||
| High school/further education | 31.9 (616) | ||
| University degree/doctorate | 22.7 (438) | ||
| Still studying | 2.0 (38) | ||
| Missing (n) | 95 | ||
| Working status | |||
| Full time | 42.3 (846) | 63.6d (31,220,000) | < 0.001 |
| Part time | 13.2 (263) | ||
| Not working | 14.7 (294) | 36.4e (17,839,000) | |
| Retired | 29.9 (597) | ||
| Missing (n) | 28 | ||
| Ethnicity | |||
| White British/Irish/other | 94.4 (1848) | 89.0 (52,233) | < 0.001 |
| Mixed Caribbean/African/Asian/other | 0.9 (17) | 1.2 (677) | |
| Indian/Pakistani/other Asian | 2.1 (41) | 5.3 (3107) | |
| Black Caribbean/African/other | 2.3 (45) | 2.6 (1531) | |
| Any other | 0.4 (7) | 1.9 (1160) | |
| Missing (n) | 70 | – |
Table 33 reports the answers given to the eight questions. A total of 477 (23.5%) people said that they had suffered a side effect from a medicine, the majority of whom (408, 85.5%) had reported it to their GP or another HCP. The main reasons why respondents did not report it to a HCP were the side effect not being serious enough or respondents expecting or knowing about the side effect. Only 172 respondents (8.5%) had heard about the YCS and, of these, only three had self-reported to the YCS the last time they had a side effect (two reported it online and one by post). Nearly 60% (n = 1295) of the sample had used the internet within the last month.
| Question | Response | % (n) |
|---|---|---|
| Have you ever had side effects from any medicine? | Yes | 23.5 (477) |
| No | 76.1 (1543) | |
| Don’t know | 0.4 (8) | |
| If yes, did you tell your GP or other HCP about it? | Yes | 85.5 (408) |
| No | 14.3 (68) | |
| Don’t know | 0.2 (1) | |
| Question not applicable n | 1551 | |
| If not, why not? | Side effect not serious enough | 25.0 (17) |
| Didn’t realise side effect due to medicine | 7.4 (5) | |
| Expected/knew about side effect | 19.1 (13) | |
| Stopped using medicine | 7.4 (5) | |
| I am a GP or HCP | 4.4 (3) | |
| Didn’t want to bother/unnecessary | 7.4 (5) | |
| Other (including ‘embarrassed’, ‘abroad’, ‘didn’t read instructions’) | 22.1 (15) | |
| Don’t know | 7.4 (5) | |
| Question not applicable | 1960 | |
| There is a scheme called the ‘YCS’ that allows people who’ve had side effects from medicine to send a report about it to the Department of Health. Were you aware of this scheme before today? | Yes | 8.5 (172) |
| No | 91.5 (1856) | |
| Thinking about last time you had side effect, did you report it yourself using YCS? | Yes | 5.9 (3) |
| No | 94.1 (48) | |
| Not applicable | (1977) | |
| When you reported the side effects using the YCS, did you report it? | Online | 66.7 (2) |
| By filling out and sending in a form | 33.3 (1) | |
| By telephone | 0 (0) | |
| Not applicable | (2025) | |
| Have you used the internet in the last month? | Yes | 63.9 (1295) |
| No | 36.1 (733) |
Table 34 shows that there was no significant association between awareness of the YCS and gender, age group, geographical region, working status and ethnicity (all p > 0.05). However, significantly more people who were aware of the YCS came from the upper or middle social grades compared with the skilled working and working class (p = 0.006). In addition, awareness of the YCS was associated with respondents having completed post secondary or university education (p < 0.001).
| Factor | Aware of YCS | p-value | |
|---|---|---|---|
| Yes (n = 172) | No (n = 1856) | ||
| Gender | |||
| Male | 41.9 (72) | 45.2 (839) | 0.399 |
| Female | 58.1 (100) | 54.8 (1017) | |
| Age group (years) | |||
| 16–24 | 3.6 (6) | 6.9 (124) | 0.128 |
| 25–34 | 7.1 (12) | 10.1 (184) | |
| 35–44 | 16.0 (27) | 17.5 (320) | |
| 45–54 | 20.7 (35) | 20.2 (368) | |
| 55–64 | 27.2 (46) | 19.6 (358) | |
| 65+ | 25.4 (43) | 25.8 (471) | |
| Geographical region | |||
| London/SE/SW | 41.9 (72) | 41.2 (764) | 0.790 |
| Wales | 5.2 (9) | 5.1 (95) | |
| E Anglia/E and W Midlands | 24.4 (42) | 21.9 (406) | |
| Yorkshire/NW/NE/ Scotland | 28.5 (49) | 31.8 (591) | |
| Social grade | |||
| AB (upper middle) | 27.2 (46) | 21.5 (388) | 0.006 |
| C1 (lower middle) | 37.3 (63) | 32.9 (594) | |
| C2 (skilled working) | 8.9 (15) | 19.3 (348) | |
| DE (working class) | 26.6 (45) | 26.3 (475) | |
| Highest level education | |||
| None/primary/few years secondary | 6.6 (11) | 8.0 (142) | < 0.001 |
| Secondary completed | 23.5 (39) | 36.7 (649) | |
| High school/further education | 35.5 (59) | 31.5 (557) | |
| University degree/doctorate | 33.7 (56) | 21.6 (382) | |
| Still studying | 0.6 (1) | 2.1 (37) | |
| Working status | |||
| Full time | 40.6 (69) | 42.5 (777) | 0.575 |
| Part time | 15.9 (27) | 12.9 (236) | |
| Not working | 12.4 (21) | 14.9 (273) | |
| Retired | 31.2 (53) | 29.7 (544) | |
| Ethnicity | |||
| White British/Irish/other | 94.7 (160) | 94.4 (1688) | 0.863a |
| Mixed Caribbean/African/Asian/other | 1.2 (2) | 0.8 (15) | |
| Indian/Pakistani/other Asian | 1.2 (2) | 2.2 (39) | |
| Black Caribbean/African/ other | 3.0 (5) | 2.2 (40) | |
| Any other | 0 | 0.4 (7) | |
Multiple logistic regression with awareness of the YCS as the dependent variable and social grade or highest level of education as independent variables confirmed that people of upper and lower middle social grades were more likely to be aware of the YCS than those in the skilled working and working grades (overall p-value = 0.009). Those with further education were more likely to be aware of the YCS than the primary school-educated people (overall p-value = 0.001)
Table 35 suggests that perceived levels of convenience for online or telephone reporting of side effects were higher than those of a form from the local pharmacy or GP. Figure 12 shows that people felt that online reporting was either very inconvenient or very convenient. Telephoning was noted to be very convenient by the majority of subjects, whereas using a paper form from the pharmacy or GP was less favoured by some.
| Method of reporting | Median (IQR) | Missing (n) |
|---|---|---|
| Online | 8 (1 to 10) | 122 |
| Telephone | 8 (5 to 10) | 80 |
| Form from local pharmacy or GP surgery | 6 (4 to 9) | 77 |
FIGURE 12.
Level of convenience of reporting future side effects for (a) online reporting, (b) telephone reporting and (c) reporting by completing and posting a paper form, where 1 = very inconvenient and 10 = very convenient.
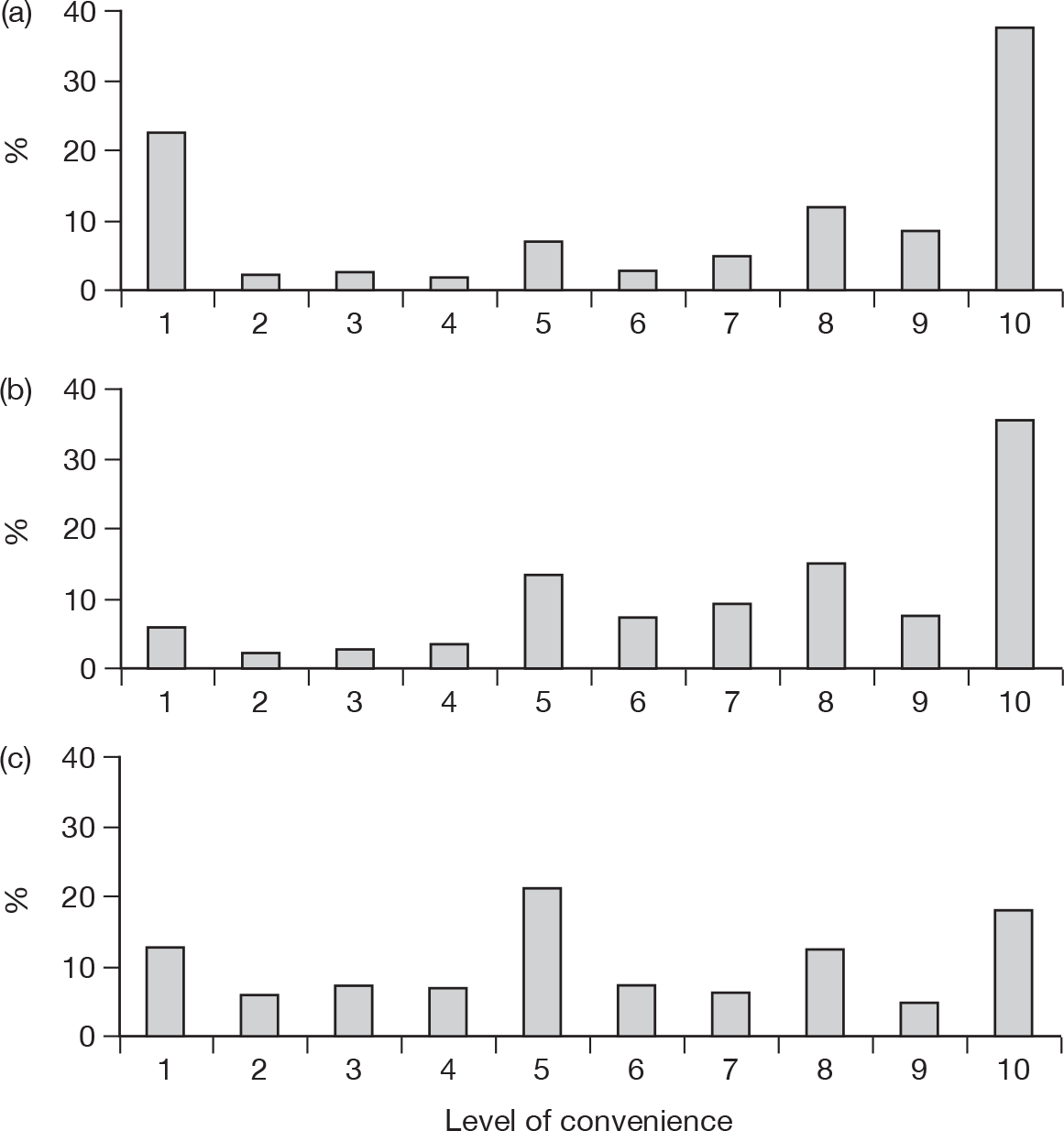
Subjects were not asked which of the three different forms of reporting they found most convenient – they were simply asked to rate each method separately. Therefore, they could give the same level of convenience to more than one method. Out of the 2028 subjects, 156 (7.7%) answered ‘don’t know’ to one, two or three of the questions, whereas 1035 (50.8%) gave equal ratings to two or three of the questions. This meant that only 837 (41%) of subjects had unique ratings to all three questions. Of these 837 subjects, 404 (48.3%) rated online as most convenient method of reporting, 287 (34.3%) the telephone and 146 (17.4%) a form from their GP/pharmacy.
There were significant associations between reported levels of convenience of each of the three methods of reporting and demographic factors.
Table 36 shows that respondents who thought that it would be convenient to report online were more likely to be male, younger, living in the Midlands/south, of middle social grade, have some form of further education and be working full time.
| Factor | Convenience of method of reporting | ||
|---|---|---|---|
| Online | Telephone | Paper | |
| Gender | |||
| Male | 8 (3 to 10) | 8 (5 to 10) | 5 (3 to 8) |
| Female | 8 (1 to 10) | 8 (5 to 10) | 6 (4 to 9) |
| p-value | 0.006 | < 0.001 | < 0.001 |
| Age group (years) | |||
| 16–24 | 9 (7 to 10) | 7 (5 to 10) | 5 (4 to 8) |
| 25–34 | 10 (7 to 10) | 8 (6 to 10) | 5 (3 to 7) |
| 35–44 | 10 (7 to 10) | 8 (5 to 10) | 5 (3 to 8) |
| 45–54 | 8 (4 to 10) | 8 (5 to 10) | 6 (3 to 9) |
| 55–64 | 7 (1 to 10) | 8 (6 to 10) | 6 (4 to 9) |
| 65+ | 1 (1 to 5) | 8 (5 to 10) | 7 (5 to 10) |
| p-value | < 0.001 | 0.052 | < 0.001 |
| Geographical region | |||
| London/SE/SW | 8 (2 to 10) | 8 (5 to 10) | 5 (3 to 8) |
| Wales | 5 (1 to 10) | 8 (5 to 10) | 5 (3 to 9) |
| E Anglia/E and W Midlands | 8 (1 to 10) | 8 (5 to 10) | 6 (4 to 9) |
| Yorkshire/NW/NE/Scotland | 7 (1 to 10) | 8 (5 to 10) | 6 (4 to 9) |
| p-value | 0.001 | 0.505 | 0.072 |
| Social grade | |||
| AB (upper middle) | 9 (7 to 10) | 8 (5 to 10) | 5 (3 to 8) |
| C1 (lower middle) | 9 (4 to 10) | 8 (5 to 10) | 5 (3 to 8) |
| C2 (skilled working) | 7 (1 to 10) | 8 (5 to 10) | 6 (4 to 8) |
| DE (working class) | 3 (1 to 8) | 9 (6 to 10) | 7 (5 to 10) |
| p-value | < 0.001 | 0.003 | < 0.001 |
| Highest level education | |||
| None/primary/few years secondary | 1 (1 to 6) | 8 (5 to 10) | 6 (4 to 9) |
| Secondary completed | 5 (1 to 9) | 8 (5 to 10) | 6 (4 to 9.8) |
| High school/further education | 8 (2 to 10) | 8 (5.5 to 10) | 6 (4 to 8) |
| University degree/doctorate | 10 (8 to 10) | 8 (5 to 10) | 5 (3 to 8) |
| Still studying | 10 (7 to 10) | 7.5 (6 to 10) | 5 (4 to 7) |
| p-value | < 0.001 | 0.134 | < 0.001 |
| Working status | |||
| Full time | 9 (6 to 10) | 8 (6 to 10) | 5 (3 to 8) |
| Part time | 8 (5 to 10) | 8 (5 to 10) | 5 (4 to 8) |
| Not working | 8 (1 to 10) | 9 (5 to 10) | 6 (5 to 10) |
| Retired | 1 (1 to 7) | 8 (5 to 10) | 7 (5 to 10) |
| p-value | < 0.001 | 0.833 | < 0.001 |
| Ethnicity | |||
| White British/Irish/other | 8 (1 to 10) | 8 (5 to 10) | 6 (4 to 9) |
| Mixed Caribbean/African/Asian/other | 8 (5 to 10) | 7 (2 to 8) | 5 (4 to 8) |
| Indian/Pakistani/other Asian | 8 (4 to 9) | 7 (5 to 9) | 5 (3 to 7) |
| Black Caribbean/African/other | 9 (3 to 10) | 7 (5 to 10) | 5 (3 to 9) |
| Any other | 10 (5 to 10) | 9.5 (1.8 to 10) | 5 (1 to 5) |
| p-value | 0.224 | 0.071 | 0.154 |
In contrast, those who thought that the telephone would be a convenient method of reporting were more likely to be female and of lower social grade.
Finally, people who thought that reporting via a paper form from their GP or pharmacy would be a convenient method tended to be female, older, of a lower social grade, have a lower level of completed education and be retired.
Summary
-
Eight questions were added to a national omnibus survey that was carried out by the BMRB by telephone over two weekends in January 2009, using a database of residential telephone numbers in Great Britain. Questions were developed to assess public knowledge of the YCS, previous experiences of side effects from medications, previous reporting of ADRs and preferred methods for reporting ADRs.
-
From the national omnibus survey of 2028 adults, only 172 (8.5%) had heard about the YCS. The preferred method of reporting varied with the characteristics of respondents.
Chapter 11 Discussion
We have successfully completed a multifaceted evaluation of patient and HCP reporting of suspected ADRs to the YCS in the UK. In this chapter we begin by discussing the findings in relation to the first two objectives of the study, drawing on the literature where appropriate. The structure of these discussions reflects the authors’ views on the importance and significance of the findings, while addressing the original research questions set out in Chapter 1.
We then discuss the strengths and limitations of our studies in detail before offering recommendations for improvement to patient reporting based on our findings and those from the literature (our third objective). We end by providing suggestions for further research.
Evaluating the pharmacovigilance impact of patient reporting to the Yellow Card Scheme
How do patients describe suspected side effects, and how does the richness of patient reports compare with those of health-care professionals?
Patients gave detailed descriptions of suspected ADRs, attributed reactions to specific medicines and provided information useful for assessing causality. Patient reports often had richer narratives than those of HCPs and rarely provided irrelevant information or ambiguities. Patient reports also often contained detailed information about the impact of the suspected ADR on the patient’s life, thus providing insights that there were comparatively rare in HCP reports.
These findings are in keeping with analyses of consumer reports of reactions to paroxetine52 compared with reports from HCPs to the YCS. 52 The study authors noted:52
Our analyses suggest that reports from patients – in their own words – communicate essential information which professional reporters can never be expected to provide. In this case, patients provided reports that were much richer in their descriptions of behavioural phenomena and feelings than the YC [Yellow Card] reports, and often much better at explaining the nature, significance and consequences of adverse drug effects.
Medawar and Herxheimer52 also identified the potential for some terms used by patients, such as electric shock sensation associated with paroxetine, to be coded as less-specific terms, such as paraesthesia. In our study, we did not formally check the text from patients against coded reaction terms, but this information was readily available when selecting the cases for qualitative analysis. Our impression was that symptom descriptions were coded accurately and comprehensively. Nevertheless, if an entirely new symptom not listed in the MedDRA dictionary was to emerge in relation to a medicine, delays would occur in detection if there was an overemphasis on coding at the expense of careful reading and consideration of reports. Also, it is important to recognise that reports from either patients or HCPs will lose their richness when converted to symptom codes. Therefore, detailed reading of reports is still an extremely important task for regulatory bodies, particularly in relation to drugs undergoing intensive monitoring.
In keeping with our findings, an earlier literature review of patient reporting of ADRs24 concluded that none of the countries with patient reporting systems had identified poor quality of patient reports as an issue. Van Grootheest and colleagues53 state that the quality of the report should be distinguished from the quality of the clinical judgement. In terms of the quality of the report, they identified the following elements needed to make a report useful: description of the adverse reaction, time of onset of the reaction, the drug involved, concomitant medication, duration of drug use, including dates of initiation and discontinuation, and any other relevant information. Most of the patient reports in our qualitative study contained this information. Quality of the judgement, relates to whether patients can distinguish drug-related complaints from other problems, for example disease-related complaints. 53 Many of the patients in our qualitative study appeared to be able to differentiate between symptoms of disease and ADRs, sometimes explaining their reasoning for this judgement.
The pharmacovigilance impact of suspected adverse drug reaction reports from patients and health-care-professionals
Spontaneous reporting of suspected ADRs by HCPs is a valuable pharmacovigilance tool1,54 and has contributed to the identification of major safety issues. 1 There is general acknowledgement that patient ADR reporting is the right thing to do and that it will enhance pharmacovigilance,6,53,55 although the specific value of ADR reports from patients has not been clear.
Although proponents have highlighted potential benefits from patient reporting of ADRs,30,56,57 others have expressed concerns. Many of these concerns have been highlighted already, including the capability of patients to distinguish between suspect ADRs and problems associated with the underlying condition being treated by the suspect drug;58 unclear ADR description;58 large numbers of reports of minor and well-known ADRs adversely affecting the ability to identify new drug safety issues;1 duplication of ADR reports;1 resources available to regulators;1 the potential influence of campaigning groups on signal generation;1 and perceived undermining of HCP status. 59
Our qualitative findings should reassure those who worry that patients may not be able to give useful information for detailed pharmacovigilance assessment, such as the detailed reading of narratives required to assess the potential causality and impact of suspected ADRs. Other issues relevant to pharmacovigilance include:
-
seriousness of reports
-
outcome of the ADR
-
timeliness of reports
-
role of patient reports in the generation of new signals.
Seriousness of reactions and patient outcomes
In our study we found no significant differences between patients and HCPs in the proportion of reports containing at least one reaction term classified as ‘serious’ by the MHRA (58.3% vs 58.8%, respectively). The observation that such a high proportion of patient reports contained a reaction term classified as serious provides reassurance to those concerned that patients might report too many relatively minor ADRs. 1
Studies from Denmark19 and the Netherlands30 also found that physicians and patients report a similar proportion of serious ADRs.
It was difficult to make a direct comparison of patient outcomes from suspected ADRs because of differences in the questions asked of patients completing a Yellow Card report compared with those of HCPs. For those categories where direct comparison was possible, HCPs were more likely than patients to report that the suspected ADR had caused hospitalisation, was considered life-threatening or caused death. This difference may be because patients (or their representatives) are not in a strong position to make a report in such circumstances because of the physical and/or emotional impact of the illness or death (a point reinforced by our advisory group). Having said this, a Dutch study showed that patients reported a higher proportion of suspected ADRs for life-threatening events than physicians. 30
Our content analysis of Yellow Card reports showed that patients were much more likely than HCPs to document the potential impact of suspected ADRs on their lives (47% vs 12% of reports) and a Dutch study showed that patients report higher proportions of ADRs associated with disability. 30
Our qualitative analysis also highlighted the detailed descriptions that patients often gave for the emotional, social and occupational impact of suspected ADRs. Although this information would not be used in signal generation analysis, it could be extremely useful for regulators undertaking more detailed assessments of drugs that are being intensively monitored or investigating drug–ADR pairings that have generated signals.
Our study has highlighted the burden of ADRs on patients’ lives and yet there may be a difference between what patients and regulatory authorities regard as serious. The concurrence, or disparity, of views between patients and regulatory authorities needs further exploration, we believe, because there is uncertainty about whether current regulatory approaches to assessing seriousness miss important reactions that may have very serious implications for patients’ lives. An example would be electric shock sensations associated with SSRI withdrawal, which was brought to prominence through analysis of patient reports to the media. 52
Time taken to report reactions
In terms of the time taken to report reactions, it should be noted that over 60% of patient reports analysed had this piece of information missing. Where information was available, patient reporters took significantly longer to report their ADR than HCPs (median 104 vs 28 days). The situation did not change when the second year of patient reporting was compared with the first.
Our questionnaire survey of patient reporters to the YCS showed that those reporting by telephone did so relatively sooner than those reporting by either of the other two methods. Those who reported by post took the longest time to report. Our telephone interviews with patients who had reported an ADR, suggests that the main reason for delay is lack of knowledge of the reporting scheme. Lack of knowledge about the YCS was also found in our omnibus survey. Delays in reporting will inevitably delay signal generation and may affect the accuracy of recall of events relevant to the report. Therefore, it is extremely important to improve awareness of the YCS among the public and to ensure that access to the different reporting methods is made as easy as possible.
Do patient reports generate ‘new’ signals?
In Australia, 20% of patient reports were for previously unknown ADRs27 and there were conflicting results regarding whether patients were more or less likely to report these than HCPs. In the Netherlands, 30% of suspected ADRs reported by patients were not mentioned in the PIL. 60
Our study went much further than previous published studies to examine the impact of patient reports on signal generation. We examined SDRs that are ‘statistical signals’, where the reporting rate for an ADR in association with a particular medicine is disproportionate to that of other products in the database. We found that the ‘patient-only’ and ‘HCP-only’ data sets produced quite different findings in terms of reactions reported and suspect drugs, with only 10% of all reported drug–ADR pairs being present in both data sets.
The potential signals (SDRs) detected by disproportionality analysis of each reporter data set were also quite different. It should be noted, however, that this type of analysis relies heavily on the coding of reactions, so some differences may have arisen because of the way in which reactions were described by the two reporter groups, and, subsequently, coded using MedDRA terminology. For example, a patient may report a ‘seizure’ with metoclopramide, whereas a HCP may report an ‘oculogyric crisis’. These are subtle, but important differences. Nevertheless, comparisons using exact, similar or related terms for a particular reaction, still found that two-thirds of the SDRs generated by the ‘patient-only’ data set were not generated in the ‘HCP-only’ data set. A similar proportion of SDRs generated in reports from either group (15%) were assessed as being ‘not documented on the product’s SPC’, suggesting that the identification of potentially important new information.
Do patient reports add weight to known signals or signals generated from reporting by health-care professionals?
Our study also found that combining patient and HCP data sets generated additional SDRs that were not generated by either data set alone (n = 508), although 10% (n = 186) of SDRs generated by the ‘HCP-only’ data set no longer appeared as SDRs. Forty-seven of the additional SDRs generated by the combined data set involved reactions that were classified as ‘serious’ and that had not been documented previously on the product’s SPC; 10 of these involved ‘black triangle’ drugs (for further details, see Appendices 11 and 12).
It would appear, therefore, that patient reports add weight to HCP reports by creating new and important SDRs. This is at the expense, however, of the loss of a smaller number of SDRs generated by HCP reports alone. SDRs for drugs labelled as ‘black triangle’ are unlikely to be missed in practice in the UK, as the MHRA would identify these using the traditional case-by-case approach. Five SDRs were ‘lost’ by the addition of patient reports to HCP reports for previously undocumented reactions to established drugs, compared with 37 being newly identified by the combination.
Those involved in the analysis of patient and HCP reports need to take these findings into account when considering how to maximise the pharmacovigilance benefit of using these two sources of information. For example, in addition to examining the combined data set, it might be helpful to conduct signal detection analysis on separate HCP and patient data sets to ensure that important new signals are not missed. Consideration of these issues will become more important over time as patient reports accumulate in national ADR databases and represent an increasing proportion of all reports available for analysis.
Recent analysis from the YCS has shown that patient reports do contribute to signal generation with examples including ‘varenicline and aggression, hyoscine and visual hallucinations and a food interactions between amlodipine and grapefruit’. 61
Causality assessment of suspected adverse drug reactions
Our study showed that most of the reports assessed for causality were judged to have a ‘possible’ causal association between the drug and the suspected ADR, regardless of reporter group. This indicates that patients provide comparable information to HCPs with respect to its usefulness for causality assessment. It is noteworthy, however, that around one-fifth of both patient and HCP reports could not be assessed. This was because of missing, conflicting or too much information, for example multiple suspect drugs and reaction terms supplied. Providing further guidance to reporters on what information to report may help strengthen the usefulness of both patient and HCP reports.
Other observations regarding patient and health-care professional reports
Demographic characteristics: age and gender
We found a similar median age of people included in patient and HCP reports as a Dutch study. 30 In common with others,19,30 we found that more patient and HCP reports were submitted for females. The reasons for higher ADR reporting in women are not simply related to higher consumption of medicines. 62 For example, a large hospital-based cohort study showed higher risk of ADRs in women than men, even when the number of drugs prescribed was taken into account. 4,63 Women were found to be particularly at risk from dose-related ADRs and ADRs related to cardiovascular drugs. 4,63 In a large hospital-based UK study, women were also found to be more susceptible to ADRs than men, although the number of medicines prescribed was the only significant predictor of ADRs when multivariate analysis was undertaken. 4 However, women may not necessarily be at increased risk of ADRs for all types of drugs. One study of self-reported side effects to anti-hypertensive drugs64 showed no increased risk in women, in contrast with the cardiovascular drug findings of Zopf and colleagues. 4,63 Clearly, the increased susceptibility of women to ADRs is not fully understood and is inadequately explained by current epidemiological studies. 4,63 There is also bias against understanding female responses to medication as women are often excluded from phase I and phase II drug studies. 65 Further studies are needed to help better understand the reasons for higher prevalence of ADRs in women.
Number and types of drugs and suspected adverse drug reactions reported
In our study, over 16% of patient Yellow Card reports contained more than one distinct suspect drug compared with 9% of HCP reports. This might be owing to patients being less able than HCPs to identify the drug(s) most likely to be associated with a particular suspected ADR.
When drugs were grouped into their ATC anatomical classifications, the three most frequently reported classes were the same for both patient and HCP reporters (nervous system, cardiovascular system, anti-infectives for systemic use), as in a Danish study. 19 In the Netherlands, patients and physicians also reported similar types of drugs to each other. 30 In our study, however, there were more marked differences between groups at individual drug level, with simvastatin and citalopram being the only two drugs in the top 10 suspect drugs for both patients and HCPs.
Health-care professionals were more likely than patients to report suspected reactions to newer ‘black triangle’ drugs. Potential signals relating to ‘black triangle’ drugs were also more commonly identified when analysing the ‘HCP-only’ data set. This was not surprising given that HCPs are encouraged to report suspected ADRs to these drugs, whereas patients are not routinely told about drugs undergoing intense surveillance. Levels of patient reporting for ‘black triangle’ drugs may be raised if patients are given more information about which drugs are flagged as ‘black triangle’ products. For example, PILs could highlight the ‘black triangle’ status of the drug, and prescribers and pharmacists could encourage patients to report suspected ADRs for these drugs in particular. Patient reporters to the Australian national scheme were more likely to report new drugs compared with HCPs. 27 It would be useful to explore why this might be the case.
In our study, patients reported significantly more suspected ADRs per Yellow Card report than HCPs. The reasons for this are not clear but may, in part, be related to patients writing more about their symptoms than health professionals (as demonstrated by our qualitative study and the three times higher word count in patient reports). A Dutch study also found that patients listed more suspected ADRs per report than HCPs. 30
The top two (LLT) reactions reported in our study by both types of reporters were nausea and headache. These reactions were in the top five in a Dutch study of patient reporting, with six of the top 10 reactions similar between patient reporters in the two studies. 30
Our study showed that patients were almost three times more likely than HCPs to report ADRs categorised as affecting the nervous system, but the differences were less marked when examining SDRs. A Danish study also found that nervous system disorders were reported slightly more often by patients than other sources (i.e. HCPs, drug manufacturers and lawyers). 19 Patient reports may, thus, be particularly valuable in helping to detect ADRs relating to the nervous system.
Word count
The median word count for patient descriptions of suspected ADRs was three times greater than that of HCPs. This may, in part, be owing to high motivation among patients to report in detail. In contrast, HCP reports may have been shorter because of limited time to complete the reports and HCPs having fewer details of the suspected ADR than patients. Another reason may be that the Yellow Card report form (in the BNF,38 which HCPs often use) has much less space for free text than either the paper YCS forms used by patients or the online Yellow Card system available to all reporters. Encouraging electronic reports by HCPs may result in more detailed reports being submitted.
Proportion of ADR reports coming from patients
Our study showed that over the period October 2005 to September 2007, patient reports constituted 19.8% of all reports from both patients and HCPs. This is comparable with published figures of 19.2% for the Netherlands. 30 In the USA, the proportion of reports from patients has risen from 15% in 200424 to over 40% in 2010. 23 Denmark and Canada have lower levels of patient reporting (see Figure 1), although the Canadian figures will be an underestimate because they do not contain reports categorised as ‘consumer and patient’.
Even though we have shown that public awareness of the YCS is low, there may be lessons that other countries can learn from the success of the Netherlands, the UK and the USA in facilitating patient reporting.
The views and experiences of patients and members of the public regarding patient reporting of adverse drug reactions
Awareness of the Yellow Card Scheme among patients and members of the public
Our national omnibus survey of 2028 adults showed that although one-quarter of the respondents had experienced a side effect from a medicine at some time, fewer than 10% had heard of the YCS and only three people had used it. Those who were aware of the scheme were more likely to be from upper or middle socioeconomic groups and to have completed education post secondary school. Most of those who had experienced a side effect had told a HCP about it (85.5%).
Our questionnaire survey of 1362 patients who had reported an ADR via the YCS found that the most likely source of information about the scheme was from a community pharmacy (49.0%) or general practice (16.2%). Most patients reported the ADR(s) by post, with a third reporting online.
Our finding that a higher proportion of respondents reporting by post learned about the scheme from a pharmacy or GP surgery probably reflects the main source of the forms. From our telephone interviews we know that some patients had seen posters about the scheme or displays of Yellow Cards in pharmacies. Some respondents commented that there appeared to be limited awareness of direct patient reporting among health professionals. Given that responses to our omnibus survey indicated that most of those experiencing a drug side effect told their HCP about it, it is important that HCPs are aware of patients’ ability to report ADRs directly and that they pass this information on to patients.
There are likely to be benefits from increasing public awareness of the YCS. These include increasing the volume of patient reports and reducing delays in reporting. Suggestions from participants about how to increase awareness of the YCS included advertising campaigns and provision of information when patients are issued with their medicines, for example by providing a leaflet with the drugs or information about the scheme on PILs. There may be a need to target publicity among minority ethnic groups and less educated members of the public, given their under-representation among reporters.
The MHRA had advertising campaigns about direct reporting to the YCS in 2005 and 2007. Information is also available on the MHRA website,14 including a video about patient reporting that can also be found on YouTube (www.youtube.com/watch?v=jExeaOpDRbE). There is some evidence from our findings that some patients heard about the YCS through television and radio, whereas others came across the MHRA website when searching the internet for information about their suspected ADRs. These results highlight the need to use multiple media for disseminating information about the scheme. Changes in awareness of the YCS following new publicity campaigns could be monitored by repeating the omnibus survey.
Patient and public views and experiences of reporting to the Yellow Card Scheme
Our questionnaire showed that although, in response to a closed question, most patient reporters found that it at least ‘fairly easy’ to make a report, some thought that the reporting systems could be improved. More detailed suggestions for enhancements arose from our usability testing sessions. In addition, the omnibus survey provided support for continuing with the three different methods of patient reporting.
Motivations for reporting
Several motivations for reporting were identified from the questionnaire survey and telephone interviews. Many patients reported for altruistic reasons accompanied by a sense that they did not want someone else to suffer like they had. Some recognised the importance of contributing to a database of reports so that adverse effects could be identified. A considerable number indicated the need for patients to be aware of possible ADRs, through the PIL or advice from HCPs, to help them make informed choices about whether or not to use medicines. A few had reported hoping that they would to be linked with similar sufferers.
Three of the telephone interviewees felt that patient reports would be different and more complete than HCP reports, thus supporting arguments made in the literature. 52,66 They suggested that patient reports would show a better understanding of the effect of the ADR on a patient’s life and that a HCP report might just consist of a list of symptoms. Participants in our focus group study argued that direct patient reporting would avoid information being reported through a professional lens, and this was backed up by comments in response to the questionnaire. This supports Basch’s66 thesis that patient self-reports of ADRs provide valuable information and capture the subjective elements of patient experiences.
Expectations and desire for feedback
Almost one-third of respondents to our questionnaire expected feedback from the MHRA and just over 60% said that they would have liked feedback. Currently, reporters do not receive individual feedback about their suspected ADR. In the Netherlands, every report submitted to the Pharmacovigilance Centre Lareb, including those made by the public, is assessed by a doctor and pharmacist before contacting the reporter with customised feedback on the ADR reported. 30 A similar system exists in Sweden, run by a non-profit organisation called Kilen. 60
Though these systems are resource intensive, it might be worth considering methods of providing individual feedback in the UK. This could be partly automated by drawing on existing data that the MHRA have on frequency of reports on different drugs and the associated suspected ADRs. Alternatively, in order to ensure that those reporting suspected ADRs do not have unrealistic expectations, the MHRA could make it clear that no personal feedback will be provided. General information about how reports are dealt with could be supplied when acknowledging reports from patients and this might go some way to addressing patient-reporters’ desire for feedback.
Perceived attitudes of health-care professionals towards patient reporting
A small minority of respondents to our questionnaire survey stated that a HCP had discouraged them from making a report and some said that a HCP refused to make a report on their behalf. Furthermore, a total of 294 questionnaire respondents (21.6%) made at least one comment about HCP lack of awareness of ADRs, dismissive attitudes towards ADRs or the lack of awareness of patient reporting. These findings were further supported by comments from our focus groups indicating dismissive attitudes from some HCPs towards patients reporting side effects to them. Similar attitudes were noted in an American study which surveyed patients about their experiences of reporting ADRs associated with statin use to their physicians;67 physicians dismissed the possibility that the suspected ADR might be linked to the statin more often than they agreed that an association was possible. The authors noted ‘Rejection of a possible connection was reported to occur even for symptoms with strong literature support for a drug connection, and even in patients for whom the symptom met presumptive literature-based criteria for probable or definite drug-adverse effect causality’.
One-half of the patients who reported to the Netherlands Pharmacovigilance Centre Lareb53 in the first 6 months of reporting stated that they did so because their health professional did not listen to their complaint about a possible ADR or they were not convinced that the HCP would report their experience. Although, a few similar reports were found in our results, these were uncommon.
Our study gives only the patients’ perspective on the attitudes of HCPs to patient reporting. Nevertheless, whereas clinicians need to make their own careful judgements about the possibility of ADRs in patients presenting to them, it is likely that some HCPs would benefit from learning more about side effects associated with drugs, how these present in patients and how they affect patients’ lives. As noted above, clinicians also need to be more aware of the availability of patient reporting and to be more proactive in informing patients about this. In addition, it would be helpful to explore in more detail, why some HCPs have dismissive attitudes towards patient reporting of suspected ADRs and whether these attitudes can be altered by exposure to the findings of studies that indicate the pharmacovigilance benefits of patient reporting.
Method of reporting
Our studies indicate that each of the three different methods of patient reporting of suspected ADRs suit different people. The omnibus data about perceived convenience of different reporting methods was particularly relevant. Given the continuing growth of internet access it is not surprising that many respondents, particularly younger ones, considered this method to be a very convenient way of reporting ADRs. It is clear, however, that for many people (particularly the elderly) this method of reporting would not be useful or possible. It is important, therefore, to continue to make available the other methods of reporting, including telephone reporting, which many people considered very convenient.
Our usability testing study identified a number of enhancements that could be made to the paper-based and online reporting systems. Suggested changes to online reports included:
-
making it easier for users to navigate through the web pages
-
reducing the complexity of the drop-down menu options for ADRs
-
allowing users to save the report as they are going through it.
Our findings suggest that the frustration that occurs when data are lost by navigating backwards when trying to complete a YCS report online may result in the reporter abandoning their attempt at reporting. Changes to the online report forms could probably be made relatively easily. There are many good examples available of user-friendly online systems, particularly those that protect data with autosave functions and allow for easy navigation between screens.
With respect to reducing the complexity of the drop-down menu options for ADRs, we recognise the advantages to the MHRA of having MedDRA terms that can be imported directly to the MHRA database. Nevertheless, many of these terms are not easily understood by patients. It would help them if it was easier to record their ADR in free text, or using higher level terms such as ‘rash’ rather than having to select from a long list of different medical terms for rash.
One thing worth noting here is the importance of the interaction between users and the different systems of reporting. Not only did we identify technical issues, but also respondents’ motivations, expectations, willingness and ability to complete. Reporting systems are sociotechnical68 and there is a strong motivational component to their successful operation, which influences the willingness and decision to report.
Modifying the online form to fit more with patient requirements, while capturing similar data from both patients and HCPs, would probably be the optimal approach to allow for collection of comparable data, but in a way that patients find straightforward.
Suggested changes to paper reports included:
-
allowing more space for the recording of multiple medications
-
having a larger font size for people with visual impairment
-
redesigning the envelope so that the report fits within it more easily.
Changes to paper reports would require a redesign and we recognise that a balance is needed with respect to the amount of space provided for answers on the form. Nevertheless, on both patient and HCP forms, there is limited room for free text, and for recording multiple morbidities and multiple medications. Whereas the forms encourage reporters to add additional sheets of information if there is not enough space, members of the public involved in the usability testing were not keen to do this.
Although our usability tests identified no problems with telephone reporting, some concerns were expressed by participants about the limited hours of availability (10 am to 2 pm on weekdays). Given that telephone reporting was a popular option in our omnibus survey, particularly among older people and those of lower socioeconomic status, consideration should be given to extending the hours of availability and increasing publicity about telephone reporting. It is noteworthy that posters previously available to promote the YCS did not mention the possibility of reporting by telephone. If it is not possible to staff the line for extended hours, then there does need to be a facility for patients to record their contact details so that their call can be followed up.
Harmonisation across reporting schemes
Our review of the literature has shown that spontaneous reporting schemes that include patient reports vary greatly. Some schemes involve government-funded organisations, for example MHRA in the UK and MedWatch in the USA, whereas others are patient organisations, for example Institute for Responsible Medicines Use (IVM) in the Netherlands (www.medicijngebruik.nl). Some countries have multiple schemes, for example Australia and the Netherlands, thereby potentially creating a risk of duplication of reports and effort, and perhaps more importantly, creating databases that cannot be compared due to different information being collected. Some systems accept reports solely for ADRs, while others include medicine and product-related problems, including errors, for example AME Line in Australia and Health Canada. What is apparent from our review is the disparity that exists across and within schemes. Whereas the USA uses the same data collection form for patients and HCPs, the UK has two different versions, one for each type of reporter, with important differences in key information, for example, categories of ‘seriousness’ of ADR.
To maximise the value derived from patient reports, national patient and HCP schemes should at least be compatible with one another; ideally with other national schemes. At the time of preparing this manuscript, changes to legislation affecting pharmacovigilance were being planned in the European Parliament. 55,69 It is timely for nations with existing patient reporting schemes to engage in a harmonisation process regarding data collection, analysis and dissemination to inform the future of patient reporting. Although the need for harmonisation in Europe has been recognised,70 a wider international approach should also be considered. There is also a need to promote pharmacovigilance in developing countries,71 preferably including patient reporting. This will require appropriate funding, training and public awareness for success.
Strengths and limitations of our studies
Success in addressing the objectives of the evaluation
Overall, we have been successful in addressing the main objectives of the evaluation and answering the research questions set out in Table 1 in Chapter 1 and in our original protocol (Appendix 23). We had only partial success in addressing our original plans for the following aspects of the work (more detailed explanations are given further below):
-
In the descriptive analysis of reports from patients and HCPs there were few categories on which we could directly compare the seriousness and outcomes of suspected side effects.
-
In the signal generation analysis:
-
– It was difficult to assess whether new signals generated by patients were for events that are expected to be of more concern to patients than HCPs.
-
– With only 2 years’ worth of data there was limited scope to make a valid judgement on whether patient reports generate new signals sooner.
-
-
When selecting cases for the qualitative analysis of Yellow Card reports:
-
– We identified and analysed 449 reports, but did not reach our original suggested target of 600 reports.
-
– There were very few reports relating to OTC medicines or complementary therapies.
-
-
For the focus groups and usability testing, originally we had planned to include patients who believed they may have experienced side effects from medications, but had not previously filled in a Yellow Card report. We decided, however, that this would make recruitment difficult and widened our selection criteria to include all adults.
Literature review and survey of international experiences of reporting schemes
Although the literature review was conducted systematically, the searches were not exhaustive. Although only studies published in English were included, it is unlikely that any major comparative study (of patient and HCP reports) has been omitted, because most countries with longer established patient reporting schemes have already published reports or studies in English.
The online version of a major review of patient reporting of suspected ADRs was published in 2006. 24 Our review has identified a number of papers published since then, including two large studies comparing patient and HCP reports from Denmark19 and the Netherlands. 30 Other studies we identified were of variable quality, with some being available only as conference abstracts.
The questionnaire was sent to 47 different countries using the pharmacovigilance contacts listed by the WHO Uppsala Monitoring Centre website (www.WHO-UMC.org). Nevertheless, there was a low response rate, despite a reminder e-mail being sent. From the responses received, however, and from the ‘15-country survey and literature review’ on direct patient reporting of ADRs,15 as well as the WHO survey of pharmacovigilance schemes in low- and middle-income countries,16 it is clear that patient reporting has been introduced to an increasing number of national pharmacovigilance schemes.
There is no single source of information showing whether a country has patient spontaneous ADR reporting. To allow for sharing of information and future comparisons, it would be helpful to have a centralised resource of all national patient reporting schemes, similar to that held by the WHO Uppsala Monitoring Centre. Nevertheless, when comparing information on patient reporting from different countries it needs to be recognised that there may be differences in the types of problems that can be reported, the available methods of reporting, the types of data collected and other context specific factors.
Analysis of reports of suspected adverse drug reactions from the UK Yellow Card Scheme
This is the first study in the UK to investigate large numbers of reports of suspected ADRs from patients and HCPs. Our descriptive analysis allows these reports to be compared with large retrospective analyses of other national reporting schemes. In addition, we undertook signal generation analyses, which enabled us to answer a number of questions raised in the literature regarding the pharmacovigilance impact of patient reports. Furthermore, our qualitative analyses of extracts from patient and HCP Yellow Card reports have provided useful information on the richness of patient reports, how patients describe ADRs, the impact of ADRs on patients’ lives, the clinical significance of patient reports and likely causality.
One strength of our study has been collaboration with the MHRA. This enabled us to obtain and analyse all ADRs reported over 2-year period, giving access to a database of > 26,000 reports including just over 5000 from patients. As a result, our study was larger than previously published major studies from Denmark19 and the Netherlands. 30 This has also provided us with a sizeable database for signal generation analysis and for the sampling of reports for qualitative analyses.
We worked carefully with the MHRA to ensure that, wherever possible, any anomalies in the database were corrected. This meant that, as far as we can tell, we had virtually complete information on key variables, such as the drugs and reactions reported by patients and HCPs. Nevertheless, many reporters failed to complete all fields and so data were missing for some variables, including indication for the drug, reaction outcome and time to report a reaction. Gender and age was missing for 3.6% and 10%, respectively, of all reports.
We noted several differences between the patient and HCP Yellow Cards. The most notable difference related to the perceived seriousness of the suspected reaction; only three of the six options were comparable. Nevertheless, we were able to directly compare the seriousness of suspected ADRs reported by patients and HCPs using the MHRA classification of seriousness based on the MedDRA dictionary.
For reasons outlined in Chapter 3, we were not able to calculate the frequency of duplicate reports in the database of patient and HCP reports. We found some evidence to suggest duplicate reports, but these appear to be uncommon; probably not at a level to invalidate the pharmacovigilance assessments.
The main limitation of our disproportionality analysis, was that the data were accumulated over only a 2-year period, soon after the launch of patient reporting. Reports from the pharmaceutical industry (normally around 40% of all reports received by MHRA each year) were also excluded, as the aim of the project was to compare direct reporting by patients and HCPs. Furthermore, as data from HCP reports received in the YCS before the study period were not included in our data set, it was not possible to analyse SDRs generated over many years. In addition, this meant that we could not investigate properly whether the introduction of patient reporting leads to earlier or later detection of signals.
In the database we used for our studies, patient reports comprised around one-fifth of all reports. In reality, the proportion of patient reports in the YCS database will be much smaller, as the data have accumulated since 1964. Hence, the contribution of patient reports to quantitative signal generation is likely to be smaller, although it will increase as patient reports accumulate.
We wished to investigate the relative contribution of patient reports to signal generation, and so we wanted every patient report to contribute as much information as possible, especially as it was clear that patient reports were different to HCP reports in terms of suspect drugs and reactions reported. The majority of the signals generated involved reactions that are not classed as ‘serious’ by the MHRA. In practice, these reactions would not routinely be prioritised by their signal detection system (unless they involved certain events on the MHRA’s ‘alert’ list or those involving a child). Nevertheless, we have shown the contribution that patient reports made to signals for reactions that are classed as ‘serious’ by MHRA. It is important to note that the signals ‘lost’ or ‘gained’ in this study were intended as examples to illustrate the potential effect of adding patient reporting to the spontaneous reporting database. It is difficult to say whether one ‘signal’ is more important than another. This would depend on a number of factors such as the level of use/exposure of the drug in the population, the type of population using the drug, the drug’s indication, the tolerability of the reaction. A detailed impact analysis for each of the drug–reaction pairs in question would be required in practice, but was beyond the scope of this project.
Another possible limitation of our signal generation analysis was that it was based only on the proportional reporting ratio method (see Chapter 5 for further details). There are several methods for disproportionality analysis and different thresholds for filtering potentially important reactions. In order to maximise the size of the data sets in this study we analysed drugs and vaccines together, rather than analysing these separately as the MHRA would do. Ideally, our results should be replicated by the MHRA using source data.
In our main qualitative analysis, we included many patient and HCP Yellow Card reports, looking at a range of purposively selected drug–ADR pairs. There was, however, sometimes an imbalance in the number of reports made by patients and HCPs available for comparison. We had planned to analyse around 600 reports in total, but the sample generated was smaller than anticipated (270 patient reports and 179 HCP reports) because although the database appeared to contain a very large number of suspect drugs and reactions that met our sampling strategy criteria, specific cases within the database were replicated many times if multiple drugs and multiple reactions had been recorded. Having completed the time-consuming process of selecting reports (which took several months) we did not have time to go back and select additional cases. Nevertheless, having undertaken the qualitative analysis of 449 reports, we judge that it is unlikely that our findings would have changed substantially with 600 reports.
We focused our attention on the medicines most commonly reported by patients and those that at the time of the study were classified by the MHRA as ‘black triangle’ drugs. For these groups of drugs we had relatively large amounts of data. For reports of medicines that were purchased OTC and complementary medicines, we had to focus on patient reports only because there were so few reports from HCPs; even then, the numbers of reports were few.
Our content analysis of reports was necessarily subjective and could have been biased because the researcher knew whether the reports were from patients or HCPs. Nevertheless, the work was checked carefully (by AA) throughout the process and very few misinterpretations, or inaccuracies in data entry, were apparent. For some categories, such as text indicating the impact of the suspected ADR on the patient, the contrast between patient and HCP reports was so marked that they clearly represent genuine differences. For other categories, such as text indicating temporal relationships between medicines and suspect ADRs, differences were less marked and did not take account of information available in other fields on the Yellow Card report database.
When considering different types of report, there was some concern among the research team that the meaning of patients’ concerns may be changed when telephone reports are transcribed at the MHRA. Reassuringly, the qualitative analysis indicated that telephone reports were not very different from internet and paper reports, often including information that was assumed to be direct patient quotes.
Our in-depth qualitative analysis of reports was, by its nature, subjective and could have been biased because we knew who the reporter was. Nevertheless, we checked the data carefully, rereading the reports several times and sought out divergent cases to counter possible biases.
Questionnaire of patient reporters and telephone interviews
Our questionnaire was the first large national study to follow-up patients or their representatives reporting via the YCS. The response rate of 68% was reasonably high; probably helped by distribution of the questionnaires directly by the MHRA. Although, we had no information on the characteristics of non-responders, the age and gender of respondents to our questionnaire was similar to those of all patient reporters in the MHRA database used for the retrospective analysis of Yellow Card reports. Therefore, it is likely that the findings are generalisable to all patient reporters at the time of our study. The high number of responses to open questions shows that reporters were willing to express their views in detail; this information has given us valuable insights into many aspects of patient reporters’ experiences of the YCS.
These more in-depth comments were supplemented by telephone interviews, which allowed us to further explore issues regarding ease of reporting, motivations for reporting and promotion of the scheme. Of the 27 interviews, we managed to interview only two people who had reported by telephone and two who had reported via the internet, so our results are biased towards those who reported using the paper Yellow Card. This imbalance occurred because of difficulties arranging interviews with patients who had reported using the internet. In addition, because of circumstances beyond our control, the data were analysed by a third person (CA) who had not done the interviews. This may have led to misinterpretation of data, although the researcher listened to the tapes and repeatedly reviewed the transcripts. The proportion of women interviewed (70%) was slightly higher than the proportion of women in the questionnaire survey (66.8%) and the median age of interviewees was also slightly higher (60 years vs 56.5 years). It should be noted, that the interviewees are likely to have been a highly motivated group of people who not only completed a Yellow Card report and a questionnaire, but also who volunteered to take part in the interviews; not surprisingly, their views about ease of reporting were different from those in the usability study.
Usability study and focus groups
The participants recruited for the focus groups and usability testing covered a range of demographic characteristics, with a similar proportion of women (67.5%) to that found in our other studies, but they were slightly older and there was a higher proportion from minority ethnic groups (15%).
The scenarios for the usability tests were ‘hypothetical’. This may have introduced some artificiality as the participants may not have been familiar with the details contained within these scenarios. It might also have lengthened the process of completing the forms by any of the three methods. Conversely, the process may have been shortened as dates for starting and finishing the medicines were included in the scenarios, whereas in real life participants would have had to recall this information, look it up or ask someone else.
For practical reasons we recruited from the Nottingham area and decided to widen our selection criteria for the focus groups and usability testing to all adults. Even so, it was a challenge to recruit 40 participants and it is unlikely that we would have achieved this number if we had restricted entry criteria to people who had experienced ADRs. Although it is possible that we would have obtained different findings using members of the public from other parts of the country, we doubt whether any important additional issues would have been detected from the usability assessments.
Nevertheless, some of the findings from this part of the study are different from our studies of patient reporters, and this may, in part, have been owing to the sampling. For example, whereas some of our focus group participants say that they would have ‘given up’ trying to report because of difficulties they had experienced with the online reporting system, it is possible that people who had suffered a suspected ADR would have been more persistent.
National omnibus survey
The sample of > 2000 members of the British public was broadly representative of the general population, although they were different to patient reporters to the YCS. The response rate was very low, a common issue with this type of survey. We were aware of this issue at the time of planning our study, but judged that the advantages of being able to conduct a national survey at relatively low-cost outweighed the difficulties of using more rigorous techniques. Although it is possible that another type of survey might have revealed some different findings to those observed in the omnibus sample, we doubt that other methods would indicate a substantially higher level of public awareness of the YCS.
One advantage of the omnibus survey was that it could be repeated at regular intervals and at low cost to track public awareness.
Conclusions
Patient reports gave detailed descriptions of suspected ADRs, attributed reactions to specific medicines and provided information useful for assessing causality. Patient reports often had richer narratives than those of HCPs and rarely provided irrelevant information or ambiguities. Patient reports also often contained detailed information about the impact of the suspected ADR on the patient’s life, thus providing insights that there were comparatively rare in HCP reports.
Patients showed different patterns of reporting of drugs and ADRs compared with HCPs. Nevertheless, similar proportions of reports contained at least one reaction term that was classified as ‘serious’ by the MHRA, demonstrating that most patients report clinically significant problems.
Signal generation analysis showed that patient reports were markedly different from those of HCPs in terms of the drug–ADR combinations reported. Patient reports add weight to HCP reports by creating new and important potential signals, although a smaller number of potential signals from HCP reports are ‘lost’ when the data sets are combined.
In relation to the existing literature, there are broad similarities between our study and large-scale Danish and Dutch studies comparing patient reports with HCP reports. In keeping with the literature, our study has also demonstrated the richness of patient reports. Whereas previous studies suggest that patient reports identify previously unknown ADRs, ours is the first large-scale study, we are aware of, that has undertaken detailed signal generation analysis.
Most patient reporters found it at least ‘fairly easy’ to report, but many had suggestions for improvements in reporting methods and publicity for the scheme, and most would have liked feedback from the MHRA. Suggestions for improvements to publicity for the YCS, and detailed comments on the design of reporting methods, came also from members of the public involved in focus groups and usability testing. All of these studies identified the need to continue with the three different modes of reporting and to redesign the paper-based and internet reporting systems. A national survey demonstrated low levels of public awareness of the YCS.
This study has demonstrated the value of patient reporting to the YCS, and provides justification for further promotion of patient reporting, and for making improvements to existing reporting systems.
Implications for patient reporting
In the authors’ opinion, the following approaches may help to improve the timeliness and value of patient reporting for pharmacovigilance; increase the number of reports from patients; and improve patient experiences of reporting:
-
increase publicity for patient reporting by:
-
– advertising campaigns targeted at the public and HCPs
-
– encouraging proactive efforts by HCPs, particularly in community pharmacy and general practice, to publicise and promote patient reporting
-
– providing information on patient reporting at the point of dispensing, ideally by incorporating this information within PILs
-
-
provide further guidance to reporters on what information to report (this may help strengthen the usefulness of both patient and HCP reports)
-
increase patient awareness of medicines where the MHRA is undertaking intensive monitoring, for example by highlighting this on PILs
-
change the design of paper reports and the online reporting system, in line with suggestions made in this report, and increase the number of hours during which telephone reports can be made
-
provide general feedback to patient reporters on what the MHRA does with reports
-
explore possibilities for providing specific feedback to patients in relation to the medicines and suspected ADRs that they report.
To aid future comparisons of reports submitted by patients and HCPs, it important that similar information is collected from both groups, particularly with respect to categories of seriousness.
According to patient accounts, some HCPs seem to be unaware that patients can submit their own ADR reports, and some appear to be dismissive of patients who report suspected ADRs. Education at undergraduate and postgraduate level might help address these issues.
Recommendations for research
In order of priority these are investigating:
-
the pharmacovigilance impact of patient reporting in a long-term study, including the identification and tracking of regulatory action taken as a result of the contribution of patient reports, such as the addition of new ADRs to PILs, SPCs and the BNF
-
the optimum approach to signal generation analysis of patient and HCP reports. It is important to establish, in practice, the true extent to which important signals may be identified or missed by combining data from patient and HCP reports for data mining purposes and the feasibility of analysing the data separately by reporter group, in addition to analysing all reports combined
-
the burden of ADRs in terms of impact on patients’ lives, and evaluating the extent to which patients’ views and experiences of the seriousness of ADRs concur with those of regulatory bodies such as the MHRA
-
the knowledge and attitudes of HCPs patient reporting of ADRs, and evaluating approaches aimed at addressing any learning needs identified
-
the value of using patient reports of ADRs to help other patients and HCPs who are seeking information on patient experiences of ADRs
-
the impact of increasing publicity and/or enhancements to reporting systems on the numbers and types of Yellow Card reports from patients. This would be especially important if patients were to be provided with information on drugs undergoing intense monitoring, for example black triangle drugs.
Acknowledgements
We thank the following:
-
Millie Kieve and other members of the advisory group who have given considerable amounts of time to the project, particularly in terms of commenting on our findings and on drafts of our report.
-
Mick Foy and colleagues at the MHRA for providing data for the project, administering the questionnaire and answering queries.
-
The referees appointed by NIHR HTA for their detailed and helpful comments and suggestions on our report.
-
Clare Randall, Administrator, University of Nottingham for administrative support during the final 18 months of the project and for considerable work formatting the report.
-
Randy Barber, Administrator, University of Nottingham for administrative support in the first 16 months of the project, including administration of the questionnaire.
-
April McCambridge, Research Unit Administrator, University of Nottingham, for importing references into endnote and for helping with referencing in the final report.
-
Barbara Rupnik, Medical Student, for assistance with analysis of the omnibus survey and aspects of the questionnaire survey (ATC coding of drugs and checking a 10% sample of reports for accuracy of data entry).
-
Vaibhav Gupta, Medical Student, University of Nottingham, for undertaking content analysis of Yellow Card reports as part of the qualitative analysis.
-
Dr Tim Payne, Research Fellow, University of Nottingham, for assisting with the sampling of Yellow Card reports for the qualitative analysis and for undertaking most of the telephone interviews.
-
Mr Andrew Hauschild, Software Developer at the DSRU for his IT support and software development throughout the study.
-
Dr Victoria Cornelius, Statistician, DSRU, for ongoing statistical support.
-
Professor Stephen Evans, Professor of Pharmacoepidemiology, London School of Hygiene and Tropical Medicine, for his valuable suggestions on the statistical elements of the signal generation analysis.
-
Dr Vanessa Marshall, Senior Research Fellow, DSRU, for her contributions to writing the initial study protocol.
-
Dr Stella Anakwe-Umeh, Researcher, University of Aberdeen, for assisting with the literature review and survey to identify countries that have introduced patient reporting of ADRs.
-
Julia Taylor, Researcher, Liverpool John Moores University, for assisting with the analysis of free-text comments from the questionnaire survey of patients who had reported to the YCS.
In addition, AA would like to thank his family (particularly Chris and Izzy) for tolerating many evenings, nights and weekends taken up with him putting together this report.
Contribution of authors
The authors of this report are listed in alphabetical order, apart from Professor Avery who, as Chief Investigator, is listed first.
The following authors were involved in the construction of the original bid including the research design: AA, CA, AB, HF, PH, JK, AL, EM, SS, MW.
All authors were part of the project management team which had regular (monthly) meetings (usually by teleconference) at which the following were discussed: study design, research methods, analysis and interpretation of findings, and the writing up and dissemination of the project.
Below we have listed the specific contributions of each author:
-
Professor AJ Avery (Academic GP and Chief Investigator) led the project, contributed to each of the studies, liaised with the MHRA and advisory group, conducted the sampling the qualitative analysis of Yellow Card reports and was responsible for producing the final report.
-
Professor C Anderson (Professor of Social Pharmacy, with expertise in qualitative research) led the studies involving qualitative analysis of Yellow Card reports, telephone interviews of patients reporting to the YCS, focus groups and usability testing. She undertook the qualitative analysis for the first two of these studies and supervised the analysis of the focus groups and usability testing. She had a major responsibility for writing the chapters relating to these studies. She also had the idea for the omnibus survey and helped to design this.
-
Professor CM Bond (Professor of Pharmacy) contributed in detail to the questionnaire design and to the interpretation of findings of the survey; contributed to omnibus questions and interpretation of findings; contributed to secondary analysis and synthesis and detailed write-up of focus groups and usability testing study; contributed to write-up of interview data and the in-depth analysis of reports; and contributed to interpretation of analysis of Yellow Card reports.
-
Dr H Fortnum (Epidemiologist and Reader in Hearing Research) contributed specifically to the design, implementation and interpretation of the questionnaire, usability testing study, and omnibus survey. She was responsible for completing the omnibus survey chapter.
-
Dr A Gifford (Lecturer in Pharmacy) conducted the qualitative research relating to usability testing of the YCS and some of the telephone interviews with individuals who had completed YCS report.
-
Professor PC Hannaford (Professor of Primary Care) provided particular input into the analysis and interpretation of the quantitative data from MHRA and questionnaire data, and interpretation of the signal generation analyses. He gave detailed input into the drafting of corresponding sections of the report.
-
L Hazell (Pharmacist and Researcher, with expertise in pharmacovigilance) liaised with MHRA regarding request for data set on Yellow Cards; checked the data set(s) and resolved queries and anomalies with the MHRA; prepared data set for quantitative analysis by Aberdeen colleagues, including mapping of drug names to ATC classification system; contributed to the plan for quantitative analysis; prepared various data sets for Nottingham colleagues to facilitate and support qualitative analysis; planned and conducted signal generation analysis; planned and performed causality assessments on a sample of reports; and was responsible for writing the chapters describing the processing of Yellow Card data and the signal generation analysis.
-
Professor J Krska (Professor of Pharmacy Practice) contributed in detail to the questionnaire design and undertook the analysis of the qualitative findings of the questionnaire survey; contributed to omnibus questions; and contributed to write-up of focus groups and usability testing study, interview data and in-depth analysis of reports. She also provided support for the establishment of the advisory group and attended all advisory group meetings.
-
Professor AJ Lee (Professor of Medical Statistics) provided statistical support for the original application including advice on design, sample size and proposed statistical analysis of the quantitative components. She supervised the Aberdeen-based statistician on the analysis of the questionnaire data and comparison of the patient and HCP Yellow Card reports. She conducted the statistical analysis of the omnibus survey.
-
Dr DJ McLernon (Research Fellow in Medical Statistics) undertook the descriptive statistical analysis of the Yellow Card data from the MHRA, and data from the questionnaire survey. He drafted the chapters of the final report relating to the descriptive analysis of Yellow Card reports and the questionnaire survey.
-
Professor E Murphy (Professor of Sociology) contributed to the design, conduct and analysis of the studies, involving qualitative analysis of Yellow Card reports, telephone interviews of patients reporting to the YCS, focus groups and usability testing, and to the design of the questionnaire.
-
Professor S Shakir (Director of the Drug Safety Research Unit) contributed to the development of the concept of the study (particularly for the quantitative analysis, including the signal generation analysis) and has overseen all of the contributions to the project by Lorna Hazell.
-
Dr MC Watson (Academic Pharmacist and Senior Research Fellow) contributed particularly to the design, evaluation and write-up of the quantitative analysis and questionnaire. In addition, she led on the conduct and analysis of the literature review and contributed to the writing of the discussion using information from the literature review.
Project advisory group
The project advisory group was chaired by Millie Kieve and included representatives of the main professional groupings making reports, and patient representatives from both patient support groups and patient advocacy groups (see Appendix 4 for the list of members of the advisory group).
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Metters J. Report of an Independent Review of Access to the Yellow Card Scheme 2004.
- International Society of Drug Bulletins (ISDB) . Berlin Declaration on Pharmacovigilance (ISDB Workshop) 2005.
- Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18,820 patients. BMJ 2004;329:15-9.
- Davies EC, Green CF, Taylor S, Williamson PR, Mottram DR, Pirmohamed M. Adverse drug reactions in hospital in-patients: a prospective analysis of 3695 patient-episodes. PLoS ONE 2009;4.
- Committee on the Safety of Medicines . Report of the Working Party on Adverse Reactions: Part 1 1983.
- World Health Organization (WHO) . Consumer reporting of adverse drug reactions. WHO Drug Information 2000;14:211-15.
- Hazell L, Shakir SAW. Under-reporting of adverse drug reactions: a systematic review. Drug Saf 2006;29:385-96.
- McGettigan P, Golden J, Conroy RM, Arthur N, Feely J. Reporting of adverse drug reactions by hospital doctors and the response to intervention. Br J Clin Pharmacol 1997;44:98-100.
- Hammond IW, Rich D. Consumers usurp spontaneous adverse event reporting in the United States. Pharmacoepidemiol Drug Saf 2005;14:S8-9.
- Ekins-Dauke S, Irvine D, Wise L, Fiddes S. The Yellow Card Scheme: evaluation of patient reporting of suspected adverse drug reactions. Pharmacoepidemiol Drug Saf 2006;15.
- Cox A. Patient reporting of adverse drug reactions. Pharmacovigilance Rev 2009;3:18-21.
- British Medical Association and Royal Pharmaceutical Society of Great Britain . British National Formulary 2009.
- Medicines and Healthcare products Regulatory Agency (MHRA) . Download Reporting Forms and Promotional Material for the Yellow Card Scheme n.d. www.mhra.gov.uk/Safetyinformation/Reportingsafetyproblems/Reportingsuspectedadversedrugreactions/Downloadreportingformsandpromotionalmaterial/index.htm (accessed 9 June 2010).
- Medicines and Healthcare products Regulatory Agency (MHRA) . What Happens to a Yellow Card n.d. www.mhra.gov.uk/Safetyinformation/Howwemonitorthesafetyofproducts/Medicines/TheYellowCardScheme/WhathappenstoaYellowCard/index.htm (accessed 9 June 2010).
- Herxheimer A, Crombag M, Alves TL. Direct Patient Reporting of Adverse Drug Reactions: A Fifteen-Country Survey and Literature Review (Paper Series Reference 01–2010 05) 2010.
- Olsson S, Pal SN, Stergachis A, Couper M. Pharmacovigilance Activities in 55 low- and middle-income countries: a questionnaire-based analysis. Drug Saf 2010;33:689-703.
- McGuire T, Moses G. What Do Consumers Contribute to Pharmacovigilance? Lessons from the AME Line n.d. www.icms.com.au/nms2006/abstract/132.htm.
- Health Canada . Adverse reaction and incident reporting – 2008. CARN 2009;19.
- Aagaard L, Lars Hougaard N, Ebba Holme H. Consumer reporting of adverse drug reactions: a retrospective analysis of the Danish Adverse Drug Reaction Database from 2004 to 2006. Drug Saf 2009;32:1067-74.
- Egberts TCG, Smulders M, de Koning FHP, Meyboom RHB, Leufkens HGM. Can adverse drug reactions be detected earlier? A comparison of reports by patients and professionals. BMJ 1996;313:530-1.
- Hazell L, Cornelius V, Shakir SAW, Avery A. Patient reporting of adverse drug reactions in the UK: a comparison of potential signals generated by patients and healthcare professionals. Drug Saf 2009;32.
- Hazell L, Cornelius V, Shakir SAW, Avery A. Patient reporting of ADRs in the UK: how do patient reports affect potential signals generated by health professionals?. Drug Saf 2009;32.
- Adverse Events Reporting System (AERS) . AERS Reporting by Healthcare Providers and Consumers by Year n.d. www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm070456.htm (accessed 9 June 2010).
- Blenkinsopp A, Wilkie P, Wang M, Routledge PA. Patient reporting of suspected adverse drug reactions: a review of published literature and international experience. Br J Clin Pharmacol 2007;63:148-56.
- Adverse Medicine Events (AME) Line n.d. www.mater.org.au/ame/HomeAME1.htm (accessed 9 June 2010).
- Hill R, Tan A. Adverse Drug Reaction Reporting and Quality Use of Medicines n.d.
- Moses G, McGuire T. Consumers Reporting Novel ADRs: A Zolpidem Case Series n.d. www.icms.com.au/nms2006/abstract/136.htm.
- Health Canada . Reporting Adverse Reactions to Drugs and Other Health Products n.d. www.hc-sc.gc.ca/dhp-mps/medeff/report-declaration/reporting-declaration-eng.php#a1 (accessed 9 June 2010).
- Netherlands Pharmacovigilance Centre (Lareb) . Annual Report from Netherlands Pharmacovigilance Centre 2005. www.lareb.nl Hertogenbosch: Lareb.
- de Langen J, van Hunsel F, Passier A, de Jong-van den Berg L, van Grootheest K. Adverse drug reaction reporting by patients in the Netherlands: three years of experience. Drug Saf 2008;31:515-24.
- Meldpunt Medicijnen n.d. www.meldpuntmedicijnen.nl (accessed 14 June 2010).
- van Geffen ECG, van der Wal SW, van Hulten R, de Groot MCH, Egberts ACG, Heerdink ER. Evaluation of patients’ experiences with antidepressants reported by means of a medicine reporting system. Eur J Clin Pharmacol 2007;63:1193-9.
- MedWatch . MedWatch Online Voluntary Reporting Form n.d. www.fda.gov/medwatch/report.htm (accessed 9 June 2010).
- Food and Drug Administration (FDA) . Annual Adverse Drug Experience Report: 1996 1997.
- Jarernsiripornkul N, Kakaew W, Loalukkana W, Krska J. Adverse drug reaction monitoring: comparing doctor and patient reporting for new drugs. Pharmacoepidemiol Drug Saf 2009;18:240-5.
- Debordeaux F, Castel M, Sailler L, Arlet P, Pierre E, Montastruc JL, et al. Characteristics of adverse drug reactions reported by patients: which difference with doctors’ reports?. Drug Saf 2009;32.
- Nasrallah-Irles D, Castot A, Thomas L, Babai S, Delorme B, Le-Louët H. Signalement d’événements indésirables par les patients: étude pilote réalisée avec la collaboration d’associations de patients [adverse drug reactions: a pilot study on patient reporting through patient associations]. Thérapie 2008;63:385-92.
- British Medical Association and Royal Pharmaceutical Society of Great Britain . British National Formulary. 2010.
- WHO Collaborating Centre for Drug Statistics Methodology . ATC Structure and Principles n.d. www.whocc.no/atc/structure_and_principles/ (accessed 9 June 2010).
- McLernon DJ, Bond CM, Hannaford PC, Watson MC, Lee AJ, Hazell L, et al. on behalf of the Yellow Card collaboration . Adverse drug reaction reporting in the UK: a retrospective observational comparison of Yellow Card reports submitted by patients and healthcare professionals. Drug Saf 2010;33:775-88.
- Hauben M, Aronson J. Defining ‘Signal’ and its subtypes in pharmacovigilance based on a systematic review of previous definitions. Drug Saf 2009;32:99-110.
- Ståhl M, Lindquist M, Edwards IR, Brown EG. Introducing triage logic as a new strategy for the detection of signals in the WHO Drug Monitoring Database. Pharmacoepidemiol Drug Saf 2004;13:355-63.
- Levitan B, Yee CL, Russo L, Bayney R, Thomas AP, Klincewicz SL. A model for decision support in signal triage. Drug Saf 2008;31:727-35.
- Bate A, Evans SJW. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf 2009;18:427-36.
- DuMouchel W. Bayesian data mining in large frequency tables, with an application to the FDA Spontaneous Reporting System. Am Stat 1999;53:177-90.
- Evans SJ, Waller PC, Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf 2001;10:483-6.
- European Medicines Agency (EMEA) . Guideline of the Use of Statistical Signal Detection Methods in the Eudravigilance Data Analysis System 2006. www.emea.europa.eu/pdfs/human/phvwp/10646406en.pdf (accessed 9 June 2010).
- Norén GN. Statistical Methods for Knowledge Discovery in Adverse Drug Reaction Surveillance 2007. http://su.diva-portal.org/smash/record.jsf?pid=diva2: 197004.
- Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239-45.
- National Statistics Opinions (Omnibus) Survey . User Guide n.d. www.statistics.gov.uk/about/services/omnibus/downloads/User_Guide.pdf (accessed 9 June 2010).
- Thomas K, Coleman P. Use of complementary or alternative medicine in a general population in Great Britain. Results from the National Omnibus survey. J Public Health 2004;26:152-7.
- Medawar C, Herxheimer A. A comparison of adverse drug reaction reports from professionals and users, relating to risk of dependence and suicidal behaviour with paroxetine. Int J Risk Safety Med 2003 2004;15:5-19.
- van Grootheest AC, de Jong-van den Berg L. Review: patients’ role in reporting adverse drug reactions. Expert Opin Drug Saf 2004;3:363-8.
- George C. Reporting Adverse Drug Reactions. A Guide for Healthcare Professionals 2006.
- Commission of the European Communities . Proposal for a Regulation of the European Parliament and of the Council Amending, As Regards Pharmacovigilance of Medicinal Products for Human Use, Regulation (EC) No 726 2004 Laying down Community Procedures for the Authorisation and Supervision of Medicinal Products for Human and Veterinary Use and Establishing a European Medicines Agency 2008.
- Foster J, van der Molen T, de Jong–van den Berg L. Patient reporting of side effects may provide an important source of information in clinical practice. Eur J Clin Pharmacol 2007;63:979-80.
- Jarernsiripornkul N, Krska J, Richards ME, Capps PAG. Patient reporting of adverse drug reactions: useful information for pain management?. Eur J Pain 2003;7:219-24.
- van Grootheest K, de Graaf L, de Jong-van den Berg LTW. Consumer adverse drug reaction reporting: a new step in pharmacovigilance?. Drug Saf 2003;26:211-17.
- Waller PC. Making the most of spontaneous adverse drug reaction reporting. Basic Clin Pharmacol Toxicol 2006;98:320-3.
- Health Action International (HAI) . Patients’ Reporting of Adverse Reactions. Outcomes of a Seminar Organised by Health Action International Europe 26 May 2005 2005. www.haiweb.org/docs2005/final_report.doc (accessed 9 June 2010).
- Cumber SL, Heffer SJ, Gandhi S, Avery AJ. The Yellow Card Scheme: Experience of patient reporting of adverse drug reactions 5 years since launch. Pharmacoepidemiol Drug Saf 2010;19.
- Perry BA, Turner LW. A prediction model for polypharmacy: are older, educated women more susceptible to an adverse drug event?. J Women Aging 2001;13:39-51.
- Zopf Y, Rabe C, Neubert A, Gassmann KG, Rascher W, Hahn EG, et al. Women encounter ADRs more often than do men. Eur J Clin Pharmacol 2008;64:999-1004.
- Bardage C, Isacson DGL. Self-reported side effects of antihypertensive drugs: an epidemiological study on prevalence and impact on health-state utility. Blood Pressure 2000;9:328-34.
- Holdcroft A. Sex differences and analgesics. Eur J Anaesthesiol 2002;19:1-2.
- Basch E. The missing voice of patients in drug-safety reporting. N Engl J Med 2010;362:865-9.
- Golomb BA, McGraw JJ, Evans MA, Dimsdale JE. Physician response to patient reports of adverse drug effects: Implications for patient-targeted adverse effect surveillance. Drug Saf 2007;30:669-75.
- Berg M. Patient care information systems and health care work: a sociotechnical approach. Int J Med Inf 1999;55:87-101.
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) . The EU Pharmacovigilance System n.d. http://ec.europa.eu/enterprise/sectors/pharmaceuticals/human-use/pharmacovigilance/ (accessed 9 June 2010).
- Moore N, Begaud B. Improving pharmacovigilance in Europe. BMJ 2010;340.
- Pirmohamed M, Atuah KN, Dodoo ANO, Winstanley P. Pharmacovigilance in developing countries. BMJ 2007;335.
Appendix 1 Yellow Card health-care professional reporting form
Appendix 2 Yellow Card patient reporting form used from September 2005 to January 2008
Appendix 3 Yellow Card patient reporting form used from February 2008
Appendix 4 Advisory group members
Millie Kieve Chairperson of the Advisory Group;* Founder/Chairperson of APRIL.
Anthony Cox Lecturer in Clinical Therapeutics, Aston University.
Meryl Davies Volunteer, Patient for Patient Safety Champions, National Patient Safety Agency (NPSA).
Andrew Herxheimer Emeritus Fellow, UK Cochrane Centre; Coconvenor, Cochrane Adverse Effects Methods Group; Co-founder, DIPEx.
Anita Holdcroft Emeritus Reader in Anaesthesia, Imperial College London.
Nigel Meadows HM Coroner, Manchester.
Hugh Middleton Clinical Associate Professor, University of Nottingham.
Kathy Piccolo Royal College of Nursing, BSc Hons (Human Physiology).
Christine Randall Senior Medicines Information Pharmacist, North West Medicines Information Centre.
Anne Roberts Registered Nurse, Diploma in Social Work.
Patricia Wilkie Social Scientist and Patient Activist; President of National Association for Patient Participation (NAPP); Chairperson of the former CSM Working Group on Patient-reporting of Adverse Drug Reaction.
*Anyone wishing to contact members of the advisory group can do this through Millie Kieve, c/o: APRIL, Room 311 Linen Hall, 162–168 Regent St, London W1B 5TD.
Project team members on advisory group
Claire Anderson Professor of Social Pharmacy, University of Nottingham.
Tony Avery Professor of Primary Care, University of Nottingham.
Janet Krska Professor and Head of Pharmacy Research Practice, Liverpool John Moores University.
Appendix 5 Questionnaire to countries regarding their adverse drug reaction reporting schemes
Name of country
| Questions | Consumer | HCPs |
|---|---|---|
| 1. Name of reporting system in your country | ||
| 2. Year of commencement of the reporting system in your country | ||
| 3. Products for which ADRs can be reported. Please tick all that apply: | ||
| ■ Prescription drugs | ◻ | ◻ |
| ■ OTC drugs | ◻ | ◻ |
| ■ Medical devices | ◻ | ◻ |
| ■ Other (please specify) | ||
| 4. What are the methods of report submission? | ||
| ■ Post | ◻ | ◻ |
| ◻ | ◻ | |
| ■ Online | ◻ | ◻ |
| ■ Telephone | ◻ | ◻ |
| Other (please specify) | ||
| 5. Who can report ADRs in your reporting system? | Drug users ◻ | Doctors ◻ |
| Family of drug user ◻ | Nurses ◻ | |
| Doctor of drug user ◻ | Pharmacists ◻ | |
| Other (please specify) | ||
| 6. Has a scientific comparison been made between the reports from consumers and HCPs using your national reporting scheme? | ||
| Yes | ◻ | ◻ |
| No | ◻ | ◻ |
| If yes, please give details of the comparisons made and the results in the next column. If the results are published, we would be glad if you could provide us with a copy or give us the source of information/citation details so that we can retrieve them. If no, proceed to Q7. | ||
| 7. What similarities occurred in the ADR reports from consumers and HCPs? | ||
| 8. What was the time lag between the occurrence of ADR and the submission of the report? | ||
| 9. Which systems affected by the ADRs were mostly reported? | ||
| ■ Central nervous | ◻ | ◻ |
| ■ Skin | ◻ | ◻ |
| ■ Gastrointestinal | ◻ | ◻ |
| ■ Respiratory | ◻ | ◻ |
| ■ Other (please specify) | ||
| 10. Which drugs were implicated in the reports? | ||
| ■ Nervous system drugs | ◻ | ◻ |
| ■ Cardiovascular drugs | ◻ | ◻ |
| ■ Anti-infective drugs | ◻ | ◻ |
| ■ Hormonal drugs | ◻ | ◻ |
| ■ Other (please specify) | ||
| 11. What were the ADRs reported? | ||
| Nausea | ◻ | ◻ |
| Vomiting | ◻ | ◻ |
| Rash | ◻ | ◻ |
| Depression | ◻ | ◻ |
| Anxiety | ◻ | ◻ |
| Itching | ◻ | ◻ |
| Other (please specify) | ||
| Which of the reporters were likely to report serious ADRs? |
Appendix 6 Search terms for electronic databases
MEDLINE
| # | Search history | Results |
|---|---|---|
| 1 | exp Adverse Drug Reaction Reporting systems/or adverse drug reaction reporting.mp. | 3384 |
| 2 | Exp Product Surveillance, post marketing/or exp Pharmacoepidemiology/or exp Adverse Drug Reaction Reporting Systems/or pharmacovigilance.mp. | 6844 |
| 3 | Side effects reporting.mp | 1 |
| 4 | Consumer adj3 report$.mp | 157 |
| 5 | Patient adj3 report$.mp | 18,752 |
| 6 | Public adj3 report$.mp | 937 |
| 7 | 3 or 2 or 1 | 6850 |
| 8 | 6 or 5 or 4 | 19,821 |
| 9 | 8 and 7 | 91 |
EMBASE
| # | Search history | Results |
|---|---|---|
| 1 | Exp Adverse Drug Reaction Reporting Systems/or adverse drug reaction reporting.mp | 6696 |
| 2 | Exp Product surveillance, Postmarketing/or exp pharmacoepidemiology/or exp adverse drug reaction reporting systems/or pharmacovigilance.mp | 10,438 |
| 3 | Side effects reporting.mp | 2 |
| 4 | Consumer adj3 report$.mp | 123 |
| 5 | Patient adj3 report$.mp | 18,874 |
| 6 | Public adj3 report$ | 704 |
| 7 | 3 or 2 or 1 | 10,472 |
| 8 | 6 or 5 or 4 | 19,675 |
| 9 | 8 or 7 | 85 |
Pharm-line
-
Simple phrase: Consumer OR public
-
Key words: adverse reaction monitoring, adverse reaction reporting, postmarketing surveillance and pharmacoepidemiology.
-
Number of hits: 189.
Appendix 7 National pharmacovigilance organisations with no patient-reporting or no response to questionnaire (n = 25)
| Country | Pharmacovigilance organisation contacted |
|---|---|
| Austria | Austrian Agency for Health and Food Safety |
| Bulgaria | Bulgarian Drug Agency |
| Colombia | National Institute of Drug and Food Surveillance |
| Croatia | Croatia National Centre for Adverse Drug Reactions Monitoring |
| Cyprus | Ministry of Health |
| Estonia | State Agency Of Medicines |
| Ethiopia | Drug Administration and Control Authority |
| Finland | National Agency for Medicines |
| Germany | Federal Institute for Drugs and Medical devices |
| Greece | National Organisation for Medicines |
| Hungary | National Institute of Pharmacy |
| India | Indian Pharmacovigilance Centre |
| Japan | Pharmaceuticals and Medical Devices Agency |
| Korea | Pharmaceutical Safety Bureau |
| Latvia | Ministry of Health |
| Malaysia | National Pharmaceutical Bureau |
| Malta | Malta Medicines Authority |
| Nepal | Department of Drug Administration |
| Nigeria | National Agency for Food and Drug Administration and Control |
| Portugal | National Institute of Pharmacy and Medicines |
| Romania | National Medicines Agency |
| Singapore | Health Sciences Authority |
| Spain | Spanish Medicines and Health Products Agency |
| Uganda | National Drug Authority |
| Ukraine | Pharmacology Centre of the Ministry of Health |
Appendix 8 Data cleaning checks performed by the research team on Yellow Card data from the Medicines and Healthcare Regulatory Authority
Table 37, below, outlines the data cleaning checks performed on the Yellow Card data from the MHRA. A number of inconsistencies and apparent anomalies were noted with the data from the MHRA. We explain here what action we took in relation to these.
| Check performed | Action after checking with the MHRA | Patient reports | HCP reports | ||
|---|---|---|---|---|---|
| No. of records checked | No. of changes made | No. of records checked | No. of changes made | ||
| Outliers of weight and height for age | Data updated in the case of coding errors, otherwise left as reported | 104 | 87 | 77 | 35 |
| Reports of gender-specific reactions among patients of the opposite gender | Data updated in the case of coding errors, otherwise left as reported | 1 | 1 | 7 | 2 |
| Date anomalies, e.g. reaction date after date report received or report signed by reporter after date report received | Data updated in the case of coding errors, otherwise left as reported | 57 | 23 | 293 | 31 |
| Missing data on reaction or drug name | Data updated in the case of coding errors, otherwise left as reported | 23 | 23 | 81 | 73 |
| Case number in both patient- and HCP-report data sets | Data from both data sets were merged and included in the data set pertaining to the primary report (as specified by the MHRA) | 2 | 2 | 5 | 5 |
| Duplicate reaction text found in reports with different case numbers | These were either deleted, merged to one report or retained as separate reports relating to two separate reaction experiences in the same patient | 30 | 8 | 132 | 5 |
| Reports coded as received from the pharmaceutical industry | These were removed from data sets or method of reporting updated in the case of a coding error | 9 | 9 | 101 | 101 |
With respect to the ‘reporter considered serious’ flag, for HCP reports this flag should be coded as ‘yes’ if the reporter specified one or more of the CIOMS flags for seriousness. This was not the case for 220 HCP reports. On the advice of the MHRA the absence of a flag was assumed to be an error and the ‘reporter considered serious’ flag was manually set to ‘yes’ for analysis purposes.
Apparent anomalies were also detected for seriousness classification in patient reports and the MHRA was contacted for clarification. For example, if one of the last three options in response to the question ‘How bad was the reaction?’ is ticked then the ‘reporter considered serious’ flag should have been set to ‘yes’. On further inspection of the data, however, it was found that in 75 reports this flag was set to ‘yes’, but without one of the last three options being ticked. The MHRA advised that it was possible for this flag to be manually set to ‘yes’ independently of the other seriousness options, but it is unknown whether this would have been done deliberately or in error. Without further information, the DSRU decided it should not update these records.
For 267 patient reports, the ‘reporter considered serious’ flag was set to ‘yes’ because one or more CIOMS flags for seriousness had been set to ‘yes’ despite the patient not being asked for this information on the Yellow Card. The MHRA were asked to check a 10% sample of these records and provided the following explanations:
-
In the database used before Sentinel, if the patient had chosen the third response to the question ‘How bad was the reaction?’ (i.e. ‘bad enough to affect everyday activities’) then, because this option was not available on the database at the time, this may have been recorded by setting the CIOMS flag ‘involved persistent or disabling incapacity’ to ‘yes’ and, hence, would have triggered the ‘reporter considered serious’ flag.
-
The patient had completed a HCP Yellow Card.
-
The case folder contained merged data from both a patient and HCP Yellow Card.
-
There was a coding error by the MHRA.
Given the complexity of these issues, the DSRU did not update the records before analysis.
Appendix 9 Rules and assumptions used in mapping suspect drug names to Anatomical Therapeutic Chemical classification system for drugs
| Mapping rule for selection of ATC code | No. of mappings |
|---|---|
| Only one ATC code considered appropriate | 2098 |
| ATC code selected based on the formulation/route of administration specified or the most common formulation/route of administration used in practice | 141 |
| ATC code selected based on the indication specified or the most common prescribed indication in practice | 67 |
| No ATC code selected – separate category for herbal or other complementary therapies | 214 |
| No direct map available, but mapped manually to closest matching ATC code (includes multiple ingredient products, excipients, non-specific drug name, e.g. ‘immunoglobulin’) | 239 |
| Unmapped | 2 |
| Total | 2761 |
Appendix 10 Yellow Card reports for suspected adverse drug reactions in children
| Characteristic | Patient report | HCP report | ||||
|---|---|---|---|---|---|---|
| Male | Female | All | Male | Female | All | |
| Age (years): median (IQR) | 4.0 (1.0 to 11.0) | 4.5 (1.0 to 10.0) | 4.0 (1.0 to 11.0) | 4.0 (1.0 to 9.0) | 3.0 (1.0 to 9.0) | 4.0 (1.0 to 9.0) |
| Total: n (%) | 74 (51.0) | 68 (46.9) | 145 (100.0) | 1265 (51.9) | 1080 (44.3) | 2439 (100.0) |
| No. of reactions | No. of patient reports (IQR) or (%) | No. of HCP reports (IQR) or (%) | p-value |
|---|---|---|---|
| Median (IQR) | 2 (2 to 4) | 2 (1 to 3) | < 0.001a |
| 1 | 32 (22.1) | 1022 (41.9) | < 0.001b |
| 2 | 41 (28.3) | 667 (27.4) | |
| 3 | 24 (16.6) | 411 (16.9) | |
| 4 or 5 | 28 (19.3) | 287 (11.8) | |
| > 5 | 20 (13.8) | 52 (2.1) | |
| Total (%) | 145 (5.6) | 2439 (94.4) |
| No. of patient-reported reactions (N = 494) | No. of HCP-reported reactions (N = 5176) | |||
|---|---|---|---|---|
| LLT name | n (% of reactions) | LLT name | n (% of reactions) | Rank for patient reports |
| Vomiting | 12 (2.4) | Redness | 136 (2.6) | 6 |
| Diarrhoea | 9 (1.8) | Vomiting | 131 (2.5) | 1 |
| High temperature | 9 (1.8) | Local reaction | 111 (2.1) | – |
| Rash | 9 (1.8) | Erythema | 108 (2.1) | 34 |
| Nausea | 8 (1.6) | Rash | 99 (1.9) | 2 |
| Crying | 7 (1.4) | Injection site reaction | 82 (1.6) | 34 |
| Headache | 7 (1.4) | Swelling | 79 (1.5) | 6 |
| Redness | 7 (1.4) | Fever | 73 (1.4) | 20 |
| Swelling | 7 (1.4) | Injection site swelling | 73 (1.4) | 34 |
| Appetite lost | 6 (1.2) | Injection site redness | 71 (1.4) | 84 |
| Difficulty sleeping | 5 (1.0) | Diarrhoea | 69 (1.3) | 2 |
| Hyperactive | 5 (1.0) | Swollen arm | 64 (1.2) | 84 |
| Stomach pain | 5 (1.0) | Urticarial rash | 57 (1.1) | – |
| Drowsiness | 4 (0.8) | Pyrexia | 55 (1.1) | 34 |
| Itching | 4 (0.8) | Crying | 53 (1.0) | 6 |
| Personality change | 4 (0.8) | Headache | 46 (0.9) | 6 |
| Screaming | 4 (0.8) | Swelling arm | 43 (0.8) | 84 |
| Sleep disturbance | 4 (0.8) | Urticaria | 39 (0.8) | – |
| Unwell | 4 (0.8) | Injection site erythema | 37 (0.7) | 84 |
| Allergic reaction | 3 (0.6) | High temperature | 36 (0.7) | 2 |
| Reaction | No. of patient reports (%)a | No. of HCP reports (%)a | Patient vs HCP: adjusted ORb (99% CI) |
|---|---|---|---|
| General disorders and administration site conditions | 65 (45.8) | 1001 (42.7) | 1.19 (0.76 to 1.87) |
| Skin and subcutaneous tissue disorders | 53 (37.3) | 829 (35.4) | 1.12 (0.71 to 1.79) |
| Nervous system disorders | 45 (31.7) | 360 (15.4) | 2.49 (1.51 to 4.12) |
| Psychiatric disorders | 41 (28.9) | 310 (13.2) | 2.64 (1.59 to 4.38) |
| Gastrointestinal disorders | 40 (28.2) | 308 (13.1) | 2.54 (1.53 to 4.23) |
| Respiratory, thoracic and mediastinal disorders | 14 (9.9) | 128 (5.5) | 1.83 (0.85 to 3.94) |
| Metabolism and nutrition disorders | 14 (9.9) | 70 (3.0) | 3.66 (1.66 to 8.08) |
| Musculoskeletal and connective tissue disorders | 10 (7.0) | 93 (4.0) | 1.77 (0.73 to 4.32) |
| Eye disorders | 8 (5.6) | 67 (2.9) | 1.89 (0.70 to 5.14) |
| Infections and infestations | 7 (4.9) | 161 (6.9) | 0.74 (0.26 to 2.05) |
| Vascular disorders | 6 (4.2) | 85 (3.6) | 1.14 (0.37 to 3.47) |
| Injury, poisoning and procedural complications | 5 (3.5) | 43 (1.8) | 1.95 (0.56 to 6.75) |
| Investigations | 4 (2.8) | 118 (5.0) | 0.52 (0.14 to 1.95) |
| Renal and urinary disorders | 3 (2.1) | 26 (1.1) | 1.89 (0.39 to 9.24) |
| Cardiac disorders | 2 (1.4) | 63 (2.7) | 0.51 (0.08 to 3.32) |
Appendix 11 Additional signals of disproportionate reporting (1)
The additional SDRs shown below were identified after combining the patient and HCP reports (they were not SDRs in either the ‘patient-only’ or ‘HCP-only’ data sets). The SDRs shown were classified as serious reactions, involving drugs flagged as ‘black triangle’ and not documented on the product’s SPC.
| Suspect drug | Preferred term |
|---|---|
| Adalimumab | Deep-vein thrombosis |
| Aripiprazole | Mania |
| Bisoprolola | Arthralgia |
| Drospirenone and oestrogen | Muscular weakness |
| Etanercept | Emphysema |
| Fluoxetinea | Hepatic failure |
| Paracetamola | Haematemesis |
| Pregabalin | Urinary retention |
| Strontium ranelate | Asthma |
| Zoledronic acid | Infection |
Appendix 12 Additional signals of disproportionate reporting (2)
The additional SDRs shown below were identified after combining the patient and HCP reports (they were not SDRs in either the ‘patient-only’ or ‘HCP-only’ data sets). The SDRs shown were classified as serious reactions, not documented on the product’s SPC and involving established drugs.
| Suspect drug | Preferred term |
|---|---|
| Acetylsalicylic acid | Blood pressure decreased |
| Amoxicillin and enzyme inhibitor | Chromaturia, faeces discoloured |
| Atorvastatin | Chromaturia |
| Cefalexin | Chest discomfort, wheezing |
| Chloramphenicol | Eyelid oedema |
| Cyproterone and oestrogen | Paranoia, withdrawal syndrome |
| Diazepam | Convulsion, muscle spasms |
| Dipyridamole | Melaena |
| Donepezil | Confusional state |
| Doxazosin | Swelling face |
| Doxycycline | Confusional state |
| Erythromycin | Dysphagia |
| Influenza vaccines | Lower respiratory tract infection, polymyalgia rheumatica |
| Lansoprazole | Haemorrhage |
| Levothyroxine sodium | Contusion |
| Loratadine | Syncope |
| Losartan | Ocular hyperaemia, skin exfoliation |
| Mirtazapine | Coordination abnormal, dystonia |
| MMR vaccine | Cellulitis |
| Natural opium alkaloids | Muscle spasms |
| Nifedipine | Dysphagia |
| Ofloxacin | Arthralgia |
| Paroxetine | Death, ocular hyperaemia |
| Ramipril | Dysphagia |
| Simvastatin | Muscle atrophy |
| Venlafaxine | Paranoia, amnesia |
| Zopiclone | Drug dependence, suicidal ideation |
Appendix 13 Causality analysis
Category 3
Summary of causality assessments on drug–reaction pairs reported by both groups, but only generated as SDRs when data sets combined.
| Drug | Reaction | Serious | Listed/label | Black triangle | Patients | HCP | Duplicates | ||
|---|---|---|---|---|---|---|---|---|---|
| No. | Causality | No. | Causality | ||||||
| Amlodipine | Arthralgia | Y | Y | N | 9 | Probable – 3 | 6 | Probable – 2 | No |
| Possible – 4 | Possible – 2 | ||||||||
| Unlikely – 0 | Unlikely – 1 | ||||||||
| Unassessable – 2 | Unassessable –1 | ||||||||
| Alendronic acid | Myalgia | Y | Ya | N | 3 | Probable – 1 | 6 | Probable – 3 | No |
| Possible – 2 | Possible – 1 | ||||||||
| Unlikely – 0 | Unlikely – 0 | ||||||||
| Unassessable – 0 | Unassessable – 2 | ||||||||
| Risperidone | Convulsion | Y | Nb | Y | 3 | Probable – 0 | 3 | Probable – 0 | No |
| Possible – 1 | Possible – 0 | ||||||||
| Unlikely – 1 | Unlikely – 2 | ||||||||
| Unassessable – 1 | Unassessable – 1 | ||||||||
| Paroxetine | Hallucination | Y | Y | N | 4 | Probable – 0 | 3 | Probable – 0 | One case reported by patient and HCP very similar |
| Possible – 3 | Possible – 2 | ||||||||
| Unlikely – 0 | Unlikely – 1 | ||||||||
| Unassessable – 1 | Unassessable – 0 | ||||||||
| Amlodipine | Cough | N | N | N | 5 | Probable – | 4 | Probable – 0 | No |
| Possible – 2 | Possible – 2 | ||||||||
| Unlikely – 2 | Unlikely – 2 | ||||||||
| Unassessable – 1 | Unassessable – 0 | ||||||||
| Atorvastatin | Disturbance in attention | N | N | N | 5 | Probable – 1 | 3 | Probable – 1 | Yes – two patient reports similar |
| Possible – 2 | Possible – 2 | ||||||||
| Unlikely – 2 | Unlikely – 0 | ||||||||
| Unassessable – 0 | Unassessable – 0 | ||||||||
| Erythromycin | Chest pain | N | Nb | N | 4 | Probable – 0 | 3 | Probable – 1 | No |
| Possible – 3 | Possible – 2 | ||||||||
| Unlikely – 0 | Unlikely – 0 | ||||||||
| Unassessable – 1 | Unassessable – 0 | ||||||||
| Simvastatin | Mobility decreased | N | N | N | 3 | Probable – 1 | 3 | Probable – 1 | No |
| Possible – 1 | Possible – 1 | ||||||||
| Unlikely – 0 | Unlikely – 1 | ||||||||
| Unassessable – 1 | Unassessable – 0 | ||||||||
| Insulin glargine | Headache | N | Ya | Y | 4 | Probable – 0 | 4 | Probable – 0 | No |
| Possible – 3 | Possible – 2 | ||||||||
| Unlikely – 0 | Unlikely – 0 | ||||||||
| Unassessable – 1 | Unassessable – 2 | ||||||||
| Olanzapine | Agitation | N | N | Y | 3 | Probable – 0 | 4 | Probable – 1 | No |
| Possible – 0 | Possible – 0 | ||||||||
| Unlikely – 1 | Unlikely – 1 | ||||||||
| Unassessable – 2 | Unassessable – 2 | ||||||||
| Risperidone | Tremor | Y | Nb | Y | 6 | Probable – 1 | 3 | Probable – 1 | Yes – two HCP reports likely duplicates |
| Possible – 3 | Possible – 2 | ||||||||
| Unlikely – 0 | Unlikely – 0 | ||||||||
| Unassessable – 2 | Unassessable – 0 | ||||||||
| Proguanil, combinations | Vomiting | N | Ya | Y | 3 | Probable – 0 | 4 | Probable – 0 | No |
| Possible – 3 | Possible – 3 | ||||||||
| Unlikely – 0 | Unlikely – 0 | ||||||||
| Unassessable – 0 | Unassessable – 1 | ||||||||
Category 4
Summary of causality assessments on drug–reaction pairs generated as SDRs by HCP diluted out in combined data set.
| Drug | Reaction | Serious | Listed/label | Black triangle | Patients | Reported by patients | Duplicates | |
|---|---|---|---|---|---|---|---|---|
| No. | Causality | |||||||
| Rimonabant | Muscle spasms | Y | Y | Y | 9 | Probable – 3 | Yes | No |
| Possible – 5 | ||||||||
| Unlikely – 0 | ||||||||
| Unassessable – 1 | ||||||||
| Infliximab | Arthralgia | Y | Y | Y | 10 | Probable – 9 | No (but flu-like illness reported) | No |
| Possible – 1 | ||||||||
| Unlikely – 0 | ||||||||
| Unassessable – 0 | ||||||||
| Duloxetine | Confusional state | Y | Ya | Y | 9 | Probable – 3 | Yes | No |
| Possible – 5 | ||||||||
| Unlikely – 1 | ||||||||
| Unassessable – 0 | ||||||||
| Atomoxetine | Depression | Y | Nb | Y | 7 | Probable – 0 | No (suicidal attempt reported) | No |
| Possible – 7 | ||||||||
| Unlikely – 0 | ||||||||
| Unassessable – 0 | ||||||||
| Hepatitis B, purified antigen | Chest pain | N | N | N | 6 | Probable – 3 | No | No |
| Possible – 1 | ||||||||
| Unlikely – 1 | ||||||||
| Unassessable – 1 | ||||||||
| Ramipril | Abnormal dreams | N | N | N | 5 | Probable – 0 | No (sleep disorder reported) | No |
| Possible – 3 | ||||||||
| Unlikely – 0 | ||||||||
| Unassessable – 2 | ||||||||
| Rimonabant | Blood pressure increased | Y | N | Y | 5 | Probable – 0 | No | No |
| Possible – 5 | ||||||||
| Unlikely – 0 | ||||||||
| Unassessable – 0 | ||||||||
| Tiotropium bromide | Dyspnoea | N | N | Y | 8 | Probable – 1 | No | No |
| Possible – 2 | ||||||||
| Unlikely – 1 | ||||||||
| Unassessable – 4 | ||||||||
| Rimonabant | Nightmare | N | Ya | Y | 9 | Probable – 5 | No (sleep terror, sleep disorder reported) | No |
| Possible – 0 | ||||||||
| Unlikely – 0 | ||||||||
| Unassessable – 4 | ||||||||
| Ezetimibe | Asthenia | N | Ya | Y | 6 | Probable – 1 | No (apathy, fatigue reported) | No |
| Possible – 3 | ||||||||
| Unlikely – 0 | ||||||||
| Unassessable – 2 | ||||||||
| Pregabalin | Gait disturbance | N | Y | Y | 5 | Probable – 2 | No (coordination abnormal, dysstasia reported) | No |
| Possible – 3 | ||||||||
| Unlikely – 0 | ||||||||
| Unassessable – 0 | ||||||||
| Atomoxetine | Insomnia | N | Y | Y | 5 | Probable – 2 | No | No |
| Possible – 1 | ||||||||
| Unlikely – 1 | ||||||||
| Unassessable – 1 | ||||||||
Signals of disproportionate reporting
Signals of disproportionate reporting generated in drugs classified by the MHRA (at least once in patient reports) as having been obtained OTC and SDRs involving complementary therapies.
| Drug | Reaction | Serious | Listed/Label | Black triangle | Patients | HCP | Duplicates | Comments | ||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Causality | No. | Causality | |||||||
| Paracetamol | Urticaria | N | Y | N | 3 | Probable – 0 | 6 | Probable – 4 | No | One patient report classified as obtained OTC – assessed as probable |
| Possible – 1 | Possible – 2 | |||||||||
| Unlikely – 0 | Unlikely – 0 | |||||||||
| Unassessable – 2 | Unassessable – 1 | |||||||||
| Paracetamol, combinations excluding psycholeptics | Syncope | Y | N | N | 5 | Probable – 0 | 0 | N/A | Yes – two patient reports likely duplicates | One patient report classified as obtained OTC – assessed as possible |
| Possible – 4 | ||||||||||
| Unlikely – 0 | ||||||||||
| Unassessable – 1 | ||||||||||
| Chloroquine | Pyrexia | N | N | N | 3 | Probable – 0 | 1 | Probable – 0 | No | One patient report classified as obtained OTC – assessed as possible |
| Possible – 3 | Possible – 0 | |||||||||
| Unlikely – 0 | Unlikely – 0 | |||||||||
| Unassessable – 0 | Unassessable – 1 | |||||||||
| Proguanil | Diarrhoea | N | Y | N | 3 | Probable – 0 | 1 | Probable – 0 | No | Two patient reports classified as obtained OTC – both assessed as possible |
| Possible – 3 | Possible – 1 | |||||||||
| Unlikely – 0 | Unlikely – 0 | |||||||||
| Unassessable – 0 | Unassessable – 0 | |||||||||
| St John's wort | Anxiety | N | – | – | 3 | Probable – 0 | 0 | N/A | ||
| Possible – 3 | ||||||||||
| Unlikely – 0 | ||||||||||
| Unassessable – 0 | ||||||||||
| Black cohosh | Abnormal liver function tests | Y | – | – | 1 | Probable – 0 | 3 | Probable – 0 | No | |
| Possible – 1 | Possible – 1 | |||||||||
| Unlikely – 0 | Unlikely – 0 | |||||||||
| Unassessable – 0 | Unassessable – 2 | |||||||||
Appendix 14 Case selection for qualitative analysis of Yellow Card reports
The reports were divided into the following categories:
-
most commonly reported drugs
-
black triangle drugs
-
drugs purchased OTC
-
complementary therapies.
The Advisory Group suggested sampling by reaction type as well as by drug. Therefore, the DSRU was asked to provide data in a Microsoft access 2003 database (Mircrosoft Corporation, Redmond, WA, USA) containing tables that allowed this to be done. For example, for the ‘most commonly reported drugs’ category and the ‘black triangle drug’ category data were provided on the top five drugs in each of these categories. Separate tables giving reactions for these drugs that were either commonly reported by patients and/or deemed to be of importance to the project team or advisory group were also provided.
Tables 42 and 43 below show the numbers of complete reports provided in the ‘most commonly reported drugs’ category and the ‘black triangle drug’ category along with the different types of reactions that were looked at in detail.
| Reaction | Citalopram | Co-cyprindiol | Paroxetine | Simvastatin | Venlafaxine | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pt | HCP | Pt | HCP | Pt | HCP | Pt | HCP | Pt | HCP | |
| Anxiety | 9 | 7 | 22 | 0 | 22 | 5 | 10 | 3a | ||
| Arthralgia | 20 | 1 | ||||||||
| Depression | 4 | 1 | 66 | 2 | 17 | 1 | 6 | 1a | ||
| Dizziness | 11 | 4 | 41 | 3 | 18 | 8 | ||||
| Drug withdrawal syndrome | 41 | 1 | 8 | 7 | ||||||
| Myalgia | 39 | 34 | ||||||||
| Paraesthesia | 4 | 2 | 32 | 1 | 8 | 3 | ||||
| Suicidal ideation | 10 | 7 | 20 | 1a | 27 | 8 | 3 | 2 | ||
| Tremor | 11 | 3 | 20 | 4 | 13 | 3 | ||||
| Totalb | 28 | 23 | 69 | 3 | 101 | 17 | 54 | 35 | 33 | 19 |
| Reaction | Insulin glargine | Levetiracetam | Olanzapine | Pregabalin | Proguanil with atovaquone | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pt | HCP | Pt | HCP | Pt | HCP | Pt | HCP | Pt | HCP | |
| Abdominal pain | 3 | 1 | ||||||||
| Anorexia | 3a | 0 | ||||||||
| Anxiety | 2 | 3 | ||||||||
| Arthralgia | 2a | 1 | 2a | 1a | ||||||
| Balance disorder | 2 | 0 | 1 | 1 | ||||||
| Blister | 2 | 2 | ||||||||
| Confusional state | 2 | 0 | ||||||||
| Constipation | 3a | 0 | ||||||||
| Convulsion | 3 | 3 | ||||||||
| Depression | 2 | 2 | ||||||||
| Diabetes inadequately controlled | 3 | 1 | ||||||||
| Diarrhoea | 2 | 3 | 8 | 6 | ||||||
| Disturbance in attention | 3 | 1 | ||||||||
| Dizziness | 1 | 2 | 6a | 12 | 3 | 1 | ||||
| Dry mouth | 2a | 3 | ||||||||
| Dyspnoea | 2 | 2 | 2 | 3 | ||||||
| Emotional distress | 3a | 0 | ||||||||
| Fatigue | 3a | 1a | ||||||||
| Headache | 3 | 3 | 5 | 8 | ||||||
| Hyperhidrosis | 3a | 0 | ||||||||
| Hypoaesthesia | 2 | 0 | ||||||||
| Hypoglycaemia | 4a | 0a | ||||||||
| Influenza-like illness | 2 | 0 | ||||||||
| Insomnia | 2 | 0 | 5 | 0 | ||||||
| Malaise | 5a | 2a | 3a | 3 | 5 | 2 | ||||
| Memory impairment | 4a | 1 | ||||||||
| Nausea | 3 | 0 | 2 | 2a | ||||||
| Pain in extremity | 4a | 0 | ||||||||
| Paraesthesia | 2a | 0 | ||||||||
| Pyrexia | 3 | 2 | ||||||||
| Rash | 4a | 3a | ||||||||
| Somnolence | 5 | 1 | 3 | 13 | ||||||
| Speech disorder | 1 | 2a | ||||||||
| Tremor | 3 | 1 | 6a | 1 | ||||||
| Vision blurred | 2 | 4 | ||||||||
| Weight increased | 7a | 0 | 13a | 5 | ||||||
| Total b | 13 | 9 | 13 | 8 | 25 | 13 | 17 | 38 | 14 | 19 |
Complete cases were defined as those containing details of:
-
report type, for example paper, internet, telephone
-
suspect drug
-
description of the type of reporter – for example patient, HCP (and type of HCP)
-
reactions of interest (in terms of ‘most commonly reported drugs’ and the ‘black triangle drug’ categories)
-
age of the patient at the time of the reaction
-
gender of the patient.
These restrictions to selection were decided upon to enable description of each case in as much detail as was (potentially) available within the database and to allow for comparing and contrasting different types of report.
A total of 286 unique drug–ADR records were identified for patients and a total of 97 for HCPs.
There was a total of 82 unique black triangle reports for patients and 87 for HCPs.
In addition, all reports available for eight drug types that were purchased OTC and the 10 most commonly reported complementary therapies were requested. There was a total of 25 patient reports for drugs that were purchased OTC and 15 patient reports for complementary therapies.
The following approach to sampling reports for the qualitative analysis was taken. First, reports across the four categories were included. Second, for the ‘most commonly reported drugs’, where there were more than 30 reports for any particular drug, up to 30 cases randomly selected. Third, as far as possible, we tried to identify similar numbers of reports for specific drugs (and the selected ADRs) for both patients and HCP reports. It should be noted that the patient reports and HCP reports related to different cases, but the sampling strategy means that there were similarities in the types of drug–ADR combinations included in the samples from each group of reporters.
For the ‘drugs most commonly reported by patients’ 30 cases from HCPs for simvastatin and all cases available for the other drugs were randomly selected (where the total was < 30).
For black triangle drugs, patient reports were compared with all the HCP reports available for the ADRs selected.
It was not possible to find HCP reports for drugs purchased OTC and there were very few for complementary therapies. Therefore, for these categories of report only the patient reports were analysed.
Cases were selected in the following way:
-
First, cases were selected from each of the drug–reaction access tables on the basis of complete data being available (see above). For some of the HCP reports, however, where numbers were low, some reports that did not have full details of the reporter type were accepted.
-
Second, data from each of the drug–reaction tables were exported to excel, where data for each drug and drug category were combined and duplicates were removed (note: duplicates were sometimes common because the same report could appear in different tables within the database).
-
Third, in line with the sampling strategy outlined above, where necessary, cases were randomly selected using a web-based computer program (www.randomizer.org/form.htm).
Table 44, below, shows the numbers of cases selected for the most commonly reported drug category and the black triangle drug category.
| Drug category | Drug | No. of reports selected | |
|---|---|---|---|
| Patients | HCPs | ||
| Most commonly reported drugs by patients | Citalopram | All 28 | All 23 |
| Co-cyprindiol (Dianette®) | Random 30 | All 3 | |
| Paroxetine | Random 30 | All 17 | |
| Simvastatin | Random 30 | Random 30 | |
| Venlafaxine | Random 30 | All 19 | |
| Total | 148 | 92 | |
| Black triangle drugs | Insulin glargine | 13 | 9 |
| Levetiracetam | 13 | 8 | |
| Olanzapine | 25 | 13 | |
| Pregabalin | 17 | 38 | |
| Proguanil with atovaquone (Malarone®) | 14 | 19 | |
| Total | 82 | 87 | |
In addition, the tables below show the numbers of patient reports analysed relating to drugs purchased OTC (Table 45), and complementary treatments (Table 46).
| Drug name | Preparation name (for ‘other cold remedies and paracetamol combinations’) | No. of patient reports |
|---|---|---|
| Cetirizine | 4 | |
| Chloroquine | 3 | |
| Fluconazole | 1 | |
| Levonorgesterol | 2 | |
| ‘Other cold remedies’ | Beechams Flu-Plus | 1 |
| Benylin Chesty Cough | 1 | |
| Benylin 4 Flu Liquid | 1 | |
| Day Nurse | 2 | |
| Lemsip | 1 | |
| Nirolex | 1 | |
| Nirolex Night Cold and Flu Comfort | 1 | |
| Paracetamol combinations | Co-codamol | 3 |
| Medised | 2 | |
| Proguanil | 3 | |
| Sumatriptan | 1 | |
| Total | 25 |
| Complementary therapy | No. of unique entries in the database |
|---|---|
| Agnus castus | 2 |
| Angelica sinensis | 2 |
| Arcitium lappa | 1 |
| Atractylodes macrocephala | 2 |
| Berberis aquifolium | 1 |
| Bupleurum chinense | 1 |
| Fructus lycii | 3 |
| Panax ginseng | 3 |
| Siberian ginseng | 3 |
| St John’s wort | 8 |
| Total unique entries for all the above complementary therapies a | 15 |
It should be noted that the numbers of cases selected for drugs purchased OTC and complementary therapies is much less than was expected because of the large number of duplicates in the database.
For example, for complementary therapies there appeared to be 163 entries in the database for the 10 preparations listed in Table 46, but a large number of these entries were multiple entries of the same record with different side effects.
Appendix 15 Yellow Card narrative codes and definitions
| High-level category | Code | Definition |
|---|---|---|
| 1. Report number | ADR number | |
| 2. Suspect drug | Drug suspected of causing reaction | |
| Description of the problem | 3. Symptoms | Presentation of subjective symptoms. In the case of the patients this will often be of the form ‘and then I felt …’ In the case of HCPs it will often be of the form ‘the patient said she/he felt …’ |
| 4. Signs | Physical signs observed by a HCP | |
| Impact of ADR on patient | 5. Any impact | Any impact noted on the patient’s life (this includes categories 6–8 below) |
| 6. Impact on daily life | How the reaction made the patient feel (e.g. sad, scared, etc. – rather than sensory experiences, such as pain) | |
| 7. Social impact | Impact of the reaction/s on the patient’s family and social activities | |
| 8. Occupational impact | Impact on the patient’s work – absence, inability to fulfil duties, etc. | |
| Involvement of health professionals | 9. Involvement of health services |
Visits by or to HCPs, ambulance called, A&E, admission, etc. The HCP reports will almost always involve health services because in order to do a report the HCP will have been contacted about the problem leading to the report |
| 10. Investigations | Any investigations carried out | |
| 11. Treatment | Any treatment carried out for the ADR (stopping the drug not included here) | |
| 12. HCP advice | Any recording of advice given by HCP about what to do about ADR | |
| Patient’s background | 13. Past medical history | Information about patient’s medical history |
| 14. General health | Information about the patient’s general health | |
| Causality | 15. Temporality 1 | Text indicating that ADR followed administration of drug |
| 16. Temporality 2 | Text indicating that ADR improved when drug discontinued – or dose was reduced | |
| 17. Temporality 3 | Text indicating that ADR occurred on discontinuation of drug (withdrawal) | |
| 18. Temporality 4 | Text indicating that ADR recurred when drug started again | |
| 19. Dose relationship | Discussion linking increasing severity of reaction to changing dose of suspect drug | |
| 20. Differential diagnosis | Consideration/discussion/rejection of possible alternative causes for reaction. Where evidence is adduced to indicate grounds for believing that the signs and symptoms were/were not the result of the drug rather than the underlying condition, some new condition or other factor. Please also tick this box where there is reference to possible alternative explanations, even when reporter does not come down on one side or another | |
| 21. Past history | Reference to any similar reactions to drugs in the past | |
| 22. Causal theorising | Discussion of possible causal pathways, e.g. possible drug interactions, change in form of drug (e.g. from injected sumatriptan to oral sumatriptan), faulty batch of drug, interaction between drug and concurrent illness, etc. | |
| 23. Objective evidence | Confirmation of adverse event by objective evidence | |
| 24. Endorsement by HCP of causal link | Where HCP is quoted as agreeing that drug may have caused reaction. This may also apply where a HCP is making the report if s/he cites another HCP as supporting the view that the reaction was caused by the drug | |
| Patient actions | 25. Self-management | Text indicating patient self-management in response to the reaction |
| Presentation of report | 26. Medicotechnical | Where reporter uses technical language (e.g. tachycardia, pyrexia, etc.) rather than lay terms |
| 27. Extreme nature of signs and symptoms | Please tick this box where the presentation of signs and symptoms stresses their extreme nature | |
| 28. ‘Elaboration’ score |
Please make a judgement about how elaborate the account is (which includes the extent to which the text tells the story of the suspected ADR) 0 = no narrative; 1 = scant narrative; 2 = moderately elaborate; 3 = very elaborate |
|
| 29. Ambiguity | Please tick this box if the account is confused or has conflicting information | |
| Coder’s remarks | 30. Apparent recording error | Please tick this box if there’s reason to think that a recording error has been made, e.g. if the report clearly comes from a physician but has been recorded as a patient report; or where the coding of a reaction appears to be based on a misinterpretation of the narrative |
| 31. Worth reading the text in more detail? | Please tick box if the text appears to provide particularly useful text for the in-depth qualitative analysis | |
| 32. Comment | Any other comment you wish to make about the report |
Appendix 16 Questionnaire and letter
Appendix 17 Initial covering letter sent out by Medicines and Healthcare Regulatory Authority for the questionnaire survey
Appendix 18 Reminder letter sent out by Medicines and Healthcare Regulatory Authority for the questionnaire survey
Appendix 19 Interview schedule for telephone interviews
The interviewer will set the scene regarding audiotaping, confidentiality, etc:
-
story of illness/event
-
story of treatment
-
story of suspected side effect
-
story of decision to report
-
story of process of reporting.
Story of illness/event
-
You’ve all ready told us a little bit about the side effect that you have reported to the MHRA using the YCS. I want use this opportunity to get you tell me a bit more about the circumstances that lead up to you reporting.
-
Firstly, can you tell me a little bit about how you came to take the medicine that caused the problem:
-
– Reason for medicine (symptoms, etc.) and how you came to take it.
-
– Prescribed or OTC.
-
– How long had you been taking the medicine before you realised there was a problem?
-
– Tell us about the side effect. Description of symptoms, etc.
-
– What was the problem? Did you stop taking the medicine?
-
– Straight away? If you didn’t stop, why not?
-
– What made you think the symptoms were related to medicine? How certain were you? Did you talk to anyone about it, if so who? What did they say?
-
-
Was this the first time you have ever experienced a side effect from a medicine you have taken?
-
On this particular occasion, how easy did you find it to link the particular side effect you experience to taking a particular medicine?
Story of decision to report
-
Can you tell me a bit more about what made you decide to report this side effect using the YCS?
-
When you experienced [the suspected side effect] did you discuss this with anyone?
-
If so what advice did they give you?
-
Were you aware of the YCS and/or the fact that patients can report before you recently experienced [the suspected side effect]?
-
How did you know about the YCS?
-
What made you decide to use paper/internet reporting?
-
How easy did you find the reporting process you used (i.e. questionnaire or internet)?
-
Did you experience any specific difficulties in using this process?
-
How easy did you find it to describe the side effects you had experienced?
-
Were you able to provide all the information that the report form asked you for?
-
How much time did it take you to fill in the form?
-
Did you need additional help in completing the form? If so, from whom did you get this help?
-
Were you contacted following your report?
-
What was the nature of the contact?
-
Was this what you were expecting? What else were you expecting, if anything?
-
Would you have liked to have had any further contact? If so what would you have liked?
-
Until recently, reports to the YCS could only be made by professionals, such as doctors, nurses and pharmacists. Do you think it’s a good idea to expand the scheme so that patients are now able to report side effects of medicines?
-
Which do you think is likely to be more useful – when patients make the reports to the scheme or when health professionals, such as doctors, pharmacists and nurses, do?
-
– What makes you say that?
-
– What do you think is the advantage of each?
-
-
What do you think is the point of reporting side effects?
-
– Who do you think will benefit? HCPS and/or patients?
-
– In what way(s) might they benefit?
-
-
Do you think the reporting system could be improved in any way?
-
Having made this report, would you consider reporting again if you experienced side effects from another medicine?
-
Would you encourage others to fill in a report if they experience a side effect from a medicine?
Appendix 20 Focus group schedule
-
Presentation about ADRs and the YCS.
-
How do you feel about reporting side effects in general?
-
What would make you report a side effect?
-
Do you think people ought to report side effects or leave it to health professionals?
-
What might put you off reporting?
-
Discuss initial preferences for paper or internet reporting.
Appendix 21 Usability scenarios
Scenario 1
Here are the details for the patient for scenario 1. Please note that this is not a real patient and all of the contact details are made up. In this scenario you are the patient.
Mr John Smith is a 56-year-old gentleman.
His address is 42 Long Acre, Beeston, Nottingham NG9 4EG. Tel: 0115 9286695. E-mail address at work: john.smith@notts.ac.uk
The doctor started him on a new medication to help with his angina in early May 2008. The drug was simvastatin 40 mg at night.
He started to get pains in the muscles in his legs and arms a few weeks after taking a new tablet. He noticed an aching in his muscles most of the time and he wasn’t able to get around quite as well as normal. The problem went away about a week after stopping taking the medicine. He tried taking paracetamol for the pains but this was not very helpful. He stopped taking the medicine after discussing the pains with his doctor.
He also uses a salbutamol inhaler for his asthma, takes aspirin for his angina and St John’s wort for low mood.
He has had angina since 2007 and has been an asthmatic since the age of 5 years.
He is allergic to penicillin.
Mr Smith weighs 13 stone and is 5 feet 9 inches tall.
His GP is Dr Mayberry, Middle Vale Surgery, Beeston, Nottingham NG9 7ET.
Scenario 2
Here are the details for the patient for scenario 2. Please note that this is not a real patient and all of the contact details are made up. In this scenario you are the son of the patient.
Mr Gerald Carr is a 52-year-old gentleman, who is concerned about the side effects that his elderly mother has had to a medicine the GP recently started her on.
His mother, Mrs Elsie Carr, is 93 years old.
She lives at 25 Main Street, Long Eaton, Derbyshire DE45 2LL. Tel.: 0116 255822.
Mrs Carr has a number of medical conditions. She has suffered from heart failure since 2002, hypertension since 1995 and she had an operation for cataracts in 2000. In 2006 she was also diagnosed with glaucoma.
Mrs Carr was given perindopril for her heart condition in February 2008, and a few days later she developed an irritating dry cough, which kept her up for much of the night. She tried a number of cough mixtures that her son bought from the chemist, but none were successful. On the fourth visit to the pharmacy, at the beginning of May 2008, the pharmacist asked Gerald about his mother’s other medication and suggested that the perindopril might be the cause of the cough.
Mrs Carr spoke to her GP who gave her an alternative to the perindopril and her cough went away about a week after stopping the medication.
The perindopril dose was 2 mg daily for heart failure.
Mrs Carr also takes:
-
bendrofluazide 2.5 mg daily
-
atenolol 25 mg daily
-
latanoprost eye drops for glaucoma
-
multivitamin supplement for elderly people – since January 2008.
She weighs 9 stone 2 pounds and is 5 feet 2 inches tall.
Her GP is Dr Linton, Green Lane Surgery, Long Eaton.
Mr Gerald Carr, the son, lives at 22 Fletcher Street, Spondon, Derbyshire DE55 6TH. Tel.: 01332 222664. E-mail: Gerald.Carr7974@hotmail.com
Scenario 3
Here are the details for the patient in scenario 3. Please note that this is not a real patient and all the contact details are made up. In this scenario you are the patient.
Your name is Ms Phoebe Foster.
Your address is 2 Church Street, Selston, Derbyshire DE56 3SS. Tel: 01773 566566. E-mail: Phoebe.Foster@hotmail.com.
You are a 32-year-old woman who has suffered from bouts of depression since having your second child in 2003.
In April 2008, you went to see your doctor again as you were feeling very low and unable to cope. She suggested you should go back on to an antidepressant. She prescribed Prozac (fluoxetine) 20 mg in the morning.
Two weeks later you developed a rash that spread over most of your body and itched dreadfully. You stopped the Prozac as it was the only thing you could think might have caused the rash, and the rash went away a few days later after using hydrocortisone cream.
Before you saw the doctor for your depression, you had taken St John’s wort for 2 months, but it had had no effect.
Additional information:
-
Your GP is Dr Shah, 10 Penny Lane, Selston.
-
You are 165 cm tall and weigh 65 kg.
Scenario 4
Here are the details for the patient in scenario 4. Please note that this is not a real patient and all the contact details are made up. Please do the report as if you are the patient.
Your name is Alan Partridge.
Your address is 12 Main Street, Long Eaton DE12 2PS. Tel.: 0115 234234. E-mail: apartridge@btinternet.com
You are a 54-year-old man and you are generally fit and healthy.
Last week, July 2008, you returned from a business trip to India and within 2 days you had diarrhoea and stomach cramps. You went to see your doctor and he prescribed an antibiotic, ciprofloxacin, which you were to take 500 mg twice a day for 5 days.
After 3 days you noticed your toes were tingling, but as you were wearing a new pair of shoes you assumed these were causing it. However, it continued and you mentioned it to the pharmacist 2 days later when you went to buy some paracetamol. They suggested that the antibiotic may have caused it, but you had finished the course the day before. When the tingling stopped two days later you realised it had probably been the tablets.
You are still taking the antimalarial tablets from your trip, which are proguanil two tablets daily and nivaquine two tablets weekly.
Additional information:
-
Your GP is Dr Walker, Green Lane Surgery, Long Eaton.
-
You are 5 feet 10 inches tall and weigh around 14 stone.
Scenario 5
Here are the details for the patient in scenario 5. Please note that this is not a real patient and all the contact details are made up. In this scenario you are the patient’s mother.
Your name is Clare Strand.
Address: 3 Queen Lane, West Bridgford, Nottingham NG24 3RR. Tel.: 0115 856767. E-mail: Clare.Strand@nottingham.ac.uk
You are concerned about a side effect suffered by your 3-year-old son.
On the 28 June your son Nathan Strand had a fever and headache, and you treated him with Nurofen syrup, using 5 ml three times a day. The next day he was coughing badly and when you took him to the doctor they prescribed amoxicillin syrup, one 5-ml spoonful three times a day.
The next day, on 30 June, he was covered in a rash when he woke up and then you noticed his face and fingers swelling. You phoned the doctor who suggested you take him immediately to casualty.
They treated him for an allergic reaction, stopped both of the syrups and gave him paracetamol and another antibiotic. He stayed in hospital over night, but came home with just the remains of the rash the next day (1 July). The rash cleared over the next few days.
Your son suffers from asthma and has to use a salbutamol inhaler occasionally.
Additional information:
-
The GP is Dr Shah, West Road Surgery, West Bridgford NG24 6TT.
-
Your son is 3 years and 5 months old and weighs 38 lbs. He is 3 feet 3 inches tall.
Scenario 6
Here are the details for the patient in scenario 6. Please note that this is not a real patient and all the contact details are made up. In this scenario you are the patient’s husband.
Your name is Roger Manning, and you live at Lilac Cottage, Lant Lane, Wollaton NG3 4FT. Tel.: 0115 9896662. E-mail: Roger.Manning@egg.com
You are reporting a side effect suffered by your wife, Jenny Manning. Jenny is 64 years old. In the middle of June, Jenny was started on metformin tablets, 500 mg twice a day, as she had been diagnosed with diabetes. She was also started on atenolol 50 mg in the morning as her blood pressure was high.
After 2 days Jenny complained she had a funny metallic taste in her mouth and her hands and feet were cold. Then within a week she was having nasty stomach pains.
She went back to the doctor who stopped the metformin and gave her gliclizide instead, and within a week, by the end of June, most of the symptoms had stopped, although she still has cold hands and feet.
Additional information:
-
The GP is Dr Joan Grieve, East Road, Wollaton NG3 5GF
-
Your wife is 162 cm tall and weighs around 70 kg.
Appendix 22 Omnibus questions
Appendix 23 Protocol, version 3: 7 June 2007 (version produced following response to referees’ comments)
Protocol for evaluation of patient-reporting to the Yellow Card System (RM05/JH30)
Version 3: 7 June 2007 (version produced following response to referees’ comments)
| Summary of research |
|---|
| Abstract of research |
| Objectives |
|
| Methods |
| We plan to address our objectives by undertaking: |
|
| Timescale |
| Proposed starting date: 1 September 2007 |
| Proposed duration: 2 years 0 months |
| Cost |
| Total research grant: £249,811 |
Details of proposed research
Evaluation of patient reporting to the Yellow Card System
Introduction
The call for an evaluation of patient reporting to the UK Yellow Card System (YCS) provides a tremendous opportunity to learn more about the role of patients in reporting suspecting adverse reactions.
In this document we present:
-
background literature on the UK YCS and its use by patients
-
background literature on other patient-reporting systems throughout the world
-
our research objectives
-
proposed methods for six studies and a further literature review aimed at addressing our research objectives and the questions raised in the National Coordinating Centre for Research Methodology (NCCRM) call for research
-
scheduling of the studies and responsibilities of coapplicants
-
proposals for the management of the project
-
justification for costings
-
references.
Background
An adverse drug reaction (ADR) is a reaction to a drug or combination of drugs that is harmful and unintended and which occurs at a dose normally used for prophylaxis, diagnosis or treatment (Metters 2004). The primary system for reporting suspected ADRs in the UK is the ‘YCS’. As a result of a Health Committee report (House of Commons Health Committee 2004–5), patients (or consumers) in the UK have been able to submit Yellow Card reports since 2005. The potential benefits of patient reporting were summarised at the First International Conference on Consumer Reports on Medicines in 2000, and included: the promotion of consumer rights and equity; acknowledging that consumers have unique perspectives and experiences; and, that health-care organisations would benefit from consumer involvement (World Health Organization 2000).
It has been suggested that direct consumer reporting avoids the filtering effect of reporting via health professionals, and that the former could contribute to drug surveillance when reports from the latter are declining (Hammond and Rich 2005), as is the situation in the USA. Consumer reporting of ADRs has also been suggested as a method of hypothesis generation that could be used in addition to health professional reports (Fernandopulle and Weerasuriya 2003).
International experience of consumer reporting systems
Other countries with consumer reporting of ADRs include the USA, Australia, Canada, Denmark, Sweden and the Netherlands. The characteristics of the European schemes are summarised in a report by Health Action International Europe (HAI 2005).The reporting schemes differ in terms of whether they are government supported or run by consumer groups.
MedWatch is the adverse event reporting system in the USA, which is provided by the US Food and Drug Agency (FDA). The scheme includes reports for adverse events, product problems and errors. Reports can be submitted by consumers either using mailed or faxed report forms, by telephone, or online (www.fda.gov/medwatch/report.htm). The number of reports from consumers has been increasing since 1993, while the number and proportion of reports from health-care professionals (HCPs) has been decreasing. In 1996, 41% and 58% of reports originated from consumers and health professionals, respectively (FDA 1997).
In Australia, consumers have access to the ‘Adverse Medicine Events (AME) Line’ to which they can report ‘suspected AMEs, possible errors or “near misses” with their medicines’ (www.mater.org.au/ame/HomeAME1.htm). [Consumers can also report to the Adverse Drug Reactions Unit (ADRU) of the Therapeutic Goods Administration (TGA) (Hill and Tan 2006).] The AME Line is operated by the Australian Council of Safety and Quality in Health Care. Suspected errors and ADRs are reported using two different forms, therefore, reporting rates for each can be analysed separately. A recent case series of consumer reports to AME relating to the use of zolpidem indicated that that memory disturbances, hallucinations and dependence were more common than was previously thought (Moses and McGuire 2006). An audit of the use of the AME Line showed that 43% of the 3415 calls that were received in a 2-year period were prompted by media publicity (McGuire and Moses 2006). Females and older consumers were more likely to call the line. One-fifth of ADRs reported by callers were previously unrecognised and 8% were related to complementary medicines. In total, 105 serious ADRs and drug-induced hospitalisations that were reported by callers had not been reported by health professionals (McGuire and Moses 2006).
The Canadian Adverse Drug Reaction Monitoring Program (CADRMP) at Health Canada is responsible for the collection and assessment of adverse reaction reports made voluntarily by health professionals and consumers. A public opinion survey on postmarketing surveillance, conducted on behalf of Health Canada, showed that consumers were more likely to believe in the safety of prescribed medicines than non-prescription medicines and natural health products (Health Canada 2003). The authors stated that reported ADRs in general (i.e. not solely ADR reports by consumers to CADRMP) were more likely to be reported by women, older consumers and by consumers with lower household incomes. Few health professionals who were surveyed had reported an ADR in the past year, and of those who had, they were more likely to be a pharmacist or a nurse compared with other health professionals. In terms of reporting ADRs, the majority of pharmacists (92%) stated that they knew how to make an ADR report, compared with 63% of physicians, 44% of nurses, 19% of naturopaths and 13% of dentists (Health Canada 2003). Over 80% of consumers believed that there should be a legal requirement for health professionals to report ADRs.
In the Netherlands, consumers can report ADRs directly to the Netherlands Pharmacovigilance Centre (Lareb) – a government-run organisation. Of the 6305 reports received in 2005, 819 (13%) were submitted by patients (Lareb 2005). As a result of greater emphasis on patient reporting, the number of reports received from patients in 2005 was 87% higher than in the previous year. In addition, there is a consumer-run reporting scheme (DGV: www.meldpuntmedicijnen.nl), which has operated since 2004. Higher numbers of reports are made to DGV compared with Lareb, however, the amount and type of information recorded by the two schemes differs, therefore the data are not entirely comparable. During the first 10 months of the DGV scheme, 49% of reports were related to side effects (HAI 2005), of which 6% were severe and 30% were not mentioned on the patient information leaflet (PIL).
A comparison was made of the reports associated with the use of paroxetine made to the Netherlands Pharmacovigilance Foundation (NPF), with those reported to a telephone medicines information service, which enabled patients to consult a pharmacist regarding the correct use of medicines and problems related to their medicine use (Egberts et al. 1996). Proportionally fewer reports were made about paroxetine via the telephone service compared with the NPF (0.5% vs 1.2%, respectively). However, ADRs were reported sooner [mean 229 days (95% CI, 160 to 298)] using the telephone service compared with the NPF. No difference was shown between the two reporting systems in terms of new suspected reactions (i.e. those not included in the PIL). Nine new ADRs were identified by both systems. Each reaction was first reported using the telephone system for all nine reactions, with a mean time lag of 273 days (95% CI, 89 days to 458 days) between the telephone and NPF system reports. The authors concluded that consumer reporting might assist in the earlier detection of both known and unknown ADRs, but that data from consumers alone were insufficient due to their ‘crude and incomplete’ nature.
Sweden also has a non-government consumer reporting system, known as KILEN (www.kilen.org). This system has been available since 1978. The HAI report (HAI 2005) states that the data collected by KILEN differ from data collected from health professionals. The KILEN system also provides feedback to individuals who submit reports.
Jarernsiripornkul et al. (2003) reported that the frequency of consumer reports for ADRs with tramadol was similar to spontaneous reports but higher than prescription event monitoring studies. Van den Bemt et al. (1999) compared doctor, nurse and patient reporting of ADRs for hospitalised patients. Patients were more likely to report ADRs with new drugs than doctors and nurses. Doctors reported more serious reactions. Fromme et al. (2004) compared patient reports using a specific instrument, with doctor reports of ADRs associated with chemotherapy. Patients reported more ADRs than doctors. There was little agreement between patient and doctor reporting. A qualitative study by Medawar and Herxheimer (2004) concluded that the quality and interpretation of data provided by health professionals in relation to ADRs associated with paroxetine was poor and might be considered inferior to that provided by consumers.
Summary The above evidence highlights the differences between ADR reports from patients and HCPs. These data demonstrate that patient reporting is important and complements ADR reports from health professionals. There is empirical evidence from non-UK studies that highlights the differences between patient and HCP reports; in particular, reports from the former may tend to be reported earlier and are more likely to include previously unidentified ADRs. The following programme of studies will explore the contribution that patients make to the YCS in the UK, compared with health professionals, as well as in comparison to existing schemes worldwide.
Objectives
Our objectives are to:
-
evaluate the pharmacovigilance impact of patient reporting to the YCS by analysing reports from patients and comparing these with reports from health professionals
-
report on patient experiences of the YCS by:
-
– following up a cohort of patients reporting to the YCS
-
– undertaking usability testing with patients of the different methods of reporting to the YCS
-
-
assess public awareness of being able to report to the YCS by conducting a national survey
-
offer recommendations for improvements to patient-reporting, based on our research findings and experience from other countries.
Methods
We plan to address our objectives by undertaking:
-
quantitative analyses of the pharmacovigilance impact of Yellow Card reports (objective 1)
-
qualitative analyses of the pharmacovigilance impact of Yellow Card reports (objective 1)
-
a questionnaire survey of patient experiences of reporting to the YCS, building on work already done by the MHRA (objectives 2 and 4)
-
telephone interviews to explore the experiences of patients who have reported to the YCS (objectives 2 and 4)
-
a national survey of public awareness of being able to report to the YCS (objective 3)
-
usability testing and focus groups with patients to help identify recommendations for improvement in the Yellow Card reporting system (objectives 2 and 4)
-
a further review of the world literature on patient-reporting systems to help supplement recommendations for improvement to the YCS (objective 4).
Each element of our proposed methods is explained in detail below. At the end of this methods section we provide a table illustrating how our research methods will address the specific issues identified in the NCCRM call for research (RM05/JH30).
Study 1: pharmacovigilance impact of Yellow Card reports – quantitative analyses
This study will:
-
identify the characteristics of patients reporting to the YCS
-
identify the types of drug, types of suspected adverse reaction and seriousness of suspected reactions reported by patients
-
explore the time lag between ADR occurrence and reporting for patients and health professionals
-
investigate the factors associated with patient reports compared with those made by health professionals
-
explore the relative contribution of patient reporting to signal generation.
The Medicines and Healthcare Regulatory Authority (MHRA) already has around 5000 Yellow Card reports generated by patients (Mick Foy, MHRA, London, May 2007, personal communication). Details from these reports are entered onto a computer database soon after they are received by the MHRA.
Our main analysis will be conducted on reports from October 2005 to September 2007 and we will ask the MHRA to provide us with a database of anonymised reports.
In addition, to allow us to undertake comparisons between patient reports and those from health professionals, we will ask the MHRA for an anonymised database of all reports submitted directly to the MHRA by health professionals over the same time period (there will be approximately 30,000 of these on the basis of MHRA figures). From this database we will use the following samples of reports for subsequent analyses:
-
For the main analysis, either a random sample of Yellow Card reports from all health professionals (number to match the numbers of reports from patients) or (if resources allow) all reports from health professionals.
-
For comparison of individual health professional groups with patient reports, subsamples of reports from the following groups: (1) doctors; (2) nurses; or (3) pharmacists.
-
Reports relating to particular classes of drug and for different professional groups for qualitative comparison with patient reports (see Study 2, below).
Our main quantitative analysis will be based on a comparison between patient reports for the period October 2005 to September 2007 and reports from health professionals.
Research questions
With such large data sets it is likely that we will be able to detect a number of differences between the characteristics of patient reports and those from health professionals. We are particularly interested in answering the following research questions:
-
What are the characteristics of patients who report ADRs through the YCS?
-
Are patients more or less likely than health professionals to report serious reactions?
-
What classes of drug are most commonly reported by patients?
-
Are patients more likely than health professionals to report suspected adverse reactions to particular classes of drug?
-
What categories of suspected adverse reaction are most commonly reported by patients?
-
Are patients more likely than health professionals to report particular categories of suspected adverse reactions?
-
Are there important differences in the time taken to report an ADR between patients and HCPs?
-
What are the outcomes of suspected side effects and do these differ across reporter groups?
Power calculation
The MHRA has provided us with data to do some illustrative power calculations and we have used differences in the proportions of Yellow Card reports graded as potentially ‘serious’ reactions as an example.
The MHRA has a system for coding Yellow Card reports as ‘serious’ on the basis of the information provided on the reports. These judgements are made in a consistent manner and the same approach is taken to reports from health professionals and patients. Approximately 59% of reports from health professionals are coded as potentially ‘serious’ reactions compared with 68% of reports from patients (Balall Naeem, MHRA, 2006, personal communication).
To illustrate the sample size of Yellow Card reports needed to identify both a 10% and a 20% difference in the proportion of reports coded as ‘serious’ between patients and health professionals, we have provided relevant calculations in the table below for different levels of power and statistical significance. Note that all calculations assume a two-tailed test.
Therefore, with a sample size of over 5000 in each group we will have over 90% power to detect a 10% difference in the proportions of reports coded as potentially ‘serious’ between patient and health professional reports at the 5% significance level.
Data
The YCS database contains a large number of fields, including:
-
category of reporter (patient, patient’s representative, doctor, nurse or pharmacist)
-
age of patients referred to on the Yellow Card
-
gender
-
mode of reporting: electronic, paper or telephone
-
numbers of drugs on each form reported as possibly causing side effects
-
name of drugs reported as possibly causing side effects
-
classification of suspected side effects (based on MHRA Medical Dictionary of Reaction Types)
-
free text used to describe suspected side effects
-
whether the drugs reported are new (‘black triangle’) drugs
-
date drug commenced
-
date that suspected side effect started
-
date that report sent to MHRA
-
reported seriousness of the suspected side effect
-
reported outcome of the suspected side effect.
| Significance level | Power (%) | Proportion of reports from health professionals coded as potentially ‘serious’ reactions (%) | Proportion of reports from patients coded as potentially ‘serious’ reactions (%) | No. needed in each group |
|---|---|---|---|---|
| 0.05 | 80 | 60 | 70 | 376 |
| 0.05 | 80 | 60 | 50 | 408 |
| 0.01 | 80 | 60 | 70 | 550 |
| 0.01 | 80 | 60 | 50 | 597 |
| 0.05 | 90 | 60 | 70 | 496 |
| 0.05 | 90 | 60 | 50 | 538 |
| 0.01 | 90 | 60 | 70 | 695 |
| 0.01 | 90 | 60 | 50 | 754 |
Completeness of data fields will be checked and validation checks on fields will be undertaken to ensure that subsequent analyses are done on clean data sets.
We will create additional data fields for subsequent analyses. In some instances we will be able to automate this process, for example calculating the time taken to report suspected adverse events. In other instances it will be necessary to undertake a more detailed review of the information contained in the Yellow Card report. These detailed reviews will be undertaken by a researcher with experience in pharmacovigilance work. A 1 in 10 sample of records will be checked for accuracy of interpretation and data entry.
As a result of this processing and analysis of Yellow Card data we will create the following additional data fields:
-
British National Formulary (or ATC) chapter of drugs reported
-
British National Formulary (or ATC) subchapter of drugs reported
-
whether the drugs are available over the counter (OTC)
-
whether the ‘drugs’ reported are complementary therapies, such as herbal preparations
-
time taken to report the suspected adverse event (based on the difference between the date that the suspected adverse event started and the date that it was reported).
Analyses
Descriptive statistics will be used to provide an overview of reports from patients and health professionals. Categorical data will be described using frequencies and percentages. Continuous data will be explored using frequencies and histograms and described using means and standard deviations if normally distributed and medians and interquartile ranges (IQRs) if non-normally distributed.
A major component of these quantitiative analyses will be a comparison between patient reports and those from health professionals. On the basis of the descriptive analysis we will decide how best to categorise the different classes of drug and different types of suspected adverse reaction experienced by patients. We will use appropriate univariate analyses to identify potential differences between patient reports and those from health professionals in terms of:
-
age and gender of patients
-
classes of drug
-
use of other drugs (number, type)
-
time lag between event and reporting of ADR
-
types of suspected adverse reaction
-
reported seriousness of the suspected adverse reaction (as coded by MHRA)
-
reported outcome of the suspected adverse reaction
-
number of words used to describe the suspected adverse reaction.
On the basis of the univariate analyses, multivariate logistic regression analyses will be undertaken to identify the most important factors associated with patient reports compared with those from those of health professionals.
As outlined above, the main analysis will be undertaken comparing all reports from patients with those from either the entire database of health professional reports, or a random sample of these (depending on the workload involved in processing all HCP reports). We will undertake similar analyses comparing patient reports with those from the different groups of health professionals (doctors, nurses and pharmacists).
Also, at the request of the commissioning panel for this research project we will make an assessment of the extent to which reports on the same incident have come through from both health professionals and patients. This assessment will be limited because we are using anonymised data. Nevertheless, we will look to see if there are matching cases in terms of age, gender and name of drug reported. We will then look in more detail at these cases to help determine whether they are likely to relate to the same incident, possibly enlisting the help of the MHRA in this. We will report the proportion of patient reports that also seem to have been reported by a health professional.
Signal detection analyses
The MHRA already undertakes signal detection analysis of Yellow Card reports. It does not, however, differentiate between patient and HCP reports.
In our study we will attempt to answer the following questions:
-
Do patient reports add ‘weight’ to known signals or signals generated by reporting by professionals?
-
Do patient reports generate ‘new’ signals?
-
If they do, are these signals for events which are expected to be of more concerns to patients than HCPs?
-
Do patient reports generate new signals sooner?
-
For children, are the events reported directly by parents different from those reported by HCPs, acknowledging possible small numbers?
Having received feedback from Dr Lesley Wise from the MHRA we recognise that, for the purposes of signal detection analysis, the data set of patient reports does not have large numbers. Also, it may be influenced by ‘noise’ (e.g. effects of consumer groups). In an attempt to address the first of these issues we will combine drugs into drug class and/or combine ADRs into ADR groups if necessary.
Our proposed signal detection methods will involve screening the two groups of data from HCP reports and patient reports, and ranking the groups of drugs in terms of number of reports for ADRs/groups of ADRs. We then plan to do disproportionality analysis between HCP reports and patient reports for groups (classes) of drugs and ADRs (or groups of ADRs dependent on numbers). We also plan to investigate paediatric reports by parents as a subset, dependent on number of reports available.
Study 2: pharmacovigilance impact of Yellow Card reports – qualitative analyses
This study will:
-
assess the extent to which patient reports are likely to capture new knowledge about ADRs (in terms of quantity and quality) and contribute to signal generation
-
explore the richness of patients’ descriptions of their suspected adverse reactions compared with health professional.
We plan to analyse several different categories of patient report based on information recorded in the database created for the quantitative analysis. We have budgeted to analyse in detail the text on at least 300 reports from health professionals and 300 reports from patients. We will create a sampling frame to ensure that the following categories of report are adequately covered:
-
patient reports by paper and internet (65% and 33% of reports, respectively: Balall Naeem, personal communication) (we will not focus on telephone reports as these make up less than 2% of the total and the MHRA try to encourage patients making contact by telephone to fill in a paper-based or online report)
-
reports from different groups of health professional (including doctors, nurses and pharmacists)
-
new ‘black triangle’ drugs
-
drugs that can be purchased OTC
-
complementary therapies (acknowledging that reports may be less common from health professionals)
-
a number of specific drugs or drug groups thought to be of interest, based on the initial quantitative analysis, signal generation and discussions with MHRA and members of the study steering group.
After stratifying for the mode of reporting, we plan to take a random sample of reports from patients within each category and to match them, where possible, with randomly sampled reports from health professionals for the same drug (stratifying for types of health professional, and mode of reporting). This means that we will be comparing reports for the same drugs, whether patients or health professionals have done the report. The process of matching will be possible using the databases of patient and professional Yellow Card reports mentioned above.
We will undertake two distinct types of qualitative analysis on the reports.
The first, will be a detailed clinical assessment of the extent to which patient reports capture potentially new knowledge compared with reports from health professionals. This analysis will be informed by whether the reports capture problems not previously recorded on summaries of products characteristics or PIL. In addition, we will assess the extent to which useful information from patients might be lost when suspected adverse effects are coded by the MHRA.
The second, will be a comparative documentary analysis of the patients’ and professionals’ reports of suspected medication side effects. We will particularly examine:
-
the ways in which patients describe suspected side effects
-
the richness of patient reports compared with those of health professionals.
We will seek to identify the features that characterise the reports then use these to create a coding framework that will be applied to all of the data for comparative purposes. Specialist software will be used as an aid to order and categorise the data. Each of three analysts will examine a subset of the data and generate tentative codes, which will then be discussed in team meetings. Once satisfied with the framework, it will be applied to the whole sample with inter-rater reliability coding checks carried out to ensure the consistency of coding. We will thus ensure that the data are fully explored and interpreted, and will identify similarities or differences between patient and health professional reports and the three methods of reporting.
Study 3: a questionnaire survey of patients reporting to the Yellow Card System
This study will obtain feedback from patients reporting to the YCS in order to address a number of questions raised in the call for research.
Yellow Cards currently submitted by patients usually contain their contact details, including postal address. The MHRA provides a reassurance to patients that their ‘personal details will not be passed to any person outside the MHRA without [their] permission’.
We propose, therefore, that the MHRA sent out questionnaires on our behalf with a covering letter explaining the involvement of the research team. We propose that patients return completed questionnaires to the Division of Primary Care at the University of Nottingham. We suggest putting code numbers on the questionnaires to allow the research team to inform the MHRA of non-responders. In turn, this will allow the MHRA to send out reminders to these patients.
To minimise recall bias it is important that we survey patients as soon as possible after they submit Yellow Cards. We propose, therefore, to ask the MHRA to send out requests on a weekly basis as reports come in.
During the time that we are collecting feedback from patients we believe that it would be best for the MHRA not to send out its electronic questionnaires to patients who have used internet reporting. This will help to avoid questionnaire fatigue in patients, which could compromise response rates.
Questionnaire design
We note that the MHRA already issues an electronic questionnaire to patients that have submitted electronic reports. We will ask the MHRA for reports on the results of these surveys to help inform our own questionnaire design, and, where possible, we will use similar questions to those already used by the MHRA.
The questionnaire will cover the following issues:
-
how patients found out about the YCS
-
how many times they have used the system
-
what method of reporting they used for their latest Yellow Card report (electronic, paper or telephone)
-
who did the report (the patient or the patient’s representative)?
-
how easy they found it to make a report
-
any difficulties encountered in making reports, including whether patients needed additional help in completing the electronic or paper-based forms
-
any suggestions for improvements in the reporting system
-
whether they informed a health professional about the suspected reaction
-
characteristics of respondents (age, gender, ethnicity and educational attainment).
In addition, we propose asking patients if they would be willing to participate in a telephone interview to explore in more detail issues around reporting to the YCS. This will require participants to volunteer their contact information if they agree to be contacted directly by the researchers. At the time they are given the opportunity to volunteer their contact information, it will be explained that this information will be kept securely and will be used only for purposes of contacting patients for telephone interviews.
Power calculation
According to the MHRA, Yellow Card reports from patients are coming in at a rate of 100–200 per month at present; approximately 66% of these are paper reports, 33% electronic and 2% reports by telephone.
As a primary outcome measure, we suggest looking for differences between patients making electronic or postal reports in the proportion rating it ‘easy to make a report’ (using a Likert scale). To detect a 10% difference in this measure between the two groups (say 50% in one group and 60% in the other) with a power of 80% and significance level of 5% we would need at least 408 patients in each group (we have suggested similar numbers of patients in each group as the proportion of patients making electronic reports may be similar to the proportion making paper reports by the time of the survey). Therefore, we would aim to obtain a total of 1200 questionnaire responses, which should be sufficient even if the ratio of paper to electronic reports remains at 2 : 1.
We propose to send out questionnaires to patients until the sample size is met. To increase the response rate we propose sending reminders to patients who have not replied within 3 weeks.
Given the current rate at which patients are reporting Yellow Cards (100–200 per month) and taking a conservative response rate of 50%, we estimate that it will take up to 18 months to complete the postal questionnaire.
Statistical analysis
Data from the questionnaires will be entered into a access database and a 1-in-10 sample will be checked for accuracy. If any problems are detected, data will be double entered for the whole sample.
Data will be exported into spss for statistical analysis. Categorical data, including many of the questionnaire responses, will be described using frequencies and percentages. Age of respondents will be described using means and standard deviations if normally distributed and medians and IQRs if non-normally distributed.
Appropriate univariate comparisons will be made between the types of Yellow Card report used by patients (electronic, paper and telephone) and their responses to the questionnaire. Multivariate analyses will adjust for potential confounding of factors such as age, gender and educational attainment.
Content analysis will be undertaken on free-text comments, such as those relating to potential improvements to the Yellow Card reporting system.
Study 4: telephone interview follow-up of patients reporting to the Yellow Card System
This study will enable us to obtain detailed feedback on current reporting systems and advice on how these could be improved.
Study design
Semistructured telephone interviews will be conducted with patients selected from those who have completed questionnaires (Study 3) and given consent to being contacted by the research team. Telephone interviews will begin 3 months after the start of the questionnaire study to allow the interview guide to be informed by preliminary analysis of the first tranche of questionnaire data (see below). Interviews will be recorded digitally, if patients consent to this, and will be transcribed verbatim.
Sampling
We plan to use maximum variation sampling (Marshall 1996) in order to obtain a wide range of opinions. The factors that we will take into account in the sampling include age, gender, ethnicity, educational attainment of patients and the mode of reporting. In addition, we will take account of issues raised in the questionnaire, such as the perceived ease of reporting.
Issues to be explored in the interviews
The semistructured telephone interviews will be conducted within 6 weeks of the receipt of the questionnaire, using an interview guide developed by the research team. The development of the guide will be informed by the preliminary analysis of the first tranche of questionnaire data and a number of foreshadowed issues identified by the project team, which include:
-
exploration of any difficulties in making Yellow Card reports and suggestions for improvement in the reporting system
-
patients’ motivations for making the report and anticipated contribution of their report
-
patients’ expectations about what would happen to their report
-
patients’ satisfaction or dissatisfaction with making a report
-
patients’ willingness to report in future.
Analysis
Interview transcripts will be analysed by the qualitative researcher responsible for the data collection in collaboration with two academics with experience in qualitative analysis (one with a pharmacy background, the other with sociology background). The data will be analysed for both anticipated and emergent themes using the method of constant comparison. Analysis and data collection will proceed simultaneously and continue until ‘data saturation’ is reached to ensure that the widest possible range of experiences has been included. We anticipate that between 30 and 50 interviews will be required to achieve this.
Study 5: national survey of public awareness of being able to report to the Yellow Card System
We plan to undertake a representative national survey of adults in England to assess public and patient awareness of being able to report suspected drug side effects using the YCS.
Survey design
We plan to cover the following issues in the survey:
-
whether respondents use any types of medicines regularly or have taken any types of medicines in the last year
-
whether respondents believe that they have experienced a side effect from a medicine (or complementary therapies) in the past and, if so, whether they told anyone about it
-
whether respondents have heard about the YCS for patient reporting of suspected side effects to medication
-
for those respondents who have experienced side effects from a medicine in the past and who had heard about the YCS for patient reporting:
-
– whether they made a report
-
– if they did not make a report, why not?
-
-
in rank order, which of the following ways of reporting suspected side effects might be most convenient to respondents:
-
– telephone
-
– online
-
– obtaining a paper form from a pharmacy (chemist) to fill in and post
-
– obtaining a paper form from a general practice to fill in and post
-
telling a health professional about the problem so that they can decide whether to send in a Yellow Card.
In addition, we will obtain information on the characteristics of respondents.
Survey administration
It can be extremely time consuming, and prohibitively expensive, to undertake national surveys of patients given current research governance arrangements. Therefore, we propose adding our research questions to a representative national survey of the public. We recognise that there may be some disadvantages to this approach, including low response rate, but believe that these are outweighed by the ease and speed with which the survey can be completed, costs and the ability of national omnibus surveys to collect reasonably representative data.
We have worked successfully with British Market Research Bureau (BMRB) Omnibus Surveys in the past (www.bmrb.co.uk: Ealing Gateway, 26–30 Uxbridge Road, Ealing W5 2BP, UK) and suggest using them for the proposed study. This company does national surveys using face-to-face, telephone and on-line methods of collecting data. Following discussions with the company, it is likely that a telephone survey will provide the most representative sample for our study. BMRB national telephone surveys run from Friday to Sunday every weekend and interview a fresh sample of 2000 nationally representative adults aged 16+ years each time. Results can be delivered within a week depending on the complexity and length of the questionnaire and the analysis required. Results are presented in tabulated format in a database programme or spreadsheet, with the responses broken down by gender, age, socioeconomic status and geographical region. The organisation is also able to break any question against other demographic details available such as age at leaving education. To give an idea of costings, a ‘yes/no’ question to 2000 people would cost £780 (+VAT) and a precoded question with up to five response options would cost £975 (+VAT). These are relatively low costs for a short national survey and would allow us to put more researcher time into other aspects of the YCS evaluation.
Statistical analysis
Data from the survey will be exported into spss and coded for subsequent analysis. Categorical data, including many of the questionnaire responses, will be described using frequencies and percentages. Age of respondents will be described using means and standard deviations if normally distributed and medians and IQRs if non-normally distributed.
Study 6: focus groups with patients followed by usability testing to help identify recommendations for improvement
We propose to combine two qualitative methods to prospectively examine patient experiences of using Yellow Cards: usability testing and focus groups. This study will enable us to identify patient views of the user friendliness, effectiveness and usability of different mechanisms of patient reporting, while also identifying potential ways in which the reporting system could be improved.
Recruitment
We shall include patients who believe they may have experienced side effects from medications, but who have not previously filled in a Yellow Card report [over 10% of patients on new medications experience side effects (Gandhi et al. 2003)]. We plan to recruit through advertisements in local media, surgeries and pharmacies in Nottingham. We aim to recruit six groups of eight patients for this study. Each group will be heterogeneous in terms of age, occupational class, gender, educational level and prior experience of using the internet. Each group will take part in a focus group and usability testing in one 2- to 3-hour session and will be provided with refreshments and an inconvenience payment of £25.
Focus groups
After a short presentation on ADRs and the YCS by the group convenor, participants will be encouraged to discuss their readiness or otherwise to use the reporting system, to identify any barriers or facilitators to making use of the system and to identify their initial preferences for using one or other method of reporting. The focus groups will be recorded digitally and fully transcribed.
Usability testing
Usability testing will take place in the pharmacy practice laboratories at the University of Nottingham. These rooms are equipped with computers with access to the internet, telephone handsets and a stock of paper Yellow Card report forms will be provided.
Each user will be presented with a number of scenarios in which a patient experiences what may be an adverse reaction to taking a drug to treat or prevent a specified condition. These scenarios will be drawn from a ‘bank’, which relates to the range of drug groupings identified by patients in our analysis of Yellow Card reports. Users will be asked to decide, in relation to each scenario, whether, in their opinion, it would be appropriate to complete a Yellow Card. Three of the scenarios for which they deem reporting to be appropriate will then be selected and the user will be invited to complete a Yellow Card, using each of the three methods for one of the scenarios. The order in which they use the three methods will be randomly assigned to avoid learning effects creating bias. As they complete the tasks, the researchers will encourage them to ‘talk aloud’ about the experience, any difficulties or uncertainties they encounter and any changes to the systems they would find helpful. The discussions will be digitally recorded. The researchers will review the recordings and identify relevant sections for selective transcription.
In order to complete each of these exercises successfully we will need at least eight researchers to observe patients and record their comments. These researchers would be drawn from our study team and we would also use PhD students at the University of Nottingham.
For the telephone reporting we will discuss with the MHRA whether we might use their own telephone reporting service for these exercises to help ensure that the experience of reporting is as close as possible to reality. Obviously, we would need to identify a way of ensuring that data from these telephone calls were not included in MHRA reports of suspected ADRs. If it is not possible to use the MHRA system, we would train researchers in the way in which MHRA record data from telephone contacts and use them to take calls from the patients undergoing usability testing.
Analysis of focus groups and usability testing
Data from the focus groups and usability testing will be analysed by a researcher at the University of Nottingham under the guidance of Professors Anderson and Murphy. Data will be analysed for both anticipated and emergent themes, using the method of constant comparison. We are particularly interested in patients’ views on:
-
the user friendliness, effectiveness and usability of the different mechanisms of patient-reporting
-
the ways in which the Yellow Card reporting system could be improved for patients.
Study 7: literature review
The published literature on patient-reporting systems in other settings will be reviewed. Search strategies will be based on the findings of the study. For example, if a lack of awareness of the patient-reporting system is identified, the search will be focused on mechanisms used for addressing this problem in other countries. Where possible comparisons will be made with the study findings and recommendations made as necessary to amend the UK system. It is expected that the literature will be wider than traditional academic papers and will include policy documents, websites and other grey literature.
How our proposed methods will address the questions raised in the call for research
The call for research raised a number of research questions on the themes of patient experiences of reporting to the YCS and pharmacoepidemiological impact. There was a request for researchers to address these themes within a framework that will allow comparisons between the three methods of patient reporting and between reports from patients and different groups of health professionals. We believe that we will be able to address the questions raised in the call for research and we illustrate how we propose to do this below.
| Research theme | Methodological approach | Outcomes |
|---|---|---|
| Patient experience | ||
| Patient’s awareness of being able to report |
|
|
| The relative effectiveness of different communication strategies to encourage patient reporting |
|
|
| Patients’ reactions to the reporting system and ability to complete Yellow Cards without assistance |
|
|
| Patient’s views on the user friendliness, effectiveness and usability of different mechanisms of reporting |
|
|
| Pharmacovigilance impact | ||
| A qualitative investigation of the ‘richness’ of patients’ descriptions of their symptoms |
|
|
| The time lag between ADR occurrence and reporting | Quantitative analysis of Yellow Card reports (Study 1) | Comparison of time lag for patient reports compared with reports from health professionals |
| The relative contribution of patient reporting to signal generation in terms of both quantity and quality |
|
A detailed evaluation of the relative contribution of patient reporting to signal generation |
| Framework for the analyses | ||
| Comparisons between the three methods of reporting for patients and reports from patients and different groups of health professionals |
Quantitative analyses of Yellow Card reports (Study 1) Qualitative analyses of Yellow Card reports (Study 2) |
|
Scheduling of the studies and responsibilities of coapplicants
The Gantt chart, below, indicates the proposed timelines for the seven studies including initial planning and final dissemination. Responsibilities of the different centres involved in the research are highlighted.
Project management
Professor Avery will have overall responsibility for the successful management of the whole project. Areas of lead responsibility of other applicants are as follows:
-
University of Nottingham:
-
– Dr Fortnum, liaison with the MHRA (together with Professor Avery) and coordination of the studies between the different sites
-
– Professors Anderson and Murphy, qualitative work led by the University of Nottingham
-
-
Drug Safety Research Unit:
-
– Professor Shakir, overall responsibility for analyses undertaken by the DSRU
-
– Dr Marshall, supervision of work of researcher at DSRU
-
– Dr Lorna Hazell, undertaking most of the analyses of Yellow Card reports that fall within the work remit of the DSRU
-
-
University of Aberdeen:
-
– Professor Hannaford, overall responsibility for quantitative analyses undertaken by the University of Aberdeen
-
– Dr Lee, finalisation of statistical analysis plans and supervision of quantitative analyses undertaken in Aberdeen
-
– Dr Watson, supervising the literature review
-
– Professor Bond, questionnaire design for studies 3 and 5 (with Professor Krska)
-
-
Liverpool John Moores University:
-
– Professor Krska, questionnaire design for studies 3 and 5 (with Professor Bond).
-
Project management group
Our project management group will include all the applicants in this bid with the leads from each institution being required to ensure that a representative is available for all relevant meetings. Through previous collaborative projects we have experience of working effectively together using regular teleconferences and we would envisage having these every fortnight at the start of the project and then once a month for the duration of the studies. We would plan to have face-to-face meetings at the beginning, middle and end of the project, and to use the final meeting to evaluate our findings and agree on recommendations for any improvements in the Yellow Card Reporting System for patients.
Project advisory group
A project advisory group will be convened to provide advice during the course of the study and to contribute to making recommendations for improvements in the Yellow Card Reporting System for patients. The group will include representatives of the MHRA, the main professional groupings making reports, and patient representatives from both patient support groups and patient advocacy groups.
Justification for costings
In order to address the research questions raised by the NCCRD and to take maximum advantage of the wealth of information available, we have proposed an ambitious, but achievable, set of seven studies. We have costed these carefully and the total comes close to the upper limit of £250,000 that applicants were invited to bid for. We believe that our bid represents good value, given the range of outputs that we will be able to deliver.
We are requesting funding to employ four people to work solely on this project.
-
A research fellow with clinical skills in pharmacovigilance [0.6 whole-time equivalent (wte)] will be required at the DSRU for 18 months in the middle of the project to undertake initial analyses for study 1 and clinical assessment for study 2 under the supervision of Dr Marshall.
-
A research assistant with quantitative skills (0.6 wte) will be required in Aberdeen for the final 16 months of the project to undertake the quantitative analyses for studies 1, 3 and 5 under the supervision of Dr Lee.
-
A research assistant with qualitative skills (0.8 wte) will be required in Nottingham for the final 18 months of the project to undertake analyses for studies 2 and 4 under the supervision of Professors Murphy and Anderson.
-
An administrator (0.5 wte) will be required in Nottingham for the duration of the project to provide general administrative support to the whole project but specifically to coordinate the questionnaire mailing and data entry in study 3, and patient recruitment and interview transcription from studies 4 and 6 under the supervision of Dr Fortnum.
In addition, the time of an information technology specialist (20 days) will be required at the DSRU in Southampton to ensure data quality.
Management of the project will be facilitated by regular team meetings. We propose to meet together face to face as a whole team on three occasions: at the beginning, middle and towards the end of the project. We request funds for travel to Nottingham and for an overnight stay of the final meeting. In addition, we propose to maintain management of the project via teleconferences: fortnightly for the first 6 months and monthly thereafter (n = 30).
Study 3 will involve the printing and mailing of up to 2000 questionnaires for which we are requesting £2000.
The Omnibus survey for Study 5 will be costed per question and we estimate that we shall include five yes/no questions and two choice questions, at a cost of £6875.
Study 6 will involve recruitment of 48 people to focus groups and we propose to pay them an inconvenience allowance of £25 each. We shall also recruit local PhD students to supervise the sessions and have requested £885 to cover these costs for eight sessions.
The time of the coapplicants in Nottingham, Aberdeen and Liverpool is included at 6 days per person over the 2-year course of the project in accordance with full economic costing as applied at each university. In addition, an equivalent allowance has been requested for Professor Shakir and Dr Marshall at the DSRU.
Estate charges and indirect costs have been calculated on the basis of Transparent Approach to Costing methodology for each of the higher education institutions.
References
-
Adverse Medicines Event (AME) Line. URL: www.mater.org.au/ame/HomeAME1.htm (accessed 11 June 2006).
-
Egberts TCG, Smulders M, de Koning FHP, Meyboom RHB, Leufkens HGM. Can adverse drug reactions be detected earlier? A comparison of reports by patients and professionals. BMJ 1996;313:530–1.
-
Fernandopulle RBM, Weerasuriya K. What can consumer adverse drug reaction reporting add to existing health professional-based systems? Focus on the developing world. Drug Saf 2003;26:219–25.
-
Food and Drug Administration (FDA). Annual adverse drug experience report: 1996. Silver Spring, MD: FDA; 1997.
-
Fromme EK, Eilers KM, Mori M, Hsieh YC, Beer TM. How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient-reported symptoms from the quality-of-life questionnaire C30. J Clin Oncol 2004;22: 3485–90.
-
Gandhi TJ, Weingart SN, Borus J, Seger AC, Peterson J, Burdick E, et al. Adverse events in ambulatory care. N Engl J Med 2003;348:1556–64.
-
Hammond IW, Rich D. Consumers usurp spontaneous adverse event reporting in the United States. Pharmacoepidemiol Drug Saf 2005;14:S8–9.
-
Health Action International (HAI). Patients’ reporting of adverse reactions. Outcomes of a seminar organised by Health Action International Europe, Amsterdam, 26 May 2005.
-
Health Canada. Public opinion survey on key issues pertaining to post-market surveillance of marketed health products in Canada. Final Report, POR# 298–02. Decima Research Institute, Canada, December 2003.
-
Hill R, Tan A. Adverse drug reaction reporting and quality use of medicines. In Conference Proceedings, National Medicines Symposium, Canberra, 2006 (www.icms.com.au/nms2006/).
-
House of Commons Health Committee. The influence of the pharmaceutical industry. Fourth Report of Session, London, 2004–5.
-
Jarernsiripornkul N, Krska J, Richards ME, Capps PAG. Patient reporting of adverse drug reactions: useful information for pain management? Eur J Pain 2003;7:219–24.
-
KILEN. URL: www.kilen.org (accessed 11 June 2006).
-
Lareb. Annual report from Netherlands Pharmacovigilance Centre. Lareb, Hertogenbosch, 2005. (www.lareb.nl)
-
Marshall MN. Sampling for qualitative research. Fam Pract 1996;13:522–5.
-
McGuire T, Moses G. What do consumers contribute to pharmacovigilance? Lessons from the AME line. In Conference Proceedings, National Medicines Symposium, Canberra, 2006 (www.icms.com.au/nms2006/).
-
Medawar C, Herxheimer A. A comparison of adverse drug reaction reports from professionals and users, relating to risk of dependence and suicidal behaviour with paroxetine. Int J Risk Saf Med 2003–4;16:5–19.
-
MedWatch Online Voluntary Reporting Form. URL: www.fda.gov/medwatch/report.htm (accessed 11 June 2006).
-
Meldpunt Medicijnen. URL: www.meldpuntmedicijnen.nl (accessed 11 June 2006).
-
Metters J. Report of an independent review of access to the Yellow Card scheme. London: The Stationery Office; 2004.
-
Moses G, McGuire T. Consumers reporting novel ADRs: a zolpidem case series. In Conference Proceedings, National Medicines Symposium, Canberra, 2006 (www.icms.com.au/nms2006/).
-
Van den Bemt PMLA, Egberts ACG, Lenderink AW, Verzijl JM, Simons KA, van der Pol WSCJM, et al. Adverse drug events in hospitalized patients. A comparison of doctors, nurses and patients as sources of reports. Eur J Clin Pharmacol 1999;55:155–8.
-
World Health Organization (WHO). First International Conference on Consumer Reports 2000 Sigtuna. Consumer reporting of adverse drug reactions. WHO Drug Inf 2000;14:211–15.
Glossary
- Adverse drug reaction (ADR)
- An ADR is a reaction to a drug, or a combination of drugs, which is harmful and unintended and that occurs at a dose normally used for prophylaxis, diagnosis or treatment. Sometimes the use of the term is restricted to reactions that have been shown to have a strong causal association with particular drugs; sometimes it is used for reactions that reporters consider might have been associated with a specific drug, for example reactions reported to pharmacovigilance schemes.
- Black triangle drug
- Medicinal products that require a period of intensive monitoring by the Medicines and Healthcare products Regulatory Authority (MHRA) to further assess their risk–benefit profile.
- Dictionary serious
- Reaction preferred terms (PTs) within the Medical Dictionary for Regulatory Affairs (MedDRA) dictionary are assessed by medically qualified personnel within the MHRA, and individually assigned a ‘dictionary serious’ status of either ‘Yes’ or ‘No.’ This classification is based on the MHRA’s own ‘in-house’ medical opinion.
- Drug–adverse drug reaction (ADR) pair
- The occurrence of a specific drug associated with a specific ADR in Yellow Card reports.
- Patient information leaflet (PIL)
- European and UK law requires all medicines to be accompanied by a PIL, setting out comprehensive information that is accessible to and understandable by those who receive it, so that patients can use their medicine safely and appropriately.
- Patient reporter
- The patient who experienced and reported the suspected ADR or a patient representative who made the report on his or her behalf.
- Pharmacovigilance
- Pharmacovigilance is the science and activities relating to the detection, assessment, understanding and prevention of adverse effects of drugs.
- Signal
- Reported information on a possible causal relationship between an adverse event and a drug, the relationship being known or incompletely documented previously.
- Signal of disproportionate reporting (SDR)
- A ‘statistical signal’, when the reporting rate for an ADR in association with a particular medicine is disproportionate to that of other products in the database.
- Summary of product characteristics (SPC)
- A reference document in relation to a medicinal product, intended for use by health-care professionals (HCPs), which provides information on how to use/prescribe the medicinal product safely and effectively.
List of abbreviations
- ADR
- adverse drug reaction
- ADROIT
- Adverse Drug Reactions Online Information Tracking
- AME
- Adverse Medicine Events (Line)
- APRIL
- Adverse Psychiatric Reactions Information Link
- ATC
- Anatomical Therapeutic Chemical classification system for drugs
- BMRB
- British Market Research Bureau
- BNF
- British National Formulary
- CI
- confidence interval
- CIOMS
- Council for International Organizations of Medical Sciences
- CSM
- Committee on Safety of Medicines
- dm+d
- Dictionary of Medicines and Devices
- DSRU
- Drug Safety Research Unit
- GP
- general practitioner
- HCP
- health-care professional
- HLGT
- high-level group term (MedDRA)
- HLT
- high-level term (MedDRA)
- IQR
- interquartile range
- LLT
- lowest-level term (MedDRA)
- MedDRA
- Medical Dictionary for Regulatory Affairs
- MeSH
- medical subject heading
- MHRA
- Medicines and Healthcare products Regulatory Authority
- MSSO
- Maintenance and Support Services Organization
- OR
- odds ratio
- OTC
- over-the-counter
- PIL
- patient information leaflet
- PRR
- proportional reporting ratio
- PT
- preferred term (MedDRA)
- SD
- standard deviation
- SDR
- signal of disproportionate reporting
- SOC
- system organ class (MedDRA)
- SPC
- summary of product characteristics
- SSRI
- selective serotonin reuptake inhibitor
- WHO
- World Health Organization
- YCS
- Yellow Card Scheme
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Professor of General Practice, Department of Primary Health Care, University of Oxford Programme Director,
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, University of Liverpool
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Deborah Ashby, Professor of Medical Statistics and Clinical Trials, Queen Mary, Department of Epidemiology and Public Health, Imperial College London
-
Professor Peter Brocklehurst, Director, National Perinatal Epidemiology Unit, University of Oxford
-
Professor John Cairns, Professor of Health Economics, London School of Hygiene and Tropical Medicine
-
Professor Peter Croft, Director of Primary Care Sciences Research Centre, Keele University
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Jonathan Green, Professor and Acting Head of Department, Child and Adolescent Psychiatry, University of Manchester Medical School
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, Head of Nuffield Department of Surgery, University of Oxford
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norris, Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor James Raftery, Chair of NETSCC and Director of the Wessex Institute, University of Southampton
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Martin Underwood, Warwick Medical School, University of Warwick
-
Professor Marion Walker, Professor in Stroke Rehabilitation, Associate Director UK Stroke Research Network, University of Nottingham
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Catherine Moody, Programme Manager, Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire Country Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Professor Carol Tannahill, Glasgow Centre for Population Health
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Sarah Tyson, Senior Research Fellow & Associate Head of School, University of Salford
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr David P Britt, Public contributor
-
Mr Sankaran ChandraSekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seamus Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor David Taggart, Consultant Cardiothoracic Surgeon, John Radcliffe Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Professor Nicholas James, Professor of Clinical Oncology, School of Cancer Sciences, University of Birmingham
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/ Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Mr John Chapman, Public contributor
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Ms Kylie Gyertson, Oncology and Haematology Clinical Trials Manager, Guy’s and St Thomas’ NHS Foundation Trust London
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Martin Shelly, General Practitioner, Silver Lane Surgery, Leeds
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation of Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Professor Bruce Campbell, Consultant Vascular & General Surgeon, Royal Devon & Exeter Hospital, Wonford
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Neill McIntosh, Edward Clark Professor of Child Life and Health, University of Edinburgh
-
Professor Rajan Madhok, Consultant in Public Health, South Manchester Primary Care Trust
-
Professor Sir Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, Director, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Philip Shackley, Senior Lecturer in Health Economics, Sheffield Vascular Institute, University of Sheffield
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Dr Nick Summerton, GP Appraiser and Codirector, Research Network, Yorkshire Clinical Consultant, Primary Care and Public Health, University of Oxford
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Dr Ross Taylor, Senior Lecturer, University of Aberdeen
-
Dr Richard Tiner, Medical Director, Medical Department, Association of the British Pharmaceutical Industry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington