Notes
Article history
The research reported in this issue of the journal was funded by the PHR programme as project number 14/67/14. The contractual start date was in December 2015. The final report began editorial review in January 2019 and was accepted for publication in August 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PHR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Amanda Avery, alongside her academic position at the University of Nottingham, also holds a consultancy position at Slimming World® (Alfreton, UK). Neither Amanda Avery nor Slimming World had access to trial data or was involved in data collection or analyses of the trial.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Bick et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Scientific background
On discharge from maternity care at around 6 to 8 weeks postnatally, two-thirds of women weigh more than their pre-pregnancy weight. 1 Women who start their next pregnancy with an overweight or obese body mass index (BMI) score have a higher risk of adverse outcomes for themselves and/or for their infants. Failure to manage postnatal weight is linked to poor health behaviours including smoking, dietary choices, lack of regular exercise and failure to breastfeed. 2–4 In the UK around half of all pregnant women have overweight or obese BMI scores, with concerns increasing about women who develop excessive gestational weight gain (EGWG) as defined using Institute of Medicine (IoM) criteria from the USA:5 > 18 kg if pre-pregnancy BMI score was < 18.5 kg/m2; > 16 kg if pre-pregnancy BMI score was 18.50–24.99 kg/m2; > 11.5 kg if pre-pregnancy BMI score was 25.00–29.99 kg/m2; and > 9 kg if pre-pregnancy BMI score was ≥ 30 kg/m2. There are currently no equivalent guidelines for the UK population. Evidence of potential use of the IoM criteria for women in the UK was considered by the National Institute for Health and Care Excellence (NICE) in 2014 to consider the need for an update to the 2010 NICE guidance on weight management before, during and after pregnancy. 6 A decision was taken not to revise recommendations owing to lack of evidence from a UK population, and it remains an area where urgent further research is needed.
Failure to lose weight within 6 months of giving birth is an important predictor of future weight gain and obesity. 7 This is a major public health issue, as postpartum weight retention contributes to long-term obesity, hypertension, diabetes and degenerative joint disease. 8 Evidence from the UK shows that a significantly greater proportion of women from areas of high deprivation have weight management problems,9 and a large cohort study in the USA found that excessive pregnancy weight gain and failure to lose weight postnatally was highly prevalent among young, ethnic minority women with low incomes. 10 The impacts of poor weight management are not confined to the woman; their infants are at risk of a high BMI score and blood pressure in childhood and young adulthood. 4 The complexity of supporting and treating individuals who have overweight or obese BMI scores remains a challenge. 11 There is a clear need for a clinically effective and cost-effective postnatal weight management intervention in UK settings, but a lack of evidence to support what this should include,12 when an intervention should be offered or how best to recruit women. 7 There is evidence in general population studies that commercial organisations may be of more benefit than NHS providers to support individuals to manage their weight. 13
Weight management interventions during pregnancy
Recent trials of diet and weight management interventions during pregnancy, some of which included postnatal outcomes, have measured the impact on risk of gestational diabetes, having a large-for-gestational-age infant and caesarean birth,14–17 but there is limited evidence of clinical effectiveness. 18 In the UK, NICE public health guidance for weight management before, during and after pregnancy6 recommended that dieting and weight loss during pregnancy should be avoided owing to concerns about their impact on neonatal outcomes, although an Australian study found no evidence of harm. 19 A UK-based multicentre trial of a behavioural intervention during pregnancy based on changing diet to foods with a lower glycaemic index and increasing physical activity aimed to reduce the risk of gestational diabetes and birth of large-for-gestational-age infants. 20 Women who had a BMI score of ≥ 30 kg/m2 were recruited between 15 and 19 weeks’ gestation and followed for up to 6 months postnatally to assess if the intervention led to sustained dietary and physical activity change. A total of 1555 women with a mean BMI score of 36.3 kg/m2 [standard deviation (SD) 4.8 kg/m2] were recruited: 772 were randomly assigned to standard antenatal care and 783 were randomly assigned to the behavioural intervention. No difference was found in rate of gestational diabetes or incidence of large-for-gestational-age babies. Gestational diabetes was reported in 172 (26%) women in the standard care arm compared with 160 (25%) women in the intervention arm [risk ratio (RR) 0.96, 95% confidence interval (CI) 0.79 to 1.16; p = 0.68]. A total of 61 (8%) out of 751 babies in the standard care arm were large for gestational age compared with 71 (9%) out of 761 babies in the intervention arm (RR 1.15, 95% CI 0.83 to 1.59; p = 0.40). A recent meta-analysis of individual participant data on over 12,000 women from 36 randomised controlled trials (RCTs) of diet and physical activity interventions on gestational weight gain and pregnancy outcomes21 found less weight gain in the intervention arm than in the control arm (mean difference –0.70 kg, 95% CI –0.92 to –0.48 kg; 33 studies, 9320 women); this effect was observed regardless of a woman’s parity, BMI score, ethnicity or pre-existing medical condition. There were no statistically significant reductions in maternal and infant composite outcomes or differential intervention effects for gestational weight.
The UK-based Healthy Eating and Lifestyle in Pregnancy (HELP) cluster RCT aimed to assess if a theory-based intervention during pregnancy for pregnant women with obesity could reduce women’s BMI score at 12 months postnatally by equipping women with knowledge and skills to make healthier choices for themselves and their unborn infants. 17 The women allocated to the intervention were offered a weekly 1.5-hour weight management group that combined expertise from Slimming World® (Alfreton, UK) with clinical advice and supervision from NHS midwives until 6 weeks postnatally. Secondary outcomes included weight gain in pregnancy, impact on diet, level of physical activity, mental health, social support, breastfeeding, and cost-effectiveness. Trial results have not yet been published.
Postnatal-only interventions
Findings from studies that have evaluated postnatal weight management interventions are equivocal. A Cochrane systematic review of diet and/or exercise for weight reduction in women after childbirth,22 in which 12 trials contributed data on 910 women to outcome analysis, found that women who exercised did not lose significantly more weight than women in usual-care arms (two trials, n = 53, mean difference –0.10 kg, 95% CI –1.90 to 1.71 kg) but women who took part in a diet (one trial, n = 45, mean difference –1.70 kg, 95% CI –2.08 to –0.132 kg) or a diet plus exercise programme (seven trials, n = 573, mean difference –1.93 kg, 95% CI –2.96 to –0.89 kg) lost significantly more weight than women in usual-care arms. Trials were included of women with obesity, overweight or who gained excessive weight in pregnancy, with trial recruitment taking place from 3 weeks to 24 months postpartum and interventions duration ranging from 10 to 24 weeks postnatally. Interventions were often delivered as a ‘package’, for example walking for a set time each day, social support and healthy cooking sessions. Only one trial was from the UK. Despite considerable heterogeneity owing to differences in the type or duration of the intervention and differences in the participants’ characteristics, the authors suggested that diet and exercise together rather than diet alone could help women to lose weight after giving birth because the former could improve their cardiovascular fitness level and preserve fat-free mass.
A systematic review on interventions to reduce postpartum weight retention across all BMI categories was carried out by van der Pligt et al. 7 Studies were selected for inclusion if postpartum weight was a primary outcome and diet and/or exercise and/or weight monitoring were intervention components. Women were recruited from 4 weeks to 12 months postpartum. Interventions were administered from 11 days to 9 months postpartum and included counselling, individualised physical activity plans, healthy eating groups and clinic visits. Of 11 studies selected for inclusion, 10 were RCTs and none were from the UK. Seven reported a decrease in postpartum weight retention, six of which included diet and physical activity delivered by health professionals. No study considered cost-effectiveness, with wide heterogeneity in approaches to how interventions were administered. Nevertheless, findings suggested that postnatal weight loss was achievable, although the best setting, approach to delivery, intervention duration and recruitment approach were unclear. Of note is that intervention retention rates in the majority of studies were high (> 80%).
A small RCT from Sweden23 of a dietitian-led postnatal intervention that targeted women 6 to 15 weeks postnatally with self-reported BMI scores of > 27 kg/m2 randomised 110 women to a diet behaviour modification arm or to a control arm the members of which were offered a healthy eating booklet. The intervention comprised a structured 12-week diet plan, supported by a dietitian, based on the Nordic Nutrition Recommendations and self-weighing three or more times per week. Outcomes included weight change after 12 weeks and at 1 year postpartum. At 12 weeks, median weight change in the intervention arm was –6.1 kg (range –8.4 to –3.2 kg) compared with –1.6 kg (range –3.5 to –0.4 kg) in the control arm (p < 0.001). Differences in weight loss were maintained at the 1-year follow-up, with high follow-up in both trial arms.
Evidence that weight management in a postpartum lifestyle intervention could impact on other health behaviours, including smoking cessation, was considered in a systematic review by Hoedjes et al. 24 Of 17 included studies, eight assessed effects on weight loss and nine on smoking cessation and relapse prevention. Of the weight loss studies, five reported significant effects of combined diet and exercise. Two of the four studies that assessed smoking relapse prevention found no evidence of effect. Four studies included interventions for both smoking prevention and prevention of relapse. One study found increased abstinence (5.9% vs. 2.7%, respectively) and reduced smoking relapse (45% vs. 55%, respectively) at 6 months’ follow-up in the intervention arm compared with the control arm, but effects were not sustained at 12 months. Compared with the control arm, one study reported significant effects on smoking cessation and smoking relapse prevention at 6 months and one study found a small benefit on smoking cessation, but not smoking relapse, at 6 months. One study found no evidence of differences between arms at 3 months’ follow-up. Although the authors recommended that existing postpartum lifestyle interventions could achieve weight loss, smoking cessation or prevent smoking relapse, caution was needed. There was wide variability in study methods, details of who completed study selection and data extraction were not provided and study quality was not assessed.
Evidence from weight management interventions in general population studies
Previous UK RCTs have assessed the clinical effectiveness of weight management programmes in primary care settings. 13 In one trial, 740 women and men who had obesity or overweight and a comorbid disorder were recruited from one primary care NHS trust that served a diverse population. Interventions of interest included weight management programmes of 12 weeks’ duration, including those provided by Slimming World, Weight Watchers (WW International, Inc, New York, NY, USA), Rosemary Online© (Digital Wellbeing Limited, Steyning, UK), group-based dietetics-led programmes and general-practice-led or pharmacy-led one-to-one counselling. Participants selected which programme they attended, with those in comparator groups offered 12 vouchers to access a local leisure centre free of charge. Weight loss at programme end was the primary outcome, with secondary outcomes including self-reported physical activity and weight loss at 1 year. Data for follow-up were available on 658 (88.9%) participants at programme end and 522 (70.5%) participants at 1 year.
All programmes achieved significant weight loss from baseline to programme end and all except general practice and pharmacy provision had better weight loss at 1 year than the control arm. The commercial weight programmes achieved better weight loss at programme end (mean difference 2.3 kg, range 1.3–3.4 kg) and were cheaper by ≈£40 per person than the primary care services. Cost data included costs of provider’s service, searches in general practice and sending letters of invitation. The lack of process evaluation coupled with the control arm receiving a different intervention (rather than standard care), which could have diluted the impact, limited the ‘learning’ from this trial. Nevertheless, it provided useful feedback on other potential benefits of commercial weight management programmes, including the positive impact of group dynamics and attending fleixibly timed and widely available weight management groups. The lack of appropriate training and time available to clinicians tasked with providing weight management advice could limit their potential role. 13
A more recent RCT25 considered the clinical effectiveness of an internet-based behavioural intervention with regular face-to-face or remote support from primary care health clinicians compared with brief advice. Individuals registered with general practices who had a BMI score of ≥ 30 kg/m2 (or ≥ 28 kg/m2 with risk factors) were recruited by postal invitation. Positive Online Weight Reduction (POWeR+) was a 24-session online weight management intervention completed over 6 months. Individuals were randomly allocated to the control intervention (n = 279), which comprised brief online information that encouraged swapping to healthier foods and increasing intake of fruit and vegetables plus 6-monthly nurse weighing, POWeR+ supplemented by face-to-face nurse support (POWeR+F) (up to seven contacts) (n = 269) or POWeR+ supplemented by remote nurse support (POWeR+R) (up to five e-mails or brief telephone calls) (n = 270). The primary outcome was average weight reduction over 12 months. A total of 818 eligible individuals were randomised. Weight change, averaged over 12 months, was documented in 666 participants (81%; control, n = 227; POWeR+F, n = 221; POWeR+R, n = 218). The control arm maintained nearly 3 kg of weight loss per person. Compared with the control arm, the estimated additional weight reduction with POWeR+F was 1.5 kg (95% CI 0.6 to 2.4 kg; p = 0.001) and with POWeR+R was 1.3 kg (95% CI 0.34 to 2.2 kg; p = 0.007).
By 12 months, mean weight loss was not statistically significantly different between arms, but 20.8% of control participants, 29.2% of POWeR+F participants (RR 1.56, 95% CI 0.96 to 2.51; p = 0.070) and 32.4% of POWeR+R participants (RR 1.82, 95% CI 1.31 to 2.74; p = 0.004) maintained a clinically significant 5% weight reduction. Maintenance of weight loss after 1 year was unknown and few (19/54) health-care staff participated in follow-up interviews. As this was an older (mean age 53 years) general population sample that included men and women, implications of the findings for women who have recently given birth are unknown.
Rationale
There is increased recognition of the importance of postnatal care as an opportunity to implement interventions to improve women’s shorter- and longer-term health, including weight management for women with high BMI scores. Evidence is accruing of the longer-term consequences on outcomes of future pregnancies and the health of their children if women with obesity or overweight at antenatal ‘booking’, or who gain excessive gestational weight, do not manage their weight. Prior to undertaking a definitive RCT of clinical effectiveness and cost-effectiveness, evidence was needed to see if such a trial could be undertaken. Evidence was needed of whether or not women who have recently given birth would be prepared to enter a trial of weight management, when would be an appropriate time to intervene to optimise postnatal weight management, the appropriate content of a pragmatic and accessible intervention for postnatal women in an inner-city area, if an intervention could have an impact on other positive health behaviours, and outcomes likely to be of most importance in a future trial.
Use of a commercial weight management programme
In line with the remit of the National Institute for Health Research (NIHR) Public Health Research (PHR) programme, which funds research ‘to generate evidence to inform delivery of non NHS interventions intended to improve the health of the public and reduce inequalities in health’, a non-NHS intervention had to be considered. 26
Following discussion with local women who had used different weight management approaches (including online resources), academic colleagues with expertise in evidence of use of commercial weight management programmes, evidence from UK general population studies13 and review of the content of the dietary and other advice offered by commercial programmes appropriate for the needs of postnatal women, Slimming World was considered to be the most appropriate intervention for several reasons. At the time of the funding application, there was some evidence that commercial weight management interventions were likely to be of more benefit than programmes provided by the NHS. 13 Indeed, work with a local patient and public involvement (PPI) group of women who had BMI scores of ≥ 25 kg/m2 to inform the original application highlighted that women did not want to attend NHS groups and felt that it was important that weight management support was offered in a peer-group setting where they had the opportunity to meet other people (and not necessarily only new mums).
Slimming World was the only commercial organisation that offered tailored support for postnatal women, including those who are breastfeeding. It has a flexible weight management and healthy eating programme, which includes a range of different food types, suitable for nursing mothers, including adequate calcium and fibre intake. Groups are standardised, and at the time of trial commencement there were over 12,000 groups available run by consultants who lived in the local area and would be aware of cultural and dietary influences. Each consultant undertakes evidence-influenced training in dietary aspects of weight management, physical activity and dietary change, and Slimming World implement rigorous quality control procedures and monitoring of group provision undertaken by the providers. If the feasibility trial achieved its aims, this meant that it would be possible to run as a multicentre trial, knowing that the intervention would be standardised and accessible to women across all settings. One issue was that Slimming World, as a commercial organisation, charges a range of fees to attend groups, determined by whether the individual is a new or an ongoing member, with a standard weekly fee of £4.95 (as of 1 December 2018). 27 To support the Supporting Women with postnAtal weight maNagement (SWAN) trial, Slimming World agreed to waive the fees for women allocated to the intervention. They also agreed that women in the control arm would be offered the chance to commence groups with fees waived for the first 12 weeks.
Chapter 2 Methods
Aim of the SWAN feasibility trial
This was a single-centre feasibility trial, the primary objective of which was to assess the feasibility of conducting a future definitive RCT to determine the clinical effectiveness and cost-effectiveness of lifestyle information and access to commercial weight management sessions for 12 weeks in relation to achieving and maintaining long-term postnatal weight management and positive lifestyle behaviour in women at risk of poor weight management in an ethnically diverse inner-city population.
This objective was supported and supplemented by the following secondary objectives:
-
assess recruitment/time to recruitment and retention
-
estimate the impact of lifestyle information and postnatal access to commercial weight management sessions on maternal weight change from first antenatal visit to 12 months postnatally
-
explore the influence of lifestyle information and postnatal access to commercial weight management sessions on secondary outcomes at 6 and 12 months, including weight management, diet, physical activity, breastfeeding, smoking cessation, alcohol consumption, physical and mental health, infant health, sleep patterns, body image, self-esteem and patient health-related quality of life
-
assess the acceptability of the intervention and trial procedures
-
assess resource impacts across different agencies likely to be of relevance and identify data appropriate for economic evaluation in a definitive RCT based on assessment of quality and completeness of economic data generated, a preliminary within-trial cost–utility analysis and review of evidence
-
decide if criteria to inform progression to a definitive RCT are met.
The methods for the main trial (to meet objectives 1–3) are described first, followed by the process evaluation (objective 4) and the health economic evaluation (objective 5).
Trial design
The SWAN trial was a single-centre, individually randomised feasibility trial that incorporated an integral mixed-methods process evaluation in line with Medical Research Council (MRC) guidance for developing complex interventions. 28 It had a target recruitment of 190 women who had a BMI score of ≥ 25 kg/m2 at antenatal booking or women who had a normal BMI score at antenatal booking but gained excessive gestational weight at 36 weeks’ gestation. It was a two-arm trial, with one arm allocated to receive standard care plus a lifestyle information leaflet and access to a commercial weight management group, commencing from 8–16 weeks postnatally, or standard care only (Figure 1).
FIGURE 1.
Trial flow diagram.
Participant eligibility
The following inclusion criteria were applied throughout participant recruitment.
Inclusion criteria
Women eligible to participate were those aged ≥ 18 years who could speak and read English, were expecting a single baby and had not accessed weight management groups in the index pregnancy.
Women who booked for their pregnancy care at the trial site were eligible to be randomised to the feasibility trial if they met the following criteria:
-
were aged ≥ 18 years
-
had sufficient understanding of spoken and written English
-
had no current diagnosis of major psychiatric disorder documented
-
had no known abnormality detected in the fetus
-
were not involved in another postnatal study (to reduce ‘burden’ of research participation)
-
had no identified medical complications (e.g. cardiac disease, type 1 diabetes)
-
had no identified eating disorders
-
had not undergone previous surgery for weight management.
Exclusion criteria
Women who did not fulfil all of the inclusion criteria were not included in the trial.
Sample population
All women who met the inclusion criteria were considered eligible to participate in the trial.
Trial setting
One NHS maternity unit in an inner-city area in the south of England. The unit provides maternity care to women living in one of the most densely populated boroughs in the UK, with high proportions of people of black African and black Caribbean ethnicity, some of the most disadvantaged populations in the UK and high levels of mobility and migration. 29 When trial funding was awarded (in 2015), 54% of the postnatal population of women who gave birth at the trial site were of white British ethnicity, 36% were women of black African or black Caribbean ethnicity and 3% were of South Asian ethnicity. In 2017, when trial recruitment was in progress, of over 7000 births at the trial site ≈50% of women were of white ethnicity, 34% were of black ethnicity and around 5% were of South Asian ethnicity. Of the women who booked for their maternity care at the unit in 2017, 15% had BMI scores classed as obese and 25% had BMI scores classed as overweight. There were lower rates of exclusive breastfeeding at 6–8 weeks among women of black Caribbean ethnicity and women of mixed Caribbean ethnicity, and around 3% of women reported that they smoked during pregnancy.
Information for women and obtaining informed consent
We used two approaches to inform women about the trial. The first was for women who had BMI scores of ≥ 25 kg/m2, who were identified from their antenatal booking information for the index pregnancy on the maternity administration system. At approximately 26 weeks of pregnancy, all identified women in this group were sent a trial letter (see Report Supplementary Material 1) that briefly explained the trial aims and advised women that a research midwife would be in contact to explain the trial further. The letter also explained how the woman could contact the team if she did not want to receive further information. Two weeks after sending the letter, the research midwife contacted women who did not ask to be removed from the contact list to explain the trial in more detail.
The second approach was for women who had a normal BMI score at antenatal booking but gained excessive weight during pregnancy as assessed against IoM criteria. 5 Women were offered the opportunity to self-refer or were referred by their midwife or obstetrician to the research midwives; posters and postcards placed at the trial site advertised the trial. Women who were interested were asked to contact the research midwives to arrange to be weighed at around 36 weeks’ gestation (or at their next nearest antenatal appointment) as routine weighing is not recommended in current NHS maternity care. 6 As women in the normal BMI score group did not respond to this approach after 3 months of recruitment, the protocol was revised and ethics approval obtained to send opt-out letters, similar to those being used for women who had a BMI score of ≥ 25 kg/m2 at antenatal booking, during the final 2 months of the planned recruitment period (see Report Supplementary Material 2). Letters were then sent to the women with normal BMI scores at antenatal booking at around 32–34 weeks’ gestation of pregnancy.
All women identified using these approaches were offered a patient information sheet (PIS) by the research midwives prior to seeking their written informed consent at around 36 weeks’ gestation (see Report Supplementary Material 3). If, after reading the PIS, women were willing to participate in the trial, informed consent was obtained. The PIS made it clear that women were free to withdraw from the trial at any time for any reason without prejudice to their future care and were under no obligation to give a reason for withdrawing from the trial. Women interested in participating were met by the research midwives at the trial site (in the obstetric unit or a community clinic) to obtain consent and complete the baseline questionnaire (see Report Supplementary Material 4). Consent was obtained by the research midwife with delegated authority from the principal investigator at the trial site. Consent comprised a dated signature from the woman and a dated signature from the research midwife. A copy of the signed informed consent document was given to the woman, a copy retained in the woman’s medical records and a copy retained in the trial site file.
Intervention arm
Women were allocated to receive standard care (see Control group), plus a positive lifestyle information leaflet (see Report Supplementary Material 5) at around 36 weeks of pregnancy on recruitment to the trial and access to 12 commercial weight management sessions provided by Slimming World, which they could commence at any time between 8 and 16 weeks after giving birth. Women could choose which group they attended, the day, date and time of attendance and when they commenced groups to fit in with their postnatal recovery, lifestyle and family demands. They were able to take their babies with them to the groups, an important consideration for any postnatal intervention.
Joining the commercial weight management group
As the intervention was to commence 8 to 16 weeks postnatally, it was important that women were reminded about the trial offer. Processes for commencing the commercial weight management intervention included a ‘welcome’ leaflet from Slimming World about joining sessions, which was given to all women allocated to the intervention arm, a ‘congratulations’ text from the research midwives on notification of the birth of the baby and reminder texts sent by the research midwives from 6 to 8 weeks postnatally about joining Slimming World. From 8 to 16 weeks postnatally the women were asked to call Slimming World member services to speak to a consultant who could register the woman, advise her where her nearest groups were located, the venues and dates/times of groups and the name of the local consultant running the group. Following this first call, if a woman was not yet ready to join (e.g. if she felt unwell), she was asked to call Slimming World member services again when she felt ready. At this call, women were asked to provide their height and current weight.
All women were given a Slimming World trial identifier (ID), which was used to ‘track’ the woman through each group she attended, including her weight as taken at each group session. This also enabled women to be tracked who attended groups beyond the trial offer and the extent to which women moved between different groups. In line with all members of Slimming World, women had a ‘swipe card’ that included their free access to up to 12 group sessions and their recorded weekly weight information and date, time and group attended. This approach also meant that women were treated as a ‘regular’ Slimming World attendee, with no potential to be identified as receiving the offer as part of a research trial.
Content of the commercial weight management group
The content of Slimming World group programmes is underpinned by behaviour change models and groups that are homogeneous with respect to content and delivery. 30 Behaviour change techniques are supported by social cognitive theory, with a focus on motivation and self-efficacy for weight management and reducing relapse from the programme. Key techniques include goal-setting, self-monitoring, social support and positive reinforcement. 31,32 Consultants receive standardised training overseen by Slimming World dietitians and nutritionists, which includes motivation to support positive lifestyle changes to manage weight, nutrition and food facts and information on the role of exercise and activity in health and weight management. Consultants repeat training every 2 years to remain up to date with the latest evidence and attend a local programme of safeguarding training approved by the NHS. Groups follow a standard format, starting with a weigh-in for members and an introduction to the programme for new members, followed by a whole-group discussion that includes discussion of group member’s experiences of weight management to help change habits and share healthy swaps (i.e. healthy alternatives to common food choices) and recipe ideas. Sessions can include basic cooking skills, taking cost, cultural preferences and time constraints into account. A food optimising system encourages adherence to healthy eating, and physical activity encouragement includes facilitation of behaviour change, redefining what ‘activity’ can include. The diet includes a combination of different food types: ≈80% combined from fruit, vegetables, carbohydrates and protein; a smaller proportion of calcium and fibre-rich foods; and an allowance for foods high in fat or sugar. Other than limiting the intake of high-fat or high-sugar foods, Slimming World does not promote a ‘restrictive’ diet.
Women allocated to the intervention were offered (with fees waived) the standard membership, namely attendance for 12 sessions run over 14 consecutive weeks to allow 2 ‘holiday’ weeks within the 12-session offer. Slimming World guidance is that, to achieve a 5% loss in weight from baseline (a difference considered to improve health outcomes), individuals need to attend at least 10 out of the 12 group sessions. Attendance was considered in the process evaluation in terms of whether women ‘attended’ for a whole group session or only attended a group session to be weighed, and if women attended ≥ 10 sessions, six to nine sessions, one to five sessions or no sessions at all. On completion of the 12-session offer, women could continue to attend weekly sessions, but Slimming World standard fees would apply.
Lifestyle information leaflet
As postnatal health planning should start in pregnancy,33 an evidence-based positive lifestyle leaflet reflecting current NICE public health guidance for women on breastfeeding, diet, smoking cessation/prevention of relapse, reducing alcohol and managing sleep11,33 was offered following recruitment and allocation to the intervention at 36 weeks’ gestation (see Report Supplementary Material 5). The leaflet was developed with the trial Expert PPI Group and written to comply with Plain English Campaign guidance,34 using pictures and tick/cross messages to enable women of all reading abilities to understand the content. Local women we consulted when developing the trial highlighted that this information was not routinely offered to women, despite recommendations that it should be. 6,33 The research midwives were asked to go through the leaflet with the woman and discuss the content with an emphasis on why the information could support women to adopt practices likely to be of benefit to their own health and the health of their infants.
Control group
Women allocated to standard care received standard NHS maternity care for 8 weeks postnatally prior to discharge from maternity care. This could include, for example, routine midwifery and health visitor contacts for infant-feeding assessment, monitoring of recovery from the birth, commencement of the infant immunisation programme, routine assessment as part of the Healthy Child Programme, parenting interventions and other contacts with the family as determined by need. A routine contact with their general practitioner (GP) at around 6–8 weeks postnatally is usually offered. On completion of trial follow-up at 12 months, women allocated to the control arm were able to take up the same Slimming World offer as the intervention arm, namely access to 12 group sessions over a 14-week period with fees waived.
Monitoring of adherence to allocation
Monitoring of adherence to allocation was used to inform feasibility in terms of whether or not women allocated to the intervention arm would attend a commercial weight management group after giving birth and, if so, how many attended the full programme (attended ≥ 10 sessions). Data were provided by Slimming World on women’s initial and ongoing adherence to the group programme, which included weekly data on women’s attendance at groups, if they stayed with the same group or changed groups (which women could do, in line with any ‘standard’ Slimming World member) and their weekly weight. Data on women in the trial who attended groups were sent in a password-protected Microsoft Excel © (Microsoft Corporation, Redmond, WA, USA) file on a weekly basis to the trial team. In addition, all women allocated to the intervention arm were sent a copy of a ‘log’ (see Report Supplementary Material 6) that they were asked to complete regarding attendance and duration of attendance at each session (e.g. whether they just went to get weighed or also stayed for the group session) as these data are not collected by Slimming World. On completion of the offer, they were asked to return the log to the trial office using a pre-paid postage envelope.
Standardisation of the weight management groups that women attended was overseen by Slimming World. To capture data on women’s experiences of adherence to allocation, women allocated to the intervention arm were asked in the follow-up questionnaires (see Report Supplementary Material 7 and 8) if they did or did not attend any groups, what informed their decision, how many groups they attended, if they stayed for each session in full or left after being weighed (which takes place at the start of each group meeting), if they found groups helpful and if they continued to attend groups after the 12-session offer. These issues were further explored with women who were interviewed immediately post intervention and again at 12 months post birth.
In follow-up questionnaires women in both trial arms were asked what other support they had accessed to help them manage their postnatal weight, and women allocated to the control arm were specifically asked about the timing of their access to additional support (to further inform the timing of commencement feasibility issue), and if they had joined Slimming World, to enable any potential contamination between trial arms to be assessed.
Randomisation
Randomisation and allocation were carried out using the InferMed MACRO (Elsevier, Amsterdam, the Netherlands) web-based data entry system hosted by King’s College London Clinical Trials Unit (www.ctu.co.uk; accessed 25 November 2019). Women were registered on the InferMed MACRO (Elsevier, Amsterdam, the Netherlands) web-based data entry system by the research midwife prior to randomisation to allocate each a unique trial patient identifier number (PIN). The research midwife accessed the system and, using the PIN, initials and date of birth, requested randomisation. E-mail confirmations were automatically generated. The unit of randomisation was individual participant, allocated in a ratio of 1 : 1 to intervention and control. Use of a web-based system protected against allocation bias as neither the women nor the research midwives were aware of the randomisation sequence or codes. Selection bias was minimised by ensuring that all women eligible and recruited had equal opportunity of being allocated to each trial arm. Use of intention to treat (ITT) analysis limited attrition and analytical bias. It was not possible to ‘blind’ the research midwife or women to allocation, but those responsible for analyses were blinded to allocation.
Trial feasibility objectives
The aims of the quantitative evaluation were to meet objectives 1, 2, 3 and 5. These objectives included:
-
recruitment/time to recruitment and retention
-
impact of lifestyle
-
information and postnatal access to commercial weight management groups on maternal weight change from first antenatal (booking) visit to 12 months postnatally
-
influence of lifestyle information and postnatal access to commercial weight management groups on secondary outcomes at 6 and 12 months
-
resource utilisation and costs outcomes.
Proposed primary outcome
The proposed primary outcome for subsequent study was difference between trial groups in weight 12 months postnatally, expressed as percentage weight change and weight loss from the woman’s antenatal booking weight. This outcome was supported by the trial Expert PPI Group, who considered that this would be viewed positively by women, as it did not suggest that they should reach their pre-pregnancy weight.
Secondary outcomes
Data using the following measures were collected at baseline (36 weeks’ gestation) and 6 and 12 months following birth (see Report Supplementary Material 4, 7 and 8). Selection of measures enabled hypothesised shorter- and longer-term outcomes that could result from a postnatal weight management intervention to be assessed. Further information on the measures and why measures were selected for use in the trial are included in Chapter 5.
-
Dietary intake: the Dietary Instrument for Nutritional Education (DINE) (University of Oxford, Oxford, UK);35 questions on soft drinks intake were developed for the trial.
-
Physical activity: the International Physical Activity Questionnaire – Short Form (IPAQ-SF). 36
-
Mental health: Edinburgh Postnatal Depression Scale (EPDS). 37
-
Breastfeeding intent, uptake and duration: questions developed for the trial.
-
Sleep patterns: questions developed for the trial.
-
Smoking: smoking status/cigarette dependence. 38
-
Alcohol consumption: Alcohol Use Disorders Identification Test. 39
-
Self-esteem: Rosenberg Self-Esteem Scale. 40
-
Infant health: questions developed for the trial.
-
Impact on body image. 41
-
Resource utilisation and costs outcome measures: the EuroQol-5 Dimensions, five-level version (EQ-5D-5L), and the Adult Service Use Schedule (AD-SUS). 42
Data collection schedule
Information at trial entry, including eligibility and relevant maternal and obstetric characteristics (antenatal booking BMI score, parity, age, ethnicity and deprivation score), was obtained from each woman’s maternity records and entered into the trial database. A baseline questionnaire that included the measures described in Secondary outcomes was completed by women at their recruitment appointment at 36 weeks’ gestation. Following the birth, data were obtained from women’s maternity records on mode of birth, infant outcomes (gestation and infant birthweight) and duration of inpatient postnatal stay. At 6 and 12 months, women were invited to meet with the research midwives at the trial maternity unit, or arrange an appointment for the research midwife to see them at home, to be weighed (women were asked to remove their shoes) and to complete, during the appointment, a self-administered questionnaire that included the same measures as included in the baseline questionnaire, with the addition of questions on body image, infant health, use of NHS primary and secondary health-care services by the woman and/or her infant and use of weight management support interventions. Women who opted for an appointment at the trial unit were sent an off-peak travel card to cover costs of attending the two appointments. If women were unable to attend an appointment for whatever reason, they could complete the questionnaire and return it to the trial office by post. In these cases, women were asked to weigh themselves (after removing their shoes) and record their current weight in the questionnaire. In terms of acceptability of trial procedures, completion rates of measures included in the questionnaires at each time point were also considered.
An overview of time points at which trial data were collected is presented in Table 1.
| Data collection | During pregnancy | Immediately after birth | Intervention-only group | 6 months | 12 months | Completion of data collection instrument |
|---|---|---|---|---|---|---|
| Eligibility screen | ✓ | Completed by research midwife from assessment of women’s maternity records | ||||
| Contact sheet at 28 weeks’ gestation | ✓ | Completed by research midwife following telephone call with women | ||||
| Baseline health measures: recruited women only | ✓ | Self-administered questionnaire completed by women | ||||
| Women’s and infants’ labour and birth data | ✓ | Completed by research midwife on all women who consented to participate | ||||
| Health questionnaire | ✓ | Self-administered questionnaire completed by all trial women | ||||
| Log book of weight management session attendance | ✓ | Self-administered and completed by women in the intervention arm | ||||
| Interviews with women allocated to the intervention arm | ✓ | Completed by researcher from SWAN trial team | ||||
| Health questionnaire | ✓ | Self-administered questionnaire completed by all trial women | ||||
| Weight measurement | ✓ | ✓ | Research midwife follow-up appointment/document in self-administered questionnaire; all trial women | |||
| Interviews with women from both trial arms | ✓ | Completed by researcher from SWAN trial team |
Sample size
The proposed sample size was 190 women. This was to allow a 30% loss to follow-up to ensure that we achieve the required sample size of 130 women. The trial was designed to establish the rates at which women could be recruited and retained in a future definitive RCT and estimate critical parameters with necessary precision to inform sample size requirements. In particular, we required estimates of the SD and design effect for the proposed primary end point, allowing for clustering by intervention arm. A total of 130 women would enable estimates of the required sample size for any given clinically important difference to within 30% of the true value. Based on published data,13,30 the mean percentage weight change following a Slimming World programme of 12 weekly groups is –5.5% (SD 3.3%). Assuming that these numbers are typical, 65 women in each group (130 in all) would be required to detect a difference of 2% between active and control arms with 90% power. At the time of submitting the funding application (in 2014), of the ≈6600 women who gave birth at the reference maternity unit over 12 months, 40% had a BMI score of ≥ 25 kg/m2, 15% of whom had a BMI score in the obese range (≥ 30 kg/m2). Data on women with EGWG were not routinely collated. Potentially 55 women booking each week would meet obese/overweight BMI score inclusion criteria. Recruiting seven to eight women each week over an 8-month (32-week) period was considered sufficient to achieve the desired sample size to meet the aims of this feasibility trial.
Trial process evaluation objectives
The process evaluation met objective 4 of the feasibility trial. The aims of the process evaluation were to assess:
-
the acceptability of the intervention and how the intervention was experienced by women, including views on timing of commencement
-
the likely variation in groups attended by women (day/time; whether or not women changed groups/consultant)
-
timing and sources of additional weight management support, including risk of contamination
-
the acceptability of trial processes and procedures.
Trial feasibility objectives reflected MRC guidelines for process evaluation of complex interventions43 with some important exceptions owing to the nature of the trial and intervention proposed. The purpose was not to evaluate the intervention itself as Slimming World weight management groups are a ‘standardised’ intervention, with robust mechanisms to ensure intervention fidelity. Owing to the robust in-built quality assurance and evidence base for the intervention, process evaluation was not designed to answer some standard questions seen in complex evaluations regarding the generalisability of the intervention to other contexts/settings, to provide assurance that implementation/delivery of the intervention has been consistent across trial sites, or to determine mechanisms of impact. This trial reflected a pragmatic trial approach, evaluating the impact of the intervention in the hands of many, where women can choose which group to attend and can switch groups if they like, exactly as they could if they were a ‘standard’ self-referred member of the commercial weight management group.
The acceptability of the intervention and trial procedures was evaluated through brief questions included in the 6- and 12-month questionnaires and through semistructured interviews with women allocated to the intervention arm at 6 months (on completion of the commercial weight management offer) and at 12 months with all women. The probable variation in groups attended by women was assessed using data provided by Slimming World on the groups attended by women (each session attended had a ‘group’ and ‘consultant’ code, and group codes had information regarding the day of the week and timing of the group), and the timing and sources of additional weight management support was assessed through questions in the 6- and 12-month questionnaires.
Postnatal questionnaires at 6 and 12 months
The content relevant to the process evaluation was included in the final section of the questionnaires (see Report Supplementary Material 4, 7 and 8). This comprised seven or eight questions (intervention arm at 6 and 12 months, respectively) or five questions (control arm); some were quantitative (binary/categorical questions) and some were free-text/open questions. The questions were the same at both time points (although at 6 months the option was given to women allocated to the intervention arm to answer that they were part-way through the commercial weight management intervention).
For women allocated to the intervention arm, the questions included a section about attendance at the commercial weight management groups, asking if they attended any sessions and, if so, how old their baby was when they attended their first group; how many sessions they had attended in total; and if they stayed for the whole-group session or just went to get weighed. Two free-text questions asked women to add an explanation regarding why they left sessions without staying for the whole session (if relevant) and how useful they found the commercial weight management sessions. Women who had not attended any sessions were asked to explain why they chose not to attend.
All women (women allocated to the intervention arm and women allocated to the control arm) were asked if they had sought any support to help manage their weight since having their baby. If they responded ‘yes’, they were asked to indicate the type of support (categorical question) and to comment on how useful they had found the support. Women allocated to the control arm were specifically asked whether or not they had joined Slimming World and were also asked about the ‘timing’ at which they sought the support (how many months postnatally).
Interviews
Interviews were conducted with 13 women allocated to the intervention arm immediately post intervention (approximately 6 to 8 months post birth) and with nine women allocated to the intervention arm (six of whom had participated in 6-month interviews) and eight women allocated to the control arm at 12 months post birth.
Diversity of the sample was considered to be more important than the number of interviews (in line with guidance about qualitative research in feasibility RCTs44), particularly as analysis regarding the acceptability of the intervention and trial procedures would also involve integration of analysis of the textual data from completed questionnaires. For these reasons we estimated that interviews with 14–20 women allocated to the intervention arm across the two time points and 8–12 women allocated to the control arm would be sufficient.
Purposive criteria for selecting women to be invited for interview immediately post intervention aimed to capture diversity in the sample in relation to attendance at the commercial weight management sessions (including women who attended ≥ 10 sessions and those who attended fewer or none at all), weight change and ethnicity.
For the interviews at the end of the trial (12 months postnatally), the same criteria were applied for women allocated to the intervention arm (including some women interviewed at 6 months to provide a longitudinal sample) and women allocated to the control arm, aiming to include diversity in relation to weight change and ethnicity. Interview topic guides are included in Report Supplementary Material 9.
Procedure
Women who met the purposive criteria were identified by the research midwives and given or sent a separate trial PIS to consider their participation in the trial (see Report Supplementary Material 10 and 11). If a women was willing to participate, written informed consent was taken by the research midwives and her contact details were confirmed and given to the researcher conducting the interviews (VB). The researcher contacted each participant to arrange a mutually convenient time for the interview, all of which were conducted over the telephone (although face-to-face options were offered). The opportunity to ask questions was provided ahead of the interview and consent was reconfirmed verbally at the start of interviews.
Interviews immediately post intervention explored motivations for participating in the trial and experiences of participating in the intervention (and also of using the lifestyle leaflet). Interviews with both groups at the end of the trial (at 12 months postnatally) were used to explore trial processes and experiences of participating, including reasons for taking part/dropping out, recruitment and randomisation (expectations/understanding of the trial and its aims), views on outcome measures, attendance for weighing appointments as part of trial follow-up and lifestyle behaviours. They were also used to explore knowledge of weight management and the role of diet and exercise in this.
For women allocated to the intervention arm, weekly data on number of weight management groups attended were obtained from Slimming World. For each woman allocated to the intervention arm, this included weekly data on their attendance, including a code for the group, for the consultant and the woman’s weight. These data enabled analysis of variability in groups attended by women and analysis of whether they stayed with the same group or changed groups (which women were free to do, in line with other Slimming World members) and of their weight change over the intervention period. It also provided information on whether women continued to attend beyond the trial period. The interviews lasted ≈45 minutes and were digitally recorded with participants’ permission.
Governance
Ethics arrangements
Favourable ethics approval for the trial was granted by the Health Research Authority (HRA) London – Camberwell St Giles Research Ethics Committee (REC) on 2 September 2016 (reference number 16/LO/1422) and HRA approval was received on 11 October 2016. Approval was obtained from the research and development department of the participating hospital. Table 2 provides details of the substantial amendments to the protocol approved by the REC. The research and development office was notified of all amendments after REC approval was received.
| Amendment | Date (version of protocol, if revised; revision date) | Description of main items in the request for approval |
|---|---|---|
| Substantial amendment 1 | 19 April 2017 (5; 29 January 2017) |
Revision to wording of postcard for recruiting women who had normal BMI scores at antenatal booking but gained excessive gestational weight. Letter about the trial to be sent to women with normal BMI scores at antenatal booking Provision of a log book for women who attended the commercial weight management groups to complete after each group meeting to provide information on whether they stayed for the whole group session or left after being weighed Revised questions about attendance at weight management groups in the 6- and 12-month questionnaires (control and intervention arms) |
| Non-substantial amendment 1 | 15 May 2017 (6) | To extend period of participant recruitment from 6 to 8 months; to focus on recruitment of women with EGWG |
| Substantial amendment 2 | 4 January 2018 (7; 13 August 2017) | It was originally planned to collate data to consider probable variation in characteristics of the commercial weight management groups in relation to both characteristics of the groups (date/time of day, size of group) and characteristics of group members [proportion of members who reached their target weight, demographics of group members (age, sex, postcode)]. Further discussions with Slimming World highlighted that these data were not routinely collected and they would not support data collection specifically for the feasibility trial |
| Non-substantial amendment 2 | 16 March 2018 | Request for a trial end date extension to 30 November 2018 following approval from the funder (NIHR) |
Trial governance
Trial Steering Committee
The Trial Steering Committee (TSC) included an independent chairperson, three independent professional members (a professor of midwifery, a statistician and a researcher with expertise of studies in obesity and weight management) and a patient representative. Non-independent members included the chief investigator. Membership of the committee was approved by the NIHR PHR Committee. The TSC agreed a charter at the first meeting, which was informed by MRC guidance for Clinical Trials Units. 45 The TSC met four times, with the final meeting held to discuss trial findings and TSC views and recommendations with respect to progressing to a definitive RCT. As this was not a Clinical Trial of an Investigational Medicinal Product, an independent data monitoring committee was not required to oversee the safety of participants in the trial and the TSC took overall responsibility for the conduct of the trial.
Serious adverse event reporting
Although no serious adverse events (SAEs) were anticipated, it was possible that these could have occurred and a system for reporting these promptly was required. All SAEs occurring during the trial observed by the investigator or reported by the participant, whether or not attributed to the trial, would be reported on the data collection form. SAEs considered to be related to the trial by the investigator would have been followed up until resolution or the event was considered stable. All related SAEs that resulted in a participant’s withdrawal from the trial, or were present at the end of the trial, would have been followed up until a satisfactory resolution occurred. No SAEs were reported.
Data handling, checking, cleaning and processing
Data collection forms, including baseline and 6- and 12-month questionnaires completed by women and consent forms for trial participation and participating in a trial interview, were returned to the trial office and date stamped. Data files received via password-protected e-mails from Slimming World were stored electronically on designated password-protected trial computers. All data were entered by the research midwives onto a bespoke trial database set up by MedSciNet (https://medscinet.com; accessed 6 November 2019). MedSciNet ran validation checks for missing data and inconsistencies in data capture. Validation errors were queried with the research midwives and chief investigator. Any errors on the women’s questionnaires were not queried with the women.
Patient and public involvement
When the commissioned call for this research was launched, we worked with a group of local women who were previously participants in a NIHR Health Technology Assessment-funded pregnancy weight management trial. 20 Based on these women’s advice and experience of weight management support during pregnancy, it was clear that a non-NHS peer-supported intervention that was flexible and to which babies could be taken was perceived as more appropriate than an NHS-provided intervention. It was also apparent from these women’s feedback that we needed to initially assess whether or not this trial could be undertaken. We advertised for local women who had given birth at the trial site and had experienced weight management issues around the time of pregnancy to join the trial Expert PPI Group to inform all stages of the work. Four women came forward to join us.
When the research team was assembled, we invited the National Childbirth Trust (NCT) (www.nct.org.uk; accessed 6 November 2019) to work with the team and included funding for a dedicated research post at the NCT to take the lead for planned qualitative work. The NCT were also asked to provide ongoing support for an ‘expert’ group of local women who would meet alongside the Core Project Team (CPT). Sarah McMullen, Head of Research at NCT, joined as a co-applicant, and her colleague Vanita Bhavnani was employed on behalf of the trial to arrange and conduct trial interviews and provide ongoing support for the trial Expert PPI Group. Both Sarah McMullen and Vanita Bhavnani and the trial Expert PPI Group commented on the intervention lifestyle behaviour information leaflet, PISs and women’s questionnaires and were involved in all stages of trial development, including the drafting and writing of this report and plans for dissemination activities.
Chapter 3 Analysis plan
The main analysis is considered (in relation to objectives 1–3) in this chapter and in Chapters 4 and 5, followed by the analysis for the process evaluation (objective 4) (see Chapter 6) and the health economic analysis (objective 5) (see Chapter 7). Implications for a future definitive RCT (objective 6) are then described (see Chapter 9).
A data analysis plan (see Appendix 2) was written and approved by the TSC at the commencement of trial recruitment and prior to commencing trial analysis.
Losses to the trial post randomisation were defined as women for whom:
-
valid consent was not obtained
-
consent to use their data was withdrawn.
Women were able to specify whether or not data collected up to the point of withdrawal could be used. If a woman’s response was ‘no’, she would be categorised as excluded post randomisation; if a woman’s response was ‘yes’ (i.e. data collected up to the point of withdrawal could be used), data would be reported as ‘missing’ for all subsequent outcomes. Numbers of exclusions are reported by randomised treatment group.
For the primary analysis, women were analysed in the arms to which they were randomly allocated. Outcomes of women allocated to the intervention were compared with those who were allocated to receive standard care only. The unit of analysis for all outcomes was the woman.
Descriptive analysis
The Consolidated Standards of Reporting Trials (CONSORT) diagram (see Figure 3) shows the flow of participants through each stage of the trial. The number of women analysed for primary assessment is also reported, as are numbers for the 12-month follow-up, including number of women lost to follow-up. Only two women withdrew: one from the control arm at 6-month follow-up and one from the intervention arm at 12-month follow-up. Neither woman asked for her data to be withdrawn. The total number of eligible women who were approached but declined participation is also reported.
Recruitment was assessed as the number of women randomised per month from the trial centre, with 95% CIs derived from the Poisson distribution. Retention was assessed as the proportion of women randomised who provided complete analysable data for primary assessment. Linear regression was used for the primary end point and other continuous measures. Where data were available, adjustment was made for corresponding measurements made pre randomisation using the Bulk Centile Calculator version 6.2 (Gestation Network, Perinatal Institute, Birmingham; www.gestation.net). Binary regression with a log link was used to assess RRs for all binary (yes/no) outcomes, adjusting for the most important potential confounders: maternal BMI score, ethnicity and parity. Following the most recent CONSORT guidelines and additional recommendations,46 risk differences were also estimated.
Significance tests were carried out to test only for differences in drop-out rates between trial arms and for estimates of treatment effects. No formal interim analysis was planned because the results of this feasibility trial were to be used to decide if a definitive trial could be undertaken.
Reduction of weight by 5% and 10% were analysed as binary variables, with RRs and risk differences presented. Maintenance of EGWG was defined as a BMI score at 12 months postpartum > 1 kg/m2 above estimated pre-pregnancy weight. Baseline measurements of aspects of healthy lifestyle and health as assessed by questionnaire at 6 and 12 months were used in the analysis as a covariate. 46
We also planned to undertake pre-planned subgroup analysis of the primary end point in women of different antenatal booking BMI categories [overweight (BMI 25–29.9 kg/m2), obese (BMI ≥ 30 kg/m2) and normal (BMI 18.5–24.9 kg/m2)] at antenatal booking who gained EGWG when weighed at 36 weeks. Interaction tests were used to determine if the treatment effect varied by subgroup.
Per-protocol analysis
To explore if women who received the intervention as intended (e.g. attended ≥ 10 weight management sessions) were more likely to have greater weight loss at 12 months postnatally than women attending < 10 groups or women in the control arm, we conducted per-protocol analyses to assess if there was a ‘dose effect’ on this outcome. We also assessed if women who did not attend 6- or 12-month follow-up appointments with the research midwives to be weighed but recorded and documented their own weight in postal questionnaires had different weight change than women who attended appointments.
Statistical software
Stata® version 15 (StataCorp LP, College Station, TX, USA) was used for all quantitative analyses.
Reliability
Data on women’s eligibility to participate, including their antenatal booking BMI score, were obtained from the woman’s maternity records, with primary end-point data (maternal weight at 12 months) obtained when women attended pre-arranged contacts with the research midwives or returned self-administered questionnaires with their self-recorded weight. Data recorded on women’s weight at each session attended were provided to the researchers for all women at weekly intervals until 12 months postnatally to enable assessment of sustainability and how long women continued to attend sessions.
Protocol violations and deviations
Failure to comply fully with the final trial protocol as approved by the REC or research department, such as non-compliance with the protocol resulting from error, fraud or misconduct, is deemed to be a protocol violation. A protocol deviation is a departure from the final trial protocol as approved by the REC. There was one protocol deviation: one woman in the control arm was offered information in error on how to join Slimming World at 6 months, rather than at 12 months, when she should have been offered the information.
Trial process evaluation
Data from questionnaire responses and interviews were first analysed separately before being integrated to answer the research questions and meet the aims of this part of the trial.
The analysis of interview data and of the free-text responses in the questionnaires was underpinned by the capability, opportunity, motivation and behaviour (COM-B) framework for understanding behaviour and behaviour change. 47 The model proposes that for someone to engage in a particular behaviour they must be physically and psychologically ‘capable’ of performing the behaviour, have the social and physical ‘opportunity’ to carry out the behaviour and be ‘motivated’ (by both reflective and automatic mechanisms that activate or inhibit behaviour) to carry out the behaviour. The COM-B framework is proposed as a simple starting point to understand behaviour because it is comprehensive, parsimonious and applicable to all behaviours.
Questionnaire analysis
Quantitative responses from the questionnaires were analysed descriptively. Free-text questions were read in full by two members of the research team, who independently noted key themes. The thematic framework was agreed and text was coded and labelled according to the three dimensions of the COM-B framework. Data were then examined using frameworks to compare patterns between different groups of women and their responses in relation to capability, motivation and opportunity (e.g. comparing women who attended different ‘doses’ of sessions).
Interview analysis
All interviews (intervention and control) were transcribed verbatim. First, transcripts were read and re-read to ensure familiarisation with the data. Second, interview data were organised and coded using the COM-B framework, initially by two researchers independently (CT and VB). Coding was compared and discussed before all data were coded. As the elements of the COM-B framework are inter-related (not mutually exclusive), data often fit several of the dimensions and, therefore, multiple codes were applied where appropriate. Coding of the remainder of the data (by VB) continued independently and a summary of all coded data was presented in a framework matrix.
Two members of the research team (CT and VB) reviewed and discussed the coded summary data in the frameworks and compared data with and between women to identify themes and patterns in and between different women, including comparisons according to the number of sessions (fewer than six, six to nine or ≥ 10 sessions) they attended. Comparisons were also made on the basis of weight change between antenatal booking weight and weight at 6 and 12 months. This enabled the identification of key factors influencing engagement with the intervention and behaviour change. Similarly, researchers compared the coded summary data and identified themes and patterns from interviews with control arm women in relation to weight loss or gain at 6 and 12 months to allow for comparison with women allocated to the intervention arm. An example framework is provided in Appendix 1.
Integration of process data from questionnaires and interviews
Questionnaire data were coded to indicate if the women also participated in interviews so that ‘double counting’ could be avoided. Themes were compared across both samples. The final thematic framework applied to both sets of data. Interview and questionnaire data are presented according to theme, with attention paid to instances where one of the data sets expanded on or contradicted the other data set (or if one data set was ‘silent’). This was the case sometimes for the questionnaire thematic data set owing to the specific nature of the questions asked.
Trial health economic analysis
The economic analysis to meet objective 5 was carried out principally from an NHS payer and provider perspective, although some service items included in the cost analysis (e.g. smoking cessation services, social worker and housing worker contacts) are paid for through local government authority budgets. The weight management programme evaluated in the feasibility trial was delivered by a private for-profit organisation and is paid for privately by those who enrol, but trial participants were offered free access. The standard costs include a one-off enrolment fee plus a weekly attendance fee. Costs of enrolment were included in the cost-effectiveness analysis on the basis that any future commissioning of the programme would potentially be paid for through either NHS or local authority commissioning budgets.
Service contacts
Service contact data were collected using an adapted version of the AD-SUS42 used to evaluate antenatal psychological interventions for women with mental health problems. 48 The SWAN version of the AD-SUS asked trial participants to self-report the number of contacts made with a prespecified list of community-based health and social care services, hospital outpatient services, accident and emergency (A&E) departments and number of admissions to hospital. An open-ended question also allowed for service contacts not covered by the prespecified services list to be recorded. The AD-SUS was initially administered as part of the baseline questionnaire in which women were asked to report service contacts over the period of their pregnancy to date of recruitment at 36 weeks’ gestation. This covered use of community- and hospital-based antenatal services and non-pregnancy-related service contacts. The AD-SUS was then administered as part of the 6- and 12-month follow-up questionnaires in which participants were asked to report service contacts over the previous 6 months, including contact with services for both mother and infant (see Report Supplementary Material 4, 7 and 8). To measure and cost medical resources allocated to birth, data on the mode of birth for each trial participant were extracted from women’s maternity records provided by the participating NHS trust.
Unit costs
Unit cost data required for costing community- and hospital-based service contacts (including admissions to hospital) and mode of birth were extracted from the publications NHS Reference Costs 2016/1749 and Unit Costs of Health and Social Care 2017. 50 To cost contact with the weight management programme for women in the intervention arm of the feasibility trial, an enrolment cost of £49.50 quoted by the service provider (Slimming World) was applied, covering registration to the programme plus 12 weeks of programme involvement irrespective of number of sessions attended. This was assumed to be charge per participant, which would be levied on a service commissioning body if a weight management programme of the type evaluated were to be subsidised by the NHS or through a local authority public health budget. All unit costs were reported and applied to service contact data at 2017 price levels.
Quality of life measurement
Quality of life data pertinent to estimating within-trial intervention impact on quality-adjusted life-years (QALYs) and cost-effectiveness at 12-month follow-up were collected using the EQ-5D-5L instrument51 administered as part of the baseline, 6- and 12-month questionnaires (see Appendix 4, Table 29).
Modelling out-of-trial programme impacts
An overview of the available evidence required to support economic modelling of the out-of-trial cost and QALY impacts of improved postnatal weight management as part of a larger definitive trial was undertaken to inform general recommendations as to how this work might proceed. Capturing longer-term impacts of postnatal weight management is likely to be complex, requiring economic modelling of one form or another. A broad assessment into the probable feasibility of modelling longer-term impacts as part of a definitive trial was completed, including the plausible time scales over which these impacts might be assessed. A rapid evidence review of the type of evidence required to develop and parameterise an economic model of this type was completed, full details of which are presented in Chapter 7.
Integration of main feasibility trial and process evaluation findings
Following the completion of the main feasibility analyses and process evaluation, we carefully examined the findings from both sources to inform the overarching aim of determining whether or not it is feasible to conduct a definitive RCT to determine clinical effectiveness and cost-effectiveness of lifestyle information and access to the commercial weight management groups for 12 weeks to support women in an ethnically diverse inner-city population to achieve and maintain postnatal weight management and positive lifestyle behaviour.
We integrated the findings by ‘following threads’ backwards and forwards from the quantitative findings to the qualitative/mixed-methods process findings (and vice versa) to identify aspects of the findings that corroborated each other, conflicted with each other and where one source was ‘silent’. 52 For example, we examined how both data sets informed us about the relationship between the intervention and weight loss, and analysis of the qualitative data led to us conducting further analysis of the quantitative data (a per-protocol analysis to test for a dose-response effect). We followed the methods described by Moffat et al. 53 to explore the potential reasons for any conflict or ‘silence’ in relation to findings that were not concordant. This included considering the following: (1) treating the methods as fundamentally different, (2) exploring the methodological rigour of each component, (3) exploring data set comparability, (4) using additional data and making further comparisons, (5) exploring whether or not the intervention worked as expected and (6) exploring whether or not the outcomes of the quantitative and qualitative components match.
Chapter 4 Trial conduct
Important feasibility outcomes included whether or not we were able to recruit women during pregnancy to a weight management trial commencing postnatally, whether or not it was possible to recruit to the required sample size within time allocated, and whether or not we could retain sufficient women to 12 months postnatally.
Recruitment
For the feasibility trial, we recruited from one NHS trust based in an inner-city area in the south of England. We initially faced severe delays with commencing recruitment (around 9 months) because of problems experienced with the recent introduction of the HRA approval process. The roll-out of the HRA approval process had a significant impact on internal processes for dealing with review of trial documentation and sponsor sign-off between the university and the NHS trust. Owing to the delays, when the recruitment could commence there was a backlog of women to contact who met the trial inclusion criteria. This meant that the recruitment rate during the first 4 months met the planned target, with some small delays thereafter to achieving the final sample size (Figure 2).
FIGURE 2.
Planned and actual recruitment at the trial site.
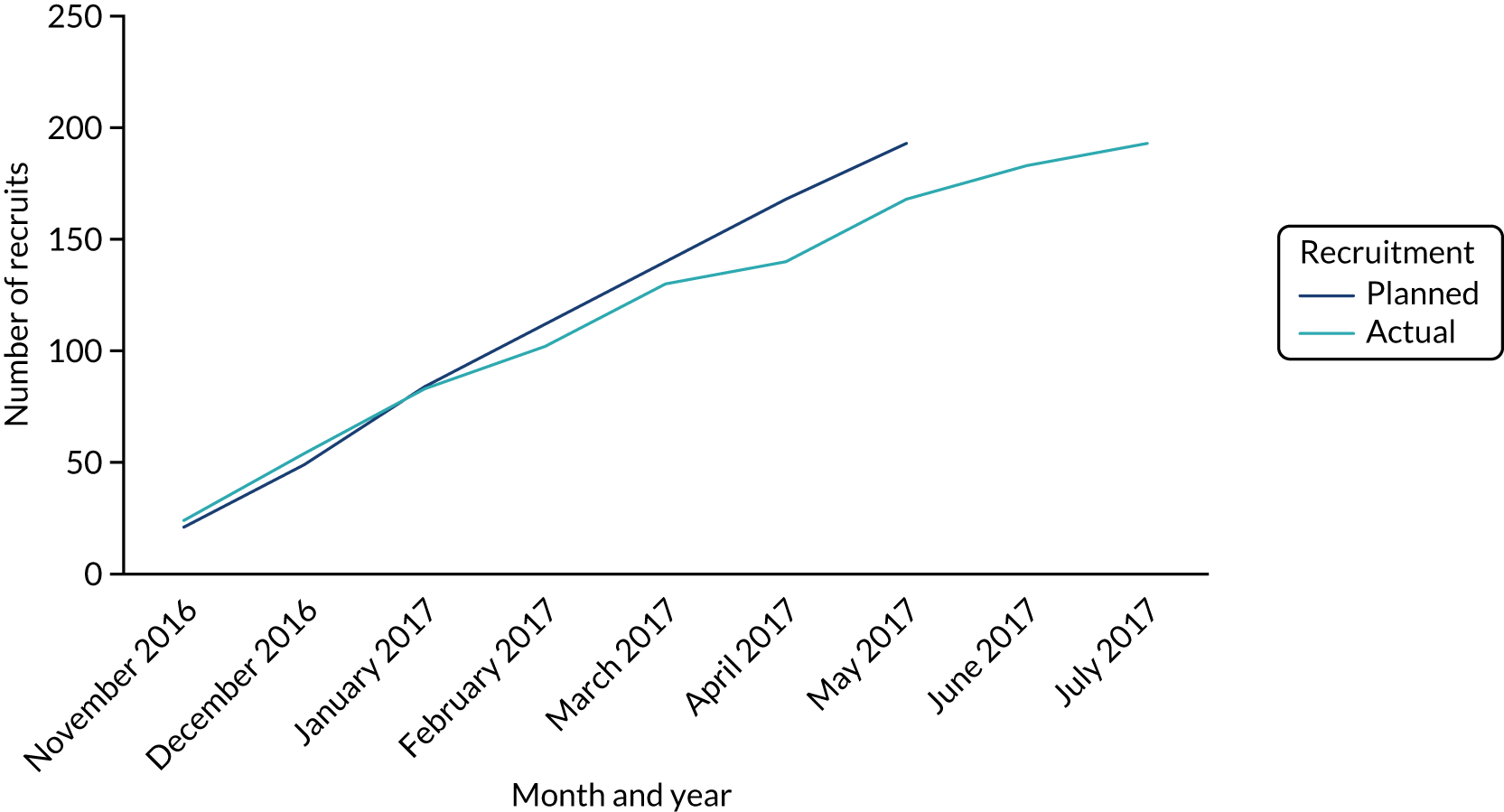
Challenges to trial recruitment
Despite recruitment commencing well, it soon became apparent that, although we were recruiting women with BMI scores of > 25kg/m2 at their antenatal booking, we were not recruiting any women with normal BMI scores at antenatal booking who gained excessive gestational weight.
The original protocol was that these women would be recruited through advertising the trial at the site via the distribution of postcards and posters. After 4 months of recruitment, and following discussion with the TSC and CPT, a substantive ethics amendment was submitted to revise the recruitment approach for women with EGWG. The content of postcards that clinicians were asked to hand to women who had a normal BMI score at antenatal booking when they were around 32–34 weeks’ gestation was revised to state that a research midwife may call the woman to discuss ‘a study of advice and support for postnatal weight management’; if women were not interested, they could ‘opt out’ by e-mailing the trial team. A letter was also sent to all women identified as having a normal BMI score at antenatal booking at around 32–34 weeks’ gestation. Other options discussed to recruit women with EGWG included asking community midwifery teams to identify and recruit women; however, owing to high workloads, this was not considered practicable.
Given concerns about lack of recruitment of women with EGWG and the need to ‘embed’ the revised recruitment processes, we sought ethics approval to extend the period of participant recruitment from 6 to 8 months, which was granted. This requirement, plus the 9-month delay at trial commencement, led to a 12-month cost-extension request to the NIHR PHR programme being made in September 2017, which was recommended for funding.
Recruitment strategies
To encourage women to attend follow-up appointments with the research midwives at the trial unit at 6 and 12 months, we offered a £10 Love2shop Gift Card (highstreetvouchers.com, Birkenhead, UK) and pre-paid travel cards to cover bus and tube fares. We also offered women the option of a home-based appointment with a research midwife, because for some women travelling with a baby and, in some cases, other small children was difficult to arrange. With the support of Slimming World we were able to offer women allocated to the control arm who completed their 12 month follow-up access to 12 sessions (over a 14-week period) free of charge. A total of 11 women (15%) took up this offer. To prevent possible trial contamination, women were not advised of this offer until after their 12 month contact. Women in the intervention arm who completed their 12-week offer as part of the trial were able to continue attending groups but had to pay the standard fee of £4.95 per week, in line with usual Slimming World membership policy.
Three contact attempts were made to speak to women who met inclusion criteria, were sent a trial letter and had not asked to opt out of further contact. This approach meant that women (and not those providing their clinical care) could make decisions about participating or not participating. As recruitment took place later in pregnancy, it also meant that women were less likely to be anxious about their pregnancies (because routine scans and other tests would be more likely to have been completed), although ‘problems in pregnancy’ were given as a reason by a small number of women for not wanting to participate.
In many cases, women did not contact the research team to discuss trial participation, despite the research midwives leaving telephone or text messages. For those who were contacted and spoken to but declined recruitment, common reasons given included lack of time, leaving the country after giving birth and not being concerned about their weight. One initial concern was that women would not want to participate owing to being embarrassed or upset by being contacted to consider joining a trial of postnatal weight management. Only two out of 1132 women identified as potentially eligible for recruitment and contacted about the trial by letter (including women with a normal BMI score contacted by letter during the last 2 months of recruitment) complained to the trial team about being contacted this way (note that we do not have data on how many women may not have received a copy of the trial letter).
Chapter 5 Feasibility trial results
Parts of this chapter are adapted from Bick et al. 54 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
This chapter presents findings from the main feasibility trial analysis. Process evaluation findings are presented in Chapter 6 and health economic data are presented in Chapter 7.
In relation to the feasibility trial analysis, data are presented on trial recruitment and retention to 12 months postnatally, baseline characteristics of the two trial arms, the trial primary outcome of the impact of attendance at commercial weight management groups on maternal weight change from antenatal booking to 12 months postnatally, and secondary outcome assessments including physical and mental health, diet, lifestyle, breastfeeding, sleep and body image at baseline, 6 months and 12 months postnatally.
Recruitment, time to recruitment and retention
Between 15 November 2016 and 11 July 2017, antenatal booking data on women from one maternity unit in an inner-city area potentially eligible to receive an invitation to join the SWAN trial were accessed by research midwives employed at the NHS site when women were at around 28 weeks’ gestation. Of the 1132 women initially eligible to be sent an invitation letter, 835 (73.5%) were not recruited and 59 (5.2%) were later ineligible (e.g. they went on to a premature birth); contact data on 43 women (3.8%) were missing. Reasons for not recruiting included that women were moving away, women were not interested, letters of contact were returned unopened or there was no response to phone calls. Of the 195 women (17.2%) who agreed to join the trial and meet the research midwives at the recruitment (baseline) appointment, two women changed their minds prior to the appointment, leaving 193 women recruited and randomised to the feasibility trial. The majority of women recruited had BMI scores ≥ 25 kg/m2. Only four women recruited had a normal BMI score at antenatal booking and EGWG at 36 weeks’ gestation.
In accordance with the prespecified protocol, all primary analysis were by ITT. We also undertook a per-protocol analysis to consider if women who completed ≥ 10 sessions were more likely to achieve weight loss at 12 months than women who attended < 10 sessions, did not attend any groups or were in the control arm, and to explore any differences between women who did and did not attend follow-up appointments with the research midwives. Consent forms were received from all 193 women; recruitment and details of participant follow-up are presented in Figure 3.
FIGURE 3.
The CONSORT flow diagram.
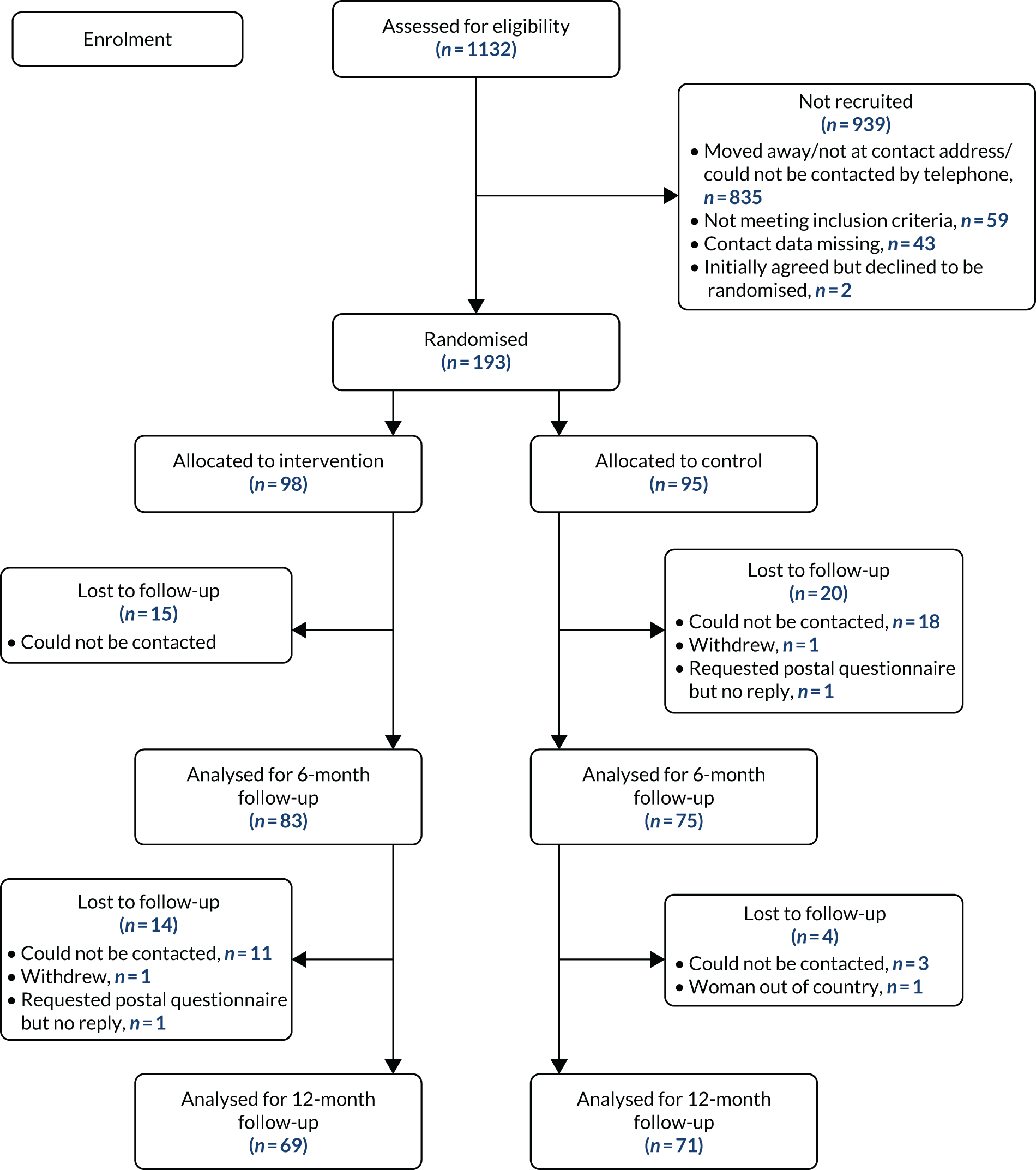
Overall follow-up at 6 and 12 months was achieved for 81.8% and 88.6% of women, respectively. The 6-month follow-ups commenced in June 2017, with 158 women (83 allocated to the intervention arm and 75 allocated to the control arm) followed up. Thirty-five (18.1%; 15 allocated to the intervention arm and 20 allocated to the control arm) of the total 193 women were lost to follow-up, a response of 82.3%. At 6 months, women lost to follow-up included 20 women (which includes the 13 women allocated to the intervention arm who were lost to follow-up) who could not be contacted, one woman who withdrew and one woman who requested a postal questionnaire (rather than having an appointment with a research midwife) but did not return it.
The 12-month follow-ups commenced in December 2017. We did not attempt to contact women lost to follow-up at 6 months because the objective was to maintain recruitment to 12 months. At 12 months, and out of the 158 women, follow-up was completed on 140 women (69 allocated to the intervention arm and 71 allocated to the control arm). A total of 18 women (11.3%) were lost to follow-up (14 allocated to the intervention arm and four allocated to the control arm). Women lost to follow-up included 11 women who could not be contacted, one woman who asked to withdraw from the trial and two women who requested a postal questionnaire (rather than having an appointment with a research midwife) but did not return it. Data from both of the women who withdrew were included as neither woman asked for her data to be excluded. Six women who were pregnant again at the time of their 12-month follow-up were included in analysis as per ITT.
Baseline characteristics
Key sociodemographic and obstetric information for both trial arms, and overall sample, are presented in Table 3. The BMI data used for trial outcome comparisons were those recorded at women’s antenatal booking and not those recorded at trial entry. Customised birthweight centiles were used, correcting the expected birthweight for maternal height, weight and ethnicity and for parity, neonatal sex and gestation at delivery using the Bulk Centile Calculator version 6.2.
| Characteristic | Trial arm | ||
|---|---|---|---|
| Intervention (N = 98) | Control (N = 95) | Combined (N = 193) | |
| Maternal | |||
| Age (years), mean (SD) | 32.44 (5.10) | 33.06 (5.37) | 32.74 (5.23) |
| Height (m), mean (SD) | 1.64 (0.07) | 1.64 (0.06) | 1.64 (0.06) |
| Weight (kg), mean (SD) | 83.77 (18.77) | 80.53 (13.17) | 82.17 (16.29) |
| Mean antenatal booking BMI score (kg/m2), mean (SD) | 31.18 (6.47) | 29.83 (4.11) | 30.51 (5.46) |
| Antenatal booking BMI score (kg/m2), n (%) | |||
| < 25; no EGWG | 0 (0.0) | 1 (1.1) | 1 (0.5) |
| 25–29.9; no EGWG | 2 (2.0) | 1 (1.1) | 3 (1.6) |
| 25–29.9; EGWG | 20 (20.4) | 31 (32.6) | 51 (26.4) |
| 30–34.9; no EGWG | 37 (37.8) | 26 (27.4) | 63 (32.6) |
| 30–34.9; EGWG | 9 (9.2) | 18 (18.9) | 27 (14.0) |
| ≥ 35; no EGWG | 14 (14.3) | 11 (11.6) | 25 (13.0) |
| ≥ 35; EGWG | 11 (11.2) | 6 (6.3) | 17 (8.8) |
| Ethnicity,a n (%) | |||
| White | 38 (38.8) | 40 (42.1) | 78 (40.4) |
| Black | 40 (40.8) | 36 (37.9) | 76 (39.4) |
| Asian | 6 (6.1) | 2 (2.1) | 8 (4.1) |
| Other | 14 (14.3) | 17 (17.9) | 31 (16.1) |
| IMD (centile scale), mean (SD) | 0.27 (0.15) | 0.28 (0.17) | 0.28 (0.16) |
| IMD quintiles, n (%) | |||
| 1 (least deprived) | 2 (2.0) | 2 (2.2) | 4 (2.1) |
| 2 | 2 (2.0) | 3 (3.2) | 5 (2.6) |
| 3 | 11 (11.2) | 15 (16.1) | 26 (13.6) |
| 4 | 49 (50.0) | 41 (44.1) | 90 (47.1) |
| 5 (most deprived) | 34 (34.7) | 32 (34.4) | 66 (34.6) |
| Infant | |||
| Gestational age at birth (weeks), mean (SD) | 39.38 (1.54) | 39.49 (3.36) | 39.43 (2.59) |
| Mode of birth,b n (%) | |||
| Vaginal (normal) | 45 (46.4) | 53 (56.4) | 98 (51.3) |
| Vaginal (assisted) | 10 (10.3) | 12 (12.8) | 22 (11.5) |
| Planned C-section | 30 (30.9) | 14 (14.9) | 44 (23.0) |
| Emergency C-section | 10 (10.3) | 14 (14.9) | 24 (12.6) |
| Birthweight (kg), mean (SD) | 33.78 (4.98) | 35.00 (5.06) | 34.38 (5.04) |
| < 10th centile,c n (%) | 14/90 (15.6) | 7/89 (7.9) | 21/179 (11.7) |
| < 3rd centile,c n (%) | 5/90 (5.6) | 2/89 (2.2) | 7/179 (3.9) |
The mean maternal age was 32 years (SD 5.2 years) and the mean maternal BMI score at antenatal booking was 30.51 kg/m2 (SD 5.4 kg/m2). Following generally recommended practice,55 we did not test for significant differences between trial arms at baseline. More women in the intervention arm had a mean BMI score of > 30 kg/m2 at their antenatal booking appointment. More women in the control arm had a spontaneous vaginal birth, with twice as many women in the intervention having a planned caesarean section. The mean gestational age at birth was 39.4 weeks (SD 2.5 weeks) and the mean infant birthweight 3.43 kg (SD 5.0 kg).
Recruited women were more likely to live in areas of highest social deprivation, based on Indices of Multiple Deprivation;56 however, total annual household income levels ranged widely, with around one-third of women in both trial arms reporting a total annual income of < £30,000 and one-third reporting a total annual income of > £60,000. The ethnicity of recruited women reflected the local maternity population, although the proportion of women of white ethnicity recruited was slightly lower than in the general maternity population (≈50% of women who booked for care in 2017 were of white ethnicity), but we had a slightly higher proportion of women of black ethnicity (34% of women who booked for care in 2017). Few women were of Asian ethnicity, reflecting the general local population. The ‘other ethnicity’ category included women from a wide range of countries, including Brazil, Lithuania, Portugal, Poland and Spain.
Differences in trial completion between trial arms
Differences in completion between trial arms were explored to assess, for example, if women with obese BMI scores were more or less likely to drop out if randomised to the control arm. Trial completion was defined as follow-up to 12 months, including useable current weight. Logistic regression was used to investigate if dropout rates were the same in each arm, with interaction tests to check for differential drop-out by relevant maternal characteristics (see Appendix 3, Table 22) including antenatal booking BMI score, parity, age and IMD score. No significant differences were found (OR –2.2, 95% CI –15.2 to 10.8).
All 193 women completed a baseline questionnaire at the trial recruitment appointment with the research midwife after providing signed consent to enter the trial and following allocation to trial arm. A high number of women in each trial arm completed a 6- and 12-month follow-up questionnaire. At 6 months, 83 out of 98 women (84.7%) allocated to the intervention arm and 75 out of 95 women (78.9%) allocated to the control arm completed a questionnaire. At 12 months, 69 out of 98 women (70.4%) allocated to the intervention arm and 71 out of 95 women (74.7%) allocated to the control arm completed questionnaires.
Responses to measures and items included in baseline and 6- and 12-month questionnaires showed generally high overall completion (> 80%), with few missing items. Some items were missing or had low completion (e.g. on extent of performing vigorous physical activity when asked at baseline; see Proposed primary and secondary outcomes); this was possibly due to a specific question not being viewed as relevant to the woman at time of completion.
Proposed primary and secondary outcomes
Trial outcomes reflected the need to clarify uncertainty in relation to various aspects of the trial to inform progression to a definitive RCT. The trial was not powered to detect statistically significant difference in primary or secondary outcomes.
Proposed primary outcome: impact of lifestyle information and commercial weight management sessions on maternal weight at 12 months postnatally
The proposed primary outcome was maternal weight change as calculated from a woman’s BMI score recorded at her first antenatal booking visit, which usually takes place before 12 weeks’ gestation, and at 12 months postnatally. Antenatal weight was estimated as weight at the antenatal booking appointment minus 1.25 kg to accommodate early pregnancy weight gain. Linear regression was undertaken to adjust for the most powerful predictors measured pre randomisation and remove any biases caused by chance imbalance at baseline.
An important feasibility question was whether to use the woman’s trial eligibility weight, which was recorded at her antenatal booking appointment, or her weight as recorded at trial entry at 36 weeks’ gestation. Using the eligibility weight, the standard error (SE) of the estimated treatment effect was 1.29 kg; using the trial entry weight, the SE was slightly smaller at 1.27 kg, in line with the expectation that weight used to define trial entry could have been subject to selection bias.
Weight loss as assessed at 12 months postnatally was greater than at 6 months (Table 4), supporting the decision to use 12 months as the primary end point for the feasibility trial.
| Variable | Trial arm | ||
|---|---|---|---|
| Intervention | Control | Combined | |
| Baseline | |||
| n | 98 | 95 | 193 |
| Mean estimated antenatal booking weight, kg (SD) | 82.52 (18.77) | 79.28 (13.17) | 80.92 (16.29) |
| Mean weight at trial recruitment, kg (SD) | 83.77 (18.77) | 80.53 (13.17) | 82.17 (16.29) |
| 6 months postnatally | |||
| n | 82 | 72 | 154 |
| Weight, kg (SD) | 83.24 (17.68) | 81.88 (12.60) | 82.60 (15.48) |
| Weight change, kg (SD) | –7.49 (9.61) | –5.38 (6.41) | –6.50 (8.31) |
| 12 months postnatally | |||
| n | 69 | 71 | 140 |
| Weight, kg (SD) | 82.35 (18.41) | 81.89 (14.60) | 82.12 (16.53) |
| Weight change, kg (SD) | –9.00 (8.18) | –6.23 (7.07) | –7.59 (7.74) |
Secondary outcomes at baseline and 6 and 12 months: influence of lifestyle information and weight management sessions on women’s health and health behaviour
Previous studies have shown that a number of health behaviours during pregnancy are associated with poorer outcomes for a woman and her child. These include tobacco smoking,57 poor nutrition,58 heavy alcohol consumption59,60 and use of illicit drugs. 61–63 These are compounded by poor health behaviours before and after pregnancy, including lack of physical exercise, having a high BMI score and not initiating or persisting with breastfeeding. 56 Secondary outcomes included a range of measures to assess the potential for the intervention, which includes a positive lifestyle information leaflet, to have an impact on women’s lifestyle and positive health behaviours after giving birth as assessed at 6 and 12 months postnatally. We also wanted to consider if the measures of health behaviour used were most appropriate to inform outcomes in a future RCT, and if women would be willing to complete these measures.
Women’s weight reduction by > 5% and > 10% of weight at trial entry
A pre-planned subgroup analysis of the primary outcome was undertaken for women who were overweight (BMI 25.0–29.9 kg/m2) and obese (BMI ≥ 30 kg/m2) at antenatal booking. Although differences were not significant, there were more women with a > 10% weight reduction at 12 months in the intervention than in control arm (Table 5). There was no evidence of an interaction effect (which would suggest that treatment may be more clinically effective in particular groups), although this test lacks power.
| Variable | Trial arm, n/N (%) | Health ratio (95% CI) | Risk difference, % (95% CI) | p-value | |
|---|---|---|---|---|---|
| Intervention | Control | ||||
| Weight reduction | |||||
| 6 months postnatally | |||||
| > 5% | 20/82 (24.4) | 10/72 (13.9) | 1.76 (0.88 to 3.50) | 10.5 (–1.8 to 22.8) | 0.101 |
| > 10% | 6/82 (7.3) | 2/72 (2.8) | 2.63 (0.55 to 12.64) | 4.5 (–2.3 to 11.3) | 0.205 |
| 12 months postnatally | |||||
| > 5% | 16/69 (23.2) | 18/71 (25.4) | 0.91 (0.51 to 1.64) | –2.2 (–16.4 to12.0) | 0.765 |
| > 10% | 9/69 (13.0) | 3/71 (4.2) | 3.09 (0.87 to 10.93) | 8.8 (–0.4 to 18.0) | 0.062 |
| Retention of EGWG: all women | |||||
| 6 months postnatally | 44/82 (53.7) | 36/72 (50.0) | 1.07 (0.79 to 1.46) | 3.7 (–12.1 to 19.5) | 0.650 |
| 12 months postnatally | 30/69 (43.5) | 33/71 (46.5) | 0.94 (0.65 to 1.35) | –3.0 (–19.5 to 13.5) | 0.721 |
| Retention of EGWG: women with EGWG at entry only | |||||
| 6 months postnatally | 34/56 (60.7) | 17/33 (51.5) | 1.18 (0.80 to 1.74) | 9.2 (–12.1 to 30.5) | 0.397 |
| 12 months postnatally | 24/50 (48.0) | 16/34 (47.1) | 1.02 (0.64 to 1.61) | 0.9 (–20.8 to 22.7) | 0.932 |
Self-reported and research-midwife-recorded maternal weight at 6 and 12 months
There were no differences in weight outcomes among women who self-reported their weight in 6- and 12-month questionnaires posted to the research team (and who did not receive follow-up appointments) and women who were weighed by the research midwives. At 6 months, the effects were –1.62 kg (95% CI –4.18 to 1.65 kg; p = 0.393) in women who had an appointment to be weighed by the research midwife and –5.76 kg (–12.7 to 1.23 kg; p = 0.101) in the self-report group (likelihood ratio interaction test: p = 0.390). At 12 months the effects were –2.22 kg (95% CI –4.97 to 0.53 kg; p = 0.122) in women who were weighed at an appointment and –4.8 kg (95% CI –10.9 to 1.3 kg) in the self-report group (likelihood ratio interaction test: p = 0.390).
Number of weight management sessions attended
At 6 months postnatally, although some women allocated to the intervention arm were still attending sessions as part of the intervention offer, there were no detectable differences in adjusted weight loss [i.e. a difference of 0 kg (95% CI –7.28 to 5.37 kg; p = 0.765)] between women who attended 10–12 sessions, women who had attended nine or fewer sessions and women in the control arm. By contrast, at 12 months postnatally there was a difference in adjusted weight loss of ≈5 kg (95% CI 1.05 to 8.93 kg; p = 0.013) between women who attended 10–12 sessions and all other women, indicating a ‘dose effect’ response in terms of ≥ 10 sessions as recommended by Slimming World.
Dietary intake
An important issue to consider with respect to the intervention was whether or not there was the potential to influence maternal dietary intake at 6 and 12 months postnatally from baseline. There was considerable discussion among the trial team (which included individuals with expertise in dietetics and nutrition) when planning the trial as to the most appropriate food frequency measure to use to capture dietary information in a population of women during and after pregnancy. We also consulted on this issue with Professor Siân Robinson, from the NIHR Southampton Biomedical Research Centre, who leads a programme of work on nutrition in older age.
The DINE, developed by Roe et al. ,64 is a measure of an individual’s intake of dietary fat and fibre and was developed for use in primary care settings. It lists 19 foods or food groups, pre-scored according to the amount of fat or fibre contained in an average portion size of the food. Scores are weighted by daily or weekly frequency of intake of the foods, and then summed to give overall fat and fibre scores. A decision was made to use the DINE but to slightly adapt foods listed as examples in some of the included categories (as recommended by the developers) to include foods more likely to be used in the local population (e.g. including ghee in ‘types of fats which may be used in cooking’ and to list beans and pulses such as lentils as starchy carbohydrates) and popular local food brands.
The DINE results showed no differences between the trial arms in overall intake of fat, fibre or unsaturated fat at baseline and 6 and 12 months (Table 6).
| Intake | Trial arm, n (%) | ||
|---|---|---|---|
| Intervention | Control | Combined | |
| Fibre | |||
| Baseline | |||
| All | 98 | 95 | 193 |
| Low | 72 (73.5) | 67 (70.5) | 139 (72.0) |
| Medium | 16 (16.3) | 20 (21.1) | 36 (18.7) |
| High | 10 (10.2) | 8 (8.4) | 18 (9.3) |
| 6 months | |||
| All | 83 | 75 | 158 |
| Low | 48 (57.8) | 47 (62.7) | 95 (60.1) |
| Medium | 15 (18.1) | 13 (17.3) | 28 (17.7) |
| High | 20 (24.1) | 15 (20.0) | 35 (22.2) |
| 12 months | |||
| All | 69 | 71 | 140 |
| Low | 43 (62.3) | 48 (67.6) | 91 (65.0) |
| Medium | 20 (29.0) | 15 (21.1) | 35 (25.0) |
| High | 6 (8.7) | 8 (11.3) | 14 (10.0) |
| Fat | |||
| Baseline | |||
| All | 98 | 95 | 193 |
| Low | 60 (61.2) | 65 (68.4) | 125 (64.8) |
| Medium | 25 (25.5) | 22 (23.2) | 47 (24.4) |
| High | 13 (13.3) | 8 (8.4) | 21 (10.9) |
| 6 months | |||
| All | 83 | 75 | 158 |
| Low | 68 (81.9) | 54 (72.0) | 122 (77.2) |
| Medium | 10 (12.0) | 13 (17.3) | 23 (14.6) |
| High | 5 (6.0) | 8 (10.7) | 13 (8.2) |
| 12 months | |||
| All | 69 | 71 | 140 |
| Low | 51 (73.9) | 57 (80.3) | 108 (77.1) |
| Medium | 14 (20.3) | 11 (15.5) | 25 (17.9) |
| High | 4 (5.8) | 3 (4.2) | 7 (5.0) |
| Unsaturated fat | |||
| Baseline | |||
| All | 98 | 95 | 193 |
| Medium | 27 (27.6) | 34 (35.8) | 61 (31.6) |
| High | 71 (72.4) | 61 (64.2) | 132 (68.4) |
| 6 months | |||
| All | 83 | 75 | 158 |
| Medium | 19 (22.9) | 22 (29.3) | 41 (25.9) |
| High | 64 (77.1) | 53 (70.7) | 117 (74.1) |
| 12 months | |||
| All | 69 | 71 | 140 |
| Medium | 26 (37.7) | 19 (26.8) | 45 (32.1) |
| High | 43 (62.3) | 52 (73.2) | 95 (67.9) |
Daily intake of soft drinks
In addition to food intake, we were interested in the sort of soft drinks women drank on a usual day, including fizzy drinks [non-diet such as Coca-Cola® (The Coca-Cola Company, Atlanta, GA, USA) and Lucozade® (Suntory Beverage & Food Limited, Osaka, Japan)], sugar-free fizzy drinks, squash (non-diet or sugar free/diet or sugar free), fruit juices and smoothies, given the calorific load some soft drinks carry. Questions were developed by the trial team after discussions with Lucilla Poston, who led a NIHR Health Technology Assessment (HTA) programme-funded study of a behavioural intervention in pregnancy in women with obesity. 20
At 6 months postnatally, women in the intervention arm were more likely to be drinking diet or sugar-free squash (OR 2.84, 95% CI 1.11 to 7.29; p = 0.029). There were no differences between the groups at baseline or 12 months (see Appendix 3, Table 23).
Physical activity
The importance of maintaining physical activity was highlighted in the intervention lifestyle information leaflet and is addressed in the commercial weight management programme. An important part of trial assessment was to consider if women allocated to the intervention were more likely to participate in physical activity than women allocated to the control arm (Table 7).
| Time point | Trial arm, mean (SD) | ||
|---|---|---|---|
| Intervention | Control | Combined | |
| Baseline | 512.57 (210.01) | 293.23 (159.04) | 367.89 (227.41) |
| 6 months | 393.11 (189.19) | 464.01 (388.57) | 423.10 (285.86) |
| 12 months | 542.65 (381.87) | 577.19 (357.65) | 557.89 (366.21) |
The IPAQ-SF was developed to measure health-related physical activity in general populations aged 15–64 years. It can be administered over the telephone or self-administered36,65 and has been tested and validated in a range of different countries and populations. 36 We used the IPAQ-SF, which includes seven items, to capture data on women’s physical activity at baseline and 6 and 12 months postnatally. On completing the IPAQ-SF scale, women were asked to consider the number of days over the previous 7 days they had undertaken vigorous activity, such as aerobics or fast cycling, and moderate activity, such as dancing or water aerobics, and how much time they had spent walking for ≥ 10 minutes at a time.
We summarised data according to methods recommended by the developers. 65 The included items provide separate scores on the activities of interest. Deriving a total score requires summation of the duration (minutes) and frequency (days) of these activities. Data can be presented as a continuous measure. Data on volume of activity can also be computed by weighting each type of activity by its energy requirements as defined in metabolic equivalents (METs) to yield a score in MET minutes, which is computed by multiplying the MET score of an activity by minutes performed. Data that women provided were calculated as METS. Other data are presented in Appendix 3, Table 24, showing that low activity levels were recorded for some items, particularly at baseline, which is not an unexpected finding. It is possible that, in many cases, the true answer was ‘no vigorous activity’ but, because women did not record a ‘0’ against the item, it was recorded as missing data.
Maternal mental health
Maternal perinatal mental health problems are commonly reported and a major public health concern given the longer-term implications of poor perinatal mental health for women, their infants and their families. 66 Risk of poor maternal mental health postnatally has been reported among women with obese BMI scores before and after their pregnancies and has been associated with postpartum weight retention. 67,68 The EPDS37 is a 10-item scale that asks women to consider how they have felt in the previous 7 days. It is a well-validated measure of women’s risk of developing depression during and after pregnancy, and a commonly used research tool in perinatal mental health studies.
We asked women to complete the EPDS at 6 and 12 months postnatally to see if there were likely to be any differences between trial arms as a result of women in the intervention arm being offered support for their weight management.
The mean EPDS scores at 6 months were 5.72 (SD 5.04) and 4.59 (SD 3.40) for the intervention and control arms, respectively (p = 0.096), and at 12 months these were 5.19 (SD 4.96) and 4.34 (SD 3.94), respectively (p = 0.26). An EPDS score of ≥ 12 is considered indicative of a woman’s risk of developing depression. 37 More women in the intervention arm had EPDS scores ≥ 12 than women in the control arm at 6 and 12 months, with a statistically significant difference at 6 months only (Table 8). Appendix 3, Table 25, shows data on all items included.
| EPDS scores | Trial arm, n/N (%) | RR (95% CI) | p-value | |
|---|---|---|---|---|
| Intervention | Control | |||
| 6 months | ||||
| ≥ 12 | 9/83 (10.8) | 1/75 (1.3) | 8.13 (1.06 to 62.69) | 0.01 |
| 12 months | ||||
| ≥ 12 | 6/69 (8.7) | 3/71 (4.2) | 2.06 (0.54 to 7.90) | 0.28 |
Breastfeeding intention, uptake and continuation
Breastfeeding is a public health priority for the UK, with robust evidence of benefits on shorter- and longer-term health of women and their infants. 69 The questions on breastfeeding were devised by the trial team and aimed to explore differences between the intervention and control arms with respect to breastfeeding as a positive behavioural intervention. There is evidence that women who have high BMI scores are less likely to commence, continue or exclusively breastfeed than women who have normal BMI scores (18.0–24.9 kg/m2),70,71 and breastfeeding could also support women with postnatal weight management. 3,72
Women were asked in the baseline questionnaire about their breastfeeding intentions. Those women who answered in the affirmative were asked if they had considered how long they planned to breastfeed for, with over half of all women saying that they hoped to breastfeed for > 6 months (Table 9). Few women stated that they planned to only formula feed their babies. More women in the intervention arm planned to offer their babies only breast milk, with over half of women in both groups planning to breastfeed their babies for > 6 months.
| Breastfeeding intention | Trial arm, n/N (%) | ||
|---|---|---|---|
| Intervention | Control | Combined | |
| How do you plan to feed your baby? | |||
| Bottle feed (formula milk) only | 1/98 (1.0) | 3/95 (3.2) | 4/193 (2.1) |
| Bottle feed and breastfeed | 24/98 (24.5) | 32/95 (33.7) | 56/193 (29.0) |
| Breastfeed only | 71/98 (72.4) | 59/95 (62.1) | 130/193 (67.4) |
| I have not decided yet | 2/98 (2.0) | 1/95 (1.1) | 3/193 (1.6) |
| If you are planning to breastfeed your baby, how long do you hope to breastfeed?a | |||
| First 1–2 weeks | 0/95 (0.0) | 1/91 (1.1) | 1/186 (0.5) |
| 1–2 months | 1/95 (1.1) | 3/91 (3.3) | 4/186 (2.2) |
| 2–3 months | 5/95 (5.3) | 5/91 (5.5) | 10/186 (5.4) |
| 4–6 months | 36/95 (37.9) | 29/91 (31.9) | 65/186 (34.9) |
| > 6 months | 53/95 (55.8) | 53/91 (58.2) | 106/186 (57.0) |
The World Health Organization currently recommends that infants are exclusively breastfed to at least 6 months of age73 to enable protective benefits to be maximised. At 6 and 12 months, women were asked if they were still breastfeeding and, if they were, if they offered their infants other fluids in addition to breast milk. If women had stopped breastfeeding, they were asked when they stopped. Women were also asked if they had introduced their baby to solid foods (such as finger foods or mashed or pureed food) and, if they had, when their baby was first introduced to solid food. At 12 months, an additional question on ‘breast plus cow’s milk’ was included, as NHS guidance is that cow’s milk should not be introduced as a drink before 12 months. 74
At 6 months, the majority (95%) of women reported that they had commenced breastfeeding (Table 10), reflecting the high breastfeeding uptake among the local population, which is reported as ≈90%. 75 More women in the control arm were still breastfeeding at 6 months and offering their infants only breast milk, including expressed breast milk. A similarly high proportion of women in both trial arms had introduced their infants to solid food.
| Breastfeeding uptake | Trial arm, n/N (%) | ||
|---|---|---|---|
| Intervention | Control | Combined | |
| 6 months | |||
| Breastfed any time | 80/83 (96.4) | 70/75 (93.3) | 150/158 (94.9) |
| Still breastfeeding | 53/83 (66.3) | 51/75 (72.9) | 104/158 (69.3) |
| Breast milk (including expressed) only | 30/83 (36.1) | 34/75 (45.3) | 64/158 (40.5) |
| Breast plus formula milk | 22/83 (26.5) | 17/75 (22.7) | 39/158 (24.7) |
| Introduced baby to solid food | 74/83 (89.2) | 66/75 (88.0) | 140/158 (88.6) |
| 12 months | |||
| Still breastfeeding | 29/69 (43.3) | 29/71 (43.3) | 58/140 (43.3) |
| Breast milk (including expressed) only | 12/69 (17.4) | 14/71 (19.7) | 26/140 (18.6) |
| Breast plus formula milk | 10/69 (14.5) | 8/71 (11.3) | 18/140 (12.9) |
| Breast plus cow’s milk | 7/69 (10.1) | 7/71 (9.9) | 14/140 (10.0) |
| Introduced baby to solid food | 69/69 (100.0) | 71/71 (100.0) | 140/140 (100.0) |
At 12 months, responses from women in both groups were similar and it was encouraging that over one-third of women continued to breastfeed. Of the women still breastfeeding, some were only offering their babies breast milk, with smaller proportions of women offering breast plus formula or cow’s milk. All women had introduced their infants to solid foods at a mean infant age of 22.2 weeks (SD 3.72 weeks) in the intervention and 23.4 weeks (SD 4.78 weeks) in the control.
When time of breastfeeding cessation was assessed, women in the intervention stopped breastfeeding at a mean of 20.0 weeks (SD 14.4 weeks) after birth compared with a mean of 24.2 weeks (SD 15.9 weeks) after birth among women in the control arm.
Maternal sleep patterns
Lack of sleep resulting in excessive fatigue is a commonly reported health issue for women who have recently given birth. 76 Sleep quality and sleep maintenance during and after pregnancy have been associated with symptoms of postnatal depression77 and were raised by members of the Expert PPI Group as potential contributors to women’s poor lifestyle behaviours after giving birth. Tiredness could result in women not making healthy food choices or participating in physical activity. In terms of feasibility, lack of sleep was understood to be an important health issue to consider.
In the absence of a specific validated postnatal measure, questions were devised by the trial team. Findings were similar across the two groups, with the exception that women allocated to the intervention arm were more likely to report that their infants slept through the night for 4–6 nights per week when assessed at 6 and 12 months, although reasons for this are hard to speculate (Table 11). At 12 months, just under one-third of women across both groups reported that their babies were not yet sleeping through the night.
| Sleep pattern | Trial arm | ||
|---|---|---|---|
| Intervention | Control | Combined | |
| 6 months | |||
| Mean maternal sleep at night in last month, hours (SD) | 5.96 (1.30) | 5.87 (1.21) | 5.92 (1.26) |
| Infant sleeping through the night (nights per week), n/N (%) | |||
| 0 | 39/83 (47.0) | 36/75 (48.0) | 75/158 (47.5) |
| 1–3 | 7/83 (8.4) | 6/75 (8.0) | 13/158 (8.2) |
| 4–6 | 20/83 (24.1) | 13/75 (17.3) | 33/158 (20.9) |
| 7 | 17/83 (20.5) | 20/75 (26.7) | 37/158 (23.4) |
| 12 months | |||
| Mean maternal sleep at night in last month, hours (SD) | 6.20 (1.29) | 5.92/71 (1.17) | 6.06 (1.23) |
| Infant sleeping through the night (nights per week), n/N (%) | |||
| 0 | 19/69 (27.5) | 20/71 (28.2) | 39/140 (27.9) |
| 1–3 | 6/69 (8.7) | 10/71 (14.1) | 16/140 (11.4) |
| 4–6 | 21/69 (30.4) | 15/71 (21.1) | 36/140 (25.7) |
| 7 | 23/69 (33.3) | 26/71 (36.6) | 49/140 (35.0) |
Tobacco smoking
Smoking during pregnancy is an important risk factor for a range of adverse maternal and infant outcomes, including premature birth, low infant birthweight, stillbirth and placental abruption. 38 Clinically effective interventions to support women to stop or reduce smoking in pregnancy and not relapse postnatally are priorities for NHS maternity services, with evidence to date inconclusive as to ‘what works’. Drug interventions to support smoking cessation such as nicotine replacement therapy (NRT) and e-cigarettes are not likely to be of benefit,78 although counselling, feedback and financial incentives offered to women in late pregnancy may have some benefit. 79 Studies of interventions to support women to stop smoking in pregnancy have reported that many women who quit recommence smoking within the first year postpartum,80 and postnatal-specific interventions have highlighted the importance of considering other aspects of women’s lifestyles given the complexity of supporting women to stop smoking. 81
We were interested in assessing whether or not the intervention could affect other aspects of women’s health behaviour, including tobacco smoking. Multiple-choice questions were adapted from Mullen et al. 81 because it was considered that these would generate more accurate information than a single ‘do you smoke?’ question, although it was recognised that some women may not report or under-report their tobacco use. Questions on NRT and use of e-cigarettes were also included; nicotine use increases metabolism and could affect weight management, and it was possible that there could be an increase in the use of e-cigarettes among postnatal women.
Only a small number of women in the sample reported that they smoked, in line with the local maternity population. In the local area in 2014–15, 3% of women acknowledged that they smoked at the end of pregnancy. 82 At baseline, eight women smoked, four of whom were occasional smokers and four of whom smoked daily (Table 12). When asked how soon after getting up these women smoked their first cigarette, of the seven women who answered the question, one woman had a first cigarette within 5 minutes, three within 5–30 minutes and three within 31–60 minutes.
| Tobacco smoking | Trial arm, n/N (%) | ||
|---|---|---|---|
| Intervention | Control | Combined | |
| Baseline | |||
| Never smoked | 75/98 (76.5) | 62/95 (65.3) | 137/193 (71.0) |
| Ex-smoker | 19/98 (19.4) | 29/95 (30.5) | 48/193 (24.9) |
| Current occasional smoker | 3/98 (3.1) | 1/95 (1.1) | 4/193 (2.1) |
| Current daily smoker | 1/98 (1.0) | 3/95 (3.2) | 4/193 (2.1) |
| 6 months | |||
| Never smoked | 65/83 (78.3) | 51/75 (68.0) | 116/158 (73.4) |
| Ex-smoker | 12/83 (14.5) | 19/75 (25.3) | 31/158 (19.6) |
| Current occasional smoker | 2/83 (2.4) | 2/75 (2.7) | 4/158 (2.5) |
| Current daily smoker | 4/83 (4.8) | 3/75 (4.0) | 7/158 (4.4) |
| 12 months | |||
| Never smoked | 56/69 (81.2) | 49/71 (69.0) | 105/140 (75.0) |
| Ex-smoker | 7/69 (10.1) | 16/71 (22.5) | 23/140 (16.4) |
| Current occasional smoker | 2/69 (2.9) | 3/71 (4.2) | 5/140 (3.6) |
| Current daily smoker | 4/69 (5.8) | 3/71 (4.2) | 7/140 (5.0) |
A greater number of women in the intervention arm had never smoked. Women in the control arm who were current smokers smoked a mean of 8.30 cigarettes per day (SD 2.89 cigarettes per day) compared with a mean of 3.50 cigarettes per day (SD 1.73 cigarettes per day) smoked by women allocated to the intervention arm. No women in the control arm were using NRT and one woman in the control arm was using e-cigarettes. At 6 months, there was a very small increase in the number of women in the intervention arm who smoked daily. Among women who smoked occasionally or daily, women reported smoking < 10 cigarettes per day. Women allocated to the control arm smoked on average 8.75 cigarettes per day (SD 6.29 cigarettes per day) and women allocated to the intervention arm smoked on average 6.17 cigarettes per day (SD 5.49 cigarettes per day). One woman in the intervention arm was using NRT, with e-cigarettes used by two women in the intervention arm and one woman in the control arm. At 12 months, findings were similar, with women who smoked smoking on average 6.25 cigarettes per day (SD 6.18 cigarettes per day) in the intervention and 6.33 cigarettes per day (SD 6.22 cigarettes per day) in the control arm. No women were using NRT and five women (two in the intervention arm and three in the control arm) were using e-cigarettes.
Alcohol consumption
Another important issue to assess was whether or not the intervention had potential to impact on women’s alcohol consumption. In the absence of a specific measure of this outcome among pregnant and postnatal women, we used questions from the Alcohol Use Disorders Identification Test Guidelines for Use in Primary Care. 39 Women were asked about their alcohol consumption in the previous 6 months, including how often they drank alcohol, how many units of alcohol they drank on a typical day when drinking and how often they had six or more units on a single occasion in the last 6 months.
At baseline, 80% of women in both groups reported not drinking any alcohol, with five women (5.1%) in the intervention arm and two (2.1%) in the control arm reporting that they drank alcohol two to four times per month (Table 13). At 6 months, women in the intervention were less likely to drink any alcohol [44 women (53.0%) allocated to the intervention arm compared with 33 women (44.6%) allocated to the control arm; a statistically significant difference (95% CI –2.719 to –0.083; p = 0.038)]. At 12 months there were no significant differences, although women in the intervention arm were still more likely to report not drinking any alcohol or drinking alcohol only monthly or less than this. Additional data are presented in Appendix 3, Table 26.
| Maternal self-esteem | Trial arm | ||
|---|---|---|---|
| Intervention | Control | Combined | |
| Baseline | |||
| n | 98 | 95 | 193 |
| Mean maternal self-rated esteem score (SD) | 32.34 (4.13) | 32.74 (3.71) | 32.53 (3.92) |
| 6 months | |||
| n | 83 | 75 | 158 |
| Mean maternal self-rated esteem score (SD) | 32.84 (4.77) | 33.81 (4.84) | 33.30 (4.81) |
| 12 months | |||
| n | 69 | 71 | 140 |
| Mean maternal self-rated esteem score (SD) | 32.77 (4.48) | 33.06 (4.84) | 33.06 (4.84) |
Maternal body image
Women who have obese or overweight BMI scores are more likely to have a poorer body image than women who have normal BMI scores. Poor body image can affect women’s willingness to participate in physical activities and undertake positive lifestyle behaviours, such as commence and continue to breastfeed, owing to fear such as of negative comments when breastfeeding in public. 83,84 A better body image among women who have higher BMI scores could be protective against the onset of depression after birth, because obesity and symptoms of depression have been found to cluster together in women. 85
To assess women’s perspectives on their body image at 6 and 12 months, we used the Eating Disorders Examination questionnaire on body image. 40 The seven questions ask women about how they have felt about their weight or shape over the last 4 weeks, using a scale of 0 to 6, and to indicate how often they have felt that way in the last 4 weeks. On one of the subscales included at 12 months, women in the intervention arm were more likely to report no dissatisfaction with their weight in the previous 4 weeks than women in the control arm (p = 0.055). There were no differences between the groups in the global score or any other subscales. Full data are included in Appendix 3, Table 27.
Maternal self-esteem
In addition to physical and mental health consequences, a range of psychosocial issues, including decreased feelings of self-worth, can be experienced by an individual who has an overweight or obese BMI score, with some general population studies reporting links between weight stigma and poor self-esteem. 86 To provide a measure of whether or not the intervention had the potential to affect women’s self-esteem (feelings of worthiness), and if this differed between trial arms, we used the Rosenberg Self-Esteem Scale40 at baseline and 6 and 12 months. The scale was developed, using data on > 5000 adolescents attending US high schools, specifically for use as a research tool. It includes 10 questions with four options (strongly agree, agree, disagree and strongly disagree) and has a total score of 40. A score of ≤ 15 is considered to indicate that an individual has a problem with their esteem. The scale has been used in previous maternity care studies, including those that have focused on aspects of postnatal maternal weight management in an ethnically diverse population. 10
The scale asked women to respond to each question with the rating that best reflected their general feelings about themselves. We found no differences in mean scores between the trial arms and women’s self-esteem scores at any of the time points. Further data are presented in Appendix 3, Table 28.
Implications of feasibility trial findings for a future definitive trial
-
Recruitment and retention strategies enabled the required sample size of women from a diverse inner-city area to be retained to 12 months postnatally and highlighted that, although overall approaches worked well with women with overweight or obese BMI scores at antenatal booking, approaches to identify and recruit women with normal BMI scores who gained EGWG would need to be reconsidered in a future trial.
-
The two planned appointments with the research midwives at 6 and 12 months to complete trial follow-up supported high rates of follow-up, high rates of completion of trial measures and a lack of difference in drop-out rates across the groups. This should be a strategy adopted in a future trial.
-
Feasibility outcomes were designed to clarify uncertainty to inform progression to a definitive RCT. This included whether or not women’s antenatal booking BMI scores should be used as the baseline measure to compare postnatal weight change. Trial findings support the use of antenatal booking BMI score as the baseline comparison, given the potential selection bias that use of a woman’s BMI score at trial entry could introduce.
-
The primary outcome was difference between the trial arms in women’s weight, as measured from antenatal booking to 12 months postnatally. Findings support use of a 12-month primary end point in a future trial.
-
The intervention included specific dietary support offered to women allocated to the intervention arm as part of the Slimming World programme. For a definitive trial, consideration should be given to identification of a more appropriate measure of dietary intake that reflects the healthy eating or nutritional content recommended as part of a trial intervention.
-
Given a lack of change in other important public health areas of interest such as breastfeeding, tobacco smoking and drinking alcohol, when developing a definitive trial specific consideration should be given to priority public health needs in the local population to be targeted. This would enable measures to be more appropriately tailored to capture improvements in outcomes of interest.
-
As findings showed minimal differences in outcomes between the trial arms (the trial was not powered to detect change), consideration should be given in a future trial to extending the intervention offer to enable any lifestyle changes to be ‘embedded’ and any subsequent changes in positive health behaviour outcomes to be detected (see the findings presented in Chapter 6).
Chapter 6 Findings from the process evaluation
Parts of this chapter have been reproduced from Taylor et al. 87 This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
This chapter presents the findings from the process evaluation element of the trial. The process evaluation sought to address objective 4: to assess the acceptability of the intervention and trial procedures. This chapter is organised according to the four specific aims; these are to assess:
-
the acceptability of the intervention and how the intervention was experienced by women, including views on timing of commencement
-
probable variation in groups attended by women (day/time; whether or not women changed groups/consultant)
-
the timing and sources of additional weight management support, including risk of contamination
-
the acceptability of trial processes and procedures.
The methods are described in full in Chapter 4. These aims were met through the use of questionnaire data (6 and 12 months), semistructured interviews and data provided by Slimming World.
Questionnaire
The overall response rates at both time points for both trial arms were high (at least 70% responded) (Table 14; see Chapter 5). At both time points, there were two open-ended questions about attendance at the commercial weight management group for women allocated to the intervention arm (‘If you left any weekly sessions before the end, could you say why?’ and ‘How useful did you find the weekly sessions?’). At least one of these questions was answered by each woman who attended at least one session. For women who did not attend any sessions there was a question asking them to explain their choice not to attend.
| Trial arm (N) | Questionnaire, n (%) | |
|---|---|---|
| 6 months | 12 monthsa | |
| Intervention (98) | 83 (85) | 69 (70) |
| Control (95) | 75 (79) | 71 (75) |
Interviews
Of the 17 women invited to participate in interviews immediately following the intervention, 13 agreed to participate. At 12 months postnatally, consent was received from all (n = 16) women allocated to the intervention arm approached to participate, though only nine completed an interview at this time point (six of whom also participated in 6-month interviews; Table 15). A total of 12 women allocated to the control arm provided written consent to participate, of whom eight completed an interview (Table 16). As outlined in Chapter 2, women were purposively selected to encompass those who attended the recommended number of sessions (≥ 10) and those who did not, those who lost weight and those who gained, and variability in demographic factors such as ethnicity and parity.
| ID | Interview time point(s), months | Demographic characteristics | Antenatal booking BMI score, kg/m2 (category) | Antenatal booking weight, kg | Weight gain at 36 weeks, kg | Age of baby at start of SW, weeks | Weight change, kg | Number of sessions attended | |
|---|---|---|---|---|---|---|---|---|---|
| 6 months | 12 months | ||||||||
| 0–5 sessions attended | |||||||||
| Crocus | 6 | Aged 26 years, white European, primigravid | 26.30 (OW) | 75.2 | 12.6 | – | 82.8 G | 86.6 G | 0 |
| Marigold | 12 | Aged 32 years, black British, multigravid | 28.00 (OW) | 72.5 | 7.5 | – | 72.0 L | 70.0 L | 0 |
| Anemone | 6 and 12 | Aged 32 years, white European, primigravid | 28.30 (OW) | 69.4 | 13.7 | 12 | 72.2 G | 72.1 G | 1 |
| Iris | 6 and 12 | Aged 32 years, black African, primigravid, pregnant again at 12 months | 29.30 (OW) | 76.8 | 3.4 | 12 | 83.6 G | 81.2 G | 1 |
| Daisy | 6 | Aged 30 years, white European, missing parity | 26.71 (OW) | 70.1 | 11.7 | 8 | 73.4 G | Withdrew | 3 |
| Allium | 12 | Aged 35 years, white European, multigravid, EGWG | 24.20 (normal) | 54.0 | 18.2 | 12 | 61.2 G | 59.0 G | 3 |
| 6–9 sessions attended | |||||||||
| Daffodil | 6 | Aged 29 years, black British, primigravid, achieved her target | 25.70 (OW) | 75.5 | 4.7 | 13–14 | 62.4 L | Missing | 7 |
| Hyacinth | 6 | Aged 20 years, white British, primigravid | 38.74 (OB) | 96.7 | 10.3 | 16 | 107.0 G | 110.4 G | 7 |
| Lavender | 6 | Aged 38 years, white British, primigravid | 28.10 (OW) | 81.2 | 14.6 | 9 | 82.0 G | 80.8 L | 9 |
| Aster | 12 | Aged 42 years, white, primigravid | 46.00 (OB) | 130.6 | 0.6 | 16 | 133.4 G | 133.4 G | 6 |
| ≥ 10 sessions attended | |||||||||
| Amaryllis | 6 | Aged 41 years, white British, multigravid | 36.57 (OB) | 102.0 | 12.4 | 16 | 100.6 L | 103.4 G | 11 |
| Azalea | 6 | Aged 31 years, white British, primigravid | 30.05 (OB) | 94.8 | 7.4 | 12 | 89.0 L | 93.9 L | 11 |
| Heather | 6 and 12 | Aged 37 years, white British, primigravid | 27.50 (OW) | 79.8 | 7.6 | ≥ 16 | 75.8 L | 77.2 L | 12 |
| Hibiscus | 6 and 12 | Aged 39 years, white Irish, multigravid | 28.70 (OW) | 79.5 | 5.7 | 10 | 77.2 L | 79.8 G | 10 |
| Orchid | 6 and 12 | Aged 38 years, white European, primigravid, continued with SW | 27.60 (OW) | 75.2 | 17.8 | 8 | 76.4 G | 71.5 L | 10 |
| Violet | 6 and 12 | Aged 28 years, black British, multigravid, continued with SW | 32.20 (OB) | 104.5 | 2.5 | 12 | 90.9 L | 87.0 L | 11 |
| ID | Demographic characteristics | Antenatal booking BMI score, kg/m2 (category) | Antenatal booking weight, kg | Weight gain at 36 weeks, kg | Weight change, kg | |
|---|---|---|---|---|---|---|
| 6 months | 12 months | |||||
| Beech | Aged 36 years, white, primigravid | 26.30 (OW) | 60.9 | 10.9 | 56.2 L | 55.0 L |
| Pine | Aged 40 years, white, primigravid | 32.30 (OB) | 87.0 | 4.6 | 83.3 L | 79.3 L |
| Birch | Aged 35 years, white, multigravid | 27.80 (OW) | 77.5 | 10.9 | 80.4 G | 83.7 G |
| Ash | Aged 40 years, black, multigravid | 29.00 (OW) | 72.5 | 9.5 | 84.0 G | 76.0 G |
| Chestnut | Aged 45 years, white, primigravid | 25.30 (OW) | 60.0 | 7.2 | 62.8 G | 58.4 L |
| Maple | Aged 41 years, black African, multigravid | 27.20 (OW) | 72.9 | 3.1 | 78.2 G | 76.0 G |
| Rowan | Aged 32 years, South Asian, primigravid | 24.91 (normal) | 72.2 | Missing | 81.0 G | 71.0 L |
| Willow | Aged 34 years, white, primigravid | 29.90 (OW) | 81.5 | 15.7 | 86.0 G | 82.6 G |
Acceptability of the intervention and how the intervention was experienced by women
Quotations throughout have been anonymised by providing each woman with a pseudonym ID (i.e. a flower). Quotation attributions contain the ID, number of sessions attended (if allocated to the intervention arm), weight change from antenatal booking and the time point (i.e. 6 or 12 months) at which data were provided via interview or questionnaire, and may contain BMI score at antenatal booking.
Attendance
Of the 98 women assigned to the intervention arm, and according to Slimming World records, 46 women (47%) attended at least one session and 52 (53%) did not did not attend any sessions. Of the women who attended at least one session, 19 (41%) attended 10–12 sessions and 19 (41%) attended only one to five sessions (Figure 4). Among those attending, the mean number of sessions attended was 6.74 (SD 3.94). Two women recorded as not attending any weight management sessions were found by the research team to be anomalies. Data could not be found by Slimming World for one woman who reported attending 12 sessions, and a second woman who reported going to a first session stated that she was not able to join the group owing to requiring information about her weight that she did not have (see Non-attendance for further details). The denominator is therefore 46 when based on data provided by Slimming World and 48 when based on questionnaire data.
FIGURE 4.
Slimming World sessions attended by women allocated to the intervention group who attended ≥ 1 session (n = 46).
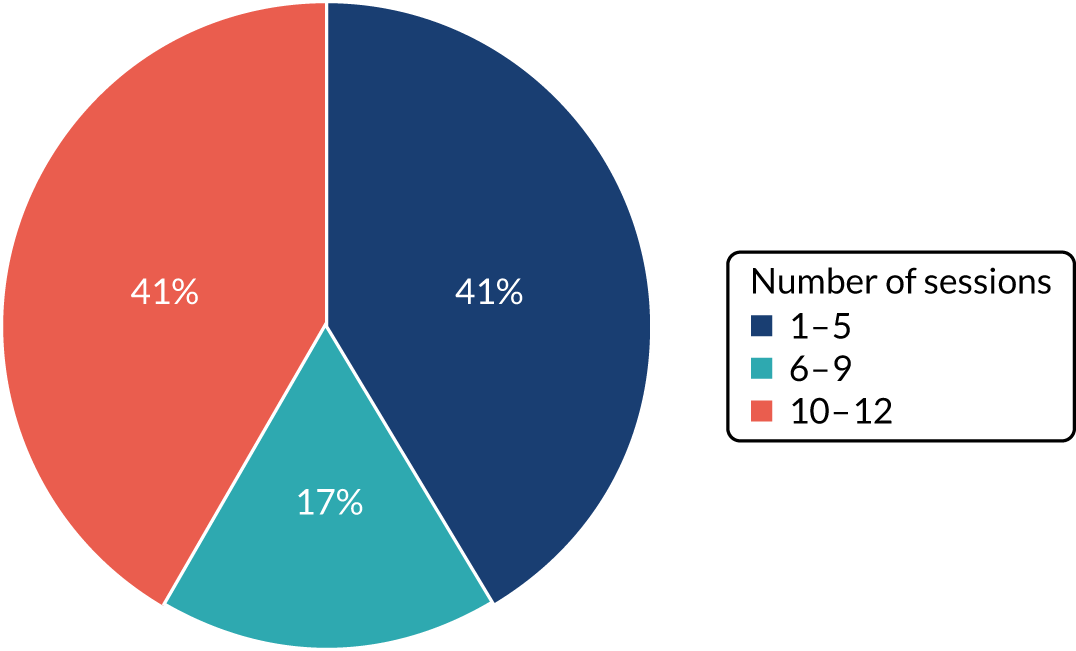
In the intervention arm, the 12-month questionnaire was completed by 62% of women (32/52 women) who did not attend any sessions and by 80% of women (37/46 women) who did attend Slimming World: 17 women who attended ≥ 10 sessions, seven women who attended six to nine sessions and 10 women who attended one to five sessions. Both of the women who attended Slimming World but did not have their data recorded by the organisation responded to both the 6- and 12-month questionnaires.
Non-attendance
Of the 52 women who did not did not attend any weight management sessions, 39 (75%) provided a reason for this in the questionnaire. Women offered several key reasons, most of which related to ‘opportunity’ issues within the COM-B framework (see Chapter 2). The most common barrier reported was finding time to attend; more than half of women who responded indicated that their circumstances did not allow them time to attend groups. Several explained that looking after their baby took up all their time or energy:
Difficulty with child care and generally getting to grips with being a new mum was more important.
Snapdragon, lost 12.8 kg, 12 months
Some specified that breastfeeding on demand made it difficult to make time, others related that they were looking after older children and several mentioned that they had no child-care support to enable them to come:
I chose to breastfeed on demand so I would not have been able to attend any scheduled sessions easily.
Rose, gained 12.0 kg, 12 months
The timing of sessions in the evening, when children were going to bed, was an additional limiting factor for a few women. Two participants commented that the groups were offered too soon after having their baby, and both indicated that 6 months post partum would have been more suitable for them [see Accessibility of the intervention (physical opportunity), Timing of commencement of the intervention].
Women also identified circumstantial and social factors that meant that they did not have the opportunity to attend. Several women said that their babies were unwell or that they themselves had physical or mental health problems after the birth that prevented them from attending. Furthermore, there were two cases where the women were not enrolled in the programme on time, reporting that there were administrative errors by Slimming World. Other reasons included, as reported by one woman, that as a non-native English speaker she was not confident enough to attend, and, as reported by a few women, that they were either out of the country for a prolonged period of time or had moved away from the city. Only a small number of women in this group found that the groups were difficult to access in terms of location.
Together with these ‘opportunity’ factors that presented barriers to participation, there were also several ‘motivational’ factors highlighted. Some women felt that the weight management group was not suitable for them: they did not feel motivated to lose weight or they lacked confidence in the effectiveness of the intervention. Some were happy with their weight or felt they had already managed to lose enough; one joined an online weight management group that she found helpful and very convenient to access and another decided not to participate because she did not agree with Slimming World’s approach (i.e. minimising fat consumption), misunderstanding that this could mean that the diet allowed high-sugar foods:
Slimming World is based around minimising fat consumption within the diet. I don’t think it is healthy to completely remove a food group from the diet. I also strongly disagree with 0%-fat products as their sugar content is very high and this has its own problems.
Freesia, lost 2.6 kg, 12 months
Finally, one participant appeared to interpret the trial as being targeted at losing pregnancy weight gain, perhaps not recognising that she had an overweight BMI score (27 kg/m2) at the start of her pregnancy. She felt very strongly that the weight management offer was not targeted at new mums and therefore was not relevant to her:
When I called to make the first appointment it became clear that Slimming World was targeted at overweight people rather than new mothers. I was asked whether I would be comfortable with people cheering if I’d lost weight, which made me think I had done something wrong rather than have a child! And also that the programme was targeted to teach me how to eat better, for example by using olive oil and not eating ready meals, which I’d never done. So it sounded more like something for unhealthy people with bad habits, not new mums.
Lily, lost 6.5 kg, 12 months
Views on the weight management intervention
Analysis of the questionnaire and interview data resulted in the identification of five key themes describing women’s experiences of the groups. Each of these is described and evidence mapped against the COM-B domains (Table 17). Themes demonstrate how women perceived and understood the intervention and highlight the salience of factors that may facilitate or act as barriers to uptake and engagement. Each is considered in turn in this section.
| Themes | COM-B domain(s) | Subthemes or descriptions |
|---|---|---|
| Weight loss aspirations | Motivation | Reasons for participating in the trial; personal evaluation of weight and reasons for overweight |
| Beliefs and expectations | Motivation (psychological capability) | Previous weight management experiences and fit of intervention or weight management through diet with weight loss beliefs |
| Understanding and implementing the intervention | Motivation (psychological and physical capability) | Implementing the Slimming World programme: ease of implementation – evidence of changes made and dietary planning; views about sustainability of the Slimming World programme; motivating factors (e.g. reinforcement) |
| The social context | Opportunity (social opportunity) and motivation |
Group identification and support: women’s views about group support and their personal comparison of themselves in relation to others attending the group The consultant–participant relationship: quality of support and interaction with consultant; personalisation of support (e.g. needs and circumstances as new mothers understood or considered) Social support (partners and others) |
| Accessibility of the intervention | Opportunity (physical opportunity) |
Timing of commencement of the intervention Group attendance (location, convenience, changing groups, joining Slimming World) Continuation beyond intervention (facilitators/barriers) |
Weight loss aspirations
Most women interviewed expressed aspirations for weight reduction and positive lifestyle changes as reasons for deciding to participate in the trial. However, the strength of these aspirations varied across the sample. Aspirations were often related to whether or not they saw weight loss as a priority after birth and to women’s personal evaluations of their weight.
Women who completed the programme by attending ≥ 10 sessions, and most women who attended between six and nine sessions, acknowledged that they had an overweight BMI score and attributed their weight gain to ‘comfort eating’, ‘eating too much’, or ‘eating rubbish’. Some talked about gaining weight during pregnancy whereas others talked about having struggled with weight for a long time during their lives. All expressed a desire to lose weight, some talked about wanting to break ‘unhealthy’ dietary habits to gain a ‘sense of control’ and others talked about gaining weight during pregnancy or wanting to get back to their pre-pregnancy weight. All were overweight at the start of their pregnancies and had gained 3–18 kg during pregnancy. They were keen to take part and felt that the programme would be beneficial:
I guess I got into the routine of comfort eating and putting on more weight. I thought that if I didn’t take this opportunity I would have really been at a loss, so it would benefit me.
Violet, 11 sessions, lost 17.5 kg, 12 months
I’ve tried SlimFast® [SlimFast, Palm Beach Gardens, FL, USA], I’ve tried Juice Plus+® [Natural Alternatives International, San Macros, CA, USA] and I’ve tried Weight Watchers [WW International, Inc., New York, NY, USA]. I’ve previously tried Slimming World. There was a lot . . . I’ve always been overweight. I thought it might have been a bit easier to lose weight after having a baby. I was told by quite a lot of people and I was quite excited to try it to see if it would work.
Hyacinth, seven sessions, gained 13.7 kg, 12 months
By contrast, for women who attended six to nine sessions but had gained weight at 6 months postnatally, or those who attended fewer than six sessions, adapting to life with a new baby in those early months was seen as more of a priority than weight management. One woman who attended six sessions acknowledged that she had an overweight BMI score but did not consider weight management to be a priority; her priority was to acclimatise to life with a new baby and her concern was her postnatal mental health and overall fitness and well-being, but not weight loss specifically. Therefore, she felt that the intervention did not address her particular needs:
There wasn’t a holistic approach in terms of the intervention, it was just, ‘right, you’ve got a baby and 3 months to get rid of the weight’. It felt a little disconnected. For me, 3 months into having had a baby, I wasn’t standing there thinking I’ve got to lose the weight. I was standing there thinking, ‘wow, how do I reacclimatise and recalibrate my life to accommodate this little person?’ So I think it was too early and again I don’t think it was the right intervention.
Aster, six sessions, gained 2.8 kg, 12 months
Women seemed less aware of having overweight BMI scores at antenatal booking and focused more on evaluating their weight on the basis of pregnancy weight gain. In this context they did not consider themselves to have an overweight BMI score:
I didn’t gain too much weight during pregnancy. Compared with the people who are at [name of Slimming World group], I really was the slimmest there. The people who are at the group really needed help, you could tell, it’s quite a big difference between me and them.
Anemone, one session, gained 2.7 kg, 12 months; BMI score at antenatal booking 28.3 kg/m2
I’m not too much on the big side. When I was pregnant the body changed and I didn’t add too much weight . . . but I think I just wanted to be able to get back to my shape, post pregnancy . . . I’ve never been overweight. I’ve just always tried to maintain a healthy [lifestyle], tried to eat healthy and tried to keep fit as much as I can.
Iris, one session, gained 6.8 kg, 12 months; BMI score at antenatal booking 29.3 kg/m2
Beliefs and expectations (motivation: psychological capability)
In interviews, most women described long histories of weight issues and prior experience of successful and unsuccessful attempts at weight loss (including weight loss to conceive or for occasions such as weddings or holidays). Previous attempts involved adopting a variety of strategies, most notably some form of exercise, and for some women adopting short-term ‘diets’ such as the SlimFast Plan® (SlimFast) or the ‘cabbage soup diet’. Three women also had prior experience of commercial weight loss programmes: Slimming World, Weight Watchers and the LighterLife® programme (LighterLife UK Ltd, Harlow, UK).
Interviews revealed that women’s previous experiences of weight loss and their beliefs about postnatal weight management influenced how they perceived the intervention and in particular their perceptions of its efficacy.
Women who completed the programme, and the one woman who attended seven sessions and reached her target weight, acknowledged that previous attempts had provided them with only short-term benefits in relation to weight loss or that their attempts either through diet or exercise had been unsuccessful. They were often surprised to learn about the role of diet and exercise in weight management, and attending sessions and losing weight facilitated a change in previously held beliefs:
I’ve gone on various diets but half-heartedly, and I’ve always done it though exercise and worked extra hard on the exercise side. So it’s a kind of revelation that it’s 90% food and 10% exercise, which is what I learnt at Slimming World. I knew that it was about being aware of what you are eating and how that can help you lose weight and also how you add exercise into the mix . . . Seeing five and a half pounds’ loss in the first week confirmed it would work. I am proud of myself.
Heather, 12 sessions, lost 2.6 kg, 12 months
At 12 months these women reflected on and described once again about learning about the role of diet and exercise in weight management as key, even if they had gained weight since completing the programme:
One thing I learnt at Slimming World is that diet is more important than exercise. Exercise alone is not going to lose the weight and the impact of cutting out calories in your diet is much bigger than going to the gym. When I was at university I was very active and did lots of sports and I couldn’t understand why I was still overweight. When I look back now to what I ate and drank at university, it’s no wonder.
Hibiscus, 10 sessions, gained 0.3 kg, 12 months
Their beliefs in the intervention were strengthened as they felt that it was safe and relevant to their needs as breastfeeding mothers:
They’ve got a handout for breastfeeding and pregnancy, so you know that the diet is safe to be on and they’ve obviously done their research.
Azalea, 11 sessions, lost 1.8 kg, 12 months
In the questionnaire, two further women who had attended ≥ 10 sessions indicated that the intervention had increased their knowledge and skills: ‘I still go off track but then I just go over the programme myself at home’ (Lotus, 10 sessions, lost 8.1 kg, 12 months); ‘Learnt a lot of substitutes and better choices of cooking foods’ (Gardenia, 11 sessions, lost 1.7 kg, 12 months). Two women mentioned the psychological benefits of attending a weight management group: ‘It made me feel like I had control back over my body’ (Azalea, 11 sessions, lost 1.8 kg, 12 months); ‘It’s been amazing for my self-confidence returning to work’ (Cyclamen, 12 sessions, lost 9.8 kg, 12 months). Both of these women lost weight over the course of the trial and said that the intervention was effective; one still attends Slimming World, and the other stopped for financial reasons.
By contrast, women who attended fewer than six sessions and gained weight tended to be more mistrustful of the intervention. One area of concern was their perception that the programme failed to prioritise exercise. One woman who had previously managed her weight by attending Zumba® (Zumba Fitness, LLC, Hallandale Beach, FL, USA) classes commented on the lack of exercise as affecting her motivation to continue:
I just felt there should also be an activity, there wasn’t any exercise classes or anything like that and maybe that side of things didn’t attract me . . . I would have also been quite motivated for me to try and go back if I knew that maybe there was some form of exercise class that would be involved in the session.
Iris, one session, gained 4.4 kg, 12 months
Two women commented on this aspect in their questionnaires too. One stated that she ‘did not agree with some of the information regarding exercise’ (Poinsettia, one session, gained 1.8 kg, 6 months) and another that the diet ‘was not geared towards people who train . . . was convoluted – prefer to focus on exercise’ (Poppy, two sessions, lost 7 kg, 12 months).
Another area of concern was the perception that Slimming World was about marketing their own products and promoting the consumption of artificial sweetener, which did not fit in with the beliefs of one woman who attended one session. She was sceptical about the focus of the plan and about the group’s intentions:
I didn’t know what to expect and I was hoping for activity and not just sitting on a chair and encouraging ourselves. I’m well educated in how to lose weight. I didn’t like the idea of having allowable ‘syns’ [points]. I saw they had some boxes [products] with allowable syns which were basically sugar . . . Everything was a Slimming World product; it was mass advertising for their products. So I was sceptical about that and the intentions as well.
Anemone, one session, gained 2.7 kg, 12 months
Several women who attended between six and nine sessions were mistrustful for a number of other reasons, most notably their belief that the plan was unsuitable for women who were breastfeeding. There was a general misconception about the need to eat fat in order to breastfeed. A woman who had previously attended a LighterLife weight management programme commented that she had been sceptical about the ethos of the plan and its promotion of sugar-free food, which she felt was not good when breastfeeding:
I was quite disappointed and quite shocked . . . I was expecting it to be quite advanced ideas, which was the LighterLife stuff, which was you look at your relationship with food. I found I was eating a lot of sugar-free stuff, which I didn’t like very much breastfeeding. Knowing that . . . it’s quite a low-sugar, low-fat diet. I was also really reluctant to cut my fat levels down too much so I still tried to keep in some full-fat milk and I would still eat more fatty avocados and stuff like that . . .
Lavender, nine sessions, lost 0.4 kg, 12 months
In questionnaire responses from those who had attended < 10 sessions, although two women thought that the advice they received in the weight management group was ‘reasonable’ and ‘common sense’ (Sunflower, two sessions, lost 2.4 kg, 6 months), two noted that it was targeted more at people with ‘no healthy eating knowledge’ (Bluebell, three sessions, lost 5.4 kg, 12 months) and that consequently they had not benefited as much: the sessions had ‘not improved my health all that much as I already had some knowledge of healthy eating and exercise’ (Geranium, three sessions, gained 9.9 kg, 12 months). One woman who was also interviewed, and who described the role of exercise in the programme as a barrier, indicated in the questionnaire that she had only partially followed the advice given at the sessions as she found it challenging: ‘some interesting advice, which I took on board, but struggle to plan diet and follow through’ (Allium, three sessions, gained 5.0 kg, 12 months).
Understanding and implementing the intervention (capability and motivation)
Capability in relation to understanding and being able to apply the Slimming World programme appeared to have a dose response effect: the more sessions women attended, the more they appeared to understand the plan and were able to incorporate it into their daily lives.
Women who completed the programme, and one woman who attended seven sessions and reached her target weight, described changes they had made to their eating habits and physical activity. These included walking more or participating in BuggyFit® (Shabbington, UK) classes with babies as well as dietary changes such as reducing cereal or bread consumption and replacing oil with low-fat sprays. They commented that adopting the plan was ‘easy’ and ‘uncomplicated’ (Begonia, 10 sessions, gained 6.3 kg, 12 months):
Slimming World was a lot easier in terms of a lot more practical for life. I liked the fact that you could have the syns, so you don’t feel guilty having them if you’ve had a terrible day, or whatever, so I liked that. Not having to weigh food makes a big difference.
Amaryllis, 11 sessions, gained 1.4 kg, 12 months
Some felt that that the plan reflected what they were already eating, but they had made some adaptations including changes to cultural foods. Others talked about specific Slimming World recipes that they found useful, e.g. Diet Coke® (The Coca-Cola Company) chicken, or using their syns when making adaptations to recipes:
It was very easy actually. I was actually quite surprised that a lot of the foods that I was eating I was able to continue eating. I’d have to make adaptions to the sizes or fat-free versions but all in all a lot of the meals that I could prepare are meals that I was used to making at home. It’s just making those little tweaks. I could still cook the barbecue chicken if I wanted to but obviously I’d have to syn the barbecue sauce or adapt to a Diet Coke chicken. I tried to stay away from the fried food. I can just bake the chicken instead of frying it.
Violet, 11 sessions, lost 17.5 kg, 12 months
Women also talked about planning meals and using online resources and the Slimming World software application (app) to help them make better decisions and choices. They attributed gains in weight to straying from the plan. However, they saw the plan as something that they could return to when this happened. Women also described the plan as unrestrictive and something that promoted empowerment and a sense of belief and control and fitted well into postnatal life:
I also knew why I’d gained; it was always the weeks when I’d gone out or eaten something that I knew I shouldn’t. I was aware of that. There were some weeks where it was like a birthday or there was something going on, but it just gave me that element of control. It was just very empowering, it was very much like, ‘you can do this. There is loads of food that is available for you to eat, you are not restricted in any way’. It honestly didn’t feel like a diet . . . If I was breastfeeding, I could just stand up and get a Babybel® [Bel Group, Paris, France] and there were things I could pre-make and just have ready for myself so I could still do the diet but still be looking after a newborn when you are tied to the sofa. It was nice to think, ‘yes, but I am not eating biscuits, I’m not out of control’.
Azalea, 11 sessions, lost 1.8 kg, 12 months
Women in this group also saw the plan as sustainable and a lifestyle change:
It felt to me to be sustainable, not a harsh diet that I was only going to do for a few weeks. I was still enjoying myself and enjoying my food and I was still losing weight, so that was very motivating.
Hibiscus, 10 sessions, gained 0.3 kg, 12 months
However, at her 12-month interview, this same woman described some difficulties with implementing the plan beyond the intervention period without the support of attending the group and continued use of the Slimming World app:
It’s easier to let yourself off the hook when nobody is checking up on you. At the time it was easy enough and I’ve been trying to keep going with some of the basic principles. One thing I used to use a lot is the app and it had a syn calculator but once your membership lapses you don’t get to access it anymore. Just to continue to have access to the app resources would have been helpful.
Hibiscus, 10 sessions, gained 0.3 kg, 12 months
Women were motivated by what they perceived as the wider benefits of adopting the plan, including benefits that they could pass on to their baby in relation to nutrition and that helped them to stay healthy to look after their baby. These women continued with Slimming World beyond the trial intervention period:
For me it was better because I knew that I was having more healthy foods, so having spinach and stuff like that incorporated into my baby even more. I know that my baby was benefitting from that.
Violet, 11 sessions, lost 17.5 kg, 12 months
Adopting the plan was also seen as beneficial to addressing other health issues, for example hypertension:
I think with this where I am is the high blood pressure and I’m an older mum, it took me 15 years to have her, so I want to enjoy this. I don’t want to be ill and having to go to the doctors and worry about my blood pressure. I’m off the pills completely now.
Orchid, 10 sessions, lost 3.7 kg, 12 months
Women in this group were motivated by seeing a positive difference in their weight and being weighed every week to keep them on track. In this context, women talked about their sense of accountability throughout the active intervention period; this included accountability to the group and to themselves, which had a motivating effect:
Also for me knowing that I have to go in and get weighed and knowing that it would get told to everyone whether I’d gained or lost kind of gave me that little check to stay on top of it.
Daffodil, seven sessions, lost 13.0 kg, 6 months
The weighing keeps you on your toes like ‘oh I’ve got to weigh in’ and you want to do well and you get in a little competition with yourself.
Orchid, 10 sessions, lost 3.7 kg, 12 months
Four women from this group were followed up at 12 months, two of whom had continued with Slimming World beyond the intervention period. They all talked about continuing to implement the principles they had learnt through attending the programme, although not having access to the Slimming World resources made it more difficult to stay on track and resulted in weight gain for women who were no longer attending. However, all women described a healthy diet as incorporating the key principles of the weight management intervention, which they could use moving forward:
A healthy diet is the one that I think a lot of the recipes at Slimming World give you. I do like a lot of naughty things but I think interspersed with, you pull out these tools out of your toolkit when you need to. It was a really really good thing to do.
Heather, 12 sessions, lost 2.6 kg, 12 months
Three further women who had attended ≥ 10 sessions also mentioned similar themes in the questionnaire response. One noticed the impact on her health [‘health has definitely improved; weight loss has noticeably improved my fitness’ (Cyclamen, 12 sessions, lost 9.8 kg, 12 months)], a second commented on lifestyle change [‘improve healthy lifestyle choices’ (Azalea, 11 sessions, lost 1.8 kg, 12 months)] and a third mentioned the impact on her family [‘the advice was very easy to follow, even used on kids dinners, and I lost over 1.5 stones and felt good’ (Pansy, 10 sessions, lost 4.5 kg, 12 months)].
Despite not attending the full programme, four women who had attended six to nine sessions and completed the questionnaire indicated that they too had learned to eat a healthier diet and how to have a healthier lifestyle, although two of these admitted to only partially following the advice given. A similar pattern was found with the interviewed women who attended six to nine sessions and gained weight: they described following the plan as difficult and commented that they had not made significant changes to their eating habits. For several women in this group, reluctance to fully implement the plan was related to beliefs about the efficacy of the intervention [see Beliefs and expectations (motivation: psychological capability)] and physical capability (i.e. the ease of following the plan, and for some, the physical opportunity in relation to timing of the intervention):
Knowing that . . . it’s quite a low-sugar, low-fat diet. I was also really reluctant to cut my fat levels down too much so I still tried to keep in some full-fat milk and I would still eat more fatty avocados and stuff like that . . . I just took the free food and went with it without actually counting syns . . . I am not convinced that sticking to the plan would actually help me maintain longer term.
Lavender, nine sessions, lost 0.4 kg, 12 months
For several women, knowing that they had gained weight (e.g. owing to holidays) affected their motivation to attend sessions:
When you know you are not losing weight then you are less likely to go. I think I didn’t go the week after the holiday because I know I’m not going to lose, so I missed the holiday week and the week after.
Lavender, nine sessions, lost 0.4 kg, 12 months
Two women, despite not attending many sessions, reported in the questionnaire that they had learned new skills that could help with their weight loss: ‘Learnt a lot, how to eat healthy, mix food types, etc. I know what I need to do’ (Carnation, three sessions, lost 4 kg, 12 months); ‘Slimming World has taught me to eat smarter; when I go back to work I would definitely like to try their recipes’ (Stock, one session, gained 4.1 kg, 12 months).
The social context (social opportunity/motivation)
The social context of being part of a group-based intervention was a key factor influencing how women perceived the intervention. There were two prominent subthemes: (1) perceived group identity and the group support approach and (2) the role of the consultant. A third theme relating to the wider social context – social support from partners – was also identified.
Group identity and the group support approach
Most of the interviewed women who completed the programme, and one who attended seven sessions and achieved her weight loss goal, had very positive views about their weight management group, in terms of both the other members attending and how the intervention was delivered. Several described group members as welcoming, friendly and accepting of them bringing along their baby, including sometimes offering practical help when they attended. Contact with other members afforded women with a safe, affirming, supportive and low-pressure environment where they felt comfortable to attend and share their experiences:
It’s that low-pressure, comfortable, happy environment that you are invited into, a hotchpotch of grannies and mums . . . Yes, it’s not just losing the weight, it’s everything that goes with it and I think that’s the Slimming World thing almost. It’s a strange club that you get invited to.
Heather, 12 sessions, lost 2.6 kg, 12 months
Drawing inspiration from others was often seen as motivating, particularly when women felt they were struggling:
The main thing is that it gives me the motivation to keep going. And seeing other people have up and down days, it wasn’t so bad when I had my up and down days. I think it was more just drawing from the inspiration of how much weight some of the other members had lost.
Violet, 11 sessions, lost 17.5 kg, 12 months
For some women, attending groups provided them with additional social benefits, for example feeling connected to others and making new friends and consequently feeling part of a community:
I was positively surprised how much the group was supportive and I’m also new in the area so it was a really good way to meet people, like meet new mums. I keep bumping into members in the supermarket and in the park exercising, enjoying the social side of things. I felt part of a little community and the ladies were all very welcoming. It was a bit like going to meet some mates at the pub without calories.
Orchid, 10 sessions, lost 3.7 kg, 12 months
For two women who attended Slimming World beyond the trial intervention period, the group continued to be a source of valued support. Both women had continued to lose weight:
Yes, I did go back . . . I wanted to stay in the same group because the consultant is really nice and I got to know the other ladies, so they became friends.
Orchid, 10 sessions, lost 3.7 kg, 12 months
They [group members] are very good . . . and we all got to know each other well and it’s just nice seeing a familiar face and all being a support for each other.
Violet, 11 sessions, lost 17.5 kg, 12 months
In the questionnaire responses, although most of the women who had attended ≥ 10 sessions concurred with these positive views about the group support, a few did not. Two women stated they did not find attending the groups useful: ‘Did not find group particularly helpful as most information available on the online support repository’ (Jasmine, 11 sessions, lost 16.8 kg, 12 months); ‘I would generally only stay if I had any concerns about my weight that week’ (Violet, 11 sessions, lost 17.5 kg, 12 months).
There were also some that were unhappy with some of the diet recommendations. For example, one woman said her consultant suggested she eat Angel Delight® (Premier Foods, St. Albans, UK) and another said that she disagreed with the attitude to losing weight presented: ‘The advice in my group wasn’t really about improving health. I’d prefer more info on this than “image therapy”, which I don’t like. Meetings mainly plugging food or how to cheat syns! Or eat treats’ (Lotus, 10 sessions, lost 8.1 kg, 12 months).
For several women who attended < 10 sessions (one to five sessions or six to nine sessions) and gained weight, the group support approach was not seen as beneficial and several felt that they did not identify with others attending:
The people who came and the host were of lower quality than I expected. I didn’t want to bond with them. I also don’t need to come every week to listen to other people saying ‘oh, I’ve lost weight this week and this is what I ate’ and then we all clap and say well done. I didn’t need that type of encouragement.
Anemone, one session, gained 2.7kg, 12 months
Women also perceived others attending their groups to have long-standing and established social connections with each other, which meant that they felt isolated. One woman compared her group to a well-known television sitcom that presented a comical and stereotypical view of weight management groups:
The meeting itself wasn’t bad, to be fair. It doesn’t quite fit with my expectations of motivation, to get motivation to slim, but it was interesting to probably hear some other people. A lot of them were older people and with all honesty I felt like this is more like a club for them. They were lovely, I can’t deny that, even the person that was hosting the event, she was lovely. But it’s still very – how can I put it? – like American funny, I don’t know, a bit like they went to one but sometimes on the comedy shows. But maybe it’s me going there with these ideas of Little Britain [BBC, 2003–7] kind of thing. I had some ideas what to expect and in the end it felt to me like at times that I was in a comedy sketch.
Allium, three sessions, gained 5 kg, 12 months
Comments on group identity/the groups were similarly mixed in the questionnaire responses. Some were very positive: ‘I think attending the sessions post pregnancy is a positive thing and encourages you to start to think with a healthier mind set’ (Tulip, six sessions, gained 3.6 kg, 12 months); ‘The groups were very useful to incentivise me to start to lose weight’ (Lavender, nine sessions, lost 0.4 kg, 12 months); ‘loved the group setting and sharing of experience’ (Carnation, three sessions, lost 4 kg, 12 months). Other women were less positive about this aspect of the weight management group. One woman described her group as ‘not entirely welcoming of small baby/noise’ and felt that it was too evangelical and that there was too much marketing of ready meals and products (Aster, six sessions, gained 2.8 kg, 12 months). Another was disappointed that the groups did not have ‘more cognitive behaviour therapy type info’ (Lavender, nine sessions, lost 0.4 kg, 12 months).
Other comments from those that attended < 10 sessions included that they felt the meetings were not useful or insightful: ‘Meetings are not that insightful or particularly helpful to me personally. Meetings also seem a bit disorganised and lack focus’ (Geranium, three sessions, gained 9.9 kg, 12 months). Several women commented specifically that they did not like sharing their experiences of weight loss and listening to others: ‘Didn’t want to spend an hour listening to others’ perspectives. It didn’t feel like a good use of my time’ (Bluebell, three sessions, lost 5.4 kg, 12 months); ‘Felt a bit like alcoholics anonymous, wasn’t my cup of tea . . . Don’t really like the idea of sitting down and talking about your problems as reasons why one is overweight. I felt like I don’t need a group setting to help me lose weight – not really my style’ (Geranium, three sessions, gained 9.9 kg, 12 months).
Several specifically mentioned that they struggled to identify with the group: ‘I couldn’t relate to any of the other people’ (Poppy, two sessions, lost 7 kg, 12 months); ‘The atmosphere was very city/work based and not welcoming to new mothers’ (Poinsettia, one session, gained 1.8 kg, 6 months).
Only 11 women (30% of those who attended the weight management sessions and responding to the questionnaire) provided information about how many times they had stayed for the whole session (only five women returned the log of attendance that they were asked to complete for each group attended, as described earlier).
All 11 women explained why they left sessions after being weighed. Most of them (n = 9) did not have the opportunity to stay because the timing did not fit with their schedules, the sessions lasted longer than they could stay, they needed to look after their baby or they had other family commitments. One woman explained that the group she attended only had weigh-ins (did not have image therapy). In several cases, women did not stay because they did not like the groups and/or the consultant running them: ‘didn’t feel the sessions were very well structured. Others also left before the end so didn’t feel the need to stay’ (Geranium, three sessions, gained 9.9 kg, 12 months).
The consultant–participant relationship
The personality and approach of the consultant and the quality of support they provided to women was also an important factor influencing their perception of the intervention. For most women who completed the programme (attended > 10 sessions), regardless of whether they gained or lost weight, and the one woman who attended only seven sessions and reached her target weight, their Slimming World consultants were perceived as being friendly, welcoming and encouraging. They perceived their consultants as skilful at being able to judge and pick out what they themselves and others needed in terms of support. Several valued what they perceived as personalised support that reflected their needs as mothers:
She offered support and encouragement. She also sends me special messages regarding breastfeeding, so she encouraged me to have the extra calcium and the other things that you are allowed when you are breastfeeding, so she just made sure I was clear with the information.
Orchid, 10 sessions, lost 3.7 kg, 12 months
However, for several women who attended between six and nine sessions and gained weight, and for one woman who attended > 10 sessions, their contact and interaction with their consultant was less positive. These women described feeling scrutinised and chastised for either not being able to stay for the whole group session (e.g. leaving after being weighed) or not losing weight. They felt that their consultant was not understanding or sensitive to their postnatal situation and the challenges of being a new mother:
The woman who ran the group wasn’t very nice, she was very critical . . . The first time I gained she said to me ‘why did you gain?’ I explained that things are harder when you have a baby and to be able to eat healthier things in the time that you get to eat, and she said ‘well, how important is your weight gain to you?’ I think you need to be able to read people because some people were fine with those sorts of questions, whereas me, I found it quite disheartening . . . It had an impact on my motivation to attend the body image therapy.
Hyacinth, seven sessions, gained 13.7 kg, 12 months
A similar pattern of responses was noted in the questionnaire responses with the majority of those who attended ≥ 10 sessions providing positive comments about their consultant: ‘the consultant is a very helpful person and very motivated’ (Nemesia, 10 sessions, gained 0.9 kg, 12 months); ‘Consultant very positive, made baby welcome and was supportive’ (Peony, lost 3.1 kg, 12 sessions, 12 months). Those attending < 10 sessions provided mixed views on this aspect, with three very dissatisfied with their consultant [describing their consultant as ‘abrupt and quite rude’ and ‘not very kind with her words when members gained’ (Hyacinth, seven sessions, gained 13.7 kg, 12 months), as having a ‘lack of empathy with new mum challenge’ (Aster, six sessions, gained 2.8 kg, 12 months) or as ‘patronising and demoralising . . . She had an attitude of “telling off” ’ (Amaryllis, 11 sessions, gained 1.4 kg, 12 months)]. The last woman persevered with attending despite this owing to her positive view about the Slimming World programme and the support she got from her mother, who also attended with her.
Social support
All participants who attended ≥ 10 sessions on the programme, and one woman who attended seven sessions and reached her target weight, described their families, most notably their partners (and for one woman her mother, who also attending Slimming World), as providing support in their weight management efforts. This was mostly described in relation to food preparation; several women described their partners as being happy with what they cooked and/or participating in cooking low-fat options.
One woman described how she and her husband negotiated the cooking to ensure that she could follow the Slimming World programme:
Well, actually, since I’ve been doing Slimming World my husband has started doing more cooking because he doesn’t mind the food but he’s like OK I need to be more creative and I think he’s trying to tell me that he doesn’t really want to do Slimming World as well, so he cooks and we have lots of curries, so the trade-off is he’ll cook reasonably low fat and I’ll eat what he’s cooking but I will bulk it up with vegetables, so like a hybrid of his cooking and me applying Slimming World.
Heather, 12 sessions, lost 2.6 kg, 12 months
Several women described their partners as also being keen to lose weight, and one had persuaded her husband to join her by the time of the 12-month interview:
I took my husband with me. He lost quite a lot of weight as well. So it was good.
Orchid, 10 sessions, lost 3.7 kg, 12 months
However, for one woman who gained weight again at 12 months, support from her partner had waned:
It’s a lot easier to do [follow the plan] on the nights he [husband] is working. I suppose he just likes certain food, especially when he works a lot of evenings and when he is off he likes to have a proper meal . . . steak, basically.
Hibiscus, 10 sessions, gained 0.3 kg, 12 months
By contrast, women who attended < 10 sessions and gained weight described partners who continued to eat as they had before. Many of these women described making small changes to their diet but generally continuing to cook food in the way they had always done:
If for example we’re going to have a pasta dish I would have mostly vegetables with a very small amount of pasta whereas my husband would have the normal combo. I would still eat more fatty avocados and stuff like that. I didn’t go down the whole slimmer’s swapping, I would just eat more of the free foods. I still needed to have a bit of oil so I was still cooking with oil.
Lavender, nine sessions, lost 0.4 kg, 12 months
In the questionnaires, two women specifically mentioned that it was hard for them to find the motivation to change their eating habits because they did not receive support from their partners and needed to accommodate their families: ‘Hard on portion control because I had to make food for the family, didn’t want to cook just for me. Husband wasn’t keen on programme restricting his diet’ (Bluebell, three sessions, lost 5.4 kg, 12 months). Several women stated that other aspects of support limited their participation. This included lack of child care (in relation to looking after their babies for the duration of the session and/or their older children). Three women felt that it was unsuitable to take their babies to the weight management group (meetings were too long, lights were too bright and the babies became irritable and cried). A further three women noted that they benefited particularly from the remote provision of advice using Slimming World’s online support repository, the Slimming World app or contacting the consultant through Facebook (Facebook, Inc., Menlo Park, CA, USA) or WhatsApp (Facebook, Inc.). These were an additional source of support when they were unable to attend or stay for the duration of the meeting because of child care needs (although one respondent stated that the online system did not recognise the extra foods she could eat while breastfeeding).
Accessibility of the intervention (physical opportunity)
Timing of commencement of the intervention
Women’s views about the timing of commencement of the intervention varied across the interview sample and some mentioned this specifically in their questionnaire responses also. Their views about this did not appear to be related to the number of sessions that they attended or changes in their weight. In the interview sample, all the women who completed the programme, two women who attended between six and nine sessions and three who attended fewer than six sessions found the timing of the intervention acceptable:
I thought it was the right time. I think I went at 7 weeks but I thought if I left it any later maybe you get too involved with your life with your baby and you just fall into looking after the baby and not looking after myself. I had recovered from my C-section so I was more mobile . . .
Orchid, 10 sessions, lost 3.7 kg, 12 months
Those who felt that timing of the intervention was not acceptable included three women who attended fewer than six sessions and two who attended between six and nine sessions. These women described the difficulties they experienced with breastfeeding, concerns over their baby’s health and not feeling ready to commit to the intervention because they were struggling with their transition to parenthood:
I simply didn’t have the capacity or the energy at times. Again, I think that reflects the timing of the intervention. Had that taken place at 5 or 6 months when I was weaning, when I’d begun weaning, then that actually might have been far easier because it’s something I could have incorporated into the weaning and the food preparation that I was going through then.
Aster, six sessions, gained 2.8 kg, 12 months
One woman felt she would have benefited from starting earlier; however, she seemed unclear that the window was between 8 and 16 weeks and started the weight management programme when her baby was 4 months old:
I would have wanted to have joined a bit sooner but I didn’t receive the information and obviously you only did it for 16 weeks onwards . . . I personally would have wanted to join it a little bit sooner because I’d already got into a routine, my routine had already changed into what it was. So, changing it again with the baby was really difficult. For me personally, maybe a week after I’d got home from hospital . . . obviously, I understand starting it straight away was really unrealistic. In the beginning I did benefit from it but even if I was just a month earlier I think I might have benefited from it.
Hyacinth, seven sessions, gained 13.7 kg, 12 months
Only four women mentioned the timing of commencement in the questionnaires, one stating that she would have preferred to start the programme during pregnancy and the other three stating that it would have been better to start later when their babies were a bit older. The third, when explaining why she did not go to more sessions and stay for the group, said the following: ‘couldn’t concentrate while had baby with me as breastfeeding . . . offer too early in postnatal period, bad timing of groups with baby, exhausted’ (Primrose, two sessions, lost 0.7 kg, 12 months).
Two women experienced barriers when attempting to join the weight management group. One woman contacted two different consultants at different groups but said that she did not receive a response from either and therefore decided not to pursue joining a group. A second woman (mentioned at the start of the chapter), who attended one session, reported that she was asked for her BMI score and weight details at joining, and as she did not have this information she was not weighed and instead asked to obtain this information before she would be allowed to register. Despite trying to get hold of this information she gave up and did not return to the group:
The issue I had with Slimming World. I think maybe when my initial, when I did try to join a group it took me a long time to actually find one that was close to me and then when I did find one they were asking me for my BMI figures and at the time I didn’t have it to hand and they said they wouldn’t weigh me because obviously I think I came through a referral but there were other people obviously attending that were paying members.
Iris, one session, gained 4.4 kg, 12 months
Group location and timing
In the interview sample, most women who completed the programme chose group locations and times that fitted with their postnatal routines or suited their preference of attending either with their baby or on their own. Only one woman mentioned that ‘sessions in the area were limited’ (Amaryllis, 11 sessions, gained 1.4 kg, 12 months). Some women chose groups that were further away from where they lived so that they could make it an outing and incorporate some exercise that they could do with their baby:
The Slimming World group that I joined was on the other side of [area] and so when I started I’d get my buggy and it was about a 45-minute walk from one side of [area] to the other which when I was doing it I really enjoyed it . . . It became a nice thing because I found a nice route to go via a park and there is a fruit shop on the way that I would pick up loads of fresh fruit and I spoke to the owner of the shop and it was a little morning thing, I made it into a whole morning’s activity.
Heather, 12 sessions, lost 2.6 kg, 12 months
However, two women who did not complete the programme described difficulties finding groups that were close enough to their home at times compatible with their postnatal routines. These women attended groups that were either further away from home but at convenient times or closer to home but at inconvenient times. Regular attendance at group sessions was often affected by holiday plans, child care difficulties that arose from changing postnatal routines, and baby’s or mother’s illness. For some of these women distance and changing postnatal routines were cited as reasons for missing sessions or not completing the programme:
That [group] was the only one within walking distance from my house . . . On most days, I didn’t stay for the after discussion . . . because of the inconvenient timing she was, by that point in time she would be really ratty and ready for sleep, so I didn’t stay. Towards the end as well I got postnatal depression so that made it harder for me to leave the house.
Hyacinth, seven sessions, gained 13.7 kg, 12 months
One woman mentioned that a terrorist attack in London meant that she could not get to her group and that she was not aware she could go to other groups:
I wasn’t sure whether I could attend other groups . . . I wasn’t sure whether I could attend in other parts of London.
Daisy, three sessions, gained 3.3 kg, 6 months
The questionnaire responses show that, even among women who managed to attend ≥ 10 sessions, some women found that the timing of groups (in the evening) coincided with their children’s bedtime, which presented a considerable barrier to them staying for the whole session, and three women who attended only one to five sessions stated that it was the timing of the groups that presented a significant barrier to attendance. The sessions were felt by some women to be too late in the day, and others stated that going to the sessions with their babies did not work as, for example, ‘the clapping was too loud’ (Poppy, two sessions, lost 7 kg, 12 months).
Continuation beyond the intervention
According to Slimming World data, a total of nine women continued to attend beyond the 12 sessions that were offered as part of the intervention. In the 12 months subsequent to them joining Slimming World, these women attended a total of 13–49 visits over a period of 20–52 weeks. All had attended at least 10 sessions in the intervention period.
In the interview sample, several women who completed the programme also talked about wanting to continue beyond the intervention period. They suggested that the intervention should last longer than 12 weeks because they felt that it took time to understand the plan and adjust to life with a baby:
I think the programme didn’t last long enough so you are running around and I know they say if you do something for 3 months then you are in the habit but I think you need 6 months, so that you get used to it. Your life starts getting in a position where you are more in control with your baby . . . I don’t feel that it went on long enough that it became second nature.
Amaryllis, 11 sessions, gained 1.4 kg, 12 months
However, the cost to continue with Slimming World after the trial was a barrier for some women, particularly when they had limited resources while on maternity leave (which was also raised in the questionnaire by another woman):
Once the 12 weeks were up and it came around to paying for it weekly, that was the time when I had no money, so £5 a week ended up being quite a lot of money that I couldn’t really afford. It all came down to money sadly and I think maybe if it was possible to do it for a year, like which is the year that the women have got their maternity leave, that would be fantastic.
Azalea, 11 sessions, lost 1.8 kg, 12 months
One of the respondents to the questionnaire specifically mentioned that the duration was not long enough: ‘My only criticism is that I don’t think 12 weeks is long enough to see any real benefits and to establish healthy eating/lose weight long term. I would say 6 months is optimal’ (Tulip, six sessions, gained 3.6 kg, 12 months).
In the 12-month survey sample there were four women (11% of the 37 women who attended) who continued attending sessions after the end of the 12-week programme. In the 12 months after the birth of their babies they had each lost between 1.7 kg and 17.5 kg (data collected by research midwives).
Women allocated to the control arm: views and experiences of weight management
In this section data collected during 12-month interviews with women who were in the control arm are presented. Three themes were identified that demonstrate the key factors influencing women’s views about weight management after birth. Women’s views are compared according to the weight change from antenatal booking to 6 months and 12 months postnatally. Quotation attributions in this section contain a pseudonym ID (i.e. a tree), weight change from antenatal booking and the time point (i.e. 6 or 12 months) at which data were provided via interview or questionnaire.
Weight loss aspirations (psychological capability/motivation)
All women interviewed expressed aspirations for weight reduction and/or positive lifestyle changes as reasons for deciding to participate in the trial. As for the women allocated to the intervention arm, the strength of these aspirations for the women in the control arm varied across the sample. Aspirations were often related to whether or not women felt that weight loss after birth was important and whether or not they perceived a need to lose weight in relation to whether or not they felt they had gained weight during pregnancy.
Two women who had lost weight at both 6 and 12 months felt that weight management after birth was important primarily because of health reasons, beliefs about the link between weight gain and postnatal depression and the longer-term impact of weight on the mother and child. One of these women had an overweight BMI score and the other had an obese BMI score at antenatal booking. In this context, these women were disappointed about being allocated to the control arm and felt that they would have benefited from having some support with weight management after birth:
Well, I have always struggled but I have PCOS [polycystic ovary syndrome], so weight was always something that I kept an eye on and also my mum put on a lot of weight when she had me and has never really lost it, so I was aware that that’s something that I didn’t want to happen. But I certainly had a bigger appetite. I’m absolutely sure that I gained quite a bit. I was slightly disappointed not to be one of the random ones selected.
Beech, lost 5.9 kg, 12 months
Two women, one who had an overweight BMI at antenatal booking (BMI 25.3 kg/m2) and the other who had a normal BMI at antenatal booking (BMI 24.9 kg/m2) and EGWG, had lost weight by 12 months. These women also felt that weight management was important and wanted to return to their pre-pregnancy weight:
I’m not skinny but I’m not fat either, I’m medium. I would say slim-ish, kind of slim, a bit slim. Normal? I’m just normal. So, yes, I wanted to go back to my normal weight.
Chestnut, lost 1.6 kg, 12 months
For one woman her immediate concern after birth was being healthy for breastfeeding but she acknowledged the importance of weight management for future pregnancy:
I was more concerned about my breast milk and just being healthy enough to feed my baby . . . The other thing is that, it’s probably relevant to the study as well, is that I’d probably like to have another baby in the next year to 18 months. So I’d like to be at the same baseline or I’d like to be a few kilos less even than I was for this pregnancy.
Rowan, lost 1.0 kg, 12 months
By contrast, most women who gained weight at both time points did not feel that they had gained much weight during their pregnancy and/or were more accepting of their body weight post birth. One woman who had an overweight BMI at antenatal booking emphasised that she had not gained much weight during her pregnancy and felt that her weight was acceptable at the time of the interview despite having gained further weight postnatally:
To be honest, when I was pregnant I didn’t really gain that much weight because I wasn’t eating well. I don’t really want to be big. With me I don’t want to be very fat, so I’d rather be slim, keep fit than to be fat . . . I said to you my weight is kind of OK, it’s kind of balanced at the moment for an African woman, for a black person, we’re mostly big, very big. But for me I’m just in-between. I was a size 16 but now I’ve gone to 14, which is good. I am very happy with that.
Maple, gained 3.1 kg, 12 months
Several of these women also felt that weight management was not a priority after giving birth. Breastfeeding and the focus on general health and well-being was more of a priority. Women referred in particular to their postnatal mental health needs, but some also had physical health issues, meaning that these women were reluctant to think about weight loss, particularly when they prioritised exercise as the preferred method for weight loss:
In the first year after having [name of son] I didn’t do any exercise and that’s mainly because I was breastfeeding and I just didn’t feel comfortable doing much exercise whilst that was happening . . . It’s not even just so much the weight I think it’s just about getting posture back, so for me I had very bad stomach muscles and a very bad back and I was recovering from PGP [pelvic girdle pain], and I put off doing exercise because I was too scared to do more damage. It’s not so much the losing weight, it’s the getting back the strength, it’s the core strength, and then I think you can only do that bit first and build on that to then lose weight.
Birch, gained 6.2 kg, 12 months
One woman acknowledged that her BMI score was in the overweight category but felt that there was a gap in her care and did not feel emotionally ready to focus on weight management after birth:
I stopped drinking in order to get pregnant and then I let myself off the hook and ate lots of cake because I figured that was better than a bottle of wine. But the focus of the study was about weight management, it was never about my health and well-being prior to becoming pregnant, during my pregnancy or post pregnancy. Post pregnancy my priorities have changed and I’m still breastfeeding, so it was more about doing activities that I could bond with my child. I was definitely not in a good physical and emotional healthy state.
Pine, lost 7.7 kg, 12 months
Weight management beliefs (capability and motivation)
Interviews revealed that women’s previous experiences of healthy lifestyle management and their understanding of what constituted a healthy diet influenced their beliefs about the most efficacious ways in which to manage weight.
Women who lost weight at both 6 months and 12 months during the trial period had a good understanding of what constituted a healthy diet and were actively engaging in managing their weight by focusing on what they ate and combining this with exercise. Women talked about using apps, going to the gym and joining fitness programmes with a dietary element included. A healthy diet was seen as including lean meat, chicken and fish, with lots of fruit and vegetables and a reduction of sugar. One woman had previous experience of Weight Watchers, so this earlier learning may well have contributed to her beliefs. For these women, there was a belief that diet and exercise contributed to their weight loss:
So I’ve managed the weight, to be honest mainly with food. Sometimes I’ve been better than others, sometimes I’m 4 lbs over but then I quite quickly pull that back. Portion sizes, cutting back on alcohol, cutting back on carbohydrates in the evening, bulking up with more vegetables and that sort of thing. I see quite immediate effects from that sort of thing.
Beech, lost 5.9 kg, 12 months
I started this weight-lifting-based programme for my back, my shoulder and my leg, that I really actively did anything like full on top of everything else. I picked that up. It’s made a huge difference to my physical well-being . . . They also have a dietitian as part of the programme, or a nutrition specialist, so they tell me what I should be eating and how many calories to help through the whole process. They recommend MyFitnessPal® [Under Armour, Inc., Baltimore, MD, USA], and I don’t track it every day, but it’s helped me work out what calories are in things. So it’s been quite helpful.
Pine, lost 7.7 kg, 12 months
Women who lost weight at 12 months had similar views. They were actively engaged in watching what they were eating and one had used the Weight Watchers app for support. A healthy diet was perceived as controlling portion sizes; focusing on fruit and salad; making food healthier by not cooking with oil; avoiding takeaway meals, sweet foodstuff and junk food; and being mindful of the importance of the longer-term goal of being healthy for your family. Participating in the trial was also seen as an incentive to lose weight. Women understood the role of diet and exercise for weight management:
In all honesty I really believe that it’s more diet weight loss is 90% and 10% exercise. I think portion control is the biggest one. I think it’s being creative with what healthy eating is. I think people need to be re-educated about what healthy eating is. I tell you one benefit of the study though: I think it gave me more incentive to get back to my normal eating and to get the weight down so that by the end of the study I was back to my normal weight.
Chestnut, lost 1.6 kg, 12 months
In the group of women who gained weight throughout the trial period, views were mixed. Two women talked a lot about focusing on exercise during the trial period, although there was some mention of changes in dietary habits. For one woman there was a recognition that her weight loss attempts through exercise had not been successful and that she needed to monitor her calorie intake:
I’ve been exercising a lot for about 6 months and I haven’t really lost any weight, so I need to look at my diet and do both together I think. It will be probably be some kind of calorie-controlled diet. I think what I’m going to do is try and have an app that tracks calories, so that I can have in the same app see how many calories I’m burning through exercise versus how many I’m eating.
Birch, gained 6.2 kg, 12 months
Two other women in this group felt that diet was important but they were not actively managing their weight in this way, although they had some idea of what they needed to do, for example cutting down on certain sweet foods and drinks such as chocolate and sugary drinks, eating more vegetables and doing some exercise. They described completing the trial questionnaires as an incentive to think about weight management:
Every time I opened the questionnaire and answered the questions about the healthy food and I remember that I have to do that . . . Sometimes when I leave work, I get the bus but I was walking in, 20–25 minutes, so I was doing that. I was reducing bread and chocolate.
Ash, gained 3.5 kg, 12 months
But with your exercise [i.e. completion of the study questionnaires] I got to ask myself, do I really need that much in a week? How many vegetables do you eat? Bread, salad, pizza, pasta? All those things, I do eat them, but I’ve never think about it, how many do I eat? But right now with the study I’ve been able to work things out myself. So that has really helped me a lot to be honest. I was kind of eating more vegetables, more fruit and I do walk 40 minutes to work and 40 minutes back home. It’s different to what I used to do.
Maple, gained 3.1 kg, 12 months
Social support (opportunity)
All women who had lost weight at 12 months described their partners as taking an active role in supporting them by sharing the same meals, offering practical support in relation to cooking, providing childcare or generally encouraging them to stay fit:
My partner is 100% supportive. He’ll eat whatever I cook. He’s very happy to follow the same sort of meal plan that I eat. He’s helped with the childcare. I wouldn’t have been able to do it [attend a fitness programme] without him. I couldn’t have done it without him. That’s in short, full stop.
Pine, lost 7.7 kg, 12 months
By contrast, women who gained weight described that their partners had been happy to eat what they were cooking but had not made significant changes to their dietary patterns:
Whatever I cook in the house is what everyone eats. My husband is happy with it, he can’t be bothered. Whatever mummy is eating is what everyone is eating.
Maple, gained 3.1 kg, 12 months
One woman felt that being back at work after birth made managing her weight more difficult:
I mean I work in a place where there is a culture that someone has to bring something every week like a cake or sweet and sometimes you really don’t want to have the cake but because someone made the effort of bringing that for us you don’t want to let anybody down, so you end up eating. I would say OK, just a little cake.
Ash, gained 3.5 kg, 12 months
Variation in group attendance (using data from Slimming World on day/time and consultant for each session attended)
The patterns in attendance were analysed to examine whether women were more or less likely to attend on weekdays or weekends, more or less likely to attend during the day or evening and whether or not they tried out different groups and/or different consultants. (The denominator is 46, rather than 48, owing to reliance on data from Slimming World, which was missing for two women.) We found that:
-
Most women (35/46; 76%) attended only one group (i.e. did not change groups at all), nine attended two groups, one attended three groups and one attended four groups.
-
Because most women attended only one group, it follows that most women (28/46; 61%) had the same consultant throughout. Despite staying in the same group, seven women had different consultants (six women had two consultants, most probably because their regular consultant was on leave) and one woman had four consultants at the same group.
-
Most women (40/46; 87%) attended groups on weekdays; five women attended at weekends and one woman, who attended 11 sessions, attended mostly on weekdays but went to one session at the weekend.
-
The time of day that women chose to go to sessions was split fairly equally between morning (09.00–14.30) and evening. Nineteen women went to groups in the morning and 20 went to groups in the evening. Seven women attended a mix of daytime and evening sessions.
Weight management support: type of support accessed, timing of commencement and risk of contamination
Access
Access to additional weight management support was assessed at 6 and 12 months postnatally. A total of 83 women (85%) allocated to the intervention arm completed the relevant question in the questionnaire at 6 months, of whom 71 also did so at 12 months. A total of 75 women (79%) allocated to the control arm completed the question at 6 months, of whom 71 also did so at 12 months.
In total, 25 out of 83 women (30%) allocated to the intervention arm and 28 out of 75 women (37%) allocated to the control arm accessed additional support at 6 months postnatally. Similar rates were reported at 12 months postnatally (32% of women allocated to the intervention arm and 37% of women allocated to the control arm). Looking at both time points, a total of 37 women allocated to the intervention arm and 39 women allocated to the control arm accessed additional weight management support at either time point. Eleven women allocated to the intervention arm and 15 women allocated to the control arm accessed additional weight management support at both time points.
Timing of commencement
Women assigned to the control arm were asked when (if at all) they had accessed additional weight management support. Support most commonly commenced between 5 and 6 months postnatally (that is, for 40% of women who accessed support), with fewer women (only 20% of women who accessed support) reporting that support commenced earlier than 5 months postnatally. This suggests that women were ready to access weight management support slightly later than the time at which the intervention commenced.
Types of additional support and risk of contamination
Joining a gym was the most popular type of weight management support for participants in the intervention and control arms (30% and 50%, respectively; Table 18). In the intervention arm, using a fitness tracker such as a Fitbit® (Fitbit, Inc., San Francisco, CA, USA) was the second most popular type of support (13%), whereas in the control arm weight management apps and Weight Watchers were each used by 15% of women who responded. In relation to risk of contamination, a total of five women allocated to the control arm joined Slimming World (three women chose to join when their baby was 6 months old and two women when their baby was 8–9 months old).
| Type of support | Arm, n (%) | |||
|---|---|---|---|---|
| Intervention | Control | |||
| 6 months | 12 months | 6 months | 12 months | |
| I joined a gym | 11 (44) | 7 (30) | 11 (39) | 13 (50) |
| I used a Fitbit or similar | 9 (36) | 3 (13) | 3 (11) | 2 (8) |
| The Body Coach88 or other website | 3 (12) | 1 (4) | 2 (7) | 2 (8) |
| Weight management app | 2 (8) | 1 (4) | 1 (4) | 4 (15) |
| Hospital weight management clinic | 1 (4) | 0 (0) | 2 (7) | 1 (4) |
| Weight Watchers | 1 (4) | 0 (0) | 1 (4) | 4 (15) |
| I used weight loss drinks | 1 (4) | 1 (4) | 0 (0) | 0 (0) |
| GP | 0 (0) | 0 (0) | 1 (4) | 1 (4) |
| NHS website (formerly NHS Choices) | 0 (0) | 0 (0) | 1 (4) | 0 (0) |
| NHS weight loss plan | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| I followed my own plan | NA | NA | 4 (14) | 5 (19) |
| Slimming World | NA | NA | 2 (7) | 5 (19) |
| Other | 10 (40) | 15 (65) | 11 (39) | 11 (39) |
In the intervention arm there were participants using the Fitness Blender website (www.fitnessblender.com; accessed 3 January 2019), the Body Boss website (www.bodyboss.com; accessed 3 January 2019), the FatSecret® app (FatSecret, Melbourne, VIC, Australia), the Samsung Health® app (Samsung, Suwon, South Korea) and the 7 Minute Workout® app (Johnson & Johnson, New Brunswick, NJ, USA). In the control arm one participant used the Sweat® app (Sweat, Adelaide, SA, Australia), Weight Watchers app and MyFitnessPal app and one women used the Body Boss website.
The ‘other’ weight management support accessed by women is presented in Table 19.
| Arm | Questionnaire | |
|---|---|---|
| 6 months | 12 months | |
|
|
|
|
|
|
Views on additional support
All women allocated to the intervention arm were positive about the additional measures they had chosen to help their weight loss, and several benefits were mentioned. Women commented that going to the gym improved their health and physical strength, and that ‘whilst my weight hasn’t gone down my measurements have’ (Poppy, two sessions, lost 7.0 kg, 12 months). It was noted by two women that the gym had the advantage of easy access and flexibility and one said that having a personal trainer gave her additional motivation. Two women mentioned that they use a gym and swimming pool at which there is a creche, which makes it easier for them to access it, with one finding that she enjoyed exercising and having that time away from her baby. Another found that attending yoga and Pilates classes were important for her core strength and pelvic floor muscles.
One woman found a Zumba class to be useful; another went to two drop-in exercise groups that were held when her daughter was at nursery, which made them easy to attend. However, there were also several comments about the convenience of personal exercise at home using online support: ‘easy to access, felt comfortable to exercise within my own space’ (Magnolia, lost 4.6 kg, 12 months); ‘as the onus is on me, I found that I made time to exercise. Committing to classes with two children is not ideal’ (Freesia, lost 2.6 kg, 12 months). There were also those who liked walking with their baby and found a fitness tracker useful to track how many steps they had done. Informal social support from friends and family was mentioned as very helpful by two women; one woman said that it also resulted in improved well-being and mental health.
In contrast to women allocated to the intervention arm, women allocated to the control arm presented mixed views about their experiences of finding ways to lose weight. Some women found useful and enjoyable ways to exercise individually, including walking to work, swimming, going to the gym or using YouTube (YouTube, LLC, San Bruno, CA, USA) videos. One woman described exercising at home with her husband as useful. One participant found Slimming World useful and easy to fit in to her lifestyle; another woman said that Weight Watchers was great for support, particularly the app she used to track what she ate (whereas one woman said she disliked the app and therefore stopped using Weight Watchers) and the motivation of attending weekly meetings:
I find going to Weight Watchers meetings really helpful as members support each other without judgement . . . However, after several good months it is easy to go back to bad habits. I am not sure it is a long-term solution as I am an emotional eater and this is frustrating.
Elm, lost 12.8 kg, 12 months
Others commented that they had seen the benefits of their activities, including weight loss, improvement in health and mood, strengthened core muscles, increased energy, and motivation to take better care of themselves. On the other hand, there was a group of participants who acknowledged that they found it difficult to continue with their activities, even though they saw the benefits:
[The Fitbit] has improved my health; however, I haven’t been constant.
Oak, gained 34.0 kg, 12 months
The inconsistency this participant described was primarily due to struggling to find time. One participant stopped due to ill health, and another was finding it expensive. One woman admitted that, despite some effort, she did not manage to lose weight: ‘the gym and step tracker helped me get my energy up but didn’t help with weight loss. Other than these, I just followed my own diet plan but still didn’t manage to lose weight’ (Poplar, lost 7.1 kg, 12 months).
Acceptability of trial processes and procedures
An important aspect of the feasibility trial was the extent to which women were willing and able to complete included measures of lifestyle behaviour and health assessment. There were no reported concerns from the research midwives of participants having difficulties responding to questions regarding their lifestyle behaviours and aspects of their own and their infant’s health. That the majority of women met with a research midwife for their 6- and 12-month follow-up undoubtedly resulted in high completion rates of baseline and follow-up measures, and women were offered a £10 voucher at the 6- and 12-month follow-ups. As reported in Chapter 5, completion of all measures was high. Most measures required women only to enter a tick-box response or complete a Likert-type scale, with a few open questions limited to exploring women’s specific views of weight management support (as reported in the process evaluation).
During the trial development phase, we worked with the trial PPI group to explore their views on the proposed tools and scales we planned to use, the appropriateness of these to meet the aims of the feasibility trial and the potential for the content of follow-up questionnaires to place additional burden on women asked to complete them. Having two follow-up visits (6 and 12 months) was not considered burdensome and was considered to be an approach that could support women’s motivation to complete follow-up. Another advantage was that trial PPI members considered that these contacts would highlight the research team’s continuing interest in their longer-term health. The PPI group also recommended that women should be asked to complete the two follow-up questionnaires with the research midwives present so that the midwives could answer any queries the women may have had about questions.
To provide further information on acceptability, interviews with nine women allocated to the intervention arm and eight allocated to the control arm were held approximately 12 months postnatally, during which women were asked about their participation in the trial. Questions were structured around the key aspects of the trial design that follow.
Understanding of the purpose of the trial
One woman allocated to the control arm admitted understanding very little about the trial, but most other participants understood that it was about supporting post-partum women to lose weight. However, only a few women understood that it was specifically about trialling Slimming World as a postnatal weight management intervention, as Slimming World was not mentioned in trial recruitment literature in order to minimise risk of contamination. Several women mentioned improving nutrition, well-being and monitoring the psychological impact of weight loss as additional objectives.
Recruitment strategy
Participants thought that the trial recruitment process was straightforward and the timing was appropriate:
I think because in the context of when you are pregnant, and you know that you are going to have to have vaccines appointments, it’s just another interaction with the health service, so it was like your mind is already thinking along those things because you are really focused on those interactions. So it’s not like maybe another time of my life when I would never need to go to a doctor or nurse, but when you are pregnant you are in and out pretty much every month for something.
Willow, gained 1.0 kg, 12 months
One woman suggested that it would have been better to have personal contact with researchers than to find out about the trial through a leaflet given with other paperwork during a midwife appointment; another suggested recruiting at the health centre where women also have antenatal appointments as well as at the hospital.
Recruitment information
Women in both the intervention and control arms had mixed views about the information provided during recruitment to the trial. Some were satisfied with it, saying it was sufficient; others said that it did not provide enough information about the trial or the intervention. More specifically, women felt there could have been more information about the rationale for the intervention and about randomisation into trial arms. They also would have wanted to know that the intervention involved Slimming World specifically and was focused on diet and that they would be expected to attend meetings:
I thought it would be around eating as well as physical activity. I did maybe have that expectation, but obviously when I went to the session I realised that it was more around the diet and the food intake. They didn’t say you couldn’t exercise, obviously, but I think that would be something that you had to do on your own.
Iris, one session, gained 4.4 kg, 12 months
One woman also commented that she would have liked a more nuanced approach to weight loss and to have been asked what her barriers are and what would personally help her.
Time to decide to participate
Most women said that they had sufficient time to make their decision about participating, knew they could opt out and did not feel pressure to agree. However, one woman allocated to the intervention arm who did not attend any sessions said that she made the decision too quickly, without thinking about it, and in hindsight thinks that she would not have agreed to participate if she had properly considered how difficult it was going to be to attend a group with a baby and her toddler.
Lifestyle leaflet
Of the nine women allocated to the intervention arm interviewed, seven were asked specifically about the lifestyle leaflet component of the intervention (provided at the beginning of the trial). Most did not recall much about the lifestyle leaflet. Five women remembered the leaflet, three of whom remembered some of the content (mainly about breastfeeding) and thought that some of it was useful but that other aspects were not relevant to them. One of these five woman did not remember any of the content, and one said that she did not read the leaflet. Two of the seven women who were asked about the leaflet could not remember receiving it.
Randomisation
Many of the trial participants understood that they were randomly assigned to two arms to assess the impact of the intervention. Two women said that they did not understand it, one thought that there were three trial arms (although could not remember what they were) and one thought that participants were allocated according to questionnaire responses.
Allocation to the control arm
Several women said that they did not mind being allocated to the control arm because they did not feel they had missed out and that it did not change anything for them. They mentioned that being weighed by the midwives and completing the questionnaires was still a positive experience. Only one woman said that she was disappointed not to be selected for the intervention but felt that the questionnaires and interviews kept her focused on her weight loss.
Women allocated to the intervention arm had more varied views. Three said that they would still have been happy to take part in the trial had they been allocated to the control arm, which would have meant ‘not having the benefit of weight loss, but research in itself is a good thing’ (Hibiscus, 10 sessions, gained 0.3 kg, 12 months). One woman said that she would have been less interested in participating; another said that she would have felt disadvantaged and would have gone to Slimming World herself to try it out. One woman who did not understand randomisation said that she would have questioned not being allocated to participate in the intervention. Two women allocated to the control arm stated that they would not have wanted to participate in the intervention. One woman hoped when she first joined the trial that she would become part of a group with other new mums, which would help her to find a social environment, and she was not too concerned about the weight loss aspect. Another said that she would prioritise using the time for exercise:
Actually now thinking about it, I think I would have struggled to go to weekly meetings with my baby [because they would be too frequent]. I think I would have been more likely to have preferred using that time to go for a swim or go to exercise class than go to a meeting where I sit around talking about my weight.
Rowan, lost 1.0 kg, 12 months
Incentives
Seven women discussed the use of incentives (vouchers given to thank women for their time in participating). Four of these women, who were allocated to the intervention arm, thought that the shopping vouchers were a good incentive:
I mean, £10 is OK. That’s alright. It’s just to say a thank you to have that opportunity to get the time out of your busy schedule to help. So to me I think it’s not that bad. I appreciate that.
Marigold, lost 2.5 kg, 12 months
However, the three women from the control arm who spoke about the vouchers did not think they were a good enough incentive to convince them to participate if they did not want to do it anyway:
I mean, probably not in terms of my time and the effort and all of that kind of thing, probably not. If I wasn’t going to do the study that wouldn’t have made me do it.
Birch, gained 6.2 kg, 12 months
I know that £10 is standard for a questionnaire and that wasn’t motivation for me, but I don’t know if that is enough motivation for a busy mum . . . If you want the really wider demographic I think you might have to incentivise, think of another way to incentivise. Maybe baby products. I know you can’t do that, but I think it’s going to have to be something else because 12 sessions and going to meetings, that’s a lot of time commitment.
Rowan, lost 1.0 kg, 12 months
There was also one participant who said she gave her vouchers away as she does all her payments contactless on her phone or Apple Watch® (Apple Inc., Cupertino, CA, USA), so for her a digital format voucher would have been better.
Choice of intervention
Two women allocated to the intervention arm spontaneously discussed the choice of intervention (i.e Slimming World) and were critical of the programme in relation to meeting the needs of women in the postnatal period. One thought that the Slimming World consultants needed to be trained to better deal with postnatal women, their dietary needs and mental health vulnerability. The other would have preferred a more holistic approach with more support:
It did feel very much as though it was a, not even a signpost, but an on-passing. So, ‘Go over there, have your intervention, see what Slimming World manage to do to you, for you, with you and then come back’. It didn’t feel particularly connected or joined up . . . It didn’t feel particularly structured or managed within that setting . . . It was just, ‘Right, you’ve got a baby and 3 months in get rid of the weight’. It felt a little disconnected.
Aster, six sessions, gained 2.8 kg, 12 months
Questionnaire
All women (from both arms) provided feedback on the questionnaires they were asked to complete. As reported in Chapter 5, there was a high completion rate of trial questionnaires and included measures. Some women liked the questionnaires and found them suitable for the trial; they specifically mentioned that they could answer about the food they had eaten, the pictures of the types of food were helpful, the length of the questionnaire was OK and they were glad that it took mental health into consideration. Two women allocated to the control arm said that responding about what they ate was helpful because it made them think more critically about it:
The questionnaires were really very good. How often do you eat starter? How often do you eat bread? How often do you eat salad? How often do you drink Coke? These are questions that I’ve never asked myself . . . But right now, with the study, I’ve been able to work things out myself. ‘OK, do I really need this much on a weekly basis? No.’ So that has really helped me a lot to be honest.
Maple, gained 3.1 kg, 12 months
However, there were many comments that suggested that the questionnaire could be improved. A number of women thought that it was too long (one needed two sittings to do it; another said that she would not have had the time to do it if she was not on maternity leave) and required a lot of mental effort. However, several women suggested that it would have been useful to add some more questions about exercise and well-being (one said that there was too much emphasis on food) and about factors that influence diet, for example support at home, birth experience and recovery, which would provide a more holistic picture:
It wasn’t really asking about health and well-being, it was asking very specifically about weight. I felt like the focus was on weight management and not on health and well-being. Maybe I had more to say about that.
Willow, gained 1 kg, 12 months
One woman allocated to the intervention arm felt that there was too little space for her comments on attending a commercial weight management group:
It was very quantitative and not much about whether I actually thought the whole programme was helpful or not. It was almost too objective . . . I actually felt that I had found the programme helpful and I had learnt things that would change my behaviour, but I just didn’t feel – the benefit that I thought I got from the intervention – I didn’t feel that was reflected in what I was answering . . . So there wasn’t really anything asking me on how good I thought the programme was or anything like that.
Hibiscus, 10 sessions, gained 0.3 kg, 12 months
Another woman said that she would have preferred to have a food diary for 2 weeks because she had no detailed memory of what she had eaten.
Many women mentioned that the questions were difficult to answer and that they needed help from the research midwives to know how to fill it in correctly for the following reasons:
-
inconsistency in question design and therefore easy to make a mistake
-
responses were not in a logical order from strongly agree to strongly disagree
-
no dairy-free food options to tick, so not possible to properly enter dairy substitutes (mentioned by two women).
Finally, two women suggested that the questionnaire could have been made available in electronic format, which would have made it more efficient and convenient for them to fill in:
So first the administration of it being paper and pen was surprising to me in this day and age. Nearly any kind of market research is done on an iPad® [Apple Inc., Cupertino, CA, USA] with a Google [Google Inc., Mountain View, CA, USA] document or something. It felt really archaic to be using paper and pen and then also I was a little, not that I would question anybody’s ability, but obviously there is room for error in that because it then has to be transcribed and re-entered into a system and so mistakes could be made or there’s just another point of potential human error that’s getting in the way.
Willow, gained 1 kg, 12 months
Contact with research midwives
Women were overall very satisfied with their contact with the research midwives, reporting that their visits provided additional reassurance in terms of their physical and mental health and that being visited and weighed was motivating. Some said that they had a choice of whether they had the meetings at hospital or at home and this was very helpful. Most felt that the meeting places were appropriate, with home visits being particularly complimented because this was felt to be very accommodating in a difficult period:
She was very nice about coming at a time that suited me. Actually the home visits are the things that I think were really agreeable. Not having to go to hospital because we’re all kind of trying to get back with our careers and stuff and I was working from home.
Heather, 12 sessions, lost 2.6 kg, 12 months
Several women mentioned that they were happy to go to hospital for their appointments as they added them on to antenatal appointments (baseline visit) or enjoyed having the excuse to go out without the baby and have time to themselves. However, two women said that they had to come to the hospital and that this was difficult for them; in one case this also made the meeting feel rushed.
Weighing frequency
Four women said that they were happy with how frequently they were weighed; however, one woman allocated to the control arm said that she could not make it in to be weighed a second time as she was working full time from 6 months postnatally onwards. Several women from both the intervention and control arms reported that they would have liked to have been weighed more often to help keep them on target or to reflect the more frequent significant fluctuations in weight that they experienced. Suggestions included that weighing could be carried out:
-
monthly
-
after baby weight checks at the clinic
-
every 3 months
-
between the two meetings with research midwives
-
at the end of the 12-week programme.
More frequent weighing of the women allocated to the control arm could have resulted in an intervention effect because this is not current standard care.
The women allocated to the intervention arm did not seem to realise that the data on their weight from Slimming World would also be taken into account and so were concerned that the fact that they did lose weight when on the programme would be lost by the time they were weighed again for the trial:
So I felt that after the 12 weeks I had lost weight and then I put some of that back on by the time I got weighed again, which I suppose is what you are trying to see if the weight loss is maintained. But at the same time I think it would be useful to have more weighs in order to see what the pattern is.
Hibiscus, 10 sessions, gained 0.3 kg, 12 months
It was also commented that the weighing was not standardised and therefore not an accurate reflection of their weight:
I was weighed at completely different times of the day. I’d just had lunch before one, I hadn’t had lunch, it was sort of like nothing was on par, like one I had a heap of jewellery on and the other one I didn’t. It was a whole lot of things like if you were to normally measure weight you would try and standardise.
Pine, lost 7.7 kg, 12 months
Slimming World voucher at end of trial (control arm)
Women who were in the control arm of the trial were given a voucher to attend 12 Slimming World sessions over a 14-week period at the end of the trial. Some were positive about this and two women were considering attending. One woman said that it was a really good incentive to look forward to; however, she had already lost weight so did not need it any more. Three women said that they would not be able to make it to meetings because they had to go back to work and it would have been better to be able to use it during maternity leave. Two women said that they were not interested in attending as they did not like the sound of it:
And that’s not really my bag. Even the brand of the title, Slimming World, I just think, it’s not something that I would be interested in. I’ve joined a CrossFit® [CrossFit, Inc., Santa Cruz, CA, USA] gym and they are kind of more my people . . . I know friends who have done it. For me it sounds like a fat-people version of AA [Alcoholics Anonymous] meetings. It just sounds sad, and the weigh-ins, and it just . . . Also there’s something very kind of middle-aged housewifey about it that just because I’m a mum does not mean that I am one of those types of mums.
Willow, gained 1 kg, 12 months
Suggestions for other forms of support
Two women (allocated to the intervention arm and control arm, respectively) offered suggestions for further support that would be useful to them: a creche that they could make use when undertaking fitness activities and having an NHS ‘centre’ where women could come for support with managing their diets.
Summary of key findings
Acceptability of intervention
-
Of the 98 women assigned to the intervention arm, 47% attended at least one session, but only 19% attended the full Slimming World recommended programme of ≥ 10 sessions.
-
Key barriers related to a lack of opportunity to attend. Women struggled to find the time, to fit attendance into their family routines or to find childcare when they felt it was inappropriate to attend with their baby/children. Motivation was also a factor, with some women either disagreeing with some aspects of the approach or not feeling that the groups provided a positive and supportive environment for them. There was also a number of women who were positive about the intervention but unable to attend many sessions owing to unforeseen circumstances.
-
According to data from Slimming World, nine women (20% of women who attended at least one session) continued to attend sessions subsequent to the 12-week intervention package. A key barrier to attending sessions after the intervention ended for others was cost, especially where the timing coincided with the end of maternity-leave benefits.
Timing of commencement of intervention
-
The majority of women (77%) who attended at least one session did so when their baby was aged ≥ 10 weeks. Feedback from women indicated that there were a significant proportion who felt that the commencement period was too early in the postnatal period and that it was difficult for them to find the time or energy to attend sessions and/or implement the proposed lifestyle changes.
-
A total of 40% of women allocated to the control arm who accessed additional support did so in the first 5–6 months postnatally, with only 20% accessing it before 5 months postnatally. This suggests that women were ready to access weight management support slightly later than the time at which the intervention commenced.
Experience of attending commercial weight management groups sessions
-
Five key themes emerged that mapped to various aspects of the COM-B framework: weight loss aspirations, beliefs and expectations, understanding and implementing the intervention, the social context, and accessibility of the intervention. Analysis compared women who completed the programme by attending ≥ 10 sessions and women who attended fewer sessions but lost weight with women who attended < 10 sessions, highlighting key differences in relation to these themes.
-
Women who completed the programme by attending ≥ 10 sessions and women who attended < 10 sessions but lost weight were more likely to perceive themselves as overweight, understood why they gained weight and were more desirous to lose weight after birth. Their experiences of the programme and resulting weight loss helped to change pre-existing ideas about how to lose weight, particularly in relation to exercise. They understood the dietary plan, found it easy to follow, planned meals and used online resources to support their weight loss journeys. They considered the plan to be unrestrictive, sustainable and compatible with their postnatal lifestyle, leading to positive benefits for not only themselves but their babies and families. These views persisted when several were interviewed again at 12 months.
-
Social context also played a positive role in facilitating women’s acceptance of the programme. Identifying with group members, forming social bonds at a time when women wanted to be out with their babies meeting others, and opportunities to share experiences in a safe environment were key. Women formed positive relationships with group consultants who provided personalised support in relation to breastfeeding needs, which enhanced their belief in the programme and therefore its acceptability. Women in this group were supported to lose weight by their partners, who helped on a practical level or were happy to share Slimming World-friendly meals. Although some women continued with Slimming World beyond the trial intervention period, cost was a key barrier for others. Several women suggested extending the intervention period. Women interviewed at 12 months were still adopting aspects of the programme, although one woman with some difficulty.
-
By contrast, most women who attended between six and nine sessions (and gained weight) acknowledged that they were overweight and understood why, but viewed weight management after birth as less of a priority. Adapting to life with a baby was perceived as more important than attending a weight management programme. These women were more mistrustful of the intervention in part because they felt it failed to prioritise exercise in line with their pre-existing beliefs; they saw it as unsuitable for breastfeeding mothers and felt that there was a gap in their care, particularly in relation to postnatal mental well-being. Despite attending sessions, the women did not appear to understand the Slimming World programme and experienced difficulties making dietary adjustments. Weight gain and lack of perceived benefit may have affected regular attendance. The social context was not seen as positive. Irregular attendance may have been why women felt they did not bond with others in the group, leading to isolation, although several felt that they did not socially identify with others. Some women in this group also felt that their group consultants were unsympathetic to their postnatal circumstances and felt scrutinised or chastised for not following the plan or gaining weight. Social support was conceptualised as partners being happy to eat what was cooked by women; however, several women did not make major adaptations to their dietary habits and used only selected aspects of the Slimming World programme.
-
There were some similarities between women who gained weight and attended between six and nine sessions and women who attended fewer than six sessions. Weight management was not a priority in either of these groups. For some women there was a degree of acceptance of their weight or they felt that they had not gained much during their pregnancies despite being classified as overweight at antenatal booking. Women in this group felt that the intervention failed to prioritise exercise, particularly when exercise was perceived as a main method of previous weight management attempts. Women in this group did not attend enough sessions to fully comprehend the principles of the Slimming World programme because most attended fewer than three sessions. Two women in this group experienced difficulties with joining Slimming World.
-
All women allocated to the control group expressed aspirations for weight reduction and positive lifestyle changes after birth. Women allocated to the control group who lost weight acknowledged the importance of weight loss after birth primarily for health reasons, of belief in the link between weight gain and postnatal depression and of weight loss for future pregnancies. They had a good understanding of what constituted a healthy diet and actively engaged with weight loss strategies through diet and exercise. They were also supported or encouraged by their partners in their weight loss attempts. By contrast, women allocated to the control group who gained weight tended to perceive that they had not gained much weight during their pregnancies or were more accepting of their weight. Breastfeeding and the focus on general health and well-being, particularly postnatal mental health, were considered more of a priority and, for several women, exercise was seen as key to weight loss. Most women made changes to their dietary patterns but this did not have an impact on their weight. Social support was conceptualised as partners being happy to eat what was cooked by the women; however, several women did not make major adaptations to their dietary habits. The work environment added an additional barrier to weight management.
Variation in group attendance
-
Most women attended only one group, with the same consultant, for the duration that they attended the weight management sessions, though some women appeared to move around groups and consultants.
-
The majority of women attended sessions on weekdays, with an almost equal split between attendance in the morning and evening.
-
Feedback indicates that some women may not have known about the opportunity to change groups/consultants when they were not satisfied with their initial choice.
Additional weight loss support
-
Around one-third of women in both arms stated they had accessed additional support for weight management. The most popular type of support was joining a gym, followed by using a fitness tracker (intervention arm) and weight management apps and Weight Watchers (control arm).
-
Women allocated to the control group were most likely to access support when their babies were aged 5–6 months (only 20% of women accessed support before then).
-
The majority of women who sought additional support (in both the intervention and control arms) were still accessing it at the time of completing the questionnaire.
Contamination between trial arms
-
There is a low risk of contamination between the arms of the trial: only five women from the control arm joined Slimming World independently (choosing to join 6–9 months postnatally).
Acceptability of trial processes and procedures
-
Most women understood the trial design but some did not realise that the intervention involved attendance at Slimming World sessions, and some had expectations of a postnatal-specific intervention focused on more than solely weight management.
-
Few women remembered the lifestyle leaflet or its contents.
-
Completion of the questionnaire was felt to be useful and acceptable by some women, but others found it too long and complicated, or commented on the absence of questions on some aspects of lifestyle behaviours that they considered important. Despite this, there were generally high completion rates of questionnaire measures other than aspects of physical activity during pregnancy.
-
The appointments with the research midwives were valued; women had no objection to being weighed and some asked for this to be more frequent.
Implications of process evaluation findings for a future definitive trial
-
Pre commencement:
-
It may be beneficial to provide women (at the point of randomisation) with more information about the intervention, including key components of the dietary advice, the role of exercise (which may or may not be discussed at weekly sessions) and that the plan is suitable for breastfeeding women.
-
Provide women with more information about what to expect at Slimming World and that it may take several sessions before they feel comfortable, which is normal; explain that there is a learning curve associated with getting to grips with the plan and feeling at ease in group sessions.
-
-
Timing of commencement: extend the window of opportunity to commence the intervention to enable women to join earlier if they wish and those who experience more difficult challenges to join later than at 4 months (based on findings, we would suggest extending the commencement window to 6 months postnatally).
-
Duration of intervention: increase the intervention duration (e.g. to 24 weeks) to enhance the likelihood of increasing capability and motivation for women to change their behaviour and sustain weight loss, and/or consider enabling women to continue with the intervention online after they complete the 12-week programme, particularly where cost to continue may be a barrier.
-
Consider a third trial arm, for example offering Slimming World online, to counteract some of the ‘opportunity’ issues reported.
-
Reinforce participants’ understanding that they can attend with their babies, that they can choose to attend different groups if the first one that they try is not ‘right’ for any reason and that it is important to attend for the duration of the programme if possible.
-
Ensure that women know how to contact the research team (using details from the participant information sheet) if they have any difficulties accessing groups or process issues to do with the intervention.
Chapter 7 Findings from the health economic evaluation
Findings in this chapter address objective 5, namely:
-
suitability of chosen economic data collection tools as a basis for facilitating an evaluation of intervention cost-effectiveness in a definitive trial
-
deliver a preliminary within-trial analysis of the cost-effectiveness of the intervention compared with standard care conducted over the 12-month follow-up period
-
conduct a rapid evidence review to assess if the wider evidence base would support economic modelling of relevant out-of-trial health and resource impacts in a future definitive trial.
Trial perspective and data
For the feasibility trial we designed the approach to data collection with a view to meeting requirements for conducting a cost–utility analysis of the intervention principally from an NHS/Personal Social Services perspective as part of any future definitive trial. A version of the AD-SUS42 was designed and administered at baseline (36 weeks’ gestation) and at 6 and 12 months postnatally to gather the data required to cost health service contacts for trial participants. It asked participants to report the number of times they had contact with different community-based health services, hospital outpatient services and A&E departments and the frequency of hospital admissions during pregnancy (baseline) and during the 6 months prior to each follow-up contact. Clinical case note data on mode of birth were extracted for costing clinical care received during labour and delivery. To support the cost–utility approach we selected the widely recognised and utilised EQ-5D-5L health state measurement instrument, developed by the EuroQol Group, as a basis for estimating QALY outcomes. This was completed by women at baseline and each follow-up point.
Assessment of suitability of approach to economic data collection
We evaluated the suitability of the selected economic data collection tools according to:
-
Data completeness with respect to individual items of service contact, measured using the AD-SUS, and health outcomes data, measured using the EQ-5D-5L.
-
The proportion of participants followed up with complete data on total costs (defined in Preliminary analysis of intervention cost-effectiveness: methods) and complete paired data on total costs and QALY outcomes (using the EQ-5D-5L) required for evaluating cost-effectiveness (details of how QALYs were estimated from EQ-5D-5L data are also provided in Preliminary analysis of intervention cost-effectiveness: methods). As part of the preliminary cost-effectiveness analysis, use of multiple imputations was explored as a means of mitigating against loss of economic data for evaluating cost-effectiveness.
-
The completeness of available unit cost data suitable for costing service contacts, measured using the AD-SUS.
Preliminary analysis of intervention cost-effectiveness: methods
A within-trial cost–utility analysis over 12 months from birth was conducted using the feasibility trial data. This was carried out principally from an NHS/Personal Social Services perspective. Costs were inclusive of contacts over the period of follow-up with community-based and hospital outpatient services (for mother and infant), hospital admissions (for mother and infant), costs of labour and birth for the index pregnancy and costs of the weight management programme. The intervention evaluated is delivered by a private for-profit organisation and paid for privately by those who enrol, although trial participants were offered free access during the trial period. We included the cost of enrolment in the economic evaluation on the basis that any future commissioning of the programme could potentially be paid for through either NHS or local authority commissioning budgets. To evaluate cost-effectiveness, the total cost of all service contacts for each trial participant who completed follow-up interviews at 6 and 12 months were estimated. Total costs are defined as the sum of all costs across all individual service items for both 6-month periods included at postnatal follow-up for each participant (inclusive of the cost of birth) in addition to the assumed cost of enrolment in the weight management programme (for those in the intervention arm of the trial).
Quality-adjusted life-years were used to evaluate programme cost-effectiveness. When a respondent completes the EQ-5D-5L instrument (in this trial at baseline and at 6 and 12 months postnatally) they are allocated a unique health state positioned within an overall descriptive system of 3125 unique states of health. 89 Each health state is characterised according to a combination of five health-related quality of life dimensions (mobility, pain, usual activities, self-care pain/discomfort and anxiety/depression), with the respondent indicating the level of problems currently experienced within each on a five-point scale. Each allocated health state has a unique community ‘utility’ weighting attached to it based on health state preferences elicited from a sample of the English general population. 89 The utility weights range from 0 (death) to 1 (full health), with an allowance for some severe health states that are considered to be worse than death and valued at < 0. To match each SWAN participant health state to its corresponding community tariff, we used an algorithm provided by the Office of Health Economics.
The QALY for each trial participant over 12 months was calculated using an ‘area under the curve’ approach,51 which estimates QALYs per participant for the first 6-month postnatal follow-up period as:(1)[(baseline utility weight–6-month utility weight)/2]×0.5,
and for the second 6-month follow-up period as:(2)[(6-month utility weight–12-month utility weight)/2]×0.5.
The sum of QALYs over both periods gives the total QALYs per participant for the 12-month postnatal follow-up period.
Intervention, comparator and time horizon
The economic evaluation assessed the total incremental costs and QALY gains of usual postnatal care combined with enrolment in the weight management programme (intervention arm) versus usual care only (control arm). Incremental costs and QALYs were estimated for the 12-month follow-up, inclusive of service contacts and quality of life changes aggregated over the two 6-month periods following the baseline assessment at trial entry (36 weeks’ gestation).
No discounting of costs or QALY impacts was undertaken given that the evaluation period did not extend beyond 12 months. The economic analysis did not consider any longer-term programme impacts linked to future pregnancies and wider health risks to mother and infant. Issues concerning the modelling of these impacts in any future definitive trial are discussed in Modelling out-of-trial programme impacts.
Analytical approach
The economic evaluation was conducted on an ITT basis including only those randomised participants who were followed up at 12 months (n = 140). Stata version 15 was used for all reported analyses. Population mean differences in total cost and total QALYs at 12 months were estimated using ordinary least squares regression modelling including a binary ‘treatment’ explanatory variable indicating whether a participant was randomised to the intervention or control arm of the feasibility trial. Both models made baseline covariate adjustments for any differences between intervention and control participants in relation to age at trial registration, ethnicity (‘black’, ‘Asian’ and ‘other’; reference group ‘white’, plus three cases recorded of ‘unknown’ ethnicity), weight (in kilograms) at trial registration, obesity identified at the first antenatal visit (defined as a BMI score of ≥ 30 kg/m2; reference group, BMI score of < 30 kg/m2), number of children prior to the birth of the index infant, EQ-5D-5L community weighting scores at baseline and costs of service contacts reported at baseline with respect to the period of pregnancy prior to 36 weeks’ gestation (pregnancy- and non-pregnancy-related hospital and community health service contacts and costs of inpatient admissions were entered as separate covariates).
Missing data
Missing data on specific service items and baseline regression covariates (excluding missingness due to lack of follow-up at 12 months) reduced the size of the potential estimation sample (from 140 to 85) for the economic analysis. Where an admission to hospital and reason for admission were reported but data on number of days spent in hospital were missing (mother or infant), the average cost of a finished consultant episode (FCE) reported in NHS Reference Costs 2016/1749 for the currency code that best matched the reason for admission was applied. More generally, to avoid discarding relevant data collected from participants and to mitigate the risk of evaluating programme cost-differences on a biased subsample of those followed up to 12 months, missing data were handled using multiple imputations. This involved using the chained equations approach,90 applying an imputations model drawing on variation in observed baseline sociodemographic data and participant weight, costs and EQ-5D-5L quality of life data measured at follow-up. Imputations were made across each trial time point for all participants followed up at 12 months. This generated 20 simulated fully imputed data sets each containing 140 observations for all relevant cost, quality of life and baseline covariates, included in the regression modelling.
Intervention cost-effectiveness
To identify the programme’s combined effect on total costs and QALYs over the follow-up period, and facilitate a full analysis of sampling uncertainty in the cost-effectiveness estimates, the regression models were non-parametrically bootstrapped by drawing 5000 data samples at random (with replacement) from each of the 20 imputed data sets, giving 100,000 (20 × 5000) paired estimates of the difference in the total cost and QALYs at 12 months between intervention and control participants. The mean of these bootstrapped estimates was used to infer from the feasibility trial data the intervention incremental impact on total cost and QALYs over 12 months.
If the intervention is found to have higher costs and to generate additional QALYs, it is conventional for this information to summarised as an incremental cost-effectiveness ratio (ICER). This measures the additional cost of each extra QALY gained, which can be used to gauge whether the additional costs fall above or below what is currently regarded as an acceptable level of incremental cost by NICE. The NICE acceptance threshold for health-care programmes currently lies at £20,000–30,000 per QALY gained. The trial intervention ICER is calculated by dividing the estimated increase in cost (if relevant) from the bootstrap simulations by the estimated increase in QALYs.
In presenting the cost-effectiveness evidence from this preliminary analysis, the net benefit of the weight management programme was determined. This was defined as the difference in QALYs over the follow-up period between the trial arms minus the implied opportunity cost of the programme expressed as the QALYs that would be displaced in a resource-limited health system in consequence of the intervention generating higher total costs per participant over the follow-up period (or conversely the QALYs saved if the programme was found to generate incremental cost savings). The opportunity cost of the programme was calculated by dividing the lower end of the current threshold used by the NICE for determining programme cost-effectiveness (£20,000 per QALY gained) by the incremental cost of the programme. This calculation is predicated on the basis that the NICE threshold should in principle identify the level of cost that will displace a single QALY. 91 A positive net QALY benefit would therefore indicate that, at 12 months, the weight management programme is cost-effective.
Sampling uncertainty
Sampling uncertainty was analysed using the distribution of bootstrapped replications of incremental cost and QALY outcome pairs. This uncertainty is presented in two stages: first, using a visual presentation of the simulated distribution of cost and QALY outcome combinations in the ‘cost effectiveness plane’ and then using this distribution to determine the proportion of simulations located in each cost-effectiveness ‘quadrant’; second, by identifying the proportion of simulated cost and QALY outcome pairs that result in a positive net QALY benefit (indicating that the programme is cost-effective) and repeating this for different assumed cost-effectiveness thresholds. This is used to trace a cost-effectiveness acceptability curve (CEAC),91 which plots the probability that the intervention is cost-effective at different assumed values for the cost-effectiveness threshold.
Additional sensitivity analysis
The appropriate level of the cost-effectiveness threshold for guiding decisions over the funding of new health-care programmes is uncertain. 92,93 Therefore, in addition to generating a CEAC, we also estimated the net programme QALY benefit across variable thresholds, some higher and some lower than the existing value adopted by NICE. The sensitivity of the main findings to the exclusion of high-cost outliers (e.g. owing to infrequent instances of unusually lengthy periods reportedly spent in hospital during the follow-up period by mother or infant) that could risk skewing findings in favour of either the weight management programme or usual care was also examined.
To explore the sensitivity of findings concerning costs and QALY outcomes to the inclusion of participants who were not successfully followed up at either 6 or 12 months, multiple imputations were used to impute missing cost and QALY data for trial dropouts. This test assumes that loss to follow-up is random (conditional on observable participant characteristics) and not related to either QALY outcomes or levels of health-care usage during the follow-up period.
Results
Quality and completeness of economic data
There were no reported concerns from the research midwives that participants had difficulties responding to questions regarding contacts with community- or hospital-based services over the follow-up period. Analysis of the data collected to meet objective 5 broadly supported collection methods used as a feasible basis for evaluating intervention cost-effectiveness in a definitive trial. Tables 31–33 in Appendix 4 provide details on completeness of cost data against individual items of service use during pregnancy and the first and second 6-month periods. Self-reported data on contact with community-based health care from the 6- and 12-months interviews were complete. Only three cases had missing data from clinical case notes for costing labour and birth. In a small number of cases, data for costing A&E contacts at 6 and 12 months were missing (6% and 3% of participants interviewed, respectively) and for outpatient service contacts (3% of participants interviewed at each time point). Missing information was more apparent for costing inpatient admissions over follow-up, the proportion of participants with missing inpatient admission costs ranging between 4% (infant admissions at 12 months) and 11% (maternal admissions at 6 months) of those who were followed up. Unit cost data suitable for use in economic evaluation and for costing service use measured via the AD-SUS (including mode of birth) were universally available across all service items included in the scope of the economic analysis. Data on self-reported health status using the EQ-5D-5L measure (for QALY estimation) were provided by all women who completed follow-up. All component items of the EQ-5D-5L were fully completed by all women followed up at 6 and 12 months, enabling identification of the relevant EQ-5D-5L health state and its associated utility weighting.
Despite high levels of complete data for most service items, aggregation across individual items of cost led to missing data on total costs and missing paired data on total costs and QALYs for 33% of participants at 6 and 12 months. Missing cost data were successfully imputed using multiple imputation methods.
Preliminary cost-effectiveness analysis: findings
Reported service use by time period
Descriptive statistics on service contacts during pregnancy and at 6- and 12-month follow-up for women allocated to either arm are shown in Tables 31–33 in Appendix 4. All the information reported in these tables refer to the sample of women who had complete data on service contacts at each time point.
During pregnancy a wide range of services were reportedly used by women in both trial arms. Midwives, GPs, GP practice nurses and obstetricians were reported to be the most frequently contacted clinical professionals. As could be expected, antenatal visits for ultrasound scans and blood tests were almost universally reported across the trial sample. Hospital admissions during pregnancy were comparatively rare in both arms: 8% and 12% of the intervention and control arm samples, respectively, reported at least one admission to hospital.
The overwhelming majority of women (> 90%) continued to report having some level of contact with GPs and midwives at the 6-month follow-up, averaging between three and four contacts over 6 months for those who reported engaging with these services. Health visitors were also widely utilised at follow-up (> 90% of women). Reported use of midwives dropped off at 12 months, though GPs and health visitors continued to be widely utilised. Social workers, mental health professionals and employment and housing workers were contacted by only a minority of participants over the entire follow-up period.
Contact with an A&E department was reported by 26% and 30% of women at 6 and 12 months, respectively, with a slightly higher reported use in the intervention arm at both follow-up points. At 6 months, a higher percentage of women allocated to the intervention arm reported at least one outpatient visit relating to their infant’s health compared with women allocated to the control arm (28% vs. 17%, respectively), though this differential narrowed at 12 months. For those who reported outpatient contact for the infant, there was a reported average of two visits.
The percentage of women reporting any contact with outpatient services relating to their own health was higher in the control arm (23% at 6 and 12 months) than in the intervention arm (18% at 6 months and 16% at 12 months), with a mean of two to three visits for those reporting at least one contact at each of the follow-up interviews.
Health-related quality of life
Utility weights applicable to the health states described by participants using the EQ-5D-5L instrument are reported in Appendix 4, Tables 31–33. Mean health state utility scores improved in both groups from baseline over the follow-up period. Differences in mean utility scores between the intervention and control arms at 6 and 12 months were negligible.
Cost-effectiveness
Pre-intervention baseline costs accrued over pregnancy prior to 36 weeks’ gestation (inclusive of imputed values where data are missing) are presented in Appendix 4, Table 34. These estimates relate exclusively to participants included in the cost-effectiveness analysis who were followed up for 12 months. The costs of pregnancy- and non-pregnancy-related service contacts were broadly similar over this period for the intervention and control participants, though costs of hospital admissions were considerably higher for control arm participants.
The mean cost of service contacts for the intervention and control arms over the follow-up period (again for cases included in the economic evaluation) are presented in Table 20, along with mean cost differences adjusted for baseline covariates. Unadjusted differences (not presented) were similar and in practice adjustment for baseline covariates made little difference to the main cost-effectiveness findings.
| Variable | Mean (SE) | Adjusted mean difference (95% CI)a | |
|---|---|---|---|
| Intervention arm (n = 69) | Control arm (n = 71) | ||
| Costs (£) | |||
| Delivery | 3133 (70) | 3110 (80) | 52 (–173 to 269) |
| At 6 months | |||
| Community-based, outpatient and A&E service contacts | 884 (71) | 758 (45) | 106 (–53 to 275) |
| Hospital admissions: baby | 846 (478) | 372 (349) | 487 (–487 to 1603) |
| Hospital admissions: mother | 224 (105) | 131 (81) | 79 (–170 to 348) |
| At 12 months | |||
| Community-based, outpatient and A&E service contacts | 362 (43) | 358 (42) | –17 (–142 to 103) |
| Hospital admissions: baby | 114 (387) | 128 (76) | –24 (–239 to 223) |
| Hospital admissions: mother | 107 (79) | 135 (122) | 8 (–318 to 263) |
| Total | 5718 (578) | 4992 (431) | 741 (–488 to 2107) |
| QALYs | |||
| At 12 months | 0.920 (0.011) | 0.924 (0.008) | 0.01 (–0.01 to 0.02) |
| Net benefit of intervention at 12 months | –0.032 (–0.10 to 0.03) | ||
| ICER | |||
| At 12 months | £74,100 per QALY gained | ||
The mean total cost per participant was £5718 in the intervention arm and £4922 in the control arm. The costs of birth contributed most to participant total costs over the follow-up period (a mean of £3000 in both groups) followed by community-based, A&E and outpatient service contacts (a mean of £884 and £758 in the intervention and control arms, respectively) and hospital admission costs. The cost of weight management programme enrolment (≈ £50 per randomised participant) was < 1% of the overall mean total cost for that group.
Total costs at 12 months (inclusive of the cost of commercial weight management group enrolment) were £741 higher in the intervention arm than in the control arm, adjusting for baseline covariates. In terms of types of service use, the biggest difference in mean costs arose in relation to infant hospital admissions at 6 months (a mean difference of £487 between intervention and control arms). The mean costs of contact with community-based and hospital outpatient services at 6 months were £106 higher in the intervention arm than in the control arm. The mean cost of delivery was also marginally higher for participants in the intervention arm. Mean cost differences across all service categories generally reduced between 6 and 12 months. At 12 months participants in the weight management group were estimated to have lived a single life-year of marginally higher quality after adjusting for health state utility scores measured at 6 and 12 months. This amounted to a mean difference of 0.01 QALYs at 12 months (see Table 20).
Table 20 also presents summary cost-effectiveness results based on the mean difference in total costs and QALYs at 12 months. The ICER for the intervention arm was estimated to be £74,100 per QALY gained. This translates into a 0.032 net QALY loss at 12 months.
Sampling uncertainty and sensitivity analysis
Figure 5 presents 100,000 simulated pairs of cost and outcome (QALY) differences in the cost-effectiveness plane along with a 95% confidence ellipse and the lower end of the threshold currently used by NICE to determine health programme value for money (£20,000 per QALY gain). A total of 63% of simulated pairs are in the north-east quadrant of the plane (the weight management programme has a higher cost and is more effective than usual care), with 26% located in the north-west quadrant (the weight management programme is cost-increasing and less effective) and a small percentage located in either the south-east or south-west quadrants (8% and 3%, respectively).
FIGURE 5.
Simulated cost and QALY pairings at 12-month follow-up.
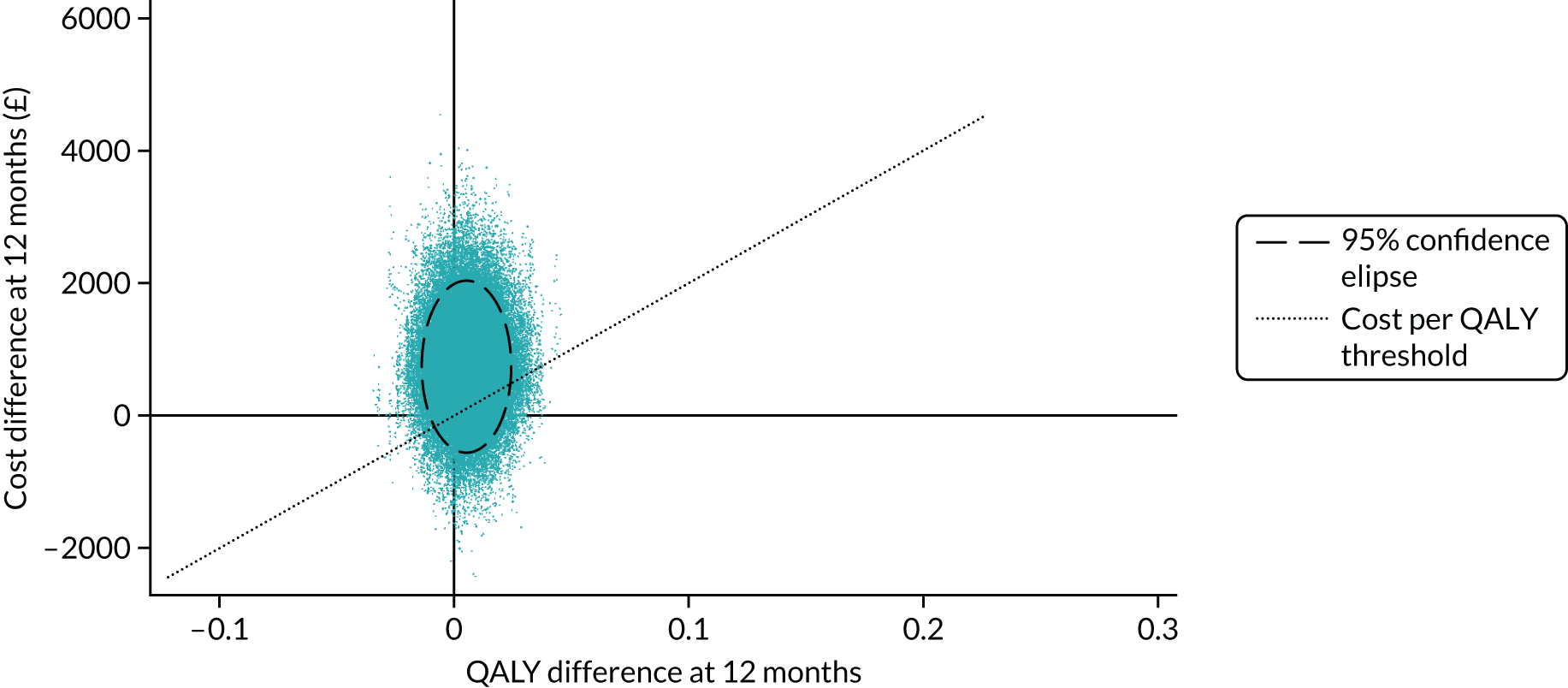
Figure 6 translates this information into a CEAC.
FIGURE 6.
Cost-effectiveness acceptability curve.
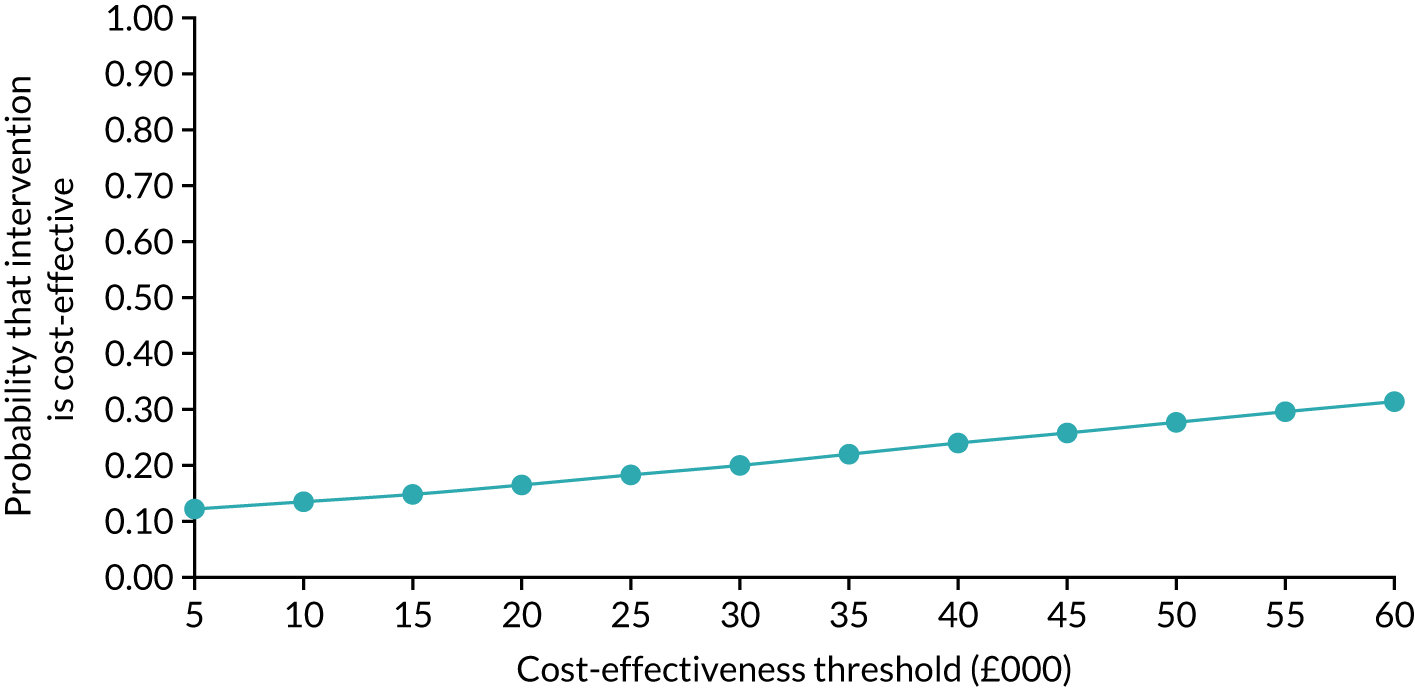
At a cost-effectiveness threshold of £20,000 per QALY gained there is an estimated 0.17 chance that the intervention will be cost-effective at 12-month follow-up. At higher thresholds this probability increases, although it remains considerably below 0.5 even when assuming a maximum threshold of £60,000 per QALY gained. Figure 7 presents varying estimates of the net QALY benefit for different cost-effectiveness thresholds. Over the selected range of thresholds, the net QALY benefit of the programme as estimated at 12 months never exceeds 0.
FIGURE 7.
Net benefit of intervention at 12 months assuming varying cost-effectiveness thresholds.
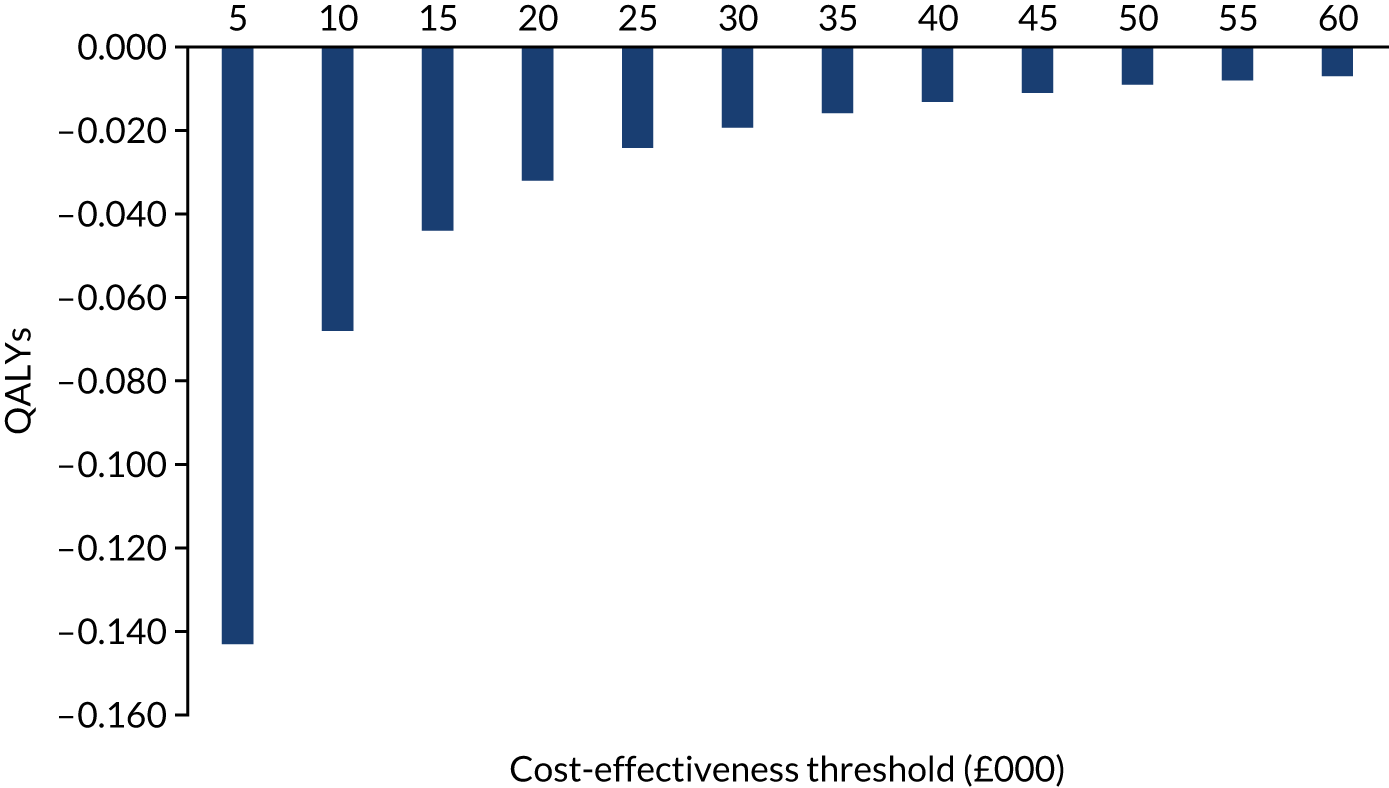
When testing the sensitivity of these findings to the exclusion of a high-cost outlier (due to infant-related hospital admission costs in excess of £15,000 at 6 months) in the intervention arm, the mean difference in total cost between both trial arms fell from £741 to £625. This did not significantly affect the estimated ICER and the overall conclusions regarding programme cost-effectiveness at 12 months. Through use of multiple imputations, there was evidence that loss to follow-up could substantially bias estimated differences in mean costs: imputing for missing data conditional on observed values of covariates at baseline and over the follow-up period, the covariate-adjusted excess total cost per participant for the intervention compared with the control arm increased from £741 to in excess of £1500. This would serve to strengthen the conclusion that, over a 12-month follow-up period, the intervention would exceed the existing NICE maximum cost per QALY thresholds.
Implications of health economic evaluation for a future definitive trial
-
A within-trial economic evaluation demonstrated the feasibility of using a combination of self-reported data on relevant service contacts combined with data extracted from women’s maternity records to evaluate the within-trial economic impacts of a weight management programme delivered postnatally.
-
Health-related quality of life measured using the EQ-5D-5L instrument, required to support the estimation of QALYs, was completed for all items and domains by all participants who were followed up postnatally.
-
There were no reported problems or concerns from women regarding the questions contained in the AD-SUS42 used to measure service contacts for costing purposes or regarding the component items included in the EQ-5D-5L instrument.
-
Although service contact data were generally complete, missing information pertinent to costing was more apparent in relation to hospital service contacts, including admissions. Given that hospital admissions, although relatively infrequent, are generally the most important item of resource use in terms of cost, with potential to influence cost comparisons across trial arms, use of hospital records should be explored as a possible alternative to capturing hospital admissions data in a future definitive trial.
-
Aggregation of costs over individual service contact items where there were missing data meant that data on total costs required for cost-effectiveness analysis (in combination with QALY outcomes) were missing for 33% of women who were followed up postnatally and eligible for inclusion in an economic evaluation. The effect of missing data was mitigated using multiple imputation. This prevented data provided by trial participants from being unused and provided a means of reducing the risk of biasing trial findings owing to the exclusion of cases from the main cost-effectiveness analysis. Strategies for understanding and handling missing data, including more detailed analysis of patterns of missingness (which we did not undertake in any great detail for this trial) and use of multiple imputation methods, are likely to be a key component of an economic evaluation in a future definitive trial.
-
The resource use measurement instruments employed in this feasibility trial were designed to quantify costs from an NHS/Personal Social Services perspective. However, given that the intervention has a strong public health rationale and that a key ‘active ingredient’ includes advice on lifestyle and behaviour to promote weight loss, we would recommend that any future definitive trial gives consideration to the additional measurement of costs borne by participants (both time and financial) in taking positive action on behaviour and lifestyle choices. This would seem particularly relevant given that these personal resource commitments could affect weight management outcomes.
-
The economic evaluation based on the feasibility trial data assessed the incremental costs and QALY outcomes of the intervention at 12 months compared with usual care. The higher mean total costs were largely driven by increased infant hospital admissions in the intervention arm at 6 months. This may have reflected a real programme effect or a consequence of underlying unobserved differences between intervention and control participants not balanced out through randomisation. Further consideration should be given to this in a future definitive trial.
Modelling out-of-trial programme impacts
In this section an overview is presented of available evidence required to support economic modelling of out-of-trial costs and QALY impacts of improved postnatal weight management as part of any larger definitive trial. When considering the cost-effectiveness of funding weight management and lifestyle support programmes, the focus of any future definitive trial on costs and outcomes measured over the trial period itself may risk understating longer-term impacts on resource use, quality of life and life expectancy. Capturing these longer-term impacts is likely to be complex and will require economic modelling of one form or another.
A broad assessment of the feasibility of modelling longer-term impacts was undertaken for a future definitive trial, including plausible time scales over which impacts might be assessed. The feasibility assessment draws on a rapid evidence review of the type of evidence required to develop and parameterise an economic model of this type, namely:
-
epidemiological evidence directly linking weight-related outcomes measurable in a clinical trial to the risk of exposure to relevant health conditions (e.g. complications and adverse outcomes in future pregnancies, type 2 diabetes, cardiovascular disease) and impacts on birth outcomes and infant development
-
economic end-points, including loss of quality-adjusted life expectancy for women and their infants linked to adverse outcomes and resource costs arising from the management of adverse health outcomes
-
wider evidence on weight management programme clinical effectiveness and cost-effectiveness and the sustainability of effect on weight outcomes beyond the period observable in a trial
-
existing economic models (e.g. in the field of obesity management) that could inform the development of an economic model applied to the evaluation of a weight management programme in a future trial.
Search strategy
A rapid review is a method of literature search and synthesis designed to provide a useful overview of the available evidence in a short period of time. It has much in common with a systematic review in that it follows a broad problem/population, intervention, comparison, outcomes (PICO) or problem/population, intervention, comparison, outcomes, study design (PICOS) approach, answers a specific question, uses inclusion and exclusion criteria to judge papers and uses critical appraisal to judge the quality of papers. The main differences between a rapid review and a systematic review are the time required for completion (a matter of weeks compared a matter of months, respectively) and the outcomes, which are simplified for a rapid review, which opts for descriptive summaries and the identification of themes in the data, compared with the meta-analysis and methodical summary approach used in a full review.
Searches were conducted using MEDLINE and EMBASE databases during October and November 2018, with no limitation on publication date and limited to the English language.
A three-pronged search approach was taken. Screening was completed using Rayyan [Qatar Computing Research Institute (Data Analytics), Doha, Qatar], a web-based literature review programme that has a flexible tagging system. Papers were included if they satisfied the main criteria, tagged with their inclusion or exclusion reasons and then tagged with each paper’s main themes and findings. These themes were then summarised to give a narrative for each topic and recommendations were made regarding the implications of the findings from the review for any future modelling of intervention impacts as part of a definitive trial.
The search strategy included the following searches.
Search 1
(Economic AND model*) AND (obes* OR overweight OR ‘excess weight’ OR ‘gestational weight gain’ OR ‘gestational obesity’) AND method*
Search 2
((obes* OR overweight OR ‘excess weight’ OR ‘gestational weight gain’ OR ‘gestational obesity’) AND (pregnancy OR birth OR natal OR partum OR intrapartum OR interpregnancy) AND (‘weight loss’ OR diet* OR ‘weight reduction’)) AND human AND (econom* OR effect OR cost OR qaly OR ‘quality of life’ OR qol OR eval*)
Search 3
(obes* OR overweight OR ‘excess weight’ OR ‘gestational weight gain’ OR ‘gestational obesity’) AND (pregnancy OR birth OR natal OR partum OR intrapartum OR interpregnancy) AND (‘long term’ OR lifetime OR ‘life course’) AND human AND outcome* AND (mother OR baby OR child OR infant)
Inclusion criteria (common to all searches):
-
English language
-
human subjects only (no animal studies)
-
journal articles only (no conference abstracts)
-
published any time
-
Organisation for Economic Co-operation and Development (OECD) countries.
Additional inclusion criteria for search 1 (PICO):
-
problem – economic modelling
-
intervention – obesity model
-
comparison – other methodologies/conventional modelling approaches
-
outcomes – modelling methodology.
Additional inclusion criteria for search 2 (PICO): problem/population, intervention, comparison, outcome
-
population – women who experience EGWG or who are obese at conception
-
intervention – any intervention to reduce bodyweight after pregnancy or between pregnancies
-
comparison – any other intervention or usual care/natural weight loss
-
outcomes – effect sizes, costs, quality of life measures.
Additional inclusion criteria for search 3 (PICOS):
-
population – women who experience EGWG or who are obese at conception and their children
-
intervention – any intervention
-
comparison – any comparison
-
outcomes – long-term outcomes in mother and child as a result of excessive weight gain
-
study design – RCT, longitudinal study.
Results
Across the three search strategies, 5405 abstracts were returned. The results were de-duplicated and analysed in Rayyan. In total, 124 abstracts were included. The main reasons for papers to be excluded were topic irrelevance (46%), wrong type of intervention (26%), wrong population (13%) and animal studies (6%). The remaining 9% were excluded for not being in English, not being full articles or for being from non-OECD countries. Of the included studies, eight provided evidence of economic end points (such as unit costs or QALY estimates), 39 covered epidemiological evidence of long-term impacts of obesity in mother and child that would be useful when designing an economic model, 34 were existing economic models and 43 concerned the clinical effectiveness and/or cost-effectiveness of postnatal interventions.
Figure 8 shows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart of the screening and inclusion process.
FIGURE 8.
The PRISMA flow chart.
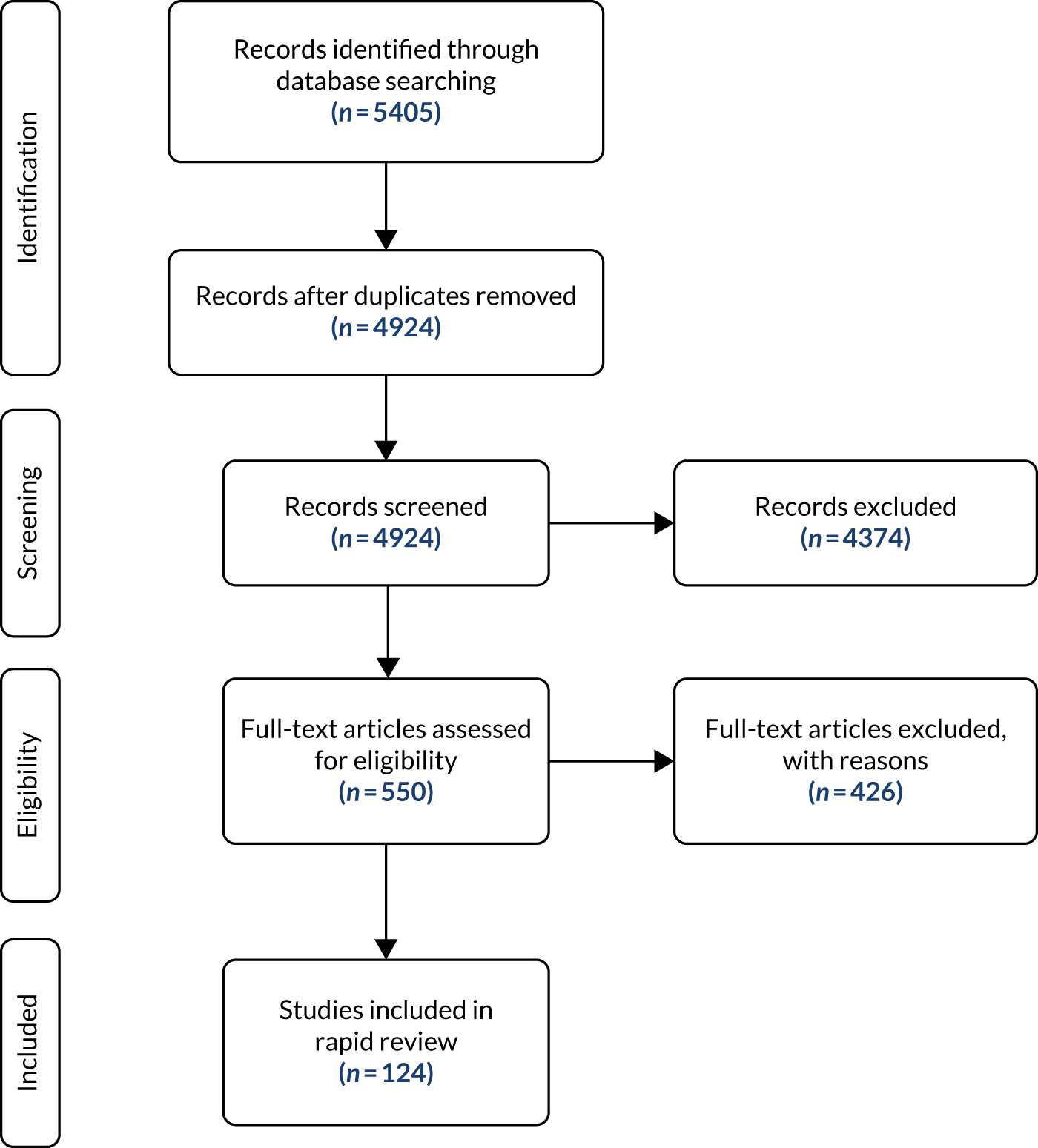
Epidemiological evidence
Because the purpose of this review was to identify key considerations for future modelling work, it was necessary to consider how the epidemiological evidence would fit into the outcomes observed as part of any definitive trial. A key piece of information is the reference measure, which establishes a link between a trial’s short- to medium-term outcomes and longer-term impacts, whether or not there is a dose–response relationship for a given effect size and how it is quantified. This might be usefully expressed as a RR, hazard ratio or odds ratio for each relationship. Existing models (such as those that underpin NICE guidance)94,95 have established evidence for general population risks of diseases stemming from long-term obesity, but these rarely have subgroups other than by sex. The aim here was to gain a general insight into what should be, on the strength of available evidence, the key adverse health outcomes (for woman or infant) in an economic model of pregnancy-related weight management.
We identified 39 studies96–134 that contained potentially relevant estimates of costs of achieving behavioural change and resource impacts linked to relevant long-term health outcomes. Fourteen studies118,135–147 reported health state utility scores relating to maternal outcomes, obesity and long-term conditions associated with weight gain.
The primary health risks for women linked to pregnancy weight gain reported in the reviewed studies were type 2 diabetes and cardiovascular disease. The evidence underpinning these comes mostly from large epidemiological registry reviews and regression models of large data sets, for example Eriksson et al. 105 There was limited evidence of elevated risks of cancer-related mortality and morbidity, though the authors warn that these effects are of only borderline statistical significance. The Eriksson et al. 105 study sample was drawn from the Finnish population; therefore, pregnancy-specific risks specific to the UK population were not addressed.
There was no evidence of a significant differential between elevated health risks linked to general weight gain and elevated health risks linked to weight gain during pregnancy. Therefore, for modelling purposes, general hazard ratios for the UK female population already used in existing general obesity impact modelling could be used for modelling long-term benefits of improved postnatal weight management.
In terms of subsequent pregnancy-related outcomes, the evidence points to an increased risk of higher maternal BMI scores on pre-eclampsia, gestational diabetes, caesarean section and other birth complications,133,134 and there is evidence that long-term weight retention in the postnatal period leads to negative outcomes in subsequent pregnancies. 7,8
The rapid evidence review did not identify a substantive pool of evidence for quantifying the link between pregnancy-related weight gain and risk to long-term infant development, suggesting that modelling these impacts will carry considerable uncertainty.
Economic end points
Estimates of long-term costs and QALY impacts attached to adverse health events of relevance to pregnancy-related weight management are well established in the UK and have been used in models underpinning NICE guidance (specifically guidelines PH25,134 PH15148 and CG181149 on cardiovascular disease, PH35 on diabetes,150 and PH5394 and NG795 on long-term models of weight loss). This review identified eight economic studies containing relevant evidence on economic points that could support modelling of long-term benefits. These included studies evaluating pharmacological approaches to delivering weight loss in patients with obese BMI scores135,151,152 and surgical interventions, including bariatric surgery. 136 The review also identified four epidemiological studies containing relevant economic data: two studies undertaken to estimate the burden of obesity118,153 and two studies that estimated the life-time burden of childhood obesity. 124,154
Programme clinical effectiveness and cost-effectiveness
We included 43 studies that estimated the clinical effectiveness and/or cost-effectiveness of behavioural programmes. 7,10,13,137,138,155–193 The primary outcome of the behavioural interventions identified in the review concerned their clinical effectiveness at reducing women’s weight in the first year post pregnancy. Most interventions identified used the absolute number of kilograms lost as the primary outcome of interest. In two studies, weight outcomes were also converted into a binary outcome identifying achievement of clinically significant weight loss postnatally, of which returning to within 5% of the woman’s pre-pregnancy weight was the most consistently used measure. 187,188 Significant 1-year outcomes in weight loss terms were found in only two studies. 189,190 One study191 was designed to capture adverse pregnancy events from a weight management programme and did not report weight loss outcomes; another study192 captured the impact of exercise interventions on other measures of cardiac and endocrine health through blood tests and waist measurement. Several other papers168,171,175,179,184,187 reported on initial acceptance of ideas or settings for interventions or provided outcomes for children rather than mothers.
Overall, the long-term clinical effectiveness and cost-effectiveness of behavioural programmes beyond the period of a clinical trial is uncertain. One paper193 offered evidence on weight maintenance over a 2-year follow-up period, with the remaining papers having follow-up periods no longer than 1 year. Evidence on longevity of effect, which is a crucial parameter for determining long-term economic outcomes, is lacking.
Existing economic models
The final search strand identified papers that would help in informing the methodological approach to modelling long-term impacts. A total of 34 papers139–147,194–219 were included. This search was deliberately broader in scope to try to capture the best practice for modelling obesity in general, not just in the postnatal population.
A total of 38% of the studies were based in the USA, 18% in the UK and 6% in the Netherlands. Canada,99,198 Germany,102,124 Ireland,115,135 Italy125,145 and Mexico195,214 each had two studies, and Australia,103 Belgium,152 Denmark,116 France,105 Greece,200 Malta,197 Portugal201 and Switzerland202 each had one study. One study98 used United Nations data to estimate worldwide impacts of obesity. The economic perspective of the studies was extracted, with 63% of papers taking a health service perspective and 37% taking a broader societal perspective.
Because this was a methodological search, there was a wider range of topics than only behavioural interventions. A total of 34% of models concerned modelling baseline or long-term epidemiological impacts of obesity without focusing on a specific intervention. Behavioural interventions were the second largest group, at 28%. A total of 22% modelled surgical interventions, one of which was to determine whether or not bariatric surgery improves pregnancy outcomes. A total of 10% modelled pharmacological interventions and 6% examined the population-level impact of policy interventions, including taxes on the sugar or fat content of food products. None of the papers covered by the review included a model dealing specifically with post-partum weight loss. One-fifth of the included studies covered only methodological approaches or were systematic reviews of A total of 32% of models used a microsimulation approach to model intervention impacts, 30% followed a Markov state-transition structure, 19% were based on regression analysis and 19% used a decision analytic approach.
Reviews of existing obesity models found that sophisticated long-term models are still rare, with modellers adopting strong assumptions about sustained duration of effect of interventions. 215–217
We identified one study218 that looked to capture the nuances of weight loss and regain in weight in key subgroups of people with obesity. This model used microsimulation methods to estimate four weight loss and gain scenarios over 4 years based on longitudinal data from a long-running diabetes study. This type of modelling structure could prove informative for extrapolating the impact of weight management programmes observed in any definitive trial given uncertainty regarding sustainability programme effects.
One review of the wider obesity modelling literature214 was identified and its key recommendations included the need to capture cardiovascular disease as a key consequence of obesity and to use modelling approaches geared to capturing the impacts of varying time to disease onset as well as disease severity and time to death. Microsimulation approaches, such as individual patient simulation or a Markov model, provide methodological frameworks for achieving this. Griffiths et al. 215 also recommend that implementation of interventions be included in the modelling, because they found that the scale-up and roll-out of small-scale interventions to population level is ‘controversial’ in the plausibility of the effects holding at population level.
Summary of review findings
Epidemiological evidence
-
The review found strong evidence for the inclusion of pre-eclampsia, gestational diabetes, cardiovascular disease and type 2 diabetes in a future economic model, with relative risks available for these diseases already established in the literature.
-
The evidence linking maternal outcomes to childhood obesity is not well established and linking a mother’s weight loss post partum to her child’s adult outcomes would require strong assumptions. Therefore, a childhood-to-adulthood model for this intervention would not be recommended.
Economic end points
-
The economic impacts of cardiovascular disease, type 2 diabetes and pregnancy complications are well established in the UK through NICE guidance and these should be used to inform the future model.
-
Relevant costs and health state utility/QALY estimates are available and widely documented in the literature for key clinical end points.
Programme clinical effectiveness and cost-effectiveness
-
Estimation of the clinical effectiveness and cost-effectiveness of weight management interventions has been largely restricted to periods of < 1 year. Evidence on long-term sustainability of any weight loss over longer periods is generally lacking, which poses challenges for modelling extrapolated intervention impacts beyond time periods that could be feasibly built into a main trial.
Existing economic models
-
The economic literature included did not contain an economic model specifically dealing with the impacts of postnatal weight management. However, many of the modelling studies included, including those that examined the impact of obesity, offer insight into appropriate methodological approaches to take when modelling impacts arising from improvement in pregnancy-related weight management and in some cases sources of evidence and data (e.g. relating to economic end points).
Implications of the rapid review for a future definitive trial
-
The modelling of long-term impacts linked to any clinically significant short-term weight loss observed in the context of a definitive trial will be feasible.
-
Modelling should prioritise the estimation of impacts where evidence is strongest with respect to adverse health outcomes. These include risks to subsequent pregnancies (specifically pre-eclampsia and gestational diabetes) and non-pregnancy-related health risks (specifically cardiovascular disease and type 2 diabetes).
-
Measurement of within-trial clinical effectiveness of the weight management intervention should account for the needs of economic modelling, specifically in using measures that can be synthesised with wider epidemiological data for determining impacts of risk of adverse events.
-
A modelling structure should be developed to account for prevailing uncertainties in the evidence base, specifically in relation to the probable sustainability of any weight loss that might be observed within the confines of a definitive trial. This should include a focus on the impacts of weight recidivism over time to avoid overestimating the long-term impacts of effective weight management, for example building on the work of Su et al. 218
-
The model should use a microsimulation structure such as discrete event simulation or Markov-based approaches. Figure 9 is a simplified example of the type of model structure that could be adopted to evaluate the long-term impacts arising from the type of weight management programme evaluated in the SWAN trial.
FIGURE 9.
Example of pathway impact model for economic evaluation of a postnatal weight management programme.
Chapter 8 Integration of findings
In this chapter we present the integration of findings from the main feasibility analyses and the process evaluation to contribute to meeting the overarching aim of the trial: to assess whether or not it is feasible to conduct a definitive RCT to determine the clinical effectiveness and cost-effectiveness of lifestyle information and access to weight management sessions for 12 weeks to support women in an ethnically diverse inner-city population to achieve long-term postnatal weight management and positive lifestyle behaviour.
In summary, the quantitative analysis comparing women allocated to the intervention and control arms regarding weight change and secondary outcomes showed that women allocated to the intervention arm had a modest increased weight loss at 12 months compared with women allocated to the control arm, with little difference in any of the other outcomes measured. By contrast, the process evaluation (in particular the interview data but also questionnaire free-text data) found that the relationship between the intervention and outcomes was more complex, with the ‘dose’ of the weight management group intervention being a key explanatory factor. This related to the three components of behaviour change: capability, motivation and opportunity. A more detailed examination of the potential reasons for disparate findings between the two data sets follows, using the framework provided by Moffat et al. 53 (Table 21).
| Approaches to explore disparities in findings53 | Application to trial |
|---|---|
| Treating methods as fundamentally different | The quantitative and qualitative components had different (although related) aims/objectives; approaches to data collection/analyses are based on fundamentally different theoretical paradigms. This could explain the disparities between findings in relation to the secondary outcomes, which were designed to assess outcomes derived from the logic model proposing how the intervention would work. The process evaluation was designed to understand how women experienced the intervention and did not specifically ask questions about these outcomes |
| Exploring the methodological rigour of each component |
Quantitative and qualitative components were conducted rigorously Quantitative: informed by a sample size calculation to assess clinically significant weight change differences. Utilised measures of outcome related to the intended outcomes of Slimming World, and other outcomes of interest (e.g. smoking, exercise). Each selected for their psychometric properties and validity in postnatal populations but many did not have strong psychometric properties or validity in postnatal populations Qualitative: the interview sample was diverse in relation to demographic and weight-related factors as well as intervention dose (including non-attenders). This was supplemented with questionnaire data from all women |
| Exploring data set comparability | The qualitative interview sample was a subsample of the women who participated in the main (quantitative) trial purposively selected for diversity, including women who did attend and women who did not attend the weight management groups. The qualitative analysis also included data from all women who completed free-text questions, so directly comparable to the quantitative sample |
| Conducting additional analyses and making further comparisons |
A critical distinguishing feature that is ‘ignored’ in the ITT analyses for the quantitative data is that half of the women allocated to the intervention arm did not attend any weight management groups at all, and of those that attended only 19% received the intervention as intended (e.g. attended ≥ 10 sessions) Based on the findings from the process evaluation that suggested a ‘dose effect’ with regard to the intervention, a per-protocol analysis was completed (see Chapter 5), showing a ‘dose effect’ with women who attended ≥ 10 sessions losing significantly more weight |
| Exploring whether or not the intervention under trial worked as expected |
Quantitative: each woman was treated as having received the same ‘dose’ of the intervention regardless of the number of sessions attended (or indeed if she attended at all) Qualitative: evidence of a dose–response effect was suggested by the data; women who attended more sessions were more likely to report positive capability, motivation and opportunity factor and to lose weight and sustain weight loss. The qualitative data suggested fidelity issues in relation to group and consultant factors that affected the acceptability of the intervention |
| Exploring whether or not the outcomes of the quantitative and qualitative components match | As mentioned above, the methods were distinct. The process evaluation (qualitative data) was not designed to assess ‘outcomes’, meaning that data sets were not directly comparable. Included quantitative measures to assess changes in some outcomes of interest need to be further considered in terms of relevance to women who have recently given birth and how best to capture data of interest |
The integration of data from all sources provided a rich explanation regarding how, why and if the intervention has potential to ‘work’. The quantitative data alone suggested a modest benefit in relation to weight change for women allocated to the intervention arm compared with women allocated to the control arm, with little difference in relation to other outcome measures. However, when examined in conjunction with the qualitative data, and with further per-protocol analysis, we found the intervention to have a ‘dose effect’, with greater understanding of the factors that could influence whether or not women will receive the full Slimming World ‘dose’.
Potential explanations for disparities in findings included that methods were fundamentally different (had different aims and questions) and ‘measures’ were not matched, and that the quantitative analyses (ITT) necessarily treated every intervention woman the same, regardless of whether or not she received the intervention as intended, and treated every group and session as the same. However, the qualitative data showed a dose–response effect, and key issues regarding group identity and consultant factors that influenced how the intervention was experienced by women.
The in-depth findings from the process evaluation regarding women’s motivations to participate in the trial and to lose weight, their beliefs about the intervention and their ability to follow the Slimming World programme – plus opportunity factors relating to the ease with which they could participate, the support they had for participation and whether or not they felt comfortable participating – were all key explanatory factors regarding whether or not women who attended ≥ 10 sessions were associated with more benefits in terms of sustainable weight loss.
This integration of findings is important for informing decisions about whether or not a definitive trial is feasible: there are clear recommendations emergent from the process evaluation that could enhance or optimise the potency of the intervention in a definitive trial. For recommendations, see Chapter 9, Recommendations for future research.
Chapter 9 Discussion and conclusion
Introduction
This trial was designed to assess the feasibility of conducting a future definitive trial to determine the clinical effectiveness and cost-effectiveness of lifestyle information and access to commercial weight management groups for 12 weeks to support women in an ethnically diverse inner-city population to achieve and maintain postnatal weight management and positive lifestyle behaviour. Findings showed that it was possible to recruit and retain women with overweight and obese BMI scores at antenatal booking, but recruitment approaches for women who had normal BMI scores who developed EGWG were not successful.
Women allocated to the intervention were more likely to have lost weight at 12 months postnatally, with evidence of a ‘dose effect’ in terms of number of sessions attended. There appeared to be minimal impact on other aspects of lifestyle behaviour, with the process evaluation findings informing why this may be the case, as well as potentially reflecting current positive health behaviours in the local population. The economic evaluation showed that it was feasible to use women’s self-reported data and data from maternity records to evaluate within-trial economic impacts and the potential importance of economic modelling of longer-term impacts.
Overview of findings
The original commissioning brief asked for a trial of the clinical effectiveness and cost-effectiveness of interventions for weight management after pregnancy. Because evidence was lacking to support a clinically effective and cost-effective intervention, including evidence of whether or not women with high BMI scores living in an ethnically diverse inner-city area would be prepared to participate in a trial or complete all trial follow-up processes or of the optimal time to commence weight management support, we proposed a feasibility trial in the first instance. Findings would inform a decision about whether or not a future definitive trial of clinical effectiveness and cost-effectiveness could be undertaken.
The majority of the feasibility trial objectives were achieved. Where objectives were not fully met as anticipated, the mixed-methods approach and integration of qualitative and quantitative trial data enabled us to consider why this may be the case and postulate how a similar objective could be optimised in a future trial. Trial objectives 1–6 are explored further below.
Objective 1
The sample size required 130 women to be followed up for 12 months postnatally. To reflect a 30% drop-out rate, because women are particularly mobile and tend to change addresses in the weeks and months after giving birth, we aimed to recruit a total of 190 women over a 6-month period. We recruited 193 women over 8 months; additional time was taken because of a revision to the trial protocol regarding recruitment of women who had normal antenatal booking BMI scores and developed EGWG. Contacting women initially by letter and asking them to opt out if they were not interested appeared to work well and meant that women could make their own decision about whether or not to participate. Of all women who received an invitation letter, only two contacted the team to express concerns about receiving a letter, indicating that women were generally happy to be contacted by a named midwife who could explain the trial further for those who were interested.
The initial plan (to advertise at the trial site to ask women who thought that they might have gained more weight than needed to contact the research midwives) only attracted a handful of women, none of whom met IoM criteria for gestational weight gain at 36 weeks. 5 A decision was made, supported by the TSC, to revise the recruitment approach and reflect the same approach used for women with overweight and obese BMI scores at antenatal booking, namely initially contacting women by letter to ask if they would be interested in joining the trial. We extended recruitment duration by 2 months to accommodate changes to the protocol and time to obtain REC/HRA approvals. Despite changes, we recruited only four women with EGWG. Although more women came forward to be weighed, they did not meet IoM criteria.
An important barrier to recruitment of these women is that women are not routinely weighed at each antenatal appointment in line with current NICE guidance,6 presenting a challenge with respect to identifying women with a normal BMI score at antenatal booking who develop EGWG. This is a population of women who give cause for concern15 because those who develop EGWG and do not lose the weight postnatally may gain more weight prior to their next pregnancy, increasing their risk of adverse outcomes such as gestational diabetes. 219 Alternative approaches to identify and support women with EGWG are needed, and, if weight management interventions are to target these women in the postnatal period, all women attending trial units who commence pregnancy with a normal BMI score may need to be weighed at each appointment or at pre-defined times during pregnancy.
Achieving high follow-up rates at 9 and 12 months in both groups was reassuring and reflected use of several approaches to follow-up, including face-to-face appointments with the research midwives at the trial unit, or in the woman’s homes, at 6 and 12 months, which women seemed to value. That so many women allocated to the control arm, and allocated to the intervention arm but who did not attend any sessions, completed follow-up was encouraging.
An important part of the process evaluation was to explore the acceptability of the intervention and trial procedures from interviews with women allocated to the intervention on completion of the commercial weight management programme and with women from both trial arms at 12 months postnatally to explore experiences of participating in the trial. We recruited as planned at both time points, selecting women for interview to reflect diversity of weight change and ethnicity and, in the intervention arm, high or low uptake or non-attendance of sessions.
Objective 2
We aimed to estimate the effect size for a probable primary trial outcome in a future definitive trial, namely difference between the trial groups in weight at 12 months postnatally, expressed as percentage weight change from antenatal booking weight. This was an outcome that the Expert PPI Group supported because longer-term weight management benefits were considered to be important. Analysis of weight data showed a modest benefit in relation to weight change at 12 months postnatally among women in the intervention arm, which was not apparent at 6 months. This is likely to be because some women allocated to the intervention arm (i.e. those who started the programme nearer to 16 weeks postnatally) were still accessing the Slimming World programme at 6 months postnatally. It may also reflect the need for women who had completed their weight management sessions to fully adapt the programme into their daily lives beyond the initial intervention period. Nevertheless, the findings confirmed the initial plan to include a primary end point at 12 months. Per-protocol analysis suggested that the intervention had a ‘dose effect’ associated with engaging with the Slimming World programme, with the greatest benefit seen among women who attended ≥ 10 sessions.
Objective 3
Supporting an individual’s lifestyle behaviour change is complex, even more so when women who have high BMI scores at antenatal booking may have multiple poor health-related behaviours, for example smoking tobacco, not taking physical exercise and not commencing breastfeeding. 70,220 When planning the feasibility trial, we had to consider if evidence-based information on positive lifestyle behaviour offered to women in the intervention arm, together with access to commercial weight management sessions postnatally, could support women to alter their lifestyle behaviours in the shorter- and longer-term, and, if so, the extent to which this was likely to occur if measures selected to assess ‘change’ were completed by women, and the most appropriate measures to use in a future definitive trial. Few of the potential measures available had been previously validated in postnatal populations because they were developed for general population use, and, for other specific areas of interest, questions were developed by the trial team because no relevant measures were identified.
Assessment of secondary outcomes showed minimal differences between trial arms. There could be several reasons for this, including that motivation for joining the trial was similar across trial arms because women who agreed to participate were interested in their weight after having a baby. It was also reassuring that a high proportion of women in both trial arms completed follow-up questionnaires, with few data missing from included measures. When data items were missing, for example on extent of physical activity at baseline, it is understandable that women at 36 weeks’ gestation may have considered this to be not relevant to their lives at the time.
Women allocated to the intervention arm were more likely to have an EPDS score of ≥ 12 at 6 months, indicating that women were more likely to be at risk of developing postnatal depression. 37 Reasons for this are difficult to speculate but could reflect baseline differences in mode of birth in the sample or that women attending weight management groups felt more anxious or concerned about their health. However, lack of difference in other outcomes compared with the control arm including body image, maternal esteem and infant sleep patterns, which could be associated with potential mental health issues,66,73 does not suggest that women were affected in other ways by the intervention. Lack of statistical power may also mean that any differences that may have been present were not detected, and that measures were not analysed per protocol.
Lifestyle and behavioural outcomes we were keen to explore in terms of whether or not the intervention was likely to make a difference included women’s dietary intake. As described in Chapter 5, the team had several discussions when designing the trial as to an appropriate food frequency questionnaire, eventually selecting DINE, which had been used previously in studies that included women of reproductive age. 35 Measures of fat, fibre and unsaturated fat showed no change between groups from baseline to 6 or 12 months, with potential reasons for this identified from the process evaluation (see Objective 4), including that the intervention was potentially not long enough to allow changes in dietary and other lifestyle behaviours to occur. If undertaking a definitive trial, it would be useful to consider further what data on diet are important to capture (i.e. patterns of healthy eating or nutritional content of dietary intake) to inform what may be a more appropriate measure. Measures used should also reflect the dietary content of the intervention of interest to capture any change in intake over time, because items included in an existing measure may not reflect dietary advice offered as part of the weight management intervention. Of note (and an unexpected finding) was that a few women described that completing the DINE questions influenced them to alter their diet as it included items that made them realise how much ‘bad’ food they were eating.
That there were no differences in important lifestyle outcomes including breastfeeding and tobacco smoking between trial arms probably reflects more positive lifestyle behaviour in the diverse trial population and population of women who uses the local maternity services generally, as well as lack of statistical power. The inner-city area from which women were recruited has traditionally had high levels of breastfeeding uptake and duration (in 2016, 91% of women in the local area commenced breastfeeding),221 which may be attributed to the ethnic mix of women in the area. Similarly, few women living in the local maternity catchment area acknowledged that they smoked in pregnancy, although slightly more women in the intervention arm reported that they had never smoked. The recent introduction of carbon monoxide monitoring at the trial site could provide data of greater accuracy on the number of women who smoke tobacco in pregnancy in a definitive trial. Fewer women allocated to the intervention arm than allocated to the control arm reported drinking any alcohol at 6 and 12 months, with a significant difference at 6 months, but low overall rates of alcohol use probably also reflects the profile of the local population of women.
Objective 4
An important aspect of the feasibility trial was to assess the acceptability of the intervention and trial procedures. This aspect of the evaluation was addressed using mixed methods, including questionnaire data, semistructured interviews with women and data provided by Slimming World. Findings highlighted the complex relationship between the intervention and some outcomes of interest.
Of the women allocated to the intervention, few women could recall the content of the lifestyle information leaflet despite this being offered to all women allocated to the intervention arm at trial recruitment. The information reflected current NICE guidance on a number of lifestyle behaviours (breastfeeding, keeping active, healthy eating and stopping smoking),6,33 which were assessed in included measures. The leaflet was developed with the support of the Expert PPI Group, but, perhaps not unsurprisingly, for women in late pregnancy and then facing the pressures of new parenthood it was unlikely that reading and remembering the content of the leaflet was a priority. For a definitive trial, the inclusion of additional information alongside a weight management support intervention would have to be considered further, as would a more user-friendly and accessible format of presentation, for example an online cartoon, blogs, infographics or social media.
All women allocated to the intervention could access a Slimming World group of their choosing at any time between 8 and 16 weeks postnatally. Just under half of the women (46; 47%) attended at least one session, most doing so when their infant was aged ≥ 10 weeks, with several key barriers to not attending sessions identified, including lack of opportunity, difficulty organising child care, timing of sessions and family illness. It would have been encouraging to have had greater uptake of weight management sessions among the intervention arm, but, given that the trial sample included women with a range of child-care and other family responsibilities who may not have encountered a similar intervention before and who live in one of the most socioeconomically deprived boroughs in England, that just under half attended one session could be viewed as positive. Analysis of the women’s experiences of attending weight management groups using the COM-B framework46 pointed to several important themes and to issues concerning why some women completed the full ‘dose’ of ≥ 10 sessions, why some attended < 10 sessions and why some did not attend any sessions. Of note is that the most common reason for not attending sessions was a lack of opportunity, although a small number of women had misbeliefs about Slimming World.
An important finding was that, based on per-protocol analysis, women who attended ≥ 10 sessions had greater weight loss at 12 months postnatally than women who attended nine or fewer sessions, did not attend any sessions or were allocated to the control arm. Of interest was evidence of a dose–response effect, which reflected that the more weight management sessions attended, the more women could adapt the programme to their daily lives. The process evaluation findings provided very useful data on how we could potentially increase uptake of sessions in a future trial of postnatal weight management support. This includes the need to offer commencement later in the postnatal period, because some women found that the time and energy required to attend a weekly session was too much and too soon after giving birth. Women expressed the potential importance of later commencement of the sessions and some considered that having only 12 sessions as part of the Slimming World programme was not long enough because they had only just got to grips with how to adapt the programme to their daily lives. Feedback also reflected the need to ensure that women were aware that they could change groups if they were not satisfied with their initial choice. Women wanted more information on trial allocation on what the Slimming World programme included, such as the role of exercise and how well the plan suited the needs of breastfeeding women.
Around one-third of women in both trial arms accessed additional support for weight management, most of whom joined a gym. There was low risk of contamination between trial arms, an outcome of particular importance to inform a definitive trial. From the 12-month interviews it was apparent that most women in both trial arms understood the aims of the trial, what being randomised meant and found recruitment approaches to be straightforward. Women generally found the trial questionnaires and included measures easy to complete and enjoyed having longer-term contact with research midwives.
Objective 5
It was feasible to generate economic data to inform a larger definitive trial using a combination of participant self-reported information and maternity records. There were no reported problems with the acceptability of questions regarding service contacts from the women and self-reported data on use of community-based services during and after pregnancy was largely complete. However, resource use information was less complete for more costly types of hospital-based service contacts, including hospital admissions for mothers and infants. A future trial should consider use of patient records data to reduce missing data for these types of service contacts.
The economic analysis highlighted a wide range of services used by women and for their infants during the 12 months after birth. Women allocated to the intervention arm had on average a higher service use cost over the first 12 months following birth than women allocated to the control arm. The average QALY lived over the follow-up period was only marginally higher for women allocated to the intervention. Combined findings suggested that the extra cost of the programme per QALY gained would be in excess of the cost threshold normally used by NICE to assess programme value for money. However, as many potential beneficial impacts of effective weight management could not be captured directly in this type of trial-based analysis (e.g. life-years gained and costs to the NHS avoided through prevention of disease linked to obesity and avoidance of complications in subsequent pregnancies), economic modelling of wider out-of-trial impacts should be included in any future definitive trial.
This was supported by the findings of the rapid review, which demonstrated that modelling of longer-term impacts linked to clinically significant short-term weight loss observed in a definitive trial would be feasible. The review identified a sufficient evidential basis for modelling out-of-trial impacts of effective weight management. The review findings suggest that priority should be given to modelling the impacts of health outcomes linked to pregnancy-related obesity and weight gain with the strongest supporting evidence, including cardiovascular disease, type 2 diabetes, gestational diabetes and health complications arising in subsequent pregnancies.
Objective 6
The criteria to proceed to a definitive trial (objective 6) were met. It was feasible to recruit and retain women with overweight or obese BMI scores at antenatal booking to a trial of a commercial weight management group plus standard care compared with standard care only after birth. Approaches to recruit women with normal BMI scores who develop EGWG and to optimise the benefit of the intervention based on the process evaluation findings need to be further considered.
Inclusion of a nested mixed-methods process evaluation enabled the integration of data from all sources to provide an in-depth explanation regarding how, why and if the intervention had potential to ‘work’ in a future definitive trial. As described with respect to objective 2, quantitative data analysis suggested a modest benefit in relation to weight change for women allocated to the intervention arm compared with women allocated to the control arm at 12 months postnatally, but little difference in relation to other lifestyle and health outcome measures of interest. To provide further context for the weight outcome finding, it was examined in conjunction with the qualitative data and per-protocol analysis, which showed that the intervention had a ‘dose effect’: greater weight loss was reported among women who attended ≥ 10 sessions than among women who attended < 10 sessions or were allocated to the control arm. The integration of findings provided greater understanding of factors that influenced women to receive the full ‘dose’ or not, which will inform a future trial.
Some disparity in trial findings was as a consequence of quantitative and qualitative methods being fundamentally different in terms of aims and questions, meaning that ‘measures’ were not matched. ITT analysis necessarily treated every intervention woman the same, regardless of whether she received the intervention as intended, and treated every weight management group and session as the same. However, the qualitative data showed a dose–response effect and that group identity and consultant factors influenced how the intervention was experienced by women.
The process evaluation identified key explanatory factors as to why some women attended ≥ 10 sessions, including their motivations to participate in the trial and to lose weight, their beliefs about the intervention, their ability to follow the Slimming World programme, opportunity factors and the support they had for participation. Integration of findings will inform decisions about a future definitive trial, in line with objective 6, with several clear recommendations emergent from the process evaluation that could optimise the potency of the intervention in a future definitive trial.
Patient and public involvement
Engagement with women in the local area with high BMI scores who had experienced a recent pregnancy was invaluable to the development of the trial, including their input regarding the aims and objectives. There was overwhelming support from the women consulted about the importance of postnatal support, because pregnancy was not viewed as an optimal time for women to consider altering lifestyles to promote weight management. Furthermore, women we consulted specifically asked for an intervention that was not led by the NHS or held in NHS settings; they wanted a group intervention led by non-NHS staff where they could meet people from their local community. The use of Slimming World was viewed positively because women were aware of the organisation, knew that it offered groups that women were likely to be able to access at a range of times and venues and very keen that it offered weight management support suitable for nursing mothers.
We did experience some difficulties with organising regular Expert PPI Group meetings when all four members could attend owing to problems related to organising child care, dealing with family illness, not being able to take time out of paid employment and, in one case, having to take time out from the group because of a subsequent pregnancy. Nevertheless, at least one of the PPI members joined six CPT meetings, which was extremely useful to ongoing discussions as the trial progressed, and an active WhatsApp group meant that we could communicate with Expert PPI Group members to check availability for meetings and to ask for advice on issues as the trial progressed. Examples of the value of this included seeking their advice on the revised approach to recruiting women with normal antenatal booking BMI scores (when it became clear that the original plan for recruitment of these women was not working) and the content of responses to concerns raised by the two women about letters inviting them to consider participation in SWAN.
On reflection, although the PPI approach was very useful and of benefit to the trial, inviting women at the outset to commit to four meetings per year over a 2-year period, plus invitations to attend more regular CPT meetings, was not feasible, given the demands on their time. It may be more efficient to establish a ‘virtual’ PPI group to invite members to provide guidance and advice as a trial progresses, with costed time for one or two PPI representatives to attend regular CPT meetings (perhaps taking it in turn to save time and costs). More information on commitment and expectations of the role of an Expert PPI Group member from the outset should also be offered.
Strengths and limitations
This single-centre feasibility trial had several strengths in that it showed the potential to recruit and retain to 12 months postnatal women from diverse ethnic backgrounds living in an inner-city area of high social deprivation to a trial of lifestyle information and access to a weight management group. The intervention assessed has national (and international) reach, meaning that women could access weight management sessions at any venue and attend sessions on a day and time that best suits their needs and lifestyles. The intervention has an evidence base and is acknowledged as being suitable for new and nursing mothers.
The nested process evaluation, which used a mixed-methods approach, enabled us to carefully evaluate and consider the experiences of women allocated to the intervention in terms of its acceptability to women, barriers to and facilitators of women completing ≥ 10 sessions as part of the intervention, and how and why some trial outcomes of interest may have been influenced by the intervention content. This approach means that if a future larger trial were to be undertaken, we have evidence of how outcomes could be optimised, including the potential need to extend the duration of the offer of access to the weight management group, and provide more information about the Slimming World programme for women as soon as they are allocated to the intervention. Furthermore, use of research midwives to recruit and arrange to meet women as part of planned follow-up was an important component of the trial approach and, together with a small financial incentive for the women, resulted in high data completion rates across trial arms.
Regarding the economic evaluation, measurement of QALYs over such a short time frame (12 months from baseline interview) may have risked a failure to capture the full range of health-related benefits linked to effective weight management that could arise though improving health and birth outcomes in subsequent pregnancies and reducing non-pregnancy-related health risks to women. The preliminary within-trial economic analysis provided no assessment of long-term cost savings to the NHS or other agencies that would be expected through avoidance of excessive weight gain; however, the rapid review findings highlighted the important role of economic modelling to inform longer-term impacts on outcomes of importance for the NHS and other agencies in a future trial.
Limitations included that we were unable to recruit women who had a normal antenatal booking BMI score and EGWG, meaning that findings should be interpreted only among women with high BMI scores (> 25 kg/m2). The issue of how best to support women with EGWG remains unanswered. That some of the survey measures used had not been validated in a postnatal population means that we cannot be confident about their validity and interpretation. However, the process evaluation provided some insight into reasons for variability in some outcomes of interest. Furthermore, as a single-centre feasibility trial, findings cannot be generalised.
Recommendations for future research
-
The content, timing and presentation of evidence-based lifestyle information needs to be further considered if women are to find information of benefit. Options for presentation of content, other than offering a paper leaflet, should be considered.
-
The process evaluation findings indicate that, if commercial weight management sessions are to support women with high BMI scores to achieve and sustain postnatal weight loss and positive lifestyle change, the duration of the intervention needs to be extended in terms of a wider window of opportunity for commencing sessions and a longer intervention period.
-
Prior to undertaking a future trial it will be important to map the barriers to uptake and retention of the intervention to behaviour change techniques and methods to mitigate these where possible, for example to tackle women’s perceptions of the importance of weight management and to provide them with information about the intervention to counteract misconceptions they may have about the safety for breastfeeding mothers or the role of exercise.
-
Consideration of inclusion of an additional arm where women are randomised to the online version of Slimming World, which could counteract some of the opportunity issues identified by women allocated to the intervention arm who did not attend any weight management sessions; however, evidence to support this option is needed.
-
Women in this trial were positive about planned follow-up contacts with the research midwives. Building in contact points for research midwives to collect follow-up data on outcomes of interest could support high follow-up and data completion rates in a future trial.
-
Research into postnatal weight management support of women with normal BMI scores and EGWG needs to consider how best to identify and recruit women.
-
A future definitive trial of lifestyle information and commercial weight management groups would need to consider inclusion of economic modelling. Economic impacts over the course of a short-term trial alone are unlikely to be sufficient to demonstrate cost-effectiveness of weight management support longer-term for women or their infants.
Conclusion
This feasibility trial aimed to assess if it would be possible to recruit and retain women from a diverse inner-city population to participate in a trial of lifestyle information and postnatal weight management support through use of Slimming World. We achieved the majority of the feasibility trial objectives but showed that recruitment of women with EGWG needs to be reconsidered. We were able to retain women who were recruited to complete trial follow-up at 12 months. Findings supported the use of a primary outcome based on comparison of antenatal booking weight and weight at 12 months postnatally. Attendance at weight management sessions appeared to support women’s postnatal weight management as assessed at 12 months postnatally compared with standard care alone, with likely greater benefit on weight outcomes from attending ≥ 10 sessions.
The potential to inform lifestyle behaviours was less clear, probably owing to lack of power and small sample size, but could reflect some positive lifestyle behaviour such as high breastfeeding commencement rates already present in the local population. Integration of quantitative and qualitative findings highlighted several key findings to optimise the potency of the Slimming World programme in a future definitive trial, including offering more information about the intervention in pregnancy, a longer intervention commencement period, a longer period of intervention and alternative approaches to presenting information on positive health behaviours.
The preliminary within-trial economic evaluation demonstrated the feasibility of using a combination of self-reported data on relevant service contacts combined with data extracted from clinical case notes on childbirth to evaluate the within-trial economic impacts of a weight management programme delivered post partum. The findings suggest that it may be difficult to demonstrate the cost-effectiveness of a weight management programme offered to women postnatally relying purely on a reference to economic impacts measured over the course of a clinical trial, and inclusion of economic modelling could prove an essential vehicle for a more complete and robust examination of programme cost-effectiveness.
It is acknowledged that women who participated may have been more motivated and interested, but follow-up and adherence was good, with important learning for developing and designing a future RCT. A further, larger trial of clinical effectiveness and cost-effectiveness of lifestyle information and commercial weight management groups is an important next step to consider how best to support weight management among women with high BMI scores who have recently given birth.
Acknowledgements
We would like to thank all women who agreed to take part in the trial, and the four members of our Expert PPI Group (Nikki-Lee Markham, Ashaki Bailey, Charlotte Littleboy and Sueann Carter) who offered their time and support for all stages of our work and gave permission to have their names cited here.
Thanks also to Linda Hoinville for her support with analysis of some of the questionnaire data.
Trial Steering Committee
Independent members
Professor Rona McCandlish (chairperson).
Professor Hora Soltani.
Dr Nicola Heslehurst.
Mrs Samantha Hainsworth (PPI representative).
Dr Richard Hooper.
Contributions of authors
Debra Bick (https://orcid.org/0000-0002-8557-7276) (Professor of Clinical Trials in Maternal Health) was lead applicant and chief investigator with overall responsibility for the grant and oversaw all aspects of writing the report.
Cath Taylor (https://orcid.org/0000-0001-6239-4744) (Reader in Health Services Research) was a co-applicant, led the process evaluation component of the trial, including the analysis and writing of the report chapter, and contributed to the design of the trial and writing of the report.
Vanita Bhavnani (https://orcid.org/0000-0001-5866-5193) (Senior Research and Evaluation Officer) led the Expert PPI Group, contributed to the process evaluation component of the trial and contributed to the writing of the report.
Andy Healey (https://orcid.org/0000-0003-2013-3161) (Senior Research Fellow) was a co-applicant, responsible for the health economics and contributed to the design of the trial and writing of the report.
Paul Seed (https://orcid.org/0000-0001-7904-7933) (Senior Lecturer in Medical Statistics) was a co-applicant, responsible for the data analysis plan, trial statistician and contributed to the writing of the report.
Sarah Roberts (https://orcid.org/0000-0002-6807-9830) (Research Associate) contributed to the health economics component of the trial and the writing of the report.
Magdalena Zasada (https://orcid.org/0000-0002-4701-0359) (Research Fellow) contributed to analysis of the process evaluation data and the writing of the report.
Amanda Avery (https://orcid.org/0000-0002-3845-6420) (Assistant Professor in Nutrition and Dietetics) was a co-applicant and contributed to the design of the trial and the writing of the report.
Victoria Craig (https://orcid.org/0000-0001-8691-4433) (Midwife) was responsible for recruitment and follow-up of women and contributed to the writing of the report.
Nina Khazaezadah (Consultant Midwife in Public Health) was a co-applicant and contributed to the design of the trial and the writing of the report.
Sarah McMullen (https://orcid.org/0000-0001-5325-0458) (Head of Knowledge, NCT) was a co-applicant, contributed to the PPI support and contributed to the design of the trial and the writing of the report.
Sheila O’Connor (https://orcid.org/0000-0003-0144-2954) (Midwife) was responsible for recruitment and follow-up of women and contributed to the writing of the report.
Bimpe Oki (https://orcid.org/0000-0002-2741-6503) (Consultant in Public Health) was a co-applicant and contributed to the writing of the report.
Eugene Oteng-Ntim (https://orcid.org/0000-0001-8867-2909) (Consultant Obstetrician) was a co-applicant and contributed to the writing of the report.
Lucilla Poston (https://orcid.org/0000-0003-1100-2821) (Professor of Maternal and Fetal Health) was a co-applicant and contributed to the writing of the report.
Michael Ussher (https://orcid.org/0000-0002-0995-7955) (Professor of Behavioural Medicine) was a co-applicant, advised on content of follow-up measures of lifestyle behaviours and contributed to the writing of the report.
Publications
Bick D, Taylor C, Avery A, Bhavnani V, Craig V, Healey A, et al. Protocol for a two-arm feasibility RCT to support postnatal maternal weight management and positive lifestyle behaviour in women from an ethnically diverse inner city population: the SWAN feasibility trial. Pilot Feasibility Stud 2019;5:117.
Bick D, Taylor C, Bhavnani V, Healey A, Seed P, Roberts S, et al. Lifestyle information and commercial weight management groups to support maternal postnatal weight management and positive lifestyle behaviour: the SWAN feasibility randomised controlled trial. BJOG 2020;127:636‒45.
Taylor C, Bhavnani V, Zasada M, Ussher M, Bick D. Barriers and facilitators to uptake and retention of inner-city ethnically diverse women in a postnatal weight management intervention: a mixed-methods process evaluation within a feasibility trial in England. BMJ Open 2020;10:e034747.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the PHR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PHR programme or the Department of Health and Social Care.
References
- Walker LO, Timmerman GM, Sterling BS, Kim M, Dickson P. Do low-income women attain their pre-pregnant weight by the 6th week of postpartum?. Ethn Dis 2004;14:119-26.
- Hilson JA, Rasmussen KM, Kjolhede CL. Excessive weight gain during pregnancy is associated with earlier termination of breast-feeding among white women. J Nutr 2006;136:140-6. https://doi.org/10.1093/jn/136.1.140.
- Vinter CA, Jensen DM, Ovesen P, Beck-Nielsen H, Tanvig M, Lamont RF, et al. Postpartum weight retention and breastfeeding among obese women from the randomized controlled Lifestyle in Pregnancy (LiP) trial. Acta Obstet Gynecol Scand 2014;93:794-801. https://doi.org/10.1111/aogs.12429.
- Poston L, Harthoorn LF, Van Der Beek EM. Contributors to the ILSI Europe Workshop. Obesity in pregnancy: implications for the mother and lifelong health of the child. A consensus statement. Pediatr Res 2011;69:175-80. https://doi.org/10.1203/PDR.0b013e3182055ede.
- Siega-Riz AM, Gray GL. Gestational weight gain recommendations in the context of the obesity epidemic. Nutr Rev 2013;71:26-30. https://doi.org/10.1111/nure.12074.
- National Institute for Health and Care Excellence (NICE) . Weight Management Before, During and After Pregnancy 2010.
- van der Pligt P, Willcox J, Hesketh KD, Ball K, Wilkinson S, Crawford D, et al. Systematic review of lifestyle interventions to limit postpartum weight retention: implications for future opportunities to prevent maternal overweight and obesity following childbirth. Obes Rev 2013;14:792-805. https://doi.org/10.1111/obr.12053.
- Rooney BL, Schauberger CW, Mathiason MA. Impact of perinatal weight change on long-term obesity and obesity-related illnesses. Obstet Gynecol 2005;106:1349-56. https://doi.org/10.1097/01.AOG.0000185480.09068.4a.
- Heslehurst N, Ells LJ, Simpson H, Batterham A, Wilkinson J, Summerbell CD. Trends in maternal obesity incidence rates, demographic predictors, and health inequalities in 36,821 women over a 15-year period. BJOG 2007;114:187-94. https://doi.org/10.1111/j.1471-0528.2006.01180.x.
- Gould Rothberg BE, Magriples U, Kershaw TS, Rising SS, Ickovics JR. Gestational weight gain and subsequent postpartum weight loss among young, low-income, ethnic minority women. Am J Obstet Gynecol 2011;204:52.e1-11. https://doi.org/10.1016/j.ajog.2010.08.028.
- National Institute for Health and Care Excellence (NICE) . Weight Management: Lifestyle Services for Overweight or Obese Adults 2014.
- Duenas A, Rawdin A, Chilcott J, Messina J, Johnson M, Campbell F, et al. The Cost Effectiveness of Weight Management Interventions Following Pregnancy. Sheffield: ScHARR Public Health Collaborating Centre; 2011.
- Jolly K, Lewis A, Beach J, Denley J, Adab P, Deeks JJ, et al. Comparison of range of commercial or primary care led weight reduction programmes with minimal intervention control for weight loss in obesity: Lighten Up randomised controlled trial. BMJ 2011;343. https://doi.org/10.1136/bmj.d6500.
- Ehrlich SF, Hedderson MM, Quesenberry CP, Feng J, Brown SD, Crites Y, et al. Post-partum weight loss and glucose metabolism in women with gestational diabetes: the DEBI Study. Diabet Med 2014;31:862-7. https://doi.org/10.1111/dme.12425.
- Haugen M, Brantsæter AL, Winkvist A, Lissner L, Alexander J, Oftedal B, et al. Associations of pre-pregnancy body mass index and gestational weight gain with pregnancy outcome and postpartum weight retention: a prospective observational cohort study. BMC Pregnancy Childbirth 2014;14. https://doi.org/10.1186/1471-2393-14-201.
- Briley AL, Barr S, Badger S, Bell R, Croker H, Godfrey KM, et al. A complex intervention to improve pregnancy outcome in obese women; the UPBEAT randomised controlled trial. BMC Pregnancy Childbirth 2014;14. https://doi.org/10.1186/1471-2393-14-74.
- John E, Cassidy DM, Playle R, Jewell K, Cohen D, Duncan D, et al. Healthy eating and lifestyle in pregnancy (HELP): a protocol for a cluster randomised trial to evaluate the effectiveness of a weight management intervention in pregnancy. BMC Public Health 2014;14. https://doi.org/10.1186/1471-2458-14-439.
- Poston L. Obesity in pregnancy: could lifestyle interventions work?. BMC Med 2014;12. https://doi.org/10.1186/s12916-014-0201-7.
- Dodd JM, Turnbull D, McPhee AJ, Deussen AR, Grivell RM, Yelland LN, et al. Antenatal lifestyle advice for women who are overweight or obese: LIMIT randomised trial. BMJ 2014;348. https://doi.org/10.1136/bmj.g1285.
- Poston L, Bell R, Croker H, Flynn AC, Godfrey KM, Goff L, et al. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol 2015;3:767-77. https://doi.org/10.1016/S2213-8587(15)00227-2.
- The International Weight Management in Pregnancy (i-WIP) Collaborative Group . Effect of diet and physical activity based interventions in pregnancy on gestational weight gain and pregnancy outcomes: meta-analysis of individual participant data from randomised trials. BMJ 2017;358. https://doi.org/10.1136/bmj.j3119.
- Amorim Adegboye AR, Linne YM. Diet or exercise, or both, for weight reduction in women after childbirth. Cochrane Database Syst Rev 2013;7. https://doi.org/10.1002/14651858.CD005627.pub3.
- Huseinovic E, Bertz F, Leu Agelii M, Hellebö Johansson E, Winkvist A, Brekke HK. Effectiveness of a weight loss intervention in postpartum women: results from a randomized controlled trial in primary health care. Am J Clin Nutr 2016;104:362-70. https://doi.org/10.3945/ajcn.116.135673.
- Hoedjes M, Berks D, Vogel I, Franx A, Visser W, Duvekot JJ, et al. Effect of postpartum lifestyle interventions on weight loss, smoking cessation, and prevention of smoking relapse: a systematic review. Obstet Gynecol Surv 2010;65:631-52. https://doi.org/10.1097/OGX.0b013e3182077f64.
- Little P, Stuart B, Hobbs FR, Kelly J, Smith ER, Bradbury KJ, et al. Randomised controlled trial and economic analysis of an internet-based weight management programme: POWeR+ (Positive Online Weight Reduction). Health Technol Assess 2017;21. https://doi.org/10.3310/hta21040.
- National Institute for Health Research . Public Health Research n.d. www.nihr.ac.uk/explore-nihr/funding-programmes/public-health-research.htm (accessed 12 December 2019).
- Slimming World . How Much Does It Cost? n.d. www.slimmingworld.co.uk/health/swor/how-much-does-it-cost.aspx (accessed 12 December 2019).
- Medical Research Council (MRC) . Developing and Evaluating Complex Interventions 2019. https://mrc.ukri.org/documents/pdf/complex-interventions-guidance/ (accessed 6 November 2019).
- NHS Lambeth Clinical Commissioning Group . NHS Lambeth Clinical Commissioning Group ‘Healthier Together’ Five Year Strategy: 2014 15 to 2018 19 2013. www.lambethccg.nhs.uk/news-and-publications/publications/Documents/Current%20plans%20and%20strategies/NHS%20Lambeth%20CCG%20Five%20Year%20Strategy%20Healthier%20Together%202014-15-18-19.pdf (accessed 5 December 2018).
- Stubbs RJ, Pallister C, Whybrow S, Avery A, Lavin J. Weight outcomes audit for 34,271 adults referred to a primary care/commercial weight management partnership scheme. Obes Facts 2011;4:113-20. https://doi.org/10.1159/000327249.
- Elfhag K, Rössner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obes Rev 2005;6:67-85. https://doi.org/10.1111/j.1467-789X.2005.00170.x.
- Wing RR, Jeffery RW. Benefits of recruiting participants with friends and increasing social support for weight loss and maintenance. J Consult Clin Psychol 1999;67:132-8. https://doi.org/10.1037//0022-006x.67.1.132.
- National Institute for Health and Care Excellence . Postnatal Care: Routine Postnatal Care of Women and Their Babies 2014.
- Plain English Campaign . How To Write in Plain English n.d. https://plainenglish.co.uk/files/howto.pdf (accessed 12 December 2019).
- Crozier SR, Inskip HM, Barker ME, Lawrence WT, Cooper C, Robinson SM. SWS Study Group . Development of a 20-item food frequency questionnaire to assess a ‘prudent’ dietary pattern among young women in Southampton. Eur J Clin Nutr 2010;64:99-104. https://doi.org/10.1038/ejcn.2009.114.
- Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International Physical Activity Questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381-95. https://doi.org/10.1249/01.MSS.0000078924.61453.FB.
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry 1987;150:782-6. https://doi.org/10.1192/bjp.150.6.782.
- Ussher M, Aveyard P, Manyonda I, Lewis S, West R, Lewis B, et al. Physical activity as an aid to smoking cessation during pregnancy (LEAP) trial: study protocol for a randomized controlled trial. Trials 2012;13. https://doi.org/10.1186/1745-6215-13-186.
- Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. AUDIT: The Alcohol Use Disorders Identification Test – Guidelines for Use in Primary Care. Geneva: World Health Organization; 2001.
- Rosenberg M. Society and the Adolescent Self-image. Princeton, NJ: Princeton University Press; 1965.
- Fairburn CG, Beglin SJ. Assessment of eating disorders: interview or self-report questionnaire?. Int J Eat Disord 1994;16:363-70.
- Barrett B, Waheed W, Farrelly S, Birchwood M, Dunn G, Flach C, et al. Randomised controlled trial of joint crisis plans to reduce compulsory treatment for people with psychosis: economic outcomes. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0074210.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- O’Cathain A, Hoddinott P, Lewin S, Thomas KJ, Young B, Adamson J, et al. Maximising the impact of qualitative research in feasibility studies for randomised controlled trials: guidance for researchers. Pilot Feasibility Stud 2015;1. https://doi.org/10.1186/s40814-015-0026-y.
- Medical Research Council (MRC) . MRC Guidelines for Good Clinical Practice in Clinical Trials 1998.
- Vickers AJ, Altman DG. Statistics notes: Analysing controlled trials with baseline and follow up measurements. BMJ 2001;323:1123-4. https://doi.org/10.1136/bmj.323.7321.1123.
- Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6. https://doi.org/10.1186/1748-5908-6-42.
- Trevillion K, Domoney J, Pickles A, Bick D, Byford S, Heslin M, et al. Depression: an exploratory parallel-group randomised controlled trial of Antenatal guided self help for WomeN (DAWN): study protocol for a randomised controlled trial. Trials 2016;17. https://doi.org/10.1186/s13063-016-1632-6.
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2016 17 2017.
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: Personal Social Services Research Unit, University of Kent; 2017.
- Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2018;27:7-22. https://doi.org/10.1002/hec.3564.
- O’Cathain A, Murphy E, Nicholl J. Three techniques for integrating data in mixed methods studies. BMJ 2010;341. https://doi.org/10.1136/bmj.c4587.
- Moffatt S, White M, Mackintosh J, Howel D. Using quantitative and qualitative data in health services research – what happens when mixed method findings conflict?. BMC Health Serv Res 2006;6. https://doi.org/10.1186/1472-6963-6-28.
- Bick D, Taylor C, Bhavnani V, Healey A, Seed P, Roberts S, et al. Lifestyle information and commercial weight management groups to support maternal postnatal weight management and positive lifestyle behaviour: the SWAN feasibility randomised controlled trial. BJOG 2020;127:636-45. https://doi.org/10.1111/1471-0528.16043.
- Altman DG, Doré CJ. Randomisation and baseline comparisons in clinical trials. Lancet 1990;335:149-53. https://doi.org/10.1016/0140-6736(90)90014-V.
- Smith T, Noble M, Wright G, Mclennan D, Plunkett E. The English Indices of Multiple Deprivation. London: Department for Communities and Local Government; 2015.
- Gray R, Bick D, Chang YS. Health in pregnancy and post-birth: contribution to improved child outcomes. J Children’s Services 2014;9:109-27. https://doi.org/10.1108/JCS-03-2014-0020.
- British Medical Association (BMA) . Smoking and Reproductive Life: The Impact of Smoking on Sexual, Reproductive and Child Health 2004.
- Painter RC, Roseboom TJ, Bleker OP. Prenatal exposure to the Dutch famine and disease in later life: an overview. Reprod Toxicol 2005;20:345-52. https://doi.org/10.1016/j.reprotox.2005.04.005.
- Riley EP, Infante MA, Warren KR. Fetal alcohol spectrum disorders: an overview. Neuropsychol Rev 2011;21:73-80. https://doi.org/10.1007/s11065-011-9166-x.
- Henderson J, Gray R, Brocklehurst P. Systematic review of effects of low-moderate prenatal alcohol exposure on pregnancy outcome. BJOG 2007;114:243-52. https://doi.org/10.1111/j.1471-0528.2006.01163.x.
- Schempf AH, Strobino DM. Illicit drug use and adverse birth outcomes: is it drugs or context?. J Urban Health 2008;85:858-73. https://doi.org/10.1007/s11524-008-9315-6.
- Minnes S, Lang A, Singer L. Prenatal tobacco, marijuana, stimulant, and opiate exposure: outcomes and practice implications. Addict Sci Clin Pract 2011;6:57-70.
- Roe L, Strong C, Whiteside C, Neil A, Mant D. Dietary intervention in primary care: validity of the DINE method for diet assessment. Fam Pract 1994;11:375-81. https://doi.org/10.1093/fampra/11.4.375.
- International Physical Activity Questionnaire . Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ) n.d. www.ipaq.ki.se/scoring.pdf (accessed 5 December 2019).
- Stein A, Pearson RM, Goodman SH, Rapa E, Rahman A, McCallum M, et al. Effects of perinatal mental disorders on the fetus and child. Lancet 2014;384:1800-19. https://doi.org/10.1016/S0140-6736(14)61277-0.
- Steinig J, Nagl M, Linde K, Zietlow G, Kersting A. Antenatal and postnatal depression in women with obesity: a systematic review. Arch Womens Ment Health 2017;20:569-85. https://doi.org/10.1007/s00737-017-0739-4.
- Molyneaux E, Poston L, Ashurst-Williams S, Howard L. Pre-pregnancy obesity and mental disorders during pregnancy and post-partum: a systematic review and meta-analysis. Pregnancy Hypertens 2014;4. https://doi.org/10.1016/j.preghy.2014.03.022.
- Victora CG, Bahl R, Barros AJ, França GV, Horton S, Krasevec J, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 2016;387:475-90. https://doi.org/10.1016/S0140-6736(15)01024-7.
- Turcksin R, Bel S, Galjaard S, Devlieger R. Maternal obesity and breastfeeding intention, initiation, intensity and duration: a systematic review. Matern Child Nutr 2014;10:166-83. https://doi.org/10.1111/j.1740-8709.2012.00439.x.
- Mäkelä J, Vaarno J, Kaljonen A, Niinikoski H, Lagström H. Maternal overweight impacts infant feeding patterns – the STEPS Study. Eur J Clin Nutr 2014;68:43-9. https://doi.org/10.1038/ejcn.2013.229.
- Baker JL, Gamborg M, Heitmann BL, Lissner L, Sørensen TI, Rasmussen KM. Breastfeeding reduces postpartum weight retention. Am J Clin Nutr 2008;88:1543-51. https://doi.org/10.3945/ajcn.2008.26379.
- World Health Organization (WHO) . The Optimal Duration of Exclusive Breastfeeding: Report of an Expert Consultation 2001.
- NHS . Drinks and Cups for Babies and Young Children: Your Pregnancy and Baby Guide n.d. www.nhs.uk/conditions/pregnancy-and-baby/drinks-and-cups-children/ (accessed 18 November 2018).
- Public Health England (PHE) . Child Health Profile, March 2016: Southwark 2016.
- Bick D, MacArthur C, Knowles H, Winter H, Bick D, MacArthur C, et al. Postnatal Care: Evidence And Guidelines for Management. Edinburgh: Churchill Livingstone; 2008.
- Dørheim SK, Bjorvatn B, Eberhard-Gran M. Can insomnia in pregnancy predict postpartum depression? A longitudinal, population-based study. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0094674.
- Coleman T, Chamberlain C, Davey MA, Cooper SE, Leonardi-Bee J. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev 2015;12. https://doi.org/10.1002/14651858.CD010078.pub2.
- Chamberlain C, O’Mara-Eves A, Porter J, Coleman T, Perlen SM, Thomas J, et al. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database Syst Rev 2017;2. https://doi.org/10.1002/14651858.CD001055.pub5.
- Mullen PD, Quinn VP, Ershoff DH. Maintenance of non-smoking postpartum by women who stopped smoking during pregnancy. Am J Public Health 1990;80:992-4. https://doi.org/10.2105/AJPH.80.8.992.
- Mullen PD, Carbonari JP, Tabak ER, Glenday MC. Improving disclosure of smoking by pregnant women. Am J Obstet Gynecol 1991;165:409-13. https://doi.org/10.1016/0002-9378(91)90105-Z.
- NHS Lambeth Clinical Commissioning Group . Annual Report 2016 2017 2017. www.lambethccg.nhs.uk/news-and-publications/publications/Annual%20reports/NHS%20Lambeth%20CCG%20Annual%20Report%202016-17.pdf (accessed 6 January 2020).
- Hauff LE, Demerath EW. Body image concerns and reduced breastfeeding duration in primiparous overweight and obese women. Am J Hum Biol 2012;24:339-49. https://doi.org/10.1002/ajhb.22238.
- Swanson V, Keely A, Denison FC. Does body image influence the relationship between body weight and breastfeeding maintenance in new mothers?. Br J Health Psychol 2017;22:557-76. https://doi.org/10.1111/bjhp.12246.
- Han SY, Brewis AA, Wutich A. Body image mediates the depressive effects of weight gain in new mothers, particularly for women already obese: evidence from the Norwegian Mother and Child Cohort Study. BMC Public Health 2016;16. https://doi.org/10.1186/s12889-016-3363-8.
- Gatineau M, Dent M. Obesity and Mental Health. Oxford: National Obesity Observatory; 2011.
- Taylor C, Bhavnani V, Zasada M, Ussher M, Bick D. Barriers and facilitators to uptake and retention of inner-city ethnically diverse women in a postnatal weight management intervention: a mixed-methods process evaluation within a feasibility trial in England. BMJ Open 2020;10. https://doi.org/10.1136/bmjopen-2019-034747.
- The Body Coach . Homepage n.d. www.thebodycoach.com (accessed 3 January 2019).
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Glick H, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2015.
- Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19140.
- Fenwick E, Byford S. A guide to cost-effectiveness acceptability curves. Br J Psychiatry 2005;187:106-8. https://doi.org/10.1192/bjp.187.2.106.
- Vallejo-Torres L, Garcia-Lorenzo B, Castilla I, Valcarcel-Nazco C, Garcia-Perez L, Linertova R, et al. On the estimation of the cost-effectiveness threshold: why, what, how?. Value Health 2016;19:558-66. https://doi.org/10.1016/j.jval.2016.02.020.
- National Institute for Health and Care Excellence . Weight Management: Lifestyle Services for Overweight or Obese Adults 2014.
- National Institute for Health and Care Excellence . Preventing Excess Weight Gain 2015.
- Andersen CS, Gamborg M, Sørensen TI, Nohr EA. Weight gain in different periods of pregnancy and offspring’s body mass index at 7 years of age. Int J Pediatr Obes 2011;6:e179-86. https://doi.org/10.3109/17477166.2010.521560.
- Baker JL. Long term health consequences of childhood obesity. Obes Facts 2016;1.
- Bala K, Perry IJ, Millar S, Dee A, Webber L. Future lifetime costs of childhood obesity and overweight. Obes Facts 2018;11:16-7.
- Begum F, Colman I, McCargar L, Bell RC. Gestational weight gain is associated with postpartum weight retention and infant anthropometrics. FASEB J 2014;26.
- Chen C, Xu X, Yan Y. Estimated global overweight and obesity burden in pregnant women based on panel data model. PLOS ONE 2018;13. https://doi.org/10.1371/journal.pone.0202183.
- Cho GJ, Yoon HJ, Kim EJ, Oh MJ, Seo HS, Kim HJ. Postpartum changes in body composition. Obesity 2011;19:2425-8. https://doi.org/10.1038/oby.2011.163.
- Danielzik S, Czerwinski-Mast M, Langnäse K, Dilba B, Müller MJ. Parental overweight, socioeconomic status and high birth weight are the major determinants of overweight and obesity in 5–7 y-old children: baseline data of the Kiel Obesity Prevention Study (KOPS). Int J Obes Relat Metab Disord 2004;28:1494-502. https://doi.org/10.1038/sj.ijo.0802756.
- Dodd JM, Louise J, Deussen AR, McPhee AJ, Owens JA, Robinson JS. Prenatal Diet and Child Growth at 18 Months. Pediatrics 2018;142. https://doi.org/10.1542/peds.2018-0035.
- Endres L, Straub H, Plunkett B, Clark-Kauffman E, Wagenaar K, Zhou Y, et al. Postpartum weight retention: risk factors and relationship to obesity at one year. Am J Obstet Gynecol 2012;208. https://doi.org/10.1016/j.ajog.2012.10.339.
- Eriksson JG, Sandboge S, Salonen MK, Kajantie E, Osmond C. Long-term consequences of maternal overweight in pregnancy on offspring later health: findings from the Helsinki Birth Cohort Study. Ann Med 2014;46:434-8. https://doi.org/10.3109/07853890.2014.919728.
- Fraser A, Tilling K, Macdonald-Wallis C, Hughes R, Sattar N, Nelson SM, et al. Associations of gestational weight gain with maternal body mass index, waist circumference, and blood pressure measured 16 y after pregnancy: the Avon Longitudinal Study of Parents and Children (ALSPAC). Am J Clin Nutr 2011;93:1285-92. https://doi.org/10.3945/ajcn.110.008326.
- Fraser A, Tilling K, Macdonald-Wallis C, Sattar N, Brion MJ, Benfield L, et al. Association of maternal weight gain in pregnancy with offspring obesity and metabolic and vascular traits in childhood. Circulation 2010;121:2557-64. https://doi.org/10.1161/CIRCULATIONAHA.109.906081.
- Gaillard R. Maternal obesity during pregnancy and cardiovascular development and disease in the offspring. Eur J Epidemiol 2015;30:1141-52. https://doi.org/10.1007/s10654-015-0085-7.
- Goosby BJ, Cheadle JE, McDade T. Birth weight, early life course BMI, and body size change: Chains of risk to adult inflammation?. Soc Sci Med 2016;148:102-9. https://doi.org/10.1016/j.socscimed.2015.11.040.
- Hayes A, Lung T, Tan A. Novel methods for simulating BMI and obesity trajectories during childhood: a life course model from Australia. Obes Facts 2018;11.
- He X, Zhu M, Hu C, Tao X, Li Y, Wang Q, et al. Breast-feeding and postpartum weight retention: a systematic review and meta-analysis. Public Health Nutr 2015;18:3308-16. https://doi.org/10.1017/S1368980015000828.
- Henriques A, Santos AC, Guimarães JT, Barros H, Azevedo A. Healthy excessive weight in Portuguese women 4 years after delivery of a liveborn. Prev Med 2015;75:49-55. https://doi.org/10.1016/j.ypmed.2015.03.009.
- Hinkle SN, Sharma AJ, Swan DW, Schieve LJ, Stein A, Ramakrishnan U. Gestational weight gain and child weight status at 5 years of age: differential effects by prepregnancy body mass index status. FASEB Journal 2012;26.
- Kac G, Benicio MH, Velásquez-Meléndez G, Valente JG. Nine months postpartum weight retention predictors for Brazilian women. Public Health Nutr 2004;7:621-8. https://doi.org/10.1079/PHN2003579.
- Keaver L, Webber L. Future trends in morbid obesity in England, Scotland, and Wales: a modelling projection study. Lancet 2016;388. https://doi.org/10.1016/S0140-6736(16)32299-1.
- Kirkegaard H, Stovring H, Sorensen TI, Nohr EA. Life course path analysis of pregnancy-related weight changes and long-term maternal obesity: a Danish cohort study. Obes Facts 2012;1.
- Lenoir-Wijnkoop I, Nuijten M, Uauy R. Health economic model for assessing the impact of high birth weight on public health. Ann Nutr Metab 2013;1.
- Lovelady CA, Garner KE, Moreno KL, Williams JP. The effect of weight loss in overweight, lactating women on the growth of their infants. N Engl J Med 2000;342:449-53. https://doi.org/10.1056/NEJM200002173420701.
- Mann JR, McDermott SW, Hardin J, Pan C, Zhang Z. Pre-pregnancy body mass index, weight change during pregnancy, and risk of intellectual disability in children. BJOG 2013;120:309-19. https://doi.org/10.1111/1471-0528.12052.
- Ng SK, Cameron CM, Hills AP, McClure RJ, Scuffham PA. Socioeconomic disparities in prepregnancy BMI and impact on maternal and neonatal outcomes and postpartum weight retention: the EFHL longitudinal birth cohort study. BMC Pregnancy Childbirth 2014;14. https://doi.org/10.1186/1471-2393-14-314.
- Ohlin A, Rössner S. Factors related to body weight changes during and after pregnancy: the Stockholm Pregnancy and Weight Development Study. Obes Res 1996;4:271-6. https://doi.org/10.1002/j.1550-8528.1996.tb00545.x.
- Parsons TJ, Power C, Logan S, Summerbell CD. Childhood predictors of adult obesity: a systematic review. Int J Obes Relat Metab Disord 1999;23:1-107.
- Schrauwers C, Dekker G. Maternal and perinatal outcome in obese pregnant patients. J Matern Fetal Neonatal Med 2009;22:218-26. https://doi.org/10.1080/14767050902801652.
- Sonntag D, Ali S, De Bock F. Estimating the lifetime indirect cost of childhood overweight and obesity: A Markov modelling study. Value Health 2015;18. https://doi.org/10.1016/j.jval.2015.09.2812.
- Tabacchi G, Giammanco S, La Guardia M, Giammanco M. A review of the literature and a new classification of the early determinants of childhood obesity: from pregnancy to the first years of life. Nutr Res 2007;27:587-604. https://doi.org/10.1016/j.nutres.2007.06.001.
- Vinter C, Jensen D, Ovesen P, Beck-Nielsen H, Lamont R, Jorgensen J. Postpartum weight retention and breastfeeding among obese women from the LiP (Lifestyle in Pregnancy) study. Acta Obstet Gynecol Scand 2012;159:141-2.
- Widen EM, Whyatt RM, Hoepner LA, Mueller NT, Ramirez-Carvey J, Oberfield SE, et al. Gestational weight gain and obesity, adiposity and body size in African-American and Dominican children in the Bronx and Northern Manhattan. Matern Child Nutr 2016;12:918-28. https://doi.org/10.1111/mcn.12174.
- Williamson DF, Madans J, Pamuk E, Flegal KM, Kendrick JS, Serdula MK. A prospective study of childbearing and 10-year weight gain in US white women 25 to 45 years of age. Int J Obes Relat Metab Disord 1994;18:561-9.
- Mostello D, Jen Chang J, Allen J, Luehr L, Shyken J, Leet T. Recurrent preeclampsia. Obstet Gynecol 2010;116:667-72. https://doi.org/10.1097/AOG.0b013e3181ed74ea.
- Lenoir-Wijnkoop I, van der Beek EM, Garssen J, Nuijten MJC, Uauy RD. Health economic modeling to assess short-term costs of maternal overweight, gestational diabetes, and related macrosomia: a pilot evaluation. Front Pharmacol 2015;6. https://doi.org/10.3389/fphar.2015.00103.
- Galvao TF, Silva MT, Silva AW, Bronstein AC, Pereira MG. Quality guidelines for poison centers: a systematic review. Clin Toxicol 2013;51.
- Tabet M, Flick LH, Tuuli MG, Macones GA, Chang JJ. Prepregnancy body mass index in a first uncomplicated pregnancy and outcomes of a second pregnancy. Am J Obstet Gynecol 2015;213:548.e1-7. https://doi.org/10.1016/j.ajog.2015.06.031.
- Vesco KK, Dietz PM, Rizzo J, Stevens VJ, Perrin NA, Bachman DJ, et al. Excessive gestational weight gain and postpartum weight retention among obese women. Obstet Gynecol 2009;114:1069-75. https://doi.org/10.1097/AOG.0b013e3181baeacf.
- National Institute for Health and Care Excellence . Cardiovascular Disease Prevention 2010.
- Lacey LA, Wolf A, O’shea D, Erny S, Ruof J. Cost-effectiveness of orlistat for the treatment of overweight and obese patients in Ireland. Int J Obes 2005;29:975-82. https://doi.org/10.1038/sj.ijo.0802947.
- Picot J, Jones J, Colquitt JL, Gospodarevskaya E, Loveman E, Baxter L, et al. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess 2009;13. https://doi.org/10.3310/hta13410.
- Hagberg LA, Brekke HK, Bertz F, Winkvist A. Cost-utility analysis of a randomized controlled weight loss trial among lactating overweight/obese women. BMC Public Health 2014;14. https://doi.org/10.1186/1471-2458-14-38.
- Rawdin AC, Duenas A, Chilcott JB. The cost-effectiveness of weight management programmes in a postnatal population. Public Health 2014;128:804-10. https://doi.org/10.1016/j.puhe.2014.07.005.
- Alsumali A, Eguale T, Bairdain S, Samnaliev M. Cost-effectiveness analysis of bariatric surgery for morbid obesity. Obes Surg 2018;28:2203-14. https://doi.org/10.1007/s11695-017-3100-0.
- Borisenko O, Lukyanov V, Johnsen SP, Funch-Jensen P. Cost analysis of bariatric surgery in Denmark made with a decision-analytic model. Dan Med J 2017;64.
- Cahill AG, Odibo A, Jungheim E, Pilliod R, Grobman W, Macones G, et al. Pre-pregnancy gastric bypass for the morbidly obese: a decision and economic analysis. Am J Obstet Gynecol 2012;1. https://doi.org/10.1016/j.ajog.2011.10.628.
- Chiang AL, Tapper EB, Thompson CC. Cost-utility analysis of obesity therapy: a comparison of lifestyle intervention to surgical, and endoscopic strategies. Gastroenterol 2016;1. https://doi.org/10.1016/S0016-5085(16)30407-3.
- Griffiths UK, Anigbogu B, Miners A, Nanchahal K. Economic evaluations of adult weight management interventions: a systematic literature review focusing on modelling approaches. Obes Rev 2011;1.
- Huang Y, Kypridemos C, Liu J, Lee Y, Collins B, Pearson-Stuttard J, et al. Quantifying the health and economic impact of the FDA added sugar labeling mandate in the us: a cost-effectiveness analysis. Circulation 2018;137.
- Iannazzo S, Zaniolo O, Pradelli L. Economic evaluation of treatment with orlistat in Italian obese patients. Curr Med Res Opin 2008;24:63-74. https://doi.org/10.1185/030079908X253591.
- Konchak C, Prasad K. Incorporating social network effects into cost-effectiveness analysis: a methodological contribution with application to obesity prevention. BMJ Open 2012;2. https://doi.org/10.1136/bmjopen-2012-001038.
- Su W, Huang J, Chen F, Iacobucci W, Mocarski M, Dall TM, et al. Modeling the clinical and economic implications of obesity using microsimulation. J Med Econ 2015;18:886-97. https://doi.org/10.3111/13696998.2015.1058805.
- National Institute for Health and Care Excellence . Cardiovascular Disease: Identifying and Supporting People Most at Risk of Dying Early 2008.
- National Institute for Health and Care Excellence . Cardiovascular Disease: Risk Assessment and Reduction, Including Lipid Modification 2014.
- National Institute for Health and Care Excellence . Type 2 Diabetes Prevention: Population and Community-Level Interventions 2011.
- Boyers D, Avenell A, Stewart F, Robertson C, Archibald D, Douglas F, et al. A systematic review of the cost-effectiveness of non-surgical obesity interventions in men. Obes Res Clin Pract 2015;9:310-27. https://doi.org/10.1016/j.orcp.2015.03.001.
- Lamotte M, Annemans L, Lefever A, Nechelput M, Masure J. A health economic model to assess the long-term effects and cost-effectiveness of orlistat in obese type 2 diabetic patients. Diabetes Care 2002;25:303-8. https://doi.org/10.2337/diacare.25.2.303.
- Oster G, Thompson D, Edelsberg J, Bird AP, Colditz GA. Lifetime health and economic benefits of weight loss among obese persons. Am J Public Health 1999;89:1536-42. https://doi.org/10.2105/AJPH.89.10.1536.
- Zomer E, Leach R, Trimmer C, Lobstein T, Morris S, James WP, et al. Effectiveness and cost-effectiveness of interventions that cause weight loss and reduce the risk of cardiovascular disease. Diabetes Obes Metab 2017;19:118-24. https://doi.org/10.1111/dom.12792.
- Althuizen E, van der Wijden CL, van Mechelen W, Seidell JC, van Poppel MN. The effect of a counselling intervention on weight changes during and after pregnancy: a randomised trial. BJOG 2013;120:92-9. https://doi.org/10.1111/1471-0528.12014.
- Amorim AR, Linne YM, Lourenco PM. Diet or exercise, or both, for weight reduction in women after childbirth. Cochrane Database Syst Rev 2007;3. https://doi.org/10.1002/14651858.CD005627.pub2.
- Chang MW, Brown R, Nitzke S. Results and lessons learned from a prevention of weight gain program for low-income overweight and obese young mothers: Mothers In Motion. BMC Public Health 2017;17. https://doi.org/10.1186/s12889-017-4109-y.
- Costa CS, Campagnolo PD, Lumey LH, Vitolo MR. Effect of maternal dietary counselling during the 1st year of life on glucose profile and insulin resistance at the age of 8 years: a randomised field trial. Br J Nutr 2017;117:134-41. https://doi.org/10.1017/S0007114516004578.
- Elliott SA, Pereira LCR, Guigard E, McCargar LJ, Prado CCM, Bell RC. Association between breastfeeding, maternal weight loss and body composition at 3 months postpartum. FASEB Journal 2016;30.
- Elliott-Sale KJ. The effects of exercise on postpartum weight retention in overweight and obese women. Curr Womens Health Rev 2017;13:11-6. https://doi.org/10.2174/1573404812666160815120213.
- Elliott-Sale KJ, Barnett CT, Sale C. Exercise interventions for weight management during pregnancy and up to 1 year postpartum among normal weight, overweight and obese women: a systematic review and meta-analysis. Br J Sports Med 2015;49:1336-42. https://doi.org/10.1136/bjsports-2014-093875.
- Herring SJ, Cruice JF, Bennett GG, Darden N, Wallen JJ, Rose MZ, et al. Intervening during and after pregnancy to prevent weight retention among African American women. Prev Med Rep 2017;7:119-23. https://doi.org/10.1016/j.pmedr.2017.05.015.
- Huseinovic E, Winkvist A, Bertz F, Bertéus Forslund H, Brekke HK. Eating frequency, energy intake and body weight during a successful weight loss trial in overweight and obese postpartum women. Eur J Clin Nutr 2014;68:71-6. https://doi.org/10.1038/ejcn.2013.200.
- Jarlenski MP, Bennett WL, Bleich SN, Barry CL, Stuart EA. Effects of breastfeeding on postpartum weight loss among U.S. women. Prev Med 2014;69:146-50. https://doi.org/10.1016/j.ypmed.2014.09.018.
- Joshi P, Quintiliani L, B, AC, Cuellar A, Mahesri M, . Behavioral weight management intervention in underserved overweight postpartum women, a randomized controlled feasibility trial: the renew study. J Gen Intern Med 2016;1:S137-S8.
- Keller C, Records K, Ainsworth B, Permana P, Coonrod DV. Interventions for weight management in postpartum women. J Obstet Gynecol Neonatal Nurs 2008;37:71-9. https://doi.org/10.1111/j.1552-6909.2007.00202.x.
- Linares J, Corvalan C, Kain J, Garmendia ML, Gonzalez L, Mericq V. Effect of pre-pregnancy BMI and gestational weight gain on the timing of adiposity rebound. Horm Res Paediatr 2013;1.
- Louzada ML, Campagnolo PD, Rauber F, Vitolo MR. Long-term effectiveness of maternal dietary counseling in a low-income population: a randomized field trial. Pediatrics 2012;129:e1477-84. https://doi.org/10.1542/peds.2011-3063.
- Lovelady C. Balancing exercise and food intake with lactation to promote post-partum weight loss. Proc Nutr Soc 2011;70:181-4. https://doi.org/10.1017/S002966511100005X.
- Moran LJ, Flynn AC, Louise J, Deussen AR, Dodd JM. The effect of a lifestyle intervention on pregnancy and postpartum dietary patterns determined by factor analysis. Obesity 2017;25:1022-32. https://doi.org/10.1002/oby.21848.
- Mustila T, Raitanen J, Keskinen P, Saari A, Luoto R. Lifestyle counseling during pregnancy and offspring weight development until four years of age: follow-up study of a controlled trial. J Negat Results Biomed 2012;11. https://doi.org/10.1186/1477-5751-11-11.
- Neville CE, McKinley MC, Holmes VA, Spence D, Woodside JV. The effectiveness of weight management interventions in breastfeeding women – a systematic review and critical evaluation. Birth 2014;41:223-36. https://doi.org/10.1111/birt.12111.
- Neville CE, McKinley MC, Holmes VA, Spence D, Woodside JV. The relationship between breastfeeding and postpartum weight change – a systematic review and critical evaluation. Int J Obes 2014;38:577-90. https://doi.org/10.1038/ijo.2013.132.
- Østbye T, Krause KM, Swamy GK, Lovelady CA. Effect of breastfeeding on weight retention from one pregnancy to the next: results from the North Carolina WIC program. Prev Med 2010;51:368-72. https://doi.org/10.1016/j.ypmed.2010.07.017.
- Patel NR, Pasupathy D, Flynn AC, Hayes L, Levin JG, Singh C, et al. The upbeat behavioural intervention in obese pregnant women-maternal and infant follow-up 6 months postpartum. Reproductive Sciences 2016;1.
- Phelan S, Phipps MG, Abrams B, Darroch F, Grantham K, Schaffner A, et al. Does behavioral intervention in pregnancy reduce postpartum weight retention? Twelve-month outcomes of the Fit for Delivery randomized trial. Am J Clin Nutr 2014;99:302-11. https://doi.org/10.3945/ajcn.113.070151.
- Ramos D, Lopez L, Resser L, Rodriguez N, Donatoni G. Choose health la MOMs: a novel approach to postpartum weight loss. Obstet Gynecol 2016;127:77S-8S. https://doi.org/10.1097/01.AOG.0000483721.14523.2f.
- Ruchat SM, Sopper MM, Giroux I, Prapavessis H, Mottola MF. Postpartum lifestyle intervention program for overweight and obese women to prevent gestational weight retention. Obesity 2011;1.
- Sen S, Carpenter AH, Hochstadt J, Huddleston JY, Kustanovich V, Reynolds AA, et al. Nutrition, weight gain and eating behavior in pregnancy: a review of experimental evidence for long-term effects on the risk of obesity in offspring. Physiol Behav 2012;107:138-45. https://doi.org/10.1016/j.physbeh.2012.04.014.
- Skreden M, Hillesund ER, Wills AK, Brantsæter AL, Bere E, Øverby NC. Adherence to the New Nordic Diet during pregnancy and subsequent maternal weight development: a study conducted in the Norwegian Mother and Child Cohort Study (MoBa). Br J Nutr 2018;119:1286-94. https://doi.org/10.1017/S0007114518000776.
- Spencer L, Rollo M, Hutchesson M, Collins C. Perceived healthy eating and physical activity factors influencing weight management in postpartum WOMEN: a mixed methods analysis. Obes Res Clin Pract 2014;1. https://doi.org/10.1016/j.orcp.2014.10.176.
- Van Der Pligt P, Willcox J, Hesketh K, Ball K, Wilkinson S, Crawford D, et al. Systematic review of lifestyle interventions to limit postpartum weight retention in women following childbirth. J Paediatr Child Health 2013;2.
- von Ruesten A, Brantsæter AL, Haugen M, Meltzer HM, Mehlig K, Winkvist A, et al. Adherence of pregnant women to Nordic dietary guidelines in relation to postpartum weight retention: results from the Norwegian Mother and Child Cohort Study. BMC Public Health 2014;14. https://doi.org/10.1186/1471-2458-14-75.
- Wall CR, Murphy R, Waldie KE, Mitchell EA, Thompson JMD. The effects of maternal and early childhood dietary patterns on BMI, percentage body fat and sleep at 7 years. Cogent Med 2016;3.
- Wilcox S, Liu J, Addy CL, Turner-McGrievy G, Burgis JT, Wingard E, et al. A randomized controlled trial to prevent excessive gestational weight gain and promote postpartum weight loss in overweight and obese women: Health In Pregnancy and Postpartum (HIPP). Contemp Clin Trials 2018;66:51-63. https://doi.org/10.1016/j.cct.2018.01.008.
- Wrieden WL, Williams H, Thompson J, McFarlane G, Barnett C, Hellman Z. Evaluation of NHS referral of postnatal women to a commercial, community-based lifestyle modification programme. Proc Nutr Soc 2013;72. https://doi.org/10.1017/S0029665113001213.
- Lau Y, Klainin-Yobas P, Htun TP, Wong SN, Tan KL, Ho-Lim ST, et al. Electronic-based lifestyle interventions in overweight or obese perinatal women: a systematic review and meta-analysis. Obes Rev 2017;18:1071-87. https://doi.org/10.1111/obr.12557.
- Wilkinson SA, van der Pligt P, Gibbons KS, McIntyre HD. Trial for Reducing Weight Retention in New Mums: a randomised controlled trial evaluating a low intensity, postpartum weight management programme. J Hum Nutr Diet 2015;28:15-28. https://doi.org/10.1111/jhn.12193.
- Mustila T, Raitanen J, Keskinen P, Saari A, Luoto R. Lifestyle counselling targeting infant’s mother during the child’s first year and offspring weight development until 4 years of age: a follow-up study of a cluster RCT. BMJ Open 2012;2. https://doi.org/10.1136/bmjopen-2011-000624.
- O’Toole ML, Sawicki MA, Artal R. Structured diet and physical activity prevent postpartum weight retention. J Womens Health 2003;12:991-8. https://doi.org/10.1089/154099903322643910.
- Bramwell RCJ. Can collaborative intervention by health professionals and slimming world help reduce the complications of obesity in pregnancy?. BJOG 2013;120:411-13.
- Brekke HK, Bertz F, Rasmussen KM, Bosaeus I, Ellegård L, Winkvist A. Diet and exercise interventions among overweight and obese lactating women: randomized trial of effects on cardiovascular risk factors. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0088250.
- Huseinovic E, Bertz F, Brekke HK, Winkvist A. Two-year follow-up of a postpartum weight loss intervention: results from a randomized controlled trial. Matern Child Nutr 2018;14. https://doi.org/10.1111/mcn.12539.
- Asseburg C, Johansen P, Nilsson A, Willis M. Impact of the Framingham Offspring Study (FOS) vs Kaiser Permanente NorthWest (KPNW) prediction equations for diabetes mellitus in economic modelling of type 2 diabetes mellitus. Diabetologia 2015;1.
- Cabra HA, Zanela OO, Anaya P, Rodriguez S. Economic evaluation of bariatric surgery as treatment for obesity and associated comorbidities-estimation by discrete event simulation. Value Health 2009;12. https://doi.org/10.1016/S1098-3015(10)75321-3.
- Cao B. Estimating the effects of obesity and weight change on mortality using a dynamic causal model. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0129946.
- Cauchi D, Gauci D, Calleja N, Marsh T, Webber L. Application of the UK foresight obesity model in Malta: Health and economic consequences of projected obesity trends. Obes Facts 2015;1.
- Che M, Kong L, Bell RC, Yuan Y. Trajectory modeling of gestational weight: a functional principal component analysis approach. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0186761.
- Chen F, Iacobucci W, Su W, Dall T. Projecting benefits from weight loss in obese populations: A microsimulation approach. Value Health 2015;18. https://doi.org/10.1016/j.jval.2015.03.1732.
- Drichoutis AC, Lazaridis P, Nayga RM, Kapsokefalou M, Chryssochoidis G. A theoretical and empirical investigation of nutritional label use. Eur J Health Econ 2008;9:293-304. https://doi.org/10.1007/s10198-007-0077-y.
- Faria GR, Preto JR, Costa-Maia J. Gastric bypass is a cost-saving procedure: results from a comprehensive Markov model. Obes Surg 2013;23:460-6. https://doi.org/10.1007/s11695-012-0816-8.
- Galani C, Schneider H, Rutten FF. Modelling the lifetime costs and health effects of lifestyle intervention in the prevention and treatment of obesity in Switzerland. Int J Public Health 2007;52:372-82. https://doi.org/10.1007/s00038-007-7014-9.
- Gaudette É, Goldman DP, Messali A, Sood N. Do statins reduce the health and health care costs of obesity?. PharmacoEconomics 2015;33:723-34. https://doi.org/10.1007/s40273-014-0234-y.
- Hoogenveen RT, Boshuizen HC, Engelfriet PM, van Baal PHM. You only die once: accounting for multi-attributable mortality risks in multi-disease models for health-economic analyses. Med Decis Making 2017;37:403-14. https://doi.org/10.1177/0272989X16658661.
- Keaver L, Webber L, Dee A, Shiely F, Marsh T, Balanda K, et al. Application of the UK foresight obesity model in Ireland: the health and economic consequences of projected obesity trends in Ireland. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0079827.
- McLawhorn AS, Southren D, Wang YC, Marx RG, Dodwell ER. Cost-effectiveness of bariatric surgery prior to total knee arthroplasty in the morbidly obese: a computer model-based evaluation. J Bone Joint Surg Am 2016;98. https://doi.org/10.2106/JBJS.N.00416.
- Pasricha A, Blackhouse G, Goeree R, Tarride JE, O’Reilly D. Cost-effectiveness of bariatric surgical procedures versus no treatment for morbid obesity. Value Health 2010;13. https://doi.org/10.1016/S1098-3015(10)73032-1.
- Retat L, Pimpin L, Webber L, Jebb S, Aveyard P. Modelling the epidemiological impact of a brief physician intervention for obesity using a microsimulation model. Obes Facts 2017;10.
- Schwer B, Nuijten M, Hiligsmann M, Evers S. Quantity and quality of external event validation procedures performed in published health economic models in obesity: outcomes of a systematic review. Value Health 2017;20. https://doi.org/10.1016/j.jval.2017.08.2116.
- Schwer B, Nuijten M, Hiligsmann M, Evers SM. Evaluation of methodological variations in the event simulation approaches of published health economic models in obesity prevention and therapy. Value Health 2016;19. https://doi.org/10.1016/j.jval.2016.09.168.
- Schwander B, Nuijten M, Hiligsmann M, Evers SMAA. Event simulation and external validation applied in published health economic models for obesity: a systematic review. Expert Rev Pharmacoecon Outcomes Res 2018;18:529-41. https://doi.org/10.1080/14737167.2018.1501680.
- Tucker DM, Palmer AJ, Valentine WJ, Roze S, Ray JA. Counting the costs of overweight and obesity: modeling clinical and cost outcomes. Curr Med Res Opin 2006;22:575-86. https://doi.org/10.1185/030079906X96227.
- Vreman RA, Goodell AJ, Rodriguez LA, Porco TC, Lustig RH, Kahn JG. Health and economic benefits of reducing sugar intake in the USA, including effects via non-alcoholic fatty liver disease: a microsimulation model. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-013543.
- Zanela OO, Cabra HA, Meléndez G, Anaya P, Rupprecht F. economic evaluation of bariatric surgery in Mexico using discrete event simulation. Value Health Reg Issues 2012;1:172-9. https://doi.org/10.1016/j.vhri.2012.09.012.
- Griffiths UK, Anigbogu B, Nanchahal K. Economic Evaluations of adult weight management interventions. Appl Health Econ Health Policy 2012;10:145-62. https://doi.org/10.2165/11599250-000000000-00000.
- Chen G, Kusel J, Buguth B, Higgins A, Belarbi S. Cost-effectiveness of bariatric surgery compared to conventional treatment from a societal perspective in Germany, France, Italy and the UK. Value Health 2017;20. https://doi.org/10.1016/j.jval.2017.08.900.
- Schwander B, Hiligsmann M, Nuijten M, Evers S. Systematic review and overview of health economic evaluation models in obesity prevention and therapy. Expert Rev Pharmacoecon Outcomes Res 2016;16:561-70. https://doi.org/10.1080/14737167.2016.1230497.
- Su W, Chen F, Dall TM, Zvenyach T, Kyle TK, Perreault L. Where can obesity management policy make the largest impact? Evaluating sub-populations through a microsimulation approach. J Med Econ 2018;21:936-43. https://doi.org/10.1080/13696998.2018.1496922.
- Holmes VA, Draffin CR, Patterson CC, Francis L, Irwin J, McConnell M, et al. Postnatal lifestyle intervention for overweight women with previous gestational diabetes: a randomized controlled Trial. J Clin Endocrinol Metab 2018;103:2478-87. https://doi.org/10.1210/jc.2017-02654.
- Lyons S, Currie S, Peters S, Lavender T, Smith DM. The association between psychological factors and breastfeeding behaviour in women with a body mass index (BMI) ≥ 30 kg/m2: a systematic review. Obesity Rev 2018;19:947-59. https://doi.org/10.1111/obr.12681.
- Public Health England . Child Health Profile: March 2016 – Lambeth n.d. https://moderngov.lambeth.gov.uk/documents/s84156/LSCB%20ChildHealthProfile2016-Lambeth.pdf (accessed 6 January 2020).
Appendix 1 Example of framework analyses using COM-B: intervention
| Capability: the individual’s physical (physical processes) and psychological (thought processes) capacity to engage in the behaviour | ||||
|---|---|---|---|---|
| Participant |
Physical capability: how have they implemented the plan and suggested lifestyle changes? [Having skills and knowledge to adapt to lifestyle changes/diet/change the way they eat: break habits (self-regulate)] |
Psychological comprehension: use and ease of use of online/other resources (e.g. Slimming World website/app/Facebook group/magazine/other Slimming World resources) | Psychological capacity to plan: are they able to plan/judge (e.g. complete food diaries, choose the right foods in supermarkets, plan meals)? | Psychological capability/comprehension: do they have an understanding of why they are overweight and why the diet would work/why they need to change eating behaviour? Did the plan fit their beliefs about weight loss and breastfeeding? |
| Anemone, attended 1 session, aged 32 years, white European, primigravid, gained weight |
|
|||
| Daisy, attended 3 sessions, aged 30 years, white European, parity missing, gained weight |
|
|
||
| Daffodil, attended 7 sessions, aged 29 years, black British, primigravid, achieved target |
|
|
|
|
| Lavender, attended 9 sessions, aged 38 years, white British, primigravid, gained weight |
|
|
|
|
| Heather, attended 12 sessions, aged 37 years, white British, primigravid, lost weight |
|
|
||
| Violet, attended ≥ 12 sessions, aged 28 years old, black British, multigravid, lost weight, continued with Slimming World beyond intervention period |
|
|
|
|
Appendix 2 Data analysis plan
Appendix 3 Feasibility trial tables
| Women’s characteristics | Trial arm | All women | Difference (95% CI) | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | |||||||
| n | % | n | % | n | % | |||
| Recruitment/retention | ||||||||
| Recruited | 98 | 95 | 193 | |||||
| Completed (followed up to 12 months) | 67 | 68.4 | 67 | 70.5 | 134 | 69.4 | –2.2 (–15.2 to 10.8) | |
| Age (years) | ||||||||
| 20–29 | 17 | 25.4 | 10 | 14.9 | 27 | 20.1 | 15.4 (–13.2 to 43.9) | |
| ≥ 30 | 50 | 74.6 | 56 | 83.6 | 106 | 79.1 | –6.2 (–20.7 to 8.2) | |
| Total | 67 | 67 | 134 | 0.181 | ||||
| Missing | 0 | 0.0 | 1 | 1.5 | 1 | 0.7 | ||
| BMI score (kg/m2) at antenatal booking | ||||||||
| 18.0–24.9 | 2 | 3.0 | 2 | 3.0 | 4 | 3.0 | 0.0 (0.0 to 0.0) | |
| 25.0–29.9 | 42 | 62.7 | 38 | 56.7 | 80 | 59.7 | 7.0 (–9.7 to 23.8) | |
| ≥ 30.0 | 23 | 34.3 | 27 | 40.3 | 50 | 37.3 | –16.0 (–37.0 to 4.9) | |
| Total | 67 | 67 | 134 | 0.097 | ||||
| EGWG | ||||||||
| No | 25 | 37.3 | 40 | 59.7 | 65 | 48.5 | –8.9 (–28.0 to 10.2) | |
| Yes | 42 | 62.7 | 27 | 40.3 | 69 | 51.5 | 3.2 (–15.3 to 21.7) | |
| Total | 67 | 67 | 134 | 0.378 | ||||
| BMI score (kg/m2) at antenatal booking and EGWG | ||||||||
| < 25.0 | ||||||||
| No EGWG | 0 | 0.0 | 1 | 1.5 | 1 | 0.7 | ||
| EGWG | 2 | 3.0 | 1 | 1.5 | 3 | 2.2 | 0.0 (0.0 to 0.0) | |
| 25.0–29.9 | ||||||||
| No EGWG | 15 | 22.4 | 20 | 29.9 | 35 | 26.1 | 10.5 (–14.9 to 35.9) | |
| EGWG | 27 | 40.3 | 18 | 26.9 | 45 | 33.6 | 3.7 (–19.1 to 26.5) | |
| ≥ 30 | ||||||||
| No EGWG | 10 | 14.9 | 19 | 28.4 | 29 | 21.6 | –29.2 (–56.4 to –1.9) | |
| EGWG | 13 | 19.4 | 8 | 11.9 | 21 | 15.7 | 1.8 (–32.1 to 35.6) | |
| Total | 67 | 67 | 134 | 0.189 | ||||
| IMD quintiles | ||||||||
| 1 (least deprived) | 1 | 1.5 | 2 | 3.0 | 3 | 2.2 | –50.0 (–119.3 to 19.3) | |
| 2 | 2 | 3.0 | 3 | 4.5 | 5 | 3.7 | 0.0 (0.0 to 0.0) | |
| 3 | 9 | 13.4 | 11 | 16.4 | 20 | 14.9 | 8.5 (–23.5 to 40.4) | |
| 4 | 33 | 49.3 | 31 | 46.3 | 64 | 47.8 | –8.3 (–26.8 to 10.3) | |
| 5 (most deprived) | 22 | 32.8 | 20 | 29.9 | 42 | 31.3 | 2.2 (–21.0 to 25.4) | |
| Total | 67 | 67 | 134 | 0.622 | ||||
| Missing | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0.0 (0.0 to 0.0) | |
| Birthweight | ||||||||
| Appropriate for gestational age | 54 | 80.6 | 58 | 86.6 | 112 | 0.3 (–13.9 to 14.5) | ||
| Small for gestational age (< 10th birthweight centile) | 10 | 14.9 | 5 | 7.5 | 15 | 0.0 (–41.0 to 41.0) | ||
| Total | 67 | 67 | 134 | 0.989 | ||||
| Missing | 3 | 4.5 | 4 | 6.0 | 7 | –29.2 (–79.6 to 21.3) | ||
| Prematurity | ||||||||
| Term | 65 | 97.0 | 67 | 100.0 | 132 | 98.5 | –3.7 (–16.7 to 9.4) | |
| Preterm (< 37 weeks) | 2 | 3.0 | 0 | 0.0 | 2 | 1.5 | 66.7 (13.3 to 120.0) | |
| Total | 67 | 67 | 134 | |||||
| Missing | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0.0 (0.0 to 0.0) | |
| Baby sex | ||||||||
| Female | 38 | 56.7 | 32 | 47.8 | 70 | 52.2 | –13.3 (–30.8 to 4.1) | |
| Male | 29 | 43.3 | 34 | 50.7 | 63 | 47.0 | 8.3 (–10.6 to 27.3) | |
| Unclassified | 0 | 0.0 | 1 | 1.5 | 1 | 0.7 | –50.0 (–119.3 to 19.3) | |
| Total | 67 | 67 | 134 | 0.1 | ||||
| Soft drink (diet or sugar free)a intake | Trial arm, n (%) | Both arms (N = 192) , n (%) | |
|---|---|---|---|
| Intervention (N = 98) | Control (N = 94) | ||
| Baseline | |||
| None | 80 (81.6) | 76 (80.9) | 156 (81.3) |
| 1–2 weekly | 7 (7.1) | (10.6) | 17 (8.9) |
| 3–6 weekly | 3 (3.1) | 2 (2.1) | 5 (2.6) |
| 1 daily | 2 (2.0) | 1 (1.1) | 3 (1.6) |
| 2–3 daily | 5 (5.1) | 1 (1.1) | 6 (3.1) |
| 4–5 daily | 0 (0.0) | 2 (2.1) | 2 (1.0) |
| ≥ 6 daily | 1 (1.0) | 2 (2.1) | 3 (1.6) |
| 6 months | N = 80 | N = 75 | N = 155 |
| None | 60 (75.0) | 64 (85.3) | 124 (80.0) |
| 1–2 weekly | 4 (5.0) | 6 (8.0) | 10 (6.5) |
| 3–6 weekly | 6 (7.5) | 2 (2.7) | 8 (5.2) |
| 1 daily | 2 (2.5) | 1 (1.3) | 3 (1.9) |
| 2–3 daily | 6 (7.5) | 1 (1.3) | 7 (4.5) |
| 4–5 daily | 2 (2.5) | 1 (1.3) | 3 (1.9) |
| 12 months | N = 69 | N = 71 | N = 140 |
| None | 56 (8.1) | 56 (78.9) | 112 (80.0) |
| 1–2 weekly | 5 (7.2) | 7 (9.9) | 12 (8.6) |
| 3–6 weekly | 1 (1.4) | 3 (4.2) | 4 (2.9) |
| 1 daily | 1 (1.4) | 1 (1.4) | 2 (1.4) |
| 2–3 daily | 4 (5.8) | 3 (4.2) | 7 (5.0) |
| 4–5 daily | 2 (2.9) | 1 (1.4) | 3 (2.1) |
| Physical activity (minutes per day) | Trial arm | Both trial arms | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | ||||||||
| Baseline | 6 months | 12 months | Baseline | 6 months | 12 months | Baseline | 6 months | 12 months | |
| Vigorous physical activities | |||||||||
| n | 9 | 21 | 21 | 11 | 21 | 19 | |||
| Mean (SD) | 20.63 (22.12) | 16.81 (8.02) | 24.56 (33.58) | 7.01 (7.60) | 16.55 (16.40) | 19.51 (17.33) | 13.14 (16.88) | 16.68 (12.75) | 22.16 (26.90) |
| Moderate physical activities | |||||||||
| n | 42 | 45 | 43 | 45 | 29 | 36 | |||
| Mean (SD) | 27.35 (28.16) | 23.19 (26.89) | 37.89 (35.62) | 31.43 (36.78) | 42.02 (46.48) | 41.96 (49.32) | 29.46 (32.78) | 30.57 (36.75) | 39.75 (42.18) |
| Walking | |||||||||
| n | 96 | 82 | 69 | 89 | 72 | 71 | |||
| Mean (SD) | 59.26 (49.56) | 60.13 (48.66) | 59.42 (47.72) | 58.70 (50.21) | 70.08 (52.67) | 66.76 (52.25) | 58.99 (49.74) | 64.78 (50.65) | 63.14 (50.02) |
| Sitting | |||||||||
| n | 9 | 6 | 6 | 5 | 13 | 3 | |||
| Mean (SD) | 325.00 (187.65) | 255.00 (113.45) | 140.00 (45.17) | 312.00 (146.36) | 177.69 (87.00) | 110.00 (34.64) | 320.36 (168.23) | 202.11 (99.92) | 130.00 (42.43) |
| Vigorous/moderate physical activity | |||||||||
| n | 8 | 15 | 19 | 9 | 13 | 15 | |||
| Mean (SD) | 57.68 (28.22) | 37.62 (23.05) | 57.37 (43.85) | 18.10 (17.58) | 55.86 (58.27) | 48.43 (36.83) | 36.72 (30.29) | 46.09 (43.25) | 53.42 (40.56) |
| Total activity | |||||||||
| Mean (SD) | 512.57 (210.01) | 393.11 (189.19) | 542.65 (381.87) | 239.23 (159.05) | 464.01 (388.57) | 577.19 (357.65) | 367.89 (227.41) | 423.10 (285.86) | 557.89 (366.21) |
| Trial arm | Both trial arms | p-value | ||||||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | |||||||
| 6 months | 12 months | 6 months | 12 months | 6 months | 12 months | 6 months | 12 months | |
| In the past week, I have been able to laugh and see the funny side of things, n (%) | ||||||||
| As much as I always could | 64 (77.1) | 53 (76.8) | 65 (86.7) | 58 (81.7) | 129 (81.6) | 111 (79.3) | ||
| Not quite so much now | 16 (19.3) | 14 (20.3) | 9 (12.0) | 12 (16.9) | 25 (15.8) | 26 (18.6) | ||
| Definitely not so much now | 2 (2.4) | 1 (1.4) | 1 (1.3) | 1 (1.4) | 3 (1.9) | 2 (1.4) | ||
| Not at all | 1 (1.2) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 1 (0.6) | 1 (0.7) | ||
| Total | 83 | 69 | 75 | 71 | 158 | 140 | 0.116 | 0.459 |
| In the past week, I have looked forward with enjoyment to things, n (%) | ||||||||
| As much as I ever did | 66 (79.5) | 54 (78.3) | 58 (77.3) | 61 (85.9) | 124 (78.5) | 115 (82.1) | ||
| Rather less than I used to | 10 (12.0) | 12 (17.4) | 15 (20.0) | 8 (11.3) | 25 (15.8) | 20 (14.3) | ||
| Definitely less than I used to | 5 (6.0) | 2 (2.9) | 2 (2.7) | 2 (2.8) | 7 (4.4) | 4 (2.9) | ||
| Hardly at all | 2 (2.4) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 2 (1.3) | 1 (0.7) | ||
| Total | 83 | 69 | 75 | 71 | 158 | 140 | 0.909 | 0.238 |
| In the past week, I have blamed myself unnecessarily when things went wrong, n (%) | ||||||||
| No, never | 20 (24.1) | 26 (37.7) | 23 (30.7) | 27 (38.6) | 43 (27.2) | 53 (38.1) | ||
| Not very often | 35 (42.2) | 23 (33.3) | 30 (40.0) | 28 (40.0) | 65 (41.1) | 51 (36.7) | ||
| Yes, some of the time | 24 (28.9) | 19 (27.5) | 20 (26.7) | 14 (20.0) | 44 (27.8) | 33 (23.7) | ||
| Yes, most of the time | 4 (4.8) | 1 (1.4) | 2 (2.7) | 1 (1.4) | 6 (3.8) | 2 (1.4) | ||
| Total | 83 | 69 | 75 | 70 | 158 | 139 | 0.345 | 0.570 |
| In the past week, I have been anxious or worried for no good reason, n (%) | ||||||||
| No, not at all | 39 (47.0) | 34 (49.3) | 30 (40.0) | 41 (57.7) | 69 (43.7) | 75 (53.6) | ||
| Hardly ever | 17 (20.5) | 13 (18.8) | 17 (22.7) | 7 (9.9) | 34 (21.5) | 20 (14.3) | ||
| Yes, sometimes | 23 (27.7) | 20 (29.0) | 27 (36.0) | 21 (29.6) | 50 (31.6) | 41 (29.3) | ||
| Yes, very often | 4 (4.8) | 2 (2.9) | 1 (1.3) | 2 (2.8) | 5 (3.2) | 4 (2.9) | ||
| Total | 83 | 69 | 75 | 71 | 158 | 140 | 0.495 | 0.531 |
| In the past week, I have felt scared or panicky for no very good reason, n (%) | ||||||||
| No, not at all | 52 (62.7) | 49 (71.0) | 48 (64.0) | 50 (70.4) | 100 (63.3) | 99 (70.7) | ||
| No, not much | 13 (15.7) | 11 (15.9) | 18 (24.0) | 11 (15.5) | 31 (19.6) | 22 (15.7) | ||
| Yes, sometimes | 16 (19.3) | 9 (13.0) | 9 (12.0) | 9 (12.7) | 25 (15.8) | 18 (12.9) | ||
| Yes, quite a lot | 2 (2.4) | 0 (0.0) | 0 (0.0) | 1 (1.4) | 2 (1.3) | 1 (0.7) | ||
| Total | 83 | 69 | 75 | 71 | 158 | 140 | 0.530 | 0.898 |
| In the past week, things have been getting on top of me, n (%) | ||||||||
| No, I have been coping as well as ever | 35 (42.2) | 27 (39.7) | 26 (34.7) | 25 (35.2) | 61 (38.6) | 52 (37.4) | ||
| No, most of the time I have coped quite well | 27 (32.5) | 20 (29.4) | 37 (49.3) | 30 (42.3) | 64 (40.5) | 50 (36.0) | ||
| Yes, sometimes I have not been coping as well as usual | 18 (21.7) | 21 (30.9) | 11 (14.7) | 14 (19.7) | 29 (18.4) | 35 (25.2) | ||
| Yes, most of the time I have not been able to cope at all | 3 (3.6) | 0 (0.0) | 1 (1.3) | 2 (2.8) | 4 (2.5) | 2 (1.4) | ||
| Total | 83 | 68 | 75 | 71 | 158 | 139 | 0.999 | 0.909 |
| In the past week, I have been so unhappy that I have had difficulty sleeping, n (%) | ||||||||
| No, not at all | 53 (63.9) | 46 (66.7) | 60 (80.0) | 53 (74.6) | 113 (71.5) | 99 (70.7) | ||
| Not very often | 18 (21.7) | 11 (15.9) | 11 (14.7) | 12 (16.9) | 29 (18.4) | 23 (16.4) | ||
| Yes, sometimes | 4 (4.8) | 11 (15.9) | 4 (5.3) | 6 (8.5) | 8 (5.1) | 17 (12.1) | ||
| Yes, most of the time | 8 (9.6) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 8 (5.1) | 1 (0.7) | ||
| Total | 83 | 69 | 75 | 71 | 158 | 140 | 0.016 | 0.216 |
| In the past week, I have felt sad or miserable, n (%) | ||||||||
| No, not at all | 41 (49.4) | 35 (50.7) | 43 (57.3) | 43 (60.6) | 84 (53.2) | 78 (55.7) | ||
| Not very often | 32 (38.6) | 25 (36.2) | 29 (38.7) | 25 (35.2) | 61 (38.6) | 50 (35.7) | ||
| Yes, quite often | 8 (9.6) | 7 (10.1) | 3 (4.0) | 2 (2.8) | 11 (7.0) | 9 (6.4) | ||
| Yes, most of the time | 2 (2.4) | 2 (2.9) | 0 (0.0) | 1 (1.4) | 2 (1.3) | 3 (2.1) | ||
| Total | 83 | 69 | 75 | 71 | 158 | 140 | 0.175 | 0.138 |
| In the past week, I have been so unhappy that I have been crying, n (%) | ||||||||
| No, never | 63 (75.9) | 49 (71.0) | 64 (86.5) | 57 (80.3) | 127 (80.9) | 106 (75.7) | ||
| Only occasionally | 16 (19.3) | 16 (23.2) | 10 (13.5) | 13 (18.3) | 26 (16.6) | 29 (20.7) | ||
| Yes, quite often | 3 (3.6) | 4 (5.8) | 0 (0.0) | 0 (0.0) | 3 (1.9) | 4 (2.9) | ||
| Yes, most of the time | 1 (1.2) | 0 (0.0) | 0 (0.0) | 1 (1.4) | 1 (0.6) | 1 (0.7) | ||
| Total | 83 | 69 | 74 | 71 | 157 | 140 | 0.076 | 0.175 |
| In the past week, the thought of harming myself has occurred to me, n (%) | ||||||||
| Never | 79 (95.2) | 68 (98.6) | 74 (98.7) | 71 (100.0) | 153 (96.8) | 139 (99.3) | ||
| Hardly ever | 4 (4.8) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 4 (2.5) | 1 (0.7) | ||
| Sometimes | 0 (0.0) | 0 (0.0) | 1 (1.3) | 0 (0.0) | 1 (0.6) | 0 (0.0) | ||
| Yes, quite often | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Total | 83 | 69 | 75 | 71 | 158 | 140 | 0.221 | 0.310 |
| EPDS score | ||||||||
| > 12, n (%) | 9 (10.8) | 6 (8.7) | 1 (1.3) | 3 (4.2) | 0.014 [RR 8.13 (95% CI 1.06 to 62.69)] | 0.281 [RR 2.06 (95% CI 0.54 to 7.90)] | ||
| Mean (SD) | 5.72 (5.04) | 5.19 (4.96) | 4.59 (3.40) | 4.34 (3.94) | 0.096 [RR 1.14 (95% CI –0.20 to 2.48)] | 0.264 [RR 0.85 (95% CI –0.65 to 2.35)] | ||
| Total | 83 | 69 | 75 | 71 | ||||
| Trial arm | Both trial arms | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | ||||||||
| Baseline | 6 months | 12 months | Baseline | 6 months | 12 months | Baseline | 6 months | 12 months | |
| Alcohol score | |||||||||
| 1, n (%) | 2 (10.0) | 1 (2.4) | 1 (2.4) | 2 (10.0) | 1 (2.5) | 1 (2.2) | 4 (10.0) | 2 (2.5) | 2 (2.3) |
| 2, n (%) | 11 (55.0) | 15 (36.6) | 14 (33.3) | 15 (75.0) | 10 (25.0) | 11 (23.9) | 26 (65.0) | 25 (30.9) | 25 (28.4) |
| 3, n (%) | 5 (25.0) | 10 (24.4) | 8 (19.0) | 2 (10.0) | 14 (35.0) | 13 (28.3) | 7 (17.5) | 24 (29.6) | 21 (23.9) |
| 4, n (%) | 2 (10.0) | 7 (17.1) | 8 (19.0) | 1 (5.0) | 5 (12.5) | 5 (10.9) | 3 (7.5) | 12 (14.8) | 13 (14.8) |
| 5, n (%) | 5 (12.2) | 6 (14.3) | 4 (10.0) | 11 (23.9) | 9 (11.1) | 17 (19.3) | |||
| 6, n (%) | 1 (2.4) | 1 (2.4) | 1 (2.5) | 1 (2.2) | 2 (2.5) | 2 (2.3) | |||
| 7, n (%) | 1 (2.4) | 2 (4.8) | 3 (7.5) | 1 (2.2) | 4 (4.9) | 3 (3.4) | |||
| 8, n (%) | 1 (2.4) | 1 (2.4) | 0 (0.0) | 2 (4.3) | 1 (1.2) | 3 (3.4) | |||
| 9, n (%) | 0 (0.0) | 0 (0.0) | 2 (5.0) | 1 (2.2) | 2 (2.5) | 1 (1.1) | |||
| 10, n (%) | 1 (2.4) | 0 (0.0) | 1 (1.1) | ||||||
| Mean (SD) | 2.35 (0.81) | 3.29 (1.52) | 3.64 (1.90) | 2.10 (0.64) | 3.70 (1.92) | 3.80 (1.80) | 2.23 (0.73) | 3.49 (1.73) | 3.73 (1.84) |
| Total | 20 | 41 | 42 | 20 | 40 | 46 | 40 | 81 | 88 |
| How often do you have a drink containing alcohol?, n (%) | |||||||||
| Never | 78 (79.6) | 44 (53.0) | 27 (39.1) | 75 (79.8) | 33 (44.6) | 25 (35.2) | 153 (79.7) | 77 (49.0) | 52 (37.1) |
| Monthly or less | 15 (15.3) | 20 (24.1) | 23 (33.3) | 16 (17.0) | 16 (21.6) | 18 (25.4) | 31 (16.1) | 36 (22.9) | 41 (29.3) |
| 2–4 times/month | 5 (5.1) | 10 (12.0) | 14 (20.3) | 2 (2.1) | 19 (25.7) | 19 (26.8) | 7 (3.6) | 29 (18.5) | 33 (23.6) |
| 2 or 3 times/week | 0 (0.0) | 9 (10.8) | 4 (5.8) | 1 (1.1) | 4 (5.4) | 7 (9.9) | 1 (0.5) | 13 (8.3) | 11 (7.9) |
| ≥ 4 times/week | 0 (0.0) | 0 (0.0) | 1 (1.4) | 0 (0.0) | 2 (2.7) | 2 (2.8) | 0 (0.0) | 2 (1.3) | 3 (2.1) |
| Total | 98 | 83 | 69 | 94 | 74 | 71 | 192 | 157 | 140 |
| How many units of alcohol do you drink on a typical day when you are drinking?, n (%) | |||||||||
| 1 or 2 | 18 (90.0) | 31 (75.6) | 28 (65.1) | 20 (95.2) | 31 (75.6) | 32 (68.1) | 38 (92.7) | 62 (75.6) | 60 (66.7) |
| 3 or 4 | 2 (10.0) | 7 (17.1) | 10 (23.3) | 1 (4.8) | 6 (14.6) | 12 (25.5) | 3 (7.3) | 13 (15.9) | 22 (24.4) |
| 5 or 6 | 0 (0.0) | 2 (4.9) | 4 (9.3) | 0 (0.0) | 4 (9.8) | 2 (4.3) | 0 (0.0) | 6 (7.3) | 6 (6.7) |
| 7–9 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.1) | 0 (0.0) | 0 (0.0) | 1 (1.1) |
| ≥ 10 | 0 (0.0) | 1 (2.4) | 1 (2.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.2) | 1 (1.1) |
| Total | 20 | 41 | 43 | 21 | 41 | 47 | 41 | 82 | 90 |
| How often have you had six or more units on a single occasion in last 6 months?, n (%) | |||||||||
| Never | 85 (97.7) | 37 (77.1) | 30 (63.8) | 86 (100.0) | 32 (68.1) | 29 (59.2) | 171 (98.8) | 69 (72.6) | 59 (61.5) |
| Less than monthly | 2 (2.3) | 10 (20.8) | 13 (27.7) | 0 (0.0) | 10 (21.3) | 15 (30.6) | 2 (1.2) | 20 (21.1) | 28 (29.2) |
| Monthly | 0 (0.0) | 1 (2.1) | 2 (4.3) | 0 (0.0) | 3 (6.4) | 4 (8.2) | 0 (0.0) | 4 (4.2) | 6 (6.3) |
| Weekly | 0 (0.0) | 0 (0.0) | 2 (4.3) | 0 (0.0) | 2 (4.3) | 1 (2.0) | 0 (0.0) | 2 (2.1) | 3 (3.1) |
| Daily or almost daily | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 87 | 48 | 47 | 86 | 47 | 49 | 173 | 95 | 96 |
| Trial arm, n (%) | Both trial arms | |||||||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | |||||||
| 6 months | 12 months | 6 months | 12 months | 6 months | 12 months | |||
| RR (95% CI) | p-value | RR (95% CI) | p-value | |||||
| Has your weight influenced how you think about (judge) yourself as a person? | ||||||||
| 0 (not at all) | 18 (21.7) | 16 (23.2) | 11 (14.7) | 17 (23.9) | ||||
| 1 | 6 (7.2) | 7 (10.1) | 8 (10.7) | 9 (12.7) | 0.59 (0.25 to 1.42) | 0.88 (0.39 to 1.98) | ||
| 2 (slightly) | 21 (25.3) | 17 (24.6) | 24 (32.0) | 19 (26.8) | 0.79 (0.54 to 1.13) | 0.98 (0.62 to 1.54) | ||
| 3 | 1 (1.2) | 6 (8.7) | 8 (10.7) | 8 (11.3) | 0.13 (0.02 to 0.90) | 0.85 (0.35 to 2.08) | ||
| 4 (moderately) | 16 (19.3) | 11 (15.9) | 6 (8.0) | 5 (7.0) | 1.33 (0.64 to 2.78) | 1.79 (0.73 to 4.39) | ||
| 5 | 9 (10.8) | 4 (5.8) | 6 (8.0) | 7 (9.9) | 0.94 (0.41 to 2.18) | 0.69 (0.23 to 2.01) | ||
| 6 (markedly) | 12 (14.5) | 8 (11.6) | 12 (16.0) | 6 (8.5) | 0.77 (0.43 to 1.38) | 1.28 (0.52 to 3.11) | ||
| Total | 83 | 69 | 75 | 71 | 0.121 | 0.701 | ||
| Has your shape influenced how you think about (judge) yourself as a person? | ||||||||
| 0 (not at all) | 20 (24.1) | 20 (29.0) | 13 (17.6) | 18 (25.4) | ||||
| 1 | 11 (13.3) | 11 (15.9) | 10 (13.5) | 9 (12.7) | 0.82 (0.42 to 1.59) | 1.06 (0.52 to 2.17) | ||
| 2 (slightly) | 14 (16.9) | 12 (17.4) | 20 (27.0) | 16 (22.5) | 0.68 (0.42 to 1.11) | 0.80 (0.45 to 1.41) | ||
| 3 | 5 (6.0) | 6 (8.7) | 7 (9.5) | 10 (14.1) | 0.57 (0.21 to 1.53) | 0.65 (0.27 to 1.53) | ||
| 4 (moderately) | 12 (14.5) | 10 (14.5) | 8 (10.8) | 5 (7.0) | 0.98 (0.49 to 1.99) | 1.53 (0.61 to 3.87) | ||
| 5 | 8 (9.6) | 4 (5.8) | 7 (9.5) | 8 (11.3) | 0.82 (0.35 to 1.88) | 0.54 (0.19 to 1.57) | ||
| 6 (markedly) | 13 (15.7) | 6 (8.7) | 9 (12.2) | 5 (7.0) | 0.96 (0.50 to 1.86) | 1.06 (0.37 to 3.02) | ||
| Total | 83 | 69 | 74 | 71 | 0.685 | 0.574 | ||
| How much would it have upset you if you had been asked to weigh yourself once per week (no more, or less, often) for the next 4 weeks? | ||||||||
| 0 (not at all) | 54 (65.1) | 50 (72.5) | 44 (58.7) | 48 (67.6) | ||||
| 1 | 10 (12.0) | 9 (13.0) | 13 (17.3) | 5 (7.0) | 0.69 (0.33 to 1.44) | 1.62 (0.58 to 4.52) | ||
| 2 (slightly) | 9 (10.8) | 4 (5.8) | 8 (10.7) | 7 (9.9) | 0.93 (0.39 to 2.24) | 0.58 (0.18 to 1.87) | ||
| 3 | 3 (3.6) | 2 (2.9) | 4 (5.3) | 6 (8.5) | 0.63 (0.15 to 2.68) | 0.35 (0.07 to 1.64) | ||
| 4 (moderately) | 1 (1.2) | 3 (4.3) | 4 (5.3) | 4 (5.6) | 0.22 (0.03 to 1.89) | 0.74 (0.17 to 3.13) | ||
| 5 | 4 (4.8) | 0 (0.0) | 1 (1.3) | 1 (1.4) | 3.10 (0.36 to 26.81) | . (. to.) | ||
| 6 (markedly) | 2 (2.4) | 1 (1.4) | 1 (1.3) | 0 (0.0) | 1.61 (0.15 to 17.16) | . (. to.) | ||
| Total | 83 | 69 | 75 | 71 | 0.601 | 0.320 | ||
| How dissatisfied have you been with your weight? | ||||||||
| 0 (not at all) | 13 (15.7) | 6 (8.7) | 10 (13.3) | 13 (18.3) | ||||
| 1 | 7 (8.4) | 6 (8.7) | 5 (6.7) | 14 (19.7) | 1.05 (0.41 to 2.67) | 0.96 (0.49 to 1.89) | ||
| 2 (slightly) | 10 (12.0) | 18 (26.1) | 8 (10.7) | 5 (7.0) | 0.98 (0.49 to 1.96) | 2.70 (1.24 to 5.89) | ||
| 3 | 11 (13.3) | 7 (10.1) | 9 (12.0) | 8 (11.3) | 0.97 (0.51 to 1.84) | 1.41 (0.67 to 2.97) | ||
| 4 (moderately) | 11 (13.3) | 11 (15.9) | 17 (22.7) | 13 (18.3) | 0.73 (0.43 to 1.23) | 1.29 (0.77 to 2.18) | ||
| 5 | 8 (9.6) | 7 (10.1) | 7 (9.3) | 5 (7.0) | 0.93 (0.42 to 2.03) | 1.94 (0.79 to 4.76) | ||
| 6 (markedly) | 23 (27.7) | 14 (20.3) | 19 (25.3) | 13 (18.3) | 0.98 (0.68 to 1.40) | 1.40 (0.87 to 2.26) | ||
| Total | 83 | 69 | 75 | 71 | 0.878 | 0.055 | ||
| How dissatisfied have you been with your shape? | ||||||||
| 0 (not at all) | 15 (18.1) | 8 (11.8) | 8 (10.7) | 13 (18.3) | ||||
| 1 | 9 (10.8) | 9 (13.2) | 10 (13.3) | 10 (14.1) | 0.68 (0.35 to 1.31) | 1.22 (0.64 to 2.32) | ||
| 2 (slightly) | 13 (15.7) | 12 (17.6) | 8 (10.7) | 13 (18.3) | 0.93 (0.49 to 1.75) | 1.20 (0.71 to 2.03) | ||
| 3 | 11 (13.3) | 10 (14.7) | 14 (18.7) | 8 (11.3) | 0.66 (0.38 to 1.15) | 1.46 (0.74 to 2.89) | ||
| 4 (moderately) | 10 (12.0) | 11 (16.2) | 15 (20.0) | 12 (16.9) | 0.61 (0.35 to 1.08) | 1.21 (0.69 to 2.11) | ||
| 5 | 7 (8.4) | 8 (11.8) | 7 (9.3) | 3 (4.2) | 0.68 (0.30 to 1.54) | 2.67 (0.86 to 8.27) | ||
| 6 (markedly) | 18 (21.7) | 10 (14.7) | 13 (17.3) | 12 (16.9) | 0.88 (0.56 to 1.39) | 1.16 (0.65 to 2.07) | ||
| Total | 83 | 68 | 75 | 71 | 0.540 | 0.719 | ||
| How uncomfortable have you felt about seeing your body (for example, seeing your shape in the mirror, in a shop reflection, while undressing or taking a bath or shower)? | ||||||||
| 0 (not at all) | 17 (20.5) | 16 (23.2) | 15 (20.0) | 22 (31.0) | ||||
| 1 | 8 (9.6) | 7 (10.1) | 8 (10.7) | 12 (16.9) | 0.92 (0.41 to 2.05) | 0.86 (0.40 to 1.86) | ||
| 2 (slightly) | 14 (16.9) | 16 (23.2) | 12 (16.0) | 8 (11.3) | 1.02 (0.57 to 1.80) | 1.87 (0.94 to 3.73) | ||
| 3 | 9 (10.8) | 6 (8.7) | 8 (10.7) | 7 (9.9) | 1.00 (0.46 to 2.15) | 1.13 (0.44 to 2.89) | ||
| 4 (moderately) | 10 (12.0) | 11 (15.9) | 12 (16.0) | 9 (12.7) | 0.83 (0.44 to 1.59) | 1.40 (0.69 to 2.87) | ||
| 5 | 6 (7.2) | 6 (8.7) | 5 (6.7) | 5 (7.0) | 1.04 (0.37 to 2.91) | 1.47 (0.52 to 4.19) | ||
| 6 (markedly) | 19 (22.9) | 7 (10.1) | 15 (20.0) | 8 (11.3) | 1.06 (0.66 to 1.69) | 1.14 (0.48 to 2.69) | ||
| Total | 83 | 69 | 75 | 71 | 0.995 | 0.526 | ||
| How uncomfortable have you felt about others seeing your shape or figure (for example, in communal changing rooms, when swimming, or wearing tight clothes)? | ||||||||
| 0 (not at all) | 17 (20.5) | 13 (18.8) | 20 (26.7) | 28 (39.4) | ||||
| 1 | 9 (10.8) | 8 (11.6) | 3 (4.0) | 10 (14.1) | 2.65 (0.82 to 8.64) | 1.45 (0.68 to 3.10) | ||
| 2 (slightly) | 14 (16.9) | 18 (26.1) | 15 (20.0) | 6 (8.5) | 1.05 (0.61 to 1.82) | 3.29 (1.50 to 7.22) | ||
| 3 | 9 (10.8) | 8 (11.6) | 10 (13.3) | 6 (8.5) | 1.04 (0.50 to 2.16) | 2.16 (0.87 to 5.35) | ||
| 4 (moderately) | 6 (7.2) | 5 (7.2) | 5 (6.7) | 6 (8.5) | 1.30 (0.46 to 3.70) | 1.57 (0.56 to 4.45) | ||
| 5 | 11 (13.3) | 8 (11.6) | 9 (12.0) | 7 (9.9) | 1.27 (0.62 to 2.58) | 1.90 (0.81 to 4.49) | ||
| 6 (markedly) | 17 (20.5) | 9 (13.0) | 13 (17.3) | 8 (11.3) | 1.27 (0.74 to 2.18) | 1.84 (0.83 to 4.06) | ||
| Total | 83 | 69 | 75 | 71 | 0.739 | 0.081 | ||
| Trial arm | Both trial arms | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | ||||||||
| Baseline | 6 months | 12 months | Baseline | 6 months | 12 months | Baseline | 6 months | 12 months | |
| On the whole, I am satisfied with myself, n (%) | |||||||||
| Strongly agree | 23 (23.5) | 24 (28.9) | 17 (24.6) | 34 (35.8) | 23 (30.7) | 24 (33.8) | 57 (29.5) | 47 (29.7) | 41 (29.3) |
| Agree | 63 (64.3) | 51 (61.4) | 47 (68.1) | 53 (55.8) | 43 (57.3) | 40 (56.3) | 116 (60.1) | 94 (59.5) | 87 (62.1) |
| Strongly disagree | 6 (6.1) | 0 (0.0) | 2 (2.9) | 2 (2.1) | 3 (4.0) | 1 (1.4) | 8 (4.1) | 3 (1.9) | 3 (2.1) |
| Disagree | 6 (6.1) | 8 (9.6) | 3 (4.3) | 6 (6.3) | 6 (8.0) | 6 (8.5) | 12 (6.2) | 14 (8.9) | 9 (6.4) |
| Total | 98 | 83 | 69 | 95 | 75 | 71 | 193 | 158 | 140 |
| At times, I think I am no good at all, n (%) | |||||||||
| Strongly agree | 1 (1.0) | 1 (1.2) | 1 (1.4) | 2 (2.1) | 2 (2.7) | 1 (1.4) | 3 (1.6) | 3 (1.9) | 2 (1.4) |
| Agree | 18 (18.4) | 13 (15.7) | 11 (15.9) | 18 (18.9) | 8 (10.7) | 6 (8.5) | 36 (18.7) | 21 (13.3) | 17 (12.1) |
| Strongly disagree | 30 (30.6) | 38 (45.8) | 24 (34.8) | 25 (26.3) | 30 (40.0) | 27 (38.0) | 55 (28.5) | 68 (43.0) | 51 (36.4) |
| Disagree | 49 (50.0) | 31 (37.3) | 33 (47.8) | 50 (52.6) | 35 (46.7) | 37 (52.1) | 99 (51.3) | 66 (41.8) | 70 (50.0) |
| Total | 98 | 83 | 69 | 95 | 75 | 71 | 193 | 158 | 140 |
| I feel that I have a number of good qualities, n (%) | |||||||||
| Strongly agree | 37 (38.1) | 45 (54.2) | 36 (52.2) | 38 (40.0) | 48 (64.0) | 42 (59.2) | 75 (39.1) | 93 (58.9) | 78 (55.7) |
| Agree | 58 (59.8) | 37 (44.6) | 31 (44.9) | 54 (56.8) | 27 (36.0) | 25 (35.2) | 112 (58.3) | 64 (40.5) | 56 (40.0) |
| Strongly disagree | 0 (0.0) | 1 (1.2) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 1 (1.4) | 0 (0.0) | 1 (0.6) | 2 (1.4) |
| Disagree | 2 (2.1) | 0 (0.0) | 1 (1.4) | 3 (3.2) | 0 (0.0) | 3 (4.2) | 5 (2.6) | 0 (0.0) | 4 (2.9) |
| Total | 97 | 83 | 69 | 95 | 75 | 71 | 192 | 158 | 140 |
| I am able to do things as well as most other people, n (%) | |||||||||
| Strongly agree | 35 (35.7) | 36 (43.4) | 33 (47.8) | 34 (35.8) | 49 (65.3) | 40 (56.3) | 69 (35.8) | 85 (53.8) | 73 (52.1) |
| Agree | 55 (56.1) | 44 (53.0) | 32 (46.4) | 57 (60.0) | 24 (32.0) | 24 (33.8) | 112 (58.0) | 68 (43.0) | 56 (40.0) |
| Strongly disagree | 3 (3.1) | 1 (1.2) | 1 (1.4) | 2 (2.1) | 1 (1.3) | 3 (4.2) | 5 (2.6) | 2 (1.3) | 4 (2.9) |
| Disagree | 5 (5.1) | 2 (2.4) | 3 (4.3) | 2 (2.1) | 1 (1.3) | 4 (5.6) | 7 (3.6) | 3 (1.9) | 7 (5.0) |
| Total | 98 | 83 | 69 | 95 | 75 | 71 | 193 | 158 | 140 |
| I feel I do not have much to be proud of, n (%) | |||||||||
| Strongly agree | 2 (2.0) | 2 (2.4) | 2 (2.9) | 2 (2.1) | 2 (2.7) | 2 (2.8) | 4 (2.1) | 4 (2.5) | 4 (2.9) |
| Agree | 8 (8.2) | 5 (6.0) | 3 (4.3) | 4 (4.2) | 6 (8.0) | 5 (7.0) | 12 (6.2) | 11 (7.0) | 8 (5.7) |
| Strongly disagree | 42 (42.9) | 42 (50.6) | 35 (50.7) | 43 (45.3) | 43 (57.3) | 31 (43.7) | 85 (44.0) | 85 (53.8) | 66 (47.1) |
| Disagree | 46 (46.9) | 34 (41.0) | 29 (42.0) | 46 (48.4) | 24 (32.0) | 33 (46.5) | 92 (47.7) | 58 (36.7) | 62 (44.3) |
| Total | 98 | 83 | 69 | 95 | 75 | 71 | 193 | 158 | 140 |
| I certainly feel useless at times, n (%) | |||||||||
| Strongly agree | 0 (0.0) | 3 (3.6) | 0 (0.0) | 2 (2.1) | 2 (2.7) | 2 (2.8) | 2 (1.0) | 5 (3.2) | 2 (1.4) |
| Agree | 24 (24.5) | 18 (21.7) | 9 (13.0) | 16 (16.8) | 12 (16.0) | 7 (9.9) | 40 (20.7) | 30 (19.0) | 16 (11.4) |
| Strongly disagree | 30 (30.6) | 29 (34.9) | 20 (29.0) | 29 (30.5) | 30 (40.0) | 26 (36.6) | 59 (30.6) | 59 (37.3) | 46 (32.9) |
| Disagree | 44 (44.9) | 33 (39.8) | 40 (58.0) | 48 (50.5) | 31 (41.3) | 36 (50.7) | 92 (47.7) | 64 (40.5) | 76 (54.3) |
| Total | 98 | 83 | 69 | 95 | 75 | 71 | 193 | 158 | 140 |
| I feel that I’m a person of worth, at least on an equal plane with others, n (%) | |||||||||
| Strongly agree | 40 (40.8) | 39 (47.0) | 33 (47.8) | 35 (36.8) | 47 (62.7) | 39 (54.9) | 75 (38.9) | 86 (54.4) | 72 (51.4) |
| Agree | 55 (56.1) | 36 (43.4) | 30 (43.5) | 55 (57.9) | 25 (33.3) | 26 (36.6) | 110 (57.0) | 61 (38.6) | 56 (40.0) |
| Strongly disagree | 2 (2.0) | 3 (3.6) | 2 (2.9) | 2 (2.1) | 2 (2.7) | 2 (2.8) | 4 (2.1) | 5 (3.2) | 4 (2.9) |
| Disagree | 1 (1.0) | 5 (6.0) | 4 (5.8) | 3 (3.2) | 1 (1.3) | 4 (5.6) | 4 (2.1) | 6 (3.8) | 8 (5.7) |
| Total | 98 | 83 | 69 | 95 | 75 | 71 | 193 | 158 | 140 |
| I wish I could have more respect for myself, n (%) | |||||||||
| Strongly agree | 0 (0.0) | 6 (7.2) | 6 (8.7) | 3 (3.2) | 9 (12.0) | 4 (5.6) | 3 (1.6) | 15 (9.5) | 10 (7.1) |
| Agree | 22 (22.4) | 20 (24.1) | 14 (20.3) | 12 (12.6) | 11 (14.7) | 14 (19.7) | 34 (17.6) | 31 (19.6) | 28 (20.0) |
| Strongly disagree | 35 (35.7) | 29 (34.9) | 19 (27.5) | 30 (31.6) | 31 (41.3) | 21 (29.6) | 65 (33.7) | 60 (38.0) | 40 (28.6) |
| Disagree | 41 (41.8) | 28 (33.7) | 30 (43.5) | 50 (52.6) | 24 (32.0) | 32 (45.1) | 91 (47.2) | 52 (32.9) | 62 (44.3) |
| Total | 98 | 83 | 69 | 95 | 75 | 71 | 193 | 158 | 140 |
| All in all, I am inclined to feel that I am a failure, n (%) | |||||||||
| Strongly agree | 0 (0.0) | 1 (1.2) | 0 (0.0) | 0 (0.0) | 2 (2.7) | 1 (1.4) | 0 (0.0) | 3 (1.9) | 1 (0.7) |
| Agree | 5 (5.2) | 3 (3.6) | 4 (5.8) | 4 (4.2) | 3 (4.0) | 6 (8.5) | 9 (4.7) | 6 (3.8) | 10 (7.1) |
| Strongly disagree | 48 (49.5) | 40 (48.2) | 32 (46.4) | 49 (51.6) | 43 (57.3) | 30 (42.3) | 97 (50.5) | 83 (52.5) | 62 (44.3) |
| Disagree | 44 (45.4) | 39 (47.0) | 33 (47.8) | 42 (44.2) | 27 (36.0) | 34 (47.9) | 86 (44.8) | 66 (41.8) | 67 (47.9) |
| Total | 97 | 83 | 69 | 95 | 75 | 71 | 192 | 158 | 140 |
| I take a positive attitude towards myself, n (%) | |||||||||
| Strongly disagree | 1 (1.0) | 1 (1.2) | 2 (2.9) | 0 (0.0) | 1 (1.3) | 2 (2.8) | 1 (0.5) | 2 (1.3) | 4 (2.9) |
| Disagree | 3 (3.1) | 9 (10.8) | 4 (5.8) | 4 (4.2) | 4 (5.3) | 6 (8.5) | 7 (3.6) | 13 (8.2) | 10 (7.1) |
| Strongly agree | 39 (39.8) | 35 (42.2) | 32 (46.4) | 45 (47.4) | 42 (56.0) | 36 (50.7) | 84 (43.5) | 77 (48.7) | 68 (48.6) |
| Agree | 55 (56.1) | 38 (45.8) | 31 (44.9) | 46 (48.4) | 28 (37.3) | 27 (38.0) | 101 (52.3) | 66 (41.8) | 58 (41.4) |
| Total | 98 | 83 | 69 | 95 | 75 | 71 | 193 | 158 | 140 |
| Total score40 | |||||||||
| Mean (SD) | 32.34 (4.13) | 32.84 (4.77) | 32.77 (4.48) | 32.74 (3.71) | 33.81 (4.84) | 33.06 (4.84) | 32.53 (3.92) | 33.30 (4.81) | 32.91 (4.65) |
Appendix 4 Health economics tables
| Trial arm | Both trial arms | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | ||||||||
| Baseline | 6 months | 12 months | Baseline | 6 months | 12 months | Baseline | 6 months | 12 months | |
| Mobility, n (%) | 98 | 83 | 69 | 95 | 75 | 71 | 193 | 158 | 140 |
| I have no problems in walking about | 73 (74.5) | 74 (89.2) | 62 (89.9) | 70 (73.7) | 62 (82.7) | 66 (93.0) | 143 (74.1) | 136 (86.1) | 128 (91.4) |
| I have slight problems in walking about | 14 (14.3) | 6 (7.2) | 6 (8.7) | 19 (20.0) | 10 (13.3) | 3 (4.2) | 33 (17.1) | 16 (10.1) | 9 (6.4) |
| I have moderate problems in walking about | 10 (10.2) | 2 (2.4) | 1 (1.4) | 4 (4.2) | 3 (4.0) | 1 (1.4) | 14 (7.3) | 5 (3.2) | 2 (1.4) |
| I have severe problems in walking about | 1 (1.0) | 0 (0.0) | 0 (0.0) | 1 (1.1) | 0 (0.0) | 0 (0.0) | 2 (1.0) | 0 (0.0) | 0 (0.0) |
| I am unable to walk about | 0 (0.0) | 1 (1.2) | 0 (0.0) | 1 (1.1) | 0 (0.0) | 1 (1.4) | 1 (0.5) | 1 (0.6) | 1 (0.7) |
| Self-care, n (%) | 98 | 83 | 69 | 95 | 75 | 71 | 193 | 158 | 140 |
| I have no problems washing or dressing myself | 77 (78.6) | 81 (97.6) | 67 (97.1) | 87 (91.6) | 74 (98.7) | 69 (97.2) | 164 (85.0) | 155 (98.1) | 136 (97.1) |
| I have slight problems washing or dressing myself | 16 (16.3) | 2 (2.4) | 2 (2.9) | 6 (6.3) | 1 (1.3) | 1 (1.4) | 22 (11.4) | 3 (1.9) | 3 (2.1) |
| I have moderate problems washing or dressing myself | 5 (5.1) | 0 (0.0) | 0 (0.0) | 2 (2.1) | 0 (0.0) | 1 (1.4) | 7 (3.6) | 0 (0.0) | 1 (0.7) |
| I have severe problems washing or dressing myself | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| I am unable to wash or dress myself | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Usual activities, n (%) | 98 | 83 | 69 | 95 | 75 | 71 | 193 | 158 | 140 |
| I have no problems doing my usual activities | 62 (63.3) | 72 (86.7) | 60 (87.0) | 58 (61.1) | 64 (85.3) | 67 (94.4) | 120 (62.2) | 136 (86.1) | 127 (90.7) |
| I have slight problems doing my usual activities | 22 (22.4) | 11 (13.3) | 5 (7.2) | 29 (30.5) | 8 (10.7) | 3 (4.2) | 51 (26.4) | 19 (12.0) | 8 (5.7) |
| I have moderate problems doing my usual activities | 10 (10.2) | 0 (0.0) | 4 (5.8) | 6 (6.3) | 3 (4.0) | 1 (1.4) | 16 (8.3) | 3 (1.9) | 5 (3.6) |
| I have severe problems doing my usual activities | 3 (3.1) | 0 (0.0) | 0 (0.0) | 1 (1.1) | 0 (0.0) | 0 (0.0) | 4 (2.1) | 0 (0.0) | 0 (0.0) |
| I am unable to do my usual activities | 1 (1.0) | 0 (0.0) | 0 (0.0) | 1 (1.1) | 0 (0.0) | 0 (0.0) | 2 (1.0) | 0 (0.0) | 0 (0.0) |
| Pain/discomfort, n (%) | 98 | 83 | 69 | 95 | 75 | 71 | 193 | 158 | 140 |
| I have no pain or discomfort | 36 (36.7) | 51 (61.4) | 41 (59.4) | 35 (36.8) | 42 (56.0) | 47 (66.2) | 71 (36.8) | 93 (58.9) | 88 (62.9) |
| I have slight pain or discomfort | 43 (43.9) | 24 (28.9) | 24 (34.8) | 37 (38.9) | 26 (34.7) | 21 (29.6) | 80 (41.5) | 50 (31.6) | 45 (32.1) |
| I have moderate pain or discomfort | 16 (16.3) | 8 (9.6) | 4 (5.8) | 20 (21.1) | 6 (8.0) | 2 (2.8) | 36 (18.7) | 14 (8.9) | 6 (4.3) |
| I have severe pain or discomfort | 3 (3.1) | 0 (0.0) | 0 (0.0) | 1 (1.1) | 1 (1.3) | 1 (1.4) | 4 (2.1) | 1 (0.6) | 1 (0.7) |
| I have extreme pain or discomfort | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (2.1) | 0 (0.0) | 0 (0.0) | 2 (1.0) | 0 (0.0) | 0 (0.0) |
| Anxiety/depression, n (%) | 98 | 83 | 69 | 95 | 75 | 71 | 193 | 158 | 140 |
| I am not anxious or depressed | 81 (82.7) | 67 (80.7) | 54 (78.3) | 74 (77.9) | 58 (77.3) | 61 (85.9) | 155 (80.3) | 125 (79.1) | 115 (82.1) |
| I am slightly anxious or depressed | 13 (13.3) | 11 (13.3) | 11 (15.9) | 19 (20.0) | 14 (18.7) | 7 (9.9) | 32 (16.6) | 25 (15.8) | 18 (12.9) |
| I am moderately anxious or depressed | 3 (3.1) | 5 (6.0) | 3 (4.3) | 2 (2.1) | 3 (4.0) | 3 (4.2) | 5 (2.6) | 8 (5.1) | 6 (4.3) |
| I am severely anxious or depressed | 0 (0.0) | 0 (0.0) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.7) |
| I am extremely anxious or depressed | 1 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 0 (0.0) | 0 (0.0) |
| Health scale, n | 98 | 83 | 69 | 95 | 75 | 71 | 193 | 158 | 140 |
| Mean (SD) | 76.94 (18.53) | 77.93 (15.83) | 78.13 (15.90) | 78.78 (17.68) | 77.64 (16.59) | 80.82 (15.50) | 77.84 (18.09) | 77.79 (16.15) | 79.49 (15.70) |
| Service | Unit cost (£) |
|---|---|
| Community-based health services | |
| GP | 32.00 per contact |
| Practice nurse | 9.30 per contact |
| Health visitor | 70.00 per contact |
| Smoking cessation | 9.30 per contact |
| Social worker | 59.00 per contact |
| Housing worker | 31.00 per contact |
| Employment worker | 31.00 per contact |
| Sexual health clinic | 31.00 per contact |
| Clinical psychologist | 55.00 per contact |
| Community psychiatrist | 215.00 per contact |
| Community psychiatric nurse | 35.85 per contact |
| Dietitian | 15.00 per contact |
| Perinatal psychiatric treatment team | 215.00 per contact |
| Psychotherapist | 55.00 per contact |
| Family support worker | 31.00 per contact |
| Antenatal/postnatal services | |
| Midwife (clinic) | 57.43 per contact |
| Midwife (home) | 57.43 per contact |
| Obstetrician | 128.82 per contact |
| Ultrasound scan | 112.00 per scan |
| Blood test | 1.00 per test |
| Glucose test | 1.00 per test |
| Diabetes clinic | 115.31 per contact |
| Physiotherapist | 48.81 per contact |
| Parent craft | 38.33 per contact |
| Day unit | 233.00 per contact |
| Pre-term clinic | 198.20 per contact |
| Early pregnancy unit | 192.98 per contact |
| Clinic for newborn | 100.00 per contact |
| Breastfeeding advisor | 57.43 per contact |
| Other outpatient visits (mother/infant) | |
| Trauma and orthopaedics | 119.19 per contact |
| Breast surgeon | 149.46 per contact |
| CAT scan | 83.00 per scan |
| Anticoagulant clinic | 31.87 per contact |
| Adult mental illness | 283.98 per contact |
| Gastroenterology | 145.57 per contact |
| Gynaecology | 140.93 per contact |
| Cardiology | 128.72 per contact |
| Oncology (ultrasound scan) | 164.00 per scan |
| Hepatology | 212.92 per contact |
| Infectious disease | 233.62 per contact |
| Pain management | 139.23 per contact |
| Medical oncology | 161.13 per contact |
| Urology | 109.40 per contact |
| Audiology | 86.83 per contact |
| Haemophilia | 491.70 per contact |
| Paediatrics | 198.20 per contact |
| A&E | |
| A&E attendance | 147.80 per contact |
| Ambulance | 247.00 per contact |
| Hospital admission (mother/baby) | |
| Antenatal admission (average) | 824.00 per night |
| Antenatal day case (average) | 257.00 per night |
| Non-elective long stay (pelvic fracture) | 400.00 per night |
| Non-elective long stay (sepsis) | 4416.00 per FCE |
| Non-elective long stay (appendectomy) | 1067.00 per night |
| Non-elective long stay (cholecystectomy) | 871.00 per night |
| Non-elective short stay (hypertension) | 1381.00 per FCE |
| Elective long stay (lower back pain) | 857.00 per night |
| Non-elective long stay (average) | 2985.00 per FCE |
| Non-elective short stay (average) | 617.00 per FCE |
| Non-elective long stay (paediatric: cardio) | 984.00 per night |
| Non-elective long stay (paediatric: major infections) | 4372.00 per FCE |
| Non-elective long stay (paediatric: all infections) | 736.00 per night |
| Non-elective short stay (paediatric: all infections) | 684.00 per FCE |
| Non-elective long stay (paediatric: musculoskeletal connective tissue disorders) | 2908.00 per FCE |
| Non-elective short stay (average paediatric) | 708.00 per FCE |
| Non-elective long stay (average paediatric) | 789.00 per night |
| Contact | Intervention (N = 98) | Control (N = 95) | ||||
|---|---|---|---|---|---|---|
| Percentage reporting using service | Mean number of contacts/hospital admissions where number of contacts (admissions) > 0 | Missing cost data, n (%) | Percentage reporting using service | Mean number of contacts/hospital admissions where number of contacts (admissions) > 0 | Missing cost data, n (%) | |
| Pregnancy-related community-based and outpatient service contacts | ||||||
| Midwife (clinic) | 94 | 5 | 3 (3) | 99 | 5 | 4 (4) |
| Midwife (home) | 9 | 5 | 0 (0) | 15 | 3 | 0 (0) |
| GP | 82 | 2 | 3 (3) | 91 | 2 | 1 (1) |
| Practice nurse | 61 | 1 | 0 (0) | 58 | 1 | 0 (0) |
| Obstetrician | 65 | 3 | 2 (2) | 68 | 3 | 0 (0) |
| Ultrasound scan | 98 | 4 | 1 (1) | 100 | 4 | 1 (1) |
| Blood test | 99 | 3 | 0 (0) | 96 | 3 | 0 (0) |
| Glucose test | 26 | 1 | 2 (2) | 23 | 1 | 1 (1) |
| Diabetes clinic | 14 | 3 | 2 (2) | 11 | 4 | 1 (1) |
| Physiotherapist | 27 | 2 | 2 (2) | 32 | 4 | 1 (1) |
| Parent craft | 38 | 2 | 3 (3) | 38 | 2 | 1 (1) |
| Health visitor | 5 | 3 | 2 (2) | 5 | 1 | 0 (0) |
| Other | 28 | 2 | 3 (3) | 35 | 2 | 1 (1) |
| Non-pregnancy-related community-based, other outpatient and A&E service contacts | ||||||
| GP | 20 | 1 | 2 (2) | 17 | 2 | 1 (1) |
| Practice nurse | 2 | 1 | 3 (3) | 2 | 1 | 1 (1) |
| Smoking cessation | 2 | 6 | 3 (3) | 3 | 5 | 1 (1) |
| Social worker | 1 | 1 | 3 (3) | 1 | 1 | 1 (1) |
| Housing worker | 5 | 2 | 3 (3) | 3 | 2 | 1 (1) |
| Employment worker | 0 | 0 | 3 (3) | 1 | 1 | 1 (1) |
| Outpatients | 6 | 2 | 1 (1) | 4 | 2 | 1 (1) |
| A&E | 13 | 1 | 1 (1) | 13 | 1 | 0 (0) |
| Other | 0 | 0 | 0 (0) | 1 | 0 | 1 (1) |
| Hospital admissions | 8 | 5 | 1 (1) | 12 | 4 | 1 (1) |
| Variable | Mean (SD) | Missing (%) | Mean (SD) | Missing (%) | ||
| EQ-5D-5L | 0.88 (0.15) | 0 | 0.88 (0.14) | 0 | ||
| Contact | Intervention (N = 83) | Control (N = 75) | ||||
|---|---|---|---|---|---|---|
| Percentage reporting using service | Mean number of contacts/hospital admissions where number of contacts (admissions) > 0 | Missing cost data, n (% of those followed up) | Percentage reporting using service | Mean number of contacts/hospital admissions where number of contacts (admissions) > 0 | Missing cost data, n (% of those followed up) | |
| Total cost: community-based, outpatient and A&E service contacts | ||||||
| Midwife | 94 | 3 | 0 (0) | 92 | 3 | 0 (0) |
| Health visitor | 98 | 3 | 0 (0) | 93 | 4 | 0 (0) |
| GP | 93 | 4 | 0 (0) | 92 | 3 | 0 (0) |
| Community psychiatrist | 1 | 1 | 0 (0) | < 1 | 1 | 0 (0) |
| Community psychiatric nurse | 2 | 2 | 0 (0) | < 1 | 1 | 0 (0) |
| Practice nurse | 54 | 3 | 0 (0) | 41 | 3 | 0 (0) |
| Smoking cessation | 1 | 3 | 0 (0) | 0 | 0 | 0 (0) |
| Social worker | 4 | 4 | 0 (0) | 0 | 0 | 0 (0) |
| Housing worker | 10 | 2 | 0 (0) | < 1 | 2 | 0 (0) |
| Employment worker | 1 | 3 | 0 (0) | 0 | 0 | 0 (0) |
| Outpatients (mother) | 18 | 3 | 1 (1) | 23 | 2 | 0 (0) |
| Outpatients (infant) | 28 | 2 | 2 (2) | 17 | 2 | 1 (1) |
| A&E | 36 | 1 | 5 (6) | 30 | 1 | 1 (1) |
| Other | 12 | 5 | 1 (1) | 14 | 3 | 1 (1) |
| Hospital admissions (mother) | 11 | 3 | 12 (14) | 5 | 2 | 6 (8) |
| Hospital admissions (infant) | 14 | 13 | 8 (10) | 8 | 3 | 6 (8) |
| Variable | Mean (SD) | Missing (%) | Mean (SD) | Missing (%) | ||
| EQ-5D-5L | 0.94 (0.07) | 0 | 0.93 (0.09) | 0 | ||
| Contact | Intervention (N = 39) | Control (N = 71) | ||||
|---|---|---|---|---|---|---|
| Percentage reporting using service | Mean number of contacts/hospital admissions where number of contacts (admissions) > 0 | Missing cost data, n (% of those followed up) | Percentage reporting using service | Mean number of contacts/hospital admissions where number of contacts (admissions) > 0 | Missing cost data, n (% of those followed up) | |
| Total cost: community-based, outpatient and A&E service contacts | ||||||
| Midwife | 0 | 0 | 0 (0) | 0 | 0 | 0 (0) |
| Health visitor | 70 | 2 | 0 (0) | 63 | 2 | 0 (0) |
| GP | 84 | 3 | 0 (0) | 72 | 3 | 0 (0) |
| Community psychiatrist | 0 | 0 | 0 (0) | 1 | 2 | 0 (0) |
| Community psychiatric nurse | 0 | 0 | 0 (0) | 0 | 0 | 0 (0) |
| Practice nurse | 23 | 2 | 0 (0) | 34 | 1 | 0 (0) |
| Smoking cessation | 1 | 5 | 0 (0) | 0 | 0 | 0 (0) |
| Social worker | 3 | 2 | 0 (0) | 0 | 0 | 0 (0) |
| Housing worker | 7 | 2 | 0 (0) | 4 | 2 | 0 (0) |
| Employment worker | 0 | 0 | 0 (0) | 1 | 1 | 0 (0) |
| Outpatients (mother) | 16 | 3 | 2 (3) | 23 | 2 | 0 (0) |
| Outpatients (infant) | 19 | 2 | 2 (3) | 17 | 2 | 0 (0) |
| A&E | 30 | 1 | 1 (1) | 29 | 1 | 3 (4) |
| Other | 6 | 3 | 0 (0) | 6 | 3 | 0 (0) |
| Hospital admissions | ||||||
| Mother | 5 | –a | 4 (6) | 2 | 7b | 5 (7) |
| Infant | 19 | 5 | 4 (6) | 17 | 2 | 2 (3) |
| Variable | Mean (SD) | Missing (%) | Mean (SD) | Missing (%) | ||
| EQ-5D-5L | 0.94 (0.09) | 0 | 0.95 (0.08) | 0 | ||
| Contact | Arm | |
|---|---|---|
| Intervention (n = 69) | Control (n = 71) | |
| Cost (£), mean (SE) | Cost (£), mean (SE) | |
| Pregnancy-related community-based and outpatient service contacts | 1176 (68) | 1284 (98) |
| Non-pregnancy-related community-based, outpatient and A&E contacts | 68 (16) | 60 (15) |
| Hospital admissions | 107 (96) | 511 (247) |
List of abbreviations
- A&E
- accident and emergency
- AD-SUS
- Adult Service Use Schedule
- app
- software application
- BMI
- body mass index
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- COM-B
- capability, opportunity, motivation and behaviour
- CONSORT
- Consolidated Standards of Reporting Trials
- CPT
- Core Project Team
- DINE
- Dietary Instrument for Nutritional Education
- EGWG
- excessive gestational weight gain
- EPDS
- Edinburgh Postnatal Depression Scale
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- FCE
- finished consultant episode
- GP
- general practitioner
- HRA
- Health Research Authority
- ICER
- incremental cost-effectiveness ratio
- ID
- identifier
- IoM
- Institute of Medicine
- IPAQ-SF
- International Physical Activity Questionnaire – Short Form
- ITT
- intention to treat
- MET
- metabolic equivalent
- MRC
- Medical Research Council
- NCT
- National Childbirth Trust
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NRT
- nicotine replacement therapy
- OECD
- Organisation for Economic Co-operation and Development
- PHR
- Public Health Research
- PICO
- problem/population, intervention, comparison, outcomes
- PICOS
- problem/population, intervention, comparison, outcomes, study design
- PIN
- patient identifier number
- PIS
- patient information sheet
- POWeR+
- Positive Online Weight Reduction
- POWeR+F
- Positive Online Weight Reduction supplemented by face-to-face nurse support
- POWeR+R
- Positive Online Weight Reduction supplemented by remote nurse support
- PPI
- patient and public involvement
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RR
- risk ratio
- SAE
- serious adverse event
- SD
- standard deviation
- SE
- standard error
- SWAN
- Supporting Women with postnAtal weight maNagement
- TSC
- Trial Steering Committee
Notes
-
Letter to women who had a BMI score of ≥ 25 kg/m 2 at antenatal booking
-
Letter to women who had a normal BMI score at antenatal booking
-
Participant information sheet and consent form for women with a BMI score of ≥ 25 kg/m 2
-
Topic guide for interviews on completion of Slimming World offer
-
Participant information sheet and consent form: interviews at 12 months – all women
-
Participant information sheet and consent form: interviews following completion of Slimming World groups
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/phr08090).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
