Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 13/115/29. The contractual start date was in May 2016. The draft report began editorial review in September 2020 and was accepted for publication in May 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
Copyright © 2022 Logan et al. This work was produced by Logan et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Logan et al.
Chapter 1 Introduction: why this study was needed
Material throughout the report has been adapted with permission from the trial protocol. 1
Why are falls important?
A fall can have a devastating impact on a person, their family and their carers, and can place demand on health and social care resources. Falls are common, with one-third of those aged > 65 years and half of those aged > 80 years falling at least once per year. 2 Ageing societies pose challenges for health and social care systems. The UK has an ageing population; there are nearly 12 million people ≥ 65 years, of whom 5.4 million are aged ≥ 75 years, 1.6 million are aged ≥ 85 years, over 500,000 are aged ≥ 90 years and 14,430 are centenarians. 3 Falls are the most common cause of emergency hospital admissions for older people. 4 In 2017/18, there were around 218,000 emergency hospital admissions related to falls among patients aged ≥ 65 years, with around 149,000 (68%) of these patients aged ≥ 80 years. 5
Consequences of a fall
Falls can cause injury, distress, pain, reduced mobility, loss of confidence or independence, and a fear of falling, leading to reduced levels of activity in daily life and increased mortality. 2 In 2017, 5048 people aged ≥ 65 years died from having a fall, equating to 14 people every day. 4 Hip fracture is the most common serious injury following a fall, and it is estimated that around one-quarter of people who are aged > 65 years and fracture their hip will consequently require long-term care in a care home. Hip fractures are the leading cause of accidental death. 6 Older adults with frailty are less able to cope and recover from accidents, physical illness or other stressful events, including falls. People living in care homes are more frail than community-based populations and their care needs merit specific attention. 7 Delivering comprehensive, consistent and structured enhanced support to those in care homes will ensure that their needs continue to be identified and met proactively. 8
Prevention and management of falls
The National Falls Prevention Coordination Group’s falls and fractures consensus statement advocates a whole-system approach to the prevention of falls that includes risk factor reduction across the life-course, case finding and risk assessment, strength and balance exercise programmes, healthy homes, high-risk care environments, fracture liaison services, and collaborative care for severe injury. 9 Identification of those at risk of falls; assessment of contributory risk factors for falling; and interventions to reverse, reduce or modify those risk factors are recommended by the National Institute for Health and Care Excellence (NICE). 10 However, these recommendations are based on people living in their own homes who have capacity to listen and react to health professional’s advice; they were not written for care home staff or residents.
Falls in care homes
Approximately 421,000 older people were living in UK care homes in 2016–17. 11 Two levels of care accommodation are available, depending on the level of care required. These are personal care and 24-hour support (provided by care homes without nursing, sometimes called residential homes), and additional on-site nursing (provided by care homes with nursing, sometimes called nursing homes). Some homes provide both levels of care (dual-registered homes). Care homes both with and without nursing match the international consensus definition of a nursing home. 12 Around 5500 different providers in the UK operate 11,300 care homes for the elderly, with 95% of beds provided by the independent sector (both for-profit and charitable providers). 13
The majority of older people living in care homes are aged > 85 years; live with cognitive impairment, multimorbidity and limited mobility; and take multiple medications. 7 The rate and risk of falls for residents of care homes is high, with falls being three times more common in care home residents than in older people living in their own homes, and those falling in care homes being 10 times more likely to suffer a serious injury. 14 One in five people living in care homes will die within a year of suffering an injurious fall. 15 Falls may often occur as a symptom of underlying frailty and illness. 16 Falls may engender feelings of anxiety in care home staff, and fear of litigation and complaints, which may have an impact on care staff’s willingness to encourage residents to be physically active. 17
Multiple diverse factors can contribute to the increased risk of falls in care home residents, including frailty, the presence of long-term conditions, physical inactivity, taking multiple medications and the unfamiliarity of the surroundings. The interaction of factors that contribute to an individual’s risk of falling is unique to them; therefore, interventions to reduce falls risk must be individualised and meaningful for individuals. Protocols used to perform risk assessments for falls in hospitals and care homes vary in quality, do not necessarily trigger individually tailored interventions to reduce risk factors and, in some cases, seek only to stratify risk. 18
A number of interventions have been applied to reduce falls in care homes, including multifactorial approaches. However, a Cochrane review found the evidence to be of low quality and inconclusive regarding effective strategies to reduce both falls rates and falls risk. 19
Implementing health-care interventions in care homes
The NICE falls guidelines and quality standards10 do not explicitly provide guidance for care home residents. Instead, care home staff and clinicians are relied on to apply research evidence from hospital in-patient and community falls trials. Interventions are often difficult to implement within a care home and it is unclear whether or not interventions can lead to cultural change that becomes embedded in care home practices so that effects are sustained or even increase after the intervention. 20
Improving the lives and health of older people living in care homes is a major UK government priority and is embedded in the NHS Long Term Plan. 21 The Enabling Research In Care Homes (ENRICH) initiative brings together care home staff, residents and researchers to facilitate the design and delivery of research in care homes. Increasingly, research has looked at providing care home staff with training in an aspect of care from experts, with the aim of increasing carers’ knowledge and expertise in caring for older people with frailty. A recurrent observation has been the need to adapt existing approaches to improvement and implementation to take account of and empower care home staff and organisations. 22 In addition, there is evidence that a number of care home-specific issues affect how ready care homes are to engage with external organisations regarding change. 23 Where care home staff and NHS professionals are required to work together, there is evidence that outcomes will be better if specific activities that encourage shared working between care home staff and visiting health-care professionals are integrated into care delivery. 24
Falls are often a consequence of undiagnosed and untreated underlying health conditions, frailty and environmental factors, including the way that care is structured. Approaches to falls prevention are therefore likely to be multiagency collaborations between care home staff and external health-care providers. Taking into account the lessons learned about effective partnership working and implementation science, adoption of early co-design and strong stakeholder involvement in the planning and design of the intervention and study was essential to create a falls prevention programme that was pragmatic and suitable to subsequent adoption, while also maximising the external validity of the study. The Medical Research Council’s framework for developing and evaluating complex interventions25 provided a framework for the development of our falls prevention programme, feasibility study and multicentre trial. The framework highlights the need to understand the context for delivery of a complex intervention, which is even more relevant in care homes as they pose a distinct challenge for the introduction of complex interventions: they vary in size, funding, workforce and culture, and house vulnerable individuals with far-reaching health and social care needs.
The feasibility study [Falls in Care Homes (FiCH)], which laid the foundation for the current Falls prevention in Care Homes (FinCH) trial, found that an effective falls prevention programme would have to use language that care home staff could understand and identify with, explain the rationale for falls prevention in ways that aligned with care home organisational priorities, and be conducted in a way that allowed for care home schedules and care regimes. 26
The GtACH programme
Our intervention, the Guide to Action for falls prevention in Care Homes (GtACH) programme,27,28 was developed to reduce falls rates by supporting care home staff to identify risk factors for falling that are pertinent for an individual and take action to reduce those risks. It was co-produced by a group of care home staff, clinicians, researchers, public, voluntary and social care organisations and includes care home staff training, support and documentation. Care home staff are trained to implement the programme in their home by an NHS falls lead over 1 hour in small groups. The NHS falls lead is a nurse, physiotherapist or occupational therapist who has specialist training, skills and knowledge in falls prevention and bone health. The homes are asked to identify a falls champion to help maintain implementation. When the GtACH programme is implemented, homes are given a copy of the GtACH manual and are supplied with the GtACH tool. The latter is a paper form, comprising an assessment component (a checklist of falls risk factors) and care planning section supported by suggested actions linked to each fall risk factor (see Appendix 1). After training, it takes an average of 20 minutes to complete the GtACH tool for each resident. Initial proof of concept work29 and a subsequent feasibility randomised controlled trial (RCT), FiCH, showed that the GtACH programme was implementable and changed staff behaviour in line with gold standard practice. 26
Limitations of previous studies
A Cochrane review published in 2018,19 which looked at the effectiveness of interventions designed to reduce falls in older people in care facilities and hospitals, found that the majority of trials were at high risk of bias in one or more domains, mostly relating to lack of blinding. With few exceptions, the quality of evidence for individual interventions in either setting was generally rated as low or very low. Risk of fracture and adverse events (AEs) were generally poorly reported and, where reported, the evidence was of very low quality. The authors concluded that there was a need for further research and, in particular, large RCTs in care facilities to inform practice in falls prevention.
Justification for current trial
The Cochrane review in 201819 concluded that further research to strengthen the evidence for multifactorial interventions for falls reduction in care homes was required as there were some individual trials that showed potentially important reductions in the rate of falls. The authors noted that a key feature of these multifactorial interventions was the individualised nature of the interventions delivered. The review stated:
[t]his implies that further research with emphasis on an individualised, standardised approach to delivery of interventions with consistent description and application within further trials is warranted, including as a clear description of existing falls prevention practices in the control arm of any trials and the interaction of the intervention arm of the trial with usual care. A mixed methods approach may be necessary to achieve this.
Reproduced with permission from Cameron et al. 19 Copyright © 2020 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution Non-Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for non-commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by-nc/4.0/
Research aims
The aims of the trial were to:
-
determine the clinical effectiveness and cost-effectiveness of the GtACH programme compared with usual care
-
complete a process evaluation to provide insight into the implementation of the GtACH programme and to contextualise trial findings.
Chapter 2 Trial design, including interventions
This chapter describes the trial as originally designed and the interventions. It is a summary of the full protocol,1 with the methods of analysis for the economic evaluation and results presented in Chapter 4. The methods and analysis for the process evaluation are presented in Chapter 5.
Trial design
The FinCH trial was a pragmatic, multicentre, cluster parallel 1 : 1 RCT to evaluate the GtACH programme compared with usual care (an absence of a systematic and co-ordinated falls prevention process) in UK care homes for older people. The allocation was at the care home level. A flow diagram of the trial design is shown in Figure 1.
FIGURE 1.
Flow diagram of the trial design.
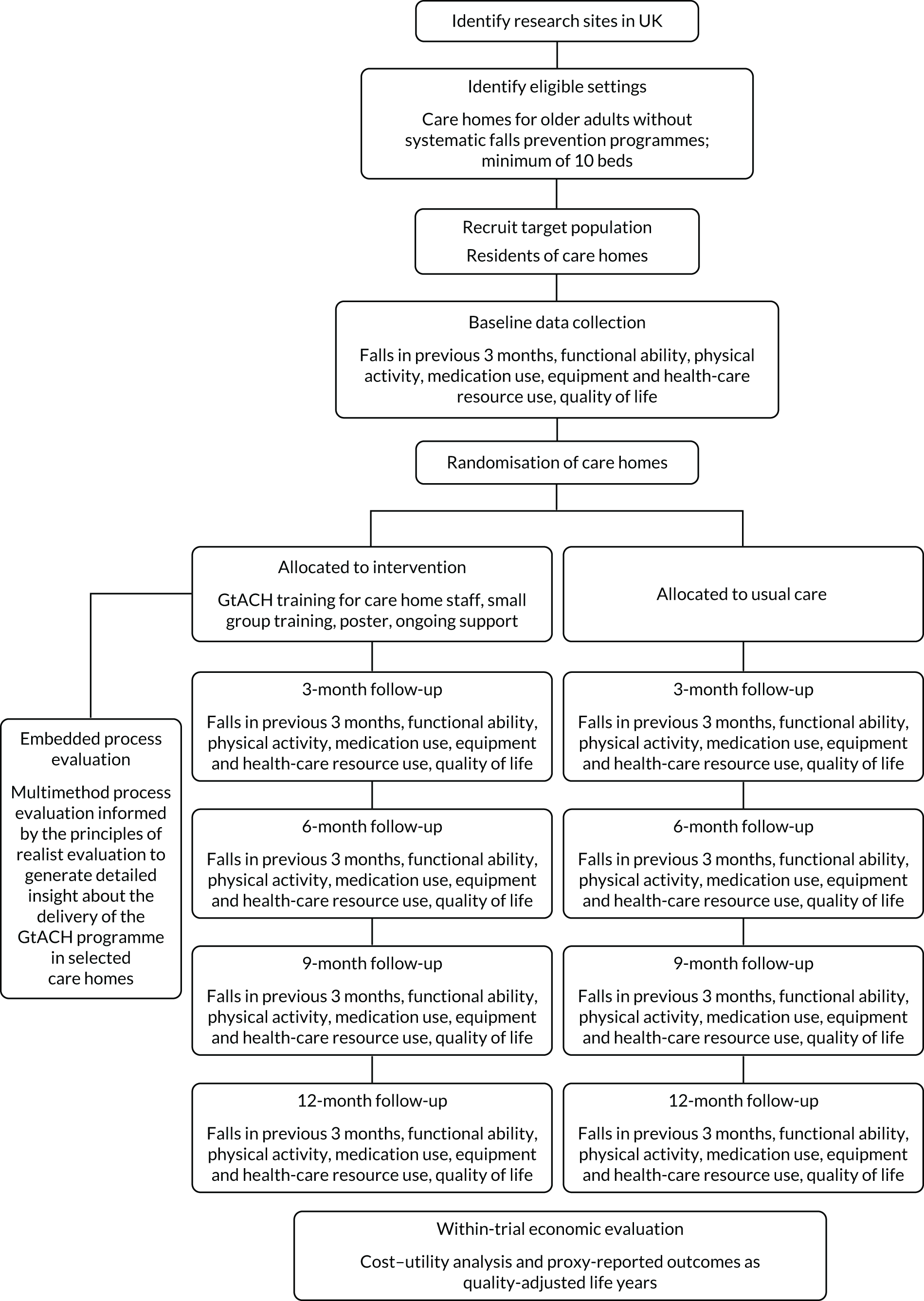
Care homes and residents took part in the study. The primary RCT health outcome was falls rates in the 90-day period between 91 and 180 days post randomisation. Secondary outcomes were collected at baseline and at 3, 6, 9 and 12 months post randomisation.
Trial population
The trial population was care homes (with and without nursing) registered with the care home regulator [the Care Quality Commission (CQC)] in England.
Eligibility criteria: care homes
Inclusion criteria:
-
long-stay care home with old age and or dementia registration
-
≥ 10 potentially eligible residents
-
falls routinely recorded in residents’ personal records and on incident sheets
-
written agreement of care home manager to comply with the protocol and identify a care home falls champion if allocated to the GtACH arm.
Exclusion criteria:
-
participated in GtACH pilot/feasibility studies
-
exclusively provided care for those with learning difficulties or substance dependency
-
contracts with health or social providers were under suspension, or were under investigation by the regulator of care homes (the CQC) or special measures at time of recruitment
-
a significant proportion of beds taken up by health-service commissioned intermediate-care services
-
existing systematic falls prevention programme.
Eligibility criteria: residents
Inclusion criteria:
-
all long-term care home residents.
Exclusion criteria:
-
residents receiving end-of-life care.
Identification of care homes and consent of care home managers
Research assistants at each site identified care homes through examining the CQC website, presenting the study at the ENRICH research network events and liaising with Clinical Research Network (CRN) staff who had experience of conducting research in care homes. Care home managers were telephoned and/or sent a letter inviting them to participate. If they responded to the invitation, the researcher posted details of the study to the care home and an appointment was made to visit the home. A researcher then visited the care home to confirm eligibility at least 24 hours later to allow adequate time for the manager to digest the written study information. Questions and an opportunity to clarify their involvement were encouraged at the visit and, if willing, informed consent from the care home manager was obtained. All care homes invited to participate in the research were offered an incentive of £200. Control homes were given this at the start of the study. Intervention homes were given £100 at the start of the study and a further £100 once the training was complete. The homes were asked to send an invoice to the University of Nottingham.
Screening, recruitment and identification of participants
Once the care home manager agreed to participation in the study, care home staff distributed (within the care home) or posted study invitation letters and participant information sheets (PISs) or consultee information sheets to residents, relatives and/or personal consultees (PCs). After a 2-week window, details of those who confirmed that they were happy to take part were provided to the researcher, who then arranged to meet with the resident (and their consultee, if appropriate) at a mutually agreeable time to provide further information about the study and obtain consent. This took place prior to randomisation of the care home.
Resident recruitment and consent procedures
All residents consented to participate or a consultee agreed to their participation. For residents who were unable to provide consent, the researcher used a short ‘picture’ version of the PIS to explain the study to the resident in the presence of their consultee.
A recommendation by the Trial Steering Committee (TSC) to change the definition of ‘fall’ on the PIS was implemented so that the control arm and GtACH arm were given the same definition.
The researcher confirmed eligibility, assessed capacity and fully explained the study to the resident, relative and/or PC. Before being enrolled in the study, informed written consent was obtained in accordance with Research Ethics Committee (REC) guidance and good clinical practice (GCP). When a consultee was required, the consultee signed a declaration if they believed that the participant would have wished to take part in the study had they had the mental capacity to state their preference. Residents who did not have capacity and whose relatives did not respond to the invitation letter within 2 weeks were also able to be enrolled in the study, as the care home manager acted as consultee in these instances. Baseline data were collected by the researcher after consent or consultee agreement was given.
The process of contacting PCs for residents who did not have capacity was identified as the main barrier to recruitment. An amendment was sought, with patient and public involvement (PPI), for a cover letter to be sent to residents’ PCs summarising the research and indicating that if a response from the consultee was not received within 2 weeks of receipt of the letter, a care home manager would act as nominated consultee on the resident’s behalf. The amendment was approved by the REC in June 2017 and implemented in July 2017.
Interventions
The GtACH programme
The GtACH programme is summarised using the Template for Intervention Description and Replication (TIDieR) checklist30 and can be obtained from the authors.
Rationale
The relatively untrained nature of care home staff, the complex nature of falls risk factors in care home residents and the need for multiple interventions to address multiple risk factors require a systematic home-wide programme, including staff education and support in the use of risk assessment and decision support tools.
Materials
-
GtACH training slides: these were used by the NHS falls lead to train care home staff in each intervention home.
-
The GtACH reference manual: given to care home staff to support implementation of the GtACH paper screening and assessment tool. It included a master copy of the paper tool, information about the study, a copy of the training session slides, falls information (including a definition of a fall, why falls are important and causes of falls), instructions on how to complete the GtACH paper screening and assessment tool, a falls incident analysis template, a medication and falls chart and information on how to obtain further expert advice or support from the local falls expert.
-
The GtACH paper screening and assessment tool comprised 33 items related to falls risk factors grouped into four domains: falls history, medical history, movement/environment and personal needs. The presence of risk factors prompts up to 30 individual staff actions (see Appendix 1).
-
Attendance certificate: given to care home staff at the end of training.
-
The GtACH poster: an A4 poster given to care homes to display in the home to act as a reminder to implement the GtACH programme in the home.
Procedures
-
Care home staff training was provided by the local NHS falls lead, lasted 1 hour per session and included the purpose of the study, purpose of the training, prevalence of falls in care homes, the GtACH programme’s history, and how to complete and where to file completed forms. It emphasised consistent delivery and referenced the materials listed above, in particular the GtACH reference manual. Case studies and role play were used. The NHS falls lead was a registered nurse, physiotherapist or occupational therapist who was trained and specialised in falls prevention and bone health. As it was not feasible for all care home staff to attend a single training session, repeated training sessions were offered according to care home staff availability.
-
A care home falls champion, whose role was raising awareness and liaising between staff and the NHS falls lead, was identified in each care home.
-
Trained staff were asked to complete the GtACH screening and assessment tool with every resident within 2 weeks of the training, in private, and involve family, friends and other care home staff as appropriate. Completed GtACH documentation was to be placed in the resident’s care records and it was expected to contribute to the care plan for each resident. Reassessment was expected if the resident developed a new health condition or fall, or every 3–6 months.
The training period for care homes was extended from 2 weeks to 4 weeks (post randomisation) to enable fall leads time to train very large homes (≥ 50 staff members).
Control intervention
The intervention in the homes randomised to the control arm was usual care. The materials and procedures described in the intervention were not used and no systematic falls prevention approach was applied.
Strategies to reduce contamination
Health-care trials of complex interventions are at risk of contamination bias as the interventions often involve multiple components, multiple stakeholders and a range of organisations that interact with the context in which they are delivered. FinCH was a complex rehabilitation trial for which the intervention and trial procedures involved interactions between clinicians, care homes, residents, researchers and wider stakeholders (e.g. commissioners and private organisations). These interactions could lead to a change in behaviour and, potentially, a change in usual care, even in the control settings where exposure to the intervention is intended to be prohibited. In the design and conduct of the FinCH trial, the following strategies were used to reduce the potential for contamination:31
-
Research staff at the set-up meetings explained the importance of continuing with usual care for the control arm to act as a comparator.
-
NHS falls leads in the sites were asked to sign a confidentiality agreement to state that they would not share the GtACH reference manual.
-
Data on the number of care home staff leaving and starting at each home were collected.
-
The GtACH reference manual was not published prior to study completion.
-
The content of the intervention was not described in detail when the study team were invited to present the ongoing trial information at conferences and high-profile impact events.
-
The study team spoke to commissioners to explain the trial timelines and confirm that all control care homes would be offered the intervention at the end of the trial.
-
All control care homes were offered the intervention at the end of the trial.
-
Therapists and nurses in the sites were given training in RCT design and ethics considerations to help them understand the issues.
Strategies to reduce bias
We aimed to minimise the risk of falls recording and ascertainment bias by care home staff through our eligibility criterion that homes were required to have a falls recording process in place to routinely record falls in residents’ personal records and on incident sheets. The GtACH programme did not include a falls recording system.
We aimed to reduce bias arising from research staff collecting outcome data by ensuring that they were not involved in and were independent of care delivery in any of the study care homes.
Further steps were:
-
All research assistants (RAs) collecting outcome data were blind to allocation of the homes.
-
When unblinding occurred, we asked the RAs to report this on an unblinding form, localised by site and collated by Norwich Clinical Trials Unit (NCTU). When site staff were available, RAs/CRN staff did not continue data collection at care homes for which they had been unblinded to trial allocation.
-
Data were collected from care home records using a standardised data collection form, and data were checked by a second researcher if there were concerns over the content of handwritten notes.
-
When possible, CRN staff collected data, as they were better trained and less emotive about the results.
-
Data were inputted into the research trial database (REDCap)32,33 by data entry technicians unrelated to the study where site capacity allowed. NCTU monitored data quality throughout the trial, reporting to sites on a weekly basis so that there was more of a chance that researchers could find the source data in the care homes to check.
Baseline and outcome measures and assessment
The following characteristics of care homes were collected:
-
number of staff in caring role
-
number of beds in care home
-
number of residents
-
falls monitoring processes.
Baseline data collected
Primary outcome
The primary RCT outcome was the rate of falls per participant in the 90-day period between 91 and 180 days post randomisation, with data collected from care home records and incident forms. The primary outcome for the economic analysis was the cost per fall prevented (cost-effectiveness) and the incremental cost per quality-adjusted life-year (QALY) (cost utility).
Secondary outcomes
The secondary outcomes were assessed at 90, 180, 270 and 360 post randomisation:
-
Falls were assessed by a researcher examining residents’ care home records for routinely collected data at each time point. Incident report forms were also examined. The date, time and source of the information for each fall was recorded on the participant’s case report form (CRF).
-
Fall injuries were assessed by a researcher using residents’ care home records, and through liaison with care home staff to source the data. A yes/no response to the ‘sustaining an injury’ question was recorded on the CRF at the same time point by the researcher. Any details of medical assistance and source of the information was collected and added to the CRF.
-
Medication administration records – consent was sought to allow clarification of medication data from general practitioner (GP) records where necessary.
-
Days in hospital were obtained from NHS Digital data.
-
Fractures per participant were collected using NHS Digital data.
-
Personal activities of daily living (ADL) were assessed using the Barthel ADL Index34 completed by care home staff and collected by the researcher.
-
Resident knowledge, skills and confidence (activation) were assessed using the Physical Activity and Mobility in Residential Care (PAM-RC)35 (completed by care home staff and collected by the researcher.
-
Quality of life (QoL) was assessed using the validated questionnaires Dementia Quality of Life utility version-5 dimensions (DEMQOL-U-5D)36 and EuroQol-5 dimensions, five-level version (EQ-5D-5L)37 completed by the resident, and the proxy measures Dementia Quality of Life Utility version, proxy complete-4 dimensions (DEMQOL-P-U-4D)38 and EuroQol-5 dimensions, five-level version, proxy complete (EQ-5D-5L-P)37 completed by care home staff. Participants were offered help to complete the QoL questionnaires by the researcher, if necessary. Dual recording of these data were undertaken to ensure that baseline data were captured in the event of a resident losing mental capacity.
-
Death was recorded using care home records.
Economic data
-
Secondary care resource use was identified from electronic records held by NHS Digital.
-
Provision of equipment to an individual resident and the item, date purchased and description of item obtained was identified from the resident’s care home records and care home staff’s knowledge.
-
Medication administration records: consent was sought to allow clarification of medication data from GP records where necessary.
-
Community health-care provision was identified from care home records.
-
Resources used to deliver the intervention (training, staff time and materials) were measured by recording the number of training sessions delivered, the number and names of staff at each training session, and the cost of the manual and printed materials.
-
Secondary care resource data were obtained from NHS Digital data.
Ethics and regulatory issues
The trial was not initiated until after the protocol, informed consent forms and PISs received approval/favourable opinion from the REC and the NHS Research and Development department. Approval was received from the NHS Health Research Authority and NHS sites. (Yorkshire and the Humber – Bradford Leeds REC, 11/04/2016, reference 16/YH/0111).
The NCTU governed the trial, monitored data collection, and completed data checking and data cleaning.
The trial was conducted in accordance with the ethics principles that have their origin in the Declaration of Helsinki, 1996;39 the principles of GCP, and the Department of Health and Social Care Research Governance Framework for Health and Social Care, 2005. 40
The trial was registered as Current Controlled Trials ISRCTN34353836 with protocol V6 14 November 2017.
Sample size
The sample size was based on the primary RCT outcome of falls rate during the 90-day period between 91 and 180 days post randomisation. The original total sample size estimate was for 1308 residents to be recruited from 66 care homes (33 in each arm). This assumed a falls rate of 2.5 falls per year (0.625 falls in 3 months) in the control arm,41 80% power and a two-sided significance level of 5%, resulting in the need to recruit 189 residents per arm to detect a 33% reduction in falls rate in the GtACH arm. A reduction rate of 33% was chosen as this was the rate achieved by community-based falls prevention interventions16 and therefore deemed clinically significant. The sample size calculation was based on information obtained from a previous care home study that had a falls rate of 15 falls per year,26 but only recruited those residents who had fallen recently. The adjustment for clustering assumed an average cluster size of 20 residents42 and an intracluster coefficient of 0.1,42 giving a sample size of 549 residents per arm. Incorporating a further 16% into the sample size to account for potential attrition gave a total sample size of 1308 residents (654 in the GtACH arm and 654 in the control arm).
During recruitment it became apparent that the average number of residents per cluster was slightly smaller than expected (19 residents) and the size of the clusters was variable (coefficient of variation 0.5). Based on this new information, the design effect for the revised sample size calculation increased from 2.9 to 3.275, leading to a total sample size of 1474 residents after the adjustment for 16% attrition rate. This led to a need to recruit 39 care homes per arm. Across all sites, it was anticipated that the rate of recruitment was five or six care homes, each with 18–19 residents.
Randomisation and blinding
Care homes were randomised on a 1 : 1 basis to one of two parallel arms: the GtACH programme or control (usual care). Participants, care home staff, site NHS falls leads and RAs undertaking the process evaluation at the care homes were not blinded to allocation.
Randomisation of homes to trial arms occurred after all participants had given consent and all baseline data had been collected. The RA who gathered the baseline information notified the local site NHS falls lead when the care home was ready to be randomised. The NHS falls lead used a remote, internet-based randomisation system to obtain the allocation for each home and informed the falls champion within the care home of the allocated GtACH arm.
Randomisation was based on a bespoke, computer-generated, pseudo-random code using variable block randomisation within strata [site, care home type (nursing/residential/dual registration)] provided by the NCTU via a secure web-based randomisation service.
The sequence of treatment allocations was concealed from the study statistician until all interventions had been assigned and recruitment, data collection and all other study-related assessments were complete.
The Trial Management Group (TMG) and the Data Monitoring Committee (DMC) were unblinded to the intervention. The chief investigators and principal investigators (PIs) had direct contact with the randomised care homes, although not with the participants.
The RAs at the sites, participating residents and staff informants were blind to allocation at recruitment and for baseline data collection because participants were recruited prior to care home randomisation. RAs collecting outcome data were not informed of allocation (occasions of unblinding were recorded). NHS Digital data were extracted blind to allocation.
Interim analyses required to populate recruitment and data monitoring for harm reports for the DMC were conducted on unblinded data by the NCTU.
Assessment of compliance
Care home records for all care homes randomised to the intervention were reviewed by the NHS falls lead during the first 3 months post randomisation to GtACH training and during the implementation period to consider broad compliance with the GtACH programme. As part of this evaluation, evidence was sought that the GtACH manual was accessible, the GtACH poster was displayed, and the GtACH paperwork was attached to care records. The number of care home staff who attended the GtACH programme training was recorded as a proportion of the total number of available staff. Compliance with the intervention was also evaluated as part of the process evaluation. We did not record the number of GtACH forms completed in the homes.
Withdrawal of participants
Residents were able to withdraw from the trial at their own request, their consultee’s request or at the discretion of the investigator. The participants were assured that withdrawal would not affect their future care. Participants and consultees, when appropriate, were made aware (through the information sheet and consent form) that should they withdraw, the data collected up to the date of withdrawal could not be erased and may still be used in the final analysis. Care home managers were able to withdraw support for the trial at their own request or at the discretion of the investigator (residents were also withdrawn following care home manager withdrawal of consent).
Adverse event reporting
Data on AEs (serious and non-serious) were not collected in this study.
This was a low-risk intervention. No specific risks, untoward incidents or AEs were reported during the feasibility work. The GtACH tool recommends that actions are taken but does not stipulate what these actions are, other than to recommend referral to health professionals as appropriate. If residents became distressed during the GtACH assessment or when intervention actions were completed, the process was halted, and the event was recorded and closely monitored until resolution, stabilisation or until it was shown that the study intervention was not the cause. The participant had the right to decline any intervention at any time.
Gentle exercises were 1 out of the 30 activities included in the action checklist. If gentle exercises were recommended after the assessment, staff in the care home were advised to refer the resident to a physiotherapist so that a programme of exercise could be put in place. It was possible that participants may have suffered an injury that they would not have had if they had not taken part in the exercise. These were recorded and monitored by the falls champion in the care home. If there was any concern, the falls champion referred to the NHS falls lead for advice. If required, the exercises were stopped.
Falls rates were monitored for harm and reported to the DMC and TSC every 3 months after they were collected. The DMC and TSC had the ability to recommend changes to the study protocol if falls rates were substantially higher than expected. The DMC reviewed unblinded safety data, including reported falls frequencies, at least yearly. These were provided by the NCTU via secure e-mail.
As the GtACH programme is copyrighted by the University of Nottingham, the University of Nottingham was responsible for any issues that arose because of the design of the intervention, training given to care home staff or any issues with the programme itself. However, in respect of the use of the GtACH programme in care homes, the care home was responsible if the programme as a whole was incorrectly used. Care home managers were asked to confirm that they had indemnity for this and, if the indemnity did not include research, they were asked to seek indemnity from their insurance providers, making clear that all individual components of the GtACH programme were currently being used in routine care but in a consistent or structured manner.
The GtACH assessments and or actions were stopped if the participant showed evidence of distress. This was documented in one participant, but the participant was not withdrawn from the study.
Data handling and record keeping
All trial staff and investigators endeavoured to protect the rights of the trial’s participants to privacy and informed consent, and adhered to the Data Protection Act 1998. 43 The CRF collected the minimum required information for the purposes of the trial only. CRFs were held securely in a locked room, or locked cupboard or cabinet. Study data were collected and managed using REDCap electronic data capture tools32,33 hosted at the University of East Anglia. REDCap is a secure, web-based software platform designed to support data capture for research studies, providing (1) an intuitive interface for validated data capture, (2) audit trails for tracking data manipulation and export procedures, (3) automated export procedures for seamless data downloads to common statistical packages and (4) procedures for data integration and interoperability with external sources. Access to the information was limited to the trial staff and investigators, and relevant regulatory authorities. Data held on computers, including the trial database, were held securely and password protected. All data were stored on a secure dedicated web server. Access was restricted by user identifiers and passwords (encrypted using a one-way encryption method). Information about the trial in participants’ care home records was treated confidentially in the same way as all other confidential medical information. Electronic data were backed up every 24 hours to both local and remote media in an encrypted format.
Statistical analysis
Analyses were undertaken on an intention-to-treat basis in which care homes were analysed in the arm to which they were allocated regardless of their compliance with the intervention. The progress of care homes and residents through the phases of the trial from the screening and enrolment of care homes to the analysis of the outcome data has been summarised in the Consolidated Standards of Reporting Trials (CONSORT) flow diagram (see Figure 6). Those who died between care home randomisation and the 3-month follow-up data collection were regarded as having been exposed to the intervention (GtACH/control) and recorded as lost to follow-up at the time of death.
Data were analysed according to a prespecified statistical analysis plan (SAP) that was finalised prior to the start of the analysis. Full details of all analyses are provided in the SAP (see Report Supplementary Material 1). Analyses were based on available case data. Two-sided tests were used to test statistical significance at the 5% level. The analysis was carried out using standard statistical software, either Stata/MP® version 16 (StataCorp LP, College Station, TX, USA), SAS® (SAS Institute Inc., Cary, NC, USA) or R (The R Foundation for Statistical Computing, Vienna, Austria).
Baseline characteristics of care homes and residents as well as outcome measures at baseline and each follow-up time point were summarised by treatment arm using descriptive statistics. The baseline falls rate was expressed as the number of falls per 1000 resident-days for each arm.
The primary outcome, the rate of falls per participating resident during the 90-day period between 91 and 180 days post randomisation, was expressed as the number of falls per 1000 participating resident-days for each arm. This period was chosen to give time for the intervention to be implemented after training, while acknowledging that people in care homes have short life expectancies. The secondary outcomes, rate of falls occurring during the 90-day period between 181 and 270 days post randomisation, and the 90-day period between 271 and 360 days post randomisation were calculated and reported in the same way as for the primary outcome.
The number of falls per resident was compared between arms using a random-effects/hierarchical two-level Poisson model with resident at level one and care home at level two, with length of residence in care home as an offset. The primary analysis adjusted for type of care home (residential, nursing, dual registration) and site.
Two additional models were fitted to assess the robustness of the model. In addition to adjusting for care home type and site, we adjusted for (1) baseline falls rate during the 3 months before the baseline assessment and (2) baseline falls rate and other variables that were thought to be associated with falling.
The falls rates during the 3-month periods prior to the 9- and 12-month follow-up were analysed and presented in the same way as for the primary outcome variable. For other secondary outcomes, arms were compared using multilevel regression analysis for continuous outcomes and multilevel logistic regression for binary outcomes. Regression coefficients and 95% confidence intervals (CIs) were presented.
Compliance with the intervention was calculated as the percentage of care-giving staff in each care home trained to use the GtACH screening and assessment tool. We calculated this as:
The percentage adherence was calculated and presented for each care home in the GtACH arm. The average adherence for all intervention care homes has also been presented.
Site support
The RAs supported the recruitment, data collection, data entry and data cleaning at each site. Delivery models included a combination of National Institute for Health Research (NIHR) CRN delivery staff, NHS research staff, NHS research therapists and academics employed by higher education institutions. RAs received training in trial processes by the NCTU. A RA network was developed to support RAs who were geographically dispersed across the 10 sites. This was led by a RA based in the same site as the chief investigator.
Monthly teleconferences were held to discuss challenges and share good practice, with any ongoing issues raised at the monthly trial management meetings. The format, content and evolution of the network was directed by the RAs. The peer support of the group allowed open discussion of challenges in a supportive environment. The primary foci of discussions were clarification of recruitment and data collection processes, identification of common barriers to recruitment through PCs, and sharing of methods to engage and sustain relationships with care homes. Face-to-face investigator meetings were held for all FinCH trial team members, including the RAs, PPI members and the teams delivering the interventions. Two meetings were held throughout the trial:
-
May 2017 (16 RAs, 4 PPI members and 10 therapists attended), which included training in the recruitment of older adults lacking capacity, open discussions on good practice and the opportunity to feed back challenges and solutions to the senior research team.
-
July 2018 (23 RAs attended), which included training in abstract and poster design, data quality and awards for sites related to recruitment outcomes.
Chapter 3 Randomised controlled trial results
Recruitment
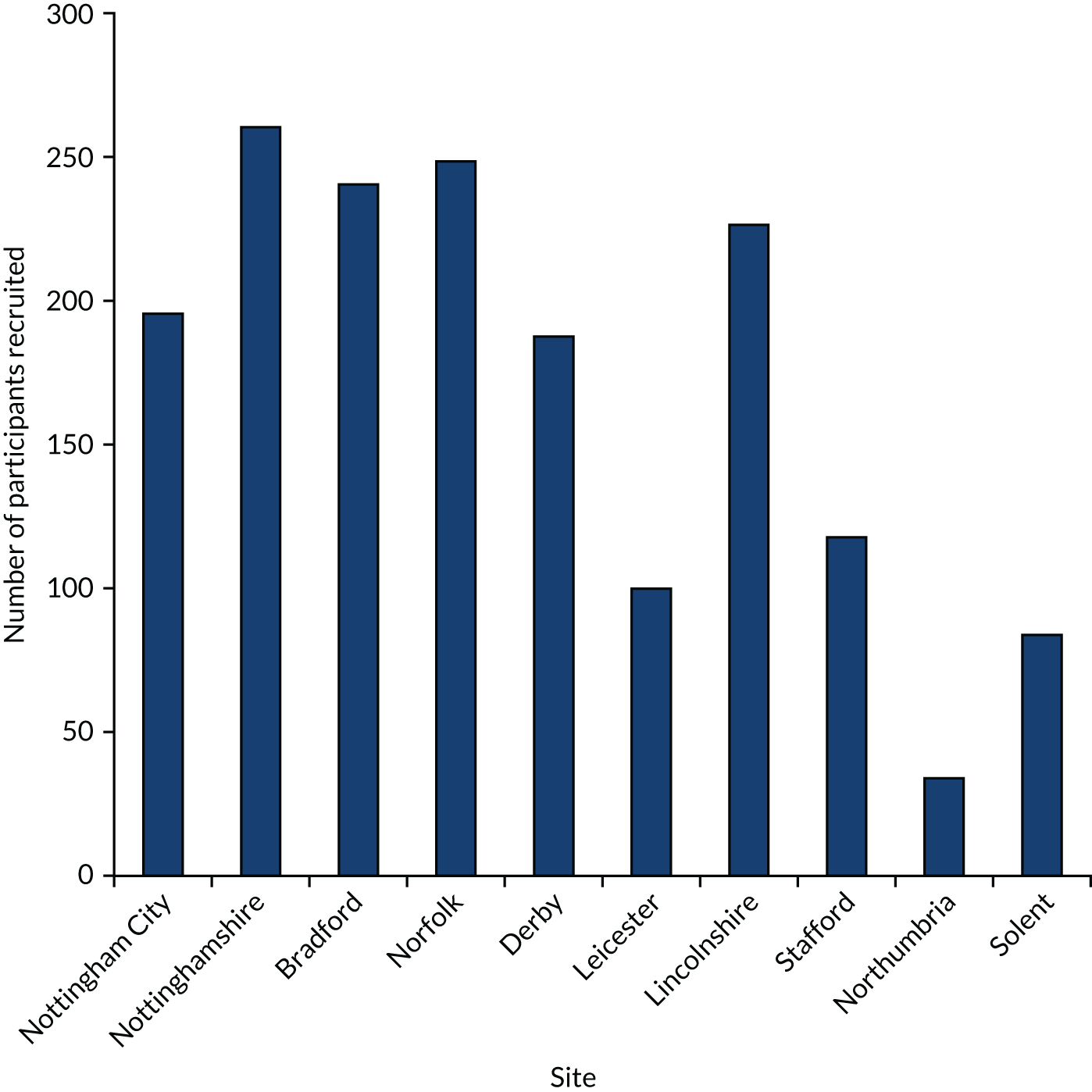
Recruitment to the FinCH trial opened on 1 November 2016. The first care home was recruited on 16 November 2016 and the first resident was consented on 23 November 2016. In total, 10 sites were recruited to the FinCH trial (Table 1). The last care home was recruited on 29 December 2017 and the last resident was recruited on 31 January 2018; all care homes were randomised by 31 January 2018.
| Site | Date recruitment started | Total care homes recruited (n) |
|---|---|---|
| Nottingham City | 16 November 2016 | 12 |
| Nottinghamshire | 21 November 2016 | 12 |
| Bradford | 2 February 2017 | 12 |
| Norfolk | 1 March 2017 | 15 |
| Derby | 7 March 2017 | 11 |
| Leicester | 24 April 2017 | 6 |
| Lincolnshire | 7 June 2017 | 7 |
| Stafford | 24 July 2017 | 6 |
| Northumbria | 2 August 2017 | 3 |
| Solent | 3 October 2017 | 3 |
| Total | 87 |
In total, 87 care homes were recruited; 84 of these care homes were randomised to either the GtACH programme or usual care (Figure 2). Over-recruitment was permitted to allow care homes that were actively engaged in the recruitment of residents by 29 December 2017 to continue through to randomisation by 31 January 2018.
FIGURE 2.
Recruitment of care homes.
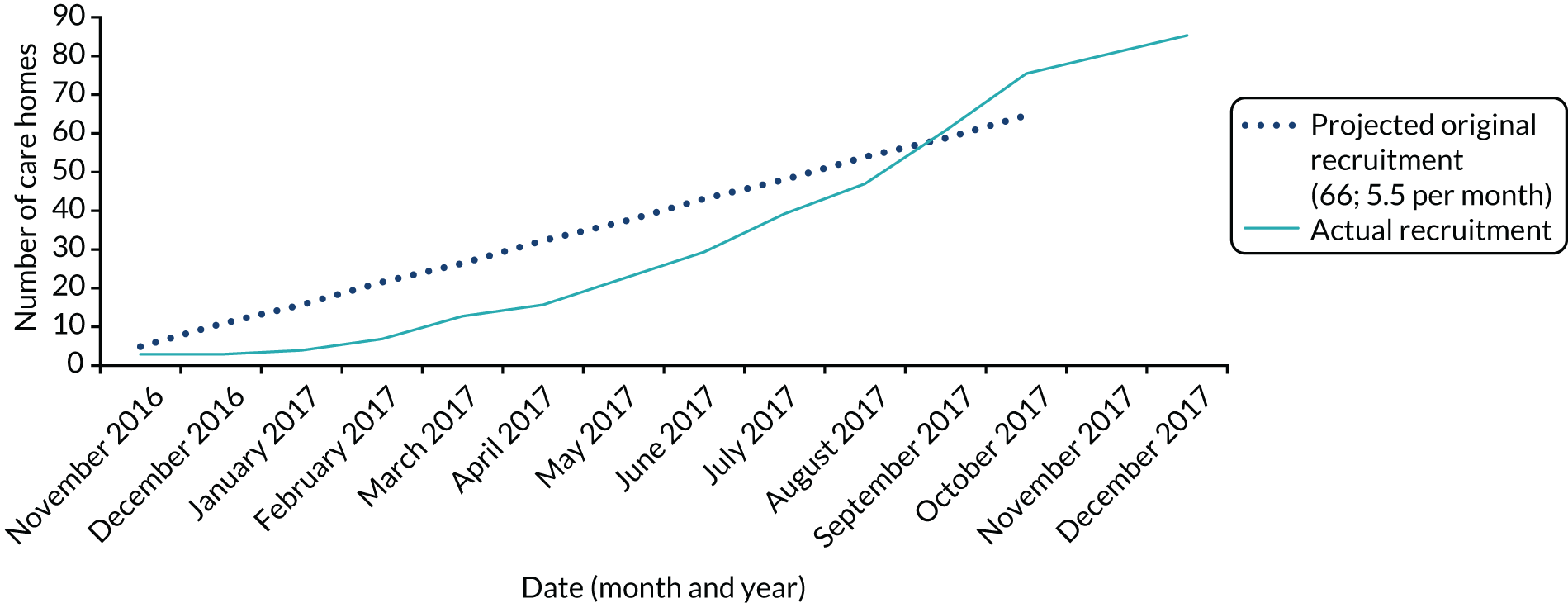
In the same period, a total of 1698 residents were recruited; again, this was higher than the adjusted target of 1482 individuals (Figure 3).
FIGURE 3.
Recruitment of residents.
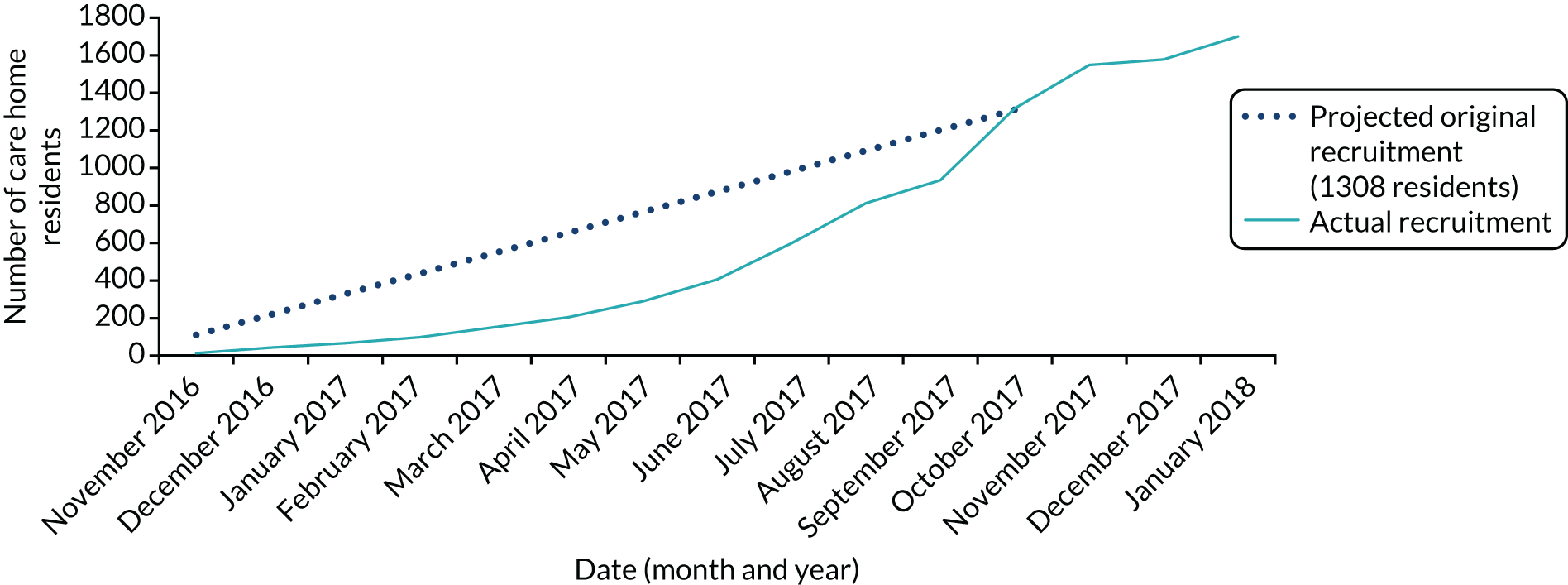
Figure 4 shows care home recruitment and resident recruitment by site. An average of 50% of residents from the participating care homes were consented and there was an average of 19.5 participants per care home. There was an average of 45 days between care home consent and randomisation. Figure 5 shows care home recruitment by site.
FIGURE 4.
Care home recruitment by site.
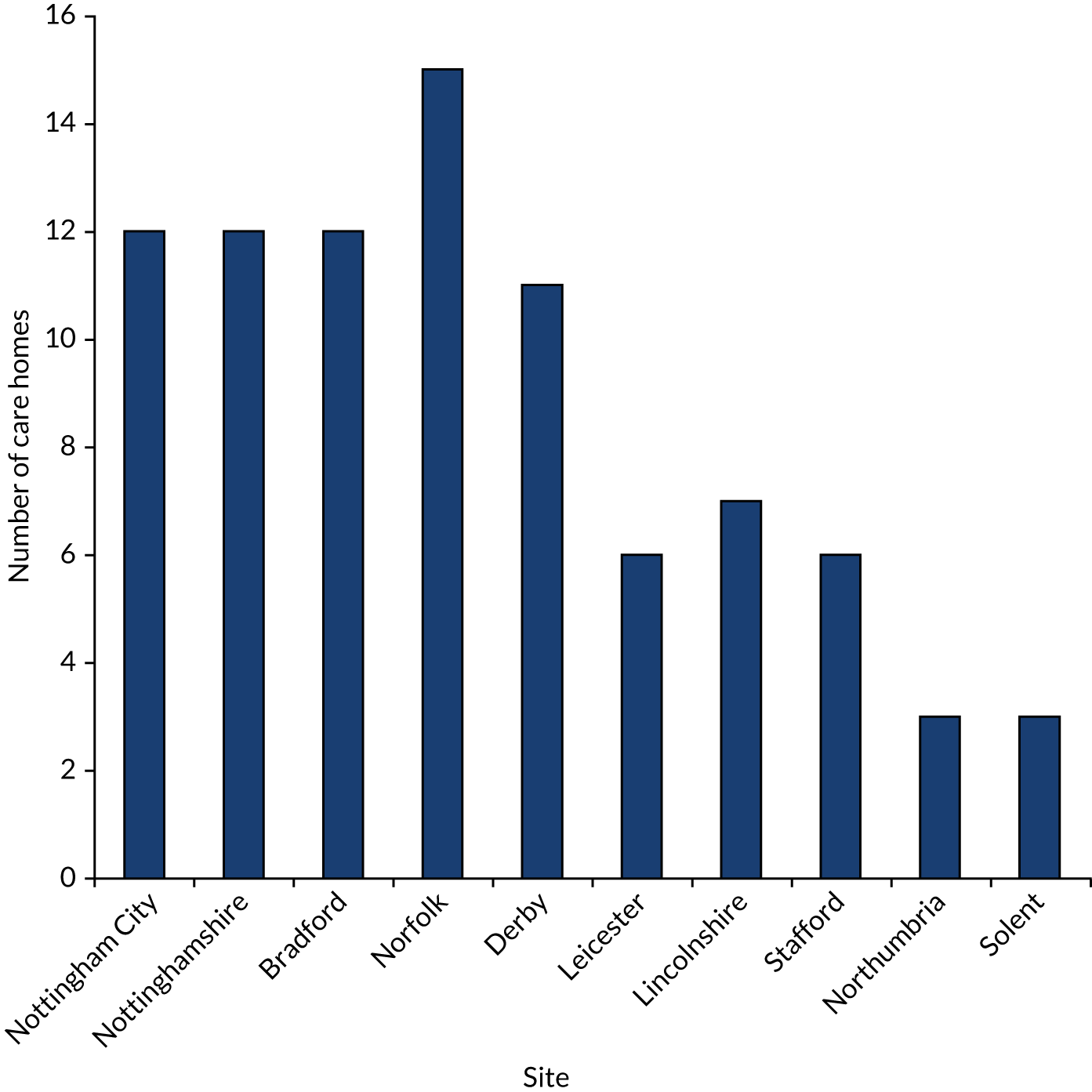
FIGURE 5.
Care home resident recruitment by site.
The CONSORT flow diagram (Figure 6) shows trial screening and recruitment to completion, as well as follow-up loss and completion. During the study there were 490 deaths, of which 459 occurred following care home randomisation. A total of 63 participants moved out of the study care home, 60 of these following randomisation. A total of 24 participants were residents in a care home that withdrew from the trial and a further eight participants were residents in a care home that was closed following a CQC inspection. Primary outcome data (falls occurring between 90 and 180 days after randomisation of the care home) were available for 1342 residents.
FIGURE 6.
The CONSORT flow diagram of the flow through the trial. The CONSORT flow diagram indicates the flow through the trial from the perspective of the availability of primary outcome variable data (falls). Availability of secondary outcome data, including QoL questionnaires is detailed in Secondary outcome analysis. a, Would not provide a falls champion (n = 24), had falls prevention in place (n = 13), had participated in FinCH feasibility trial (n = 4), home exclusively to learning disability/substance misuse (n = 3), in special measures (n = 1), did not wish to participate (no reason given) (n = 10). b, Wished to participate but did not have time (n = 1), initially indicated willingness but then stopped communicating with researcher (n = 8), initially indicated willingness but adopted a local falls intervention prior to consent (n = 3), wished to participate but were not recruited (no reason given) (n = 7).

Intervention adherence
Intervention adherence was defined as the percentage of care-giving staff trained to use the GtACH programme. Table 2 shows intervention adherence for each care home randomised to the GtACH arm. Average adherence to the training per care home was 71% (70% per site; minimum 17%, maximum 130%). A total of 14 (36%) care homes achieved adherence of ≥ 80%.
| Site | Number of care-giving staff at time of care home recruitment | Number (%) of care-giving staff trained to use the GtACH programme |
|---|---|---|
| Lincolnshire | ||
| 01/01 | 75 | 67 (89.3) |
| 01/07 | 48 | 38 (79.2) |
| Total | 123 | 105 (85.3) |
| Derby | ||
| 02/01 | 40 | 26 (65.0) |
| 02/03 | 16 | 16 (100.0) |
| 02/07 | 41 | 29 (70.7) |
| 02/08 | 32 | 20 (62.5) |
| 02/09 | 18 | 11 (61.1) |
| 02/11 | 24 | 19 (79.2) |
| Total | 171 | 121 (70.8) |
| Northumbria | ||
| 03/02 | 27 | 22 (81.5) |
| 03/03 | 33 | 43 (130.3)a |
| Total | 60 | 65 (108.3) |
| Leicester | ||
| 04/01 | 37 | 18 (48.6) |
| 04/02 | 54 | 42 (77.8) |
| Total | 91 | 60 (65.9) |
| Stafford | ||
| 05/02 | 16 | 9 (56.3) |
| 05/03 | 59 | 25 (42.4) |
| 05/06 | 66 | 11 (16.7) |
| Total | 141 | 45 (31.9) |
| Norwich | ||
| 06/02 | 55 | 40 (72.7) |
| 06/05 | 30 | 24 (80.0) |
| 06/07 | 36 | 30 (83.3) |
| 06/09 | 39 | 30 (76.9) |
| 06/12 | 38 | 30 (78.9) |
| 06/13 | 22 | 18 (81.8) |
| 06/15 | 18 | 10 (55.6) |
| Total | 238 | 182 (76.5) |
| Nottingham City | ||
| 07/01 | 25 | 22 (88.0) |
| 07/03 | 39 | 24 (61.5) |
| 07/04 | 45 | 30 (66.7) |
| 07/05 | 48 | 22 (45.8) |
| 07/08 | 16 | 19 (118.8)a |
| 07/11 | 12 | 10 (83.3) |
| Total | 185 | 127 (68.6) |
| Nottinghamshire | ||
| 08/03 | 59 | 47 (79.7) |
| 08/04 | 67 | 51 (76.1) |
| 08/06 | 23 | 22 (95.7) |
| 08/10 | 22 | 12 (54.5) |
| 08/11 | 49 | 37 (75.5) |
| Total | 220 | 169 (76.8) |
| Bradford | ||
| 09/01 | 26 | 10 (38.5) |
| 09/06b | 38 | 17 (44.7) |
| 09/08 | 53 | 43 (81.1) |
| 09/09 | 50 | 40 (80.0) |
| 09/10 | 33 | 29 (87.9) |
| 09/11 | 62 | 38 (61.3) |
| Total | 262 | 177 (67.6) |
| Solent | ||
| No care homes allocated to intervention | ||
| All | ||
| Overall compliance | 1491 | 1051 (70.5) |
Baseline characteristics
Baseline characteristics of care homes
The characteristics of the 84 randomised care homes are given in Table 3. Overall, just under half of the homes had dual registration (nursing and residential), 96% were reported by the care home owner to be privately owned, and the average number of staff per care home was 43. Care homes were fairly well balanced between the two arms in size and registration, although homes in the control arm reported a larger number of care-giving staff.
| Characteristic | Overall (N = 84) | GtACH (N = 39) | Control (N = 45) |
|---|---|---|---|
| Number of care homes by site, n (%) | |||
| Lincolnshire | 7 (8) | 2 (5) | 5 (11) |
| Derby | 10 (12) | 6 (15) | 4 (9) |
| Northumbria | 3 (4) | 2 (5) | 1 (2) |
| Leicester | 5 (6) | 2 (5) | 3 (7) |
| Stafford | 5 (6) | 3 (8) | 2 (4) |
| Norwich | 15 (18) | 7 (18) | 8 (18) |
| Nottingham City | 12 (14) | 6 (15) | 6 (13) |
| Nottinghamshire | 12 (14) | 5 (13) | 7 (16) |
| Bradford | 12 (14) | 6 (15) | 6 (13) |
| Solent | 3 (4) | 0 (0) | 3 (7) |
| Number of care homes by type, n (%) | |||
| Nursing | 11 (13) | 5 (13) | 6 (13) |
| Residential | 34 (40) | 16 (41) | 18 (40) |
| Dual registration | 39 (46) | 18 (46) | 21 (47) |
| Number of care homes by ownership | |||
| Charity, n (%) | 3 (4) | 2 (5) | 1 (2) |
| Private, n (%) | 81 (96) | 37 (95) | 44 (98) |
| Total number of care-giving staff | 3609 | 1491 | 2118 |
| Mean (SD) care-giving staff per home | 42.9 (41.0) | 38.2 (16.4) | 47.1 (53.8) |
| Total number of beds | 4112 | 1912 | 2200 |
| Mean (SD) beds per home | 49.0 (25.1) | 49.0 (21.3) | 48.9 (28.2) |
| Total number of residents | 3561 | 1672 | 1889 |
| Mean (SD) residents per home | 42.4 (21.9) | 42.9 (19.4) | 42.0 (24.1) |
Baseline characteristics of participants
Table 4 shows the characteristics of the 1657 trial participants. Overall, the average age was 85 years and the majority of residents were female. The median time spent in the care home at baseline was just under 19 months. Just under one-third of residents experienced one or more falls in the 3 months prior to randomisation (baseline period). Resident characteristics were reasonably well balanced between arms.
| Characteristic | Overall (N = 1657) | GtACH (N = 775) | Control (N = 882) |
|---|---|---|---|
| Age at consent to FinCH trial (years), mean (SD) | 85.04 (9.28) | 86.03 (8.64) | 84.16 (9.74) |
| Male, n (%) | 532 (32.1) | 231 (29.8) | 301 (34.1) |
| Consent, n (%) | |||
| Resident | 387 (23.4) | 186 (24.0) | 201 (22.8) |
| Consultee | 1270 (76.6) | 589 (76.0) | 681 (77.2) |
| Time in care home (months), median (IQR) | 18.6 (8.3–36.4) | 18.8 (8.1–36.5) | 18.1 (8.6–35.8) |
| Recorded diagnosis, n (%) | |||
| Dementia | 1109 (67.0) | 506 (65.4) | 603 (68.4) |
| Diabetes | 320 (19.3) | 150 (19.4) | 170 (19.3) |
| Stroke | 262 (15.8) | 118 (15.2) | 144 (16.3) |
| Coronary heart disease | 234 (14.1) | 100 (12.9) | 134 (15.2) |
| Number of falls during period 3 months prior to baseline data collection, n (%) | |||
| None | 1138 (68.8) | 546 (70.6) | 592 (67.1) |
| 1 | 299 (18.1) | 134 (173) | 165 (18.7) |
| 2 | 92 (5.6) | 42 (5.4) | 50 (5.7) |
| 3 | 55 (3.3) | 26 (3.4) | 29 (3.3) |
| 4 | 25 (1.5) | 10 (1.3) | 15 (1.7) |
| 5 | 6 (0.4) | 2 (0.3) | 4 (0.5) |
| 6 | 8 (0.5) | 2 (0.3) | 6 (0.7) |
| 7 | 5 (0.3) | 3 (0.4) | 2 (0.2) |
| 8 | 5 (0.3) | 1 (0.1) | 4 (0.5) |
| 9 | 9 (0.5) | 2 (0.3) | 7 (0.8) |
| 10 | 2 (0.1) | 0 (0.0) | 2 (0.2) |
| 11 | 3 (0.2) | 2 (0.3) | 1 (0.1) |
| 12 | 2 (0.1) | 1 (0.1) | 1 (0.1) |
| 13 | 2 (0.1) | 0 (0.0) | 2 (0.2) |
| 15 | 1 (0.1) | 1 (0.1) | 0 (0.0) |
| 16 | 1 (0.1) | 0 (0.0) | 1 (0.1) |
| 20 | 1 (0.1) | 1 (0.1) | 0 (0.0) |
| 31 | 1 (0.1) | 0 (0.0) | 1 (0.1) |
| Number of falls per person during period 3 months prior to baseline data collection | |||
| No falls, n (%) | 1138 (68.8) | 546 (70.6) | 592 (67.1) |
| 1–5 falls, n (%) | 477 (28.8) | 214 (27.7) | 263 (29.8) |
| 6–10 falls, n (%) | 29 (1.8) | 8 (1.0) | 21 (2.4) |
| 11–15 falls, n (%) | 8 (0.5) | 4 (0.5) | 4 (0.5) |
| ≥ 16 falls, n (%) | 3 (0.2) | 1 (0.1) | 2 (0.2) |
| Mean (SD) number of falls per person during the 3 months prior to baseline data collection | 0.71 (1.82) | 0.61 (1.57) | 0.79 (2.02) |
| Number of medications in the 3 months prior to baseline data collection, n (%) | |||
| None | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| One to three | 56 (3.4) | 26 (3.4) | 30 (3.4) |
| Four or more | 1601 (96.6) | 749 (96.6) | 852 (96.6) |
| Physical activity (PAM-RC) score at baseline, mean (SD) | 8.61 (6.09) | 8.57 (5.95) | 8.66 (6.21) |
| ADL (Barthel) score at baseline, mean (SD) | 8.57 (6.05) | 8.86 (6.12) | 8.30 (5.99) |
| DEMQOL at baseline | 0.82 (0.16) | 0.83 (0.16) | 0.81 (0.16) |
| DEMQOL-P at baseline | 0.74 (0.12) | 0.74 (0.12) | 0.74 (0.12) |
| EQ-5D-5L self-completion at baseline | 0.49 (0.36) | 0.52 (0.36) | 0.46 (0.35) |
| EQ-5D-5L proxy at baseline | 0.35 (0.37) | 0.36 (0.37) | 0.34 (0.36) |
Unblinding rates
The RAs responsible for recruiting care homes, consenting patients and carers, and collecting care home and patient-level outcome data were to remain blinded to the home allocation. In some instances, the RAs became unblinded, typically being unblinded by the care home manager.
Unblinding occurred in 26 out of the 84 participating care homes when the RA entered the care home 3 months after randomisation to collect the primary outcome measure and 3-month data. Of these 26 homes, 16 were GtACH care homes and 10 were control care homes.
Primary outcome: falls between 90 and 180 days
A negative binomial regression model [generalised estimating equation (GEE)] showed that the falls rate in the GtACH arm was reduced compared with that in the control arm in both the unadjusted and the adjusted analyses. Over the period of the primary outcome assessment (a 90-day period between 91 and 180 days after randomisation), the falls rate was 6.0 per 1000 residents in the GtACH arm and 10.4 per 1000 residents in the control arm (Table 5). Results of other approaches to the analysis of the primary outcome may be seen in Appendix 2, Tables 22–26.
| Time point | GtACH | Control | Unadjusted | Adjusted for baseline falls | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| At risk, n | Falls, n (SD) | Falls rate, n (SD) | At risk, n | Falls, n (SD) | Falls rate, n (SD) | IRR (95% CI) | p-value | IRR (95% CI) | p-value | |
| Pre randomisationa | 773 | 0.61 (1.57) | 6.97 (17.67) | 882 | 0.79 (2.02) | 9.48 (24.14) | ||||
| 0–90 days | 708 | 0.55 (1.36) | 6.93 (20.56) | 826 | 0.88 (2.37) | 10.24 (27.26) | 0.6 (0.49 to 0.73) | < 0.001 | 0.74 (0.60 to 0.92) | 0.006 |
| 91–180 days | 630 | 0.49 (1.13) | 6.04 (14.02) | 712 | 0.89 (2.60) | 10.38 (29.52) | 0.57 (0.45 to 0.71) | < 0.001 | 0.63 (0.52 to 0.78) | < 0.001 |
| 181–270 days | 547 | 0.60 (1.29) | 7.28 (16.67) | 633 | 0.73 (1.85) | 9.21 (28.77) | 0.85 (0.69 to 1.05) | 0.128 | 0.91 (0.74 to 1.12) | 0.369 |
| 271–360 days | 502 | 0.55 (1.14) | 6.22 (12.88) | 573 | 0.79 (2.37) | 9.22 (27.36) | 0.79 (0.60 to 1.03) | 0.078 | 0.93 (0.71 to 1.22) | 0.614 |
Secondary outcome analysis
Falls rates and fallers over other periods
There was a significant reduction in falls rates in the GtACH arm in the 0- to 90-day period, but there was no significant difference between the arm’s falls rates for either of the 3-month follow-up periods between 6 and 9 months or between 9 and 12 months (Table 5).
There was no difference in the proportion of residents who fell on one or more occasion (i.e. “fallers”) during any of the outcome time periods (Table 6).
| Time point | GtACH | Control | Unadjusted | Adjusted for baseline | ||||
|---|---|---|---|---|---|---|---|---|
| At risk (N) | Fell, n (%) | At risk (N) | Fell, n (%) | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Pre randomisationa | 773 | 227 (29.4) | 882 | 290 (32.9) | ||||
| 0–90 days | 708 | 194 (27.4) | 826 | 266 (32.2) | 0.7 (0.50 to 1.00) | 0.048 | 0.75 (0.53 to 1.05) | 0.09 |
| 91–180 days | 630 | 167 (26.5) | 712 | 216 (30.3) | 0.76 (0.56 to 1.03) | 0.078 | 0.81 (0.60 to 1.10) | 0.179 |
| 181–270 days | 547 | 165 (30.2) | 633 | 187 (29.5) | 1.00 (0.73 to 1.37) | 0.986 | 1.06 (0.78 to 1.45) | 0.697 |
| 271–360 days | 502 | 147 (29.3) | 573 | 175 (30.5) | 0.88 (0.60 to 1.29) | 0.516 | 0.94 (0.65 to 1.37) | 0.752 |
Activities of daily living: Barthel Index
There was no difference in the 20-point Barthel (ADL) Index scores between the arms at any of the time points considered (Table 7).
| Time point | GtACH | Control | Unadjusted | Adjusted for baseline | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | |
| Pre randomisationa | 768 | 8.86 (6.12) | 854 | 8.30 (5.99) | ||||
| 0–90 days | 643 | 8.24 (6.12) | 726 | 7.87 (5.94) | 0.08 (–0.96 to 1.13) | 0.874 | –0.03 (–0.69 to 0.64) | 0.937 |
| 91–180 days | 584 | 8.12 (6.05) | 648 | 7.54 (5.86) | 0.16 (–0.89 to 1.20) | 0.766 | –0.02 (–0.48 to 0.43) | 0.924 |
| 181–270 days | 514 | 8.52 (6.17) | 576 | 7.18 (5.98) | 0.90 (–0.29 to 2.10) | 0.138 | 0.46 (–0.10 to 1.01) | 0.11 |
| 271–360 days | 447 | 8.11 (6.20) | 519 | 6.86 (5.92) | 0.82 (–0.32 to 1.96) | 0.159 | 0.44 (–0.26 to 1.15) | 0.214 |
Physical activity and mobility: Physical Activity and Mobility in Residential Care
There was no difference in the PAM-RC scores between the arms at any of the time points considered (Table 8).
| Time point | GtACH | Control | Unadjusted | Adjusted for baseline | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | |
| Pre randomisationa | 773 | 8.57 (5.95) | 878 | 8.66 (6.21) | ||||
| 0–90 days | 652 | 7.99 (6.01) | 736 | 8.16 (5.98) | –0.41 (–1.51 to 0.69) | 0.468 | –0.1 (–0.55 to 0.35) | 0.662 |
| 91–180 days | 578 | 8.11 (6.05) | 633 | 7.74 (6.08) | 0.07 (–1.04 to 1.17) | 0.908 | 0.23 (–0.28 to 0.75) | 0.376 |
| 181–270 days | 491 | 8.13 (5.98) | 576 | 7.59 (6.12) | 0.32 (–0.90 to 1.54) | 0.61 | 0.43 (–0.24 to 1.10) | 0.209 |
| 271–360 days | 439 | 7.96 (5.63) | 520 | 7.19 (6.03) | 0.45 (–0.57 to 1.47) | 0.39 | 0.49 (–0.16 to 1.14) | 0.141 |
Inpatient days in hospital
There was no difference in inpatient hospital days between the arms at either baseline to 6 months post randomisation or at 6 to 12 months post randomisation using a GEE approach (Table 9). A Poisson regression of these data (see Appendix 3, Table 27) yielded similar results.
| Time point | GtACH | Control | Unadjusted | Adjusted for baseline | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | |
| Pre randomisationa | 773 | 0.46 (2.62) | 877 | 0.60 (2.69) | ||||
| 0–180 days | 697 | 1.54 (5.36) | 793 | 1.61 (4.85) | 0.91 (0.64 to 1.28) | 0.588 | 0.94 (0.67 to 1.32) | 0.725 |
| 181–360 days | 532 | 1.08 (4.04) | 620 | 1.58 (6.03) | 0.66 (0.40 to 1.08) | 0.101 | 0.63 (0.38 to 1.06) | 0.081 |
Quality of life
EuroQol-5 Dimensions, five-level version, proxy complete
There was no difference in the EQ-5D-5L-P scores between the arms at any of the time points considered (Table 10).
| Time point | GtACH | Control | Unadjusted | Adjusted for baseline | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | |
| Pre randomisationa | 766 | 0.36 (0.37) | 878 | 0.34 (0.36) | ||||
| 0–90 days | 728 | 0.30 (0.38) | 802 | 0.30 (0.36) | –0.01 (–0.08 to 0.06) | 0.851 | 0 (–0.05 to 0.04) | 0.854 |
| 91–180 days | 717 | 0.26 (0.36) | 817 | 0.22 (0.34) | 0.02 (–0.05 to 0.08) | 0.588 | 0.02 (–0.03 to 0.07) | 0.483 |
| 181–270 days | 693 | 0.25 (0.36) | 823 | 0.20 (0.33) | 0.03 (–0.03 to 0.10) | 0.288 | 0.04 (–0.01 to 0.08) | 0.083 |
| 271–360 days | 674 | 0.21 (0.32) | 809 | 0.16 (0.31) | 0.04 (–0.01 to 0.08) | 0.105 | 0.03 (0.00 to 0.07) | 0.083 |
Dementia Quality of Life Utility version, proxy complete
There was no difference in Dementia Quality of Life Utility version, proxy complete (DEMQOL-P-U), scores between the arms at any of the time points considered (Table 11).
| Time point | GtACH | Control | Unadjusted | Adjusted for baseline | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | |
| Pre randomisationa | 764 | 0.74 (0.12) | 877 | 0.74 (0.12) | ||||
| 0–90 days | 716 | 0.66 (0.25) | 807 | 0.67 (0.23) | –0.02 (–0.05 to 0.02) | 0.355 | –0.02 (–0.05 to 0.02) | 0.315 |
| 91–180 days | 698 | 0.59 (0.31) | 805 | 0.57 (0.31) | 0.02 (–0.04 to 0.08) | 0.511 | 0.02 (–0.04 to 0.07) | 0.565 |
| 181–270 days | 694 | 0.52 (0.34) | 807 | 0.52 (0.35) | 0.00 (–0.07 to 0.07) | 0.977 | –0.01 (–0.07 to 0.05) | 0.85 |
| 271–360 days | 673 | 0.48 (0.36) | 809 | 0.47 (0.36) | –0.01 (–0.07 to 0.06) | 0.868 | –0.01 (–0.07 to 0.05) | 0.779 |
Deaths
There was no difference between the arms in the number of deaths occurring at any time during the trial (Table 12).
| GtACH | Control | Unadjusted | ||||
|---|---|---|---|---|---|---|
| n | Deaths | n | Deaths | OR (95% CI) | p-value | |
| Overall deaths | 775 | 233 (30.1%) | 882 | 281 (31.9%) | 0.93 (0.73 to 1.20) | 0.576 |
Fractures
There was no difference between the arms in the number of hip fractures, wrist fractures or any fractures occurring between baseline and 6 months. There was a significantly lower rate of fractures between 6 and 12 months (Table 13); however, note that the actual numbers were small and there was no corresponding reduction in falls rates over this period. A list of fractures included in these analyses is provided in Appendix 4.
| Time point | Fracture | Arm, n (%) | OR (95% CI) | p-value | ||
|---|---|---|---|---|---|---|
| Type | Number | GtACH | Control | |||
| Baseline to 180 days | Hip | 0 | 758 (97.8) | 867 (98.3) | ||
| 1 | 12 (1.5) | 8 (0.9) | 1.4 (0.67 to 2.96) | 0.371 | ||
| 2 | 5 (0.6) | 7 (0.8) | ||||
| Wrist | 0 | 772 (99.6) | 880 (99.8) | |||
| 1 | 3 (0.4) | 2 (0.2) | 1.63 (0.26 to 10.2) | 0.603 | ||
| Any | 0 | 742 (95.7) | 850 (96.4) | |||
| 1 | 22 (2.8) | 17 (1.9) | 1.19 (0.70 to 2.01) | 0.527 | ||
| 2 | 10 (1.3) | 12 (1.4) | ||||
| 3 | 1 (0.1) | 2 (0.2) | ||||
| 4 | 0 (0.0) | 1 (0.1) | ||||
| 181–360 days | Hip | 0 | 591 (98.5) | 662 (96.6) | ||
| 1 | 9 (1.5) | 23 (3.4) | 0.38 (0.17 to 0.85) | 0.019 | ||
| Wrist | 0 | 600 (100.0) | 685 (100.0) | NA | NA | |
| Any | 0 | 591 (98.5) | 659 (96.2) | |||
| 1 | 7 (1.2) | 11 (1.6) | 0.34 (0.15 to 0.75) | 0.007 | ||
| 2 | 2 (0.3) | 15 (2.2) | ||||
Changes to the analysis plan during the trial
Although we had specified that multiple imputation (MI) would be used to account for missing data, this was not undertaken because the primary reason for missing data was that the patient had died. However, we did collect and analyse the falls data until the date of death. We have presented medians and interquartile ranges for time spent in the care home, rather than means and standard deviations (SDs), because of the skewed distribution of these data.
Chapter 4 Economic evaluation
Overview
The aim of this chapter is to report the within-trial economic evaluation undertaken to estimate the cost-effectiveness of delivering the GtACH programme in care homes from an NHS and personal social services (PSS) perspective. The primary analysis was a cost–utility analysis and presents proxy-reported outcomes as QALYs. A cost-effectiveness analysis (CEA) based on cost per falls averted was also conducted so that the GtACH programme can be directly compared with other interventions aimed at reducing falls.
Methods
The health economics analysis plan (HEAP) was written and approved by the TMG prior to the data being locked. The HEAP is available as Report Supplementary Material 3.
Measuring resource use and estimating costs
In line with NICE guidelines,44 we estimated costs from a health and PSS perspective. This included the cost of implementing the GtACH programme, any health resource use (primary care, secondary care, medications and ADL equipment) and social services received as part of routine care.
GtACH programme resource use and costs
The specific technology under investigation was the GtACH programme, which was delivered to care home residents by trained and supported care home staff. 26–28 The GtACH programme is a systematic falls risk assessment and action process, co-designed by care home and NHS staff, and based on NICE clinical guidelines. 44
The GtACH programme was delivered by care home staff who had received training from NHS falls leads. NHS falls leads were health-care professionals (generally occupational therapists or physiotherapists) recruited in each location to provide on-site training for care home staff.
The GtACH programme costs included the senior trial team training the NHS falls leads, NHS falls leads then delivering GtACH training sessions to care home staff, care home staff delivering the GtACH programme to residents, and support provided by the falls prevention leads in the first 3 months of delivery. Specific training details were recorded by the NHS falls leads at each care home. Additional costs of the delivery and receipt of GtACH training included travel time and consumables, but we excluded the cost of developing the tool itself, as this had been developed previously and was considered a sunk cost. 26
We assumed that, in the base case, every resident was assessed using the GtACH tool once, as this was reflective of observations by the process evaluation team. The estimated time required for the GtACH programme was 30 minutes, based on observations from process evaluation and discussion with the senior trial team.
The per-protocol delivery of the GtACH programme would be for the tool to be used after every fall. We surmised that, given staffing pressures, the maximum number of repeat GtACH programmes each care home could provide would be one per month. Therefore, in sensitivity analysis, we included the cost of up to 11 extra intervention sessions (after the initial session in month 1) that would take place for an individual experiencing any falls in the previous 30 days, continuing to trial end.
To calculate the total cost of staff time, an hourly wage was estimated for a typical NHS falls lead and care home staff based on Agenda for Change wage rates45 (see Appendix 5, Table 28). The training costs and costs of delivering the GtACH programme were calculated for each care home and then divided among the residents recruited in that care home to produce an estimated cost per resident.
Usual-care resource use and costs
The comparator to the intervention was usual care, where usual care was defined as the absence of a systematic and co-ordinated falls prevention process. The control care homes had the option to receive the GtACH training at the end of the trial, but the cost of this has not been included as this occurred after the trial follow-up period.
Health and social services resource use
To estimate the cost of primary care, community health, and social services visits, data on resource use incurred during the previous 3 months were extracted from care home residents’ care plans by study RAs at 3, 6, 9 and 12 months post randomisation. Baseline resource use (90 days pre randomisation) data were also collected to control for prior health resource use in analyses because past resource use may predict future costs. A copy of the health resource use questionnaires used as part of the CRF are provided in Report Supplementary Material 2. For secondary care [inpatient stays, accident and emergency (A&E), and outpatient attendances], we requested linked data from NHS Digital, receiving one data transfer covering the entire trial after the trial had closed.
Unit costs, in 2017/18 Great British pounds (the most recent year available at the time of analysis), were applied based on annually published national sources including the National Schedule of Reference Costs46 and the Personal Social Services Research Unit’s Unit Costs of Health and Social Care. 45
Medication costs
Researchers collected data on all medications recorded in care home records as ongoing at baseline or as being taken in the preceding 90 days. At each subsequent quarterly data collection point, researchers reported whether the medications were stopped or new medications were started. Medications were mapped to the Prescription Cost Analysis47 (price year 2017/18) to apply unit costs for each individual preparation used, assuming one item was prescribed per month during the period a resident was recorded as using the medication.
Equipment costs
Residents use of any equipment to help them cope with a health problem was recorded. Items deemed to be shared among residents (for instance stair lift or hoists) were not costed. For larger ADL equipment (for instance wheelchairs or profile beds), costs were annuitised to reflect the expected lifespan of the piece of equipment (assuming an expected lifespan of 5 years). 48 Unit costs were derived from NRS Healthcare (Coalville, UK) where possible49 (see Appendix 5, Table 29).
Secondary care costs
Hospital Episode Statistics (HES) data were requested from NHS Digital for all inpatient stays, outpatient attendances and A&E attendances for all residents for the period they were in the trial. Costs were applied by mapping the HES-provided Health Research Group code to the NHS National Tariff, using 2017/18 prices regardless of activity date. These costs were provided in the HES data received from NHS digital. Further details on costing HES data are reported in Report Supplementary Material 4, and unit costs and sources are reported in Appendix 5, Table 28.
The mean costs per resident in the GtACH arm and per resident in the control arm were estimated by summing intervention costs and wider NHS and PSS costs, and then dividing by the number of residents in the trial arm.
Outcomes
The main outcome measure in the economic evaluation was QALYs accrued for the resident over the 12 months’ follow-up period as valued using the DEMQOL-P-U and EQ-5D-5L proxy. For both instruments, responses were obtained from proxies (care home staff) at baseline and 3-monthly intervals. Responses were converted into a utility using published UK tariff values; for the DEMQOL-P-U this was the valuation sets published by Mulhern et al. 38 and Rowen et al. ,50 and for the EQ-5D-5L-P this was in line with current recommendations51 to use the ‘cross-walk’ valuation set published by van Hout et al. 52 in 2012. These utilities represent residents’ overall health-related quality of life (HRQoL) at single time points. These utilities were employed to generate QALYs using linear interpolation and area under the curve analysis with baseline adjustment, adjusted for age at randomisation and sex. 53 If residents died, their utility value (and costs) were assumed to be zero from the subsequent assessment point and their data were retained in the analyses.
If residents had sufficient mental capacity they were also asked to complete the EQ-5D-5L and Dementia Quality of Life Utility version (DEMQOL-U) (while also having these measures captured by proxy respondents). This secondary analysis was important because of uncertainties regarding how best to capture health utilities in this population. 38,50,54
Economics analysis
The primary economic analysis was a within-trial cost–utility analysis comparing the GtACH intervention with usual care without a systematic and co-ordinated falls prevention process in place, with outcomes expressed in QALYs. As the clinical analyses used falls rates as the primary end point, a secondary CEA based on difference in falls rates over 12 months was also conducted. Analysis was undertaken based on the intention-to-treat principle, including all randomised residents with data available. As follow-up did not continue past 12 months, discounting of costs or outcomes was not undertaken.
The mean cost and outcomes data were combined to calculate an incremental cost-effectiveness ratio (ICER) from the NHS/PSS perspective. As randomisation was clustered by care home, analysis should reflect the increased uncertainty of randomising clusters rather than individuals. Several approaches have been proposed for taking this into account, with each method found to generate similar findings. 55,56 The use of regression analysis is advocated to account for potential baseline differences and/or confounders when comparing costs and outcomes between treatment arms, and is essential to formally account for the cluster randomised design. We chose the GEE regression model to analyse cost and outcomes, adjusting for age, sex, site and baseline measures for costs/outcomes, respectively. To account for correlation between cost and outcomes measures, standard errors were estimated by repeatedly re-estimating the equations on non-parametric bootstrap replications of the data, retaining the coefficient on treatment arm for each measure from each bootstrap replicate. The mean (SD) cost, QALYs and falls per resident per randomised arm were estimated. The mean (95% CI) difference in costs, QALYs and falls between arms were estimated, with adjusted and unadjusted results reported. The bootstrapped estimates were used to generate a graphical representation of the sampling uncertainty, presented as a scatterplot of incremental cost–outcome pairs and the cost-effectiveness acceptability curve (CEAC).
Sensitivity analyses
Sensitivity analyses were undertaken to explore uncertainties surrounding key parameters in the economic evaluation to investigate the robustness of findings. The following sensitivity analyses were undertaken:
-
In the base case, the cost of undertaking a single GtACH assessment per resident was included in the cost. The per-protocol delivery of the GtACH programme would have required the GtACH tool to be applied again, refreshed, for each resident after each fall. To test the impact of this on the cost of the GtACH programme, the cost of refresher GtACH tools (assuming that if a participant fell at least once in a month they would receive a refresher GtACH tool in that month, meaning that participants could receive up to 11 refresher GtACH tools) were included in sensitivity analyses.
-
The base-case analysis did not include the costs of final hospital stays when a resident left the study following the stay (for instance, when the resident transferred to another care home). A sensitivity analysis was undertaken including these costs.
-
The base case was adjusted for baseline variables. In sensitivity analyses, we present both raw, unadjusted results and an adjusted analysis with missing data imputed using MI. The MI model included predictors of secondary and non-secondary care costs (baseline and full follow-up); EQ-5D-5L-P- and DEMQOL-P-U-based QALYs; treatment arm; care home; age; and sex. The imputation generated values for missing data at each follow-up using ordinary least squares, generating 50 data sets. The GEE models were then run on each of these data sets and the outputs pooled using Rubin’s rules. This provided paired cost and outcome data for the entire study population. This was repeated 200 times, with bootstrap replications of the original data.
All regression analysis was conducted in Stata/MP version 16, with some figures and determination of the appropriate number of bootstrap replications performed in R. The code is available from the corresponding author on request.
Results
Participants and data completeness
The final data set for the economic analysis comprised 1603 participants (GtACH, n = 732 participants; usual care, n = 871 participants). Completion rates for data were very high, with a maximum of 283 out of 1603 (17.7%) items missing from any individual variable [Dementia Specific Quality of Life (DEMQOL)-based QALYs] and complete data sets available for 1260 out of 1603 (78.6%) participants. A total of 6 out of 1603 (0.4%) participants were missing cost data, 13 and 15 out of 1603 (0.8% and 0.9%) participants were missing baseline EQ-5D-5L and DEMQOL utility data, respectively, and 262 and 283 out of 1603 (16.3% and 17.7%) participants were missing EQ-5D-5L and DEMQOL-based QALYs, respectively.
During development of the MI model (sensitivity analysis 3), visual inspection confirmed that the imputed means and SDs of most parameters with missing data achieved stability within 10 or 20 chained cycles. However, the exception was the estimates of total cost, which failed to achieve stability even after 1000 cycles and the use of various estimation methods (including predictive mean matching). This is most likely to be because of the skewed nature of costs, where outliers can severely influence the mean and SD cost. Given that there were only six observations with missing cost data (0.4% of the data set), these were dropped from the data set for the MI analysis, leaving a sample size of 1597 observations for this sensitivity analysis.
Although completion rates for the proxy measures (as completed by care home staff on behalf of the resident) were high, for self-reported measures, completion rates were much lower, with only 12% of participants having full data at each assessment point from which to calculate QALYs. The number of participants self-completing the measures decreased gradually over the 12 months’ follow-up (see Report Supplementary Material 5). Owing to this, the self-reported measures are not discussed further in this chapter.
GtACH programme intervention costs
Total costs of delivering the GtACH programme are outlined in Table 14. In total, 1211 care home staff were provided with GtACH training by NHS falls leads over 146 group sessions. The cost per intervention resident to receive one GtACH tool assessment each, without any refreshers, was estimated as £87.57 per resident. This increased to a mean cost of £108.91 per resident when the refresher sessions were added, assuming a refresher GtACH was refreshed a maximum of once per month only if the resident fell in that month. Costs varied depending on how many times NHS falls leads visited the care home and how many residents were recruited per care home, with the range of costs per resident varying between £54–208 (or £54–357 when the costs of refresher GtACH tool assessments were included).
| GtACH intervention costing | Costing details/assumptions | Total | Mean | Minimum | Maximum | Component cost per GtACH resident |
|---|---|---|---|---|---|---|
| Number of staff trained per care home | Taken from GtACH training log: attendees recorded job title and attendance/did not attend | 1211 | 31.05 | 10 | 70 | – |
| Number of residents recruited per care home | Taken from baseline electronic CRF | 790 | 20.26 | 10 | 65 | – |
| Maximum number of NHS falls leads sessions in care home | NHS falls leads’ travel costs incurred for each session | 146 | 3.74 | 1 | 13 | – |
| Cost components | ||||||
| Train-the-trainer day costs | 1 day in Nottingham and 4 days on site. Includes trial staff time, NHS falls leads’ time and travel costs reimbursement | £11,295 | £289.62 | Flat fee per care home | £14.30 | |
| Training care home staff costs | Each session costed according to number of staff attending and their roles | £31,701 | £812.85 | £283.00 | £2025.00 | £40.13 |
| NHS falls leads delivering training to care homes | Band 6. 60-minute training plus 30 minutes of preparation | £9636 | £247.08 | £66.00 | £858.00 | £12.20 |
| NHS falls leads’ travel to care homes | Assume 5 miles, 40 pence per mile, 30 minutes of travel | £3504 | £89.85 | £24.00 | £312.00 | £4.44 |
| Cost of consumables | Two manuals per care home, two GtACH tools per resident, attendance certificate per staff member | £2381 | £61.04 | £3.01 | ||
| Undertaking the GtACH programme: care home staff time | Assume undertaken by care home worker (£27 per hour) – 30 minutes per participant (NB higher than per protocol – evidence from process evaluation) | £10,665 | £273.46 | £135.00 | £877.50 | £13.50 |
| Residents who fall: refresher GtACH tools – sensitivity analysis | Assumes that if the resident fell then the 30-minute checklist process (£13.50) would be repeated. Realistic number of refresher sessions one per month, maximum 11 extra sessions (maximum £14) | £16,861 | £21.34 | £0.00 (no falls) | £148.50 (11 extra sessions) | £21.34 |
| GtACH programme cost per care home (GtACH arm only) | £69,182 | £1773.88 | £858.62 | £4530.62 | ||
| GtACH programme cost per GtACH-arm resident | £87.57 | £53.75 | £208.46 | |||
| GtACH programme cost per resident plus refresher | £108.91 | £53.75 | £356.96 | |||
Given the large number of care homes in this study, we believe that the training costs estimated in this study would be generalisable if the intervention were to be rolled out using the methods employed here. It may be possible to change the format of training and, by implication, the costs of the training and achieve the same outcomes, but this was not tested in this study.
Health and social care resource use and costs (excluding intervention)
Resource use and costs were broadly similar in the GtACH programme and control care homes. Tables 15 and 16 provide estimates of mean resource use per resident pre intervention and during follow-up, respectively. The provision of the GtACH programme to care homes was not associated with changes in the level of wider health-care use by residents, and the cost of the intervention was not offset by lower costs in wider health-care use (e.g. GP visits, inpatient stays or contact with other therapists). Participants had frequent visits from GPs, district nurses and podiatrists, whereas visits to A&E, outpatient appointments and inpatient stays were relatively infrequent. Despite their relative infrequency, hospital stays were an important cost driver because of their high cost (see Table 16), as were medication costs.
| Resource category | Arm, mean (SD) | Mean difference (GtACH – control) (95% CI) | |
|---|---|---|---|
| GtACH (n = 732) | Control (n = 871) | ||
| Secondary care | 462.60 (1681.93) | 509.50 (1599.67) | –46.90 (–208.24 to 114.45) |
| Inpatient | 399.44 (1625.17) | 431.26 (1524.84) | –31.82 (–186.64 to 122.99) |
| A&E | 29.91 (77.66) | 35.03 (88.13) | –5.13 (–13.35 to 3.10) |
| Outpatient | 33.26 (87.91) | 43.20 (109.88) | –9.95 (–19.84 to –0.06) |
| Primary and community care | 216.83 (263.64) | 187.79 (200.49) | 29.04 (6.23 to 51.85) |
| Equipment | 77.19 (108.70) | 81.34 (113.07) | –4.15 (–15.09 to 6.80) |
| Medications | 332.70 (300.34) | 363.41 (307.53) | –30.72 (–60.69 to –0.75) |
| Total costs | 1089.32 (1780.41) | 1142.04 (1694.86) | –52.72 (–223.59 to 118.15) |
| Resource category | Arm, mean (SD) | Mean difference (GtACH – control) (95% CI) | |
|---|---|---|---|
| GtACH (n = 732) | Control (n = 871) | ||
| Intervention cost without refresher GtACH tools | 88.55 (26.87) | 0.00 (0.00) | 88.55 (86.76 to 90.34) |
| Intervention cost with refresher GtACH tools | 111.46 (47.44) | 0.00 (0.00) | 111.46 (108.29 to 114.62) |
| Secondary care without mortality costs | 1790.20 (3436.28) | 1814.74 (3336.26) | –24.54 (–357.74 to 308.65) |
| Secondary care including mortality costs | 1938.87 (3634.96) | 1927.09 (3459.34) | 11.78 (–337.02 to 360.58) |
| Inpatient without mortality costs | 1567.76 (3249.07) | 1581.07 (3148.96) | –13.30 (–328.06 to 301.45) |
| Inpatient including mortality costs | 1716.43 (3447.82) | 1693.42 (3269.41) | 23.02 (–307.21 to 353.25) |
| A&E | 119.67 (207.99) | 126.25 (210.89) | –6.58 (–27.22 to 14.07) |
| Outpatient | 102.76 (255.68) | 107.42 (212.46) | –4.66 (–27.64 to 18.32) |
| Primary and community care | 728.46 (795.49) | 646.80 (734.36) | 81.66 (6.50 to 156.81) |
| Equipment | 17.30 (53.34) | 20.34 (51.21) | –3.04 (–8.18 to 2.10) |
| Medications | 1330.79 (1201.37) | 1453.66 (1230.13) | –122.87 (–242.76 to –2.99) |
| Total cost: base case | 3955.29 (3949.38) | 3935.54 (3879.90) | 19.76 (–365.88 to 405.39) |
| Total cost: with refresher GtACH tools | 3978.20 (3955.87) | 3935.54 (3879.90) | 42.66 (–343.32 to 428.64) |
| Total cost: including extra mortality costs | 4103.96 (4121.02) | 4047.89 (3989.66) | 56.08 (–343.70 to 455.85) |
| Total cost: including refresher GtACH tools and extra mortality costs | 4126.87 (4127.10) | 4047.89 (3989.66) | 78.98 (–321.12 to 479.09) |
During the trial, costs were higher in the GtACH arm (including intervention costs) but the difference was very small, with wide CIs (see Table 16).
It can be seen that the GtACH arm had higher costs for nursing (£285 per resident compared with £170 per resident in the control arm), but the GtACH arm received fewer allied health professional contacts than the control arm (£94 compared with £127). It is possible that additional care requested as a result of the GtACH programme would fall on district nurses (e.g. for continence and medication reviews) as opposed to physiotherapists or occupational therapists.
Medication costs were estimated for items reported in the GtACH arm. Pre intervention, GtACH residents had slightly lower medication costs than control residents (mean £332.70 per GtACH resident vs. mean £363.41 per control resident) and this difference widened by 12 months post intervention (£1330.79 vs. £1453.60 for GtACH residents and control residents, respectively). The mean number of prescription items over the 12-month trial period was 10.28 (SD 21.72) in the GtACH arm and 10.23 (SD 1.65) in the control arm, giving a difference of 0.04 (95% CI –0.12 to 0.21). Equipment costs were similar between the study arms both pre and post intervention. The mean number of equipment items during the 12-month trial period was 3.02 (SD 2.31) in the GtACH arm and 3.01 (SD 2.27) in the control arm, giving a difference of 0.01 (95% CI –0.21 to 0.24).
Secondary care resource use and costs were similar between the study arms pre and post intervention. There were 1296 unique inpatient stays in the 12-month trial period; the cost of these can be seen in Table 16.
Table 17 shows the mean (SD) utility estimates at each time point, for both arms, using the DEMQOL-P-U and EQ-5D-5L-P, as well as the estimated QALYs and mean difference (95% CI) of each.
| Utility instrument | GtACH | Control | Mean difference | ||||
|---|---|---|---|---|---|---|---|
| n (N = 732) | Mean | SD | n (N = 871) | Mean | SD | ||
| DEMQOL-P-U | |||||||
| Baseline | 722 | 0.740 | 0.123 | 866 | 0.737 | 0.124 | 0.003 (–0.009 to 0.015) |
| 3 months | 699 | 0.655 | 0.248 | 799 | 0.669 | 0.230 | –0.001 (–0.013 to 0.012) |
| 6 months | 683 | 0.585 | 0.306 | 798 | 0.573 | 0.312 | 0.012 (–0.010 to 0.016) |
| 9 months | 687 | 0.523 | 0.336 | 801 | 0.524 | 0.346 | –0.001 (–0.006 to 0.010) |
| 12 months | 666 | 0.477 | 0.357 | 804 | 0.476 | 0.358 | 0.001 (–0.036 to 0.038) |
| DEMQOL-P-U-based QALYs at 12 months | 611 | 0.578 | 0.240 | 708 | 0.581 | 0.235 | –0.003 (–0.028 to 0.023) |
| EQ-5D-5L-P | |||||||
| Baseline | 723 | 0.367 | 0.369 | 867 | 0.344 | 0.360 | 0.021 (–0.015 to 0.057) |
| 3 months | 711 | 0.301 | 0.379 | 794 | 0.300 | 0.364 | –0.001 (–0.038 to 0.037) |
| 6 months | 702 | 0.260 | 0.361 | 810 | 0.223 | 0.343 | 0.036 (0.000 to 0.072) |
| 9 months | 686 | 0.250 | 0.358 | 817 | 0.198 | 0.328 | 0.051 (0.016 to 0.085) |
| 12 months | 667 | 0.210 | 0.321 | 803 | 0.162 | 0.313 | 0.047 (0.014 to 0.079) |
| EQ-5D-5L-P-based QALYs at 12 months | 622 | 0.266 | 0.317 | 718 | 0.232 | 0.291 | 0.034 (0.002 to 0.067) |
Outcomes
Dementia Quality of Life, proxy complete
The mean Dementia Quality of Life, proxy complete (DEMQOL-P), utilities declined in both arms over time at a virtually identical rate. Thus, accumulated QALYs over time are virtually identical (Figure 7 and Table 17).
FIGURE 7.
The DEMQOL-P-U scores over the trial period. Complete case, n = 1453.
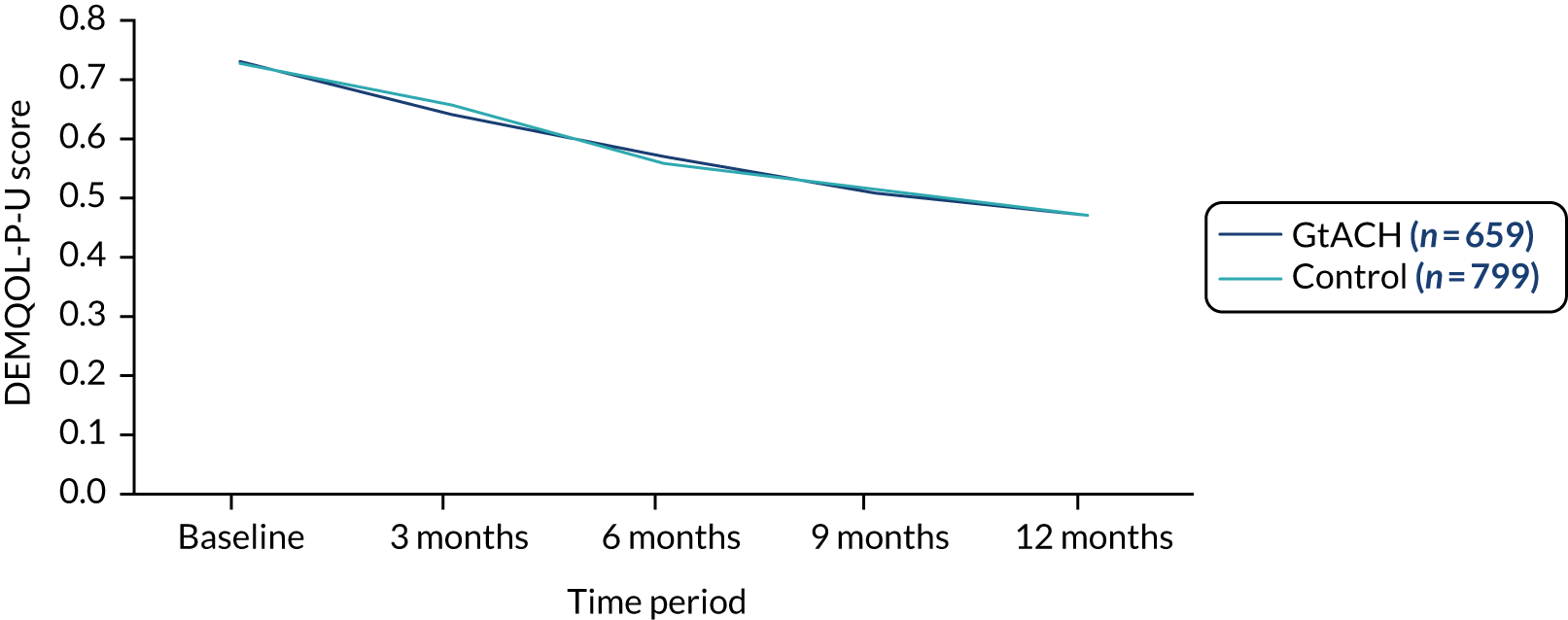
EuroQol-5 Dimensions, five-level version, proxy complete
The EQ-5D-5L-based utilities were consistently higher in the GtACH arm than in the control arm (Figure 8 and Table 17). This leads to larger observed mean QALYs. These raw unadjusted comparisons of the data illustrate the importance of adjusting for baseline utility.
FIGURE 8.
The EQ-5D-5L-P scores over the trial period. Complete case, n = 1453.
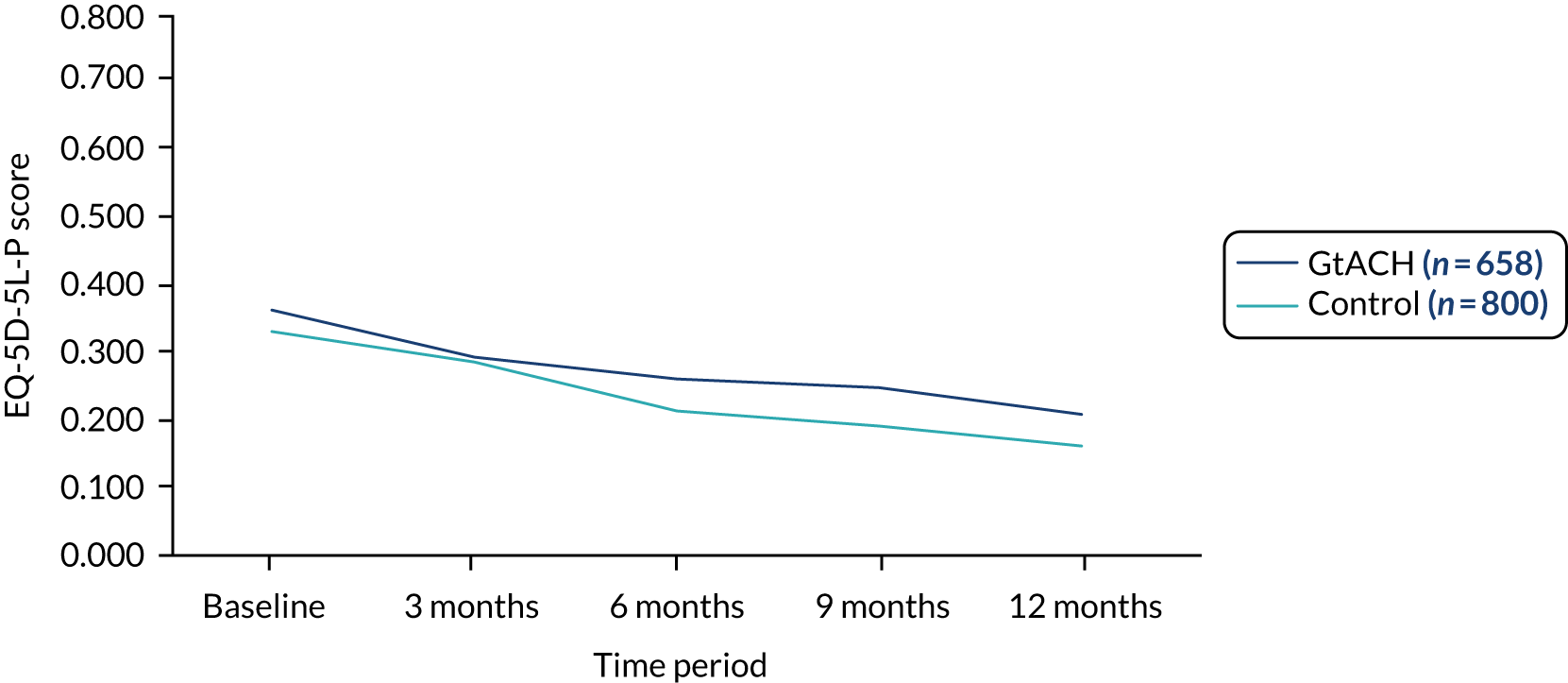
Falls
The primary outcome for the trial was based on the number of falls in months 4–6; analysis of the primary outcome showed a substantial difference between arms, with more falls in the control arm than in the GtACH arm. The economic evaluation measures the number of falls over the full 12-month period and, as Figure 9 shows, the difference between arms became smaller in the second half of the trial. Reflecting the RCT results, the intervention appeared to be more effective in the first 6 months than in the final 6 months.
FIGURE 9.
Mean number of falls per quarter over the trial period.
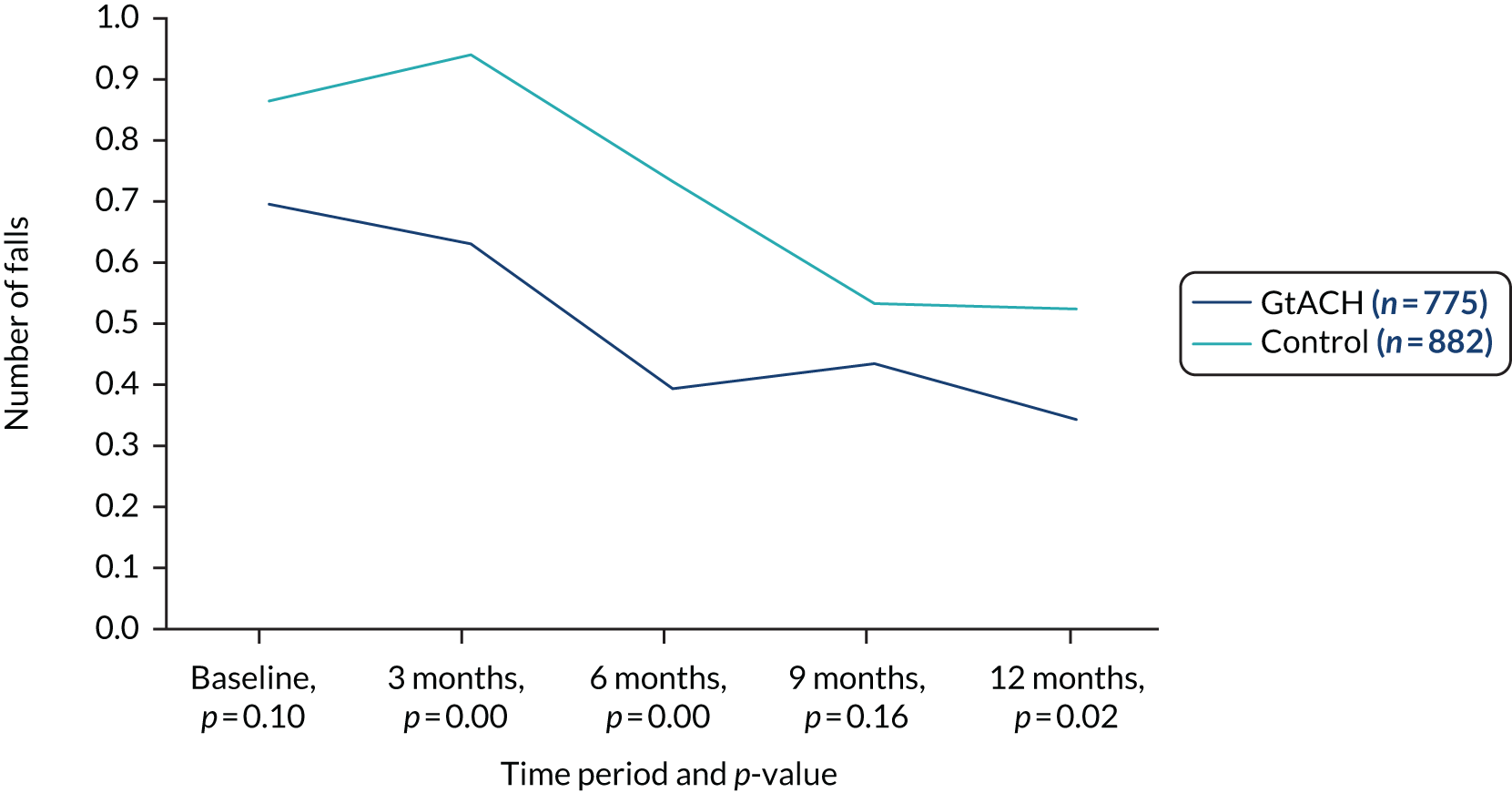
There was a mean of 1.89 (SD 3.66) falls recorded per resident in the GtACH arm and a mean of 2.77 (SD 7.44) falls per resident in the control arm, giving a mean difference of –0.877 (95% CI –1.469 to –0.285).
Cost-effectiveness
Results of the cost-effectiveness analyses are shown in Table 18. The raw incremental cost difference between arms was £19.76. However, this figure is unadjusted for baseline imbalances in cost and potential influences on cost other than the intervention. Adjusting for these yields an incremental cost difference of £108.26 (95% CI –£271 to £488) (‘adj, bs’ analysis). Adding in the cost of additional refresher sessions (sensitivity analysis 1; see Table 6) increases the incremental cost difference to £131.81 (95% CI –£248 to £511). Adding in the cost of mortality (sensitivity analysis 2, see Table 6) increases the incremental cost to £148.52 (95% CI –£245 to £542). The MI sensitivity analysis yields an identical mean cost, but narrower 95% CIs (see the final column, rows 1–4 of Table 6).
| Parameter | GtACH | Control | Incremental mean (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | Raw | adj, bs (primary analysis) | MI, adj, bs | ||
| Cost | ||||||||
| 1 | Base case | 732 | £3955.29 (£3949.38) | 865 | £3935.54 (£3879.90) | £19.76 (–£365.88 to £405.39) | £108.26 (–£271.06 to £487.58) | £108.26 (–£232.89 to £449.41) |
| 2 | With refresher GtACH tools | 732 | £3978.20 (£3955.87) | 865 | £3935.54 (£3879.90) | £42.66 (–£343.32 to £428.64) | £131.81 (–£247.77 to £511.40) | £131.81 (–£209.28 to £472.90) |
| 3 | Including extra mortality costs | 732 | £4103.96 (£4121.02) | 865 | £4047.89 (£3989.66) | £56.08 (–£343.70 to £455.85) | £124.98 (–£268.68 to £518.64) | £124.98 (–£230.84 to £480.80) |
| 4 | Including refresher GtACH tools and extra mortality costs | 732 | £4126.87 (£4127.10) | 865 | £4047.89 (£3989.66) | £78.98 (–£321.12 to £479.09) | £148.52 (–£245.40 to £542.45) | £148.52 (–£207.33 to £504.38) |
| Outcomes | ||||||||
| 5 | DEMQOL-P-U-based QALYs | 611 | 0.578 (0.24) | 708 | 0.581 (0.235) | –0.003 (–0.028 to 0.023) | 0.005 (–0.019 to 0.03) | 0.005 (–0.018 to 0.029) |
| 6 | EQ-5D-5L-P-based QALYs | 622 | 0.266 (0.317) | 718 | 0.232 (0.291) | 0.034 (0.002 to 0.067) | 0.024 (0.004 to 0.044) | 0.023 (0.003 to 0.043) |
| 7 | Falls | 732 | 1.889 (3.662) | 871 | 2.747 (7.414) | –0.858 (–1.417 to –0.299) | –0.568 (–0.97 to –0.166) | –0.574 (–0.961 to –0.186) |
| ICERs | Analysis | |||||||
| 1/5 | Base case, incremental cost per DEMQOL-P-U-based QALY | –£7226.47 | £20,889.42 | £20,557.80 | ||||
| 2/5 | With refresher GtACH tools, incremental cost per DEMQOL-P-U-based QALY | –£15,605.59 | £25,433.80 | £25,030.04 | ||||
| 3/5 | With extra mortality cost, incremental cost per DEMQOL-P-U-based QALY | –£20,513.22 | £24,115.39 | £23,732.56 | ||||
| 4/5 | With extra GtACH tools and mortality cost, incremental cost per DEMQOL-P-U-based QALY | –£28,892.34 | £28,658.26 | £28,203.32 | ||||
| 1/6 | Base case, incremental cost per EQ-5D-5L-P-based QALY | £575.01 | £4543.69 | £4651.63 | ||||
| 2/6 | With refresher GtACH tools, incremental cost per EQ-5D-5L-P-based QALY | £1241.73 | £5532.14 | £5663.56 | ||||
| 3/6 | With extra mortality cost, incremental cost per EQ-5D-5L-P-based QALY | £1632.22 | £5245.37 | £5369.98 | ||||
| 4/6 | With extra GtACH tools and mortality cost, incremental cost per EQ-5D-5L-P-based QALY | £2298.94 | £6233.50 | £6381.58 | ||||
| 1/7 | Base case, incremental cost per fall averted | £23.02 | £190.62 | £188.72 | ||||
| 2/7 | With refresher GtACH tools, incremental cost per fall averted | £49.72 | £232.09 | £229.77 | ||||
| 3/7 | With extra mortality cost, incremental cost per fall averted | £65.35 | £220.06 | £217.86 | ||||
| 4/7 | With extra GtACH tools and mortality cost, incremental cost per fall averted | £92.05 | £261.52 | £258.91 | ||||
Point estimate, unadjusted, DEMQOL-based QALYs are –0.003 per care home resident. However, adjusting for baseline values and other predictors of QALYs yields an estimate of 0.005 attributable to the intervention (95% CI –0.019 to 0.030). The (adjusted) incremental gain in EuroQol-5 Dimensions (EQ-5D)-based QALYs was larger at 0.024, with the 95% CI excluding zero (0.004 to 0.044). Likewise, the incremental gain in the number of falls was –0.568 per person over the 12-month period (–0.970 to –0.166). Note that a negative value indicates fewer falls in the GtACH arm than in the control arm. As per the cost estimates, imputing missing values does not materially affect the results.
Depending on the costs included (i.e. extra mortality and refresher sessions), point estimate ICERs range between £20,889 and £28,658 per DEMQOL-based QALY gained, with a 53–57% probability that the ICER is below £20,000 (Figures 10 and 11), and between £4544 and £6234 per EQ-5D-based QALY gained, with an 88.8–91.6% probability of being below £20,000 per QALY (Figures 12 and 13). The incremental cost per fall prevented is between £190 and £262, with a 98.6% probability of being cost-effective as long as the willingness to pay to avoid a fall is above £2000 (Figures 14 and 15).
FIGURE 10.
Scatterplot of DEMQOL-P-U-based QALYs: base-case costs.
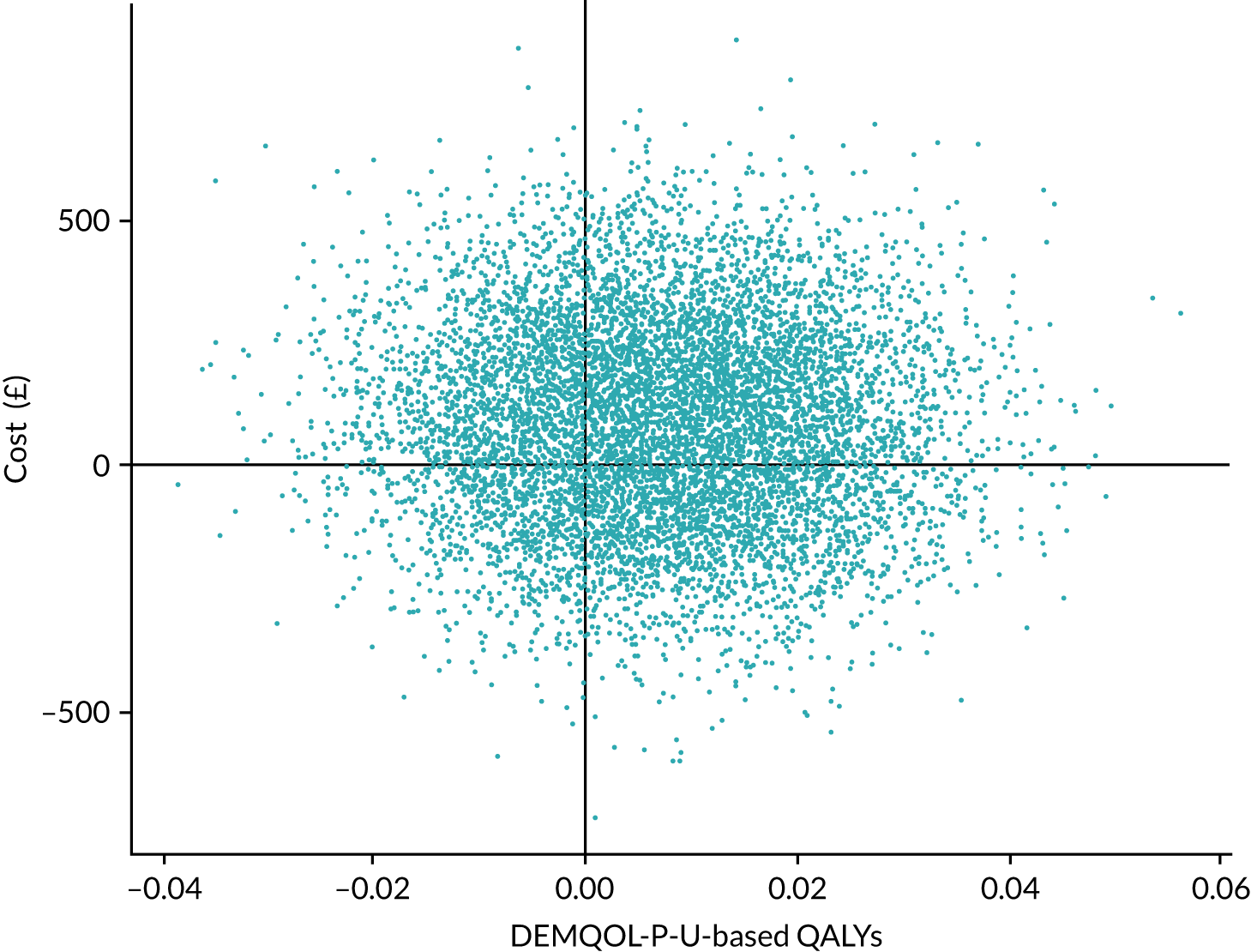
FIGURE 11.
The CEAC of DEMQOL-P-U-based QALYs: base-case costs.
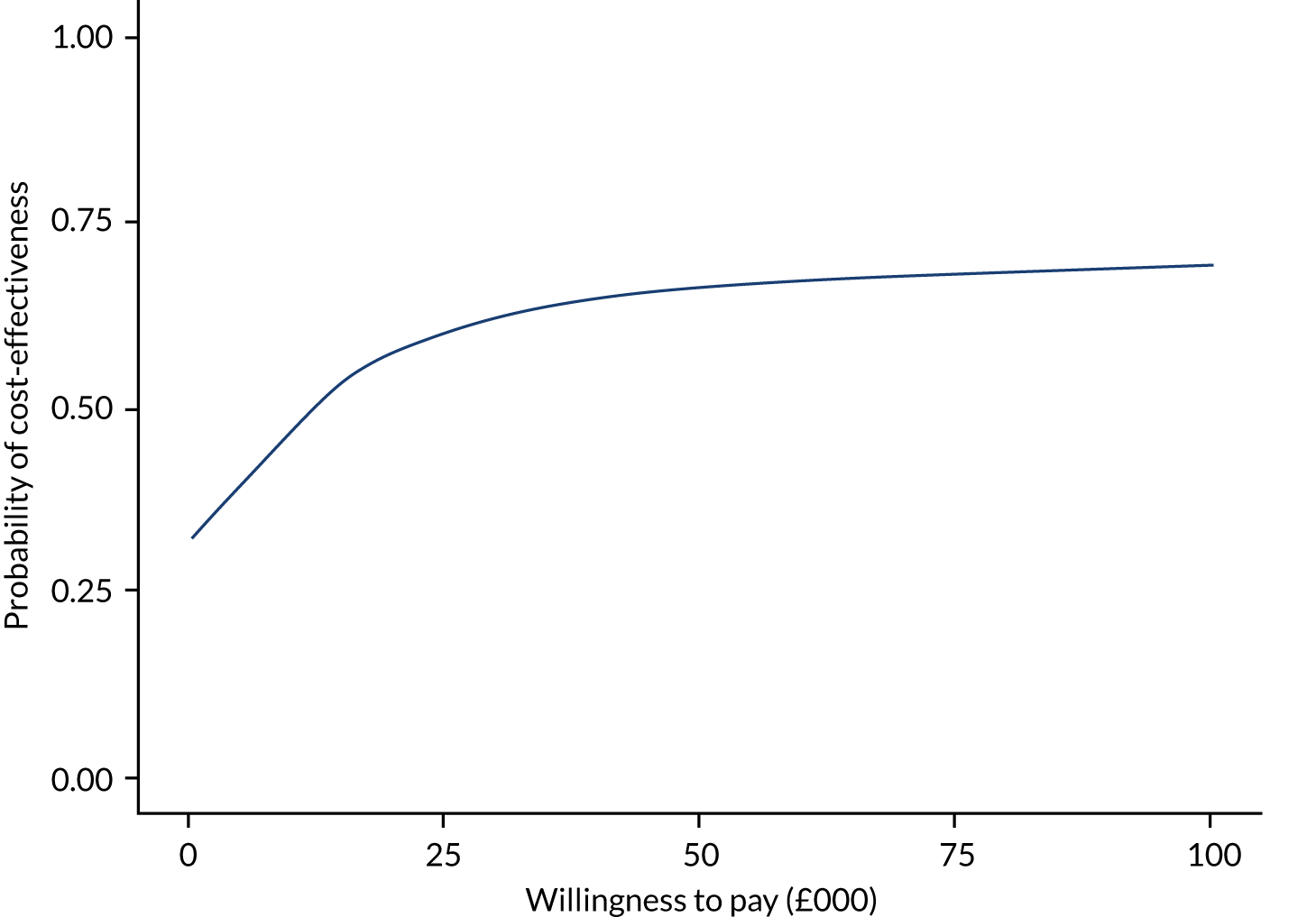
FIGURE 12.
Scatterplot of EQ-5D-5L-P-based QALYs: base-case costs.
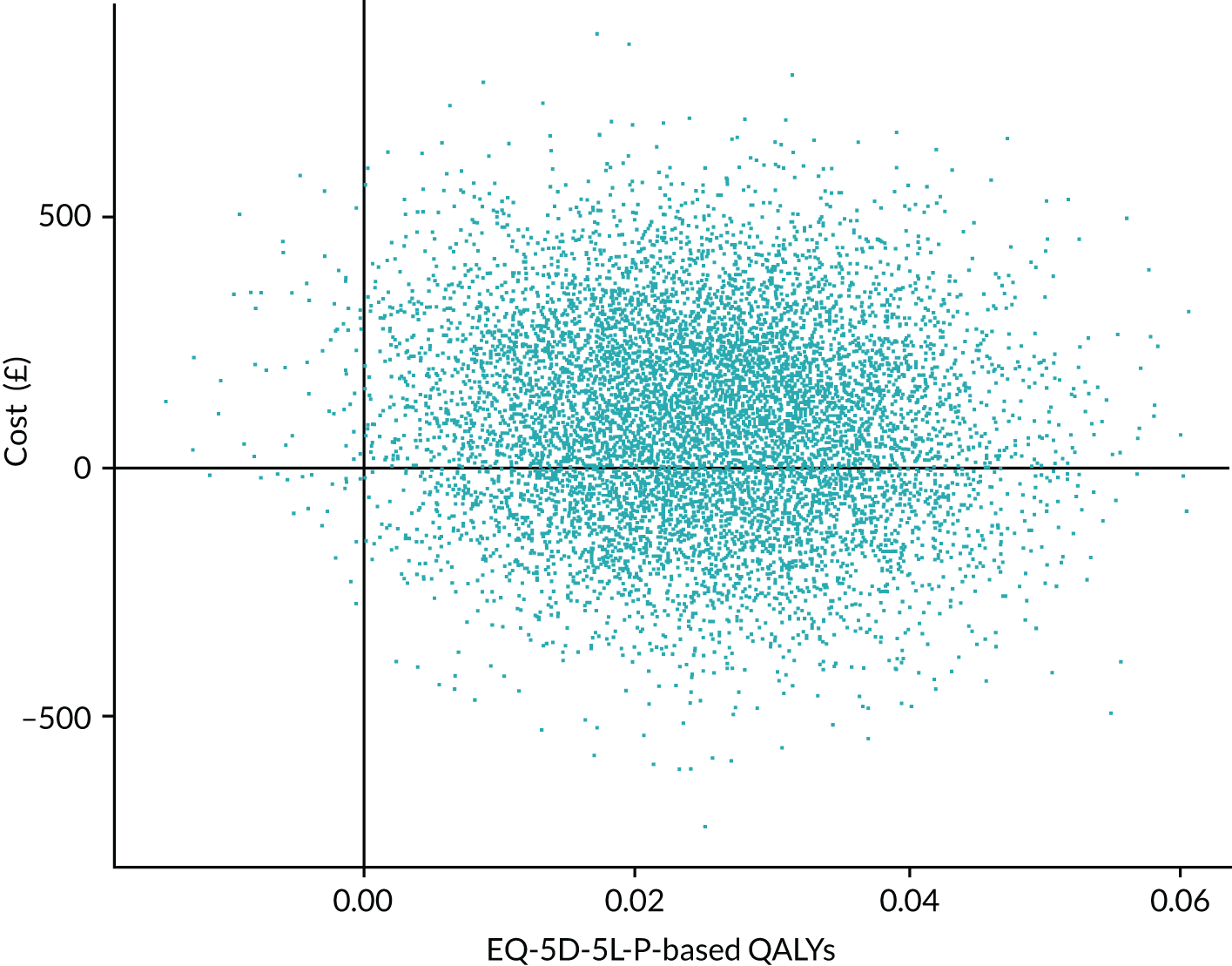
FIGURE 13.
The CEAC of EQ-5D-5L-P-based QALYs: base-case costs.

FIGURE 14.
Scatterplot of falls: base-case costs. Note that negative falls = the GtACH programme prevents falls.
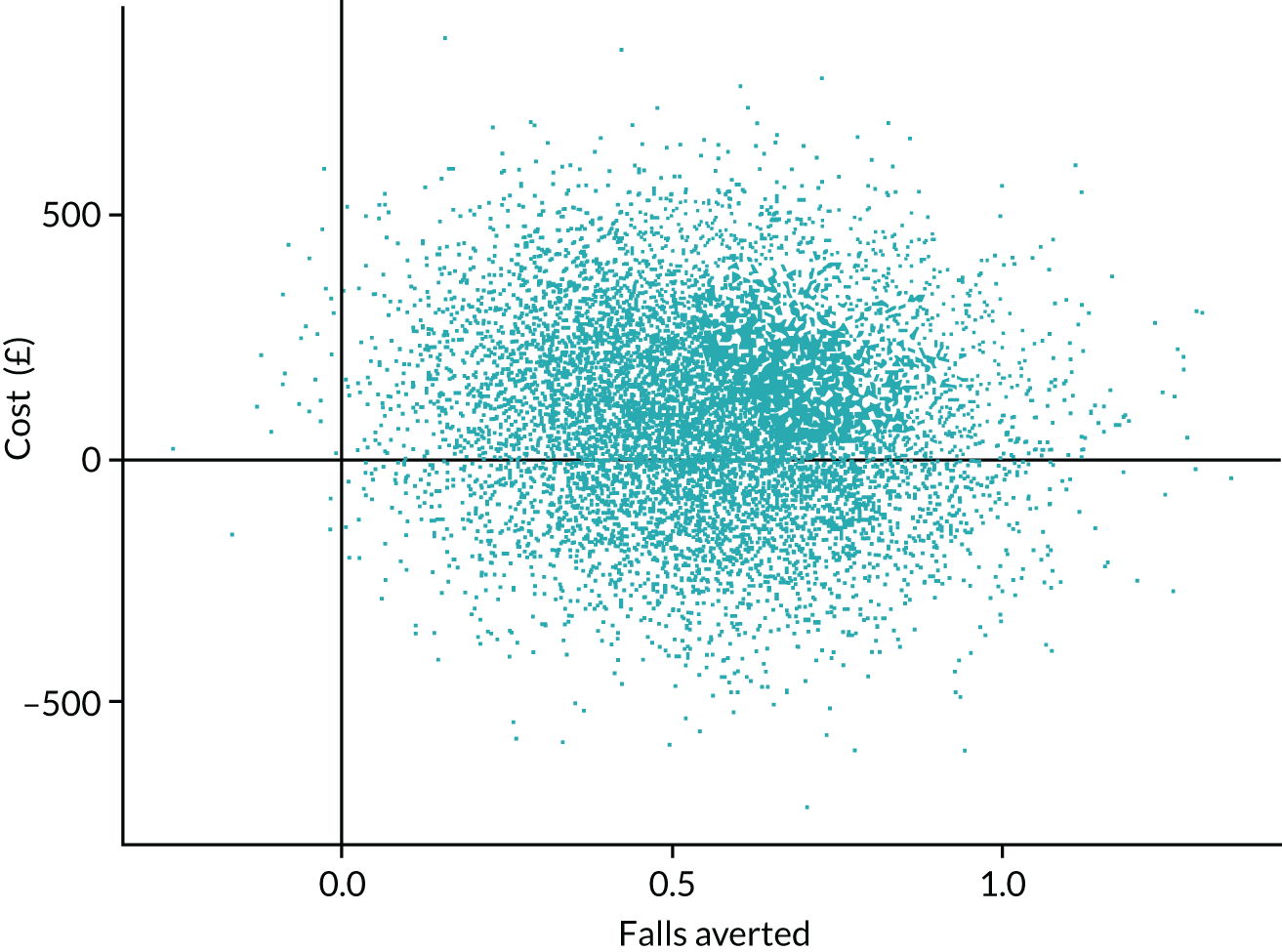
FIGURE 15.
The CEAC of falls: base-case costs. Note that negative falls = the GtACH programme prevents falls.
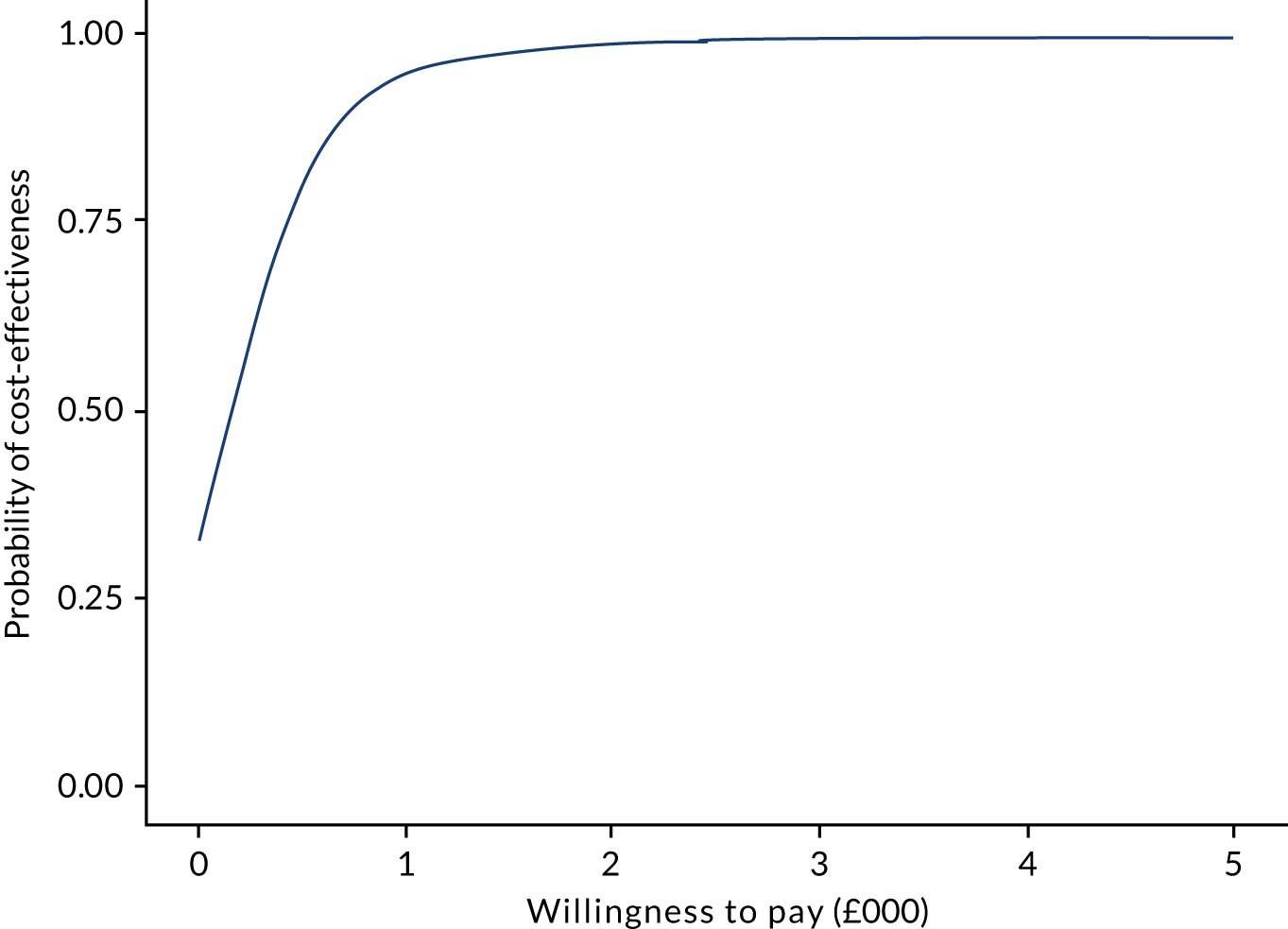
Discussion
Interpretation of results
Overall, the GtACH programme showed a benefit in terms of EQ-5D-5L-P-based QALYs and falls. However, DEMQOL-P-U-based QALYs yielded almost identical results; point estimates were marginally worse in the GtACH arm than in the control arm, but this disappeared after adjustment for baseline parameters. There was very little difference in health service costs between the arms. There was no evidence of an increase in ADL costs associated with the intervention; this is consistent with care homes already being well fitted out with relevant equipment.
When measuring QALYs with the DEMQOL-P-U, the GtACH programme was of borderline cost-effectiveness at the willingness-to-pay threshold for a QALY of £20,000–30,000 commonly employed in England. 44 The ICER was between approximately £20,900 and 28,700, depending on the choice of cost scenario, with around 53–57% probability of being below the lower threshold of £20,000. When measuring QALYs based on the EQ-5D-5L-P, point estimate ICERs were well within the range normally considered cost-effective in England at between £4500 and £6200 per QALY gained, with approximately 89–92% probability of being below £20,000. The cost to prevent one extra fall with the GtACH programme was approximately £190–260.
In designing this economic evaluation there was always an uncertainty regarding which preference-based measure to use to assess QoL among care home residents. This uncertainty reflects a number of issues involved in measuring health-related QoL in this population (most notably who should rate it50 and which instrument should be used57), and although the number of studies looking at these issues has increased substantially in the last year or two, it is still a question researchers would face in designing a similar study from scratch today. 57 We included both the generic EQ-5D-5L and a dementia-specific outcome measure, the DEMQOL-U. 38,50 The relative merits of each are discussed below (see Strengths and limitations of the economic evaluation). Ultimately it is for the decision-maker to decide whether DEMQOL-P-U- or EQ-5D-5L-P-based QALYs are more appropriate, and to interpret the results accordingly. If DEMQOL-P-U-based QALYs are chosen, the GtACH programme is of borderline cost-effectiveness; if EQ-5D-5L-P-based QALYs are chosen, it is solidly within conventional limits.
Finally, the incremental cost per fall prevented is of note, and may appear subjectively ‘reasonable’, at £190–260. The willingness to pay for preventing a fall is less clearly established and, furthermore, does not take into account the varying severity of falls. This is a major advantage of the QALY, which (theoretically) captures the health consequences of events, so may be considered a more useful metric in this regard.
Strengths and limitations of the economic evaluation
A major strength of this study is the quality and completeness of the data collected. Data were collected at quarterly follow-up points. These relatively frequent collections were needed as discussion with care home managers revealed that care records tend to be archived very quickly following deaths and thus less frequent collection could have caused us to lose data on falls, resource use and outcomes from residents who died or transferred homes between assessment points. The final data set for the economic analysis was 1603 participants (GtACH arm, n = 732 participants; control arm, n = 871 participants), with no missing data on falls and only six missing observations for cost data. Completion rates for DEMQOL-P-U and EQ-5D-5L at each time point were such that QALYs were calculable for at least 82.3% of these.
A major limitation was uncertainty as to which health-related quality-of-life measure to use. Neither the EQ-5D-5L nor the DEMQOL-P-U are perfect; the utility value sets for the DEMQOL-U and DEMQOL-P-U are fairly recently published so have not been extensively used or validated in funded trials. In contrast, there were concerns about the valuation of the EQ-5D-5L. Thus, although the EQ-5D is the preferred instrument of NICE,44 the position statement published by NICE in 201951 raised concerns about use of the -5L variant. However, there was some evidence to support the use of proxy scores instead of self-report scores on the EQ-5D for care home residents. 58
A decision was made to use the DEMQOL-P-U in the base-case analysis at the time the HEAP (see Report Supplementary Material 3) was signed off; however, there was not a strong conviction for this choice. Therefore, this chapter presented the cost–utility analysis based on the DEMQOL-P-U first, followed by the cost–utility analysis based on the EQ-5D-5L-P and finally the CEA based on falls outcomes. In interpreting these results, it is necessary to acknowledge the growing evidence that the choice to use the DEMQOL in the base case may not have been the most appropriate choice. As the HEAP was signed off, concerns that its use in care home populations may be inappropriate led to it being adapted for use in this population. 59 At the same time, several economic evaluations in care home populations have been published that show the EQ-5D can be used in this population20,60 and may be more responsive than the DEMQOL-P-U. 59 Recently, however, a qualitative study comparing six preference-based measures (in terms of face and content validity) in the context of dementia found that no single instrument was favoured,61 such that choice of a preference-based measure remains on the research agenda.
A further limitation was that although we recorded how many staff in each care home received training, it was not feasible to record whether or not each resident actually received a completed GtACH tool. However, even if the GtACH programme was not delivered per resident as planned, educating staff on falls risks would be expected to have had an impact. To explore this uncertainty, in the base case we assumed each resident had one session, with the cost of refresher sessions if the resident experienced a fall added in sensitivity analysis. Even with the refresher, the GtACH intervention was low cost compared with other care home interventions. In the base case this cost was £88, rising to £109 in sensitivity analysis, although this does increase the DEMQOL-based ICER by approximately £4600 and the EQ-5D-based ICER by £1000.
Future research
In the analysis reported, utility is derived from the DEMQOL-P-U and EQ-5D-5L-P. Both instruments have self-complete versions available. A previous study58 found that the proxy version of the EQ-5D was an acceptable source of data for utility index scores. Given the anticipated difficulties in collecting self-report data for QoL, because of cognitive difficulties, the trial collected proxy estimates for all residents from their main carer in the care home. Those with capacity were also asked to self-report QoL; however, completion rates were much lower for the self-reported versions (see Report Supplementary Material 5 for data completion figures for the DEMQOL-U and EQ-5D-5L). Future work should be undertaken to compare the proxy and self-report data collected for those residents with both available.
Conclusion
The evidence suggests that, depending on the choice of HRQoL measure, the GtACH programme was either of borderline cost-effectiveness or well within conventional thresholds of cost-effectiveness. Implementation of the GtACH programme was associated with a reduction in falls in care homes and improvements in EQ-5D-based QALYs. Future research could explore the relative validity and appropriateness of different health-related QoL measures in care home residents.
Chapter 5 Process evaluation
Introduction
Context: a realist process evaluation
Process evaluation promises insight into fidelity and quality of implementation, provides information about causal mechanisms and contextual factors, and supports an understanding of why an intervention fails or has unexpected consequences. 25 The more recent development of the Medical Research Council (MRC) guidelines has stressed the importance of theory in this. 25
Realist evaluation is one such theoretical approach. Based on the work of Pawson and Tilley,62–65 realist evaluation demonstrates a concern for causality and change mechanisms, postulating the ‘contextually contingent nature of these’ and challenging how ‘hypothesised causal chains play out in the implementation of a complex intervention’. 25 Put more simply, a realist approach considers the GtACH programme to be a resource that enables change to happen. The GtACH programme in itself does not reduce falls but rather provokes a response or creates mechanisms for change to happen. Change may come (for example) in the form of individual knowledge, awareness, confidence or organisational structures and it is these changes that lead to difference in falls outcome.
A key tenet of realist evaluation is that different mechanisms will be triggered in different contexts. The GtACH programme may not work in all places and, if it does work, it may work in different ways depending on which mechanisms (organisational structure, individual knowledge, self-confidence, etc.) are triggered. Understanding the contextual circumstances of GtACH programme delivery and identifying those mechanisms that are triggered in different settings is key to understanding how the GtACH programme might be implemented in the future.
Aims and objectives
The aim of this realist evaluation was to generate detailed insight into the delivery of the GtACH programme to (1) identify those contexts in which it is easily adopted and (2) recognise those mechanisms that lead to positive outcomes.
The evaluation considered consistency of the GtACH programme’s use within and across care home settings and illustrated the views and opinions of key stakeholders about the adoption of the GtACH programme. Specifically, it assessed:
-
fidelity in GtACH training
-
fidelity in the use of the GtACH screening and assessment paper tool
-
acceptability of the GtACH programme (training and tool)
-
impact of the GtACH programme on falls rate.
Background
Care homes pose a distinct challenge for the introduction of complex interventions: they vary in size, funding, workforce and culture, and house vulnerable individuals with far-reaching health and social care needs. This heterogeneity of organisational context and uncertainty of individual need is an inherent (and unavoidable) barrier to effective innovation. 24,66–70 Although the delivery of programmes such as the GtACH programme is intended to be consistent, with justifiable variation only, it may be that the needs of local residents and the preference of local staff create situated and specific variations in how programmes are delivered.
Our starting point in this (as with all realist evaluation) was to consider how the GtACH programme was intended to work. In the realist tenet, programmes are not simply treatments or interventions, but rather ‘Programmes are “theories incarnate”. Every programme has a theoretical underpinning, whether it is made explicit or not’. 65 A programme such as GtACH rests on some theorised causal relationship that has a broader reach and application than its specific components; previous realist research illuminates the type of programme theories that might underpin initiatives in care homes. 71
In a recent realist review,24 three broad programme theories were recognised in the delivery of health care to care home residents. Incentives, targets and sanctions might motivate GPs to engage more routinely in the delivery of health care to this group; greater involvement of experts might make for more appropriate provision of elderly health care; and health outcomes might be improved by better relational working that spans care home staff and external health professionals. (Relational working may already exist; this is more about improving relational working.)
More recent research by the same authors66 identified similar programme theories. Improved relational working might underpin better outcomes in the delivery of health care; dedicated (financial) investment can trigger more appropriate provision; and wrap-around care, manifest in referral networks for external services, can support care home staff in accessing appropriate specialist care. The benefit of dementia-specialist services is a final focus for improved care home health care.
The PEACH (Proactive hEAlthcare of older people in Care Homes) programme67 looked to programme theories derived from the quality improvement collaborative literature to examine the implementation of comprehensive geriatric assessment (CGA) in care homes.
The FIRE (Facilitating Implementation of Research Evidence) evaluation68 speculated that the implementation of incontinence recommendations is mediated by organisational context, a pertinent theory of action, and staff support for change.
The GtACH programme had its own programme theories (see Chapter 5, Initial programme theories), but these examples demonstrate how broader theories and causal relationships might underpin the specific components of an intervention. They highlight that it is not (simply) the adequacy of the incontinence recommendations, the appropriateness of the CGA approach or the health care delivered, but rather that it might be incentives, better relational working,24,66 sharing best practice67 or the fit with organisational context68 that govern the impact of an intervention.
Initial programme theories
In accordance with this approach, this evaluation looked beyond the individual elements of the GtACH programme to identify initial programme theories that could be tested in the evaluation. These programme theories were derived from previous published work relating to the creation of the GtACH programme27,28 and its early testing,26 and were verified by the FinCH trial TMG (February 2017).
Programme theory 1: connecting falls risk to remedial actions
In care homes, falls are a constant risk because of the complex mix of individual, organisational and environmental factors; this complexity and variety has made falls management difficult. Prior approaches to falls management have stressed the inter-relationship of different risk factors and have sought to quantify an individual’s risk of falling. Prior strategies have often focused on generating combined risk scores for individual care home residents, with less concern for the measures that might be taken to limit falls risk.
The GtACH screening and assessment paper tool isolates and disaggregates individual risk factors and connects them with specific actions to reduce risk. The GtACH programme is based on the value of considering each category of falls risk independently and the importance of generating solutions as well as understanding risks.
Programme theory 2: supporting all staff in falls risk management
Care home staff are heterogeneous in expertise, experience, training and skills; they will be more or less aware of falls risk and those measures that might reduce it. Consequently, care home staff may be more or less able and prepared to utilise the GtACH paper tool, which has implications for the effective delivery of the GtACH programme.
Specialist staff (local falls champions and regional NHS falls leads) are intended to support implementation by offering direct support to individual staff and by providing greater knowledge and expertise about falls risk management. Specialist staff are intended to ensure that there is consistent and appropriate delivery of the GtACH programme.
Methods
Study design
This was a multimethod process evaluation run concurrently with (but independently of) the main trial. It was informed by the principles of realist evaluation62–65 and was characterised by a concern for testing the programme theories described in Initial programme theories.
The evaluation incorporated a number of distinct but inter-related stages: (1) the formulation of initial programme theories (see Initial programme theories), (2) theoretical sampling to identify the most appropriate environments to test these theories, (3) the adaptation of these theories or creation of new programme theories, (4) recognising patterns in these revisions and (5) identifying a mid-range theory that explains these patterns.
Review and revision of the initial programme theory takes the form of context–mechanism–outcome (CMO) configurations that are the mainstay of the realist approach. 62,63,65 Context here relates to those individual or organisational features that predate the introduction of the GtACH programme, including (among a variety of care home-specific details) the size and ownership of the care home, the nature of its provision (residential and/or nursing) and will also include characteristics of both staff and residents. Outcome describes that which results from the introduction of the GtACH programme, including a concern for fidelity and acceptability, as well as any measurable change in the frequency or consequence of resident falls.
Mechanism is perhaps the most complex element of this equation64 and is seen here as a mediating factor that illuminates the causal relationship between the introduction of a programme into a specific context and the precise outcomes that result therein. Mechanisms are more than the resources introduced; they also encapsulate the individual or organisational reaction to or reasoning about the resources invested. 62 As this suggests, a realist approach acknowledges that mechanism might just as equally be a subjective response to the GtACH programme as it might be an objective change to practice.
Multiple CMO configurations are the likely output of any realist evaluation and it is evidence of recurrent patterns across these CMOs that is suggestive of more certain causal relationships. 72 Although such demi-regularities do not represent undeniable causality, they do offer a layer of explanatory power that aids understanding of the outcomes achieved. Reflecting on these demi-regularities completes the evaluation cycle and it is in these recurrent patterns that the strengths and shortcomings of the initial programme theories are made explicit. Further explanatory power might be achieved in the application of some more general mid-range theory to unpick these patterns.
Participants
Only those care homes randomised to the GtACH programme were included in this process evaluation and consent for the evaluation was taken independently of that for the main trial.
Care homes were selected purposively73 from those that expressed a willingness to participate. Selection was driven by the realist agenda of testing programme theories against a range of contextual features that initially were considered pertinent to delivery of the GtACH programme. This included the size of the care home, its ownership and the presence or absence of nursing staff.
Where the care home manager consented, all pertinent staff were approached and those willing were consented to the process evaluation. Residents who were identified as able were also approached and consented when they were willing.
Care homes were recruited in different geographic areas so as to capture any variation in local practice/policy, and regional NHS falls leads (who delivered GtACH training) were also involved in the process evaluation.
Data collection
Finally, for each care home involved, falls rate data were also included. This necessarily includes falls data for those residents who were not able (or unwilling) to consent to the process evaluation, but this was collected as part of the main trial and was not specifically collected for the process evaluation.
Data were collected using a combination of interviews, focus groups, fidelity observations, documentary review and falls rate reviews. Data were primarily collected during a 3-month period following the introduction of the GtACH programme, with an additional care home visit made 6 months after the introduction of the GtACH programme.
GtACH training was observed in each care home using a checklist to assess fidelity with the training protocol (see Report Supplementary Material 6). The primary training session was observed by two researchers, with additional sessions observed by at least one researcher.
The implementation of the GtACH paper tool was observed in each care home using a checklist to assess fidelity with the GtACH programme protocol (for the fidelity checklist, see Report Supplementary Material 6). In each care home, multiple researchers would record this process with a number of different residents. Evidence of use of the GtACH tool was also sought in a review of care home records.
A staff focus group was held immediately post GtACH training that considered their experience of training and their expectations of the GtACH programme. At the 6-month visit, a second focus group was organised that reflected on staff experiences and their thoughts about future use of the GtACH programme (for focus group guides, see Report Supplementary Material 7).
During the evaluation, a number of key stakeholders were interviewed. This included care home staff, the care home manager, the care home falls champion and the regional NHS falls lead; where possible, care home residents were also interviewed. Interviews focused on the local experience of the GtACH programme (for interview topic guides, see Report Supplementary Material 7). All interviews were recorded using digital audio equipment.
Falls data for care homes included in the process evaluation were sought to inform the outcome element of our CMO configurations. Practicalities of trial management (maintaining blinding, etc.) meant that this was provided as a single data set after all other process evaluation data had been collected, and it did not include adjustments informed by HES data about resident hospitalisation.
Data analysis
All interview and focus group data were transcribed in full, anonymised and handled using the NVivo (QSR International, Warrington, UK) software package. All data were coded by at least two researchers and the organisation of themes and the structure of the coding book was agreed by the process evaluation team. Initial coding was also verified by the FinCH trial PPI group. Fidelity checklists were reviewed by at least two researchers.
The focus of analysis in this realist evaluation was on the iterative development of those programme theories that aid understanding of the implementation and impact of the GtACH programme; it is focused through the lens of three conceptual tools: context, mechanism and outcome.
Thematic analysis74 of interview and focus group data added specific detail to the context by exposing existing practice, process and priorities. Baseline falls data also supplemented our understanding of the context.
Thematic analysis74 exposed those mechanisms triggered by the introduction of the GtACH programme that were manifest in stakeholders’ descriptions of its delivery.
Descriptive statistics for each process evaluation care home, for all intervention care homes and for all control care homes were produced to illuminate the outcome of the GtACH programme. These findings were mapped to the main trial baseline and primary data outcome time points. Thematic analysis and fidelity checks also aided our understanding of the outcome, highlighting stakeholder assessment of acceptability and demonstrating fidelity of use.
Data were synthesised in the form of multiple, specific CMO configurations for each care home. In each configuration, context and outcome were considered fixed, with the mechanism ascribed the causal power to explain why/how specific outcomes emerged in a context. CMO configurations were reviewed by the process evaluation team and recurrent patterns across multiple care homes (demi-regularities) were identified.
Results
Care home characteristics
Six care homes were recruited to the process evaluation from different parts of the country (to reflect different local practice); they ranged in size, included both residential and nursing homes and demonstrated different models of ownership and management (Table 19).
| Home | Registration | Size (number of residents recruited to trial) | Home ownership | Number of focus groups | Number of staff interviews |
|---|---|---|---|---|---|
| A | Residential | 71 beds (18) | Part of large national corporate chain | 4 | 7 |
| B | Dual registered | 48 beds (16) | Part of small local corporate chain | 2 | 1 |
| C | Residential | 46 beds (29) | Part of small local charitable chain | 2 | 10 |
| D | Residential | 40 beds (10) | Part of large national corporate chain | 1 | 10 |
| E | Residential | 17 beds (12) | Independent (business with one home) | 0 | 8 |
| F | Residential | 53 beds (42) | Part of small local corporate chain | 2 | 8 |
Across these settings, 88 participants consented to take part in the evaluation, 44 stakeholders were interviewed and 11 focus groups took place. Overall, 7 managers, 4 deputy managers, 1 nurse, 3 falls champions, 1 floor manager, 22 senior caring staff, 38 caring staff, 6 residents and 6 NHS falls leads took part in the evaluation.
Falls rate data
A total of 194 independent codes were identified in the data. These were organised within a simple thematic structure (consisting of 14 broad themes) that reflects a pragmatic concern for delivering the GtACH programme. Themes included the GtACH tool, the falls champion, GtACH training and GtACH programme implementation. The complete code book is presented in Appendix 6.
The data presented in Chapter 3 suggest that the GtACH programme (training and tool) offered benefit to those care homes in which it was introduced. The process evaluation was completed in six of the intervention care homes, with the falls rates for these homes shown in Table 20, alongside the average from all the homes.
| Falls details | Control (all – average) | GtACH (all – average) | Care home A | Care home B | Care home C | Care home D | Care home E | Care home F |
|---|---|---|---|---|---|---|---|---|
| Number of falls | ||||||||
| Baseline period | 8.09 | 7.13 | 12 | 6 | 14 | 8 | 9 | 12 |
| Primary end point | 14.13 | 7.97 | 19 | 4 | 20 | 16 | 3 | 20 |
| Change | +6.04 | +0.85 | +7.0 | –2.0 | +6.0 | +8.0 | –6.0 | +8.0 |
| Falls rate | ||||||||
| Baseline period | 4.76 | 4.17 | 7.41 | 4.21 | 5.42 | 8.97 | 8.33 | 3.19 |
| Primary end point | 9.29 | 5.49 | 12.44 | 3.53 | 8.85 | 18.29 | 3.03 | 6.93 |
| Change | +4.53 | +1.32 | +5.03 | –0.68 | +3.43 | +9.32 | –5.3 | +3.74 |
Counts of the number of falls in each setting (unadjusted for the size of the care home or the nature of the care offered) suggest a distinct trend, which we describe here to allow the data from the process evaluation to be compared with the whole sample. At baseline (90 days prior to randomisation), the number of falls recorded in participants from all homes ranged from 0 to 25 falls. For the period 91–180 days post randomisation, the number of falls ranged from 1 to 116 in control settings and from 0 to 28 in those homes where the GtACH programme was introduced. No care home that received the GtACH programme recorded > 30 falls in a 90-day period (at either baseline or post randomisation); in the control arm, three care homes recorded > 50 falls in the period 91–180 days post randomisation. An unadjusted count of falls in all care homes is presented in Appendix 7, Table 30. In the control arm, the rate of falls increased by 4.53 falls per 1000 resident-days at 91–180 days post randomisation (see Table 20); in the GtACH arm the increase was less pronounced, at only 1.32 more falls per 1000 resident-days.
Of those care homes included in the process evaluation, the falls rates decreased in care homes B and E; in care homes C and F, the rate of falls increased at a slower rate than in the control arm; and in care homes A and D the rate of falls increased at a greater rate than in the control arm.
Care home experiences
Results for each home are described with a full list of CMO configurations for all care homes in Appendix 8, Table 31, and summarised in Table 21.
| Contexts | Mechanism | Outcome |
|---|---|---|
| Care home A (0803) | ||
| Knowledgeable staff | Little motivation for change | Persistence of existing practice |
| Effective falls systems in place | Inertia (inhibits innovation) | Persistence of existing practice |
| Demarcation of staff roles in falls management – not all staff manage falls | Inflexibility in job roles | Persistence of existing practice – falls champion role not adopted |
| Existing administrative/paperwork burden | Little appetite for more paperwork | Persistence of existing practice – GtACH tool not adopted |
| Internal and external management systems | Change processes not owned locally | Persistence of existing practice – change requires corporate approval |
| A large proportion of residents have dementia, and a consequent higher than average risk of falls | Staff believing that residents with dementia will fall, and so they are not motivated to introduce change for these residents | Persistence of existing practice for residents with dementia – falls rate does not decrease |
| Care home B (0703) | ||
| Existing administrative/paperwork burden | Reluctance to introduce additional burden | Persistence of existing practice – GtACH tool explicitly not adopted |
| Nursing staff part of the care home team | Nurses take ownership and lead falls awareness initiative | Changes to existing practice – all staff encouraged/supported to take part in falls risk management |
| Demarcation of staff roles in falls management – not all staff manage falls | Cascade of falls risk information (from training) to all staff | Changes to existing practice – broader range of staff engaged in falls management activities |
| Demarcation of staff roles in falls management – not all staff manage falls | Cascade of falls risk information (from training) to all staff | Changes to existing practice – broader range of staff confident about falls management |
| Demarcation of staff roles in falls management – not all staff manage falls | Shared responsibility for falls recognised across a broader group of staff | Changes to existing practice – staff more proactive in identifying and responding to falls risks |
| Care home C (0402) | ||
| Falls systems in place – staff working at capacity | No appetite for practice change | Persistence of existing practice – GtACH tool not adopted |
| Existing administrative/paperwork burden – staff working at capacity | No appetite for more paperwork | Persistence of existing practice – GtACH tool not adopted |
| Demarcation of staff roles in falls management – not all staff manage falls | Staff reluctant to take on new responsibilities | Persistence of existing practice – GtACH tool not adopted |
| Demarcation of staff roles in falls management – not all staff manage falls | Staff anxious about completing paperwork | Persistence of existing practice – GtACH tool not adopted |
| External management systems | Change process not owned locally | Long-term adoption of the GtACH programme unlikely |
| A large proportion of residents with a higher than average risk of falls (residents who are visually impaired and/or have dementia) | Staff believe that residents will fall irrespective of what they do, and so they are not motivated to introduce change | Persistence of existing practice – falls rate does not decrease |
| Care home D (0302) | ||
| Falls systems in place – staff working at capacity | No appetite for practice change | Persistence of existing practice – GtACH tool not adopted |
| External management systems | Change process not owned locally | Long-term adoption of the GtACH programme unlikely |
| Demarcation of staff roles in falls management – not all staff manage falls | Staff reluctant to take on new responsibilities | Persistence of existing practice – GtACH tool not adopted |
| Demarcation of staff roles in falls management – not all staff manage falls | Staff anxious about completing paperwork | Persistence of existing practice – GtACH tool not adopted |
| Knowledgeable staff who had received internal training on falls prevention | No motivation to change paperwork or systems | Persistence of existing practice – GtACH tool not adopted |
| A large proportion of residents with dementia, and a consequent higher than average risk of falls | Staff believe that residents with dementia will fall, and so they are not motivated to introduce change for these residents | Persistence of existing practice for residents with dementia – falls rate does not decrease |
| Care home E (0209) | ||
| Independent residents. Few with dementia | Lack of perceived need for change | Persistence of existing practice – GtACH tool not adopted |
| Independent residents. Few with dementia | GtACH tool considered inappropriate | Persistence of existing practice – GtACH tool not adopted |
| Demarcation of staff roles in falls management – not all staff manage falls | Staff anxious about completing paperwork | Persistence of existing practice – GtACH tool not adopted |
| Staff know the residents well | Lack of motivation to adopt a tool that duplicates, rather than adds information, about residents | Persistence of existing practice – GtACH tool not adopted |
| A staff group that had received limited prior training in falls risk management | GtACH training brings improved knowledge about falls risks | Staff more engaged in falls management activities |
| A staff group that had received limited prior training in falls risk management | GtACH training brings improved confidence in dealing with falls risk | Staff more engaged in falls management activities |
| Care home F (0107) | ||
| Frequent changes in management affecting working practices in the home | Lack of staff ownership with documentation | Persistence of existing practice – the GtACH programme will only be adopted if there is management ownership |
| Demarcation of staff roles in falls management – not all staff manage falls | Staff anxious about completing paperwork | Persistence of existing practice – GtACH tool not adopted |
| Demarcation of staff roles in falls management – not all staff manage falls | Staff reluctant to take on new responsibilities | Persistence of existing practice – GtACH tool not adopted |
| The NHS falls lead was not trained alongside the other NHS falls leads and weaknesses were identified with the training | Lack of confidence to use the GtACH programme following training | Persistence of existing practice – GtACH tool not adopted |
| A staff group that had received limited prior training in falls risk management | GtACH training brings improved knowledge about falls risks | Staff more engaged in falls management activities |
| A large proportion of residents with dementia, and a consequent higher than average risk of falls | Staff believe that residents with dementia will fall, and so are not motivated to introduce change for these residents | Persistence of existing practice with residents with dementia – falls rate does not decrease |
Care home A (0803)
Context
This was a 71-bed care home providing residential dementia care. The home was corporately owned and was part of a large national chain.
Outcome
GtACH training was delivered in accordance with the training guidelines but implementation of the GtACH screening and assessment paper tool was poor (only six GtACH tools were completed for the 18 recruited participants). Only senior staff used the GtACH tool correctly, as per the training and intervention manual; carers rarely used it independently (only doing so when observed by a researcher) and did not complete it in full. The GtACH tool was not being used at the end of the 6-month period.
Both the number of falls and the falls rate increased during the observation period: the number of falls increased from 12 in the baseline period to 19 in the primary outcome period, and the falls rate increased from 7.41 falls per 1000 resident-days to 12.44 falls per 1000 resident-days.
Commentary
The staff reported that falls prevention provision was well established before the study, and staff felt knowledgeable about falls and confident in their management. Staff were reluctant to adopt new ways of working alongside (and in addition to) the home’s existing systems. Managerial changes during the study had a negative impact on the implementation of the GtACH programme and change was not driven by either senior managers or care home staff. The home did not actively instigate the falls champion role.
Care home B (0703)
Context
This was a 48-bed, dual-registered, nursing-led home providing residential, dementia and nursing care. The home was corporately owned by a small chain.
Outcome
The GtACH training was delivered in accordance with the training guidelines. Implementation fidelity was poor. A number of GtACH assessments were completed by a single member of nursing staff, but only in anticipation of a process evaluation interview. Otherwise, the tool was not used during or after the process evaluation observation period.
Both the number of falls and the falls rate decreased during the observation period: the number of falls decreased from six falls (baseline) to four falls (primary outcome period), and the falls rate decreased from 4.21 falls per 1000 resident-days to 3.53 falls per 1000 resident-days.
Commentary
A change of manager at the outset of the study was marked by a reluctance to introduce new systems at a time of change. The new manager would not sanction additional paperwork alongside existing home systems and processes. By contrast, the GtACH training was well received and valued by staff and management alike. Staff described feeling more aware of falls risk and more confident in addressing them; management described changes to staff behaviour, with staff becoming more proactive with falls management.
Care home C (0402)
Context
This was a 46-bed residential home, which was part of a small local chain run by a charity that specialised in supporting people with sight loss and/or dementia.
Outcome
The GtACH training was delivered in accordance with the training guidelines. Twenty-four GtACH screening and assessment paper tools were completed during the observation period, although most of these (n = 14) were completed when researchers were present. Few of those observed were actually completed correctly. It was considered unlikely that the GtACH programme would be continued post study.
The number of falls and the falls rate increased during the observation period: the number of falls increased from 14 falls (baseline) to 20 falls (primary outcome period), and the falls rate decreased from 5.42 falls per 1000 resident-days to 8.85 falls per 1000 resident-days.
Commentary
An enthusiastic falls champion involved all grades of staff in the completion of the GtACH programme and staff reported that it was more in-depth than the home’s own documentation. Despite (or, perhaps, because of) this, care staff in this setting were uncomfortable and lacked confidence when faced with the GtACH programme; some of them did not consider ‘paperwork’ to be part of their job and some were anxious about their ability to complete the tool correctly. Longer term, it was felt it was unlikely that the GtACH programme would be used, as any change in paperwork had to be adopted by all homes in the chain.
Care home D (0302)
Context
This was a 40-bed residential home with a high number of residents with dementia. Only residents with dementia were recruited into the trial.
Outcome
Training was delivered in accordance with the GtACH training guidelines. Implementation fidelity was poor. Only one observation was completed as a result of cancelled visits, and fidelity was assessed using filed tools – in all cases completion of the GtACH tool was judged to be poor. The GtACH programme was not continued post study period.
Both the number of falls and the falls rate increased during the observation period: the numbers of falls increased from 8 falls (baseline) to 16 falls (primary outcome period), and the falls rate increased from 8.97 falls per 1000 resident-days to 18.29 falls per 1000 resident-days.
Commentary
This home was part of a very large national chain. The manager had previously worked as the falls awareness trainer for the chain and had trained the staff in falls prevention. Staff felt knowledgeable and confident in falls management. It was reported that most falls occurred in the evenings and that this may be attributed to increased confusion as a result of dementia. The staff perceived the GtACH programme as a useful prompt, but felt that it could not be used as a standalone tool without the entire chain changing its practice and procedures.
Care home E (0209)
Context
This was a small independent residential home with 17 beds and 19 staff.
Outcome
Training was delivered in accordance with the GtACH training guidelines. Implementation fidelity was poor – three tools were completed during the observation period, but none of the completed GtACH tools was judged to have met fidelity standards. It was reported that it would be unlikely that use of the GtACH tools would be continued post study.
Both the number of falls and the falls rate decreased during the observation period: the number of falls decreased from nine falls (baseline) to three falls (primary outcome period), and the falls rate decreased from 8.33 falls per 1000 resident-days to 3.03 falls per 1000 resident-days.
Commentary
In contrast to the other homes, residents here were more physically able and independent in their day-to-day lives; some residents were observed leaving the home to walk around a local park. This home was not registered for dementia care. Few of the residents were considered to be at high risk of falling.
This independent care home had previously received in-house falls prevention training only, and the external training provided as part of the FinCH trial was received with enthusiasm. By contrast, perhaps because residents were more mobile and independent, the GtACH tools were not considered appropriate for the residents’ needs.
Care home F (0107)
Context
This was a 53-bed residential home, which was part of a small, family-operated chain providing care across five homes.
Outcome
Training was not observed in this setting, so it is not possible to comment on the fidelity of the training. Some negative feedback about the training was received and it should be noted that the NHS falls lead did not participate in the in-depth training, but was introduced to the GtACH programme at the site initiation visit. The GtACH tool was not inserted into residents’ notes until the later end of the process evaluation period and, consequently, the implementation of the GtACH tools could not be observed.
Both the number of falls and falls rate increased during the observation period: the number of falls increased from 12 falls (baseline) to 20 falls (primary outcome period), and the falls rate increased from 3.19 falls per 1000 resident-days to 6.93 falls per 1000 resident-days.
Commentary
There was a change of management in this home, with the new (temporary) management having little knowledge of the FinCH trial. This meant that the falls champion role was not adopted and the implementation of the GtACH programme was delayed. Previous training in this home had been largely limited to in-house training. Staff were reported to be keen to attend falls awareness training. However, there was a lack of staff confidence around completing the GtACH programme.
Recurring patterns (demi-regularities)
The effectiveness of the GtACH programme is predicated on two notions: (1) that falls risks are better managed when they are identified and specifically rectified (rather than simply quantified); and (2) that care home staff may benefit from training and peer support in managing falls. Here, we introduce five recurring patterns that illuminate the extent to which these notions are fulfilled in the data generated here.
The relevance of prior practice
All settings included in this evaluation demonstrated the existence of falls management systems prior to the introduction of the GtACH programme, and no setting totally adopted the GtACH tool and removed their own process. The tool was more often used by the evaluation team rather than being adopted into routine practice. Care home staff indicated satisfaction and familiarity with their existing systems, which meant that they were not motivated to adapt their practice and incorporate a new tool:
I don’t think I’d feel any better or, I don’t feel I’d do my job any better filling this in every time. The form we’ve got is adequate.
Senior carer 0209 417
Staff pointed to capacity issues (and to the duplication of effort) associated with the implementation of the GtACH tool:
It’d be the time element, we wouldn’t be able to fill one out three times because it’d be three times for the same thing. We wouldn’t have the time to do that because we’ve already got the action tools to fill out, then we’ve got the 24-hour obs[ervation] to fill out. Erm, so realistically, you know, we wouldn’t be filling that out.
Carer 0803 502
Because our paperwork, as it is, takes a lot of our day up, especially when it comes to a fall, you know . . . to then have to fill out more paperwork, and to duplicate it however many times it happens, it can be time consuming to us, and it takes us away from doing the rest of our work, you know, that’s the concern for me.
Senior carer 0302 413
In accordance with programme theory 1, staff did recognise the value of identifying and specifically rectifying falls risks, but felt that their existing systems already achieved this without the need for new tools.
The relevance of training
The benefits of the GtACH training were recognised across all settings, with a clear recognition among more experienced staff that its benefits reached beyond and are distinct from using the GtACH tool:
. . . I liked the training. It was a refresher for myself and the other qualified [staff] . . . I think, again, it made us look a bit beyond what, why, you know, what medication are they on, have they got an infection? I think we pretty much do that anyway. But there was factors on there that I perhaps didn’t think of myself. You know, because it does tell you through the list of other things to look for. I think, we have struggled filling the paperwork in but the knowledge has stayed in our head. I don’t know if that’s the right or wrong thing to say but the knowledge is certainly there and we do talk and look at why people are falling, but I think some of the care staff struggled with the paperwork.
Falls champion 0703
Training was considered particularly beneficial in those settings where prior training had been lacking or had been internally delivered (e.g. care homes B, E and F) and in those settings where parts of the staff group had not previously managed falls (e.g. care home B). In these settings, training generated greater knowledge of and confidence regarding falls management, and more acceptance of shared responsibility for managing residents’ falls.
Comments in care home F expressed a disappointment that more had not been covered in the training:
Thought it was very good, really. I thought it was going to be a bit more about falls in general, rather than just the form [tool], it seemed to just cover the form [tool], and I thought it was, well, it was just kinda sold to us wrong as staff.
Deputy manager 0107 203
It is manifest in other aspects of the evaluation that the GtACH training did not sufficiently encourage a broad use of the GtACH tool (as, perhaps, was intended). However, it acted as a refresher for experienced staff and provided new knowledge for the less experienced; training encouraged engagement with falls management, if not with the GtACH tool itself.
Staff roles
Where not all staff manage falls (see Table 21), the GtACH programme potentially brings changes to staff roles and responsibilities – falls are no longer the domain of nursing staff or senior staff alone, but become a concern for all. Training might encourage carers to engage in falls management, but this process is more effectively cemented when local staff take ownership of the GtACH programme and support its use. In care home B, where the falls rate decreased, it was nursing staff who acted as advocates for the GtACH programme; in care home C, the falls champion sought to engage all staff in its use:
. . . because the carers care for the people, and they know them more than what we probably do, and what their daily living is, that’s why we’re getting involved with the carers with this as well . . .
Falls champion 0402 0602
When (less experienced) staff indicated that they might become engaged with falls, they often made a distinction between providing care and completing paperwork:
. . . we want to provide practical care and support, etc., and, unfortunately, it’s like in the hospitals, there’s more and more going in, on to, you know, the computer, on to paper, and it’s time-consuming, it does take you away from looking after the ladies and gentlemen.
Carer 533 0107
Anxiety about completing paperwork was communicated in all care homes and many carers felt that completing formal records was beyond their level of qualification and experience:
I think is better for someone who is more . . . higher from me. I am not confident with fill this everything. I think is better job for them, and I think, because, exactly, they have better contact with GP, doctors, everything. They know more better about like, some forms, documents, I mean.
Carer focus group 0803
The introduction of the GtACH programme challenges care home staff to review their roles and responsibilities with regard to residents’ falls. With the provision of training and the support of peers (programme theory 2), such changes seem acceptable to care staff; however, this acceptance does not extend to incorporating completing paperwork into their roles.
The significance of residents with dementia
The presence (or absence) of residents with dementia would seem significant in the implementation of the GtACH programme: care homes A (with a high proportion of residents with dementia) and D (where only those residents with dementia were recruited) demonstrated the greatest increase in falls rate; and care home E (where no residents were registered with dementia) showed the greatest reduction in falls rate. The GtACH tool was not adopted in any of these settings, but for quite different reasons.
In care homes A, D and C (with residents who have dementia and/or were visually impaired), falls were considered an inevitable consequence of residents’ health. Implementing the GtACH programme for residents with dementia could not change this underlying factor and was thus considered to be of little value with these residents:
. . . it’s silly questions to me, because I know the gentleman has got, probably, the end journey of dementia, he’s not going to be able to tell us, you know. He knows, if he gets up, he’s not aware of what’s around him, you know, and you’re asking me these questions where I’m thinking, oh my God, you know, you lot, you know, people, whatever, you know, I know him that well, he doesn’t acknowledge what time of day it is, what’s around him or anything, you’re asking me these quest– it just doesn’t help.
Carer 0402 518
This directly challenges the notion that falls risks can be mitigated and managed by appropriate actions, as proposed in programme theory 1. By contrast, the circumstances in home E led staff to make a similar assessment, but in this case it was because no resident displayed a constant and significant risk of falling:
. . . even though we’re relatively small as care homes go, we do have quite a lot of able-bodied residents, at least half or so, with capacity, so they make their own decisions around their own risks. And it’s something that we’re very keen on here, that we don’t restrain anybody with moving around the home with freedom. So we do have quite a lot of falls, we have periods where we’ll have, you know, one or two people that, for whatever reason, do have a number of falls in a short space of time . . .
Care home manager 0209 104
Although residents fell in this home, the more independent and self-caring nature of the residents meant that risks were perceived differently:
[Residents] take their own risks, and it’s something that, that we train the staff to, to support residents to explain what the risks are, but actually then make them, allow them to make that decision.
Care home manager 0209 104
Both the presence and absence of residents with dementia undermined the perceived utility of the GtACH tool; in one setting, residents were beyond assistance, while in the other they did not require assistance.
Care home ownership and operation
All bar one of the care homes were part of broader organisations: care homes A and D were part of large, national care home groups, homes B and F were part of smaller, regional groups and care home C was operated by a national charity; only care home E was independently operated. External management potentially inhibits the freedom with which a home might adapt its local practice, and/or might place restrictions on what might be changed so as to maintain consistency across a number of care settings:
As an organisation, across the four homes, because there’s four homes, if we want to change anything or do anything, we have to do it as an organisation. So it would not be sort of, if you like, correct for us to suddenly stop using what we already use, and to take on board a different tool, unless we could get that tool approved for the rest of the organisation, particularly around falls and falls prevention.
Care home manager 0402
Of more immediate impact within this study was the requirement for homes to continue using systems and paperwork; the GtACH programme might be used alongside, but not instead of, existing systems and processes. With internal processes being a requirement for all staff, their motivation to utilise the GtACH programme was somewhat diminished by the sense that it would duplicate their efforts and double their workload:
I feel like, well, first of all, we have to fill out the accident form, the legal one. Then we’ve got our own that we have to fill out. Then we have to go on to the system, and update all the care plans due to the accident, we have to write all what, everything that I’ve wrote on this, I put on the system anyway, so I just feel like I’m duplicating myself all the time.
Senior carer 0402
I know that the seniors think that it’s a lot, it’s a lot of, sort of, it’s, if they were doing one or the other, if they were doing, I think they wouldn’t mind doing it, but because they’re having to fill two lots of documentation in at the moment, they sort of do pull a face and say ‘Oh, I’ve got another one to fill in, another, more document, paperwork’.
Manager 0302
The implementation of the GtACH programme is premised on programme theories that make sense in relation to individual care settings (programme theory 1, identify and act on falls risks; programme theory 2, support staff in this), but which might be inhibited by the organisation of broader management systems.
Discussion
Key points
Several general points might be made about the care home experiences of the GtACH programme:
-
The impact of the GtACH programme might vary in different settings. In our evaluation, the falls rates decreased in two homes, stayed stable in two homes and increased in two homes.
-
It is pertinent to reflect that (1) awareness of the intervention, (2) taking part in training, (3) completing the tool and (4) taking action to reduce falls might be distinct activities that are not mutually dependent. A commitment to falls management and fidelity in training might have a positive impact on falls rates without the GtACH tool being widely used in a care home, as was evident in some settings here.
-
Different aspects of the programme sparked different mechanisms. Training was viewed as a refresher by some, empowering others and broadening engagement with falls management. The tool was viewed with indifference, considered a duplication of local systems and was a source of anxiety for some. In some homes, local champions encouraged innovation in practice, while in others external management inhibited local ownership of change and were a barrier to long-term integration.
-
Despite these variations, the initial programme theories still have broad application. Falls would seem best managed when specific systematic strategies are aligned with specified risks. However, there was a strong feeling that falls are viewed as unavoidable in people with dementia and efforts to manage these falls are considered pointless.
-
The evaluation also demonstrates the value of specialist and peer support to care home staff – this may not take the form of a formal falls champion, but might be more informally managed.
Interpreting the results
The final element in our realist evaluation is to utilise normalisation process theory (NPT)75,76 to reflect on our findings. NPT is a mid-range sociological theory that supports understanding of how innovation becomes normalised in everyday routines in practice. For the GtACH programme to become part of everyday practice, it needs to be understood by stakeholders (coherence) and valued by them (cognitive participation); individuals should be able to enact the work associated with the GtACH programme (collective action) and the outcome of the GtACH programme should be clear and observable (reflexive monitoring). Failure to achieve any one of these building blocks is a barrier to the GtACH programme becoming part of normal practice.
Coherence
A recurrent observation was that staff (and some managers) found it difficult to differentiate the GtACH programme from already existing falls management initiatives (evident in demi-regularities, see The relevance of prior practice and The relevance of training). GtACH training was identified as a refresher for previously undertaken training, or was viewed as disappointing and too limited in its focus on the GtACH programme (rather than on falls generically). The GtACH tool was viewed in some places as an unwelcome duplication of existing paperwork. It is important to recognise this in the future implementation of the GtACH programme.
It is notable that in care home E, where no previous external training had been received, GtACH training was recognised as novel and as improving local understanding; this setting witnessed the largest improvement in falls rate of the included homes.
In care home C, the GtACH tool was identified as more detailed than local documentation; here, despite a population at a high risk of falling, the increase in falls rate was more marginal and was better than in the control arm of the study.
Communicating the value and distinctiveness of the GtACH programme is, perhaps, an important element of any future implementation; distinguishing it from more routine falls assessment will help stakeholders to more readily accept it into local practice. Communicating that the GtACH programme is a more appropriate form of provision might also support adoption, and mirrors strategies identified in the management of health care in care homes. 24
Cognitive participation
We have noted above that dementia is an important contextual feature that might challenge the underlying legitimacy of the GtACH programme (see also The significance of residents with dementia). This is most explicit in care home D where (evening) falls were directly attributed to dementia and in care home C, where carers questioned the value of using the GtACH tool with residents who had dementia. In care home E, it was the absence of dementia that challenged the value of the GtACH programme.
The pertinence of dementia-specialist services has been identified in other realist care home research66 and highlighting the GtACH programme’s place in this research might aid stakeholders in recognising its appropriateness for all care home residents.
A second area in which the legitimacy of the GtACH tools has been commonly challenged is that some staff consider forms to be a distraction from the act of caring for residents or view forms to be outside their job role [see Results, Recurring patterns (demi-regularities), Job roles]. To this we might add staff who lack confidence in completing paperwork. This was manifest across all homes and perhaps suggests that a simpler form of paperwork is required for the future implementation of the GtACH programme, or that different types of the GtACH tool are required for different levels of care staff.
Collective action
The persistence of local, organisational falls management systems after the introduction of the GtACH programme was perhaps the single most significant barrier to the GtACH programme becoming normalised in those homes. Introducing the GtACH programme alongside existing systems and paperwork (see Care home ownership and operation) undermined the contextual integration of the GtACH tool into the actual work undertaken by care home staff. Staff faced the unenviable dilemma of either duplicating their efforts (for ostensibly the same ends) or ignoring one system.
We have noted above that the distinctiveness of the GtACH programme should be stressed to help establish it. For future implementation, GtACH may have greater influence where it is adopted as a single, coherent falls management system rather than alongside other falls management approaches. Recognising the appropriateness of the GtACH approach will help staff to adopt it as normal practice. 24
Although the GtACH tool did not integrate well into the work undertaken by staff, this does not mean that the knowledge gained and the increased awareness of falls made no practical difference. Such differences were most clearly manifest in care home B (where the falls rate decreased) and care home C (where the rate of falls increased at a slower rate than in the control group). In both locations, local individuals championed the GtACH programme and encouraged all staff to change their practice (see Staff roles). The role of the falls champion and other informal advocates of the GtACH programme should be stressed in future implementation; these individuals have a critical role in translating the GtACH programme into workable local practice and in supporting other staff to adapt to new ways of working. 24
Reflexive monitoring
Changes in management in care homes A, B and F undermined the coherence of the implementation of the GtACH programme either in part (a reluctance to use the tool) or totally (not wishing to introduce new things at a time of managerial uncertainty). Without the commitment of and, importantly, monitoring by senior management, the delivery and impact of the GtACH programme is uncertain; changes to practice are not rewarded and benefits of new approaches are not recorded. Without locally observed evidence of the GtACH programme positively contributing to residents’ well-being, it is difficult for it to become established. The success of the future implementation of the GtACH programme rests not only in integrating new ways of working, but in the effective monitoring of its impact.
Of more general concern is the role of external management systems in governing local practice (see Care home ownership and operation). With the exception of care home E (where the most improvement was manifest), all other homes had some form of external management system that might impose its own incentives, targets and sanctions. 24 For the GtACH programme to become normalised, it needs to sit within these wider systems and demonstrate value to the broader corporate group. Future implementation needs to consider how the GtACH programme maps to broader organisational priorities and stress how it serves these metrics.
Process evaluation reflections: strengths and limitations
This process evaluation offers a detailed, contextualised understanding of how the GtACH programme was delivered and illuminates the experiences and opinions of the involved stakeholders. Using different methods, it complements the clinical and economic data reported in Chapters 3 and 4 and provides a framework for others to interpret these findings. It offers more textured results than the overarching RCT and provides insight about how best to implement the GtACH programme in the future, supporting stakeholders in considering which elements of the GtACH programme might work best in their setting, what adaptations might be required, and which elements might be ignored. In this way, it demonstrates the value of a process evaluation aligned with a RCT in the trajectory of developing and evaluating complex interventions. 25
In line with more recent recommendations,25 a theorised approach to evaluation is taken here, with initial programme theories being a focus for testing and sampling decisions. The adoption of a realist approach provides a distinctive flavour in this: highlighting contextual variation in how the GtACH programme might work and recognising that subjective responses can be as important as objective change in the delivery of the GtACH programme. This evaluation demonstrates a pragmatic application of the different stages of a realist evaluation: initial programme theories, sampling to test theories, revised theories (and emergent mechanisms), recurrent patterns in CMO configurations, and mid-range theories to explore these patterns.
The evaluation was delivered by a multidisciplinary team and was managed independently of the main study. This was to ensure that care home allocation remained blinded and was not revealed to other parts of the FinCH trial,31 and to ensure that early insight did not lead to any change in practice in either the control or intervention arms of the trial. 77 Each home was visited by multiple researchers on multiple occasions and all data were reviewed by at least two members of the team. Researchers were flexible and responsive to the needs of the care home, accepting that caring responsibilities were more important than our research. Other practical challenges might be taken as limitations: few homes had private space where interviews or focus groups could take place; in some homes, staff could participate when on their break or off-shift only; in some, management governed which staff participated; and research visits and research activities were sometimes cancelled or curtailed at short notice because of staffing and/or resident issues in the homes. 78
We should also acknowledge some limitations with the realist method as applied here. 77 Resources allowed us to evaluate in one care home at a time only, recruiting sequentially, and this affected our purposive sampling strategy. Rather than being able to recruit from a broad population (the 39 care homes randomised to the GtACH arm) at any one point in time, a more restricted choice was possible: those recently recruited to the trial, who were randomised to the GtACH arm but had not yet received training and who were willing to participate in the process evaluation. The window of recruitment to GtACH training made local investigation of, for example, prior falls history or staff knowledge (which might have productively directed our purposive sampling) impossible. Consequently, our sampling used simpler and more restricted characteristics (size, ownership, nursing provision, etc.) to govern where we tested the programme theories.
Difficulties accessing outcome data also had an impact on the realist evaluation. Seeking data for specific homes risked identifying to the trial team that these homes were receiving the GtACH programme. Consequently, hard outcome data were not used in the CMO configurations; rather, these incorporated softer, process concerns for fidelity, acceptability and evidence of use. In addition, primary outcome data (falls incidence in days 90–181 post randomisation, adjusted for hospitalisations) were not available until all trial data collection had been completed; consequently, the process evaluation pragmatically used unadjusted data in reviewing care home outcomes. Both of these barriers had an impact on the completeness of data available to the process evaluation when finalising CMO configurations, prioritising mechanisms and in sampling care homes for inclusion.
Despite these issues, this evaluation demonstrates the potential of a realist approach and contributes to recent debates about the integration of realist evaluation into RCTs. 68,79–81 To be explicit, for the realist evaluation to sit within the FinCH RCT, some methodological compromises were necessary, most notably (although not exclusively) in sampling and access to outcome data. However, these compromises do not undermine the insight generated here. 77 A realist approach uncovered those contexts where the GtACH programme had the most impact, and identified the reasons and responses to the GtACH programme that make it successful; a realist approach has extended our understanding beyond that which would have been possible with a trial alone.
Chapter 6 Patient and public involvement
Introduction
Patient and public involvement was embedded in the FinCH trial to enhance the design, conduct and dissemination of the trial, and future implementation findings. The PPI team was instrumental in securing funding, influencing the trial set-up and advocating for care home residents throughout the trial. An adaptation of the research cycle (see Appendix 9, Figure 17) was examined to plan PPI involvement in each stage of the study, as advocated by INVOLVE. 82 The aim was to ensure that the trial was relevant to care home residents and care home interested parties, as well as to the public. The GRIPP2 (Guidance for Reporting Involvement of Patients and the Public, revised version) short form83 framework was used to ensure consistency. This approach captures the unique perspective of patients and the public experience at each stage of the research cycle. This co-designed model with people skilled and willing to enhance the FinCH trial had a PPI budget of £21,252.00. This chapter first describes and then explores the acceptability and appropriateness of the model.
Methods
Description of the hub-and-spoke approach
A hub-and-spoke organisational approach was used,84 in which there is a central anchor (hub) and spokes located at trial sites (Figure 16). The success of this approach is evidenced in health-care practice and services. 85,86
FIGURE 16.
Hub-and-spoke approach. Orange indicates subgroup.
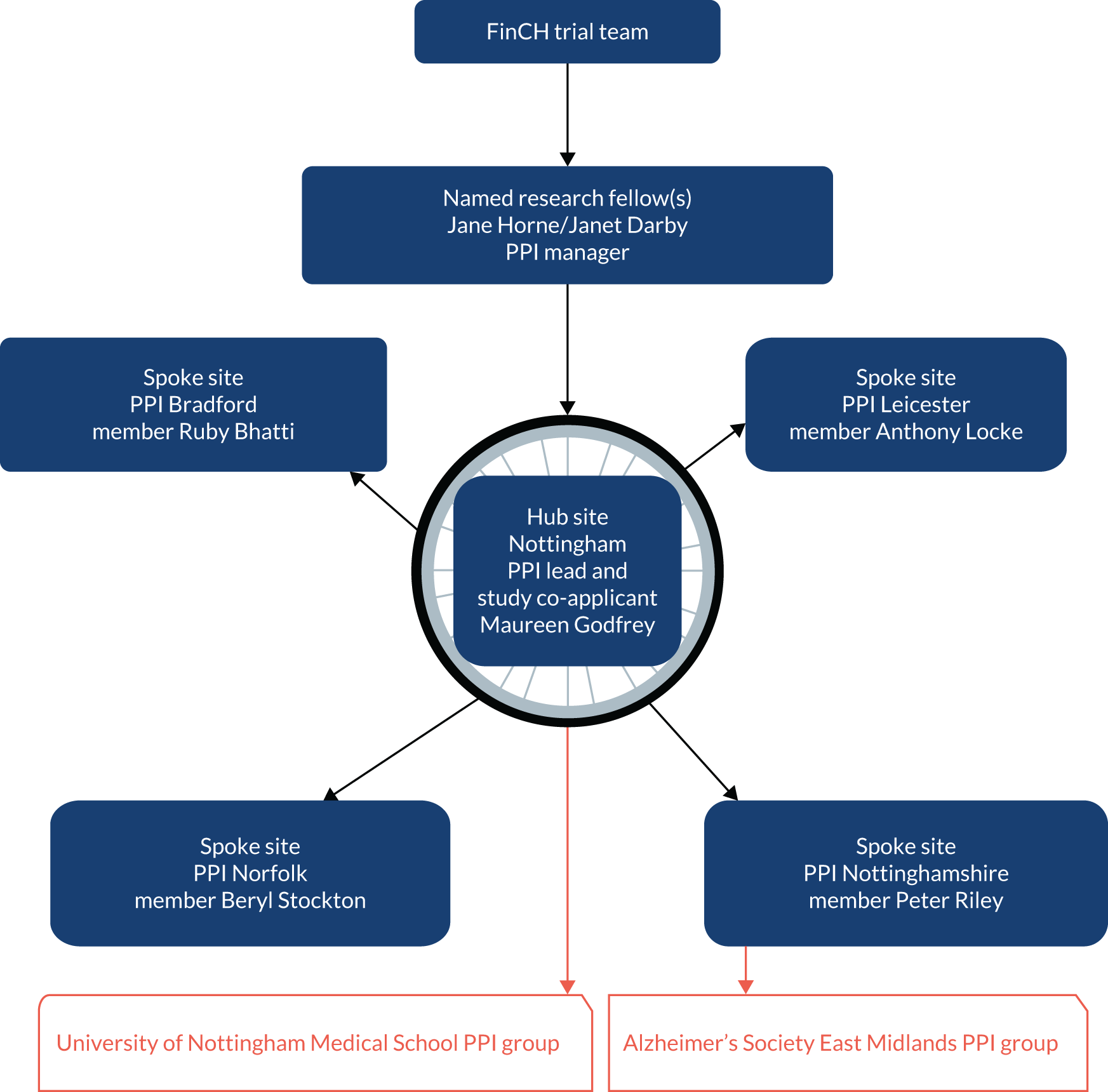
Hub role in the FinCH trial
A job description focusing on this leadership role was produced and research networks were approached to identify interested people with the appropriate skills and training. A PPI member who had previous experience of being a carer for a care home resident and experience of a range of research projects, including RCTs, expressed an interest in the hub role. They were appropriately trained and had leadership experience. They contributed to the monthly TMG meetings to oversee the conduct of the study from a PPI perspective and to represent the broader PPI team. This role was supported and managed by a named PPI researcher. Specific support for the role included communication throughout the trial by e-mail, by telephone, at one-one meetings (both formal and informal), and through open-door access to the wider trial team (including the chief investigator and a trial administrator who provided administrative support, including the processing of expenses) to foster professional relationships.
Spoke role in the FinCH trial
The research team recruited ‘spoke’ members. The PPI researcher asked the FinCH trial networks and PIs to identify individuals who were willing to contribute their local trial perspectives for a minimum of 1 hour bimonthly. The PPI lead worked with the PPI researcher to recruit four spoke members with a range of experience. The majority had worked on assessing grant proposals and had PPI roles on research committees. A retired care home manager and nurse (female); a retired medic with experience in a caring role (male); a carer who had previous experience of caring for a partner with dementia (male); and a patient research ambassador (female) who was a lay chairperson for NIHR joined the FinCH trial team. The PPI researcher completed a telephone interview with these people and appropriate training was provided in line with the national standards for public involvement. 87
Patient and public attendance at FinCH trial meetings
Annual investigators’ meetings were organised as a communication mechanism to engage all trial team members, including the PPI team, by providing an opportunity to interact and connect with the national trial research team, keynote speakers and invited guests. These events included research methods training on topics such as gaining consent and writing an abstract.
Monitoring the GtACH programme’s delivery
Two members of the PPI team observed the GtACH training in care homes and fed back their perspectives to the process evaluation team in the form of written and vocal reports, and to the trial team through the TMG.
Undertaking data analysis for the process evaluation
The PPI team participated in the qualitative data analysis with the trial process evaluation team. The PPI members were asked for their perspectives on emerging themes to broaden data validation. These perspectives were then embedded in the process evaluation findings. The lead PPI member received training organised at the University of Nottingham (Nottingham, UK). Spoke members received training materials by e-mail/post and received support from a qualitative research expert (JD) by telephone and e-mail. All five members contributed to the data analysis.
Patient and public contribution to dissemination of results
The PPI team attended the investigators’ meeting to reflect on the results and celebrate successful completion, and supported workshops on dissemination and ways to add impact. The PPI team contributed to resources to ensure that plain, accessible language was used when the key findings were relayed to forums, for example at ENRICH, a research-ready care home network. The Plain English summary for the final report was written by the hub lead and this chapter was co-written with the PPI members.
Evaluation of the hub-and-spoke approach
We evaluated the hub-and-spoke approach to understand the PPI members’ everyday experiences of participating in the trial, making sense of any surprising experiences that arose during the main trial and the process evaluation component.
Data collection
Participants took part in a 1-hour focus group in August 2019. A prompt sheet was devised to explore the barriers to and facilitators of the hub-and-spoke approach. Those participants who were unable to dial in were offered a one-to-one telephone interview or to provide written responses using the same prompt sheet. Responses were recorded digitally and transcribed verbatim. Postal responses were collated and filed with the transcription data prior to analysis.
Data analysis
Data analysis was conducted using a framework analysis method. 88 The focus group (n = 2), one-to-one interview data (n = 1) and postal (n = 2) data were examined by two experienced qualitative researchers. A process of familiarisation occurred, notes on the transcripts and a simple indexing method was independently conducted. A thematic framework was constructed and two researchers met to discuss these initial findings.
The next stage resulted in indexing and amalgamating the researchers’ frameworks, ideas and differences into one agreed index, and data from the transcripts was applied. This ensured that patterns in the data could be easily identified and any differences and gaps noted. The data were summarised using the language of the PPI members. Mapping and interpretation were initially undertaken by the qualitative researchers. These draft themes were then sent out to participants for comment, views, perceptions and interpretations of the final themes. All five PPI team members participated in the evaluation.
Findings and emergent themes
The five principal themes
Initially, 12 themes and 50 subthemes were generated from the data. The final mapping and interpretation of the data identified five principal themes:
-
team cohesion, communication and engagement
-
confidence and personal development
-
support and training
-
motivation, commitment and responsibility
-
identified needs.
Theme 1: team cohesion, communication and engagement
Participants referred to the process of forming and developing the PPI team. Initial anxieties revealed uncertainties about whether or not the hub-and-spoke approach could work, given that members were in different locations:
I value the diversity and depth of experience from my peer lay members. Embedding their experience at every stage of the study is strengthened by their practical commitment and involvement.
P1
I was looked after, but I was definitely on my own. Geographically, I was away from the action and unable to connect to the wider research team.
P4
However, the benefit of telephone conferences, regular communication by group e-mail and individual e-mail, and postal lay summaries of the trial monthly management minutes appeared to facilitate team cohesion and their performance as a group. The descriptive data suggest that, regardless of some initial anxieties, participants perceived the teleconferences to be an effective mechanism for communication. One idea that emerged from the data was to rotate the teleconference around the sites, making sites feel more included and, potentially, enhancing the spoke member role through them chairing the teleconferences:
. . . it has felt very rewarding. And it feels like a new model we are trying, and there is always something nice about trying something new, isn’t there?
P1
Specific tasks that stimulated me and made me feel even more involved was the process evaluation [data analysis]. I particularly liked that because I think as a team we also contributed. So that gave me a sense of team membership.
P2
One spoke member chose not to engage in the telephone conferences, as the telephone was not their preferred method of communication. This was established early in the trial and alternatives, such as posting an accessible agenda for comment prior to the telephone conference and posting minutes after the conference for information and comment, facilitated inclusion:
Even though based in a different part of the country, felt involved, informed via e-mail and the telephone conference provided a connection to the team.
P5
. . . your university can get PPI members not just in Nottingham or where you are, you can get PPI members from all over the country or all around the world and we can do this and it works.
P5
Theme 2: confidence and personal development
Participants described the positive reinforcement from the hub lead, their peers and the research team; feeling that they were heard and being listened to improved their confidence as PPI members:
. . . and in the telephone conference [pause] because I may not have got the right end of the stick about something. But there is a kindly tolerance and indulgence of someone not quite comprehending [laughs] what was written or said. But there was an openness which allowed me to make a contribution.
P2
There was an acknowledgement that members had differing levels of confidence that depended on their role. The data indicated a strong self-belief in their expertise when contributing to areas in which they felt that they had previous experience:
I feel valued because I could contribute from a hands-on perspective. My strengths were ‘I have been there and done that’.
P4
Although all PPI members offered to analyse the process evaluation data, they required training, which then helped with improving confidence and led to further personal development:
It [the training] was two days and I think what was so useful about it was that I just like to learn from other people and feel more confident when I am looking at a piece of work, you know? I was able to undertake valuable qualitative research training and have used these skills in FinCH and other studies that I am now working on.
P1
Theme 3: support and training
The data highlighted that effective leadership and support from the PPI researcher were crucial to the work of the PPI team:
Throughout I felt valued and supported by everyone in the study.
P1
So what I am really saying is there is a place for people to be involved who have minimal or limited experience. There was appropriate training.
P2
Patient and public involvement members identified that they would have liked more background information on the topic, and one participant felt that the training was not adequate enough to allow them to confidently complete the qualitative data analysis, but that telephone support filled this gap:
It wasn’t the training for me [that was most helpful], it could have been for other people. If you wanted more PPI [member]s to be involved that have not done it before [analysed qualitative data] than someone like myself who has, where you could have done with a bit more support. For me personally, the benefit that I had, was to speak to the team [qualitative researcher in the team who delivered the telephone training].
P5
Theme 4: motivation, commitment and responsibility
The PPI team members felt that their past experiences were crucial to the role. However, while there was merit in their experience, the data suggest that PPI members without such experience could be equally committed and valuable to a study:
It is quite important to involve people who do not have a great deal of direct understanding, so you achieve two things. You get a better mix of public and patient involvement, and you have people who will ask what seem to be naive questions, but they are the kind of questions that the engrossed researcher may not have thought to ask.
P2
The opportunity to conduct data analysis and observe the care home training appeared to be key activities that were particularly meaningful and motivational to PPI members. These activities enhanced their roles and developed their research knowledge, enabling these skills to be used in other studies:
Because we weren’t just talking or sat on the outside of the project. Doing that [data analysis] was very useful. Because we were able to understand it better and get involved in the data side and give the input that could help.
P5
When participants did not engage in these activities, they expressed regret, said that they had more to give (particularly regarding the care home training) and described how they were keen to get involved with future studies. Members described observing the care home training as hugely motivational for them. Although all members volunteered to engage in this activity, timing was described as an issue. Expanding the observation role to include observation of the intervention, in addition to the training, was a common suggestion from PPI members:
I was disappointed only to do one care home visit.
P4
If this project came up, or an extension of this project, I would be definitely wanting to put myself forward. Without hesitation, I would get involved.
P5
I could have added more value there for you [by visiting a care home] probably missed out because I couldn’t get there . . . I think [name of geographical area] has an interesting diversity, so it would have been an interesting insight into staff, staff members who were new to English, you know, a different angle to it, which I feel was missed out.
P5
Observation in the care homes to see the implementation of the tool might be one omission, but ethically the participant’s privacy must be respected.
P1
Theme 5: identified needs
Leadership and direction as the trial progressed were considered key in terms of keeping the PPI team informed and engaged. A clear understanding of the PPI roles and responsibilities throughout the research cycle was one area that the PPI team felt could have been improved. Although they recognised that this is difficult, they felt that the stages of the research cycle used by the main trial could have been reinforced to the PPI team:
I think you have followed that cycle . . . it would be nice if we [the PPI team] touched base with that cycle as well. It would have been really good, because you could go back over ‘we’re on this part, we’re here on the cycle’ in our telephone conferences as well, a bit more . . .
P5
The need to be flexible throughout the trial was voiced, ensuring that members had time to plan their research activity around their often busy lives. The idea that PPI was becoming increasingly complex was also expressed. The need for improved induction training or an improved pack was evident in the data, especially when members joined the team after the study had started:
Increasingly, as I get involved in the studies, they become more and more complex. The role that is asked of PPI is expanding, the process analysis [data analysis] is an example. But I really feel strongly, if you are out of your depth it’s best to actually say you are out of your depth.
P1
Although finance and budgets were not explicitly highlighted in the data, there were a few covert references to these, such as an acknowledgement of the issue of limited funding in general for PPI. An understanding that intensive training (in qualitative analysis) was costly emerged from the data, and there was an acceptance from lay representatives that such funds were limited.
Impact and influences on the trial
Effective two-way communication was established, ensuring that the five PPI members contributed their diverse knowledge by attending and contributing to 10 telephone conferences. This resulted in 20 written lay statements of the monthly trial management meetings being distributed to improve the accessibility of information. There was also independent representation at an international PPI conference and the presentation of a poster at a scientific conference. 89
The participant-facing materials were improved with the PPI team’s suggestion of pictures and accessible wording. The PPI team were instrumental in helping the research team to secure additional funding, in helping with our funding monitoring committee and in adapting our recruitment methods. The PPI team suggested that care home managers were best placed to understand their residents’ wishes in terms of participating in this research, which resulted in a 3% increase in recruitment per care home.
Independent perspectives when observing the intervention in four care homes, in addition to analysing the qualitative data, was undertaken by four out of the five PPI members and enriched the qualitative findings.
Discussion
Main findings
In the evaluation, the PPI members described their positive experiences of the hub-and-spoke approach. The data suggested that this was an acceptable and workable approach to employ in future trials. There is evidence that hub-and-spoke approaches have been used successfully in other areas of health-care service and delivery research. 84
The enhanced role offered to PPI members, to view the development of thematic data in the qualitative analysis and offer their perspective, was not only valued but embraced by all PPI members. The volition to participate and the enthusiasm of all PPI members to undertake this role was a surprising finding to the research team, who had assumed that not all of the PPI members would want to participate. It was reported that the quality of the PPI members’ comments added a valued perspective. There is substantial evidence to suggest that PPI has an impact on improving retention and recruitment in trials, but there is limited reporting on the impact of PPI in the later stages of trials, with few studies involving older people in data collection and analysis. 90
Contributing to the data analysis and observing the intervention training resulted in issues being identified that were pertinent to care home staff and residents, but that the trial team had not thought about, which is the purpose of PPI. These contributions have the potential to add to the impact of the trial, in addition to adding value to the PPI role.
Although training was given to PPI members, not all PPI members attended face-to-face training as there was a limited budget for this. It is recommended that future trials include such training in the PPI budget at the proposal stage, to build knowledge, skills and confidence.
Participants also valued the opportunity for enhanced roles, for example, willingly volunteering for roles that required them to observe the intervention in a care home. Finding suitable dates, often at short notice, proved challenging in some sites. In one case, the observation did not happen, despite trying very hard to facilitate a date that worked for the trainer, the care home manager/staff and the PPI member. Future trials should plan research activities well in advance to enable wider participation.
The research cycle was used throughout the trial and guided the trial team in ensuring that the co-production was evident at each stage of the trial. As a result, the voices of care home residents and their families were represented throughout the trial. Our approach enabled an integration of PPI views and ideas throughout the study that challenged thinking and, therefore, raised ethics standards. 91
Job descriptions were used to establish role expectations. This process appeared formal when recruiting experts who were volunteering their time willingly. However, the process helped to establish clear expectations, as it is argued that PPI works well when goals are clear. 92 In addition, it also gave both the hub and the spoke members a baseline to work with, which ensured clarity from the outset. However, the trial was not able to recruit a PPI member in every site. This questions whether or not there is a pool of adequately skilled personnel with lived experiences willing to undertake this role. There is evidence in the literature to suggest that recruiting these experts by experience is a challenging process93 and there are not always people willing to take up these roles.
The regular PPI telephone conferences provided a means of supporting both experienced and less experienced members, and appeared to be the mechanism that ensured team cohesion, coupled with the opportunity to meet face to face. Telephone conferences were evaluated as being cost-effective, able to generate good relationships and able to provide an opportunity for regular member engagement. The ‘forming’ and ‘storming’ stages of establishing a group were evident in the initial telephone conferences prior to entering the ‘norm’ and ‘perform’ stages of Tuckman’s small group development model. 94 Tuckman describes these as ‘necessary stages’ as a group matures. 94 The inclusion of an induction pack and being sensitive to people’s potential anxiety at the start of a project could guide PPI members through these initial stages of group formation.
Cost was a factor in our decision to implement a hub-and-spoke approach. By operating a network approach, linking members from various sites to the hub by telephone conferences and e-mail, ensured that travel costs for attending training and the FinCH trial meetings were kept to a minimum. Members reported that they felt that they had sufficient information to keep them involved and active without high travel costs and cost to the environment.
Once the telephone conferences were established, there was commitment and buy-in from PPI members. When time enabled them to participate, they dialled in, contributed to the agenda items, followed up with ideas, and demonstrated a respect and humour evident in the content of the calls. They described having a voice, being able to talk and be heard. They appeared to value this respect, evidenced by feeling that their suggestions were discussed and actioned by the research team.
Critical reflection
Using The UK Standards for Public Involvement in Research as a framework,82 we present what we did, what we might do differently and what other researchers might learn from our experience.
Inclusive opportunities
We involved key PPI members from the very early stages of the project’s development and design, and the lead PPI member was a co-applicant on the grant. Some PPI members were recruited later. These members were provided with information and a job description; an induction pack may have helped to clarify the role and relieve any initial anxieties about their role in the study.
Working together
The investigators’ annual meetings included targeted PPI sessions and quality external training. A planned budget meant that transport could be provided and PPI members were encouraged to attend, resulting in members feeling that they were a cohesive group that were listened to and valued. In future studies, we would increase our efforts to encourage every member to attend as it facilitated integrated working.
Support and learning
We sought PPI perspectives on emerging themes in the qualitative data after analysis had been conducted in the first three homes. Face-to-face data analysis training was not provided for all members because of limited budgets. We would aim to provide this training in future trials to enhance the PPI role in undertaking data analysis. There was an appetite to engage in this activity; however, training would be essential.
Communication
The regular telephone conferences ensured that all members were included and communicated with at each stage of the trial. A two-way open communication mechanism was a useful engagement tool. For members who were not comfortable with technology, alternatives such as post and paper were used. This ensured that they were not excluded. Future trials might include similar mechanisms or other digital platforms such as Zoom (Zoom Video Communications, San Jose, CA, USA) or Microsoft Teams (Microsoft Corporation, Redmond, WA, USA) for this purpose.
Impact
A collaborative poster (research team/PPI) illustrating the hub-and-spoke approach and how this worked in a RCT won the best Allied Health Professional prize at the 2016 British Geriatric Society spring conference. 95
Governance
The lead hub PPI member attended the ethics committee meeting at the beginning of the project, demonstrating that both lay and academic perspectives were being presented. The lead hub PPI member contributed to the funders’ oversight and monitoring meetings. We would recommend adopting this approach as it demonstrates leadership, accountability and shared responsibility.
Chapter 7 Discussion
What this trial shows
Care homes in which the GtACH programme was implemented experienced significantly lower falls rates than care homes in which the programme was not used. The primary RCT outcome result showed an unadjusted incidence rate ratio (IRR) of 0.57 (95% CI 0.45 to 0.71; p < 0.01) in favour of the GtACH programme. The falls rate over this period was 6.0 per 1000 residents in the GtACH arm and 10.4 per 1000 residents in the control arm. This translates to a falls rate per participant per year of 2.2 for the GtACH arm and 3.8 for the control arm. The secondary RCT results saw a significantly lower falls rate in the GtACH arm for the 1- to 3-month period, but not in the 7- to 9-month or 10- to 12-month periods. There were no differences between arms in any of the other secondary outcomes. In the base-case analysis, the mean cost per resident was £3955 in the GtACH arm and £3935 in the control arm, with an adjusted mean difference in cost of £108 (95% CI –£271.06 to £487.58) representing the mean additional cost per resident in the GtACH arm. 96
In the base case, the DEMQOL-based QALYs were 0.578 in the GtACH arm and 0.581 in the control arm, with incremental QALYs of 0.005 (95% CI –0.019 to 0.03). The EQ-5D-5L-based QALYs were 0.266 and 0.232 for the GtACH and control arms, respectively, with incremental QALYs of 0.024 (95% CI 0.004 to 0.044). The incremental cost per DEMQOL-based QALY was £20,889 and the incremental cost per EQ-5D-5L-based QALY was £4544. The base-case incremental cost per fall averted was £191.
The GtACH programme was feasible: 69% of care homes achieved acceptable levels of staff training, with 80% of staff who worked in a caring role being trained. The programme was also likely to be affordable: the mean cost per resident was £88. The process study showed that the GtACH programme was widely accepted by stakeholders. Care home staff valued the way that the GtACH programme helped them address specific falls risks directly, and the emphasis on training and peer support. They also valued the fact that different elements of the GtACH programme could work independently of each other and be of benefit to the care home residents. The improved knowledge acquired through training or the increased awareness of falls gained from taking part in the GtACH programme did not always trigger completion of the GtACH tool. However, action was taken to limit falls risks, which is the likely mechanism for the reduction in falls rates.
Comparison with other studies
Prior to the FinCH trial, there was an indication in the Cochrane care home and falls prevention review19 that multifactorial interventions could prevent falls, but the evidence for effectiveness in care homes was limited and of low quality. FinCH was the largest care home RCT completed to date to evaluate a multifactorial falls prevention intervention and it has added a large number of data to the evidence base (increasing it by over 50%).
The Cochrane review19 recommended further research into falls in care homes, with an emphasis on evaluating an individualised, standardised approach to the delivery of interventions that are adequately described and, therefore, easily replicated or implemented, and on using a mixed-methods approach. The FinCH trial met these recommendations by using a published and standardised falls prevention programme, successfully replicating delivery across 87 care homes (control homes were given the training at the end of the study), as well as measuring effectiveness through a RCT that followed CONSORT guidelines; a process evaluation; an economic evaluation; and resident, care home staff and public collaboration.
In closer comparison to the existing evidence, the Cochrane review19 identified 13 studies that trialled multifactorial interventions in care homes. This included studies ranging from 31 to 682 participants. The FinCH trial was considerably larger, with 1657 recruited participants. The FinCH trial reported the rate of falls in the same way as the Cochrane review19 by measuring the total number of falls per unit of person-time. The studies reported a range of falls rates, from 1.7 falls per person per year to 2.51 falls per person per year. 19 This FinCH trial found slightly higher falls rates, with the GtACH arm having a falls rate of 2.2 falls per participant per year and the control arm a falls rate of 3.8 falls per participant per year. These falls rates were similar to those seen in the FiCH feasibility study,26 and could indicate that residents in care homes are falling more than in previous years, that care home residents in the UK are more likely to fall than those in Canada or the USA (where the Kennedy97 and Rubenstein98 studies were conducted) or that some intrinsic selection factors recruited those residents who were at a greater risk of falls.
To be able to compare the results of the FinCH trial to those of other studies, it is useful to compare the populations on a range of outcomes. Hospital admission rates usually indicate that a person is unwell and, although it is a blunt measure, it can be objectively measured and is needed for health economic evaluations. The mean number of hospital inpatient days for participants in the FinCH trial was 1.8 days per year, which is lower than that reported by Gordon et al. ,7 who found that a cohort of 227 participants had a mean of 2.2 days in hospital. This could indicate that the FinCH trial participants were more stable than those recruited by Gordon et al. 7 or that interventions undertaken across the UK to reduce the number of admissions of care home residents to hospital may be working.
The Barthel Index was used in the FinCH trial to measure participants’ ADL ability and although there was no difference between arms, the mean score of 8 out of 20 points indicates that most of the population could walk around the home with the use of aids, could feed themselves and were continent. Another large care home study of a rehabilitation intervention, the Occupational Therapy in Care Homes Trial (OTCH),99 which recruited 1042 residents with stroke from 227 care homes, found that 70% of the participants recruited were classified as severely limited on the Barthel Index at baseline, with 50% in the very severe (0–4) category. However, OTCH was primarily focused on patients with stroke-related disability, with most of the patient participants immobile and dependent, as less impaired patients with stroke are discharged home. The FinCH trial, meanwhile, focused on residents at risk of falls who were, by definition, ambulant, with this being the likely explanation for the difference between the cohorts recruited for these two large studies.
Strengths and limitations
To our knowledge, this is the largest RCT of a falls prevention intervention in care homes in the UK to date. The study was well powered to detect meaningful differences in falls rates, even when allowing for inflation for the power calculation and adjustment of recruitment targets during the study. We used clinically plausible outcome measures that were likely to be affected by the GtACH programme. We adhered to best practice for resident and public involvement, randomisation, allocation, outcome assessment and analysis. We took overt steps to reduce contamination bias. 31 RAs collecting outcome data were blinded and less than one-third of residents (29%) were accidentally unblinded as the study progressed.
A potential limitation was that the care home staff and participants were not blind to allocation owing to the nature of the intervention.
As with all falls trials, a limitation is the possibility of falls ascertainment bias: care home staff may have been sensitised to falls and have been more likely to record them in the GtACH arm because of the awareness-raising aspect of the GtACH programme. We believe that we minimised this risk by recruiting only those care homes in which there was a well-established falls recording system in place before randomisation. It is not clear if such bias occurred or, if it did, what effect it may have had on our results. We assume that if there was an effect, it would have increased falls reporting in the homes allocated to the GtACH programme and thereby attenuated our observed effect size. For this reason, we believe that our primary study’s positive finding is unlikely to be due to bias.
Another potential limitation was that the two trial arms were not completely balanced at baseline with regards to the mean falls rate. However, as the analysis included adjusting for baseline falls rate, we do not feel that this confounds the results.
The care homes we recruited were generally representative of UK care homes and, hence, our findings are likely to be generalisable within the UK and settings with similarly sized and run care homes. Ten sites in England took part, spread across urban, suburban and rural locations, and 87 out of the 186 homes that we approached took part. However, we acknowledge that the impact of the GtACH programme may differ in other countries and settings, such as nursing homes where medical and therapy staff are on site and regularly contribute to care. In such settings, the GtACH programme may contribute little, but it is likely that some kind of structured and evidence-based approach to falls management will be associated with a reduction in the incidence of falls. Although the GtACH programme was implemented across all residents in an allocated care home, we were not able to recruit all residents in all homes. Residents who lacked the mental capacity to provide consent and for whom no suitable consultee was available were a particularly under-recruited group, as with other care home studies. 100 However, we have no reason to think that the programme would be more or less effective in the residents we were unable to recruit.
A limitation of the GtACH programme itself is that it served as a prompt to care home staff, rather than the wider health-care team. We did not specifically collect data on lying and standing blood pressures, nor did we collate data on medication changes during the study in a way that would have enabled us to understand the effect of the intervention on polypharmacy. There are, however, prompts in the GtACH programme to ensure that care home staff trigger a medical assessment of residents who fell and we did not detect any evidence that staff were unable to follow this advice or trigger such assessment. If such assessment was not triggered, then this represents an opportunity to further improve the treatment effect seen with the GtACH programme and we will focus on this issue in future research studies.
A further issue is that because we did not collect data on lying and standing blood pressures at baseline, our intervention and control arms could have been imbalanced with regard to these risk factors. We hope that randomisation will have dealt with this issue. There was no evidence that the prescription of medications was different between intervention and control arms.
Another strength of this study is that our process evaluation was undertaken by a research team that was independent of the RCT team. This not only helped to illuminate the mechanisms by which the intervention led to the improved outcomes, but also identified where the intervention’s implementation could be improved. The findings of the process evaluation will be valuable in optimising the subsequent adoption of this intervention beyond this trial setting.
We recognise that measuring QoL in care home residents sufficiently for economic analyses is challenging, largely because of the high prevalence of cognitive impairment in this population that makes it difficult for them to complete assessment schedules asking questions about abstract concepts such as QoL. For this reason, we chose two approaches to measuring QALYs: the widely used EQ-5D-5L and the more recently developed DEMQOL. Our estimates of cost utility differed between these two approaches – using the EQ-5D-5L, the GtACH programme was conventionally cost-effective, but using the DEMQOL it was only of borderline cost-effectiveness. When we finalised our analysis plan, we chose the DEMQOL over the EQ-5D-5L, although this decision was finely balanced. Since then, limitations of the DEMQOL have led to the development of a care home-specific version, the DEMQOL-CH (Dementia Quality of Life Care Home version). Although there are limitations of using the EQ-5D-5L in this setting, it has been consistently evaluated better than other QoL measures in care homes. 59 Given the uncertainty of the methods available to assess cost utility in care home residents, while we cannot conclude that the GtACH programme was unequivocally cost-effective, we have presented evidence that suggests it is soundly cost-effective when using the EQ-5D-5L, but of borderline cost-effectiveness when using the (original) DEMQOL. We would argue that decision-makers should not rely solely on one effectiveness or economic statistic to make funding decisions, and that the overall evidence presented here supports a decision to implement the GtACH programme more widely.
Adoption and implementation
Given our findings, we believe that further trials of falls prevention interventions versus usual care in care homes are no longer required or justified, although this and any other trials recently and yet to be completed should be added to existing meta-analyses. We believe that it is now important to put these findings into practice as widely and swiftly as possible so that the GtACH programme, or other programmes derived from it, become part of usual care in care homes. The GtACH programme was extensively developed and delivered before being evaluated in this trial and, hence, is inherently designed to be suitable for implementation. Our process evaluation sheds a light on how it could be implemented even more effectively, for example through greater engagement with care home provider organisations to encourage the adaptation of the GtACH programme and documentation to the systems and processes of different care homes.
There are very few published papers of care home research and implementation. A recent rapid review and consensus study23 around implementation in care homes recommended consistent, regular, fluid conversations across diverse care home settings, paying close attention to understanding when care homes are ready for change and what measures have to be taken to enable them to be ready. An increasing body of evidence suggests that implementation and improvement in care homes must be led by care home staff, with NHS colleagues playing a facilitatory role, with the aim being to support the development of improvement capacity in care homes in parallel with implementation. 23
Stacey et al. 101 looked to determine the use of patient decision aids in clinical practice following RCTs. Only 44% of trial authors indicated some level of subsequent use of patient decision aids following trials. In 2019, Douglas and Affoo102 explored the difficulty of translating evidence-based health-care innovations for clinical practice settings (skilled nursing facilities), and examined the barriers to and facilitators of implementation, finding that the engagement of managers was crucial and time for investment was needed.
A key issue for implementation of the GtACH programme is if the short-term effect on reducing falls rate could be extended to reducing falls rates over a longer term or if the short-term effect of a relatively inexpensive intervention is enough to persuade people to implement the GtACH programme. It is important to consider the fact that the reduction in falls rates was not maintained beyond 6 months after randomisation. The process evaluation findings suggested that the training was beneficial, increasing staff knowledge and awareness of falls risks and providing the skills to reduce these risks, but it also indicated that one component of the programme (i.e. the paper assessment) was not completed as regularly as expected. This appeared to be because of a number of barriers, including care staff not being allowed to write in care records and a lack of ongoing support and training from the GtACH programme trainers. By not reinforcing the learning through physically completing the paper assessment, the longer-term implementation of the GtACH programme may have been lost. Investigating ways to better embed learning into longer-term practice will be an important component of follow-on work.
The study identified a number of contextual features that are pertinent to how the GtACH programme is received and used. Existing falls management processes, prior staff training in falls, strongly demarcated staff roles, external management systems and the high proportion of residents with dementia might have an impact on how the GtACH programme is implemented. Subsequent long-term implementation of the GtACH programme will need to address these issues, but not through a series of external interventions by NHS staff. Rather, in keeping with care home implementation research, they can only be addressed if care home staff see value in doing so. Dissemination materials must look at packaging the important learning from the FinCH trial in a way that is accessible and immediately useable for care home staff, and in a way that the work can be seen to align with their organisation and personal priorities, as well as achieving immediate, recognisable results. Normalisation process theory103 suggests that minimising additional work is essential for implementation. How this is done depends on leadership by the care home sector, as it is care home staff who understand their own organisational processes and routines.
Essentially, if the GtACH programme is to be implemented, the following actions should be considered:
-
The care home community should be supported to lead the implementation process and any implementation research. The GtACH programme had a greater impact when it was championed by local staff (formally or informally).
-
Information aimed at care home owners, managers, staff and residents should highlight the ways in which the benefits of the GtACH programme align with individual and organisational priorities.
-
The context of the care home should be assessed in a sensitive way to understand any barriers to training or use of the GtACH programme. For example, in care homes that were willing to be recruited to the study, care home staff indicated a willingness to take part in the research, attend training, explore quality improvement concepts and assist with data collection. It may be that these homes had a different context to homes that did not agree to take part in the research study. In addition, when no prior training had been received or where training had been solely managed internally, care home staff were more keen to adopt the GtACH programme. When not all care home staff were allowed to write in care records, the GtACH paper assessments were not completed as regularly as in other homes. But, again, the emphasis should be on enabling care home managers and staff to assess and respond to their own organisational contexts.
-
The role for NHS staff would be championship, a long-established approach for work in care homes. NHS falls leads may be well placed to do this, but will be particularly enabled when care home work is recognised and protected in their job plans.
-
The GtACH programme consists of awareness, training, a tool and action; although we acknowledge that its elements may be independently important, it is the combination of the components that makes it a programme. The evidence suggests that the only justifiable conclusion based on the FinCH trial is that the GtACH programme should be delivered as a whole to gain similar results.
-
The strongest impact of the GtACH programme was seen following its introduction, when it remained a novelty and was a focus of attention. Beyond this period, existing systems may re-emerge as the normal way of working. For future implementation, this means that the distinctiveness and benefit of the GtACH programme (beyond standard falls management) need to be better communicated to encourage stronger engagement.
There are some important areas for further research to be conducted in tandem with implementation. The GtACH training increased knowledge and awareness in the short term; however, to maintain knowledge, we know that learning is better established when it is enacted on a regular basis, but we don’t know how to reinforce such activities. The GtACH programme content may need to be provided in different formats, as some homes use electronic records only and some use paper only. Online, electronic or smartphone digital platforms that host the components of the GtACH programme may need to be developed. For future implementation, the role of the GtACH programme’s associated paper resources should be considered – it may be that the GtACH programme can make a difference without all staff completing dedicated paperwork. Alternatively, it could be that revised or simplified versions of the GtACH programme are created for less experienced staff to complete. Given the above points about minimising effort to support implementation, minimising the labour associated with the GtACH programme will be an important consideration. In homes with a high number of residents with dementia, falls were considered inevitable and initiatives such as the GtACH programme were considered ineffectual and not worth implementing; therefore, future implementation requires appropriate adaptation of the GtACH programme for residents with dementia or clearer communication of the pertinence/application of the GtACH programme to residents with dementia. Targeted, co-designed work with care home staff would be a powerful means by which to achieve this.
Lessons learnt
Care homes are keen to take part in research studies: residents were recruited and reliable data were collected. The processes for claiming costs to deliver interventions that are under evaluation and the costs for staff to attend training and help with data collection may have discouraged some homes from taking part. Few care home staff had any research experience. Care home staff learn about research by taking part and this is central to capacity development. Life expectancy of residents in care homes is short, so studies need relevant primary outcomes to be measured in a timely manner. Data from NHS Digital were difficult to obtain, meaning that many participants had died before the results could be analysed.
Conclusions
The intervention showed a significant reduction in falls rate. This was achieved without a reduction in residents’ activity levels or an increase in dependency. The evidence suggests that the GtACH programme was likely to be cost-effective and was accepted by care home staff and residents. The programme should be widely implemented through a programme designed to empower care home staff to lead its implementation. There are a number of important research questions regarding implementation that should be addressed in parallel with this process.
Acknowledgements
We would like to thank all the nursing home managers, staff, residents and informal carers who generously gave their time and participated in the study, without whom this study would not have been possible. We would also like to thank the research teams and CRN staff across all participating sites who worked diligently to deliver the trial at the local level. We would like to thank Wynne Williams (care home manager and co-applicant) who contributed to the grant application and study protocol.
The trial was conducted in collaboration with NCTU, the staff of which supported the design, conduct and analysis of the trial, including data management (Martin Pond, Antony Colles, Cecile Guillard), clinical trial operations and quality assurance (Erika Sims, Veronica Bion), statistics (Allan Clark, Sue Stirling) and health economics (Tracey Sach, Lisa Irvine and Ed Wilson).
Contributions of authors
Philippa A Logan (https://orcid.org/0000-0002-8585-478X) (Professor of Rehabilitation) was the chief investigator from the concept of the study through to lead authorship of the final report.
Jane C Horne (https://orcid.org/0000-0003-0985-8110) (Senior Research Fellow and Occupational Therapist) was the intervention trial manager, providing clinical advice and support to the NHS falls leads delivering the intervention. She also co-trained the NHS falls leads (therapists), managed the PPI members, co-authored Chapter 6 and contributed important intellectual content to the report.
Frances Allen (https://orcid.org/0000-0001-7265-0676) (Research Fellow) jointly analysed qualitative interviews for process evaluation, jointly prepared the qualitative results for publication and contributed intellectual content to the report.
Sarah J Armstrong (https://orcid.org/0000-0002-6789-6169) (Professor of Medical Statistics) oversaw the statistical analysis of the trial, jointly prepared the statistical results for publication, contributed to the development of the trial application and trial protocol, and contributed important intellectual content to the report.
Allan B Clark (https://orcid.org/0000-0003-2965-8941) (Senior Statistician) jointly prepared the statistical plan, analysis and results for publication; advised the oversight committees on statistical procedures and analysis; and contributed important intellectual content to the report.
Simon Conroy (https://orcid.org/0000-0002-4306-6064) (Professor of Geriatric Medicine, Geriatrician) contributed to the grant application and protocol, and contributed important intellectual content to the report.
Janet Darby (https://orcid.org/0000-0002-6702-6956) (Research Fellow) jointly conducted the qualitative interviews and analysis for the process evaluation; jointly prepared the qualitative results for publication; and contributed to the grant application and trial protocol, and contributed intellectual content to the report.
Chris Fox (https://orcid.org/0000-0001-9480-5704) (Professor of Clinical Psychiatry) contributed to the study design and interpretation of data, and contributed intellectual content to the final report.
John RF Gladman (https://orcid.org/0000-0002-8506-7786) (Professor of the Medicine of Older People) contributed to the grant application and protocol, and contributed important intellectual content to the report.
Maureen Godfrey (https://orcid.org/0000-0001-8081-2188) (Patient and Public Representative) contributed to the grant application and protocol, observed the intervention, contributed to the qualitative data analysis, co-authored Chapter 6 and wrote the Plain English summary for the final report.
Adam L Gordon (https://orcid.org/0000-0003-1676-9853) (Professor of the Care of Older People) contributed to the study protocol and contributed important intellectual content to the report.
Lisa Irvine (https://orcid.org/0000-0003-1936-3584) (Research Fellow) jointly conducted the health economics analysis and contributed to the preparation of the health economics results for publication.
Paul Leighton (https://orcid.org/0000-0001-5208-0274) (Associate Professor of Applied Health Services Research) conducted the process evaluation and contributed to the data analysis, jointly prepared the qualitative results for publication, contributed to the trial protocol and contributed important intellectual content to the report.
Karen McCartney (https://orcid.org/0000-0003-2027-6031) (Research Fellow) prepared the protocol for publication and contributed to the preparation of the final report.
Gail Mountain (https://orcid.org/0000-0002-5417-7691) (Professor of Applied Dementia Research) contributed to the study protocol and contributed important intellectual content to the final report.
Kate Robertson (https://orcid.org/0000-0002-0922-3126) (Consultant Occupational Therapist and Honorary Assistant Professor) contributed to the grant application and study protocol, designed and jointly delivered training to the fall leads (therapists) and contributed important intellectual content to the final report.
Katie Robinson (https://orcid.org/0000-0003-1458-8186) (AHP Clinical Academic Lead) contributed to the study protocol and contributed important intellectual content to the final report.
Tracey H Sach (https://orcid.org/0000-0002-8098-9220) (Professor of Health Economics) jointly conducted the health economics analysis and prepared the health economics results for publication, contributed to the development of the grant application and trial protocol, and contributed important intellectual content to the final report.
Susan Stirling (https://orcid.org/0000-0001-6663-7846) (Statistician) jointly conducted the statistical analysis of the trial, prepared the statistical results for publication and contributed important intellectual content to the report.
Edward CF Wilson (https://orcid.org/0000-0002-8369-1577) (Senior Lecturer in Health Economics) jointly conducted the health economics analysis, prepared the health economics results for publication and contributed important intellectual content to the final report.
Erika J Sims (https://orcid.org/0000-0002-7898-0331) (Operations Manager at NCTU) was the Trial Manager, contributed to the development of the trial protocol, led the trial delivery and contributed important intellectual content to the final report.
Publications
Horne JC, Darby J, Godfrey M, Riley P, Jabber R, Stockton B, et al. Using a Hub and Spoke Approach to Enhance a Trial. British Geriatric Society Spring Conference, Nottingham, 2016.
Horne JC, Darby J, Godfrey M, Riley P, Jabber R, Stockton B, et al. Using a Hub and Spoke Approach to Enhance a Trial. Public, Patient Involvement International Conference, Manchester, 2017.
Izza MAD, Lunt E, Gordon AL, Gladman JRF, Armstrong S, Logan P. Polypharmacy, benzodiazepines, and antidepressants, but not antipsychotics, are associated with increased falls risk in UK care home residents: a prospective multi-centre study. Eur Geriatr Med 2020;11:1043–50.
Robinson K, Allen F, Darby J, Fox C, Gordon AL, Horne P, et al. Contamination in complex healthcare trials: the falls in care homes (FinCH) study experience. BMC Med Res Methodol 2020;20:46.
Logan PA, Horne JC, Gladman JRF, Gordon AL, Sach T, Clark A, et al. Multifactorial falls prevention programme compared with usual care in UK care homes for older people: multicentre cluster randomised controlled trial with economic evaluation. BMJ 2021;7:e066991.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Logan P, McCartney K, Armstrong S, Clarke A, Conroy S, Darby J, et al. Evaluation of the Guide to Action Care Home Fall Prevention Programme in Care Homes for Older People: Protocol for a Multi-Centre, Single Blinded, Cluster Randomised Controlled Trial (FinCH) 2019. https://nottingham-repository.worktribe.com/output/1500801 (accessed 1 March 2021).
- Public Health England . Falls: Applying All Our Health n.d. www.gov.uk/government/publications/falls-applying-all-our-health/falls-applying-all-our-health (accessed 7 January 2020).
- Age UK . Later Life in the United Kingdom 2015. www.ageuk.org.uk/globalassets/age-uk/documents/reports-and-publications/later_life_uk_factsheet.pdf/ (accessed 10 January 2021).
- NHS England . Injuries Due to Falls in People Aged 65 and Over 2018. www.nhs.uk/Scorecard/Pages/IndicatorFacts.aspx?MetricId=8135 (accessed 12 January 2021).
- Public Health England . Hip Fractures in People Aged 65 and Over. 2018. https://fingertips.phe.org.uk/search/hip%20fractures#page/3/gid/1/pat/6/par/E12000004/ati/102/are/E06000015/iid/41401/age/27/sex/4 (accessed 11 January 2021).
- Royal College of Physicians . National Hip Fracture Database (NHFD) Annual Report n.d. www.rcplondon.ac.uk/projects/outputs/national-hip-fracture-database-nhfd-annual-report-2018 (accessed 11 January 2021).
- Gordon AL, Franklin M, Bradshaw L, Logan P, Elliott R, Gladman JR. Health status of UK care home residents: a cohort study. Age Ageing 2014;43:97-103. https://doi.org/10.1093/ageing/aft077.
- NHS England . Ageing Well and Supporting People Living With Frailty 2019. www.england.nhs.uk/ourwork/clinical-policy/older-people/frailty/ (accessed 12 January 2021).
- Public Health England . Falls and Fractures: Consensus Statement and Resources Pack. 2017. www.gov.uk/government/publications/falls-and-fractures-consensus-statement (accessed 12 January 2021).
- NICE . Falls in Older People: Assessing Risk and Prevention. 2013. www.nice.org.uk/guidance/cg161/resources/falls-in-older-people-assessing-risk-and-prevention-pdf-35109686728645 (accessed 1 March 2021).
- Laing W. Care Homes for Older People Market Analysis and Projections 2017. www.laingbuissonevents.com/wp-content/uploads/2017/05/William-COP.pdf (accessed 1 March 2021).
- Sanford AM, Orrell M, Tolson D, Abbatecola AM, Arai H, Bauer JM, et al. An international definition for ‘nursing home’. J Am Med Dir Assoc 2015;16:181-4. https://doi.org/10.1016/j.jamda.2014.12.013.
- Competition and Markets Authority . Care Homes Market Study: Final Report 2017. www.gov.uk/cma-cases/care-homes-market-study (accessed 12 January 2021).
- Department of Health and Social Care . Falls and Fractures: Effective Interventions in Health and Social Care. 2009. www.laterlifetraining.co.uk/wp-content/uploads/2011/12/FF_Effective-Interventions-in-health-and-social-care.pdf (accessed 1 March 2021).
- Leibson CL, Tosteson ANA, Gabriel SE, Ransom JE, Melton LJ. Mortality, disability, and nursing home use for persons with and without hip fracture: a population-based study. J Am Geriatrics Soc 2002;50:1644-50. https://doi.org/10.1046/j.1532-5415.2002.50455.x.
- Gillespie LD, Robertson MC, Gillespie WJ, Sherrington C, Gates S, Clemson LM, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev 2012;9. https://doi.org/10.1002/14651858.CD007146.pub3.
- Robertson K, MacDonald A. Preventing Falls in Care Homes 2015. www.gmjournal.co.uk/preventing-falls-in-care-homes (accessed 1 March 2021).
- Oliver D, Healey F. Falls risk prediction tools for hospital inpatients: do they work?. Nurs Times 2009;105:18-21.
- Cameron ID, Dyer SM, Panagoda CE, Murray GR, Hill KD, Cumming RG, et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev 2018;9. https://doi.org/10.1002/14651858.CD005465.pub4.
- Livingston G, Barber J, Marston L, Stringer A, Panca M, Hunter R, et al. Clinical and cost-effectiveness of the Managing Agitation and Raising Quality of Life (MARQUE) intervention for agitation in people with dementia in care homes: a single-blind, cluster-randomised controlled trial. Lancet Psychiatry 2019;6:293-304. https://doi.org/10.1016/S2215-0366(19)30045-8.
- NHS England . NHS Long Term Plan n.d. www.longtermplan.nhs.uk (accessed 20 September 2021).
- Marshall M, de Silva D, Cruickshank L, Shand J, Wei L, Anderson J. What we know about designing an effective improvement intervention (but too often fail to put into practice). BMJ Qual Saf 2017;26:578-82. https://doi.org/10.1136/bmjqs-2016-006143.
- Bunn F, Goodman C, Corazzini K, Sharpe R, Handley M, Lynch J, et al. Setting priorities to inform assessment of care homes’ readiness to participate in healthcare innovation: a systematic mapping review and consensus process. Int J Environ Res Public Health 2020;17. https://doi.org/10.3390/ijerph17030987.
- Goodman C, Dening T, Gordon AL, Davies SL, Meyer J, Martin FC, et al. Effective health care for older people living and dying in care homes: a realist review. BMC Health Serv Res 2016;16. https://doi.org/10.1186/s12913-016-1493-4.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and Evaluating Complex Interventions. London: Medical Research Council; 2008.
- Walker GM, Armstrong S, Gordon AL, Gladman J, Robertson K, Ward M, et al. The Falls In Care Home study: a feasibility randomized controlled trial of the use of a risk assessment and decision support tool to prevent falls in care homes. Clin Rehabil 2016;30:972-83. https://doi.org/10.1177/0269215515604672.
- Robertson K, Logan PA, Conroy S, Dods V, Gordon A, Challands L, et al. Thinking falls – taking action: a guide to action for falls prevention. Br J Community Nurs 2010;15:406-10. https://doi.org/10.12968/bjcn.2010.15.8.76117.
- Robertson K, Logan P, Ward M, Pollard J, Gordon A, Williams W, et al. Thinking falls – taking action: a falls prevention tool for care homes. Br J Community Nurs 2012;17:206-9. https://doi.org/10.12968/bjcn.2012.17.5.206.
- Logan PA, Robertson K. Does Identifying Fall Risk Factors Lead to Appropriate Interventions? n.d.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: Template for Intervention Description and Replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Robinson K, Allen F, Darby J, Fox C, Gordon AL, Horne JC, et al. Contamination in complex healthcare trials: the Falls in Care Homes (FinCH) study experience. BMC Med Res Methodol 2020;20. https://doi.org/10.1186/s12874-020-00925-z.
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377-81. https://doi.org/10.1016/j.jbi.2008.08.010.
- Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: building an international community of software partners. J Biomed Inform 2019;95. https://doi.org/10.1016/j.jbi.2019.103208.
- Collin C, Wade DT, Davies S, Horne V. The Barthel ADL Index: a reliability study. Int Disabil Stud 1988;10:61-3. https://doi.org/10.3109/09638288809164103.
- Whitney J. Defining Falls Risk Factors in Older Adults With Cognitive Impairment Living in Residential Care 2013. https://kclpure.kcl.ac.uk/portal/files/32160428/2013_Whitney_Julie_0854187_ethesis.pdfey (accessed 13 September 2021).
- Smith SC, Lamping DL, Banerjee S, Harwood RH, Foley B, Smith P, et al. Development of a new measure of health-related quality of life for people with dementia: DEMQOL. Psychol Med 2007;37:737-46. https://doi.org/10.1017/S0033291706009469.
- Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2018;27:7-22. https://doi.org/10.1002/hec.3564.
- Mulhern B, Rowen D, Brazier J, Smith S, Romeo R, Tait R, et al. Development of DEMQOL-U and DEMQOL-PROXY-U: generation of preference-based indices from DEMQOL and DEMQOL-PROXY for use in economic evaluation. Health Technol Assess 2013;17. https://doi.org/10.3310/hta17050.
- World Medical Association . Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191-4. https://doi.org/10.1001/jama.2013.281053.
- Health Research Authority . UK Policy Framework for Health and Social Care Research n.d. www.hra.nhs.uk/planning-and-improving-research/policies-standards-legislation/uk-policy-framework-health-social-care-research/uk-policy-framework-health-and-social-care-research/ (accessed 5 November 2021).
- Whitney J, Close J, Lord SR, Jackson S. Identification of high risk fallers among older people living in residential care facilities: a simple screen based on easily collectable measures. Arch Gerontol and Geriatr 2012;55:690-5. https://doi.org/10.1016/j.archger.2012.05.010.
- Dyer CA, Taylor GJ, Reed M, Dyer CA, Robertson DR, Harrington R. Falls prevention in residential care homes: a randomised controlled trial. Age Ageing 2004;33:596-602. https://doi.org/10.1093/ageing/afh204.
- Great Britain . Data Protection Act 1998 1998.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal. 2013. www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781 (accessed 7 January 2021).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018. www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2018/ (accessed 5 March 2020).
- NHS Improvement . National Schedule of Reference Costs 2019. https://improvement.nhs.uk/resources/reference-costs/ (accessed 10 November 2020).
- Prescribing and Medicines Team, NHS Digital . Prescription Cost Analysis – England 2018 2018. https://digital.nhs.uk/data-and-information/publications/statistical/prescription-cost-analysis/2018 (accessed 7 January 2021).
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- NRS Healthcare . NRS Healthcare n.d. www.nrshealthcare.co.uk/ (accessed March 2020).
- Rowen D, Mulhern B, Banerjee S, van Hout B, Young TA, Knapp M, et al. Estimating preference-based single index measures for dementia using DEMQOL and DEMQOL-Proxy. Value Health 2012;15:346-56. https://doi.org/10.1016/j.jval.2011.10.016.
- National Institute for Health and Care Excellence (NICE) . Position Statement on Use of the EQ-5D-5L Value Set for England (Updated October 2019). 2019. www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l (accessed 7 January 2021).
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Gomes M, Ng ES, Grieve R, Nixon R, Carpenter J, Thompson SG. Developing appropriate methods for cost-effectiveness analysis of cluster randomized trials. Med Decis Making 2012;32:350-61. https://doi.org/10.1177/0272989X11418372.
- Gomes M, Grieve R, Nixon R, Ng ES, Carpenter J, Thompson SG. Methods for covariate adjustment in cost-effectiveness analysis that use cluster randomised trials. Health Econ 2012;21:1101-18. https://doi.org/10.1002/hec.2812.
- Ratcliffe J, Flint T, Easton T, Killington M, Cameron I, Davies O, et al. An empirical comparison of the EQ-5D-5L, DEMQOL-U and DEMQOL-Proxy-U in a post-hospitalisation population of frail older people living in residential aged care. Appl Health Econ Health Policy 2017;15:399-412. https://doi.org/10.1007/s40258-016-0293-7.
- Devine A, Taylor SJ, Spencer A, Diaz-Ordaz K, Eldridge S, Underwood M. The agreement between proxy and self-completed EQ-5D for care home residents was better for index scores than individual domains. J Clin Epidemiol 2014;67:1035-43. https://doi.org/10.1016/j.jclinepi.2014.04.005.
- Hughes LJ, Farina N, Page TE, Tabet N, Banerjee S. Adaptation of the DEMQOL-Proxy for routine use in care homes: a cross-sectional study of the reliability and validity of DEMQOL-CH. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2018-028045.
- Meads DM, Martin A, Griffiths A, Kelley R, Creese B, Robinson L, et al. Cost-effectiveness of dementia care mapping in care-home settings: evaluation of a randomised controlled trial. Appl Health Econ Health Policy 2020;18:237-47. https://doi.org/10.1007/s40258-019-00531-1.
- Engel L, Bucholc J, Mihalopoulos C, Mulhern B, Ratcliffe J, Yates M, et al. A qualitative exploration of the content and face validity of preference-based measures within the context of dementia. Health Qual Life Outcomes 2020;18. https://doi.org/10.1186/s12955-020-01425-w.
- Pawson R, Tilley N. Realistic Evaluation. London: SAGE Publications Ltd; 1997.
- Pawson R. The Science of Evaluation – A Realist Manifesto. London: SAGE Publications Ltd; 2013.
- Emmel N, Greenhalgh J, Manzano A, Monaghan M, Dalkin S. Doing Realist Research. London: SAGE Publications Ltd; 2018.
- Pawson R. Evidence Based Policy: A Realist Perspective. London: SAGE Publications Ltd; 2006.
- Gordon AL, Goodman C, Davies SL, Dening T, Gage H, Meyer J, et al. Optimal healthcare delivery to care homes in the UK: a realist evaluation of what supports effective working to improve healthcare outcomes. Age Ageing 2018;47:595-603. https://doi.org/10.1093/ageing/afx195.
- Devi R, Meyer J, Banerjee J, Goodman C, Gladman JRF, Dening T, et al. Quality improvement collaborative aiming for Proactive HEAlthcare of Older People in Care Homes (PEACH): a realist evaluation protocol. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2018-023287.
- Rycroft-Malone J, Seers K, Eldh AC, Cox K, Crichton N, Harvey G, et al. A realist process evaluation within the Facilitating Implementation of Research Evidence (FIRE) cluster randomised controlled international trial: an exemplar. Implement Sci 2018;13. https://doi.org/10.1186/s13012-018-0811-0.
- Goodman C, Davies SL, Gordon AL, Dening T, Gage H, Meyer J, et al. Optimal NHS service delivery to care homes: a realist evaluation of the features and mechanisms that support effective working for the continuing care of older people in residential settings. Health Serv Deliv Res 2017;5. https://doi.org/10.3310/hsdr05290.
- Robbins I, Gordon A, Dyas J, Logan P, Gladman J. Explaining the barriers to and tensions in delivering effective healthcare in UK care homes: a qualitative study. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-003178.
- Devi R, Chadborn NH, Meyer J, Banerjee J, Goodman C, Dening T, et al. How quality improvement collaboratives work to improve healthcare in care homes: a realist evaluation. Age Ageing 2021;50:1371-81. https://doi.org/10.1093/ageing/afab007.
- Pawson R, Vaessen J, Leeuw F. Mind the Gap: Evaluation and the Disciplines. Abingdon-on-Thames: Routledge; 2010.
- Lavrakas PJ. Encyclopedia of Survey Research Methods. Thousand Oaks, CA: SAGE Publications Ltd; 2008.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- May C, Finch T. Implementing, embedding, and integrating practices: an outline of normalization process theory. Sociology 2009;43:535-54. https://doi.org/10.1177/0038038509103208.
- May C. Towards a general theory of implementation. Implement Sci 2013;8. https://doi.org/10.1186/1748-5908-8-18.
- Leighton P, Darby J, Allen F, Evley R, Logan P. PS9D–03 What worked for us in which circumstances, and what didn’t; reflections upon incorporating a realist evaluation within a clinical trial of a complex intervention. Trials 2019;20.
- Allen F, Darby J, Cook M, Evley R, Godfrey M, Horne J, et al. Learning from a successful process evaluation in care homes. Age Ageing 2021;5:1850-3. https://doi.org/10.1093/ageing/afab139.
- Belle SV, Wong G, Westhorp G, Pearson M, Emmel N, Manzano A, et al. Can ‘realist’ randomised controlled trials be genuinely realist?. Trials 2016;17. https://doi.org/10.1186/s13063-016-1407-0.
- Fletcher A, Jamal F, Moore G, Evans RE, Murphy S, Bonell C. Realist complex intervention science: applying realist principles across all phases of the Medical Research Council framework for developing and evaluating complex interventions. Evaluation 2016;22:286-303. https://doi.org/10.1177/1356389016652743.
- Bonell C, Fletcher A, Morton M, Lorenc T, Moore L. Realist randomised controlled trials: a new approach to evaluating complex public health interventions. Soc Sci Med 2012;75:2299-306. https://doi.org/10.1016/j.socscimed.2012.08.032.
- UK Public Involvement Standards Development Partnership . The UK Standards for Public Involvement in Research 2019. https://sites.google.com/nihr.ac.uk/pi-standards/home (accessed 1 March 2021).
- Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. Res Involv Engagem 2017;3. https://doi.org/10.1186/s40900-017-0062-2.
- Elrod JK, Fortenberry JL. The hub-and-spoke organization design: an avenue for serving patients well. BMC Health Serv Res 2017;17:25-33. https://doi.org/10.1186/s12913-017-2341-x.
- Millar L. Use of hub and spoke model in nursing students’ practice learning. Nurs Stand 2014;28:37-42. https://doi.org/10.7748/ns.28.49.37.e8616.
- Huddleston P, Zimmermann MB. Stroke care using a hub and spoke model with telemedicine. Crit Care Nurs Clin North Am 2014;26:469-75. https://doi.org/10.1016/j.ccell.2014.08.014.
- Staniszewska S, Denegri S, Matthews R, Minogue V. Reviewing progress in public involvement in NIHR research: developing and implementing a new vision for the future. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2017-017124.
- Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-117.
- Horne JC, Darby J, Godfrey M, Riley P, Jabber R, Stockton B, et al. Using a Hub and Spoke Approach to Enhance a Trial n.d.
- Fudge N, Wolfe CDA, McKevitt C. Involving older people in health research. Age Ageing 2007;36:492-500. https://doi.org/10.1093/ageing/afm029.
- Boivin A, Richards T, Forsythe L, Grégoire A, L’Espérance A, Abelson J, et al. Evaluating patient and public involvement in research. BMJ 2018;363. https://doi.org/10.1136/bmj.k5147.
- Bagley HJ, Short H, Harman NL, Hickey HR, Gamble CL, Woolfall K, et al. A patient and public involvement (PPI) toolkit for meaningful and flexible involvement in clinical trials – a work in progress. Res Involv Engagem 2016;2. https://doi.org/10.1186/s40900-016-0029-8.
- Dudley L, Gamble C, Allam A, Bell P, Buck D, Goodare H, et al. A little more conversation please? Qualitative study of researchers’ and patients’ interview accounts of training for patient and public involvement in clinical trials. Trials 2015;16. https://doi.org/10.1186/s13063-015-0667-4.
- Tuckman BW, Jensen MAC. Stages of small-group development revisited. Group Organ Manag 1977;2:419-27. https://doi.org/10.1177/105960117700200404.
- Horne JC, Darby J, Godfrey M, Riley P, Jabber R, Stockton B, et al. Using a Hub and Spoke Approach to Enhance a Trial n.d.
- Logan PA, Horne JC, Gladman JRF, Gordon AL, Sach T, Clark A, et al. Multifactorial falls prevention programme compared with usual care in UK care homes for older people: multicentre cluster randomised controlled trial with economic evaluation. BMJ 2021;375. https://doi.org/10.1136/bmj-2021-066991.
- Kennedy CC, Ioannidis G, Thabane L, Adachi JD, Marr S, Giangregorio LM, et al. Successful knowledge translation intervention in long-term care: final results from the vitamin D and osteoporosis study (ViDOS) pilot cluster randomized controlled trial. Trials 2015;16. https://doi.org/10.1186/s13063-015-0720-3.
- Rubenstein LZ, Robbins AS, Josephson KR, Schulman BL, Osterweil D. The value of assessing falls in an elderly population: a randomized clinical trial. Ann Int Med 1990;113:308-16. https://doi.org/10.7326/0003-4819-113-4-308.
- Sackley CM, van den Berg ME, Lett K, Patel S, Hollands K, Wright CC, et al. Effects of a physiotherapy and occupational therapy intervention on mobility and activity in care home residents: a cluster randomised controlled trial. BMJ 2009;339. https://doi.org/10.1136/bmj.b3123.
- Luff R, Laybourne A, Ferreira Z, Meyer J. A guide to research with care homes. Qual Ageing 2015;16:186-94. https://doi.org/10.1108/QAOA-06-2015-0027.
- Stacey D, Suwalska V, Boland L, Lewis KB, Presseau J, Thomson R. Are patient decision aids used in clinical practice after rigorous evaluation? A survey of trial authors. Med Decis Making 2019;39:805-15. https://doi.org/10.1177/0272989X19868193.
- Douglas N, Affoo R. Certified nursing assistants want to use external memory aids for residents with dementia: survey results within an implementation science framework. Am J Speech Lang Pathol 2019;28:591-8. https://doi.org/10.1044/2018_AJSLP-18-0118.
- Murray E, Treweek S, Pope C, MacFarlane A, Ballini L, Dowrick C, et al. Normalisation process theory: a framework for developing, evaluating and implementing complex interventions. BMC Med 2010;8. https://doi.org/10.1186/1741-7015-8-63.
Appendix 1 GtACH tool
Appendix 2 Sensitivity analysis
| Time point | GtACH | Control | Unadjusted | Adjusted for baseline | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number at risk | Number of falls | Falls rate | Number at risk | Number of falls | Falls rate | IRR (95% CI) | p-value | IRR (95% CI) | p-value | |
| Pre randomisationa | 773 | 0.61 (1.57) | 6.97 (17.67) | 882 | 0.79 (2.02) | 9.48 (24.14) | ||||
| 0–90 days | 708 | 0.55 (1.36) | 6.93 (20.56) | 826 | 0.88 (2.37) | 10.24 (27.26) | 0.62 (0.44 to 0.87) | 0.005 | 0.73 (0.55 to 0.98) | 0.033 |
| 91–180 days | 630 | 0.49 (1.13) | 6.04 (14.02) | 712 | 0.89 (2.60) | 10.38 (29.52) | 0.59 (0.43 to 0.83) | 0.002 | 0.68 (0.51 to 0.9) | 0.007 |
| 181–270 days | 547 | 0.60 (1.29) | 7.28 (16.67) | 633 | 0.73 (1.85) | 9.21 (28.77) | 0.9 (0.66 to 1.22) | 0.503 | 0.96 (0.74 to 1.25) | 0.778 |
| 271–360 days | 502 | 0.55 (1.14) | 6.22 (12.88) | 573 | 0.79 (2.37) | 9.22 (27.36) | 0.86 (0.58 to 1.28) | 0.46 | 0.94 (0.67 to 1.33) | 0.738 |
| Time point | GtACH | Control | Unadjusted | Adjusted for baseline | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number at risk | Number of falls | Falls rate | Number at risk | Number of falls | Falls rate | IRR (95% CI) | p-value | IRR (95% CI) | p-value | |
| Pre randomisationa | 773 | 0.61 (1.57) | 6.97 (17.67) | 882 | 0.79 (2.02) | 9.48 (24.14) | ||||
| 0–90 days | 708 | 0.55 (1.36) | 6.93 (20.56) | 826 | 0.88 (2.37) | 10.24 (27.26) | 0.61 (0.52 to 0.71) | < 0.001 | 0.68 (0.58 to 0.79) | < 0.001 |
| 91–180 days | 630 | 0.49 (1.13) | 6.04 (14.02) | 712 | 0.89 (2.60) | 10.38 (29.52) | 0.55 (0.46 to 0.65) | < 0.001 | 0.62 (0.53 to 0.72) | < 0.001 |
| 180–270 days | 547 | 0.60 (1.29) | 7.28 (16.67) | 633 | 0.73 (1.85) | 9.21 (28.77) | 0.81 (0.69 to 0.96) | 0.014 | 0.91 (0.78 to 1.08) | 0.288 |
| 271–360 days | 502 | 0.55 (1.14) | 6.22 (12.88) | 573 | 0.79 (2.37) | 9.22 (27.36) | 0.71 (0.58 to 0.87) | 0.001 | 0.84 (0.68 to 1.03) | 0.090 |
| Time point | GtACH | Control | Unadjusted | Adjusted for baseline | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number at risk | Number of falls | Falls rate | Number at risk | Number of falls | Falls rate | IRR (95% CI) | p-value | IRR (95% CI) | p-value | |
| Pre randomisationa | 773 | 0.61 (1.57) | 6.97 (17.67) | 882 | 0.79 (2.02) | 9.48 (24.14) | ||||
| 0–90 days | 708 | 0.55 (1.36) | 6.93 (20.56) | 826 | 0.88 (2.37) | 10.24 (27.26) | 0.85 (0.66 to 1.09) | 0.197 | 0.83 (0.66 to 1.06) | 0.14 |
| 91–180 days | 630 | 0.49 (1.13) | 6.04 (14.02) | 712 | 0.89 (2.60) | 10.38 (29.52) | 0.85 (0.66 to 1.11) | 0.24 | 0.87 (0.68 to 1.11) | 0.254 |
| 181–270 days | 547 | 0.60 (1.29) | 7.28 (16.67) | 633 | 0.73 (1.85) | 9.21 (28.77) | 1.02 (0.79 to 1.33) | 0.853 | 1.05 (0.82 to 1.34) | 0.693 |
| 271–360 days | 502 | 0.55 (1.14) | 6.22 (12.88) | 573 | 0.79 (2.37) | 9.22 (27.36) | 1.06 (0.77 to 1.45) | 0.724 | 1.06 (0.78 to 1.44) | 0.707 |
In Stata, the results for the negative binomial model (see Table 24) were very different from those obtained in the negative binomial GEE and the Poisson models. However, using R (results not shown) rather than Stata yielded similar results to the Poisson regression model.
The ordinal logistic regression model, looking at 0, 1, 2 or ≥ 3 falls, provided evidence of a difference between arms for falls occurring between baseline and 3 months, and between 3 and 6 months.
| Time point | Ordinal OR (95% CI) | p-value | Ordinal OR (95% CI) | p-value |
|---|---|---|---|---|
| Pre randomisationa | ||||
| 0–90 days | 0.67 (0.47 to 0.94) | 0.023 | 0.68 (0.49 to 0.95) | 0.024 |
| 91–180 days | 0.69 (0.51 to 0.94) | 0.02 | 0.76 (0.58 to 1) | 0.047 |
| 181–270 days | 0.98 (0.71 to 1.35) | 0.899 | 1.06 (0.79 to 1.41) | 0.713 |
| 271–360 days | 0.89 (0.6 to 1.32) | 0.556 | 0.98 (0.67 to 1.44) | 0.913 |
A cluster summary analysis also provided evidence of a difference in falls occurring between 3 and 6 months.
| Time point | GtACH | Control | Unadjusted | Adjusted for baseline | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | |
| Pre randomisationa | 39 | 7.55 (6.30) | 45 | 8.94 (7.09) | ||||
| 0–90 days | 38 | 6.74 (6.50) | 45 | 9.84 (9.04) | 0.64 (0.43 to 0.97) | 0.036 | 0.85 (0.61 to 1.17) | 0.314 |
| 91–180 days | 38 | 5.99 (4.91) | 45 | 10.39 (10.63) | 0.59 (0.41 to 0.85) | 0.005 | 0.69 (0.49 to 0.97) | 0.033 |
| 181–270 days | 37 | 6.93 (4.25) | 45 | 7.99 (8.09) | 1.08 (0.74 to 1.58) | 0.669 | 1.17 (0.83 to 1.64) | 0.357 |
| 271–360 days | 37 | 6.10 (5.51) | 45 | 8.08 (9.51) | 0.83 (0.53 to 1.31) | 0.418 | 0.95 (0.62 to 1.45) | 0.796 |
Appendix 3 Poisson regression analysis of hospital admissions
| Time point | GtACH | Control | Unadjusted | Adjusted for baseline | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | |
| Pre randomisationa | 773 | 0.46 (2.62) | 877 | 0.60 (2.69) | ||||
| 0–180 days | 697 | 1.54 (5.36) | 793 | 1.61 (4.85) | 0.94 (0.64 to 1.38) | 0.75 | 0.98 (0.67 to 1.43) | 0.919 |
| 181–360 days | 532 | 1.08 (4.04) | 620 | 1.58 (6.03) | 0.81 (0.49 to 1.33) | 0.403 | 0.81 (0.49 to 1.32) | 0.394 |
Appendix 4 Fractures
Hip fractures
-
S72.0 Fracture of neck of femur.
-
S72.00 Fracture of unspecified part of neck of femur.
-
S72.1 Pertrochanteric fracture.
-
S72.10 Unspecified trochanteric fracture of femur.
-
S72.2 Subtrochanteric fracture.
-
S72.20 Not found in ICD10 (International Statistical Classification of Diseases and Related Health Problems, Tenth Revision), but occurs seven times in data set.
Wrist fractures
-
S52.5 Fracture of lower end of radius.
-
S52.50 Unspecified fracture of the lower end of radius.
-
S52.6 Fracture of lower end of both ulna and radius.
-
S52.60 Unspecified fracture of lower end of ulna.
-
S62.0 Fracture of navicular (scaphoid) bone of hand.
-
S62.1 Fracture of other carpal bone.
-
S62.4 Multiple fractures of carpal bones.
-
S62.40 Not found in ICD10, but occurs once in data set.
-
S62.8 Fracture of other and unspecified parts of wrist and hand.
-
S62.80 Not found in ICD10, but occurs twice in data set.
Any fracture
-
S02.0 Fracture of vault of skull.
-
S02.00 Not found in ICD10, but occurs twice in data set.
-
S02.1 Fracture of base of skull.
-
S02.10 Unspecified fracture of base of skull.
-
S02.2 Fracture of nasal bones.
-
S02.20 Not found in ICD10, but occurs twice in data set.
-
S02.4 Fracture of malar and maxillary bones.
-
S02.40 Fracture of malar, maxillary and zygoma bones, unspecified.
-
S02.5 Fracture of tooth (traumatic).
-
S02.50 Not found in ICD10, but occurs once in data set.
-
S02.6 Fracture of mandible.
-
S02.7 Multiple fracture involving skull and facial bones.
-
S02.8 Fractures of other skull and facial bones.
-
S02.9 Fracture of skull and facial bones, part unspecified.
-
S22.0 Fracture of thoracic vertebra.
-
S22.00 Fracture of unspecified thoracic vertebra.
-
S22.1 Multiple fractures of thoracic spine.
-
S22.10 Not found in ICD10, but occurs once in data set.
-
S22.2 Fracture of sternum.
-
S22.3 Fracture of one rib.
-
S22.30 Not found in ICD10, but occurs 11 times in data set.
-
S22.4 Multiple fractures of ribs.
-
S22.40 Not found in ICD10, but occurs 13 times in data set.
-
S22.5 Flail chest.
-
S22.8 Fracture of other parts of bony thorax.
-
S22.9 Fracture of bony thorax, part unspecified.
-
S32 Fracture of lumbar spine and pelvis.
-
S32.0 Fracture of lumbar vertebrae.
-
S32.00 Fracture of unspecified lumbar vertebra.
-
S32.1 Fracture of sacrum.
-
S32.2 Fracture of coccyx.
-
S32.3 Fracture of ilium.
-
S32.30 Unspecified fracture of ilium.
-
S32.4 Fracture of acetabulum.
-
S32.40 Unspecified fracture of acetabulum.
-
S32.5 Fracture of pubis.
-
S32.5 Not in ICD10, but occurs five times in data set.
-
S32.50 Unspecified fracture of pubis.
-
S32.7 Multiple fractures of lumbar spine and pelvis.
-
S32.8 Fracture of other and unspecified parts of lumbar spine and pelvis.
-
S42.0 Fracture of clavicle.
-
S42.00 Fracture of unspecified part of clavicle.
-
S42.1 Fracture of scapula.
-
S42.2 Fracture of upper end of humerus.
-
S42.20 Unspecified fracture of upper end of humerus.
-
S42.3 Fracture of shaft of humerus.
-
S42.30 Unspecified fracture of shaft of humerus.
-
S42.30 Not in ICD10, but occurs three times in data set.
-
S42.4 Fracture of lower end of humerus.
-
S42.7 Multiple fractures of clavicle, scapula and humerus.
-
S42.8 Fracture of other parts of shoulder and upper arm.
-
S42.9 Fracture of shoulder girdle, part unspecified.
-
S42.90 Fracture of unspecified shoulder girdle, part unspecified.
-
S52.0 Fracture of upper end of ulna.
-
S52.1 Fracture of upper end of radius.
-
S52.2 Fracture of shaft of ulna.
-
S52.3 Fracture of shaft of radius.
-
S52.4 Fracture of shafts of both ulna and radius.
-
S52.5 Fracture of lower end of radius.
-
S52.6 Fracture of lower end of both ulna and radius.
-
S52.7 Multiple fractures of forearm.
-
S52.8 Fracture of other parts of forearm.
-
S52.9 Fracture of forearm, part unspecified.
-
S62.0 Fracture of navicular (scaphoid) bone of hand.
-
S62.1 Fracture of other carpal bones.
-
S62.2 Fracture of first metacarpal bone.
-
S62.3 Fracture of other metacarpal bone.
-
S62.30 Unspecified fracture of other metacarpal bone.
-
S62.4 Multiple fractures of carpal bones.
-
S62.40 Not in ICD10, but occurs once in data set.
-
S62.5 Fracture of thumb.
-
S62.50 Fracture of unspecified phalanx of thumb.
-
S62.6 Fracture of other finger.
-
S62.60 Fracture of unspecified phalanx of finger.
-
S62.61 Displaced fracture of proximal phalanx of finger.
-
S62.7 Multiple fracture of fingers.
-
S62.8 Fracture of other and unspecified parts of wrist and hand.
-
S62.80 Not in ICD10, but occurs twice in data set.
-
S72.0 Fracture of neck of femur.
-
S72.00 Fracture of unspecified part of neck of femur.
-
S72.1 Pertrochanteric fracture.
-
S72.10 Unspecified pertrochanteric fracture.
-
S72.2 Subtrochanteric fracture.
-
S72.20 Not in ICD10, but occurs seven times in data set.
-
S72.3 Fracture of shaft of femur.
-
S72.4 Fracture of lower end of femur.
-
S72.40 Unspecified fracture of lower end of femur.
-
S72.7 Multiple fractures of femur.
-
S72.8 Fractures of other parts of femur.
-
S72.9 Fracture of femur, part unspecified.
-
S82.0 Fracture of patella.
-
S82.00 Unspecified fracture of patella.
-
S82.1 Fracture of upper end of tibia.
-
S82.10 Unspecified fracture of upper end of tibia.
-
S82.2 Fracture of shaft of tibia.
-
S82.20 Unspecified fracture of shaft of tibia.
-
S82.3 Fracture of lower end of tibia.
-
S82.31 Torus fracture of lower end of tibia.
-
S82.4 Fracture of fibula alone.
-
S82.40 Unspecified fracture of shaft of fibula.
-
S82.5 Fracture of medial malleolus.
-
S82.6 Fracture of lateral malleolus.
-
S82.7 Multiple fracture of lower leg.
-
S82.8 Fractures of other parts of lower leg.
-
S82.9 Fracture of lower leg, part unspecified.
Appendix 5 Unit costs for staff and equipment
| Description | Unit cost in 2017/18 GBP | Source of unit cost |
|---|---|---|
| Ambulance hear and treat or refer | 37.00 | Reference Costs 2017–18 46 |
| Ambulance see and treat or refer | 192.00 | Reference Costs 2017–18 46 |
| Advanced clinical practitioner, ANP | 66.00 | Assume AfC band 8a |
| Community mental health team (including CPN and DOT) | 160.00 | Reference Costs 2017–18,46 other mental health specialist teams, adult and elderly |
| Dentist | 164.00 | Reference Costs 2017–18 46 |
| Dietitian | 86.00 | Unit Costs of Health and Social Care 45 |
| District nurse (community matron) | 38.00 | Reference Costs 2017–18 46 |
| NHS falls lead | 44.00 | Assume AfC band 6 |
| FinCH trial research staff ‘Train the Trainer’ | 63.00 | Assume AfC band 8a |
| Any nurse (telephone only) | 19.00 | Reference Costs 2017–18 46 |
| GP | 34.00 | Unit Costs of Health and Social Care 45 |
| GP (telephone only) | 15.10 | Unit Costs of Health and Social Care 45 |
| GP (OOH service) | 34.00 | Unit Costs of Health and Social Care 45 |
| Hearing test | 63.00 | Audiology |
| Home care manager | 40.00 | Unit Costs of Health and Social Care 45 |
| Home care worker | 27.00 | Unit Costs of Health and Social Care 45 |
| Optician | 55.00 | Assume AfC band 7 |
| Occupational therapist | 78.00 | Unit Costs of Health and Social Care 45 |
| Pharmacist | 55.00 | Assume AfC band 7 |
| Phlebotomist | 32.00 | Assume AfC band 4 |
| Physiotherapist and falls team | 54.00 | Unit Costs of Health and Social Care 45 |
| Podiatrist | 41.00 | Reference Costs 2017–18 46 |
| Practice nurse | 10.50 | Unit Costs of Health and Social Care 45 |
| SALT | 97.00 | Unit Costs of Health and Social Care 45 |
| Social worker (adult services) | 84.00 | Unit Costs of Health and Social Care 45 |
| Specialist nurse | 79.00 | Reference Costs 2017–18 46 |
| GP surgery administrator | 8.00 | Assume AfC band 4 |
| Social services | 84.00 | Unit Costs of Health and Social Care 45 |
| Support and outreach worker | 23.00 | Unit Costs of Health and Social Care 45 |
| Funding assessment | 84.00 | Assume social worker |
| Equipment (various; see Table 29) | 6.79–3500.00a | NRS or CCS |
| Item | Number recorded in CRF | Unit cost (£) | Source | Annualised costs | Personal or shared |
|---|---|---|---|---|---|
| Hoists, etc. | |||||
| Hoist | 555 | 639.95 | Not costed | Shared | |
| Slide sheet | 239 | 16.94 | NRSa | No | Personal |
| Sling | 199 | 99.69 | NRSa | Yes | Personal |
| Stand aid | 91 | 785.00 | Not costed | Shared | |
| Rotunda | 79 | 359.95 | Not costed | Shared | |
| Handling belt | 9 | 49.63 | NRSa | Yes | Personal |
| ARJOb sara stedy | 4 | 1209.00 | Not costed | Shared | |
| Large care home equipment | |||||
| Stair climber | 13 | 1900.00 | Not costed | Shared | |
| Hand rail | 11 | 6.79 | Not costed | Shared | |
| Stair lift | 9 | 1900.00 | Not costed | Shared | |
| Brackets | 2 | 73.35 | Not costed | Shared | |
| Ramp | 2 | 47.19 | Not costed | Shared | |
| Mobility | |||||
| Basic wheelchair | 728 | 114.45 | NRSa | Yes | Personal |
| Walking frame | 571 | 23.33 | NRSa | Yes | Personal |
| Rollator frame | 179 | 41.95 | NRSa | Yes | Personal |
| Walking stick | 166 | 9.39 | NRSa | Yes | Personal |
| Electric wheelchair | 30 | 1235.00 | CCSc | Yes | Personal |
| Mobility scooter | 12 | 649.00 | CCSc | Yes | Personal |
| Sensor/alarms | |||||
| Sensor mat | 269 | 27.05 | NRSa | Yes | Personal |
| Crash mat | 103 | 27.05 | NRSa | Yes | Personal |
| Pendant alarm | 57 | 31.45 | NRSa | Yes | Personal |
| PIR sensor | 24 | 12.55 | NRSa | Yes | Personal |
| Call bell | 22 | 42.95 | NRSa | Yes | Personal |
| Motion sensor | 12 | 12.55 | NRSa | Yes | Personal |
| Chair sensor | 4 | 103.65 | NRSa | Yes | Personal |
| Sitting | |||||
| Cushion | 312 | 24.95 | NRSa | No | Personal |
| Recliner chair | 43 | 652.15 | NRSa | Yes | Personal |
| Pro-pad cushion | 36 | 52.45 | NRSa | No | Personal |
| Repose wedge | 14 | 83.95 | NRSa | No | Personal |
| Hydrotilt chair | 13 | 1800.00 | CCSc | Yes | Personal |
| Kirton chair | 13 | 1611.43 | NRSa | Yes | Personal |
| Deepdale chair | 10 | 185.00 | CCSc | Yes | Personal |
| Perching stool | 3 | 41.95 | NRSa | Yes | Personal |
| Rollator frame | 3 | 23.33 | NRSa | Yes | Personal |
| Sleeping | |||||
| Profile bed | 395 | 498.75 | NRSa | Yes | Personal |
| Bed rails | 269 | 31.55 | NRSa | Yes | Personal |
| Pressure mattress | 219 | 111.59 | NRSa | Yes | Personal |
| Airflow mattress | 172 | 625.00 | CCSc | Yes | Personal |
| Bed bumpers | 54 | 72.75 | NRSa | Yes | Personal |
| Repose mattress | 32 | 113.65 | NRSa | Yes | Personal |
| Pro-pad mattress | 29 | 125.95 | CCSc | Yes | Personal |
| Bed sensor | 14 | 111.99 | NRSa | Yes | Personal |
| Bed lever | 7 | 98.00 | NRSa | Yes | Personal |
| Electric mattress | 5 | 984.00 | NRSa | Yes | Personal |
| Washing/toilet | |||||
| Shower chair | 240 | 84.95 | NRSa | Yes | Personal |
| Bath hoist | 181 | 209.95 | Not costed | Shared | |
| Commode | 151 | 24.95 | NRSa | Yes | Personal |
| Bath chair | 102 | 19.85 | NRSa | Yes | Personal |
| Raised toilet seat | 28 | 16.55 | NRSa | Yes | Personal |
| Bath seat | 22 | 19.85 | NRSa | Yes | Personal |
| Parker bath | 18 | 3500.00 | Not costed | Shared | |
| Non-slip mat | 7 | 11.23 | NRSa | Yes | Personal |
| Lap strap | 4 | 84.00 | NRSa | Yes | Personal |
Appendix 6 Analytic code book
Appendix 7 Unadjusted falls count at baseline and primary end point
| Care home ID (control arm) | Total number of falls | Care home ID (GtACH arm) | Total number of falls | ||
|---|---|---|---|---|---|
| 90 days prior to randomisation | 91–180 days post randomisation | 90 days prior to randomisation | 91–180 days post randomisation | ||
| 102 | 7 | 16 | 101 | 19 | 19 |
| 103 | 5 | 10 | 107 | 12 | 20 |
| 104 | 17 | 28 | 201 | 12 | 5 |
| 105 | 10 | 13 | 203 | 7 | 7 |
| 106 | 8 | 12 | 207 | 6 | 2 |
| 204 | 8 | 20 | 208 | 10 | 6 |
| 205 | 3 | 1 | 209 | 9 | 3 |
| 206 | 5 | 5 | 211 | 1 | 0 |
| 210 | 11 | 12 | 302 | 8 | 16 |
| 301 | 12 | 16 | 303 | 8 | 15 |
| 404 | 6 | 2 | 401 | 4 | 1 |
| 405 | 1 | 3 | 402 | 14 | 20 |
| 406 | 18 | 11 | 502 | 1 | 2 |
| 504 | 20 | 8 | 503 | 2 | 6 |
| 505 | 2 | 3 | 506 | 0 | 0 |
| 601 | 5 | 7 | 602 | 12 | 9 |
| 603 | 7 | 9 | 605 | 6 | 9 |
| 604 | 11 | 32 | 607 | 9 | 4 |
| 606 | 12 | 27 | 609 | 3 | 9 |
| 608 | 11 | 7 | 612 | 11 | 18 |
| 610 | 11 | 15 | 613 | 9 | 2 |
| 611 | 8 | 35 | 615 | 1 | 3 |
| 614 | 5 | 9 | 701 | 2 | 0 |
| 702 | 0 | 3 | 703 | 6 | 4 |
| 706 | 3 | 3 | 704 | 7 | 8 |
| 707 | 5 | 10 | 705 | 3 | 9 |
| 709 | 4 | 4 | 708 | 3 | 13 |
| 710 | 6 | 9 | 711 | 2 | 3 |
| 712 | 3 | 5 | 803 | 12 | 19 |
| 801 | 15 | 50 | 804 | 8 | 1 |
| 802 | 22 | 116 | 806 | 5 | 9 |
| 805 | 3 | 3 | 810 | 9 | 10 |
| 807 | 7 | 11 | 811 | 19 | 28 |
| 808 | 10 | 9 | 901 | 5 | 2 |
| 809 | 6 | 8 | 906 | 7 | 0 |
| 812 | 25 | 53 | 908 | 9 | 11 |
| 902 | 7 | 18 | 909 | 7 | 4 |
| 903 | 5 | 3 | 910 | 6 | 1 |
| 904 | 4 | 2 | 911 | 4 | 13 |
| 905 | 7 | 1 | |||
| 907 | 5 | 5 | |||
| 912 | 8 | 4 | |||
| 1001 | 5 | 11 | |||
| 1002 | 0 | 3 | |||
| 1003 | 11 | 4 | |||
Appendix 8 Longlist of context–mechanism–outcome configurations
| Code | Environment | Context | Mechanism | Outcome | Evidence |
|---|---|---|---|---|---|
| 0803 care home A | |||||
| 1 | Corporate, large home | Knowledgeable staff | Little motivation to change | GtACH programme not adopted. Persistence of existing practice | Weak |
| 2a | Corporate, large home | Effective falls systems in place with which staff are happy | Inertia (inhibits innovation) | GtACH programme not adopted or used when observed only | Strong |
| 2b | Corporate, large home | Effective falls systems in place that staff are happy with, therefore no perceived benefit of using the GtACH programme and no risk to residents if the GtACH programme is not used | No incentive to change | GtACH programme not adopted or used when observed only | Strong |
| 3 | Corporate, large home | Existing heavy administrative/paperwork burden | Lack of appetite for more paperwork | GtACH programme not adopted as seen as extra to existing paperwork | Moderate |
| 4a | Corporate, large home | Staff respond reactively rather than proactively to falls | Staff accepting of current practices | Partial adoption of the GtACH programme – used reactively only | Moderate |
| 4b | Corporate, large home | Staff respond reactively rather proactively to falls | Staff are accountable for current paperwork only, which is used after falls | Partial adoption of the GtACH programme – used reactively following home’s paperwork only | Moderate |
| 5a | Corporate, large home | Clear internal and external management hierarchy | Lack of ownership on the part of staff | GtACH programme not adopted because of lack of authority/drive | Weak |
| 5b | Corporate, large home | Clear internal and external management hierarchy | Lack of ownership on the part of local management | GtACH programme not adopted because of lack of authority – changes need corporate approval | Weak |
| 5c | Corporate, large home | Clear internal and external management hierarchy | Lack of awareness at corporate level | GtACH programme not adopted. Lack of authority – changes need corporate approval | Weak |
| 6 | Corporate, large home | Home has not adopted champion roles. Staff did not feel that a falls champion would be a useful role | Lack of appetite by staff at all levels to adopt the falls champion role | Falls champion role will not be adopted | Moderate |
| 7a | Corporate, large home | Staff allocated clear roles. Only senior staff complete documentation and medication. Carers complete hands-on care and daily logs | Inflexibility in structured job roles | Partial or no adoption. GtACH programme will be adopted only if instigated by management and will be used by senior staff only | Moderate |
| 7b | Corporate, large home | Staff allocated clear roles. Only senior staff complete documentation. Carers complete hands-on care and daily logs | Staff inhibited from breaking system | Partial or no adoption. GtACH programme will be adopted only if instigated by management and will be used by senior staff only | Moderate |
| 7c | Corporate, large home | Staff allocated clear roles. Only senior staff complete documentation. Carers complete hands-on care and daily logs | Lack of care staff ownership of the intervention | Partial or no adoption. GtACH programme will be adopted only if instigated by management and will be used by senior staff only | Moderate |
| 7d | Corporate, large home | Staff allocated clear roles. Only senior staff complete documentation Carers complete hands-on care and daily logs. Carers feel that time spent doing paperwork would be detrimental to their caring role | Lack of incentive to change | Partial or no adoption. GtACH programme will be adopted only if instigated by management and will be used by senior staff only | Moderate |
| 0703 care home B | |||||
| 1a | Corporate (small local chain), medium-sized home | The FinCH trial increased awareness and knowledge of falls risks and prevention among all staff (including domestic staff and cooks). Staff became more mindful of falls risks | Increased appetite to report falls risks | The training, rather than the GtACH programme (as a tool), may result in a reduction in the number of falls in the home as a result of raised awareness and knowledge of falls risks | Moderate |
| 1b | Corporate (small local chain), medium-sized home | The FinCH trial increased awareness and knowledge of falls risks and prevention among all staff (including domestic staff and cooks). Staff became more mindful of falls risks | Increased staff confidence to identify and report falls risks | The training, rather than the GtACH programme (as a tool), may result in a reduction in the number of falls in the home as a result of raised awareness and knowledge of falls risks | Moderate |
| 2a | Corporate (small local chain), medium-sized home | GtACH programme was similar to home’s existing paperwork and seen as secondary. Staff felt that they had too much existing documentation already following a fall and that the GtACH programme added to this | Lack of motivation to implement GtACH programme over and above existing documentation | GtACH programme may be adopted during the trial only and may not be used long term – home will complete its own documentation in preference to the GtACH programme | Strong |
| 2b | Corporate (small local chain), medium-sized home | GtACH programme was similar to home’s existing paperwork and seen as secondary. Staff felt that they had too much existing documentation already following a fall and the GtACH programme added to this, leaving less time for care | Lack of incentive to change | GtACH programme may be adopted during the trial only and may not be used long term – home will complete its own documentation in preference to the GtACH programme | Strong |
| 3a | Corporate (small local chain), medium-sized home | Clear internal hierarchy. Staff allocated clear roles: only senior staff/nurses complete documentation, carers complete hands-on care and daily logs | Inflexibility in structured job roles | Partial adoption. GtACH programme will be adopted only if management take ownership and then will be used by senior staff/nurses only | Weak |
| 3b | Corporate (small local chain), medium-sized home | Clear internal hierarchy. Staff allocated clear roles: only senior staff/nurses complete documentation, carers complete hands-on care and daily logs | Staff inhibited from breaking system | Partial adoption. GtACH programme will be adopted only if management take ownership and then will be used by senior staff/nurses only | Weak |
| 3c | Corporate (small local chain), medium-sized home | Clear internal hierarchy. Staff allocated clear roles: only seniors/nurses complete documentation, carers complete hands-on care and daily logs | Lack of care staff ownership | Partial adoption. GtACH programme will only be adopted if management take ownership and then only used by seniors/nurses | Weak |
| 4 | Corporate (small local chain), medium-sized home | Staff respond reactively rather than proactively to falls as per existing practice in the home | Staff lacked incentive to change current practices | Partial adoption of GtACH programme – if the GtACH programme is adopted it will be used reactively only, rather than proactively | Strong |
| 5 | Corporate (small local chain), medium-sized home | Recent changes in management resulted in a heavy workload for staff taking over the managerial role | Lack of motivation by management to take on new practices or documentation during period of upheaval | GtACH programme will be adopted only if the new management take ownership of it | No evidence |
| 6 | Corporate (small local chain), medium-sized home | The home has not adopted champion roles and staff did not feel that the falls champion would be a useful role | Lack of motivation by staff at all levels to adopt the falls champion role | Falls champion role will not be adopted | Weak |
| 0402 care home C | |||||
| 1a | Corporate local chain, medium-sized home | Systems/documentation are filtered down through the external chain. Any changes to systems/documentation require organisation approval and for the entire chain to adopt the changes | Lack of staff ownership | GtACH programme may be adopted during the trial, but it is unlikely to be used long term as its continued use requires adoption by the chain | Moderate |
| 1b | Corporate local chain, medium-sized home | Systems/documentation are filtered down through the external chain. Any changes to systems/documentation require organisation approval and for the entire chain to adopt the changes | Lack of management ownership | GtACH programme may be adopted during the trial, but it is unlikely to be used long term as its continued use requires adoption by the chain | Moderate |
| 1c | Corporate local chain, medium-sized home | Systems/documentation are filtered down through the external chain. Any changes to systems/documentation require organisation approval and for the entire chain to adopt the changes | Lack of awareness at corporate level | GtACH programme may be adopted during the trial, but it is unlikely to be used long term as its continued use requires adoption by the chain | Moderate |
| 2a | Corporate local chain, medium-sized home | Staff reported that the practical component of the training session was insufficient to enable independent use of the GtACH programme | Staff felt daunted and overwhelmed by the GtACH programme | Partial adoption – not all staff will use the GtACH programme | Moderate |
| 2b | Corporate local chain, medium-sized home | Staff reported that the practical component of the training session was insufficient to enable independent use of the GtACH programme | Lack of confidence among all grades of staff to use the GtACH programme | Partial adoption – not all staff will use the GtACH programme | Moderate |
| 3 | Corporate local chain, medium-sized home | Staff (including the falls champion) thought that they were going to receive falls awareness training, not specific GtACH training. Some of the staff struggled to recall the training and elements of the training had not been processed | Staff felt daunted and bewildered by the prospect of completing the GtACH | Partial adoption of GtACH programme – it will be used by only those staff who feel that they gained enough knowledge and skills from the training session to complete the GtACH programme | Weak |
| 4 | Corporate local chain, medium-sized home | Effective falls prevention systems/procedures/documentation already in place | No incentive to use new paperwork | GtACH programme completed during researcher observations only. Unlikely to be adopted post study | Weak |
| 5 | Corporate local chain, medium-sized home | Existing systems working at full capacity – heavy administrative and/or paperwork burden | Lack of appetite for more paperwork | GtACH programme not adopted – existing documentation and systems/procedures take precedence. The GtACH programme duplicates rather than adds new information | Strong |
| 6 | Corporate local chain, medium-sized home | Staff respond reactively rather than proactively to falls as per existing practice in the home | Staff accepting of current practices | Partial adoption of GtACH programme – if the GtACH programme is adopted it will only be used reactively rather than proactively | Weak |
| 7 | Corporate local chain, medium-sized home | The falls champion was a senior staff member who was also the Moving and Handling Lead and was very influential on whether or not the GtACH programme was completed | Adoption of GtACH programme was influenced by enthusiasm from those above (i.e. those in the falls champion role or respected senior staff/management) | GtACH programme will be adopted only if advocated for by senior/management level staff in the home | Strong |
| 8a | Corporate local chain, medium-sized home | Clear internal hierarchy of responsibility, with staff allocated clear roles. The senior staff complete all documentation. Carers did not have access to information required for completion of the GtACH programme | Inflexibility in structured job roles | Partial adoption | Strong |
| 8b | Corporate local chain, medium-sized home | Clear internal hierarchy of responsibility, with staff allocated clear roles. The senior staff complete all documentation. Carers did not have access to information required for completion of the GtACH programme | Care staff inhibited from breaking system | GtACH programme – if adopted, the GtACH programme will be completed by senior staff only | Strong |
| 8c | Corporate local chain, medium-sized home | Clear internal hierarchy of responsibility, with staff allocated clear roles. The senior staff complete all documentation. Carers did not have access to information required for completion of the GtACH programme | Lack of care staff ownership | Partial adoption | Strong |
| 8d | Corporate local chain, medium-sized home | Clear internal hierarchy of responsibility, with staff allocated clear roles. The senior staff complete all documentation. Carers did not have access to information required for completion of the GtACH programme | Care staff were anxious about the responsibility of completing documentation | GtACH programme– if adopted, the GtACH programme will be completed by senior staff only | Strong |
| 0302 care home D | |||||
| 1a | National corporate chain, medium-sized home | Systems/paperwork filtered down through the external chain. Clear internal hierarchy of management and responsibility. Only the senior staff complete the care plans | Lack of staff ownership | GtACH programme will be adopted during the trial only. It is unlikely to be used long term as its continued use requires adoption by the national chain | Moderate |
| 1b | National corporate chain, medium-sized home | Systems/paperwork filtered down through the external chain. Clear internal hierarchy of management and responsibility. Only the senior staff complete the care plans | Lack of ownership on the part of local management | GtACH programme will be adopted during the trial only. It is unlikely to be used long term as its continued use requires adoption by the national chain | Moderate |
| 1c | National corporate chain, medium-sized home | Systems/paperwork filtered down through the external chain. Clear internal hierarchy of management and responsibility. Only the senior staff complete the care plans | Lack of awareness at corporate level | GtACH programme will be adopted during the trial only. It is unlikely to be used long term as its continued use requires adoption by the national chain | Moderate |
| 2a | National corporate chain, medium-sized home | Established/fixed roles | Inflexibility in structured job roles | Partial adoption of GtACH programme – if the GtACH programme is used, it will be used by senior staff/management only | Strong |
| 2b | National corporate chain, medium-sized home | Established/fixed roles | Staff inhibited from breaking system | Partial adoption of GtACH programme – if the GtACH programme is used it will only be used by seniors/management | Strong |
| 2c | National corporate chain, medium-sized home | Established/fixed roles | Staff lack the access/authority for elements of the GtACH programme | Partial adoption of GtACH programme if the GtACH programme is used it will only be used by seniors/management | Strong |
| 3 | National corporate chain, medium-sized home | Effective and thorough systems already in place | Inertia: long-standing systems and processes inhibit innovation | Partial adoption of GtACH programme if the GtACH programme is used, it is likely that only some elements of the tool will be used, alongside the pre-existing paperwork/systems | Strong |
| 4 | National corporate chain, medium-sized home | Staff respond reactively rather than proactively to falls and few falls had occurred (during the day) during the study | Staff accepting of current role | Partial adoption of GtACH programme | Strong |
| 5 | National corporate chain, medium-sized home | Knowledgeable staff who had received internal training on falls prevention | Little motivation for change (inertia?) | Partial adoption – persistence of existing practice | Strong |
| 6 | National corporate chain, medium-sized home | GtACH training increased knowledge base of staff | Staff may use the GtACH programme as a prompt/reminder to identify risks/actions, rather than as a stand-alone tool. Staff accepting of existing paperwork/systems | Partial adoption – GtACH programme used as a visual prompt/reminder only | Strong |
| 7 | National corporate chain, medium-sized home | Residential home with no nursing provision. Some of the GtACH programme components are perceived as inappropriate for the residential care setting (i.e. measuring blood pressure) | Staff reluctant to complete the GtACH programme through fear it will be completed incorrectly | Partial adoption – reluctance by some senior staff to complete GtACH programme through fear of completing incorrectly | No evidence |
| 0209 care home E | |||||
| 1 | Small independent home | Effective system already in place. These systems take precedence, with stipulated deadlines for completion | Inertia | Partial adoption of GtACH programme – if the GtACH programme is used, it is likely that management will pick and choose elements to improve existing paperwork | Strong |
| 2a | Small independent home | Established/fixed roles | Inflexibility in structured job roles | Partial adoption of GtACH programme – if the GtACH programme is used it will be used by senior staff/management only | No evidence |
| 2b | Small independent home | Established/fixed roles | Staff inhibited from breaking system | Partial adoption of GtACH programme – if the GtACH programme is used, it will be used by senior staff/management only | No evidence |
| 2c | Small independent home | Established/fixed roles | Accountability for paperwork rests with senior staff – care staff do not want to be responsible | Partial adoption of GtACH programme – if the GtACH programme is used it will be used by senior staff/management only | No evidence |
| 3a | Small independent home | Top-down approach to management (manager led). Experienced manager in place for 3 years and is visible on the floor. Manager did not attend the GtACH training | Lack of staff motivation to implement GtACH programme | GtACH programme will be adopted during the trial only and is unlikely to be used long term | Weak |
| 3b | Small independent home | Top-down approach to management (manager led). Experienced manager in place for 3 years and is visible on the floor. Manager did not attend the GtACH training | Lack of ownership on the part of staff | GtACH programme will be adopted during the trial only and is unlikely to be used long term | Weak |
| 4 | Small independent home | Many high-functioning residents who self-care and have few medical needs. Few residents with dementia. Few residents considered to be at a high risk of falling | Inertia | Partial adoption of GtACH programme – not used as intended (i.e. as a proactive tool) | Moderate |
| 5 | Small independent home | Staff know the residents well. Staff support residents to be independent and make choices/take risks | Inertia: little motivation to adopt a tool that duplicates rather than adds to information about the residents | Partial adoption of GtACH programme | Moderate |
| 6 | Small independent home | Paperwork not standardised by a chain: adopt their own paperwork, and pick up ideas and tools from external sources | Staff appetite to seek out new tools that can either be adopted or adapted | GtACH programme may be implemented if found favourable once trialled or may be adapted to incorporate into existing tools | Strong |
| 7 | Small independent home | Isolated from other homes. Fewer opportunities for training and support because it is an isolated home. Manager/deputy attend external training and cascade down to staff | Staff motivated to attend training | Good attendance at the GtACH training (as training was in-house) | Moderate |
| 8 | Small independent home | Carers lack confidence in completing paperwork. Residential setting so not comfortable with nursing tasks (i.e. blood pressure monitoring) and medical terminology. Need reassurance that they are completing the GtACH programme correctly | Staff reluctant to complete the GtACH programme through fear it will be incorrect and they will be accountable | Partial adoption – GtACH programme not completed as intended. Only senior staff/management will adopt the GtACH programme | Contextual evidence only |
| 9 | Small independent home | Established/fixed roles. Senior staff write care plans, not carers | Inflexibility in structured job roles | Moderate | |
| 0107 care home F | |||||
| 1 | Small local chain, large home | Clear internal hierarchy of responsibility, with staff allocated clear roles. Frequent changes in management affected working practices in the home | Lack of staff ownership on the part of staff working on the floor. Practices determined by management | GtACH programme will be adopted only if management take ownership. Staff have no authority to use the GtACH programme unless management insert the GtACH programme into the residents’ notes | Moderate |
| 2a | Small local chain, large home | Established/fixed roles. Only staff in senior or management roles complete documentation. Care staff provide the hands-on care and report back to the staff in senior or management roles | Inflexibility in structured job roles | Partial adoption of GtACH programme – GtACH programme will be used by staff in senior or management roles only | Moderate |
| 2b | Small local chain, large home | Only staff in senior or management roles complete documentation. Care staff provide the hands-on care and report back to the staff in senior or management roles | Lack of incentive to change working systems | Partial adoption of GtACH programme – GtACH programme will be used by staff in senior or management roles only | Moderate |
| 2c | Small local chain, large home | Established/fixed roles. Only staff in senior or management roles complete documentation. Care staff provide the hands-on care and report back to the staff in senior or management roles | Staff lack the access/authority for elements of the GtACH programme | Partial adoption of GtACH programme – GtACH programme will be used by staff in senior or management roles only | Moderate |
| 3 | Small local chain, large home | GtACH programme perceived as more robust than existing documentation and as useful for information provision when liaising with medical professionals | Staff motivated to use GtACH programme as a memory aid and a prompt to complete own documentation | Partial adoption of GtACH programme – GtACH programme is likely to be used alongside rather than instead of pre-existing paperwork/systems | Strong |
| 4a | Small local chain, large home | Staff respond reactively rather than proactively to falls | Staff accepting of current practices | Partial adoption of GtACH programme | Strong |
| 4b | Small local chain | Staff respond reactively rather than proactively to falls, as their responsibility is for corporate and government reporting of AEs | Lack of incentive to change practice | Partial or no adoption of GtACH programme | Strong |
| 5a | Small local chain, large home | Neither the acting compliance manager nor the permanent manager were working in the home at the initiation of the process evaluation or attended the training | Lack of incentive to implement GtACH programme | GtACH programme will be adopted only if management take ownership and filter it down to the staff on the floor | Contextual evidence only |
| 5b | Small local chain, large home | Neither the acting compliance manager nor the permanent manager were working in the home at the initiation of the process evaluation or attended the training | Lack of ownership on the part of staff | GtACH programme will be adopted only if management take ownership and filter it down to the staff on the floor | Contextual evidence only |
| 5c | Small local chain | Neither the acting compliance manager nor the permanent manager were working in the home at the initiation of the process evaluation or attended the training | Lack of staff autonomy | GtACH programme will be adopted only if management take ownership and filter it down to the staff on the floor | Contextual evidence only |
| 6a | Small local chain, large home | Staff were keen to attend the training as there is little falls awareness training provided in the locality. The training sessions were prioritised in the home | Staff motivated to attend training | Partial adoption – not all staff will use the GtACH programme, with some preferring to continue using familiar documentation | Weak |
| 6b | Small local chain, large home | Staff were keen to attend the training as there is little falls awareness training provided in the locality. The training sessions were prioritised in the home | Staff anxious completing the GtACH programme as it represents new and unfamiliar documentation | Partial adoption – not all staff will use the GtACH programme, with some preferring to continue using familiar documentation | Weak |
| 7a | Small local chain, large home | This was a site that joined the study late and the NHS falls lead was not trained alongside the other NHS falls leads, receiving their training at the site initiation visit | Lack of confidence to use the GtACH programme as a result of insufficient knowledge | Partial adoption – not all staff will use the GtACH programme | Weak |
| 7b | Small local chain, large home | This was a site that joined the study late and the NHS falls lead was not trained alongside the other NHS falls leads, receiving their training at the site initiation visit | Lack of confidence to use the GtACH programme as a result of insufficient support | Partial adoption – not all staff will use the GtACH programme | Weak |
| 7c | Small local chain, large home | This was a site that joined the study late and the NHS falls lead was not trained alongside the other NHS falls leads, receiving their training at the site initiation visit | Inertia to use the GtACH programme because of a misperception that it is limited to the study rather than a broader tool for reducing falls risks | Partial adoption – not all staff will use the GtACH programme | Weak |
| 8a | Small local chain, large home | Successive/late changes in management at this home at the beginning of the study | Uncertainty/lack of leadership to implement study | Late adoption. GtACH programme not placed in residents’ notes until 5 months into the study | No evidence |
| 8b | Small local chain, large home | Changes in management resulted in slow implementation of the GtACH programme in this home, with the GtACH programme not inserted into the residents’ notes until 5 months into the process evaluation | Lack of knowledge at management level | Late adoption – the GtACH programme will only be adopted once management take ownership of the GtACH programme and insert it into the residents’ notes | Contextual evidence only |
| 8c | Small local chain, large home | Changes in management resulted in slow implementation of the GtACH programme in this home, with the GtACH programme not inserted into the resident’s notes until 5 months into the process evaluation | Lack of ownership at management level | Late adoption – the GtACH programme will only be adopted once management take ownership of the GtACH programme and insert it into the residents’ notes | Contextual evidence only |
| 8d | Small local chain | Changes in management resulted in slow implementation of the GtACH programme in this home, with the GtACH programme not inserted into the resident’s notes until 5 months into the process evaluation | Lack of staff autonomy | Late adoption – the GtACH programme will only be adopted once management take ownership of the GtACH programme and insert it into the residents’ notes | Contextual evidence only |
Appendix 9 Adapted research cycle
FIGURE 17.
Adapted research cycle.
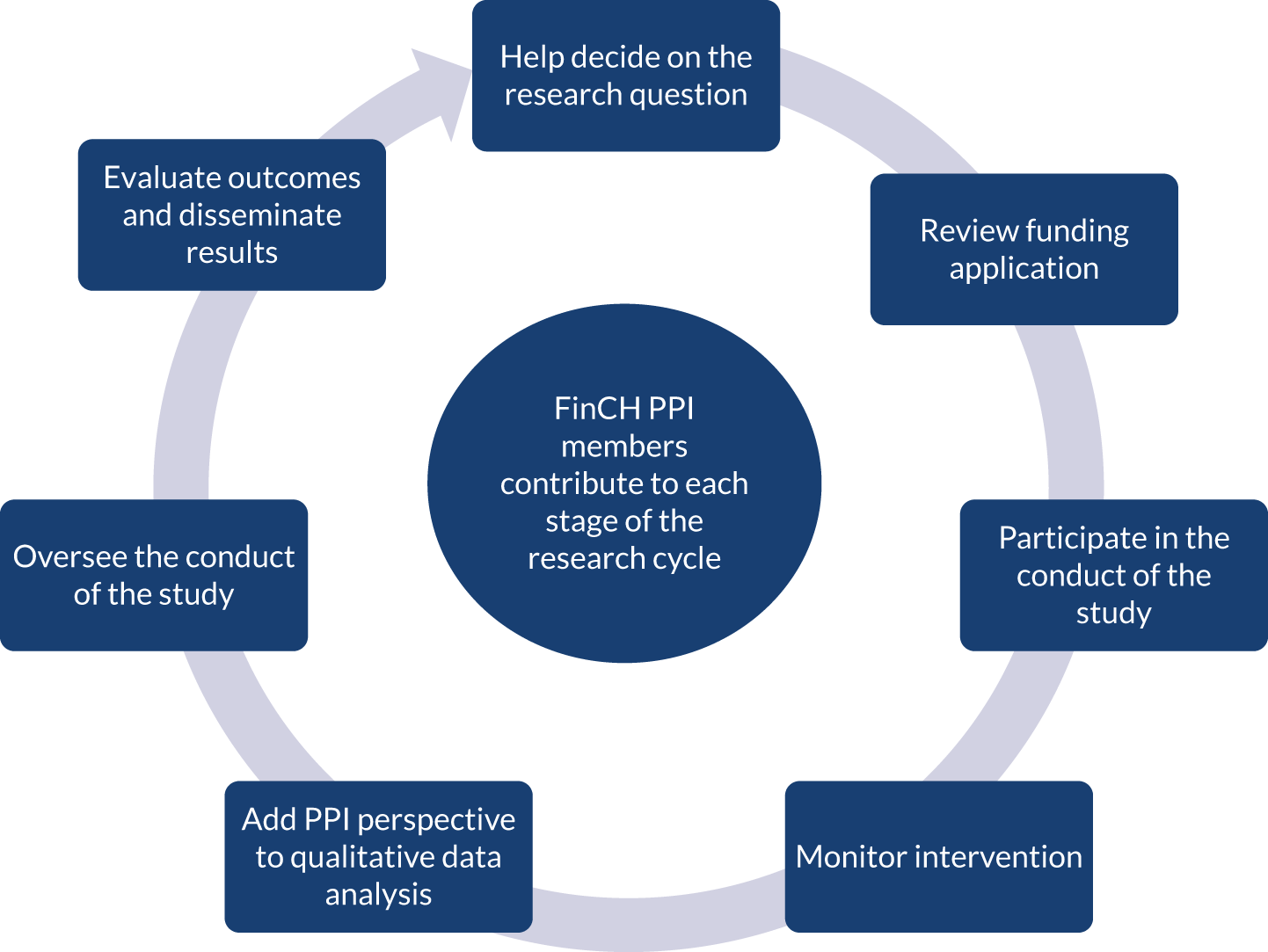
Glossary
- Guide to Action for falls prevention in Care Homes poster
- A poster to display in the care home to promote and maintain fall prevention awareness.
- Guide to Action for falls prevention in Care Homes programme
- Training and support for care home staff in the use of an individualised resident falls risk factor identification and decision support tool for the prevention and management of falls in care homes. The programme includes the Guide to Action for falls prevention in Care Homes training and support, Guide to Action for falls prevention in Care Homes tool, Guide to Action for falls prevention in Care Homes reference manual and Guide to Action for falls prevention in Care Homes poster.
- Guide to Action for falls prevention in Care Homes reference manual
- A reference manual for use during and after the training, including copies of slides and the Guide to Action for falls prevention in Care Homes tool, information regarding medications that can increase risk of falls and a case study with a completed Guide to Action for falls prevention in Care Homes tool for guidance and reference.
- Guide to Action for falls prevention in Care Homes tool
- A paper-based individualised resident falls risk factor identification and decision support tool supported by printed materials (Guide to Action for falls prevention in Care Homes reference manual and Guide to Action for falls prevention in Care Homes poster).
- Guide to Action for falls prevention in Care Homes training and support
- Training and support for care home staff in the use of the Guide to Action for falls prevention in Care Homes tool.
List of abbreviations
- A&E
- accident and emergency
- ADL
- activities of daily living
- AE
- adverse event
- CEA
- cost-effectiveness analysis
- CEAC
- cost-effectiveness acceptability curve
- CGA
- comprehensive geriatric assessment
- CI
- confidence interval
- CMO
- context–mechanism–outcome
- CONSORT
- Consolidated Standards of Reporting Trials
- CQC
- Care Quality Commission
- CRF
- case report form
- CRN
- Clinical Research Network
- DEMQOL
- Dementia Specific Quality of Life
- DEMQOL-P
- Dementia Quality of Life, proxy complete
- DEMQOL-U
- Dementia Quality of Life Utility version
- DEMQOL-P-U
- Dementia Quality of Life Utility version, proxy complete
- DMC
- Data Monitoring Committee
- ENRICH
- Enabling Research In Care Homes
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- EQ-5D-5L-P
- EuroQol-5 Dimensions, five-level version, proxy complete
- FiCH
- Falls in Care Homes
- FinCH
- Falls prevention in Care Homes
- GCP
- good clinical practice
- GEE
- generalised estimating equation
- GP
- general practitioner
- GtACH
- Guide to Action for falls prevention in Care Homes
- HEAP
- health economics analysis plan
- HES
- Hospital Episode Statistics
- HRQoL
- health-related quality of life
- ICD10
- International Statistical Classification of Diseases and Related Health Problems, Tenth Revision
- ICER
- incremental cost-effectiveness ratio
- IRR
- incidence rate ratio
- ITT
- intention to treat
- MI
- multiple imputation
- NCTU
- Norwich Clinical Trials Unit
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- PAM-RC
- Physical Activity and Mobility in Residential Care
- PC
- personal consultee
- PI
- principal investigator
- PIS
- participant information sheet
- PPI
- patient and public involvement
- PSS
- personal social services
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RA
- research assistant
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SD
- standard deviation
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/CWIB0236).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
