Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number DTC-RP-PG-0311-12001. The contractual start date was in January 2014. The final report began editorial review in May 2019 and was accepted for publication in March 2021. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
Copyright © 2021 O’Brien et al. This work was produced by O’Brien et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 O’Brien et al.
SYNOPSIS
Introduction
Lewy body dementia (LBD) is a term used to describe two closely related conditions: dementia with Lewy bodies (DLB) and Parkinson’s disease dementia (PDD). They form the second most common neurodegenerative dementia (after Alzheimer’s disease), but it is recognised that they are underdiagnosed. Diagnosis has important implications for management, as those with LBD present with a range of variable clinical features, including cognitive impairment, motor symptoms, autonomic symptoms, sleep problems and neuropsychiatric features.
We undertook this programme because little is known regarding the prevalence or diagnostic practice of LBD in the UK and, although national management guidelines [e.g. National Institute for Health and Care Excellence (NICE) 20181] do recognise the importance of both DLB and PDD and make some limited management recommendations, there are no systematic approaches to management or recognised care pathways. DIAMOND-Lewy (Improving the DIAgnosis and Management of Neurodegenerative Dementia of Lewy body type) was a comprehensive, multidisciplinary 5-year programme of work seeking to investigate and implement ways to improve both the diagnosis and management of LBD (i.e. both DLB and PDD) within the NHS.
We achieved this through a number of interlinked work packages (WPs), with a very strong patient and public involvement (PPI) core running throughout the programme. In this synopsis, we first describe our approach to PPI and how this influenced the design of the programme. In WP1, we undertook a retrospective case note study to investigate current practice in secondary care NHS services with regard to the diagnosis and management of LBD. In WP2, we undertook systematic reviews of pharmacological and non-pharmacological management of LBD and used this evidence, supplemented by a Delphi study of expert clinicians, to produce an evidence-based management toolkit. In WP3, we developed assessment toolkits for the diagnosis of LBD and, incorporating the management toolkit from WP2, piloted these assessment and management toolkits in routine NHS memory assessment and movement disorder services. In WP4, we introduced the assessment and management toolkits more broadly within a pilot cluster randomised trial. WP5 was a series of qualitative studies with three components: (1) an investigation of the barriers to and facilitators of making a diagnosis and managing LBD, (2) exploration of views on the assessment and management toolkits and (3) exploration of implementation of the assessment and management toolkits in clinical practice. Finally, in WP1 repeated (WP1R), we undertook a repeat assessment of DLB and PDD diagnostic rates following introduction of the assessment toolkits in some of the same services that had participated in WP1.
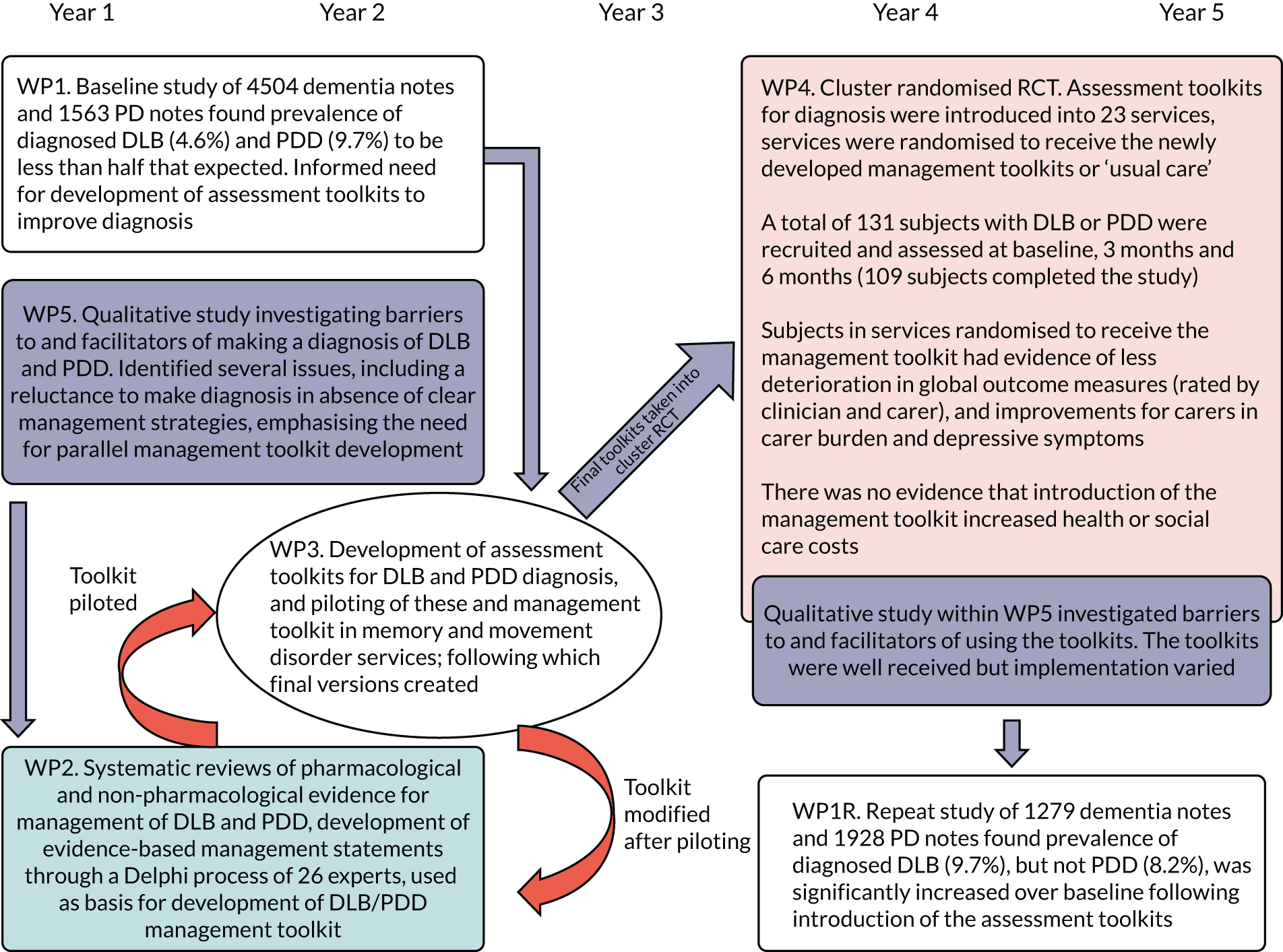
A research pathway diagram showing how these WPs linked together is provided (Figure 1). Each WP will now be discussed in turn after a description of the PPI core running throughout the programme.
FIGURE 1.
Research pathway diagram. PD, Parkinson’s disease; RCT, randomised controlled trial.
Patient and public involvement throughout the DIAMOND-Lewy programme
Introduction
Patient and public involvement is seen as fundamentally important to the quality and relevance of research. In the light of calls for greater transparency and consistency in reporting PPI, we have followed the GRIPP2-SF (Guidance for Reporting Involvement of Patients and the Public 2–short form) reporting guidelines, highlighting the impacts of PPI and critically reviewing our approach to PPI. 2–4 Much of this section of the report was drafted collaboratively with members of the PPI group.
Aim
The aim was to use PPI to inform all phases of the study from patient and carer perspectives.
Methods
Patient and public involvement in this programme started very early, during the development of the proposal, with one PPI member (Derek Forster) being a co-applicant. Once the programme started, we established a PPI group that met regularly throughout the programme. Potential volunteers were sought through a public meeting organised by the North East Dementias and Neurodegenerative Research Network (DeNDRoN) in July 2013 and through the existing networks of Derek Forster and the DeNDRoN PPI co-ordinator. Three PPI representatives agreed to join the group with Derek Forster, and all four members contributed throughout the programme. All members’ lives have been touched by neurodegenerative diseases, either as a patient or as a carer. In addition, members were motivated by their desire to ‘give something back’, interest in medical research and enthusiasm to help progress research into LBD. Experience of PPI varied. One member had been involved in PPI for > 10 years; however, for another this was their first PPI experience. All members received training on research through DeNDRoN. This was supplemented by an introductory session on LBD by Ian McKeith. One of the co-applicants (CB) facilitated the group and meetings were held on university premises. We followed the INVOLVE guidance on reimbursing group members for their time and expenses. 5
The first PPI meeting focused on how to achieve the desired aim. This led to agreement that the group would:
-
share personal knowledge and experience of DLB, Parkinson’s disease (PD) and PDD
-
comment on the practicalities and pressures that participating in the research could create for patients and carers (safeguarding)
-
ensure that information was user-friendly and did not alarm potential participants
-
ensure that a patient/carer focus was included in developing the management toolkit
-
contribute to study newsletters.
Members agreed that the timing and duration of PPI meetings should reflect the needs of the programme. Although the group thought it was unnecessary to link individual PPI members to specific WPs, they were keen to embed PPI into the programme management structures. To achieve this, Derek Forster attended both the PPI group and the Programme Management Group (PMG) and Valerie Argent joined the Programme Steering Committee (PSC) as a lay member.
In addition to the PPI group, two PPI workshops were convened to inform the development of the management toolkit (see Work package 2: development of management toolkits for Lewy body dementia). Potential participants were identified by team members through patient and carer interviews conducted during WP5 and local contacts. One member of the PPI group (Anne Lister) also attended the PPI workshops for WP2. The content of the workshops is described in Work package 2: development of management toolkits for Lewy body dementia.
Outcomes of patient and public involvement
The PPI group met 20 times, with full attendance at nearly all meetings. The number of meetings ranged from one to five per year. Members often prepared for meetings by reading documents, and commented on documents by e-mail between meetings. The initial expectation of group members and the research team was that the group would adopt a consultation approach, with an emphasis on ensuring that documents would make sense to patients and carers, including advising on the feasibility and acceptability of the study procedures. Over time, however, the group grew in confidence and were proactive in developing and expanding their role, adopting collaborative and user-controlled approaches at different times. 6 For example, rather than commenting on the public version of the study newsletter (which was initially drafted by the research team), members took ownership of the newsletter by redesigning the layout, introducing a new structure and writing much of the content themselves.
A user-controlled approach was adopted to obtain feedback on participants’ experiences of taking part in the pilot trial. Rather than inviting a single participant to describe their experience at the PPI dissemination event in the North East, members were keen to present a broader range of experiences. This led to the development and inclusion of some open questions about study participation with the letter inviting participants to the PPI event. Overall, 28 detailed responses were received. Two members of the group (VA and AL) reviewed and collated the information and presented their findings at the PPI dissemination event. Key themes were the interpersonal skills of the interviewers and having an opportunity to talk about their situation. Everybody found the home visits convenient and > 70% of respondents said that they would be willing to take part in a similar study. These comments provided useful feedback for the researchers and highlight aspects of study design, which may maximise recruitment and retention in future studies. Furthermore, the comments highlighted the lack of support available after diagnosis, suggesting new avenues for research and intervention.
Key activities undertaken by both the PPI group and the PPI workshops in WP2 are related to the research cycle7 and are summarised in Table 1.
| Stage of the research cycle7 | Example outcomes |
|---|---|
| Identifying and prioritising the research agenda | Future research priorities were highlighted by the PPI workshops for WP2 and the feedback collected for the PPI event |
| Study design |
Advised on consent procedures for clinic observation (WP5) and for notes review (WP1) Reviewed outcome measures to ensure that they were appropriate and relevant (WP4) |
| Development of the grant proposal |
Reviewed draft proposal Contributed to lay summary Included as a co-applicant (DF) |
| Undertaking/managing research |
Reviewed PIS and consent forms (WPs 1, 2, 4 and 5) Advised on most appropriate order of questions in assessment toolkit (WP3) and outcome measures (WP4) Shared experiences of PD and LBD with a new researcher to raise awareness and understanding prior to data collection (WP5) Advised on eliciting feedback on the toolkits (WP5) Identified key principles to underpin the management toolkit (WP2) Advised on appropriate ways of thanking study participants (WP4) Wrote a thank-you letter for study participants (WP4) Member of PMG (DF) Member of PSC (VA) |
| Analysing and interpreting |
Reviewed qualitative interview data (WP5) Collected and analysed data on participants’ experiences of the pilot trial (WP4) |
| Dissemination and implementation |
Produced biannual programme newsletters Produced lay summaries of published papers for the programme website Drafted annual progress reports and this section of the report Contributed to the organisation of PPI events Presented at the North East PPI event |
| Monitoring and evaluation |
Reflected on their role Made recommendations for PPI in future research |
Having highlighted some of the successful contributions made by PPI during the programme, we also acknowledge that its impact in some areas was limited. Owing to uncertainty about which outcome measures would prove sensitive to changes resulting from the introduction of the management toolkit, the team proposed a range of measures. Reconciling the perspectives of PPI members and those of the research team was sometimes challenging, for example in relation to the number of outcome measures and the perceived burden of these on participants, and in relation to the wording of questions in the assessment toolkit, some of which were considered to be inaccessible to patients and carers. However, there was limited scope to make changes because they were validated measures.
Discussion and conclusions
Using more than one approach to PPI in the programme proved effective. While the PPI group provided continuity throughout the programme, the workshops enabled the inclusion of a broader range of perspectives, including those of people with dementia. By combining a ‘fully intertwined’ approach to PPI and the ‘one-off’ approach for the workshops, we were able to include patients and carers who did not wish to commit to ongoing input. 8 The inclusion of PPI members on the PMG and PSC ensured opportunities for communicating with the full team. The detailed understanding of the programme developed through the PPI group, facilitated the ability of the PPI members to contribute confidently and effectively to meetings.
All members of the PPI group continued to be involved throughout the programme. This continuity helped the group to develop in confidence and expertise. Although we have benefited from stable group membership throughout the programme, members recognise that this may be unusual, as the health or circumstances of PPI members may deteriorate over a 5-year period, particularly if they are directly affected by the condition.
A limitation of the PPI group was the homogeneity of members in terms of ethnicity and class, and the lack of ability to alter some validated questions that members thought contained complex and inaccessible terms. Previous studies have emphasised the challenges of ensuring diversity in PPI, in particular the inclusion of people with dementia. 9 None of the members of the PPI group had dementia (although two had PD). Although caring for a spouse with LBD clearly provides significant insight into living with the condition, this is not the same as first-hand experience. 9 Despite the efforts of the DeNDRoN PPI co-ordinator, no people with dementia were identified for the PPI group. This partly reflects the low diagnostic rates and delays in diagnosing LBD (see Work package 1: baseline study of the diagnosis and management of Lewy body dementia). The situation was exacerbated by the high demand for PPI members in Newcastle upon Tyne, a Centre of Excellence in LBD research.
Reflections
Recent publications on PPI have emphasised the need for increased understanding of effective approaches to PPI. 4,8,10 Unfortunately, we did not obtain formal feedback from workshop participants, although the attendance of several participants at the subsequent PPI dissemination event suggested an ongoing interest in the programme. The perception of the PPI group was that although not everyone in the research team initially recognised the potential value of PPI, their contributions were increasingly valued as the programme progressed. Although no formal evaluation or opportunities for reflection were planned at the outset, the process of drafting newsletters, giving presentations at the PPI event and drafting this section for the final report prompted the group to recognise their wide-ranging contributions and reflect on their experiences of this approach to PPI. Key factors thought to contribute to the success of the PPI group were as follows:
-
having a simple two-page overview of the programme to help members understand the different WPs and how they were interlinked
-
having regular meetings to maintain interest and understanding of the programme
-
working together in a group and sharing ideas
-
having a facilitator
-
attending to practical issues, for example ease of access, free parking, timely reimbursement of expenses and flexibility over the timing of meetings
-
having access to a computer and large screen to enable the group to produce or edit documents collaboratively during meetings
-
having stability of group membership
-
ensuring that meetings were business-like, but enjoyable.
Several of these factors are consistent with those identified in previous studies. 8 Three suggestions were made for facilitating PPI contributions to future projects. The first concerned the importance of all team members recognising and valuing the different perspectives offered by PPI members, even when these challenged their own views. The second related to greater clarity over the responsibilities and remit of the PPI member within the PSC and finally the group emphasised the need for the chairperson of the PSC to explicitly invite comments from the PPI member.
After 5 years, members of the PPI group are surprised at their continuing levels of enthusiasm and commitment. Reflecting on this, members attribute their loyalty to the relevance of the programme to their lives, compatibility of members and the appreciative facilitation. In terms of personal benefit, members have valued the opportunity to keep up with research, maintain mental agility and develop new skills. Although the programme has now ended, the PPI group is planning to share their experiences to wider audiences through a conference presentation and an article for lay audiences.
In summary, the inclusion of PPI throughout the programme was extremely valuable and added very positively to the design of the DIAMOND-Lewy programme, its conduct and outcome, with PPI members making key contributions that affected the design and administration of the assessment and management toolkits. PPI also played a key role in dissemination and feedback of the study to participants, with members taking ownership of content for regular study newsletters, designing suitable methods for thanking participants and playing key roles in the co-design and delivery of the programme dissemination events.
Work package 1: baseline study of the diagnosis and management of Lewy body dementia
The aim of this WP was to undertake a baseline study of diagnosis and management of LBD in NHS secondary care services.
Introduction
The accurate recognition of dementia and the diagnosis of dementia subtype helps ensure appropriate management, and are central to improving patient care. LBD, comprising both DLB and PDD, is the second most common cause of neurodegenerative dementia in older people, accounting for between 15% and 20% of all cases of dementia, according to autopsy studies. 11 However, clinical and epidemiological studies suggest rates of only 5–7%. 12 This suggests that a considerable number of people with LBD go undiagnosed and so are not offered potentially beneficial management strategies. More worryingly, incorrect diagnosis risks inappropriate management with drugs such as antipsychotics, which, even at a low dose, can have serious side effects and cause mortality in those with LBD. 11
Currently, the proportion of cases of dementia diagnosed with DLB and PDD in NHS secondary care services is not known, nor has there been any systematic study of diagnostic practice or management in secondary care, although a US-based retrospective survey indicated frequent erroneous diagnosis and delays to a final correct diagnosis. 13 This is an essential first step to improving care. Therefore, this WP sought to establish the baseline for current diagnostic rates for DLB and PDD, providing a foundation for the other WPs. The WP was split into two parts: (1) WP1A examined diagnostic and management practice for DLB and took place in old age psychiatry and memory services, and (2) WP1B examined diagnostic and management practice for PDD. This latter element was undertaken in movement disorders services run by neurologists and geriatricians.
Initially, the plan had been to undertake WP1 solely in services in one geographic region of the UK (the north-east of England). However, there arose an opportunity to undertake a much more informative two-region study, whereby diagnostic rates for DLB and PDD could be ascertained in both the North East and East Anglia, two representative but clearly separate UK regions. This change also allowed some comparison of whether or not any variation in diagnostic rates and practice existed between regions.
Work package 1A: baseline study of the diagnosis and management of dementia with Lewy bodies
Methods
This WP took place over the first 2 years of the programme, with the aim of comparing rates of current clinical diagnosis of DLB with expected prevalence figures, confirming the accuracy of DLB diagnosis and comparing diagnostic and management pathways of those with DLB with those with non-DLB dementia. The hypothesis was that the actual rate of diagnosis of DLB would be ≤ 5% and that pathways to diagnosis of DLB would be longer and more complex than for non-DLB dementia.
The study had two phases: (1) a case note-screening phase to obtain information on consecutive referrals to services and (2) a phase involving detailed case note examination by clinical members of the research team (specialty registrars). Ethics approval was obtained for both phases from National Research Ethics Service Committee North East – Newcastle and North Tyneside 1 (reference 13/NE/0268). In addition, we obtained approval from the Confidentiality and Advisory Group to allow the extraction of some information during phase 1 without the explicit consent of the person with dementia [reference CAG 8.03(PR8)/2013].
We included nine old age psychiatry/memory services across four NHS trusts (two in East Anglia and two in the North East). Approvals were obtained from all trust research and development departments. Referral lists were obtained over an 18-month period and consecutive case notes screened by the research team, with key data extracted, including age, sex, diagnosis and Mini Mental State Examination (MMSE) score. Those with a DLB diagnosis who were alive and felt to be well enough by the clinical team (105/207 cases identified) were approached for their consent to be included in a more detailed study of their notes, with the extraction of information regarding diagnostic and management pathways collected using a standardised case report form. To describe the management pathway for each participant, data were collected on the use of services, spanning several years of clinical records before and after final diagnosis. Data collected included the number of appointments before and after diagnosis, and the number and type of diagnostic investigations undertaken before and after diagnosis.
For each DLB participant who consented, a non-DLB control participant was recruited by selecting the next participant seen in the respective service who was diagnosed with non-DLB dementia, matched for age (± 5 years), sex and MMSE score (in three bands: 0–10, 11–20 and 21–30) and who consented to participate. To assess the accuracy of the clinical diagnosis of DLB, a panel of three expert clinicians (not involved in the original diagnoses) independently reviewed clinical documentation and applied the 2005 international DLB consensus criteria to each case,14 with a primary diagnosis of probable or possible DLB taken as agreement with a clinician diagnosis of DLB. The expert panel agreed with the clinical diagnosis in 99% of cases of DLB (74/75) and 97% of cases with non-DLB dementia (72/74). One DLB case was excluded because the panel felt that there was insufficient evidence to assign any dementia subtype. Two non-DLB cases were excluded: one because the panel felt that the case had a diagnosis of mild cognitive impairment (MCI) and the other because the diagnosis was thought most likely to be PDD.
The data on use of services were combined with unit cost data taken from routine sources, including NHS reference costs15 and Personal Social Services Research Unit costs. 16 This enabled the cost of service use to be estimated and comparison between the cost of pathways to diagnosis and post-diagnosis care for DLB participants compared with control participants. Full details of the methods for this are included in Appendix 1. This study (and WP1B) were approved by the North East – Newcastle & North Tyneside 1 Research Ethics Committee (reference 13/NE/0268) and the Confidentiality and Advisory Group [reference 8.03(PR8)/2013].
Statistical analysis
Continuous data were assessed using t-tests, with chi-squared tests used for categorical data. Differences in costs between DLB and non-DLB participants were explored using independent-samples t-tests with unequal variance for normally distributed variables, Wilcoxon rank-sum tests for continuous non-normally distributed variables and chi-squared tests were used to explore differences in proportions. Differences in average cost between those with DLB and controls in the costs of diagnosis and of post-diagnosis treatment were estimated using marginal effects from a generalised linear model of costs. The generalised linear model was specified with a gamma distribution and a log-link function to account for skewness in the distribution of costs.
Results
We identified 9449 consecutive referrals, of whom 4504 received a diagnosis of dementia. Of those patients receiving a diagnosis of dementia, 207 (4.6%) received a diagnosis of DLB and of these, 74 DLB patients and 72 non-DLB dementia control patients consented for more detailed study of their notes (see Appendix 1, Table 8, for details of cohort characteristics).
Dementia with Lewy bodies was diagnosed in 4.6% of all those with dementia and there was evidence of a difference in diagnostic rates between the two regions, with the rate in the North East being higher (5.6%) than in East Anglia (3.3%) (χ2 = 13.6; p < 0.01). There were differences in the number of core diagnostic features for DLB between the North East and East Anglia, with more clinical features being present in DLB cases diagnosed in East Anglia, suggesting a higher ‘threshold’ for diagnosis, which would be consistent with the lower rates of diagnosis. Use of biomarkers, such as dopaminergic imaging, was also significantly different between the two areas, with the use of biomarkers being much less common in East Anglia. Overall, there was evidence of an impact of age, with younger participants taking longer to receive an accurate DLB diagnosis than older patients. Those with DLB were more likely to have received another diagnosis before DLB (mean alternative diagnoses before final dementia diagnosis 1.1 vs. 0.6; p = 0.003). DLB participants also had significantly more clinical assessments and imaging tests prior to their diagnosis than those with non-DLB dementia (Table 2). Data on costs were estimated over a time of 6.4 years for DLB participants compared with 5.1 years for non-DLB participants. Overall, the average cost of care for DLB participants was £6557, compared with £3425 for non-DLB participants [mean difference £2868, 95% confidence interval (CI) –£68 to £4013; p = 0.055] (see Appendix 1, Table 9). After controlling for sex, time since diagnosis, total resource use time and other patient characteristics, having a DLB diagnosis resulted in an increase of £3600 in total costs compared with non-DLB participants (p < 0.001). For DLB participants, medical history costs represented the largest contribution, with an average across participants of 40% of total costs compared with 27% for non-DLB participants (p = 0.042) (see further details in Appendix 1, Tables 10 and 11).
| Results: regional diagnostic variation | North East, n | East Anglia, n | Statistic | p-value |
|---|---|---|---|---|
| Core features of DLB at time of diagnosis (mean) | 1.5 | 2.1 | –2.78 (Student’s t-test) | 0.007 |
| Suggestive features of DLB at time of diagnosis, including DaT scans (mean) | 0.8 | 0.4 | 2.63 (Student’s t-test) | 0.0011 |
| Abnormal DaT scans prior to diagnosis | 24 | 1 | 12.9 (chi-squared test) | 0.001 |
| DaT scans prior to diagnosis (including normal) | 31 | 1 | 20.6 (chi-squared test) | < 0.001 |
| Total diagnostic features (core and suggestive) of DLB at time of diagnosis | 2.4 | 2.6 | 0.80 (Student’s t-test) | 0.42 |
| Time between first secondary care appointment and final diagnosis (years) | 1.4 | 0.9 | 1.03 (Student’s t-test) | 0.31 |
Work package 1B: baseline study of the diagnosis and management of Parkinson’s disease dementia
This study, also undertaken over the first 2 years of the programme, looked at diagnostic and management practice in those with PDD, with the aim of establishing baseline diagnostic rates for PDD and comparing investigations, assessment and management of those with PDD with control participants (i.e. patients with PD without dementia).
Methods
We included five PD or movement disorder services, each from a separate trust, comprising two geriatric medicine services and three services that combined geriatric medicine and neurology expertise. The research team reviewed notes of all referrals to services over an 18-month period. Those patients identified with PDD who were alive and felt to be well enough by the clinical team (44/151 identified) were approached for consent for a more detailed study of their notes, with the extraction of information with regard to diagnostic and management pathways collected using a case report form. For each PDD participant who consented, a person with PD but without dementia (control) was recruited by selecting the next case seen in the respective service who was diagnosed with PD, matched for age (± 5 years) and sex, and who consented to participate.
Similar methods of analysis as those described for WP1A (see Work package 1A: baseline study of the diagnosis and management of dementia with Lewy bodies) were used to collect and analyse data, and ethics, Confidentiality Advisory Group and research and development approvals were sought and obtained.
Results
The research team identified 2263 referral patients, of whom 1563 had a diagnosis of PD. Of these patients, 151 (9.7%) had received a diagnosis of PDD and of these, 38 PDD patients and 35 PD control patients were approached and consented for detailed review of their notes (see Appendix 1, Table 8, for details of cohort characteristics). For PDD, unlike DLB, there was no evidence of a difference in diagnostic rates between the two regions (p = 0.2), although mean MMSE scores at time of diagnosis were lower in East Anglia than in the North East (p = 0.008).
Examining the management pathways, there was evidence that dementia was probably present well before the diagnosis of PDD was made. For example, 46% of those with PDD had impaired activities of daily living due to cognitive impairment, with a mean intervening time of 1.5 years prior to diagnosis. Cognitive impairment in multiple domains was present in 57% of patients at a mean period of 2 years before diagnosis and 39% of patients had received an antidementia drug at an average of 1.75 years before a dementia diagnosis.
Data on costs were available for an average of 9.3 and 7.8 years of management for PDD patients and PD control patients, respectively. There was no evidence of a difference in average total costs between PDD and PD controls (average difference £2024, 95% CI –£3598 to £5548; p = 0.462) (see Appendix 1, Table 9). After controlling for sex, time since diagnosis, total resource use time and other patient characteristics, PDD participants had an average total cost of care of £7655 more than PD controls (95% CI £3676 to £11,634; p < 0.001). PDD participants had higher average costs of investigations (£250 more, on average, 95% CI £237 to £306; p = 0.001) and higher post-diagnosis management costs (£570 more, 95% CI £281 to £808; p = 0.001) (see Appendix 1, Tables 10 and 11).
Discussion
Our results showed, in keeping with our expectations, that DLB and PDD were diagnosed at around half (or less) the rates that one would expect them to be, based on prior epidemiological and pathological studies. 12 There was clear evidence of differences in diagnostic practice between different services and different regions, with a higher threshold for diagnosing DLB being, unsurprisingly, associated with lower diagnostic rates. For PDD, there was evidence that the diagnosis was delayed, with those diagnosed by services in one region having a significantly lower MMSE score at point of diagnosis than the other, and those diagnosed with PDD had evidence of dementia being present up to 2 years before the actual diagnosis was made. Our study had limitations, including the use of retrospective data and sampling of only some services in two geographical regions. Services were not randomly selected, but selected on the basis of geography. For the detailed case note assessment of subjects, we were able to obtain only a modest number of cases and there may have been selection bias, as we could consent only people who were alive and not deemed too unwell by their clinical teams to approach. The diagnostic rate findings have been fully published17 and the differences in pathways to diagnosis and management are currently in preparation. In terms of costs for both DLB and PDD groups, there was evidence that after controlling for sex, time since diagnosis, total resource use time and other patient characteristics that the costs of care were higher for both DLB and PDD groups than their respective controls.
Work package 2: development of management toolkits for Lewy body dementia
Objectives
The objectives of WP2 were to (1) complete a systematic review of evidence about the management of LBD and (2) use this to develop a clinical guideline, using a Delphi process incorporating the views of a multidisciplinary panel of independent experts combined with two facilitated PPI workshops.
Systematic reviews
At the time that these reviews were undertaken and to the best of our knowledge, no evidence-based reviews of the comprehensive management of LBD existed. As a consequence, little formal management guidance for LBD was offered in the NICE dementia guideline used at that time,18 despite the fact that this group of patients has multiple symptoms requiring treatment. Clinical guidance at the time appeared solely in the form of pharmacological reviews and expert consensus opinion statements. 14,19,20 This was an unsatisfactory situation for prescribers, health-care providers and recipients of care. We therefore conducted systematic reviews on pharmacological and non-pharmacological management of DLB.
Methods
The first of our two systematic reviews21 examined research on treatment effects and costs, and patient and carer views of pharmacological management strategies for LBD. Studies were identified through bibliographic databases, trials registers, grey literature, reference lists and experts. The review protocol is provided in Appendix 2. 22 In brief, we used the keywords ‘Lewy or parkinson’ and ‘dementia’, conducting searches until March 2015, without restrictions on publication date or language. Titles and abstracts were screened, with non-English-language papers screened by native speakers, and criteria used were (1) participants who had a diagnosis of DLB, PDD or LBD, (2) studies examined pharmacological strategies and (3) outcome measures and scores were specified. No restrictions were placed on study design, but opinion papers were excluded. Data were extracted in relation to participant demographic characteristics, selection criteria, study design, management strategies, outcome measures and scores, adverse events and withdrawals. Studies were grouped and analysed according to pharmacological strategy. Methodological quality was assessed using the Quality Assessment Tool for Quantitative Studies (QATQS) [URL: www.ephpp.ca/tools.html (accessed April 2021)]. The QATQS examines selection bias, study design, confounders, blinding, data collection methods, withdrawals and drop-outs. Domains are rated as being of weak, moderate or strong quality, which feeds into an overall rating of study quality.
Meta-analyses were conducted and, when studies could not be combined, summaries were provided. The level of evidence and grade of recommendations for each management strategy were assessed using the Oxford Centre for Evidence-Based Medicine criteria. 23
Forty-four studies examining 22 strategies were included in the review. Meta-analysis indicated beneficial effects of donepezil and rivastigmine for cognitive and psychiatric symptoms. Rivastigmine, but not donepezil, was associated with greater risk of adverse events. Meta-analysis of memantine suggested that it is well tolerated, but with few benefits. Descriptive summaries provided some evidence of benefits for galantamine, modafinil, levodopa, rotigotine, clozapine, duloxetine, clonazepam, ramelteon, gabapentin, zonisamide and yokukansan. Piracetam, amantadine, selegiline, olanzapine, quetiapine, risperidone and citalopram did not appear to be effective. Methodological quality was rated as weak for 41% of included studies, moderate for 39% and strong for 20%. This review concluded that high-level evidence related to pharmacological strategies for managing LBD is rare and that strategies for important areas of need in LBD, such as autonomic symptoms and carer burden, had not been investigated, nor had the views of patients and carers about pharmacological strategies.
This review21 of pharmacological management was published in the American Journal of Psychiatry (impact factor = 13.6) and by the end of 2018 had been cited 92 times, with an Altmetric Attention Score of 26 placing it in the top 5% of all research outputs scored. It formed the basis for the subsequent development of the management guideline and the first round of statements for Delphi panel evaluation.
Our second systematic review in WP2 regarded the non-pharmacological management of LBD. 24 Details of the search strategy are described in Appendix 3. As with pharmacological management, the literature and guidance available about LBD management available to clinicians and families at that time was virtually non-existent and mainly accessed through not-for-profit support organisations. Bibliographic databases were searched using a wide range of search terms and no restrictions were placed on study design, language or clinical setting. We used the search terms [(Lewy OR Park*) and Dementia]. Interventions were any non-pharmacological treatment and identified using a wide range of terms covering individual non-pharmacological therapies: activit*, acupuncture, alternative, animal, aromatherapy, art therapy, assisted, balance, behav*, bicycle, calisthenics, carer intervention, caregiver intervention, CBT, Chi gong, cognit*, cognitive behavioral therapy, cognitive behavioural therapy, counsel*, creative arts, dance, dancing, diet, direct current stimulation, drama, ECT, educat*, electroconvulsive therapy, enhanc*, environmental intervention, environmental modification, exercise, flexibility, humor therapy, humour therapy, hydrotherapy, intervention*, leisure, light therapy, management, martial arts, massage, meditation, Montessori, multisensory, music, non-pharm*, nonpharm*, nutrition, occupational therapy, pet therapy, physical activity, physical therapy, physiotherapy, pilates, psychoeducation, psychol*, psychosocial, psychotherapy, Qi gong, reality orientation, recreation*, reminiscence, resistance training, run*, sensory, simulated presence, stimulation, Snoezelen, support*, support group*, swim*, tai chi, therap*, therapeutic activity, TMS, training, training carers, training caregivers, transcranial magnetic stimulation, treatment*, validation, weight training, yoga. Searches were conducted on 30 October 2016.
The search identified 21 studies [including two randomised controlled trials (RCTs) with available subgroup data], seven case series and 12 case studies. Most studies reported beneficial effects of the interventions used, although the only sizeable study was on dysphagia, showing a benefit of honey-thickened liquids. Given the heterogeneity of interventions and poor quality of the studies overall, no quantitative synthesis (meta-analysis) was possible. Overall, identified studies suggested possible benefits of non-pharmacological interventions in LBD, but the small sample sizes and low quality of studies meant that no definite recommendations could be offered. This work underscored the clear and urgent need for future research on this topic.
The review of non-pharmacological management was published in Psychological Medicine24 (impact factor = 5.4) and was also subsequently incorporated into the development of the management toolkit.
Public–patient workshops
Two public–patient workshops were held, with the participation of 38 people with LBD and their family/carers. The first event focused on identifying best practice in LBD clinical management, based on their own experiences. The content of the workshops is shown in Figure 2. The emergent themes were developed further in the second event and refined into a set of guiding principles.
FIGURE 2.
Workshops and content.
Some of the key themes that were held to be important are listed in Table 3. The themes were structured in such a way as to help the clinical team deliver the guideline recommendations in a user-friendly format.
| Management principle | Summary of discussion point |
|---|---|
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
|
| 5 |
|
| 6 |
|
| 7 |
|
From our work, a core element was the recognition that the carer is often the expert on the person with dementia and they need to feel that this is recognised. Although people want to be told what the clinician feels would be best for them, they also expressed concern about doctors treating what they know best, rather than following patient and carer preferences, or seeking help from other types of expert. Carer diaries were often mentioned as a useful way of dealing with these issues. Opinions were divided as to whether or not the patient and carer should be seen together by the clinical team, separately or offered both. Some issues, such as increased carer stress, which is high in LBD, probably need to be explicitly discussed with the carer on their own.
Although the advantages of developing and working through a problem list were recognised, it was also said that a long list of problems could be demoralising, something that is particularly relevant for LBD where there are often many symptoms, not all of which may need to be formulated as problems. It was noted as helpful that an expert experienced in LBD is often able to offer reassurance that these symptoms can be expected as part of the disorder and not part of another illness.
Carer and peer support groups are very valuable for support and practical help, providing people have similar conditions. Support groups are one source of practical information at an early stage on important general matters, including power of attorney, attendance allowance and council tax. Where geographical distance is a problem, telephone or e-mail contact may still be helpful.
A clear message throughout the PPI consultations was that although they appreciated the levels of care and concern that clinical teams offered, they often had difficulty in obtaining and understanding information about LBD. A lack of post-diagnostic support, advice and counselling was frequently mentioned, as was a shortage of easily accessible materials. As a result, the guideline that we produced contained links to what were judged to be the best-quality information sources at the time of writing, and it is hoped that these will be made freely available to those attending our NHS services and that they will, in turn, be replaced by improved versions and adapted for local use.
Guideline development
Using the systematic reviews and public–patient feedback, an initial draft of the guidelines was developed by two authors (J-PT and IMcK). Statements relating to symptom domains were created and, using an anonymised online platform, reviewed by the Delphi panel. The panel comprised experts in the field (n = 26), including psychology, geriatrics, psychiatry, neurology, primary care, physiotherapy, nursing and academic experts and some key international opinion leaders (see Appendix 4 for list of contributors), who were identified through consultation with relevant stakeholder groups and supported by an extensive publication search and/or their role as keynote speakers on management of LBD at conferences. The Delphi process was undertaken over three rounds. A high level of agreement was sought across the three rounds (85% for rounds 1 and 2, and 75% for round 3). Controversial statements were modified on the basis of feedback and rerun in the subsequent round or removed. Of 252 original statements, 161 were kept, with 78 of these (48.4%) gaining full consensus panel agreement for inclusion, 52 (32.3%) with 90–99% consensus agreement and 31 (19.3%) agreed by 75–89% of the panel. After this process, the guideline statements were re-collated and formulated into one document. More controversial statements (but still meeting majority consensus opinion of > 50%) were included as clarifying footnotes, where appropriate, in the guideline document.
The guideline statements were then supplemented by summary management sheets (one overall summary figure and separate summary figures for each key symptom domain, produced by J-PT and IMcK, with input from the DIAMOND-Lewy team) that accompanied the written guideline, with the whole package being called the management toolkit. This toolkit was then subjected to further evaluation and feedback in WP3 and typological corrections or clarification of guideline statements (although no change to content or meaning) were made based on the feedback from clinicians.
The management toolkit was finalised at this point and put forward for evaluation in WP4 (see Report Supplementary Material 1). The toolkit comprised an overview (see Appendix 5), brief summaries for each symptom area and the management guideline.
Dissemination of the toolkit
Our major output has been the acceptance of an authoritative management review,25 which contains reference to the Delphi statements and the evidence base used to produce the management toolkit, in Lancet Neurology (impact factor = 27.14). This has made the findings from our guidelines accessible for adaption and implementation in clinical services internationally.
Specific to the UK and NHS context, we have a dissemination plan, including:
-
making the management toolkit freely available via our website
-
embedding the use of the management toolkit in significant national educational fora (e.g. the British Association of Psychopharmacology)
-
developing an online learning resource with video discussing management approaches in LBD.
Work package 3: development of assessment toolkits for the diagnosis of Lewy body dementia
Introduction
Work package 3 was developed because the accurate recognition of LBD is crucial to ensuring appropriate management and, therefore, central to improving patient care in dementia. 26–28
Prior to this programme, there was no single simple-to-administer toolkit that incorporated assessment of all the symptoms needed to make a diagnosis of DLB and PDD. Therefore, WP3 aimed to develop such assessment toolkits to improve the recognition and diagnosis of both DLB and PDD.
Methods
We developed the final LBD assessment toolkits in three stages: (1) identifying validated assessments for different symptoms and signs needed for a LBD diagnosis to make a pilot instrument, (2) obtaining feedback on the acceptability and feasibility of the toolkit through interviews with clinicians and the programme PPI group, and (3) piloting the toolkits in memory and movement disorder services prior to producing a final version to be used in WP4.
Stage 1: development of pilot assessment toolkits
Following funding, the DIAMOND-Lewy PMG first discussed the basic approach to the problem and, in particular, whether one overall assessment and assessment toolkit should be developed or two separate ones, one for DLB (aimed at memory services) and one for PDD (aimed at movement disorder services or neurology and geriatric medicine services that also see people with movement disorders). It was concluded that two different instruments would be needed that matched the international diagnostic criteria for DLB14 and PDD. 29 The clinical experts in the DIAMOND-Lewy programme (AT, J-PT, IMcK, LA, DB and JOB) then reviewed the published literature and supplemented this with their expert knowledge to identify available validated assessment instruments26–28,30 that would form the contents of the toolkits. The PMG identified appropriate components for each toolkit from the identified assessment instruments.
For the DLB toolkit, the aim was to improve identification of the core and suggestive diagnostic symptoms, as diagnosis of cognitive impairment and dementia in specialist memory services is not a concern. At the time of toolkit development, there were six core and suggestive symptoms for diagnosing DLB. One was dopaminergic imaging, which would not form part of a clinical assessment tool, and another was neuroleptic sensitivity, which can only be identified following exposure to antipsychotic drugs, which is now a rare event. 31,32 Therefore, the focus was on assessment of the four remaining symptoms: (1) persistent complex visual hallucinations, (2) spontaneous cognitive fluctuations, (3) spontaneous parkinsonism and (4) rapid eye movement (REM) sleep behaviour disorder. The following components of the instrument were identified:
-
cognitive fluctuation – four questions to carers from the Dementia Cognitive Fluctuation Scale28
-
REM sleep behaviour disorder – a single screening question to carers from the Mayo Sleep Questionnaire27
-
visual hallucinations – two core questions for patients and two for carers from the North East Visual Hallucinations Inventory30
-
motor features of parkinsonism – five items from the Unified Parkinson’s Disease Rating Scale. 26
For the PDD toolkit, the aim was to improve identification of cognitive impairment and to facilitate matching of symptoms to the PDD diagnostic criteria. 29 The Montreal Cognitive Assessment (MoCA) was identified as most appropriate for brief cognitive assessment in this context. 33
Stage 2: feedback and modification of toolkits
During 2014, as WP3 lead, Alan Thomas put together the two instruments and there followed a period of iteration with the rest of the PMG. This led to the formulation of draft versions of the toolkits, which were then sent to the PPI panel for feedback in early 2015. PPI panel feedback was incorporated into a revised version, which was again commented on by the PMG and PPI members until final agreed versions of the toolkits and text were agreed for the pilot study. These iterative rounds led to changes in the wording of questions to try to improve clarity. In addition, a major change suggested was to separate the PDD toolkit into one version for use with carers and one for use with patients, based on how patients present to PD services where they may or may not have a carer present. After final iterations between PMG and PPI groups, three final assessment toolkits were produced for the pilot and feasibility study.
Stage 3: pilot and feasibility of assessment toolkits
During early 2015, as the final versions of the toolkits were developed and by mid-2015, the necessary research ethics approval was obtained from Yorkshire and The Humber – Bradford Leeds Research Ethics Committee (reference 15/NE/0028). Trust research and development approval was also obtained prior to the start of the study.
In the autumn of 2015, we conducted the pilot study of the feasibility of the assessment toolkits in memory and PD services at Gateshead Health NHS Foundation Trust. Details are published in Thomas et al. 34 Briefly, we obtained engagement from medical and nursing staff in these services who administered the toolkits to > 20 patients, with individual clinicians using the toolkits between zero and seven times in patients with and without LBD. Feedback on acceptability and thoughts on improvement were obtained by direct comments to the WP lead (AT) and the research nurse by comments written on the toolkits and from qualitative interviews with patients, carers and clinicians.
Clinicians found the toolkits straightforward to use, although in the PD service they found attempting the cognitive assessment to be a problem because of time constraints. These clinicians were also keen to have the questions about core symptoms of DLB included in the toolkit for PD services. Patients and carers had no concerns about their use or how the questions were phrased. Therefore, following the pilot, a revision of the toolkits was produced that took these comments into account. Specifically, we produced a single assessment toolkit (the LBD toolkit) for PD services, which included the questions from the DLB toolkit for identifying core DLB features. Finally, by this time, the revised DLB consensus diagnostic criteria were being published,11 which led to a few final minor changes to these toolkits to ensure that they were aligned with the revised criteria. Details of these changes were published. 35
Use of the toolkits in work package 4
These final published versions of the DIAMOND-Lewy toolkits (see Report Supplementary Material 2) were utilised in WP4 of the programme, with all services, whether randomised to intervention or usual care, being supported in using the toolkits. The toolkits were published online in 2016 and in print in 2017,34 and this contributed to making these toolkits publicly available through open access. In parallel, the toolkits have also been made available through the DIAMOND-Lewy study website [URL: https://research.ncl.ac.uk/diamondlewy/ (accessed April 2021)].
Work package 4: a pilot cluster randomised trial of the management toolkit
This WP involved the introduction of the assessment toolkits (developed in WP3) to memory/old age psychiatry and PD/movement disorder services, with the aim of facilitating diagnosis of DLB and PDD, and the introduction through a cluster randomised trial (randomised at the individual service level) of the management toolkit (developed in WP2) into half of the services taking part, with the other half continuing with standard care. The objectives of the pilot RCT were to (1) see if such a study was feasible [i.e. that we could recruit subjects with DLB and PDD (target n = 120) and retain them for 6 months] and (2) obtain data to inform power calculations for future studies.
Methods
We included 11 services in four NHS trusts in the North East and 12 services in four NHS trusts in East Anglia. The trial was supported by the Newcastle Clinical Trials Unit, which undertook the randomisation via a statistician blinded to other aspects of the study. One service in the North East was subsequently unable to recruit any patients and withdrew part-way through the study. This study was approved by the West Midlands – Coventry and Warwickshire Research Ethics Committee (reference 16/WM/0025).
Assessment toolkits and, for services randomised to the intervention arm, the management toolkit were introduced during an in-person site initiation visit undertaken by the research team. The site initiation visits comprised standardised presentations and handouts followed by a question and answer session. The site initiation visits included as many clinical team members as were able to attend and all those involved in the diagnosis and management of those with DLB and PDD were encouraged to use the diagnostic and management toolkits. Follow-up support and further sessions were provided as required, and the study team maintained regular contact with all services during the course of the recruitment and follow-up period.
Assessment and management toolkits were provided as paper copies, with laminated copies of the overview and symptom summary sheets for the management toolkit. Some sites requested electronic [Portable Document Format (PDF)] versions and, where these were requested, they were supplied. If services had questions regarding the assessment or management toolkits, they were free to ask members of the research team. Regular trial newsletters that contained a ‘frequently asked questions’ section were sent to services to facilitate engagement with the study. Questionnaires regarding the value and use of the management toolkit were sent to services in the intervention arm halfway through the study and at the study end. Qualitative interviews (see Work package 5: qualitative studies throughout the DIAMOND-Lewy programme) were conducted to ascertain barriers to and facilitators of the implementation of both the diagnostic and management toolkits.
In all services, patients with DLB and PDD were recruited for data collection at baseline, 3 months and 6 months. A carer/informant was recruited for all but two patients. The full study protocol, which includes all the assessments undertaken, is available on the DIAMOND-Lewy website. 36 In brief, assessments included those of cognition (MMSE and MoCA), neuropsychiatric symptoms (Neuropsychiatric Inventory and Geriatric Depression Scale), fluctuations (Cognitive Fluctuation Scale), activities of daily living (Bristol Activities of Daily Living Scale), motor symptoms [Unified Parkinson’s Disease Rating Scale Part 3 (UPDRS)] and quality of life [DEMQOL (Dementia Quality of Life) and EuroQol-5 Dimensions, five-level version (EQ-5D-5L)]. Current management and use of health care and personal/social care services were collected on a bespoke questionnaire. Global outcome scales were completed by the visiting researcher and the carer/informant. Informant assessments included quality of life of the carer (EQ-5D-5L), carer burden (Zarit Burden Interview) and the Hospital Anxiety and Depression Scale.
All baseline and 3- and 6-month assessments were undertaken by members of the Clinical Research Network DeNDRoN teams in the North East and East Anglia, who were unaware of the service allocation (to management toolkit or standard care).
Data entry was completed by those undertaking the assessments, and central checking and quality assurance followed double-entry at local sites with an error rate of < 0.1% (all errors noticed were, of course, corrected). Database integrity was therefore felt to be very good. Identical shell databases were completed in the North East and East Anglia and then merged and sent to the study statistical team.
Results
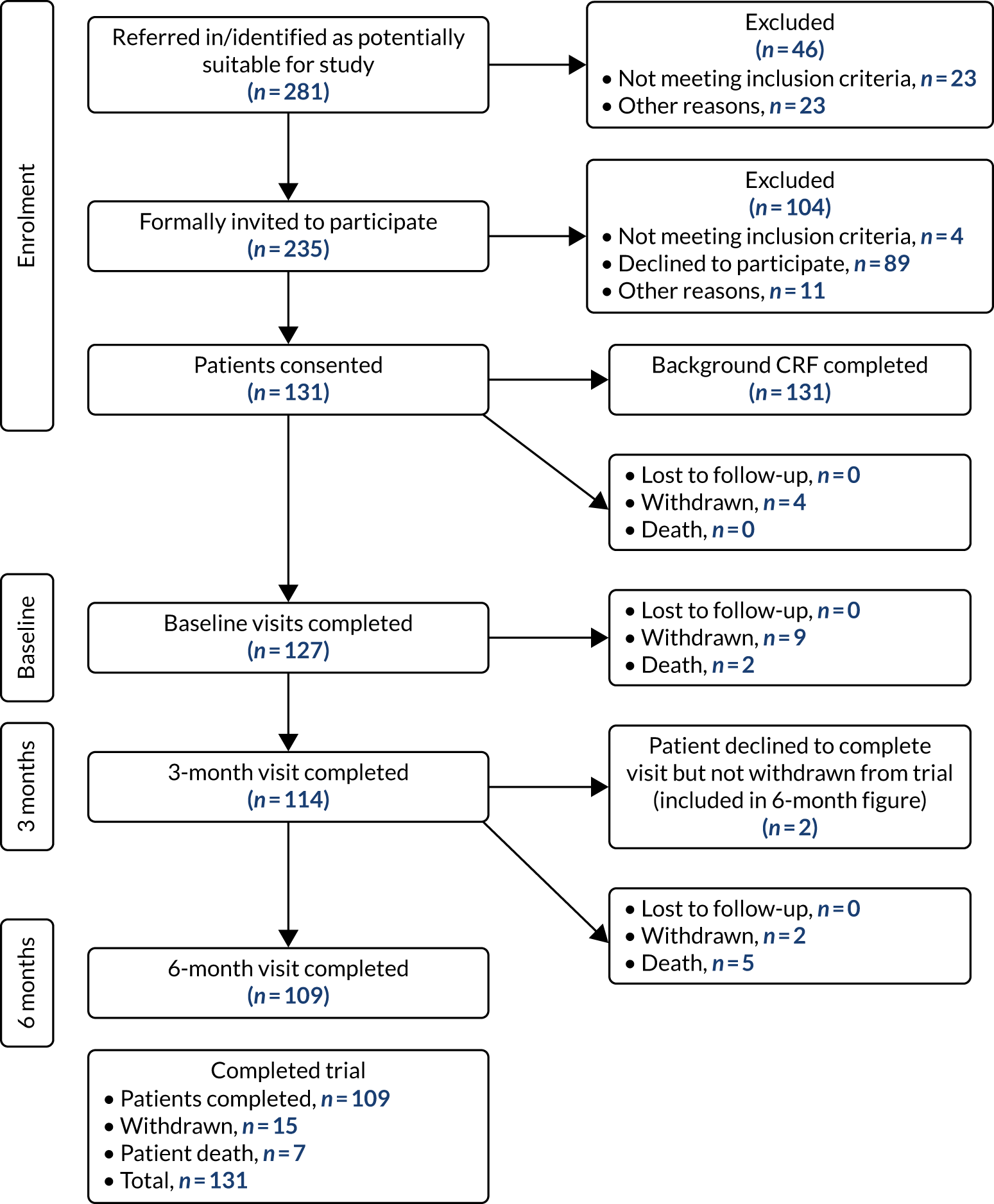
One hundred and thirty-one participants consented to take part in the study. A CONSORT (Consolidated Standards of Reporting Trials) flow diagram, indicating patient progression through the study, is shown in Figure 3. One hundred and twenty-seven participants underwent a baseline assessment, and 6-month data were available for 109 participants (86% of those who completed baseline, 83% of all participants who consented). Eighteen participants were lost to follow-up, seven of whom died (four in the intervention arm, three in the control arm). Those lost to follow-up were similar in demographic characteristics to those remaining in the study except for age, with those lost to follow-up being significantly older (median age of 83 vs. 77 years).
FIGURE 3.
DIAMOND-Lewy WP4 CONSORT flow diagram of participants’ completion rate (27 March 2019). CRF, case report form.
The recruitment target was more than achieved. We aimed to recruit 120 patients and actually recruited 131. Patient characteristics at baseline are shown in Table 4. Participants randomised to receive the intervention did not differ significantly from those randomised to the control group on any of the baseline measures except for carer-reported DEMQOL and carer anxiety symptoms as assessed on the Hospital Anxiety and Depression Scale.
| Characteristic | Control (N = 52) | Intervention (N = 75) | p-valuea |
|---|---|---|---|
| Number of sites | 11 | 12 | |
| Age (years) | |||
| Mean (SD) | 77.0 (7.59) | 79.3 (6.97) | 0.086 |
| Median | 77.0 | 79.0 | 0.094 |
| Interquartile range | 72.0–83.0 | 75.0–84.0 | |
| Diagnosis, n (%) | 0.846 | ||
| DLB | 31 (59.6) | 46 (61.3) | |
| PDD | 21 (40.4) | 29 (38.7) | |
| Sex, n (%) | 0.642 | ||
| Female | 10 (19.2) | 17 (22.7) | |
| Male | 42 (80.8) | 58 (77.3) | |
| DEMQOL | |||
| Mean (SD) | 0.76 (0.13) | 0.78 (0.12) | 0.229 |
| Median | 0.78 | 0.80 | 0.215 |
| Interquartile range | 0.70–0.82 | 0.70–0.88 | |
| Carer DEMQOL-proxy | |||
| Mean (SD) | 0.70 (0.14) | 0.76 (0.12) | 0.021 |
| Median | 0.67 | 0.79 | 0.026 |
| Interquartile range | 0.55–0.82 | 0.67–0.85 | |
| Neuropsychiatric Inventory | |||
| Mean (SD) | 25.0 (17.5) | 20.0 (18.0) | 0.125 |
| Median | 22.0 | 15.0 | 0.038 |
| Interquartile range | 12.0–31.0 | 9.0–24.0 | |
| UPDRS | |||
| Mean (SD) | 43.7 (19.1) | 38.2 (18.6) | 0.112 |
| Median | 41.0 | 35.5 | 0.137 |
| Interquartile range | 28.0–55.0 | 26.0–51.0 | |
| Cornell Scale for Depression in Dementia | |||
| Mean (SD) | 9.31 (6.10) | 7.41 (4.85) | 0.056 |
| Median | 9.0 | 7.0 | 0.104 |
| Interquartile range | 4.0–13.0 | 4.0–11.0 | |
| Geriatric Depression Scale | |||
| Mean (SD) | 5.7 (3.5) | 5.6 (3.3) | 0.899 |
| Median | 5.0 | 5.0 | 0.952 |
| Interquartile range | 3.0–7.0 | 3.0–7.0 | |
| MMSE | |||
| Mean (SD) | 20.8 (6.1) | 21.4 (6.1) | 0.581 |
| Median | 22.0 | 22.0 | 0.503 |
| Interquartile range | 17.0–25.0 | 19.0–26.0 | |
| MoCA | |||
| Mean (SD) | 15.1 (4.9) | 15.6 (6.0) | 0.644 |
| Median | 15.5 | 16.0 | 0.690 |
| Interquartile range | 12.0–19.0 | 12.0–19.0 | |
| EQ-5D-5L | |||
| Mean (SD) | 0.67 (0.27) | 0.67 (0.21) | 0.998 |
| Median | 0.74 | 0.73 | 0.516 |
| Interquartile range | 0.55–0.85 | 0.57–0.80 | |
| EQ-5D-5L (proxy) | |||
| Mean (SD) | 0.55 (0.27) | 0.56 (0.27) | 0.769 |
| Median | 0.62 | 0.62 | 0.929 |
| Interquartile range | 0.37–0.73 | 0.40–0.77 | |
| Hospital Anxiety and Depression Scale: anxiety | |||
| Mean (SD) | 6.7 (4.2) | 5.2 (4.1) | 0.052 |
| Median | 6.0 | 4.0 | 0.037 |
| Interquartile range | 3.0–9.0 | 2.0–8.0 | |
| Hospital Anxiety and Depression Scale: depression | |||
| Mean (SD) | 4.6 (3.8) | 4.2 (3.5) | 0.553 |
| Median | 3.0 | 3.5 | 0.610 |
| Interquartile range | 1.0–7.0 | 1.0–7.0 | |
| Zarit Burden Interview | |||
| Mean (SD) | 27.5 (15.6) | 22.6 (15.3) | 0.082 |
| Median | 26.0 | 18.0 | 0.070 |
| Interquartile range | 14.5–38.5 | 10.0–33.0 | |
| Carer EQ-5D-5L | |||
| Mean (SD) | 0.80 (0.20) | 0.81 (0.19) | 0.822 |
| Median | 0.84 | 0.82 | 0.902 |
| Interquartile range | 0.72–1.0 | 0.69–1.0 | |
Health economics
The main objective of the economic component of the pilot RCT was to rehearse the methods for a future definitive economic evaluation of the new management toolkit compared with the usual care provided for dementia. As is typical for pilot trials, the modest sample size meant that a full economic evaluation was not appropriate because estimates would be both imprecise and unreliable. Therefore, the focus of the economic component was restricted to provide a descriptive analysis of the costs and outcomes. Data on costs and outcomes for each patient sample (DLB and PDD) were examined separately, as it was expected that the different diagnoses would lead to a differential use of services and outcomes.
Deviations from protocol
The protocol, and the analysis plan (see Report Supplementary Material 3), which was based on the protocol, stated that a cost-effectiveness analysis would be conducted, reporting an incremental cost-effectiveness ratio. A stochastic analysis, reporting results in the form of cost-effectiveness acceptability curves and cost–quality-adjusted life-year (QALY) plots, was also planned. However, because of the smaller number of data collected than expected, this analysis was not conducted to avoid reporting imprecise and unreliable results.
A full report of the economic component can be found in Appendix 6. A summary of the key results is presented below.
Costs
Costs were examined from the perspectives of the UK NHS and Personal Social Services, which includes costs of medications and health and social service use. Costs incurred by other sectors (e.g. local authorities) were not included; however, private costs incurred by patients and carers for attending health-care services were included. Data on costs using a service use questionnaire administered to the carer were collected at baseline and at the 3- and 6-month follow-ups. Data on private costs to patients were collected using a time and travel questionnaire at the 6-month follow-up.
Health care and social service resource use
In both patient samples, mean costs associated with health and social service use decreased in the intervention arms between baseline and the 6-month follow-up. However, in the control groups, costs increased. There is a substantial amount of imprecision around the service use cost data, however, and median values are considerably smaller than the mean values, which suggests that there is a substantial right skew to the data, with some participants incurring much higher costs than the remainder of the sample (see Appendix 6, Table 12).
Medication costs
There was little variation in mean medication costs between baseline and the 6-month follow-up in both patient samples. There was a trend towards increasing costs for both arms over the follow-up period. A greater increase in mean medication costs can be observed in the intervention arms of both patient samples than in the control arms. However, the increase is larger for the PDD patients (see Appendix 6, Table 13).
Intervention delivery costs
The cost of the intervention included the production of the management toolkits and training staff to use them. The total delivery cost was divided by the number of participants in the intervention arm in both patient groups combined (n = 75) to estimate a mean delivery cost per participant receiving the intervention. The mean intervention delivery cost was estimated at £76.32 per participant in the intervention arms and £0 for those not receiving the intervention (i.e. control arms) (see Appendix 6, Table 14).
Total costs
Total costs included health and social service use, medication costs and intervention delivery costs (to illustrate cumulative costs at that time point they were added to the cumulative costs, estimated at the 3- and 6-month follow-ups only). The health and social care use costs made up the largest proportion of the total cost. In both patient samples, there was an overall increase in total costs between baseline and the 6-month follow-up in the control arms, whereas total costs at the 6-month follow-up were lower than baseline in both sample intervention arms (see Appendix 6, Table 15). There remains substantial imprecision in the total cost values because of the small sample size and, therefore, the total costs are, at best, illustrative.
Private costs
Private costs included the cost of travelling to inpatient services, outpatient appointments, general practitioner (GP) visits and community dementia service visits for both patients and carers who may have accompanied patients. The cost of time spent travelling to each appointment and the cost of time spent at each appointment were also estimated. Costs were attributed to time based on activity rates for various activities, using estimates published by the Office for National Statistics (ONS)37 (see Appendix 6 for further details). For both patients and carers, mean travel costs decreased between baseline and the 6-month follow-up in both trial arms and patient samples. An exception to this was the control group of the PDD sample, which incurred higher mean costs at the 6-month follow-up than at baseline.
With reference to the cost of patients’ time spent attending health-care services, the intervention groups of both patient samples incurred lower mean costs over the 3-month period between the 3- and 6-month follow-ups than during the 3-month period prior to baseline data collection, whereas the control groups had a higher mean cost for the same 3-month period prior to the 6-month follow-up than at baseline (see Appendix 6, Table 16). A similar pattern was observed for costs to carers, with the exception that carers in the control group of the DLB sample incurred lower mean costs at the 6-month follow-up than at baseline (see Appendix 6, Table 17).
Quality of life
Patients’ generic quality of life was derived from the responses to the EQ-5D-5L questionnaire,38 completed at baseline and at the 3- and 6-month follow-ups (missing EQ-5D-5L data at each time point are recorded in Appendix 6, Table 18). Responses to the EQ-5D-5L questionnaire were transformed into health state utilities, using tariffs derived from the UK population. 39 Using the change from baseline approach, the health state utilities were used to estimate generic QALYs for each participant so that the change in QALYs between baseline and the 6-month follow-up was estimated.
Overall, changes in QALYs in each trial arm for each sample were small to modest, but with consistent decreases in mean and median scores for those with DLB, indicating a benefit for those in the intervention arm. An extremely small decrease in QALYs was observed in the intervention arm of the PDD sample, compared with a small increase in mean QALYs for the control arm (see Appendix 6, Table 19).
Discussion
This study demonstrated the feasibility of successfully recruiting a large number of participants with DLB and PDD over an 18-month period from 23 sites, and we more than achieved the recruitment target (target, n = 120; recruited, n = 131). Follow-up rates were good (85%), given the condition under study, as LBD is known to be associated with more rapid functional decline and increased mortality than other dementias. 11,40 Completion rates of assessments for both patients and carers were high.
Using results from this study to inform a power calculation (80% power, alpha 0.05) gives a required sample size of 726 for outcome based on the Neuropsychiatric Inventory {18.8 [standard deviation (SD) 19.3] in the intervention and 22.6 [SD 16.3] in the control group}. Assuming 80% completion rates, the recruitment sample would need to be 908 for the Neuropsychiatric Inventory. However, given that the toolkits are already evidence based and associated with some positive outcomes, even in this pilot study, it is not clear that a larger trial based around demonstrating efficacy of the toolkits is needed. Instead, an approach focused more on their routine implementation, through service improvement or other approaches, may be preferable.
Taking account of the cost of implementing the management toolkits, the health economic analysis indicated that the total costs for both DLB and PDD increased over the course of the study in the control arms and decreased in the intervention arms, despite a slight increase in medication costs in intervention groups. With such a modest sample size, data were insufficient to draw conclusions, although there is no indication that the intervention increased costs. Data indicated that further study on health economic consequences in a larger sample is needed.
The qualitative studies (see Work package 5: qualitative studies throughout the DIAMOND-Lewy programme) indicate that implementation and use of the management toolkit varied considerably between individual clinicians and between services. Limitations of the study are that we were not able to standardise or measure this, nor – because this was randomised at a cluster level – could we directly associate the impact of the management toolkit on patient outcomes at the individual patient level. Further study should investigate these issues, but our results strongly support the need for a large, definitive trial of the management toolkit and indicate that its introduction is not associated with increased costs.
Work package 5: qualitative studies throughout the DIAMOND-Lewy programme
The reasons for underdiagnosis and suboptimal management of LBD have not previously been empirically investigated using qualitative methods. Research has focused on clarifying diagnostic criteria, identifying biomarkers or screening tools to facilitate diagnosis, with little attention to potential barriers to diagnosis. The emphasis has therefore been on identifying technical solutions, rather than considering the full range of factors that might contribute to the underdiagnosis of LBD. There is growing recognition of the contribution qualitative research can make to feasibility studies, pilot trials and RCTs,41,42 for example by understanding the context into which interventions are to be introduced, optimising interventions, understanding factors influencing the implementation of the intervention and facilitating the interpretation of findings. 41,43,44 In the DIAMOND-Lewy programme, the objectives of the qualitative work were to:
-
understand current practice in diagnosing and managing LBD (WP5.1)
-
explore stakeholder views on the acceptability of the toolkits (WP5.2)
-
explore the implementation of the assessment and management toolkits in routine NHS practice (WP5.3).
A range of theories are available to assess the likelihood of a new intervention being successfully embedded into routine practice. 45 Normalisation process theory (NPT) is a well-established theory of implementation, which has been used extensively in studies of health-care interventions. 46 NPT considers factors that affect implementation in relation to four key areas: (1) how people make sense of a new practice (coherence), (2) the willingness of people to sign-up and commit to the new practice (cognitive participation), (3) their ability to take on the work required of the practice (collective action) and (4) activity undertaken to monitor and review the practice (reflexive monitoring) [URL: www.normalizationprocess.org (accessed April 2021)]. 47
The qualitative work aimed to generate practical knowledge to inform subsequent stages of the programme (e.g. by providing feedback that could be used in revising toolkits, proposing an implementation strategy to address key barriers and proposing change mechanisms for the study findings). Ethics and research governance approvals were sought in two phases. Favourable ethics opinion for phase 1 was obtained from the Newcastle and North Tyneside 1 Research Ethics Committee (reference 13/NE/0322). Subsequent research and development and Caldicott approvals were granted from each participating site. Favourable ethics opinion for phase 2 was obtained from Wales Research Ethics Committee 5 (reference 16/WA/0098). Health Research Authority approval for phase 2 was obtained on 29 June 2016.
Methods
We used ethnographic methods, including semistructured interviews, focus groups and observations of clinical practice, throughout WP5. Structured questionnaires were used to collect information on current practice, confidence in diagnosing and managing LBD, and (in WP5.3) views on the assessment and management toolkits (Table 5).
| WP | Methods | Participants |
|---|---|---|
| WP5.1: understanding current practice | Clinician questionnaire (T0) | 146/336 completed questionnaires |
| Clinician interviews | 20 | |
| Clinician focus groups | Two (16 participants) | |
| Observation of routine practice |
17 clinical sessions (44 patients and 36 carers) Three clinical discussions (eight patients and eight carers) Two multidisciplinary team meetings (five patients and two carers) |
|
| WP5.2: acceptability of assessment and management toolkits | Clinician interviews | 60 (across whole study) |
| Observation of acceptability of toolkits in feasibility study | 0 | |
| Interviews with patients and carers with whom the toolkits had been used |
Six patients Four carers |
|
| WP5.3: implementation of assessment and management toolkits | Clinician questionnaires (T1 towards the start of WP5 and T2 after the pilot trial) |
60/124 completed T1 questionnaires 52/140 completed T2 questionnaires |
| Clinician interviews | 40 (including nine from the feasibility study) | |
| Clinician focus groups | Five (32 participants) | |
| Observation of routine practice | 25 clinical sessions | |
| Interviews with patients and carers were only planned in the event of any indication of the toolkits having had an impact on consultations | None |
Participants
Participants included clinicians working in memory and movement disorder services (including geriatricians and/or neurologists) and patients attending participating services together with any companions (hereafter ‘carers’). We aimed to recruit maximum variation samples throughout (with the exception of WP5.2 where we recruited all available participants). Relevant clinician characteristics were professional background (doctors, nurses, allied health professionals), specialty (e.g. geriatrics, neurology, old age psychiatry), geographical location (East Anglia, North East) and, in WP5.3, study arm (control, intervention). Only 22 services took part in WP5.3. The remaining service involved in WP4 dropped out prior to the start of WP5.3.
All clinicians in participating services were eligible for all components of WP5, although WP5.3 questionnaires were targeted primarily at those who had been exposed to the toolkits. All patients and carers seen by participating clinicians on the dates of observation were eligible. Patients and carers for interview in WP5.2 were identified by participating clinicians and the local research nurse. Recruitment continued until no new themes or issues emerged from further interviews or observation. In WP5.3 interviews with patients and carers were only planned in the event of any indication of the toolkits having had an impact on consultations.
Data collection and analysis
Data collection and analysis were iterative to ensure that insights from observation informed the interviews and vice versa. Clinician interviews and focus groups explored current practice, the perceived relevance of the assessment and management toolkits, and facilitators of and barriers to their implementation (see Report Supplementary Material 4 for topic guides). The guides were informed by NPT, but allowed additional issues to be raised. Interviews and focus groups were audio-recorded, transcribed, checked and anonymised prior to analysis. Interviews with patients and carers explored their perceptions of the assessment toolkit and also used cognitive interviewing techniques to obtain feedback on question wording. 48 Observation provided insight into clinic organisation, content of consultations, team roles and the use of standardised tests. In the pilot trial, observation focused primarily on if and how the toolkits were used. Anonymised field notes were written as soon as possible after observation.
Questionnaires explored the views of clinicians on diagnosis and management of LBD and on implementation in the pilot trial. Postal (WP5.1) and electronic questionnaires (WP5.3) were used, with one reminder to non-responders. For full details of analysis, response rates and respondent characteristics see Appendix 7, Tables 20–24.
Qualitative data were analysed thematically,49 with themes from WP5.3 subsequently mapped to the core constructs of NPT. Questionnaire data were analysed using simple descriptive statistics using SPSSX version 22 (IBM SPSS Statistics, Armonk, NY, USA).
The final data set for the qualitative work comprised 60 interviews with clinicians, seven focus groups (with 48 clinicians), observation of 42 clinical sessions, four clinical discussions, two multidisciplinary team meetings and interviews with six patients and four carers (see Table 5).
Results
Understanding current practice in the diagnosis and management of Lewy body dementia (work package 5.1)
Factors influencing the diagnosis and management of LBD were grouped into four overarching themes: (1) complexity of LBD, (2) service organisation, (3) skills, training and knowledge, and (4) clinician attitudes and values (Table 6). Each comprised a number of subthemes (those identified as particularly relevant to LBD are listed first and those relevant to dementia in general are shown in italics in Table 6). The complexity of LBD and service organisation were relevant to both diagnosis and management, whereas training and attitudes primarily related to diagnosis. The relative importance of each theme and subtheme in movement disorder and memory services is explored below. For further illustrative quotations and details of ID numbers see Appendix 8, Tables 25 and 26.
| Theme | Subtheme |
|---|---|
| Complexity of LBD | Variability in presentation |
| Lack of a definitive test | |
| Balancing treatments | |
| Shift to earlier presentation | |
| Availability of an informant | |
| Service organisation | Shift to nurse-led memory services |
| Follow-up arrangements | |
| Fragmented care | |
| Training, knowledge and experience | Awareness of LBD and cognitive biases |
| If and how core symptoms were covered in consultations | |
| Interpreting cognitive tests | |
| Knowledge of dementia subtypes | |
| Attitudes and values | Perceived prevalence and status of LBD |
| Perceived value of diagnosing LBD | |
| Perceived responsibility for LBD | |
| Perceived value of diagnosing dementia subtypes |
Complexity of Lewy body dementia
Variability in presentation within and between patients with LBD, together with a trend towards earlier presentation to memory services, meant that diagnosing LBD was not straightforward. Several old age psychiatrists (OAPs) commented on the subtleties of presentation, which were easy to miss unless explicitly explored during assessments:
. . . this is a very special kind of dementia, which is very difficult to diagnose in the first stages of the illness. Because the cognitive function is not that prominent, it’s mostly a sleep problem and falls, unsteadiness. Sometimes, they are almost the same as the Alzheimer’s type [. . .] In the early stages, it’s not easy to diagnose.
0501, trainee OAP
Fluctuating cognition, a characteristic of LBD, meant that key symptoms were not necessarily present during a single assessment or short follow-up appointment. Several clinicians in movement disorder services commented on the difficulties in distinguishing dementia and delirium and identifying dementia subtypes, as symptoms were not exclusively associated with a particular diagnosis. Although scans could assist with diagnosis, access to some types of scans varied. The lack of a specific, definitive test for LBD made it difficult for clinicians to develop confidence in their diagnostic skills.
Complexity also influenced the management of LBD. Sensitivity to medication required a cautious approach to introducing new treatments to minimise side effects. Eliciting patient priorities for treatment was crucial to ensure that symptoms of most relevance to the patient (or carer) were addressed. Understanding how patients had responded to new treatments in the context of fluctuation posed a further challenge and made the availability of an informant particularly important.
Service organisation
The increasing demand on services, in a context of stable or diminishing resources, had a significant impact on the diagnosis and management of LBD. In some memory services, new referrals were triaged, with ‘straightforward’ patients being allocated for assessment by a nurse or memory assessor. This could lead to DLB being missed, particularly when such staff had not received adequate training.
The limited time for follow-up appointments in movement disorder services influenced the extent to which clinicians proactively explored memory problems. Although cognitive function was routinely assessed (usually annually) in some services, this was not feasible in less well-resourced services: a further difficulty related to disclosing a diagnosis of PDD in a short follow-up appointment.
Strategies adopted to support the diagnosis of PDD included using nursing staff to conduct annual cognitive tests and developing a separate cognitive clinic with longer appointment times for patients with suspected PDD. Some clinicians approached the possible diagnosis of dementia over a number of visits to prepare patients and their families for disclosure gradually. Others emphasised the need to educate patients at an early stage about the potential cognitive impairment associated with PD.
Current service organisation emerged as a key barrier to the effective management of LBD, with considerable inequity of provision. Where patients developed cognitive symptoms first and were diagnosed with DLB by memory services, most were discharged back to GP care following diagnosis and treatment initiation. Referrals were then made as and when needed, resulting in episodic care with limited proactive management. PD patients who developed cognitive problems were typically managed in line with the NICE PD guideline, with reviews every 6–12 months. 50 Many PDD patients had access to a specialist nurse and few were discharged. Clinicians in movement disorder services nevertheless identified time as a barrier to holistic management of PDD patients:
The most frustrating thing is seeing a patient you may have known for a long time or who has been under follow-up for a long time and then you have a 10-minute slot to deal with them when they have got advanced Parkinson’s with dementia. It is ridiculous really but that is what happens [. . .] And so the most needy patients actually get the least attention and time which is very frustrating.
2101, consultant neurologist
Although some experienced geriatricians preferred to manage all symptoms to ensure continuity of care or expedite treatment, the complexity of LBD meant that most patients were referred between old age psychiatry, older people’s medicine and neurology departments. In the UK NHS, joint working or shared care by different specialties was difficult to achieve in practice because of funding arrangements, lack of shared geographical boundaries, incompatible information technology systems and limited direct consultant–consultant communication (with communication typically routed via the GP).
Although clinicians described a number of strategies to improve shared care of people with LBD, few had proved sustainable. These included joint meetings between services about complex patients, and adapting the care pathway to provide rapid access to interventions offered by memory services for patients already diagnosed with PDD by a movement disorder specialist without further diagnostic assessment. Although informal approaches, where clinicians in different specialties were co-located and had worked together for a number of years, appeared most successful, such arrangements were rare.
Training, knowledge and experience
Training, knowledge and experience were identified as key barriers to the diagnosis of LBD, but were less often discussed in relation to management. The questionnaires indicated that clinicians in memory assessment services were less confident in diagnosing DLB and PDD than other types of dementia (with the exception of frontotemporal dementia, see Appendix 7, Figure 7). Similarly, few clinicians were confident in managing both cognitive and physical symptoms (see Appendix 7, Figures 8 and 9), reflecting the need for shared care by different specialists.
Cognitive biases (where clinicians anticipated a default diagnosis of Alzheimer’s disease and framed questions in ways that would confirm their expectation) were thought to contribute to underdiagnosis of DLB:
I suppose I used to get told in medical school, that what the mind does not know, the eye will not see. So, unless you actually have this as a probable differential in the back of your mind, you’re not going to consider it, or then check to make sure that you aren’t missing something.
0601, consultant OAP, author’s emphasis in bold
In addition to awareness of LBD, clinicians needed to ask the right questions to elicit relevant symptoms. Observation of memory services indicated that although symptoms such as hallucinations, sleep and fluctuation were often explored, questions tended to be non-specific and/or leading. The lack of formal neurological examination was also confirmed, although participants often described assessing parkinsonism informally, for example by observing or asking about gait, blink rate and facial expression, and looking for tremor. Even when clinicians were aware of the need to explore core symptoms, such as fluctuation, they were sometimes unsure how to do so. In movement disorder services, observation confirmed the variable assessment of cognitive function. In addition, interviews suggested that, even when cognitive tests were completed, declining scores were sometimes simply documented without formally making a diagnosis of PDD.
The main facilitator to developing skills and confidence in diagnosing DLB was working alongside experienced clinicians and exposure to DLB patients:
I think experience is the biggest thing and working with somebody who is experienced who can point things out. [. . .] I think it’s quite nuanced. I think it is pattern recognition. The more you see, the more comfortable you are with ‘this is . . .’ or ‘this isn’t . . .’ You just have a bit of a hunch with things.
2307, trainee, older people’s medicine
Staff attitudes and values
There was little evidence that staff attitudes or values influenced management beyond personal preferences for managing certain symptoms themselves or referring patients to other specialties. Staff attitudes, however, had an impact on the diagnosis of LBD, particularly in movement disorder services. Where clinical experience of the number of patients with DLB contradicted published prevalence rates, some clinicians acknowledged that they might be missing cases or wondered whether or not DLB patients were being referred to other services. Others questioned the prevalence figures, for example referring to DLB as ‘the Newcastle disease’.
Although clinicians in movement disorder services did not dispute the prevalence of PDD, some expressed reservations over the value of making a formal diagnosis. Clinicians’ own views and attitudes clearly influenced if and how they explored cognition in PD patients, and how they labelled and explained any problems to patients. A range of benefits and disadvantages of formal diagnosis of PDD were identified. Potential benefits were increased patient and carer understanding of symptoms, opportunities for planning for the future and ensuring that potentially dangerous treatments were avoided. Formal diagnosis was sometimes avoided because of the difficulties of disclosing dementia in the context of an ongoing relationship, concerns over the potential negative impacts for patients and the stigma associated with dementia. Clinicians in movement disorder services also expressed varied views concerning the responsibility for diagnosis and management of LBD. Although some staff were confident to make the diagnosis themselves, others thought confirmation of a diagnosis of dementia in PD by a specialist memory service was essential. Views over responsibility for management similarly varied.
Although some clinicians questioned the value of diagnosing dementia subtypes, others argued that a clear diagnosis was important in terms of understanding and access to support:
I think it [diagnosis] matters to carers if they are going to get a diagnosis because they can put the symptoms into a context. They can obviously get frustrated and stressed seeing all these different symptoms and not understanding, and if you can say ‘that’s because of their diagnosis of Lewy bodies, that’s completely normal and you can expect this kind of prognosis’ then that is helpful for them to know what to expect in the future. So we can support the carers better if they’ve got a proper diagnosis.
0904, community nurse, memory service
Stakeholder perspectives on the acceptability of the toolkits (work package 5.2)
Clinician views
Clinicians generally held positive views towards the concept of the toolkits. Potential benefits of the assessment toolkits included increased awareness of core and suggestive features of LBD and a more consistent and systematic approach to assessment. The management toolkit successfully translated academic papers to a format and style suitable for practice:
I think the good thing about it is it’s all in one place, and it’s really clear. The instructions and the advice are really clear, which certainly, when I was training, Parkinson’s and Lewy body dementia always seemed quite complicated illnesses to look after. You felt like you might not know quite what the current guidance was. Whereas, at least with that, it’s a one-stop shop, which tells you everything that you need to know.
0701, consultant OAP
Feedback on the assessment and management toolkits covered a range of areas (for illustrative quotations see Appendix 9, Tables 27 and 28). The majority of the feedback on the management toolkit related to the version used in the pilot trial, as the format changed substantially after the feasibility study. Issues relating to the assessment toolkits (already covered in Work package 3: development of assessment toolkits for the diagnosis of Lewy body dementia) are not reiterated here, with the exception of question wording where additional comments were made during the pilot trial.
Views on layout and presentation
The assessment toolkit for movement disorder services began with questions about relevant symptoms and ended with diagnostic criteria. In contrast, the toolkit for memory services began with the diagnostic criteria, followed by specific questions. Although few comments were made about the order of the PDD toolkit, some participants thought that the order of the DLB toolkit was counterintuitive. Furthermore, non-medical staff who did not make the diagnosis could find the first page off-putting.
Although few participants explicitly commented on signposting in the assessment toolkits, several did not realise that the diagnostic criteria in the DLB toolkit were followed by specific questions to explore core and suggestive features, or that instructions on how to perform and score the five-item UPDRS were provided. The structure of the PDD assessment toolkit was widely misunderstood, with many participants thinking that they were required to perform a formal cognitive test with all patients, whereas this was relevant only if responses to the initial questions indicated potential problems.
The management toolkit was organised in three levels (Figure 4). A one-page overview provided a brief, accessible summary of the symptom areas covered and the general principles for managing LBD. The colour coding of the overview then carried through to the next level, which comprised one-page summaries of recommendations for each of the five symptom areas. The final level comprised the full reference guidelines.
FIGURE 4.
Formats of the management toolkit.

Clinicians valued the colour coding, which facilitated navigation to different levels of detail. The majority of clinicians found the symptom summaries most useful, although some relied primarily on the overview.
Format
Participants suggested a number of alternative formats to facilitate use of the toolkits, most commonly integration with existing information technology systems or paperwork. Using the local intranet to display the toolkits automatically for patients with relevant diagnostic codes was also suggested. Applications were identified as an alternative way to ensure that the toolkits were easily accessible. For the assessment toolkit, participants suggested portable summary ‘prompts’ for each section (similar to the symptom summaries).
Several clinicians requested an A5 version of the overview and/or symptom summaries from the management toolkit to make it more portable. Others recommended displaying a poster of the overview to maintain awareness of the management toolkit. Another suggestion was to embed the management toolkit within an e-learning module for trainee doctors.
Some clinicians in movement disorder services suggested that the initial questions on memory and executive function from the assessment toolkit could form a separate document to be completed by patients and carers before their consultation. Other clinicians, however, thought that this would be too burdensome for patients and carers.
The common perception that the toolkits were more relevant to doctors led to suggestions that separate versions be produced for non-medical staff. This would enable non-medical staff to focus on those aspects of the toolkits most relevant to their work and skills (e.g. questions on core and suggestive criteria in the DLB toolkit and non-pharmacological interventions in the management toolkit). Although some consultants saw the assessment toolkits as more valuable to nurses or trainees than themselves, others thought that it was a useful reminder even for experienced staff. Some participants thought that the management toolkit would be useful in other settings, notably primary care or inpatient wards.
Content
Feedback on the content of the toolkits related to five themes, some of which were more relevant to specific toolkits.
The inclusion of the diagnostic criteria was typically seen as a useful reminder and prompt by medical staff. A number of clinicians, however, asked why key features they associated with LBD (e.g. falls) were not included in the assessment toolkits. Some clinicians in movement disorder services queried the absence of questions on hallucinations and fluctuations, as they saw these as essential for differentiating between LBD and other dementias in PD. Although the toolkit for movement disorder services was modified after the feasibility study, questions on hallucinations, REM sleep behaviour disorder and fluctuations were not integrated into the toolkit for patients with established PD.
Some clinicians queried the omission of key areas, such as carer support, psychosocial interventions and information for patients and carers, from the management toolkit. These comments suggest that the rationale for focusing on selected areas should be included in the toolkits.
Comments on terminology and question wording related mainly to the assessment toolkits, particularly the DLB version. Unfamiliar terminology (such as ‘kinetic tremor’, ‘spontaneous parkinsonism’ and specific types of scans) was alienating, particularly for non-medical staff.
Although some clinicians emphasised the need for standardised questions, some questions were thought to be inappropriate for patients and carers, or to lack sensitivity. Less experienced clinicians valued the questions on REM sleep behaviour disorder, as they were sometimes unsure how to explore this symptom. Few comments were made about wording in the management toolkit, although greater clarity about incontinence (i.e. whether faecal or urinary) was suggested.
Several clinicians valued being able to use the symptom summaries to check drug dosages. For drug treatments used infrequently, some clinicians suggested providing more detailed protocols:
Clozapine is such a complicated drug. People like psychiatrists are afraid to touch it. You put it in one line saying clozapine. There is some evidence for clozapine and I think clozapine should be written out in like almost a protocol how to use it [. . .] Nobody really knows how often you are supposed to do the blood test. When do you start and what is the best maximum dose. So, unless you de-mystify it and put it in there [. . .] I think people will be worried to [use it].
1901, consultant geriatrician
The recommendations regarding melatonin were inconsistent with the clinical experience of some participants, and several reported that GPs were reluctant to prescribe melatonin. To address this issue, one clinician suggested including an information leaflet to support prescribing in primary care.
Although few comments were made about the five-item UPDRS during the feasibility study, this was the most contentious aspect of the DLB assessment toolkit in the pilot trial. Three main issues with the five-item UPDRS were highlighted: (1) the rationale for focusing on these five items, (2) uncertainty over the value of scoring each item and (3) the challenges of introducing the scale in services where non-medical staff were responsible for assessments.
The toolkits used in the feasibility study and pilot trial gave no explanation for using the five-item UPDRS. Understanding that the items in the scale had been demonstrated to be useful in distinguishing between DLB and AD26 could have increased staff buy-in to the scale. Although the current version of the toolkits (v3) includes a brief statement, it is unclear whether or not this is enough to counterbalance the negative attitudes to this scale.
Clinicians frequently questioned the need to score each item on the UPDRS on a five-point scale, preferring to record simply the presence or absence of symptoms. Common arguments against scoring related to the difficulties in scoring accurately without extensive training, the additional time needed and the perception that scoring was more relevant to research than to clinical practice.
Although non-medical staff in memory services were often confident in informally assessing for parkinsonism, most saw physical examination as outside their area of expertise and role:
Because you haven’t got a full caseload of clients with Lewy body dementia you’re doing it occasionally. So, for me, I need to be doing something all the time for it to click. I’m fine with asking the questions, speaking to the carers and speaking to the patients, but when it comes to the actual of doing some of the assessment here I lack confidence.
0904, community nurse, memory service
Consultants had varied views on whether or not it was feasible and/or appropriate to train non-medical staff to perform the five-item UPDRS. Some were keen to train their colleagues, whereas others felt that physical examination was a medical responsibility.
Some clinicians thought that their ongoing contact with patients facilitated identification of emerging cognitive problems, but others argued that symptoms could easily be missed without a formal assessment. Several clinicians in movement disorder services described (or were observed) using selected items from formal cognitive tests to explore cognitive impairment. Most clinicians in movement disorder services showed little interest in changing their existing approach often because of time constraints:
I just think as a concept it is really frightening to try and commit to doing it [the assessment toolkit] in a standard clinic. I think if you are going to do it, you would have to set up a stand-alone clinic [. . .] but that’s the problem isn’t it? The sort of balancing the desirable against the practical.
2402, consultant geriatrician
Patient and carer views of the assessment toolkits
A number of patients and carers invited to take part in an interview declined because they felt unable to comment on the assessment toolkit because they had been unable to distinguish it from the rest of the consultation.
Interviews with six patients and four carers confirmed that the assessment toolkit was ‘invisible’ to them. None of the questions from the assessment toolkit stood out as being inappropriate, unclear or in any way different from the rest of the consultation. In view of the consistency of these findings, data collection ceased before achieving the target sample size. Cognitive interviews with patients and carers highlighted some issues with question wording, typically with the same questions identified as problematic by clinicians.
Implementation of the toolkits (work package 5.3)
The implementation of the toolkits is summarised in Table 7, using the framework of NPT (for illustrative quotations see Appendix 10, Tables 29 and 30). Comments relating to all of the main NPT constructs were made about both the assessment and management toolkits and by staff working in memory and movement disorder services. Lack of investment in the assessment toolkits in many movement disorder services meant that few of these clinicians had experience relating to collective action or reflexive monitoring. They were able to comment, however, on both of these areas when discussing the management toolkit.
| Making sense of the toolkits (coherence) | All toolkits |
Translation of technical information into more practical formats Similarity to existing practice |
| Assessment toolkit | Improve consistency | |
| Management toolkit | Confidence in robustness of toolkit | |
| Investing in the toolkits (cognitive participation) | All toolkits | Perceived as more appropriate for ‘others’:
|
| Lack of investment as a team | ||
| Adopting the toolkits (collective action) | All toolkits | Lack of integration with existing paperwork or information technology system |
| Assessment toolkits | Selective use when LBD suspected (memory services) | |
| Lack of resource for implementation | ||
| Management toolkit | Insufficient alone to change practice | |
| Assessing impacts of the toolkits (reflexive monitoring) | All toolkits | Increased awareness of LBD |
| Assessment toolkits |
Increased confidence in diagnosing LBD Increased exploration and documentation of core and suggestive features (memory services) |
|
| Management toolkit |
More holistic practice (memory services) Increased confidence Use to offer suggestions to GP for drug treatment or referrals |
Making sense of the toolkits (coherence)
The concept of the toolkits was relatively straightforward to grasp and clinicians valued the translation of technical knowledge into practical tools. Key potential benefits of the assessment toolkits were improving the consistency of assessment (both within and between clinicians) and increasing diagnostic accuracy.
Although many clinicians reported that their current practice was similar to the assessment and management toolkits, this did not necessarily detract from their value. Most consultant and trainee doctors were confident in the recommendations in the management toolkit because of the expertise of the study team. Non-medics, however, were not necessarily familiar with the authors and some would have valued more detail on the background and references to source papers. The need to update the toolkits was emphasised by several participants, in part prompted by changes to the diagnostic criteria for DLB11 and to the NICE guidelines for management of PD50 during the pilot trial.
Investing in the toolkits (cognitive participation)
The generally positive views towards the toolkits did not, however, necessarily translate into a commitment to implementation. Consistent with earlier findings on the acceptability of the toolkits, they were often seen as more relevant to ‘other’ clinicians, typically junior doctors and non-medical staff. In addition, some clinicians queried whether or not the toolkits were relevant to their specialty, service or geographical location. Some movement disorder clinicians viewed diagnosis and management of LBD (particularly DLB) as more appropriate for memory services. Participants in some memory services argued that the management toolkit was of limited relevance, as their focus was primarily on diagnosis.
In three memory services, the lead clinician successfully made concerted and ongoing efforts to engage staff with the toolkits. In movement disorder services, such efforts were less evident, with only two services making efforts to engage some of the staff team. The majority of services, therefore, were characterised by a striking lack of collaborative work regarding the implementation of both toolkits. In these services, it was largely up to individual members of staff to decide if and how to use the toolkits:
For me, it was because we weren’t all invested as a team together. If you are trying to work with a team it is really important to know, ‘This is what we are all going to sign up to. This is what we are all going to do. This is how we are going to work together. This is how it will or won’t work’. You just feel as a team we never had those conversations about, ‘How can this be used in practice? How are we going to implement it?’
2602, specialist PD nurse
For some non-medical staff, the lack of perceived relevance of the toolkits limited their willingness to engage with the pilot trial.
Putting the toolkits into practice (collective action)
Implementation of both toolkits varied within and between services. Although the lack of integration with existing electronic or paper documentation was identified as a significant barrier to implementation, several clinicians reported incorporating some questions from the assessment toolkits into their routine practice:
What I’ve done is, actually, I’ve taken some of those criteria that are easy and then I have prepared my own clinic pro forma. So that when I see someone I have a piece of paper and the kind of things I need to ask. And part of my standard assessment question is to cover those criteria. That’s how I use them.
0102, trainee OAP
Although views varied on the additional time required to use the assessment toolkits, most participants in memory services used the toolkits only when LBD was suspected, and often did so after a consultation or during supervision, rather than embedding the questions into routine practice. The training on the five-item UPDRS provided by the clinical research associates to staff in some services in the pilot trial was well received, but insufficient to change practice on its own. The questionnaire explored the extent to which respondents explored core and suggestive features of DLB in consultations with new patients. Although over three-quarters of respondents from memory services reported that they always conducted a cognitive test and explored fluctuation and hallucinations, fewer than half explored REM sleep behaviour disorder, parkinsonism or used diagnostic criteria (see Appendix 7, Figure 10). Use of the assessment toolkits was limited in movement disorder services, with most services continuing with their usual approach to exploring cognition, although several individuals reported incorporating the initial questions on executive function into their consultations.
The management toolkit was similarly typically used after consultations, partly because of the logistics of accessing the documents but also because of concerns over patient reactions. Some clinicians appeared reluctant to change their practice purely on the basis of the management toolkit, preferring to continue with tried and tested prescriptions (even if the evidence for these was less robust):
We had that discussion this morning about clozapine being the most helpful but we don’t tend to use that, we tend to go for quetiapine.
Unidentified female participant, memory service 07
The questionnaires also explored views on the likelihood of embedding the toolkits into routine practice. Similar views were expressed about integration and sustainability of the assessment and management toolkits, although respondents had slightly more positive views about the management toolkit becoming part of their normal practice (see Appendix 7, Figure 11). Data also indicated that more positive views towards integration of the assessment toolkit were associated with having access to the management toolkit (see Appendix 7, Figure 12) and working in memory services (see Appendix 7, Figure 13).
Assessing impacts of the toolkits (reflexive monitoring)
For many clinicians, a key outcome of both toolkits was increased awareness of LBD. Where lead consultants in memory services had encouraged implementation of the DLB assessment toolkit, they reported improved documentation of core and suggestive features by non-medical staff. Other changes resulting from use of the assessment toolkit identified by staff in memory services included more consistent documentation of negative findings and a more holistic approach to assessment. Even when non-medical staff did not perform the five-item UPDRS, the assessment toolkit was thought to have increased awareness of the range of possible signs of parkinsonism.
We tried to identify possible mechanisms through which the management toolkit could lead to improved outcomes. Qualitative data from clinicians suggested that only a small number had adopted new treatments and there was little evidence of increased management of new symptom areas. We included questions on confidence in managing common LBD symptoms in all three WP5 questionnaires administered during the programme (see Appendix 7). Comparison over time indicated that confidence in managing three symptom areas (autonomic symptoms, parkinsonism and REM sleep behaviour disorder) increased significantly in control practices during the programme (Figure 5). Clinicians who received the management toolkit showed small, but (statistically) significant, changes in confidence in managing all but two symptom areas (Figure 6). This suggests that a possible mechanism of change may be the accumulation of marginal gains in confidence over a wide range of areas. However, the results should be interpreted with caution, as the respondents at each time point are overlapping, but not identical.
FIGURE 5.
Mean confidence ratings for symptom management for respondents in control services. *p < 0.05, **p < 0.01, ***p < 0.001. T0, initial data; T1, follow-up data 1; T2, follow-up 2.
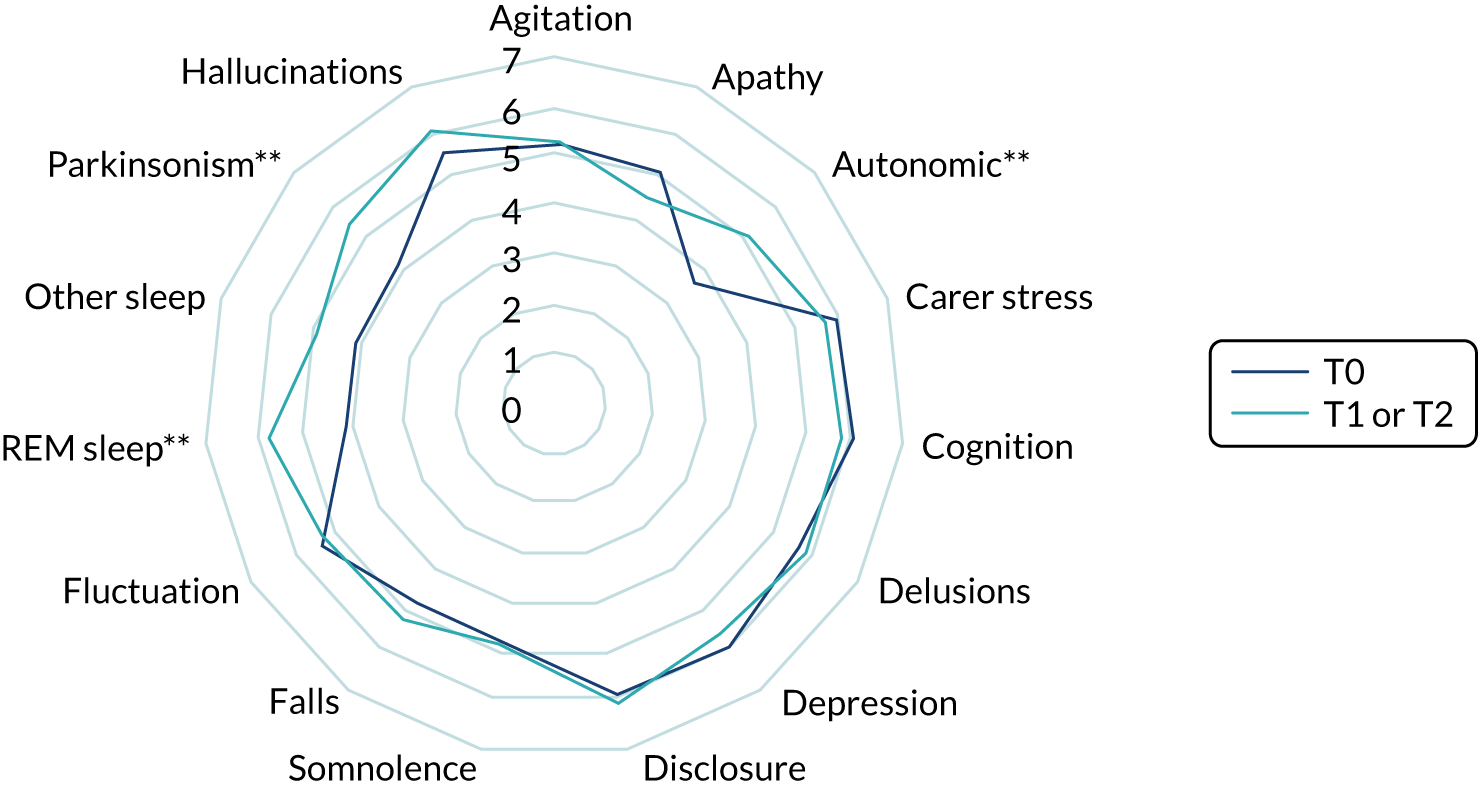
FIGURE 6.
Mean confidence ratings for symptom management for respondents in intervention services. *p < 0.05, **p < 0.01, ***p < 0.001. T0, initial data; T1, follow-up data 1; T2, follow-up 2.
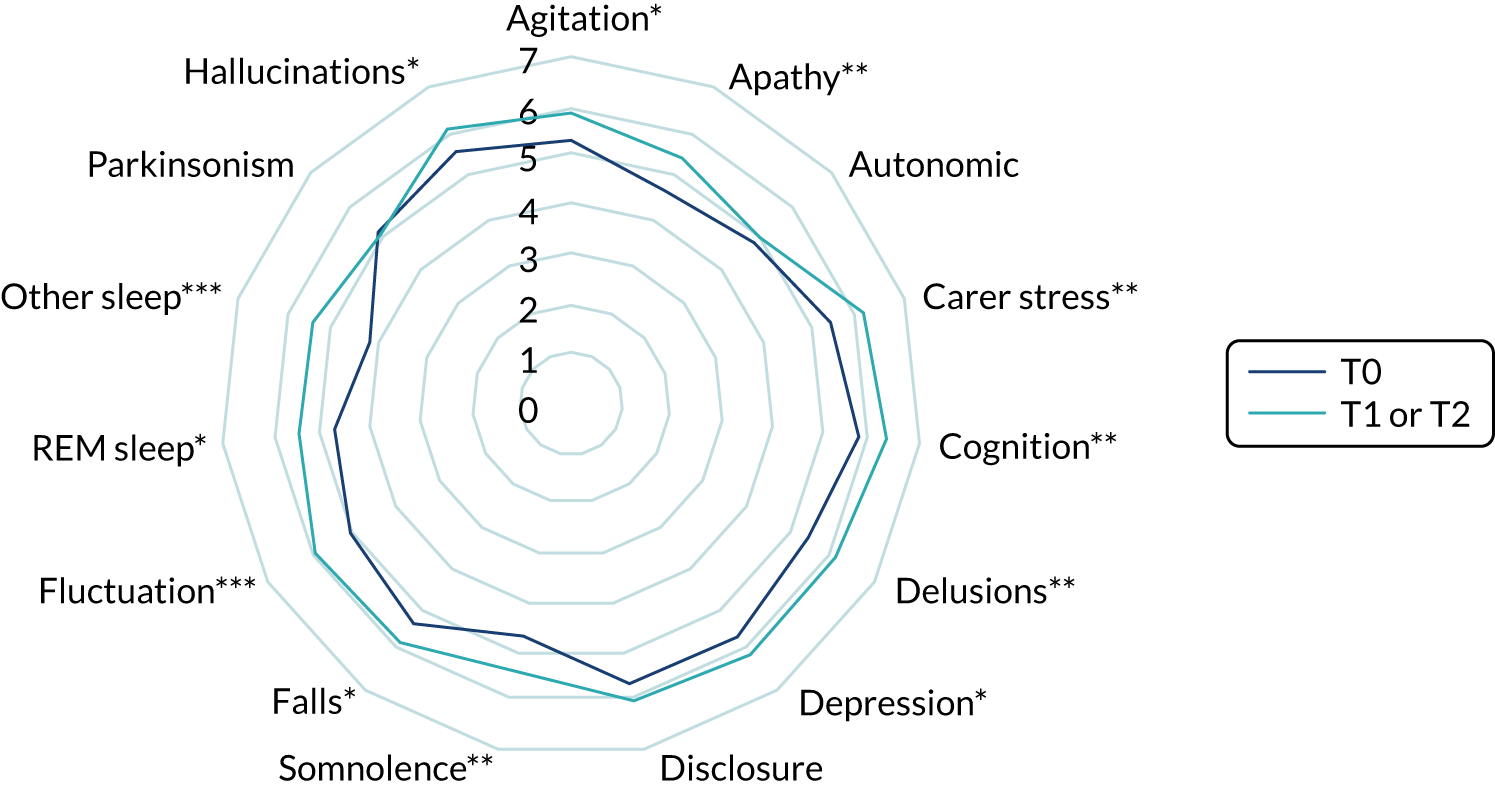
An alternative mechanism may be that change is achieved via indirect routes. For example, some clinicians reported that they were now more willing to suggest possible changes of medication or referral to other specialties to the patient’s GP. This more holistic approach to management is potentially a mechanism through which change could be achieved:
It gives guidelines for things that I wouldn’t consider treating in a memory service [. . .] So, I’m not really starting medications for constipation and even things like hypotension, but they might trigger me to ask the falls clinic or somebody else to see people.
1601, consultant OAP
Views on implementation
The insights from the qualitative work in WP5.1 and WP5.2 were used to develop an implementation strategy for the pilot trial (see Appendix 11, Tables 31–34). The extent to which this was implemented during the pilot trial was limited because of the restricted time and resources available, concerns over the risk of challenging and potentially alienating professionals, availability of credible professionals to address some issues (particularly for movement disorder services) and lack of skills within the research team to develop local implementation strategies.
During interviews and focus groups, we explored participant views on implementation in the pilot trial and alternative ways to support toolkit use. The interviews and focus groups were often the first opportunity for clinicians to reflect on if and how they were using the toolkits. Participants thought that implementation had been influenced by local leadership and tailored education sessions. In a small number of sites, a lead professional adopted the role of a local champion, typically acting as a focal point for the intervention, engaging the wider team and maintaining momentum (e.g. by adding the toolkits to the agenda of regular meetings). Some participants suggested that the lack of a lead clinician had been a factor contributing to poor implementation:
What I would say in retrospect, is I’m not sure that we’ve maximised the value of this within the service. But to do that, we would have actually needed a more sustained input, I think, from the team. And somebody, one or two of us, actually, really taking a role in terms of getting hold of this and running with it. You, actually, need somebody in the team who is going to take a real lead to try and sort of keep it on people’s minds. Find out what people’s experience was, address any issues, advocate for its value, which, to be fair, I don’t think there was somebody who took on that role.
1209, consultant OAP
The above quotation also mentions the need for sustained input from the research team. Tailored educational sessions were delivered by the study team to some sites (usually at their request). Feedback on the sessions indicated that they were valued by practitioners and often provided new insights into the assessment or management of LBD:
We had your colleague who came along and did the training on a Wednesday morning – which was excellent, I learned loads from that [. . .] [He] was much better at assessing for increased tone and did a far greater range of movement than I would have been routinely doing.
1101, consultant OAP
Although the site initiation visit provided an overview of the study and toolkits, several participants would have preferred a more interactive approach in which they could have reviewed the toolkits in detail with a knowledgeable clinician from the study team. This would have allowed queries to be raised and discussed and could explicitly have addressed the misperception among many movement disorder clinicians that the assessment toolkit required them to perform a cognitive assessment on all patients. A final strategy suggested by several participants was to use team meetings or peer learning groups to identify divergences between their current practice and the assessment or management toolkits as a way of highlighting scope for change.
Discussion
The detailed qualitative work provided new insights into the complex and inter-related factors influencing the diagnosis and management of LBD. The inherent complexities of the disease itself required input from different specialties, leading to potential fragmentation of services. Other important factors identified were a workforce with variable levels of training and confidence in LBD, and persisting negative attitudes among some clinicians towards diagnosis and disclosure of dementia in patients with pre-existing PD.
Observation confirmed the lack of routine assessment of core and suggestive features of LBD in memory services. In movement disorder services, resources were a key factor in determining whether or not cognitive function was formally assessed. The assessment toolkits potentially address these issues by including standardised questions for memory services and brief screening questions for movement disorder services to determine whether or not formal cognitive assessment is required. The management toolkit could potentially facilitate management of the complex and multiple symptoms in LBD. Other barriers to diagnosis and management were not amenable to change through the toolkits and some acted as barriers to their implementation.
Most clinicians welcomed the concept of the toolkits to facilitate the diagnosis and management of LBD. Key potential benefits were increased awareness of LBD, more consistent and comprehensive assessment and evidence-based management. The assessment toolkits were thought to be particularly beneficial to junior doctors and non-medical staff.
Qualitative data highlighted aspects of the assessment toolkits requiring further revision. It is now clear that training non-medical staff to administer the five-item UPDRS26 is neither feasible nor acceptable. However, with nurse-led models of memory assessment services becoming more common, alternative ways to conduct a simple neurological screen for non-medical staff are needed. Other issues related to question wording, signposting and the rationale underpinning the diagnostic criteria and choice of symptoms.
The positive feedback on the presentation of the management toolkit suggests that applying the same design principles to the assessment toolkits may make them more appealing and easier to use in practice. Ideally, the toolkits would be available in a variety of formats to suit personal preferences.
Implementation of the assessment toolkits in the pilot trial varied within and between sites. In many movement disorder services, key barriers related to cognitive participation or willingness to invest in the assessment toolkits. This resulted in limited use of the assessment toolkits (which is likely to explain the unchanged diagnostic rates of PDD observed in WP1R, see Work package 1 repeated: re-assessment of diagnostic rates for Lewy body dementia after introduction of the assessment toolkits for diagnosis). In three memory services in the North East, the lead clinician successfully encouraged widespread adoption throughout the team. Although this was attempted in two further memory services (both in East Anglia), uptake in these teams remained patchy. In other memory services, there was little collective work to support implementation and it seemed largely up to individual members of staff to decide if and how to implement the toolkits.
Uptake of the management toolkit varied, with some participants reporting that their practice was already consistent with the toolkit. Barriers to implementation were that few LBD patients were seen and that (memory) services focused on diagnosis with little involvement in management. Identifying possible mechanisms through which the management toolkit could lead to improved outcomes was challenging. Few participants reported changing their prescribing habits or managing additional symptom areas. However, the use of the toolkit appeared to raise awareness of the range of symptom areas affected in LBD and to increase confidence in managing common symptoms, and this may have facilitated a more holistic approach to care. In any future implementation study, we recommend the collection of data on prescriptions and referrals, and content analysis of letters to GPs, as it is possible that some benefits accrue from changes made by GPs, rather than directly by the specialist clinicians using the management toolkit.
Limitations
The increase in the number of services in WP4 (from 8 to 22), and the inclusion of East Anglia as a second geographical region, meant that resources for the qualitative component were stretched. One consequence was that a follow-up interview was conducted with only a single clinician in WP4 and, therefore, we have limited data on how the implementation and views of the toolkits changed over time. It proved impossible to engage four services in the qualitative work in WP4. The response rates to the questionnaires were acceptable, although disappointing. The views of less engaged clinicians are likely to be under-represented in our data set and consequently we may not have identified all barriers to implementing the toolkits.
Although only a small number of patients and carers provided feedback on the toolkits, the consistency of their comments means that we do not consider this a significant limitation. We achieved data saturation with a small number of interviews and there was no indication in the pilot trial that further data collection from patients and carers was merited.
We developed an implementation strategy for the pilot trial based on the initial qualitative data; however, because of limited resources, many suggestions were not adopted. Participants in the pilot trial identified additional strategies to inform future implementation.
Some services reported limited opportunities to use the management toolkit, as they focused on diagnosis and discharged patients shortly afterwards. This suggests that additional criteria may be required to identify potential services for a future trial.
There were occasionally some tensions between research paradigms, in particular in relation to managing qualitative feedback on question wording in the assessment toolkits, with the value given to ‘validated’ questions derived from clinical research.
Work package 1 repeated: re-assessment of diagnostic rates for Lewy body dementia after introduction of the assessment toolkits for diagnosis
One of the aims of the programme was to seek to improve the diagnosis of DLB and PDD by facilitating diagnosis so that more cases receive a correct diagnosis. To this end, approximately 2.5 years after WP1 was undertaken, and following the development and piloting of the assessment toolkits in WP3 (see Work package 3: development of assessment toolkits for the diagnosis of Lewy body dementia), they were introduced to 23 services as part of the pilot cluster randomised trial of the management toolkit undertaken in WP4.
Some of the same services that took part in WP4, where the assessment toolkits were introduced, also participated in WP1, which established the baseline diagnostic rates for DLB and PDD (see Work package 1: baseline study of the diagnosis and management of Lewy body dementia). The aim of WP1R was therefore to undertake, using exactly the same methodology as baseline, a revised study over an 18-month period, following the introduction of the assessment toolkits to examine diagnostic rates for DLB and PDD. Our hypothesis was that the introduction of the assessment and diagnostic toolkits would result in significantly increased diagnostic rates for DLB and PDD. This study was approved by the North East – Newcastle & North Tyneside 1 Research Ethics Committee (reference 17/NE/0362) and the Confidentiality and Advisory Group (reference 17/CAG/0188).
Methods
We used exactly the same methods as in WP1 (see Work package 1: baseline study of the diagnosis and management of Lewy body dementia) to ensure comparability of findings. For DLB, case notes of patients in four of the same memory services in three different NHS trusts from the two regions were examined. For PDD, case notes were reviewed from three movement disorder services in three NHS trusts in the two regions.
Results
Of 2058 referrals to the memory services over an 18-month period, 1279 received a diagnosis of dementia, of whom 6.2% received a diagnosis of DLB.
This rate, found after introduction of the assessment toolkits, was significantly higher than the 4.6% found at baseline before the introduction of the toolkits (χ2 = 5.3; p = 0.021). On further examination, diagnostic practice in East Anglia was unchanged, whereas rates had significantly increased in the North East (9.7% vs. 5.6% at baseline, χ2 = 14; p = 0.000019).
A total of 3405 referrals to movement disorder services were identified, of whom 1968 received a diagnosis of PD. Of these, 8.2% received a diagnosis of PDD. This diagnostic rate, after introduction of the assessment and diagnostic toolkits, was not significantly different from the 9.7% found at baseline (χ2 = 2.8; p = 0.09). For PDD, there was a difference between regions, with the North East showing a significant decrease in PDD rates (7.8% vs. 10.5%; p = 0.006); however, East Anglia showed a significant increase (13.3% vs. 8.3% at baseline; p = 0.046).
Discussion
The introduction of assessment and diagnostic toolkits for DLB and PDD was associated with a significant increase in diagnostic rates for DLB, but not PDD. However, there were significant differences between services and regions, with some services increasing their diagnostic rates and others not. Differences between regions were also noted for WP1 in terms of baseline rates for DLB diagnosis, although it is not entirely clear what factors drive these differences. There was diversity both within and between North East and East Anglia in terms of service organisation. Both sites included memory services where initial assessments of ‘straightforward’ patients were conducted by non-medical staff. Access to scans in the North East appeared to be better. In East Anglia, issues with scans reflected both the significant travelling required by some patients in rural areas and the informal limits described by some participants on the ‘appropriate’ number of scans. The North East has a long track record of research in DLB and many clinicians working in the North East have been exposed to DLB, either during training and/or through their involvement in research studies. This was reflected in a number of comments made by participants in the qualitative work on the North East being particularly sensitised to DLB. There were, however, clinicians in East Anglia with a long-standing interest in DLB who had similarly been involved in training medical staff over a period of years. Interest in DLB was therefore by no means limited to the North East.
The strengths of this study include the inclusion of the same services for which we ascertained baseline diagnostic rates in WP1, and use of exactly the same methodology for determining cases and diagnosis. Limitations include that, as this was not a controlled study, we cannot definitely conclude that the introduction of the assessment toolkits was causally related to the change in diagnostic rates. Other factors may have been involved, including generally heighted awareness of DLB over time and/or service or other changes. Finally, unlike WP1, in WP1R we did not undertake a repeat of the more detailed case note study and so we could not determine whether or not the factors associated with the diagnosis of DLB and PDD found at baseline had been moderated in any way. We did not collect data on the additional time taken to administer the toolkits and so cost-effectiveness of the toolkits for increasing diagnostic rates cannot be determined.
The qualitative studies (WP5) provide some insights as to why diagnostic rates for PDD (and DLB in East Anglia) were unchanged, but further research is required to better understand whether the unchanged diagnostic rates were linked to variation in implementation or whether additional factors were involved. Further exploration of ways to tackle the barriers to diagnosis identified in WP5 and to improve implementation of the assessment toolkit, especially in sites with low diagnostic rates at baseline, is required.
Overall programme discussion
The DIAMOND-Lewy programme achieved all its core aims. We demonstrated in WP1 that diagnostic rates for DLB and PDD were below, less than half, of those expected. For DLB, we found significant variation between regions and services. There appeared to be several contributing factors to this, including variations in availability of diagnostic tests, differences in service organisation and a great awareness of some clinical staff to DLB and PDD as a diagnostic possibility. We could not examine all these factors in detail within this programme. WP1 was retrospective in nature and used case records. Although this was a strength in terms of examining an unselected group of referrals to secondary care, there was some selection bias, as we could include people in the more detailed case note study only if they were alive.
In WP2, we developed evidence-based management toolkits. One major limitation was that the evidence base was thin, with most pharmacological studies focusing on a few agents, largely cholinesterase inhibitors and memantine, and there were very few non-pharmacological studies. To address these gaps, we used a Delphi approach with an expert clinical panel to develop a final management toolkit. Assessment toolkits were assembled within WP3 and, together with the management toolkit, trialled in a busy NHS trust that provided both memory assessment and movement disorder services. The toolkits were well received, but the extra time needed to undertake cognitive assessments in movement disorder services (where they are not standard) was a limitation. The toolkits had been designed to be as brief as possible, while also being consistent with the evidence base informing their development. This will always be a tension, and a limitation of WP3, as well as the pilot cluster randomised trial, is that we did not formally gather information on the time taken to administer the toolkits. In WP4, we successfully undertook a pilot cluster randomised trial of their implementation, demonstrating that such a study was possible, and we achieved the recruitment target of 120 participants (n = 131 recruited) within 23 services. Our qualitative studies (WP5) showed that implementation was variable, and one key lesson learned was that a clear implementation plan is needed for future studies or introduction of the toolkits.
Power calculations showed that a main trial would need to be of around 900 people, allowing for attrition and using an outcome measure such as the Neuropsychiatric Inventory. This would require a substantial investment and would be a complex trial to undertake. There are alternative models to implementing the assessment and management toolkits, including through service quality improvement initiatives and working with services using principles from implementation science to tailor implementation to the local context. In WP1R we used the same methodology as in WP1 to reassess diagnostic rates for DLB and PDD after introduction of the assessment toolkits in services where we had baseline data from WP1. We found a significant increase in diagnostic rates for DLB but not PDD; however, rates did not increase in every service. One limitation is that we were not able to examine, in detail, factors associated with a change in diagnostic rates. It appeared, contrary to expectation, that services that already had higher baseline rates of DLB diagnosis showed the greatest increase in diagnostic rates. This may be because the toolkits were introduced on the background of a high level of knowledge and interest in DLB diagnosis. It also suggests that in areas of low diagnostic activity for DLB, the introduction of the assessment toolkits themselves will not be sufficient to increase rates. Diagnostic rates for PDD did not change following introduction of the assessment toolkits. Qualitative work indicated that this may be because of a combination of insufficient time to always use these, as well as continued negative attitudes to making the diagnosis of dementia in PDD. Further work needs to examine how to improve diagnostic rates for PDD.
Conclusions and recommendations for research
The DIAMOND-Lewy programme has provided considerable new knowledge about diagnostic and management practice of DLB and PDD in the NHS, has produced evidence-based assessment and management toolkits, and has shown, through a pilot cluster randomised trial, the feasibility of undertaking a study following their introduction into NHS services. In addition, introduction of the assessment toolkits was associated with an increase in diagnostic rates for DLB, although we cannot definitely conclude a causal link.
Implications for practice and any lessons learned
The findings of the programme have a number of important implications for clinical practice:
-
In the regions we studied, DLB and PDD appeared to be underdiagnosed compared with expected rates, with variability in diagnostic rates between services. This suggests that improvements may be needed in the way in which clinicians assess people for symptoms and make diagnoses.
-
We found that the reluctance of some clinicians to make a formal diagnosis of dementia could be a significant factor contributing to the underdiagnosis of LBD (particularly PDD). This implies that negative attitudes to disclosure may need to be challenged, possibly through supervision, appraisal or local audits to examine the diagnostic rate of PDD.
-
In eliciting symptoms, observation suggested that leading questions were not uncommon and that some symptom areas (e.g. sleep) were often explored only superficially during memory assessments. Existing clinical practices, such as supervision, may provide an opportunity to address both of these areas. An iterative process of discussing and sharing ways of asking questions may enable staff to critically evaluate their existing approach and develop more consistent ways of approaching certain symptoms or issues. The concerns expressed over whether or not non-medical staff had received adequate training for their role in assessment imply that additional on-the-job training on dementia subtypes may improve diagnostic accuracy.
-
There may be benefits for more training to enhance awareness in secondary care clinical teams of how to make a diagnosis, and of why, including raising awareness of the opportunities for evidence-based management.
-
Using a structured method, like the assessment toolkit, for diagnosis may help increase diagnostic rates.
-
The complexities of managing LBD may require input from a range of specialties, which has the potential to lead to fragmentation of care pathways. Successful strategies for joint working included telephone multidisciplinary team meetings for complex patients, informal consultation arrangements across specialties where clinicians were co-located and adapting a memory service pathway to enable patients diagnosed with LBD by a geriatrician or neurologist to access support without further assessment. These initiatives highlight the potential value of developing tailored, local strategies to address barriers to cross-specialty working.
Recommendations for research
Findings from the programme highlight a number of areas for future research:
-
Further study to ascertain whether or not unchanged diagnostic rates, despite access to the toolkits, reflect poor implementation of the assessment toolkits or additional factors.
-
Research into how best to co-ordinate multispecialty input to patients with LBD is needed to streamline management and facilitate a holistic approach.
-
Further work is needed to better understand how assessment toolkits for PDD can be integrated into practice to improve diagnostic rates. Qualitative studies indicate that there remain important barriers and negative attitudes to diagnosis and management, and further work is needed to see how these are best addressed.
-
The evidence base informing the management of LBD is limited, especially for non-pharmacological interventions. More therapeutic studies are needed, especially well-designed RCTs for both cognitive and non-cognitive symptoms.
-
Future research should investigate the specific domains contained within the management toolkit and the extent to which global improvements are due to specific symptom improvement, as opposed to a number of marginal gains in several areas.
-
Our successful pilot demonstrates that a larger, more comprehensive trial of introducing the management toolkit could be undertaken, but it would need to include a minimum of 410 (and up to 908) people, depending on the primary outcome.
Acknowledgements
We are extremely grateful to the members of the PPI group for their valuable insights and contributions throughout the 5 years of the programme: Valerie Argent, Andrew Brown, Derek Forster and Anne Lister.
We thank DeNDRoN within the North East and Eastern Clinical Research Networks for their support with this study.
Contributions of authors
John T O’Brien (https://orcid.org/0000-0002-0837-5080) was chief investigator of the programme, lead for WPs 1A, 1R and 4A, generated the first draft of this report, commented and made suggested revisions and subsequently approved the final version of the report.
John-Paul Taylor (https://orcid.org/0000-0001-7958-6558) was co-lead for WP2, generated the first draft of this report, commented and made suggested revisions and subsequently approved the final version of the report.
Alan Thomas (https://orcid.org/0000-0002-6667-9533) was lead for WP3, generated the first draft of this report, commented and made suggested revisions and subsequently approved the final version of the report.
Claire Bamford (https://orcid.org/0000-0003-2885-801X) was co-lead for WP5, led and supported the PPI work, generated the first draft of this report, commented and made suggested revisions and subsequently approved the final version of the report.
Luke Vale (https://orcid.org/0000-0001-8574-8429) led the health economic analysis, generated the first draft of this report, commented and made suggested revisions and subsequently approved the final version of the report.
Sarah Hill (https://orcid.org/0000-0002-5408-2473) undertook the health economic analysis, generated the first draft of this report, commented and made suggested revisions and subsequently approved the final version of the report.
Louise Allan (https://orcid.org/0000-0002-8912-4901) advised on various aspects of study design and strategy, commented and made suggested revisions and subsequently approved the final version of the report.
Tracy Finch (https://orcid.org/0000-0001-8647-735X) was co-lead for WP5, commented and made suggested revisions and subsequently approved the final version of the report.
Richard McNally (https://orcid.org/0000-0001-6685-6467) led statistical aspects of the study, commented and made suggested revisions and subsequently approved the final version of the report.
Louise Hayes (https://orcid.org/0000-0001-6685-6467) supported statistical aspects of the study, generated the first draft of this report, commented and made suggested revisions and subsequently approved the final version of the report.
Ajenthan Surendranathan (https://orcid.org/0000-0003-3809-1545) undertook data collection and analysis for WPs 1A, 1B, 1R, 4A and 4B, commented and made suggested revisions and subsequently approved the final version of the report.
Joseph Kane (https://orcid.org/0000-0002-8479-9977) undertook data collection and analysis for WPs 1A, 1B, 1R, 4A and 4B, commented and made suggested revisions and subsequently approved the final version of the report.
Alexandros E Chrysos (https://orcid.org/0000-0002-4568-4202) undertook the health economic analysis, commented and made suggested revisions and subsequently approved the final version of the report.
Allison Bentley (https://orcid.org/0000-0001-9673-580X) undertook data collection and analysis for WPs 1A, 1B, 1R, 4A and 4B, commented and made suggested revisions and subsequently approved the final version of the report.
Sally Barker (https://orcid.org/0000-0002-2981-3748) undertook data collection and analysis for WPs 1A, 1B, 1R, 4A and 4B, commented and made suggested revisions and subsequently approved the final version of the report.
James Mason (https://orcid.org/0000-0001-9210-4082) advised on various aspects of study design and strategy, commented and made suggested revisions and subsequently approved the final version of the report.
David Burn (https://orcid.org/0000-0001-7658-1209) was lead for WPs 1B and 4B, commented and made suggested revisions and subsequently approved the final version of the report.
Ian McKeith (https://orcid.org/0000-0002-9250-0568) was co-lead for WP2, led and supported the PPI work, commented and made suggested revisions and subsequently approved the final version of the report.
Publications
Stinton C, Bamford C, Cambridge V, Lafortune L, Mak E, Mason J, et al. A Systematic Review of Management Strategies for Lewy Body Dementia (Dementia with Lewy Bodies and Parkinson’s Disease Dementia). PROSPERO 2014:CRD42014007180. URL: www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=7180 (accessed April 2021).
Stinton C, McKeith I, Taylor JP, Lafortune L, Mioshi E, Mak E, et al. Pharmacological management of Lewy body dementia: a systematic review and meta-analysis. Am J Psychiatry 2015;172:731–42. https://doi.org/10.1176/appi.ajp.2015.14121582
Thomas AJ, Taylor JP, McKeith I, Bamford C, Burn D, Allan L, O’Brien J. Development of assessment toolkits for improving the diagnosis of the Lewy body dementias: feasibility study within the DIAMOND Lewy study. Int J Geriatr Psychiatry 2017;32:1280–304. https://doi.org/10.1002/gps.4609
Connors MH, Quinto L, McKeith I, Brodaty H, Allan L, Bamford C, et al. Non-pharmacological interventions for Lewy body dementia: a systematic review. Psychol Med 2018;48:1749–58. https://doi.org/10.1017/S0033291717003257
Kane JPM, Surendranathan A, Bentley A, Barker SAH, Taylor JP, Thomas AJ, et al. Clinical prevalence of Lewy body dementia. Alzheimers Res Ther 2018;10:19. https://doi.org/10.1186/s13195-018-0350-6
Thomas AJ, Taylor JP, McKeith I, Bamford C, Burn D, Allan L, O’Brien J. Revision of assessment toolkits for improving the diagnosis of Lewy body dementia: the DIAMOND Lewy study. Int J Geriatr Psychiatry 2018;33:1293–304. https://doi.org/10.1002/gps.4948
Surendranathan A, Kane JP, Bentley A, Barker SA, Taylor JP, Thomas AJ, et al. Clinical diagnosis of Lewy body dementia. BJPsych Open 2020;6:1–8.
Taylor JP, McKeith IG, Burn DJ, Boeve B, Weintraub D, Bamford C, et al. New evidence on the management of Lewy body dementia. Lancet Neurol 2020;19:157–69.
O’Brien JT, McKeith IG, Thomas AJ, Bamford C, Vale L, Hill S, et al. Introduction of a management toolkit for Lewy body dementia: a pilot cluster randomised trial. Mov Disord 2021;36:143–51.
Surendranathan A, Kane J, Bentley A, Barker S, McNally R, Bamford C, et al. Introduction of an assessment toolkit associated with increased rate of DLB diagnosis. Alzheimer Res Ther 2021;13.
Data-sharing statement
We shall make data available to the scientific community with few restrictions as feasible, while retaining exclusive use until the publication of major outputs. Anonymised data can be obtained by contacting the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health and Social Care.
References
- National Institute for Health and Care Excellence (NICE) . Dementia: Assessment, Management and Support for People Living With Dementia and Their Carers. NICE Guideline (NG97) 2018. https://www.nice.org.uk/guidance/ng97 (accessed 9 April 2019).
- Brett J, Staniszewska S, Mockford C, Herron-Marx S, Hughes J, Tysall C, et al. Mapping the impact of patient and public involvement on health and social care research: a systematic review. Health Expect 2014;17:637-50. https://doi.org/10.1111/j.1369-7625.2012.00795.x.
- Mathie E, Wilson P, Poland F, McNeilly E, Howe A, Staniszewska S, et al. Consumer involvement in health research: a UK scoping and survey. Int J Consum Stud 2014;38:35-44. https://doi.org/10.1111/ijcs.12072.
- Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ 2017;358. https://doi.org/10.1136/bmj.j3453.
- INVOLVE . Policy on Payment of Fees and Expenses for Members of the Public Actively Involved With INVOLVE n.d. www.invo.org.uk/wp-content/uploads/2016/05/INVOLVE-internal-payment-policy-2016-final-1.pdf (accessed April 2021).
- INVOLVE . Briefing Notes for Researchers: Public Involvement in NHS, Public Health and Social Care Research n.d. www.invo.org.uk/wp-content/uploads/2012/04/INVOLVEBriefingNotesApr2012.pdf (accessed April 2021).
- NIHR Research Design Service . Patient and Public Involvement in Health and Social Care Research: A Handbook for Researchers n.d. www.rds-yh.nihr.ac.uk/wp-content/uploads/2015/01/RDS_PPI-Handbook_2014-v8-FINAL-11.pdf (accessed April 2021).
- Wilson P, Mathie E, Keenan J, McNeilly E, Goodman C, Howe A, et al. ReseArch with Patient and Public invOlvement: a RealisT evaluation – the RAPPORT study. Health Serv Deliv Res 2015;3. https://doi.org/10.3310/hsdr03380.
- Bethell J, Commisso E, Rostad HM, Puts M, Babineau J, Grinbergs-Saull A, et al. Patient engagement in research related to dementia: a scoping review. Dementia 2018;17:944-75. https://doi.org/10.1177/1471301218789292.
- Domecq JP, Prutsky G, Elraiyah T, Wang Z, Nabhan M, Shippee N, et al. Patient engagement in research: a systematic review. BMC Health Serv Res 2014;14. https://doi.org/10.1186/1472-6963-14-89.
- McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 2017;89:88-100. https://doi.org/10.1212/WNL.0000000000004058.
- Vann Jones SA, O’Brien JT. The prevalence and incidence of dementia with Lewy bodies: a systematic review of population and clinical studies. Psychol Med 2014;44:673-83. https://doi.org/10.1017/S0033291713000494.
- Galvin JE, Duda JE, Kaufer DI, Lippa CF, Taylor A, Zarit SH. Lewy body dementia: the caregiver experience of clinical care. Parkinsonism Relat Disord 2010;16:388-92. https://doi.org/10.1016/j.parkreldis.2010.03.007.
- McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65:1863-72. https://doi.org/10.1212/01.wnl.0000187889.17253.b1.
- NHS . National Schedule of Reference Costs 2017 18 2018. https://improvement.nhs.uk/documents/1973/2_-_National_schedule_of_reference_costs_v2.xlsx (accessed 22 November 2018).
- Curtis LA. Unit Costs of Health and Social Care 2014. Canterbury: Personal Social Services Research Unit, University of Kent; 2014.
- Kane JPM, Surendranathan A, Bentley A, Barker SAH, Taylor JP, Thomas AJ, et al. Clinical prevalence of Lewy body dementia. Alzheimers Res Ther 2018;10. https://doi.org/10.1186/s13195-018-0350-6.
- National Institute for Health and Care Excellence . Dementia: Supporting People With Dementia and Their Carers in Health and Social Care. (Clinical Guideline CG42) 2006. www.nice.org.uk/guidance/cg42 (accessed 1 April 2019).
- Rolinski M, Fox C, Maidment I, McShane R. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson’s disease dementia and cognitive impairment in Parkinson’s disease. Cochrane Database Syst Rev 2012;3. https://doi.org/10.1002/14651858.CD006504.pub2.
- O’Brien JT, Burns A. BAP Dementia Consensus Group . Clinical practice with anti-dementia drugs: a revised (second) consensus statement from the British Association for Psychopharmacology. J Psychopharmacol 2011;25:997-1019. https://doi.org/10.1177/0269881110387547.
- Stinton C, McKeith I, Taylor JP, Lafortune L, Mioshi E, Mak E, et al. Pharmacological management of Lewy body dementia: a systematic review and meta-analysis. Am J Psychiatry 2015;172:731-42. https://doi.org/10.1176/appi.ajp.2015.14121582.
- Stinton C, Bamford C, Cambridge V, Lafortune L, Mak E, Mason J, et al. A Systematic Review of Management Strategies for Lewy Body Dementia (Dementia With Lewy Bodies and Parkinson’s Disease Dementia) n.d. www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=7180 (accessed April 2021).
- OCEBM Levels of Evidence Working Group . Oxford Centre for Evidence-Based Medicine 2011 Levels of Evidence 2011. www.cebm.net/wp-content/uploads/2014/06/CEBM-Levels-of-Evidence-2.1.pdf (accessed 1 April 2019).
- Connors MH, Quinto L, McKeith I, Brodaty H, Allan L, Bamford C, et al. Non-pharmacological interventions for Lewy body dementia: a systematic review. Psychol Med 2018;48:1749-58. https://doi.org/10.1017/S0033291717003257.
- Taylor JP, McKeith IG, Burn DJ, Boeve B, Weintraub D, Bamford C, et al. New evidence on the management of Lewy body dementia. Lancet Neurol 2020;19:157-69. https://doi.org/10.1016/S1474-4422(19)30153-X.
- Ballard C, McKeith I, Burn D, Harrison R, O’Brien J, Lowery K, et al. The UPDRS scale as a means of identifying extrapyramidal signs in patients suffering from dementia with Lewy bodies. Acta Neurol Scand 1997;96:366-71. https://doi.org/10.1111/j.1600-0404.1997.tb00299.x.
- Schenck CH, Boeve BF. The strong presence of REM sleep behavior disorder in PD: clinical and research implications. Neurology 2011;77:1030-2. https://doi.org/10.1212/WNL.0b013e31822e14d7.
- Lee DR, McKeith I, Mosimann U, Ghosh-Nodial A, Grayson L, Wilson B, et al. The dementia cognitive fluctuation scale, a new psychometric test for clinicians to identify cognitive fluctuations in people with dementia. Am J Geriatr Psychiatry 2014;22:926-35. https://doi.org/10.1016/j.jagp.2013.01.072.
- Emre M, Aarsland D, Brown R, Bum DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 2007;22:1689-707. https://doi.org/10.1002/mds.21507.
- Mosimann UP, Collerton D, Dudley R, Meyer TD, Graham G, Dean JL, et al. A semi-structured interview to assess visual hallucinations in older people. Int J Geriatr Psychiatry 2008;23:712-18. https://doi.org/10.1002/gps.1965.
- Walker Z, Possin KL, Boeve BF, Aarsland D. Lewy body dementias. Lancet 2015;386:1683-97. https://doi.org/10.1016/S0140-6736(15)00462-6.
- Seibyl JP, Kupsch A, Booij J, Grosset DG, Costa DC, Hauser RA, et al. Individual-reader diagnostic performance and between-reader agreement in assessment of subjects with Parkinsonian syndrome or dementia using 123I-ioflupane injection (DaTscan) imaging. J Nucl Med 2014;55:1288-96. https://doi.org/10.2967/jnumed.114.140228.
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695-9. https://doi.org/10.1111/j.1532-5415.2005.53221.x.
- Thomas AJ, Taylor JP, McKeith I, Bamford C, Burn D, Allan L, et al. Development of assessment toolkits for improving the diagnosis of the Lewy body dementias: feasibility study within the DIAMOND Lewy study. Int J Geriatr Psychiatry 2017;32:1280-304. https://doi.org/10.1002/gps.4609.
- Thomas AJ, Taylor JP, McKeith I, Bamford C, Burn D, Allan L, et al. Revision of assessment toolkits for improving the diagnosis of Lewy body dementia: the DIAMOND Lewy study. Int J Geriatr Psychiatry 2018;33:1293-304. https://doi.org/10.1002/gps.4948.
- O’Brien J. Improving the Diagnosis and Management of Neurodegenerative Dementia of Lewy Body Type in the NHS. Work Package 4A and 4B: A Pilot Cluster Randomised Study of the Management Toolkit in the NHS Secondary Care Services 2020. https://research.ncl.ac.uk/diamondlewy/about/ (accessed 3 April 2020).
- Office for National Statistics . Employee earnings in the UK: 2018. Stat Bull n.d.:1-16.
- EuroQol Group . EQ-5D-5L About 2009. https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/ (accessed 26 February 2019).
- EuroQol Group . Crosswalk Index Value Calculator 2018. https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/valuation-standard-value-sets/crosswalk-index-value-calculator/ (accessed 14 February 2019).
- Price A, Farooq R, Yuan JM, Menon VB, Cardinal RN, O’Brien JT. Mortality in dementia with Lewy bodies compared with Alzheimer’s dementia: a retrospective naturalistic cohort study. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2017-017504.
- O’Cathain A, Thomas KJ, Drabble SJ, Rudolph A, Hewison J. What can qualitative research do for randomised controlled trials? A systematic mapping review. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-002889.
- O’Cathain A, Hoddinott P, Lewin S, Thomas KJ, Young B, Adamson J, et al. Maximising the impact of qualitative research in feasibility studies for randomised controlled trials: guidance for researchers. Pilot Feasibility Stud 2015;1. https://doi.org/10.1186/s40814-015-0026-y.
- Oakley A, Strange V, Bonell C, Allen E, Stephenson J. RIPPLE Study Team . Process evaluation in randomised controlled trials of complex interventions. BMJ 2006;332:413-16. https://doi.org/10.1136/bmj.332.7538.413.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Tabak RG, Khoong EC, Chambers DA, Brownson RC. Bridging research and practice: models for dissemination and implementation research. Am J Prev Med 2012;43:337-50. https://doi.org/10.1016/j.amepre.2012.05.024.
- May CR, Cummings A, Girling M, Bracher M, Mair FS, May CM, et al. Using normalization process theory in feasibility studies and process evaluations of complex healthcare interventions: a systematic review. Implement Sci 2018;13. https://doi.org/10.1186/s13012-018-0758-1.
- May C, Finch T. Implementing, embedding, and integrating practices: an outline of normalization process theory. Sociology 2009;43:535-54. https://doi.org/10.1177/0038038509103208.
- Willis G. Cognitive Interviewing: A Tool for Improving Questionnaire Design. Thousand Oaks, CA: SAGE Publications Ltd; 2005.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- National Institute for Health and Care Excellence . Parkinson’s Disease in Adults: Diagnosis and Management: Full Guideline 2017. www.nice.org.uk/guidance/ng71/evidence/full-guideline-pdf-4538466253 (accessed 6 March 2019).
- Zhu CW, Scarmeas N, Stavitsky K, Albert M, Brandt J, Blacker D, et al. Comparison of costs of care between patients with Alzheimer’s disease and dementia with Lewy bodies. Alzheimers Dement 2008;4:280-4. https://doi.org/10.1016/j.jalz.2008.02.008.
- McKeith IG, Ballard CG, Perry RH, Ince PG, O’Brien JT, Neill D, et al. Prospective validation of consensus criteria for the diagnosis of dementia with Lewy bodies. Neurology 2000;54:1050-8. https://doi.org/10.1212/wnl.54.5.1050.
- Stinton C, Bamford C, Cambridge V, Lafortune L, Mak E, Mason J, et al. A systematic review of management strategies for lewy body dementia (dementia with lewy bodies and parkinson’s disease dementia). PROSPERO 2014. www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=7180.
- Effective Public Health Practice Project . Quality Assessment Tool for Quantitative Studies 2010. https://merst.ca/wp-content/uploads/2018/02/quality-assessment-tool_2010.pdf (accessed 1 April 2019).
- Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011.
- National Institute for Health and Care Excellence . The Social Care Guidance Manual n.d. www.nice.org.uk/process/pmg10 (accessed April 2021).
- Beecham J, Knapp M, Thornicroft G. Measuring Mental Health Needs. London: Gaskell Publishing; 2001.
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: PSSRU, University of Kent; 2018.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2019.
- Newcastle University . Newcastle University Print Services n.d. www.ncl.ac.uk/library/services/print-bind-copy/print-services/ (accessed 16 November 2018).
- Department of Transport . TAG Data Book 2018. www.gov.uk/government/publications/tag-data-book (accessed 5 February 2020).
- Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: An EQ-5D-5L value set for England. Health Econ 2018;27:7-22. https://doi.org/10.1002/hec.3564.
- National Institute for Health and Care Excellence . NICE Position Statement on Use of EQ-5D-5L Valuation Set 2018. www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l (accessed 15 February 2019).
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. ValueHealth 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Edwards RT HB, Russell D, Russell I, Williams N, Linck P. QALY Calculation Alongside Randomised Controlled Trials: From the Torch to the Traffic Light 2004.
- Morris S, Devlin N, Parkin D. Economic Analysis in Health Care. Hoboken, NJ: John Wiley & Sons; 2007.
Appendix 1 Health economic analysis for work package 1
Comparison of health-care service utilisation and costs between Lewy body and non-Lewy body dementia patients: the DIAMOND-Lewy study
Introduction
This appendix describes the methods and results of the economics component of WP1. The aim of this component was to compare the cost of the pathway to final diagnosis and costs of care for those with DLB with non-DLB dementia (WP1A), and those with PDD with those with PD without dementia (WP1B). For both comparisons, the main objective of the study was to explore differences in the cost of assessments, appointments and diagnostic investigations undertaken, as well as hospital inpatient/day hospital resources and treatment.
Methods
Study sample and recruitment
Patients for the study were recruited into a DLB cohort and a PDD cohort. All participants were recruited from four UK NHS trusts in two different geographical regions of England (North East and East Anglia). DLB patients were recruited from nine psychiatry of old age/memory services from January 2013 to December 2014. PDD patients were recruited between August 2015 and February 2016 across eight movement disorder clinics (four neurology services and four geriatric medicine services). For each of the DLB and the PDD patient groups, control groups were also recruited (non-DLB dementia and PD without dementia, respectively), identified as the next control patient seen that could be matched. These controls were matched for sex, age (± 5 years) and MMSE score (in three bands 0–10, 11–20 and 21–30) to match for dementia severity. 51
At the point of recruitment, all participants had had their cognitive function assessed and had been diagnosed with dementia and/or PD 12 months prior to the start date of the study. This reflects the fact that the determination of diagnosis can take several months, especially for DLB. Diagnoses were confirmed by an independent expert panel of three clinicians (a method previously validated as an acceptable approach to discriminating incorrect diagnoses, compared with autopsy and imaging measures). 52
Data
For the DLB cohort, the research team screened the clinical notes of 9449 consecutive people seen within old age psychiatry/memory assessment services. Of these 9449 people, 4504 (47.7%) had a dementia diagnosis. Of these, 207 (4.6%) received a clinical diagnosis of DLB during this period and 105 were approached to participate in the study. Seventy-four people consented and granted researchers further access to their clinical records. Seventy-two non-DLB dementia control participants were also recruited into the study.
For the PDD cohort, consecutive clinical notes of 2263 patients in movement disorder neurology and geriatric medicine services were screened. Of these 2263 patients, 151 (6.7%) received a clinical diagnosis of PDD. Out of these 151 cases, 53 patients were approached for consent and 38 were recruited into the study. A total of 1474 people received a diagnosis of PD. Forty-four people were approached to take part in the study, with 35 providing consent.
Measures
The records of consenting participants in both the DLB and PDD cohorts were reviewed and extracted into case report forms tailored to each patient cohort. For each participant, data were collected on the use of services spanning several years of clinical records before and after final diagnosis. Data collected included the number of appointments before and after diagnosis and the number and type of diagnostic investigations undertaken before and after diagnosis.
To estimate the cost of service use and to compare differences between the pathway to diagnosis and the post-diagnosis cost of care for LBD (DLB and PDD) participants compared with control participants, the data on use of services were combined with unit cost data taken from routine sources, such as NHS reference costs15 and Personal Social Services Research Unit costs. 16 These data were then used to estimate the total cost of diagnosis and management for each participant. All costs were reported in Great British pounds (£) for the year 2017. Costs were categorised into components of total cost of diagnosis and care as ‘medical history costs’, ‘medication costs’, ‘investigation costs’, ‘pathway to diagnosis costs’ and ‘post-diagnosis costs’. ‘Investigation costs’ excluded neuropsychology and tests of cognition and function (e.g. MMSE, Addenbrooke's Cognitive Examination Revised and Bristol Activities of Daily Living Scale) because the costs of these were thought to be negligible. Investigation costs did include blood tests, brain imaging and other imaging examinations (such as ultrasounds and computerised tomography, radiographs, electrocardiograms, echocardiograms, etc.). ‘Pathway to diagnosis costs’ covered the cost of home visits by specialists (e.g. OAP, physiotherapist, social worker), clinic appointments (e.g. specialist PD nurse, physiotherapist, geriatric physician) and appointments with secondary care specialists (e.g. Community Mental Health Team, clinical psychologist, memory support service). ‘Medical history costs’ included operations, hospitalisations and attendances at accidents and emergency departments after the date of final diagnosis. ‘Medication change costs’ were based on the only available complete list of medications recorded for each patient in the case report form and refers to post-diagnosis medication changes. Finally, ‘post-diagnosis costs’ included home visits by specialists and clinic appointments, but excluded services associated with dementia management strategies, as these would have already been captured in other cost categories.
Statistical analyses
Demographic and clinical characteristics of the sample were summarised using proportions and measures of central tendency. Differences between DLB and non-DLB participants and PDD and PD without dementia participants were explored using independent-samples t-tests with unequal variance for normally distributed variables, Wilcoxon rank-sum tests for continuous non-normally distributed variables and chi-squared tests to explore differences in proportions. Differences between cases and controls in average costs of the different components of diagnosis and care costs and differences in the proportion to the total of each cost category were also estimated. A third set of analyses explored the contribution of patient characteristics to total costs.
The dispersion around the average was estimated using 95% CIs, with bias correction from 1000 bootstrap replications clustered by study site. The difference between patient groups was explored using Wilcoxon rank-sum tests. Finally, the marginal effects from a generalised linear model, using a gamma distribution with log-link function, adjusting by time of resource use and clustered standard errors by study site, were used to explore the effect of participant demographic and clinical characteristics on the total cost of diagnosis and care.
Results
Cohort characteristics
For the DLB comparison, the proportion of men in the sample was comparable between the DLB and non-DLB dementia groups at around 60% of participants (Table 8). The average age of participants across the two groups was 77 years and almost all participants reported a white British background. DLB participants had received a larger number of previous diagnoses before achieving a final diagnosis, and presented with more clinical features of LBD (e.g. fluctuating cognition, recurrent visual hallucinations, features of parkinsonism) than non-DLB participants.
| Characteristic | DLB (n = 74) | Non-DLB dementia (n = 72) | Difference between DLB groups, p-value (test statistic) | PDD (n = 38) | Non-PDD (n = 35) | Difference between PDD groups, p-value (test statistic) |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 59 | 58 | 73 | 74 | ||
| Female | 41 | 42 | 0.890 (χ2 = 0.019) | 27 | 26 | 0.899 (χ2 = 0.016) |
| Ethnicity | ||||||
| White British | 95 | 98 | 96 | 95 | ||
| Other | 5 | 2 | 0.370 (χ2 = 0.802) | 4 | 5 | 0.947 (χ2 = 0.004) |
| Age (years),a mean (SD) | 77.6 (8.4) | 77.2 (8.0) | 0.757 (t = 0.311) | 66.3 (19.7) | 68.1 (15.2) | 0.661 (t = –0.441) |
| Number of previous diagnosesb | ||||||
| Mean (SD) | 1.1 (1.0) | 0.6 (0.8) | 0.004 (z = 2.911) | 1.4 (1.1) | 0.5 (0.6) | < 0.001 (z = 3.837) |
| None | 31 | 51 | 24 | 51 | ||
| One | 39 | 36 | 24 | 43 | ||
| Two to four | 30 | 13 | 0.012 (χ2 = 8.856) | |||
| Two to five | 53 | 6 | < 0.001 (χ2 = 19.136) | |||
| Living arrangementsc | ||||||
| Home or family | 56 | 68 | 49 | 100 | ||
| Institution | 44 | 32 | 0.145 (χ2 = 2.121) | 51 | 0 | < 0.001 (χ2 = 23.839) |
| Use of care supportc | ||||||
| No | 0 | 3 | 75 | 45 | ||
| Yes | 82 | 75 | 6 | 52 | ||
| Not applicable (%) | 18 | 22 | 0.282 (χ2 = 2.534) | 19 | 3 | < 0.001 (χ2 = 18.805) |
| Time of resource use (years), mean (SD) | 6.4 (4.7) | 5.1 (4.6) | 0.052 (t = 1.637) | 9.2 (3.5) | 7.8 (4.4) | 0.129 (t = 1.537) |
| Time since diagnosis (years), mean (SD) | 1.8 (1.4) | 1.7 (1.9) | 0.290 (t = 0.554) | 3.2 (4.3) | 5.9 (3.9) | 0.007 (t = –2.797) |
| Number of clinical features of DLB or PDDd | ||||||
| Mean (SD) | 3.5 (2.1) | 0.9 (1.3) | < 0.001 (z = 7.848) | 7.2 (3.4) | 1.8 (2.0) | < 0.001 (z = 5.732) |
| None | 4e | 46e | 8f | 20f | ||
| One or two | 36 | 44 | ||||
| Three or more | 59 | 10 | < 0.001 (χ2 = 52.249) | < 0.001 (χ2 = 41.043) | ||
| One to five | 16 | 77 | ||||
| Six or more | 16 | 77 | < 0.001 (χ2 = 41.043) | |||
For the PDD cohort (across the 38 PDD and 35 non-PDD participants) the proportion of men in the sample was comparable between the two groups, with mean age across the two PDD cohort groups of 67 years. Those with PDD had received a larger number of previous diagnoses before reaching a final diagnosis than those with non-PDD dementia, and presented with more clinical features (e.g. cognitive impairment, dementia impact on activities of daily living, cognitive deficits). The proportion of participants who were receiving support from a formal or informal carer was higher for those in the non-PDD group than for with those with PDD.
Cost of diagnosis and care
Dementia with Lewy bodies participants had higher costs arising from medication changes, investigations and the use of primary and secondary care services associated with the pathway to diagnosis (Table 9). Overall, the average cost of care for DLB participants was £6557, compared with £3425 for non-DLB participants (mean difference £2868, 95% CI –£68 to £4013; p = 0.055).
| Cost | Mean | DLB (£) | PDD (£) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SD | Median | Maximum | Minimum | Mean | SD | Median | Maximum | Minimum | ||
| Medical history costs | ||||||||||
| Case | 4715 | 6901 | 1547 | 29,081 | 0 | 5303 | 8828 | 537 | 32,667 | 0 |
| Control | 2398 | 5327 | 1408 | 32,860 | 0 | 5054 | 9010 | 0 | 35,114 | 0 |
| Difference (95% CI) | 2202 (–2035 to 3164) | 285 (–6876 to 4344) | ||||||||
| Difference p-value | 0.170 | 0.957 | ||||||||
| Medication changes costs | ||||||||||
| Case | 208 | 348 | 97 | 2433 | 0 | 2244 | 3695 | 473 | 16,325 | 23 |
| Control | 86 | 170 | 52 | 1245 | 0 | 1439 | 3235 | 221 | 13,282 | 0 |
| Difference (95% CI) | 104 (28 to 137) | 828 (–1235 to 1777) | ||||||||
| Difference p-value | 0.002 | 0.580 | ||||||||
| Investigation costs | ||||||||||
| Case | 462 | 333 | 22 | 2313 | 28 | 473 | 448 | 311 | 2287 | 43 |
| Control | 278 | 174 | 0 | 997 | 18 | 212 | 229 | 186 | 994 | 0 |
| Difference (95% CI) | 153 (5 to 216) | 257 (237 to 306) | ||||||||
| Difference p-value | 0.042 | < 0.001 | ||||||||
| Pathway to diagnosis costs | ||||||||||
| Case | 480 | 633 | 305 | 4351 | 0 | 889 | 778 | 674 | 2949 | 0 |
| Control | 206 | 248 | 126 | 1515 | 42 | 869 | 494 | 863 | 1988 | 0 |
| Difference (95% CI) | 250 (160 to 311) | 33 (–323 to 350) | ||||||||
| Difference p-value | < 0.001 | 0.886 | ||||||||
| Post-diagnosis costs | ||||||||||
| Case | 693 | 750 | 517 | 3449 | 0 | 656 | 734 | 594 | 3555 | 0 |
| Control | 458 | 598 | 309 | 4027 | 0 | 39 | 174 | 0 | 995 | 0 |
| Difference (95% CI) | 176 (–2 to 281) | 574 (281 to 808) | ||||||||
| Difference p-value | 0.062 | < 0.001 | ||||||||
| Total of all costs | ||||||||||
| Case | 6557 | 7303 | 3745 | 30,954 | 274 | 9567 | 9738 | 6556 | 43,437 | 744 |
| Control | 3425 | 5514 | 1186 | 33,977 | 60 | 7614 | 9769 | 2886 | 38,646 | 330 |
| Difference (95% CI) | 2868 (–62 to 4013) | 2024 (–3598 to 5548) | ||||||||
| Difference p-value | 0.055 | 0.462 | ||||||||
Parkinson’s disease dementia participants also had higher average costs of investigations, at around £250 per patient (95% CI £237 to £306; p = 0.001) and higher post-diagnosis management costs (average difference of £570 per patient, 95% CI £281 to £808; p = 0.001). However, there was no evidence of a difference in overall costs between those in the non-PDD group (£2024, 95% CI –£3598 to £5548; p = 0.462) and those with PDD.
Table 10 shows the contribution to total cost of the different cost subcategories associated with pathway to diagnosis, medical history post diagnosis and post-diagnosis treatment. For DLB participants, medical history costs represented the largest contribution, with an average across patients of 40% of total costs (27% for non-DLB participants) (p = 0.042). The share of investigation costs was lower for DLB participants (20%) than for non-DLB participants (26%) (p = 0.042), although, on average, investigation costs were higher for DLB patients (see Table 9), probably reflecting the effect of the higher medical history costs on the share of total DLB costs.
| Cost subcategory | Mean DLB | 95% CI | Mean non-DLB | 95% CI | Difference | |
|---|---|---|---|---|---|---|
| z-value | p-value | |||||
| Total cost of care | 100.0 | 100.0 | ||||
| Medical history | 40.0 | 38.1 to 41.9 | 26.7 | 11.8 to 41.5 | 2.034 | 0.042 |
| Medications | 6.0 | 5.6 to 6.4 | 4.6 | 4.3 to 4.9 | 0.861 | 0.389 |
| Investigations | 19.5 | 16.9 to 22 | 25.9 | 17.1 to 34.7 | –2.035 | 0.042 |
| Pathway to diagnosis | 15.5 | 15 to 16.1 | 17.6 | 14.9 to 20.3 | –0.799 | 0.425 |
| Post diagnosis | 19.0 | 18.6 to 19.5 | 25.2 | 22.1 to 28.2 | –1.675 | 0.094 |
| Mean PDD | Mean non-PDD | |||||
| Total cost of care | 100.0 | 100.0 | ||||
| Medical history | 33.3 | 15.5 to 51.2 | 33.1 | 4.4 to 61.9 | 0.667 | 0.505 |
| Medications | 22.8 | 17.4 to 28.3 | 19.0 | 11.4 to 26.6 | 1.204 | 0.229 |
| Investigations | 10.3 | 9.9 to 10.7 | 11.0 | 5.7 to 16.3 | 1.027 | 0.304 |
| Pathway to diagnosis | 18.8 | 15.1 to 22.5 | 35.9 | 22.3 to 49.5 | –2.866 | 0.004 |
| Post diagnosis | 14.8 | 6.7 to 22.9 | 0.9 | 0.0 to 2.03 | 6.826 | < 0.001 |
No evidence of differences was observed between PDD and non-PDD participants, except for the share of cost of pathway to diagnosis and the post-diagnosis costs. For pathway to diagnosis, these represented 19% of total costs for PDD participants [a lower share than for those in the non-PDD group (36%; p = 0.004)]. For the post-diagnosis management costs, these were higher in the PDD group (see Table 9), which is also reflected in the much higher proportion of total cost of post-diagnosis management in PDD participants than in control participants (15% vs. 1%; p < 0.001).
Factors influencing costs of diagnosis and care
Table 11 shows the estimated difference in total costs of diagnosis and care between participants with DLB and participants with non-DLB dementia (see Table 11, top rows, panels A and B), and between participants with PDD and participants with PD but without dementia, after taking into account the influence on costs of differences in sex, time since diagnosis, total resource use time and other patient characteristics (see Table 11, bottom rows, panels A and B).
| Factor | A | B | ||||||
|---|---|---|---|---|---|---|---|---|
| Coefficient (£) | SE | p-value | 95% CI | Coefficient (£) | SE | p-value | 95% CI | |
| DLB | ||||||||
| Non-DLB dementia | (Reference) | |||||||
| DLB dementia | 3638 | 1028 | < 0.001* | 1478 to 5507 | ||||
| DLB with two or less clinical features | 5763 | 1699 | 0.001* | 2432 to 9093 | ||||
| DLB with three or more clinical features | 2248 | 298 | < 0.001* | 1664 to 2833 | ||||
| Number of previous diagnoses | 2247 | 421 | < 0.001* | 1421 to 3072 | 2315 | 382 | < 0.001* | 1566 to 3064 |
| Institution care | 3373 | 1067 | 0.002* | 1281 to 5465 | 3312 | 1079 | 0.002* | 1198 to 5426 |
| Sex | –562 | 601 | 0.350 | –1740 to 616 | –456 | 700 | 0.515 | –1827 to 916 |
| Total resource use time | –299 | 166 | 0.072 | –623 to 27 | –324 | 106 | 0.002* | –532 to –116 |
| Time since diagnosis | 5565 | 546 | < 0.001* | 4495 to 6636 | 5278 | 451 | < 0.001* | 4395 to 6161 |
| n | 136a | 136a | ||||||
| AIC | 2507 | 2503 | ||||||
| PDD | ||||||||
| PD without dementia | (Reference) | |||||||
| PDD | 7655 | 2030 | < 0.001* | 3676 to 11,634 | ||||
| PDD with five or fewer clinical features | 6856 | 1600 | < 0.001* | 3721 to 9991 | ||||
| PDD with six or more clinical features | 8691 | 2612 | 0.001* | 3571 to 13,810 | ||||
| Number of previous diagnoses | –625 | 3411 | 0.855 | –7311 to 6061 | –706 | 3461 | 0.838 | –7489 to 6076 |
| Institution care | –3394 | 3753 | 0.366 | –10,749 to 3961 | –3809 | 3446 | 0.269 | –10,563 to 2946 |
| Sex | –5235 | 1365 | < 0.001* | –7909 to –2561 | –4391 | 2576 | 0.088 | –9441 to 658 |
| Total resource use time | 618 | 270 | 0.022* | 90 to 1146 | 610 | 265 | 0.021* | 90 to 1130 |
| Time since diagnosis | 3177 | 3895 | 0.415 | –4458 to 10,811 | 3319 | 3893 | 0.394 | –4310 to 10,948 |
| n | 69b | 69b | ||||||
| AIC | 1367 | 1367 | ||||||
Results show that for DLB participants, having a DLB diagnosis represented higher total costs of approximately £3683 (95% CI £1478 to £5507; p < 0.001) than for non-DLB participants (see Table 11, top panel A). When disaggregating the DLB group according to the number of clinical features associated with DLB (recorded before final diagnosis), participants with two or fewer clinical features before diagnosis (n = 30) had costs at an average of £5763 higher than those of non-DLB participants (95% CI £2432 to £9093; p = 0.001), and DLB participants with three or more clinical features had, on average, higher costs than non-DLB participants (of around £2482, 95% CI £1664 to £2833; p < 0.001) (see Table 11, top panel B).
Average costs across all patient types in the DLB cohort (i.e. participants with DLB and non-DLB dementia) were influenced by the number of previous diagnoses and type of delivery of care. Total costs of diagnosis and care increased, on average, around £2250 for each time a participant was diagnosed with a dementia-related condition before receiving a final diagnosis (p < 0.001). Those participants who were residing in a care institution at the time of the study had, on average, £3300 higher total costs of care than those residing at home or with a family member (p = 0.002). As time over which resource use data increased, total cost declined, although this effect was small and not always significant. In contrast, there was strong evidence of a positive relationship between time since diagnosis and total costs.
Participants with PDD had, on average, higher total costs of diagnosis and care of approximately £7650 than non-PDD participants (95% CI £3676 to £11,634; p < 0.001) (see Table 11, bottom panel A). When disaggregating the PDD group according to the number of clinical features recorded before final diagnosis (five or fewer, or six or more), those participants with five or fewer clinical features before diagnosis (n = 24) had, on average, £6800 higher costs than non-PDD patients (95% CI £3721 to £9919; p < 0.001), and PDD participants with six or more clinical features had, on average, higher costs than non-PDD cases (of around £8700, 95% CI £3571 to £13,810; p = 0.001) (see Table 11, bottom panel B).
In the PDD cohort, across all participant types, there was no evidence of an impact of the number of previous diagnoses on total costs, on residing in a care institution at the time of recruitment to the study or on time since diagnosis. Each additional year of recorded service use increased total costs by an average of £610 (p = 0.021–0.022). Finally, there was some evidence of a difference in cost between men and women, with women having lower costs (between £4400 and £5200) than men (p < 0.10).
Appendix 2 Review protocol for systematic reviews
Reproduced with permission, June 2021. 53
PROSPERO: international prospective register of systematic reviews
A systematic review of management strategies for Lewy body dementia (dementia with Lewy bodies and Parkinson’s disease dementia)
Chris Stinton, Claire Bamford, Victoria Cambridge, Louise Lafortune, Elijah Mak, James Mason, Ian McKeith, John-Paul Taylor, Alan Thomas, John O’Brien.
Citation
Stinton C, Bamford C, Cambridge V, Lafortune L, Mak E, Mason J, et al. A Systematic Review of Management Strategies for Lewy Body Dementia (Dementia with Lewy Bodies and Parkinson’s Disease Dementia). PROSPERO 2014:CRD42014007180. URL: www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=7180.
Review question(s)
What are the benefits, harms and costs of management strategies for Lewy body dementia delivered to (1) patients and (2) carers?
How, when and where should management strategies for Lewy body dementia be implemented? What views do patients and carers have of management strategies for Lewy body dementia?
Searches
We will search the following electronic bibliographic databases: AgeInfo, Allied and Complementary Medicine, Applied Social Sciences Index and Abstracts (ASSIA), BiblioMap, British Nursing Index (BNI), Campbell Collaboration, CenterWatch, ClinicalTrials.gov, Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Database of Abstracts of Reviews of Effects (DARE), Database of Systematic Reviews, Cochrane Dementia and Cognitive Improvement Group Specialised Register, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Database of Promoting Health Effectiveness Reviews (DoPHER), EMBASE, Educational Resources Information Centre (ERIC), Health Services Research Projects in Progress, Health Technology Assessment database, IFPMA Clinical Trials Portal, Lilacs: Latin American and Caribbean Health Sciences database, MEDLINE, metaRegister of Controlled Trials, NHS Economic Evaluation Database (NHS EED), NHS Evidence, PROSPERO, PsycInfo, Scopus, Social Care Online, Trials Register of Promoting Health Interventions, UK Clinical Research Network Portfolio database, UMIN Clinical Trials Registry, WHO International Clinical Trials Registry Platform.
The search strategy will include terms relating to Lewy body dementia.
We will also search the grey literature (i.e. data reported outside peer-reviewed publications), reference lists of studies included in the review and seek expert input from within the extended review team.
There will be no restrictions on language or publication period.
Where the function is available, we will set up auto-alerts in databases to capture any new data arising after we have conducted our searches.
Types of study to be included
There are no restrictions on the types of study designs for this review, but opinion papers will be excluded.
Condition or domain being studied
Lewy body dementia (LBD) is the second most common cause of neurodegenerative dementia after Alzheimer’s, accounting for around 15% of cases. It is characterised by psychiatric symptoms (e.g. hallucinations), impairments and fluctuations in cognition, and parkinsonism. LBD comprises two related disorders: dementia with Lewy bodies (DLB) and Parkinson’s disease dementia (PDD). DLB and PDD are distinguished on the basis of the temporal sequence of the onset of motor symptoms; a diagnosis of PDD is applied when motor symptoms occur at least one year before dementia and a diagnosis of DLB when dementia precedes motor symptoms or they arise within one year of one another.
Participants/population
Patients: We will include study participants who have received a formal diagnosis of LBD on the basis of operationalised criteria or clinical judgement.
Carers: We will include participants who provide care (formal or informal) to people who have received a diagnosis of LBD.
Intervention(s), exposure(s)
Any management strategy, or combination of strategies, that are intended to address the needs (medical, social, economic, etc.) of people who have LBD or their carers. These will include, but not be limited to, pharmacotherapy, psychotherapy, physiotherapy, education, speech and language therapy, support services.
Comparator(s)/control
We will include studies with and without comparison/control groups.
Context
We will place no restriction on the settings in which managements strategies are delivered.
Outcome(s)
Primary outcomes
The primary outcome variables of interest are cognition and psychiatric and motor symptoms.
Secondary outcomes
The secondary outcome variables of interest will include carer burden, costs, sleep disorder, autonomic symptoms, falls, activities of daily living, quality of life, hospitalisation, institutionalisation, mortality, acceptability of management strategies, and harms/side-effects (e.g. physical, psychological, financial).
Data extraction, (selection and coding)
Selection of studies
Titles and abstracts will be screened independently by two reviewers. Studies that are considered by either reviewer to be relevant (or potentially relevant) will be obtained in full. Full-text articles will be assessed by each of the reviewers for inclusion in the review. Any discrepancies between the studies selected for inclusion by the reviewers will be resolved through discussion, with the involvement of a third reviewer if necessary.
Data extraction
Data from studies will be extracted into forms designed by the reviewers. We will collect information relating to eligibility, quality, participant details, study methods, management strategies employed, outcomes, results and author conclusions.
Risk of bias (quality) assessment
The assessment of quality of studies will be conducted independently by two reviewers using the Quality Assessment Tool for Quantitative Studies (Effective Public Health Practice Project, 1998), the NICE Methodology Checklist: Qualitative Studies (NICE, 2009), and the Drummond Checklist for Economic Evaluations (Drummond & Jefferson, 1996). Any disagreement between the reviewers will be resolved through discussion and, if necessary, by consulting a third reviewer. Where reviewers identify significant lack of quality, these studies will be excluded.
Strategy for data synthesis
Quantitative and qualitative data will be treated as separate streams in the initial phase of the review. Qualitative evidence will subsequently be used to aid in the interpretation of quantitative evidence.
We will distinguish between published and unpublished literature and assess the impact that publication status has on the results of the review using sensitivity analysis and/or descriptively.
Quantitative data
Where studies of management strategies employ quantitative methods we will select appropriate data against a hierarchy of evidence (i.e. giving preference to RCTs over studies with cohort designs) and synthesise the results using meta-analysis. Where we identify significant heterogeneity, we will employ random-effect models. If there is no significant heterogeneity we will used fixed-effect models. Where we are not able to combine data from different studies, we will provide a descriptive summary of the results.
Qualitative data
We anticipate that qualitative data will be synthesised thematically. Descriptive analysis will be conducted if this is not possible.
Ongoing studies
Data from ongoing studies will not be available for quantitative or thematic analyses but we will provide a list of ongoing research to inform on the gaps in the evidence.
Analysis of subgroups or subsets
If sufficient data are available, we will consider data for participants who have DLB and those who have PDD separately.
If sufficient data are available, we will conduct subgroup analyses based on dementia severity.
Dissemination plans
The findings will be disseminated to NHS professionals, NHS commissioners, Social Care, and patient/public organisations. Scientific findings will be published in academic journals and presented at conferences.
Contact details for further information
Chris Stinton
Organisational affiliation of the review
University of Cambridge, University of Newcastle, University of Durham.
Review team
Dr Chris Stinton, University of Cambridge.
Ms Claire Bamford, University of Newcastle.
Dr Victoria Cambridge, University of Cambridge.
Dr Louise Lafortune, University of Cambridge.
Mr Elijah Mak, University of Cambridge.
Professor James Mason, University of Durham.
Professor Ian McKeith, University of Newcastle.
Dr John-Paul Taylor, University of Newcastle.
Professor Alan Thomas, University of Newcastle.
Professor John O’Brien, University of Cambridge.
Anticipated or actual start date
16 September 2013.
Anticipated completion date
30 April 2014.
Funding sources/sponsors
NIHR Programme Grants for Applied Research (Project ref. DTC-RP-PG0311-12001) – Improving the diagnosis and management of neurodegenerative dementia of Lewy body type in the NHS (DIAMOND-Lewy).
Conflicts of interest
None known.
Language
English.
Country
England.
Subject index terms status
Subject indexing assigned by CRD.
Subject index terms
Humans; Lewy Body Disease.
Any other information
This review is being conducted as part of a programme of research to improve the care of people who have LBD within routine NHS secondary care.
| Date of registration in PROSPERO: 16 January 2014 | ||
| Date of publication of this revision: 16 January 2014 | ||
| Stage of review at time of this submission | Started | Completed |
|---|---|---|
| Preliminary searches | Yes | Yes |
| Piloting of the study selection process | Yes | Yes |
| Formal screening of search results against eligibility criteria | Yes | No |
| Data extraction | No | No |
| Risk of bias (quality) assessment | No | No |
| Data analysis | No | No |
Appendix 3 Search strategies for systematic reviews
Work package 2 was underpinned by two major systematic reviews conducted by the Diamond-Lewy study group. The reviews focused on pharmacological21 and non-pharmacological24 management strategies in LBD. Details of the search strategy, study selection and data synthesis are described below.
Pharmacological management strategies21
Search strategy
We searched the following electronic bibliographic databases: AgeInfo, Allied and Complementary Medicine Database (AMED), Applied Social Sciences Index and Abstracts (ASSIA), BiblioMap, British Nursing Index (BNI), The Campbell Collaboration Social, Psychological, Educational, and Criminological Trials Register, CenterWatch, ClinicalTrials.gov, Cochrane Central Register of Controlled Trials (CENTRAL), Database of Abstracts of Reviews of Effects (DARE), Database of Systematic Reviews, Cochrane Dementia and Cognitive Improvement Group Specialised Register, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Database of Promoting Health Effectiveness Reviews (DoPHER), EMBASE™ (Elsevier, Amsterdam, the Netherlands) Educational Resources Information Center (ERIC), Health Services Research Projects in Progress, Health Technology Assessment database, International Federation of Pharmaceutical Manufacturers & Associations (IFPMA) Clinical Trials Portal, Latin American and Caribbean Health Sciences Literature (LILACS), MEDLINE, metaRegister of Current Controlled Trials, NHS Economic Evaluation Database (NHS EED), NHS Evidence, PROSPERO, PsycInfo® (American Psychological Association, Washington, DC, USA), Scopus® (Elsevier), Social Care Online, Trials Register of Promoting Health Interventions, UK Clinical Research Network Portfolio database, University hospital Medical Information Network (UMIN) Clinical Trials Registry and the World Health Organization’s International Clinical Trials Registry Platform. The search strategy included terms relating to LBD. We also searched grey literature (i.e. data reported outside peer-reviewed publications), reference lists of studies included in the review, and sought expert input from within the extended review team. There were no restrictions on language or publication period. Where the function was available, we set up auto-alerts in databases to capture any new data arising after we conducted our searches.
Study selection
Titles and abstracts were screened independently by four of the authors, with non-English-language papers screened by native speakers. Discrepancies were resolved through discussion between screeners. Potentially relevant studies were obtained in full and examined in detail by the first author against the following criteria: (1) participants had a diagnosis of DLB, PDD or LBD (or were the carers of patients with these diagnoses), (2) studies examined pharmacological strategies and (3) outcome measures and scores were specified. No restrictions were placed on study design, but opinion papers were excluded.
Data extraction
Data were extracted by two reviewers and recorded in an a Microsoft Excel® spreadsheet (Microsoft Corporation, Redmond, WA, USA). We collected information relating to participant demographic characteristics, selection criteria, study design, management strategies, outcome measures and scores, adverse events and withdrawals.
Data synthesis
Studies were grouped and analysed according to pharmacological strategy. For each strategy, studies of the highest level of evidence were included in the review. Classification of level of evidence was determined using guidelines from the Oxford Centre for Evidence-Based Medicine. 23
Methodological quality
Methodological quality was assessed by three of the authors using the QATQS,54 which was developed to assess quality across study designs, aiding consistency and clarity of reporting. The QATQS examines selection bias, study design, confounders, blinding, data collection methods, withdrawals and drop-outs. Domains are rated as being of weak, moderate or strong quality, which feed into an overall rating of study quality. The reliability and validity of the QATQS have been demonstrated. 6
Statistical analysis
Meta-analysis was conducted using RevMan version 5.3 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark), employing the inverse variance method. Heterogeneity was assessed using the chi-squared and I2-statistics and considered significant with p-values < 0.10 for chi-squared and > 40% for I2. We employed random-effects models when there was significant study heterogeneity and fixed-effect models when heterogeneity was not significant. Missing data were sought from study authors. For data that were not obtainable, values were estimated using methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions. 55 We estimated risk ratio with 95% CIs for dichotomous outcomes and weighted mean difference or standardised mean difference with 95% CIs for continuous outcomes. Descriptive summaries were provided when studies could not be combined.
Non-pharmacological management strategies24
Search strategy
The search identified studies through bibliographic databases, trial registers and the grey literature. Bibliographic databases and trial registers included the following: MEDLINE (1946–present), PreMEDLINE, PubMed, EMBASE (1974–present), Scopus, the Web of Science™ (Clarivate Analytics, Philadelphia, PA, USA) (1900–current), PsycInfo (1806–present), CINAHL (1981–present), Cochrane libraries [Cochrane Database of Systematic Reviews (2005–October 2016), CENTRAL (August 2016) and the Cochrane Methodology Register (third quarter of 2012), other evidence-based medicine databases [American College of Physicians® (ACP)] Journal Club (1991–September 2016), DARE (first quarter of 2015), Health Technology Assessment database (third quarter of 2016) and NHS EED (first quarter of 2015)]; AgeLine (1978–present); ALOIS (ALzheimer’s and cOgnitive Improvement Studies register); AMED (1985–present); Physiotherapy Evidence Database (PEDro) (1929–present); Social Work Abstracts (1968–present) and the National Association of Social Workers (NASW) clinical register (14th edition). The grey literature was searched using such resources as the System for Information on Grey Literature in Europe (SIGLE), National Technical Information Service (NTIS) database and PsycExtra® (American Psychological Association) (1908–present).
The search strategy used only population and intervention terms to maximise the likelihood of identifying relevant studies. Comparator and outcome terms were not used. The population was people with LBD or their carers and this was identified using the search terms: [(Lewy OR Park*) and Dementia]. Interventions were any non-pharmacological treatment and identified using a wide range of terms: (activit*, acupuncture, alternative, animal, aromatherapy, art therapy, assisted, balance, behav*, bicycle, calisthenics, carer intervention, caregiver intervention, CBT, Chi gong, cognit*, cognitive behavioral therapy, cognitive behavioural therapy, counsel*, creative arts, dance, dancing, diet, direct current stimulation, drama, ECT, educat*, electroconvulsive therapy, enhanc*, environmental intervention, environmental modification, exercise, flexibility, humor therapy, humour therapy, hydrotherapy, intervention*, leisure, light therapy, management, martial arts, massage, meditation, Montessori, multisensory, music, non-pharm*, nonpharm*, nutrition, occupational therapy, pet therapy, physical activity, physical therapy, physiotherapy, pilates, psychoeducation, psychol*, psychosocial, psychotherapy, Qi gong, reality orientation, recreation*, reminiscence, resistance training, run*, sensory, simulated presence, stimulation, Snoezelen, support*, support group*, swim*, tai chi, therap*, therapeutic activity, TMS, training, training carers, training caregivers, transcranial magnetic stimulation, treatment*, validation, weight training, yoga). Searches were conducted on 30 October 2016.
In addition to bibliographic database searches, the reference lists of papers included in the review and previous systematic reviews on both LBD and non-pharmacological interventions were checked for relevant papers. Advice was also sought from experts in the field.
Study selection
Two reviewers independently assessed search results for inclusion by title and abstract. All articles deemed relevant by either reviewer were obtained in full. Both reviewers then independently evaluated full-text articles for inclusion. Any disagreements were resolved through discussion or, if necessary, with a third reviewer.
Data extraction
Two reviewers independently extracted relevant data from publications using a standardised form. This included participant details (e.g. demographics, number, recruitment, clinical context, dementia severity), intervention type, study design, measures and results. Qualitative data were also collated.
The primary outcomes were measures of cognition, function, neuropsychiatric symptoms and motor symptoms. The secondary outcomes were measures of any other clinically relevant outcomes, such as quality of life, carer burden, financial costs, other symptoms (e.g. sleep or autonomic disturbances) and objective end points (e.g. falls, hospitalisation, institutionalisation, mortality). Secondary outcomes also included the perceived acceptability of treatments, reported side effects and drop-out rates (a measure of treatment acceptability).
Appendix 4 List of Delphi panel contributors
| Contributor | Location |
|---|---|
| Dag Aarsland | King’s College London, London, UK |
| Neil Archibald | South Tees Hospitals NHS Foundation Trust, Middlesbrough, UK |
| Louise Allan | University of Exeter Medical School, Exeter, UK |
| Clive Ballard | University of Exeter Medical School, Exeter, UK |
| Bob Barber | Newcastle University, Newcastle upon Tyne, UK |
| Roger Barker | University of Cambridge, Cambridge, UK |
| Brad Boeve | Mayo Clinic, Rochester, MN, USA |
| Daniel Collerton | Newcastle University, Newcastle upon Tyne, UK |
| Tom Dening | University of Nottingham, Nottingham, UK |
| Murat Emre | Istanbul University, Istanbul, Turkey |
| Duncan Forsyth | Cambridge University Hospitals Foundation Trust, Cambridge, UK |
| John Hindle | Bangor University, Bangor, UK |
| Clive Holmes | University of Southampton, Southampton, UK |
| Clare Lawton | Cambridgeshire and Peterborough NHS Foundation Trust (retired), Cambridge, UK |
| Ian Maidment | Aston University, Birmingham, UK |
| Eneida Mioshi | University of Cambridge, Cambridge, UK |
| Sean Page | Bangor University, Bangor, UK |
| Jill Rasmussen | The Lewy Body Society, London, UK |
| Sharon Reading | Newcastle Hospitals NHS Foundation Trust, Newcastle upon Tyne, UK |
| Louise Robinson | Newcastle University, Newcastle upon Tyne, UK |
| Lynn Rochester | Newcastle University, Newcastle upon Tyne, UK |
| Ben Underwood | Cambridgeshire and Peterborough NHS Foundation Trust, Cambridge, UK |
Appendix 5 Overview of management guideline

Appendix 6 Health economic analysis for work package 4
Introduction
The main objective of the economic component of the pilot cluster RCT was to rehearse the methods for a definitive economic evaluation of the new management toolkit for DLB and PDD compared with usual care. For a definitive study, a within-trial economic evaluation is anticipated. Such a study would seek to estimate costs and outcomes for each trial participant over the duration of the trial follow-up. These data would then be used to estimate cost-effectiveness. As is typical for pilot trials, because of the modest sample size available for each randomised patient group, a full economic evaluation was not conducted. The small sample size would not provide reliable conclusions on the cost-effectiveness of the management toolkit. Therefore, the focus of the economic component deviated slightly from protocol and the analysis aimed rather to provide a descriptive analysis of the costs and outcomes. Data on costs and outcomes for each patient sample (DLB and PDD) were examined separately, as it was expected that different diagnoses would lead to a differential use of services and outcomes. Suggestions for reducing the number of missing data will be made and methods that would be employed in a full evaluation of a definitive trial will also be discussed.
Methods
Costs
Costs were examined from the perspectives of the NHS and Personal Social Services, which includes costs of medications and health and social care use. Costs incurred by other sectors (e.g. local authorities) were not included. Data on use of health and social care were captured by using a bespoke service utilisation questionnaire that was developed based on questions included in the Client Service Receipt Inventory (CSRI). 57 Data were collected at baseline and at the 3- and 6-month follow-ups. The participants were asked about their use of a broad range of services, including inpatient services, outpatient services, day activity services and community care services, over the preceding 3 months. All costs are reported in GBP (£) for the financial year 2017/18. As the study follow-up was < 12 months, no discounting was performed.
Health care and social service resource use
Unit costs were estimated using the National Schedule of Reference Costs 2017/1815 and Unit Costs of Health and Social Care 2018. 58 Resources from the voluntary sector (e.g. dementia cafes) and local authority-run services (e.g. local authority day activity services) were not included in the NHS and Personal Social Services perspective; however, these services would be included in a broader societal perspective.
Resource use costs per patient at each time point (baseline and at the 3- and 6-month follow-ups) were estimated by multiplying total reported use of each service by each relevant unit cost. A total resource use cost for each participant was calculated by summing all individual service use costs for the individual. Mean and median resource use costs were then estimated by trial arm for each patient group.
Medication costs
Data were collected at each visit (baseline and at the 3- and 6-month follow-ups) on all medications taken by participants for the previous 3 months. Data on dose, frequency and duration taken were collected for each medication. Unit costs for medications were obtained from the British National Formulary (BNF). 59
Over-the-counter supplements (e.g. cod liver oil) were excluded from the analysis, as these were not assumed to be provided as part of NHS care and the costs would be considered ‘out-of-pocket’ costs for the participant. Medications that were insufficiently specific to obtain costs for (e.g. general antibiotics or steroids) were also excluded, as costs vary depending on the precise medication prescribed. Where data were missing on either dosage or frequency, recommended dosage was taken from the BNF where possible. Where this was not possible, the use of that medication was excluded, as no assumptions could be made on the average use of those medications. Therefore, where data were missing on dose, frequency or duration, and data could not be estimated using the BNF, costs could not be calculated.
Intervention delivery costs
The delivery of the intervention incurred costs from the production of the management toolkit and training staff to use it. The cost of producing the toolkit included materials and staff time. Unit costs for each element of production were obtained from invoices provided by the trial support team and service costs listed on the Newcastle University print services website. 60 The service costs stated by print services were assumed to include labour time.
The cost of training staff to use the toolkit was estimated from data collected on staff attending training sessions and approximate duration of the sessions, in addition to staff time required to develop training sessions. Unit costs for staff were obtained from publicly available pay scales for university staff and the Unit Costs of Health and Social Care 2018. 58
Total costs
Costs from each area of resource use (medications and health and social care) were combined at each time point. For the cumulative totals at the 3- and 6-month follow-ups, intervention delivery costs are also added to reflect the additional cost of receiving the intervention. In a definitive trial, cost differences between trial arms would be estimated using regression analysis, which would estimate total follow-up costs at 6 months, adjusting for baseline costs (which may differ between trial arms despite randomisation). To avoid double counting in the regression analysis, delivery costs would be included only once in the total follow-up costs, which would combine cost data collected at both the 3- and 6-month time points.
Patient and carer private costs
Costs incurred by patients and by carers who accompanied patients to appointments were also measured using data collected from a time and travel questionnaire combined with data on use of resources. The time and travel questionnaire was administered at the 6-month follow-up and elicited information on patients’ and carers’ most recent inpatient attendance, outpatient appointment, GP appointment and community dementia specialist appointment (i.e. community neurologist, OAP or geriatrician). Information on the mode of transport used to travel to the appointment, the time taken to travel and the time spent at the appointment (or days spend in inpatient care) was elicited. Where public transport or taxis were used to travel to an appointment, the cost of travel one-way was recorded. Where a private car was used, the cost of travel was estimated from the distance travelled using a cost of £0.45 per mile taken from the 2018 TAG Data Book61 and any additional parking charges. If both patient and carer travelled by taxi or private car, it was assumed that this journey was taken together and, therefore, the cost of travel was calculated for only the patient to avoid double counting.
The cost of time spent attending an appointment for both the patient and carer was calculated based on activity rates for various activities published by the ONS, according to the activity that either the patient or carer reported they would have been doing at the time of attending the appointment. Paid work, child care, caring for a relative or friend and voluntary work was valued as £13.88, which is the average hourly wage, according to the ONS. 37 Housework and leisure activities were valued at £10.10 an hour. Time spent in unemployment, retirement or in education was valued as £6.04 an hour.
The mean unit costs of time and travel for both patients and carers were calculated and multiplied by the number of attendances or appointments reported at each time period.
Quality of life
Patients’ generic quality of life was derived from the responses to the EQ-5D-5L questionnaire, completed at baseline and at the 3- and 6-month follow-ups. The EQ-5D-5L asks about a patient’s general quality of life over five dimensions (i.e. mobility, pain, usual activities, anxiety and depression, and self-care) and five levels. Responses to the EQ-5D-5L questionnaire were transformed into health state utilities using tariffs derived from the UK population. Values for the EQ-5D-5L were recently derived by Devlin et al. ;62 however, there is some debate over the accuracy of values in this new value set. Therefore, NICE63 recommends applying index values from a ‘crosswalk’ between the original EuroQol-5 Dimensions, the three-level version and the new five-level version, until the concerns over the new value set have been addressed. Therefore, the ‘crosswalk’ value set was used in this study to derive utility values for each participant. The crosswalk index value calculator, developed by EuroQoL Foundation39 and based on the index values estimated by van Hout et al.,64 was used to estimate the health state utilities for each participant.
The health state utilities were then used to estimate generic QALYs for each participant using the change from baseline approach. 65 This approach was chosen because the difference in baseline utilities was very small between trial arms. 66 In a full evaluation, QALYs would be calculated using the area under the curve approach67 and QALYs at the 6-month follow-up would be adjusted using regression analysis, including baseline utilities as a covariate. As unadjusted values are being reported here, the change from baseline approach was used because it accounts for differences at baseline, which the area under the curve approach does not, in the absence of regression analysis.
Results
Costs
Missing cost data
For the data from the service utilisation questionnaire, substantially more participants for the PDD sample were missing all service use data in the intervention arm than in the control arm at the 6-month follow-up (Table 12). It is possible that participants were missing because they were unable to complete follow-up visits because of deterioration in their health, which would imply that the intervention arm costs are lower than they should be. Conversely, there are more participants missing all service use data in the control arm in the DLB patient sample. Therefore, higher costs would be expected in the control arm than are observed.
| Time point | DLB | PDD | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Missing, na (%) | Mean (SD) (£) | Median (IQR) (£) | N | Missing, na (%) | Mean (SD) (£) | Median (IQR) (£) | |
| Baseline | ||||||||
| Control | 33 | 0 (0) | 1454 (2118) | 561 (790) | 18 | 1 (5) | 685 (812) | 410 (385) |
| Intervention | 44 | 0 (0) | 2369 (5007) | 648 (1055) | 31 | 0 (0) | 1312 (2697) | 359 (631) |
| 3 months | ||||||||
| Control | 25 | 8 (24) | 2852 (5759) | 535 (1134) | 18 | 1 (5) | 905 (1378) | 345 (1026) |
| Intervention | 40 | 4 (9) | 1393 (2745) | 411 (845) | 28 | 3 (10) | 2148 (3917) | 502 (1080) |
| 6 months | ||||||||
| Control | 26 | 7 (21) | 2091 (4738) | 520 (1585) | 18 | 1 (5) | 1089 (1637) | 322 (499) |
| Intervention | 40 | 4 (9) | 1070 (1472) | 519 (951) | 24 | 7 (23) | 783 (1119) | 369 (603) |
A similar pattern of missing data was observed when examining data collected for each individual component of service utilisation across all visits. In the PDD patient sample, 11% of data across all areas of service use was missing in the intervention arm compared with 6% in the control arm. For the DLB patient sample, 7% of data across all areas of service use was missing in the intervention arm compared with 16% in the control arm. The majority of these missing data can be attributed to participants missing at each visit.
The pattern of missing data on all medication use is almost identical to that identified for health and social care resource use (Table 13). Data missing on dose, frequency or duration (therefore preventing the calculation of a cost for the medication reported) were also examined. For participants with a diagnosis of DLB, 7% of data were missing in the intervention group and 9% of data in the control group. For the PDD sample, the corresponding proportions were 3% and 4%, respectively.
| Time point | DLB | PDD | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Missing, na (%) | Mean (SD) (£) | Median (IQR) (£) | N | Missing, na (%) | Mean (SD) (£) | Median (IQR) (£) | |
| Baseline | ||||||||
| Control | 33 | 0 (0) | 225 (268) | 141 (230) | 19 | 0 (0) | 195 (182) | 126 (190) |
| Intervention | 43 | 1 (2) | 218 (220) | 156 (197) | 31 | 0 (0) | 231 (182) | 180 (269) |
| 3 months | ||||||||
| Control | 26 | 7 (21) | 222 (200) | 161 (239) | 18 | 1 (5) | 210 (193) | 153 (167) |
| Intervention | 39 | 5 (11) | 264 (247) | 225 (276) | 28 | 3 (10) | 269 (195) | 248 (282) |
| 6 months | ||||||||
| Control | 27 | 6 (18) | 245 (216) | 166 (253) | 18 | 1 (5) | 206 (192) | 156 (171) |
| Intervention | 40 | 4 (9) | 272 (258) | 223 (263) | 24 | 7 (23) | 331 (331) | 264 (329) |
Health and social care resource use
Table 12 suggests a difference in mean health and social care resource use costs between the trial arms at baseline in both patient groups, although the small sample size cautions against any conclusions being drawn. The mean costs in the intervention arm for the DLB sample decreased at each follow-up visit, compared with costs calculated at baseline, whereas costs increased for the control arm. For the PDD sample, intervention arm costs also decreased at the 6-month follow-up compared with costs incurred between baseline and 3 months, whereas control group costs increased at each follow-up visit. The SDs for both randomised arms, both groups and at all time periods are larger than the mean values, suggesting substantial imprecision. The median values are considerably smaller than the mean values, which suggests that there is a substantial right skew to the data, with some participants incurring very much higher costs.
Medication costs
Table 13 shows little variation in both mean and median medication costs between baseline and the 6-month follow-up for both patient samples. There is a trend towards increasing medication costs for both arms over the 6-month follow-up period. Given the degenerative nature of both dementia and PD, this is unsurprising.
A greater increase in mean medication costs is observed for the intervention arms of both patient samples than in the control arms. However, the increase is greater for the PDD sample than for the DLB sample (see Table 13). Alternative rationales for this could be advanced. For example, the data may indicate that the management toolkit encouraged increased medication prescribing. An alternative explanation is that the larger proportion of missing observations in the intervention arm of PDD patients was a result of these participants being less well and, therefore, less able to complete follow-up visits. However, the small sample size prevents any robust conclusions being drawn.
Intervention delivery costs
Table 14 shows the breakdown of the resource costs associated with the intervention delivery. The total delivery cost was divided by the number of participants in the intervention arm in both patient groups combined (n = 75) to estimate a mean delivery cost per participant receiving the intervention. Mean intervention delivery cost was estimated at £76.32 per participant in the intervention arms. Participants in the control arms did not receive the intervention and, therefore, delivery costs are £0 for the control groups.
| Resource | Cost (£) |
|---|---|
| Total staff preparation time | 133 |
| Total staff training time | 2338 |
| Total materials | 3252 |
| Total | 5724 |
| Total per person with dementia | 76.32 |
Total costs
Table 15 shows the total costs for each trial arm at all time points. Total costs include health and social service use, medication costs and intervention delivery costs (for the intervention arms at the 3- and 6-month follow-ups). The health and social care use costs make up the largest proportion of the total cost. In both patient samples, there is an overall increase in total costs between baseline and the 6-month follow-up in the control arms, whereas there is an overall decrease in total costs in the DLB sample intervention arm. In the PDD sample, an increase in costs occurs between baseline and 3 months; however, a decrease in costs occurs between the 3- and 6-month follow-ups, and the mean 6-month cost is lower than the baseline cost for the control arm.
| Time point | DLB | PDD | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Missing, na (%) | Mean (SD) (£) | Median (IQR) (£) | N | Missing, na (%) | Mean (SD) (£) | Median (IQR) (£) | |
| Baseline | ||||||||
| Control | 33 | 0 (0) | 1679 (2154) | 797 (1237) | 19 | 0 (0) | 844 (793) | 593 (677) |
| Intervention | 44 | 0 (0) | 2582 (5076) | 806 (1214) | 31 | 0 (0) | 1543 (2769) | 572 (913) |
| 3 months | ||||||||
| Control | 26 | 7 (21) | 2964 (5681) | 797 (1203) | 18 | 1 (5) | 1114 (1385) | 591 (928) |
| Intervention | 40 | 4 (9) | 1726 (2807) | 783 (1040) | 28 | 3 (10) | 2493 (3961) | 770 (1314) |
| 6 months | ||||||||
| Control | 27 | 6 (18) | 2259 (4763) | 574 (1645) | 18 | 1 (5) | 1295 (1607) | 600 (653) |
| Intervention | 40 | 4 (9) | 1419 (1506) | 947 (1207) | 24 | 7 (23) | 1191 (1196) | 730 (917) |
The median costs imply a right skew to the total cost data, indicating that a small number of participants incurred much higher costs than the remainder of the sample. The SDs are larger than the mean values for each reported cost, which demonstrates imprecision. The imprecision is probably caused by the small sample size. For this reason, these total costs should be considered only illustrative.
Private costs
Table 16 shows the mean combined travel costs incurred by patients from travelling to all inpatient, outpatient, GP and community dementia specialist appointments for each time point. Table 16 also shows the mean combined time costs for the time spent in attendance at each of the four aforementioned areas of health-care services. There is an overall decrease in mean travel costs between baseline and the 6-month follow-up for both intervention and control groups, with the exception of the control group of the PDD sample whose mean travel costs increased at the 6-month follow-up. For both patient samples, there is an observed increase in mean time costs between baseline and the 6-month follow-up for the control group, yet a decrease in mean time costs for the intervention group. However, the median time costs imply a strong right-skew, which indicates that a small number of patients incurred much higher costs than the remainder of the sample.
| Time point | DLB | PDD | ||||||
|---|---|---|---|---|---|---|---|---|
| Travel costa | Time costb | Travel costa | Time costb | |||||
| Mean (SD) (£) | Median (IQR) (£) | Mean (SD) (£) | Median (IQR) (£) | Mean (SD) (£) | Median (IQR) (£) | Mean (SD) (£) | Median (IQR) (£) | |
| Baseline | ||||||||
| Control | 33 (42) | 27 (43) | 255 (671 | 22 (30) | 36 (58) | 25 (29) | 26 (40) | 18 (20) |
| Intervention | 44 (40) | 33 (40) | 378 (729) | 33 (73) | 32 (39) | 17 (38) | 121 (383) | 16 (30) |
| 3 months | ||||||||
| Control | 21 (16) | 20 (20) | 260 (573) | 16 (24) | 18 (21) | 12 (16) | 13 (17) | 8 (11) |
| Intervention | 29 (35) | 17 (39) | 174 (468) | 12 (38) | 19 (19) | 16 (25) | 342 (764) | 14 (35) |
| 6 months | ||||||||
| Control | 27 (36) | 17 (16) | 314 (757) | 14 (25) | 53 (138) | 19 (28) | 121 (370) | 14 (19) |
| Intervention | 30 (33) | 19 (33) | 213 (516) | 15 (38) | 21 (25) | 16 (23) | 79 (313) | 12 (17) |
Table 17 shows the mean and median travel and time costs incurred by carers for attending appointments with patients, including the time cost of visiting patients who were hospital inpatients. The mean travel costs are lower for carers (see Table 17) than for patients (see Table 16), largely because of the assumption that patients incur the cost of travel if a carer has accompanied a patient in a taxi or private car.
| Time point | Carers of DLB patients | Carers of PDD patients | ||||||
|---|---|---|---|---|---|---|---|---|
| Travel costa | Time costb | Travel costa | Time costb | |||||
| Mean (SD) (£) | Median (IQR) (£) | Mean (SD) (£) | Median (IQR) (£) | Mean (SD) (£) | Median (IQR) (£) | Mean (SD) (£) | Median (IQR) (£) | |
| Baseline | ||||||||
| Control | 9 (13) | 6 (13) | 21 (24) | 15 (26) | 10 (18) | 5 (8) | 17 (31) | 8 (15) |
| Intervention | 13 (12) | 9 (12) | 28 (26) | 23 (37) | 9 (11) | 4 (11) | 16 (22) | 9 (19) |
| 3 months | ||||||||
| Control | 6 (5) | 5 (7) | 15 (15) | 9 (16) | 5 (8) | 2 (5) | 9 (13) | 3 (9) |
| Intervention | 9 (11) | 4 (11) | 18 (21) | 7 (27) | 5 (6) | 3 (7) | 16 (20) | 5 (25) |
| 6 months | ||||||||
| Control | 8 (11) | 4 (7) | 20 (28) | 9 (18) | 15 (40) | 6 (6) | 28 (71) | 9 (11) |
| Intervention | 9 (11) | 5 (9) | 20 (22) | 9 (26) | 6 (8) | 4 (6) | 11 (15) | 7 (12) |
There is a slight decrease in mean travel costs for the carers of both patient samples in both trial arms between baseline and the 6-month follow-up, with the exception of carers of PDD patients in the control arm whose mean travel costs increased between baseline and the 6-month follow-up. The same pattern is observed for the mean time costs, which decrease between baseline and the 6-month follow-up for carers of patients in both trial arms and patient samples, except for the control group of PDD patients.
The mean travel and time costs for carers of DLB patients are typically slightly higher for carers of patients in the intervention group than for carers of patients in control group. The opposite is observed for carers of PDD patients, as both mean time and travel costs are typically higher for carers of patients in the control group than for carers of patients in the intervention group.
Quality of life
Missing EQ-5D-5L data
The DLB group had a larger proportion of missing observations for the EQ-5D-5L utilities at the 6-month follow-up than the PDD group, although the difference was not large (4 percentage points). Both groups had between 30% and 35% data missing at the final follow-up (Table 18).
| Sample | Baseline | 3-month follow-up | 6-month follow-up | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DLB | PDD | DLB | PDD | DLB | PDD | |||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| Control | 3 | 9 | 2 | 11 | 11 | 33 | 4 | 21 | 12 | 36 | 4 | 21 |
| Intervention | 3 | 7 | 4 | 13 | 8 | 18 | 5 | 16 | 11 | 25 | 9 | 29 |
| Total | 6 | 8 | 6 | 12 | 19 | 25 | 9 | 18 | 23 | 30 | 13 | 26 |
Health state utilities and quality-adjusted life-years
Using the change from baseline method, the difference in QALYs between baseline and the 6-month follow-up was calculated for each arm. Table 19 shows the mean and median utility score at each time point and the QALY change from baseline at the 6-month follow-up for each patient group. As the sample size was small, these values should be considered only illustrative. Overall, changes in QALYs in each trial arm for each sample were small to modest, but with consistent decreases in mean and median scores for those in the DLB sample. A similar pattern was not evident for the PDD sample. However, with such small samples, data are insufficient to draw conclusions.
| Utility score | DLB | PDD | ||||
|---|---|---|---|---|---|---|
| n | Mean (SD) | Median (IQR) | n | Mean (SD) | Median (IQR) | |
| Health state utilities | ||||||
| Control: baseline | 30 | 0.681 (0.267) | 0.763 (0.295) | 17 | 0.640 (0.283) | 0.739 (0.298) |
| Intervention: baseline | 41 | 0.658 (0.214) | 0.707 (0.211) | 27 | 0.678 (0.219) | 0.746 (0.217) |
| Control: 3 months | 22 | 0.658 (0.190) | 0.699 (0.195) | 15 | 0.743 (0.184) | 0.742 (0.19) |
| Intervention: 3 months | 36 | 0.612 (0.278) | 0.657 (0.295) | 26 | 0.694 (0.159) | 0.735 (0.223) |
| Control: 6 months | 21 | 0.592 (0.291) | 0.619 (0.391) | 15 | 0.696 (0.180) | 0.720 (0.227) |
| Intervention: 6 months | 33 | 0.683 (0.206) | 0.714 (0.246) | 22 | 0.613 (0.228) | 0.663 (0.185) |
| QALYs | ||||||
| Control | 18 | –0.029 (0.068) | –0.015 (0.065) | 15 | 0.007 (0.060) | 0.000 (0.037) |
| Intervention | 30 | –0.009 (0.039) | –0.001 (0.040) | 22 | –0.001 (0.057) | –0.001 (0.023) |
Discussion
Robust conclusions cannot be drawn on the impact of the intervention on costs and quality of life, given the small sample size of the trial. In a definitive trial, the impact on cost would be more reliably assessed in conjunction with the impact on quality of life. The impact of the intervention on quality of life, indicated by this pilot study, is small and appears to differ between patient samples. As with costs, the impact of the intervention on QALYs cannot be reliably assessed from this sample.
Given the disease area being studied in this trial, a substantial number of missing data might be expected because of difficulties in collecting data from people with dementia. Within a definitive trial, precision of results could be improved through the use of statistical methods to replace missing data with appropriate values where some data collection was unsuccessful. Numerous methods exist for handling missing data. The choice between these methods depends on the nature and pattern of missing data.
A substantial number of quality-of-life data were missing from responses to the EQ-5D-5L. If participants are unable to complete the questionnaire themselves, as a sensitivity analysis, proxy utilities from EQ-5D-5L responses completed by a carer on behalf of the patient participant could be used. Statistical tests for correlation between proxy and self-reported EQ-5D-5L can be conducted to assess whether or not the proxy values are sufficiently similar to the self-reported values (where both are available). If the tests indicate that proxy and self-report EQ-5D-5L utilities are not strongly correlated, using proxy values would introduce bias to the outcome. In a base-case analysis, imputation may be more reliable if there is sufficient data for this to be a reliable option.
Lessons learned
During the analysis of this pilot study data, lessons have been learned on how to approach a full economic evaluation of a definitive trial. These are briefly discussed below.
In a definitive trial, it would be important to include all costs associated with the health and social service use, including voluntary and local authority sectors, as they have an opportunity cost that could be relevant to NHS costs. For instance, if voluntary care services were discontinued, it would be likely that NHS resource use may increase, as NHS-provided services absorb the voluntary service users, or the burden would be placed on informal carers. This would have an impact on informal carers’ time available for work or leisure and may affect their own health, resulting in further NHS resource use. However, costs associated with voluntary sector and some community resources (e.g. educational classes) are not readily available. Therefore, these would have to be estimated via direct contact with services for a definitive trial.
In addition, informal care from relatives and friends is common for people with dementia and, therefore, it would be beneficial to assess the impact of the intervention on carers if a broader societal perspective is to be considered for the definitive analysis. If the intervention reduces or increases the need for informal care then this has a societal impact in terms of productivity (i.e. if the carer is still an active member of the workforce) and an opportunity cost in terms of care being provided informally, as opposed to via the formal care sector. Therefore, the costs (both time and financial) associated with care responsibilities and carer quality of life would be analysed in a definitive analysis.
The difficulty in conducting reliable analysis imposed by a small initial sample and compounded by missing data indicates that a much larger sample would be needed in a definitive trial. The number of total missing data identified in this pilot analysis could be used to estimate a sample size that would allow for a sufficiently large sample, given the expected numbers of participant drop-off across follow-up visits.
Appendix 7 Analysis of clinician questionnaires from work package 5
During the programme, three questionnaires were sent to professionals working in participating services to supplement the qualitative work. The aim of the first questionnaire (T0, distributed during WP5.1) was to explore self-reported confidence in diagnosing and managing conditions typically seen in memory and/or movement disorder services. The second questionnaire (T1, distributed towards the start of the pilot trial) aimed to collect baseline data on assessment practices and views of the assessment toolkits (and management toolkit in intervention services). The final questionnaire (T2, distributed after the end of the pilot trial) aimed to explore views on the toolkits, assessment practices, implementation and sustainability of the toolkits. A set of questions on confidence in managing common LBD symptoms was repeated in all three questionnaires. Details of potential explanatory variables (e.g. role, region and experience/training) were also collected. We hoped that using questionnaires would enable us to achieve greater coverage of respondents than was possible through interviews and observation.
Data collection
In January 2015, the first questionnaire was sent by post to staff working in most participating services, along with a prepaid envelope. One reminder was sent, including a copy of the questionnaire and a prepaid envelope, after approximately 3 weeks. As one NHS trust was unwilling to release staff details, we had to rely on local contacts to distribute the questionnaires. Although some attempts were made to send reminders within this trust, the process was hampered by a lack of information on individual participants and limited local resources to support distribution of the questionnaires.
The intention was to distribute the second questionnaire (T1) shortly after completion of all site initiation visits for the pilot trial. As there was insufficient capacity within the team to send out questionnaires to each site as approvals were obtained, distribution was delayed until all approvals were in place. Unfortunately, approvals for the qualitative work were not necessarily processed in parallel with approval for the pilot trial and obtaining approval from one trust was a protracted process. As a result, by the time T1 questionnaires were sent out (in January 2017), some sites had been using the toolkits for some time. The final questionnaire (T2) was sent out after the end of the pilot trial, as planned (in October 2018). The T1 and T2 questionnaires were administered using SurveyMonkey® (Palo Alto, CA, USA), with one electronic and one paper reminder.
Questionnaires completed on paper were entered into SPSS version 16 (IBM, New York, NY, USA). Questionnaires completed electronically were transferred to SPSS for analysis. All questionnaires were identified by a unique identifier.
Analysis
The questionnaires were analysed using simple descriptive statistics. Analysis of variance was used to explore the impact of potential explanatory variables. The reasons for selecting potential plausible explanatory variables are summarised in Table 20. It is evident that these potential explanatory variables are not independent, with region and role, in particular, being confounded.
| Explanatory variable | Rationale |
|---|---|
| Specialty | The marked differences in organisation of movement disorder and memory services suggested that staff views might systematically differ. Furthermore, the introduction of the assessment toolkits had different implications for the services, which might influence views of their acceptability and feasibility |
| Region | Newcastle upon Tyne is a leading centre in LBD research and doctors who have trained in the region are likely to have experienced greater exposure to LBD. There are also marked regional differences in the organisation of memory services, with nurse-led models predominating in East Anglia and a higher proportion of consultant-led services in the North East |
| Role | Knowledge of and training in LBD are likely to differ systematically between doctors and other allied health professionals |
| Study arm (relevant to T1 and T2 only) | Access to the management toolkit may influence perceptions of the value and purpose of the assessment toolkits, as respondents may feel that there are greater benefits in identifying and diagnosing people with LBD if they have resources to support better management |
| Time | Impacts on practice and perceptions of the toolkits may change over time, either as a result of successful embedding into practice or because of growing realisation of the difficulties of integrating the toolkits into routine practice |
There were difficulties in examining changes over time, as, although the respondents to the three questionnaires overlapped, they were not identical and only seven individuals completed questionnaires at all three time points. For the analysis of confidence in symptom management (for which information was available at all time points), we maximised the number of observations for analysis by constructing a file containing people who responded to the T0 questionnaire and either the T1 and/or T2 questionnaires (where respondents completed both questionnaires, the T2 response took precedence).
Response rates
Response rates to each questionnaire are summarised in Table 21. Undelivered questionnaires are excluded from calculation of the response rate. Overall response rates were similar for each of the questionnaires (ranging from 37% to 48%). Data from SurveyMonkey indicated that, overall, nearly two-thirds of questionnaires delivered electronically (62%) were unopened, highlighting the need to provide copies of the questionnaire by post as well as e-mail.
| Response | T0, n (%) | T1, n (%) | T2, n (%) |
|---|---|---|---|
| Distributed | 346 | 127 | 145 |
| Not delivered | 0 | 3 | 5 |
| No response/incomplete | 193 (56) | 64 (51.6) | 88 (62.9) |
| Completed | 153 (44) | 60 (48.4) | 52 (37.1) |
Data were available to compare response rates to each questionnaire by region and specialty. There was no evidence of differential response rates by region. Response rates were significantly higher for movement disorder than for memory services at T0 (p < 0.01) and T2 (p < 0.001), but not at T1 (Table 22). Response rates were examined by study arm for T1 and T2 questionnaires. There was no difference at baseline, but a trend for a better response rate from the control arm at T2 (54.5% vs. 31.8%; p < 0.05). This is likely to reflect the unequal distribution of larger teams between study arms.
| Questionnaire returned | T0, n (%) | T1, n (%) | T2, n (%) |
|---|---|---|---|
| Memory services | |||
| Yes | 109 (40.5) | 48 (45.7) | 31 (28.7) |
| No | 160 (59.5) | 57 (54.3) | 77 (71.3) |
| Total | 269 | 105 | 108 |
| Movement disorder services | |||
| Yes | 44 (57.1) | 12 (63.2) | 21 (65.6) |
| No | 33 (42.9) | 7 (36.8) | 11 (34.4) |
| Total | 27 | 19 | 32 |
Characteristics of respondents
Respondent characteristics at each time point are shown in Table 23. Respondents had a range of roles, although the majority were either consultants or nurses. To facilitate subsequent analyses, role was recoded into doctors (including trainees) and allied health professionals (including nurses).
| Characteristic | T0 (N = 153), n (%) | T1 (N = 60), n (%) | T2 (N = 52), n (%) |
|---|---|---|---|
| Sex | |||
| Male | 53 (34.6) | 20 (33.3) | 22 (42.3) |
| Female | 100 (65.4) | 40 (66.7) | 30 (57.7) |
| Specialty | |||
| Memory service | 110 (71.9) | 48 (80) | 31 (59.6) |
| Movement disorder service | 43 (28.1) | 12 (20) | 21 (40.4) |
| Role | |||
| Consultants and trainees | 72 (47.1) | 29 (48.3) | 31 (59.6) |
| Nurses | 56 (36.6) | 28 (46.7) | 18 (34.6) |
| Others | 25 (16.3) | 3 (5.0) | 3 (5.8) |
| Region | |||
| East Anglia | 104 (68.0) | 34 (56.7) | 24 (46.2) |
| North East | 49 (32.0) | 26 (43.3) | 28 (53.8) |
| Study arm | |||
| Intervention | NA | 44 (73.3) | 34 (65.4) |
| Control | 16 (26.7) | 18 (34.6) | |
At T0, information was collected on experience of working with patients with dementia and/or PD. This varied from < 1 to 40 years, with an average of 13 years’ experience with dementia (median 12 years) and just under 10 years’ experience of working with PD patients (median 9 years). The T1 and T2 questionnaires asked more specifically about the training in LBD received by respondents. The response categories were chosen on the basis of comments made by interview participants that emphasised the value of exposure to LBD through working with experts in the field. Data were combined to explore training received by each unique participant to T1 and/or T2 questionnaires (n = 80). A series of binary variables was constructed and we explored whether or not training varied systematically by professional background (doctors vs. others), specialty (movement disorder vs. memory services), region (East Anglia vs. North East) or study arm (intervention vs. control). Overall, around one-third of respondents (33.8%) had received only basic training, a similar proportion (32.5%) reported self-directed study (through reading or attendance at conferences) and nearly half (45.0%) had worked with a team with expertise in LBD (the totals do not sum to 100, as some respondents reported both self-directed study and working with an expert team).
No differences in training were found by specialty or study arm. Respondents in East Anglia were more likely to report only basic training than those in the North East (43.2% vs. 22.2%; p < 0.05). Although working with an expert team was not associated with professional background, doctors were more likely to report self-directed study and less likely to have received only basic training (Table 24). The findings suggest that those completing and returning the questionnaires may have considerable knowledge of and expertise in LBD, and highlight the need to provide additional training on LBD for non-medical staff.
| Training in LBD | Doctors, n (%) | Non-medics, n (%) |
|---|---|---|
| Basic training only | 7 (17.5) | 20 (50) |
| Self-directed study, conferences and/or LBD research | 23 (57.5) | 3 (7.5) |
| Worked in a team with LBD expertise | 16 (40.0) | 20 (50.0) |
| Total | 40 | 40 |
Confidence in diagnosing selected conditions (T0 only)
A seven-point scale was used to assess confidence in diagnosing and managing a range of conditions commonly seen in memory and/or movement disorder services. A not applicable column was included, as some staff were not responsible for making diagnoses or for managing certain conditions (the number of respondents therefore varies for different conditions). Figure 7 presents the mean level of confidence (where 1 = low and 7 = high confidence).
FIGURE 7.
Confidence in diagnosing selected conditions by specialty at T0 (n = 98–120). *p < 0.01, **p < 0.001. AD, Alzheimer’s disease; FTD, frontotemporal dementia; HD, Huntingdon’s disease; MS, multiple sclerosis; PSP, progressive supranuclear palsy; VaD, vascular dementia.
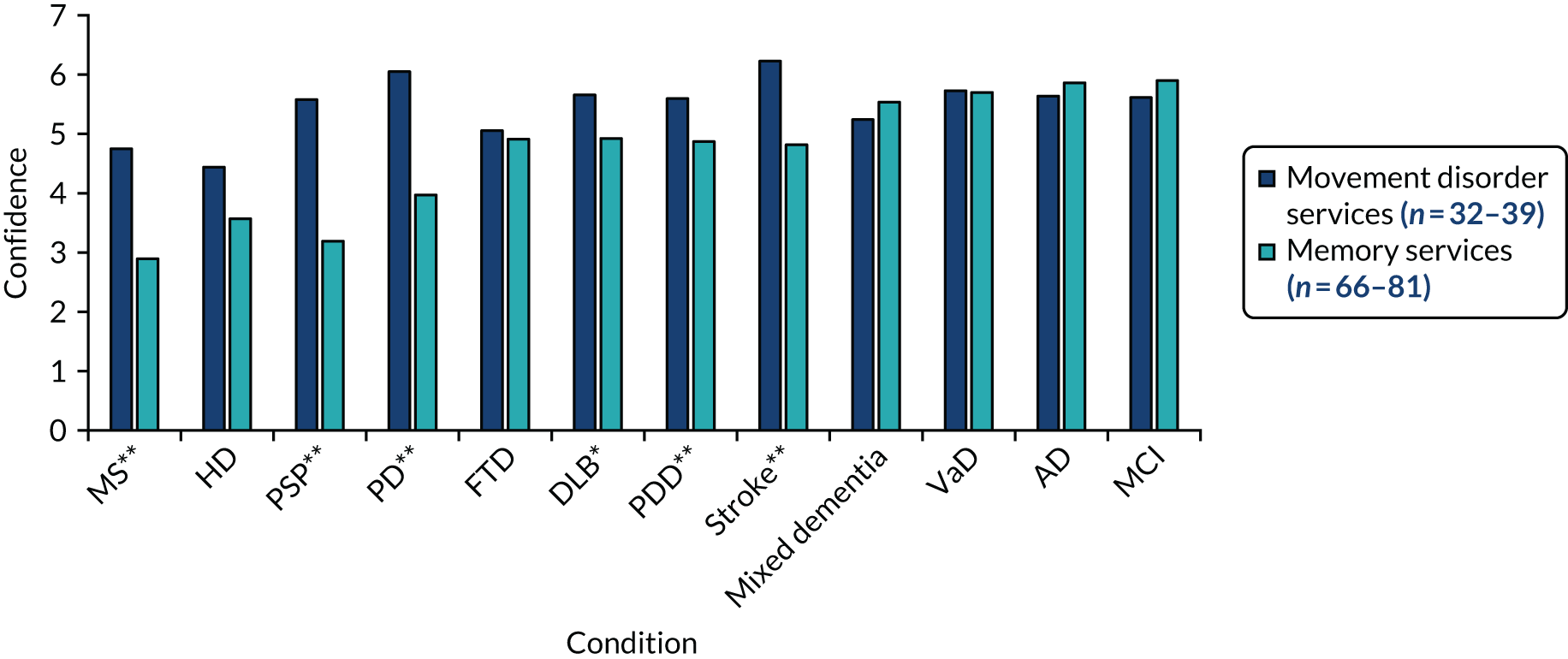
With the exception of FTD, clinicians were, generally, less confident in diagnosing DLB and PDD than other types of dementia. There were significant differences between specialties in confidence in diagnosing six conditions (see Figure 7). Greater confidence in diagnosing predominantly physical conditions would be anticipated for staff in movement disorder services (i.e. multiple sclerosis, progressive supranuclear palsy, PD and stroke, all p < 0.001). Staff in these services also rated their confidence higher in diagnosing both PDD and DLB than those in memory services (p < 0.001 and p < 0.01, respectively).
Confidence in managing selected conditions (T0 only)
Parallel analyses were conducted for confidence in managing the same set of conditions (Figure 8). Significant differences between specialties were generally as would be anticipated. Professionals in movement disorder services were more confident in managing more physical conditions (multiple sclerosis, p < 0.01; progressive supranuclear palsy, PD and stroke, all p < 0.001), whereas those in memory services were more confident in managing some dementias, including frontotemporal dementia, mixed dementia, Alzheimer’s disease and MCI (all p < 0.001). Staff in both specialties reported similar levels of confidence in managing DLB and PDD. Examination of confidence in managing symptoms common in these conditions suggests a possible explanation for this.
FIGURE 8.
Confidence in managing selected conditions by specialty at T0 (n = 130–150). *p < 0.01, **p < 0.001. AD, Alzheimer’s disease; FTD, frontotemporal dementia; HD, Huntingdon’s disease; MS, multiple sclerosis; PSP, progressive supranuclear palsy; VaD, vascular dementia.
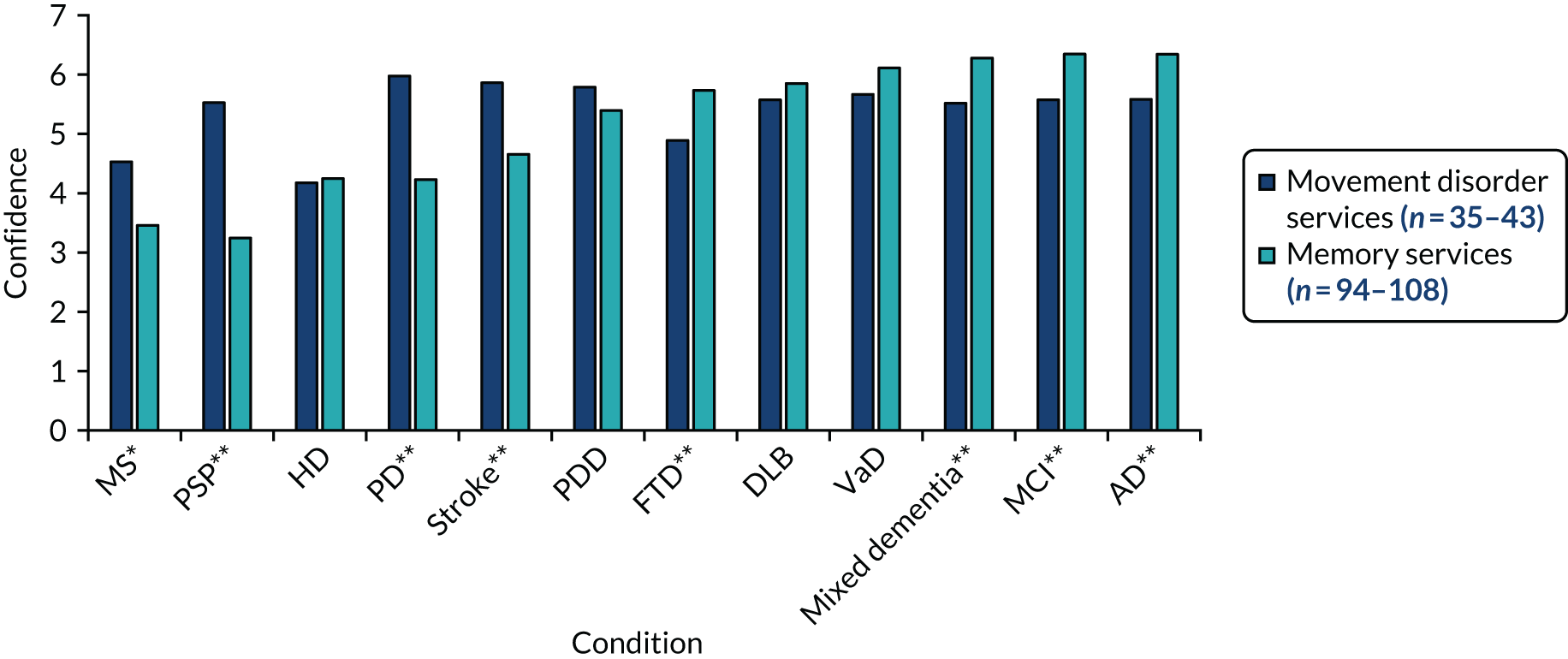
Confidence in managing symptoms common in Lewy body dementia (all time points)
The questionnaire also included a section on confidence in managing common symptoms in LBD (again rated on a seven-point scale). Figure 9 shows that both specialties have areas of expertise, but neither are confident in managing the full range of symptoms associated with LBD. The pattern of responses underpins the dual pathway experienced by many people with LBD.
FIGURE 9.
Confidence in managing common LBD symptoms by specialty at T0 (n = 136–150). *p < 0.01, **p < 0.001. RBD, rapid eye movement sleep behaviour disorder.
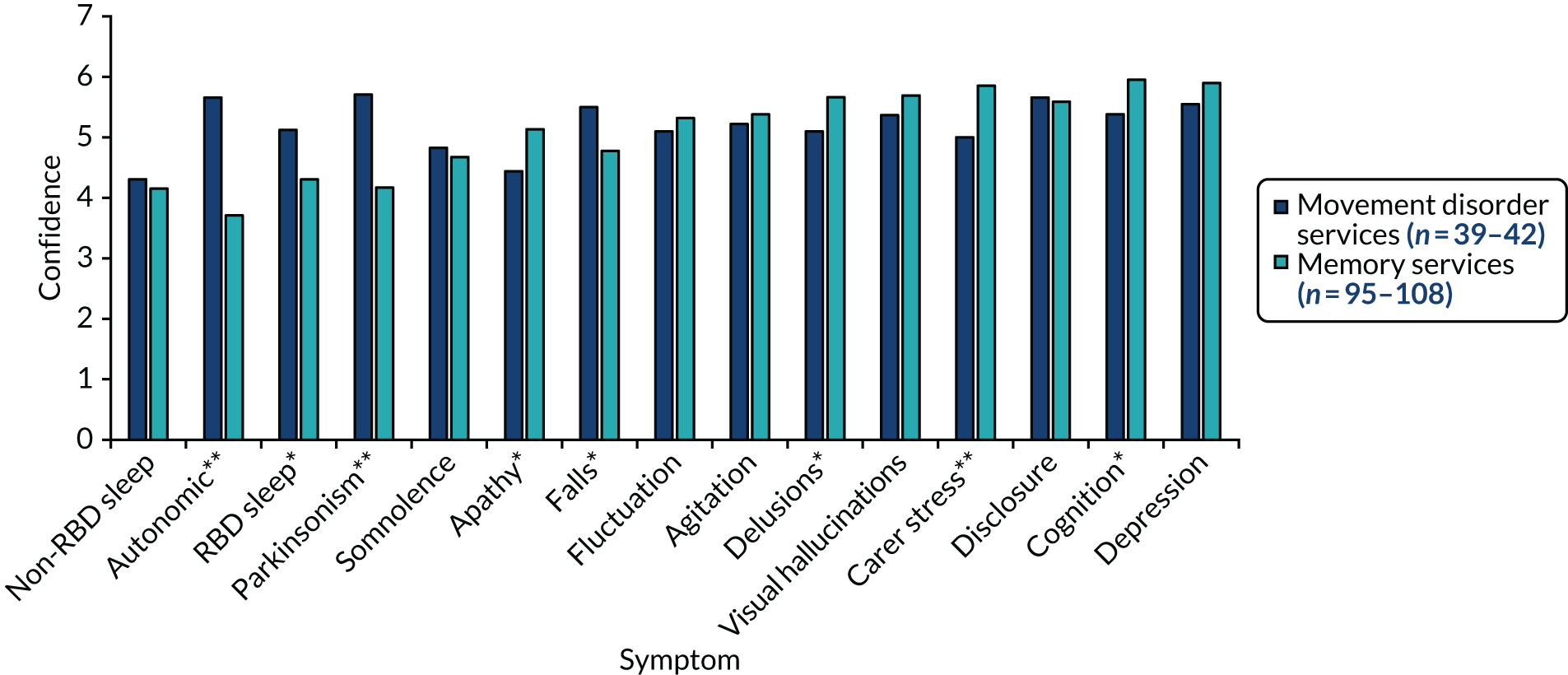
As this set of questions was included in all three questionnaires, responses at T0 were compared with those at either T1 or T2. Radar plots were used to illustrate changes in confidence in managing common LBD symptoms between the beginning and end of the programme for respondents in the control and intervention arms (see Figures 5 and 6). The plots indicated that confidence in symptom management reported by staff in intervention services later in the programme was significantly higher for all but three symptom areas than at the beginning of the programme. For these figures, we have included changes significant at a p-value < 0.05, as these suggest a trend towards greater confidence in some areas. Although, as previously emphasised, interpretation is hindered by the lack of matched samples, these findings suggest that including questionnaire data may help in understanding the mechanisms through which changes occur, particularly if larger samples are available with more consistent respondents at both time points. The findings lend some support to the hypothesis that the positive outcomes achieved in the pilot trial may result from the accumulation of marginal gains.
Approach to new assessments (T1 and T2 only)
The questionnaires administered during the pilot trial asked respondents how frequently they explored key LBD symptoms, completed formal cognitive assessments and used formal diagnostic criteria. These items related to the content of the assessment toolkits. As the focus of the toolkits varied for clinicians in movement disorder and memory services, the findings are presented by specialty. Analysis of variance indicated no significant changes between responses to T1 and T2 questionnaires relating to new assessments. Figure 10 therefore presents data from only T1, which indicated that staff working in movement disorder services more consistently reported exploring parkinsonism and REM sleep behaviour disorder, whereas those in memory services were more likely to report using formal cognitive tests and applying diagnostic criteria.
FIGURE 10.
Percentage of T1 respondents reporting always covering specific areas in new consultations by specialty. **p < 0.001.
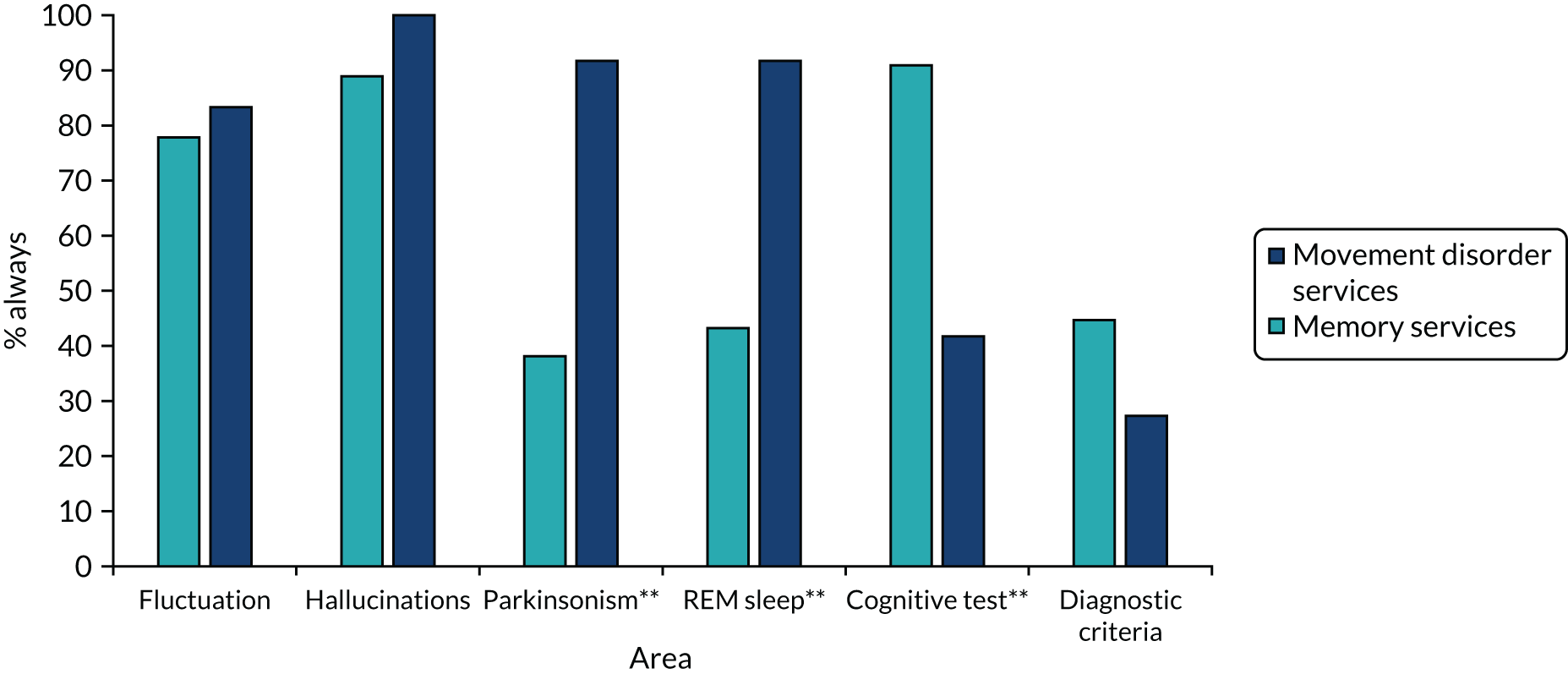
These results suggest that, despite the availability of the assessment toolkits, staff working in memory services were not routinely exploring all key features of DLB in new patient assessments. With respect to diagnosing PDD, it would not be anticipated that formal cognitive testing would be conducted at every new consultation, as clinicians often emphasised that there was insufficient time and that it was not always appropriate to look at cognition in the first appointment. In the absence of data on practice prior to the introduction of the assessment toolkits, it is difficult to interpret these results.
Integration of the toolkits into routine practice (T1 and T2 only)
Questions related to NPT explored the perceived value of the toolkits, whether or not participants believed that the toolkits were a legitimate part of their role, ease of integrating the toolkits into their work and whether or not they thought that the toolkits would become part of their normal practice. Parallel questions were asked about the assessment and management toolkits, with responses on a seven-point scale (where higher scores indicate greater agreement with each statement). Responses from T1 have been coded into three groups (agree, neutral and disagree) in Figure 11. Views on the assessment and management toolkits were generally similar, although respondents had slightly more positive views about the management toolkit becoming part of their normal practice. Only one person disagreed with any of the items, and this was on the item relating to whether or not the assessment toolkit was likely to become part of their normal practice.
FIGURE 11.
Views on integration of the management and assessment toolkits into routine practice at T1. AT, assessment; MT, management.
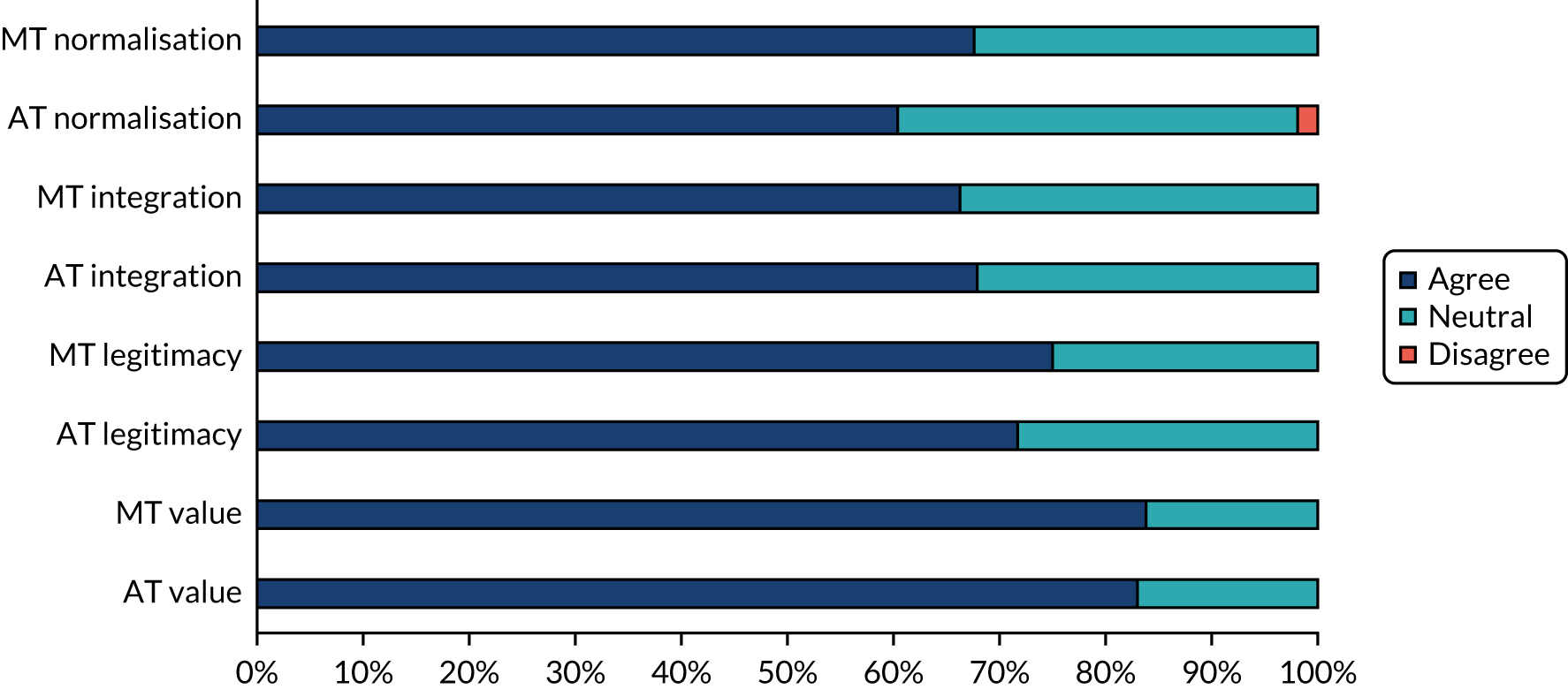
Further analyses explored whether or not views on integration of the assessment toolkits were related to role, specialty and study arm or changed between T1 and T2. There were trends for views on implementation to become less positive over time and for allied health professionals to express less positive views on whether or not the assessment toolkit was a legitimate part of their role (both p < 0.05). The mean values on the seven-point scale for each NPT item by study arm and specialty are shown in Figures 12 and 13.
FIGURE 12.
Mean scores on NPT questions relating to the assessment toolkit by study arm (T1). *p < 0.01, **p < 0.001.
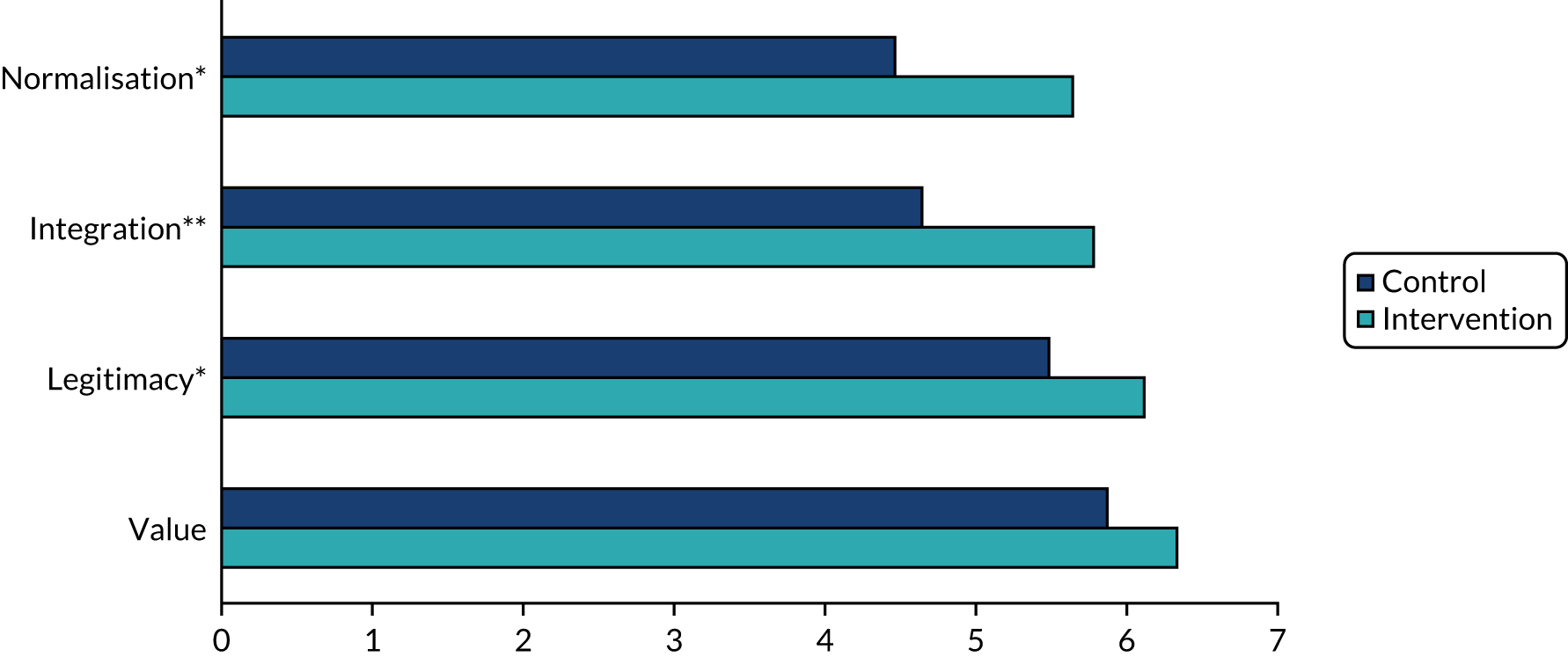
FIGURE 13.
Mean scores on NPT questions relating to assessment toolkit by specialty. *p < 0.01, **p < 0.001.
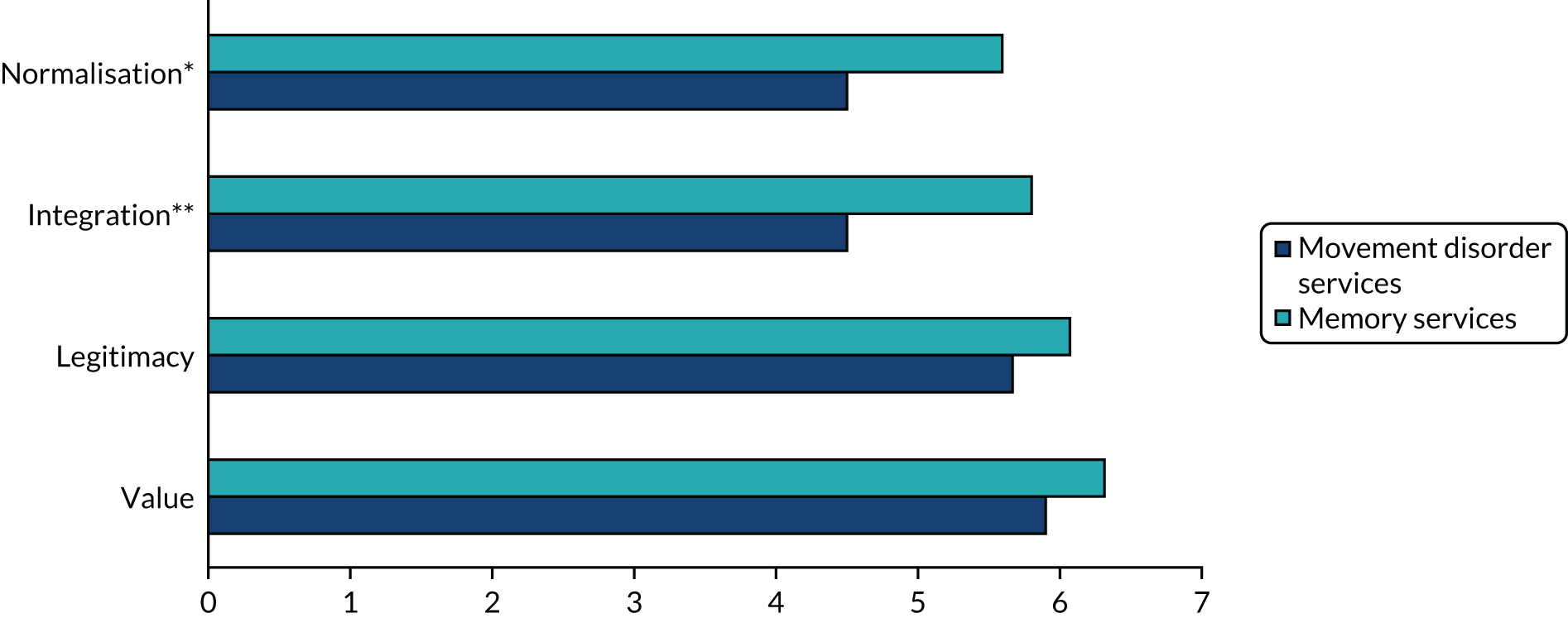
Respondents in the intervention arm generally held more positive views towards implementation of the assessment toolkits than those in the control arm. Although rarely explicitly mentioned during interviews, the availability of the management toolkit may have created more positive views towards accurate diagnosis of LBD and, therefore, towards the assessment toolkit. Respondents in memory services had more positive views towards implementation of the assessment toolkits than their colleagues in movement disorder services (see Figure 13). These findings are consistent with the views expressed in qualitative interviews when professionals in movement disorder services often expressed concerns over the feasibility of implementation of the assessment toolkits.
Parallel analyses were conducted for the NPT questions about the integration of the management toolkit. There were no significant differences in views expressed according to region, role or specialty (study arm was not relevant, as only those in the intervention arm received the management toolkit).
Overall views on toolkits (T2 only)
On the final questionnaire, we asked if and how the toolkits had an impact on the respondents’ work. These questions were included in only the intervention questionnaire (n = 34). Six respondents had not used the assessment toolkits (17.6%), half reported that it had changed their approach to new or follow-up assessments (50.0%), five were unsure (14.7%) and the remaining six felt that it had not changed their approach (17.6%).
Three respondents did not answer the question relating to the impact of the management toolkit and nine reported that they had not used it. The remaining respondents had most commonly used the management toolkit for reference (19/22, 86.4%), with smaller numbers using it to explore new treatment options (9/22, 40.9%) or with trainees (8/22, 36.4%). (As respondents could use the toolkit in more than one way, the percentages do not sum to 100.)
Comments on open questions (T1 and T2 only)
Overall, just under half (45.5%) of respondents made at least one comment on either the T1 or the T2 questionnaire. Analysis of the comments indicated that they were consistent with the analysis of qualitative interviews and focus groups. As some questionnaire respondents were based in services where it had not been possible to conduct any observation or interviews, this provided useful triangulation of the qualitative analysis from new perspectives.
Summary
Response rates to the questionnaires were acceptable and staff with a variety of roles took part. The large number of questionnaire invitations left unopened when delivered electronically suggests that both electronic and paper copies of questionnaires should be sent to maximise response rates. The findings from the T0 questionnaire confirm that confidence in diagnosing and managing DLB and PDD is lower than for most other subtypes of dementia. This supports the need for assessment and management toolkits. Confidence in managing common LBD symptoms varied according to specialty and underpinned the dual pathway, with referrals to both movement disorder and memory services, experienced by many people with LBD. Consistent, small but (statistically) significant changes in confidence in managing these common symptoms were found between the first questionnaire and those administered during or after the pilot trial for respondents based in services that received the management toolkit, lending support to the hypothesis that the potential mechanism for change is through the accumulation of marginal gains. Caution is, however, needed in interpreting differences over time, as the respondents at each time point are overlapping, but not identical.
Appendix 8 Factors influencing the diagnosis and management of Lewy body dementia: illustrative quotations from work package 5
Quotations from interviews and focus groups shown in italics and identified by a unique speaker identifier. The first two digits indicate the service (memory services are represented by 01–16 and movement disorder services by 17–26). Services 01–08 and 17–21 are in East Anglia, and services 09–16 and 22–26 are in the North East. The third and fourth digits indicate the individual within the service.
| Theme | Subtheme | Illustrative quotation |
|---|---|---|
| Complexity of LBD | Variability in presentation | The fluctuating nature of Lewy Body, I think, meant that when he [consultant] saw her – his ward rounds were in the morning – [. . .] he found that she was pretty good and quite plausible. Then, you’d see her later in the afternoon or evening and she’d be struggling2307, trainee geriatrician |
| Lack of a definitive test and restricted access to scans | The only thing we can’t do DaTscans. I mean for DaTscans we probably have to send it to [hospital 1]. I mean to be honest, I don’t use DaTscan a lot. I’ve probably one done one DaT for the last 3 years2001, consultant geriatrician | |
| Shift to earlier presentation | . . . this is a very special kind of dementia, which is very difficult to diagnose in the first stages of the illness. Because the cognitive function is not that prominent, it’s mostly a sleep problem and falls, unsteadiness. Sometimes, they are almost the same as the Alzheimer’s type [. . .] In the early stages, it’s not easy to diagnose0501, trainee OAP | |
| Availability of an informant | Some people will say ‘yes, I can do everything’ and you’re pretty sure they probably can’t but, again, it’s difficult to know what they’re doing for themselves [. . .] it’s quite tricky with that generation as well, with older people. The way their relationships were set up, often women did do everything around the house. Often, retired older men don’t do masses of activities of daily living that you can easily assess2307, trainee geriatrician | |
| Service organisation | Shift to nurse-led memory services | For a start, who are we asking to make the diagnosis of our patients? Is it people who are properly trained and who have good clinical expertise, who’ve seen a range of different dementias? Or is it people who are trained, who have had exposure to, maybe, lots of Alzheimer’s disease? And their clinical repertoire is very narrow0901, consultant OAP |
| Follow-up arrangements | Let’s say you have a follow-up review, you have 15 minutes, roughly. All the other things you have to address and then how do you in that time say ‘oh, by the way, I think you have got dementia?’ It is a huge thing to be diagnosed with dementia, a massive thing. So I think you don’t do people justice just throwing it in on the way out ‘you have got dementia’. So very rarely do people do that in that setting2306, trainee neurologist | |
| Training, knowledge and experience | Awareness of LBD and cognitive biases | I think also one of the problems is that when we think about dementia we would – you’re never surprised of a symptom, we put everything down to dementia. So a lot of the things that you see there you just say, ‘Well it’s their dementia’. And you may not pick out some of those little nuances of Lewy body0208, community worker, memory service |
| If and how LBD symptoms were covered in consultations | I’ve never really clearly understood what the nature of the fluctuation is despite talking to various people and trying to read up about it, because everybody’s cognitive fluctuation, you know, everybody’s cognitive function fluctuates a bit, [. . .] is it a kind of regular fluctuation pattern, but how quickly as well? Are you talking days, or weeks, or hours?1402, consultant OAPI always ask ‘Have you noticed any shakiness or tremor?’ So that’s a question I always ask and I’m always observing for that as well0403, memory assessment nurse | |
| Interpreting cognitive tests | I also think that differentiating between MCI and dementia is something that people find more difficult to do, so what we may find in the notes is that a lot of people on their problem list have Parkinson’s disease, cognitive impairment. But those people who have cognitive impairment listed could have an MMSE anywhere from 24 to 122201, consultant geriatrician | |
| Knowledge of dementia subtypes | Unfortunately is quite difficult to characterise, whether it’s PD dementia or Lewy body dementia or it’s Alzheimer’s dementia with Parkinson’s1701, consultant geriatrician | |
| Attitudes and values | Perceived prevalence and status of LBD | I don’t think we see that many people with Lewy body dementia, which might suggest that it’s underdiagnosed or else they’re just not coming through to our service. And I wonder whether some of them might be going to neurology instead0209, community worker, memory service |
| Perceived value of diagnosing LBD | I’m sure a psychiatrist will say ‘we must know what every sort of dementia is’. You know, and perhaps we should. They’re probably right, but I’ve never felt it makes a great deal of difference to the patient really. [The patient] doesn’t care whether she’s got Lewy body or Alzheimer’s or whatever else really2401, consultant geriatricianI guess it [disclosing dementia] gives them a rough idea how the disease might progress of how their mental function might progress in the next few months so that they’re aware of how things might go, as well as the carers and their family, explain that there are possible drugs, if they meet the criteria, to go on temporarily, may to halt progression of the cognitive impairment for some time. It may not improve things, but it might hold the decline of it. Also, I guess once you label people with dementia they’re more entitled to some of the support services and benefits and allowances and things like that1701, consultant geriatrician | |
| Perceived responsibility for diagnosing LBD | But you still want the most authoritative person [. . .] Being in the medical paradigm everything goes to the diagnosis. So what you want is the person who is going to give you a diagnosis and then suggest what to do2402, consultant geriatrician. . . you have fantastic movement disorder people and then just a few rooms further down are fantastic dementia people, but the overlap is still not good there. There are not many, if any at all, movement disorder people with an interest in dementia and vice versa2306, trainee neurologist | |
| Perceived value of diagnosing dementia subtypes | I suppose the other practical thing as well, what difference does it make? And actually it doesn’t make that much difference, if it’s either Lewy body disease or Alzheimer’s, because you’ll be treating them pretty much the same way anyway1402, consultant OAPThere’s not that many common dementias, we should at least be able to diagnose the most common three or four, shouldn’t we?1601, consultant OAP |
| Theme | Subtheme | Illustrative quotation |
|---|---|---|
| Complexity of LBD | Balancing treatments | I think it’s getting the balance between the cognitive and motor issues. So cutting back on the motor treatments and the balance between that, hallucinations and the cholinesterase inhibitors2305, consultant geriatrician |
| Service organisation | Follow-up arrangements | The way things are at the moment, with the changes to monitoring as well, people are being discharged much more early to GPs than they used to be. We used to have 6-monthly monitoring to go on indefinitely and now it’s that people are discharged back to GP0501, trainee OAP |
| Fragmented care pathways | From a technical, diagnostic and pharmacological, perspective, I am happy to diagnose and manage both DLB and PDD. The difficulty that I have, or where I come unstuck, is if I do that I then, quite often, can’t get patients into community mental health services because they’ve been furnished with a diagnosis and therefore, the piece of work is done. It takes longer to get them into services if there are crises, which clearly there quite often are, from a psychological perspective. So I can’t, then, get access to Community Mental Health Team, CPN [community psychiatric nurse] support, medical support for those individuals. If I make that diagnosis, and manage that diagnosis initially, they don’t get the other softer stuff around the dementia diagnosis that community mental health teams are commissioned to provide2703, consultant geriatrician. . . it becomes a lot harder to coordinate care for really complex people who need their care coordinated. What happens then is a little bit of indecision, because nobody wants to take the wrong step if they don’t know what either side is doing2301, trainee geriatrician | |
| Skills, training and knowledge | I haven’t got enough knowledge about sleep hygiene. I mean, I can offer basic things about people trying to avoid sleeping through the day and taking a regular pattern, but I don’t think I have enough knowledge to advise people on a sleep hygiene0503, specialist PD nurse | |
| Attitudes and values | Perceived responsibility for managing LBD | But obviously DLB – the thing is, a lot of people are on things like anticholinergics for urinary dysfunction. It’s not up to us to avoid centrally acting anticholinergics because they’re always started by other people, by urologists. It’s not us, we never start them, but we come across people on them0404, consultant OAP |
Appendix 9 The acceptability of the assessment and management toolkits: illustrative quotations from work package 5
| Theme | Comment | Illustrative quotation |
|---|---|---|
| Layout and presentation | General appearance | I think it’s [assessment toolkit] quite nicely formatted and visually it doesn’t look too crowded or too dense. So I think that’s always important [. . .] it’s not one of those annoying pieces of paper that you feel ‘oh, I can’t even read what is written here’. It’s very clear and easy to go through. It sort of flows naturally0601, consultant OAP |
| Signposting and rationale | I could be doing things that aren’t necessary, potentially [. . .] I could literally trim it down to these [five-item UPDRS] and these are the ones that are the most evidence based as being the most discriminatory [. . .] That’s a helpful thing to know1213, GPSI, memory service | |
| Format | Integration with existing pro formas | Then there’s a question of, for any toolkits, remembering where they are because they’re not incorporated into our computer system. If I complete a toolkit, I’ve got to have a pen with me because I would have to do it as a paper version. What do I then do about getting it into our computer system? I’m completing the questions I want to ask, which I want to be in the computer system1801, consultant geriatrician |
| Local intranet | . . . if the assessment toolkit was made available on, say, the shared drive where people could access [it]0601, consultant OAP | |
| A self-completion version for patients and carers (PDD toolkit only) | I guess that would be one option, just screen everyone, hand them out while they wait. Give them those things so you have got something when they walk in, you can check already, ‘OK, you don’t have to worry about it’. Or I do have to worry about it and dig in a bit further2306, trainee neurologist | |
| Brief laminated summaries of sections for reference | We thought about making a smaller thing that we could cover in our assessments and then that was always something you could pull out1104, community psychiatric nurse | |
| Separate versions for medical and non-medical staff | I’m just thinking that the therapists ask a lot of the questions, and if we pull the nursing assessment to pieces and put that in place1708, specialist nurse, movement disorder service | |
| Content | Perceived relevance of content | And I guess the other reason is that we might not always recognise them. So, we might be seeing someone with a different type of dementia but we wouldn’t know. So, I thought it would be a really good tool for us to remind us of the diagnostic criteria, what questions to ask0101, consultant OAP. . . there is nothing in here [toolkit for movement disorder services] that mentions hallucinations, or the fluctuating nature. And actually, I think we might, if we’d used this we might have missed that2403, specialist PD nurse |
| Terminology and question wording | With the hallucinations at the moment, all you have to those two questions to the information is ‘yes’ or ‘no’ answers. There’s a thing about the frequency, the duration, the distress. There’s just something else which might give you a feel for how significant this area is. Because my understanding of Lewy Body disease is that [. . .] it’s not just the presence or absence of hallucinations; it’s the duration of them, it’s the frequency, it’s the intensity of those1301, consultant OAPWe probably wouldn’t ask ‘Does the patient show moderate changes in their level of functioning during the day?’ Not with that sentence. People wouldn’t know what it was on about0901, consultant OAP | |
| Inclusion of five-item UPDRS (memory services) | I think that is useful in terms of awareness. Because a lot of people, certainly in my team I think, could spot parkinsonism from a tremor point of view, but might not have necessarily from the bradykinesia and the facial expression. As being as important as tremor. I think that’s the message, isn’t it? Parkinson’s disease isn’t just about tremor. So I think that there is an awful lot of value in this0901, consultant OAPIf you think there’s Parkinsonism, it doesn’t matter whether it’s mild or severe, does it, for DLB? Do you see what I mean? Does it matter? Do you need to go into that amount of detail? It’s just whether it’s there or not [. . .] Because we’re not Parkinson specialists, we just need to know whether there is enough to meet the diagnostic criteria for DLB not Parkinson’s1503, consultant OAP | |
| Inclusion of formal cognitive assessment (movement disorder services) | I have yet to be convinced that a nurse with good instinct won’t pick it up, as part of her general assessment. It’s difficult to know what would be gained by routinely doing it because most patients, although I dare say the Carslake study and everything after it showed you get very high cumulative instance of dementia in Parkinson’s, it’s not everybody. I think they could pick it up instinctively first, rather than screening2501, consultant neurologistSo, briefly, I’ll probably do an AMTS [Abbreviated Mental Test] and I do a clock drawing and an intersecting pentagon, just to cover, get a bit of visuospatial things. So, that’s why I do the clock drawing, clocks and a pentagon thing. And with the AMTS, I know the AMTS is rubbish in Parkinson’s. It’s not going to give us anything, but I don’t have time to do a detailed thing2001, consultant geriatrician |
| Theme | Comment | Illustrative quotation |
|---|---|---|
| Layout and presentation | Use of colour and ability to access information at different levels was valued | I think the fact that it’s broken down into three levels in itself is a help, because if we’d gone straight to the tome [reference guidelines] it would absolutely go over my head, but this breaks it down quite well1106, community psychiatric nurse, focus groupThe colouring is terrible. People have problems with eyesight. Sorry but the contrast is not so good0501, trainee OAPWe need to look at everything. When you go out to see someone in the community, they’re going to describe to you something that they’ve noticed or they’re concerned about and it could be anything. So we don’t necessarily have the answers, but if you can look at this [one page summary] and say ‘Oh actually, with Lewy body dementia, that’s quite a common sort of symptom’. To me, then I’d find that really useful0710, social worker, memory service |
| Format | Integration with existing electronic records | In an ideal world, you’d be seeing somebody with Lewy body in clinic, and there would be a link from their patient records to say, ‘Your toolkit’, so that you can just click on it0701, consultant OAP |
| Access via computer or application | I guess if you put it on the computer, you can sort of look at things while you are talking to someone without it being so in your face, if it is easy to access2302, trainee neurologistHaving availability of guidance on the management tool I see would fit potentially quite nicely into the sort of guidance that we have on our intranet and put into bite-sized chunks1801, consultant geriatrician | |
| Small A5 versions for portability | The only thing is perhaps the size of it. Maybe if you could make it kind of pocket size, so we can carry it around0702, consultant OAP | |
| Tailored versions for medical and non-medical staff | I think maybe splitting it and having one that was more about the management role of CPNs [community psychiatric nurses] and one that was more tailored to the medics1104, community psychiatric nurse | |
| Version for patients would be useful | I think it would be helpful to have something that you could give to the patient as well, not just something we can use for our own things to ask, but actually something you could physically give to the person as well to go through and explain it a bit more. So rather than it just being something that we can be aware of, ask and talk about, but something that we can actually give out as well1103, community psychiatric nurse | |
| Relevance to other settings | Where I most commonly find mismanagement of Lewy body disease is in primary care [. . .] I think the people for whom computerisation would be especially important, would be in GP surgeries, where, when they are seeing someone with Parkinson’s disease, flagging up the things and the different pages that they need to be mindful of1101, consultant OAP | |
| Content | Perceived relevance of content | But then they probably go out of date quite quickly, so you’d need an online version to keep up to date with it. That would be my only concern, because treatments change1501, consultant OAPThere’s no point saying there’s no evidence for it because if somebody’s got low mood, you’re going to give them something. You can’t ignore it. I’m not going to say to the patient, ‘There’s no evidence for use of antidepressants’, because if it’s really impeding their function and causing distress, I will treat [. . .] It’s about alleviating their distress and their symptoms. I don’t know that it’s that helpful. You might want to say, ‘If using antidepressants, just avoid significant anticholinergic things’1503, consultant OAPThey seem to push melatonin for poor sleep. Firstly, GPs are not familiar with melatonin [. . .] A lot of people will start feeling sick, elderly people feel sick on it and can’t tolerate it. So you have to warn them that might happen. Because it says, given the relatively benign side effect profile, but in this high dose, it does make them nauseous [. . .] I can’t get to grips with melatonin. I have tried it several times and everybody seems to come back sick [. . .] But maybe because we are working with people who are frail1901, consultant geriatrician |
| Appropriate for other professionals | I think they are something for a GP, for somebody that is more involved in the long-term management, would be helpful [. . .] I think that’s quite educational for GPs as a whole, that it affects sleep, it affects hypotension, constipation, urinary dysfunction, all these sorts of things that you expect sometimes to happen in the elderly, but you wouldn’t necessarily put together as being part of the dementia syndrome1213, GPSI, memory service | |
| Dosage information | I think it’s excellent and I think it’s really useful, especially the more detailed ones actually. I like the fact that they actually tell you doses to use as well, which I think most guidelines don’t. Most guidelines shy away from actually saying, ‘Use this specific dose’, or, ‘Between these doses’. [. . .] Actually, people who use things want to be told what they can use and have some support. The doses are very similar to what I’d use. It gives you that additional confidence that you’re using the right doses1601, consultant OAPI know we’ve got the BNF and everything but that’s really just quite dry. I think you’d need something a little bit more practical, for example, maybe an information sheet that you could give to patients about melatonin so that both you and they know what to expect from it. Also, just some specific advice on dosage and when to review somebody, how long do you wait for the therapeutic effect to kick in, etc., etc. Yes, I think a well-designed information sheet for using melatonin in DLB would help1213, GPSI, memory service |
Appendix 10 Implementation of the assessment and management toolkits: illustrative quotations from work package 5
| NPT construct | Illustrative quotation |
|---|---|
| Coherence | For me, the toolkit is a really good idea that we’ve got some commonality that we all should be asking the same questions. We should all be working towards the same guidelines [. . .]2602, specialist PD nurseI thought it was very useful because as part of our training we have a lot of exams, a lot of basic knowledge and then a lot of practical knowledge, which we gain by experience or seeing other people. But to gain this practical knowledge, there is no specific way of doing it because it is different reading in books and its different doing the things. I think those sorts of toolkits are in the middle. They bring the criteria to day-to-day practice, so I find them very useful0102, trainee OAPBefore I actually saw the paperwork of the toolkit, I thought it was a good idea, because I think it’s something that people often make errors in diagnosis with. Either they can be too keen and any patient who presents with confusion and hallucinations, people sort of start thinking ‘oh, could this be Lewy body dementia?’ Or, sometimes, equally it gets completely missed because people haven’t asked all the questions. So I did think it would be quite useful, but then, I suppose when I actually looked at the toolkit for assessment, I felt that it didn’t add anything to what I already knew. But it was useful in the sense of there being a checklist and that you’re forced to go through all of the items and you’re then sure that you don’t miss anything0601, consultant OAP |
| Cognitive participation | I think that the toolkits are potentially useful for people who don’t feel so confident [. . .] as a guide for them as to sorts of questions they might need to be asking. As somebody who feels confident, my view on the toolkits has been I can see how they can be useful for others. They don’t necessarily help me that much because I think they cover all of the questions that I think I’m asking but I don’t necessarily ask them in the way that they’re written in the toolkit1801, consultant geriatricianI run the clinic with my registrar, it was easy to train him quite quickly [. . .] it was easy to get my specialist nurse up to speed [. . .] We are kind of all united on this assessment tool, so we don’t have to discuss and everybody has different opinions [. . .] it is ultimately uniting the approach in making assessment more straightforward1901, consultant geriatricianI think it’s difficult when we’re not getting referrals for Lewy body dementia every week, I think that makes it more difficult to keep your interest and momentum going1106, community psychiatric nurseWhat I would say in retrospect, is I’m not sure that we’ve maximised the value of this within the service. But to do that, we would have actually needed a more sustained input, I think, from the team. And somebody, one or two of us, actually, really taking a role in terms of getting hold of this and running with it. You, actually, need somebody in the team who is going to take a real lead to try and sort of keep it on people’s minds. Find out what people’s experience was, address any issues, advocate for its value, which, to be fair, I don’t think there was somebody who took on that role1209, consultant OAP |
| Collective action | Unless it’s incorporated into our computer system it adds another level of complexity, which is just another barrier for me1801, consultant geriatricianIt is just a bit of extra work. I mean just asking a couple more questions and maybe getting into the habit of doing a physical examination for all patients1505, trainee OAPI honestly wouldn’t have the time to go through all the questions. And to be honest with you, most of them, we do ask anyway, like, ‘Do you have hallucinations? Do you have that? Do you have a problem with planning? Do you forget familiar names?’ We kind of ask that anyway, but as a quick screening thing, we just generally go, ‘Oh, yes, any problems with memory?’ and I usually turn to the [patient’s] wife: ‘Have you noticed anything with the memory?’ rather than go, formulaic, through the individual2301, trainee geriatrician |
| Reflexive monitoring | What I’ve been particularly impressed with . . . Because really this is about team work, I very rarely would see the patients independently. Information is presented to me much earlier in the pathway than it would have been before. I’m not having to ask these symptoms, they’re already there. The team are already asking the relatives, ‘What is this patient like when they’re asleep at night? Are they all over?’ They are following and picking up, particularly, those kinds of symptoms0901, consultant OAPPersonally, I think having had this, it’s probably made me document that there’s, like no REM sleep behaviour disorder or no visual. Do you see what I mean? [. . .] And that’s maybe me assuring myself that I’m asking, you know, at a later date, if somebody, you know if they come across this person and wondered, I wonder whether – say they develop DLB later on or something like that and you just think, well ‘How long have they had it?’, then it’s a good account for the future1503, consultant OAPIt improved my understanding of DLB, and maybe I’m thinking more about DLB now. Because I think as a movement disorder service you always tend to focus on Parkinson’s dementia, but now with DLB we’re doing more of DLB now, I would think, or diagnosing more, definitely1701, consultant geriatricianAnd maybe the heuristic that I use should be different. Because like, I said, I do this if I have a suspicion they might have fluctuation, Parkinsonian symptoms, or visual hallucinations. So, if they have none of these, I’m not going to do this form. So maybe – well, I don’t know – I was thinking maybe I should do the form anyway, on everyone, but we don’t. [Later in the interview]. But it’s just a niggling thought in my mind now. Maybe we’re missing some0101, consultant OAP |
| NPT construct | Illustrative quotation |
|---|---|
| Coherence | I think it is, at a glance, by all means it is a very complicated condition with a lot of little branches. Again, none of the textbooks will tell you exactly what medication to use on which condition. Although we were doing very much what it says here, before this toolkit came forward [. . .] this comes in a very supportive way, explaining that you are doing the right thing, this is exactly what we use, so it was very, very helpful1901, consultant geriatricianObviously, coming from a consensus from the best experts available, we trust the material. So it’s definitely very useful [. . .] you regard this as the expert opinion, evidence-based expert opinion. So, you consult it because you are even expecting to find things in it that you wouldn’t find in the BNF, or you wouldn’t find in some of the other sources that you look to usually0101, consultant OAP |
| Cognitive participation | We haven’t got round on our monthly mentoring sessions to specifically doing one where I would have thought about bringing these in. Some of the mentoring sessions have been about some aspects of the management of dementia, but the questions they’ve had have been more about which drugs and why. There have been some questions within that about behaviour, so I probably could have used some of it, now I think back1801, consultant geriatricianI think the first problem is, would we manage them at all? [. . .] because to me conceptually it falls into the province of old age psychiatry2402, consultant geriatricianI think this would be quite useful for juniors. I think our SHOs [senior house officers] would really find this very informative and practically very useful. I think that would be the case, not just at SPR [specialist registrar] level but also the SHOs, VTS [vocational training scheme] trainees that we have, they’d find it very useful. I think nursing staff, community mental health nurses, memory clinics, they’d find it useful just to give a broad awareness of the various symptoms and management. I think they’d find it useful as well1504, consultant OAP |
| Collective action | I think one thing is you know if you’re sort of flicking through sheets and things it looks a bit rubbish and it also kind of looks like you don’t know what you are doing. So, and if they don’t know what the diagnosis is and you’ve got like ‘neuropsychiatric’ symptoms and stuff, then that’s a bit stressful2302, trainee neurologistVery occasionally, when I’ve had somebody, where they’ve had a problem and you’ve tried one or two things, I might just refer to it and say ‘have I done what the toolkit would say?’0701, consultant OAPSo when I’m supervising the trainees, I’m supervising memory clinic nurses and they come to me with a specific problem, then we get this out of the cupboard and have a read and make a decision. Sometimes, but very rarely, when the patient is in the room as well0101, consultant OAP |
| Reflexive monitoring | I suppose I feel like most people did when I first looked at it. You think, ‘Is that what I do? Is that what I not do? Should I do be doing it differently?’1101, consultant OAPIt adds more awareness of other things which might be worrying people, not just cognition and neuropsychiatric and sleep. There are also other things. It sort of gives me an indication of some of the other medical issues which can affect quality of life. I think it also allows me to set the signposts better. [. . .] I could ask the GP to do things a bit more, like constipation and sexual dysfunction, that sort of thing [. . .] I guess it’s raising awareness of it and raising the profile of it1502, consultant OAPLike the melatonin thing, I certainly wouldn’t feel confident about that [. . .] I wouldn’t feel that it is appropriate for me to prescribe it. It might prompt ‘I have heard melatonin might be used sometimes in this situation’2402, consultant geriatrician |
Appendix 11 Proposed implementation strategy for work package 4
| Content | Who | Duration | How |
|---|---|---|---|
| Introduction to the programme, study team and WP4 | WP1 team | 5–10 minutes | Presentation supported by documentation (e.g. PIS) |
| Study background and summary | WP1 team/CTU | ||
| Objectives – cRCT and process evaluation | WP1 team/CTU/Claire Bamford | ||
| What study participation involves: for patients and for clinicians | WS1 team/CTU | 5 minutes | Presentation supported by flow chart(s) |
| Discussion of evidence base for the assessment toolkits and potential benefits of improved diagnosis of LBD | Credible professional | 10 minutes | Brief presentation on process of developing assessment tool and provision of supplementary materials (e.g. references for validated questions included in the assessment toolkits; rationale for recommending MoCA). Case studies to highlight benefits of improved diagnosis |
| Explore potential benefits of assessment toolkits (consistency, validated questions, documenting negatives) | CB (feedback from WS2 interviews) | 5 minutes | Presentation |
| Clarify when assessment toolkits could be used and how clinicians can ensure that the assessment toolkit is readily available (e.g. how copies will get into patients’ notes) | Facilitator | 10 minutes | Group discussion |
| Highlight variances from usual practice and focus on planning how to integrate new aspects into assessments | Facilitator | 10 minutes | Reflection, group discussion to first identify barriers and then strategies to overcome these. Identify additional training needs to be met through web-based materials and/or local training sessions |
| Training on UPDRS and MoCA | Appropriate (local) professional | Separate session if required | Video/demonstration, practice sessions with feedback, supplementary resources (e.g. web-based materials, document with FAQs) |
| Content | Who | Duration | How |
|---|---|---|---|
| Discussion of evidence base for the management toolkit | Credible professional | 5 minutes | Brief presentation on process of developing management guidelines; provision of supplementary materials (e.g. systematic review) |
| Highlight specific issues regarding management of LBD (include feedback from patients/carers regarding unmet needs and worse outcomes) | Credible professional/Claire Bamford | 5 minutes | Summary of literature and case studies |
| Explore how the management guidelines could be used in practice and consider ways of ensuring that the guidelines are readily available (e.g. is it feasible to include copy of the overview diagram in paper notes of patients with LBD?) | Facilitator | 10 minutes | Group discussion: sharing ideas (e.g. use as reference document when writing letters, have a copy of overview diagram in notes of LBD patients to check key aspects covered in consultation) |
| Identify variances from usual practice and problem-solve to overcome potential barriers to use of management guidelines | Facilitator | 15 minutes | Case studies, group discussion (requires time to review the guidelines, identify barriers and strategies to overcome these) |
| Content | Who | How |
|---|---|---|
| Physical prompts: laminated management overview diagram for office wall; DIAMOND-Lewy coasters, pencils, Post-its® (3M, Cynthiana, KY, USA); inserts for diaries with management overview diagram | Project team | Send out at regular intervals |
| Agenda item at regular team meetings to review use and identify problems | Local services | Local team member to liaise with lead clinician to obtain feedback and to attend occasional meetings to problem-solve, address queries, etc. |
| Audit and feedback of use of assessment toolkits, either through small-scale notes review or through case discussions | Local services | Local team member to liaise with lead clinician to check progress, etc. |
| Case-based discussions to discuss use of management guidelines and identify any impacts on management and any queries | Local services | Local team member to liaise with lead clinician to check progress, etc. |
| Resource | Aim | Availability | For |
|---|---|---|---|
| Copy of assessment toolkit | Ensure clinicians are able to access copies as required | ✓ | C/I |
| Copy of management guidelines |
Ensure clinicians are able to access guidelines To incorporate user-friendly navigation system to facilitate use |
✓ | I |
| Clinician PIS for cRCT | Easy access to a reminder about the design of the study and what participation involves | ✓ | C/I |
| Clinician PIS for qualitative study | Easy access to a reminder about the components of the qualitative study | ✓ | C/I |
| Summary of key points from SIV | This may cover the previous two points to identify which resource would be most useful to clinicians | C/I | |
| Videos of UPDRS | To facilitate skill development and provide a reminder if needed | ✓ | C/I |
| Videos of MoCA | To facilitate skill development and provide a reminder if needed | ✓ | C/I |
| FAQs for assessment toolkits | To provide easily accessible information on common questions about how to use the assessment toolkits | C/I | |
| FAQs for management guidelinesa | To provide easily accessible information on common questions about how to use the assessment toolkits | I | |
| Published literature review |
To enable keen clinicians to review source material for guidelines To demonstrate credibility of management guidelines |
✓ | I |
| Copies of training materials on assessment toolkits (e.g. case studies) |
To facilitate further discussion within teams if required To enable clinicians who were unable to attend the briefing/training session(s) to work through the materials |
C/I | |
| Copies of training materials on management guidelines (e.g. case studies) | I |
List of abbreviations
- AMED
- Allied and Complementary Medicine Database
- BNF
- British National Formulary
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CONSORT
- Consolidated Standards of Reporting Trials
- DARE
- Database of Abstracts of Reviews of Effects
- DEMQOL
- Dementia Quality of Life
- DeNDRoN
- Dementias and Neurodegenerative Research Network
- DIAMOND-Lewy
- Improving the DIAgnosis and Management of Neurodegenerative Dementia of Lewy body type
- DLB
- dementia with Lewy bodies
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GP
- general practitioner
- LBD
- Lewy body dementia
- MCI
- mild cognitive impairment
- MMSE
- Mini Mental State Examination
- MoCA
- Montreal Cognitive Assessment
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NPT
- normalisation process theory
- OAP
- old age psychiatrist
- ONS
- Office for National Statistics
- PD
- Parkinson’s disease
- PDD
- Parkinson’s disease dementia
- PMG
- Programme Management Group
- PPI
- patient and public involvement
- PSC
- Programme Steering Committee
- QALY
- quality-adjusted life-year
- QATQS
- Quality Assessment Tool for Quantitative Studies
- RCT
- randomised controlled trial
- REM
- rapid eye movement
- SD
- standard deviation
- UPDRS
- Unified Parkinson’s Disease Rating Scale Part 3
- WP
- work package
- WP1R
- work package 1 repeated
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/pgfar09070).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
