Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-1210-12004. The contractual start date was in January 2013. The final report began editorial review in March 2019 and was accepted for publication in November 2020. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. This report has been published following a shortened production process and, therefore, did not undergo the usual number of proof stages and opportunities for correction. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2021 Dalal et al. This work was produced by Dalal et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 Dalal et al.
SYNOPSIS
Research summary
Our original proposal stated that the Rehabilitation Enablement in Chronic Heart Failure (REACH-HF) programme of research would include four linked work packages (WPs) that aim to:
-
develop an evidence-informed, home-based, self-care cardiac rehabilitation (CR) programme for people with heart failure (HF) and their caregivers (‘the REACH-HF intervention’)
-
conduct a pilot trial to assess the feasibility of a definitive trial of the REACH-HF intervention in heart failure with preserved ejection fraction (HFpEF)
-
conduct a randomised controlled trial (RCT) to assess the clinical effectiveness and cost-effectiveness of the REACH-HF intervention versus usual care in people with heart failure with reduced ejection fraction (HFrEF) and their carers
-
undertake evidence synthesis/modelling to assess the clinical effectiveness and cost-effectiveness of the REACH-HF intervention versus centre-based CR in people with HFrEF.
Our original research questions were:
-
WP1 –
-
What are the necessary intervention components of a home-based, self-care manual for patients with HF?
-
What are the necessary intervention components of a home-based, self-care manual for caregivers of patients with HF?
-
-
WP2 –
-
How feasible is the REACH-HF intervention in patients with HFpEF?
-
-
WP3 –
-
What are the clinical effectiveness and cost-effectiveness of the REACH-HF intervention compared with usual care in patients with HFrEF?
-
What is the impact for caregivers of using the intervention versus usual care?
-
-
WP4 –
-
What are the clinical effectiveness and cost-effectiveness of the REACH-HF intervention versus centre-based CR in patients with HFrEF?
-
What is the expected value of information for future research, including a RCT of the REACH-HF intervention versus centre-based CR in patients with HFrEF?
-
Over the 6 years of our programme of applied research, we were able to complete all four WPs as per our original research questions, although we had to modify our economic evaluation in WP4. Given the paucity of direct head-to-head RCTs in the HF population identified by the updated Cochrane review1 of home- versus hospital-based CR (four RCTs in a total of 295 patients, with only one single-centre study undertaken in a UK setting) and that the data were too limited to directly estimate the longer-term cost-effectiveness of home- versus hospital-based CR, we decided to restrict our economic modelling to examine the questions of the longer cost-effectiveness of:
-
the REACH-HF intervention versus usual care
-
home-based CR versus usual care
-
centre-based CR versus usual care.
In summary, the only change that we made to our original proposal was to WP4, for which we modified the economic modelling, as we were not able to make a direct comparison of the effectiveness of the REACH-HF intervention versus centre-based CR in people with HFrEF. Instead, our modelling compared home- and centre-based CR versus usual care.
Setting the scene
The burden of heart failure
Chronic HF is a burgeoning global health challenge that affects about 64 million people worldwide,2,3 including nearly 5 million people in the USA4 and about 900,000 people in the UK. 5 In the Western world, 1–2% of adults are living with the condition,6 and the prevalence is predicted to increase with the ageing population. 3,7 Low- and middle-income countries are also seeing an increase in prevalence, as people in these countries adopt Western lifestyles, leading to higher rates of obesity, diabetes and hypertension – morbidities that contribute to development of HF. 8,9
An editorial in The Lancet highlighted the negative impact of HF, describing it as ‘a dangerous, debilitating, and common disease, subjecting patients, carers, and doctors to a substantial burden. The need for admission to hospital and provision of specialist care, often for extended periods, means that the costs to health systems are correspondingly great’. 3 In 2002, the annual NHS spend on HF was £716M – just less than 2% of the total NHS expenditure of nearly £40B. Globally, the economic burden is predicted to grow to more than US$108B [£82B, conversion as at 27 June 2018: 1 US$ =$ 0.760133 Great British pounds (GBP)] as the population ages and nations become more industrialised. Hospital admissions are a key driver of the rising costs related to HF in high-income countries: in people > 65 years of age, it is the most common reason for hospital admission in the USA, where about 1 million admissions per year are related to HF, which is comparable to annual admissions for HF in Europe. 3
With the prevalence of HF predicted to increase as a result of increased life expectancy and a corresponding increase in the elderly population,3 care needed by elderly people, especially those with long-term conditions and multimorbidities, will also increase. Nearly 80% of patients with HF have three or more comorbidities, with the most common preceding a diagnosis of HF being hypertension, ischaemic heart disease, osteoarthritis and atrial fibrillation (AF). 7 A recent population-based study in the UK assessed the interval need (a measure of dependency and an assessment of demand) that was being met by carers for generational cohorts of elderly people and identified a change in care needs between 1991 and 2011, with an increase in elderly people being cared for in the community, creating more responsibilities for family and friends. 10
People with HF are commonly categorised on the basis of their left ventricular ejection fraction (LVEF): patients with HF with reduced ejection fraction (HFrEF) (typically LVEF < 40–45%) have ‘systolic’ HF due to impaired left ventricular contraction, while those with normal LVEF (typically > 45–50%) have HF with preserved ejection fraction (HFpEF). 11,12 Although individuals with HFpEF are thought to account for more than half of all patients with HF, most trials have recruited only patients with HFrEF – probably because patients with HFpEF tend to be older and have multiple comorbidities and historically there has been a lack of consensus on the exact definition of HFpEF. The prevalence of HFrEF is decreasing, whereas the prevalence of HFpEF is increasing.
Both forms of chronic HF can cause several symptoms, including shortness of breath, fatigue, fluid retention, impaired cognitive function and appetite disturbance. People with HF consequently experience exercise intolerance, poor health-related quality of life (HRQoL), increased hospital admissions, increased mortality and higher health-care costs, which seem to be similar for those with HFrEF and HFpEF. 4,13 Historically, 5-year survival rates for patients with HF have been worse than for the most common cancers. With the increasing cost of hospitalisations, the focus of treatment over the past two decades has been on pharmacological therapies and devices (biventricular pacemakers and implanted cardiac defibrillators), which have resulted in improvements in survival and fewer hospitalisations, mainly in patients with HFrEF. 3
Poor HRQoL in people with HF13 is multifaceted and involves complex interactions across a range of measures, including severity of HF, extent of comorbidity (e.g. pulmonary disease and chronic kidney disease), exercise capacity, physical activity status, activities of daily living14,15 and depressive symptoms, which alone are evident in up to 42% of patients with HF. 16 As survival rates in people with HFrEF have improved, HRQoL is becoming a key patient-reported outcome measure. 3,17,18 Better management of HF can reduce uncertainty and anxiety and may improve quality of life. 19
The evidence base for cardiac rehabilitation in heart failure
Cardiac rehabilitation has, in its broadest sense, been defined as the sum of activities required to:
. . . influence favourably the underlying cause of cardiovascular disease, as well as to provide the best possible physical, mental and social conditions, so that the patients may, by their own efforts, preserve or resume optimal functioning in their community and through improved health behaviour, slow or reverse progression of disease.
Although exercise training remains a key component of CR, current practice guidelines consistently recommend ‘comprehensive rehabilitation’ programmes that contain the core components necessary to optimise cardiovascular risk reduction, foster health promotion behaviour patterns and their maintenance over time, reduce disability, and promote an active lifestyle. Guideline-based CR consists of three key elements: exercise to rebuild physical capacity and heart health, psychological support to help manage the emotional consequences of living with a heart condition, and support for key self-care behaviours. In the context of HF, key self-care behaviours include taking medications, engaging in (long-term) exercise, managing stress/anxiety and monitoring/managing fluid build-up. 21 Friends and family can have a key role in supporting patients to engage in and maintain these key self-care behaviours. CR for HF supports patients and carers to achieve or maintain good HRQoL as part of managing HF symptoms and HF-related self-care.
In 2004, the first Cochrane systematic review22 of exercise-based interventions for HF in 29 studies (1126 randomised participants) with up to 12-month follow-up reported that exercise training significantly improved exercise capacity compared with no exercise training. Only one study examined the effect of exercise on mortality and morbidity, and only nine out of 29 RCTs measured HRQoL, with seven of these reporting an improvement in exercise capacity. An updated Cochrane review14 published in 2010 focused on trials with follow-up of 6 months or longer that reported on HRQoL and clinical events – that is, mortality and hospitalisation. This review, which included data from a trial-level aggregate of 19 RCTs involving 3647 participants, found no difference in short- or long-term all-cause mortality between exercise and no exercise; however, exercise therapy resulted in a reduction in HF-related hospitalisations [risk ratio 0.72, 95% confidence interval (CI) 0.52 to 0.99] and an improvement in patient-reported HRQoL (standardised mean difference 20.63, 95% CI 20.37 to 20.80). However, most of the trials recruited predominantly men (median 87%) and, although more recent studies have included women, they were limited to people with mild or moderate HF – that is, class II or III of the New York Heart Association (NYHA) classification, which is used to categorise the severity of HF based on symptoms and functional exercise capacity, but not severe disease. 11,23 None of the trials included people with HFpEF, and the interventions in 14 out of 19 RCTs were delivered in centre-based settings.
A further update of the Cochrane review in 2014 reassessed the effectiveness of exercise-based rehabilitation on mortality, hospital admissions, morbidity and HRQoL in people with HF compared with no exercise training. 24 It also sought to identify additional evidence, including cost and cost-effectiveness, for those individuals poorly represented in previous reviews (i.e. older people, women and people with HFpEF) and the interventions specifically delivered in a home- or community-based setting. This review, which included 33 trials involving 4740 people with HF, identified no difference in pooled mortality between exercise-based rehabilitation and no exercise in trials with up to 12-months’ follow-up [relative risk (RR) 0.93, 95% CI 0.69 to 1.27]. However, there was a trend towards a reduction in mortality for exercise-based rehabilitation alone after more than 12 months of follow-up (RR 0.88, 95% CI 0.75 to 1.02). Compared with no CR, exercise training reduced the rate of overall hospitalisation (RR 0.75, 95% CI 0.62 to 0.92) and HF-specific hospitalisation (RR 0.61, 95% CI 0.46 to 0.80). A change of 5 points in the Minnesota Living with Heart Failure Questionnaire (MLHFQ) score is considered clinically important (higher scores indicate poorer HF-related HRQoL), and inclusion of exercise in rehabilitation programmes resulted in a greater improvement in this disease-specific measure of HRQoL (13 trials, 1270 participants, mean difference –5.8 points, 95% CI –9.2 to –2.4 points). Univariate meta-regression analysis showed that the benefits of exercise-based CR are independent of participants’ age, sex or degree of left ventricular dysfunction, type of CR (exercise only versus comprehensive), mean dose of exercise intervention, length of follow-up, overall risk of bias and trial publication date. Most of the participants included in this review had HFrEF and were categorised as having NYHA class II or III disease. 15,24 The more recent trials included people with HFpEF and NYHA class IV disease and greater proportions of women and older patients. Evidence from two trials supported the cost-effectiveness of exercise-based CR in terms of gain in quality-adjusted life-years (QALYs) and life-years saved. 25,26 Although Cochrane reviews of RCTs are not conclusive about the mortality benefits of CR, data from national audits show that patients referred to CR have better survival; however, observational analyses like these should be interpreted with caution, as they are subject to selection bias and confounding.
On the basis of evidence accumulating on the clinical benefits of CR, in 2010 the National Institute for Health and Care Excellence (NICE) recommended supervised, group-format, exercise-based rehabilitation for people with HF. 27 Similarly, other international guidelines on the management of HF, including those published by the American College of Cardiology/American Heart Association and the European Society of Cardiology (ESC), recommend CR as an effective and safe intervention. 11,28 The ESC recommends that CR must be integrated into the overall provision of care for patients with HF based on class IA evidence, which shows that regular aerobic exercise improves functional capacity and symptoms in people with HFrEF and also reduces the risk of hospitalisations in these patients. 11
Current provision of cardiac rehabilitation
Historically, CR after myocardial infarction (MI) and revascularisation (percutaneous coronary intervention or coronary artery bypass graft) has been provided for groups of patients in centre-based settings, such as hospitals. The 2017 annual statistical report from the National Audit of Cardiac Rehabilitation (NACR) states that, overall, 51% of eligible patients who had a MI, percutaneous coronary intervention or coronary artery bypass graft participated in CR in England, Northern Ireland and Wales. 29 Lower rates of uptake in these groups led to innovative ways of delivering CR, such as home-based CR using the Heart Manual. 30 This step-by-step guide is supported by a trained nurse facilitator and directs the patient through a 6-week programme of exercise, stress management and education (www.theheartmanual.com; accessed 20 February 2020). Supervised group-based interventions, as recommended by NICE,5,31 may be difficult for patients to access,32 and home-based CR programmes, such as the Heart Manual, are no less clinically effective than centre-based programmes but equally cost-effective. Patients like to have a choice of CR, and studies have shown that choice can enhance uptake.
The CR needs of people with HF can be more complex than for patients after uncomplicated MI or revascularisation – the settings in which CR was first used. People with HF are prone to greater levels of disability, as disease progression leads to increasing incapacity and deconditioning. 33 Activities of daily living are limited by symptoms such as shortness of breath and fatigue, thus affecting HRQoL. 13
The 2017 National Heart Failure Audit stated that there is underprovision of CR in the UK, with < 20% of patients with HF being referred to CR during hospitalisation. 34 According to the 2016 and 2017 reports from the NACR, people with HF make up < 10% of all patients who participate in CR. 35 The 2017 standards of the British Association for Cardiac Prevention (BACPR) recommend that HF should be a priority area and that CR providers should consider offering alternatives to centre-based CR to improve the current low uptake of CR in people with HF. 20
Importance and relevance of programme
The underprovision of CR has been evident for several years, with a national survey undertaken as part of the programme development application for the REACH-HF programme showing that only one in seven (35/224, 16%) CR centres in England, Wales and Northern Ireland provided a dedicated HF programme in 2009–10. A European survey conducted around the same time showed that < 20% of patients with HF were involved in CR. A recent registry study from the USA reported that only 10% of eligible patients with HF were referred for CR after hospital discharge, although this proportion increased between 2005 and 2014. 36
Despite good evidence (see The evidence base for cardiac rehabilitation in heart failure), the importance of CR in HF is not acknowledged in major clinical reviews. A key potential strategy for commissioners to improve on the current poor provision of CR in HF and address this important unmet need is to offer individuals choice by including a home-based CR intervention that is evidence based and provides an alternative to centre-based CR.
Involvement of caregivers
Despite recommendations that family members or caregivers should be included in discussions about care, few evaluations of supportive interventions for individuals caring for someone with HF have been published to date. There have, however, been calls for research to develop interventions with caregivers to improve quality of care, reduce costs and improve patients’ HRQoL. 37
Caregivers have an important role in supporting the management of chronic disease, although this often comes at considerable personal cost, and many caregivers are uncertain about how best to provide this support. Most caregivers have health and well-being problems of their own – either pre-existing conditions or issues arising from the burden of their caregiving activities. Caregiver burden is due to the tasks of caring (objective burden) and the distress associated with the role (subjective burden). The objective burden comes from activities that include physical tasks such as washing, dressing, feeding and assistance with mobility, which are often performed at night. Other caregiving activities support self-care of chronic conditions such as HF, including assisting with identification of signs and symptoms of deterioration, taking action during an emergency, and assisting with blood pressure monitoring and medication management. Caregivers also support adherence to dietary restrictions, support emotional needs, promote exercise and physical activity, provide transport, maintain safety, and liaise with health-care professionals (HCPs).
Innovation
We set out to design and develop a novel HF rehabilitation intervention/approach that would (1) provide information that is individually tailored rather than a one-size-fits-all approach; (2) include facilitation and careful monitoring of the development of self-care skills and strategies by a specially trained facilitator; (3) offer near-patient care in the patient’s home rather than requiring attendance at hospital clinics; (4) employ theoretically informed and evidence-based practice to support behaviour change; and (5) provide an intervention strongly informed by service-user consultation at every stage of its development. Overall, the intervention package was expected to offer a higher standard of care, and we hypothesised that it would result in improved self-care. 38
Original aims, objectives and outputs
The overarching aim of this programme was to improve the body of evidence-based knowledge of CR for HF to enhance the current low uptake of CR in HF and to inform future service commissioning.
Our specific objectives were to:
-
develop an evidence-informed, home-based, self-care CR programme for patients with HF and their caregivers (‘the REACH-HF intervention’)
-
conduct a pilot trial to assess the feasibility of a full trial to assess the clinical effectiveness and cost-effectiveness of the REACH-HF intervention in addition to usual care in patients with HFpEF
-
assess the clinical effectiveness and cost-effectiveness of the REACH-HF intervention in addition to usual care in patients with HFrEF and their caregivers
-
assess cost-effectiveness over the longer term of the REACH-HF intervention, other home-based CR and centre-based CR versus usual care in patients with HFrEF.
To meet these objectives, we developed a series of inter-related WPs (Figure 1):
-
WP1 – to develop a novel, evidence-informed, facilitated, self-care home-based rehabilitation intervention (‘the REACH-HF intervention’) for people with HF (see WP1A) and their caregivers (see WP1B) and undertake an uncontrolled feasibility study in patients with HfrEF.
-
WP2 – to conduct a single-centre, pilot RCT of the REACH-HF intervention to determine the feasibility of a full trial of its clinical effectiveness and cost-effectiveness in addition to usual care in patients with HFpEF (see Work package 2: single-centre, pilot randomised controlled trial of the REACH-HF intervention in patients with heart failure with preserved ejection fraction).
-
WP3 – to assess the clinical effectiveness and cost-effectiveness of the REACH-HF intervention in addition to usual care in patients with HFrEF and their caregivers in a multicentre RCT (see Work package 3: multicentre randomised controlled trial of the REACH-HF intervention in patients with heart failure with reduced ejection fraction).
-
WP4 – to assess the longer-term cost-effectiveness of the REACH-HF intervention, other home-based CR and centre-based CR versus usual care in people with HFrEF by using evidence synthesis and modelling methods to bring together evidence on home- and centre-based CR (see Work package 4: model-based cost-effectiveness analysis).
FIGURE 1.
Diagram depicting the REACH-HF programme and its inter-relationships. a, Adapted for patients with HFpEF for pilot RCT.
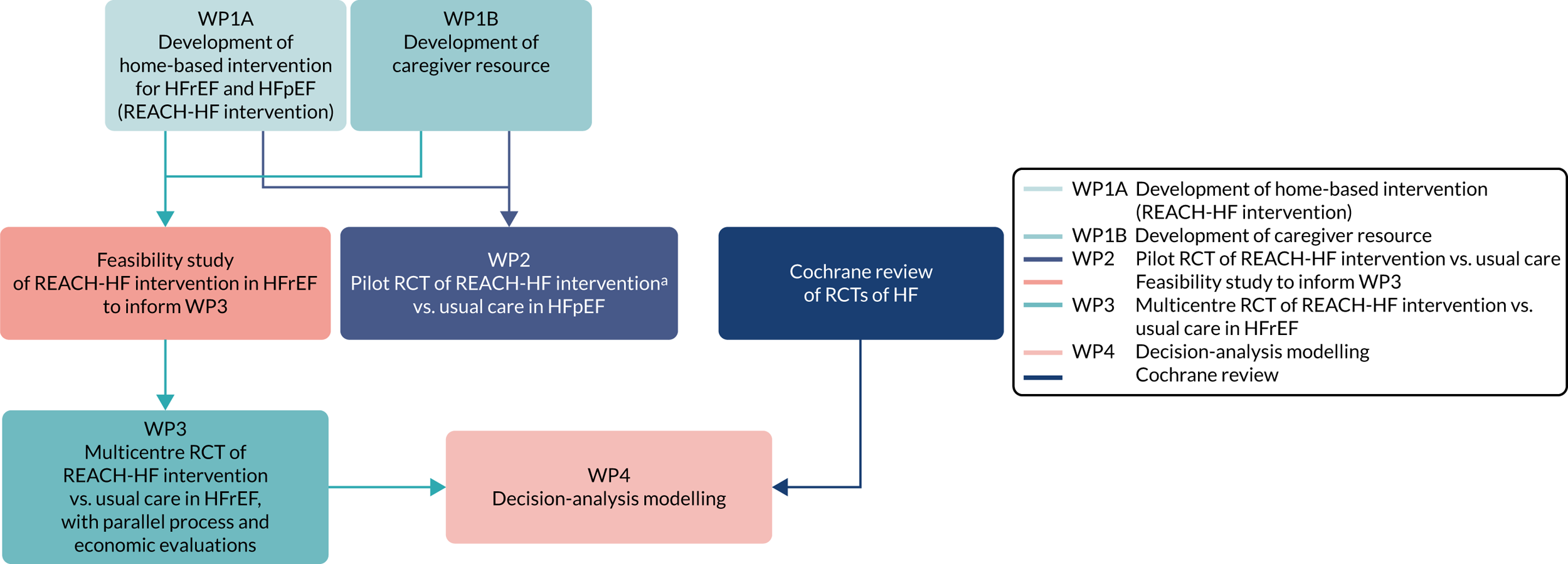
We were able to fully meet the programme objectives:
-
We co-designed the REACH-HF intervention with stakeholders including patients, caregivers, clinicians and commissioners.
-
Both WP2 and WP3 trials recruited to target and had excellent retention.
-
We successfully completed an updated evidence synthesis and economic modelling of the clinical effectiveness and cost-effectiveness of home- and centre-based CR.
Table 1 summarises the publication outputs from this programme.
| Programme objectives | Synopsis: research development | Programme outputs |
|---|---|---|
| WP1 – to develop an evidence-informed, home-based, self-care CR programme for patients with HF and their caregivers (‘the REACH-HF intervention’) |
WP1A: systematic intervention mapping methods to develop a facilitated home-based, self-help intervention for patients with HFrEF (‘the REACH-HF intervention’) WP1B: individual qualitative interviews and focus group interviews in participants’ homes to systematically develop a theoretically robust, home-based resource for caregivers of people with HF The REACH-HF intervention was also evaluated in a feasibility study in patients with HFrEF with parallel process evaluation |
REACH-HF intervention, comprising HF Manual, participant Progress Tracker booklet, Family and Friends Resource for caregivers, and facilitation by cardiac nurses or physiotherapists (details available from investigator team) Greaves et al. 39 (see Appendix 1) Wingham et al. 40 (see Appendix 1) |
| WP2 – to conduct a pilot trial to assess the feasibility of undertaking a full trial to assess the clinical effectiveness and cost-effectiveness of the REACH-HF intervention in addition to usual care in patients with HFpEF | WP2: pilot trial to assess the effectiveness of the REACH-HF intervention in patients with HFpEF |
Eyre et al. 41 (see Appendix 1) Lang et al. 42 (see Appendix 1) Smith et al. 43 (see Appendix 1) |
| WP3 – to assess the clinical effectiveness and cost-effectiveness of the REACH-HF intervention in addition to usual care in patients with HFrEF and their caregivers | WP3: RCT and process evaluation to assess the clinical effectiveness and cost-effectiveness of the REACH-HF intervention in addition to usual care vs. usual care alone in patients with HFrEF |
Taylor et al. 38 (see Appendix 1) Dalal et al. 44 (see Appendix 1) Wingham et al. 10 (see Appendix 1) Frost et al. 45 (see Appendix 1) Report on trial-based cost-effectiveness analysis (unpublished report, see Appendix 3) |
| WP4 – to assess cost-effectiveness over the longer term of the REACH-HF intervention, home-based CR and centre-based CR vs. usual care in people with HFrEF | WP4: evidence synthesis/modelling to assess the cost-effectiveness over the longer term of the REACH-HF intervention, home-based CR and centre-based CR vs. usual care in people with HFrEF |
Long et al. 1 (see Appendix 1) Taylor et al. 46 (see Appendix 1) Report on model based on cost-effectiveness analysis (unpublished report, see Appendix 3) |
Work package 1: intervention development and feasibility study
Some parts of these sections have been reproduced with permission from Greaves et al. 39 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated. The text includes minor additions and formatting changes to the original text.
Work package 1: overview
We used a systematic intervention development framework – intervention mapping47 – to develop a novel, evidence-informed, facilitated, home-based, self-care manual for people with HFrEF (‘the REACH-HF intervention’) (see WP1A) and their caregivers (see WP1B) and evaluated this intervention in a feasibility study in patients with HFrEF. This included the use of formal and informal literature reviewing, individual qualitative interviews, focus groups and workshops with a range of stakeholders (patients, caregivers, service providers and experts in the field) to develop a model of targets for change and intended processes of change (a logic model). Following this, we identified and ‘mapped’ change techniques to each intended process of change and strategically organised the intervention components. This resulted in a comprehensive, theory-based, user-centred, home-based, self-care support programme for people with HF and their caregivers. We collaborated closely with our patient and public involvement (PPI) advisory group throughout, such that the intervention can be considered to have been co-created with patients with HF and their caregivers. The resulting REACH-HF intervention includes three core printed components (i.e. the Heart Failure Manual, a Family and Friends Resource for caregivers and a Progress Tracker booklet), a choice of two exercise programmes for patients and a training course for facilitators. The intervention was delivered by trained facilitators (cardiac nurses or physiotherapists with experience of delivering CR programmes). We stipulated that, in most cases, patients should receive a minimum of two face-to-face sessions and around six contacts in total over the course of 12 weeks. The methods used to develop the novel REACH-HF intervention and a detailed description of the intervention and its theoretical basis have been published. 39 This paper included a supplemental file with data on the feasibility and acceptability of the intervention for patients and caregivers but with data on changes in outcome measures redacted (these data were considered sensitive at the time, as the main trial was under way). We now include the previously unpublished results of the feasibility study in Report Supplementary Material 2. The REACH-HF intervention was trialled in a feasibility study in patients with HFrEF and was used successfully in both of our RCTs (see Work package 2: single-centre, pilot randomised controlled trial of the REACH-HF intervention in patients with heart failure with preserved ejection fraction and Work package 3: multicentre randomised controlled trial of the REACH-HF intervention in patients with heart failure with reduced ejection fraction). In both studies, it was well accepted by study participants and HCP facilitators.
Work package 1: introduction
To manage HF effectively, patients need to engage in a number of self-care behaviours including exercise-based CR, which is recommended nationally and internationally. 48 However, participation in CR remains suboptimal in people with HF, which has been attributed to difficulties in patients accessing hospital- or centre-based CR and lack of support for caregivers. Consequently, there have been calls for alternative ways of delivering CR for patients with HF to improve the poor uptake.
Work package 1 concerned the development and feasibility testing of a novel, home-based intervention – ‘the REACH-HF intervention’ – for people with HF (WP1A) and their caregivers (WP1B). A detailed report of the development process and content of the intervention and its theoretical basis has been published (see Appendix 1). Report Supplementary Material 2 provides the results of a single-arm feasibility study designed to test the acceptability of the REACH-HF intervention in patients with HFrEF and the research methods for the main trial (see Work package 3: multicentre randomised controlled trial of the REACH-HF intervention in patients with heart failure with reduced ejection fraction) and to identify ways to improve/optimise the intervention ready for further evaluation. The intervention’s development and its content are further described below.
Work package 1: research aims
Intervention development aims (work packages 1A and 1B combined)
The aims of the intervention development were to:
-
establish the support needs of people with HF and their caregivers
-
use a systematic intervention development framework (intervention mapping) to develop a home-based CR intervention that is theory based and evidence informed, involving people with HF and their caregivers
-
develop a training course to support intervention delivery.
Feasibility study aims
The aims of the feasibility study were to:
-
assess the feasibility and acceptability of adding the REACH-HF intervention to usual care for patients with HFrEF, their caregivers and intervention facilitators
-
assess the fidelity (quality) of delivery of the REACH-HF intervention
-
assess the feasibility of outcome data collection processes and measure completion/attrition rates
-
identify any changes needed in the REACH-HF intervention or facilitator training.
Work package 1: methods
Framework for intervention development
Following the UK Medical Research Council guidance for developing complex health-care interventions,49 we used a systematic, evidence-informed approach to develop the REACH-HF intervention. Our approach was based on intervention mapping – a six-step systematic framework for intervention development:50
-
Step 1 – ‘needs assessment’ to identify targets for change.
-
Step 2 – building matrices to ‘map’ change targets against determinants of the desired changes.
-
Step 3 – selection of appropriate behaviour change techniques and strategies to address each determinant identified in step 2.
-
Step 4 – production of detailed intervention and training materials.
-
Step 5 – anticipating adoption and implementation of the intervention.
-
Step 6 – plans for evaluation of processes and effects.
Intervention mapping seeks to ground the intervention in the context and the population to be targeted, as well as the existing evidence base. A key element of the intervention development process was the inclusion of the REACH-HF PPI group, which consisted of six people from Cornwall with a range of experiences with HF and three caregivers of people with HF. An overview of the intervention development process is provided in Figure 2, and the following sections provide a summary of the first five steps of the intervention mapping process. The process evaluation plans (step 6) are described elsewhere (see Report Supplementary Material 3 and Work package 3: process evaluation). 39
FIGURE 2.
Development of the REACH-HF intervention. CG, caregivers; Cm, commissioners; HP, health-care providers (e.g. general practitioners, cardio specialists, nurses); P, patients; qual, qualitative; quant, quantitative.
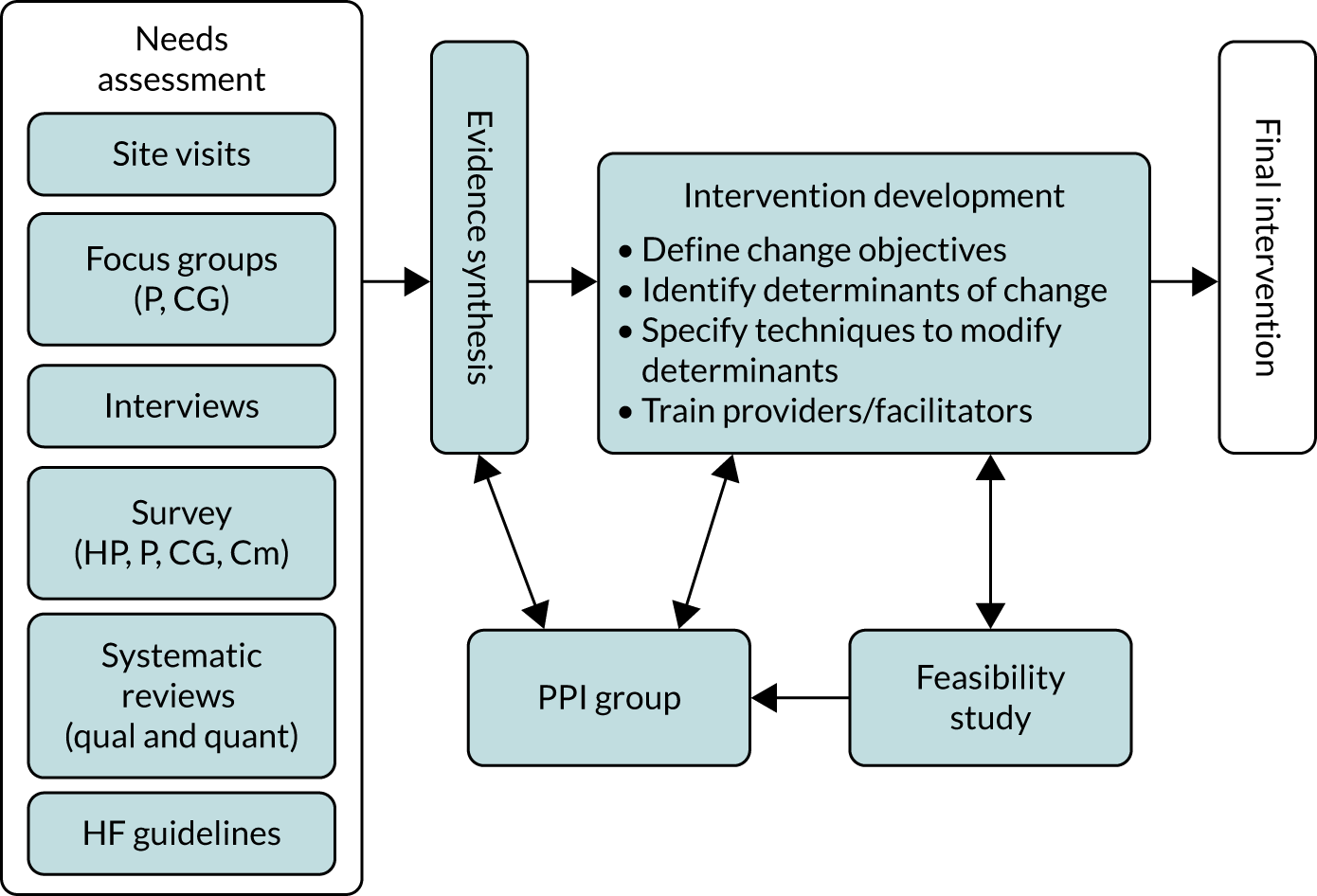
Step 1: needs assessment/identifying targets for change
The process began by assessing the needs of patients with HF, their caregivers and the service providers. This included gathering information on the problem, its causes and the target population and then developing a ‘causal model’ outlining the main modifiable factors that might contribute to an improvement in HRQoL for people with HF (see Figure 2).
Reviews of qualitative and quantitative literature provided a starting point for assessing the self-care support needs of patients and caregivers. An ongoing literature search (updated every 2–3 months) identified published reviews of self-care and rehabilitation interventions for people with HF from 1994 onwards. In addition, two de novo systematic reviews were undertaken by the project team: a meta-ethnographic synthesis of qualitative literature on the attitudes, beliefs and expectations of people with HF receiving CR51 and a systematic review and meta-analysis of the efficacy and safety of CR in people with HFpEF. 52 We also reviewed national and international clinical guidelines for HF recommended by our project management group, including the ESC21 and NICE practice guidelines. 27 The key recommendations on behaviour change, information needs or other changes needed to improve the HRQoL of patients or caregivers were extracted, along with potential self-care strategies and potential determinants of such changes.
A number of systematic reviews and guidelines highlighted the importance of exercise-based rehabilitation as a central element in driving positive outcomes in HF. 14,21,27,52 As a result, a specialist working subgroup of project team members (PD, SS, KJ, JA, CG and RT) met several times (along with extensive e-mail interaction) to develop and refine the exercise and physical activity components of the intervention.
We conducted focus group interviews with two community-based HF support groups and attendees at a hospital-based rehabilitation class. Each group included 12–20 patients and 6–10 caregivers. The main topic areas were ‘coming to terms with heart failure’; benefits of and barriers to exercise/physical activity; problems and solutions associated with taking medications; information and support needs; advice for family members or caregivers; and how the REACH-HF intervention should be delivered.
To further elicit the views of key stakeholders, a needs assessment questionnaire was circulated to 10 people with HF and 24 other experts in the field, including two behavioural scientists, 14 specialist nurses (HF, CR and primary care cardiac nurses), two cardiologists, two general practitioners (GPs), two exercise physiologists with CR experience and two pharmacists. This was an opportunity sample based on contacts known to the REACH-HF project management group and people in the focus groups who had volunteered to complete the questionnaire. The questionnaire (which is published alongside the intervention development paper39) included questions about what outcomes are important for people with HF; self-care behaviours that should be targeted; information and support needs; suggested content and delivery formats; and who might deliver the intervention. Respondents were also asked how the manual could be adapted for a range of users, including those with HFpEF.
The REACH-HF PPI group helped to design the topic guide for the focus group interviews, completed and commented on the needs assessment survey and commented on summaries of information from the focus groups. The group met every 2 months throughout the 12-month needs assessment stage, with additional e-mail and postal correspondence between meetings.
A qualitative research study involving face-to-face, semistructured interviews with a purposive sample of 26 caregivers of people with HF with a range of sex, age and socioeconomic status was also conducted to specifically identify caregivers’ needs. 40
Understanding the context or community in which an intervention is delivered is another important aspect of needs assessment. 50 A member of the research team (Wendy Armitage from the Heart Manual Department) conducted site visits to HF treatment centres and a range of staff at four sites (Truro, York, Birmingham and Abergavenny) and administered a questionnaire on current service provision. 53 This identified existing strengths, limitations, competencies and capacities of potential providers.
Two team members (Carolyn Deighan and Michelle Clark from the Heart Manual Department) reviewed further literature to identify evidence on the effectiveness of relaxation and mindfulness interventions for people with HF (and other chronic illnesses) to inform the stress management component of the Heart Failure Manual. Finally (just prior to implementation in the feasibility study), a training needs questionnaire was sent to HCPs who had been selected to deliver the intervention to assess their current state of knowledge/expertise with regard to key elements of the intervention. This was used to tailor the training course in step 4.
A key challenge was to summarise and integrate the data and ideas from many diverse sources. We did this using a framework for mixed-mode evidence synthesis called triangulation protocol. 54 First, a thematic synthesis of the needs assessment documents and recordings was used to generate a ‘needs assessment’ table, which listed the key recommendations from each evidence component. We then considered where the recommendations from each source agreed (convergence), offered complementary information on the same issue (complementarity) or seemed to be contradictory (dissonance). Where there was dissonance, we resolved this through further discussion with the members of the project management and PPI groups. The focus of the data synthesis was on identifying (1) targets for change and (2) modifiable determinants of the changes suggested. This analytical process was conducted separately for patients and caregivers.
The themes identified by the above synthesis were organised into a logic model55 for the intervention (Figure 3). This was developed by grouping the targets for change into broad themes (behavioural, environmental, social and psychological) and mapping them onto a generic causal modelling framework for intervention development (the PRECEDE model50,56). It was acknowledged that environmental and contextual factors (e.g. home environment, social support networks) might affect HRQoL directly or indirectly (via interaction with behavioural or psychological factors).
FIGURE 3.
Logic model/behavioural specification for the REACH-HF intervention. ADL, activities of daily living.
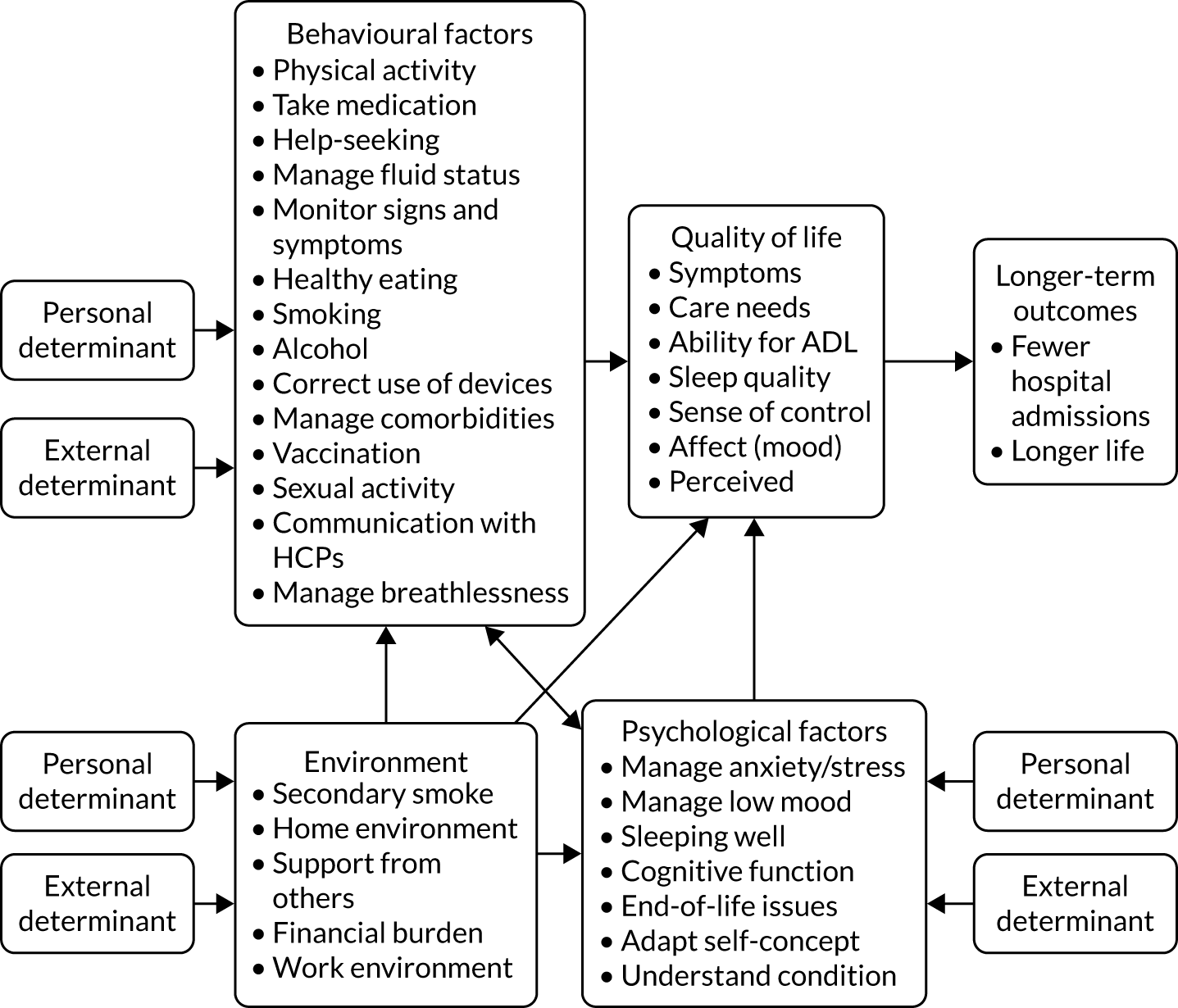
The targets for change (Box 1) were then prioritised through a process that included noting the level of agreement between stakeholders from the needs assessment table, consultation with the project PPI group and further discussion within the project team. We took into account the strength of the evidence base and the potential for improving HRQoL. The highest priority targets for change (shown in dark blue in Box 1) were then grouped into the following five categories:
-
engaging in exercise training to build (and maintain) cardiovascular fitness
-
managing stress, breathlessness and anxiety
-
HF symptom monitoring (and associated help-seeking), particularly in terms of managing fluid status
-
taking prescribed medications
-
understanding HF.
Engage in exercise training (to improve cardiovascular fitness).
Longer-term physical activity (to maintain cardiovascular fitness).
Manage stress/anxiety.
Manage fluid status (over and under hydration).
Monitor signs and symptoms: seek help appropriately.
Take medications.
Manage breathlessness.
Understand HF.
Manage low mood.
Manage fatigue.
Healthy eating.
Manage/live with uncertainty (about possible deterioration/decompensation events/end of life).
Sleep well.
Maintain social activities/social roles.
Weight management.
Manage severe depression.
Stop smoking.
Manage alcohol intake.
Manage/organise the home and work environments.
Manage financial burden/organising benefits.
Manage comorbidities (other illnesses) that might affect the ability to manage HF.
Manage and respond appropriately to devices (e.g. implantable cardiac defibrillator, cardiac resynchronisation therapy), including managing anxiety around devices.
Understand medications/treatments.
Get vaccinations.
Engage in sexual activity if desired.
Dark blue font indicates full coverage (core topic and important for all).
Light blue font indicates needs-based intervention (topic important for some but not all patients).
Orange font indicates case management approach (topic important for some but needing external input).
Light orange font indicates information only (topic peripheral or of relatively minor importance in most cases).
Item 5 (understanding HF) was included following step 3 (see below), as it was identified as a core determinant underpinning engagement with the first four targets for change.
The project management group and PPI group agreed that the above core priorities should receive strong focused support from the intervention facilitator and that the intervention manual should contain interactive elements to support change in these areas (e.g. for exercise training, we included a choice of a walking programme or chair-based exercise programme, as well as interactive tools for goal-setting and self-monitoring).
A second set of targets (the light blue text in Box 1) were identified as important for some but not all patients (e.g. smoking cessation and healthy eating). It was agreed that these aspects should be assessed and (briefer) intervention from the facilitator provided if needed.
A third set of targets, although important for some patients, were deemed to be outside the remit of the provider (e.g. management of severe depression) or possible to address through existing services (e.g. smoking cessation). It was agreed that these topics would be dealt with using a case-management approach.
A fourth set of targets were categorised as peripheral or minor topics, such as vaccination. For these topics, the patient was assessed, given some information and, if needed, signposted to further agencies or information (e.g. websites).
The core priorities for the caregiver resource were to:
-
facilitate improvement in HRQoL for the person with HF by helping them to achieve the core priorities for change for patients (see Box 1)
-
improve the quality of life for caregivers by acting to maintain their own health and well-being.
The target of ‘understanding heart failure’ was also felt to be of core importance in underpinning engagement with the above targets. The targets for change for caregivers that emerged from the needs assessment process are shown in Box 2. A focus group with four caregivers was conducted to prioritise the targets in the same way as described for the patient manual (see Analysis and integration of needs assessment data).
Support monitoring and management of signs/symptoms of HFa (e.g. when to ‘step in’).
Understand HF. a
Understand and manage medicines. a
Exercise/physical activity promotiona (facilitate the HF Manual exercise programme as well as maintenance).
Provide emotional support to help manage patient’s mood, anxiety, stress.
Manage caregiver stress and emotional consequences.
Manage own physical healtha (including physical activity/lifestyle, health problems, safe lifting).
Become a caregiver (psychological and practical adaptation, role negotiation).
Sleep well.
Deal with emergencies (including resuscitation issues).
Communication with health professionals.
Manage/organise the home and work environments – daily hassles.
Maintain own social activities/social roles.
Monitor signs/symptoms of depression of cared-for person. a
Understand and manage comorbidities. a
Provide and engage social support (e.g. offer information, encouragement and practical support; engage friends, relatives, carer groups).
Live with uncertaintya (e.g. possible deterioration/decompensation events, end-of-life issues).
Attend to physical needs of the cared-for person (practical tips).
Manage and respond appropriately to devices (e.g. implantable cardioverter defibrillator, cardiac resynchronisation therapy), including managing anxiety around devices. a
Engage social services (e.g. carer needs assessment).
Manage financial burden/organising benefits for caregiver.
These self-care issues are also dealt with in the HF Manual, and relevant sections are referenced from the caregiver resource.
Dark blue font indicates full coverage (core topic and important for all).
Light blue font indicates brief, needs-based intervention (topic important for some but not all patients).
Orange font indicates case management approach (topic important for some but needing external input).
Step 2: specifying performance objectives/identifying determinants of change
The behavioural, environmental, social and psychological targets for change resulting from needs assessment (see Boxes 1 and 2) were broken down into more proximal ‘performance objectives’. Performance objectives are statements of who needs to change and what behaviours or thought processes need to be changed (and in what circumstances) to achieve each target. 50 For each performance objective, modifiable determinants of change were identified using several parallel methods:
-
Existing evidence (e.g. process evaluations in rehabilitation studies).
-
Theories of behaviour change and psychological adaptation. 51,57–63
-
Evidence identified during the needs assessment stage (e.g. qualitative data, needs assessment questionnaire).
-
A structured 1-day workshop with a panel of experts in the field (two exercise/rehabilitation specialists, two cardiac specialist nurses, two GPs with cardiac special interest, two cardiologists and three behavioural scientists).
-
Similar structured workshops (three separate 2-hour sessions) with the PPI group. The consultation workshops focused on the ‘core priority’ change targets.
In the workshops, the ‘core priority’ targets for change and their associated performance objectives were presented to the expert panel and the PPI group. For each performance objective, the panel were asked:
-
What will help people to achieve this target?
-
What will stop people achieving this target? What will get in the way?
-
How could we help people to overcome any barriers and achieve this target?
A facilitated group discussion resulted in a list of modifiable determinants (barriers and facilitators) relating to each objective.
The performance objectives and determinants were then used to construct a set of ‘mapping matrices’ or tables. The first two columns in Table 2 show the performance objective and determinants for performance objective 1: ‘Engage in exercise training sessions 2–3 times per week’ as an example. Separate matrices of performance objectives and determinants were constructed for the caregiver intervention.
| Performance objective | Modifiable determinants | Change techniques | Strategies |
|---|---|---|---|
| 1. Engage in exercise training sessions two or three times per weekb |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
The intervention design team specified a theoretical basis that (1) provided sufficient range to incorporate all the determinants of change identified for each change target, and (2) was as parsimonious as possible.
Step 3: specification of change techniques and strategies
Step 3 of the intervention mapping process involved the selection of change techniques (e.g. behaviour change and psychological intervention techniques) targeting each of the determinants of change identified in step 2. In addition to expert opinion and experience, this work drew on an existing taxonomy of behaviour change techniques64 and the expertise of the REACH-HF collaborators in developing disease management programmes and CR programmes to identify potentially successful strategies for patients with HF and their caregivers. The PPI group were asked about the strategies that they had found to be successful and reviewed the selected change strategies (and the final programme materials) to ensure that they were likely to be feasible and acceptable for patients and caregivers.
Step 4: production of detailed intervention and training materials
The outputs from the first three stages of the intervention mapping process were used to generate detailed intervention materials and a training course for facilitators. These are described in Work package 1: results. The PPI group commented on the materials in terms of both format and content.
Step 5: anticipating adoption and implementation issues – the REACH-HF feasibility study
We conducted a single-arm feasibility study (ISRCTN25032672) with a parallel process evaluation across four sites (Birmingham, Cornwall, Gwent and York) to (1) assess the feasibility and acceptability of adding the REACH-HF intervention to usual care for patients with HFrEF, their caregivers and intervention facilitators, (2) help refine the intervention in advance of the main trial and (3) assess the quality of intervention delivery. 39
The REACH-HF intervention was delivered to 23 patients (and 12 caregivers) by seven trained intervention facilitators at four sites (Cornwall, Abergavenny, Birmingham and York) over a period of 12 weeks in addition to usual care. Process data to help assess feasibility, acceptability and quality of intervention delivery (intervention fidelity) were collected from multiple sources:
-
Qualitative methods were used to explore the stakeholder experiences of using and delivering the intervention. Brief semistructured interviews with 13 patients and seven caregivers about their experiences of receiving the REACH-HF intervention were conducted by telephone or face to face. These were undertaken at 6 weeks (halfway through the intervention period) and at around 15 weeks (at the end of the intervention period). The seven intervention facilitators completed feedback forms at the end of each session. They documented what went well or not well and also participated in up to three (audio-recorded) debriefing/feedback sessions during intervention delivery and a focus group at the end of the intervention period. Topic guides (see Report Supplementary Material 1) were developed in consultation with the intervention development team, the REACH-HF investigators and our PPI Advisory Group.
-
A 13-item intervention fidelity checklist was applied to audio-recordings of all intervention sessions for 18 participants to assess the quality of intervention delivery (see Appendix 1). This produced a score of 0–6 for each (predefined) key component of the intervention process. Two members of the intervention design team (from a pool of three team members) independently rated intervention fidelity for each recording. To improve inter-rater reliability, prior to rating all the recordings, the three coders (Anna Sansom, Colin J Greaves and Jennifer Wingham) coded the same four audio-recordings and had debrief meetings to compare scores and agree principles to identify examples of adequate, excellent or poor delivery for scoring going forwards. A detailed instruction set was developed and is available in Appendix 1. 39
-
Patient and caregiver outcome measures planned for the main RCT were collected before and after the intervention (3 months after baseline) to assess their feasibility and acceptability. Serious adverse events (SAEs) and adverse events that were potentially related to the intervention were recorded for patients (not for caregivers).
Work package 1: analysis
The recorded interview data, facilitator feedback forms and other qualitative data were subjected to simple thematic analysis. 65 Data were transcribed verbatim and analysed to extract concepts, which were then grouped into higher-level themes using a constant comparison approach. Data were organised using NVivo version 11.0 (QSR International, Warrington, UK). Two researchers independently analysed a sample of interviews and then conferred to develop an initial coding framework (see Report Supplementary Material 3). Quantitative outcome measures were summarised using basic descriptive statistics, with mean pre–post change scores for outcomes presented alongside CIs, as well as completion rates. Fidelity scores were summarised across participants for each checklist item and also broken down by facilitator.
Work package 1: results
Feasibility study results
A summary of the findings is available in Appendix 1. The feasibility study recruited 23 patients and 12 caregivers, and seven intervention facilitators provided the intervention.
-
Patients and caregivers were highly satisfied with the REACH-HF intervention and attendance at sessions was high (all patients attended at least three face-to-face sessions and typically received four telephone contacts).
-
A number of potential modifications to the content of the manual and facilitator training were identified.
-
Intervention fidelity scoring of all of the delivered consultations for 18 cases indicated adequate delivery for most aspects of the intervention by all facilitators [mean scores ranged from 2.6 (item 11, caregiver health and well-being) to 5.0 (item 1, person-centred delivery)] and are presented in in the feasibility study report (see Report Supplementary Material 1, Table A).
-
Two items [addressing emotional consequences of being a caregiver (item 10) and caregiver health and well-being (item 11)] had suboptimal delivery–fidelity scores.
-
We also computed fidelity scores collated per facilitator, and these are presented in Table 5. These represent delivery for only three patients per facilitator and so should be treated with caution. Despite this, we observed reasonable consistency between facilitators on most items.
-
Levels of outcome completion were generally excellent, and patients/caregivers perceived relatively low measurement burden.
-
A number of patient and caregiver outcomes following the REACH-HF intervention showed evidence of improvement (with all of the caveats of a small population of selected participants and uncontrolled comparisons).
-
No patient or caregiver safety concerns were identified.
-
Physical fitness [measured through the Incremental Shuttle Walk Test (ISWT)] was successfully carried out by the majority of patients. As per previous studies, older patients with multiple comorbidities showed some improvement, but this did not achieve statistical significance over time.
A number of ideas for improving the text of the HF Manual and the training were identified, such as changing the name of the original ‘Caregiver Resource’ to ‘Family and Friends Resource’ to promote engagement with the intervention, as many cohabitees did not identify themselves as a ‘caregiver’. Following processing of the above data, the HF Manual (including the Family and Friends Resource and Progress Tracker) and training course were substantially revised. This final version was then evaluated in Work package 3: multicentre randomised controlled trial of the REACH-HF intervention in patients with heart failure with reduced ejection fraction.
The REACH-HF intervention
The REACH-HF intervention is a comprehensive self-care support programme comprising the ‘HF Manual’, including a choice of two exercise programmes for patients, a Family and Friends Resource for caregivers, a Progress Tracker booklet and a facilitator training course. For patients, the main self-care targets are engaging in exercise training, monitoring for symptom deterioration, managing stress and anxiety, managing medications and understanding HF (see Box 1 for an expanded list). Secondary targets include managing low mood and smoking cessation (where relevant). For caregivers, the main intervention targets are supporting self-care for the patient and looking after their own physical and mental health (see Box 2). The intervention is facilitated by trained HCPs with specialist cardiac experience (e.g. a HF nurse or physiotherapist) over 12 weeks via home and telephone contacts. The four main REACH-HF intervention elements as described in Figure 4.
FIGURE 4.
The REACH-HF intervention materials.

Heart Failure Manual
This is a self-help resource for use by patients and their caregivers. The HF Manual includes patient-focused/plain English explanations of what HF is and how people can learn to live with the condition to maximise their quality of life. The text aims to improve understanding of HF in terms of the identity of the illness, as well as causes, consequences, control/treatment and timeline, in line with Leventhal’s common sense model of illness. 61 It includes sections on taking medications, fluid management (including a traffic-light guide on recognising when to seek help), managing stress, managing changes in symptoms and a choice of two structured exercise programmes (see REACH-HF exercise programme). The manual also includes a compact disc (CD) with relaxation and breathing control exercises from the existing Heart Manual30 and appendices offering self-care advice on other self-care behaviours, such as smoking cessation, implanted devices, managing low mood and healthy eating.
Progress Tracker
This is an interactive booklet designed to facilitate learning from experience/over time and build understanding about the impact self-care activities have on symptoms, emotional well-being and quality of life through practice, self-monitoring of progress and (facilitated) problem-solving.
Family and Friends Resource
This is a manual for use by caregivers. It aims to increase caregiver understanding and skills both to help the person with HF and to look after their own physical and mental well-being. The resource is divided into three main sections: (1) supporting the patient’s self-management of HF (‘Providing support’), (2) caring for the caregiver (‘Being a caregiver’) and (3) practical advice, including mobilising social support, accessing benefits and other formal and voluntary support (‘Getting help’).
Training course for facilitators
A training manual/syllabus for a 3-day training course for the REACH-HF intervention facilitators was developed. Facilitators were defined as professionals with experience in CR or cardiac nursing. The facilitation role is crucial to the success of the REACH-HF programme. As well as being the main delivery process, it enables tailoring of the REACH-HF intervention resources to the individual needs of patients and their caregivers. The course includes the theory and process of facilitation (building rapport using patient-centred counselling techniques,66 empowerment and support of self-management, building understanding of the condition61); using behaviour change techniques; techniques for managing stress and anxiety; contents of the manual; supporting exercise and physical activity using the intervention materials; and facilitation of the Family and Friends Resource and medical/nursing issues. The training was linked by three case studies of patients with HF and opportunities to practise facilitation techniques and to problem-solve potentially difficult situations.
A set of quotations or ‘patient voices’ selected by patients and caregivers in the PPI group from our prior qualitative work was incorporated throughout the written resources in the form of speech bubbles to help illustrate key points (Figure 5).
FIGURE 5.
Extract from the HF Manual showing a ‘patient voice’ callout.
REACH-HF exercise programme
Patients were offered a choice of two exercise programmes:
-
A chair-based exercise programme and digital versatile disc (DVD) that had been previously developed by Professor Patrick Doherty, who is a REACH-HF co-investigator and was principal investigator at the York trial site. The DVD includes seven levels of aerobic and resistance exercise with progressively increasing intensity. The exercises are designed to avoid undue breathlessness, build cardiovascular fitness, improve efficiency of movement and strengthen muscles to facilitate functional activities associated with daily living. The chair-based exercise programme was integrated into the REACH-HF intervention and facilitators received training on how to support patients in using the chair-based exercise DVD. The intellectual property for the chair-based exercise programme belongs to Professor Doherty who, as part of the REACH-HF and NIHR intellectual property agreement, licensed the chair-based exercise programme DVD to REACH-HF for the main trial and subsequent dissemination of REACH-HF.
-
A progressive walking-training programme based on increasing walk duration and intensity over time to build cardiovascular fitness and lower limb muscle strength.
The starting level (for the DVD) or walking time/distance (for the walking programme) was agreed (by patient and facilitator) based on results from an ISWT in conjunction with data and metabolic equivalent (MET) values for each of the known fitness level (METs) for each of the seven chair-based exercise programme levels. The proposed chair-based exercise level would start patients at around 65–70% of their ISWT MET, which is an expression of physical fitness. Values for ISWT (e.g. metres walked or speed achieved) were used to guide the walking exercise programme, using mostly distance-walked targets or speed of walking, where that suited the patient’s ability and goals.
Patients were also given pragmatic instructions on how to work at a moderate intensity. We wanted to ensure that the initial exercise prescription for patients was in the range of 65–70% of their maximal fitness derived from the ISWT. In addition, we sought to avoid reliance on the use of heart rate monitors by helping patients to become familiar with and skilful at using a self-rating scale of perceived effort (scored 1–10 from ‘nothing’ to ‘exhausted’) and advised patients to work at an effort level of 4–6. We also encouraged them to monitor their breathing and to work at a level that resulted in breathing heavier, feeling warmer and having a faster heartbeat. Instructions for warming up and cooling down were given, and, where possible, the facilitator observed the patient doing some exercise (using the DVD or walking) to ensure that the patient understood the level of intensity required. We advised that patients should be a little out of breath but still able to carry on a conversation. Patients were allowed to ‘mix and match’ between the two programmes if desired and were encouraged to engage with one of the exercise options every other day, or at least three times per week.
Intervention delivery
Based on existing CR practice and clinical guidelines, 12 weeks was considered an appropriate duration for delivery, with a minimum of three face-to-face contacts with a facilitator (plus telephone contacts) during this time. The face-to-face contacts were designed to be delivered in the patient’s home. The number of contacts was not specified exactly to allow tailoring of the delivery to patient needs, and no maximum number of sessions was stipulated. However, we stated that, in most cases, patients should receive a minimum of two face-to-face sessions and about six contacts in total over the course of 12 weeks. In practice (see Work package 3: multicentre randomised controlled trial of the REACH-HF intervention in patients with heart failure with reduced ejection fraction), a mean of four face-to-face contacts and a mean of 6.5 contacts were delivered in total.
Theoretical basis
As different barriers and enablers (processes of change) were identified for different change targets, the resulting intervention drew on multiple theoretical perspectives. Despite this, several common theoretical processes for supporting the targeted changes in behaviour and psychological processes were identified (Table 3). Key principles included building an understanding of the condition to provide a rationale for change (Leventhal’s common sense model61); building intrinsic motivation and promoting autonomy (self-determination theory57); promoting adaptation to living with HF and an active approach to coping;51,63 and encouraging learning from experience through engagement in self-care activities (control theory68). The elements were aimed at managing stress and anxiety used psychological intervention processes based on cognitive–behavioural therapy70 and mindfulness therapy. 71,72
| Process (and theoretical basis) | Key features and intervention facilitation techniques |
|---|---|
| Active patient involvement (motivational interviewing66/self-determination theory57) | The facilitator should encourage the participant to be actively involved in the consultation. The idea is to maximise the participant’s autonomy as the main agent of change, developing intrinsic rather than extrinsic motivation. However, the consultation should be guided. Empathy-building skills (open questions, affirmation, reflective listening, summaries) and individual tailoring should be used throughout the consultations. Reflective listening may be used to direct the conversation or highlight key strengths or barriers. A collaborative/shared decision-making style is appropriate, and the facilitator may share their own expertise and ideas. The Ask–Tell–Discuss technique should be used to exchange information (e.g. to address misconceptions or offer helpful new information). Overall, the participant should be increasingly empowered to take control of her/his self-care behaviour. Interactions should be encouraging, respectful and non-judgemental. The interaction should also be individually tailored to the patient’s specific information needs, beliefs, skills, and priorities |
| Assessing the patient’s current situation and needs (motivational interviewing,66 individual tailoring67) | The facilitator should use patient-centred communication techniques (as above), which may include the Ask–Tell–Discuss and open-ended questions to explore the patient’s current situation. This should include all of the following:
|
| Formulating an individualised treatment plan (self-regulation/control theory,68 individual tailoring67) | The facilitator should use patient-centred communication techniques (as above) to formulate an appropriate treatment plan based on the patient’s current situation (as assessed above). The treatment plan will be staged over time, aiming to work on a few topics initially and introducing other elements as the programme continues. This should be set up as an experiment to see how feasible the proposed actions are and whether or not they help the patient’s situation. An element of guiding to ensure the inclusion of clinical priorities (e.g. medication issues, exercise), as well as patient priorities, may be appropriate. The facilitator and participant should formulate a specific written action plan (using the template in the Progress Tracker) for exercise-training based on a choice of the two REACH-HF exercise-training programmes. The patient and caregiver should be ‘signposted’ to relevant sections of the manual. The facilitator may also employ some problem-solving techniques at this stage to pre-empt and address potential problems |
| Building the patient’s understanding of HF/their situation (Leventhal’s common sense model,61 theories of illness adaptation63) |
The facilitator should elicit the patient’s and caregiver’s current understanding of HF and seek to build their ‘illness model’ in terms of understanding the identity, causes, consequences, cure/control options and timeline associated with the condition. This process may take several weeks and should be reinforced as the programme progresses Facilitators will signpost the patient and caregiver to relevant sections of the manual, including the ‘Understanding heart failure’ section and use patient-centred communication techniques (as above) to elicit and build understanding. The Ask–Tell–Discuss technique and reflective listening will be used to exchange information to reinforce elements of the patient’s understanding that predispose positive self-care behaviours (e.g. understanding the link between physical fitness and symptoms of HF). The facilitator should seek to reframe negative attitudes and exchange information to address misconceptions or address important gaps in understanding. Learning should be reflected on/reinforced at subsequent sessions |
| Supporting self-regulation skills (self-regulation/control theory,68 relapse prevention,69 theories of illness adaptation63) |
The facilitator should discuss and encourage the use of the ‘Progress Tracker’ workbook in the HF Manual to keep track of progress and as a way of recording and addressing any problems completing the activities and any benefits that might be associated with the planned activities. At subsequent meetings, the facilitator and participant should review progress with all planned changes to exercise/physical activity and other self-care activities. The facilitator should reinforce and reflect on any successes. The participant and facilitator should discuss any setbacks, encourage identification and problem-solving of barriers to self-care, and the patient’s plans should be revised accordingly. Reframing should be used to normalise setbacks and see them as an opportunity to learn from experience (trial and error) rather than as failures Problem-solving should use OARS and information exchange (Ask–Tell–Discuss) techniques to identify barriers and explore ways to overcome them. Problem-solving may specifically focus on issues of connectedness (social influences, involvement of others in supporting activities) and long-term sustainability or on breaking the problem down into more manageable chunks |
| Addressing emotional consequences of HF (cognitive–behavioural therapy,70 mindfulness,71 theories of illness adaptation63) | The facilitator should help the patient recognise and address any significant stress, anxiety, anger or depression that is related to having HF. They should seek to normalise such feelings and help the patient to access and facilitate use of the cognitive–behavioural therapy techniques and stress management techniques within the manual. If depression, anxiety or other emotional problems are severe, a referral to appropriate clinical services should be facilitated |
| Caregiver involvement (if applicable) (literature on caregiver needs40) |
The facilitator should engage the caregiver as much as possible as a co-facilitator of the intervention. The facilitator should tailor the intervention to work with the caregiver’s abilities and availability. Person-centred counselling techniques (OARS) should be used for caregiver assessment and to exchange information to build the caregiver’s understanding of the situation and help them recognise and manage their own health needs, including mental health, physical health and social needs. The facilitator should facilitate a conversation between the patient and the caregiver to agree their roles and responsibilities and how these might change if the patient’s condition declines. Attention should be given to the caregiver’s needs and concerns about being a caregiver/providing care, as well as those of the patient The facilitator should help the caregiver to recognise and address any significant stress, anxiety, anger or depression related to supporting someone with HF and facilitate the use of the cognitive–behavioural therapy techniques and stress management techniques within the manual, as needed. This includes facilitating a referral for a carer’s assessment if the caregiver wishes, as well as referral to other relevant care services as appropriate The facilitator should help the caregiver to prioritise and look after their own health and well-being |
| Bringing the programme to a close (Leventhal’s common sense model,61 theories of illness adaptation,63 self-regulation/control theory,68 relapse prevention69) | Progress should be consolidated and reinforced. Plans for long-term sustainability of activities and strategies learned for managing HF should be discussed. The facilitator will review progress since the start of the intervention and reinforce what has been learnt. Useful strategies that were helpful should be identified. Plans to stay well/prevent relapse should be discussed, as well as ‘cues for action’ and plans to revisit the manual in the future. The facilitator will discuss plans to sustain any new activities, identifying any potential problems and coping strategies to overcome these. The possibility of good and bad days should be discussed and normalised |
Work package 1: discussion
Summary
Intervention mapping gave a clear structure and process for developing the REACH-HF intervention and the associated training programme for intervention facilitators. The process took into account the needs of a range of stakeholders, including patients with HF, their caregivers, HCPs, potential facilitators and health-care commissioners.
The construction of a causal model as part of the intervention mapping method (see Figure 2) was useful as a framework to integrate the identified needs and define the intervention’s ‘targets for change’. However, as reported by other studies that have used intervention mapping to develop complex health-care interventions,73–75 the overall process was time-consuming and resource intensive.
Successes
To our knowledge, this is the only publication that provides a detailed description of the theoretical and evidentiary basis, intervention techniques and strategies for an intervention to promote quality of life in people with HF. The intervention was co-developed with patients, caregivers, clinicians and academics to optimise it for use with patients with HF in real-world CR settings, and this was a particular strength of the research. In particular, PPI ensured that the intervention was tailored to individual needs based on a diverse range of patient backgrounds, knowledge levels and severity of HF.
Limitations
The complexity of the intervention development process may affect replicability, and it is unlikely that a different team of collaborators using the same methods would have produced exactly the same intervention.
Conclusions
We developed a comprehensive, evidence-informed, theoretically driven, self-care and rehabilitation intervention that is grounded in the needs of patients and caregivers. The intervention was well received by patients and caregivers and was feasible for and acceptable to the nurses and physiotherapists who delivered it. The intervention was delivered to an acceptable standard, although some revisions to the intervention and its associated training course were suggested. After these revisions, the REACH-HF intervention was ready for evaluation in a full-scale trial.
Work package 2: single-centre, pilot randomised controlled trial of the REACH-HF intervention in patients with heart failure with preserved ejection fraction
Some parts of these sections have been reproduced with permission from Eyre et al. 41 This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/. The text includes minor additions and formatting changes to the original text.
Some parts are also reproduced with permission from Lang et al. 42 and Smith et al. ,43 both of which are Open Access articles distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
Work package 2: overview
We undertook a pilot RCT and a process evaluation in a single centre in Dundee, in which there is specialist interest in the care of patients with HFpEF, to assess the feasibility of a definitive trial of the clinical effectiveness of the REACH-HF intervention in addition to usual care in patients with HFpEF. The trial recruited 50 patients and their caregivers who were identified from outpatient clinics and a Scottish register/database. Twenty-five participants were randomised to receive a slightly modified form of the REACH-HF intervention to include information relevant for patients with HFpEF (see Work package 1: intervention development and feasibility study) in addition to usual medical management for HF (according to national and local guidelines) and 25 participants were randomly allocated to receive usual care alone. Potentially favourable impacts of the REACH-HF intervention on caregiver mental health and measures of burden were observed in this pilot study. The findings support the feasibility of delivering the facilitated home-based REACH-HF intervention for patients with HFpEF and their caregivers and the progression to a full multicentre RCT to test its clinical effectiveness and cost-effectiveness. The protocol and quantitative results have been published (see Appendix 1) and qualitative results were pre-print published in 2020 and the definitive peer-reviewed paper was published in 202143 (see Appendix 1).
Work package 2: patient outcomes
Introduction
About 50% of patients with clinical features of HF are estimated to have HFpEF. 76 Moreover, the prevalence of HFpEF is increasing, and both HFpEF and HFrEF pose a substantial burden on patients in terms of reduced exercise intolerance, poor HRQoL and mortality. 77 For the NHS, this is evident through increased hospital admissions and higher health-care costs.
Recent systematic reviews and meta-analyses have shown promising evidence for the benefit of exercise-based CR in HFpEF. The CR programmes undertaken in these trials were predominantly group-based, supervised and delivered in centre-based settings. There is increasing recognition of the possibility of alternative delivery models of CR, such as home-based programmes, to overcome the suboptimal rates of CR uptake seen with HF.
A single-centre pilot RCT (ISRCTN78539530) was conducted in Dundee to assess the feasibility and acceptability of the novel REACH-HF intervention for patients with HFpEF and their caregivers. Detailed reports of the rationale and protocol of this pilot RCT and its findings have been published (see Appendix 1) and a report of the process evaluation has been pre-print published43 (see Appendix 1).
Research aims
The main aim of this pilot study was to assess the feasibility of undertaking a definitive RCT to assess the clinical effectiveness and cost-effectiveness of the REACH-HF intervention in patients with HFpEF and their caregivers. Our objectives were to:
-
assess the acceptability of the study design and procedures to participants (patients and caregivers)
-
assess the feasibility and experience of the delivery of intervention for participants and HCP facilitators
-
identify barriers to participation in the intervention and study procedures
-
inform a definitive study sample size
-
assess methods for the collection of data, including resource use and costs
-
assess the fidelity of delivery of the REACH-HF intervention by cardiac nurses.
Methods
A total of 225 patients were approached, of whom 50 were randomised (1 : 1) to the REACH-HF intervention plus usual care (intervention group) or to usual care alone (control group) (see Report Supplementary Material 1, Figure B). Participants were randomly allocated in a 1 : 1 ratio to either intervention or control group arms without stratification or minimisation. Randomisation numbers were computer-generated and assigned in strict sequence. At the point of randomisation, participants were assigned the next randomisation number in the sequence. To maintain concealment and minimise selection bias, randomisation was performed after the baseline visit by a member of Peninsula Clinical Trials Unit (CTU), independent from investigator teams, using a secure, web-based randomisation system.
As described earlier, the REACH-HF intervention is a home-based comprehensive self-care CR programme that comprises patient and caregiver manuals with supplementary tools and is delivered by trained health-care facilitators over a 12-week period. The REACH-HF intervention was originally designed for patients with HFrEF in terms of coverage of medication and explanations of the condition, as there was limited evidence to guide the development of the REACH-HF intervention for patients with HFpEF. Following a review of clinical guidance and consultation with the expert cardiology, primary care and physiology/CR specialist members of our project management group, the intervention was adapted for this feasibility study to allow evaluation in patients with HFpEF. Modifications were minor and were as follows: (1) we excluded information on beta-blockers, angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor antagonists, as these drugs have not been shown to be of prognostic benefit in patients with HFpEF, and (2) we changed text relating to the description of the condition to include a more comprehensive explanation of what HFpEF is, what causes it and what treatments are commonly offered (and why). The self-care advice in all other sections of the REACH-HF intervention was considered to be relevant to all patients with HF and corresponds to national HF guidelines. 5,21 Patient outcomes were collected by blinded assessors at baseline and at 3 and 6 months after randomisation, and included HRQoL (primary) and psychological well-being, exercise capacity, physical activity and HF-related hospitalisation (secondary). Outcomes were also collected for caregivers. The parallel process evaluation includes interviews with patients and caregivers (see Work package 2: process evaluation).
Analysis
Our sample size was adequate to enable an estimate of attrition, estimates of the standard deviation (SD) of the primary and secondary outcomes to inform power for a future definitive trial and sufficient numbers of participants for qualitative interviews. We report the mean and SD (or relevant summary statistics) for both groups for all patient and caregiver outcomes at each follow-up point and the mean (and 95% CIs) for between-group differences in outcomes at 6-month follow-up using linear regression models adjusting for baseline outcome.
Key findings
The study recruited 50 symptomatic patients with HFpEF (LVEF ≥ 45%) (mean age 73.9 years, 54% female) and 21 caregivers. Study retention and intervention uptake were excellent (90% and 92%, respectively) (see Report Supplementary Material 1, Figure B).
At 6 months, data from 45 patients showed a mean between-group difference in favour of the intervention group for the primary outcome, MLHFQ score, although the CI was wide and included 0 (between-group mean difference −11.5, 95% CI −22.8 to 0.3). A total of 11 patients (four in the intervention group and seven in the control group) were admitted to hospital over the 6 months of follow-up, with four of these admissions (all in the control group) being related to HF. In the context of this study being a single-centre pilot and not fully powered to demonstrate between-group differences in patients or caregivers, these findings should be considered indicative.
These findings support the feasibility of and rationale for delivering the facilitated, home-based REACH-HF intervention for patients with HFpEF and their caregivers and progression to a full multicentre RCT to test its clinical effectiveness and cost-effectiveness (see Report Supplementary Material 4).
Successes
We recruited to target (Figure 6); the intervention was well received by patients, caregivers and health-care facilitators, and intervention adherence was good. At follow-up, the primary outcome (MLHFQ) showed a significant improvement, and a number of other patient outcomes showed a direction of effect in favour of the intervention group over control, although this pilot trial lacked power to detect a statistically significant between-group difference.
FIGURE 6.
Work package 2: target vs. actual number of participants randomised for REACH-HFpEF pilot trial.
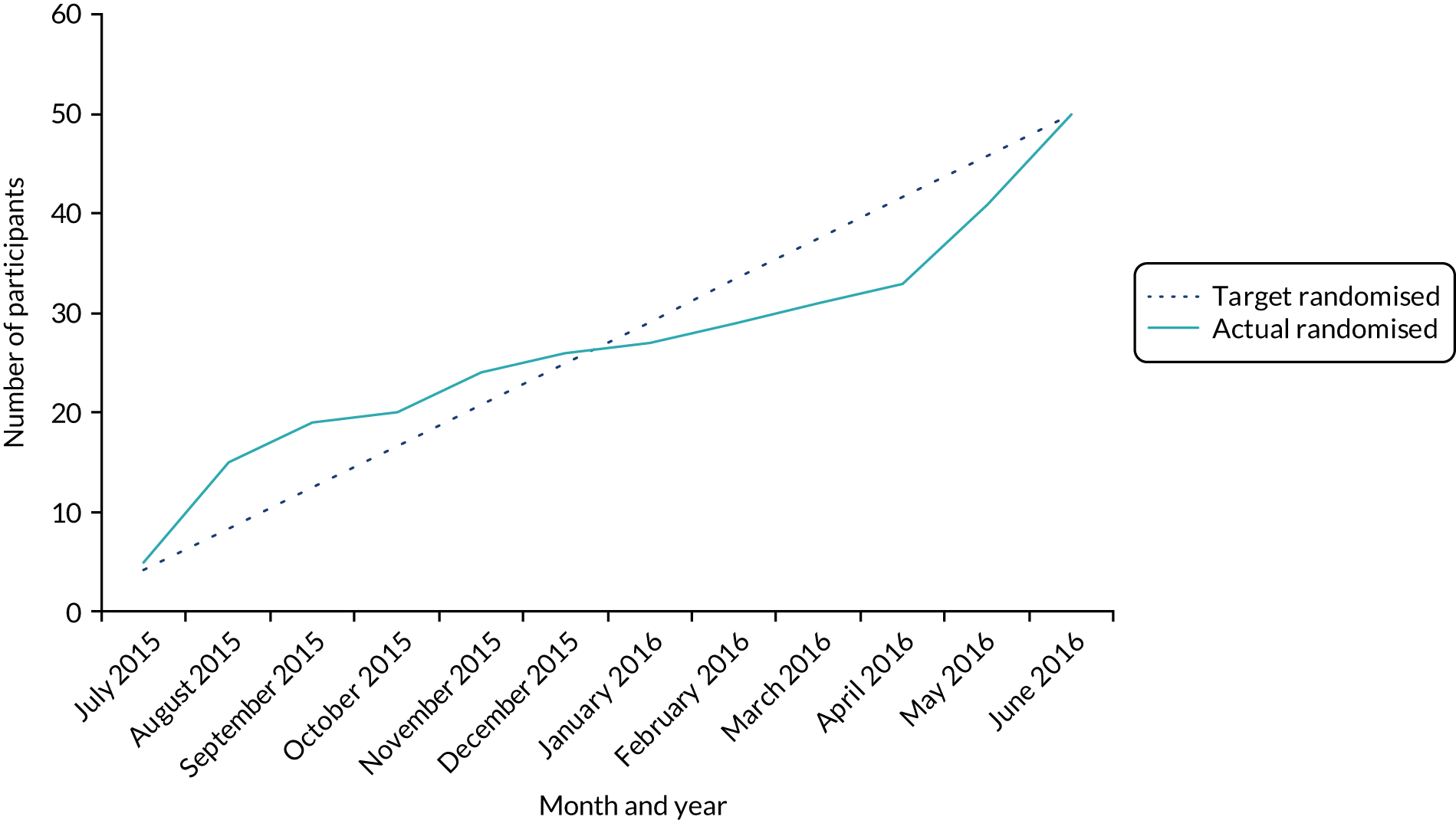
Limitations
Given that this was a pilot RCT, there were several limitations:
-
Originally, this study was going to be run in Birmingham, but the local NHS trust was facing challenges in agreeing to recruit research staff during a staff recruitment freeze, so the research team enrolled a centre in Dundee that holds the Scottish database for patients with HF, including patients with HFpEF. An excellent rate of initial recruitment faltered as the number of patients from the database who were potential participants diminished, and we had to seek patients from outpatient medical clinics, which enabled us to recruit to target after extending the recruitment period by 3 months (see Figure 6).
-
As the trial was conducted in a single centre in a population lacking ethnic diversity, the generalisability of our findings is limited.
-
Patient and clinician blinding was not possible in this study because of the nature of the intervention, although we did show that it was possible to blind outcome assessors to group allocation.
-
An issue raised in patient interviews was the method of exercise testing in the trial, as several patients found that the ISWT exacerbated symptoms such as fatigue and shortness of breath – 27% were unable to undertake the ISWT at final follow-up.
-
The open-label design of the study may have resulted in improvements in patient-reported outcomes in intervention participants because of placebo effects.
Recommendations for future research
This pilot study produced compelling evidence for a full multicentre RCT to assess the clinical effectiveness and cost-effectiveness of the REACH-HF intervention in patients with HFpEF and their caregivers.
Trial registration
This trial is registered as ISRCTN78539530.
Work package 2: process evaluation
Introduction
Process evaluation is becoming an important part of research when assessing complex interventions such as the REACH-HF intervention (see Work package 1: intervention development and feasibility study). Currently, evidence is lacking about the experience and needs of patients living with HFpEF and their caregivers, the factors that influence their self-care decision-making and the provision of support by caregivers. 78 The complexities of living with HF in the real world and the challenges of self-care have not been adequately addressed in the literature. 79
Research aims
The aims of this process evaluation were to:
-
assess the intervention fidelity delivered by the facilitators to the patients with HFpEF
-
explore the experiences of patients and caregivers who participated in the REACH-HF, single-centre pilot RCT (see Work package 1: intervention development and feasibility study).
Methods
A mixed-methods process evaluation was completed alongside the single-centre pilot REACH-HFpEF RCT (see Work package 1: intervention development and feasibility study). The process evaluation consisted of a quantitative assessment of the fidelity of intervention delivery and a qualitative exploration of HFpEF patients’ and their caregivers’ experiences of the REACH-HF intervention involving semistructured interviews.
Data collection
Data were collected in parallel with the REACH-HFpEF pilot RCT (see Work package 1: intervention development and feasibility study).
Quantitative data to assess intervention fidelity
Facilitators’ interactions with all participants were audio-recorded and intervention fidelity was assessed through a 13-item checklist developed by the research team (see Appendix 1) in a purposively selected subset of six participants (three from each facilitator). Each item of the checklist related to the delivery of a key component or delivery process of the intervention, as defined by the intervention design team (see Appendix 1, Work package 1, and Greaves et al. 39 for full details of the checklist). Adequate delivery of the REACH-HF intervention was defined as a score of ≥ 3. A detailed instruction set was developed and is available in the supplementary file of the published intervention development paper (see Appendix 1). Intervention exposure was measured in terms of both intervention engagement (contact hours and number of each type of session attended) and quality of intervention delivery (intervention fidelity) in this subsample of six patients.
Qualitative data
After the 12 weeks required to complete the REACH-HF intervention, a purposive sample of 15 patients and seven caregivers were approached and interviewed in participants’ homes (n = 21) or by telephone (n = 1 caregiver). The interviews explored the experiences of participants living with HFpEF and their caregivers, the perceived benefits of the various elements of the intervention, maintenance of self-care behaviours and coping skills.
Analysis
Quantitative data
Descriptive statistics were used to summarise the fidelity of the intervention.
Qualitative data
Individual interviews were completed and transcribed verbatim and a thematic analysis using a constant comparative method was completed. 65 Data familiarisation was achieved by listening to audio-recordings and repeated reading of the transcripts. Initial codes were produced in a systematic fashion across all data sets – those with common features were grouped together into emerging themes before finally being assigned to overarching themes. The participant quotations included under Key findings illustrate some of the themes.
Key findings
Fidelity of intervention delivery
The fidelity assessment indicated adequate delivery (scores ≥ 3) for most of the key intervention components by the facilitators. Lower scores (< 3) indicating inadequate delivery were mainly related to caregiver issues, particularly the need to address caregiver health and well-being, indicating that there is room for improvement with respect to the involvement of and support provided to caregivers by the facilitators. The mean score for session closure was also < 3 (see Smith et al. 43), indicating potential for improvement in reinforcing and summarising specifically agreed plans.
Audio-recordings from the six patients included in the fidelity analysis provided a total of 41 interactions, 34 of which were face to face (with a mean duration of 63 minutes). Short telephone contacts were reported on only seven occasions, lasting an average of 6 minutes.
Semistructured qualitative interviews
Three overarching themes became apparent (see Report Supplementary Material 1, Table A):
-
understanding their condition
-
emotional consequences of HF
-
response to the intervention and facilitator.
The REACH-HF intervention also enabled positive changes in health-related behaviour, particularly in relation to exercise and diet, not only among patients but also for some caregivers.
A report on the process evaluation of the pilot study has been pre-print published by Smith et al. 43 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
Successes
Overall, this process evaluation suggested that the REACH-HF intervention was generally well delivered and was well received by patients with HFpEF. Qualitative data also captured some excellent examples of good practice in education, engagement and support of these patients and their caregivers by the facilitators. The findings highlight a genuine need for support in a population currently excluded from many HF and CR services.
Limitations
The small number of patients included in the fidelity analysis (n = 6), the characteristics of the participants (who were predominantly of white ethnic origin) and the single-centre setting limit the potential generalisability of the findings. Translating complex interpersonal interactions into numerical scores within the fidelity analysis does not fully illustrate some of the excellent examples of good practice. Facilitators often demonstrated high levels of skill and competence in providing tailored educational and psychological support, enabling patients to reframe negative thoughts, engage in appropriate exercise and participate in self-care. The study also highlighted how involving caregivers was challenging at times, so more could be done to enhance facilitator training on supporting caregivers.
Recommendations for future research
Given the small numbers in this study, we recommend repeating the process evaluation as part of a large multicentre RCT of the REACH-HF intervention in patients with HFpEF.
Trial registration
This trial is registered as ISRCTN78539530.
Work package 3: multicentre randomised controlled trial of the REACH-HF intervention in patients with heart failure with reduced ejection fraction
Some parts of these sections have been reproduced with permission from Dalal et al. ,44 Wingham et al. 80 and Frost et al. 45 These are Open Access articles distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permit others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/. The text includes minor additions and formatting changes to the original text.
Some parts have also been reproduced with permission from Taylor et al. 38 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
Work package 3: overview
To assess the clinical effectiveness and cost-effectiveness of the REACH-HF intervention in addition to usual care in people with HFrEF and their caregivers, our RCT recruited 216 people with HFrEF from four centres in the UK: Birmingham, Cornwall, Gwent and York. Participants were randomly allocated to the REACH-HF intervention described above plus usual care or to usual care alone. Patients randomised to the REACH-HF group usually had a home-based consultation with a CR or HF nurse or physiotherapist (trained in facilitating the REACH-HF intervention), who assessed the participant’s individual needs and provided instruction in use of the appropriate sections of the HF Manual. During the 12 weeks after randomisation, the nurse facilitator was on hand to answer specific questions from individuals with HF or their caregivers during home visits or by e-mail or telephone. The primary outcome was disease-specific HRQoL measured using the MLHFQ at 12 months. Overall, the novel REACH-HF home-based facilitated intervention for HFrEF was clinically superior, in terms of disease-specific HRQoL at 12 months, to usual care alone. Secondary outcomes included death or admissions with decompensated HF or acute coronary syndrome, N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels, ISWT, psychological well-being, physical activity level, generic HRQoL, caregiver outcome, health-care utilisation and adverse events. Papers on the trial protocol, main trial results and process evaluation and on cost-effectiveness have been published (see Appendix 1). Full details of the trial-based cost-effectiveness analysis are presented in Appendix 3. A comparison of psychological well-being, quality of life, self-care activities and burden between caregivers in the intervention and control groups of the REACH-HF multicentre RCT was also undertaken (see Appendix 1, Work package 4, and Taylor et al. 46). In addition, a trial-based economic analysis compared the costs associated with health and social care resource use and HRQoL of the REACH-HF intervention and control groups. 46
Work package 3: patient outcomes
Introduction
Chronic HF is a burgeoning global health challenge that affects 1–2% of adults in the Western world. 3,6 Globally, the prevalence of HF is increasing,3 with the annual economic burden predicted to grow to more than US$108B (£82B, conversion as at 27 June 2018: 1 USD = 0.760133 GBP), as the population ages. Hospital admissions are a key driver of the rising costs,3 with the emphasis of treatment over the past two decades on pharmacological therapies and devices.
A Cochrane systematic review and meta-analysis of exercise-based CR in patients with HF showed improvements in HRQoL and a reduction in rehospitalisations. In 2010, national guidance in the UK recommended centre-based CR (supervised group exercise) for patients with HFrEF,27 with similar recommendations in Europe and the USA. 11,81 However, < 20% of people with HF in Europe and < 10% in the USA participate in CR, prompting a call to explore newer strategies to improve participation in CR and explore the clinical effectiveness of alternatives to centre-based CR.
Home-based CR programmes can widen access and have been shown to be as clinically effective as centre-based CR after MI and coronary revascularisation and with similar costs. 82 The high cost of treating people with HF is well documented,3 but little evidence is available on the cost and cost-effectiveness of home-based CR in HF. 83
We therefore developed a home-based CR programme – ‘the REACH-HF intervention’ – for people with HF and their caregivers.
Research aims
The primary aim of this multicentre RCT was to test the hypothesis that addition of the REACH-HF intervention to usual care would improve disease-specific HRQoL for patients with HFrEF at 12 months’ follow-up compared with usual care alone.
Methods
We targeted men and women aged ≥ 18 years with a confirmed diagnosis of HFrEF on echocardiography or angiography (LVEF < 45%). Patients were recruited from primary and secondary care settings in four centres in the UK (Birmingham, Cornwall, Gwent and York), excluding those who had undertaken CR within 12 months prior to the study and those with a contraindication to exercise testing or exercise training. Participants were randomly allocated in a 1 : 1 ratio, stratified by investigator site and baseline plasma NT-proBNP levels (≤ 2000 pg/ml vs. > 2000 pg/ml), using minimisation to facilitate balance between the groups. Randomisation numbers were computer-generated and assigned in strict sequence at the point of randomisation. To maintain concealment, the Peninsula CTU used a password-protected, web-based randomisation system to allocate participants after consent was obtained and baseline assessment data were entered.
Between January 2015 and February 2016, we approached 1161 patients, 216 of whom were randomised to the intervention (REACH-HF intervention plus usual care) or the control (usual care alone) (see Report Supplementary Material 1, Figures D and E). Participants were randomly allocated (see Report Supplementary Material 1, Figure D) and stratified by investigator site and baseline plasma NT-proBNP levels.
The REACH-HF intervention was uniquely co-developed with key stakeholders: patients, caregivers and clinicians (see Work package 1: intervention development and feasibility study). Briefly, the components of this complex intervention (see Work package 1: intervention development and feasibility study for more detail) included the HF Manual for patients, a Progress Tracker for the patient, a Family and Friends Resource for caregivers and facilitation by cardiac nurses or physiotherapists. Development of the intervention took just over 12 months before it was fully evaluated in our multicentre RCT.
In the trial, participants in both the intervention group and the control group continued with usual medical management and care of HF according to local and national guidelines. In most places in the UK, including the four centres participating in our RCT, usual care involved follow-up by the GP with input from a HF specialist nurse and, in some cases, a cardiologist.
Data collection
The primary outcome was disease-specific HRQoL at 12 months measured using the MLHFQ. Secondary outcomes were death, hospitalisation, generic quality of life [EuroQol-5 Dimensions, five-level version (EQ-5D-5L), scale], psychological well-being [Hospital Anxiety and Depression Scale (HADS)], exercise capacity (ISWT) and physical activity assessed using GeneActiv accelerometer. Additional secondary measures included the HeartQoL questionnaire, Self-Care of Heart Failure Index (SCHFI) and health-care utilisation in terms of primary and secondary care contacts, social care contacts and relevant drug use. 39
Outcome data were collected from participants during three clinic visits – one each at baseline and at 4 and 12 months – and by postal questionnaire at 6 months. At the baseline clinic visit, after obtaining written consent, we collected sociodemographic data and information on past medical history from the participants’ hospital and primary care records, including key comorbidities, NYHA classification, concomitant cardiac drugs and presence of an implantable cardioverter defibrillator.
Analysis
The sample size was based on an effect size that represented the minimal clinically important difference in our primary outcome measure, that is 5 points on the MLHFQ (higher scores indicate a poorer outcome). 84 With a type I error of 0.05 and a power of 90%, 85 participants per group were required to detect a 5-point difference in the MLHFQ score. With an attrition rate of 20% (in accordance with the level of attrition seen in previous trials),82 108 participants were required per group.
The primary analyses for all participant outcomes were based on a between-group (intervention vs. control) intention-to-treat approach, using observed data collected at 12 months. Continuous outcomes were analysed using linear regression methods. Adjustment was made for baseline scores and randomisation variables [site, NT-proBNP concentration (≤ 2000/> 2000 pg/ml) and past MI and diagnosis of AF/atrial flutter (unbalanced at baseline and considered to be predictive of outcome)]. Mortality was reported descriptively and also analysed using logistic regression (with adjustments as for the linear regression models, with the exception of adjustment for baseline score); hospital admissions were reported descriptively, analysed using a negative binomial model and dichotomised according to whether or not the participant experienced at least one admission to facilitate analysis using logistic regression.
Key findings
The primary analysis included 92 (86%) patients in the REACH-HF group and 93 (85%) in the control group. Table 4 summarises primary and secondary patient-reported outcomes and secondary objective outcomes at baseline and follow-up. Compared with control, the REACH-HF intervention was associated with a reduction in total MLHFQ score (i.e. improved HF-related HRQoL) at 12 months: between-group difference –5.7 (95% CI –10.6 to –0.7; p = 0.025). At 12 months, there had been eight deaths (four in each group, 4% of the total). The number of participants with at least one hospital admission was 19 in the REACH-HF group, compared with 24 participants in the control group (odds ratio 0.72, 95% CI 0.35 to 1.51; p = 0.386). Overall, there were 33 hospital admissions in the REACH-HF group (four related to HF) and 35 (10 related to HF) in the control group. The maintenance score on the SCHFI, a measure of self-care, was in favour of the REACH-HF intervention group at 12 months (p < 0.001). Within-group improvements from baseline were seen in the REACH-HF group for HADS anxiety and depression, ISWT and SCHFI (management and confidence) but did not reach statistical significance compared with the control group at 12 months. No differences were seen in the other secondary outcomes [i.e. EuroQol-5 Dimensions (EQ-5D), HeartQoL and physical activity]. The mean cost of the REACH-HF intervention was £418.39 per participant.
| Outcome | Baseline | Follow-up | Between-group difference (95% CI) | p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4 months | 6 months | 12 months | ||||||||
| REACH-HF | Control | REACH-HF | Control | REACH-HF | Control | REACH-HF | Control | |||
| Primary and secondary patient-reported outcomes | ||||||||||
| MLHFQ (primary) | ||||||||||
| Overall | 32.8 (23.8) 107 | 28.3 (22) 109 | 22.7 (18.4) 96 | 27.8 (23.2) 100 | 28.8 (20.5) 90 | 29.5 (21.8) 94 | 24.1 (20.9) 92 | 27.5 (23.2) 93 | –5.7 (–10.6 to –0.7) | 0.025 |
| Physical | 16.5 (11.5) 107 | 14.7 (11.2) 109 | 11.7 (9.0) 96 | 14.5 (11.3) 100 | 14.7 (10.7) 90 | 14.9 (11.2) 94 | 12.2 (10.8) 92 | 14.5 (11.8) 93 | –3.2 (–5.7 to –0.6) | 0.016 |
| Emotional | 7.7 (7.3) 107 | 6.8 (6.6) 109 | 4.8 (5.8) 96 | 6.4 (6.9) 100 | 6.2 (6.2) 90 | 6.8 (6.8) 94 | 5.1 (5.8) 92 | 5.5 (6.4) 93 | –0.8 (–2.2 to 0.6) | 0.273 |
| HADS | ||||||||||
| Anxiety | 5.1 (4.4) 107 | 5.7 (4.3) 109 | 4.4 (3.9) 95 | 5.2 (4.2) 101 | 4.7 (3.7) 89 | 5.4 (4.3) 94 | 4.2 (3.8) 88 | 4.7 (4.5) 92 | 0.1 (–0.8 to 1.0) | 0.829 |
| Depression | 4.4 (3.5) 107 | 4.6 (3.3) 109 | 3.6 (2.7) 95 | 4.5 (3.5) 101 | 4.6 (3.2) 89 | 4.7 (3.6) 94 | 3.6 (3.1) 88 | 3.9 (3.4) 92 | –0.2 (–1.1 to 0.6) | 0.563 |
| HeartQoL | ||||||||||
| Global | 1.8 (0.7) 107 | 1.8 (0.7) 109 | 2.0 (0.7) 95 | 1.9 (0.8) 101 | 1.8 (0.8) 89 | 1.8 (0.8) 91 | 1.9 (0.8) 88 | 1.9 (0.9) 92 | 0.0 (–0.2 to 0.2) | 0.823 |
| Physical | 1.7 (0.8) 107 | 1.7 (0.8) 109 | 1.9 (0.8) 95 | 1.7 (0.9) 101 | 1.6 (0.8) 90 | 1.7 (0.9) 92 | 1.8 (0.9) 88 | 1.7 (0.9) 92 | 0.0 (–0.2 to 0.2) | 0.869 |
| Emotional | 2.1 (0.9) 107 | 2.2 (0.8) 109 | 2.3 (0.8) 95 | 2.2 (0.8) 101 | 2.2 (0.8) 89 | 2.1 (0.8) 93 | 2.3 (0.8) 88 | 2.3 (0.8) 92 | 0.0 (–0.2 to 0.3) | 0.683 |
| EQ-5D-3L | 0.739 (0.234) 106 | 0.723 (0.236) 108 | 0.758 (0.223) 95 | 0.753 (0.219) 101 | 0.708 (0.265) 88 | 0.733 (0.217) 92 | 0.752 (0.240) 88 | 0.739 (0.263) 92 | –0.024 (–0.091 to 0.044) | 0.487 |
| EQ-5D VAS (0 to 100) | 69 (20) 97 | 71 (20) 97 | 73 (17) 90 | 74 (17) 93 | 72 (18) 80 | 70 (19) 85 | 74 (18) 85 | 73 (22) 84 | 1 (–5 to 6) | 0.859 |
| SCHFI | ||||||||||
| Maintenance | 55.8 (16.5) 107 | 54.5 (14.5) 109 | 68.3 (13.6) 96 | 55.7 (17.0) 101 | 65.4 (14.4) 89 | 54.7 (16.0) 94 | 63.8 (17.0) 87 | 55.2 (16.8) 92 | 8.0 (3.6 to 12.4) | < 0.001 |
| Management | 43.1 (25.9) 47 | 40.4 (21) 59 | 46.8 (24.2) 33 | 42.0 (21.0) 48 | 52.1 (18.8) 42 | 41.9 (21.6) 37 | 53.8 (23.4) 39 | 43.4 (20.1) 40 | 9.4 (–4.0 to 22.8) | 0.165 |
| Confidence | 61.7 (25.0) 107 | 65.3 (23.8) 108 | 67.0 (22.3) 94 | 64.7 (21.7) 101 | 65.4 (22.8) 85 | 62.5 (22.7) 93 | 70.3 (21.8) 88 | 66.4 (21.3) 92 | 5.6 (–0.1 to 11.3) | 0.056 |
| Secondary objective outcomes | ||||||||||
| ISWT (m) | 262.3 (153.4) 99 | 239.7 (152.4) 103 | 328.5 (181.3) 66 | 294.3 (215.5) 75 | – | – | 328.5 (181.3) 66 | 294.3 (215.5) 75 | 0.1 (–33.3 to 33.5) | 0.995 |
| Number of days/week with at least 10 minutes/day activity > 100 milli-ga | 5.8 (2.3) 99 | 5.9 (1.9) 103 | 5.6 (2.4) 78 | 5.5 (2.6) 84 | – | – | 5.6 (2.4) 78 | 5.5 (2.6) 84 | 0.2 (–0.4 to 0.7) | 0.601 |
| Average time/day (minutes) | ||||||||||
| ≤ 20 milli-ga | 1,104 (102) 99 | 1106 (114) 103 | 1107 (110) 88 | 1092 (116) 93 | – | – | 1092 (124) 78 | 1103 (118) 84 | –7 (–29 to 15) | 0.534 |
| 21–40 milli-ga | 141 (35) 99 | 136 (35) 103 | 140 (35) 88 | 138 (30) 93 | – | – | 142 (39) 78 | 138 (34) 84 | –1 (–9 to 8) | 0.880 |
| 41–60 milli-ga | 80 (25) 99 | 80 (27) 103 | 80 (27) 88 | 82 (26) 93 | – | – | 81 (30) 78 | 81 (28) 84 | 0 (–6 to 6) | 0.901 |
| 61–80 milli-ga | 45 (21) 99 | 46 (21) 103 | 45 (22) 88 | 48 (22) 93 | – | – | 48 (23) 78 | 46 (22) 84 | 2 (–2 to 5) | 0.372 |
| 81–100 milli-ga | 26 (16) 99 | 27 (16) 103 | 26 (16) 88 | 28 (17) 93 | – | – | – | – | – | – |
| > 100 milli-ga | 42 (34) 99 | 46 (40) 103 | 43 (37) 88 | 51 (46) 93 | – | – | – | – | – | – |
Successes
Behaviour theory underpinned the design of all components of the intervention, which was co-developed with patients, caregivers and clinicians and subjected to the rigours of a multicentre RCT. We recruited to target on time and achieved 86% retention at 12 months (see Report Supplementary Material 1, Figure D). The magnitude of the mean between-group difference in our primary outcome measure – total MLHFQ score – was not only statistically significant but also clinically meaningful (i.e. a reduction of > 5 points) and consistent with effects found in other exercise-based CR interventions for people with HF. The cost of the REACH-HF intervention (£418.39 per participant) is below the NHS tariff for CR in England of £477 per patient.
Limitations
Lack of blinding was a key limitation, as the complex nature of the intervention and the control meant that we could not mask participants to treatments, introducing the possibility of patient expectation bias. Another limitation was the 15% missing data for the primary outcome measure at the 12-month follow-up. Assessment of adherence was also a limitation given the self-directed nature of the home-based intervention, and we were not able to capture consistent patient-level data on adherence to the exercise training component of the intervention.
There is an emerging hypothesis that it is the act of exercise training and not the gain in fitness that improves outcomes for patients. For many patients with HF, there are no or very limited physiological mechanisms for improved fitness, as cardiac output (heart rate × stroke volume) and difference in the extent by which oxygen is extracted at the active muscle interface [a-vO2 diff (arteriovenous oxygen difference)] are severely hindered by HF. Using the EQ-5D, our trial-based analysis showed no difference in QALY gain between groups at 12 months and we were therefore unable to show that REACH-HF was cost-effective in the short term according to current willingness to pay (WTP) cost per QALY thresholds in the UK. 31 However, it is important to note that a growing body of evidence is showing that the EQ-5D lacks sensitivity in the patient population of our trial (i.e. patients with mild to moderate HF). Extrapolation of our trial findings for long-term outcomes estimated via model-based cost-effectiveness analysis results showed a high likelihood that the REACH-HF intervention is cost-effective (see Work package 4: model-based cost-effectiveness analysis). The key characteristics and findings of this trial are summarised in Report Supplementary Material 1, Figure F.
Recommendations for future research
With the growing availability of evidence-based CR interventions delivered at home, systematic collection of real-world data utilising vehicles, such as the NACR database in the UK, are needed to allow the tracking of potential future changes in uptake of and adherence with CR. In addition, there is a need for further well-conducted RCTs of home-based CR interventions to collect and report long-term outcomes, including hospital admissions and health-care costs.
Trial registration
This trial is registered as ISRCTN86234930.
Work package 3: caregiver outcomes
Introduction
Caregivers have an important role in supporting chronic disease management, although this often comes at considerable personal cost and many caregivers are uncertain about how to best provide this support. Most caregivers have health and well-being problems of their own – either pre-existing or arising from the burden of their caregiving activities. 85 However, there is paucity of evidence on interventions that support caregivers in their role. 78 The needs of caregivers in the management of HF and their responses have been depicted in two recent qualitative studies. 40,86 Caregivers may also assist patients in appraising and responding to symptoms of HF. 87
Research aims
The aims of this evaluation were to:
-
compare psychological well-being, quality of life, self-care activities and burden between caregivers in the intervention and control groups of the REACH-HF multicentre RCT
-
explore the views of caregivers on their experience of using the REACH-HF caregiver manual (Family and Friends Resource) and its influence on their confidence in caring for patients with HF and themselves.
Methods
Patients aged ≥ 18 years with a confirmed diagnosis of HFrEF (LVEF < 45%) were recruited from four centres in the UK (Birmingham, Cornwall, Gwent and York) and invited to participate. At study entry, patients were asked whether or not they had a caregiver, that is a partner, other relative or friend. If the patient did have a caregiver who was aged ≥ 18 years, the caregiver was invited to participate in the trial alongside the patient. Participating caregivers provided unpaid support to patients who could otherwise not manage without such support. Caregivers were allocated to the REACH-HF group or control group in accordance with the random allocation of their accompanying patient.
Data collection
Face-to-face or telephone interviews were conducted at 4 and 12 months after recruitment to the trial. The intervention had been completed prior to the first interview. Where possible, all interviews were conducted with caregivers alone. A topic guide (see Report Supplementary Material 1, Table C) was reviewed throughout the study so that the questions were informed by relevant emerging topics. The data set included facilitator fidelity scores and qualitative notes on the scores, facilitator contact sheets, baseline questionnaire data, field notes and interview transcripts.
Caregiver outcomes included HADS, HRQoL assessed by EQ-5D-5L questionnaire, Family Caregiver Quality of Life (FamQol), Caregiver Burden Questionnaire for Heart Failure (CBQ-HF) and Caregiver’s Contribution to Self-Care of HF Index (CC-SCHFI). Outcome data were collected during three clinic visits – one each at baseline and at 4 and 12 months – and by postal questionnaire at 6 months. Sociodemographic data were also collected at the baseline clinic visit. Data were collected by research nurses who were blinded to the patients’ and caregivers’ group allocations.
Analysis
Caregiver outcomes were analysed and reported using the same methods as for continuous patient outcomes. Constant comparative techniques were used to compare the individual 4- and 12-month interviews and all caregiver participants’ interviews.
Key findings
The REACH-HF group included 107 patients and the control group included 109 patients. A total of 97 patients declared having a caregiver, with 53 caregivers allocated to the REACH-HF group and 44 to the control group. At 12 months, data were available for 45 (85%) caregivers in the REACH-HF group and 37 (84%) in the control group.
Improvements from baseline at 4, 6 and 12 months were seen for a number of outcomes in the REACH-HF group, including HADS anxiety; CBQ-HF physical, social life and lifestyle; and all SCHFI dimension scores. However, only SCHFI confidence at 12 months was statistically significant (p < 0.05) compared with the control. Based on interviews of 17 caregivers, the REACH-HF intervention was acceptable and was associated with an increase in confidence in their caregiving role.
Successes
Mean between-group differences favoured the REACH-HF intervention for caregiver outcomes of anxiety, perceived burden and self-care management, although the results were not statistically significant. The SCHFI confidence score outcome achieved statistical significance at the 12-month follow-up. Most caregivers who were interviewed following the intervention made changes to how they supported the person with HF for whom they were caring and gained confidence.
Limitations
As caregivers were not themselves randomised to the intervention and control groups and the study sample size was powered on the between-group difference in patient outcomes rather than caregiver outcomes, we did not generally find significant differences in caregiver HRQoL compared with the control. Non-random allocation of caregivers increased the risk of selection bias between the REACH-HF and the control groups, which means that our results are subject to confounding. We also found that facilitators need additional training on how best to involve caregivers in interventions such as REACH-HF. There is potential to revise the intervention to improve caregiver and facilitator engagement.
Recommendations for future research
The quantitative and qualitative findings for caregivers (see Work package 3: process evaluation) suggest the need to better engage caregivers in CR and self-care interventions for patients with HF. Our results suggest that caregivers require skilled and personalised facilitation by HCPs. Facilitators may need a caregiver assessment tool to help plan care. 40 Further high-quality evidence is required to confirm the benefits of actively involving caregivers in the development and delivery of CR and self-care interventions for HF and other chronic diseases.
Trial registration
This trial is registered as ISRCTN86234930.
Work package 3: process evaluation
Introduction
As mentioned earlier (see Work package 2: process evaluation), process evaluation is becoming an important part of research when assessing complex interventions, such as the REACH-HF intervention (see Work package 1: intervention development and feasibility study). However, RCTs of CR for HF have not included process evaluation, so evidence is lacking about the characteristics of CR programmes that consistently affect the success of self-care interventions across different outcomes, how different elements of the elements of CR programme interact, and the complexity of living with HF and the burden of both self-care and adherence to intervention regimens.
Research aims
The aims of this process evaluation were to:
-
assess the intervention fidelity and patients’ and caregivers’ experiences (see Work package 3: caregiver outcomes) of participation in the REACH-HF multicentre RCT (see Work package 3: patient outcomes44)
-
investigate the change processes that may be responsible for changes in the primary outcome measure of the RCT – MLHFQ.
Methods
A mixed-methods process evaluation used linked data to build individual case studies with links between different available data, paying particular attention to fidelity (the consistency of what is implemented with the planned intervention) and adaptations (alterations made to an intervention in order to achieve contextual fit) (see Report Supplementary Material 1, Figure G).
Data collection
Data were collected in parallel with the REACH-HF multicentre trial (see Work package 3: patient outcomes44).
Quantitative data
A 13-domain fidelity checklist was developed and piloted during the feasibility study and used in the process evaluation to assess the facilitators’ delivery of the intervention.
Qualitative data
For the 19 patients who constituted our sampling frame (two or three patients per facilitator across the four recruiting sites), all face-to-face meetings and subsequent telephone contacts with the facilitator were audio-recorded. Face-to-face or telephone interviews were conducted at 4 and 12 months after recruitment to the trial, allowing changes over time to be explored. The intervention had been completed prior to the first interview. A topic guide (see Report Supplementary Material 1) was reviewed throughout the study so that the questions were informed by relevant emerging topics. The data set included facilitator fidelity scores and qualitative notes of the scores, facilitator contact sheets, baseline questionnaire data, field notes and interview transcripts.
Analysis
Quantitative data
Descriptive statistics were used to summarise dose and fidelity.
Qualitative data
Individual cases were created for each respondent, including interview transcripts, contact sheets, audio-recordings of facilitator–patient interactions and associated fidelity scores, summaries of the intervention sessions, field notes, clinical data such as case report forms, and patient-reported outcome measures. Interview transcripts were initially analysed using evaluative coding, which assigned a judgement about the merit or significance of the intervention from the participant’s perspective. On the basis of these multimodal and longitudinal data, each case was coded on the basis of intervention fidelity and tailoring of the intervention. Audio-recordings, sampled on the basis of the fidelity scores and position in the typology, allowed closer analysis of relevant sections of the intervention sessions.
Key findings
The process evaluation sample was broadly representative of study participants. On average, 7.05 (SD 2.6) facilitated sessions were provided per process evaluation participant, of which 4 (SD 1.8) were face to face and 2.94 (SD 2.6) were by telephone.
Fidelity of intervention delivery
Audio-recordings of most facilitator contacts were available (n = 110) and assessed by two investigators (Jennifer Wingham and Colin J Greaves) using the fidelity checklist (Table 5) applied to the audio-recordings of all of the available (n = 110) facilitator–patient contacts. Fidelity was deemed adequate (≥ 3) in most domains (see Table 5). However, there was room for improvement, particularly with respect to the involvement of and support provided to caregivers (items 10–12 in Table 5), for which the mean scores were well below the acceptable standard (see Table 5). In addition, given that scores for many items were only marginally above (or just below) the defined standard for adequacy, this indicates room for improvement across the board. The fact that the range of scores varied widely also indicates that it is possible to achieve higher scores in every domain (again indicating scope for improvement).
| Characteristic | Mean | Minimum | Maximum | n |
|---|---|---|---|---|
| 1. Participant involvement | 3.3 | 1.3 | 4.9 | 20 |
| 2. Assessment of individual needs | 4.1 | 2.5 | 5.0 | 15 |
| 3. Tailored treatment plan | 2.9 | 1.8 | 5.1 | 20 |
| 4. Building understanding of HF | 3.3 | 1.5 | 5.0 | 17 |
| 5. Support progress monitoring | 2.9 | 1.8 | 5.0 | 20 |
| 6. Review progress | 3.2 | 2.2 | 4.8 | 18 |
| 7. Physical activity plan | 4.2 | 3.0 | 5.5 | 15 |
| 8. Address patient emotion | 3.4 | 1.0 | 5.0 | 17 |
| 9. Medication issues | 3.3 | 2.0 | 5.0 | 17 |
| 10. Involve caregiver | 1.6 | 0.0 | 3.1 | 16 |
| 11. Address caregiver emotion | 2.2 | 0.0 | 4.0 | 14 |
| 12. Address caregiver well-being | 1.1 | 0.0 | 3.3 | 16 |
| 13. Closure | 3.4 | 2.0 | 5.0 | 14 |
Successes
Intervention attendance was high, with > 90% of participants receiving the minimum intended dose; this compares well with six group-based CR programmes, which typically report attendance levels of about 80% of intended contacts. How well the intervention was perceived by the participant depended not on the ‘dose’ (i.e. the contact time with the facilitator) but on other factors related to delivery of the REACH-HF intervention (Table 6). The appropriate ‘dose’ of the REACH-HF intervention was contingent on fidelity and skilled facilitation rather than the duration or frequency of contact alone. Importantly, in the case studies that we analysed, the fidelity score at the initial ‘assessment’ session was indicative of the overall fidelity for an individual participant over the course of the intervention. Intervention effectiveness was dependent on the facilitator’s style of engagement and the extent to which the trial materials were matched or adapted to the existing beliefs and goals of participants.
| Finding | Participants’ commentsa |
|---|---|
| One participant, Mary, received one of the lowest doses of the REACH-HF intervention (293 minutes over three face-to-face and three telephone-based intervention sessions), but her fidelity scores were high (≥ 5) at baseline and remained about 4–5 after the 12 weeks of the intervention | 4 months into the programme, Mary explained how the support of the facilitator combined with the walking programme had re-orientated her understanding of HF:I’ve learnt a lot and I think it is a very good, um manual, I think it’s excellent and I think, er, given to people – I wished I’d had it right at the beginning. Because it has changed my attitude . . . |
| Another participant, Dorothy, received a larger dose of the intervention (440 minutes over four face-to-face and two telephone sessions); however, her fidelity scores remained low (< 2.5) at baseline and after 12 weeks of REACH-HF. Her lack of engagement was attributed to her lack of insight, which was repeatedly reinforced by poor facilitation of the intervention, resulting in no change in understanding or behaviour | After 12 months of the REACH-HF programme, Dorothy said:I can’t seem to get the seriousness of it . . . [exercise] is recreation, isn’t it? Mainly . . . it keeps, you know, things going . . . I can’t explain it really now . . . I did have a diary at first ‘cos they said keep a diary, but even that I don’t tend to . . . no, have got a bit complacent . . . |
| To some, the diagnosis came as a relief because it normalised and explained their symptoms (e.g. tiredness and breathlessness) making them feel less anxious | |
| Several patients liked the description of HF in the HF Manual, along with the facilitator’s explanation, which aided their understanding of HF, thus better equipping them to untangle, identify and act on symptoms of HF | One caregiver stated:I just feel once he started to understand more about heart failure, with the [HF] Manual, that, yes, he sort of – I don’t know, sort of maybe accepted it more . . . I think sometimes he sort of panics, thinking, oh, you know, should I be feeling this way? Whereas having the [HF] Manual has, I think, sort of made him realise, yes, this is normal for me to feel like this and be like this |
Limitations
Several factors were identified as possible reasons why participants may not have benefited from the intervention. Behaviour change seemed sustained when the facilitator adapted the intervention to the needs of the participant. The facilitators’ competence was key to participants’ acquisition of skills and changes in behaviour. Most participants had multiple comorbidities. Some participants valued the simplicity of the REACH-HF intervention, specifically the accessibility of the information provided. Socioeconomic factors influenced the level of engagement with the intervention – sustaining change seemed easier for participants with favourable financial or social circumstances. The main REACH-HF RCT (see Work package 3: patient outcomes) and this process evaluation included very few (< 3%) ethnic minority participants.
The fact that data were not collected during the facilitator training and that interviews with facilitators were not recorded meant that we had limited insight into the interaction between a practitioner’s existing professional repertoire and their engagement with and delivery of the REACH-HF intervention.
Recommendations for future research
Future research could explore ways of optimising the ‘workability’ of the REACH-HF intervention and integration of its components into the facilitators’ clinical practice to ensure that fidelity and adaptation are maximised in the dynamic and complex context of managing patients with HF. Given the low uptake of CR among people from minority ethnic backgrounds,88 future research should also attempt to understand the specific support needs of people from more diverse ethnic backgrounds and the extent to which cultural beliefs may moderate or mediate the effectiveness of home-based CR interventions, such as the REACH-HF intervention.
Trial registration
This trial is registered as ISRCTN86234930.
Work package 3: economic evaluation
Introduction
In addition to information on the effectiveness of interventions, it is important to assess the cost-effectiveness of interventions to inform health policy decision-making within the context of the NHS in the UK. This section reports a cost-effectiveness analysis of the REACH-HF intervention versus usual care, which was carried out alongside the main trial (see Work package 3: patient outcomes).
Research aims
The aim of the trial-based economic analysis was to assess whether or not addition of the REACH-HF intervention to usual care alone (REACH-HF group) was cost-effective compared with usual care alone (control group) based on resource use, costs and HRQoL outcomes recorded for participants with HFrEF over the 12 months of the trial.
Methods
The trial-based economic analysis compared the costs associated with health and social care resource use and HRQoL of the REACH-HF intervention and control groups. Statistical analyses were used to estimate between-group differences in estimated costs and QALYs. Bootstrapping was used to provide estimates of uncertainty. Primary analysis was based on complete cases, with sensitivity analyses using imputed data, subgroups, caregiver QALYs, and informal care costs and varying the intervention cost. Full methods for the trial-based analysis are reported in Appendix 3.
Data collection
Data on resource use related to health and social care were collected from REACH-HF trial participants with HFrEF over the course of the 12 months of the trial and were costed using published estimates and national data sets. 89,90 Data on staff time, training and consumables used in providing the intervention, collected within the trial, were used to estimate the cost of the REACH-HF intervention. Data on HRQoL were collected by self-report from trial participants using the EQ-5D questionnaire.
Cost-effectiveness analyses
Cost-effectiveness was reported in terms of the incremental cost-effectiveness ratio [incremental cost-effectiveness ratio (ICER) cost per QALY] estimated over the 12 months of trial follow-up.
Key findings
The mean estimated total cost for delivery of the REACH-HF intervention was £418.39 per patient with HFrEF. We found an increase of £345 per patient for health and social care services for the REACH-HF group compared with the control group over the 12 months of the trial, although the difference was not statistically significant. No statistically significant difference was seen between the groups in EQ-5D or QALYs. With an expected additional cost associated with the addition of the REACH-HF intervention to usual care and no reported health gains, usual care alone dominates. Full results of the trial-based cost-effectiveness analysis are reported in Appendix 3.
Successes
Retention in the trial was good, with 86% of participants recruited to the trial completing and providing data for the full 12 months.
Limitations
The REACH-HF trial did not show a significant short-term improvement in generic HRQoL (EQ-5D) and it was not found to be cost-effective over the 12 months of the trial, as costs were higher in the REACH-HF group. Although meta-analyses of randomised trials studies have shown that CR can reduce all-cause and HF-specific hospital admissions, the main REACH-HF trial was not powered to detect such a change. This short-term analysis, therefore, was not able to assess whether or not there would be longer-term benefits to people with HFrEF from receiving the REACH-HF intervention.
Recommendations for future research
Robust, long-term, real-world estimates of the effectiveness of home-based CR for HFrEF in terms of hospitalisations and deaths are required to further inform the cost-effectiveness of home-based CR in patients with HF.
Work package 4: model-based cost-effectiveness analysis
Some parts of these sections have been reproduced with permission from Taylor et al. 46 This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/. The text includes minor additions and formatting changes to the original text.
Work package 4: overview
To assess the longer-term cost-effectiveness of the REACH-HF intervention, home-based CR and centre-based CR versus usual care in people with HFrEF, our model-based analyses used an appropriate model type and analytical framework to capture disease progression using health states that represent the important event-related activity of HF. An updated Cochrane systematic review of the evidence for exercise-based CR (both home and centre based) was undertaken as part of our programme-informed data inputs to the evidence synthesis (see Appendix 1). The detailed costs of the REACH-HF intervention were obtained through WP3 (see full report in Appendix 3). We estimated the cost-effectiveness of home-based CR versus usual care, the REACH-HF intervention versus usual care and centre-based CR versus usual care46 (see Appendices 1 and 3). Using the common comparator of usual medical care, it was possible to compare (indirectly) the trial-based evidence for centre-based CR and the findings for the REACH-HF intervention in WP3 to derive the relative effect of centre-based CR versus the REACH-HF intervention. The cost-effectiveness of centre-based CR versus usual care was considered, for consistency, using the common decision-analysis framework that we developed (see Work package 4: model-based cost-effectiveness analysis). Modelling predicts a reduction in hospital admissions, which is consistent with the literature on trials of home-based CR, and the results indicate the interventions are likely to be highly cost-effective. The results of the updated Cochrane review of exercise-based CR for HF1 and the economic modelling have been published (see Appendix 1).
Work package 4: introduction
Among patients with HF, the risk of deterioration leading to hospitalisation is high. Hospitalisation is not only an indicator of greater morbidity but is associated with an increased risk of death, higher health-care costs and significant impairments in HRQoL. Systematic reviews and meta-analyses of exercise-based CR in patients with HF have shown improvements in HRQoL and a reduction in rehospitalisations. 1,14,15 Reducing acute events and hospital admissions may have the knock-on effect of slowing disease progression and reducing mortality, as patients are at increased risk of death during and immediately after admission. It therefore is important to understand the long-term cost-effectiveness of a home-based CR intervention, such as that trialled in the REACH-HF programme, and this can be done using a decision-analytic economic model. Given the paucity of direct head-to-head RCTs in the HF population identified by our updated Cochrane review of home- versus centre-based CR (four RCTs in a total of 315 patients, with only one single-centre study undertaken in a UK setting),91 we decided to restrict our economic modelling to examine the questions of the long-term cost-effectiveness of:
-
REACH-HF versus usual care
-
home-based CR versus usual care
-
centre-based CR versus usual care.
The data were too limited to directly estimate the longer-term cost-effectiveness of home- versus centre-based CR.
Work package 4: research aims
The aim of this model-based economic evaluation was to assess cost-effectiveness over the longer term of the REACH-HF intervention, other home-based CR and centre-based CR versus usual care in people with HFrEF (see Appendix 1). Additional analyses, including assessment of the cost-effectiveness of centre-based CR versus usual care, are reported in Appendix 3.
Work package 4: methods
The long-term model-based economic evaluation used a Markov cohort model with a 1-month cycle, which captured the impact of the intervention on (1) all-cause hospitalisations and (2) HF-related hospital admissions and related increases in mortality for the lifetime of people with HF. Development of the model involved a systematic review of the literature on model-based economic evaluations for HF (see Report Supplementary Material 1 for more details), exploring model structure and data inputs, building on previously published literature and input from a stakeholder group. The perspective was of a third-party payer (i.e. the NHS in the UK). Full probabilistic and deterministic sensitivity analyses were used to test the sensitivity of the results to both uncertainty and changes in model structure and parameterisation. Detailed methods for the model-based analysis are included in a published paper (see Appendix 1). 46
Work package 4: data collection
The model was parameterised using data on hospital admissions, mortality, costs and HRQoL from published studies and national data sets. Effectiveness estimates in terms of odds ratios for hospital admission for HF were drawn from two sources: (1) a systematic review and Cochrane meta-analysis of randomised trials of the effectiveness of centre- and home-based CR versus usual care (see Appendix 1) and (2) the REACH-HF trial results (see Work package 3: multicentre randomised controlled trial of the REACH-HF intervention in patients with heart failure with reduced ejection fraction). Full details of model parameters are set out in a published paper (see Appendix 1).
Work package 4: cost-effectiveness analyses
The model estimated the lifetime cost-effectiveness of home-based CR (from our meta-analysis) plus usual care versus usual care alone and the REACH-HF intervention plus usual care versus usual care alone in terms of the incremental cost per QALY. The key outcomes of the model were costs in GBP and health outcomes measured in terms of QALYs based on EQ-5D values.
Work package 4: key findings
Although not cost-effective over the 12 months of the trial, over the lifetime of patients with HFrEF both the REACH-HF intervention and the home-based CR (from our meta-analysis) were potentially cost-effective, with longer survival, increased HRQoL and slightly increased costs. The model-based economic evaluation, extrapolating results from the multicentre trial in HFrEF patient over a time horizon of 4 years, found that an estimated mean cost per patient of £15,452 (95% CI £14,240 to £16,780) and a mean QALY gain of 4.47 (95% CI 3.83 to 4.91) for the REACH-HF intervention plus usual care, compared with a mean cost of £15,051 (95% CI £13,844 to £16,289) and a mean QALY gain of 4.24 (95% CI 4.05 to 4.43) with usual care alone, resulting in an incremental cost per QALY of £1721. Probabilistic sensitivity analysis indicated a 78% probability that the REACH-HF intervention plus usual care is cost-effective versus usual care alone at a threshold of £20,000 per QALY (see Figure 8, Appendix 3). The full results of the model-based cost-effectiveness analysis are reported in a published paper (see Appendix 1). The results of the analysis of centre-based CR versus usual care are reported in Appendix 3.
Work package 4: successes
Consistent with previous research in this area, our modelling work suggests that the reduced incidence of HF-related admissions seen with home-based CR led to reduced mortality and improved HRQoL. An average reduction of 2% in HF-related admissions was seen over 12 months, with the difference in numbers of HF-related admissions falling to 16% after 5 years and 9% at 10 years. Although evidence of a reduction in HF-related admissions is uncertain (i.e. wide 95% CIs), when this is taken into account, we predicted that both the addition of the REACH-HF intervention and home-based CR to usual care were cost-effective in more than 73% of cases compared with usual care alone.
Work package 4: limitations
The main challenge was to assess cost-effectiveness of home-based CR given the limited amount of current randomised trial evidence for home-based CR and the small number of HF-related admissions. Using the best available evidence, we used a decision-analytic framework to provide model-based analyses, applying the reported uncertainty in effectiveness of home-based CR versus usual care in reducing HF-related hospital admissions. In addition, we did not undertake a network meta-analysis to estimate indirectly the cost-effectiveness of the REACH-HF intervention versus centre-based programmes.
Work package 4: recommendations for future research
Robust, long-term, real-world estimates of the clinical effectiveness of home-based CR for HF in terms of hospitalisations and deaths are needed to further inform the cost-effectiveness of home-based CR in HF. Insensitivity of the EQ-5D in patients with mild HF in other studies has prompted some health economists to recommend using the Short Form questionnaire-6 Dimensions (SF-6D) in those with milder disease. Future studies should consider using the SF-6D [from Short Form questionnaire-12 items (SF-12)] alongside the EuroQol-5 Dimensions, three-level version (EQ-5D-3L).
Patient and public involvement in REACH-HF
In the REACH-HF programme, PPI was defined as the involvement of members of the public who had a diagnosis of chronic HF or were caring for relatives or partners with HF. The term ‘public’ is defined using guidelines from INVOLVE, where it is understood to include people of different ages, ethnicities, sexes and disabilities and with different needs and concerns. PPI is an active partnership between the researchers and the public, which takes the form of consultation and collaboration. The members of the PPI Advisory Group contributed their own experiences of coping with HF – from the perspective of a person with HF or a person acting as a caregiver for someone with HF.
Recruitment of the patient and public involvement Advisory Group
Patients with HF and their caregivers were highly involved in our programme of research from its conception. The first formal meeting of the PPI Advisory Group in March 2011 was chaired by a representative of the NIHR Collaborations for Leadership in Applied Health Research and Care South West Peninsula (PenCLAHRC) and included five people with HF, one caregiver and two co–investigators/REACH investigators (Jennifer Wingham and Hasnain Dalal). The PPI Advisory Group met three or four times per year and more frequently during the intervention development phase to review the various WPs. The group provided detailed comments on aspects, such as the proposed choice of patient-reported outcome measures. In the summer of 2011, members of the PPI Advisory Group contributed in particular to the development of the home-based REACH-HF intervention [helping to select the core intervention targets, identifying barriers to change and ways to facilitate change, and reviewing the entire content of the intervention as it developed as part of the Programme Development Grant (RP-DG-0709-10111)]. From the outset, the PPI Advisory Group fed directly into the Programme Steering Committee (PSC), with the PPI chairperson (Kevin Paul) acting as the patient representative and included as a co-applicant for the Programme Grant.
Embedding patient and public involvement in the REACH-HF research programme
The PPI Advisory Group reported directly to the Programme Management Group and had a representative on that committee – the chairperson of the PPI Advisory Group fulfilled this role from 2012. The Programme Management Group meetings had a standard agenda item for PPI activities. The chairperson reported on PPI activities and kept members of the PPI Advisory Group informed about discussions and the progress of the REACH-HF programme. The PPI members were keen to have feedback about how their recommendations were used; for example, they suggested having two types of exercise programmes (chair based and walking) and they contributed to wording on patient-facing documents to encourage participants in the control group to continue with the trial.
The lay chairperson, independent of the research team, also reported to the PPI representative on the PSC (Liz Clark).
Process and impact of patient and public involvement in the REACH-HF programme
Using the methods described in WP1 (see Work package 1: methods), the PPI Advisory Group helped the REACH-HF research team to develop a new facilitated self-care manual – ‘the REACH-HF intervention’ – for people with HF and their caregivers to help them manage the condition. 39
Over the 6 years to June 2018, the REACH-HF PPI Advisory Group consisted of up to eight active members – patients and caregivers – who regularly contributed to the development of the research study through consultation and collaborative approaches. Some members who joined at the start of the study had to leave the group because of deterioration in their health, with others replacing them as the study progressed. We developed a whole-systems model in which communication was expected to flow as a two-way process across all elements of the teams/groups. PPI informed the design of the intervention and contributed towards the research cycle, including revision of the intervention following a feasibility study in 2014. The PPI Advisory Group was involved in the dissemination events in 2018 and have continued to advise on dissemination of the REACH beyond June 2019 at the end of the NIHR Programme Grant. Some members of the group have become involved in ongoing projects related to the digitisation of the REACH-HF intervention in 2020–21.
The work of the PPI Advisory Group was presented as an oral poster presentation at the NHS Research Forum in May 2016. 92
Kevin Paul, chairperson of the PPI Advisory Group, described the impact of the REACH-HF project for him and other members of the group over the course of the project:
From the outset, the management team have been welcoming and willing to take on board comments from the PPI [Advisory] Group, ensuring that the [HF] Manual is easy to read for the patients and caregivers. The genuine interest taken in the feedback from the group throughout the project has been both gratifying and encouraging.
On 24 November 2017, members of the PPI Advisory Group met with Rod S Taylor and Hasnain Dalal, the co-chief investigators, as well as Jennifer Wingham (investigator) and Suzanne Nunn (project administrator). Rod S Taylor and Hasnain Dalal thanked the PPI Advisory Group members for their contribution and gave a presentation on the REACH-HF study and its key findings. Members of the group were the first to hear about the main results of both RCTs. They appreciated being involved at every stage of the REACH-HF project and agreed to continue to help with dissemination of the results. Members of the PPI Advisory Group joined Rod S Taylor, Hasnain Dalal, Jennifer Wingham and Suzanne Nunn for a meal after the presentation. Some members of the PPI Advisory Group accepted an invitation to attend the REACH-HF dissemination event in Falmouth on 6 April 2018. A further meeting in Truro on 26 June 2018 was convened by Professor Colin J Greaves (investigator) to seek the views of the PPI Advisory Group on updating the REACH-HF intervention prior to possible implementation within the NHS.
Conclusions
The REACH-HF research programme focused on developing and evaluating a novel home-based self-care rehabilitation intervention for patients with HF and their caregivers. Our aim was to create a clinically effective and cost-effective alternative to centre- or hospital-based CR that could increase the current low uptake of CR in people with HF.
Our research was conducted across NHS secondary and primary care centres in England, Wales and Scotland. Over the 5 years of our research programme, we achieved our stated overall aim and objectives across four WPs.
In the first 15 months of the programme, we successfully co-designed and developed a novel, home-based, self-care rehabilitation intervention (‘the REACH-HF intervention’), with stakeholders including patients with HF (see WP1A), their caregivers (see WP1B), clinicians, NHS commissioners and academics with an interest in CR and HF. This resulting REACH-HF intervention is a comprehensive, theory-based, user-centred, home-based, self-care support programme for people with HF and their caregivers comprising three core components (i.e. the REACH-HF Manual, a Participant ‘Progress Tracker’ booklet and a ‘Family and Friends Resource’ for caregivers) and a training course for facilitators. This is one of the few studies to describe in detail the theoretical and evidentiary basis, intervention techniques and strategies for an intervention to promote the quality of life of people with HF and their caregivers. The main limitation was the complexity of the process, which affects replicability and requires considerable resources. Despite a transparent audit trail and documentation of all of the processes involved, it is unlikely that a different team of collaborators using the same methods would have produced exactly the same intervention. The implementation could have taken a number of different forms, as ‘judgement calls’, decisions and selection of appropriate methods or theoretical approaches were required at many stages during the process. Although a panel of experts ensured that judgement calls involved multiple stakeholders and decisions were based on either evidence or appropriate expertise, there was often no clear ‘best solution’ and a different group of experts may have made different decisions. Therefore, intervention development remains as much an art as a science, depending on the individual expertise, experience, instincts and knowledge of the team, as well as on team dynamics and collective decision-making. Intervention mapping, along with strong service user involvement, was a resource-intensive, but rigorous, method that allowed the development of a comprehensive, evidence-informed, theoretically driven, facilitated self-care and rehabilitation intervention that is grounded in the needs of patients with HF, caregivers and service providers. 39
The REACH-HF intervention was tested in a feasibility study in patients with HFrEF, in which it was well accepted by the 23 patients, 12 caregivers and health-care facilitators who took part, with high attendance and adequate delivery of most aspects of the intervention. Levels of outcome completion were generally excellent, and patients/caregivers perceived a relatively low measurement burden. A number of patient and caregiver outcomes showed evidence of improvement after the REACH-HF intervention. Analysis of the quality of intervention delivery, based on applying a checklist to recordings of all the consultations for 18 cases, suggested that the components of the intervention were mostly delivered as intended and with reasonably high quality. However, there was room for improvement in terms of addressing caregiver health and emotional health. Potential modifications to the content of the manual and facilitator training were identified, and, after these revisions, the REACH-HF intervention was ready for evaluation in a full-scale trial.
The main component of our programme was evaluation of the REACH-HF intervention in a multicentre RCT (see Work package 3: multicentre randomised controlled trial of the REACH-HF intervention in patients with heart failure with reduced ejection fraction) in patients with HFrEF from Birmingham, Cornwall, Gwent and York. We recruited to target (n = 216) and on time, with excellent retention (86%) at the 12-month follow-up. We found a statistically significant and clinically meaningful (> 5 points) mean between-group difference in the primary outcome measure, MLHFQ score, in favour of the REACH-HF group at the 12-month follow-up. With the exception of patient self-care (p < 0.001), no significant difference was seen in other secondary outcomes, including clinical events (p > 0.05), at follow-up compared with usual care. The maintenance score on the SCHFI was in favour of the REACH-HF intervention at 12 months (p < 0.001). Within-group improvements from baseline were seen in the REACH-HF group for HADS anxiety and depression, ISWT and SCHFI (management and confidence), but these were not statistically significant when compared with the control group at 12 months. We found no evidence of a significant subgroup treatment interaction on the primary outcome at 12 months by NT-proBNP level, presence of caregiver, recruitment site or duration of HF. The absence of significant between-group differences in exercise capacity and physical activity and no differences in most of our secondary outcomes may reflect the fact that the trial was not formally powered on these outcomes. Furthermore, the mean age of 70 years and multimorbidities of our trial population may have had a negative impact on the outcomes of exercise capacity and physical activity. In terms of caregiver outcomes, only the SCHFI confidence score achieved between-group statistical significance at follow-up. This may reflect that caregivers were not randomised and the study sample size was powered on the between-group difference in patient outcomes rather than caregiver outcomes. We calculated that the mean cost of the REACH-HF intervention was £418.39 per participant, which is less than the current NHS tariff for CR of £477. Our evidence suggests that the novel REACH-HF home-based facilitated intervention for HFrEF was, thus, clinically superior in terms of disease-specific HRQoL at 12 months and offers an affordable alternative to traditional centre-based programmes to address current low CR uptake rates for HF. 44
In a cost-effectiveness analysis using data from our multicentre RCT, we used model-based analyses to capture disease progression using health states that represent important event-related activity of HF. An updated Cochrane systematic review of the evidence for exercise-based CR in adults with HF, which was undertaken as part of our programme and includes data from our multicentre RCT, informed data inputs to the evidence synthesis. Taking a UK National Health and Personal Social Services perspective, we estimated the incremental cost per QALY gained and assessed uncertainty using probabilistic and deterministic sensitivity analyses. We estimated the cost-effectiveness of home-based CR versus usual care, the REACH-HF intervention versus usual care and centre-based CR versus usual care. Using the results from the main RCT and the EQ-5D, we saw no difference in QALY gain between groups, with usual care dominant, so the REACH-HF intervention was not cost-effective using this short-term perspective.
The health-economic findings from our main RCT and the lack of responsiveness of the EQ-5D in other studies support the use of an alternative to the EQ-5D, such as the SF-6D, for future health-economic evaluations in patients with less severe heart disease. The small number of direct head-to-head RCTs of centre- versus home-based CR in HF mean that data were too limited to directly estimate the longer-term cost-effectiveness of home- versus centre-based CR, so we focused on comparisons of home- and centre-based CR versus usual care (see Work package 4: model-based cost-effectiveness analysis and Appendix 3). In the base-case analysis, the REACH-HF intervention was associated with per patient mean QALY gain of 0.23 and an increased mean cost of £401 compared with usual care, resulting in a cost per QALY of £1721. Overall, our economic modelling using evidence synthesis and taking a longer-term view (see Work package 4: model-based cost-effectiveness analysis) suggests that the REACH-HF intervention was cost-effective compared with usual care. Findings of the REACH-HF intervention evaluation indicate effectiveness on one set of clinical outcomes but not all, and while the economic analysis conducted alongside the full RCT did not produce significant differences in EQ-5D or QALYs, economic modelling of the REACH-HF intervention against usual care suggested greater cost-effectiveness of the REACH-HF intervention.
In the process evaluation of the main multicentre RCT, intervention attendance was high, with > 90% of participants receiving the minimum intended dose, which contrasts with group-based CR programmes for which typical attendance levels are about 80% of intended contacts. 45 How well the REACH-HF intervention was perceived by the participants seemed to depend not on the ‘dose’ of the intervention (i.e. the contact time with the facilitator), but on other factors related to the delivery of the intervention. Multimodal and longitudinal qualitative data analysis suggested that behaviour change might be more likely to be sustained when the facilitator adapts the intervention to a participant’s needs. The facilitators’ competence also seemed to be a key factor in participants acquiring skills and changing behaviour. Most caregivers engaged with the intervention and made changes to how they support the person with HF for whom they were caring and gained in confidence. Lower intervention fidelity scores were also related to caregiver issues, suggesting that the support provided to caregivers by facilitators should be improved in a future, revised, intervention. However, based on qualitative data across WP2, WP3 and the feasibility study, components of the programme patients valued highly or that seemed to be associated with effectiveness included the Progress Tracker, the facilitators, adaptation of the intervention to individual needs, addressing emotional consequences (especially for the HFpEF sample), information in the HF Manual, understanding what to do in an emergency, support for caregivers (where this was given) and the exercise programme.
We also conducted a single-centre pilot RCT to assess the feasibility and acceptability of the REACH-HF intervention for patients with HFpEF and their caregivers. The REACH-HF intervention was originally designed for patients with HFrEF in terms of coverage of medication and explanations of the condition, as there was limited evidence to guide development of the REACH-HF intervention for patients with HFpEF. Following a review of clinical guidance and consultation with the expert cardiology, primary care and physiology/CR specialist members of our project management group, the intervention required only minor modification for patients with HFpEF. This review and consultation included removing specific information on certain medications, such as beta-blockers, ACE inhibitors and angiotensin receptor antagonists, which have not been shown to be of prognostic benefit in patients with HFpEF, and changing the description of the condition to better cover causes and management of HFpEF. In contrast to the neutral outcomes of device and drug trials in HFpEF, promising evidence indicates the potential for exercise-based CR to benefit patients with HFpEF. The revised REACH-HF intervention was well received by patients, caregivers and health-care facilitators, and adherence to the intervention was good. At follow-up, a number of patient outcomes, including our primary outcome measure, the MLHFQ score, showed a direction of effect in favour of the intervention group over control, although this study was not designed or powered to assess definitively the efficacy or safety of the REACH-HF intervention in HFpEF. The findings of this pilot study have informed a funding application for a fully powered, multicentre RCT in patients with HFpEF. Patient interviews from a nested qualitative study showed that the ISWT was not feasible as a method of assessing exercise tolerance for some participants, with more than one in four unable to undertake the exercise test at final follow-up. A fidelity assessment that was part of the process evaluation suggested that the intervention was generally well delivered by the facilitators, although lower scores were related to caregiver issues, indicating room for improvement with respect to the involvement of and support provided to caregivers by facilitators. Semistructured qualitative interviews gave an insight into the patient’s understanding of HF, the emotional consequences of HF and the patient’s response to the REACH-HF intervention, including the facilitator.
In the main multicentre RCT (WP3) the lack of blinding was a key limitation, as the complex nature of the intervention and control meant that we could not mask participants to treatments, introducing the possibility of patient expectation bias. Another limitation was the 15% missing data for the primary outcome measure at the 12-month follow-up. Assessment of adherence was also a limitation given the self-directed nature of the home-based intervention, and we were not able to capture consistent patient-level data on adherence to the exercise training component of the intervention. Moreover, interpretation of our findings should take account of the fact that, compared with the general population of patients with HF in the UK, our study population had a lower mean age (70 vs. 76 years) and included a higher proportion of males (78% compared with an almost equal sex balance); in addition, we recruited only a very small number (n = 7) of people from ethnic minorities.
We successfully completed all components of our programme on time and under budget. We attribute the smooth running of our programme to several factors. We were able to establish a team of investigators with a track record of conducting some of the major RCTs of CR in the UK when we were awarded our NIHR Programme Development Grant in 2010. We had good working relationships within our internal and external committees and the benefit of active high-quality guidance from our PSC and PPI Advisory Group. Support from the UKCRC-registered Peninsula CTU helped to ensure that our study centres were able to recruit to target and on time and achieve high standards of data management and reporting.
Key conclusions
The REACH-HF multicentre trial44 has been acknowledged as ‘one of the first CR studies to systematically develop a rehabilitation intervention using gold standard methods for developing complex interventions’. 93 Other key attributes of the study include co-development of the intervention with patients, caregivers, clinicians and academics with expertise in health behaviour change, psychology and exercise. The intervention was evaluated in a fully powered multicentre RCT in patients with HFrEF and a single-centre pilot RCT in patients with HFpEF. The results of the multicentre RCT showed that the addition of a home-based rehabilitation and self-care intervention to usual care resulted in a clinically important improvement in the HRQoL of patients with HFrEF. Our model-based economic evaluation, extrapolating results from the multicentre trial in patients with HFrEF over a time horizon of 4 years, found that an estimated mean cost per patient of £15,452 (95% CI £14,240 to £16,780) and a mean QALY gain of 4.47 (95% CI 3.83 to 4.91) for the REACH-HF intervention plus usual care, compared with a mean cost of £15,051 (95% CI £13,844 to £16,289) and a mean QALY gain of 4.24 (95% CI 4.05 to 4.43) with usual care alone, resulting in an incremental cost per QALY of £1721. Probabilistic sensitivity analysis indicated a 78% probability that the REACH-HF intervention plus usual care is cost-effective versus usual care alone at a threshold of £20,000 per QALY. The mean cost of the REACH-HF intervention was £418 per participant and within the current NHS tariff for CR of £477 per patient. 44
The REACH-HF may be the first alternative to supervised group-based intervention for patients with HFrEF which has indicated effectiveness in terms of HRQoL and patient self-care. As attendance at group-based CR for people with HF is poor, ‘the 90% adherence rate achieved in this study is important. The evidence for an affordable home-based programme as an alternative to other settings is growing and may have future workforce implications’. 94 Our findings provide an important contribution to the evidence base supporting home-based approaches to delivery of CR in HF, such as the REACH-HF intervention, which can provide a patient-centred alternative to centre-based CR and could help to address the current suboptimal uptake of CR for HF in the UK and elsewhere. It also addresses the recommendations in the most recent NICE clinical guideline for the management of HF (NG106),5,19 which has stated the importance of home-based CR programmes and recommends offering patients with chronic heart failure an easily accessible personalised programme of exercise-based rehabilitation, which may be at home, in the hospital or in the community. A 4-minute video demonstrates our intervention and summarises the findings of our main RCT (https://reachhf.co.uk/; accessed 11 February 2020).
Recommendations for future research
Specific recommendations for future research include the following:
-
Assess the impact of different models of CR on uptake and adherence (e.g. does introducing home-based CR increase uptake and adherence by patients with HF compared with traditional centre-based CR alone?).
-
Assess the impact of CR in people with HFpEF (e.g. is home-based CR clinically effective and cost-effective in patients with HFpEF?).
-
Assess the impact of digital enhancements to delivery of home-based CR (e.g. are internet-/app-based CR models clinically effective and cost-effective for patients with HF?).
-
Improve equity in CR delivery (e.g. how do we improve the uptake and delivery of CR in hard-to-reach groups, including ethnic minorities?).
-
Explore models of CR to address multimorbidity (e.g. how do we best adapt existing CR models to deliver the health needs of cardiac patients with multimorbidity?).
-
Capture the health utility of therapies for HF {e.g. given the current evidence of the potential insensitivity of the EQ-5D in patients with HF, especially those with mild disease severity, can we use instruments to better capture HRQoL utility, such as SF-6D [from the SF-12 or Short Form questionnaire-36 items (SF-36)]?}.
Discussion
In the UK, < 20% of patients discharged from hospital after a diagnosis of HF are referred for CR. 34 The availability of the REACH-HF intervention gives clinicians the opportunity to refer patients to a potentially clinically effective and cost-effective evidence-based intervention that can be delivered in their home.
Low referral rates to CR are compounded by poor uptake. In 2017–18, 68,266 patients were admitted to hospital with a primary diagnosis of HF95 but < 5000 attended at least one session of CR,29 despite guidance recommending CR for HF from NICE dating back to 2010. 5,35 However, recent NACR reports reflect an increase in the number of patients with HF attending CR,29 suggesting that there may be more programmes offering dedicated CR to patients with HF than those reported in 2012. 96 The majority of REACH-HF patients were multimorbid, experiencing a number of cardiovascular and non-cardiovascular comorbidities. Despite the high mean age (≈ 70 years) and presence of multiple comorbidities, adherence to the REACH-HF programme exceeded 85% in both trials. 42,44
The REACH-HF intervention may also offer direct benefits to caregivers. Our qualitative process evaluation showed that the REACH-HF intervention facilitated communication between the patient and the caregiver. The inclusion of family/friends was identified as valuable (by patients and caregivers) in the provision of social support in HF self-care for both patients and caregivers. Patients and caregivers valued having the Progress Tracker because it enabled them to measure the patient’s progress. Caregivers who were low in knowledge and confidence at the beginning of the REACH-HF programme reported increased knowledge and enhanced caregiving skills from REACH-HF to maintain their role and contribute to the management and maintenance of HF self-care.
Patients valued the simplicity of the REACH-HF programme, and specifically the accessibility of the information provided. Based on the qualitative data across WP2, WP3 and the feasibility study, the components of the intervention that patients valued highly and seemed to be associated with effectiveness included the Progress Tracker, high-quality facilitation, adaptation for the intervention to individual needs, addressing emotional consequences (especially for the HFpEF sample), the relevance of the information in the HF Manual, understanding what to do in an emergency, support for caregivers (where this was given) and the exercise programme. For patients with HFrEF and their caregivers, the intervention was considered to be adequately delivered, was well received and could be implemented more widely. That this could be implemented more widely should be considered in the context of the small number of drop-outs in the main trial, which was attributed to withdrawal (< 10%) as some participants did not wish to continue because of their deteriorating health and the modest number of deaths (4%, n = 8, four in the REACH-HF intervention group and four in the control group). The time commitment was not reported as a significant issue by patients or caregivers. Although the lack of significant between-group difference in outcomes other than the total MLHFQ and the SCHFI may reflect no true difference in these outcomes, it may also reflect the fact that the main trial was not formally powered to detect differences in secondary outcomes, especially clinical events. Furthermore, the REACH-HF intervention is comprehensive and multifactorial, with individual patients likely to have experienced different pathways to improved HRQoL, which may include reduced stress or anxiety; improved pacing of physical activity, exercise capacity or sleep quality; and better medication management. Variations in participants’ application of the REACH-HF intervention to their own requirements, as well as variations in treatment effect, may have precluded the observation of statistically significant between-group differences in secondary outcomes. In addition, the baseline characteristics of our study population indicated high levels of comorbidity. The lack of impact on exercise capacity and physical activity may, therefore, be attributed to the ‘heavy burden of comorbid disease’ that can affect outcomes in older patients with HF.
Successful development of the REACH-HF Manual/Intervention for people with HF involved the collaboration of four parties: the University of Exeter, the Heart Manual Department (NHS Lothian), the Royal Cornwall Hospitals NHS Trust and Professor Patrick Doherty (who developed the chair-based exercise DVD). A legally executed collaboration agreement regarding the intellectual property rights of the REACH-HF Manual/Intervention was signed by the four parties, with an equal share in the intellectual property rights to cover the duration of the study (2013–18). In February 2019, the four parties signed a memorandum of understanding, in which the parties agreed to explore the possibility of commercially exploiting the REACH-HF Manual/Intervention.
Implications for practice
Our clinical and economic findings suggest that a home-based CR programme, such as REACH-HF, may help to improve the HRQoL of patients with HFrEF. Although there was no evidence of impact on hospital admissions or mortality at 12 months within the RCT, economic modelling showed that the REACH-HF intervention plus usual care is cost-effective versus usual care alone at a threshold of £20,000 per QALY. 44,46 The clinical characteristics of patient participants were consistent with the wider population of HF patients in terms of age only; however, most of our participants were male (78%) and we recruited only a very small number (n = 7) of people from ethnic minorities. In the UK, usual care involves follow-up by the GP or specialist nurse and, for some patients, a cardiologist. In addition, we have tested the REACH-HF intervention across a range of geographical sites in UK that included rural (Cornwall and Gwent) and urban (Birmingham and York) centres with people from ethnic minorities (Birmingham). However, in applying the results of the REACH-HF programme, it is important to acknowledge the limitations stated above in terms of sex inequality and ethnicity. 45
Given the successful dissemination of our findings and enhanced dissemination through the beacon sites (funded from an underspend of this Programme Grant), at the time of writing (February 2020), the four beacon sites set up in 2019 have delivered the REACH-HF intervention to > 100 patients, and outcome data are being collected from the NACR database as part of ongoing research. 97 Furthermore, funding to set up and evaluate another four beacon sites in Scotland in 2020 has been obtained from Heart Research UK. 98
We are continuing to disseminate our findings: see Appendices 1 and 2, and Report Supplementary Material 1 and 7. To gain maximum impact, we are making our findings publicly available in various formats tailored to the specific audience (Box 3).
In addition to publication in scientific peer-reviewed journals, we also aim to reach audiences including clinicians, commissioners, service users, policy-makers, methodologists, research participants, patients, caregivers, members of the public and academics involved in teaching and research.
We worked with a professional health-care video company to make a brief film involving patients, a caregiver and the lead researchers to showcase the findings of the REACH-HF RCT to enhance future dissemination. This video of the intervention and summary of the study results can be viewed at https://reachhf.co.uk/ (accessed 11 February 2020).
In October 2018, the NIHR Central Commissioning Facility approved our request for enhanced dissemination by allowing us to use an underspend that we had accrued over the duration of the PGfAR (2013–18).
-
A variation to the contract has allowed us to engage in enhanced dissemination to maximise the opportunity for the NHS to take up the intervention going forward.
-
Specifically, we sought to use the funding to undertake a range of additional dissemination activities that would increase the value and use of this intervention through the recruitment of four beacon CR centres within the NHS.
-
All CR centres in the NHS were invited, through an open competition, to apply to become beacon sites and engage in enhanced dissemination of the updated REACH-HF intervention from summer 2019.
-
In return for free training of three facilitators per beacon site and the provision of 50 free REACH-HF intervention packs, each site will be expected to recruit 50 patients with HF and their caregivers over a 12-month period.
-
A dedicated post-doctoral research associate from the University of Exeter will liaise with the NACR to collect pre- and post-CR outcomes of the patients receiving the REACH-HF intervention so that we can assess the ‘real-world’ outcomes of the REACH-HF intervention (and compare with what we have seen in our RCTs).
-
In addition, beacon sites will allow visits from other interested CR centres and providers to see first-hand how the REACH-HF intervention is delivered in their location.
-
A PhD student under the guidance of one of the investigators (Colin J Greaves) is also looking at the barriers to implementation of a novel intervention such as REACH-HF in the NHS. 97
-
PGfAR, Programme Grants for Applied Research; PhD, Doctor of Philosophy.
The NACR has developed a specific REACH-HF Manual data field as part of the national audit, which was available to more than 200 CR programmes by the end of 2019. From June 2019, CR programmes have been able to record REACH-HF as a specific mode of delivery using the NHS Digital/NACR system, and data are being collected by the national audit team for reporting in the 2020 NACR report. This has allowed us to track uptakes from 2019 and will be reported in future NACR annual quality and outcomes reports.
In October 2020, REACH-HF won the BMJ Award for Stroke and Cardiovascular Team of the Year for our patient involvement and ‘excellent teamwork producing a real-world solution’. 99,100
Acknowledgements
The full research team was Hasnain M Dalal (co-lead), Rod S Taylor (co-lead), Charles Abraham, Wendy Armitage, Jackie Miles, Nicky Britten, Sarah Buckingham, Michelle Clark, Russell C Davis, Patrick Doherty, Victoria Eyre, Lorna Geach, Colin J Greaves, Chris Hayward, Melvyn Hillsden, Kate Jolly, Chim Lang, Suzanne Nunn, Kevin Paul, Susannah Sadler, Sally Singh, Karen Smith, Louise Taylor, Robin van Lingen, Fiona Warren and Jennifer Wingham. For part of the study, we acknowledge input from Beth Barnes, Lucy Moore and Anna Sansom. Colin Green and Susannah Sadler both contributed to the health economic aspects of the study; Colin Green requested that his involvement in the study be terminated in September 2018.
Acknowledgements by work package and publication
Work package 1
Optimising self-care support for people with heart failure and their caregivers: development of the REACH-HF intervention using intervention mapping
Ethics approval and consent to participate
The feasibility study was approved by National Research Ethics Service (NRES) Committee South Central, Berkshire (REC reference 13/SC/0640), and all participants consented to participate. Prior qualitative work had separate ethics approval, as described in the papers. No ethics approval was sought for other elements of the intervention development work described here. This is because these elements consisted of service development work not falling under the NRES/Health Research Authority definition of research (www.hra.nhs.uk/documents/2016/06/defining-research.pdf; accessed 20 February 2020) in that we were not attempting to generate generalisable new knowledge (we merely sought to gather data to inform choices during the development of our specific intervention). Hence, we did not consider that ethics approval was needed. All of the activities involving service users are commonly used in PPI work, which is not normally considered to constitute research. This decision was validated through discussion with the programme chief investigators and the REACH-HF Project Management Group.
Acknowledgements
We would like to thank the members of our PPI Advisory Group, the independent Project Steering Committee (chaired by Professor Martin Cowie) and everyone who contributed data or their expert opinion and advice to the study. Mr Brian Begg from the Aneurin Bevan Health Board Cardiac Rehabilitation team also provided advice and input to the exercise and physical activity working group.
The full REACH-HF research team is Rod S Taylor (Programme Co-Lead), Hasnain Dalal (Programme Co-Lead), Charles Abraham, Jackie Austin, Nicky Britten, Sarah Buckingham, Russell C Davis, Patrick Doherty, Lorna Geach, Colin J Greaves, Colin Green, Heart Manual Department Edinburgh (Louise Taylor, Carolyn Deighan, Wendy Armitage, Jennifer Elliott, Michelle Clark), Kate Jolly, Kevin Paul, Chris Hayward, Victoria Eyre, Sally Singh, Robin van Lingen and Jennifer Wingham.
Needs of caregivers in heart failure management: a qualitative study
Ethics approval and consent to participate
Ethics approval for the study protocol was obtained from NRES Committee South Central – Southampton B 12/SC/0643.
Acknowledgements
We would like to thank all of the caregivers who gave their time and valuable insights into their needs and Dave Turner (qualitative researcher), the CR departments, specialist community HF nurses, support groups in Cornwall, Birmingham and Leicester, and the Cardiomyopathy Association for their help in identifying caregivers.
Work package 2
REACH-HF: a facilitated self-care rehabilitation intervention in patients with heart failure with preserved ejection fraction and their caregivers – rationale and protocol for a single-centre pilot randomised controlled trial
Ethics approval and consent to participate
The study was approved by the East of Scotland Research Ethics Service (reference 15/ES/0036). Findings will be disseminated via journals and presentations to clinicians, commissioners and service users.
Acknowledgements
The REACH-HFpEF pilot trial was designed by Chim C Lang, Karen M Smith, Hasnain M Dalal, Rod S Taylor, Jennifer Wingham, Kate Jolly, Russell C Davis, Patrick J Doherty, Jackie Miles, Robin van Lingen, Sally Singh, Charles Abraham, Nicky Britten, Colin J Greaves, Kevin Paul and Sarah Buckingham. Kate Jolly, Rod S Taylor, Russell C Davis and Hasnain M Dalal developed the original idea for REACH-HFpEF. Chim C Lang, Karen M Smith, Hasnain M Dalal, Rod S Taylor, Kate Jolly and Russell C Davis developed the protocol for the mechanistic substudy elements. Victoria Eyre undertook the first draft of the manuscript that was then edited by Christopher Hayward, Rod S Taylor and Hasnain M Dalal. All authors provided critical evaluation and revision of the manuscript and have given final approval of the manuscript accepting responsibility for all aspects.
A randomised controlled trial of a facilitated home-based rehabilitation intervention in patients with heart failure with preserved ejection fraction and their caregivers: the REACH-HFpEF pilot study
Ethics approval and consent to participate
Scotland A Research Ethics Committee (ISRCTN57596739). Patient consent was obtained.
Acknowledgements
The REACH-HFpEF pilot trial was designed by Chim C Lang, Karen M Smith, Hasnain M Dalal, Rod S Taylor, Jennifer Wingham, Kate Jolly, Russell C Davis, Patrick J Doherty, JM, Robin van Lingen, Sally Singh, Charles Abraham, Nicky Britten, Colin J Greaves, Kevin Paul, Melvyn Hillsdon, Sally Singh and Christopher Hayward. Kate Jolly, Rod S Taylor, Russell C Davis and Hasnain M Dalal developed the original idea for REACH-HFpEF. Fiona C Warren and Colin J Greaves analysed the data. Victoria Eyre and Christopher Hayward were responsible for study and data collection management. Chim C Lang undertook the first draft of the manuscript that was then edited by Jennifer Wingham, Fiona C Warren, Rod S Taylor and Hasnain M Dalal. All authors were involved in critical evaluation and revision of the manuscript and have given final approval of the manuscript accepting responsibility for all aspects.
The authors thank the research nurses (Lynn Rutherford and Fatima Baig), intervention facilitators (Anona Cranston and Gillian Smith) and patients and their caregivers in Dundee and Tayside who participated in this study. The authors also thank the data team at Peninsula CTU (University of Plymouth), Lisa Price (University of Exeter), who assisted with the analysis of accelerometry data, Karen Coyle (University of Dundee), who assisted with qualitative interviews and intervention fidelity checking, and Carolyn Deighan, Jennifer Elliott and Louise Taylor and her team at the Heart Manual Office Heart Office (Astley Ainslie Hospital, Edinburgh) for their assistance with the preparation and development of the REACH-HF intervention.
Patient and caregiver experiences of the REACH-HF in patients with heart failure with preserved ejection fraction (REACH-HFpEF) study: a process evaluation
Ethics approval and consent to participate
Ethics approval was obtained from the East of Scotland Research Ethics Committee A (11/AL/0250). Patient consent was obtained.
Acknowledgements
The REACH-HFpEF pilot trial was designed by Chim C Lang, Karen M Smith, Hasnain M Dalal, Rod S Taylor, Jennifer Wingham, Kate Jolly, Russell C Davis, Patrick J Doherty, Jackie Miles, Robin van Lingen, Sally Singh, Charles Abraham, Nicky Britten, Colin J Greaves, Kevin Paul, Melvyn Hillsdon, Sally Singh and Christopher Hayward. Kate Jolly, Rod S Taylor, Russell C Davis and Hasnain M Dalal developed the original idea for REACH-HFpEF. Joanne Coyle and Karen M Smith were responsible for study data collection and management. Joanne Coyle, Karen M Smith and Fiona C Warren analysed the data. Karen M Smith undertook the first draft of the manuscript that was then edited by Jennifer Wingham, Fiona C Warren, Rod S Taylor, Fiona C Warren and Hasnain M Dalal. All authors were involved in critical evaluation and revision of the manuscript and have given final approval of the manuscript accepting responsibility for all aspects.
The authors thank the research nurse/assistants (Lynn Rutherford and Fatima Baig), the intervention facilitators (Anona Cranston and Gillian Smith), and patients and their caregivers in Dundee and Tayside who participated in this study. The authors also thank the teams at TP Transcription Limited for transcription of the audio-recorded interviews and the data team at Peninsula CTU (University of Plymouth), Lisa Price (University of Exeter) who assisted with the analysis of accelerometry data, Joanne Coyle (University of Dundee) who assisted with qualitative interviews and intervention fidelity checking, and Louise Taylor and her team at the Heart Manual Office (Astley Ainslie Hospital, Edinburgh) for their assistance with preparation and development of the REACH-HF intervention.
Work package 3
Clinical effectiveness and cost-effectiveness of the REACH-HF facilitated self-care rehabilitation intervention in heart failure patients and caregivers: rationale and protocol for a multicentre randomised controlled trial
Ethics approval and consent to participate
The study was approved by the North West Lancaster Research Ethics Committee (ref 14/NW/1351). Findings will be disseminated via journals and presentations to publicise the research to clinicians, commissioners and service users. Patient consent was obtained.
Acknowledgements
The REACH-HF trial was designed by Hasnain M Dalal, Rod S Taylor, Nicky Britten, Jackie Miles, Russell C Davis, Patrick J Doherty, Kate Jolly, Jennifer Wingham, Robin van Lingen, Kevin Paul, Charles Abraham and Colin J Greaves. Christopher Hayward undertook the first draft of the manuscript that was then edited by Rod S Taylor and Hasnain M Dalal. All authors provided critical evaluation and revision of the manuscript and have given final approval of the manuscript accepting responsibility for all aspects.
Home-based rehabilitation for heart failure with reduced ejection fraction: the REACH-HF multicentre randomised controlled trial
Ethics approval and consent to participate
This trial was approved by the North West Lancaster Research Ethics Committee (14/NW/1351). All participants provided written informed consent.
Acknowledgements
We thank all participants, facilitators, clinicians, researchers and administrators in Birmingham, Cornwall, Gwent, HM Department NHS Lothian (Carolyn Deighan and Jenny Elliott), Peninsula CTU, Royal Cornwall Hospitals Trust (Research, Development and Innovation and Clinical Chemistry departments), the Programme Steering Committee [Martin Cowie (chairperson), Graham Dunn, Suzanna Hardman, Roger Boyle and Liz Clark], the Data Monitoring Committee [Ann Dorthe-Zwisler (chairperson), Alan Montgomery and Gill Furze] and independent adjudicators (Iain Squire, Sern Lim and Paco Leyva). We thank Jemma Lough for technical editing and John Cleland, John Campbell and Tony Mourant for comments on earlier drafts of this manuscript.
Funding
The funders’ peer-review process informed the trial protocol. The sponsor of the trial had no role in trial design, data collection, data analysis, data interpretation or writing of the report. The corresponding author had full access to all of the data in the trial and had final responsibility for the decision to submit.
Facilitated self-care and rehabilitation for patients with reduced ejection fraction heart failure (REACH-HF trial): impact on caregiver outcomes
Ethics approval and consent to participate
The investigation conformed to the principles outlined in the Declaration of Helsinki and was approved by the North West Lancaster Research Ethics Committee (14/NW/1351). Written informed consent was obtained from both patient and caregiver participants.
Acknowledgements
We thank all caregivers, patients, facilitators and clinicians involved in the trial and acknowledge the vital contributions of trial researchers and administrators in Birmingham, Cornwall, Gwent, the Heart Manual Office (Carolyn Deighan and Jenny Elliott for the facilitator training), the Peninsula CTU, the Royal Cornwall Hospitals Trust (Research, Development and Innovation and Clinical Chemistry departments), the Programme Trial Steering Committee [Martin Cowie (chairperson), Graham Dunn, Suzanna Hardman, Roger Boyle and Liz Clark] and the Data Monitoring Committee [Ann Dorthe-Zwisler (chairperson), Alan Montgomery and Gill Furze]. We thank Jemma Lough for help with technical editing of the manuscript and Lucy Moore for conducting three of the caregiver interviews.
Process evaluation of the REACH HF multicentre randomised controlled trial
Ethics approval and consent to participate
Ethics approval was granted by the North West Lancaster Research Ethics Committee (reference 14/NW/1351). Patient consent was obtained.
Acknowledgements
We thank the patients and caregivers who took part in the REACH-HF trial and process evaluation. We thank Kevin Paul for his contribution as PPI representative. We acknowledge the contribution of the Heart Manual Office at NHS Lothian, which contributed to the design of the intervention and facilitator training and the Peninsula CTU for study management.
We would also like to thank all facilitators and clinicians involved in the study and acknowledge the vital contributions of study researchers and administrators in Birmingham, Cornwall, Gwent, the Heart Manual Office (Carolyn Deighan and Jenny Edwards for the facilitator training), the Peninsula CTU, the Royal Cornwall Hospitals Trust (Research, Development and Innovation and Clinical Chemistry departments), the PSC (Martin Cowie, Graham Dunn, Suzanna Hardman, Roger Boyle and Liz Clark), the Data Monitoring Committee (Alan Montgomery, Ann Dorthe-Zwisler and Gill Furze) and independent adjudicators (Iain Squire, Sern Lim and Paco Leyva). Lucy Moore conducted a small number of interviews.
Work package 4
Exercise-based rehabilitation for adults with heart failure (review)
Ethics approval and consent to participate
None given.
Acknowledgements
We thank Simon Briscoe who performed searches in the previous update review.
Exercise-based rehabilitation for heart failure: Cochrane systematic review, and meta-analysis and trial sequential analysis
Ethics approval and consent to participate
None given.
Acknowledgements
We thank the Cochrane Heart Editorial and reviewers for comments on drafts of the Cochrane review on which this manuscript is based.
The cost-effectiveness of REACH-HF and home-based cardiac rehabilitation in the treatment of heart failure with reduced ejection fraction: a decision model-based analysis
Acknowledgements
The following are the authors’ current affiliations: Rod S Taylor – Institute of Health and Wellbeing, School of Medicine, Dentistry and Nursing, University of Glasgow, Glasgow, UK; Colin J Greaves – School of Sport, Exercise and Rehabilitation Sciences, University of Birmingham, Birmingham, UK; Charles Abraham – School of Psychological Sciences, Faculty of Medicine, Dentistry and Health Sciences, University of Melbourne, Parkville, VIC, Australia.
We thank all REACH-HF study participants, facilitators, clinicians, researchers and administrators who contributed data or their expert opinion and advice to the study in Birmingham, Cornwall, Exeter, Gwent, York, Heart Manual Department NHS Lothian (Carolyn Deighan, Louise Taylor and Jenny Elliott), Peninsula CTU, Royal Cornwall Hospitals Trust (Research, Development and Innovation and Clinical Chemistry departments), PPI Advisory Group [Kevin Paul (chairperson)], PSC [Martin Cowie (chairperson), Graham Dunn, Suzanna Hardman, Roger Boyle and Liz Clark], Data Monitoring Committee [Ann Dorthe-Zwisler (chairperson), Alan Montgomery and Gill Furze], and independent adjudicators (Iain Squire, Sern Lim and Paco Leyva).
Funding
The funders’ peer-review process informed the trial protocol. The sponsor of the trial had no role in trial design, data collection, data analysis, data interpretation or writing of the report.
This report is based on a programme of work that included multiple contributions from several authors.
Contributions of others
We thank all participants, facilitators, clinicians, researchers and administrators who contributed data or their expert opinion and advice to the study in Birmingham, Cornwall, Gwent, Heart Manual Department NHS Lothian (Carolyn Deighan, Louise Taylor and Jenny Elliott), Peninsula CTU, Royal Cornwall Hospitals Trust (Research, Development and Innovation and Clinical Chemistry departments), PPI Advisory Group [Kevin Paul (chairperson)], PSC [Martin Cowie (chairperson), Graham Dunn, Suzanna Hardman, Roger Boyle and Liz Clark], Data Monitoring Committee [Ann Dorthe-Zwisler (chairperson), Alan Montgomery and Gill Furze], and independent adjudicators (Iain Squire, Sern Lim and Paco Leyva).
Mr Brian Begg from Aneurin Bevan Health Board Cardiac Rehabilitation team also provided advice and input to the exercise and physical activity working group.
John Cleland, John Campbell, Tony Mourant and Miriam Noonan provided comments on the manuscript drafts. Jemma Lough provided technical editing support. Sinead McDonagh and Samantha van Beurden engaged in the enhanced dissemination.
Contributions of authors
Hasnain M Dalal (https://orcid.org/0000-0002-7316-7544), Rod S Taylor (https://orcid.org/0000-0002-3043-6011), Jennifer Wingham (https://orcid.org/0000-0002-3342-1202), Colin J Greaves (https://orcid.org/0000-0003-4425-2691), Kate Jolly (https://orcid.org/0000-0002-6224-2115), Russell C Davis (https://orcid.org/0000-0002-4530-3078), Patrick J Doherty (https://orcid.org/0000-0002-1887-0237), Jackie Miles (https://orcid.org/0000-0001-7914-1110), Charles Abraham (https://orcid.org/0000-0002-0901-1975), Nicky Britten (https://orcid.org/0000-0002-7533-414X) and Sally Singh (https://orcid.org/0000-0002-9834-0366) designed the study, were responsible for its conduct and obtained the study funding.
Robin van Lingen was responsible for the conduct of the study and obtained the study funding.
Fiona C Warren (https://orcid.org/0000-0002-3833-0182), Susannah Sadler (https://orcid.org/0000-0001-5935-8507) and Melvyn Hillsdon (https://orcid.org/0000-0003-2818-3278) analysed the data, with Susannah Sadler conducting the health economic evaluation.
Christopher Hayward (https://orcid.org/0000-0002-2666-5073) and Victoria Eyre (https://orcid.org/0000-0003-0927-6815) were responsible for study and data collection management.
Kevin Paul (https://orcid.org/0000-0003-2972-9594) provided PPI input.
Colin J Greaves led the design of the intervention, with contributions from Jennifer Wingham, Patrick Doherty, Jackie Miles, Charles Abraham, Julia Frost, Sally Singh, Kate Jolly, Kevin Paul, Russell C Davis, Hasnain M Dalal and Rod S Taylor.
Hasnain Dalal wrote the first draft of this report, with contributions from Rod S Taylor.
Work package leads contributed to other sections: Colin J Greaves on WP1, Jennifer Wingham on WP1 and WP3, Chim C Lang (https://orcid.org/0000-0002-4530-3078) on WP2, Karen M Smith (https://orcid.org/0000-0001-6663-985X) on WP2, Julia Frost (https://orcid.org/0000-0002-3503-5911) on WP3, and Susannah Sadler on WP4.
All named authors reviewed and commented on drafts of the report. Investigators making specific contributions are listed above.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review. Requests can also be made to Rod S Taylor (as the joint lead of the REACH HF Study: Rod.Taylor@glasgow.ac.uk).
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health and Social Care.
References
- Long L, Mordi IR, Bridges C, Sagar VA, Davies EJ, Coats AJ, et al. Exercise-based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst Rev 2019;1. https://doi.org/10.1002/14651858.CD003331.pub5.
- Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail 2020;22:1342-56. https://doi.org/10.1002/ejhf.1858.
- Braunwald E. The war against heart failure: the Lancet lecture. Lancet 2015;385:812-24. https://doi.org/10.1016/S0140-6736(14)61889-4.
- Liao L, Anstrom KJ, Gottdiener JS, Pappas PA, Whellan DJ, Kitzman DW, et al. Long-term costs and resource use in elderly participants with congestive heart failure in the Cardiovascular Health Study. Am Heart J 2007;153:245-52. https://doi.org/10.1016/j.ahj.2006.11.010.
- National Institute for Health and Care Excellence (NICE) . Chronic Heart Failure in Adults: Diagnosis and Management 2018.
- Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart 2007;93:1137-46. https://doi.org/10.1136/hrt.2003.025270.
- Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet 2018;391:572-80. https://doi.org/10.1016/S0140-6736(17)32520-5.
- Huffman MD, Prabhakaran D. Heart failure: epidemiology and prevention in India. Natl Med J India 2010;23:283-8.
- Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014;63:1123-33. https://doi.org/10.1016/j.jacc.2013.11.053.
- Kingston A, Wohland P, Wittenberg R, Robinson L, Brayne C, Matthews FE, et al. Cognitive Function and Ageing Studies collaboration . Is late-life dependency increasing or not? A comparison of the Cognitive Function and Ageing Studies (CFAS). Lancet 2017;390:1676-84. https://doi.org/10.1016/S0140-6736(17)31575-1.
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891-975. https://doi.org/10.1002/ejhf.592.
- Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014;370:1383-92. https://doi.org/10.1056/NEJMoa1313731.
- Weinberger M, Oddone EZ, Henderson WG. Does increased access to primary care reduce hospital readmissions?. N Engl J Med 1996;334:1441-7. https://doi.org/10.1056/NEJM199605303342206.
- Davies EJ, Moxham T, Rees K, Singh S, Coats AJ, Ebrahim S, et al. Exercise based rehabilitation for heart failure. Cochrane Database Syst Rev 2010;4. https://doi.org/10.1002/14651858.CD003331.pub3.
- Sagar VA, Davies EJ, Briscoe S, Coats AJ, Dalal HM, Lough F, et al. Exercise-based rehabilitation for heart failure: systematic review and meta-analysis. Open Heart 2015;2. https://doi.org/10.1136/openhrt-2014-000163.
- Tu RH, Zeng ZY, Zhong GQ, Wu WF, Lu YJ, Bo ZD, et al. Effects of exercise training on depression in patients with heart failure: a systematic review and meta-analysis of randomized controlled trials. Eur J Heart Fail 2014;16:749-57. https://doi.org/10.1002/ejhf.101.
- Calvert MJ, Freemantle N, Cleland JG. The impact of chronic heart failure on health-related quality of life data acquired in the baseline phase of the CARE-HF study. Eur J Heart Fail 2005;7:243-51. https://doi.org/10.1016/j.ejheart.2005.01.012.
- Lewis EF, Johnson PA, Johnson W, Collins C, Griffin L, Stevenson LW. Preferences for quality of life or survival expressed by patients with heart failure. J Heart Lung Transplant 2001;20:1016-24. https://doi.org/10.1016/S1053-2498(01)00298-4.
- National Institute for Health and Care Excellence (NICE) . Quality Standard for Chronic Heart Failure 2016.
- British Association for Cardiovascular Prevention and Rehabilitation (BACPR) . The BACPR Standards and Core Components for Cardiovascular Disease Prevention and Rehabilitation 2017 2017. www.bacpr.com/resources/BACPR_Standards_and_Core_Components_2017.pdf (accessed January 2021).
- McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012;14:803-69. https://doi.org/10.1093/eurjhf/hfs105.
- Rees K, Taylor RS, Singh S, Coats AJ, Ebrahim S. Exercise based rehabilitation for heart failure. Cochrane Database Syst Rev 2004;3. https://doi.org/10.1002/14651858.CD003331.pub2.
- New York Heart Association . Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels 1994.
- Taylor RS, Sagar VA, Davies EJ, Briscoe S, Coats AJ, Dalal H, et al. Exercise-based rehabilitation for heart failure. Cochrane Database Syst Rev 2014;4. https://doi.org/10.1002/14651858.CD003331.pub4.
- Georgiou D, Chen Y, Appadoo S, Belardinelli R, Greene R, Parides MK, et al. Cost-effectiveness analysis of long-term moderate exercise training in chronic heart failure. Am J Cardiol 2001;87:984-8. https://doi.org/10.1016/S0002-9149(01)01434-5.
- Flynn KE, Piña IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, et al. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009;301:1451-9. https://doi.org/10.1001/jama.2009.457.
- National Institute for Health and Care Excellence (NICE) . Chronic Heart Failure: Management of Chronic Heart Failure in Adults in Primary and Secondary Care 2010.
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, et al. 2013 ACCF/AHA Guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;128:e240-e327.
- British Heart Foundation (BHF) . The National Audit of Cardiac Rehabilitation: Quality and Outcomes Report 2017 2017.
- Lewin B, Robertson IH, Cay EL, Irving JB, Campbell M. Effects of self-help post-myocardial-infarction rehabilitation on psychological adjustment and use of health services. Lancet 1992;339:1036-40. https://doi.org/10.1016/0140-6736(92)90547-G.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013.
- Beswick AD, Rees K, Griebsch I, Taylor FC, Burke M, West RR, et al. Provision, uptake and cost of cardiac rehabilitation programmes: improving services to under-represented groups. Health Technol Assess 2004;8. https://doi.org/10.3310/hta8410.
- Piña IL, Apstein CS, Balady GJ, Belardinelli R, Chaitman BR, Duscha BD, et al. Exercise and heart failure: a statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation 2003;107:1210-25. https://doi.org/10.1161/01.cir.0000055013.92097.40.
- Donkor A, McDonagh T, Hardman S. National Heart Failure Audit. London: Healthcare Quality Improvement Partnership; 2017.
- British Heart Foundation (BHF) . The National Audit of Cardiac Rehabilitation: Quality and Outcomes Report 2016 2016.
- Golwala H, Pandey A, Ju C, Butler J, Yancy C, Bhatt DL, et al. Temporal trends and factors associated with cardiac rehabilitation referral among patients hospitalized with heart failure: findings from Get With The Guidelines–Heart Failure Registry. J Am Coll Cardiol 2015;66:917-26. https://doi.org/10.1016/j.jacc.2015.06.1089.
- Clark AM, Reid ME, Morrison CE, Capewell S, Murdoch DL, McMurray JJ. The complex nature of informal care in home-based heart failure management. J Adv Nurs 2008;61:373-83. https://doi.org/10.1111/j.1365-2648.2007.04527.x.
- Taylor RS, Hayward C, Eyre V, Austin J, Davies R, Doherty P, et al. Clinical effectiveness and cost-effectiveness of the Rehabilitation EnAblement in CHronic Heart Failure (REACH-HF) facilitated self-care rehabilitation intervention in heart failure patients and caregivers: rationale and protocol for a multicentre randomised controlled trial. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-009994.
- Greaves CJ, Wingham J, Deighan C, Doherty P, Elliott J, Armitage W, et al. Optimising self-care support for people with heart failure and their caregivers: development of the Rehabilitation EnAblement in CHronic Heart Failure (REACH-HF) intervention using intervention mapping. Pilot Feasibility Stud 2016;2. https://doi.org/10.1186/s40814-016-0075-x.
- Wingham J, Frost J, Britten N, Jolly K, Greaves C, Abraham C, et al. REACH-HF research investigators . Needs of caregivers in heart failure management: a qualitative study. Chronic Illn 2015;11:304-19. https://doi.org/10.1177/1742395315574765.
- Eyre V, Lang CC, Smith K, Jolly K, Davis R, Hayward C, et al. Rehabilitation EnAblement in CHronic Heart Failure – a facilitated self-care rehabilitation intervention in patients with Heart Failure with preserved Ejection Fraction (REACH-HFpEF) and their caregivers: rationale and protocol for a single-centre pilot randomised controlled trial. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-012853.
- Lang CC, Smith K, Wingham J, Eyre V, Greaves CJ, Warren FC, et al. A randomised controlled trial of a facilitated home-based rehabilitation intervention in patients with heart failure with preserved ejection fraction and their caregivers: the REACH-HFpEF Pilot Study. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2017-019649.
- Smith K, Lang C, Wingham J, Frost J, Greaves C, Abraham C, et al. Process evaluation of a randomised pilot trial of home-based rehabilitation compared to usual care in patients with heart failure with preserved ejection fraction and their caregiver’s. Pilot Feasibility Stud 2021;7. https://doi.org/10.1186/s40814-020-00747-2.
- Dalal HM, Taylor RS, Jolly K, Davis RC, Doherty P, Miles J, et al. The effects and costs of home-based rehabilitation for heart failure with reduced ejection fraction: the REACH-HF multicentre randomized controlled trial. Eur J Prev Cardiol 2019;26:262-72. https://doi.org/10.1177/2047487318806358.
- Frost J, Wingham J, Britten N, Greaves C, Abraham C, Warren FC, et al. Home-based rehabilitation for heart failure with reduced ejection fraction: mixed methods process evaluation of the REACH-HF multicentre randomised controlled trial. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2018-026039.
- Taylor RS, Sadler S, Dalal HM, Warren FC, Jolly K, Davis RC, et al. The cost effectiveness of REACH-HF and home-based cardiac rehabilitation compared with the usual medical care for heart failure with reduced ejection fraction: a decision model-based analysis. Eur J Prev Cardiol 2019;26:1252-61. https://doi.org/10.1177/2047487319833507.
- Eldredge KL, Bartholomew CM, Markham GK, Ruiter RAC, Parcel GS. Planning Health Promotion Programs: An Intervention Mapping Approach. San Francisco, CA: John Wiley & Sons, Inc.; 2016.
- Piepoli MF, Conraads V, Corrà U, Dickstein K, Francis DP, Jaarsma T, et al. Exercise training in heart failure: from theory to practice. A consensus document of the Heart Failure Association and the European Association for Cardiovascular Prevention and Rehabilitation. Eur J Heart Fail 2011;13:347-57. https://doi.org/10.1093/eurjhf/hfr017.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Bartholomew LK, Parcel GS, Kok G. Planning Health Promotion Programs. An Intervention Mapping Approach. San Francisco, CA: Jossey-Bass; 2011.
- Wingham J, Harding G, Britten N, Dalal H. Heart failure patients’ attitudes, beliefs, expectations and experiences of self-management strategies: a qualitative synthesis. Chronic Illn 2014;10:135-54. https://doi.org/10.1177/1742395313502993.
- Taylor RS, Davies EJ, Dalal HM, Davis R, Doherty P, Cooper C, et al. Effects of exercise training for heart failure with preserved ejection fraction: a systematic review and meta-analysis of comparative studies. Int J Cardiol 2012;162:6-13. https://doi.org/10.1016/j.ijcard.2012.05.070.
- Armitage W. Review of Current Health Care Services for Those Living with Heart Failure – REACH-HF Project Geographical Areas Evaluation. Edinburgh: Heart Manual Service, NHS Lothian; 2013.
- O’Cathain A, Murphy E, Nicholl J. Three techniques for integrating data in mixed methods studies. BMJ 2010;341. https://doi.org/10.1136/bmj.c4587.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Green L, Kreuter M. Health Program Planning: An Educational and Ecological Approach. New York, NY: McGraw-Hill Education; 2005.
- Deci EL, Ryan RM. Intrinsic Motivation and Self-Determination in Human Behavior. New York, NY: Plenum Publishing Company; 1985.
- Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, CO: Prentice Hall; 1986.
- Carver CS, Scheier MF. Autonomy and self-regulation. Psychol Inq 2000;11:284-91.
- Greaves CJ, Reddy P, Sheppard K, Schwarz P RP, Greaves CJ, . Diabetes Prevention in Practice. Dresden: TUMAINI Institute for Prevention Management; 2010.
- Leventhal H, Diefenbach M, Leventhal EA. Illness cognition: using common sense to understand treatment adherence and affect cognition interactions. Cognit Ther Res 1992;16:143-63. https://doi.org/10.1007/BF01173486.
- French SD, Green SE, O’Connor DA, McKenzie JE, Francis JJ, Michie S, et al. Developing theory-informed behaviour change interventions to implement evidence into practice: a systematic approach using the Theoretical Domains Framework. Implement Sci 2012;7. https://doi.org/10.1186/1748-5908-7-38.
- Taylor SE. Adjustment to threatening events: a theory of cognitive adaptation. Am Psychol 1983;38:1161-73. https://doi.org/10.1037/0003-066X.38.11.1161.
- Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychol 2008;27:379-87. https://doi.org/10.1037/0278-6133.27.3.379.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Miller RW, Rollnick S. Motivational Interviewing: Preparing People for Change. New York, NY, USA: Guildford Press; 2002.
- Denford S, Campbell JL, Frost J, Greaves CJ. Processes of change in an asthma self-care intervention. Qual Health Res 2013;23:1419-29. https://doi.org/10.1177/1049732313507376.
- Carver CS, Scheier MF. On the Self-Regulation of Behavior. New York, NY: Cambridge University Press; 1998.
- Marlatt G, Marlatt GA, Gordon JR. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviours. New York, NY: Guildford Press; 1985.
- Beck AT. Cognitive Therapy and the Emotional Disorders. London: Penguin; 1989.
- Hofmann SG, Sawyer AT, Witt AA, Oh D. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. J Consult Clin Psychol 2010;78:169-83. https://doi.org/10.1037/a0018555.
- Sullivan MJ, Wood L, Terry J, Brantley J, Charles A, McGee V, et al. The Support, Education, and Research in Chronic Heart Failure Study (SEARCH): a mindfulness-based psychoeducational intervention improves depression and clinical symptoms in patients with chronic heart failure. Am Heart J 2009;157:84-90. https://doi.org/10.1016/j.ahj.2008.08.033.
- McEachan RR, Lawton RJ, Jackson C, Conner M, Lunt J. Evidence, theory and context: using intervention mapping to develop a worksite physical activity intervention. BMC Public Health 2008;8. https://doi.org/10.1186/1471-2458-8-326.
- Lloyd JJ, Logan S, Greaves CJ, Wyatt KM. Evidence, theory and context – using intervention mapping to develop a school-based intervention to prevent obesity in children. Int J Behav Nutr Phys Act 2011;8. https://doi.org/10.1186/1479-5868-8-73.
- Gillison F, Greaves C, Stathi A, Ramsay R, Bennett P, Taylor G, et al. ‘Waste the Waist’: the development of an intervention to promote changes in diet and physical activity for people with high cardiovascular risk. Br J Health Psychol 2012;17:327-45. https://doi.org/10.1111/j.2044-8287.2011.02040.x.
- Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2017;14:591-602. https://doi.org/10.1038/nrcardio.2017.65.
- Lam CS, Donal E, Kraigher-Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail 2011;13:18-2. https://doi.org/10.1093/eurjhf/hfq121.
- Buck HG, Harkness K, Wion R, Carroll SL, Cosman T, Kaasalainen S, et al. Caregivers’ contributions to heart failure self-care: a systematic review. Eur J Cardiovasc Nurs 2015;14:79-8. https://doi.org/10.1177/1474515113518434.
- Greenhalgh T, A’Court C, Shaw S. Understanding heart failure; explaining telehealth – a hermeneutic systematic review. BMC Cardiovasc Disord 2017;17. https://doi.org/10.1186/s12872-017-0594-2.
- Wingham J, Frost J, Britten N, Greaves C, Abraham C, Warren FC, et al. Caregiver outcomes of the REACH-HF multicentre randomized controlled trial of home-based rehabilitation for heart failure with reduced ejection fraction. Eur J Cardiovasc Nurs 2019;18:611-20. https://doi.org/10.1177/1474515119850011.
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147-239. https://doi.org/10.1016/j.jacc.2013.05.019.
- Dalal HM, Zawada A, Jolly K, Moxham T, Taylor RS. Home based versus centre based cardiac rehabilitation: Cochrane systematic review and meta-analysis. BMJ 2010;340. https://doi.org/10.1136/bmj.b5631.
- Reed SD, Whellan DJ, Li Y, Friedman JY, Ellis SJ, Piña IL, et al. Economic evaluation of the HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) randomized controlled trial: an exercise training study of patients with chronic heart failure. Circ Cardiovasc Qual Outcomes 2010;3:374-81. https://doi.org/10.1161/CIRCOUTCOMES.109.907287.
- American Thoracic Society . Minnesota Living With Heart Failure Questionnaire 2004. http://qol.thoracic.org/sections/instruments/ko/pages/mlwhfq.html (accessed 31 January 2018).
- Pressler SJ, Gradus-Pizlo I, Chubinski SD, Smith G, Wheeler S, Sloan R, et al. Family caregivers of patients with heart failure: a longitudinal study. J Cardiovasc Nurs 2013;28:417-28. https://doi.org/10.1097/JCN.0b013e3182563877.
- Wingham J, Frost J, Britten N. Behind the smile: qualitative study of caregivers’ anguish and management responses while caring for someone living with heart failure. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-014126.
- Lee CS, Vellone E, Lyons KS, Cocchieri A, Bidwell JT, D’Agostino F, et al. Patterns and predictors of patient and caregiver engagement in heart failure care: a multi-level dyadic study. Int J Nurs Stud 2015;52:588-97. https://doi.org/10.1016/j.ijnurstu.2014.11.005.
- Dalal HM, Doherty P, Taylor RS. Cardiac rehabilitation. BMJ 2015;351. https://doi.org/10.1136/bmj.h5000.
- EuroQoL G . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: Personal Social Services Research Unit, University of Kent; 2016.
- Buckingham SA, Taylor RS, Jolly K, Zawada A, Dean SG, Cowie A, et al. Home-based versus centre-based cardiac rehabilitation: abridged Cochrane systematic review and meta-analysis. Open Heart 2016;3. https://doi.org/10.1136/openhrt-2016-000463.
- Palmer J, Wingham J. An Evaluation of the Patient and Public Involvement (PPI) Work in Designing and Delivering a Research Project. Stratford-upon-Avon: National NHS R&D Forum; 2016.
- Olsen Zwisler AD. Home-based rehabilitation interventions aimed at congestive heart failure: are we there yet?. Euro J Prevent Cardiol 2019;26:259-61. https://doi.org/10.1177/2047487318816783.
- National Institute for Health Research (NIHR) . Home-Based Cardiac Rehabilitation for Heart Failure Has High Rates of Participation n.d. https://evidence.nihr.ac.uk/alert/home-based-cardiac-rehabilitation-for-heart-failure-has-high-rates-of-participation-/?source=chainmail (accessed January 2021).
- National Cardiac Audit Programme . National Heart Failure Audit: 2019 Summary Report (2017 18 Data) 2019.
- Dalal HM, Wingham J, Palmer J, Taylor R, Petre C, Lewin R. REACH-HF investigators . Why do so few patients with heart failure participate in cardiac rehabilitation? A cross-sectional survey from England, Wales and Northern Ireland. BMJ Open 2012;2. https://doi.org/10.1136/bmjopen-2011-000787.
- Daw P, van Beurden SB, Greaves C, Veldhuijzen van Zanten JJCS, Harrison A, Dalal H, et al. Getting evidence into clinical practice: protocol for evaluation of the implementation of a home-based cardiac rehabilitation programme for patients with heart failure. BMJ Open 2020;10. https://doi.org/10.1136/bmjopen-2019-036137.
- Purcell C, Daw P, Kerr C, Cleland J, Cowie A, Dalal HM, et al. Protocol for an implementation study of an evidence-based home cardiac rehabilitation programme for people with heart failure and their caregivers in Scotland (SCOT:REACH-HF). BMJ Open 2020;10. https://doi.org/10.1136/bmjopen-2020-040771.
- Limb M. The BMJ Awards 2020: showcase of this year’s winning teams. BMJ 2020;371. www.bmj.com/content/bmj/371/bmj.m4341.full.pdf (accessed January 2021).
- The BMJ Awards . The 2020 BMJ Awards Showcase n.d. http://thebmjawards.bmj.com/showcase/ (accessed January 2021).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
- Curtis L. Unit Costs of Health and Social Care 2010. Canterbury: Personal Social Services Research Unit, University of Kent; 2010.
- Department of Health and Social Care . Reference Costs 2015–16 2016.
- Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011;364:11-2. https://doi.org/10.1056/NEJMoa1009492.
- NHS Improvement . Making the Case for Cardiac Rehabilitation: Modelling Potential Impact on Readmissions 2013.
- Joint Formulary Committee . BNF Online 2017.
- EBM DataLab . OpenPrescribing.Net 2017.
- Joint Formulary Committee . British National Formulary 2017.
- OpenPrescribing . Explore England’s Prescribing Data n.d. http://OpenPrescribing.net (accessed January 2021).
- HM Revenue and Customs . Distribution of Median and Mean Income and Tax by Age Range and Gender: 2014 to 2015 2017.
- Goehler A, Geisler BP, Manne JM, Jahn B, Conrads-Frank A, . Schnell-Inderst . Decision-analytic models to simulate health outcomes and costs in heart failure: a systematic review. PharmacoEconomics 2011;29:753-69. https://doi.org/10.2165/11585990-000000000-00000.
- Thokala P, Baalbaki H, Brennan A, Pandor A, Stevens JW, Gomersall T, et al. Telemonitoring after discharge from hospital with heart failure: Cost-effectiveness modelling of alternative service designs. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-003250.
- Kansal AR, Cowie M, Kielhorn A, Krotneva S, Taffazzoli A, Zheng Y, et al. Cost-effectiveness of ivabradine as a treatment for systolic chronic heart failure in the United States. Circulation 2015;132.
Appendix 1 Links to full text of published studies and list of other published studies
Work package 1
Greaves CJ, Wingham J, Deighan D, Doherty P, Elliott J, Armitage W, et al. Optimising self-care support for people with heart failure and their caregivers: development of the Rehabilitation Enablement in Chronic Heart Failure (REACH-HF) intervention using intervention mapping. Pilot Feasibility Stud 2016;2:37.
Needs assessment questionnaire: supplemental file to the intervention development paper39
https://static-content.springer.com/esm/art%3A10.1186%2Fs40814–016–0075-x/MediaObjects/40814_2016_75_MOESM1_ESM.pdf (accessed 20 February 2020).
REACH-HF needs assessment summary: supplemental file to the intervention development paper
https://static-content.springer.com/esm/art%3A10.1186%2Fs40814–016–0075-x/MediaObjects/40814_2016_75_MOESM2_ESM.pdf (accessed 20 February 2020).
Extracts from intervention maps for stress management, medication management and managing symptoms: supplemental file to the intervention development paper
https://static-content.springer.com/esm/art%3A10.1186%2Fs40814–016–0075-x/MediaObjects/40814_2016_75_MOESM3_ESM.pdf (accessed 20 February 2020).
The REACH-HF facilitation process: supplemental file to the intervention development paper
https://static-content.springer.com/esm/art%3A10.1186%2Fs40814–016–0075-x/MediaObjects/40814_2016_75_MOESM4_ESM.pdf (accessed 20 February 2020).
End-of-study feasibility report supplemental file to the intervention development paper
https://static-content.springer.com/esm/art%3A10.1186%2Fs40814–016–0075-x/MediaObjects/40814_2016_75_MOESM5_ESM.pdf (accessed 20 February 2020).
Note that the outcome data at follow-up were redacted from the published version of this additional file but are included separately in this report in Report Supplementary Material 1.
Wingham J, Frost J, Britten N, Jolly K, Greaves C, Abraham C, et al. Needs of caregivers in heart failure management: a qualitative study. Chronic Illn 2015;11:304–19.
Work package 2
Eyre V, Lang CC, Smith K, Jolly K, Davis R, Hayward C, et al. Rehabilitation Enablement in Chronic Heart Failure – a facilitated self-care rehabilitation intervention in patients with heart failure with preserved ejection fraction (REACH-HFpEF) and their caregivers: rationale and protocol for a single-centre pilot randomised controlled trial. BMJ Open 2016;5:e012853.
Lang CC, Smith K, Wingham J, Eyre V, Greaves CJ, Warren FC, et al. A randomised controlled trial of a facilitated home-based rehabilitation intervention in patients with heart failure with preserved ejection fraction and their caregivers: the REACH-HFpEF pilot study. BMJ Open 2018;8:e019649.
Smith K, Lang C, Wingham J, Frost J, Britten N, Greaves C, et al. Patients with heart failure with preserved ejection fraction and their caregiver’s experiences of home-based rehabilitation (REACH-HFpEF): mixed methods process evaluation of the REACH-HFpEF single centre pilot randomised controlled trial [published online ahead of print November 6 2020]. Pilot Feasibility Stud 2020.
Work package 3
Dalal HM, Taylor RS, Jolly K, Davis RC, Doherty P, Miles J, et al. The effects and costs of home-based rehabilitation for heart failure with reduced ejection fraction: the REACH-HF multicentre randomized controlled trial. Eur J Prev Cardiol 2019;26:262–72.
Taylor RS, Hayward C, Eyre V, Austin J, Davis R, Doherty P, et al. Clinical effectiveness and cost-effectiveness of the Rehabilitation Enablement in Chronic Heart Failure (REACH-HF) facilitated self-care rehabilitation intervention in heart failure patients and caregivers: rationale and protocol for a multicentre randomised controlled trial. BMJ Open 2015;5:e009994.
Wingham J, Frost J, Britten N, Greaves C, Abraham C, Warren FC, et al. Caregiver outcomes of the REACH-HF multicentre randomized controlled trial of home-based rehabilitation for heart failure with reduced ejection fraction. Eur J Cardiovasc Nurs 2019;18:611–20.
Frost J, Wingham J, Britten N, Greaves C, Abraham C, Taylor R, et al. Home-based rehabilitation for heart failure with reduced ejection fraction: mixed methods process evaluation of the REACH-HF multicentre randomised controlled trial. BMJ Open 2019;9:e026039.
Work package 4
Long L, Mordi IR, Bridges C, Sagar VA, Davies EJ, Coats AJS, et al. Exercise-based rehabilitation for adults with heart failure. Cochrane Database Syst Rev 2019;1:CD003331.
Taylor RS, Sadler S, Dalal HM, Warren FC, Jolly K, et al. The cost effectiveness of REACH-HF and home-based cardiac rehabilitation compared with usual medical care for heart failure with reduced ejection fraction: a decision model-based analysis. Eur J Prev Cardiol 2019;26:1252–61.
Appendix 2 Other outputs arising from this programme
Oral presentations
Greaves C, on behalf of the REACH-HF investigators. The REACH Heart Failure Manual: A Complex Intervention to Support Rehabilitation and Self-care for People with Heart Failure. Society for Academic Primary Care (SAPC), Edinburgh, 2014.
Dalal H, on behalf of the REACH-HF investigators. Engaging with Patients, Clinicians and Academics to Improve the Uptake of Rehabilitation: An Overview of the REACH-HF Programme of Research. Your Heart Our Passion Conference, Cardiff, 2015.
Taylor R, on behalf of the REACH-HF investigators. The Evidence Base for Exercise Training in Heart Failure and COPD. NIHR CLAHRC Northwest London meeting, London, 2015.
Austin J, on behalf of the REACH-HF investigators. Engaging with Patients, Clinicians and Academics to Improve Care: Back to the Future. 6th Aneurin Bevan University Health Board R&D Conference, Newport, 2015.
Wingham J, on behalf of the REACH-HF investigators. Supporting Carers in Heart Failure Management: A Qualitative Study to Identify Carer Needs. International Carers Conference, Gothenburg, 2015.
Taylor R, on behalf of the REACH-HF investigators. What do the Latest Cochrane Reviews Say About Rehab? British Association for Cardiovascular Prevention and Rehabilitation (BACPR) conference, Manchester, 2015.
Dalal H, on behalf of the REACH-HF investigators. The Rehabilitation Enablement in Chronic Heart Failure (REACH-HF): An Update on a Home Based Intervention for People with Heart Failure. South West Society for Academic Primary Care Conference (SWSAPC), Cardiff, 2016.
Taylor R, on behalf of the REACH-HF investigators. Does Exercise-Based Rehabilitation Reduce the Risk of Death and Hospitalisation in Patients with Heart Failure? An Individual Patient Data Meta-analysis. European Society of Cardiology (ESC) Heart Failure conference, Florence, 2016.
Taylor R, on behalf of the REACH-HF investigators. Early Results from REACH-HF-pEF. British Association for Cardiovascular Prevention and Rehabilitation (BACPR) Conference, London, 2017.
Taylor R, on behalf of the REACH-HF investigators. A Facilitated Home Based Rehabilitation Intervention: Impact on Well-Being and Quality of Life in Patients with Heart Failure with Preserved Ejection Fraction. American Heart Association (AHA) Scientific Sessions, Anaheim, CA, 2017.
Mills J, on behalf of the REACH-HF investigators. A Review of RCT Trials of Exercise in Heart Failure. British Society for Heart Failure, London, 2017.
Dalal H, on behalf of the REACH-HF investigators. A Facilitated Home Based Rehabilitation Intervention: Impact on Well-Being and Quality Of Life in Patients with Heart Failure with Preserved Ejection Fraction. South West Society for Primary Care, Plymouth, 2017.
Dalal H, on behalf of the REACH-HF investigators. REACH-HFpEF Pilot Study: A Randomised Controlled Trial of a Facilitated Home-Based Rehabilitation Intervention in Patients with Heart Failure with Preserved Ejection Fraction and Their Caregivers. British Journal of General Practice (BJGP) Research Conference, London, 2018.
Greaves C, on behalf of the REACH-HF investigators. Rehabilitation Enablement in CHronic Heart Failure (REACH-HF): Findings and Delivery Quality Issues in a Multicentre Randomised Controlled Trial of a Facilitated, Exercise Based Rehabilitation Intervention in Heart Failure. International Society of Behavioural Nutrition and Physical Activity (ISBNPA), Wan Chai, 2018.
Wingham J, on behalf of the REACH-HF investigators. Rehabilitation Enablement in Chronic Heart Failure Self-Management Intervention: Process Evaluation of the Impact on Caregivers. European Society for Health and Medical Sociology (ESHMS), Lisbon, 2018.
Wingham J, on behalf of the REACH-HF investigators. Optimising the Role of Patients and Their Carers/Family: I Am Not Just A Patient, I Am Part Of A Family – The Role of Patients and Their Caregivers in Heart Failure Self-Management. EuroHeartCare, Dublin, 2018.
Poster presentations
Dalal H, on behalf of the REACH-HF investigators. Rehabilitation Enablement in Chronic Heart Failure (REACH-HF) – A National Institute for Health Research (NIHR) Research Programme. Society for Academic Primary Care (SAPC), Southampton, 2013.
Wingham J, on behalf of the REACH-HF investigators. Involving People and Caregivers Living with an Unpredictable Condition in Research: Rehabilitation Enablement in Chronic Heart Failure (REACH-HF). British Association for Cardiovascular Prevention and Rehabilitation (BACPR) Conference, Solihull, 2013.
Wingham J, on behalf of the REACH-HF investigators. Involving People and Caregivers Living with an Unpredictable Condition in Research: Rehabilitation Enablement in Chronic Heart Failure (REACH-HF). Collaborations for Leadership in Applied Health Research and Care (CLAHRC) for the South West Peninsula (PenCLAHRC) Patient and Public Involvement Conference, Buckfastleigh, 2013.
Wingham J, on behalf of the REACH-HF investigators. Cardiac Rehabilitation: An Overview of Cochrane Systematic Reviews. Where Should the Cochrane Reviews be Going in the Future? British Association for Cardiovascular Prevention and Rehabilitation (BACPR) Conference, Londonderry, 2014.
Wingham J, on behalf of the REACH-HF investigators. So What About the Caregivers? Designing a Heart Failure Rehabilitation Programme to Include Caregivers. British Association for Cardiovascular Prevention and Rehabilitation (BACPR) Conference, Londonderry, 2014.
Wingham J, on behalf of the REACH-HF investigators. The Heart Failure Manual: A Complex Intervention to Support Rehabilitation and Self Care for People with Heart Failure. British Association for Cardiovascular Prevention and Rehabilitation (BACPR) Conference, Londonderry, 2014.
Singh S, on behalf of the REACH-HF investigators. The Heart Failure Manual: A Complex Intervention to Support Rehabilitation and Self Care for People with Heart Failure. British Heart Foundation National Centre (BHFNC) Conference, Loughborough, 2015.
Palmer J, Wingham J, on behalf of the REACH-HF investigators. An Evaluation of the Patient and Public Involvement (PPI) Work in Designing and Delivering a Research Project. National NHS R&D Forum, Stratford-upon-Avon, 2016.
Taylor R, on behalf of the REACH-HF investigators. A Facilitated Home Based Rehabilitation Intervention: Impact on Well-Being and Quality of Life in Patients with Heart Failure with Preserved Ejection Fraction. American Heart Association (AHA) Scientific Sessions, Anaheim, CA, 2017.
Dalal H, on behalf of the REACH-HF investigators. A Facilitated Home Based Rehabilitation Intervention: Impact on Well-Being and Quality of Life in Patients with Heart Failure with Preserved Ejection Fraction. South West Society for Primary Care (SWSPC), Plymouth, 2018.
Taylor RS, Dalal HM, on behalf of the REACH-HF investigators. Home-based Rehabilitation for Heart Failure with Reduced Ejection Fraction: Randomised Controlled Trial. European Society of Cardiology (ESC) Heart Failure Conference, Vienna, 2018.
Dalal HM, Taylor RS, Jolly K, Davis RC, Doherty P, Austin J, et al. Rehabilitation Enablement in Chronic Heart Failure (REACH-HF) – A Multicentre Randomised Controlled Trial of Facilitated Self-Care Rehabilitation Intervention in Heart Failure with Reduced Ejection Fraction. British Cardiovascular Society (BCS), Manchester, 2018.
Appendix 3 Economic analysis
Introduction
The aims of the economic analysis were to estimate the cost-effectiveness in patients with HFrEF of:
-
the REACH-HF intervention plus usual care versus usual care alone
-
home-based CR plus usual care versus usual care alone.
In this appendix, we present trial-based cost-effectiveness analyses of the REACH-HF intervention versus usual care undertaken alongside the REACH-HF RCT and based on the trial follow-up period of 12 months. We estimate the resource use and cost associated with the delivery of the REACH-HF intervention and compare resource use, costs and outcomes across treatment and control groups to inform cost-effectiveness analyses.
Model-based cost-effectiveness analyses with primary analyses on home-based CR versus usual care and secondary analyses reporting cost-effectiveness of REACH-HF intervention versus usual care are described in a published paper (see Appendix 1). 46 These analyses model longer-term costs and outcomes using a decision-analytic model. In this appendix, we present additional model-based analyses that were not included in the submitted manuscript, including:
-
analysis of both the REACH-HF intervention and the home-based CR versus usual care using effectiveness in terms of all-cause hospital admissions as opposed to HF-related hospital admissions
-
analysis of the cost-effectiveness of centre-based CR (based on both HF-related and all-cause admissions).
Trial-based cost-effectiveness analysis
Here we present the cost-effectiveness analysis undertaken alongside the REACH-HF RCT in patients with HFrEF (see Work package 3: multicentre randomised controlled trial of the REACH-HF intervention in patients with heart failure with reduced ejection fraction and Appendix 1) to estimate the cost-effectiveness of the REACH-HF intervention plus usual care versus usual care alone over the 12 months of the trial.
Methods
Setting and perspective
The setting is the English health-care system and the perspective is that of the English NHS and Personal Social Services.
Target population and subgroups
Adult patients with confirmed HFrEF were enrolled (n = 216) and those who completed all elements of data collection for resource use and HRQoL at all follow-up points, including the last at 12 months, formed the participant group for the base-case analysis. The trial protocol specified subgroup-level analyses by trial centre, severity of HF (defined by NT-proBNP levels < 2000 vs. ≥ 2000 pg/ml), time since diagnosis of HF (< 1 year, 1–2 years or ≥ 2 years) and whether or not the patient had a participating carer.
Intervention cost
The REACH-HF intervention is a nurse-facilitated, home-based CR programme designed to enhance quality of life and self-care of people with HF and their caregivers. The intervention is described in full in Work package 1: intervention development and feasibility study. The REACH-HF intervention was delivered over 12 weeks. The trial methods, including economic analysis, were set out in the published trial protocol. 38 Resource use associated with delivery of the REACH-HF intervention was informed by early development research, with the primary item of resource use being the time input of the HF facilitator who delivered the intervention. Other resource use comprises consumables and some allowance for training of the HF facilitators. Data on each of these areas of resource use were collected within trial, with HF facilitator time input collected using a ‘facilitator contact sheet’ (see Report Supplementary Material 5) capturing contact time with participants and also non-contact time (e.g. planning and preparation) related to delivery of the intervention.
Unit costs for facilitation staff were based on the grade equivalent to ‘community nurse’ (including district nursing sister and district nurse) and nurse specialist (community) from Unit Costs of Health and Social Care for 2016. 90 These are based on Agenda for Change band 6, including salary, salary on-costs, overheads (management costs and non-staff costs including travel/transport) capital overheads and excluding costs for qualifications.
Training costs per facilitator, specific to delivery of the REACH-HF intervention, were estimated based on resource use data collected within trial by the trial co-ordinator.
Consumables costs (manuals and DVDs) were based on resource use data collected within trial by the trial co-ordinator.
Health, social care and other resource use and cost
Data on resource use related to health and social care were collected using a standardised resource use questionnaire (RUQ) (see Report Supplementary Material 6) at baseline (referring to the previous 6 months), at 4 months (referring to the 4 months since the trial started) and at 12 months (referring to the 8 months since the last assessment). The RUQ was developed and tested in early development research on REACH-HF. Data from assessments at 4 and 12 months were combined to calculate the total resource use during the 12 months of follow-up. Although resource use data for hospital admissions were collected in the self-reported RUQ, data on hospital admissions were reconciled against SAE reporting (with SAE reporting being the primary data source). Data on SAEs were used as the primary source of data on hospital admissions. In addition, the RUQ captured wider societal costs, including time off work for participants and caregivers, and unpaid caring activities carried out by the participant’s main caregiver and by other people.
Costs are reported in 2016 GBP. Unit costs per item of resource use (Table 7) were obtained from published estimates and, where necessary, inflated to 2016 prices using the Healthcare and Community Health Services index (see Report Supplementary Material 1). 90 These unit costs were then applied to the resource use reported at participant level to estimate the delivery costs associated with the REACH-HF intervention and the total costs associated with health and social care at baseline and over the 12 months of follow-up.
| Resource use item/category | Unit cost (2016 GBP) | Basis of calculation |
|---|---|---|
| Primary care appointment/visit | ||
| GP (surgery) | 31.0090 | 9.22-minute (average length) appointment |
| GP (home) | 74.98101 | 11.4-minute home visit plus travel time |
| GP (telephone) | 22.29101 | 7.1-minute telephone appointment |
| Practice nurse (surgery) | 11.1190,101 | 15.5-minute surgery contact at £43/hour |
| Practice nurse (home) | 18.8090,101,102 | 25-minute home visit at £43/hour + travel cost |
| Practice nurse (telephone) | 4.3090,101 | 6-minute telephone consultation (nurse advanced) at £43/hour |
| HF nurse | 22.1190 | 15.5-minute surgery/25-minute home appointment at £44/hour (AfC band 6) + travel |
| Physiotherapist | 77.5290,102 |
Community physiotherapist with hourly cost of £47.92 Estimated 83% home and 17% clinic appointments at 60 and 30 minutes, respectively, + travel |
| Occupational therapist | 75.1090,102 |
Community occupational therapist with hourly cost of £46.42 Estimated 83% home and 17% clinic appointments at 60 and 30 minutes, respectively |
| Community/district nurse | 39.5190,102 | Community nurse with hourly cost of £46.14 and 20-minute appointments + travel |
| Health visitor | 27.22101,102 | 20-minute visit at (£76/hour of patient-related work + travel) |
| Secondary care | ||
| Hospital admission (HF) | 3805.00103 | Weighted average cost of a single spell for HRGs (EB03A-D) |
| Hospital admission (non-HF) | 2208.00103,104 | Using breakdown of rate of admission by type from EMPHASIS HF trial and weighted mean cost of a hospital spell for that category from the national schedule of reference costs 2015/16 |
| Hospital admission (all cause) | 2510.95 | Weighted all-cause cost based on 18.97% of (followed up) admissions in REACH-HF being HF-related |
| A&E attendance | 137.82103 | Mean cost across all A&E attendances excluding dentistry |
| Day hospital attendance | 319.33103 | Based on the weighted average cost of hospital day cases for HF |
| Outpatient cardiology | 135.68103 | Unit cost of cardiology outpatient attendance (consultant led) |
| Outpatient cardiac or HF nurse | 102.96103 | Unit cost of cardiology outpatient attendance (non-consultant led) |
| Other outpatient contact | 116.54103 | Unit cost of a non-cardiology outpatient attendance (consultant and non-consultant led) |
| CR classes | 477.00105 | Based on tariff from national tariff payment system 2017–18 and 2018–19 for rehabilitation post discharge where provider provides both acute and community care |
| Social and community care visits | ||
| Social worker | 79.0090 | 60-minute appointment at £79/hour of client-related work |
| Home care/home help | 12.0090 | 30-minute visit at £24/hour (weekday) for face-to-face social service-provided home care |
| Voluntary agency | 10.0090 | 30-minute visit at £24/hour (weekday) for face-to-face independent sector-provided home care |
| Day care | 46.0090 | £46 per client session lasting 3.5 hours |
| Drop-in club | 13.0090 | 1-hour attendance at day care cost of £13/hour |
| Other day-care service | 46.0090 | £46 per client session lasting 3.5 hours |
| Medication | ||
| Angiotensin II receptor antagonist | 30.24106,107 | Drug costs taken from BNF 2017108 assuming recommended dosage, or where dose up-titration was recommended assuming half the maximum dose. Generics used where available, and where multiple products fell within a single average cost was estimated by weighting based on usage data from OpenPrescribing,109 assuming that prescribing patterns are the same across all conditions |
| ACE inhibitor | 13.80106,107 | |
| Aldosterone receptor antagonist | 126.12106,107 | |
| Anticoagulant | 16.68106,107 | |
| Beta-blocker | 12.24106,107 | |
| Digoxin | 36.00106,107 | |
| Ivabradine | 516.48106,107 | |
| Loop diuretic | 15.96106,107 | |
| Nitrate | 554.64106,107 | |
| Thiazide diuretic | 19.20106,107 | |
| Informal care | ||
| Caregiver hours per week | 24.0090 | Cost per hour of face-to-face home care worker time (weekday daytime) provided to private purchasers |
| Non-caregiver hours per week | 24.0090 | Cost per hour of face-to-face home care worker time (weekday daytime) provided to private purchasers |
| Caregiver days off work | 122.31110 | Mean income for all ages/sexes at £31,800 per annum, assuming 260 working days per year |
| Non-caregiver days off work | 122.31110 | Mean income for all ages/sexes at £31,800 per annum, assuming 260 working days per year |
| Patient days off work | 66.35110 | Mean participant age was 71 years, mean income in this age group before tax was £25,000. Assumed 260 working days per year. Multiplied by 69%, the average %FTE reported by trial participants |
Note that the unit cost of a hospital admission represents the expected cost (published cost) of a typical HF or non-HF hospital admission in patients with HF (see Table 7) rather than the specific cost of the hospital admission events experienced by patients in the trial, as reported in SAEs. However, where mean length of stay in the trial was longer than that reported in the NHS reference cost item for hospital admission (HF related and non-HF), extra costs for additional bed-days were applied. For non-HF hospital admissions, we used data reported on type of non-HF hospital admission for people with HF in a large HF RCT and cost data from NHS reference costs103 to estimate a weighted cost for non-HF hospital admissions (see Table 7 and Report Supplementary Material 1).
Medication costs were estimated using data from the RUQ on self-report use (yes/no response) of key medication types (e.g. beta-blockers, ACE inhibitors and calcium channel blockers) and recommended dosage and medication costs reported in the British National Formulary (2016–17). We used recommend dosage or we applied half of the maximum dose where dose uptitration was relevant. Unit costs were those for generic products, where available; when multiple products were available in a single category (e.g. ACE inhibitors), we used a weighted cost based on prescribing information available from OpenPrescribing (2017),109 assuming that prescribing patterns reported were relevant for HF.
Where we have put a monetary value on informal care in sensitivity analyses, we used a replacement cost approach and assumed that the value of an hour of informal care was the same as the cost of an hour of care provided by social care/social services (£24 per hour; see Table 7).
Health-related quality of life
Data on HRQoL were collected by self-report for both the participants and their caregivers using the EQ-5D-5L health questionnaire, and health state values were derived using the mapping/crosswalk values for the UK to provide EQ-5D-3L values from EQ-5D-5L states. This is the method currently recommended by NICE for assessment of EQ-5D/QALYs in economic evaluation. 31 Estimates of QALYs for the 12 months of the trial were calculated using EQ-5D-5L data at baseline and at 4, 6 and 12 months, using the area under the curve method.
The primary economic end point was the QALY (derived from EQ-5D-5L) estimated over the 12 months of follow-up. The primary perspective taken here for analyses is that of the NHS and social care sector (third-party payer), although data collection on resource use provides an opportunity to consider wider participant level and societal impacts.
Cost-effectiveness
The trial-based analysis assessed the cost-effectiveness of the REACH-HF intervention plus usual care compared with usual care alone over the 12 months of the trial. No discounting of future costs or outcomes was required. Primary analyses were based on intention-to-treat complete-case analyses, consistent with the trial main statistical analysis plan (see the statistical analysis plan, which is available here: www.journalslibrary.nihr.ac.uk/programmes/pgfar/rp-pg-1210-12004/#/documentation; accessed December 2020).
We used statistical analyses to estimate between-group differences in estimated costs and QALYs. Generalised linear modelling regression models (with appropriate family and link functions) were used with adjustment for baseline values and prespecified covariates (see the statistical analysis plan, which is available here: www.journalslibrary.nihr.ac.uk/programmes/pgfar/rp-pg-1210-12004/#/documentation; accessed December 2020). Given the expected nature of cost data, non-parametric bootstrap methods were used to estimate CIs. Bootstrapping was used to resample (with replacement) 1000 times (i.e. simulating 1000 trials) from the empirical trial data, providing estimates of uncertainty around the modelled estimates of incremental cost, incremental effect and ICER. We produced a cost-effectiveness acceptability curve, plotting expectation of the REACH-HF intervention being cost-effective compared with usual care at a range of estimates of WTP per QALY gained. In the UK, using NICE guidance, if the cost of an intervention is below £20,000 to £30,000 per QALY gained it is considered value for money for the NHS.
In addition to the primary (complete-case) analysis, the following sensitivity analyses were carried out:
-
per protocol [see the statistical analysis plan, URL: www.journalslibrary.nihr.ac.uk/programmes/pgfar/rp-pg-1210-12004/#/documentation (accessed January 2021)]
-
using multiple imputation of missing values for aggregate costs
-
by subgroups:
-
time since HF diagnosis (< 1 year, 1–2 years or ≥ 2 years)
-
severity of HF (BNP levels < 2000 vs. ≥ 2000 pg/ml)
-
trial centre
-
whether or not the patient had a participating carer
-
-
including caregiver QALYs
-
considering a wider societal perspective (including informal care and time off work costs)
-
including intervention cost variation, based on estimated range of intervention cost.
A multiple imputation by chained equations approach was used, with predictive mean matching owing to the constrained (non-zero) nature of the cost data.
In addition to the above sensitivity analyses, we present a ‘post hoc’ sensitivity analysis, in which the EQ-5D-5L health state values and related QALY estimates are based on use of a ‘mapping’ algorithm to predict EQ-5D health state values from participant data on the MLHFQ. In this sensitivity analysis, estimates for the difference in EQ-5D/QALY were not derived from the directly collected/measured EQ-5D-5L scores (health status) but from the MLHFQ scores in the RCT using an unpublished mapping algorithm previously developed by Edlin et al. obtained via personal communication (Edlin R, Tsuchiya A, Brazier J. Mapping the Minnesota Living with Heart Failure Questionnaire to the EQ-5D index; 2002. Reproduced with permission from Brazier J, personal communication, 2018). This post hoc analysis was requested by the REACH-HF Trial Steering Committee.
Results
Intervention cost
Facilitator contact sheets were completed and available for 613 contacts in 94 intervention group participants who received the REACH-HF intervention. Table 8 reports the mean number of contacts by type (face to face or telephone) and mean duration of contacts and related non-contact activity. The mean number of contacts was 6.52, and total contact time and non-contact time inputs were 5.3 hours (319 minutes) and 2.9 hours (176 minutes), respectively, with overall time input at 8.25 hours (495 minutes). Using the unit cost of £44 per hour (based on a salary of £32,114 per annum90), the estimated mean cost of facilitator input was £363. Training cost per facilitator, specific to delivery of the REACH-HF intervention, was estimated at £1547 [3 days’ training at £352 per day; cost for trainers per trainee at £366 (assuming eight trainees per 3-day course) and cost for REACH-HF facilitator manuals at £50 each plus an estimate of consumables for training sessions]. These were distributed across the first 100 participants who received the intervention to give mean costs attributed to each participant of £15.47. The patient’s HF Manual was costed at £40, based on one hard copy manual at £25 and two DVDs at £7.50 each. This gave a mean estimated total cost for delivery of the REACH-HF intervention of £418.39 (95% CI £203.64 to £729.78) per participant.
| Contacts/item | Contacts (number) | Duration (minutes) | |
|---|---|---|---|
| Contact | Non-contact (planning) | ||
| Contacts, mean (SD) [minimum, maximum] | |||
| Face-to-face contact | 3.96 (1.2) [2, 7] | 69.15 (28.4) [8, 170] | 35.96 (34.1) [5, 200] |
| Telephone contact | 2.51 (2.37) [0, 9] | 18.89 (9.06) [2, 50] | 13.68 (12.8) [0, 100] |
| Total contact | 6.52 (2.63) [3, 13] | ||
| Total time, mean (SD) | |||
| Face-to-face contact | 271.45 (93.6) | ||
| Telephone contact | 47.41 (49.6) | ||
| Planning/non-contact time, face to face | 141.91 (89.9) | ||
| Planning/non-contact time, telephone | 34.35 (42.2) | ||
| Overall total time input, mean (SD) [minimum, maximum]a | 495.13 (180) [175, 1190] | ||
| Costs (£) | |||
| aCost per hour90 | 44.00 | ||
| Estimated total delivery cost (HF facilitator), mean (SD) a | 362.93 (131.96) | ||
| Other resource use/costsb | |||
| Consumables (1 × HF Manual) | 25.00 | ||
| DVDs (two at £7.50 each) | 15.00 | ||
| Distribution of HF Facilitator training costs per participantb | 15.47 | ||
| Estimated total delivery cost for the REACH-HF intervention (mean, estimated 95% CIc) | 418.39 (207.14 to 733.28) | ||
Resource use
Resource use for primary and secondary care and social services is reported in Table 9. Use of prescribed medications is reported in Table 10. Numbers of all-cause and HF hospital admissions and length of stay are reported in Table 11. Informal care is summarised in Table 12.
| Resource | Baseline (6 months) | Follow-up (12 months) | ||||||
|---|---|---|---|---|---|---|---|---|
| REACH-HF | Usual care | REACH-HF | Usual care | |||||
| n | Mean (SD) [range] appointments/visits per person | n | Mean (SD) [range] appointments/visits per person | n | Mean (SD) [range] appointments/visits per person | n | Mean (SD) [range] appointments/visits per person | |
| Primary care appointment/visit | ||||||||
| GP (surgery) | 106 | 2.89 (2.67) [0–12] | 109 | 3.43 (3.46) [0–26] | 84 | 3.73 (3.81) [0–18] | 88 | 3.88 (3.53) [0–17] |
| GP (home) | 107 | 0.09 (0.51) [0–4] | 109 | 0.11 (0.66) [0–6] | 84 | 0.10 (0.43) [0–3] | 88 | 0.03 (0.18) [0–1] |
| GP (telephone) | 107 | 0.59 (1.12) [0–6] | 109 | 0.89 (1.74) [0–12] | 84 | 0.94 (1.92) [0–11] | 88 | 1.11 (2.05) [0–10] |
| Practice nurse (surgery) | 106 | 2.70 (4.61) [0–36] | 109 | 3.16 (4.99) [0–30] | 83 | 6.92 (16.54) [0–144] | 88 | 6.02 (9.21) [0–61] |
| Practice nurse (home) | 107 | 0.01 (0.10) [0–1] | 109 | 0.06 (0.58) [0–6] | 83 | 0.04 (0.24) [0–2] | 88 | 0.08 (0.48) [0–4] |
| Practice nurse (telephone) | 107 | 0.07 (0.48) [0–4] | 109 | 0.10 (0.59) [0–5] | 84 | 0.27 (1.60) [0–14] | 88 | 0.40 (1.33) [0–9] |
| HF nurse | 107 | 1.26 (2.39) [0–14] | 109 | 0.89 (1.57) [0–7] | 84 | 1.10 (2.11) [0–12] | 88 | 1.33 (2.91) [0–14] |
| Physiotherapist | 107 | 0.20 (0.82) [0–6] | 109 | 0.49 (2.51) [0–24] | 84 | 0.39 (1.61) [0–12] | 88 | 0.75 (3.56) [0–24] |
| Occupational therapist | 107 | 0.01 (0.10) [0–1] | 109 | 0.00 (0.00) [0–0] | 84 | 0.01 (0.11) [0–1] | 88 | 0.00 (0.00) [0–0] |
| Community/district nurse | 107 | 0.34 (1.39) [0–9] | 109 | 0.30 (2.23) [0–20] | 84 | 0.14 (1.20) [0–11] | 88 | 0.31 (1.88) [0–16] |
| Health visitor | 107 | 0.00 (0.00) [0–0] | 109 | 0.00 (0.00) [0–0] | 84 | 0.00 (0.00) [0–0] | 88 | 0.00 (0.00) [0–0] |
| Secondary care | ||||||||
| Hospital admission (all cause) | 107 | 0.29 (0.50) [0–2] | 109 | 0.40 (1.14) [0–10] | 84 | 0.31 (0.84) [0–5] | 88 | 0.35 (0.84) [0–6] |
| A&E attendance | 107 | 0.16 (0.39) [0–2] | 109 | 0.26 (0.55) [0–3] | 84 | 0.19 (0.55) [0–4] | 88 | 0.20 (0.63) [0–4] |
| Day hospital attendance | 107 | 0.92 (7.54) [0–78] | 109 | 0.28 (0.65) [0–4] | 84 | 0.23 (0.59) [0–4] | 88 | 0.34 (0.95) [0–6] |
| Outpatient cardiology | 107 | 0.80 (0.87) [0–4] | 109 | 0.88 (0.92) [0–4] | 84 | 1.19 (1.29) [0–5] | 88 | 0.85 (1.31) [0–7] |
| Outpatient cardiac or HF nurse | 107 | 0.87 (1.35) [0–6] | 109 | 0.68 (1.19) [0–6] | 84 | 0.81 (1.26) [0–6] | 88 | 0.77 (1.98) [0–16] |
| Other outpatient | 107 | 0.37 (1.13) [0–8] | 109 | 0.61 (1.42) [0–10] | 84 | 0.89 (1.69) [0–9] | 88 | 1.26 (3.66) [0–32] |
| CR classes | NA | NA | NA | NA | 84 | 0.19 (0.40) [0–1] | 88 | 0.03 (0.18) [0–1] |
| Social and community care visits | ||||||||
| Social worker | 107 | 0.03 (0.29) [0–3] | 109 | 0.00 (0.00) [0–0] | 84 | 0.02 (0.15) [0–1] | 88 | 0.01 (0.11) [0–1] |
| Home care/home help | 107 | 3.38 (24.49) [0–180] | 109 | 3.67 (26.24) [0–208] | 84 | 19.54 (93.37) [0–672] | 88 | 3.86 (27.60) [0–240] |
| Day care | 107 | 0.22 (2.32) [0–24] | 108 | 0.00 (0.00) [0–0] | 84 | 0.49 (4.36) [0–40] | 88 | 0.00 (0.00) [0–0] |
| Drop-in club | 107 | 0.07 (0.59) [0–6] | 108 | 0.24 (2.50) [0–26] | 84 | 0.00 (0.00) [0–0] | 88 | 0.18 (1.71) [0–16] |
| Other-day-care service | 107 | 0.00 (0.00) [0–0] | 109 | 0.00 (0.00) [0–0] | 84 | 0.01 (0.11) [0–1] | 88 | 0.13 (0.91) [0–8] |
| Other | ||||||||
| Voluntary agency | 107 | 0.00 (0.00) [0–0] | 109 | 0.24 (2.49) [0–26] | 84 | 0.02 (0.15) [0–1] | 88 | 0.01 (0.11) [0–1] |
| Drug | Baseline (6 months) | Follow-up (12 months) | ||||||
|---|---|---|---|---|---|---|---|---|
| REACH-HF | Usual care | REACH-HF | Usual care | |||||
| n | % prescribed, mean (SD) | n | % prescribed, mean (SD) | n | % prescribed, mean (SD) | n | % prescribed, mean (SD) | |
| Angiotensin II receptor antagonist | 107 | 0.29 (0.46) | 109 | 0.22 (0.42) | 84 | 0.29 (0.44) | 88 | 0.23 (0.41) |
| ACE inhibitor | 107 | 0.64 (0.48) | 109 | 0.68 (0.47) | 84 | 0.62 (0.47) | 88 | 0.66 (0.45) |
| MRA | 107 | 0.60 (0.49) | 109 | 0.48 (0.50) | 84 | 0.61 (0.47) | 88 | 0.52 (0.49) |
| Anticoagulant | 107 | 0.47 (0.50) | 109 | 0.50 (0.50) | 84 | 0.44 (0.48) | 88 | 0.53 (0.49) |
| Beta-blocker | 107 | 0.84 (0.37) | 109 | 0.83 (0.38) | 84 | 0.85 (0.34) | 88 | 0.81 (0.38) |
| Digoxin | 107 | 0.19 (0.39) | 109 | 0.13 (0.34) | 84 | 0.16 (0.36) | 88 | 0.16 (0.36) |
| Ivabradine | 107 | 0.04 (0.19) | 109 | 0.06 (0.25) | 84 | 0.04 (0.19) | 88 | 0.06 (0.22) |
| Loop diuretic | 107 | 0.65 (0.48) | 109 | 0.62 (0.49) | 84 | 0.62 (0.47) | 88 | 0.59 (0.49) |
| Nitrate | 107 | 0.11 (0.32) | 109 | 0.14 (0.35) | 84 | 0.10 (0.27) | 88 | 0.12 (0.31) |
| Thiazide diuretic | 107 | 0.00 (0.00) | 109 | 0.02 (0.13) | 84 | 0.02 (0.10) | 88 | 0.01 (0.11) |
| Baselinea (6 months) | Follow-upb (12 monthsc) | |||||
|---|---|---|---|---|---|---|
| REACH-HF | Usual care | Total | REACH-HF | Usual care | Total | |
| Admissions (all cause) | 31 | 44 | 75 | 26 | 31 | 57 |
| Days per admission | 6.5 | 3.1 | 4.5 | 3.2 | 6.2 | 4.8 |
| Admissions due to HF | NA | NA | NA | 2 | 9 | 11 |
| Baseline (6 months) | Follow-up (12 months) | |||||||
|---|---|---|---|---|---|---|---|---|
| REACH-HF | Usual care | REACH-HF | Usual care | |||||
| n (number affected) | Mean (SD) [range] time per person | n (number affected) | Mean (SD) [range] time per person | n (number affected) | Mean (SD) [range] time per person | n (number affected) | Mean (SD) [range] time per person | |
| Hours per week | ||||||||
| Caregiver | 106 (37) | 4.11 (9.63) [0–50] | 108 (30) | 6.07 (17.19) [0–98] | 83 (35) | 6.10 (16.72) [0–114] | 87 (23) | 6.89 (19.70) [0–99] |
| Non-caregiver | 106 (27) | 1.68 (4.58) [0–25] | 109 (31) | 2.32 (6.33) [0–35] | 84 (33) | 2.25 (4.72) [0–28] | 88 (28) | 2.21 (6.41) [0–35] |
| Days off work | ||||||||
| Caregiver | 107 (8) | 0.85 (5.11) [0–50] | 109 (3) | 0.06 (0.39) [0–3] | 84 (4) | 1.19 (10.47) [0–96] | 87 (1) | 0.02 (0.21) [0–2] |
| Non-caregiver | 107 (7) | 0.39 (2.44) [0–21] | 109 (4) | 0.24 (1.49) [0–14] | 84 (4) | 0.15 (0.92) [0–8] | 88 (2) | 0.13 (0.98) [0–9] |
| Patient | 107 (13) | 4.68 (22.00) [0–191] | 109 (8) | 4.53 (22.80) [0–176] | 84 (8) | 3.64 (15.62) [0–105] | 88 (4) | 4.09 (32.28) [0–300] |
Costs
NHS and Personal Social Services costs are reported by allocation in Table 13. Informal care costs are reported in Table 14.
| Resource | Baseline (6 months) | Follow-up (12 months) | ||||||
|---|---|---|---|---|---|---|---|---|
| REACH-HF | Usual care | REACH-HF | Usual care | |||||
| n | Mean (SD) cost/person (£) | n | Mean (SD) cost/person (£) | n | Mean (SD) cost/person (£) | n | Mean (SD) cost/person (£) | |
| Primary care appointment/visit | ||||||||
| GP (surgery) | 106 | 89.49 (82.67) | 109 | 106.37 (107.25) | 84 | 115.51 (118.10) | 88 | 120.13 (109.31) |
| GP (home) | 107 | 7.01 (37.89) | 109 | 8.26 (49.30) | 84 | 7.14 (32.13) | 88 | 2.56 (13.68) |
| GP (telephone) | 107 | 13.13 (25.06) | 109 | 19.84 (38.89) | 84 | 20.97 (42.85) | 88 | 24.83 (45.78) |
| Practice nurse (surgery) | 106 | 29.97 (51.17) | 109 | 35.06 (55.44) | 83 | 76.82 (183.75) | 88 | 66.90 (102.35) |
| Practice nurse (home) | 107 | 0.18 (1.82) | 109 | 1.21 (10.94) | 83 | 0.68 (4.59) | 88 | 1.50 (9.11) |
| Practice nurse (telephone) | 107 | 0.28 (2.07) | 109 | 0.43 (2.55) | 84 | 1.18 (6.88) | 88 | 1.71 (5.70) |
| HF nurse | 107 | 27.90 (52.82) | 109 | 19.68 (34.75) | 84 | 24.22 (46.77) | 88 | 29.40 (64.39) |
| Physiotherapist | 107 | 15.21 (63.39) | 109 | 37.69 (194.40) | 84 | 30.45 (125.06) | 88 | 58.14 (275.94) |
| Occupational therapist | 107 | 0.70 (7.26) | 109 | 0 | 84 | 0.89 (8.19) | 88 | 0 |
| Community/district nurse | 107 | 13.29 (54.79) | 109 | 11.96 (87.93) | 84 | 5.64 (47.56) | 88 | 12.12 (74.18) |
| Health visitor | 107 | 0 | 109 | 0 | 84 | 0 | 88 | 0 |
| Primary care total | 105 | 193.42 (183.89) | 109 | 240.49 (351.91) | 83 | 284.24 (316.82) | 88 | 317.28 (380.86) |
| Secondary care | ||||||||
| Hospital admission (all cause) | 107 | 727 (1244) | 109 | 1014 (2861) | 84 | 777 (2098) | 88 | 885 (2121) |
| A&E attendance | 107 | 22 (54) | 109 | 35 (76) | 84 | 26 (76) | 88 | 28 (87) |
| Day hospital attendance | 107 | 292 (2407) | 109 | 88 (208) | 84 | 72 (188) | 88 | 109 (302) |
| Outpatient cardiology | 107 | 109 (119) | 109 | 119 (125) | 84 | 162 (176) | 88 | 116 (178) |
| Outpatient cardiac or HF nurse | 107 | 89 (139) | 109 | 70 (122) | 84 | 83 (129) | 88 | 80 (204) |
| Other outpatient | 107 | 44 (132) | 109 | 71 (166) | 84 | 104 (197) | 88 | 147 (427) |
| CR classes | – | – | – | – | 84 | 85.90 (178.16) | 88 | 15.37 (82.31) |
| Secondary care total | 107 | 1284 (2934) | 109 | 1397 (2965) | 84 | 1311 (2265) | 88 | 1379 (2390) |
| Social and community care visits | ||||||||
| Social worker | 107 | 2.21 (22.91) | 109 | 0 | 84 | 1.88 (12.12) | 88 | 0.90 (8.42) |
| Home care/home help | 107 | 40.60 (293.89) | 109 | 44.04 (314.83) | 84 | 234.43 (1120.40) | 88 | 46.36 (331.23) |
| Day care | 107 | 10.32 (106.73) | 108 | 0 | 84 | 22.45 (200.76) | 88 | 0.00 (0.00) |
| Drop-in club | 107 | 0.85 (7.63) | 108 | 3.13 (32.52) | 84 | 0 | 88 | 2.36 (22.17) |
| Other day-care service | 107 | 0 | 109 | 0 | 84 | 0.55 (5.02) | 88 | 5.75 (41.74) |
| Social care total | 107 | 53.98 (326.86) | 108 | 47.57 (341.43) | 84 | 259.31 (1136.89) | 88 | 55.58 (379.20) |
| Other | ||||||||
| Voluntary agency | 107 | 0 | 109 | 0.24 (2.49) [0–26] | 84 | 0.02 (0.15) [0–1] | 88 | 0.01 (0.11) [0–1] |
| All health and social care visits total | 105 | 1552 (3032) | 108 | 1702 (3023) | 83 | 1860 (2619) | 88 | 1751 (2554) |
| Medications | ||||||||
| Angiotensin II receptor antagonist | 107 | 4.38 (6.89) | 109 | 3.33 (6.29) | 84 | 8.76 (13.35) | 88 | 6.99 (12.54) |
| ACE inhibitor | 107 | 4.39 (3.34) | 109 | 4.68 (3.24) | 84 | 8.54 (6.51) | 88 | 9.15 (6.22) |
| Aldosterone receptor antagonist | 107 | 37.72 (31.06) | 109 | 30.08 (31.64) | 84 | 76.57 (58.79) | 88 | 64.97 (61.77) |
| Anticoagulant | 107 | 3.90 (4.18) | 109 | 4.21 (4.19) | 84 | 7.41 (8.02) | 88 | 8.78 (8.20) |
| Beta-blocker | 107 | 5.15 (2.25) | 109 | 5.05 (2.33) | 84 | 10.44 (4.22) | 88 | 9.97 (4.71) |
| Digoxin | 107 | 3.36 (7.05) | 109 | 2.31 (6.05) | 84 | 5.86 (12.97) | 88 | 5.59 (12.99) |
| Ivabradine | 107 | 9.65 (49.22) | 109 | 16.58 (63.60) | 84 | 18.45 (96.42) | 88 | 29.35 (114.44) |
| Loop diuretic | 107 | 5.22 (3.81) | 109 | 4.98 (3.88) | 84 | 9.82 (7.45) | 88 | 9.43 (7.77) |
| Nitrate | 107 | 31.10 (87.92) | 109 | 38.16 (95.98) | 84 | 57.22 (149.06) | 88 | 69.33 (171.23) |
| Thiazide diuretic | 107 | 0 | 109 | 0.18 (1.29) | 84 | 0.30 (1.96) | 88 | 0.22 (2.05) |
| All medications total | 107 | 104.87 (107.78) | 109 | 109.57 (120.58) | 84 | 203.38 (183.43) | 88 | 213.77 (208.42) |
| All health and social care total | 105 | 1763 (3048) | 108 | 1917 (3087) | 83 | 2064 (2636) | 88 | 1966 (2585) |
| Baseline (6 months) | Follow-up (12 months) | |||||||
|---|---|---|---|---|---|---|---|---|
| REACH-HF | Usual care | REACH-HF | Usual care | |||||
| n (number affected) | Mean (SD) cost per person (£) | n (number affected) | Mean (SD) cost per person (£) | n (number affected) | Mean (SD) cost per person (£) | n (number affected) | Mean (SD) cost per person (£) | |
| Caregiver hours per week | 106 (37) | 98.72 (231.23) | 108 (30) | 145.78 (412.63) | 83 (35) | 146.51 (401.37) | 87 (23) | 165.24 (472.87) |
| Non-caregiver hours per week | 106 (27) | 40.30 (109.93) | 109 (31) | 55.71 (151.89) | 84 (33) | 54.10 (113.35) | 88 (28) | 53.00 (153.84) |
| Total caring hours per week | 139 | 202 | 201 | 217 | ||||
| Total caring hours per 6 months | 3601 | 5252 | 10,465 | 11,285 | ||||
| Caregiver days off work | 107 (8) | 104.02 (624.96) | 109 (3) | 7.85 (47.88) | 84 (4) | 145.60 (1280.87) | 87 (1) | 2.81 (26.23) |
| Non-caregiver days off work | 107 (7) | 48.01 (298.53) | 109 (4) | 29.17 (182.25) | 84 (4) | 18.93 (113.11) | 88 (2) | 15.29 (119.91) |
| Total caregiver days off work | 152 | 37 | 165 | 18 | ||||
| Patient days off work | 107 (13) | 310.65 (1459.52) | 109 (8) | 300.69 (1512.37) | 84 (8) | 241.69 (1036.65) | 88 (4) | 271.42 (2141.55) |
| Patients in FTE (%) | 81 | 84 | 69 | 110 | ||||
| Patient time off work | 251.71 | 253.58 | 165.69 | 297.96 | ||||
Health state values (EuroQol-5 Dimensions, five-level version) and quality-adjusted life-years
Table 15 summarises the estimated mean health state values and QALY estimates, derived from participant and caregiver reports for EQ-5D-5L health states (based on EQ-5D-3L values per tariff). We report data for assessments at baseline and at 4 and 12 months.
| EQ-5D-3L health state value | REACH-HF | Usual care | ||
|---|---|---|---|---|
| n | Mean (SD) [range] | n | Mean (SD) [range] | |
| Participants | ||||
| Baselinea | 106 | 0.74 (0.23) [–0.26–1.00] | 108 | 0.72 (0.24) [0.06–1.00] |
| Month 4 | 95 | 0.76 (0.22) [–0.07–1.00] | 101 | 0.75 (0.22) [0.16–1.00] |
| Month 6 | 88 | 0.71 (0.27) [–0.43–1.00] | 92 | 0.73 (0.22) [–0.21–1.00] |
| Month 12 | 88 | 0.75 (0.24) [–0.10–1.00] | 92 | 0.74 (0.26) [–0.31–1.00] |
| Estimated 12-month QALYsb | 81 | 0.74 (0.22) [–0.04–1.00] | 84 | 0.76 (0.21) [–0.06–1.00] |
| Caregivers | ||||
| Baseline | 53 | 0.83 (0.19) [0.30–1.00] | 44 | 0.80 (0.15) [0.55–1.00] |
| Month 4 | 46 | 0.85 (0.17) [0.35–1.00] | 36 | 0.80 (0.20) [0.02–1.00] |
| Month 6 | 44 | 0.83 (0.15) [0.39–1.00] | 36 | 0.78 (0.16) [0.40–1.00] |
| Month 12 | 42 | 0.83 (0.15) [0.42–1.00] | 33 | 0.78 (0.19) [0.09–1.00] |
| Estimated 12-month QALYsb | 41 | 0.84 (0.14) [0.40–1.00] | 30 | 0.79 (0.16) [0.44–1.00] |
Cost-effectiveness: primary analyses
Table 16 summarises the primary statistical analyses undertaken to estimate the mean differences in costs (by cost category and for total cost) plus differences in participant QALYs between the REACH-HF intervention and usual care groups. The table reports unadjusted and adjusted analyses, including adjustment for baseline values and other covariates (trial centre, NT-proBNP group, previous MI and previous AF).
| REACH-HF | Usual care | Unadjusted difference (intervention – control) | Mean (95% CIa) adjusted differenceb (intervention – control) | |||
|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |||
| Primary care (£) | 83 | 284 (317) | 88 | 317 (381) | –33 | –15.81 (–113.29 to 81.66) |
| Secondary care (£) | 84 | 1311 (2265) | 88 | 1379 (2390) | –69 | 83.09 (–638.00 to 804.00) |
| Social and community care (£) | 84 | 259 (1137) | 88 | 56 (379) | 204 | 201.54 (–716.46 to 1119.54) |
| Medications (£) | 84 | 203 (183) | 88 | 214 (208) | –10 | 6.97 (–34.79 to 48.73) |
| NHS and Personal Social Services costs (£) | 83 | 2064 (2636) | 88 | 1966 (2585) | 98 | 345 (–459 to 1150) |
| Intervention cost (£) | 418.39 | 0 | 418 | |||
| Total cost (£) | 2482 | 1966 | 516 | 763 | ||
| Participant QALYs (EQ-5D-3L) | 0.74 (0.22) | 0.76 (0.21) | –0.018 | –0.024 (–0.059 to 0.010) | ||
| ICER (cost per QALY) | REACH-HF intervention ‘dominated’ by usual care (higher cost, no QALY benefit) | |||||
Excluding the intervention cost for the REACH-HF intervention, we report a difference of £345 in total costs for health and social care service use, with participants who used the REACH-HF intervention having a higher cost than usual care, although this was not statistically significant. When including the REACH-HF intervention cost in the analyses, we see an unadjusted difference in cost between the REACH-HF intervention and usual care of £516, with this estimated cost difference increasing to £763, higher cost for REACH-HF intervention participants, after adjustment.
Usual care had a better QALY profile than the REACH-HF intervention (i.e. a mean reduction of –0.024 QALYs in the REACH-HF intervention participants), but this difference was not statistically significant.
The REACH-HF intervention, with a higher expected mean cost and an absence of QALY gains, was therefore dominated by usual care and did not seem to be a value-for-money investment for the NHS in the UK in the short term.
When considering uncertainty using the bootstrapped data, Figure 7 illustrates the cost-effectiveness plane, showing that the incremental cost and QALY data (pairs) from the bootstrapped simulations are predominantly in the north-west quadrant, indicating additional costs and reduced QALYs. Figure 8 reports the cost-effectiveness acceptability curve, indicating that the REACH-HF intervention is expected to be cost-effective versus usual care only in a small number of trials/simulations (< 10%) regardless of the WTP threshold (per QALY gained) applied.
FIGURE 7.
Cost-effectiveness plane illustrating the results of 1000 bootstrap resamples of incremental cost and effectiveness with the REACH-HF intervention compared with usual care. Blue circles reflect bootstrap resample estimates and the orange diamond represents central value (mean QALY difference and mean cost difference across all samples).
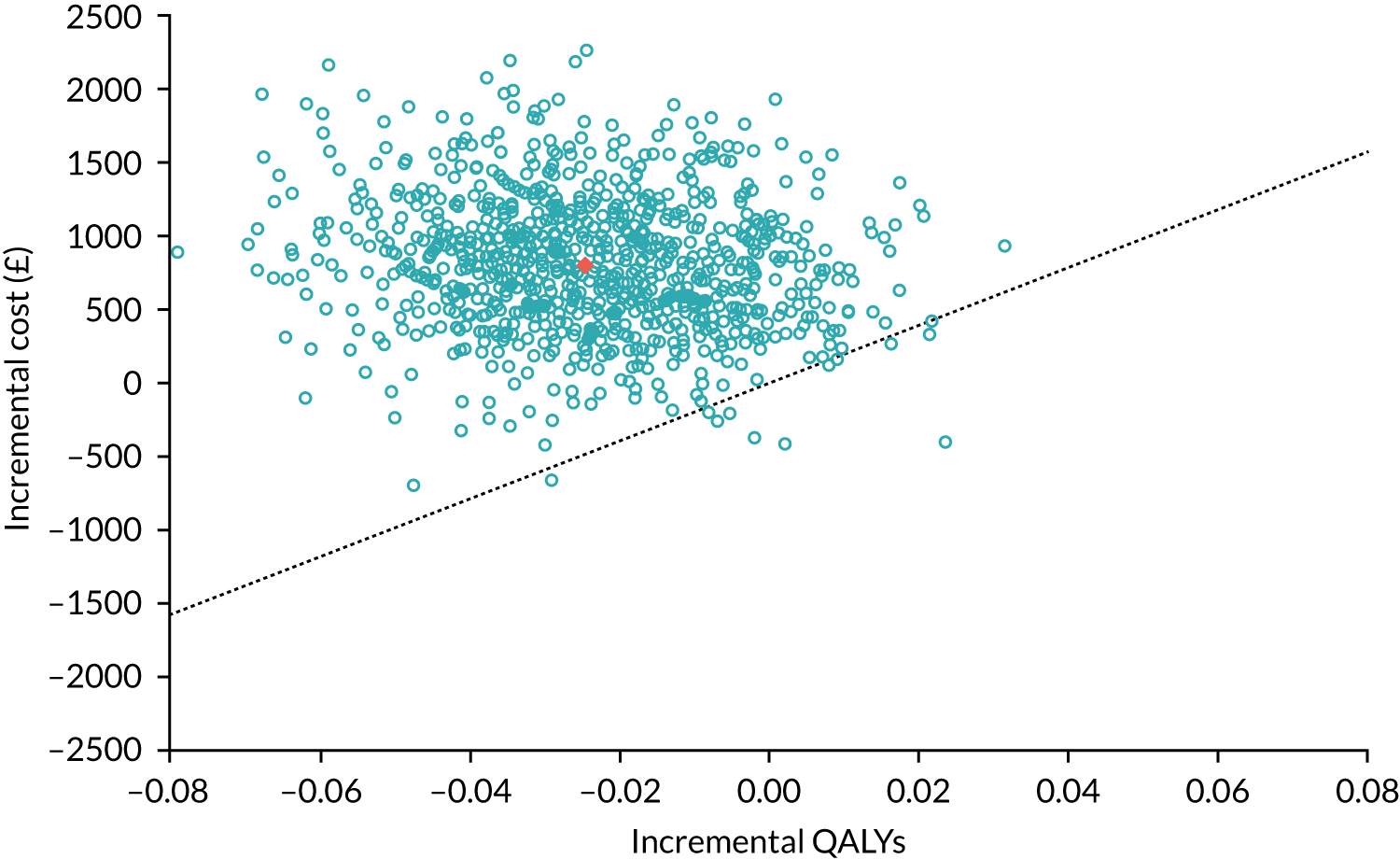
FIGURE 8.
Cost-effectiveness acceptability curve showing probability that the REACH-HF intervention is cost-effective (probability the net benefit statistic > 0) compared with usual care at each WTP threshold.
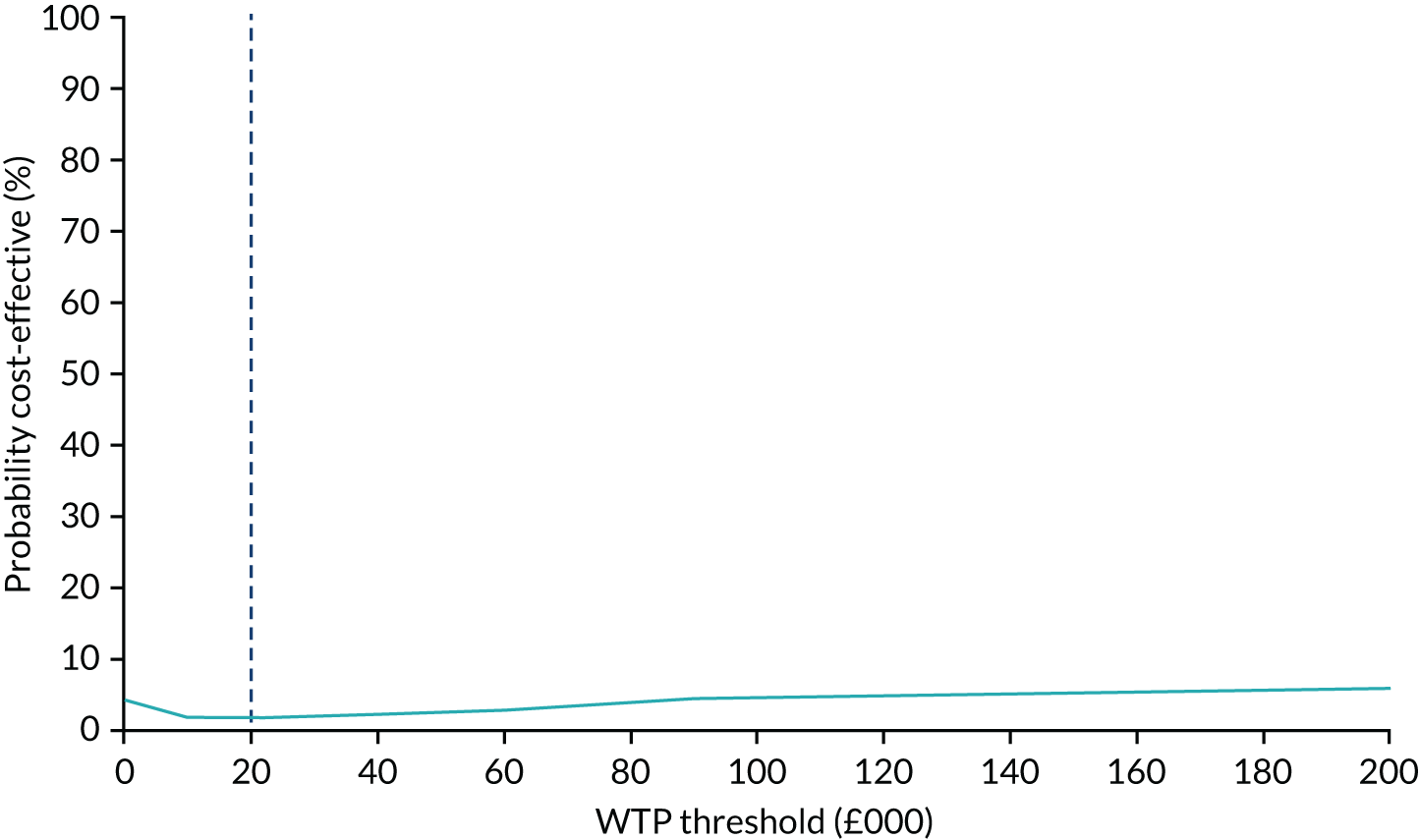
Cost-effectiveness: sensitivity analyses
Table 17 presents summary statistics for the sensitivity/scenario analyses undertaken to assess the sensitivity of the primary base-case result to alternative assumptions/scenarios.
| Analysis | Mean (SD) values per participant | Adjusted mean (95% CI) between-group difference | Intervention cost (£) | Total cost (£) difference | ICER | ||||
|---|---|---|---|---|---|---|---|---|---|
| QALYs | Costs (£) | Participant QALYs (EQ-5D-3L) | NHS and Personal Social Services costs | ||||||
| REACH-HF | Usual care | REACH-HF | Usual care | ||||||
| Complete cases (base case) | 0.74 (0.22) | 0.76 (0.21) | 2064 (2636) | 1966 (2585) | –0.024 (–0.059 to 0.010) | 345 (–459 to 1150) | 418 | 763 | Dominated |
| Per-protocol participants | 0.74 (0.22) | 0.76 (0.21) | 2135 (2682) | 1966 (2585) | –0.025 (–0.060 to 0.011) | 411 (–423 to 1245) | 418 | 829 | Dominated |
| Multiple imputationa | 0.69 (0.23) | 0.68 (0.23) | 2267 (2591) | 2126 (2461) | –0.024 (–0.059 to 0.010) | 187 (–606 to 981) | 418 | 605 | Dominated |
| Duration (years) | |||||||||
| < 1 | 0.76 (0.23) | 0.79 (0.19) | 2879 (3465) | 2323 (3432) | –0.045 (–0.107 to 0.017) | 1610 (–1359 to 4579) | 418 | 2028 | Dominated |
| 1–2 | 0.71 (0.18) | 0.79 (0.17) | 1141 (3769) | 1827 (2497) | 0.000 (–0.080 to 0.079) | 216 (–997 to 1430) | 418 | 634 | Dominated |
| > 2 | 0.73 (0.23) | 0.72 (0.24) | 1694 (2751) | 1775 (1889) | –0.021 (–0.073 to 0.030) | –79 (–938 to 780) | 418 | 339 | Dominated |
| Severity | |||||||||
| Low NT-proBNP | 0.75 (0.22) | 0.75 (0.23) | 1751 (2736) | 2035 (1878) | –0.025 (–0.064 to 0.014) | 257 (–545 to 1059) | 418 | 675 | Dominated |
| High NT-proBNP | 0.68 (0.21) | 0.79 (0.13) | 3479 (4273) | 2315 (2552) | –0.033 (–0.130 to 0.065) | 841 (–2878 to 4560) | 418 | 1259 | Dominated |
| Centre | |||||||||
| 1 | 0.79 (0.14) | 0.77 (0.20) | 2137 (3333) | 3330 (1684) | –0.026 (–0.085 to 0.033) | 483 (–492 to 1458) | 418 | 901 | Dominated |
| 2 | 0.65 (0.24) | 0.71 (0.22) | 2124 (1947) | 1947 (1512) | –0.014 (–0.079 to 0.051) | 634 (–328 to 1597) | 418 | 1052 | Dominated |
| 3 | 0.71 (0.25) | 0.71 (0.16) | 1506 (2215) | 2046 (2471) | –0.017 (–0.098 to 0.064) | –283 (–2588 to 2023) | 418 | 135 | Dominated |
| 4 | 0.78 (0.23) | 0.80 (0.25) | 2339 (2516) | 2622 (3774) | –0.024 (–0.108 to 0.059) | 52 (–2092 to 2196) | 418 | 470 | Dominated |
| Caregiver | |||||||||
| Participating | 0.73 (0.24) | 0.71 (0.24) | 2106 (2417) | 2417 (2083) | –0.043 (–0.101 to 0.015) | 339 (–666 to 1344) | 418 | 757 | Dominated |
| Not participating | 0.76 (0.19) | 0.78 (0.19) | 2011 (2919) | 1902 (2839) | –0.003 (–0.043 to 0.038) | 46 (–1202 to 1293) | 418 | 464 | Dominated |
| Including caregiver QALYs | 1.57 (0.29) | 1.50 (0.30) | 2064 (2636) | 1966 (2585) | –0.012 (–0.108 to 0.084) | 345 (–459 to 1150) | 418 | 763 | Dominated |
| Including informal care costs | 0.74 (0.22) | 0.76 (0.21) | 12,248 (22,197) | 13,260 (26,131) | –0.024 (–0.059 to 0.010) | 6514 (–2012 to 15,040) | 418 | 6932 | Dominated |
| Including informal care and time off work costs | 0.74 (0.22) | 0.76 (0.21) | 12,588 (22,213) | 13,517 (26,143) | –0.024 (–0.059 to 0.010) | 4894 (–4096 to 13,884) | 418 | 4894 | Dominated |
| Lower intervention cost | 0.74 (0.22) | 0.76 (0.21) | 2064 (2636) | 1966 (2585) | –0.024 (–0.059 to 0.010) | 345 (–459 to 1150) | 204 | 549 | Dominated |
| Higher intervention cost | 0.74 (0.22) | 0.76 (0.21) | 2064 (2636) | 1966 (2585) | –0.024 (–0.059 to 0.010) | 345 (–459 to 1150) | 730 | 1075 | Dominated |
| MLHFQ mapping to EQ-5Da | 0.76 (0.12) | 0.76 (0.14) | 2064 (2636) | 1966 (2585) | 0.026 (0.005 to 0.047) | 345 (–442 to 1133) | 418 | 763 | 29,346 |
In all scenarios, except the MLHFQ–EQ-5D mapping scenario, the REACH-HF intervention remains dominated by usual care, with no reported QALY gains associated with the REACH-HF intervention.
Where we present analysis with EQ-5D values and QALYs derived from the indirect, participant-level MLHFQ mapping algorithm, we report a statistically significant difference in mean estimated QALYs of 0.026 (95% CI 0.005 to 0.047), favouring the intervention. When this approach is used alongside the analysis of costs, with a mean incremental cost of £763, we have an incremental cost per QALY of £29,346 for the REACH-HF intervention versus usual care. Figures 9 and 10 report the cost-effectiveness plane and cost-effectiveness acceptability curve for this analysis, where, when using the net benefit statistic approach, we see the probability that the REACH-HF intervention is cost-effective at WTP threshold values of £20,000 per QALY and £30,000 per QALY, respectively, at 31.3% and 50.3%.
FIGURE 9.
Cost-effectiveness plane showing 1000 bootstrapped resampled estimates of incremental cost and incremental QALY change, in the scenario where QALYs were derived from mapping between MLHFQ and EQ-5D scores using the algorithm developed by Edlin et al. (personal communication, 2018).
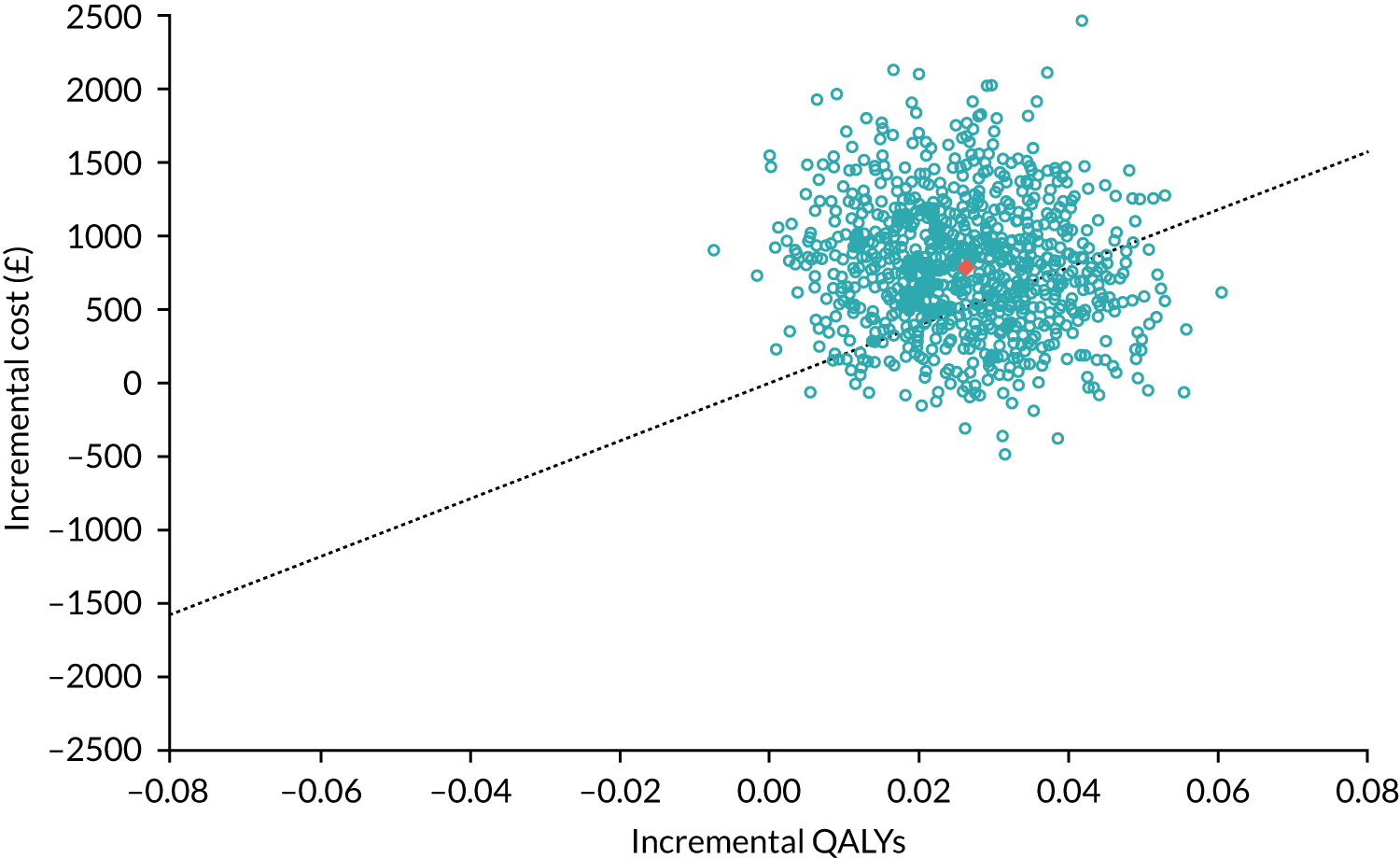
FIGURE 10.
Cost-effectiveness acceptability curve showing the probability that REACH-HF is cost-effective over a range of WTP thresholds, in the scenario where QALYs were derived from mapping between MLHFQ and EQ-5D scores using the algorithm developed by Edlin et al. (personal communication, 2018).
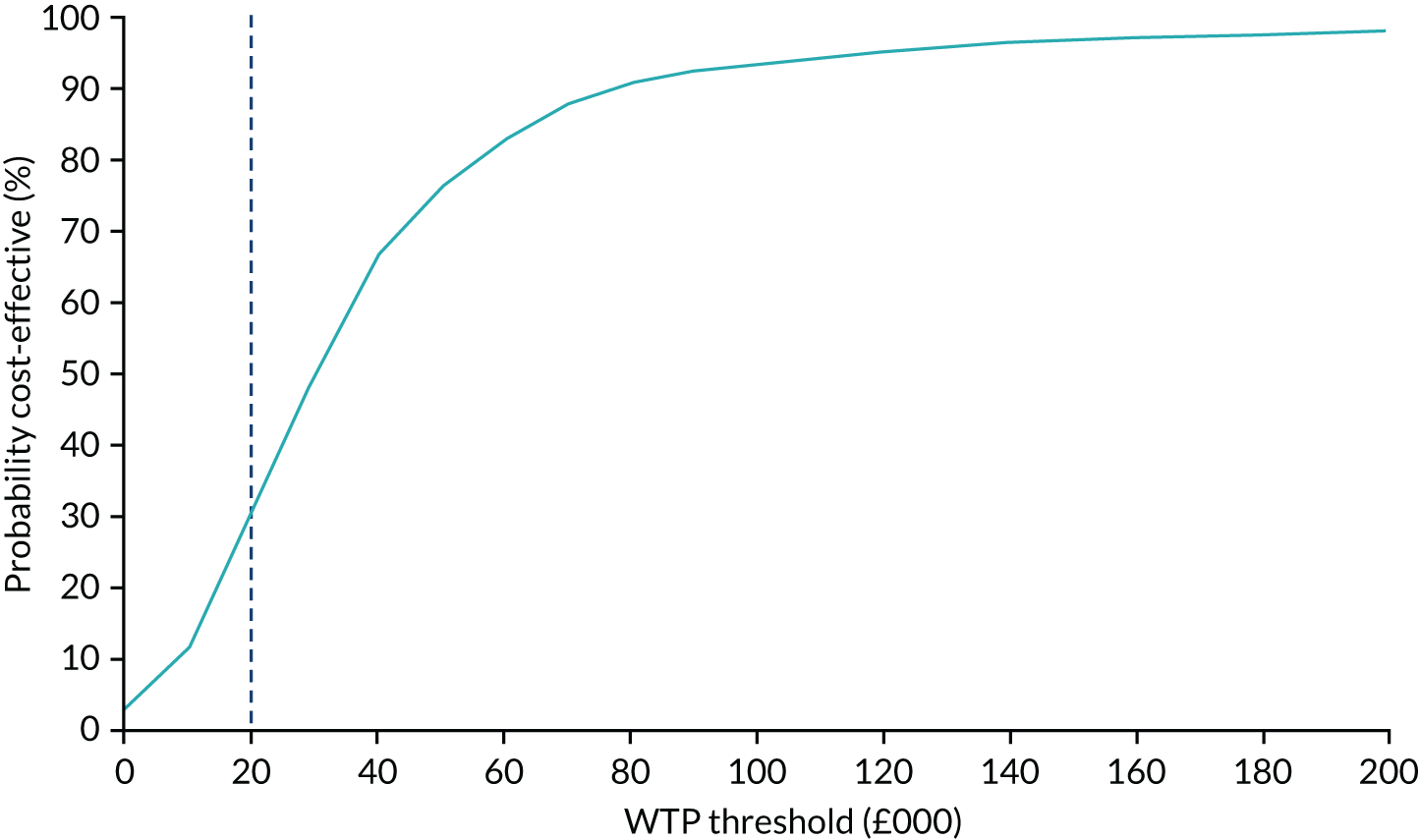
Discussion
We have reported the trial-based cost-effectiveness analysis of the REACH-HF intervention versus usual care. We find health and social care costs to be similar over the 12 months of follow-up, with no significant difference in adjusted analyses, and we find no difference in QALYs; however, we do expect an additional cost associated with the intervention. The results suggest that the REACH-HF intervention is dominated by usual care.
A key strength of this analysis is that it is undertaken alongside the REACH-HF RCT and uses effectiveness data and cost estimates derived from the RCT, which followed up patients over 12 months from enrolment, with excellent retention (86%). However, despite the trial finding a statistically significant and clinically meaningful difference in the primary outcome measure of disease-specific HRQoL using the MLHFQ, the trial did not detect a significant change in EQ-5D, the generic HRQoL measure currently recommended for use in the assessment of cost-effectiveness of health interventions in the UK. Therefore, the short-term assessment of cost-effectiveness did not show the REACH-HF intervention to be cost-effective compared with usual care.
A limitation of the trial-based cost-effectiveness analysis is that it assesses cost-effectiveness in the short term using HRQoL (EQ-5D, QALY) data from the RCT, and this may exclude longer-term benefits such as slowing symptomatic progression, reducing hospital admissions and related mortality impacts. In the broader literature, the cost-effectiveness of interventions for HF has been commonly assessed using model-based analyses, considering longer-term (e.g. lifetime) costs and consequences. We present this form of analysis in Work package 4: model-based cost-effectiveness analysis, with detailed primary analyses described in a published paper (see Appendix 1)46 and additional analyses below.
Conclusions
The results from our cost-effectiveness analyses undertaken alongside the REACH-HF RCT suggest that the REACH-HF intervention is not cost-effective versus usual care.
Model-based cost-effectiveness analyses
Introduction
The model-based cost-effectiveness analysis is described in full in a published paper46 (see Appendix 1). This section sets out additional detail not included in that manuscript, which includes:
-
Review of modelling studies in HF – details of the review of cost-effectiveness modelling studies in HF that informed the development of the model.
-
Model-based cost-effectiveness analysis based on all-cause hospital admissions – here, in a form of sensitivity analysis (structural uncertainty), we examined the effect of changing the mechanism of intervention effect from impact on HF-related hospital admissions to all-cause hospital admissions. The HF-specific hospital admissions were used as the base-case analysis, because it was considered most likely that the intervention would affect HF-related hospital admissions (based on literature review and stakeholder input) and limiting the effect to these hospital admissions only was a conservative assumption. However, we also considered scenarios in which a rehabilitation intervention is shown to be effective on ‘all-cause’ hospital admissions, given that the intervention has a potential impact on a range of hospital admissions (e.g. other types of cardiac admissions or admissions for associated symptoms or comorbidities that are worsened by HF).
-
Model-based cost-effectiveness analysis of centre-based CR versus usual care – centre-based rehabilitation, although not available to all patients owing to geographical and transport limitations, is the main form currently available to patients with HF in the UK but is accessed only by < 10% of patients with HF. The cost-effectiveness of centre-based CR compared with usual care (which is assumed to include no CR) was an important context for the cost-effectiveness analysis. This was assessed based on the impact of the intervention on HF-related hospital admissions as the base case and all-cause hospital admissions as a sensitivity analysis.
Review of cost-effectiveness modelling studies in heart failure
To inform the modelling approach, a systematic review of the literature on HF economic modelling was performed. A previous review published in 2011 was identified. 111 A systematic search was, therefore, carried out in MEDLINE, EMBASE, the Cochrane Library, EconLit and CINAHL (Cumulative Index to Nursing and Allied Health Literature) databases for cost-effectiveness studies of HF covering the period from 2010 to September 2016. Search terms included those related to HF, as well as costs, economics, cost-effectiveness or decision modelling. Full search details are reported in Report Supplementary Material 1.
Studies available in full text and in English were included. Automatic and manual deduplication was performed, and titles and abstracts were screened for inclusion. Inclusion criteria were studies reporting on the use of a decision-analytic or mathematical model of costs and consequences associated with HF treatment or management over time. This included Markov, decision tree and discrete event simulation models, as well as those based on sets of mathematical equations. Studies were excluded if they did not include a model, were not specific to HF (this included studies that reported a model of a related cardiovascular condition where, for example, HF comprised just one state in a larger model), reported on only the short-term cost-effectiveness alongside a clinical trial or study, were animal studies or were specific to HF prevention as opposed to treatment.
Review of literature
Through the searches, 6393 studies were identified. A total of 890 were duplicates and were removed, and 2010 were removed as they had been published prior to the end date of the previous systematic review. This left 3493 studies, of which 3350 were removed at the abstract screening stage because they did not include a health-care evaluation model (n = 2434), were not carried out on those with HF (n = 815), reported only short-term cost-effectiveness alongside a clinical trial (n = 63), were animal studies (n = 21) or were about HF prevention and not treatment (n = 17). Sixty-seven studies were then excluded because they were not available in full text in the English language (e.g. conference abstracts).
The remaining 76 full-text papers were reviewed and 24 were excluded because they did not meet inclusion criteria (no model, 14 papers) or were systematic reviews of modelling studies (10 papers), which left 52 papers. Of these, 50 described a unique economic model or assessment associated (two were additional reports from existing studies).
The results of the literature search are illustrated in Figure 11.
FIGURE 11.
Results of the literature search.
Summary of the review of heart failure modelling literature
The review identified a range of studies with varying decision contexts, modelling approaches, model structures, settings and parameters. In terms of model types, the reviewed models most commonly used Markov cohort models. Models for pharmaceutical or service-level interventions (as opposed to those for surgical devices, which tended to include a broader range of states) tended to use one of three main approaches: using a simple model with two basic disease states, alive (with HF) and dead, an alive/dead model plus additional states for one or more hospital admission, or modelling disease progression via NYHA class. Thokala et al. 112 included hospital admission (for HF and other causes) and additionally adjusted mortality for the time from hospital discharge to reflect the increased risk of death soon after discharge. Some models allowed both for progression in the underlying condition, based on NYHA grades, and for hospital admission, but this was less common. For example, Kansal et al. 113 reported a Markov cohort model with NYHA progression, as well as additional hospital admission states for both HF and other-cause hospital admissions for up to three events, allowing utility to decline with multiple hospital admissions. Two studies used an adaptation of a Markov cohort approach but modelled specific patient subgroups separately using relevant risk equations allowing for differences in treatment effect, disease progression, cost or mortality within different subgroups. Alternative approaches were used by some authors – for example, seven studies reported a discrete event simulation approach following individual simulated patients based on time-to-event data.
Model-based cost-effectiveness analysis based on all-cause hospital admissions
The primary analyses (see Appendix 1) are based on effectiveness estimates for changes in HF-related hospital admissions (Table 18). Here, in an additional scenario analysis, we examine the effect of changing the basis of intervention effectiveness from impact on HF-related hospital admissions to all-cause hospital admissions.
| Intervention | Odds ratio (vs. usual care) | 95% confidence interval | Source |
|---|---|---|---|
| Home-based CR | 0.79 | 0.54 to 1.16 | Cochrane review and meta-analysis1 |
| REACH-HF intervention | 0.72 | 0.35 to 1.51 | REACH-HF trial (see Work package 3: patient outcomes) |
The cost for all-cause hospital admissions was based on the cost of HF-related hospital admissions and other-cause hospital admissions, weighted by the proportion of HF-related and other hospital admissions observed in the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS) trial. 104
The results (Table 19) suggest that home-based rehabilitation would dominate usual care, with the home-based CR resulting in cost savings (–£236 vs. usual care) because of a reduction in hospital admissions and a similar profile on health outcomes (QALYs), with no difference reported in incremental QALYs. The results suggest that REACH-HF would dominate usual care if the intervention effect was to reduce all-cause hospital admissions. The REACH-HF intervention was estimated to be cost saving at a level of £506 per person, with no difference in incremental QALYs.
| Usual care | Intervention | |
|---|---|---|
| Home-based CR based on all-cause hospitalisation rate | ||
| Discounted costs (£) | 12,082 | 11,846 |
| Discounted QALYs | 4.06 | 4.06 |
| Incremental costs (£) | –236 | |
| Incremental QALYs | 0.00 | |
| Cost/QALY gain (£) | Dominating | |
| REACH-HF based on all-cause hospitalisation rate | ||
| Discounted costs (£) | 12,082 | 11,576 |
| Discounted QALYs | 4.06 | 4.06 |
| Incremental costs (£) | –£506 | |
| Incremental QALYs | 0.00 | |
| Cost/QALY gain (£) | Dominating | |
Model-based cost-effectiveness analysis of centre-based cardiac rehabilitation versus usual care
The cost-effectiveness of centre-based CR provides important context to the analysis of the cost-effectiveness of home-based CR in the UK. Effectiveness estimates for centre-based CR on HF-related hospital admissions were drawn from the recent Cochrane systematic review and meta-analysis (Table 20). 1
| Intervention | Odds ratio (vs. usual care) | 95% confidence interval | Source |
|---|---|---|---|
| Centre-based CR | 0.36 for HF admissions | 0.18 to 0.73 | Cochrane review and meta-analysis1 |
| Centre-based CR | 0.60 for all-cause admissions | 0.40 to 0.90 | Cochrane review and meta-analysis1 |
The results of the cost-effectiveness analysis indicate that centre-based CR is cost-effective versus usual care, with a mean QALY gain of 0.46, a mean incremental cost of £656, and an incremental cost per QALY of £8828. The mean health gains are much higher than seen for home-based CR versus usual care (0.20) and REACH-HF versus usual care (0.30), but all interventions are predicted to be cost-effective versus usual care.
The results of the sensitivity analysis indicate that centre-based CR dominates usual care, with no change in QALYs but a mean incremental cost reduction of £528 when all-cause hospital admissions were the intervention effect (Table 21). Similar to home-based CR and the REACH-HF intervention, when modelled with all-cause hospital admissions (and, therefore, no mortality effect), there were no QALY gains, but cost savings with centre-based CR were similar to those achieved with the REACH-HF intervention (£523) and greater than those achieved with home-based CR (£247).
| Centre-based CR | Usual care (mean) | Intervention (mean) |
|---|---|---|
| Based on HF-specific hospitalisation rate | ||
| Discounted costs (£) | 15,119 | 15,418 |
| Discounted QALYs | 4.26 | 4.71 |
| Incremental costs (£) | 656 | |
| Incremental QALYs | 0.46 | |
| Cost/QALY gain (£) | 8828 | |
| Based on all-cause hospitalisation rate | ||
| Discounted costs (£) | 14,101 | 13,573 |
| Discounted QALYs | 4.06 | 4.06 |
| Incremental costs (£) | –528 | |
| Incremental QALYs | 0.00 | |
| Cost/QALY gain (£) | Dominating | |
Overall conclusions of the cost-effectiveness analysis
Trial-based cost-effectiveness analyses, using shorter-term outcomes on HRQoL, indicated that the REACH-HF intervention was not cost-effective when compared with usual care. However, when using model-based analyses, with evidence synthesis, to estimate longer-term costs and outcomes based on effectiveness data suggesting a reduction in hospital admissions, the cost-effectiveness analyses indicate that both the REACH-HF intervention and home-based CR more generally are likely to be cost-effective when compared with usual care. Sensitivity analyses, against uncertainty in input parameters (data inputs) and structural uncertainty, suggest that the findings that interventions are cost-effective when compared with usual care are robust to changes in assumptions used and to uncertainty in data inputs.
List of abbreviations
- ACE
- angiotensin-converting enzyme
- AF
- atrial fibrillation
- CBQ-HF
- Caregiver Burden Questionnaire for Heart Failure
- CI
- confidence interval
- CLAHRC
- Collaborations for Leadership in Applied Health Research and Care
- CR
- cardiac rehabilitation
- CTU
- Clinical Trials Unit
- DVD
- digital versatile disc
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- ESC
- European Society of Cardiology
- GBP
- Great British pounds
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- HCP
- health-care professional
- HF
- heart failure
- HFpEF
- heart failure with preserved ejection fraction
- HFrEF
- heart failure with reduced ejection fraction
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ISWT
- Incremental Shuttle Walk Test
- LVEF
- left ventricular ejection fraction
- MET
- metabolic equivalent
- MI
- myocardial infarction
- MLHFQ
- Minnesota Living with Heart Failure Questionnaire
- NACR
- National Audit of Cardiac Rehabilitation
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NRES
- National Research Ethics Service
- NT-proBNP
- N-terminal pro-B-type natriuretic peptide
- NYHA
- New York Heart Association
- PenCLAHRC
- Collaborations for Leadership in Applied Health Research and Care South West Peninsula
- PGfAR
- Programme Grants for Applied Research
- PPI
- patient and public involvement
- PSC
- Programme Steering Committee
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- REACH-HF
- Rehabilitation Enablement in Chronic Heart Failure
- RR
- relative risk
- RUQ
- resource use questionnaire
- SAE
- serious adverse event
- SCHFI
- Self-Care of Heart Failure Index
- SD
- standard deviation
- SF-6D
- Short Form questionnaire-6 Dimensions
- SF-12
- Short Form questionnaire-12 items
- WP
- work package
- WTP
- willingness to pay
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/pgfar09010).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
