Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0606-1128. The contractual start date was in September 2007. The final report began editorial review February 2013 and was accepted for publication in November 2013. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Bradford Teaching Hospitals NHS Foundation Trust received additional funding for the submitted work from The Stroke Association. AF received funding from the NIHR Stroke Research Network and The Stroke Association for activities outside of the submitted work. Funding was provided by a Stroke Association Junior Research Fellowship for NA to undertake a PhD - a realist evaluation of the LoTS care trial. JRTF 2009/03.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Forster et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
There are over 900,000 people in England who have had a stroke, of whom 300,000 live with moderate to severe disability. 1 As such, stroke generates considerable health and social care costs. Costs are estimated at £7B a year, which includes £2.8B in direct costs to the NHS, £2.4B in informal care costs and £1.8B in income lost to productivity and disability. 1 Unplanned general practitioner (GP) visits and hospital readmissions generate an economic burden to the NHS and cause stress and discomfort to the patient. Previously viewed in a nihilistic way, stroke care is now characterised by a more dynamic and positive approach supported by the development of stroke medicine as a clinical specialty, rigorous reviews of services and production of evidence-based guidelines. 2 Although successful preventative measures (e.g. carotid endarterectomy) and drug interventions (e.g. thrombolysis) have been identified, they are appropriate for only a minority of stroke patients, and rehabilitation remains the cornerstone of treatment for many.
The recommended stroke care pathway in the first weeks after stroke is becoming established. Well-described service components include neurovascular clinics, stroke units and early supported discharge schemes. Despite these service developments, approximately one-third of stroke survivors are left with some physical impairment,3,4 one-third of stroke survivors are depressed,5 inactivity is common, participation levels are low6 and health-related quality of life deteriorates post stroke. 7
Community-based observational studies over three decades have left little doubt about the daily struggle for stroke survivors and their families as they come to terms with the longer-term consequences of a stroke illness. 8–10 Many stroke survivors require assistance from informal carers, often family members, for activities of daily living, including bathing, dressing and toileting. 11,12 This burden of care has an important effect on carers’ physical and psychosocial well-being,13,14 with up to 48% of carers reporting health problems, two-thirds reporting a decline in their social life and high self-reported levels of strain. 14
In recognition of the pressing need for a ‘comprehensive revolution’ in stroke care the National Stroke Strategy (NSS) was produced in 2007. 15 This included an emphasis on longer-term stroke care.
Despite this recognition of the importance of longer-term stroke care, surveys undertaken demonstrate that current service responses are not appropriately developed as stroke survivors have a range of unmet needs 1–5 years after stroke. In one study including 1251 participants,4 half reported some unmet needs. These related to information provision (54%) mobility problems (25%), falls (21%), incontinence (21%), pain (15%) and fatigue (43%).
The National Service Framework for Older People16 and the NSS15 emphasised the need for post-discharge support, in particular the need for ‘good-quality, appropriate, tailored and flexible rehabilitation’ (p. 35). 16 Previous service models for post-stroke care have been associated with equivocal,17–21 and sometimes adverse, outcomes. 20 There was a requirement therefore for innovative models of care to be developed, more closely tailored to the expressed needs of patients and their carers, and embedded in local stroke services. 8,22 A primary care orientation to assess, support and co-ordinate services might be more helpful in minimising longer-term stroke morbidity.
To address this we developed (through previously funded work; see Appendix 1) a systematic approach (termed a system of care) to longer-term stroke care based on the expressed needs of patients and carers23–25 and a monitoring tool to identify longer-term unmet needs after stroke. The twofold aim of the current programme was to enhance the care of stroke survivors and their carers in the first year after stroke and to gain insights into the process of adjustment.
Development of the system of care
Previously no systematic approach has been developed for routine monitoring, problem identification and co-ordination of services to assist stroke patients and their families as they continue to recover from their stroke and make life adjustments to its consequences. The system of care is based on a systematic review and synthesis of the available qualitative literature reporting interviews with stroke patients and carers in which longer-term stroke-related issues were discussed. Approximately 500 patients and 180 carers had participated in the 23 studies included in the review. 25 The review identified 203 patient- and carer-centred problems, which were clustered into five domains (hospital experience, transfer of care, communication and information, service provision and social and emotional difficulties) encompassing 12 main problem areas. A complementary review of 27 quantitative stroke surveys including 6000 patients and 3000 carers assessed the prevalence of these problem areas. 24 A further two prevalent problem areas (falls and sexual problems) were identified. To confirm content validity, our emerging findings were checked and refined by stroke patients and carers in individual interviews and in focus groups, leading to the addition of two more problem areas. 26 This approach has ensured that the proposed new service model is targeted at the most common stroke-related problems of central importance to stroke patients and their carers. The final 16 problem areas were transfer of care, communication and information, medicines and general health, pain, mobility/falls, personal hygiene and dressing, shopping and meal preparation, house and home, cognition, driving and general transport, finances and benefits, continence, sexual functioning, patient mood, patient social needs (and employability) and carer social and emotional needs.
Validated assessment tools (e.g. The Camberwell Assessment of Need for the Elderly27 and EASY-Care28) were appraised to map relevant questions to the identified problem areas. A number of questions required modification to ensure that they accurately reflected the stroke-specific problem areas identified in the reviews. Additionally, some problem areas were not represented by the assessment tools and thus assessment questions were developed by the research team. From this process, patient and carer action plans were devised consisting of 15 questions (patient) and 12 questions (carer) representing the problem areas identified and an additional question to capture ‘other’ stroke-related problems.
Creation of problem-specific reference guides
Having identified the key problem areas and operationalised them through appropriate assessment questions, a range of literature, both within and beyond stroke, was reviewed to identify effective evidenced-based service interventions. The 16 problem-specific reference guides (relating to the 15 patient questions plus one carer-specific question) contain educational text with supporting assessment/treatment algorithms and checklists. The algorithms guide problem-solving as many apparently straightforward problems become multilayered on closer examination. For example, the activity of shopping might be impeded by physical barriers (mobility, lack of suitable transport), cognitive problems (poor comprehension, short-term memory loss) or psychological problems (fear, embarrassment). The final stage in this development phase was to obtain feedback from a range of local community and primary care professionals. This involved presenting and discussing the model during two workshops. Feedback from the workshops enabled us to frame our model into a product that was considered acceptable to primary care professionals. The resulting system of care is presented in a manual.
Manual presentation
Evidence on the implementation of clinical guidelines suggests that presentation can influence uptake into practice. The National Institute for Health and Care Excellence (NICE)29 details five key principles – language and style, bulleted lists, tables and figures, abbreviations and algorithms – and these were used to structure the components of the system of care. The aim of these principles is to make the information clear, accessible to non-specialists, unambiguous, succinct and guiding, but not prescriptive.
The resulting manual comprises patient- and carer-structured assessments representing the identified problem areas linked to, a reference guide and treatment algorithm; patient and carer goal and action plans; a directory of service information; and a selection of validated assessment scales for specific areas such as depression30 and cognitive impairment,31 included as appendices. Thus, we have created a manualised system for longer-term stroke care that is comprehensive (encompasses all areas of potential concern to patient and carer) but individualised (patient-specific action plans constructed).
Clinical implementation
The next stage in the development of the system for longer-term stroke care was to identify means of clinical implementation. Our previous work has identified a need for a service embedded in the community, where staff would have a greater awareness of locally available services. 22 Other work has identified that nurse-delivered interventions have been effective. 32 A survey of local district nurses demonstrated that nurses had relevant insights and skills but would appreciate additional training. 33
The National Service Framework for Older People16 identified a new role of ‘stroke care co-ordinator’ (SCC) to provide advice, arrange reassessment when necessary and co-ordinate long-term support. But who should be involved and how this role might be fulfilled are ill-defined. Through national presentations of our work, through links with the Stroke Association and through the National Stroke Nursing Forum, we were aware that a number of centres were developing SCC roles. To explore this further we undertook a national survey of all UK stroke services. For the purposes of this survey, a SCC was defined as ‘a qualified health professional in regular contact with stroke patients in the community, co-ordinating care inputs on their behalf’.
Pilot work: feasibility of the system for longer-term stroke care
Pilot work was conducted to investigate the practical implementation and feasibility of the proposed system of care. 23 A training programme was provided to community-based staff recruited to use the system of care in clinical practice. In total, 47 stroke patients and 21 carers were assessed using the new system of care. Analysis of care plans, 3 months after the initial assessment, indicated that the process was successful in picking up patient and carer problems. Of 219 problems, 75% had been resolved 3 months after assessment. Patient and carer participants thought that the review process would be more valuable if conducted sooner after hospital discharge (undertaken 8 months after stroke in the pilot study). We were able to demonstrate that a systematic assessment approach incorporated in a disease-specific manual is feasible to implement and was successful in identifying problems and triggering interventions.
Objectives
Projects 1 and 2 of the programme grant described here continue this work:
-
project 1 – an update of the evidence-based treatment algorithms using a structured protocol for searching and assessing the available literature (see Chapter 2)
-
project 2 – a cluster randomised controlled trial (RCT) of the system of care as delivered by SCCs, evaluating its impact on patients’ and carers’ psychological and physical outcomes and its cost-effectiveness (see Chapter 3).
Since this programme grant was awarded in 2007, there have been considerable changes in stroke service provision across the UK. Our system of care is very much in keeping with current developments. Similar approaches have been developed, for example the Greater Manchester Stroke Assessment Tool (GM-SAT) for 6-month reviews. 34 This indicates that our thinking is feasible, practical and appropriate for ongoing service development.
Assessment of unmet needs after stroke
In interlinked work we had undertaken preliminary development of a tool to assess and monitor patient longer-term unmet needs after stroke [the Longer-term Unmet Needs after Stroke (LUNS) tool].
We, and others, have demonstrated that the problems faced by stroke patients and their families in the longer term encompass physical, social and mental well-being. 4,24,25 There is no currently available measure that provides a ‘good fit’ across all of these outcome domains in the special context of longer-term stroke care. The common compromise in research, in which greater insights are required, is to use a basket of measures, all addressing different components of the stroke experience, or to create and use one larger outcome tool (e.g. the Stroke Impact Scale35). In clinical practice such approaches are impractical, expensive and time-consuming and there is a need for a short, easy to complete monitoring tool. We developed a comprehensive monitoring tool to measure unmet needs in stroke patients by converting the assessment questions (based on patient- and carer-identified problems) contained in our proposed system of care into statements of need. Preliminary psychometric testing was undertaken. The draft, 28-item version of the measure, was reviewed by experts in the field and refined in collaboration with our Consumer Research Advisory Group (CRAG), which contributed to various aspects of the measure, including layout, presentation and wording of questions. The monitoring tool therefore has face and content validity. We have undertaken preliminary work (through existing funding) to investigate the reliability of the measure. Patients (n = 29) were assessed with the measure in their home on two occasions, approximately 1 week apart. Statistical analysis demonstrated poor reliability for 12 questions and, in the light of this, further work was undertaken with the CRAG, members of other local stroke groups and stroke physicians. All aspects of the measure, including layout, presentation and wording of questions, were reviewed. The number of items was reduced from 28 to 21 and the questions were grouped according to whether they addressed informational needs or practical needs.
Objectives
Project 3 in this programme continues this work:
-
further development and psychometric testing of a measure to assess and monitor patient longer-term met/unmet stroke-related needs (the LUNS questionnaire) (see Chapter 4).
Identification of needs after stroke has become increasingly topical during the implementation of this programme grant. The Stroke Association commissioned a survey of post-stroke unmet needs and co-applicants of this grant (Forster and Young) contributed to the development of this survey,4 which included some questions from the LUNS tool.
‘Failure to thrive’ patients
A complementary approach to the described projects is to work with patients and their families to gain a greater understanding of the mechanisms of adjustment to stroke. It is reported in the literature, and often cited anecdotally, that many patients with good physical recovery are paradoxically socially inactive; however there is little information on the prevalence or cause of poor social recovery.
We were fortunate to have a number of data sets available to us from previous community stroke studies,18,36,37 on which we undertook some exploratory analysis. By comparing Barthel Index38 scores at various time points with social activity scores [measured by the Frenchay Activities Index (FAI)39] we have been able to identify statistically a small subgroup of patients who do not do as well as we would expect, that is, they are more socially restricted than would be expected for their level of disability. To investigate why there is such a variation in social recovery for patients with good physical recovery, this subgroup was investigated further. Unsurprisingly, previous activity level, age and living in institutional care were all related to poor social activity at 12 months after stroke, even when physical recovery is good. A review of a number of our previous data sets indicates that approximately 10% of the patients in each of the samples had ‘unexplained’ poorer outcomes (i.e. more socially restricted than expected for their level of disabilty but not explained by age or living in institutional care or pre-stroke activity). Although this group of patients is small, it is possible that they have attributes that have impeded their recovery that are present to a lesser extent in the wider stroke population and therefore have been difficult to detect. A specific study of this smaller group may therefore provide insights of relevance to the wider stroke population and thereby inform future service development.
Objectives
Project 4 undertook further exploratory work:
-
identify factors contributing to patients’ poor adjustment after stroke through a qualitative and quantitative case–control study nested in the randomised trial (see Chapter 5) [it was clarified during the review of the grant application that the design is not in fact a nested case–control study but rather a qualitative substudy (with purposive sampling based on the quantitative trial data)].
Programme management
This programme grant has been undertaken and completed by a committed team of co-applicants. The management structure is summarised in Appendix 2. During implementation one of the co-applicants (Val Steele) retired and another (Joanna Powell) left clinical practice. The update of the system of care (project 1) and further development of the LUNS tool (first part of project 3) were completed before the system of care trial (project 2), which then ran until the end of the programme grant; the LUNS tool was included in the trial outcome measures. Psychometric evaluation of the LUNS tool (second part of project 3) was carried out in parallel with project 2. Exploration of poor adjustment (project 4) was carried out in parallel with the 12-month follow-up in project 2.
Clinical engagement
Clinical engagement has been sustained throughout the programme through:
-
Yorkshire Stroke Research Network (YSRN) therapy meetings – held three times a year and open to all allied health professionals in Yorkshire
-
the attendance of the chief investigator at the quarterly Clinical Stroke Network meetings, where research progress is reported as a regular agenda item
-
the ongoing contact of the chief investigator, clinical lead for the YSRN, with all local stroke services and robust links with national colleagues.
Patient and public involvement
Terry Brady (co-applicant and stroke survivor) has been involved at all stages of programme development. He is a member of the CRAG, which has met bimonthly throughout the duration of the programme. The CRAG has been provided with regular updates on the progress of the programme and has contributed at all stages.
Terry Brady is also on the organising committee for the annual consumer conference, which is hosted by the YSRN and attracts over 70 stroke survivors and their carers each year. Updates on ongoing research are provided at the conference and this includes progress reports on this programme of research. In addition, advice and guidance were sought on specific components of the research through ‘round table’ discussions, for example outcome assessment for the cluster trial and layout of the outcome assessment booklet (project 2); considerable input into the development of the LUNS tool, both content and layout (project 3); and contribution to the adjustment after stroke study and consideration of the results (project 4).
Colleagues in the CRAG have become experienced in the review of stroke research. One of our members, Mick Speed, is now a member of the Stroke Association research panel.
Collaborations
Professors Anne Forster and John Young were members of the Stroke Association UK Stroke Survivor Needs Survey study team and this survey included some of the questions from the LUNS questionnaire.
Chapter 2 Project 1: update of the system of care documentation
Abstract
Background
Our purposely developed system of care was framed around patient- and carer-identified problems. To support treatment delivery a manual was created that included algorithms to assist SCCs in clarifying problems and directing patients to treatment options. To ensure that all of the treatment algorithms were up to date and evidence based, they were rigorously reviewed and updated.
Methods
In collaboration with information specialists at the University of Leeds, a hierarchical, comprehensive structured protocol for identifying evidence in each of the 16 problem areas was developed. This protocol included identifying relevant stroke- and problem-specific guidelines; meta-analyses and systematic reviews; and, if necessary, individual RCTs. Two researchers independently reviewed all outputs. Guidelines, reviews and papers identified for inclusion were assessed for quality using standard tools. Drafted treatment algorithms were peer reviewed by external experts.
Results
Over 71,000 articles were identified by the initial searches. Robust guidelines were identified for three problem areas; for one problem area, information provision, we are the authors of the Cochrane review, which we updated. For the 12 remaining problem areas detailed searches were implemented. Following the review procedure algorithms were updated in accordance with the identified evidence and, following external peer review, they were incorporated into the system of care manual. Presentation of the manual was informed by expert opinion and feedback from clinical colleagues.
Conclusion
The system of care manual was created using robust methods to ensure that advice and guidance provided were up to date and evidence based.
Introduction
The system of care was formulated around the post-stroke problems identified by patients and their carers. These were translated into a series of assessment questions to elicit the problems and to enable the SCC to work with each patient and his or her carer to develop goals and action plans. Goal setting is considered an essential part of clinical rehabilitation,40 and there has been growing emphasis on the need for interventions with patients to be goal orientated. Our intent was that the system of care would more appropriately address patient unmet needs. Documentation involved with the delivery of this system of care included:
-
the care plan: assessment questions (formulated around problem areas) and goal and action planner
-
the manual: providing information about the problem area (reference guide) linked to an evidence-based treatment algorithm and other supporting material
-
national and local service information.
All required refining and updating before the planned trial to evaluate the system of care (see Chapter 3).
The care plan
The care plan included questions addressing all problem areas supported by additional prompts. It also included goal and action planners for the patient/carer, which aimed to encourage and support shared decision-making about goals and the prioritisation of goals according to patient (carer) preference and joint problem-solving approaches. The layout and presentation of the assessment questions for patients and their carers were initially reviewed by the study management group (SMG) and clinical colleagues. The care plan was further refined by SCCs taking part in the trial, before the start of the trial (see Chapter 3, Intervention arm: system of care).
Part of the care plan is shown in Appendix 3.
The manual
The manual associated with our system of care was created in 2003. The manual included introductory text, reference guides with associated detailed treatment algorithms, which are centred around the patient- and carer-identified problems, and general advice on patient assessment. On commencement of the programme grant the manual needed updating to ensure that it remained a relevant and reliable resource.
Methods
Updating the manual
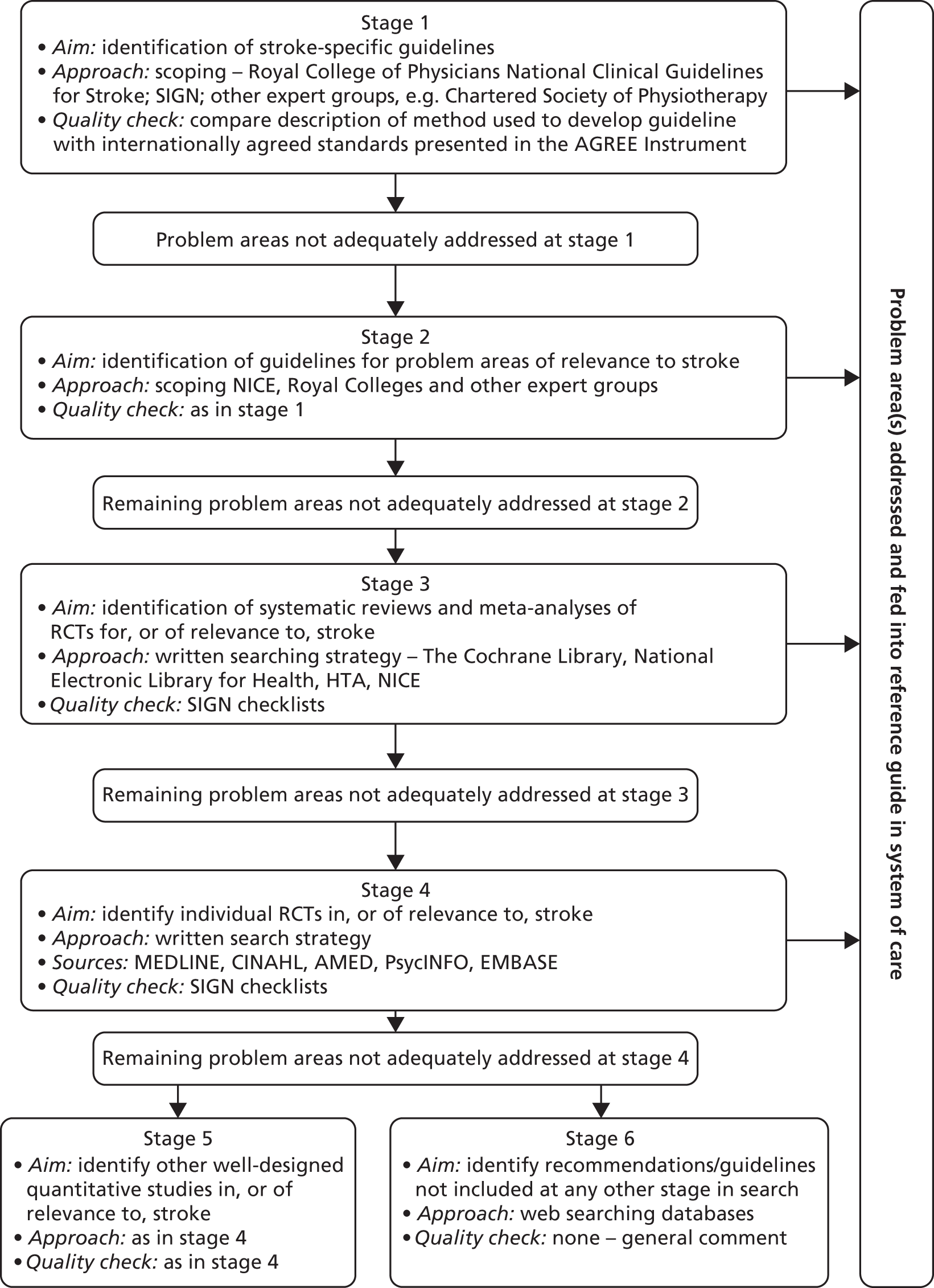
To ensure that all of the treatment algorithms were up to date and evidence based, in collaboration with information specialists at the University of Leeds, a hierarchical, comprehensive, structured protocol for identifying evidence in each problem area was developed (Figure 1).
FIGURE 1.
Protocol for identifying evidence-based interventions for stroke problem areas. AGREE, Appraisal of Guidelines Research and Evaluation; AMED, Applied and Complementary Medicine Database; CINAHL, Cumulative Index to Nursing and Allied Health Literature; HTA, Health Technology Assessment; SIGN, Scottish Intercollegiate Guidelines Network.
The reference guides linked to treatment algorithms addressed the following problem areas:
-
transfer of care
-
communication and information
-
medicines and general health
-
pain
-
mobility/falls
-
personal hygiene and dressing
-
shopping and meal preparation
-
house and home
-
cognition
-
driving and general transport
-
finances and benefits
-
continence
-
sexual functioning
-
patient mood
-
patient social needs (and employability)
-
carer social and emotional needs.
The protocol started (stage 1) with searches to identify all potentially relevant evidence-based stroke guidelines (e.g. the National Clinical Guidelines for Stroke2,41). This progressed to the identification of guidelines for problem areas of relevance to stroke but that were not necessarily stroke specific (stage 2) and the identification of systematic reviews and meta-analyses (stage 3). If it was considered that the problem area had not been adequately addressed (e.g. through the availability of national guidelines) then a comprehensive electronic search strategy was developed and implemented to identify all RCTs of effective interventions evaluated for stroke and for other diseases with similar experiences to those of stroke (stage 4).
The search strategy was developed iteratively. Each problem area was discussed between the researchers (A Forster, J Murray and R Breen) and the information specialists and consensus agreement was reached about scope (e.g. age limits, key words, databases to be searched). When available, guidance on search terms was taken from existing reviews (e.g. the Cochrane review on the prevention and treatment of urinary incontinence after stroke in adults42). Example search strategies for pain for MEDLINE and EMBASE are provided in Appendix 4 and other search strategies are available on request from the authors. All searches were restricted to the English language only, with a date restriction from 1995 onwards, and included appropriate methodological filters for guidelines, consensus statements, systematic and other reviews and RCTs. The information specialists then conducted initial searches with the results reviewed by J Murray and A Forster to verify that appropriate titles were being identified. Following further amendments as necessary, the full search was undertaken, with the results downloaded into EndNote (version 5; Thomson Reuters, CA, USA). All searches were undertaken in MEDLINE, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), Evidence-based Medicine Reviews, PsycINFO and EMBASE. A member of the research team was designated as lead reviewer for each problem area.
The overall purpose was not to develop guidelines but to identify and present the evidence in a format that will aid the clinical application of the structured assessment system, triggering appropriate, evidenced-based interventions. These interventions had to be relevant to SCC practices; therefore, for example, in medicines management the focus was on knowledge rather than the SCC being an active prescriber of drugs. Similarly, when considering the problem area of mobility, interventions that require specialist equipment (such as a treadmill) were not considered as the SCC would refer to a physiotherapist for such specialist treatment.
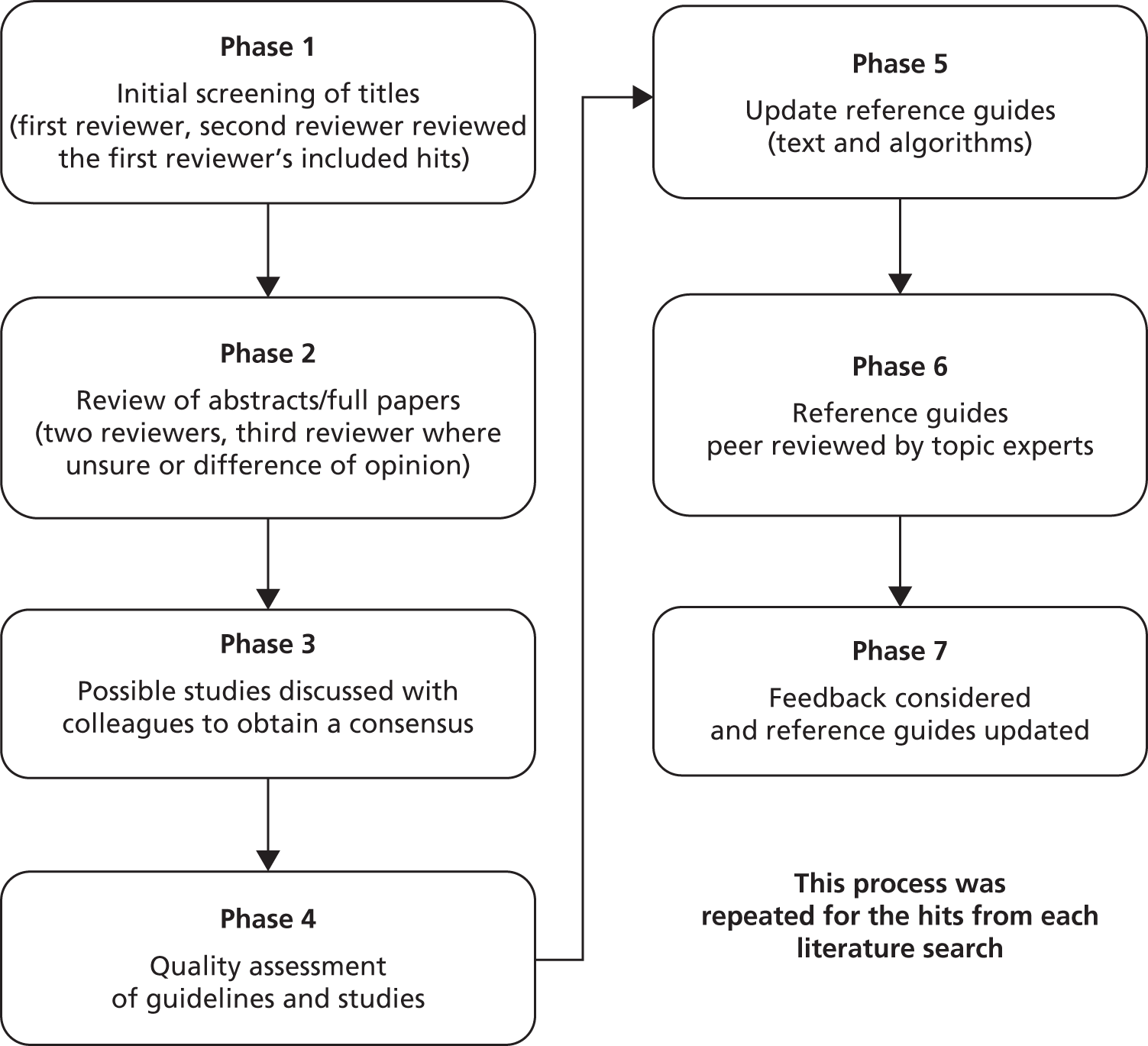
The outputs from stages 1–3 were initially reviewed by one researcher (J Murray) and presented and discussed at regular SMG meetings (monthly then moving to fortnightly). For stage 4 (Figure 2), initial screening of the titles was conducted in two phases: the first reviewer carried out an initial screen to eliminate any obviously irrelevant papers and then the second reviewer screened all remaining papers. The abstracts/full papers were then considered by two reviewers independently using a standard proforma. A third reviewer was required when there was a conflicting opinion or both reviewers were unsure about study inclusion. Following discussions a consensus of studies for inclusion was reached.
FIGURE 2.
Written search strategy used in stage 4.
Quality assessment
The quality of the guidelines was assessed using the Appraisal of Guidelines for Research and Evaluation (AGREE) Instrument (see www.agreetrust.org/about-the-agree-enterprise/agree-research-teams/agree-collaboration/). Quantitative studies were assessed using quality checklists developed by the Scottish Intercollegiate Guidelines Network (see www.sign.ac.uk).
Synthesis of papers
Evidence identified through the structured protocol was presented to the SMG by the lead reviewer for each problem area. The evidence (identified guidelines/studies, etc.) was used to inform development of the reference guide (supporting text) and treatment algorithm. Existing algorithms were reviewed and amended as necessary. A synthesis of study results through meta-analysis was not undertaken. Drafts of the reference guides and algorithms for each problem area were discussed and reviewed in SMG meetings.
Once agreement on content was reached by the SMG, the reference guides were then sent out for peer review to topic experts in the UK and their feedback was incorporated into the updated reference guides.
Presentation of the manual
Concurrent with content development, advice was sought regarding presentation, to facilitate ease of use. This was sought through a review of the relevant literature and from the Medical Illustration Department of Bradford Teaching Hospitals NHS Foundation Trust. In addition, the SMG considered what components were essential for delivery of the system of care.
The Cochrane review of information provision for stroke patients and carers
The Cochrane review of information provision for patients and their carers after stroke has been updated twice during the programme. The systematic review was conducted using The Cochrane Collaboration-recommended methodology for undertaking systematic reviews of RCTs and full details of the methods and outcomes have been published. 43–45
Studies were included if there was random assignment of participants (patients with a clinical diagnosis of stroke and/or their identified carers) to intervention groups (one of which involved the provision of information and/or education) and to a control group. In addition, studies using a matched pairs design and studies in which strict randomisation procedures were not adopted were also considered for inclusion.
We excluded trials in which information giving was only one component of a more complex rehabilitation intervention, for example family support worker trials (e.g. Forster and Young18 and Dennis et al. 20), which are the subject of a separate Cochrane review. 46
The primary outcomes used to assess the effectiveness of information provision were patient and carer knowledge about stroke and stroke services, and impact on health, especially mood. Secondary outcomes were activities of daily living; handicap; social activities; perceived health status; quality of life; satisfaction with information; hospital admissions, service contacts or health professional contacts; compliance with treatment/rehabilitation; death and/or institutionalisation; and cost to health and social services.
Relevant trials were identified in the Cochrane Stroke Group Specialised Trials Register. Additional intervention-based search strategies were developed for the Cochrane Central Register of Controlled Trials (CENTRAL/CCTR, formerly Cochrane Controlled Trials Register), MEDLINE, EMBASE, CINAHL, ISI Citation Index, ISI Web of Science Service, ASLIB Index to Theses, Dissertation Abstracts International, Applied Social Sciences Index and Abstracts and PsychLIT/PsycINFO. We also searched registers of trials in progress and bibliographies of retrieved papers, articles and books.
Results
Updating the manual
Following a review of all problem areas it was agreed that each category of problem should be subjected to a separate search, with the approach and range of the search varying according to the problem. Broadly three approaches to the search strategy were undertaken:
-
an inclusive general search of the problem area
-
the search was restricted to ‘stroke’
-
the search was restricted to stroke and other similar long-term conditions, which we termed a ‘chronic illness filter’.
A summary of the search methods used is presented in Table 1.
| Reference guide | Search strategy |
|---|---|
| Transfer of care | It was felt that a full literature search of this area was outwith the scope of this model. A systematic review and guideline were available |
| Communication and information | A Cochrane review on information provision was conducted as part of this programme (see an update of the Cochrane review on information provision for stroke patients and carers44,45) |
| Medicines and general health | The National Clinical Guidelines for Stroke2 had comprehensively covered this problem type |
| Pain | Stroke-specific search, specifically including shoulder pain and neuropathic pain |
| Mobility/falls | Mobility: stroke/exercise guidelines; excluded equipment-related therapy (treadmills/robots) as SCCs would not deliver this treatment Falls: stroke-specific search – for general falls a Cochrane review is available47 |
| Driving and general transport | Search combined because of considerable overlap. General search then limited using terms (stroke, traumatic brain injury, disabled persons, chronic disease) supported by text word searches for physical disabilities |
| Continence | General search: aware that a Cochrane review of urinary incontinence is available42 |
| Sexual functioning | General search then limited to stroke, chronic heart disease, traumatic brain injury, disabled persons |
| Shopping and meal preparation | Problem areas combined. General search excluding anorexia/bulimia/children, then limited to stroke, traumatic brain injury, disabled persons, chronic disease and text word searches for physical disabilities |
| House and home | General search (excluding children) |
| Finances and benefits | General search |
| Personal hygiene and dressing | General search then limited using chronic illness filter |
| Cognition | Search terms tailored based on existing Cochrane reviews;48–50 additional search for compensation devices, e.g. aids/devices to compensate for memory loss |
| Patient mood | A recent systematic review and guidelines were available51 |
| Patient social needs (and employability search) | General search |
| Carer social and emotional needs | General search followed by stroke-specific search |
Over 71,000 titles were indentified by the individual searches, details are provided in Table 2. In total, 12 problem types required a review of evidence-based interventions from stage 3 (see Figure 1). Two problem areas, shopping and meal preparation and house and home, were addressed by a combined search and one problem area, patient social needs (and employability), was addressed by two separate searches.
| Reference guide | Guidelines/reviews/RCTs identified | Number of papers after first review | Number of papers after final review | |
|---|---|---|---|---|
| Transfer of care | – | – | – | |
| Communication and information | – | – | – | |
| Medicines and general health | – | – | – | |
| Pain | 215 guidelines, 1619 reviews, 1565 RCTs | 190 | 7 | |
| Mobility/falls | 193 guidelines, 141 reviews, 109 RCTs | 17 | 8 | |
| Driving and general transport | 131 guidelines, 364 reviews, 1028 RCTs | 49 | 49 | |
| Continence | 867 guidelines, 5793 reviews, 12,139 RCTs | Guidelines sufficient | Guidelines sufficient | |
| Sexual functioning | Not recorded | 243 | 21 | |
| Shopping and meal preparation | } | 313 guidelines, 1377 reviews, 4213 RCTs | 40 | 16 |
| House and home | ||||
| Finances and benefits | 640 guidelines, 1296 reviews, 4101 RCTs | 80 | 10 | |
| Personal hygiene and dressing | 195 guidelines, 742 reviews, 830 RCTs | 43 | 17 | |
| Cognition | Not recorded | 83 | 13 | |
| Patient mood | – | – | – | |
| Patient social needs (and employability) | 94 guidelines, 489 reviews, 1757 RCTs | 209 (395) | 25 (21) | |
| Carer social and emotional needs | 3483 general, 485 stroke | 420 | 52 | |
Identified guidelines and papers were subject to appropriate quality assessment. Relevant evidence-based interventions from guidelines and studies found to be of high quality were then used to update treatment algorithms. Each was drafted by a lead reviewer and then reviewed by the SMG before external review. Final versions were further revised and formatted for inclusion in the manual.
The completed reference guides and treatment algorithms with supporting text are included in the manual; an example (for pain) is provided in Appendix 5.
Manual content and layout
In discussion with clinical colleagues and colleagues from the Medical Illustration Department of Bradford Teaching Hospitals NHS Foundation Trust, presentation of the manual was agreed. Introductory text was finalised and advice and guidance on use of the manual were provided. Each reference guide has a number of sections (‘The problem’, ‘The evidence’ and ‘Addressing the problem’) followed by the algorithm. A frequency table was also provided for each of the problem areas (e.g. 88% of stroke patients have a fear of falling), to provide insight for SCCs and also assist them in informing/reassuring the patient. A list of screening tools that might be of use was included in the manual, with the tool reviewed to ensure that there were no copyright issues. All documentation was reviewed by members of the SMG before being finalised.
The essential components of the system of care were considered by the SMG. These were described as principles and were included in the manual. They were as follows:
-
patient-centred comprehensive coverage of problems identified as important by patients and carers
-
all assessment questions asked
-
follow-up on actions and review goals.
In addition, national information about services available for patients after stroke was collated, with the intention that this would be used alongside information about local services.
Review of the evidence
Updating these treatment algorithms provided an opportunity to review evidence gaps. Available evidence was reviewed for each problem area. The results were tabulated and summarised to include available evidence and recommendations (if available) made in the Evidence-Based Review of Stroke Rehabilitation (see www.ebrsr.com), a relevant Cochrane review, Royal College of Physicians (RCP) guidelines,2 Australian guidelines52 or Canadian guidelines53 or identified through searches undertaken for the manual update. We also highlighted when a relevant RCT was ongoing. The resulting output is 85 pages long. Although the work was completed, we did not promote it as we were mindful that NICE was developing stroke rehabilitation guidelines (issued June 2013)54 that could replicate the work and the RCP was similarly updating its guidelines (issued September 2012). 55
The Cochrane review of information provision for stroke patients and their carers
For the first update44 we identified 228 abstracts, of which 63 studies were potentially relevant to the review. In total, 17 studies, involving 1773 patients and 1058 carers, were included (published 2009).
For the most recent update45 we reviewed 28,110 titles including 134 full papers, resulting in the inclusion of four additional studies. The current review includes 21 trials from seven countries involving 2289 patients and 1290 carer participants.
Meta-analyses of reported outcomes showed a significant effect in favour of the intervention on patient knowledge, carer knowledge and patient satisfaction with information provision. There was also an effect on patient depression; however, the reduction was small and may not be clinically relevant. There was no effect on the number of cases of anxiety or depression or on patient mortality. Qualitative analyses found no strong evidence in favour of the intervention for other outcomes relevant to stroke recovery, including independence, participation in social activities, service use or modification of health-related behaviours.
Post hoc subgroup analyses demonstrated that, when information was provided in a format that more actively involved patients and carers, for example by offering repeated opportunities to ask questions, it had more effect on patient mood than information provided on one occasion only. However, as we saw no effect on the dichotomous end points of anxiety or depression (number of cases), the effects may be small. The specific component of the active information provision that may provide beneficial effects requires further investigation. There was no evidence that active information provision strategies were effective for other outcomes.
Discussion
The content and delivery of the developed system of care were comprehensively reviewed. Reviewing the literature for evidence-based treatment options focusing on patient-identified problems is a novel approach. This work focused on handicap rather than impairments and intended to create a more patient-centred service.
We took expert advice and guidance in the development of the treatment algorithms, using recognised methods to review the literature, and involved external peer review. The work was rigorous and comprehensive. However, some topic areas, for example house and home, are huge and may not be addressed in the more medical, impairment-focused literature. However detailed our searching, we may have missed some pertinent literature in less mainstream journals.
Information provision is reported as the most common unmet need and we undertook and published the Cochrane review relating to information provision for stroke patients twice during this programme. 43,45
Chapter 3 Project 2: cluster randomised controlled trial to evaluate the clinical effectiveness and cost-effectiveness of a system of longer-term stroke care
Abstract
Trial design
A pragmatic, multicentre, cluster RCT compared a new system of care with usual practice delivered by SCCs.
Methods
Stroke services were randomised to the intervention or the control. Patients with new stroke living at home post stroke and referred to a SCC, and their informal carers, were eligible for recruitment before their first SCC assessment. The primary objective was to determine whether the intervention improved patient psychological outcomes (General Health Questionnaire-12, GHQ-12) at 6 months; secondary objectives included further functional outcomes for patients, carer outcomes (if registered) and cost-effectiveness. Follow-up was through self-completed postal questionnaires at 6 and 12 months after registration.
Results
In total, 32 randomised stroke services (29 participated), including 800 patients (399 control, 401 intervention) and 208 carers (100 control, 108 intervention), were recruited. In intention-to-treat (ITT) analysis the adjusted difference in patient GHQ-12 mean scores at 6 months was −0.6 points [95% confidence interval (CI) −1.8 to 0.7 points; p = 0.394], indicating no evidence of a statistically significant difference between treatment arms; analyses of secondary end points and of the per-protocol population resulted in the same conclusion. The intervention involved no additional SCC time. Quality-adjusted life-years and total health and social care costs at 6 months, 12 months and over 1 year were similar between arms. Societal costs were higher for the intervention arm (+£1163 at 6 months, 95% CI £56 to £3271).
Conclusions
This robust trial demonstrated no benefit for clinical effectiveness or cost-effectiveness outcomes for the system of care compared with usual SCC practice.
Trial registration
This trial was registered as ISRCTN67932305.
Introduction
Background
The diversity of longer-term problems experienced by stroke patients and their carers has long been recognised,56 but they remain poorly addressed by existing services. 57 There are persuasive arguments that a community-based orientation to post-stroke care delivered by primary care in the patient’s local community, to assess, support and co-ordinate relevant services, might be more helpful in minimising longer-term stroke morbidity. 22,58
To address this we have developed an evidence-based system of care that aims to meet the longer-term needs of stroke survivors and their carers living at home in the community. The system of care incorporates structured assessment focused on patient- and carer-centred problems and associated evidenced-based treatment algorithms.
We aimed to evaluate the clinical effectiveness and cost effectiveness of the system of care when delivered by SCCs compared with usual practice in a cluster RCT [Longer-Term Stroke care (LoTS care) trial]. The methodology for the trial was informed by our survey of 39 SCCs. 59 The survey, undertaken in 2006, revealed that approximately 40% of SCCs reviewed the patient within 1 week of discharge from hospital. Most SCCs (n = 27) saw patients more than twice after discharge from hospital, but the number of contact visits was often dependent on patient need (as opposed to defined time points). The majority of SCCs appeared to refer on to other services for carer assessments.
Objectives
Primary objective
The primary objective of the trial was to determine whether the system of care improved psychological outcomes for stroke patients living at home.
Secondary objectives
The secondary objectives were to:
-
determine whether the provision of the system of care improved functional outcomes for stroke patients living at home
-
determine whether the provision of the system of care improved psychological and functional outcomes for carers of stroke patients living at home
-
assess whether the system of care was cost-effective based on patient outcomes from both health/social care and societal perspectives.
Methods
A summary of the protocol has been published. 60 The study was approved by Leeds West and Scotland A Research Ethics Committees.
Trial design
The trial has been designed as a pragmatic, multicentre, cluster RCT. The unit of randomisation was at the level of the stroke service, with SCCs (single practitioners or part of a community-based team) randomised to deliver the new system of care to all patients (and their carers, if appropriate) or to continue to deliver current practice as determined through local SCC policy and practices. Follow-up was through postal questionnaires at 6 and 12 months after recruitment.
A prospective cluster RCT design was used to reduce between-group contamination. The training component of the intervention was designed to impact on the skills, knowledge and clinical practice of SCCs and therefore the risk of contamination would be high if randomisation was at the individual patient level. If a service had more than one SCC it was likely that the SCCs would meet on a regular basis and work as a team, sharing caseloads. For these reasons the unit of randomisation was at the level of the service.
The system of care was evaluated as a whole to avoid a type III error,61 in which deconstruction of the component parts of a complex intervention may result in the loss of benefits of the interactions between components.
We recognised that the intervention may be effective on a number of domains. We therefore chose a range of outcome measures. The primary outcome measure was the General Health Questionnaire-12 (GHQ-12),62 to reflect patient mood, which would be influenced by a range of service inputs (including overall co-ordination of care). We aimed to capture psychosocial outcomes rather than just physical abilities as reflected in activities of daily living. The GHQ-12 has been used in previous stroke research and is sensitive to change.
Eligibility
Stroke services
The trial evaluated a complex intervention delivered to patients by a SCC within a stroke service; therefore, eligibility criteria were applied at three levels – the stroke service, the stroke unit and the SCC.
A stroke service encompasses primary and secondary care over a defined geographical area within the UK. As treatment in a stroke unit is the recommended care pathway for all patients after stroke,16,63 a stroke service was considered for inclusion in the trial only if it included a stroke unit that fulfilled the RCP’s41 definition of a stroke unit, that is, it fulfilled four out of the five following criteria:
-
it had a consultant physician with responsibility for stroke
-
it had formal links with patient and carer organisations
-
it had multidisciplinary meetings at least weekly to plan patient care
-
it provided information to patients about stroke
-
it provided continuing education programmes for staff.
A SCC was eligible if he or she fulfilled the following criteria:
-
is a registered health-care professional with documented experience in stroke care
-
undertakes a community-based/liaison or co-ordinating role for stroke patients (i.e. providing care for patients living in the community after discharge from hospital)
-
is in contact with patients and co-ordinates a range of longer-term care inputs on their and their carers’ behalf (e.g. signposting, carrying out assessments)
-
works within a stroke service as above.
If SCCs worked as a team and shared caseloads then they were classed as one ‘stroke service’ for the purpose of the trial randomisation. A SCC was classified as working in a team if he or she attended regular (i.e. weekly/fortnightly) community multidisciplinary team (MDT) meetings.
Participants
In keeping with the pragmatic trial design, the patient eligibility criteria were broad to be inclusive of a clinically meaningful variety of stroke pathology and severity affecting patients and reflecting current clinical practice.
Patients were eligible for the trial if they:
-
had a confirmed primary diagnosis of new stroke
-
were referred to a SCC on discharge home from hospital or within 6 weeks of stroke and were still waiting for their first home or outpatient SCC assessment
-
were able to provide written informed consent or consultee declaration.
Patients were excluded from the trial only if:
-
they had a planned permanent admission to, or were already resident in, a nursing or residential care home
-
their main requirement was palliative care
-
they had been previously registered to the trial.
Patients involved in other Stroke Research Network (SRN)-adopted studies could be recruited into this trial unless they were first recruited into another study that was considered to overlap, as listed in the excluded studies list [Assessing Communication Therapy in the North West (ActNoW), LUNS, Oxford Vascular Study (OXVASC), Surgical Trial in Lobar Intracerebral Haemorrhage (STICH) II and Training Caregivers After Stroke (TRACS)].
Recruitment of carers was optional. Carers were eligible for the trial if:
-
they were identified by the patient as the main informal carer who provides the patient with practical support a minimum of once per week.
Carers were excluded from the trial if their patient did not consent to the trial.
Randomisation
Randomisation was undertaken by the University of Leeds Clinical Trials Research Unit (CTRU). Stroke services were randomised on a 1 : 1 basis to either the intervention group or the control group. A detailed overview of the stroke services was obtained before randomisation to inform stratification.
Randomisation was stratified by the quality of the stroke unit [National Stroke Audit (NSA) score based on 2006 data64], the annual number of referrals, whether SCCs work alone or within a community-based MDT and the Strategic Health Authority (SHA). When a SCC received referrals from more than one stroke unit, a weighted average of the NSA scores reflecting referral rates was used.
A method of obtaining a balanced design from the covariates available at the start of the trial, as proposed by Carter and Hood,65 was used. This method uses an imbalance measure to compare the extent to which all possible designs balance important covariates between the arms of the trial and then randomly selects one member from the class of designs that achieves maximum balance. The advantage of the method is that it allows randomisation in multiple phases, conditioning on previous allocations. Because of the complexities of the current research approvals process, which created variable time delays (see Appendix 6), the services were randomised in two phases.
Blinding
As this was a cluster trial, all patients within the services randomised to the intervention received the new system of care and all patients within the services randomised to the control received usual care.
Recruitment of trial participants was by members of the independent reseach team and the SCCs were unaware which of their patients had consented to participate. Members of the research team were blinded to whether they were recruiting within an intervention service or a control service. This trial design has the advantage of reducing the potential for selection bias from differential recruitment, inherent if the SCCs were responsible for patient identification, and it minimises altering of SCCs’ clinical activity for trial participants.
Interventions
All patients received the services of a SCC in a community stroke service. SCCs within the intervention arm provided care according to the new system of care. Control SCCs continued with usual practice.
Control arm
Patients who received care from the control SCCs received current community-based care as determined by local policy and practices.
Intervention arm: system of care
The development and description of the system of care is provided in Chapter 1 and Appendix 1. For implementation in the trial, the system of care comprised the following components:
-
A care plan containing a structured assessment (assessment questions linked to reference guides in the manual) and a goal and action planner for each contact (patients and carers) (see Appendix 3). In discussion with the SCCs it was agreed that the assessment documentation should incorporate the patient details that they collected in delivery of their service, the intent being to create a single care plan that would replace currently used patient records, thus facilitating it becoming embedded in their service. The care plan was piloted by the SCCs (between the two training days; see below) before the final version was produced.
-
An optional checklist detailing the content of the assessment to be given to patients before the SCC visit.
-
A manual containing reference guides with evidence-based treatment algorithms, a frequency table of longer-term problems after stroke, a service directory and recommended assessment scales.
-
National information about services available for patients after stroke was collated and provided to the SCCs at the first training day. Each SCC was given a large box file including leaflets of relevant services (e.g. Disabled Holiday Directory, Age Concern). The SCCs were asked to further develop a resource inventory of local services.
Training in the delivery of the system of care was provided for each of the SCC services randomised to the intervention before commencement of patient recruitment. Training was delivered through two centrally based Royal College of Nursing-accredited training days approximately 1 month apart and was supported by detailed documentation and a purposely produced CD. The first training day included details of the system of care [from the Academic Unit of Elderly Care and Rehabilitation (AUECR) research team, involved in its development, and clinicians involved in the pilot work], guidance on problem-solving techniques and the principles of the intervention, as described below:
-
patient centred (comprehensive coverage of problems identified by patients and carers)
-
provide assessment areas (checklist) before assessment whenever possible
-
ask all assessment questions
-
keep accurate records
-
problem-solving approach with collaborative goal setting
-
follow up on actions
-
review goals
-
non-prescriptive – individual creativity
-
according to local services/resources
-
within patient’s own environment whenever possible
-
timing/duration of intervention (according to national recommendations)
-
cut-off time (problem-solving approach leads to reduced problems over time)
-
flexible approach to carer assessment.
The SCCs then used the system of care with several patients in their service before the second training day, which reviewed the use of the system of care and problem solving and in addition provided training in specific areas such as pain and benefits. After the second training day SCCs began to use the system of care for all patients referred to their service. Recruitment was not opened until all parties were satisfied that the system of care was being delivered. This was assessed during site set-up visits at which a sample of care plans was reviewed to ensure that all assessment questions were asked and that identified problems were being addressed in the goal and action planner.
Documentation of interventions
Before randomisation, all SCC services completed a survey and a semistructured interview; these were used both to assess eligibility and inform stratification and to describe the services and the nature of their interactions with patients. Changes in service set-up and SCC practice were captured by semistructured interviews midway through recruitment and after the end of the 12-month follow-up and by a second survey after the end of follow-up. The second survey also requested estimates of typical SCC input times, to be used in the economic analysis. Interviews that took place during recruitment were designed and performed so that they did not influence SCC practice.
After randomisation, control SCCs were asked to complete time logs for all patients, documenting the number and duration of contacts and the time spent co-ordinating actions, note writing and discussing patients in MDT meetings. Time logs were collected for trial patients after the end of the 12-month follow-up so as not to unblind SCCs to trial patients during follow-up. Once trained in use of the system of care, intervention SCCs were asked to use the care plan for all contacts with all patients (and carers if carer assessments were performed). After the end of the 12-month follow-up, equivalent data to those contained in the time logs and additional information on the use of the structured assessment and goal and action planner were collected for trial patients by transcribing the appropriate information from the care plans at site. This was carried out by two researchers from AUECR who were familiar with the intervention, to ensure consistency.
Assessments
Recruitment
The trial reflected usual referral pathways of patients to SCCs, determined during site set-up visits, involving the SCCs and the hospital-based clinical team and clinical research team. Patients were referred to SCCs in three main ways: by in-hospital referral (the majority of patients), by referral post discharge or by referral of patients not admitted to local hospitals. The recruitment process was facilitated by close liaison between the clinical research team and the clinical team, but patients were recruited by independent members of the clinical research team.
Anonymous screening data (demographic data and modified Rankin Scale score66) were collected for all patients referred to a participating SCC. Written informed consent for baseline and follow-up assessments was obtained from patients and carers (if appropriate) before any trial-specific procedures were carried out. In the event that a patient lacked the capacity to consent, the patient’s family member, carer or friend was asked to act as the consultee and provide a consultee declaration.
The clinical research team collected baseline demographic data, which included assessment of cognition [six-item Cognitive Impairment Test (6CIT)],67 language ability, pre-stroke disability (Barthel Index)38,68 and the six factors from the Edinburgh stroke case mix adjuster. 69
Patients and carers completed the baseline questionnaires predominantly before discharge from hospital. Patients completed the GHQ-12,62 the FAI,39 the Barthel Index (post-stroke disability), the European Quality of Life-5 Dimensions (EQ-5D)70–72 and the Client Service Receipt Inventory (CSRI). 73–75 Carers completed the GHQ-12.
Follow-up
Patients and carers were followed up by the CTRU using postal questionnaires at 6 and 12 months post recruitment. These included the questionnaires completed at baseline and in addition the LUNS questionnaire (see Chapter 4) for patients and the Caregiver Burden Scale (CBS)76 for carers. This was supported by postal and telephone reminders if questionnaires were not returned within 2 weeks. If necessary (following postal and telephone reminders), patients were contacted by telephone to complete the primary outcome measure (GHQ-12)62 at 6 months and at 12 months if no 6-month data had been obtained. The clinical research team recorded deaths, emergency outpatient treatment and hospital admissions at 6 and 12 months.
Questionnaire scoring
-
The GHQ-12 is measure of current mental health. It is a 12-item scale with a total score ranging from 0 to 36; higher scores are indicative of greater psychological distress.
-
Modified Rankin Scale – 0, no symptoms; 1, no significant disability; 2, slight disability; 3, moderate disability; 4, moderately severe disability; 5, severe disability.
-
The Barthel Index is a measure of daily functioning, specifically the activities of daily living and mobility. The total score ranges from 0 to 20, with lower scores indicating increased disability. Barthel Index (categorical):77 independent 20, mild disability 15–19, moderate disability 10–14, severe disability 0–9.
-
6CIT – the total score ranges from 0 to 28; a score of ≤ 7 indicates normal cognitive function whereas a score of > 7 indicates cognitive impairment.
-
The Edinburgh stroke case mix adjuster predicts the probability of survival free of dependency at 6 months.
-
The EQ-5D is a five-item scale able to identify 243 unique health states. The EQ-5D index varies from –0.59 for the worst possible health status to 1.0 for perfect health, with 0 on the scale representing the state of being dead.
-
The CBS is a 22-item scale measuring subjective burden score, with higher scores indicating greater carer distress. Each domain has a possible score of 1–4, with the overall score ranging from 22 to 88.
-
The FAI is a measure of instrumental activities of daily living for use with patients recovering from stroke. It is a 15-item scale with the total score ranging from 0 to 45; a low score is indicative of a low level of activity.
-
The LUNS questionnaire is a monitoring tool that has been developed to assess unmet needs consisting of 22 items, with a ‘yes’ response indicating unmet need.
The CSRI measures use of health, social care and informal resources (see Resource use data).
Outcomes
In common with other stroke rehabilitation trials78,79 the primary outcome point was at 6 months, with the final follow-up at 12 months to assess whether any intervention effect was sustained.
Primary outcome
The primary end point was the psychological outcome for stroke patients living at home as measured by the patient-reported GHQ-12 at 6 months after recruitment.
Secondary outcomes
Patients
The following patient-reported secondary outcomes were measured at 6 and 12 months after recruitment: social activity (FAI), disability (Barthel Index), health state (EQ-5D), unmet stroke-related needs (LUNS questionnaire; see Chapter 4), death, hospital readmission and institutionalisation. In addition, the psychological outcome for patients was measured using the GHQ-12 at 12 months after recruitment.
Carers
Secondary outcomes also included the following carer end points: carer self-reported GHQ-12 and CBS scores, death and institutionalisation, all measured at 6 and 12 months after recruitment.
Sample size
Initial sample size calculations, based on the primary outcome measure, GHQ-12 at 6 months, indicated that recruitment of 800 patients from 40 services would provide 90% power at the 5% significance level to detect a clinically relevant difference of 2.5 GHQ-12 points [standard deviation (SD) 7 points], as defined in the Trial of Occupational Therapy and Leisure (TOTAL). 78 This sample size accounted for loss to follow-up and clustering; the inflation factor of 1.95 was derived from a maximum cluster size of 20 and an intracluster correlation coefficient (ICC) of no greater than 0.05. 80,81 The TOTAL study achieved an 80% response rate to postal questionnaires at 6 months and a 71% response rate at 12 months. We therefore estimated the maximum loss to follow-up at 6 months as 25%. Losses to follow-up are likely to increase over time78 and the interpretation and credibility of results are difficult if losses exceed 30%. Therefore, follow-up was limited to 12 months post recruitment and the primary outcome was defined as 6 months, when there will be a higher follow-up rate.
The SCCs indicated that they were likely to receive two or three new referrals per week, of which one or two were likely to consent to inclusion in the trial. The initial aim was to recruit 40 SCC services; if 40 services recruited an average of 20 patients, this would result in the required number of patients (800) and the trial having 90% power to detect the clinically relevant difference (2.5 GHQ-12 points). Overall, 32 services were randomised, each with the aim of recruiting 25 patients, providing a power of 88%. To minimise unequal recruitment, the maximum number of patients per service was capped at 45, maintaining 85% power.
Statistical methods
Statistical analyses were based on the ITT population. All statistical testing was performed at a two-sided 5% significance level.
The ITT population was defined as all patients registered for active follow-up regardless of non-compliance with the intervention. All patients within a stroke service were analysed according to the intervention that that stroke service was randomised to. The ITT population was used for both primary and secondary analyses.
Per-protocol analysis was also undertaken in which major protocol violators or patients not receiving care from a SCC were excluded from the ITT population. The following groups of patients were excluded from the per-protocol population:
-
non-eligible patients
-
patients registered but then not referred to the SCC
-
patients referred to the SCC at baseline but who were not known to the SCC as a referred patient
-
patients not receiving any care from the SCC for the following reasons: patient declined the SCC service, the SCC was unable to contact the patient and the patient was registered but died before the first SCC assessment.
As the trial was a cluster RCT, outcome measures at follow-up were compared between the intervention group and the control group using a two-level multilevel model, with patients nested within stroke services.
Both primary analysis and secondary analysis involved a two-level linear model. The models were adjusted for the patient-level covariates (level 1) [baseline Barthel Index score (pre and post stroke); gender; age; living circumstances (living alone vs. living with carer); stroke severity, reflected by speech and language impairment (normal/impaired) and baseline 6CIT score (normal/impaired), and patient baseline score for the outcome measure] and the following stroke unit-level covariates (level 2): quality of stroke unit (NSA score), referral rate and SCCs working alone compared with working within a community MDT.
Two-level linear models for analyses of carer outcomes were adjusted for carer covariates (level 1) (carer baseline score for the outcome measure, gender, carer education, patient baseline Barthel Index score) and stroke unit covariates (level 2) [quality of stroke unit (NSA score), referral rate and SCCs working alone vs. working within a community MDT].
No interim analyses were undertaken.
All losses to follow-up because of death, dropouts and loss of contact are fully reported.
Details of patient deaths and hospital readmissions, carer deaths, any serious adverse events and related and unexpected serious adverse events are reported for each treatment group.
Sensitivity analyses to examine the robustness of the conclusion of the primary analysis were employed. Each of these analyses was compared with the primary analysis separately. The following sensitivity analyses were performed:
-
Including patients who died – patients who died were assumed to have a GHQ-12 score of 36 (worst possible outcome).
-
Only including patients returning postal questionnaires at 6 months; patients who provided primary outcome data by telephone were excluded.
-
Repeating analysis without proxy responses (the proxy response was when the whole questionnaire was completed on the patient’s behalf without consulting him or her) to assess the impact of proxy responses on the analysis of the primary end point.
-
Using data collected at 12 months for patients who did not send their questionnaires back at 6 months.
-
Accounting for all participants in the ITT population assuming data missing at random using multiple imputation. Information based on Edinburgh stroke case mix adjuster, clinical classification of stroke, patient status in terms of response compared with non-response (alive, died, too poorly) and prespecified model covariates was used to impute the missing outcomes. The analysis with imputed values was repeated using the same model as the model in the primary analysis.
The relationship between the adjusted primary outcome and compliance with care plans and completion of time logs was explored graphically.
Economic evaluation
Study question
Is SCC care under the new system of care cost-effective compared with SCC care according to usual practice, from either a health and social care perspective or a societal perspective?
Selection of alternatives
The economic evaluation was embedded within the trial and incorporated the same comparators (system of care vs. usual care) as for the outcome evaluation and the same overall design (same sample size, participants, randomisation, recruitment, etc.).
Form of evaluation
The economic evaluation was based on individual-level data collected within the trial. It assessed cost-effectiveness based on the GHQ-12 and cost–utility based on quality-adjusted life-years (QALYs) derived from the EQ-5D.
Time horizon
In keeping with the outcome evaluation, the primary end point for the economic evaluation was 6 months. We also explored findings at 12 months and over 1 year to determine whether any advantages were sustained in the longer term. The 1-year costs were estimated as the sum of the costs from the 6-month and 12-month assessments and were linked with (1) GHQ-12 values at 12 months and (2) the sum of the QALYs from the 6-month and 12-month assessments.
Effectiveness data
The GHQ-12 scores were measured as described earlier. For the estimation of QALYs, health states were measured using the EQ-5D at baseline, 6 months and 12 months. Utility weights from a UK general population survey82 were attached to these. QALYs were estimated using linear interpolation to calculate the area under the QALY curve as follows:
Resource use data
Individual-level data on use of health and social care resources and informal care were collected using a CSRI specifically adapted for this study from versions used successfully in previous large stroke rehabilitation trials. 73–75 It was administered retrospectively as a self-complete questionnaire alongside other measures, predominantly in hospital for the baseline assessment (which measured the previous 3 months) and as a postal questionnaire at 6 months and 12 months (each of which measured the previous 6 months).
We also prospectively measured the duration of SCC inputs (of both a contact and a non-contact nature) at the individual patient level in both the intervention group and the control group. In the intervention group, these inputs were measured as part of the care plan. In the control group, staff recorded equivalent inputs into a specifically designed time log. Staff were also asked to report their pay band to enable costs to be estimated by staff level.
We further conducted a general survey of SCCs at all services to gather information about typical inputs (e.g. typical duration of a patient assessment, typical duration spent discussing a patient in a MDT meeting) across all of their patients, not just trial participants, to obtain service-relevant imputation values in the event of missing trial data for trial participants because of non-completed/partially completed or misplaced care plans or time logs.
Unit costs
Unit cost estimates, their sources and any assumptions made for their estimation are detailed in Appendix 7 and summarised in Table 3. Unit costs were standardised at 2010/11 levels and national estimates were used when possible to represent the geographical spread of the sites and facilitate the generalisability of the results.
| Category | Unit | Unit cost (£, 2010/11) |
|---|---|---|
| Residential care home stay | Night | 75 |
| Nursing home stay | Night | 76 |
| Inpatient services | Bed-day | Range 315–1213 |
| Day hospital/day case services | Activity | Range 230–1190 |
| Outpatient services | Visit | Range 3–772 |
| Primary care/community-based services | Contact/hour/item | Range 9–152 |
| Value of caregiver time – average wage | Hour | 15 |
| Value of caregiver time – leisure time | Hour | 5 |
| SCC | Hour | Range 19–78 |
| Stroke MDT meeting | Hour | 284 |
Hospital admission costs were estimated by mapping participant-reported specialty or reason for the admission to Healthcare Resource Groups (HRGs) and then applying weighted average non-elective long-stay bed-day costs for each of those HRGs (or across HRGs when multiple specialties were reported for one admission). An average cost across all HRGs was applied when specialty and reason were missing or could not be readily allocated to a specific HRG. Outpatient costs were estimated using the same approach.
The CSRI included a question asking respondents to report the use of any other services not covered by the previous questions. Many responses to this question were for services already itemised in the instrument. We report these total ‘other’ costs separately rather than amalgamate them into the specific resource categories.
Informal care inputs were valued using the opportunity cost approach to represent the opportunities forgone by carers because of time spent caregiving. For patients with a carer enrolled in the trial, we distinguished opportunity costs as either lost employment or lost leisure on the basis of a carer’s employment status at each assessment. When it was assumed that the carer could otherwise have been working (those working part-time or unemployed seeking work), we applied the national average wage. When it was assumed that the carer was unlikely to instead be working (those working full-time, at home not seeking work, retired, redundant/early retired, unable to work and students), we applied an estimate of the cost of leisure time. We applied the average of the two unit costs when carer employment status was ‘other’ or missing and for carers outside the trial. We assumed that, if the main carer lived with the patient, all reported live-in informal care inputs were by that carer and all live-out inputs were provided by others. Conversely, if the main carer did not live with the patient, we assumed that all live-out informal care inputs were provided by that person and that all live-in inputs were provided by others.
Costs and perspectives
Unit costs were multiplied by individual-level resource use quantities at each assessment point. The total costs for each individual were computed from (1) health and social care and (2) societal perspectives. Health and social care costs included the costs of nursing/residential care; hospital inpatient, hospital outpatient, day hospital and accident and emergency (A&E) services; and primary care/community-based health/social care services. Societal costs included all of these categories plus informal care costs. It was not necessary to discount costs or outcomes because the evaluation covered only 1 year.
Cost-effectiveness and cost–utility analyses
Given the two cost perspectives and the two outcomes of interest, we examined the following four cost–outcome combinations at the primary end point of 6 months: (1) GHQ-12 and health and social care costs, (2) GHQ-12 and societal costs, (3) QALYs and health and social care costs and (4) QALYs and societal costs. Considering cost-effectiveness and cost–utility at 12 months and over 1 year involved examining the same cost–outcome combination set twice more. There were thus a total of 12 cost–outcome combinations to consider.
We aimed to calculate incremental cost-effectiveness ratios (ICERs) only for any cost–outcome combinations for which there were statistically significant higher costs and greater benefits for one group compared with the other.
We further examined uncertainty around the cost-effectiveness and cost–utility of the system of care using cost-effectiveness planes and cost-effectiveness acceptability curves (CEACs) based on the net benefit approach. 83
Cost-effectiveness planes plot between-group mean differences in total health and social care costs, GHQ-12 scores and QALYs at 6 months. Differences were calculated using bootstrapped regressions (5000 replications) with an adjustment for cluster and the same patient-level baseline covariates as used for other group comparisons (see Statistical analyses).
Net benefits provide a single summary monetary measure of costs and outcomes for each individual (removing the need to examine ICERs, which have limitations as they are based on point estimates and it is difficult to estimate CIs around them). Net benefits account for the value (λ) that a decision-maker would be willing to pay for a greater net benefit and are calculated as follows: net benefit = (λ × outcome) – cost. For each cost–outcome combination, we calculated a series of net benefits for a range of relevant λ values (£0–2000 per point improvement on the GHQ-12 and £0–50,000 per QALY gain). Net benefits were then compared by randomisation group using bootstrapped regressions (5000 replications) of study group on net benefit, with an adjustment for cluster and the same patient-level baseline covariates as used for other group comparisons (see Statistical analyses). For each value of λ, the proportion of iterations indicating a higher net benefit for the intervention group was calculated and plotted as a CEAC. CEACs are an alternative to CIs around ICERs and show the probability of the system of care being cost-effective (or optimal) compared with usual care for a range of values that a decision-maker may be willing to pay for an additional unit gain in the GHQ-12 or QALYs.
Statistical analyses
All cost and QALY data are reported as mean values with SDs. To accommodate a cluster randomisation design, differences in costs and QALYs between groups were tested by multilevel modelling using the xtreg procedure in Stata 10.1 (StataCorp LP, College Station, TX, USA), from which we report 95% CIs and p-values for the differences in means. Relevant baseline patient characteristics were included as covariates for comparisons at follow-ups: Barthel Index (pre and post stroke), gender, age, living circumstances (living alone vs. living with carer), stroke severity as represented by speech and language impairment (normal/impaired) and 6CIT score (normal/impaired), utility and GHQ-12 score (and total cost from the relevant cost perspective for the comparison of costs). Data were analysed using the same ITT approach as for the outcome evaluation, with individuals analysed according to the group to which they were randomised regardless of compliance with the intervention. Resource use differences were not compared statistically to avoid problems associated with multiple testing.
Missing data
As for the outcomes evaluation, the base-case economic evaluation was a completers analysis without imputation for loss to follow-up under the assumption that loss to follow-up was at random. We report CSRI and EQ-5D completion rates and describe baseline characteristics of those with and without these data at the primary end point, 6 months. We also report rates for the combined availability of CSRI/GHQ-12 and CSRI/EQ-5D data for the cost-effectiveness and cost–utility analyses respectively.
Missing GHQ-12 and EQ-5D data were not imputed. Resource use data from the CSRI formed the basis of the total cost calculations for each participant. Self-completed applications of such complex instruments inevitably include missing items on returned questionnaires and, to allow the computation of total costs that reflect variations in resource use rather than variations in data completeness, we imputed missing cost items. For returned questionnaires, if there was no report of use of a particular resource, we assumed that it was not used and thus imputed a zero cost. When use of a resource was reported without specifying quantity, we imputed the cost of that resource based on the mean cost for participants with data for that item at the same assessment point and in the same randomisation group (or the other randomisation group if there were no valid cases to impute from in the same group). In the case of hospital admissions, we used an average admission cost from NHS reference costs,82 rather than mean values from within the sample. All such imputations were made to cost estimates, rather than to the resource use data, so any descriptions of resource use data include no imputations.
It was similarly necessary to impute missing data related to SCC inputs. Costs for each SCC input were imputed separately (rather than the total SCC cost) to make use of any input data that were present. For patients in both groups, we assumed a zero cost if a care plan or time log was not completed for the following reasons: referral not received by the SCC service, patient declined the SCC service, patient died before receiving the SCC service, SCC unable to contact the patient or patient withdrew from all follow-up. If a care plan/time log was completed and the duration of input was missing, we imputed values from the SCC survey when available (by service) or else used the within-group mean cost for those with care plans/time logs and data for the relevant component. If a patient received a SCC service but no care plan/time log was completed, we imputed the mean cost for those with data in the control group.
For carers in the intervention group, we assumed a zero cost if an assessment did not take place, if it was unknown whether one took place or if there was no consenting carer. If an assessment took place but the duration was missing, we allocated the mean cost for those with completed assessments and data for that component. Data for inputs to non-consenting carers were found to be complete across all components. We assumed a zero cost in the absence of any identified non-consenting carer.
Sensitivity analyses
We examined the effect of loss to follow-up by imputing missing health and social care costs and QALYs at the primary end point, 6 months, and comparing group differences with those in the base-case analyses. We report the same statistics around means and mean differences (using the same covariates for comparisons of means) as for the base-case analyses and recomputed alternative ICERs and CEACs based on the alternative values if group comparisons suggested different cost or QALY conclusions compared with the base-case analyses. For those who were lost to follow-up because of death, we computed QALY gains assuming a utility value of zero at 6 months and included costs of SCC inputs while assuming that all other health and social care costs were zero. For those lost to follow-up for any other reason, we imputed total costs and QALYs using the multiple imputation procedure in Stata 10.1. Imputations were based on key baseline variables expected to predict follow-up costs and QALYs. These were the same variables used as covariates for the comparisons of costs and QALYs for those with data, plus randomisation group and three service-level variables: at the patient level – baseline Barthel Index (pre and post stroke), gender, age, living circumstances (living alone vs. living with carer), stroke severity as represented by speech and language impairment (normal/impaired) and 6CIT score (normal/impaired), utility and GHQ-12 score (and total cost from the relevant cost perspective for the prediction of costs); at the service level – quality of stroke unit (NSA score), referral rate and SCCs working alone compared with working within a community MDT.
Results
The trial was a pragmatic, multicentre, cluster RCT of 800 stroke patients requiring community-based longer-term stroke care and their carers if appropriate. The unit of randomisation was at the level of the stroke service.
Participant flow
The Consolidated Standards of Reporting Trials (CONSORT) flow diagram depicting the progress of participants and clusters through the phases of the trial, including assessment of eligibility of clusters, cluster randomisation, participant screening, recruitment and follow-up and the ITT populations, is provided in Figure 3, with additional detail given in Appendix 8 (see Tables 76–79).
FIGURE 3.
CONSORT flow diagram. PI, principal investigator.
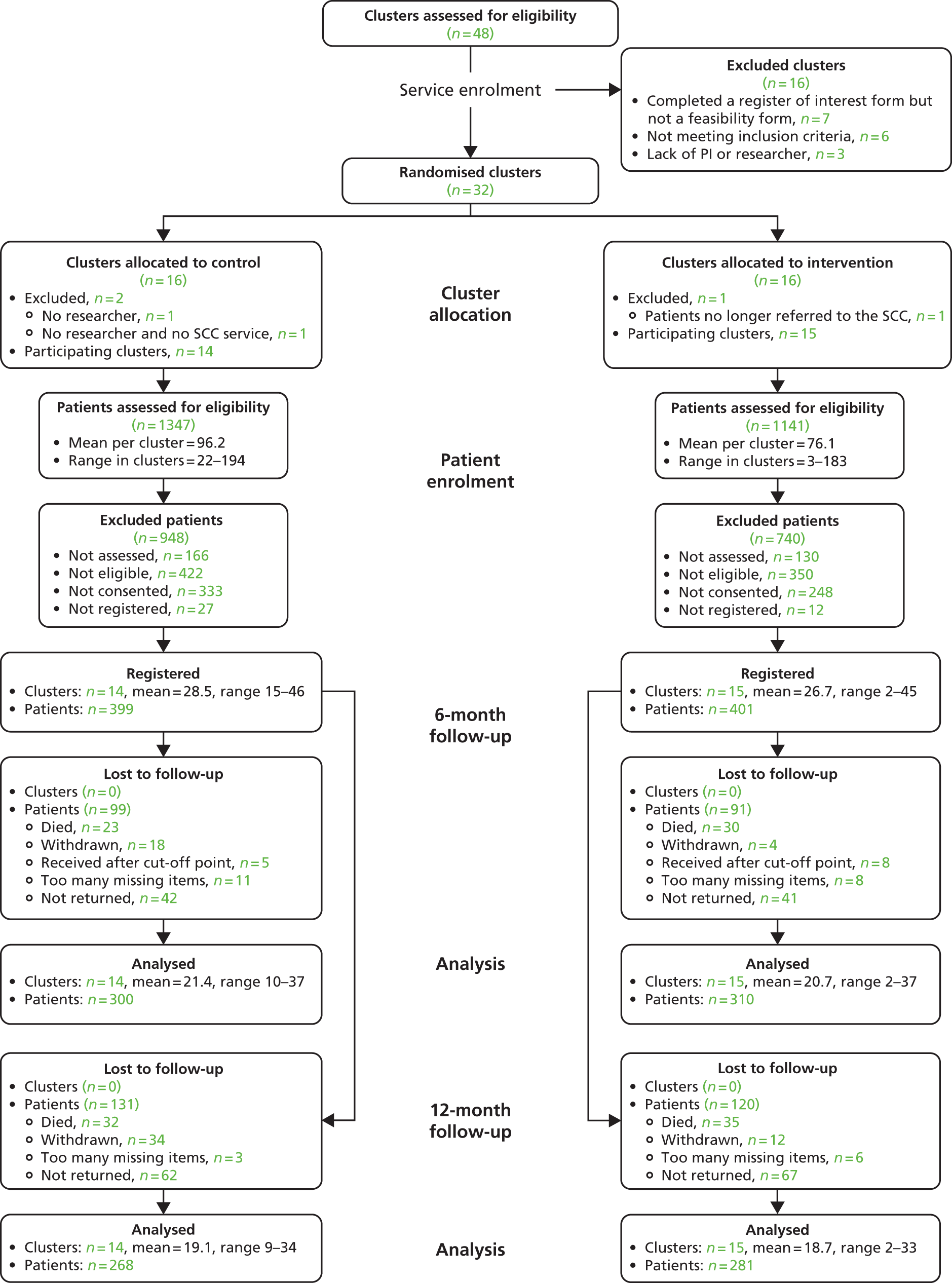
In total, 32 clusters were randomised and, of these, 29 participated (14 control clusters and 15 intervention).
During screening, 2488 participants were screened for eligibility, 1347 in the control group and 1141 in the intervention group.
The numbers of registered patients were balanced between the two arms (399 control, 401 intervention). At all time points the number of clusters remained the same (14 control, 15 intervention). The numbers of registered carers were also balanced (100 carers were recruited in the control group and 108 in the intervention group).
Response rates for patient-reported outcomes at 6 months were 75.2% (300 patients) in the control group and 77.3% (310 patients) in the intervention group; these dropped to 67.2% (268 patients) and 70% (281 patients), respectively, at 12 months’ follow-up.
Response rates for carer-completed outcomes at 6 months were 88% (88 carers) in the control group and 82.4% (89 carers) in the intervention group; these dropped to 71% (71 carers) and 67.3% (73 carers), respectively, at 12 months’ follow-up.
Recruitment
Service eligibility and randomisation were undertaken in two phases. Fourteen services were randomised in phase 1 in January 2009. In phase 2, in September 2009, an additional 18 services were randomised. Following the training and implementation period (intervention services), participant recruitment began in July 2009 in phase 1 and in March 2010 in phase 2, which is reflected in the steep increase in monthly accrual (Figure 4). Overall, 800 patients were registered from July 2009 to March 2011, 399 patients from 14 control services and 401 patients from 15 intervention services. Follow-up was completed in May 2012.
FIGURE 4.
Accrual into the trial.
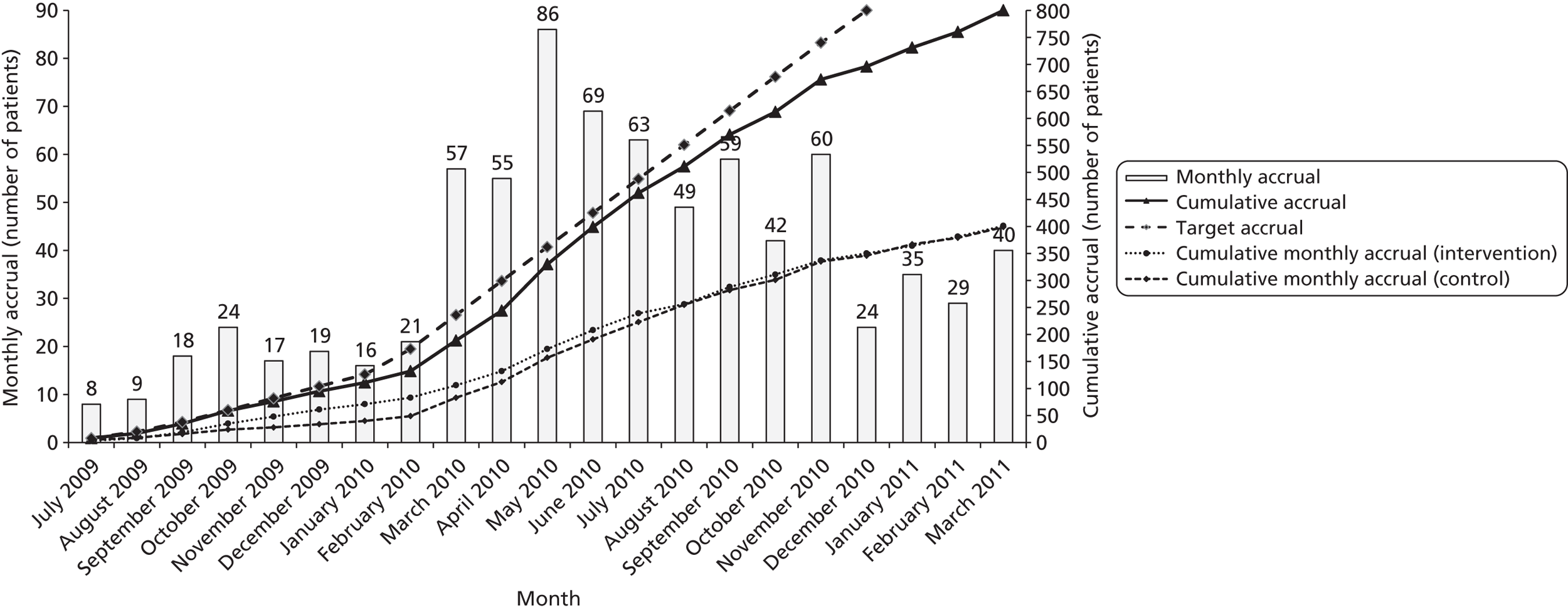
Randomised services
The characteristics of the randomised services are shown in Table 4. Both arms are balanced in terms of SCC teamworking, referral rate and NSA 2006 score. Balance based on SHA was difficult to achieve because of the requirement to randomise 32 services from 11 SHAs. Of 16 control services, two clusters dropped out post randomisation. One had no researcher and one had neither a researcher nor a SCC service. Of 16 intervention clusters, one cluster dropped out as patients were no longer referred to the SCC.
| Centre characteristic | Control (N = 16), n (%) | Intervention (N = 16), n (%) | Total (N = 32), n (%) |
|---|---|---|---|
| SHA | |||
| North East | 1 (6.3) | 2 (12.5) | 3 (9.4) |
| North West | 2 (12.5) | 4 (25.0) | 6 (18.8) |
| Northern Ireland | 2 (12.5) | 1 (6.3) | 3 (9.4) |
| Wales | 0 (0.0) | 2 (12.5) | 2 (6.3) |
| West Midlands | 2 (12.5) | 1 (6.3) | 3 (9.4) |
| South East Coast | 2 (12.5) | 1 (6.3) | 3 (9.4) |
| South Central | 1 (6.3) | 0 (0.0) | 1 (3.1) |
| South West | 3 (18.8) | 3 (18.8) | 6 (18.8) |
| East of England | 1 (6.3) | 1 (6.3) | 2 (6.3) |
| Scotland | 1 (6.3) | 1 (6.3) | 2 (6.3) |
| East Midlands | 1 (6.3) | 0 (0.0) | 1 (3.1) |
| SCC | |||
| Individual | 4 (25.0) | 4 (25.0) | 8 (25.0) |
| Team | 12 (75.0) | 12 (75.0) | 24 (75.0) |
| Referral rate | |||
| Mean (SD) | 190.3 (88.63) | 178.9 (126.16) | 184.6 (107.41) |
| Median (range) | 185.0 (44.0–304.0) | 148.5 (38.0–539.0) | 161.5 (38.0–539.0) |
| NSA 2006 score | |||
| Mean (SD) | 63.8 (11.04) | 62.4 (10.26) | 63.1 (10.50) |
| Median (range) | 64.0 (49.0–80.0) | 65.0 (42.0–76.0) | 64.5 (42.0–80.0) |
Patient screening
Anonymised information for each patient referral to the participating SCC was reported to the CTRU on a monthly basis. Each service screened patients for eligibility to participate in the trial, with 1347 patients screened in the control services and 1141 screened in the intervention services (Table 5). Details of participant non-registration were collected, including reasons for participants not being screened, assessed, eligible, consented and registered (see Appendix 8, Tables 67–70).
| Control (N = 1347), n (%) | Intervention (N = 1141), n (%) | Total (N = 2488), n (%) | |
|---|---|---|---|
| Assessed | 1181 (87.7) | 1011 (88.6) | 2192 (88.1) |
| Eligible | 759 (56.3) | 661 (57.9) | 1420 (57.1) |
| Consented | 426 (31.6) | 413 (36.2) | 839 (33.7) |
| Registered | 399 (29.6) | 401 (35.1) | 800 (32.2) |
Of the screened patients, 399/1347 (29.6%) were registered in control services and 401/1141 (35.1%) were registered in intervention services. There were more patients screened and subsequently found not to be eligible in control services than in intervention services; three control sites (3, 14 and 15) screened large numbers of participants (see Appendix 8, Table 67) but a higher than average percentage of patients from these sites were not registered in the trial (see Appendix 8, Tables 68–70).
The demographics of screened patients including patient age, gender, ethnicity and modified Rankin Scale score were collected and are presented in Tables 6 and 7. These data show balance between the two treatment arms. Demographic screening data were also collected before the start of recruitment and are shown in Appendix 8 (see Tables 65 and 66); there were no major differences between screening data pre and post recruitment.
| Characteristic | Control (N = 1347), n (%) | Intervention (N = 1141), n (%) | Total (N = 2488), n (%) |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 75.1 (12.77) | 74.3 (13.17) | 74.7 (12.95) |
| Median (range) | 78.0 (23.0–100.0) | 76.0 (22.0–100.0) | 77.0 (22.0–100.0) |
| Missing | 8 | 3 | 11 |
| Gender | |||
| Male | 668 (49.6) | 562 (49.3) | 1230 (49.4) |
| Ethnicity | |||
| White | 1278 (94.9) | 1101 (96.5) | 2379 (95.6) |
| Modified Rankin Scale score | |||
| 0 | 44 (3.3) | 41 (3.6) | 85 (3.4) |
| 1 | 233 (17.3) | 167 (14.6) | 400 (16.1) |
| 2 | 203 (15.1) | 140 (12.3) | 343 (13.8) |
| 3 | 250 (18.6) | 221 (19.4) | 471 (18.9) |
| 4 | 352 (26.1) | 380 (33.3) | 732 (29.4) |
| 5 | 170 (12.6) | 156 (13.7) | 326 (13.1) |
| Missing | 95 (7.1) | 36 (3.2) | 131 (5.3) |
| Modified Rankin Scale score | Control (N = 399), n (%) | Intervention (N = 401), n (%) | Total (N = 800), n (%) |
|---|---|---|---|
| 0 | 24 (6.0) | 13 (3.2) | 37 (4.6) |
| 1 | 99 (24.8) | 81 (20.2) | 180 (22.5) |
| 2 | 76 (19.0) | 81 (20.2) | 157 (19.6) |
| 3 | 90 (22.6) | 92 (22.9) | 182 (22.8) |
| 4 | 82 (20.6) | 102 (25.4) | 184 (23.0) |
| 5 | 4 (1.0) | 21 (5.2) | 25 (3.1) |
| Missing | 24 (6.0) | 11 (2.7) | 35 (4.4) |
Baseline data
Patients
The clinical research team recorded patients’ baseline characteristics before registration. Patients’ characteristics at baseline related to their demographic data, living circumstances, education, employment, characteristics of current stroke and current verbal, physical and cognitive ability, are shown in Tables 8–11 (see also Table 15).
| Characteristic | Control (N = 399), n (%) | Intervention (N = 401), n (%) | Total (N = 800), n (%) |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 72.5 (12.84) | 70.9 (13.18) | 71.7 (13.03) |
| Median (range) | 74.8 (23.2–98.8) | 72.6 (22.9–95.7) | 73.6 (22.9–98.8) |
| Missing | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| Ethnicity | |||
| White | 389 (97.5) | 388 (96.8) | 777 (97.1) |
| Mixed – white and black Caribbean | 0 (0.0) | 2 (0.5) | 2 (0.3) |
| Asian – Indian | 3 (0.8) | 4 (1.0) | 7 (0.9) |
| Asian – Pakistani | 2 (0.5) | 0 (0.0) | 2 (0.3) |
| Asian – Bangladeshi | 0 (0.0) | 1 (0.2) | 1 (0.1) |
| Other Asian background | 1 (0.3) | 1 (0.2) | 2 (0.3) |
| Black – Caribbean | 2 (0.5) | 3 (0.7) | 5 (0.6) |
| Other black background | 1 (0.3) | 1 (0.2) | 2 (0.3) |
| Other ethnic group | 1 (0.3) | 1 (0.2) | 2 (0.3) |
| Gender | |||
| Male | 218 (54.6) | 215 (53.6) | 433 (54.1) |
| Female | 181 (45.4) | 186 (46.4) | 367 (45.9) |
| Preferred language | |||
| English | 398 (99.7) | 391 (97.5) | 789 (98.6) |
| Other | 1 (0.3) | 10 (2.5) | 11 (1.4) |
| If other, can read and understand English? | |||
| Yes | 0 (0.0) | 7 (70.0) | 7 (63.6) |
| No | 1 (100.0) | 3 (30.0) | 4 (36.4) |
| Lived alone before stroke? | |||
| Yes | 149 (37.3) | 132 (32.9) | 281 (35.1) |
| No | 249 (62.4) | 269 (67.1) | 518 (64.8) |
| Missing | 1 (0.3) | 0 (0.0) | 1 (0.1) |
| What are living arrangements post stroke? | |||
| Live alone | 138 (34.6) | 118 (29.4) | 256 (32.0) |
| Cohabit with identified carer | 105 (26.3) | 114 (28.4) | 219 (27.4) |
| Cohabit with other | 153 (38.3) | 169 (42.1) | 322 (40.3) |
| Missing | 3 (0.8) | 0 (0.0) | 3 (0.4) |
| Characteristic | Control (N = 399), n (%) | Intervention (N = 401), n (%) | Total (N = 800), n (%) |
|---|---|---|---|
| Has the patient had any formal education? | |||
| Yes | 383 (96.0) | 380 (94.8) | 763 (95.4) |
| No | 15 (3.8) | 21 (5.2) | 36 (4.5) |
| Missing | 1 (0.3) | 0 (0.0) | 1 (0.1) |
| If yes, at what age did the patient leave education? | |||
| ≤ 16 years | 282 (73.6) | 319 (83.9) | 601 (78.8) |
| 17–20 years | 83 (21.7) | 48 (12.6) | 131 (17.2) |
| ≥ 21 years | 14 (3.7) | 12 (3.2) | 26 (3.4) |
| Unknown | 4 (1.0) | 1 (0.3) | 5 (0.7) |
| If yes, has the patient received more education since leaving school? | |||
| Yes | 107 (27.9) | 131 (34.5) | 238 (31.2) |
| No | 264 (68.9) | 245 (64.5) | 509 (66.7) |
| Missing | 12 (3.1) | 4 (1.1) | 16 (2.1) |
| Main employment before stroke | |||
| Working full time (≥ 30 hours per week) | 46 (11.5) | 44 (11.0) | 90 (11.3) |
| Working part time (< 30 hours per week) | 23 (5.8) | 20 (5.0) | 43 (5.4) |
| At home and not looking for work (e.g. looking after home/family) | 15 (3.8) | 15 (3.7) | 30 (3.8) |
| Unemployed and looking for work | 7 (1.8) | 8 (2.0) | 15 (1.9) |
| Retired | 295 (73.9) | 287 (71.6) | 582 (72.8) |
| Made redundant/took early retirement | 2 (0.5) | 5 (1.2) | 7 (0.9) |
| Unable to work (for medical and/or other reasons) | 10 (2.5) | 20 (5.0) | 30 (3.8) |
| Other | 1 (0.3) | 2 (0.5) | 3 (0.4) |
| Characteristic | Control (N = 399), n (%) | Intervention (N = 401), n (%) | Total (N = 800), n (%) |
|---|---|---|---|
| Source of referral to the SCC | |||
| Prior to hospital discharge (including community hospital) | 369 (92.5) | 354 (88.3) | 723 (90.4) |
| After discharge from hospital (including community hospital) | 30 (7.5) | 42 (10.5) | 72 (9.0) |
| From the community | 0 (0.0) | 1 (0.2) | 1 (0.1) |
| Missing | 0 (0.0) | 4 (1.0) | 4 (0.5) |
| Pathological classification of current stroke | |||
| Cerebral infarction | 343 (86.0) | 341 (85.0) | 684 (85.5) |
| Primary intracerebral haemorrhage | 39 (9.8) | 50 (12.5) | 89 (11.1) |
| Unclassified | 17 (4.3) | 10 (2.5) | 27 (3.4) |
| Clinical classification of stroke symptoms | |||
| Left hemiparesis | 179 (44.9) | 178 (44.4) | 357 (44.6) |
| Right hemiparesis | 165 (41.4) | 164 (40.9) | 329 (41.1) |
| Brain stem | 9 (2.3) | 11 (2.7) | 20 (2.5) |
| Unclassified | 46 (11.5) | 48 (12.0) | 94 (11.8) |
| Has the patient had a previous stroke? | |||
| Yes | 60 (15.0) | 84 (20.9) | 144 (18.0) |
| No | 336 (84.2) | 315 (78.6) | 651 (81.4) |
| Missing | 3 (0.8) | 2 (0.5) | 5 (0.6) |
| Overall time from admission to discharge for patients with a hospital stay related to this stroke incident (days) | |||
| Mean (SD) | 29.5 (34.9) | 38.9 (44.4) | 34.2 (40.2) |
| Median (range) | 16 (1–305) | 23 (1–306) | 20 (1–306) |
| Missing | 2 (0.5) | 2 (0.5) | 4 (0.5) |
| No. of patients with a hospital stay related to this stroke | 389 (97.5) | 391 (97.5) | 780 (97.5) |
| Hospital stay not related to this stroke | 3 (0.8) | 3 (0.7) | 6 (0.8) |
| Hospital admission did not include a stay in a stroke unit | 5 (1.3) | 5 (1.3) | 10 (1.3) |
| Characteristic | Control (N = 399), n (%) | Intervention (N = 401), n (%) | Total (N = 800), n (%) |
|---|---|---|---|
| Can the patient talk and is he or she orientated? | |||
| Yes | 387 (97.0) | 378 (94.3) | 765 (95.6) |
| No | 12 (3.0) | 22 (5.5) | 34 (4.3) |
| Missing | 0 (0.0) | 1 (0.2) | 1 (0.1) |
| Can the patient lift both arms off the bed? | |||
| Yes | 341 (85.5) | 333 (83.0) | 674 (84.3) |
| No | 56 (14.0) | 68 (17.0) | 124 (15.5) |
| Missing | 2 (0.5) | 0 (0.0) | 2 (0.3) |
| Can the patient walk without help from others? | |||
| Yes | 262 (65.7) | 257 (64.1) | 519 (64.9) |
| No | 135 (33.8) | 144 (35.9) | 279 (34.9) |
| Missing | 2 (0.5) | 0 (0.0) | 2 (0.3) |
| Is the patient’s language ability normal? | |||
| Yes | 323 (81.0) | 295 (73.6) | 618 (77.3) |
| No | 76 (19.0) | 104 (25.9) | 180 (22.5) |
| Missing | 0 (0.0) | 2 (0.5) | 2 (0.3) |
| If not, does the patient have dysphasia? | |||
| Yes | 56 (73.7) | 80 (76.9) | 136 (75.6) |
| No | 20 (26.3) | 24 (23.1) | 44 (24.4) |
| If the patient has dysphasia, what type of dysphasia? | |||
| Receptive | 3 (5.4) | 1 (1.3) | 4 (2.9) |
| Expressive | 42 (75.0) | 65 (81.3) | 107 (78.7) |
| Global | 5 (8.9) | 4 (5.0) | 9 (6.6) |
| Missing | 6 (10.7) | 10 (12.5) | 16 (11.8) |
| If not, does the patient have dysarthria? | |||
| Yes | 29 (38.2) | 30 (28.8) | 59 (32.8) |
| No | 47 (61.8) | 74 (71.2) | 121 (67.2) |
| Was the patient able to answer the 6CIT? | |||
| Yes | 366 (91.7) | 361 (90.0) | 727 (90.9) |
| No | 33 (8.3) | 40 (10.0) | 73 (9.1) |
| If not, why not? | |||
| Stroke-related communication problems | 30 (90.9) | 31 (77.5) | 61 (83.6) |
| Other | 3 (9.1) | 9 (22.5) | 12 (16.4) |
The mean patient age in control services was 72.5 years (SD 12.84 years) and in intervention services was 70.9 years (SD 13.18 years). The majority of patients were of white ethnic origin [389 (97.5%) control, 388 (96.8%) intervention]. The observed ethnic breakdown is comparable to the ethnic breakdown of the population aged ≥ 65 years in England in 2001. 85 There were more men than women registered to the trial [218 (54.6%) control, 215 (53.6%) in intervention]. In total, 383 (96.0%) in the control services and 380 (94.8%) in the intervention services had received formal education and 282 (73.6%) in the control services and 319 (83.9%) in the intervention services left education at the age of ≤ 16 years.
Before stroke, 149 (37.3%) patients in control services and 132 (32.9%) patients in intervention services lived alone; post stroke, 138 (34.6%) patients in control services and 118 (29.4%) patients in intervention services planned to live alone. The majority of patients in both treatment arms suffered a cerebral infarction [343 (86.0%) control, 341 (85.0%) intervention]. In control services, 179 (44.9%) patients had left hemiparesis and 165 (41.4%) patients had right hemiparesis; in intervention services, 178 (44.4%) patients had left hemiparesis and 164 (40.9%) patients had right hemiparesis. In total, 60 (15.0%) patients in control services and 84 (20.9%) patients in intervention services had had a previous stroke.
Patient language ability was classified as normal in 323 (81.0%) patients in control services and 295 (73.6%) patients in intervention services. Cognitive function was measured using the 6CIT, with 267 (66.9%) patients in control services and 229 (57.1%) patients in intervention services having normal cognitive function.
Both treatment arms are balanced across the majority of baseline variables; differences in age when left education (see Table 9), language ability (see Table 11) and cognitive ability (see Table 15) are accounted for in statistical modelling of primary and secondary end points. Differences in the length of inpatient stay (see Table 10) could not be used as a covariate as not all patients had an inpatient stay.
The baseline demographic characteristics of patients with carers registered in the trial are shown in Appendix 8 (see Tables 71–75).
Carers
In total, 208 carers consented to participate in the trial, 108 in the intervention group and 100 in the control group. Carer baseline demographics in terms of age, gender, ethnicity, relationship to patient and characteristics of carer education and employment are balanced between the two arms (Tables 12–14).
| Characteristic | Control (N = 100), n (%) | Intervention (N = 108), n (%) | Total (N = 208), n (%) |
|---|---|---|---|
| Carer age (years) | |||
| Mean (SD) | 61.4 (14.07) | 61.0 (15.02) | 61.2 (14.54) |
| Median (range) | 62.8 (19.4–87.4) | 60.7 (23.6–89.6) | 62.3 (19.4–89.6) |
| Missing | 1.0 (1.0) | 0.0 (0.0) | 1.0 (0.5) |
| Ethnicity | |||
| White | 99 (99.0) | 105 (97.2) | 204 (98.1) |
| Asian – Indian | 0 (0.0) | 1 (0.9) | 1 (0.5) |
| Asian – Pakistani | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Asian – Bangladeshi | 0 (0.0) | 1 (0.9) | 1 (0.5) |
| Other ethnic group | 0 (0.0) | 1 (0.9) | 1 (0.5) |
| Gender | |||
| Male | 32 (32.0) | 35 (32.4) | 67 (32.2) |
| Female | 68 (68.0) | 73 (67.6) | 141 (67.8) |
| Preferred language | |||
| English | 99 (99.0) | 107 (99.1) | 206 (99.0) |
| Other | 1 (1.0) | 1 (0.9) | 2 (1.0) |
| If other, can read and understand English? | |||
| Yes | 1 (100.0) | 0 (0.0) | 1 (50.0) |
| No | 0 (0.0) | 1 (100.0) | 1 (50.0) |
| Characteristic | Control (N = 100), n (%) | Intervention (N = 108), n (%) | Total (N = 208), n (%) |
|---|---|---|---|
| Carer–patient relationship | |||
| Partner (married/never married/divorced/separated) | 67 (67.0) | 70 (64.8) | 137 (65.9) |
| Daughter/son (including in-law, stepchild) | 29 (29.0) | 33 (30.6) | 62 (29.8) |
| Grandchild | 0 (0.0) | 1 (0.9) | 1 (0.5) |
| Other relative | 3 (3.0) | 3 (2.8) | 6 (2.9) |
| Friend/neighbour | 1 (1.0) | 1 (0.9) | 2 (1.0) |
| Carer place of residence | |||
| Did the carer reside with the patient in the last 12 months? | |||
| Yes | 75 (75.0) | 81 (75.0) | 156 (75.0) |
| No | 25 (25.0) | 27 (25.0) | 52 (25.0) |
| Is the planned residence of the carer with the patient? | |||
| Yes | 78 (78.0) | 85 (78.7) | 163 (78.4) |
| No | 22 (22.0) | 23 (21.3) | 45 (21.6) |
| Characteristic | Control (N = 100), n (%) | Intervention (N = 108), n (%) | Total (N = 208), n (%) |
|---|---|---|---|
| Has the carer had any formal education? | |||
| Yes | 97 (97.0) | 103 (95.4) | 200 (96.2) |
| No | 3 (3.0) | 5 (4.6) | 8 (3.8) |
| If yes, age that the carer left full-time education | |||
| ≤ 16 years | 68 (70.1) | 76 (73.8) | 144 (72.0) |
| 17–20 years | 19 (19.6) | 21 (20.4) | 40 (20.0) |
| ≥ 21 years | 7 (7.2) | 6 (5.8) | 13 (6.5) |
| Unknown | 3 (3.1) | 0 (0.0) | 3 (1.5) |
| If yes, has the carer received further education since? | |||
| Yes | 40 (41.2) | 45 (43.7) | 85 (42.5) |
| No | 53 (54.6) | 58 (56.3) | 111 (55.5) |
| Missing | 4 (4.1) | 0 (0.0) | 4 (2.0) |
| Carer main employment in the last 3 months before patient stroke | |||
| Working full time (≥ 30 hours per week) | 19 (19.0) | 20 (18.5) | 39 (18.8) |
| Working part time (≤ 30 hours per week) | 11 (11.0) | 13 (12.0) | 24 (11.5) |
| At home and not looking for work (e.g. looking after home/family) | 13 (13.0) | 15 (13.9) | 28 (13.5) |
| Unemployed and looking for work | 3 (3.0) | 2 (1.9) | 5 (2.4) |
| Retired | 48 (48.0) | 47 (43.5) | 95 (45.7) |
| Made redundant/took early retirement | 2 (2.0) | 2 (1.9) | 4 (1.9) |
| Unable to work (for medical and other reasons) | 4 (4.0) | 9 (8.3) | 13 (6.3) |
In general, carers were younger than patients, with a mean carer age of 61.4 years (SD 14.07 years) in the control group and 61.0 years (SD 15.02 years) in the intervention group (see Table 12).
Researcher-completed baseline questionnaires
The clinical research team completed additional baseline questionnaires with patients including the pre-stroke Barthel Index, the 6CIT and items necessary to derive the Edinburgh stroke case mix adjuster. Scores on these measures are summarised in Table 15. Differences in 6CIT scores are accounted for in the statistical modelling of primary and secondary end points.
| Measure | Control (N = 399), n (%) | Intervention (N = 401), n (%) | Total (N = 800), n (%) |
|---|---|---|---|
| Pre-stroke Barthel Index | |||
| Mean (SD) | 19.2 (1.98) | 19.2 (2.22) | 19.2 (2.10) |
| Median (range) | 20.0 (7.0–20.0) | 20.0 (3.0–20.0) | 20.0 (3.0–20.0) |
| Missing | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| Pre-stroke Barthel Index (categorical) | |||
| Independent | 311 (77.9) | 317 (79.1) | 628 (78.5) |
| Mild disability | 70 (17.5) | 67 (16.7) | 137 (17.1) |
| Moderate disability | 14 (3.5) | 12 (3.0) | 26 (3.3) |
| Severe disability | 4 (1.0) | 5 (1.2) | 9 (1.1) |
| 6CIT | |||
| Normal cognitive function | 267 (66.9) | 229 (57.1) | 496 (62.0) |
| Cognitive impairment | 99 (24.8) | 132 (32.9) | 231 (28.9) |
| Communication problems | 33 (8.3) | 40 (10.0) | 73 (9.1) |
| Edinburgh stroke case mix adjuster | |||
| Mean (SD) | 0.59 (0.277) | 0.59 (0.295) | 0.59 (0.286) |
| Median (range) | 0.66 (0.00–0.98) | 0.68 (0.00–0.98) | 0.67 (0.00–0.98) |
| Missing | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) |
Numbers analysed
All analyses and data summaries were carried out using the ITT population. Per-protocol analysis was undertaken for analyses of primary and secondary end points. Numbers of patients and carers in each population are shown in Table 16.
| Population | Control, n | Intervention, n | Total, n |
|---|---|---|---|
| ITT – patients | 399 | 401 | 800 |
| Per protocol – patients | 300 | 301 | 601 |
| ITT – carers | 100 | 108 | 208 |
| Per protocol – carers | 73 | 82 | 155 |
Outcomes and estimation
Patient-reported outcomes
Patient-reported outcomes were collected at three time points: baseline, 6 months and 12 months.
Unadjusted scores
Summaries of unadjusted scores for primary and secondary patient-reported outcomes at baseline and 6 and 12 months are shown in Table 17. Baseline unadjusted scores are balanced between treatment arms.
| Questionnaire | Baseline, [mean (SD)] | 6 months, [mean (SD)] | 12 months, [mean (SD)] | |||
|---|---|---|---|---|---|---|
| Control | Intervention | Control | Intervention | Control | Intervention | |
| Barthel Index n | 15.2 (4.48) 398 | 14.4 (5.06) 401 | 16.2 (4.24) 296 | 15.5 (4.48) 307 | 16.3 (3.91) 266 | 15.7 (4.26) 282 |
| GHQ-12 n | 15.2 (7.26) 396 | 15.9 (7.03) 397 | 15.3 (7.32) 305 | 16.2 (7.17) 318 | 14.5 (6.78) 268 | 14.3 (6.87) 281 |
| EQ-5D n | 0.56 (0.340) 382 | 0.51 (0.378) 381 | 0.61 (0.339) 288 | 0.56 (0.322) 301 | 0.61 (0.316) 259 | 0.54 (0.318) 270 |
| FAI n | 28.1 (9.71) 398 | 27.8 (9.70) 399 | 20.3 (11.41) 293 | 18.4 (11.27) 304 | 21.5 (11.30) 266 | 18.8 (11.53) 281 |
Adjusted scores
Two-level hierarchical modelling was used to compare the intervention and control groups, with patient-level covariates (baseline Barthel Index score, baseline GHQ-12 score, gender, age, living circumstances, stroke severity and baseline 6CIT score) being level 1 and service-level covariates (quality of stroke unit, referral rate, SCC working alone or in a team) being level 2. In all models we allowed for different variances between the treatment arms.
A summary of the adjusted scores, differences in scores with 95% CIs, p-values and adjusted ICCs for each treatment arm for the primary and secondary end points at 6 and 12 months is provided in Table 18.
| Questionnaire | Control, mean (SE) n | Intervention, mean (SE) n | Difference (SE) | 95% CI of the difference | p-value | Adjusted ICC | |
|---|---|---|---|---|---|---|---|
| Control | Intervention | ||||||
| 6 months | |||||||
| GHQ-12 | 14.9 (0.60) 300 | 15.5 (0.60) 310 | −0.6 (0.65) | −1.8 to 0.7 | 0.394 | 0.013 | 0.025 |
| Barthel Index | 15.8 (0.33) 296 | 15.3 (0.28) 307 | 0.5 (0.33) | −0.2 to 1.1 | 0.133 | 0.022 | 0.000 |
| EQ-5D | 0.58 (0.025) 288 | 0.55 (0.022) 301 | 0.03 (0.025) | −0.02 to 0.08 | 0.252 | 0.014 | 0.059 |
| FAI | 19.0 (0.76) 293 | 18.0 (0.76) 304 | 1.0 (0.80) | −0.6 to 2.5 | 0.229 | 0.000 | 0.014 |
| 12 months | |||||||
| GHQ-12 | 14.4 (0.58) 268 | 13.9 (0.72) 281 | 0.5 (0.73) | −0.9 to 2.0 | 0.454 | 0.000 | 0.063 |
| Barthel Index | 15.6 (0.36) 266 | 15.4 (0.30) 282 | 0.2 (0.37) | −0.5 to 0.9 | 0.585 | 0.049 | 0.023 |
| EQ-5D | 0.56 (0.030) 259 | 0.51 (0.028) 270 | 0.05 (0.033) | −0.02 to 0.11 | 0.167 | 0.050 | 0.044 |
| FAI | 20.2 (0.78) 266 | 18.7 (0.84) 281 | 1.5 (0.87) | −0.2 to 3.2 | 0.078 | 0.000 | 0.025 |
The primary and secondary analyses were based on a complete case analysis with no substitution for missing patient-level outcome data; numbers of patients analysed for each end point are shown in Table 18.
In the analysis of the primary end point, the adjusted GHQ-12 mean score at 6 months was 14.9 [standard error (SE) 0.6] in the control group and 15.5 (SE 0.6) in the intervention group, a difference of −0.6 points (95% CI −1.8 to 0.7 points), with a p-value of 0.394 and an adjusted ICC of 0.013 for the control group and 0.025 for the intervention group, indicating that there is no statistical evidence of a significant difference between the treatment groups in psychological outcomes for stroke patients living at home at 6 months, as measured by the GHQ-12.
Results for secondary end points (Barthel Index, EQ-5D and FAI at 6 and 12 months and GHQ-12 at 12 months; see Table 18) also indicate that there are no statistically significant differences between the treatment groups for any of the secondary end points.
Longer-term Unmet Needs after Stroke questionnaire
Summaries of the patient-completed LUNS questionnaire at 6 and 12 months, showing the number of unmet needs out of a possible 22, are provided in Tables 19 and 20 respectively. The numbers and types of unmet needs reported were similar for the control group and the intervention group at 6 and 12 months. A median of 2.5 unmet needs were reported, and the prevalence of individual unmet needs ranged from 2% to 51% of those completing the questionnaire (Figure 5). The most commonly reported unmet need was for more information; this was followed by unmet needs in the areas of falls, memory/concentration, accessible holidays and pain, all reported by over one-fifth of responders.
| No. of unmet needs at 6 months | Control (N = 399), n (%) | Intervention (N = 401), n (%) | Total (N = 800), n (%) |
|---|---|---|---|
| 0 | 57 (14.3) | 43 (10.7) | 100 (12.5) |
| 1 | 46 (11.5) | 46 (11.5) | 92 (11.5) |
| 2 | 44 (11.0) | 41 (10.2) | 85 (10.6) |
| 3 | 25 (6.3) | 39 (9.7) | 64 (8.0) |
| 4 | 28 (7.0) | 31 (7.7) | 59 (7.4) |
| 5 | 15 (3.8) | 16 (4.0) | 31 (3.9) |
| 6 | 18 (4.5) | 17 (4.2) | 35 (4.4) |
| 7 | 15 (3.8) | 18 (4.5) | 33 (4.1) |
| 8 | 13 (3.3) | 17 (4.2) | 30 (3.8) |
| 9 | 8 (2.0) | 8 (2.0) | 16 (2.0) |
| 10 | 3 (0.8) | 10 (2.5) | 13 (1.6) |
| 11 | 3 (0.8) | 5 (1.2) | 8 (1.0) |
| 12 | 7 (1.8) | 1 (0.2) | 8 (1.0) |
| 13 | 4 (1.0) | 7 (1.7) | 11 (1.4) |
| 14 | 2 (0.5) | 1 (0.2) | 3 (0.4) |
| 15 | 1 (0.3) | 1 (0.2) | 2 (0.3) |
| 16 | 0 (0.0) | 1 (0.2) | 1 (0.1) |
| 17 | 1 (0.3) | 0 (0.0) | 1 (0.1) |
| 18 | 0 (0.0) | 1 (0.2) | 1 (0.1) |
| Missing | 109 (27.3) | 98 (24.4) | 207 (25.9) |
| No. of unmet needs at 12 months | Control (N = 399), n (%) | Intervention (N = 401), n (%) | Total (N = 800), n (%) |
|---|---|---|---|
| 0 | 62 (15.5) | 62 (15.5) | 124 (15.5) |
| 1 | 35 (8.8) | 36 (9.0) | 71 (8.9) |
| 2 | 34 (8.5) | 43 (10.7) | 77 (9.6) |
| 3 | 29 (7.3) | 31 (7.7) | 60 (7.5) |
| 4 | 20 (5.0) | 28 (7.0) | 48 (6.0) |
| 5 | 21 (5.3) | 11 (2.7) | 32 (4.0) |
| 6 | 11 (2.8) | 12 (3.0) | 23 (2.9) |
| 7 | 12 (3.0) | 19 (4.7) | 31 (3.9) |
| 8 | 10 (2.5) | 8 (2.0) | 18 (2.3) |
| 9 | 10 (2.5) | 7 (1.7) | 17 (2.1) |
| 10 | 6 (1.5) | 7 (1.7) | 13 (1.6) |
| 11 | 4 (1.0) | 3 (0.7) | 7 (0.9) |
| 12 | 2 (0.5) | 6 (1.5) | 8 (1.0) |
| 13 | 3 (0.8) | 2 (0.5) | 5 (0.6) |
| 14 | 1 (0.3) | 1 (0.2) | 2 (0.3) |
| 15 | 1 (0.3) | 0 (0.0) | 1 (0.1) |
| 18 | 1 (0.3) | 0 (0.0) | 1 (0.1) |
| 21 | 0 (0.0) | 1 (0.2) | 1 (0.1) |
| Missing | 137 (34.3) | 124 (30.9) | 261 (32.6) |
FIGURE 5.
Prevalence of individual unmet needs: (a) 6 months; and (b) 12 months.
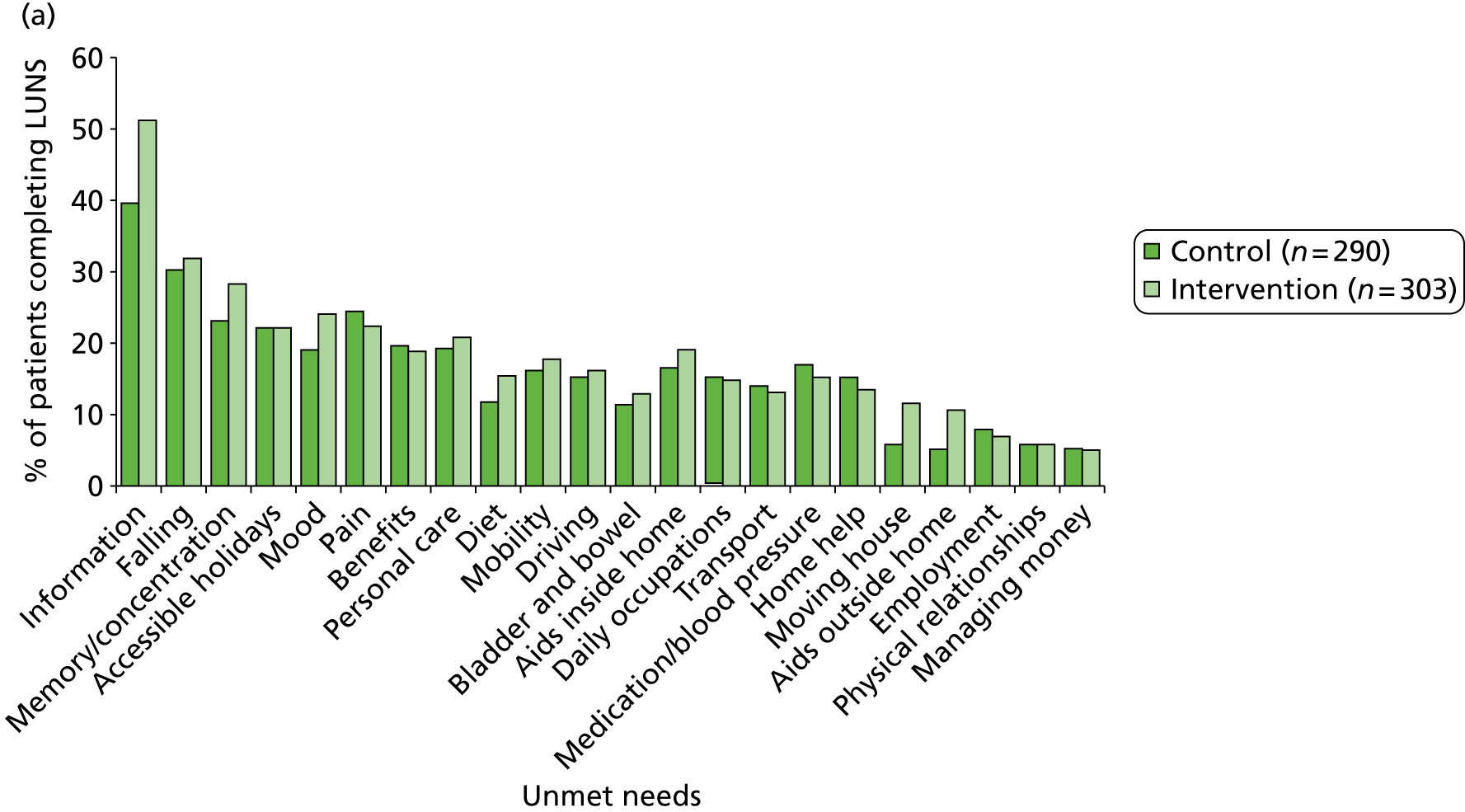
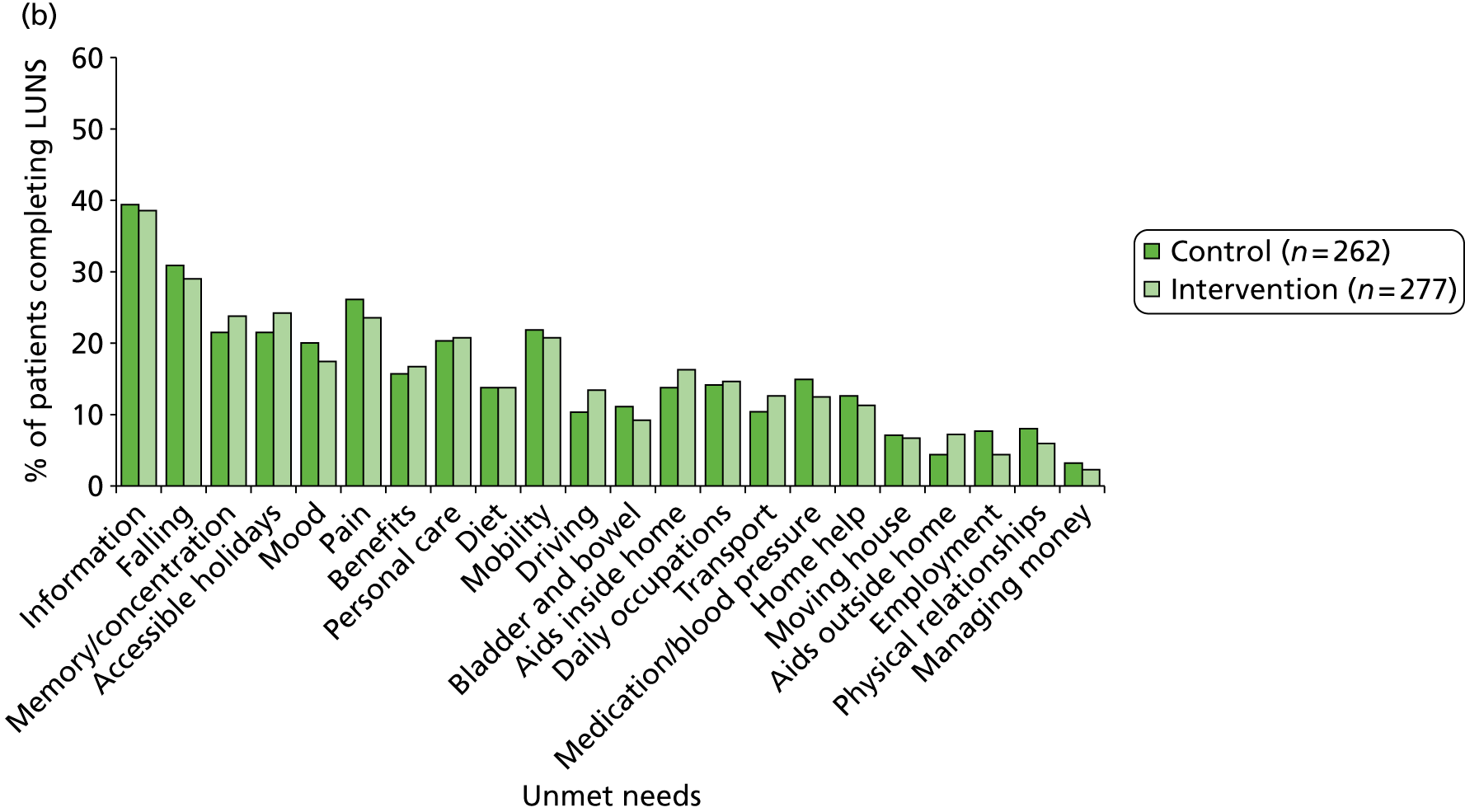
Carer-reported outcomes
Unadjusted scores
Carer unadjusted outcome scores are shown in Table 21. The carer baseline GHQ-12 score is 1.7 points higher in the intervention group than in the control group. This difference is accounted for in the statistical modelling of the carer GHQ-12 end point.
| Questionnaire | Baseline, mean (SD) n | 6 months, mean (SD) n | 12 months, mean (SD) n | |||
|---|---|---|---|---|---|---|
| Control | Intervention | Control | Intervention | Control | Intervention | |
| GHQ-12 | 14.0 (6.61) 100 | 15.7 (6.30) 108 | 12.8 (6.00) 82 | 15.3 (6.23) 80 | 13.0 (5.68) 71 | 14.3 (5.77) 73 |
| CBS | N/A | N/A | 44.8 (14.58) 81 | 48.7 (13.82) 80 | 44.3 (15.23) 71 | 48.2 (15.15) 72 |
| General strain | N/A | N/A | 2.23 (0.789) 82 | 2.48 (0.793) 80 | 2.19 (0.853) 71 | 2.45 (0.805) 72 |
| Isolation | N/A | N/A | 2.17 (0.808) 81 | 2.27 (0.772) 80 | 2.20 (0.768) 71 | 2.28 (0.840) 72 |
| Disappointment | N/A | N/A | 2.15 (0.790) 82 | 2.33 (0.812) 80 | 2.12 (0.886) 71 | 2.34 (0.807) 73 |
| Emotional involvement | N/A | N/A | 1.61 (0.625) 81 | 1.69 (0.632) 79 | 1.58 (0.585) 71 | 1.63 (0.638) 72 |
| Environment | N/A | N/A | 1.61 (0.640) 81 | 1.80 (0.688) 80 | 1.60 (0.686) 71 | 1.75 (0.725) 72 |
Adjusted scores
Carer-reported outcomes were also assessed using two-level hierarchical models allowing for different variances between the treatment arms, with carer-level covariates (carer gender, education, patient baseline Barthel Index score) being level 1 and service-level covariates (quality of stroke unit, referral rate, SCC working alone or in a team) being level 2. A summary of the adjusted scores, differences between the scores, 95% CIs, p-values and adjusted ICCs for each treatment arm at 6 and 12 months is provided in Table 22.
| Questionnaire | Control, mean (SE) n | Intervention, mean (SE) n | Difference (SE) | 95% CI of the difference | p-value | Adjusted ICC | |
|---|---|---|---|---|---|---|---|
| Control | Intervention | ||||||
| 6 months | |||||||
| GHQ-12 | 12.5 (0.69) 82 | 14.2 (0.79) 80 | −1.7 (0.91) | −3.5 to 0.1 | 0.061 | 0.000 | 0.000 |
| CBS | 44.5 (2.19) 81 | 48.4 (1.80) 80 | −3.9 (2.51) | −8.8 to 1.1 | 0.125 | 0.055 | 0.000 |
| 12 months | |||||||
| GHQ-12 | 13.5 (1.11) 71 | 13.9 (0.85) 73 | −0.4 (1.26) | −2.9 to 2.1 | 0.747 | 0.255 | 0.000 |
| CBS | 43.9 (2.85) 71 | 48.7 (2.12) 72 | −4.8 (3.17) | −11.1 to 1.5 | 0.132 | 0.137 | 0.000 |
Comparison of adjusted scores for the carer-reported outcomes of GHQ-12 and CBS at 6 and 12 months indicates that there is no evidence of statistical differences in either of these outcomes between the intervention group and the control group.
Ancillary analyses
Per-protocol analyses
Per-protocol analyses were undertaken as there were a considerable number of protocol violators and patients not receiving care from SCCs. Per-protocol analyses were also undertaken for carers of patients from the per-protocol population.
Patients
In total, 601 patients were included in the per-protocol population, 300 in the control group and 301 in the intervention group (see Table 16).
Analyses on the per-protocol population were conducted for all end points and the results of these analyses are consistent with those of the ITT analyses, indicating that there is no evidence of a statistically significant difference in any patient-reported outcomes at 6 or 12 months between the treatment arms for this population of patients (Table 23).
| Questionnaire | Control, mean (SE) n | Intervention, mean (SE) n | Difference (SE) | 95% CI of the difference | p-value | Adjusted ICC | |
|---|---|---|---|---|---|---|---|
| Control | Intervention | ||||||
| 6 months | |||||||
| GHQ-12 | 14.0 (0.69) 228 | 14.8 (0.78) 248 | −0.8 (0.76) | −2.3 to 0.7 | 0.280 | 0.000 | 0.058 |
| Barthel Index | 15.7 (0.35) 228 | 15.4 (0.34) 244 | 0.3 (0.33) | −0.3 to 1.0 | 0.310 | 0.000 | 0.000 |
| EQ-5D | 0.57 (0.028) 223 | 0.56 (0.025) 228 | 0.01 (0.026) | −0.04 to 0.07 | 0.602 | 0.007 | 0.000 |
| FAI | 18.9 (0.90) 225 | 17.9 (0.87) 467 | 1.0 (0.87) | −0.7 to 2.7 | 0.240 | 0.000 | 0.011 |
| 12 months | |||||||
| GHQ-12 | 13.8 (0.70) 201 | 12.9 (0.85) 227 | 0.8 (0.85) | −0.9 to 2.5 | 0.336 | 0.000 | 0.097 |
| Barthel Index | 15.6 (0.35) 203 | 15.6 (0.36) 226 | 0.0 (0.37) | −0.8 to 0.7 | 0.933 | 0.000 | 0.036 |
| EQ-5D | 0.55 (0.033) 199 | 0.52 (0.032) 215 | 0.03 (0.034) | −0.03 to 0.1 | 0.328 | 0.034 | 0.046 |
| FAI | 20.0 (0.93) 201 | 18.2 (0.95) 225 | 1.7 (0.94) | −0.1 to 3.6 | 0.069 | 0.000 | 0.016 |
Carers
In total, 155 carers were included in the per-protocol population, 73 in the control group and 82 in the intervention group. Results from the analyses of end points based on the per-protocol population indicate that, for this population of carers, there is no evidence of any statistically significant differences between the treatment arms (Table 24).
| Questionnaire | Control, mean (SE) n | Intervention, mean (SE) n | Difference (SE) | 95% CI of the difference | p-value | Adjusted ICC | |
|---|---|---|---|---|---|---|---|
| Control | Intervention | ||||||
| 6 months | |||||||
| GHQ-12 | 12.6 (0.82) 58 | 14.0 (0.90) 67 | −1.4 (1.04) | −3.5 to 0.7 | 0.181 | 0.000 | 0.000 |
| CBS | 45.5 (2.64) 57 | 49.7 (2.25) 67 | −4.3 (3.02) | −10.2 to 1.7 | 0.162 | 0.073 | 0.037 |
| 12 months | |||||||
| GHQ-12 | 13.7 (1.18) 51 | 14.2 (0.95) 61 | −0.50 (1.34) | −3.2 to 2.1 | 0.707 | 0.145 | 0.000 |
| CBS | 45.1 (2.80) 51 | 50.1 (2.46) 60 | −5.0 (3.22) | −11.4 to 1.3 | 0.120 | 0.054 | 0.025 |
Sensitivity analyses
Sensitivity analyses were undertaken to examine the robustness of the conclusions of the primary analysis. The results from these analyses are shown in Table 25 and support the conclusions of the primary analysis, that is, that there is no evidence of any statistically significant differences between the control group and the intervention group in terms of GHQ-12 scores.
| Analysis | Control, mean (SE) n | Intervention, mean (SE) n | Difference (SE) | 95% CI of the difference | p-value | Adjusted ICC | |
|---|---|---|---|---|---|---|---|
| Control | Intervention | ||||||
| Patients who died (GHQ-12 score assigned to be 36) | 17.5 (0.67) 325 | 18.3 (0.61) 342 | −0.8 (0.68) | −2.1 to 0.6 | 0.263 | 0.004 | 0.000 |
| Excluding those who provided the primary end point by telephone | 15.0 (0.62) 294 | 15.5 (0.60) 308 | −0.5 (0.66) | −1.8 to 0.8 | 0.436 | 0.018 | 0.027 |
| Excluding proxy responses | 14.7 (0.62) 296 | 15.2 (0.63) 294 | −0.5 (0.63) | −1.7 to 0.7 | 0.427 | 0.009 | 0.021 |
| Data from 12 months used for patients who did not return their questionnaires at 6 months | 14.9 (0.57) 319 | 15.7 (0.61) 331 | −0.7 (0.64) | −2.0 to 0.5 | 0.251 | 0.007 | 0.031 |
| Multiple imputation | 15.4 (0.59) 399 | 15.9 (0.58) 401 | −0.5 (0.59) | −1.7 to 0.6 | 0.370 | N/Aa | N/Aa |
Adverse events
Deaths, hospital readmissions, institutionalisation and treatment on an emergency outpatient basis were reported at 6 and 12 months’ follow-up by the clinical research team using health and social care records.
Deaths
Throughout the trial, no safety issues were highlighted. Overall, 67 patient deaths were reported, 32 in the control group and 35 in the intervention group; most of these occurred in hospital and within the first 6 months of registration, as shown in Table 26 and Figure 6.
| Time/Location of death | Control (N = 399), n (%) | Intervention (N = 401), n (%) | Total (N = 800), n (%) |
|---|---|---|---|
| Deaths | |||
| 6 monthsa | 27 (6.8) | 31 (7.7) | 58 (7.3) |
| 12 months | 5 (1.3) | 4 (1.0) | 9 (1.1) |
| After 12-month follow-upb | 2 (0.5) | 2 (0.5) | 4 (0.5) |
| Total | 32 (8.0) | 35 (8.7) | 67 (8.4) |
| Place of death | |||
| Home | 2 (0.5) | 4 (1.0) | 6 (0.8) |
| Hospital | 23 (5.8) | 19 (4.7) | 42 (5.3) |
| Nursing home | 3 (0.8) | 3 (0.7) | 6 (0.8) |
| Unknown | 3 (0.8) | 7 (1.7) | 10 (1.3) |
| Other | 1 (0.3) | 2 (0.5) | 3 (0.4) |
FIGURE 6.
Time between registration and patient death.
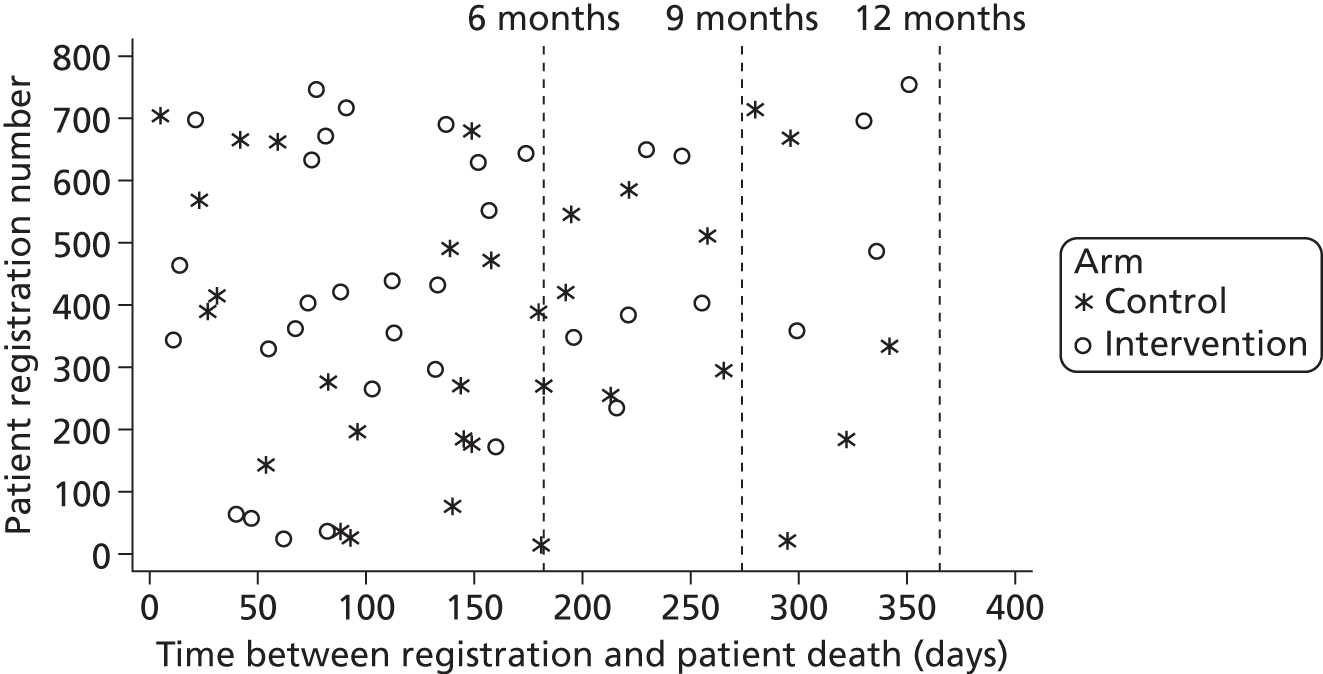
One carer death was reported in the control group, which occurred 7 days after the carer was registered to the trial.
Treatment on an emergency outpatient basis and hospital admissions
Information on further treatment on an emergency outpatient basis and hospital admissions as reported by researchers at 6 and 12 months, is summarised in Tables 27 and 28 respectively. In the first 6 months after registration, 105 (26.3%) patients in the control group and 113 (28.2%) patients in the intervention group visited an A&E department and 113 (28.3%) patients in the control group and 97 (24.2%) patients in the intervention group were admitted to hospital overnight. Between 6 and 12 months post registration, 71 (17.8%) patients in the control group and 77 (19.2%) patients in the intervention group visited an A&E department and 74 (18.5%) patients in the control group and 77 (19.2%) patients in the intervention group were admitted to hospital overnight.
| 6-month summary | Control (N = 399), n (%) | Intervention (N = 401), n (%) | Total (N = 800), n (%) |
|---|---|---|---|
| Follow-up form completed? | |||
| Yes | 395 (99.0) | 400 (99.8) | 795 (99.4) |
| Withdrawn | 4 (1.0) | 1 (0.2) | 5 (0.6) |
| Patient visited A&E in the past 6 months? | |||
| Yes | 105 (26.3) | 113 (28.2) | 218 (27.3) |
| No | 290 (72.7) | 261 (65.1) | 551 (68.9) |
| Missing | 4 (1.0) | 27 (6.7) | 31 (3.9) |
| Number of A&E visits per patient | |||
| Mean (SD) | 0.4 (0.95) | 0.4 (0.83) | 0.4 (0.89) |
| Median (range) | 0.0 (0.0–8.0) | 0.0 (0.0–7.0) | 0.0 (0.0–8.0) |
| Patient admitted to hospital overnight in the past 6 months? | |||
| Yes | 113 (28.3) | 97 (24.2) | 210 (26.3) |
| No | 282 (70.7) | 303 (75.6) | 585 (73.1) |
| Missing | 4 (1.0) | 1 (0.2) | 5 (0.6) |
| Number of hospital admissions | |||
| Mean (SD) | 0.4 (0.93) | 0.3 (0.66) | 0.4 (0.81) |
| Median (range) | 0.0 (0.0–10.0) | 0.0 (0.0–5.0) | 0.0 (0.0–10.0) |
| Number of patients who died during hospital admission | 13 (3.3) | 10 (2.5) | 23 (2.9) |
| Number of patients who spent time in a specialist care unit | 8 (2.0) | 4 (1.0) | 12 (1.5) |
| Number of times admitted to a specialist care unit | |||
| Mean (SD) | 0.0 (0.20) | 0.0 (0.13) | 0.0 (0.17) |
| Median (range) | 0.0 (0.0–3.0) | 0.0 (0.0–2.0) | 0.0 (0.0–3.0) |
| Time spent in a specialist care unit (days) | |||
| Mean (SD) | 0.2 (1.36) | 0.0 (0.63) | 0.1 (1.06) |
| Median (range) | 0.0 (0.0–20.0) | 0.0 (0.0–11.0) | 0.0 (0.0–20.0) |
| 12-month summary | Control (N = 399), n (%) | Intervention (N = 401), n (%) | Total (N = 800), n (%) |
|---|---|---|---|
| Follow-up form completed? | |||
| Yes | 374 (93.7) | 374 (93.3) | 748 (93.5) |
| Died | 21 (5.3) | 26 (6.5) | 47 (5.9) |
| Withdrawn | 4 (1.0) | 1 (0.2) | 5 (0.6) |
| Patient visited A&E in the past 6 months? | |||
| Yes | 71 (17.8) | 77 (19.2) | 148 (18.5) |
| No | 303 (75.9) | 275 (68.6) | 578 (72.3) |
| Missing | 25 (6.3) | 49 (12.2) | 74 (9.3) |
| Number of A&E visits per patient | |||
| Mean (SD) | 0.3 (1.17) | 0.3 (0.66) | 0.3 (0.95) |
| Median (range) | 0.0 (0.0–19.0) | 0.0 (0.0–4.0) | 0.0 (0.0–19.0) |
| Patient admitted to hospital overnight in the past 6 months? | |||
| Yes | 74 (18.5) | 77 (19.2) | 151 (18.9) |
| No | 300 (75.2) | 297 (74.1) | 597 (74.6) |
| Missing | 25 (6.3) | 27 (6.7) | 52 (6.5) |
| Number of hospital admissions | |||
| Mean (SD) | 0.3 (0.73) | 0.3 (0.68) | 0.3 (0.71) |
| Median (range) | 0.0 (0.0–5.0) | 0.0 (0.0–4.0) | 0.0 (0.0–5.0) |
| Number of patients who died during hospital admission | 3 (0.8) | 5 (1.2) | 8 (1.0) |
| Number of patients who spent time in a specialist care unit | 2 (0.5) | 4 (1.0) | 6 (0.8) |
| Number of times admitted to a specialist care unit | |||
| Mean (SD) | 0.0 (0.07) | 0.0 (0.13) | 0.0 (0.11) |
| Median (range) | 0.0 (0.0–1.0) | 0.0 (0.0–2.0) | 0.0 (0.0–2.0) |
| Time spent in a specialist care unit (days) | |||
| Mean (SD) | 0.0 (0.29) | 0.0 (0.56) | 0.0 (0.45) |
| Median (range) | 0.0 (0.0–5.0) | 0.0 (0.0–10.0) | 0.0 (0.0–10.0) |
Related and unexpected serious adverse events
No related and unexpected serious adverse events were reported.
Withdrawals
Withdrawals of patients and carers from the trial are shown in Tables 29 and 30 respectively. Overall, there were 49 withdrawals among the patients and more patients and carers withdrew in the control group than in the intervention group (35 patients and 8 carers in the control group and 14 patients and two carers in the intervention group).
| Control (N = 399), n (%) | Intervention (N = 401), n (%) | Total (N = 800), n (%) | |
|---|---|---|---|
| Withdrawals | |||
| 6 months | 21 (5.3) | 6 (1.5) | 27 (3.4) |
| 12 months | 14 (3.5) | 8 (2.0) | 22 (2.8) |
| After 12-month follow-upa | 3 (0.8) | 2 (0.5) | 5 (0.6) |
| Total | 35 (8.8) | 14 (3.5) | 49 (6.1) |
| Type of withdrawal | |||
| Patient withdrew consent for postal follow-up | 31 (7.8) | 13 (3.2) | 44 (5.5) |
| Patient withdrew consent for all follow-up | 4 (1.0) | 1 (0.2) | 5 (0.6) |
| Control (N = 100), n (%) | Intervention (N = 108), n (%) | Total (N = 208), n (%) | |
|---|---|---|---|
| 6 months | 5 (5.0) | 1 (0.9) | 6 (2.9) |
| 12 months | 3 (3.0) | 1 (0.9) | 4 (1.9) |
| Total | 8 (8.0) | 2 (1.9) | 10 (4.8) |
Process data: implementation of control and intervention services
Completion of process data
Control services were asked to complete a time log for all patients summarising the contacts. Intervention services were asked to complete the care plan for all contacts with all patients. Table 31 shows the completion of the time logs and Table 32 shows the completion of the care plans. Of patients registered to the trial, 314 (78.7%) and 318 (79.3%) received the SCC service in the control and intervention arms respectively. Reasons for not receiving the service include the SCC not receiving a referral, the patient declining to receive the service, patient death or patient not contactable. SCCs completed time logs for 207 (51.9%) patients in the control arm and completed care plans for 283 (70.6%) patients in the intervention arm. Of the 283 completed care plans, three had all contacts completed > 12 months after patient registration to the trial.
| Service | Registered, n (%) | Time log completed | Reasons not completed | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes, n (%) | No, n (%) | Referral not received by SCC, n (%) | Patient declined SCC service, n (%) | Patient died before receiving SCC service, n (%) | SCC unable to contact patient, n (%) | Patient withdrew from all follow-up, n (%) | Received SCC service, no time log completed, n (%) | ||
| Total | 399 (100.0) | 207 (51.9) | 192 (48.1) | 67 (34.9) | 9 (4.7) | 3 (1.6) | 2 (1.0) | 4 (2.1) | 107 (55.7) |
| S001 | 15 (3.8) | 8 (53.3) | 7 (46.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 7 (100.0) |
| S003 | 18 (4.5) | 8 (44.4) | 10 (55.6) | 4 (40.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (10.0) | 5 (50.0) |
| S004 | 32 (8.0) | 20 (62.5) | 12 (37.5) | 1 (8.3) | 1 (8.3) | 1 (8.3) | 0 (0.0) | 1 (8.3) | 8 (66.7) |
| S008 | 25 (6.3) | 22 (88.0) | 3 (12.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (100.0) |
| S010 | 17 (4.3) | 13 (76.5) | 4 (23.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (25.0) | 3 (75.0) |
| S011 | 45 (11.3) | 18 (40.0) | 27 (60.0) | 13 (48.1) | 7 (25.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 7 (25.9) |
| S014 | 29 (7.3) | 5 (17.2) | 24 (82.8) | 7 (29.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 17 (70.8) |
| S015 | 46 (11.5) | 35 (76.1) | 11 (23.9) | 1 (9.1) | 0 (0.0) | 1 (9.1) | 1 (9.1) | 0 (0.0) | 8 (72.7) |
| S018 | 35 (8.8) | 4 (11.4) | 31 (88.6) | 14 (45.2) | 1 (3.2) | 0 (0.0) | 1 (3.2) | 0 (0.0) | 15 (48.4) |
| S019 | 45 (11.3) | 33 (73.3) | 12 (26.7) | 4 (33.3) | 0 (0.0) | 1 (8.3) | 0 (0.0) | 0 (0.0) | 7 (58.3) |
| S022 | 19 (4.8) | 3 (15.8) | 16 (84.2) | 13 (81.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (18.8) |
| S025 | 18 (4.5) | 7 (38.9) | 11 (61.1) | 4 (36.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 7 (63.6) |
| S029 | 41 (10.3) | 25 (61.0) | 16 (39.0) | 6 (37.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 10 (62.5) |
| S032 | 14 (3.5) | 6 (42.9) | 8 (57.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 7 (87.5) |
| Service | Registered, n (%) | Care plan completed | Reasons not completed | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes, n (%) | No, n (%) | Referral not received by SCC, n (%) | Patient declined SCC service, n (%) | Patient died before receiving SCC service, n (%) | SCC unable to contact patient, n (%) | Patient withdrew from all follow-up, n (%) | Received SCC service, no care plan completed, n (%) | ||
| Total | 401 (100.0) | 283 (70.6) | 118 (29.4) | 45 (38.1) | 21 (17.8) | 14 (11.9) | 2 (1.7) | 1 (0.8) | 35 (29.7) |
| S002 | 27 (6.7) | 20 (74.1) | 7 (25.9) | 0 (0.0) | 0 (0.0) | 2 (28.6) | 0 (0.0) | 1 (14.3) | 4 (57.1) |
| S005 | 19 (4.7) | 15 (78.9) | 4 (21.1) | 0 (0.0) | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (75.0) |
| S006 | 30 (7.5) | 26 (86.7) | 4 (13.3) | 3 (75.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (25.0) |
| S007 | 31 (7.7) | 28 (90.3) | 3 (9.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (100.0) |
| S009 | 17 (4.2) | 16 (94.1) | 1 (5.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) |
| S012 | 45 (11.2) | 25a (55.6) | 20 (44.4) | 8 (40.0) | 4 (20.0) | 2 (10.0) | 0 (0.0) | 0 (0.0) | 6 (30.0) |
| S013 | 45 (11.2) | 33b (73.3) | 12 (26.7) | 8 (66.7) | 1 (8.3) | 2 (16.7) | 0 (0.0) | 0 (0.0) | 1 (8.3) |
| S016 | 27 (6.7) | 19 (70.4) | 8 (29.6) | 2 (25.0) | 1 (12.5) | 4 (50.0) | 1 (12.5) | 0 (0.0) | 0 (0.0) |
| S020 | 2 (0.5) | 1 (50.0) | 1 (50.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| S023 | 13 (3.2) | 11 (84.6) | 2 (15.4) | 0 (0.0) | 1 (50.0) | 1 (50.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| S024 | 27 (6.7) | 10 (37.0) | 17 (63.0) | 4 (23.5) | 1 (5.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 12 (70.6) |
| S026 | 45 (11.2) | 29 (64.4) | 16 (35.6) | 8 (50.0) | 5 (31.3) | 0 (0.0) | 1 (6.3) | 0 (0.0) | 2 (12.5) |
| S027 | 26 (6.5) | 26 (100.0) | 0 (0.0) | N/A | N/A | N/A | N/A | N/A | N/A |
| S030 | 29 (7.2) | 10 (34.5) | 19 (65.5) | 10 (52.6) | 5 (26.3) | 3 (15.8) | 0 (0.0) | 0 (0.0) | 1 (5.3) |
| S031 | 18 (4.5) | 14 (77.8) | 4 (22.2) | 2 (50.0) | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (25.0) |
For control services, the relationship between the adjusted mean primary outcome and the mean percentage of completed time logs (of registered patients) by service is shown in Figure 7. There is no linear trend between the percentage of completed time logs observed and services’ adjusted means.
FIGURE 7.
Adjusted differences of each service from adjusted control mean in GHQ-12 scores ordered by service completion of time logs: control.
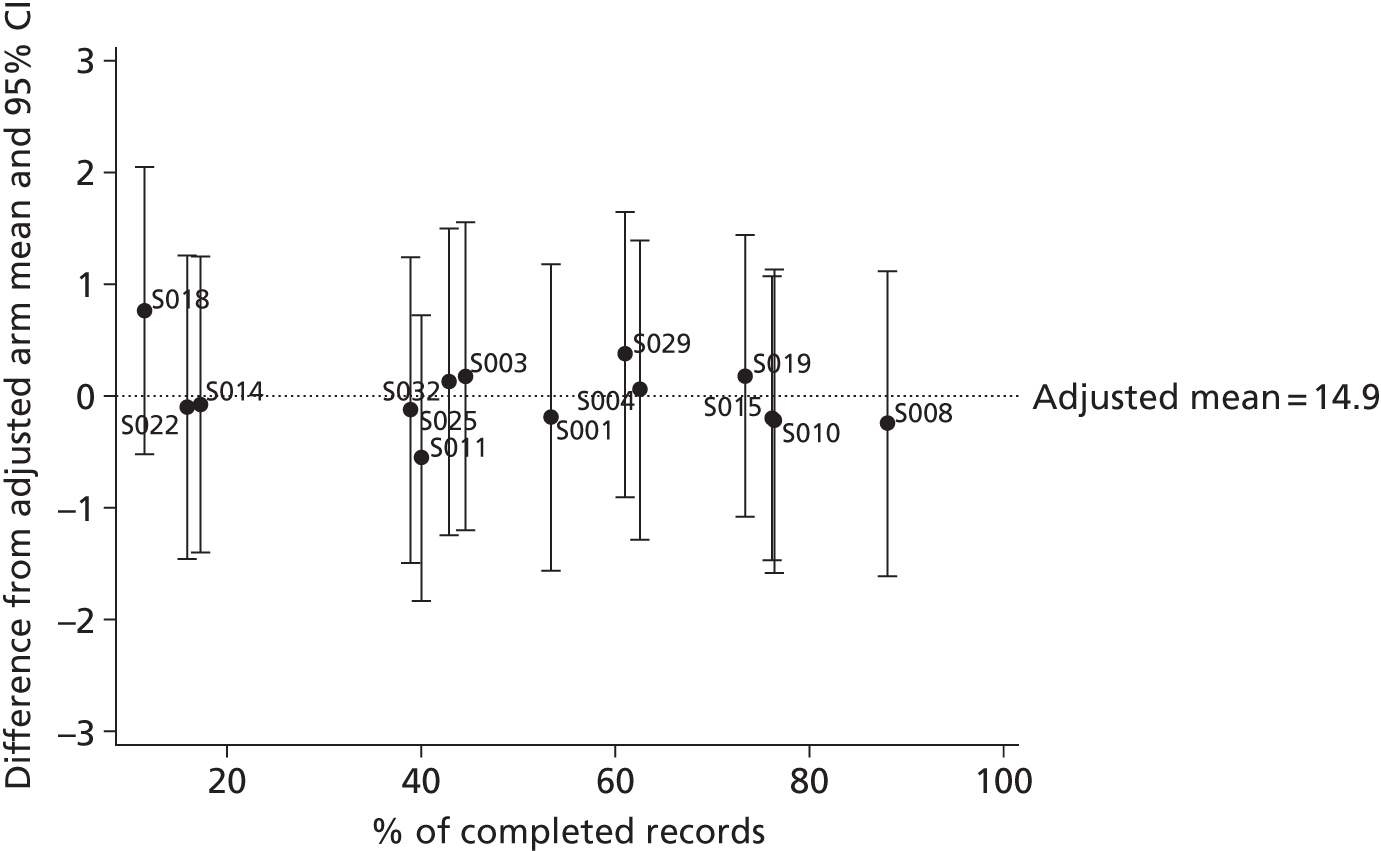
Compliance with the intervention
Compliance with the intervention assessed from completed care plans is shown by service in Table 33. Compliance with the intervention was defined as at least 12 of 16 (75%) assessment areas (each linked to a reference guide in the manual) asked on patient contact 1 of the care plan (with at least one question in the assessment area being asked). In total, 10 care plans were not compliant and 269 (96.1%) met the definition of compliance.
| Service | Compliant record, n (%) | Non-compliant record, n (%) | Missing, n (%) | Care plan completed, n (%) |
|---|---|---|---|---|
| S002 | 19 (95.0) | 0 (0.0) | 1a (5.0) | 20 (7.1) |
| S005 | 15 (100.0) | 0 (0.0) | 0 (0.0) | 15 (5.4) |
| S006 | 25 (96.2) | 1 (3.8) | 0 (0.0) | 26 (9.3) |
| S007 | 28 (100.0) | 0 (0.0) | 0 (0.0) | 28 (10.0) |
| S009 | 16 (100.0) | 0 (0.0) | 0 (0.0) | 16 (5.7) |
| S012 | 24 (100.0) | 0 (0.0) | 0 (0.0) | 24 (8.6) |
| S013 | 31 (100.0) | 0 (0.0) | 0 (0.0) | 31 (11.1) |
| S016 | 18 (94.7) | 1 (5.3) | 0 (0.0) | 19 (6.8) |
| S020 | 1 (100.0) | 0 (0.0) | 0 (0.0) | 1 (0.4) |
| S023 | 11 (100.0) | 0 (0.0) | 0 (0.0) | 11 (3.9) |
| S024 | 10 (100.0) | 0 (0.0) | 0 (0.0) | 10 (3.6) |
| S026 | 28 (96.6) | 1 (3.4) | 0 (0.0) | 29 (10.4) |
| S027 | 20 (76.9) | 6 (23.1) | 0 (0.0) | 26 (9.3) |
| S030 | 9 (90.0) | 1 (10.0) | 0 (0.0) | 10 (3.6) |
| S031 | 14 (100.0) | 0 (0.0) | 0 (0.0) | 14 (5.0) |
| Total | 269 (96.1) | 10 (3.6) | 1 (0.4) | 280 (100.0) |
For intervention services, the relationship between the adjusted mean primary outcome and the mean percentage of compliant care plans (of registered patients) by service is shown in Figure 8. There is larger variation in adjusted scores for services with higher compliance, but no linear trend is observed between the percentage of compliant care plans and services’ adjusted mean scores.
FIGURE 8.
Adjusted differences of each service from adjusted intervention mean in GHQ-12 scores ordered by service compliance with care plans: intervention.
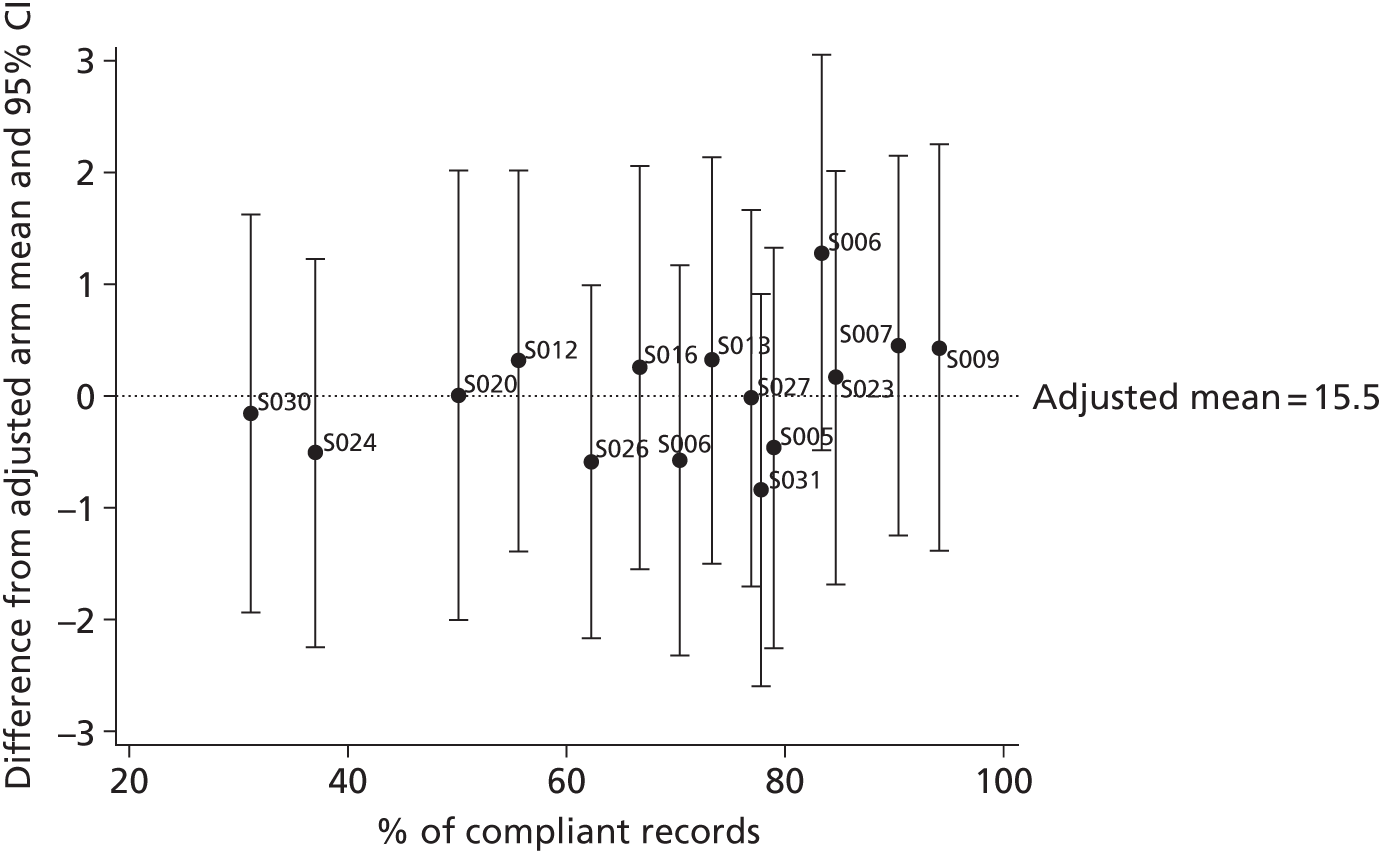
Number of patient contacts
The numbers of SCC contacts within 12 months of registration to the trial for patients with completed time logs or completed care plans are shown in Table 34 (it should be noted that in some cases SCCs continued to see patients post 12 months; only data up to 12 months are included). Both control services and intervention services had a median of two SCC contacts with patients. The median number of contacts per service ranged from 1 to 4 for control services and from 1 to 4.5 for intervention services (see Appendix 9). The median duration from registration to the first/last contact was 14/57.5 days and 27/103 days in the control services and intervention services respectively. In total, 76% of control service contacts were face to face and 94% of intervention service contacts were face to face. In addition, 68% of intervention service contacts took place in the patient’s home (these data were not collected for control services).
| Patient contacts | Control (N = 207) | Intervention (N = 280) |
|---|---|---|
| Number of patient contacts within 12 months of registration | ||
| Mean (SD) | 2.3 (1.46) | 2.1 (1.15) |
| Median (range) | 2.0 (1.0–7.0) | 2.0 (1.0–6.0) |
| Missing | 3 | 1 |
| Number of patient contacts within 12 months of registration, n (%) | ||
| Missing | 3 (1.4) | 1 (0.4) |
| 1 | 81 (39.1) | 98 (35.0) |
| 2 | 49 (23.7) | 99 (35.4) |
| 3 | 35 (16.9) | 47 (16.8) |
| 4 | 19 (9.2) | 23 (8.2) |
| 5 | 10 (4.8) | 8 (2.9) |
| 6 | 9 (4.3) | 4 (1.4) |
| 7 | 1 (0.5) | |
Delivery of the intervention
The overall proportion of assessment areas asked at contact 1 was 94% and ranged from 80% to 93% at subsequent contacts. A median of three problem areas were identified in the assessment at contact 1, dropping to one or fewer at subsequent contacts (Table 35); this is the same as the median number of problems addressed in the goal and action planner. A median of three goals/actions were set at contact 1, dropping to two at contact 2 and one or less at subsequent contacts; these comprise both SCC and patient goals/actions. Data were collected on the number of goals/actions achieved; however, this section of the care plan was poorly completed by SCCs and therefore has not been reported.
| Care plan summary | Contact 1 (n = 279) | Contact 2 (n = 181) | Contact 3 (n = 82) | Contact 4 (n = 37) | Contact 5 (n = 12) | Contact 6 (n = 4) |
|---|---|---|---|---|---|---|
| Problem areas identified in the assessment | ||||||
| Mean (SD) | 3.9 (2.90) | 1.8 (1.71) | 1.4 (1.34) | 0.9 (1.16) | 0.5 (1.73) | 0.5 (0.58) |
| Median (range) | 3.0 (0.0–14.0) | 1.0 (0.0–7.0) | 1.0 (0.0–6.0) | 0.0 (0.0–4.0) | 0.0 (0.0–6.0) | 0.5 (0.0–1.0) |
| Problems addressed in the goal planner | ||||||
| Mean (SD) | 3.3 (2.46) | 1.6 (1.48) | 1.4 (1.37) | 0.8 (0.97) | 0.7 (1.72) | 0.5 (0.58) |
| Median (range) | 3.0 (0.0–12.0) | 1.0 (0.0–7.0) | 1.0 (0.0–6.0) | 0.0 (0.0–3.0) | 0.0 (0.0–6.0) | 0.5 (0.0–1.0) |
| Total goals/actions in the goal planner | ||||||
| Mean (SD) | 3.7 (2.73) | 2.0 (2.14) | 1.7 (1.60) | 0.7 (0.87) | 0.6 (1.44) | 0.5 (0.58) |
| Median (range) | 3.0 (0.0–12.0) | 2.0 (0.0–10.0) | 1.0 (0.0–8.0) | 0.0 (0.0–3.0) | 0.0 (0.0–5.0) | 0.5 (0.0–1.0) |
| Patient goals/actions in the goal planner | ||||||
| Mean (SD) | 1.2 (1.84) | 1.1 (1.80) | 1.0 (1.29) | 0.4 (0.63) | 0.0 (0.00) | 0.0 (0.00) |
| Median (range) | 1.0 (0.0–8.0) | 0.0 (0.0–9.0) | 0.5 (0.0–6.0) | 0.0 (0.0–2.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
Apart from assessment area 13 (sexual functioning), at least 90% of patients were asked each of the assessment areas at contact 1. In total, 63% of patients were asked about sexual functioning at contact 1, increasing to 76–100% at subsequent contacts. At least 80% of patients were asked about the other assessment areas at contacts 2–4; this value was slightly lower for some of the assessment areas at contacts 5 and 6 because these areas had already been addressed. Table 36 shows the assessment areas in which problems were identified. At contact 1, the most common problem areas, affecting at least one-third of patients, were medicines/general health, mobility/falls, personal hygiene/dressing, mood and ‘other’ problems (including speech and language, limb weakness/numbness, visual problems, swallowing, sleep/fatigue and employment). Apart from mood and sexual functioning, the prevalence of all problem areas had substantially reduced by contact 2 and generally continued to reduce at subsequent contacts. The prevalence of mood (and sexual functioning) problems remained similar until contact 3, after which a reduction was observed. After an initial reduction, the prevalence of social need problems remained constant from contact 2 to contact 5.
| Assessment area (related reference guide) | Number (%) of patients with problem | |||||
|---|---|---|---|---|---|---|
| Contact 1 (n = 279) | Contact 2 (n = 181) | Contact 3 (n = 82) | Contact 4 (n = 37) | Contact 5 (n = 12) | Contact 6 (n = 4) | |
| 1. Transfer of care | 17 (6.1) | N/A | N/A | N/A | N/A | N/A |
| 2. Communication and information | 60 (21.5) | 5 (2.8) | 1 (1.2) | 2 (5.4) | 0 (0.0) | 0 (0.0) |
| 3. Medicines and general health | 103 (36.9) | 39 (21.5) | 16 (19.5) | 4 (10.8) | 1 (8.3) | 0 (0.0) |
| 4. Pain | 56 (20.1) | 20 (11.1) | 11 (13.4) | 2 (5.4) | 1 (8.3) | 0 (0.0) |
| 5. Mobility/falls | 143 (51.3) | 52 (28.7) | 18 (22.0) | 2 (5.4) | 0 (0.0) | 0 (0.0) |
| 6. Personal hygiene and dressing | 92 (33.0) | 27 (15.0) | 6 (7.3) | 1 (2.7) | 1 (8.3) | 0 (0.0) |
| 7. Shopping and meal preparation | 74 (26.5) | 19 (10.5) | 1 (1.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 8. House and home | 38 (13.6) | 9 (5.0) | 1 (1.2) | 1 (2.7) | 0 (0.0) | 0 (0.0) |
| 9. Cognition | 64 (22.9) | 19 (10.6) | 4 (4.9) | 1 (2.7) | 0 (0.0) | 0 (0.0) |
| 10. Driving and general transport | 73 (26.2) | 21 (11.7) | 9 (11.0) | 3 (8.1) | 0 (0.0) | 0 (0.0) |
| 11. Finances and benefits | 66 (23.7) | 15 (8.3) | 3 (3.7) | 1 (2.7) | 0 (0.0) | 0 (0.0) |
| 12. Continence | 27 (9.7) | 4 (2.2) | 1 (1.2) | 2 (5.4) | 1 (8.3) | 0 (0.0) |
| 13. Sexual functioning | 7 (2.5) | 4 (2.2) | 3 (3.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 14. Patient mood | 99 (35.5) | 53 (29.3) | 26 (31.7) | 6 (16.2) | 1 (8.3) | 0 (0.0) |
| 15. Patient social needs | 53 (19.0) | 11 (6.1) | 5 (6.1) | 3 (8.1) | 1 (8.3) | 0 (0.0) |
| 16. Other | 110 (39.4) | 33 (18.3) | 8 (9.8) | 4 (10.8) | 0 (0.0) | 2 (50.0) |
The problem areas that have been addressed in the goal and action planner are shown in Table 37 (differences between services are discussed in Appendix 9). Apart from a few exceptions (contact 1 – transfer of care; contact 2 – continence, mood; contact 3 – cognition, finances/benefits, social needs; contact 4 – transport, other), at least 80% of problem areas identified in the assessment were subsequently addressed in the goal and action planner. In some cases, a problem area was addressed in the goal and action planner without having been documented in the assessment. The types of goals/actions used to address these problems are summarised in Table 38. At contact 1, the most common goals/actions were referral to physiotherapy, referral to occupational therapy or ‘other referral’, provision of information/advice or ‘other goals/actions’. ‘Other referrals’ include referrals to other health-care services (e.g. podiatry, psychology, stroke clinic, community rehabilitation team or smoking cessation) and local authority and third-sector services (e.g. the Stroke Association, benefits review, social services, exercise classes). ‘Other goals/actions’ include patient goals and SCC actions (e.g. discussing with colleague or chasing up appointment). The numbers of patients requiring referrals, information/advice or signposting were substantially reduced at subsequent contacts, whereas the number of ‘other goals/actions’ remained high and the number requiring GP appointments increased.
| Assessment area (related reference guide) | No. (%) of patients with problem addressed | |||||
|---|---|---|---|---|---|---|
| Contact 1 (n = 279) | Contact 2 (n = 181) | Contact 3 (n = 82) | Contact 4 (n = 37) | Contact 5 (n = 12) | Contact 6 (n = 4) | |
| 1. Transfer of care | 11 (3.9) | N/A | N/A | N/A | N/A | N/A |
| 2. Communication and information | 56 (20.1) | 5 (2.8) | 2 (2.4) | N/A | N/A | N/A |
| 3. Medicines and general health | 106 (38.0) | 38 (21.0) | 20 (24.4) | 6 (16.2) | 2 (16.7) | N/A |
| 4. Pain | 50 (17.9) | 18 (9.9) | 11 (13.4) | 2 (5.4) | 1 (8.3) | N/A |
| 5. Mobility/falls | 139 (49.8) | 50 (27.6) | 20 (24.4) | 4 (10.8) | N/A | N/A |
| 6. Personal hygiene and dressing | 95 (34.1) | 27 (14.9) | 11 (13.4) | 1 (2.7) | 2 (16.7) | N/A |
| 7. Shopping and meal preparation | 78 (28.0) | 23 (12.7) | 6 (7.3) | N/A | N/A | N/A |
| 8. House and home | 38 (13.6) | 11 (6.1) | 1 (1.2) | 1 (2.7) | N/A | N/A |
| 9. Cognition | 51 (18.3) | 18 (9.9) | 2 (2.4) | 1 (2.7) | N/A | N/A |
| 10. Driving and general transport | 63 (22.6) | 20 (11.0) | 9 (11.0) | 1 (2.7) | N/A | N/A |
| 11. Finances and benefits | 59 (21.1) | 15 (8.3) | 2 (2.4) | 2 (5.4) | N/A | N/A |
| 12. Continence | 24 (8.6) | 3 (1.7) | 1 (1.2) | 2 (5.4) | 1 (8.3) | N/A |
| 13. Sexual functioning | 6 (2.2) | 4 (2.2) | 4 (4.9) | N/A | N/A | N/A |
| 14. Patient mood | 89 (31.9) | 41 (22.7) | 24 (29.3) | 6 (16.2) | 1 (8.3) | N/A |
| 15. Patient social needs | 53 (19.0) | 18 (9.9) | 7 (8.5) | 3 (8.1) | 1 (8.3) | N/A |
| 16. Other | 100 (35.8) | 31 (17.1) | 9 (11.0) | 2 (5.4) | N/A | 2 (50.0) |
| Goal/action | No. (%) of patients with individual goal/action | |||||
|---|---|---|---|---|---|---|
| Contact 1 (n = 279) | Contact 2 (n = 181) | Contact 3 (n = 82) | Contact 4 (n = 37) | Contact 5 (n = 12) | Contact 6 (n = 4) | |
| Referral to physiotherapist | 95 (34.1) | 16 (8.8) | 3 (3.7) | |||
| Referral to occupational therapy | 78 (28.0) | 14 (7.7) | 1 (1.2) | |||
| Referral to speech and language therapy | 43 (15.4) | 4 (2.2) | 1 (1.2) | 1 (2.7) | ||
| Referral to dietitian | 14 (5.0) | 2 (1.1) | ||||
| Referral to community nurse | 22 (7.9) | 6 (3.3) | ||||
| Other referrala | 115 (41.2) | 31 (17.1) | 14 (17.1) | 4 (10.8) | 1 (8.3) | |
| GP appointment | 19 (6.8) | 17 (9.4) | 9 (11.0) | 4 (10.8) | ||
| Information and advice | 87 (31.2) | 22 (12.2) | 10 (12.2) | 2 (5.4) | 2 (16.7) | |
| Signpostinga | 34 (12.2) | 6 (3.3) | 2 (2.4) | 1 (2.7) | ||
| Other goals/actionsa | 175 (62.7) | 97 (53.6) | 49 (59.8) | 12 (32.4) | 2 (16.7) | 2 (50.0) |
An optional component of the intervention was the provision to patients, before the assessment with the SCC, of a checklist of the assessment areas to be discussed. Data from the care plans showed that SCCs provided the checklist to patients in advance in 14% of contacts.
The intervention included an optional carer assessment. Of the 279 patients with a care plan, 178 (64%) had a carer and, of these, 58 (33%) had a carer assessment. The most common reasons for not completing a carer assessment were that the assessment was provided by another service, the carer was not present or the carer declined. All of the carers who underwent an assessment had a single contact with the SCC except for one carer who had two contacts; 97% of contacts were face to face.
Of the carers registered to the trial, 26 (24%) had a carer assessment. For these carers, 90% of the assessment areas were asked at contact 1 and a median of one problem area was identified (range 0–6). As with patients, the sexual functioning assessment was asked less frequently (58% of carers) than other assessment areas (88–96% of carers). Problems were identified across the assessment areas, most commonly in the areas of communication/information, mood and social/emotional needs, all identified in 23% of carers. A median of one problem (range 0–5) was addressed in the goal and action planner, leading to a median of one (range 0–3) goal/action. These included the provision of information (11 carers), referrals to health-care/social care/third-sector services (three carers), a GP appointment (one carer) and other goals/actions (eight carers).
Overview of stroke care co-ordinator practice
A description of the control and intervention services based on surveys and semistructured interviews with SCCs is given in Appendix 9.
Overall, the intervention SCCs seem to feel that the trial or manual has not changed their practice. Specifically, four services said that the care plan did not change their assessment and five services said that the manual did not change their practice (no data for other services). However, 10 SCCs gave examples during interview of how their assessment or practice has changed (as a result of either the training day or using the system of care). These included more in-depth thinking about problem-solving; enabling patients to identify their resources or coping strategies; a more consistent and well-structured assessment; covering new areas such as sexual functioning; and delegating more responsibility for actions to the patient. Some services have subsequently adapted their documentation to include areas from the system of care.
The control and intervention arms are similar in that, generally, they aimed to assess all areas at initial assessment; actions rather than goals were recorded by SCCs; goals were reviewed in similar ways; the co-ordinator role was seen as giving information, secondary prevention and/or co-ordinating referrals; and responses to problems were largely informed by experience, local knowledge and discussions. Local services that were felt to be lacking were generally similar between the control arm and the intervention arm, with access to psychology being the most problematic. There was no difference in arms between involving the family in patient assessments and encouraging patients to carry out actions for themselves.
There are two clear differences between the control arm and the intervention arm. First, there were differences in the components of the assessment. Seven intervention SCCs provided patients with the LoTS care checklist before assessment, whereas no control SCCs mentioned an equivalent in their service. Control SCCs also did not report using an evidenced-based manual whereas 10 of the intervention SCCs used the LoTS care manual to at least some extent. Second, although control SCCs intended to provide a fully holistic initial assessment, it is not clear from interviews whether they actually did, and three services reported not covering all areas for all patients, whereas the intervention SCCs appeared to provide a holistic assessment more consistently.
Economic evaluation
Client Service Receipt Inventory return rate and European Quality of Life-5 Dimensions completion rate
Table 39 summarises the numbers of patients returning the CSRI and completing the EQ-5D at each assessment point. Both measures had similar rates of return/completion at each assessment point and rates were balanced between the intervention arm and the control arm. Table 40 characterises those with and without the necessary cost and outcome data at 6 months for inclusion in the CEAC-based analyses at the primary end point. Although differences were not explored statistically, the baseline characteristics of patients with the necessary data at 6 months appeared similar to those of the full sample. Therefore, the results based on those followed up are likely to generally be representative of the full sample.
| Group | CSRI, n (%) | EQ-5D, n (%) | ||||
|---|---|---|---|---|---|---|
| Baseline | 6 months | 12 months | Baseline | 6 months | 12 months | |
| Intervention (N = 401) | 401 (100) | 307 (77) | 283 (71) | 381 (95) | 301 (75) | 270 (67) |
| Control (N = 399) | 398 (99) | 295 (74) | 268 (67) | 382 (96) | 288 (72) | 259 (65) |
| Total (N = 800) | 799 (99) | 602 (75) | 551 (69) | 763 (95) | 589 (74) | 529 (66) |
| Characteristic | Full sample (N = 800) | Subsample with both cost and GHQ-12 data at 6 months (N = 589) | Subsample with both costs and QALY data at 6 months (N = 564) |
|---|---|---|---|
| Age (years, mean) | 72 | 72 | 72 |
| Male, n (%) | 433 (54) | 324 (55) | 310 (55) |
| Mean baseline health and social care cost (£) | 750 (valid n = 799) | 699 | 713 |
| Mean baseline GHQ-12 total score | 16 (valid n = 793) | 15 (valid n = 585) | – |
| Mean baseline utility score | 0.53 (valid n = 763) | – | 0.54 |
Resource use
Tables 41–43 show resource use at each assessment point. For brevity, these tables are limited to all inpatient services and all informal care plus other health and social care resources used by at least 10% of responders in either trial arm at that time point. Full resource use data are provided in Appendix 10. Use of health and social care resources appeared broadly comparable between the two groups at baseline, 6 months and 12 months. As could be expected, use of inpatient services (following the index stroke admission), outpatient services, hospital-based physiotherapy and hospital-based occupational therapy increased during the post-stroke period compared with baseline. Greater numbers of people accessed a wider range of community-based services in the first 6 months after recruitment but numbers were reduced in the final 6 months of follow-up. In comparison with formal care inputs, use of informal care was very high, with care from non-resident informal carers increasing at each time point.
| Resource | Unit | Intervention (n = 401) | Control (n = 398) | ||||
|---|---|---|---|---|---|---|---|
| Users (%) | Meanb | SD | Users (%) | Meanb | SD | ||
| Index stroke admissionc | Bed-days | 99 | 39 | 44 | 99 | 30 | 35 |
| Inpatient services | Bed-days | 7 | 11 | 24 | 10 | 7 | 6 |
| A&E | Visit | 7 | 11 | 24 | 10 | 7 | 6 |
| Outpatient services | Visit | 8 | 2 | 4 | 10 | 1 | 1 |
| Community-based services | |||||||
| GP | |||||||
| Surgery visit | Visit | 49 | 2 | 1 | 44 | 2 | 2 |
| Home visit | Visit | 9 | 2 | 1 | 11 | 2 | 2 |
| Telephone call | Call | 10 | 2 | 1 | 12 | 2 | 1 |
| Repeat prescription | Occurrence | 47 | 2 | 1 | 49 | 2 | 1 |
| Practice nurse | Visit | 26 | 2 | 1 | 23 | 2 | 2 |
| Chiropodist | Contact | 10 | 2 | 1 | 12 | 1 | 1 |
| Dentist | Contact | 12 | 1 | 1 | 12 | 1 | 1 |
| Optician | Contact | 12 | 1 | < 1 | 12 | 1 | < 1 |
| Informal care from coresidents | |||||||
| Personal care | Hour | 3 | 47 | 43 | 3 | 219 | 320 |
| Providing transport | Hour | 3 | 78 | 205 | 6 | 37 | 56 |
| Preparing meals | Hour | 4 | 138 | 175 | 6 | 134 | 141 |
| Housework/laundry | Hour | 4 | 147 | 186 | 6 | 95 | 93 |
| DIY | Hour | 2 | 97 | 247 | 3 | 40 | 65 |
| Gardening | Hour | 3 | 94 | 207 | 4 | 31 | 44 |
| Shopping | Hour | 4 | 81 | 184 | 5 | 41 | 47 |
| Outings | Hour | 2 | 106 | 231 | 3 | 44 | 63 |
| Socialising | Hour | 3 | 415 | 604 | 3 | 202 | 428 |
| Help managing finances | Hour | 3 | 77 | 214 | 4 | 31 | 40 |
| Informal care from non-residents | |||||||
| Personal care | Hour | 1 | 39 | 29 | 1 | 171 | 281 |
| Providing transport | Hour | 6 | 25 | 23 | 4 | 39 | 48 |
| Preparing meals | Hour | 3 | 66 | 72 | 2 | 48 | 65 |
| Housework/laundry | Hour | 4 | 43 | 39 | 4 | 24 | 20 |
| DIY | Hour | 3 | 19 | 19 | 2 | 10 | 7 |
| Gardening | Hour | 4 | 18 | 14 | 3 | 18 | 14 |
| Shopping | Hour | 5 | 24 | 15 | 4 | 28 | 21 |
| Outings | Hour | 5 | 28 | 56 | 3 | 23 | 16 |
| Socialising | Hour | 5 | 95 | 115 | 5 | 78 | 100 |
| Help managing finances | Hour | 2 | 19 | 14 | 2 | 12 | 7 |
| Resource | Unit | Intervention (n = 307) | Control (n = 295) | ||||
|---|---|---|---|---|---|---|---|
| Users (%) | Meanb | SD | Users (%) | Meanb | SD | ||
| Inpatient services | Bed-days | 19 | 15 | 18 | 19 | 14 | 27 |
| Day hospital/day cases | Activity | 12 | 1 | 1 | 8 | 1 | 1 |
| A&E | Visit | 17 | 2 | 2 | 14 | 2 | 1 |
| Outpatient services | Visit | 44 | 3 | 3 | 40 | 3 | 5 |
| Physiotherapist, hospitalc | Visit | 12 | 8 | 8 | 17 | 7 | 8 |
| Community-based services | |||||||
| GP | |||||||
| Surgery visit | Visit | 57 | 3 | 2 | 60 | 3 | 2 |
| Home visit | Visit | 24 | 2 | 2 | 22 | 2 | 1 |
| Telephone call | Call | 20 | 2 | 2 | 21 | 2 | 3 |
| Repeat prescription | Occurrence | 52 | 3 | 4 | 51 | 5 | 3 |
| Practice nurse | Visit | 33 | 3 | 3 | 40 | 3 | 3 |
| Physiotherapist | Home visit | 28 | 8 | 9 | 22 | 8 | 10 |
| Occupational therapist | Visit | 26 | 5 | 8 | 24 | 6 | 7 |
| Speech and language therapist | Home visit | 11 | 4 | 3 | 13 | 4 | 4 |
| Social worker | Home visit | 12 | 2 | 1 | 11 | 2 | 1 |
| Community/district nurse | Contact | 23 | 7 | 21 | 23 | 4 | 9 |
| Chiropodist | Contact | 18 | 2 | 2 | 16 | 2 | 1 |
| Dentist | Contact | 21 | 2 | 1 | 14 | 1 | 1 |
| Optician | Contact | 20 | 1 | 1 | 22 | 1 | 1 |
| Informal care from coresidents | |||||||
| Personal care | Hour | 15 | 385 | 834 | 14 | 242 | 324 |
| Providing transport | Hour | 15 | 108 | 140 | 14 | 120 | 126 |
| Preparing meals | Hour | 19 | 249 | 234 | 14 | 249 | 200 |
| Housework/laundry | Hour | 18 | 244 | 305 | 14 | 204 | 163 |
| DIY | Hour | 9 | 87 | 224 | 4 | 49 | 51 |
| Gardening | Hour | 11 | 80 | 94 | 8 | 60 | 52 |
| Shopping | Hour | 16 | 115 | 122 | 15 | 95 | 113 |
| Outings | Hour | 14 | 116 | 142 | 9 | 93 | 87 |
| Socialising | Hour | 14 | 728 | 1125 | 9 | 489 | 488 |
| Help managing finances | Hour | 13 | 89 | 123 | 11 | 124 | 225 |
| Informal care from non-residents | |||||||
| Personal care | Hour | 8 | 110 | 96 | 6 | 110 | 102 |
| Providing transport | Hour | 14 | 63 | 82 | 14 | 58 | 58 |
| Preparing meals | Hour | 8 | 104 | 135 | 6 | 85 | 98 |
| Housework/laundry | Hour | 9 | 80 | 82 | 7 | 45 | 43 |
| DIY | Hour | 6 | 20 | 20 | 4 | 24 | 35 |
| Gardening | Hour | 9 | 26 | 36 | 7 | 32 | 37 |
| Shopping | Hour | 11 | 48 | 48 | 11 | 44 | 35 |
| Outings | Hour | 13 | 57 | 98 | 11 | 54 | 62 |
| Socialising | Hour | 13 | 156 | 163 | 9 | 146 | 218 |
| Help managing finances | Hour | 7 | 60 | 69 | 5 | 33 | 36 |
| Resource | Unit | Intervention (n = 283) | Control (n = 268) | ||||
|---|---|---|---|---|---|---|---|
| Users (%) | Meanb | SD | Users (%) | Meanb | SD | ||
| Inpatient services | Bed-days | 16 | 9 | 15 | 15 | 8 | 9 |
| Day hospital/day cases | Activity | 8 | 2 | 1 | 10 | 1 | < 1 |
| A&E | Visit | 12 | 2 | 1 | 10 | 2 | 1 |
| Outpatient services | Visit | 36 | 3 | 3 | 37 | 3 | 5 |
| Physiotherapist, hospitalc | Visit | 14 | 6 | 6 | 12 | 6 | 8 |
| Community-based services | |||||||
| GP | |||||||
| Surgery visit | Visit | 53 | 3 | 3 | 60 | 3 | 2 |
| Home visit | Visit | 15 | 3 | 4 | 13 | 3 | 6 |
| Telephone call | Call | 18 | 2 | 1 | 13 | 3 | 3 |
| Repeat prescription | Occurrence | 49 | 5 | 3 | 54 | 5 | 2 |
| Practice nurse | Visit | 35 | 3 | 4 | 44 | 3 | 4 |
| Community/district nurse | Contact | 15 | 8 | 27 | 14 | 4 | 5 |
| Chiropodist | Contact | 18 | 2 | 1 | 18 | 2 | 2 |
| Dentist | Contact | 27 | 2 | 1 | 22 | 2 | 1 |
| Optician | Contact | 21 | 1 | < 1 | 24 | 1 | 1 |
| Other services | Occurrence | 6 | 4 | 6 | 3 | 2 | 1 |
| Informal care from coresidents | |||||||
| Personal care | Hour | 11 | 564 | 1695 | 6 | 460 | 1035 |
| Providing transport | Hour | 12 | 202 | 357 | 10 | 134 | 278 |
| Preparing meals | Hour | 14 | 317 | 336 | 9 | 213 | 203 |
| Housework/laundry | Hour | 14 | 339 | 769 | 11 | 210 | 235 |
| DIY | Hour | 7 | 157 | 455 | 4 | 67 | 83 |
| Gardening | Hour | 9 | 137 | 390 | 8 | 81 | 160 |
| Shopping | Hour | 11 | 175 | 349 | 10 | 80 | 141 |
| Outings | Hour | 11 | 173 | 378 | 7 | 123 | 176 |
| Socialising | Hour | 11 | 550 | 776 | 7 | 343 | 488 |
| Help managing finances | Hour | 9 | 169 | 403 | 8 | 63 | 117 |
| Informal care from non-residents | |||||||
| Personal care | Hour | 5 | 209 | 356 | 3 | 186 | 349 |
| Providing transport | Hour | 12 | 53 | 66 | 11 | 42 | 40 |
| Preparing meals | Hour | 7 | 106 | 170 | 4 | 87 | 84 |
| Housework/laundry | Hour | 9 | 107 | 154 | 7 | 60 | 67 |
| DIY | Hour | 5 | 46 | 43 | 7 | 34 | 44 |
| Gardening | Hour | 6 | 38 | 37 | 6 | 31 | 36 |
| Shopping | Hour | 8 | 62 | 72 | 8 | 44 | 39 |
| Outings | Hour | 11 | 63 | 135 | 9 | 47 | 46 |
| Socialising | Hour | 12 | 222 | 356 | 8 | 116 | 140 |
| Help managing finances | Hour | 5 | 49 | 64 | 4 | 44 | 37 |
Costs and quality-adjusted life-years
The mean cost of SCC inputs was similar between the groups: £277 in the intervention group and £239 in the control group (mean difference £42; 95% CI – £30 to £116) (Table 44). This is the mean across the whole group, including zero costs when SCC inputs were not received. The system of care therefore did not substantially add to the average cost of SCC inputs. The majority of SCC costs were related to patient assessments and time spent discussing patients in MDT meetings. Non-contact activities (actions related to assessments and note writing) accounted for a substantial proportion of the total cost, at 66% of the cost of patient assessments in the intervention group and 58% of the cost of patient assessments in the control group. The costs of inputs to carers in the intervention group were minimal.
| Input | Intervention (n = 401) | Control (n = 399) | Intervention – controla | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean difference | 95% CI | p-value | |
| Patient inputs | |||||||
| In-hospital contact | 3 | 7 | 2 | 6 | |||
| Assessment | 104 | 86 | 100 | 72 | |||
| Actions arising from assessment | 33 | 34 | 36 | 30 | |||
| Note writing | 36 | 29 | 22 | 19 | |||
| MDT meetings | 90 | 104 | 78 | 41 | |||
| Consenting carer inputsb | |||||||
| Assessment | 3 | 8 | – | – | |||
| Actions arising from assessment | 1 | 2 | – | – | |||
| Note writing | 3 | 7 | – | – | |||
| Non-consenting carer inputsb | |||||||
| Assessment | 2 | 6 | – | – | |||
| Actions arising from assessment | 1 | 4 | – | – | |||
| Note writing | 1 | 3 | – | – | |||
| Total | 277 | 207 | 239 | 146 | 42 | –30 to 116 | 0.258 |
There were no differences in specific categories of health and social care costs (Table 45) or in total health and social care costs (Table 46). Informal care costs notably increased after baseline and were significantly higher in the intervention group at 6 months, 12 months and over 1 year. Although informal care costs fell between 6 months and 12 months in the control group, they increased over the same period in the intervention group. These costs overshadowed health and social care costs by a factor of 2.4 in the intervention group and 1.9 in the control group at 6 months. This pattern was reflected in total societal costs, with the intervention group having significantly higher societal costs at 6 months, 12 months and over 1 year. Higher informal care costs in the intervention group may suggest that these patients had ongoing care needs for a longer period of time or that they accessed more informal care because of the goal-setting element of the intervention.
| Resource category | Intervention (N = 401) | Control (N = 399) | Intervention – controla | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Valid n | Mean | SD | Valid n | Mean | SD | Mean difference | 95% CI | p-value | |
| SCC inputs | 401 | 277 | 207 | 399 | 239 | 146 | 42 | –30 to 116 | 0.258 |
| Institutionalisation | |||||||||
| Baseline | 401 | 1 | 27 | 398 | 0 | 0 | 1 | –1 to 4 | 0.319 |
| 6 months | 307 | 294 | 1438 | 295 | 317 | 1462 | –62 | –287 to 164 | 0.590 |
| 12 months | 283 | 351 | 1777 | 268 | 451 | 2333 | –137 | –473 to 200 | 0.426 |
| Secondary care | |||||||||
| Baseline | 401 | 504 | 2507 | 398 | 495 | 1486 | 9 | –277 to 295 | 0.950 |
| 6 months | 307 | 1599 | 3726 | 295 | 1608 | 4902 | –18 | –708 to 671 | 0.959 |
| 12 months | 283 | 932 | 2529 | 268 | 820 | 1993 | 99 | –286 to 484 | 0.614 |
| Community-based services | |||||||||
| Baseline | 401 | 188 | 400 | 398 | 286 | 705 | –99 | –181 to –18 | 0.016 |
| 6 months | 307 | 1100 | 1557 | 295 | 953 | 1476 | 106 | –142 to 353 | 0.404 |
| 12 months | 283 | 1017 | 2031 | 268 | 692 | 1390 | 207 | –67 to 481 | 0.139 |
| Other health and social care services | |||||||||
| Baseline | 401 | 20 | 298 | 398 | 6 | 80 | 14 | –16 to 45 | 0.353 |
| 6 months | 307 | 76 | 738 | 295 | 41 | 241 | 25 | –69 to 118 | 0.605 |
| 12 months | 283 | 109 | 1101 | 268 | 5 | 31 | 49 | –50 to 147 | 0.334 |
| Informal care | |||||||||
| Baseline | 401 | 1937 | 3451 | 398 | 1508 | 3014 | 429 | –20 to 878 | 0.061 |
| 6 months | 307 | 8217 | 10,404 | 295 | 6176 | 6381 | 1586 | –252 to 2918 | 0.020 |
| 12 months | 283 | 11,152 | 21,493 | 268 | 5686 | 8786 | 3958 | 836 to 7081 | 0.013 |
| Intervention (N = 401) | Control (N = 399) | Intervention – controla | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Valid n | Mean | SD | Valid n | Mean | SD | Mean difference | 95% CI | p-value | |
| Total health and social care costs | |||||||||
| Baseline | 401 | 713 | 2636 | 398 | 787 | 1709 | –74 | –382 to 234 | 0.639 |
| 6 monthsb | 307 | 3369 | 4735 | 295 | 3171 | 5942 | 98 | –721 to 917 | 0.814 |
| 12 months | 283 | 2408 | 4161 | 268 | 1967 | 3726 | 291 | –316 to 898 | 0.347 |
| 1 yearb | 263 | 5442 | 6837 | 252 | 4462 | 6415 | 706 | –335 to 1748 | 0.184 |
| Total societal costs | |||||||||
| Baseline | 401 | 2651 | 4401 | 398 | 2296 | 3661 | 355 | –206 to 917 | 0.215 |
| 6 monthsb | 307 | 11,586 | 11,981 | 295 | 9347 | 9269 | 1663 | 56 to 3271 | 0.043 |
| 12 months | 283 | 13,560 | 22,383 | 268 | 7653 | 9472 | 4135 | 618 to 7652 | 0.021 |
| 1 yearb | 263 | 24,450 | 28,055 | 252 | 16,359 | 15,034 | 5809 | 1884 to 9734 | 0.004 |
There were no differences in QALYs between the two groups at any assessment point (Table 47).
| Intervention (N = 401) | Control (N = 399) | Intervention – controla | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Valid n | Mean | SD | Valid n | Mean | SD | Mean difference | 95% CI | p-value | |
| Utility scores | |||||||||
| Baseline | 381 | 0.51 | 0.38 | 382 | 0.56 | 0.34 | –0.04 | –0.12 to 0.03 | 0.246 |
| 6 months | 301 | 0.56 | 0.32 | 288 | 0.61 | 0.34 | –0.03 | –0.07 to 0.02 | 0.206 |
| 12 months | 270 | 0.54 | 0.32 | 259 | 0.61 | 0.32 | –0.04 | –0.10 to 0.01 | 0.140 |
| QALYs | |||||||||
| 6 months | 289 | 0.27 | 0.15 | 276 | 0.29 | 0.15 | –0.004 | –0.02 to 0.01 | 0.436 |
| 12 months | 247 | 0.28 | 0.14 | 238 | 0.31 | 0.15 | –0.01 | –0.03 to 0.01 | 0.233 |
| 1 year | 239 | 0.56 | 0.28 | 228 | 0.61 | 0.28 | –0.01 | –0.04 to 0.02 | 0.492 |
Sensitivity analyses
Imputing missing health and social care costs and QALYs at 6 months did not alter the base-case conclusion of no difference in costs or QALYs between the groups (Table 48).
| Sensitivity analysis | Intervention (N = 401) | Control (N = 399) | Intervention – controla | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Valid n | Mean | SD | Valid n | Mean | SD | Mean difference | 95% CI | p-value | |
| Effect on total health and social care costs (£) at 6 months of imputing missing total costs at 6 months | |||||||||
| Base case | 307 | 3369 | 4735 | 295 | 3171 | 5942 | 98 | –721 to 917 | 0.814 |
| Imputed data | 401 | 3208 | 4324 | 399 | 2999 | 5215 | 70 | –565 to 704 | 0.829 |
| Effect on QALY gains at 6 months of imputing missing QALY gains at 6 months | |||||||||
| Base case | 289 | 0.27 | 0.15 | 276 | 0.29 | 0.15 | –0.004 | –0.02 to 0.01 | 0.436 |
| Imputed data | 401 | 0.25 | 0.15 | 399 | 0.28 | 0.14 | –0.005 | –0.01 to 0.004 | 0.264 |
Cost-effectiveness and cost–utility
Of the 12 cost–outcome combinations examined for the cost-effectiveness and cost–utility analyses, none suggested that there were statistically significant between-group differences for both cost and outcome elements. Therefore, we have not calculated ICERs.
Cost-effectiveness planes (Figures 9 and 10) show that differences in health and social care costs between the groups at 6 months are fairly strongly centred around zero, that is, no difference, whereas outcome differences suggest a disadvantage for the intervention group in terms of less improvement on the GHQ-12 and QALY losses rather than gains. This conclusion is further represented in Figures 11 and 12, which show the probabilities that the intervention group is cost-effective compared with the control group. The probabilities of cost-effectiveness were low based on both outcome measures, not exceeding 30% for the threshold ranges examined. Probabilities based on the societal perspective were particularly low as these costs are dominated by informal care costs, which were significantly higher in the intervention group. Therefore, the intervention is unlikely to be cost-effective over a 6-month period at current policy thresholds of £20,000–30,000 per QALY gain. It is unclear what the willingness to pay for a GHQ-12 point improvement would be in practice.
FIGURE 9.
Cost-effectiveness plane of incremental total health and social care costs and point changes on the GHQ-12 at 6 months. Note: GHQ-12 scores have been reversed so that a positive difference is an improvement.
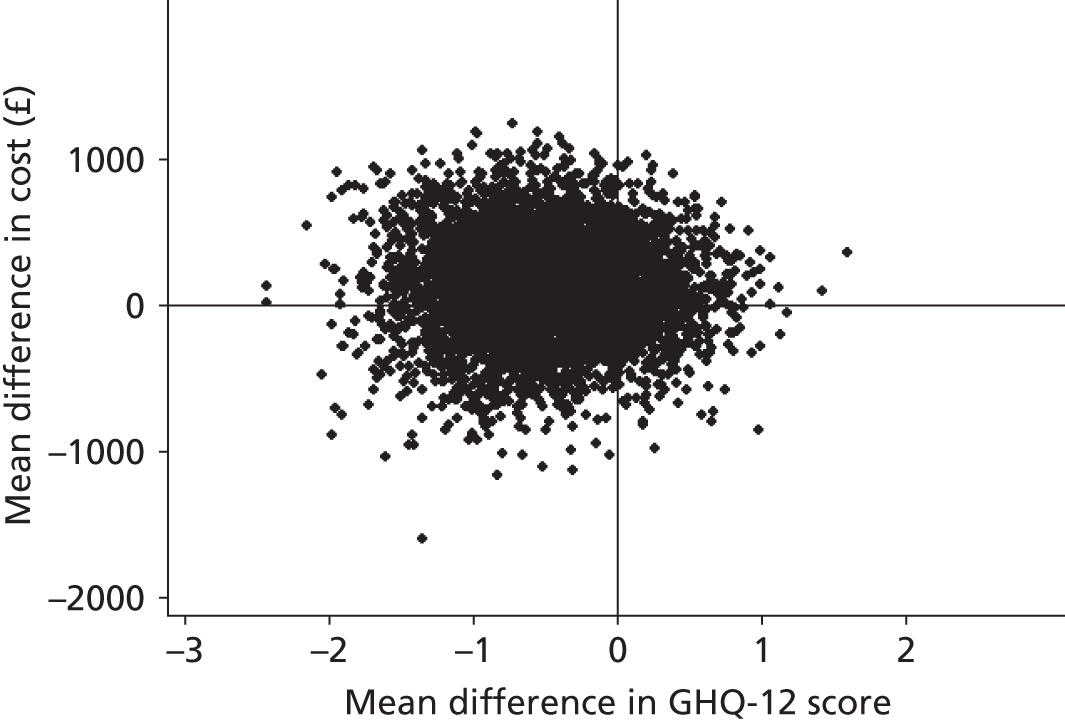
FIGURE 10.
Cost-effectiveness plane of incremental total health and social care costs and QALYs at 6 months.
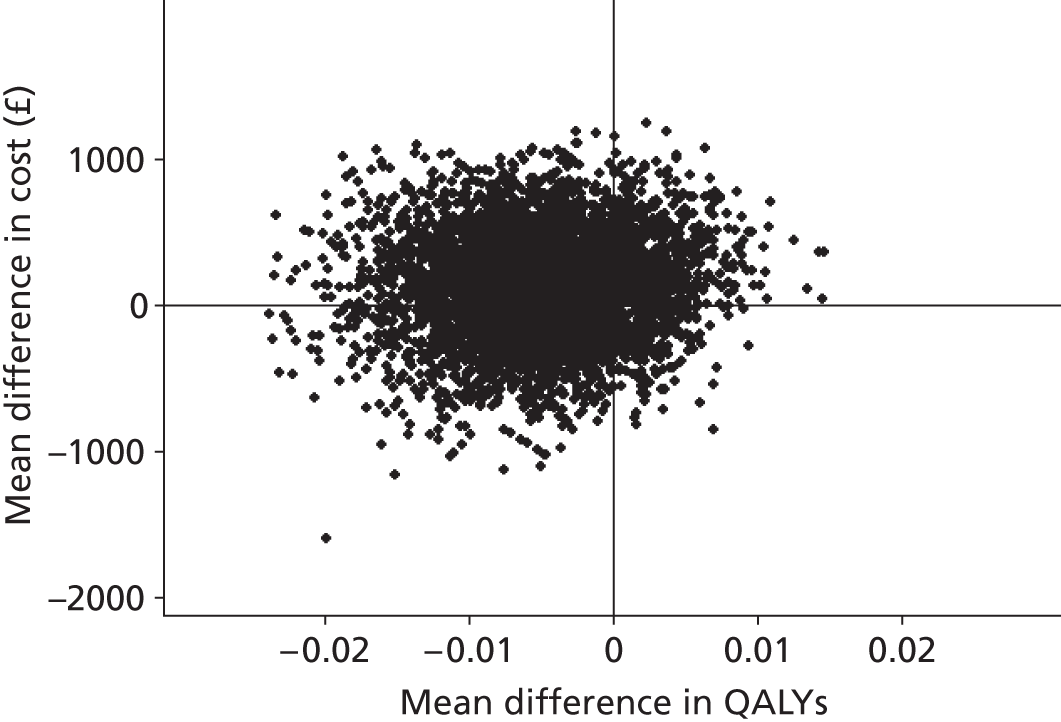
FIGURE 11.
Probability that the intervention is cost-effective compared with the control at 6 months, from each cost perspective, for a range of willingness-to-pay values for an additional point improvement on the GHQ-12.
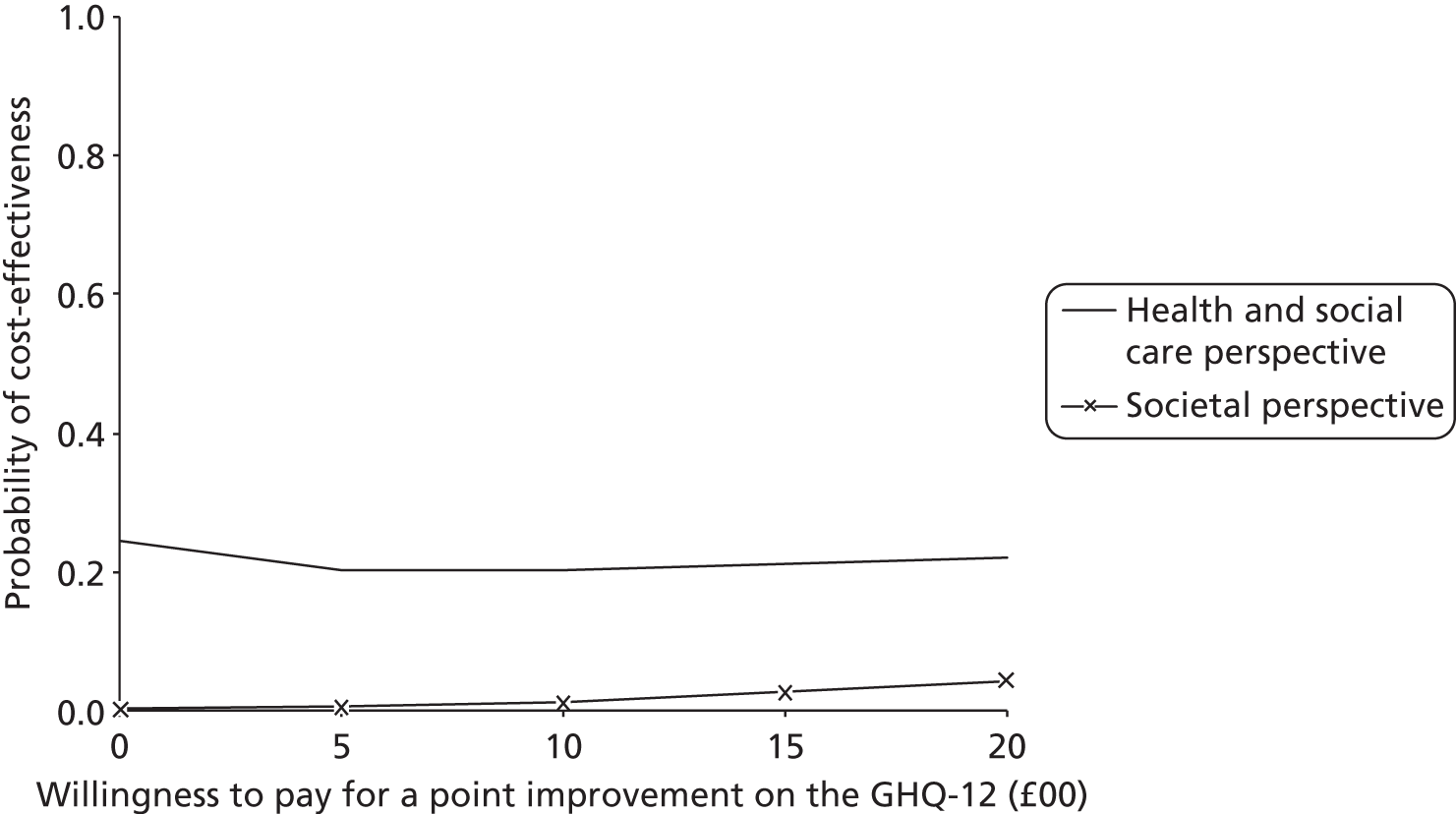
FIGURE 12.
Probability that the intervention is cost-effective compared with the control at 6 months, from each cost perspective, for a range of willingness-to-pay values for an additional QALY.
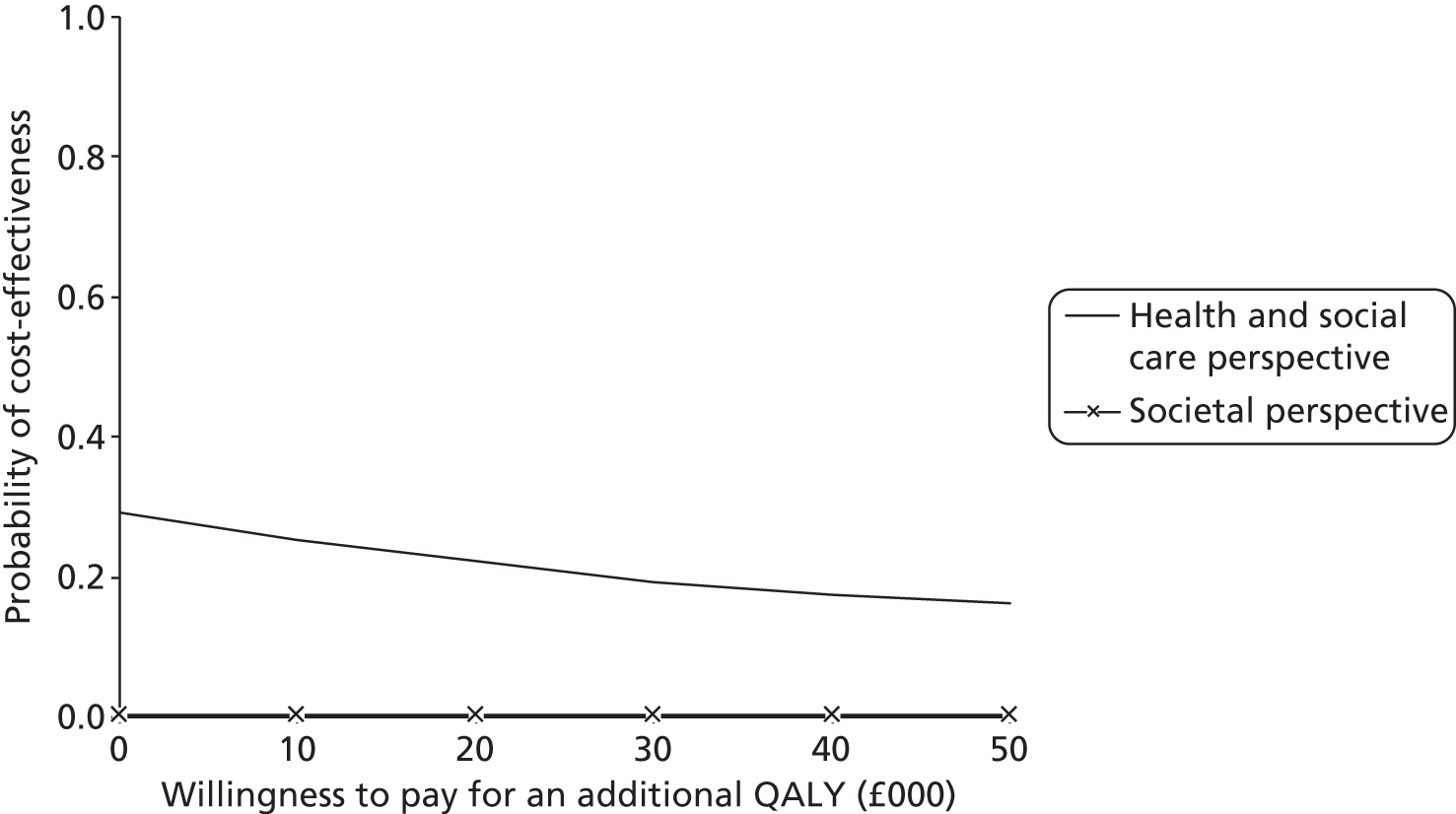
Discussion
Key findings
This trial was a pragmatic multicentre cluster RCT of a complex intervention. The trial was designed to evaluate a newly developed system of care compared with usual practice when implemented by stroke services, specifically SCCs, across the UK. The system of care was predicated on patients’ and carers’ needs and was developed through systematic reviews and the views of patients and carers, with input from primary health-care providers. The intervention was manualised and the treatment algorithms were developed through robust, evidence-based methodologies.
Services randomised to the intervention were trained in using the system of care whereas control services continued with usual practice. Patients (and optionally carers) were recruited on referral to the service and patient and carer outcomes were assessed using postal questionnaires at 6 and 12 months post recruitment. The primary outcome was patient psychological health (GHQ-12) at 6 months and secondary outcomes included patient functional health, carer psychological and functional health, and cost-effectiveness.
In total, 800 patients and 208 carers were recruited from 29 stroke services in England, Scotland, Northern Ireland and Wales. SCCs in 14 services provided the intervention whereas those in 15 services continued with usual practice. Patient groups were well matched between the two arms in demographic and clinical characteristics. There was no evidence of statistically significant differences between the arms in primary or secondary outcomes using either ITT analysis or per-protocol analysis. There was no difference in adverse events between the two groups.
The costs of SCC inputs (mean difference £42; 95% CI – £30 to £116) and total health and social care costs at 6 months, 12 months and over 1 year were similar between the groups. Societal costs were higher in the intervention group (mean difference at 6 months £1163; 95% CI £56 to £3271) because of greater use of informal care. There was no difference in QALYs between the groups and the probability of the intervention being cost-effective at 6 months was low at the current policy threshold.
Strengths and limitations of the study
This trial is one of the largest stroke rehabilitation trials completed to date (worldwide). It successfully recruited to time and target and involved centres from a wide geographical area. Implementation was supported by the National Institute for Health Research (NIHR) SRN and demonstrates the importance of the network in facilitating set-up and recruitment.
Study design
The trial followed closely Medical Research Council guidance on the evaluation of a complex intervention. 86 A cluster RCT design was chosen as the most appropriate design for the evaluation of this service-level intervention; outcome measures were carefully considered; an economic evaluation was conducted; and process data were collected.
We elected to collect outcomes by post, in keeping with our patient- and carer-centred approach. This also helped mitigate against bias, particularly as unblinding of a researcher to one patient would inevitably result in unblinding of the whole cluster. Outcome assessments for stroke patients are challenging and postal assessments are no different in this regard. There are some missing data but sensitivity analysis suggests that this does not affect the trial outcome.
Internal validity
Clusters
A total of 32 clusters were randomised equally between the control arm and the intervention arm, stratified by quality of the stroke unit (NSA score), referral rate, SCCs working alone compared with working within a community MDT, and SHA. This was fewer than the 40 services anticipated. In total, 48 services expressed an initial interest, with seven of these deciding not to pursue their involvement further and the remaining nine not being suitable to participate, either because the SCC service did not fulfil the eligibility criteria (six) or because we were unable to identify a principal investigator or researcher provision (three).
Three randomised clusters were unable to participate in the trial because of changes in SCC service or researcher provision, and some services struggled to achieve the target recruitment of 25 patients. However, low recruitment in some services was compensated for by increasing the recruitment target in services with high levels of recruitment, with only a small loss of power.
Recruitment
The cluster trial was rigorously implemented. Recruitment of patients and carers commenced after cluster randomisation of the services. The trial was carefully designed to avoid selection bias. The SRN researchers assisted with the successful minimisation of selection bias in the trial, allowing recruitment by research staff who were independent of the clinical MDTs. The SRN researchers were unaware of whether they were recruiting to an intervention cluster or a control cluster. Comprehensive screening and recruitment data were collected from all participating services and reviewed by the trial management group on a monthly basis to assess any potential bias in selection procedures.
Fewer carers were recruited to the trial than anticipated. Recruitment of carers is difficult as they may not be readily available to meet and talk through trial documentation and, when they are present, their focus will be on their relative. These lower than expected numbers are likely to be a reflection of these practical issues set in the context of SRN staff being performance managed only on number of patients recruited. These factors should be considered by researchers planning other similar studies.
Procedures were set in place to ensure that the SCCs were unaware of which patients had consented to provide trial data. The SCCs in the intervention arm delivered the system of care to all referrals regardless of trial participation. The intervention documentation (care plan) was designed so that it replaced the intervention SCCs’ previous patient documentation and thus became embedded in their standard practice.
These design features proved successful. Pre- and post-recruitment screening data show no systematic bias in favour of ‘better’ patients. Among all patients, 22% had a modified Rankin Scale score of 0–1 and 7% had a modified Rankin Scale score of 5 in pre-recruitment screening, compared with 27% and 3%, respectively, in patients recruited to the trial. Both the numbers of participants recruited and their baseline characteristics were on the whole well balanced between the study arms, demonstrating a lack of selection bias in the recruitment of participants. In the control arm there were more people who had participated in higher education and fewer with language or cognitive impairments than in the intervention arm (accounted for in statistical modelling). There was also a difference in length of inpatient stay, which was shorter in the control arm, possibly influenced by the introduction of early supported discharge services. The follow-up rate for patients of 75% at 6 months required for the power calculation was achieved in the trial (actual follow-up rate 76%).
Outcomes
The selection of outcome measures is always problematic and was subject to considerable discussion within the trial team and trial steering committee. It may be that the GHQ-12 was not sufficiently sensitive to change to pick up outcome differences in our study population. We felt that this measure was consistent with a patient-centred model of stroke recovery in which adjustment to disability is seen as a critical issue, reflects the high prevalence of psychological symptoms after stroke,25 and that psychological problems become more prevalent with time. 87 We also used a range of secondary outcomes, which similarly demonstrated little between-group differences.
Economic evaluation
It was necessary to rely on self-reported data (CSRI) in the context of factors that served as major strengths: large sample, broad evaluation perspective and spread of the sample across geographical areas/health regions. There was no feasible way to collect the same level of data by other means within the available time, staffing and monetary resources, as we lack nationally common electronic recording systems that cover different care sectors. Any limitations associated with this approach, because of inaccuracies in recollection of service use, can be assumed to apply equally to both the control group and the intervention group.
There were different patterns of informal care costs between the groups, with these costs rising over time in the intervention group but tending to fall in the control group. This may suggest that the intervention group had ongoing care needs for a longer period of time or that the intervention group may have accessed more informal care because of the goal-setting element of the intervention. However, caution must be exercised in interpreting the informal care data, which were based on retrospective self-reports. Questions about inputs were categorised to aid recall but it cannot be ruled out that responders double-counted or overestimated inputs. However, although this would affect the quantification of care inputs and their costs, any such bias could be expected to be equal across the groups. The size of the informal care costs in comparison to the formal care costs highlights the need for more attention to be paid to the development of appropriate and feasible methods for the measurement and valuation of such inputs.
Generalisability
Including a wide range of disparate geographical regions ensured a good representation of different health-care settings. The eligibility criteria were kept to a minimum, in keeping with the pragmatic trial design, to ensure that a stroke patient population representative of referrals to SCCs was recruited, including patients with language and cognitive impairments. Of those patients eligible for the trial, 56% were recruited. In total, 29% of patients recruited had a cognitive impairment and 9% were unable to complete the cognitive test as a result of communication difficulties.
Implementation of a complex intervention
As a pragmatic trial the intervention was implemented as any service initiative within the NHS would have been. The challenges of implementation were considered carefully in designing the delivery of the training and included choosing a method that would be acceptable and feasible to MDT staff and NHS management and which could easily be replicated across the UK at the end of the trial.
Training was provided at national training days, which SCCs from all intervention services attended. Because of the nature of a cluster trial the SCCs randomised to the intervention were unaware of what the new service model consisted of before these training days. The system of care was well received when presented by the trial team, demonstrating ‘face validity’ for the intervention. In the first training day the system of care was introduced and a workshop was led by Professor Allan House on assessment and problem-solving techniques. Practical issues of implementation were addressed, specifically the design of the care plans and manual. The content of subsequent training days was informed by the needs of the SCCs. Thus, a lecture on pain management was provided by Professor Tony Rudd and a discussion on benefits was led by a colleague from the Department for Work and Pensions. The format and layout of the care plan were further reviewed and refined following feedback from the SCCs. The intent was to ensure that the paperwork captured all of the information that SCCs might require to deliver their service and that it was therefore able to replace the current documentation.
The training was supported by a CD of the training day. The SCCs implemented the new system of care in their service over a period of months and when they were comfortable with implementation (demonstrated through a review of completed care plans) trial recruitment began. We feel that this process was thorough and comprehensive. Some SCCs left and were replaced during the trial and, in services that included MDTs, all SCCs did not necessarily attend the training. The intent was that the training would be cascaded down to other staff in the team by staff who had attended the training days. It may be that this commonly used ‘cascade’ method was not as effective as we would have wished.
Trial procedures
Service engagement in the trial was preceded by completion of an extensive survey, individual interviews with SCCs and visits to every service. In addition, an ‘organisational’ visit took place at every service, which included all staff directly involved in the trial and post-discharge care pathway. This included the inpatient services trial principal investigator (often the stroke consultant), the community principal investigator, the SCC(s), SRN staff and researchers. At this meeting the practicalities of trial recruitment were discussed in relation to the patient flow and agreement was reached on the most efficient processes. At these meetings it was also emphasised that the SCCs should not be aware of which patients were trial participants and researchers should not know whether the service was an intervention service or a control service. Despite this, however, when visits were undertaken to collect process data after the end of follow-up, it emerged at some services that patients fulfilling the entry criteria of ‘referred to stroke care co-ordinator’ had not been seen by a SCC (approximately 20% of patients across all services). There were a range of reasons for this, which included patients never actually being referred or being referred but living outside the catchment area for the service and the referral therefore not being accepted. These glitches in the service caused those SCCs affected to review their referral pathways. Of patients seen by a SCC, 89% in the intervention arm had a completed care plan compared with 66% in the control arm with a completed time log (completed only for the purposes of the trial), illustrating the difficulty of ensuring completion of additional trial paperwork.
Intervention compliance
We set a high bar for intervention compliance, defined as at least 12 of 16 assessment areas (75%) (each linked to a reference guide in the manual) discussed at patient contact 1 of the care plan. Of all care plans completed, 96% were compliant according to this definition, with on average 94% of assessment areas discussed at contact 1, indicating that the assessment was delivered as expected and that this is an appropriate approach to implement evidence into practice.
Although it seems that the structured assessment was delivered to the majority of participants who received the SCC service, the basic tenet of the approach, what is more difficult to assess is whether this translated into a change of practice. In keeping with the pragmatic trial design, we collected limited data about processes of care. A process evaluation may have provided more in-depth information.
The number of intervention patient assessment contacts ranged from one to six, with the majority (70%) of patients having one or two (comparable to the number in control services). This reflects the pre-trial survey and service models, in which a SCC undertakes initial assessment before referral to other members of the MDT (75% of the services in the trial included team rather than individual SCCs). It may be that in this service model the notion of comprehensive holistic assessment becomes dissipated by individual allied health professionals delivering their single discipline input, for example therapists prioritising mobility problems. Although the number of assessment contacts was not prescribed, a key principle of the intervention is that goals are reviewed at subsequent contacts, which will necessarily be limited if the number of contacts is limited.
It was observed that, in some services, care plans were clearly different from patient to patient and gave an impression of a patient’s personality, overall situation and priorities. In other services, care plans were more uniform, being difficult to distinguish from one patient to another, and it appeared that the SCCs were focusing the assessment on certain areas for all patients, despite asking questions in all of the assessment areas. These observations might have several interpretations (see Appendix 9): they may reflect wider service pressure, for example a service-level focus on secondary prevention and medical management could mean (however unintentionally) prioritising that aspect of the assessment, but may also represent the extent to which the assessment was patient led and holistic and based on a collaborative discussion with the patient. This highlights the challenge of implementing interventions in rehabilitation that are a complex interaction between context, patient and professional.
Despite regular MDT meetings the original care plan assessments were not always revisited to check that actions had been undertaken and goals reached. It does appear from the LUNS monitoring tool that needs remain unmet at 6 and 12 months. A proportion of these may be intractable difficulties for which there is no solution available, or needs that have emerged after the end of SCC service provision, whereas others may reflect a lack of access to appropriate services to address the need. No service will necessarily be able to successfully address all problem areas or predict what difficulties may occur in the future.
The SCCs gave examples during interview of how their assessment or practice has changed (as a result of either the training day or using the system of care). These included more in-depth thinking about problem-solving; enabling patients to identify their resources or coping strategies; a more consistent and well-structured assessment; covering new areas such as sexual functioning; and delegating more responsibility for actions to the patient. It is possible that delegated responsibility may move from the patient into additional burden for the carer, contributing to the observed increase in informal care costs discussed earlier. SCCs used the care manual to at least some extent. There were a number of similarities between the practice of control SCCs and the practice of intervention SCCs; however, although control SCCs intended to provide a fully holistic initial assessment, it is not clear from interviews whether they actually did, and three services reported not covering all areas for all patients, whereas the intervention SCCs appeared to provide a holistic assessment more consistently. Although elements of evidence-based practice were evident across all settings, the intervention ensures that all key issues are covered. The lack of effectiveness of the intervention may be in part because there were not big enough differences in the work of SCCs between the control setting and the intervention setting.
Improving outcomes following comprehensive assessment can be successful only if appropriate care and treatment are available for the problems identified. Although all of the reference guides are evidence based, the evidence points to more effective interventions for certain problems than for others. This may be particularly relevant in relation to psychological problems. Interviews with the SCCs after the trial indicated that access to psychological care was particularly patchy, with 12 services across control and intervention services reporting extremely limited psychological services. This in turn impinges on our main outcome measure, which assessed mood. Access to other services was generally good, with limitations mentioned by no more than one service; however, limited voluntary and community resources such as social groups were reported by a number of services, two services reported a lack of services to address employment and two services reported a lack of services to address sexual functioning problems. In intervention services, mood was identified as one of the most prevalent problem areas at the first SCC contact and the most prevalent problem area at later contacts.
The challenge in multicentre rehabilitation trials is to provide some guidance on the intervention to capture the main features, enhance external validity and improve generalisability while making it sufficiently flexible for it to be acceptable to staff and deliverable in a range of service models. Our approach was to manualise the intervention, supported by training days, with an opportunity to practise delivery before the start of patient recruitment. The care plan was well completed but we were perhaps less successful in changing the behaviours and mindset of the SCCs, as illustrated by the observation discussed earlier, that some SCCs focused the assessment on certain areas for all patients. Further work should explore how to embed behaviour change techniques in both intervention training and delivery and the intervention itself.
Context
Although the role of the SCC had been recommended in the National Service Framework for Older People,16 our pre-trial survey revealed that relatively few services had adopted this model and even fewer used a dedicated SCC role. It might be suggested therefore that the services that were recruited to the trial were particularly forward thinking and slightly atypical compared with other services across the UK. Since this programme grant was awarded, in 2007, there have been considerable changes in stroke service provision across the UK. Our system of care is very much in keeping with current developments. Similar approaches have been developed (e.g. GM-SAT for 6-month reviews34). This indicates that our thinking is feasible, practical and appropriate for ongoing service development. However, because of the increased awareness of the longer-term needs of stroke patients and carers, as promoted through the work of the Stroke Improvement Programme, the SCCs in the control arm may have adapted their practice accordingly. However, none of the control SCCs reported using structured review tools in the survey and interview that they completed after the end of the trial.
It would seem that longer-term outcomes for patients after stroke do remain quite poor. A Stroke Association unmet needs survey4 demonstrated the range and depth of unmet needs. Our own work reported similar outcomes (see Chapter 4).
The trial was not designed to evaluate whether the SCC service was effective but whether the structured patient-centred system of care improved patient outcomes. Our hypothesis was that the system of care configured around patient needs would be more holistic and more responsive in addressing the well-recognised unmet needs of patients after stroke.
Further analysis is ongoing to determine whether some subsets of patients, possibly over selected time periods, did have an enhanced outcome from the intervention approach, or whether service/process factors contributed to differences in outcomes. A realist evaluation of the trial has also been undertaken,88 which may shed further light on how the SCCs have implemented the system of care.
The trial results apply to a heterogeneous population. It might be that the SCC service should be targeted more towards patients (and carers) with specific needs, leading to a more specialised bespoke service.
Chapter 4 Project 3: Longer-term Unmet Needs after Stroke study
Abstract
Aim
The study aim was to finalise the development of the LUNS questionnaire and evaluate the acceptability, test–retest reliability and validity of the resulting 22-item questionnaire for measuring longer-term unmet needs of patients living at home post stroke.
Methods
Patients with a primary diagnosis of stroke being discharged to their own home or that of a carer, after a minimum 3-day hospital stay, were recruited from 40 stroke units across England. A questionnaire pack including the LUNS questionnaire, GHQ-12, FAI and Short Form questionnaire-12 item (SF-12) was posted to participants 3 or 6 months after stroke to assess acceptability and validity. The LUNS questionnaire was re-sent 1 week after receiving back the first pack to assess test–retest reliability. Semistructured interviews were conducted with participants who reported low unmet need on the LUNS questionnaire to explore whether they genuinely considered this to be the case.
Results
In total, 850 patients, including 199 (23%) with impaired communication and/or cognition, were recruited. A total of 529 pack 1s were returned (69% of those sent), with 3.5% missing LUNS items, comparable with the concurrent measures. The median number of unmet needs was four (range 0–19) and participants reporting low unmet need verified this to be the case and that they did not have other unmet needs not captured by the LUNS questionnaire. Identification of an unmet need was consistently associated with poorer outcomes on concurrent measures. The test–retest reliability of the LUNS questionnaire (n = 326) was moderate to good (77.9–98.8% item agreement, κ = 0.45–0.67).
Conclusions
The LUNS questionnaire shows suitable acceptability, test–retest reliability and validity for identifying longer-term unmet needs after stroke.
Introduction
Background
Stroke is the leading cause of complex disability in adults. 89 Research into the longer-term experiences of stroke survivors has found that they experience a range of difficulties, including, for example, incontinence, depression, lack of information, pain, and restrictions in social and household activities. 5,90–93 Many of these problems are highly prevalent and exist even a decade after stroke,94 suggesting that they are not appropriately identified or addressed by service providers. To address the diverse and complex difficulties that stroke survivors face, effective methods are required to identify their unmet needs. Regular patient reviews, as part of an integrated longer-term stroke pathway, are recommended2,15 and could incorporate needs assessment. Measuring unmet need is also a way of identifying stroke service deficiencies95 and this may enable effective commissioning and provision of services. Assessment of needs can be defined as the ability to benefit from health (or social care)96 and unmet needs may be defined as ‘expressed needs that are not satisfied by current service provision’ (p. 1052). 97
Existing instruments to measure problems after stroke, such as the FAI,98 tend to deal with limited aspects of the stroke experience and therefore do not capture the range of domains pertinent to longer-term life after stroke. A recent survey in the UK identified the unmet needs of stroke survivors but the survey instrument was not evaluated for reliability. 4 Tools designed for measuring unmet needs in people recovering from stroke have been developed for research purposes but are long and have poor response rates. 99–101 There is a need for a short, easy-to-complete tool for identifying unmet needs after stroke.
Development of the Longer-term Unmet Needs after Stroke questionnaire
We developed a 22-item questionnaire (termed ‘LUNS’) to provide a method for identifying the longer-term unmet needs of stroke survivors. A multistage iterative process was used to develop, refine and carry out preliminary testing of the LUNS questionnaire, summarised in Figure 13 and described in detail below.
FIGURE 13.
Tool development summary.
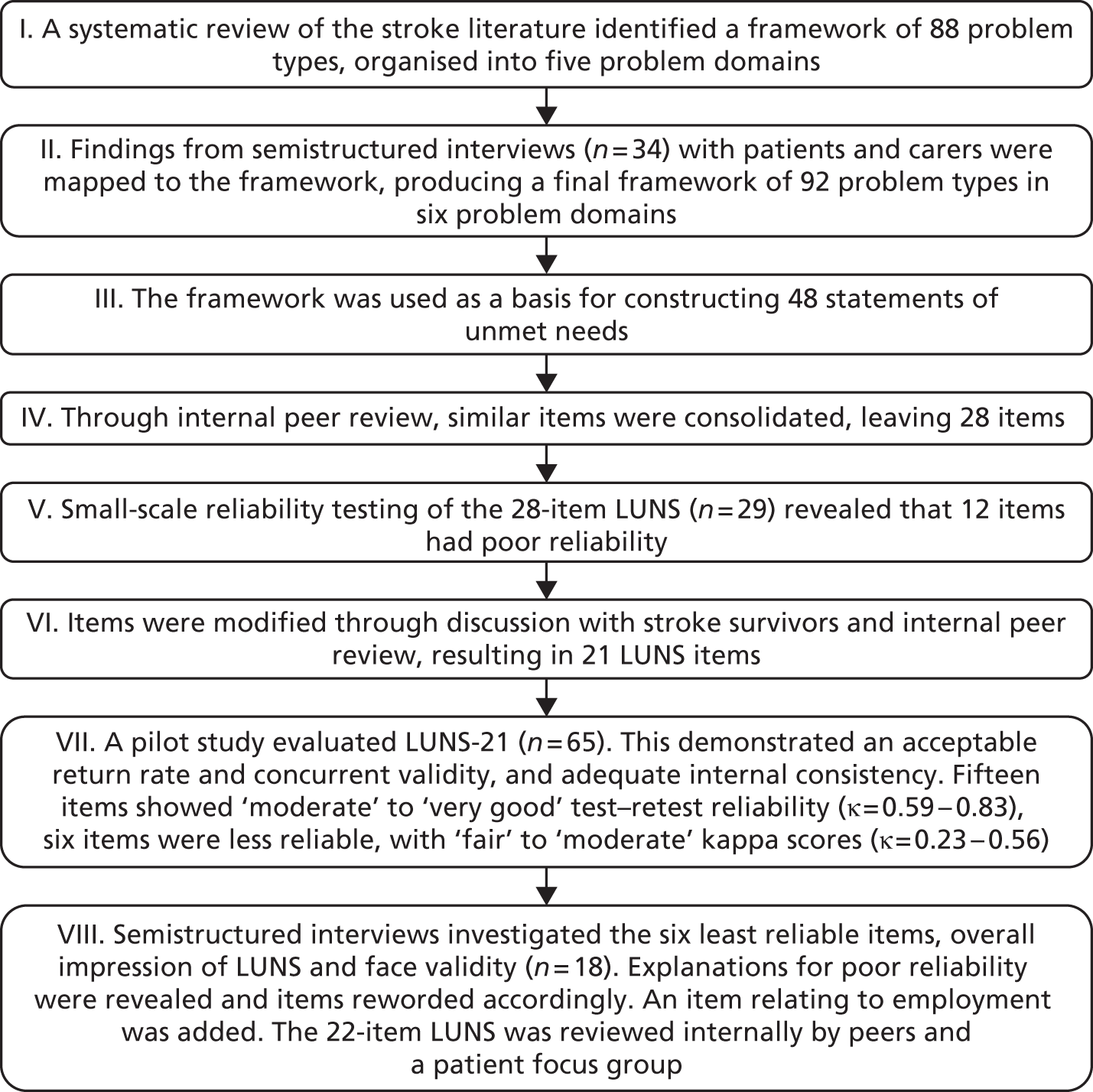
Items for the LUNS tool were identified through systematic reviewing of qualitative and quantitative literature reporting the longer-term stroke experience24,25 and, subsequently, semistructured interviews with stroke patients and carers. 26 The data were represented in a framework comprising 92 longer-term stroke problem types organised into six domains (transfer of care, information, services, social and emotional consequences, caring role, and health problems and related areas).
This framework was used as a basis for constructing an initial series of 48 statements representing patient unmet needs (items relating to the domain of caring role were excluded). Through internal peer review, similar items were consolidated (such as bathing and dental into personal care) and statements were removed, leaving 28 items. The 28-item LUNS tool underwent small-scale reliability testing with 29 stroke patients, revealing that 12 items had poor test–retest reliability. Further modification to each item within the tool was facilitated by discussion with members of two stroke clubs, a patient research advisory group and internal peer review. This resulted in a 21-item LUNS tool.
Pilot study
To further evaluate the 21-item LUNS tool it was combined in a self-completion A4 booklet, along with the Hospital Anxiety and Depression Scale (HADS)102 and the Nottingham Extended Activities of Daily Living (NEADL) Index. 103 The style of the questionnaire booklet used here was informed by previous work104 and the booklet was reviewed by members of the CRAG. The acceptability, reliability and validity of the 21-item LUNS tool were evaluated in a three-centre pilot study. A total of 65 patients living at home at 2–21 months post stroke were sent the booklet at two time points. Of these, 48 patients (74%) completed both booklets at a median interval of 10 days, with only 0.1% of the LUNS items not completed (after chasing missing data). The 21-item LUNS tool showed good discriminant validity with HADS, with cases of anxiety or depression reporting more unmet needs, and acceptable concurrent validity with respect to HADS and NEADL (correlation coefficients were −0.424 for NEADL, 0.588 for HADS anxiety and 0.547 for HADS depression). Internal consistency was adequate with a Cronbach’s alpha of 0.814. Of the 21 LUNS items, 15 showed moderate to very good test–retest reliability (88–98% agreement between the two time points, κ = 0.59–0.83). The remaining six items were less reliable [77–90% agreement, kappa fair for two items (κ = 0.23–0.24) and moderate for four items (κ = 0.50–0.56)].
These items were investigated in semistructured interviews with 18 patients, following their completion of the LUNS questionnaire. The six items were included in the interview topic guide, along with four general questions (scoping all other LUNS items) relating to ease of completion, face validity, sensitivity and general impression of the LUNS tool. The recorded completion time for the 21-item LUNS tool was a median of 6 minutes (range 2–12 minutes). All interviewees reported that the LUNS tool was acceptable and unobtrusive and reflected their experiences of stroke. Possible explanations for poor reliability were revealed during the interviews and the six items were reworded accordingly. An item relating to employment after stroke was added.
The resulting 22-item LUNS tool was internally peer reviewed and then circulated to members of a patient group for feedback in a subsequent focus group. The focus group agreed that employment was an important issue and should be included. In the final 22-item LUNS tool, each item has a ‘yes/no’ response, with the ‘no’ option applying to either no need or the need is met. Problem domains are information, services, social and emotional consequences, and health problems and related areas. The LUNS questionnaire is provided in Appendix 11.
Objectives
The primary objective of the LUNS study was to evaluate the acceptability, test–retest reliability and validity of the LUNS questionnaire for identifying longer-term unmet needs of patients living at home post stroke.
Secondary objectives were to:
-
evaluate whether the LUNS questionnaire has suitable properties to be used to give a score for measuring the level of unmet need (internal consistency, dimensionality)
-
explore perceptions of unmet need using qualitative methods.
Methods
This study was approved by the Bradford Research Ethics Committee.
Study design
The LUNS questionnaire was evaluated in a large multicentre study. It was administered by post at 3–6 months post stroke in a self-completion questionnaire pack of the same style as that used in the pilot study. The pack contained the LUNS questionnaire and four validated questionnaires on health status, which were used as concurrent measures to assess the validity of the LUNS questionnaire (see Data collection). There are no measures of unmet need capable of capturing broad life areas in this population with which we could evaluate the concurrent validity of the LUNS tool; therefore, the LUNS tool was compared with health measures that could reflect the level of need. The LUNS questionnaire was also administered at a second time point to assess test–retest reliability.
The study was conducted in two phases. Factors such as cognitive impairment and unfamiliarity with the language may affect ability to accurately complete a questionnaire. To assess properties of the LUNS tool without interference from these factors, in phase 1 a selected population of English-speaking patients without communication or cognitive impairment was selected. Interim analysis of phase 1 data was conducted to ensure adequate acceptability, test–retest reliability and validity of the tool in this optimised group before proceeding to phase 2. To ensure inclusion of a sample with a broad range of stroke impairments in phase 2, we recruited patients who had spent longer in hospital (likely to be more disabled) and set a target of 50% of patients with cognitive or communication impairment; patients who did not speak English were also included in this group because it was expected that they would have difficulty completing an English-language questionnaire. This target was reduced to 40% during recruitment to meet target timelines.
Participants
Patients were recruited by SRN staff from stroke units across England. Eligibility criteria were purposefully broad: a diagnosed new stroke, age ≥ 16 years and patient being discharged to his or her own home or that of his or her carer. Patients were not eligible if concurrent illness required palliative care or if permanent discharge to a nursing or residential home was planned.
In addition to the above, patients were eligible for phase 1 only if they were English speaking, had no cognitive or communication impairment and had undergone a minimum hospital stay of 72 hours. In the second phase, patients who were unable to read and understand English were recruited if they had an English-speaking carer, patients with a communication or cognitive impairment were included and the minimum hospital stay was increased to 14 days.
With verbal consent, cognition and communication were assessed in both phases using 6CIT65 or the Frenchay Aphasia Screening Test (FAST)105 respectively. Patients who scored ≥ 8 on 6CIT were considered to have cognitive impairment and patients who scored ≤ 6 for FAST comprehension or ≤ 3 for FAST reading were considered to have communication impairment. The group assessed as having no communication or cognitive impairment and who are English speaking are described as the ‘no impairment group’ whereas those assessed as having a cognitive or communication impairment or who are not English speaking are described as the ‘impairment group’ (not speaking English could be considered an impairment in relation to completing an English-language questionnaire).
Written informed consent was obtained from patients before collection of baseline data or, when applicable in the second phase, a relative or friend of a patient lacking capacity was asked to consider the patient’s wishes and provide a consultee declaration.
Recruited patients who became ineligible (e.g. moved to a nursing home) were withdrawn from follow-up and analysis.
Data collection
Baseline data were collected on the ward by SRN staff. Demographic information, stroke details, pre- and post-stroke Barthel Index scores38 and comorbidities106 were recorded.
After confirming patients’ contact details and survival status through GP or NHS database records, the LUNS tool was posted to the participants’ home address in a self-completion questionnaire pack. The first pack (pack 1) contained the LUNS tool and the following validated and reliable measures:
-
Impairment Manikin107 – This asks whether the respondent has completely recovered from stroke and includes a diagram of a body on which patients indicate ongoing problems, for example difficulties with their leg or vision.
-
GHQ-1262 – A measure of emotional health and well-being. A higher score indicates a poorer level of emotional well-being.
-
FAI98 – A measure of extended activities of daily living. The FAI is scored from 0 to 45 with a higher score indicating a higher activity level.
-
SF-12108 – A measure of health-related quality of life. The two component scores of the SF-12 [mental component score (MCS) and physical component score (PCS)] were used. Scores are standardised to a 0–100 scale, with 50 representing the norm value in the general population, < 50 indicating health worse than the norm and > 50 indicating health better than the norm.
The second pack (pack 2) included the LUNS and SF-12 tools. Both packs included a question asking whether any help was needed with completion of the questionnaires and pack 2 included a question asking whether the patient had experienced any health changes since completing pack 1. Proxy responses were allowed.
In phase 1, pack 1 was posted to study participants at 3 or 6 months post stroke (dependent on their date of recruitment into the study). This was to enable timely completion of data collection and the interim analysis. In phase 2 all pack 1s were posted at 6 months post stroke when patients were more likely to be home from hospital (considering the increased length of stay and the possibility of cognitive or communication impairment). Using these time points allowed patients a period of time living at home so that they could understand their longer-term needs. Patients were asked to complete the pack and return it to the research team using a prepaid addressed envelope. Patients who had not returned their pack to the study team within 2 weeks were telephoned to check receipt and if necessary the pack was re-sent. If questionnaires were missing < 50% of the data, patients were telephoned to complete the missing data. Questionnaires missing ≥ 50% of the data were re-sent. To assess test–retest reliability, pack 2 was sent approximately 1 week after the fully completed pack 1 was received, or if missing data in pack 1 had not been returned within 2 weeks.
Sample size
A sample size of 350 was required in phase 1 for the interim analysis evaluating whether the LUNS tool was suitable for this optimised group. The sample size in phase 2 was 500 to give a total sample size of 850 to assess the full psychometric properties of the LUNS tool. For the psychometric Rasch analysis,109 a sample size of 243 is required to generate 99% confidence that item calibrations are stable within half a logit, even with poor scale targeting. 106 In phase 1, with an estimated response rate of 70%, a sample size of 350 would generate 245 cases, which is adequate. Phase 2 targeted a more impaired sample and so a lower response rate was anticipated. A response rate of 50% was estimated within this target population, meaning that a sample size of 500 would generate 250 cases. Phase 1 and phase 2 data can also be pooled to create a more powerful analysis.
Statistical methods
Baseline data were summarised using descriptive statistics and compared using Mann–Whitney U-test, chi-squared and independent t-tests for responders and non-responders to pack 1. The number and types of unmet needs reported in pack 1 were summarised and individual unmet needs were compared between the impairment group and the no-impairment group using Pearson’s chi-squared test.
The acceptability of the LUNS tool was determined by the percentage of pack 1s returned and the amount of non-completed items on initial receipt.
The test–retest reliability of the LUNS tool was assessed by comparing the percentage agreement for pack 1 and pack 2 and kappa statistics for individual items. 111 SF-12 test–retest results were calculated to provide context to the LUNS results. Patients who identified a change in their health status between packs 1 and 2 were excluded from the test–retest reliability assessment. The strength of agreement for kappa was defined as ranging from poor (κ = 0–0.20) to fair (κ = 0.21–0.40), moderate (κ = 0.41–0.60), good (κ = 0.61–0.80) and to very good (κ = 0.81–1). 112
The Mann–Whitney U-test was used to test differences in health status (GHQ-12, FAI, SF-12) between the group who identified unmet need and the group who identified no unmet need on each LUNS item. FAI total scores were calculated only for patients who were sent pack 1 at 6 months post stroke because of the inclusion of questions regarding the preceding 3 and 6 months.
Whether the LUNS questionnaire measures a single underlying dimension or multiple separate dimensions was investigated. Factor analysis allows us to produce an internally reliable scale by identifying the items that most correlate with each other. 113 It was first hypothesised that the LUNS tool would fit one dimension and would be tested using confirmatory factor analysis; should this fail, exploratory factor analysis would be used to explore potential dimensions. Factor analysis was carried out using MPlus computer software (version 6; Muthén & Muthén, Los Angeles, CA, USA).
A Cronbach’s coefficient alpha value114 was also calculated for the item set. Cronbach’s coefficient alpha is referred to as a measure of internal consistency reliability and is the proportion of variance in a set of scores that can be attributed to a common influence on the scores of the individual items. 115 However, it has also been argued that alpha is unrelated to the internal structure of the test and should therefore be viewed with caution. 116
Additionally, Rasch methods109 were used to formally test the LUNS tool against a unidimensional measurement model. Rasch analysis provides a unified framework to assess a number of measurement characteristics of individual items, as well as the total scale. Rasch analysis was carried out using RUMM2030 computer software (RUMM Laboratory, Perth, WA, Australia). Full details of Rasch analysis and the various tests of fit available can be found elsewhere. 117,118 Briefly, we examined individual person and item fit, with fit residuals between ± 2.5 displaying adequate fit. Chi-squared and analysis of variance (ANOVA) item fit statistics were also used to identify measurement anomalies. Differential item functioning (DIF) was examined to identify any bias by sample subgroup, considering age, gender, living arrangements (alone/with someone) and cognitive status (impairment or no impairment). Response dependency occurs when the response to one item has a direct influence on the response to another item, indicated by a positive residual correlation > 0.2. Targeting was considered to assess the relative distributions of the item and person locations. The person separation index (PSI) and Cronbach’s alpha statistics were used to assess the internal consistency (reliability) of the LUNS questionnaire.
Qualitative evaluation of unmet needs
During phase 2, a substudy was designed to further understand whether stroke patients who self-reported having no unmet needs on the LUNS questionnaire genuinely feel that they have no unmet needs or whether their unmet needs were missed by the questionnaire. Purposive sampling selected people who self-reported no (0) or low-level (1) unmet needs within a single region (Yorkshire) and semistructured interviews were conducted. A topic guide was written to ask about current problems people may have, how these are managed and how they may be identified as unmet needs. Data analysis used a thematic approach, working through the data until a small number of themes were identified that sufficiently describe the data.
Results
Key results have also been published elsewhere. 119
Participant flow
A total of 350 participants were recruited from 29 sites in phase 1. In total, 237 pack 1s and 200 pack 2s were returned and included in the interim analysis. Interim analysis of phase 1 data found that the LUNS tool demonstrated adequate acceptability, test–retest reliability and validity to progress to phase 2 of the study. In phase 2, 500 participants were recruited from 40 sites. All results are reported for the combined study population of 850. Recruitment, questionnaire returns, losses and withdrawals for the combined phase 1 and phase 2 study population are summarised in Figure 14.
Recruitment
Recruitment to phase 1 was conducted between 11 December 2008 and 1 May 2009. Phase 1 follow-up was completed on 26 September 2012. Recruitment to phase 2 was conducted between 1 February 2010 and 10 January 2011. Follow-up was completed on 29 June 2011.
Baseline data
Of the 850 patients recruited, 199 (23%) had a communication or cognitive impairment or did not speak English. The 6CIT identified cognitive impairments in 138 patients, the FAST identified communication difficulties in 56 patients, three people did not speak English and two people could not provide a 6CIT or FAST score but were assessed as having a cognitive impairment. The median age of the population was 73 years and the median time in hospital was 12 days in phase 1 and 42 days in phase 2. Comorbidities are shown in Table 49 and additional baseline characteristics are shown in Table 50.
| Comorbidity | Problem identified, n (%) |
|---|---|
| Heart problem | 249 (29) |
| High blood pressure | 496 (58) |
| Lung disease | 108 (13) |
| Diabetes | 158 (19) |
| Ulcer or stomach disease | 91 (11) |
| Kidney disease | 41 (5) |
| Liver disease | 6 (1) |
| Anaemia/blood disease | 66 (8) |
| Cancer | 70 (8) |
| Depression | 126 (15) |
| Arthritis | 337 (40) |
| Back pain | 242 (29) |
| Characteristic | Responders (N = 529), n (%) | Non-responders (N = 231), n (%) | All recruited (N = 850), n (%) |
|---|---|---|---|
| Age (years), median (range) | 73 (28–96) | 69 (29–98) | 73 (28–98) |
| Gender: male | 288 (54) | 129 (56) | 458 (54) |
| Living alone pre stroke | 201 (38) | 102 (44) | 343 (40) |
| Days in hospital, median | 25 | 28 | 27 |
| First-ever strokea | 439 (83) | 169 (73) | 687 (81) |
| Ethnicity: whitea | 521 (99) | 220 (95) | 827 (97) |
| Have communication or cognitive impairmenta | 91 (17) | 63 (27) | 199 (23) |
| Post-stroke Barthel Index scorea | |||
| Median score (range) | 18 (0–20) | 17 (0–20) | 17 (0–20) |
| 20 (independent) | 148 (28) | 43 (19) | 201 (24) |
| 15–19 (mild disability) | 204 (39) | 104 (45) | 333 (39) |
| < 15 (moderate to very severe disability) | 176 (33) | 84 (36) | 311 (37) |
| Stroke: pathological classification | |||
| Cerebral infarction | 472 (89) | 207 (90) | 761 (90) |
| Primary intracerebral haemorrhage | 49 (9) | 20 (9) | 74 (9) |
| Other | 8 (2) | 4 (2) | 14 (2) |
| Stroke: clinical classification | |||
| Left hemiparesis | 262 (50) | 94 (41) | 397 (47) |
| Right hemiparesis | 195 (37) | 106 (46) | 341 (40) |
| Brain stem | 14 (3) | 5 (2) | 22 (3) |
| Other | 58 (11) | 26 (11) | 89 (11) |
Table 50 also shows the baseline demographic and clinical characteristics of patients returning or not returning questionnaire pack 1 (responders and non-responders respectively). Overall, responders had a lower level of cognitive and physical impairment than non-responders. Significant differences (p < 0.01) were observed for number of previous strokes, ethnicity, communication or cognitive impairment, and post-stroke Barthel Index score. No other significant differences were found when comparing responders with non-responders.
The baseline characteristics of the no-impairment and impairment groups were broadly similar, except that the impairment group was slightly older, had a longer hospital stay and was more disabled according to the Barthel Index score; in addition, a greater proportion of the impairment group had suffered a haemorrhage or had right hemiparesis (Table 51).
| Characteristic | No-impairment responders (N = 438), n (%) | Impairment responders (N = 91), n (%) |
|---|---|---|
| Age (years), median (range) | 72 (30–96) | 79 (28–96) |
| Gender: male | 243 (56) | 45 (50) |
| Living alone pre stroke | 168 (38) | 33 (36) |
| Days in hospital, median (range) | 22 (4–214) | 46 (15–267) |
| First-ever stroke | 366 (84) | 73 (80) |
| Ethnicity: white | 431 (98) | 90 (99) |
| Post-stroke Barthel Index score | ||
| Median score (range) | 18 (0–20 | 15 (0–20 |
| 20 (independent) | 137 (31) | 11 (12) |
| 15–19 (mild disability) | 168 (38) | 36 (40) |
| < 15 (moderate to very severe disability) | 132 (30) | 44 (49) |
| Stroke: pathological classification | ||
| Cerebral infarction | 394 (90) | 78 (86) |
| Primary intracerebral haemorrhage | 36 (8) | 13 (14) |
| Other | 8 (2) | 0 (0) |
| Stroke: clinical classification | ||
| Left hemiparesis | 228 (52) | 24 (37) |
| Right hemiparesis | 151 (35) | 44 (48) |
| Brain stem | 13 (3) | 1 (1) |
| Other | 46 (11) | 12 (13) |
Numbers analysed
In total, 529 pack 1s were returned. Of these, 20 were excluded because of missing LUNS items and 509 were included to assess acceptability, for comparison with concurrent measures, for calculation of Cronbach’s alpha and for factor analysis (423 in the no-impairment group and 86 in the impairment group). Of the pack 2s, 460 were returned and, of these, 124 were excluded because of a reported change in health status and 336 were included in the test–retest analysis (275 in the no-impairment group and 61 in the impairment group).
Rasch analysis was based on the responses of 449 participants (phases 1 and 2 are included but only the pack 1 data from each phase). All 529 response sets were read into the analysis but one person had no LUNS data (all missing) and 79 persons were removed from the analysis as they were judged to be ‘extreme’. An extreme person is someone who is either at the floor or the ceiling of the scale, meaning that they have scored either maximally or minimally on the scale. Extreme patients are removed from the analysis as they do not offer any information; they are beyond the measurement parameters of the scale.
In this case, all of the extreme patients were at the floor of the scale, meaning that they had no unmet needs.
Response to questionnaire packs
Packs were sent out for completion at 3 or 6 months post stroke, with the median time between stroke and completion 3 months (range 3–6 months) (n = 115) and 6 months (range 5–10 months) (n = 414) respectively.
Of all patients who returned pack 1, 213 (40%) reported that they had received some kind of help to complete the pack. The majority (68–76%) of those receiving help reported help with ticking boxes or reading or discussing the questions; in addition, 14 (6%) reported that someone had completed the pack on their behalf without consulting them (proxy completion) and two (1%) reported help with translating the questions. Proxy completion represented 2.6% of all patients who returned pack 1.
Of the responders with no impairment, 32% reported receiving help to complete pack 1, compared with 78% in the impairment group of responders. In the no-impairment group, 1.3% of the packs were completed on the patients’ behalf by proxy, compared with 10.1% in the impairment group.
The median number of unmet needs as measured by the LUNS questionnaire was four (range 0–19), with the prevalence of individual unmet needs ranging from 3% to 58%. The most commonly reported unmet need was for more information; this was followed by unmet needs in the areas of falls and memory/concentration.
In total, 16% (n = 69) of the no-impairment group and 12% (n = 10) of the impairment group reported no unmet needs (first pack only). The total number of unmet needs reported was higher on average for the impairment group than for those with no impairment (Figure 15). The median number of unmet needs for the no-impairment group was four and the mode was zero (range 0–19). For the impairment group, the median number of unmet needs was five and the mode was five (range 0–17).
FIGURE 15.
Total number of unmet needs reported by individuals.
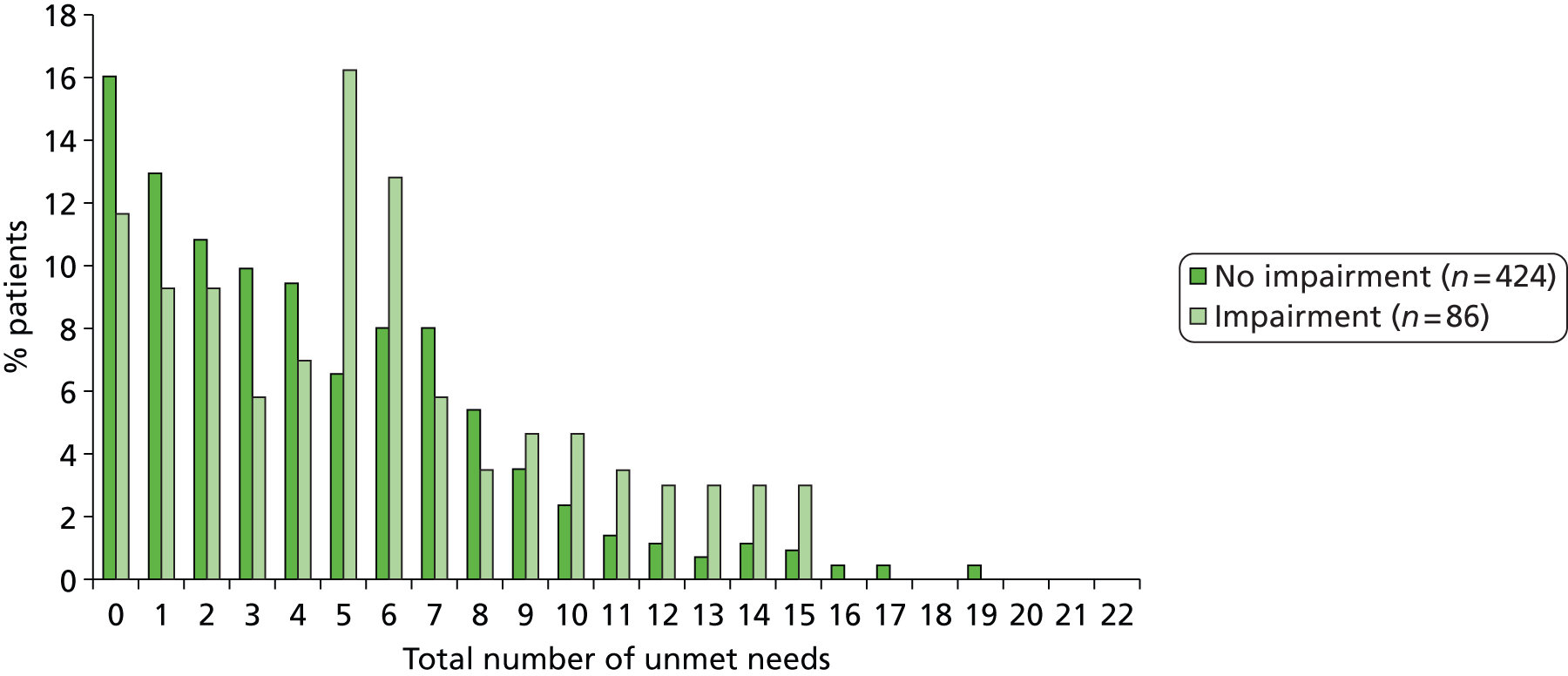
A significantly higher proportion of patients in the group with impairment than in the group with no impairment had unmet needs in the areas of memory/concentration, incontinence and accessible holidays (Figure 16). The proportion of responders who identified memory/concentration as an unmet need was 26% (n = 114) in the no-impairment group and 45% (n = 41) in the impairment group.
FIGURE 16.
Types of unmet need in the impairment and no-impairment groups. a, p < 0.005; b, p < 0.05.
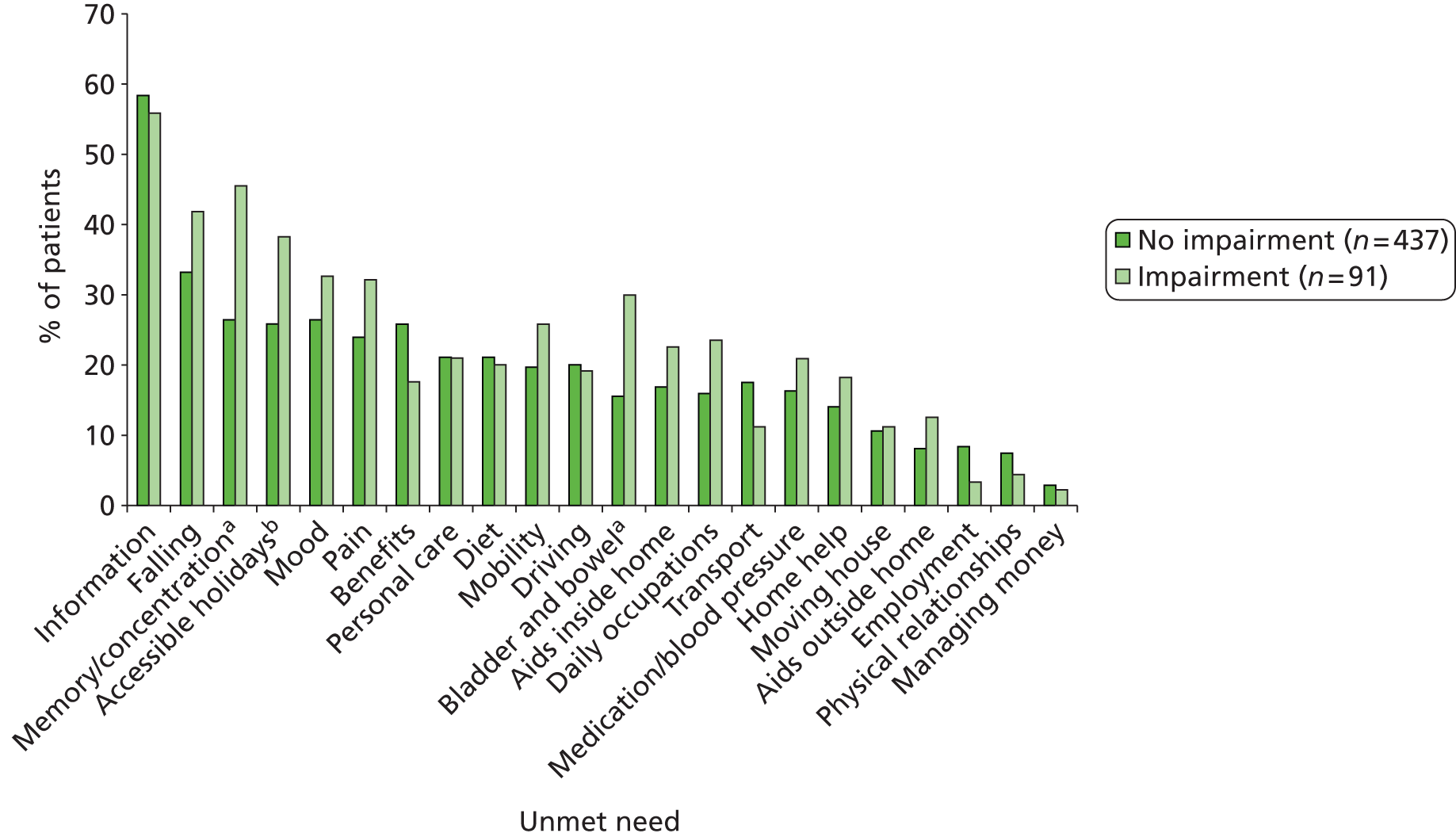
On the Impairment Manikin, responders reported impairments as listed in Table 52. A lower proportion of the no-impairment group than of the impairment group reported difficulties with speech and thinking.
| Impairment of | No impairment, n (%) | Impairment, n (%) |
|---|---|---|
| Completely recovered from stroke | 81 (19) | 5 (6) |
| Thinking | 136 (31) | 46 (51) |
| Speech | 106 (24) | 40 (44) |
| Vision | 97 (22) | 28 (31) |
| Swallowing | 58 (13) | 11 (12) |
| Right arm | 101 (23) | 44 (48) |
| Left arm | 156 (36) | 26 (29) |
| Right leg | 115 (26) | 34 (37) |
| Left leg | 166 (38) | 27 (30) |
Primary analysis: evaluation of acceptability, validity and test–retest reliability
Acceptability
Responses to pack 1 were received from 529 patients (69% of those sent) (see Figure 14). On initial receipt, 85% of the returned questionnaires were fully completed and, overall, 3.5% of the LUNS items were not completed. Table 53 shows that acceptability of the LUNS questionnaire was comparable to that of the concurrent measures.
| Questionnaire | Questionnaires returned completed (before prompting for missing data) (% of responders) | Items missing (before prompting for missing data) (% of all items received) | Prompts sent (% of all items received) | Items completed after prompting for missing data (% of total prompts sent) |
|---|---|---|---|---|
| LUNS | 451 (85) | 412 (3.5) | 385 (3.3) | 299 (78) |
| GHQ-12 | 474 (90) | 168 (2.7) | 141 (2.2) | 108 (77) |
| FAI | 466 (88) | 162 (2.2) | 119 (1.2) | 103 (87) |
| SF-12 | 442 (84) | 254 (4.0) | 204 (3.2) | 161 (79) |
Test–retest reliability
Of the pack 2 responders, 336 (73%) reported no health changes between completing pack 1 and completing pack 2. Test–retest reliability was assessed in this group only. The median time between completion of packs 1 and 2 was 14 days (range 3–96 days). The percentage agreement for individual items was between 77.9% and 98.8%, with 19 items having > 85% agreement (Table 54). Kappa values were between 0.445 and 0.673, with 14 items showing moderate agreement of between 0.445 and 0.593 and eight items showing good agreement of between 0.611 and 0.673. As a comparison, assessment of test–retest reliability of individual items of the SF-12 resulted in kappa scores between 0.34 and 0.57 and a percentage agreement between 44% and 73%.
| Item | Kappa | Agreement (%) |
|---|---|---|
| Physical relationships | 0.673a | 95.8 |
| Managing money | 0.661a | 98.8 |
| Accessible holidays | 0.660a | 88.1 |
| Pain | 0.653a | 88.0 |
| Driving | 0.630a | 89.8 |
| Memory/concentration | 0.620a | 86.6 |
| Information | 0.616a | 80.9 |
| Employment | 0.611a | 95.2 |
| Benefits | 0.593a | 86.6 |
| Daily occupations | 0.583a | 90.7 |
| Bladder/bowel | 0.562a | 89.3 |
| Mood | 0.554a | 84.5 |
| Aids/adaptations outside | 0.554a | 93.7 |
| Diet | 0.549a | 86.0 |
| Personal care | 0.548a | 87.1 |
| Home help | 0.546a | 90.3 |
| Moving house | 0.545a | 93.1 |
| Transport | 0.514a | 86.5 |
| Aids/adaptations inside | 0.493a | 85.8 |
| Falling | 0.492a | 77.9 |
| Mobility | 0.452a | 85.5 |
| Medication/blood pressure | 0.445a | 85.7 |
Comparison of unmet need with health status
For each individual LUNS item, the group of responders who identified unmet need had significantly poorer health scores on the GHQ-12 than those who identified no unmet need (p < 0.05). This significant difference in health status between those who did and those who did not identify unmet need was also observed for 21 LUNS items using the SF-12 MCS, 15 LUNS items using the SF-12 PCS and 14 LUNS items using the FAI. Table 55 shows the LUNS items for which the group with unmet need had significantly poorer health than the group with no unmet need (p < 0.05) on the concurrent measures.
| LUNS items | Concurrent measure |
|---|---|
| Pain | GHQ-12 FAI SF-12 PCS SF-12 MCS |
| Mobility | |
| Falls | |
| Aids/adaptations inside | |
| Transport | |
| Home help | |
| Moving home | |
| Personal care | |
| Bladder/bowel | |
| Memory | |
| Mood/concentration | |
| Daily occupations | |
| Accessible holidays | |
| Driving | GHQ-12 SF-12 MCS SF-12 PCS |
| Benefits | |
| Aids/adaptations outside | GHQ-12 FAI SF-12 MCS |
| Information | GHQ-12 SF-12 MCS |
| Medication | |
| Diet | |
| Money | |
| Physical relationships | |
| Employment | GHQ-12 |
Subgroup analyses
Of the no-impairment group, 71% (n = 438) of those sent the first questionnaire pack returned it, compared with 8% (n = 91) in the impairment group. Missing data on first receipt of the LUNS questionnaire for the respective groups was 3.4% (no impairment) and 4.1% (impairment) of all items, compared with 2.0–3.9% and 2.5–6.0% missing data on the concurrent questionnaires in the no-impairment and impairment groups respectively. In total, 86% of the no-impairment group LUNS questionnaires and 84% of the impairment group LUNS questionnaires were returned fully completed; ranges for the concurrent questionnaires were 84–93% for the no impairment group and 81–88% of the impairment group.
Test–retest was assessed in 275 responders with no impairment and in 61 responders with impairment who reported no health changes between pack 1 and pack 2. The percentage agreement for individual items was > 85% for 20 items for the no-impairment group and for 10 items for the impairment group. Kappa values ranged between 0.423 and 0.855 in the no-impairment group (Table 56) and between 0.253 and 0.673 in the impairment group (Table 57).
| Agreement | No. of items | Agreement (%) | Kappa |
|---|---|---|---|
| Moderate | 10 | 78–95 | 0.42–0.60 |
| Good | 11 | 82–96 | 0.61–0.69 |
| Very good | 1 | 100 | 0.86 |
| Agreement | No. of items | Agreement (%) | Kappa |
|---|---|---|---|
| Fair | 7 | 78–95 | 0.25–0.38 |
| Moderate | 12 | 75–92 | 0.41–0.56 |
| Good | 3 | 87–97 | 0.65–0.67 |
The results of testing for significant differences (p < 0.05) in the concurrent measure scores between the group who identified unmet need and the group who did not identify unmet need (item by item) are summarised in Table 58. More items have a significant difference for those with no impairment compared with those with impairment.
| Measure | No. of LUNS items (out of a possible 22) on which the group of responders who identified unmet need had a significantly poorer health score (p < 0.05) | |
|---|---|---|
| No impairment | Impairment | |
| SF-12 PCS | 15 | 2 |
| SF-12 MCS | 21 | 4 |
| GHQ-12 | 22 | 8 |
| FAI | 18 | 1 |
When looking at health scores, there are fewer LUNS items for which there is a significant difference between the group who identified unmet need and the group who had no unmet need (according to LUNS) on the physical health measures (SF-12 PCS and FAI), and more LUNS items for which there is a significant difference between the groups on the mental health measures (SF-12 MCS and GHQ-12).
Ancillary analyses: evaluation of internal consistency and dimensionality
Cronbach’s alpha
The Cronbach’s alpha value for the 22 LUNS items was 0.815.
Factor analysis
A confirmatory factor analysis on one dimension failed. Exploratory analysis was conducted to reveal that a three- or four-dimension solution could be taken (root-mean-square error of approximation of a three-factor solution = 0.034; root-mean-square error of approximation of a four-factor solution = 0.027); however, both are affected by response dependency within the items. LUNS-17 (identified by Rasch analysis; see following section) also failed a single-dimension confirmatory factor analysis, and exploratory factor analysis suggested that four dimensions were present.
Rasch analysis
The LUNS-22 PSI was 0.66 and overall chi-squared probability was < 0.0001. Three items (information, driving and daily occupations) were found to be poor fitting because of under- or overdiscrimination (all displayed significant chi-squared and ANOVA item misfit). There were response dependencies between items relating to aids/adaptations inside and aids/adaptations outside (0.224) and between mood and memory/concentration (0.223). DIF analysis showed a bias by age for the item relating to employment and a bias by age and gender for the item relating to physical relationships. Removing the five problematic items (with regard to fit indices and DIF) and accounting for the apparent response dependency within the scale results in a 17-item solution (LUNS-17), which displayed reasonable model fit as none of the items were individually misfitting. However, this 17-item tool remains unsuitable for use as a scale because of poor targeting and reliability/internal consistency issues (PSI = 0.52), The low PSI also reduces the power of the tests of fit, meaning that the observed adequate fit may be misleading. The Cronbach’s alpha value at this point is 0.77, but this does not take into account the targeting, or the response dependency that is present. The targeting plot can be seen in Figure 17, in which it is apparent that there is a skew between the locations of the items and the locations of the sample that is being measured.
FIGURE 17.
The LUNS Rasch targeting plot, displaying the relative distributions of person and item logit locations on the underlying ‘unmet need’ continuum. Grouping set to Interval length of 0.25 making 28 groups. n = 528, mean = –1.711, SD = 1.189.

Qualitative evaluation of unmet needs
In total, 10 semistructured interviews were conducted. Eight interviewees were male and the median age was 78 years. Nine interviewees lived with their husband/wife and one lived alone. During the LUNS study, six participants had identified no unmet needs, three participants identified one unmet need in either the first or the second pack and one participant identified one unmet need in both the first and the second packs. The qualitative study found that most people felt that they genuinely had no unmet needs. Despite this, all interviewees were able to identify current problems. However, interviewees often rejected the word ‘problem’ and therefore these are termed ‘identified issues’. Identified issues may relate to stroke, comorbidities and/or ageing. The type, impact and importance of these issues varied. People can be living with significant limitations yet still identify no unmet needs. People may be in the process of seeking help for their issues or may feel that they are managing them. Issues were grouped according to their effect on life (number of people who reported each in brackets):
-
leg, arm or hand pain, weakness, spasticity or other limitation (nine)
-
walking, getting out, mobility (nine)
-
other (nine) (e.g. feeling slow, frustrated, experiencing a changed or strained relationship with partner, weight gain, breathlessness)
-
balance or falls (eight)
-
hobbies, leisure (seven)
-
feeling tired (six)
-
mood, anxiety (five)
-
domestic activities (four)
-
social life (four)
-
memory and/or concentration (three)
-
vision (two)
-
throat and voice (two)
-
communication (two)
-
bladder and bowel (two)
-
personal care (two)
-
holiday (one)
-
crying (one)
-
sleeping (one).
The following themes emerged:
-
Level of acceptance – relates to adjusted expectations; high levels of acceptance were common and this links with experience of ageing and may explain how issues can become redefined in the context of current life.
-
Support received – people are often receiving support from their partner; family, friends or services also provide support.
-
Relative situation – people compare themselves to other people and report feeling fortunate and valuing information from peers.
-
Presentation of character – some people identify themselves as proud, being independent and/or determined.
-
Experience of frustration – frustration was common and includes limitations in relation to carrying out tasks or things taking longer to do, for example.
-
Experience of services – people have experiences and expectations about services, including what treatment is available and acceptable to ask for.
Discussion
Interpretation
The LUNS questionnaire was developed to identify the longer-term unmet needs of stroke survivors. It consists of 22 items, covering information, services, social and emotional consequences and health problems and related areas. The study aim was to evaluate the acceptability, test–retest reliability and validity of the LUNS questionnaire in a broad sample of patients living at home post stroke. The response rate (69%) and minimal missing data, comparable to those for concurrent measures, suggest that the LUNS questionnaire is acceptable to the target group. Individual-item test–retest reliability was good and compares favourably to that for SF-12. It was found that identification of an individual unmet need was consistently associated with poorer outcomes on the concurrent health measures, demonstrating validity. Interestingly, unmet need was more strongly associated with lower mental health than with lower physical health or activity.
The acceptability of the LUNS questionnaire was lower in stroke survivors with cognitive or communication impairment than in those with no impairment. However, the acceptability in stroke survivors with cognitive or communication impairment was comparable to the acceptability of concurrent measures in this group. The test–retest reliability of LUNS items was slightly lower for the impairment group and comparison with concurrent measures suggests a reduced association between LUNS items and health status. Cognitive and communication impairments were assessed at baseline; however, Impairment Manikin results suggest that cognitive and communication impairment caused by stroke may persist up to 3 or 6 months post stroke for some people.
A secondary aim was to evaluate the internal consistency and dimensionality of the LUNS questionnaire. An initial Cronbach’s alpha value of 0.815 suggests a reasonable level of internal consistency among the 22 LUNS items. However, additional factor and Rasch analysis revealed that the LUNS questionnaire does not function as a scale because of multidimensionality and poor targeting; therefore, items cannot be added up to give a total score for level of unmet need.
A substudy interviewed a sample of patients who identified a low level of unmet need (no or one unmet need) to further understand self-reported unmet need following stroke. People in this study felt that they genuinely had no unmet needs, yet all participants identified current issues or problems, which may relate to stroke, comorbidities or ageing. Six themes demonstrate the importance of adjusting: help received from others; comparisons to other people; characteristics such as pride, independence and determination; frustration when engaging in activities; and experiences and expectations of services. This adds to the understanding of how problems are managed and what shapes the identification and perceptions of unmet needs.
To put the LUNS questionnaire in context, few suitable tools exist to assess an individual’s unmet needs following stroke. The Stroke Association needs survey consists of 44 items and has not been psychometrically evaluated. 4 The Southampton Needs Assessment Questionnaire has not been evaluated for test–retest reliability99 and the postal response rate was low at 49%. 100 This questionnaire was evaluated only in people aged ≤ 65 years, yet older adults make up the majority of the stroke population. 120 The Stroke Impact Scale is long (59 multiple choice items) and low postal response rates of 41%104 and 63%101 have been reported.
Generalisability and limitations
Comparison with data from the UK NSA population120 indicates that the LUNS study sample is reasonably representative of the wider UK stroke population (age, gender, time in hospital, post-stroke Barthel Index score). The ethnicity of the sample is representative of the ethnicity of the population aged 65+ years in England in 2001. 85 By setting a target for the proportion of patients with communication and cognitive impairments, and allowing proxy responses, the LUNS questionnaire has been evaluated on a sample with varying stroke severity. The target recruitment of the impairment group was reduced to meet study deadlines. Inherent barriers exist to the recruitment and inclusion in research of stroke survivors with cognitive and communication impairments. Therefore, stroke survivors with communication or cognitive impairment should be actively included, especially with regard to health or needs assessment and questionnaire evaluation. Lower test–retest reliability and problems relating to validity may be properties of all questionnaires when including stroke survivors with cognitive or communication impairment.
The LUNS questionnaire demonstrated a response rate and median number of unmet needs that are similar to those found for a UK stroke needs survey (response rate 60–78%, median unmet needs four). 4 A median of two unmet needs has been reported during post-stroke reviews using GM-SAT. 34 The LUNS questionnaire is a pragmatic tool which can identify the unmet needs of stroke survivors that relate to either stroke or comorbidities. These include unmet needs that may require inputs from health providers and other providers such as local authorities and third-sector organisations. Results from the LoTS care trial (see Chapter 3, Overview of stroke care co-ordinator practice) indicate that SCCs currently refer/signpost stroke patients to such whole system services. It is possible that the LUNS questionnaire may also have wider relevance beyond stroke care, to capture general issues that affect older people with long-term conditions.
Use of dichotomous responses is considered a strength as it is simple and results in an unambiguous response. 121 The potential complexity of double-barrelled questions was managed by providing instructions to tick ‘no’ if there is no need or if the need has been met. As a result, the LUNS questionnaire is quick for stroke survivors to complete and easy to interpret; items ticked ‘yes’ indicate the type and number of unmet needs that an individual has. The results show that, for the majority of individual items, responders commonly tick ‘no’; as such, this may result in vulnerability to a floor effect.
When assessing validity we were unable to compare the LUNS questionnaire with validated benchmark measures of unmet need as no such tools exist. Therefore, health status questionnaires were chosen that could reflect health-related needs.
It is acknowledged that some topics do not specifically map onto the LUNS questionnaire, for example fatigue, vision and communication. However, tool development incorporated stroke patient- and carer-identified problems and, furthermore, the qualitative exploration of the unmet needs study did not find that the LUNS questionnaire was missing these or any other needs. Iterative development and robust methods were used to capture evidence from the literature and the views of experts and > 150 patients. The subjectivity of unmet needs meant that patient involvement was an important part of the development of the LUNS questionnaire, and patient understanding of items demonstrates provenance.
Recommendations for research and implications for health care
We have demonstrated that the LUNS questionnaire has adequate acceptability, test–retest reliability and validity to provide a simple method for identifying longer-term unmet needs after stroke. Policy recommends that stroke patients are reviewed in the longer term15,55 and that this includes measurement of unmet needs. The LUNS questionnaire is suitable to be used during individual patient consultations to guide service responses. It is also suitable to be used to promote service development, compare different services or inform commissioning, for example evaluating whether services are meeting patients’ needs by measuring the percentage of the local stroke population whose mobility needs are unmet. Further research is needed to evaluate the sensitivity to change of the LUNS questionnaire, to determine whether it can be used to monitor change at a patient or service level over time. Results from factor and Rasch analysis suggest that the LUNS questionnaire should be used at the individual-item level rather than to give a score representing the burden of unmet need.
The observed lower test–retest reliability and validity of the LUNS questionnaire in patients with cognitive or communication impairment suggests that it may be important to research the accuracy of proxy responses and potentially to develop a version of the questionnaire that is suitable for people with communication impairment.
The subtlety and complexity of measuring unmet needs described in the substudy interviews should be considered when planning stroke reviews and using questionnaire tools, for example to ensure that individual circumstances are considered when assessing unmet need.
Chapter 5 Project 4: adjustment after stroke study
Abstract
Aim
The study aimed to investigate longer-term recovery and adjustment post stroke with a particular focus on the processes and mechanisms that contribute to social inactivity and/or social activity.
Study design
A qualitative substudy of the system of care trial was undertaken, drawing on grounded theory techniques.
Methods
A purposive sampling strategy was adopted to identify trial participants who were less or more socially active than anticipated based on social activity and physical disability outcome measures and to ensure variation in key characteristics known to shape adjustment post stroke (age, socioeconomic status, living alone). A combination of qualitative methods was used, including multiple semistructured interviews, limited observations, solicited diaries, and informal and formal support mapping techniques.
Results
In total, 22 stroke survivors and 12 carers/significant others participated in the study. From participants’ accounts, four different post-stroke recovery trajectories have been identified: (1) disruption followed by adjustment and acceptance; (2) cycles of disruption followed by adjustment and acceptance; (3) disruption without adjustment and acceptance; (4) stroke as a continuation of ongoing decline. The different trajectories illustrate how multiple interacting factors shape the process and meaning of recovery and adjustment over time and therefore social participation. The factors that shape the different trajectories were identified.
Conclusions
Knowledge of different recovery trajectories and the factors that shape adjustment and social participation post stroke may enable stroke survivors, caregivers and professionals to identify factors that act as barriers to adjustment and social participation. This may help stroke survivors shape their own recovery trajectory and facilitate professionals in better tailoring services provided.
Introduction
There has been great interest in the experience of and meaning attributed to recovery and adjustment following stroke. Much existing research, however, has focused on particular aspects of recovery and adjustment rather than examining the process in its complexity over time. Furthermore, studies have tended to focus on the first 12 months after stroke. This qualitative study aimed to explore the interacting factors that shaped the meaning and process of recovery and adjustment of stroke survivors who had had a stroke at least 12 months previously. In particular, the study aimed to understand the social dimensions of recovery and adjustment, focusing on social activity and participation post stroke.
Background
Many factors are known to affect the process of adjustment following stroke and these factors may impact on social participation. They include:
-
physical and functional improvement
-
emotional factors
-
the understanding given to the stroke, rehabilitation and disability
-
stigma
-
formal and informal support
-
various contextual factors.
Physical and functional recovery shapes longer-term outcomes after stroke. 25,122,123 Stroke-related impairments and the resulting changed relationship that people have with their body can result in people experiencing their body as being unreliable. 123 In addition to the physical and functional implications of stroke, various emotional and psychological consequences have been highlighted in the literature, including depression, low mood, apathy, aggression, irritability and anxiety. 10,124,125
People’s perceptions of stroke, rehabilitation and disability also shape longer-term outcomes following stroke and the process of adjustment. Chronic conditions can disrupt previous roles, relationships and structures of everyday life as well as expectations for the future. 126 The disruption can be meaningful both in terms of its significance to a person’s sense of self and biography and in terms of its effect on a person’s ability to carry out everyday activities. 126,127
Although little has been written specifically about experiences of stigma post stroke, the existing literature details how some people are concerned about how others (including strangers) perceive them, that relationships and friendships change and that people may become anxious about engaging in social situations and may experience agoraphobia. 125 People may also try to cover up the effects of their stroke. 122,128
Social support from families, friends, neighbours and support groups has been shown to be extremely important in terms of instrumental, informational and emotional support post stroke. 129–131 Formal services, including access to and withdrawal from these, also shape longer-term outcomes. 122,132,133 In addition, socioeconomic factors, health inequalities, patterns of health service use and local resource allocation have implications for life after stroke. 133
Social inactivity and social isolation are experienced by many people who have had stroke. 25 The wider literature indicates that those who perceive themselves to be socially isolated are more likely to experience anxiety and stress,134–136 which may increase their vulnerability to physical and mental health problems. 134 Living alone and low social participation are risk factors for disability among older men, and dissatisfaction with social relationships is significantly associated with disability for both older men and older women. 136 Social isolation and loneliness may therefore have implications for rehabilitation following stroke. Furthermore, stroke-related impairments may restrict a person’s ability to engage in social activities and may contribute to him or her becoming socially isolated. 25 Thus, stroke-related impairments may themselves compound isolation and loneliness. Existing data,18,37 however, suggest that even those who recover physically and functionally well following stroke may still become socially inactive. There is a need, therefore, to further explore the relationship between the process of recovery, including physical and functional improvement, and social participation post stroke.
Although the existing literature provides an understanding of multiple factors that influence life after stroke, the complex process of recovery and adjustment is not fully understood. Furthermore, there is a lack of clarity as to the processes and mechanisms that influence social activity and social participation, as well as the relationship between recovery and social participation. This study addresses these gaps by exploring the process of recovery and the role of social participation in that process from the perspective of stroke survivors. By understanding the process of recovery and adjustment over time, steps can be taken to further support the adjustment of stroke survivors and their carers and help to improve services and longer-term outcomes.
Aims and objectives
The study aimed to investigate longer-term recovery and adjustment post stroke with a particular focus on social activity and participation. The primary and secondary objectives are:
-
primary objective: to understand the processes and mechanisms that contribute to stroke survivors’ social inactivity and/or social activity post stroke
-
secondary objectives:
-
to comprehend what stroke survivors (and carers) understand to be a good recovery post stroke
-
to understand stroke survivors’ (and carers’) experiences of the stroke and the process of adjustment (within the context of their ongoing lives/biography)
-
to comprehend stroke survivors’ (and carers’) experiences of the social and cultural context in which adaptation occurs post stroke
-
to develop a better definition of poor adjustment post stroke and determine the prevalence of poor adjustment among the stroke population.
-
Methods
Study design
The adjustment after stroke study is a substudy of the stroke system of care trial (see Chapter 3). The study adopted a grounded theory approach138–140 and a combination of qualitative methods was used to understand the process of adjustment following stroke, including semistructured interviews, limited observations and solicited diaries.
This study used data collected by the trial to purposively sample stroke survivors and study their recovery and adjustment over time. As the study aimed to explore adjustment after stroke and in particular to understand the processes and mechanisms that contributed to social participation following stroke, the study recruited stroke survivors who were less or more socially active than anticipated. Analysis was conducted on an existing data set with a similar population to that of the stroke system of care trial36 to identify categories based on pre-stroke and 12-month post-stroke Barthel Index scores and FAI scores. Three categories were identified: better than expected, failure to thrive and doing as expected (doing well). Table 59 provides definitions of the categories and Appendix 12 provides further information regarding the development of these categories.
| Category | Reduction in Barthel Index score (between pre stroke and 12 months post stroke) | Reduction in FAI score (between pre stroke and 12 months post stroke) | Excluding |
|---|---|---|---|
| A: better than expected | > 4 | ≤ 16 | Those who have a 12-month FAI score of 0 |
| B: failure to thrive | ≤ 4 | >16 | Not applicable |
| C: doing as expected (doing well) | ≤ 4 | ≤ 7 | Those who have a 12-month FAI score of 0 |
This study was approved by Bradford and Scotland A Research Ethics Committees. A SMG was established to oversee the running of the project. If researchers had any concerns with regard to emerging ethical issues (including the safety of participants) they were able to discuss this with the chief investigator and, if deemed appropriate, the wider SMG to reach a consensus on how to proceed. Pseudonyms are used throughout the document.
Eligibility criteria
All participating stroke survivors had taken part in and completed the stroke system of care trial by returning the 12-month questionnaire pack and therefore were subject to the trial inclusion and exclusion criteria at the point of recruitment into the trial (see Chapter 3 for the trial eligibility criteria).
Stroke survivors who were less or more socially active than anticipated, as defined by the categories discussed earlier, were identified by screening the trial database (see Appendix 12 for further details).
Stroke survivors were excluded from this substudy if they had moved into 24-hour care following recruitment to the trial, as this is already known to shape adjustment after stroke and is associated with a decrease in social activity as well as an increase in social isolation and loneliness.
Although the primary focus of the study was on the process of adjustment from the perspective of the stroke survivor, it is acknowledged that carers (and the dynamic between the stroke survivor and the carer) are important to the process. Therefore, carers who consented to take part in the trial and whose relative/friend was identified as a potential participant for this substudy were also approached to take part. Those stroke survivors who were identified as potential participants and whose carer did not take part in the trial were asked if they had a carer or significant other (who may not be a carer) who would be interested in taking part. Stroke survivors receiving palliative care and their carers/significant others were also excluded.
Sampling strategy
A purposive sampling strategy was adopted to identify stroke survivors who were less or more socially active than anticipated. Because of the exploratory nature of this study, the sample was also purposively selected to ensure variation in relation to key characteristics that the evidence suggests may shape adjustment and social participation post stroke. These included age, socioeconomic status and whether the stroke survivor lived alone or with others. In addition, both male and female stroke survivors were sampled.
Following the identification of potential participants, a researcher telephoned them to assess eligibility and interest in the study. Suitable participants were sent an information sheet and this was followed by a telephone call several days later, at which the first interview was arranged if appropriate. Written consent and/or consultee declaration for those lacking the capacity to consent was obtained face to face during this first visit.
Data collection methods
A combination of qualitative methods was used, including semistructured interviews, solicited diaries, observations and mapping of formal and informal support networks. Figure 18 illustrates the data collection procedure.
FIGURE 18.
Data collection procedure for adjustment after stroke study.
Initial interview
The initial interview addressed topics including life before stroke, the stroke event itself and being in hospital, life post discharge home and life since (up to and including the present). Participants discussed their understanding and perception of the stroke, recovery and adjustment; the physical, functional, emotional and social consequences of the stroke; and changes that they had experienced in their lives since the stroke. Limited observations were also carried out by the researchers. These comprised environmental observations, which included neighbourhood observations (description of the location of the residence and nearby environment, rural/urban/suburban setting, local amenities, availability of public transport, evidence of social problems, accessibility issues, etc.) and household observations (type of home, description of aids and adaptations made to home, use of space including if participant is still sleeping downstairs, etc.). These observations provided contextual information regarding the immediate environment and adaptations made to the home, which informed the topic guide for the follow-up interview (specifically with regard to discussions concerning managing stroke-related impairments and engaging in everyday, leisure and social activities). In addition, observations were also conducted alongside the interview to record contextual information, for instance interactions with and between participants, gestures and mannerisms, physical demonstrations of impairments and use of objects by participants during the interview. Notes of non-verbal actions were added to the verbatim interview transcripts to aid understanding and interpretation.
Diary
At the end of the first interview stroke survivors and their carers/significant others were asked if they would be willing to keep either a written or audio diary for a period of 4 weeks (this was optional). The diary–interview method141–146 has been used previously in health research with people with chronic illnesses. Diaries can capture the ‘contemporaneous flow of public and private events’ (p. 170),147 ‘transient phenomena’ (p. 265)141 and hidden accounts that do not often emerge in one-off face-to-face interviews.
Participants were asked to record meaningful events and experiences as well as their thoughts and feelings. They were free to decide what information they chose to include, how frequently they recorded an entry and how much time they spent on the diary. The researchers telephoned participants weekly during the 4 weeks that they kept the diary as this has been shown to remind participants about the diary and provide an additional source of data. 141
In the diaries participants chose to record information relating to their day-to-day experiences and difficulties; factors that facilitated and/or hindered social participation; their social support networks; their physical and functional impairments; their feelings; and other contextual information.
Follow-up interview
Participants’ first interviews and diaries were transcribed and analysed before the follow-up interviews. In accordance with a grounded theory approach,138–140 the topic guides for the second interviews were developed and refined based on the ongoing analysis of the first interviews, observations and diaries. The majority of follow-up interviews took place 3–4 months after the first interview.
To explore participants’ informal and formal support networks in greater detail the researchers undertook mapping exercises during the follow-up interviews. The exercises enabled the participants to illustrate and discuss informal and formal support that had been important to them since the stroke. The approach used in this study drew on the hierarchical mapping technique. 148 Participants were given a diagram in which they were depicted at the centre of three concentric circles, which represented different levels of support. Participants were then asked to place onto the concentric circles people/services who they felt had offered support, with the most supportive being placed in the circle closest to the centre. The researchers engaged participants in conversation throughout the exercise to explore the maps produced in depth. Mapping informal support allowed the researchers to gain an understanding of participants’ experiences of their (changing) social relationships and the support that these different relationships provided. Formal support mapping involved participants recording services used (including health and social care services, volunteer services and peer support groups), how helpful participants found such support and how formal support changed over time.
Data analysis
A grounded theory approach to data analysis was taken, which entailed simultaneous data collection and analysis. 138–140,149 Analysis of the data involved careful rereading of the transcripts of interviews and diaries. This was followed by three phases of coding to facilitate the development of themes and theories: open coding involved descriptively coding the data line by line (open codes were numerous and included ‘daily routine’, ‘getting out of the house’, ‘pain’, ‘frustration’, ‘family encourages to do things and go out’, ‘does not go out’ and ‘sees some improvement’); focused coding involved grouping the descriptive codes and forming categories (focused codes included ‘activities – pre stroke’, ‘activities – post stroke’, ‘loss and uncertainty’, ‘everyday life work’, ‘meaning as consequence’, ‘meaning as significance’, ‘relationships over time’, ‘sense of recovery’ and ‘role of informal support’); theoretical coding involved examining the relationships between the categories and developing an explanation of the process of adjustment (the theoretical codes were a typology of recovery trajectories). 138,140 The software program NVivo 8 (QSR International, Southport, UK) was used to store, organise and code the data. Memos concerning coding and emerging themes and theories were also recorded.
The interpretations made were tested by the use of ‘constant comparison’,139 whereby data segments and the developing codes and categories are compared. In this study, data segments from different data collection methods were also compared (interview, diary and observation data) to add depth and complexity to the analysis. This constant comparison was carried out, first, within case (so data segments from the same participant were compared) to produce an understanding of each individual’s process of recovery and adjustment over time and, second, across cases (so data segments were compared across participants) to provide an understanding of similarities and differences in the process of recovery and adjustment over time for different participants.
As the research aimed to explore the meaning and process of recovery post stroke, and thus the interacting factors that shaped activity and social participation over time, time became an important analytical device. As analysis progressed and the researchers began to explore individual and across-case recovery and adjustment, concepts of the illness trajectory and recovery trajectory became particularly important. 150–153 The concept of the illness trajectory comprises several interacting elements: the course of the chronic illness; work related to managing and living with that chronic illness (including illness work, everyday life work, biographical work); and the impact that the chronic illness and such work have on the person with the chronic illness and their relationships with others. Of importance, therefore, is the dynamic between subjective experience, the course of the chronic illness and the management of that illness. In this instance, recovery trajectories post stroke can be understood as the interplay between the course of the stroke and rehabilitation; the work required to manage and live with stroke-related impairments; and the subjective experience and meanings given to the stroke, stroke-related impairments, the process of rehabilitation and the work to manage and live with the stroke-related impairments and the impact that these have on the stroke survivor and their relationships with others. Analysis, therefore, led to the identification of different types of recovery trajectory (a typology of recovery trajectories) as well as the exploration of the different processes and mechanisms that shaped these different recovery trajectories.
Standard approaches to demonstrating trustworthiness and quality in qualitative research were used. 154–157 Throughout the data collection and analysis process, data, codes and emerging categories and theories were presented to and discussed with the study steering group at regular intervals. The emerging findings were also presented to academic audiences and therapy staff who currently work with stroke survivors as well as stroke survivors and their carers. Comments received were considered alongside ongoing analysis.
Results
Recruitment
In total, 22 stroke survivors and 12 carers/significant others were recruited to the study from November 2010 to December 2011. The period of time between stroke and the first interview ranged from 14 to 24 months, with most participants being interviewed for the first time at either 14 months (five stroke survivors) or 15 months (eight stroke survivors) post stroke. For most participants the second interview took place 3–4 months after the first interview. Those stroke survivors whose carers/significant others also participated in the study were given the option of being interviewed together or separately; all decided to be interviewed together. Table 60 details the numbers of stroke survivors and carers/significant others who participated in each phase of data collection. Two stroke survivors and one carer/significant other were lost to follow-up.
| Participants | First interview and observations | Diary | Second interview and mapping |
|---|---|---|---|
| Stroke survivors | 22a | 12 | 20 |
| Carers | 12 | 3 | 11 |
Table 61 summarises some of the characteristics of the participating stroke survivors (age at time of stroke, living circumstances, life and health before stroke and stroke-related impairments). Table 62 summarises the forms of support that participating stroke survivors reported receiving, including formal support following discharge home from the hospital, formal support provision at the time of the interviews and informal support.
| Name (pseudonym) | Age at time of stroke (years) | Category (from Barthel Index/FAI scores) | Living circumstances | Summary of life pre stroke (as reported during interviews) | Stroke impairments (as reported at the time of the first interview, unless indicated otherwise) |
|---|---|---|---|---|---|
| Matt | 57 | Better than expected | Lives with wife and son in their own house | Independent, restricted mobility and fatigue because of pre-existing chronic condition | Impaired mobility, problems with balance, walks with frame/two sticks, some memory loss |
| Olive | 88 | Better than expected | Lives alone in supported bungalow | Independent, pre-existing chronic conditions but did not present these as having a big impact on her life | Impaired mobility, short-term memory problems, sight and speech temporarily impaired following stroke but now almost fully returned |
| Eric | 65 | Better than expected | Lives alone in his own house | Independent, registered blind | Weakened right side, mild expressive aphasia, impaired mobility, limited function in right hand |
| Alf | 64 | Better than expected | Lives alone in a rented ground floor flat | Independent, generally healthy, arthritis | Weakened left side, impaired mobility, reduced function in left hand, impaired memory, speech temporarily impaired following stroke |
| Bernard | 62 | Better than expected | Lives alone in sheltered accommodation | Lived in sheltered accommodation and had paid carers before stroke; had a range of pre-existing chronic health conditions | Weakened side, impaired mobility, fatigue, uses mobility scooter and/or walks with stick, speech temporarily impaired following stroke |
| Barry | 63 | Better than expected | Lives with wife in their own house | Independent, working full time, generally healthy | Weakened right side, limited function in right arm and hand, impaired mobility (walks with stick), depression |
| Sal | 65 | Better than expected | Lives alone in a council-owned flat | Independent, working full time (volunteer role), arthritis and another chronic condition that had resulted in some mobility impairment, but walked unaided | Weakened right side, limited use of right hand, impaired mobility, problems with balance, pins and needles/burning sensation in right side, impaired vision (improved over time), short-term memory problems |
| Rita | 67 | Better than expected | Lives alone in a flat | Independent, generally healthy, transient ischaemic attacks before stroke | Weakened left side, memory problems, minor sight problems, problems with balance |
| Peter | 81 | Better than expected | Lives with wife in their own bungalow | Independent, restrictions because of pre-existing chronic condition requiring regular hospital visits | Impaired vision (lost virtually all sight in his right eye), short-term memory problems |
| Dave | 57 | Better than expected | Lives with wife in their own house | Other health problems and disabilities, profoundly deaf | Weakened left side, limited function in left arm, mobility impairments, fatigue |
| Greg | 71 | Better than expected | Lives with wife in their own house | Life restricted because of pre-existing chronic condition, rarely left house, impaired mobility | Weakened left side, impaired function in left arm/hand, impaired function in left leg, incontinent, decreased concentration |
| John | 61 | Better than expected | Lives with wife in their own house | Independent, diabetes, other chronic health condition | Some numbness and mild weakness on right side, impaired mobility |
| June | 66 | Doing as expected (doing well) | Lives with her husband in their own bungalow | Independent, working part time, cares for dependent adult son | Some problems with short-term memory, initially had problems tolerating some of the medication, loss of stamina, fatigue |
| Emily | 77 | Doing as expected (doing well) | Lives with husband in their own house | Generally healthy, transient ischaemic attack before stroke | Weakened right side, impaired mobility, fatigue |
| Lyn | 55 | Failure to thrive | Lives with brother | Independent, mobility slightly impaired because of pre-existing condition | Weakened right side, weak right leg, impaired mobility (walks with four-pronged stick/wheelchair user), paralysed right arm, speech slightly impaired |
| Joseph | 77 | Failure to thrive | Lives with wife in their own bungalow | Independent, still working part time/odd jobs | Weakened right side, aphasia, limited function in right arm/hand, weakened right leg, impaired mobility (walks short distances inside with stick/wheelchair user), needs support with most activities of daily living |
| Phil | 52 | Failure to thrive | Lives with wife | Independent, working full time, generally healthy, on medication for high blood pressure | Weakened left side, impaired mobility (walks with stick/wheelchair user), limited function in hand, impaired vision (left eye) |
| May | 65 | Failure to thrive | Lives with her husband in their own house | Independent, restrictions because of impairments from previous stroke (12 years previously) including some visual impairment | Weakened left side, limited function in left arm, weakened left leg, impaired mobility (three-pronged walking stick/wheelchair user) |
| Harry | 85 | Failure to thrive | Lives alone in bungalow | Independent, tremor in hand, impaired mobility (minor) | Weakened left side, impaired mobility, problems with balance, weakened facial muscles |
| Jack | 69 | Failure to thrive | Lives with wife in their own house | Independent, generally healthy | Weakened right side, aphasia, paralysed right arm/hand, impaired mobility |
| Margaret | 72 | Failure to thrive | Lives alone in her own house | Independent, previous acute health problems including a heart attack | Impaired mobility (uses walking frame in the house/stair lift to get upstairs) |
| Jim | 73 | Failure to thrive | Lives with wife in their own house | Independent, diabetes, arthritis | Weakened left side, mobility impairments (needs walking frame when outside) |
| Name (pseudonym) | Formal support post discharge | Formal support at time of interview | Informal support |
|---|---|---|---|
| Matt | Physiotherapist (6 months), occupational therapist (6 months), paid carers (6 weeks), Stroke Association worker | No formal support | Wife, son, members of extended family, neighbours |
| Olive | ‘Hospital team’ helped to prepare house for discharge, paid carers (every day for 4 weeks) | Paid carer (one visit per week), day centre, exercise class, transport provided to/from centre and class | Hairdresser, friends, neighbours, granddaughter |
| Eric | Physiotherapist, sensory team, housing authority (help with gardening) | Sensory team | Sister (lives nearby), friends |
| Alf | Physiotherapist, outpatient stroke clinic, Age Concern | No formal support | Daughter, son, sister, friends |
| Bernard | Physiotherapist, paid carers | Paid carers, painting class, sheltered housing social events | Friend, sister, other residents |
| Barry | Physiotherapist (6 weeks), occupational therapist (6 weeks), rehabilitation centre, paid carers (6 weeks) | Re-referred to rehabilitation centre and physiotherapist, pharmacist, chiropodist | Wife, daughter, son and daughter-in-law, grandchildren, mother-in-law, siblings, friends, neighbours |
| Sal | Stroke nurse (visit once every 6–8 weeks) | No formal support, GP had referred her to community physiotherapist | Brother, neighbours, friend’s daughter |
| Rita | Physiotherapist, paid carer, Stroke Association worker, social services | Paid carer | Friends, son (mostly over telephone), daughter (mostly over telephone) |
| Peter | Outpatient stroke consultant appointment once every 3 months | Outpatient stroke consultant appointment once every 3 months | Wife, son, friends |
| Dave | Physiotherapist, occupational therapist, paid carers | Physiotherapist | Wife, sons, sister and brother-in-law, son’s girlfriend |
| Greg | Physiotherapist, occupational therapist, paid carers (four visits per day) | Paid carers (three/four visits per day) | Wife, sister, neighbour |
| John | Physiotherapist, outpatient visits to stroke clinic | No formal support | Wife, son, daughter, daughter-in-law, son-in-law |
| June | No formal support | No formal support | Husband, best friend, son, siblings, friends |
| Emily | Outpatient physiotherapy clinic | No formal support | Husband, sons, friends and neighbours, church community, guides |
| Lyn | Physiotherapist (12 months), paid carers | Paid carers, day centre | Brother, ex sister-in-law, friends, extended family |
| Joseph | Physiotherapist (re-referred following bad fall) | Volunteer physiotherapist, day centre, bowling group, transport (to and from activities), sitting service, volunteer to support him using mobility scooter, carer support group | Wife, son, neighbours, friends |
| Phil | Occupational therapist visits (regular), Different Strokes, charitable organisation that helps people get back to work | Occupational therapist visits (less frequent), gym for stroke survivors held at open prison | Wife (works), daughter |
| May | Unclear | No formal support | Husband, daughter, son, some members of extended family, neighbour |
| Harry | Physiotherapist, occupational therapist, paid carers (6 weeks), Stroke Association worker, person from a local charity visited (6 weeks), support from social services to fill in financial forms | No formal support | Daughter and her partner, son and daughter-in-law |
| Jack | Physiotherapist, speech and language therapist, paid carers, home assistance | Paid carer (reduced), referred to speech and language therapist | Wife, daughter and son-in-law, son and daughter-in-law, brother, friends |
| Margaret | Physiotherapist, emergency alarm | No formal support | Stepson, neighbour (and neighbour’s daughter), church community (visit very occasionally) |
| Jim | Physiotherapist, emergency alarm, health checks with nurse | No formal support | Wife, daughter, son, friend |
Recovery trajectories
We examined the process of recovery and adjustment over time (which we have termed recovery trajectories) of 22 stroke survivors. From the accounts of participating stroke survivors and their carers/significant others, different recovery trajectories have been identified, as well as the inter-related factors that participants perceived as having shaped these trajectories, and what was meaningful in terms of recovery and adjustment.
This section will explore the different recovery trajectories from the perspective of those adjusting to and living with the effects of stroke. From participants’ accounts, four different recovery trajectories have been identified: (1) disruption followed by adjustment and acceptance; (2) cycles of disruption followed by adjustment and acceptance; (3) disruption without adjustment and acceptance; and (4) stroke as a continuation of ongoing decline.
Stroke experienced as a disruptive event
For participants in three of the four trajectory categories (the 20 stroke survivors in trajectories 1, 2 and 3), the stroke was experienced as being a disruptive event. Participants spoke of this disruption in similar terms and tended to experience this sense of disruption most acutely in the period post discharge home. This disruption was meaningful for participating stroke survivors both in terms of its significance (the effects that the stroke had on their identity and biography) and in terms of its consequences (the difficulties and challenges that the stroke impairments presented in terms of their day-to-day activities).
Following discharge home, participating stroke survivors presented their lives as having changed dramatically and they experienced a period of loss, a reduction in their ability to carry out everyday activities, an unreliable body, changed roles and relationships and feelings of not belonging in their home and surrounding environment. Even those who had previously experienced an acute and/or a chronic illness viewed the stroke as being a big event in their life:
It [the stroke] is very hard, I’ve had serious illnesses in my life when it has been touch and go and I’ve got over it, but even that, it’s not like a stroke, I never thought it, it’s not like a stroke.
Olive
Many spoke about being unable to carry out everyday tasks and activities, which they had taken for granted before the stroke:
I couldn’t do two things at a time. I used to get in a terrible muddle. I mean, I’ve always been used to, because my son’s got a special diet, serving up two or three different meals at the same time. But, I’d get to Sunday dinner and I couldn’t handle plating up, you know, for six people. I’d forget the sprouts on one, I’d go, ‘oh’. That really threw me.
June
Such mundane activities were imbued with meaning and, because of their inability to carry them out, became markers of the disruptive nature of the stroke and their changed body, relationships and self.
With regard to their bodies, participants spoke of their stroke-related physical and cognitive impairments, of feeling strange in their body (bodily strangeness) and of instances when their body was unreliable and embarrassing. Many spoke of losing confidence as a result of their unpredictable and impaired body:
‘But I mean it [mobility problems] knocked my confidence something terrible at first’
Matt
Many also spoke of feeling unfamiliar or of not belonging in their immediate physical environment post discharge. Of particular importance was feeling ‘lost’ or awkward in their own home:
‘You don’t get the same feeling at the end of it [acute illness] that you get with a stroke when you come home, that, that terrible lost feeling . . . you feel utterly lost in your own place’
Olive
Some lived downstairs following discharge home, which marked a significant change in the way that they lived in their home.
Recovery trajectory 1: disruption followed by adjustment and acceptance
This trajectory was characterised by participants experiencing the stroke as a disruptive event and then over time experiencing a process of recovery, adjustment and acceptance. Nine of the 22 stroke survivors presented this trajectory: Matt, Olive, Eric, Alf, Bernard, June, Lyn, Joseph and Phil.
Process of meaningful recovery over time
Initially, following discharge home, participants focused on their physical and functional rehabilitation and felt that they experienced some improvements in the months after their return home. For many, this sense of physical/functional improvement occurred in the first 6 months post stroke. Some experienced a more rapid improvement than others, with one participant stating that she had almost completely rehabilitated by 16 months post stroke, whereas another experienced significant improvement between 24 and 31 months post stroke. The ‘speed’ of this initial phase of physical/functional improvement was dependent on the severity of the impairment as well as the support received.
This initial period of physical/functional improvement was meaningful to participating stroke survivors as they understood this as enabling them to return to carrying out some activities of daily living (e.g. washing and dressing themselves). The process of recovery, therefore, was shaped not only by physical/functional improvements but also by the subjective meanings and importance given to the improvements experienced – these stroke survivors, therefore, perceived this to be a meaningful recovery that enabled them to return to carrying out some tasks:
So just talking about from when you first came out of hospital till now, have you seen any changes?
Oh yeah, majorly, yeah. I mean I was in bed when I first came home.
Couldn’t really do any, you couldn’t dress yourself or anything could you really?
For many, there was a sense of relief and achievement that they had managed to regain some function and were able to return to carrying out such tasks.
Stroke survivors described being housebound when first leaving hospital and in some cases being restricted to one room of the house. For those who initially moved into a room downstairs on their return from hospital, being able to get upstairs to their bedroom was often a major achievement. This was often a result of some physical/functional improvement, but also sometimes required certain adaptations to be made, for instance the installation of hand rails, grab bars or a banister on both sides of the stairs. Phil could not use the stairs when he first came home and had a bed moved to the dining room. He spoke of how this room became a space in which he ate, slept and went to the toilet. For Phil, being able to climb the stairs, return to his bed and use the upstairs bathroom was described as a milestone in his recovery.
Leaving the home was also described as an important milestone in this initial phase of recovery. Leaving the house was almost always facilitated by formal support – family or friends at first – before the stroke survivor gained the confidence to venture out alone:
I didn’t go out of the house for about what, three month, you know, and then I just, Fred me mate, I said, ‘Fred come on, walk me to the shops’, and it was a job to walk to the shops, but I stuck with it, you know, I stuck with it so I got better and better, now I can go out on me own.
Eric
For some, leaving the house continued to require the assistance of others, which was facilitated by having family live nearby or having friends and neighbours to help. For instance, Phil described his sister as his ‘chauffeuress’ during the day whereas Alf’s daughter took him out several times a week.
For these stroke survivors, therefore, the process of recovery in the period post discharge was shaped not only by physical/functional improvements but also by the meaning given to such improvements and by working to achieve meaningful milestones through one’s own action, as well as through the initiative and support of others.
Adjusting to and managing impairments
Most stroke survivors had residual impairments and their bodies could be problematic and sometimes unreliable; however, over time, they gained knowledge about how to manage their bodies and became more familiar with their impairments. Through experiencing some improvement, gaining knowledge about how to manage their altered body and by ‘having a go’, stroke survivors began, over time, to regain a degree of confidence in their body and ability to undertake everyday activities:
Yeah, I don’t feel too bad [going out on his own], if I take my time like, I have a little walking frame with wheels on that’s in the car. [. . .] And so I’ll get my sticks and I’ve got some clips on, I can stick clips in and push that round, yeah, a bit more confident than I was. I was glad they were there before [his wife and son], you know, but now I’m sort of I have to have a go myself, got to try and do it myself.
Matt
Stroke survivors spoke at length about taking charge of their situation, of adjustments they had made and about how they managed their impairments. Stroke survivors would pace their activities and schedule in time for rest. Many would meticulously plan their activities to enable them to carry out everyday tasks (i.e. activities of daily living, domestic and household tasks) and engage in social events.
Stroke survivors within this trajectory attempted to return to a range of previously valued activities with mixed success; however, most were able to engage in some meaningful activities by adapting activities, substituting activities or taking up new activities. Some were able to return to previously valued activities that had importance for their identity and role within their family and/or community (e.g. being able to prepare a family meal, carrying out DIY) as well as leisure and social activities (e.g. going to the pub, going out for a meal, visiting friends and family). The ability to return to such activities was determined in part by the degree and nature of their impairments, the level of support that they received and the types of activities that they engaged in prior to the stroke. For instance, Phil was unable to return to physically demanding activities following his stroke; however, with support from his family and accessible facilities he was able to go to the pub and attend football matches, both of which had also been of importance to him before his stroke.
Some stroke survivors had also taken the opportunity to experience new activities. For instance, Olive had started going to an exercise class once a week, Phil attended a gym for stroke survivors at an open prison, Matt took up gardening and Lyn took up card making:
I made loads [greetings cards], yeah, I made one for Julie for her niece’s birthday and I made one for my, for Mary for her friend’s birthday and it’s got ‘Best Friend’ on it, I made one for her, and I made some Easter cards for Julie and Sammi, because Sammi going to treasure it, and oh I’ve made lots, yeah, it’ll be surprising how many cards I’ve made, yes. I did a Mother’s Day card for Julie, yeah, well the carer had to help me for Mother’s Day card, yeah, but I done all sorts, yes I did.
Lyn
For some, taking up new activities provided an opportunity for social interaction. In addition to the benefits for his physical health and recovery, Phil spoke of attending the gym as an activity that he enjoyed and of the ‘banter’ he would have with the men who helped him (paralleling how he described his relationships with his work colleagues prior to the stroke). For many, the importance of relationships, interactions and social contact was emphasised:
Even though I find that very, you know [laughs] it’s very hard, I’m in a lot of pain when I come home [from the exercise class]. But that, there again, I enjoy that because there’s men go there as well and they are so funny. They have you in stitches laughing [laughs] and it’s the best, laughter is the best tonic in the world
Olive
Olive lived alone and, although her stroke-related impairments made it more difficult for her to go out and visit her friends, because of reduced mobility and not being able to drive, she found new ways of engaging with friends and took up opportunities offered to her to interact with others.
Many of these new activities had been initiated and/or were facilitated by formal support, for instance Olive was transported to and from her class by a local charity, Matt was encouraged and supported to take up gardening by his occupational therapist and Lyn started making cards at a day centre. Many also drew on support from family or friends to facilitate them in undertaking activities.
Returning to and taking up new activities gave meaning and purpose to stroke survivors’ daily lives. Such activities enabled them to maintain (albeit perhaps in a slightly altered form) existing roles and relationships. Matt spoke of how he had grown closer to his wife since his stroke, as they now did more everyday things together.
A changed but meaningful life
Important to the process of recovery and adjustment over time was the ability to come to terms with the changed self. All but one stroke survivor who presented this trajectory spoke of their lives since the stroke as being different from their pre-stroke lives and they still spoke of loss. In particular, they spoke of the loss of freedom, independence and spontaneity in their lives. Stroke survivors missed activities that they were no longer able to do. For instance, Lyn wished to be able to go into town and window shop with her friends, as opposed to her friends visiting her at home:
Now my friends, because they come to see me, you know, but I don’t go nowhere now
Lyn
Matt missed being able to go out and visit family and friends by himself without his wife and/or son. Olive missed being able to do things spontaneously and being able to drive to visit her friends who lived some distance away. Phil said:
I used to be very independent, I just used to fly off here, there and everywhere else, go whenever or wherever I wanted to which I can’t achieve any more.
Eric spoke of how he would stay locally since the stroke and would limit him to places that were served by local bus routes:
I used to do a lot of walking, a hell of a lot of walking, every day, sometimes I’d walk ten or 12 miles a day, but three times a week, you know, but I can’t do that anymore, you know, can’t do that anymore.
Eric
Eric described life after stroke as ‘more difficult’, ‘slower’ and ‘quieter’, but to a certain extent accepted a quieter and slower pace of life as an inevitable part of getting older:
I mean I’m 66 now so I’d have to slow down anyway, you know, so just slowing down a bit more that’s all, you know, but like I say, I don’t sit feeling sorry for myself or getting depressed
Eric
These stroke survivors continued to face challenges and difficulties from living with their stroke-related impairments and many were aware of their fragile independence and/or their dependence on others. Despite these losses and challenges, however, the overall picture presented was one of disruption followed by a process of recovery, adjustment and acceptance over time. Stroke survivors felt that they were managing to live a life that was acceptable and meaningful to them and their families.
Recovery trajectory 2: cycles of disruption followed by adjustment and acceptance
This trajectory follows a similar pattern to the previous trajectory in that stroke survivors presented a similar account of experiencing the stroke as a disruptive event followed by a process of recovery, adjustment and acceptance. Their lives were interrupted again, however, by a further disruptive event that impacted on the adjustment process. Five of the 22 participating stroke survivors fell into this recovery trajectory: Barry, Sal, Rita, Emily and Jim.
For all stroke survivors in this trajectory the second disruptive event was connected to their stroke or to comorbid conditions interacting with their stroke-related impairments. Emily had a bad fall and dislocated her shoulder. Barry had an acute illness, which meant that he was unable to manage the depression he had experienced following his stroke. Both Sal’s and Jim’s stroke-related impairments interacted with their worsening arthritis and Rita experienced a small second stroke.
For Barry and Sal the second disruptive event was associated not only with their stroke-related impairments interacting with a new or worsening pre-existing health condition, but also with shifts in their social world. Barry had managed his post-stroke depression by keeping active and talking about his feelings with his daughter. His acute illness and reduced contact with his daughter meant that he was unable to manage his depression.
Sal began to feel embarrassed by her stroke-related impairments and, combined with her worsening mobility, this contributed to her resigning from her full-time volunteer post:
I went to read the minutes at one of the meetings down in the office, ‘cos I was the chairperson, and I couldn’t get the minutes out, I couldn’t read them fast enough to get them out. I was tripping over myself and that was embarrassing and it was two weeks after that that I resigned. [. . .] I thought, ‘well, I’m not doing my job if I’m not, you know, reading the minutes out’ so, well, that’s part of my job, so um I just resigned.
Sal
This second period of disruption meant that stroke survivors felt that they had gone ‘backwards’ in terms of their recovery post stroke and it also exacerbated their feelings of vulnerability and knocked their confidence. It therefore impacted on the process of adjustment and acceptance. Emily experienced a disruption to the process of adjustment when she tripped and fell in her home, dislocating her shoulder. Emily described how it had put her ‘back to how it was when I had the stroke’:
Well you knew I dislocated my shoulder so that’s put me back quite a bit but I am now going forward again because I lost my confidence, obviously, didn’t dare walk without my husband and I was just starting to do that and then snow came so I didn’t do it then obviously [laughs] and but since then I’m now going down the town by myself and going out to the shops by myself . . . so it really, it put me back to how it was when I had the stroke in other words. My mind said ‘oh no, I’ve gone back a whole year’ but now it’s back to normal, to how I was I think almost.
Emily
Emily’s husband Ken explained that it was as if she’d ‘gone back to square one’. Ken described the aftermath of the fall as similar to that of the stroke, stating that he had to take over all household roles and responsibilities again.
Emily, Rita and Barry all felt that following this second period of disruption they began to improve physically and functionally once more; however, most claimed that it had put back their recovery and prevented further progress over the following few months. Barry, in particular, explained how it was taking a long time to recover following his acute illness and 3 months later he was still not back to where he was before the acute event:
Up to Christmas I were doing fine, then Christmas . . . it just, I just wanted to die, top and bottom I didn’t want to be here. And then when, I got over it [acute illness], didn’t I? It’s too a long time to get back to how I were . . . but, it’s hard to explain. Even now, I’m still not 100% like I was [before acute illness]
Barry
Rita attributed her smaller second stroke to her stressful journey home from Spain, which involved long flight delays that deprived her of rest. Rita experienced this as a major knock to her confidence, which represented a step backwards in her recovery and adjustment. Her subsequent fear of flying also isolated her from her family who lived in Ireland. Rita described how a family crisis and self-determination, however, led her to overcome her fear:
When my daughter said what had happened [she had been diagnosed with breast cancer] and nobody could do it for me, so I just had to dig the heels in and go over there on my own. It was frightening but, did it.
Rita
Rita’s positive experience of receiving assistance at the airport and her successful engagement in a range of social activities while with her family helped her to grow in confidence again.
However, two participants, Jim and Sal, did not speak about improvements following their second period of disruption. It was during the second interview that Jim spoke of his worsening mobility. In between the two interviews he had experienced a bad fall (he had fallen out of bed) and was struggling to get upstairs. At this time he felt that he was still ‘going backwards’ and did not speak of any improvement.
Sal differed slightly in the way that she experienced the second disruptive event in that, unlike the others, she did not feel that it caused her to go ‘backwards’ in her recovery but that it led to a much bigger change in her life. Sal felt that she had recovered and adjusted quickly following her stroke and she returned to her full-time volunteer role only 3 months after discharge home. However, 11 months later she claimed that her work had become too much for her and she went from having a full-time job to only leaving her flat once a week to drive to the supermarket. Not only had Sal’s stroke-related impairments caused her embarrassment at work, her mobility declined because of her worsening arthritis, compounding the effects of her stroke, and she became very wary of falling. Giving up her full-time volunteer role was very disruptive to her life and sense of self and Sal felt that this was the biggest impact that the stroke had had on her life. Sal’s life, therefore, changed quite dramatically over a period of a few weeks, which she was struggling to comprehend during the first interview. However, 3 months later Sal had begun to make sense of these changes. Unlike the other stroke survivors in this trajectory, though, she did not talk about improving following this second disruptive event, but was adjusting to and starting to accept her less active lifestyle. She was more accepting of help offered by friends and neighbours and talked of how she needed to slow down as she aged:
I suppose I’ve realised that when I was told to slow down before, I should have slowed down, you can’t always do what you want to do, there comes a stage where you have to cut something out and not add things onto your list, do you know what I mean, which I was always doing, adding different things onto my list.
Sal
For stroke survivors in this trajectory changes in their health and social support networks, as well as incidents such as a fall, created new instances of disruption that impacted on their process of recovery and adjustment.
Recovery trajectory 3: disruption without adjustment and acceptance
Unlike those in trajectories 1 and 2, for participants in this trajectory the disruption caused by the stroke was not followed by a process of recovery, adjustment and acceptance. Instead, stroke survivors presented their lives as continuing to be disrupted and unmanageable. Six of the 22 participating stroke survivors presented this trajectory: Peter, Dave, May, Harry, Jack and Margaret.
Whereas other stroke survivors talked of a period of some physical/functional improvement following discharge home, stroke survivors who narrated this trajectory focused on their residual impairments. The severity of the stroke and residual impairments varied amongst this group and did not necessarily differ from those in the other trajectories. The importance and consequences attributed to their stroke-related impairments, however, meant that they were understood as having a great impact on their lives (including those that could be considered quite mild impairments).
Some seemed unable to envision a recovery or meaningful life without regaining function in their affected limbs, which prevented them from resuming their previous life. For instance, Dave had a paralysed left arm following his stroke. Despite being told by physiotherapists that, although he may see some further improvement, it is unlikely that he will regain function in his arm, Dave was unable to accept this:
I keep asking the same question, is everything going to come back to normal? And the question [sic] they keep giving me, “no”, and I can’t take no for an answer [bangs right hand on the table]
Dave
Several stroke survivors had other health conditions and impairments that had constrained their lives in some way before their stroke; however, the stroke was still viewed as causing significant and dramatic restrictions in their lives. For instance, Peter’s only impairment was the loss of sight in his right eye, which meant that he was unable to drive. He was unable to visit family members and engage in activities that he used to enjoy. Interestingly, although his wife spoke about how their lives had become restricted before his stroke because of his other health conditions, Peter felt that his stroke was the cause of his restrictions as the stroke took away his only remaining option for getting out and about (driving).
A focus on returning to their pre-stroke bodies and lives shaped this recovery trajectory. Many of the stroke survivors continued to compare their bodies and their lives with how they were before the stroke and focused on the losses that they had experienced and their desire to return to their ‘old’ self and life:
I know I have so far to go till I get back anywhere near my old self
May
This lack of meaningful functional improvement and preoccupation with returning to their ‘normal’ pre-stroke bodies contributed to the ongoing sense of disruption caused by the stroke.
Although they had made some changes and adaptations, overall their focus remained on what they were unable to do and what they had lost rather than what they were now able to do. The small improvements some experienced in their lives and their ability to manage their impairments were not marked with the same sense of achievement as for those in the other trajectories. Overall, there was a sense of ongoing restriction and lack of recovery. These stroke survivors were struggling to come to terms with the impact of the stroke and create a meaningful life for themselves:
So that was like cutting my legs off because I’d been driving for sixty odd years and then suddenly to lose it, you know, you lose your means of transport and we can’t get anywhere now, this is the problem.
Peter
Many needed support to get out of the house, which, although not uncommon among the stroke survivors interviewed, was a particular source of frustration for these participants. Peter felt that he was stuck in the house a lot more now:
you can’t do the things you’d like to do obviously and get around as much as you used to, you know, I’m housebound a lot more
Peter
In contrast to those in the other trajectories, those who experienced ongoing disruption were often unable to return to, or had not succeeded in returning to, activities and occupations that they felt were meaningful and important. Neither did they manage to adapt activities that they had previously enjoyed to enable them to continue to participate in them in some, albeit altered, form.
Stroke survivors in this trajectory were also unlikely to take up new activities following their stroke. May was one of the few participants in this trajectory who tried something new following her stroke by joining a stroke group; however, she did not enjoy the activities that they engaged in and soon stopped going.
These stroke survivors therefore tended to feel that their days were extremely repetitive and lacked meaningful activities. Following her stroke, Margaret was unable to knit and was not able to leave the house independently. She described her day as consisting of coming downstairs, making a coffee, watching television in her chair, having her dinner and going to bed. She highlighted the monotony and boredom of this routine through repetitive entries in an audio diary, such as the following:
Up, coffee, telly, [next-door neighbour’s daughter] came in and did a bit of cleaning, telly all afternoon, tea, bed by six. Another lovely day [said in a sarcastic tone] and I am totally, totally bored to death.
(Margaret, 1 December)
Had another exciting day [said in a sarcastic tone]. Up, coffee, telly, tea, bed.
(Margaret, 4 December)
Stroke survivors felt that the stroke had ruined their life. Margaret claimed that she was no longer living but rather ‘existing from day to day’:
It’s just ruined me really, totally . . . I was so active before, y’know, because as I say, y’know, I can’t turn me own fire on, I can’t put a plug in the bath, I can’t pick up me kettle, y’know. They might seem damn silly things but they’re so frustrating, y’know, that you can’t do them. No, it hasn’t been very nice [Margaret begins to cry]. I say me prayers every night, I say ‘Don’t let me wake up when it’s morning’, but he ain’t listening to me. I’d just like to go to bed and, y’know, not wake up, because y’know it isn’t living, y’know, you’re just existing, that’s all it is. I don’t know. I keep saying I’m going to save up £10,000 and go to Sweden [laughs] and have a cocktail over there . . . it’s just ruined my life really. It isn’t living, it’s just, you’re just existing from day to day.
Margaret
Being unable to undertake everyday and meaningful activities impacted on their relationships with family and friends and their ability to engage socially with others. Some felt unable to establish a role within their family or were unable to visit and spend time with their family. May, in particular, felt that her inability to fulfil her ‘role’ in her marriage with regard to the housework, managing the finances, cooking for her husband and being physically intimate had had a negative impact on her marriage:
I gave Bob [her husband] a bad time, I think he so good to put up with me, but it is not doing our marriage much good
May
She spoke quite openly about the difficulties and tensions that had developed in their relationship since her stroke and the daily arguments that they now had.
Some stroke survivors felt that they had withdrawn from their social life by stopping going out and/or inviting people to their house to visit. This self-withdrawal was often connected to feelings of embarrassment and/or shame about either their stroke-related impairments or the effect that the impairments had had on their ability to carry out everyday tasks (e.g. cleaning the house and/or using a knife or fork). This impacted on their ability and willingness to leave the house and/or engage in social activities. Several stroke survivors had stopped going out for meals as they had difficulty eating and found this embarrassing. For instance, the stigma associated with his paralysed arm prevented Dave from engaging in a number of social and relational activities, including going out for a meal and holding his grandchild. May was unable to tidy and clean her home and she described her husband as being unable to do these kinds of tasks to an acceptable standard. She was ashamed of the ‘state’ of her house and so she did not like to invite her extended family and friends over to visit. As she was also restricted in terms of getting out and about and was unable to visit others, this meant that she had less contact with some family members and friends:
because the house is in such a state, and we know what state it is, there’s not getting away from it, [May] doesn’t like having people in the house because, obviously . . .
I’m ashamed of it.
May also described the negative emotions that she experienced and the reactions from other people when she used her wheelchair, which meant that she was reluctant to go outside.
Some stroke survivors also felt that others had withdrawn from them and some felt very let down by friends who they expected to have been more supportive. During the mapping exercise, May specifically asked where she could place those friends whom she felt had let her down. Margaret also described her isolation following her stroke as being due to the abandonment or withdrawal of others, stating that:
when you’re actually disabled nobody really wants to know you, y’know, where you thought you had friends you don’t have ‘em anymore
Several stroke survivors spoke about the emotional impact of the stroke and the restrictions that it had caused. May described feeling very angry about the stroke and the impact that it had had on her life. She also spoke about disliking the reactions that she gets from other people. She feels that they are either overly sympathetic or that they do not acknowledge her impairments and limitations:
So I don’t like to gain sympathy, I don’t want sympathy, like I said before, if people aren’t helpful and it’s obvious I need that if, I get cross, and if they’re too helpful, I get cross, I told you that in the diary, so I mean it is a stupid mindset, I know that, but I’m sure I’m not the only one, I’m sure I’m not the only one who reacts like that, you know. You want to be normal, you don’t want to be treated different, but you do need help sometimes.
May
Dave described the stroke as having affected him ‘mentally’, that he gets very down but doesn’t like others to know this. He described his life as deteriorating and spoke of death several times during the two interviews:
My life to me it’s started to deteriorate. I have always said, I’ve got this, this and this, the next thing what’s going to happen that’ll be it [makes a cut throat gesture], end of story
Dave
Although other participating stroke survivors mentioned the limitations of the formal support that they had received following their stroke, this was particularly apparent in the accounts of those in this trajectory. Many felt disappointed and let down by the formal support that they had received, which may be because of the quality and type of support received and/or because of their high expectations of recovery and services. Both Peter and May describe receiving very little formal support following discharge home. Margaret expressed disappointment with the rehabilitation services that she had received, although she also suggested that she may have refused rehabilitation both in hospital (as she wanted to return home) and while at home (because of the pain that she experienced):
the physios were coming but when they’d been me leg just felt like jelly and twice, well three times I nearly fell in there and at night-time the pain in me leg was horrible. So one of em came one day, I said, ‘No, don’t do it’, and so I’ve never seen em since.
Margaret
Harry felt abandoned after formal services had withdrawn:
It’s all the same ‘cause you don’t get them [services], that’s what I’m saying, times when you need them they stop them. You’re going on alright when you come out of hospital, yeah felt great, but gradually I got worse, but they don’t bother with you when you’re getting worse, they say, ‘No, dig hole in the garden and chuck him in’.
Harry
Margaret suggested that she also had a lack of knowledge about navigating health and social care services:
Somebody once said, ‘who’s your social worker?’ I said, ‘I haven’t got one’, y’know. I don’t know who to ring or anything . . . So you don’t know what’s out there, y’know, or anything.
Margaret
Most felt uneasy with their level of dependence on their partners and/or adult children and, as they did not feel that they had recovered this degree of dependency continued over time. May was very dependent on her husband and felt extremely uncomfortable that her husband emptied her commode and did various personal care tasks for her. Dave had very limited function in his left arm, had some difficulty walking without the aid of a stick, was unable to fully wash or dress himself and said that he felt physically tired a lot more, despite engaging in little physical activity. This meant that he depended on others to do the jobs around the house that he used to be able to do, which he described as ‘lowering’ himself:
I did all the rooms and tiling and painting and, I mean all these jobs I could be doing meself. I don’t like asking other people to do it but at end of the day you’ve got to lower yourself and say, ‘Will you do me that?’
Dave
Other stroke survivors spoke of their dependence on others and their desire to retain some independence. Both May and Dave spoke about the support that they received from family and friends in very emotive terms.
Although many of the factors discussed above were familiar to those who presented an alternative trajectory, for these stroke survivors these issues were emphasised, remained unresolved and shaped an ongoing sense of disruption. The meaning given to their stroke-related impairments and their focus on regaining their previous lives and bodies meant that they did not experience a meaningful recovery. They were unable, therefore, to go through a process of meaningful recovery, adjustment and acceptance over time. Lives were presented as being disrupted.
Recovery trajectory 4: stroke as a continuation of ongoing decline
The fourth trajectory, of continuing ongoing decline, is characterised by a long and gradual decline in health, everyday activities and social participation. There were only two participating stroke survivors who presented this trajectory: Greg and John.
For stroke survivors in this trajectory, the stroke was not understood as being a disruptive event. For Greg, the stroke was one of a series of factors that contributed to an ongoing decline. On reflection, Greg and his wife Ann identified numerous events (both health related and social events) that had contributed to this decline, starting in the mid-1980s. These included the onset of his degenerative condition and his subsequent early retirement from work on medical grounds; Ann’s retirement, which led to Greg giving up the domestic tasks and walking the dog; Greg giving up driving because he did not feel that his legs were strong enough to drive, which is when he stopped going out of the house; and finally his stroke, which contributed to his worsening mobility and restricted his movement within the house:
It all seemed to start when, as I say, when we finished with the motor and getting out and about with it, driving a bit, and ever since then it seemed to quietly go down, you know, sort of go downhill if you will [. . .] I don’t know, it’s a weird sort of thing if you will, but as I say, the telly’s here, telly’s on.
Greg
John had diabetes and suffered from kidney problems and minor visual impairments. He spent most of the interview talking of his other health conditions. For John, the stroke appeared to be understood as just another complication of his diabetes:
I mean from a medical point of view, yeah well diabetic you know which will lead to this, which will lead to this, which will lead to this so it’s obvious that as a diabetic you’re going to have trouble with your eyes, you’re going to have trouble with your feelings and things like that and you’re going to have all this, that and the other and you’re going to, you know, be more liable to have a stroke or a heart attack or whatever.
John
Neither Greg nor John felt that the impairments caused by the stroke had a big impact on their lives. Greg identified several consequences of the stroke, including his weakened left arm and subsequent lack of confidence in using his Zimmer frame™ (Zimmer Holdings Inc., Warsaw, IN, USA), the further reduction in his mobility, a lack of concentration and incontinence. Despite these impairments, however, both Greg and his wife Ann felt that there had been little change in his life following the stroke. Greg felt that the biggest difference was that he was no longer able to go up and down the stairs, so he had his bedroom in the front room. Although he talked of sometimes feeling trapped in the front room at night, he also said that he had got used to living downstairs. The stroke had not impacted greatly on his life nor changed how he felt his future would pan out. When asked about the impact that the stroke has had on his life, Greg replied:
I mean it weren’t as though we were dashing about a hundred miles an hour [before the stroke] was it?
Greg
John also identified stroke-related impairments but he described himself as having ‘got away very lightly’ with the stroke. He felt that he had only minimal long-term physical impairments. John was left with a limp and some numbness in his left hand side but presented himself as able to accept any limitations from the stroke. One of the major problems affecting his social participation, however, is fatigue, which he attributes to his diabetes rather than the stroke.
Although the stroke did mark the end of paid employment for John, which could have been viewed as having a big impact on his life, his other medical problems had already resulted in a decline in the duties that he was able to perform:
I thoroughly enjoyed [my job] you know until this, I started to get the medical problems due to the diabetic thing which were getting to the stage where, you know, when I couldn’t operate as efficiently shall we say that, was still doing the job but I’m getting more and more on light duties you know. And then of course when this happened that was the end of it all.
John
In fact, John felt that he was already too ill to work prior to the stroke and he had been medically assessed for incapacity benefits but was judged to be ‘not ill enough’ to give up work.
For Greg, his physical decline coincided with a gradual disengagement with everyday activities and the social and physical worlds beyond the house. Greg presented himself as being content and accepting of his life and did not desire to go outside or participate in any particular activity. He did not talk of loss or of missing being able to do things or go to different places. He acknowledged that this frustrated Ann and spoke of trying to get out of the house and going out in the future, but reiterated that he was happy with life as it was. When he talked of going outside he identified the potential problems, difficulties and barriers associated with this. Ann felt that he had got in a ‘rut’ and that he was ‘laid back’ and ‘happy in his own environment’.
This trajectory is therefore marked by a gradual decline in health. The stroke was not viewed as being disruptive and the impact on the stroke survivors’ lives was considered to be minimal. They were accepting of the consequences of the stroke and viewed these as being part of other health conditions and/or the process of declining health. Life post stroke was therefore understood as being much the same as life before the stroke.
Processes and mechanisms shaping recovery trajectories
The different trajectories illustrate how multiple interacting factors shape the process and meaning of recovery and adjustment over time and therefore social participation. The key processes and mechanisms that shaped the different recovery trajectories are summarised in the following sections.
Recovery, adjustment and acceptance over time
When discussing their lives post stroke and their recovery trajectory, stroke survivors identified multiple and interacting factors that shaped this process. This suggests that the process of recovery, adjustment and acceptance is complex and multifaceted.
Interacting factors that supported a process of recovery, adjustment and acceptance over time included:
-
A process of recovery that included physical/functional improvements that were understood as being meaningful, and the achievement of important milestones through actively taking charge of their situation and the initiative and support of formal services, family and friends.
-
This process of meaningful recovery contributed to an increase in confidence and a knowledge about managing their impairments. This enabled them to plan and undertake different tasks and activities.
-
Stroke survivors engaging in meaningful activities by returning to previously valued activities, substituting activities or taking up new activities. Engaging in everyday tasks and activities enabled stroke survivors to maintain a role in their family/community and ‘do’ relationships (i.e. enabled them to maintain and establish relationships through the doing of everyday activities/tasks). Such activities also gave pleasure and structure to stroke survivors’ lives.
-
Stroke survivors successfully managing loss and creating a different but meaningful life.
Lack of recovery, adjustment and acceptance over time (ongoing disruption)
Interacting processes and mechanisms that affected the process of recovery, adjustment and acceptance and contributed to a sense of ongoing disruption included:
-
Stroke survivors focusing on their lives before the stroke and the significance and consequences of the stroke and related impairments. These stroke survivors did not feel as if they had experienced a meaningful recovery.
-
This lack of meaningful recovery, which included the sense that they had not reached important milestones, contributed to an ongoing sense of uncertainty and a lack of confidence in their abilities.
-
Stroke survivors being unable to undertake previously valued activities, to substitute other activities or to take up new activities. This often impacted on social contact, relationships with others and the person’s role in the household.
-
Stroke survivors experiencing tensions in their relationships and/or struggling to establish/maintain supportive relationships.
-
Withdrawing from social situations because of feelings of embarrassment associated with their stroke-related impairments. Although feelings of embarrassment were experienced by stroke survivors across the different recovery trajectories, those who reported adjustment and acceptance either felt that this had lessened over time or still engaged in social activities despite such feelings.
What is a good (social) recovery post stroke?
For those who experienced disruption following stroke (stroke survivors in trajectories 1, 2 and 3), there seem to be two understandings of a good (or acceptable) recovery post stroke. For those in trajectory 3, who experienced ongoing disruption, their understanding of an acceptable recovery was one that would enable them to return to their pre-stroke lives and bodies. This focus on recovery as returning to how they were before the stroke (both physically and socially) contributed to their inability to adjust, accept and create a meaningful life post stroke.
For those in trajectories 1 and 2, however, recovery was not necessarily about their ability to return to how they (and their lives) were before their stroke. What constituted a good (acceptable) recovery was a process of adjustment and acceptance that enabled them to manage and come to terms with the losses that they had experienced and to create a different but meaningful new life. This involved a complex process comprising some physical/functional improvement that was understood as being meaningful; the achievement of milestones that were important to them; actively taking charge of their situation; the initiative and support of formal support, family and friends; and engagement in everyday tasks and activities that enabled them to maintain and enact (and sometimes establish) relationships.
Critical reflection on the eligibility criteria and sampling strategy (developing a better definition of poor adjustment post stroke)
It was anticipated that the criteria used for sampling stroke survivors (based on the change between pre-stroke and 12-month post-stroke Barthel Index and FAI scores) would be associated with the recovery trajectories identified; however, this was not the case. For example, those who were categorised as ‘doing better than expected’ (more socially active than anticipated) did not necessarily have a different recovery trajectory from those who were categorised as less socially active than anticipated (‘failing to thrive’). Nor did the category predict the recovery trajectory identified through the analysis of the qualitative data (see Appendix 12, Figure 25).
It is important to note that a purposive sampling strategy was used and therefore the stroke survivors interviewed were not randomly selected to represent their associated category (i.e. those interviewed who were categorised as ‘doing better than expected’ were not necessarily representative of the population of those ‘doing better than expected’). This means that caution needs to be taken in interpreting the observation that the outcome measures were not clearly linked to the recovery trajectory identified. This lack of apparent connection perhaps emphasises the complexity of assessing recovery and adjustment after stroke, because of the multiple and interacting subjective and objective factors that stroke survivors identify as influencing their recovery and adjustment. It was anticipated by the study team that the Barthel Index and FAI could be used to define poor adjustment post stroke; however, based on the findings of this study these outcome measures alone probably cannot tell us enough about the experiences of stroke survivors post stroke to allow us to conclude whether they are recovering and adjusting well or not.
Discussion
Summary and discussion of key findings
This qualitative substudy examined the process of recovery and adjustment over time of 22 stroke survivors and 12 carers. From participants’ accounts four different recovery trajectories have been identified: (1) disruption followed by adjustment and acceptance; (2) cycles of disruption followed by adjustment and acceptance; (3) disruption without adjustment and acceptance; and (4) stroke as a continuation of ongoing decline.
Participants’ accounts of their lives post stroke comprised complex and interacting factors that shaped recovery and adjustment. The existing literature has previously discussed important aspects of recovery and adjustment following stroke,10,23,25,121–125,128–133 including pre-existing factors (age, other health conditions and previous knowledge of stroke) that may mitigate the disrupting impact of the stroke. 158,159 Although insightful, such research has often treated post-stroke adjustment as a static event (i.e. stroke survivors either experience disruption following stroke or do not experience disruption) rather than as a complex multifaceted ongoing process. This study contributes to this wider body of literature by identifying different types of recovery trajectories through examination of the process and meaning of recovery and adjustment over time. Furthermore, this study has explored how certain factors interact and shape the meaning and process of recovery and adjustment over time, and thus stroke survivors’ social participation.
Interestingly, for those stroke survivors who experienced meaningful recovery, adjustment and acceptance (those in trajectories 1 and 2), recovery was not about returning to their pre-stroke lives but was a process that enabled them to manage loss and create a different but meaningful life. For those stroke survivors who experienced ongoing disruption, however, the focus was on returning to their pre-stroke bodies and lives. This has implications for existing literature in which recovery post stroke is understood as being linked to the extent to which stroke survivors are able to re-establish continuity with their previous self and life. 160 This study found that stroke survivors develop different understandings of what constitutes recovery and therefore a more nuanced notion of recovery needs to be acknowledged and explored in the stroke literature. Furthermore, the findings suggest that those who experienced meaningful recovery, adjustment and acceptance over time did so not only by establishing some markers of continuity with their previous self but also by managing loss and creatively establishing new elements of their self and life. For some, therefore, the process of meaningful recovery and adjustment following stroke involves more than establishing markers of continuity with their pre-stroke body, identity and life.
A further point of interest is the importance of everydayness in stroke survivors’ discussions of their life post stroke and the significance that being able to participate in meaningful but everyday tasks has for enacting and maintaining relationships. Interviews were often dominated by talk about everyday activities and how stroke survivors managed (or not) their stroke-related impairments to enable them to engage in everyday tasks and activities. Although everyday activities and the importance of meaningful activities have been discussed in the stroke literature,25,122 the meaning and role of everyday tasks and activities in enacting relationships and social participation have been overlooked. Although mundane, everyday tasks and activities, and one’s ability to carry them out, are important components of ‘doing’ relationships (i.e. actively maintaining relationships) and fulfilling roles within the household/social sphere (i.e. being a father, mother and/or friend). The ability of stroke survivors to manage their impairments to engage in everyday tasks and meaningful activities shaped the meaning and process of recovery post stroke and thus their social participation.
Strengths and weaknesses
This substudy drew on a grounded theory approach to qualitative research138–140 and made use of multiple qualitative methods, including semistructured interviews, limited observations, solicited diaries and support network mapping techniques, to understand the process of adjustment following stroke. In total, 22 stroke survivors and 12 carers/significant others participated in the study. A total of 42 interviews were conducted and 15 diaries were completed. A further strength of the study was that participating stroke survivors were purposively sampled to include those who were less or more socially active than anticipated (based on their pre-stroke and 12-month post-stroke Barthel Index and FAI scores). The sample was also purposively selected to ensure variation in key characteristics that are known to shape adjustment and social participation post stroke. These included age, socioeconomic status and whether the stroke survivor lived alone or with others. This enabled the exploration of recovery and adjustment in a varied, data-rich sample.
Although the purposive sampling strategy enabled the recruitment of a diverse group of participants, we were unable to conduct secondary theoretical sampling often undertaken in grounded theory studies. This was because of time constraints and data limitations of the quantitative trial (i.e. the information collected for the trial did not contain information required for the theoretical sampling of potential participants). Themes and theories developed in the ongoing analysis were therefore tested through the refinement of interview topic guides, ongoing data collection and the follow-up interviews. 138
The categories based on the Barthel Index and FAI scores did not appear to be associated with the recovery trajectories identified from the qualitative data and therefore we were unable to develop a definition of poor adjustment post stroke based on these measures to be used to evaluate the prevalence of poor adjustment.
Implications for health and social care
This study suggests that, ideally, health and/or social care professionals should routinely monitor stroke survivors’ progress during the 6- and 12-month reviews by incorporating into the reviews a discussion around the factors identified that shape adjustment post stroke (and in particular barriers to adjustment). They would then be able to refer stroke survivors for further specialist support if required. Such support services would work with stroke survivors and their carers to find creative ways of managing impairments and coping with loss to enable meaningful activity, and thereby sustain relationships and enable social interaction, to facilitate stroke survivors through a process of meaningful recovery, adjustment and acceptance, as well as helping to prevent and/or address ongoing and/or further instances of disruption. Changes in stroke survivors’ social support and health, as well as incidents such as a fall, can create new challenges that need to be overcome and/or can result in stroke survivors going ‘backwards’ in their process of recovery and adjustment post stroke. This suggests the need for services to be able to respond to such potentially disrupting events and changing needs. As GPs are often the first point of call for many, it would be appropriate to devise a tool that prompts GPs to discuss the adjustment process, possible barriers and changes in health and support during their consultations with stroke survivors.
Case studies illustrating the different recovery trajectories may be informative for stroke survivors and carers and may also be useful as a resource for training health and social care professionals who work with stroke survivors in the longer term.
The findings have been discussed with therapy staff as well as with stroke survivors and their carers. Through these workshops several further implications of this exploratory study were identified. These included how health-care professionals could support stroke survivors to adjust and adapt to life post stroke by moving from an initial focus on functional improvement to working with patients to create a meaningful life post stroke and mobilising stroke survivors’ informal support networks (thus supporting the recovery trajectory of disruption followed by adjustment and acceptance); supporting stroke survivors to come to terms with the likelihood that their lives will be different post stroke; and signposting stroke survivors and their families to alternative emotional and relational support, such as Relate. Stroke survivors and their carers felt that it was important that there should be a single health/social care professional who they could contact and/or who would contact them from time to time to provide ongoing advice and support into the longer term.
Recommendations for future research
A number of participating stroke survivors felt their lives continued to be disrupted, even over a year after the stroke. They felt unable to manage the stroke-related impairments and engage in activities to create a meaningful life. It is worth noting that half of these stroke survivors were still receiving some form of formal support for their stroke-related impairments at the time of data collection, but that most felt dissatisfied with the support that they received, some were unaware of other support available and some may have initially rejected support that had been offered. Stroke survivors who experience ongoing disruption may therefore be hard to engage but perhaps have the most to gain from longer-term tailored support. We plan to further explore resource use of participating stroke survivors using the trial data from the economic analysis. It may also be informative to compare stroke survivors’ recovery trajectories with unmet needs, identified using the LUNS questionnaire and GHQ-12 scores, collected as part of the trial data.
Future research is needed to further explore the different recovery trajectories and how, in routine practice, the process of adjustment can be monitored and supported and how potential barriers to adjustment can be identified and overcome.
Further research is also needed to examine how health and social care professionals can accurately identify stroke survivors’ recovery trajectories, in particular those who experience ongoing disruption or a period of further disruption following their stroke. There is the potential to identify stroke survivors who may be vulnerable to experiencing ongoing and/or further disruption while they are inpatients, while they are receiving support at home following discharge and/or during their 6- and 12-month reviews.
Chapter 6 Conclusions/recommendations
This programme grant has been completed as specified in the original application, on time and with all objectives met. In projects 3 and 4 we have delivered considerably more than originally intended. Through a programme of interlinked studies we have developed and evaluated interventions and tools that aim to improve the longer-term outcomes for stroke patients and their carers and have explored adjustment post stroke. This was achieved through the production of evidence-based treatment algorithms focused on problems identified by patients and carers; the robust evaluation of a new system of care; the development and evaluation of a monitoring tool for longer-term unmet needs after stroke; and qualitative exploration of barriers that impede stroke recovery.
In project 1 we updated previously developed evidence-based treatment algorithms addressing identified and reported patient and carer post-stroke problems. Evidence relating to 16 patient- and/or carer-centred problem areas was comprehensively searched through the development and implementation of a structured search protocol. The methodology that we adopted supported the practical implementation of evidence and is transferable to other clinical areas. This review of the evidence also served the dual purpose of allowing us to identify patient-centred evidence gaps. We plan to review the evidence gaps in the context of the 2013 NICE stroke rehabilitation guidelines. 161
These updated treatment algorithms replaced the existing algorithms that underpinned our manualised system of care. Working with community-based SCCs across England, Scotland, Wales and Northern Ireland, we undertook a cluster RCT evaluation of this new system of care to determine the effects on patient and carer outcomes and its cost-effectiveness. The system of care included a care plan containing a structured assessment and goal and action planner, a manual containing the treatment alogorithms linked to the assessment questions and a checklist of the assessment areas for patients, underpinned by a problem-solving approach.
In the trial evaluation of the new system of care it was clearly important to facilitate implementation by the SCCs randomised to the intervention. This was supported by working with the SCCs on the intervention documentation. By gaining their input into the design, content and layout, the intervention care plan replaced their previously used systems, thus embedding the structured assessment system within routine practice. We preset a high bar for compliance – that 75% of the structured assessment was delivered. Of patients seen by the intervention SCCs, 89% received at least one structured assessment and 96% of the initial assessments achieved compliance.
The trial design was a pragmatic one in that we sought to implement the new system of care as it would have been implemented in usual practice. Implementation was carefully thought through and planned. It was based on a survey of services undertaken, both before and after services were recruited into the trial, and detailed discussion, including site visits, during set-up. Implementation of a multicentre cluster trial was extremely challenging against the backdrop of changing bureaucracy related to the NHS approval system. The case study we submitted to the Academy of Medical Sciences review162 (included in Appendix 6) demonstrates the difficulties that we faced. We required 69 research and development (R&D) approvals from 62 trusts to cover the 32 SCC services involved in the trial. The time taken to receive all R&D approvals for a service ranged from 1 to 10 months. Despite this, the trial (with the support of the UK SRNs) was completed to time and target and we achieved our sample size of 800. This makes it one of the world’s largest completed stroke rehabilitation trials. The data generated, including the health economic data, provide insight into the problems faced and resources utilised by a large cohort of stroke patients and their carers from across the UK.
The trial was rigorously implemented and had a robust design. The inclusion of sites in England, Scotland, Wales and Northern Ireland supports the generalisability of the results. The return rate of outcome measures and loss to follow-up were in line with our sample size predictions, with > 75% of patients completing outcome measures at 6 months.
In project 3 we tested and demonstrated the validity, test–retest reliability and acceptability of a novel monitoring tool (LUNS) to identify individual- and service-level unmet needs relevant to longer-term stroke care. This component of the programme grant was enlarged significantly from our submitted grant application. The sample size was increased to provide a more robust evaluation of the questionnaire.
In project 4 we undertook a study to explore quantitatively and qualitatively the adjustment of patients and carers to stroke. However, the methodology was enhanced in that, instead of the proposed one-off interview, two interviews were conducted either side of a period of diary keeping. This enabled a much richer picture of life after stroke to emerge and the identification of recovery trajectories and facilitators of, and barriers to, adjustment.
The limitations of the individual projects are discussed in the specific chapters. Overall, we believe that we have delivered a complex programme successfully.
In conducting a large multicentre cluster RCT across the UK we have demonstrated that this methodology (appropriately conducted) is feasible in stroke rehabilitation research. By the engagement of 29 clinical stroke services, including community stroke services, many of which had not been involved in research previously, we have increased the clinical capacity for and understanding of research. We hope that the conduct of large trials such as this marks a step change in stroke rehabilitation research and will facilitate future large trials to address important clinical questions. Our research illuminated some ongoing challenges, which include the identification of appropriate outcome measures. With the support of our trial steering committee we have undertaken a 1-day workshop with colleagues from stroke care and statistics (the First Leeds Colloquium on Stroke Rehabilitation Research) to share our ‘lessons learnt’ and debate methodological issues in stroke research, specifically in relation to the evaluation of complex interventions. A summary of the discussions is being prepared for publication.
Foreseen challenges in delivering the programme included the protracted approvals process for studies, the complexities of running a huge multicentre study across the UK and undertaking research with a generally older, vulnerable patient group. Unforeseen challenges included a national postal strike (inconvenient when awaiting the return of postal outcomes), organising training on a day that had ‘the worst weather this decade’ and working with sites that included isolated rural communities (one SCC used a ferry, plane and bus to reach the national training day and the trial manager was unable to undertake a planned site visit as a result of ferry cancellations because of bad weather). The programme was implemented with the assistance of the hugely supportive NIHR SRN in England and the research networks in Scotland, Wales and Northern Ireland, which enable the recruitment of large numbers of participants to studies.
Stroke is a family illness that can have devastating consequences for the patient, their family and society. Despite tremendous advances in the treatment of acute (and hyperacute) stroke, rehabilitation remains the cornerstone of treatment for many and the longer-term outcome remains poor. Our research programme was in part predicated on the recommendation for continuing post-stroke care in the National Service Framework for Older People and the recommendation for implementing a SCC role. 16 This has been supported by subsequent reports, most specifically the NSS published in December 2007. 15 Our thinking and the contents of this programme of work, which was commissioned in summer 2007, were innovative and novel at that time and are absolutely in keeping with the recommendations of the NSS. The strategy, supported by the Stroke Improvement Programme, highlighted the need for a review 6 months after stroke, and other review tools (e.g. the GM-SAT34) have been developed that are similar to our own structured assessment. Despite these advances, however, the survey of McKevitt and colleagues4 (which utilised some of the LUNS questions) of 1257 stroke survivors demonstrated that many stroke survivors still have unmet needs. The range and number of unmet needs are similar to those reported in our own work (projects 2 and 3). The unmet needs identified are similar to the problems addressed in the system of care structured assessment, confirming that the assessment had internal validity.
We undertook a cluster RCT of this complex intervention in the hope that positive evaluation would lead to widespread commissioning of this system of care. It is disappointing that the purposely developed patient- and carer-centred approach did not result in improved outcomes for this group of patients or their carers, with total health and social care costs similar between the control group and the intervention group. This highlights the complexity and heterogeneity of post-stroke problems and the difficulty in addressing these.
We have outlined the detailed work that we undertook to develop the treatment manual and structured assessment. These were focused on patient- and carer-centred problems and we conducted a rigorous review of the evidence supported by peer review to update the treatment algorithms. The manual was well received by the SCCs and acknowledged as a useful resource, particularly for staff new to the role. There was good compliance with the structured assessment, with on average > 90% of the assessment areas discussed, and this involved no additional SCC time compared with usual practice. In total, 96% of SCCs reported that the assessment was both easy to use and useful. The assessment questions and treatment manual are therefore a useful resource that underpins the implementation of evidence-based treatment for post-stroke patients and their carers. This wider more holistic model with an emphasis on patient-identified problems is in keeping with the current drive for integration of care, considering and addressing both health and social well-being. The manual promotes interdisciplinary planning and supports staff to move from multidisciplinary to more interdisciplinary work.
Our planned further analyses and the in-depth qualitative exploration undertaken in project 4 go some way to not only explaining the trial results but also providing indicators for future research. It is interesting that our suggestion that outcomes assessment of functional and social activity would be a reasonable indicator of ‘poorer’ or ‘better than expected’ adjustment proved to be unfounded. This indicates (perhaps not surprisingly) that such outcome measures are not necessarily an accurate marker of someone’s recovery from and adjustment after stroke. But our interpretation, as we suspected, was narrow in focus; the measure of someone’s recovery after stroke is not necessarily about their ability to live busy, active and hectic (social) lives, or even to return to how they were (and their lives were) before their stroke. To stroke survivors in project 4, what constituted a ‘good’/’acceptable’ recovery was the ability to find ways to carry out everyday tasks and activities that enabled them to maintain and enact (and sometimes establish) relationships that were important and meaningful to them, that is, the ability to find ways to ‘be’ a person and to ‘do’ relationships.
That information provision is the most commonly reported unmet need is in keeping with previous work. 4 As discussed earlier, words used do not necessarily fully capture meaning. Although ‘information’ is commonly required, this can reflect a range of thought processes including that stroke survivors may feel unsure whether they are fully informed and, despite being in receipt of considerable information, feel that they should remain open to further information if it is available. ‘Information’ may act as a surrogate marker for ‘wanted something but not sure what’. The provision of appropriate and timely information remains a challenge. It is likely that information needs will change over time and ‘generic’ leaflets become less applicable. Our Cochrane review45 suggested slight benefits for structured information provision (e.g. information booklets with an opportunity for feedback) in terms of improving outcomes for patients and carers after stroke. It is interesting, however, that stroke survivors have gained benefits from the provision of information even 3 years after stroke. 163 Our research43 and that of others164 indicates that the provision of information must be an integral part of all services provided to people with a long-term condition such as stroke and it has recently been suggested that a more intensive individually tailored approach may provide some benefit. 165 The improved provision of information to stroke survivors about their condition and the services available to them will support self-management, which can be developed as they adjust to their new health state. 166
We hoped that the system of care would be delivered by the SCCs through a more problem-solving approach to post-stroke care. Although some SCCs did adopt this new mode of delivery, it appeared that not all did so. The emphasis on supporting stroke patients and carers to help themselves was occasionally lost in a more predominant ‘doing for’ service model. A more iterative training and education programme for staff would help to address this issue. This would also contribute to the formalisation of a more targeted treatment plan.
We acknowledge that the lack of improvement in patient and carer outcomes may be attributable to insufficient provision in the area of need. This is particularly the case with respect to the management of psychological issues. Mood was one of the most prevalent and persistent problem areas identified yet most SCCs reported that difficulties exist for addressing this problem, with 12 services reporting an extreme lack of appropriate psychological services. Both mood and cognition were common unmet needs. Co-applicant Allan House has been instrumental in the recent development of the stepped care model for psychological interventions after stroke. 167 SCCs would be well placed to provide level 1 and 2 support (addressing mild/moderate symptoms of mood and cognition). A number of services also reported that there were limited voluntary and community resources such as social groups.
The heterogeneity of stroke survivors, who have a wide range of problems and unmet needs, as revealed in our projects, indicates that targeted more bespoke interventions may be the way forward. We believe that the structured assessment is a useful tool but greater consideration should be given to delivery and formulation of a subsequent treatment plan. Stratification of the patient group may be required. Some patient (and carer) needs may be so complex that they necessitate a tailored case management approach; for others, the system of care as described may be appropriate, enabling identification of specific problems that require addressing, such as pain management; other patients (and carers) might require minimum further involvement but could benefit from a programme of supported self-management. Such programmes are effective in other long-term conditions168 and there is a suggestion of benefit in stroke. 169,170 Building on our assessment tools and qualitative research exploring adjustment after stroke, there is the potential to identify stroke survivors who may be vulnerable to experiencing ongoing and/or further disruption. In addition, it would be beneficial to use theory-based predictors of behaviours that impact on longer-term stroke outcomes. Further work is required to refine these models and scope content (e.g. the self-management materials) appropriate for this heterogeneous client group.
Family members are key supporters for stroke survivors; further synthesis of this programme’s outputs and other work171 is required to determine how best to prepare them for this role to facilitate longer-term adjustment. It is interesting in this work that stroke survivors emphasised the importance of being able to undertake activities of daily living, not only for the practical benefits but also for the emotional and social benefits of being able to interact in the ‘normal’ world.
There have been considerable research and clinical gains in stroke care during the implementation of this programme. The emphasis on the delivery of acute, hyperacute and early rehabilitation treatment has been addressed recently with the publication of the NICE guidelines on rehabilitation after stroke. 159 However, this programme of work is a timely reminder that stroke is a challenging condition for many and that further work is required to refine the longer-term care pathway to support stroke survivors and their families in achieving optimal outcomes.
Recommendations for research
In project 1, updating of the treatment algorithms enabled us to produce a comprehensive review of evidence gaps. Our detailed programme of research has explored the difficulties facing patients and their carers in the first year after stroke. Through this work a number of research recommendations have emerged.
When evaluated using a robust cluster RCT design, the system of care was not more effective than usual care at improving patient and carer outcomes. The research team will continue to explore the large data set available [informed by the outputs of the adjustment after stroke study (project 4)] to investigate whether subgroups of patients may have shown evidence of benefit.
In addition, we recommend that:
-
Further research should be undertaken to explore the approach of stratifying patients after initial assessment, with subsequent input being tailored to level of need.
-
The role of supported self-management should be further investigated.
-
The LUNS tool is appropriate to identify unmet need and that a similar tool for carers should be developed. Consideration should also be given to the evaluation of proxy responses and development of an aphasia-friendly version of the LUNS tool.
-
Future research is needed to further explore the different recovery trajectories and how, in routine practice, the process of adjustment can be monitored and supported and how potential barriers to adjustment can be identified and overcome.
Research methodology
We have demonstrated that cluster trials are feasible in rehabilitation research. However, they require rigorous implementation and are preferably (as with this trial) delivered through a CTRU. We believe that this research has highlighted the exciting new opportunities available with the establishment of the NIHR SRN in England and the research networks in Scotland, Wales and Northern Ireland. For the first time large cohorts of patients can be recruited, facilitating higher-quality and more timely research. 172 Overcoming the barriers of small sample sizes is a major step forward. It is now timely to reflect on and refine methods:
-
The value of tandem process and realist evaluations is acknowledged and approaches to synthesising the evidence should be further refined.
-
The spectrum of pragmatic to explanatory trials needs to be carefully considered in trial design. 173
-
It is essential that there is discussion and debate about methods, procedures for trial implementation and factors such as therapy effects, to inform planning of the next generation of rehabilitation research.
-
The UK SRNs provided tremendous support to our research team and enabled us to deliver two large studies. It is important that researchers engage with the networks as soon as possible when planning a study to gain realistic insights into what is feasible and what additional resources may be required.
-
The mechanics of how the clinical research network might influence the study sample must be considered. For example, we purposefully amended the entry criteria for participants in project 3 to ensure that sufficient patients with cognitive or communication impairments were recruited. The difficulties of recruiting carers when they are not included in the network performance management statistics have been acknowledged.
-
Screening data are particularly important in cluster trials to enable monitoring of bias. This is likely to be an additional burden for clinical research network staff and the resources required should be considered at the planning stage.
-
Our studies have again highlighted the challenges of outcome assessment in this population group and we have indicated the dissonance between objective outcome assessment and patients’ subjective views. Further work is required to successfully address this.
Implications for health care
Policy recommends that stroke patients are reviewed in the longer term15,55 and that this includes measurement of unmet needs. The LUNS questionnaire has been shown to be suitable for identifying longer-term unmet needs post stroke and therefore could be used for this purpose. It is possible that the LUNS tool may have wider relevance beyond stroke care, to capture general issues that affect older people with long-term conditions.
A structured assessment based on stroke patient- and carer-identified problems and linked to evidence-based treatment algorithms has been developed and implemented in a range of UK stroke services. The assessment and the associated manual can be used in clinical practice to enhance evidence-based care, for example the assessment provides a structured way of ensuring that an evidence-based post-stroke review is carried out and the manual serves as a repository of evidence-based practice, which could be useful for training inexperienced staff. They may be of particular use for developing services in sites currently without organised long-term care for stroke. The manual would support staff to form a broader professional base to facilitate moving from multidisciplinary to interdisciplinary work.
Consideration should be given to the stratification of patients following the initial post-discharge assessment to facilitate targeting of appropriate treatment. Some patient (and carer) needs may be so complex that they necessitate a tailored case management approach; for others, the system of care as described may be appropriate; other patients (and carers) might require minimum further involvement but could benefit from a programme of supported self-management.
We anticipate that case studies illustrating the different recovery trajectories identified in the adjustment after stroke study (project 4) may be useful as a resource both for stroke survivors/carers and for health and social care professionals who work with stroke survivors.
Implications for commissioners
Service provision
The work reported here will inform commissioning strategies. We have identified evidence gaps and unmet needs and have provided case studies of the challenges that patients and their families face after stroke.
Continuous audit of the community-based care of stroke patients, in particular covering the transfer of care, would ensure that patients referred to community services received those services. This was highlighted as an area of concern in the system of care trial in which (despite extensive discussions and planning meetings) a number of patients either were incorrectly referred or did not receive the expected referral to the SCC.
Commissioners need to be aware of gaps in service provision in the stroke care pathway. The LUNS questionnaire could be used as a survey tool to assess the extent to which community services are meeting the needs of their stroke patients, for example measuring the percentage of the local stroke population whose mobility needs are not met. Such data could be used to compare different services, promote service development or guide commissioning of services to enhance outcomes. It is fundamental to any assessment of needs that there are mechanisms in place to address those needs.
There should be a greater emphasis on integrated working at the level of the individual and the level of the service. For the health professional an emphasis on a problem/handicap rather than an impairment-focused approach to assessment of patient and carer needs is required. Services should be interlinked appropriately rather than operating independently.
Training and education
Unidisciplinary staff should be supported through appropriate training and education to broaden their skills and understanding to enable them to more successfully address patient and carer needs. We consider that interdisciplinary working is important for the success of the system of care methodology, and the system of care trial highlighted that, although some participating SCCs worked in this way, others focused their assessment on a limited number of areas for all patients. Such training might include case studies of the different recovery trajectories (project 4) and guidance on how to provide psychological support as outlined in the stepped care model for psychological interventions after stroke. This may mitigate against the observed lack of access to psychological services for stroke patients.
Acknowledgements
This programme grant was implemented with the help, support and good humour of over a thousand people. Our thanks go to them all.
A huge thank you to all of the stroke patients and their carers who participated in the studies. We also appreciate that 136 stroke survivors have expressed a wish to be informed of any future research projects in which they may participate.
Thanks to the NIHR SRN, particularly the managers and researchers, who worked so hard to enable us to achieve the sample size for two of our projects (projects 2 and 3) – a massive effort from all.
Thanks to Terry Brady and friends and colleagues in the CRAG who provided endless advice and guidance on the implementation of the research.
Many thanks to colleagues at the University of Leeds CTRU and Bradford Teaching Hospitals NHS Foundation Trust [including the Academic Unit of Elderly Care and Rehabilitation, the Finance Office (Alec Cowell) and human resources staff] who provided support and advice throughout the lifetime of this programme.
Project 1
With thanks to Ruth Lambley who initially co-ordinated this project.
Thanks also to Deirdre Andre and colleagues, University of Leeds Health Sciences Library, for undertaking literature searching throughout the programme grant, for the reference guides in project 1, two updates of the Cochrane review on information provision and repeated reviews of the literature related to longer-term outcomes after stroke.
We thank the external peer reviewers of the references guides and Kate Hill, Jane Smith and John Green for reviewing literature from the searches.
Project 2
Thanks to Dr Peter Wanklyn and Dr Tony Rudd who assisted with the intervention training.
The trial involved hundreds of people across the 32 sites. This included staff from R&D, clinical teams and administrative staff at the different sites.
Thanks go to a hugely supportive and helpful trial steering committee: Professor Helen Rodgers (chair), Professor Jonathan Mant, Professor Kerry Hood, Professor Avril Drummond, Professor Peter Langhorne and Mrs Joan Firth (lay representative).
Thanks to Renee Romeo for assistance during the design of the economic evaluation and Margaret Heslin for assistance with unit cost collation.
Thanks go to Andrew Carter and Alison Ferguson for data management support, Maria Efthymiou for statistical input, Joanne Copeland for supervision of trial co-ordination, Nick Hearfield and Muna Matouk for administration of patient questionnaires and Danielle Beaumont for assistance with trial co-ordination.
Project 3
We thank Sue Bogle (Aysgarth Statistics) for statistical support in the pilot study, Chung Fu for data management support, Nick Preston for assistance with statistical analyses and Jane Smith for assistance with study co-ordination.
Project 4
We thank the stroke survivors who provided advice and feedback at the YSRN stroke survivor and carer annual events and members of the YSRN Therapy Group.
Thanks to Ivana Holloway for providing reports of potential participants from the trial database.
Intellectual property
All intellectual property arising from the research is owned by Bradford Teaching Hospitals NHS Foundation Trust.
The intellectual property arising from the research includes:
-
the LUNS questionnaire (UK Copyright Service Registration No: 284656482)
-
the LoTS care manual and treatment algorithms
-
the LoTS care structured assessment
-
the case studies of recovery trajectories.
Reuse is permitted free of charge for academic and non-commercial research purposes; for teaching and training purposes relating to the provision of health and social care; and for the evaluation and provision of health and social care. Any and all reuse requires prior written permission from Bradford Teaching Hospitals NHS Foundation Trust (contact corresponding author).
Contributions of authors
Major contributors to the programme
Anne Forster (Professor of Stroke Rehabilitation) was the programme lead, the chief investigator for project 2, the lead for project 1 and the chief investigator for project 3 (from January 2009).
Kirste Mellish was the programme manager from September 2009 to December 2012.
Amanda Farrin (Professor of Clinical Trials and Evaluation of Complex Interventions) was the programme statistician and the supervising statistician for project 2.
Bipin Bhakta (Professor of Rehabilitation Medicine) had input across the programme and was the outcome measure evaluation lead for project 3.
Allan House (Professor of Liaison Psychiatry) had input across the programme and contributed to the intervention training in project 2.
Jenny Hewison (Professor of the Psychology of Healthcare) had input across the programme.
Jenni Murray (Senior Research Fellow) was the lead for project 1 and the chief investigator for project 3 (to December 2008) and made substantial contributions to the development of the intervention.
Anita Patel (Reader) was the health economist for project 2.
Martin Knapp (Professor of Health Economics) was the supervising health economist for project 2.
Rachel Breen was the programme manager from September 2007 to June 2009.
John Young (Professor of Elderly Care Medicine) had input across the programme and shared clinical expertise.
Major contributors to individual projects
Project 2
Katie Chapman was the trial manager; Ivana Holloway was the trial statistician; Jane Nixon was the lead for the CTRU; and Rosemary Shannon and Natasha Alvarado were responsible for the qualitative process data; Shamaila Anwar was the trial co-ordinator.
Project 3
Rosemary Shannon was the study co-ordinator and the research fellow for the qualitative study; Mike Horton was the project statistician; Natasha Alvarado was the research assistant (pilot study); and Alan Tennant was the supervising statistician.
Project 4
Rebecca Hawkins was the senior research fellow and chief investigator; Adam Jowett was the research fellow; and Mary Godfrey was the supervising qualitative researcher.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health.
Publications
Forster A, Young J, Nixon J, Chapman K, Murray J, Patel A, et al. Protocol of a cluster randomized trial evaluation of a patient and carer-centered system of longer-term stroke care (LoTS care) [published online ahead of print 19 February 2013]. Int J Stroke 2013. doi:10.1111/ijs.12038
LoTS care LUNS study team. Validation of the longer-term unmet needs after stroke (LUNS) monitoring tool: a multicentre study. Clin Rehabil 2013;27:1020–8.
References
- Reducing Brain Damage: Faster Access to Better Stroke Care. London: National Audit Office; 2005.
- National Clinical Guidelines for Stroke. London: Royal College of Physicians; 2008.
- Feigin VL, Barker-Collo S, Parag V, Senior H, Lawes CM, Ratnasabapathy Y, et al. Auckland Stroke Outcomes Study. Part 1: gender, stroke types, ethnicity, and functional outcomes 5 years poststroke. Neurology 2010;75:1597-607. http://dx.doi.org/10.1212/WNL.0b013e3181fb44b3.
- McKevitt C, Fudge N, Redfern J, Sheldenkar A, Crichton S, Rudd AR, et al. Self-reported long-term needs after stroke. Stroke 2011;42:1398-403. http://dx.doi.org/10.1161/STROKEAHA.110.598839.
- Hackett ML, Yapa C, Parag V, Anderson CS. Frequency of depression after stroke: a systematic review of observational studies. Stroke 2005;36:1330-40. http://dx.doi.org/10.1161/01.STR.0000165928.19135.35.
- Patel MD, Tilling K, Lawrence E, Rudd AG, Wolfe CD, McKevitt C. Relationships between long-term stroke disability, handicap and health-related quality of life. Age Ageing 2006;35:273-9. http://dx.doi.org/10.1093/ageing/afj074.
- Kwok T, Pan JH, Lo R, Song X. The influence of participation on health-related quality of life in stroke patients. Disabil Rehabil 2011;33:1990-6. http://dx.doi.org/10.3109/09638288.2011.553709.
- Hare R, Rogers H, Lester H, McManus R, Mant J. What do stroke patients and their carers want from community services?. Fam Pract 2006;23:131-6. http://dx.doi.org/10.1093/fampra/cmi098.
- Salter K, Hellings C, Foley N, Teasell R. The experience of living with stroke: a qualitative meta-synthesis. J Rehabil Med 2008;40:595-602. http://dx.doi.org/10.2340/16501977-0238.
- Murray J, Young J, Forster A. Review of longer-term problems after a disabling stroke. Rev Clin Gerontol 2007;17:277-92. http://dx.doi.org/10.1017/S0959259808002608.
- Anderson CS, Linto J, Stewartwynne EG. A population-based assessment of the impact and burden of caregiving for long-term stroke survivors. Stroke 1995;26:843-9. http://dx.doi.org/10.1161/01.STR.26.5.843.
- Guidetti S, Andersson K, Andersson M, Tham K, Von Koch L. Client-centred self-care intervention after stroke: a feasibility study. Scand J Occup Ther 2010;17:276-85. http://dx.doi.org/10.3109/11038120903281169.
- Rigby H, Gubitz G, Phillips S. A systematic review of caregiver burden following stroke. Int J Stroke 2009;4:285-92. http://dx.doi.org/10.1111/j.1747-4949.2009.00289.x.
- Greenwood N, Mackenzie A, Cloud GC, Wilson N. Informal carers of stroke survivors – factors influencing carers: a systematic review of quantitative studies. Disabil Rehabil 2008;30:1329-49. http://dx.doi.org/10.1080/09638280701602178.
- National Stroke Strategy. London: Department of Health; 2007.
- National Service Framework for Older People. London: Department of Health; 2001.
- Mant J, Carter J, Wade DT, Winner S. Family support for stroke: a randomised controlled trial. Lancet 2000;356:808-13. http://dx.doi.org/10.1016/S0140-6736(00)02655-6.
- Forster A, Young J. Specialist nurse support for patients with stroke in the community: a randomised controlled trial. BMJ 1996;312:1642-6. http://dx.doi.org/10.1136/bmj.312.7047.1642.
- Boter H, De Haan RJ, Rinkel GJ. Clinimetric evaluation of a Satisfaction-with-Stroke-Care questionnaire. J Neurol 2003;250:534-41. http://dx.doi.org/10.1007/s00415-003-1031-2.
- Dennis M, O’Rourke S, Slattery J, Staniforth T, Warlow C. Evaluation of a stroke family care worker: results of a randomised controlled trial. BMJ 1997;314:1071-6. http://dx.doi.org/10.1136/bmj.314.7087.1071.
- Lincoln NB, Francis VM, Lilley SA, Sharma JC, Summerfield M. Evaluation of a stroke family support organiser: a randomized controlled trial. Stroke 2003;34:116-21. http://dx.doi.org/10.1161/01.STR.0000047850.33686.32.
- Young JB. The primary care stroke gap. Br J Gen Pract 2001;51:787-8.
- Murray J, Young J, Forster A, Herbert A, Ashworth R. Feasibility study of a primary care-based model for stroke aftercare. Br J Gen Pract 2006;56:775-80.
- Murray J, Young J, Forster A, Ashworth R. Developing a primary care-based stroke model: the prevalence of longer-term problems experienced by patients and carers. Br J Gen Pract 2003;53:803-7.
- Murray J, Ashworth R, Forster A, Young J. Developing a primary care-based stroke service: a review of the qualitative literature. Br J Gen Pract 2003;53:137-42.
- Murray J. The Development of a Primary Care-Based Model for After Stroke Care 2007.
- Orrell M, Hancock G. The Camberwell Assessment of Need for the Elderly. London: Gaskell; 2004.
- Philp I. EASY-Care: a systematic approach to the assessment of older people. Geriatr Med 2000;30:15-9.
- National Institute for Health and Care Excellence . The Guidelines Manual, April 2007 n.d. www.nice.org.uk/niceMedia/pdf/GuidelinesManualAllChapters.pdf (accessed June 2014).
- Kroenke K, Spitzer RL. The PHQ-9: a new depression and diagnostic severity measure. Psychiatr Ann 2002;32:509-21. http://dx.doi.org/10.3928/0048-5713-20020901-06.
- Hodkinson HM. Evaluation of a mental test score for assessment of mental impairment in the elderly. Age Ageing 1972;1:233-8. http://dx.doi.org/10.1093/ageing/1.4.233.
- Allen KR, Hazelett S, Jarjoura D, Wickstrom GC, Hua K, Weinhardt J, et al. Effectiveness of a postdischarge care management model for stroke and transient ischemic attack: a randomized trial. J Stroke Cerebrovasc Dis 2002;11:88-9. http://dx.doi.org/10.1053/jscd.2002.127106.
- Murray J, Young J, Forster A, Ashworth R. A survey to investigate the role of the district nurse in stroke care. Br J Community Nurs 2004;9:318-24. http://dx.doi.org/10.12968/bjcn.2004.9.8.15352.
- Rothwell K, Boaden R, Bamford D, Tyrrell PJ. Feasibility of assessing the needs of stroke patients after six months using the GM-SAT. Clin Rehabil 2013;27:264-71. http://dx.doi.org/10.1177/0269215512457403.
- Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The stroke impact scale version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke 1999;30:2131-40. http://dx.doi.org/10.1161/01.STR.30.10.2131.
- Forster A, Young J, Green J, Patterson C, Wanklyn P, Smith J, et al. Structured re-assessment system at 6 months after a disabling stroke: a randomised controlled trial with resource use and cost study. Age Ageing 2009;38:576-83. http://dx.doi.org/10.1093/ageing/afp095.
- Green J, Forster A, Bogle S, Young J. Physiotherapy for patients with mobility problems more than 1 year after stroke: a randomised controlled trial. Lancet 2002;359:199-203. http://dx.doi.org/10.1016/S0140-6736(02)07443-3.
- Collin C, Wade DT, Davies S, Home V. The Barthel ADL Index: a reliability study. Int Disability Studies 1988;10:61-3.
- Wade DT, Legh-Smith J, Langton Hewer R. Social activities after stroke: measurement and natural history using the Frenchay Activities Index. Int Rehabil Med 1985;7:176-81.
- Wade DT. Goal setting in rehabilitation: an overview of what, why and how. Clin Rehabil 2009;23:291-5. http://dx.doi.org/10.1177/0269215509103551.
- National Clinical Guidelines for Stroke. London: Royal College of Physicians; 2004.
- Thomas LH, Barrett J, Cross S, French B, Leathley M, Sutton C, et al. Prevention and treatment of urinary incontinence after stroke in adults. Cochrane Database Syst Rev 2005;3.
- Forster A, Smith J, Young J, Knapp P, House A, Wright J. Information provision for stroke patients and their caregivers. Cochrane Database Syst Rev 2001;3.
- Smith J, Forster A, Young J. Cochrane Group for information provision after stroke . Cochrane review: information provision for stroke patients and their caregivers. Clin Rehabil 2009;23:195-206. http://dx.doi.org/10.1177/0269215508092820.
- Forster A, Brown L, Smith J, House A, Knapp P, Wright JJ, et al. Information provision for stroke patients and their caregivers. Cochrane Database Syst Rev 2012;11. http://dx.doi.org/10.1002/14651858.CD001919.pub3.
- Ellis G, Mant J, Langhorne P, Dennis M, Winner S. Stroke liaison workers for stroke patients and carers: an individual patient data meta-analysis. Cochrane Database Syst Rev 2010;5. http://dx.doi.org/10.1002/14651858.CD005066.pub2.
- Gillespie LD, Robertson MC, Gillespie WJ, Sherrington C, Gates S, Clemson LM, et al. Intervention for preventing falls in older people living in the community. Cochrane Database Syst Rev 2012;9.
- Nair RD, Lincoln NB. Cognitive rehabilitation for memory deficits following stroke. Cochrane Database Syst Rev 2007;3.
- Bowen A, Lincoln NB. Cognitive rehabilitation for spatial neglect following stroke. Cochrane Database Syst Rev 2007;2.
- Lincoln NB, Majid MJ, Weyman N. Cognitive rehabilitation for attention deficits following stroke. Cochrane Database Syst Rev 2000;4.
- Hackett ML, Anderson CS, House A, Haltech C. Interventions for preventing depression after stroke. Cochrane Database Syst Rev 2008;3.
- Clinical Guidelines for Stroke Management. Melbourne, VIC: National Stroke Foundation; 2010.
- Lindsay P, Bayley M, McDonald A, Graham ID, Warner G, Phillips S. Toward a more effective approach to stroke: Canadian Best Practice Recommendations for Stroke Care. CMAJ 2008;178:1418-25. http://dx.doi.org/10.1503/cmaj.071253.
- National Institute for Health and Care Excellence . Stroke Rehabilitation. Long-Term Rehabilitation After Stroke 2013. www.nice.org.uk/nicemedia/live/14182/64098/64098.pdf (accessed June 2014).
- National Clinical Guidelines for Stroke. London: Royal College of Physicians; 2012.
- Forster A, Young J. Stroke rehabilitation: can we do better?. BMJ 1992;305:1446-7. http://dx.doi.org/10.1136/bmj.305.6867.1446.
- Young JM, Murray J, Forster A. Review of longer-term problems after disabling stroke. Rev Clin Gerontol 2003;13:55-6. http://dx.doi.org/10.1017/S0959259803013157.
- Young J. Is stroke better managed in the community? Community care allows patients to reach their full potential. BMJ 1994;309:1356-7. http://dx.doi.org/10.1136/bmj.309.6965.1356.
- Murray J, Forster A, Young J. Survey to investigate the role of the community stroke care coordinator. Br J Community Nurs 2008;13:31-6. http://dx.doi.org/10.12968/bjcn.2008.13.1.27981.
- Forster A, Young J, Nixon J, Chapman K, Murray J, Patel A, et al. Protocol of a cluster randomized trial evaluation of a patient and carer-centred system of longer-term stroke care (LoTS care) [published online ahead of print 19 February 2013]. Int J Stroke 2014. http://dx.doi.org/10.1111/ijs.12038.
- Wade DT. Research into the black box of rehabilitation: the risks of a type III error. Clin Rehabil 2001;15:1-4. http://dx.doi.org/10.1191/026921501675961253.
- Goldberg D, Williams P. A User’s Guide to the General Health Questionnaire. Windsor: NFER-Nelson; 1992.
- Stroke Unit Trialists’ Collaboration . Collaborative systematic review of the randomised trials of organised inpatient (stroke unit) care after stroke. BMJ 1997;314:1151-9. http://dx.doi.org/10.1136/bmj.314.7088.1151.
- National Sentinel Audit for Stroke. London: Royal College of Physicians; 2006.
- Carter BR, Hood K. Balance algorithm for cluster randomized trials. BMC Med Res Methodol 2008;8. http://dx.doi.org/10.1186/1471-2288-8-65.
- van Swieten J, Koudstaal P, Visser M, Schouten H, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604-7. http://dx.doi.org/10.1161/01.STR.19.5.604.
- Brooke P, Bullock R. Validation of a 6 item cognitive impairment test with a view to primary care usage. Int J Geriatr Psychiatry 1999;14:936-40. http://dx.doi.org/10.1002/(SICI)1099-1166(199911)14:11<936::AID-GPS39>3.0.CO;2-1.
- Gompertz P, Pound P, Ebrahim S. A postal version of the Barthel Index. Clin Rehabil 1994;8:233-9. http://dx.doi.org/10.1177/026921559400800308.
- Dennis M. Performance of a statistical model to predict stroke outcome in the context of a large, simple, randomized, controlled trial of feeding. Stroke 2003;34:127-33.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. http://dx.doi.org/10.1016/0168-8510(96)00822-6.
- The EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. http://dx.doi.org/10.1016/0168-8510(90)90421-9.
- Krabbe P, Weijnen T, Brooks R, Rabin R, De Cahrro F. The Measurement and Valuation for Health Status using EQ-5D: a European Perspective (Evdience from the EuroQol BIOMED Research Programme). Dordrecht: Kluwer Academic Publishers; 2003.
- Patel A, Knapp M, Evans A, Perez I, Kalra L. Training care givers of stroke patients: economic evaluation. BMJ 2004;328:1102-4A. http://dx.doi.org/10.1136/bmj.328.7448.1102.
- Patel A, Knapp M, Perez I, Evans A, Kalra L. Alternative strategies for stroke care – cost-effectiveness and cost–utility analyses from a prospective randomized controlled trial. Stroke 2004;35:196-203. http://dx.doi.org/10.1161/01.STR.0000105390.20430.9F.
- Forster A, Young J, Nixon J, Kalra L, Smithard D, Patel A, et al. A cluster randomized controlled trial of a structured training programme for caregivers of inpatients after stroke (TRACS). Int J Stroke 2012;7:94-9. http://dx.doi.org/10.1111/j.1747-4949.2011.00722.x.
- Elmstahl S, Malmberg B, Annerstedt L. Caregiver’s burden of patients 3 years after stroke assessed by a novel caregiver burden scale. Arch Phys Med Rehabil 1996;77:177-82. http://dx.doi.org/10.1016/S0003-9993(96)90164-1.
- Wade DT, Hewer RL. Functional abilities after stroke: measurement, natural history and prognosis. J Neurol Neurosurg Psychiatry 1987;50:177-82. http://dx.doi.org/10.1136/jnnp.50.2.177.
- Parker CJ, Gladman JRF, Drummond AER, Dewey ME, Lincoln NB, Barer D, et al. A multicentre randomized controlled trial of leisure therapy and conventional occupational therapy after stroke. Clin Rehabil 2001;15:42-5. http://dx.doi.org/10.1191/026921501666968247.
- Walker MF, Gladman JRF, Lincoln NB, Siemonsma P, Whiteley T. Occupational therapy for stroke patients not admitted to hospital: a randomised controlled trial. Lancet 1999;354:278-80. http://dx.doi.org/10.1016/S0140-6736(98)11128-5.
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JA, Burney PG. Methods for evaluating area-wide and organisation-based interventions in health and health care: a systematic review. Health Technol Assess 1999;3.
- Campbell MK, Mollison J, Grimshaw JM. Cluster trials in implementation research: estimation of intracluster correlation coefficients and sample size. Stat Med 2001;20:391-9. http://dx.doi.org/10.1002/1097-0258(20010215)20:3<391::AID-SIM800>3.0.CO;2-Z.
- Dolan P, Gudex C, Kind P, Williams A. A Social Tariff for EuroQol: Results from a UK Population Survey. York: University of York; 1995.
- van Hout BA, Al MJ, Gordon GS, Rutten FF. Costs, effects and C/E-ratios alongside a clinical trial. Health Econ 1994;3:309-19. http://dx.doi.org/10.1002/hec.4730030505.
- Department of Health . NHS Trust Reference Cost Schedules, 2010–2011 n.d. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_131140 (accessed 28 August 2012).
- Katbamna S, Matthews R. Ageing & Ethnicity in England. A Demographic Profile of BME Older People in England. London: Age Concern; 2007.
- Medical Research Council n.d. www.mrc.ac.uk/complexinterventionsguidance (accessed 26 August 2014).
- Dowswell G, Lawler J, Dowswell T, Young J, Forster A, Hearn J. Investigating recovery from stroke: a qualitative study. J Clin Nurs 2000;9:507-15. http://dx.doi.org/10.1046/j.1365-2702.2000.00411.x.
- Alvarado N. The LoTS (Longer-Term Stroke) System of Care: An Evaluation of Its Local Implementation at Two Community Stroke Services 2013.
- Adamson J, Beswick A, Ebrahim S. Is stroke the most common cause of disability?. J Stroke Cerebrovasc Dis 2004;13:171-7. http://dx.doi.org/10.1016/j.jstrokecerebrovasdis.2004.06.003.
- Jorgensen L, Engstad T, Jacobsen BK. Self-reported urinary incontinence in noninstitutionalized long-term stroke survivors: a population-based study. Arch Phys Med Rehabil 2005;86:416-20. http://dx.doi.org/10.1016/j.apmr.2004.05.011.
- Caring for People after They Have Had a Stroke: a Follow up Survey of Patients. London: Healthcare Commission; 2006.
- Jonsson AC, Lindgren I, Hallstrom B, Norrving B, Lindgren A. Prevalence and intensity of pain after stroke: a population based study focusing on patients’ perspectives. J Neurol Neurosurg Psychiatry 2006;77:590-5. http://dx.doi.org/10.1136/jnnp.2005.079145.
- Hartman-Maeir A, Soroker N, Ring H, Avni N, Katz N. Activities, participation and satisfaction one-year post stroke. Disabil Rehabil 2007;29:559-66. http://dx.doi.org/10.1080/09638280600924996.
- Wolfe CD, Crichton SL, Heuschmann PU, McKevitt CJ, Toschke AM, Grieve AP, et al. Estimates of outcomes up to ten years after stroke: analysis from the prospective South London Stroke Register. PLOS Med 2011;8. http://dx.doi.org/10.1371/journal.pmed.1001033.
- Fair Care for Older People: Care Development Group Report. Edinburgh: Scottish Executive; 2001.
- Wright J, Williams R, Wilkinson JR. Development and importance of health needs assessment. BMJ 1998;316. http://dx.doi.org/10.1136/bmj.316.7140.1310.
- Heinemann AW, Sokol K, Garvin L, Bode RK. Measuring unmet needs and services among persons with traumatic brain injury. Arch Phys Med Rehabil 2002;83:1052-9. http://dx.doi.org/10.1053/apmr.2002.34283.
- Carter J, Mant F, Mant J, Wade D, Winner S. Comparison of postal version of the Frenchay Activities Index with interviewer-administered version for use in people with stroke. Clin Rehabil 1997;11:131-8. http://dx.doi.org/10.1177/026921559701100206.
- Kersten P, McLellan L, George S, Smith JA. The Southampton Needs Assessment Questionnaire (SNAQ): a valid tool for assessing the rehabilitation needs of disabled people. Clin Rehabil 2000;14:641-50. http://dx.doi.org/10.1191/0269215500cr373oa.
- Kersten P, Low JT, Ashburn A, George SL, McLellan DL. The unmet needs of young people who have had a stroke: results of a national UK survey. Disabil Rehabil 2002;24:860-6. http://dx.doi.org/10.1080/09638280210142167.
- Duncan PW, Reker DM, Horner RD, Samsa GP, Hoenig H, LaClair BJ, et al. Performance of a mail-administered version of a stroke-specific outcome measure, the Stroke Impact Scale. Clin Rehabil 2002;16:493-505. http://dx.doi.org/10.1191/0269215502cr510oa.
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983;67:361-70. http://dx.doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Nouri F, Lincoln N. An extended activities of daily living scale for stroke patients. Clin Rehabil 1987;1:301-5. http://dx.doi.org/10.1177/026921558700100409.
- Murray J, Young J, Forster A. Response and completion rates for postal outcomes booklets for use in stroke rehabilitation. Int J Ther Rehabil 2007;14:440-5. http://dx.doi.org/10.12968/ijtr.2007.14.10.27395.
- Enderby PM, Wood VA, Wade DT. Frenchay Aphasia Screening Test. Chichester: John Riley; 2006.
- Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum 2003;49:156-63. http://dx.doi.org/10.1002/art.10993.
- Geddes JM, Fear J, Tennant A, Pickering A, Hillman M, Chamberlain MA. Prevalence of self reported stroke in a population in northern England. J Epidemiol Community Health 1996;50:140-3. http://dx.doi.org/10.1136/jech.50.2.140.
- Ware J, Kosinski M, Keller SD. A 12-item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220-33. http://dx.doi.org/10.1097/00005650-199603000-00003.
- Rasch G. Probabilistic Models for Some Intelligence and Attainment Tests. Copenhagen: Danish Institute for Educational Research; 1960.
- Linacre JM. Sample size and item calibration stability. Rasch Meas Trans 1994;7.
- Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas 1960;20:37-46. http://dx.doi.org/10.1177/001316446002000104.
- Altman DG. Practical Statistics for Medical Research. London: Chapman and Hall; 1991.
- Fitzpatrick R, Davey C, Buxton MJ, Jones DR. Evaluating patient-based outcome measures for use in clinical trials. Health Technol Assess 1998;2.
- Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika 1951;16:297-334. http://dx.doi.org/10.1007/BF02310555.
- DeVelliss RF. Classical test theory. Med Care 2006;44:S50-9. http://dx.doi.org/10.1097/01.mlr.0000245426.10853.30.
- Sijtsma K. On the use, the misuse, and the very limited usefulness of Cronbach’s alpha. Psychometrika 2009;74:107-20. http://dx.doi.org/10.1007/s11336-008-9101-0.
- Tennant A, Conaghan P. The Rasch measurement model in rheumatology: what is it and why use it? When should it be applied, and what should one look for in a Rasch paper?. Arthritis Rheum 2007;57:1358-62. http://dx.doi.org/10.1002/art.23108.
- Pallant JF, Tennant A. An introduction to the Rasch measurement model: an example of using the Hospital Anxiety and Depression Scale (HADS). Br J Clin Psychol 2007;46:1-18. http://dx.doi.org/10.1348/014466506X96931.
- LoTS care LUNS study team . Validation of the longer-term unmet needs after stroke (LUNS) monitoring tool: a multicentre study. Clin Rehabil 2013;27:1020-8. http://dx.doi.org/10.1177/0269215513487082.
- Stroke Improvement National Audit Programme (SINAP). London: Royal College of Physicians; 2012.
- Kline P. A Psychometrics Primer. London: Free Association Books; 2000.
- Robison J, Wiles R, Ellis-Hill C, McPherson K, Hyndman D, Ashburn A. Resuming previously valued activities post-stroke: who or what helps?. Disabil Rehabil 2009;31:1555-66. http://dx.doi.org/10.1080/09638280802639327.
- Ellis-Hill C, Payne S, Ward C. Self-body split: issues of identity in physical recovery following stroke. Disabil Rehabil 2000;22:725-33. http://dx.doi.org/10.1080/09638280050191990.
- Burvill PW, Johnson GA, Jamrozki KD, Anderson CS, Stewart-Wynne, Chakera TMH. Prevalence of depression after stroke: the Perth Community Stroke Study. Br J Psychiatry 1995;166:320-7. http://dx.doi.org/10.1192/bjp.166.3.320.
- Burvill PW, Johnson GA, Jamrozki KD, Anderson CS, Stewart-Wynne, Chakera TMH. Anxiety disorders after stroke: results from the Perth Community Stroke Study. Br J Psychiatry 1995;166:328-32. http://dx.doi.org/10.1192/bjp.166.3.328.
- Bury M. Chronic illness as biographical disruption. Sociol Health Illn 1982;4:167-82. http://dx.doi.org/10.1111/1467-9566.ep11339939.
- Sanders C, Donovan J, Dieppe P. The significance and consequences of having painful and disabled joints in older age: co-existing accounts of normal and disrupted biographies. Sociol Health Illn 2002;24:227-53. http://dx.doi.org/10.1111/1467-9566.00292.
- Pound P, Gompertz P, Ebrahim S. Social and practical strategies described by people living at home with stroke. Health Soc Care Community 1999;7:120-8. http://dx.doi.org/10.1046/j.1365-2524.1999.00168.x.
- Lackner S, Goldenberg S, Arrizza G, Tjosvold I. The contingency of social support. Qual Health Res 1994;4:224-43. http://dx.doi.org/10.1177/104973239400400206.
- Morgan M, Patrick DL, Charlton JR. Social networks and psychosocial support among disabled people. Soc Sci Med 1984;19:489-97. http://dx.doi.org/10.1016/0277-9536(84)90044-3.
- Glass TA, Maddox GL. The quality and quantity of social support: stroke recovery as psycho-social transition. Soc Sci Med 1992;34:1249-61. http://dx.doi.org/10.1016/0277-9536(92)90317-J.
- Sabari JS, Meisler J, Silver E. Reflections upon rehabilitation by members of a community based stroke club. Rehabil Pract 2000;22:330-6.
- Mold F, McKevitt C, Wolfe C. A review and commentary of the social factors which influence stroke care: issues of inequality in qualitative literature. Health Soc Care Community 2003;11:405-14. http://dx.doi.org/10.1046/j.1365-2524.2003.00443.x.
- Wenger GC, Davies R, Shahtahmasebi S, Scott A. Social isolation and loneliness in old age: review and model refinement. Ageing Soc 1996;16:333-58. http://dx.doi.org/10.1017/S0144686X00003457.
- House JS. Social isolation kills, but how and why?. Psychosom Med 2001;63:273-4. http://dx.doi.org/10.1097/00006842-200103000-00011.
- Cacioppo JT, Hawkley LC. Social isolation and health, with an emphasis on underlying mechanisms. Perspect Biol Med 2003;46:S39-52. http://dx.doi.org/10.1353/pbm.2003.0049.
- Lund R, Nilsson CJ, Avlund K. Can the higher risk of disability onset among older people who live alone be alleviated by strong social relations? A longitudinal study of non-disabled men and women. Age Ageing 2010;39:319-26. http://dx.doi.org/10.1093/ageing/afq020.
- Charmaz K. Constructing Grounded Theory: A Practical Guide through Qualitative Analysis. London: Sage; 2006.
- Glaser BG, Strauss AL. The Discovery of Grounded Theory: Strategies for Qualitative Research. Chicago, IL: Aldine Publishing; 1967.
- Charmaz K, Denzin NK, Lincoln YS. Handbook of Qualitative Research. London: Sage; 2000.
- Furness PJ, Garrud P. Adaptation after facial surgery: using the diary as a research tool. Qual Health Res 2010;20:262-72. http://dx.doi.org/10.1177/1049732309357571.
- Plummer K. Documents of Life 2: An Invitation to Critical Humanism. London: Sage; 2001.
- Corti L, Surrey UO. Social Research Update. Guildford: University of Surrey; 1993.
- Elliott H. The use of diaries in sociological research on health experience. Sociol Res Online 1997;2. http://dx.doi.org/10.5153/sro.38 (accessed March 2010).
- Jones RK. The unsolicited diary as a qualitative research tool for advanced research capacity in the field of health and illness. Qual Health Res 2000;10:555-67. http://dx.doi.org/10.1177/104973200129118543.
- Jacelon CS, Imperio K. Participant diaries as a source of data in research with older adults. Qual Health Res 2005;15:991-7. http://dx.doi.org/10.1177/1049732305278603.
- Plummer K. Documents of Life. London: George Allen and Unwin; 1983.
- Antonucci TC. Hierarchical mapping technique. Generations 1986;10:10-2.
- Charmaz K, Mitchell RG, Atkinson P, Coffey A, Delamont S, Lofland J, et al. Handbook of Ethnography. London: Sage; 2001.
- Godfrey M, Townsend J. Older people in transition from illness to health: trajectories of recovery. Qual Health Res 2008;18:939-51. http://dx.doi.org/10.1177/1049732308318038.
- Glaser BG, Strauss AL. Time for Dying. New Brunswick, NJ: Aldine De Gruyter; 2007.
- Strauss AL, Corbin J, Fagerhaugh S, Glaser BG, Maines D, Suczek B, et al. Chronic Illness and the Quality of Life. St Louis, MO: Mosby; 1984.
- Corbin J, Strauss A. Managing chronic illness at home: three lines of work. Qual Sociol 1985;8:224-47. http://dx.doi.org/10.1007/BF00989485.
- Seale C, Seale C, Gobo G, Gubrium JF, Silverman D. Qualitative Research Practice. London: Sage; 2004.
- Silverman D, Miller G, Dingwall R. Context and Method in Qualitative Research. London: Sage; 1997.
- Silverman D. Interpreting Qualitative Data. London: Sage; 2006.
- Mays N, Pope C. Qualitative research in health care: assessing quality in qualitative research. BMJ 2000;320. http://dx.doi.org/10.1136/bmj.320.7226.50.
- Pound P, Gompertz P, Ebrahim S. Illness in the context of older age: the case of stroke. Sociol Health Illn 1998;20:489-506. http://dx.doi.org/10.1111/1467-9566.00112.
- Faircloth CA, Boylstein C, Rittman M, Young ME, Gubrium J. Sudden illiness and biographical flow in narratives of stroke recovery. Sociol Health Illn 2004;26:242-61. http://dx.doi.org/10.1111/j.1467-9566.2004.00388.x.
- Becker G, Kaufman SR. Managing an uncertain illness trajectory in old age: patients’ and physicians’ views of stroke. Med Anthropol Q 1995;9:165-87. http://dx.doi.org/10.1525/maq.1995.9.2.02a00040.
- Dworzynski K, Ritchie G, Fenu E, Macdermott K, Playford ED. Rehabilitation after stroke: summary of NICE guidance. BMJ 2013;346. http://dx.doi.org/10.1136/bmj.f3615.
- Academy of Medical Science . A New Pathway for the Regulation and Governance of Health Research 2011. www.acmedsci.ac.uk/download.php?file=/images/project/130734957423.pdf (accessed September 2014).
- Johnson J, Pearson V. The effects of a structured education course on stroke survivors living in the community. Rehabil Nurs 2000;25:59-65. http://dx.doi.org/10.1002/j.2048-7940.2000.tb01864.x.
- Cameron JI, Gignac MAM. ‘Timing it right’: a conceptual framework for addressing the support needs of family caregivers to stroke survivors from the hospital to the home. Patient Educ Couns 2008;70:305-14. http://dx.doi.org/10.1016/j.pec.2007.10.020.
- Eames S, Hoffmann T, Worrall L, Read S, Wong A. Randomised controlled trial of an education and support package for stroke patients and their carers [published online ahead of print 8 May 2013]. BMJ Open 2013;3. http://bmjopen.bmj.com/content/3/5/e002538.full (accessed October 2013).
- Corben S, Rosen R. Self-Management for Long-Term Conditions. London: The King’s Fund; 2005.
- Anonymous . Psychological Care After Stroke. The Stepped Approach n.d. www.improvement.nhs.uk/stroke/Psychologicalcareafterstroke/Stepped.aspx (accessed October 2013).
- de Silva D. Evidence: Helping People to Help Themselves: a Review of the Evidence of Considering Whether it is Worthwhile to Support Self-Management. London: Health Foundation; 2011.
- Cadilhac DA, Hoffmann S, Kilkenny M, Lindley R, Lalor E, Osborne RH, et al. A phase II multicentered, single-blind, randomized, controlled trial of the stroke self-management program. Stroke 2011;42:1673-9. http://dx.doi.org/10.1161/STROKEAHA.110.601997.
- Lennon S, McKenna S, Jones F. Self-management programmes for people post stroke: a systematic review. Clin Rehabil 2013;27:867-78. http://dx.doi.org/10.1177/0269215513481045.
- Forster A, Dickerson J, Young J, Patel A, Kalra L, Nixon J, et al. A cluster randomised controlled trial and economic evaluation of a structured training programme for caregivers of inpatients after stroke: the TRACS trial. Health Technol Assess 2013;17. http://dx.doi.org/10.3310/hta17460.
- Forster A, Young J. Research networks for stroke rehabilitation: opportunities and barriers. Clin Med 2005;5:42-6. http://dx.doi.org/10.7861/clinmedicine.5-1-42.
- Thorpe KE, Zwarenstein M, Oxman AD, Treweek S, Furberg CD, Altman DG, et al. A pragmatic explanatory continuum indicator summary (PRECIS): a tool to help trial designers. CMAJ 2009;180:E47-57. http://dx.doi.org/10.1503/cmaj.090523.
Appendix 1 Development of the system of care
Development of a primary care-based service for stroke aftercare
Submitted November 1999; funded by the Stroke Association.
Purpose
Recent research has clarified aspects of acute hospital care for stroke patients but important longer-term issues remain and can be addressed adequately only in the primary care setting. 1,2 These include continuing rehabilitation to minimise handicap; provision of home adaptations, equipment and access to services; psychological support; and responding to intercurrent events. At present, involvement of the primary care health team in stroke is inconsistent. No systematic approach has been developed for routine monitoring, problem identification and co-ordination of services to assist stroke patients and their families as they continue to recover from their stroke and make life adjustments to its consequences. The lack of engagement of primary care in stroke is in particular contrast to the situation with other chronic diseases such as asthma and diabetes, for which a strategic shift from secondary to primary care has occurred. In this initial developmental project the elements of a primary care-based service for stroke will be systematically defined and tested by pilot implementation with process change measurement.
Background
Stroke as a community care issue
Community-based observational studies over three decades have left little doubt about the daily life struggle for victims and their families as they grapple with the longer-term consequences of their stroke illness. 3–7 The practical difficulties include lack of information about their condition,8 poor knowledge of the services and benefits available4,9 and fragmented community services10 that are poorly focused. 11 Social activities may be restricted despite apparently good physical recovery. 5,12 Falls and consequent morbidity are also common. 13 Three inter-related themes can be proposed as important stroke challenges: longer-term perspective; greater emphasis on handicap rather than disability; and addressing psychosocial and adjustment needs. 1 Ideally, all require a primary care perspective.
Primary care approach to longer-term stroke management
The role of the primary care health team in the longer-term support of stroke patients and their families has received scant attention amongst the vastness of contemporary stroke literature. Currently, primary care health teams behave reactively, responding to ‘crises’ rather than putting into place a proactive preventative strategy. The challenge is to engage the primary care health team in post-stroke care to assess, support and co-ordinate relevant services to minimise longer-term stroke morbidity. This is a difficult task as primary care is hard pressed. Yet opportunities exist, particularly in respect of practice nurses, nurse practitioners and the extended role of community nurses, within the new structure of the primary care group.
Questions to be answered
-
To define the types of longer-term problems most frequently encountered, methods for systematic assessment, and optimal management based on best available evidence.
-
To develop a generalisable primary care-based structure capable of supporting a process of routine and systematic management for post-acute stroke care for both patients and their carers.
-
To develop a post-acute assessment system with supporting training for use by primary health-care teams to promote continuing rehabilitation, access and co-ordination of supporting services, and psychosocial support.
Plan of investigation
Simple and single interventions are rarely effective in rehabilitation. 14 We are therefore seeking to develop a complex intervention embedded in a clearly described organisational structure for delivery. The work has been broken down into stages progressed and co-ordinated by a project group comprising the grant applicants, a patient representative from a local stroke club, Dr Robert Ashworth (GP with academic links), and the project research assistant.
Stage 1: preparatory work (9 months)
This will entail producing a report identifying the most frequently encountered post-acute stroke problems and their optimal management based on best available evidence. This will be achieved by (a) literature searching; (b) existing collaboration with the Glasgow-based systematic review of community rehabilitation stroke studies; (c) current work reviewing models of community care (Nuffield Institute for Health); (d) examples of good practice: the Stroke Association has identified six community care stroke projects that demonstrate successful innovative practice and these are under descriptive evaluation by Professor Chamberlain, who has kindly made the interim reports available to us; and (e) interviews with regional Stroke Association staff who have daily contact with stroke patients.
Stage 2: user involvement (4 months)
We will form focus groups of 10–12 patients and carers in at least three localities: Bradford, Sheffield and Leeds. The groups will receive a presentation of the stage 1 findings and suggestions from the project group to date and be invited to make a critical appraisal of these ideas. We wish to examine for areas of agreement and disagreement: to determine the extent to which the conclusions from stage 1 ‘fit’ with their experiences. Facilitation of the groups will be by Nuffield Institute for Health staff, who have considerable experience with this type of sensitive exploratory research. The discussions and dynamics of the focus groups will be recorded by contemporaneous note taking by a research assistant. Some individual interviews with assistance from carers will also be necessary to ensure that people with dysphasia are included. Other individual interviews will be arranged if particular areas of ambiguity emerge that require greater clarification.
Stage 3: consolidation (3 months)
The distillations from the focus groups will be synthesised around three key areas:
-
the main patient and carer stroke problems to be encompassed
-
guidance on the optimum responses to those problems
-
an outline format and content for the proces of co-ordinating the inputs from the primary care team.
Stage 4: involvement of the primary care team (4 months)
This would begin with a series of half-day workshops in four areas [Leeds, Bradford, Sheffield and Skipton, North Yorkshire (rural)] for invited primary care professionals – mixed groups of GPs, nurses, therapists and social service staff. Within each workshop there would be a facilitated debate about what primary care teams currently do for stroke patients and what they would like to do in the light of the ‘good practice’ literature formulated in stage 3. More specifically, we will address two key questions:
-
Who can take lead responsibility? (e.g., GP, practice nurse, nurse practitioner, community nurse, therapist)
-
How will this be organised?
-
Patient perspective: visiting frequency, telephone checks
-
Primary care perspective: co-ordination of community agencies, a reliable system for case finding, assessing and reviewing needs, nature and range of inputs required.
-
The workshop conclusions will be summarised and returned to the participants for consultation and refinements.
Stage 5: development of materials and training (4 months)
Implementation of a primary care-based stroke service requires well-presented documentation describing content, process and organisation, and supporting knowledge and skills training. At present, we anticipate a 2-day residential course: 1 day to discuss the nature and consequences of stroke and 1 day to work through the new primary care-based stroke service. Follow-up work will include the participating primary care teams collating lists of local agencies and disability services and developing an initial case load of (say) four patients. These practical experiences will be used for discussion at a second training school session 6 weeks after the first. The training will be organised at the Nuffield Institute for Health, which is well equipped for residential courses and near to the proposed pilot site.
Stage 6: pilot implementation (12 months)
The primary care-based stroke service will be established as a pilot project in north Bradford where provisional access has been obtained. Ethics committee approval for this phase of the study will be requested. The purpose of the pilot is:
-
to test the feasibility of the primary care stroke service
-
to determine if the new intervention influences the process of care (not clinical outcomes at this stage)
-
to prepare for a future evaluation of the primary care-based stroke service by a multisite RCT.
Feasibility study
This will begin with the training of the participating primary health-care teams using the system designed in stage 5. Over 4 months 50 patients will be recruited at various stages of the ‘stroke career’: post discharge to 1-year post stroke onset. Acceptability of the assessment process for patients and families will be determined by regular discussion between research and primary care staff, by non-responses to assessment questions and by interviews with patients. At the end of the feasibility study a workshop will be held for the participating staff to discuss their experiences, identify key issues and problems and suggest refinements. Similarly, we will organise a focus group with participating patients selected on the basis of positive and negative experiences.
References
- Young JB, Gladman J. Future directions in stroke rehabilitation. Rev Clin Gerontol 1995;5:46-54.
- Young JB. Is stroke better managed in the community?. BMJ 1994;309:1356-8.
- Forster A, Young JB. Stroke rehabilitation: can we do better?. BMJ 1992;305:1446-7.
- Anderson R. The Aftermath of Stroke. Cambridge University Press; 1992.
- Kettle M, Chamberlain MA. The stroke patient in an urban environment. Clin Rehab 1989;3:131-8.
- Wade D, Langton-Hewer R. Effects of living with and looking after survivors of stroke. Br Med J (Clin Res Ed) 1986;293:418-20.
- Pound P, Gompertz P, Ebrahim S. A patient centred study of stroke and its consequences. Clin Rehab 1998;12:338-47.
- Hanger HC, Mulley GP. Questions people ask about stroke. Stroke 1993;24:536-8.
- Greveson G, James O. Improving long-term outcome after stroke – the views of patients and carers. Health Trends 1991;23:161-2.
- Garraway WM, Akhtar AJ, Hockey L, Prescott RJ. Management of acute stroke in the elderly: follow up of a controlled trial. Br Med J 1980;281:827-9.
- Ebrahim S, Barer D, Nouri F. An audit of follow-up services for stroke patients after discharge from hospital. Int Disabil Stud 1987;9:103-5.
- Thorngren M, Westling B, Norrving B. Outcome after stroke inpatients discharged to independent living. Stroke 1990;21:236-40.
- Young JB, Forster A. Incidence and consequences of falls due to stroke. BMJ 1995;311:83-6.
- Sinclair A, Dickinson E. Effective Practice in Rehabilitation: the Evidence of Systematic Review. London: The King’s Fund; 1998.
Appendix 2 Programme management structure
Appendix 3 Project 1: sample (contact 1) of the care plan
Appendix 4 Project 1: example search strategies (pain)
Database(s): Ovid MEDLINE(R)
Date range searched: 1995 to February week 3 2008.
Date of search: 4 March 2008.
Search strategy
| # | Searches |
|---|---|
| 1 | cerebrovascular disorders/ |
| 2 | exp basal ganglia cerebrovascular disease/ |
| 3 | exp brain ischemia/ |
| 4 | exp carotid artery diseases/ |
| 5 | cerebrovascular accident/ |
| 6 | exp brain infarction/ |
| 7 | exp cerebrovascular trauma/ |
| 8 | exp hypoxia-ischemia, brain/ |
| 9 | exp intracranial arterial diseases/ |
| 10 | intracranial arteriovenous malformations/ |
| 11 | exp “intracranial embolism and thrombosis”/ |
| 12 | exp intracranial hemorrhages/ |
| 13 | vasospasm, intracranial/ |
| 14 | vertebral artery dissection/ |
| 15 | aneurysm, ruptured/ |
| 16 | brain injuries/ |
| 17 | brain injury, chronic/ |
| 18 | exp carotid arteries/ |
| 19 | endarterectomy, carotid/ |
| 20 | endarterectomy/ |
| 21 | *heart septal defects, atrial/ |
| 22 | *atrial fibrillation/ |
| 23 | (stroke or poststroke or post-stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva or apoplex$ or isch?emi$ attack$ or tia or tias or neurologic$ deficit or SAH or AVM).tw. |
| 24 | ((brain$ or cerebr$ or cerebell$ or cortical or vertebrobasilar or hemispher$ or intracran$ or intracerebral$ or infratentorial or supratentorial or MCA or anterior circulation or posterior circulation or basal ganglia) adj10 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypox$ or vasospasm or obstruction or vasculopathy)).tw. |
| 25 | ((lacunal or cortical) adj5 infarct$).tw. |
| 26 | ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or subarachnoid or putaminal or putamen or posterior fossa) adj10 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. |
| 27 | ((brain or cerebral or intracranial or communicating or giant or basilar or vertebral artery or berry or saccular or ruptured) adj10 aneurysm$).tw. |
| 28 | (vertebral artery dissection or cerebral art$ disease$).tw. |
| 29 | ((brain or intracranial or basal ganglia or lenticulostriate) adj10 (vascular adj5 (disease$ or disorder or accident or injur$ or trauma$ or insult or event))).tw. |
| 30 | ((isch?emic or apoplectic) adj5 (event or events or insult or attack$)).tw. |
| 31 | ((cerebral vein or cerebral venous or sinus or sagittal) adj5 thrombo$).tw. |
| 32 | (CVDST or CVT).tw. |
| 33 | ((intracranial or cerebral art$ or basilar art$ or vertebral art$ or vertebrobasilar or vertebral basilar) adj5 (stenosis or isch?emia or insufficiency or arteriosclero$ or atherosclero$ or occlus$)).tw. |
| 34 | ((venous or arteriovenous or brain vasc$) adj5 malformation$).tw. |
| 35 | ((brain or cerebral) adj5 (angioma$ or hemangioma$ or haemangioma$)).tw. |
| 36 | carotid$.tw. |
| 37 | (patent foramen ovale or PFO).tw. |
| 38 | ((atrial or atrium or auricular) adj fibrillation).tw. |
| 39 | asymptomatic cervical bruit.tw. |
| 40 | exp aphasia/ or anomia/ or hemiplegia/ or hemianopsia/ or exp paresis/ or deglutition disorders/ or dysarthria/ or pseudobulbar palsy/ or muscle spasticity/ |
| 41 | (aphasi$ or apraxi$ or dysphasi$ or dysphagi$ or deglutition disorder$ or swallow$ disorder$ or dysarthri$ or hemipleg$ or hemipar$ or paresis or paretic or hemiaop$ or hemineglect or spasticity or anomi$ or dysnomi$ or acquired brain injur$ or hemiball$).tw. |
| 42 | ((unilateral or visual or hemispatial or attentional or spatial) adj10 neglect).tw. |
| 43 | or/1-42 |
| 44 | limit 43 to english language |
| 45 | exp Pain/ |
| 46 | pain$.tw. |
| 47 | arm/ |
| 48 | shoulder/ |
| 49 | Shoulder Joint/ |
| 50 | Shoulder Dislocation/ |
| 51 | Shoulder Pain/ |
| 52 | (arm$ or shoulder$).tw. |
| 53 | (upper limb$ or upper extremit$).tw. |
| 54 | allodynia.tw. |
| 55 | Complex regional pain syndromes/ |
| 56 | crps$2.tw. |
| 57 | ((post-traumatic or posttraumatic) adj2 dystrophy).tw. |
| 58 | (reflex adj2 sympathetic adj2 dystrophy).tw. |
| 59 | (sympathetic adj2 dystrophy adj2 syndrome).tw. |
| 60 | (sympathetic adj2 reflex adj2 dystrophy).tw. |
| 61 | or/45-60 |
| 62 | 44 and 61 |
| 63 | (exp child/ or exp infant/) not exp adult/ |
| 64 | 62 not 63 |
| 65 | guideline.pt. |
| 66 | practice guideline.pt. |
| 67 | Practice Guidelines as Topic/ |
| 68 | guideline$.tw. |
| 69 | Guidelines as topic/ |
| 70 | consensus development conference, NIH.pt. |
| 71 | nih consensus statement.jn. |
| 72 | consensus development conference.pt. |
| 73 | consensus.tw. |
| 74 | Consensus/ |
| 75 | Consensus Development Conferences as topic/ |
| 76 | Consensus Development Conferences, NIH/ |
| 77 | or/65-76 |
| 78 | (review or review, tutorial or review, academic).pt. |
| 79 | (medline or medlars or embase).tw,sh. |
| 80 | (scisearch or psycinfo or psychinfo).tw,sh. |
| 81 | (psychlit or psyclit).tw,sh. |
| 82 | cinahl.tw,sh. |
| 83 | ((hand adj2 search$) or (manual$ adj2 search$)).tw,sh. |
| 84 | (electronic database$ or bibliographic database$ or computeri?ed database$ or online database$).tw,sh. |
| 85 | (pooling or pooled or mantel haenszel).tw,sh. |
| 86 | (peto or dersimonian or der simonian or fixed effect).tw,sh. |
| 87 | or/78-86 |
| 88 | meta-analysis.pt. |
| 89 | meta-analysis as topic/ |
| 90 | (meta-analys$ or meta analys$ or metanalys$).tw,sh. |
| 91 | (systematic$ adj5 review$).tw,sh. |
| 92 | (systematic$ adj5 overview$).tw,sh. |
| 93 | (quantitativ$ adj5 review$).tw,sh. |
| 94 | (quantitativ$ adj5 overview$).tw,sh. |
| 95 | (quantitativ$ adj5 synthesis$).tw,sh. |
| 96 | (methodologic$ adj5 review$).tw,sh. |
| 97 | (methodologic$ adj5 overview$).tw,sh. |
| 98 | (integrative research review$ or research integration).tw. |
| 99 | or/88-98 |
| 100 | randomized controlled trial.pt. |
| 101 | controlled clinical trial.pt. |
| 102 | randomized.ab. |
| 103 | placebo.ab. |
| 104 | drug therapy.fs. |
| 105 | randomly.ab. |
| 106 | trial.ab. |
| 107 | groups.ab. |
| 108 | or/100-107 |
| 109 | humans.sh. |
| 110 | 108 and 109 |
| 111 | 64 and 77 |
| 112 | limit 111 to yr=”1995 - 2008” |
| 113 | 64 and (87 or 99) |
| 114 | 113 not 112 |
| 115 | limit 114 to yr=”1995 - 2008” |
| 116 | 64 and 110 |
| 117 | 116 not (112 or 115) |
| 118 | limit 117 to yr=”1995 - 2008” |
Databases: EMBASE Classic + EMBASE
Date range searched: 1995 to 2008 week 09.
Date of search: 4 March 2008.
Search strategy
| # | Searches |
|---|---|
| 1 | exp cerebrovascular disease/ |
| 2 | stroke$.tw. |
| 3 | cerebrovascular$.tw. |
| 4 | (cerebral or cerebellar or brainstem or vertebrobasilar).tw. |
| 5 | (infarct$ or isch?emi$ or thrombo$ or emboli$).tw. |
| 6 | 4 and 5 |
| 7 | carotid$.tw. |
| 8 | (cerebral or intracerebral or intracranial or parenchymal).tw. |
| 9 | (brain or intraventricular or brainstem or cerebellar).tw. |
| 10 | (infratentorial or supratentorial or subarachnoid).tw. |
| 11 | 8 or 9 or 10 |
| 12 | (haemorrhage or hemorrhage or haematoma or hematoma).tw. |
| 13 | (bleeding or aneurysm).tw. |
| 14 | 12 or 13 |
| 15 | 11 and 14 |
| 16 | thrombo$.tw. |
| 17 | (intracranial or (venous adj5 sinus) or (sagittal adj5 venous) or sagittal vein).tw. |
| 18 | 16 and 17 |
| 19 | transient isch?emic attack$.tw. |
| 20 | reversible isch$ neurologic$.tw. |
| 21 | venous malformation$.tw. |
| 22 | arteriovenous malformation$.tw. |
| 23 | 21 or 22 |
| 24 | 11 and 23 |
| 25 | exp aphasia/ |
| 26 | exp dysphasia/ |
| 27 | hemianopia/ |
| 28 | hemiplegia/ |
| 29 | hemiparesis/ |
| 30 | (aphas$ or dysphas$ or hemianop).tw. |
| 31 | (hemipleg$ or hemipar$).tw. |
| 32 | exp carotid artery surgery/ |
| 33 | or/1-3,6-7,15,18-20,24-32 |
| 34 | human/ |
| 35 | nonhuman/ |
| 36 | 34 and 35 |
| 37 | 35 not 36 |
| 38 | 33 not 37 |
| 39 | exp Pain/ |
| 40 | pain$.tw. |
| 41 | ARM MOVEMENT/ or ARM/ or arm muscle/ or ARM WEAKNESS/ |
| 42 | SHOULDER DISLOCATION/ or SHOULDER PAIN/ or SHOULDER/ or shoulder injury/ or shoulder girdle/ or shoulder hand syndrome/ or frozen shoulder/ |
| 43 | (arm$ or shoulder$).tw. |
| 44 | upper limb$.tw. |
| 45 | upper extremit$.tw. |
| 46 | Allodynia/ |
| 47 | allodynia.tw. |
| 48 | exp Complex regional pain syndrome/ |
| 49 | crps$2.tw. |
| 50 | ((post-traumatic or posttraumatic) adj2 dystrophy).tw. |
| 51 | (reflex adj2 sympathetic adj2 dystrophy).tw. |
| 52 | (sympathetic adj2 dystrophy adj2 syndrome).tw. |
| 53 | (sympathetic adj2 reflex adj2 dystrophy).tw. |
| 54 | or/39-53 |
| 55 | 38 and 54 |
| 56 | (exp child/ or exp infant/) not (exp adult/ or exp adolescent/) |
| 57 | 55 not 56 |
| 58 | limit 57 to english language |
| 59 | ((clinical or practice) adj2 guideline$).tw. |
| 60 | (consensus adj3 statement$).tw. |
| 61 | CONSENSUS/ |
| 62 | exp practice guideline/ |
| 63 | guideline$.ti. |
| 64 | guidelines/ |
| 65 | (consensus adj2 development).mp. |
| 66 | (evidence adj2 based adj2 (protocol$ or guideline$)).tw. |
| 67 | (clinical adj2 protocol$).tw. |
| 68 | nursing protocol/ |
| 69 | consensus conference.tw. |
| 70 | or/59-69 |
| 71 | exp review/ |
| 72 | (medline or medlars or embase or pubmed).ti,ab,sh. |
| 73 | (scisearch or psychlit or psyclit).ti,ab,sh. |
| 74 | (psycinfo or psychinfo).ti,ab,sh. |
| 75 | cinahl.ti,ab,sh. |
| 76 | ((hand adj2 search$) or (manual$ adj search$)).tw. |
| 77 | ((electronic adj database$) or (bibliographic adj database$)).tw. |
| 78 | ((pooled adj analys$) or pooling).tw. |
| 79 | (peto or dersimonian or (fixed adj effect) or mantel haenszel).tw. |
| 80 | RETRACTED ARTICLE/ |
| 81 | or/72-80 |
| 82 | 71 and 81 |
| 83 | exp meta analysis/ |
| 84 | meta?analys$.tw,sh. |
| 85 | (systematic$ adj5 review$).tw,sh. |
| 86 | (systematic$ adj5 overview$).tw,sh. |
| 87 | (quantitativ$ adj5 review$).tw,sh. |
| 88 | (quantitativ$ adj5 overview$).tw,sh. |
| 89 | (methodologic$ adj5 review$).tw,sh. |
| 90 | (methodologic$ adj5 overview$).tw,sh. |
| 91 | ((integrative adj5 research adj5 review$) or (research adj5 integration)).tw. |
| 92 | (quantitativ$ adj5 synthesi$).tw,sh. |
| 93 | or/83-92 |
| 94 | 82 or 93 |
| 95 | clinical trial/ |
| 96 | randomized controlled trial/ |
| 97 | randomization/ |
| 98 | single blind procedure/ |
| 99 | double blind procedure/ |
| 100 | crossover procedure/ |
| 101 | placebo/ |
| 102 | randomi?ed controlled trial$.tw. |
| 103 | rct.tw. |
| 104 | random allocation.tw. |
| 105 | randomly allocated.tw. |
| 106 | allocated randomly.tw. |
| 107 | (allocated adj2 random).tw. |
| 108 | single blind$.tw. |
| 109 | double blind$.tw. |
| 110 | ((treble or triple) adj blind$).tw. |
| 111 | placebo$.tw. |
| 112 | prospective study/ |
| 113 | or/95-112 |
| 114 | case study/ |
| 115 | case report.tw. |
| 116 | abstract report/ |
| 117 | letter/ |
| 118 | or/114-117 |
| 119 | 113 not 118 |
| 120 | 58 and 70 |
| 121 | limit 120 to yr=”1995 - 2008” |
| 122 | 58 and 94 |
| 123 | limit 122 to yr=”1995 - 2008” |
| 124 | 123 not 121 |
| 125 | 58 and 119 |
| 126 | limit 125 to yr=”1995 - 2008” |
| 127 | 126 not (121 or 124) |
Appendix 5 Project 1: example reference guide (pain)
Reproduced with permission from Bradford Teaching Hospitals NHS Foundation Trust. Please note this is provided as an example only – this reference quide may now be out of date.
Appendix 6 Project 2: evidence submitted to the Academy of Medical Sciences for the review on research governance
The following case study was submitted as evidence to the Academy of Medical sciences for the review on research governance. 162
Case study: cluster randomised trial evaluation of a patient- and carer-centred system of longer-term stroke care (Longer-Term Stroke care trial)
Introduction
The LoTS care trial is part of a NIHR-funded programme of research that aims to improve outcomes after stroke by addressing the longer-term needs of patients and carers living at home in the community. It is a cluster RCT evaluating the clinical effectiveness and cost-effectiveness of a new system of care (LoTS care intervention) in comparison to usual practice, as delivered by SCCs, for 800 stroke patients (with and without carers) living at home in the community. The system of care involves the use of a structured assessment linked to a manual containing reference guides and evidence-based treatment algorithms to identify individual patient and carer needs and create action plans for those patients and carers.
Trial organisation
The trial is managed by the Bradford Teaching Hospitals NHS Foundation Trust and the University of Leeds and is sponsored by the Bradford Teaching Hospitals NHS Foundation Trust. It is adopted by the SRN (prior to the NIHR co-ordinated system for gaining NHS permission). The trial involves 32 services across the UK, each of which is composed of a SCC-led community service and the hospitals that refer patients to them, such that 62 separate trusts are involved. After all approvals are in place, SCC services are randomised to either the LoTS care intervention or the control (usual practice) (cluster randomisation); after a training period for intervention services to embed this practice into their service, services then use the practice to which they have been randomised with all patients referred to them throughout the duration of the trial. Patients are recruited to the trial in hospital (for the majority of patients) or in the community by a stroke researcher who is separate from the clinical team, before their first appointment with the SCC service. Patients (and carers) who consent to the trial have a baseline assessment undertaken by the researcher and are asked to complete questionnaires assessing psychological and functional outcomes at baseline and 6 and 12 months after recruitment.
Trial timelines
The NIHR-funded research programme runs for 5 years from October 2007 until September 2012. The initial application for ethics approval for the trial was made in January 2008 and the first patient was recruited in July 2009 (phase 1 services) and February 2010 (phase 2 services). The steps leading up to the first patient being recruited are summarised in Table 63 and are discussed in more detail below. Recruitment of 800 patients is required to be completed by the end of 2010, with 12 months’ follow-up completed in January 2012, allowing time for analysis and writing up of results by the end of the programme. The original programme timescale was 55 months; however, this was extended to 60 months to accommodate the protracted set-up and approval times at the start of the trial.
| 2008 | 2009 | 2010 | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| J | F | M | A | M | J | J | A | S | O | N | D | J | F | M | A | M | J | J | A | S | O | N | D | J | F | ||
| Ethics (England, Wales, Northern Ireland) | Submission | ||||||||||||||||||||||||||
| Resubmission | |||||||||||||||||||||||||||
| Approval | ✗ | ||||||||||||||||||||||||||
| Ethics (Scotland) | Submission | ||||||||||||||||||||||||||
| Resubmission | |||||||||||||||||||||||||||
| Approval | ✗ | ||||||||||||||||||||||||||
| Phase 1a | Local set-up | ||||||||||||||||||||||||||
| R&D approvals | |||||||||||||||||||||||||||
| Randomisation | ✗ | ||||||||||||||||||||||||||
| Training (intervention) | |||||||||||||||||||||||||||
| First patient recruited | ✗ | ||||||||||||||||||||||||||
| Phase 2a | Local set-up | ||||||||||||||||||||||||||
| R&D approvals | |||||||||||||||||||||||||||
| Randomisation | ✗ | ||||||||||||||||||||||||||
| Training (intervention) | |||||||||||||||||||||||||||
| First patient recruited | ✗ | ||||||||||||||||||||||||||
Ethics approval
The review process was efficient (1–2 months). However, approval was delayed because of initial rejections, which appeared to be based on a lack of understanding of the cluster randomised design of the trial. In addition, separate ethics approvals were required for Scotland and the rest of the UK because of the inclusion of adults lacking capacity.
Local set-up
This involved establishing interest and the suitability of SCC services and the hospitals from which they received referrals, determining which acute trusts and primary care trusts are involved, identifying principal and co-investigators and preparing R&D applications. In the majority of services the principal investigator was required to take responsibility for the trial over the whole service, with co-investigators at each of the sites involved. In some services, the principal investigator was able to take responsibility for the trial only at an individual trust, resulting in more than one principal investigator for the service. The intervention is delivered in the primary care trust whereas the majority of patients are recruited from acute trusts, so agreement was required as to who would take responsibility. Delays were caused by differing trust policies regarding who could be principal investigator; many acute trusts would accept principal investigators employed by their own trust only and others required that the principal investigator must be a consultant, despite this being a non-CTIMP (Clinical Trial of an Investigational Medicinal Product) study delivered by stroke research nurses and SCCs. There were also delays in the issuing of honorary contracts for those principal investigators not employed by the acute trusts. Set-up times ranged from 0.5 to 12.2 months, with a median time of 4.1 months. Set-up times for individual services are given in Tables 64a and b.
| SRN (at time of trial set-up) | Service | Number of trusts | Set-up (months) | Trust | R&D approval trust (months) | R&D approval service (months) | Set-up and approval (months) |
|---|---|---|---|---|---|---|---|
| None | Aylesbury | 2 | 3.7 | Buckinghamshire Hospitals NHS Trust | 2.6 | 2.6 | 6.3 |
| Buckinghamshire PCT | 0.6 | ||||||
| North-east | Hartlepool | 2 | 6.2 | North Tees and Hartlepool NHS Foundation Trust | 2.6 | 2.6 | 8.8 |
| Hartlepool PCT | 1.8 | ||||||
| Peterlee | 4 | 6.1 | North Tees and Hartlepool NHS Foundation Trust | 2.6 | 2.6 | 8.7 | |
| City Hospitals Sunderland NHS Foundation Trust | 2.5 | ||||||
| County Durham and Darlington NHS Foundation Trust | 0.5 | ||||||
| County Durham PCT and Darlington PCT | 2.4 | ||||||
| Stockton-on-Tees | 2 | 5.5 | North Tees and Hartlepool NHS Foundation Trust | 2.6 | 2.6 | 8.1 | |
| North Tees PCT | 1.8 | ||||||
| Northern Ireland | Dungannon | 2 | 2.8 | Southern Health and Social Care Trust | 2.8 | 3.0 | 5.8 |
| Western Health and Social Care Trust | 3.0 | ||||||
| Newry | 1 | 4.1 | Southern Health and Social Care Trust | 2.8 | 2.8 | 6.9 | |
| Omagh | 1 | 4.7 | Western Health and Social Care Trust | 3.0 | 3.0 | 7.7 | |
| North-west | Manchester | 3 | 2.2 | Central Manchester and Manchester Children’s University Hospitals NHS Trust | 2.6 | 2.6 | 4.8 |
| Pennine Acute Hospitals NHS Trust | 2.6 | ||||||
| Manchester PCT | 2.0 | ||||||
| South-east | Surrey | 2 | 4.6 | Ashford and St Peter’s Hospitals NHS Trust | 1.1 | 1.7 | 6.3 |
| Surrey PCT | 1.7 | ||||||
| Wales | Cardiff | 1 | 3.2 | Cardiff and Vale NHS Trust | 3.5 | 3.5 | 6.7 |
| Swansea | 1 | 4.2 | Abertawe Bro Morgannwg University Health Board | 3.1 | 3.1 | 7.3 | |
| West Midlands | Coventry | 2 | 4.2 | University Hospitals Coventry and Warwickshire NHS Trust | 4.2 | 4.4 | 8.6 |
| Coventry Teaching PCT | 4.4 | ||||||
| Walsall | 2 | 3.2 | Walsall Hospitals NHS Trust | 3.5 | 3.5 | 6.7 | |
| Walsall PCT | 2.6 | ||||||
| Wolverhampton | 2 | 0.9 | The Royal Wolverhampton Hospitals NHS Trust | 2.1 | 2.1 | 3.0 | |
| Wolverhampton City PCT | 0.6 |
| SRN (at time of trial set-up) | Service | Number of trusts | Set-up (months) | Trust | R&D approval trust (months) | R&D approval service (months) | Set-up and approval (months) |
|---|---|---|---|---|---|---|---|
| None | Cheltenham | 2 | 4.0 | Gloucestershire Hospitals NHS Foundation Trust | 9.3 | 9.3 | 13.3 |
| NHS Gloucestershire | 9.3 | ||||||
| Gloucester | 2 | Gloucestershire Hospitals NHS Foundation Trust | 9.3 | 9.3 | 13.3 | ||
| NHS Gloucestershire | 9.3 | ||||||
| Ipswich | 2 | 3.3 | Ipswich Hospital NHS Trust | 9.1 | 9.1 | 12.4 | |
| Suffolk PCT | 8.0 | ||||||
| Lyndhurst | 3 | 4.0 | Southampton University Hospitals NHS Trust | 9.1 | 9.1 | 13.1 | |
| Royal Bournemouth and Christchurch Hospitals NHS Foundation Trust | 2.5 | ||||||
| Hampshire Community Health Care | 8.5 | ||||||
| Peninsula | Cornwall | 4 | 7.3 | Royal Cornwall Hospitals NHS Trust | 2.6 | 2.6 | 9.9 |
| Cornwall and Isles of Scilly PCT | 1.5 | ||||||
| Plymouth Teaching PCT | 1.2 | ||||||
| Plymouth Hospitals NHS Trust | 1.8 | ||||||
| Exeter | 2 | 3.0 | Royal Devon and Exeter NHS Foundation Trust | 3.0 | 3.0 | 6.0 | |
| Devon PCT | 3.0 | ||||||
| Williton | 1 | 0.5 | Somerset PCT | 1.8 | 1.8 | 2.3 | |
| Scotland | Dunoon | 2 | 10.6 | NHS Greater Glasgow and Clyde | 2.5 | 2.5 | 13.1 |
| NHS Highland | 1.7 | ||||||
| Oban | 1 | 11.7 | NHS Highland | 1.7 | 1.7 | 13.4 | |
| Trent | Chesterfield | 2 | 9.4 | Chesterfield Royal Hospital NHS Foundation Trust | 2.7 | 4.4 | 13.8 |
| Derbyshire County PCT | 4.4 | ||||||
| Norwich | 2 | 3.6 | Norfolk and Norwich University Hospitals NHS Foundation Trust | 1.8 | 2.5 | 6.1 | |
| NHS Norfolk | 2.5 | ||||||
| North-west | Blackpool | 2 | 0.6 | Blackpool PCT | 2.9 | 2.9 | 3.5 |
| Blackpool, Fylde and Wyre Hospitals NHS Foundation Trust | 1.9 | ||||||
| Ellesmere Port | 1 | 11.3 | NHS Western Cheshire | 2.8 | 2.8 | 14.1 | |
| East Lancashire | 4 | 0.6 | East Lancashire Hospitals NHS Trust | 0.5 | 1.3 | 1.9 | |
| Airedale NHS Trust | 1.3 | ||||||
| Pennine Acute Hospitals NHS Trust | 1.2 | ||||||
| NHS East Lancashire | 0.7 | ||||||
| Preston | 3 | 9.3 | Southport and Ormskirk Hospital NHS Trust | 6.9 | 6.9 | 16.2 | |
| Lancashire Teaching Hospitals NHS Foundation Trust | 1.7 | ||||||
| NHS Central Lancashire | 1.2 | ||||||
| St Helens | 3 | 12.2 | St Helens and Knowsley Teaching Hospitals NHS Trust | 2.7 | 2.7 | 14.9 | |
| Warrington and Halton Hospitals NHS Foundation Trust | 1.9 | ||||||
| NHS Halton and St Helens | 2.2 | ||||||
| South-east | Gravesend | 4 | 10.2 | Maidstone and Tunbridge Wells NHS Trust | 2.7 | 2.7 | 12.9 |
| Dartford and Gravesham NHS Trust | 1.7 | ||||||
| NHS West Kent | 0.8 | ||||||
| Guy’s and St Thomas’ NHS Foundation Trust | 0.8 | ||||||
| Worthing | 2 | 2.7 | Western Sussex Hospitals NHS Trust | 10.4 | 10.4 | 13.1 | |
| West Sussex PCT | 1.4 |
Research and development approval (NHS permission)
Sixty-nine R&D approvals from 62 trusts were required to cover the 32 SCC services involved in the trial. The number of trusts involved in a service ranged from one to four. Because of the unpredictability of R&D approval timelines, it was required that all approvals at a service had to be in place before that service could be randomised. The randomisation had to be staggered into two phases according to the time taken to obtain R&D approvals, with 14 services in phase 1 and 18 services in phase 2.
All R&D applications were submitted after ethics approval (Scotland or the rest of the UK as appropriate) was in place. The time taken to receive all R&D approvals for a service ranged from 1.3 to 10.4 months (median 2.8 months), with R&D approvals from individual trusts taking between 0.5 and 10.4 months (median 2.5 months). In total, 42% of trusts gave approval in < 2 months, 35% in 2–3 months, 10% in 3–4 months and the remaining 13% in 7–10 months. R&D approval times for individual services and trusts are given in Table 64a and b.
Common problems that were encountered included:
-
lack of understanding of the cluster randomised trial design
-
changes requested to the model non-commercial agreement (Schedule 2: division of responsibilities) and delays in sign-off
-
local requirements for documentation in addition to the standard forms and documents
-
reluctance to provide approval without a named stroke researcher; in some cases this was necessary as the SRN/trial funds could not provide a researcher until approval was in place
-
insistence that consent could be taken only by a physician rather than by a stroke researcher
-
requirement to provide documentary evidence of indemnity despite the trial having a NHS sponsor
-
pressure from R&D departments (and the NHS sponsor) to prevent researchers from implementing ethics-approved amendments until R&D departments have also approved the amendments, and delays in approving amendments by R&D departments.
Conclusions
The LoTS care trial is a publicly funded trial with the potential to improve the longer-term care of stroke patients and carers. Substantial delays were caused in the time to first patient recruited by the time taken to obtain R&D approvals from the large number of trusts involved and local policies disproportionate to the risk of the study. It is suggested that a target of R&D approval within 2 months be implemented. Of the 62 trusts involved in the LoTS care trial, 77% gave R&D approval within 3 months and 42% within 2 months, suggesting that 2 months is an achievable target, in addition to bringing R&D approval in line with ethics and Medicines and Healthcare products Regulatory Agency approval timelines.
Appendix 7 Project 2: unit costs
| Item | Unit | Unit cost (£, 2010/11 prices) | Sourcea | Notes |
|---|---|---|---|---|
| Residential and nursing home | ||||
| Residential care home | Night | 75 | 1 | |
| Nursing home | Night | 76 | 1 | |
| Inpatient services | ||||
| A – Nervous system | Bed-day | 356 | 2 | Tab TNEI_L: weighted mean of codes A |
| B – Eyes and periorbita | Bed-day | 587 | 2 | Tab TNEI_L: weighted mean of codes B |
| C – Mouth, head, neck and ears | Bed-day | 503 | 2 | Tab TNEI_L: weighted mean of codes C |
| D – Respiratory system | Bed-day | 316 | 2 | Tab TNEI_L: weighted mean of codes D |
| E – Cardiac surgery and primary cardiac conditions | Bed-day | 438 | 2 | Tab TNEI_L: weighted mean of codes E |
| F – Digestive system | Bed-day | 415 | 2 | Tab TNEI_L: weighted mean of codes F |
| G – Hepatobiliary and pancreatic systems | Bed-day | 385 | 2 | Tab TNEI_L: weighted mean of codes G |
| H – Musculoskeletal system | Bed-day | 471 | 2 | Tab TNEI_L: weighted mean of codes H |
| J – Skin, breast and burns | Bed-day | 391 | 2 | Tab TNEI_L: weighted mean of codes J |
| K – Endocrine and metabolic system | Bed-day | 317 | 2 | Tab TNEI_L: weighted mean of codes K |
| L – Urinary tract and male reproductive systems | Bed-day | 339 | 2 | Tab TNEI_L: weighted mean of codes L |
| M – Female reproductive system and assisted reproduction | Bed-day | 580 | 2 | Tab TNEI_L: weighted mean of codes M |
| N – Obstetrics | Bed-day | 792 | 2 | Tab TNEI_L: weighted mean of codes N |
| P – Diseases of childhood and neonates | Bed-day | 559 | 2 | Tab TNEI_L: weighted mean of codes P |
| Q – Vascular system | Bed-day | 457 | 2 | Tab TNEI_L: weighted mean of codes Q |
| R – Radiology and nuclear medicine | Bed-day | 497 | 2 | Tab TNEI_L: weighted mean of codes R |
| S – Haematology, chemotherapy, radiotherapy and specialist palliative care | Bed-day | 434 | 2 | Tab TNEI_L: weighted mean of codes S |
| W – Immunology, infectious diseases and other contacts | Bed-day | 444 | 2 | Tab TNEI_L: weighted mean of codes W |
| Geriatric | Bed-day | 336 | 2 | Costed as ‘stroke rehabilitation’ |
| Oncology/cancer | Bed-day | 425 | 2 | Costed as ‘general medical’ |
| Surgery | Bed-day | 425 | 2 | Costed as ‘general medical’ |
| Intensive care | Bed day | 1213 | 2 | Index tab TCCSALCCU: Critical Care Services – Adult: Critical Care Unit |
| Acute stroke/stroke unit | Bed-day | 315 | 2 | Tab TNEI_L: weighted average of ischaemic and haemorrhagic stroke |
| Stroke rehabilitation/stroke/stroke ward | Bed-day | 336 | 2 | Tab TREHAB_CSRS_LEVEL_1_BEDDAY_APC: VC04Z (rehabilitation for stroke) cost. Assumption this is per bed-day |
| General medical | Bed-day | 425 | 2 | Tab TNEI_L: weighted average of all costs |
| Inpatient services | ||||
| A – Nervous system | Stay | 2500 | 2 | Tab TNEI_L: weighted mean of codes A |
| B – Eyes and periorbita | Stay | 2249 | 2 | Tab TNEI_L: weighted mean of codes B |
| C – Mouth, head, neck and ears | Stay | 1966 | 2 | Tab TNEI_L: weighted mean of codes C |
| D – Respiratory system | Stay | 1999 | 2 | Tab TNEI_L: weighted mean of codes D |
| E – Cardiac surgery and primary cardiac conditions | Stay | 1996 | 2 | Tab TNEI_L: weighted mean of codes E |
| F – Digestive system | Stay | 2341 | 2 | Tab TNEI_L: weighted mean of codes F |
| G – Hepatobiliary and pancreatic systems | Stay | 2523 | 2 | Tab TNEI_L: weighted mean of codes G |
| H – Musculoskeletal system | Stay | 3720 | 2 | Tab TNEI_L: weighted mean of codes H |
| J – Skin, breast and burns | Stay | 2617 | 2 | Tab TNEI_L: weighted mean of codes J |
| K – Endocrine and metabolic system | Stay | 1789 | 2 | Tab TNEI_L: weighted mean of codes K |
| L – Urinary tract and male reproductive systems | Stay | 2370 | 2 | Tab TNEI_L: weighted mean of codes L |
| M – Female reproductive system and assisted Reproduction | Stay | 2050 | 2 | Tab TNEI_L: weighted mean of codes M |
| N – Obstetrics | Stay | 2330 | 2 | Tab TNEI_L: weighted mean of codes N |
| P – Diseases of childhood and neonates | Stay | 1721 | 2 | Tab TNEI_L: weighted mean of codes P |
| Q – Vascular system | Stay | 4057 | 2 | Tab TNEI_L: weighted mean of codes Q |
| R – Radiology and nuclear medicine | Bed-day | 5328 | 2 | Tab TNEI_L: weighted mean of codes R |
| S – Haematology, chemotherapy, radiotherapy and specialist palliative care | Stay | 2686 | 2 | Tab TNEI_L: weighted mean of codes S |
| W – Immunology, infectious diseases and other contacts | Stay | 4717 | 2 | Tab TNEI_L: weighted mean of codes W |
| Oncology/cancer | Stay | 2378 | 2 | Costed as ‘general medical’ |
| Surgery | Stay | 2378 | 2 | Costed as ‘general medical’ |
| Acute stroke/stroke unit | Stay | 2884 | 2 | Tab TNEI_L: weighted average of ischaemic and haemorrhagic stroke |
| General medical | Stay | 2378 | 2 | Tab TNEI_L: weighted average of all costs |
| Day hospital/day cases | ||||
| A – Nervous system | Activity | 633 | 2 | Tab TDC: weighted mean of codes A |
| B – Eyes and periorbita | Activity | 762 | 2 | Tab TDC: weighted mean of codes B |
| C – Mouth, head, neck and ears | Activity | 761 | 2 | Tab TDC: weighted mean of codes C |
| D – Respiratory system | Activity | 578 | 2 | Tab TDC: weighted mean of codes D |
| E – Cardiac surgery and primary cardiac conditions | Activity | 1190 | 2 | Tab TDC: weighted mean of codes E |
| F – Digestive system | Activity | 560 | 2 | Tab TDC: weighted mean of codes F |
| G – Hepatobiliary and pancreatic systems | Activity | 949 | 2 | Tab TDC: weighted mean of codes G |
| H – Musculoskeletal system | Activity | 980 | 2 | Tab TDC: weighted mean of codes H |
| J – Skin, breast and burns | Activity | 685 | 2 | Tab TDC: weighted mean of codes J |
| K – Endocrine and metabolic system | Activity | 379 | 2 | Tab TDC: weighted mean of codes K |
| L – Urinary tract and male reproductive systems | Activity | 494 | 2 | Tab TDC: weighted mean of codes L |
| M – Female reproductive system and assisted reproduction | Activity | 719 | 2 | Tab TDC: weighted mean of codes M |
| N – Obstetrics | Activity | 230 | 2 | Tab TDC: weighted mean of codes N |
| P – Diseases of childhood and neonates | Activity | 692 | 2 | Tab TDC: weighted mean of codes P |
| Q – Vascular system | Activity | 750 | 2 | Tab TDC: weighted mean of codes Q |
| R – Radiology and nuclear medicine | Activity | 956 | 2 | Tab TDC: weighted mean of codes R |
| S – Haematology, chemotherapy, radiotherapy and specialist palliative care | Activity | 451 | 2 | Tab TDC: weighted mean of codes S |
| V – Multiple trauma, emergency medicine and rehabilitation | Activity | 991 | 2 | Tab TDC: weighted mean of codes V |
| W – Immunology, infectious diseases and other contacts | Activity | 407 | 2 | Tab TDC: weighted mean of codes W |
| Overall day hospital | Activity | 664 | 2 | Index tab: Day Cases HRG Data – TDC |
| Surgery | Activity | 664 | 2 | Costed as ‘overall day hospital’ |
| Stroke | Activity | 664 | 2 | Costed as ‘overall day hospital’ |
| Outpatient services | ||||
| A&E | Activity | 113 | 2 | Total – OPATT Tab: Service code 180 |
| General surgery | Activity | 119 | 2 | Total – OPATT Tab: Service code 100 |
| Urology | Activity | 102 | 2 | Total – OPATT Tab: Service code 101 |
| Breast surgery | Activity | 127 | 2 | Total – OPATT Tab: Service code 103 |
| Colorectal surgery | Activity | 106 | 2 | Total – OPATT Tab: Service code 104 |
| Hepatobiliary and pancreatic surgery | Activity | 156 | 2 | Total – OPATT Tab: Service code 105 |
| Upper gastrointestinal surgery | Activity | 111 | 2 | Total – OPATT Tab: Service code 106 |
| Vascular surgery | Activity | 133 | 2 | Total – OPATT Tab: Service code 107 |
| Ear, nose and throat | Activity | 92 | 2 | Total – OPATT Tab: Service code 120 |
| Ophthalmology | Activity | 83 | 2 | Total – OPATT Tab: Service code 130 |
| Oral surgery | Activity | 110 | 2 | Total – OPATT Tab: Service code 140 |
| Restorative dentistry | Activity | 114 | 2 | Total – OPATT Tab: Service code 141 |
| Orthodontics | Activity | 112 | 2 | Total – OPATT Tab: Service code 143 |
| Maxillofacial surgery | Activity | 109 | 2 | Total – OPATT Tab: Service code 144 |
| Neurosurgery | Activity | 157 | 2 | Total – OPATT Tab: Service code 150 |
| Plastic surgery | Activity | 89 | 2 | Total – OPATT Tab: Service code 160 |
| Burns care | Activity | 160 | 2 | Total – OPATT Tab: Service code 161 |
| Cardiothoracic surgery | Activity | 211 | 2 | Total – OPATT Tab: Service code 170 |
| Cardiac surgery | Activity | 230 | 2 | Total – OPATT Tab: Service code 172 |
| Thoracic surgery | Activity | 204 | 2 | Total – OPATT Tab: Service code 173 |
| Anaesthetics | Activity | 84 | 2 | Total – OPATT Tab: Service code 190 |
| Pain management | Activity | 128 | 2 | Total – OPATT Tab: Service code 191 |
| Critical care medicine | Activity | 139 | 2 | Total – OPATT Tab: Service code 192 |
| General medicine | Activity | 153 | 2 | Total – OPATT Tab: Service code 300 |
| Endocrinology | Activity | 140 | 2 | Total – OPATT Tab: Service code 302 |
| Clinical haematology | Activity | 152 | 2 | Total – OPATT Tab: Service code 303 |
| Clinical physiology | Activity | 53 | 2 | Total – OPATT Tab: Service code 304 |
| Clinical pharmacology | Activity | 139 | 2 | Total – OPATT Tab: Service code 305 |
| Hepatology | Activity | 202 | 2 | Total – OPATT Tab: Service code 306 |
| Diabetic medicine | Activity | 130 | 2 | Total – OPATT Tab: Service code 307 |
| Haemophilia | Activity | 772 | 2 | Total – OPATT Tab: Service code 309 |
| Audiological medicine | Activity | 92 | 2 | Total – OPATT Tab: Service code 310 |
| Clinical genetics | Activity | 672 | 2 | Total – OPATT Tab: Service code 311 |
| Clinical cytogenetics and molecular genetics | Activity | 185 | 2 | Total – OPATT Tab: Service code 312 |
| Clinical immunology and allergy | Activity | 161 | 2 | Total – OPATT Tab: Service code 313 |
| Rehabilitation | Activity | 122 | 2 | Total – OPATT Tab: Service code 314 |
| Palliative medicine | Activity | 191 | 2 | Total – OPATT Tab: Service code 315 |
| Clinical immunology | Activity | 215 | 2 | Total – OPATT Tab: Service code 316 |
| Allergy | Activity | 124 | 2 | Total – OPATT Tab: Service code 317 |
| Respite care | Activity | 17 | 2 | Total – OPATT Tab: Service code 319 |
| Cardiology | Activity | 134 | 2 | Total – OPATT Tab: Service code 320 |
| Clinical microbiology | Activity | 206 | 2 | Total – OPATT Tab: Service code 322 |
| Spinal injuries | Activity | 253 | 2 | Total – OPATT Tab: Service code 323 |
| Anticoagulant service | Activity | 21 | 2 | Total – OPATT Tab: Service code 324 |
| Dermatology | Activity | 95 | 2 | Total – OPATT Tab: Service code 330 |
| Respiratory medicine | Activity | 148 | 2 | Total – OPATT Tab: Service code 340 |
| Respiratory physiology | Activity | 161 | 2 | Total – OPATT Tab: Service code 341 |
| Infectious diseases | Activity | 241 | 2 | Total – OPATT Tab: Service code 350 |
| Tropical medicine | Activity | 228 | 2 | Total – OPATT Tab: Service code 352 |
| Genitourinary medicine | Activity | 153 | 2 | Total – OPATT Tab: Service code 360 |
| Nephrology | Activity | 164 | 2 | Total – OPATT Tab: Service code 361 |
| Medical oncology | Activity | 123 | 2 | Total – OPATT Tab: Service code 370 |
| Nuclear medicine | Activity | 99 | 2 | Total – OPATT Tab: Service code 371 |
| Neurology | Activity | 168 | 2 | Total – OPATT Tab: Service code 400 |
| Clinical neurophysiology | Activity | 170 | 2 | Total – OPATT Tab: Service code 401 |
| Rheumatology | Activity | 138 | 2 | Total – OPATT Tab: Service code 410 |
| Geriatric medicine | Activity | 205 | 2 | Total – OPATT Tab: Service code 430 |
| Dental medicine specialties | Activity | 101 | 2 | Total – OPATT Tab: Service code 450 |
| Medical ophthalmology | Activity | 89 | 2 | Total – OPATT Tab: Service code 460 |
| Obstetrics | Activity | 112 | 2 | Total – OPATT Tab: Service code 501 |
| Gynaecology | Activity | 118 | 2 | Total – OPATT Tab: Service code 502 |
| Gynaecological oncology | Activity | 134 | 2 | Total – OPATT Tab: Service code 503 |
| Midwife episode | Activity | 63 | 2 | Total – OPATT Tab: Service code 560 |
| Podiatry | Activity | 43 | 2 | Total – OPATT Tab: Service code 653 |
| Orthoptics | Activity | 54 | 2 | Total – OPATT Tab: Service code 655 |
| Clinical oncology | Activity | 126 | 2 | Total – OPATT Tab: Service code 800 |
| Diagnostic imaging | Activity | 30 | 2 | Total – OPATT Tab: Service code 812 |
| Chemical pathology | Activity | 67 | 2 | Total – OPATT Tab: Service code 822 |
| Audiology | Activity | 113 | 2 | Total – OPATT Tab: Service code 840 |
| Trauma and orthopaedics: non-trauma | Activity | 99 | 2 | Total – OPATT Tab: Service code 110N |
| Trauma and orthopaedics: trauma | Activity | 101 | 2 | Total – OPATT Tab: Service code 110T |
| Medical gastroenterology | Activity | 128 | 2 | Total – OPATT Tab: Service code 301M |
| Surgical gastroenterology | Activity | 116 | 2 | Total – OPATT Tab: Service code 301S |
| Endocrine surgery | Activity | 123 | 2 | Total – OPATT Tab: Service code 302S |
| Physiotherapy | Activity | 38 | 2 | Total – OPATT Tab: Service code 650A |
| Occupational therapy | Activity | 56 | 2 | Total – OPATT Tab: Service code 651A |
| Speech and language therapy | Activity | 98 | 2 | Total – OPATT Tab: Service code 652A |
| Dietetics | Activity | 55 | 2 | Total – OPATT Tab: Service code 654A |
| Breast MDT meetings | Activity | 89 | 2 | Total – OPATT Tab: Service code CMDT_B |
| Colorectal MDT meetings | Activity | 137 | 2 | Total – OPATT Tab: Service code CMDT_C |
| Local gynaecology MDT meetings | Activity | 111 | 2 | Total – OPATT Tab: Service code CMDT_LG |
| Specialist gynaecology MDT meetings | Activity | 120 | 2 | Total – OPATT Tab: Service code CMDT_SpG |
| Specialist UGI meetings | Activity | 125 | 2 | Total – OPATT Tab: Service code CMDT_SpU |
| Radiography | Activity | 29 | 2 | Total – OPATT Tab: Service code DAPF |
| Sexual and reproductive health clinic (previously referred to as family planning clinic) | Activity | 65 | 2 | Total – OPATT Tab: Service code FPC |
| HIV/AIDS | Activity | 515 | 2 | Total – OPATT Tab: Service code H/A |
| Stroke | Activity | 168 | 2 | Costed as ‘neurology’ |
| Mental health | Activity | 179 | 2 | A weighted average of all hospital outpatient services was calculated. Index tab: variables: TMHCSOPFAF, TMHCSOPFANF, TMHCSOPFUAF, TMHCSOPFUANF, TMHCSOPSSFAF, TMHCSOPSSFANF, TMHCSOPSSFUAF, TMHCSOPSSFUANF |
| Blood tests | Activity | 3 | 2 | TDAPS Tab: DAP839 code |
| Radiology | Activity | 109 | 2 | Index tab: TRADTHPY_TREAT_OP: Radiotherapy treatment: outpatient |
| Community-based services | ||||
| GP, surgery | Visit | 30 | 3 | Average surgery consultation lasting 11.7 minutes. Includes direct care staff costs; excludes qualification costs (p. 149) |
| GP, home | Visit | 99 | 3 | Average home visit lasting 23.4 minutes. Includes travel time and direct care staff costs; excludes qualification costs (p. 149) |
| GP, telephone consultation | Call | 18 | 3 | Average telephone consultation lasting 7.1 minutes. Includes direct care staff costs; excludes qualification costs (p. 149) |
| Practice nurse, surgery | Visit | 11 | 3 | Cost per consultation based on per-hour face-to-face × duration of contact. Excludes qualification costs (p. 146) |
| Practice nurse, telephone call | Call | 9 | 3 | Assume ratio of time spent on telephone consultation compared with face-to-face consultation is same as for GP (83.07%). On this basis, a face-to-face nurse consultation of 15.5 minutes at £11.10 translates to a 12.88-minute telephone consultation at £9.22 – (pp. 146 and 149) |
| Repeat prescription request (without nurse/doctor contact) | Request | 13 | 3 | Assume 5 minutes of GP time, i.e. 5 × £2.56 per surgery/clinic minute. Includes direct care staff costs; excludes qualification costs (p. 149) |
| Physiotherapist home | Visit | 56 | 3, 4 | Excludes qualification costs (p. 133). Based on the length of contact (60 minutes) and travel time and cost from PSSRU 2010 (p. 151) as none available in 2011 (£31 per hour for 60 minutes) |
| Physiotherapist surgery | Visit | 26 | 3, 4 | Excludes qualification costs (p. 133). Based on the length of contact (30 minutes) from PSSRU 2010 (p. 151) as none available in 2011 (£31 per hour for 30 minutes) |
| Physiotherapist visit elsewhere (not private) | Visit | 38 | 2 | Costed as ‘hospital outpatient visit’ |
| Occupational therapist, home | Visit | 56 | 3, 4 | Excludes qualification costs (p. 132). Based on the length of contact (60 minutes) and travel time and cost from PSSRU 2010 (p. 152) as none available in 2011 (£31 per hour for 60 minutes) |
| Occupational therapist, surgery | Visit | 26 | 3 | Excludes qualification costs (p. 132). Based on the length of contact (30 minutes) from PSSRU 2010 (p. 152) as none available in 2011 (£31 per hour for 30 minutes) |
| Occupational therapist visit elsewhere (not private) | Visit | 56 | 2 | Costed as ‘hospital outpatient visit’ |
| Speech and language therapist, home | Visit | 56 | 3, 4 | Excludes qualification costs (p. 135). Based on the length of contact (60 minutes) and travel time and cost from PSSRU 2010 (p. 153) as none available in 2011 (£31 per hour for 60 minutes) |
| Speech and language therapist, surgery | Visit | 26 | 3, 4 | Excludes qualification costs (p. 135). Based on the length of contact (30 minutes) from PSSRU 2010 (p. 153) as none available in 2011 (£31 per hour for 30 minutes) |
| Speech and language therapist visit elsewhere (not private) | Visit | 98 | 2 | Costed as ‘hospital outpatient visit’ |
| Community or district nurse | Visit | 34 | 3, 4 | Excludes qualification costs (p. 141). Based on the length of contact (20 minutes) and travel time and cost from PSSRU 2010 (p. 159) as none available in 2011 (£44 per hour for 20 minutes) |
| Health visitor | Visit | 23 | 3, 4 | Excludes qualification costs (p. 143). Based on the length of contact (20 minutes) and travel cost from PSSRU 2010 (p. 161) as none available in 2011 (£64 per hour of home visiting for 20 minutes) |
| Geriatrician | Contact | 46 | 3 | Assume 20-minute contact with medical consultant (£137 per contract-hour). Excludes qualification costs (p. 203) |
| Psychiatrist | Contact | 131 | 2 | Index tab: Mental Health Consultant Services (Community Setting) – Follow Up Contact Face to Face – TMHCSCFUAF |
| Psychologist | Contact | 135 | 3 | Assume 1-hour contact (£135 per hour of client contact) (p. 137) |
| Chiropodist | Visit | 11 | 3, 4 | £22 per hour (p. 136). Based on half-hour clinic visit stated in PSSRU 2010 as no information in 2011 version |
| Chiropractor | Contact | 28 | 9 | Assume mid-point cost per session from range of £20–35 per 30-minute appointment |
| Osteopath | Contact | 43 | 8 | Assume mid-point cost per session from range of £35–50 per 30- to 40-minute contact |
| Dentist | Activity | 78 | 2 | Tab TOCS: Community Dental Services – CN20 |
| Optician | Eye test | 20 | 7 | Eye test rate (£20) at Boots Opticians (as at 15 November 2011) |
| Day hospital | Half-day | 75 | 2 | Tab TDCFRAD: half the cost of day-care facilities for elderly patients (DCF20) |
| Social club | Session | 36 | 3 | Local authority day care for older people (p. 28) |
| Lunch club | Visit | 12 | 4 | Voluntary day care for older people. Meals cost £1.90 per client-day and other running costs amount to £38.90 per client-day. Assumes £1.90 per meal and one-quarter of other running costs (p. 55). 2009/10 costs uprated using PSS pay and prices inflation rate |
| Drop-in centre | Session | 36 | 4 | Local authority day care for older people (p. 28) |
| Meals on wheels | Meal | 6 | 3 | Weekly cost of £43, assume one meal per day (p. 103) |
| Frozen meals | Meal | 3 | 3 | Assume half the cost of meals on wheels |
| Home care worker | Hour | 24 | 3 | Local authority home-care worker per hour of face-to-face contact, weighted average accounting for different rates for day/evening/weekday/weekends (p. 160) |
| Social worker | Hour | 152 | 3 | Per hour of face-to-face contact. Excludes qualification costs (p. 156) |
| Social worker, telephone call | Call | 13 | 3 | Assume 15 minutes of social worker time based on £53 per hour of client-related work. Excludes qualification costs (p. 156) |
| Social services day-care centre | Visit | 12 | 3 | Local authority day care for older people (p. 28). Session cost £36. Assumed session lasts 3 hours to derive hourly cost of £12 |
| Intermediate care team | Contact | 15 | 3 | Based on a scheme for people having difficulty managing at home or who have been recently discharged from hospital or who are considering entry to a residential care home (p. 31). Weekly cost £427. Assumed quarter day contact |
| Value of time | ||||
| National average wage (opportunity cost) | Hour | 15 | 5 | Table 1.5a: hourly gross pay for all employees, UK, 2010. Mean, not affected by absence = £14.60 |
| Leisure time cost (opportunity cost) | Hour | 5 | 6 | Table 2: £4.80 per hour of non-working time per person (resource cost, non-commuting reasons); 2010 rate inflated to 2011 rate using PSS pay and prices inflation rate |
| Other services | ||||
| NHS Direct | Call | 16 | 10 | |
| SCC | ||||
| NHS Agenda for Change band 1 | Hourly cost/travel cost | 19/9 | 11 | Assuming: (a) 25.44% employer contribution to National Insurance and superannuation for NHS band 7 staff as per the unit cost for a nurse team manager (4). Includes travel/transport cost (b) 65.46% indirect and capital overheads for NHS band 7 staff as per the unit cost for a nurse team manager (2). Includes travel/transport cost (c) 41.3 weeks per annum (taking off 27 days annual leave, eight bank holidays, five study/training days and 12 sickness days) and a 37.5-hour working week as per the unit cost for a nurse team manager (4) (d) 30 minutes’ travel time for non-clinic contacts. Travel costs are included in staff overheads |
| NHS Agenda for Change band 2 | Hourly cost/travel cost | 20/10 | 11 | |
| NHS Agenda for Change band 3 | Hourly cost/travel cost | 23/11 | 11 | |
| NHS Agenda for Change band 4 | Hourly cost/travel cost | 27/13 | 11 | |
| NHS Agenda for Change band 5 | Hourly cost/travel cost | 32/16 | 11 | |
| NHS Agenda for Change band 6 | Hourly cost/travel cost | 39/20 | 11 | |
| NHS Agenda for Change band 7 | Hourly cost/travel cost | 47/24 | 11 | |
| NHS Agenda for Change BAND 8 | Hourly cost/travel cost | 78/39 | 11 | |
| Stroke MDT meetings | Hour | 284 | 4 | Assuming that the team consists of one each of a medical consultant, band 7 nurse, physiotherapist, occupational therapist and speech and language therapist. Based on unit costs for hospital-based staff, excluding qualification costs |
Appendix 8 Project 2: additional results
Pre-recruitment screening data
Data are from 26 (out of 32) randomised services and were collected before the recruitment of participants in these services.
| Demographic characteristics | Control (N = 730) | Intervention (N = 427) | Total (N = 1157) |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 73.6 (12.58) | 72.9 (12.72) | 73.4 (12.64) |
| Median (range) | 76.0 (29.0–101.0) | 75.0 (32.0–100.0) | 75.0 (29.0–101.0) |
| Missing | 13 | 3 | 16 |
| Gender, n (%) | |||
| Male | 372 (51.0) | 220 (51.5) | 592 (51.2) |
| Ethnicity, n (%) | |||
| White | 681 (93.3) | 411 (96.3) | 1092 (94.4) |
| Modified Rankin Scale score | Control (N = 730), n (%) | Intervention (N = 427), n (%) | Total (N = 1157), n (%) |
|---|---|---|---|
| 0 | 59 (8.1) | 15 (3.5) | 74 (6.4) |
| 1 | 114 (15.6) | 70 (16.4) | 184 (15.9) |
| 2 | 138 (18.9) | 91 (21.3) | 229 (19.8) |
| 3 | 169 (23.2) | 132 (30.9) | 301 (26.0) |
| 4 | 114 (15.6) | 73 (17.1) | 187 (16.2) |
| 5 | 38 (5.2) | 42 (9.8) | 80 (6.9) |
| Missing | 98 (13.4) | 4 (0.9) | 102 (8.8) |
Screening data: non-registered patients by service and treatment arm
| Site | Out of screened, n (%) | Reasons not assessed – out of not assessed, n (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Assessed | Not Assessed | Missed by clinical research team | Patient died | SCC referral withdrawn because of sudden acute illness | Transferred to another health-care setting | LoTS care trial closed to recruitment | Missing | |
| Control sites | ||||||||
| 1 | 78 (91.8) | 7 (8.2) | 7 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 176 (90.7) | 18 (9.3) | 1 (5.6) | 17 (94.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 85 (96.6) | 3 (3.4) | 3 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 8 | 49 (98.0) | 1 (2.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 10 | 62 (82.7) | 13 (17.3) | 12 (92.3) | 1 (7.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 11 | 59 (100.0) | 0 (0.0) | ||||||
| 14 | 127 (80.4) | 31 (19.6) | 23 (74.2) | 5 (16.1) | 1 (3.2) | 2 (6.5) | 0 (0.0) | 0 (0.0) |
| 15 | 149 (89.8) | 17 (10.2) | 16 (94.1) | 0 (0.0) | 1 (5.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 18 | 39 (100.0) | 0 (0.0) | ||||||
| 19 | 86 (97.7) | 2 (2.3) | 1 (50.0) | 0 (0.0) | 1 (50.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 22 | 57 (76.0) | 18 (24.0) | 13 (72.2) | 2 (11.1) | 3 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 25 | 22 (100.0 | 0 (0.0) | ||||||
| 29 | 99 (79.2) | 26 (20.8) | 24 (92.3) | 1 (3.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.8) |
| 32 | 93 (75.6) | 30 (24.4) | 28 (93.3) | 2 (6.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 1181 (87.7) | 166 (12.3) | 129 (77.7) | 28 (16.9) | 6 (3.6) | 2 (1.2) | 0 (0.0) | 1 (0.6) |
| Intervention sites | ||||||||
| 2 | 67 (89.3) | 8 (10.7) | 8 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 76 (82.6) | 16 (17.4) | 16 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 47 (85.5) | 8 (14.5) | 6 (75.0) | 2 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 96 (99.0) | 1 (1.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 9 | 53 (82.8) | 11 (17.2) | 11 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 12 | 88 (94.6) | 5 (5.4) | 4 (80.0) | 1 (20.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 13 | 88 (95.7) | 4 (4.3) | 3 (75.0) | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 16 | 59 (88.1) | 8 (11.9) | 4 (50.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (50.0) | 0 (0.0) |
| 20 | 3 (100.0) | 0 (0.0) | ||||||
| 23 | 24 (80.0) | 6 (20.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 4 (66.7) | 1 (16.7) | 0 (0.0) |
| 24 | 46 (62.2) | 28 (37.8) | 27 (96.4) | 1 (3.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 26 | 171 (93.4) | 12 (6.6) | 8 (66.7) | 4 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 27 | 35 (94.6) | 2 (5.4) | 1 (50.0) | 0 (0.0) | 1 (50.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 30 | 61 (100.0) | 0 (0.0) | ||||||
| 31 | 97 (82.2) | 21 (17.8) | 16 (76.2) | 0 (0.0) | 0 (0.0) | 4 (19.0) | 0 (0.0) | 1 (4.8) |
| Total | 1011 (88.6) | 130 (11.4) | 105 (80.8) | 9 (6.9) | 2 (1.5) | 8 (6.2) | 5 (3.8) | 1 (0.8) |
| Site | Out of assessed, n (%) | Reasons not eligible – out of not eligible, n (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eligible | Not eligible | No primary diagnosis of stroke | Not referred to a SCC on discharge or within 6 weeks of stroke | Had first home or outpatient SCC assessment | Unlikely to survive > 3 months | Nursing or residential care | Previously registered into the LoTS care trial | In another study overlapping with the LoTS care trial | Discharged out of SCC area | Reason not known | Other | Missing | |
| Control sites | |||||||||||||
| 1 | 40 (51.3) | 38 (48.7) | 2 (5.3) | 3 (7.9) | 0 (0.0) | 7 (18.4) | 13 (34.2) | 0 (0.0) | 0 (0.0) | 13 (34.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 | 98 (55.7) | 78 (44.3) | 2 (2.6) | 1 (1.3) | 0 (0.0) | 19 (24.4) | 30 (38.5) | 0 (0.0) | 25 (32.1) | 1 (1.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 54 (63.5) | 31 (36.5) | 16 (51.6) | 0 (0.0) | 3 (9.7) | 2 (6.5) | 6 (19.4) | 0 (0.0) | 0 (0.0) | 3 (9.7) | 1 (3.2) | 0 (0.0) | 0 (0.0) |
| 8 | 45 (91.8) | 4 (8.2) | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (75.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 10 | 30 (48.4) | 32 (51.6) | 3 (9.4) | 0 (0.0) | 0 (0.0) | 2 (6.3) | 25 (78.1) | 0 (0.0) | 0 (0.0) | 2 (6.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 11 | 57 (96.6) | 2 (3.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (50.0) | 1 (50.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 14 | 96 (75.6) | 31 (24.4) | 12 (38.7) | 0 (0.0) | 0 (0.0) | 7 (22.6) | 1 (3.2) | 0 (0.0) | 0 (0.0) | 8 (25.8) | 3 (9.7) | 0 (0.0) | 0 (0.0) |
| 15 | 86 (57.7) | 63 (42.3) | 20 (31.7) | 1 (1.6) | 0 (0.0) | 10 (15.9) | 19 (30.2) | 0 (0.0) | 2 (3.2) | 11 (17.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 18 | 39 (100.0) | 0 (0.0) | |||||||||||
| 19 | 61 (70.9) | 25 (29.1) | 5 (20.0) | 1 (4.0) | 1 (4.0) | 2 (8.0) | 15 (60.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (4.0) | 0 (0.0) | 0 (0.0) |
| 22 | 42 (73.7) | 15 (26.3) | 2 (13.3) | 0 (0.0) | 1 (6.7) | 1 (6.7) | 7 (46.7) | 0 (0.0) | 2 (13.3) | 1 (6.7) | 1 (6.7) | 0 (0.0) | 0 (0.0) |
| 25 | 22 (100.0) | 0 (0.0) | |||||||||||
| 29 | 54 (54.5) | 45 (45.5) | 3 (6.7) | 4 (8.9) | 0 (0.0) | 3 (6.7) | 24 (53.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 10 (22.2) | 0 (0.0) | 1 (2.2) |
| 32 | 35 (37.6) | 58 (62.4) | 3 (5.2) | 2 (3.4) | 0 (0.0) | 0 (0.0) | 27 (46.6) | 0 (0.0) | 0 (0.0) | 26 (44.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 759 (64.3) | 422 (35.7) | 69 (16.4) | 12 (2.8) | 5 (1.2) | 54 (12.8) | 171 (40.5) | 0 (0.0) | 29 (6.9) | 65 (15.4) | 16 (3.8) | 0 (0.0) | 1 (0.2) |
| Intervention sites | |||||||||||||
| 2 | 38 (56.7) | 29 (43.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (10.3) | 21 (72.4) | 0 (0.0) | 0 (0.0) | 2 (6.9) | 3 (10.3) | 0 (0.0) | 0 (0.0) |
| 5 | 43 (56.6) | 33 (43.4) | 5 (15.2) | 0 (0.0) | 0 (0.0) | 6 (18.2) | 22 (66.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 37 (78.7) | 10 (21.3) | 1 (10.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (40.0) | 0 (0.0) | 1 (10.0) | 4 (40.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 55 (57.3) | 41 (42.7) | 23 (56.1) | 1 (2.4) | 5 (12.2) | 4 (9.8) | 8 (19.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 9 | 33 (62.3) | 20 (37.7) | 8 (40.0) | 0 (0.0) | 0 (0.0) | 2 (10.0) | 10 (50.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 12 | 72 (81.8) | 16 (18.2) | 2 (12.5) | 0 (0.0) | 0 (0.0) | 2 (12.5) | 11 (68.8) | 0 (0.0) | 0 (0.0) | 1 (6.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 13 | 57 (64.8) | 31 (35.2) | 5 (16.1) | 0 (0.0) | 0 (0.0) | 3 (9.7) | 9 (29.0) | 0 (0.0) | 0 (0.0) | 1 (3.2) | 13 (41.9) | 0 (0.0) | 0 (0.0) |
| 16 | 47 (79.7) | 12 (20.3) | 1 (8.3) | 0 (0.0) | 0 (0.0) | 2 (16.7) | 5 (41.7) | 0 (0.0) | 0 (0.0) | 3 (25.0) | 1 (8.3) | 0 (0.0) | 0 (0.0) |
| 20 | 2 (66.7) | 1 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 23 | 23 (95.8) | 1 (4.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0 | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 24 | 44 (95.7) | 2 (4.3) | 1 (50.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (50.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 26 | 71 (41.5) | 100 (58.5) | 37 (37.0) | 1 (1.0) | 0 (0.0) | 20 (20.0) | 37 (37.0) | 1 (1.0) | 0 (0.0) | 3 (3.0) | 0 (0.0) | 1 (1.0) | 0 (0.0) |
| 27 | 33 (94.3) | 2 (5.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (50.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (50.0) | 0 (0.0) | 0 (0.0) |
| 30 | 46 (75.4) | 15 (24.6) | 2 (13.3) | 0 (0.0) | 0 (0.0) | 2 (13.3) | 8 (53.3) | 0 (0.0) | 0 (0.0) | 3 (20.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 31 | 60 (61.9) | 37 (38.1) | 3 (8.1) | 0 (0.0) | 0 (0.0) | 2 (5.4) | 21 (56.8) | 0 (0.0) | 2 (5.4) | 9 (24.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 661 (65.4) | 350 (34.6) | 88 (25.1) | 2 (0.6) | 5 (1.4) | 46 (13.1) | 159 (45.4) | 1 (0.3) | 3 (0.9) | 27 (7.7) | 18 (5.1) | 1 (0.3) | 0 (0.0) |
| Site | Out of eligible, n (%) | Reasons not consented – out of not consented, n (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Consented | Not consented | Patient refused | Consultee declaration refused | Patient died | Patient does not have the capacity to provide consent and no consultee | Patient not approached/deemed unsuitable for this study | Does not speak English | Missed by clinical research team | Unable to complete follow-up questionnaires | Other | Missing | |
| Control sites | ||||||||||||
| 1 | 17 (42.5) | 23 (57.5) | 10 (43.5) | 5 (21.7) | 0 (0.0) | 6 (26.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (8.7) |
| 3 | 19 (19.4) | 79 (80.6) | 50 (63.3) | 5 (6.3) | 0 (0.0) | 11 (13.9) | 6 (7.6) | 3 (3.8) | 0 (0.0) | 4 (5.1) | 0 (0.0) | 0 (0.0) |
| 4 | 32 (59.3) | 22 (40.7) | 16 (72.7) | 1 (4.5) | 0 (0.0) | 4 (18.2) | 0 (0.0) | 1 (4.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 8 | 28 (62.2) | 17 (37.8) | 10 (58.8) | 1 (5.9) | 0 (0.0) | 6 (35.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 10 | 18 (60.0) | 12 (40.0) | 6 (50.0) | 0 (0.0) | 3 (25.0) | 3 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 11 | 46 (80.7) | 11 (19.3) | 6 (54.5) | 1 (9.1) | 0 (0.0) | 4 (36.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 14 | 32 (33.3) | 64 (66.7) | 42 (65.6) | 2 (3.1) | 0 (0.0) | 8 (12.5) | 2 (3.1) | 1 (1.6) | 6 (9.4) | 1 (1.6) | 2 (3.1) | 0 (0.0) |
| 15 | 50 (58.1) | 36 (41.9) | 30 (83.3) | 1 (2.8) | 0 (0.0) | 2 (5.6) | 0 (0.0) | 1 (2.8) | 0 (0.0) | 2 (5.6) | 0 (0.0) | 0 (0.0) |
| 18 | 36 (92.3) | 3 (7.7) | 3 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 19 | 46 (75.4) | 15 (24.6) | 12 (80.0) | 0 (0.0) | 0 (0.0) | 3 (20.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 22 | 23 (54.8) | 19 (45.2) | 7 (36.8) | 4 (21.1) | 0 (0.0) | 6 (31.6) | 2 (10.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 25 | 20 (90.9) | 2 (9.1) | 2 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 29 | 42 (77.8) | 12 (22.2) | 7 (58.3) | 3 (25.0) | 0 (0.0) | 2 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 32 | 17 (48.6) | 18 (51.4) | 15 (83.3) | 0 (0.0) | 0 (0.0) | 3 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 426 (56.1) | 333 (43.9) | 216 (64.9) | 23 (6.9) | 3 (0.9) | 58 (17.4) | 10 (3.0) | 6 (1.8) | 6 (1.8) | 7 (2.1) | 2 (0.6) | 2 (0.6) |
| Intervention sites | ||||||||||||
| 2 | 28 (73.7) | 10 (26.3) | 9 (90.0) | 1 (10.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 19 (44.2) | 24 (55.8) | 12 (50.0) | 0 (0.0) | 4 (16.7) | 8 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 31 (83.8) | 6 (16.2) | 2 (33.3) | 2 (33.3) | 0 (0.0) | 2 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 34 (61.8) | 21 (38.2) | 12 (57.1) | 1 (4.8) | 0 (0.0) | 7 (33.3) | 0 (0.0) | 1 (4.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 9 | 17 (51.5) | 16 (48.5) | 11 (68.8) | 0 (0.0) | 0 (0.0) | 5 (31.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 12 | 45 (62.5) | 27 (37.5) | 23 (85.2) | 2 (7.4) | 0 (0.0) | 2 (7.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 13 | 46 (80.7) | 11 (19.3) | 10 (90.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (9.1) | 0 (0.0) | 0 (0.0) |
| 16 | 27 (57.4) | 20 (42.6) | 12 (60.0) | 4 (20.0) | 2 (10.0) | 1 (5.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.0) | 0 (0.0) |
| 20 | 2 (100.0) | 0 (0.0) | ||||||||||
| 23 | 14 (60.9) | 9 (39.1) | 5 (55.6) | 0 (0.0) | 0 (0.0) | 2 (22.2) | 0 (0.0) | 0 (0.0) | 2 (22.2) | 0 (0.0) | 0 (0.0 | 0 (0.0) |
| 24 | 27 (61.4) | 17 (38.6) | 14 (82.4) | 0 (0.0) | 0 (0.0) | 1 (5.9) | 0 (0.0) | 2 (11.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 26 | 45 (63.4) | 26 (36.6) | 11 (42.3) | 0 (0.0) | 0 (0.0) | 3 (11.5) | 6 (23.1) | 0 (0.0) | 3 (11.5) | 2 (7.7) | 0 (0.0) | 1 (3.8) |
| 27 | 27 (81.8) | 6 (18.2) | 5 (83.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) |
| 30 | 33 (71.7) | 13 (28.3) | 8 (61.5) | 0 (0.0) | 4 (30.8) | 1 (7.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 31 | 18 (30.0) | 42 (70.0) | 9 (21.4) | 2 (4.8) | 0 (0.0) | 13 (31.0) | 8 (19.0) | 0 (0.0) | 9 (21.4) | 1 (2.4) | 0 (0.0) | 0 (0.0) |
| Total | 413 (62.5) | 248 (37.5) | 143 (57.7) | 12 (4.8) | 10 (4.0) | 45 (18.1) | 14 (5.6) | 3 (1.2) | 14 (5.6) | 4 (1.6) | 2 (0.8) | 1 (0.4) |
| Site | Out of consented, n (%) | Reasons not registered – out of not registered, n (%) | ||||
|---|---|---|---|---|---|---|
| Registered | Not registered | Baseline forms not completed/retrieved | Patient died | No longer eligible | Missing | |
| Control sites | ||||||
| 1 | 15 (88.2) | 2 (11.8) | 0 (0.0) | 0 (0.0) | 2 (100.0) | 0 (0.0) |
| 3 | 18 (94.7) | 1 (5.3) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 | 32 (100.0) | 0 (0.0) | ||||
| 8 | 25 (89.3) | 3 (10.7) | 3 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 10 | 17 (94.4) | 1 (5.6) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 11 | 45 (97.8) | 1 (2.2) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 14 | 29 (90.6) | 3 (9.4) | 2 (66.7) | 1 (33.3) | 0 (0.0) | 0 (0.0) |
| 15 | 4 (92.0) | 4 (8.0) | 4 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 18 | 35 (97.2) | 1 (2.8) | 0 (0.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) |
| 19 | 45 (97.8) | 1 (2.2) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 22 | 19 (82.6) | 4 (17.4) | 4 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 25 | 18 (90.0) | 2 (10.0) | 0 (0.0) | 0 (0.0) | 1 (50.0) | 1 (50.0) |
| 29 | 41 (97.6) | 1 (2.4) | 0 (0.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) |
| 32 | 14 (82.4) | 3 (17.6) | 2 (66.7) | 0 (0.0) | 1 (33.3) | 0 (0.0) |
| Total | 399 (93.7) | 27 (6.3) | 19 (70.4) | 1 (3.7) | 6 (22.2) | 1 (3.7) |
| Intervention sites | ||||||
| 2 | 27 (96.4) | 1 (3.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) |
| 5 | 19 (100.0) | 0 (0.0) | ||||
| 6 | 30 (96.8) | 1 (3.2) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 | 31 (91.2) | 3 (8.8) | 2 (66.7) | 0 (0.0) | 1 (33.3) | 0 (0.0) |
| 9 | 17 (100.0) | 0 (0.0) | ||||
| 12 | 45 (100.0) | 0 (0.0) | ||||
| 13 | 45 (97.8) | 1 (2.2) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 16 | 27 (100.0) | 0 (0.0) | ||||
| 20 | 2 (100.0) | 0 (0.0) | ||||
| 23 | 13 (92.9) | 1 (7.1) | 1 (100.0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 24 | 27 (100.0) | 0 (0.0) | ||||
| 26 | 45 (100.0) | 0 (0.0) | ||||
| 27 | 26 (96.3) | 1 (3.7) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 30 | 29 (87.9) | 4 (12.1) | 1 (25.0) | 0 (0.0) | 3 (75.0) | 0 (0.0) |
| 31 | 18 (100.0) | 0 (0.0) | ||||
| Total | 401 (97.1) | 12 (2.9) | 7 (58.3) | 0 (0.0) | 4 (33.3) | 1 (8.3) |
Patients with carers: baseline characteristics
| Demographic characteristics | Control (N = 100), n (%) | Intervention (N = 108), n (%) | Total (N = 208), n (%) |
|---|---|---|---|
| Patient age (years) | |||
| Mean (SD) | 72.8 (11.51) | 72.5 (12.06) | 72.6 (11.77) |
| Median (range) | 73.1 (38.3–98.8) | 75.1 (28.3–95.7) | 74.5 (28.3–98.8) |
| Missing | 0 | 0 | 0 |
| Ethnicity | |||
| White | 97 (97.0) | 103 (95.4) | 200 (96.2) |
| Mixed – white and black Caribbean | 0 (0.0) | 1 (0.9) | 1 (0.5) |
| Asian – Indian | 0 (0.0) | 1 (0.9) | 1 (0.5) |
| Asian – Pakistani | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Asian – Bangladeshi | 0 (0.0) | 1 (0.9) | 1 (0.5) |
| Other Asian background | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Black – Caribbean | 0 (0.0) | 1 (0.9) | 1 (0.5) |
| Other black background | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Other ethnic group | 0 (0.0) | 1 (0.9) | 1 (0.5) |
| Gender | |||
| Male | 58 (58.0) | 58 (53.7) | 116 (55.8) |
| Female | 42 (42.0) | 50 (46.3) | 92 (44.2) |
| Education/employment | Control (N = 100), n (%) | Intervention (N = 108), n (%) | Total (N = 208), n (%) |
|---|---|---|---|
| Has the patient had any formal education? | |||
| Yes | 97 (97.0) | 99 (91.7) | 196 (94.2) |
| No | 2 (2.0) | 9 (8.3) | 11 (5.3) |
| Missing | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| If yes, at what age did the patient leave education? | |||
| ≤ 16 years | 75 (77.3) | 83 (83.8) | 158 (80.6) |
| 17–20 years | 20 (20.6) | 11 (11.1) | 31 (15.8) |
| ≥ 21 years | 1 (1.0) | 4 (4.0) | 5 (2.6) |
| Unknown | 1 (1.0) | 1 (1.0) | 2 (1.0) |
| If yes, has the patient received more education since leaving school? | |||
| Yes | 26 (26.8) | 38 (38.4) | 64 (32.7) |
| No | 64 (66.0) | 60 (60.6) | 124 (63.3) |
| Missing | 7 (7.2) | 1 (1.0) | 8 (4.1) |
| Main employment before stroke | |||
| Working full time (≥ 30 hours per week) | 14 (14.0) | 12 (11.1) | 26 (12.5) |
| Working part time (< 30 hours per week) | 3 (3.0) | 3 (2.8) | 6 (2.9) |
| At home and not looking for work (e.g. looking after home/family) | 6 (6.0) | 3 (2.8) | 9 (4.3) |
| Unemployed and looking for work | 2 (2.0) | 1 (0.9) | 3 (1.4) |
| Retired | 72 (72.0) | 84 (77.8) | 156 (75.0) |
| Made redundant/took early retirement | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Unable to work (for medical and/or other reasons) | 2 (2.0) | 5 (4.6) | 7 (3.4) |
| Stroke characteristics | Control (N = 100), n (%) | Intervention (N = 108), n (%) | Total (N = 208), n (%) |
|---|---|---|---|
| Source of referral to the SCC | |||
| Before hospital discharge (including community hospital) | 94 (94.0) | 100 (92.6) | 194 (93.3) |
| After discharge form hospital (including community hospital) | 6 (6.0) | 7 (6.5) | 13 (6.3) |
| Missing | 0 (0.0) | 1 (0.9) | 1 (0.5) |
| Pathological classification of current stroke | |||
| Cerebral infarction | 81 (81.0) | 95 (88.0) | 176 (84.6) |
| Primary intracerebral haemorrhage | 12 (12.0) | 12 (11.1) | 24 (11.5) |
| Other | 7 (7.0) | 1 (0.9) | 8 (3.8) |
| Clinical classification of stroke symptoms | |||
| Left hemiparesis | 47 (47.0) | 41 (38.0) | 88 (42.3) |
| Right hemiparesis | 42 (42.0) | 51 (47.2) | 93 (44.7) |
| Brain stem | 4 (4.0) | 4 (3.7) | 8 (3.8) |
| Other | 7 (7.0) | 12 (11.1) | 19 (9.1) |
| Has the patient had a previous stroke? | |||
| Yes | 17 (17.0) | 28 (25.9) | 45 (21.6) |
| No | 81 (81.0) | 80 (74.1) | 161 (77.4) |
| Missing | 2 (2.0) | 0 (0.0) | 2 (1.0) |
| Ability after stroke | Control (N = 100), n (%) | Intervention (N = 108), n (%) | Total (N = 208), n (%) |
|---|---|---|---|
| Can the patient talk and are they orientated? | |||
| Yes | 92 (92.0) | 93 (86.1) | 185 (88.9) |
| No | 8 (8.0) | 14 (13.0) | 22 (10.6) |
| Missing | 0 (0.0) | 1 (0.9) | 1 (0.5) |
| Can the patient lift both arms of the bed? | |||
| Yes | 74 (74.0) | 80 (74.1) | 154 (74.0) |
| No | 26 (26.0) | 28 (25.9) | 54 (26.0) |
| Can the patient walk without help from others? | |||
| Yes | 56 (56.0) | 50 (46.3) | 106 (51.0) |
| No | 44 (44.0) | 58 (53.7) | 102 (49.0) |
| Is patient’s language ability normal? | |||
| Yes | 76 (76.0) | 72 (66.7) | 148 (71.2) |
| No | 24 (24.0) | 36 (33.3) | 60 (28.8) |
| If not, does the patient have dysphasia? | |||
| Yes | 18 (75.0) | 31 (86.1) | 49 (81.7) |
| No | 6 (25.0) | 5 (13.9) | 11 (18.3) |
| If the patient has dysphasia, what type of dysphasia? | |||
| Receptive | 2 (11.1) | 0 (0.0) | 2 (4.1) |
| Expressive | 11 (61.1) | 23 (74.2) | 34 (69.4) |
| Global | 3 (16.7) | 1 (3.2) | 4 (8.2) |
| Missing | 2 (11.1) | 7 (22.6) | 9 (18.4) |
| If not, does the patient have dysarthria? | |||
| Yes | 8 (33.3) | 7 (19.4) | 15 (25.0) |
| No | 16 (66.7) | 29 (80.6) | 45 (75.0) |
| Was the patient able to answer the 6CIT? | |||
| Yes | 85 (85.0) | 81 (75.0) | 166 (79.8) |
| No | 15 (15.0) | 27 (25.0) | 42 (20.2) |
| If not, why not? | |||
| Stroke-related communication problems | 12 (80.0) | 22 (81.5) | 34 (81.0) |
| Other | 3 (20.0) | 5 (18.5) | 8 (19.0) |
| Inpatient stay | Control (N = 100), n (%) | Intervention (N = 108), n (%) | Total (N = 208), n (%) |
|---|---|---|---|
| Overall time from admission to discharge for patients with a hospital stay related to this stroke incident (days) | |||
| Mean (SD) | 42.2 (42.0) | 51.6 (49.8) | 47.1 (46.3) |
| Median (range) | 29 (2–181) | 37 (1–220) | 33 (1–220) |
| Missing | 0 | 0 | 0 |
| n | 100 | 108 | 208 |
Follow-up
Categories in these tables are mutually exclusive and their content contains information that explains the CONSORT diagram in more detail.
| Questionnaire completion | Control (N = 399), n (%) | Intervention (N = 401), n (%) | Total (N = 800), n (%) |
|---|---|---|---|
| Primary end point questionnaires | |||
| Questionnaire completed | 294 (73.7) | 308 (76.8) | 602 (75.3) |
| Completed primary outcome call | 6 (1.5) | 2 (0.5) | 8 (1.0) |
| Patient died | 23 (5.8) | 30 (7.5) | 53 (6.6) |
| Patient withdrew | 18 (4.5) | 4 (1.0) | 22 (2.8) |
| Questionnaire returned after cut-off point | 5 (1.3) | 8 (2.0) | 13 (1.6) |
| Questionnaire returned but too many missing items | 11 (2.8) | 8 (2.0) | 19 (2.4) |
| Questionnaire not returned | 42 (10.5) | 41 (10.2) | 83 (10.4) |
| Reason questionnaire not returned | |||
| Too poorly | 4 (1.0) | 2 (0.5) | 6 (0.8) |
| Could not get hold of participant | 22 (5.5) | 22 (5.5) | 44 (5.5) |
| Confirmed received questionnaire and would return | 6 (1.5) | 6 (1.5) | 12 (1.5) |
| No recollection of participation | 0 (0.0) | 2 (0.5) | 2 (0.3) |
| Other | 10 (2.5) | 9 (2.2) | 19 (2.4) |
| Questionnaire completion | Control (N = 399), n (%) | Intervention (N = 401), n (%) | Total (N = 800), n (%) |
|---|---|---|---|
| Patient GHQ-12 questionnaire | |||
| Questionnaire completed | 265 (66.4) | 280 (69.8) | 545 (68.1) |
| Completed primary outcome call | 3 (0.8) | 1 (0.2) | 4 (0.5) |
| Patient died | 32 (8.0) | 35 (8.7) | 67 (8.4) |
| Patient withdrew | 34 (8.5) | 12 (3.0) | 46 (5.8) |
| Questionnaire returned but too many missing items | 3 (0.8) | 6 (1.5) | 9 (1.1) |
| Questionnaire not returned | 62 (15.5) | 67 (16.7) | 129 (16.1) |
| Reason questionnaire not returned | |||
| Too poorly | 3 (0.8) | 3 (0.7) | 6 (0.8) |
| Could not get hold of participant | 29 (7.3) | 35 (8.7) | 64 (8.0) |
| Confirmed received questionnaire and would return | 9 (2.3) | 10 (2.5) | 19 (2.4) |
| No time to complete the questionnaire | 0 (0.0) | 1 (0.2) | 1 (0.1) |
| No recollection of participation | 0 (0.0) | 1 (0.2) | 1 (0.1) |
| Other | 21 (5.3) | 17 (4.2) | 38 (4.8) |
| Questionnaire completion | Control (N = 100), n (%) | Intervention (N = 108), n (%) | Total (N = 208), n (%) |
|---|---|---|---|
| Carer GHQ-12 questionnaire | |||
| Questionnaire completed | 82 (82.0) | 80 (74.1) | 162 (77.9) |
| Patient or caregiver died | 6 (6.0) | 9 (8.3) | 15 (7.2) |
| Patient or caregiver withdrew | 3 (3.0) | 0 (0.0) | 3 (1.4) |
| Questionnaire not returned | 9 (9.0) | 19 (17.6) | 28 (13.5) |
| Reason questionnaire not returned | |||
| Could not get hold of participant | 3 (3.0) | 6 (5.6) | 9 (4.3) |
| Confirmed received questionnaire and would return | 2 (2.0) | 6 (5.6) | 8 (3.8) |
| Other | 4 (4.0) | 7 (6.5) | 11 (5.3) |
| Questionnaire completion | Control (N = 100), n (%) | Intervention (N = 108), n (%) | Total (N = 208), n (%) |
|---|---|---|---|
| Carer GHQ-12 questionnaire | |||
| Questionnaire completed | 71 (71.0) | 73 (67.6) | 144 (69.2) |
| Patient or caregiver died | 11 (11.0) | 11 (10.2) | 22 (10.6) |
| Patient or caregiver withdrew | 8 (8.0) | 3 (2.8) | 11 (5.3) |
| Questionnaire not returned | 10 (10.0) | 21 (19.4) | 31 (14.9) |
| Reason questionnaire not returned | |||
| Could not get hold of participant | 4 (4.0) | 8 (7.4) | 12 (5.8) |
| Confirmed received questionnaire and would return | 2 (2.0) | 8 (7.4) | 10 (4.8) |
| Other | 4 (4.0) | 5 (4.6) | 9 (4.3) |
Patient-reported outcomes
FIGURE 19.
Time between registration and receiving questionnaires: patients.
Carer-reported outcomes
FIGURE 20.
Time between registration and receiving questionnaires: carers. Carer registration number is the same as the registration number of their patient.
Withdrawals
FIGURE 21.
Time between registration and patient withdrawal.
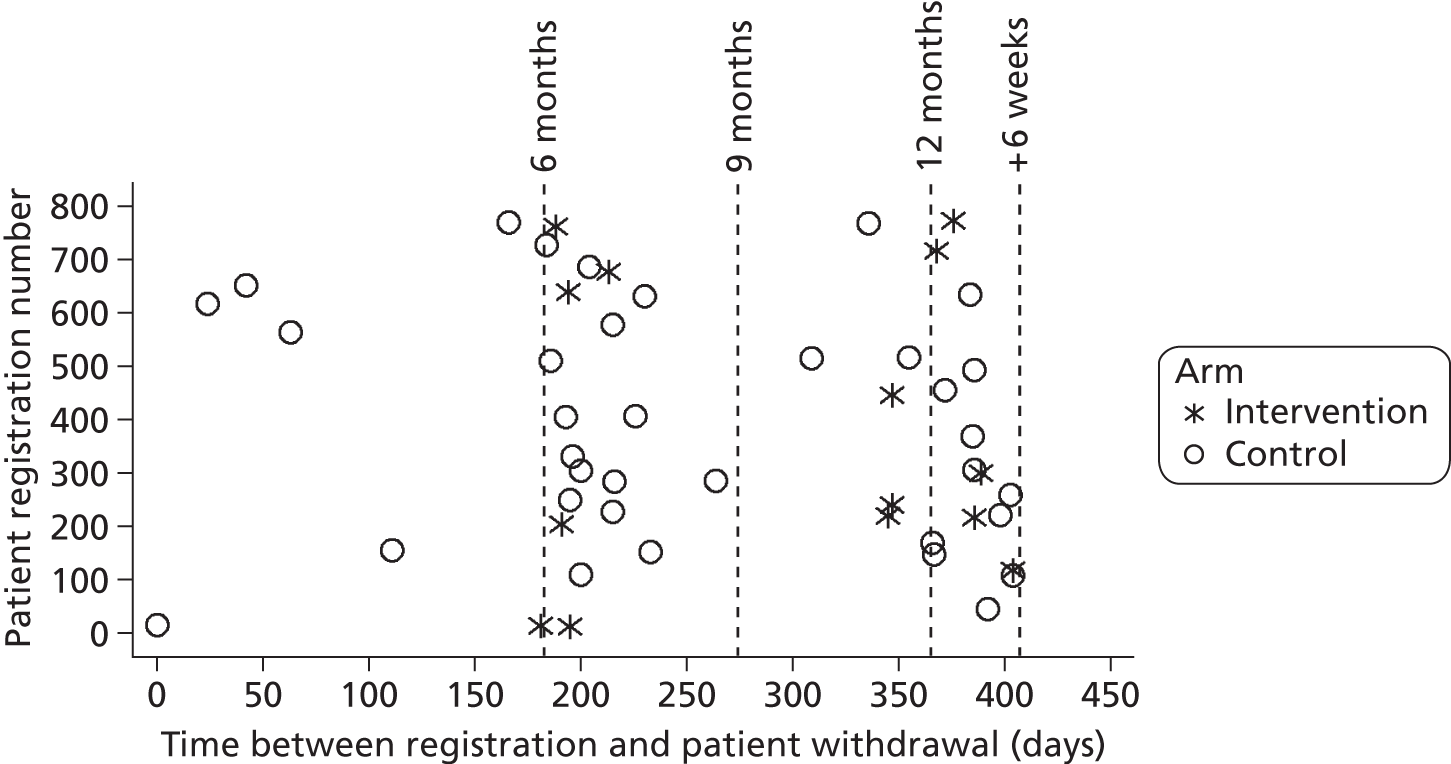
FIGURE 22.
Time between registration and carer withdrawal. Carer registration number is the same as the registration number of their patient.
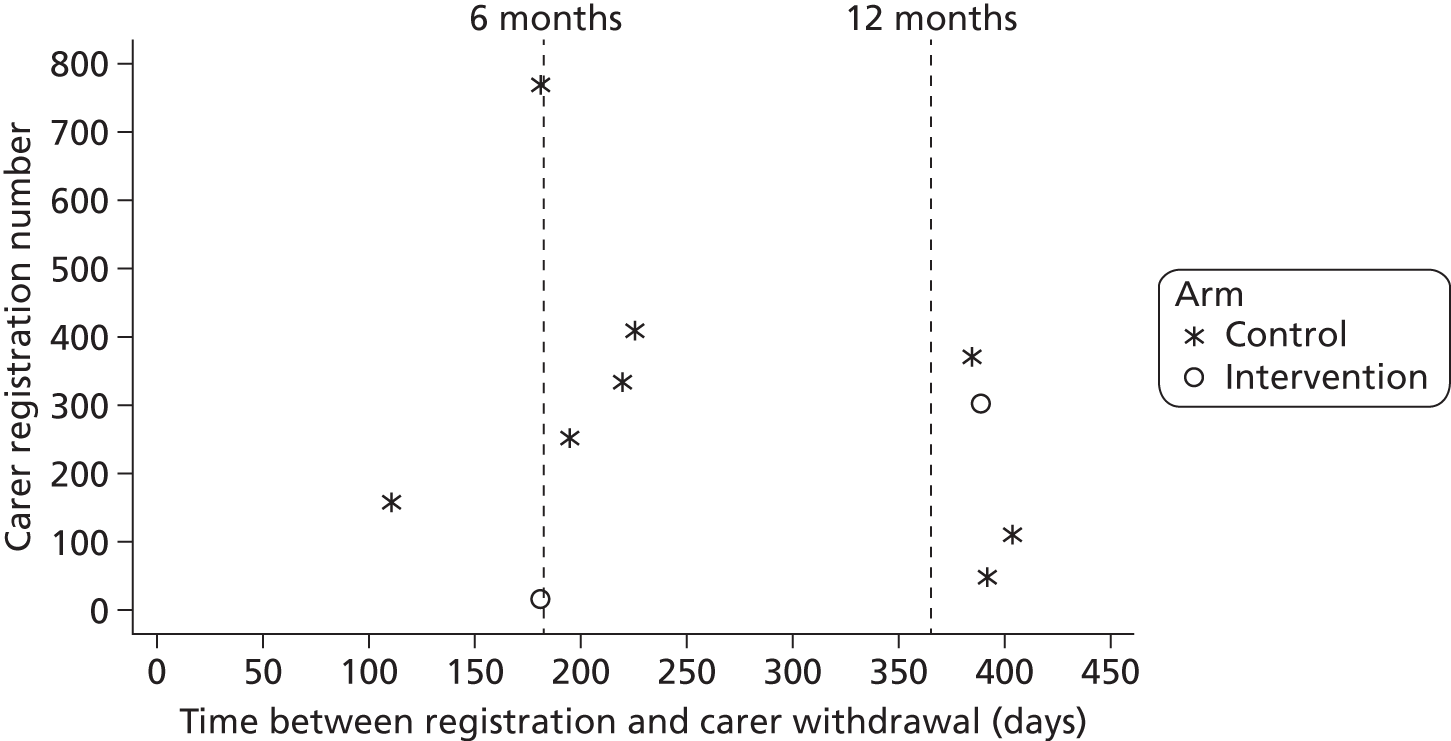
Appendix 9 Project 2: implementation of control and intervention services
Differences in delivery of the intervention between services
Quantitative data to describe delivery of the intervention (see Chapter 3, Process data: implementation of control and intervention services) were transcribed from the care plans by two researchers. During this process, informal observations were made on the way that the care plans were completed. It was observed that, in some services, care plans were clearly different from patient to patient and gave an impression of the patients’ personalities, overall situations and priorities. Each patient had a unique combination of problems identified in the assessment and addressed in the goal and action planner and individualised goals and actions. This can be illustrated by the wide range of problem areas addressed in the goal and action planner (Figure 23a). In other services, care plans were more uniform, being difficult to distinguish from one patient to another, and it appeared that the SCC was focusing the assessment on certain areas for all patients, despite asking questions in all of the assessment areas. Patients tended to have a similar range of problems identified in the assessment and addressed in the goal and action planner and goals and actions were more standardised. This is illustrated by the problems addressed in the goal and action planner being clustered in a limited number of areas (Figure 23b). In this example, the majority of problems addressed relate to medicines and general health (assessment area 3), including medications, blood pressure, smoking cessation and reduction of alcohol consumption. When services focused on certain areas, these areas were general health/secondary prevention, mobility and/or personal and domestic activities of daily living. Such variations in the apparent needs of patients between services appeared to be greater than would be likely from any variations in case mix.
FIGURE 23.
Problem areas addressed in the goal and action planner at contact one in two example services compared with all patients. (a) Example of a service (S013) addressing a wide range of problem areas; and (b) example of a service (S007) addressing a limited range of problem areas.
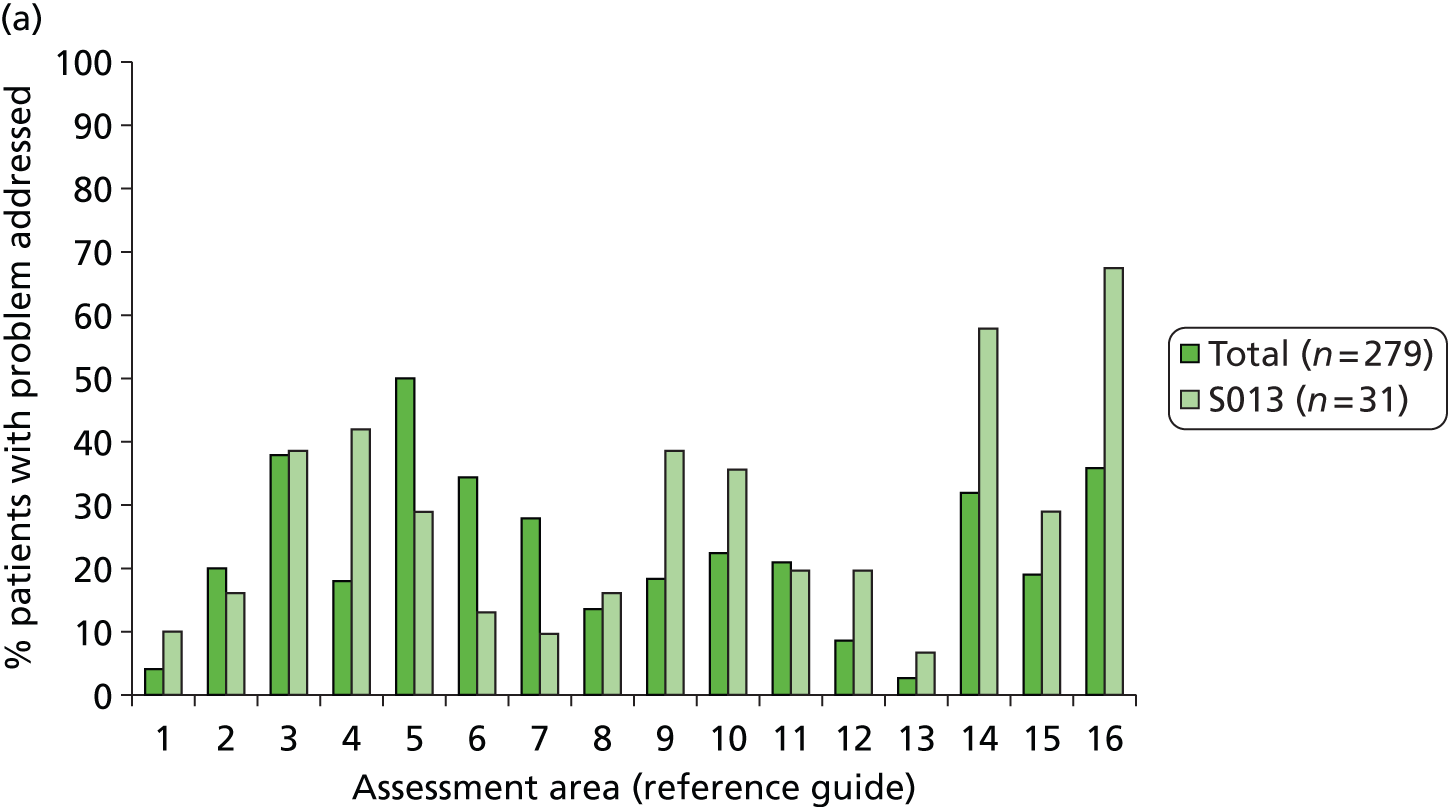
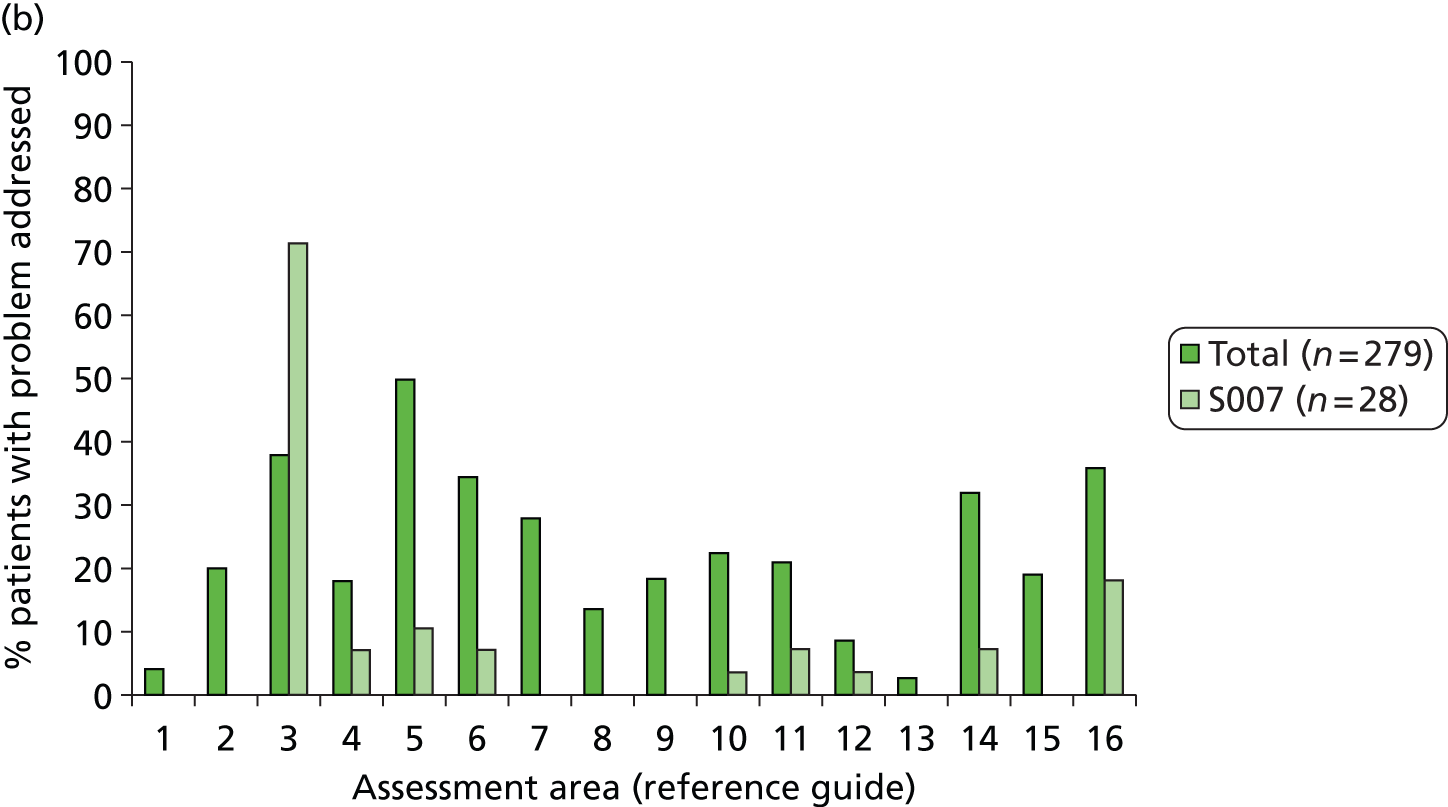
These observations have several possible interpretations relating to how the intervention has been delivered:
-
Differences in the extent to which the assessment was patient led and holistic and based on a collaborative discussion with the patient.
-
Differences in the approach to documentation. The implications of this are dependent on how and by whom the document is subsequently used in the service.
-
The assessment and follow-up may be limited by boundaries of the SCC role, for example SCC responsibility may end with an onward referral to a therapist, who is then the one to set patient-centred goals.
-
It is possible that problems are identified and addressed only in areas in which a service is available to meet the need, depending on existing processes and the availability of onward referrals and resources. For example, mood may be addressed only if a psychology service is available.
-
Specific areas of the assessment may be focused on as a result of local priorities and commissioner requirements. Such requirements may also dictate the number of contacts that a SCC has with a patient, with some services having only one contact with the majority of patients (Table 80).
-
The assessment is implemented by the SCC using existing knowledge, skills and experience; therefore, the areas focused on may be influenced by familiarity with and expertise in those areas.
| Service | Mean (SD) | Median (range) |
|---|---|---|
| S002 (n = 19) | 1.6 (0.60) | 2.0 (1.0–3.0) |
| S005 (n = 15) | 1.3 (0.49) | 1.0 (1.0–2.0) |
| S006 (n = 26) | 1.0 (0.20) | 1.0 (1.0–2.0) |
| S007 (n = 28) | 2.2 (0.79) | 2.0 (1.0–4.0) |
| S009 (n = 16) | 1.6 (1.09) | 1.0 (1.0–5.0) |
| S012 (n = 24) | 1.8 (0.72) | 2.0 (1.0–4.0) |
| S013 (n = 31) | 3.0 (1.02) | 3.0 (1.0–5.0) |
| S016 (n = 19) | 3.2 (1.62) | 3.0 (1.0–6.0) |
| S020 (n = 1) | 2.0 | 2.0 (2.0–2.0) |
| S023 (n = 11) | 3.2 (1.08) | 4.0 (1.0–4.0) |
| S024 (n = 10) | 1.9 (0.57) | 2.0 (1.0–3.0) |
| S026 (n = 29) | 2.5 (0.91) | 3.0 (1.0–4.0) |
| S027 (n = 26) | 1.8 (0.75) | 2.0 (1.0–4.0) |
| S030 (n = 10) | 3.9 (1.79) | 4.5 (1.0–6.0) |
| S031 (n = 14) | 1.4 (0.50) | 1.0 (1.0–2.0) |
| Total (n = 279) | 2.1 (1.16) | 2.0 (1.0–6.0) |
Description of control and intervention services based on stroke care co-ordinator interviews and surveys
For all services randomised, SCCs completed a survey and semistructured interview before randomisation. A second semistructured interview was conducted with SCCs in all services that recruited participants (29 services, 31 SCCs), midway through recruitment. A second survey and third semistructured interview were completed by 25 services (12 control, 13 intervention; 27 SCCs: 14 control, 13 intervention) after the end of the 12-month follow-up (some SCCs were no longer in post by this time). The interviews were informed by SCC responses to the surveys. These data have been reviewed, focusing on the final interview, to describe practice in control services and summarise how intervention services used the system of care and whether it changed their practice.
Control services
Patients were generally seen at home but outpatient clinics and telephone calls were also used. Family would be included in the patient assessment when present and carer reviews were conducted ad hoc (e.g. if the carer appeared to be struggling).
All services aimed to provide a holistic initial assessment, covering broad areas of physical, social and psychological health, activities of daily living, and so on. Methods for capturing this usually involved documentation that had been developed locally by the SCC or team and which had evolved over time. Some services mentioned using a conversational manner, for example asking about daily routines and how a patient was managing after stroke, responding to the patient rather than following the documentation in order. Documentation was based on an integrated pathway tool (two services), a form based on the local neurology rehabilitation outpatients documentation (one service) or a copy of documentation used by other community staff in the area (one service). Two services described focusing their initial visit on high-priority areas such as medical stability and risks at home. Eight services reported using additional standardised tools where relevant (e.g. HADS, Carer Strain Index, Barthel Index, modified Rankin Scale).
Three services identified that some issues were not routinely covered (e.g. finances, sexual functioning and employment) but would be discussed if raised by a patient, or the SCC would raise it if he or she felt that it was relevant (e.g. asking younger people about employment – one service). Some issues would be raised at later meetings either because it might not be appropriate at the first visit (sexual functioning and mood) or because there was not enough time during the first visit (four services). Some SCCs viewed assessment as ongoing and needing time to build a rapport.
Stroke care co-ordinators often write an action list rather than specific patient goals. Six services identified that patients agree goals or actions for them to work on. Actions and goals are reviewed at each visit (two services) and may be chased by ringing either the patient or the place where the referral was made to (nine services). Most services report focusing on how patients perceive their problems and what they want to achieve. Three services reported encouraging patients to carry out actions themselves, including self-referral. One service would sometimes offer the Bridges stroke self-management booklet (see www.bridges-stroke.org.uk/) to help motivate patients and encourage them to set their own goals, and another service routinely gave patients ‘hand-held’ records in which health-care information was recorded and they could note their own goals.
At reviews, the majority of services (10 SCCs) do not provide a full holistic review but instead focus on changes, reviewing goals or asking about the main areas using a shortened version of the initial assessment. Two services may be providing full holistic assessments (no data for the remaining four services).
The SCC role is seen as providing education and secondary prevention advice, gathering information about patient problems and co-ordinating onward referrals. Services report that their practice is informed by experience, which has built up over years, by local knowledge and from speaking to others either within or without the team. Five services also reported being influenced by national or profession-specific guidelines (such as those from RCP and NICE). Seven services use an organised local resource list or manual to facilitate referrals they make or to provide to patients for self-referral.
Several services reported that major difficulties exist for addressing problems relating to mood, with extremely limited psychological services available in seven services. Services to address employment, benefits, younger stroke, and speech and language therapy were also lacking (each reported by one service). Several services noted that local social groups and day centre services are either lacking or more limited than previously, possibly because places are full. Three services reported largely good links for making patient referrals. Most services report that patients are aware that they can call to ask for help or to re-refer.
Intervention services
For seven services the LoTS care checklist was usually/sometimes sent to patients and usually/sometimes used by patients; this includes a minority who gave the checklist out at (rather than before) the first visit. Five services did not give the checklist out, largely because of the practicalities of getting the checklist to patients before the first visit. Services who used the checklist commented that it was positive for one or more of the following reasons: giving the patient ownership or time to prepare; helping the patient know what to expect; helping to manage awkward questions; contributing to a more successful assessment; helping to get to the crux of the matter quicker (but with no reduction in overall assessment time).
Five services reported that patients are normally seen in outpatient clinics and four services reported that patients are normally seen at home.
When it was mentioned, family would normally be included in the patient assessment when present. Carer reviews may be conducted ad hoc; two services provided carer assessments more consistently and three services did not provide carer assessments (because this was covered by another service, e.g. the Stroke Association).
Three services focus their initial visit on high-priority areas such as risk and immediate patient-identified problems. Three services guided assessment primarily using conversation and then using the care plan to ensure that all areas are covered. The care plan was used at both the initial assessment and the reviews for at least five services. At least eight other services were using the care plan but it is not clear whether this was at the initial assessment or at both the initial assessment and the reviews. It is often not clear whether all questions were asked; however, one service commented that it carried out holistic reviews because patients can deteriorate or their situation can change.
Eight services reported that they might not cover everything at the initial visit (although they felt that all areas would be addressed at some point) because of one or more of the following reasons: there is a lot to ask and they do not want to overwhelm the patient; patients may be fatigued; the initial visit is shortly after hospital discharge and so it may not be appropriate to perform a full assessment; the assessment is seen as ongoing. In total, 10 services highlighted that the sexual function question was often not appropriate (e.g. with family present, without having time to build a relationship with the patient) and some SCCs felt uncomfortable asking this question. These SCCs felt that patients need time to get to know the SCC and so this area might be addressed later on. However, this area may not be addressed for all patients and some SCCs would rely on patients having seen this question on the checklist and raising it themselves.
Most services used the goal and action planner, mainly to record actions (rather than patient-specific goals). Actions and goals are reviewed at each visit (minimum nine services) and may be chased by ringing either the patient or the place where the referral was made to (minimum eight services). One service reported using the client goal planner from the manual.
The benefits of using the care plan include it being an organised formalised system, being able to ask specific questions and potentially capturing problems sooner. A disadvantage of the care plan is that it does not cover all areas (e.g. speech and language therapy, limb function, vision, allergies). SCCs found their own ways of managing this by taking paper prompts.
For some services the care plan data do not reflect the full care given to patients, for example formal reviews being carried out by a nursing outpatient clinic not using the system of care.
The SCC role is seen as providing information and secondary prevention advice to patients (minimum five services) and co-ordinating overall care and referrals (minimum 11 services). Services report that their practice is informed by experience, which has built up over years, by local knowledge and from speaking to others either within or without the team (12 services). One service reported that it also tries to keep up to date with research and evidence-based practice. Two services use an organised local resource list to facilitate referrals.
When asked whether the manual was used, three services reported regularly using the manual, two services used the manual at first but felt that they got to know its contents, another five services may have used the manual a little and four services reported not using the manual. Regarding the manual algorithms, two services initially used these on visits but found them too cumbersome and so they stopped taking the manual to visits but might look at it on return to the office. Two services found the frequency table about fatigue helpful to reassure their patients and some found the reference guide on sexual function useful.
Nine services reported that the manual might be useful for providing ideas and confidence when considering how to respond to a problem, particularly for a SCC who is not used to dealing with the problem or who wants to consider other ideas or check his or her thinking. Six services said that the manual would be valuable for junior staff.
Difficulties exist for addressing problems relating to mood for most services, with extremely limited psychological services for five services. Seven services reported that other services were difficult to access or were limited, including voluntary/community resources, services to address employment, services to address transport, therapy, addiction treatment, podiatry, social work, optometry (each reported by one service) and service responses for sexual functioning (two services).
Most SCCs report focusing on what is important for patients, letting them raise issues through conversation and trying to incorporate their perspective. Most services involve patients in action planning, either through collaborative problem-solving, writing actions together or encouraging them to decide on their priorities. One service reported that the system of care helped them implement patient-centred practice. Four services reported encouraging patients to carry out actions themselves, including self-referral. Three services routinely gave patients a copy of their care plan or goals, whereas another three did not routinely give patients any such documentation.
Stroke care co-ordinators may share the dual role of working in the co-ordinator and profession-specific role (at least six services). One of these commented that patients can get confused about who they have seen and SCCs feel that their roles can become merged or the co-ordinator role takes over the profession-specific role. A minority of services did not identify as having a coordinator role, with co-ordination limited to the first visit (formal reviews are not provided by their service).
Most services will approach discharge through collaborative discussion with the patient. Patients are usually able to call SCCs to ask for help or re-refer themselves. Three services can either use inactive caseloads or offer patients a break and visit them in the future.
Appendix 10 Project 2: resource use
| Resource | Unit | Intervention (n = 401) | Control (n = 398) | ||||
|---|---|---|---|---|---|---|---|
| Users (%) | Meana | SD | Users (%) | Meana | SD | ||
| Index stroke admissionb | Bed-days | 99 | 39 | 44 | 99 | 30 | 35 |
| Residential care home | Night | 0 | – | – | 0 | – | – |
| Nursing home | Night | 0 | – | – | 0 | – | – |
| Inpatient services | Bed-day | 7 | 11 | 24 | 10 | 7 | 6 |
| Day hospital/day cases | Activity | 9 | 1 | 1 | 6 | 1 | < 1 |
| A&E | Visit | 7 | 11 | 24 | 10 | 7 | 6 |
| Outpatient services | Visit | 8 | 2 | 4 | 10 | 1 | 1 |
| Physiotherapist, hospitalc | Visit | 3 | 3 | 4 | 4 | 2 | 2 |
| Occupational therapist, hospitalc | Visit | < 1 | 2 | 1 | 1 | 3 | 2 |
| Speech and language therapist, hospitalc | Visit | 1 | 3 | 2 | 1 | 2 | 1 |
| Community-based services | |||||||
| GP | |||||||
| Surgery visit | Visit | 49 | 2 | 1 | 44 | 2 | 2 |
| Home visit | Visit | 9 | 2 | 1 | 11 | 2 | 2 |
| Telephone call | Call | 10 | 2 | 1 | 12 | 2 | 1 |
| Repeat prescription | Occurrence | 47 | 2 | 1 | 49 | 2 | 1 |
| Practice nurse | |||||||
| Surgery visit | Visit | 26 | 2 | 1 | 23 | 2 | 2 |
| Telephone call | Call | 3 | 2 | 1 | 3 | 1 | 1 |
| Physiotherapist | |||||||
| Home visit | Visit | 1 | 4 | 5 | 1 | 7 | 4 |
| Surgery visit | Visit | 1 | 2 | 2 | 2 | 3 | 2 |
| Elsewhere | Visit | < 1 | 1 | 0 | 0 | – | – |
| Occupational therapist | |||||||
| Home visit | Visit | 1 | 1 | 1 | 1 | 1 | 1 |
| Surgery visit | Visit | < 1 | 3 | 11 | < 1 | 3 | – |
| Elsewhere | Visit | 0 | – | – | 0 | – | – |
| Speech and language therapist | |||||||
| Home visit | Visit | < 1 | 1 | – | 0 | – | – |
| Surgery visit | Visit | 0 | – | – | < 1 | 3 | – |
| Elsewhere | Visit | 0 | – | – | 0 | – | – |
| Social worker | |||||||
| Home visit | Visit | 1 | 2 | 1 | 2 | 2 | 1 |
| Telephone call | Call | 1 | 2 | 2 | 1 | 2 | 1 |
| Community/district nurse | Contact | 5 | 3 | 3 | 6 | 5 | 10 |
| Health visitor | Contact | < 1 | 3 | 2 | 1 | 2 | 1 |
| Geriatrician | Contact | 0 | – | – | 1 | 1 | 0 |
| Psychiatrist | Contact | < 1 | 2 | – | 0 | – | – |
| Psychologist | Contact | < 1 | 1 | – | 0 | – | – |
| Chiropodist | Contact | 10 | 2 | 1 | 12 | 1 | 1 |
| Chiropractor | Contact | < 1 | 1 | – | < 1 | 1 | – |
| Osteopath | Contact | < 1 | 2 | – | 0 | – | – |
| Dentist | Contact | 12 | 1 | 1 | 12 | 1 | 1 |
| Optician | Contact | 12 | 1 | < 1 | 12 | 1 | < 1 |
| Day hospital | Half-day | 1 | 2 | 1 | 1 | 1 | < 1 |
| Social club | Half-day | 3 | 9 | 7 | 3 | 12 | 12 |
| Lunch club | Visit | 1 | 7 | 9 | 1 | 4 | 2 |
| Drop-in centre | Visit | 1 | 2 | 1 | 1 | 4 | 5 |
| Meals on wheels | Meal | 0 | – | – | < 1 | 5 | – |
| Frozen meals | Meal | < 1 | 4 | 4 | 1 | 4 | 4 |
| Home help, personal care | Visit | 1 | 42 | 46 | 2 | 53 | 55 |
| Home help, household care | Visit | 1 | 30 | 43 | 2 | 20 | 28 |
| Home help, shopping care | Visit | < 1 | 2 | 1 | 1 | 8 | 5 |
| Social services day-care centre | Hour | 0 | – | – | < 1 | 90 | – |
| Intermediate care team | Contact | < 1 | 3 | 1 | 0 | – | – |
| Other services | Occurrence | 2 | 5 | 11 | 2 | 1 | < 1 |
| Informal care from co-residents | |||||||
| Personal care | Hour | 3 | 47 | 43 | 3 | 219 | 320 |
| Providing transport | Hour | 3 | 78 | 205 | 6 | 37 | 56 |
| Preparing meals | Hour | 4 | 138 | 175 | 6 | 134 | 141 |
| Housework/laundry | Hour | 4 | 147 | 186 | 6 | 95 | 93 |
| DIY | Hour | 2 | 97 | 247 | 3 | 40 | 65 |
| Gardening | Hour | 3 | 94 | 207 | 4 | 31 | 44 |
| Shopping | Hour | 4 | 81 | 184 | 5 | 41 | 47 |
| Outings | Hour | 2 | 106 | 231 | 3 | 44 | 63 |
| Socialising | Hour | 3 | 415 | 604 | 3 | 202 | 428 |
| Help managing finances | Hour | 3 | 77 | 214 | 4 | 31 | 40 |
| Informal care from non-residents | |||||||
| Personal care | Hour | 1 | 39 | 29 | 1 | 171 | 281 |
| Providing transport | Hour | 6 | 25 | 23 | 4 | 39 | 48 |
| Preparing meals | Hour | 3 | 66 | 72 | 2 | 48 | 65 |
| Housework/laundry | Hour | 4 | 43 | 39 | 4 | 24 | 20 |
| DIY | Hour | 3 | 19 | 19 | 2 | 10 | 7 |
| Gardening | Hour | 4 | 18 | 14 | 3 | 18 | 14 |
| Shopping | Hour | 5 | 24 | 15 | 4 | 28 | 21 |
| Outings | Hour | 5 | 28 | 56 | 3 | 23 | 16 |
| Socialising | Hour | 5 | 95 | 115 | 5 | 78 | 100 |
| Help managing finances | Hour | 2 | 19 | 14 | 2 | 12 | 7 |
| Resource | Unit | Intervention (n = 307) | Control (n = 295) | ||||
|---|---|---|---|---|---|---|---|
| Users (%) | Meana | SD | Users (%) | Meana | SD | ||
| Residential care home | Night | 3 | 58 | 62 | 4 | 39 | 48 |
| Nursing home | Night | 2 | 59 | 49 | 2 | 63 | 58 |
| Inpatient services | Bed-day | 19 | 15 | 18 | 19 | 14 | 27 |
| Day hospital/day cases | Activity | 12 | 1 | 1 | 8 | 1 | 1 |
| A&E | Visit | 17 | 2 | 2 | 14 | 2 | 1 |
| Outpatient services | Visit | 44 | 3 | 3 | 40 | 3 | 5 |
| Physiotherapist, hospitalb | Visit | 12 | 8 | 8 | 17 | 7 | 8 |
| Occupational therapist, hospitalb | Visit | 5 | 3 | 3 | 9 | 8 | 13 |
| Speech and language therapist, hospitalb | Visit | 6 | 4 | 3 | 8 | 3 | 3 |
| Community-based services | |||||||
| GP | |||||||
| Surgery visit | Visit | 57 | 3 | 2 | 60 | 3 | 2 |
| Home visit | Visit | 24 | 2 | 2 | 22 | 2 | 1 |
| Telephone call | Call | 20 | 2 | 2 | 21 | 2 | 3 |
| Repeat prescription | Occurrence | 52 | 3 | 4 | 51 | 5 | 3 |
| Practice nurse | |||||||
| Surgery visit | Visit | 33 | 3 | 3 | 40 | 3 | 3 |
| Telephone call | Call | 7 | 2 | 2 | 8 | 2 | 2 |
| Physiotherapist | |||||||
| Home visit | Visit | 28 | 8 | 9 | 22 | 8 | 10 |
| Surgery visit | Visit | 3 | 4 | 3 | 4 | 3 | 3 |
| Elsewhere | Visit | 1 | 15 | 22 | 6 | 8 | 11 |
| Occupational therapist | |||||||
| Home visit | Visit | 26 | 5 | 8 | 24 | 6 | 7 |
| Surgery visit | Visit | 1 | 2 | 2 | 1 | 2 | 1 |
| Elsewhere | Visit | 1 | 14 | 23 | 3 | 9 | 14 |
| Speech and language therapist | |||||||
| Home visit | Visit | 11 | 4 | 3 | 13 | 4 | 4 |
| Surgery visit | Visit | 2 | 3 | 2 | 1 | 3 | 3 |
| Elsewhere | Visit | 1 | 10 | 8 | 2 | 3 | 2 |
| Social worker | |||||||
| Home visit | Visit | 12 | 2 | 1 | 11 | 2 | 1 |
| Telephone call | Call | 8 | 3 | 2 | 5 | 3 | 2 |
| Community/district nurse | Contact | 23 | 7 | 21 | 23 | 4 | 9 |
| Health visitor | Contact | 5 | 2 | 1 | 8 | 10 | 27 |
| Geriatrician | Contact | 1 | 3 | 1 | 1 | 1 | 1 |
| Psychiatrist | Contact | 2 | 2 | 1 | 1 | 2 | 1 |
| Psychologist | Contact | 3 | 3 | 1 | 3 | 2 | 1 |
| Chiropodist | Contact | 18 | 2 | 2 | 16 | 2 | 1 |
| Chiropractor | Contact | < 1 | 3 | – | 1 | 1 | 1 |
| Osteopath | Contact | < 1 | 4 | – | < 1 | 1 | – |
| Dentist | Contact | 21 | 2 | 1 | 14 | 1 | 1 |
| Optician | Contact | 20 | 1 | 1 | 22 | 1 | 1 |
| Day hospital | Half-day | 6 | 8 | 18 | 4 | 6 | 6 |
| Social club | Half-day | 5 | 12 | 16 | 3 | 8 | 8 |
| Lunch club | Visit | 2 | 6 | 4 | 2 | 11 | 8 |
| Drop-in centre | Visit | 2 | 7 | 5 | < 1 | 6 | – |
| Meals on wheels | Meal | 0 | – | – | < 1 | 5 | – |
| Frozen meals | Meal | 2 | 21 | 24 | 1 | 30 | 14 |
| Home help, personal care | Visit | 5 | 49 | 67 | 5 | 78 | 138 |
| Home help, household care | Visit | 3 | 61 | 127 | 2 | 42 | 63 |
| Home help, shopping care | Visit | 2 | 12 | 13 | 1 | 13 | 12 |
| Social services day-care centre | Hour | < 1 | 6 | – | < 1 | 4 | 3 |
| Intermediate care team | Contact | 5 | 57 | 167 | 3 | 7 | 11 |
| Other services | Occurrence | 8 | 6 | 15 | 8 | 9 | 16 |
| Informal care from co-residents | |||||||
| Personal care | Hour | 15 | 385 | 834 | 14 | 242 | 324 |
| Providing transport | Hour | 15 | 108 | 140 | 14 | 120 | 126 |
| Preparing meals | Hour | 19 | 249 | 234 | 14 | 249 | 200 |
| Housework/laundry | Hour | 18 | 244 | 305 | 14 | 204 | 163 |
| DIY | Hour | 9 | 87 | 224 | 4 | 49 | 51 |
| Gardening | Hour | 11 | 80 | 94 | 8 | 60 | 52 |
| Shopping | Hour | 16 | 115 | 122 | 15 | 95 | 113 |
| Outings | Hour | 14 | 116 | 142 | 9 | 93 | 87 |
| Socialising | Hour | 14 | 728 | 1125 | 9 | 489 | 488 |
| Help managing finances | Hour | 13 | 89 | 123 | 11 | 124 | 225 |
| Informal care from non-residents | |||||||
| Personal care | Hour | 8 | 110 | 96 | 6 | 110 | 102 |
| Providing transport | Hour | 14 | 63 | 82 | 14 | 58 | 58 |
| Preparing meals | Hour | 8 | 104 | 135 | 6 | 85 | 98 |
| Housework/laundry | Hour | 9 | 80 | 82 | 7 | 45 | 43 |
| DIY | Hour | 6 | 20 | 20 | 4 | 24 | 35 |
| Gardening | Hour | 9 | 26 | 36 | 7 | 32 | 37 |
| Shopping | Hour | 11 | 48 | 48 | 11 | 44 | 35 |
| Outings | Hour | 13 | 57 | 98 | 11 | 54 | 62 |
| Socialising | Hour | 13 | 156 | 163 | 9 | 146 | 218 |
| Help managing finances | Hour | 7 | 60 | 69 | 5 | 33 | 36 |
| Resource | Unit | Intervention (n = 283) | Control (n = 268) | ||||
|---|---|---|---|---|---|---|---|
| Users (%) | Meana | SD | Users (%) | Meana | SD | ||
| Residential care home | Night | 2 | 81 | 84 | 2 | 46 | 76 |
| Nursing home | Night | 1 | 92 | 91 | 1 | 103 | 112 |
| Inpatient services | Bed-day | 16 | 9 | 15 | 15 | 8 | 9 |
| Day hospital/day cases | Activity | 8 | 2 | 1 | 10 | 1 | <1 |
| A&E | Visit | 12 | 2 | 1 | 10 | 2 | 1 |
| Outpatient services | Visit | 36 | 3 | 3 | 37 | 3 | 5 |
| Physiotherapist, hospitalb | Visit | 14 | 6 | 6 | 12 | 6 | 8 |
| Occupational therapist, hospitalb | Visit | 4 | 1 | 1 | 7 | 3 | 2 |
| Speech and language therapist, hospitalb | Visit | 6 | 8 | 7 | 2 | 2 | 1 |
| Community-based services | |||||||
| GP | |||||||
| Surgery visit | Visit | 53 | 3 | 3 | 60 | 3 | 2 |
| Home visit | Visit | 15 | 3 | 4 | 13 | 3 | 6 |
| Telephone call | Call | 18 | 2 | 1 | 13 | 3 | 3 |
| Repeat prescription | Occurrence | 49 | 5 | 3 | 54 | 5 | 2 |
| Practice nurse | |||||||
| Surgery visit | Visit | 35 | 3 | 4 | 44 | 3 | 4 |
| Telephone call | Call | 6 | 2 | 2 | 8 | 3 | 3 |
| Physiotherapist | |||||||
| Home visit | Visit | 7 | 9 | 14 | 7 | 5 | 7 |
| Surgery visit | Visit | 4 | 2 | 1 | 4 | 2 | 2 |
| Elsewhere | Visit | 2 | 12 | 12 | 1 | 16 | 26 |
| Occupational therapist | |||||||
| Home visit | Visit | 6 | 2 | 2 | 7 | 5 | 6 |
| Surgery visit | Visit | 1 | 3 | 2 | 1 | 2 | 0 |
| Elsewhere | Visit | <1 | 5 | – | 1 | 4 | 3 |
| Speech and language therapist | |||||||
| Home visit | Visit | 3 | 3 | 4 | 3 | 2 | 1 |
| Surgery visit | Visit | 1 | 1 | 0 | 0 | – | – |
| Elsewhere | Visit | 0 | – | – | <1 | 6 | – |
| Social worker | |||||||
| Home visit | Visit | 8 | 2 | 1 | 4 | 2 | 1 |
| Telephone call | Call | 5 | 3 | 1 | 2 | 1 | 1 |
| Community/district nurse | Contact | 15 | 8 | 27 | 14 | 4 | 5 |
| Health visitor | Contact | 5 | 2 | 1 | 3 | 2 | 1 |
| Geriatrician | Contact | 1 | 1 | 0 | 1 | 1 | 0 |
| Psychiatrist | Contact | 2 | 6 | 8 | 1 | 2 | 1 |
| Psychologist | Contact | 2 | 2 | 1 | 2 | 2 | 1 |
| Chiropodist | Contact | 18 | 2 | 1 | 18 | 2 | 2 |
| Chiropractor | Contact | <1 | 1 | – | 1 | 2 | 1 |
| Osteopath | Contact | 1 | 2 | – | <1 | 3 | – |
| Dentist | Contact | 27 | 2 | 1 | 22 | 2 | 1 |
| Optician | Contact | 21 | 1 | <1 | 24 | 1 | 1 |
| Day hospital | Half-day | 5 | 5 | 6 | 6 | 4 | 4 |
| Social club | Half-day | 4 | 13 | 12 | 3 | 13 | 15 |
| Lunch club | Visit | 2 | 9 | 8 | 2 | 14 | 11 |
| Drop-in centre | Visit | 1 | 2 | 1 | 2 | 10 | 10 |
| Meals on wheels | Meal | 0 | – | – | <1 | 150 | – |
| Frozen meals | Meal | 1 | 5 | 5 | 1 | 24 | 23 |
| Home help, personal care | Visit | 2 | 194 | 306 | 2 | 163 | 169 |
| Home help, household care | Visit | 1 | 5 | 6 | 1 | 9 | 3 |
| Home help, shopping care | Visit | <1 | 1 | – | <1 | 5 | – |
| Social services day-care centre | Hour | 2 | 56 | 64 | 1 | 20 | 10 |
| Intermediate care team | Contact | 0 | – | – | 1 | 5 | 5 |
| Other services | Occurrence | 6 | 4 | 6 | 3 | 2 | 1 |
| Informal care from co-residents | |||||||
| Personal care | Hour | 11 | 564 | 1695 | 6 | 460 | 1035 |
| Providing transport | Hour | 12 | 202 | 357 | 10 | 134 | 278 |
| Preparing meals | Hour | 14 | 317 | 336 | 9 | 213 | 203 |
| Housework/laundry | Hour | 14 | 339 | 769 | 11 | 210 | 235 |
| DIY | Hour | 7 | 157 | 455 | 4 | 67 | 83 |
| Gardening | Hour | 9 | 137 | 390 | 8 | 81 | 160 |
| Shopping | Hour | 11 | 175 | 349 | 10 | 80 | 141 |
| Outings | Hour | 11 | 173 | 378 | 7 | 123 | 176 |
| Socialising | Hour | 11 | 550 | 776 | 7 | 343 | 488 |
| Help managing finances | Hour | 9 | 169 | 403 | 8 | 63 | 117 |
| Informal care from non-residents | |||||||
| Personal care | Hour | 5 | 209 | 356 | 3 | 186 | 349 |
| Providing transport | Hour | 12 | 53 | 66 | 11 | 42 | 40 |
| Preparing meals | Hour | 7 | 106 | 170 | 4 | 87 | 84 |
| Housework/laundry | Hour | 9 | 107 | 154 | 7 | 60 | 67 |
| DIY | Hour | 5 | 46 | 43 | 7 | 34 | 44 |
| Gardening | Hour | 6 | 38 | 37 | 6 | 31 | 36 |
| Shopping | Hour | 8 | 62 | 72 | 8 | 44 | 39 |
| Outings | Hour | 11 | 63 | 135 | 9 | 47 | 46 |
| Socialising | Hour | 12 | 222 | 356 | 8 | 116 | 140 |
| Help managing finances | Hour | 5 | 49 | 64 | 4 | 44 | 37 |
Appendix 11 Project 3: The Longer-term Unmet Needs after Stroke questionnaire
Reproduced with permission from Bradford Teaching Hospitals NHS Foundation Trust.
The Longer-term Unmet Needs after Stroke questionnaire (PDF download)
© Copyright 2008 Anne Forster on behalf of Bradford Teaching Hospitals NHS Foundation Trust. All rights reserved. May not be reproduced or transmitted in any form or by any means without prior written permission of Bradford Teaching Hospitals NHS Foundation Trust.
Appendix 12 Project 4: categories of social activity
Description of the categories used in the purposive sampling strategy for the adjustment after stroke study
The adjustment after stroke study aimed to explore adjustment and recovery post stroke, including the processes and mechanisms that contributed to social participation following stroke. To achieve this, the study was designed to enable a purposeful sample of stroke survivors to be recruited, comprising those who were less or more socially active than anticipated for their level of functional ability.
Before being recruited to the adjustment after stroke study, participating stroke survivors had to complete the LoTS care stroke system of care trial. The trial database contained information about each trial participant, including their Barthel Index and FAI scores pre stroke and 12 months post stroke. The Barthel Index measures current ability to carry out basic activities of daily living (e.g. continence, ability to get dressed, mobility, feeding). The FAI measures the frequency of extended activities of daily living (e.g. leisure activities, social occasions, housework, travelling). These could be used to help identify potential participants for the substudy.
The trial was ongoing at the time that the sampling strategy for the adjustment after stroke study was designed. Analysis was therefore conducted on an existing data set,36 with a similar population to that of the stroke system of care trial, to identify criteria to enable us to distinguish between stroke survivors in terms of their social activity level (based on Barthel Index and FAI scores). In this population the median (interquartile range) reduction in Barthel Index score and FAI score from pre stroke to 12 months post stroke was 4 (1–8) and 16 (7–26) respectively. Three main categories of stroke survivors were defined: ‘doing better than expected’, ‘failing to thrive’ and ‘doing as expected (doing well)’. Figure 24 plots the reduction in Barthel Index and FAI scores from pre stroke to 12 months post stroke for the existing data set and highlights the three different categories identified. Table 84 provides the definitions for the three different categories as well as any exclusions made to take account of the potential floor effect in FAI score. The trial database was screened on a monthly basis to identify potential participants who fell into each of the three categories for recruitment to the substudy. Initially, the recruitment of those in the ‘doing better than expected’ and ‘failure to thrive’ categories was prioritised, with an attempt made to recruit those in the extremes of these categories to enable maximum variation within the sample.
FIGURE 24.
Reduction in Barthel Index and FAI scores from pre stroke to 12 months post stroke. A, better than expected; B, failure to thrive; C, doing as expected (doing well).
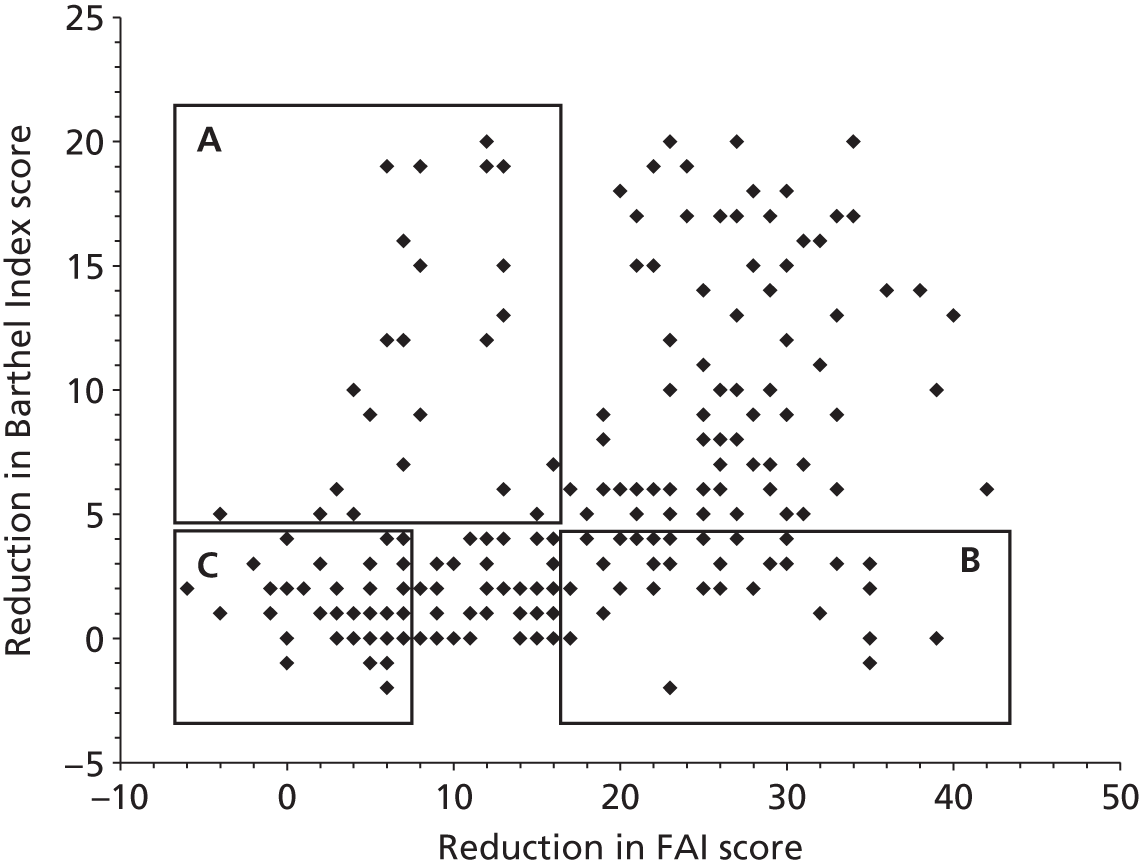
| Category | Reduction in Barthel Index score (between pre stroke and 12 months post stroke) | Reduction in FAI score (between pre stroke and 12 months post stroke) | Excluding |
|---|---|---|---|
| A: better than expected | > 4 | ≤ 16 | Those who have a 12-month FAI score of 0 |
| B: failure to thrive | ≤ 4 | >16 | Not applicable |
| C: doing as expected (doing well) | ≤ 4 | ≤ 7 | Those who have a 12-month FAI score of 0 |
The relationship between the categories and identified recovery trajectories
It was anticipated that the categories, based on changes in Barthel Index and FAI scores from pre stroke to 12 months post stroke, would be indicative of stroke survivors’ adjustment after stroke. These objective measures, however, did not predict the recovery trajectory that each participating stroke survivor identified. Table 85 summarises the numbers of participating stroke survivors who fell into each of the four recovery trajectories, arranged according to the different categories: doing better than expected, failure to thrive and doing as expected (doing well). Figure 25 shows the reductions in FAI and Barthel Index scores from pre stroke to 12 months post stroke for participating stroke survivors in the different recovery trajectories.
| Category | No. of stroke survivors recruited | Recovery trajectory (identified from analysis of qualitative data) | No. of stroke survivors in each trajectory by category |
|---|---|---|---|
| A: doing better than expected | 12 | Disruption followed by adjustment and acceptance | 5 |
| Cycles of disruption followed by adjustment and acceptance | 3 | ||
| Disruption without adjustment and acceptance | 2 | ||
| Stroke as continuation of ongoing decline | 2 | ||
| B: failure to thrive | 8 | Disruption followed by adjustment and acceptance | 3 |
| Cycles of disruption followed by adjustment and acceptance | 1 | ||
| Disruption without adjustment and acceptance | 4 | ||
| Stroke as continuation of ongoing decline | 0 | ||
| C: doing as expected (doing well) | 2 | Disruption followed by adjustment and acceptance | 1 |
| Cycles of disruption followed by adjustment and acceptance | 1 | ||
| Disruption without adjustment and acceptance | 0 | ||
| Stroke as continuation of ongoing decline | 0 |
FIGURE 25.
Reduction in FAI and Barthel Index scores from pre stroke to 12 months post stroke for participating stroke survivors in the different recovery trajectories.
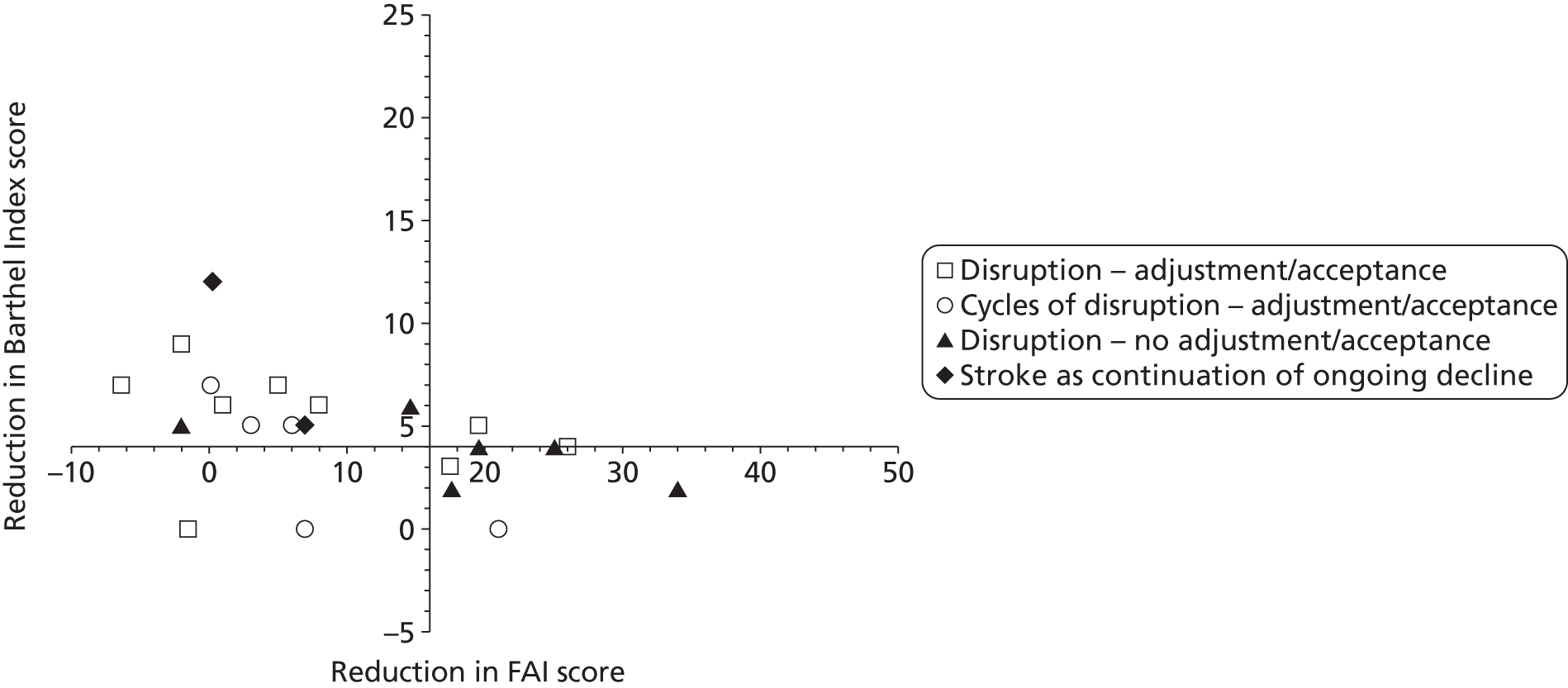
It is important to note that a purposive sampling strategy was used and therefore the stroke survivors interviewed were not randomly selected to represent their associated category (i.e. those interviewed who were categorised as ‘doing better than expected’ were not necessarily representative of the population of those ‘doing better than expected’). There is, however, no clear relationship between the participating stroke survivors’ category and the recovery trajectory identified from the qualitative data. This is perhaps unsurprising given that participants’ narratives indicate that multiple, interacting factors shape the process of recovery, adjustment and acceptance over time. This demonstrates that we cannot reduce our understanding of recovery and adjustment to outcome measures such as the Barthel Index and the FAI.
Appendix 13 Dissemination activities
Publications directly attributable to the programme
Published
Forster A, Young J, Nixon J, Chapman K, Murray J, Patel A, et al. Protocol of a cluster randomized trial evaluation of a patient and carer-centered system of longer-term stroke care (LoTS care) [published online ahead of print 19 February 2013]. Int J Stroke 2013. doi:10.1111/ijs.12038
LoTS care LUNS study team. Validation of the longer-term unmet needs after stroke (LUNS) monitoring tool: a multicentre study. Clin Rehabil 2013;27:1020–8.
Planned
-
Update of the LoTS care stroke system of care reference guides.
-
Clinical effectiveness and cost-effectiveness of the LoTS care stroke system of care: results of the cluster RCT.
-
Implementation of the LoTS care stroke system of care in the trial.
-
Exploratory analyses of patient and service factors and trial outcomes.
-
Evaluation of the LUNS tool as a scale (internal consistency, dimensionality).
-
Comparison of the LUNS tool results in patients with and without cognitive/language impairments.
-
Qualitative evaluation of longer-term unmet needs after stroke.
-
Correlation of longer-term unmet needs after stroke with other variables.
-
Adjustment after stroke study: methodology.
-
Adjustment after stroke study: results.
-
Adjustment after stroke study: literature review on factors influencing adjustment post stroke.
Cofunded publications
Published
Smith J, Forster A, Young J. On behalf of the Cochrane Group for information provision after stroke. Cochrane review: information provision for stroke patients and their caregivers. Clin Rehabil 2009;23:195–206.
Murray J, Young J, Forster A. Measuring outcomes in the longer term after a stroke. Clin Rehabil 2009;23:918–21.
McKevitt C, Fudge N, Redfern J, Sheldenkar A, Crichton S, Rudd AR, et al. Self-reported long-term needs after stroke. Stroke 2011;42:1398–403.
Forster A, Brown L, Smith J, House A, Knapp P, Wright J, et al. Information provision for stroke patients and their caregivers. Cochrane Database Syst Rev 2012;11:CD001919.
Planned
-
Overcoming the obstacles for multicentre trials in rehabilitation [LoTS care/Training Caregivers After Stroke (TRACS) trials].
-
Design challenges in cluster RCTs (LoTS care/TRACS trials).
-
Appropriate safety monitoring in stroke rehabilitation trials (LoTS care/TRACS trials).
-
Recruitment of carers to stroke trials (LoTS care/TRACS trials).
-
Follow-up response rates in postal questionnaires sent to stroke patients and their carers (LoTS care/TRACS trials).
-
Statistical methodology (LoTS care/TRACS trials).
-
Realist evaluation of the LoTS care system of care trial.
Other dissemination activities
-
A summary of all results and links to publications will be published on the LoTS care trial website (www.lotscare.co.uk).
-
A summary of the results of the LoTS care system of care trial and the LUNS study have been sent to those participants who requested this (307 and 229 participants in the two studies respectively).
-
Results will be disseminated at appropriate national and international conferences, including those in the fields of stroke, health services research and medical sociology.
-
Results of the LoTS care system of care trial and the LUNS study will be disseminated to participating SRNs and clinical staff through regional meetings.
-
The LUNS questionnaire for identifying longer-term unmet needs after stroke is available free of charge for non-commercial use on request from the corresponding author.
-
We anticipate that case studies illustrating the different recovery trajectories identified in the adjustment after stroke study may be useful as a resource both for stroke survivors/carers and for health and social care professionals who work with stroke survivors.
-
Although the LoTS care system of care was not demonstrated to improve clinical outcomes, components of it may have utility, for example the assessment provides a structured way of ensuring that an evidence-based post-stroke review is carried out and the manual serves as a repository of evidence-based practice that could be useful for training inexperienced staff; these may be of particular use for developing services in sites currently without organised long-term care for stroke.
Glossary
- Activities of daily living
- Tasks that are carried out on a daily basis, such as dressing, eating, toileting and walking.
- Analysis of variance (ANOVA) fit statistics (within Rasch analysis)
- A fit statistic used at the individual item level. The person sample is partitioned into a number of discrete ability groups and fit residual values are calculated for these discrete groups. The ANOVA fit statistics assess whether there is a difference in fit residuals between these discrete ability groups.
- Aphasia
- Difficulty using or understanding spoken or written language. Mild forms of aphasia can be referred to as dysphasia.
- Barthel Index
- An index measuring independent function in activities of daily living. The total score ranges from 0 to 20, with lower scores indicating increased disability.
- Caregiver Burden Scale
- A 22-item scale measuring subjective carer burden. The overall score ranges from 22 to 88, with higher scores indicating a greater carer burden.
- Carer
- A relative or friend who provides regular care to another person. Carers were eligible for the Longer-Term Stroke care trial if they were the main person providing practical support to the patient a minimum of once per week.
- Cerebral infarction
- A stroke caused by deprivation of the blood supply to the brain, for example because of a clot, leading to death of part of the brain.
- Chi-squared fit statistics
- Fit statistics based on whether the observed frequency distribution differs from the theoretical distribution of a particular model.
- Client Service Receipt Inventory
- A measure of use of health, social care and informal resources. The Longer-Term Stroke care trial used an adapted version of this measure.
- Clinically important difference
- A clinically important difference represents a change (e.g. the smallest change in outcome score) that would be considered meaningful and worthwhile by a patient or health care professional.
- Cluster randomised controlled trial
- A type of randomised controlled trial in which clusters of individuals (such as services or geographical areas), as opposed to individual subjects, are randomly assigned to either the treatment or the control group.
- Community based
- An activity that is organised and takes place locally, for example care and support provided to stroke survivors by their local stroke service in their own home or local community.
- Confidence interval
- A confidence interval gives an estimated range of values that are likely to include an unknown population parameter, the estimated range being calculated from a given set of sample data. A wide confidence interval indicates a lack of certainty about the true effect of the test or treatment; a narrow confidence interval indicates a more precise estimate.
- Confirmatory factor analysis
- A statistical analysis that confirms whether a set of items fall within a predefined number of factors/dimensions.
- Consolidated Standards of Reporting Trials (CONSORT)
- The CONSORT statement is an evidence-based minimum set of recommendations for reporting randomised controlled trials.
- Content validity
- The extent to which a measure represents all aspects of a given concept (such as the concept of unmet need).
- Cost-effectiveness acceptability curve
- A method used to represent the uncertainty of cost-effectiveness results. A cost-effectiveness acceptability curve presents the probability that one treatment is more cost-effective than another treatment.
- Cronbach’s alpha
- A value used to rate the internal consistency or correlation of items in a test. A correlation coefficient is given between 0 and 1, with higher values representing higher levels of internal consistency.
- Differential item functioning
- A form of item bias in which item difficulty changes depending on which group is responding. For example, men and women with the same level of anxiety may consistently respond differently to an item in an anxiety questionnaire.
- Dimensionality
- The number of dimensions (also known as factors, components or constructs) that are being measured by a scale or set of items.
- Dysarthria
- A motor speech disorder. Patients suffering from dysarthria have problems in controlling the muscles used for speaking.
- Dysphasia
- Difficulty using or understanding spoken or written language. A mild form of aphasia.
- Early supported discharge
- A service model that aims to bring forward hospital discharge and provide a more continuous rehabilitation process by transferring stroke patients from an inpatient setting to continue rehabilitation in primary care.
- Edinburgh stroke case mix adjuster
- Predictive model that can be used to predict stroke outcomes using six factors (age, pre-stroke independence, pre-stroke living circumstances, verbal subsection of the Glasgow Coma Scale, ability to lift both arms off the bed, independent walking). The model used in the Longer-Term Stroke care trial predicts the probability of dependency-free survival at 6 months.
- European Quality of Life-5 Dimensions health state measure
- A five-item scale to identify health state. The total score varies from −0.59 for worst health to 1.0 for perfect health, with a score of 0 representing the state of being dead.
- Exploratory factor analysis
- A statistical analysis that explores how many factors/dimensions a set of items partition into.
- Fit indices
- Indicators of the extent to which observed data agree with a particular statistical model. These can be reported at an overall scale level or on an individual item basis.
- Fit residuals
- A fit statistic relating to the discrepancies between model-predicted expected data values and what is actually observed. The reported values are z-standardised.
- Frenchay Activities Index
- A 15-item measure of activities of daily living for use with stroke survivors. The overall score ranges from 0 to 45, with lower scores indicating lower activity levels.
- Frenchay Aphasia Screening Test
- A screening tool for identifying communication or language impairment. The test assesses comprehension and reading and uses written and pictorial stimuli.
- General Health Questionnaire-12
- A 12-item measure of emotional and mental health and well-being. The overall score ranges from 0 to 36, with higher scores indicating greater psychological distress.
- Hemiparesis
- Weakness of one side of the body that may be caused by stroke and can be accompanied by sensory or other neurological deficits.
- Intention-to-treat analysis
- An analysis conducted on participants based on the group that they were initially (and randomly) allocated to regardless of whether they dropped out, fully complied with the treatment or switched to an alternative treatment. Intention-to-treat analyses are used to assess clinical effectiveness because they mirror actual practice.
- Internal consistency
- A type of reliability that reflects the extent to which items of a test measure aspects of the same characteristic (and nothing else) (see Cronbach’s alpha).
- Intracluster correlation coefficient
- The intracluster correlation coefficient is a measure of the relatedness of data within a cluster. It accounts for the relatedness of clustered data by comparing the variance within clusters with the variance between clusters. Values of the intracluster correlation coefficient range from 0 to 1.
- Kappa
- A measure of (non-random) agreement between multiple measurements of the same variable.
- Length of stay
- Duration of admission in hospital.
- Longer-term Unmet Needs after Stroke
- A 22-item tool developed to assess unmet needs, in which a ‘yes’ response indicates unmet need.
- Modified Rankin Scale
- A single-item measure of functional independence. The total score ranges from 0 (perfect health) to 6 (death).
- Multidisciplinary team
- A team of professionals, including those from different disciplines such as nursing or physiotherapy, who work together to co-ordinate and provide patient care.
- National Stroke Audit
- A report outlining where progress in stroke care has been made and providing recommendations for improving stroke outcomes and reducing costs. Produced by the National Audit Office.
- National Stroke Strategy
- A report providing a national strategy for stroke services with the intention of making improvements in stroke care, providing guidance to commissioners and informing the expectations of patients and families. Produced by the Department of Health.
- Per-protocol analysis
- Analysis that includes only participants who complete the trial according to the protocol are included in the final results (see Intention-to-treat analysis).
- Person separation index
- Similar to the Cronbach’s alpha statistic, this is a measure of reliability/internal consistency. However, the person separation index is also influenced by scale targeting. Again, the person separation index is given a value between 0 and 1, with higher values representing higher levels of internal consistency.
- Primary intracerebral haemorrhage
- A stroke caused by the rupture of a blood vessel within the brain, usually an artery.
- Primary objective
- The most important aim of a study, evaluated using primary outcomes or end points.
- Psychometric properties
- How well a scale/test/set of items measures the construct of interest. These broadly consist of the reliability of the scale (how consistently the scale measures) and the validity of the scale (whether the scale is measuring what it is supposed to measure).
- Quality-adjusted life-year
- A measure of disease burden, including quality and quantity of life lived, used in assessing the value for money of clinical interventions.
- Rasch analysis
- A statistical method that allows evaluation of a scale measuring a particular latent attribute. Observed data are fitted within a Rasch unidimensional measurement model framework to assess the measurement characteristics of the scale.
- Response dependency
- Response to one item (question) has a direct impact on the response to another item, over and above what is explained by the underlying trait.
- Root-mean-square error of approximation
- A goodness-of-fit index used within confirmatory factor analysis and structural equation modelling. Values of < 0.08 are widely regarded as acceptable and values of < 0.05 are classed as good.
- Secondary objectives
- Aims that are secondary to the primary objective, evaluated using secondary outcomes or end points.
- Short Form questionnaire-12 items
- A 12-item tool measuring health-related quality of life. The total score ranges from 0 to 100, with a score of < 50 indicating health worse than the norm and a score of > 50 indicating health better than the norm.
- Six-item Cognitive Impairment Test
- A screening tool for identifying cognitive impairment. The overall score ranges from 0 to 28, with a score of ≤ 7 indicating normal cognitive function and a score of > 7 indicating cognitive impairment, with higher scores indicating greater cognitive impairment.
- Standard deviation
- A measure that shows how much variation exists from the average (mean) or expected value.
- Stroke Association
- A UK charity that campaigns for better care of people affected by stroke. Includes research and support services.
- Stroke care co-ordinator
- Registered health professional with experience in stroke care, working in a community-based co-ordinating role for stroke patients.
- Stroke Research Network
- Part of the National Institute for Health Research, the Stroke Research Network supports both quality and delivery of stroke research.
- Stroke unit
- A specialised hospital unit with a dedicated stroke team and stroke resources (e.g. care pathway, educational material, monitored beds).
- System of care
- A structured assessment linked to evidence-based treatment algorithms and reference guides contained in a manual.
- Targeting
- A comparison of the distribution of item difficulty level within a scale relative to the distribution of person abilities within a particular sample.
- Test–retest reliability
- The extent to which a scale or measure that is repeated in the same stable population yields the same result.
- Transient ischaemic attack
- A stroke-like event that fully recovers within 24 hours of the start of symptoms.
List of abbreviations
- 6CIT
- six-item Cognitive Impairment Test
- A&E
- accident and emergency
- ANOVA
- analysis of variance
- AUECR
- Academic Unit of Elderly Care and Rehabilitation
- CBS
- Caregiver Burden Scale
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CONSORT
- Consolidated Standards of Reporting Trials
- CRAG
- Consumer Research Advisory Group
- CSRI
- Client Service Receipt Inventory
- CTRU
- Clinical Trials Research Unit
- DIF
- differential item functioning
- EQ-5D
- European Quality of Life-5 Dimensions
- FAI
- Frenchay Activities Index
- FAST
- Frenchay Aphasia Screening Test
- GHQ-12
- General Health Questionnaire-12
- GM-SAT
- Greater Manchester Stroke Assessment Tool
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- HRG
- Healthcare Resource Group
- ICC
- intracluster correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- ITT
- intention to treat
- LoTS care
- Longer-Term Stroke care trial
- LUNS
- Longer-term Unmet Needs after Stroke
- MCS
- mental component score (of the SF-12)
- MDT
- multidisciplinary team
- NEADL
- Nottingham Extended Activities of Daily Living
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NSA
- National Stroke Audit
- NSS
- National Stroke Strategy
- PCS
- physical component score (of the SF-12)
- PSI
- person separation index
- QALY
- quality-adjusted life-year
- R&D
- research and development
- RCP
- Royal College of Physicians
- RCT
- randomised controlled trial
- SCC
- stroke care co-ordinator
- SD
- standard deviation
- SE
- standard error
- SF-12
- Short Form questionnaire-12 items
- SHA
- Strategic Health Authority
- SMG
- study management group
- SRN
- Stroke Research Network
- TOTAL
- Trial of Occupational Therapy and Leisure
- TRACS
- Training Caregivers After Stroke
- YSRN
- Yorkshire Stroke Research Network