Notes
Article history
The research reported in this issue of the journal was funded by the PHR programme as project number 15/05/28. The contractual start date was in October 2016. The final report began editorial review in December 2019 and was accepted for publication in April 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PHR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2021 Cockayne et al. This work was produced by Cockayne et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 The authors
Chapter 1 Introduction
Material throughout the report has been reproduced from Cockayne et al. 1,2 These are Open Access articles distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Slips, trips and falls on the same level (STFL) are a major contributor to the burden of workplace injury in Great Britain (GB; refers to England, Scotland and Wales). They are the most common cause of non-fatal injury to employees, accounting for around 30% of those injuries reported to GB’s Health and Safety Executive (HSE). 3 It is estimated that there are around 100,000 non-fatal workplace injuries due to STFL every year. 4
The consequences of work-related STFL are rarely fatal, but the resulting injuries are not always trivial. Nearly 40% of the STFL injuries reported to the HSE in 2017/18 were ‘specified’ injuries,5 which means that the injury appeared on a predefined list that tends to represent the more serious workplace injuries,6 with the vast majority being fractures. 7 Fractures can sometimes have long-lasting effects, such as nerve damage, decreased strength and increased discomfort around the site of the fracture. It is estimated that, on average, a worker takes around 9 days off work if injured by a workplace STFL, which equates to nearly 1 million working days lost through STFL injuries every year. 4
Furthermore, the workforce is ageing and the employment rate for those aged ≥ 65 years has doubled over the last 20 years. 8 Older workers are more susceptible to falls and tend to sustain more serious injuries when they occur. 9,10 In the workplace, normal age-related physiological factors, such as diminished balance, vision changes and hearing loss, can contribute to increased STFL work hazards. 11 Hence, workplace STFL are an increasing concern, particularly within the context of an ageing workforce.
Slips in the workplace and the role of slip-resistant footwear
Injury-reporting systems rarely differentiate between slips, trips and other causes of falls on the same level, but it is thought that slipping is a major contributor. Courtney et al. 12 reviewed six reporting systems in the USA, the UK and Sweden, and found that just three (in the USA and Sweden) provided sufficient information to attempt to isolate the contribution of slipping to the overall burden of STFL. The authors12 suggested that slipping contributed to between 40% and 50% of STFL-related injuries.
Workplace slips and their resulting falls happen for a number of reasons. Factors that can contribute to slips in the workplace include the floor surface, the presence and characteristics of any contamination of the floor (wet or dry), the cleaning regime, the level and type of pedestrian activity, footwear choice, the working environment (e.g. lighting or noise levels) and human factors (how people interact with their working environment). 13 A multitude of factors may be present at the same time and so it is rarely just one factor that causes a specific slip incident.
In the UK, employers have a duty to assess risks (including slip risk) and, when necessary, take action to address them. This is done through a risk assessment, when the employer considers what workplace risks may lead to slip injuries and decide what suitable and effective control measures could prevent them. In many instances, straightforward measures can readily control risks (e.g. ensuring that the floor surface is kept clean and dry or by installing a floor surface that is sufficiently slip resistant when contaminated). However, when it is not practicable to prevent the floor surface becoming slippery, employers may consider the use of slip-resistant footwear.
The choice of slip-resistant footwear is important and the selection of inappropriate footwear (i.e. with poor slip resistance) could potentially add to the problem rather than provide a solution. Choosing appropriate footwear can be difficult, as there is a lack of robust testing and reliable information to help guide the decision-making process.
It has been suggested that testing footwear under more lifelike conditions will provide a more accurate assessment of the slip-resistant properties of the footwear. 14,15 In response to this, and to help guide the selection process, the HSE developed the ‘GRIP’ rating scheme. 16 This scheme utilises a test regime designed to reflect the biomechanics of walking, and categorises footwear in accordance with the level of slip resistance it provides on a smooth surface contaminated with (1) water and (2) a 75% volume per volume glycerol solution (aqueous). ‘GRIP’ star ratings, ranging from one to five stars, are assigned to the footwear soling units on the basis of the results obtained, with five stars being awarded to those with the highest level of slip resistance. The risk assessment process can help guide the employer on which star rating is required for their workplace.
Current evidence relating to the use of slip-resistant footwear
A number of publications have described studies investigating the influence of footwear on slip risk using a range of laboratory tests. The evidence suggests that the level of slip resistance provided by different types of footwear varies considerably and that the effectiveness of footwear at preventing slips and falls depends on factors such as the footwear material, the tread design, the type of contamination and the characteristics of the floor. 17–19
The HSE has developed tests to accurately assess the slip resistance of footwear and has helped to inform the footwear selected by some companies that have subsequently seen a reduction in accidents and personal liability claims. For example, one case study20 showed how the test method could be used to remove inadequate footwear from use and identified alternatives. As a consequence, the risk of workplace accidents was reduced. 20 However, these are individual, non-peer-reviewed before-and-after case studies and the findings are not in the context of a randomised controlled trial (RCT).
There is, however, some evidence to support this suggestion in studies that have measured a reduction in slips occurring in the workplace by the use of appropriate slip-resistant footwear. Bell et al. 21 evaluated a comprehensive slip, trip and fall prevention programme for hospital employees in the USA. The programme included slip-resistant footwear for certain employee subgroups, but also included many other prevention strategies, such as hazard assessments, changes to housekeeping, general awareness campaigns and flooring changes. The study suggested that the programme reduced workers’ compensation claims for slips, trips and falls by 58%, but it was not possible to disentangle the specific effect of slip-resistant footwear from the other preventative interventions. 21
An uncontrolled before-and-after study among fishermen in Denmark suggested that slip-resistant boots led to a reduction in self-reported slips, trips and falls. 22 In contrast, a cross-sectional study23 of 125 fast-food restaurant workers in the USA did not find an association between slipping and use of footwear perceived to be slip resistant. The authors suggested that the null finding could have been due to the method of classifying footwear as slip resistant (through visual inspection of the tread), possibly leading to misclassification and the potential for reverse causality (people who had experienced a slip or fall in the past being more likely to wear slip-resistant footwear).
More recently, in the USA, a prospective cohort study24 of 475 fast-food restaurant workers found that the use of slip-resistant footwear was associated with a 54% reduction in the reported rate of slipping. A case-crossover study nested within the same cohort found that rushing and walking increased the rate of slipping, but that use of slip-resistant footwear reduced the effects of rushing and walking on slip risk when on a contaminated floor. 25 Further analysis of the cohort indicated that slip-resistant shoes worn for < 6 months were marginally more effective at preventing slips than slip-resistant shoes worn for > 6 months. 26
We are aware of only one RCT27 assessing the effectiveness of slip-resistant footwear. This cluster randomised trial of food service workers from 226 school districts in the USA found that the probability of a slipping injury fell by 67% in the intervention group, who received slip-resistant footwear. By contrast, the control group (who wore slip-resistant footwear that they purchased themselves in accordance with company policy) saw a slight increase in the probability of slipping injury during the follow-up period.
Overall, there seems to be promising evidence that slip-resistant footwear can substantially reduce the burden of accidents at work, but much of this evidence has been gathered for footwear with slip-resistant properties that are ill-defined. It was therefore considered important to test the clinical effectiveness and cost-effectiveness of appropriately specified slip-resistant footwear in a large pragmatic trial within a UK setting.
Trial setting
It is estimated that the health and social care sector employs 1 in 10 of the working population28 and has one of the highest numbers of non-fatal STFL compared with other industries in GB. It is estimated that every year around 14,000 workers in this industry sector are injured as a result of STFL [Health and Safety Executive. Estimated Incidence of All Self-Reported Workplace Non-Fatal Injury Resulting in a Slip, Trip or Fall on the Same Level, Sustained in Current or Most Recent Job, By Industry, for People Working in the Last 12 Months, Averaged 2014/15–2016/17 (Unpublished data). London: Labour Force Survey; 2018].
The NHS is the biggest employer in the sector and the fifth biggest employer in the world. 29 NHS sites, particularly hospitals, present challenging working environments with respect to slip risk. For example, the floor is generally smooth and not carpeted to aid regular cleaning, there are multiple sources and types of contamination, there is a relatively high level of pedestrian activity and of varied types (e.g. walking, pushing and pulling) and people frequently walk at speed, often multitasking or focused on a more critical objective than the environment around them. Many large hospital sites are a conglomeration of buildings and so people often switch between indoor and outdoor environments, and community staff are mainly offsite with no control over their surrounding environment. In such circumstances, it would seem potentially beneficial for staff to consider wearing slip-resistant footwear.
It is for these reasons that NHS staff were considered to be a good population on which to base research into the potential effectiveness of appropriately specified (in this study, 5-star, GRIP-rated) slip-resistant footwear. In addition, the NHS includes different working subpopulations (e.g. nursing staff, kitchen staff, porters and people who work in the community). This will help to generalise any research to other industries that share many of the same risk factors, such as retail, hospitality, education and manufacturing, and maximise the impact of the research. One other potential benefit of undertaking the trial within the NHS is that its staff are generally used to the research environment although it is usually patients rather than the staff who take part in research. However, it seemed likely that, for this reason, participation in this research would be higher among NHS staff than among those of other employers.
Objectives
Primary objective
The primary objective of this research was to assess whether or not the offer and provision of 5-star, GRIP-rated, slip-resistant footwear to NHS employees working in general, clinical or catering areas leads to a reduction in the incidence rate of self-reported slips.
Secondary objectives
The secondary objectives of this study were to:
-
undertake an internal pilot RCT to (1) check the feasibility of the study, including if it is possible to recruit, randomise and follow up 800 participants; (2) check the sample size calculation assumptions and the attrition rate; and (3) explore and address any issues regarding footwear compliance
-
assess if the provision and use of 5-star, GRIP-rated footwear leads to a reduction in slips and is cost-effective
-
disseminate the findings of this study using the HSE, NHS trust managers and health and safety managers (in addition to publishing the results of the study in key journals and in this Public Health Research monograph).
Chapter 2 Methods
Study design
The Stopping Slips among Health-care Workers (SSHeW) trial was a pragmatic, two-arm, open RCT with an internal pilot trial, economic evaluation and qualitative study. Participants were randomised to either the intervention group and were offered a pair of 5-star, GRIP-rated, slip-resistant shoes to wear at work (free of charge to themselves), or to the waiting list control group and were asked to wear their usual work footwear for the trial period and offered a pair of the slip-resistant shoes at the end of their participation in the trial (free of charge to themselves). The trial included an economic evaluation (see Chapter 4) and a nested qualitative study to explore the experiences of participants in the trial (see Chapter 5). The SSHeW trial also served as a host trial for an embedded study within a trial (SWAT) to evaluate the effectiveness, on response rate, of enclosing a pen with the 14-week post-randomisation postal follow-up questionnaire.
Ethics approval and research governance
Ethics approval for the study was obtained from the University of York, Department of Health Sciences Research Governance Committee (DHSRGC), on 28 November 2016. The study was approved by the Health Research Authority (HRA) on 7 March 2017 (HRA reference number 17/HRA/0435). The HRA did not require Research Ethics Committee approval for the study, as the participants were NHS staff (as opposed to NHS patients). Approval and ‘confirmation of capacity and capability’ was obtained for each participating trust prior to commencement of the trial at that site (see Appendix 1). Substantial amendments to the protocol were submitted to the DHSRGC, the HRA and each site’s research and development office during the course of this study.
The trial was registered with the International Standard Randomised Controlled Trial Number registry as ISRCTN33051393. The trial protocol is published. 1
Setting
Recruitment of participants took place in seven NHS trusts in England. The participating trusts ranged from large acute hospital trusts to smaller trusts, and included district general hospitals serving local communities, a trust that provided mental health services and a community trust.
Recruitment procedure
Recruitment of participants into the study took place in multiple sites within participating NHS trusts, facilitated by members of the research and development teams, and assisted by members of the HSE team and University of York researchers in some sites. Sites used a variety of recruitment strategies to recruit participants, which were developed during the pilot phase of the study. These included holding ‘shoe shops’ (where interested staff could learn more about the study, and see and try on samples of 5-star, GRIP-rated, slip-resistant footwear) and stands promoting the trial in convenient hospital locations, such as reception foyers or the canteen, or at staff conferences or induction days. Other recruitment activities included promotion of the trial via posters, information on the staff intranet, screensavers and weekly staff bulletins. Direct staff recruitment occurred by members of the research team attending staff meetings and ward areas, particularly at shift handover time, and via word of mouth as participants discussed the trial with colleagues who then approached research staff to discuss participation.
At recruitment, research staff provided information about the study and demonstrated the 5-star, GRIP-rated, slip-resistant shoes being tested in the trial. Sample styles and sizes were available for interested staff to try on. Research staff briefly assessed eligibility of interested staff by asking if they were employed by the recruiting trust, their area of work and the average number of hours they worked per week, before giving them a recruitment pack. The recruitment pack contained an invitation letter from the principal investigator at the NHS trust (see Report Supplementary Material 1), a participant information sheet, which included contact details for the research team should they have had any queries about the study (see Report Supplementary Material 2), a flier with pictures of the shoe styles being offered at the trust (see Appendix 2 for an example of this document offered at one trust), a baseline questionnaire including more detail on the eligibility criteria (see Appendix 3), a consent form (see Report Supplementary Material 3) and a prepaid envelope for returning the completed paperwork.
Consent process
Participation in the SSHeW trial was voluntary. Participants who wished to take part in the trial after receiving information about the trial from a member of the research team and/or reading the participant information sheet provided in the recruitment pack were asked to complete the consent form by initialling that they had read the items listed, and signing and dating the form.
The consent form also asked if participants were willing to take part in a telephone interview about the study (for the qualitative component of the study, further consent was obtained from the participant by post prior to undertaking a telephone interview). Participation in the SSHeW trial was not affected if the member of staff did not consent to participate in a telephone interview.
Baseline assessment
On receipt of completed written consent, researchers at the York Trials Unit (YTU) assessed the participants’ responses to the baseline questionnaires for eligibility in accordance with the following criteria.
Participant eligibility
Inclusion criteria
-
Aged ≥ 18 years.
-
Employed by (named) participating NHS trust.
-
Were required to adhere to a dress code.
-
Worked in a clinical, general or catering area.
-
Worked at least 30 hours per week [80% working-time equivalent (WTE)] (reduced mid-way through the trial to 22.5 hours/week or 60% WTE).
-
Had a mobile phone and were willing to receive and send text messages for trial data collection.
Exclusion criteria
-
Not employed by the participating NHS trust.
-
Did not have a mobile phone, or were unwilling or unable to receive and send text messages.
-
Were provided with, and required to wear, protective footwear by their employer (e.g. steel toe-capped boots).
-
Were agency staff or staff with < 6 months remaining on their employment contract.
-
Worked < 60% WTE (22.5 hours/week).
-
Were predominantly office or theatre based.
Note that the eligibility criterion with respect to the number of working hours was changed from the initial requirement of working, on average, at least 80% WTE (equivalent to 30 hours/week) to working, on average, at least 60% WTE (equivalent to 22.5 hours/week) (protocol version 5.0, dated 26 April 2018 and approved 2 September 2018). This change was made to facilitate the recruitment of participants to meet the required sample size. Participating sites had saturated the eligible workforce and found that the number of hours worked was the predominant factor limiting others from taking part. The required working hours were dropped to no lower than 22.5 hours to ensure that participants were working a sufficient amount of time to (1) provide a sufficient work-related slip rate and (2) retain the ability to detect a reduction in this slip rate. It was felt that if we reduced the working hours further then we would not have been able to do this.
Participants who were assessed as being ineligible for the study were notified in writing and no further correspondence was sent. Participants who were deemed eligible were entered into the trial and sent a welcome text message that said:
Welcome to the SSHeW trial. We very much value your agreement to participate. You will shortly start to receive text messages asking about any slips you have at work. These texts will always come from this number and will begin with the word SSHeW so that you can recognise them. Thank you.
All participants who were eligible to take part were sent a copy of their signed consent form and a paper diary enabling them to record slips, falls and injuries when they occurred, and any time off work as a result (see Appendix 4). This was intended as a memory aid to help participants complete the text message responses and the 14-week questionnaire.
Pre-randomisation phase
After the welcome message was sent, all eligible participants were sent a text message each Sunday at 18.30 for 4 weeks, unless they requested that these be stopped. We chose to send text messages at this time as we anticipated that fewer participants would be at work, which would lead to higher response rates. This message read:
SSHeW trial: How many slips did you have at work between [DD/MM/20YY] and [DD/MM/20YY]? Please provide a single number (e.g. 2) or 0 if you did not slip. Thank you.
The dates were populated with the previous Monday and the current date. Those who provided a valid response (a numerical value or written number, rather than a written text response or other message, such as a symbol) to at least two of the slip data collection text messages were randomised into the trial.
It became apparent during the trial pilot phase that some eligible participants had misunderstood the trial process. Instead of providing slip data in response to their text messages, they reported not having received their trial shoes as they mistakenly thought that they needed to report slip data only once they had received their trial shoes. Therefore, the protocol was amended (substantial amendment 6, approved 20 June 2018) to allow two additional text messages to be sent to participants who had not responded to their pre-randomisation text messages, along with a reminder that they needed to reply to these messages if they wished to be included in the trial.
Randomisation
Participants who fulfilled the eligibility criteria, provided written consent to take part in the study, completed a baseline questionnaire and returned at least two of the weekly text messages providing slip data were eligible for randomisation. Participants were randomised using the YTU’s secure web-based randomisation system based on an allocation sequence generated by an independent data systems manager at the YTU. The systems manager was not involved in the recruitment of participants. Block randomisation, stratified by trust, was used with variable block sizes. Participants were randomised at a particular site in batches of 1 : 1 between the intervention and control group (depending on when sites had capacity to order and deliver footwear). The block size was equal to the number of participants to be randomised in each ‘batch’. Participants were notified of their group allocation by letter from the YTU.
Blinding of trial participants to group allocation was not possible because of the nature of the intervention. Members of the study team, including the statistician, health economist and those involved in the administration of the study, were not blinded to group allocation.
Group allocation
Intervention
Individuals allocated to the intervention arm were offered a pair of 5-star, GRIP-rated, slip-resistant shoes. The footwear was provided by a single manufacturer [Shoes For Crews (Europe) Ltd] and the cost covered by the NHS trust. A range of intervention shoes were offered as part of the trial. The shoes included a variety of styles, to suit different people and job roles, including dress shoes, casual styles and ‘trainer’-type designs. The shoes had either a water-resistant leather or synthetic mesh upper (the synthetic materials being vegan). Each individual trust reviewed the footwear available in terms of style; to offer a range of footwear representative of that already worn by staff; health and safety issues, for example potential for needlestick injuries; potential infection control issues; and cost. They then produced a list of footwear which was deemed suitable for staff at their trust to wear. All participants were asked to indicate on their baseline questionnaire, prior to randomisation, which style and size of trial shoe they would like to wear. This facilitated swift ordering of the shoes by the trust for participants subsequently randomised to the intervention group. The indicated shoes were ordered for the participant, who was then provided with instructions about how to collect their trial shoes. Further information was provided to participants about how to exchange their shoes if required. (Participants were able to exchange their shoes if they were found to be unsuitable, for example incorrect size, as long as the shoes were unworn and returned in their original box.) We had hoped that this process would facilitate shoes being available for intervention participants to collect no later than 2 weeks after randomisation; however, we know that in some sites, and at certain times, there were delays in ordering and/or delivery of the trial shoes. In addition, we know that not all intervention participants collected their shoes if they were delivered to a ‘collection point’ at the hospital, and those that did may not have done so in a timely manner. More information on receipt, and timing of receipt, of trial footwear is provided in Chapter 3, Receipt of shoes.
The shoes used in the trial had been assigned a 5-star GRIP rating from the HSE. The GRIP rating scheme was developed by the HSE to allow footwear users to identify slip-resistant footwear to help prevent slipping accidents. During GRIP-rating assessment, the soling of footwear is tested under very challenging conditions (i.e. a smooth surface contaminated first with water and then with a glycerol solution). Slip resistance of shoes is rated one to five stars on the basis of the results, with five stars being awarded to the highest performing footwear. The shoes used as the intervention in the trial had a rubber sole with an intricate tread pattern that provided higher levels of slip resistance than most other types of footwear on surfaces that are contaminated with non-clogging substances (Figure 1). This level of slip resistance is achieved by the soles providing a relatively large surface contact area, while also effectively dispersing any surface contamination. When walking surfaces are clean and dry the trial footwear would be expected to behave like any other footwear; however, their slip resistance is not compromised to the same extent as other footwear when walking on surfaces that are contaminated.
FIGURE 1.
Example of sole of intervention footwear. Photograph received and reproduced with permission from Shoes for Crews (Europe) Ltd.

Control
Participants allocated to the control arm were asked to continue to wear their usual work shoes for the 14-week trial period. They were informed that they would receive a free pair of 5-star, GRIP-rated, slip-resistant shoes at the end of the 14-week trial period, the size and style of which they indicated on their baseline questionnaire. The footwear was provided by a single manufacturer (Shoes For Crews Ltd) and the cost covered by the NHS trust. Control group participants were also sent a copy of their signed consent form and a slip diary, the same as the intervention group, at the start of their participation in the trial.
Participant follow-up
All participants in the SSHeW trial were sent weekly text messages for 14 weeks post randomisation to collect slip data (i.e. the number of slips the participant had experienced at work in the past week). As with the pre-randomisation messages, these were sent on a Sunday evening (18.30) and the wording was the same, with dates inserted relative to the past week:
SSHeW trial. How many slips did you have at work between [DD/MM/20YY] and [DD/MM/20YY]? Please provide a single number (e.g. 2) or 0 if you did not slip. Thank you.
These messages started, for all participants, the first Sunday after they were randomised, regardless of whether or not the intervention participants had received their shoes.
Non-responders did not receive reminder text messages, as there was limited time to send these because of the short time frame of 1 week before the next text message was sent. Participants were also sent a group-specific postal questionnaire at 14 weeks post randomisation to collect data on slips, falls, injuries, time off work, health-care resource use, if participants were worried about slipping and falling, how often they had worn the trial shoes while at work and if participants had any views on trial shoes (intervention group only) (see Appendices 5 and 6). Participants who did not return this questionnaire within 3 weeks were sent a postal reminder. Participants who reported a slip in their weekly text response were sent a questionnaire for further details regarding their first reported slip, including whether or not they were wearing the trial shoes when they slipped, any resultant injury, health service use and time off work. Furthermore, participants reporting an injury were sent an injury follow-up questionnaire.
Slip data collection sheet
On reporting their first slip via a weekly post-randomisation text message, participants were posted a questionnaire asking for further details about that slip, with reference to the week in which the slip occurred (see Appendix 7). This collected information about the date of the slip, whether or not the slip resulted in a fall, any consequence of the slip (such as superficial or other injury), whether or not the participant sought health-care advice and if the participant required any time off work. The questionnaire also included the EuroQol-5 Dimensions, five-level version (EQ-5D-5L), health-related quality-of-life (HRQoL) measure. 30 A reminder letter and further copy of the questionnaire was sent to those participants who had not returned the questionnaire after 2 weeks. Initially, data were going to be collected over the telephone, rather than by post, for each slip that occurred; however, it quickly became apparent that this would place too great a burden on the participant and study team.
Injury follow-up
An injury follow-up questionnaire was sent when a trial participant reported an injury. A participant may have reported an injury via one of several means: by text message, direct telephone call or e-mail to either a member of the trial team at the YTU or a member of the research team at participating sites, reported on a returned slip data collection sheet (SDCS) or reported on the 14-week questionnaire. If the participant reported an injury between randomisation and the 14-week questionnaire, an injury questionnaire was sent asking for EQ-5D-5L data, whether or not the participant had fully recovered from the injury and the date they recovered (see Appendix 8). If the participant had not fully recovered from the injury, a further injury follow-up questionnaire was sent 1 month later. This continued until the injury was resolved, the participant no longer wished to be contacted or the trial ended. If the participant reported an injury after completion of the 14-week questionnaire then they were also sent an injury follow-up questionnaire (see Appendix 9). This questionnaire collected the same data as the injury form sent out during the trial (i.e. EQ-5D-5L scores and details of any time required off work) and also included questions asking about health-care services used for the injury after the 14-week trial period. This continued until the injury was resolved, the participant no longer wished to be contacted or the trial ended.
Compliance text message follow-up
In addition to the weekly text messages collecting data on the number of slips per week, participants in the intervention arm of the trial were sent a text message at 6, 10 and 14 weeks post randomisation to gather information about how often the participant was wearing their trial shoes:
SSHeW trial. In the past month, how often have you worn your trial shoes at work? Reply 0, 1 or 2 (0 = none of the time, 1 = some of the time, 2 = all of the time).
Wear testing
The soles of all footwear degrade over time and so it was important to have a concept of how long the slip resistance of the trial footwear lasted in a real-world setting. This information could help determine any replacement schedule, and results from this fed into the health economic analysis for the trial.
To assess the serviceable life of the intervention footwear, we asked eligible participants from three trusts who had continued to wear their trial shoes beyond the trial period to return them for assessment. Participants from the intervention group who had been in possession of the trial footwear for 6, 9 or 12 months since randomisation, and had reported wearing the footwear either all of the time or most of the time in response to the 14-week questionnaire, were contacted to establish if they had continued to wear the footwear. Those who had were then asked if they would be willing to return their trial footwear in exchange for a new pair. The exchange of footwear was co-ordinated by the participating trusts.
The soles of each pair of footwear were cleaned, rinsed and allowed to dry prior to the assessment. A visual inspection was carried out to assess the condition of the footwear and identify any defects. Each pair of footwear was inspected by one member of the HSE research team and a HSE colleague who was not involved in the study to provide layperson corroboration. The condition of the upper was rated as good, reasonable or poor, and any defects were recorded. The depth of tread was measured to determine the approximate proportion of sole area that had tread depth below the 2 mm minimum recommended by the manufacturer.
The slip resistance of the worn footwear was then tested on the HSE’s simulated slip test31 and the results compared with those generated for the same styles of footwear when new. Each pair was tested in line with the conditions set out in the HSE GRIP scheme handbook. 32
Outcomes
Primary outcome
Parts of this text have been reproduced with permission from Cockayne et al. 2 This is an Open Access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
The primary outcome in this study was the incidence rate of self-reported slips, not necessarily resulting in a fall or injury, in the workplace over a 14-week period, as reported via weekly text messages. A slip was defined as ‘a loss of traction of your foot on the floor surface, which may or may not result in a fall’. A fall was defined as ‘an unexpected event in which you come to rest on the ground, floor or lower level’.
Participants were also asked on the 14-week questionnaire, ‘How many times did you slip (with or without falling) while at work in the past 14 weeks?’. Responses to this question were used when the participant did not provide any text message data.
Secondary outcomes
-
The incidence rate of falls resulting from a slip in the workplace over 14 weeks (14-week questionnaire).
-
The incidence rate of falls not resulting from a slip in the workplace over 14 weeks (14-week questionnaire).
-
Proportion of participants who reported a slip over 14 weeks (weekly slip text message and 14-week questionnaire).
-
Proportion of participants who reported a fall (whether or not resulting from a slip) over 14 weeks (14-week questionnaire and SDCS).
-
Proportion of participants who reported a fracture over 14 weeks (SDCS).
-
Time to first slip (number of days between randomisation and date of first slip, as reported via text message).
-
Time to first fall (number of days between randomisation and date of first fall, as reported on the 14-week questionnaire or SDCS).
-
Health-related utility (measured by the EQ-5D-5L) and cost-effectiveness.
Sample size
Parts of this text have been reproduced with permission from Cockayne et al. 2 This is an Open Access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
There were limited published data on which to base a sample size calculation for this trial. A prospective cohort study24 found that 49 of 422 (11.6%) workers in a restaurant setting in the USA reported at least one ‘major’ (i.e. resulting in a fall and/or injury) slip over a 12-week follow-up period. The exact number who experienced any type of slip was not reported in that study, but could reasonably be expected to be higher than this. For our sample size calculation, we required an estimate of the proportion of individuals in the control group who would experience at least one slip over a 14-week follow-up period; we conservatively assumed a proportion of 10%. We proposed to randomise 4400 participants using 1 : 1 allocation (i.e. 2200 per group) to have 90% power to show a 30% relative reduction in the proportion of participants who report at least one slip over a 14-week period (a 3-percentage-point absolute reduction from 10% to 7%), allowing for 20% attrition. This sample size would also give us 80% power to see an absolute reduction of 2 percentage points in the risk of falls, from 5.5% to 3.5%, allowing for 20% attrition. Although we based the sample size calculation on detecting a difference in proportions, the primary outcome is the incidence rate of slips over the 14 weeks and so we used a mixed-effects negative binomial regression model to compare this outcome between the two groups. As this analysis uses more information than a simple binary outcome, we expected it to still be adequately powered.
Trial completion and exit
Participants completed the trial if they completed the 14-week trial period post randomisation. Participants exited the trial if they had completed the 14-week follow-up period, had fully withdrawn from the trial (no further follow-up by text or post), were lost to follow-up or had died.
Participant change of status and participant withdrawal
Participants could withdraw from the trial at any time without giving a reason. If, however, a reason was provided, then it was recorded. If we were informed that a participant wanted to withdraw from the trial, a member of the research team would attempt to clarify to what extent they wished to withdraw (i.e. fully or partially). Participants could partially withdraw from the trial in two ways; first, by withdrawing from the intervention only, meaning that they would not wear the trial shoes but continue to provide text and questionnaire data; and, second, participants could withdraw from data collection either by declining to respond to text messages or by declining to complete the follow-up questionnaires. Data were retained for withdrawn participants unless a participant requested specifically that their data be removed. A change of circumstance form was completed by a member of the research team for any participant changing status during the trial (see Report Supplementary Material 4).
Adverse events
Adverse events (AEs) were reported by participants to the research team at the YTU via telephone or text message or by writing free-text comments in the postal questionnaires. AEs could also be reported through the local research teams at the participating sites. Details of AEs were recorded on a SSHeW AE form (see Report Supplementary Material 5). The AE reporting period started at the point when the participant gave their consent to be in the trial and ended at 14 weeks after they were randomised.
Serious AEs that were related to being in the study and were unexpected were recorded. Non-serious AEs were not recorded, unless they were related to being in the study or were related to the intervention.
A serious AE was defined as any untoward occurrence that:
-
resulted in death
-
was life-threatening
-
required hospitalisation or prolongation of existing hospitalisation
-
consisted of a congenital anomaly or birth defect
-
was otherwise considered medically significant by the investigator.
Expected events
Slips and falls were not recorded as AEs in this trial, as these were recorded as trial outcomes. It was expected that some participants could experience foot problems associated with the new footwear. These could range from minor superficial problems associated with skin irritation and pressure, such as blisters or calluses, to more mechanical foot complaints, such as plantar fasciitis and tendonitis. In severe cases, when shoe styles were not suited to the participant’s foot shape, changes or exacerbation of foot structure, such as toe deformities, could be expected.
The occurrence of AEs during the trial was reported and monitored by the combined Trial Steering Committee (TSC) and Data Monitoring and Ethics Committee (DMEC). The TSC and DMEC were immediately sent all serious AEs that were thought to be related to treatment.
The SSHeW internal pilot trial
An internal pilot trial was conducted during the first 6 months of the study (March–September 2017). The objectives of the pilot trial were to:
-
test and refine recruitment strategies for the study
-
check the sample size calculation assumptions by reviewing the proportion of participants in the control group who experienced a slip
-
check the attrition rate
-
explore and address any issues regarding footwear compliance.
To progress to the main SSHeW trial, the pilot phase had to meet the following criteria:
-
at least 400 participants recruited in 6 months
-
80% of participants contributing at least 50% of the requested follow-up text data (i.e. responding to 7 of the 14 weekly post-randomisation text messages)
-
90% of participants responding to at least one post-randomisation text message
-
a slip rate in the control group of at least 7%.
Data analysis
Analysis
There were two analyses: (1) a descriptive analysis of the internal pilot data and (2) a single effectiveness analysis of the trial data at the end of follow-up of all participants. All analyses were conducted in Stata v15 (StataCorp LP, College Station, TX, USA) and followed the principles of intention to treat, with participants’ outcomes analysed in accordance with their original randomised group, irrespective of deviations based on non-compliance. Statistical tests are two-sided at the 5% significance level and 95% confidence intervals (CIs) are used.
The trial is reported in accordance with the Consolidated Standards of Reporting Trials (CONSORT) guidelines for parallel-group, randomised trials [URL: www.consort-statement.org/ (accessed 19 May 2020)]. The flow of participants through each stage of the trial, including reasons for non-eligibility, is presented in a CONSORT diagram (see Figure 2).
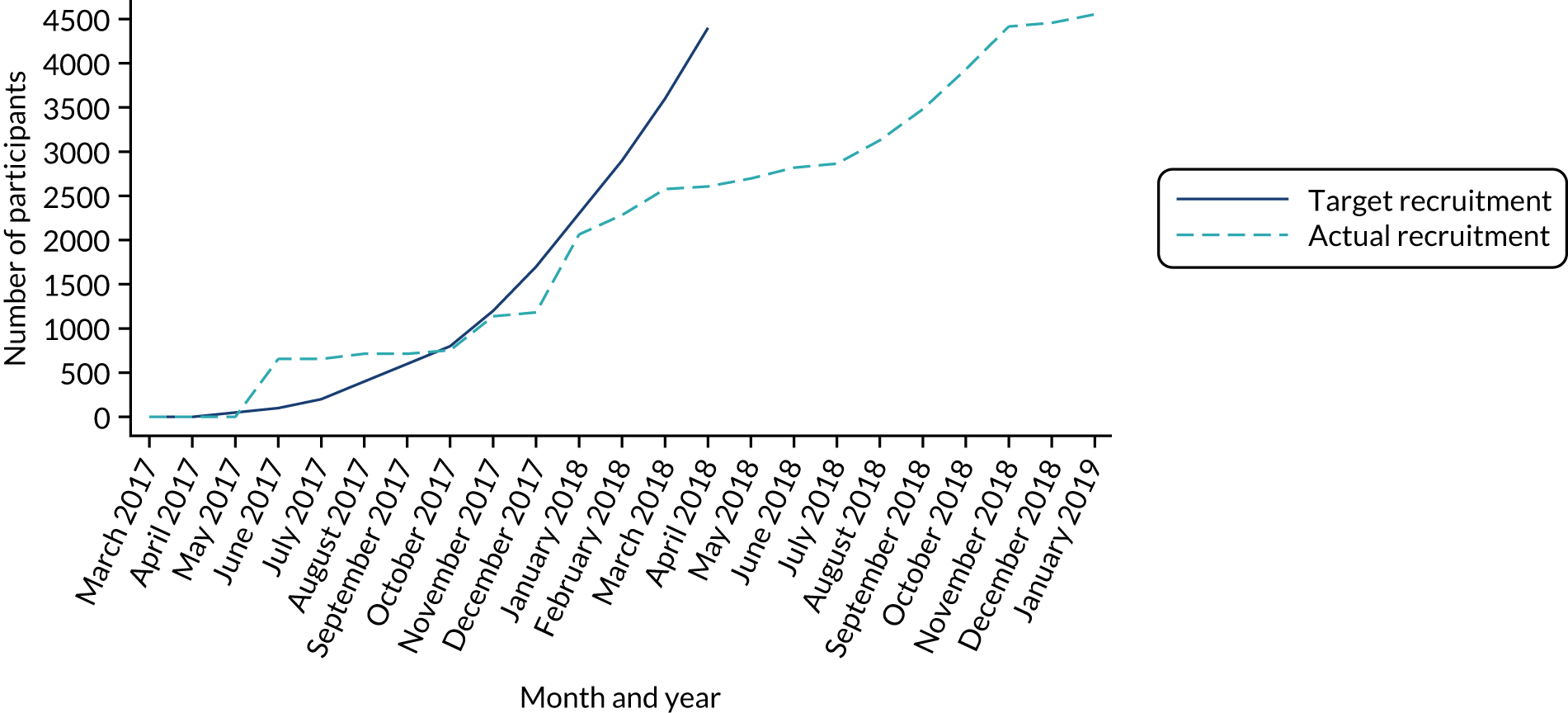
Figure 3 presents the overall recruitment by month, and the actual compared with target recruitment.
Follow-up response rates to the weekly text messages (both pre and post randomisation), the compliance text messages (for the intervention group only), SDCSs and the 14-week participant questionnaire (including time to response) are summarised overall and by treatment group when appropriate.
The number of intervention participants receiving a pair of trial shoes and the time taken from randomisation to receiving the shoes is summarised. The date provided by the participant on their 14-week questionnaire was the primary source of data for the date when shoes were received, with the date provided by the sites used, when available, if participants did not provide a date. We primarily used the participant-reported date, as the date provided by the site may be the date the shoes were delivered to the shoe collection point for the participant and not necessarily the date the participant collected the shoes, which could potentially have been significantly later.
Withdrawal
Type and timing of withdrawals are presented overall and by randomised group, with reasons when available.
Baseline data
Baseline data are summarised descriptively overall and by randomised arm. Continuous measures are reported using descriptive statistics [mean, standard deviation (SD), median, minimum and maximum], whereas categorical data are reported as counts and percentages. No formal statistical comparisons of baseline data were undertaken between the trial arms. Comparisons are made based on visual observation only.
Primary analysis
The difference in slip rate between the intervention and control groups over 14 weeks was analysed using a mixed-effect negative binomial regression model, adjusting for gender (three categories: male; female; prefer not to say or missing), age at enrolment (continuous) and job role [four categories: administration and information technology (administrator/receptionist/secretarial, ward clerk); facilities workers (catering, porter); direct patient care (doctor/consultant, qualified nurse/midwife, physiotherapist, occupational therapist, health-care assistant, pharmacist/pharmacy technician, social worker, support worker, podiatrist, other qualified staff/health-care professional); other/missing (imaging, other)], and baseline weekly slip rate ascertained from the pre-randomisation text message responses as fixed effects. Trial site was included as a random effect to account for potential clustering by recruitment site. The total number of hours that participants worked in the weeks for which they provided slip data was calculated (by multiplying the number of weeks the participant provided valid post-randomisation text message slip data by the number of hours they indicated they worked, on average, per week on the baseline questionnaire). This amount of time was accounted for in the negative binomial model (using the exposure option in Stata).
The incidence rate ratio (IRR) and associated 95% CI and p-value for the treatment effect are presented.
This analysis primarily included slip data from the weekly text message responses. When no post-randomisation responses were returned, data from the 14-week questionnaire were used for a participant, when available.
The primary analysis included all participants who had full baseline covariate data. The model could accommodate participants who did not provide a valid response to any weekly text messages or the 14-week questionnaire by considering that they reported zero slips over a negligible exposure time of 0.1 hours.
The primary analysis was checked by a second statistician (GF), who reviewed the analysis syntax, the derivation of variables required for the analysis, and the reporting and interpretation of the results.
Sensitivity analysis
Missing data
In our prespecified analysis plan, we said that if > 5% of randomised participants did not provide any slip data then, to account for possible selection bias in the primary analysis, a mixed-effect logistic regression would be run to predict non-response (no post-randomisation slip text message responses or 14-week questionnaire received), including all baseline variables collected prior to randomisation and trial site as a random effect. The primary analysis would then have been repeated including as covariates all variables found to be significantly predictive of non-response, to determine if this affected the parameter estimates. However, as < 5% of randomised participants did not provide slip data, this analysis was not performed.
Intervention compliance
Information on how often participants reported wearing their shoes, as recorded for the intervention group in the compliance text messages at 6, 10 and 14 weeks post randomisation and on the 14-week questionnaire, are summarised. Differences between responses provided via the different methods are discussed.
Complier-average causal effect (CACE) analysis to assess the impact of non-compliance on treatment estimates was undertaken. CACE analysis allows an unbiased treatment estimate of, in this case, the IRR of slips between the two groups in the presence of non-compliance with the shoes to be obtained. It is less prone to biased estimates than the more commonly used approaches of per-protocol or ‘on-treatment’ analysis, as it preserves the original randomisation. A two-stage instrumental variable approach was used, with randomised group as the instrumental variable.
The difficulty with this type of analysis is precisely how ‘compliance with the intervention’ is defined and quantified. This requires identifying a measure of how well a participant followed the intended elements of the intervention that can be applied to all participants. In this scenario, the intervention was the offer and provision of the trial slip-resistant footwear within the follow-up period. ‘Compliance’ was therefore defined, initially, in two very simplistic ways in separate compliance analyses, receiving the trial shoes:
-
during the first 7 weeks of the follow-up period
-
within the 14-week follow-up period.
However, these analyses do not account for how often the shoes were actually worn. A third and more complex definition of exposure to the trial shoes was therefore defined based on receiving the shoes and the responses to the 6-, 10- and 14-week compliance text messages (and response to the compliance question on the 14-week questionnaire when compliance text responses were missing):
-
A score between 0 and 12 was calculated based on the number of weeks the participant had the shoes, assigning a score of 0 for each week that, according to the participants, the shoes were worn ‘none of the time’, a score of 0.5 for each week the shoes were worn for ‘some of the time’ and a score of 1 for each week the shoes were worn ‘all of the time’ (this analysis allows for 2 weeks immediately after randomisation for participants to receive their shoes).
The CACE analysis was repeated using this continuous ‘compliance score’.
Subgroup analysis
We considered whether or not the intervention effect differed by gender and area of work by repeating the primary analysis and by including the factor and an interaction term between the factor and group allocation in the model. Area of work was categorised as areas with higher risk of spillages or contamination (food preparation areas, clinical rooms/areas, wards, indoor hospital grounds/corridors, theatres, laboratories) and areas with lower risk (community, pharmacy, office, podiatry).
Secondary analysis
All secondary outcomes and other important collected data (including data from the SDCSs) are summarised descriptively overall and by trial arm.
The IRR of falls (both resulting and not resulting from a slip) over 14 weeks was analysed in the same way as described for the primary outcome of slips (not including the exposure option in the model as the follow-up is time fixed).
The following outcomes were analysed using mixed-effects logistic regression, adjusting for the same fixed-effect covariates as the primary analysis, with NHS trust as a random effect. The first outcome was the proportion of participants who slipped at least once over 14 weeks (two separate analyses: one according to weekly slip text message data or 14-week questionnaire if no text message data were provided, and one using only 14-week questionnaire data), and the second was the proportion of participants who fell at least once during the 14 weeks. The number of participants who reported a fracture was very small and so no formal analyses of this outcome were undertaken (see Chapter 4). Odds ratios (ORs) and their associated 95% CIs are provided.
Time to first slip and the time to first fall were calculated. Participants who did not report a slip were treated as censored at their date of trial exit (completion of follow-up or withdrawal). The proportion of participants yet to experience a slip is summarised by a Kaplan–Meier survival curve for each group. Time to first slip was analysed using Cox proportional hazards regression with shared centre frailty and adjusting for the same covariates as in the primary analysis model. Hazard ratios (HRs) and their associated 95% CIs are provided. The proportional hazards assumption was evaluated using Schoenfeld residuals. Time to first fall was not analysed via a Cox proportional hazards regression model for two reasons: (1) relatively few falls were reported and (2) the date of the fall was poorly reported for any falls that were reported.
Adverse events
Adverse events are summarised descriptively by treatment arm.
Patient and public involvement in research
The SSHeW trial was informed by the involvement of NHS staff from diverse roles. These included nurses, health-care assistants, radiographers, physiotherapists, catering staff, and maintenance and housekeeping staff of different ages (age range 20–71 years) and genders. The study design was discussed with ward managers at their ward management task and finish group meeting and among NHS staff. The staff provided feedback about the rationale for the trial, the range of shoe styles to be offered in the trial, the use of text messages to collect data, the use of a slip diary and the length of the follow-up period. We had originally planned to follow-up participants for 12 months. However, the patient and public involvement (PPI) group felt that 12 months was too onerous and that participants would not provide outcome data for this length of time. We therefore reduced this to 3 months (12 weeks), but added an additional 2 weeks to allow for the ordering and delivery of shoes. During the trial, further views on the footwear, footwear buying habits and testing of staff’s usual shoes to determine slip resistance took place. In total, 30 pairs of shoes were tested.
Changes to the trial protocol
Minor changes to the trial protocol were submitted during the trial. These are listed in Appendix 10.
Study within a trial: pen substudy
To add to the body of evidence relating to making trial processes more efficient, a SWAT33 was conducted. The aim of this SWAT was to evaluate the effectiveness of enclosing a YTU, University of York pen with the 14-week postal questionnaire, on the response rates to the questionnaire.
Participants in the SSHeW trial who were to receive their 14-week questionnaire were randomised using simple randomisation in the ratio of 1 : 1 (receive a pen or not receive a pen). Generation of the allocation sequence was conducted by the trial statistician, who was not involved with sending the questionnaires.
The pen substudy was embedded into the trial part-way through recruitment. All participants in the main trial who were to be sent their 14-week questionnaire between 4 July 2018 and 12 February 2019 were included in this SWAT (unless they had withdrawn from follow-up). Participants in the main trial who had already been sent their 14-week questionnaire before the SWAT was introduced were excluded. No pens were included with questionnaires sent after 12 February 2019.
The primary outcome measure for the pen substudy was the proportion of participants in each group who returned the questionnaire. Secondary outcome measures included length of time taken to respond to the questionnaire, number of items completed and whether or not a reminder notice to return the questionnaire was required. As is usual with an embedded trial, no formal power calculation was undertaken, as the sample size was constrained by the number of participants to be sent the 14-week questionnaire. Binary data were compared using logistic regression, time to response by a Cox proportional hazards model and number of items completed by a linear regression model. All models were adjusted for the main trial allocation.
Chapter 3 Effectiveness results
Pilot phase
The findings from the pilot phase of the trial evaluating the progression criteria are presented in Table 1. As all of the progression criteria were met, the trial continued seamlessly.
| Criterion | Evaluation | Conclusion |
|---|---|---|
| Recruit at least 400 participants in 6 months (March–September 2017) | 714 participants recruited and randomised | Fulfilled |
| 80% of the participants will provide a valid response to at least 50% of the follow-up text data (i.e. 7 of the 14 weekly post-randomisation text messages) | 335 participants sent all 14 of their post-randomisation text messages, of whom 302 (90%) provided a valid response to at least 50% (n = 7) of messages | Fulfilled |
| 90% of the participants will respond to at least one post-randomisation text | 714 participants sent at least one post-randomisation text message (minimum five messages sent), of whom 707 (99%) responded to at least one message | Fulfilled |
| The slip rate in the control group will be at least 7% | Considering the post-randomisation text message responses from all participants in the control group (n = 357), regardless of the length of time they had been in the trial, 137 participants reported at least one slip (38%, 90% CI 34% to 43%). Across the 168 participants who had completed the trial (i.e. sent all 14 post-randomisation text messages) 53 (32%) reported at least one slip (90% CI 26% to 38%) | Fulfilled |
Recruitment
Participants were enrolled into the SSHeW trial from seven NHS trusts in England: (1) Cheshire and Wirral Partnership NHS Trust (CWP), (2) Leeds Teaching Hospitals NHS Trust, (3) York Teaching Hospital NHS Foundation Trust, (4) Lancashire Care NHS Foundation Trust, (5) Nottingham University Hospitals NHS Trust, (6) Harrogate and District NHS Foundation Trust and (7) University Hospitals of Derby and Burton NHS Foundation Trust. York Teaching Hospital NHS Foundation Trust was split into two geographical locations. Theses were considered as two distinct populations (Scarborough and Bridlington areas, and York and Malton areas) and formed eight trial sites. A total of 8524 recruitment packs were estimated to have been handed out in the eight sites between March 2017 and November 2018 (Figure 2), with follow-up occurring until June 2019. Varying numbers of recruitment packs were sent to the sites from YTU to be handed out to potential participants, depending on the size of the site and its capacity. The number of unused packs was reported to the YTU at the end of the trial. The number of packs handed out was estimated at 8524 by subtracting the number reported unused from the number originally sent to the site, on the assumption that the rest were distributed (CWP, n = 2029; Leeds, n = 1686; York, n = 642; Scarborough, n = 775; Lancashire, n = 1251; Nottingham, n = 1240; Harrogate, n = 401; and Derby, n = 500).
FIGURE 2.
A CONSORT flow diagram of participants in the SSHeW trial. a, n = 1930 (85%) received trial shoes within the 14-week trial follow-up. Reproduced with permission from Cockayne et al. 2 This is an Open Access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
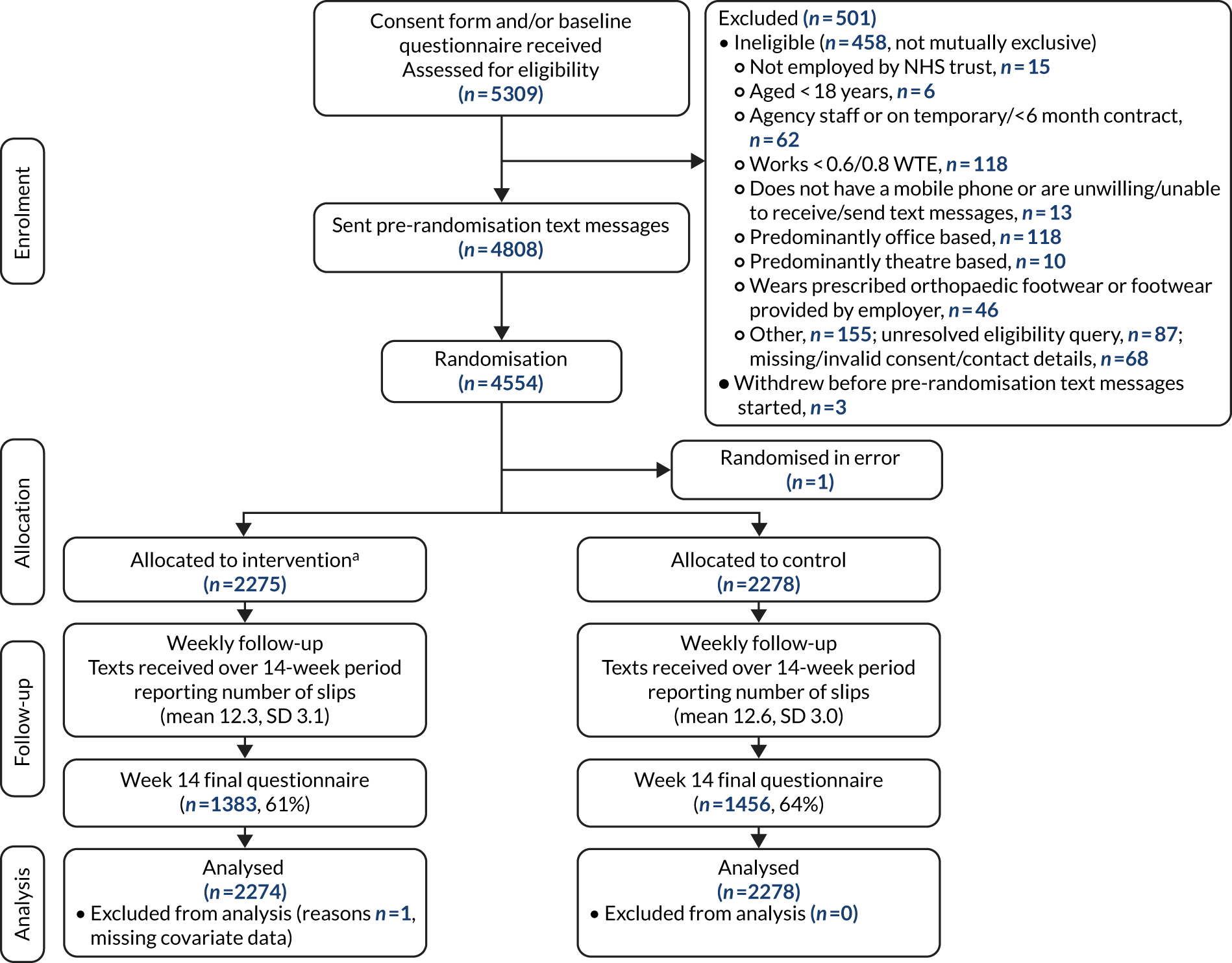
A completed baseline form was received at YTU from 5309 (62.3%) potential participants, of whom 498 (9.4%) were ineligible. Reasons for ineligibility were not mutually exclusive. One of the most common reasons was not working enough hours, on average, per week. Initially, the inclusion criteria specified that potential participants had to work, on average, at least 80% WTE (30 hours/week, assuming 37.5 working hours/week as full time). When it was observed that this was a predominant reason for ineligibility, it was, with the agreement of the TSC, DMEC and the funder, decided to lower this to 60% WTE (22.5 hours/week).
A total of 4811 participants were eligible to be sent the pre-randomisation text messages. Three participants withdrew before these text messages were started (one participant moved out of the trust, one participant was discovered to be a duplicate and one participant had an issue with their mobile telephone number that was never resolved), and 254 participants did not provide a valid response to at least two text messages. Therefore, 4554 participants were randomised (a slight increase of 154 participants on the 4400 planned). One participant was discovered to be ineligible after randomisation and so was immediately withdrawn (their data are not included in summaries of randomised participants). The flow of participants is illustrated in a CONSORT diagram (see Figure 2).
Pre-randomisation text message response rates
A total of 4808 participants were deemed eligible for the trial and were sent at least one pre-randomisation text message. Participants could request that the text messages be stopped at any time and so not all were sent at least four messages. Send and response rates to the initial four text messages were all > 86% (Table 2). A total of 4605 (95.8%) participants responded to at least one text message. A total of 260 participants were sent a fifth text (108 replied, 41.5%) and 245 were sent a sixth text (103 replied, 42.0%). Overall, 87 of 245 (36%) replied to both messages.
| Week | n received/N sent (%) |
|---|---|
| 1 | 4222/4808 (87.8) |
| 2 | 4256/4806 (88.6) |
| 3 | 3716/4249 (87.5) |
| 4 | 3044/3524 (86.4) |
| 5 | 108/260 (41.5) |
| 6 | 103/245 (42.0) |
All but one of the 4555 participants who replied to at least two pre-randomisation text messages (either two of the first four messages, or both the fifth and sixth messages) were randomised into the trial (one participant withdrew prior to randomisation).
Randomisation
Our required sample size was 4400 participants, which we aimed to achieve by the end of April 2018. However, recruitment was slightly slower than anticipated and we agreed with the trial sponsor, funder and HRA to extend recruitment to late 2018 to meet the target.
The first participants were randomised on 1 June 2017 and the last on 10 January 2019. Over this 20-month period, 4554 participants were randomised into the trial (2276 to the intervention group and 2278 to the control group); however, one participant (intervention group) was randomised in error, so their data have not been used post randomisation. A median of 529 (range 299–1014) participants were recruited from each site. Figure 3 presents the target compared with actual recruitment.
FIGURE 3.
Target vs. actual participant recruitment.
Baseline data
Baseline data are presented by randomised group and overall in Tables 3–6. One participant, allocated to the intervention group, withdrew shortly after they were randomised and requested that all their data be removed; they are included in the denominators in Tables 3–6, but all their data are missing.
| Characteristic | Intervention group (N = 2275) | Control group (N = 2278) | Total (N = 4553) |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 42.7 (11.5) | 42.7 (11.3) | 42.7 (11.4) |
| Median (minimum, maximum) | 44.0 (19.0, 74.0) | 44.0 (18.0, 71.0) | 44.0 (18.0, 74.0) |
| Gender, n (%) | |||
| Male | 325 (14.3) | 355 (15.6) | 680 (14.9) |
| Female | 1948 (85.6) | 1921 (84.3) | 3869 (85.0) |
| Prefer not to say | 1 (0.0) | 2 (0.1) | 3 (0.1) |
| Missing | 1 (0.0) | 0 (0.0) | 1 (0.0) |
| BMI (kg/m2) | |||
| Mean (SD) | 27.0 (5.5) | 27.0 (5.2) | 27.0 (5.3) |
| Median (minimum, maximum) | 25.9 (14.4, 56.5) | 26.3 (16.1, 54.2) | 26.1 (14.4, 56.5) |
| Education after school leaving age, n (%) | |||
| Yes | 1839 (80.8) | 1844 (80.9) | 3683 (80.9) |
| No | 421 (18.5) | 423 (18.6) | 844 (18.5) |
| Missing | 15 (0.7) | 11 (0.5) | 26 (0.6) |
| Degree/equivalent professional qualification, n (%) | |||
| Yes | 1512 (66.5) | 1522 (66.8) | 3034 (66.6) |
| No | 747 (32.8) | 741 (32.5) | 1488 (32.7) |
| Missing | 16 (0.7) | 15 (0.7) | 31 (0.7) |
| Ethnic group, n (%) | |||
| White/white British | 2008 (88.3) | 1982 (87.0) | 3990 (87.6) |
| Asian/Asian British | 154 (6.8) | 176 (7.7) | 330 (7.2) |
| Black/black British | 84 (3.7) | 82 (3.6) | 166 (3.6) |
| Mixed/multiple | 24 (1.1) | 28 (1.2) | 52 (1.1) |
| Other | 2 (0.1) | 3 (0.1) | 5 (0.1) |
| Missing | 3 (0.1) | 7 (0.3) | 10 (0.2) |
| Average number of hours worked per week | |||
| Mean (SD) | 35.8 (4.3) | 35.8 (4.5) | 35.8 (4.4) |
| Median (minimum, maximum) | 37.5 (22.5, 63.0) | 37.5 (15.0, 62.0) | 37.5 (15.0, 63.0) |
| Job characteristic | Intervention group (N = 2275) | Control group (N = 2278) | Total (N = 4553) |
|---|---|---|---|
| Job type, n (%) | |||
| Qualified nurse/midwife | 952 (41.8) | 985 (43.2) | 1937 (42.5) |
| Support worker | 286 (12.6) | 276 (12.1) | 562 (12.3) |
| Health-care assistant | 272 (12.0) | 246 (10.8) | 518 (11.4) |
| Other qualified staff/health-care professional | 150 (6.6) | 163 (7.2) | 313 (6.9) |
| Domestic services | 115 (5.1) | 110 (4.8) | 225 (4.9) |
| Administrator/receptionist/secretarial | 78 (3.4) | 100 (4.4) | 178 (3.9) |
| Occupational therapist | 65 (2.9) | 65 (2.9) | 130 (2.9) |
| Imaging staff | 64 (2.8) | 53 (2.3) | 117 (2.6) |
| Physiotherapist | 68 (3.0) | 36 (1.6) | 104 (2.3) |
| Pharmacist/pharmacy technician | 50 (2.2) | 53 (2.3) | 103 (2.3) |
| Ward clerk | 44 (1.9) | 49 (2.2) | 93 (2.0) |
| Doctor/consultant | 38 (1.7) | 42 (1.8) | 80 (1.8) |
| Catering staff | 29 (1.3) | 38 (1.7) | 67 (1.5) |
| Phlebotomist | 13 (0.6) | 17 (0.7) | 30 (0.7) |
| Podiatrist | 16 (0.7) | 9 (0.4) | 25 (0.5) |
| Laboratory staff | 7 (0.3) | 10 (0.4) | 17 (0.4) |
| Facilities | 9 (0.4) | 6 (0.3) | 15 (0.3) |
| Social worker | 6 (0.3) | 4 (0.2) | 10 (0.2) |
| Porter | 3 (0.1) | 5 (0.2) | 8 (0.2) |
| Other | 9 (0.4) | 11 (0.5) | 20 (0.4) |
| Missing | 1 (0.0) | 0 (0.0) | 1 (0.0) |
| Areas worked in, n (%)a | |||
| Ward | 1261 (55.4) | 1199 (52.6) | 2460 (54.0) |
| Clinical room/area | 722 (31.7) | 755 (33.1) | 1477 (32.4) |
| Community | 260 (11.4) | 268 (11.8) | 528 (11.6) |
| Indoor hospital grounds/corridors | 160 (7.0) | 183 (8.0) | 343 (7.5) |
| Office | 91 (4.0) | 113 (5.0) | 204 (4.5) |
| Food preparation/serving area | 77 (3.4) | 87 (3.8) | 164 (3.6) |
| Pharmacy | 40 (1.8) | 49 (2.2) | 89 (2.0) |
| Laboratory | 21 (0.9) | 47 (2.1) | 68 (1.5) |
| Theatre | 9 (0.4) | 14 (0.6) | 23 (0.5) |
| Podiatry | 10 (0.4) | 8 (0.4) | 18 (0.4) |
| Required to work in the community, n (%) | |||
| Yes | 552 (24.3) | 554 (24.3) | 1106 (24.3) |
| No | 1688 (74.2) | 1689 (74.1) | 3377 (74.2) |
| Missing | 35 (1.5) | 35 (1.5) | 70 (1.5) |
| Time spent on feet at work, n (%) | |||
| Most of the time | 1843 (81.0) | 1829 (80.3) | 3672 (80.7) |
| Some of the time | 385 (16.9) | 400 (17.6) | 785 (17.2) |
| A little of the time | 11 (0.5) | 18 (0.8) | 29 (0.6) |
| Missing | 36 (1.6) | 31 (1.4) | 67 (1.5) |
| Slips and falls in past 12 months | Intervention group (N = 2275) | Control group (N = 2278) | Total (N = 4553) |
|---|---|---|---|
| Have you had slip at work?, n (%) | |||
| Yes | 850 (37.4) | 885 (38.8) | 1735 (38.1) |
| No | 1318 (57.9) | 1297 (56.9) | 2615 (57.4) |
| Do not know | 101 (4.4) | 91 (4.0) | 192 (4.2) |
| Missing | 6 (0.3) | 5 (0.2) | 11 (0.2) |
| If yes, how many? | |||
| Median (minimum, maximum) | 2 (1, 400) | 2 (1, 300) | 2 (1, 400) |
| Have you suffered injury from any of these slips?, n (%) | 96 (11.3) | 89 (10.1) | 185 (10.7) |
| Have you had a fall at work?, n (%) | |||
| Yes | 188 (8.3) | 192 (8.4) | 380 (8.3) |
| No | 2039 (89.6) | 2040 (89.6) | 4079 (89.6) |
| Do not know | 29 (1.3) | 31 (1.4) | 60 (1.3) |
| Missing | 19 (0.8) | 15 (0.7) | 34 (0.7) |
| If yes, how many? | |||
| Median (minimum, maximum) | 1 (1, 52) | 1 (1, 20) | 1 (1, 52) |
| Have you suffered injury from any of these falls?, n (%) | 69 (36.9) | 74 (39.5) | 144 (38.2) |
| How often do you worry about slipping or falling in the workplace?, n (%) | |||
| All of the time | 57 (2.5) | 63 (2.8) | 120 (2.6) |
| Most of the time | 149 (6.5) | 121 (5.3) | 270 (5.9) |
| Some of the time | 734 (32.3) | 746 (32.7) | 1480 (32.5) |
| A little of the time | 798 (35.1) | 818 (35.9) | 1616 (35.5) |
| None of the time | 454 (20.0) | 461 (20.2) | 915 (20.1) |
| Missing | 83 (3.6) | 69 (3.0) | 152 (3.3) |
| Footwear | Intervention group (N = 2275) | Control group (N = 2278) | Total (N = 4553) |
|---|---|---|---|
| How many months do your usual work shoes last before they need replacing? | |||
| Median (IQR) | 12 (6–12) | 12 (6–12) | 12 (6–12) |
| Normally buy your work shoes from, n (%)a | |||
| High street store | 2113 (92.9) | 2091 (91.8) | 4204 (92.3) |
| Online specialist shop/catalogue | 194 (8.5) | 225 (9.9) | 419 (9.2) |
| Brand of shoe usually worn at work, n (%)a | |||
| General high street brand | 1893 (83.2) | 1888 (82.9) | 3781 (83.0) |
| General sports shop brand | 400 (17.6) | 402 (17.6) | 802 (17.6) |
| Any/various/depends on cost | 15 (0.7) | 23 (1.0) | 38 (0.8) |
| Shoes for Crews Ltd | 10 (0.4) | 13 (0.6) | 23 (0.5) |
| Magnum (Portsmouth, UK) | 9 (0.4) | 8 (0.4) | 17 (0.4) |
| Other safety/uniform shoe brand | 3 (0.1) | 7 (0.3) | 10 (0.2) |
| Alexandra (Bristol, UK) | 6 (0.3) | 3 (0.1) | 9 (0.2) |
| J&M Medical (Sunderland, UK) | 3 (0.1) | 6 (0.3) | 9 (0.2) |
| Other | 39 (1.7) | 41 (1.8) | 80 (1.8) |
| Style of shoes normally worn at work, n (%)a | |||
| Trainers | 671 (29.5) | 661 (29.0) | 1332 (29.2) |
| Pumps | 357 (15.7) | 361 (15.8) | 718 (15.8) |
| Work/safety boots | 39 (1.7) | 47 (2.1) | 86 (1.9) |
| Clogs | 82 (3.6) | 100 (4.4) | 182 (4.0) |
| Heeled shoes/boots | 90 (4.0) | 119 (5.2) | 209 (4.6) |
| Flat shoes/boots | 1115 (49.0) | 1098 (48.2) | 2213 (48.6) |
| Casual/dress shoe or boot | 404 (17.8) | 404 (17.7) | 808 (17.7) |
| Other | 81 (3.6) | 79 (3.5) | 160 (3.5) |
| Does your usual style of shoe have a secure fastening over the top of foot (e.g. laces or velcro strap)? n (%) | |||
| Yes | 1309 (57.5) | 1308 (57.4) | 2617 (57.5) |
| No | 932 (41.0) | 943 (41.4) | 1875 (41.2) |
| Missing | 34 (1.5) | 27 (1.2) | 61 (1.3) |
The recruited participants were predominantly female (n = 3869, 85.0%) and the average age was 42.7 (range 18–74) years. Participants worked a median of 37.5 hours per week and qualified nurse or midwife was the most represented job role (n = 1937, 42.5%). One participant who reported working 15 hours per week (less than the required amount) was randomised in error into the trial. Options to manage this participant were discussed with the TSC and DMEC and it was agreed that this participant should be retained in the trial and their data included in the analyses (as the impact of this one participant would be negligible).
Just over one-third of participants reported experiencing a slip at work in the previous 12 months (median of two slips), of whom 11% had suffered an injury as a result of one of these slips.
Via the pre-randomisation slip text messages, a total of 3891 slips were reported (2035 from participants who were subsequently randomised to the intervention group and 1856 from participants subsequently randomised to the control group). Both groups provided pre-randomisation text message slip data for an average of 3.4 (SD 0.8, median 4, range 2–4) weeks. Overall, 1627 (35.3%) participants (of the 4605 participants who responded to at least one text) reported at least one slip in the pre-randomisation text messages. The average number of reported slips was 0.89 (SD 2.00, median 0, range 0–22) in the intervention group and 0.81 (SD 1.76, median 0, range 0–26) in the control group. The baseline slip rate was calculated as the average weekly number of slips: 0.27 (SD 0.59, median 0, range 0–7) in the intervention group and 0.24 (SD 0.51, median 0, range 0–6.5) in the control group.
The treatment groups, as randomised, appear to be comparable on all measured baseline data.
Post-randomisation text message response rates
Following randomisation, participants were sent up to 14 weekly slip text messages. At any time, participants could request not to be sent any further messages. Response rates to the post-randomisation text messages are presented in Table 7 by group allocation. Of the 4553 participants sent at least one post-randomisation text message, a valid response to at least one message was received from 4494 (98.7%). Participants in the intervention group responded to an average of 12.3 (SD 3.1, median 14) text messages and participants in the control group to an average of 12.6 (SD 3.0, median 14) text messages.
| Week post randomisation | Intervention group (n = 2275), n received/N sent (%) | Control group (n = 2278), n received/N sent (%) | Total (n = 4553), n received/N sent (%) |
|---|---|---|---|
| 1 | 2109/2275 (92.7) | 2112/2278 (92.7) | 4221/4553 (92.7) |
| 2 | 2117/2272 (93.2) | 2101/2278 (92.2) | 4218/4550 (92.7) |
| 3 | 2079/2270 (91.6) | 2094/2278 (91.9) | 4173/4548 (91.8) |
| 4 | 2052/2269 (90.4) | 2075/2276 (91.2) | 4127/4545 (90.8) |
| 5 | 2063/2267 (91.0) | 2068/2275 (90.9) | 4131/4542 (91.0) |
| 6 | 2033/2265 (89.8) | 2047/2272 (90.1) | 4080/4537 (89.9) |
| 7 | 1967/2265 (86.8) | 2055/2271 (90.5) | 4022/4536 (88.7) |
| 8 | 1963/2261 (86.8) | 2028/2271 (89.3) | 3991/4532 (88.1) |
| 9 | 1962/2261 (86.8) | 2034/2271 (89.6) | 3996/4532 (88.2) |
| 10 | 1936/2259 (85.7) | 2012/2271 (88.6) | 3948/4530 (87.2) |
| 11 | 1948/2259 (86.2) | 1996/2271 (87.9) | 3944/4530 (87.1) |
| 12 | 1926/2256 (85.4) | 1988/2271 (87.5) | 3914/4527 (86.5) |
| 13 | 1921/2255 (85.2) | 1996/2271 (87.9) | 3917/4526 (86.5) |
| 14 | 1924/2253 (85.4) | 1985/2269 (87.5) | 3909/4522 (86.4) |
Withdrawals
There were 35 (0.8%) full, post-randomisation withdrawals [n = 26 (1.1%) from the intervention group, n = 9 (0.4%) from the control group]. The majority (n = 28) were requested during the 14-week follow-up period (median of 35 days after randomisation, range 6–93 days). Reasons for these withdrawals included participant left the trust or was on long-term absence (e.g. sickness, adoption leave) (n = 15); did not want to take part, issue with trial shoes (n = 7); unhappy with sizing and/or appearance of shoe (n = 5); shoes were causing trips and blisters (n = 1); saw colleague trip on carpet in trial shoes and participant works in similar area and concerned about tripping (n = 1); and unknown reason (n = 6). Seven of the full withdrawals were requested after the 14-week questionnaire was sent and none of the questionnaires was returned. Six of these withdrawals were in the intervention group, with four participants reporting issues with the shoes as a reason for not completing the 14-week questionnaire and choosing to formally withdraw. Issues reported included being unable to select suitable footwear (n = 1), did not wear shoes as they did not fit (n = 1), shoes caused trips (n = 1) or shoes were unsuitable after a change in job role (n = 1). The other two withdrawals did not provide a reason for withdrawing. One further participant withdrew from postal follow-up 84 days into their participation in the trial (no reason recorded) and so was not sent a 14-week questionnaire, but continued to receive text messages.
There were two reported deaths [one in each group (0.04%); both deaths were reported after sending the 14-week questionnaire, which was not returned, and in both cases the date of death was unknown].
Final (14-week) questionnaire response rates
The first set of 14-week questionnaires was sent out on 7 September 2017. A total of 4524 (99.4%) questionnaires were sent out [intervention group, 2254/2275 (99.1%); control group, 2270/2278 (99.7%)], of which 2839 [62.8%; intervention group, 1383/2254 (61.4%); control group, 1456/2270 (64.1%)] were returned. Overall, a questionnaire was received from a slightly higher proportion of randomised participants in the control group (63.9%) than the intervention group (60.8%). The median time between the questionnaire being sent and completed was 6 days in the control group (range 1–140 days) and 7 days in the intervention group (range 1–146 days).
Primary outcome
Raw data
In total, 4494 (98.7%) trial participants provided a valid response to at least one slip text message following randomisation [intervention group, n = 2254 (99.1%); control group, n = 2240 (98.3%)], with 2689 (59.1%) participants returning a complete 14 weeks’ worth of responses [intervention group, n = 1289 (56.7%); control group, n = 1400 (61.5%)].
Of the 59 participants who did not provide a response to at least one post-randomisation text message, 11 provided data for number of slips experienced on the 14-week questionnaire. These data were used for these participants in the analysis and therefore post-randomisation slip data were available for 4505 randomised participants [98.9%; intervention group, n = 2258 (99.3%); control group, n = 2247 (98.6%)]. In total, 6743 slips were reported: 2633 slips in the intervention group (mean of 1.17 per participant, SD 3.0, median 0, range 0–36) and 4110 in the control group (mean of 1.83 per participant, SD 4.7, median 0, range 0–83) (Figure 4).
FIGURE 4.
Histogram displaying the distribution of the number of reported slips, by randomised group (participants with at least 1 week of slip text message data). (a) Intervention; and (b) control.
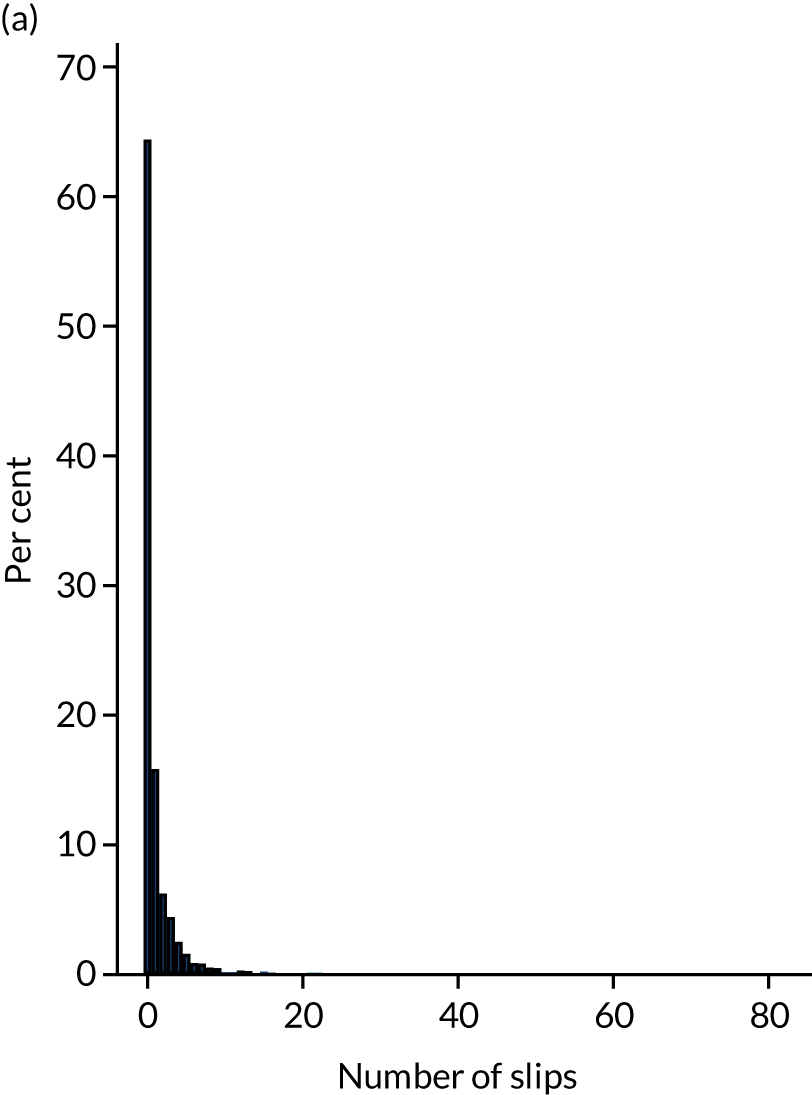
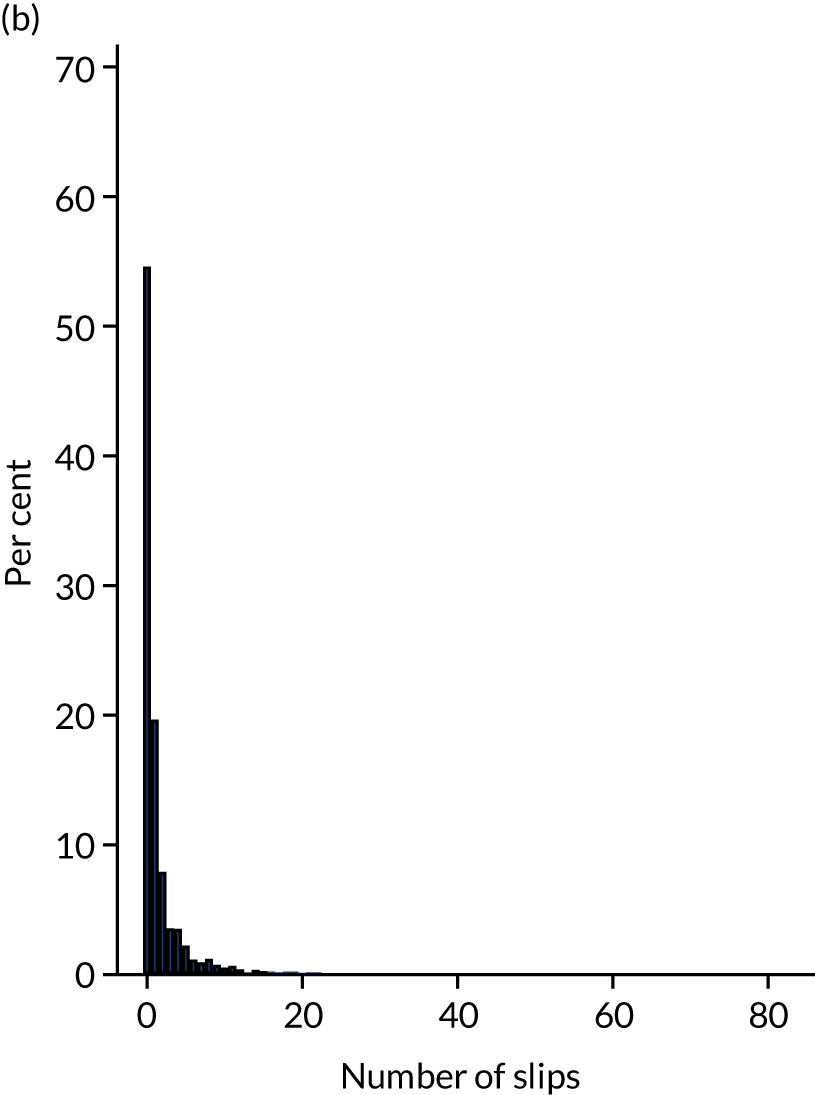
For the analysis, participants who did not provide any follow-up slip data were ‘censored’ immediately following randomisation (i.e. they were recorded as having reported zero slips over a minimal follow-up time of 0.1 hours). Therefore, all randomised participants were accounted for in the primary analysis model except those missing relevant covariate data (summary statistics for slips per group in the analysis: intervention group – mean of 1.16 per participant, SD 2.9, median 0, range 0–36; control group – mean of 1.80 per participant, SD 4.6, median 0, range 0–83).
Covariates
The primary analysis model controlled for gender, age at randomisation, job role and baseline weekly slip rate ascertained from the pre-randomisation text message responses. Covariate data were missing for one participant and so the primary model was based on 4552 participants (2274 participants in the intervention group and 2278 participants in the control group).
Slip data collection sheet: response rates and data
Slip data collection sheets were sent for the first slip reported via a post-randomisation slip text message. In total, 1821 (40.0%) participants reported at least one slip post randomisation via text message [intervention group, n = 803 (35.3%); control group, n = 1018 (44.7%)]. A slip data collection form was received for 1159 (63.6%) of those participants [intervention group, n = 497 (61.9%); control group, n = 662 (65.0%)]. Participants predominantly reported slips without falling (n = 1033, 89.1%) and participants in the intervention group tended not to be wearing the trial shoes when they slipped (n = 363, 73.0%) (Table 8). Nearly all slips occurred on a ward or other clinical area and most were on a smooth surface. The vast majority of slips did not result in an injury (95.1%, assuming that missing data implies no injury).
| Characteristic | Intervention group (N = 497) | Control group (N = 662) | Total (N = 1159) |
|---|---|---|---|
| Hours worked in week of first slip | |||
| Mean (SD) | 35.7 (7.8) | 35.6 (7.0) | 35.6 (7.4) |
| Median (minimum, maximum) | 37.5 (3.5, 75.0) | 37.5 (5.0, 75.0) | 37.5 (3.5, 75.0) |
| Type of slip, n (%) | |||
| Slip without falling (however minor) | 437 (87.9) | 596 (90.0) | 1033 (89.1) |
| Slip and fall | 12 (2.4) | 23 (3.5) | 35 (3.0) |
| Missing | 48 (9.7) | 43 (6.5) | 91 (7.9) |
| Wearing trial shoes when slipped, n (%) | |||
| Yes | 82 (16.5) | ||
| No | 363 (73.0) | ||
| Do not know | 3 (0.6) | ||
| Missing | 49 (9.9) | ||
| Location of slip, n (%) | |||
| On a ward or other clinical area in a hospital | 479 (96.4) | 644 (97.3) | 1123 (96.9) |
| In a non-clinical area in a hospital (e.g. office, corridor) | 5 (1.0) | 3 (0.5) | 8 (0.7) |
| In an catering area where food is prepared or served | 1 (0.2) | 0 (0.0) | 1 (0.1) |
| Outside | 4 (0.8) | 6 (0.9) | 10 (0.9) |
| Inside a patient’s home | 1 (0.2) | 0 (0.0) | 1 (0.1) |
| Other | 7 (1.4) | 9 (1.4) | 16 (1.4) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Slipped on . . . , n (%) | |||
| Smooth surface | 341 (68.6) | 532 (80.4) | 873 (75.3) |
| Textured surface | 92 (18.5) | 77 (11.6) | 169 (14.6) |
| Missing | 64 (12.9) | 53 (8.0) | 117 (10.1) |
| Injuries suffered, n (%)a | |||
| None | 419 (84.3) | 582 (87.9) | 1001 (86.4) |
| Superficial wound | 12 (2.4) | 17 (2.6) | 29 (2.5) |
| Broken bone | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Pulled muscle/sprained ligament | 10 (2.0) | 12 (1.8) | 22 (1.9) |
| Other | 5 (1.0) | 3 (0.5) | 8 (0.7) |
| Missing | 52 (10.5) | 49 (7.4) | 101 (8.7) |
Primary analysis
In total, the intervention group contributed 28,002 person-working weeks of slip data and the control group contributed 28,595 person-working weeks of slip data. The incidence rates of slips per person-working week were approximately 0.10 (95% CI 0.09 to 0.11) in the intervention group and 0.15 (95% CI 0.14 to 0.17) in the control group. The median working week was 37.5 hours; however, at baseline participants reported working up to 63 hours per week. Poisson regression is a common method used to analyse count data; however, it is limited in that it assumes that the mean and variance of the data are equal (i.e. that the data are not overdispersed). Negative binomial regression is the recommended alternative in such cases, as it does not share this assumption and so allows for overdispersion. The variance for number of slips per person over the 14-week follow-up period was larger than the mean, which gave an indication that the data were overdispersed. Therefore, a mixed-effect negative binomial model was run within which, in Stata, a likelihood ratio test of the overdispersion parameter (alpha) is conducted. A significant p-value for the likelihood ratio test indicated that the data were overdispersed and therefore that Poisson regression was not appropriate. Additionally, a key assumption of Poisson regression is that the counts are of independent events; however, it is reasonable to assume that slips in this population are not independent, as a person who slips once is more likely to slip again. Negative binomial regression allows for this lack of independence.
The adjusted negative binomial model indicated a statistically significant reduction in the slip rate in the intervention group relative to the control group (IRR 0.63, 95% CI 0.57 to 0.70; p < 0.001). Pre-randomisation slip rate was seen to be a significant predictor in the model, such that a higher baseline slip rate was associated with an increased post-randomisation slip rate (IRR 5.73, 95% CI 5.05 to 6.50; p < 0.001). Females also had a higher post-randomisation slip rate than males (IRR 1.23, 95% CI 1.05 to 1.43; p = 0.01). Age and job role were not statistically significant factors in the model.
Sensitivity analysis
Receipt of shoes
The 14-week questionnaire asked participants allocated to the intervention group if, and when, they received their trial shoes. Of the 1383 intervention participants who returned a 14-week questionnaire, 1320 (95.4%) reported having received a pair of trial shoes, 62 said that they had not received their trial shoes (4.5%) and one did not provide a response (0.1%). Of the 1320 participants who reported having received a pair of trial shoes, 960 (72.7%) provided a date for when they received the shoes. Unfortunately, perhaps owing to recall difficulties or recording errors on the part of the participant, some of the dates provided were invalid (before randomisation or after the date that the 14-week questionnaire was completed), or were inconsistent with responses to the week 6, 10 and 14 compliance text messages.
Sites were also asked to supply the date that they provided the participant with their trial shoes. A date from the site was provided for 1414 intervention participants (62.2%). In five of the sites, virtually all their intervention participants were accounted for in these data (Leeds, York, Scarborough, Nottingham and Harrogate). The majority of the participants were accounted for in Lancashire, but limited data were provided by CWP and no data were provided by Derby.
The participant-reported date was considered primarily, but this was replaced with the date provided by the site when the participant-reported date was missing or invalid. This resulted in a date for 1718 (75.5%) intervention participants (mean 33.4, SD 29.3, median 24, range 1–239 days from randomisation).
For the remaining participants, some post hoc data decisions were made to estimate the time between randomisation and receipt of shoes based on responses to the compliance text messages, when provided. A time of 42, 70 and 98 days was imputed when the participant indicated that they wore the shoes some or all of the time in response to the week 6, 10 and 14 compliance messages, respectively. Following this imputation, there remained 34 participants who had reported receiving a pair of trial shoes on their 14-week questionnaire but for whom period from randomisation to receipt of shoes was missing; in these cases a value of 24 days (the median time) was assigned. This left 273 intervention participants whom we cannot be sure received a pair of intervention shoes; we conservatively assume that they did not. (Certainly, 48 participants indicated on the 14-week questionnaire that they had not received a pair by the time they completed the questionnaire.) Therefore, we estimate that 2002 (88.0%) intervention participants received a pair of trial shoes within an average of 36 (SD 29, median 27, range 1–239) days of randomisation, 1523 (66.9%) intervention participants received a pair of trial shoes within 7 weeks of randomisation and 1930 (84.8%) intervention participants received a pair of trial shoes within 14 weeks.
Compliance text messages
Text messages were sent to participants in the intervention group 6, 10 and 14 weeks after randomisation, asking the following question:
SSHeW trial. In the past month, how often have you worn your trial shoes at work? Reply 0, 1 or 2 (0 = none of the time, 1 = some of the time, 2 = all of the time).
On occasion, participants responded to tell us that they had not yet received their shoes or that they had been on leave or off sick during the time we asked them about; these responses were not considered valid. Valid responses are summarised in Table 9. At 14 weeks, 79% of the intervention participants who were sent a compliance text provided a valid response. Of these, just under half reported that they were wearing the trial shoes all of the time, just under one-quarter some of the time and just over one-quarter none of the time.
| Week | n valid received/N sent (%) | Frequency of wear | ||
|---|---|---|---|---|
| All of the time, n (%) | Some of the time, n (%) | None of the time, n (%) | ||
| 6 | 1451/2173 (66.8) | 623 (42.9) | 386 (26.6) | 442 (30.5) |
| 10 | 1621/2166 (74.8) | 815 (50.3) | 369 (22.8) | 437 (27.0) |
| 14 | 1694/2159 (78.5) | 830 (49.0) | 397 (23.4) | 467 (27.6) |
On the 14-week questionnaire, intervention participants were asked how often they had typically worn the trial shoes while at work over the previous 14 weeks. Just over half (n = 704, 55.0%) responded ‘all of the time’, 258 (20.2%) responded ‘most of the time’, 168 (13.1%) responded ‘some of the time’, 106 (8.3%) responded ‘a little of the time’ and 44 (3.4%) responded ‘none of the time’.
Complier-average causal effect analysis
The CACE estimate for receiving a pair of shoes within 7 weeks was similar to the intention-to-treat estimate (IRR 0.65, 95% CI 0.58 to 0.73; p < 0.001). The CACE estimate for receiving a pair of shoes within 14 weeks was virtually identical to the 7-week estimate (IRR 0.65, 95% CI 0.59 to 0.73; p < 0.001).
When a continuous measure of exposure to the footwear was used, which considered the timing of the receipt of shoes and the amount of time they were reported to have been worn, participants in the intervention group had an average overall ‘compliance score’ of 5.2 (SD 4.4, median 5.1, range 0–12). A higher score indicates greater compliance. Participants in the control group all had a score of zero. Among those participants in the intervention group whom we estimate received a pair of trial shoes within 14 weeks from randomisation, the average compliance score was 6.1 (SD 4.1, median 6, range 0–12), indicating that some participants reported that they did not wear the shoes at all. The CACE estimate for this analysis was an adjusted IRR of 0.95 (95% CI 0.94 to 0.97; p < 0.001). This indicates that, for every unit increase in compliance score, the rate of slipping was reduced by approximately 5%.
As data on the dates that participants received their shoes were limited and untidy, the reliability of these analyses is likely compromised and the results should be interpreted with due caution.
Subgroup analyses
Gender
When an interaction between gender (male/female) and treatment allocation was included in the primary model, a weak effect of the interaction was observed (female IRR 1.09, 95% CI 0.89 to 1.33, p = 0.41; intervention IRR 0.49, 95% CI 0.37 to 0.65, p < 0.001; interaction IRR 1.33, 95% CI 0.98 to 1.82, p = 0.07). Post hoc exploratory analysis of the intervention effect in males and females separately indicates a greater effect of the intervention in males (males IRR 0.49, 95% CI 0.36 to 0.66, vs. females IRR 0.65, 95% CI 0.58 to 0.73).
Area of work
When an interaction between work area (low/high risk of contamination) and treatment allocation was included in the primary model, a weak effect of the interaction was observed (high-risk work area IRR 1.16, 95% CI 0.92 to 1.46, p = 0.20; intervention IRR 0.81, 95% CI 0.60 to 1.10, p = 0.17; interaction IRR 0.75, 95% CI 0.55 to 1.04, p = 0.08). Separate post hoc exploratory analysis of the intervention effect in participants working in high-risk areas compared with low-risk areas indicates a greater effect of the intervention in high-risk areas (high-risk area IRR 0.61, 95% CI 0.54 to 0.68, vs. low-risk area IRR 0.80, 95% CI 0.61 to 1.06).
Secondary outcomes
Slips in the workplace over 14 weeks as reported on the 14-week questionnaire
In total, 2793 participants provided data to the question on the 14-week questionnaire asking how many times they slipped (with or without falling) while at work in the past 14 weeks. The number of participants who reported at least one slip was 336 of 1360 (24.7%) in the intervention group, compared with 549 of 1433 (38.3%) in the control group. One participant in the control group reported experiencing 650 slips; this appeared to be the number written on the paper questionnaire but it was not possible to confirm this figure with the participant. As this was such a significant outlier from the next largest number of slips (n = 92), the analysis was performed with and without this participant. All 2793 participants had full covariate data and so were included in the analysis models.
Including outlier
The mean number of reported slips was 0.79 in the intervention group (SD 2.5, median 0, range 0–32) and 2.06 in the control group (SD 17.9, median 0, range 0–650). There was a statistically significant difference in the rate of slips between the two groups (adjusted IRR 0.44, 95% CI 0.37 to 0.53; p < 0.001).
Excluding outlier
The mean number of reported slips was 0.79 in the intervention group (SD 2.5, median 0, range 0–32) and 1.61 in the control group (SD 5.3, median 0, range 0–92). There was a statistically significant difference in the rate of slips between the two groups (adjusted IRR 0.56, 95% CI 0.48 to 0.66; p < 0.001).
Falls resulting from a slip in the workplace over 14 weeks (14-week questionnaire)
Of the 885 participants who reported having at least one slip at work in the past 14 weeks on the 14-week questionnaire, all but nine (1.0%) provided a valid response to the follow-on question asking how many of these slips resulted in a fall. The number of participants who said that at least one of their workplace slips resulted in a fall was lower in the intervention group (19 of 332; 5.7%) than in the control group (38 of 544; 7.0%) but the difference was not statistically significant (IRR 0.72, 95% CI 0.40 to 1.29; p = 0.27). After imputing zero falls (from a slip) for those participants who reported zero slips, participants in the intervention group reported marginally fewer falls resulting from a slip over the 14-week follow-up period [mean 0.02 (SD 0.22)] than participants in the control group [mean 0.03 (SD 0.23)]. This difference was statistically significant (IRR 0.51, 95% CI 0.28 to 0.92; p = 0.03).
Falls not resulting from a slip in the workplace over 14 weeks (14-week questionnaire)
In total, 2681 participants provided data in response to the question on the 14-week questionnaire asking how many times they fell at work for reasons other than a slip in the past 14 weeks. In the intervention group, 61 of 1302 (4.7%) participants reported at least one fall, compared with 75 of 1379 (5.4%) participants in the control group. The mean number of reported falls not resulting from a slip was 0.13 in the intervention group (SD 0.9, median 0, range 0–18) and 0.22 in the control group (SD 2.1, median 0, range 0–48). There was no evidence of a difference in this fall rate between the two groups from the adjusted analysis (IRR 0.82, 95% CI 0.50 to 1.34; p = 0.44).
Proportion of participants who report a slip over 14 weeks (weekly slip text or 14-week questionnaire)
Of the 4505 participants who provided slip data via at least one post-randomisation weekly text message or the 14-week questionnaire, 1824 (40.5%) reported at least one slip [intervention group, 804/2258 (35.6%); control group, 1020/2247 (45.4%)]. The adjusted OR for the intervention was 0.58 (95% CI 0.50 to 0.66; p < 0.001).
Proportion of participants who report a slip over 14 weeks (14-week questionnaire)
In total, 2793 participants provided data in response to the question on the 14-week questionnaire asking how many times they slipped (with or without falling) while at work in the past 14 weeks: 336 of 1360 (24.7%) participants in the intervention group and 549 of 1433 (38.3%) participants in the control group reported at least one slip (OR 0.45, 95% CI 0.38 to 0.54; p < 0.001).
Proportion of participants who report a fall (whether or not resulting from a slip) over 14 weeks (14-week questionnaire and slip data collection sheet)
Overall, 2824 participants provided a response to one of the questions on the 14-week questionnaire asking how many falls (related to a slip or other reason) they had experienced at work in the past 14 weeks, of whom 181 reported at least one fall [6.4%; intervention group, 77/1297 (5.6%); control group, 104/1346 (7.2%)]. A further six participants reported a slip that resulted in a fall on a SDCS (three in each group). There was a statistically significant difference in overall likelihood of falling in the two groups (adjusted OR 0.73, 95% CI 0.54 to 0.99; p = 0.04).
Time to first slip
Figure 5 presents the Kaplan–Meier survival curves for time to first slip for the two groups. The two lines appear to follow one another reasonably closely until about 2 or 3 weeks, when they start to diverge. At this point, about one-quarter of participants in each group have had an event. Indeed, the time for one-quarter of participants to report a slip following randomisation was 18 (95% CI 13 to 19) days in the control group and 20 (95% CI 18 to 25) days in the intervention group. The time for one-third of participants to report a slip following randomisation was 27 (95% CI 25 to 32) days in the control group and 46 (95% CI 34 to 60) days in the intervention group. The intervention group was found to have a lower risk of slipping than control group (HR 0.73, 95% CI 0.67 to 0.80; p < 0.001).
FIGURE 5.
Kaplan–Meier survival plot for time to first slip, by randomised group.
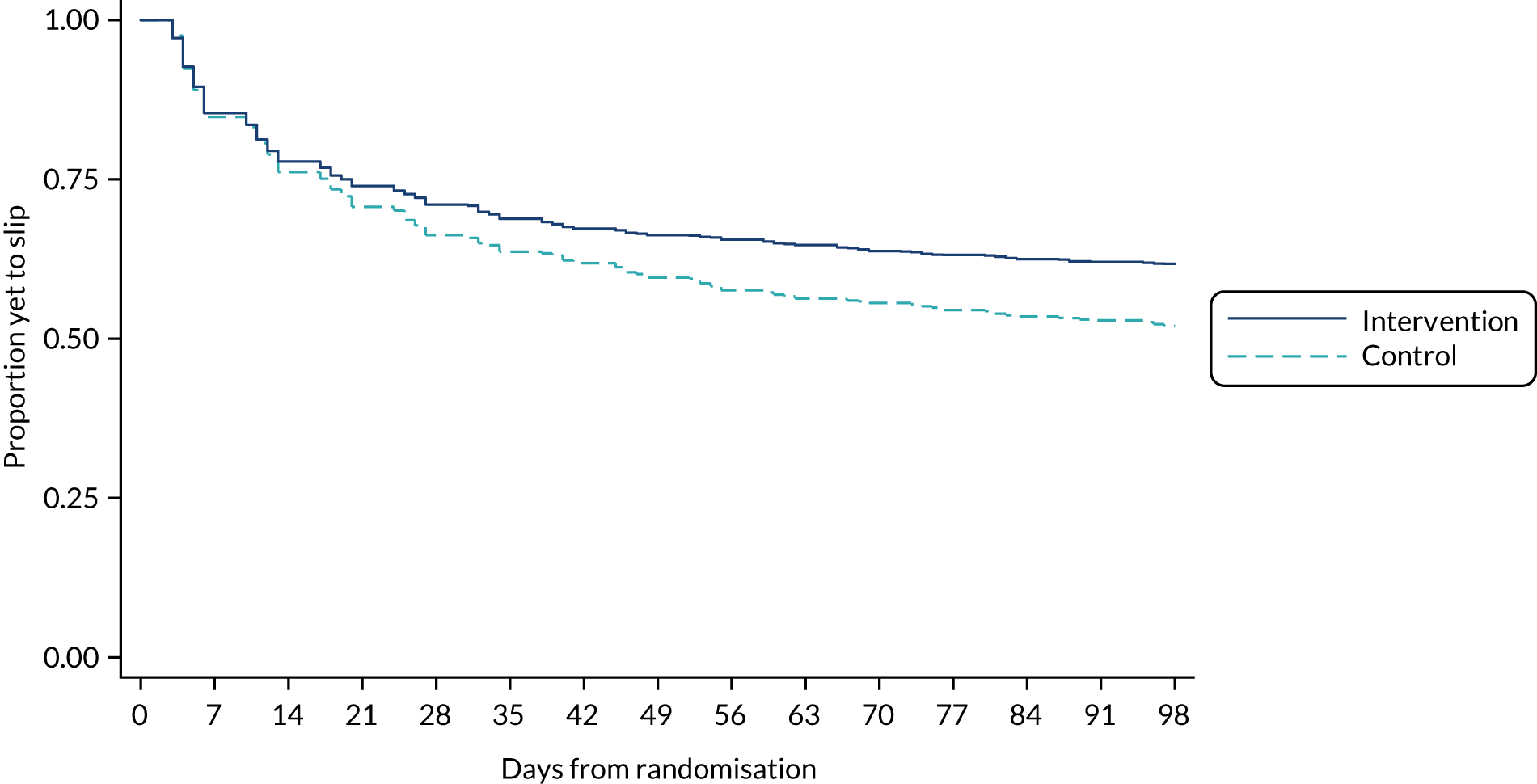
Time to first fall
Of the 187 participants who reported at least one fall, a valid date for the fall was provided for 118 (63.1%). The falls were reported a median of 34 days after randomisation in the intervention group (n = 53, range 0–98 days) and 41 days after randomisation in the control group (n = 65, range 1–97 days). As there were relatively few falls with a valid date, no formal time-to-event analyses were conducted.
Adverse events
Serious adverse events
No related serious AEs were reported. During the course of the trial, the research team was notified of two deaths. Limited information was available about these deaths, but it could be ascertained that neither was related to the intervention. One participant was in the control group. This participant had responded to all 14 post-randomisation text messages, but not the 14-week questionnaire; we were informed of this death shortly after the 14-week questionnaire was sent. The other participant was in the intervention group. This participant did not respond to any of the weekly post-randomisation text messages or the 14-week questionnaire, and did not collect their shoes. We were informed of this death after the 14-week questionnaire was sent to the participant, but their date of death is unknown.
Non-serious adverse events
Eleven non-serious AEs that were deemed to be at least possibly related to any of the research procedures are summarised in Table 10. These were all in the intervention group. Events were recorded on an AE form if they were reported to the research team through the course of the trial (e.g. in response to a slip or compliance text, or on a SDCS). We found that a few participants were reporting very minor problems from wearing the shoes (e.g. blisters); however, these were somewhat expected with new shoes and, therefore, with the agreement of the TSC and DMEC, these were not reported as AEs. Instead, we added some items to the 14-week questionnaire to ask about experiences of wearing the trial shoes (see Blisters, calluses and other problems caused by shoes).
| Non-serious AE | Intervention group (N = 2275) |
|---|---|
| Total number of non-serious AEs | 11 |
| Number of participants with one or more non-serious AEs, n (%)a | 11 (0.5) |
| Action, n (%)b | |
| None | 7 (63.6) |
| Study treatment interrupted/halted | 3 (27.3) |
| Therapy prescribed | 1 (9.1) |
| Outcome, n (%)b | |
| Resolved | 4 (36.4) |
| Resolved with sequelae | 1 (9.1) |
| Ongoing | 6 (54.5) |
| Relationship to treatment, n (%)b | |
| Possibly | 3 (27.3) |
| Probably | 4 (36.4) |
| Definitely | 4 (36.4) |
| Expectedness, n (%)b | |
| Expected | 8 (72.7) |
| Unexpected | 3 (27.3) |
The non-serious AEs recorded on an AE form were pain in hip after wearing trial shoes; shoes caused blisters, redness and/or burning of toes; bony lump at base of big toe corresponding to area where the shoe felt tight; worsening of plantar fasciitis; pain in heels after wearing trial shoes; foot pain after wearing trial shoes; loss of toenail from big toe perhaps due to pressure of the shoes; infection and athlete’s foot; slipped on icy flags causing bruising to forearm and lacerations; fell and bruised nose, face, arms and leg; and fell and hurt knee.
Blisters, calluses and other problems caused by shoes
In the initial stages of the trial, a question was included on the 14-week questionnaire which asked intervention participants if their trial shoes had caused blisters, calluses or any other foot problems. Forty-four (20.1%) participants reported that they had experienced an issue of this nature. All 44 of these participants reported on the 14-week questionnaire that they had received their shoes.
From February 2018, new versions of the 14-week questionnaire were sent to participants. The new version of the questionnaire aimed to collect further information on problems caused by their footwear. Questions were added to the version sent to intervention participants which asked whether or not they had experienced problems and, if so, if (1) they sought treatment for them and (2) they had resolved. Control participants were also asked these questions in relation to their experience of wearing their usual work shoes. Responses to these questions can be found in Table 11. The proportion of participants in the intervention group reporting having experienced a problem with their trial shoes (19.5%) was similar to the proportion of participants in the control group reporting having experienced a problem with their usual work footwear (17.8%). More participants in the control group sought treatment for the problem (control group 14.8% vs. intervention group 8.8%); however, participants in the intervention group were more likely to report that the problem had resolved (intervention group 78.4% vs. control group 51.6%).
| Problem with shoes | Intervention group (N = 1164) | Control group (N = 1219) | Total (N = 2383) |
|---|---|---|---|
| Experienced blisters/callouses/other problems, n (%) | 227 (19.5)a | 217 (17.8) | 444 (18.6) |
| If yes . . . , n (%) | |||
| Treatment sought? | 20 (8.8) | 32 (14.8) | 52 (11.7) |
| Problems resolved? | 178 (78.4) | 112 (51.6) | 290 (65.3) |
SSHeW trial footwear testing
Intervention footwear testing
A total of 52 pairs of footwear were recovered, and these were reported to have been worn either all of the time or most of the time, over periods ranging from 6 to 18 months. However, it later transpired during a telephone interview that one recovered pair had seldom been worn and so this pair was subsequently excluded from the assessment. Of the 51 remaining pairs, 23 pairs were recovered from nurse or midwifes, six pairs from support workers, five pairs from health-care assistants, four pairs from physiotherapists, three pairs from occupational therapists, two pairs from doctors and one pair from each of the following roles: mental health practitioner, health visitor, administrator, radiographer, caterer, researcher and fitness instructor. One further pair of shoes was recovered from a participant whose role could not be determined. A summary of the findings from our assessment of the remaining 51 pairs of recovered footwear is presented in Table 12.
| Approximate wear period (months) | Pairs tested, n | Styles tested, n | Reasonable/good appearance of upper, n (%) | Defects (e.g. holes or cracks in upper or lining), n (%) | Approximate % of sole < 2 mm tread depth, minimum, maximum | Slip resistance equivalent to when new, n (%) | Usable, n (%) |
|---|---|---|---|---|---|---|---|
| 6 | 7 | 4 | 7 (100) | 2 (29) | 0, 1 | 7 (100) | 7 (100) |
| 7–9 | 21 | 8 | 20 (95) | 6 (29) | 0, 13 | 20 (91) | 20 (95) |
| 10–12 | 13 | 5 | 13 (100) | 9 (69) | 0, 6 | 13 (100) | 11 (85) |
| 18 | 10 | 6 | 7 (70) | 7 (70) | 0, 17 | 6 (60) | 7 (70) |
As shown in Table 12, the external appearance of the footwear uppers was considered to be reasonable or good in most cases (n = 47, 92%). However, four pairs of footwear (8%) had uppers that were considered to have a poor external appearance: one pair in the 7–9-months group and three pairs in the 18 months group. Defects were noted on 24 pairs of footwear (47%). In the majority of cases, these defects related to the internal lining of the footwear, which tended to be minor and were not considered to render the footwear unusable, provided they did not lead to discomfort. Six pairs of footwear (12%) were considered to be unusable because of the extent of damage to the internal lining and/or the presence of other defects: one pair had been worn for 9 months, two pairs had been worn for 12 months and three pairs had been worn for 18 months.
Damage to the internal lining did not appear to be related to a specific style and appeared to be influenced by the user as well as the period of wear. For example, the most extensive wear was found on footwear that had been submitted with its laces tied, which suggested that the user had been treating them as slip-ons. The extent of wear on the soles also varied from user to user and was concentrated in different areas of the sole. As expected, the soles of footwear that had been worn for longer periods (> 12 months) tended to have the greatest level of wear; however, only five pairs of footwear (10%) had tread depths below the manufacturer’s recommendations (across > 5% of the sole’s area). Furthermore, the results of our testing showed that most of the worn footwear provided similar slip resistance to when it was new, with only five pairs (10%) generating lower results. However, it is important to bear in mind that individual users would vary in the way that they initially load weight on the shoe and how their foot subsequently functions in the shoe with each step. This may also vary between the left and right side, depending on overall mechanical factors and their walking pattern. This would result in specific areas of wear for the user, and our test, as well as all other known laboratory tests, cannot replicate an individual’s foot placement and concentrate forces in the same way that a user would. The results are therefore likely to suggest better slip resistance than the user might experience in reality.
Determining a serviceable duration for footwear is very difficult, and our analysis shows that it will vary from one individual to another and is also likely to vary between roles and workplace environments. Consideration was given to using a PPI exercise to obtain the views of NHS staff regarding the recovered footwear; however, this was ruled out because of concerns relating to infection control. Judgements on condition and usability were therefore based on the subjective opinion of our research team member and a lay colleague. However, it was also self-evident that the NHS staff from whom we recovered the footwear considered them to be usable up to the point of submission.
Only one pair of footwear with a wear period of < 12 months was considered to be unusable, although a further two pairs in the 12-month group demonstrated a reduction in slip resistance from when they were new and so their usability would need to be determined via a risk assessment. In contrast, there were five pairs of footwear that had been worn for approximately 18 months that were considered usable and demonstrated no reduction in slip resistance. On the basis of our assessments, we consider it is reasonable to expect a typical serviceable duration for the footwear to be 12 months, but acknowledge that some footwear would need to be replaced sooner and some would last longer.
Usual footwear testing
In total, 31 pairs of usual work footwear worn by NHS staff were provided for assessment of slip resistance, of which 29 pairs were suitable for testing. (Two pairs were unable to be tested: one was actually the intervention footwear and the other pair could not be fitted securely to the simulated slip test rig.) The results of the testing are reported in Table 13. The average GRIP rating was found to be one star, with a significant proportion of the footwear (28%) failing to meet the minimum requirements to achieve a one-star rating.
| GRIP star rating (n) | Role (n) | Gender (n) | Footwear brand (n) | Condition (n) |
|---|---|---|---|---|
| 0 (8) | Domestic (4) | Female (5) | High street brand (2) | Heavily worn (3) |
| Clinical (2) | Male (3) | Skechers (Manhattan Beach, CA, USA) (2) | Worn (2) | |
| Porter (1) | Adidas (Herzogenaurach, Germany) (1) | Reasonable (1) | ||
| IT (1) | Asda (Leeds, UK) (1) | Good (2) | ||
| Clarks (Somerset, UK) (1) | ||||
| Nike (Beaverton, OR, USA) (1) | ||||
| 1 (10) | Clinical (5) | Female (6) | Clarks (3) | Worn (1) |
| Domestic (2) | Male (4) | High street brand (3) | Reasonable (1) | |
| Administration (1) | Skechers (1) | Good (8) | ||
| Chaplin (1) | Adidas (1) | |||
| Driver (1) | Nike (1) | |||
| Timberland (Stratham, NH, USA) (1) | ||||
| 2 (8) | Domestic (2) | Male (5) | Clarks (2) | Worn (4) |
| Clinical (2) | Female (3) | Dr. Martens (Wollaston, UK) (1) | Reasonable (1) | |
| Driver (2) | High street brand (1) | Good (3) | ||
| Chaplin (1) | Karrimor (Shirebrook, UK) (1) | |||
| Facilities (1) | Regatta (Manchester, UK) (1) | |||
| Tesco (Welwyn Garden City, UK) (1) | ||||
| Timberland (1) | ||||
| ≥ 3 (3) | Domestic (2) | Female (3) | Asda (1) | Good (3) |
| Clinical (1) | Ecco (Bredebro, Denmark) (1) | |||
| Hotter (Skelmersdale, UK) (1) |
Study within a trial: pen substudy results
A total of 1453 participants were included in this substudy: 725 participants were allocated to the control group (no pen) and 728 participants to the intervention group, who were sent a pen with their 14-week questionnaire.
Questionnaire response rates
The total number of SWAT participants who returned their 14-week questionnaire was 962 (66.2%). There was no evidence of a difference in return rate between the groups [pen, 493/728 (67.7%); no pen, 469/725 (64.7%); OR 1.15, 95% CI 0.92 to 1.43; p = 0.22].
Time to response
The median time to return the 14-week questionnaire (among 962 returned) was 16 [interquartile range (IQR) 9–35] days overall: 18 (IQR 9–37) days in the no pen group and 15 (IQR 9–33) days in the pen group. There was weak evidence of a difference in the time to response between the two groups (HR 1.12, 95% CI 0.98 to 1.27; p = 0.09).
Completeness of responses
Rounded to one decimal place, the mean number of completed items (out of five) was the same in both groups, at 4.9 (SD 0.4). There was no evidence of a difference in the number of completed items between the two groups (adjusted mean difference –0.01, 95% CI –0.06 to 0.05; p = 0.77).
Reminders sent
Of the 1453 participants in this SWAT, 708 (48.7%) were sent a reminder [pen group, 339/728 (46.6%); no pen group, 369/725 (50.9%)]. There was weak evidence of a difference in the proportion of participants requiring a reminder between those who received a pen with their original 14-week questionnaire and those who did not receive a pen (OR 0.83, 95% CI 0.68 to 1.02; p = 0.08).
Discussion
This substudy evaluated the effectiveness of including a pen to improve response rates of NHS staff to a postal questionnaire within the SSHeW trial. The key finding from this study was that including a pen did not improve response rates to a postal questionnaire in this population, although there was weak evidence that the time to response and number of reminders required were reduced.
Chapter 4 Economic evaluation
Introduction
As stated in the Scientific summary and Chapter 1, the primary objective of this research was to assess whether or not the offer of slip-resistant footwear to NHS staff would lead to a reduction in the incidence rate of self-reported slips over 14 weeks. A secondary objective was to assess whether or not the provision of the footwear would be cost-effective.
To address this, an economic evaluation was conducted alongside the SSHeW trial. The aim of this economic analysis was to help decision-making in determining whether or not the intervention represents a cost-effective means of mitigating the risk of slips among health-care workers in the UK NHS.
Methods
Overview
Individual participant data collected from the SSHeW trial and a wide range of secondary data were used to perform a within-trial economic analysis that comprised (1) a cost–utility analysis, in terms of the incremental cost per quality-adjusted life-year (QALY); and (2) cost-effectiveness analyses (CEAs), in terms of the incremental cost per averted slip (i.e. using the primary effectiveness outcome of the trial) and cost per averted injury. Costs are presented in GBP at 2018 prices and the analysis was undertaken in Microsoft Excel® 2016 (Microsoft Corporation, Redmond, WA, USA).
Base-case analysis
Overview of approach
The primary outcome measure for the economic evaluation base case is injuries due to slips, as slips that do not result in injury are unlikely to lead to material economic costs. The rarity of injurious events among SSHeW trial participants means that data collected from the trial are likely to be insufficient to enable us to directly infer a relationship between slips and injuries. Therefore, we have modelled the impact of slip-resistant footwear on slip-related injuries.
To model the effect on injuries due to the intervention, it was necessary to determine (1) the appropriate baseline injury rate (see Determining the baseline injury rate) and (2) the estimated reduction in injuries attributable to the intervention footwear (see Determining effectiveness in reducing injuries).
Determining the baseline injury rate
Three different baseline measures were considered:
-
an injury rate derived from Labour Force Survey (LFS) data for injuries due to STFL for the relevant occupation and industry classifications
-
an injury rate derived from responses to a question on the SSHeW trial baseline questionnaire, which asked participants if they had suffered an injury in the past 12 months due to a slip at work
-
the observed injury rate in the SSHeW trial control group.
The LFS was initially the preferred source of injury rate data in this population, given it is a high-quality, nationally representative survey used to derive official statistics, and is the HSE’s preferred data source for workplace injuries. 34 LFS data are self-reported: members of sampled households are asked questions covering many topics, including whether or not they have had an injury at work in the past 12 months, and the nature of the injury. The HSE LFS team derived an injury rate of 0.45 (95% CI 0.33 to 0.58) per 100 workers for STFL for Standard Industry Code 86101 (‘Hospital Activities’), averaged across years 2011/12–2017/18.
The injury rate differs markedly from that reported in the SSHeW trial baseline questionnaire (4.2/100 workers), which also asked about injuries due to slips at work over the past 12 months (see Baseline injuries). Comparison of the LFS and trial baseline data suggests that individuals were more likely to report less severe injuries in the trial than in the LFS. Although the reason for this cannot be determined from the data, it is possible that trial participants answering the baseline questionnaire were primed to recall and report all injuries, including very minor incidents, as a consequence of participating in a trial focused on slips alone. By contrast, the LFS is a large survey covering many aspects of household employment circumstances, which may not elicit the same level of recall and reporting regarding injuries.
Given the large difference from the observed trial data (both the baseline questionnaire and follow-up reporting), the LFS injury rate was not considered suitable for application in the analysis. Therefore, the primary baseline for the analysis is the rate derived from the trial baseline questionnaire, for which we had > 4000 responses. The injury rate observed in the trial control group is used as an alternative baseline measure.
Determining effectiveness in reducing injuries
The base case applies the primary estimate of the effectiveness of the trial intervention, as determined by the adjusted negative binomial model applied in the statistical analysis, which implies a reduction in slip rate of around 37% (central estimate). This assumes that the reduction in the injury rate is the same as the reduction in the slip rate. The effect of alternative levels of injury reduction on cost-effectiveness is tested in sensitivity analysis.
Estimating unit costs per injury avoided and total avoided costs
Given the expected small number of injuries, data on the outcomes of slips (work absence, health-care use, etc.) were likely to be limited, which meant that comparisons between the control and intervention groups were subject to a high degree of random variability. We therefore pooled data across both groups to derive a mean unit treatment cost across all injuries. Results based on the difference in mean outcomes between treatment groups are reported in sensitivity analysis.
Mean unit costs per injury were estimated by aggregating the outcome data by each cost component, set out in Costing framework, across the control and intervention groups, then dividing by the total number of injuries observed in the trial, presented in Trial injury data. Outcome data by treatment group are also presented in the results (see Sensitivity analysis).
Mean costs per injury were multiplied by the expected change in injuries due to the intervention (see Costing framework) to derive total estimated avoided costs attributable to the intervention across the trial population. These are subtracted from intervention costs to estimate total net intervention costs.
Cost-effectiveness analysis
The focus of the economic evaluation was a CEA, with results presented in the form of incremental cost-effectiveness ratios (ICERs) (i.e. the additional cost per extra unit of benefit). The ICERs were calculated as follows:
The following ICERs were estimated, taking net intervention costs over three different outcome measures:
-
cost per averted slip
-
cost per averted injury
-
cost per QALY.
These were estimated from the perspective of both the NHS budget and society. Impacts were modelled over the expected lifetime of the footwear, which is assumed to be 12 months, based on the trial footwear testing analysis presented in SSHeW trial footwear testing.
The ICER was compared with the willingness-to-pay threshold (i.e. the amount a decision-maker is willing to pay for an additional QALY). A willingness-to-pay threshold of £30,000 was used to determine cost-effectiveness, in line with National Institute for Health and Care Excellence (NICE) recommendations. 35 If the estimated cost per QALY was below this threshold, the intervention was considered to be cost-effective. A threshold of £0 was also assessed, which represents the point at which the cost savings from the intervention equal the costs and result in a financial ‘break-even’ for the NHS.
All prices were converted to a 2017/18 base year using the appropriate price index. For health-care costs, the Hospital and Community Health Service Pay and Prices Index36 was used for all years, except 2017/18, when the Personal Social Services Research Unit Health Services Index was used. 36 For NHS pay rates, the NHS spring 2018 pay settlement was used. 37 For all other costs, HM Treasury gross domestic product deflators were used. 38 No discounting of costs or benefits was required, given that the appraisal period is 1 year. Results in the text are presented to an accuracy of three significant figures, which may result in rounding errors in presented values. All calculations were undertaken using non-rounded values.
Economic data collection
Data for outcomes and resource use for the economic analysis were collected prospectively from a wide range of secondary sources. Health service usage and loss of working time was measured using participant-reported questionnaires at 14 weeks and monthly follow-ups after the 14-week trial participation period, until the injury had resolved, the participant wished not to be contacted or the data collection period ended.
Health-related quality of life
Selection of EuroQol-5 Dimensions instrument and value set
The QALY is the metric used to quantify the change in HRQoL due to the intervention. As recommended by NICE, the EuroQol-5 Dimensions (EQ-5D) instrument was used to measure participant health states. 30 The five-level version of the questionnaire was used, with the intention of using utility values for the English population derived for the EQ-5D-5L instrument. 39 However, at the time of writing, NICE does not recommend using the Devlin et al. 39 value set because of concerns raised during quality assurance. 35
Instead, NICE recommends that, when data are gathered using the EQ-5D-5L descriptive system, utility values should be calculated by mapping the five-level descriptive system data onto the three-level valuation set using the function developed by van Hout et al. 40 (also known as the ‘crosswalk’ approach). The EuroQol Group publishes the full crosswalk value sets for several countries, providing recommended utility values mapped to the three-level value set for each of the 3125 health states in the five-level descriptive system. The UK value set has been applied in the present study. 41
To reduce the burden on trial participants, EQ-5D data were collected for the first slip reported (whether or not an injury had occurred and whether in the control or intervention group) and then during follow-up at monthly intervals for those reporting an injury, until the injury was resolved (rather than from all participants at all time points). This was on the basis that the individuals were in employment and working, so were likely to report high health state scores reflective of the generally healthy working population.
Baseline health utility for the trial population was estimated using EQ-5D data from participants reporting no injury with complete EQ-5D responses on the SDCS (n = 1002) (see Baseline health utility). This was preferred to published population norm values (e.g. Kind et al. 42) because of the high specificity of the trial baseline data to the trial population.
Overview of quality-adjusted life-year estimation approach
The total change in QALYs due to the intervention was estimated by:
-
estimating total QALYs for the duration of each reported injury at the participant level, when complete data on EQ-5D are available, along with dates of injury and form completion, which were used to calculate duration
-
estimating baseline QALYs for the duration of injury reported by each participant, calculated by applying baseline health utility
-
subtracting total QALYs lived from baseline QALYs for each injury to estimate per injury QALY loss, enabling calculation of the mean QALY loss per injury
-
multiplying the mean QALY loss per injury by the expected change in injuries due to the intervention derived in Measures of effectiveness in reducing injuries.
Utilities derived from EQ-5D were converted to QALYs based on the area under the curve method, following the trapezium rule and assuming linear interpolation between monthly follow-up points. 43
Data exclusions
Observations were excluded if the following data were incomplete:
-
EQ-5D data at all follow-up points when an injury was reported and not resolved
-
data required to calculate duration: date of slip (on the SDCS) or injury (for monthly follow-ups), form completion dates and days since fully recovered (when the participant indicates that their injury has fully resolved during the last period).
EuroQol-5 Dimensions observations were also excluded if the number of fully recovered days was greater than the number of days elapsed since last observation or since the participant had reported perfect health (11111), both indicating that the participant had fully recovered prior to completing the form.
Calculating duration
For observations with complete data, duration of the observed health state is calculated as follows:
-
for observations on the SDCS, the difference between the slip date (when an injury was reported) and the form completion date
-
for monthly follow-ups when the participant indicated that they had recovered, the difference between the previous observation and the number of days since fully recovered
-
for monthly follow-ups when the participant had not fully recovered, the difference between the last observation and the form completion date.
Further considerations in assigning utility values
Several issues with the data required judgements regarding the assignment of utility values. In all cases there was a lag between the reported slip date and the date of completion of the SDCS, which collected for first reported slips data on the nature of any injury and HRQoL (EQ-5D). The analysis assumes that HRQoL for the period between injury and reporting on SDCS is as per the value at SDCS, as the health state at the point of injury is not observed. The mean elapsed time between slip date and date of the SDCS across the sample was 17 (SD 16, minimum 3, maximum 66) days, so it is likely that many participants would have partially or fully recovered from their inury in this time, meaning that QALY loss during this period is underestimated.
Additionally, participants were sent a SDCS only for the first reported slip. The primary route to monthly follow-up was for participants reporting injury on the SDCS (although injured participants were also identified in several other ways, such as by text message, direct telephone call or e-mail to a member of the York trial team or local research team at participating sites). Therefore, many follow-up injuries are different to those reported on the SDCS. To estimate the correct duration and interpolation of utility weight between observations, and to allow for some recall and reporting error on the part of participants, the analysis treated follow-up observations as pertaining to the same injury as reported on the SDCS if the difference between the slip date reported on the SDCS and the injury date reported on the follow-up was < 10 days.
Costing framework
The conceptual framework applied in the CEA (Figure 6) is based on the HSE’s costs of illness model,44 which is used to produce annual national statistics on the costs of workplace injuries and illnesses in GB.
FIGURE 6.
Summary of cost-effectiveness model.
Intervention costs
NHS trusts implementing the footwear intervention will primarily incur two types of costs: (1) the cost of the footwear and (2) resources expended in distributing the footwear to staff.
Footwear purchase costs
Data from the trial on the number and style of footwear purchased, along with the purchase cost from the trial footwear supplier, were used to estimate a weighted average purchase cost per pair. This was then multiplied by the number of employees in the trial intervention group to estimate the purchase cost from the NHS perspective. From the societal perspective, the provision of the intervention footwear displaces footwear that would otherwise be purchased and worn (or, if own footwear is worn occasionally, as observed in the trial, then own footwear is replaced less frequently because of reduced wear). If prior to the intervention employees purchase their own footwear, the intervention represents a transfer from the NHS employer to the member of staff (which, depending on the relative purchase prices, may entirely cancel out). Data on the costs and longevity of footwear usually worn were gathered from 311 trial participants at two trusts during recruitment to the trial to estimate the costs differential between intervention and baseline footwear.
Distribution costs
The trial protocol proposed not to quantify the costs of distributing footwear to staff, on the basis that the NHS has existing dress requirements and provides staff uniform, and any staff time incurred in distributing the footwear to staff in addition to existing processes for uniform would be negligible.
Discussions with a small number of staff at participating trusts involved in distributing footwear during the trial indicated that this had, in some cases, been burdensome, because of the nature of a large number of orders being created and handled in a short period of time. Other, more cost-effective, methods are likely to be applied in practice. The ordering and distribution of shoes could be staggered to minimise logistical costs. An alternative model, confirmed by the footwear supplier as operating in other sectors [T Swinney, Shoes For Crews (Europe) Ltd, 19 September 2019, personal communication], would be for NHS staff to obtain the shoes directly from the supplier, using a unique code to pass the charge on to the NHS, with the footwear delivered to the employee’s home address or other chosen location. The supplier would then charge the NHS on a periodic basis for all shoes supplied. Delivery and returns would be free of charge (as confirmed by the supplier).
Therefore, distribution costs are treated as negligible and accounted for in the existing footwear purchase price.
Health-care resource use
Health-care resource use data were collected via participant-reported questionnaires at 14 weeks and, for injuries unresolved after 14 weeks, during the monthly injury follow-up questionnaire (collected until either the participant reported that the injury had fully resolved or the trial data collection ended).
Participants were asked to provide information on their use of the following services: (1) primary and community care [e.g. general practitioner (GP) and nurse attendance, physiotherapy and occupational therapist visits] and (2) secondary and hospital care (e.g. inpatient, accident and emergency, day case and outpatient attendances, podiatry care). Reported episodes of care were valued using the relevant NHS reference costs45 and Personal Social Services Research Unit unit costs of health and social care,36 summarised in Table 14.
| Episode of care | Unit cost (£) | Source |
|---|---|---|
| GP at a general practice or at home | 43.40 | a Unit Costs of Health and Social Care 201836 |
| Nurse at a general practice or at home | 12.61 | a Unit Costs of Health and Social Care 201836 |
| Occupational therapist | 74.87 | a Unit Costs of Health and Social Care 201836 |
| Physiotherapist | 50.99 | a Unit Costs of Health and Social Care 201836 |
| Podiatrist | 44.48 | a Unit Costs of Health and Social Care 201836 |
| Clinic outpatient visit | 125.00 | NHS Reference Costs 2017/18 45 |
| A&E visit | 160.00 | NHS Reference Costs 2017/18 45 |
| Hospital day case | 742.00 | NHS Reference Costs 2017/18 45 |
| Non-elective inpatient | 1603.00 | NHS Reference Costs 2017/18 45 |
To derive the unit health-care costs per injury, the total costs of health-care resource use observed in the trial were divided by the relevant number of trial injuries. For the base case, the calculation included health-care use relating to injuries resulting from slips and the denominator was the total number of injuries resulting from slips reported on the 14-week questionnaire. The estimated unit health-care costs per injury were multiplied by the expected change in the number of injuries due to the intervention across the trial population, to estimate the total expected avoided health-care costs.
Absenteeism costs
From the NHS perspective, worker absence because of injury gives rise to costs associated with temporarily replacing the injured worker and sickness payments made to the injured worker (in lieu of the worker’s wages).
Data on time off work were collected on the SDCS, 14-week questionnaire and at monthly follow-ups after this period. To avoid double counting, time off work data from the SDCS were not included, as this period was covered by the 14-week questionnaire.
Hourly pay rates, including on-wage costs (pensions and National Insurance contributions), were obtained from one NHS trust involved in the trial for each of the occupation types and checked against published NHS earnings data.
Sickness payments were modelled based on the NHS Terms and Conditions of Service Handbook. 46 The rate and duration of sickness pay depends on length of service. Given that 69% of hours lost in the trial occurred within the first month of absence, no absences were > 4 months, and data on the duration of service for trial participants were not available, we have assumed for simplicity that all participants receive sickness payment equivalent to full pay for the duration of their absence.
The NHS can take a range of actions to ensure continuity of service in the event of worker absence due to injury, including hiring replacement bank or agency staff, seeking overtime cover from existing staff or absorbing the absence if staffing levels are adequate. A range of scenarios were considered; however, modelling these would add considerable complexity to the analysis. Therefore, we made a further simplifying assumption that all absences were covered by overtime, at the standard NHS rate of 1.5 times pay, which should provide a reasonable approximation of the likely costs. 46
At the societal level, the principal economic cost of worker absenteeism caused by injury is the loss of output or production due to one less worker (temporarily or permanently) in the labour force. We apply the conventional assumption that the worker’s marginal productivity is at least equal to their costs of employment. 47 This is equal to (1) the worker’s wage plus (2) on-wage costs (National Insurance and pensions contributions). This assumes that the replacement worker would otherwise be doing some other productive activity (i.e. is not involuntarily unemployed). This is reasonable given (1) the current historically high levels of employment and (2) the high vacancy rate in the NHS relative to other public sector occupations.
The analysis did not account for presenteeism costs; that is, instances of when staff returned to work following injury, but before they were fully recovered. This can result in staff being less productive for some period, because of reduced strength or mobility. Quantifying the incidence of presenteeism, and its effects on productivity, is challenging,48 and was not undertaken to avoid adding complexity and uncertainty to the data collection and analysis.
Compensation costs
Workers who suffer an injury caused by a slip or fall at work may submit a claim for compensation. NHS Resolution handles these claims on behalf of NHS trusts, via the Liabilities to Third Parties Scheme. The Liabilities to Third Parties Scheme claims are subject to excesses, with NHS trusts responsible for funding below-excess claims. However, after discussions with NHS Resolution and with a NHS claims manager who was working in one of the participating trusts, it appears that, in practice, NHS Resolution handles the vast majority of claims. Damages paid for settled claims represent a transfer from the NHS to the claimant worker, with additional costs arising from legal resources expended on behalf of the claimant and defendant.
Data on total non-clinical claims and associated claims cost for injuries with ‘slip or trip’ as the primary reason code for NHS employees between 2007/8 and 2016/17 were obtained from NHS Resolution via a freedom of information request (Table 15). Total costs to the NHS are the sum of claimants’ recoverable legal costs (see Table 15, column 2), total damages paid (see Table 15, column 3) and total defence costs (see Table 15, column 4). From this, the compensation cost per claim can readily be calculated; there were on average 528 settled claims per year over the period, resulting in an average annual total claim cost of £12.7M (or £24,000 per claim). However, this is likely to differ from the cost per injury, as many injuries will not result in a claim.
| Year of notification | Number of settled claims closed | Total damages paid (£) | Total defence costs (£) | Total claimant costs paid (£) | Sum of total paid (£) |
|---|---|---|---|---|---|
| 2007/8 | 608 | 5,908,829 | 1,585,870 | 5,846,194 | 13,340,893 |
| 2008/9 | 603 | 5,826,471 | 1,569,863 | 5,612,495 | 13,008,829 |
| 2009/10 | 459 | 4,645,895 | 1,131,722 | 4,276,821 | 10,054,437 |
| 2010/11 | 482 | 4,720,081 | 891,907 | 4,306,962 | 9,918,950 |
| 2011/12 | 801 | 8,732,507 | 1,369,108 | 7,260,387 | 17,362,002 |
| 2012/13 | 588 | 7,027,166 | 1,149,515 | 6,094,007 | 14,270,688 |
| 2013/14 | 552 | 7,270,611 | 1,207,061 | 6,425,411 | 14,903,084 |
| 2014/15 | 444 | 6,807,880 | 1,062,790 | 5,535,280 | 13,405,950 |
| 2015/16 | 410 | 5,190,604 | 867,058 | 4,917,494 | 10,975,156 |
| 2016/17 | 332 | 4,582,955 | 790,836 | 3,909,156 | 9,282,947 |
| Total | 5279 | 60,712,999 | 11,625,731 | 54,184,207 | 126,522,937 |
| Average annual | 528 | 6,071,300 | 1,162,573 | 5,418,421 | 12,652,294 |
Claim costs per injury were derived by dividing the average annual total claim cost by the total annual injuries due to slips across the NHS. The latter was estimated by applying the estimated baseline annual injury rate from the trial (of 4.06 per 100 workers, see Baseline injuries) to the 1.22 million NHS employees,49 giving an estimated 49,800 annual slip-related injuries. Using this as the denominator results in an estimated compensation claim cost per injury of £254.
At the societal level, the damages paid were excluded as a transfer, leaving average annual costs of £6.66M (total defence costs plus total claimant costs), or £132 per injury.
Sensitivity analysis and uncertainty
The main sources of uncertainty in the economic evaluation relate to effectiveness of the intervention in reducing injuries; mean impact per injury averted, in terms of unit costs and loss of QALYs; intervention costs, depending on the model of distribution adopted; and sampling variability, owing to the rarity of injuries.
Given the limited number of data on injury outcomes from the trial, probabilistic sensitivity analysis was not undertaken. Instead, the effect of uncertainty in input parameters was assessed through one- and two-way sensitivity analyses. These tested the change in values from the base case required to result in costs per QALY equivalent to £30,000 (i.e. the NICE threshold) and £0 (i.e. financial break-even). To simplify the analysis, combined mean cost savings per averted injury were tested, rather than the individual cost components (absenteeism, health-care costs and compensation), as these would be highly correlated. Although this form of sensitivity analysis does not account for all sources of uncertainty simultaneously, it facilitates a qualitative assessment of the likelihood that the intervention is cost-effective by placing bounds on the magnitude of parameters required to meet alternative thresholds.
A further uncertainty relating to the base-case modelling approach is that it assumes that the main effect of the intervention footwear is to reduce the frequency of injuries and that severity of injuries is essentially unchanged. Given this, the results from a direct comparison of outcomes by treatment group is also presented in the sensitivity analysis, which, in principle, accounts for both effects, although it is subject to a high degree of random variability because of the small injury numbers.
Validation of results
The Microsoft Excel analysis was quality assured by a second HSE economist.
Results
Intervention effectiveness in reducing injuries
Trial injury data
Injuries due to slips
Table 16 summarises the data on injuries reported in the trial via the SDCS and the end-of-trial 14-week questionnaire. Via the SDCS, Thirty-one participants (1.4%) in the control group reported an injury on the SDCS, compared with 26 participants (1.1%) in the intervention group. Forty participants (1.8%) in the control group reported an injury on the 14-week questionnaire, and reported 49 separate incidents in total (a rate of 2.2%), compared with 23 (1.0%) participants reporting injury in the intervention group, reporting 29 incidents in total (1.3%).
| Injury | Control group (N = 2275) | Intervention group (N = 2278) | Total (N = 4553) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Injuries due to slips reported on SDCS | ||||||
| Participants reporting injury (% of participants in group) | 31 | 1.4 | 25 | 1.1 | 56 | 1.2 |
| Number of injury typesa | 32 | 26 | 58 | |||
| Injury reported (% of all injury types) | ||||||
| Superficial wounds | 17 | 53.1 | 12 | 46.2 | 29 | 50.0 |
| Broken bones | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Pulled muscles/sprained ligaments | 12 | 37.5 | 10 | 38.5 | 22 | 37.9 |
| Other injuries | 3 | 9.4 | 4 | 15.4 | 7 | 12.1 |
| Injuries due to slips reported on the 14-week questionnaire | ||||||
| Participants reporting injury (% of participants in group) | 40 | 1.8 | 22 | 1.0 | 62 | 1.4 |
| Number of slips resulting in injuryb | 49 | 2.2 | 26 | 1.1 | 75 | 1.6 |
| Number of injury typesa | 50 | 33 | 83 | |||
| Injury reported (% of all injury types) | ||||||
| Superficial wounds | 24 | 48.0 | 16 | 48.5 | 40 | 48.2 |
| Broken bones | 1 | 2.0 | 0 | 0.0 | 1 | 1.2 |
| Pulled muscles/sprained ligaments | 21 | 42.0 | 8 | 24.2 | 29 | 34.9 |
| Other injuries | 4 | 8.0 | 9 | 27.3 | 13 | 15.7 |
| Injuries due to falls not resulting from a slip reported on the 14-week questionnaire | ||||||
| Participants reporting injury (% of participants in group) | 5 | 0.2 | 4 | 0.2 | 9 | 0.2 |
| Number of falls not due to slips resulting in injuryb | 6 | 0.3 | 4 | 0.2 | 10 | 0.2 |
| Number of injury typesa | 16 | 10 | 26 | |||
| Injury reported (% of all injury types) | ||||||
| Superficial wounds | 7 | 14.0 | 5 | 15.2 | 12 | 14.5 |
| Broken bones | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Pulled muscles/sprained ligaments | 4 | 8.0 | 2 | 6.1 | 6 | 7.2 |
| Other injuries | 5 | 10.0 | 3 | 9.1 | 8 | 9.6 |
Superficial wounds were the most common type of injury across both groups, accounting for 48.0% of injuries in the control group and 47.1% of injuries in the intervention group at 14 weeks. Pulled muscles and sprained ligaments were the second most commonly reported injury overall, accounting for 42.0% of injuries in the control group and 23.5% of injuries in the intervention group at 14 weeks. One broken bone was reported, by a participant in the control group.
A total of 32 participants reported an injury on the SDCS but did not report injury at 14 weeks. Of these participants, 23 had completed at least some of the 14-week questionnaire.
Injuries due to falls not resulting from a slip
Of the nine participants reporting an injury from a fall not resulting from a slip, five were in the control group (0.2% of control group participants) and four were in the intervention group (0.2% of intervention group participants). As with injuries due to slips, superficial wounds were the most common reported injury, accounting for 14.5% of injuries reported across both groups.
As the trial statistical analysis did not find a statistically significant reduction in the rate of falls not due to slips, data relating to these are not accounted for further in the economic evaluation.
Baseline injuries
Table 17 shows the estimates of baseline injuries applied in the economic evaluation. Of the 4553 participants providing trial baseline questionnaire data, 185 (4.06 per 100 participants) reported an injury due to a slip at work in the past 12 months. In the trial, 49 injuries were reported on the 14-week questionnaire (a rate of 2.15 per 100 control group participants). Scaling this to an annual rate (making no adjustment for seasonal variation, given that participant involvement in the trial was spread throughout the year) suggests an annual injury rate of 8.00 per 100 participants in the control group, giving an expected 364 baseline injuries across the trial population.
| Baseline option | Number of injuries at 14 weeks, n (%) of participants | Annual injury rate per 100 people | Expected injuries for trial population over 1 year |
|---|---|---|---|
| Trial baseline survey (n = 4553) | n/a | 4.06 | 185 |
| Trial control group (n = 2775) | 49 (2.15) | 8.00 | 364 |
Both baselines are used in the analysis to construct alternative scenarios.
Measures of effectiveness in reducing injuries
The primary measure of effectiveness is based on the proportional reduction in the rate of slips due to the intervention, as estimated in the primary statistical analysis. This is estimated as an intention-to-treat IRR of 0.63 (central estimate), implying a reduction of 37% in the rate of slips in the intervention group. Applying this to the expected number of annual injuries over the trial population gives an expected reduction in annual injuries over the trial population of 68 under baseline 1 and 135 under baseline 2.
Health-related quality of life
Baseline health utility
The baseline health utility weight was based on responses to the 1002 SDCSs completed by participants who provided complete EQ-5D data and did not report an injury (Table 18). Applying the crosswalk approach set out in van Hout et al. 40 gave a mean utility of 0.911 (SD 0.130, minimum 0.088, maximum 1).
| Level | EQ-5D-5L domension | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mobility | Self-care | Usual activities | Pain/discomfort | Anxiety/depression | ||||||
| n | % | n | % | n | % | n | % | n | % | |
| 1 | 907 | 91 | 995 | 99 | 930 | 93 | 683 | 68 | 817 | 82 |
| 2 | 81 | 8 | 7 | 1 | 62 | 6 | 234 | 23 | 133 | 13 |
| 3 | 11 | 1 | 0 | 0 | 9 | 1 | 71 | 7 | 44 | 4 |
| 4 | 3 | 0 | 0 | 0 | 0 | 0 | 12 | 1 | 5 | 0 |
| 5 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 3 | 0 |
Quality-adjusted life-years and quality-adjusted life-year loss during the trial
Total QALY loss associated with an injury was estimated from the differences between baseline QALYs and observed QALYs for each injured participant with complete QALY data, for the duration of each reported injury. Mean QALY loss per injury was estimated by dividing total QALY loss by the number of injuries with complete QALY data.
Total QALY loss over the duration of the trial was 0.817 QALYs, with 0.521 QALY loss (63.8% of total) occurring in the control group and 0.296 QALY loss (36.2% of total) occurring in the intervention group (Table 19). Mean loss per injury across both groups was 0.017 (SD 0.028) QALYs, with a higher mean loss in the control group (0.019 QALYs, SD 0.032 QALYs) than in the intervention group (0.016 QALYs, SD 0.022 QALYs).
| QALY loss data | Control group | Intervention group | Total | Difference (intervention – control) |
|---|---|---|---|---|
| On SDCS | ||||
| Number of injured participants with complete data, minus exclusions | 21 | 15 | 36 | –6.00 |
| Number of complete observations | 21 | 15 | 36 | –6.00 |
| Total duration of period (years)a | 1.02 | 0.80 | 1.82 | –0.21 |
| Mean duration of period per participant, years (SD) | 0.05 (0.05) | 0.05 (0.04) | 0.05 (0.05) | 0.01 |
| Total QALYs lived for injured participants | 0.78 | 0.59 | 1.37 | –0.19 |
| Weighted average injured HRQoL weight for period | 0.76 | 0.73 | 0.75 | –0.03 |
| Total QALYs lived: baseline | 0.93 | 0.73 | 1.66 | –0.19 |
| Total QALY loss | 0.150 | 0.142 | 0.292 | –0.008 |
| Average QALY loss per injury with complete data, minus exclusions (SD) | 0.007 (0.009) | 0.009 (0.017) | 0.008 (0.013) | 0.002 |
| On monthly follow-ups | ||||
| Number of injured participants with complete data, minus exclusions | 15 | 8 | 23 | –7.00 |
| Number of complete observations | 20 | 23 | 43 | 3.00 |
| Total duration of period (years)a | 1.77 | 0.74 | 2.51 | –1.03 |
| Mean duration of period per participant, years (SD) | 0.12 (0.04) | 0.08 (0.04) | 0.10 (0.04) | –0.04 |
| Total QALYs lived for injured participants | 1.24 | 0.52 | 1.77 | –0.72 |
| Weighted average injured HRQoL weight for period | 0.70 | 0.70 | 0.70 | 0.00 |
| Total QALYs lived: baseline | 1.61 | 0.68 | 2.29 | –0.94 |
| Total QALY loss | 0.371 | 0.154 | 0.525 | –0.218 |
| Average QALY loss per injury with complete data, minus exclusions (SD) | 0.025 (0.039) | 0.017 (0.021) | 0.023 (0.033) | –0.008 |
| Over the trial | ||||
| Number of injured participants with complete data, minus exclusions | 28 | 18 | 46 | –10 |
| Number of complete observations | 41 | 38 | 79 | –3 |
| Total duration of period (years)a | 2.79 | 1.55 | 4.33 | –1.43 |
| Mean duration of period per participant, years (SD) | 0.10 (0.14) | 0.08 (0.08) | 0.09 (0.12) | –0.02 |
| Total QALYs lived for injured participants | 2.02 | 1.11 | 3.13 | –0.91 |
| Weighted average injured HRQoL weight for period | 0.72 | 0.72 | 0.72 | 0.00 |
| Total QALYs lived: baseline | 2.54 | 1.41 | 3.95 | –1.13 |
| Total QALY loss | 0.521 | 0.296 | 0.817 | –0.226 |
| Average QALY loss per injury with complete data, minus exclusions (SD) | 0.019 (0.032) | 0.016 (0.022) | 0.017 (0.028) | –0.003 |
Calculation of quality-adjusted life-years: anomalies and exclusions
Quality-adjusted life-years were estimated up to the point of final follow-up (when the injury was resolved, the participant no longer wished to be contacted or the trial data collection ended). Nine of the 16 participants who provided complete data on monthly injury follow-ups had not fully recovered by the time of their final follow-up observation. This suggests that estimated QALY loss for these participants is incomplete and will be underestimated for participants as a whole. The paucity of data precludes modelling QALY loss beyond the period of data collection.
As described in Health-related quality of life, observations with complete data for which participants have reported injury and perfect health (11111) have been excluded, as this indicates that the participant has recovered from the injury and otherwise results in estimated QALY gains due to injury, relative to baseline HRQoL. This removed 10 participants at the SDCS stage and six participants from the monthly follow-ups. Including these participants would reduce the mean QALY loss per injury across control and intervention groups to 0.010 (SD 0.026) QALYs.
Two participants were followed up and provided complete data to estimate QALYs but reported no injury on either the SDCS or the 14-week questionnaire. Both participants were excluded from the analysis. One participant stated explicitly on the follow-up questionnaire that they were injured from a trip at home, whereas the other participant did not provide sufficient data to determine whether or not an injury occurred at all and, if so, if this was due to a slip or fall at work, or some other cause.
Health-care resource use
Health-care use reported on the 14-week and post-14-week follow-up questionnaires
Table 20 reports total health-care use by follow-up point and treatment group. Over the duration of the trial, a total of 13 participants required health care for an injury caused by a slip at work [seven participants in the control group and six participants in the intervention group (one participant in the intervention group reported health-care use in the post-14-week period but not at 14 weeks)]. Physiotherapy was the most commonly used care (39 visits in the control group and 10 visits in the intervention group), followed by a nurse appointment at a general practice (15 visits in the control group and seven visits in the intervention group) and accident and emergency attendance (14 visits in the control group and four visits in the intervention group).
| Health-care resource use | Control group, n | Intervention group, n | Total, n | Difference, n (intervention – control) |
|---|---|---|---|---|
| On 14-week questionnaire | ||||
| Total participants reporting health-care use | 6 | 6 | 12 | 0 |
| GP visit | 4 | 6 | 10 | 2 |
| Nurse at general practice | 0 | 0 | 0 | 0 |
| Occupational therapist | 0 | 0 | 0 | 0 |
| Physiotherapist | 13 | 10 | 23 | –3 |
| Podiatrist | 1 | 0 | 1 | –1 |
| Outpatient | 2 | 1 | 3 | –1 |
| A&E | 5 | 4 | 9 | –1 |
| Hospital visit as a day case | 0 | 0 | 0 | 0 |
| Days inpatient stay | 0 | 0 | 0 | 0 |
| Total costs of care (£) | 1931 | 1535 | 3466 | –396 |
| Post-14-week monthly follow-ups | ||||
| Total participants reporting health-care use | 3 | 1 | 4 | –2 |
| GP visit | 11 | 1 | 12 | –10 |
| Nurse at general practice | 0 | 0 | 0 | 0 |
| Occupational therapist | 1 | 0 | 1 | –1 |
| Physiotherapist | 26 | 0 | 26 | –26 |
| Podiatrist | 0 | 0 | 0 | 0 |
| Outpatient | 0 | 0 | 0 | 0 |
| A&E | 9 | 0 | 9 | –9 |
| Hospital visit as a day case | 4 | 0 | 4 | –4 |
| Days inpatient stay | 2 | 0 | 2 | –2 |
| Total costs of care (£) | 9492 | 43 | 9536 | –9449 |
| Over the trial | ||||
| Total participants reporting health-care use | 6 | 7 | 13 | 1 |
| GP visit | 15 | 7 | 22 | –8 |
| Nurse at general practice | 0 | 0 | 0 | 0 |
| Occupational therapist | 1 | 0 | 1 | –1 |
| Physiotherapist | 39 | 10 | 49 | –29 |
| Podiatrist | 1 | 0 | 1 | –1 |
| Outpatient | 2 | 1 | 3 | –1 |
| A&E | 14 | 4 | 18 | –10 |
| Hospital visit as a day case | 4 | 0 | 4 | –4 |
| Days inpatient stay | 2 | 0 | 2 | –2 |
| Total costs of care (£) | 11,423 | 1579 | 13,002 | –9844 |
| Number of injuries at 14 weeks | 49 | 26 | 75 | –23 |
| Mean costs of care per injury (£) | 233 | 61 | 173 | –172 |
Table 20 also reports the associated costs of health care, calculated by applying the unit costs in Table 14. Total costs of care across the duration of the trial were £13,000, of which the vast majority (£11,400, 87.9%) occurred in the control group. Three participants in the control group accounted for £10,500, or 80.4% of total health-care costs.
Average health-care costs per injury were £233 in the control group, £61 in the intervention group and £173 across all participants.
The following anomalies and exclusions should be noted:
-
Six respondents selected ‘other’ health-care use and provided information. It was evident that all but one of these respondents duplicated information provided in the specified categories and so these responses were removed. One respondent reported five sessions of sports therapy, which were recoded as physiotherapy.
-
One participant answered ‘yes’ to needing health care, but did not provide details. The injury was a sprain and the participant indicated on the 14-week questionnaire that they had recovered from injury. No health-care use was included for this participant.
-
One participant provided a large number of health-care use data but was excluded from the results because the participant’s comments on the questionnaire indicated that the injury was due to a trip at home.
Additional health-care use reported on the slip data collection sheet
Eight participants reported health-care use on the SDCS that was not reported on the 14-week questionnaire or post-14-week follow-ups, amounting to £3800 in resource costs. These costs were not included in the CEA, as per the methodology, but are discussed as follows:
-
Four participants did not report an injury on either the SDCS or 14-week questionnaire and were not followed up for injury.
-
One of the eight participants reported a slip-injury on the 14-week questionnaire, although the comments suggested that the reported outpatient appointment related to a pre-existing injury.
-
Of the remaining three participants, one participant reported a fall injury not related to a slip on the 14-week questionnaire, with a similar date to the slip reported on the SDCS. The reported health-care use and costs (£1110) would be taken as relating to a fall not caused by slipping and so are not included in the analysis. The remaining two participants reported an injury on SDCS, but did not complete the 14-week questionnaire. Given that the associated health-care use and costs for these were very low (£145), they would not have made a material difference to the results.
Of those participants who reported health-care use on both the SDCS and during later follow-up questionnaires, none reported higher costs on the SDCS.
Absenteeism costs
The data on self-reported absence from work because of injuries caused by slips at work are summarised in Table 21. Most participants suffering an injury due to a slip at work reported taking no time off work. A total of 57 participants reported no absence (37 participants in the control group, amounting to 86% of those reporting injury, and 20 participants in the intervention group, amounting to 80% of those reporting injury). A total of 11 participants reported taking time off work (six participants in the control group and five participants in the intervention group).
| Self-reported lost working hours | Control group | Intervention group | Total | Difference (intervention – control) |
|---|---|---|---|---|
| Reported on 14-week questionnaire | ||||
| Total lost working hours | 520 | 388 | 908 | –132 |
| Number of participants reporting absence | 5 | 5 | 10 | 0 |
| Of those reporting absence | ||||
| Mean duration (hours) per participant (SD) | 104 (113) | 78 (74) | 91 (91) | –26 |
| Median (minimum, maximum) hours | 60 (2, 263) | 30 (16, 180) | 45 (2, 263) | –30 |
| Post-14-week follow-up | ||||
| Total lost working hours | 643 | 0 | 643 | –643 |
| Number of participants reporting absence | 3 | 0 | 3 | –3 |
| Of those reporting absence | ||||
| Mean duration (hours) per participant | 214 (137) | 0 | 214 (137) | –214 |
| Median (minimum, maximum) hours | 263 (60, 320) | 0 | 263 (60, 320) | –263 |
| Total over the trial | ||||
| Total lost working hours | 1162 | 388 | 1550 | –774 |
| Number of participants reporting absence | 6 | 5 | 11 | –1 |
| Number of participants reporting injury but no absence | 37 | 20 | 57 | |
| Of those reporting absence | ||||
| Mean duration (hours) per participant (SD) | 194 (200) | 78 (74) | 141 (161) | –17 |
| Median (minimum, maximum) hours | 150 (2, 525) | 30 (16, 180) | 120 (2, 525) | –120 |
Control group participants reported a total of 1162 lost working hours because of an injury caused by a slip (compared with 388 lost working hours in the intervention group). Injuries in the control group resulted, on average, in a much longer absence (194 hours, SD 200 hours) than injuries in the intervention group (78 hours, SD 74 hours). Over half of the hours lost in the control group occurred during the long-term monthly follow-ups, whereas no participants reported hours lost past 14 weeks in the intervention group. One injury in the control group accounted for almost half (45.2%) of the total time off work in that group.
Table 22 summarises the distribution of time off work for injuries due to slips at work taken by participants over the duration of the trial, by treatment group. Overall, over the period of data collection, the vast majority of participants (84% across both groups) who reported an injury resulting from a slip reported no time off work due to the injury. Participants in the control group were more likely to have reported longer absence from work, although this is based on a small number of observations and so is subject to a high degree of random variation.
| Time off work | Control group | Intervention group | Total | |||
|---|---|---|---|---|---|---|
| Participants, n | % total | Participants, n | % total | Participants, n | % total | |
| No time off | 37 | 86 | 20 | 80 | 57 | 84 |
| Up to 1 week | 2 | 5 | 3 | 12 | 5 | 7 |
| Between 1 and 2 weeks | 0 | 0 | 0 | 0 | 0 | 0 |
| Between 2 weeks and 1 month | 1 | 2 | 1 | 4 | 2 | 3 |
| > 1 month | 3 | 7 | 1 | 4 | 4 | 6 |
| Total | 43 | 100 | 25 | 100 | 68 | 100 |
Table 23 summarises the number of lost working hours and hourly wage rates (including on-wage costs) by occupation. Other qualified staff and health-care professionals account for the largest absence (525 hours, 33.9% of lost working hours due to slips), resulting from the absence of one participant. Health-care assistants accounted for the second largest amount of time off work (two participants, 300 hours, 19.4% of total hours). Qualified nurses and midwives accounted for the largest number of participants reporting time off. All participants reporting their occupation as ‘other’ were recoded to the nearest category. None of the four participants who failed to complete the occupation field (i.e. missing data) reported injury or time off work.
| Occupation | Participants reporting absence, n | Total lost working hours, n | Lost hours per participant, n | Hourly staff cost (basic wage + on-wage costs, £) |
|---|---|---|---|---|
| Doctor/consultant | 0 | 0 | 0 | 68 |
| Qualified nurse/midwife | 5 | 257 | 51 | 17 |
| Physiotherapist | 0 | 0 | 0 | 21 |
| Porter | 0 | 0 | 0 | 11 |
| Occupational therapist | 0 | 0 | 0 | 21 |
| Health-care assistant | 2 | 300 | 150 | 12 |
| Ward clerk | 0 | 0 | 0 | 12 |
| Pharmacist/pharmacy technician | 0 | 0 | 0 | 30 |
| Social worker | 0 | 0 | 0 | 21 |
| Support worker | 1 | 132 | 132 | 12 |
| Podiatrist | 0 | 0 | 0 | 21 |
| Administrator/receptionist/secretarial | 2 | 336 | 168 | 11 |
| Other qualified staff/health-care professional | 1 | 525 | 525 | 21 |
| Catering | 0 | 0 | 0 | 12 |
| Imaging | 0 | 0 | 0 | 47 |
| Total | 11 | 1550 | 141 |
Table 24 presents the total estimated sickness payments, overtime payments and value of lost working time due to slip-related injuries. The total value of lost working time in the control group was £19,100, compared with £4850 in the intervention group. Overtime payments were similarly larger in the control than in the intervention group (£28,700 vs. £7270, respectively). The costs of sickness payments are equivalent to the value of lost working time, given the modelling assumption that workers are paid their full wage when absent.
| Absenteeism | Control group | Intervention group | Total | Difference (intervention – control) |
|---|---|---|---|---|
| Total sickness payments (£) | 19,117 | 4846 | 23,963 | –14,270 |
| Total cost of overtime staff (£) | 28,675 | 7270 | 35,945 | –21,405 |
| Total value of lost working time (marginal employee cost) (£) | 19,117 | 4846 | 23,963 | –14,270 |
| Total cost to the NHS (£) | 28,675 | 7270 | 35,945 | –21,405 |
| Total cost to society (£) | 19,117 | 4846 | 23,963 | –14,270 |
| Total injuries (n) | 49 | 26 | 75 | –23 |
| Mean sickness payments per injury (£) | 390 | 186 | 320 | –204 |
| Mean overtime payments per injury (£) | 585 | 280 | 479 | –306 |
| Mean staff costs not paid for injured workers per injury (£) | 390 | 186 | 320 | –204 |
| Mean cost to the NHS per injury (£) | 585 | 280 | 479 | –306 |
| Mean cost to society per injury (£) | 390 | 186 | 320 | –204 |
Table 24 also shows the estimated mean costs per injury by treatment group. The estimated mean unit cost per injury to the NHS was £479 across both groups, £585 in the control group and £280 in the intervention group. The total mean cost to society per injury was £320 (£390 in the control group and £186 in the intervention group).
Two participants reported needing time off work due to a slip-related injury (one superficial injury, one not specified); however, the participants did not provide the number of hours. Therefore, it was assumed that no time off was taken in both cases.
A comparison of the absence data identified that 7.5 hours’ absence reported on the SDCS was missing from the 14-week questionnaire. Given that the participant completed some of the 14-week questionnaire but did not report an injury at that point, and to avoid further complication to the analysis, these hours are excluded.
Compensation costs
Compensation costs estimated that the mean compensation cost per slip-related injury to the NHS was £254, based on secondary data. Applying this value, the number of injuries observed in the trial gave a total compensation cost of £19,100 to the NHS, with £12,400 incurred in the control group and £6600 in the intervention group.
Total mean costs per averted injury
Accounting for all cost components, total mean cost per injury was £907 from the NHS perspective across both treatment groups (£1072 in the control group and £594 in the intervention group) (Table 25) and £625 from the societal perspective (£755 in the control group and £379 in the intervention group). Absenteeism costs accounted for over half of the total mean costs from both the NHS and societal perspectives.
| Mean costs per injury | Control (n = 49) | Treatment (n = 26) | Total (n = 75) | Difference (intervention – control) |
|---|---|---|---|---|
| NHS | ||||
| Health-care costs (£) | 233 | 61 | 173 | –172 |
| Absenteeism costs (£) | 585 | 280 | 479 | –306 |
| Compensation costs (£) | 254 | 254 | 254 | 0 |
| Total mean costs per injury (£) | 1072 | 594 | 907 | –478 |
| Society | ||||
| Health-care costs (£) | 233 | 61 | 173 | –172 |
| Absenteeism costs (£) | 390 | 186 | 320 | –204 |
| Compensation costs (£) | 132 | 132 | 132 | 0 |
| Total mean costs per injury (£) | 755 | 379 | 625 | –376 |
Intervention costs
Costs to the NHS
Data on the frequency and prices of slip-resistant footwear styles ordered for the trial were available for 2201 pairs of footwear, from which a weighted average purchase cost of £23.80 per pair was derived. Based on the testing of the trial footwear, presented in Chapter 3, SSHeW trial footwear testing, we assumed that the footwear would be replaced on average every 12 months. Across the 2278 intervention group participants, this resulted in an annual footwear purchase cost to the NHS of £54,200. As per Costing framework, Distribution costs, the distribution costs are assumed to be negligible and so total intervention costs equal the footwear purchase cost.
Costs to society
From a societal perspective, the purchase of the trial footwear would displace footwear that employees would otherwise purchase and wear. Based on data from 312 participants on the costs and longevity of baseline footwear gathered during recruitment to the trial, we estimated mean baseline footwear costs of £44.52 (SD £19.88) and mean time to replacement of 1.47 (SD 1.20) years, giving an estimated cost of £30.20 per year. This exceeded the mean cost of the trial footwear by £6.40, suggesting a saving to society from each pair of intervention footwear provided. Applied across all control group participants, this gives a total purchase cost saving of £14,600.
Cost–utility analysis
An incremental cost per QALY from the NHS perspective was estimated at £38,900 when applying baseline 1 (Table 26). The equivalent value when applying a higher annual baseline injury rate of 8.00 per 100 workers based on the trial control group was –£5900 (i.e. cost saving).
| Cost–utility analysis | Injury baseline 1 | Injury baseline 2 |
|---|---|---|
| NHS perspective: inputs | ||
| Estimated averted annual injuries over trial population, n | 68 | 135 |
| Cost savings per averted injury (£) | 907 | 907 |
| QALY gain per averted injury (£) | 0.017 | 0.017 |
| Intervention costs per averted injury (£) | 1583 | 804 |
| Societal perspective: inputs (if different to NHS perspective) | ||
| Cost savings per averted injury (£) | 625 | 625 |
| Intervention costs per averted injury (£) | –425 | –216 |
| Cost per QALY | ||
| NHS perspective (£) | 38,900 | –5902 |
| Societal perspective (£) | –60,412 | –48,372 |
The intervention was substantially more cost-effective from the societal perspective. The purchase of intervention footwear offsets more expensive baseline footwear, resulting in a net saving in purchase cost. As a result, intervention costs are effectively negative and the intervention is unequivocally cost-effective, with cost per QALY values of –£60,400 under baseline 1 and –£48,400 under baseline 2.
Sensitivity analysis
Direct comparison by treatment group
The base-case results are not based on a direct comparison of outcomes between treatment groups, due to limited data. The difference in mean costs per participant between treatment group was compared in sensitivity analysis. In principle, this captures the effect of the intervention in reducing the severity as well as the frequency of injuries, although it is subject to a high degree of random variability. The incremental cost per QALY based on this comparison is –£99,670. This implies a mean cost saving per averted injury, prior to intervention costs, of £1610, 77.8% higher than the base-case analysis.
One-way sensitivity analysis: threshold tests on unit cost values
Table 27 reports one-way sensitivity analysis to test the proportional change from base mean values (cost savings and QALY gains per averted injury) required to meet a £30,000 cost-per-QALY threshold, and for zero net intervention costs. As these values will be positively correlated, Table 27 will overestimate the extent of change required to achieve thresholds.
| Parameter | Base value | Threshold (£) | Applying injury baseline 1 | Applying injury baseline 2 | ||
|---|---|---|---|---|---|---|
| New value | % change | New value | % change | |||
| Mean cost saving per averted injury | £907 | 0 | £1583 | 75 | –11 | |
| 30,000 | £1061 | 17 | –69 | |||
| Mean QALY gain per averted injury | 0.017 | 30,000 | 0.023 | 30 | (Cost saving) | |
| Intervention cost per participant | £24 | 0 | £14 | –43 | £27 | 13 |
| 30,000 | £21 | –12 | £42 | 76 | ||
| Baseline injury rate (per 100 people) | 4.06 (baseline 1) | 0 | 7.09 | 75 | n/a | |
| 30,000 | 4.50 | 11 | n/a | |||
Cost savings per averted injury at around one-fifth higher would meet the £30,000 NICE threshold under baseline 1, whereas unit cost savings could be around 70% lower under baseline 2, as the base-case estimate was below the £30,000 threshold. QALY loss per avoided injury at around 30% higher than base case would produce a cost-effective result at the £30,000 threshold (results under baseline 2 were cost saving, so QALY loss was not tested).
Sensitivity analysis also conducted threshold tests on the intervention cost per employee. Cost-effectiveness results are highly sensitive to changes in this parameter (a ± 20% change from the base value results in a ± 47% change in the cost-per-QALY estimate). The threshold test suggests that an intervention cost per employee of £20 would result in £30,000 costs per QALY under baseline 1, with costs of up to £40 producing a cost-effective outcome at the higher baseline injury rate.
A baseline injury rate of 4.50 per 100 participants (11% higher than the baseline derived from the trial baseline questionnaire) would result in a cost per QALY of £30,000. This is considerably lower than the injury rate of 8.00 per 100 participants observed in the trial control group.
Two-way sensitivity analysis
Table 28 reports two-way sensitivity analysis, testing the percentage reduction in injuries required to achieve zero net intervention costs (i.e. a financial break-even) from the NHS perspective, at varying magnitudes of avoided injury cost. In this analysis, the effect of the intervention on QALYs and the uncertainty associated with their estimation are irrelevant. The percentage reduction can be compared with the estimated 95% CIs for intervention effectiveness in reducing slips in the primary analysis (IRR 0.57–0.70, implying a reduction in the slip rate between 30% and 43%), assuming a commensurate reduction in the injury rate.
| Parameter | Change in cost per injury avoided (mean cost per injury avoided with percentage change) | |||||||
|---|---|---|---|---|---|---|---|---|
| 40% lower (£544) | 20% lower (£725) | With base costs (£907) | 20% higher (£1088) | 40% higher (£1269) | 60% higher (£1451) | 80% higher (£1632) | 100% higher (£1813) | |
| Base case (distribution to home address), % | > 100 | 81 | 65 | 54 | 46 | 40 | 36 | 32 |
The intervention would deliver net cost savings to the NHS in the lower bound of the effectiveness CI, with unit costs savings per averted injury at 60% higher (around £1450). This is lower than the cost saving per averted injury derived from the direct comparison of treatment groups (£1610).
Cost-effectiveness analysis
Costs per slip or injury averted may be of interest to decision-makers for comparison purposes, although there is no established cost-effectiveness threshold for these measures. For brevity, the values are presented for the injury baseline 1. From the NHS perspective, cost per slip averted was estimated to be £27 and cost per injury averted was £676.
These values are negative from a societal perspective, at –£43 per slip averted and –£1050 per injury averted.
Chapter 5 Qualitative evaluation
A qualitative study was undertaken to explore participants’ experiences and acceptability of wearing the slip-resistant shoes, their thoughts on slips and falls in the workplace, and their experiences of being in the trial.
Qualitative methods
Design
Semistructured interviews were used to gather information from trial participants. These interviews were conducted over the telephone after completion of the trial with participants who were allocated to the intervention group.
Sampling
A purposive sampling strategy was used to select participants to be invited to take part in a telephone interview. This was to ensure that there was variation in the sample in terms of job roles, both clinical (nursing, medical and other health-care professional staff) and non-clinical (domestic services and catering), shoe types and participating sites. We planned to interview a total of 30–40 intervention participants.
Recruitment and consent
All participants who had agreed to be invited to take part in a one-to-one telephone interview on their SSHeW trial consent form and who had been in the intervention group (i.e. been offered a pair of trial shoes to wear for the intervention period) were eligible to take part in the qualitative study.
Following sampling, selected participants were sent an invitation letter, a qualitative study participant information sheet and a qualitative study consent form (see Report Supplementary Material 6 and 7), along with a prepaid return envelope. The invitation letter informed participants that they would be contacted by a researcher from the YTU (SC or RCB) by telephone, text or e-mail a few days later. If, when contacted, the participant indicated a willingness to take part in an interview, a suitable time was arranged. The response to these invitations was poor and, consequently, the protocol was amended to allow members of the local research teams at participating trusts to approach participants directly and ask if they were willing to take part. It was felt that the local research team were in a good position to identify possible participants because of their contact with them during the day-to-day running of the trial. The protocol was also changed to include the provision of a £20 high street voucher, by way of a thank you, for participants who completed a telephone interview. Additionally, some SSHeW trial participants who contacted the Trial Research Team at YTU regarding issues with their shoes were also invited to take part in an interview.
Prior to the start of the interview, the researcher explained the aims of the interview and gave participants an opportunity to ask questions. Participants were reminded that the interview would be recorded for transcription purposes and confidentiality and anonymity was assured. Informed consent was gained by the researcher before the start of the interview.
Data collection
Semistructured interviews with trial participants were carried out over the telephone by researchers at the University of York, between May 2018 and July 2019. A total of 35 interviews were carried out. The calls lasted on average 25 minutes (ranging from 10 to 35 minutes). A topic guide (see Appendix 11) was used to provide a framework for the interview and to ensure that all participants were asked similar questions. This allowed comparisons to be made in the analysis. However, flexibility in the wording and order of the questions allowed adaptation to the unique conversations and enabled participants to raise any issues important to them. All of the interviews were audiotaped, transcribed verbatim and anonymised before data analysis. Analysis was carried out on 34 transcripts as unfortunately, for technical reasons, one interview failed to record.
Data analysis
Transcripts were checked for accuracy against the original recordings. The data were analysed independently by two researchers; one researcher (RCB) used NVivo software (version 12, QSR International, Warrington, UK) to assist data analysis, the other (SC) used Microsoft Word® (2016, Microsoft Corporation, Redmond, WA, USA). An initial thematic analysis was carried out based on the stages outlined by Braun and Clarke. 50
The transcripts were read and re-read to ensure familiarisation with the data. An initial coding framework was developed based on a priori themes related to issues covered in the topic guide. Emerging themes and subthemes were discussed, reviewed and agreed by both researchers. Three themes were identified and named. Data were reported with quotes to illustrate the underlying points, ensuring that both overall views and individual perspectives were considered.
Both researchers were academic research fellows, had previously worked in health-care settings and were involved in the management of the SSHeW trial, but had no connection with the shoe manufacturer.
To ensure quality, the data collection and analysis process was transparent. There was comprehensive data analysis, with interpretations supported by illustrative quotes. Interpretations of the data were shared with members of the trial team for confirmation of the findings. The sample was selected to include a range of job roles and working environments, and specifically to include some participants who had experienced issues with their trial shoes so that we could explore these problems in more detail. The researchers had declared their involvement in the management of the trial and were therefore aware of how this connection would have shaped their approach to the data.
Qualitative results
Participants
Qualitative semistructured interviews were conducted with 35 SSHeW trial participants who had been allocated to the intervention arm of the trial and had therefore been offered the slip-resistant shoes to wear during the 14-week trial period. The sample comprised 30 women and five men, and represented a range of job roles across all seven participating NHS trusts. Further details of the participants who took part in the qualitative interview are provided in Table 29.
| Participant ID | Gender | Age (years) | Job role | Shoe style |
|---|---|---|---|---|
| 1 | Female | 46 | Research nurse | Mary Jane |
| 2 | Female | 53 | Specialist nurse | Mary Jane |
| 3 | Male | 34 | Practice education facilitator | Cambridge |
| 4 | Female | 41 | Ward manager | Old School III |
| 5 | Female | 58 | Dental nurse | Old School III |
| 6 | Female | 56 | Community staff nurse | Falcon II |
| 7 | Female | 57 | Domestic | Old School III |
| 8 | Male | 45 | Domestic | Evolution II |
| 9 | Female | 40 | Research co-ordinator | Mary Jane |
| 10 | Female | 36 | Qualified nurse | Mary Jane |
| 11 | Female | 56 | Domestic | Revolution II |
| 12 | Female | 40 | Occupational therapist | Jenni |
| 13 | Male | 34 | Doctor | Falcon II |
| 14 | Female | 35 | Physiotherapist | Revolution II |
| 15 | Female | 32 | Research midwife | Mary Jane |
| 16 | Female | 48 | Research nurse | Revolution II |
| 17 | Female | 58 | Clerical officer | Mary Jane |
| 18 | Female | 41 | Specialist nurse | Mary Jane |
| 19 | Female | 53 | Ward clerk | Mary Jane |
| 20 | Female | 29 | Specialist nurse | Old School III |
| 21 | Female | 64 | Specialist nurse | Jenni |
| 22 | Female | 50 | Pathology team | Ollie II |
| 23 | Male | 58 | Health roster co-ordinator | Executive Wing Tip |
| 24 | Female | 56 | Community specialist nurse | Mary Jane |
| 25 | Female | 35 | Support worker | Carter |
| 26 | Female | 49 | Specialist nurse | Mary Jane |
| 27 | Female | 47 | Research nurse | Mary Jane |
| 28 | Male | 33 | Specialist nurse | Evolution II |
| 29 | Female | 30 | Physiotherapist | Falcon II |
| 30 | Female | 43 | Research radiographer | Old School III |
| 31 | Female | 59 | Community therapy assistant | Mary Jane |
| 32 | Female | 55 | Specialist nurse | Willa |
| 33 | Male | 45 | Assistant practitioner | Ollie II |
| 34 | Female | 57 | Health visitor | Pegasus |
| 35 | Female | 56 | Health visitor | Mary Jane |
During the interviews participants discussed their job roles; their thoughts about, and personal experiences of, slips and slip prevention within the NHS workplace; work footwear, including their experience of the slip-resistant shoes; and their experiences of being involved in the trial. Three themes were identified in the interviews: (1) slips and slip prevention, (2) footwear and (3) experience of being a trial participant. These themes were shaped by the topic guide (see Appendix 11). Each theme is discussed as follows.
Theme 1: slips and slip prevention
Awareness and experience of slips, trips and falls in the workplace
Although most participants had not considered slips and falls to be a particular issue among NHS staff, some, through prior experience, were aware of the issue. Many reported minor slips, describing them as a ‘skid’ or ‘loss of footing’; however, some described more serious incidents that resulted in an injury and/or a fall:
I have had my foot go from under me while coming out of the lift and not seeing the patch of fluid and just had my foot go, not actually fallen and hurt myself but it’s sort of jarred the pelvis, almost done the splits.
Participant 9
We had a room deep cleaned and it was left really, really wet, literally there was a big layer of water on the surface. They did put a cone out to say, careful wet floors but somebody moved it around the corner . . . and went absolutely flying. I had a very, very swollen painful knee . . . spent the entire weekend sitting with my leg up with ice on it but I couldn’t kneel, you know, for a few months, it was very painful.
Participant 22
Several of the participants interviewed who had worked in the community had experienced slips outside when undertaking home visits:
I had one and what happened was I was coming away from my car and the ground was quite wet, slippy but I didn’t have the shoes on that you provided me with, it was before the trial and it was quite a nasty slip. I gouged my knee and my elbow but that was before we started.
Participant 24
Others had become aware of the issue of slips in the workplace by witnessing colleagues slipping or through professional experience of dealing with the consequences of slips, trips and falls among staff. One participant had worked in staff rota management and was therefore aware of sickness absences as a result of slips, trips and falls at work. Another participant, a physiotherapist, had previously treated staff who had sustained musculoskeletal injuries at work, including those who had fallen.
Although participants did not identify that slips and falls were a particular problem for staff, they did consider them an issue for patients:
Not in staff groups no. I’m a ward nurse by background, very familiar with slips and falls in patients but not particularly in staff to be honest.
Participant 28
Some staff were more concerned about other risks encountered when undertaking their duties at work, rather than the risk of slips, trips and falls:
I didn’t realise it [the issue of slips, trips and falls among NHS staff] was such a problem. I’m always alerted to back problems and things like that being a nurse . . . so more from that point of view. I wouldn’t have automatically brought to my mind slips, trips and falls really.
Participant 27
The weekly text messages asked specifically for the number of slips. Although the definitions of a slip and a fall were provided in the participant documentation, we wanted to explore whether or not participants understood the difference between slips and trips. All participants who were asked this felt that the distinction was clear. One went on to describe how she differentiated these events:
Well to me you slip if something is caused by something underfoot like you stand on something or it’s slippy and you slip. Whereas a trip means you literally fall over something, a piece of equipment or a bag or whatever.
Participant 31
Risks for slips in the workplace: environment
The participants interviewed reported a large range of potential slip hazards when undertaking their roles in different NHS settings. Flooring was one area of particular concern. Staff described the flooring in their work area as ‘smooth’, ‘hard’, ‘shiny’ and ‘highly polished’. Wet floors were a major concern: floors could become wet through cleaning or as a result of spillages from drinks, food or bodily fluids. Participants described how weather conditions can also pose a risk for slips (e.g. wet floors at entrances and slippery paths outside).
Participants reported that warning signs were widely used to alert them to wet floors during the cleaning process. However, these signs could present a hazard in themselves when incorrectly placed:
The biggest hazard, other than the terrible carpet in the office, is the actual non-slip signs. They just get left in the most stupid places, so like behind a door and you fall over them.
Participant 30
One particular job role posed quite a specific slip risk because of the nature of the procedures being undertaken; this occurred in the endoscopy suite:
There certainly can be fluids yes and every day the scopes spit out water, so water is an issue. Obviously it’s more wires is the issue in endoscopy, but yes fluids there is a problem as well.
Participant 26
Risks for slipping were not the only hazards staff encountered in their work environment as trips were also raised as a cause for concern by several participants. Trip hazards arose from uneven flooring, rips and tears in carpets, slopes, overcrowding, clutter or equipment related to clinical care:
No, not slipping. I mean trip hazards all over the place but I would say more of a thing there’s not enough room and equipment being left out, so more of a trip hazard but not slipping, no.
Participant 12
Risks for slips in the workplace: behaviours
Participants described a variety of behaviours that could increase their risk of slips, trips and falls. These included rushing, carrying too much equipment or being so focused on patient care that they were not concentrating on their surroundings:
I think I just because I walk fast, I don’t know. I just want to do things as quickly as possible, get as much done, so I walk a lot up and down, so I think I have more slips than normal.
Participant 10
Staff wearing unsuitable shoes was identified as another potential risk factor for slips at work by a participant who commented:
Yes there are some people that wear heels for work and some people wear shoes without a grip because you’re not provided with shoes, so a shoe suitable for work everyone has got their own ideas haven’t they?
Participant 34
Preventing slips at work
Participants reported several ways to prevent slips. These included avoiding running to incidents (even emergencies) and a personal responsibility to clear up spillages rather than leaving for cleaning staff. A few staff recalled undertaking health and safety training which included slips, trips and falls prevention, and one member of staff reported a risk assessment being carried out before entering a potentially hazardous area. Preventing slips was seen as important in avoiding injuries. These injuries have an effect on individual’s health, short and long term, and also have the potential to reduce staffing levels, which could ultimately have an impact on patient care:
Yeah obviously when I was a manager on the ward, you know, you did have people that slipped and back injuries and that sort of thing, so that can impact on your staffing. Like the job that I do if I was to [go off sick] then nobody else sees my patients, if you know what I mean, so from that point of view yeah and also for your own long-term health.
Participant 32
Theme 2: footwear
Two subthemes were identified: (1) usual work shoes and (2) slip-resistant shoes provided as part of the trial, referred to as ‘trial’ shoes.
Usual work shoes
When choosing their shoes for work, participants considered several criteria, including comfort, compliance with dress code, the appearance and the sole of the shoe. Comfort was of primary importance; however, other considerations included whether or not the shoes were sturdy, easy to clean, would last well and cost:
Well I think I look for comfort mostly . . . I think when you’re wearing them all day for a long time, yeah so I look for comfort.
Participant 19
Definitely comfort and then like a decent sole, you know, something that feels like it’s actually cushioning your foot.
Participant 30
Something that is robust, is hopefully going to last a long time . . . So they have to be closed toe. They have to be wipeable and I think comfort is a big thing as well because we are on our feet for such a long time, you know, something that isn’t going to cause you any pain or, you know, sensible shoes really.
Participant 29
Participants’ choice was also shaped by the need to comply with dress code requirements, meaning that work shoes had to be ‘black’ and ‘low heeled’ and have a ‘closed toe’. However, some participants mentioned that the dress code was not always adhered to. The appearance of work shoes was also important, as some participants wanted their shoes to be smart and professional looking, but at the same time fashionable. For others the appearance or style was less important:
Both because I wouldn’t want something that’s completely flat underneath and thin, you know, the type where you can feel the floor through it. That’s not my thing and then I’d definitely look for how it looks as well, maybe. The trendiness of the shoe [laugh] . . . and not look too old fashioned.
Participant 30
Yeah I’m not really bothered about what things look like as long as they were comfy.
Participant 32
Although most participants did not consider slip resistance a priority when choosing shoes for work, they did make an assessment of the sole. They judged the grip by looking at the texture and the tread. Participants also believed a thick sole would ‘cushion the foot’, whereas a thin sole was associated with slipping:
Well I wouldn’t get something that’s completely flat, you know, because I would slip in that case. But I certainly wouldn’t prioritise oh that’s got a really good grip, I’ll go for those.
Participant 20
In addition, one participant also considered a thin sole to be noisy. This participant looked for a ‘rubbery’ sole to be quiet around patients. As a consequence of being in the trial, some participants would now consider the slip-resistant properties of shoes when purchasing footwear in the future.
Generally, participants preferred to purchase their shoes from high street retailers, as opposed to online, so that they could try them on. The amount of money spent on work shoes varied from £10 to £90, with a majority of participants spending between £40 and £50. Many participants also mentioned waiting for sales before making their purchase. Work shoes lasted between 3 months and 3 years, with most participants saying they lasted around 1 year:
I obviously try to spend as little as possible but I think because I’m in them so much and so often I would normally go up to about £50 if I couldn’t find anything cheaper.
Participant 30
For work shoes I would normally spend £30/£40 just because I want them to be comfy, I’m in them for so long.
Participant 12
The trial shoes
The majority of participants were very happy with the pair of slip-resistant shoes they were given as part of the trial, describing them as ‘brilliant’, ‘lovely’, ‘smart’ and ‘comfortable’. One participant commented that they were a better-quality shoe than they would normally buy:
They were comfortable and I really loved them, I’m never out of them.
Participant 9
Yeah I thought it was a suitable work shoe, it must be comfortable and it would look smart and yeah definitely grippy.
Participant 20
Participants in the trial could select a style of shoe from a range offered at their trust. They often selected shoes similar to their usual work shoes. Other participants saw the trial as an opportunity to try a style that they would not normally wear for work, such as a trainer:
Yeah. I personally think a trainer looks a bit scruffy with a uniform but I think for how long we are on our feet and how many miles we cover a day, I think it’s better. They normally feel much more comfortable to be in, so I can see why people have got . . . I’ve never had a trainer style before, so I can now see why people do have trainers.
Participant 30
Although some of the participants thought that the range of shoes offered was good and varied, others, both male and female, felt that it was limited, particularly in offering smarter styles rather than a more casual shoe:
I was quite surprised actually because I only thought you’d have one choice. One for girls and one for boys. It was quite nice to be able to have different choices of design for the shoes that was good.
Participant 4
Yeah I thought there was a really good range. Different styles to suit different people as well.
Participant 20
. . . I thought there was a good choice . . . There were some that I wouldn’t feel comfortable wearing because I do wear a dress at times, you know, the trainer type ones I didn’t feel I could wear those with my dress. So my options were kind of limited to those ones that looked a bit smarter and that would work with both.
Participant 18
It could probably be a little bit wider. People do tend to care a lot about what they wear on their feet in terms of style and stuff like that don’t they, so I think some more streamlined versions would be more appealing but to be honest I’m still quite happy with them.
Participant 3
There were conflicting opinions regarding the appearance of the shoes. Some participants thought that they looked good, whereas others described them as ‘old-fashioned’ or ‘clunky’. Others felt that some styles were not smart enough for work. A few participants commented that the shoes looked better than they expected:
They have been fairly comfy and they look better than I thought they would. I expected to them sort of look like, you know, orthopaedic correction shoes or something like that but they look pretty good and it was good that there was a range of choice that you could choose from.
Participant 3
Experience of wearing the trial shoes
Overall, experiences of wearing the trial shoes were positive, with the shoes being described as comfortable and practical. However, some found that the shoes made their feet hot or were heavy to wear. The style of one shoe was a particular issue with one participant who felt that they were going to fall when wearing the shoes:
I couldn’t wear them because they hurt my feet so much and they . . . the shoes were lovely, don’t get me wrong, but they made my feet tilt in and it was like I was gonna fall.
Participant 17
Some participants experienced discomfort for a short time when first wearing the shoes; however, it was felt that it was no more than expected for new shoes. In the minority of cases participants were unable to persevere with wearing the shoes because of persistent foot problems. A few participants felt that the shoes altered the way they walked; however, this settled in the majority of cases as they adapted to the new shoes:
Yeah I think they did but I don’t think it was any more than I would expect from any other pair of work shoes really. I think because we do wear them such a lot you kind of, whenever I’ve bought new work shoes I’ve thought, god I’ve got blisters or whatever. Yeah they were a bit uncomfortable at the beginning but they’ve soon worn in.
Participant 29
As much as it sounds silly I think sometimes, and I know other colleagues had the same thing, the actual non-slip took a bit of getting used to, you kind of felt like you were going to slip, I know that sounds mad but it did feel like because it’s like . . . I’m trying to think of the word. You put your foot down, there is no give if you’re not used to that so at first I felt like I was going to fall over.
Participant 32
The slip-resistant sole
Participants noticed a difference in both the appearance and the performance of the slip-resistant soles, compared with their usual shoes, commenting on how well they gripped and prevented slips:
I know you could tell straightaway by looking at the bottom that they were a different, I’ve never seen a grip like that on the bottom and when you walked on them there was no give in them, you know, you did feel safe walking in them.
Participant 34
There was a situation . . . where I’d gone to a residential setting with two other professionals and the floor had been mopped but there was no sign and I walked in and walked straight along the corridor. The person behind me slipped, really slipped. So it was then I thought, oh my gosh, you know, it made me think about the shoes, you know, she slipped and as I say I went ahead of her and she slipped and she had flat shoes on but she slipped. That’s when I became aware and thought probably these shoes prevented that.
Participant 24
Participants noticed that the increased grip on the soles posed a problem when walking on carpeted areas. This caused feet to stick, resulting in trips or stumbles. However, participants quickly adapted to this:
They are very grippy particularly on carpet because my office is carpet. At first, but actually you start to think about it and actually you pick your feet up a bit more and don’t drag.
Participant 28
The deep tread itself caused a few issues, particularly for staff who went outside:
The only one thing I found with them is if I walked anywhere that was muddy, because they’ve got quite an intense grip on them, they’re quite a deep tread and then when the mud has dried off, like I’d be walking on the wards and I’d be leaving little piles of mud where I’ve walked.
Participant 1
The only thing that’s a negative about them is in the snow, snow seems to clump up underneath them and I think it might have made them even more slippy because it kind of went slippy underneath and then when I got in my car and put my feet on the pedals, it was slippy and I had to scrape it off.
Participant 6
Many participants continued to wear their shoes beyond the 14-week intervention period. They reported a few signs of wear and tear, such as scuffs and wear on straps, but they did not notice any difference in the sole over that time. Owing to their positive experience of wearing the shoes, most participants would consider buying slip-resistant shoes again for work, depending on the price and styles available, and some would recommend the shoes to colleagues.
Theme 3: experiences of being a trial participant
Participants generally had a positive experience of being in the trial. Participants reported hearing about the trial via various routes at their NHS trust, including advertising, e-mails, posters, staff bulletins, or by word of mouth from colleagues or the research staff themselves. Most participants found signing up for the trial straightforward. Issues were reported in trying on sample shoes, which sometimes were in short supply at busy recruitment events:
There were queues and if you went in your break and then they didn’t have maybe the size of another style and you didn’t really have the same kind of patience or time to try two or three pairs on and walk around with them on the floor for a couple of minutes to see which ones were the most comfortable.
Participant 22
The predominant reason given by interviewed participants for taking part in the trial was the prospect of getting a pair of shoes. Other reasons cited included an interest in the research area, the need to provide an evidence base and wanting to support colleagues:
I think one that you got a free pair of shoes [laugh] that you don’t have to pay for and two I suppose as physios [physiotherapists] we read a lot about research and evidence and stuff and I think I was just quite keen to help, you know.
Participant 29
Although participants reported mainly positive experiences of being in the trial, one participant reported some feelings of resentment from some colleagues who, because of their job, were ineligible for the trial:
I think there was an issue with some people not being eligible and I do think that was an issue, yes and particularly with the porters because they’ve got a new contract and, you know, staff that are in that category and I think that made it quite hard for the staff that were running it. But otherwise I’ve seen people, I’ve been onto wards and you know they’ve said, oh you’re in the shoe trial too, they’ve looked at my shoes and recognised them.
Participant 27
Despite the research team being aware of delays with some participants receiving their trial shoes, the majority of participants interviewed experienced no problems. The participants described either collecting their shoes or having them delivered directly to them at work. When delays were experienced, they were due to stock issues, ordering or delivery problems, or participants requiring an exchange.
Data collection
Participants generally found the methods of data collection acceptable: questionnaires were reported as being clear and easy to complete, and the use of text messaging was particularly well received. Participants reported that text messages were quick, convenient and immediate. Participants found it helpful that the text messages were sent weekly, meaning that they had to recall slips that occurred over only the previous week:
It was really good. It was quick. Obviously because you’re working, you’re on the go all the time, you don’t want anything that’s going to take time. It was quick, easy. It was good.
Participant 24
In addition to the weekly slip data collection text message, participants were also sent a compliance text message at three time points during the intervention period. One participant did report getting confused between these text messages, despite them being sent on different days:
Yeah and I think if you put zero for slips, trips and falls but you could put zero if I’m never wearing them and I think on the text I glanced your text, thought it was asking me about slips, trips and falls and answered it zero for not wearing the shoes and I think I did text back, I think, and say I didn’t mean, you know, whatever at some point. So I think the texts were quite similar.
Participant 27
Summary
Although participants had not considered slips and falls to be a particular issue for staff in the workplace, they identified many potential hazards (e.g. wet floors, clutter from equipment and rushing). Some participants had experienced slips and described the impact of these for both themselves and others (e.g. in terms of staffing levels). Staff interviewed reported little training in slips, trips and falls prevention and felt that any health and safety training received focused more on manual handling safety to prevent back injury.
Participants took several factors into account when choosing work shoes. The main factor was comfort; however, appearance was also considered important, as was the shoes meeting dress code requirements. The consideration of slip resistance was not a predominant thought, although some participants did make an assessment of the sole. Many trial participants were happy with the slip-resistant shoes provided to wear in the trial, finding them comfortable, hard-wearing and smart. A few participants, however, reported problems with them and were unable to wear them. Participants noticed a difference when walking in the trial shoes (associated with the grip) and some reported slips being prevented.
Participants generally had a positive experience of being in the trial. The trial was well promoted within the trusts, making signing up easy. The predominant reason given for taking part was the prospect of receiving a free pair of shoes. Participants found the methods of data collection acceptable and particularly liked the use of text messages to collect data.
Interviews were undertaken via the telephone. This was more practical and allowed easy communication with dispersed participants at convenient and flexible times. This method also minimised costs. We acknowledge that conducting the interviews via the telephone would have prevented us from picking up on non-verbal cues.
Chapter 6 Discussion
Here we report the results of a large RCT assessing the clinical effectiveness and cost-effectiveness of 5-star, GRIP-rated, slip-resistant footwear for preventing slips in NHS staff. There is some evidence to indicate the potential effectiveness of appropriately specified slip-resistant footwear to prevent slips. 22,24,27 However, only one of these studies, which reported a 67% reduction in the number of slips, was a RCT,27 which is considered the ‘gold-standard’ experimental design for evaluating effectiveness. In this discussion, we summarise our key findings, compare these with previous studies, and discuss the strengths and limitations of our study.
Summary of main findings
Trial population
This large multicentre RCT included NHS staff aged ≥ 18 years, working at least 22.5 hours per week in clinical, general or catering areas who owned a mobile phone. The majority (85%) of the trial population were female and the average age was 42 years. We included a wide age range in the trial, from 18 to 74 years. Approximately one-third of participants reported at baseline that they had experienced at least one slip at work in the previous 12 months. Indeed, a similar proportion reported at least one slip in the pre-randomisation text messages; this was over a much smaller time frame of up to 6 weeks, which indicates that slipping is a genuine problem in this population.
Primary outcome
Compared with usual footwear, the offer and provision of slip-resistant footwear reduced the mean number of slips over the 14-week period by roughly one-third. The primary, intention-to-treat analysis showed a statistically significant reduction in the slip rate in the intervention group relative to the control group (IRR 0.63, 95% CI 0.56 to 0.70; p < 0.001). Pre-randomisation slip rate was seen to be a significant predictor of post-randomisation slip incidence rate. For every increase of 1 in the average weekly number of slips a person experienced, their post-randomisation incidence rate of slips was predicted to increase by a factor of 5.7 (95% CI 5.0 to 6.5). Gender was also a significant factor and females were, on average, 23% more likely than males to experience a slip (IRR 1.23, 95% CI 1.06 to 1.43). Analyses indicated that although beneficial for both males and females, the intervention had a greater effect in males. This is surprising, given that females had a higher slip rate and therefore had more potential for improvement. This may have been a chance finding. Alternatively, we could hypothesise that the shoe styles worn by males tended to have a larger sole surface area and so were more effective than the female styles.
When compliance with the intervention was accounted for using an instrumental variable CACE analysis approach, receipt of the shoes by week 7 was seen to have a similar benefit as in the intention-to-treat analysis (IRR 0.65, 95% CI 0.58 to 0.73). This was expected, as most (two-thirds of) intervention participants had received their shoes by this point. Compliance text messages indicated that, once they had received their trial shoes, approximately 50% of intervention participants wore them all of the time when at work. Further CACE analysis indicated that participants with more exposure to the trial shoes (i.e. received them earlier and/or wore them more often) were likely to benefit more from the intervention than those with less exposure.
Secondary outcomes
Statistically significant results were also observed in the incidence rate of slips as reported on the 14-week questionnaire (IRR 0.56, 95% CI 0.48 to 0.66); the incidence rate of falls resulting from a slip (IRR 0.51, 95% CI 0.28 to 0.92); the proportion of participants reporting a slip over 14 weeks (OR 0.58, 95% CI 0.50 to 0.66); the proportion of participants who reported a fall (whether or not resulting from a slip) (OR 0.73, 95% CI 0.54 to 0.99); and time to first slip (HR 0.73, 95% CI 0.67 to 0.80). There was little evidence of a difference in the incidence rate of falls not resulting from a slip in the workplace (IRR 0.82, 95% CI 0.50 to 1.34).
Cost-effectiveness
Under the base-case scenario, the cost per QALY from the NHS perspective was £38,900 (–£5900 when applying the higher baseline injury rate). From the societal perspective, the trial footwear displaces usual footwear, meaning that the intervention was unequivocally cost-effective at –£60,400 cost per QALY (–£48,400 when applying a higher baseline injury rate).
Sensitivity analysis indicates that the intervention would be cost-effective from the NHS perspective at a £30,000 cost-per-QALY threshold if the cost savings per averted injury were roughly one-fifth higher. This is plausible, given that the costing method is likely to underestimate the cost savings attributable to the intervention and that the intervention could also result in net cost savings to the NHS (i.e. a positive return on investment) within plausible levels of effect in reducing injuries.
The CEA is based on limited data: the number of injuries due to slips observed in the trial was small (n = 79), with the majority of slips being minor and not requiring health-care treatment or time off work. To mitigate a high level of random variability between treatment groups and maximise available data, the costing approach aggregated injury outcome data across both groups to derive mean values per injury averted. If the intervention reduced both the frequency and severity of injuries due to slips, the costing approach would underestimate the resulting reduction in injury costs. A direct comparison of costs in the intervention and control groups was undertaken in sensitivity analysis and indicated an improved incremental cost per QALY from the NHS perspective of –£99,700 (i.e. cost saving), suggesting that costs in the base-case analysis may be underestimated. Improved cost-effectiveness should also be observed in practice if higher compliance with the footwear is achieved (e.g. if it is compulsory) with all else equal.
Qualitative findings
The qualitative study explored participants’ thoughts about slips and falls in their workplace and their experience of being in the trial, particularly with respect to their views of the slip-resistant shoes provided.
Through exploration of participants’ job roles and working environments, we were able to understand the risks for slips and falls that these health professionals were aware of when undertaking their work. Slip and fall hazards were encountered from cleaning activities and through clinical care, as well as from busy, often cluttered, work spaces. Participants described many different types of flooring in their workplaces and reported that they would often traverse several types in a working day. Although most of their time was spent on typical ‘hard, shiny’ hospital flooring, some participants crossed onto carpet in certain hospital areas, such as their offices. Furthermore, community staff encountered carpets in patients’ homes. A few participants described some difficulties due to the grip of the trial shoes in carpeted areas.
Many participants went outside in the course of their work, either in community work or in moving from one part of the hospital to another. This external environment was seen to present slip risks that were unavoidable because of the weather conditions and the slip-resistant shoes were seen to be of benefit in these areas.
Participants described many features they sought when choosing shoes for work and the slip-resistant shoes offered in the trial met many of these requirements: they were comfortable, practical and complied with the trusts’ dress codes. Participants noticed the increased grip when wearing the slip-resistant shoes and reported instances of slip avoidance. Most participants were happy with the slip-resistant shoes and continued to wear them beyond the 14-week intervention period. However, the shoes were not suitable for all participants and some would have preferred a wider range which included smarter styles.
Participants described that the trial was well promoted and received in their NHS trusts, and found trial processes for recruitment and data collection straightforward and not too burdensome. The collection of slip data by text messaging was particularly well received.
The qualitative sample was purposefully selected to include participants from several different job roles and working in different health-care environments. The sample also included participants who had reported some issues with the trial shoes, so we could explore this in greater detail. Owing to modification to the recruitment strategy involving the research teams at site, the resultant qualitative sample included several health-care workers who worked in the research field. These participants may have had a better understanding of the trial processes, including familiarity with questionnaires and the importance of accurate data.
Comparison with other studies
Our results corroborate the limited evidence to support the potential effectiveness of appropriately specified slip-resistant footwear to prevent slips in the workplace. A longitudinal study by Bell et al. 21 indicated a 58% reduction in the number of workers’ compensation claims when a comprehensive slip, trip and fall prevention programme (which included slip-resistant footwear among other strategies) for hospital employees was introduced in the USA. A before-and-after study22 in Denmark suggested that new slip-resistant boots reduced slips, trips and falls compared with older boots by around 80% among fishermen. Additionally, a prospective cohort study of 475 fast food restaurant workers found that the use of slip-resistant footwear was associated with a 54% reduction in the reported rate of slipping. 24 To the best of knowledge, there has been only one other RCT27 of slip-resistant footwear. In this cluster trial, undertaken in the USA, 226 school districts were randomised to receive appropriately specified slip-resistant footwear or continue with the usual company policy, which generally required staff to purchase slip-resistant footwear but did not specify performance requirements. The participants in the study were food service workers and a 67% reduction in slipping injuries was observed (adjusted OR 0.33, 95% CI 0.17 to 0.63). Our population was different to this trial in that only 1.5% of our population included food service workers. In contrast, a cross-sectional study of 125 fast food workers in the USA23 did not find an association between slipping and use of footwear perceived to be slip resistant. However, this finding may have been as a result of the way in which footwear was classified as slip resistant, which was by visual inspection of the tread only (as opposed to any scientific evaluation of slip resistance).
Strengths and limitations of the study
To the best of our knowledge, this is the largest pragmatic trial evaluating the clinical effectiveness and cost-effectiveness of 5-star, GRIP-rated slip-resistant shoes conducted to date, randomising a total of 4554 participants. The trial was well powered. We had anticipated that the proportion of participants in the control group who slipped would be 10% and powered the trial at 90% to detect a 30% relative reduction, to 7%, in the intervention group. In the event, of the 4505 participants who provided slip data, 36% in the intervention group and 45% in the control group reported at least one slip, so the power for this outcome was increased.
The trial design had several strengths: a short data collection period (14 weeks), use of text messages to collect slip and compliance outcomes, and use of a run-in period with outcome data collection, which could have reduced the incidence of post-randomisation attrition. The use of text messages to collect primary outcome and compliance data was acceptable to trial participants. Response rates to the primary outcome were high. Both groups responded in a similar way, with the intervention group responding to an average of 12.3 of the possible 14 (SD 3.1, median 14) text messages and the control group responding to an average of 12.6 (SD 3.0, median 14) text messages. Participants reported that it was a quick way to report outcomes with minimal effort. Text messages were sent weekly to minimise the risk of recall bias.
There were some limitations to the study. Exposure to the intervention was perhaps lower than intended. It was hoped that most intervention participants would receive their footwear within 2 weeks of randomisation; however, the actual median time to receipt of shoes was approximately 4 weeks. The logistics of ordering and distributing large numbers of shoes to staff working varying schedules and over geographically dispersed areas was greater than trusts had anticipated, leading to delays. In addition, despite their best efforts, the trusts could not always get every intervention participant to collect their shoes in a timely manner, if at all. Further delays were experienced if participants needed to exchange their shoes, despite the shoe manufacturer amending their usual returns policy to accommodate swift exchanges.
Once the trial shoes were received, around 50% of intervention participants reported wearing them all of the time when at work, which means that about 50% of participants wore the shoes some, or even none, of the time. This is not necessarily a problem for the trial, as we were primarily testing the offer and provision of slip-resistant footwear without mandating and checking that participants were wearing the footwear. However, it does affect the generalisability of results to other work environments where use of appropriate footwear may be more closely monitored. The primary analysis was based on intention to treat and so may underestimate the true benefit of the slip-resistant footwear. Secondary analysis indicated that intervention participants who showed greater compliance with wearing the slip-resistant footwear had a lower incidence of slipping in the workplace than participants with less or no exposure to the trial shoes. However, we cannot necessarily conclude that a larger effect size would have been produced if all intervention participants wore the shoes all the time when at work. Within the trial population, those with greater ‘compliance’ are likely to be different from those with lower ‘compliance’ (e.g. have a higher or lower slip risk).
Another potential issue is the possibility of contamination. The control group could have seen colleagues wearing their trial shoes and purchased shoes from the same manufacturer, or other 5-star, GRIP-rated shoes, for themselves. Although we do not have data on contamination rates, we used a waiting list control group design whereby control participants were informed that they would receive their shoes soon after their participation in the study ended. Anecdotally, we know from correspondence with control participants that several participants delayed purchasing new footwear as they waited for their trial shoes.
There were some limitations to using text messages. Some NHS staff who would have been eligible for participation could not be recruited as they were unable or unwilling to send and receive text messages. It is difficult to estimate how significant a problem this was. A small number of participants (n = 13) who returned a screening form were ineligible because they explicitly reported that they did not have a mobile phone or were unable or unwilling to send and receive text messages, although this question was asked on an early version of the baseline form only. Following this, we assumed that if participants provided a valid mobile phone number on the contact sheet then they were willing and able to send and receive text messages.
Up to a further 68 screened individuals were ineligible as they had missing or invalid consent or contact details, which may have involved an issue with their mobile phone number. What we cannot know is the number of potential participants in the hospital who did not even express an interest in the trial because they would be unable or unwilling to send and receive text messages, or the number that were ‘screened out’ before being handed a recruitment pack to complete. We tried initially to encourage sites to retain a screening log to record the reason why potential participants who expressed an interest in the trial were not handed a recruitment pack because they were deemed ineligible. However, owing to the overwhelming interest in the trial, and often frenetic recruitment activities, this proved infeasible.
Another limitation was that the text messages were automated and, because of the timing, some text messages were sent out on Christmas Eve and New Year’s Eve 2017, resulting in some messages not being delivered. There is also the possibility that some participants in the intervention group may have incorrectly responded to their compliance text, believing it to be their slip data text. Although participants were requested to send only a numerical reply in response to their text messages requesting slip data, many participants sent non-numeric responses (e.g. messages to the study team).
Another potential limitation to the study is that the outcome data are participant self-reported, which may have led to inaccuracies in the reported slip data. Although we asked participants to report any slip regardless of how ‘minor’, some participants may have reported only slips that were memorable for some reason (e.g. those resulting in an injury or a fall). This could have resulted in a lower slip number being reported. However, as that the slip rate observed in the trial is higher than we initially thought, it would seem unlikely that recall bias occurred, especially given the short time frame over which the data were collected.
It may also be the case that a very small number of people experienced a major injury as a result of a slip at work and may not have been able or willing to complete the follow-up questionnaires, thereby further reducing the number of reported slips. However, it is likely that the research team would have found out about such injuries because of the trusts’ reporting requirements. Conversely, although participants were provided with a definition of a slip and a fall on the participant information sheet, participant diary and, when possible, at recruitment, some participants may have reported some trips as slips, resulting in an overestimate of the number of slips. At set-up, the study team did consider this potential issue and considered sending participants multiple text messages per week. The first text would ask if the participant had been in work that week. If the participant indicated that they had, three further text messages would be sent asking for the number of slips, trips and falls separately. Unfortunately, the text messaging service was not sophisticated enough to dynamically respond to large numbers of participants in this way and so it was decided to send participants one simple text asking if they had experienced any slips.
This raises another potential issue. We have assumed that a zero response via text message means that the participant had worked at least some hours that week and had not experienced any slips when at work. However, it is feasible that people could have replied zero, but not actually been at work. This could potentially underestimate the slip rate; however, we hope that this issue was minimal (indeed, some people replied to the slip text message to explicitly report that they had not been in work that week) and would therefore have a limited impact on the effect size of the intervention. We asked on the 14-week questionnaire whether or not participants had taken time off work for any reason in the last 14 weeks and, if so, how much time they had off. The amount of time taken off was similar between the two groups and therefore this allays some concerns about selective reporting between the two groups. A further issue which could have affected the accuracy of the slip data is that it was not possible to blind participants to their group allocation. Responses could have been affected by the knowledge that they were or were not wearing potentially slip-resistant footwear.
The rate of response to the week 14 questionnaires, which were posted to participants’ homes, was low, at 62.8%. This was despite reminders being sent to non-responders and a SWAT being implemented in the trial to improve response rates to this questionnaire. The response rate was slightly higher in the control group than in the intervention group (64.1% vs. 61.4%, respectively). A potential reason for this is that the intervention group had already received their shoes by the time the questionnaire was sent to them and so there may have been a level of disengagement in the study by this point. On the other hand, participants in the control group were still to receive their shoes and so perhaps more likely to remain engaged in data collection. In general, a contributing factor to the low response rate overall might be that participants had forgotten to expect a postal, paper questionnaire (between being informed at trial enrolment and 14 weeks post randomisation) and were used to providing outcome data via text messages only. Additionally, this was a busy, working population and, as such, the are likely to struggle to find the time to complete questionnaires. It is possible, although difficult to measure, that there is selection bias introduced in that responders are likely to be different to non-responders.
Generalisability of the results
The SSHeW trial was a pragmatic RCT across seven trusts within England, including small and large acute trusts, and mental health and community trusts. The trial included participants who were undertaking a wide variety of jobs, and included clinical, administration, domestic and catering staff. They worked in diverse environments, such as wards, offices, food preparation areas, laboratories and in the community, and walked on a range of floorings, including lino, carpet, slip-resistant surfaces and outside areas, such as car parks, paths and patients’ homes. We were, however, unable to include all staff working in all of the hospitals. Some staff, such as porters and operating theatre staff, in some trusts had to wear protective shoes provided by the trust, which made them ineligible for the study. The number of catering staff and domestic staff who took part in our study is low. This was because in some of the participating hospitals catering staff and domestic staff were employed by a subsidiary trust and could not be enrolled in the trial as a consequence of indemnity issues.
Eighty-five per cent of participants were female. Certainly, we know that in one of our included trusts their male to female ratio is split at approximately 20% male to 80% female and so our population may be reasonably representative in terms of the gender distribution of NHS staff. In this same trust, approximately 11% of their workforce are aged 16–29 years, 23% are aged 30–39 years, 28% are aged 40–49 years, 30% are aged 50–59 years and 8% are aged > 60 years. In our trial, the corresponding proportions are 17%, 21%, 28%, 29% and 5%, respectively. Therefore, except perhaps being marginally skewed to the youngest ages, our population was representative of NHS staff in terms of age. Our trial population was representative in terms of ethnicity as, on average, the ethnicity in our participating trusts was approximately 87% white or white British.
The findings of our study are applicable to footwear that has been tested using the HSE GRIP rating scheme and is appropriately specified for the workplace and role. Although the footwear used in our trial was awarded the highest 5-star rating, it is important to note that this rating may not be appropriate for all roles and workplaces. It is the friction generated between the footwear and floor surface that prevents slips, and footwear with lower GRIP ratings would be expected to provide similar levels of friction in environments that are less challenging (i.e. where there are fewer contaminants or where the floor surface has a lower slip potential). Some roles are also likely to have a lower slip risk than some of the roles included in our trial. For example, they may involve shorter walking distances, less rushing, and no pushing and pulling. Similar results may therefore be obtained with other types of slip-resistant footwear, provided they have been appropriately specified for the role and workplace environment by undertaking a risk assessment and a meaningful evaluation of the footwear’s performance. The results may not be applicable to other footwear that claim to be ‘slip resistant’, but have not been appropriately evaluated and rated with the GRIP rating scheme.
Implications for health care
The preference would always be to try to minimise or adequately control a potential slip risk for all individuals it could affect (workers and members of the public). This can be done by eliminating, for example, floor surface contamination or by specifying floor surfaces that remain sufficiently slip resistant when they are contaminated. However, this may not always be possible. In this situation, employers or staff may consider the use of slip-resistant footwear. Our results suggest that there is a role for appropriately specified footwear in reducing slips in the workplace. The qualitative work has shown that these shoes would be acceptable to staff.
Further research
Although the staff represented a working group that was very diverse, our findings may not generalise to all workplaces. It may therefore be worth considering replicating the trial in other high-risk environments, such as some manufacturing and food-processing facilities, or in settings which have different surfaces, such as in the construction industry, where surfaces are more likely to include loose gravel or mud.
The use of text messages to collect outcome data, for which a single number response is required, was acceptable to both the participants and the researchers conducting the study. From the participants’ perspective, text messages were an easy and convenient way to provide data. For the researchers, text messages were a cheap way of collecting a large number of data from a wide geographical area. The data were collected quickly, with high response rates which were similar between the two groups. As participants did not find it burdensome to respond to the text messages, text messages could be sent weekly, which minimised the possibility of recall bias being introduced. However, we would recommend caution if using text messages to collect more than one variable. In this study there was the possibility of participants confusing the text messages requesting slip and compliance data, even though the text messages were sent out on different days. In future trials, researchers may wish to consider using other technologies, such as accelerometers, to collect outcome data.
Further qualitative research could be undertaken to explore how the introduction of the slip-resistant footwear could be implemented in different trusts.
In this study we took the opportunity to undertake a SWAT, which contributed to the body of evidence regarding how to run RCTs more efficiently. The SWAT, which evaluated an intervention to improve response rates to postal questionnaires, was cheap and easy to implement. We would recommend researchers consider embedding a SWAT in their research. Guidance about conducting SWATs can be found at the Trial Forge website [URL: www.trialforge.org/trial-forge-centres/ (accessed 26 May 2020)].
Conclusion
The SSHeW trial has shown clear evidence that 5-star, GRIP-rated footwear can reduce slips and falls resulting from slips in the NHS workplace. The results indicate that the intervention could also be cost-effective at the £30,000 per QALY threshold from NHS perspective and cost saving from a societal perspective if the footwear is distributed to the employee’s home address rather than at NHS premises. Participants found the shoes to be acceptable. In cases when it is not practicable to prevent floor surfaces becoming slippery in the workplace, employers or staff may consider the use of appropriately specified slip-resistant footwear.
Acknowledgements
The authors would like to thank the participants for taking part in the trial, the members of the trust involved in recruiting participants, ordering and distributing the footwear, and the principal investigators at each site for co-ordinating the study. We would also like to thank the staff at CWP who acted as our PPI group throughout the study. In addition, we would like to thank the NHS trusts or trust charities for funding the trial footwear.
Our thanks are also given to the independent members of the TSC and DMEC for their guidance and support throughout the study. Professor Avril Drummond (University of Nottingham) chaired the TSC and DMEC, Dr Farina Hashmi (Lecturer in Podiatry, University of Salford), Mr Mark Thomas (consultant on slips and trips, previously worked as a policy adviser for the HSE), Kate Chapman (Nurse Professional Advisor at Cheshire and Wirral Partnership NHS Foundation Trust) and Isabelle Smith (statistician, University of Leeds dependent member). We would like to acknowledge the support of the National Institute for Health Research Clinical Research Network. We would also like to thank the Patient Involvement Group, Kevin Hallas and Jo Ellwood from the HSE for their work on the study, and Shaun Donaghy and Kyran Donald (also from the HSE) for their review of the health economics.
We would specifically like to thank Linda Bamford, Tom Beanland, Claire Beard, Zorba Begum, Alison Bowry, Louise Bramley, Andrew Conway, Joanne Cooper, Kinga Dwornik, Jill Foster, Richard Furnival, Katie Glickman, Lydia Harris, Barbara Heath, Andrea Houlding, James Hughes, Dianne Jones, Lisa Jones, Cate Laven, Helen Leyland, Beverley Lowe, Jack Mellor, Pat Mottram, Belinda Oakley, Karen Palmer, Jo Pearson, Maggie Peat, Laura Penver, Rob Shaw, Sarah Sheath, Jacqui Smith, Willemijn Spoor, Claudia Tarr, Joanne Thornhill, Theresa Whittingham and Lianne Wright, who were members of the trusts and who either recruited participants or distributed footwear to trial participants.
Contributions of authors
Sarah Cockayne (https://orcid.org/0000-0002-1288-5497) (Research Fellow, Health Sciences) was co-chief investigator and the SSHeW trial manager. She contributed to the overall study design and was the lead for study management. She undertook some of the qualitative interviews and analysis, and was involved in writing the initial version of the report. She was responsible for co-ordinating the compilation, formatting, proofreading of the final report and gave final approval of the manuscript.
Caroline Fairhurst (https://orcid.org/0000-0003-0547-462X) (Statistician, Health Sciences) was a co-investigator, contributed to the overall study design, wrote the statistical analysis plan, conducted the statistical analysis, contributed to writing the initial version of the report and gave final approval of the manuscript.
Michael Zand (https://orcid.org/0000-0001-9340-7958) (Economic Adviser, HSE) was a co-investigator, contributed to the overall study design, wrote the health economics analysis plan, led the health economics analysis, wrote the health economics section of the report and gave final approval of the manuscript.
Gillian Frost (https://orcid.org/0000-0003-3339-8898) (Senior Epidemiologist) was a co-investigator, contributed to the overall study design, undertook PPI work, recruited participants, checked the primary statistical analysis, contributed to writing the initial version of the report and gave final approval of the manuscript.
Mark Liddle (https://orcid.org/0000-0001-9332-129X) (risk and human factor) was a co-investigator, contributed to the overall study design, undertook PPI work, recruited participants, co-ordinated the footwear assessments, liaised with the shoe manufacturer, contributed to writing the initial version of the report and gave final approval of the manuscript.
Rachel Cunningham-Burley (https://orcid.org/0000-0001-8724-8276) (Research Fellow, Health Sciences) was the trial co-ordinator, assisted with the day-to-day management of the study, undertook qualitative interviews and analysis, and was involved in writing the initial version of the report and gave final approval of the manuscript.
Catherine Hewitt (https://orcid.org/0000-0002-0415-3536) (Professor in Statistics, Health Sciences) was a co-investigator, contributed to the overall study design and implementation, supervised the statistical analyses and gave final approval of the manuscript.
Heather Iles-Smith (https://orcid.org/0000-0002-0520-2694) (Leeds Teaching Hospitals NHS Trust) was a co-investigator, contributed to the overall study design, co-ordinated recruitment at the trust and gave final approval of the manuscript.
Lorraine Green (https://orcid.org/0000-0003-1620-2443) (Research Podiatrist) was a co-investigator, contributed to the overall study design and gave final approval of the manuscript.
Emily Bain (https://orcid.org/0000-0002-7173-797X) (Assistant Health Economist, HSE) assisted with the health economic analysis and gave final approval of the manuscript.
Misbah Mogradia (https://orcid.org/0000-0003-2792-6455) (Student Health Economist, HSE) assisted with the health economic analysis and gave final approval of the manuscript.
David J Torgerson (https://orcid.org/0000-0002-1667-4275) (Professor, Director of YTU, University of York) was the lead applicant and chief investigator for the SSHeW trial. He had overall responsibility for the design and implementation of the trial, was responsible for the writing of the report and had final approval of the report submission.
Publications
Cockayne S, Fairhurst C, Frost G, Hewitt C, Liddle M, Zand M, et al. SSHeW study protocol: does slip resistant footwear reduce slips among healthcare workers? A randomised controlled trial. BMJ Open 2018;8:e026023.
Cockayne S, Fairhurst C, Frost G, Liddle M, Cunningham-Burley R, Zand M, et al. Slip-resistant footwear reduces slips among National Health Service workers in England: a randomised controlled trial [published online ahead of print January 15 2021]. Occup Environ Med 2021.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the PHR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PHR programme or the Department of Health and Social Care.
References
- Cockayne S, Fairhurst C, Frost G, Hewitt C, Liddle M, Zand M, et al. SSHeW study protocol: does slip resistant footwear reduce slips among healthcare workers? A randomised controlled trial. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2018-026023.
- Cockayne S, Fairhurst C, Frost G, Liddle M, Cunningham-Burley R, Zand M, et al. Slip-resistant footwear reduces slips among National Health Service workers in England: a randomised controlled trial [published online ahead of print January 15 2021]. Occup Environ Med 2021. https://doi.org/10.1136/oemed-2020-106914.
- Health and Safety Executive . Non-Fatal Injuries at Work in Great Britain 2018. www.hse.gov.uk/statistics/causinj/index.htm (accessed 12 February 2019).
- Health and Safety Executive . Workplace Injuries – Kind of Accident (LFSINJKND 2018. www.hse.gov.uk/statistics/lfs/lfsinjknd.xlsx (accessed 12 February 2019).
- Health and Safety Executive . RIDKIND: RIDDOR Reported Injuries by Kind of Accident and Broad Industry Group 2018. www.hse.gov.uk/statistics/tables/ridkind.xlsx (accessed 12 February 2019).
- Great Britain . The Reporting of Injuries, Diseases and Dangerous Occurrences Regulations 2013 n.d. www.legislation.gov.uk/uksi/2013/1471/pdfs/uksi_20131471_en.pdf (accessed December 2019).
- Health and Safety Executive . RIDNAT: RIDDOR Reported Injuries by Nature of Injury 2018. www.hse.gov.uk/statistics/tables/ridnat.xlsx (accessed 23 October 2019).
- Office for National Statistics . Living Longer: How Our Population Is Changing and Why It Matters 2018. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/ageing/articles/livinglongerhowourpopulationischangingandwhyitmatters/2018-08-13 (accessed 8 October 2019).
- National Institute for Health and Care Excellence (NICE) . Falls in Older People: Assessing Risk and Prevention (CG161). 2013.
- Rubenstein LZ. Falls in older people: epidemiology, risk factors and strategies for prevention. Age Ageing 2006;35:ii37-ii41. https://doi.org/10.1093/ageing/afl084.
- Phillips JA, Miltner R. Work hazards for an aging nursing workforce. J Nurs Manag 2015;23:803-12. https://doi.org/10.1111/jonm.12217.
- Courtney TK, Sorock GS, Manning DP, Collins JW, Holbein-Jenny MA. Occupational slip, trip, and fall-related injuries – can the contribution of slipperiness be isolated?. Ergonomics 2001;44:1118-37. https://doi.org/10.1080/00140130110085538.
- Health and Safety Executive . Slips and Trips: Causes and Prevention – Slips 2018. www.hse.gov.uk/slips/preventing.htm (accessed 31 August 2018).
- Hallas K, Thorpe S, Liddle M, Thomas M. The Role of Safety Footwear in Slipping Accidents n.d.
- Hsu J, Shaw R, Novak A, Li Y, Ormerod M, Newton R, et al. Slip resistance of winter footwear on snow and ice measured using maximum achievable incline. Ergonomics 2016;59:717-28. https://doi.org/10.1080/00140139.2015.1084051.
- Health and Safety Executive . GRIP – Taking Steps Against Workplace Slips 2019. www.hsl.gov.uk/publications-and-products/grip (accessed 16 July 2019).
- Jones T, Iraqi A, Beschorner K. Performance testing of work shoes labeled as slip resistant. Appl Ergon 2018;68:304-12. https://doi.org/10.1016/j.apergo.2017.12.008.
- Liu LW, Lee YH, Lin CJ, Li KW, Chen CY. Shoe sole tread designs and outcomes of slipping and falling on slippery floor surfaces. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0068989.
- Li KW, Chen CY, Chen CC, Liu L. Assessment of slip resistance under footwear materials, tread designs, floor contamination, and floor inclination conditions. Work 2012;41:3349-51. https://doi.org/10.3233/WOR-2012-0604-3349.
- Health and Safety Executive . Case Study: Reducing Slipping Accidents for BP 2019. www.hsl.gov.uk/resources/case-studies/reducing-slipping-accidents-for-bp (accessed 16 July 2019).
- Bell JL, Collins JW, Wolf L, Gronqvist R, Chiou S, Chang WR, et al. Evaluation of a comprehensive slip, trip and fall prevention programme for hospital employees. Ergonomics 2008;51:1906-25. https://doi.org/10.1080/00140130802248092.
- Jensen OC, Laursen LH. Reduction of slips, trips and falls and better comfort by using new anti-slipping boots in fishing. Int J Inj Contr Saf Promot 2011;18:85-7. https://doi.org/10.1080/17457300.2010.487156.
- Courtney TK, Verma SK, Huang YH, Chang WR, Li KW, Filiaggi AJ. Factors associated with worker slipping in limited-service restaurants. Inj Prev 2010;16:36-41. https://doi.org/10.1136/ip.2009.022749.
- Verma SK, Chang WR, Courtney TK, Lombardi DA, Huang YH, Brennan MJ, et al. A prospective study of floor surface, shoes, floor cleaning and slipping in US limited-service restaurant workers. Occup Environ Med 2011;68:279-85. https://doi.org/10.1136/oem.2010.056218.
- Verma SK, Lombardi DA, Chang WR, Courtney TK, Huang YH, Brennan MJ, et al. Rushing, distraction, walking on contaminated floors and risk of slipping in limited-service restaurants: a case – crossover study. Occup Environ Med 2011;68:575-81. https://doi.org/10.1136/oem.2010.056226.
- Verma SK, Zhao Z, Courtney TK, Chang WR, Lombardi DA, Huang YH, et al. Duration of slip-resistant shoe usage and the rate of slipping in limited-service restaurants: results from a prospective and crossover study. Ergonomics 2014;57:1919-26. https://doi.org/10.1080/00140139.2014.952348.
- Bell JL, Collins JW, Chiou S. Effectiveness of a no-cost-to-workers, slip-resistant footwear program for reducing slipping-related injuries in food service workers: a cluster randomised trial. Scand J Work Environ Health 2019;45:194-202. https://doi.org/10.5271/sjweh.3790.
- The King’s Fund . Overview of the Health and Social Care Workforce 2019. www.kingsfund.org.uk/projects/time-think-differently/trends-workforce-overview (accessed 16 July 2019).
- Forbes . The World’s Biggest Employers [Infographic] 2015. www.forbes.com/sites/niallmccarthy/2015/06/23/the-worlds-biggest-employers-infographic/#4ee73bd1686b (accessed 13 February 2019).
- Kind P. The EuroQoL instrument: an index of health-related quality of life. Qual Life Pharmacoecon Clin Trials 1996:191-20.
- Lewis SR, Hallas K, Keen B, Hunwin G, Shaw R, Carrié MJ. Development of a new shoe/floor slip resistance test rig. Tribology International 2020;4. https://doi.org/10.1016/j.triboint.2020.106500.
- Health and Safety Laboratory . The GRIP Scheme. Footwear Slip Resistance Ratings, Handbook for Participants 2014. www.hsl.gov.uk/media/435727/the%20grip%20scheme%20handbook%20v1.1.pdf (accessed 8 November 2019).
- Treweek S, Bevan S, Bower P, Campbell M, Christie J, Clarke M, et al. Trial Forge guidance 1: what is a study within a trial (SWAT)?. Trials 2018;19. https://doi.org/10.1186/s13063-018-2535-5.
- Health and Safety Executive . Table of Preferred Sources for Injuries and Ill Health 2017. www.hse.gov.uk/statistics/preferred-data-sources.pdf (accessed 11 November 2019).
- National Institute for Health and Care Excellence (NICE) . Developing NICE Guidelines: The Manual. 2014. www.nice.org.uk/media/default/about/what-we-do/our-programmes/developing-nice-guidelines-the-manual.pdf (accessed 11 November 2019).
- Personal Social Services Research Unit . Unit Costs of Health and Social Care 2018 n.d. www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2018/ (accessed 8 August 2019).
- NHS . NHS Spring 2018 Settlement n.d. www.nhspay.org/what-does-the-deal-mean-for-me (accessed August 2020).
- GOV.UK. National Statistics . GDP Deflators at Market Prices, and Money GDP March 2019 (Spring Statement) n.d. www.gov.uk/government/statistics/gdp-deflators-at-market-prices-and-money-gdp-march-2019-spring-statement (accessed August 2020).
- Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2018;27:7-22. https://doi.org/10.1002/hec.3564.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- EQ-5D . EQ-5D-5L Valuation, Crosswalk Index Value Calculator 2019. https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/valuation-standard-value-sets/crosswalk-index-value-calculator/ (accessed 11 November 2019).
- Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D 1999. www.york.ac.uk/che/pdf/DP172.pdf (accessed 26 May 2020).
- Billingham LJ, Abrams KR, Jones DR. Methods for the analysis of quality-of-life and survival data in health technology assessment. Health Technol Assess 1999;3. https://doi.org/10.3310/hta3100.
- Health and Safety Executive . Costs to Britain of Workplace Fatalities and Self-Reported Injuries and Ill Health, 2017 18 n.d. www.hse.gov.uk/statistics/pdf/cost-to-britain.pdf (accessed 22 May 2020).
- NHS Improvement . NHS Reference Costs 2017 18 n.d. https://improvement.nhs.uk/resources/reference-costs/#rc1718 (accessed 9 August 2019).
- NHS Employers . NHS Terms and Conditions of Service Handbook 2019. www.nhsemployers.org/tchandbook (accessed 11 November 2019).
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Brooks A, Hagen SE, Sathyanarayanan S, Schultz AB, Edington DW. Presenteeism: critical issues. J Occup Environ Med 2010;52:1055-67. https://doi.org/10.1097/JOM.0b013e3181f475cc.
- NHS Digital . NHS Workforce Statistics – May 2019 2019. https://digital.nhs.uk/data-and-information/publications/statistical/nhs-workforce-statistics/may-2019 (accessed 11 November 2019).
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
Appendix 1 Approval and confirmation of capacity and capability
| Research site | Date of research and development approval |
|---|---|
| Cheshire and Wirral Partnership NHS Foundation Trust | 7 March 2017 |
| Leeds Teaching Hospitals NHS Trust | 16 March 2017 |
| York Teaching Hospital NHS Foundation Trust | 27 September 2017 |
| Lancashire Care NHS Foundation Trust | 24 October 2017 |
| Nottingham University Hospitals NHS Trust | 13 June 2018 |
| University Hospitals of Derby and Burton NHS Foundation Trust | 14 September 2018 |
Appendix 2 Sample shoe styles
Figures reproduced with permission from Shoes for Crews (Europe) Ltd, 2019, personal communication.


Appendix 3 SSHeW trial baseline questionnaire
Health & Safety Executive logo reproduced with permission, 2020, personal communication.
Appendix 4 SSHeW trial slip diary
Appendix 5 SSHeW trial 14-week (final) follow-up questionnaire: control group
Health & Safety Executive logo reproduced with permission, 2020, personal communication.
Appendix 6 SSHeW trial 14-week (final) follow-up questionnaire: intervention group
Health & Safety Executive logo reproduced with permission, 2020, personal communication.
Appendix 7 SSHeW trial slip data collection sheet
Pages 129 and 130 of this data collection sheet have been reproduced with permission from the EuroQol Research Foundation. EQ-5D™ is a trade mark of the EuroQol Research Foundation. © EuroQol Research Foundation. Reproduction of this version is not allowed. For reproduction, use or modification of the EQ-5D (any version), please register your study by using the online EQ registration page: www.euroqol.org.
Health & Safety Executive logo reproduced with permission, 2020, personal communication.
Appendix 8 SSHeW trial injury follow-up questionnaire: during the trial
Pages 136 and 137 of this follow-up questionnaire have been reproduced with permission from the EuroQol Research Foundation. EQ-5D™ is a trade mark of the EuroQol Research Foundation. © EuroQol Research Foundation. Reproduction of this version is not allowed. For reproduction, use or modification of the EQ-5D (any version), please register your study by using the online EQ registration page: www.euroqol.org.
Health & Safety Executive logo reproduced with permission, 2020, personal communication.
Appendix 9 SSHeW trial post-14-week injury follow-up
Pages 142 and 143 have been reproduced with permission from the EuroQol Research Foundation. EQ-5D™ is a trade mark of the EuroQol Research Foundation. © EuroQol Research Foundation. Reproduction of this version is not allowed. For reproduction, use or modification of the EQ-5D (any version), please register your study by using the online EQ registration page: www.euroqol.org.
Health & Safety Executive logo reproduced with permission, 2020, personal communication.
Appendix 10 Changes to trial protocol
| Protocol | Date | Changes made |
|---|---|---|
| SSHeW protocol v1.0 | 31 October 2016 | This version was submitted to the DHSRGC prior to HRA approval |
| SSHeW protocol v2.0 | 4 January 2017 | Version submitted with initial HRA application. Changes made included simplifying data collection about falls by not asking for these data in a weekly text message (so one text will be sent each week), but asking for these data in the final 14-week questionnaire instead; clarifications to some secondary outcomes; update of contact details of new trial co-ordinator; clarification of exclusion criteria regarding orthoses/modification of footwear; adding long-term follow-up data collection and injury follow-up plan with EQ-5D and resource use for health economics; and correcting HSL to HSE |
| SSHeW protocol v3.0 | 20 June 2017 | Minor clarifications: wording of welcome text when participants join the trial and wording of compliance text sent three times during the trial; clarification about eligibility criteria; clarification about data query process; and clarification about data collected from a participant who reports an injury |
| SSHeW protocol v4.0 | 3 August 2017 |
Allowing a reminder to be sent to participants who have not returned their slip data collection questionnaire or 14-week questionnaire Allowing two additional pre-randomisation text messages to be sent to participants who may have misunderstood the trial and not responded to enough of the pre-randomisation text messages to be eligible for randomisation (including sending then a letter explaining the trial process and that they will receive two further text messages) |
| SSHeW protocol v5.0 | 26 April 2018 |
Inclusion of a SWAT to evaluate if inclusion of a pen with the final questionnaire increases response rates Change in eligibility criteria to include participants who work 60% WTE, rather than 80% WTE Clarifications to secondary outcomes and method of randomisation |
| SSHeW protocol v6.0 | 9 August 2018 | Clarification of 60% WTE to mean 22.5 hours per week (i.e. 60% of full-time NHS week, which is 37.5 hours) |
| SSHeW protocol v7.0 | 20 November 2018 |
Extension of the randomisation period Addition of a questionnaire to help identify participants who would be willing to return their shoes for wear testing |
| SSHeW protocol v8.0 | 14 February 2019 | Changes to qualitative recruitment to ask local research teams at sites to help identify willing volunteers to take part in an interview and the addition of a £20 high street voucher to be given as a thank you to those who undertake an interview |
Appendix 11 Qualitative topic guide
SSHEW trial qualitative topic guide: interviews with participants as part of the SSHEW trial
Invitation e-mail/text/telephone call procedure to arrange interview
-
The qualitative researcher will introduce themselves to the participant.
-
The researcher will ask the participant if they received the invitation text, e-mail or letter.
-
The researcher will explain the reason for calling (i.e. to see if the participant would like to take part in the interview study).
-
The researcher will answer questions and/or explain the study.
-
The researcher will determine if the participant would like to take part in the study.
-
If the participant is willing to take part, the researcher will thank them and arrange a convenient date and time. If the participant is not willing to take part, the researcher will thank them for their time. If the participant is unsure then the researcher will answer any questions and give the participant more time to consider participation and arrange to contact them again (at least 24 hours later).
Interview topic guide for SSHEW trial participants
Approximately 40 participants will be interviewed. This topic guide summarises the main areas to be explored in each interview about slips and falls in the workplace and acceptability of the trial footwear. As with any qualitative interviews, these headings are intended as a starting point to ensure that the primary issues are covered, while allowing flexibility for new issues to emerge. Preliminary analysis of data from earlier interviews will shape the topics covered in later interviews.
Introduction
-
The researcher introduces themselves.
-
The researcher explains the background of the study and purpose of the interview.
-
The researcher emphasises confidentiality, reminds the participant that the interview will be audio-recorded and that they can stop the interview at any time if they wish.
-
The researcher will remind the participant that the information from the research will be published as a report for the Public Health Research programme and as other reports.
-
The researcher will check if the participant has any questions about the study or interview before the start.
-
The researcher will confirm that the participant is still willing to take part.
Understanding reducing slips and falls in the workplace
-
General background information.
-
How long have you worked at the trust? (Number of hours worked, role, where they work, e.g. ward, community.)
-
Is there a history of slips and falls in the workplace? How much of a problem do you perceive slips and falls in the workplace to be in general? What are the consequences of slips and falls? Is reducing or preventing slips and falls important to you?
-
How were you made aware, or were you aware, of the purpose of the intervention?
-
Are staff in this organisation aware of the purpose of the intervention?
-
-
What footwear did you wear prior to the study? Did you consider them to be slip resistant? What criteria would they use to judge if footwear were slip resistant?
-
What are your thoughts on how the shoes may reduce slips and falls in the workplace?
-
What is your understanding of ‘risk factors’ for slipping and falling at work?
Compliance
-
Had you heard of slip-resistant footwear before the start of the study?
-
Did you think they were a good idea?
-
Did you think wearing the shoes provided by the study would prevent slips and falls?
-
What did you think about the footwear that was provided?
-
What did you think about the choice of footwear on offer?
-
Did you have any concerns about the footwear?
-
How did the new shoes differ from the ones you wore before the start of the study?
-
How often do you wear the footwear?
-
Where do you wear the shoes? For example, at work only, on the way to work, outside work?
-
Did you have any problems when wearing the shoes? (Explore fitting, comfort, issues with underlying foot pathologies.)
-
What benefits and disadvantages have you seen since wearing the shoes?
-
Do you feel more confident when wearing the shoes?
-
Do you worry less about slipping and falling when you wear the shoes?
-
Would you buy these shoes for work after the study has finished and when your current shoes need replacing?
-
Would you consider trying a different style of shoe in the slip-resistant range?
-
Would you recommend these shoes to other members of staff?
-
Would you consider buying this range of shoes to wear day to day in your personal life?
Participating in the study
-
Explore the participant’s views on their experience of being involved in the SSHEW trial (e.g. was it something that they really wanted to do, have they ever taken part in a study before, would they take part in a research study again).
-
How did you feel about filling in the screening form you were asked to complete? How did you find it? Was it easy to understand? Did you like the layout? Could it have been improved in any way?
-
How did you feel about responding to the text messages collecting slip and falls information?
-
Views on being randomised to the intervention group (e.g. experiences of randomisation process and understanding of this).
-
Views about value of the trial.
Any other issues
-
Any other issues or questions the participant would like to raise?
-
The researcher will clarify what happens next in terms of the SSHEW trial.
-
The researcher will thank the participant for their time.
List of abbreviations
- AE
- adverse event
- CACE
- complier-average causal effect
- CEA
- cost-effectiveness analysis
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CWP
- Cheshire and Wirral Partnership NHS Trust
- DHSRGC
- Department of Health Sciences Research Governance Committee
- DMEC
- Data Monitoring and Ethics Committee
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GB
- Great Britain
- GP
- general practitioner
- HR
- hazard ratio
- HRA
- Health Research Authority
- HRQoL
- health-related quality of life
- HSE
- Health and Safety Executive
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- IRR
- incidence rate ratio
- LFS
- Labour Force Survey
- NICE
- National Institute for Health and Care Excellence
- OR
- odds ratio
- PPI
- patient and public involvement
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SD
- standard deviation
- SDCS
- slip data collection sheet
- SSHeW
- Stopping Slips among Health-care Workers
- STFL
- slips, trips and falls on the same level
- SWAT
- study within a trial
- TSC
- Trial Steering Committee
- WTE
- working-time equivalent
- YTU
- York Trials Unit
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/phr09030).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
