Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0610-10114. The contractual start date was in June 2012. The final report began editorial review in December 2018 and was accepted for publication in December 2020. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2021 Bennett et al. This work was produced by Bennett et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 Bennett et al.
SYNOPSIS
Research summary
During the research programme, we made one significant modification to the original protocol. Specifically, we amended the recruitment strategy in our randomised controlled trial (RCT) (work package 4) after the trial commenced to increase accrual. Our trial opened in October 2015 at six sites in which we aimed to screen for oncology outpatients and for those with pain. We wanted to randomise participants to usual care (UC) or to an intervention that consisted of early referral to palliative care, educational support and electronic pain monitoring. We realised that the design had inadvertently inhibited recruitment and caused trial recruitment to fall behind the target. Our screening process for the trial identified patients with pain who would not have been identified as such in routine care. This screening process alerted the oncology teams, who were then reluctant to enter patients into a trial in which the control arm was to continue with UC (i.e. no specific support). We recognised this issue early and in February 2016 began to discuss amending the design so that all patients identified with pain could be randomised to standard palliative care support or enhanced palliative care support, which included several of our interventions. We moved to secure all sponsor and Health Research Authority approvals as quickly as possible, and the new protocol was in place by 12 April 2016, supported by a new round of site visits for additional training. There was a clear increase in recruitment following the protocol change and sites were much more comfortable entering patients to the new design. The inevitable delays in our project plan necessitated an application for a trial extension, which we discussed with our National Institute for Health Research (NIHR) Programme Grants for Applied Research (PGfAR) manager and which was supported by our independent Trial Steering Committee.
Background
Pain is the most common presenting feature at the diagnosis of cancer and is the symptom that patients with this disease fear most. 1,2 This fear is supported by studies of pain prevalence and intensity. A systematic review by van den Beuken-van Everdingen3 showed that pain prevalence rises with disease progression and that pain affects approximately 64% of patients with advanced cancer; a large European survey1 showed that 45% of all patients with advanced cancer experienced pain of moderate to severe intensity (at least 5 on a pain rating scale of 0–10). 3 More recently, in an update to these data that was published almost 10 years later,4 these estimates were 66% and 55%, respectively, suggesting that little progress had been made in cancer pain management during this period. Each year in England and Wales, 150,000 people die from cancer; these data suggest that 110,000 of these patients will suffer from cancer pain.
Greco et al. 5 estimated the adequacy of treatment for cancer pain and identified that approximately 32% of patients did not received analgesia proportionate to their pain severity. This potentially represents an improvement on an earlier estimate,6 which suggested that 43% of cancer patients were undertreated. Nevertheless, a significant number of patients with cancer pain are not well managed.
Aims and objectives
We aimed to develop and evaluate pain self-management interventions for community-based patients with advanced cancer. We conducted a programme of mixed-methods intervention development work leading to a pragmatic multicentre RCT of a multicomponent intervention for pain management compared with UC, including an assessment of cost-effectiveness (Figure 1).
FIGURE 1.
Programme summary: research pathway diagram.

Our specific objectives were to:
-
model and test a cancer pain pathway for patients with advanced cancer that optimises support and advice, delivers brief educational interventions and can be delivered to promote self-management
-
develop systems for capturing and communicating clinical and patient-reported outcomes on pain assessment that can be integrated into the routine practice of community-based health professionals
-
determine whether or not key aspects of medicines management, such as prescribing practice and access to analgesia, can be modified to ensure that patients with cancer pain benefit from timely intervention
-
implement and evaluate the clinical effectiveness and cost-effectiveness of a cancer pain pathway (based on objectives 1–3) in reducing pain and related distress, and on reducing pain-related hospital admissions.
Work package 1: can we model and test a cancer pain pathway for patients with advanced cancer in which support and advice are optimised, and in which brief educational interventions can be delivered to promote self-management?
Work package (WP) 1 (Figure 2) explored patient, caregiver and health professional perspectives on existing care pathways for pain management in advanced cancer. Previous research7 highlighted the complexity of advanced cancer pain, with pain control often understood by patients as whether or not activities or tasks can be completed and if relationships with family or friends are maintained. Gaining a greater understanding of patient interaction with health services and barriers to pain management at the level of health professional or health system is crucial when refining cancer pain pathways. Furthermore, an understanding of existing pathways can inform the timing and format of interventions seeking to support and improve pain management in patients with advanced cancer.
FIGURE 2.
Work package 1.
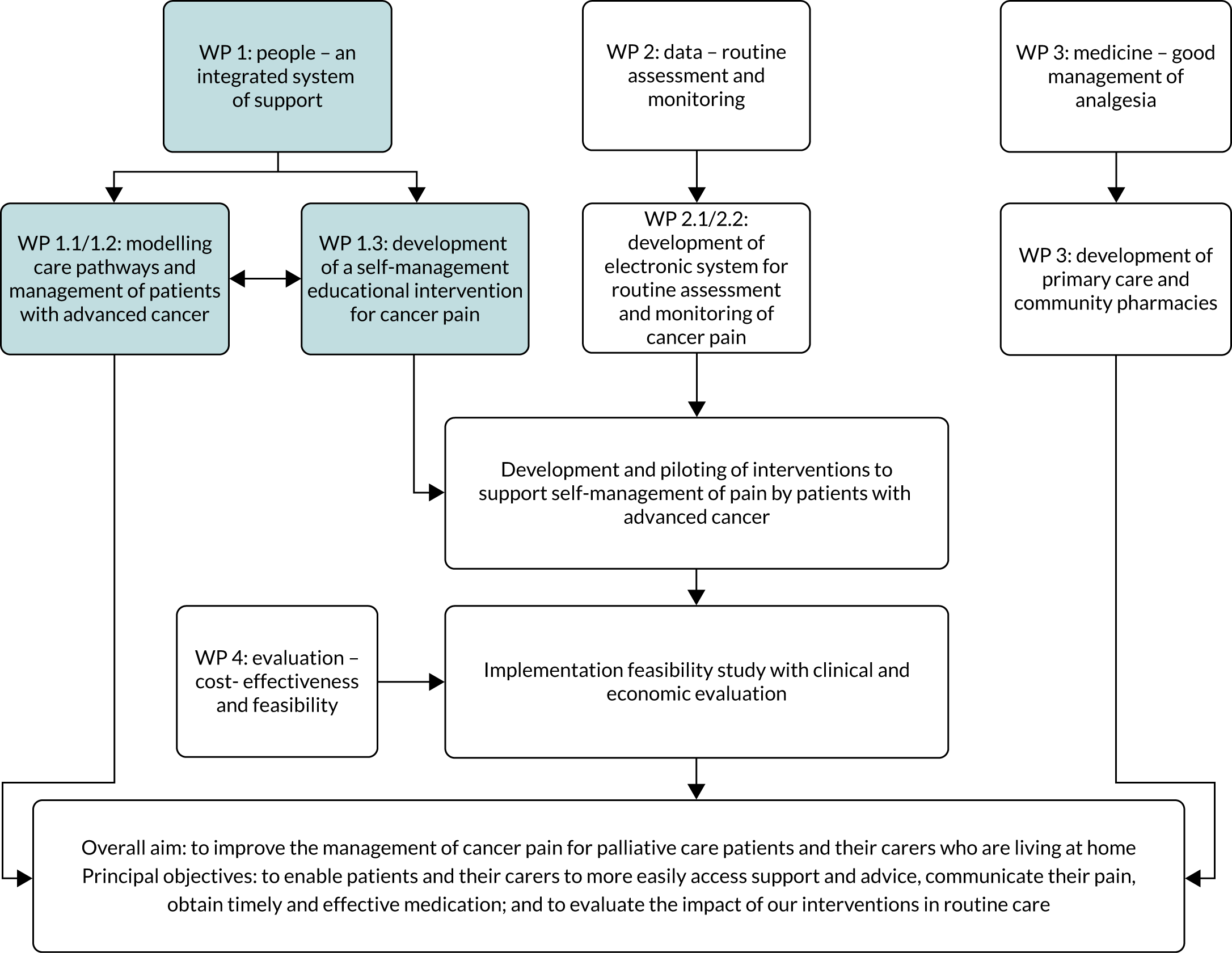
Work package 1: people – an integrated system of support
Three key areas of activity were undertaken in this WP, underpinned by the following research questions:
-
Which mechanisms exist within current systems of cancer care for identifying and co-ordinating support and treatment for cancer-related pain and what barriers exist at the health professional and system levels?
-
What is the optimal timing for palliative care interventions for patients with advanced cancer and their carers?
-
What is the evidence for educational approaches in the self-management of cancer pain and how can this be used to develop resources for patients with advanced cancer?
The first two questions were addressed through research activities to determine factors that influence access to and interaction with services to support cancer pain management. The third question involved the design and development of a self-management education tool for pain management in patients with advanced cancer. By ‘palliative care services’, we mean those services that provide specialist multidisciplinary palliative care (hospice inpatient units, day-care centres and outpatient and community nursing services, as well as hospital-based palliative care teams). These services are characterised by multidisciplinary teams whose members have undergone recognised training in palliative care. This is different from a generalist palliative care approach used by non-specialist professionals, such as general practitioners (GPs), oncologists and district nurses.
Work package 1.1: modelling a care pathway
Understanding the perception of services to support pain management by patients with advanced cancer and their carers
Hackett et al.8
We interviewed patients and carers at two time points to explore their experiences of pain in advanced cancer and the processes in which they engaged to manage pain. Patients were drawn from oncology outpatient clinics in a tertiary cancer centre and a hospice palliative care service. In total, 35 patients (29 patient–caregiver dyads and six patients on their own), of whom 21 were first interview participants and 14 were second interview participants, were interviewed. Four open-ended audio diaries were completed. A grounded theory analytic approach was employed. We used the method of constant comparison within cases over time and across cases to explore patterns of variation in the experience of pain and the strategies employed to manage it, as these were shaped by the course of illness, disease process, treatment regimen and access to supportive care, including for pain.
We found that pain in advanced cancer is complex, multidimensional and dynamic: patients and caregivers reported varying types and sources of pain, experienced both singly and together, that altered over the advanced illness course. For patients, pain management is an active, dynamic process, involving considerable ongoing work, so that developing expertise in pain management is not a one-off achievement. The amelioration of pain is uncertain and involves much trial and error (including by professionals) in the combinations of pharmacological and other therapeutic options, particularly because bone pain and neuropathic pain, common here, are more difficult to treat. Thus, a medication regimen often involves different types and combinations of drugs alongside prophylaxis medication to manage side effects. For most patients, managing pain is about securing ‘good enough’ relief that is consistent with balancing the side effects of medication and sustaining what they value in their lives. Three distinct patterns of pain were identified, varying in their degree of complexity, severity, transience/persistence and perceived control over pain, which are of practical and theoretical significance. This research has been published. 8
Determining factors that influence the timing of palliative care interventions for patients with advanced cancer and their caregivers
With a focus on access to services to support patients with advanced cancer, we sought to determine at which point before death patients are referred to palliative care services. Through a retrospective data analysis, we determined the timing of referrals to palliative care services prior to death in a large UK city, before extending our work to determine the timing of referrals across the UK.
Emerging evidence indicates the benefits that can arise from contact with palliative care, including improvements in symptom control and quality of life, better outcomes for families, and increased satisfaction with care. 9 Early referral to palliative care has also been shown to lead to multiple benefits for patients and their families. 10 The drive to increase referrals at an earlier stage in the disease trajectory has been notable for patients with cancer through, for example, the integration of oncology and palliative care services. 11 This was highlighted recently by the American Society of Clinical Oncology and the European Society for Medical Oncology, which suggested that, for those with advanced cancer, palliative care should form part of care alongside active treatment. 12,13 The literature on the benefits of early referral for patients with conditions other than cancer also highlights multiple benefits and supportive needs across the illness trajectory. 14 A current research priority is to understand approaches to early referral and the barriers to the integration of palliative care. 15 A key starting point is to understand current practice.
A marker of the quality of palliative care, reflecting how services are operating, can be the timing of referrals to services. 16 There are, however, very few data on the length of time that patients have access to palliative care at a population level, limiting efforts to understand how this access can vary based on the patient’s diagnosis or other characteristics. 17 Data are also limited beyond the UK, with wide variation across the literature when reporting the time between referral to a service and death. Reports that are available show a varied picture both across and within countries. For example, when looking across the Republic of Korea, the USA and the UK, figures vary from 12 to 21 days,18–20 which can then be compared with figures in Canada of 60–70 days. 21 Through a series of studies, we sought to determine and report, with greater accuracy, the duration of palliative care received by patients in the UK both regionally and nationally.
Timing and duration of palliative care in a large UK city
Bennett et al.20
We sought to determine when referrals to palliative care occur by exploring the length of time between an initial referral and death. A retrospective cohort study was undertaken that sought to determine the timing of referrals to three specialist palliative care services: two hospice-based community palliative care services and one hospital-based inpatient palliative care service. For each patient who had been referred to any of the three services, we identified the date on which they were first referred to the service and then calculated the median number of days between the first referral and the patient’s death. We also examined variation in the timing of referral before death across a range of characteristics of the patient (age, sex and diagnosis) and the service to which they were referred. Data were included for 4650 patients referred to specialist palliative care services in Leeds between April 2012 and March 2014. The median age of the sample was 75 years, and 3903 (84.0%) of the patients had a diagnosis of cancer. Overall, the median duration from referral to death for all patients was 34 days. This varied by hospital-based (20 days) and community-based palliative care (46 days). Age, diagnosis and place of referral were significant predictors of the duration of palliative care before death. We found that age was independently associated with the duration of palliative care, regardless of diagnosis. For patients aged > 75 years, 29 fewer days of palliative care were received than for patients aged < 50 years. Furthermore, patients with conditions other than cancer were found to receive 13 fewer days of palliative care than patients with cancer. When looking at services, we found that patients referred to hospital palliative care were receiving 24.5 fewer days of palliative care than patients being referred to community palliative care services. The study suggests that the current timing of referral to palliative care may limit the benefits to patients in terms of improvements in end-of-life care, particularly for older patients and patients with conditions other than cancer. Given that retrospective routinely collected clinical data were used, we do not have data on date of diagnosis, which prevents us from relating the duration of palliative care services to the duration of clinical awareness of disease. Late diagnosis may be a key factor in late referral, although the almost universally short duration of palliative care in our cohort suggests that this factor is unlikely to account for the observed referral pattern. This research has been published. 20
Timing and duration of palliative care across the UK
Allsop et al.22
Having identified that patients in Leeds were receiving a median of 34 days of palliative care prior to death [when community (46 days) and hospital (20 days) service data were combined], we sought to understand the national picture. Working in partnership with Hospice UK (London, UK), the research team conducted a national retrospective cohort study of the timing of referral to hospice-based specialist palliative care. The aim of the study was to identify patient and organisational factors that influence the duration of hospice-based palliative care in the UK prior to death. Overall, 64 UK hospices (i.e. one-third of all UK hospices) providing specialist palliative care inpatient beds and community services extracted data for all adult decedents (aged > 17 years) with progressive advanced disease who had received a prior referral (e.g. inpatient, community teams and outpatient), who died between 1 January 2015 and 31 December 2015. Data were requested for factors relating to both the patient and the hospice site. In total, data for 42,758 decedents were included in the analysis. The overall median time from referral to death was 48 days. Significant differences in referral to death days were found for those with cancer (53 days) and for conditions other than cancer (27 days). As age increases, the median number of days from referral to death decreases (those aged > 50 years, 78 days; those aged 50–74 years, 59 days; and those aged ≥ 75 years, 39 days). An adjusted multivariable negative binomial model demonstrated that increasing age was a significant predictor of fewer days of hospice care, as was being male, having a missing ethnicity classification and having a non-cancer diagnosis. This work provided a broader context for earlier findings on the timing of referral, with more than half of all patients being referred to palliative care < 7 weeks before death. This provided the national context in which to understand the Leeds data. The median of 46 days between referral and death for community-based patient in Leeds aligns with the 48 days found in national data relating to referrals to hospices before death.
The national survey highlights that, despite increasing rhetoric around the need for early referral to palliative care, patients with advanced disease across the UK receive referrals to hospice specialist palliative care very late in their illness trajectory. Age and diagnosis persist as determinants of duration of hospice specialist palliative care before death. Recent projections of the demand for palliative care by 2040 suggest that health-care systems need to adapt to the age-related growth in deaths from chronic illness, with dementia and cancer likely to be the main drivers of increased need. 23 Findings from this study suggest that there may be a need for reorientation of services to both older age groups and non-cancer conditions; both groups are associated with limited duration of hospice-based palliative care prior to death. The remit of palliative care has expanded to have relevance for any patient at an early stage in the disease process whose death can be medically anticipated. 24 Such a broad remit, when people are living longer with an increased illness burden, suggests that many patients will need primary, secondary and specialist palliative care. With increasing calls to reduce hospital bed-days in the last months of life and to support patient preferences for home death, it is particularly important that community-based specialist palliative care can consider how provision might match demand. This research has been published. 22
Work package 1.2: early identification of patients by health professionals
Exploration of oncology health professionals’ perceptions of the advanced cancer trajectory
We explored oncology health professionals’ perceptions of the advanced cancer trajectory, and identified transition points requiring mobilisation of supportive and palliative care and the mechanisms in place to effect transition. Interviews were conducted with 16 health professionals in a tertiary cancer centre: nine medical and clinical oncologists and seven clinical nurse specialists (CNSs) providing treatment and support to patients in respect of eight cancer types (multiple myeloma, colorectal, breast, prostate, gynaecological, head and neck, lung, and renal). Data were analysed using a grounded theory approach.
We found that, although the term ‘advanced cancer’ was commonly understood as ‘active’, non-curable cancer among oncology health professionals, it concealed considerable variability in the advanced cancer trajectory. The varied pattern of survival across cancer types, including new treatment modalities that extend life, means that the trajectory projected for advanced cancer may extend for years at one end of the spectrum (typical of breast and prostate) to months at the other (lung), with varying patterns of oncology involvement from intermittent to continuous with advance cancer patients. Drawing on professionals’ accounts and prior interviews with patients and carers, and time to death, we delineated three broad advanced cancer trajectories that varied in duration and shape, and that informed the pattern of engagement of cancer specialists with patients and the overall work organisation. Within each trajectory, critical transitions were identified, requiring mobilisation of support and resources including support with symptom management from palliative care services alongside treatment. The length of time that patients may be involved with oncology services and the regularity of their engagement mean that these services provide a level of continuity of support, perceived expertise and accessibility for patients in crises, such that they are regarded as the ‘key’ services.
Alongside oncologists, CNSs provided emotional support and comfort, provided clinical expertise in illness and symptom management, and navigated patients to services and support. We did not find that oncology professionals were reluctant to involve palliative care alongside treatment or to facilitate transition to end-of-life care. CNSs in particular assumed a proactive role in encouraging patients to accept such assistance, although patients themselves were often reluctant, viewing this acceptance as ‘giving up’. Apparently at odds with the ‘big data’ picture, this applied only to those patients in receipt of treatment to control their advanced cancer, which tends to exclude those with frailty and who are unable to tolerate treatment toxicities and those who refuse further treatment.
Exploration of primary palliative care teams on co-ordinating and managing people with advanced cancer
Hackett et al.25
Alongside patients’, caregivers’ and oncologists’ perspectives, we engaged with primary health-care teams, which are a key component of care delivery to patients with advanced cancer during the last year of life. The Gold Standards Framework is proposed as a mechanism for co-ordinating and guiding identification, assessment and support. There are still considerable variations in practice despite its introduction. The aim of this qualitative study was to improve understanding of variations in practice by exploring the perspectives and experiences of members of primary health-care teams involved in the care of patients with advanced cancer. Qualitative, semistructured interviews, focus groups and non-participatory observations involving 67 members of primary health-care teams providing palliative care were undertaken. Data were analysed using a grounded theory approach. We identified distinct differences in the drivers of and barriers to community advanced cancer care co-ordination, which relate to identification and management, and access to effective pain management, and go some way to explaining variations in practice. These include proactive identification processes, time and resource pressures, unclear roles and responsibilities, poor multidisciplinary working, and inflexible models for referral and prescribing. These provide valuable insights into how health professionals work together and independently within an infrastructure that can both support and hinder the provision of effective community palliative care. Although the Gold Standards Framework is a guide for good practice, alone it is not a mechanism for change. Rather, it provides a framework for describing quality of practice that was already occurring. Consequently, there will continue to be variations in practice. This research has been published. 25
Information provision for patients with cancer
Taylor et al.26
Increasing evidence suggests that, for patients with a range of advanced cancers, earlier integration of palliative care should be an essential component of their care. 27–31 Communication is an important element of the provision of advanced cancer care32 and may play a role in facilitating awareness of and access to palliative care services. However, a high level of unmet information needs exists among patients whose care has become palliative in focus. 33 We conducted a study to understand the current evidence on the information provision for patients with advanced cancer while exploring where deficits in provision may exist. The study was conducted in three distinct phases: (1) a literature review, (2) a regional audit of patient information and (3) a critique of patient information. The literature review was conducted as part of a scoping exercise to focus the direction of future research projects. The review highlighted particular issues of interest around patient information that were investigated further in the audit. The literature review identified patient-related barriers to earlier integration of palliative care, misconceptions of what palliative care is and a lack of understanding of the role and breadth of services available. 34 The lack of information available to patients about palliative care and their unmet information needs was evident in the literature, which led us to conduct an audit of patient palliative care information in our geographical area. Our regional audit of patient information resources found that patient information relating to palliative care is not widely available to cancer patients. Our audit showed that < 13% of all inpatient units, 7% of outpatient departments and 25% of chemotherapy day units had written information available for patients regarding palliative care. Despite this, > 90% of palliative care teams that were surveyed said that information leaflets had been produced but were not in routine circulation. Our audit shows that, although information resources exist within the trusts, these are often distributed to patients only after they have received a palliative care referral. Our review of the content of patient information resources, where these exist, shows considerable variation. The majority of the resources fail to describe the referral process and what patients can expect once they have been referred. None of the information leaflets explain that palliative care can be integrated alongside oncology care. Patient and health professionals’ understanding of this concept is key to achieving an integrated service in which patients can receive appropriate palliative care input alongside cancer treatment. 35 More research is required to explore ways to disseminate information about palliative care effectively and sensitively. This research has been published. 26
Work package 1.3: promoting self-management
Developing educational approaches in the self-management of cancer pain for patients with advanced cancer
A simultaneous component of the WP was focused on developing education materials to support patient self-management of pain. Supporting self-management has become a standard approach for health professionals working with people who experience chronic non-cancer pain,21,22 and many studies and reviews have reported that patient-focused educational interventions, including self-management, can also improve pain control in patients with advanced cancer. 23–30 We sought to determine the optimal content, format and timing of an educational intervention for patients and carers to support the self-management of cancer pain. This is consistent with National Institute for Health and Care Excellence (NICE) guidance on prescribing strong opioids for pain in adult palliative care, which describes the importance of providing written and verbal information to support patients. 36
Systematic review of evidence for self-management education interventions for patients with cancer
Flemming et al.37
A review of evidence drawn from systematic reviews of complex interventions was combined with a synthesis of qualitative research to identify the key components of a self-management intervention for advanced cancer pain. We identified four systematic reviews examining interventions for the self-management of advanced cancer pain. Although attributes of a pain management intervention were recommended in each of the reviews, the essential key components could not be determined. As part of subsequent qualitative evidence syntheses, a further three systematic reviews were identified and integrated with the effectiveness reviews. The key components of a self-management intervention following the integration included the importance of addressing patients’ knowledge, skills, individualised approaches to care and attitudes towards pain management, alongside the significance of the role of team approaches and interdisciplinary working in the management of pain. Mapping the findings of each paper onto a behaviour change framework led us to identify the contextual and intervention components that are essential for the development of successful educational programmes. We concluded that educational interventions to promote the self-management of advanced cancer pain by patients and that their carers should seek to include content that addresses how individuals manage their pain in the context of their situation and that of their health professionals, while also focusing on those intervention functions that are known to influence behaviour. This research has been published. 37
Understanding patient, carer and health professionals’ perspectives on the role of educational interventions for self-management of cancer pain
Hughes et al.38
This study was undertaken to ascertain the views of specialist palliative care health professionals on patient self-management of cancer pain to inform the development of a new educational intervention. Focus group interviews were conducted with 17 health professionals [community CNSs, n = 6; complementary therapists, n = 3; hospice nurses, n = 5; hospice social worker, n = 1; hospice spiritual care co-ordinator, n = 1; and palliative care consultant physician, n = 1). The aim of the focus groups was to elicit experiential perspectives that would help us to extend and refine an educational intervention that focused on the use of strong opioids for control of cancer pain, which was produced and tested during earlier work by the team. 39 Participants viewed self-management of cancer pain as desirable and achievable, but also as something that could be problematic. Challenges to self-management were perceived in patient attitudes and behaviours, health professionals’ own beliefs and actions and the wider social system. Practitioners showed awareness of potential tension between their espoused views (the desirability that patients manage pain autonomously) and their tacit views (the undesirability of patients managing pain in ways that conflict with health professionals’ knowledge and identity). This research has been published. 38
Developing an educational self-management resource for patients with advanced cancer
Following on from the systematic review and health professional engagement, outlined above, two stages of patient engagement occurred: (1) focus group interviews with patients and family caregivers to understand perceptions of ‘self-management’ for cancer pain and methods for delivery, and (2) a feasibility study with community-dwelling adults with pain from advanced cancer that evaluated the use of an educational self-management intervention for cancer pain. The first stage informed the design of an educational self-management resource for patients with pain from advanced cancer and their caregivers, and the second stage informed the feasibility of using this resource.
Focus group interviews
We conducted a qualitative descriptive study by undertaking focus group interviews with patients and family caregivers. We conducted four focus group interviews between August 2013 and January 2014: three with patients and one with family caregivers. We recruited patients (n = 8) through day-care services at two local hospices and through patient support groups at a cancer information centre. We gained access to carers (n = 4) through a bereavement support group at one of the hospices. Patients were aged 40–67 years (average age 58 years; five male, three female) with cancers of brain (n = 1), breast (n = 2), bowel (n = 2), and prostate and throat (n = 2), and metastatic disease reported in the bones and liver (n = 1). Family caregivers were aged 63–68 years (average age 65 years; all female). All had been bereaved, and their partners had died from cancers of the stomach (n = 1), prostate (n = 1), lung (n = 1) and skin (n = 1).
Patients and family caregivers reported varied perspectives on pain management, many of which were incorporated directly into the educational materials that we created to support self-management. For example, patients described their own self-management practices, including relaxation, distraction, comfort measures and medication scheduling. The family caregivers we interviewed spoke about their own activities to try to help relieve their partners’ pain, typically by administering pain medication, rather than their partners’ self-management behaviours. One defining characteristic of the experience that they reported was their partners’ unwillingness to talk about their pain. We concluded that education to support self-management of cancer pain in advanced disease should incorporate practitioner, patient and family caregiver perspectives.
Educational self-management resource
Tackling Cancer Pain: A Toolkit for Patients and Families is informed by a wider body of international research that has investigated patient education for managing cancer pain, and by our own research with patients, carers and health professionals. The programme outlined in the resource is based on principles of adult learning and is available in a multimedia format. The intervention is targeted at patients who have cancer pain that is not relieved by their current medication regime or by other pain control measures. It is also available for family members of people with cancer who are closely involved with the patient’s pain experience. The toolkit provides an information resource that may be used independently by patients and families, or with the guidance of a health professional. Tackling Cancer Pain consists of five sections: understanding cancer pain, talking about pain, using drugs to manage pain, additional approaches to managing pain and getting more help. Each section contains information and self-directed learning activities along with sources of further information. The toolkit has been formatted at the suggestion of patients and their families in a loose-leaf ring binder with an accompanying digital versatile disc (DVD); they considered that this gave them the time to explore the resource and to reflect on and revisit it as required, using it very much as a reference. It can be readily transferred into a web resource in the future but that format was not preferred at the time of undertaking the work. Importantly, it is written in easily understandable lay persons’ language.
Feasibility study
We recruited community-dwelling adults with pain from advanced cancer, taking baseline measures of pain intensity and frequency [using the Brief Pain Inventory (BPI)]40 along with assessing participants’ knowledge and experience of cancer pain [using the Patient Pain Questionnaire (PPQ)]. 41 We explained the content, format and purpose of the Tackling Cancer Pain: A Toolkit for Patients and Families resource and gave a copy to each participant. We asked them to watch or read (or both) self-selected parts of Tackling Cancer Pain and to carry out associated learning activities from the resource that were related to pain management for a period of 4 weeks. At 4 weeks, we conducted face-to-face interviews with participants to understand the feasibility of the intervention in terms of the acceptability of both its content and the mode of delivery. After a further 4 weeks we interviewed the participants again and repeated the baseline measures.
Twenty-two patients consented and completed baseline measures. Seven patients were lost to follow-up before the first interview (died, n = 2; too unwell, n = 2; admitted to hospice, n = 1; unable to contact, n = 2). Fifteen patients completed a first follow-up interview (4 weeks after baseline). A further five patients were lost to follow-up before the second interview (died, n = 1; too unwell, n = 1; unable to contact, n = 3). Ten patients completed a second interview (baseline + 8 weeks), although only nine of these patients provided complete data. Of the 15 participating patients, four nominated a family caregiver who was willing to take part. Three family caregivers took part in a joint interview with the patient and one caregiver was interviewed separately. Two family caregivers completed both interviews; all were female. Three caregivers were spouses and one was a close friend of the patient. The average age of the patients interviewed was 66.9 years (range 45–89 years). Six patients were female and nine were male. The primary cancer diagnoses included stomach (n = 1), myeloma (n = 1), prostate (n = 5), breast (n = 3), throat (n = 1), ovary (n = 1), endometrium (n = 1) and colon (n = 1) (and one undisclosed). The time since diagnosis ranged from 13 years to < 1 year. The average age of the 22 participants (female, n = 12; male, n = 10) who consented and completed the baseline measures (missing data for one participant) was 71 years. The average age of the seven participants (female, n = 6; male, n = 1) who completed the baseline measures but were not interviewed was 83 years (missing data for one participant).
Of the nine patients who provided complete data, four patients reported (using the BPI) having less pain, on average, at the end of the study period and three patients reported having more pain. For two patients, the average pain score (using the BPI) was unchanged. On the PPQ Experience subscale, three patients reported less pain, three patients reported more pain and three reported no change. Five patients indicated, according to the PPQ Knowledge subscale scores, that their knowledge of cancer pain had increased. There was no change for two patients and the remaining two patients reported having less knowledge at 8 weeks than at baseline (this may have been an artefact of variation in the interpretation of the questions). Average scores for the overall utility of Tackling Cancer Pain changed from 7.5 out of 10.00 at baseline to 7.2 out of 10.00 at 8 weeks.
We recognise the small number of patients who were able to contribute data to this stage of development of the educational resource. However, we intended to understand only feasibility of delivery and acceptability at this stage, as we considered our randomised clinical trial a more definitive evaluation of this component of our complex intervention.
Commendation
In September 2016, Tackling Cancer Pain, our educational self-management resource, was highly commended at the British Medical Association (BMA) Patient Information Awards. The BMA reviewers described it as an excellent resource.
Work package 2: can systems for capturing and communicating clinical and patient-reported outcomes on pain assessment be integrated into routine practice by community-based health professionals?
In the UK, the application of information and communication technology (ICT) in health-care settings has been highlighted as a means of improving patient outcomes42 and ensuring that patients receive high-quality care. 43 ICT can include all digital technologies that facilitate the electronic capture, processing, storage and exchange of information. 44 Electronic systems have been developed that use ICT to facilitate the capture of clinical data directly from patients; these approaches are deemed acceptable by patients. 45 This workstream carried out user engagement with patients, caregivers and health professionals to inform the development of an ICT system (Figure 3). The ICT system, which was developed with a technology partner, aimed to facilitate routine reporting and management of pain for patients with advanced cancer.
FIGURE 3.
Work package 2.
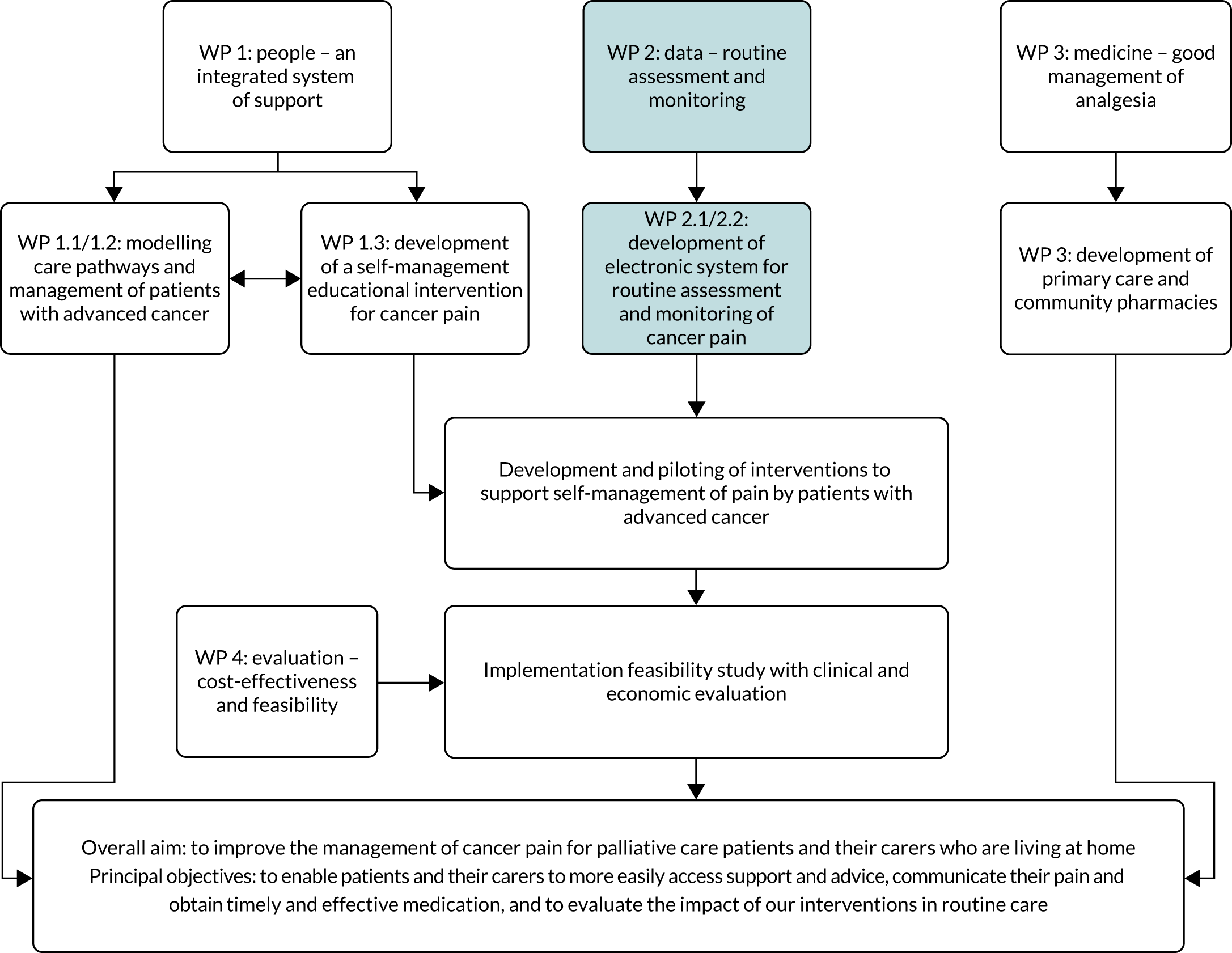
The ICT systems have been developed to support different stages of the illness trajectory in cancer, although their use to capture clinical data specifically in palliative care is at an early stage. 46 Patients in palliative care experience multiple physical symptoms47 that affect their quality of life (QoL) and psychological well-being. 48 Pain is reported by 66.4% of patients with advanced cancer,4,47 but this is commonly undertreated. 36 Frequently cited barriers to adequate pain management include knowledge deficits, inadequate pain assessment and misconceptions regarding pain. 49 The use of ICT is one approach to addressing pain management barriers by facilitating greater communication between patients and their palliative care health professionals. 50 There is opportunity to develop and implement online palliative care symptom reporting systems, with the aim of supporting timely communication. 51
Work package 2: data – routine assessment and monitoring of pain
This WP addressed the following research questions:
-
What are the requirements of patients and health professionals in the design of ICT systems for pain management in advanced cancer?
-
How should a system to support cancer pain self-management be designed and evaluated prior to implementation?
-
Can ICT systems to support routine pain monitoring and assessment be implemented as part of palliative care for patients with advanced cancer?
Three key areas of research activity were undertaken to address these questions:
-
user engagement with patients, caregivers and health professionals to understand their requirements for the development of an ICT system for pain reporting
-
a complementary WP that involved the design and usability testing of an ICT platform developed in response to identified user needs
-
a third strand of work evaluating the completed ICT system in the context of a trial.
Work package 2.1: developing an information management system
Developing an information and communication technology platform to support pain reporting and assessment by community-based palliative care patients and their health professionals
To develop an ICT platform to facilitate pain reporting and assessment, we worked alongside a software development company. We began by identifying the existing approaches to ICT use in pain management in palliative care services through a systematic review. 52 The team shared the requirements that were identified from user engagement activities with developers to inform the architecture of an ICT system. We tested the underlying infrastructure of the system to ensure its integrity for data collection. This work was conducted with participants with chronic pain. 53 We then completed usability testing of a prototype system with patients with advanced cancer and health professionals prior to the inclusion of the ICT system in the feasibility study. 54 This work is described across three publications, as follows. 52–54
Allsop et al.52
Our aim in this systematic review was to review existing ICT systems developed for pain management in palliative care. The majority of the literature identified in our systematic review was of a non-experimental research design. ICT systems included in the review were at various stages of development (planning, analysis, design and evaluation), with no systems implemented into routine care. Most ICT systems measured pain as part of a quality-of-life measurement and there was wide variation in the approaches used to assess pain. We concluded that future development of ICT systems needs to increase the quality and scale of development work, consider how recommendations for pain measurement can be integrated, and explore how to effectively use system feedback with patients. This research has been published. 52
Taylor et al.53
We recruited 34 participants with chronic pain who were asked to complete twice-daily pain assessments over two consecutive days. Participants alternated their mode (personal computer/laptop, smartphone, short messaging service or tablet computer) of accessing the ICT platform. Most participants completed all four assessments. The system was reported to be useful for reporting pain. Participants suggested that the platform could be useful to improve recall and monitoring of pain, promote self-management and improve links with health professionals. Perceived benefits for health professionals included increased understanding of pain experiences, improvements in pain monitoring and management, and enhanced communication. This research has been published. 53
Taylor et al.54
We undertook usability testing of the ICT system with 29 participants (advanced cancer patients, n = 13; GPs, n = 4; CNSs, n = 4; district nurses, n = 3; palliative care doctors, n = 5). Patients and health professionals were quickly able to understand and use the ICT system with very limited information about what the system was designed to do or how it worked. Both patients and health professionals were generally positive about PainCheck and found it easy to understand. Participants did have some concerns about how PainCheck might work in clinical practice. Their concerns were largely related to process issues, such as whether or not health professionals would have time to use the ICT system and how a lack of response may have an impact on patient care. Patients’ and health professionals’ ability to engage and use the technology was also mentioned as a potential barrier to ICT system integration. This research has been published. 54
Work package 2.2: ensuring engagement of patients and health professionals
Understanding user needs and perspectives to inform the development of an information and communication technology system for pain reporting and assessment
Our goal was to adopt a patient-centred approach throughout all stages of the research programme, working with a strong group of patient and public involvement contributors to bring their lived experiences and perspectives of managing cancer pain central to the programme. We sought to engage with end-user populations to determine the initial requirements of an ICT system for pain management. Our earlier programme development work highlighted the complexities of and barriers to the routine capture of data on pain and related distress focused on locations of care, circles of support and management and sharing of data. 50
Allsop et al.55
Face-to-face interviews were conducted with 13 patients with advanced cancer who were receiving palliative care. Patients described technology as peripheral to existing processes of care. Simple approaches that employ well-established technologies may be a preferred starting point. For system content to have relevance for a patient with advanced cancer, it needs to take account of the complexity of pain experiences and existing relationships with health professionals. Future research is required to understand how ICT systems can be positioned flexibly within existing delivery models of palliative care. This research has been published. 55
Taylor et al.56
We carried out face-to-face interviews with 15 health professionals managing the palliative care of patients living in the community. Participants included GPs, CNSs, district/community nurses and palliative care doctors. Within our work, even the most sceptical of health professionals acknowledged the potential benefits of implementing an electronic patient-reported pain monitoring system. Health professionals had reservations about how PainCheck would work in practice. For optimal use, an ICT system would need to be embedded within existing electronic health records. Electronic pain monitoring systems were reported to have the potential to enable health professionals to support patients’ pain management more effectively but only when barriers to implementation are appropriately identified and addressed. This research has been published. 56
An eHealth intervention for routine assessment of pain: PainCheck
Allsop et al.57
We adopted a research-led development process that sought to develop an ICT system that met the needs of patients and health professionals in real clinical settings and that was fit for clinical trial. Agile software development methods were combined with health science research methods and ‘participatory design,’ including diary studies, face-to-face interviews, questionnaires, observations of clinical settings, prototyping, think-aloud, agile sprints, process reviews, requirements clustering and pilots. Three iterations of ICT system development were necessary to prepare the tool for the feasibility study.
PainCheck, our information and communication intervention for the management of pain, was built by a private company, X-Lab (Leeds, UK). X-Lab used a University of Leeds platform called QTool. QTool is an electronic online questionnaire management software suite. QTool is used by health-care practitioners and researchers to build and schedule complex questionnaires that can be completed by patients and clinical staff. QTool has been used for patient-reported outcomes in cancer survivors and self-report and management of adverse events during cancer treatment. QTool was selected as a starting point for the development of PainCheck. This research has been published. 57
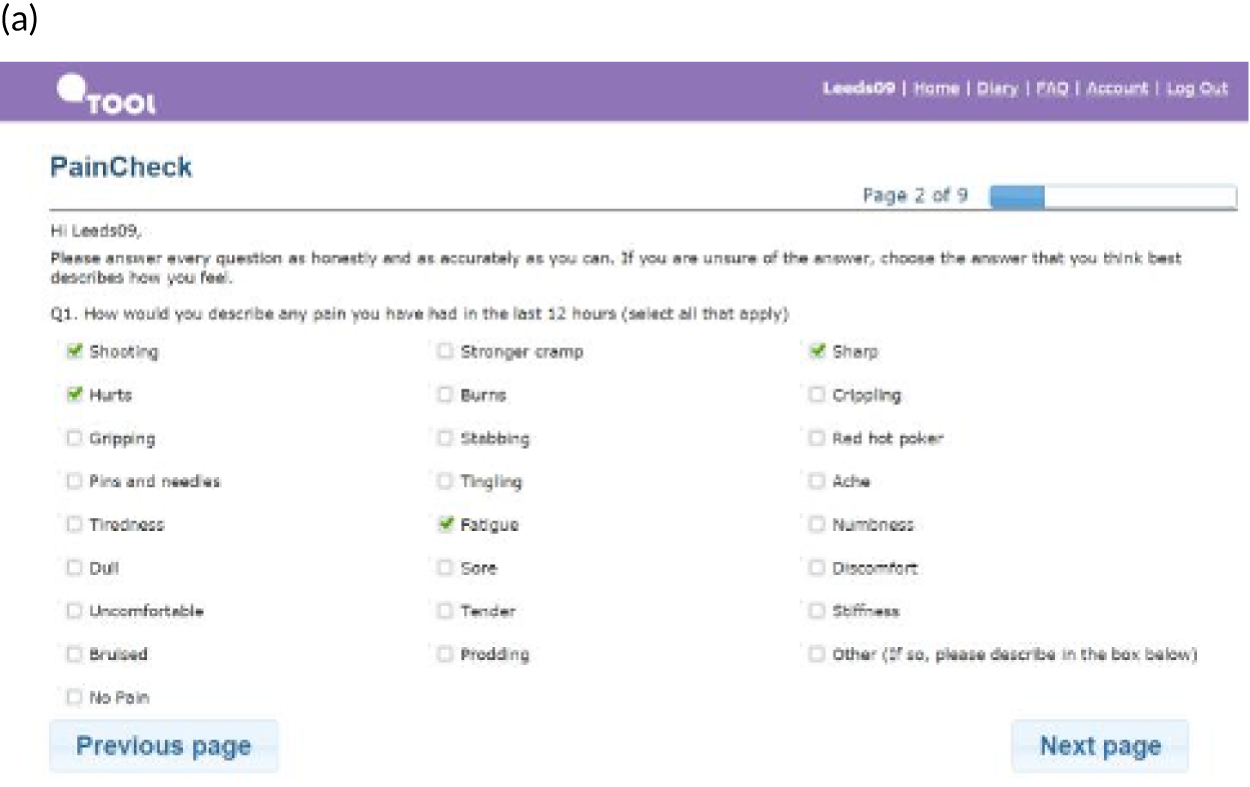
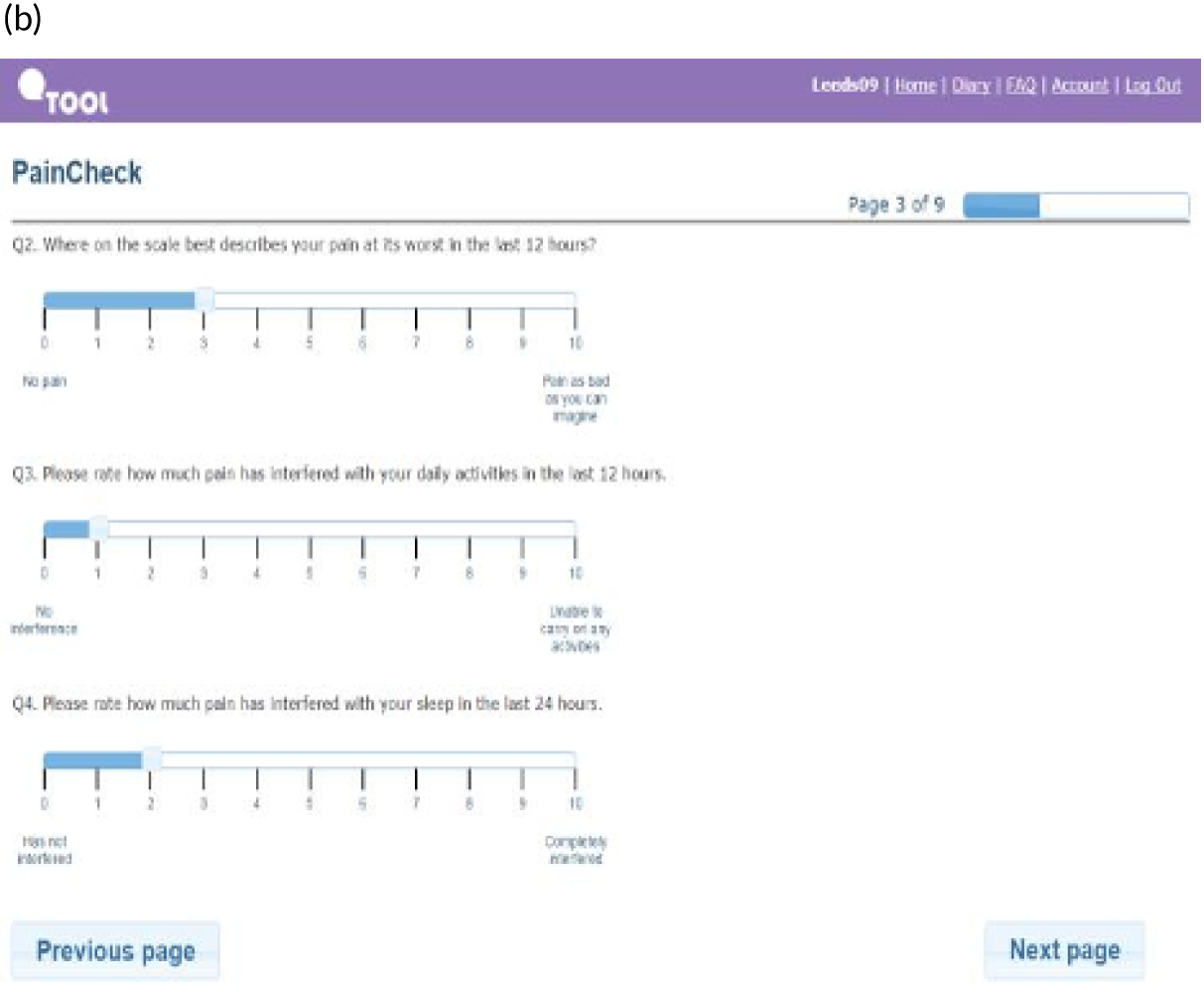
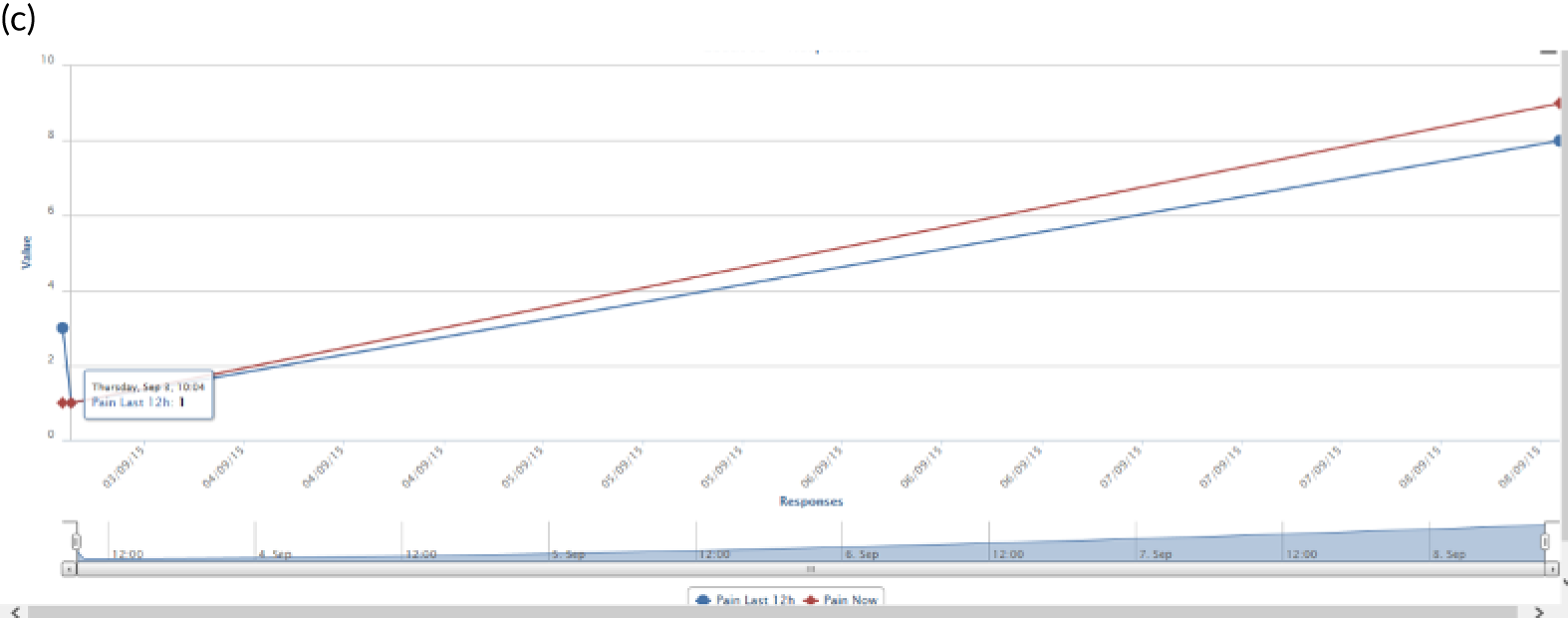
PainCheck was accessed by patients and health professionals through a website hosted by the University of Leeds. PainCheck allows patients to record their pain and gives them access to personalised pain management advice. Patients are asked to respond to various questions, including providing a description of their pain (Figure 4a); providing a rating of the intensity of current pain and the intensity of pain in the last 12 hours (see Figure 4b); and giving the perception of control of their pain and how pain has interfered with daily activities and their sleep. The majority of items seeking this information were taken from the BPI.
FIGURE 4.
Examples of PainCheck user interface. (a) Qualitative description of pain; (b) numerical sliding scales rating pain and interference; (c) questions exploring what patients have undertaken to control their pain and if they were helpful (the second page included self-help measure such as use of hot/cold, having a bath, exercise); and (d) patient feedback.
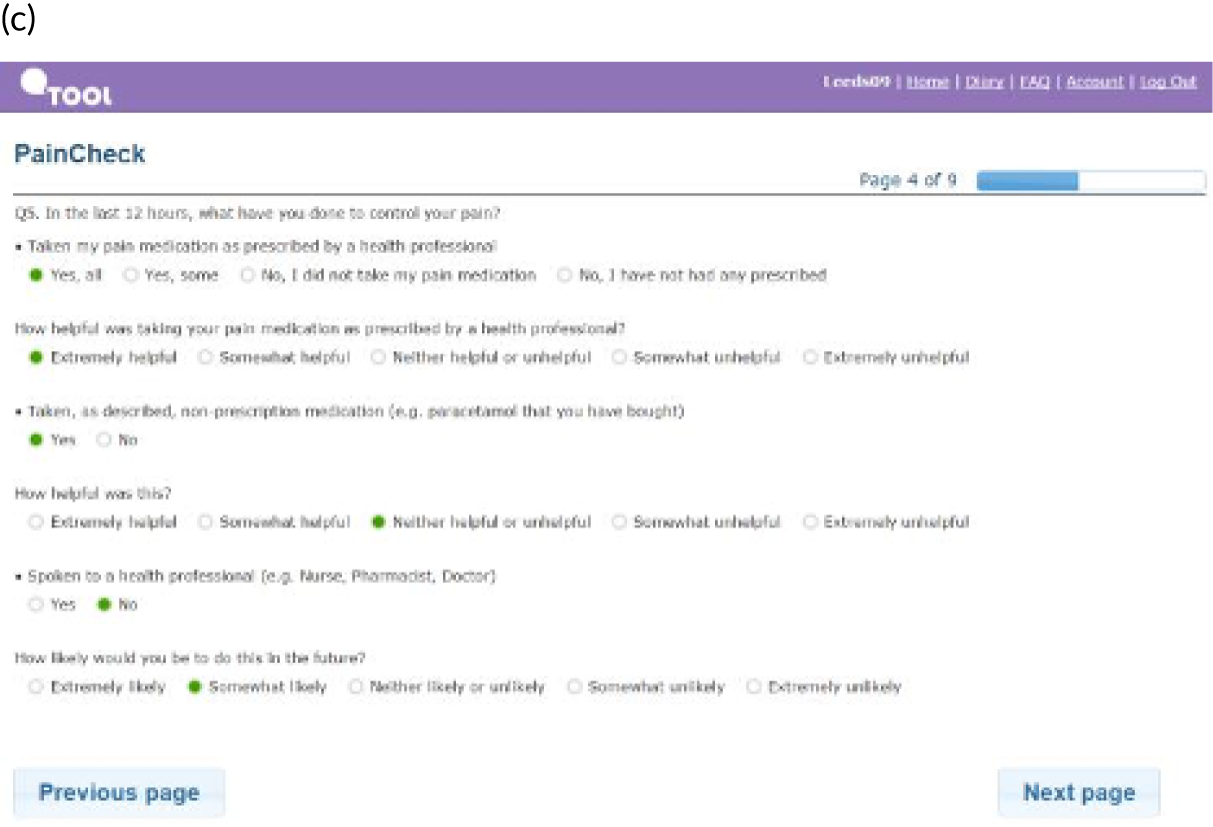
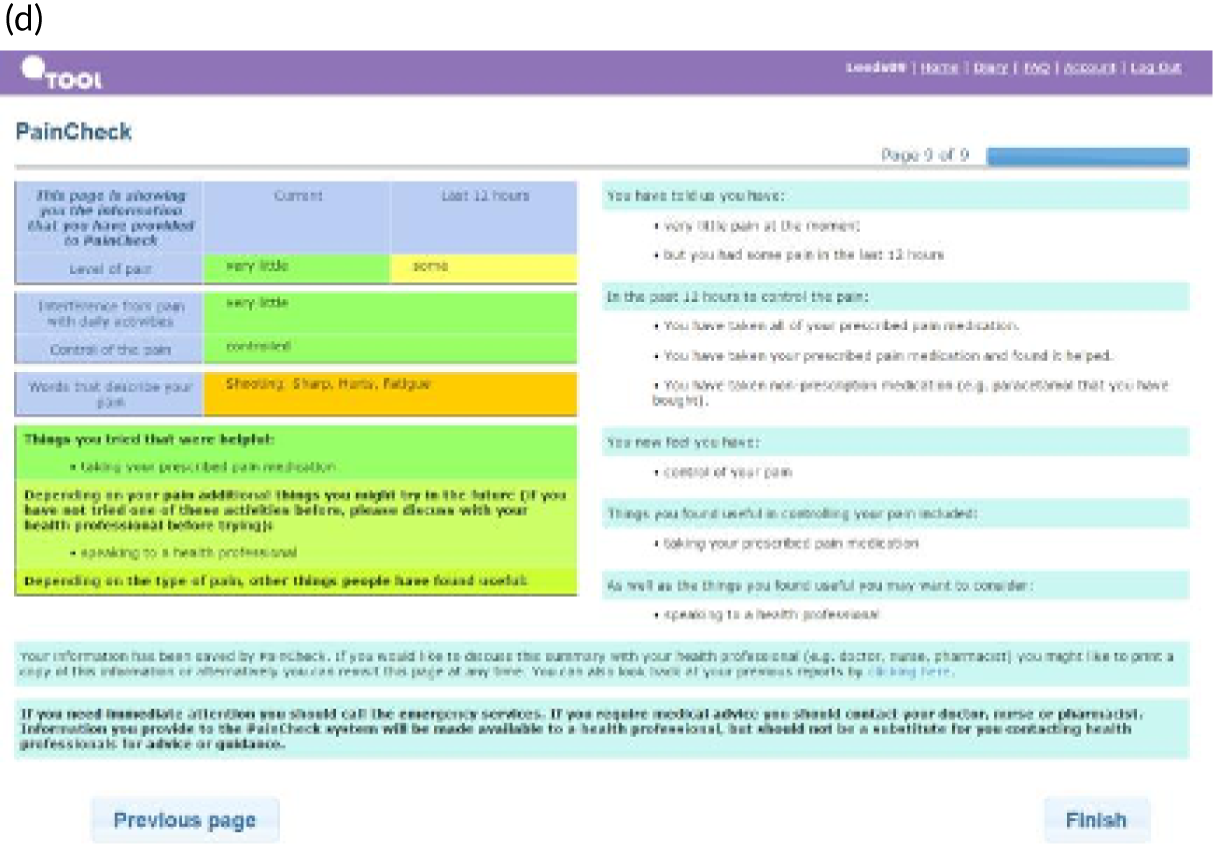
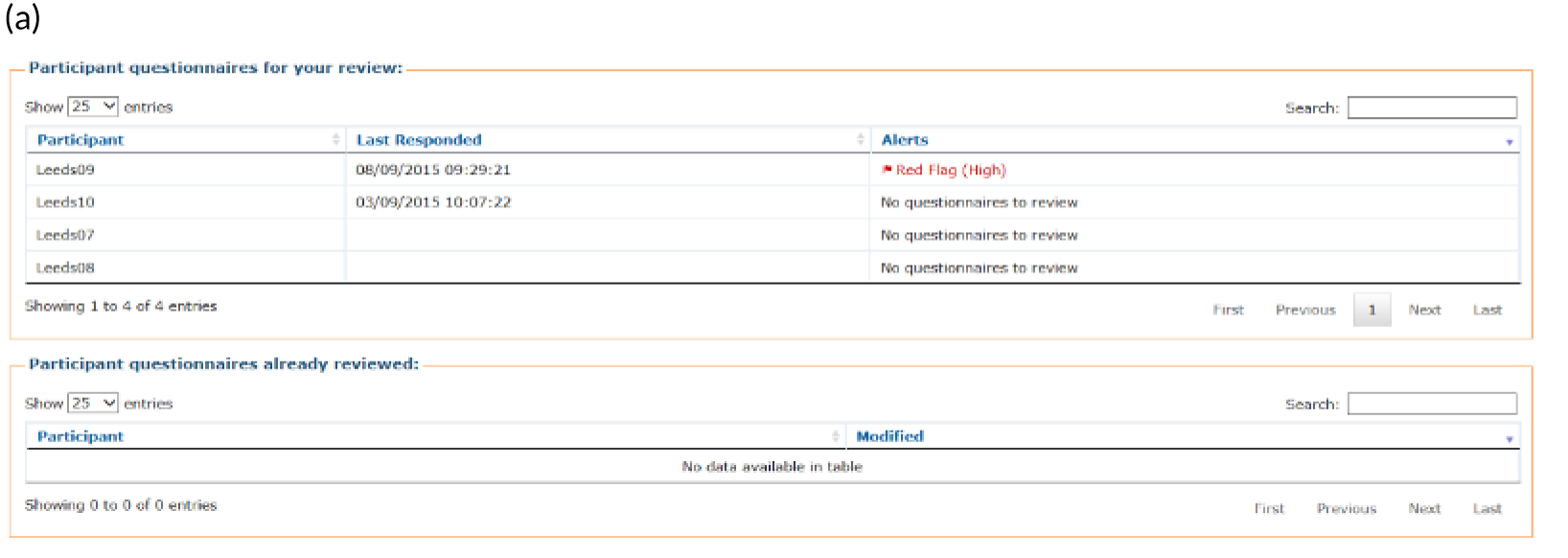
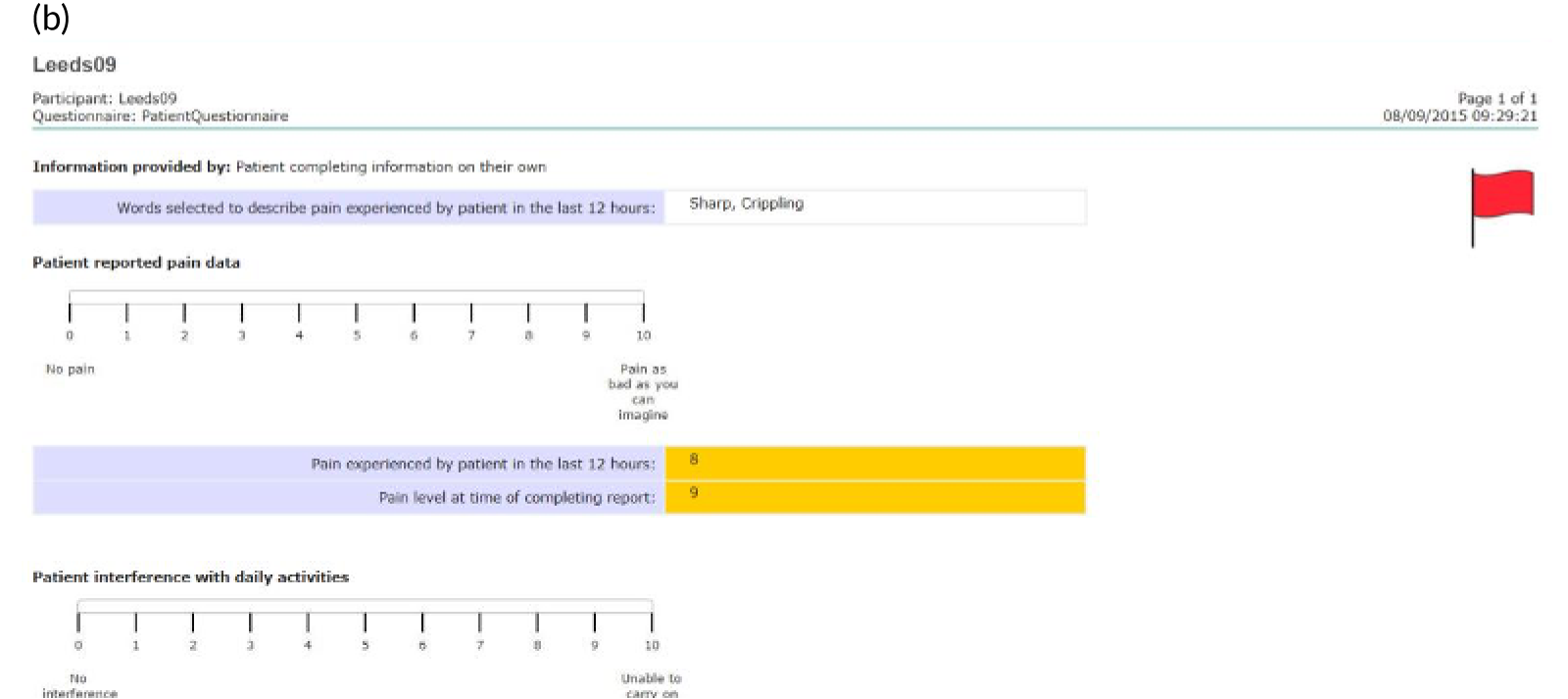
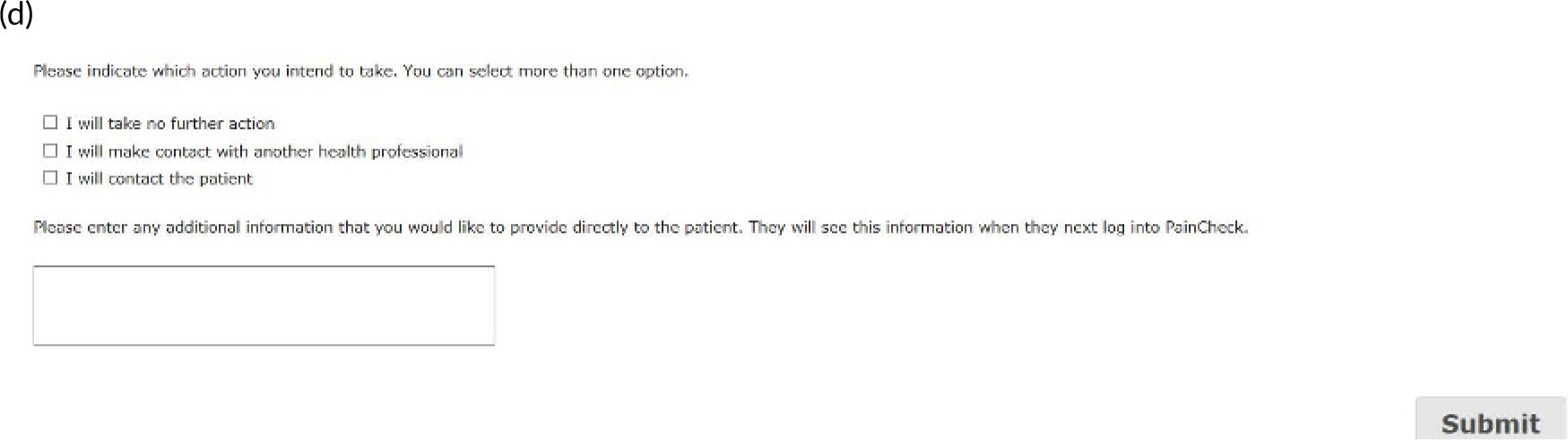
Patients are asked about pain management techniques, which of these techniques were helpful and how likely they are to try them in the future (see Figure 4c). Various question response options are used, including multiple choice, numerical slider scales (0–10 or 0–6) and free text. After completion, patients are provided with a summary of their results and suggestions of pain management techniques that they may want to try in the future (see Figure 4d). Health professionals can log in to PainCheck and view all patients registered on PainCheck and see who has completed reports (Figure 5a). They are then able to select a patient and view their responses to individual questions (see Figure 5b). Health professionals are presented with a graph that tracks patients’ current pain and their pain in the last 12 hours over time (see Figure 5c). Patients are given a ‘red flag’ in the health professional system if they reach certain thresholds for current pain and pain control. After reading the patient report, health professionals can decide what action, if any, they would like to take (see Figure 5d). Health professionals have the option of contacting patients through PainCheck to provide information and advice. All data entered into PainCheck can be exported into Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) in comma-separated value format.
FIGURE 5.
Screenshots of health professional dashboard. (a) Health professional dashboard; (b) individual patient report; (c) patient scores over time; and (d) record of health professional action.
Testing of PainCheck for pain self-management as part of a feasibility study
The ICT system was included as part of the supported self-management (SSM) intervention in the feasibility study. Two pieces of related work were subsequently undertaken to support this work: a process evaluation of the ICT system as part of the multicentre RCT and a commentary on the current state of ICT systems for pain management in palliative care. The commentary is outlined below, with details of the process evaluation outlined alongside the multicentre RCT findings in the summary of WP 4.
International workshop
In February 2017, an international workshop was organised by the IMPACCT team. Invited speakers from the UK, the Netherlands, Germany and Ireland attended, alongside delegates from research teams in Leeds, palliative care health professionals and software developers. An open discussion following presentations focused on current issues in ICT development in palliative care and approaches that could support the advancement of the research field. Key priorities for future development of ICT systems were identified, including the need to better understand and define how user engagement with ICT systems is understood and measured. In addition, the need for flexible approaches to the evaluation of ICT systems was highlighted. Ideally, this would enable ICT systems to continue to undergo iterative development in response to user feedback throughout periods of evaluation.
Work package 3: can the most important aspects of medicines management, such as prescribing practice and access to analgesia, potentially be modified to ensure that patients with cancer pain benefit from timely intervention?
Strong opioids, especially morphine, are the principal treatment for pain related to advanced and progressive disease, and their use has increased significantly in the primary care setting. However, many patients with advanced cancer experience pain that is poorly controlled. Although effective and safe titration of opioids can have a major impact on patient comfort, advice to prescribers has been varied and sometimes conflicting, and the wide range of formulations and preparations can be confusing. Together with the complex web of attitudes, knowledge and communication skills of prescribers, patients and the public, these factors have resulted in underdosing and avoidable pain, or overdosing and distressing adverse effects. Furthermore, both prescribers and patients may be concerned that opioids may reduce survival time, another barrier to optimising pain management.
A network of health professionals may be involved in the prescribing of strong opioids to manage cancer pain, and we were keen to understand the components of current provision and to identify opportunities for improving practice.
The overarching aim of this work package (Figure 6) was to explore ways of ensuring that patients benefit from improved pain control through better management of medicines, including timely access to both a prescriber and analgesia, and a greater opportunity for patients to discuss their medicines with a health professional.
FIGURE 6.
Work package 3.
Work package 3: medicines – good management of analgesic drugs
This WP planned to address the following research questions:
-
What is the pharmacoepidemiology of prescribed medicines and prescription pathways among cancer patients during their last year of life?
-
What are the effects of non-medical prescribing (NMP) by nurses and pharmacists on timely access to prescriptions for analgesics and what opportunities for intervention can be modelled?
-
What is the potential for intervention at community pharmacy level to improve cancer pain management?
These three questions, covering the prescribing and subsequent access to analgesia and information/advice for cancer pain management, were explored under three strands of activity: (1) mapping pathways of prescriptions among cancer patients during their last year of life; (2) evaluating the impact of NMP by nurses and pharmacists; and (3) conducting exploratory research to understand the feasibility of community-based pharmacy medicine consultation interventions.
Work package 3.1: primary care-based interventions
Primary care-based interventions
Mapping pathways of prescriptions among cancer patients during their last year of life
We began by seeking to understand existing patterns of prescriptions for strong opioid treatment in patients with cancer and to identify current practice. We investigated this through a regional analysis of prescribing data on patients who had died from cancer.
In parallel, we undertook a systematic review to synthesise the research evidence on the relationship between strong opioid analgesia and survival in patients with advanced cancer. We hypothesised that one of the reasons that patients with advanced cancer pain are often undertreated may be that the patient or their clinician perceive that strong opioids used in the terminally ill population can hasten death. By undertaking this systematic review, we hoped that it would be possible to substantiate or refute this perception.
Opioid prescribing for patients with cancer in their last year of life
Ziegler et al.58
We originally set out to understand the prescription pathways in a population of 400 deceased cancer patients during their last year of life, including the analgesics prescribed (e.g. paracetamol, codeine, strong opioids) and the adjuvant analgesics, such as antidepressants and antiepileptics, often used for cancer neuropathic pain. We were able to substantially expand the original scope and increased the study population to 6800 patients, accordingly increasing the power of our study to detect and test associations. We used an innovative data linkage system (i.e. Openpseudonymiser) to link data derived from the primary care electronic patient record system (SystmOne) within the electronic system within a large acute NHS trust (Patient Pathway Manager) and data from the Northern and Yorkshire Cancer Registry. Comprehensive linked data on 6080 patients who died from cancer over the 7-year period (2005–12) were retrieved and analysed. To the best of our knowledge, this is the most comprehensive data set of its type and is of international significance to the field.
For all patients included in the linked cohort, all prescriptions for analgesics were identified for the period 12 months prior to their death. These prescriptions were analysed to determine to what extent and for what duration strong opioids were provided, exploring any differences that arose when analysing prescriptions by clinical and patient characteristics. Strong opioids were prescribed for 48% of patients in the last year of life (Figure 7). The median interval between the first prescription of a strong opioid and death was 9 weeks (interquartile range 3–23 weeks). Prescribing was not influenced by cancer type, duration of illness or sex, but we found a strong association with patients’ age, with older patients much less likely to be prescribed a strong opioid. Patients who died in a hospital were 60% less likely to have received a strong opioid in primary care before admission than patients who died in a hospice [relative risk ratio 0.4, 95% confidence interval (CI) 0.3 to 0.5; p < 0.01].
FIGURE 7.
Cumulative proportion of patients (%) prescribed analgesics week by week for the last year of life.
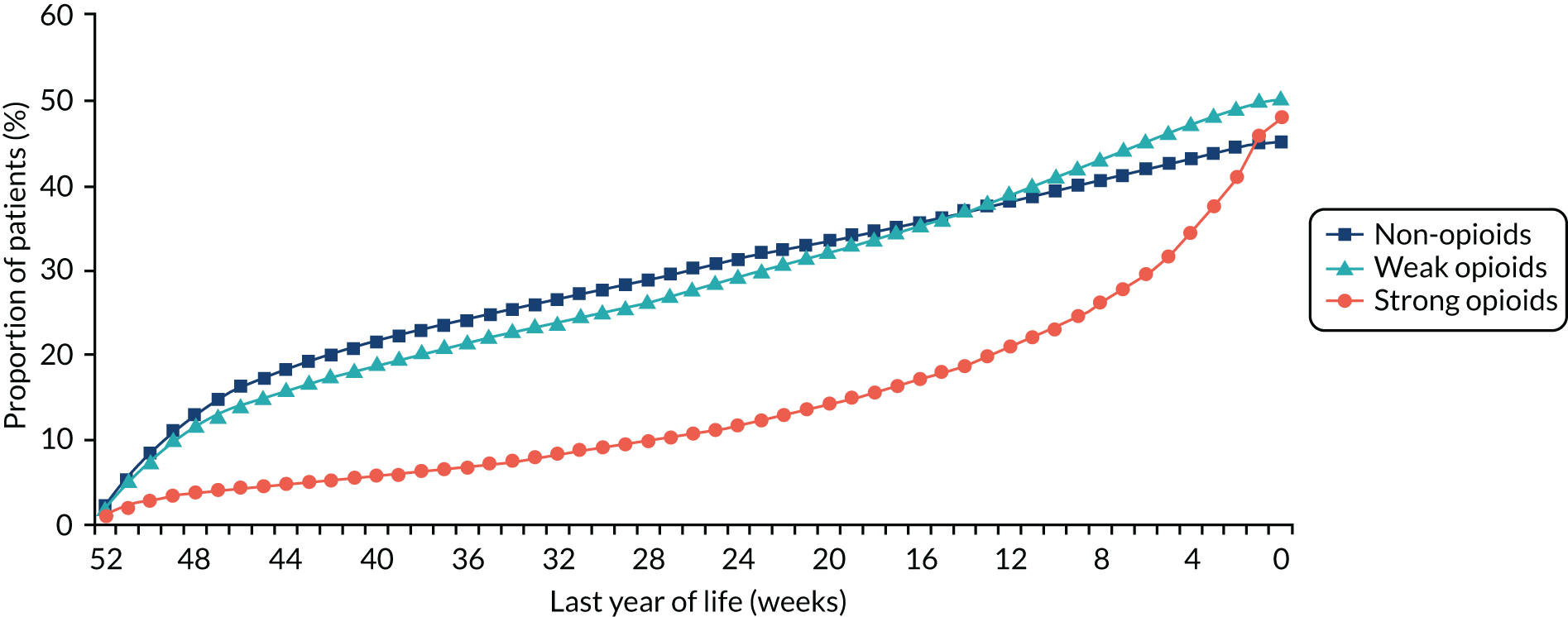
The study provides the first detailed analysis of the relatively late onset and short duration of strong opioid treatment in patients with cancer before death in a representative UK cohort. This pattern of prescribing does not match epidemiological data, which point to the earlier onset of pain. 5 Although the persistent under-treatment of cancer pain is well documented, this study suggests that strategies for earlier pain assessment and the initiation of strong opioid treatment in community-based patients with cancer could help to improve pain outcomes. In addition, our finding of unexplained variation in the prescribing of opioids for younger and older patients indicates a previously unknown inequity in access to pain relief. This research has been published. 58
Association between regular systemic opioid analgesia and survival in adult patients with cancer
Boland et al.59
The effects of opioids can be concerning for some clinicians and patients, with specific concerns relating to the impact of opioids on length of survival. These concerns can lead to reductions in opioid prescribing and issues with patient compliance, culminating in a detrimental impact on symptom control. 60 This study was conducted to determine whether or not there is an association between opioid analgesia and shorter survival, specifically for adult patients with cancer. We systematically searched for studies that assessed the effect of regular systemic opioid analgesia on survival. We identified 526 unique records, with 20 articles meeting the inclusion criteria. Thirteen end-of-life studies (11 of which were very low-quality retrospective studies) did not find a consistent association between opioid analgesic treatment and survival. The findings from this review should be interpreted with caution owing to the low quality of most of the studies that were included. In total, the included studies comprised three RCTs and two prospective studies. Six of these studies indicated that opioids were likely to be associated with shorter survival, none of which was powered to assess the effect of opioids on survival as a primary end point. In view of this, no definitive conclusions can be made as to whether or not opioids affect survival in patients with cancer. These data suggest that, although opioid analgesia does not affect survival at the end of life, in the context of longer-term treatment higher-quality studies, with survival as a primary end point, are required to confirm any independent association between opioid analgesia and shorter survival. An important limitation of research in this field is that the relationship between greater analgesic requirements and shorter survival may be mediated by painful progressive cancer; this mediation is not examined in research. This research has been published. 59
Non-medical prescribing by nurses and pharmacists to support timely access to prescriptions for analgesics
Our second set of studies investigated the impact of a policy change in NMP that has the potential to improve timely access to analgesia for cancer patients through community palliative care nurse and pharmacist prescribers. During our programme development grant (PDG) (RP-DG-1108-10010), we identified that the role of non-medical prescribers working in palliative care was due to expand, and in 2012, the year that this programme grant began, the UK Department of Health and Social Care issued legislation that enabled nurses and pharmacists with appropriate NMP qualifications to prescribe controlled drugs to patients within their field of professional expertise. Prescribers anecdotally reported improvements in patient care and patient safety, better use of health professionals’ skills and an increase in the amount of flexible team working. However, there was a lack of empirical evidence of the clinical and economic impacts, limiting the understanding of the future role of non-medical prescribers in a health-care system serving an increasing number of people with palliative care needs. We sought to measure the impact of this legislation and concurrent nurse prescribing in the UK through regional and national surveys of nurse and pharmacist prescribers and to assess the extent of NMP within overall palliative care prescribing.
Surveys of non-medical prescribing in palliative care
Ziegler et al.61
We undertook what is, to our knowledge, the first study of NMP in palliative care in almost one decade to explore the current position in the UK and the impact of the 2012 legislative changes on practice. An online survey of nurses in a regional cancer network was conducted in mid-2013, to which 37 nurses responded. We also undertook a national survey of pharmacist prescribers in the palliative care pharmacist network (n = 71). Although these surveys found that non-medical prescribers embraced the 2012 legislative changes and prescribed a wide range of drugs for cancer pain, we also identified scope to maximise the economic and clinical benefits by improving the transition from qualified to active non-medical prescriber by reducing the time interval between the two. Our findings indicate that nurses who are considering undertaking training to be a non-medical prescriber may be encouraged by the provision of adequate study leave and support to cover clinical work. We did not identify any substantial barriers to NMP in either survey. In our original grant application, we planned to conduct case studies to understand the health system-wide factors facilitating or inhibiting NMP. However, the lack of a national NMP sampling frame precluded a robust sampling strategy and, therefore, we decided not to proceed. Participants in our multistakeholder event, at which the findings of the NMP surveys were discussed, recommended amending our research plan with an additional study to chart any changes in NMP activity in palliative care nationally, and establish a better understanding of the proportion of NMP activity in relation to medical prescribing undertaken in community palliative care. This research has been published. 61
Analysis of growth and impact of non-medical prescribing
Zielger et al.62
We developed a novel methodology to establish the level of non-medical prescribers’ activity in palliative care across England and to consider the likely overall contribution of non-medical prescribers at a national level in this context in relation to medical prescribing. A ‘basket’ of 10 palliative care drugs was co-developed with experts in prescribing research and a range of clinicians. All prescriptions for these ‘core’ palliative care drugs prescribed by GPs, nurses and pharmacists in England and dispensed in the community between April 2011 and April 2015 were extracted by NHS Digital from the electronic Prescribing Analysis Cost Tool (ePACT) system. The data were broken down by type of prescriber and a basic descriptive analysis of prescription frequencies by opioid, non-opioids and total prescriptions by year was undertaken. To evaluate the yearly growth of NMP, the total number of prescriptions was compared by year for each of nurses, pharmacists and GPs.
We found that, overall, the total number of total prescriptions issued by NMPs rose by 28% per year compared with 9% of those issued by medical prescribers, demonstrating some impact of the 2012 legislative change. In addition, there was an increase in the total number of opioid prescriptions that had been issued by NMPs, which had risen by around 30% year on year. This rose by 31% in 2012–13, by 28% in 2013–14 and by 33% in 2014–15. Similar to the increase in NMP for opioids, non-opioid prescriptions also showed an increasing trend, with a 32% increase during 2012–13. During ensuing comparative years, there was a slowing rate of increase, with a 12% increase during 2013–14 and a 10% increase during 2014–15. This suggests that, during 2011–15, increases in the total number of prescriptions from non-medical prescribers can be largely attributed to increases in prescriptions for opioids.
However, the annual growth in non-medical prescribers’ prescriptions represented < 1% per year of total community palliative care prescribing activity in England. We identified unexplained geographical variation with a small number of ‘hot spots’ of palliative care prescribing by non-medical prescribers. At the current rate of growth, it would be 20 years before non-medical prescribers were prescribing 25% of all drugs issued in community palliative care. Although our findings confirm that more patients are receiving prescriptions in palliative care from non-medical prescribers, the findings indicate a significant gap between policy and implementation. Prior to our study, to our knowledge, the only evidence on patterns and trends in non-medical prescribing was from local service-level audits. This study demonstrates, for the first time, that, although a growth in NMP is evident, the number of prescriptions issued in palliative care remains small in relation to medical prescribing and there is potential for further change. Non-medical prescribers manage a full range of drugs and their handling of opioids is proportionally greater than that of doctors. Furthermore, although their total number of prescriptions may be small relative to all prescriptions, a large proportion of this activity relates to end-of-life care and pain management. This research has been published. 62
Work package 3.2: pharmacy-based interventions
Pharmacy-based interventions
Although community pharmacists are well placed to provide advice and support in pain management, our PDG work demonstrated that, in practice, they are not currently part of the palliative care team, they lack access to even the most basic NHS information (so do not know if a patient has cancer), and there is little communication between professions about patients with cancer pain. 1 Furthermore, although community pharmacists can provide a NHS medicines consultation [Medicines Use Review (MUR)], this was rarely carried out for patients with cancer pain. To explore the potential for intervention at the community pharmacy level, we sought to model methods of enhanced communication between pharmacies and members of primary care and palliative care teams, examine continuity of pharmacy use and model a MUR for patients with cancer pain and their carers.
Modelling methods of enhanced communication between pharmacies and members of primary care and palliative care teams
We originally planned to explore the effects of both ‘soft’ (health professional personal referrals to and from community pharmacies) and ‘hard’ (ICT integration) networking on patient-centred communication between health professionals. We recruited five GP practices and nine community pharmacies that the practices knew dispensed many of their prescriptions. At the time that we submitted our PGfAR report results, the NHS had introduced a demonstrator site in a single area in which community pharmacies and general practice clinical systems were linked, and Bradford was set to be the second site. In the event this did not proceed because of a NHS ICT policy change, so we focused on the development of personal referrals and of a patient-held cancer pain medicines record.
Survey of health professionals’ views on community pharmacy services for patients with cancer pain
Edwards et al.63
We explored the knowledge, experience and opinions of health professionals regarding community pharmacist input for patients with cancer pain through a structured online survey in two clinical commissioning groups (CCGs). GPs and nurses from those practices, a local practice pharmacist group and community and outpatient-based palliative care nurses from the city hospital and hospice were invited to take part. The 40 respondents, who represented all health professional groups, were divided in their opinion of whether or not medicinal support needs were already being adequately met by the palliative care team. Although a large majority of respondents agreed that community pharmacists should become part of the palliative care team, additional training for cancer pain management and consultation skills was thought to be needed. Lack of access to patient records was viewed by most as a barrier to community pharmacist involvement. There was strong support for read access and slightly less so for write access to the GPs’ clinical system. Remote provision of medicine consultations by telephone was strongly supported, but Skype™ (Microsoft Corporation, Redmond, WA, USA) consultations less so. Concerns raised by some respondents in additional comments were potential duplication of services and the perception that if the patient was already under specialist palliative care services then community pharmacists would provide little benefit.
The results showed a mixed picture, with some appetite for closer working with community pharmacists, acknowledgement of the need for shared access to patient records and questions about community pharmacists’ consultation skills and knowledge of cancer pain management. This research has been published. 63
Evaluating recruitment methods used in a pharmacist-led cancer pain medicines consultation study
Edwards et al.64
In this part of the work package we also attempted to establish pathways to identify and refer patients to a community pharmacy for a MUR in our planned feasibility study. Recruitment of patients with advanced cancer into health services research studies is known to be challenging. Our aim was to assess the effectiveness and efficiency of different recruitment methods, and the study also enabled us to observe the extent to which health professionals engaged in referral of patients for a medicines consultation. The methods of recruitment were community based (general practice computer search and letter of invitation, health professional identification and referral of patients, and hospital oncology outpatient clinic list search followed by postal invitation) and hospice based (hospice staff introducing the study to inpatients and day-care patients). Recruitment via general practice computer searches and letter of invitation was potentially efficient but with a low rate of recruitment, compounded by restrictions of NHS regulations for the MUR service. We found a lack of engagement by health professionals, with only two GPs and no community specialist nurses referring patients. In the hospice setting, the personal involvement of hospice staff and the presence of the researcher to answer any questions facilitated recruitment. The overall recruitment rate was in line with that of other studies in this patient group. Our methods were less successful at recruiting patients who were not already engaged with hospice services and this remains a challenge for future research. This study has been published. 64
Patient views of pharmacist medicines consultation
Edwards et al.65
We conducted a qualitative study to explore how patients with pain from advanced cancer currently use community pharmacies and their attitudes towards medicines consultations from pharmacists delivered in a pharmacy or remotely by telephone or Skype. Purposive sampling of GP clinical information systems was used to recruit patients with advanced cancer; 13 patients took part in a semistructured interview that was audio-recorded and transcribed verbatim. Around half of the patients were receiving palliative care services. All patients reported using a single regular community pharmacy; several had changed their pharmacy during the course of their cancer treatment, mainly to improve timely supply of their medicines. Patients’ expectations of what community pharmacists and their teams might provide were low, with convenience, service and staff friendliness as influential factors. Despite MURs having been available for over 10 years, only one patient reported that they had received a MUR, and awareness of MURs was generally low. Nevertheless, the idea of a community pharmacy medicines consultation was acceptable to most patients, with telephone consultations positively received but Skype or other electronic media not being feasible or acceptable for most. Patients perceived a hierarchy of health professionals from whom they might actively seek advice about pain management, with those patients in contact with specialist palliative care nurses placing those nurses at the top (owing to their combined knowledge of their condition and medicines), followed by GPs and then community pharmacists. Patients who were receiving specialist palliative care described pain that was better controlled and thought that medicines consultations with a pharmacist would have been useful prior to their referral for palliative care. Nevertheless, the interviews indicated that both patients who were not receiving palliative care services and patients who were receiving palliative care services had unresolved medicines-related problems (MRPs). The study findings showed a need for pain medicines support for patients with advanced cancer, and unmet need appears greater for those not under the care of specialist services. Medicines consultations, in principle, are acceptable to patients both in person and over the telephone, particularly for patients less able to leave the house. This research has been published. 65
Modelling a Medicines Use Review for patients with cancer pain and their carers
Systematic review of pharmacist educational interventions for cancer pain management
Edwards et al.66
We conducted a systematic review and meta-analysis; of the 989 studies identified, four were included (three from China and one from the UK) and comprised 944 patients. Study interventions ranged from 5 to 12 pharmacist consultations, with a follow-up period of between 8 days and 6 months. Studies were of varying quality and bias was detected in all. All studies included pain assessment as an outcome. Meta-analysis found a reduction in pain intensity following the intervention of 0.76 (on a 0–10 pain scale) in the intervention arm compared with the control arm, with a 95% CI. Improvements in patient knowledge, reductions in side effects and increased patient satisfaction were also found. The results indicate that pharmacist educational interventions are effective in reducing pain from cancer, but this finding should be treated with caution because of the low study quality. The review demonstrated that more studies of better quality and with homogeneous outcome measures are needed. This research has been published. 66
Feasibility and acceptability of pharmacist medicines consultation
Edwards et al.67
We held a multistakeholder workshop to explore whether or not existing community pharmacy medicines consultations might be used for patients with pain from advanced cancer. Participants included community and general practice pharmacists, palliative care health professionals, patients, commissioners, researchers and educators. We found that many of the health professionals who attended knew little about MURs but were positive about them, and they identified methods of integrating MURs into patient pathways in advanced cancer and of identifying signposting and referral routes to and from pharmacies.
We recruited patients with advanced cancer living in the community using the methods described in Patient views of pharmacist medicines consultation, Edwardset al. , on page 28. 67 Of a total of 128 patients identified, 47 met the inclusion criteria and 23 agreed to take part. Most (n = 17) of the 19 patients who completed the study were from a hospice setting. In the course of the study, very few patients met the NHS criteria for eligibility for MUR in our nine pharmacies (because they had their prescriptions dispensed at other pharmacies), so we decided to introduce research pharmacist-provided telephone medicines consultations. In addition to increasing the number of patients who could take part, this meant that we could test a medicines consultation with follow-up, a model analogous to the New Medicines Service.
Consultations involved either one face-to-face consultation with a community pharmacist or two telephone consultations with a research pharmacist. Community pharmacists are already trained and accredited to provide MURs. We asked them to attend an interactive learning session designed to address barriers identified in our earlier research: fears about talking to patients with advanced cancer and being disconnected from the local palliative care team. Pharmacists spent time at workstations where a pre-briefed palliative care nurse, a patient and a representative from a local cancer charity drop-in centre raised these issues, encouraged participants to voice concerns and offered suggestions and tips. Five patients subsequently had a community pharmacist-delivered consultation and 14 had consultations conducted by the research pharmacist. Patients completed questionnaires about pain levels, self-perceived knowledge and the acceptability of the pharmacist consultation before and after the consultations. Pharmacists documented details of MRPs identified and these were categorised and analysed using a validated typology.
In total, the 19 patients had 33 consultations in which 47 MRPs were identified (range 0–7; median × per patient). Pharmacists resolved most (n = 38) MRPs with the patient by providing advice. There were 10 referrals to other health professionals. The most common MRPs identified were pain and constipation of varying severity. Tiredness, other side effects and concerns about concomitant medicines were also identified. Most referrals to other health professionals were to initiate treatment with laxatives or to change pain medicines. Eleven patients, most of whom said that they would recommend the consultation to others, completed both the pre- and the post-consultation questionnaires. A small number of patients reported that their knowledge about pain medicines had increased.
These findings suggest that many patients with advanced cancer have unresolved MRPs, even when they are receiving specialist palliative care. Medicines consultations with a pharmacist resolved many of the problems through advice and education, and enabled referral to prescribers to optimise treatment and manage side effects. The vast majority of MRPs required ‘generalist’ rather than ‘specialist’ knowledge; therefore, they fell within the current expected knowledge and scope of practice of community pharmacists, confirming the finding from our PDG study that there is not a knowledge deficit. 68
Some aspects of acceptability and feasibility of delivering medicines consultations were demonstrated. We were able to recruit patients to the study and to retain four in five of them. However, only one in two patients completed both pre- and post-consultation questionnaires, limiting the conclusions that we could draw about patients’ experiences. Other limitations of the study include the small number of patients who were not receiving palliative care services; the difficulty of establishing a workable referral pathway from GP practices to community pharmacies; and that most consultations were conducted by the research pharmacist, hence limiting generalisability. Further research is needed to refine the intervention and delivery pathway prior to a future trial.
Work package 4: can a cancer pain pathway (based on work packages 1–3) be clinically effective and cost-effective in reducing pain and related distress, and on reducing pain-related hospital admissions?
Work package 4 (Figure 8) sought to determine whether or not a cancer pain pathway (comprising pain self-management interventions developed across prior work packages) could be clinically effective and cost-effective in reducing pain and related distress, and reduce pain-related hospital admissions. The overarching research question for this work package was ‘Can a cancer pain pathway (based on work packages 1–3) be clinically effective and cost-effective in reducing pain and related distress, and on reducing pain-related hospital admissions?’
FIGURE 8.
Work package 4.
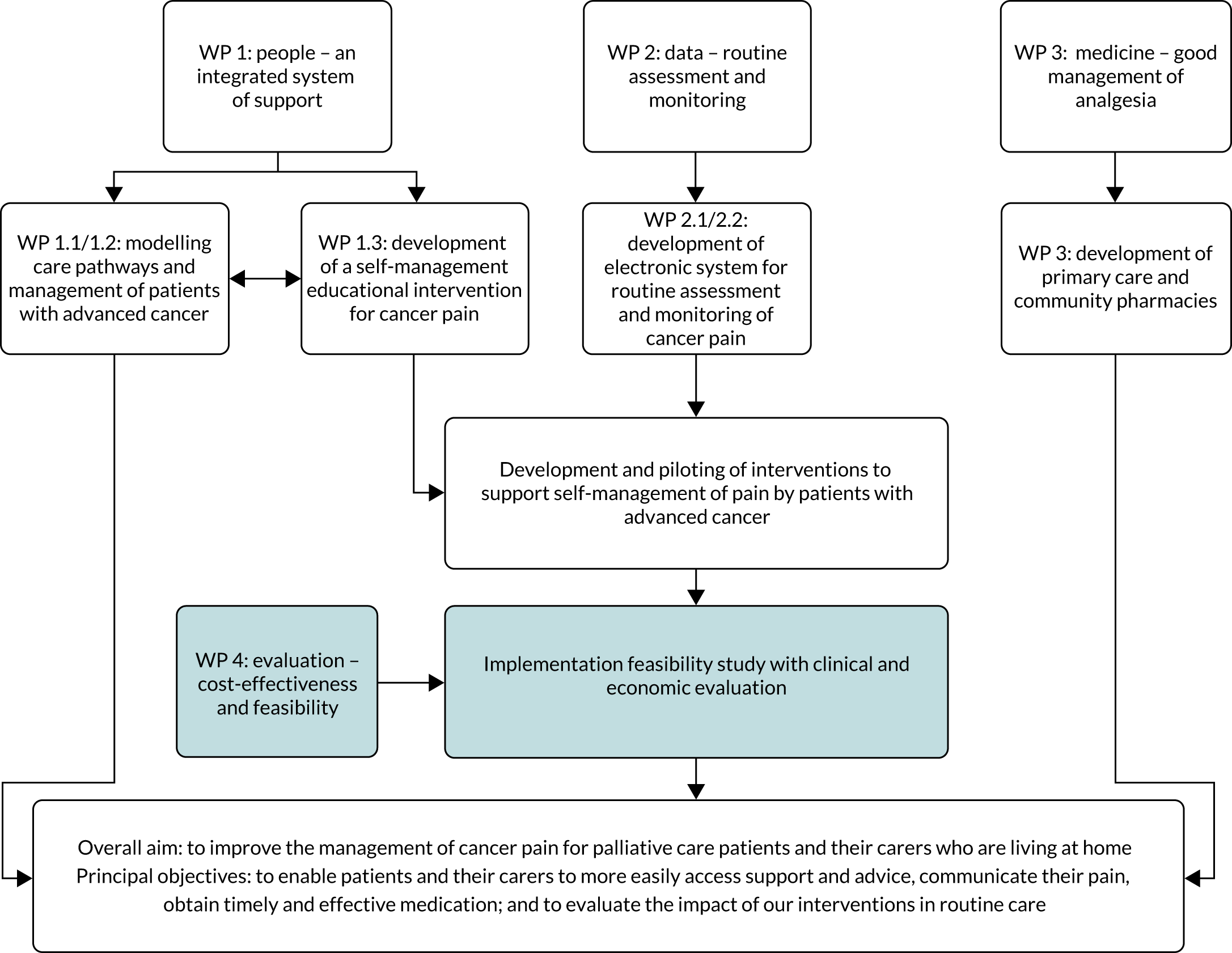
Work package 4: evaluation – cost-effectiveness and feasibility
Research activities were broken down into two strands of activity, with the following underpinning research questions:
-
What is the most appropriate measure of utility and the relative importance of different aspects of cancer pain management to service users?
-
Can the implementation of evidence-based interventions into routine clinical practice be evaluated in terms of fidelity and impact on pain and health-care resource use?
This first half of this chapter relates to the first of these subquestions, outlining the development of the most appropriate measure of utility and the relative importance of different aspects of cancer pain management to service users. This work was carried out to inform the development of an economic model for use in an implementation feasibility trial, detailing the incremental cost-effectiveness ratio (ICER) for a pain self-management intervention compared with standard care for pain management in the treatment of people with advanced cancer. The second half of the chapter focuses on the findings from a multicentre RCT, assessing the clinical effectiveness and cost-effectiveness of a pain self-management multicomponent intervention developed through the research activities outlined in earlier chapters.
Work package 4.1: developing a model for economic evaluation
Prior to the start of the clinical trial evaluating SSM, we conducted work to inform how QoL (utility) and resource use might best be captured to enable a full economic evaluation of trial data. We approached this by asking patients and carers to complete a survey that included a resource use questionnaire, pain assessments and several ways of capturing health state utility. We also included in this a stated preference survey [discrete choice experiment (DCE)] to understand which aspects of pain management services were most important to them.
These survey data in turn were used to inform the development of a decision model to estimate the cost-effectiveness of SSM. The purpose of the model was not only to enable an economic evaluation of the interventions prior to the completion of the trial, but also to enable any costs and benefits observed in the trial, to be extrapolated forward for a longer time horizon. A separate trial-based economic evaluation was undertaken following completion of the multicentre RCT.
Costs and quality of life associated with pain in patients with advanced cancer
We conducted a cross-sectional survey with patients with cancer pain and their carers to establish the feasibility of utility and health-care resource use data collection in this group; to explore the validity of alternative measures of utility [including the EuroQol-5 Dimensions (EQ-5D), ICECAP-A (ICEpop CAPability measure for Adults) and EORTC-8D (European Organisation for Research and Treatment of Cancer – 8 Domains)]; to generate utility and cost parameter values for a decision model; and to determine whether or not proxies could report on behalf of patients. Two-hundred and forty-eight patients completed the survey. There were minimal missing data and no ceiling/floor effects on the utility measures. There were more missing data on the resource use measure. All utility measures correlated well and all discriminated between pain severity groups (no/mild, moderate, severe) but the EQ-5D appeared better able to do this than the ICECAP-A and EORTC-8D. Although formal and informal carer reports of utility correlated with those of the patient, there were non-trivial differences between these reports and the informal carers represented a better proxy than formal carers. Results suggest that patients are able to complete measures at the end of life and that proxy reports may not be necessary or desirable.
We were able to use the survey data to generate cost and utility parameter values for a decision model and inform the data collection of the IMPACCT trial to enable an economic evaluation. Given the degree of missing data in patient-reported resource use we experienced here, we made use of administrative data to a larger degree in the trial.
Understanding patient preferences
We conducted a DCE with patients to understand their preferences for various aspects of pain management services. This also provided information on patient preferences for alternative pain management service types.
Meads et al.69
A DCE and focus groups with patients were undertaken to understand their preferences for pain management services and to inform service development. Focus groups were used to develop the attributes and levels of the DCE. The attributes were waiting time, type of health professional, out-of-pocket costs, side effect control, quality of communication, quality of information and pain control. Patients completed the DCE along with clinical and health-related quality-of-life (HRQoL) questions. Conditional and mixed logit models were used to analyse the data. Patients with cancer pain (n = 221) and within palliative care services completed the survey (45% female; mean age 64.6 years; age range 21–92 years). The most important aspects of pain management were good pain control, zero out-of-pocket costs and good side-effect control. Poor or moderate pain control and £30 out-of-pocket costs were the least preferred aspects. Respondents preferred control of side-effects and provision of better information and communication over access to certain health professionals. Those with a lower HRQoL were less willing to wait for treatment and more willing to incur higher costs than those with a higher HRQoL. The presence of a carer influenced preferences. Outcome attributes were more important than process attributes but the latter were still valued. Thus, supporting self-management, for example by providing better information on pain, may be a worthwhile endeavour. However, service provision may need to account for individual characteristics, given the heterogeneity in preferences. This research has been published. 69
Health economic model development
Meads et al.70,71
We constructed a decision-analytic model to estimate the cost-effectiveness of supported self-management (SSM) interventions compared with UC. The model would enable an estimate of cost-effectiveness prior to the completion of the trial and would allow the possibility of evaluating the cost-effectiveness of other interventions and of testing scenarios. However, the main motivation for the model development was to facilitate the extrapolation of costs and benefits from the trial over a longer time horizon (should that be warranted). The model was a Markov model structured around pain severity health states (no/mild, moderate, severe pain), each of which had associated costs and QoL/utility estimates (which were informed by the patient survey). It estimated costs and quality-adjusted life-years (QALYs) for a cohort of individuals with cancer pain over 1 year. We evaluated PainCheck (online pain reporting system) and the Tackling Cancer Pain toolkit (an education self-management resource) separately against UC and took the effectiveness of these (measured in terms of change in pain rating scores) from published meta-analyses of similar interventions. 37 This research has been published. 70,71
The results indicated that both PainCheck and the Tackling Cancer Pain toolkit would be cost saving and cost-effective but, owing to the higher implementation costs and lower assumed effectiveness of the former, the latter intervention offered the greater value for money. These results were relatively robust to a series of sensitivity analyses. However, it should be noted that the conclusions from the modelling may have been different had the meta-analysis results it incorporated been updated with the WP 4.2 trial results.
Work package 4.2: pragmatic multicentre randomised controlled trial
Aims and research questions
Having developed two evidence-based interventions, an educational resource (Tackling Cancer Pain) and a pain monitoring system (PainCheck) in earlier work streams, we sought to assess the feasibility of implementation, effects and cost-effectiveness in a RCT. A protocol for the implementation feasibility RCT has been published previously. 70
The trial is registered with the ISRCTN registry, with reference 18281271. Details of the registration can be found at the following link: https://doi.org/10.1186/ISRCTN18281271.
The combined intervention (UC plus SSM), delivered by community palliative care, aimed to:
-
improve the management of cancer pain for palliative care patients who are living at home
-
enable patients to more easily access support and advice, communicate their pain and obtain timely and effective medication
-
educate patients on Tackling Cancer Pain through an educational intervention.
Trial methods
Summary of trial design
IMPACCT is a pragmatic multicentre RCT in patients with active and incurable cancer that aimed to evaluate the delivery and implementation of evidence-based interventions into routine clinical practice, developed and piloted as part of the IMPACCT programme grant.
We aimed to recruit 160 participants at the point of identification for referral into palliative care. Participants were randomised on a 1 : 1 basis to receive either the intervention [UC plus SSM delivered within the oncology clinic and palliative care services by locally assigned community palliative care nurses (health professional), consisting of self-management/educational support and pain monitoring], or UC. Participants were identified and recruited in the oncology (or related) clinic by the research nurse in consultation with the patients’ clinician/treating team. All participants were referred to their local palliative care team for pain management. The recruiting team were not involved in subsequent intervention delivery.
The follow-up period of the trial was 12 weeks, with patient-reported outcome measures collected by post at 6 and 12 weeks following randomisation. When required, the blinded IMPACCT trial researcher collected these data by telephone. Medical data were obtained directly from patient hospital and palliative care records by the research nurse and palliative care team, respectively. All participants were followed up for overall survival until the final participants’ 12-week follow-up.
A subsample of consenting participants and health professionals were invited to take part in an interview at approximately 6 or 12 weeks post randomisation (see Process evaluation).
Protocol amendment
A substantial amendment to the protocol and trial design was implemented early on in the trial, 6 months after opening to recruitment and after 13 participants had been recruited (Table 1). The original protocol (v3.0) had an additional aim to evaluate early screening and referral. However, early screening and recruitment methods were scrutinised and it was not feasible to implement an early referral pathway into the existing cancer pain pathways in the RCT. Once our screening procedures had identified cancer patients with significant pain (≥ 4 on a rating scale of 0–10), oncology staff were reluctant to allow patients to be randomised, potentially to UC, and felt obliged to make a clinical referral to palliative care. With the support of the TSC, we agreed an amendment to recruit at the point of referral so that all patients eligible for community-based palliative care could be recruited and randomised, with community palliative care referral in both trial arms, essentially removing the early referral pathway of the intervention.
| Pre-amendment (protocol v3.0, n = 13) | Post-amendment (protocol v4.0, n = 148) | |
|---|---|---|
| Eligibility criteria | ||
| Inclusion criteria | Has the patient been diagnosed with advanced incurable cancer in one of the following disease areas:
|
Has the patient been diagnosed with advanced incurable cancer? |
| Exclusion criteria | Has the patient previously been referred to the palliative care team? | Has the patient previously received or is currently receiving community palliative care? |
| Trial arms | ||
| Intervention arm | Pain management support
|
UC plus SSM
|
| Control arm | Standard care
|
UC
|
Recruitment, setting and participants
For full details of recruitment procedures, see Allsop et al. 70 The inclusion criteria for patients were that they (1) were male or female aged ≥ 16 years; (2) had a diagnosis of advanced incurable cancer (locally advanced or metastatic) and were experiencing cancer-related pain (tumour or treatment related), with a pain score of ≥ 4 on the ‘average pain’ item of the BPI; (3) had the potential to benefit from pain management; (4) had an expected prognosis of ≥ 12 weeks; (5) were living at home; (6) lived in the local catchment area of a participating hospice; and (7) were able and willing to provide written informed consent. The exclusion criteria were patients who (1) were currently receiving or had previously received community palliative care support; (2) had insufficient literacy or proficiency in English to contribute to the data collection required for the research; (3) lacked capacity to provide informed consent to this trial; or (4) had dominant chronic pain that was not cancer related (tumour or treatment).
Randomisation and blinding
A computer-generated minimisation programme incorporating a random element was used to randomise participants to either the UC plus SSM arm or the UC arm. Randomisation was completed on a 1 : 1 basis. The randomisation process ensured that trial arms were well balanced for both the recruiting site and the participants’ average pain scores at baseline using the BPI score ranges (4–6, 7–10).
Participants, clinicians, research nurses in the recruiting clinics and palliative care nurses were, of necessity, aware of treatment allocation, but the collection of patient outcomes via the IMPACCT trial researcher was blinded.
Intervention
The intervention components are outlined in Table 2, reporting according to the Template for Intervention Description and Replication (TIDieR) checklist. 79
| Intervention components | Description |
|---|---|
| UC (received by both arms) |
Referral to community palliative care: screening of patients with pain from advanced cancer will be implemented by optimising entry points to the care pathway via oncology (or related) outpatient services and will facilitate the flow of patients to appropriate pain support as and when required. Oncology research nurses will refer trial participants to the local community palliative care team, at which point the locally assigned palliative care nurse will endeavour to arrange an initial visit/appointment with the participant within 1 week of randomisation Appointment into palliative care: this will take place with a locally assigned community palliative care nurse. Routine practice will be followed, including an assessment of the participants’ other palliative care needs. For those participants allocated to receive UC and SSM, the nurse will be trained in the trial interventions and will introduce and deliver the trial interventions described below, alongside their UC |
| Patient self-management educational intervention |
Name: Tackling Cancer Pain: A Toolkit for Patients and Families Why: Tackling Cancer Pain: A Toolkit for Patients and Families is based on a review of current evidence and on focus group interviews with patients, family caregivers and health professionals working in specialist palliative care. 37,38 Providing information to patients with cancer pain and addressing concerns regarding pain and analgesia are effective interventions that support self-management and lead to improvements in pain outcomes72–76 What: Tackling Cancer Pain: A Toolkit for Patients and Families is formatted as a loose-leaf ring binder with an accompanying DVD. It consists of five sections: understanding cancer pain, using drugs to manage pain, additional approaches to managing pain, talking about pain and getting more help. Each section contains information and self-directed learning activities along with sources of further information. It is written in easily understandable lay persons’ language. The booklet and DVD contain essentially the same information but in different formats and are structured so that they can be used independently by patients or family members. Guidance is given in each chapter about how to use the information presented. Step-by-step tuition is provided on non-pharmacological pain relief measures such as relaxation and visualisation, and on how to initiate and conduct conversations about pain with health professionals Who provides: Tackling Cancer Pain: A Toolkit for Patients and Families will be introduced to participants by their trained locally assigned community palliative care nurse within 1 week of randomisation, and subsequent participant questions on the booklet and DVD can be addressed to this palliative care nurse. Training on the content and use of Tackling Cancer Pain: A Toolkit for Patients and Families will be provided by the trial researchers. The training will include written instructions on how to train participants in the interventions and a contact for any future queries How: Tackling Cancer Pain: A Toolkit for Patients and Families is accessed by the patient or their family by reviewing the information contained in the loose-leaf ring binder and on the accompanying DVD Where: the expectation is that Tackling Cancer Pain: A Toolkit for Patients and Families will be accessed by patients and their families while in the community setting (i.e. in their usual place of residence) When and how much: participants are not provided with details of schedule, duration, intensity or dose for using Tackling Cancer Pain: A Toolkit for Patients and Families. Instead participants are free to use the resource as they would like Modifications: no modifications to the intervention were made during the study How well (planned): intervention data collection (baseline) will identify that the patient received the intervention. Semistructured end-of-trial interviews with patients and health professionals will also inform how the intervention is used How well (actual): overall, baseline data determined that 72 patients were introduced to the intervention |
| eHealth intervention for routine pain assessment and monitoring in patients with advanced cancer |
Name: ‘PainCheck’ Why: the process of assessing pain and presenting data to physicians prior to consultation, who then use those within discussions, significantly improves pain outcomes and QoL for patients. 77 PainCheck was developed to facilitate this communication by enabling patients to routinely report and share pain data for health professionals to access. Its development was informed by patient, caregiver and health professional involvement52–55 What: PainCheck allows patients to record their pain and gives them access to personalised pain management advice. Patients are asked to answer questions about their pain, including providing a description of their pain, rating current pain intensity and intensity in the last 12 hours, pain control, interference and sleep. Items were taken from the BPI40 and the Coping Strategies Questionnaire. 78 Patients are asked about pain management techniques, which of these are helpful and how likely they are to try them in the future. A variety of question response options are used, including multiple choice, numerical (0–10 or 0–6) slider scales and free text. After completion, patients are provided with a summary of their results and suggestions of pain management techniques that they may want to try in the future. Health professionals can log in to PainCheck and view all patients registered on PainCheck and see who have completed reports. They are then able to select a patient and view responses to individual questions. Health professionals are presented with a graph that tracks patients’ current pain and pain in the last 12 hours over time. Patients are given a ‘red flag’ in the health professional system if they reach thresholds for current pain and pain control. After reading the patient report, health professionals can decide what action, if any, they would like to take as a result. Health professionals have the option to contact patients through PainCheck to provide information and advice PainCheck was developed using QTool. QTool is a secure online system, which can be integrated in real time with the electronic patient records, enabling the collection of patient-reported information (such as symptoms, treatment side effects, pre-clinic questions, satisfaction surveys). QTool was developed by the collaborative efforts of multiple research groups based at the University of Leeds, pooling approximately £400,000 of research funding. The version of PainCheck used in the trial did not utilise linkage with existing electronic clinical record systems; instead PainCheck was run as a standalone intervention Who provides: PainCheck is introduced to participants by their locally assigned community palliative care nurse within 1 week of randomisation. Training on using PainCheck with patients is provided by trial researchers to community palliative care nurses. Training includes instructions for training the participants in the interventions, which include a demonstration for the participant on how to log on and use the routine pain assessment and monitoring system at the initial visit How: PainCheck is introduced to patients by their locally assigned community palliative care nurse. The introduction involves participants logging in to the system (using unique login details that will be provided by the community palliative care nurse) and working through an assessment using the instruction leaflet as a guide. The palliative care nurse oversees the participant’s first access and use of PainCheck and provides additional support/guidance if necessary. At induction into the system, participants are made aware that PainCheck should not be used to request urgent or emergency help; when urgent or emergency help is required participants are told to contact the emergency services. On visiting PainCheck participants are reminded, by use of an on-screen message, that should they need immediate medical attention they should call the emergency services. If they require urgent medical advice they are advised to contact their doctor, nurse or pharmacist. This reminder is provided near the beginning and at the end of each PainCheck session. Following an introduction to PainCheck, participants (or a person submitting responses on a participant’s behalf) are expected to complete pain assessments without the community palliative care nurse present. Each pain assessment asks for clarification on who is entering data (i.e. a patient or someone on their behalf) which is reflected in reports when viewed by health professionals. Based on reports submitted by participants, the PainCheck system provides two types of e-mail alert to health professionals: high priority (generated immediately when a patient submits a report indicating high pain and/or low levels of control) and low priority (a weekly e-mail sent when participants are interacting with PainCheck but reporting very low levels of pain or no pain). After reviewing reports submitted to PainCheck, a health professional is asked to record in the system what action they took: (1) no further action, (2) contact another health professional, (3) contact the patient or (4) other. PainCheck can be accessed by patients and health professionals using any device that enables access to the internet (e.g. using laptop, smartphone or tablet computer) Where: the expectation is that PainCheck would be accessed by patients or their families while in the community setting, which could include usual place of residence or alternative chosen location When and how much: following the introduction of routine completion of PainCheck in the community, participants are encouraged to use the system at least once per day, with additional entries being encouraged when/if pain events occur. PainCheck is available to participants until 14 days after their 12-week follow-up assessment. Participants are notified in writing that access to PainCheck is ending Modifications: no modifications to the intervention were made during the study How well (planned): intervention data collection (at baseline) is used to confirm that a patient received details to access PainCheck. Data captured by PainCheck can provide insight into the frequency of use by patients, alongside identifying interaction between health professionals and patients that occurs through PainCheck (i.e. messages sent to patients by health professionals). Semistructured end-of-trial interviews with patients and health professionals are used to inform how the intervention is used How well (actual): baseline data showed that, of the 78 participants recruited and willing to participate in the trial, 47 (58.8%) are introduced to PainCheck. Of those introduced to PainCheck, 32 participants (51.6%) logged in. In total, 23 participants (40%) are deemed engaged with the intervention, logging in and submitting a pain report three or more times |
Trial data collection
For a full description of trial data collection, see Allsop et al. 70
Objectives
Objectives relate to both the feasibility of delivery and the implementation of interventions into routine practice and an assessment of the effectiveness of the intervention.
Primary objectives
-
Delivery and implementation of the intervention: the primary implementation objective is to evaluate adherence in terms of the uptake and retention rate of each intervention.
-
Potential effectiveness of the intervention: the primary effectiveness objective is to assess the effectiveness of the intervention compared with UC, as measured by pain severity on the BPI (the mean and the proportion of responders with a ≥ 30% reduction in the BPI pain score) 6 and 12 weeks after randomisation.
Secondary objectives
-
To assess differences in patient-rated pain, as measured using the BPI pain interference and the 7-point global rating of change in pain at 6 and 12 weeks.
-
To assess health-care use in each arm, in particular hospital admissions within 12 weeks of randomisation.
-
To assess patients’ pain knowledge and experience, as measured using the PPQ at 6 and 12 weeks.
-
To assess the differences in patients’ general and cancer-specific QoL as measured using the EQ-5D and EORTC QLQ-C30 at 6 and 12 weeks.
-
To document the cost-effectiveness of the interventions.
Furthermore, we aimed to report on the delivery and implementation of the intervention through a process evaluation using qualitative data to explore participant and palliative care nurse views.
Statistical methods
Sample size
To assess the effectiveness of the intervention, the power calculation was based on the difference in mean pain severity on the BPI at 12 weeks. With 80% power and a two-sided type I error rate of 0.05, we estimated that 128 patients (64 per arm) were required to detect a moderate intervention effect size of 0.580 between the intervention and the control arms.
In addition, a relative reduction of ≥ 30% in pain severity (BPI) is an accepted threshold for clinically significant improvement in pain trials. 81 Results from a previous study involving automated symptom modelling found a 27% difference in such improvement rates at 12 weeks. 82 Our estimated sample size also provided 80% power to detect a similar difference in rate.
As our patient sample was drawn from a generally frail population, we allowed for an attrition rate of 20% and, therefore, aimed to recruit 160 participants (80 per arm).
Data analyses
No interim analyses were conducted and all analyses were planned prior to the final analysis. Analyses were conducted on the intention-to-treat population, with all participants included in the analysis according to allocation regardless of non-compliance with the intervention. An overall two-sided 5% significance level was used for all statistical analyses.
The completion and scoring of patient-reported questionnaires were analysed in line with scoring manuals including guidance on how to handle missing items. If no direction was given, then scores were prorated if ≥ 50% of the items were completed, and the ‘worst’ response was taken when multiple consecutive responses were recorded.
We used descripitive statistics and CI estimations to evaluate the primary implementation outcome.
We compared the primary and secondary effectiveness patient-reported questionnaire outcomes using linear (and logistic regression, where appropriate) mixed-effects regression models with repeated measures (6 and 12 weeks) using an unstructured covariance pattern correlation structure. Analyses were adjusted for randomisation stratification factors and average pain (4–6/7–10), and baseline response. We undertook appropriate regression diagnostics to check the validity of the statistical modelling.
Primary analysis handled missing outcome data using multiple imputation. 83 The characteristics of participants with and participants without the primary outcome were summarised to explore the missing data mechanism. Logistic regression was used to determine whether or not missing data differed by participants’ baseline characteristics, and forward selection (with 10% significance level) was used to identify key participant characteristics predictive of missing data at 6 or 12 weeks for inclusion in a multiple imputation model. Sensitivity analysis were conducted to the availability of data.
For further details of the statistical methods, see Allsop et al. 70
Key findings
Study summary
Figure 9 presents the overall trial Consolidated Standard of Reporting Trials (CONSORT) flow diagram depicting the flow of participants through the study from screening to analysis, summarising screening, accrual, intervention receipt and participant follow-up at 6 and 12 weeks (see Report Supplementary Material 1, Table 14, for further details).
FIGURE 9.
Trial CONSORT flow diagram.
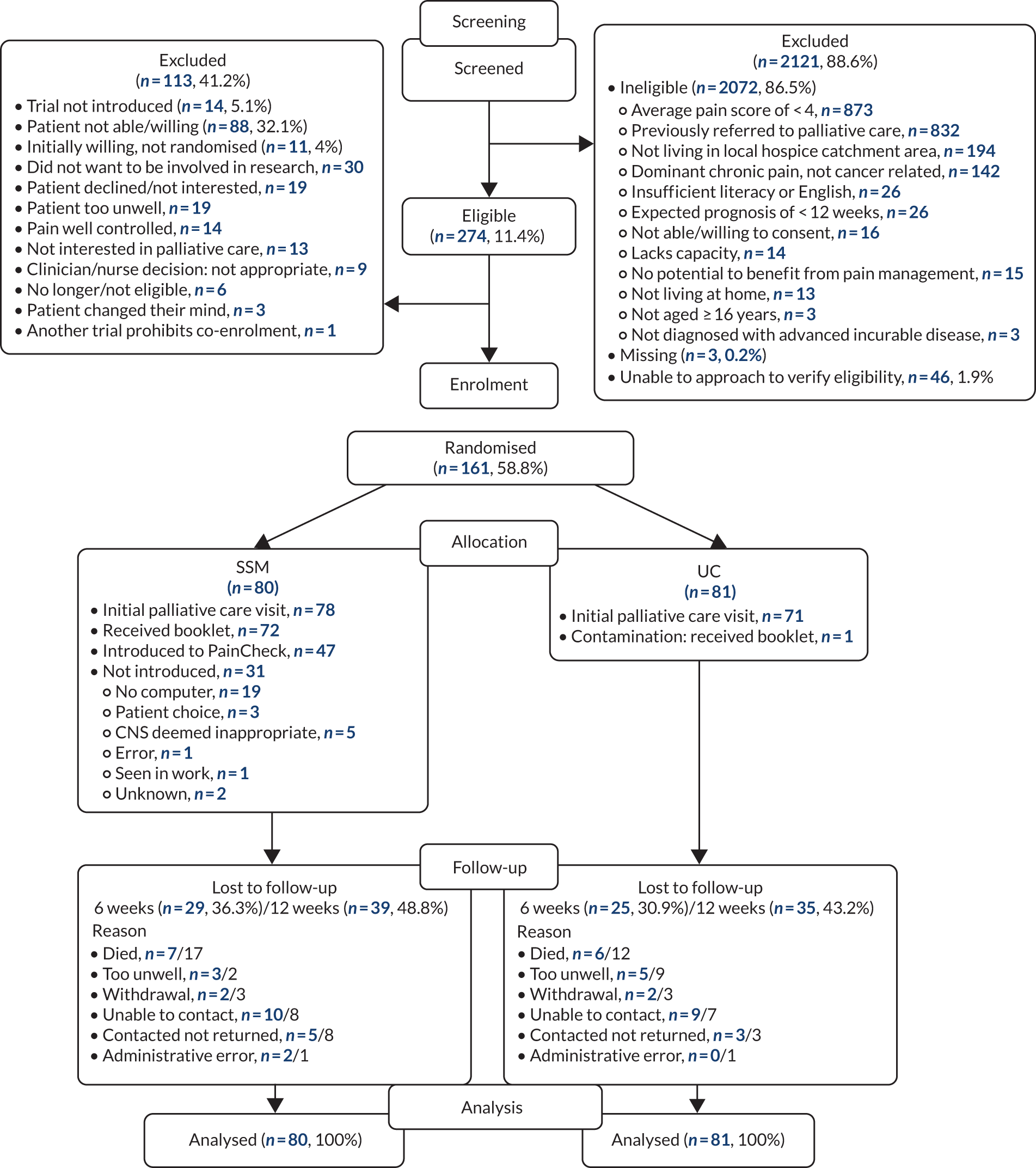
Screening and recruitment
Screening and recruitment took place between October 2015 and January 2018 across eight hospital sites (Figure 10; see Report Supplementary Material 1, Table 15). A total of 2395 patients were screened: 274 (11.4%) were eligible and 161 (58.8% of those eligible; 6.7% of those screened) were randomised into the trial (see Figure 9; see Report Supplementary Material 1, Figure 16); 81 participants were allocated to the UC arm and 80 were allocated to the SSM arm.
FIGURE 10.
Recruitment graph.
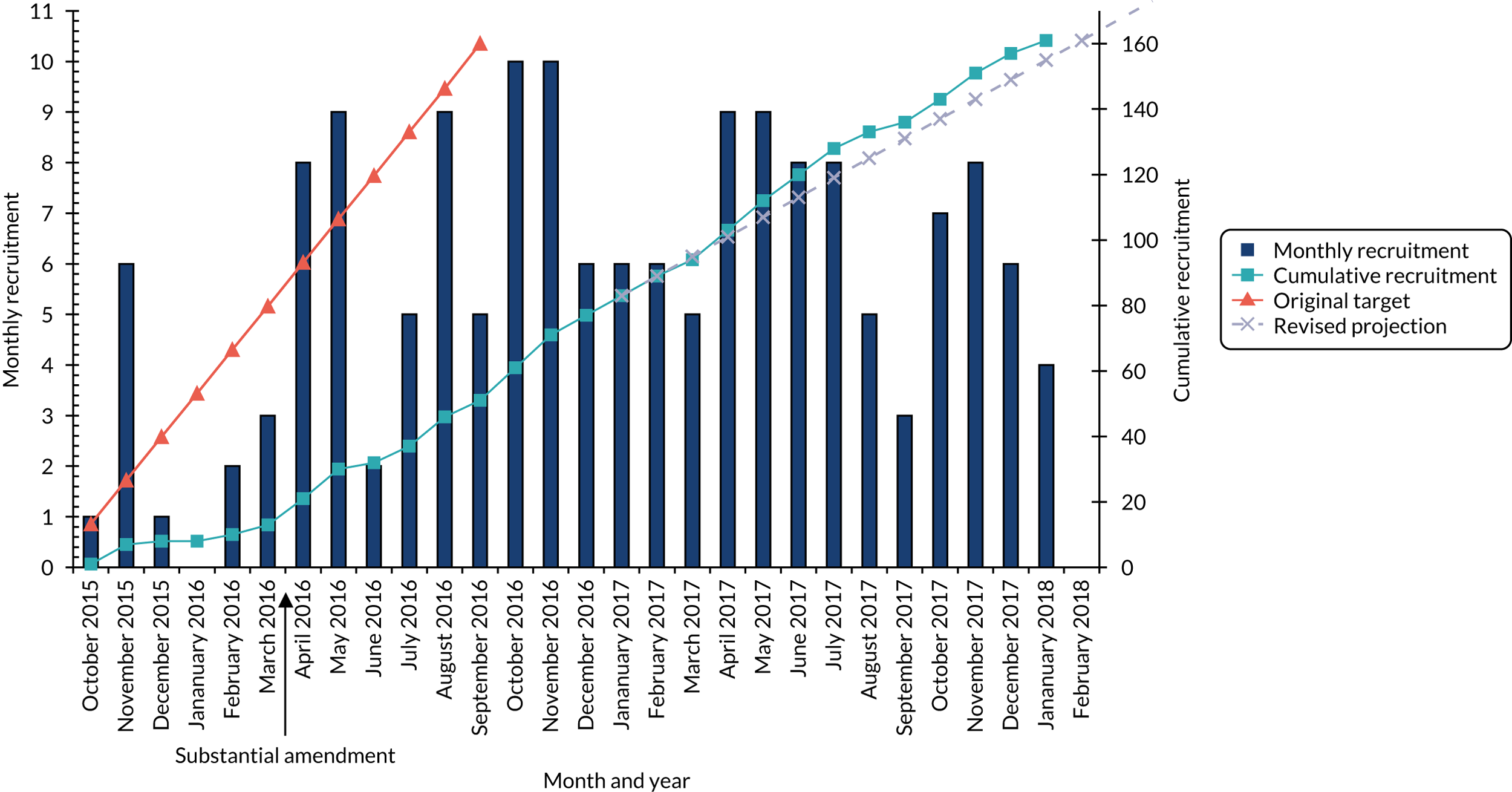
A total 2121 patients (88.6% of screened) were not eligible. The most common reasons for ineligibility were having an average pain score of < 4 (41.2% of those ineligible), having a previous palliative care referral (39.2%), living outside the local hospice/community palliative care team catchment area (9.1%) and their dominant chronic pain not being cancer related (6.7%) (see Figure 9).
Of the 274 eligible patients, 260 (94.9%) were introduced to the trial, 172 (62.8%) were able and willing to consent, and 161 (58.8%) were randomised. A total of 113 (41.2%) eligible patients were, therefore, not recruited to the trial, largely because the patient did not want to be involved in research or palliative care; the patient declined or was not interested in taking part; the patient was too unwell; the patient or clinician felt that the patient’s pain was already well controlled; the clinician deemed it not appropriate for the patient to take part; the patient became ineligible during screening; or the patient changed their mind (see Figure 9).
There was variation across hospitals in the number and proportion of patients screened, eligible and recruited (Table 3). The largest recruiting site screened 58.7% of patients and recruited 70.8% of all participants. The eligibility rate was, however, the lowest in this site (9.6%), whereas the proportion of eligible patients who went on to be randomised was the highest (84.4%). This highlights the differences in screening procedures, investment and resources across the sites; the highest recruiting sites had dedicated research nurses focusing almost exclusively on the screening and identification of potential subjects.
| Site | Participants, N = 2395 | ||||||
|---|---|---|---|---|---|---|---|
| Screened, n (%) | Eligible (n = 274) | Randomised (n = 161) | |||||
| n | Total eligible participants (%) | Eligible patients from site (%) | n | Total randomised participants (%) | Eligible patients who were randomised from site (%) | ||
| Leeds | 1407 (58.7) | 135 | 49.3 | 9.6 | 114 | 70.8 | 84.4 |
| Scarborough | 37 (1.5) | 12 | 4.4 | 32.4 | 4 | 2.5 | 33.3 |
| Huddersfield | 38 (1.6) | 22 | 8.0 | 57.9 | 9 | 5.6 | 40.9 |
| Bradford | 74 (3.1) | 7 | 2.6 | 9.5 | 2 | 1.2 | 28.6 |
| York | 444 (18.5) | 45 | 16.4 | 10.1 | 15 | 9.3 | 33.3 |
| Nottingham | 358 (14.9) | 37 | 13.5 | 10.3 | 8 | 5.0 | 21.6 |
| Grimsby | 31 (1.3) | 11 | 4.0 | 35.5 | 6 | 3.7 | 54.5 |
| Oxford | 6 (0.3) | 5 | 1.8 | 83.3 | 3 | 1.9 | 60.0 |
Protocol amendment
Recruitment to the trial was originally planned to be for 1 year; however, the staggered start of recruitment across sites and the lower than expected monthly recruitment led to a substantial amendment to the protocol (see Table 1). The substantial amendment was implemented in April 2016 following retraining and the launch of the revised protocol (see Figure 2); a total of 13 participants were recruited pre amendment (protocol v3.0) and 148 were recruited post amendment (protocol v4.0) (see Report Supplementary Material 1, Table 16). A funded recruitment extension was also obtained with a projected recruitment rate of six participants per month, allowing recruitment to complete to target after a total of 2 years and ≈ 3 months.
Eligibility violators
One participant allocated to the intervention arm was identified as not fulfilling the eligibility criteria owing to the ongoing receipt of community palliative care prior to trial entry. One participant allocated to the UC arm (pre amendment, protocol v3.0) was identified as not fulfilling the eligibility criteria owing to a previous un-actioned referral to community palliative care prior to trial entry (note that this participant would have been eligible post amendment, protocol v4.0).
Screening characteristics
Patient characteristics were broadly similar across the patients screened, eligible and randomised in terms of age, ethnicity, other types of cancer, stage of disease and time since diagnosis; therefore, the recruited participants can be considered a representative sample (see Report Supplementary Material 1, Table 17). There was, however, a slightly higher proportion of male patients in randomised patients (55.3% vs. 47.2%) and patients with haematological cancer (9.3% vs. 2.1%) than in all those screened.
Participant characteristics
Randomisation resulted in balanced trial arms for the randomisation strata, centre and average pain item on the BPI (see Report Supplementary Material 1, Table 18). Three-quarters of participants were randomised with an average pain score on the BPI of 4–6 (representing mild/moderate pain), whereas one-quarter were randomised with a higher pain score of 7–10 (representing severe pain).
Participant demographics and disease characteristics were generally well balanced across the trial arms (Tables 4 and 5). Across all participants, 55.3% were male, the mean age was 64.1 years [standard deviation (SD) 11.59 years], 93.2% were white British and 76.4% had access to a computer.
| Demographic | Trial arm | Total (N = 161) | |
|---|---|---|---|
| SSM (N = 80) | UC (N = 81) | ||
| Local hospice,a n (%) | |||
| St Gemma’s Hospice, Leeds | 18 (22.5) | 25 (30.9) | 43 (26.7) |
| Wheatfields Hospice, Leeds | 31 (38.8) | 28 (34.6) | 59 (36.6) |
| Mid Yorkshire Hospitals NHS Trust | 2 (2.5) | 3 (3.7) | 5 (3.1) |
| Kirkwood Hospice, Huddersfield | 6 (7.5) | 7 (8.6) | 13 (8.1) |
| Marie Curie, Bradford | 4 (5.0) | 1 (1.2) | 5 (3.1) |
| St Leonard’s Hospice, York | 8 (10.0) | 7 (8.6) | 15 (9.3) |
| Nottingham CityCare Partnership | 4 (5.0) | 4 (4.9) | 8 (5.0) |
| Care Plus Group, Grimsby | 3 (3.8) | 3 (3.7) | 6 (3.7) |
| St Catherine’s Hospice, Scarborough | 2 (2.5) | 2 (2.5) | 4 (2.5) |
| Sobell House Hospice, Oxford | 2 (2.5) | 1 (1.2) | 3 (1.9) |
| Gender, n (%) | |||
| Male | 42 (52.5) | 47 (58.0) | 89 (55.3) |
| Female | 38 (47.5) | 34 (42.0) | 72 (44.7) |
| Age at randomisation (years) | |||
| Missing | 0 | 0 | 0 |
| Mean (SD) | 62.5 (11.73) | 65.7 (11.29) | 64.1 (11.59) |
| Median (range) | 64.5 (19–84) | 68 (33–85) | 66 (19–85) |
| Ethnicity, n (%) | |||
| White British | 73 (91.3) | 77 (95.1) | 150 (93.2) |
| Non-white British | 4 (5.0) | 2 (2.5) | 6 (3.7) |
| Missing | 3 (3.8) | 2 (2.5) | 5 (3.1) |
| Does patient have access to a computer,b n (%) | |||
| Yes | 61 (76.3) | 62 (76.5) | 123 (76.4) |
| Willing to use PainCheck | 59 (73.8) | 61 (75.3) | 120 (74.5) |
| Not willing to use PainCheck | 1 (1.3) | 0 (0.0) | 1 (0.6) |
| Missing | 1 (1.3) | 1 (1.2) | 2 (1.2) |
| No | 19 (23.8) | 19 (23.5) | 38 (23.6) |
| Demographic | Trial arm | Total (N = 161) | |
|---|---|---|---|
| SSM (N = 80) | UC (N = 81) | ||
| ECOG performance status,a n (%) | |||
| 0 | 3 (3.8) | 0 (0.0) | 3 (1.9) |
| 1 | 48 (60.0) | 45 (55.6) | 93 (57.8) |
| 2 | 23 (28.8) | 29 (35.8) | 52 (32.3) |
| 3 | 6 (7.5) | 7 (8.6) | 13 (8.1) |
| Type of advanced cancer, n (%) | |||
| Breast | 20 (25.0) | 16 (19.8) | 36 (22.4) |
| Prostate | 11 (13.8) | 16 (19.8) | 27 (16.8) |
| Colon or rectal | 11 (13.8) | 10 (12.3) | 21 (13.0) |
| Upper GI | 10 (12.5) | 9 (11.1) | 19 (11.8) |
| Haematological | 6 (7.5) | 9 (11.1) | 15 (9.3) |
| Non-small-cell lung | 7 (8.8) | 6 (7.4) | 13 (8.1) |
| Lung (other/not specified) | 4 (5.0) | 5 (6.2) | 9 (5.6) |
| Urological (other/not prostate) | 6 (7.5) | 4 (4.9) | 10 (6.2) |
| Gynaecological | 2 (2.5) | 0 (0.0) | 2 (1.2) |
| Soft and connective tissue | 1 (1.3) | 1 (1.2) | 2 (1.2) |
| Unknown | 2 (2.5) | 5 (6.2) | 7 (4.3) |
| Years since original diagnosis (to randomisation) | |||
| Mean (SD) | 4.0 (5.09) | 3.7 (4.77) | 3.8 (4.92) |
| Median (range) | 2.1 (0–21.3) | 1.5 (0–20.2) | 1.9 (0–21.3) |
| Patient currently (or within the past month) receiving active cancer treatment, n (%) | |||
| Yes | 54 (67.5) | 56 (69.1) | 110 (68.3) |
| Chemotherapy | 32 (40.0) | 35 (43.2) | 67 (41.6) |
| Radiotherapy | 7 (8.8) | 8 (9.9) | 15 (9.3) |
| Hormone therapy | 14 (17.5) | 19 (23.5) | 33 (20.5) |
| Surgery | 1 (1.3) | 0 (0.0) | 1 (0.6) |
| Immunotherapyb | 2 (2.5) | 2 (2.5) | 4 (2.5) |
| Inhibitors of bone painb | 4 (5.0) | 3 (3.7) | 7 (4.3) |
| No | 26 (32.5) | 25 (30.9) | 51 (31.7) |
| Strongest pain medication, n (%) | |||
| Strong opioid | 48 (60.0) | 47 (58.0) | 95 (59.0) |
| Weak opioid | 23 (28.8) | 24 (29.6) | 47 (29.2) |
| Non-opioid | 9 (11.3) | 9 (11.1) | 18 (11.2) |
| No medication | 0 (0.0) | 1 (1.2) | 1 (0.6) |
| Number of pain medications | |||
| Mean (SD) | 2.4 (0.82) | 2.4 (0.98) | 2.4 (0.90) |
| Median (range) | 2 (1–4) | 2 (0–5) | 2 (0–5) |
Over half of all participants (57.8%) were reported to have a European Cooperative Oncology Group (ECOG) performance status of 1, whereas 32.3% were status 2 and 8.1% were status 3 (see Table 5). Breast cancer was the most common type of advanced disease (22.4% of participants), followed by prostate (16.8%), lung (non-small-cell, small-cell or unspecified; 13.7%), colon or rectal (13%), upper GI (11.8%) and haematological (9.3%) cancer. The median time from the participant’s original diagnosis to their randomisation was 1.9 years; however, this was highly variable, ranging from 2 weeks to 21.3 years. Over two-thirds of participants were receiving (or had recently received) active cancer treatment (68.3%), comprising mainly chemotherapy or hormone therapy. All participants but one were receiving pain medication; participants were taking a mean of 2.4 different pain medications (SD 0.9) and 59% of participants were taking a strong opioid. See Report Supplementary Material 1, Table 19 for further disease characteristics,
The mean worst pain, as reported on a 0–10 numerical rating scale on the BPI, was 7.7 (SD 1.76), and three-quarters of participants reported a severe level of worst pain (7–10) (Table 6). Mean scores for composite outcomes of overall pain severity and pain interference, also reported on the BPI, were 5.0 (SD 1.47) and 5.5 (SD 2.37), respectively. On the patient pain knowledge and experience questionnaire, participants reported a mean score of 38.2 (SD 15.3) out of a possible 90 on the knowledge subscale, and 41.5 (SD 9.3) out of a possible 70 on the experience subscale, with higher scores indicating poorer experience of pain, knowledge and attitudes. On the cancer-specific QoL questionnaire, the EORTC QLQ-C30, the mean summary score was 55.7 (SD 16.9) out of a possible 100, with higher scores indicating high QoL.
| Questionnaire | Trial arm | Total (N = 161)a | |
|---|---|---|---|
| SSM (N = 80)a | UC (N = 81)a | ||
| BPI (scores 0–10; higher score = increased pain) | |||
| Worst pain | |||
| Mean (SD) | 7.7 (1.84) | 7.6 (1.70) | 7.7 (1.76) |
| Median (range) | 8 (0–10) | 8 (4–10) | 8 (0–10) |
| Worst pain category, n (%) | |||
| No pain | 1 (1.3) | 0 (0.0) | 1 (0.6) |
| Mild pain (1–4) | 1 (1.3) | 1 (1.2) | 2 (1.2) |
| Moderate pain (5–6) | 15 (18.8) | 22 (27.2) | 37 (23.0) |
| Severe pain (7–10) | 62 (77.5) | 58 (71.6) | 120 (74.5) |
| Least pain | |||
| Mean (SD) | 2.5 (1.97) | 2.8 (1.94) | 2.6 (1.95) |
| Median (range) | 2 (0–9) | 2.0 (0–10) | 2.0 (0–10) |
| Average pain | |||
| Mean (SD) | 5.6 (1.36) | 5.4 (1.47) | 5.5 (1.42) |
| Median (range) | 5 (2–10) | 5 (2–10) | 5 (2–10) |
| Pain right now | |||
| Mean (SD) | 4.0 (2.33) | 4.2 (2.41) | 4.1 (2.37) |
| Median (range) | 4 (0–10) | 4 (0–10) | 4 (0–10) |
| Overall pain severity score | |||
| Mean (SD) | 4.9 (1.39) | 5.0 (1.56) | 5.0 (1.47) |
| Median (range) | 4.8 (2–8.8) | 4.5 (2–10) | 4.8 (2–10) |
| Pain interference score | |||
| Mean (SD) | 5.2 (2.48) | 5.7 (2.25) | 5.5 (2.37) |
| Median (range) | 5.3 (0–10) | 5.9 (1.4–10) | 5.6 (0–10) |
| PPQ (higher score = poorer experience of pain) | |||
| Knowledge subscale (scores 0–90) | |||
| Mean (SD) | 38.2 (13.52) | 38.1 (16.94) | 38.2 (15.30) |
| Median (range) | 35 (10–80) | 38 (5–70) | 37 (5–80) |
| Experience subscale (scores 0–70) | |||
| Mean (SD) | 41.3 (9.70) | 41.7 (8.96) | 41.5 (9.30) |
| Median (range) | 40 (18–61) | 42 (27–61) | 40.5 (18–61) |
| Total score (scores 0–160) | |||
| Mean (SD) | 79.4 (16.50) | 79.9 (20.22) | 79.7 (18.42) |
| Median (range) | 79 (43–124) | 81 (34–123) | 80 (34–124) |
| EORTC QLQ-C30 summary score (scores 0–100; higher score = high QoL and functioning) | |||
| Mean (SD) | 57.4 (17.20) | 54.1 (16.53) | 55.7 (16.89) |
| Median (range) | 58.9 (20–91.6) | 53.7 (13.6–91.3) | 56.2 (13.6–91.6) |
Participant follow-up
Withdrawals
Nine (5.6%) participants withdrew from trial processes: four (5%) who were allocated to the SSM arm and five who were (6.2%) allocated to the UC arm (see Report Supplementary Material 1, Table 20). All withdrew from questionnaire completion via researcher contact and all but one withdrew from postal questionnaire completion. All remained willing for further data to be collected from their medical records. The majority of withdrawals were because the participant was too unwell and were clustered around the 6- and 12-week follow-ups.
Participant questionnaires
A total of 115 (71.4%) participants had at least one successful follow-up, with questionnaires completed at 6 or 12 weeks post randomisation: 56 (70.0%) in the SSM arm and 59 (72.8%) in the UC arm (see Report Supplementary Material 1, Table 21).
Baseline questionnaires were completed face to face with the recruiting researcher and were returned for all but one participant (see Report Supplementary Material 1, Table 21, and Table 6). The majority of participants completed follow-up questionnaires via the post; over one-quarter had help completing the questionnaires at 6 weeks and just under 20% had help at 12 weeks, with help provided primarily by the participants’ partner or child (see Report Supplementary Material 1, Table 22). Questionnaire packs were completed for 107 (66.5%) participants at the 6-week follow-up and 87 (54.4%) participants at the 12-week follow-up (Table 7). Questionnaires were not returned at 12 weeks owing to participant death (n = 29, 39.2%), illness (n = 11, 14.9%), and withdrawal (n = 6, 8.1%). Questionnaires were also not returned when these had been sent by post and the participant was contacted by telephone to offer telephone completion (n = 11, 14.9%), when there was difficulty contacting the participant by telephone (n = 15, 20.3%) or when the participant was not contacted by telephone (n = 2, 2.7%).
| Baselinea | 6-week follow-up | 12-week follow-up | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SSM | UC | Total | SSM | UC | Total | SSM | UC | Total | |
| Questionnaire pack completed, n (%) | |||||||||
| Yes | 79 (98.8) | 81 (100.0) | 160 (99.4) | 51 (63.8) | 56 (69.1) | 107 (66.5) | 41 (51.3) | 46 (56.8) | 87 (54.0) |
| No | 1 (1.3) | 0 (0.0) | 1 (0.6)b | 29 (36.3) | 25 (30.9) | 54 (33.5) | 39 (48.8) | 35 (43.2) | 74 (46.0) |
| Reason not completed, n (%) | |||||||||
| Participant withdrawal | NA | NA | NA | 2 (6.9) | 2 (8.0) | 4c (7.4) | 3 (7.7) | 3 (8.6) | 6c (8.1) |
| Participant died | NA | NA | NA | 7 (24.1) | 6 (24.0) | 13 (24.1) | 17 (43.6) | 12 (34.3) | 29 (39.2) |
| Participant too unwell | NA | NA | NA | 3 (10.3) | 5 (20.0) | 8 (14.8) | 2 (5.1) | 9 (25.7) | 11 (14.9) |
| Contacted not returned | NA | NA | NA | 5 (17.2) | 3 (12.0) | 8 (14.8) | 8 (20.5) | 3 (8.6) | 11 (14.9) |
| Unable to contact | NA | NA | NA | 10 (34.5) | 9 (36.0) | 19 (35.2) | 8 (20.5) | 7 (20.0) | 15 (20.3) |
| Administrative error | 1 (100.0) | NA | 1 (100.0) | 2 (6.9) | 0 (0.0) | 2 (3.7) | 1 (2.6) | 1 (2.9) | 2 (2.7) |
| Method of completion, n (%) | |||||||||
| Face to face | 79 (100.0) | 81 (100.0) | 160 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.4) | 1 (2.2) | 2 (2.3) |
| Telephone | NA | NA | NA | 1 (2.0) | 1 (1.8) | 2 (1.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Post | NA | NA | NA | 50 (98.0) | 55 (98.2) | 105 (98.1) | 40 (97.6) | 45 (97.8) | 85 (97.7) |
| All questionnaires completed? n (%)d | |||||||||
| Yes | 79 (100.0) | 81 (100.0) | 160 (100.0) | 48 (94.1) | 55 (98.2) | 103 (96.3) | 37 (90.2) | 45 (97.8) | 82 (94.3) |
| No | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (5.9) | 1 (1.8) | 4 (3.7) | 4 (9.8) | 1 (2.2) | 5 (6.8) |
| Timing of completion (weeks) | |||||||||
| Mean (SD) | –0.0 (0.33) | –0.0 (0.21) | –0.0 (0.28) | 6.2 (0.88) | 6.3 (1.05) | 6.3 (0.97) | 12.1 (1.28) | 12.0 (0.97) | 12.1 (1.12) |
| Median (range) | 0.0 (–1.4–2.4) | 0.0 (–1.9–0.0) | 0.0 (–1.9–2.4) | 6.0 (4.9–9.4) | 6.0 (4.7–9.4) | 6.0 (4.7–9.4) | 11.9 (10.7–18.1) | 11.6 (11.0–15.3) | 11.7 (10.7–18.1) |
| Completed within 2 weeks, n (%) | |||||||||
| Yes | 78 (98.7) | 81 (100.0) | 159 (99.4) | 50 (98.0) | 51 (91.1) | 101 (94.4) | 38 (92.7) | 43 (93.5) | 81 (93.1) |
| No | 1 (1.3) | 0 (0.0) | 1 (0.6) | 1 (2.0) | 5 (8.9) | 6 (5.6) | 3 (7.3) | 3 (6.5) | 6 (6.9) |
The majority of questionnaires were completed within a 2-week time window: 94.4% at 6 weeks and 93.1% at 12 weeks (see Table 6; and Report Supplementary Material 1, Figure 17).
Comparison of baseline characteristics between participants with and participants without follow-up (primary outcome completion; see Report Supplementary Material 1, Table 23) indicated that those not completing follow-up at 6 or 12 weeks were more likely to be male, younger and taking a strong opioid and to have a higher ECOG performance status; had more recently received their original diagnosis; had lower baseline QoL scores (EORTC-8D, EQ-5D-3L); and had worse baseline measures on the BPI (worst pain, pain severity and interference), PPQ (Patient Pain Questionnaire) (experience scale) and QLQc30 (Quality of Life Questionnaire c30) (global health status, physical, social and role functioning).
The completeness of questionnaire data, including missing item level data, outcome scores and multiple item responses, is summarised in Report Supplementary Material 1, Tables 24 and 25.
Researcher unblinding
Researcher unblinding occurred for three participants by their 6-week follow-up; all of the participants were allocated to the SSM arm and were recruited under the original v3 protocol. Two instances occurred as the participant discussed the intervention during the telephone follow-up (follow-up completed) and one occurred as the researcher became aware of palliative care contact following nurse contact (follow-up not completed).
Safety
No related and unexpected serious adverse events (RUSAEs) were reported during the trial.
Deaths were recorded throughout the trial up to the last participant’s 12-week follow-up. A total of 92 (57.1%) participants were reported to have died (see Table 26, Figure 11), with an overall median survival of 53.3 weeks (95% CI 40.9 to 59.6 weeks) and similar estimates across trial arms. A total of eight (5%, 95% CI 1.6% to 21.1%) participants had died by 6 weeks post randomisation, and 25 (15.5%, 95% CI 9.9% to 21.1%) participants had died by 12 weeks post randomisation. Almost half of the participants who died did so in a hospice (44.6%), 28.3% died at home and 21.7% died in hospital.
FIGURE 11.
Kaplan–Meier survival estimates.
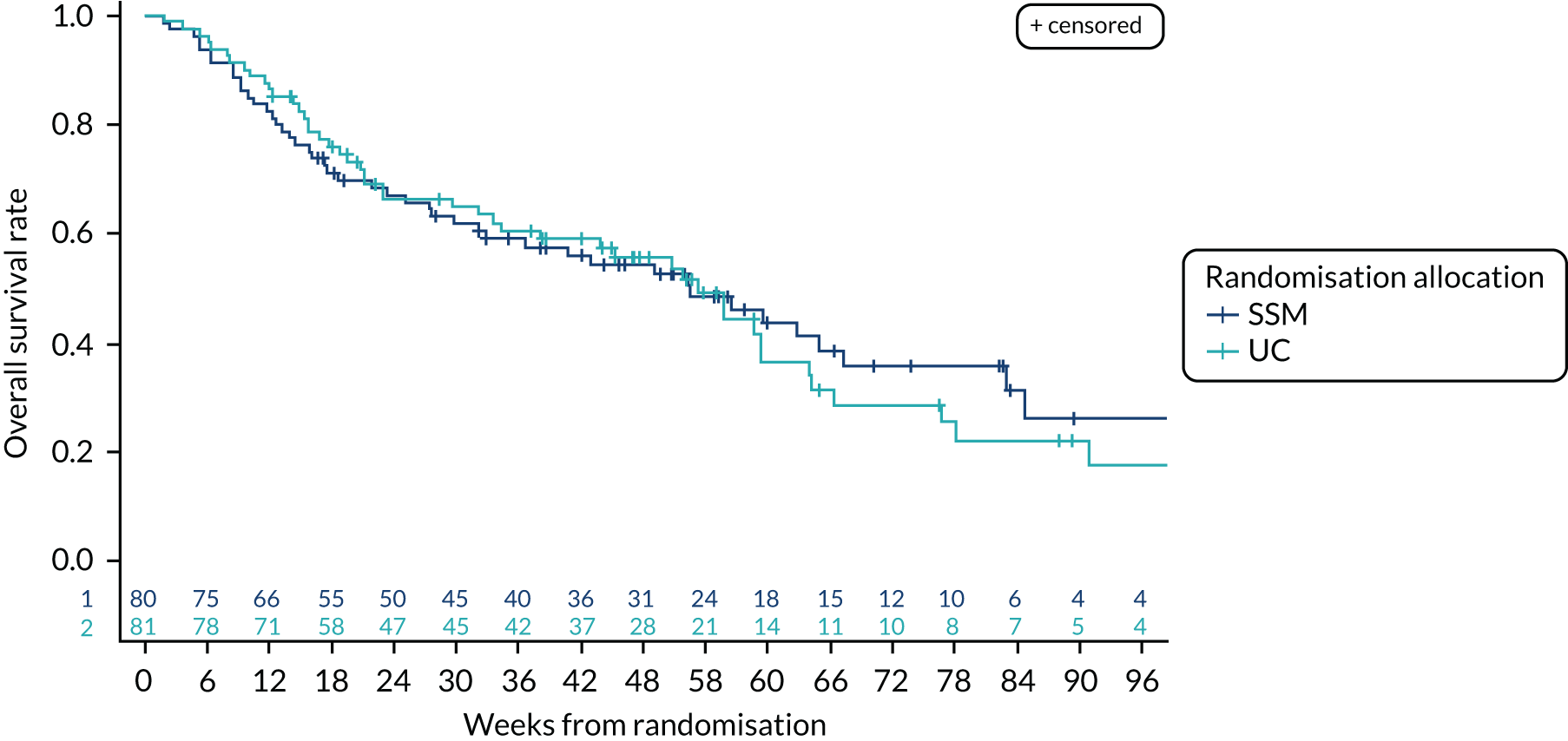
Intervention delivery
Protocol amendment
In accordance with the original protocol (v3.0), six participants allocated to the UC arm were not referred to community palliative care, whereas the 75 (92.6%) participants allocated to UC in accordance with the amended protocol (v4.0) were referred to community palliative care as part of UC (see Report Supplementary Material 1, Table 16).
Intervention training
Intervention training in each hospice took place face to face prior to the hospital site opening to recruitment, and ranged in duration from 20 minutes to 2 hours 20 minutes (median 1 hour 15 minutes) (see Report Supplementary Material 1, Table 27). Refresher training was offered to all sites during the trial; 5 out of 11 hospice sites received further face-to-face training, with a maximum of four face-to-face refresher training sessions taking place for a site. The number of health professionals (palliative care nurses, health professionals) trained across the hospices (with recruited participants) ranged from 5 to 16.
Usual care
An initial palliative care visit took place for 78 (97.5%) participants in the SSM arm and 71 (87.7%) participants in the UC arm (see Report Supplementary Material 1, Table 28). The visits mainly took place in participants’ own homes (95.3%) a median of 1 week following randomisation, and lasted a median of 75 minutes in both arms.
During the initial visit, the following palliative care needs were addressed with similar rates across trial arms (see Report Supplementary Material 1, Table 29): pain in 94.6% of participants, additional symptoms in 57.5%, psychological needs in 47.7%, carer concerns in 20.1% and other care needs in 14.1%. A recommended change to pain medication was made for 67.1% of participants: 65.4% in the SSM arm and 69.0% in the UC arm.
A total of 58 health professionals were involved in the 149 participants’ initial palliative care visits (Figure 12); 46 health professionals for 78 participants in the SSM arm and 38 health professionals for 71 participants in the UC arm. Of these, 26 health professionals saw participants across trial arms for 44 SSM and 56 UC participants.
FIGURE 12.
Health professional delivery of the initial palliative care visit.
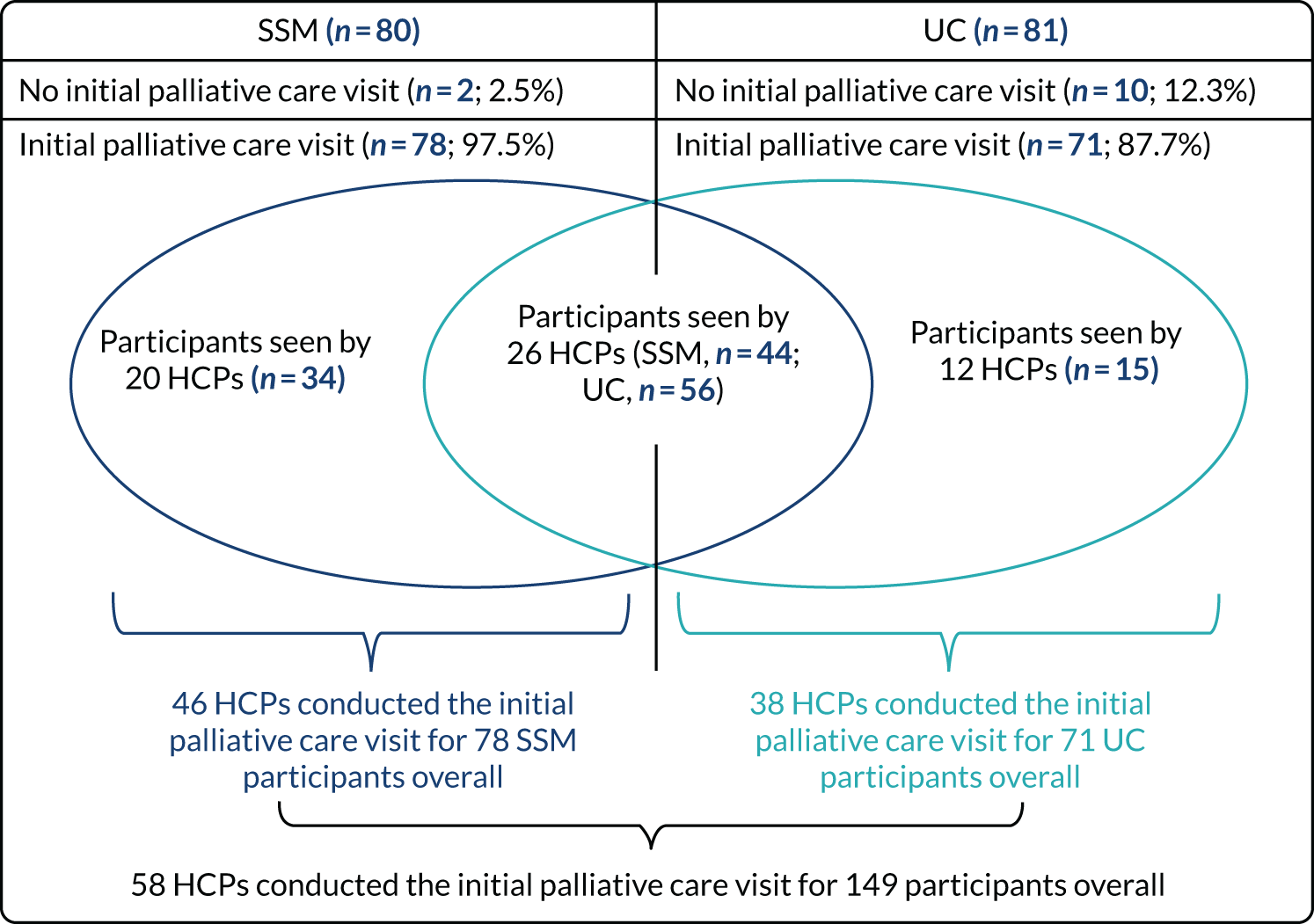
For further details of palliative care receipt during the trial, see Health-care use (see Table 13; see Report Supplementary Material 1, Tables 40 and 41).
Supported self-management
Primary intervention implementation outcome
Of the 80 participants allocated to the SSM arm, the Tackling Cancer Pain booklet and DVD were introduced and accepted by 72 (90.0%, 95% CI 81.2% to 95.6%) participants (see Figure 8).
The PainCheck online monitoring system was introduced to 47 (58.8%, 95% CI 47.2% to 69.7%) participants (see Figure 8). The main reasons that PainCheck was not introduced were that the participant did not have a computer (n = 19), the health professional felt that it was not appropriate (n = 5) and the participant chose not to use the computer (n = 3). A total of 32 (40%, 95% CI 29.2% to 51.6%) participants logged on to PainCheck and 23 (28.8%, 95% CI 19.2% to 40%) were found to have engaged with the intervention, having used PainCheck on three or more occasions.
Of the 32 participants using PainCheck, the median total time spent on PainCheck over the 12-week trial period was just under 1 hour, with times ranging from 6.5 minutes to > 10 hours. Participants logged on a median of five times, ranging from one time to 82 times when the participant used PainCheck daily (see Figures 8 and 9). A health professional also used PainCheck for 21 (65.6%) of the 32 participants using PainCheck (see Report Supplementary Material 1, Table 30). The median number of times that a health professional logged on was one time, ranging from one time to 55 times (see Figures 8 and 9). Of the health professionals using PainCheck, the median total time spent on PainCheck per participant (n = 21) was 25.7 minutes, ranging from 3.1 minutes to > 7 hours.
Contamination
Contamination was reported for one participant allocated to UC for whom the Tackling Cancer Pain booklet and DVD were provided to the participant in error.
Qualitative process evaluation
Process evaluation during implementation of multicomponent intervention for pain management
To understand the process of implementation surrounding the pain self-management intervention, we conducted semistructured interviews with 12 patients and 12 hospice-based palliative care nurses. Participants were interviewed at their home and at their place of work. Interviews were guided conversations to elicit accounts of participants’ experiences in their own words of taking part in the trial using a topic guide. Key themes that emerged from the process evaluation are outlined below.
Emotional barriers to referral to palliative care
There were emotional barriers associated with referral to palliative care that influenced the perceived acceptability of the trial. About two-thirds of health professionals believed palliative care to be appropriate, particularly for those patients who were in severe pain, for whom pain was not their only issue and for whom there was clear specialist need. However, one-third of health professionals described palliative care as symbolising something that they thought patients were not psychologically ready for and said that it was inappropriate for the patients’ stage in the disease process. Health professionals believed that patients’ symptoms should be managed by the hospital-based palliative care nurse because the patients were still embedded within the hospital system and receiving active treatment. For half of patients, the association of palliative care with closeness to death was a barrier to accessing supportive care for pain. They believed that they were not yet at that stage and ‘felt a fraud’. Despite this, being engaged with palliative care reassured these patients that help would be easily accessible when their disease progressed. Although they were reluctant to acknowledge the appropriateness of their referral to palliative care, they were engaged with participating in the trial.
Impact of interventions on delivery of care
Half of health professionals did not think that being involved with the trial had added to their workload and discussed how they could use the eHealth component of the intervention (PainCheck) to proactively manage their workload to save time in the future. However, all health professionals felt that this could become burdensome if a larger number of patients on their caseload were using the eHealth tool. Patients felt that taking part in the trial had increased their care and provided them with a support system. They no longer felt isolated but were connected to and embedded within services. CNSs were fulfilling a role that previously did not exist, and patients felt reassured that help was there if they needed it. Both health professionals and patients asserted that both interventions improved patients’ self-management of pain. They described how the education resource (Tackling Cancer Pain) was a good resource for patients to use at their own pace and refer to, and resulted in positive changes to self-management. PainCheck then provided a good baseline for patients and acted as a reminder to engage in self-management strategies. For some, it provided the opportunity to reflect on previous pain scores, both providing context for their present pain and enabling them to re-evaluate past pain as less intense than they had remembered.
Acceptability, engagement and feasibility of eHealth intervention
Health professionals were the gatekeepers to patient acceptability of and access to PainCheck. However, many health professionals also reported a lack of knowledge about, understanding of and familiarity with PainCheck. This affected the degree to which health professionals encouraged and facilitated patient usage. Patient acceptability of the interventions was largely determined by their health professionals, with CNSs facilitating the introduction to and monitoring of PainCheck. Some patients were not introduced to the PainCheck because CNSs wanted to avoid what they perceived as an unnecessary additional burden for them. For patients, a lack of familiarity with ICT or having no internet connection at home influenced the perceived value to them of using PainCheck.
Engagement with interventions
Participants who used the eHealth tool and the educational resources described the interventions as straightforward, easy to use, quick, user-friendly and unobtrusive. The eHealth tool was considered by participants as a simple tool to aid with monitoring their symptoms and communicating with professionals. Those who had limited engagement with PainCheck were not regular users of technology and computers, and often did not have or were unable to use internet connections. They found engaging with PainCheck to be stressful and subsequently were not interested in integrating it into their daily lives.
Feasibility of eHealth intervention
Health professionals felt that there was a place for PainCheck in current practice if its use was streamlined (Figures 13 and 14). Some felt that it easily supported their current way of working by adding in another layer of detail, which they could use to monitor patients’ pain. Others felt that it enhanced the care that they provided because it enabled them to think about other aspects of pain management. Although digital technologies were viewed as becoming more pervasive within health care, health professionals believed that these would not replace their current way of working. This research is now published and available at http://eprints.whiterose.ac.uk/155107/.
FIGURE 13.
Intervention uptake.
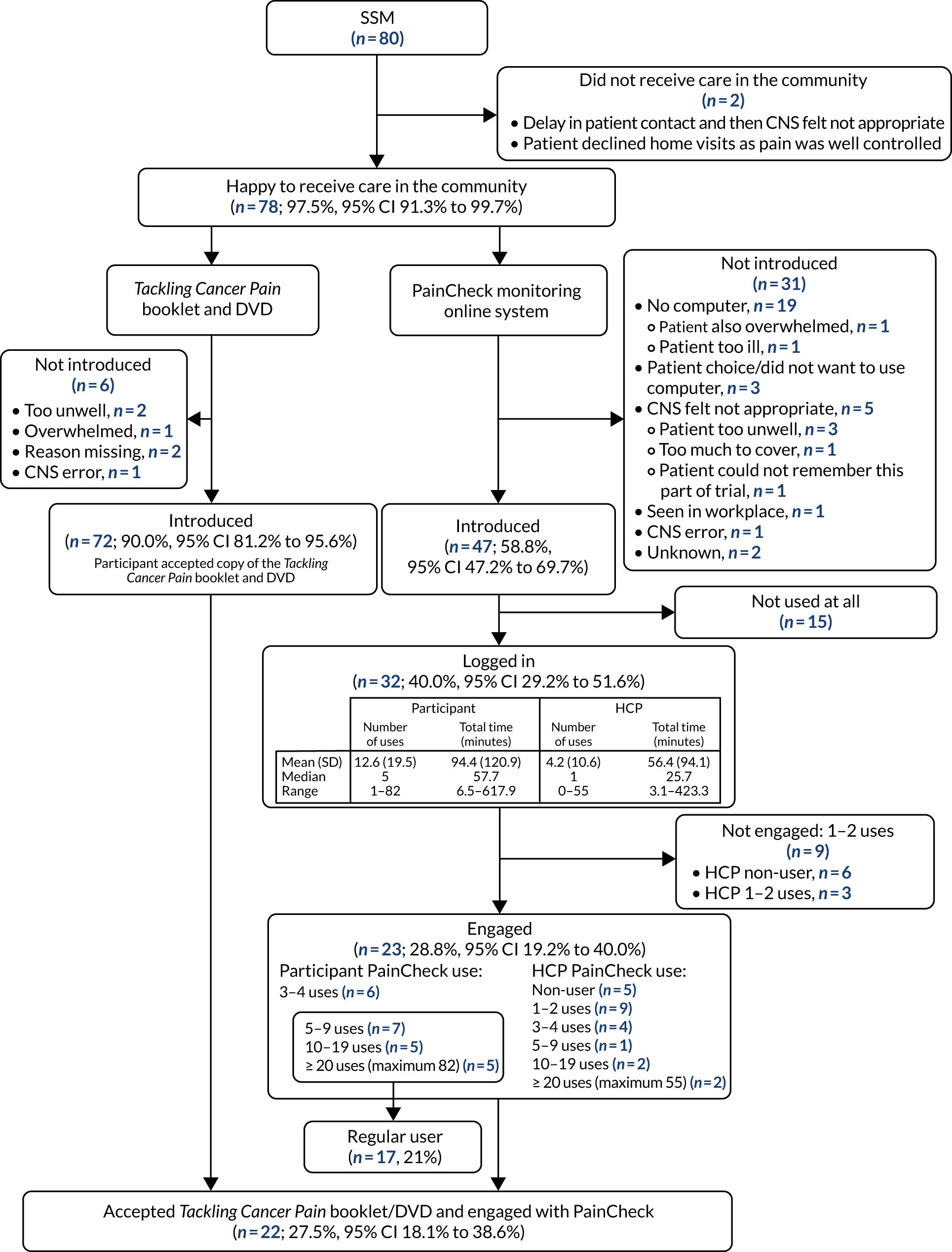
FIGURE 14.
Participant and health professional PainCheck use.
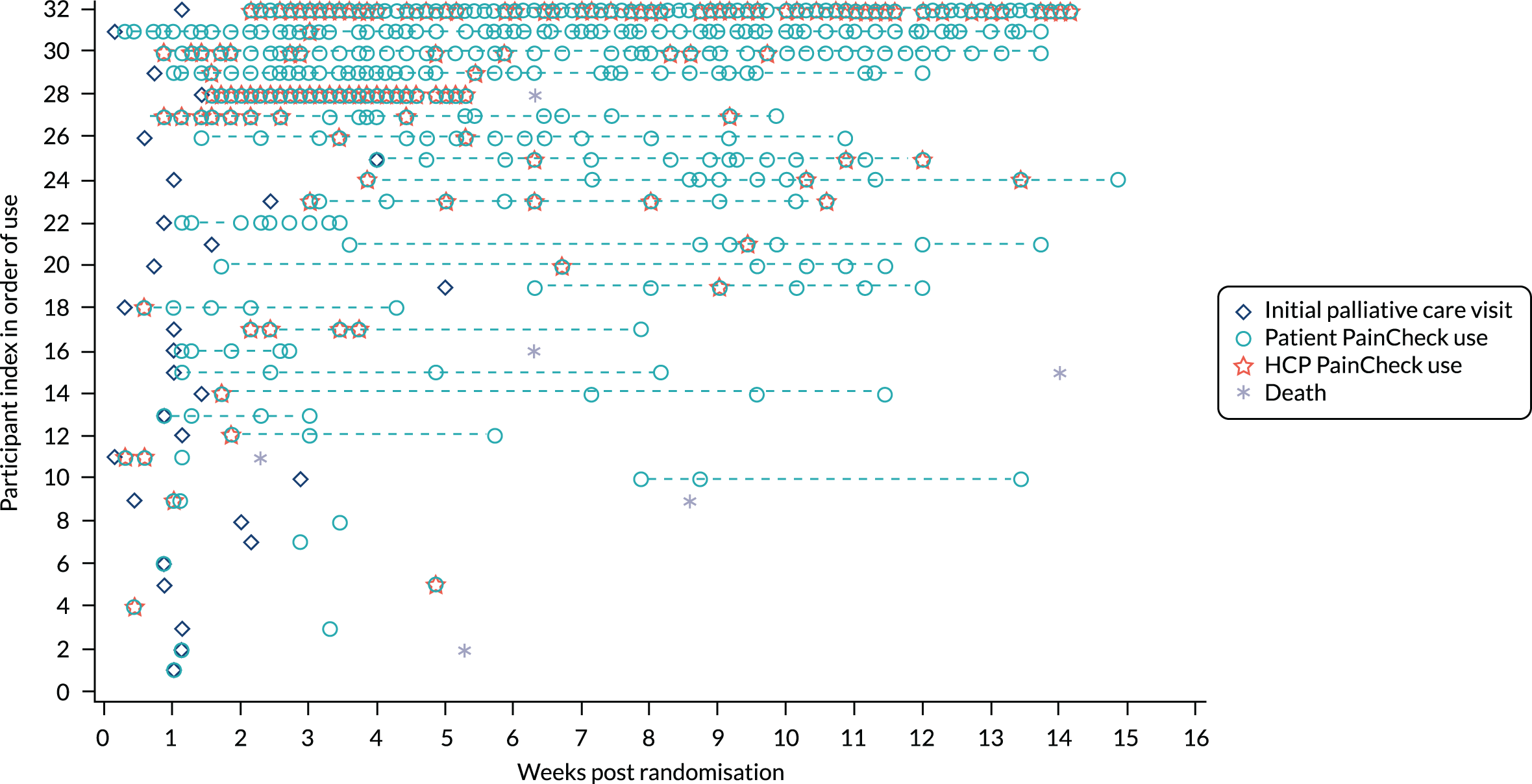
Effectiveness analysis
Missing data
The primary outcome was available for all time points for 78 (48.4%) participants, for at least one follow-up time point for 37 (23.0%) participants and for baseline only for 45 (28.0%) participants, and was not available at any time point for one (0.6%) participant (Table 8). Forward selection identified BPI worst pain, sex, age, centre, opioid use, health today and QoL from the EuroQol-5 Dimensions, three-level version (EQ-5D-3L), as key participant characteristics predictive of missing data at 6 or 12 weeks in multivariable models (see Report Supplementary Material 1, Tables 31 and 32). Multiple imputation for primary analysis, therefore, used the above predictive variables (worst pain on the BPI replaced by the relevant baseline value for secondary outcomes) in addition to randomisation strata (dichotomised average pain response and centre) and allocation. A total of 52 imputations were made for all models, corresponding to the overall percentage of missing data (i.e. 52% missing at least one time point).
| Baseline | 6 weeks | 12 weeks | BPI, n (%) | PPQ, n (%) | QLQc30 summary score | 7-point change in paina | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Worst pain | Pain severity | Pain interference | Knowledge | Experience | Total | |||||
| O | O | O | 78 (48.4) | 76 (47.2) | 78 (48.4) | 75 (46.6) | 78 (48.4) | 75 (46.6) | 74 (46.0) | 76 (47.2) |
| O | O | Missing | 29 (18.0) | 31 (19.3) | 28 (17.4) | 31 (19.3) | 29 (18.0) | 31 (19.3) | 28 (17.4) | 29 (18.0) |
| O | Missing | O | 8 (5.0) | 8 (5.0) | 9 (5.6) | 9 (5.6) | 8 (5.0) | 9 (5.6) | 9 (5.6) | 9 (5.6) |
| O | Missing | Missing | 45 (28.0) | 45 (28.0) | 45 (28.0) | 45 (28.0) | 45 (28.0) | 45 (28.0) | 45 (28.0) | 47 (29.2) |
| Missing | O | O | 1 (0.6) | NA | ||||||
| Missing | O | Missing | 1 (0.6) | NA | ||||||
| Missing | Missing | Missing | 1 (0.6) | 1 (0.6) | 1 (0.6) | 1 (0.6) | 1 (0.6) | 1 (0.6) | 3 (1.9) | NA |
Primary outcome
Pain severity: worst pain (Brief Pain Inventory)
The mean worst pain was 7.7 points (SD 1.76 points, n = 160) at baseline, representing a severe level of pain. This was 5.8 points (SD 2.35 points, n = 107) at the 6-week follow-up and 4.7 points (SD 3.09 points, n = 86) at the 12-week follow-up, each representing a reduced moderate level of pain and an increase in variability given the high level of loss to follow-up (see Report Supplementary Material 1, Table 33 and Figure 18). Three-quarters of participants reported a severe level of worst pain at baseline (7–10 points), reducing to 43% of participants at 6 weeks and 34% of participants at 12 weeks. Mean scores were slightly higher in the SSM arm than in the UC arm and an increased proportion of participants reported severe pain at 6 and 12 weeks.
Based on participants’ change in worst pain compared with baseline, there was a mean reduction of 1.5 points (SD 2.62 points, n = 107) at 6 weeks and 2.7 points (SD 3.54 points, n = 86) at 12 weeks (see Report Supplementary Material 1, Table 34). A total of 41.1% of participants were classed as a responder at 6 weeks and 57% at 12 weeks, having had a reduction in worst pain score of ≥ 2 points or ≥ 30%, with similar rates across trial arms.
The primary and sensitivity analyses found no significant treatment differences for the primary outcome or for other secondary outcomes of pain severity items on the BPI (Table 9, and see Figure 10 and Report Supplementary Material 1, Tables 35 and 36). The mean adjusted difference between the trial arms (SSM – UC) at 12 weeks was 0.5 (95% CI –0.7 to 1.8; p = 0.4102), representing no significant difference between the trial arms. There was similarly no significant difference in the odds of a response, with an odds ratio of 0.84 (95% CI 0.39 to 1.82; p = 0.6558) (Table 10).
| BPI pain severity outcomea | 6 weeks, mean (95% CI), SE; p-value | 12 weeks, mean (95% CI), SE; p-value | ||||
|---|---|---|---|---|---|---|
| SSM | UC | Differenceb | SSM | UC | Differenceb | |
| Primary outcome: worst pain | 6.7 (5.9 to 7.6), 0.44 | 6.3 (5.4 to 7.3), 0.47 | 0.4 (–0.4 to 1.2), 0.42; 0.3304 | 5.8 (4.6 to 6.9), 0.57 | 5.2 (4.2 to 6.3), 0.54 | 0.5 (–0.7 to 1.8), 0.65; 0.4102 |
| Secondary pain severity outcomes | ||||||
| Least pain | 3.5 (2.6 to 4.4), 0.47 | 3.2 (2.2 to 4.1), 0.47 | 0.3 (–0.4 to 1.1), 0.39; 0.3830 | 3.3 (2.3 to 4.3), 0.51 | 2.9 (1.9 to 3.8), 0.50 | 0.5 (–0.4 to 1.4), 0.47; 0.3122 |
| Average pain | 4.7 (3.7 to 5.6), 0.48 | 4.4 (3.6 to 5.3), 0.44 | 0.3 (–0.4 to 1.0), 0.35; 0.4650 | 4.0 (3.0 to 5.1), 0.52 | 4.0 (3.1 to 4.9), 0.46 | –0.0 (–1.0 to 1.0), 0.50; 0.9925 |
| Pain right now | 4.0 (3.0 to 5.0), 0.52 | 4.1 (3.1 to 5.1), 0.53 | –0.1 (–1.0 to 0.8), 0.45; 0.8587 | 3.7 (2.7 to 4.8), 0.54 | 3.6 (2.6 to 4.7), 0.53 | 0.1 (–1.0 to 1.2), 0.55; 0.8856 |
| Overall pain severity score | 4.7 (3.8 to 5.6), 0.45 | 4.4 (3.6 to 5.2), 0.42 | 0.3 (–0.4 to 1.0), 0.36; 0.4510 | 4.1 (3.2 to 5.1), 0.49 | 3.9 (3.0 to 4.8), 0.48 | 0.2 (–0.8 to 1.2), 0.50; 0.6515 |
| Time point (weeks) | Mean proportion (95% CI), SE | Odds (95% CI) | Odds ratio (95% CI); p-value | ||
|---|---|---|---|---|---|
| SSM | UC | SSM | UC | SSM vs. UC | |
| 6 | 0.40 (0.25 to 0.56), 0.08 | 0.45 (0.31 to 0.58), 0.07 | 0.68 (0.36 to 1.29) | 0.81 (0.47 to 1.40) | 0.84 (0.39 to 1.82); 0.6558 |
| 12 | 0.55 (0.38 to 0.72), 0.08 | 0.57 (0.42 to 0.72), 0.08 | 1.23 (0.62 to 2.42) | 1.34 (0.72 to 2.51) | 0.91 (0.39 to 2.15); 0.8357 |
Exploratory subgroup analysis
Based on summary statistics, participants in the intervention arm who engaged in the use of PainCheck (n = 23) had slightly increased worse pain scores at baseline, 6 weeks and 12 weeks compared with those who did not engage (n = 57); there was no evidence of a significant difference (Figure 15; see Report Supplementary Material 1, Figure 19).
FIGURE 15.
Estimated mean worst pain scores with 95% CIs for unadjusted, adjusted primary and adjusted sensitivity analyses. Primary analysis – multiple imputation; sensitivity analysis – to availability of data.
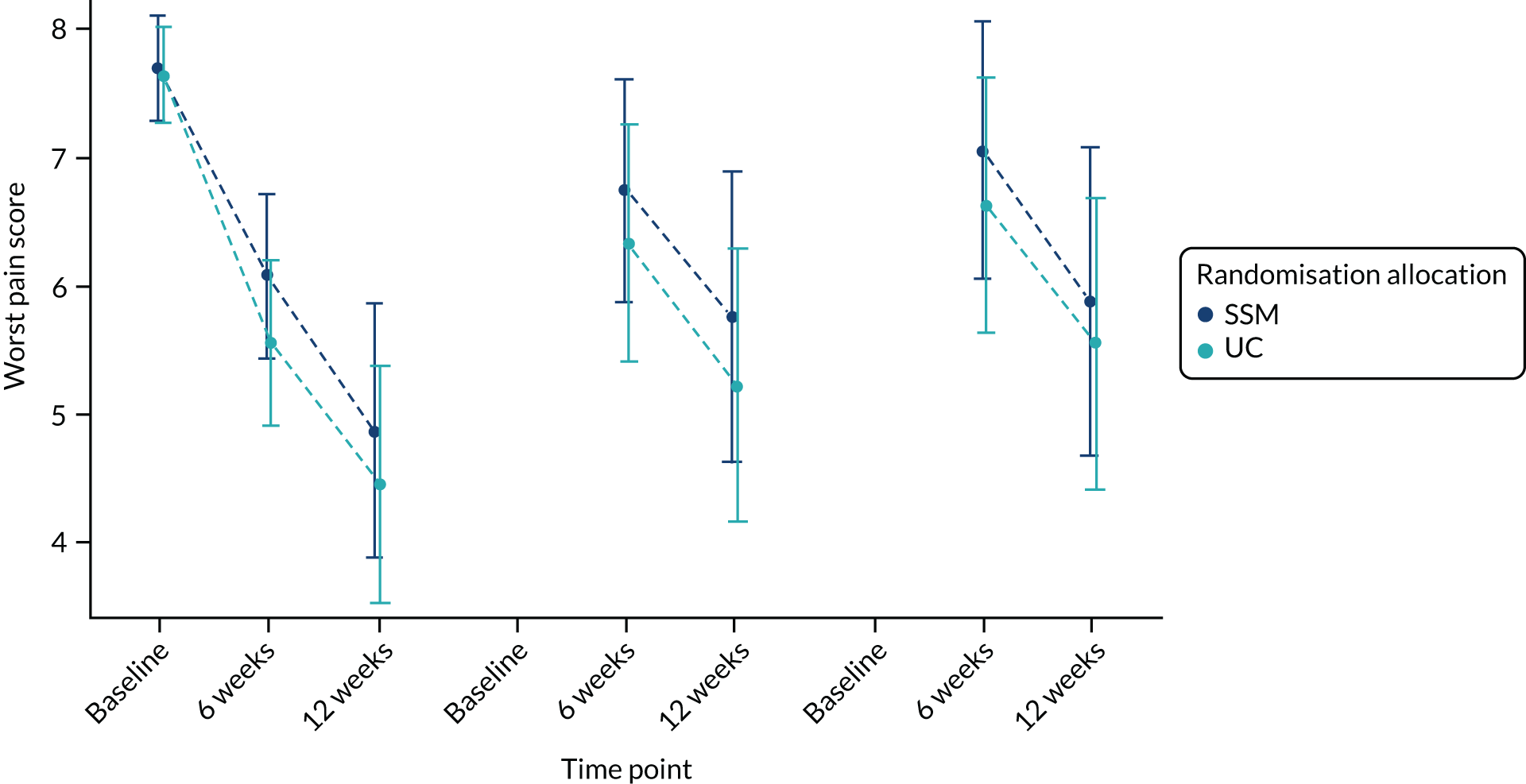
Secondary outcomes
Mean scores improved in both arms across all secondary outcomes of pain severity (BPI), pain interference (BPI), patient pain knowledge and experience (PPQ), and cancer-specific QoL (QLQc-30) (see Report Supplementary Material 1, Figures 20–22 and Table 37). Unadjusted mean scores suggested slightly worse pain severity in the SSM arm than in the UC arm at 6 and 12 weeks, slightly better pain knowledge and experience in the SSM arm than in the UC arm at 6 and 12 weeks, and slightly better pain interference and QoL in the SSM arm than in the UC arm from baseline to the 6- and 12-week follow-ups. There was, however, no evidence of a significant difference in trial arms in primary or sensitivity analysis for the secondary outcomes (Table 11; see Report Supplementary Material 1, Table 38) at 6- or 12-week follow-up.
| Secondary outcome | 6 weeks, mean (95% CI), SE; p-value | 12 weeks, mean (95% CI), SE; p-value | ||||
|---|---|---|---|---|---|---|
| SSM | UC | Differencea | SSM | UC | Differencea | |
| BPI pain interferenceb | 5.1 (4.2 to 6.0), 0.46 | 5.1 (4.2 to 6.0), 0.47 | 0.0 (–0.9 to 0.9), 0.45; 0.9597 | 4.3 (3.4 to 5.2), 0.46 | 4.3 (3.3 to 5.3), 0.49 | 0.0 (–1.0 to 0.9), 0.50; 0.9547 |
| PPQ pain knowledge subscalec | 36.9 (30.7 to 43.0), 3.14 | 35.2 (28.6 to 41.8), 3.35 | 1.7 (–4.4 to 7.8), 3.13; 0.5877 | 37.6 (30.4 to 44.8), 3.64 | 35.0 (28.9 to 41.2), 3.14 | 2.6 (–4.0 to 9.1), 3.32; 0.4424 |
| PPQ pain experience subscaled | 39.6 (34.9 to 44.3), 2.40 | 38.2 (33.5 to 43.0), 2.42 | 1.4 (–2.7 to 5.4), 2.06; 0.5027 | 35.5 (29.7 to 41.4), 2.95 | 34.8 (29.6 to 40.0), 2.63 | 0.7 (–4.9 to 6.4), 2.87; 0.8033 |
| PPQ total scoree | 77.3 (69.2 to 85.5), 4.15 | 74.7 (66.6 to 82.7), 4.09 | 2.7 (–5.0 to 10.4), 3.91; 0.4946 | 74.4 (65.6 to 83.2), 4.48 | 70.6 (62.1 to 79.1), 4.33 | 3.8 (–4.6 to 12.3), 4.31; 0.3751 |
| EORTC QLQc-30 summary scoref | 54.6 (48.7 to 60.5), 2.99 | 54.0 (48.5 to 59.5), 2.81 | 0.6 (–4.3 to 5.6), 2.52; 0.8067 | 60.0 (53.7 to 66.3), 3.20 | 59.5 (53.3 to 65.7), 3.15 | 0.5 (–5.4 to 6.4), 3.01; 0.8739 |
Global rating of change
An increase in pain on the 7-point global rating of change in pain was reported for 31 (29.5%) participants at 6 weeks and 27 (31.8%) participants at 12 weeks, and a reduction in pain was reported for 59 (56.2%) participants at 6 weeks and 43 (50.6%) participants at 12 weeks (see Report Supplementary Material 1, Table 39). A higher rate of increase in pain and a lower rate of reduction in pain was observed in the SSM arm than in the UC arm at 6 weeks. However, this difference was not observed at 12 weeks; instead, a higher rate of reduction in pain was observed in the SSM arm than in the UC arm.
Health-care use
Hospital attendances
Hospital outpatient appointments were reported during the 12-week trial period for the majority of participants (95%), with a similar rate across trial arms (Table 12). The number of visits per participant was larger in the UC arm, with a mean of 7.6 visits (SD 5.42 visits) compared with 5.6 visits (SD 4.56 visits) in the SSM arm. Over three-quarters of appointments were for cancer treatment and routine follow-up. Accident and emergency (A&E) attendances were reported for 14.9% of participants overall, with similar rates across the trial arms. A&E attendances were most frequently for disease complications (66.7%), and a higher proportion were owing to cancer-related pain (22.2%) than outpatient appointments (6.3%). Hospital admissions were reported for almost half of participants: 38.8% in the SSM arm and 48.1% in the UC arm. A maximum of four admissions were reported per participant. The most frequent reasons for admission were disease complications (46.3%) and cancer-related pain (35.2%).
| Hospital use | SSM (N = 80) | UC (N = 81) | Total (N = 161) |
|---|---|---|---|
| Outpatient visit | |||
| Yes, n (%) | 75 (93.8) | 78 (96.3) | 153 (95.0) |
| Outpatient visits per participant | |||
| Visits (n) | 449 | 615 | 1064 |
| Mean (SD) | 5.6 (4.56) | 7.6 (5.42) | 6.6 (5.09) |
| Median (range) | 4 (0–20) | 6 (0–27) | 6 (0–27) |
| Reason for outpatient visit (not mutually exclusive), n (%) | |||
| Cancer-related pain | 30 (6.7) | 37 (6.0) | 67 (6.3) |
| Cancer treatment and routine follow-up | 324 (72.2) | 482 (78.4) | 806 (75.8) |
| Assessment/scan/review | 83 (18.5) | 100 (16.3) | 183 (17.2) |
| Intervention/treatment | 24 (5.3) | 23 (3.7) | 47 (4.4) |
| Disease complications | 10 (2.2) | 11 (1.8) | 21 (2.0) |
| A&E attendance, n (%) | |||
| Yesa | 11 (13.8) | 13 (16.0) | 24 (14.9) |
| A&E attendances per participant | |||
| Visitsb (n) | 12 | 15 | 27 |
| Mean (SD) | 0.2 (0.39) | 0.2 (0.45) | 0.2 (0.42) |
| Median (range) | 0 (0–2) | 0 (0–2) | 0 (0–2) |
| Reason for A&E attendance (not mutually exclusive), n (%) | |||
| Cancer-related pain | 2 (16.7) | 4 (26.7) | 6 (22.2) |
| Intervention/treatment | 0 (0.0) | 4 (26.7) | 4 (14.8) |
| Disease complications | 10 (83.3) | 8 (53.3) | 18 (66.7) |
| Admission/inpatient stay | |||
| Yes,c n (%) | 31 (38.8) | 39 (48.1) | 70 (43.5) |
| Admission/inpatient stays per participant | |||
| Visitsb, n | 45 | 63 | 108 |
| Mean (SD) | 0.6 (0.81) | 0.8 (1.00) | 0.7 (0.91) |
| Median (range) | 0 (0–3) | 0 (0–4) | 0 (0–4) |
| Reason for inpatient stay (not mutually exclusive), n (%) | |||
| Cancer-related pain | 17 (37.8) | 21 (33.3) | 38 (35.2) |
| Cancer treatment and routine follow-up | 3 (6.7) | 11 (17.5) | 14 (13.0) |
| Assessment/scan/review | 1 (2.2) | 6 (9.5) | 7 (6.5) |
| Intervention/treatment | 7 (15.6) | 8 (12.7) | 15 (13.9) |
| Disease complications | 25 (55.6) | 25 (39.7) | 50 (46.3) |
Palliative care contacts
All participants in the SSM arm and the majority (92.6%) in the UC arm received some palliative care contact, including telephone contact, home visits, day or outpatient hospice attendances and inpatient stays, during the 12-week trial period (see Report Supplementary Material 1, Table 40; Table 13).
| Type and number of contacts | Trial arm | Total (N = 161) | |
|---|---|---|---|
| SSM (N = 80) | UC (N = 81) | ||
| Telephone calls | |||
| Yes, n (%) | 78 (97.5) | 72 (88.9) | 150 (93.2) |
| Mean (SD) | 5.0 (3.91) | 5.5 (4.27) | 5.3 (4.09) |
| Median (range) | 4 (0–21) | 5 (0–16) | 4 (0–21) |
| Home visits | |||
| Yes, n (%) | 76 (95.0) | 71 (87.7) | 147 (91.3) |
| Mean (SD) | 2.8 (2.30) | 2.7 (2.13) | 2.8 (2.21) |
| Median (range) | 2 (0–10) | 2 (0–8) | 2 (0–10) |
| Reason for home visit, n (%) | |||
| Cancer-related pain | 71 (31.7) | 69 (31.1) | 140 (31.4) |
| Initial visit | 74 (33.0) | 68 (30.6) | 142 (31.8) |
| Cancer-related pain and other reason | 59 (26.3) | 65 (29.3) | 124 (27.8) |
| Other | 20 (8.9) | 20 (9.0) | 40 (9.0) |
| Day/outpatient visit | |||
| Yes, n (%) | 14 (17.5) | 17 (21.0) | 31 (19.3) |
| Mean (SD) | 0.3 (0.87) | 0.6 (1.59) | 0.5 (1.28) |
| Median (range) | 0 (0–4) | 0 (0–9) | 0 (0–9) |
| Reason for day/outpatient visit, n (%) | |||
| Cancer-related pain | 9 (33.3) | 11 (22.4) | 20 (26.3) |
| Initial visit | 1 (3.7) | 3 (6.1) | 4 (5.3) |
| Cancer-related pain and other reason | 8 (29.6) | 19 (38.8) | 27 (35.5) |
| Other | 9 (33.3) | 16 (32.7) | 25 (32.9) |
| Inpatient stays | |||
| Yes,a n (%) | 10 (12.5) | 7 (8.6) | 17 (10.6) |
| Mean (SD) | 0.2 (0.46) | 0.1 (0.28) | 0.1 (0.38) |
| Median (range) | 0 (0–2) | 0 (0–1) | 0 (0–2) |
| Reason for inpatient stay, n (%) | |||
| Cancer-related pain | 2 (15.4) | 1 (14.3) | 3 (15.0) |
| Cancer-related pain and other reason | 6 (46.2) | 1 (14.3) | 7 (35.0) |
| Deteriorating condition | 4 (30.8) | 4 (57.1) | 8 (40.0) |
| Other | 1 (7.7) | 1 (14.3) | 2 (10.0) |
| Days per palliative care inpatient stay | |||
| n | 13 | 7 | 20 |
| Mean (SD) | 14.9 (13.79) | 10.9 (9.34) | 13.5 (12.31) |
| Median (range) | 11 (2–50) | 12 (0–27) | 11.5 (0–50) |
Over half of participants continued within palliative care at the end of the trial, with a slightly larger number of SSM participants (62.5%) than UC participants (53.1%). One-fifth were discharged from palliative care at the end of the trial: 17.5% in the SSM arm and 22.2% in the UC arm. The remaining participants were discharged before 12 weeks, largely owing to the participants’ death (see Report Supplementary Material 1, Table 40).
Almost all participants (93.2%) received telephone palliative care contact, with a median of four contacts (see Table 34). A median of two home visits took place in both arms, ranging up to 10 in the SSM arm and eight in the UC arm. The reason for home visits were mainly because of the initial palliative care visit, cancer-related pain or cancer-related pain with other reasons.
The number of participants with a day/outpatient visit and an inpatient stay and the number of visits/stays was similar between arms. Just under one-fifth of participants had a day/outpatient visit: 17.5% in the SSM arm attended up to four times and 21% in the UC arm attended up to nine times. A total of 10.6% of participants had an inpatient stay: 12.5% of participants in the SSM arm attended on up to two occasions and 8.6% of participants in the UC arm attended once. Inpatient stays were on average a median of 11.5 days, ranging up to 50 days.
Pain medications
All participants but one were receiving pain medication at baseline and all but four participants (alive, with available data) were receiving pain medication at 6 and 12 weeks (see Report Supplementary Material 1, Table 41). Participants were taking a mean of 2.4 different medications (ranging from 0 to 5 medications) at baseline, increasing to a mean of 2.8 and 2.9 medications (ranging from 0 to 7 medications) at 6 and 12 weeks, respectively. The proportion of participants on a strong opioid increased from a baseline rate of 59% to 72.4% at 6 weeks and 71.8% at 12 weeks. Medication use was similar across trial arms, albeit with a slightly higher rate for all classes of medication in the SSM arm than in the UC arm.
Cost-effectiveness analysis of the IMPACCT trial
The cost-effectiveness analysis of the IMPACCT trial was based on within-trial outcomes, with costs and HRQoL data collected at baseline, 6 weeks and 12 weeks. The decision model presented in WP 4.1 was not used here to extrapolate the outcomes of the trial over a longer time horizon, principally because the statistical analysis of the trial data found no difference between the trial arms in terms of the primary outcome measure (pain). Without a differential in pain outcome, there could be no differential in the costs and effects in the model. Thus, the time horizon for the evaluation was 12 weeks. Where possible, we followed the National Institute for Health and Care Excellence’s reference case, reporting cost per incremental QALY [based on EuroQol-5 Dimensions, 5-level version (EQ-5D-5L), and EORTC-8D].
Our primary analysis was based on the EQ-5D-5L direct valuation84 with supplementary analyses presented based on the EQ–5D-5L crosswalk to the EQ-5D-3L85 and the EORTC-8D, derived from the QLQ-C30. 86
The QALY calculation combined HRQoL and survival information and, thus, was based on a linear interpolation, area-under-curve approach. Given that some patients were observed to die between follow-up waves, some further assumptions were required for such cases. For patients who died with their last HRQoL observation as positive, we calculated the aggregate HRQoL measure on the basis that such patients had a linear fall in this measure from this point to death, imposing the assumption that HRQoL deteriorates in a linear fashion until the value zero is reached at death. For patients who died with their last EQ-5D observation as negative, we calculated the aggregate HRQoL observation measure on the basis that such patients had this constant level of HRQoL according to this measure from this point until death, imposing the assumption that HRQoL does not improve from this level until death.
Costs
We used a combination of the IMPACCT patient-completed questionnaires and administratively collected records to estimate the health resource use of each patient within the trial. We prioritised data from the latter source because patient-completed questionnaires rely on both accurate recall and the individual being willing and able to provide information at the time of the 6- and 12-week follow-ups.
Our cost estimates were formed of four components: primary care costs, hospital and hospice secondary care costs, prescribed medication costs and programme costs. For all but primary care costs and programme costs, we derived activity estimates for each of these from administratively completed records. The administratively completed records specified whether or not hospital visits and admissions were pain related. Although the a priori assumption would be that data on the former would be more specific and reduce noise in the results, discussions with nurses collating administrative data, and examination of the text responses made alongside these records, indicated that these data were potentially confounded. For example, frequently, although resource use was indicated to have been pain related, text responses indicated that there were multiple, including non-pain-related, reasons for admission. That being the case, we elected to use all reported resource use and not just those indicated to be pain related. Prescribed medication details (both specific type/brand of medication and dosage) were also derived from administrative records.
Health-care resource use (primary and secondary care) was costed using the most recently available data from national resource reports. The unit costs employed, sources and the year from which these costs were taken are documented in Table 14. We inflated all such costs to 2018/19 prices using the NHS Pay & Prices index for years up to 2015/1691 and Monitor’s published Economic Assumptions92 for 2016/17 onwards, with the inflator used for data from each calendar year documented in Table 15. Prescribed medication costs were assigned from the August 2018 NHS Electronic Drugs Tariff,93 with adjustments made for dosage as applicable, with the modal dosage assumed in cases for which this was missing. Programme costs, arising from staff costs and the production of digital and physical information materials to support pain self-management, amounted to a one-off per-patient cost of £63.35 and a weekly per-patient cost of £15.17.
| Item | Cost (£) (2018/19 prices) | Source | Inflated from year |
|---|---|---|---|
| Contact with health services, section 1 | |||
| GP | |||
| Surgery visit (face to face) | 37.74 | Unit Costs of Health and Social Care 2017 87 | 2017 |
| Telephone/e-mail (assumed to be the same for any values entered for telephone contacts in the final column for all of ‘GP surgery visit,’ ‘GP home visit,’ ‘GP out of hours home visit’) | 28.97 | Unit Costs of Health and Social Care 2017,87 Unit Costs of Health and Social Care 201588 | 2017 |
| Home visit (face to face) | 95.47 | Unit Costs of Health and Social Care 2017 87 | 2017 |
| Out-of-hours home visit (face to face) | 124.06 | Unit Costs of Health and Social Care 2017,87 National Audit Office89 | 2017/2013 |
| Pharmacist | |||
| Face to face | 6.74 | Unit Costs of Health and Social Care 2017 87 | 2017 |
| Telephone/e-mail | 5.19 | Unit Costs of Health and Social Care 2017 87 | 2017 |
| District nurse | |||
| Face to face | 38.61 | ‘District Nurse, Adult, Face to face’89 | 2016 |
| Telephone/e-mail | 17.74 | ‘District Nurse, Adult, Non face to face’89 | 2016 |
| Health visitor | |||
| Face to face | 57.39 | ‘Health Visitor, Other Clinical Intervention’89 | 2016 |
| Telephone/e-mail | 24.12 | ‘Health Visitor, Other Clinical Intervention,’89 adjusted for ratio of face-to-face to non-face-to-face costs | 2016 |
| Hospice nurse | |||
| Home visit (face to face) | 84.84 | ‘Hourly cost of Community Palliative Nursing Visit (e.g. as provided by Marie Curie)’90 | 2012 |
| Telephone/e-mail | 8.48 | ‘Hourly cost of Community Palliative Nursing Visit (e.g. as provided by Marie Curie)’90 | 2012 |
| Counsellor (face to face)a | 63.78 | Unit Costs of Health and Social Care 2015 88 | 2013 |
| Psychologist | |||
| Face to face | 175.98 | ‘Clinical Psychology outpatient’89 | 2016 |
| Telephone/e-mail | 83.54 | ‘Clinical Psychology, Non-Admitted Non-Face-to-Face Attendance, Follow-up,89 using volume-weighted average of consultant-led and non-consultant led costs | 2016 |
| Physiotherapist | |||
| Face to face | 55.81 | ‘Physiotherapist, Adult, One to One’89 | 2016 |
| Telephone/e-mail | 32.41 | ‘Physiotherapy, Non-Admitted Non-Face-to-Face Attendance, Follow-up,’89 using volume-weighted average of consultant-led and non-consultant led costs | 2016 |
| Occupational therapist | |||
| Face to face | 80.06 | ‘Occupational Therapist, Adult, One to One’89 | 2016 |
| Telephone/e-mail | 42.78 | ‘Occupational therapy, Non-Admitted Non-Face-to-Face Attendance, Follow-up,’89 (consultant-led and non-consultant led reference costs identical) | 2016 |
| Contact with health services, section 2 | |||
| Hospital inpatient stay, per day | 415.30 | ‘Inpatient Specialist Palliative Care, 19 years and over’89 | 2016 |
| Hospice stay, per day | 468.26 | ‘Inpatient Day in Hospice Care’90 | 2012 |
| Hospital day centre, per visit | 206.61 | ‘Inpatient Specialist Palliative Care, Same Day, 19 years and over’89 | 2016 |
| Hospital outpatient clinic, per visit | 199.25 | ‘Palliative Medicine, Total Outpatient attendances’89 | 2016 |
| Hospital accident and emergency, per visit | 154.43 | ‘Unit costs, by point of delivery: A&E’89 | 2016 |
| Year | Inflator applied (3 dp) |
|---|---|
| 2012 | 1.102 |
| 2013 | 1.100 |
| 2014 | 1.090 |
| 2015 | 1.076 |
| 2016 | 1.043 |
| 2017 | 1.020 |
Missing data
Given the substantial number of cases for which we did not have observations for all dependent and independent variables (a ‘complete case’), we carried out multiple imputation on missing outcomes. Given that our cost data were predominantly based on administrative records, we encountered missing data with patient recall of primary care usage only: this occurred in 32% of cases. In such cases, we imputed costs related to primary care usage only and added this to other costs derived from administrative data. This category amounted to under 6% of all costs included in our analysis and, thus, should not unduly influence results.
We determined relevant variables to be used in imputation by whether they were predictive of either or both outcomes or missingness, and also included baseline values of costs and HRQoL. In addition to these baseline values and in line with methods used in the main statistical analysis, we included sex, age, a grouped variable of the site at which the patient was recruited, the patient’s self-reported health out of 100 according to the EQ-5D visual analogue scale measure and the individual’s baseline pain stratum as measured by the BPI score, as well as whether or not and what type of opioid pain relief the patient received.
Analysis
We primarily estimated costs and HRQoL outcomes using seemingly unrelated regression (SUR), accounting for correlation between observed costs and utilities, as well as a three-stage least squares complier average causal effect (CACE) analysis, as proposed by DiazOrdaz et al. 94 This treated compliers as being those who engaged with the PainCheck software three or more times, effectively using treatment allocation as our identifying restriction for compliance. We have presented results from a generalised linear model regression using a log-link function and gamma distribution.
We subsequently used the results of these regressions to take 10,000 draws from implied parametric distributions to graphically illustrate the uncertainty around this estimate using cost-effectiveness planes and cost-effectiveness acceptability curves. We carried out this analysis for both complete cases and our multiply imputed data set, using base-case control variables of the relevant baseline outcome (costs or HRQoL), the site of patient recruitment, the patient’s baseline pain stratum and the patient’s status as alive at each of 4, 8, 12, 16, 20, 24, 28 and 32 weeks from baseline. Although the inclusion of these time-to-death indicator variables is unusual in such cost-effectiveness analyses, we included them on the basis that the intervention employed in this case was not intended or expected to extend life. Consequently, we considered these variables both exogenous in our analyses, and likely to be relevant in explaining hospital costs given existing evidence on the relationship between time-to-death and medical expenditures, and the degree to which time-to-death proxies for underlying morbidity in such relationships. 95–98
Results regarding the cost-effectiveness of an intervention are most commonly presented as ICERs, representing the ratio of incremental costs and incremental effects, each of which were generally positive for a given intervention. The interpretation of such a combination (positive costs, positive effects) was to compare the derived ICER with a benchmark threshold, with a value below this threshold deemed to represent a cost-effective intervention. 99
This interpretation of the ICER was somewhat different in cases in which either or both incremental costs and incremental benefits were negative. In cases in which negative ICERs resulted from a combination of negative incremental costs and positive incremental QALYs, this implied a dominant intervention, both cost saving and health improving. In cases in which negative ICERs resulted from a combination of positive incremental costs and negative incremental QALYs, however, this implied a dominated intervention, both more costly and health reducing. In cases in which both costs and effects were negative, however, point estimates implied positive ICERs and a positive value above the threshold is deemed to represent a cost-effective intervention, that is, although there may be a negative impact on HRQoL from the intervention, the cost saving occasioned by it implied a net gain in population health.
Given these well-established issues regarding the use of ICERs, which were particularly pertinent in this case, we present our results primarily in terms of a point estimate of net monetary benefit (NMB) per patient treated. This was calculated as:
where E is effects, C is costs and λ is the relevant threshold, with the intervention deemed to be cost-effective if, and only if, the derived value of this is positive. 100
All analyses were conducted using Stata® 15 (StataCorp LP, College Station, TX, USA).
Results
Characteristics (at baseline for all bar survival) of the UC and SSM arms are presented in Table 16. The UC arm was on average slightly older and contained slightly more male participants than the SSM arm.
| Characteristic | Trial arm, mean (SD) | |
|---|---|---|
| UC | SSM | |
| Age (years) | 65.68 (11.29) | 62.29 (11.7) |
| Male | 0.58 (0.497) | 0.519 (0.503) |
| BPI score of > 6 | 0.222 (0.418) | 0.203 (0.404) |
| Survival at (weeks) | ||
| 4 | 0.975 (0.156) | 0.975 (0.158) |
| 8 | 0.938 (0.242) | 0.911 (0.286) |
| 12 | 0.877 (0.331) | 0.823 (0.384) |
| 16 | 0.741 (0.441) | 0.759 (0.43) |
| 20 | 0.667 (0.474) | 0.658 (0.477) |
| 24 | 0.58 (0.497) | 0.633 (0.485) |
| 28 | 0.58 (0.497) | 0.595 (0.494) |
| 32 | 0.556 (0.5) | 0.57 (0.498) |
Base case
The mean costs in each trial arm of each of the cost components, including imputed costs (for primary care resource use only), are presented in Table 17. The programme costs were estimated to be £232 per person on average. Although the UC arm on average incurred lower hospice costs, it also incurred significantly higher hospital costs than the SSM arm. Differences between arms in terms of primary care and medication costs are relatively small.
| Cost type | Trial arm (£) | |
|---|---|---|
| UC | SSM | |
| Primary care | 348.47 | 269.79 |
| Hospice | 439.35 | 1206.66 |
| Hospital | 4960.12 | 3196.27 |
| Programme | 0.00 | 232.25 |
| Medication | 100.11 | 114.59 |
| Total | 5848.05 | 5019.56 |
Table 18 presents the average EQ-5D-5L scores at baseline, 6 weeks and 12 weeks, broken down by trial arm. The SSM arm exhibited on average higher HRQoL than the UC arm at each of baseline, 6 weeks and 12 weeks.
| Time point | Trial arm | |
|---|---|---|
| UC | SSM | |
| Baseline | 0.539 | 0.596 |
| 6 weeks | 0.655 | 0.667 |
| 12 weeks | 0.697 | 0.719 |
Table 19 presents the average total QALYs attained by those in each trial arm over the time period observed. Minimal differences were observed, with the SSM arm again exhibiting marginally higher values than the UC arm.
| Questionnaire/valuation | Trial arm | |
|---|---|---|
| UC | SSM | |
| EQ-5D-5L (direct) | 0.135 | 0.137 |
| EQ-5D-3L (crosswalk from EQ-5D-5L) | 0.110 | 0.114 |
| EORTC-QLQ (direct) | 0.134 | 0.134 |
The results from the SUR base-case analysis implied, for the trial duration, a point estimate (SSM vs. UC) of a reduction in costs of £587 (p = 0.54) and a point estimate QALY increase of 0.0018 (p = 0.67). This implied an ICER with a point estimate of –£321,795, indicating that the IMPACCT programme dominated standard care (having lower costs and higher QALYs). The NMB per patient was estimated at £623 at a threshold of £20,000. The probability of SSM being cost-effective was estimated at 73.86% at a threshold of £20,000. The probability of SSM being cost saving was estimated at 72.9%. Figures 16 and 17 show the level of uncertainty around the base-case results in the form of the cost-effectiveness plane and the cost-effectiveness acceptability curve, respectively. Figure 18 illustrates the distribution of the estimated difference in costs between SSM and UC.
FIGURE 16.
Cost-effectiveness plane and ellipse: IMPACCT vs. no IMPACCT (SUR, base case).
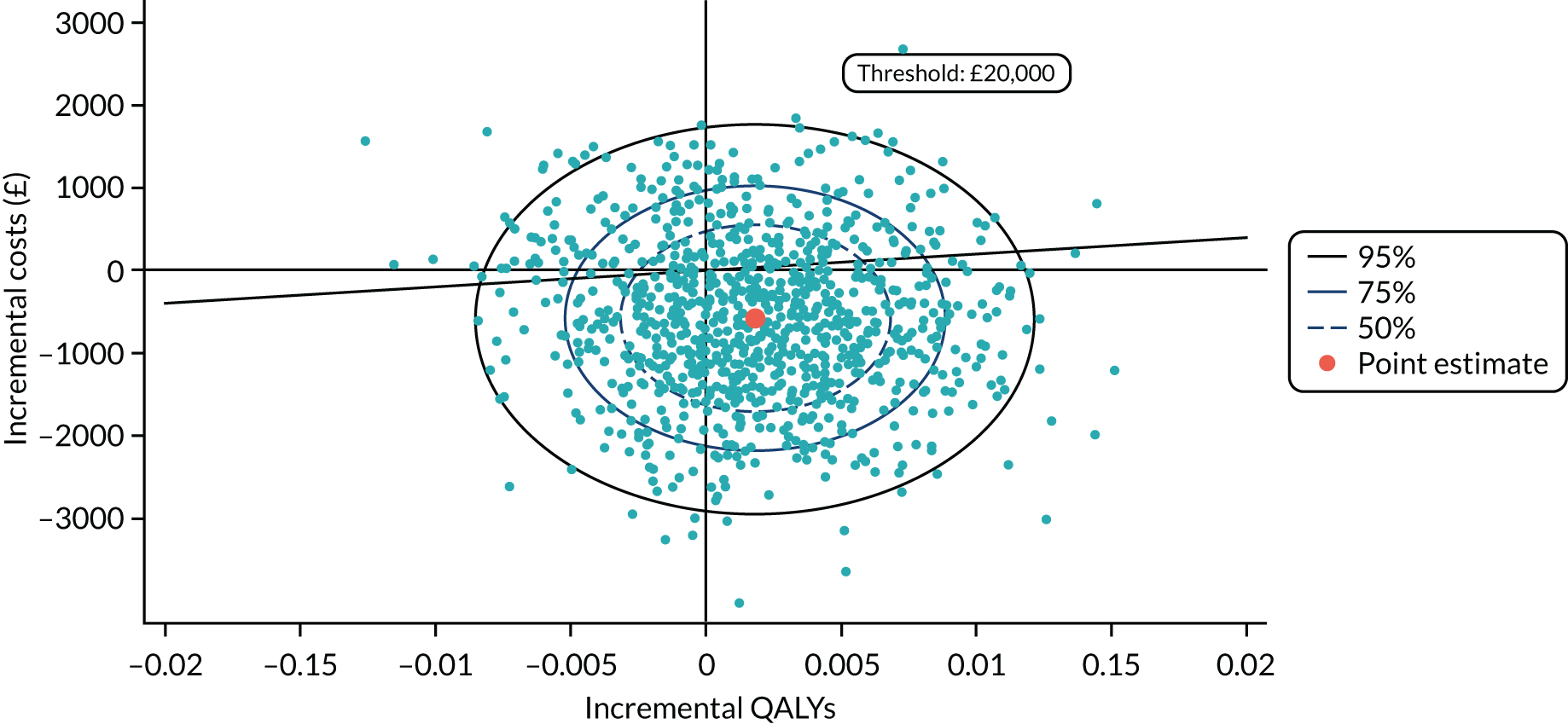
FIGURE 17.
Cost-effectiveness acceptability curve (SUR, base case).
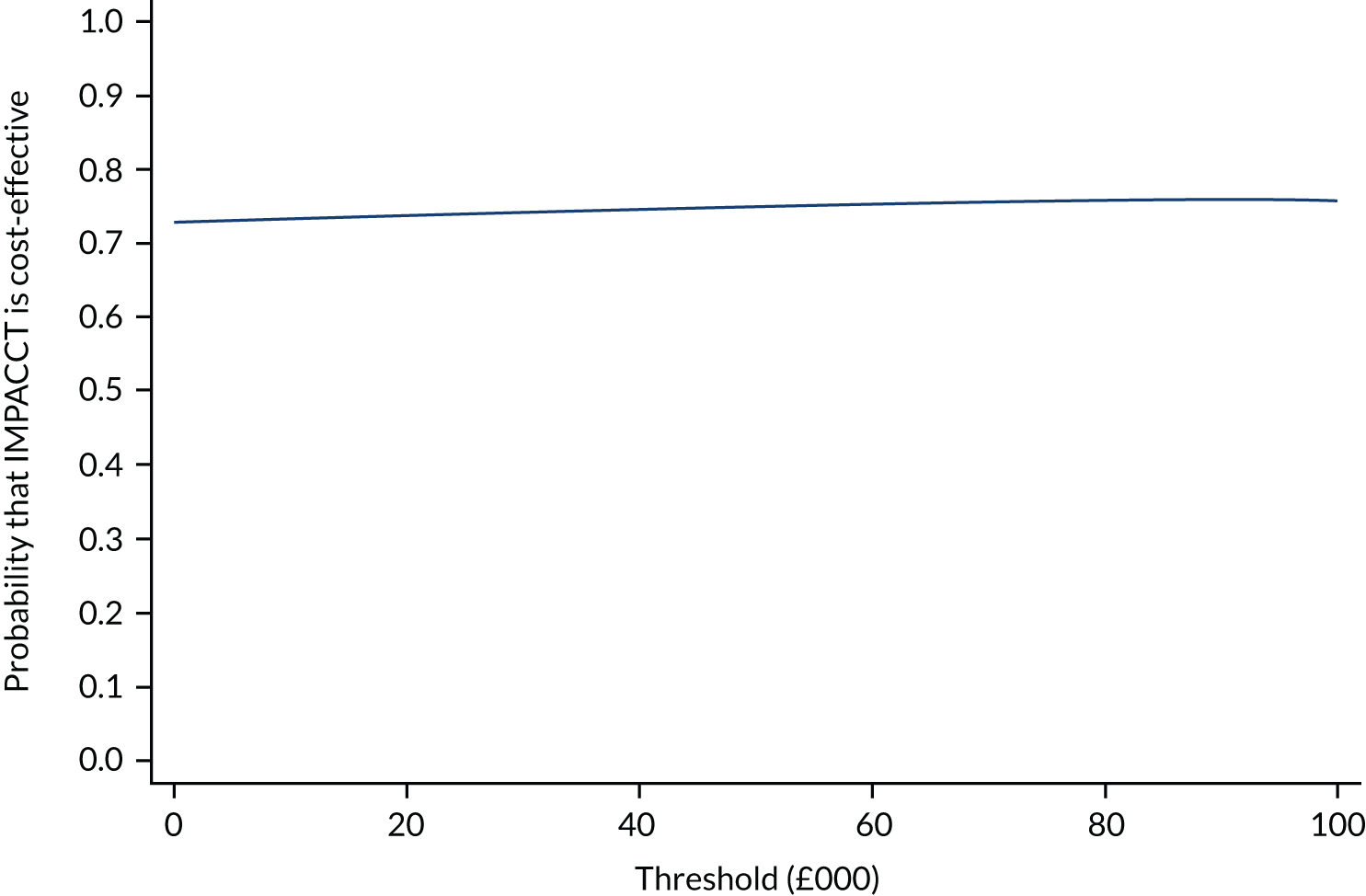
FIGURE 18.
Distribution of estimated cost difference (negative represents SSM cost-saving). Mean = –£587. Probability of cost-saving (shaded area) = 72.9%.
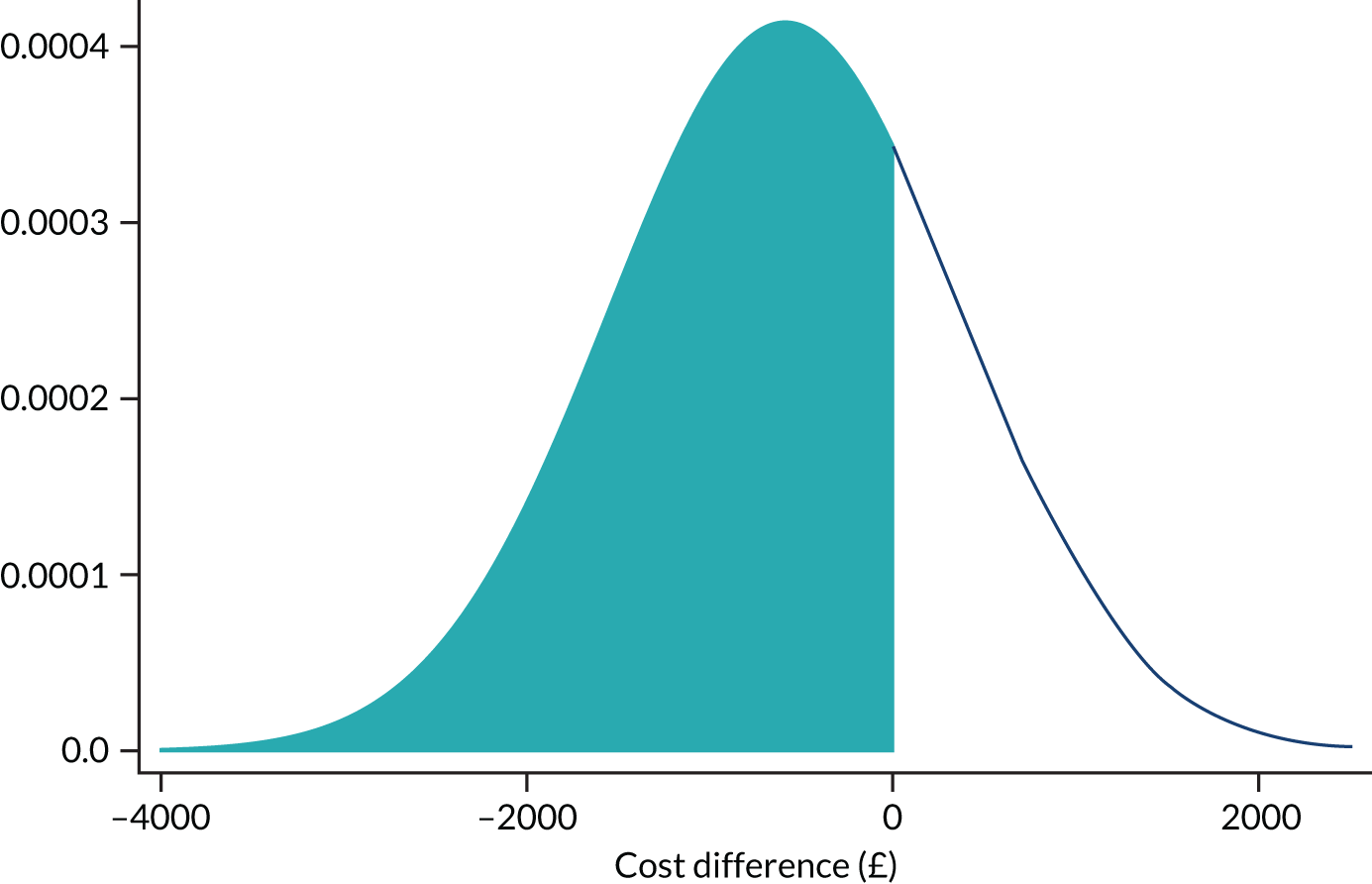
The majority of our results from alternative model specifications and measures of HRQoL suggested a positive NMB from IMPACCT, implying that it was more likely than not to be cost-effective, with a probability varying from 67.88% to 76.33% in our SUR analyses of multiply imputed data (Table 20) and 94.72% to 98.76% when using complete cases only (Table 21). These results were driven by the results of the cost side of our regressions, which generally exhibited greater statistical significance than the QALY side.
| HRQoL measure | Controls | Incremental costs (£) | Incremental QALYs | NMBa (£) | Probability cost-effectivea (%) |
|---|---|---|---|---|---|
| EQ-5D-5L | Baseline values | –716.12 | –0.0023 | 671.10 | 73.75 |
| Baseline values, pain stratum, site | –747.92 | –0.0018 | 711.30 | 75.99 | |
| Baseline values, pain stratum, site, time to death | –586.76 | 0.0018 | 623.23 | 73.86 | |
| EQ-5D-3L crosswalk | Baseline values | –716.28 | –0.0019 | 679.19 | 74.05 |
| Baseline values, pain stratum, site | –748.05 | –0.0014 | 720.77 | 76.33 | |
| Baseline values, pain stratum, site, time to death | –586.64 | 0.0016 | 617.77 | 73.71 | |
| EORTC-8D | Baseline values | –605.04 | –0.0051 | 502.13 | 67.88 |
| Baseline values, pain stratum, site | –716.09 | –0.0047 | 623.04 | 72.61 | |
| Baseline values, pain stratum, site, time to death | –499.06 | 0.0004 | 507.04 | 69.30 |
| HRQoL measure | Controls | Incremental costs (£) | Incremental HRQoL | NMBa (£) | Probability cost-effectivea (%) |
|---|---|---|---|---|---|
| EQ-5D-5L | Baseline values | –2341.95 | 0.0010 | 2361.29 | 98.07 |
| Baseline values, pain stratum, site | –2309.11 | 0.0020 | 2349.50 | 98.43 | |
| Baseline values, pain stratum, site, time to death | –1797.59 | 0.0019 | 1834.86 | 95.56 | |
| EQ-5D-3L crosswalk | Baseline values | –2406.97 | –0.0020 | 2367.70 | 98.17 |
| Baseline values, pain stratum, site | –2426.88 | 0.0001 | 2429.26 | 98.76 | |
| Baseline values, pain stratum, site, time to death | –1895.49 | –0.0006 | 1882.77 | 96.09 | |
| EORTC-8D | Baseline values | –2058.90 | 0.0001 | 2061.13 | 96.07 |
| Baseline values, pain stratum, site | –2246.90 | 0.0000 | 2246.90 | 97.57 | |
| Baseline values, pain stratum, site, time to death | –1840.13 | 0.0003 | 1845.25 | 94.72 |
Following multiple imputation for missing data, we estimated cost and effects using SUR. In a further set of results, we presented the outcome of a CACE analysis. The economic evaluation demonstrated that the most important driver of cost-effectiveness was the level of pain reduction, and our interventions resulted in lower health-care costs. Using a cost-effectiveness threshold of £20,000 per QALY, there was a 67% chance that our interventions were optimal in our base-case analysis. Substantially higher estimates of this probability – in excess of 93% – were derived when a CACE analysis was conducted. This result was driven chiefly by differences in estimated costs per patient between arms of the trial, with our base case implying a saving of £586.76 with the IMPACCT intervention, and our CACE analysis implying a saving of £4356.36. Although the SSM arm appeared to incur greater hospice use cost than the UC arm, this is outweighed by lower hospital visit/stay costs in the SSM arm. Most analyses suggest that IMPACCT was dominant; however, there was minimal difference in QALYs between arms.
Limitations of the trial
Study design
We had originally intended to evaluate early screening and referral to palliative care as well the effectiveness of our two interventions. However, after opening the trial with the original protocol, it became clear that it was not feasible to implement an early referral pathway into the existing cancer pain pathways within the RCT. Once our screening procedures had identified cancer patients with significant pain (≥ 4 on a 0–10 rating scale), oncology staff were reluctant to allow patients to be randomised, potentially to UC, and felt obliged to make a clinical referral to palliative care. Instead, we randomised all patients to early palliative care with or without SSM. In effect, the revised study design forced the UC arm to have earlier access to palliative care services and this might explain the lack of observed benefit. Greater differences between the arms in relation to pain management might have been apparent had we been able to continue with the original protocol.
Although cancer pain remains common, severe and undertreated, our trial showed that routine screening for pain in oncology patients can prompt earlier referral to specialist palliative care support and in itself be an important intervention in relation to improving outcomes for patients. The difficulty that faces patients is that access to specialist palliative care support in routine care often occurs very late and only weeks before death. Our trial patients were recruited and referred to palliative care a median of 53 weeks before their death compared with referral in routine care around a median of 7 weeks before death. 22 This resulted in significant improvements in pain for our trial participants in both trial arms because they were able to access a widely acknowledged good standard of palliative care services in the UK. 101 Other research by our team using national data has also shown that for patients at home, good pain relief is significantly associated with contact with community palliative care services. 102
Fidelity of intervention
Despite recognising the potential role that our interventions might have in routine care, health professionals acted as gatekeepers to patient acceptability of, and access to, PainCheck in particular. We identified an ambivalence towards digital technologies in palliative care; some health professionals felt that it easily supported their current way of working whereas others felt that PainCheck needed to be streamlined, and they were concerned that it could become burdensome if they had a larger number of patients on their caseload who were using it. Many believed that it would not replace their current way of working.
Patients who used PainCheck and educational resources described the interventions as straightforward, easy to use, quick, user-friendly and unobtrusive, with the eHealth tool considered a simple tool to aid with monitoring their symptoms and communicating with professionals. By contrast, patients who had limited engagement with PainCheck were not regular users of technology or computers, and often did not have or were unable to use internet connections. They found engaging with it stressful and subsequently were not interested in integrating it into their daily lives.
Overall, there was low engagement with PainCheck and possibly with Tacking Cancer Pain too, and this low fidelity might also have contributed to a lack of difference in pain outcomes between the two trial arms. We do not regard the low engagement as a failure of the research study, but as a finding: engagement with digital technology in routine palliative care was low.
Policy context
During the course of our research programme, NHS England introduced a new initiative called ‘Enhanced Supportive Care’, which is designed to provide earlier integration of supportive care for patients with cancer (at any stage of disease). A number of pilot sites have been set up in larger cancer centres, although none of these was at the hospitals involved in our trial. This type of initiative may in future help to encourage earlier referral of patients with pain from cancer to community palliative care services, although the delivery and outcomes of this initiative have not been fully evaluated. One challenge is that, although some cancer centres have in-hospital ‘supportive care’ teams, there is no such community equivalent. Although palliative care services are well established, this is not the case for supportive care services and so earlier referral to palliative care (as opposed to referral to supportive care) is advocated. Currently, most of these initiatives are being delivered by existing palliative care teams within hospitals and we have indicated that it is possible to provide early palliative care in this context to community-based patients.
Economic analysis
The difference in QALYs between the two trial arms was negligible and this was not in line with our expectations or the decision model that we had developed. Our a priori hypothesis was that SSM would increase pain control. Given that this was not the case, we were unable to use the decision model to extrapolate benefits forward because it was structured around mutually exclusive pain severity health states. It may have been possible to adapt the model to capture cost savings associated with the SSM intervention only but, given that this was already highly likely to be cost-effective in 12 weeks, the additional modelling was not considered informative.
The decision modelling (WP 4.1) indicated that information and feedback interventions similar to the SSM intervention developed in IMPACCT can be cost-effective. However, the trial-based economic evaluation (WP 4.2, which did not include the model) found SSM to be cost-effective but for different reasons: it did not improve pain relief but did reduce health-care resource use costs.
The intervention did not significantly affect survival or HRQoL. It is possible that the HRQoL measurement schedule missed important pain experiences and events and future research should consider more frequent pain assessments, especially when area under the curve analyses are being estimated. Although evidence regarding the cost-effectiveness of palliative care from RCTs remains scant, our results add to the growing body of evidence of the cost-saving nature of palliative care interventions and point to the importance of carrying out robust studies of cost-effectiveness even when there is little evidence of clinical effectiveness.
Supported self-management appears to lead to non-trivial cost savings per patient which, if factored up across commissioning areas, could be substantial. One interpretation of our result is that patients felt better able to manage pain at home and/or with the help of hospice staff and were therefore less likely to visit, and be admitted to, hospital. Additional qualitative research and exploration of the process evaluation may elucidate this point. Should this shift of resource use from secondary care to hospice be a real effect, there may be resource allocation implications to be considered.
Conclusions and recommendations
Our research programme aimed to improve the management of cancer pain for palliative care patients and their carers who are living at home. We were successful in developing interventions that enabled patients and their carers to more easily access support and advice, communicate the patient’s pain, and obtain timely and effective support for the patient’s medication. We evaluated the impact of our interventions in routine care. The programme recruited over 1400 participants during the development work and clinical trial. We also built on the research within IMPACCT to secure external project funding from the National Institute for Health Research (NIHR) [Research for Patient Benefit (RfPB), Health Technology Assessment (HTA) and Health Services and Delivery Research (HSDR) awards], as well as securing four externally funded senior research fellowships. This has provided longer term sustainability for the Academic Unit of Palliative Care at the University of Leeds and secured increased research capacity within this field nationally.
Our interviews with patients, carers and health professionals revealed several new perspectives on managing cancer pain. We identified that for patients, managing their pain is a constant daily challenge involving trade-offs between pain, adverse effects of analgesia and sustaining what is valued in terms of life quality. In primary and community care, we found that health professionals and the systems in which they work vary considerably in the extent to which they identify patients with cancer pain who need more help. The complexity of cancer as an advanced disease with features of chronic illness, albeit life-threatening, meant that a simplistic linear cancer pain pathway could not be modelled easily. A mixed picture emerged regarding oncologists’ perception of optimal timing of referral to palliative care. From the qualitative studies, engagement of early supportive care alongside treatment for managing symptoms was regarded as optimal by cancer specialists, although patients were often reluctant to accept referral. This may reflect variable access to such support depending on which point in the service system patients are located and the extent to which practices are in process of change.
Our data analyses from Leeds and from our national hospice survey revealed for the first time, to our knowledge, the relatively short duration of palliative care that many patients with advanced progressive diseases receive before they die. Importantly, we demonstrated that older patients and those with non-cancer diseases receive even less palliative care than younger patients and those with cancer, highlighting significant inequality in service access and provision. Our earlier qualitative research helped us to understand these data in terms of the challenges in identifying patients who need referral.
We analysed systematic reviews to determine the key components of an education intervention underpinned by behaviour change theory that might support self-management. We also explored health professionals’ views on this concept. We combined these insights, together with those from our longitudinal patient and carer interviews, to develop and evaluate our own intervention, Tackling Cancer Pain. This was regarded as an excellent resource by the BMA Patient Information Awards, and was accepted and liked by patients. We recognise that we did not conduct a definitive evaluation of this resource as a single intervention to establish its efficacy before combining this with PainCheck for our complex intervention. We considered that this was not necessary (and would delay the RCT) given the systematic review evidence showing broad effectiveness of this approach and our careful development of Tacking Cancer Pain using patient, carer and health professional input. However, this may be regarded as a limitation.
During the development of PainCheck, we learned that patients with advanced cancer and their health professionals recognised the benefits of an electronic system to monitor pain but had reservations about how such a system might work in practice. We developed and tested a prototype PainCheck system that users were generally positive about and found easy to understand, albeit with some concerns about implementation. We recognised that any ICT system should be embedded within the electronic record to maximise usefulness, although we were not able to achieve this within the life of the programme.
For those patients who used PainCheck within the clinical trial, it was found to be helpful and supportive. However, our process evaluation showed that the perceptions of PainCheck by specialist nurses, as either a useful adjunct to practice or an unnecessary burden, directly influenced patients’ attitudes. Although we trained the teams in the delivery of PainCheck, we may not understand its real value unless we can embed electronic assessments into health-care records and in routine care, and better engage health-care staff in the value of PainCheck. We recognise that not all patients want to or can manage electronic reporting systems and so we need to determine how to engage patients who are most likely to use it. Despite the assertions of patients and staff in the development phases, we identified that in practice, engagement was much lower than expected.
Our medicines management research showed for the first time, to our knowledge, in a large sample of patients with advanced cancer, that many will be prescribed a strong opioid only a few weeks before death, that older patients are less likely than younger patients to receive opioids and that patients admitted to hospital are less likely to have been prescribed an opioid in the community. These findings bring new insights into the reasons underlying uncontrolled pain from advanced cancer in the last year of life. The pattern of opioid prescribing does not match epidemiological data, which point to earlier onset of pain. Although persistent undertreatment of cancer pain is well documented, this study suggests that strategies for earlier pain assessment and the initiation of strong opioid treatment in community-based patients with cancer could help to improve pain outcomes.
We had expected that more NMPs would prescribe for pain in palliative care after the enabling legislation was implemented in 2012. Through our novel methodology we were able to confirm this hypothesis but our analysis also showed that 5 years later NMP prescribing still made only a small contribution to total palliative care prescribing in the community. Our findings suggest that there is still much scope for NMPs to improve access to pain medicines; future research should identify and evaluate good practice.
Our research on the potential for role of pharmacist interventions showed that other health professionals’ concerns about duplication of work were not corroborated. Indeed, even patients receiving palliative care services were found to have unresolved MRPs, many of which could be resolved through discussion with a community pharmacist. Importantly, a medicines consultation with a pharmacist enabled referral to the prescriber in which a change in pain medicines was needed to improve pain control or treat side effects of strong opioids. Most patients who received a medicines consultation said that it could have been more helpful earlier in their cancer pain journey at the time when opioids were first prescribed. We were not able to find a reliable method of easily identifying patients prior to their referral to palliative care but did find that many patients were not aware that their community pharmacist could provide this service, indicating the need for awareness raising. A limitation of this study was the small numbers of patients who were not receiving palliative care services. Future research could explore how this intervention might be applied to patients when they are first prescribed opioids and perhaps before they are referred to palliative care services.
Overall, our findings can inform prescribing practice and prompt better utilisation of existing medicines optimisation services by pharmacists. Changes in NHS policy have altered the landscape of the use of ICT and the deployment of pharmacists in different primary care settings. During the latter part of our PGfAR study, access to patients’ medicines lists in their summary care record was piloted by NHS Digital and then rolled out across England. This was a step forward in making it possible for community pharmacists to see patients’ prescribed medicines during a MUR. Community pharmacists do not currently have access permissions for information about diagnosis so are limited in their ability to identify patients with cancer.
During the final year of our PGfAR study, a national NHS England programme began to deploy 2000 clinical pharmacists into GP practices by 2020 in response to GP shortages. This new medicines optimisation resource has untested potential to be utilised in cancer pain management and to strengthen links with local community pharmacies. Future research should investigate the role of interventions by general practice pharmacists. This increasing emphasis within UK health policy for pharmacists to be more closely involved in supporting patients meant that our work with community pharmacists was timely.
We were able to identify aspects of pain management that were of greatest value to patients and used these data to develop a health economic model. Our economic modelling based on systematic review data indicated that self-management interventions were likely to be cost-effective in this setting. Our clinical trial showed that enhancing existing community palliative care support with our Tackling Cancer Pain and PainCheck interventions did not result in additional clinical benefit. The economic evaluation demonstrated that the most important driver of cost-effectiveness was level of pain reduction, and our interventions resulted in lower health-care costs. Using a cost-effectiveness threshold of £20,000 per QALY, there was a 67% chance that our interventions were optimal in our base-case analysis.
Limitations
In the RCT, the low fidelity of the interventions and the challenge of the study design that forced the UC arm to have earlier access to palliative care services might explain the lack of observed benefit. We do not regard the low engagement as a failure of the research study, but as a finding: engagement with digital technology in routine palliative care was low.
We noted that the initial trial protocol (which aimed to randomise patients to early palliative care or UC) was not feasible because once our screening procedures had identified cancer patients with significant pain (≥ 4 on a 0–10 rating scale), oncology staff were reluctant to allow patients to be randomised, potentially to UC, and felt obliged to make a clinical referral to palliative care. Instead, we randomised all patients to early palliative care with or without SSM. Greater differences between the arms in relation to pain management might have been apparent had we been able to continue with the original protocol. Our cost-effectiveness literature review did not include null results from the trial within this programme and so we cannot be certain that our interventions were cost-effective. We did not focus our research on patients with learning disabilities or cognitive impairment and so are unable to comment on meeting the palliative care needs of these patients.
The trial demonstrated that patients were experiencing high levels of pain around 1 year before they died. Earlier integration of palliative care (involvement in the trial a median of 53 weeks before death compared with routine care a median of 7 weeks before death) resulted in significant improvements in pain for participants in both trial arms. The recent NHS England initiative ‘Enhanced Supportive Care’ was designed to provide earlier integration of supportive care for patients with cancer (at any stage of disease), and our trial findings demonstrate the need for this. However, the delivery and outcomes of this initiative have not been fully evaluated and supportive care as a recognised service is not widely available. We recognise that there is little consensus regarding the terminology that surrounds supportive and palliative care; palliative care may be regarded as one aspect of supportive care, or it may be regarded as a separate approach. What is clear, however, is that although palliative care services are well established, this is not the case for supportive care services, and so earlier referral to palliative care (as opposed to referral to supportive care) is advocated. Currently, most of these initiatives are being delivered by existing palliative care teams and we have shown that it is possible to provide early palliative care in this context.
Patient and public involvement in the programme
We established a PPI panel for the NIHR IMPACCT that consistently supported the research and championed our work at external meetings and conferences, and has been a model for subsequent projects. PPI members attended quarterly investigator meetings at which they freely commented and advised on our work, and they have closely supported the development of specific WPs based on their individual skills and interests. From individual experiences of our PPI members we have learnt that enabling early access to the right care and a focus on supporting self-management of symptoms are priorities for patients approaching the end of life and their carers.
Our PPI members were actively engaged in the workstreams as well as in the overall programme. Some examples include the following:
-
Provided advice in the development of protocols and patient recruitment strategies.
-
Reviewed patient information sheets and consent forms to determine whether or not wording was likely to be appropriate and adequately sensitive for the patient population.
-
Reviewed ethics forms and documentation for NHS ethics applications.
-
Helped the WP1 research team map a clinical pathway for a specific cancer type based on personal experience.
-
In WP2, provided personal perspectives as a carer to inform the early design of the IMPACCT electronic system, and completed a ‘walkthrough’ of the electronic system to explore how well it related to the reality of a patient’s pain experience from a carer’s perspective.
-
Attended a WP3 multistakeholder meeting at which they provided valuable contributions to inform the design of research methodology and patient materials for the pharmacy-delivered intervention.
-
In WP4, helped to design the DCE vignettes and helped our discussions regarding protocol amendments for the main trial.
-
Informed NIHR fellowship proposals related to IMPACCT, and provided input into research project grant applications aligned with IMPACCT [Marie Curie, Yorkshire Cancer Research (Harrogate, UK) and the NIHR RfPB funding stream].
-
Our academic unit hosted a European research seminar in October 2015 in which the focus was on pain management. Over 70 international delegates attended and our IMPACCT work was presented (judged independently in an open competition). We encouraged and funded Christine Allmark and Diana Robinson to attend the 2-day meeting to see the broader international context. They found the meeting very helpful for supporting dissemination throughout their own PPI networks.
Our PPI panel feedback (included in our annual report to NIHR): ‘it has been really positive for me to observe the widening inclusion of more lay colleagues in the trial management and development processes,’ and ‘since my initial involvement at the grant application stage, I have been impressed by the IMPACCT team’s commitment to getting input and perspectives from patients and carers who are affected by cancer’ (PPI member, personal communication).
Developing and supporting PPI members has been crucial to securing new grant awards for the research team. We have ensured that PPI members have been supported to attend related conferences and workshops relevant to cancer pain management to help the design of future applications. Our PPI panel (of which Jean Gallagher and Diana Robinson were subsequent PPI co-applicants) helped secure the SMARTE (Self-Management and related Treatments at End of life) HTA award, the STEP (Supporting Timely Engagement with Palliative care) RfPB award and most recently the RESOLVE programme grant award from Yorkshire Cancer Research.
Recommendations for future research
Our programme generated a number of research recommendations for future work that relate to some of the priorities identified by the James Lind Alliance (Southampton, UK) for research in palliative and end-of-life care, as well as for living with and beyond cancer:103,104
-
How can access to palliative care services be improved for everyone regardless of where they are in the UK?
-
What are the best ways to make sure that palliative care patients receive adequate pain and symptom relief?
-
What are the benefits and best ways of providing care in the patient’s home and how can home care be maintained as long as possible? Does good co-ordination of services affect this?
-
Are outcomes (e.g. symptom control and incidental prolonging of life) better for terminally ill patients the sooner palliative care is introduced and services are accessed?
-
What are the best ways to manage persistent pain caused by cancer or cancer treatments?
-
There is a need for further research to understand and improve triggers that prompt health professionals to consider earlier integration of palliative care and pain management within oncology services.
-
There is a need to determine the optimal timing of technologies for self-management in the context of pathways for progressive illness. Patients with advanced cancer were able to participate throughout the development of PainCheck and used technology to communicate with friends and family, but there remained uncertainty about its role in palliative care delivery.
-
During the development and subsequent testing of PainCheck there was a lack of research literature guiding how engagement with systems could be conceptualised. There is a need to develop better approaches to measuring and understanding engagement with technologies and how to use this information to inform their development.
-
We cannot directly comment on whether or not self-management approaches that use information and communication technologies lead to improvements in pain management for patients with advanced cancer. PainCheck will require further research and evaluation as a single intervention to better understand its role in supporting pain management for patients with advanced cancer. Gaining a better understanding of the mechanisms of action underpinning PainCheck could help to inform the potential of its role in supporting pain management in advanced cancer.
-
Studies to change prescriber and patient behaviour to achieve earlier pain assessment, initiation and use of strong opioid treatment in community-based patients with cancer are recommended.
-
The novel methodology developed in our study of community palliative care prescribing could be researched further to examine GP and NMP since 2015. Future research could investigate how the data could be used to provide audit/feedback reports at general practice level for CCGs and to measure the effects on prescribing.
-
We have established that patients with pain from advanced cancer have unresolved MRPs amenable to community pharmacist intervention and we identified barriers to referral by other health professionals. A challenge prior to a future pragmatic trial would be to establish whether or not a workable referral pathway in primary care is feasible. Alternative models should be explored including hospice-based pharmacist telephone outreach and general practice pharmacist involvement.
-
Our health economic evaluation demonstrated that the most important driver of cost-effectiveness was level of pain reduction and further research to better understand this relationship in more detail would inform future intervention delivery.
-
A key limitation of the clinical trial was the fidelity of the intervention delivered by community palliative care nursing teams. Our development research in WPs 2–3 suggested that health professionals recognised the value of the interventions but had reservations regarding their implementation in routine care. Further research, particularly regarding attitudes towards digital technology within palliative care, is needed.
-
Our trial screening experience and our process evaluation revealed that there remains significant reluctance to discuss palliative or supportive care approaches with cancer patients. Research that explores ways to overcome these barriers is needed.
-
Implications for practice and lessons learnt
Research generated within the IMPACCT programme has several implications for practice:
-
Outcomes for patients in relation to cancer pain management may need to reflect the trade-offs that patients make between acceptable pain relief and acceptable cognitive side effects from analgesia, rather than simple pain intensity scales.
-
Access to palliative care occurs relatively late for many patients with cancer (and even later for those with non-cancer diseases). Earlier access to palliative care for patients with pain who need help is associated with better outcomes and is consistent with recently published guidance. 105 Our clinical trial demonstrated that screening for pain and integrating palliative care support a median of 53 weeks before death (compared with only 7 weeks in clinical practice) led to important improvements in patient outcomes.
-
Digital technology will be an increasingly important aspect of palliative care provision if projected increased demand is to be met with limited resources.
-
Our finding that access to strong opioid analgesia occurs relatively late before death suggests the importance of screening for pain within primary and secondary care.
-
Our clinical trial failed to show an added benefit of our interventions to enhance existing community palliative care support, although both the decision model and the economic evaluation of the trial indicated that SSM could be cost-effective. Based on these, the correct implications for practice remain as directed within the Care Quality Commission inspection framework for end-of-life care: patients should be offered verbal or written support, particularly regarding their medicines and patients should have their pain assessed when seen by a health professional and have easy access to pain monitoring.
Perhaps the key lesson learnt was that it is possible to recruit patients with advanced cancer and palliative care needs to a randomised clinical trial, and achieve an overall follow-up rate of 70% at either 6 weeks or 12 weeks. To our knowledge, this is one of the largest and longest RCTs in palliative care in the UK and demonstrates that it is feasible to engage this population in large-scale research.
Acknowledgements
Important information
We gratefully acknowledge the NIHR PGfAR programme funding that enabled us to undertake this programme of research. We thank all patients, caregivers, health professionals and clinical teams that contributed to the development and implementation of the programme of research. We also acknowledge the very valuable comments and guidance of our Project Steering Group and, in particular, all of our PPI representatives.
Contributions of authors
Michael I Bennett (https://orcid.org/0000-0002-8369-8349) (St. Gemma’s Professor of Palliative Medicine) was principal investigator for the programme.
S José Closs (https://orcid.org/0000-0002-3257-5277) (Professor of Nursing) led WP1 and worked with Kate Flemming (https://orcid.org/0000-0002-0795-8516) (Professor of Hospice Practice and Evidence Synthesis), Robbie Foy (https://orcid.org/0000-0003-0605-7713) (Professor of Primary Care), Mary Godfrey (https://orcid.org/0000-0002-2408-534X) (Reader in Health and Social Care), Julia Hackett (https://orcid.org/0000-0003-1720-6665) (Research Fellow), Nicholas Hughes (https://orcid.org/0000-0003-0129-922X) (Senior Research Fellow) and Geoff Hall (https://orcid.org/0000-0002-8864-5932) (Professor of Digital Health) to understand patient pathways and develop the educational intervention.
Bridgette M Bewick (https://orcid.org/0000-0001-5752-5623) (Associate Professor in Psychological Health and Wellbeing) led WP2 and worked with Matthew J Allsop (https://orcid.org/0000-0002-7399-0194) (University Academic Fellow), Richard Jones (Professor of Chemical Pathology and Health Informatics) and Sally Taylor (https://orcid.org/0000-0001-9026-1789) (Research Fellow in Applied Health) to develop PainCheck.
Alison Blenkinsopp (https://orcid.org/0000-0001-8872-2769) (Professor of the Practice of Pharmacy) led WP3 and worked with Zoe Edwards (https://orcid.org/0000-0001-6525-8190) (Research Fellow), Matthew R Mulvey (https://orcid.org/0000-0002-6357-3848) (University Academic Fellow) and Lucy Ziegler (https://orcid.org/0000-0001-9563-5014) (Associate Professor in Palliative Care) to understand prescribing practices.
Julia Brown (https://orcid.org/0000-0002-2719-7064) (Professor of Clinical Trials Research) led WP4 and worked with Kath Black (NIHR Research Nurse), Marie Fletcher (https://orcid.org/0000-0001-7545-1314) (Senior Trial Co-ordinator), Suzanne Hartley (https://orcid.org/0000-0003-2346-9461) (Head of Trial Management – Complex Interventions), Angela Wray (https://orcid.org/0000-0002-7075-9253) (Research Nurse), Alexandra Wright-Hughes (https://orcid.org/0000-0001-8839-6756) (Senior Medical Statistician) to deliver the randomised trial.
The health economic analyses were conducted by Daniel Howdon (https://orcid.org/0000-0001-8052-2893) (Research Fellow), Claire Hulme (https://orcid.org/0000-0003-2077-0419) (Chairperson of Health Economics), David Meads (https://orcid.org/0000-0003-1369-2483) (Associate Professor in Health Economics) and John O’Dwyer (https://orcid.org/0000-0002-7212-6089) (NIHR Research Fellow).
Sue H Pavitt (https://orcid.org/0000-0001-7447-440X) (Professor of Translational and Applied Health Research) supported PPI engagement and worked with Peter Allen, Christine Allmark, Peter Rainey and Diana Robinson (Lay Members).
All authors participated in programme management, WP management or dissemination meetings. All authors provided a critical review and final approval of the report.
Publications
Ziegler L, Bennett MI, Blenkinsopp A, Coppock S. Non-medical prescribing in palliative care: a regional survey to explore analgesic practice. Palliat Med 2015;29:177–81.
Boland JW, Ziegler L, Boland EG, McDermid K, Bennett MI. Is regular systemic opioid analgesia associated with shorter survival in adult patients with cancer? A systematic literature review. Pain 2015;156:2152–63. https://doi.org/10.1097/j.pain.0000000000000306
Allsop MJ, Taylor S, Mulvey MR, Bennett MI, Bewick BM. Information and communication technology for managing pain in palliative care: a review of the literature. BMJ Support Palliat Care 2015;5:481–9. https://doi.org/10.1136/bmjspcare-2013-000625
Taylor S, Allsop MJ, Shaw J, Bennett MI, Jones R, Bewick BM. The feasibility of collecting patient reported pain data using a system delivered across four modes of technology. Pain Med 2015;16:2212–13.
Ziegler L, Mulvey M, Blenkinsopp A, Petty D, Bennett MI. Opioid prescribing for patients with cancer in the last year of life: a longitudinal population cohort study. Pain 2016;157:2445–51. https://doi.org/10.1097/j.pain.0000000000000656
Flemming K, Closs SJ, Hughes ND, Bennett MI. Using qualitative research to overcome the shortcomings of systematic reviews when designing of a self-management intervention for advanced cancer pain. Int J Qual Meth 2016;15:1–11.
Hackett J, Godfrey M, Bennett MI. Patient and caregiver perspectives on managing pain in advanced cancer: A qualitative longitudinal study. Palliat Med 2016;30:711–19. https://doi.org/10.1177/0269216316628407
Hughes ND, Closs SJ, Flemming K, Bennett MI. Supporting self-management of pain by patients with advanced cancer: views of palliative care professionals. Support Care Cancer 2016;24:5049–57. https://doi.org/10.1007/s00520-016-3372-2
Bennett MI, Ziegler L, Allsop M, Daniel S, Hurlow A. What determines duration of palliative care before death for patients with advanced disease? A retrospective cohort study of community and hospital palliative care provision in a large UK city. BMJ Open 2016;6:e012576. https://doi.org/10.1136/bmjopen-2016-012576
Taylor S, Allsop MJ, Bekker HL, Bennett MI, Bewick BM. Identifying professionals’ needs in integrating electronic pain monitoring in community palliative care services: an interview study. Palliat Med 2017;31:661–70. https://doi.org/10.1177/0269216316677470
Meads DM, O’Dwyer JL, Hulme CT, Chintakayala P, Vinall-Collier K, Bennett MI. Patient preferences for pain management in advanced cancer: results from a discrete choice experiment. Patient 2017;10:643–51. https://doi.org/10.1007/s40271-017-0236-x
Hackett J, Ziegler L, Godfrey M, Foy R, Bennett MI. Primary palliative care team perspectives on coordinating and managing people with advanced cancer in the community: a qualitative study. BMC Fam Pract 2018;19:177. https://doi.org/10.1186/s12875-018-0861-z
Allsop MJ, Ziegler LE, Mulvey MR, Russell S, Taylor R, Bennett MI. Duration and determinants of hospice-based specialist palliative care for patients in the UK. A national retrospective cohort study. Palliat Med 2018;32:1322–33.
Edwards Z, Blenkinsopp A, Ziegler L, Bennett MI. How do patients with cancer pain view community pharmacy services? An interview study. Health Soc Care Community 2018;26:507–18. https://doi.org/10.1111/hsc.12549
Ziegler L, Bennett MI, Mulvey M, Hamilton T, Blenkinsopp A. Characterising the growth in palliative care prescribing 2011-2015: analysis of national medical and non-medical activity. Palliat Med 2018;32:767–74. https://doi.org/10.1177/0269216317739805
Allsop MJ, Wright-Hughes A, Black K, Hartley S, Fletcher M, Ziegler LE, et al. Improving the management of pain from advanced cancer in the community: study protocol for a pragmatic multi-centre randomised controlled trial. BMJ Open 2018;8:e021965.
Taylor SS, Wyld L, Ziegler L, Bennett MI. Is patient information on palliative care good enough? A literature review and audit. Cancer Nursing Practice 2019;18:44–49.
Edwards Z, Bennett MI, Blenkinsopp A. A community pharmacist medicines optimisation service for patients with advanced cancer pain: a proof of concept study. Int J Clin Pharm 2019;41:700–10. https://doi.org/10.1007/s11096-019-00820-8
Allsop MJ, Johnson O, Taylor S, Hackett J, Allen P, Bennett MI, Bewick BM. Multidisciplinary software design for the routine monitoring and assessment of pain in palliative care services: the development of PainCheck. JCO Clin Cancer Inform 2019;3:1–17. https://doi.org/10.1200/CCI.18.00120
Meads DM, O’Dwyer JL, Hulme CT, Lopez RR, Bennett MI. Cost-effectiveness of pain management strategies in advanced cancer. Int J Technol Assess Health Care 2019;35:141–9.
Edwards Z, Bennett MI, Petty D, Blenkinsopp A. Evaluating recruitment methods of patients with advanced cancer: a pragmatic opportunistic comparison. Int J Pharm Pract 2019;27:536–44. https://doi.org/10.1111/ijpp.12562
Edwards Z, Ziegler L, Craigs C, Blenkinsopp A, Bennett MI. Pharmacist educational interventions for cancer pain management: a systematic review and meta-analysis. Int J Pharm Pract 2019;27:336–45. https://doi.org/10.1111/ijpp.12516
Allsop MJ, Taylor S, Bennett MI, Bewick BM. Understanding patient requirements for technology systems that support pain management in palliative care services: a qualitative study. Health Inform J 2019;25:1105–15. https://doi.org/10.1177/1460458217740724
Taylor S, Allsop MJ, Bennett MI, Bewick BM. Usability testing of an electronic pain monitoring system for palliative cancer patients: a think-aloud study. Health Inform J 2019;25:1133–47. https://doi.org/10.1177/1460458217741754
Hackett, Allsop MJ, Taylor S, Bennett MI, Bewick BM. Using information and communication technologies to improve the management of pain from advanced cancer in the community: qualitative study of the experience of implementation for patients and health professionals in a trial. Health Inform J 2020;26:2435–45.
Data-sharing statement
We shall make data available to the scientific community with as few restrictions as feasible, while retaining exclusive use until the publication of major outputs. Requests for access to data can be made through contacting the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health and Social Care.
References
- Breivik H, Cherny N, Collett B, de Conno F, Filbet M, Foubert AJ, et al. Cancer-related pain: a pan-European survey of prevalence, treatment, and patient attitudes. Ann Oncol 2009;20:1420-33. https://doi.org/10.1093/annonc/mdp001.
- British Medical Association (BMA) . End-of-Life Care and Physician-Assisted Dying 2015.
- van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol 2007;18:1437-49. https://doi.org/10.1093/annonc/mdm056.
- van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, Tjan-Heijnen VC, Janssen DJ. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manage 2016;51:1070-90.e9. https://doi.org/10.1016/j.jpainsymman.2015.12.340.
- Greco MT, Roberto A, Corli O, Deandrea S, Bandieri E, Cavuto S, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol 2014;32:4149-54. https://doi.org/10.1200/jco.2014.56.0383.
- Deandrea S, Montanari M, Moja L. Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol 2008;19:1985-91. https://doi.org/10.1093/annonc/mdn419.
- Gibbins J, Bhatia R, Forbes K, Reid CM. What do patients with advanced incurable cancer want from the management of their pain? A qualitative study. Palliat Med 2014;28:71-8. https://doi.org/10.1177/0269216313486310.
- Hackett J, Godfrey M, Bennett MI. Patient and caregiver perspectives on managing pain in advanced cancer: a qualitative longitudinal study. Palliat Med 2016;30:711-19. https://doi.org/10.1177/0269216316628407.
- Gaertner J, Siemens W, Meerpohl JJ, Antes G, Meffert C, Xander C, et al. Effect of specialist palliative care services on quality of life in adults with advanced incurable illness in hospital, hospice, or community settings: systematic review and meta-analysis. BMJ 2017;357. https://doi.org/10.1136/bmj.j2925.
- Davis MP, Temel JS, Balboni T, Glare P. A review of the trials which examine early integration of outpatient and home palliative care for patients with serious illnesses. Ann Palliat Med 2015;4:99-121. https://doi.org/10.3978/j.issn.2224-5820.2015.04.04.
- Bauman JR, Temel JS. The integration of early palliative care with oncology care: the time has come for a new tradition. J Natl Compr Canc Netw 2014;12:1763-71. https://doi.org/10.6004/jnccn.2014.0177.
- Ferrell BR, Temel JS, Temin S, Alesi ER, Balboni TA, Basch EM, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology Clinical Practice guideline update. J Clin Oncol 2017;35:96-112. https://doi.org/10.1200/JCO.2016.70.1474.
- Jordan K, Aapro M, Kaasa S, Ripamonti CI, Scotté F, Strasser F, et al. European Society for Medical Oncology (ESMO) position paper on supportive and palliative care. Ann Oncol 2018;29:36-43. https://doi.org/10.1093/annonc/mdx757.
- Siouta N, van Beek K, Preston N, Hasselaar J, Hughes S, Payne S, et al. Towards integration of palliative care in patients with chronic heart failure and chronic obstructive pulmonary disease: a systematic literature review of European guidelines and pathways. BMC Palliat Care 2016;15. https://doi.org/10.1186/s12904-016-0089-4.
- Aldridge MD, Hasselaar J, Garralda E, van der Eerden M, Stevenson D, McKendrick K, et al. Education, implementation, and policy barriers to greater integration of palliative care: a literature review. Palliat Med 2016;30:224-39. https://doi.org/10.1177/0269216315606645.
- Ziegler L, Craigs C, West R, Carder P, Hurlow A, Millares-Martin P, et al. Is palliative care support associated with better quality end-of-life care indicators for patients with advanced cancer? A retrospective cohort study. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2017-018284.
- Tanuseputro P, Budhwani S, Bai YQ, Wodchis WP. Palliative care delivery across health sectors: a population-level observational study. Palliat Med 2017;31:247-57. https://doi.org/10.1177/0269216316653524.
- Baek YJ, Shin DW, Choi JY, Kang J, Mo HN, Kim YH, et al. Late referral to palliative care services in Korea. J Pain Symptom Manage 2011;41:692-9. https://doi.org/10.1016/j.jpainsymman.2010.06.019.
- Cheung WY, Schaefer K, May CW, Glynn RJ, Curtis LH, Stevenson LW, et al. Enrollment and events of hospice patients with heart failure vs. cancer. J Pain Symptom Manage 2013;45:552-60. https://doi.org/10.1016/j.jpainsymman.2012.03.006.
- Bennett MI, Ziegler L, Allsop M, Daniel S, Hurlow A. What determines duration of palliative care before death for patients with advanced disease? A retrospective cohort study of community and hospital palliative care provision in a large UK city. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-012576.
- McWhinney IR, Bass MJ, Orr V. Factors associated with location of death (home or hospital) of patients referred to a palliative care team. CMAJ 1995;152:361-7.
- Allsop MJ, Ziegler LE, Mulvey MR, Russell S, Taylor R, Bennett MI. Duration and determinants of hospice-based specialist palliative care: a national retrospective cohort study. Palliat Med 2018;32:1322-33. https://doi.org/10.1177/0269216318781417.
- Etkind SN, Bone AE, Gomes B, Lovell N, Evans CJ, Higginson IJ, et al. How many people will need palliative care in 2040? Past trends, future projections and implications for services. BMC Med 2017;15. https://doi.org/10.1186/s12916-017-0860-2.
- Gott M, Seymour J, Ingleton C, Gardiner C, Bellamy G. ‘That’s part of everybody’s job’: the perspectives of health care staff in England and New Zealand on the meaning and remit of palliative care. Palliat Med 2012;26:232-41. https://doi.org/10.1177/0269216311408993.
- Hackett J, Ziegler L, Godfrey M, Foy R, Bennett MI. Primary palliative care team perspectives on coordinating and managing people with advanced cancer in the community: a qualitative study. BMC Fam Pract 2018;19. https://doi.org/10.1186/s12875-018-0861-z.
- Taylor S, Wyld L, Ziegler L, Bennet MI. Is patient information on palliative care good enough? A literature review and audit. Cancer Nursing Practice n.d. https://doi.org/10.7748/cnp.2018.e1506.
- Rabow MW, Dibble SL, Pantilat SZ, McPhee SJ. The comprehensive care team: a controlled trial of outpatient palliative medicine consultation. Arch Intern Med 2004;164:83-91. https://doi.org/10.1001/archinte.164.1.83.
- Brumley R, Enguidanos S, Jamison P, Seitz R, Morgenstern N, Saito S, et al. Increased satisfaction with care and lower costs: results of a randomized trial of in-home palliative care. J Am Geriatr Soc 2007;55:993-1000. https://doi.org/10.1111/j.1532-5415.2007.01234.x.
- Bakitas M, Lyons KD, Hegel MT, Balan S, Barnett KN, Brokaw FC, et al. The project ENABLE II randomized controlled trial to improve palliative care for rural patients with advanced cancer: baseline findings, methodological challenges, and solutions. Palliat Support Care 2009;7:75-86. https://doi.org/10.1017/S1478951509000108.
- Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;363:733-42. https://doi.org/10.1056/NEJMoa1000678.
- Zimmermann C, Swami N, Krzyzanowska M, Hannon B, Leighl N, Oza A, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet 2014;383:1721-30. https://doi.org/10.1016/S0140-6736(13)62416-2.
- Stajduhar KI, Thorne SE, McGuinness L, Kim-Sing C. Patient perceptions of helpful communication in the context of advanced cancer. J Clin Nurs 2010;19:2039-47. https://doi.org/10.1111/j.1365-2702.2009.03158.x.
- Harrison JD, Young JM, Price MA, Butow PN, Solomon MJ. What are the unmet supportive care needs of people with cancer? A systematic review. Support Care Cancer 2009;17:1117-28. https://doi.org/10.1007/s00520-009-0615-5.
- Tomlinson K, Barker S, Soden K. What are cancer patients’ experiences and preferences for the provision of written information in the palliative care setting? A focus group study. Palliat Med 2012;26:760-5. https://doi.org/10.1177/0269216311419988.
- Von Roenn JH, Voltz R, Serrie A. Barriers and approaches to the successful integration of palliative care and oncology practice. J Natl Compr Canc Netw 2013;11:11-6. https://doi.org/10.6004/jnccn.2013.0209.
- Bennett MI, Graham J, Schmidt-Hansen M, Prettyjohns M, Arnold S. Guideline Development Group . Prescribing strong opioids for pain in adult palliative care: summary of NICE guidance. BMJ 2012;344. https://doi.org/10.1136/bmj.e2806.
- Flemming K, Closs SJ, Hughes ND, Bennett MI. Using qualitative research to overcome the shortcomings of systematic reviews when designing of a self-management intervention for advanced cancer pain. Int J Qual Meth 2016;15:1-11. https://doi.org/10.1177/1609406916670656.
- Hughes ND, Closs SJ, Flemming K, Bennett MI. Supporting self-management of pain by patients with advanced cancer: views of palliative care professionals. Support Care Cancer 2016;24:5049-57. https://doi.org/10.1007/s00520-016-3372-2.
- Capewell C, Gregory W, Closs S, Bennett M. Brief DVD-based educational intervention for patients with cancer pain: feasibility study. Palliat Med 2010;24:616-22. https://doi.org/10.1177/0269216310371704.
- Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore 1994;23:129-38.
- City of Hope Pain & Palliative Care Resource Center . The Patient Pain Questionnaire (PPQ) 2012 n.d. http://prc.coh.org/ (accessed 10 January 2018).
- Liddell A, Adshead S, Burgess E. Technology in the NHS: Transforming the Patient’s Experience of Care. London: The King’s Fund; 2008.
- Department of Health and Social Care . Equity and Excellence: Liberating the NHS 2010. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_117353 (accessed 10 January 2018).
- Gagnon MP, Desmartis M, Labrecque M, Car J, Pagliari C, Pluye P, et al. Systematic review of factors influencing the adoption of information and communication technologies by healthcare professionals. J Med Syst 2012;36:241-77. https://doi.org/10.1007/s10916-010-9473-4.
- Johansen MA, Berntsen G, Shrestha N, Bellika JG, Johnsen JA. An exploratory study of patient attitudes towards symptom reporting in a primary care setting. Benefits for medical consultation and syndromic surveillance?. Methods Inf Med 2011;50:479-86. https://doi.org/10.3414/ME11-02-0005.
- Hjermstad MJ, Lie HC, Caraceni A, Currow DC, Fainsinger RL, Gundersen OE, et al. Computer-based symptom assessment is feasible in patients with advanced cancer: results from an international multicenter study, the EPCRC-CSA. J Pain Symptom Manage 2012;44:639-54. https://doi.org/10.1016/j.jpainsymman.2011.10.025.
- Kirkova J, Rybicki L, Walsh D, Aktas A. Symptom prevalence in advanced cancer: age, gender, and performance status interactions. Am J Hosp Palliat Care 2012;29:139-45. https://doi.org/10.1177/1049909111410965.
- Cleeland CS. Symptom burden: multiple symptoms and their impact as patient-reported outcomes. J Natl Cancer Inst Monogr 2007;37:16-21. https://doi.org/10.1093/jncimonographs/lgm005.
- Oldenmenger WH, Sillevis Smitt PA, van Dooren S, Stoter G, van der Rijt CC. A systematic review on barriers hindering adequate cancer pain management and interventions to reduce them: a critical appraisal. Eur J Cancer 2009;45:1370-80. https://doi.org/10.1016/j.ejca.2009.01.007.
- Dempster PG, Bewick BM, Jones R, Bennett MI. Management of cancer pain in the community: perceptions of current UK information technology systems and implications for future development. Health Informatics J 2012;18:284-93. https://doi.org/10.1177/1460458212445341.
- Kreps G, Moore RJ. Handbook of Pain and Palliative Care: Biobehavioural Approaches for the Life Course. New York, NY: Springer; 2012.
- Allsop MJ, Taylor S, Mulvey MR, Bennett MI, Bewick BM. Information and communication technology for managing pain in palliative care: a review of the literature. BMJ Support Palliat Care 2015;5:481-9. https://doi.org/10.1136/bmjspcare-2013-000625.
- Taylor S, Allsop MJ, Shaw J, Bennett MI, Jones R, Bewick BM. The feasibility of collecting patient reported pain data using a system delivered across four modes of technology. Pain Med 2015;16:2212-13. https://doi.org/10.1111/pme.12811.
- Taylor S, Allsop MJ, Bennett MI, Bewick BM. Usability testing of an electronic pain monitoring system for palliative cancer patients: a think aloud study. Health Informatics J 2019;25:1133-47. https://doi.org/10.1177/1460458217741754.
- Allsop MJ, Taylor S, Bennett MI, Bewick BM. Understanding patient requirements for technology systems that support pain management in palliative care services: a qualitative study. Health Informatics J 2019;25:1105-15. https://doi.org/10.1177/1460458217740724.
- Taylor S, Allsop MJ, Bekker HL, Bennett MI, Bewick BM. Identifying professionals’ needs in integrating electronic pain monitoring in community palliative care services: an interview study. Palliat Med 2017;31:661-70. https://doi.org/10.1177/0269216316677470.
- Allsop MJ, Johnson O, Taylor S, Hackett J, Allen P, Bennett MI, et al. Multidisciplinary software design for the routine monitoring and assessment of pain in palliative care services: the development of PainCheck. JCO Clin Cancer Inform 2019;3:1-17. https://doi.org/10.1200/CCI.18.00120.
- Ziegler L, Mulvey M, Blenkinsopp A, Petty D, Bennett MI. Opioid prescribing for patients with cancer in the last year of life: a longitudinal population cohort study. Pain 2016;157:2445-51. https://doi.org/10.1097/j.pain.0000000000000656.
- Boland JW, Ziegler L, Boland EG, McDermid K, Bennett MI. Is regular systemic opioid analgesia associated with shorter survival in adult patients with cancer? A systematic literature review. Pain 2015;156:2152-63. https://doi.org/10.1097/j.pain.0000000000000306.
- Halabi S, Vogelzang NJ, Kornblith AB, San-San O, Kantoff PW, Dawson NA, et al. Pain predicts overall survival in men with metastatic castration-refractory prostate cancer. J Clin Oncol 2008;26:2544-9. https://doi.org/10.1200/jco.2007.15.0367.
- Ziegler L, Bennett M, Blenkinsopp A, Coppock S. Non-medical prescribing in palliative care: a regional survey. Palliat Med 2015;29:177-81. https://doi.org/10.1177/0269216314557346.
- Ziegler L, Bennett MI, Mulvey M, Hamilton T, Blenkinsopp A. Characterising the growth in palliative care prescribing 2011–2015: analysis of national medical and non-medical activity. Palliat Med 2017;32:767-74. https://doi.org/10.1177/0269216317739805.
- Edwards Z, Blenkinsopp A, Bennett M. A Survey of Healthcare Professionals’ Views on Community Pharmacy Services for Patients With Cancer Pain n.d.
- Edwards Z, Bennett MI, Petty DR, Blenkinsopp A. Evaluating recruitment methods of patients with advanced cancer: a pragmatic opportunistic comparison. Int J Pharm Pract 2019;27:536-44. https://doi.org/10.1111/ijpp.12562.
- Edwards Z, Blenkinsopp A, Ziegler L, Bennett MI. How do patients with cancer pain view community pharmacy services? An interview study. Health Soc Care Community 2018;26:507-18. https://doi.org/10.1111/hsc.12549.
- Edwards Z, Ziegler L, Craigs C, Blenkinsopp A, Bennett MI. Pharmacist educational interventions for cancer pain management: a systematic review and meta-analysis. Int J Pharm Pract 2019;27:336-45. https://doi.org/10.1111/ijpp.12516.
- Edwards Z, Bennett MI, Blenkinsopp A. A community pharmacist medicines optimisation service for patients with advanced cancer pain: a proof of concept study. Int J Clin Pharm 2019;41:700-10. https://doi.org/10.1007/s11096-019-00820-8.
- Savage I, Blenkinsopp A, Closs SJ, Bennett MI. Like doing a jigsaw with half the parts missing: community pharmacists and the management of cancer pain in the community. Int J Pharm Pract 2013;21:151-60. https://doi.org/10.1111/j.2042-7174.2012.00245.x.
- Meads DM, O’Dwyer JL, Hulme CT, Chintakayala P, Vinall-Collier K, Bennett MI. Patient preferences for pain management in advanced cancer: results from a discrete choice experiment. Patient 2017;10:643-51. https://doi.org/10.1007/s40271-017-0236-x.
- Allsop MJ, Wright-Hughes A, Black K, Hartley S, Fletcher M, Ziegler LE, et al. Improving the management of pain from advanced cancer in the community: study protocol for a pragmatic multicentre randomised controlled trial. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2018-021965.
- Meads DM, O’Dwyer JL, Hulme CT, Lopez RR, Bennett MI. Cost-effectiveness of pain management strategies in advanced cancer. Int J Technol Assess Health Care 2019;35:1-9. https://doi.org/10.1017/S0266462319000114.
- Lovell MR, Luckett T, Boyle FM, Phillips J, Agar M, Davidson PM. Patient education, coaching, and self-management for cancer pain. J Clin Oncol 2014;32:1712-20. https://doi.org/10.1200/JCO.2013.52.4850.
- Bennett MI, Bagnall AM, Closs JS. How effective are patient-based educational interventions in the management of cancer pain? Systematic review and meta-analysis. Pain 2009;143:192-9. https://doi.org/10.1016/j.pain.2009.01.016.
- Cummings GG, Olivo SA, Biondo PD, Stiles CR, Yurtseven O, Fainsinger RL, et al. Effectiveness of knowledge translation interventions to improve cancer pain management. J Pain Symptom Manage 2011;41:915-39. https://doi.org/10.1016/j.jpainsymman.2010.07.017.
- Gorin SS, Krebs P, Badr H, . Meta-analysis of psychosocial interventions to reduce pain in patients with cancer. J Clin Oncol 2012;10:539-47. https://doi.org/10.1200/JCO.2011.37.0437.
- Koller A, Miaskowski C, De Geest S, Opitz O, Spichiger E. A systematic evaluation of content, structure, and efficacy of interventions to improve patients’ self-management of cancer pain. J Pain Symptom Manage 2012;44:264-84. https://doi.org/10.1016/j.jpainsymman.2011.08.015.
- Trowbridge R, Dugan W, Jay SJ, Littrell D, Casebeer LL, Edgerton S, et al. Determining the effectiveness of a clinical-practice intervention in improving the control of pain in outpatients with cancer. Acad Med 1997;72:798-800. https://doi.org/10.1097/00001888-199709000-00016.
- Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. Pain 1983;17:33-44. https://doi.org/10.1016/0304-3959(83)90125-2.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Mahwah, NJ: Lawrence Erlbaum Associates Inc.; 1988.
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008;9:105-21. https://doi.org/10.1016/j.jpain.2007.09.005.
- Kroenke K, Theobald D, Wu J, Norton K, Morrison G, Carpenter J, et al. Effect of telecare management on pain and depression in patients with cancer: a randomized trial. JAMA 2010;304:163-71. https://doi.org/10.1001/jama.2010.944.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. Toronto, ON: John Wiley & Sons, Inc.; 1987.
- Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2018;27:7-22. https://doi.org/10.1002/hec.3564.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Rowen D, Brazier J, Young T, Gaugris S, Craig BM, King MT, et al. Deriving a preference-based measure for cancer using the EORTC QLQ-C30. Value Health 2011;14:721-31. https://doi.org/10.1016/j.jval.2011.01.004.
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: Personal Social Services Research Unit, University of Kent; 2017.
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
- National Audit Office . Out-of-Hours GP Services in England 2014. www.nao.org.uk/report/hours-gp-services-england-2/2019 (accessed September 2019).
- Public Health England . End of Life Care Economic Tool 2017. www.gov.uk/government/publications/end-of-life-care-economic-tool (accessed September 2019).
- Department of Health and Social Care . HCHS Pay &Amp; Price Inflation 2016. www.info.doh.gov.uk/doh/finman.nsf/af3d43e36a4c8f8500256722005b77f8/360a47827991d10a80258036002d8d9f/$FILE/2015.16%20Pay%20%26%20Price%20series.xlsx2019 (accessed September 2019).
- Monitor, NHS Trust Development Authority . Economic Assumptions 2016 17 to 2020 21 2016. www.gov.uk/government/publications/economic-assumptions-201617-to-202021 (accessed September 2019).
- NHS Prescription Services . NHS Electronic Drug Tariff 2018. www.drugtariff.nhsbsa.nhs.uk/#/00616707-DB/DB00616702/Home2019 (accessed September 2019).
- DiazOrdaz K, Franchini AJ, Grieve R. Methods for estimating complier average causal effects for cost-effectiveness analysis. J R Stat Soc Ser A Stat Soc 2018;181:277-97. https://doi.org/10.1111/rssa.12294.
- Howdon D, Rice N. Health care expenditures, age, proximity to death and morbidity: implications for an ageing population. J Health Econ 2018;57:60-74. https://doi.org/10.1016/j.jhealeco.2017.11.001.
- Werblow A, Felder S, Zweifel P. Population ageing and health care expenditure: a school of ‘red herrings’?. Health Econ 2007;16:1109-26. https://doi.org/10.1002/hec.1213.
- Zweifel P, Felder S, Meiers M. Ageing of population and health care expenditure: a red herring?. Health Econ 1999;8:485-96. https://doi.org/10.1002/(SICI)1099-1050(199909)8:6<485::AID-HEC461>3.0.CO;2-4.
- Zweifel P, Felder S, Werblow A. Population ageing and health care expenditure: new evidence on the ‘red herring.’. Geneva Pap Risk Insur – Issues Pract 2004;29:652-66. https://doi.org/10.1111/j.1468-0440.2004.00308.x.
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making 1998;18:68-80. https://doi.org/10.1177/0272989X98018002S09.
- The Economist Intelligence Unit . The 2015 Quality of Death Index: Ranking Palliative Care Across the World 2015.
- ElMokhallalati Y, Woodhouse N, Farragher T, Bennet MI. Specialist palliative care support is associated with improved pain relief at home during the last 3 months of life in patients with advanced disease: analysis of 5-year data from the national survey of bereaved people (VOICES). BMC Med 2019;17. https://doi.org/10.1186/s12916-019-1287-8.
- James Lind Alliance . Palliative and End of Life Care Priority Setting Partnership 2015. www.jla.nihr.ac.uk/priority-setting-partnerships/palliative-and-end-of-life-care/ (accessed September 2019).
- James Lind Alliance . Living With and Beyond Cancer 2018. www.jla.nihr.ac.uk/priority-setting-partnerships/living-with-and-beyond-cancer/top-10-priorities.htm (accessed September 2019).
- Faculty of Pain Medicine . Framework for Provision of Pain Services for Adults Across the UK With Cancer or Life-Limiting Disease 2019. https://beta.fpm.ac.uk/sites/fpm/files/documents/2019-07/Framework%20for%20pain%20services%20cancer%20and%20life%20limiting%20disease%202019.pdf (accessed September 2019).
List of abbreviations
- A&E
- accident and emergency
- BMA
- British Medical Association
- BPI
- Brief Pain Inventory
- CACE
- complier average causal effect
- CCG
- clinical commissioning group
- CI
- confidence interval
- CNS
- clinical nurse specialist
- CONSORT
- Consolidated Standard of Reporting Trials
- DCE
- discrete choice experiment
- DVD
- digital versatile disc
- ECOG
- Eastern Cooperative Oncology Group
- EORTC-8D
- European Organisation for Research and Treatment of Cancer – 8 Domains
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- HTA
- Health Technology Assessment
- ICECAP-A
- ICEpop CAPability measure for Adults
- ICER
- incremental cost-effectiveness ratio
- ICT
- information and communication technology
- MRP
- medicines-related problem
- MUR
- Medicines Use Review
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMB
- net monetary benefit
- NMP
- non-medical prescribing
- PDG
- programme development grant
- PGfAR
- Programme Grants for Applied Research
- PPQ
- Patient Pain Questionnaire
- QALY
- quality-adjusted life-year
- QLQc30
- Quality of Life Questionnaire c30
- QoL
- quality of life
- RCT
- randomised controlled trial
- RfPB
- Research for Patient Benefit
- SD
- standard deviation
- SSM
- supported self-management
- SUR
- seemingly unrelated regression
- TIDieR
- Template for Intervention Description and Replication
- UC
- usual care
- WP
- work package
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/pgfar09150).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
