Notes
Article history paragraph text
The research reported in this issue of the journal was funded by the HTA programme as project number 09/146/01. The contractual start date was in August 2010. The draft report began editorial review in July 2012 and was accepted for publication in January 2013. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors' report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
ARP has received reporting fees from Nuada Medical Prostate Care for patients with prostate cancer who were scanned at their medical facilities. JR has received consultancy honoraria and payment for lectures, expert testimony and travel/accommodation/meetings expenses from GlaxoSmithKline, and consultancy honoraria, payment for lectures and travel/accommodation/meetings expenses from Ipsen. TS has received payment for travel/accommodation/meeting expenses from multiple sources and his institution has received payment from Siemens Healthcare for a research collaboration.
Permissions
Copyright statement
© Queen's Printer and Controller of HMSO 2013. This work was produced by Mowatt et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Executive summary
Background
In the UK, prostate cancer (PC) is the most common cancer in men. Many men find themselves with the dilemma of having an elevated prostate-specific antigen (PSA) level and a negative prostate biopsy, and the best way for doctors to manage these patients remains uncertain. The strategy of further repeat biopsies for these men remains controversial, with uncertainties surrounding the optimal number of cores, which area of the prostate to target, and imaging modality for guidance. This has led to the introduction of new imaging techniques. Conventional standard (T2-weighted) magnetic resonance imaging (T2-MRI) can be performed with add-on modalities, including three-dimensional magnetic resonance spectroscopy (MRS), dynamic contrast-enhanced MRI (DCE-MRI) and diffusion-weighted MRI (DW-MRI).
Objectives
This review aims to assess the diagnostic accuracy of MRS and enhanced MRI techniques (DCE-MRI, DW-MRI) and the clinical effectiveness and cost-effectiveness of strategies involving their use in aiding the localisation of prostate abnormalities for biopsy in patients with prior negative biopsy in whom there remains a clinical suspicion that they are harbouring malignancy.
Methods
Electronic databases searched included MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations, EMBASE, Bioscience Information Service (BIOSIS), Science Citation Index (SCI), Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Database of Systematic Reviews (CDSR), Database of Abstracts of Reviews of Effects (DARE), Medion, Health Technology Assessment database, conference abstracts from the American Society for Clinical Oncology (ASCO) and current research registers. Searches were carried out from 1995 to March 2012. Types of studies considered were direct studies or randomised controlled trials reporting absolute numbers of true- and false-positives and true- and false-negatives, allowing the calculation of sensitivity, specificity or predictive values. The population was men with suspected PC and elevated PSA level but previously negative biopsy. Index tests were MRS, DCE-MRI and DW-MRI, and comparator tests were standard T2-MRI and transrectal ultrasonography (TRUS). The reference standard was histopathological assessment of biopsied tissue obtained via transrectal needle biopsy, saturation biopsy, transperineal template biopsy or from prostatectomy specimens.
Two reviewers independently screened the titles and abstracts of all reports identified by the search strategy and full-text papers were subsequently obtained for assessment. Data extraction was undertaken by one reviewer and checked by a second. Two reviewers independently assessed the risk of bias of the diagnostic studies using a modified version of the QUADAS-2 (quality assessment of diagnostic accuracy studies, version 2) instrument.
The results of the individual studies were tabulated and sensitivity, specificity and their 95% confidence intervals (CIs) presented for each test or combination of tests at both patient and biopsy level. The presence of heterogeneity was assessed by visual examination of pairs of forest plots of sensitivity and specificity. Separate summary receiver operating characteristic (SROC) curves were derived for different levels of analysis. Meta-analysis models were fitted using hierarchical SROC (HSROC) curves. Summary sensitivity, specificity, positive and negative likelihood ratios and diagnostic odds ratios for each model were reported as median and 95% CI. An indirect comparison of tests was also undertaken.
An economic model was developed to assess the cost-effectiveness of using alternative MRS/MRI sequences to direct TRUS-guided biopsies (TRUS/Bx), compared with the standard practice of relying on systematic extended TRUS-guided biopsies (in patients with a previous negative biopsy). The alternative diagnostic pathways were embedded in a Markov model simulating the progression of undiagnosed cancer and the downstream impact of diagnosis and treatment on survival and health-related quality of life (QoL). Costs incorporated in the model included the costs associated with obtaining the final diagnosis (cancer/no cancer), management of biopsy complications, cancer staging, cancer treatment, and the management of complications resulting from cancer treatment. Survival benefits of diagnosis were captured through the application of relative risk parameters reflecting the benefit of appropriate treatment by stage of underlying cancer. Health-state utilities associated with cancer stage and the occurrence of treatment complications were incorporated in the model to estimate quality-adjusted life-years (QALYs). Experimental strategies were compared incrementally with standard practice in terms of their incremental cost per life-year and QALY gained.
Results
Number and quality of studies
Fifty-one studies (39 full text and 12 abstracts) were included, involving over 10,000 men. Only full-text studies were assessed for risk of bias, the majority of which were considered to have a low risk of bias for the patient selection (74%, 29/39), index test (100%, 39/39) and flow and timing (92%, 36/39) domains. In the reference standard domain, the majority of studies (64%, 25/39) were considered at high risk of bias owing to a lack of follow-up.
Summary of benefits and risks
In meta-analyses of the individual tests, sensitivity was highest for MRS at 92% (95% CI 86% to 95%), followed by T2-MRI at 86% (95% CI 74% to 93%) and DCE-MRI at 79% (95% CI 69% to 87%), whereas specificity was highest for TRUS (used as an imaging test) at 81% (95% CI 77% to 85%), followed by MRS at 76% (95% CI 61% to 87%). In pooled estimates for combinations of tests, sensitivity was highest for ‘MRS or T2-MRI’ at 96% (95% CI 90% to 98%) followed by ‘DCE-MRI or T2-MRI’ at 88% (95% CI 80% to 96%), whereas specificity was highest for ‘MRS and T2-MRI’ at 74% (95% CI 65% to 84%). Only one small study involving 43 patients reported DW-MRI, with sensitivity of 100% (specificity not reported). The results of the indirect comparison broadly reflected those of the meta-analyses of the individual tests and combinations of tests.
Summary of costs
The base-case analysis showed average discounted lifetime costs to range between £3895 using systematic TRUS-guided biopsies and £4056 using positive findings on either T2-MRI or DCE-MRI to determine and direct biopsies (60-year-old cohort, cancer prevalence 24%). The corresponding figures for the same strategies in a 70-year-old cohort were £3199–3660. Using T2-MRI to direct biopsies represented the least costly approach in low-prevalence (10%) cohorts.
Summary of cost-effectiveness
Survival and QALY differences between strategies were very small but these favoured more sensitive approaches. Under base-case parameter values and assumptions (with underlying cancer prevalence 24%), the incremental cost-effectiveness ratio (ICER) for T2-MRI was < £30,000 per QALY in comparison with systematic extended-cores TRUS/Bx (all cohorts) and T2-MRI was found to dominate extended-cores TRUS/Bx in low-prevalence cohorts. However, probabilistic sensitivity analysis demonstrated a high degree of uncertainty surrounding the incremental cost-effectiveness of T2-MRI compared with extended-cores TRUS/Bx in the moderate prevalence cohorts. The cost-effectiveness of MRS compared with T2-MRI was less favourable under base-case assumptions, although its ICER did fall to < £30,000 compared with extended-cores TRUS/Bx in the moderate prevalence 60-year-old cohort, and also compared with T2-MRI-directed biopsy in the high-prevalence 60-year-old cohort. The ICER for MRS, or any of the other more sensitive strategies, did not fall to < £30,000 in any of the 70-year-old cohorts under base-case assumptions.
Sensitivity analyses
Base-case findings were found to be highly sensitive to a number of uncertain parameters and assumptions. The cost-effectiveness of using MRS to direct biopsies was found to be particularly sensitive to the cost of prostate biopsies relative to the cost of obtaining a MRS sequence. When the cost of obtaining biopsies was raised by ∼£115 relative to the cost of MRS, MRS-directed biopsy was found to dominate extended-cores TRUS/Bx in all of the cohorts, and its ICER dropped to < £30,000 in comparison with the T2-MRI-directed approach in the moderate- and high-prevalence 60-year-old cohorts (although it remained > £30,000 in all of the 70-year-old cohorts). The cost-effectiveness of MRS was also crucially sensitive to its modelled ability to discriminate between low- and moderate-/high-risk cancer. When all of its false-negative findings were modelled to occur in patients with low-risk disease, its cost-effectiveness improved substantially in the moderate- and high-prevalence 60-year-old cohorts, although its ICERs remained less favourable in the 70-year-old cohorts. Factors undermining the cost-effectiveness of MRS included the application of lower disease progression rates and lower relative risk reductions associated with diagnosis and treatment. Although a lack of available evidence precluded its inclusion in our base-case analysis, if DW-MRI could be shown to perform similarly to MRS in terms of diagnostic accuracy, it would probably be favoured over MRS for its lower cost.
Discussion
Strengths, limitations of the analyses and uncertainties
In terms of strengths, a comprehensive literature search was undertaken. A HSROC model was used, which takes account of the trade-off between true/false-positives and models between-study heterogeneity. Pooled estimates were performed at both patient and biopsy level and an indirect comparison of tests was undertaken. In terms of limitations, non-English-language studies were excluded. Few studies reported DCE-MRI or DW-MRI or included a period of follow-up as part of the reference standard. The index and comparator tests were not independent of the reference standard.
In terms of uncertainties, where studies reported an ‘equivocal’ results category, this was classed with positive rather than negative results, increasing sensitivity and decreasing specificity, whereas the reverse would have been the case if ‘equivocal’ had been classed with negative results. There was only limited evidence available of the ability of MRS and other MRI techniques to detect clinically significant disease. In studies reporting MRS or other MRI techniques a systematic TRUS/Bx was also undertaken and in most of these studies it was unclear how this contributed to sensitivity and specificity values reported.
Generalisability of the findings
All studies included in the pooled estimates reported men with suspected PC and elevated PSA level but previously negative biopsy, and therefore these findings would be broadly generalisable to patients meeting the above criteria. However, in one study the spectrum of patients was not representative (all had atypical small acinar proliferation). In two studies imaging was MR-guided (rather than TRUS-guided), a method not generally used in the UK. Six studies reporting TRUS-guided systematic biopsies were large screening studies, which is not representative of how men are detected with PC in the UK.
Conclusions
Implications for service provision
Given the level of uncertainty surrounding several key model inputs, it is difficult to arrive at definitive conclusions on the cost-effectiveness of using different MRS/MRI sequences to aid the localisation of prostate abnormalities for biopsy. However, our modelling suggests that, under certain circumstances, T2-MRI may be considered cost-effective in comparison with systematic TRUS/Bx, and if MRS and DW-MRI can be shown to have high sensitivity for detecting moderate-/high-risk cancer, while negating the need for patients with no cancer or low-risk disease to undergo biopsy, then their use could represent a cost-effective approach to diagnosis.
The introduction of MRS and other MRI techniques (T2-MRI, DCE-MRI, DW-MRI) for evaluation of men with negative TRUS/Bx but in whom there remains suspicion of cancer would have a range of implications for the NHS. These would arise primarily because of a shift in the test–treatment pathway for this group, with changes in the method of making diagnosis resulting in changes to the types of patients being treated, offered patient options and timings of treatments. This would have consequential effects on service provision, costs and training. If urological and/or radiological services were to undertake targeted biopsies of MRS-/MRI-suspicious regions then extra provision would be required for this. A new generation of equipment and software would be needed to enable accurate, documentable biopsies to be obtained from all regions of the prostate. If MRS/MRI identified more patients with localised disease with intermediate and high risk of progression then this would increase the proportion of patients considered eligible for radical therapies. If MRS or MRI detected few patients with low risk of disease progression then fewer patients in this category would undergo perhaps inappropriate radical therapies. Thus, the total number of patients undergoing radical therapies would be appropriately decreased, requiring a rebalancing of resources currently allocated to surgical and radiation therapy services. Furthermore, if MRS or MRI contributed to the more accurate classification of patients with a low risk of progression, this would lead to an increase in the proportion of appropriately selected patients who are likely to undergo ‘active surveillance’, helping to mitigate the current high dropout rate of this approach. The implications for the follow-up of active surveillance patients would include repeated PSA testing, repeated interval biopsies and follow-up clinics (much of this work is protocol driven and could be nurse practitioner led). Taken together, earlier, more accurate diagnoses and more appropriate treatments of PC may improve patient outcomes by reducing treatment-related morbidity, improving survival and, in the longer term, reducing the requirement for end-of-life and palliative care services. There would be cost implications of these service reconfigurations and for changes in treatment patterns mentioned above. Implementation would also result in the need for further training of all staff involved in delivering care to patients with PC.
Suggested research priorities
Prospective studies are required in men with suspected PC in whom PSA level is elevated but a previous biopsy has been negative, comparing the utility of the individual and combined components of a multiparametric magnetic resonance (MR) approach (MRS, DCE-MRI and DW-MRI) with both a MR-guided or -directed biopsy session and an extended 14-core TRUS/Bx scheme against a reference standard of histopathological assessment of biopsied tissue obtained via saturation biopsy, template biopsy or prostatectomy specimens. A follow-up time of 12 months should form part of the reference standard. Investigations of DW-MRI should be encouraged, as it is already gaining widespread acceptance in the clinic owing to its relatively easy use. These studies should also report the sensitivity of the tests in detecting clinically significant disease (Gleason score of ≥ 7 and/or volume > 0.5 ml). In addition to diagnostic outcomes, adverse event data and impact of the tests on subsequent physician attitudes to patient management should also be obtained, as well as cost-effectiveness data including impact of testing on health-related QoL.
Uncertainties surrounding cost-effectiveness could be significantly reduced by future research focusing on generating comparable estimates of (1) the sensitivity of MRI-/MRS-directed and systematic approaches to TRUS/Bx (using a robust and common reference standard); (2) the prospective sensitivity or specificity of MRS or MRI sequences for detecting different grades of localised disease in the repeat biopsy setting; and (3) the full economic costs of MRI sequences and systematic approaches to TRUS/Bx based on different numbers of cores.
Further, with the survival and QALY differences between strategies being so small, and of questionable clinical significance, the choice between strategies might be better informed by patient or public preferences for process of care factors to which the standard QALY model may be insensitive. Scope exists to carry out preference elicitation studies to identify and value the key factors influencing patients’ preferences for alternative diagnostic, monitoring, and subsequent treatment pathways.
Study registration
This study is registered as PROSPERO CRD42011001376.
Funding
Funding for this study was provided by the Health Technology Assessment programme of the National Institute for Health Research.
Chapter 1 Background
Description of health problem
Brief statement describing the health problem
The diagnosis of prostate cancer (PC) is based on a combination of measuring the serum prostate-specific antigen (PSA) level, performing a digital rectal examination (DRE) to palpate the prostate, and a prostate biopsy. Men with an elevated PSA level and/or abnormal DRE undergo a prostate biopsy, which is normally performed using a transrectal probe guided by greyscale ultrasound [or transrectal ultrasound-guided biopsy (TRUS/Bx)]. The prostate biopsy procedure is associated with some morbidity,1 including risk of infection, discomfort during the procedure, blood in urine (i.e. haematuria), rectal bleeding, blood in semen (i.e. haematospermia), risk of precipitating acute urinary retention, and perineal pain afterwards. In some cases, the TRUS/Bx will not show cancer and a repeat biopsy may be necessary. The strategy of repeat biopsies remains controversial, with TRUS/Bx-based protocols often resulting in high adverse effect profiles2 or low diagnostic accuracy. In order to overcome some of the current limitations, new imaging modalities and technologies such as magnetic resonance spectroscopy (MRS) and enhanced magnetic resonance imaging (MRI) techniques have been introduced. This present review was tasked with evaluating MRS and enhanced MRI techniques in aiding the localisation of prostate abnormalities for biopsy in men with suspected PC and elevated PSA level but previously negative biopsy, from the perspective of the NHS.
Aetiology and pathology
The prostate is located in the pelvis, lying below the bladder and encompassing the prostatic urethra (Figure 1). In a normal young adult male the gland is approximately 3 cm long and weighs approximately 20 g. 3 Histologically, the prostate consists of glandular epithelial cells and fibromuscular stroma, and is surrounded by a capsule. There are three glandular regions: peripheral zone (PZ), central zone (CZ) and transition zone (TZ). 4 The vast majority of PCs originate from glandular epithelial cells; hence, they are adenocarcinomas. Up to 70% of cancers arise in the PZ, 15–20% arise in the CZ, and 10–15% arise in the TZ. 5 The aetiology of PC remains controversial, although several risk factors have been identified. The most important risk factors include family history, ethnicity (especially men of black African, African American or black Caribbean ancestry6) and increasing age.
FIGURE 1.
Location of the prostate. Taken from CancerHelp UK, the patient information website of Cancer Research UK: http://cancerhelp.cancerresearchuk.org.
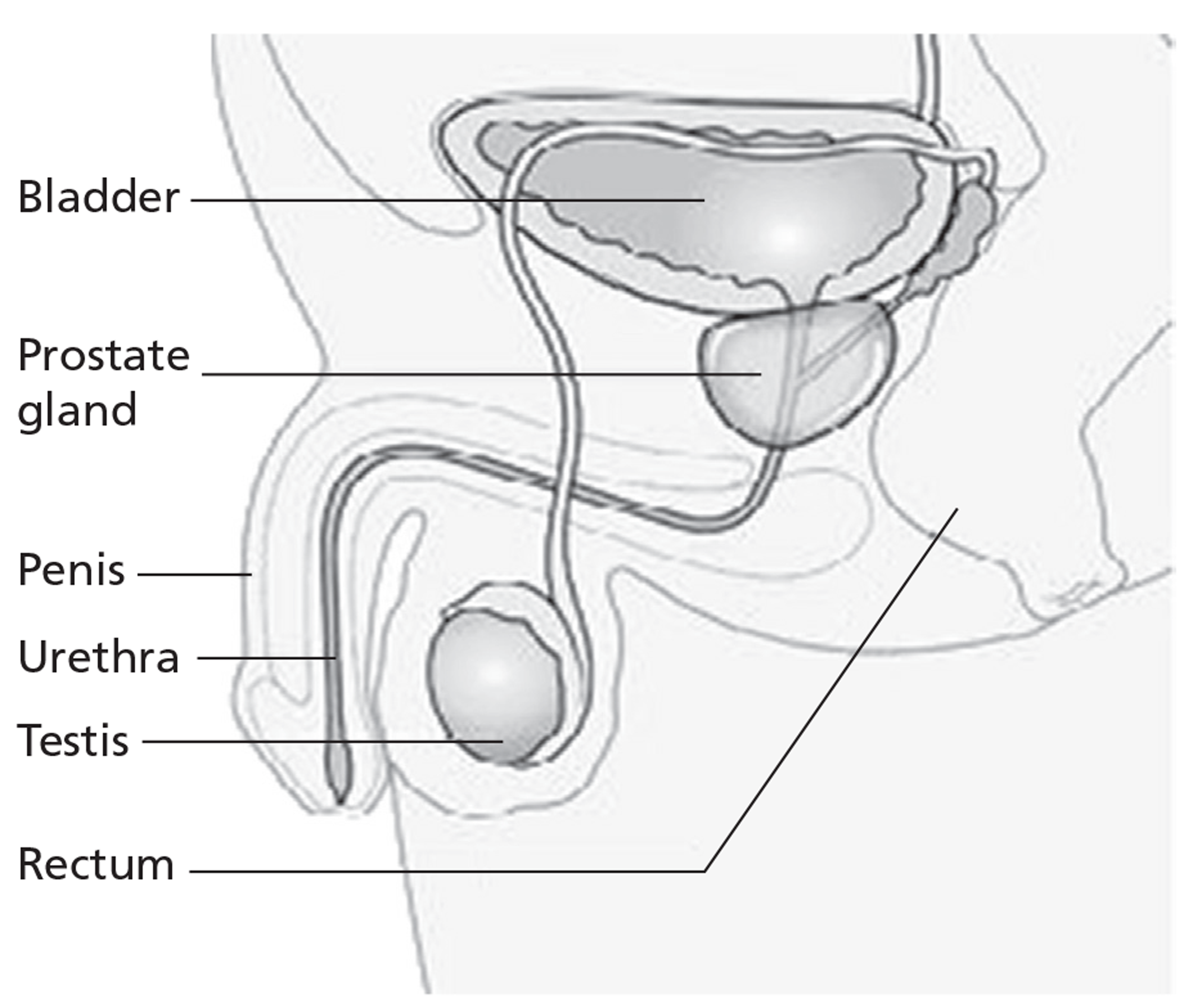
Cancer spread occurs by three possible routes: direct (or local) spread to the rectum or bladder, spread through the lymphatic channels to the pelvic lymph nodes, or spread through the blood vessels to solid organs, especially bone. Clinically, the extent of spread can be classified as localised (i.e. confined to the prostate gland), locally advanced (i.e. spread outside the capsule of the prostate gland), metastatic (i.e. distantly spread from site of origin) or hormone refractory (i.e. when the cancer becomes unresponsive to hormonal manipulation).
Epidemiology and prognosis
In the UK, PC is the commonest cancer in men and the second most common cause of cancer death in men after lung cancer. 7 Each year around 35,000 men in the UK are diagnosed with PC and more than 10,000 men die from it. 7 At the end of 2006, the number of men in the UK still living with the disease up to 10 years after diagnosis was estimated at 181,463. 7
The prognosis of patients with PC depends on several factors, especially stage of disease (i.e. extent of spread), grade of disease (i.e. histological assessment of aggressiveness, measured by the Gleason sum score), PSA level, and extent and volume of disease determined by biopsy. Since the advent of PSA testing, there has been a gradual stage migration towards the earlier stages of the disease, such that the majority of men (i.e. 80%) with PC are diagnosed when the disease is at the localised stage. 8 It has been estimated that, of asymptomatic men in whom PC is detected by prostate biopsy following PSA measurement, around 50% do not require active treatment. 9 Nearly half of patients with clinically diagnosed organ-confined disease have extraprostatic disease pathologically, whereas one-third of patients with clinically diagnosed extraprostatic disease have organ-confined disease pathologically. 10,11 With the introduction of MRI in clinical management of PC, these numbers are very likely to change. 12
Impact of health problem
Many men find themselves with the dilemma of having a persistently elevated PSA level, or persistent suspicion of cancer, and a negative biopsy. There are two possible explanations: either cancer has been missed (i.e. false-negative) or there is no cancer (i.e. true-negative). This situation can be a source of considerable uncertainty and anxiety for patients, families and friends, resulting in reduced QoL. Some patients may have friends or relatives who have PC, which may further increase anxiety. In part, anxiety is caused by a perceived delay in diagnosis and subsequent treatment. 13–15
Most men in whom there is suspicion of cancer but a previous biopsy was negative are asymptomatic. Symptoms occur when a tumour causes the prostate gland to enlarge to a significant degree or cancer spreads to areas beyond the prostate. A range of symptoms can result, including increased frequency of urination, problems starting or stopping urination, a painful burning sensation or blood in urine. 16
From a health-care services perspective, a significant amount of time and resources are directed at managing men with a suspicion of cancer but negative biopsy. These men are usually monitored either 3 or 6 monthly with PSA tests. Significant numbers of men will undergo further biopsies, either immediately or subsequently. For these men there is a risk of the diagnosis being delayed, possibly leading to disease progression (and hence compromising cure), increased morbidity and the need for more costly services.
Measurement of disease
Diagnosis of prostate cancer
Men with an elevated PSA level and/or abnormal DRE undergo a prostate biopsy, which is normally performed using TRUS/Bx. Some men with negative biopsies will require a repeat biopsy, either immediately [owing to suspicious features on histology, such as atypical small acinar proliferation (ASAP)] or subsequently (owing to a further rise in PSA, persistently raised PSA or rapidly rising PSA). 17 Achieving a diagnosis at repeat biopsy can be challenging either because they have an enlarged central prostate gland due to benign prostatic hyperplasia or because cancer is present in locations difficult to biopsy. 18 Recently, promising alternatives have emerged, which include MRS and enhanced MRI techniques. Lesions identified on MRS/MRI are sampled either by MRI-directed biopsy (tissue obtained under direct MRS/MRI imaging) or by TRUS guidance (TRUS/Bx used to identify and biopsy suspicious lesions on MRS/MRI).
Staging
Staging is performed to determine the extent of disease spread. Information from staging is essential, because it influences treatment decisions and affects prognosis.
Pre-treatment imaging staging of PC is usually individualised according to risk stratification based on clinical parameters that are predictive of the likelihood of extraprostatic disease. These clinical parameters normally include pre-treatment PSA level and rate of rise or doubling time, Gleason score, clinical T staging and volume of disease detected on biopsy. Imaging potentially improves these general estimates of risk by specifically identifying lesions with anatomical abnormalities. The most commonly used imaging modalities for staging of PC are MRI, computed tomography (CT), isotope bone scan and positron emission tomography.
Staging can be divided into local, regional and distant categories. Local staging is usually performed by DRE and MRI; regional staging is performed by either CT or MRI; and distant staging is performed by CT, bone scanning and plain bone radiography. In addition, measurement of PSA level in the blood19–21 and Gleason sum score22 can also yield useful information regarding stage. Pathological staging determines the actual extent of spread (i.e. if it is either confined to, or spread outwith, the prostate gland, or if resected lymph nodes have cancer) through histological examination. The staging system most commonly used is the tumour, node, metastasis (TNM) staging system. 23 This describes the local extent of the primary tumour (T stage), the absence or presence of spread to nearby lymph nodes (N stage) and the absence or presence of metastasis (M stage).
Grading
Grading is the histological assessment of cancer tissue to determine its aggressiveness. This is done on either biopsy tissue, resected tissue (e.g. from transurethral resection of prostate) or surgical specimens. Pathologists usually assign a grade from 1 to 5 to the most common tumour pattern observed and then a second 1–5 grade to the next most common tumour pattern. The Gleason score is the sum of these two grade assignments. 24 This scoring system describes a score between 2 and 10, with ‘2’ being the least aggressive and ‘10’ being the most aggressive,25 although most pathologists now group scores 1–6 as Gleason 6. 26
Use of nomograms to predict treatment outcomes
Nomograms are a means of predicting the probability of important outcomes following treatment using pre-treatment variables as predictors. For PC, several nomograms exist, which predict various outcomes following treatment for men with localised PC, based on pre-treatment variables such as PSA, clinical stage and Gleason score. The outcomes predicted include the probability of biochemical disease recurrence following curative treatment (Kattan nomograms21,27,28 and the D'Amico nomogram29) and the probability of various pathological stages following surgery (Partin tables30). These nomograms may be used by clinicians and health-care professionals with patients and their families to facilitate decision-making. Use of some of these nomograms has enabled the stratification of men with localised PC into risk groups according to their risk of biochemical recurrence if they were treated with radical treatment, such as radical prostatectomy or external beam radiotherapy (EBRT) (Table 1). 31 Studies have shown the added value of MRS and/or MRI in enhancing the value of normograms. 32–34
| Risk | PSA (ng/ml) | Gleason score | Clinical stage | ||
|---|---|---|---|---|---|
| Low | < 10 | and | ≤ 6 | and | T1–T2a |
| Intermediate | 10–20 | or | 7 | or | T2b–T2c |
| High | > 20 | or | 8–10 | or | T3–T4 |
Monitoring of disease following treatment
Men who have undergone curative treatments are monitored via PSA measurements, to ensure eradication of disease. Patients who develop disease recurrence will have gradual rises in their PSA level (i.e. biochemical recurrence). In addition, men with suspected local recurrence (i.e. in the pelvis) may be imaged with either MRI or CT scans, or undergo TRUS-guided prostate biopsy to confirm local disease recurrence. However, the benefit of these investigations remains controversial. 17 Patients with more rapid rises in PSA level may have disease outside of the pelvis and more extensive investigations are performed, including a bone scan.
Current service provision
Management of disease
Management of localised prostate cancer
A range of treatment options exist for men with localised PC, ranging from active surveillance for low-risk disease, whereby treatment is deferred until the cancer progresses or becomes more aggressive, to minimally invasive treatments that ablate a part of the prostate [such as high-intensity focused ultrasound (HIFU) and cryotherapy] and to immediate curative treatments (including invasive treatments such as radical prostatectomy, radiation treatment or brachytherapy). 35 Curative treatments may result in significant side effects, including urinary incontinence (UI), erectile dysfunction (ED) or troublesome urinary symptoms. 36
Based on current National Institute for Health and Care Excellence (NICE) guidance,9 Table 2 outlines the alternative treatment modalities recommended by PC stage at time of diagnosis. It has been noted that the vast majority of patients identified from second biopsies have localised cancer and few fall into the high-risk group. 2,37,38 Based on routinely collected data on hospital episodes in Scotland (Dr Karina Laing, MSc in Surgical Sciences thesis, University of Edinburgh, May 2012, personal communication), it is estimated that the majority of patients with localised disease receive active surveillance (40%), radical prostatectomy (35%) or EBRT (25%) in the first year following diagnosis.
| Treatment options | Cancer stage (risk stratification) | |||||
|---|---|---|---|---|---|---|
| Localised (low risk) | Localised (intermediate risk) | Localised (high risk) | Locally advanced | Metastatic | Hormone refractory | |
| Watchful waiting | ✓ | ✓ | ✓ | |||
| Active surveillance | ✓a | ✓ | ||||
| Prostatectomy | ✓ | ✓a | ✓a | |||
| EBRT | ✓ | ✓a | ✓a | |||
| Brachytherapy | ✓ | ✓ | ||||
| Cryotherapy | ||||||
| HIFU | ||||||
| EBRT + neoadjuvant/adjuvant hormone therapy | ✓ | |||||
| Hormone therapy (first, second lines) | ✓ | ✓ | ||||
| Chemotherapy | ✓ | |||||
Management of locally advanced prostate cancer
The vast majority of patients with locally advanced PC will undergo potentially curative hormone manipulation [castration, luteinising hormone-releasing hormone (LHRH) agonists or antagonists] for a minimum of 2 years plus radiotherapy. In the UK, for radical radiotherapy, most men receive 72 grays (Gy) in 36–37 fractions.
A small percentage of men may undergo radical prostatectomy for previously unsuspected T3 disease, T3 disease with severe lower urinary tract symptoms or patient preference where radiotherapy is contraindicated or problematic. Some will be cured by their surgery but those who are not will mostly be offered adjuvant radiotherapy. Men who would not benefit from radical treatment because of comorbidities are usually offered immediate or deferred hormone manipulation.
Management of metastatic disease
Patients who are initially diagnosed with metastatic disease receive first-line treatment with hormone manipulation. When first-line treatment fails, second-line hormone manipulation with the addition of an anti-androgen is usually initiated. If this is unsuccessful, those who are fit enough are offered chemotherapy. If unsuitable for chemotherapy, or after unsuccessful chemotherapy, third-line hormonal treatment may be initiated. Timing of third-line hormonal treatment, with respect to chemotherapy, varies throughout the UK and may change with the introduction of abiraterone (Zytiga®, Janssen Biotech). 39
Current service cost
It is difficult to estimate current PC diagnosis costs in the UK owing to limitations in the reporting of biopsies carried out as outpatient procedures. However, the number of new PC cases diagnosed in 2009 was 40,841. If we assume that approximately 25%40 of these cancers were detected by repeat TRUS-guided needle biopsies, and the cancer detection rate is approximately 25%,14,38 then it is not unreasonable to assume that approximately 41,000 repeat biopsies were performed in the UK in 2009. The 2009–10 NHS reference cost for the Healthcare Resource Group (HRG) to which needle biopsy of the prostate maps (LB27Z, outpatient procedure) was £212. 41 This would suggest an absolute lower limit for the cost of repeat prostate biopsies to the NHS of ∼£8.7M in 2009. In reality, this will be higher as a significant proportion of biopsies will have been reimbursed as day-case activity, and commissioning practice may vary by location. Given the limitations of outpatient reporting, it is difficult to ascertain exactly what this proportion is.
Considering the impact of diagnosing localised disease, the estimated first-year costs of receiving treatment under the modalities reported in Table 2 are presented in Table 3 (see Chapter 5 for details). Assuming again that 25% (10,205) of cancers diagnosed in the UK each year are identified through repeat biopsies, and that all are treated with these modalities in the proportions derived from routine Scottish data, then the approximate first-year costs to the NHS of treating this cohort would equate to approximately £30M.
Variation in services and/or uncertainty about best practice
A degree of variation has been brought about by government targets, meaning that in some centres patients undergo a standard T2-weighted magnetic resonance imaging (T2-MRI) of the prostate prior to biopsy for lesion detection and staging purposes (the latter just in case a cancer is eventually found). The MRI before biopsy strategy in some centres is done so that the wait for a staging MRI after biopsy is removed. Most centres still perform their staging MRI post biopsy at around 3–6 weeks to allow time for post-biopsy haemorrhage to resolve but this may lead to breaches in national targets.
There are a number of different diagnostic pathways for patients who have an initial negative biopsy. If histopathological assessment indicates suspicion of cancer or abnormalities, most centres would proceed to a further biopsy, either a repeat 10- to 12-core TRUS/Bx or extended 14–16 core. Some centres would perform a pre-biopsy MRI, enhanced MRI techniques or MRS, to assist in targeting larger lesions. Where available, some centres may also use TRUS-guided transperineally obtained template biopsies, or TRUS-guided transrectally obtained saturation biopsies, dependent upon physician preference, the latter usually after a second negative TRUS/Bx.
Further variation in services will depend upon:
-
local policy
-
interpretation of national policy (MRI pre biopsy in some centres)
-
access to prostate biopsy services
-
access to MRI, enhanced MRI and MRS facilities
-
access to template biopsy equipment.
Relevant national guidelines, including National Service Frameworks
The 2008 NICE PC guideline9 states that men with high-risk localised and locally advanced PC who are being considered for radical treatment should have pelvic imaging with either MRI or CT, if MRI is contraindicated. Qualifying statement: ‘there is evidence from observational studies to support making this recommendation’. Furthermore, ‘MRS is not recommended for men with PC except in the context of a clinical trial’. Qualifying statement: ‘there is no evidence to support routine use of MRS’.
The Prostate Cancer Risk Management Programme in 2006 issued guidance for prostate biopsies recommending a 10- to 12-core scheme at first biopsy, which samples the mid-lobe PZ and the lateral PZ only (NHS Cancer Screening Programmes. Undertaking a transrectal ultrasound guided biopsy of the prostate. 2006. URL: www.cancerscreening.nhs.uk/prostate/pcrmp-guide-1.html). Directed cores should also be sampled from any hypoechoic areas identified during the procedure. Anterior/TZ samples may be appropriate at a repeat biopsy. However, no comments were made on the number of cores on repeat biopsies or any other methods of guiding the biopsy protocol.
The European Association of Urology (EAU) guidelines state17 ‘if clinical suspicion for prostate cancer persists in spite of negative prostate biopsies, MRI may be used to investigate the possibility of an anterior located prostate cancer, followed by TRUS or MRI-guided biopsies of the suspicious area’.
The European Society of Urogenital Radiology (ESUR) guidelines for MRI in PC,42 issued in April 2012, recommend that when TRUS biopsy is negative, and an interval rise in PSA justifies further investigation, enhanced MRI using the ‘detection protocol’ must be applied before further TRUS/Bx. In this context, the detection protocol consists of T2-MRI, diffusion-weighted magnetic resonance imaging (DW-MRI) and dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI), with MRS being an option.
The UK Royal College of Radiologists (RCR) guidelines43 recommend the use of MRI for staging known PC. The use of MRI to detect PC is indicated only in specific circumstances, making the comment that ‘MRI is capable of detecting prostatic carcinoma when clinical suspicion is high but transrectal US-guided biopsy negative. Focal areas of abnormal signal can be targeted for biopsy or repeat biopsy under ultrasound guidance’. Guidance published in 2006 by the RCR outlined in detail the usage of MRI in PC emphasising T2-MRI. DCE-MRI and MRS were mentioned as techniques that could be useful for staging, therapy planning and for detecting recurrent disease. The 2006 RCR guidance is currently being updated under the Cancer Staging Proforma Reporting Project (CASPAR),44 which is a pilot programme to test the design and utility of proforma-based reporting for a number of cancers. The CASPAR PC imaging proforma provides guidance on the use of T2-MRI, DW-MRI and DCE-MRI (Dr Gina Brown, Project Lead, 27 February 2012, personal communication). No mention is made of the clinical utility of MRS in this setting. The RCR in its guidance does not detail a strategy for evaluating patients with negative TRUS biopsy.
Description of technologies under assessment
Summary of technologies (index tests)
This review is concerned with three technologies: MRS, DCE-MRI and DW-MRI.
Magnetic resonance spectroscopy
Further to imaging of water and lipids, which is normally performed with MRI, MRS is a technique that provides detail on protons of molecules other than water and lipids. MRS makes use of the slight differences in chemical environment of protons attached to small metabolites present in the tissue or organ of interest. Signals of the different protons in these molecules are presented in a spectrum, in which the position on the x-axis is representative for the exact so-called chemical shift of the protons at hand (which molecule), and the intensity on the y-axis represents the amount of that particular proton pool present (how much of that molecule is present). In this way, MRS can give quantitative information on the presence and quantity of metabolites in the prostate. Magnetic resonance spectroscopy imaging (MRSI) does the same, but also provides this information according to spatial location of spectra superimposed on an imaginary two- or three-dimensional grid over the prostate.
In the prostate, three-dimensional MRSI is the current standard of doing spectroscopy, providing spectra of the whole organ with a spatial resolution in the order of 0.5 cc. 45–47 In the prostate the relative concentrations of four metabolites are routinely detectable:
-
citrate, an intermediate of the Krebs cycle, which accumulates in the luminal space of healthy prostate tissue
-
choline, free and phosphorylated choline compounds, which are involved in the phospholipid metabolism of the cell, elevated in cancer tissue
-
creatine, involved in the energy metabolism of cells
-
polyamines (spermine, spermidine and more), accumulating in the luminal space.
As the chemical shifts of choline, polyamines and creatine do not differ greatly, these resonances cannot always be separated, and are therefore incorporated into one clinically useful biomarker for the presence of PC: the choline (+ polyamines) + creatine to citrate ratio (CC/C). After spectral fitting of the different metabolites, this CC/C ratio can be calculated and used either qualitatively48 or quantitatively49 in the so-called standardised threshold approach50,51 to estimate the presence and aggressiveness of cancer in prostate tissue. 52
Differences in the concentrations of these metabolites between normal and malignant prostate tissues allow for increasing the accuracy of staging among less-experienced readers, and decreasing interobserver variability. 53 Furthermore, correlations have been demonstrated between the metabolic signal pattern and a pathological Gleason score, suggesting the potential for a non-invasive assessment of tumour aggressiveness. 52,54
Dynamic contrast enhanced magnetic resonance imaging
Dynamic contrast-enhanced MRI is a fast T1 weighted imaging technique that dynamically measures a bolus pass of an intravenously administrated MR contrast agent through the prostate. For its nutrient and oxygen supply, a tumour forms new vessels made through the process of neoangiogenesis. In tumour tissue these vessels are often leaky or incomplete, which makes it easier for a contrast agent to extravasate into the extravascular extracellular space. In this extracellular space, the gadolinium-based contrast agent increases the signal intensity of T1 weighted images. In this way, tissues with increased perfusion and vessel leakage stand out with respect to normally perfused tissue, which enhances less.
Three-dimensional DCE-MRI measures the time course of the contrast agent passing through the prostate by repeatedly acquiring three-dimensional T1 weighted images at high temporal resolution (in the order of seconds), providing a signal enhancement curve for every voxel of the three dimensional MRI data sets. These time-curves can be described semiquantitatively or modelled into pharmacokinetic parameters, which gives either descriptive measures of the enhancement curve (start of enhancement, wash-in gradient, maximum enhancement, time to peak, washout gradient, area under the gadolinium curve, etc.) or model parameters (forward leakage rate, washout rate constant and leakage space) usual after the fitting to a pharmacokinetic model. 55 For an accurate assessment of the model parameters, an arterial input function (AIF) is required that describes the shape of the contrast bolus arriving at the prostate. The semiquantitative parameters do not need such an AIF. Tumour tissue in the prostate is characterised by increased pharmacokinetic parameters compared with healthy tissue. Unfortunately, especially in the TZ of older men, benign diseases such as proliferative benign prostatic hyperplasia or prostatitis also show marked enhancement after contrast agent administration, making DCE-MRI less specific in the TZ of the prostate. Very recently, recommendations have been published on how this technique can best be used. 56
Dynamic contrast-enhanced MRI has been shown to be of use in detection and staging of PC within a multiparametric protocol57–59 and is especially useful in follow-up after treatment, when normal prostate anatomy is either not present60 or disturbed after radiotherapy. 61
Diffusion-weighted magnetic resonance imaging
Diffusion-weighted MRI is a technique that evaluates the microscopic mobility of water molecules in tissue. Impeded water movements within cellularly dense tissues, such as tumours, appear as high-signal regions on diffusion-weighted images and as darker signals on apparent diffusion coefficient (ADC) maps. In glandular spaces (healthy prostate luminal spaces) or large extracellular spaces, water motion is less impeded, leading to larger signal attenuation (low signal on diffusion-weighted images) and to higher ADC values. In addition to its value in the detection of cancer,62,63 DW-MRI has also been shown to be a promising marker of tumour aggressiveness, with good correlation between ADC values and Gleason score in the PZ of the prostate. 64
Current usage in the NHS
As a result of the aforementioned guidelines (see Relevant national guidelines, including National Service Frameworks, above), MRI is widely used to evaluate the stage of PC in the UK. Most centres have 1.5 T (tesla) scanners, although 3-T machines are found in major teaching hospitals and more recently have appeared in non-teaching hospitals. Endorectal coil usage is found only at selected centres. Most centres use T2-MRI and DW-MRI routinely for PC imaging, although the quality of DW-MRI is variable on currently installed equipment in many centres. Centres with a high volume of PC referrals do perform DCE-MRI in selected patients, including patients with prior negative TRUS/Bx and for suspect locally recurrent disease. There are very few centres in the UK with prostate MRS experience. Systematic proforma reporting is beginning to appear at selected expert centres but this is likely to expand more widely once the findings and recommendations of joint RCR/National Cancer Intelligence Network (NCIN) CASPAR project (see Relevant national guidelines, including National Service Frameworks, above) are implemented nationwide.
Anticipated costs associated with the intervention
The anticipated costs associated with the use of MRS/MRI in the diagnostic pathway will depend on the specific sequences used. Diagnostic imaging scans of the prostate using T2-MRI, DW-MRI and MRS all map to the HRG RA01Z (Magnetic Resonance Imaging Scan, one area, no contrast), whereas sequences involving the use of DCE-MRI map to RA03Z (Magnetic Resonance Imaging Scan, one area, pre and post contrast). The national average NHS reference costs for RA01Z and RA03Z were £174 and £229, respectively, in 2009–10. 41 If all 41,000 patients in our estimated annual cohort undergoing a repeat biopsy were to receive an MRI scan prior to biopsy (0.4 with pre and post contrast, 0.6 without) then this would equate to a cost of approximately £8M to the NHS. If it is assumed that the results of MRI are used to direct TRUS biopsies in patients with a visible lesion, while those with no visible lesion receive a systematic TRUS/Bx instead, then this £8M represents the additional cost to the NHS of using MRI compared with using TRUS alone to guide biopsies. Of course there would be anticipated benefits in terms of improved detection rates, reduced need for further biopsies and timely intervention. An alternative way of using MRS/MRI could be to use it to safely filter out patients with no visible lesion, such that biopsy costs and associated complications would be reduced at the population level. Both these models for its use are explored in the chapter on cost-effectiveness. Although the reference costs used in the above calculations broadly reflect the cost to the NHS of commissioning different types of MRI, they do not capture more subtle differences in costs between different MRI sequences. For this reason we have carried out some bottom-up costing of the sequences and combinations of them to inform the cost-effectiveness analysis reported in Chapter 5.
Comparator tests
Standard (T2-weighted) magnetic resonance imaging
T2-weighted MR images are usually obtained in two to three planes, with axial and coronal planes being the minimum. The axial T2-weighted MRI sequence must cover the entire prostate and seminal vesicles with section thicknesses of 3–4 mm. An endorectal coil (ERC) is not an absolute requirement for T2-MRI performed on 1.5-T or 3-T scanners but a pelvic phased-array external coil with a minimum of 16 channels is required to produce high-quality images. T2-weighted MRI provides the best depiction of the prostate's zonal anatomy, seminal vesicles and the prostatic capsule. T2-MRI is mostly used for PC staging but also has some utility for lesion detection and localisation.
It is not recommended that T2-MRI should be used on its own for detection and localisation; it should, in general, be used with other enhanced MRI or MRS techniques because their combined use improves both sensitivity and specificity. 42 PC typically manifests as a round or ill-defined, low-signal-intensity focus in the PZ on T2-MRI. However, various conditions [such as prostate intraepithelial neoplasia, prostatitis (infection or inflammation), haemorrhage, glandular atrophy, scars from previous infections and biopsies, and post-treatment changes] can mimic cancer on T2-MRI in the PZ. The high frequency of non-cancer prostate conditions and their ability to affect T2-MRI appearances accounts for the high sensitivity but low specificity of T2-MRI for tumour detection and localisation.
Tumours located in the TZ are more challenging to detect on T2-MRI, as the signal intensity characteristics of the normal TZ and cancer usually overlap. 65 TZ tumour often is shown as a homogeneous signal mass, with indistinct margins with lenticular shapes if anteriorly located.
High-grade PCs tend to be larger, more infiltrative and to have lower signal intensity than low-grade cancers on T2-MRI, which makes high-grade disease easier to detect. 66,67 T2-MRI can be ineffective for detecting low-risk PC (small volume disease or sparse variants of Gleason 3 + 3 cancer) because of imaging overlaps with non-cancer conditions mentioned above.
Transrectal ultrasound guided prostate biopsy
The main role of TRUS is to direct biopsies in order to obtain a systematic sampling of the prostate gland rather than to target specific lesions, because of the unreliability of greyscale ultrasonography to visualise cancer. 68,69 A systematic TRUS biopsy simply means that the cores are obtained in an organised manner. Template biopsy is a type of systematic biopsy, and uses a grid-based method to guide the random core biopsies. A saturation biopsy aims to sample the entire prostate and would routinely use 20 or more cores. It should be noted that these techniques are not performed in a targeted manner but rather randomly, albeit in a systematic fashion.
It is unclear how repeat biopsies should be performed. 70 The standard approach would be to repeat the biopsies transrectally under TRUS guidance, increasing the number of cores, and including samples from other zones.
As the majority of cancers arise from the PZ of the gland, initial biopsies are targeted at this area. 17 The sensitivity and specificity of TRUS-guided prostate biopsies in diagnosing cancer vary depending on several factors, including the threshold of PSA level used to justify a biopsy, the area of the prostate targeted, and the number of prostate tissue cores. Although the patient-level diagnostic accuracy is increased by increasing the number of tissue cores,71 this strategy invariably results in more side effects.
Transrectal ultrasound prostate biopsy is usually performed under local anaesthetic as an outpatient procedure. Due to the risk of sepsis from the procedure a dose of antibiotic is administered prior to the procedure, with one to three doses supplied to the patient postoperatively. The patient is commonly positioned in the left lateral position. The scans are performed with either an end- or side-fire transrectal probe scanning between 7.5 and 9 MHz. A disposable guide is attached over the probe prior to its placement in the rectum. Scans are performed in the transverse and longitudinal direction, sometimes simultaneously.
Transperineal biopsies are typically performed under a short general anaesthetic. Where the patient has a rectum and an anus, a transrectal probe is then introduced to either guide the biopsy needle freehand or, in the case of template biopsies, a grid is placed over the perineum and the ultrasound transducer is placed into the rectum via a housing that keeps the probe in the correct position. Biopsies are then taken using the template with standard 18-gauge needle from predetermined sections of the gland. These can then be processed separately to allow a map of the disease to be built up. Biopsies performed in this way allow the anterior and apical portions of the gland, which are more difficult to target on transrectal biopsies, to be sampled more easily. Where the patient has no rectum the biopsies can be guided using a transabdominal probe.
Care pathways
In developing the care pathways, we used a combination of current clinical guidelines and expert opinion to devise alternative diagnostic and treatment pathways for the economic modelling reported in Chapter 5. The general diagnostic pathway is outlined in Figure 2.
FIGURE 2.
General diagnostic pathway.
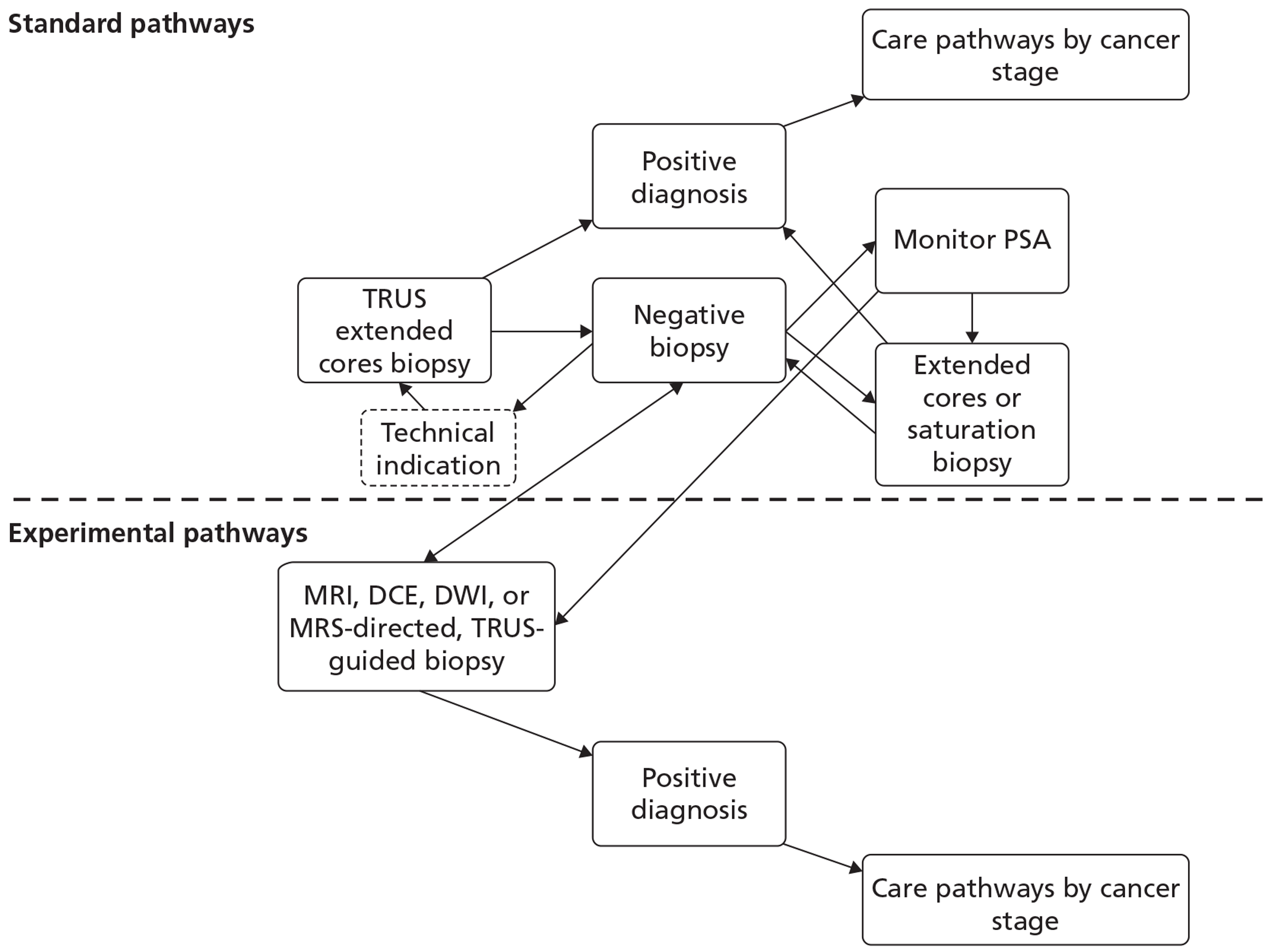
The options for patients following a previous negative biopsy are divided into standard pathways and experimental pathways. For the purposes of this review the use of any MRI sequence to direct TRUS/Bx is considered experimental, whereas the use of systematic TRUS-guided biopsies is considered standard practice. Under standard practice, the options for patients with a previous negative biopsy are to monitor PSA and other measures predictive of PC, perform a further standard cores biopsy (10–12 cores based on expert clinical opinion) if there is a technical reason to do so, or perform an extended-cores biopsy for patients where suspicion of PC remains. For the purposes of the economic modelling carried out in Chapter 5, we take patients selected for a repeat biopsy as the starting point for the analysis, and for this cohort the consensus among clinical experts on our team was that a systematic extended-cores biopsy (14–16 cores) would be the appropriate comparator under standard practice. The use of MRS/MRI to direct TRUS/Bx at this stage offers the alternative experimental approach.
Following a negative result from a second biopsy, patients can remain cycling within the diagnostic pathway, with further monitoring of PSA and further repeat biopsies. Clinical opinion within the research team was that, in the case of patients selected for a third biopsy, a systematic saturation biopsy would probably be performed at this stage. Thus, the economic modelling applied the simplifying assumption that any patients with underlying cancer missed by the second biopsy would have persistently elevated PSA level and would progress to a TRUS-guided saturation biopsy within 12 months. This last procedure is considered the reference standard for the presence of PC. For those patients with no underlying cancer (disease negative on a reference standard), the assumption was made that PSA monitoring would continue indefinitely and that no further biopsies would be undertaken unless incident cancer developed. Although this may seem clinically unrealistic, the proportion of patients with no cancer and their downstream management would probably remain constant between the experimental and control arms of the model following the index repeat biopsy. Hence, their subsequent treatment, outcomes and costs would not influence the decision problem in hand, of whether or not MRS/MRI should be used in men with a previous negative biopsy to direct the next biopsy, i.e. we do not model the ongoing use of MRI to direct all further repeat biopsies in men who remain negative following their initial MRI-directed TRUS/Bx.
Following a positive diagnosis from any biopsy procedure, staging and subsequent treatment is implemented in line with the current guidance by stage and grade of cancer present (see Table 3). The Markov model developed to simulate the progression of undiagnosed and diagnosed cancer, and its subsequent treatment by stage and grade, is described in detail in Chapter 5.
Chapter 2 Definition of the decision problem
Decision problem
The purpose of this review is to assess the diagnostic accuracy of MRS, DCE-MRI and DW-MRI and the clinical effectiveness and cost-effectiveness of strategies involving their use in men with suspected PC and elevated PSA level but previously negative biopsy.
Interventions
As data allow, the following tests are considered, alone or in combination:
-
MRS-guided biopsy
-
DCE-MRI-guided biopsy
-
DW-MRI-guided biopsy.
In addition, the above tests are considered in combination with standard (T2-weighted) MRI. In situations when both tests are required to be positive for the combination to be positive, the test combination is linked by ‘and’. When only one of the tests is required to be positive for the combination to be positive, the test combination is linked by ‘or’.
Population including subgroups
The population concerned is men with suspected PC and elevated PSA level of up to 20 ng/ml but previously negative biopsy.
The setting considered is secondary or tertiary care.
Where data allow, a subgroup of participants with prostatic intraepithelial neoplasia (PIN) and ASAP diagnosed at first biopsy is considered.
Relevant comparators
The comparator tests considered are:
-
standard (T2-weighted) MRI
-
TRUS.
Reference standard
The reference standard is histopathological assessment of biopsied tissue. Tissue samples may be obtained by transrectal needle biopsy, saturation biopsy, transperineal template biopsy or from prostatectomy specimens.
A maximum follow-up time of 12 months was incorporated into the reference standard. This was to distinguish between tumours missed by the index/comparator test (detected before 12 months) and interval tumours that were not missed (detected after 12 months).
Outcomes
The following outcomes are considered:
-
Diagnostic performance of MRS, DCE-MRI and DW-MRI in the localisation of abnormalities of the prostate.
These outcomes are considered at both patient-level and biopsy level, where data allow.
The reported Gleason score of the patients diagnosed with PC is presented to assess if index/comparator tests detect different grades of tumour.
In studies reporting the above outcome, the following outcomes are also considered, if reported:
-
altered treatment as a result of the tests
-
acceptability of the tests
-
interpretability of the tests
-
effect of testing on QoL (disease-specific and generic instruments)
-
adverse effects of testing.
Key issues
There are several key issues. First, does a single test or a combination of tests provide the greatest diagnostic accuracy and cost-effectiveness? MRS, DCE-MRI, DW-MRI or standard (T2-weighted) can be used in combination. If a combination of tests is used, is greatest benefit derived when both tests are required to be positive or when only one test is required to be positive? Second, are there patient groups for which MRS, DCE-MRI and DW-MRI are more effective, for example patients who are diagnosed with PIN or ASAP on initial biopsy? Third, does MRS, DCE-MRI or DW-MRI detect more clinically significant tumours?
Two significant challenges are worth noting. First, the reference standard (histopathological assessment of biopsied tissue) is linked with one of the comparator tests (TRUS). Most studies use TRUS to obtain histopathological samples. TRUS can be used to either obtain a systematic, predefined set of biopsies (TRUS/Bx) and/or identify suspicious areas. When TRUS is used in a systematic, predefined manner, a template is usually used and areas in the prostate are not diagnosed as ‘normal’ or ‘abnormal’. Therefore, diagnostic outcomes cannot be measured. However, when TRUS is used to identify ‘abnormal’ areas and a subsequent biopsy obtained, diagnostic outcomes can be measured. A number of studies combine these two uses of TRUS; suspicious lesions are biopsied and subsequently a systematic, predefined set of biopsies is obtained. The situation is further complicated because there is variation in the number and pattern of cores obtained on systematic biopsy.
Second, there is no widely accepted definition of ‘guided’, ‘directed’ and ‘targeted’. After a lesion is identified on MRS, DCE-MRI, DW-MRI or standard T2-MRI, biopsies can subsequently be obtained using a MRI compatible device or TRUS/Bx. For the purposes of this review, the term ‘MRI-guided’ is used when biopsies are obtained using a MRI compatible device. The term ‘MRI-directed TRUS-guided’ is used when lesions are identified using MRI, but biopsies are obtained using TRUS.
Overall aims and objectives of assessment
This review assessed the diagnostic accuracy of MRS, DCE-MRI and DW-MRI and the clinical effectiveness and cost-effectiveness of strategies involving their use in men with suspected PC and elevated PSA level but previously negative biopsy. Subsidiary questions to be addressed relating to these techniques included:
-
In which patient group are they most clinically effective?
-
Can they identify cases where PC is present but further procedures are unnecessary?
-
Does their use lead to changes in patient management?
Chapter 3 Methods for reviewing diagnostic accuracy
Methods were in accordance with the protocol, which is presented in Appendix 1.
Identification of studies
Comprehensive electronic searches were conducted to identify reports of published studies. Highly sensitive search strategies were designed including appropriate subject headings and text word terms relating to PC, biopsy and the tests under consideration. Searches were restricted to years from 1995 onwards, reflecting the time of introduction of the tests, and non-English-language publications were excluded. MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, BIOSIS, Science Citation Index (SCI) and the Cochrane Controlled Trials Register (CENTRAL) were searched for primary studies, while the Cochrane Database of Systematic Reviews (CDSR), Database of Abstracts of Reviews of Effects (DARE), MEDION and the Health Technology Assessment (HTA) databases were searched for reports of evidence syntheses. Recent conference abstracts (2009–11) from the American Society of Clinical Oncology (ASCO) meetings were also searched. The date of the last searches was March 2012.
Reference lists of all included studies were scanned in order to identify additional potentially relevant reports. The expert panel provided details of any additional potentially relevant reports. Ongoing studies were identified through searching Current Controlled Trials (CCT), Clinical Trials, WHO International Clinical Trials Registry Platform (ICTRP) and NIH Reporter. Full details of the search strategies used are detailed in Appendix 2.
Inclusion and exclusion criteria
Types of studies
For diagnostic accuracy of MRS, DCE-MRI and DW-MRI the following types of studies were included:
-
direct (head-to-head) studies in which index test(s), comparator test(s) and reference standard test were done independently in the same group of people
-
randomised controlled trials in which people were randomised to the index and comparator test(s) and all received the reference standard test.
In the event that there was insufficient evidence from direct and randomised studies, we considered undertaking indirect (between-study) comparisons by meta-analysing studies that compared each single test or combination of tests with the reference standard test, and making comparisons between meta-analyses of the different tests. However, this type of study design is less reliable than direct studies as differences in diagnostic accuracy are susceptible to confounding factors between studies. The following types of studies were considered:
-
Observational studies, including case series, in which the sample is created by identifying all people presenting at the point of testing (without any reference to the test results).
-
Case–control studies in which two groups are created, one known to have the target disease and one known not to have the target disease, where it is reasonable for all included to go through the tests. We excluded case–control studies comparing severely diseased people with very healthy control subjects or studies excluding people with other urological disease such that the spectrum of disease and non-disease was unlike that to be encountered in practice.
The following types of report were excluded:
-
reviews, editorials and opinions
-
case reports
-
reports investigating technical aspects of a test
-
non-English-language reports.
Types of participants
The types of participants considered were men with suspected PC and elevated PSA level but previously negative biopsy. Studies were also included in which the participants with previously negative biopsy had elevated PSA level and/or abnormal DRE. Studies whose populations included subgroups of men meeting these criteria were also included. Studies that included men diagnosed with ASAP or high-grade prostatic intraepithelial neoplasia (HGPIN) were included. The setting considered was secondary or tertiary care.
Index tests
The index tests considered were MRS, DCE-MRI or DW-MRI, alone or in combination.
Given sufficient data, we planned to undertake sensitivity analysis around when the studies took place, to assess the effects of changes in the technology over time. This was possible only for MRS and T2-MRI.
Comparator tests
The comparator tests considered were standard (T2-weighted) MRI and transrectal ultrasound (TRUS) guided prostate biopsy (greyscale only).
Reference standard
The reference standard considered was histopathological assessment of biopsied tissue. Tissue samples could be obtained by transrectal needle biopsy, saturation biopsy, transperineal template biopsy or from prostatectomy specimens.
A follow-up time of 12 months was specified in the protocol as part of the reference standard. The reason for this was to help distinguish between tumours missed by the index/comparator tests (subsequently detected within this 12-month period) and interval tumours that were not missed (and subsequently detected after the 12-month follow-up period). However, few studies reported a follow-up, and this criterion was relaxed to allow those that did not report a period of follow-up but otherwise met the remaining inclusion criteria to be included in the review.
Types of outcomes
Studies had to report the diagnostic performance of MRS, DCE-MRI or DW-MRI in the localisation of abnormalities of the prostate. In included studies, outcomes relating to altered treatment as a result of the tests, acceptability of the tests, interpretability of the tests, effect of testing on QoL and adverse effects of testing were also considered.
All included studies reported relevant and interpretable data including the absolute numbers of true-positives, false-positives, false-negatives and/or true-negatives, or provided information allowing their calculation such that at least one indicator of diagnostic performance [i.e. sensitivity, specificity, predictive values or likelihood ratio (LR)] was calculable. In addition to studies that reported patient-level analysis, we also considered those that reported only a biopsy-level analysis on the basis that these might also provide potentially useful information.
Data extraction strategy
Two reviewers (from MC, JF, KR, PS) independently screened the titles (and abstracts if available) of all reports identified by the search strategy. Full-text copies of all studies deemed to be potentially relevant were obtained and two reviewers (from MC, JF, GM, KR, PS) independently assessed them for inclusion. Any disagreements were resolved by consensus or arbitration by a third party.
A data extraction form was developed and piloted. One reviewer extracted details of study design, participants, index, comparator and reference standard tests and outcome data, and a second reviewer checked the data extraction. Any disagreements were resolved by consensus.
Critical appraisal strategy
Two reviewers (from MC, JF, GM, KR) independently assessed the risk of bias and applicability concerns of all included full-text diagnostic studies using the updated quality assessment of diagnostic accuracy studies (QUADAS-2) checklist. The original QUADAS checklist was developed for use in systematic reviews of diagnostic studies72 and was designed to be adapted to make it more applicable to a specific review topic. QUADAS was developed through a formal consensus method and was based on empirical evidence. Following anecdotal reports and feedback which suggested problems with QUADAS, the QUADAS-2 tool was developed. QUADAS-2 consists of four key domains: (1) patient selection, (2) index test, (3) reference standard, and (4) flow of patients through the study and timing of the index test(s) and reference standard. Each domain is assessed in terms of the risk of bias. The first three domains are also assessed for concerns regarding their applicability in terms of whether (1) the participants and setting; (2) the index test, its conduct or interpretation; and (3) the target condition as defined by the reference standard match the question being addressed by the review.
For this review, QUADAS-2 was modified to make it more appropriate for assessing the quality of studies of tests for detecting PC. Domains 1 (patient selection) and 4 (flow and timing) were retained in their entirety. The title of Domain 2 was amended to ‘index & comparator test(s)’ to accommodate all the specified tests. One item was added to the risk of bias section of Domain 2 to assess whether or not tests that required subjective interpretation were interpreted by a suitably experienced person. Two items were added to the risk of bias section of Domain 3 (reference standard) to assess whether or not (1) the results of the reference standard test were interpreted by a suitably experienced person and (2) a follow-up was included in the reference standard. The modified tool consisted of 14 items.
Prior to completing the QUADAS-2 tool some decision rules were agreed between reviewers. In general, if a particular point was not mentioned in a paper, then the relevant signalling item was marked as ‘unclear’. Responses to the risk of bias and applicability questions were based upon the three or four relevant signalling questions; in each case, the majority response to signalling questions dictated the overall risk of bias or applicability response. There were some exceptions to this. For the Domain 1 (patient selection) applicability item ‘Is there concern that the included patients do not match the review question?’, the primary criterion was previously negative biopsy, followed by elevated PSA level. The item was classed as ‘Low’ if all patients had a previously negative biopsy and > 10% of the sample had elevated PSA level. For Domain 2 [index & comparator test(s)], responses of ‘yes’ to the ‘Were the index test results interpreted without knowledge of the results of the reference standard?’, ‘not available (N/A)’ to the item ‘If a threshold was used, was it pre-specified?’, and ‘unclear’ to the item ‘For a test requiring subjective interpretation, was it interpreted by someone experienced in interpreting such tests?’ were classed as ‘low’ risk of bias. For the Domain 2 applicability item, studies that explicitly did not image or analyse the entire prostate were classed as high concern for applicability. Otherwise, it was assumed that the entire prostate had been imaged and analysed, and studies were classed as low concern for applicability on this item. For Domain 3 (reference standard), a ‘no’ response to the item ‘Were the reference standard results interpreted without knowledge of the index test?’ and histopathological specimens which had been labelled (as suspicious or not) led to risk of bias being classed as ‘high’, regardless of responses to the remaining signalling items. In addition, a ‘no’ response to the item ‘Was a follow-up included in the reference standard?’ led to an automatic classification of high risk of bias. Risk of bias for the Domain 4 (flow and timing) item ‘Were all patients included in the analysis?’ was classed as ‘low’ if the proportion of participants included in the analysis was ≥ 90%.
Each item was worded so that a rating of ‘Yes’ was always optimal in terms of methodological quality. Any disagreements were resolved by consensus or arbitration by a third party. A sample QUADAS-2 checklist used in this review is presented in Appendix 3.
Methods of data synthesis
Data from each study were summarised in a 2 × 2 table of true-positive (TP), false-positive (FP), false-negative (FN) and true-negative (TN) according to the type of test and whether the primary study analysis was based on patient or biopsy level. These 2 × 2 tables were then entered into RevMan 5 software (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark) and SAS version 9.2 (SAS Institute Inc., Cary, NC, USA). All statistical analyses and graphical plots were undertaken in RevMan.
The sensitivity, specificity and their 95% confidence intervals (CIs) were calculated for each 2 × 2 table and presented for each test or combination of tests at both patient- and biopsy-level analysis. We investigated the presence of heterogeneity by visual examination of pairs of forest plots of sensitivity and specificity.
Sensitivity describes the proportion of those with disease who have positive test results, whereas specificity is the proportion of those without disease who have negative test results. A positive predictive value (PPV) describes the proportion of those with positive test results who have the disease, whereas a negative predictive value (NPV) is the proportion of those with negative test results who do not have the disease. A positive LR describes how many more times more likely it is that a person with disease will receive a positive test than a person without disease, whereas a negative LR describes how many more times more likely it is that a person with disease will receive a negative test result than a person without disease. A diagnostic odds ratio (DOR) is a single indicator of test performance and is the ratio of the odds of testing positive in those with the disease relative to the odds of testing positive in those without the disease. It can be calculated from the sensitivity and specificity values. The DOR summarises the results into a single indicator of test performance; however, information contained in sensitivity and specificity is lost and in particular a DOR cannot distinguish between tests with high sensitivity and low specificity and vice versa.
We undertook meta-analysis, where adequate data were available, using METADAS macro73 to fit hierarchical summary receiver operating characteristic (HSROC) models in SAS. HSROC models including random effects terms for variation in accuracy and threshold between studies, and non-symmetrical underlying receiver operating characteristic (ROC) curves, were fitted. The average operating point for each test was identified on each curve, and average sensitivities and specificities computed. Comparisons between tests were made by adding a covariate for test type to the accuracy and threshold parameters assuming a common underlying shape.
The comparative analysis was between all tests with three or more studies with relevant data. Comparative analysis consisted of uncontrolled/indirect comparison where all tests with relevant data were compared by adding covariates for a test type to the threshold and accuracy assuming a common underlying shape. A second comparative analysis of paired design where patients received both tests was also conducted.
Given sufficient evidence, we planned to undertake sensitivity analysis to assess the impact of the different number of biopsy cores taken (< 10 cores and ≥ 10 cores) on the accuracy of the tests. However, there was insufficient evidence to undertake such an analysis.
Chapter 4 Assessment of diagnostic accuracy
This chapter is structured as follows. The next section (see Quantity of research available, below) provides information on the quantity of research available, including characteristics and risk of bias of the included studies. The section Results: assessment of diagnostic accuracy reports the diagnostic accuracy results: individual index and comparator tests (see Magnetic resonance spectroscopy, Dynamic contrast-enhanced magnetic resonance imaging, Diffusion-weighted magnetic resonance imaging, T2-weighted magnetic resonance imaging and Transrectal ultrasonography); studies directly comparing two or more tests (see Studies directly comparing tests); combinations of tests (see Studies reporting combinations of tests) and indirect comparison of tests (see Indirect comparison). Meta-analyses are included where appropriate and feasible, and patient- and biopsy-level analyses are reported separately. Information on false-positives is provided in False-positive results and information on the detection of clinically significant disease is provided in Detection of clinically significant disease. The section Results: assessment of non-diagnostic outcomes provides information on non-diagnostic outcomes, followed by a chapter summary (see Summary).
Quantity of research available
Number and type of studies included
Appendix 4 lists the 51 studies, published in 65 reports (41 full-text papers57,74–113 and 24 abstracts114–137) that were included in the review of diagnostic accuracy. Figure 3 shows a flow diagram outlining the screening process, with reasons for exclusion of full-text papers.
FIGURE 3.
Screening process.
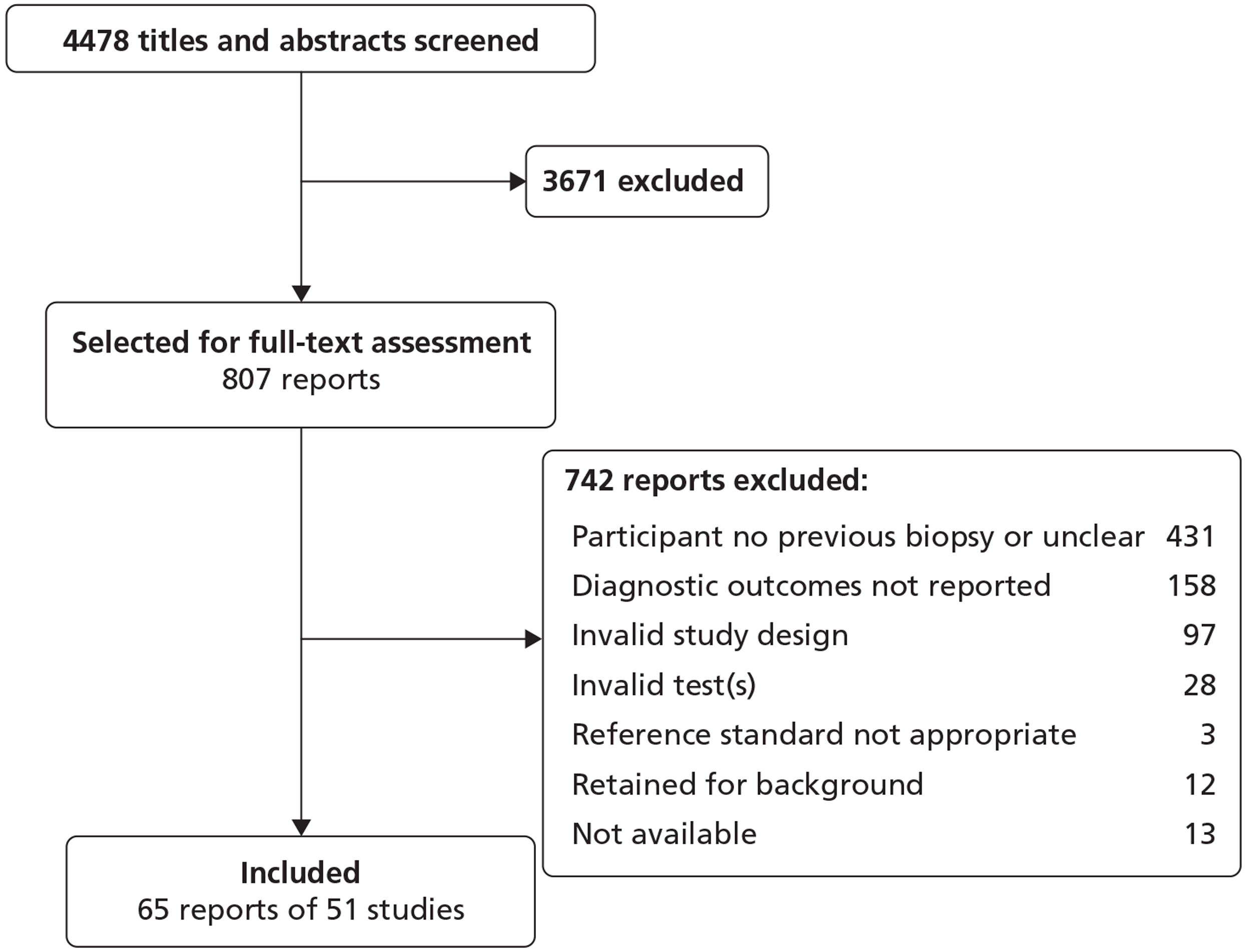
Number and type of studies excluded
A list of full-text papers that were excluded along with the reasons for their exclusion is given in Appendix 5. These reports were excluded because they failed to meet one or more of the inclusion criteria in terms of the type of study, participants, test, reference standard or outcomes reported.
Characteristics of the included studies
Appendix 6 displays the characteristics of the 51 included studies. Table 4 presents summary information for the included studies. There were 39 full-text papers. 57,74–84,86–93,95–113 Twenty-seven studies involved consecutive samples. 57,74,76,78,79,81,84,86,87,89–93,95,97,100,101,104–106,108,111,112,118,127,133 Twenty-four studies did not report this information. 75,77,80,82,83,88,96,98,99,102,103,107,109,110,113,115–117,120,126,130,134,136,137 There were 41 prospective studies57,74–76,79–84,86,88–90,92,93,95,99–101,103–106,108,109,111–113,115–118,120,126,127,130,133,134,136,137 and 10 retrospective studies. 77,78,87,91,96–98,102,107,110
| Characteristic | No. | No. of studies |
|---|---|---|
| Patients | ||
| Enrolleda | 92,588 | 50 |
| Analysed | 10,264 | 51 |
| Age (years) | ||
| Median (range) of means | 63.5 (60.3 to 68.1) | 24 (47%) |
| Median (range) of medians | 66 (62 to 69) | 7 (14%) |
| Other format/not reported | – | 20 (39%) |
| Baseline PSA (ng/ml) | ||
| Median (range) of means | 10.8 (6.4 to 16) | 17 (33%) |
| Median (range) of medians | 10 (5.5 to 19.5) | 8 (16%) |
| Other format/not reported | – | 26 (51%) |
| Participants at initial biopsy with | ||
| ASAP | 217 (2%) | 4 (8%) |
| HGPIN | 199 (2%) | 5 (10%) |
| Test results reported | ||
| MRS | 772 (8%) | 18 (35%) |
| DCE-MRI | 1094 (11%) | 12 (23%) |
| DW-MRI | 1021 (10%) | 11 (22%) |
| T2-MRIb | 1615 (16%) | 26 (51%) |
| TRUS | 8105 (79%) | 22 (43%) |
| Biopsy guidance | ||
| T2-MRI | 538 (5%) | 7 (14%) |
| TRUS | 9726 (95%) | 44 (86%) |
| Prostate size (cc) | ||
| Median (range) of means | 53.9 (42.5 to 59.3) | 4 (8%) |
| Median (range) of medians | 54.9 (41 to 67) | 3 (6%) |
| Other format/not reported | – | 44 (86%) |
Fourteen studies included a follow-up in the reference standard: Campodonico et al. 77 and Ukimura et al. 107 did not report the length of follow-up; Hoeks et al. 87 reported follow-up of 5 months; Lin et al. 91 reported a total follow-up of 18 months with only the initial 12 months taken into account for the present review; Lopez-Corona et al. 92 reported follow-up of up to 97 months; Pepe et al. 97 reported follow-up of up to 22 months; Philip et al. 98 reported follow-ups of 3 and 6 months; Quinlan et al. 102 reported follow-up of up to (a mean of) 50 months; Yanke et al. 110 reported a mean period of 30 months between first and last biopsy. Djavan et al. , Keetch et al. , Pinsky et al. , Roehl et al. and Zackrisson et al. reported population-based screening studies in which participants with negative biopsies were followed up every 6 or 8 weeks, 6 months, 12 months, 6 months and 2 years, respectively. 81,88,99,103,113
Eighteen studies74,76,79,84,95,100,101,104–106,108,111,115,117,118,120,130,134 reported diagnostic test accuracy for MRS (alone or in combination with other tests). Twelve studies78,84,86,87,89,90,95,100,104,105,109,133 reported diagnostic test accuracy for DCE-MRI (alone or in combination with other tests). Eleven studies84,86,87,89,96,100,104,109,116,126,133 reported diagnostic test accuracy for DW-MRI (alone or in combination with other tests). Twenty-six studies57,74,76,78,79,82,84,86,87,89,90,100,104,106,108,109,112,116–118,126,127,130,134,136,137 reported diagnostic test accuracy for T2-MRI (alone or in combination with other tests). Twenty-two studies57,75,77,80,81,83,88,91–93,96–99,102,103,107,110,111,113,120,136 reported diagnostic test accuracy for TRUS (alone or in combination with other tests).
Seven studies82,84,86,87,104,108,109 involved MRI-guided biopsies and 44 studies57,74–81,83,88–93,95–103,105–107,110–113,115–118,120,126,127,130,133,134,136,137 involved TRUS-guided biopsies.
Of the 18 studies84,104,112,115,117,120,130 that involved MRS, seven did not report a threshold for a positive test. Four studies74,79,100,118 reported a threshold of the CC/C ratio of > 0.86. Two studies95,105 used a threshold of CC/C > 0.80. Two studies101,106 reported a threshold of CC/C ratio more than three standard deviations (SDs) above the mean healthy value. Bhatia et al. 76 reported using the mean healthy CC/C to adjust a primary score to obtain a final voxel score. Wefer et al. 134 reported abnormal metabolism as areas with four or more voxels with a CC/C ratio more than two SDs. Wetter et al. 108 used a threshold of CC/C > 0.6.
Twelve studies were undertaken in the USA,75,88,90,92,99,103,107,110,127,130,134,136 eight in Italy,77,79,95,97,105,106,118,133 six in Germany,57,82,84,89,104,108 four each in France74,78,100,137 and the Netherlands,80,86,87,109 three in Republic of Korea,96,116,126 two each in Singapore,111,112 Spain115,117 and Turkey83,93 and one each in Brazil,101 Ireland,102 Sweden,113 Taiwan, Province of China,91 Thailand,76 Islamic Republic of Iran120 and the UK. 98 One multicentre study was undertaken in Austria, Belgium, France and Poland. 81
The 51 diagnostic studies enrolled 92,588 participants, with 10,264 included in the analysis. In 18 studies, the number of participants analysed was less than the number of participants enrolled. Of these, six81,88,97,99,103,113 were large-scale screening studies in which only some of the participants matched the inclusion criteria of this review and were reported separately. The differences between the numbers enrolled (86,749) and the much smaller numbers matching the inclusion criteria for this review (5771) in these six studies81,88,97,99,103,113 largely accounted for the difference in numbers between those enrolled and those analysed shown in Table 4. In five studies,57,105,108,127,136 not all participants had a previous negative biopsy (those with a previous negative biopsy were reported separately). Two studies84,86 involved participants withdrawing because of comorbidities. The study by Destefanis et al. 118 was an ongoing study in which not all enrolled participants had reached the point of analysis. Hoeks et al. 87 analysed only participants who underwent a follow-up MR-guided biopsy. The study by Panebianco et al. 95 involved analysing urine samples, not all of which were successful. In the study by Testa et al. ,106 data from four participants were not analysed because of poor MRS quality. Yakar et al. 109 analysed only participants in whom scanning revealed cancer-suspicious regions.
Across 24 studies reporting mean age,57,74–76,78–82,86,92,93,95,96,98–100,105–107,112,120,136,137 the median (range) of means was 63.5 years (60.3 to 68.1 years). Seven studies84,87,90,101,104,108,109 reported median values for age and the median (range) of medians was 66 years (62 to 69 years). Eight studies77,83,89,97,102,111,126 reported age in other formats. Twelve studies88,91,103,113,115–118,127,130,133,134 did not report this information.
Across 17 studies57,74–76,78,79,81,82,92,93,95,96,100,101,106,120,136 reporting mean baseline PSA, the median (range) of means was 10.8 ng/ml (6.4 to 16 ng/ml). Eight studies80,84,86,87,90,104,108,109 reported median baseline PSA levels, the median (range) of medians being 10 ng/ml (5.5 to 19.5 ng/ml). Eleven studies83,88,89,97,99,102,105,110,111,112,126 reported baseline PSA in other formats. The remaining 15 studies did not report baseline PSA levels.
At initial biopsy, four studies75,90,110,118 reported a total of 217 participants with ASAP and five studies75,79,83,90,110 reported a total of 199 participants with HGPIN. In the study by Destefanis et al. ,118 all participants had been diagnosed with ASAP on enrolment. 118 One study96 included three participants (out of 43 participants analysed) with a history of radiation therapy for PC.
Four studies75,78,81,106 reported mean prostate size, with the median (range) of means being 53.9 cc (42.5 to 59.3 cc). Three studies87,90,104 reported median prostate size, with the median (range) of medians being 54.9 cc (41 to 67 cc). Seven studies79,80,83,126,105,110,111 reported prostate size in other formats. The remaining 37 studies did not report prostate size.
Eight studies57,76,82,88,91,103,108,113 reported six or fewer cores taken in the previous biopsy scheme. Eleven studies74,77,80,81,86,93,96,97,105,116,126 reported between 8 and 12 cores taken in the previous biopsy scheme. Eskicorapci et al. 83 and Yuen et al. 111 reported six or 10 cores, and Yanke et al. 110 reported 6 or 12 cores taken in the previous biopsy scheme. Twenty-nine studies75,78,79,84,87,89,90,92,95,98–102,104,106,107,109,112,115,117,118,120,127,130,133,134,136,137 did not report this information.
Risk of bias of the included studies
All 39 full-text papers were assessed using a modified version of the QUADAS-2 tool containing 14 items. Figure 4 presents a summary of the results for the risk of bias and concerns for applicability QUADAS-2 domains across the 39 full-text papers. Appendix 7 presents results of risk of bias and applicability concerns for the individual studies.
FIGURE 4.
Summary of risk of bias and applicability domains.
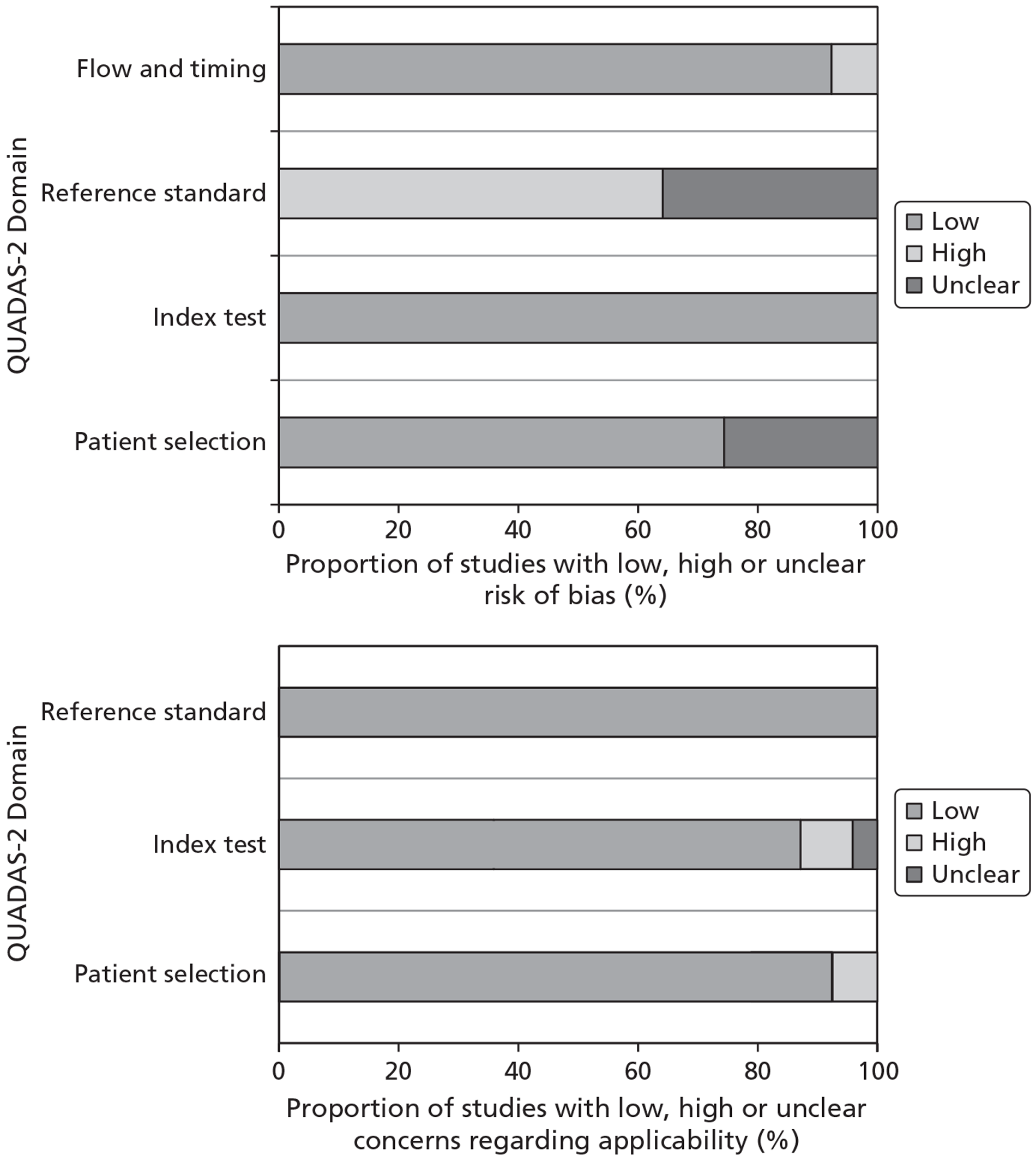
The majority of studies were considered to have a low risk of bias for the patient selection (74%, 29/39), index test (100%, 39/39) and flow and timing (92%, 36/39) domains. The 10 studies for which risk of bias for patient selection was unclear did not report exclusion criteria or whether or not the sample was consecutive. 75,77,82,83,88,96,98,102,103,110 Three studies (8%) were considered at high risk of bias for the flow and timing domain; patients did not all receive a reference standard and all patients were not included in the analysis. 57,87,109 In two studies (5%) patients did not all receive the same reference standard. 87,109
In the reference standard domain, the majority of studies (64%, 25/39) were considered at high risk of bias, although the risk of bias for the remaining 14 (36%) studies was considered unclear. All 25 studies were classed as high risk of bias in this domain owing to having no follow-up included in the reference standard. 57,74–76,78–80,82–84,86,89,90,93,95,96,100,101,104–106,108,109,111,112 Five of these studies78,83,89,93,101 also involved the reference standard not being interpreted without knowledge of the index test. None of the 14 studies77,81,87,88,91,92,97–99,102,103,107,110,113 that did include a follow-up in the reference standard reported whether or not the reference standard was interpreted without knowledge of results of the index test. In addition, 13 (33%) studies77,81,88,91,92,97–99,102,103,107,110,113 did not report whether or not the reference standard was interpreted by an experienced person.
All 39 studies had low concern for applicability for the reference standard domain and the majority had low concerns for applicability for the patient selection domain (95%, 37/39). The study by Labanaris et al. 89 was classed as high concern for applicability for patient selection due to specification of the inclusion criteria as ‘one of the following’ (p. 66), which may have resulted in some participants having a suspicious DRE but not a raised PSA level. 89 The study by Yanke et al. 110 was also classed as high concern in this domain as patient preference was one of the inclusion criteria. Thus, patients with normal PSA levels and DRE may have opted to have a biopsy, albeit all had undergone a previous negative biopsy. 110
A majority of studies had low concern for applicability for the index test domain (87%, 34/39). One study76 was classed as unclear in this domain as both normal and equivocal index tests were categorised as negative for malignancy. 76 There was therefore the possibility that some test results classed as equivocal may ultimately have been positive. Four studies79,100,101,111 for which there was high concern for applicability for the index test did not report findings relating to the entire prostate; three studies79,100,111 involved the PZ only and one study101 did not include the central gland.
Results: assessment of diagnostic accuracy
Individual study results are presented in Appendix 8.
Magnetic resonance spectroscopy
Patient-level analysis
Ten studies74,76,79,101,105,106,108,112,115,120 involving 438 patients reported the diagnostic accuracy of MRS and provided sufficient information for inclusion in a meta-analysis. All used a (10- or 12-core) TRUS-guided approach plus additional targeted cores on MRS equivocal or suspicious areas, apart from the study by Wetter et al. ,108 which used a MRI-guided approach. Four studies reported the CC/C ratio used as the cut-off for a positive test result, which ranged from > 0.6108 to > 0.86. 79
Across the studies the median (range) prevalence of PC was 34.5% (9.5% to 48.9%). The number of previous biopsy sessions the participants had undergone ranged from one105 to two to six. 101 Most studies reported that participants had undergone one to three, or one to four, previous biopsy sessions. The number of cores extracted in the previous biopsy session ranged from six76,108 to (a mean of) 16. 106
The studies were judged to have low risk of bias for the patient selection, index test and flow of timing domains. All studies were judged to have a high risk of bias for the reference standard domain owing to a lack of follow-up. All studies were judged to have low applicability concerns for the patient selection, index test and reference standard domains, apart from, for the index test domain, Cirillo et al. 79 (only PZ assessed) and Prando et al. 101 (central gland not assessed).
Figure 5 shows the sensitivity and specificity of the individual studies, pooled estimates and SROC curve. The pooled (95% CI) estimates for sensitivity and specificity were 92% (86% to 95%) and 76% (61% to 87%), respectively.
FIGURE 5.
Magnetic resonance spectroscopy – patient-level analysis: sensitivity, specificity, pooled estimates and SROC curve.
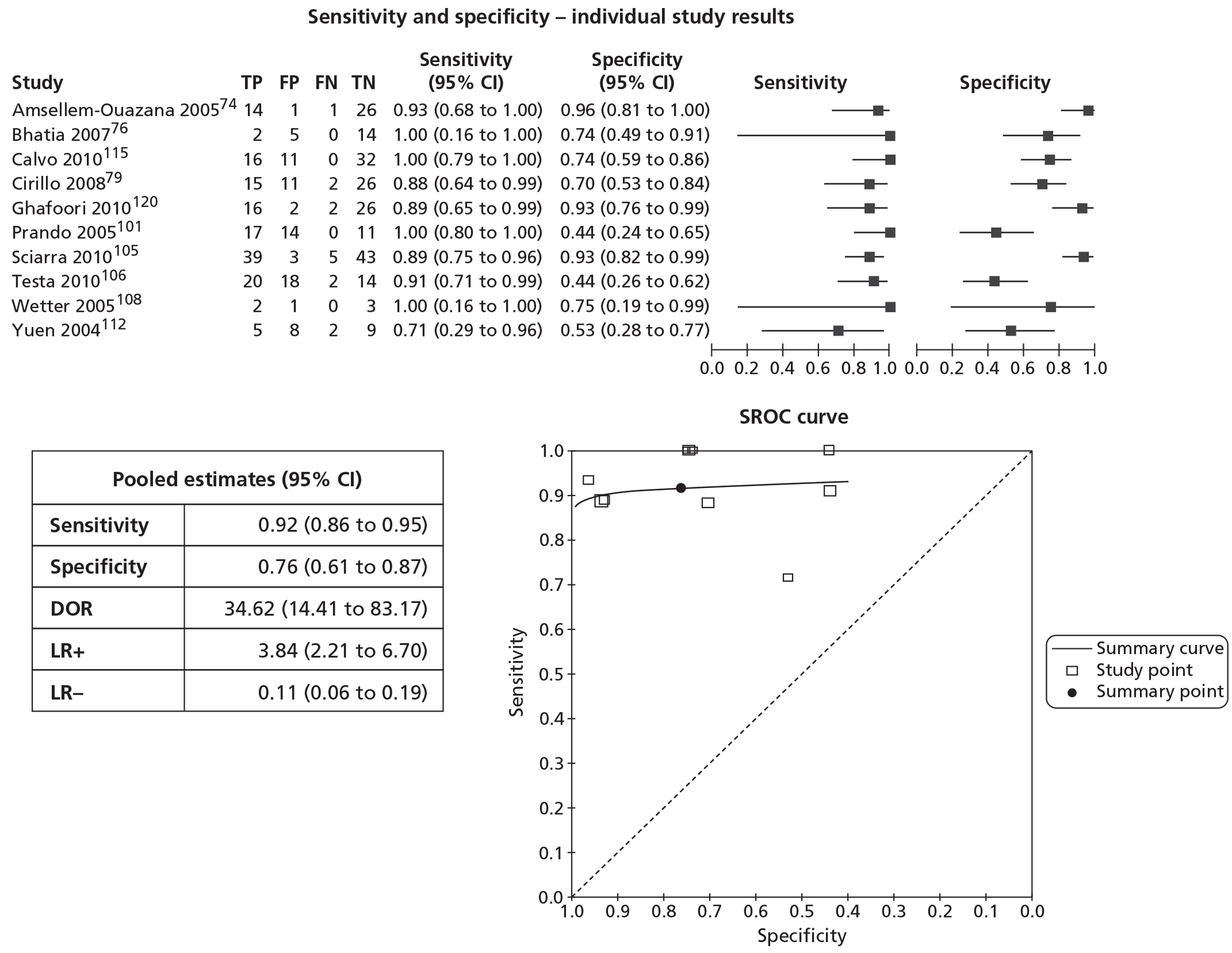
All of the studies reported sensitivity of ≥ 88% apart from Yuen et al. 112 (71%). Yuen et al. 112 suggested that contributory factors to the low sensitivity reported might have been (1) difficulties in ensuring the correspondence of TRUS biopsy spatial accuracies to suspicious areas on MRS and (2) that MRS did not cover the entire PZ of the gland. The studies by Prando et al. 101 and Testa et al. 106 reported low specificity (both 44%). Prando et al. 101 reported results either when a voxel score of 4 or 5, or just 5, was used as a cut-off for a positive test result. The results using the cut-off of 4 or 5 were included in the pooled estimates. However, if the results using a cut-off of just 5 had been used this would have increased the specificity to 84% but reduced the sensitivity from 100% to 70.6%. Testa et al. 106 suggested that the low specificity in their study was probably determined by the lower CC/C ratio used (actual value not reported) compared with cut-offs used by other studies.
A sensitivity analysis comparing pooled estimates of the results of earlier studies (pre 2007) with those of studies published more recently (2007 onwards) found no significant differences between the two subgroups. The pooled (95% CI) estimates for sensitivity and specificity were pre 2007, 93% (80% to 98%) and 71% (43% to 89%); 2007 onwards, 91% (84% to 95%) and 79% (60% to 90%) (see Appendix 9).
Biopsy-level analysis
Six studies76,79,100,101,106,112 reported the diagnostic accuracy of MRS at biopsy or other non-patient-level analysis and provided sufficient information for inclusion in a meta-analysis. Figure 6 shows the sensitivity and specificity of the individual studies, pooled estimates and SROC curve. The units of analyses reported by the studies included biopsy,76 site,79 segment,100 region106 and core. 112 The pooled (95% CI) estimates for sensitivity and specificity were 66% (46% to 82%) and 89% (86% to 92%), respectively.
FIGURE 6.
Magnetic resonance spectroscopy – biopsy-level analysis: sensitivity, specificity, pooled estimates and SROC curve.
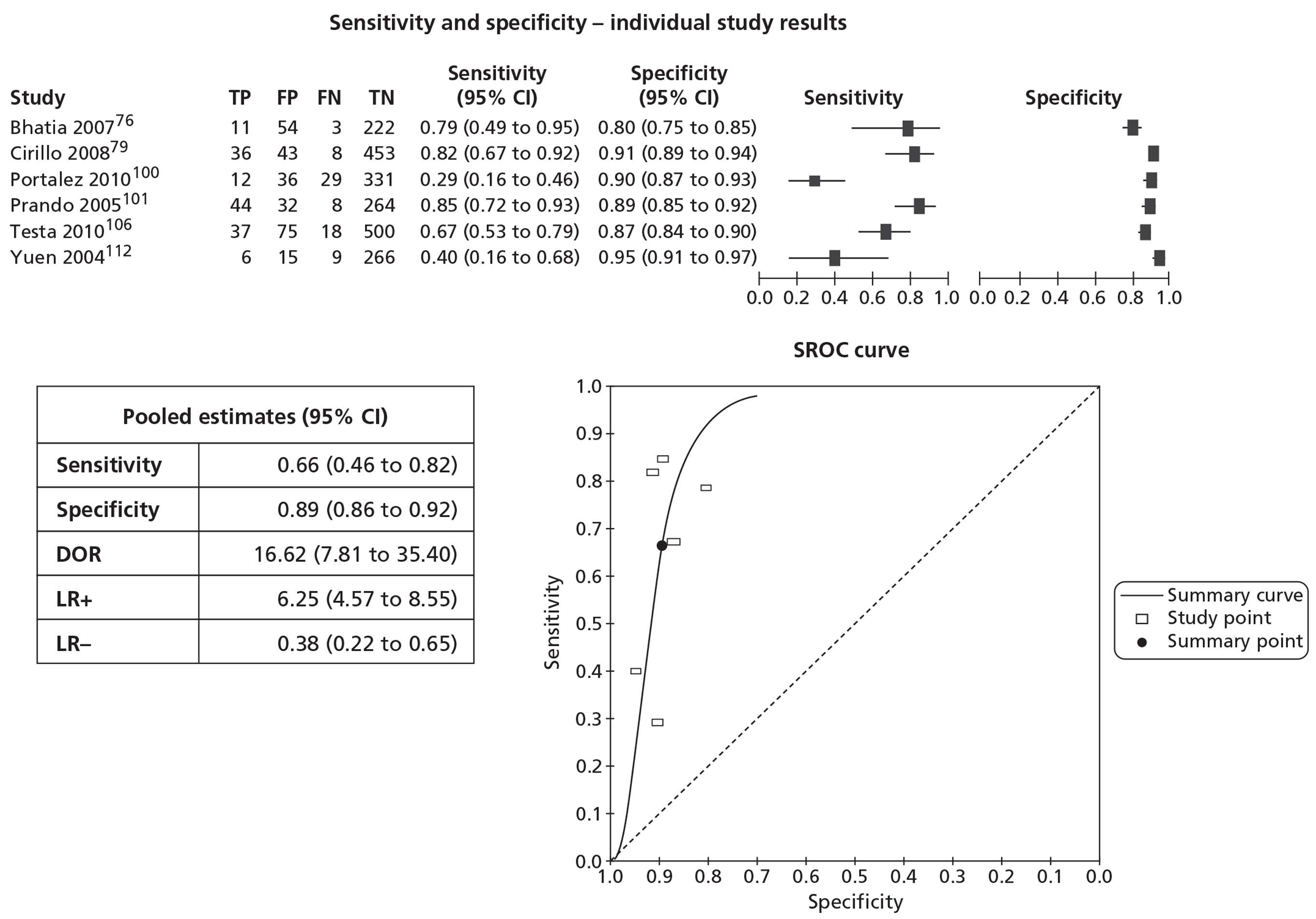
Testa et al. 106 also reported region-based analysis separately for the PZ (sensitivity 64.9%, specificity 85.8%) and the TZ (sensitivity 72.2%, specificity 93.2%).
Dynamic contrast-enhanced magnetic resonance imaging
Patient-level analysis
Three studies78,90,105 involving 209 patients reported the diagnostic accuracy of DCE-MRI and provided sufficient information for inclusion in a meta-analysis. All used a (10-core or at least 12-core) TRUS-guided approach plus additional targeted cores from suspicious areas on the imaging test.
Across the studies the median (range) prevalence of PC was 48.9% (24.7% to 53.8%). The number of previous biopsy sessions the participants had undergone ranged from one105 to one to twelve. 90 The number of cores extracted in the previous biopsy session was 10,105 and (a mean of) 12.6,78 although this information was not reported by Lattouf et al. 90 The studies were judged to have low risk of bias and applicability concerns for all domains, apart from the reference standard domain where all three were judged to be at high risk of bias due to a lack of follow-up.
Figure 7 shows the sensitivity and specificity of the individual studies, pooled estimates and SROC curve. The pooled (95% CI) estimates for sensitivity and specificity were 79% (69% to 87%) and 52% (14% to 88%), respectively. Compared with the other two studies, the study by Sciarra et al. 105 reported high specificity (91%). This study actually reported sensitivity of 84.6% and specificity of 82.3%; however, using the actual 2 × 2 data presented in the paper led to a calculation of 79.5% for sensitivity and 91.3% for specificity, and these were the data used in the pooled estimates. However, there was no obvious explanation for the large difference in specificity values between this study and the other two studies.
FIGURE 7.
Dynamic contrast-enhanced MRI – patient-level analysis: sensitivity, specificity, pooled estimates and SROC curve.
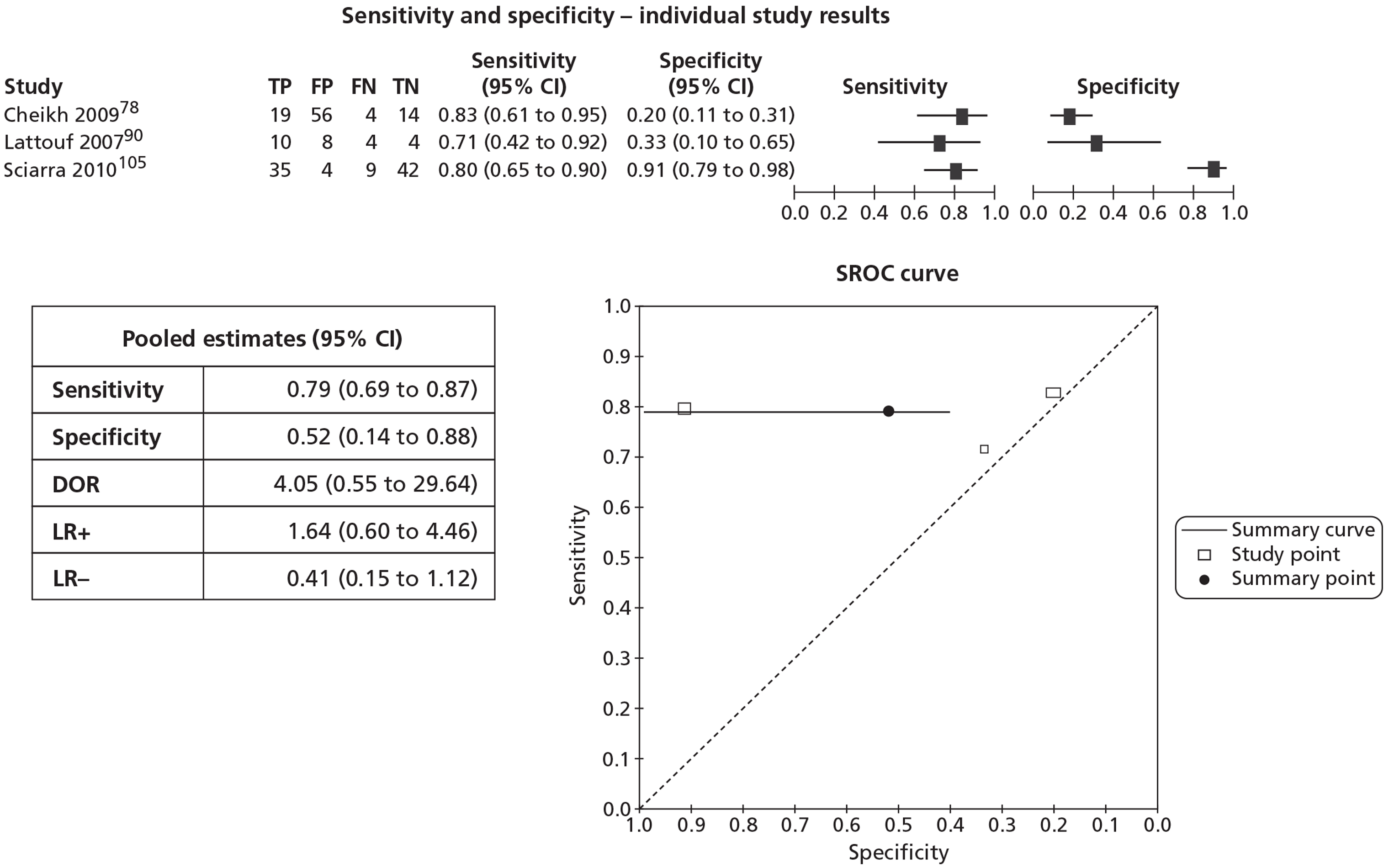
Diffusion-weighted magnetic resonance imaging
Patient-level analysis
One study, by Park et al. ,96 reported a patient-level analysis for DW-MRI. This study, involving 43 patients, employed an MRI-directed, TRUS-guided approach, with at least two cores from suspicious DW areas followed by a 6-, 8- or 10-core biopsy. The study reported a sensitivity of 100% (specificity not reported).
Biopsy-level analysis
Three studies reported DW-MRI at biopsy or other non-patient-level analysis. 96,100,133 The study by Portalez et al. 100 used a 12- to 34-core TRUS-guided approach plus two biopsies of suspicious MRI areas. In a segment level analysis (n = 408) they reported a sensitivity of 39.0% and specificity of 96.0%. Valentini et al. 133 used a 24-core TRUS/Bx (transperineal) approach plus additional biopsies of suspicious MRI areas. In a biopsy-level analysis (number of biopsies not stated) they reported a sensitivity of 60% (specificity not reported). In the study by Park et al. 96 reporting a core level analysis (number of cores not stated), from the information provided it was possible to calculate PPV (78.9%) but not sensitivity or specificity.
T2-weighted magnetic resonance imaging
Patient-level analysis
Fifteen studies57,74,76,78,79,84,90,101,106,108,112,128,134,136,137 involving 620 patients reported the diagnostic accuracy of T2-MRI and provided sufficient information for inclusion in a meta-analysis. All used a (mostly 10- or 12-core) TRUS-guided approach plus additional targeted cores on T2-MRI equivocal or suspicious areas, apart from the studies by Franiel et al. 84 and Wetter et al. ,108 which used a MRI-guided approach.
Across the studies the median (range) prevalence of PC was 35.7% (9.5% to 53.8%). The number of previous biopsy sessions the participants had undergone ranged from 1108 to 1–12. 90 Most studies reported that participants had undergone somewhere in the region of between one to six previous biopsy sessions. The number of cores extracted in the previous biopsy session ranged from 4 or 657 to (a mean of) 12–14. 112
The studies were judged to have low risk of bias for the patient selection, index test and flow of timing domains, apart from, for the flow and timing domain, Beyersdorff et al. 57 (not all patients were included in the analysis). 57 All studies were judged to have a high risk of bias for the reference standard domain owing to a lack of follow-up. All studies were judged to have low applicability concerns for the patient selection, index test and reference standard domains, apart from, for the index test domain, Bhatia et al. 76 (both normal and equivocal results were classed as negative), Cirillo et al. 79 (only PZ assessed) and Prando et al. 101 (central gland not assessed).
Figure 8 shows the sensitivity and specificity of the individual studies, pooled estimates and SROC curve. The pooled (95% CI) estimates for sensitivity and specificity were 86% (74% to 93%) and 55% (44% to 66%), respectively.
FIGURE 8.
T2-weighted magnetic resonance imaging – patient-level analysis: sensitivity, specificity, pooled estimates and SROC curve.
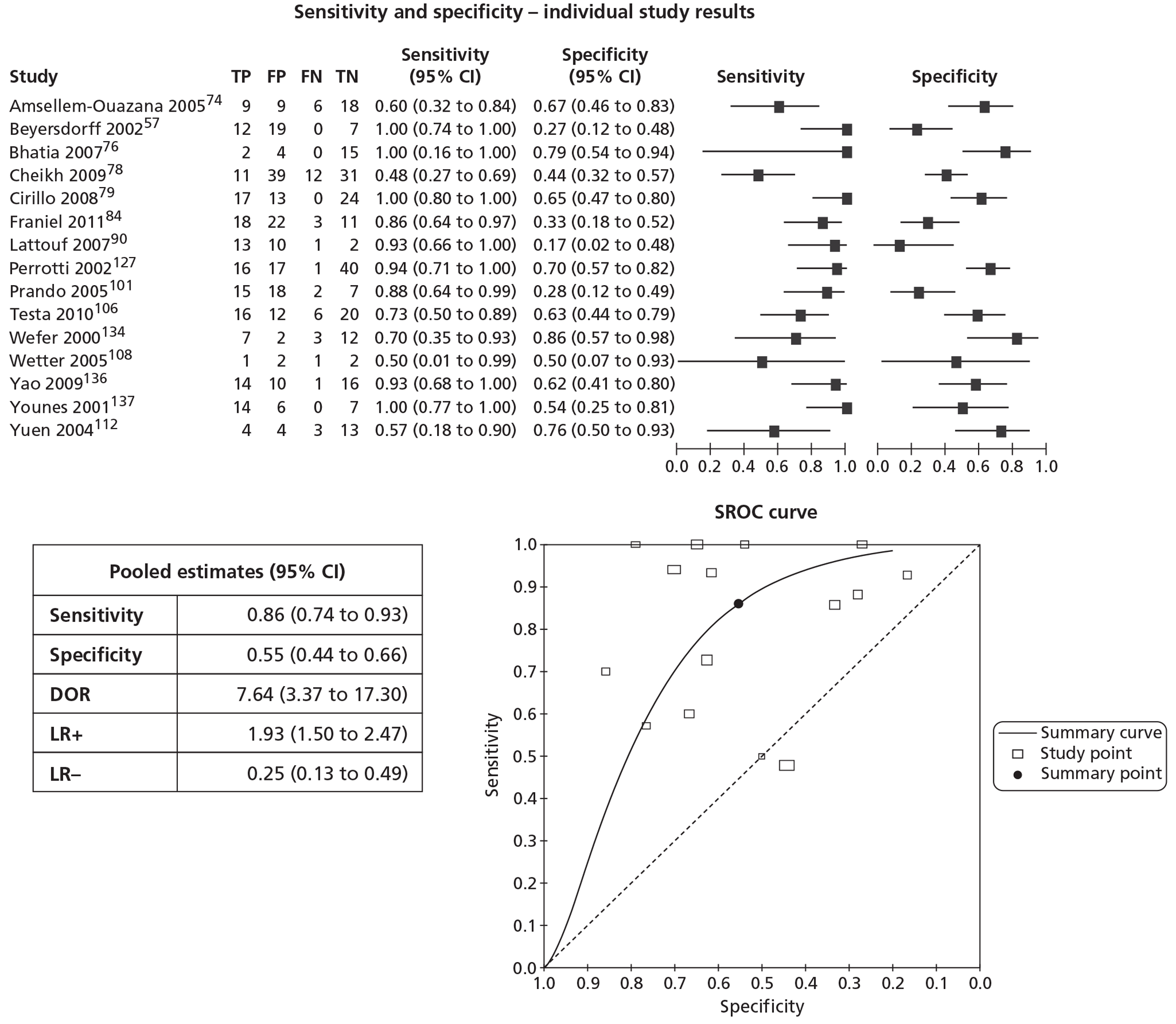
Four studies74,78,108,112 reported sensitivity of 60% or lower. There was no obvious explanation for this in the studies by Amsellem-Ouazana et al. 74 or Cheikh et al. 78 In the study by Wetter et al. 108 only six patients had undergone a previously negative biopsy and this study also extracted biopsies transgluteally. 108 Yuen et al. 112 suggested that contributory factors to the low sensitivity reported might have been (1) difficulties in ensuring the correspondence of TRUS biopsy spatial accuracies to suspicious areas on MRS and (2) that MRS did not cover the entire PZ of the gland. Four studies57,84,90,101 reported specificity of 35% or lower. There was no obvious explanation for this in the studies by Franiel et al. 84 and Prando et al. 101 Beyersdorff et al. 57 reported results either when suspicious and inconclusive, or just suspicious, were used as a cut-off for a positive test. The results using the cut-off of suspicious and inconclusive were included in the pooled estimates. However, if the results using a cut-off of just suspicious had been used this would have increased the specificity to 61.5% but reduced the sensitivity from 100% to 83.3%. The study by Lattouf et al. 90 reported sensitivity of 40% and specificity of 69.5%; however, using the actual 2 × 2 data presented in the paper led to a calculation of 92.9% for sensitivity and 16.7% for specificity, and these were the data used in the pooled estimates. 90
A sensitivity analysis comparing pooled estimates of the results of earlier studies (pre 2007) with those of studies published more recently (2007 onwards) found no significant differences between the two subgroups. The pooled (95% CI) estimates for sensitivity and specificity were pre 2007, 83% (63% to 94%) and 56% (39% to 71%) and 2007 onwards, 88% (72 to 95%) and 55% (41 to 69%) (see Appendix 10).
Biopsy-level analysis
Eight studies57,76,78,79,84,100,106,112 reported the diagnostic accuracy of T2-MRI at biopsy or other non-patient-level analysis and provided sufficient information to be included in a meta-analysis. Figure 9 shows the sensitivity and specificity of the individual studies, pooled estimates and SROC curve. The units of analyses reported by the studies included biopsy,57,76 sector,78 site,79 region,84,106 segment100 and core. 112 The pooled (95% CI) estimates for sensitivity and specificity were 54% (42% to 66%) and 87% (75% to 94%), respectively.
FIGURE 9.
T2-weighted magnetic resonance imaging – biopsy-level analysis: sensitivity, specificity, pooled estimates and SROC curve.
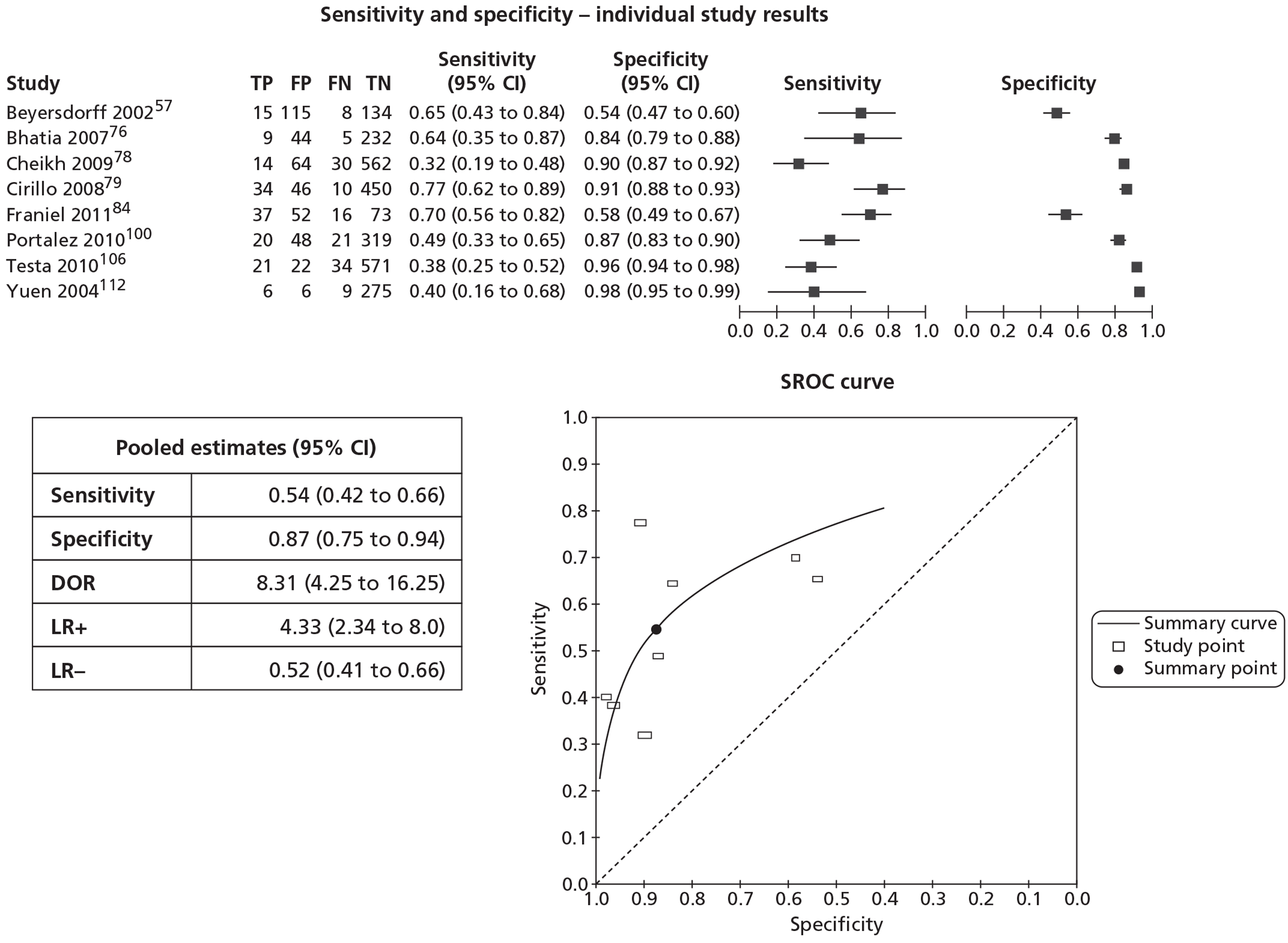
Testa et al. 106 also reported region-based analysis separately for the PZ (sensitivity 27.0%, specificity 95.8%) and the TZ (sensitivity 61.1%, specificity 98.9%).
Transrectal ultrasonography
Patient-level analysis
Twenty-one studies57,75,77,80,81,83,88,91–93,96–99,102,103,107,110,111,113,120 involving 8393 patients reported the sensitivity and/or specificity of systematic TRUS-guided biopsies. See Appendix 11 for the individual study results.
Eleven of these studies57,75,80,81,83,92,93,96,103,107,111 included the use of TRUS as an imaging test, of which six,57,75,80,83,93,111 involving 782 patients, provided sufficient information for inclusion in a meta-analysis. The number of cores extracted ranged from 8 or 1280 to 15. 93
Across the six studies57,75,80,83,93,111 the median (range) prevalence of PC was 26.5% (14.4% to 31.6%). The number of previous biopsy sessions the participants had undergone ranged from 180,93 to 1–6. 57 The number of cores extracted in the previous biopsy session ranged from 4 or 657 to 12. 93
Most studies were judged to have low risk of bias for the patient selection, index test and flow of timing domains. The study by Babaian et al. 75 was judged to be of unclear risk of bias for patient selection (did not report whether or not participant sample was consecutive or provide exclusion criteria) and for the index test (did not report whether or not the test was interpreted by an experienced person). The study by Eskicorapci et al. 83 was also judged to be of unclear risk of bias for patient selection (did not report whether or not participant sample was consecutive or provide exclusion criteria). The study by Beyersdorff et al. 57 was judged to be at high risk of bias for the flow and timing domain (not all participants received a reference standard and not all were included in the analysis). All studies were judged to have a high risk of bias for the reference standard domain owing to a lack of follow-up. All studies were judged to have low applicability concerns for the patient selection, index test and reference standard domains, apart from, for the index test domain, Yuen et al. ,111 which was judged to have high applicability concerns (only PZ assessed).
Figure 10 shows the sensitivity and specificity of the individual studies, pooled estimates and SROC curve. The pooled (95% CI) estimates for sensitivity and specificity were 27% (16% to 42%) and 81% (77% to 85%), respectively.
FIGURE 10.
Transrectal ultrasonography – patient-level analysis: sensitivity, specificity, pooled estimates and SROC curve.
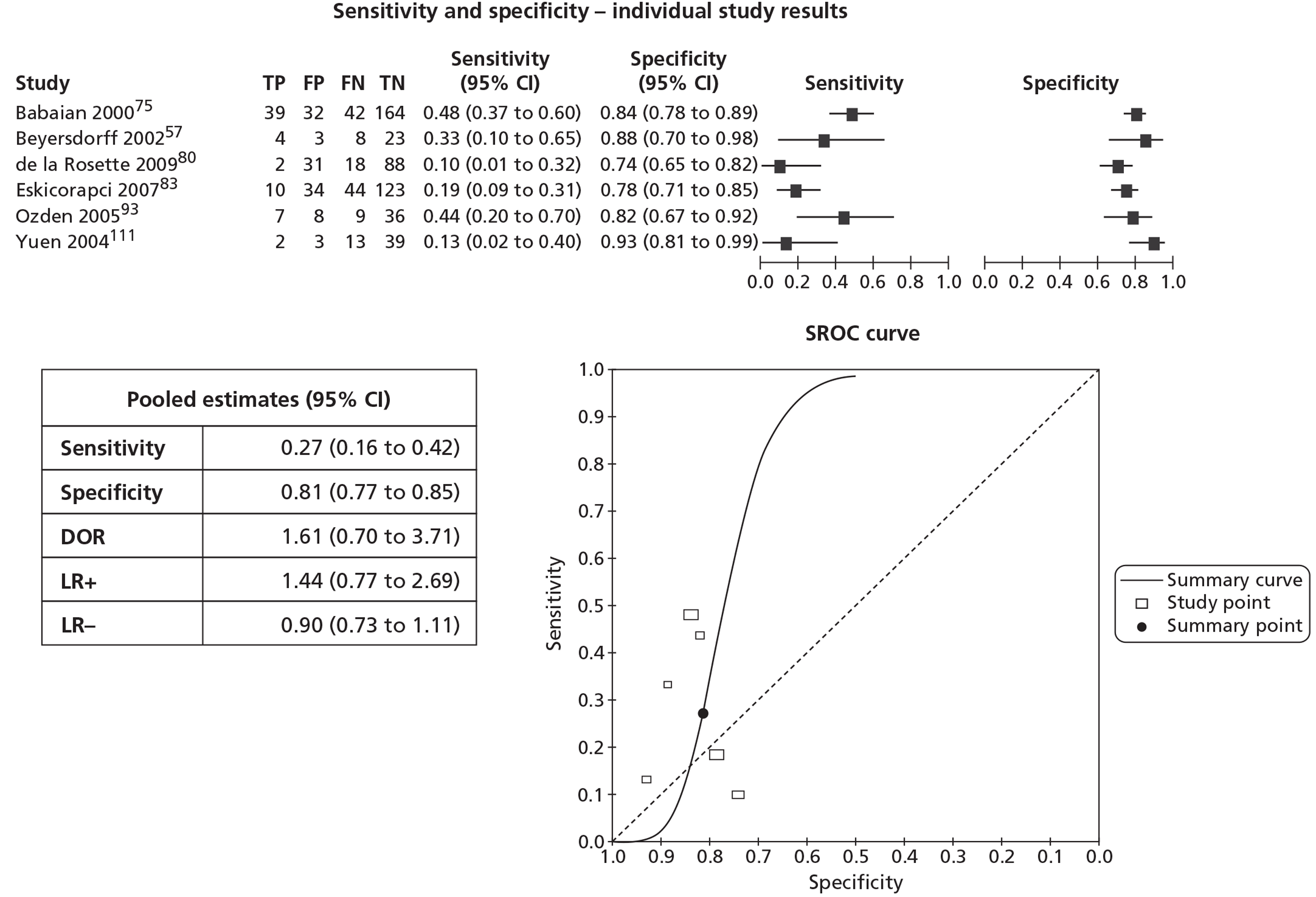
Six large-scale population screening studies enrolling 86,749 participants provided information on the performance of systematic biopsies using a TRUS-guided approach on a subset of their populations who had a previously negative biopsy (n = 5771). 81,88,97,99,103,113 The number of cores taken ranged from 4–688,103 to 16–21. 97 It was not possible to calculate specificity because the procedure merely extracted cores for histopathological assessment and therefore there were no positive or negative test results as such. It was possible to calculate sensitivity on the basis that, for participants with a first negative biopsy, cores taken during the second biopsy session and assessed histopathologically as positive were considered true-positive. For those patients negative on the second biopsy, cores taken during subsequent biopsy sessions and assessed histopathologically as positive were considered to be false-negative on the second biopsy session, thereby allowing sensitivity to be calculated. Across these studies the median (range) sensitivity was 72.5% (60.6% to 96.3%). In effect these studies provided an indication of the sensitivity of the reference standard, which is influenced by the method by which tissue samples are obtained. Across all of the 10 non-imaging TRUS/Bx studies,77,88,91,97–99,102,110,113,120 the median (range) sensitivity was 72.5% (59.3% to 96.3%). The number of cores taken ranged from 4–688 to 16–21. 97
Biopsy-level analysis
No studies reported sensitivity or specificity at a biopsy or other non-patient-level analysis. In the study by Lee et al. ,126 from the information provided it was possible to calculate PPV (3.5%) but not sensitivity or specificity. This study did not report the number of cores taken per patient but did report the overall number of cores sampled (n = 903 from 87 patients, average of 10 cores per patient).
Studies directly comparing tests
Seventeen studies57,74,76,78,79,84,90,96,100,105,106,108,112,120,133,134,136 directly compared two or more tests (see Appendix 12 for details of which studies reported which tests).
Magnetic resonance spectroscopy compared with T2-weighted magnetic resonance imaging
Six studies74,76,79,106,108,112 involving 201 patients reported MRS compared with T2-MRI and provided sufficient information for inclusion in a meta-analysis. All used a (10- or 12-core) TRUS-guided approach plus additional targeted cores on MRS/T2-MRI equivocal or suspicious areas, apart from the study by Wetter et al. ,108 which used a MRI-guided approach. 108 Three studies reported the CC/C ratio used as a cut-off for a positive test result for MRS, which ranged from > 0.6108 to > 0.86. 79
Across the studies the median (range) prevalence of PC was 32.4% (9.5% to 40.7%). The number of previous biopsy sessions the participants had undergone ranged from 1108 to 1–4. 74,106 The number of cores extracted in the previous biopsy session ranged from 676,108 to (a mean of) 12–14. 112 The studies were judged to have low risk of bias for the patient selection, index test and flow of timing domains. All studies were judged to be at high risk of bias for the reference standard domain owing to a lack of follow-up. All studies were judged to have low applicability concerns for patient selection, index test and reference standard domains, apart from, for the index test domain, Bhatia et al. 76 (unclear concern for applicability: both normal and equivocal test results categorised as negative) and Cirillo et al. 79 (high concern for applicability: only PZ assessed).
For the HSROC analysis, we made the assumption that the underlying shape parameter varies with the threshold and accuracy parameters. This is because using the original assumption of a common underlying shape made our models unstable. We provide the results of a sensitivity analysis with the original assumption in Appendix 13.
Figure 11 shows the sensitivity and specificity of the individual studies, pooled estimates and SROC plot with 95% confidence region. The pooled (95% CI) estimates for sensitivity and specificity were 89% (79% to 95%) and 71% (51% to 85%) for MRS, and 77% (55% to 90%) and 68% (59% to 75%) for T2-MRI.
FIGURE 11.
Magnetic resonance spectroscopy compared with T2-MRI patient-level analysis: sensitivity, specificity, pooled estimates and SROC curve.
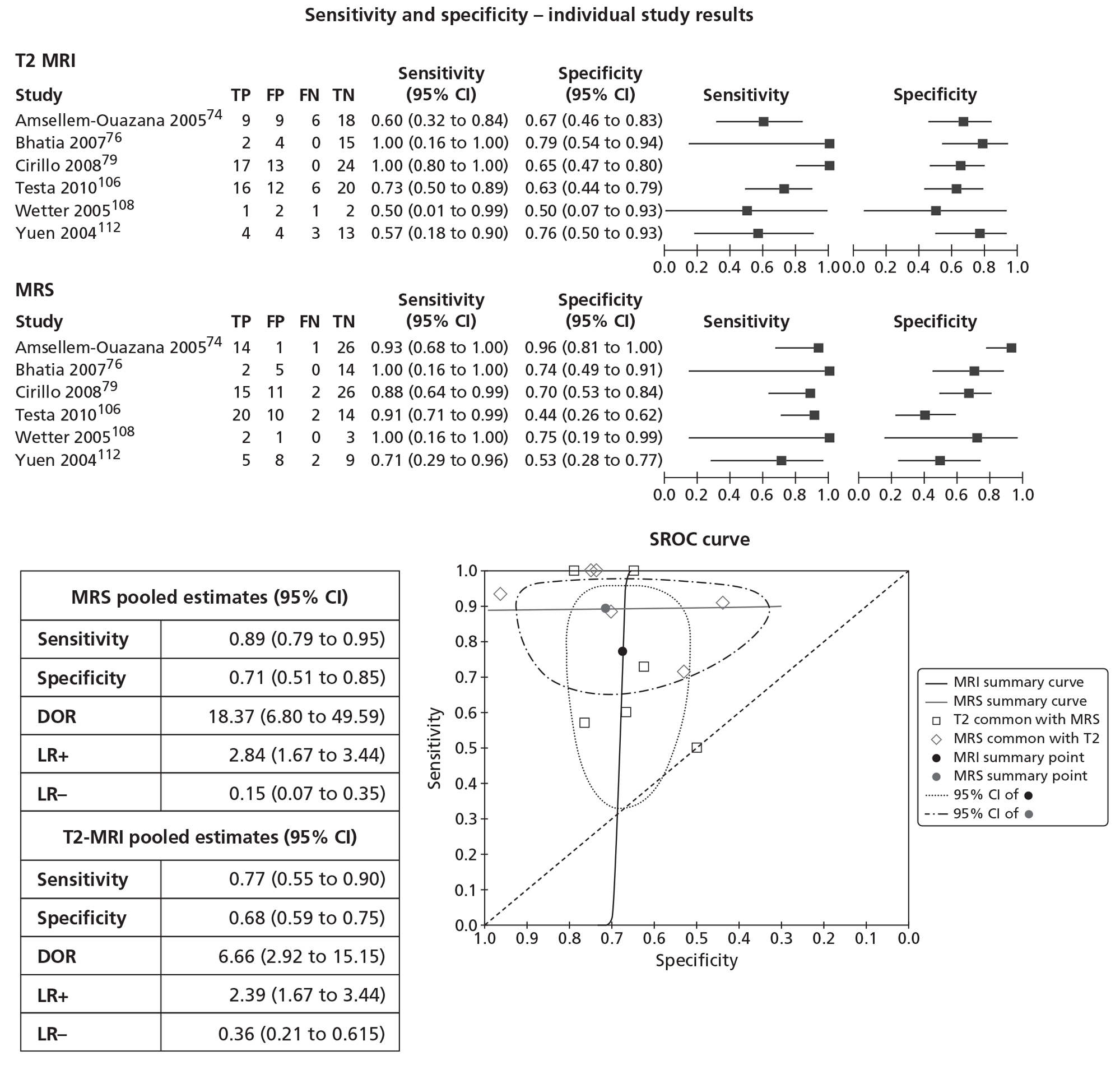
Magnetic resonance spectroscopy compared with dynamic contrast-enhanced magnetic resonance imaging
Two studies100,105 involving 158 patients reported MRS compared with DCE-MRI (Table 6). Portalez et al. 100 reported segment-level but not patient-level analysis, whereas Sciarra et al. 105 reported both patient- and core-level analysis. 105 In the study by Portalez et al. 100 the sensitivity of the tests was similar but low, whereas specificity was also similar but high. In the study by Sciarra et al. 105 MRS had higher sensitivity than DCE-MRI at both patient- and core-level analysis, with broadly similar specificity, which was higher for patient-level analysis compared with core-level analysis. With regard to the low sensitivity reported by Portalez et al. ,100 the authors stated that in order to visualise the early phase of cancer enhancement and retain spatial resolution, they resorted to the shortest possible time with their MRI unit, which proved to yield adequate specificity but suboptimal sensitivity. 100
Dynamic contrast-enhanced magnetic resonance imaging compared with T2-weighted magnetic resonance imaging
Three studies78,90,100 involving 187 patients compared DCE-MRI with T2-MRI (Table 7). In the two studies reporting patient-level analysis,78,90 DCE-MRI had higher sensitivity and lower specificity than T2-MRI in one, with lower sensitivity and higher specificity in the other. Cheikh et al. 78 reported low specificity for DCE-MRI, whereas Lattouf et al. 90 reported low specificity for both DCE-MRI and T2-MRI. The test combination ‘DCE-MRI or T2-MRI’ resulted in similar or increased sensitivity compared with the individual tests but reduced specificity, whereas the combination ‘DCE-MRI and T2-MRI’ reduced sensitivity with a moderate increase in specificity. In the two studies reporting non-patient-level analysis, DCE-MRI had higher sensitivity and slightly lower specificity than T2-MRI in one and lower sensitivity and slightly higher specificity in the other.
| Study ID | Unit of analysis | No. analysed | DCE-MRI | T2-MRI | DCE or T2 | DCE and T2 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | Sensitivity | Specificity | Sensitivity | Specificity | |||
| Cheikh 200978 | Patient | 93 | 82.6 | 20.0 | 47.8 | 44.3 | 82.6 | 15.7 | 47.8 | 51.4 |
| Sector | 670 | 52.3 | 83.5 | 31.8 | 89.8 | 52.3 | 83.1 | 31.8 | 92.3 | |
| Lattouf 200790 | Patient | 26 | 71.4 | 33.3 | 92.9 | 16.7 | 100.0 | 16.7 | 64.3 | 33.3 |
| Portalez 2010100 | Segment | 408 | 29.3 | 93.5 | 48.8 | 87.0 | NR | NR | NR | NR |
Dynamic contrast-enhanced magnetic resonance imaging compared with diffusion-weighted magnetic resonance imaging
Two studies100,133 involving 79 patients compared DCE-MRI with DW-MRI (Table 8). Both reported non-patient-level analysis. DCE-MRI had higher sensitivity than DW-MRI in one study133 and lower sensitivity100 in the other, whereas the sensitivity reported for both DCE-MRI and DW-MRI was much higher in the study by Valentini et al. 133 than it was in the study by Portalez et al. 100 Portalez et al. 100 reported similarly high specificity for both tests.
Studies reporting combinations of tests
The following combinations of tests were reported:
-
MRS or T2-MRI (eight studies)
-
MRS and T2-MRI (five studies)
-
MRS or DCE-MRI (two studies)
-
MRS and DCE-MRI (one study)
-
MRS or DCE-MRI or T2-MRI (one study)
-
MRS or DW-MRI or T2-MRI (one study)
-
MRS or DCE-MRI or DW-MRI or T2-MRI (one study)
-
DCE-MRI or T2-MRI (three studies)
-
DCE-MRI and T2-MRI (two studies)
-
DCE-MRI or DW-MRI (one study)
-
DCE-MRI or DW-MRI or T2-MRI (four studies)
-
DCE-MRI and DW-MRI and T2-MRI (one study)
-
DW-MRI or T2-MRI (three studies).
No studies reported MRS combined with DW-MRI.
In combinations linked by ‘or’ only one of the tests has to be positive for the result of the combination to be considered positive, while in combinations linked by ‘and’ all tests in the combination have to be positive before the result for the combination is considered positive. Combinations linked by ‘or’ generally result in higher sensitivity and lower specificity compared with the individual tests while the reverse is the case for combinations linked by ‘and’.
Magnetic resonance spectroscopy or T2-weighted magnetic resonance imaging
Patient-level analysis
Eight studies74,79,84,106,108,112,118,130 involving 316 patients reported the diagnostic accuracy of MRS or T2-MRI and provided sufficient information for inclusion in a meta-analysis. All used a (mostly 10- or 12-core) TRUS-guided approach plus additional targeted cores on MRS/T2-MRI equivocal or suspicious areas, apart from Franiel et al. 84 and Wetter et al. ,108 who used a MRI-guided approach. In the study by Destefanis et al. 118 all participants (n = 26) had ASAP. Four studies reported the CC/C ratio used as the cut-off for a positive test result, which ranged from > 0.6108 to > 0.86. 79,118
Across the studies the median (range) prevalence was 35.2% (29.2% to 40.7%). The number of previous biopsy sessions the participants had undergone ranged from 1108,118 to 1–6. 84 The number of cores extracted in the previous biopsy session ranged from 6108 to (a mean of) 16. 106
The studies were judged to have low risk of bias for the patient selection, index test and flow of timing domains. All studies were judged to be at high risk of bias for the reference standard domain owing to a lack of follow-up. All studies were judged to have low applicability concerns for the patient selection, index test and reference standard domains, apart from, for the index test domain, Cirillo et al. 79 (only PZ assessed).
Figure 12 shows the sensitivity and specificity of the individual studies, pooled estimates and SROC curve. The pooled (95% CI) estimates for sensitivity and specificity were 96% (90% to 98%) and 31% (21% to 42%), respectively.
FIGURE 12.
Magnetic resonance spectroscopy or T2-MRI patient-level analysis: sensitivity, specificity, pooled estimates and SROC curve.
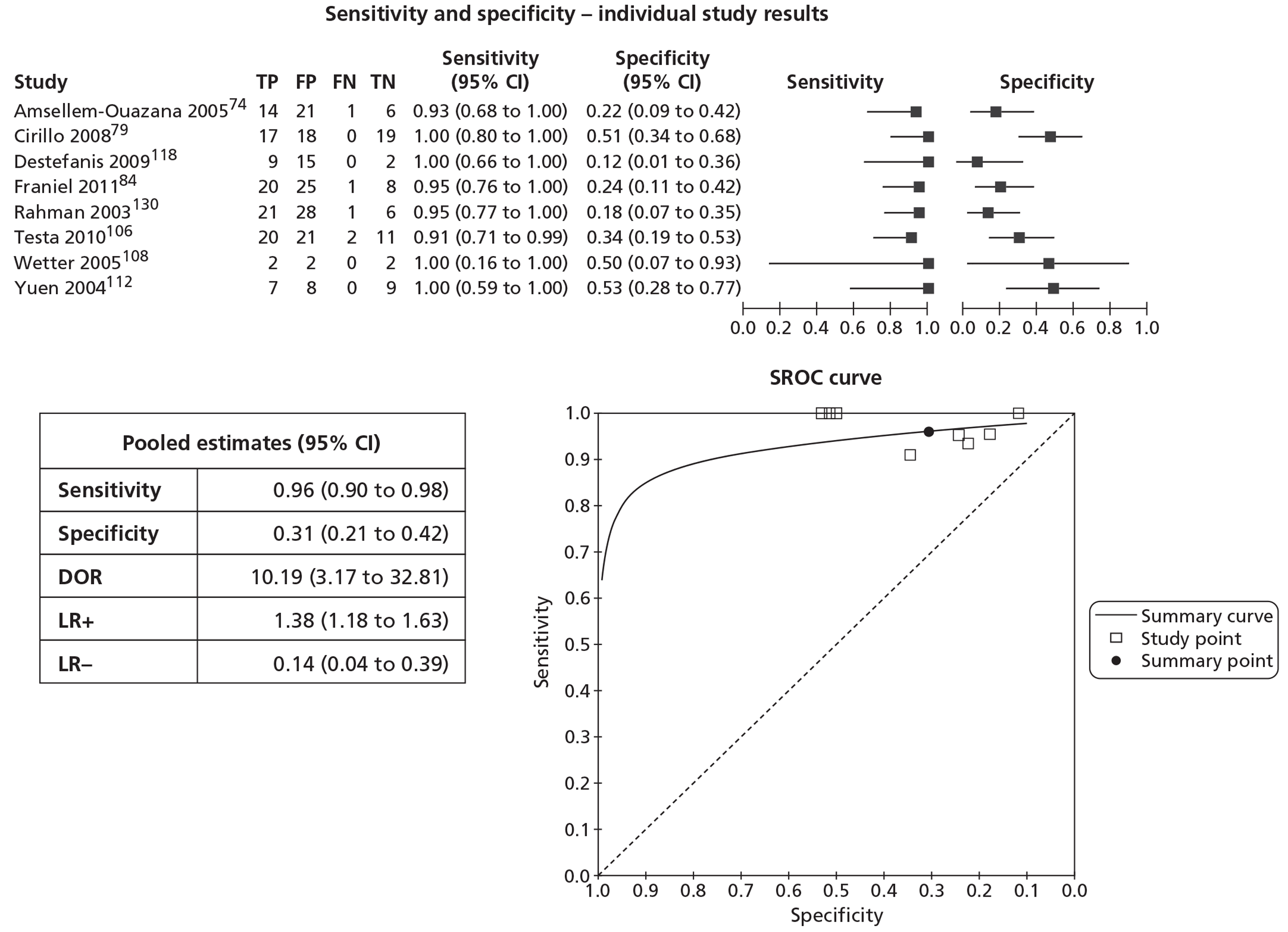
Biopsy-level analysis
Three studies79,84,106 reported MRS or T2-MRI and provided sufficient information for inclusion in a meta-analysis. The units of analyses reported by the studies included site79 and region. 84,106 Figure 13 shows the sensitivity and specificity of the individual studies, pooled estimates and SROC curve. The pooled (95% CI) estimates for sensitivity and specificity were 79% (71% to 86%) and 74% (45% to 90%), respectively. Testa et al. 106 also reported region-based analysis separately for the peripheral and TZs. For the PZ (540 regions analysed), sensitivity was 70.3% and specificity 83.3%, whereas for the TZ (108 regions analysed) sensitivity was 72.2% and specificity 92.2%.
FIGURE 13.
Magnetic resonance spectroscopy or T2-MRI biopsy-level analysis: sensitivity, specificity, pooled estimates and SROC curve.
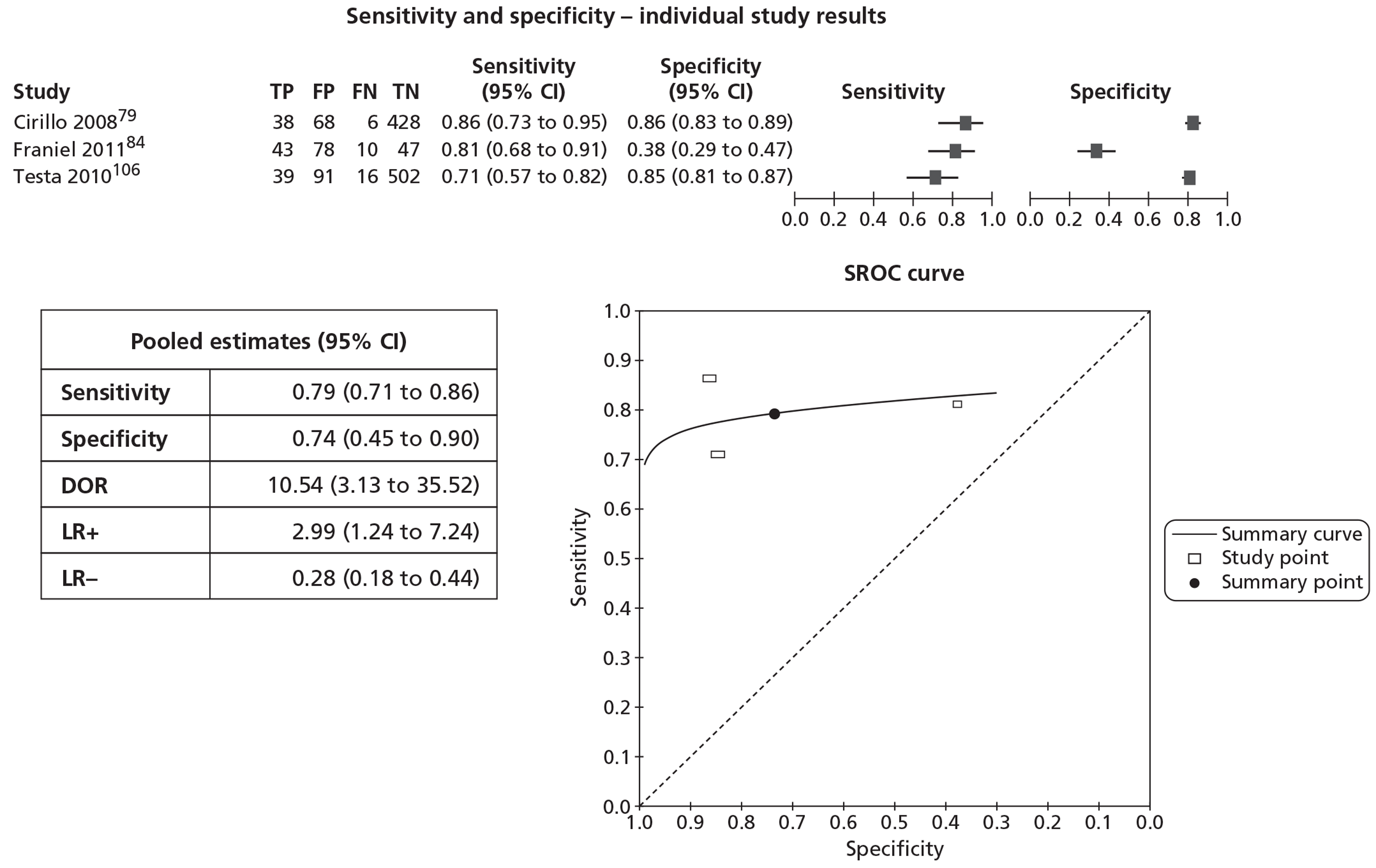
Magnetic resonance spectroscopy and T2-weighted magnetic resonance imaging
Patient-level analysis
Five studies76,106,108,112,134 involving 129 patients reported the diagnostic accuracy of MRS and T2-MRI and provided sufficient information for inclusion in a meta-analysis. All used a (mostly 10- or 12-core) TRUS-guided approach plus additional targeted cores on MRS/T2-MRI equivocal or suspicious areas, apart from Wetter et al. ,108 which used a MRI-guided approach and extracted cores transgluteally. None of the studies reported the CC/C ratio value used as the cut-off for a positive test result.
Across the studies the median (range) prevalence was 33.3% (9.5% to 41.7%). The number of previous biopsy sessions the participants had undergone ranged from 1108 to 1–4. 106 The number of cores extracted in the previous biopsy session ranged from 676,108 to (a mean of) 16. 106
The studies were judged to have low risk of bias for the patient selection, index test and flow of timing domains. All studies were judged to be at high risk of bias for the reference standard domain owing to a lack of follow-up. All studies were judged to have low applicability concerns for the patient selection, index test and reference standard domains, apart from, for the index test domain, Bhatia et al. 76 (normal and equivocal tests were categorised as negative for malignancy).
Figure 14 shows the sensitivity and specificity of the individual studies and pooled estimates (this analysis required to be undertaken without random effect parameters as otherwise the model would not converge and consequently it was not possible to produce a ROC curve). The pooled (95% CI) estimates for sensitivity and specificity were 60% (46% to 75%) and 74% (65% to 84%), respectively.
FIGURE 14.
Magnetic resonance spectroscopy and T2-MRI patient-level analysis: sensitivity, specificity and pooled estimates.
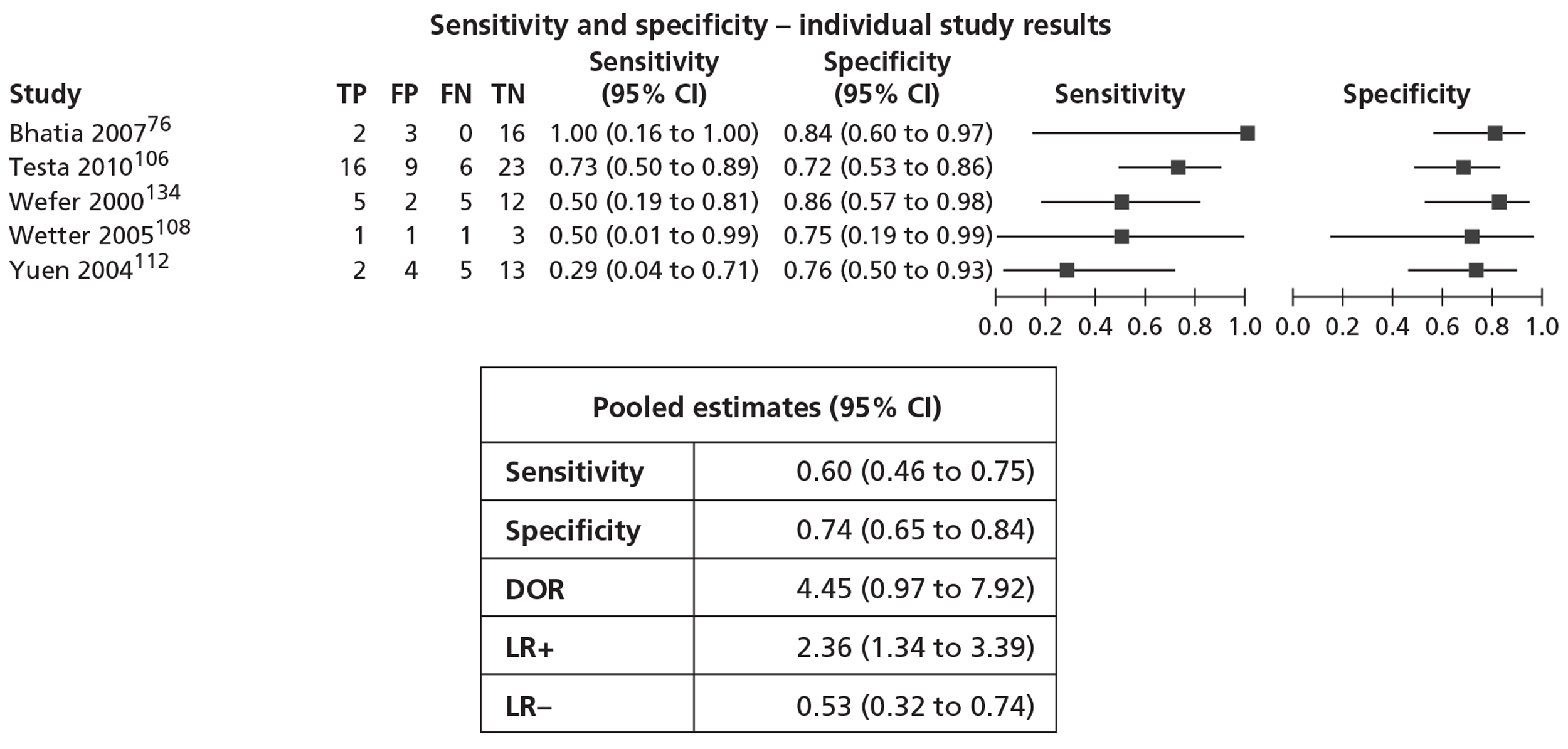
Biopsy-level analysis
Two studies reported the diagnostic accuracy of MRS and T2-MRI at biopsy or other non-patient-level analysis. 76,106 In a biopsy-level analysis (n = 290), Bhatia et al. 76 reported sensitivity of 64.3% and specificity of 91.7%, whereas in a region-based analysis (n = 648) Testa et al. 106 reported sensitivity of 34.5% and specificity of 98.8%, as well as region-based analysis separately for the PZ and TZ. 106 For the PZ (540 regions analysed), sensitivity was 21.6% and specificity 98.6%, whereas for the TZ (108 regions analysed) sensitivity was 61.1% and specificity 100%.
Magnetic resonance spectroscopy or dynamic contrast-enhanced magnetic resonance imaging
Magnetic resonance spectroscopy and dynamic contrast-enhanced magnetic resonance imaging
Patient-level analysis
One study, by Sciarra et al. 105 involving 90 patients reported sensitivity of 75% and specificity of 93.5% for MRS and DCE-MRI.
Biopsy-level analysis
Sciarra et al. 105 reported sensitivity of 89.7% and specificity of 80.4% for core-level analysis.
Other combinations involving magnetic resonance spectroscopy
Franiel et al. 84 reported other combinations of tests involving MRS, both at patient-level (n = 54) and region-level (n = 178) analysis (Table 9).
| Study ID | Test combination | Patient-level analysis | Region-level analysis | ||
|---|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | Sensitivity (%) | Specificity (%) | ||
| Franiel 201184 | MRS or DCE or T2-MRI | 95.2 | 9.1 | 90.6 | 14.4 |
| MRS or DW or T2-MRI | 100.0 | 3.0 | 94.3 | 19.2 | |
| MRS or DCE or DW or T2-MRI | 100.0 | 0.0 | 100.0 | 0.0 | |
In the study by Roethke et al. 104 reporting MRS or T2-MRI or DCE-MRI or DW-MRI (n = 100), from the information provided it was possible to calculate PPV (52%), but not sensitivity or specificity.
Dynamic contrast-enhanced magnetic resonance imaging or T2-weighted magnetic resonance imaging
Patient-level analysis
Three studies78,84,90 involving 173 patients reported the diagnostic accuracy of DCE-MRI or T2-MRI and provided sufficient information for inclusion in a meta-analysis. The studies by Cheikh et al. 78 and Lattouf et al. 90 used a 12-core TRUS-guided approach plus additional targeted cores from suspicious areas on the imaging tests. 78,90 The study by Franiel et al. used a MRI-guided approach. 84
Across the studies the median (range) prevalence of PC was 38.9% (24.7% to 53.8%). The number of previous biopsy sessions the participants had undergone ranged from 1–578 to 1–12. 90 Only Cheikh et al. 78 reported the number of cores extracted in the previous biopsy session (mean of 12.6). The studies were judged to have low risk of bias and applicability concerns for all domains apart from the reference standard domain, for which all three were judged to be at high risk of bias owing to a lack of follow-up.
Figure 15 shows the sensitivity and specificity of the individual studies, pooled estimates and SROC curve. The pooled (95% CI) estimates for sensitivity and specificity were 88% (80% to 96%) and 14% (8% to 20%), respectively.
FIGURE 15.
Dynamic contrast-enhanced MRI or T2-MRI patient-level analysis: sensitivity, specificity, pooled estimates and SROC curve.
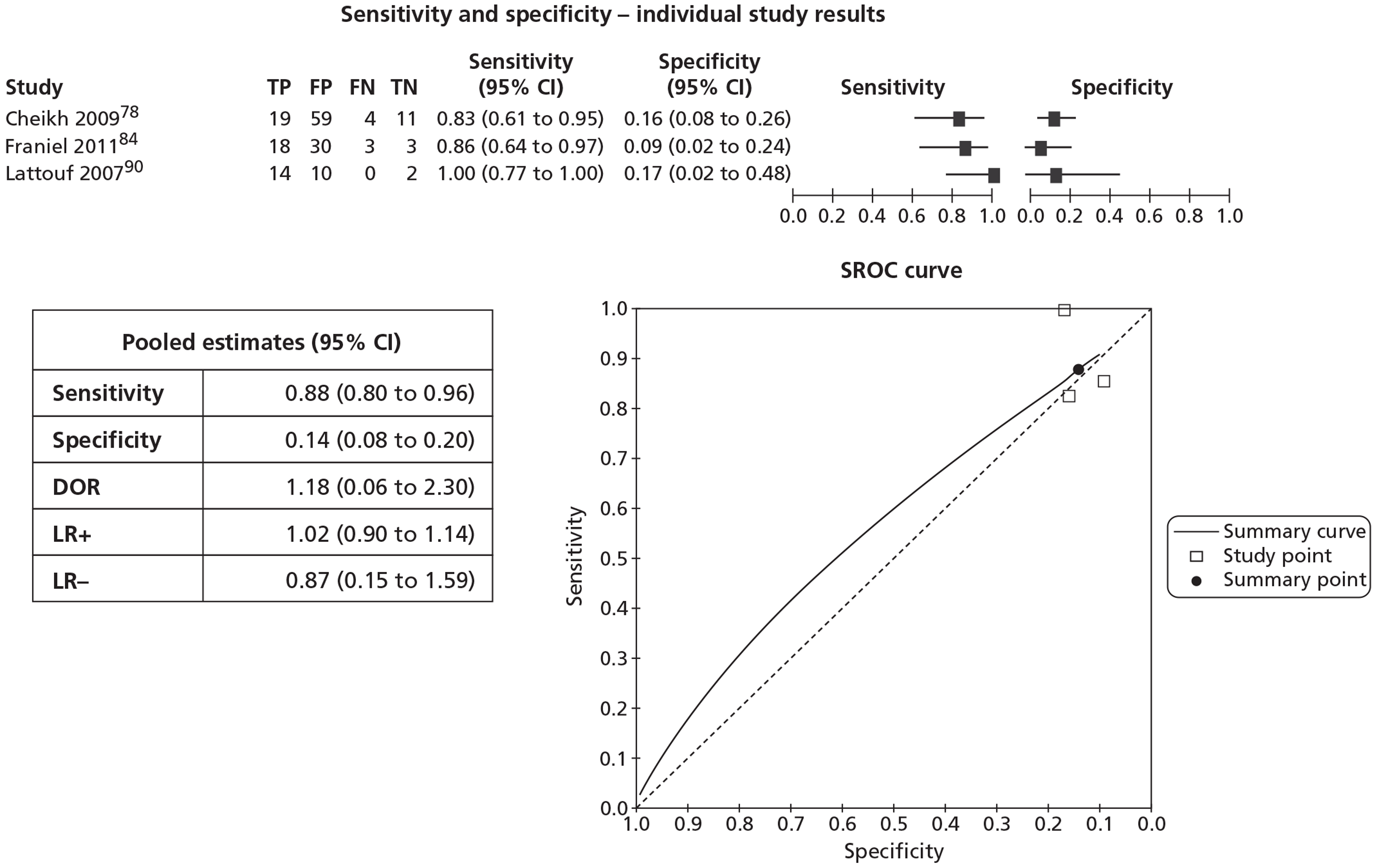
Dynamic contrast-enhanced magnetic resonance imaging and T2-weighted magnetic resonance imaging
Dynamic contrast-enhanced magnetic resonance imaging or diffusion-weighted magnetic resonance imaging
Biopsy-level analysis
No study reported a patient-level analysis of DCE-MRI combined with DW-MRI. Valentini et al. ,133 in a study involving 11 patients, reported a biopsy-level analysis (number of biopsies not reported) for DCE-MRI or DW-MRI. This study used a TRUS-guided approach (24 cores plus additional biopsies of suspicious MRI areas). From the information provided in the study it was possible to calculate PPV (17.2%) but not sensitivity or specificity.
Dynamic contrast-enhanced magnetic resonance imaging or diffusion-weighted magnetic resonance imaging or T2-weighted magnetic resonance imaging
Patient-level analysis
Four studies84,86,87,109 involving 395 patients reported DCE-MRI or DW-MRI or T2-MRI. Franiel et al. 84 reported sensitivity of 100% and specificity of 0%. However, from the information provided in the other three studies86,87,109 it was possible to calculate PPV (100%,86 40.9%,87 55.6%109) but not sensitivity or specificity.
Biopsy-level analysis
Three studies84,87,109 reported DCE-MRI or DW-MRI or T2-MRI at a region-level analysis. However, only the study by Franiel et al. 84 reported sensitivity (94.3%) and specificity (16.0%). From the information provided in the other two studies87,109 it was possible to calculate PPV (33.4%,87 46.2%109) but not sensitivity or specificity.
Dynamic contrast-enhanced magnetic resonance imaging and diffusion-weighted magnetic resonance imaging and T2-weighted magnetic resonance imaging
Patient-level analysis
One study, by Labanaris et al. 89 involving 260 patients reported sensitivity of 88.1% and specificity of 62.4% for DCE-MRI and DW-MRI and T2-MRI. No studies reported biopsy-level analysis.
Diffusion-weighted magnetic resonance imaging or T2-weighted magnetic resonance imaging
Patient-level analysis
Two studies84,126 involving 141 patients reported DW-MRI or T2-MRI at a patient-level analysis. The study by Franiel et al. 84 used a MRI-guided approach. Franiel et al. 84 reported sensitivity of 100% and specificity of 3.0%, whereas Lee et al. 126 reported sensitivity of 95.7% and specificity of 7.3%.
Biopsy-level analysis
Three studies84,116,126 reported DW-MRI or T2-MRI at a biopsy or other non-patient-level analysis. Chung et al. 116 reported sensitivity of 82.8% and specificity of 68.9%, whereas Franiel et al. 84 reported sensitivity of 84.9% and specificity of 36.8%. From the information provided in the study by Lee et al. 54 it was possible to calculate PPV (12.7%) but not sensitivity or specificity. 126
Indirect comparison
Table 10 shows the results of the indirect comparison (see also Appendix 13).
| Parameter | Estimate | 95% CI | Ratio (95% CI) | p-value | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Sensitivity for T2-MRI | 83 | 75 | 89 | 1 | |
| Sensitivity for DCE | 87 | 74 | 94 | 1.04 (0.93 to 1.17) | 0.499 |
| Sensitivity for MRS | 93 | 87 | 97 | 1.12 (1.03 to 1.22) | 0.008 |
| Sensitivity for T2-MRI and MRS | 71 | 50 | 85 | 0.85 (0.67 to 1.07) | 0.172 |
| Sensitivity for T2-MRI or DCE | 92 | 81 | 97 | 1.10 (1.00 to 1.21) | 0.046 |
| Sensitivity for T2-MRI or MRS | 97 | 91 | 99 | 1.16 (1.07 to 1.26) | 0.001 |
| Sensitivity for TRUS | 24 | 13 | 39 | 0.28 (0.16 to 0.50) | < 0.001 |
| Specificity for T2-MRI | 57 | 47 | 67 | 1 | |
| Specificity for DCE | 40 | 25 | 56 | 0.70 (0.49 to 0.98) | 0.041 |
| Specificity for MRS | 64 | 52 | 75 | 1.12 (0.95 to 1.32) | 0.194 |
| Specificity for T2-MRI and MRS | 73 | 58 | 85 | 1.28 (1.06 to 1.55) | 0.011 |
| Specificity for T2-MRI or DCE | 24 | 13 | 39 | 0.42 (0.26 to 0.68) | < 0.001 |
| Specificity for T2-MRI or MRS | 34 | 23 | 46 | 0.59 (0.44 to 0.78) | < 0.001 |
| Specificity for TRUS | 88 | 79 | 94 | 1.54 (1.27 to 1.86) | < 0.001 |
Patient-level analysis
For the patient-level estimates of tests with three or more studies, comparing T2-MRI against all the other tests showed statistically significant differences (p < 0.001). Compared with DCE-MRI, T2-MRI was observed to have lower sensitivity and significantly higher specificity. Sensitivity (95% CI) for DCE-MRI was 87% (74% to 94%) compared with 83% (75% to 89%) for T2-MRI (p = 0.499). Specificity (95% CI) for DCE was 40% (25% to 56%) compared with 57% (47% to 67%) for T2-MRI (p = 0.041).
Magnetic resonance spectroscopy was observed to have higher sensitivity and specificity than T2-MRI. Sensitivity for MRS was 93% (87% to 97%) compared with 83% (75% to 89%) for T2-MRI (p = 0.008). Specificity for MRS was 64% (52% to 75%) compared with 57% (47% to 67%) for T2-MRI (p = 0.194).
When T2-MRI was used in combination with MRS (‘T2-MRI and MRS’, both tests had to be suspicious for the combination to be considered positive) T2-MRI used alone was observed to have higher sensitivity but significantly lower specificity. Sensitivity for ‘T2-MRI and MRS’ was 71% (50% to 85%) compared with 83% (75% to 89%) for T2-MRI (p = 0.172). Specificity for ‘T2-MRI and MRS’ was 73% (58% to 85%) compared with 57% (47% to 67%) for T2-MRI (p = 0.011).
When T2-MRI was used in combination with DCE-MRI (‘T2-MRI or DCE-MRI’, if either test is suspicious the combination is considered positive), T2-MRI used alone was observed to have lower sensitivity but significantly higher specificity. Sensitivity for ‘T2-MRI or DCE-MRI’ was 92% (81% to 97%) compared with 83% (75% to 89%) for T2-MRI (p = 0.046). Specificity for ‘T2-MRI or DCE-MRI’ was 24% (13% to 39%) compared with 57% (47% to 67%) for T2-MRI (p < 0.001).
When T2-MRI was used in combination with MRS (‘T2-MRI or MRS’, if either test is suspicious the combination is considered positive), this combination had significantly higher sensitivity than T2-MRI alone but significantly lower specificity. Sensitivity for ‘T2-MRI or MRS’ was 97% (91% to 99%) compared with 83% (75% to 89%) for T2-MRI (p = 0.001). Specificity for ‘T2-MRI or MRS’ was 34% (23% to 46%) compared with 57% (47% to 67%) for T2-MRI (p < 0.001).
Compared with TRUS used as an imaging test, T2-MRI was observed to have significantly higher sensitivity but significantly lower specificity. Sensitivity for TRUS was 24% (13% to 39%) compared with 83% (75% to 89%) for T2-MRI (p < 0.001). Specificity for TRUS was 88% (79% to 94%) compared with 57% (47% to 67%) for T2-MRI (p < 0.001).
These differences are based on between-study comparisons, so may have been due to differences between the studies rather than true differences between the tests.
For the estimates comparing T2-MRI with other tests, in terms of relative sensitivity, the direction of effect favoured (1) MRS; (2) ‘T2-MRI or DCE’; and (3) ‘T2-MRI or MRS’ over T2-MRI, while favouring T2-MRI over (1) DCE-MRI; (2) ‘T2-MRI and MRS’; and (3) TRUS, although the only results that were statistically significant were for ‘T2-MRI or MRS’ compared with T2-MRI (‘T2-MRI or MRS’ better) and T2-MRI compared with TRUS (T2-MRI better). See Appendix 13.1 for further details.
In terms of relative specificity the direction of effect favoured (1) MRS; (2) ‘T2-MRI and MRS’; and (3) TRUS over T2-MRI, while favouring T2-MRI over (1) DCE-MRI; (2) ‘T2-MRI or DCE-MRI’; and (3) ‘T2-MRI or MRS’, although the only results that were statistically significant were for ‘T2-MRI and MRS’ compared with T2-MRI (‘T2-MRI and MRS’ better), T2-MRI compared with TRUS (TRUS better), ‘T2-MRI or DCE-MRI’ compared with T2-MRI (T2-MRI better) and ‘T2-MRI or MRS’ compared with T2-MRI (T2-MRI better). See Appendix 13.1 for further details.
Biopsy-level analysis
The highest sensitivity (95% CI) was for the combination ‘T2-MRI or MRS’ at 75% (61% to 86%), whereas the highest specificity was for T2-MRI at 87% (78% to 93%), with MRS also reporting a similarly high specificity at 84% (72% to 91%). See Appendix 13.2 for further details.
For the estimates comparing T2-MRI with other tests, in terms of relative sensitivity both (1) MRS and (2) ‘T2-MRI or MRS’ had statistically significantly higher sensitivity than T2-MRI, whereas for specificity the direction of effect favoured T2-MRI over both (1) MRS and (2) ‘T2-MRI or MRS’, although only the comparison with ‘T2-MRI or MRS’ was statistically significant. See Appendix 13.2 for further details.
False-positive results
Eleven studies57,74,76,78,79,101,106,108,109,133,137 provided further information on the MR-imaging false-positive results in their studies (see Appendix 14 for individual study details). The false-positive rate for patient-level analysis (six studies74,76,79,101,108,137) ranged from 2.4%74 to 100%108 and for biopsy or other non-patient-level analysis (five studies57,78,106,109,133) ranged from 13.0%106 to 46.2%. 57 High-grade PIN and prostatitis accounted for a substantial proportion of the false-positive results. 109 Cirillo et al. 79 presented this information separately for MRS and T2-MRI. For MRS (11 false-positives), there was PIN in six (54.5%), fibrosis in four (36.4%) and normal prostatic tissue in one (9.1%); for T2-MRI (13 false-positives), there was PIN in three (23.0%), fibrosis in five (38.5%) and normal prostatic tissue in five (38.5%). 79 Beyersdorff et al. 57 concluded that the T2-MRI technique used in their study did not enable reliable differentiation of PC from prostatitis, fibrosis or PIN.
Detection of clinically significant disease
Twenty-nine studies74,76,78–80,82,84,86,87,90,91,95,96,104–106,108,109,111,112,136 reported the Gleason score based on the biopsy results of patients diagnosed with PC (see Appendix 15 for individual study details). Most studies reported a median Gleason score of ≥ 6. The percentage of patients diagnosed with PC who had a Gleason score of ≥ 7 ranged from 20.3%86 to 66.7%. 109 In 13 studies74,80,81,83,87,91,96–98,102,111,113,136 it was not possible to calculate this information.
Six MRI studies reported a median Gleason score of > 6 (Table 11). In these studies the percentage of patients diagnosed with PC who had a Gleason score of ≥ 7 ranged from 50.0%90,108 to 66.7%. 109
| Study ID | Test(s) | No. analysed | No. with PC | Prevalence (%) | Median (range) Gleason score | Percentage with Gleason score ≥ 7 |
|---|---|---|---|---|---|---|
| Amsellem-Ouazana 200574 | T2-MRI/MRS | 42 | 15 | 35.7 | 6.6 (5 to 9) | NR |
| Lattouf 200790 | T2-MRI/DCE | 26 | 14 | 53.8 | 6.5 (5 to 9) | 50.0 |
| Park 200896 | DW-MRI | 43 | 17 | 39.5 | 7 (6 to 9) | NR |
| Roethke 2012104 | T2-MRI/MRS/DCE-MRI/DW-MRI | 100 | 52 | 52.0 | 7 (5 to 9) | 59.7 |
| Wetter 2005108 | T2-MRI/MRS | 6 | 2 | 33.3 | (6, 7) | 50.0 |
| Yakar 2011109 | T2-MRI/DCE-MRI/DW-MRI | 9 | 5 | 55.6 | 7 (6 to 8) | 66.7 |
Results: assessment of non-diagnostic outcomes
Altered treatment as a result of the tests
No studies reported information on altered treatment as a result of the tests.
Acceptability of the tests
No studies provided information on the acceptability of the tests used.
Interpretability of the tests
Three studies78,101,106 reported the interpretability of the tests used. Cheikh et al. ,78 in a study using T2-MRI and DCE-MRI, reported that in 1 (1.1%) of 93 patients analysed, DCE images could not be interpreted because of inadequate quality due to artefacts induced by a hip prosthesis. This patient was 1 of 23 diagnosed with cancer.
In a study that employed T2-MRI and MRS, Prando et al. 101 stated that suitable spectroscopic voxels were rated as optimal, fair or poor on the basis of spectral quality (Table 12). 101 They reported that, out of 42 patients analysed, the quality of spectral data was rated as optimal in 23 (55%), fair in 10 (24%) and poor in 9 (21%).
| Spectral quality | Definition |
|---|---|
| Optimal | Signal–noise ratio of all metabolites > 10, all metabolic resonances well resolved, no baseline distortions due to residual water or lipids |
| Fair | Signal–noise ratio of all metabolites 8–10, all metabolic resonances reasonably well resolved, or minimal baseline distortions owing to residual water or lipids |
| Poor | Lower signal–noise ratios and substantial lipid contamination |
Testa et al. ,106 in a study using T2-MRI and MRS, reported that 4 (7%) of 58 patients were excluded because more than one-third of the prostate was not included in the MRI volume of interest, or more than one-third of spectroscopic voxels were not interpretable owing to lipid contamination or presented low spectral resolution. Out of the remaining 54 patients included in the analysis, in 18 (3%) of 648 regions MRS imaging was not interpretable (corresponding to six patients) and these regions were excluded from the analysis. None of these 18 regions was in the TZ. 106
Effect of testing on quality of life
No studies reported the effects of the tests on QoL.
Adverse effects of testing
Ten studies57,76,81,82,86,87,89,109,111,112 reported adverse events, all of which appeared to be related to TRUS-guided biopsies, with one of the most frequently reported adverse events being transient haematuria (see Appendix 16 for individual study details). Of the other more serious adverse events reported, Beyersdorff et al. 57 reported that two patients (5%) experienced haemorrhage in the prostate; Djavan et al. 81 reported that 1.4% experienced moderate to severe vasovagal episodes, 0.5% experienced severe haematuria and 0.1% major rectal bleeding (numbers of patients not reported); Hoeks et al. 87 reported that one patient (0.4%) experienced sepsis with hospitalisation and four (1.5%) experienced a vasovagal reaction; Labanaris et al. 89 reported that 190 patients (73%) experienced macroscopic haematuria; Yuen et al. 111 reported that three patients (1.4%) experienced macroscopic haematuria, five (2.3%) experienced fever and five (2.3%) experienced acute retention of urine (all 13 treated conservatively as inpatient), while one patient (0.5%) experienced rectal bleeding, requiring admission to hospital. None of the studies provided information on injuries resulting from multiple biopsies over time. Neither did any study report the extremely rare adverse event of biopsy leading to disease seeding along needle tracks.
Summary
Sixty-five reports of 51 studies met the inclusion criteria (39 full text, 12 abstracts). The majority of studies were considered to have a low risk of bias for the patient selection (74%, 29/39), index test (100%, 39/39) and flow and timing (92%, 36/39) domains. In the reference standard domain, the majority of studies (64%, 25/39) were considered at high risk of bias due to a lack of follow-up. All 39 studies had low concern for applicability for the reference standard domain and the majority also had low concerns for applicability for the patient selection (95%, 37/39) and index test (87%, 34/39) domains.
The sensitivity and specificity of the tests (patient-level analysis) are summarised in Table 13 (results of the meta-analyses for the individual tests, combinations of tests and for those studies directly comparing MRS with T2-MRI), Table 14 (results for the tests and combinations of tests for which it was not considered appropriate or feasible to include in a meta-analysis) and Table 15 (pooled estimates for the individual tests and combinations of tests included in the indirect comparison).
| Test(s) | No. of studies | No. of participants | Sensitivity: pooled estimate, % (95% CI) | Specificity: pooled estimate, % (95% CI) |
|---|---|---|---|---|
| Individual tests | ||||
| MRS | 10 | 438 | 92 (86 to 95) | 76 (61 to 87) |
| DCE-MRI | 3 | 209 | 79 (69 to 87) | 52 (14 to 88) |
| T2-MRI | 15 | 620 | 86 (74 to 93) | 55 (44 to 66) |
| TRUS (imaging test) | 6 | 782 | 27 (16 to 42) | 81 (77 to 85) |
| Combinations of tests | ||||
| MRS or T2-MRI | 8 | 316 | 96 (90 to 98) | 31 (21 to 42) |
| MRS and T2-MRI | 5 | 129 | 60 (46 to 75) | 74 (65 to 84) |
| DCE-MRI or T2-MRI | 3 | 173 | 88 (80 to 96) | 14 (8 to 20) |
| Studies directly comparing MRS with T2-MRI | ||||
| MRS | 6 | 201 | 89 (79 to 95) | 71 (51 to 85) |
| T2-MRI | 77 (55 to 90) | 68 (59 to 75) | ||
| Test(s) | No. of studies | No. of participants | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| DW-MRI | 1 | 43 | 100 | NR |
| MRS or DCE | 2 | 131 | 93, 93 | 87, 91 |
| MRS and DCE | 1 | 90 | 75 | 94 |
| MRS or DCE-MRI or T2-MRI | 1 | 54 | 95 | 9 |
| MRS or DW-MRI or T2-MRI | 1 | 54 | 100 | 3 |
| MRS or DCE-MRI or DW-MRI or T2-MRI | 1 | 54 | 100 | 0 |
| DCE-MRI and T2-MRI | 2 | 119 | 48, 64 | 51, 33 |
| DCE-MRI or DW-MRI or T2-MRI | 1 | 54 | 100 | 0 |
| DCE-MRI and DW-MRI and T2-MRI | 1 | 260 | 88 | 62 |
| DW-MRI or T2-MRI | 2 | 141 | 96, 100 | 7, 3 |
| Test(s) | Sensitivity: pooled estimate, % (95% CI) | Specificity: pooled estimate, % (95% CI) |
|---|---|---|
| MRS | 93 (87 to 97) | 64 (52 to 75) |
| DCE-MRI | 87 (74 to 94) | 40 (25 to 56) |
| T2-MRI | 83 (75 to 89) | 57 (47 to 67) |
| MRS or T2-MRI | 97 (91 to 99) | 34 (23 to 46) |
| MRS and T2-MRI | 71 (50 to 85) | 73 (58 to 85) |
| DCE-MRI or T2-MRI | 92 (81 to 97) | 24 (13 to 39) |
| TRUS (imaging test) | 24 (13 to 39) | 88 (79 to 94) |
In the meta-analyses for the individual tests, sensitivity was highest for MRS (92%), followed by T2-MRI (86%) and DCE-MRI (79%), whereas specificity was highest for TRUS (used as an imaging test) (81%), followed by MRS (76%). TRUS used as an imaging test had poor sensitivity (27%). In the pooled estimates for combinations of tests, sensitivity was highest for ‘MRS or T2-MRI’ (96%) followed by ‘DCE-MRI or T2-MRI’ (88%), whereas specificity was highest for ‘MRS and T2-MRI’ (74%). The gain in sensitivity from MRS as a single test (92%) to the combination ‘MRS or T2-MRI’ (96%) was offset by a large decrease in specificity from 76% to 31%.
In the meta-analysis of the six studies74,76,79,106,108,112 directly comparing MRS with T2-MRI, sensitivity and specificity for MRS was 89% and 71%, respectively, compared with 77% and 68% for T2-MRI.
Only one small study96 involving 43 patients reported DW-MRI, with sensitivity of 100% (specificity not reported). A number of other combinations of tests were reported, mostly by single studies.
The results of the indirect comparison broadly reflected those of the meta-analyses of the individual tests and combinations of tests. In the indirect comparison, the highest sensitivity reported was for the combination of ‘MRS or T2-MRI’ (97%), followed by MRS (93%) and ‘DCE-MRI or T2-MRI’ (92%). TRUS as an imaging test had poor sensitivity (24%). However, TRUS had the highest specificity (88%), followed by the combination of ‘MRS and T2-MRI’ (73%) and MRS (64%).
Six large-scale population screening studies81,88,97,99,103,113 provided information on the performance of systematic biopsies using a (non-imaging) TRUS-guided approach on a subset of their patient populations with a previous negative biopsy (n = 5771). Across these studies the median (range) sensitivity was 72.5% (60.6% to 96.3%). Across all of the 10 non-imaging TRUS/Bx studies,77,88,91,97–99,102,110,113,120 the median (range) sensitivity was 72.5% (59.3% to 96.3%).
Eleven studies57,74,76,78,79,101,106,108,109,133,137 provided information on the MR-imaging false-positive results, with the false-positive rate for patient-level analysis (six studies74,76,79,101,108,137) ranging from 2.4% to 100%. High-grade PIN and prostatitis accounted for a substantial proportion of the false-positive results. Twenty-nine studies74,76,78–84,86,87,90,91,95–98,102–106,108–113,136 reported the Gleason score based on the biopsy results of patients diagnosed with PC, with most reporting a median Gleason score of ≥ 6. The percentage of patients diagnosed with PC that had a Gleason score of ≥ 7 ranged from 20.3% to 66.7%. Ten studies57,76,81,82,86,87,89,109,111,112 reported adverse events related to TRUS-guided biopsies, with one of the most frequently reported adverse events being transient haematuria.
Chapter 5 Assessment of cost-effectiveness
The purpose of this chapter is to assess the cost-effectiveness of utilising different MRI sequences to direct prostate biopsy following a previous negative biopsy.
The specific economic objectives are to estimate:
-
the costs of standard practice (i.e. repeated TRUS/Bx) and the alternative, directed biopsies in the form of T2-MRI, MRS, DCE-MRI and DW-MRI techniques in the diagnosis of prostate abnormalities
-
the cost-effectiveness of T2-MRI, MRS, DCE-MRI and DW-MRI in comparison with standard practice in men with suspected PC.
Structured review of cost-effectiveness studies
Although a systematic literature review of cost-effectiveness studies was not included as part of the protocol for this study, a systematic search was undertaken to locate studies considering the cost-effectiveness of MRS and enhanced MRI techniques for aiding the localisation of prostate abnormalities for biopsy. A broader search for health-state utility data and existing economic modelling studies in the area of PC, to inform subsequent cost-effectiveness modelling, was conducted simultaneously. Databases searched included the NHS Economic Evaluation Database (NHS EED) (November 2011), the IDEAS Economics and Finance Research database (November 2011), MEDLINE, the NHS Economic Evaluation Database (NHS EED), and the Cost-effectiveness Analysis Registry (CEA Registry) (specifically for health utilities). Details of the full search strategies used are given in Appendix 2.
Efforts were made to identify papers reporting full economic evaluations on the use of MRS/MRI techniques to direct/guide prostate biopsies. A total of 1315 titles and abstracts were screened for possible relevance but only one non-English-language paper was found comparing both the costs and consequences of alternatives of interest. From review of the available English-language abstract of the latter paper, this study used modelling techniques to assess the cost-effectiveness of using MRI (type not specified) to determine and direct prostate biopsies compared with the standard practice of systematic TRUS/Bx for all patients. 138 The authors reported their results in terms of a hypothetical cohort of 100,000 patients, and concluded that although the use of MRI could prevent the need for 64,000 unnecessary biopsies, it would result in increased costs to the health insurer for only a small increase in quality-adjusted life-years (QALYs). They concluded that their estimates did not permit a clear recommendation for or against the use of MRI in the diagnosis of PC. The abstract also stated that the use of MRI was being evaluated in the context of patients undergoing their first biopsy, so the results are not directly applicable to the decision problem being addressed in this report.
Independent economic assessment
Based on consideration of existing economic modelling studies and trial-based evidence, a de novo economic model was developed to assess the cost-effectiveness of using alternative MRS/MRI sequences to direct TRUS-guided biopsies, compared with the standard practice of relying on systematic TRUS-guided biopsies (in patients with a previous negative biopsy). The alternative diagnostic pathways were embedded in a Markov model simulating the progression of undiagnosed cancer and the downstream impact of diagnosis and treatment on survival and health-related QoL.
After considering a number of existing economic models of treatment and screening strategies for PC,9,139–145 we chose to adopt a Markov cohort approach similar to that used in a model developed to inform NICE clinical guidance on prostatectomy for localised cancer. 9 However, we included a greater number of states so as to capture the risk stratified natural history of localised PC, associated treatment effects, and treatment complications. Costs incorporated in the model included the costs associated with obtaining the final diagnosis (cancer/no cancer), management of biopsy complications, cancer staging, cancer treatment, and the management of complications resulting from cancer treatment.
Survival benefits of diagnosis were captured through the application of relative risk parameters reflecting the effects of appropriately targeted radical treatment by stage of underlying cancer. It was assumed that via a risk targeted approach, the observed benefits of radical treatment over observation could be achieved for diagnosed cohorts without the need to treat all patients immediately. Limited, high-quality randomised evidence was identified for the effect of radical treatment compared with observation in men with localised PC. A recently updated Cochrane review on prostatectomy compared with watchful waiting146 identified only two randomised trials for inclusion: the VACURG trial,147 which was judged to be of poor quality, and the SPCG-4 trial,148 which was judged to be of good quality. The SPCG-4 trial,148 carried out in Sweden, provides up to 15 years’ follow-up on 695 men with localised PC randomised to either radical prostatectomy (n = 347) or watchful waiting (n = 348). It recruited patients prior to the widespread introduction of PSA screening, and, as such, uncertainty exists regarding its applicability to men with localised disease identified through PSA screening. However, as systematic PSA screening is not policy in the UK, and as no more contemporary randomised data on the effect of radical prostatectomy compared with watchful waiting were available at time of model development, we based our modelled progression risks and relative treatment effects (post diagnosis) on this trial. Late in our study period, the PIVOT trial149 published preliminary results on the effect of radical prostatectomy compared with watchful waiting in men with localised disease identified through PSA screening. 149 As such, we also performed a sensitivity analysis with the model recalibrated to the progression rates and treatment effects observed in this more recent study.
Health-state utilities associated with cancer stage and the occurrence of treatment complications were incorporated in the model to estimate QALYs. Experimental strategies were compared incrementally with standard practice in terms of their incremental cost per life-year (LY) and QALY gained. For each cost per QALY analysis, the strategy with the highest net monetary benefit (NMB) was identified using the formula: NMB = (E × rc) – C, where NMB is the net monetary benefit of a strategy, E is the mean effect (in terms of QALYs), rc represents decision-makers’ maximum willingness to pay for a QALY, and C is the mean cost of the strategy. A value of £30,000 was applied for rc.
Methods
Relevant patient population
The modelled cohorts consisted of men with suspected PC with a prior negative/inconclusive biopsy, with indications for repeat biopsy (i.e. sustained suspicion of PC as a result of clinical and/or pathological findings). 40 We carried out several analyses applying cancer prevalence rates consistent with those observed in the literature for different subgroups defined by factors that influence disease prevalence at repeat biopsy. The base-case analysis was carried out using a prevalence of 24%, which is consistent with cancer detection rate (with 24-core saturation biopsy) reported for a cohort of patients with a previous benign biopsy result but persistently elevated PSA (> 4 ng/ml) and/or abnormal DRE. 38 Further analyses were carried out, with the prevalence of underlying cancer set at a higher level consistent with that reported for patients with ASAP or percentage free to total PSA level of < 10% (i.e. 50%). Further, we also set the cancer prevalence at the lower level of 10% to represent a lower risk cohort selected for repeat biopsy.
Analyses were conducted separately for men aged 60 years and men aged 70 years at time of repeat biopsy, as age influences the cost-effectiveness of diagnosing and treating PC. It was assumed that a PC diagnosis would not be aggressively pursued in men aged 75 years and over. Men with cancer were initially spread across the undiagnosed cancer states in the model (Table 16), based on the reported Gleason scores of tumours detected during the studies included in the systematic review, and other available data on the clinical and/or pathological stages/grades of cancers detected at second biopsy. 2,37,38 Although the frequency of higher grade cancer may increase with age and underlying prevalence, data limitations precluded adjustment of the proportions by selection criteria for repeat biopsy. As such, results of subgroup analyses (by age and prevalence) should be treated with caution. If a higher proportion of older men have more advanced or higher risk tumours, this would serve to improve the cost-effectiveness of more sensitive strategies in comparison with the base-case estimates we provide for 70-year-old men.
| Parameter | Proportion (ranges across studies) | Sources (references) |
|---|---|---|
| Cancer prevalence | 0.24 (0.10 to 0.50) | Stewart 2001,2 Campos-Fernandez 2009,37 Scattoni 201138 (assumptions) |
| Localised disease | 0.878 (0.767 to 0.938) | Stewart 2001,2 Campos-Fernandez 2009,37 Scattoni 201138 (assumptions) |
| Risk status of localised disease | ||
| Low | 0.540 (0.330 to 1.000) | Bhatia 2007,76 Cheikh 2009,78 Cirrillo 2008,79 Engelhard 2006,82 Franiel 2011,84 Hambrock 2008,85 Lattouf 2007,90 Testa 2010,106 Wetter 2005,108 Yakar 2011,109 Yuen 2004,112 Panebianco 2011,95 Roethke 2012,104 Sciarra 2010105 |
| Intermediate | 0.301 (0.000 to 0.500) | |
| High | 0.159 (0.000 to 0.330) | |
| Locally advanced | 0.122 (0.052 to 0.233) | Stewart 2001,2 Campos-Fernandez 2009,37 Scattoni 201138 (assumptions) |
Diagnostic strategies to be evaluated
The experimental strategies chosen for evaluation were selected based on the availability of data from the systematic review of diagnostic accuracy (Table 17). It was not possible to obtain comparable pooled sensitivity/specificity estimates for all sequences of clinical interest. Further, the majority of studies included in the diagnostic accuracy review assessed the accuracy of MRI sequences for directing TRUS-guided biopsies, rather than for directly guiding the biopsy. Thus, the economic analysis focused on evaluating the use of MRS/MRI in this context, i.e. using it to identify areas of the prostate for targeting in a subsequent TRUS/Bx. As the patient-level sensitivities obtained from the diagnostic accuracy meta-analysis reflect detection rates achieved when both targeted cores and a number of systematic cores (8 to 12) are taken from patients with positive findings on MRS/MRI, we modelled targeted biopsies to proceed in this same manner in the economic model. Insufficient data were available to ascertain how patient-level sensitivity would be affected if only targeted cores were obtained from patients positive on imaging. As a consequence, we assumed that MRI/imaging prior to biopsy would not alter the cost of the biopsy procedure in the base-case analysis. We also assumed that patients with no visible pathology on MRS/MRI would not proceed to biopsy. The model was specified to simulate the use of MRS/MRI in the index repeat biopsy only. It was assumed that any patients missed by the index repeat biopsy (false-negatives), would have persistently elevated PSA level, which would trigger the offer of a saturation biopsy (> 24 cores) 12 months later, and that acceptance would be high (assumption based on attitudes to repeat biopsy reported by Rosario et al. 150). These conservative assumptions, which favour less-sensitive cancer detection strategies, were subjected to sensitivity analysis.
| Strategy | Sensitivity | Specificity | Source |
|---|---|---|---|
| Systematic extended-core TRUS/Bx | 0.832 (0.78 to 0.88) | 1.00 | Scattoni 201138 |
| T2-MRI | 0.86 (0.74 to 0.93) | 0.55 (0.44 to 0.66) | Systematic review |
| MRS | 0.92 (0.86 to 0.95) | 0.76 (0.61 to 0.87) | Systematic review |
| DCE-MRI | 0.79 (0.69 to 0.87) | 0.52 (0.14 to 0.88) | Systematic review |
| T2-MRI or MRS | 0.96 (0.90 to 0.98) | 0.31 (0.21 to 0.42) | Systematic review |
| T2-MRI or DCE-MRI | 0.88 (0.80 to 0.96) | 0.14 (0.08 to 0.20) | Systematic review |
A systematic TRUS-guided extended-cores (14–16 cores) biopsy for all, carried out in an outpatient setting, was selected as the base comparator against which to assess the cost-effectiveness of using MRS/MRI (see Table 17). A limitation of the available literature is that no existing studies have directly assessed the relative sensitivity/specificity of MRI-directed biopsies in comparison with systematic biopsy sampling schemes with different numbers of cores. Thus, in modelling the comparison we were forced to rely on diagnostic accuracy data for the comparator and index tests derived from different sources using different reference standards. The sensitivity of the systematic extended-cores biopsy was derived from a study assessing the proportion of cancers detected by systematic biopsy schemes with variable numbers of cores,38 using the results of a TRUS-guided saturation biopsy as the reference standard. The MRS/MRI sensitivities/specificities were derived from the systematic review (see Chapter 4), where the reference standard was histopathological assessment of biopsied tissue but the number of cores taken varied from study to study. Sensitivity analysis was performed to assess the sensitivity of findings to variation in these parameters.
How care pathways were determined and modelled, including an illustration of the model
The diagnostic and care pathways were determined based on a review of guidelines, expert opinion, and the availability of data. A schematic review of the diagnostic pathways was provided in Chapter 1 (see Figure 2). Figure 16 shows the tree structure used to model the index repeat biopsy within the economic model.
FIGURE 16.
Diagnostic pathways for index re-biopsy. The base-case analysis assumed that all patients negative on MRS/MRI would not proceed to biopsy, but we also assessed the impact of assuming these patients would proceed to an extended-cores TRUS/Bx. FN, false-negative; FP, false-positive; sens, sensitivity; spec, specificity; TN, true-negative; TP, true-positive.
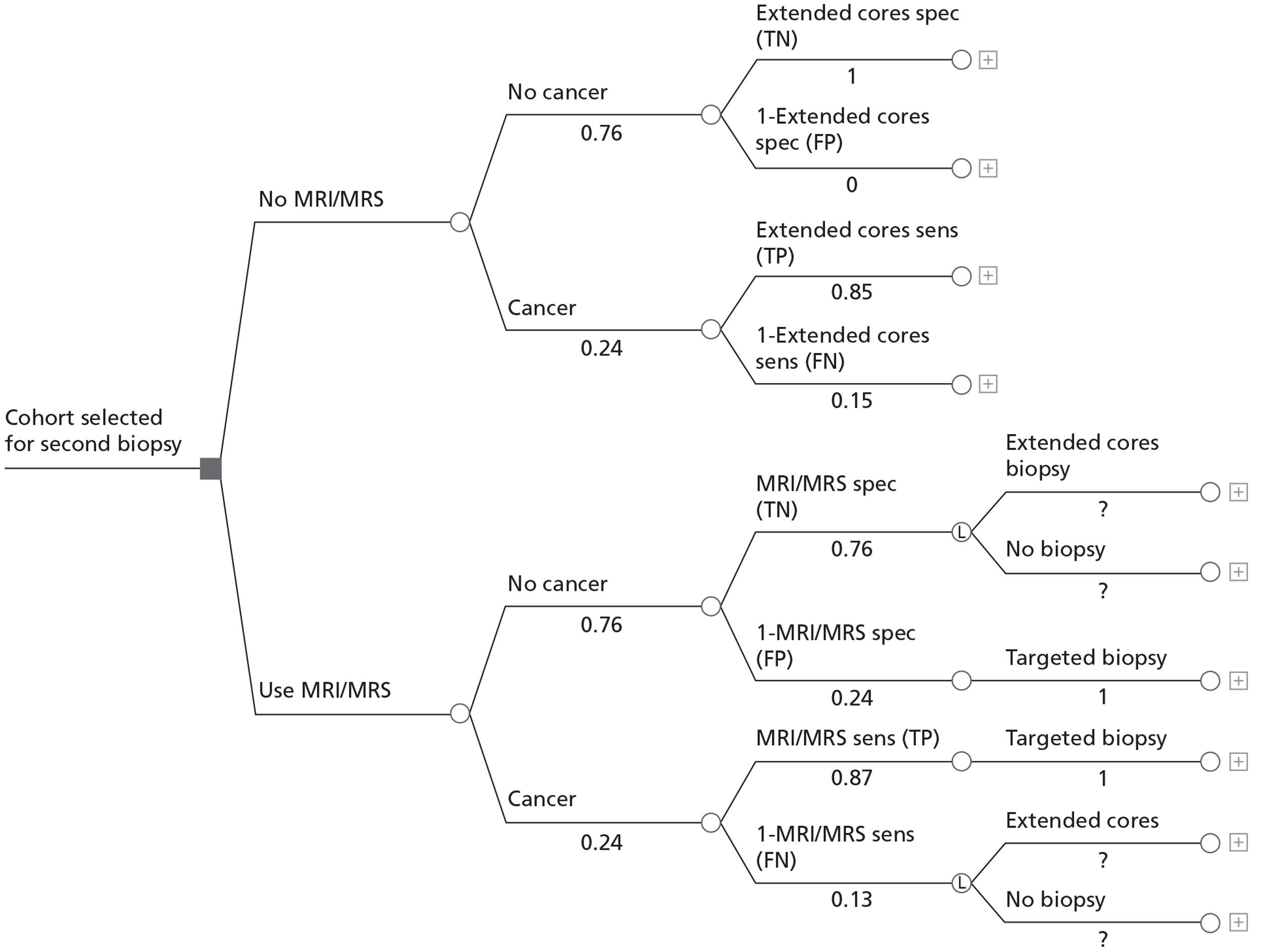
The diagnostic pathways were embedded in a Markov model developed to simulate the progression of diagnosed (treated) and undiagnosed PC (Figure 17). Seven basic states were used to model the natural history of PC: (1) no or undetectable cancer; (2) localised (T1–T2) PC (low risk); (3) localised PC (intermediate risk); (4) localised PC (high risk); (5) locally advanced cancer (T3); (6) metastatic cancer; and (7) PC death. Patients with localised and locally advanced disease were modelled to progress towards metastatic disease based on age, tumour risk status, and whether or not their cancer was diagnosed and appropriately treated. To begin with, patients with suspected PC following a first negative biopsy were spread across the undiagnosed states (using the proportions in Table 16). In the first cycle of the model, all patients were modelled to undergo their repeat biopsy, either by standard means or directed by one of the MRS/MRI sequences. Patients with underlying cancer (undetected) identified by the second biopsy as having disease, as determined by the sensitivity of the biopsy procedure, were modelled to transit to the appropriate diagnosed cancer state for the subsequent model cycle. Those with undetected cancer missed by the second negative biopsy remained in the appropriate undiagnosed state. Those remaining undiagnosed faced a higher risk of progression to metastases (based on progression rates observed for patients under watchful waiting), whereas those detected were modelled to progress at rates observed for patients receiving radical treatments. The model was cycled on a 3-monthly basis, such that probabilities of progression and costs of treatment and monitoring were expressed in terms of this constant cycle length.
FIGURE 17.
Model structure. a, Patients with localised cancer were risk stratified by cancer grade (low, intermediate and high) and modelled to progress to metastatic disease at different rates. Prop recurrence, proportion with local recurrence following treatment.
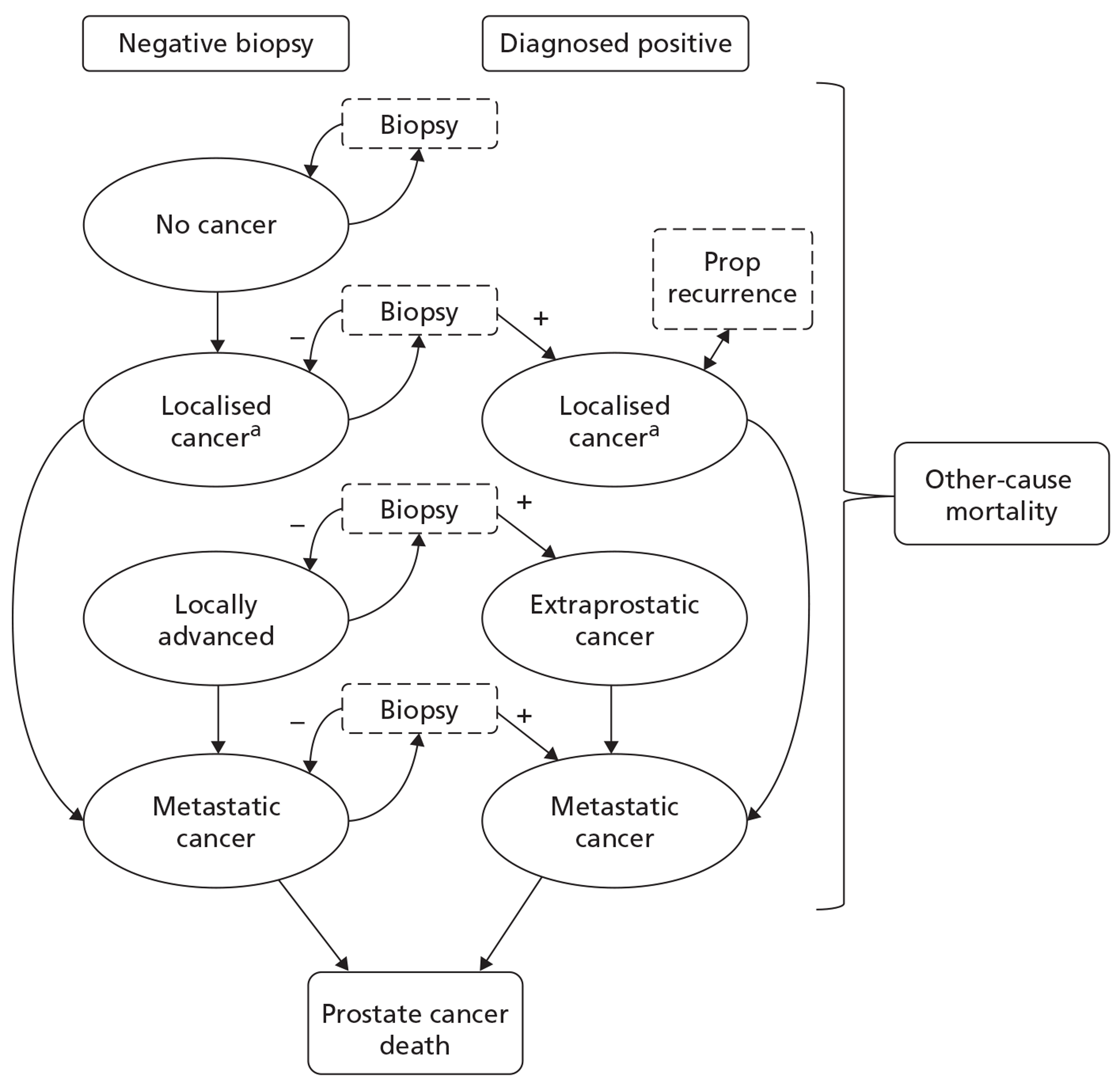
Patients remaining in an undiagnosed cancer state after the index repeat biopsy were modelled to have their PSA levels monitored on a 6-monthly basis. An assumption was made that these patients would have persistently elevated PSA level and would therefore be selected for a further biopsy 12 months later. It was assumed that a saturation biopsy (≥ 24 cores) would be offered at this stage, that there would be 90% uptake,150 and that the biopsy would have 98% sensitivity for detecting the remaining undiagnosed cancers (based on clinical opinion within the team). For patients without underlying PC, it was assumed that no further biopsies would be indicated unless incident PCs developed. These assumptions were subjected to sensitivity analysis.
For every biopsy undertaken, modelled patients also faced an associated risk of complications (bleeding, infection, urinary retention). Patients crossing to the diagnosed states were modelled to receive appropriate staging, treatment and monitoring. In addition, patients receiving treatment faced a risk of experiencing complications, which incurred further health service costs and quality-of-life decrements. Following treatment for localised disease, a proportion of the cohort was modelled to experience tumour recurrence, triggering further treatment and costs.
Costs associated with biopsy procedures, PSA monitoring, staging, treatment and disease monitoring were incorporated into the model based on the application of unit costs to procedures and treatment protocols (derived from expert opinion and current guidelines). Utilities associated with the different cancer states were used to quality adjust the time spent by patients in each state, and utility decrements associated with complications arising from treatment were also applied. Thus the model enabled cumulative costs, LYs and QALYs to be tracked over the lifetime of modelled cohorts under alternative diagnostic strategies. The model captures the potential trade-offs between increased short-term costs associated with incorporating MRI sequencing into the care pathways and any cost savings and potential survival gains resulting from fewer repeat biopsies and earlier cancer treatment. The model also accounts for the fact that treatment may have a detrimental impact on patients’ health-related QoL.
Complications of biopsy
The occurrence of biopsy complications was modelled on the basis of two data sources: the ProtecT trial150 and a cohort study reported by Nam et al. 151 The resultant probabilities are provided in Table 18. Costs associated with these complication events were estimated and incorporated into the model.
| Event | Probability (95% CI) | Distribution for PSA | Source |
|---|---|---|---|
| Biopsy complication | |||
| 0.117 (0.100 to 0.137) | Beta | Rosario 2012150 | |
| Alpha: 134 | |||
| Beta: 1013 | |||
| Probability of hospital admission given biopsy complication | |||
| 0.112 (0.069 to 0.176) | Beta | Rosario 2012150 | |
| Alpha: 15 | |||
| Beta: 119 | |||
| Reasons for hospital admission | |||
| Dirichlet | Nam 2010151 | ||
| Urinary infection related | 0.716 (0.675 to 0.738) | Alpha: 556 | |
| Urinary bleeding related | 0.194 (0.166 to 0.221) | Alpha: 151 | |
| Urinary obstruction related | 0.090 (0.081 to 0.124) | Alpha: 79 | |
| Biopsy-related consultation given complication | |||
| 0.888 (0.824 to 0.931) | Beta | Rosario 2012150 | |
| Alpha: 119 | |||
| Beta: 15 | |||
| Location of consultation | |||
| Dirichlet | Rosario 2012150 | ||
| GP | 0.773 (0.690 to 0.839) | Alpha: 92 | |
| Urology department nurse | 0.118 (0.071 to 0.188) | Alpha: 14 | |
| Other – NHS Direct | 0.109 (0.065 to 0.178) | Alpha: 13 | |
Risk of cancer progression (undiagnosed and diagnosed patients)
A simplifying assumption of the model was that all men in a cancer state are at risk of disease progression, and that men progress towards metastatic disease. It was also assumed that all cancer-related deaths occur following transition to metastatic disease. Given a lack of comparable data on the rate of transition from localised to locally advanced disease, and from locally advanced to metastatic disease (and the relative effect of diagnosis and treatment on these transitions), the model structure was simplified such that progression from localised disease to metastases was modelled in a single step (using a Weibull function fitted to observed published data for this transition).
Men were initially spread across the ‘no cancer’, ‘localised cancer’ and ‘locally advanced cancer’ states (see Table 16). They were then modelled to progress according to their cancer and diagnostic status using observed follow-up data on the cumulative incidence of metastatic disease combined with estimates of relative treatment effects (i.e. baseline transition risks were adjusted downwards to reflect the impact of appropriate treatment in those receiving a diagnosis). The progression risk for localised cancer was modelled based on data reported by Bill-Axelson et al. 148 whereas the progression risk for men starting in the locally advanced state was modelled based on data from a European Organisation for Research and Treatment of Cancer (EORTC) study reported by Bolla et al. 152
Regression methods153 were used to fit Weibull functions to the observed metastases-free survival probabilities reported for men receiving watchful waiting over a 15-year follow-up period;148 separate functions were fitted for men of < 65 years of age and men aged ≥ 65 years. The estimated parameters of the Weibull functions were then used to derive 3-monthly transition probabilities for the risk of developing metastatic disease from undiagnosed localised cancer. In order to risk-stratify the probabilities of progression, separate functions were determined for patients with low-, moderate- and high-risk localised cancer. This was achieved by adjusting the rate parameters of the Weibull functions to yield the cumulative incidence of metastases or PC mortality observed for cohorts with low-148 and high-risk154 localised cancer. The cumulative incidence rates of metastatic disease reported for the two age-specific cohorts (< 65/≥ 65) as a whole by Bill-Axelson et al. were taken to represent the risk of progression for moderate risk patients in each respective modelled age group. Transition probabilities for developing metastatic disease following diagnosis and treatment were estimated by multiplying the rate parameters of the Weibull functions by published relative risk estimates associated with radical prostatectomy. 148 The resultant modelled cumulative incidence of metastases in treated and untreated patients is shown in Figure 18 compared with the observed values derived from published sources. As a sensitivity analysis, we calibrated the model transition rates to yield the PC-specific survival probabilities (by risk status) observed for patients (> 12 years of follow-up) in the control group of a recently published randomised controlled trial of radical prostatectomy compared with observation for localised disease. 149 In addition, we applied the corresponding relative risk estimates obtained from this trial.
FIGURE 18.
Modelled and observed cumulative incidence of metastases. WW, watchful waiting; RP, radical prostatectomy.
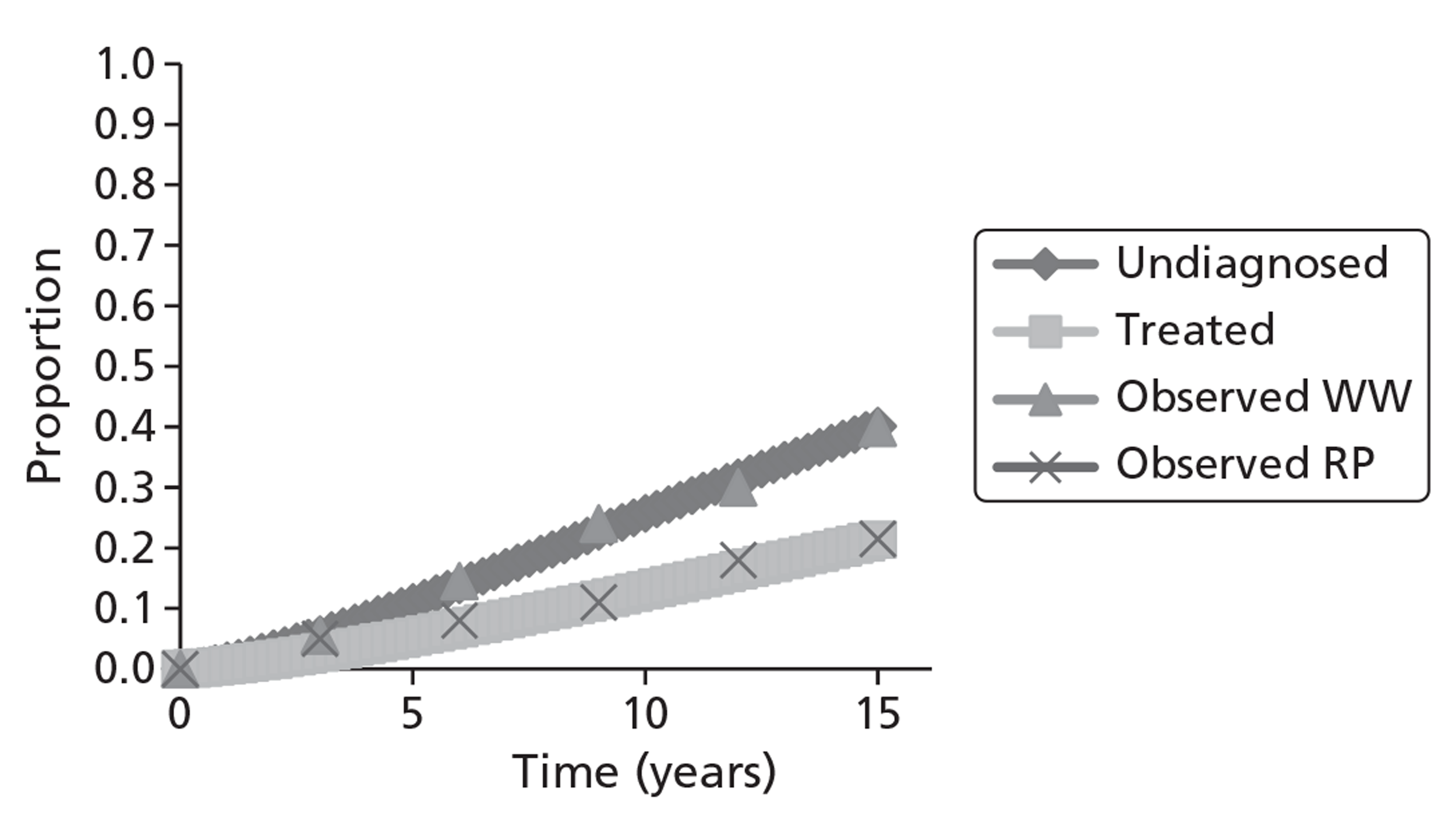
For those starting the model in the locally advanced cancer stage, a similar approach as above was used to model the risk of progression to metastatic disease. However, given the lack of contemporary data on the risk of developing metastases from untreated locally advanced disease, we applied the metastases-free survival data reported for a cohort of patients treated with EBRT alone. These rates were then adjusted downwards for diagnosed patients using the relative risk reduction associated with EBRT combined with adjuvant hormone therapy. 152 All of the relative risk parameters applied in the model are presented in Table 19.
| Parameter | Value (95% CI) | Distribution for PSA | Source |
|---|---|---|---|
| Localised disease | |||
| Relative risk of metastases (< 65 years, low risk) | 0.41 (0.18 to 0.95) | Log-normal; | Bill-Axelson et al. 2011148 |
| Ln mean: −0.8916 | |||
| Ln SE: 0.424364 | |||
| Relative risk of metastases (< 65 years, intermediate/high risk) | 0.47 (0.32 to 0.70) | Log-normal: | Bill-Axelson et al. 2011148 |
| Ln mean: −0.75502 | |||
| Ln SE: 0.199684 | |||
| Relative risk of metastases (≥ 65 years, low risk) | 0.46 (0.19 to 1.11) | Log-normal: | Bill-Axelson et al. 2011148 |
| Ln mean: −0.77653 | |||
| Ln SE: 0.450278 | |||
| Relative risk of metastases (≥ 65 years, intermediate/high risk) | 0.77 (0.51 to 1.15) | Log-normal: | Bill-Axelson et al. 2011148 |
| Ln mean: −0.026136 | |||
| Ln SE: 0.207425 | |||
| Locally advanced disease | |||
| Relative risk of metastases | 0.28 (0.18 to 0.46) | Log-normal: | Bolla et al. 2002152 |
| Ln mean: −1.27297 | |||
| Ln SE: 0.239354 | |||
For those with metastatic disease, a constant 3-monthly risk of death from PC was estimated from English observational data155 and applied in the model. The age-specific risk of death from other causes was also incorporated based on age- and sex-specific interim UK life tables. 156
Resource use and unit cost estimation
All costs were estimated based on resource-use inputs and unit costs for the 2009–10 financial year.
Standard transrectal ultrasound-guided biopsy
The cost of a TRUS-guided needle biopsy was taken from the NHS reference costs41 using the appropriate HRG (LB27Z). There is some uncertainty as to how hospitals in England and Wales are, or would be, reimbursed for repeat biopsies using the systematic extended-cores or MRI-/MRS-directed approach. Although both approaches can be carried out as outpatient procedures without general anaesthetic, it is likely that at least some organisations commission these as day-case procedures. As a result of outpatient procedure coding being non-mandatory, it is not possible to accurately ascertain the proportion of procedures carried out in each care setting, and this is likely to vary from trust to trust. Thus, adopting a conservative approach in favour of less sensitive less costly diagnostic strategies, we initially assumed that all index repeat biopsies would be carried as outpatient procedures, incurring an average cost of £212.
Note, however, that although this tariff-based cost should reflect the budget impact on NHS primary care trusts of commissioning such procedures, it might not fully capture the opportunity cost that hospitals face in delivering the procedure, particularly for extended-cores biopsies that can substantially increase pathology time over standard TRUS/Bx (10–12 cores). We therefore assessed the impact of increasing this cost through sensitivity analysis.
In the base case we also made the conservative assumption that the use of MRS/MRI would not influence the cost of the biopsy procedure itself. This was due to a lack of certainty as to how the patient-level pooled sensitivity estimates obtained for MRS/MRI imaging (from the systematic review) would be affected if only targeted cores were taken in the subsequent biopsy. However, we explored the impact of increasing the cost of extended-cores biopsies, but not the cost of MRI-/MRS-targeted biopsies.
For patients with underlying cancer missed by the index re-biopsy, we assumed that a saturation biopsy (≥ 24 cores) would be indicated at 12 months, and applied the day-case NHS reference cost for all these procedures (£447). We also explored the impact of increasing this cost to reflect potential underestimation of histopathology costs associated with obtaining larger numbers of cores (for further deterministic scenario analyses, see Results, below).
Magnetic resonance imaging sequences for guiding biopsy
The costs of performing alternative MRI sequences to guide prostate biopsy were estimated using a bottom-up approach. Radiographer and radiologist time associated with the performance of different sequences was estimated by asking all of the radiologists involved in the project to provide estimates of time inputs they deemed to be representative of standard practice. Within these estimates, allowance was made for preparation (getting the patient into the machine) and scanning time (two radiographers) and reading/reporting time (one consultant radiologist). The average reported time inputs for sequences included in the economic model are outlined in Table 20. Unit costs obtained from the Unit Costs of Health and Social Care157 were applied to these resource-use inputs. These unit costs included salaries, on-costs (employer superannuation and national insurance contributions) and an apportionment of capital space and overhead costs to capture the opportunity cost of space and overheads attributable to the alternative procedures. Capital equipment required for the alternative MRI sequences was costed by applying current market prices obtained from NHS Grampian. These initial outlay costs were annuitised over the useful working lifespan of the piece of equipment in question, applying an annual discount rate of 3.5% to account for the opportunity cost of the investment over time. The equivalent annual cost of each piece of equipment was divided through by its estimated running time to give a cost per minute estimate. The scanning time estimates associated with alternative MRI sequences were then multiplied by the appropriate equipment cost per minute estimates to give estimates of the capital equipment costs attributable to each different MRI sequence. Costs of equipment used only for DCE-MRI (pump) or MRS (MRS software) were only allocated to sequences involving these procedures. The annual equivalent costs of these items were divided through by the number of uses per year (Dr Lutfi Kurban, Aberdeen Royal Infirmary, March 2012, personal communication; Dr Anwar Padhani, Mount Vernon Cancer Centre, April 2012, personal communication) to give cost per use estimates, which were then applied to sequences incorporating these procedures. Finally, consumables associated with DCE-MRI (contrast, pump pack, others) were costed using unit prices provide by NHS Grampian.
| Sequence | Grade/band | Patient preparation time (minutes) | Time per patient (minutes) | Cost per hour (£) | Staff cost per patient (£) | Equipment cost per patient (£) | Total cost per patienta (£) |
|---|---|---|---|---|---|---|---|
| T2-MRI | |||||||
| Radiographer 1 | 6 + 7 + 7 | 10.00 | 14.33 | 48.33 | 19.60 | ||
| Radiographer 2 | 7 | 10.00 | 14.33 | 50.00 | 20.28 | ||
| Radiologist | Consultant | 5.00 | 162.00 | 13.50 | |||
| Totals | 53.38 | 46.90 | 106.29 | ||||
| DW-MRI (+ T2-MRI) | |||||||
| Radiographer 1 | 6 + 7 + 7 | 10.00 | 21.33 | 48.33 | 25.24 | ||
| Radiographer 2 | 7 | 10.00 | 21.33 | 50.00 | 26.11 | ||
| Radiologist | Consultant | 8.67 | 162.00 | 23.40 | |||
| Totals | 74.75 | 60.65 | 141.30 | ||||
| DCE-MRI (+ T2-MRI) | |||||||
| Radiographer 1 | 6 + 7 + 7 | 12.00 | 22.67 | 48.33 | 27.93 | ||
| Radiographer 2 | 7 | 12.00 | 22.67 | 50.00 | 28.89 | ||
| Radiologist | Consultant | 10.00 | 162.00 | 27.00 | |||
| Totals | 83.81 | 71.21 | 189.71 | ||||
| MRS (+ T2-MRI) | |||||||
| Radiographer 1 | 6 + 7 + 7 | 10.00 | 27.33 | 48.33 | 30.07 | ||
| Radiographer 2 | 7 | 10.00 | 27.33 | 50.00 | 31.11 | ||
| Radiologist | Consultant | 16.67 | 162.00 | 45.00 | |||
| Totals | 106.19 | 73.93 | 185.68 | ||||
| T2-MRI + DW-MRI + DCE-MRI | |||||||
| Radiographer 1 | 6 + 7 + 7 | 12.00 | 31.33 | 48.33 | 34.91 | ||
| Radiographer 2 | 7 | 12.00 | 31.33 | 50.00 | 36.11 | ||
| Radiologist | Consultant | 16.67 | 162.00 | 45.00 | |||
| Totals | 116.02 | 88.42 | 239.06 | ||||
| T2-MRI + DW-MRI + MRS | |||||||
| Radiographer 1 | 6 + 7 + 7 | 10.00 | 37.67 | 48.33 | 38.40 | ||
| Radiographer 2 | 7 | 10.00 | 37.67 | 50.00 | 39.72 | ||
| Radiologist | Consultant | 20.33 | 162.00 | 54.90 | |||
| Totals | 133.02 | 94.61 | 233.18 | ||||
| T2-MRI + DCE-MRI + MRS | |||||||
| Radiographer 1 | 6 + 7 + 7 | 12.00 | 37.33 | 48.33 | 39.74 | ||
| Radiographer 2 | 7 | 12.00 | 37.33 | 50.00 | 41.11 | ||
| Radiologist | Consultant | 21.67 | 162.00 | 58.50 | |||
| Totals | 139.35 | 101.71 | 275.34 | ||||
| T2-MRI + DW-MRI + DCE-MRI + MRS | |||||||
| Radiographer 1 | 6 + 7 + 7 | 12.00 | 46.00 | 48.33 | 46.72 | ||
| Radiographer 2 | 7 | 12.00 | 46.00 | 50.00 | 48.33 | ||
| Radiologist | Consultant | 25.33 | 162.00 | 68.40 | |||
| Totals | 163.46 | 118.92 | 316.60 | ||||
As the MRI costs represent the opportunity costs to hospitals of providing alternative scan sequences, they are well suited to assessing the relative cost-effectiveness of using alternative sequences. However, the estimated costs for some of the simpler scans may underestimate the costs of commissioning such activity. This makes them somewhat less comparable with the tariff-based cost estimate for TRUS/Bx. However, we did not adjust these costs further in the base-case analysis given the concurrent conservative approach to costing TRUS/Bx. As a sensitivity analysis we adjusted the costs of sequences by setting the cost of T2 + DCE-MRI equal to the NHS reference cost for HRG RA03Z (Magnetic Resonance Imaging Scan, one area, pre and post contrast) (£229)41 and maintained the incremental differences in cost between sequences as estimated from the bottom-up calculations.
Biopsy complication costs
Standard practice for repeat biopsy in the UK is systematic TRUS-guided extended-cores or saturation biopsy. The incidence of adverse events post biopsy was determined from the literature150,151 and categorised into hospital admissions or biopsy-related consultations (see Table 18). A risk of death from biopsy complications was experienced only by patients who developed an infection (p = 0.0009) and all other patients were assumed to recover after initial treatment.
Hospital admissions resulting from biopsy complications were reported by Nam151 and Rosario150 as being due to one of three urological diagnoses: urinary infection; urinary bleeding (haematuria); or urinary obstruction (Table 21). For inpatient admissions due to urinary tract infection (UTI) we applied the NHS reference cost for HRG LA04G (Kidney or Urinary Tract Infections with length of stay 1 day or less) (£401). Admission for haematuria was assumed to require insertion of a haematuria catheter for bladder irrigation HRG LB18Z (Attention to Suprapubic Bladder Catheter) at a cost of £567 per patient. 41 Urinary retention was assumed to be temporary and was modelled to incur the cost of inserting and subsequently removing a urethral catheter: day-case HRGs LB09Z (Ureter Intermediate Endoscopic Procedures) and LB15E (Bladder Minor Procedure 19 years and over) at £652 and £368, respectively. 41 It was further assumed that the NHS would incur the daily cost of an overnight catheter bag and the weekly cost of a leg bag (apart from in the first week when two leg bags would be required) over the course of 1 month (£6.47 and £12.61, respectively).
| Procedure | Unit cost (£) | Assumptions | Lower/higher estimates (distribution) | Source |
|---|---|---|---|---|
| Hospital admissions | ||||
| Gamma | ||||
| UTI | 401 | HRG LA04G (Kidney or Urinary Tract Infections with length of stay 1 day or less) | £286/£466 | Department of Health 201141 |
| (Alpha: 8.91; beta: 45.00) | ||||
| Urinary bleeding (haematuria) | 567 | HRG LB18Z (Attention to Suprapubic Bladder Catheter) | £293/£635 | Department of Health 201141 |
| (Alpha: 4.94; beta: 114.88) | ||||
| Urinary obstruction | 1039.08 | HRG LB09Z (Ureter Intermediate Endoscopic Procedures) and LB15E (Bladder Minor Procedure 19 years and over) + cost of catheter bags | £595/£1225 | Department of Health 2011;41 Ramsay et al. 2012144 |
| (Alpha: 4.88; beta: 212.73) | ||||
| Biopsy-related consultations | ||||
| GP visit | 36.27 | 11.7 minutes for surgery consultation | Curtis 2011157 | |
| Urology department nurse visit | 70 | £46/£85 (Alpha: 5.78; beta: 12.10) | Department of Health 201141 | |
| Call to NHS Direct | 20.98 | Applied deterministically | ||
Rosario et al. 150 also reported the 35-day incidence of consultations with general practitioners, urology department nurses, and ‘other sources of medical advice’ (e.g. NHS Direct). The cost associated with a general practitioner (GP) consultation was derived from the Unit Costs of Health and Social Care. 157 The average duration of a GP consultation is 11.7 minutes157 at a cost of £3.10 per surgery minute,157 giving a unit cost of £36.27 per consultation. The cost of a consultation with a urology department nurse was derived from the relevant NHS tariff – non-consultant-led follow-up attendance, non-admitted, face to face – at cost of £70. 41 The cost per NHS direct contact was derived from the NHS Direct National Health Service Trust Annual Report and Accounts 2009–10, and was based on the total reported staff wages divided by the number of calls logged, giving a cost of £20.98 per call.
Prostate cancer treatment costs for localised disease
Potential treatment pathways by cancer stage were derived from the current NICE guidance. 9 The costs associated with implementing alternative treatment pathways, on an ongoing 3-monthly basis, were estimated using data from a variety of sources including the Department of Health NHS reference costs,41 the Unit Costs of Health and Social Care,157 and a recently completed technology assessment report (TAR) evaluating the cost-effectiveness of robotic radical prostatectomy compared with laparoscopic prostatectomy144 for localised PC. Clinical opinion was relied upon to enable an appropriate estimation of timelines for treatment pathways.
It is typical practice in the UK to monitor PSA level for the duration of the patient's life post treatment; every 3 months for the first 2 years and then every 6 months thereafter (based on clinical opinion within the research team). The cost of PSA level monitoring was thus estimated, based on a consultation with a practice nurse (£12)157 plus £5.91 for laboratory services,144 and included in the model (Table 22).
| Procedure | Unit cost (£) | Source |
|---|---|---|
| PSA test | 5.91 | Ramsay et al.144 |
| Practice nurse | 12 per consultation | Unit Costs of Health and Social Care 157 |
| PSA unit cost | 17.91 |
Patients with localised PC were modelled to follow one of three alternative treatment pathways: (1) active surveillance; (2) radical prostatectomy followed by PSA level monitoring; or (3) EBRT followed by PSA level monitoring. The proportion of patients receiving each management strategy, by D'Amico Risk category,31 was derived from routine Scottish health episode data (Dr Karina Laing, MSc in Surgical Sciences thesis, University of Edinburgh, May 2012, personal communication). It was assumed that the alternative treatment modalities would be applied appropriately based on the risk of progression and that, as such, the observed risk reduction associated with radical prostatectomy 148 could be achieved at the level of the cohort as a whole.
The cost of active surveillance was estimated based on the cost of PSA testing (see Table 22) on a 3-monthly basis, followed by a repeat TRUS/Bx41 at 12 months, and every 3 years thereafter (based on clinical opinion within the research team).
The cost of radical prostatectomy was taken as the NHS reference cost for HRG LB21Z (Bladder Neck Open Procedures – Male). Of the two most common approaches to radical surgery (open and laparoscopic radical prostatectomy) the overall activity reported for open procedures was higher,41 and, as such, the cost for this procedure was applied in the model.
The cost associated with a programme of EBRT was calculated on the basis of 37 sessions within a 7.5-week time frame (expert opinion) at a cost per session of £129. 41 EBRT treatment is generally accompanied by a course of androgen deprivation therapy. Although all patients with localised PC were modelled to receive 3 months of hormone therapy from commencement of EBRT, hormone therapy prior to EBRT treatment was assumed to occur only for those with intermediate- or high-risk disease. Before commencing EBRT, these patients were initially modelled to receive a 21-day course of bicalutamide (Casodex®, AstraZeneca: £96.00), followed by a 3-month course of the LHRH agonist triptorelin (Decapeptyl® SR, Ipsen: 11.25-mg 3-month injection) at a cost of £207. 158 As localised low-risk patients do not generally receive hormone therapy prior to EBRT treatment, it was assumed that the costs of hormone treatment for these patients would be incurred in the first 3-month cycle following diagnosis, concurrently with the EBRT sessions. It was assumed in all cases that triptorelin would be administered by a practice nurse in a primary care setting, at a cost of £12 per visit.
Treatment costs associated with locally advanced disease
External beam radiotherapy with adjuvant hormone therapy was identified as the most appropriate treatment option for patients with locally advanced PC upon diagnosis. 9 A small proportion of men were also modelled to receive radical prostatectomy. The cost streams and timelines for these treatments were assumed to be consistent with those outlined above for patients with moderate- to high-risk localised disease, with the exception that hormone therapy was continued to 2 years post EBRT.
Costs associated with local progression following treatment for localised disease
A proportion of the cohort was modelled to experience biochemical recurrence following radical treatment for localised cancer. These patients were modelled to receive either salvage EBRT or hormone therapy alone.
Salvage EBRT is delivered at lower gray, with fewer sessions (33 sessions within a 6.5-week time frame). As such, we applied the NHS reference cost (£107) for the appropriate HRG (SC22Z) to each treatment session. 41 In addition, hormone treatment for patients receiving salvage EBRT for biochemical recurrence was extended for a period of 2 years post EBRT treatment.
The 3-monthly cost of hormone therapy was assumed to correspond to the cost of hormone therapy administered pre and post EBRT (21-day course of bicalutamide, followed by 3-monthly injections of triptorelin). However, treatment was assumed to extend for the duration of the patient's lifetime when initiated for biochemical relapse.
Costs associated with metastatic disease
Upon transiting to the metastatic disease state, it was initially assumed that all patients would be treated with hormone therapy, incurring a continuous 3-monthly cost of £219 (Table 23). Without explicitly modelling the initiation and impact of chemotherapy, we also assumed that 50% of patients developing metastatic disease would undergo a first-line docetaxel-based chemotherapy regimen (£10,450) and that 70% of these patients would go on to receive a second-line abiraterone-based regimen (£24,670) prior to death, as per the assumptions used in the costing template for the NICE abiraterone technical appraisal. 39
| Procedure | Unit cost (£) | Assumption | Lower/higher quartile (distributions) | Source |
|---|---|---|---|---|
| Active surveillance | ||||
| TRUS/Bx | 212 | HRG = LB27Z (outpatient) | £137/£295 | Department of Health 201141 |
| (Gamma; alpha: 3.23, beta: 65.58) | ||||
| Radical treatment | ||||
| Open radical prostatectomy | 4614 | HRG = LB21Z | £3650/£5408 | Department of Health 201141 |
| (Gamma; alpha: 12.37; beta: 373.04) | ||||
| EBRT: 37 sessions | 4773 | HRG = SC23Z £129 × 37 | £3848/£5439 | Department of Health 201141 |
| (Gamma; alpha: 16.16; beta: 295.35) | ||||
| Salvage treatment | ||||
| EBRT: 33 sessions | 3531 | HRG = SC22Z £107 × 33 | £2211/£4983 | Department of Health 201141 |
| (Gamma; alpha: 2.91; beta: 1211.93) | ||||
| Hormone therapy | ||||
| A 21-day course of bicalutamide | 96 | 50 mg per day | Applied deterministically | BNF 63158 |
| Three months' decapeptyl | 219 | Drugs + administration: £207 + £12 | Applied deterministically | BNF 63;158 Curtis 2011157 |
| Two years' hormones | 1752 | £219 × 8 | Applied deterministically | BNF 63;158 Curtis 2011157 |
Costs of complications arising from treatment
Radical prostatectomy
Three common adverse events following radical prostatectomy were modelled: (1) bladder neck contracture; (2) urinary incontinence (UI); and (3) ED (Table 24). The probability of experiencing bladder neck contracture following surgery was taken from the systematic review of a recently completed technology assessment review. 144 All patients were assumed to recover from bladder neck contracture and incur the one-off inpatient admission cost for a bladder neck minor endoscopic procedure (HRG LB27Z). Increases in the proportions of patients suffering from ED and/or UI at different time points following radical prostatectomy were derived from cohort studies (Sacco et al. ;159 Stanford et al. 160). Patients experiencing UI were assumed to enter a continuous period of self-management using containment pads at a 3-monthly cost of £65.90. 144 Additionally, 10% of patients experiencing UI were modelled to incur the cost of oxybutynin hydrochloride (Ditropan, Sanofi Aventis) (clinical opinion) at a 3-monthly cost of £36.66. 158 Patients recovering urinary continence were modelled to receive no further management costs for this complication, whereas those remaining incontinent continued to incur the costs of containment pads.
| Long-term complications | Probability (95% CI) | Distribution | Unit cost of treatment (£) | Source | ||
|---|---|---|---|---|---|---|
| < 65 years | ≥ 65 years | < 65 years | ≥ 65 years | |||
| Radical prostatectomy | ||||||
| Urinary stricture | 0.022 | 0.022 | 1112 (one-off) | Ramsay144 | ||
| Urinary incontinence | ||||||
| 3 months | 0.318 (0.289 to 0.348) | 0.318 (0.289 to 0.348) | Alpha: 305 | 65.90 (every 3 months) | Sacco159 | |
| Beta: 653 | ||||||
| 6 months | 0.220 (0.195 to 0.247) | 0.220 (0.195 to 0.247) | Alpha: 211 | |||
| Beta: 747 | ||||||
| 12 months | 0.131 (0.110 to 0.154) | 0.131 (0.110 to 0.154) | Alpha: 125 | |||
| Beta: 833 | ||||||
| ED | ||||||
| Baseline | 0.115 (0.094 to 0.140) | 0.262 (0.228 to 0.300) | Alpha: 83 | Alpha: 149 | 232.08 (every 3 months) | Stanford160 |
| Beta: 640 | Beta: 419 | |||||
| 12 months | 0.763 (0.728 to 0.794) | 0.840 (0.802 to 0.872) | Alpha: 488 | Alpha: 352 | ||
| Beta: 152 | Beta: 67 | |||||
| 24 months | 0.656 (0.619 to 0.692) | 0.790 (0.748 to 0.826) | Alpha: 420 | Alpha: 331 | ||
| Beta: 220 | Beta: 88 | |||||
| EBRT (late toxicity) | ||||||
| Beta | ||||||
| Urinary stricture | 0.072 (0.050 to 0.102) | 0.072 (0.050 to 0.102) | Alpha: 27 | 1112 (one-off) | Ataman161 | |
| Beta: 350 | ||||||
| Urinary incontinence | 0.053 (0.035 to 0.081) | 0.053 (0.035 to 0.081) | Alpha: 20 | 65.90 (every 3 months) | Ataman161 | |
| Beta: 357 | ||||||
| Bowel problems | 0.119 (0.090 to 0.156) | 0.119 (0.090 to 0.156) | Alpha: 45 | 18 (every 3 months) | Ataman161 | |
| Beta: 332 | ||||||
| ED | 0.45 | 0.45 | Applied deterministically | 232.08 (every 3 months) | Heidenreich162 | |
Patients suffering from ED were modelled to receive sildenafil (Viagra®, Pfizer) (84%) or alprostadil (MUSE®, Astra) (16%). Proportions of patients using both were identified in the literature and the weighted average cost was applied to estimate the 3-monthly treatment cost. All of the unit costs for treatment complications are provided in Table 25.
| Health-state utility | Utility value | Distribution for PSA | Source |
|---|---|---|---|
| Cancer states | |||
| Beta: mean (SEM) | |||
| Localised (undiagnosed) | 0.89 | 0.89 (0.0133) | Korfage 2005163 |
| Localised (diagnosed) | Korfage 2005163 | ||
| < 6 months | 0.89 | 0.89 (0.0133) | |
| 6–12 months | 0.91 | 0.91 (0.014427) | |
| 12–51 months | 0.90 | 0.90 (0.015328) | |
| ≥ 52 months | 0.88 | 0.88 (0.018276) | |
| Locally advanced (undiagnosed) | 0.81 | 0.81 (0.014625) | Korfage 2005163 |
| Locally advanced (diagnosed) | Korfage 2005163 | ||
| < 6 months | 0.81 | 0.81 (0.0146) | |
| 6–12 months | 0.83 | 0.83 (0.0156) | |
| 12–51 months | 0.82 | 0.82 (0.0149) | |
| ≥ 52 months | 0.76 | 0.76 (0.0205) | |
| Metastases | 0.635 | 0.635 (0.04) | Volk et al. 2004166 |
| Treatment complications | |||
| Urinary incontinence | 0.84 | Applied deterministically | Shimizu et al. 2008165 |
| Bowel problems | 0.83 | Applied deterministically | Krahn et al. 2003164 |
| ED | 0.88 | Applied deterministically | Shimizu et al. 2008165 |
External beam radiotherapy
Four common complications (see Table 24) following EBRT treatment were identified from the EUA Guidelines on Prostate Cancer:161,162 urinary stricture, UI, ED, and bowel problems. An identical assumption was made for patients diagnosed with urinary stricture as for those diagnosed with bladder neck contracture following radical prostatectomy; i.e. all patients were assumed to recover following a minor bladder neck endoscopic procedure carried out in an inpatient setting (£1112). The cost of managing UI following EBRT was assumed to correspond to that reported for radical prostatectomy, as were the costs of treating ED.
Health measurement and valuation
Cancer states
The model was used to estimate cumulative costs and LYs over the lifetime of the simulated cohorts. Attempts were then made to identify appropriate utility weights (Table 25) for the different cancer states, so as to enable the estimation of QALYs. A similar approach to the one taken in the Robotic report144 was used to adjust time spent in PC health states. For localised cancer, we used the European Quality of Life-5 Dimensions (EQ-5D) utility weights reported for a cohort of patients undergoing prostatectomy at baseline, 6 months, 1 year and 4 years. We assumed that patients with no cancer or undiagnosed localised cancer would have the same health-state utility as prostatectomy patients at baseline. For PC found to be locally advanced upon diagnosis, and for local recurrence following initial treatment, we applied further utility weights reported by Korfage et al. 163 for a cohort of patients undergoing EBRT. This cohort of patients was slightly older on average, with more advanced disease. For patients with metastatic disease, we applied the average of the time trade-off weights for metastatic and castration resistant metastatic disease – elicited from a sample of 45- to 70-year-old married males (with no history of PC) presenting at a primary care medical facility in the USA. 144
Biopsy and treatment complications
The EQ-5D weights reported by Korfage et al. 163 were the mean values reported for cohorts where a substantial proportion of patients experienced the main complications of prostatectomy or EBRT but nevertheless reported high levels of health-related QoL on the EQ-5D. 163 As such in the base-case analysis we made no further adjustment to health-related QoL for those modelled to experience treatment complications. However, we did explore the impact of applying further disutilities for complications through sensitivity analyses.
In order to do this we applied utilities reflecting the presence of mild/moderate bowel problems,164 UI,9,165 and ED9,165 in a multiplicative fashion, such that if a modelled patient had localised cancer and UI, then their overall utility pay-off was equal to the product of the utilities for localised cancer and UI.
Discount rate (costs and benefits)
Costs and benefits (LYs and QALYs) were discounted at the treasury recommended rate of 3.5% per annum. 167 We also assessed the impact of discounting benefits at the rate of 1.5% per annum, while maintaining a discount rate of 3.5% for costs, as suggested by NICE in instances where treatment effects ‘… are both substantial in restoring health and sustained over a very long period (normally at least 30 years)’. 167
List of assumptions
-
All patients were initially spread across the states: no cancer, localised cancer (low, intermediate, high risk) or locally advanced cancer.
-
Imaging test sensitivities were not adjusted by grade and stage of underlying cancer in the base-case analysis, but the observed correlation between MRS and DW-MRI test performance and tumour grade was explored through sensitivity analysis.
-
All patients with cancer were modelled to be at risk of progression to metastatic disease based on their D'Amico risk status (low, intermediate, high).
-
All cancer deaths occurred through distant metastases.
-
Diagnosed patients experienced a reduction in the risk of progression to metastases in line with that observed for patients receiving radical prostatectomy (favours more sensitive strategies). Although not all patients with localised disease were modelled to receive radical treatment upon diagnosis, it was assumed that appropriate risk-based targeting of treatment could maintain the relative treatment effects observed for radical prostatectomy, without the need to implement radical treatment immediately for all patients.
-
Given the lack of contemporary data on the risk of progressing to metastatic disease from locally advanced disease without treatment, progression was modelled to occur at the rate observed for patients receiving EBRT alone. Progression in those diagnosed was modelled to occur at the rate observed for patients receiving EBRT with adjuvant hormone therapy.
-
The starting point for the model was the first repeat biopsy, and it was assumed that patients with cancer missed by this biopsy would have persistently elevated PSA level, which would trigger a further definitive saturation biopsy 12 months later (base case).
-
For patients without underlying cancer, the assumption was made that management beyond the first repeat biopsy would remain the same, regardless of which strategy was used for the first repeat biopsy. No further biopsies were modelled for this group in the base-case analysis, unless incident PC developed.
-
A TRUS-guided systematic extended 14- to 16-core biopsy scheme was used as the comparator against which the cost-effectiveness of MRI-/MRS-directed TRUS/Bx was assessed in the base case.
-
It was assumed in the base case that MRI-/MRS-directed biopsy would not reduce the cost of the biopsy procedure or the risk of biopsy-related complications relative to the TRUS-guided extended-cores biopsy.
Time horizon
Once it was established that the model made internally consistent predictions of cancer-related mortality over the period to which the observed input data related (15 years), the analysis proceeded over a 30-year time horizon. By this stage the majority of the modelled cohorts were dead and the additional QALYs per cycle had fallen to < 0.001.
Internal validation
To assess the internal validity of the model, Figures 19 and 20 show the Markov traces for treated and untreated patients with localised cancer (men aged 60 years) over a 15-year follow-up period. The modelled cumulative incidence of PC death does not match the data reported by Bill-Axelson et al. 148 exactly (23% vs 26% for untreated; 12% vs 16% for treated) owing to the application of UK age-specific rates of death from other causes and the application of a constant UK-specific risk of death from metastatic PC. However, the cumulative PC mortality rate is generally consistent with the data reported by Bill-Axelson and other similar cohorts; for example, Albertson et al. 168 estimated prostate-specific mortality of ∼20% at 15 years in men aged 60–64 years with a Gleason score of 6. 168 However, our modelled rates are significantly higher than those reported in the recently published PIVOT trial,149 which identified men through PSA screening. As such, sensitivity analysis was undertaken to assess the impact of recalibrating the model to yield the PC mortality rates observed by Wilt et al. 149
FIGURE 19.
Markov trace for undiagnosed/untreated local cancer in patients aged 60 years.
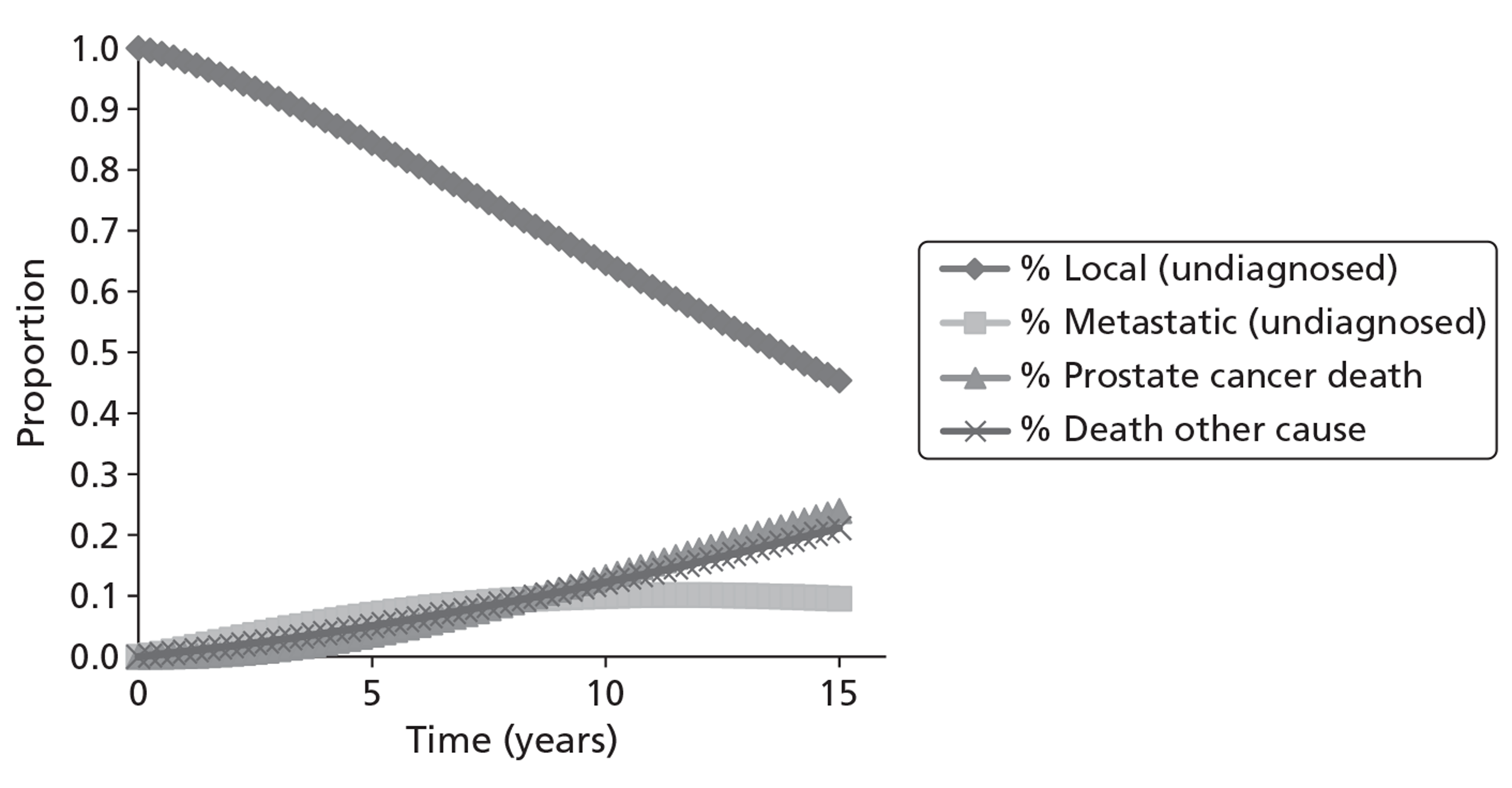
FIGURE 20.
Markov trace for diagnosed/treated local cancer for patients aged 60 years.
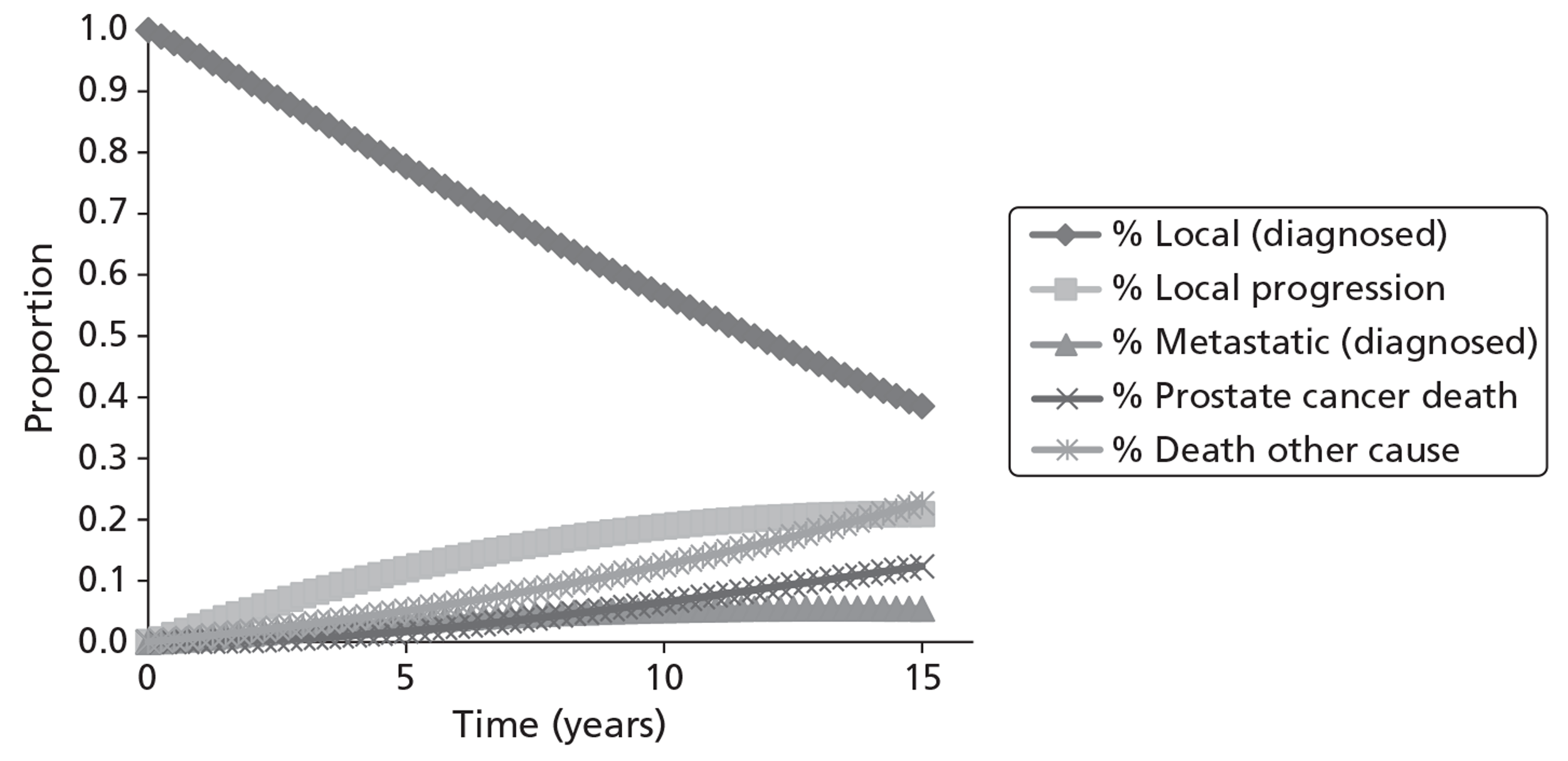
Analysis
The model was first of all analysed deterministically, and the impact of altering key parameters and structural assumptions was demonstrated using deterministic sensitivity analysis. A probabilistic analysis was also undertaken, whereby Monte Carlo simulation was used to randomly draw a value for each model parameter from its assigned probability distribution for each of 1000 runs. The NMB approach was used to generate cost-effectiveness acceptability curves (CEACs) using the output from this probabilistic sensitivity analysis. One thousand probabilistic iterations were found to produce stable CEACs. Although the mean values obtained from probabilistic sensitivity analysis provide a more appropriate estimate of expected costs and effects for non-linear models, the analysis was found to be too computationally intensive to demonstrate the impact of all deterministic uncertainties on the mean probabilistic results. For this reason, the mean results from probabilistic sensitivity analysis are presented for only the main base-case analyses in 60- and 70-year-old men.
For the PSA, beta or Dirichlet distributions were used to represent uncertainty surrounding probabilities and proportions; beta distributions were assigned for health-state utilities, gamma distributions were used for costs, and log-normal distributions were assigned for relative risk parameters (see parameter tables, above – Tables 19, 21, 23, 24 and 25). To reflect the joint uncertainty surrounding the estimated sensitivity/specificity of each MRI sequence, the logit of the sensitivity/specificity of each sequence was modelled to follow a bivariate normal distribution (derived from the meta-analysis), with negative correlation specified between sensitivity and specificity on the logit scale. As insufficient data were available to estimate the correlation between sensitivity and specificity for each sequence, correlation (−0.3), obtained from the bivariate meta-analysis model for T2-MRI (the sequence with most information available for estimating correlation), was applied to all sequences. Underlying cancer prevalence and the initial proportional spread of the cohorts across cancer stages and risk strata were omitted from the PSA. This was due to uncertainty as to how the estimated variability of these parameters (see Table 16) reflected heterogeneity rather statistical impression. Instead, the impact of uncertainty surrounding these parameters was addressed using subgroup analysis and deterministic sensitivity analysis.
Results
Mean costs and mean effects, and incremental analysis
Tables 26 and 27 present the mean costs, mean LYs, and incremental cost per LY gained for each strategy in men aged 60 years at the time of repeat biopsy (based on deterministic and the probabilistic analyses, respectively), assuming a prevalence of underlying cancer of 24%. Tables 28 and 29 present the same analyses using QALYs as the measure of effect. A breakdown of strategy costs into diagnosis and pre-diagnosis monitoring costs, biopsy complication costs, and cancer treatment and treatment complication costs is provided in Appendix 17. Appendix 17 also provides a summary of the expected numbers of unnecessary and appropriate biopsies undertaken with each strategy. Figures 21 and 22 present the findings of the cost per LY and cost per QALY analyses graphically on the cost-effectiveness plane. Strategies falling above and behind the lines plotted through the cost-effectiveness planes represent options that are more costly and less effective than other strategies or combinations of strategies. Strategies falling on the lines (the cost-effectiveness frontier) represent potentially cost-effective options, dependent on decision-makers' willingness to pay per LY or QALY gained.
| Strategy | Average cost (£) | Incremental costa (£) | Average LYs | Incremental LYsa | Incremental cost per LYa (£) | ICER vs common baseline (£) |
|---|---|---|---|---|---|---|
| Syst. TRUS | 3895 | – | 14.16796 | – | – | – |
| T2-MRI | 3902 | 7 | 14.16890 | 0.00094 | 7447 | 7447 |
| MRS | 3952 | 49 | 14.17081 | 0.00191 | 25,849 | 19,796 |
| DCE-MRI | 3984 | 32 | 14.16669 | –0.00412 | Dominated | Dominated |
| T2-MRI or MRS | 4031 | 80 | 14.17203 | 0.00122 | 65,208 | 33,425 |
| T2-MRI or DCE-MRI | 4056 | 25 | 14.16949 | –0.00254 | Dominated | 105,351 |
| Strategy | Average cost (£) | Incremental costa (£) | Average LYs | Incremental LYsa | Incremental cost per LYa (£) | ICER vs common baseline (£) |
|---|---|---|---|---|---|---|
| Syst. TRUS | 3910 | – | 14.15935 | – | – | – |
| T2-MRI | 3916 | 7 | 14.16013 | 0.00078 | 8512 | 8512 |
| MRS | 3967 | 51 | 14.16189 | 0.00176 | 28,715 | 22,535 |
| DCE | 3999 | 32 | 14.15802 | −0.00387 | Dominated | Dominated |
| MRI or MRS | 4045 | 78 | 14.16313 | 0.00124 | 63,393 | 35,903 |
| MRI or DCE | 4069 | 23 | 14.16065 | −0.00248 | Dominated | 122,575 |
| Strategy | Average cost (£) | Incremental costa (£) | Average QALYs | Incremental QALYsa | Incremental cost per QALYa (£) | ICER vs common baseline (£) |
|---|---|---|---|---|---|---|
| Syst. TRUS | 3895 | – | 12.48432 | – | – | – |
| T2-MRI | 3902 | 7 | 12.48498 | 0.00066 | 10,626 | 10,626 |
| MRS | 3952 | 49 | 12.48630 | 0.00132 | 37,382 | 28,502 |
| DCE-MRI | 3984 | 32 | 12.48346 | −0.00285 | Dominated | Dominated |
| T2-MRI or MRS | 4031 | 80 | 12.48714 | 0.00083 | 95,481 | 48,367 |
| T2-MRI or DCE-MRI | 4056 | 25 | 12.48538 | −0.00175 | Dominated | 152,323 |
| Strategy | Average cost (£) | Incremental costa (£) | Average QALYs | Incremental QALYsa | Incremental cost per QALYa (£) | ICER vs common baseline (£) |
|---|---|---|---|---|---|---|
| Syst. TRUS | 3910 | – | 12.47303 | – | – | – |
| T2-MRI | 3916 | 7 | 12.47357 | 0.00054 | 12,315 | 12,315 |
| MRS | 3967 | 51 | 12.47478 | 0.00121 | 41,927 | 32,811 |
| DCE-MRI | 3999 | 32 | 12.47213 | −0.00264 | Dominated | Dominated |
| T2-MRI or MRS | 4045 | 78 | 12.47562 | 0.00084 | 92,865 | 52,378 |
| T2-MRI or DCE-MRI | 4069 | 23 | 12.47392 | −0.00170 | Dominated | 178,746 |
FIGURE 21.
Cost-effectiveness frontier based on the cost per LY analysis (men aged 60 years, underlying cancer prevalence 24%).
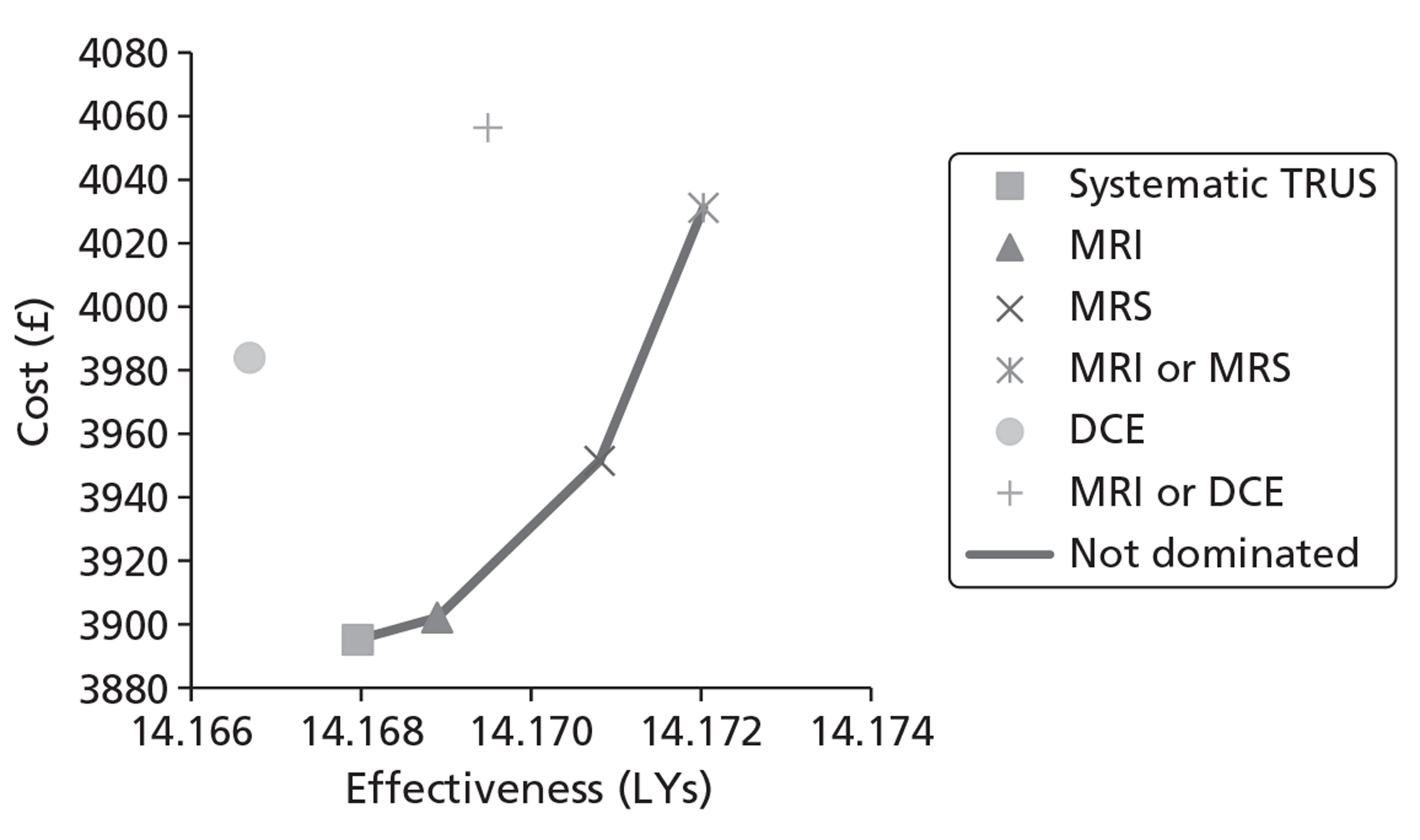
FIGURE 22.
Cost-effectiveness frontier based on the cost per QALY analysis (men aged 60 years, underlying cancer prevalence 24%).
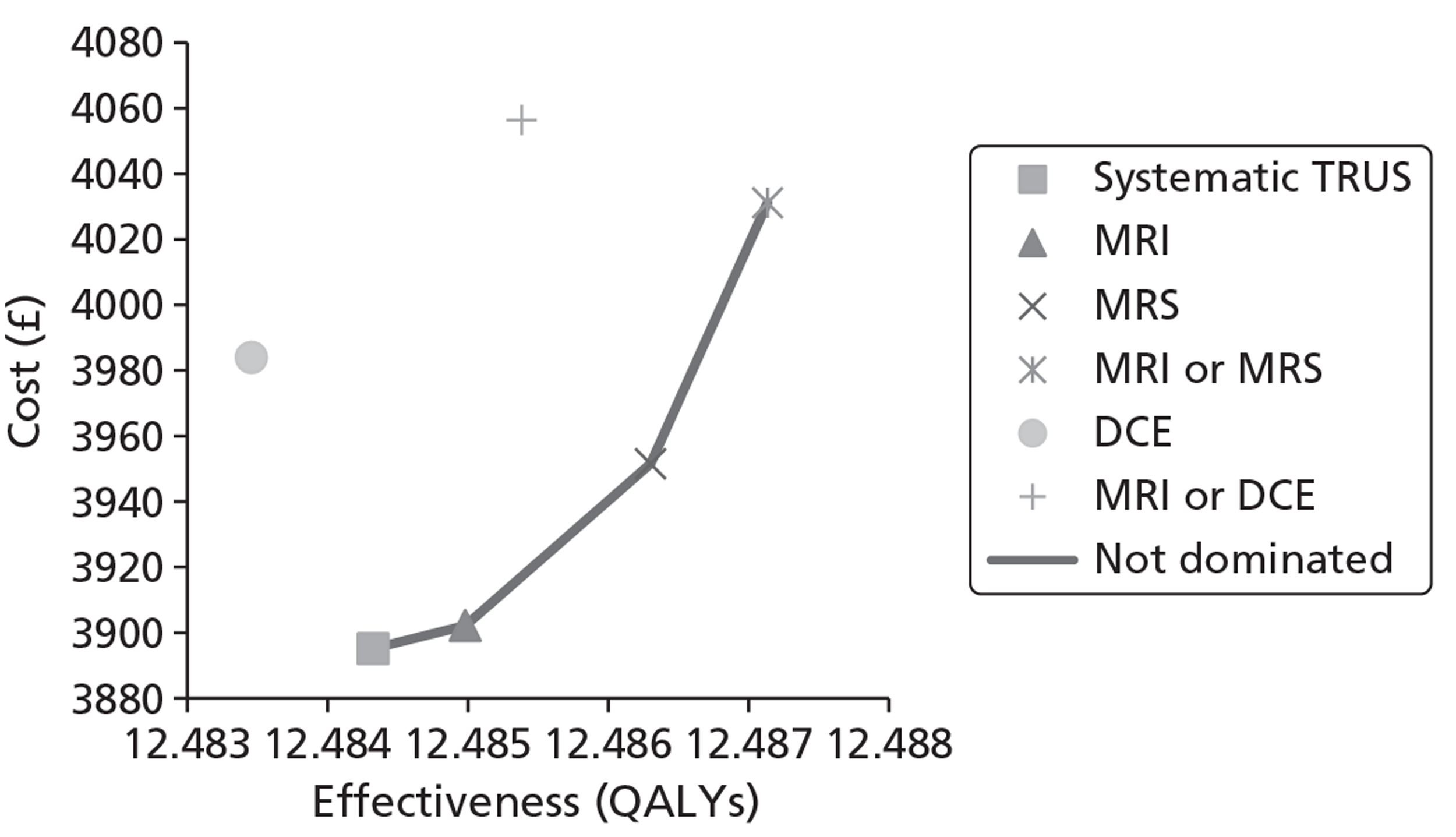
The base-case results show systematic extended-core TRUS/Bx to be the least costly option. However, using T2-MRI to determine which patients to biopsy (and to subsequently direct the biopsy) increases the costs by only a very small margin, with corresponding very small survival and QALY gains. Although these differences are very small and insignificant, T2-MRI-directed biopsy does have a favourable incremental cost per LY and QALY gained in comparison with systematic TRUS/Bx.
Using MRS to determine and direct biopsies results in a further cost increase and survival gain over T2-MRI but its incremental cost-effectiveness ratios (ICERs) are somewhat less favourable; although the incremental cost per QALY gained with MRS compared with systematic TRUS/Bx is just < £30,000 (deterministic analysis), it is > £30,000 in comparison with T2-MRI. Using positive findings on T2-MRI or MRS to determine and direct biopsies again increases costs, LYs and QALYs further. However, the ICERs (for LYs and QALYs) for using any visible abnormalities detected on T2-MRI or MRS, compared with only using abnormalities detected on MRS alone, are well above £30,000. This is due to a substantial loss of specificity associated with combined strategy, compared with using the findings on MRS alone to guide biopsy (31% for T2-MRI or MRS vs 76% for MRS alone), for only a small gain in sensitivity (96% vs 92%).
Tables 30 and 31 presents the incremental cost per LY analysis for men aged 70 years at the time of repeat biopsy, assuming a prevalence of underlying cancer of 24%. Tables 32 and 33 present the same analysis but use QALYs as the unit of outcome (see Appendix 17 for a breakdown of strategy costs by component categories). Figures 23 and 24 present the findings of the respective analyses graphically on the cost-effectiveness plane.
| Strategy | Average cost (£) | Incremental cost (£)a | Average LYs | Incremental LYsa | Incremental cost per LY (£) | ICER vs common baseline (£) |
|---|---|---|---|---|---|---|
| Syst. TRUS | 3199 | – | 10.55176 | – | – | – |
| T2-MRI | 3206 | 7 | 10.55233 | 0.00057 | 12,569 | 12,569 |
| MRS | 3256 | 50 | 10.55347 | 0.00115 | 43,305 | 33,121 |
| DCE-MRI | 3287 | 31 | 10.55100 | −0.00247 | Dominated | Dominated |
| T2-MRI or MRS | 3336 | 80 | 10.55420 | 0.00073 | 109,800 | 55,916 |
| T2-MRI or DCE-MRI | 3360 | 25 | 10.55268 | −0.00152 | Dominated | 175,340 |
| Strategy | Average cost (£) | Incremental cost (£)a | Average LYs | Incremental LYsa | Incremental cost per LY (£) | ICER vs common baseline (£) |
|---|---|---|---|---|---|---|
| Syst. TRUS | 3187 | – | 10.54702 | – | – | – |
| T2-MRI | 3194 | 7 | 10.54748 | 0.00046 | 14,696 | 14,696 |
| MRS | 3245 | 51 | 10.54854 | 0.00105 | 48,305 | 38,088 |
| DCE-MRI | 3275 | 31 | 10.54624 | −0.00229 | Dominated | Dominated |
| T2-MRI or MRS | 3323 | 78 | 10.54926 | 0.00073 | 107,834 | 60,716 |
| T2-MRI or DCE-MRI | 3346 | 23 | 10.54780 | −0.00147 | Dominated | 205,281 |
| Strategy | Average cost (£) | Incremental costa (£) | Average QALYs | Incremental QALYsa | Incremental cost per QALYa (£) | ICER vs common baseline (£) |
|---|---|---|---|---|---|---|
| Syst. TRUS | 3199 | – | 9.30639 | – | – | – |
| T2-MRI | 3206 | 7 | 9.30677 | 0.00038 | 18,727 | 18,727 |
| MRS | 3256 | 50 | 9.30752 | 0.00075 | 65,825 | 50,010 |
| DCE-MRI | 3287 | 31 | 9.30590 | −0.00162 | Dominated | Dominated |
| T2-MRI or MRS | 3336 | 80 | 9.30799 | 0.00047 | 170,109 | 85,071 |
| T2-MRI or DCE-MRI | 3360 | 25 | 9.30699 | −0.00100 | Dominated | 266,423 |
| Strategy | Average cost (£) | Incremental costa (£) | Average QALYs | Incremental QALYsa | Incremental cost per QALYa (£) | ICER vs common baseline (£) |
|---|---|---|---|---|---|---|
| Syst. TRUS | 3187 | – | 9.29963 | – | – | – |
| T2-MRI | 3194 | 7 | 9.29993 | 0.00030 | 22,677 | 22,677 |
| MRS | 3245 | 51 | 9.30061 | 0.00068 | 74,586 | 58,798 |
| DCE-MRI | 3275 | 31 | 9.29914 | −0.00147 | Dominated | Dominated |
| T2-MRI or MRS | 3323 | 78 | 9.30108 | 0.00047 | 167,637 | 93,943 |
| T2-MRI or DCE-MRI | 3346 | 23 | 9.30013 | −0.00095 | Dominated | 316,854 |
FIGURE 23.
Cost-effectiveness frontier based on the cost per LY analysis (men aged 70 years, underlying cancer prevalence 24%).
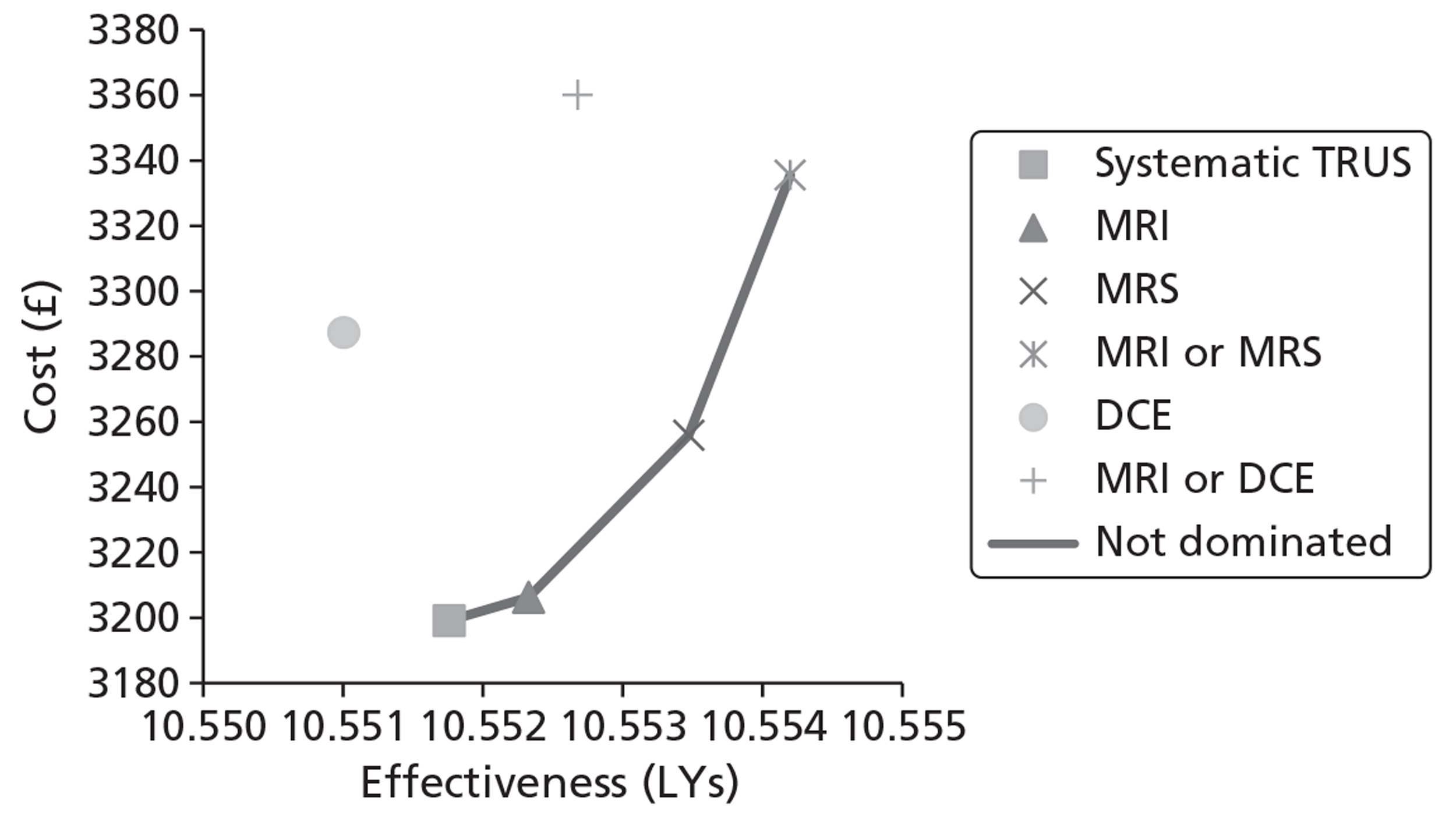
FIGURE 24.
Cost-effectiveness frontier based on the cost per QALY analysis (men aged 70 years, underlying cancer prevalence 24%).
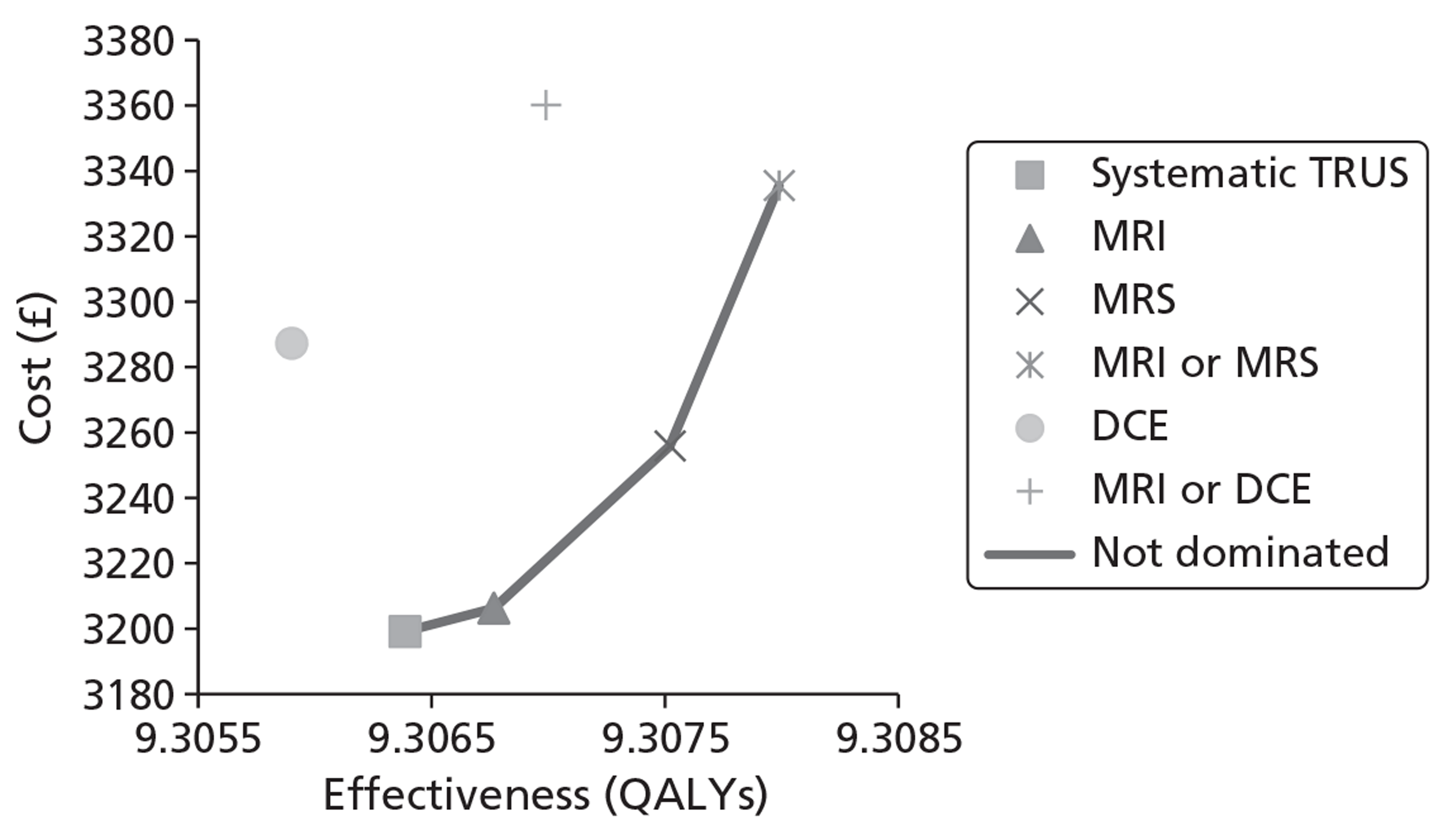
A similar pattern of results is observed as for the cohort of men aged 60 years, but the survival benefit associated with the more sensitive strategies is smaller in the older cohort, owing to there being a higher risk of death from other causes (a competing risk for death from PC) and a smaller relative risk reduction associated with radical treatment in older men. As a consequence, the additional costs per LY and QALY gained with T2-MRI, MRS, and ‘T2-MRI or MRS’, are higher. However, the ICERs for T2-MRI compared with systematic TRUS/Bx remain < £30,000 despite the very small survival/QALY benefits.
Differential results for subgroups according to disease prevalence
Although few data were available to ascertain how diagnostic accuracy parameters vary by risk status of the cohort and underlying prevalence of cancer, Tables 34 and 35 present the incremental cost per LY and QALY findings for a high-prevalence cohort (50%) and low-prevalence cohort (10%) of men aged 60 and 70 years, respectively (assumes patient-level sensitivities not influenced by cancer prevalence). The findings seem to indicate that, for the 60-year-old cohorts (see Table 34), the more sensitive, more costly strategies have a higher chance of being considered cost-effective if used to direct biopsies in groups at higher risk of harbouring PC (e.g. men with ASAP on first biopsy). In the lower prevalence cohort, the T2-MRI strategy dominates systematic TRUS/Bx as a result of its specificity taking on greater significance with the underlying cancer prevalence set at only 10%.
| Strategy | Average cost (£) | Incremental cost (£)a | Average LYs/QALYs | Incremental LYs/QALYsa | ICER (£) | ICER vs common baseline (£) |
|---|---|---|---|---|---|---|
| Prevalence 50%; unit of effect LYs | ||||||
| Syst. TRUS | 6372 | – | 13.82903 | – | – | – |
| T2-MRI | 6404 | 32 | 13.83091 | 0.00188 | 16,929 | 16,929 |
| MRS | 6472 | 68 | 13.83486 | 0.00395 | 17,086 | 17,035 |
| DCE-MRI | 6477 | 5 | 13.82631 | −0.00855 | Dominated | Dominated |
| T2-MRI or MRS | 6529 | 58 | 13.83746 | 0.00260 | 22,176 | 18,620 |
| T2-MRI or DCE-MRI | 6537 | 7 | 13.83220 | −0.00527 | Dominated | 51,871 |
| Prevalence 50%; unit of effect QALYs | ||||||
| Syst. TRUS | 6372 | – | 12.06553 | – | – | – |
| T2-MRI | 6404 | 32 | 12.06683 | 0.00131 | 24,402 | 24,402 |
| MRS | 6472 | 68 | 12.06956 | 0.00273 | 24,757 | 24,642 |
| DCE-MRI | 6477 | 5 | 12.06366 | −0.00590 | Dominated | Dominated |
| T2-MRI or MRS | 6529 | 58 | 12.07135 | 0.00179 | 32,256 | 26,981 |
| T2-MRI or DCE-MRI | 6537 | 7 | 12.06772 | −0.00363 | Dominated | 75,120 |
| Prevalence 10%; unit of effect LYs | ||||||
| T2-MRI | 2555 | – | 14.35089 | – | – | – |
| Syst. TRUS | 2561 | 6 | 14.35046 | −0.00043 | Dominated | Dominated |
| MRS | 2595 | 40 | 14.35170 | 0.00081 | 48,866 | 48,866 |
| DCE-MRI | 2641 | 47 | 14.34997 | −0.00173 | Dominated | Dominated |
| T2-MRI or MRS | 2686 | 91 | 14.35218 | 0.00048 | 191,367 | 101,707 |
| T2-MRI or | 2721 | 35 | 14.35111 | −0.00107 | Dominated | 756,814 |
| Prevalence 10%; unit of effect QALYs | ||||||
| T2-MRI | 2555 | – | 12.71014 | – | – | – |
| Syst. TRUS | 2561 | 6 | 12.70983 | −0.00031 | Dominated | Dominated |
| MRS | 2595 | 40 | 12.71070 | 0.00056 | 70,309 | 70,309 |
| DCE-MRI | 2641 | 47 | 12.70950 | −0.00120 | Dominated | Dominated |
| T2-MRI or MRS | 2686 | 91 | 12.71102 | 0.00032 | 285,797 | 148,351 |
| T2-MRI or DCE-MRI | 2721 | 35 | 12.71028 | −0.00074 | Dominated | 1,164,444 |
| Strategy | Average cost (£) | Incremental cost (£)a | Average LYs/QALYs | Incremental LYs/QALYsa | ICER (£) | ICER vs common baseline (£) |
|---|---|---|---|---|---|---|
| Prevalence 50%; unit of effect LYs | ||||||
| Syst. TRUS | 5287 | – | 10.36606 | – | – | – |
| T2-MRI | 5319 | 32 | 10.36719 | 0.00113 | 28,394 | 28,394 |
| MRS | 5388 | 68 | 10.36956 | 0.00237 | 28,791 | 28,662 |
| DCE-MRI | 5391 | 4 | 10.36444 | −0.00512 | Dominated | Dominated |
| T2-MRI or MRS | 5446 | 58 | 10.37111 | 0.00155 | 37,381 | 31,342 |
| T2-MRI or DCE-MRI | 5452 | 6 | 10.36796 | −0.00315 | Dominated | 86,624 |
| Prevalence 50%; unit of effect QALYs | ||||||
| Syst. TRUS | 5287 | – | 9.06143 | – | – | – |
| T2-MRI | 5319 | 32 | 9.06218 | 0.00075 | 42,942 | 42,942 |
| MRS | 5388 | 68 | 9.06373 | 0.00155 | 43,891 | 43,583 |
| DCE-MRI | 5391 | 4 | 9.06037 | −0.00336 | Dominated | Dominated |
| T2-MRI or MRS | 5446 | 58 | 9.06474 | 0.00101 | 57,324 | 47,782 |
| T2-MRI or DCE-MRI | 5452 | 6 | 9.06268 | −0.00207 | Dominated | 131,943 |
| Prevalence 10%; unit of effect LYs | ||||||
| T2-MRI | 2068 | – | 10.65202 | – | – | – |
| Syst. TRUS | 2075 | 6 | 10.65175 | −0.00026 | Dominated | Dominated |
| MRS | 2108 | 40 | 10.65250 | 0.00049 | 81,213 | 81,213 |
| DCE-MRI | 2154 | 46 | 10.65146 | −0.00104 | Dominated | Dominated |
| T2-MRI or MRS | 2199 | 91 | 10.65278 | 0.00028 | 326,605 | 170,521 |
| T2-MRI or DCE-MRI | 2234 | 35 | 10.65214 | −0.00064 | Dominated | 1,321,142 |
| Prevalence 10%; unit of effect QALYs | ||||||
| T2-MRI | 2068 | – | 9.43847 | – | – | – |
| Syst. TRUS | 2075 | 6 | 9.43829 | −0.00018 | Dominated | Dominated |
| MRS | 2108 | 40 | 9.43879 | 0.00032 | 122,508 | 122,508 |
| DCE-MRI | 2154 | 46 | 9.43811 | −0.00069 | Dominated | Dominated |
| T2-MRI or MRS | 2199 | 91 | 9.43897 | 0.00018 | 522,072 | 262,608 |
| T2-MRI or DCE-MRI | 2234 | 35 | 9.43854 | −0.00042 | Dominated | 2,219,778 |
A similar pattern of results is observed for the older cohort (see Table 35), with the cost-effectiveness of MRS improving relative to systematic TRUS/Bx and T2-MRI in the high-prevalence cohort. However, the ICER for MRS does not drop below £30,000 per QALY in this cohort. Moreover, the cost-effectiveness of T2-MRI rises above £30,000 in this cohort owing to the lower influence of specificity with high cancer prevalence combined with only very small gain in sensitivity with T2-MRI compared with systematic TRUS/Bx. Thus, none of the MRS/MRI sequences appears cost-effective in this older cohort.
For the 70-year-old low-prevalence cohort, the same finding is observed as for the low-prevalence 60-year-old cohort, i.e. T2-MRI dominates systematic TRUS-guided extended-cores biopsy for all.
Illustrative analysis incorporating diffusion-weighted-magnetic resonance imaging-directed biopsy
Although the lack of sensitivity/specificity estimates for DW-MRI in repeat biopsy cohorts precluded its incorporation in the base-case analysis, it was still felt to be a relevant alternative based on evidence from other cohorts coupled with its lower cost compared with MRS. As such, an illustrative analysis was undertaken to assess how it would compare in terms of cost-effectiveness if it could be demonstrated to have sensitivity at least equal to that of MRS (92%) and specificity at least equal to that of T2-MRI (55%). Table 36 presents the cost per QALY findings from this analysis for a 60-year-old cohort.
| Strategy | Average cost (£) | Incremental costa (£) | Average QALYs | Incremental QALYsa | Incremental cost per QALY (£)a | ICER vs common baseline (£) |
|---|---|---|---|---|---|---|
| Scenario 1. DW-MRI incorporated assuming sensitivity 0.92/specificity 0.55 | ||||||
| Syst. TRUS | 3895 | – | 12.48432 | – | – | – |
| T2-MRI | 3902 | 7 | 12.48498 | 0.00066 | 10,626 | 10,626 |
| DW-MRI | 3943 | 41 | 12.48629 | 0.00130 | 31,061 | 24,221 |
| MRS | 3952 | 9 | 12.48630 | 0.00002 | 529,885b | 28,502 |
| DCE-MRI | 3984 | 32 | 12.48346 | −0.00285 | Dominated | Dominated |
| T2-MRI or MRS | 4031 | 88 | 12.48714 | 0.00085 | 104,032 | 48,367 |
| T2-MRI or DCE-MRI | 4056 | 25 | 12.48538 | −0.00175 | Dominated | 152,323 |
| Scenario 2. DW-MRI incorporated assuming sensitivity 0.92/specificity 0.55 (and that all false-negatives with DW-MRI and MRS occur in individuals with low-risk cancer) | ||||||
| Syst. TRUS | 3895 | – | 12.48432 | – | – | – |
| T2-MRI | 3902 | 7 | 12.48498 | 0.00066 | 10,626 | 10,626 |
| DW-MRI | 3947 | 45 | 12.48734 | 0.00236 | 19,008 | 17,186 |
| MRS | 3956 | 9 | 12.48736 | 0.00002 | 529,885 | 20,013 |
| DCE-MRI | 3984 | 28 | 12.48346 | −0.00390 | Dominated | Dominated |
| T2-MRI or MRS | 4031 | 75 | 12.48714 | −0.00023 | −334,729 | 48,367 |
| T2-MRI or DCE-MRI | 4056 | 100 | 12.48538 | −0.00198 | Dominated | 152,323 |
The findings indicate that if DW-MRI could be shown to achieve this level of diagnostic accuracy then it would be preferred on grounds of cost-effectiveness over MRS in this cohort of patients [see Table 32 (scenario 1) and Figure 25]. Under this scenario, DW-MRI is also borderline cost-effective compared with T2-MRI (incremental cost per QALY gained: £30,298).
FIGURE 25.
Comparison with DW-MRI included (assuming 92% sensitivity, 55% specificity).
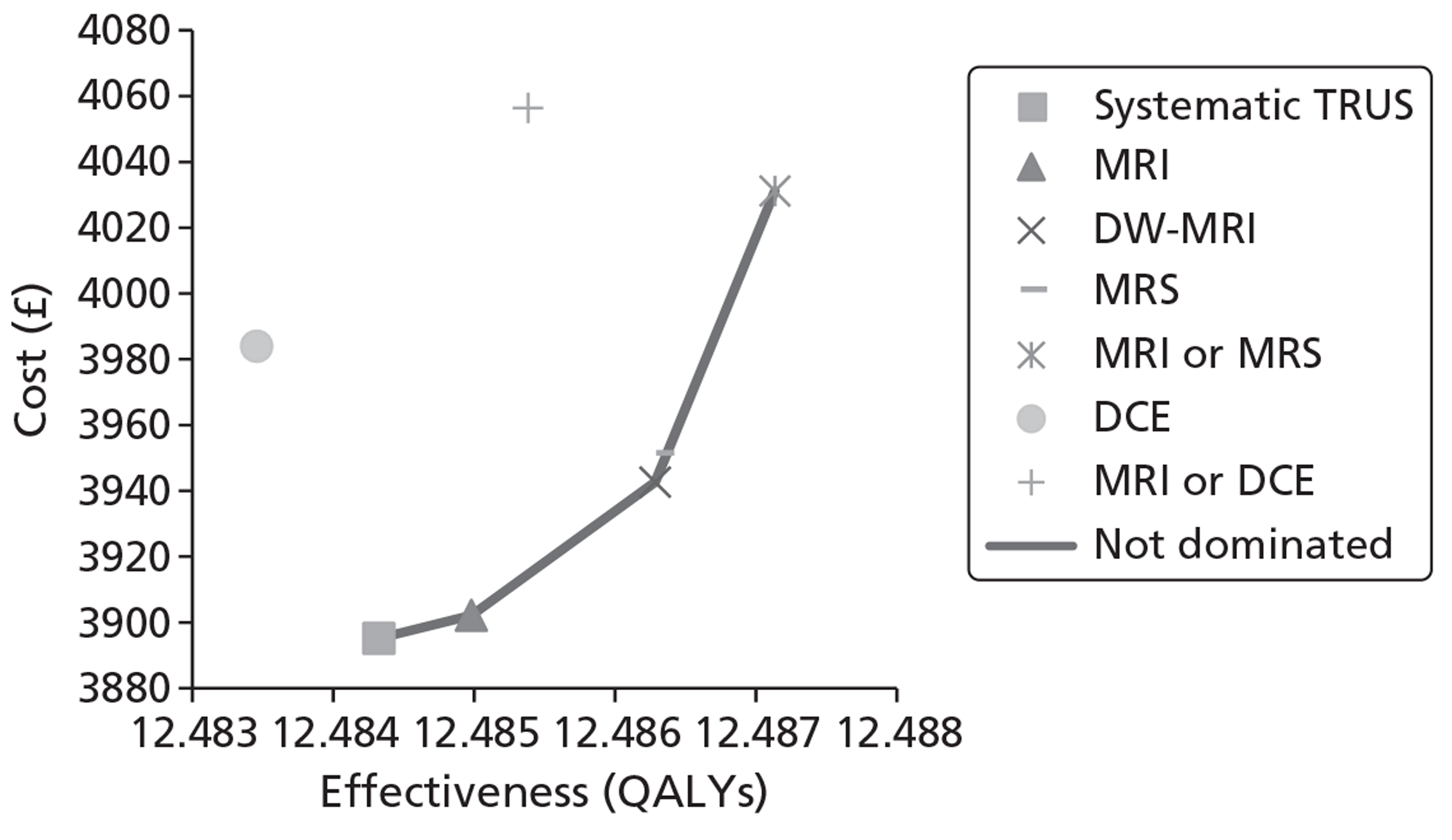
When the sensitivities of DW-MRI and MRS are adjusted by cancer grade so that all false-negatives arising with these strategies occur in patients with low-risk cancer – to reflect the observation from other cohorts that MRS and DW-MRI positivity is highly correlated with tumour Gleason score – the ICER for DW-MRI compared with MRI falls to £18,260 [see Table 32 (scenario 2) and Figure 26], i.e. below the £20,000–£30,000 per QALY range often used to make judgements on cost-effectiveness. This analysis gives an indication of sensitivity/specificity requirements for DW-MRI to be considered cost-effective for directing biopsies (holding all other model parameters constant at base-case values).
FIGURE 26.
Comparison with DW-MRI included (assuming 92% sensitivity, 55% specificity, and that DW-MRI and MRS miss only low-risk cancers).
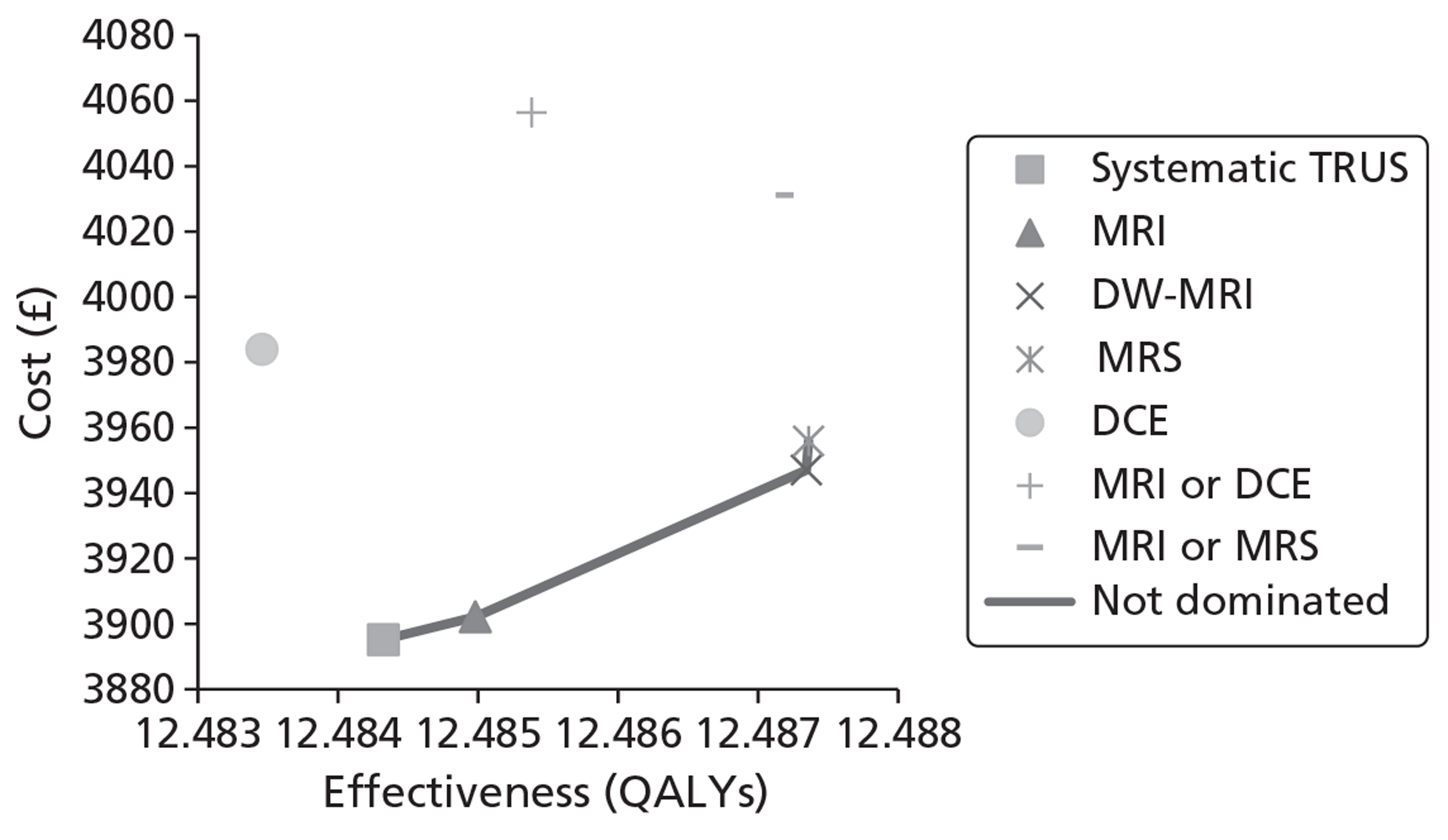
Deterministic sensitivity analysis scenarios (60-year-old cohort)
Several deterministic analyses were carried out to assess the sensitivity of the base-case cost per QALY findings to assumptions surrounding the incorporation of health-state utilities. We assessed the impact of applying a utility decrement of 0.035 (half of the disutility associated with having moderate anxiety rather than no anxiety on the EQ-5D) to patients with undiagnosed cancer, to reflect potential disutility resulting from raised anxiety associated with having a high PSA but no diagnosis. Further, we tested a multiplicative utility model whereby utility levels for diagnosed cancer states were set equal to the product of the cancer state utility and the utility of any treatment complications experienced. Finally, we assessed the impact of applying both of these modifications simultaneously. These deterministic sensitivity analyses were carried out only for the 60-year-old cohort, owing to there being a higher likelihood of changes to base-case assumptions affecting the cost-effectiveness of strategies in this group.
Table 32 shows the cost per QALY findings to be highly sensitive to these alterations. Under the first adjustment (utility decrement associated with undiagnosed cancer), the cost-effectiveness of MRS compared with T2-MRI improves substantially. However, applying the multiplicative model to further adjust utility for complications associated with radical treatment results in all of the MRS/MRI sequences having unfavourable incremental cost per QALY ratios in comparison with the less-sensitive systematic TRUS.
Applying both changes simultaneously (see Table 37, scenario 3) results in a pattern of findings more in keeping with the base-case analysis.
| Strategy | Average cost (£) | Incremental costa (£) | Average QALYs | Incremental QALYsa | ICER (£)a | ICER vs common baseline (£) |
|---|---|---|---|---|---|---|
| Scenario 1. Additional utility decrement for persistently elevated PSA without a diagnosis | ||||||
| Syst. TRUS | 3895 | – | 12.47968 | – | – | – |
| T2-MRI | 3902 | 7 | 12.48059 | 0.000914 | 7633 | 7633 |
| MRS | 3952 | 49 | 12.48246 | 0.001873 | 26,373 | 20,229 |
| DCE-MRI | 3984 | 32 | 12.47842 | −0.00404 | Dominated | Dominated |
| T2-MRI or MRS | 4031 | 80 | 12.48366 | 0.001201 | 66,262 | 34,098 |
| T2-MRI or DCE-MRI | 4056 | 25 | 12.48118 | −0.00249 | Dominated | 107,510 |
| Scenario 2. Multiplicative model to further adjust for adverse treatment effects | ||||||
| Syst. TRUS | 3895 | – | 12.32446 | – | – | – |
| T2-MRI | 3902 | 7 | 12.32466 | 0.000202 | 34,521 | 34,521 |
| MRS | 3952 | 49 | 12.32501 | 0.000348 | 141,776 | 102,408 |
| DCE-MRI | 3984 | 32 | 12.32427 | −0.00074 | Dominated | Dominated |
| T2-MRI or MRS | 4031 | 80 | 12.32519 | 0.000185 | 429,592 | 184,823 |
| T2-MRI or DCE-MRI | 4056 | 25 | 12.32474 | −0.00046 | Dominated | 575,872 |
| Scenario 3. Combination of scenarios 1 and 2 | ||||||
| Syst. TRUS | 3895 | – | 12.31981 | – | – | – |
| T2-MRI | 3902 | 7 | 12.32027 | 0.00046 | 15,182 | 15,182 |
| MRS | 3952 | 49 | 12.32117 | 0.00090 | 54,886 | 41,468 |
| DCE-MRI | 3984 | 32 | 12.31923 | −0.00193 | Dominated | Dominated |
| T2-MRI or MRS | 4031 | 80 | 12.32172 | 0.00055 | 143,966 | 71,109 |
| T2-MRI or DCE-MRI | 4056 | 25 | 12.32053 | −0.00119 | Dominated | 223,564 |
Further deterministic scenario analyses
The process of populating the model required a number of parameter and structural assumptions. To further assess the influence of these assumptions on findings, the following deterministic sensitivity analyses were undertaken:
-
Costs adjusted such that pathology costs for TRUS/Bx are increased by £86 to reflect a 25-minute increase in pathologist time for biopsies involving more than 10 cores, and MRI costs are adjusted to the NHS reference costs.
-
Sensitivity of MRS adjusted so that it misses only low-risk localised disease.
-
Comparator for MRS/MRI assumed to be a standard TRUS/Bx (10–12 cores) with sensitivity 60% (the lowest estimated sensitivity value obtained for systematic TRUS/Bx from studies assessed for inclusion in the systematic review) (see Appendix 11).
-
Application of the sensitivity/specificity estimates obtained from the indirect comparison (see Appendix 13.10).
-
Assumed a 14-core TRUS/Bx is £86 more costly than a MRI-/MRS-directed biopsy, and £112 more costly than obtaining a MRS scan.
-
Assumed that MRI-/MRS-directed biopsy reduces the risk of biopsy complications by 50% because fewer cores are obtained per patient.
-
Subsequent repeat biopsies (i.e. following a first repeat biopsy) have 95% sensitivity with 80% uptake (repeat offered every 12 months for those remaining with undiagnosed cancer).
-
Application of lower discount rate for health benefits (1.5% for QALYs vs 3.5% for costs).
-
Application of lower baseline risks of progression and less significant diagnosis treatment effects, based on new trial evidence on the effect of radical prostatectomy compared with watchful waiting. 149
-
Assumed all patients who are negative on MRI proceed with an extended 14-core TRUS biopsy.
Table 38 presents the results of these scenarios using QALYs as the unit of outcome. Appendix 17, Table 43, presents the results for these same scenarios using LYs as the unit of outcome.
| Strategy | Average cost (£) | Incremental costa (£) | Average QALYs | Incremental QALYsa | ICERa (£) | ICER vs common baseline (£) |
|---|---|---|---|---|---|---|
| Scenario 1. Biopsy costs inflated to account for additional pathology time associated with more than 10 cores; MRS/MRI costs also adjusted to the NHS reference costs | ||||||
| Syst. TRUS | 4018 | – | 12.48432 | – | – | – |
| T2-MRI | 4024 | 7 | 12.48498 | 0.00066 | 10,271 | 10,271 |
| MRS | 4060 | 35 | 12.48630 | 0.00132 | 26,848 | 21,347 |
| DCE-MRI | 4108 | 49 | 12.48346 | −0.00285 | Dominated | Dominated |
| T2-MRI or MRS | 4169 | 109 | 12.48714 | 0.00083 | 130,497 | 53,719 |
| T2-MRI or DCE-MRI | 4205 | 37 | 12.48538 | −0.00175 | Dominated | 177,295 |
| Scenario 2. Sensitivity of MRS adjusted to miss only low-grade cancer | ||||||
| Syst. TRUS | 3895 | – | 12.48432 | – | – | – |
| T2-MRI | 3902 | 7 | 12.48498 | 0.000656 | 10,626 | 10,626 |
| MRS | 3956 | 54 | 12.48736 | 0.00238 | 22,602 | 20,013 |
| DCE-MRI | 3984 | 28 | 12.48346 | −0.0039 | Dominated | Dominated |
| T2-MRI or MRS | 4031 | 75 | 12.48714 | −0.00023 | Dominated | 48,367 |
| T2-MRI or DCE-MRI | 4056 | 100 | 12.48538 | −0.00198 | Dominated | 152,323 |
| Scenario 3. Sensitivity of comparator reduced to 60% | ||||||
| Syst. TRUS | 3882 | – | 12.47825 | – | – | – |
| T2-MRI | 3899 | 16 | 12.48395 | 0.00571 | 2858 | 2858 |
| MRS | 3948 | 49 | 12.48527 | 0.00132 | 37,399 | 9353 |
| DCE-MRI | 3981 | 32 | 12.48243 | −0.00285 | Dominated | 23,455 |
| T2-MRI or MRS | 4028 | 80 | 12.48611 | 0.00083 | 95,498 | 18,490 |
| T2-MRI or DCE-MRI | 4053 | 25 | 12.48435 | −0.00175 | Dominated | 27,926 |
| Scenario 4. Application of sensitivity/specificity estimates obtained from the indirect comparison (T2-MRI sensitivity 0.84/specifcity 0.58; MRS sensitivity 0.92/specifcity 0.65) | ||||||
| Syst. TRUS | 3895 | – | 12.48432 | – | – | – |
| T2-MRI | 3895 | 0 | 12.48455 | 0.00022 | 240 | 240 |
| MRS | 3970 | 75 | 12.48629 | 0.00175 | 42,903 | 38,052 |
| DCE-MRI | 3986 | 16 | 12.48346 | −0.00284 | Dominated | Dominated |
| T2-MRI or MRS | 4029 | 59 | 12.48735 | 0.00106 | 55,218 | 44,066 |
| T2-MRI or DCE-MRI | 4052 | 23 | 12.48669 | −0.00066 | Dominated | 66,176 |
| Scenario 5. Biopsy costs uplifted for systematic TRUS (assumes 14-core TRUS biopsy is £86 more costly than MRI-/MRS-directed biopsy, and £112 more costly than MRS) | ||||||
| T2-MRI | 3907 | – | 12.48498 | – | – | – |
| MRS | 3955 | 48 | 12.48630 | 0.00132 | 36,200 | 36,200 |
| DCE-MRI | 3991 | 36 | 12.48346 | −0.00285 | Dominated | Dominated |
| Syst. TRUS | 3991 | 36 | 12.48432 | −0.00198 | Dominated | Dominated |
| T2-MRI or MRS | 4034 | 79 | 12.48714 | 0.00083 | 94,233 | 58,653 |
| T2-MRI or DCE-MRI | 4061 | 27 | 12.48538 | −0.00175 | Dominated | £382,270 |
| Scenario 6. MRI reduces the risk of biopsy complications by 50% | ||||||
| Syst. TRUS | 3895 | – | 12.48432 | – | – | – |
| T2-MRI | 3899 | 4 | 12.48501 | 0.00068 | 6063 | 6063 |
| MRS | 3949 | 50 | 12.48632 | 0.00131 | 38,173 | 27,182 |
| DCE-MRI | 3981 | 32 | 12.48348 | −0.00284 | Dominated | Dominated |
| T2-MRI or MRS | 4027 | 78 | 12.48717 | 0.00085 | 91,288 | 46,354 |
| T2-MRI or DCE-MRI | 4052 | 25 | 12.48543 | −0.00175 | Dominated | 142,198 |
| Scenario 7. Subsequent repeat biopsy offers have 80% uptake (repeat offer every 12 months for those remaining with undiagnosed cancer) | ||||||
| Syst. TRUS | 3888 | – | 12.483 | – | – | – |
| T2-MRI | 3895 | 8 | 12.48377 | 0.00077 | 9753 | 9753 |
| MRS | 3946 | 51 | 12.48534 | 0.00157 | 32,261 | 24,839 |
| DCE-MRI | 3976 | 30 | 12.48196 | −0.00338 | Dominated | Dominated |
| T2-MRI or MRS | 4026 | 80 | 12.48634 | 0.00100 | 80,567 | 41,506 |
| T2-MRI or DCE-MRI | 4050 | 24 | 12.48425 | −0.00208 | Dominated | 129,183 |
| Scenario 8. QALYs discounted at 1.5% per annum | ||||||
| Syst. TRUS | 3895 | – | 15.13056 | – | – | – |
| T2-MRI | 3902 | 7 | 15.13138 | 0.000815 | 8553 | 8553 |
| MRS | 3952 | 49 | 15.13302 | 0.001643 | 30,052 | 22,923 |
| DCE-MRI | 3984 | 32 | 15.12948 | −0.00354 | Dominated | Dominated |
| T2-MRI or MRS | 4031 | 80 | 15.13406 | 0.001038 | 76,676 | 38,882 |
| T2-MRI or DCE-MRI | 4056 | 25 | 15.13188 | −0.00218 | Dominated | 122,465 |
| Scenario 9. Disease progression calibrated to PC mortality rates observed in the PIVOT trial149 | ||||||
| Syst. TRUS | 3751 | – | 12.57939 | – | – | – |
| T2-MRI | 3758 | 7 | 12.57969 | 0.000303 | 23,054 | 23,054 |
| MRS | 3808 | 49 | 12.58026 | 0.000563 | 87,749 | 65,132 |
| DCE-MRI | 3840 | 32 | 12.57906 | −0.0012 | Dominated | Dominated |
| T2-MRI or MRS | 3887 | 80 | 12.58059 | 0.000328 | 242,585 | 113,923 |
| T2-MRI or DCE-MRI | 3913 | 25 | 12.57984 | −0.00074 | Dominated | 356,787 |
| Scenario 10. Use extended-cores biopsy for all patients negative on MRS/MRI | ||||||
| Syst. TRUS | 3895 | – | 12.48432 | – | – | – |
| T2-MRI | 4007 | 112 | 12.48747 | 0.003144 | 35,561 | 35,561 |
| MRS | 4087 | 80 | 12.48769 | 0.000219 | 362,957b | 56,913 |
| DCE-MRI | 4087 | 80 | 12.48783 | 0.000366 | 218,506 | 54,618 |
| T2-MRI or MRS | 4090 | 3 | 12.48721 | −0.00062 | Dominated | 67,370 |
| T2-MRI or DCE-MRI | 4090 | 3 | 12.48754 | −0.00029 | Dominated | 60,667 |
These analyses demonstrate that T2-MRI-directed biopsy dominates systematic TRUS/Bx under several scenarios; specifically, when the sensitivity and specificity of T2-MRI are set equal to the values obtained from the indirect comparison (see Table 38, scenario 3), and when it is assumed that T2-MRI direction reduces the cost of the subsequent biopsy procedure relative to the cost of an extended-cores biopsy (scenario 4). The ICER for MRS compared with systematic TRUS also remains at < £30,000 for most scenarios. The cost-effectiveness of MRS compared with T2-MRI appears less sensitive to scenarios presented in Table 38. The ICER for MRS-directed biopsy (relative to T2-MRI) falls below £30,000 when (1) the cost of all biopsies is increased (scenario 1); (2) when it is assumed that MRS misses only low-risk cancer (scenario 2); and (3) when the discount rate applied to health benefits is reduced to 1.5% (scenario 7). Although increasing the costs of systematic extended-cores TRUS/Bx (but not MRI-/MRS-directed TRUS/Bx) results in both T2-MRI and MRS being more effective and less costly than systematic TRUS/Bx, this specific scenario has little impact on the comparison between MRS and T2-MRI. Application of the sensitivity/specificity estimates obtained from the indirect comparison also undermines the cost-effectiveness of MRS in relation to T2-MRI, owing to a substantial decline in the specificity of MRS.
The application of lower relative diagnosis/treatment effects and lower baseline cancer progression rates (scenario 8) undermine the cost-effectiveness of MRS compared with T2-MRI and systematic TRUS/Bx. The incremental cost per QALY gained with the most sensitive imaging strategy (positive on either T2-MRI or MRS) does not fall below £30,000 for any of the scenarios assessed. Further, none of the MRI strategies compares very favourably in terms of cost-effectiveness when it is assumed that all patients who are negative on MRI proceed to an extended-cores TRUS/Bx regardless (scenario 9).
In addition to the scenarios presented in Table 38, threshold analysis would suggests that MRS would dominate systematic extended-cores TRUS/Bx in contexts where the cost saving per biopsy averted is ≥ ∼£115 more than the cost of obtaining the MRS sequences to determine whether or not a biopsy should proceed.
Probabilistic sensitivity analysis
To assess the impact of joint uncertainty surrounding all model parameters and inputs, appropriate distributions were assigned and randomly sampled for each of 1000 iterations of the base-case analysis. The results were used to estimate the probability of each diagnostic strategy being preferred on grounds of cost-effectiveness for different values of decision-makers' willingness to pay for a QALY. The resultant CEAC is displayed in Figure 27. The results indicate that under base-case parameter values and assumptions, none of the strategies demonstrates a high probability of being the preferred option at the threshold value of £30,000 per QALY gained. The cost-effectiveness acceptability frontier (Figure 28) displays the probability of the strategy with the highest NMB, at different values of decision-makers’ willingness to pay per QALY gained, being cost-effective. At a threshold ratio of £30,000 per QALY, T2-MRI provides the option with the highest NMB but with only a 34% probability. The expected value of perfect information at this threshold (i.e. of eliminating uncertainty) is £27.30 per patient.
FIGURE 27.
Cost-effectiveness acceptability curves under base-case assumptions.
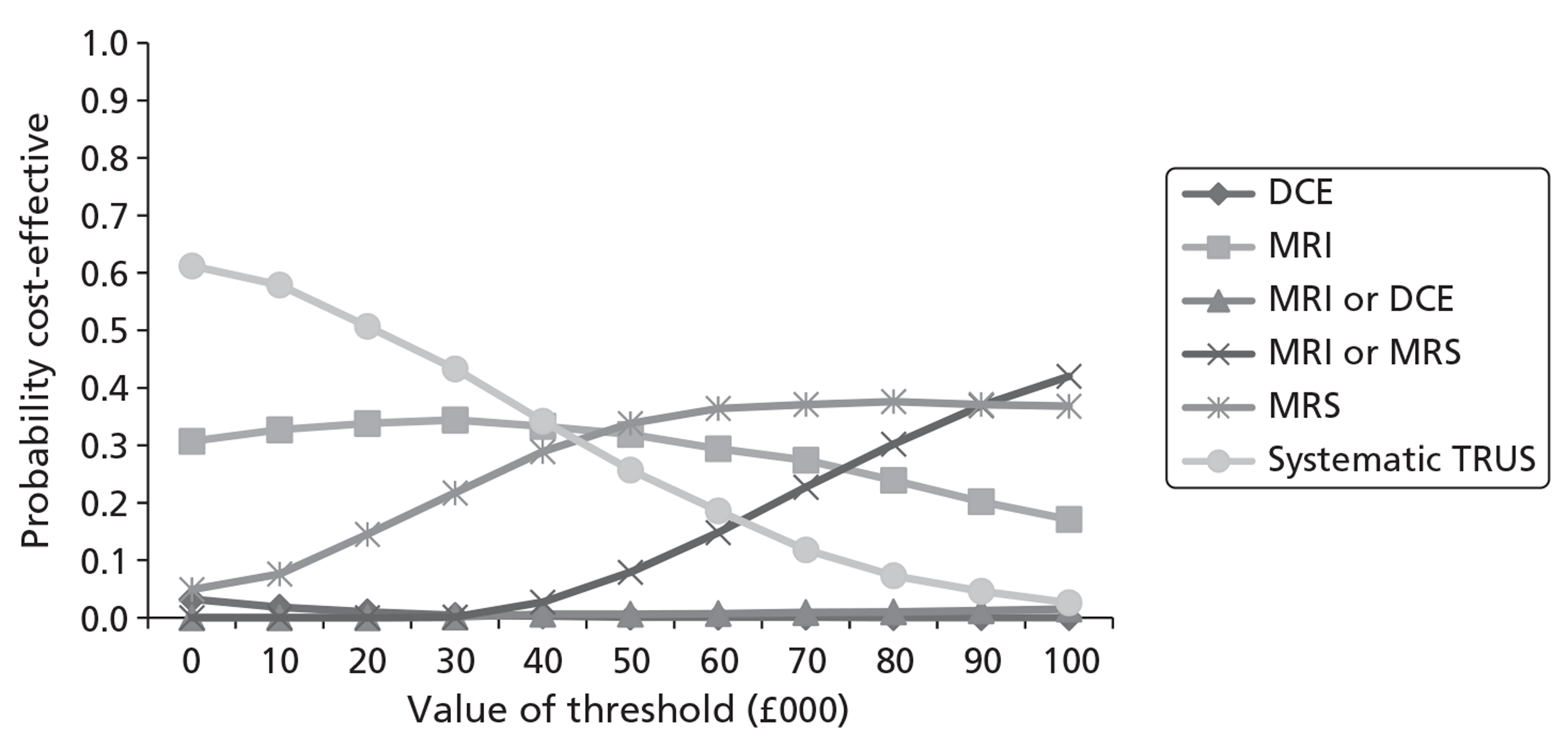
FIGURE 28.
Cost-effectiveness acceptability frontier under base-case assumptions.
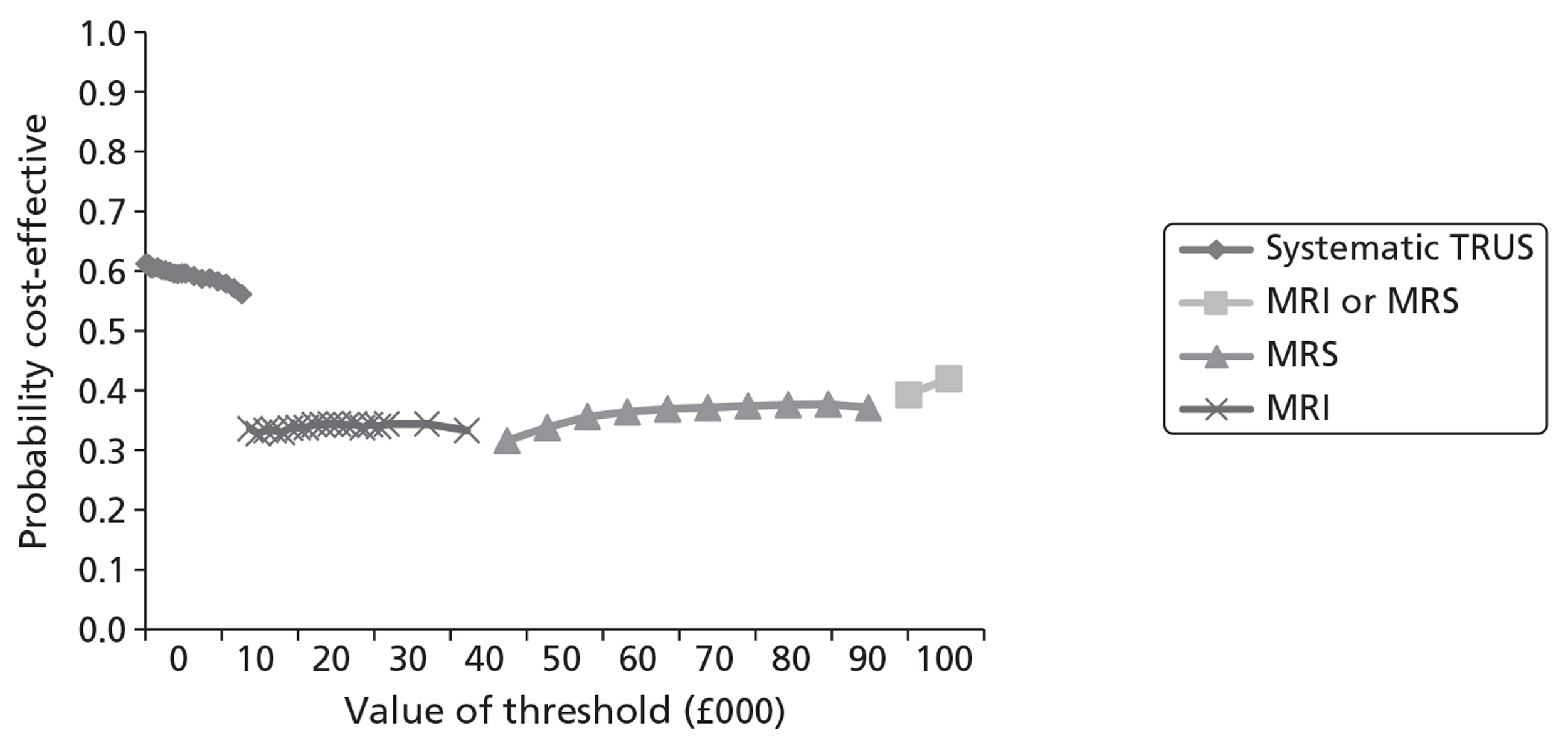
In order to assess the sensitivity of the probabilistic analysis findings to several base-case assumptions, the analysis was repeated with the biopsy costs inflated to account for the possibility of higher pathology time requirements and the MRS/MRI costs were adjusted to the reference cost for direct access DCE-MRI (see Table 38, scenario 1). Further, DW-MRI was incorporated under the assumption that it could achieve sensitivity equal to that of MRS and specificity equal to that of T2-MRI. In addition, it was assumed that MRS and DW-MRI would miss only low-risk cancer (see Table 38, scenario 2). Under this specification, there is 74% probability of either DW-MRI or MRS being cost-effective at a willingness-to-pay threshold of £30,000 per QALY gained (Figure 29). MRS in fact retains the higher probability of being cost-effective in this scenario because, with biopsy costs set at a higher level, the superior specificity of MRS (over the assumed specificity of DW-MRI, which was set at 55% purely for illustrative purposes) outweighs the additional cost of running the sequence.
FIGURE 29.
Cost-effectiveness acceptability curves, assuming DW-MRI has sensitivity equal to that of MRS and specificity equal to that of T2-MRI, and that MRS and DW-MRI miss only low-risk cancer.
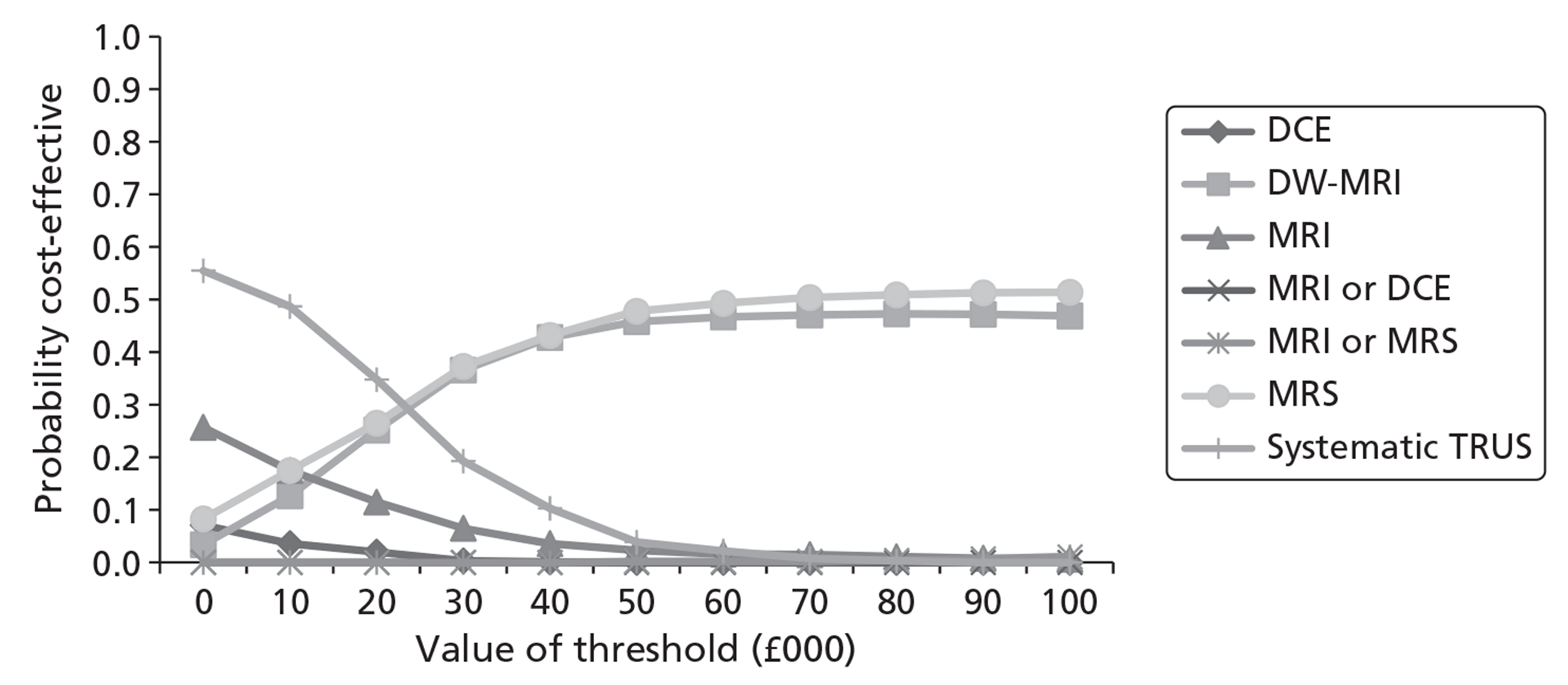
Discussion
Summary of key results
The results of the deterministic economic modelling suggest that, when considering LYs as the unit of outcome, the use of T2-MRI, to determine and direct biopsies, may be cost-effective in comparison with systematic TRUS-guided extended-cores biopsy. This results from its modest implementation cost and slightly improved sensitivity over systematic extended-cores TRUS/Bx (14–16 cores). At the same time its specificity would suggest that it could avert the need for 55% of patients without cancer having to undergo a biopsy. The base-case incremental cost per QALY estimates for the more sensitive enhanced MRS/MRI techniques are somewhat less favourable (i.e. are > £30,000 per QALY gained in comparison with the next less costly option). However, the ICER for MRS compared with T2-MRI does fall below £30,000 in the high-prevalence (50%) 60-year-old cohort. In the lower-prevalence (10%) cohorts, T2-MRI was found to dominate systematic TRUS/Bx (i.e. be less costly and more effective) owing to its specificity, resulting in more biopsies being averted in this group.
Moreover, the deterministic sensitivity analysis shows the cost-effectiveness of MRS compared with T2-MRI (and systematic TRUS/Bx) to be particularly sensitive to two key parameters. The ICER for MRS falls below £30,000 when (1) the cost of biopsies is increased to £298 and (2) when MRS is modelled to detect all moderate- and high-risk cancer (only missing low-risk disease). The latter assumption is in keeping with data from case series, which suggest high levels of correlation between MRS positivity and tumour Gleason scores. 52,54 The cost-effectiveness of MRS also improves considerably when the accuracy of and compliance with subsequent repeat biopsies decreases. Thus, our findings would suggest that the use of MRS may well be cost-effective in certain contexts, for example in settings where the cost of TRUS/Bx exceeds the cost of obtaining a MRS sequence by ∼£115. It is likely that practice and costs will vary substantially locally.
Unfortunately, there were insufficient data on the diagnostic accuracy of DW-MRI in the population of interest, and as such we did not include it as a comparator in the base-case analysis. With its lower cost in comparison with MRS, our modelling suggests it could represent a cost-effective approach if it could be shown to have sensitivity similar to that of MRS and specificity similar to that of T2-MRI. At these levels of sensitivity/specificity, however, it might also need to be able to discriminate between low-risk and moderate/high-risk disease (so that false-negatives would be concentrated in the low-risk cases) to be cost-effective in comparison with T2-MRI alone. Evidence from other case series suggest it may be able to do this. 169,170
By the same token, changes to some of the model assumptions also undermine the cost-effectiveness of strategies that improve cancer detection rates at increased costs over standard practice and T2-MRI. These include reductions in the baseline risk of disease progression and reductions in the relative risk reductions associated with diagnosis and treatment.
Considering the probabilistic sensitivity analysis, when parameter distributions are centred on the base-case point estimates and all of the strategies are compared simultaneously, none of the strategies achieves a high probability of being preferred on grounds of cost-effectiveness (see Figure 27) at the threshold ceiling ratio of £30,000 per QALY gained. Although the point estimate of the ICER of T2-MRI compared with systematic TRUS/Bx is favourable, its probability of being cost-effective at £30,000 per QALY is only ∼60% in comparison with TRUS/Bx and only 34% considering all strategies simultaneously (see Figure 28).
However, Figure 29 demonstrates how the uncertainty surrounding the relative cost-effectiveness of strategies reduces if the average cost of biopsies is increased to £300 (the upper quartile reference cost reported for biopsies carried out in an outpatient setting) and MRS and DW-MRI are modelled to miss low-risk cancer only. Under this alternative model specification, the choice between strategies becomes one between MRS and DW-MRI above a willingness-to-pay threshold of ∼£20,000 per QALY gained. Note this still assumes disease progression rates and relative diagnosis/treatment effects in line with Bill-Axelson et al. 148
Generalisability of results
In developing the model we have attempted to use data applicable to the UK setting as far as possible. However, many of the data on disease progression and relative treatment effects were derived from a European cohort identified in the pre-PSA era. 148 Although we have tried as far as possible to model the risk of progression by clinical grade (low, intermediate, high), it is possible that the progression rates observed for low-risk patients in this pre-PSA cohort are higher than would be observed for low-risk patients identified in clinical practice in the UK today. This is potentially important as reducing progression rates and treatment effects for low-risk patients reduces the cost-effectiveness of more sensitive and more costly diagnostic strategies, and further emphasises the potential importance of further research to assess the sensitivity of alternative imaging sequences by tumour grade in cohorts of patients undergoing biopsy. The ongoing PROMIS trial may help address this question. 171
The modelling also relied on health-state utility data from a number of sources outwith the UK. Attempts were made to identify EQ-5D data from UK cohorts for the modelled states and treatment complications of interest, but limited data were identified. A decision was made to use the EQ-5D utilities observed for a European cohort post diagnosis and at varying time points post treatment with radical prostatectomy or EBRT. As these average utilities were obtained from cohorts with proportions experiencing complications, we chose not to further adjust utility for modelled treatment complications in the base-case analysis. Given a lack of EQ-5D-based utility estimates for metastatic and castration resistant metastatic disease, for men modelled to progress to metastatic disease we applied the average time trade-off values elicited for these states from a sample of US men. 166
Although there is uncertainty surrounding the wider applicability of some of the progression rates, treatment effects and utility values applied in the model, the cost inputs were based on the estimation of resource use in UK settings according to current guidelines. National average unit costs were applied to resource-use estimates wherever possible, making individual cost inputs generalisable across the UK. However, there is uncertainty as to how hospitals are reimbursed for TRUS-guided biopsies at the local level and also the true opportunity cost of TRUS/Bx compared with that of running MRS/MRI sequences. It would be useful if future prospective studies could estimate these more accurately using patient-level data.
Strengths and limitations of the analysis
Strengths
Attempts have been made to use the best available evidence to model both the diagnostic pathways and subsequent treatment pathways and outcomes. The model provides a flexible framework allowing the comparison of many different diagnostic strategies in the context of the patient's lifetime. It captures the trade-off between the increased upfront costs of imaging and the reduction in subsequent biopsies, and also the trade-off between earlier diagnosis and potential utility decrements associated with treatment side-effects. Further, the model is risk stratified to allow for comparison of strategies that vary in their ability to differentiate between tumours of different stage and grade. The model can easily be updated to incorporate new, more detailed evidence as it becomes available.
Limitations
The modelling was hampered by limited availability of data on the diagnostic accuracy of alternative diagnostic strategies, comparable cost estimates for alternative procedures, the natural history of cancer detected at repeat biopsy, and also the impact of diagnosis and treatment on disease progression.
The systematic review uncovered limited data on the relative sensitivity (cancer detection rates) of MRI-/MRS-targeted biopsy techniques and systematic TRUS-guided approaches. In particular, there were no identified studies providing head-to-head comparisons of MRI-/MRS-targeted approaches and systematic sampling schemes based on different numbers of cores. Further, it was not possible to obtain pooled estimates for the sensitivity/specificity of alternative MRS/MRI sequences by grade of tumour, which is potentially an important parameter for informing cost-effectiveness. There is therefore a need for a large comparative study assessing the sensitivity of systematic approaches and MRS/MRI sequences for detecting cancer by D'Amico risk strata.
A high degree of uncertainty also exists regarding the impact of a false-negative result at repeat biopsy on the time to final diagnosis, and also on the impact of any delay on disease progression. The base-case analysis relied on the assumption that all patients experience a relative risk reduction for progression to metastatic disease upon referral, but recent data suggest that risk reductions associated with radical treatment for low-risk patients (and even moderate-risk patients) may be small and insignificant. 149 If this is the case it will undermine the cost-effectiveness of strategies that increase cancer detection rates over standard practice.
The more sensitive and more costly enhanced imaging techniques were found to be associated with small cost increases, for even smaller survival and QALY gains compared with T2-MRI and systematic TRUS/Bx. This results in the incremental cost per QALY ratios being very sensitive to the baseline risks of progression and relative treatment effects. Recent data suggest that the underlying risk of progression for low-risk cancer, identified in the PSA era, may be lower than reported for the low-risk subgroup identified by Bill-Axelson et al. 148 However, the above point also highlights the potential benefit of utilising a pre-biopsy imaging test that could differentiate between low-, moderate- and high-risk cancer, so that only those patients in the latter categories could be targeted for biopsy. Our modelling suggests that if DW-MRI or MRS could be shown to provide such discrimination in the cohort of patients with elevated PSA but previously negative biopsy, these tests could achieve levels of cost-effectiveness considered acceptable.
More detailed studies are required to assess the diagnostic accuracy of different sequences by stage/grade of cancer in order to address this question.
Finally, a further issue contributing to the uncertainty surrounding the cost-effectiveness of alternative diagnostic approaches, and indeed the cost-effectiveness of radical treatment,9 is the impact of treatment on health-related QoL. In our base-case specification we have applied utility values that suggest that radical treatment complications do not impact heavily on health-related QoL as measured by the EQ-5D. 163 However, the incremental cost per QALY findings are highly sensitive to changes to the applied utility assumptions. When health-state utilities are adjusted downwards for those patients experiencing treatment complications, the cost-effectiveness of strategies that improve cancer detection rates decreases substantially. This highlights the potential importance of risk stratifying treatment appropriately, so that only those patients likely to experience a significant survival benefit receive radical treatments. However, current data suggest that a substantial proportion of low-risk patients still elect for radical treatment, which undermines the cost-effectiveness of diagnostic strategies that result in more low-risk patients being diagnosed.
Summary/conclusions
To summarise, the level of uncertainty surrounding model inputs and structural assumptions makes it difficult to arrive at a definitive conclusion on the cost-effectiveness of using MRS/MRI techniques to aid the localisation of prostate abnormalities for biopsy. However, our modelling shows that under certain circumstances T2-MRI may be considered cost-effective in comparison with TRUS/Bx, and if MRS and DW-MRI can be shown to have high sensitivity for picking up moderate/high-risk cancer, while negating the need for patients with no cancer or low-risk disease to undergo biopsy, then their use could represent a cost-effective approach to diagnosis. Data from subgroup analysis would also suggest that the use of more sensitive and more expensive sequences is more likely to be cost-effective in subgroups of patients who are more likely to be harbouring cancer. Future research should focus on generating comparable estimates of (1) the sensitivity of MRI-/MRS-directed and systematic approaches to TRUS/Bx; (2) the sensitivity/specificity of MRS/MRI sequences for detecting different grades of localised disease in the repeat biopsy cohort; and (3) the full economic costs of MRI sequences and systematic approaches to TRUS/Bx based on different numbers of cores.
Chapter 6 Assessment of factors relevant to the NHS and other parties
The introduction of MRS and other MRI techniques (T2-MRI, DCE-MRI, DW-MRI) for evaluation of men with a TRUS-guided negative biopsy but in whom there remains suspicion of cancer would have a range of implications for the NHS, patients and other parties. These arise primarily because of a shift in the test–treatment pathway for this group. This shift is caused by changes in the method of making the diagnosis and changes the options and timings of treatments, complications and outcomes of patients. There are consequential effects on service delivery, health-care professionals and wider society.
Factors relevant to the NHS
Increased sensitivity and specificity of the diagnostic pathway would lead to more patients being correctly diagnosed with PC and diagnosed at an earlier stage and fewer patients wrongly diagnosed as having no cancer. This would result in a shift in the numbers and stages of patients diagnosed with PC. For the NHS this change in distribution of disease would have implications for service configuration, cost and training.
Service reconfiguration, including the purchase of high-end MRI scanners, would be needed to ensure that radiology departments have sufficient capacity and the means to offer high-quality MRI testing within adequate timescales. Local diagnostic pathways would require updating to ensure compliance with national targets because of the persistent suspicion of undiagnosed cancers in these subjects. Occasional local disruptions may occur if MRI equipment suffers technical failures although most scanners have up-times of > 95%.
The requirements of urological and/or radiological services to undertake targeted biopsies of MRS/MRI-suspicious regions will reduce the number of patients undergoing repeat biopsies. However, there would need to be extra provision for undertaking targeted biopsies, whether MRS/MRI guided (within MRI scanners under direct visualisation as an outpatient procedure) or MRI/MRS directed (outside MRI scanner using MRS/MRI information fused to ultrasound images). The latter could be undertaken as outpatient procedures if only a few targeted biopsies were undertaken (per rectally) or an inpatient procedure if the template transperineal route was chosen. MRI-/MRS-targeted or -directed biopsy will require the purchase of a new generation of hardware equipment and software to enable accurate biopsies to be obtained from all regions of the prostate (particularly from commonly missed anterioapical areas). This equipment is capable of operating within MRI scanners or can be used for biopsy via the transperineal or transrectal routes and is beginning to appear in the marketplace. In the future, the move towards targeted biopsy may reduce the number of biopsy cores taken per biopsy session (approximately two to five cores per session) and this will likely result in cost savings especially when compared with saturation or template biopsies (often 20–30 cores per session).
Earlier positive diagnosis of patients who would also be more accurately staged and whose risk stratification was more definitively known would have the benefit of them being appropriately triaged to several therapy options. It is likely that MRS/MRI will identify more patients with localised disease with intermediate and high risk of progression and this would increase the number of patients who would be eligible for radical therapies (including prostatectomy, brachytherapy and external beam therapy). Therefore, surgical and radiation therapy services may require more resources. Furthermore, MRI would provide more accurate preoperative imaging, which may alter the type of radical therapy being undertaken (e.g. the decision to resect or preserve neurovascular bundles at surgery). Preoperative imaging might prevent positive resection margins around large tumours and might also prevent unnecessary morbidity by predicting the preservation of neurovascular bundles if cancer foci locations permitted this. MRS/MRI may identify fewer patients with low risk of progression, which would help the problem of over-diagnosis of indolent PCs. More accurate and confident diagnoses of patients with low risk of progression disease will increase the proportion of patients likely to undergo ‘active surveillance’ programmes. This would have implications for the follow-up of these patients including increased utilisation of repeated PSA-testing, repeated interval biopsies and follow-up clinics (much of this work could be nurse practitioner led). Taken together, earlier, more accurate diagnoses and treatment of PC may improve survival and reduce the requirements on end-of-life and palliative care services.
There will be cost implications of these service reconfigurations and for changes in treatment patterns mentioned above. There will be significant cost implications for procuring and maintaining new MRI equipment. Although some centres may already have access to the high-end equipment required for this purpose, other centres may have to upgrade, purchase or rent new equipment because of access considerations. There are several other costs associated with implementing MRI testing, which are outlined in the cost-effectiveness chapter.
Implementation would result in the need for further training for radiology staff. Radiographers and radiologists would require additional training to ensure adequate technical skills for performing these tests and diagnostic skills to read the MRS/MRI scans. There is a learning curve effect for all staff when more sophisticated MRS/MRI techniques are being implemented; this is particularly true for MRS and DCE-MRI. Adequate quality control and quality assurance programmes would be needed in order to maintain high standards of data acquisition and reporting. However, these new skills and equipment would be transferable for future use in other PC subgroups (e.g. staging of known PC, therapy planning and suspected relapsed disease), and to other pathologies for which MRS/MRI are known to be useful.
Factors relevant to patients and other parties
Many men find themselves in the difficult situation of having a persistently raised serum PSA level but a negative biopsy. They and their physicians know that there is a substantial risk of an undiagnosed, perhaps life-threatening, PC that has not yet been found and as a consequence cannot be treated. This can cause anxiety for patients and their family. The anxiety and stress may substantially reduce an individual's QoL with many men seeking clarity about their status in order to be able to move forward. Increased certainty of diagnosis influences an individual's decision-making about life choices, such as employment, insurance and family issues. Any test that improves the diagnostic certainty in this group of men may reduce anxiety. Additionally, patients may feel more reassured if they have different tests that point to the same diagnosis. Although earlier diagnosis may reduce the anxiety of uncertainty, it may also cause psychological harm if effective treatments are unavailable or if the discovered cancer is indolent and unlikely to cause health deterioration over the course of an individual's life. DW-MRI and MRS may be better at detecting intermediate and high risk of progression cancers, which may have a positive effect in this regard. Active surveillance programmes for patients with indolent cancer types can have high dropout rates, partly because many patients find it difficult to have a diagnosis of cancer without commencing disease-limiting or curative treatment. If more evidence were to become available that allowed the discrimination between low-risk/less aggressive cancer and intermediate- and high-risk cancer, then urologists would not need to treat asymptomatic men with clinically insignificant cancer, curing them from a disease that might never have harmed them, with collateral morbidity as a result. Perhaps over time clinically insignificant PC may also come to be more accepted by the patient.
Magnetic resonance imaging techniques may reduce the number of patients undergoing several repeat biopsies, avoiding the discomfort, side effects and possible complications of this. Patients who have chosen not to undergo repeat biopsy, because of the unpleasantness of the procedure, may find MRI investigation more acceptable. However, some patients who suffer from claustrophobia may find MRI more unacceptable than repeat biopsy. Patient refusal to undergo MRI scanning is likely to decrease in the future with the increasing availability of wider, short-bore scanners. MRI cannot always be used in patients who have metallic foreign objects in their bodies or in those with implanted non-MRI compatible pacemakers.
Patients and carers may need to travel further to access appropriate MRI testing in scanners that have the appropriate high levels of sophistication capable of performing high-quality MRS/MRI (T2-MRI, DW-MRI and DCE-MRI) examinations. Inequalities in access may arise as services undergo reconfiguration at different rates, depending on pre-existing equipment age and capability as well as operator and interpretation expertise. If MRI technologies are not implemented across the NHS, income inequalities may arise as some patients seek investigations through private health-care companies. Private UK providers are already preparing to provide this service with some providing MRI detection only and others offering MRI detection and biopsy services. These patients may then re-present to the NHS for their further care.
Health professionals are likely to prefer the increased certainty, reproducibility and anatomic capability of diagnoses made with MRS/MRI. Subsequently, medical staff would be more confident about negative diagnoses, knowing that an intermediate or high risk of progression cancer is unlikely to be present.
Earlier treatment may result in greater medical and societal success and improved patient functioning [physiological (urinary, rectal, erectile) and psychological]. These may, in turn, reduce the requirements on end-of-life and social care.
Chapter 7 Discussion
Diagnostic accuracy
Statement of principal findings
The included diagnostic studies reported sensitivity, specificity or predictive values for the index tests of MRS, DCE-MRI and DW-MRI and the comparator tests of T2-MRI and TRUS against a reference standard of histopathological assessment of biopsied tissue. Studies that reported true/false-positive and true/false-negative results or provided information that allowed these data to be calculated were considered for inclusion in the pooled estimates (meta-analyses). Meta-analyses were performed at both patient and biopsy level. In addition to the meta-analyses models of the diagnostic accuracy of the individual tests, combinations of tests and also of six studies directly comparing MRS with T2-MRI, an indirect comparison of all tests was also undertaken.
In terms of methodological quality, the majority of the 39 full-text studies were considered to have a low risk of bias for the patient selection (29/39, 74%), index test (39/39, 100%) and flow and timing (36/39, 92%) domains. Three studies (8%) were considered at high risk of bias for the flow and timing domain. In the reference standard domain, 25 studies (64%) were considered at high risk of bias because of a lack of follow-up, and in 14 (36%) the risk of bias was considered unclear. In terms of the applicability of the studies to the review question, all studies had low concern for applicability for the reference standard domain, whereas the majority had low concern for applicability for the patient selection (37/39, 95%) and index test (34/39, 87%) domains.
Although biopsy-level analysis was reported by a number of studies, patient-level data are more useful in determining management, and more clinically relevant. Most studies took multiple biopsies from participants, leading to clustering within participants. We were unable to account for this clustering in the biopsy-level analysis and therefore estimates from the biopsy-level analysis will be to some extent artificially precise.
In the patient-level pooled estimates for the individual tests, although both sensitivity and specificity (95% CI) of MRS [92% (86% to 95%), 76% (61% to 87%)] were higher than that of T2-MRI [86% (74% to 93%), 55% (44% to 66%)], the difference was greater for specificity. However, the reverse was the case in the meta-analysis of the six studies that directly compared the two tests, where the sensitivity and specificity of MRS was 89% (95% CI 79% to 95%) and 71% (95% CI 51% to 85%) compared with 77% (95% CI 55% to 90%) and 68% (95% CI 59% to 75%) for T2-MRI, with the difference being greater for sensitivity. There was statistical evidence (p = 0.004) that accuracy varied with threshold for the direct comparison analysis of T2-MRI and MRS; however, to make inferences on how these two tests compared with each other would require the assumption that accuracy did not vary with threshold.
A sensitivity analysis for MRS and T2-MRI comparing the pooled estimates for earlier studies (pre 2007) with those published more recently (2007 onwards) found no significant differences between the two time periods for either test (see Appendices 9 and 10).
Combining the two tests so that a positive result for either was considered a positive result for the combination led to an increase in sensitivity [96% (95% CI 90% to 98%)] but at the expense of a large decrease in specificity [31% (95% CI 21% to 42%)]. In a meta-analysis of three studies reporting DCE-MRI, the pooled estimates for both sensitivity [79% (95% CI 69% to 87%)] and specificity [52% (95% CI 14% to 88%)] were lower than that reported for either MRS or T2-MRI. DW-MRI was reported only by one small study96 involving 43 patients, and only for sensitivity, although this was 100%. In pooled estimates for six studies reporting TRUS as an imaging test,57,75,80,82,93,111 sensitivity and specificity were 27% (95% CI 16% to 42%) and 81% (95% CI 77% to 85%), respectively.
Across six large-scale population screening studies81,88,97,99,103,113 that provided information on the performance of systematic biopsies using a TRUS-guided approach on a subset of their patient populations who had a previous negative biopsy (n = 5771) sensitivity was 72.5% (range 60.6% to 96.3%). Pepe et al. 97 reported that a median of 23 cores (range 20 to 38) were taken. 97 The other studies reported the number of cores to be taken as follows: four to six cores,88 at least four or six cores,103 six cores113 and eight cores. 81 Pinsky et al. 99 did not report the number of cores taken.
In the indirect comparison the highest sensitivity was reported for MRS at 93% (95% CI 87% to 97%), whereas the highest specificity was for TRUS (used as an imaging test) at 88% (95% CI 79% to 94%). The combination of tests that produced the highest sensitivity was for ‘T2-MRI or MRS’ at 97% (95% CI 91% to 99%), whereas the combination of tests that produced the highest specificity was for ‘T2-MRI and MRS’ at 73% (95% CI 58% to 85%). There was marginal evidence that accuracy varied with threshold in the indirect comparison model (p = 0.065); however, to make comparative inferences would require the assumption that accuracy did not vary with threshold.
For the estimates from the indirect comparison model comparing T2-MRI with other tests, in terms of relative sensitivity, the direction of effect favoured (1) MRS, (2) ‘T2-MRI or DCE’ and (3) ‘T2-MRI or MRS’ over T2-MRI, while favouring T2-MRI over (1) DCE-MRI, (2) ‘T2-MRI and MRS’ and (3) TRUS. However, the only results that were statistically significant were for ‘T2-MRI or MRS’ compared with T2-MRI (‘T2 or MRS’ better) and T2-MRI compared with TRUS (T2-MRI better). In terms of relative specificity the direction of effect favoured (1) MRS, (2) ‘T2-MRI and MRS’ and (3) TRUS over T2-MRI, while favouring T2-MRI over (1) DCE-MRI, (2) ‘T2-MRI or DCE-MRI’ and (3) ‘T2-MRI or MRS’. The only results that were statistically significant were for ‘T2-MRI and MRS’ compared with T2-MRI (‘T2-MRI and MRS’ better), T2-MRI compared with TRUS (TRUS better) and ‘T2-MRI or MRS’ compared with T2-MRI (T2-MRI better). However, it should be noted that in practice MRS is acquired in combination with T2-MRI and would not usually be interpreted without taking into account this information.
In summary, the evidence from patient-level pooled estimates suggests that MRS has higher sensitivity (92%) and specificity (76%) than T2-MRI (86%, 55%), while combining both tests so that when either is positive the combination is positive further increases sensitivity (96%) but at the expense of specificity (31%). DCE-MRI has lower sensitivity (79%) and specificity (52%) than either MRS or T2-MRI, although this was based on only three studies. 78,90,105 TRUS used as an imaging test has low sensitivity (27%) but high specificity (81%). Only one small study96 reported patient-level estimates for DW-MRI and only for sensitivity, which, however, was 100%.
Strengths and limitations of the assessment
In terms of strengths, a broad, robust literature search was undertaken with double screening of titles and double checking of data extraction. Risk of bias was assessed using a modified QUADAS-2 questionnaire, tailored to the needs of this review. A HSROC model was used, which takes account of the trade-off between true/false-positives and models between-study heterogeneity. 172 Pooled estimates were performed at both patient and biopsy level and an indirect comparison of tests was also undertaken. Homogeneity was improved by having a robust inclusion criterion for meta-analysis and indirect comparison, and by performing an additional analysis using only those studies that directly compared tests. Indirect comparison allows relative estimation of sensitivity and specificity for each comparison by including all tests in one model.
In terms of limitations, there was variation in the use of tests, methodology and reporting of included studies. We could not test for the effects of covariates such as the number of previous biopsies on the results because the data captured were not conducive for such analysis. Non-English-language studies were excluded. Few studies were identified reporting DCE-MRI or DW-MRI, and few studies included a period of follow-up as part of the reference standard, thereby potentially failing to identify a proportion of patients who might have been false-negative on the tests. The index and comparator tests were not independent of the reference standard (incorporation bias), as they provided the biopsy cores for the reference standard to assess for the presence or absence of disease.
Uncertainties
Dichotomising test results where studies reported an ‘equivocal’ category
Some studies reported suspicious results (test-positives), normal results (test-negatives) and a third category, neither positive nor negative, such as ‘equivocal’. In order to incorporate these results into a 2 × 2 table and for inclusion in meta-analyses they had to be dichotomised as either positive or negative. Our position was to class equivocal results along with positive results rather than with negative results. This approach increased sensitivity and decreased specificity, whereas the reverse would have been the case if equivocal results had been classed along with negative results. In Appendix 8, when studies have reported an ‘equivocal’ category, where possible, we have shown sensitivity and specificity both for equivocal cases classed with positive results and for equivocal cases classed with negative results. For example, in the study by Yuen et al. ,112 for MRS, when equivocal was classed as ‘suspicious’ sensitivity was 71.4% and specificity was 52.9%, whereas when equivocal was classed as ‘normal’ sensitivity was 57.1% and specificity was 82.4%.
False-positives
Eleven studies57,74,76,78,79,101,106,108,109,133,137 provided some additional information on the nature of their false-positive results (i.e. the test detected an abnormal area but the histopathological assessment of the biopsy cores taken from that area was negative for cancer). The false-positive rate for patient-level analysis (six studies74,76,79,101,108,137) ranged from 2.4% to 100% and for biopsy-level analysis (five studies57,78,106,109,133) ranged from 13.0% to 46.2%. High-grade PIN and prostatitis accounted for a substantial proportion of false-positives. One study presented this information separately for MRS and T2-MRI. 79 For MRS (11 false-positives), PIN accounted for six (54.5%), fibrosis for four (36.4%) and normal prostatic tissue for one (9.1%). For T2-MRI (13 false-positives) PIN accounted for three (23.0%), fibrosis for five (38.5%) and normal prostatic tissue for five (38.5%).
True-negatives
An extended TRUS/Bx procedure may miss cancers, as the transrectal approach renders sampling of apex tumours and anterior TZ tumours difficult. Using this as the approach to provide the biopsies for the reference standard has its limitations, especially if not combined with long-term follow-up. The number of true-negatives in these types of study could therefore potentially be lower than reported.
Detection of clinically significant disease
Using comprehensive (saturation) biopsy protocols based on TRUS/Bx may reduce the likelihood of missing cancers. However, although saturation biopsies may improve the detection rate of PC, solely increasing the number of biopsy cores may also lead to an increase in the detection of clinically insignificant disease. In addition, saturation biopsies have the disadvantage of possibly requiring anaesthesia and increasing the risk of adverse events. 105 On the other hand Scattoni et al. 173 reported that the detection rates of protocols including 20–38 cores ranged from 14% to 41% without significantly increasing the likelihood of detecting clinically insignificant cancers compared with initial or repeat biopsy.
One of the suggested advantages of MRS and other MRI techniques is the ability to detect clinically significant disease (Gleason score of ≥ 7). An explanation put forward for this in relation to MRS is that it is unable to detect the lowest grade of PC due to partial voluming of healthy surrounding tissue included in a spectroscopic voxel of spatial resolution 0.32 cm. 106
Twenty-nine studies74,76,78–84,86,87,90,91,95–98,102–106,108–113,136 reported the Gleason score based on the biopsy results of patients diagnosed with PC. Most studies reported a median Gleason score of ≥ 6 and in the one study96 reporting DW-MRI the median Gleason score was 7. Across six MRI studies reporting a median Gleason score of > 6,74,90,96,104,108,109 the percentage of patients with a Gleason score of ≥ 7 ranged from 50% to 66.7%. The limited evidence suggests that, potentially, a substantial number of cancers detected by MRS and other MRI techniques in patients with raised PSA levels and previous negative biopsy may be clinically significant (Gleason score of ≥ 7 and/or volume > 0.5 ml).
The experience of clinical experts (Anwar Padhani, Mount Vernon Cancer Centre, July 2012, personal communication) suggests that DW-MRI is capable of detecting more clinically significant disease than TRUS/Bx, and that cancers missed on DW-MRI that are detected on TRUS/Bx are generally not clinically significant. However, there is a lack of evidence on the detection of clinically significant disease by DW-MRI in the population of men with a previously negative biopsy who still have raised PSA levels and a continuing suspicion for cancer. There is some evidence on the ability of DW-MRI to detect clinically significant disease in the wider population of men with PC; for example, Hambrock et al. 64 undertook DW-MRI of 51 patients before prostatectomy and found that the median ADC in the tumours was negatively correlated with Gleason score in the PZ of the prostate. However, further research is needed to assess the extent to which this finding applies to men with suspected PC but a previously negative biopsy. In the TZ, although there is a small significant difference in ADC between Gleason 3 + 3 and Gleason 4 + 4 cancers, the overlap in ADC between the two cancer groups is so large that discrimination on an individual level is not possible (Tom Scheenen, Radboud University Nijmegen Medical Centre, July 2012, personal communication).
Cancer detection in the transition zone by magnetic resonance spectroscopy and other magnetic resonance imaging techniques
Hoeks et al. 87 commented that MRS had problems imaging the TZ and also noted that different choline–creatine ratios were needed for cut-offs for a positive test result for the PZ and TZ. The authors stated that their relatively high detection rates of PC in the TZ in men with one or more negative TRUS-guided biopsies agreed with the results of Hambrock et al. 86 (57% in the TZ). However, they noted that, in other reports by Roethke et al. 104 and Franiel et al. ,84 TZ cancer detection rates (47% and 35%, respectively) were lower than PZ cancer detection rates (53% and 64%, respectively). Testa et al. ,106 in a region-based analysis, also reported sensitivity and specificity separately for the PZ and TZ for MRS and T2-MRI. For the PZ, MRS sensitivity and specificity were 64.9% and 85.8%, respectively, compared with 72.2% and 93.3% for the TZ. T2-MRI sensitivity and specificity for the PZ were 27.0% and 95.8% respectively, compared with 61.1% and 98.9% for the TZ.
Heterogeneity across the studies
Across the studies, the prevalence of PC ranged from 9.5%76 to 100%. 117 The original biopsy scheme used will influence the prevalence of PC in patients with raised PSA and previously negative biopsy; the more cores taken during the original biopsy scheme(s), the more cancers are likely to be detected at this stage and consequently the prevalence of cancer in subsequent biopsies will be lower. The previous biopsy schemes reported by the studies ranged from four or six core57 to 12 core. 74,78,93,116 The number of previous biopsy sessions reported by the studies for their patient populations ranged from 1 to 9, with different numbers of sessions occurring within studies as well as across studies.
Transrectal ultrasonography: imaging compared with obtaining biopsies
Transrectal ultrasonography is used to either visualise the prostate in order to obtain a systematic, predefined biopsy (TRUS/Bx) or inspect the prostate for evidence of cancer and biopsy highly suspicious areas. Based on advice from clinical experts, the most common use for TRUS in the NHS is to obtain a systematic, predefined biopsy. Therefore, TRUS sensitivity could mean:
-
The proportion of patients with prostate cancer correctly identified on systematic predefined biopsy This is complicated by the fact that TRUS is not independent from the reference standard, as discussed elsewhere. Therefore, to obtain the false-negatives necessary to calculate sensitivity, at least one repeat biopsy is needed. In studies that use this method, low-risk patients do not usually undergo repeat biopsies. This can lead to a falsely high sensitivity, as it assumes that all patients who did not undergo a biopsy did not have cancer. Furthermore, if there is a considerable interval between biopsies, there is the potential that an individual may have developed cancer in the intervening period.
Alternatively, TRUS sensitivity could mean:
-
The proportion of patients with prostate cancer correctly identified when TRUS is used to identify suspicious lesions This scenario is complicated because often both systematic and targeted biopsies are taken and in reported studies is it unclear if the sensitivity refers to the combination or just the targeted lesions.
Systematic biopsies used in conjunction with magnetic resonance spectroscopy and other magnetic resonance imaging techniques
In studies reporting the sensitivity and specificity of MRS or other MRI techniques, a number of cores were targeted for biopsy, based on suspicious areas identified by the imaging test and a systematic (generally 10- or 12-core) TRUS/Bx was also undertaken. In most studies it was unclear how the results from the systematic biopsy contributed to the sensitivity and specificity values reported for the imaging technique, i.e. it was unclear whether the sensitivity and specificity reported were for the imaging test alone or the imaging test plus the systematic biopsies.
Subsidiary questions from the protocol
There was insufficient information from the included studies to address the subsidiary questions of (1) identifying specific patient groups in which MRS and other MRI techniques are most clinically effective; (2) whether or not these techniques can identify cases in which PC is present but further procedures are unnecessary and (3) evidence of use of MRS and other MRI techniques leading to a change in patient management.
Other relevant factors
Ongoing studies
The search strategy identified a few ongoing studies, although none focused on our population of interest – men with suspected PC and elevated PSA level but previously negative biopsy. The largest of the ongoing studies identified was the UK multicentre study ‘PROstate MRI Imaging Study: evaluation of multiparametric magnetic imaging (MP-MRI) in the diagnosis and characterisation of PC (PROMIS)’ that was anticipated to start in March 2012 and end in April 2015. 171 The objectives of this study are to (1) determine the ability of MP-MRI to identify men who can safely avoid biopsy; (2) assess the ability of the MP-MRI-based diagnostic pathway to improve the rate of detection of clinically significant cancer compared with TRUS/Bx; and (3) estimate the cost-effectiveness of an MP-MRI-based diagnostic pathway. The study design is described as a prospective validating paired cohort study. Participant inclusion criteria are men who (1) are aged ≥ 18 years who are at risk of PC and have been advised to have a prostate biopsy; (2) have a PSA level value of ≤ 15 ng/ml in the last 3 months; (3) have suspected stage of ≤ T2 on rectal examination (organ confined); and (4) are fit for general/spinal anaesthesia and all study procedures. Exclusion criteria include a previous history of prostate biopsy. The intervention is MP-MRI scan and combined prostate biopsy procedure (template prostate mapping biopsy) followed by TRUS/Bx. The primary outcome measures are (1) proportion of men who could safely avoid biopsy; (2) proportion of men correctly identified by MP-MRI to have clinically significant PC; and (3) primary definition of cancer according to biopsy: dominant Gleason pattern of ≥ 4 and/or cancer core length of ≥ 6 mm.
Another ongoing study is the ‘Prostate Cancer Localization With a Multiparametric Magnetic Resonance (MR) Approach’. 174 This is a prospective, observational, international, multicentre study that started in June 2010. Its primary objective is to prove the diagnostic accuracy of in vivo 3-T multimodality MRI (high-resolution T2-MRI, DCE-MRI, MRS and DW-MRI techniques) in distinguishing carcinoma from other prostate tissue. Specific objectives include (1) determining the diagnostic accuracy of 3-T multimodality non-endorectal coil MR imaging in localising PC and (2) proving that multimodality MR data allow for predicting tumour grade. Inclusion criteria include men with biopsy-proven diagnosis of PC in whom radical prostatectomy and histopathological examination are planned.
Comparison of our results with other systematic reviews
Our searches identified four other systematic reviews70,175–177 that assessed MRI techniques for detecting PC in men, although only the review by Lawrentschuk and Fleshner176 focused on men with previous negative biopsies and elevated PSA levels.
Umbehr et al. 70 undertook a systematic review and meta-analysis of MRI combined with MRS in the diagnosis of PC. Thirty-one studies were included, seven of which recruited participants with a previous negative biopsy. Six74,76,79,100,101,112 of these seven studies74,76,79,100,101,112,178 were included in our review; the seventh178 was excluded as the participants did not have a previous negative biopsy or this was unclear. The authors performed a meta-analysis of seven studies57,74,76,79,100,101,112 examining patients with suspected PC and found a sensitivity of 82% (95% CI 59% to 95%) and specificity of 88% (95% CI 80% to 95%). However, in this meta-analysis only four74,76,79,112 of the seven studies57,74,76,79,100,101,112 involved men with a previously negative biopsy.
Lawrentschuk and Fleshner176 undertook a review of studies of MRI or MRS which recruited participants with a previous negative biopsy and persistently elevated PSA. Six studies were included, five of which were included in our review;57,74,79,101,112 the sixth179 was excluded as the test used was outwith our inclusion criteria. The authors did not statistically pool the results, but rather narratively presented each study. For MRI or combined MRI and MRS, they reported a sensitivity of 57% to 100% and a specificity of 44% to 96%. The authors found that 54% of patients (34/63) were diagnosed with cancer solely on the basis of a MRI-targeted biopsy.
Wang et al. 177 undertook a meta-analysis of PC studies that used MRS as a diagnostic tool. The inclusion criteria were not limited to men with suspected PC and previously negative biopsy. Seven studies were included, of which two were included in our review;101,112 the remaining five18,51,53,180,181 were excluded as the types of participants were outwith our inclusion criteria. The authors reported the sensitivity and specificity of MRS using a CC/C ratio cut-off of 0.75 as 82% and 68% respectively, and with a cut-off of 0.86 as 64% and 86%, respectively.
Engelbrecht et al. 175 performed a systematic review of local staging of PC using MRI. The authors included 76 studies and calculated the area under the ROC curve using trapezium methodology. It was not reported how many of these studies included participants with a previous negative biopsy and raised PSA and the list of included studies was not included in the list of references. On the ROC curve the joint maximum sensitivity and specificity of MRI was at 71%; however, the authors found unexplained heterogeneity throughout the results.
Future technological developments
Magnetic resonance-guided biopsies are not usually carried out in the UK, resulting in challenges in ensuring the correspondence of TRUS/Bx spatial accuracies to suspicious areas identified by MRS/MRI. Hoeks et al. 87 stated that the clinical use of MR-guided biopsies was currently restricted by limited availability and long procedure times. They commented that the application of MRI–ultrasound fusion techniques, needle-guided tracking sequences, and implementation of robotics may improve these drawbacks in the near future and that, when these issues were resolved, multiparametric-MRI- and MR-guided biopsies could be applied on a larger scale for PC detection in patients with an elevated PSA level and one or more negative TRUS/Bx sessions.
Cost-effectiveness
Statement of principal findings
The economic modelling found the cost-effectiveness of strategies to be highly sensitive to a number of key parameters and assumptions, as well as context. Our findings suggest that when the average cost of TRUS/Bx is ∼£115 greater than the cost of obtaining a T2-MRI + MRS sequence (i.e. ∼£300 per patient when holding the base-case T2-MRI + MRS cost estimate constant) then T2-MRI- and MRS-directed approaches dominate the systematic extended-cores approach in both 60- and 70-year-old cohorts (with cancer prevalence at 24%). In addition, the ICER for MRS compared with T2-MRI falls to < £30,000 in the 60-year-old cohort (although not in the 70-year-old cohort). Such a difference in costs between TRUS/Bx and obtaining a MRS/MRI scan might be expected in hospitals where a significant proportion of biopsies are carried out as day-case activity, or if the average outpatient HRG cost significantly underestimates histopathology costs, as some personal communication suggests.
In both the 60- and 70-year-old low-prevalence cohorts, T2-MRI and MRS again dominate extended-cores TRUS/Bx when the cost of biopsies is inflated to £300, although MRS does not achieve an ICER of < £30,000 in either age group. In the high-prevalence cohorts, this biopsy cost increase results in MRS having an ICER of ∼£22,000 per QALY in 60-year-old men, whereas the ICER remains above £30,000 per QALY in 70-year-old men.
Under the assumption that all index repeat biopsies are carried out as outpatient procedures, at the lower cost of £212, we found the following:
-
The use of T2-MRI (for directing TRUS/Bx) may be considered cost-effective in comparison with systematic TRUS-guided extended-cores biopsy (60-year-old cohort with prevalence at 24%), but there is a high degree of uncertainty at the £30,000-per-QALY ceiling ratio.
-
T2-MRI dominates extended TRUS/Bx in cohorts in which the prevalence of cancer is low (10%).
-
MRS is borderline cost-effective in comparison with systematic TRUS/Bx in the 60-year-old cohort with prevalence set at 24%, and its cost-effectiveness improves in the high-prevalence 60-year-old cohort. The ICER for MRS compared with systematic TRUS/Bx remains > £30,000 for all 70-year old cohorts.
-
The ICER for MRS compared with T2-MRI is above £30,000 in both the low and the moderate prevalence 60- and 70-year-old cohorts but < £30,000 for the high-prevalence 60-year-old cohort.
-
The findings for point 4 (above) hold when it is assumed that MRI-/MRS-directed biopsies require fewer cores and so are carried out as outpatient procedures, whereas systematic extended-cores biopsies are carried out at higher cost.
-
Threshold cost analysis shows that when the cost of biopsy is on average ∼£90 higher than the cost of obtaining an MRS sequence, the ICER for MRS falls to < £30,000 per QALY compared with T2-MRI (60-year-old cohort, cancer prevalence 24%).
When applying the lower outpatient costs to biopsies, the cost-effectiveness of MRS compared with T2-MRI was found to be particularly sensitive to the ability of MRS to discriminate between low-, moderate- and high-risk cancer. Applying the assumption that MRS detects all moderate- and high-risk disease (with false-negatives concentrated in the low-risk group) the ICER for MRS fell to < £30,000. By the same token, reducing the baseline risk of disease progression, and applying lower relative risk reductions for diagnosis and treatment, undermined the cost-effectiveness of MRS.
A probabilistic sensitivity analysis was carried out for the 60-year-old cohort, applying the lower outpatient procedure cost to all index repeat biopsies (MRS/MRI-targeted and non-targeted), with all other parameter distributions centred on their base-case point estimates. Under this scenario, none of the strategies showed a high probability of being preferred on grounds of cost-effectiveness (see Figure 28) at a threshold willingness-to-pay ratio of £30,000 per QALY gained. However, increasing the average index repeat biopsy cost (to £298, as described above) and adjusting the sensitivity of MRS by underlying grade of disease demonstrated how these changes would give rise to a higher probability (∼57%) of MRS being cost-effective at the £30,000-per-QALY threshold (assuming that base-case progression rates and diagnoses/treatment effects hold). (Note: the 57% is calculated from the data behind Figure 29.)
Finally, while we were unable to accurately assess the diagnostic accuracy of DW-MRI in this cohort, sensitivity analysis demonstrates that its lower cost could make it preferable to MRS if it could be shown to have similar diagnostic accuracy.
Strengths and limitations of the assessment
Strengths
Attempts have been made to use the best available evidence to model both the diagnostic pathways and subsequent treatment pathways and outcomes. The model provides a flexible framework allowing the comparison of many different diagnostic strategies in the context of the patient's lifetime. It captures the trade-off between the increased upfront costs of imaging and the reduction in subsequent biopsies, and also the trade-off between earlier diagnosis and potential utility decrements associated with treatment side-effects. Further, the model is risk stratified to allow for comparison of strategies that vary in their ability to differentiate between tumours of different stage and grade. The model can easily be updated to incorporate new, more detailed evidence as it becomes available.
Limitations
The modelling was hampered by limited availability of data on the diagnostic accuracy of alternative diagnostic strategies, the natural history of cancer detected at repeat biopsy, and the impact of diagnosis and treatment on disease progression and health-related QoL.
The systematic review uncovered limited data on the relative sensitivity (cancer detection rates) of MRI-/MRS-targeted biopsy techniques and systematic TRUS-guided approaches. In particular, there were no identified studies providing head-to-head comparisons of MRI-/MRS-targeted approaches and systematic TRUS-guided sampling schemes based on different numbers of cores. Further, it was not possible to obtain pooled estimates for the sensitivity/specificity of alternative MRS/MRI sequences by grade of tumour, which is potentially an important parameter for informing cost-effectiveness. There is therefore a need for a large comparative study to prospectively assess the sensitivity of systematic approaches and MRS/MRI sequences for detecting cancer by D'Amico risk strata. It would also be beneficial for follow-up to be built into such a cohort study to ascertain how contemporary cohorts are treated, how they progress over time, and how their health-related QoL is affected by diagnosis and subsequent treatment with different modalities.
Although the model attempted to capture all the important clinical and cost events, it was not possible to capture and/or value all the important factors that might influence cost-effectiveness. For example, we were not able to ascertain and assign utility decrements for pain and short-lived complications associated with undergoing biopsy. Further, we did not have a good source of EQ-5D utility weights for a UK-based cohort of patients undergoing repeat biopsy and follow-up, making it necessary to draw on alternative sources. It would be beneficial to incorporate a measure of health-state utility into future cohort studies assessing the accuracy of alternative approaches to diagnosis. In addition, with the survival and QALY gains being so small, and of questionable clinical significance, the choice between strategies might be better informed by patient or public preferences for process of care factors to which the standard QALY model may be insensitive.
Uncertainties
Uncertainty exists regarding the way that hospitals across England and Wales are, or would be, reimbursed for repeat biopsy procedures using the TRUS-guided extended-cores approach and MRI-/MRS-directed approaches. Although it is difficult to ascertain the average picture across the UK, it is clear to see from the analysis that the use of MRS/MRI is likely to be cost-effective if a high proportion of the biopsies averted by its use would otherwise be reimbursed as day-case procedures. Sensitivity analysis suggests that, in settings where the average cost of biopsies averted is ∼£115 more than the cost of obtaining a MRS sequence, the use of MRS might be cost-effective; the greater the saving from biopsies averted, the higher the likelihood of MRS being considered cost-effective.
In contexts/settings where index repeat biopsies averted (using standard TRUS guidance and/or MRS/MRI direction) would otherwise only incur the outpatient procedure cost (£212), there is less certainty surrounding the relative cost-effectiveness of MRI-/MRS-directed approaches.
A high degree of uncertainty exists regarding the impact of a false-negative result at repeat biopsy on the time to final diagnosis, and also on the impact of any delay on disease progression. The base-case analysis relied on the assumption that all patients experience a relative risk reduction for progression to metastatic disease upon referral. However, recent data suggest that risk reductions associated with radical treatment for low-risk patients (and even moderate-risk patients) may be small and insignificant. 149 If this is the case, it might undermine the cost-effectiveness of strategies that increase cancer detection rates and costs over standard practice, unless those strategies are able to discriminate by grade of tumour.
With index repeat biopsies costed as outpatient procedures, the more sensitive and more costly enhanced imaging techniques were found to be associated with small cost increases, for even smaller survival and QALY gains compared with T2-MRI and systematic TRUS/Bx. This results in the incremental cost-per QALY ratios being very sensitive to the baseline risks of progression and relative treatment effects. Recent data suggest that the underlying risk of progression for low-risk cancer, identified in the PSA era, may be lower than that reported for the low-risk subgroup identified by Bill-Axelson et al. 148 However, the above point also highlights the potential benefit of utilising a pre-biopsy imaging test that could differentiate between low-, moderate- and high-risk cancer, so that only those patients in the moderate- and high-risk categories could be selected for biopsy. Our modelling suggests that if DW-MRI or MRS could be shown to provide such discrimination in the cohort of patients with elevated PSA level but previously negative biopsy, these tests could achieve levels of cost-effectiveness considered acceptable, even at the lower biopsy procedure costs. More detailed studies are required to assess the diagnostic accuracy of different sequences by stage/grade of cancer in order to address this question.
A key driver in the cost-effectiveness analysis was the high sensitivity/specificity of systematic TRUS-guided extended-core biopsy carried out in the outpatient setting compared with MRS/MRI. As there were no available literature data directly comparing the relative accuracy of MRI-directed biopsies with this method for obtaining biopsies, we were forced to rely on a study in which the sensitivity of the systematic extended-cores biopsy was modelled using the results of a saturation biopsy as the reference standard. There is a degree of uncertainty about the assumption of saturation biopsy as the reference standard in this study because of the following reasons: a large number of cancers modelled were found to be of low risk; there was variable correlation with prostatectomy specimens; and a considerable risk exists of missing apex and anterior TZ tumours. If this derived high level of test accuracy for systematic TRUS-guided extended biopsy is not achieved in actual clinical practice within the NHS then the cost-effectiveness of the approach would be negatively altered and correspondingly the MRS/MRI approach would be improved. To mitigate operator-dependent variability of performing outpatient systematic TRUS-guided extended biopsy, it would be advantageous to be able to record by ultrasound the actual locations where cores are obtained as a quality measure.
Finally, a further issue contributing to the uncertainty surrounding the cost-effectiveness of alternative diagnostic approaches, and indeed the cost-effectiveness of radical treatment,9 is the impact of treatment on health-related QoL. In our base-case specification, we have applied utility values that suggest radical treatment complications do not impact heavily on health-related QoL as measured by the EQ-5D. 163 However, the incremental cost per QALY findings are highly sensitive to changes to these applied utility assumptions. When health-state utilities are adjusted downwards for those patients experiencing treatment complications, the cost-effectiveness of strategies that improve cancer detection rates, at an increased cost to the health service, decreases substantially. This highlights the potential importance of risk stratifying treatment appropriately, so that only those patients likely to experience a significant survival benefit receive radical treatments. However, current data suggest that a substantial proportion of low-risk patients still elect for radical treatment, which undermines the cost-effectiveness of diagnostic strategies that result in more low-risk patients being diagnosed. This emphasises the potential benefit of reducing overdiagnosis of low-risk cancers, which MRS/MRI might be able to do.
Other relevant factors
The modelled differences in survival between strategies were found to be extremely small. Despite this, the more sensitive strategies do achieve a high probability of being more effective (in terms of LYs and QALYs) than less sensitive strategies. This is due to the application of a constant and significant relative treatment effect (at least in the 60-year-old cohort). However, the QALY model in this instance may fail to capture other important process-of-care factors that have important influences on patients' preferences for alternative approaches to diagnosis and monitoring. Scope exists to carry out preference elicitation studies to identify and value the key factors influencing patients' preferences for alternative diagnostic, monitoring, and subsequent treatment pathways. For example, one could design a preference elicitation study to directly assess patients' willingness to trade between factors such as chance of a positive diagnosis being made, risk of biopsy complications, treatment options and likely survival benefit if diagnosed, risk of treatment complications, risk of progression if undiagnosed, frequency of monitoring if diagnosed/undiagnosed, and need for repeat biopsies if undiagnosed. If the value ascribed by patients to these alternative attributes could be measured using a common numéraire such as willingness to pay, these values could then be applied within a decision analysis framework to help identify the optimal approach from the patient perspective in the modern NHS.
Chapter 8 Conclusions
Implications for service provision
The evidence from the patient-level pooled estimates suggests that MRS has higher sensitivity (92%; 95% CI 86% to 95%) and specificity (76%; 95% CI 61% to 87%) than T2-MRI [sensitivity 86% (95% CI 74% to 93%), specificity 55% (95% CI 44% to 66%)] in detecting PC in men with elevated PSA level but a previous negative biopsy. Combining both tests so that when either is positive the combination is considered positive further increases sensitivity (96%; 95% CI 90% to 98%) but at the expense of specificity (31%; 95% CI 21% to 42%). The advantages of higher sensitivity (fewer false-negatives) have to be weighed against the disadvantages of lower specificity. As the combination of MR methods works as guidance for biopsies, which need to provide the final positive diagnosis, the lower specificity may be acceptable. The limited evidence for DCE-MRI (three studies) suggests that it has lower sensitivity (79%; 95% CI 69% to 87%) and specificity (52%; 95% CI 44% to 66%) than either MRS or T2-MRI. Only one small study reported patient-level estimates for DW-MRI and only for sensitivity, which, however, was high at 100%. TRUS used as an imaging test has low sensitivity (27%; 95% CI 16% to 42%) but high specificity (81%; 95% CI 77% to 85%). The results from the indirect comparison of tests were broadly reflective of those of the pooled estimates of the individual tests.
Transrectal ultrasonography is no longer routinely used as an imaging test but rather is used to visualise the prostate in order to obtain a systematic predefined set of biopsies (TRUS/Bx). Six large population screening studies81,88,97,99,103,113 allowed the calculation of the sensitivity of TRUS used in this manner on a subset of their participants with a previously negative biopsy and continuing suspicion of cancer. The reference standard in these studies81,88,97,99,103,113 was a second, third or more, possibly extended-core, transrectal biopsy session. Across these studies81,88,97,99,103,113 the median sensitivity was moderately high at 72.5% (range 60.6% to 96.3%). However, it should be borne in mind that in these studies,81,88,97,99,103,113 patients classed as low risk do not usually proceed to further repeat biopsies. All of the remaining patients, therefore, will have a high suspicion of cancer and this could potentially lead to the sensitivity values reported being artificially high. Moreover, cancer foci that are difficult to sample transrectally in the apex or anteriorly in the TZ of the prostate could remain undetected for quite a long time.
Although saturation biopsies, through removing a higher number of cores than standard or extended biopsy schemes, may potentially improve cancer detection rates compared with these schemes, solely increasing the number of biopsy cores may also lead to an increase in the detection of clinically insignificant disease. Most of the MRS and other MRI imaging studies reported a median Gleason score of ≥ 6 and the one DW-MRI study96 reported a median Gleason score of 7. Across six studies74,90,96,104,108,109 reporting a median Gleason score of > 6, the percentage of patients with a Gleason score of ≥ 7 ranged from 50.0% to 66.7%. The limited evidence suggests that, potentially, a substantial number of cancers detected by MRS and other MRI techniques in men with raised PSA level and previously negative biopsy may be clinically significant (Gleason score of ≥ 7 and/or volume of > 0.5 ml).
The cost-effectiveness modelling showed the relative cost-effectiveness of alternative strategies to be highly sensitive to a number of key parameters and structural assumptions. Given the level of uncertainty surrounding these key inputs, it is difficult to arrive at a definitive conclusion on the cost-effectiveness of using different MRS/MRI sequences to aid the localisation of prostate abnormalities for biopsy. However, our modelling suggests that under certain circumstances T2-MRI may be considered cost-effective in comparison with systematic TRUS/Bx. In addition, if MRS and DW-MRI can be shown to have high sensitivity for detecting moderate/high-risk cancer, while negating the need for patients with no cancer or low-risk disease to undergo biopsy, their use could represent a cost-effective approach to diagnosis.
The cost-effectiveness of using MRS rather than T2-MRI to direct biopsies was also found to be sensitive to the cost of prostate biopsies relative to the cost of obtaining the MRS sequence. Threshold analysis suggests that MRS may be considered cost-effective in moderate prevalence cohorts (24%) in settings where the cost of obtaining the MRS sequence is at least ∼£90 less than the average cost of any biopsies averted (holding all other base-case parameter values constant). The greater the cost of biopsies relative to the cost of MRS, the more cost-effective it becomes. Data from subgroup analysis also show that the use of MRS is more likely to be cost-effective in subgroups harbouring a higher prevalence of cancer, and also in younger cohorts. In cohorts harbouring a low prevalence of cancer, T2-MRI may be preferred over TRUS/Bx and MRS. The most sensitive strategy of targeting all patients who are positive on either T2-MRI or MRS for biopsy did not compare favourably in terms of cost-effectiveness compared with using MRS findings alone. This is due to the significant drop in specificity for only a small increase in sensitivity compared with MRS.
The introduction of MRS and other MRI techniques (T2-MRI, DCE-MRI, DW-MRI) for evaluation of men with TRUS-guided negative biopsies but in whom there remains suspicion of cancer would have a range of implications for the NHS. These would arise primarily because of a shift in the test–treatment pathway for this group, with changes in the method of making diagnosis resulting in changes to the types of patients being treated, offered patient options and timings of treatments. This would have consequential effects on service provision, costs and training. If urological and/or radiological services were to undertake targeted biopsies of MRI-/MRS-suspicious regions then extra provision would be required for this. A new generation of equipment and software would be needed to enable accurate, documentable biopsies to be obtained from all regions of the prostate. If MRS/MRI identified more patients with localised disease with intermediate and high risk of progression, this would increase the proportion of patients considered eligible for radical therapies. If MRS/MRI detected few patients with low risk of progression disease then fewer patients in this category would undergo perhaps inappropriate radical therapies. Thus the total number of patients undergoing radical therapies would be appropriately decreased, thus requiring a rebalancing of current resources currently allocated to surgical and radiation therapy services. Furthermore, if MRS/MRI contributed to the more accurate classification of low-risk of progression patients, this would lead to an increase in the proportion of appropriately selected patients likely to undergo ‘active surveillance’ helping to mitigate the current high dropout rate of this approach. The implications for the follow-up of active surveillance patients would include utilisation of repeated PSA testing, repeated interval biopsies and follow-up clinics (much of this work is protocol driven and could be nurse practitioner led). Taken together, earlier, more accurate diagnoses and more appropriate treatments of PC may improve patient outcomes by reducing treatment-related morbidity, improved survival and, in the longer term, reduce the requirements on end-of-life and palliative care services. There would be cost implications of these service reconfigurations and for changes in treatment patterns mentioned above. Implementation would also result in the need for further training of all staff involved in delivering care to patients with PC.
Suggested research priorities
Although there is some evidence available for the sensitivity and specificity of MRS and standard T2-MRI for the detection of PC in men with suspected PC and elevated PSA level but previously negative biopsy, less evidence is available for DCE-MRI and even less for DW-MRI. More evidence is also needed for all of these tests of the extent to which they can differentiate between clinically significant and insignificant disease.
Therefore, prospective studies are required comparing the utility of the individual and combined components of a multiparametric magnetic resonance (MR) approach (MRS, DCE-MRI and DW-MRI) with both a MR-guided or -directed biopsy session and an extended 14-core TRUS/Bx scheme (the test currently most often used in the UK for a second biopsy where the first was negative but the patient still has a suspicion for PC) against a reference standard of histopathological assessment of biopsied tissue obtained via saturation biopsy, template biopsy or prostatectomy specimens. A follow-up time of 12 months should be incorporated as part of the reference standard. Investigations of DW-MRI should be encouraged as it is already gaining widespread acceptance in normal radiological practice for investigating prostate diseases. These studies could take the form of fully paired direct (head-to-head) comparisons where all of the study population receives the index test(s), comparator test(s) and reference standard, or a randomised direct comparison in which study participants are randomly allocated to receive the index test or the comparator and all receive the reference standard.
These studies should also report the sensitivity of the tests in detecting clinically significant disease (Gleason score of ≥ 7 and/or volume of > 0.5 ml). In addition to diagnostic outcomes, adverse event data and impact of the tests on subsequent physician attitudes to patient management should also be obtained, and also cost-effectiveness data including impact of testing on health-related QoL.
Uncertainties surrounding cost-effectiveness could be significantly reduced by future research focusing on generating comparable estimates of (1) the sensitivity of MRI-/MRS-directed and systematic approaches to TRUS/Bx (using a robust and common reference standard); (2) the prospective sensitivity/specificity of MRS/MRI sequences for detecting different grades of localised disease in the repeat biopsy cohort; and (3) the full economic costs of MRI sequences and systematic approaches to TRUS/Bx based on different numbers of cores.
Acknowledgements
We thank Lara Kemp for secretarial support; Pawana Sharma for helping to develop the protocol, screening titles and abstracts and assessing full-text papers; Kieran Rothnie for screening titles and abstracts, assessing full-text papers, data extraction and risk of bias assessment; Jonathan Cook for providing statistical advice; and Rob Pickard, Newcastle University, David Evans, Newcastle upon Tyne Hospitals NHS Foundation Trust, Marie O'Donnell, Western General Hospital, Edinburgh, and Ann-Marie Pollock, Raigmore Hospital, Inverness for assisting with queries relating to histopathology aspects of the review. The Health Services Research Unit and Health Economics Research Unit, Institute of Applied Health Sciences, University of Aberdeen are core funded by the Chief Scientist Office of the Scottish Government Health and Social Care Directorates.
Contribution of authors
Cynthia Fraser developed and ran the search strategies, managed the reference database and formatted references.
Charles Boachie conducted the statistical analysis.
Moira Cruickshank and John Ford screened titles and abstracts, assessed full-text papers and undertook data extraction and risk of bias assessment.
Graham Mowatt assessed full-text papers, undertook risk of bias assessment and co-ordinated the project.
Lutfi Kurban, Thomas Lam, Anwar Padhani, Justine Royle and Tom Scheenen provided expert advice on clinical aspects of the review.
Those responsible for the initial drafting of the report chapters were:
Executive summary (Graham Mowatt and Graham Scotland).
Background (John Ford, Lutfi Kurban, Thomas Lam, Anwar Padhani, Justine Royle, Graham Scotland and Tom Scheenen).
Decision problem (John Ford and Graham Scotland).
Methods (Charles Boachie, Moira Cruickshank and Cynthia Fraser).
Diagnostic accuracy (Moira Cruickshank, John Ford, Cynthia Fraser and Graham Mowatt).
Cost-effectiveness (Graham Scotland and Emma Tassie).
Assessment of factors relevant to the NHS (John Ford).
Discussion (Graham Mowatt and Graham Scotland).
Conclusions (Graham Mowatt and Graham Scotland).
All authors assisted in preparing the manuscript and commenting on drafts.
Disclaimer
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Loeb S, Carter HB, Berndt SI, Ricker W, Schaeffer EM. Complications after prostate biopsy: data from SEER-Medicare. J Urol 2011;186:1830-4. http://dx.doi.org/10.1016/j.juro.2011.06.057.
- Stewart CS, Leibovich BC, Weaver AL, Lieber MM. Prostate cancer diagnosis using a saturation needle biopsy technique after previous negative sextant biopsies. J Urol 2001;166:86-91. http://dx.doi.org/10.1016/S0022-5347(05)66083-1.
- Muruve NA. Prostate anatomy. New York, NY: Medscape; 2011.
- McNeal JE, Redwine EA, Freiha FS, Stamey TA. Zonal distribution of prostatic adenocarcinoma. Correlation with histologic pattern and direction of spread. Am J Surg Pathol 1988;12:897-906. http://dx.doi.org/10.1097/00000478-198812000-00001.
- McNeal JE. Normal histology of the prostate. Am J Surg Pathol 1988;12:619-33. http://dx.doi.org/10.1097/00000478-198808000-00003.
- Cancer facts and figures for African Americans 2009–2010. Atlanta, GA: American Cancer Society; 2010.
- Prostate cancer – UK incidence statistics 2009. London: Cancer Research UK; 2009.
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin 2010;60:277-300. http://dx.doi.org/10.3322/caac.20073.
- NICE CG58 prostate cancer: full guidance. London: NICE; 2008.
- Partin AW, Kattan MW, Subong EN, Walsh PC, Wojno KJ, Oesterling JE, et al. Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer. A multi-institutional update. JAMA 1997;277:1445-51. http://dx.doi.org/10.1001/jama.1997.03540420041027.
- Spigelman SS, McNeal JE, Freiha FS, Stamey TA. Rectal examination in volume determination of carcinoma of the prostate: clinical and anatomical correlations. J Urol 1986;136:1228-30.
- Futterer JJ, Heijmink SW, Scheenen TW, Jager GJ, Hulsbergen-van de Kaa CA, Witjes JA, et al. Prostate cancer: local staging at 3-T endorectal MR imaging – early experience. Radiology 2006;238:184-91. http://dx.doi.org/10.1148/radiol.2381041832.
- Katz DA, Jarrard DF, McHorney CA, Hillis SL, Wiebe DA, Fryback DG. Health perceptions in patients who undergo screening and workup for prostate cancer. Urology 2007;69:215-20. http://dx.doi.org/10.1016/j.urology.2006.09.059.
- Kirby R, Fitzpatrick JM. Optimising repeat prostate biopsy decisions and procedures. BJU Int 2012;109:1750-4. http://dx.doi.org/10.1111/j.1464-410X.2011.10809.x.
- McGovern PM, Gross CR, Krueger RA, Engelhard DA, Cordes JE, Church TR. False-positive cancer screens and health-related quality of life. Cancer Nurs 2004;27:347-52. http://dx.doi.org/10.1097/00002820-200409000-00003.
- Johansson JE, Andren O, Andersson SO, Dickman PW, Holmberg L, Magnuson A, et al. Natural history of early, localized prostate cancer. JAMA 2004;291:2713-19. http://dx.doi.org/10.1001/jama.291.22.2713.
- Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol 2011;59:61-7. http://dx.doi.org/10.1016/j.eururo.2010.10.039.
- Wefer AE, Hricak H, Vigneron DB, Coakley FV, Lu Y, Wefer J, et al. Sextant localization of prostate cancer: comparison of sextant biopsy, magnetic resonance imaging and magnetic resonance spectroscopic imaging with step section histology. J Urol 2000;164:400-4. http://dx.doi.org/10.1016/S0022-5347(05)67370-3.
- Chybowski FM, Keller JJ, Bergstralh EJ, Oesterling JE. Predicting radionuclide bone scan findings in patients with newly diagnosed, untreated prostate cancer: prostate specific antigen is superior to all other clinical parameters. J Urol 1991;145:313-18.
- Rana A, Karamanis K, Lucas MG, Chisholm GD. Identification of metastatic disease by T category, Gleason score and serum PSA level in patients with carcinoma of the prostate. Br J Urol 1992;69:277-81. http://dx.doi.org/10.1111/j.1464-410X.1992.tb15528.x.
- Stephenson AJ, Scardino PT, Eastham JA, Bianco FJ, Dotan ZA, Fearn PA, et al. Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Natl Cancer Inst 2006;98:715-17. http://dx.doi.org/10.1093/jnci/djj190.
- Bruwer G, Heyns CF, Allen FJ. Influence of local tumour stage and grade on reliability of serum prostate-specific antigen in predicting skeletal metastases in patients with adenocarcinoma of the prostate. Eur Urol 1999;35:223-7. http://dx.doi.org/10.1159/000019850.
- Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. Oxford: Wiley-Blackwell; 2009.
- Gleason DF, Mellinger GT. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol 1974;111:58-64.
- Gleason DF, Tannenbaum M. Urologic pathology: the prostate. Philadelphia, PA: Lea and Febiger; 1977.
- Epstein JI. Gleason score 2-4 adenocarcinoma of the prostate on needle biopsy: a diagnosis that should not be made. Am J Surg Pathol 2000;24:477-8. http://dx.doi.org/10.1097/00000478-200004000-00001.
- Kattan MW, Zelefsky MJ, Kupelian PA, Scardino PT, Fuks Z, Leibel SA. Pretreatment nomogram for predicting the outcome of three-dimensional conformal radiotherapy in prostate cancer. J Clin Oncol 2000;18:3352-9.
- Kattan MW, Potters L, Blasko JC, Beyer DC, Fearn P, Cavanagh W, et al. Pretreatment nomogram for predicting freedom from recurrence after permanent prostate brachytherapy in prostate cancer. Urology 2001;58:393-9. http://dx.doi.org/10.1016/S0090-4295(01)01233-X.
- D’Amico AV, Whittington R, Malkowicz SB, Fondurulia J, Chen MH, Kaplan I, et al. Pretreatment nomogram for prostate-specific antigen recurrence after radical prostatectomy or external-beam radiation therapy for clinically localized prostate cancer. J Clin Oncol 1999;17:168-72.
- Makarov DV, Trock BJ, Humphreys EB, Mangold LA, Walsh PC, Epstein JI, et al. Updated nomogram to predict pathologic stage of prostate cancer given prostate-specific antigen level, clinical stage, and biopsy Gleason score (Partin tables) based on cases from 2000 to 2005. Urology 2007;69:1095-101. http://dx.doi.org/10.1016/j.urology.2007.03.042.
- D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998;280:969-74. http://dx.doi.org/10.1001/jama.280.11.969.
- Fuchsjager MH, Shukla-Dave A, Hricak H, Wang L, Touijer K, Donohue JF, et al. Magnetic resonance imaging in the prediction of biochemical recurrence of prostate cancer after radical prostatectomy. BJU Int 2009;104:315-20. http://dx.doi.org/10.1111/j.1464-410X.2009.08406.x.
- Shukla-Dave A, Hricak H, Akin O, Yu C, Zakian KL, Udo K, et al. Preoperative nomograms incorporating magnetic resonance imaging and spectroscopy for prediction of insignificant prostate cancer. BJU Int 2012;109:1315-22. http://dx.doi.org/10.1111/j.1464-410X.2011.10612.x.
- Wang L, Hricak H, Kattan MW, Chen HN, Scardino PT, Kuroiwa K. Prediction of organ-confined prostate cancer: incremental value of MR imaging and MR spectroscopic imaging to staging nomograms. Radiology 2006;238:597-603. http://dx.doi.org/10.1148/radiol.2382041905.
- Nguyen CT, Jones JS. Focal therapy in the management of localized prostate cancer. BJU Int 2011;107:1362-8. http://dx.doi.org/10.1111/j.1464-410X.2010.09975.x.
- Comparative effectiveness of therapies for clinically localized prostate cancer: health care bulletin no. 13. Rockville, MD, USA: AHRQ; 2008.
- Campos-Fernandes JL, Bastien L, Nicolaiew N, Robert G, Terry S, Vacherot F, et al. Prostate cancer detection rate in patients with repeated extended 21-sample needle biopsy. Eur Urol 2009;55:600-6. http://dx.doi.org/10.1016/j.eururo.2008.06.043.
- Scattoni V, Raber M, Capitanio U, Abdollah F, Roscigno M, Angiolilli D, et al. The optimal rebiopsy prostatic scheme depends on patient clinical characteristics: results of a recursive partitioning analysis based on a 24-core systematic scheme. Eur Urol 2011;60:834-41. http://dx.doi.org/10.1016/j.eururo.2011.07.036.
- Prostate cancer (metastatic, castration resistant) – abiraterone (following cytoxic therapy): final appraisal determination guidance. London: NICE; 2012.
- Zaytoun OM, Jones JS. Prostate cancer detection after a negative prostate biopsy: lessons learnt in the Cleveland Clinic experience. Int J Urol 2011;18:557-68. http://dx.doi.org/10.1111/j.1442-2042.2011.02798.x.
- NHS reference costs 2009–10. London: Department of Health; 2011.
- Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, et al. ESUR prostate MR guidelines. Eur Radiol 2012;22:746-57. http://dx.doi.org/10.1007/s00330-011-2377-y.
- iREFER guidelines. London: Royal College of Radiologists; 2012.
- CASPAR – The Cancer Staging Proforma Reporting Project. London: Royal College of Radiologists; 2012.
- Kurhanewicz J, Vigneron DB, Hricak H, Narayan P, Carroll P, Nelson SJ. Three-dimensional H-1 MR spectroscopic imaging of the in situ human prostate with high (0.24–0.7-cm3) spatial resolution. Radiology 1996;198:795-80.
- Scheenen TW, Klomp DW, Roll SA, Futterer JJ, Barentsz JO, Heerschap A. Fast acquisition-weighted three-dimensional proton MR spectroscopic imaging of the human prostate. Magn Reson Med 2004;52:80-8. http://dx.doi.org/10.1002/mrm.20103.
- Verma S, Rajesh A, Futterer JJ, Turkbey B, Scheenen TW, Pang Y, et al. Prostate MRI and 3D MR spectroscopy: how we do it. AJR Am J Roentgenol 2010;194:1414-26. http://dx.doi.org/10.2214/AJR.10.4312.
- Klijn S, De Visschere PJ, De Meerleer GO, Villeirs GM. Comparison of qualitative and quantitative approach to prostate MR spectroscopy in peripheral zone cancer detection. Eur J Radiol 2012;81:411-16. http://dx.doi.org/10.1016/j.ejrad.2010.12.017.
- Scheenen TW, Futterer J, Weiland E, van Hecke P, Lemort M, Zechmann C, et al. Discriminating cancer from noncancer tissue in the prostate by 3-dimensional proton magnetic resonance spectroscopic imaging: a prospective multicenter validation study. Invest Radiol 2011;46:25-33. http://dx.doi.org/10.1097/RLI.0b013e3181f54081.
- Futterer JJ, Scheenen TW, Heijmink SW, Huisman HJ, Hulsbergen-van de Kaa CA, Witjes JA, et al. Standardized threshold approach using three-dimensional proton magnetic resonance spectroscopic imaging in prostate cancer localization of the entire prostate. Invest Radiol 2007;42:116-22. http://dx.doi.org/10.1097/01.rli.0000251541.03822.bb.
- Jung JA, Coakley FV, Vigneron DB, Swanson MG, Qayyum A, Weinberg V, et al. Prostate depiction at endorectal MR spectroscopic imaging: investigation of a standardized evaluation system. Radiology 2004;233:701-8. http://dx.doi.org/10.1148/radiol.2333030672.
- Kobus T, Hambrock T, Hulsbergen-van de Kaa CA, Wright AJ, Barentsz JO, Heerschap A, et al. In vivo assessment of prostate cancer aggressiveness using magnetic resonance spectroscopic imaging at 3T with an endorectal coil. Eur Urol 2011;60:1074-80. http://dx.doi.org/10.1016/j.eururo.2011.03.002.
- Scheidler J, Hricak H, Vigneron DB, Yu KK, Sokolov DL, Huang LR, et al. Prostate cancer: localization with three-dimensional proton MR spectroscopic imaging – clinicopathologic study. Radiology 1999;213:473-80.
- Zakian KL, Sircar K, Hricak H, Chen HN, Shukla-Dave A, Eberhardt S, et al. Correlation of proton MR spectroscopic imaging with Gleason score based on step-section pathologic analysis after radical prostatectomy. Radiology 2005;234:804-14. http://dx.doi.org/10.1148/radiol.2343040363.
- Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging 1999;10:223-32. http://dx.doi.org/10.1002/(SICI)1522-2586(199909)10:3⟨223::AID-JMRI2⟩3.0.CO;2-S.
- Leach MO, Morgan B, Tofts PS, Buckley DL, Huang W, Horsfield MA, et al. Imaging vascular function for early stage clinical trials using dynamic contrast-enhanced magnetic resonance imaging. Eur Radiol 2012;22:1451-64. http://dx.doi.org/10.1007/s00330-012-2446-x.
- Beyersdorff D, Taupitz M, Winkelmann B, Fischer T, Lenk S, Loening SA, et al. Patients with a history of elevated prostate-specific antigen levels and negative transrectal US-guided quadrant or sextant biopsy results: value of MR imaging. Radiology 2002;224:701-6. http://dx.doi.org/10.1148/radiol.2243011553.
- Futterer JJ, Heijmink SW, Scheenen TW, Veltman J, Huisman HJ, Vos P, et al. Prostate cancer localization with dynamic contrast-enhanced MR imaging and proton MR spectroscopic imaging. Radiology 2006;241:449-58. http://dx.doi.org/10.1148/radiol.2412051866.
- Hara N, Okuizumi M, Koike H, Kawaguchi M, Bilim V. Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) is a useful modality for the precise detection and staging of early prostate cancer. Prostate 2005;62:140-7. http://dx.doi.org/10.1002/pros.20124.
- Cirillo S, Petracchini M, Scotti L, Gallo T, Macera A, Bona MC, et al. Endorectal magnetic resonance imaging at 1.5 Tesla to assess local recurrence following radical prostatectomy using T2-weighted and contrast-enhanced imaging. Eur Radiol 2009;19:761-9. http://dx.doi.org/10.1007/s00330-008-1174-8.
- Haider MA, Chung P, Sweet J, Toi A, Jhaveri K, Menard C, et al. Dynamic contrast-enhanced magnetic resonance imaging for localization of recurrent prostate cancer after external beam radiotherapy. Int J Radiat Oncol Biol Phys 2008;70:425-30. http://dx.doi.org/10.1016/j.ijrobp.2007.06.029.
- Turkbey B, Shah VP, Pang Y, Bernardo M, Xu S, Kruecker J, et al. Is apparent diffusion coefficient associated with clinical risk scores for prostate cancers that are visible on 3-T MR images?. Radiology 2011;258:488-95. http://dx.doi.org/10.1148/radiol.10100667.
- van As NJ, de Souza NM, Riches SF, Morgan VA, Sohaib SA, Dearnaley DP, et al. A study of diffusion-weighted magnetic resonance imaging in men with untreated localised prostate cancer on active surveillance. Eur Urol 2009;56:981-7. http://dx.doi.org/10.1016/j.eururo.2008.11.051.
- Hambrock T, Somford DM, Huisman HJ, Van Oort IM, Witjes JA, Hulsbergen-van de Kaa CA, et al. Relationship between apparent diffusion coefficients at 3.0-T MR imaging and Gleason grade in peripheral zone prostate cancer. Radiology 2011;259:453-61. http://dx.doi.org/10.1148/radiol.11091409.
- Oto A, Kayhan A, Jiang Y, Tretiakova M, Yang C, Antic T, et al. Prostate cancer: differentiation of central gland cancer from benign prostatic hyperplasia by using diffusion-weighted and dynamic contrast-enhanced MR imaging. Radiology 2010;257:715-23. http://dx.doi.org/10.1148/radiol.10100021.
- Villeirs GM, De Meerleer GO, De Visschere PJ, Fonteyne VH, Verbaeys AC, Oosterlinck W. Combined magnetic resonance imaging and spectroscopy in the assessment of high grade prostate carcinoma in patients with elevated PSA: a single-institution experience of 356 patients. Eur J Radiol 2011;77:340-5. http://dx.doi.org/10.1016/j.ejrad.2009.08.007.
- Villers A, Puech P, Mouton D, Leroy X, Ballereau C, Lemaitre L. Dynamic contrast enhanced, pelvic phased array magnetic resonance imaging of localized prostate cancer for predicting tumor volume: correlation with radical prostatectomy findings. J Urol 2006;176:2432-7. http://dx.doi.org/10.1016/j.juro.2006.08.007.
- Ellis WJ, Chetner MP, Preston SD, Brawer MK. Diagnosis of prostatic carcinoma: the yield of serum prostate specific antigen, digital rectal examination and transrectal ultrasonography. J Urol 1994;152:1520-5.
- Lee F, Torp-Pedersen ST, Siders DB, Littrup PJ, McLeary RD. Transrectal ultrasound in the diagnosis and staging of prostatic carcinoma. Radiology 1989;170:1-15.
- Umbehr M, Bachmann LM, Held U, Kessler TM, Sulser T, Weishaupt D, et al. Combined magnetic resonance imaging and magnetic resonance spectroscopy imaging in the diagnosis of prostate cancer: a systematic review and meta-analysis. Eur Urol 2009;55:575-90. http://dx.doi.org/10.1016/j.eururo.2008.10.019.
- Eskew LA, Bare RL, McCullough DL. Systematic 5 region prostate biopsy is superior to sextant method for diagnosing carcinoma of the prostate. J Urol 1997;157:199-202. http://dx.doi.org/10.1016/S0022-5347(01)65322-9.
- Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3. http://dx.doi.org/10.1186/1471-2288-3-25.
- Takwoingi Y. METADAS: diagnostic test accuracy meta-analysis – bivariate and HSROC model. Cochrane Collaboration Diagnostic Test Accuracy Group; 2008.
- Amsellem-Ouazana D, Younes P, Conquy S, Peyromaure M, Flam T, Debre B, et al. Negative prostatic biopsies in patients with a high risk of prostate cancer. Is the combination of endorectal MRI and magnetic resonance spectroscopy imaging (MRSI) a useful tool? A preliminary study. Eur Urol 2005;47:582-6. http://dx.doi.org/10.1016/j.eururo.2005.01.015.
- Babaian RJ, Toi A, Kamoi K, Troncoso P, Sweet J, Evans R, et al. A comparative analysis of sextant and an extended 11-core multisite directed biopsy strategy. J Urol 2000;163:152-7. http://dx.doi.org/10.1016/S0022-5347(05)67993-1.
- Bhatia C, Phongkitkarun S, Booranapitaksonti D, Kochakarn W, Chaleumsanyakorn P. Diagnostic accuracy of MRI/MRSI for patients with persistently high PSA levels and negative TRUS-guided biopsy results. J Med Assoc Thai 2007;90:1391-9.
- Campodonico F, Casarico A, Gavazzi L, Calcagno T, Capponi G, Canepa G, et al. Cancer detection with TRUS-guided 10-core biopsy of the prostate. An institutional assessment at the first, repeated and surgical specimen biopsy. Arch Ital Urol Androl 2006;78:39-43.
- Cheikh AB, Girouin N, Colombel M, Marechal JM, Gelet A, Bissery A, et al. Evaluation of T2-weighted and dynamic contrast-enhanced MRI in localizing prostate cancer before repeat biopsy. Eur Radiol 2009;19:770-8. http://dx.doi.org/10.1007/s00330-008-1190-8.
- Cirillo S, Petracchini M, Della MP, Gallo T, Tartaglia V, Vestita E, et al. Value of endorectal MRI and MRS in patients with elevated prostate-specific antigen levels and previous negative biopsies to localize peripheral zone tumours. Clin Radiol 2008;63:871-9. http://dx.doi.org/10.1016/j.crad.2007.10.020.
- de la Rosette JJ, Wink MH, Mamoulakis C, Wondergem N, ten Kate FJ, Zwinderman K, et al. Optimizing prostate cancer detection: 8 versus 12-core biopsy protocol. J Urol 2009;182:1329-36. http://dx.doi.org/10.1016/j.juro.2009.06.037.
- Djavan B, Ravery V, Zlotta A, Dobronski P, Dobrovits M, Fakhari M, et al. Prospective evaluation of prostate cancer detected on biopsies 1, 2, 3 and 4: when should we stop?. J Urol 2001;166:1679-83. http://dx.doi.org/10.1016/S0022-5347(05)65652-2.
- Engelhard K, Hollenbach HP, Kiefer B, Winkel A, Goeb K, Engehausen D. Prostate biopsy in the supine position in a standard 1.5-T scanner under real time MR-imaging control using a MR-compatible endorectal biopsy device. Eur Radiol 2006;16:1237-43. http://dx.doi.org/10.1007/s00330-005-0100-6.
- Eskicorapci SY, Guliyev F, Islamoglu E, Ergen A, Ozen H. The effect of prior biopsy scheme on prostate cancer detection for repeat biopsy population: results of the 14-core prostate biopsy technique. Int Urol Nephrol 2007;39:189-95. http://dx.doi.org/10.1007/s11255-006-9009-5.
- Franiel T, Stephan C, Erbersdobler A, Dietz E, Maxeiner A, Hell N, et al. Areas suspicious for prostate cancer: MR-guided biopsy in patients with at least one transrectal US-guided biopsy with a negative finding – multiparametric MR imaging for detection and biopsy planning. Radiology 2011;259:162-72. http://dx.doi.org/10.1148/radiol.10101251.
- Hambrock T, Futterer JJ, Huisman HJ, Hulsbergen-van de Kaa C, van Basten JP, van Oort I, et al. Thirty-two-channel coil 3T magnetic resonance-guided biopsies of prostate tumor suspicious regions identified on multimodality 3T magnetic resonance imaging: technique and feasibility. Invest Radiol 2008;43:686-94. http://dx.doi.org/10.1097/RLI.0b013e31817d0506.
- Hambrock T, Somford DM, Hoeks C, Bouwense SA, Huisman H, Yakar D, et al. Magnetic resonance imaging guided prostate biopsy in men with repeat negative biopsies and increased prostate specific antigen. J Urol 2010;183:520-7. http://dx.doi.org/10.1016/j.juro.2009.10.022.
- Hoeks CMA, Schouten MG, Bomers JGR, Hoogendoorn SP, Hulsbergen-van de Kaa CA, Hambrock T, et al. Three-Tesla magnetic resonance-guided prostate biopsy in men with increased prostate-specific antigen and repeated, negative, random, systematic, transrectal ultrasound biopsies: detection of clinically significant prostate cancers. Eur Urol 2012;62:902-9. http://dx.doi.org/10.1016/j.eururo.2012.01.047.
- Keetch DW, Catalona WJ, Smith DS. Serial prostatic biopsies in men with persistently elevated serum prostate specific antigen values. J Urol 1994;151:1571-4.
- Labanaris AP, Engelhard K, Zugor V, Nützel R, Kühn R. Prostate cancer detection using an extended prostate biopsy schema in combination with additional targeted cores from suspicious images in conventional and functional endorectal magnetic resonance imaging of the prostate. Prostate Cancer Prostatic Dis 2010;13:65-70. http://dx.doi.org/10.1038/pcan.2009.41.
- Lattouf JB, Grubb RL III, Lee SJ, Bjurlin MA, Albert P, Singh AK, et al. Magnetic resonance imaging-directed transrectal ultrasonography-guided biopsies in patients at risk of prostate cancer. BJU Int 2007;99:1041-6. http://dx.doi.org/10.1111/j.1464-410X.2006.06690.x.
- Lin CC, Huang WJ, Wu LJ, Chang YH, Lin AT, Chen KK. Diagnosis of prostate cancer: repeated transrectal prostate biopsy or transurethral resection. JCMA 2008;71:448-54.
- Lopez-Corona E, Ohori M, Scardino PT, Reuter VE, Gonen M, Kattan MW. A nomogram for predicting a positive repeat prostate biopsy in patients with a previous negative biopsy session. J Urol 2003;170:1184-8. http://dx.doi.org/10.1097/01.ju.0000087451.64657.fa.
- Ozden E, Turgut AT, Yaman O, Gulpinar O, Baltaci S. Follow-up of the transrectal ultrasonographic features of the prostate after biopsy: does any ultrasonographically detectable lesion form secondary to the first biopsy?. J Ultrasound Med 2005;24:1659-63.
- Panebianco V, Sciarra A, Ciccariello M, Lisi D, Bernardo S, Cattarino S, et al. Role of magnetic resonance spectroscopic imaging ([1H]MRSI) and dynamic contrast-enhanced MRI (DCE-MRI) in identifying prostate cancer foci in patients with negative biopsy and high levels of prostate-specific antigen (PSA). Radiol Med (Torino) 2010;115:1314-29. http://dx.doi.org/10.1007/s11547-010-0575-3.
- Panebianco V, Sciarra A, De Berardinis E, Busetto GM, Lisi D, Buonocore V, et al. PCA3 urinary test versus 1H-MRSI and DCEMR in the detection of prostate cancer foci in patients with biochemical alterations. Anticancer Res 2011;31:1399-405.
- Park BK, Lee HM, Kim CK, Choi HY, Park JW. Lesion localization in patients with a previous negative transrectal ultrasound biopsy and persistently elevated prostate specific antigen level using diffusion-weighted imaging at three Tesla before rebiopsy. Invest Radiol 2008;43:789-93. http://dx.doi.org/10.1097/RLI.0b013e318183725e.
- Pepe P, Candiano G, Fraggetta F, Galia A, Grasso G, Aragona F. Is transition zone sampling at repeated saturation prostate biopsy still useful?. Urol Int 2010;85:324-7. http://dx.doi.org/10.1159/000320108.
- Philip J, Hanchanale V, Foster CS, Javle P. Importance of peripheral biopsies in maximising the detection of early prostate cancer in repeat 12-core biopsy protocols. BJU Int 2006;98:559-62. http://dx.doi.org/10.1111/j.1464-410X.2006.06325.x.
- Pinsky PF, Crawford ED, Kramer BS, Andriole GL, Gelmann EP, Grubb R, et al. Repeat prostate biopsy in the prostate, lung, colorectal and ovarian cancer screening trial. BJU Int 2007;99:775-9. http://dx.doi.org/10.1111/j.1464-410X.2007.06708.x.
- Portalez D, Rollin G, Leandri P, Elman B, Mouly P, Jonca F, et al. Prospective comparison of T2w-MRI and dynamic-contrast-enhanced MRI, 3D-MR spectroscopic imaging or diffusion-weighted MRI in repeat TRUS-guided biopsies. Eur Radiol 2010;20:2781-90. http://dx.doi.org/10.1007/s00330-010-1868-6.
- Prando A, Kurhanewicz J, Borges AP, Oliveira EM, Figueiredo E. Prostatic biopsy directed with endorectal MR spectroscopic imaging findings in patients with elevated prostate specific antigen levels and prior negative biopsy findings: early experience. Radiology 2005;236:903-10. http://dx.doi.org/10.1148/radiol.2363040615.
- Quinlan MR, Casey RG, Flynn R, Grainger R, McDermott TE, Thornhill JA. A review of repeat prostate biopsies and the influence of technique on cancer detection: our experience. Ir J Med Sci 2009;178:287-90. http://dx.doi.org/10.1007/s11845-009-0362-0.
- Roehl KA, Antenor JA, Catalona WJ. Serial biopsy results in prostate cancer screening study. J Urol 2002;167:2435-9. http://dx.doi.org/10.1016/S0022-5347(05)64999-3.
- Roethke M, Anastasiadis AG, Lichy M, Werner M, Wagner P, Kruck S, et al. MRI-guided prostate biopsy detects clinically significant cancer: analysis of a cohort of 100 patients after previous negative TRUS biopsy. World J Urol 2012;30:213-18. http://dx.doi.org/10.1007/s00345-011-0675-2.
- Sciarra A, Panebianco V, Ciccariello M, Salciccia S, Cattarino S, Lisi D, et al. Value of magnetic resonance spectroscopy imaging and dynamic contrast-enhanced imaging for detecting prostate cancer foci in men with prior negative biopsy. Clin Cancer Res 2010;16:1875-83. http://dx.doi.org/10.1158/1078-0432.CCR-09-2195.
- Testa C, Schiavina R, Lodi R, Salizzoni E, Tonon C, D’Errico A, et al. Accuracy of MRI/MRSI-based transrectal ultrasound biopsy in peripheral and transition zones of the prostate gland in patients with prior negative biopsy. NMR Biomed 2010;23:1017-26. http://dx.doi.org/10.1002/nbm.1522.
- Ukimura O, Durrani O, Joseph BR. Role of PSA and its indices in determining the need for repeat prostate biopsies. Urology 1997;50:66-72. http://dx.doi.org/10.1016/S0090-4295(97)00116-7.
- Wetter A, Hubner F, Lehnert T, Fliessbach K, Vorbuchner M, Roell S, et al. Three-dimensional 1H-magnetic resonance spectroscopy of the prostate in clinical practice: technique and results in patients with elevated prostate-specific antigen and negative or no previous prostate biopsies. Eur Radiol 2005;15:645-52. http://dx.doi.org/10.1007/s00330-004-2562-3.
- Yakar D, Schouten MG, Bosboom DG, Barentsz JO, Scheenen TW, Futterer JJ. Feasibility of a pneumatically actuated MR-compatible robot for transrectal prostate biopsy guidance. Radiology 2011;260:241-7. http://dx.doi.org/10.1148/radiol.11101106.
- Yanke BV, Salzhauer EW, Colon I. Is race a positive predictor of cancer on repeat prostate biopsy?. J Urol 2006;176:1114-17. http://dx.doi.org/10.1016/j.juro.2006.04.041.
- Yuen JS, Lau WK, Ng LG, Tan PH, Khin LW, Cheng CW. Clinical, biochemical and pathological features of initial and repeat transrectal ultrasonography prostate biopsy positive patients. Int J Urol 2004;11:225-31. http://dx.doi.org/10.1111/j.1442-2042.2003.00772.x.
- Yuen JS, Thng CH, Tan PH, Khin LW, Phee SJ, Xiao D, et al. Endorectal magnetic resonance imaging and spectroscopy for the detection of tumor foci in men with prior negative transrectal ultrasound prostate biopsy. J Urol 2004;171:1482-6. http://dx.doi.org/10.1097/01.ju.0000118380.90871.ef.
- Zackrisson B, Aus G, Bergdahl S, Lilja H, Lodding P, Pihl CG, et al. The risk of finding focal cancer (less than 3 mm) remains high on re-biopsy of patients with persistently increased prostate specific antigen but the clinical significance is questionable. J Urol 2004;171:1500-3. http://dx.doi.org/10.1097/01.ju.0000118052.59597.83.
- Busetto GM, Panebianco V, Sciarra A, De Berardinis E, Tommaso B, Danilo L, et al. PCA3 urinary test versus 3T 1H-MRS and DCE-MRI in the detection of prostate cancer foci in patients with biochemical alterations. Anticancer Res 2011;31.
- Calvo N, Henriquez Lopez I, Pujol F, Milia L, Pont A, Grio J, et al. Utility of magnetic resonance spectroscopy and guided biopsies in patients with previous negative biopsies and suspicious of prostate cancer. Radiother Oncol 2010;96.
- Chung MS, Lee SH, Oh CK, Park SU, Rha KH, Oh YT, et al. MRI is important before repeat targeted biopsy in men with prior negative prostatic biopsy. Eur Urol 2010;9. http://dx.doi.org/10.1016/S1569-9056(10)61106-2.
- Comet-Batlle J, Vilanova-Busquets J, Maroto-Genover A, Bucar-Terrades S, Lopez-Bonet E, Barcelo-Obregon J, et al. Targeting prostate cancer in the central gland with endorectal MRI and spectroscopy. Eur Urol Suppl 2004;3. http://dx.doi.org/10.1016/S1569-9056(04)90137-6.
- Destefanis P, Bosio A, De Maria C, Bisconti A, Cugiani A, Negro CLA, et al. Targeted needle re-biopsy of the prostate after combination of endorectal MRI (ENDOMRI) and magnetic resonance spectroscopy (MRS) in patients with atypical small acinar proliferation (ASAP). Eur Urol Suppl 2009;8. http://dx.doi.org/10.1016/S1569-9056(09)60921-0.
- Di Silverio F, Salciccia S, Busetto GM, Panebianco V, Sciarra A, Lisi D, et al. Is prostate biopsy still necessary?. Anticancer Res 2011;31:1929-30.
- Ghafoori M, Moradi M, Shakiba M, Hosseini K, Alavi M. Targeted TRUS guided biopsy of prostate with the aid of MR spectroscopy in patients with elevated PSA and negative previous systematic biopsy. J Med Imag Radiat 2010;54.
- Hambrock T, Somford D, Futterer J, Van Oort I, Van Basten J-P, Witjes A, et al. Value of 3 TESLA multi-modality directed MR guided biopsy to detect prostate cancer in patients after at least two previous negative biopsies and elevated PSA. J Urol 2009;181. http://dx.doi.org/10.1016/S0022-5347(09)61974-1.
- Hambrock T, Somford DM, Futterer JJ, Hoeks CMA, Hulsbergen-van de Kaa CA, Van Oort IM, et al. Value of 3 tesla multimodality MR guided biopsy (MRGB) to detect prostate cancer in patients after at least two previous negative biopsies and an elevated PSA. Eur Urol Suppl 2009;8. http://dx.doi.org/10.1016/S1569-9056(09)60303-1.
- Hambrock T, Somford DM, Hoeks H. Magnetic resonance imaging guided prostate biopsy in men with repeat negative biopsies and increased prostate specific antigen. J Vasc Interv Radiol 2010;21.
- Labanaris AP, Engelhard K, Smiszek R, Nützel R, Kühn R. Prostate cancer detection using an extended prostate biopsy schema in combination with additional targeted cores from suspicious images in conventional and functional endorectal magnetic resonance imaging of the prostate. J Urol 2009;181:711-12. http://dx.doi.org/10.1016/S0022-5347(09)61988-1.
- Labanaris AP, Engelhard K, Nützel R, Smiszek R, Kühn R. Endorectal magnetic resonance imaging of the prostate. A useful tool in the detection of anterior prostate cancer. J Urol 2010;183. http://dx.doi.org/10.1016/j.juro.2010.02.1791.
- Lee SH, Yeom CD, Park KK, Chung MS, Chung BH, Rha KH. Hard to detect prostate cancer diagnosed by targeted biopsy using combined T2 weighted and diffusion weighted magnetic resonance imaging. J Urol 2011;185:e849-50. http://dx.doi.org/10.1016/j.juro.2011.02.2320.
- Perrotti M, Ankem MK, Weiss RE, Epstein R, Decarvalho VS, Kattan M, et al. Endorectal MRI: adjunct to repeat biopsy in prostate cancer detection. The smart biopsy. J Urol 2002;167.
- Perrotti M, Shurtleff B, Rabbani F, Epstein R, Kennedy E, Weiss R, et al. Endorectal MRI-prospective localization of prostate tumor foci in men with prior negative prostatic biopsies: 60 consecutive cases. J Urol 2000;163. http://dx.doi.org/10.1016/S0022-5347(05)67589-1.
- Portalez D, Rollin G, Leandri P, Elman B, Mouly P, Jonca F, et al. Prospective comparison of T2w-MRI and dynamic-contrast-enhanced MRI, 3D-MR spectroscopic imaging or diffusion-weighted MRI in repeat TRUS-guided biopsies. Eur Urol Suppl 2010;9:197-8. http://dx.doi.org/10.1016/S1569-9056(10)60576-3.
- Rahman NU, Franks JH, Suhta A, Kurhanewicz J, Shinohara K. Utility of MRI/MRS in predicting prostate cancer for patients with multiple prior negative biopsies. J Urol 2003;169.
- Sciarra A, Panebianco V, Salciccia S, Gentilucci A, Parente U, Alfarone A, et al. Value of magnetic resonance spectroscopy imaging (MRSI) and dynamic contrast-enhanced imaging MR (DCEMR) for the detection of prostate adenocarcinoma foci in men with prior negative prostate biopsy and elevated prostate specific antigen (PSA) levels. Eur Urol Suppl 2010;9. http://dx.doi.org/10.1016/S1569-9056(10)60693-8.
- Sciarra A, Panebianco V, Salciccia S, Alessandro G, Ulderico P, Andrea A, et al. Value of magnetic resonance spectroscopy imaging (MRSI) and dynamic contrast-enhanced imaging MR (DCEMR) for the detection of prostate adenocarcinoma foci in men with prior negative prostate biopsy and elevated prostate specific antigen (PSA) levels. J Urol 2010;183. http://dx.doi.org/10.1016/j.juro.2010.02.1790.
- Valentini AL, Pinto F, Totaro A, Sacco E, Volpe A, Racioppi M, et al. Dynamic contrast-enhanced and diffusion-weighted magnetic resonance imaging and prostate cancer detection: preliminary results. Anticancer Res 2010;30.
- Wefer AE, Hricak H, Okuno W, Carroll P, Kurhanewicz J. Magnetic resonance imaging and spectroscopy: targeted prostate biopsy. J Urol 2000;163:280-1.
- Winkelmann B, Beyersdorff D, Taupitz M, Deger S, Tuerk I, Loening SA. Value of high-resolution MR-imaging in patients with elevated PSA levels and negative TRUS-guided quadrant or sextant biopsy. J Urol 2001;165.
- Yao DF, DeWolf WC, Sanda MG, Bloch BN, Genega EM, Berry AM, et al. Increased positive yield of clinically significant prostate cancer with MRI prompted biopsies. J Urol 2009;181:782-3. http://dx.doi.org/10.1016/S0022-5347(09)62179-0.
- Younes P, Zerbib M, Saighi D, Conquy S, Thiounn N, Flam T, et al. Interest of endorectal MRI for patients with a high risk of prostate cancer but negative sextant biopsies. Eur Urol 2001;39.
- Stadlbauer A, Bernt R, Salomonowitz E, Plas E, Strunk G, Eberhardt K. Health-economic evaluation of magnetic resonance imaging before biopsy for diagnosis of prostate cancer. Rofo 2011;183:925-32. http://dx.doi.org/10.1055/s-0031-1281601.
- Hummel S, Simpson EL, Hemingway P, Stevenson MD, Rees A. Intensity-modulated radiotherapy for the treatment of prostate cancer: a systematic review and economic evaluation. Health Technol Assess 2010;14.
- Calvert NW, Morgan AB, Catto JW, Hamdy FC, Akehurst RL, Mouncey P, et al. Effectiveness and cost-effectiveness of prognostic markers in prostate cancer. Br J Cancer 2003;88:31-5. http://dx.doi.org/10.1038/sj.bjc.6600630.
- Parker C, Muston D, Melia J, Moss S, Dearnaley D. A model of the natural history of screen-detected prostate cancer, and the effect of radical treatment on overall survival. Br J Cancer 2006;94:1361-8. http://dx.doi.org/10.1038/sj.bjc.6603105.
- Etzioni R, Gulati R, Falcon S, Penson DF. Impact of PSA screening on the incidence of advanced stage prostate cancer in the United States: a surveillance modeling approach. Med Decis Making 2008;28:323-31. http://dx.doi.org/10.1177/0272989X07312719.
- Cowen ME, Chartrand M, Weitzel WF. A Markov model of the natural history of prostate cancer. J Clin Epidemiol 1994;47:3-21. http://dx.doi.org/10.1016/0895-4356(94)90029-9.
- Ramsay C, Pickard R, Robertson C, Close A, Vale L, Armstrong N, et al. Systematic review and economic modelling of the relative clinical benefit and cost-effectiveness of laparoscopic surgery and robotic surgery for removal of the prostate in men with localised prostate cancer. Health Technol Assess 2012;16.
- Zhang J, Denton BT, Balasubramanian H, Shah ND, Inman BA. Optimization of PSA screening policies: a comparison of the patient and societal perspectives. Med Decis Making 2012;32:337-49. http://dx.doi.org/10.1177/0272989X11416513.
- Hegarty J, Beirne PV, Walsh E, Comber H, Fitzgerald T, Wallace KM. Radical prostatectomy versus watchful waiting for prostate cancer. Cochrane Database Syst Rev 2010;11.
- Iversen P, Madsen PO, Corle DK. Radical prostatectomy versus expectant treatment for early carcinoma of the prostate. Twenty-three year follow-up of a prospective randomized study. Scand J Urol Nephrol Suppl 1995;172:65-72.
- Bill-Axelson A, Holmberg L, Ruutu M, Haggman M, Andersson SO, Bratell S, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 2011;364:1708-17. http://dx.doi.org/10.1056/NEJMoa1011967.
- Wilt TJ. VA/NCI/AHRQ Cooperative Studies Program #407: Prostate cancer Intervention Versus Observation Trial (PIVOT): main results from a randomized trial comparing radical prostatectomy to watchful waiting in men with clinically localized prostate cancer. American Urological Association; 2011.
- Rosario DJ, Lane JA, Metcalfe C, Donovan JL, Doble A, Goodwin L, et al. Short term outcomes of prostate biopsy in men tested for cancer by prostate specific antigen: prospective evaluation within ProtecT study. BMJ 2012;344. http://dx.doi.org/10.1136/bmj.d7894.
- Nam RK, Saskin R, Lee Y, Liu Y, Law C, Klotz LH, et al. Increasing hospital admission rates for urological complications after transrectal ultrasound guided prostate biopsy. J Urol 2010;183:963-8. http://dx.doi.org/10.1016/j.juro.2009.11.043.
- Bolla M, Collette L, Blank L, Warde P, Dubois JB, Mirimanoff RO, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet 2002;360:103-6. http://dx.doi.org/10.1016/S0140-6736(02)09408-4.
- Tappenden P, Chilcott J, Ward S, Eggington S, Hind D, Hummel S. Methodological issues in the economic analysis of cancer treatments. Eur J Cancer 2006;42:2867-75. http://dx.doi.org/10.1016/j.ejca.2006.08.010.
- Albertsen PC, Hanley JA, Penson DF, Barrows G, Fine J. 13-year outcomes following treatment for clinically localized prostate cancer in a population based cohort. J Urol 2007;177:932-6. http://dx.doi.org/10.1016/j.juro.2006.10.051.
- SWPHO Briefing 4: Prostate cancer survival by stage. Bristol: SWPHO; 2008.
- Life expectancy at birth, UK, 1980–82 to 2008–2010, UK interim life tables. London: ONS; 2012.
- Curtis L. Unit costs of health and social care. Canterbury: Personal Social Services Research Unit; 2010.
- British national formulary. London: BMA and RPS; 2012.
- Sacco E, Prayer-Galetti T, Pinto F, Fracalanza S, Betto G, Pagano F, et al. Urinary incontinence after radical prostatectomy: incidence by definition, risk factors and temporal trend in a large series with a long-term follow-up. BJU Int 2006;97:1234-41. http://dx.doi.org/10.1111/j.1464-410X.2006.06185.x.
- Stanford JL, Feng Z, Hamilton AS, Gilliland FD, Stephenson RA, Eley JW, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA 2000;283:354-60. http://dx.doi.org/10.1001/jama.283.3.354.
- Ataman F, Zurlo A, Artignan X, van Tienhoven G, Blank LE, Warde P, et al. Late toxicity following conventional radiotherapy for prostate cancer: analysis of the EORTC trial 22863. Eur J Cancer 2004;40:1674-81. http://dx.doi.org/10.1016/j.ejca.2003.12.027.
- Heidenreich A, Bolla M, Joniau S, Mason MD, Matveev V, Mottet M, et al. Guidelines on benign prostate cancer. Arnhem, The Netherlands: European Association of Urology; 2012.
- Korfage IJ, Essink-Bot ML, Borsboom GJ, Madalinska JB, Kirkels WJ, Habbema JD, et al. Five-year follow-up of health-related quality of life after primary treatment of localized prostate cancer. Int J Cancer 2005;116:291-6. http://dx.doi.org/10.1002/ijc.21043.
- Krahn M, Ritvo P, Irvine J, Tomlinson G, Bremner KE, Bezjak A, et al. Patient and community preferences for outcomes in prostate cancer: implications for clinical policy. Med Care 2003;41:153-64. http://dx.doi.org/10.1097/00005650-200301000-00017.
- Shimizu F, Fujino K, Ito YM, Fukuda T, Kawachi Y, Minowada S, et al. Factors associated with variation in utility scores among patients with prostate cancer. Value Health 2008;11:1190-3. http://dx.doi.org/10.1111/j.1524-4733.2008.00336.x.
- Volk RJ, Cantor SB, Cass AR, Spann SJ, Weller SC, Krahn MD. Preferences of husbands and wives for outcomes of prostate cancer screening and treatment. J Gen Intern Med 2004;19:339-48. http://dx.doi.org/10.1111/j.1525-1497.2004.30046.x.
- Guide to the methods of technology appraisal. London: NICE; 2008.
- Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA 2005;293:2095-101. http://dx.doi.org/10.1001/jama.293.17.2095.
- Doo KW, Sung DJ, Park BJ, Kim MJ, Cho SB, Oh YW, et al. Detectability of low and intermediate or high risk prostate cancer with combined T2-weighted and diffusion-weighted MRI. Eur Radiol 2012;22:1812-19. http://dx.doi.org/10.1007/s00330-012-2430-5.
- Haider MA, Van Der Kwast TH, Tanguay J, Evans AJ, Hashmi AT, Lockwood G, et al. Combined T2-weighted and diffusion-weighted MRI for localization of prostate cancer. AJR Am J Roentgenol 2007;189:323-8. http://dx.doi.org/10.2214/AJR.07.2211.
- Emberton M. MRI in diagnosing prostate cancer. Bethesdaa, MD: US National Institutes of Health; 2012.
- Leeflang MM, Deeks JJ, Gatsonis C, Bossuyt PM. Systematic reviews of diagnostic test accuracy. Ann Intern Med 2008;149:889-97.
- Scattoni V, Zlotta A, Montironi R, Schulman C, Rigatti P, Montorsi F. Extended and saturation prostatic biopsy in the diagnosis and characterisation of prostate cancer: a critical analysis of the literature. Eur Urol 2007;52:1309-22. http://dx.doi.org/10.1016/j.eururo.2007.08.006.
- Scheenen TW, Futterer J. Prostate cancer localization with a multiparametric magnetic resonance (MR) approach. Bethesdaa, MD: US National Institutes of Health; 2012.
- Engelbrecht MR, Jager GJ, Laheij RJ, Verbeek AL, van Lier HJ, Barentsz JO. Local staging of prostate cancer using magnetic resonance imaging: a meta-analysis. Eur Radiol 2002;12:2294-302.
- Lawrentschuk N, Fleshner N. The role of magnetic resonance imaging in targeting prostate cancer in patients with previous negative biopsies and elevated prostate-specific antigen levels. BJU Int 2009;103:730-3. http://dx.doi.org/10.1111/j.1464-410X.2008.08205.x.
- Wang P, Guo YM, Liu M, Qiang YQ, Guo XJ, Zhang YL, et al. A meta-analysis of the accuracy of prostate cancer studies which use magnetic resonance spectroscopy as a diagnostic tool. Kor J Radiol 2008;9:432-8. http://dx.doi.org/10.3348/kjr.2008.9.5.432.
- Costouros NG, Coakley FV, Westphalen AC, Qayyum A, Yeh BM, Joe BN, et al. Diagnosis of prostate cancer in patients with an elevated prostate-specific antigen level: role of endorectal MRI and MR spectroscopic imaging. AJR Am J Roentgenol 2007;188:812-16. http://dx.doi.org/10.2214/AJR.06.0165.
- Perrotti M, Han KR, Epstein RE, Kennedy EC, Rabbani F, Badani K, et al. Prospective evaluation of endorectal magnetic resonance imaging to detect tumor foci in men with prior negative prostatic biopsy: a pilot study. J Urol 1999;162:1314-17. http://dx.doi.org/10.1016/S0022-5347(05)68275-4.
- Mueller-Lisse UG, Vigneron DB, Hricak H, Swanson MG, Carroll PR, Bessette A, et al. Localized prostate cancer: effect of hormone deprivation therapy measured by using combined three-dimensional 1H MR spectroscopy and MR imaging: clinicopathologic case-controlled study. Radiology 2001;221:380-90. http://dx.doi.org/10.1148/radiol.2211001582.
- Yu KK, Scheidler J, Hricak H, Vigneron DB, Zaloudek CJ, Males RG, et al. Prostate cancer: prediction of extracapsular extension with endorectal MR imaging and three-dimensional proton MR spectroscopic imaging. Radiology 1999;213:481-8.
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol 2000;12:191-200.
Appendix 1 Protocol
Appendix 2 Search strategies
Appendix 3 Quality assessment of diagnostic accuracy studies (version 2) checklist
Quality assessment of diagnostic accuracy studies (version 2) checklist (PDF download)
Appendix 4 List of included studies
Appendix 5 List of excluded studies: full-text papers
Appendix 6 Characteristics of the included studies
Appendix 7 Results of risk of bias and applicability for the individual full-text studies (n = 39)
Results of risk of bias and applicability for the individual full-text studies ( n = 39) (PDF download)
Appendix 8 Individual study results (n = 51)
Appendix 9 Sensitivity analysis of magnetic resonance spectroscopy subgrouped into year of publication
Sensitivity analysis of magnetic resonance spectroscopy subgrouped into year of publication (PDF download)
Appendix 10 Sensitivity analysis of T2-weighted magnetic resonance imaging subgrouped into year of publication
Sensitivity analysis of T2-weighted magnetic resonance imaging subgrouped into year of publication (PDF download)
Appendix 11 Transrectal ultrasonography individual study results (patient-level analysis)
Transrectal ultrasonography individual study results (patient-level analysis) (PDF download)
Appendix 12 Studies directly comparing two or more tests
Appendix 13 Results of the indirect comparison
Appendix 14 False-positives
Appendix 15 Gleason scores reported by the studies
Appendix 16 Adverse events related to transrectal ultrasonography-guided biopsy
Adverse events related to transrectal ultrasonography-guided biopsy (PDF download)
Appendix 17 Supplementary results from cost-effectiveness analysis
Supplementary results from cost-effectiveness analysis (PDF download)
Glossary
- Atypical small acinar proliferation
- A diagnosis of ‘atypical small acinar proliferation’ signifies an unusual cellular appearance. Atypical small acinar proliferation is not, in general, considered a pre-malignant condition, but is an indication for repeat biopsy.
- Benign prostatic hyperplasia
- Non-malignant increase in number of cells in the prostate.
- Case series
- Descriptive study of a group of people with the same disease or the same treatment. This type of study cannot determine how people with the disease compare with those without the disease or those who are treated differently.
- Case–control study
- This type of study compares a group of people who have the disease and a group who do not have it.
- Central zone
- Located at the centre of the prostate. Surrounds the ejaculatory ducts.
- Clinical T staging
- Four categories for describing the local extent of a prostate tumour, ranging from T1 to T4. T1 represents localised tumours with no spread, whereas T4 represents tumours that have spread.
- Comparator
- The best diagnostic test currently available.
- Cross-sectional study
- A study in which data are collected at one point in time and relationships between factors are explored.
- Diagnostic odds ratio
- The ratio of the odds of testing positive in those with the disease relative to the odds of testing positive in those without the disease.
- Direct head-to-head study
- A study in which people receive both index and comparator tests and the tests are therefore evaluated in the same participants.
- False-negative/true-negative/false-positive/true-positive
- In terms of diagnostic accuracy, indicators of index test results compared with the reference standard: negative index test, positive reference standard/negative index test, negative reference standard/positive index test, negative reference standard/positive index test, positive reference standard, respectively.
- Gleason score
- A system of grading prostate cancer tissue based on how it looks under a microscope. Gleason scores range from 2 to 10 and signify the likelihood of a tumour spreading. Lower Gleason scores mean that the cancer tissue is similar to normal prostate tissue and the tumour is less likely to behave aggressively; higher Gleason scores mean that the cancer tissue is very different from normal tissue and the tumour is more likely to behave aggressively.
- (High-grade) prostatic intraepithelial neoplasia
- An abnormality of prostate cells. Associated with a finding of prostate cancer on repeat biopsies.
- Hypoechoic
- In ultrasonography, describes areas of abnormally low echoes due to pathological changes in tissue density. Hypoechoic lesions are commonly found to be malignant.
- Index- or reference test-directed biopsy
- This refers to the method used to identify suspicious areas prior to biopsy, i.e. where one or more index tests are used to identify cancer-suspicious areas for use in a subsequent biopsy.
- Index- or reference test-guided biopsy
- This refers to the method used at the time of obtaining tissue samples, i.e. where the specified test is used to locate previously identified cancer-suspicious areas as part of the biopsy procedure.
- Index test
- The diagnostic test that is being evaluated.
- Isoechoic
- In ultrasonography, describes similarity between two or more tissues. Isoechoic lesions are less likely than hypoechoic lesions to be malignant.
- Likelihood ratio
- A description of how many times more likely it is that a person with the disease will receive a particular test result than a person without the disease.
- Meta-analysis
- The quantitative pooling of data from two or more studies.
- Meta-analysis
- The quantitative pooling of data from two or more studies.
- Nomogram
- A prognostic indicator incorporating multiple risk variables to produce mathematical models that predict the likelihood of disease recurrence or progression.
- Observational study
- A study in which people are observed without input from the researchers.
- Peripheral zone
- Located around the outside of the prostate gland, next to the rectum.
- Positive predictive value
- The proportion of those with positive test results who actually have the disease.
- Prostate-specific antigen
- A protein manufactured by the prostate which aids the liquefaction of semen and is released and detectable in the bloodstream in a number of conditions related to the prostate.
- Randomised controlled trial
- A study in which people are randomly allocated to receive – or not receive – a particular treatment or intervention. This is said to be the best study type to determine effectiveness of a treatment.
- Reference standard
- The best available method for establishing the presence or absence of the disease.
- Sclerotic
- Hard or hardening (of tissue).
- Sensitivity
- The proportion of those who actually have the disease and who are correctly identified with positive test results.
- Specificity
- The proportion of those who actually do not have the disease and who are correctly identified with negative test results.
- TNM staging system
- This describes the local extent of the primary tumour (T stage), the absence or presence of spread to nearby lymph nodes (N stage) and the absence or presence of metastasis (M stage).
- Transition zone
- Located in the interior of the prostate. Surrounds the proximal urethra.
List of abbreviations
- ADC
- apparent diffusion coefficient
- AIF
- arterial input function
- ASAP
- atypical small acinar proliferation
- ASCO
- American Society of Clinical Oncology
- CC/C
- choline-plus-creatine– citrate ratio
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CT
- computed tomography
- CZ
- central zone
- DCE-MR
- dynamic contrast-enhanced magnetic resonance imaging
- DOR
- diagnostic odds ratio
- DRE
- digital rectal examination
- DW-MRI
- diffusion-weighted magnetic resonance imaging
- EAU
- European Association of Urology
- EBRT
- external beam radiotherapy
- ED
- erectile dysfunction
- EQ-5D
- European Quality of Life-5 Dimensions
- GP
- general practitioner
- HGPIN
- high-grade prostatic intraepithelial neoplasia
- HIFU
- high-intensity focused ultrasound
- HRG
- Healthcare Resource Group
- HSROC
- hierarchical summary receiver operating characteristic
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- LHRH
- luteinising hormone-releasing hormone
- LR
- likelihood ratio
- MeSH
- medical subject heading
- MR
- magnetic resonance
- MRI
- magnetic resonance imaging
- MRS
- Magnetic resonance spectroscopy
- MRSI
- magnetic resonance spectroscopy imaging
- N/A
- not available
- NICE
- National Institute for Health and Care Excellence
- NMB
- net monetary benefit
- NPV
- negative predictive value
- PC
- prostate cancer
- PIN
- prostatic intraepithelial neoplasia
- PPV
- positive predictive value
- PSA
- prostate-specific antigen
- PZ
- peripheral zone
- QoL
- quality of life
- QUADAS-2
- quality assessment of diagnostic accuracy studies, version 2
- RCR
- Royal College of Radiologists
- ROC
- receiver operating characteristic
- SD
- standard deviation
- SROC
- summary receiver operating characteristic
- T2-MRI
- T2-weighted magnetic resonance imaging
- TAR
- technology assessment report
- TNM
- tumour, node, metastasis staging system
- TRUS
- transrectal ultrasonography
- TRUS/Bx
- transrectal ultrasound-guided biopsy
- TZ
- transition zone
- UI
- urinary incontinence
- UTI
- urinary tract infection
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.