Notes
Article history paragraph text
The research reported in this issue of the journal was funded by the HTA programme as project number 09/11/02. The contractual start date was in June 2010. The draft report began editorial review in January 2012 and was accepted for publication in September 2012. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors' report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
DAL has received an investigator-initiated educational grant from Bayer Healthcare and honoraria from Boehringer Ingelheim, Bayer HealthCare, Bristol-Myers Squibb, Sanofi-aventis and Pfizer. In addition, DAL is a panellist on the ninth edition of the American College of Chest Physicians guidelines on antithrombotic therapy in atrial fibrillation. DAF has received honoraria from Boehringer Ingelheim, Sanofi-aventis, and AstraZeneca
Permissions
Copyright statement
© Queen's Printer and Controller of HMSO 2013. This work was produced by Lane et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Description of the underlying health problem
Atrial fibrillation (AF), the most common abnormality of the heart's rhythm (cardiac arrhythmia) seen in clinical practice,1 is characterised by unco-ordinated and rapid beating of the upper chambers of the heart (atria). 2
Owing to the irregularity in the beating of the heart, the flow of blood is affected and there is an increased risk of formation of blood clots in the atria. If these clots are subsequently displaced, they can travel in the blood to other parts of the body and may block blood vessels, thereby disrupting blood flow, leading to an embolism. The most common site of embolism in patients with AF is the brain, resulting in a stroke. Patients with AF have an increased risk of stroke compared with individuals without AF. 3 AF is responsible for 15% of all strokes and one-quarter of strokes in people aged > 80 years. 4 Furthermore, AF confers a 1.5- and 1.9-fold increased risk of mortality in men and women, respectively,5 and is associated with elevated risk of developing heart failure (HF)2 and impairment of quality of life. 6,7
Incidence/prevalence
Atrial fibrillation is the most common cardiac arrhythmia in clinical practice1,8,9 and the prevalence increases markedly with older age, from 0.5% at 40–50 years to 5% in those aged ≥ 65 years and almost 10% in people aged ≥ 80 years. 10,11 AF is slightly more prevalent in men than in women. 8–10 The lifetime risk of developing AF aged ≥ 40 years is approximately one in four. 8,9
The Screening for Atrial Fibrillation in the Elderly (SAFE) study,12 a randomised controlled trial (RCT) of systematic screening (targeted and total population screening) compared with routine practice for the detection of AF in people aged ≥ 65 years in the UK involving 15,000 patients, revealed that the prevalence of AF was 7.2%, with a higher prevalence evident in men (7.8%) and those aged ≥ 75 years (10.3%). The incidence of AF ranged from 1.04% to 1.64% per year. The incidence and prevalence of AF are increasing and are projected to rise exponentially as the population ages and the prevalence of cardiovascular risk factors increases. 10
Impact of the health problem
The major complication of AF is stroke. AF is associated with a fivefold increased risk of stroke compared with age- and sex-matched patients in sinus rhythm,3 and doubles the risk of stroke after adjustment for other risk factors. 1 In addition, when a stroke occurs in a patient with AF it is more severe, more likely to recur, and more likely to result in death or disability than strokes in patients without AF. 13–15 Further, stroke survivors with AF face persistent neurological deficits and permanent disability, having a significant negative impact on their quality of life and increasing the burden of care for their family and the health services. 16
Information from The Office of Health Economics17 demonstrates the huge economic burden of AF to the NHS. In 2008, patients with AF accounted for 5.7 million bed-days, at a cost to the NHS of £1873M. In addition, other inpatient costs accounted for an extra £124M and outpatient costs (such as electrocardiography, monitoring anticoagulant treatment and post-discharge attendance) a further £205M. However, this figure does not take into account the significant societal costs, days of work lost, informal care, and the impact of AF on the patient and his or her family. The cost of AF appears to have increased dramatically since the turn of the century, given that a previous study estimated that the direct cost of AF to the NHS in 2000 was £45M, equivalent to 0.97% of total NHS expenditure. 18
Risk of stroke
The risk of stroke among patients with AF is heterogeneous, with risk dependent on associated comorbidities. The Stroke Risk in Atrial Fibrillation Working Group19 conducted a systematic review to identify independent predictors of stroke in patients with AF and found that a previous stroke or transient ischaemic attack (TIA) was consistently and independently associated with an augmented risk of a subsequent stroke, conferring a 2.5-fold increased risk. Increasing age also independently predicted stroke risk, with a 1.5-fold greater risk with each decade of life. In addition, a history of hypertension or elevated systolic blood pressure (> 160 mmHg) and diabetes mellitus doubled the stroke risk. Half of the studies that examined sex as a risk factor for stroke demonstrated that women had a 1.6-fold greater risk than men. 19 A history of HF and coronary artery disease (CAD) were not identified as independent risk factors for stroke by this systematic review, although systolic dysfunction (evidenced by echocardiography) was found to be a risk factor. 19 The risk of stroke in patients with AF is significantly reduced with anticoagulation therapy,20–23 and antiplatelet treatment also decreases the risk of stroke compared with placebo. 20
Current service provision
Antithrombotic management of atrial fibrillation
The management of AF consists of a rate and/or rhythm control strategy in combination with antithrombotic therapy (ATT). The aim of the former is to control the heart rate without attempting to restore the heart's normal rhythm (sinus rhythm), whereas the latter attempts to re-establish and maintain sinus rhythm. Regardless of which strategy is implemented, all patients should be assessed for individual stroke risk and receive appropriate ATT. Clinical guidelines2,24 recommend oral anticoagulant for patients who are at high risk of stroke, and either oral anticoagulation or antiplatelet(s) for those deemed to be at intermediate risk, although the European Society of Cardiology (ESC) guidelines2 prefer oral anticoagulation over antiplatelet(s) therapy in this group. Among those patients who are at low risk of stroke (those < 65 years of age with no stroke risk factors), the National Institute for Health and Care Excellence (NICE)24 recommends antiplatelet therapy (APT), whereas the ESC guidelines2 recommend APT or no treatment, with a preference for no therapy. 2,24
In order to determine the most appropriate ATT for each patient, his or her individual risk of stroke should be assessed. The main risk factors for stroke among patients with AF are described above (see Risk of stroke), but include previous stroke or TIA, age ≥ 65 years, HF, hypertension and diabetes mellitus, which together constitute the widely used stroke risk assessment tool, the CHADS2 (Congestive heart failure, Hypertension, Age ≥ 75 years, Diabetes mellitus, and prior Stroke or TIA or thromboembolism) score,25 although there are numerous other stroke risk stratification schemas available19 (Table 1).
| Risk stratification scheme, year | Risk | ||
|---|---|---|---|
| High | Moderate | Low | |
| AFI, 199426 | Previous stroke/TIA, hypertension, diabetes mellitus | Aged ≥ 65 years with no other risk factors | Aged < 65 years |
| SPAF Investigators, 199927 | Previous stroke/TIA, women aged > 75 years, men aged > 75 years with hypertension | Hypertension, diabetes mellitus | No risk factors |
| CHADS2, 2001 (classic)28 | Score of 3–6 | Score of 1–2 | Score of 0 |
| CHADS2, 2001 (revised)25 | Score of 2–6 | Score of 1 | Score of 0 |
| Framingham study, 200329 | Score of 16–31 | Score of 8–15 | Score of 0–7 |
| NICE guidelines, 200624 | Previous stroke/TIA/TE, aged ≥ 75 years with hypertension, diabetes mellitus, or vascular disease, clinical evidence of valve disease, HF, of LV dysfunction on echocardiography | Aged < 75 years with hypertension, diabetes mellitus, or vascular disease | Aged < 65 years with no moderate- or high-risk factors |
| Aged ≥ 65 years with no high risk factors | |||
| ACC/AHA/ESC guidelines, 20061 | Previous stroke/TIA/TE, or: | Aged ≥ 75 years, or hypertension, or HF, or LVEF ≤ 35%, or diabetes mellitus | No risk factors |
| or ≥ 2 moderate risk factors: age ≥ 75 years, hypertension, HF, LVEF ≤ 35%, diabetes mellitus | |||
| Eighth ACCP guidelines, 200830 | Previous stroke/TIA/TE, or: | Aged > 75 years, or hypertension, or moderately or severely impaired LVEF and/or HF, or diabetes mellitus | No risk factors |
| Two or more moderate risk factors: aged ≥ 75 years, hypertension, moderately or severely impaired LVEF and/or HF, or diabetes mellitus | |||
| CHA2DS2-VASc, 201031 | Score of ≥ 2 | Score of 1 | No risk factors |
| ESC guidelines, 20102 | Previous stroke/TIA/SE or aged ≥ 75 years, or: | Score of 1 | No risk factors |
| Two or more ‘clinically relevant non-major’ risk factors: HF or LVEF ≤ 40%, hypertension, diabetes mellitus, vascular disease,a aged 65–74 years, female sex | |||
In the UK, the NICE guidelines24 currently recommend aspirin 75–300 mg daily (unless contraindicated) for patients aged < 65 years with no moderate- or high-risk factors and who, thus, are deemed to be at low risk (≤ 1% annual risk) of stroke. For patients at moderate risk (4% annual risk), namely those aged < 75 years with hypertension, diabetes mellitus or vascular disease (CAD or peripheral artery disease) and those ≥ 65 years without any high-risk factors, NICE24 suggests anticoagulation or aspirin. Among patients at high risk (12% annual risk) of stroke, i.e. those with a previous stroke/TIA or thromboembolism (TE), clinical evidence of valve disease, HF, or impaired left ventricular (LV) function on echocardiography, or aged ≥ 75 years with hypertension, diabetes mellitus or vascular disease, NICE24 recommends anticoagulation with warfarin. The ESC guidelines2 have adopted a risk factor-based approach to determine appropriate thromboprophylaxis (Figure 1 and Table 1) and these guidelines have superseded the NICE recommendations in clinical practice in the UK. 2
FIGURE 1.
Clinical flow chart for the use of ATT in patients with AF. Redrawn from the ESC guidelines. 2 a, Congestive HF, hypertension, age ≥ 75 years, diabetes mellitus (1 point for each), stroke/TIA/TE (2 points); b, other clinically relevant non-major risk factors: age 65–74 years, female sex, vascular disease.
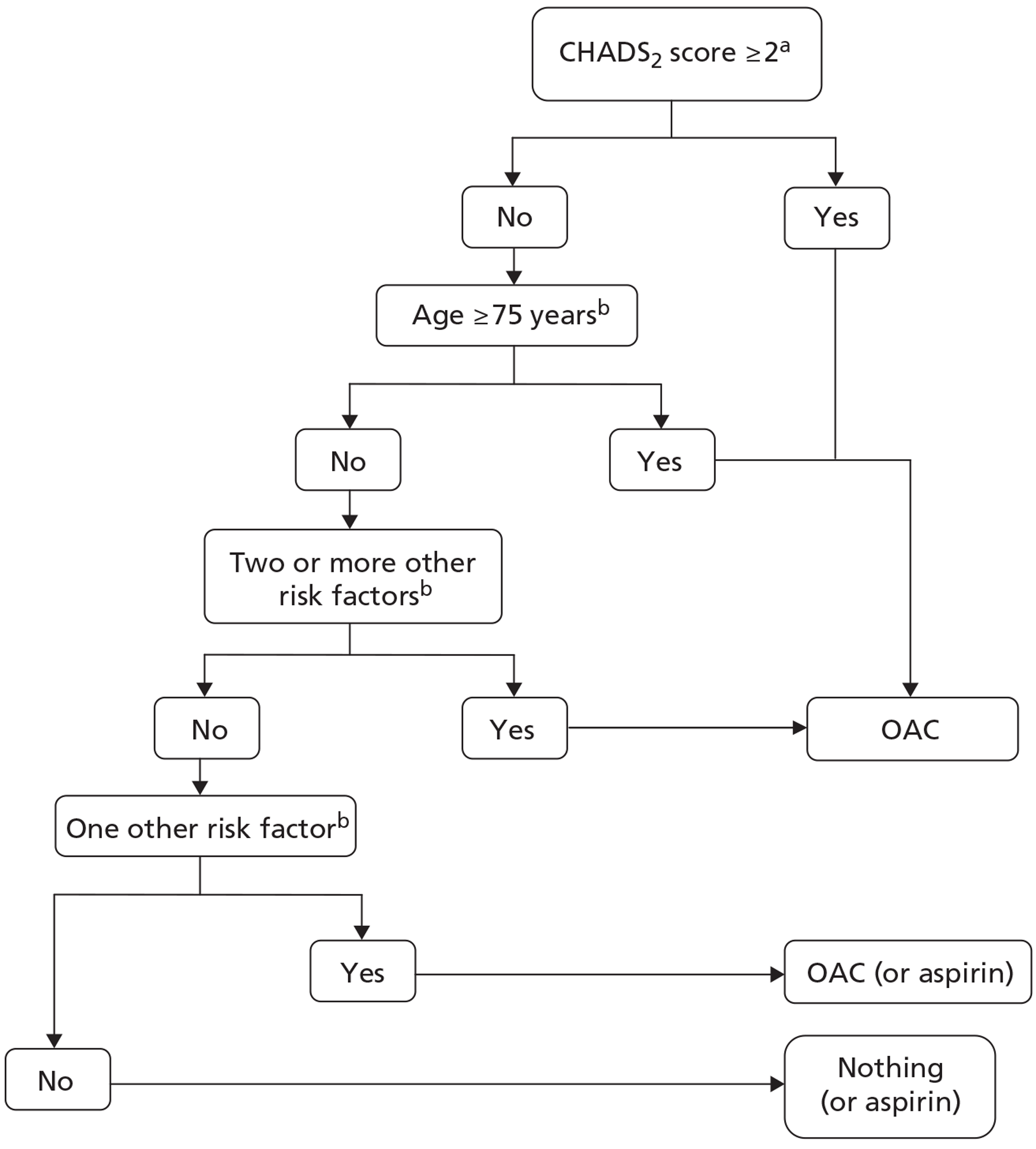
In patients with AF who have no risk factors for stroke, the ESC guidelines2 recommend either aspirin 75–325 mg daily or no ATT, with a preference for no treatment over aspirin. 2 For those with one ‘clinically relevant non-major’ risk factor [HF or left ventricular ejection fraction (LVEF) ≤ 40%, hypertension, diabetes mellitus, vascular disease, age 65–74 years, female sex], the ESC advises that oral anticoagulation or aspirin (75–325 mg) should be administered, with an oral anticoagulant (OAC) preferred over aspirin. Among those patients with one ‘major’ (previous stroke/TIA/TE or aged ≥ 75 years) or two or more ‘clinically relevant non-major’ risk factors, a OAC is recommended. Where a OAC is recommended, this includes adjusted-dose warfarin (INR 2.0–3.0) or one of the new anticoagulant drugs (see Description of technology under assessment).
In addition, CAD is also increasing in prevalence as a consequence of the improvements in survival due to advances in medical therapy and the ageing population. 30 Between 30% and 40% of patients with AF have concomitant CAD,11 and some of these patients may also require percutaneous coronary intervention (PCI) with stent implantation. Patients with AF and CAD are at increased risk of both stroke and further coronary events. An increasingly common management problem arises when faced with an anticoagulated patient with AF who presents with acute coronary syndrome (ACS) or those who require PCI with stent implantation. 32
Current guidelines for antithrombotic therapy in atrial fibrillation patients with acute coronary syndrome or undergoing percutaneous coronary intervention or stenting
The joint American College of Cardiology (ACC)/American Heart Association (AHA)/ESC 2006 guidelines on the management of AF recommend that following PCI or revascularisation surgery in patients with AF, low-dose aspirin (< 100 mg/day) and/or clopidogrel (75 mg/day) may be given concurrently with anticoagulation to prevent myocardial ischaemic events,1 although it is acknowledged that these strategies have not been thoroughly evaluated and are associated with an increased risk of bleeding. The 2006 ACC/AHA/ESC guidelines also suggest that clopidogrel should be given for a minimum of 1 month after implantation of a bare-metal stent, ≥ 3 months for a sirolimus (CYPHER™, Cordis)-eluting coronary stent-P020026, ≥ 6 months for a paclitaxel (ION™, Boston Scientific)-eluting coronary stent system-P100023, and ≥ 12 months in selected patients, following which warfarin may be continued as monotherapy in the absence of a subsequent coronary event. 1 Broadly similar recommendations are made in the eighth ACCP guidelines,33 which suggest that a low dose of aspirin (< 100 mg per day) or clopidogrel (75 mg per day) may be given with anticoagulation, although the risk of bleeding may be increased, particularly in elderly patients. The UK NICE guidelines24 do not address this topic, although acknowledging that adding aspirin to warfarin increases bleeding, and that it is a matter for individual assessment of the risk–benefit ratio in prescribing aspirin plus warfarin in patients with associated CAD.
Furthermore, all of the published guidelines do not address the issue of a presentation with ACS (where PCI is often performed) and bleeding risk. Given the need to balance stroke prevention, recurrent cardiac ischaemia and/or stent thrombosis, two more recent consensus documents,34,35 based on systematic reviews of patients on OAC undergoing PCI and stenting, advocate initial triple therapy (with OAC, aspirin and clopidogrel) in such patients, and the use of bare-metal stents (owing to the need for prolonged multiple-drug ATT with drug-eluting stents). However, triple ATT is associated with a higher risk of major bleeding and this risk must be considered before treatment initiation. 34,36 Therefore, the ESC Working Group on Thrombosis consensus guidelines35 recommend limiting triple ATT to 2–4 weeks in patients who are at high risk of haemorrhage (Table 2).
| Haemorrhagic risk | Clinical setting | Stent implanted | Recommendations |
|---|---|---|---|
| Low or intermediate | Elective | Bare metal |
|
| Elective | Drug eluting |
|
|
| ACS | Bare metal/drug eluting |
|
|
| High | Elective | Bare metalc |
|
| ACS | Bare metalc |
|
Description of technology under assessment
Anticoagulant therapy (ACT) is recommended for patients with AF who are at high risk of stroke. The main type of ACT used for patients with AF is a vitamin K antagonist (VKA), most commonly warfarin, to maintain a therapeutic international normalised ratio (INR) value of 2.0–3.0. Other classes of anticoagulants include heparins (low-molecular-weight heparins), hirudins, and, more recently, the novel anticoagulant drugs, direct oral thrombin inhibitors (ximelagatran and dabigatran), and factor Xa inhibitors [idraparinux, apixaban (Eliquis®, Bristol-Myers Squibb), rivaroxaban (Xarelto®, Bayer) and endoxaban] (Table 3). APT is also used for stroke thromboprophylaxis in patients with AF. Antiplatelet agents currently used include aspirin (non-proprietary; typically), clopidogrel (Plavix®, Sanofi-aventis), ticlopidine, dipyridamole (Persantin®, Boehringer Ingelheim) and triflusal (Table 3).
| Anticoagulants | Antiplatelet agents |
|---|---|
VKAs
|
|
| Heparins | |
|
|
| Hirudins | |
|
|
| Direct oral thrombin inhibitors | |
|
|
| Factor Xa inhibitors | |
|
Anticoagulation, antiplatelet or combined therapy in high-risk patients with atrial fibrillation
Among patients with AF, there is evidence that thromboprophylaxis with warfarin reduces the risk of TE (by 64%) compared with placebo or aspirin (by 39%). 20 Aspirin reduces the risk of TE in patients with AF by 22% compared with placebo. 20
However, it is currently unclear whether or not there is any additional benefit in adding APT to ACT in high-risk patients with AF in terms of reduction in vascular events, including stroke.
The available data from individual studies are conflicting, apart from the consistent message that combining APT with oral anticoagulation increases the risk of major bleeding. There is currently no definitive answer to the question of whether or not combination anticoagulant and antiplatelet (mono- and dual-antiplatelet) therapy is beneficial in patients with AF and concomitant CAD/vascular disease, and those undergoing PCI and stent implantation. The available evidence from observational cohort studies and registry analyses suggests a reduction in TEs with combination and triple therapy, given for a short duration, in patients with AF and concomitant CAD/vascular disease with stent implantation. However, the risk reduction in TEs is offset by an increased risk of major bleeding. 35
The aim of the current study is therefore to identify the benefits of adding APT in a subgroup of high-risk patients with AF who are receiving ACT, in whom this can be justified in terms of the balance of reducing vascular events without increasing bleeding.
Chapter 2 Methods
Aim
To determine if the addition of APT to ACT is beneficial compared with ACT alone in patients with AF who are considered to be at high risk of TEs.
Objective
To undertake a systematic review of studies comparing ACT alone with ACT in combination with APT in patients with AF.
Definitions
The Background chapter describes AF. For the purposes of this review, the definition of AF used was that determined by the authors of studies.
The Background chapter describes ACT and APT used to treat AF. For the purposes of this review, no limits were placed on the type of therapies that could be chosen as being anticoagulant or antiplatelet agents.
High-risk patients of special interest include patients with AF with previous myocardial infarction (MI) or ACS, those undergoing PCI and stent implantation, those with diabetes mellitus, and those with a CHADS2 score of ≥ 2. However, no restrictions were placed on the determinants of high risk.
Relevant study designs
Given the likely paucity of directly relevant RCTs, the steering group for this project was consulted at an early stage about whether or not evidence from a wider selection of study designs should be reviewed. The steering group decided that this should be the case.
Review methods
Standard systematic review methodology was used, consisting of searches to identify available literature, sifting and the application of specific criteria to identify relevant studies, assessment of the quality of these studies, and the extraction and synthesis of relevant data from them. The review was guided by a protocol that was prepared a priori (see Appendix 1) and externally reviewed prior to use.
Search strategies
The following resources were searched for relevant studies:
-
Bibliographic databases: The Cochrane Library [Cochrane Central Register of Controlled Trials (CENTRAL)] 2010 Issue 3; MEDLINE (Ovid) 1950 to September week 1 2010; MEDLINE In-Process and Other Non-Indexed Citations from inception to 27 September 2010; and EMBASE (Ovid) 1980 to September 2010. Searches were based on index and text words that encompassed the population: atrial fibrillation and the interventions; combined anticoagulation and antiplatelet therapy.
-
Ongoing trials were sought in ClinicalTrials.gov, National Institute for Health Research (NIHR) Clinical Research Network Portfolio, Current Controlled Trials (CCT) and the WHO International Clinical Trials Registry Platform (ICTRP).
-
Reference lists from identified systematic reviews were checked.
-
Citations of relevant studies were examined.
-
Further information was sought from clinical experts.
All study types were sought. Searches were not limited by language or date and were carried out during September 2010 by an information specialist.
Search strategies used in the bibliographic databases can be found in Appendix 2.
Scoping searches were undertaken to identify completed and ongoing systematic reviews from the following resources: The Cochrane Library [Cochrane Database of Systematic Reviews (CDSR), Database of Abstracts of Reviews of Effects (DARE), Health Technology Assessment (HTA) database, CENTRAL and NHS Economic Evaluation Database (NHS EED)], Aggressive Research Intelligence Facility (ARIF) database of reviews, HTAi portal, MEDLINE (Ovid) 1950 onwards and EMBASE (Ovid) 1980 onwards. The systematic reviews were used to check if there were additional relevant studies.
Study selection
All records identified in the searches were imported into a Reference Manager database (Reference Manager v.11, Thomson ResearchSoft, San Francisco, CA, USA). Duplicate entries were allowed to be removed by the inbuilt feature in Reference Manager and also removed when encountered by reviewers.
Owing to the number of retrieved records and the complexity of the publications, a three-stage process was used to select the studies for review.
Stage 1
The aim was to exclude obviously irrelevant records. The titles of all records were scanned by one reviewer and the record retained if it was about an article/study that met ANY of the following criteria:
-
any AF study
-
any stroke study
-
any study with a group of patients on ACT, APT or both.
Study design or publication type was not an exclusion criterion for this stage.
Stage 2
Based on the title and abstract where available, records were retained if they were about an article/study that adhered to all of the following criteria:
-
any AF population receiving ACT, APT, or both
-
indicated effectiveness data were reported.
Study design or publication type was not an exclusion criterion for this stage.
In the first instance, this stage was undertaken by two reviewers independently; however, it became clear that complexity of the information in the records and particularly absence of detail were leading to far from ideal agreement between the two reviewers (Cohen's kappa coefficient = 0.51). For this reason all records for which discord occurred were screened independently by two further reviewers and any disagreements at this level were resolved by discussion.
All articles progressing through to this stage were obtained in hard copy.
Stage 3
The hard copies were assessed for inclusion in the review against the following criteria. All criteria had to be met to warrant inclusion.
-
Study design RCTs, non-randomised comparisons, cohort studies, case series or registries, longitudinal studies, systematic reviews and meta-analyses, and conference abstracts published after 2008.
-
Population Patients with AF, aged ≥ 18 years. Publications were included, even if a subgroup of patients in the study conformed to this criterion.
-
Intervention Publications were included only if there was a subgroup of, or complete cohort of, patients on combined ACT and APT. Publications in which the INR of ACT was not specified were also included.
-
Comparator ACT alone or ACT plus placebo.
-
Outcomes All-cause mortality and/or at least one vascular event(s) [non-fatal and fatal ischaemic stroke, TIA, systemic embolism (SE)] SE (pulmonary/peripheral arterial embolism), MI, in-stent thrombosis, vascular death, bleeding (major, non-major, minor), reported for both intervention and comparator groups.
If any of the following criteria were met, then the article was excluded:
-
Study design: All case studies, bridging therapy studies with heparin, rationale or study design papers, ecological studies, case–control studies, cross-sectional studies (surveys), conference abstracts published before 2008, commentaries, and letters or communications were excluded.
-
Population: Articles that specified a population as having a CHADS2 score of < 2 or stroke patients with AF for whom outcomes were retrieved retrospectively, or a population with valve replacement or mechanical heart valves. If CHADS2 scoring or any other stroke risk scoring was not specified, then this was not a reason to exclude an article.
Part-translation of articles not fully published in the English language was obtained to facilitate selection.
The criteria were applied by two reviewers independently and disagreements were resolved by discussion and with the involvement of a third reviewer if required. The reason(s) for the exclusion of articles were recorded.
Where there was more than one unique article from a single study the articles were grouped together for reviewing purposes.
Systematic reviews and meta-analyses that met the inclusion criteria were not reviewed but were utilised to identify further articles. Articles identified in this way were entered in to the Reference Manager database and subjected to the same selection process outlined above.
Data extraction
Data were extracted into a standard form in Microsoft Excel 2007 v.12 (Microsoft Corporation, Redmond, WA, USA) from the main and supporting publications (where relevant) of all included primary studies by one reviewer. A second reviewer checked the accuracy of extracted information. Disagreements were resolved by consensus or by referral to a third reviewer if necessary.
Information regarding study design (including intervention/comparators) and characteristics of study participants was extracted. This included antithrombotic regimens used [anticoagulant ± antiplatelet(s) or placebo], type of ATT used and dose, target INR values used, indication for ATT (e.g. AF ± ACS or stent implantation), study setting (country), study design, sample size, patient inclusion and exclusion criteria, patient characteristics (e.g. age, sex, type and duration of AF, anticoagulant naive or experienced), comparability of patients between different arms (for RCTs and non-randomised trials), primary outcome measures, secondary outcome measures, length of follow-up, statistical methods used, effect sizes and uncertainty.
Data on the following outcomes were sought from included studies.
Primary outcome measures
Vascular event – stroke (non-fatal and fatal ischaemic), TIA, SE (pulmonary embolism, peripheral arterial embolism), MI, in-stent thrombosis and vascular death (from any of the aforementioned vascular events).
Secondary outcome measures
All-cause mortality and bleeding (major bleeding events, clinically relevant non-major bleeding events, minor bleeding), health-related quality of life, major adverse events (composite of all-cause mortality, non-fatal MI and stroke), revascularisation procedures (e.g. PCI, coronary artery bypass graft surgery, embolectomy) and percentage of time in therapeutic INR range.
Definitions of these outcomes as used in each study were also extracted where reported.
Data for any outcomes other than those listed above were also extracted if it was considered relevant to this report.
Quality assessment
The quality of included studies was assessed by one reviewer. A second reviewer checked the accuracy of extracted information. Disagreements were resolved by consensus or by referral to a third reviewer if necessary.
The methodological quality of RCTs was assessed in terms of the randomisation process, allocation concealment (adequate, unclear, inadequate or not used), degree of blinding, particularly of the outcome assessors, and patient attrition rate, using the Cochrane Collaboration risk of bias assessment tool. 37
The quality assessment of studies undertaking non-randomised comparisons was undertaken using the Centre for Reviews and Dissemination (CRD)'s checklist for cohort studies. 38 Information on the following was captured: method of outcome measurement, blinding of assessors, whether or not outcome definitions were clearly explained, and which parts of the study were prospective. In addition, the following topic-specific data that were considered relevant to the quality of the studies were assessed: ‘Were the indications for use of APT given?’ and ‘Was it clear whether patients were on APT at the start or commenced such therapy during the observation period?’
Data from randomised studies that were obtained from non-randomised comparisons were classed and treated as non-randomised data. For example, when data from a subset of patients in two or more arms of a RCT were combined to compare with data from another subset of patients obtained from these or other arms of the same study.
From non-randomised comparisons the potential for confounding by indication was ever present; whereby APT was added to ACT, based on clinical judgement of a potential risk of adverse outcomes in some patients if such therapy was not given. Conversely, in those without such perceived risk APT may not have been given. Thus, the patients receiving anticoagulation alone would differ from those receiving the combined therapy, and thus any comparison between the two would be confounded.
Data analysis/synthesis
Outcomes of interest
Selected outcomes of interest were specified in the review protocol, based, in part, on the briefing document produced by the NIHR. These were as shown below.
Primary outcome measures
-
Vascular events:
-
non-fatal and fatal ischaemic stroke
-
TIA
-
SE (pulmonary embolism, peripheral arterial embolism)
-
MI
-
in-stent thrombosis
-
vascular death (from any of the above mentioned vascular events).
-
Secondary outcome measures
-
All-cause mortality.
-
Bleeding:
-
major bleeding events
-
clinically relevant non-major bleeding events
-
minor bleeding.
-
-
Health-related quality of life.
-
Major adverse events.
-
Revascularisation procedures.
-
Percentage of time within therapeutic INR range (where available).
Although definitions of these outcomes could have been described rigidly for this review (such as using the definitions of the International Society on Thrombosis and Haemostasis39) it was decided to retain and record the definitions used in the original papers and to group data accordingly. Setting aside issues around non-reporting or poor reporting of definitions, for most outcomes this was fairly straightforward. However, there were instances for which judgement was required. For example, for the outcome of SE a few studies referred to TE and it was assumed from the definitions of outcomes provided by the studies that TE referred to arterial TE, not venous TE, and thus data from these studies were grouped with SE from similar studies.
For the outcomes of interest, data were not available for all.
Handling data and presentation of results
Owing to the paucity of evidence from randomised studies, data from non-randomised and/or observational designs were also included in this review. Evidence from different study designs was not combined.
The comparison of interest was between combined anticoagulation and APT and ACT alone.
For dichotomous outcomes, data from randomised studies are presented as proportions, percentages and relative risks (RRs) [± 95% confidence interval (CI)] for comparisons. RRs and 95% CIs were calculated using Review Manager (RevMan v.5.1: The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark). Dichotomous data from non-randomised comparisons are not presented as RRs, given the potential for confounding by indication within such studies. If continuous outcome data had been encountered, they would be represented as differences in means or means.
Where available, data were presented for the longest follow-up available in each study. Data for follow-up assessments less than this are also presented, where appropriate. In many cases only mean/median follow-up durations were reported by studies.
Studies were considered to directly compare anticoagulation plus APT with ACT alone if the anticoagulant was the same in both arms, and there were no other treatment-related differences between arms.
Different anticoagulation therapies were considered separately. Different APTs were also considered separately. As ACT can be a fixed or adjusted dose it was decided a priori on clinical advice to report these regimes separately. A priori it was decided that only the following groups could be considered as classes of intervention. VKAs were considered as a class of intervention and, thus, reported together and where possible pooling of data across the class was considered if there was sufficient methodological and clinical homogeneity between studies. Oral direct thrombin inhibitors (ODTIs) were also considered as a class of intervention. None of the APTs was considered as a class.
Although planned, pooling of results was not attempted for the assessment of effectiveness of individual technologies because of the substantial clinical and methodological heterogeneity between studies and the confounding by indication inherent in the observational studies.
Assessment of publication bias
The number of relevant studies for a given comparison was too small to allow formal assessment of publication bias.
Ongoing studies
A number of ongoing studies were identified in the searches. They were not included in the systematic review, but discussed in Chapter 4 (see Strengths and limitations, Ongoing studies) to aid updating and extension of this review.
Sensitivity and subgroup analysis
Although the number of subgroups and/or sensitivity analyses might have been possible in this report, none was undertaken owing to lack of data.
Changes to protocol
The protocol specified that, where possible, the relevant target INR for the combined ACT-plus-APT treatment arm should be 2.0–3.0 as recommended by ESC guidelines. 2 However, it was felt that this criterion might be too restrictive or the range not reported. Therefore, this criterion was relaxed to allow inclusion of studies with either a different target range or an unspecified target INR range.
It was intended and specified in the protocol that an individual participant data (IPD) meta-analysis would be performed to specifically address the effect of APT added to ACT compared with ACT alone on (1) time to first vascular event; (2) time to first major haemorrhage or clinically relevant bleed; (3) death; and (4) time within therapeutic INR range. Predefined subgroup analyses were to be developed to possibly include the following: (1) stent type (bare metal vs drug eluting); (2) warfarin-naive subjects compared with warfarin-experienced subjects; (3) short- and long-term outcomes; (4) patients with diabetes mellitus; and (5) a CHADS2 score of ≥ 2 and < 2. Data were to be requested either in electronic or paper from triallists and subjected to consistency checks.
However, there was a paucity of evidence from the included studies for many of these analyses, and where some data were available it was clear that the methodological heterogeneity between studies, and the clinical heterogeneity within and between studies, was against such analyses. It was therefore agreed with the NIHR not to perform the IPD analysis (for further explanation, see Chapter 4, Strengths and limitations).
An additional stage of study selection was added (Stage 2 is described above – see Study Selection) because of the high yield of relevant studies from the preceding stages. In this new stage, selection criteria were based on those determined a priori for the whole review and thus unbiased. This new selection stage came before obtaining full copies of articles and the application of all of the inclusion/exclusion criteria for the review.
Reporting findings
In the following sections based on clinical input, the findings of the review are structured by outcome (and subcategories of outcome where relevant) and then for each outcome by intervention–comparison (including division by whether ACT was by adjusted or fixed dosing), with further subdivision by risk attributed to the populations where relevant. Data from randomised comparisons are the primary evidence presented with supplementary information given from pooled analyses and/or non-randomised comparisons where this information adds to that from the randomised comparisons (i.e. longer follow-up). However, caution is applied with the use of non-randomised data given that the findings are highly likely to be confounded by indication. A summary section is provided where the findings are presented by intervention and comparator, and then for each of these the data for the review outcomes are presented. Presenting the data in both ways allows access to information depending on whether the perspective required is that of the outcomes or the comparisons.
Chapter 3 Results
Quantity and quality of research available
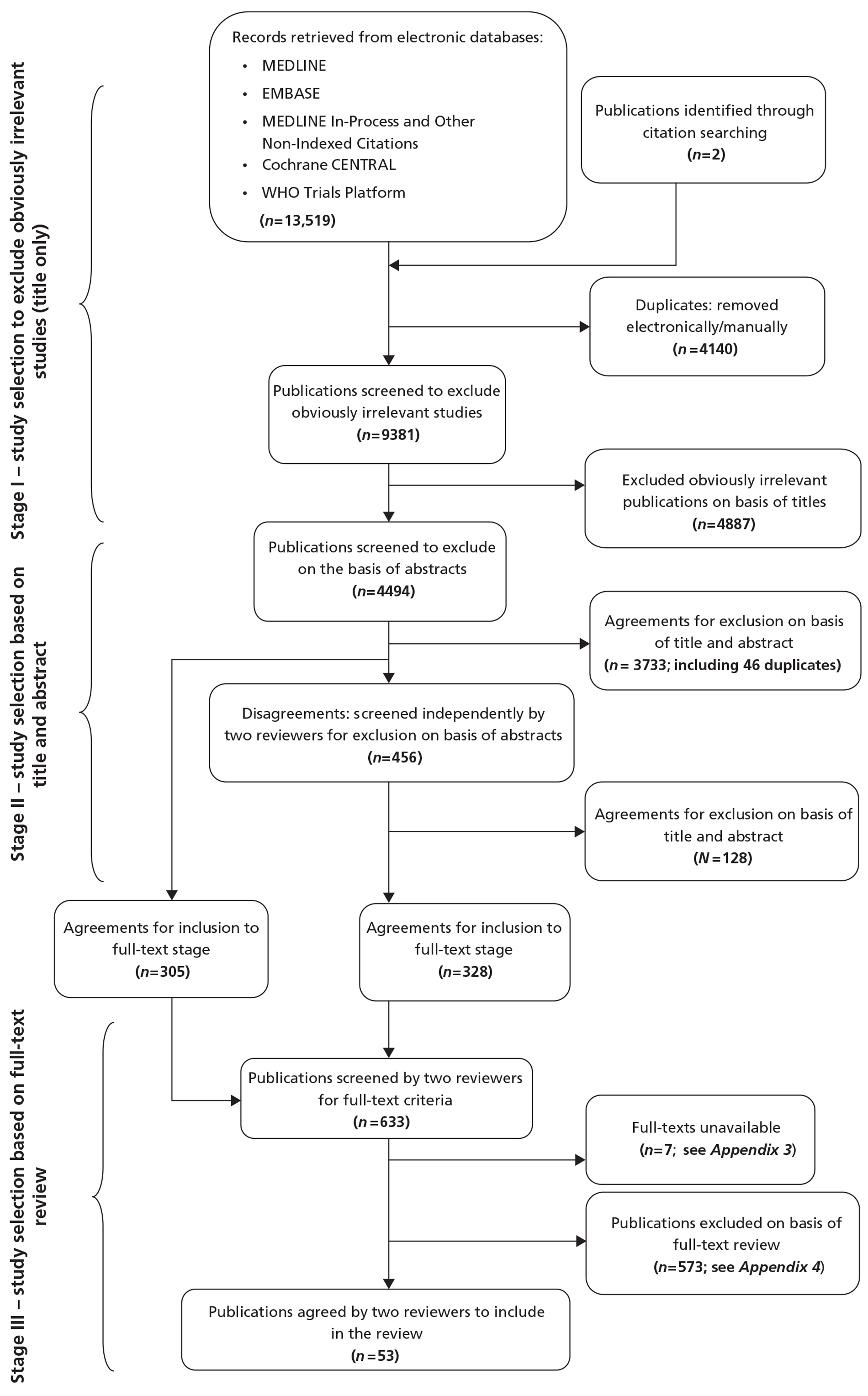
Figure 2 illustrates the study selection stages. The combined bibliographic database search yielded 13,519 citations. After the removal of records for non-relevant articles and duplicate entries, full texts of 633 potentially relevant articles were sought. The authors of 12 studies were contacted, as copies of the study reports were difficult to obtain. Seven of these were still unobtainable after this procedure. Details of these studies are presented in Appendix 3. The 626 full articles were assessed against the criteria for inclusion in the review by two reviewers independently. A total of 53 publications met the criteria (see Figure 2). A list of excluded publications along with reason(s) for their exclusion can be found in Appendix 4.
FIGURE 2.
Study selection.
No ongoing studies comparing combined ACT plus APT with ACT alone were identified in the searches. In the discussion chapter (see Chapter 4), there is a section on the pre-defined subgroup analysis of the ongoing or recently completed Phase III clinical trials identified by the steering committee.
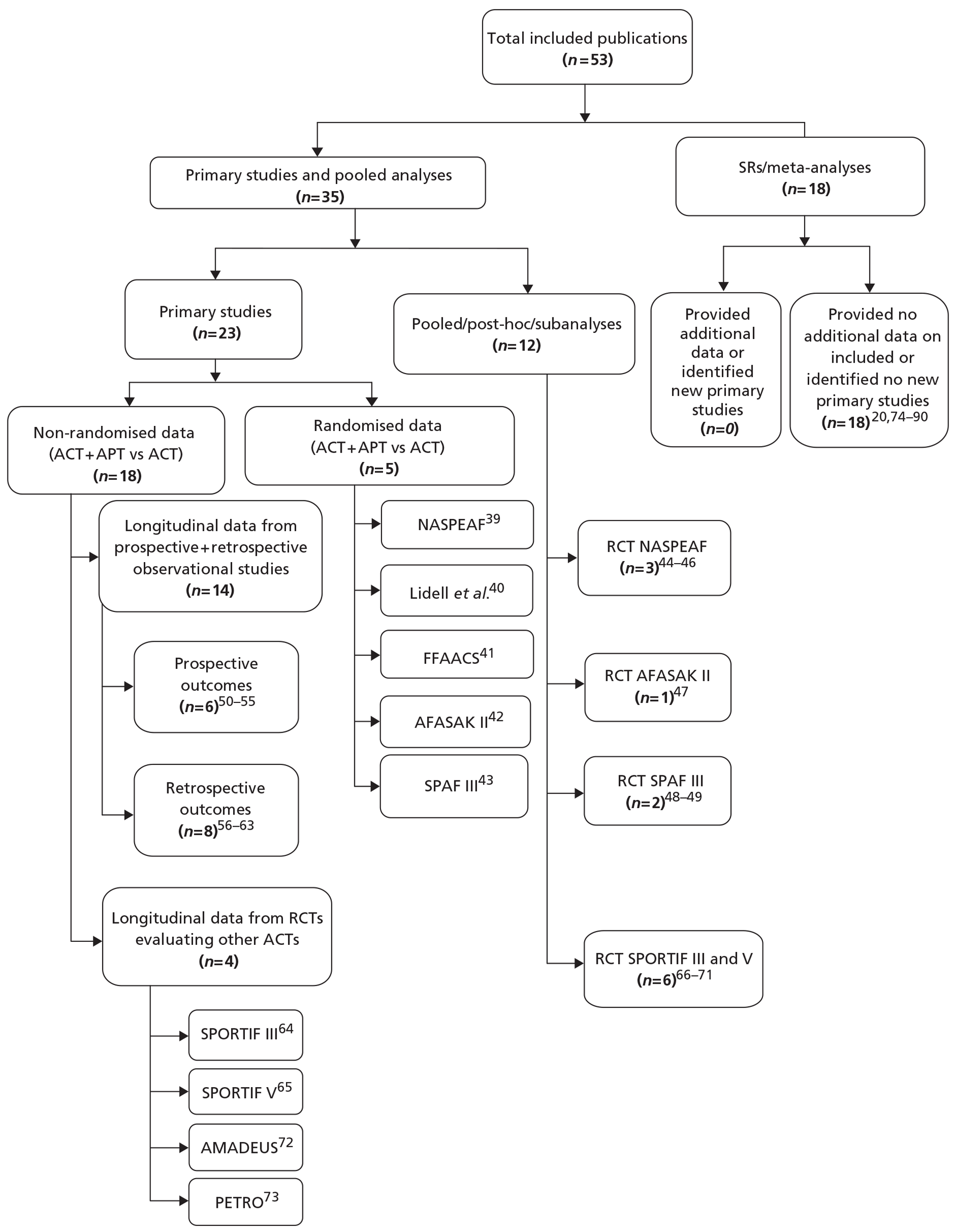
Characteristics of included studies
Of the 53 included publications (Figure 3),20,39–90 18 were reports of systematic reviews or meta-analyses20,74–90 which added no further data to the remaining 35 articles (see Figure 2 and Appendix 5). 39–73 Of the latter, five articles39–43 each reported randomised controlled studies between ACT plus APT and ACT alone. Three of these RCTs were supported by post hoc, subgroup or pooled analyses reported in a further six articles. 44–49 The characteristics of these studies and their quality assessment are reported in Tables 4 and 5, respectively, and in Appendix 6.
FIGURE 3.
Included studies. AFASAK II, Second Copenhagen Atrial Fibrillation, ASpirin and Anticoagulation Study; AMADEUS, Comparison of fixed-dose idraparinux with conventional anticoagulation by dose-adjusted oral vitamin K antagonist therapy for prevention of thromboembolism in patients with atrial fibrillation; FFAACS, Fluindione, Fibrillation Auriculaire, Aspirin et Contraste Spontané study; NASPEAF, NAtional Study for Prevention of Embolism in Atrial Fibrillation; PETRO, dabigatran with or without concomitant aspirin compared with warfarin alone in patients with non-valvular atrial fibrillation study; SPAF III, Stroke Prevention in Atrial Fibrillation III study; SPORTIF, Stroke Prevention using an ORal Thrombin Inhibitor in atrial Fibrillation.
| Author, date (name of trial), location, no. of centres | Study duration (mean), randomisation design, no. of patients randomised | Intervention (ACT + APT), no. of patients | Comparator (ACT only or ACT + placebo), no. of patients | Inclusion criteria; stroke risk | Age (years): mean (SD, range), % male |
|---|---|---|---|---|---|
| aPérez-Gómez et al., 2004 (RCT – NASPEAF), multicentre39 | 33 months, parallel, open label, n = 1209 | Adjusted-dose acenocoumarol (INR 1.25–2.0) + triflusal (600 mg), n = 222 (intermediate risk) | Adjusted-dose acenocoumarol (INR 2.0–3.0), n = 237 | Age ≥ 18 years; high risk of strokeb or intermediate risk of strokec | 68.6;d 45.6 |
| Adjusted-dose acenocoumarol (INR 1.4–2.4) + triflusal (600 mg), n = 223 (high risk) | |||||
| Lidell et al., 2003, Sweden, four centres40 | 22 days, parallel, double blind, placebo controlled, n = 43 | Adjusted-dose warfarin (INR 2.0–3.0) + clopidogrel (75 mg); n = 20 | Adjusted-dose warfarin (INR 2.0–3.0) + placebo, n = 23 | Age 35–75 years, NVAF, receiving warfarin for ≥ 2 months; no stroke risk factors reported | 66.6;d 81.4 |
| Lechat et al., 2001, (RCT – FFAACS), France, multicentre41 | 0.84 years, parallel, double blind, placebo controlled, n = 157 | Fluindione (INR 2.0–2.6) + aspirin (100 mg), n = 76 | Fluindione (INR 2.0–2.6) + placebo, n = 81 | NVAF; high risk of strokee | 73.7;d 50 |
| fGullov et al., 1998, (RCT – AFASAK II), Denmark, single centre42 | 42 months, parallel, open label, n = 677 | Fixed-dose warfarin (1.25 mg) + aspirin (300 mg); n = 171 | Fixed-dose warfarin (1.25 mg/day), n = 167 | Age ≥ 18 years with chronic NVAF; no stroke risk factors reported | 76.5 (6.9, 44–89), 60 |
| Adjusted-dose warfarin (INR 2.0–3.0), n = 170 | |||||
| gStroke Prevention in Atrial Fibrillation Investigators, 1996 (RCT – SPAF III), USA and Canada, 20 sites43 | 1.1 years, parallel, open label, n = 1044 | Fixed-dose warfarin (INR 1.2–1.5) + aspirin (325 mg), n = 521 | Adjusted-dose warfarin (INR 2.0–3.0), n = 523 | Age ≥ 18 years with NVAF, eligible 30 days from occurrence of stroke/TIA; high risk of strokeh | 72 (9); 61 |
| Author, date (name of the trial) duration (mean) | Truly random allocation and sequence generation, method | Adequate allocation concealment | Blinding | Use of ITT | Dropouts and withdrawals, n (%) | Percentage on ACT before study | Comments |
|---|---|---|---|---|---|---|---|
| aPérez-Gómez et al., 2004 (RCT – NASPEAF), 33 months39 | Yes, computer generated, centrally administered | Nob | Yesc | No | Withdrawals, 18.3%; lost to follow-up, 50 (4.14) | NR | Withdrawals resulted in switch over from combined treatment to ACT in 56 patients |
| Lidell et al., 2003, 22 days40 | Not clear, NR | Unclear | Unclear | Yes | Withdrawals and lost to follow-up, 0 | NR | Arbitrary sample size used |
| Lechat et al., 2001 (RCT – FAACS), 0.84 years41 | Yes, centrally performed randomisation through fax transmission of the inclusion form | Yes | Yesc | No | Withdrawals,30; deaths, 6; lost to follow-up, 0 | 85 | Small sample size; premature termination of trial due to low event rate and recruitment rate |
| dGullov et al., 1998, (RCT – AFASAK II), 42 months42 | Yes, computerised randomisation | Nob | Yesc | Yes | Withdrawals, 112 (16.5%); dropout, 58 (8.6) | 0 | Premature termination after publication of SPAF III43 results |
| eStroke Prevention in Atrial Fibrillation Investigators, 1996 (RCT – SPAF III), 1.1 years43 | Yes, stratified by study centre and sequence could not be previewed | Nob | Not clearf | Yes | Withdrawals, 72 (6.9%); lost to follow-up: 0 | 56 | Multiple laboratories with reagents of varying sensitivities used for INR measurements; trial terminated in interim analysis (after mean follow-up of 1.1 years) as adjusted-dose warfarin was found superior to combined therapy; diabetes mellitus not considered as one of the stroke risk factors |
The remaining 24 articles50–73 consisted of 18 primary studies reporting non-randomised comparisons for the therapies of interest. Of these, 14 studies50–63 (in 14 articles) reported data from observational designs, both prospective50–55 and retrospective56–63 in nature. The remaining four studies in 10 articles64–73 were originally designed to assess the effectiveness of an anticoagulant without additional APT. However, these were included because they reported data on a subgroup of patients treated with combined anticoagulant plus APT. The characteristics of these studies and their quality assessment are reported in Tables 6 and 7, respectively.
| Author, date; study source; location; no. of centres | Study duration mean (SD, range); prospective/retrospective; no. of patients | ACT (INR or dose) + APT (dose), no. of patients | ACT only (INR or dose), no. of patients | Inclusion criteria; stroke risk | Age (years): mean (SD, range), percentage males |
|---|---|---|---|---|---|
| Hansen et al., 2010; registry; Denmark; nationwide registries63 | 3.3 (2.6) years; retrospective; n = 118,606 | Warfarina + aspirin,a 18,345 Warfarina + clopidogrel,a 1430 Warfarina + aspirina + clopidogrel,an = 1261 | Warfarin,an = 50,919 | Age ≥ 30 years, surviving first-time hospitalisation for primary or secondary diagnosis of AF, discharge prescription of warfarin, aspirin, clopidogrel | 73.7 (12.3); 52.4 |
| Stroke risk NR | |||||
| bBover et al. 2009 to n = 574; 4.2 years54 | 4.92 years; prospective; n = 574 | Acenocoumarol (INR 1.9–2.5) + triflusal (600 mg), n = 155 | Acenocoumarol (INR 2.0–3.0), n = 265 | Patients who had undergone at least 12 months of follow-up | 68.6; 45.6 |
| Acenocoumarol (INR 1.9–2.5) + triflusal (300 mg), n = 120 | |||||
| Acenocoumarol (INR 1.9–2.5) + aspirin (100 mg), n = 34 | |||||
| Lopes et al., 2009; cohort of RCT – APEX AMI; USA, Europe, Australia, NZ and Canada; 296 sites50 | 90 days; prospective; n = 276 | Warfarina + aspirina + clopidogrel,an = 37 | Warfarin,an = 59 | Age ≥ 18 years | 52–81 |
| Stroke risk highc | |||||
| Abdelhafiz and Wheeldon, 2008; anticoagulation clinic referrals; UK; one hospital51 | 19 (8.1, 1–31) months; prospective; n = 402 | Adjusted-dose warfarin (INR 2.0–3.0) + aspirin,an = 8 | Adjusted-dose warfarin (INR 2.0–3.0), n = 394 | New NVAF patients referred by GP | 72.3; 55.72 |
| Stroke risks NR | |||||
| Amadeus Investigators, 2008, cohort of RCT – AMADEUS; Australia, Canada, Denmark France, Italy, New Zealand, Poland, Netherlands, UK and the USA; 165 centres72 | 311 days; prospective; n = 4576 | Idraparinux or adjusted-dose VKAd (INR 2.0–3.0) + aspirin (≤ 100 mg), n = 971 | Idraparinux (2.5 mg), n = 2283; adjusted-dose VKAd (INR 2.0–3.0), n = 2293 | NVAF and indication for long-term anticoagulation ≥ 1 stroke risk factore | 70.1 (9.1); 66.5 |
| Ezekowitz et al., 2007; cohort of RCT – PETRO; Denmark, the Netherlands, Sweden, and the USA; 53 centres73 | 12 weeks, prospective; n = 502 | Dabigatran (50 mg b.i.d.) + aspirin (81 mg), n = 21 | Dabigatran (50 mg), n = 105 | Documented AF + CAD ≥ 1 stroke risk criteriaf | 70 (8.3); 81.9 |
| Dabigatran (50 mg b.i.d.) + aspirin (325 mg), n = 27 | Dabigatran (150 mg), n = 166 | ||||
| Dabigatran (150 mg b.i.d.) + aspirin (81 mg), n = 36 | Dabigatran (300 mg), n = 161 | ||||
| Dabigatran (150 mg b.i.d.) + aspirin (325 mg), n = 33 | |||||
| Dabigatran (300 mg b.i.d.) + aspirin (81 mg), n = 34 | |||||
| Dabigatran (300 mg b.i.d.) + aspirin (325 mg), n = 30 | |||||
| Suzuki et al., 2007; database of cardiovascular clinic; Japan; one centre56 | 1 year, retrospective; n = 667 | Adjusted-dose warfarin (INR 1.6–2.6) + aspirin,an = 210 | Adjusted-dose warfarin (INR 1.6–2.6), n = 457 | NVAF patients on warfarin Stroke risks NR | 68.4 (10.6); 66.6 |
| Burton et al., 2006; patient records from GPs; Scotland; 27 practices57 | 42 months; retrospective; n = 601 | Adjusted-dose warfarin (INR 2.0–3.0) + aspirin,an = 18 | Adjusted-dose warfarin (INR 2.0–3.0), n = 309 | Patients with persistent AF | 77; 51.1 |
| Stroke risks NR | |||||
| Stenestrand et al., 2005; registry; Sweden; 72 hospitals58 | 1–8 years; retrospective; n = 5616 | OACa,g + aspirin,an = 479 | OAC,a,gn = 1369 | AF on the discharge ECG and AMI as final diagnosis; stroke risk highh | 77.7; 62.43 |
| SPORTIF V investigators, 2005; cohort of RCT – SPORTIF V; USA, Canada; 409 sites65 | 20 months (5.1, 0–31); prospective; n = 3992 | Ximelagatran (36 mg b.i.d.) + aspirin (< 100 mg), unclear | Ximelagatran (36 mg b.i.d.), n = 1960 | Persistent or paroxysmal NVAF patients; high riske | 72 (9.1); 69 |
| Adjusted-dose warfarin (INR 2.0–3.0) + aspirin (≤ 100 mg), unclear | Adjusted-dose warfarin (INR 2.0–3.0), n = 1962 | ||||
| SPORTIF III Investigators, 2003; cohort of RCT – SPORTIF III cohort; Europe, Asia, Australasia; 259 hospitals64 | 17.4 (4.1) months; prospective; n = 3407 | Ximelagatran (36 mg b.i.d.) + aspirin (< 100 mg), unclear | Ximelagatran (36 mg b.i.d.), n = 1704 | Persistent or paroxysmal NVAF; high riske | 70 (9); 69.1 |
| Adjusted-dose warfarin (INR 2.0–3.0) + aspirin (≤ 100 mg), unclear | Adjusted-dose warfarin (INR 2.0–3.0), n = 1703 | ||||
| Teitelbaum et al., 2008; pooled SPORTIF III and V cohort on warfarin; Asia, EU, Australasia, Canada and the USA; 409 sites and 259 hospitals66 | 16.6 (6.3) months; prospective; n = 7329 | Ximelagatran (36 mg b.i.d.) + aspirin (< 100 mg), unclear | Ximelagatran (36 mg b.i.d.), 3664 | Persistent or paroxysmal NVAF; high riske | |
| Warfarin (INR 2.0–3.0) + aspirin (≤ 100 mg), unclear | Warfarin (INR 2.0–3.0), 3665 | ||||
| Akins et al., 2007; pooled data RCTs – SPORTIF III and V; Asia, EU, Australasia, Canada and the USA; 409 sites and 259 hospitals67 | 16.6 (6.3) months; prospective; n = 1539 | Ximelagatran (36 mg b.i.d.) + aspirin (≤ 100 mg), n = 157 | Ximelagatran (36 mg b.i.d.), n = 629 | Persistent or paroxysmal NVAF | NR |
| Adjusted-dose warfarin (INR 2.0–3.0) + aspirin (≤ 100 mg), n = 186 | Adjusted-dose warfarin (INR 2.0–3.0), n = 567 | Patients with prior stroke | |||
| White et al., 2007; pooled SPORTIF III and V of cohort on Warfarin, Asia, EU, Australasia, Canada and the USA; 409 sites and 259 hospitals68 | 16.6 (6.3) months; prospective; n = 3587 | Adjusted-dose warfarin (INR 2.0–3.0) + aspirin (≤ 100 mg), n = 475 | Adjusted-dose warfarin (2.0–3.0), n = 3112 | Persistent or paroxysmal NVAF high riske | NR |
| Halperin, 2005; post hoc analysis RCT – SPORTIF III; Europe, Asia and Australasia; 259 hospitals71 | 17.4 (4.1) months; prospective; n = 3407 | Ximelagatran (36 mg b.i.d.) + aspirin (< 100 mg), n = 337 | Ximelagatran (36 mg b.i.d.), n = 1367 | Persistent or paroxysmal NVAF high riske | 70 (9); 69.1 |
| Adjusted-dose warfarin (INR 2.0–3.0) + aspirin (≤ 100 mg), n = 290 | Adjusted-dose warfarin (INR 2.0–3.0), n = 1413 | ||||
| Flaker et al., 2006; pooled data RCT – SPORTIF III and V; Asia, EU, Australasia, Canada and the USA; 409 sites and 259 hospitals69 | 16.6 (6.3) months; prospective; n = 7304i | Ximelagatran (36 mg b.i.d.) + aspirin(≤ 100 mg), n = 531 | Ximelagatran (36 mg b.i.d.), n = 3120 | Persistent or paroxysmal NVAF high riska | NR |
| Adjusted-dose warfarin (INR 2.0–3.0) + aspirin (≤ 100 mg), n = 481 | Adjusted-dose warfarin (INR 2.0–3.0), n = 3172 | ||||
| Douketis et al., 2006; pooled data RCT-SPORTIF III and V; Asia, EU, Australasia, Canada and the USA; 409 sites and 259 hospitals70 | 16.6 (6.3) months; prospective; n = 7329 | Ximelagatran (36 mg b.i.d.) + aspirin (< 100 mg), unclear | Ximelagatran (36 mg b.i.d.), n = 3664 | Persistent or paroxysmal NVAF high riske | NR |
| Warfarin (INR 2.0–3.0) + aspirin (≤ 100 mg), unclear | Adjusted-dose warfarin (INR 2.0–3.0), n = 3665 | ||||
| Johnson et al., 2005; hospital records; Australia; four hospitals59 | 28 months; retrospective; n = 228 | Adjusted-dose warfarin (INR 2.0–3.0) + APT,a,g NR | Adjusted-dose warfarin (INR 2.0–3.0), n = 228 | Age ≥ 76 years, warfarin at admission and discharge diagnosis of AF | 81.1 (76–94); 41.7 |
| Stroke risk NR | |||||
| Blich et al., 2004; primary physician clinics; Israel; 23 clinics61 | 7.2 (5.2, 2–40) years; retrospective; n = 506 | Adjusted-dose warfarin (INR 2.0–3.0) + aspirin (100 mg), NR | Adjusted-dose warfarin (INR 2.0–3.0), NR | Chronic or recurrent paroxysmal NVAF (> 48 hour duration) diagnosed ≥ 2 yearsStroke risk NR | 75.7 (8.08, 35–100); 55.7 |
| Shireman et al., 2004, database of inpatients discharged from acute-care hospitals; USA; countrywide60 | 90 days or 180 days; retrospective; n = 10,093 | Warfarin a + aspirin/clopidogrel/ticlopidine/dual APT,an = 1962 | Warfarin,an = 8131 | Age ≥ 65 yearsWarfarin on discharge; AF diagnosis on discharge | 77.2; 49.6 |
| Stroke risk NR | |||||
| Klein et al., 2003; cohort of RCT – ACUTE; international sites; 7062 | 8 weeks; prospective; n = 1222 | Warfarin/heparin (INR 2.0–3.0) + aspirin,an = 560 | Warfarin (INR 2.0–3.0), n = 444 | Age > 18 years, AF of > 2 days' duration, candidates for cardioversion, patients with atrial flutter who have a history of AF | 65.1; 66.67 |
| Heparin (INR 2.0–3.0), n = 524 | |||||
| Warfarin + heparin adjusted dose (INR 2.0–3.0), n = 249 | Stroke risk NR | ||||
| Hart et al., 2000; cohort of SPAF III; USA and Canada; 20 sites55 | 2 years; prospective; n = 2012 | Adjusted-dose warfarin (INR 1.2–1.5) + aspirin (325 mg/day), n = 81 | Adjusted-dose warfarin (INR 2.0–3.0), n = 91 | High risk of strokej | 69 (10); 72 |
| Toda et al., 1998; hospitalised patients; Japan; one hospital52 | 7.2 (5.1, 1–23) years; retrospective; n = 288 | Warfarina + APT,a,gn = 30 | Warfarin alone,an = 10 | Chronic or paroxysmal NVAF; stroke risk NR | 54.6 (13.3, 8–82); 59.0 |
| Albers et al., 1996; hospitalised patient records; USA; six hospitals53 | NR; retrospective, n = 309 | Adjusted-dose warfarin (INR 2.0–3.0) + aspirina at admission, n = 9 | Adjusted-dose warfarin (INR 2.0–3.0) on admission, n = 62 | AF documented on admission or during hospitalization | 71.6 (12.7); 51 |
| Adjusted-dose warfarin (INR 2.0–3.0) + aspirina at discharge, n = 22 | Warfarin (INR 2.0–3.0) at discharge, n = 83 | Stroke risk NR |
| Study, total no., mean follow-up (SD) | Method of outcome measurement | Blinding of assessors | Outcome definitions clearly explained? | Indications of APT in the study | Time of APT employment | Which parts of the study were prospective?a | Comments |
|---|---|---|---|---|---|---|---|
| Hansen et al., 2010, n = 118,606, 3.3 (2.6) years63 | Events identified from registry through ICD-10 coded diagnosesb | Yes | Yes | Physician's discretion | Unclear | All stages retrospective | Previous warfarin/aspirin/clopidogrel treatment: 12.8%/16.7%/1.0%, respectively |
| Bover et al., 2009, n = 574, 4.2 years54 | Hospital follow-up and INR measurement in laboratories | No | Yes | Physician's discretion or patient preference | During follow-up | All stages prospective | 70% of patients recruited from NASPEAF cohort; patients on combined therapy reported at a higher risk of strokec |
| Lopes et al., 2009, n = 276, 90 days50 | Telephone contact at 30 and 90 days | Unclear | Yes | NR | At discharge | All stages prospective | Analysis of patients enrolled in another trial91 to compare outcomes in patients with new-onset AF vs those diagnosed with AF at discharge |
| Abdelhafiz and Wheeldon, 2008, n = 402, 19 months51 | Telephone interview every 4–6 weeks with medical notes review | Yes | Yes | Physician's discretion and presence of IHD | NR | All stages prospective | |
| Amadeus Investigators, 2008, n = 4576, 339 days72 | Follow-up at week 1, 2, 6, 13 and every 3 months thereafter, or when event occurred | Yes | Yes | Physician's discretion | Unclear | All stages prospective | Trial stopped after randomisation because of excessive bleeding in patients on idraparinux; 76% of patients reported on VKA before entry into trial |
| Ezekowitz et al., 2007 (RCT – PETRO), n = 502, 22 weeks73 | Outpatient follow-up at 1, 2, 4, 8, and 12 weeks after randomisation (to dabigatran or warfarin) | Yes | Yes | NR | During the study | All stages prospective | After entry of approximately half of the patients, the requirement for CAD was removed to facilitate recruitment; all patients treated with VKA for ≥ 8 weeks prior to inclusion |
| Suzuki et al., 2007, n = 667, 1 year56 | Database review | Yes | Yes | Other cardiovascular diseases | Unclear | All stages retrospective | Study conducted on Japanese AF patients attending a hospital for cardiovascular diseases |
| Burton et al., 2006, n = 601, 42 months57 | Record review and patient contact through letters | Yes | Yes | Physician's discretion | Any time during follow-up | All stages retrospective | |
| Stenestrand et al., 2005, n = 5616, 1–8 years58 | Review of hospital records | Yes | No | Physician's discretion | At discharge | All stages retrospective | Name of OAC not specified in the study, 18% on ACT before admission |
| Flaker et al., 2006; n = 7304, 16.6 months69 | Stroke assessment every 6 months and after an event | Yes | Yes | Age ≥ 65 years with CAD with or without diabetes mellitus | Unclear | All stages prospective | Significant baseline differences in those receiving ACT + APT and ACT alone; 73.4% received anticoagulation and 20.7% were taking aspirin prior to study entry |
| Johnson et al., 2005, n = 228, 28 months59 | Patient contacted on telephone and questionnaires when event occurredd | Yes | Yes | NR | NR | All prospective | 44.3% on warfarin (n = 101/228) for varying lengths of time before their index admission |
| Blich et al., 2004, n = 506, 7.2 years61 | Review of patient records and interview with patient's GP | Yes | No | Physician's discretion | At diagnosis, during follow-up, or before TE event | All stages retrospective | 26.9% of patients receiving warfarin at diagnosis, 6.5% were young or had no stroke risk factors |
| Shireman et al., 2004, n = 10,093, 180 days60 | Review of Medicare hospital claims for events with ICD-9-CM coding | Yes | Yes | Presence of CHD | After discharge | All stages retrospective | |
| Klein et al., 2003, RCT – ACUTE cohort, n = 1222, 8 weeks62 | Weekly INR testing and TEE at 4 weeks | Unclear | Yes | NR | At enrolment | All stages prospective | No. of patients on ACT or ACT + APT not reported |
| No indication if patients took aspirin throughout the study period | |||||||
| Hart et al., 2000, n = 2012, 2 years55 | Clinic follow-up every 3–6 months | unclear | Yes | Randomisede | During follow-up | All stages prospective | Incomplete information on only a few patients on combined therapy and ACT alone reported from authors of the randomised study43 |
| Toda et al., 1998; n = 288, 7.2 years52 | Review of patient records, or patient questionnaires, supplemented with GP contact | Yes | Yes | Physician's discretion | At baseline and before event | Outcome assessment | Study conducted on hospitalised Japanese patients with AF aged 8–82 years |
| Cranial CT scan and/or angiography used to assess outcomes | |||||||
| Albers et al.,1996; n = 30953 | Chart reviews performed by HCPs in consultation with physician | Yes | No | Physician's discretion | Admission and discharge | Outcome assessment – prospective | 18% had no risk factors for stroke and 44% had contraindications for ACT on admission |
| 23.6% took warfarin before admission | |||||||
| 77% white population |
Of the included studies, three RCTs40,42,43 and 14 other studies reporting non-randomised comparisons summarised data for warfarin therapy in different regimes plus an APT compared with warfarin. 50–53,55–57,59–65 One RCT39 and one non-randomised study54 reported data on acenocoumarol (Sinthrome®, Alliance) plus an APT compared with acenocoumarol alone. The remaining one RCT41 reported data on fluindione plus aspirin compared with fluindione plus placebo. 41 One study72 reporting non-randomised comparisons used idraparinux, and one used dabigatran (Pradaxa®, Boehringer Ingelheim) as anticoagulant agent,73 whereas two studies64,65 reported data on ximelagatran plus warfarin compared with ximelagatran alone. Doses of APT varied between studies.
Of the included RCTs, three39,42,43 used therapies in an open-label fashion, whereas this information was not clear in one. 40 Assessors were blinded in three39,41,42 out of five RCTs,39–43 and intention-to-treat (ITT) analysis was undertaken in three studies. 40,42,43 However, two of these studies were terminated prematurely. 41,42 The sample size varied from 43 to 1209 participants in the RCTs,39,40 with variable periods of follow-up (22 days40 to 42 months42).
Of the studies reporting non-randomised comparisons, six were retrospective,56–58,60–63 and the time of APT use varied between the studies. The majority of these studies consisted of a retrospective review of medical records where prior knowledge of allocation of therapy was not possible. 50–53,56–61,63 However, all but five studies50,55,59,62,73 clearly reported the criteria by which APT was used in the study. Of note is the study by Ezekowitz et al. 73 [PETRO (Dabigatran with or without concomitant aspirin compared with warfarin alone in patients with non-valvular atrial fibrillation)], in which it was difficult to identify if APT was used at random or indicated in a subgroup. For this reason, the study is classified as a non-randomised comparison of ACT plus APT and ACT only.
Between-study differences
The subsequent sections will report the event rates for each outcome. Methodological heterogeneity exists between the included studies that may explain any differences in the event rates reported. Rather than repeat these methodological differences for each and every outcome of interest, the reader will be referred to the following discussion of these differences. Where specific differences in the methodology between the included studies are apparent, which are important to highlight and/or only pertinent to that particular outcome, these differences will be specified under that outcome.
The differences in the event rates reported by the included studies may reflect differences in the population risk profile, with some studies including high-risk AF populations (three RCTS39,41,43 and seven non-randomised comparisons50,55,58,64,65,72,73) and/or intermediate-risk patients with AF (one RCT39), whereas other studies did not report the risk profile of included patients (two RCTs40,42) and 11 other non-randomised comparisons. 51–54,56,57,59–63
The sample size also varied considerably between included studies, from 43 participants in one RCT40 to 118,606 in a large non-randomised comparison. 63 As a result of the overall sample size, the number of patients receiving combined ACT and APT and the comparator also varied considerably, with only 34 patients receiving the combination therapy in Bover et al. ,54 between 21 and 36 patients receiving the various permutations of ACT plus APT in the PETRO study,73 and 76 patients receiving combination therapy and 81 receiving ACT alone in the FFAACS (Fluindione, Fibrillation Auriculaire, Aspirin et Contraste Spontané) trial,41 which will have influenced the reported event rates for each outcome.
Further, the included studies comprise both randomised and non-randomised data. Among non-randomised comparisons there is the potential for confounding by indication with the use of APT, as this was often given at the discretion of the treating physician, with patients at high risk of a vascular event and/or those less likely to bleed receiving combination therapy. Indeed, Bover et al. 54 reported that the patients receiving combined therapy were at a higher risk of stroke than those who were administered adjusted-dose acenocoumarol (INR 2.0–3.0) alone. Moreover, the number of patients with previous experience of an anticoagulant agent or APT in each study may also affect the event rate, for example patients who can tolerate either ACT or APT will continue on such therapy and therefore may be less likely to bleed on treatment than those who experience a bleed and therefore discontinue such therapy – ‘ATT survivor’.
The included studies also compared different types of anticoagulant and APT in various permutations, which makes comparison of event rates across studies using different interventions and comparators difficult. Studies compared a VKA, either warfarin,40,42,43,50–53,55–57,63–65,72 acenocoumarol,39,54 or fluindione41 in combination with either aspirin41–43,50,51,53–58,60–65,72,73 or other antiplatelet agents, such as triflusal,39,54 clopidogrel,40,63 or dual APT of aspirin plus clopidogrel. 63 Furthermore, two other studies compared an ODTI (anticoagulant) – either ximelagatran64,65 or dabigatran73 – in combination with aspirin (in different doses) or alone.
Among those studies comparing VKAs plus aspirin to a VKA alone,39–43,50–54,54–57,59–65,67,69,72 different VKA regimes were used in the combination therapy arm, either fixed dose (1.25 mg42) or adjusted dose to maintain a target INR range [e.g. INR 1.2–1.5,43 INR 2.0–3.0,40,64,65 INR 1.9–2.5,54 INR 2.0–2.6,41 INR 1.4–2.4 (high risk) and INR 1.25–2.0 (intermediate risk)39]. Of the included RCTs, therapies were administered in either an open-label42,43 or in a double-blind fashion. 41 In addition, the APT also varied (aspirin, triflusal, clopidogrel, and aspirin plus clopidogrel). In the studies reporting randomised comparisons, aspirin was utilised in different doses (300 mg,42 325 mg43 and 100 mg41), and also in non-randomised comparisons (≤ 100 mg,64,65,72 100 mg,61 81 or 325 mg73 and dose not specified in others51,53,56–58,62). Similarly, other antiplatelets were used in different doses such as triflusal (600 mg,39 600 mg and 300 mg54), clopidogrel (75 mg:40 dose not specified63) and dual APT of aspirin plus clopidogrel (dose not specified50,63), which makes direct comparison between studies difficult.
In addition, some randomised studies used the same target INR range in both the intervention and comparator arm (RCTs40,41 besides non-randomised comparisons54,51,53, 56,57,59,61,62,64,65,72), whereas others did not (RCTs39,42,43 and non-randomised comparisons54,55), again making difficult the direct comparison between the intervention and comparator arms within the studies. However, the majority of studies did use the standard therapeutic INR target of 2.0–3.0 in the comparator arm40,41,51,53–55,57,59,61,62,64,65,72 whereas others did not, although only four studies39,40,43,54 reported time in therapeutic range (TTR).
There were also differences across studies in the definitions of the outcomes of interest used and these differences are discussed, where relevant, under each outcome.
Furthermore, the considerable variation in the length of follow-up (e.g. 22 days40 to 4.92 years54) in each of the included studies may have influenced event rates. The combination of a short duration of follow-up for outcomes that are not particularly common together with a small sample size may have resulted in studies being underpowered. Of note the AFASAK II study (Second Copenhagen Atrial Fibrillation, Aspirin and Anticoagulation Study)42 was prematurely terminated when results of the SAAF III (Stroke Prevention in Atrial Fibrillation) trial,43 demonstrating the superiority of adjusted-dose warfarin (INR 2.0–3.0) alone, over combination of adjusted-dose warfarin (INR 1.2–2.5) and aspirin 325 mg in preventing stroke or SE, were published. Further, the FFAACS study41 was also terminated early due to poor recruitment. It should also be noted, that Bover et al. 54 was a non-randomised comparison that followed up of a proportion of the patients enrolled in the NASPEAF (National Study for Prevention of Embolism in Atrial Fibrillation) study39 (although it is not clear how many patients from NASPEAF were included in Bover et al. , within each arm of the latter study), with addition of newly recruited participants, over a longer period of time.
Moreover, the temporal changes in the management of AF over the last 20 years may have influenced the event rate reported in studies enrolling patients in the early 1990s (AFASAK II42 and SPAF III43) compared with those from 2000 onwards. 39,40,41,54,63,64,65,73
Outcomes
Not all of the studies measured or reported information for the primary and secondary outcomes of the review.
Table 8 details the outcomes reported in each study. Not surprisingly, bleeding, stroke and/or mortality-related outcomes were the most frequently reported. The time in therapeutic INR range was infrequently measured. To some extent this might be due to the nature of the anticoagulant agents used in some studies and thus the absence of a need for this outcome. Patient quality of life, in-stent thrombosis and revascularisation procedures were not reported in any of the studies.
| Author, year (name of study) | Outcomes | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary outcome measures | Secondary outcome measures | ||||||||||||
| Stroke–anya | TIA | SE | Stroke + SEa | AMI | In-stent thrombosis | Death – vascular | Death – all cause | Bleeding | Quality of life | Adverse eventsb | Revascularisation procedures (e.g. PCI) | Percentage time in INR range | |
| Randomised comparisonsc | |||||||||||||
| Pérez-Gómez et al., 2004 (RCT – NASPEAF)39 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Pérez-Gómez et al., 2007 (RCT – NASPEAF)44 | ✓ | ✓ | ✓ | ✓ | |||||||||
| Pérez-Gómez et al., 2006 (RCT – NASPEAF)45 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Pérez-Gómez et al., 2006 (RCT – NASPEAF)46 | ✓ | ||||||||||||
| Lidell et al., 2003,40 | ✓ | ✓ | |||||||||||
| Lechat et al., 2001 (RCT – FFAACS)41 | ✓ | ✓ | ✓ | ||||||||||
| Gullov et al., 1998, (RCT – AFASAK II)42 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Gullov et al., 1999, (RCT – AFASAK II review)47 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| SPAF investigators, 1996, (RCT – SPAF III)43 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Hart et al., 2000 (SPAF I, II, III pooled)48 | ✓ | ||||||||||||
| Blackshear et al., 1999 (RCT – SPAF-III)49 | |||||||||||||
| Non-randomised comparisons | |||||||||||||
| dHansen et al., 201063 | ✓ | ✓ | |||||||||||
| d,eBover et al., 200954 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Lopes et al., 200950 | ? | ||||||||||||
| Abdelhafiz and Wheeldon, 200851 | ✓ | ||||||||||||
| Amadeus Investigators, 200872 | ✓ | ||||||||||||
| dEzekowitz et al., 2007 (RCT – PETRO)73 | ✓ | ✓ | |||||||||||
| Suzuki et al., 200756 | ✓ | ||||||||||||
| Burton et al., 200657 | ✓ | ||||||||||||
| Stenestrand et al., 200558 | ✓ | ||||||||||||
| SPORTIF V investigators, 200565 | ✓ | ||||||||||||
| SPORTIF III Investigators, 200364 | ✓ | ||||||||||||
| Teitelbaum et al., 200866 | ✓ | ✓ | |||||||||||
| d Akins et al., 2007 6 | ✓ | ✓ | |||||||||||
| White HD et al., 2007 68 | ✓ | ✓ | ✓ | ✓ | |||||||||
| Halperin, 2005 71 | ✓ | ||||||||||||
| dFlaker et al., 200669 | ✓ | ✓ | ✓ | ✓ | |||||||||
| Douketis et al., 200670 | ✓ | ||||||||||||
| Johnson et al., 200559 | ✓ | ||||||||||||
| Blich et al., 200461 | ✓ | ✓ | |||||||||||
| Shireman et al., 200460 | ✓ | ||||||||||||
| Klein et al., 200362 | ✓ | ||||||||||||
| fHart et al., 200055 | ✓ | ||||||||||||
| Toda et al., 199852 | ✓ | ||||||||||||
| Albers et al., 199653 | ✓ | ||||||||||||
Methodological issues
Twenty-three studies in 35 articles39–73 reported the outcomes of interest for combined anticoagulant plus APT compared with ACT alone in patients with AF. Of these, 5 studies in 11 articles39–49 reported randomised comparisons, whereas 18 studies in 24 articles50–73 reported non-randomised comparisons. The characteristics of these studies have been reported previously in Tables 4 and 6.
Not all of the included studies provided non-randomised data that added information to the robust randomised data. Data were extracted from these studies, but not reported in this review. Reasons for non-inclusion of study data from such studies have been reported in Appendix 7. A few studies did not report the number of events50,56,62,68,70,72 or did not clearly report the number of participants in each therapy group,57,58,61,64,65 whereas a few other publications reported duplicate data from included primary studies. 44–47,49,71 A few studies reported non-randomised data that did not add any new information to the data available from other studies, either because of a very small sample size51 or because they did not specify the name of the APT in the combination anticoagulation plus antiplatelet arm. 52,59 Other studies that furnished complete and tangible data were included.
An example of such studies are the Stroke Prevention using an ORal Thrombin Inhibitor in atrial Fibrillation (SPORTIF) studies. 64–71 The original articles of SPORTIF III64 and SPORTIF V65 did not specify the number of events and number of participants in the interventions of interest (anticoagulant plus antiplatelet and anticoagulant alone). Six articles66–71 reported pooled post hoc analyses of these two studies. 64,65 Of these, two pooled analyses, by White et al. ,68 and Douketis et al. ,70 did not report data on the number of events or the number of participants for either intervention group; however, this information was reported in pooled analyses by Flaker et al. 69 and Akins et al. 67 Two other publications66,71 reported data for stroke, or stroke and bleeding outcomes, which were also reported in pooled analyses. 67,69 Flaker et al. 69 reported data on bleeding, mortality, stroke, and combined stroke and SE events, with detailed information on the number of events and participants in the SPORTIF cohorts. Therefore, this pooled analysis was reported in the review. Akins et al. 67 furnished data for bleeding, stroke and SE events specifically for patients with previous embolic events in the SPORTIF trials. Therefore, this study consisting of a population who were at a high risk of stroke was reported in the review.
Primary outcomes of the review
Outcome 1: stroke
Thirteen articles yielded outcome data for stroke. 42–45,47–50,54,55,63,66,69 Of these, three studies in seven articles42–45,47–49 reported randomised comparisons. The findings of these are reported in Table 9. The remaining five articles50,54,55,63,66,69 reported non-randomised comparisons, of which four were primary studies,50,54,55,63 and two were secondary analyses66,69 of the SPORTIF III and SPORTIF V studies. Table 10 presents the findings of these studies.
| Author, year, study name | Stroke risk, follow-up (mean) | ACT + APT, n | No. of events/participants in ACT + APT arm (%) | ACT (alone or ACT + placebo), n | No. of events/total participants in ACT arm (%) | RR (95% CI) |
|---|---|---|---|---|---|---|
| aPérez-Gómez et al., 2004, RCT – NASPEAF 39 | High risk,b 2.95 years | Adjusted-dose acenocoumarol (1.4–2.4) + triflusal (600 mg), n = 223 | Non-fatal: 6/223 (2.7) | Adjusted-dose acenocoumarol (INR 2.0–3.0), n = 247 | Non-fatal: 6/247 (2.4) | 1.11 (0.36 to 3.38) |
| Intermediate risk,c 2.6 yearsd | Adjusted-dose acenocoumarol (1.25–2.0) + triflusal (600 mg), n = 222 | Non-fatal: 3/222 (1.4) | Adjusted-dose acenocoumarol (INR 2.0–3.0), n = 232 | Non-fatal: 3/232 (1.3) | 1.05 (0.21 to 5.12) | |
| eGullov et al., 1998 RCT – AFASAK II42 | Risk NR, 3.5 years | Fixed-dose warfarin (1.25 mg) + aspirin (300 mg), n = 171 | All: 11/171 (6.4) | Adjusted-dose warfarin (INR 2.0–3.0), n = 170 | All: 10/170 (5.9) | 1.09 (0.48 to 2.51) |
| Non-infarct: 3/171 (1.8) | Non-infarct: 3/170 (1.8) | 0.99 (0.20 to 4.86) | ||||
| Minor: 4/171 (2.3) | Minor: 0/170 (0) | 8.95 (0.49 to 164.92) | ||||
| Disabling: 4/171 (2.3) | Disabling: 3/170 (1.8) | 1.33 (0.30 to 5.83) | ||||
| Fatal: 0/171 (0) | Fatal: 0/170 (0) | Not estimable | ||||
| Haemorrhagic: 0/171 (0) | Haemorrhagic: 1/170 (0.6) | 0.33 (0.01 to 8.08) | ||||
| Ischaemic: 8/171 (4.7) | Ischaemic: 3/170 (1.8) | 2.65 (0.72 to 9.82) | ||||
| Non disabling: 3/171 (1.8) | Non-disabling: 4/170 (2.4) | 0.75 (0.17 to 3.28) | ||||
| Fixed-dose warfarin (1.25 mg), n = 167 | All: 13/167 (7.8) | 0.83 (0.38 to 1.79) | ||||
| Non infarct: 6/167 (3.6) | 0.49 (0.12 to 1.92) | |||||
| Minor: 3/167 (1.8) | 1.30 (0.30 to 5.73) | |||||
| Disabling: 2/167 (1.2) | 1.95 (0.36 to 10.52) | |||||
| Fatal: 2/167 (1.2) | 0.20 (0.01 to 4.04) | |||||
| Haemorrhagic: 0/167 (0) | Not estimable | |||||
| Ischaemic: 5/167 (2.9) | 1.56 (0.52 to 4.68) | |||||
| Non-disabling: 4/167 (2.4) | 0.73 (0.17 to 3.22) | |||||
| fSPAF investigators to 1996 RCT – SPAF43 | High risk,g 1.1 years | Adjusted-dose warfarin (INR 1.2–1.5) + aspirin (325 mg), n = 521 | Disabling:d 31/521 (5.9) | Adjusted-dose warfarin (INR 2.0–3.0), n = 523 | Disabling:d 10/523 (1.9) | 2.83 (1.44 to 5.57) |
| Ischaemic: 43/521 (8.3) | Ischaemic: 11/523 (2.1) | 3.92 (2.05 to 7.52) | ||||
| Ischaemic – fatal: 5/521 (0.9) | Ischaemic – fatal: 1/523 (0.2) | 5.02 (0.59 to 42.81) |
| Author, year | Stroke risk, follow-up | ACT + APT, n | No. of events/total participants in ACT + APT group (%) | ACT (alone), n | No. of events/total participants in ACT group (%) |
|---|---|---|---|---|---|
| aBover et al., 200954 | Risk NR, 4.92 years | Adjusted-dose acenocoumarol (1.9–2.5) + triflusal (600 mg), n = 155 | All:b 5/155 (3.2) | Adjusted-dose acenocoumarol (2.0–3.0), n = 265 | All:b 15/265 (5.7) |
| Haemorrhagic: 1/155 (0.6) | Haemorrhagic: 5/265 (1.9) | ||||
| Lethal: 2/155 (1.3) | Lethal: 4/265 (1.5) | ||||
| Adjusted-dose acenocoumarol (1.9–2.5) + triflusal (300 mg), n = 121 | All:b 8/120 (6.7) | ||||
| Haemorrhagic: 0/120 (0) | |||||
| Lethal: 3/120 (2.5) | |||||
| Adjusted-dose acenocoumarol (1.9–2.5) + aspirin (100 mg), n = 34 | All:b 1/34 (2.9) | ||||
| Haemorrhagic: 1/34 (2.9) | |||||
| Lethal: 1/34 (2.9) | |||||
| cFlaker et al., 200669 | High risk,d 16.5 months | Adjusted-dose warfarin (2.0–3.0) + aspirin (≤ 100 mg), n = 481 | All: 11/481 (2.3) | Adjusted-dose warfarin (2.0–3.0), n = 3172 | All: 67/3172 (2.1) |
| Ximelagatran (36 mg b.i.d.) + aspirin (≤ 100 mg), n = 531 | All: 11/531 (2.1) | Ximelagatran (36 mg b.i.d.), n = 3120 | All: 50/3120 (1.6) |
The study by Hansen et al. 63 reported stroke outcomes for a large number of patients with AF (118,606) over a long follow-up (3.3 years); however, neither the number of stroke events nor the details of the antiplatelet and ACT were reported. Therefore, this study is not reported in this section. Of the studies that reported non-randomised comparisons, Lopes et al. ,50 Teitelbaum et al. 66 and Hart et al. 55 are not mentioned further in this section. The reasons for these have been reported in Appendix 7. The characteristics of these studies have been reported previously (see Table 6).
Stroke events were reported either on their own (stroke alone) or in conjunction with other events such as embolism or bleeding in the included studies. In those studies that reported stroke alone, strokes were frequently classified as non-fatal, fatal, haemorrhagic, ischaemic or disabling. A precise definition of these groupings or subclassifications of stroke was not always supplied in the study reports and/or the definitions may have varied between studies for the same subclassification.
The findings of the included studies for each of these composite and/or subclassifications of stroke are detailed below.
Stroke: all
One randomised comparison42 and two non-randomised comparisons54,69 compared a VKA plus aspirin, to a VKA alone. The pooled analysis of the SPORTIF III and V trials69 and the longitudinal follow-up study by Bover et al. ,54 add data to the randomised comparisons on the risk of stroke for patients receiving VKA plus aspirin compared with VKA alone.
The AFASAK II study42 and the pooled analysis of SPORTIF trials by Flaker et al. 69 defined stroke as an acute onset of focal neurological deficit lasting ≥ 24 hours. Bover et al. 54 did not report a precise a definition of stroke in their study.
The AFASAK II42 study compared combined fixed-dose warfarin (1.25 mg) plus aspirin (300 mg daily), with either adjusted-dose warfarin (target INR 2.0–3.0) alone or with fixed-dose warfarin (1.25 mg daily) alone. The findings of this study42 have been reported in Table 9. The risk profile of the patients enrolled in this study was not specified. There were no significant differences in the rate of stroke between patients receiving the combination of fixed-dose warfarin and aspirin, and either those receiving fixed-dose warfarin alone [11/171 (6.4%) vs 13/167 (7.8%), respectively] with a RR of 0.83 (95% CI 0.38 to 1.79), or those receiving adjusted-dose warfarin alone [11/171 (6.4%) vs 10/170 (5.9%), respectively], RR 1.09 (95% CI 0.48 to 2.51), over a mean follow-up period of 3.5 years. 42
The pooled analysis of the SPORTIF studies by Flaker et al. 69 compared adjusted-dose warfarin (INR 2.0–3.0) plus aspirin (100 mg) with adjusted-dose warfarin (INR 2.0–3.0) alone, over a mean follow-up period of 16.5 months. The rate of stroke was similar in patients in the combined therapy group compared with those on adjusted-dose warfarin (INR 2.0–3.0 alone) [11/481 (2.3%) vs 67/3172 (2.1%)], respectively. The rate of stroke was much higher in the AFASAK II study42 for patients receiving combination fixed-dose warfarin plus aspirin than for those with adjusted-dose warfarin plus aspirin in the SPORTIF studies;69 11 out of 171 (6.4%) compared with 11 out of 481 (2.3%), respectively. The stroke rate was also much higher in patients receiving either adjusted-dose, 10 out of 170 (5.9%) or fixed-dose, 13 out of 167 (7.8%) warfarin alone in the AFASAK II42 study than for those receiving adjusted-dose warfarin alone in the SPORTIF studies,69 67 out of 3172 (2.1%).
Bover et al. 54 compared adjusted-dose acenocoumarol (INR target 1.9–2.5) plus aspirin (100 mg) with adjusted-dose acenocoumarol (INR 2.0–3.0) alone, over a mean follow-up period of 4.92 years. The combination of acenocoumarol with aspirin demonstrated fewer stroke events [1/34 (2.9%)] than with acenocoumarol alone [15/265 (5.7%)].
Bover et al. 54 also compared combination adjusted-dose acenocoumarol (INR 1.9–2.5) plus two different regimes of triflusal (600 and 300 mg) with adjusted-dose acenocoumarol (INR 2.0–3.0) alone. Fewer strokes were observed with the combination of acenocoumarol plus triflusal 600 mg than for acenocoumarol alone, 5 out of 155 (3.2%) compared with 15 out of 265 (5.7%), respectively. However, stroke rates were higher in those receiving acenocoumarol plus triflusal 300 mg than in those receiving with acenocoumarol alone, 8 out of 120 (6.7%) and with 15 out of 265 (5.7%), respectively. However, there were population complexities in this non-randomised study (see Between-study differences, above).
The pooled analysis of the SPORTIF trials by Flaker et al. 69 also compared ximelagatran (36 mg twice daily) plus additional aspirin (100 mg) with ximelagatran (36 mg) alone, over a mean follow-up period of 16.5 months. A higher rate of stroke was observed in patients on combined therapy group than in those on ximelagatran alone [11/531 (2.1%) vs 50/3120 (1.6%), respectively]. However, it is to be noted that aspirin use was based on clinical need and, thus, the comparison may be confounded by indication. 65,68
Overall, there were few stroke events reported and there is conflicting evidence regarding the benefit of anticoagulation plus APT over anticoagulation alone in the reduction of all stroke events, with two studies42,69 (one randomised42 and one non-randomised69) reporting no differences, whereas another non-randomised study54 reports equivocal data, demonstrating fewer strokes with two combination regimes of ACT plus APT over ACT alone [with acenocoumarol plus aspirin (although only 34 patients received this combination) and acenocoumarol plus triflusal 600 mg] but more strokes with acenocoumarol plus triflusal 300 mg. 54
Fatal stroke
The AFASAK II42 and SPAF III43 studies reported randomised comparisons for the outcome of fatal stroke comparing different regimes of combined warfarin plus aspirin, with warfarin alone, whereas Bover et al. ,54 reported non-randomised data comparing acenocoumarol plus aspirin with acenocoumarol alone. The findings of these studies are reported in Tables 9 and 10, respectively.
In both studies reporting randomised comparisons (AFASAK II42 and SPAF III43), stroke was defined as a focal neurological deficit of presumed vascular genesis lasting more than 24 hours, where stroke assessment was undertaken using neuroimaging. However, Bover et al. 54 did not report a precise definition of stroke in their study.
The AFASAK II study42 compared fixed-dose warfarin (1.25 mg) plus aspirin (300 mg daily), with either adjusted-dose warfarin (target INR 2.0–3.0) alone or with fixed-dose warfarin (1.25 mg daily) alone (see Table 9). The risk profile of the patients enrolled in this study was not specified. No fatal strokes were reported among patients receiving either combined warfarin and aspirin or those receiving warfarin alone. However, two fatal strokes were reported in patients receiving fixed-dose warfarin alone [2/167 (1.2%) vs 0/171 (0%), respectively, RR 0.20 (95% CI 0.01 to 4.04)] over a mean follow-up period of 3.5 years. 42
The SPAF III study43 compared adjusted-dose warfarin (INR 1.2–1.5) plus aspirin (325 mg) with adjusted-dose warfarin (target INR 2.0–3.0) alone in high-risk patients with AF. A non-significant but higher incidence of fatal stroke was observed in the combined therapy arm than in those treated with warfarin alone [5/521 (0.9%) vs 1/523 (0.2%), respectively, RR 5.02 (95% CI 0.59 to 42.81)] over a mean follow-up period of 1.1 years. 43
Only eight fatal strokes occurred in these two RCTs. Among those receiving combined therapy, the rate of fatal stroke was 0.9% (5/521) in the SPAF III43 study compared with 0% (0/171) in the AFASAK II study. 42 The rate of fatal stroke was similar but numerically higher among those receiving adjusted-dose warfarin alone in the SPAF III study,43 1/523 (0.2%), than 0/170 (0%) in the AFASAK II study,42 and higher among those receiving fixed-dose warfarin alone in the AFASAK II42 study [2/167 (1.2%) vs 1/523 (0.2%), respectively].
Bover et al. 54 reported a non-randomised comparison for the incidence of fatal stroke comparing adjusted-dose acenocoumarol (INR 1.9–2.5) plus an antiplatelet in three different regimes (triflusal 600 mg, triflusal 300 mg and aspirin 100 mg) with adjusted-dose acenocoumarol (INR 2.0–3.0) alone. The combination of acenocoumarol plus aspirin and acenocoumarol plus triflusal 300 mg demonstrated a higher proportion of fatal strokes than acenocoumarol alone [1/34 (2.9%), 3/120 (2.5%) vs 4/265 (1.5%), respectively] during a mean follow-up of 4.92 years. Rates of fatal stroke were similar among those receiving combination acenocoumarol plus triflusal 600 mg and acenocoumarol alone [2/155 (1.3%) vs 4/265 (1.5%), respectively].
Very few fatal stroke events were reported. Two randomised studies42,43 found no significant reduction in the risk of fatal stroke with ACT plus APT over ACT alone. One non-randomised study54 also reported no benefit of combination therapy over anticoagulation alone in lowering the risk of fatal stroke.
Non-fatal stroke
One study (NASPEAF39) reported a randomised comparison for non-fatal stroke comparing adjusted-dose acenocoumarol (INR 1.4–2.4) plus triflusal (600 mg) with acenocoumarol alone (INR 2.0–3.0) in high-risk patients, and a combination of adjusted-dose acenocoumarol (INR 1.2–2.0) plus additional triflusal (600 mg) with acenocoumarol alone (INR 2.0–3.0) in intermediate-risk patients. Table 9 presents the findings of this study. Stroke was defined as a focal neurological deficit lasting more than 24 hours, where neuroimaging was used to define the ischaemic or intracranial aetiology. 39
Similar rates of non-fatal stroke occurred with combination therapy and anticoagulation alone in the high-risk patients [6/223 (2.7%) vs 6/247 (2.4%), respectively], RR 1.11 (0.36–3.38), during a median follow-up of 2.95 years. Analogous rates were observed in the intermediate-risk group in both the combination therapy and anticoagulation alone group [3/222 (1.4%) vs 3/232 (1.3%), respectively], RR 1.05 (95% CI 0.21 to 5.12), after a median follow-up of 2.6 years.
There was no non-randomised evidence identified for non-fatal stroke.
Combination therapy did not decrease the risk of non-fatal stroke compared with anticoagulation alone in one randomised study. 39
Haemorrhagic stroke
The AFASAK II study42 reported randomised data, and Bover et al. 54 reported a non-randomised comparison for the outcome of haemorrhagic stroke.
The AFASAK II study42 compared fixed-dose warfarin (1.25 mg) plus aspirin (300 mg) with fixed-dose warfarin (1.25 mg) or adjusted-dose warfarin (INR 2.0–3.0) alone. The risk profile of the patients enrolled in this study was not specified. No haemorrhagic strokes were reported in either those patients on combination therapy or in those receiving fixed-dose warfarin alone, over a mean follow-up period of 3.5 years. One haemorrhagic stroke occurred in a patient receiving adjusted-dose warfarin [1/170 (0.6%); RR 0.33 (95% CI 0.01 to 8.08)42 compared with combination therapy] (see Table 9).
Bover et al. 54 compared adjusted-dose acenocoumarol (INR 1.9–2.5) plus an antiplatelet in three different regimes (triflusal 600 mg, triflusal 300 mg, aspirin 100 mg) with adjusted-dose acenocoumarol (INR 2.0–3.0) alone, over a mean follow-up period of 4.92 years.
Fewer haemorrhagic strokes were observed in patients in all three combination therapy arms (triflusal 600 mg, triflusal 300 mg, aspirin 100 mg) than in those patients receiving acenocoumarol alone, 1/155 (0.6%), 0/120 (0%), 1/34 (2.9%) versus 5/265 (1.9%), respectively. 54
Ischaemic stroke
The AFASAK II42 and SPAF III43 studies reported randomised comparisons for the outcome of ischaemic stroke. The findings of these studies have been reported in Table 9. There was no non-randomised evidence available for the outcome of ischaemic stroke.
The AFASAK II study,42 comparing fixed-dose warfarin (1.25 mg) plus aspirin (300 mg) with adjusted-dose (INR 2.0–3.0) or fixed-dose (1.25 mg) warfarin alone, reported a non-significant but higher incidence of ischaemic stroke in the combined therapy arm [8/171 (4.7%) compared with either adjusted dose 3/170 (1.8%), RR 2.65 (95% CI 0.72 to 9.82)] or fixed-dose [5/167 (2.9%); RR 1.56 (95% CI 0.52 to 4.68)] warfarin alone. The SPAF III study43 reported significantly higher rates of ischaemic stroke in the combined therapy arm [adjusted-dose warfarin (INR 1.2–1.5) plus aspirin 325 mg) than in those with adjusted-dose warfarin (INR 2.0–3.0) alone [43/521 (8.3%) vs 11/523 (2.1%), respectively, RR 3.92 (95% CI 2.05 to 7.52)] in high-risk patients with AF over a mean follow-up period of 1.1 years.
The rate of ischaemic stroke varied between these two RCTs. 42,43 In patients receiving combination therapy, the risk of ischaemic stroke was much higher in the SPAF III43 study than in the AFASAK II42 study [43/521 (8.3%) vs 8/171 (4.7%), respectively]. Among those receiving dose-adjusted warfarin (INR 2.0–3.0), the rate of ischaemic stroke was similar in both SPAF III43 and AFASAK II studies42 [11/523 (2.1%) vs 3/170 (1.8%), respectively]. The rate of ischaemic stroke was higher in those receiving fixed-dose warfarin in the AFASAK II42 study than in those receiving dose-adjusted warfarin in either AFASAK II42 or SPAF III43 study [5/167 (2.9%) vs 3/170 (1.8%), 11/523 (2.1%), respectively]. The differences in the rates may reflect the heterogeneity between the included studies (see Between-study differences).
There is conflicting evidence regarding the benefit of combination ACT plus APT compared with ACT alone in the reduction of ischaemic stroke, with one randomised study42 demonstrating no significant difference, whereas another randomised study43 suggests a significantly increased risk of ischaemic stroke with combination therapy.
Disabling stroke
The AFASAK II42 and SPAF III43 studies reported randomised comparisons for the outcome of disabling stroke. The findings of these studies are reported in Table 9. There was no non-randomised evidence available for this outcome.
The SPAF III study43 defined disabling stroke as stroke that was graded ≥ 2 on the modified Rankin scoring system, whereas the AFASAK II study42 did not specify a definition for disabling stroke.
The AFASAK II study42 reported a non-significant but higher incidence of disabling stroke in the combined therapy arm [fixed-dose (1.25 mg) warfarin plus aspirin 300 mg] than in either adjusted-dose warfarin (target INR 2.0–3.0) alone [4/171 (2.3%) vs 3/170 (1.8%), respectively, RR 1.33 (95% CI 0.30 to 5.83)] or fixed-dose warfarin (1.25 mg) alone [4/171 (2.3%) vs 2/167 (1.2%), respectively] (see Table 9) over a mean follow-up period of 3.5 years. The risk profile of the patients enrolled in this study42 was not specified.
The SPAF III study43 reported significantly higher rates of disabling stroke in the combined therapy arm [adjusted-dose warfarin (INR 1.2–1.5) plus aspirin 325 mg] than in the adjusted-dose warfarin (INR 2.0–3.0) alone group [31/521 (5.9%) vs 10/523 (1.9%) respectively, RR 2.83 (95% CI 1.44 to 5.57)] in high-risk patients with AF over a mean follow-up period of 1.1 years. 43
The rate of disabling strokes was much higher in patients receiving combination therapy in the SPAF III43 study than in the AFASAK II42 study [31/521 (5.9%) vs 4/171 (2.3%), respectively]. Similar rates of disabling stroke were evident in patients receiving adjusted-dose warfarin alone in both SPAF III43 and AFASAK II42 studies [10/523 (1.9%) vs 3/170 (1.8%), respectively], and those receiving fixed-dose warfarin alone in the AFASAK II42 study [2/167 (1.2%)]. Such differences reflect significant heterogeneity between the included studies (see Between-study differences, above).
There is conflicting evidence regarding the benefit of combination ACT plus APT compared with ACT alone in the reduction of disabling stroke, with one randomised study42 demonstrating no significant difference, whereas another randomised study43 suggests a significantly increased risk of disabling stroke with combination therapy.
Other stroke definitions
The AFASAK II study42 also reported the incidence of minor, non-disabling and non-infarct strokes. The findings of this study are reported in Table 9. The definitions of these subclassifications have not been reported in the study. 42 Fixed-dose warfarin (1.25 mg) plus aspirin (300 mg) demonstrated a non-significant but higher risk of minor stroke than with either adjusted-dose warfarin (INR 2.0–3.0) alone [4/171 (2.3%) vs 0/170 (0%), respectively, RR 8.95 (95% CI 0.49 to 164.92)] or fixed-dose warfarin alone [4/171 (2.3%) vs 3/167 (1.8%), respectively, RR 1.30 (95% CI 0.30 to 5.73)]. 42
This study also demonstrated similar rates of non-disabling stroke among those receiving combination therapy [3/171 (1.8%)], adjusted-dose warfarin alone [4/170 (2.4%)] and fixed-dose warfarin alone [4/167 (2.4%)]. 42 The rate of non-infarct stroke was the same among those receiving combination therapy and adjusted-dose warfarin alone [3/171 (1.8%) vs 3/170 (1.8%), respectively] but was twice as high in those receiving fixed-dose warfarin alone [6/167 (3.6%)]42 (see Table 6).
There was no non-randomised evidence available for these three subclassifications of stroke.
The differences in stroke outcomes reported in the included studies may reflect the methodological differences between these studies discussed above (see Between-study differences). In addition, although four studies39,42,43,69 used the same definition of stroke, one non-randomised study54 did not provide a specific definition of stroke, and the stroke subtypes reported varied and were not always clearly defined by each study, which may account for variation in the reported event rates. The likelihood of stroke is increased when INR is < 2.0 and, therefore, it is possible that studies using INR targets of < 2.0 in the combination therapy arm may have experienced higher rates of stroke than those using standard INR targets (2.0–3.0), particularly in high-risk populations. Furthermore, only three studies (two randomised39,43 and one non-randomised54) reported TTR for ACT plus APT and ACT alone. TTR is associated with the incidence of stroke events; when TTR is good (≥ 58%), the likelihood of adverse events (ischaemic and haemorrhagic strokes) is reduced. 92 Therefore, differences in the TTR may help to explain differences in the event rates reported.
Outcome 2: transient ischaemic attack
Three studies, reported in five articles39,42,43,45,47 yielded outcome data for TIA (Table 11). Of these, all three reported randomised comparisons,39,42,43 supported by two subgroup analyses. 45,47 No non-randomised comparisons reported TIA separately as an outcome.
| Author, year, study name | Follow-up | ACT + APT, n | No. of events/total participants in ACT + APT arm (%) | ACT (alone or ACT + placebo), n | No. of events/total participants in ACT arm (%) | RR (95% CI) |
|---|---|---|---|---|---|---|
| aPérez-Gómez et al., 2004, RCT – NASPEAF39 | High risk,b 2.95 year | Adjusted-dose acenocoumarol (INR 1.4–2.4) + triflusal (600 mg), n = 223 | TIA: 2/223 (0.9) | Adjusted-dose acenocoumarol (INR 2.0–3.0), n = 247 | TIA: 3/247 (1.2) | 0.74 (0.12 to 4.38) |
| Intermediate risk,c 2.6 years | Adjusted-dose acenocoumarol (INR 1.25–2.0) + triflusal (600 mg), n = 222 | TIA: 0/222 (0) | Acenocoumarol (2.0–3.0), n = 232 | TIA: 0/232 (0) | Not estimable | |
| dGullov et al., 1998, RCT – AFASAK II42 | Risk NR, 3.5 years | Fixed-dose warfarin (1.25 mg) + aspirin (300 mg), n = 171 | TIA: 2/171 (1.2) | Adjusted-dose warfarin (INR 2.0–3.0), n = 170 | TIA: 1/170 (0.6) | 1.99 (0.18 to 21.72) |
| Fixed-dose warfarin (1.25 mg), n = 167 | TIA: 4/167 (2.4) | 0.49 (0.09 to 2.63) | ||||
| SPAF investigators, 1996, RCT – SPAF III43 | High risk,e 1.1 years | Adjusted-dose warfarin (INR 1.2–1.5) + aspirin (325 mg), n = 521 | TIA: 23/521 (4.4) | Adjusted-dose warfarin (INR 2.0–3.0), n = 523 | TIA: 15/523 (2.9) | 1.54 (0.81 to 2.92) |
Transient ischaemic attack was similarly defined in the NASPEAF39 and AFASAK II42 studies as an acute onset of focal neurological deficit of presumed vascular genesis lasting < 24 hours, regardless of computerised tomography (CT)/magnetic resonance imaging (MRI) findings (AFASAK II) or confirmed by neurological imaging (NASPEAF). The SPAF III43 study did not define TIA.
Both the AFASAK II42 and SPAF III43 studies compared warfarin plus aspirin with warfarin alone, but the warfarin and aspirin regimes differed between the studies.
The AFASAK II42 study compared fixed-dose warfarin (1.25 mg daily) plus aspirin (300 mg daily) with adjusted-dose warfarin (INR 2.0–3.0) or fixed-dose warfarin (1.25 mg daily). The risk profile of the patients enrolled in this study was not specified. The rate of TIA among patients receiving the combination of warfarin plus aspirin was twice that of patients receiving adjusted-dose warfarin (INR 2.0–3.0) alone [2/171 (1.2%) vs 1/170 (0.6%), respectively, RR 1.99 (95% CI 0.18 to 21.72)] and half that of patients receiving fixed-dose warfarin (1.25 mg daily) alone [2/171 (1.2%) vs 4/167 (2.4%), respectively, RR 0.49 (95% CI 0.09 to 2.63)] over a mean 3.5-year follow-up period.
The SPAF III43 study compared adjusted-dose warfarin (INR 1.2–1.5) plus aspirin (325 mg daily) with adjusted-dose warfarin (INR 2.0–3.0) alone in high-risk patients with AF. A non-significant but numerically higher number of TIAs were observed in the combined therapy arm than in those receiving adjusted-dose warfarin alone [23/251 (4.4%) vs 15/523 (2.9%), respectively, RR 1.54 (95% CI 0.81 to 2.92)] over a mean 1.1-year follow-up period.
The TIA event rate was different in these two randomised comparisons. In the combination of adjusted-dose warfarin (INR 1.2–1.5) plus aspirin (325 mg) arm of the SPAF III43 study, the rate of TIA was 4.4% (23/521) compared with 1.2% (2/171) among those receiving combination fixed-dose warfarin (1.25 mg) plus aspirin (300 mg) in the AFASAK II42 study. The rate of TIA was also higher in those receiving adjusted-dose warfarin (INR 2.0–3.0) alone in the SPAF III43 study [15/523 (2.9%)] than in those receiving either adjusted- (INR 2.0–3.0) or fixed-dose warfarin (1.25 mg) alone in the AFASAK II42 study [1/170 (0.6%) and 4/167 (2.4%), respectively].
The NASPEAF39 randomised comparison compared adjusted-dose acenocoumarol (INR 1.4–2.4) in combination with triflusal 600 mg with adjusted-dose acenocoumarol (INR 2.0–3.0) alone in high-risk patients and adjusted-dose acenocoumarol (INR 1.2–2.0) and triflusal 600 mg in combination compared with adjusted-dose acenocoumarol (INR 2.0–3.0) alone in intermediate-risk patients.
In the high-risk population, a similar rate of TIA was observed with adjusted-dose acenocoumarol (INR 1.4–2.4) in combination with triflusal 600 mg compared with adjusted-dose acenocoumarol (INR 2.0–3.0) alone [2/223 (0.9%) vs 3/247 (1.2%), respectively, RR 0.74 (95% CI 0.12, 4.38)] after a median follow-up of 2.95 years. No TIAs occurred during the median 2.6 years' follow-up in the intermediate-risk patients. 39
Two further articles (AFASAK II,47 NASPEAF45) provided subgroup analyses on the AFASAK II42 and NASPEAF39 studies; however, these articles simply reported duplicate data from the original studies.
No studies of non-randomised comparisons provided further evidence on TIA.
The differences in TIA outcomes reported in the included studies may reflect the methodological differences between these studies discussed in detail above (see Between-study differences).
Outcome 3: stroke and systemic embolism
Five studies, reported in 10 articles,39,42–45,47,67–69,71 yielded outcome data for the combination of stroke and SE. Of these, three studies in six articles,39,42–44,45,47 reported randomised comparisons (Table 12). Two studies in four articles67–69,71 reported pooled analyses of non-randomised comparisons using data from two randomised studies (SPORTIF III and V). The characteristics of the randomised and non-randomised comparison studies have been presented previously in Tables 4 and 6, respectively.
| Author, year, study name | Follow-up | ACT + APT, n | No. of events/total participants in ACT + APT arm (%) | ACT [alone or ACT + placebo), n | No. of events/total participants in ACT arm (%) | RR (95% CI) |
|---|---|---|---|---|---|---|
| aPérez-Gómez et al., 2004, RCT – NASPEAF39 | High risk,b 2.95 years | Adjusted-dose acenocoumarol (INR 1.4–2.4) + triflusal (600 mg), n = 223 | Strokec/any embolism: 12/223 (5.4) | Adjusted-dose acenocoumarol (INR 2.0–3.0), n = 247 | Strokec/any embolism: 20/247 (8.1) | 0.66 (0.33 to 1.33) |
| Strokec/fatal embolism: 4/223 (1.8) | Strokec/fatal embolism: 8/247 (3.2) | 0.55 (0.17 to 1.81) | ||||
| Intermediate risk,d 2.6 years | Adjusted-dose acenocoumarol (INR 1.25–2.0) + triflusal (600 mg), n = 222 | Strokec/any embolism: 3/222 (1.4) | Adjusted-dose acenocoumarol (INR 2.0–3.0), n = 232 | Strokec/any embolism: 7/232 (3.0) | 0.45 (0.12 to 1.71) | |
| Strokec/fatal embolism: 0/222 (0) | Strokec/fatal embolism: 3/232 (1.3) | 0.15 (0.01 to 2.87) | ||||
| eGullov et al., 1998 RCT – AFASAK II42 | Risk NR, 3.5 years | Fixed-dose warfarin (1.25 mg) + aspirin (300 mg), n = 171 | Strokef + TE:g 12/171 (7.0) | Adjusted-dose warfarin (INR 2.0–3.0), n = 170 | Strokef + TE:g 12/170 (7.1) | 0.99 (0.46 to 2.15) |
| Fixed-dose warfarin (1.25 mg), n = 167 | Strokef + TE:g 14/167 (8.4) | 0.84 (0.40 to 1.76) | ||||
| SPAF investigators, 1996, RCT – SPAF III43 | High risk,h 1.1 years | Adjusted-dose warfarin (INR 1.2–1.5) + aspirin (325 mg), n = 521 | Strokei/SE: 44/521 (8.4) | Adjusted-dose warfarin (INR 2.0–3.0), n = 523 | Strokei/SE: 11/523 (2.1) | 4.02 (2.10 to 7.69) |
A precise definition of stroke was given in all the study reports, but the definitions of stroke that were used varied between the studies. Although the three randomised comparisons39,42,43 and two pooled analyses of the SPORTIF III and V trials67,69 defined stroke as an acute onset of focal neurological deficit lasting ≥ 24 hours, NASPEAF39 also included TIA, AFASAK II42 included fatal strokes, SPAF III43 included only ischaemic strokes, whereas the SPORTIF III and V trials67,69 included both ischaemic strokes and intracranial haemorrhage (ICH) in their definition. Three studies, NASPEAF,39 SPAF III43 and SPORTIF,67,69 defined SE as an abrupt vascular insufficiency related to arterial occlusion, without previous clinical symptoms (NASPEAF39) or previous evidence of obstructive disease (SPAF III43); SPORTIF III and V67,69 required clinical and radiological evidence of arterial occlusion in the absence of another possible mechanism, and in the presence of atherosclerotic peripheral vascular disease, diagnosis of embolism required angiographic demonstration of acute arterial occlusion. The AFASAK II42 study did not define SE, but specified the sites of the event and required verification using angiography, surgery, scintigraphy or autopsy. From a clinical perspective, it was assumed that these different definitions of embolism were broadly similar and considered the same for the purposes of this review.
For the purpose of this review we are considering SE and TE as the same. It is assumed from the definitions of outcomes provided by the studies that TE refers to arterial TE not venous TE. From this point onwards the term systemic embolism (SE) will be used, but the original terms reported by the studies will be retained in the tables.
Two randomised comparisons42,43 and the two pooled non-randomised comparisons67,69 (Table 13) compared warfarin plus aspirin with warfarin alone in different regimes. The AFASAK II42 study compared fixed-dose warfarin (1.25 mg daily) plus aspirin (300 mg daily) with adjusted-dose warfarin (INR 2.0–3.0) or fixed-dose warfarin (1.25 mg daily). The risk profile of the patients enrolled in the AFASAK II study42 was not specified. The rate of stroke and systemic embolism (including fatal strokes) was the same among patients receiving the combination therapy and patients receiving adjusted-dose warfarin (INR 2.0–3.0) alone [12/171 (7.0%) vs 12/170 (7.1%), respectively, RR 0.99 (95% CI 0.46 to 2.15)] after a median follow-up period of 3.5 years. 42 A non-significant but numerically lower number of people experienced a stroke and systemic embolism among those receiving combination therapy than in those receiving fixed-dose warfarin alone [12/171 (7.0%) vs 14/167 (8.4%), respectively, RR 0.84 (95% CI 0.40 to 1.76)] during the median 3.5-year follow-up period.
| Author, year | Stroke risk, follow-up | ACT + APT, n | No. of events/total participants in ACT + APT arm (%) | ACT (alone or ACT + placebo), n | No. of events/total participants in ACT arm (%) |
|---|---|---|---|---|---|
| Flaker et al., 2006, pooled analysis of SPORTIF III and V69 | High risk,a 16.5 months | Adjusted-dose warfarin (INR 2.0–3.0) + aspirin (≤ 100 mg), n = 481 | Strokeb/SE: 11/481 (2.3) | Adjusted-dose warfarin (INR 2.0–3.0), n = 3172 | Strokeb/SE: 69/3172 (2.2) |
| Ximelagatran (36 mg) + aspirin (≤ 100 mg), n = 531 | Strokeb/SE: 12/531 (2.3) | Ximelagatran (36 mg), n = 3120 | Strokeb/SE: 58/3120 (1.9) | ||
| Akins et al., 2006, pooled analysis of SPORTIF III and V cohort with previous embolic event67 | High risk,c 16.6 months | Adjusted-dose warfarin (INR 2.0–3.0) + aspirin (≤ 100 mg), n = 156 | Stroked/SE: 13/186 (6.9) | Adjusted-dose warfarin (INR 2.0–3.0), n = 567 | Stroked/SE: 23/567 (4.1) |
| Ximelagatran (36 mg) + aspirin (≤ 100 mg), n = 157 | Stroked/SE: 11/157 (7.0) | Ximelagatran (36 mg), n = 629 | Stroked/SE: 22/629 (3.5) |
The SPAF III43 study compared adjusted-dose warfarin (INR 1.2–1.5) plus aspirin (325 mg daily) with adjusted-dose warfarin (INR 2.0–3.0) alone in high-risk patients with AF. The study43 reported significantly more ischaemic strokes and systemic emboli among those receiving combination therapy than in those receiving adjusted-dose warfarin (INR 2.0–3.0) alone [44/521 (8.4%) vs 11/523 (2.1%), respectively; RR 4.02 (95% CI 2.10 to 7.69)] over a mean 1.1-year follow-up period.
The pooled analyses of the SPORTIF trials67,69 compared combination adjusted-dose warfarin (INR 2.0–3.0) plus aspirin ≤ 100 mg with adjusted-dose warfarin (INR 2.0–3.0) alone, in a pooled analysis of SPORTIF III and V69 and in a subgroup analysis of the pooled SPORTIF III and V67 among those who had experienced an embolic event prior to enrolment. For the whole cohort, the rate of stroke and systemic embolism was very similar in patients receiving the combination therapy to those receiving adjusted-dose warfarin (INR 2.0–3.0) alone, 11 out of 481 (2.3%) versus 69 out of 3172 (2.2%), respectively, during the mean 16.5-month follow-up period. 69
In the pooled analysis restricted to those patients with a previous embolic event prior to randomisation, the rate of stroke and systemic embolism was higher, but not significantly so, among those receiving combination therapy than in those receiving adjusted-dose warfarin (INR 2.0–3.0) alone [13/186 (6.9%) vs 23/567 (4.1%)] during the mean 16.6-month follow-up period. 67
The rate of stroke and systemic embolism was much higher in the AFASAK II42 and SPAF III43 studies than in the pooled analysis of the SPORTIF trials69 for those receiving combination therapy compared with warfarin alone. In the AFASAK II42 and SPAF III43 studies the rate of stroke and systemic embolism were 12 out of 171 (7.0%) and 44 out of 521 (8.4%), respectively, compared with 11 out of 481 (2.3%) in the pooled analysis of SPORTIF. 69
The rate of stroke and systemic embolism was very similar among those receiving adjusted-dose warfarin alone in the SPAF III43 study and the pooled analysis of the SPORTIF69 trial [11/523 (2.1%) and 69/3172 (2.2%), respectively]. However, the rate of stroke and systemic embolism was much higher in the AFASAK II42 study for those receiving adjusted-dose warfarin (INR 2.0–3.0) alone or fixed-dose warfarin (1.25 mg) alone [12/170 (7.1%) and 14/167 (8.4%), respectively] compared with SPAF III43 and the pooled analysis of SPORTIF. 69
The rate of stroke and systemic embolism was very similar in AFASAK II42 but higher in SPAF III43 when compared with the pooled subgroup analysis of SPORTIF III and V restricted to patients with a previous embolic event,67 for those receiving combination warfarin plus aspirin [12/171 (7.0%), 44/521 (8.4%) and 13/186 (6.9%), respectively]. The rate of stroke and systemic embolism was much higher among patients receiving either fixed or adjusted-dose warfarin alone in AFASAK II,42 14/167 (8.4%) and 12/170 (7.1%), respectively, during a median 3.5 year follow-up, and lower in SPAF III43 for those receiving warfarin alone, 11/523 (2.1%) compared with those receiving warfarin alone in the pooled subgroup analysis of SPORTIF,67 23/567 (4.1%) during a mean/median 16.6 month follow-up. The variations in the rates may reflect the heterogeneity between included studies, as discussed above (see Between-study differences, above).
One randomised comparison (NASPEAF39) compared adjusted-dose acenocoumarol (INR 1.4–2.4) in combination with triflusal (600 mg) with adjusted-dose acenocoumarol (INR 2.0–3.0) alone in high-risk patients, and adjusted-dose acenocoumarol (INR 1.25–2.0) and triflusal (600 mg) in combination compared with adjusted-dose acenocoumarol (INR 2.0–3.0) alone in intermediate-risk patients. 39
In the high-risk population, adjusted-dose acenocoumarol (INR 1.4–2.4) in combination with triflusal 600 mg was associated with a non-significant but numerically lower number of stroke and systemic embolism than adjusted-dose acenocoumarol (INR 2.0–3.0) alone [12/223 (5.4%) vs 20/247 (8.1%), respectively, RR 0.66 (95% CI 0.33 to 1.33)], after a median follow-up of 2.95 years. 39 Similarly, when analyses involved only stroke and fatal systemic embolism, adjusted-dose acenocoumarol (INR 1.4–2.4) in combination with triflusal 600 mg was associated with a non-significant but numerically lower number of stroke and systemic emboli than adjusted-dose acenocoumarol (INR 2.0–3.0) alone [4/223 (1.8%) vs 8/247 (3.2%), respectively, RR 0.55 (95% CI 0.17 to 1.81)]. 39
In the intermediate-risk population, adjusted-dose acenocoumarol (INR 1.25–2.0) in combination with triflusal 600 mg was also associated with a non-significant but numerically lower number of stroke and systemic embolism than adjusted-dose acenocoumarol (INR 2.0–3.0) alone [3/222 (1.4%) vs 7/232 (3.0%), respectively, RR 0.45 (95% CI 0.12 to 1.71)] after a median follow-up of 2.6 years. 39 Similarly, when analyses involved only stroke and fatal systemic embolism, adjusted-dose acenocoumarol (INR 1.25–2.0) in combination with triflusal 600 mg was associated with a non-significant but numerically lower number of stroke and systemic emboli than adjusted-dose acenocoumarol (INR 2.0–3.0) alone [0/222 (0%) vs 3/232 (1.3%), respectively, RR 0.15 (95% CI 0.01 to 2.87)]. 39
Three further articles provided post hoc analyses on the NASPEAF44,45 and AFASAK II47studies; however, these papers simply reported duplicate data from the original studies.
In addition to the data on warfarin plus aspirin compared with warfarin alone, the pooled analyses of the SPORTIF III and V studies67,69 also provide data on the risk of stroke and systemic embolism for patients receiving ximelagatran 36 mg given twice daily plus aspirin ≤ 100 mg compared with ximelagatran 36 mg alone. 67,69
In the pooled analyses including all SPORTIF patients,69 combination therapy yielded a slightly higher, but non-significant, rate of stroke and systemic embolism than in those receiving ximelagatran alone, 12/531 (2.3%) versus 58/3120 (1.9%), respectively, during the 16.5-month follow-up period. 69
In just those patients with a previous embolic event, combination therapy yielded a rate of stroke and systemic embolism that was twice that of those receiving ximelagatran alone [11/157 (7.0%) vs 22/629 (3.5%), respectively], during a median 16.6-month follow-up, although this difference was not significant (RR 2.00, 95% CI 0.99 to 4.04). 67
Other pooled analyses of the SPORTIF III and V68,71 trials are not presented in the table to avoid duplication of data.
The differences in stroke and systemic embolism outcomes reported in the included studies may reflect the methodological differences between these studies discussed in detail above (see Between-study differences).
There is no evidence, from two randomised39,42 and two non-randomised67,69 studies, of any benefit for combination therapy over anticoagulation alone in the reduction of the combined end point of stroke and SE. One randomised study suggests a significant increased risk of stroke and SE with the combination of ACT and APT compared with ACT alone. 43
Outcome 4: systemic embolism
Eight studies, reported in 11 articles39–45,47,52,54,61,73 yielded outcome data for SE alone. Of these, four studies39–43 reported randomised comparisons (Table 14), supported by three subgroup analyses. 44,45,47 However, these subgroup analyses did not provide additional data for this outcome and, thus, are not considered further in this section (see Appendix 7).
| Author, year | Stroke risk, follow-up | ACT + APT, n | No. of events/total participants in ACT + APT arm (%) | ACT (alone or ACT + placebo), n | No. of events/total participants in ACT arm (%) | RR (95% CI) |
|---|---|---|---|---|---|---|
| aPérez-Gómez et al., 2004, RCT – NASPEAF39 | High risk,b 2.95 years | Adjusted-dose acenocoumarol (INR 1.4–2.4) + triflusal (600 mg), n = 223 | SE – non-fatal: 0/223 (0) | Adjusted-dose acenocoumarol (INR 2.0–3.0), n = 247 | SE non-fatal: 3/247 (1.2) | 0.16 (0.01 to 3.05) |
| Intermediate risk,c 2.6 year | Adjusted-dose acenocoumarol (INR 1.25–2.0) + triflusal (600 mg), n = 222 | SE – non-fatal: 0/222 (0) | Acenocoumarol l adjusted dose (INR 2.0–3.0), n = 232 | SE non-fatal: 1/232 (0.4) | 0.35 (0.01 to 8.50) | |
| Lechat et al., 2001, RCT – FFAACS41 | High risk,d 0.84 years | Adjusted-dose fluindione (INR 2.0–2.6) + aspirin (100 mg), n = 76 | TEe: 2/76 (2.6) | Adjusted-dose fluindione (INR 2.0–2.6) + placebo, n = 81 | TE:e 1/81 (1.2) | 2.13 (0.20 to 23.03) |
| fGullov et al., 1998, RCT – AFASAK II42 | Risk NR, 3.5 years | Fixed-dose warfarin (1.25 mg) + aspirin (300 mg), n = 171 | TEe – all: 1/171 (0.6) | Adjusted-dose warfarin (INR 2.0–3.0), n = 170 | TE:e all: 2/170 (1.2) | 0.50 (0.05 to 5.43) |
| TE:e fatal: 0/170 (0) | 2.98 (0.12 to 72.70) | |||||
| TEe – fatal: 1/171 (0.6) | Fixed-dose warfarin (1.25 mg), n = 167 | TE:e all: 1/167 (0.6) | 0.98 (0.06 to 15.49) | |||
| TEe – fatal: 1/167 (0.6) | 0.98 (0.06 to 15.49) | |||||
| SPAF investigators, 1996 RCT – SPAF III43 | High risk,g 1.1 years | Adjusted-dose warfarin (INR 1.2–1.5) + aspirin (325 mg), n = 521 | SE: 1/521 (0.2) | Adjusted-dose warfarin (INR 2.0–3.0), n = 523 | SE: 0/523 (0) | 3.01 (0.12 to 73.75) |
Four studies52,54,61,73 reported non-randomised comparisons; however, data from Blich et al. 61 and Toda et al. 52 are not reported further in this section (Table 15). The reasons for this can be found in Appendix 7.
| Author, year, study name | Stroke risk, follow-up | ACT + APT, n | No. of events/total participants in ACT + APT arm (%) | ACT (alone or ACT + placebo), n | No. of events/total participants in ACT arm (%) |
|---|---|---|---|---|---|
| Bover et al., 200954 | Risk NR, 4.92 years | Adjusted-dose acenocoumarol (INR 1.9–2.5) + triflusal (600 mg), n = 155 | SE: 0/155 (0) | Adjusted-dose acenocoumarol (INR 2.0–3.0), n = 265 | SE: 7/265 (2.6) |
| Acenocoumarol (INR 1.9–2.5) + triflusal (300 mg), n = 120 | SE: 2/120 (1.7) | ||||
| Acenocoumarol (INR 1.9–2.5) + aspirin (100 mg), n = 34 | SE: 0/34 (0) | ||||
| aEzekowitz et al., 2007, PETRO73 | High risk,b 22 weeks | Dabigatran (50 mg) + aspirin (81 mg), n = 21 | TE:c 1/21 (4.8) | Dabigatran 50 mg (b.i.d.), n = 59 | TE:c 1/59 (1.7) |
| Dabigatran (50 mg) + aspirin (325 mg), n = 27 | TE:c 0/27 (0) | ||||
| Dabigatran (150 mg) + aspirin (81 mg), n = 36 | TE:c 0/36 (0 | Dabigatran 150 mg (b.i.d.), n = 100 | TE:c 0/100 (0) | ||
| Dabigatran (150 mg) + aspirin (325 mg), n = 33 | TE:c 0/33 (0) | ||||
| Dabigatran (300 mg) + aspirin (81 mg), n = 34 | TE:c 0/34 (0) | Dabigatran 300 mg (b.i.d.), n = 105 | TE:c 0/105 (0) | ||
| Dabigatran (300 mg) + aspirin (325 mg), n = 30 | TE:c 0/30 (0) |
A precise definition of SE was not always given in the study reports and/or the definitions vary between studies. The NASPEAF,39 FFAACS41 and SPAF III43 studies defined SE as an abrupt vascular insufficiency related to arterial occlusion, without previous clinical symptoms39 or previous evidence of obstructive disease,43 with one specifying the site of occlusion as affecting the mesenteric, renal, splenic or limb arteries. 41 The AFASAK II42 study did not define a systemic embolic event, but specified the sites of the event and required verification using angiography, surgery, scintigraphy or autopsy. Of the two non-randomised comparisons, PETRO73 defined a SE as an acute non-intracerebral or non-coronary vascular event, whereas Bover et al. 54 did not define SE.
Four studies,41–43,54 three randomised comparisons41,42,43 and one non-randomised comparison54 compared a VKA plus aspirin with a VKA alone. The AFASAK II42 and SPAF III43 studies both compared warfarin plus aspirin with warfarin alone, although the warfarin and aspirin regimes differed between the studies. The FFAACS41 study compared fluindione plus aspirin to fluindione alone, whereas one non-randomised comparison54 compared acenocoumarol plus aspirin with acenocoumarol alone.
The AFASAK II42 study compared fixed-dose warfarin (1.25 mg daily) plus aspirin (300 mg daily) with adjusted-dose warfarin (INR 2.0–3.0) or fixed-dose warfarin (1.25 mg daily). The risk profile of the patients enrolled in this study was not specified. The rates of SE were very small and there were no differences between groups during the median 3.5 years of follow-up; combination therapy compared with adjusted-dose warfarin (INR 2.0–3.0) alone [1/171 (0.6%) vs 2/170 (1.2%), respectively, RR 0.50 (95% CI 0.05 to 5.43)] and compared with fixed-dose (1.25 mg) warfarin alone [1/171 (0.6%) vs 1/167 (0.6%), RR 0.98 (95% CI 0.06 to 15.49)]. The rates of fatal SE were also presented, but given the very low rates of all SE these do not add anything meaningful. 42
The SPAF III43 study compared adjusted-dose warfarin (INR 1.2–1.5) plus aspirin (325 mg daily) with adjusted-dose warfarin (INR 2.0–3.0) alone in high-risk patients with AF. One patient receiving combination therapy experienced a SE compared with no patients who received warfarin alone [(1/521 (0.2%) vs 0/523 (0%), respectively, RR 3.01 (95% CI 0.12 to 73.75)] during the mean 1.1-year follow-up period. 43
The FFAACS41 study compared adjusted-dose fluindione (INR 2.0–2.6) plus aspirin 100 mg with adjusted-dose fluindione (INR 2.0–2.6) alone. The rate of SE among patients receiving combination therapy was twice that of patients receiving fluindione alone [2/76 (2.6%) vs 1/81 (1.2%), respectively, RR 2.13 (95% CI 0.20 to 23.03)] during a mean 0.84-year follow-up, although this difference was not significant.
The non-randomised study by Bover et al. 54 provided additional data on the effect of a VKA plus aspirin compared with a VKA alone. This study compared adjusted-dose acenocoumarol (INR 1.9–2.5) in combination with aspirin (100 mg) with adjusted-dose acenocoumarol (INR 2.0–3.0). There were fewer systemic emboli during a mean 4.92-year follow-up in those receiving combination therapy than in those receiving acenocoumarol alone [0/34 (0%) vs 7/265 (2.6%), respectively; RR 0.51 (95% CI 0.03 to 8.68)], but the difference was not significant. 54
In each study there were very few systemic embolic events. The rate was similar between the four studies41–43,54 and between those receiving combination VKA plus aspirin and those receiving VKA therapy alone,41–43,54 despite methodological and clinical differences between these studies (see Between-study differences).
The NASPEAF39 randomised comparison compared adjusted-dose acenocoumarol (INR 1.4–2.4) in combination with triflusal 600 mg with adjusted-dose acenocoumarol (INR 2.0–3.0) alone in high-risk patients, and adjusted-dose acenocoumarol (INR 1.2–2.0) and triflusal 600 mg in combination compared with adjusted-dose acenocoumarol (INR 2.0–3.0) alone in intermediate-risk patients.
In both comparisons, no systemic embolic events occurred in patients receiving acenocoumarol in combination with triflusal, but a small number of patients in both the high- and intermediate-risk groups experienced a systemic embolic event with acenocoumarol alone [3/247 (1.2%) vs 1/232 (0.4%), respectively]. There were no statistically significant differences between combination therapy and anticoagulation treatment alone in either the high-risk (RR 0.16; 95% CI 0.01 to 3.05) or intermediate-risk (RR 0.35; 95% CI 0.01 to 8.50) populations after a median of 2.95 and 2.6 years of follow-up, respectively. 39
One non-randomised study54 compared adjusted-dose acenocoumarol (INR 1.9–2.5) in combination with triflusal (600 mg) with adjusted-dose acenocoumarol (INR 2.0–3.0) alone, and adjusted-dose acenocoumarol (INR 1.9–2.5) and triflusal (300 mg) in combination compared with adjusted-dose acenocoumarol (INR 2.0–3.0) alone. This study adds data to the randomised comparison in the NASPEAF39 trial above.
Combination acenocoumarol (INR 1.9–2.5) with either triflusal 600 mg or triflusal 300 mg was associated with lower rates of SE, 0 out of 155 (0%) and 2 out of 120 (1.7%), respectively, than acenocoumarol alone, 7 out of 265 (2.6%), after a mean 4.92-year follow-up. 54
One additional study, PETRO,73 reported non-randomised comparisons for the outcome of SE.
The PETRO study73 contained three comparisons: (1) dabigatran 50 mg (twice daily) plus aspirin (either 81 mg or 325 mg daily) compared with dabigatran 50 mg twice daily; (2) dabigatran 150 mg (twice daily) plus aspirin (either 81 mg or 325 mg daily) compared with dabigatran 150 mg twice daily; and (3) dabigatran 300 mg (twice daily) plus aspirin (either 81 mg or 325 mg daily) compared with dabigatran 300 mg twice daily.
Systemic emboli occurred only in patients receiving combination dabigatran 50 mg (once/twice daily) plus aspirin 81 mg and dabigatran 50 mg twice daily alone. The proportion experiencing a SE was higher in patients receiving the combination therapy than in those receiving dabigatran alone [1/21 (4.8) vs 1/59 (1.7), respectively] after a 22-week follow-up period. 73
The differences in SE outcomes reported in the included studies may reflect the methodological differences between these studies discussed in detail above (see Between-study differences).
Outcome 5: acute myocardial infarction
Five studies reported in nine articles39,42–45,47,54,68,69 yielded outcome data for acute myocardial infarction (AMI) (or ACS). Of these, three studies in six articles39,42–45,47 reported randomised comparisons. The key characteristics of these studies have been previously reported previously in Table 4.
The remaining three articles54,68,69 reported non-randomised comparisons; one a primary study by Bover et al. 54 and two secondary analyses of the SPORTIF III and SPORTIF V studies by White et al. 68 and Flaker et al. 69 The characteristics of the studies reporting non-randomised comparisons have been reported previously in Table 6.
Only data from five of the included studies39,42,43,54,69 have been reported in this section. Reasons for non-inclusion of data from other studies have been reported in Appendix 7.
The findings of the studies that report randomised comparisons are shown in Table 16 and non-randomised comparisons in Table 17.
| Author, year, study name | Risk, follow-up | ACT + APT, n | No. of events/total participants in ACT + APT arm (%) | ACT (alone or ACT + placebo), n | No. of events/total participants in ACT arm (%) | RR (95% CI) |
|---|---|---|---|---|---|---|
| aPérez-Gómez et al., 2004, RCT – NASPEAF39 | High risk,b 2.95 years | Adjusted-dose acenocoumarol (INR 1.4–2.4) + triflusal (600 mg), n = 223 | AMI:d 0/223 (0) | Adjusted-dose acenocoumarol (INR 2.0–3.0), n = 247 | AMI:d 0/247 (0) | Not estimable |
| Intermediate risk,c 2.6 years | Adjusted-dose acenocoumarol (INR 1.25–2.0) + triflusal (600 mg), n = 222 | AMI:d 0/222 (0) | Adjusted-dose acenocoumarol (INR 2.0–3.0), n = 232 | AMI:d 0/232 (0) | Not estimable | |
| eGullov et al., 1998, RCT – AFASAK II42 | Risk NR, 3.5 years | Fixed-dose warfarin (1.25 mg) + aspirin (300 mg), n = 171 | AMI: 0/171 (0) | Adjusted-dose warfarin (INR 2.0–3.0), n = 170 | AMI: 4/170 (2.4) | 0.11 (0.01 to 2.04) |
| Fixed-dose warfarin (1.25 mg), n = 167 | AMI: 6/167 (3.6) | 0.08 (0.00 to 1.32) | ||||
| SPAF investigators, 1996, RCT – SPAF III43 | High risk,f 1.1 years | Adjusted-dose warfarin (INR 1.2–1.5) + aspirin (325 mg), n = 521 | AMI: 10/521 (1.9) | Adjusted-dose warfarin (INR 2.0–3.0), n = 523 | AMI: 5/523 (1.0) | 2.01 (0.69 to 5.83) |
| Author, year | Stroke risk, follow-up | ACT + APT, n | No. of events/total participants in ACT + APT arm (%) | ACT (alone or ACT + placebo), n | No. of events/total participants in ACT arm (%) |
|---|---|---|---|---|---|
| aBover et al., 2009 54 | Risk NR, 4.92 years | Adjusted-dose acenocoumarol (INR 1.9–2.5) + triflusal (600 mg), n = 155 | AMI: 0/155 (0) | Adjusted-dose acenocoumarol (INR 2.0–3.0), n = 265 | AMI: 5/265 (1.9) |
| Adjusted-dose acenocoumarol (INR 1.9–2.5) + triflusal (300 mg), n = 120 | AMI: 1/120 (0.8) | ||||
| Adjusted-dose acenocoumarol (INR 1.9–2.5) + aspirin (100 mg), n = 34 | AMI: 0/34 (0) | ||||
| Flaker et al., 2006 69 | High risk,b 16.5 months | Adjusted-dose warfarin (INR 2.0–3.0) + aspirin (≤ 100 mg), n = 481 | AMI: 4/481 (0.8) | Adjusted-dose warfarin (INR 2.0–3.0), n = 3172 | AMI: 46/3172 (1.5) |
| Ximelagatran (36 mg) + aspirin (≤ 100 mg), n = 531 | AMI: 10/531 (1.9) | Ximelagatran (36 mg), n = 3120 | AMI: 40/3120 (1.3) |
A precise definition of AMI and its subclassification was not always supplied in the study reports and/or the definitions varied between the studies. Among the included studies the AFASAK II trial42 and the analysis of the SPORTIF III and SPORTIF V studies by Flaker et al. ,69 defined AMI by presence of any two assessment criteria, i.e. history of typical chest pain, serial creatine kinase MB isozyme changes typical of AMI, or electrocardiogram changes typical of AMI. The NASPEAF trial39 reported data for non-fatal AMI. Definition of AMI was not specified in the SPAF III trial,43 NASPEAF study39 or in the study by Bover et al. 54
The AFASAK II42 and SPAF III43 studies reported randomised comparisons for different regimes of combined warfarin plus additional aspirin compared with warfarin alone. The findings of these studies have been reported in Table 16.
The AFASAK II42 study reported no AMI events among patients receiving the combination of fixed-dose warfarin (1.25 mg) plus aspirin (300 mg). The AMI event rate was lower but not significantly so among those receiving combination therapy than in those receiving either fixed-dose warfarin (1.25 mg) alone [0/171 (0%) vs 6/167 (3.6%), RR 0.08 (95% CI 0.00 to 1.32)] or adjusted-dose warfarin alone (INR 2.0–3.0) [0/171 (0%) vs 4/170 (2.4%), RR 0.11 (95% CI 0.01 to 2.04)] over a mean follow-up period of 3.5 years. The risk profile of the patients enrolled in this study42 was not specified.
The SPAF III43 study reported a non-significant but higher incidence of AMI events in the combined therapy group than in those receiving adjusted-dose warfarin (INR 2.0–3.0) alone [10/521 (1.9%) vs 5/523 (1.0%), respectively], RR 2.01 (95% CI 0.69 to 5.83), in high-risk patients over a mean follow-up period of 1.1 years. 43
The AMI rate was different in these two RCTs. Rates of AMI were higher in the combined therapy arm of the SPAF III study than those receiving combination therapy in the AFASAK II42 study [1.9% (10/521) vs 0% (0/171), respectively]. However, the AMI rates were lower in those receiving adjusted-dose warfarin (INR 2.0–3.0) alone in the SPAF III study43 [5/523 (1.0%)] than in those receiving either adjusted-dose warfarin alone or fixed-dose warfarin alone [(4/170 (2.4%) and 6/167 (3.6%), respectively] in the AFASAK II study. 42
Flaker et al. ,69 in their post hoc analysis of non-randomised comparisons from the SPORTIF III and V studies, reported fewer AMI events in the combined therapy than in those on adjusted-dose warfarin (INR 2.0–3.0) alone [4/481 (0.8%) vs 46/3172 (1.5%), respectively] over a mean follow-up period of 16.5 months. However, aspirin was indicated in patients with previous CAD in the SPORTIF studies. 64,65
Bover et al. 54 compared adjusted-dose acenocoumarol (INR 1.9–2.5) plus aspirin (100 mg) with adjusted-dose acenocoumarol alone (INR 2.0–3.0) over a mean follow-up period of 4.92 years. This study also compared combination acenocoumarol and two different regimes of triflusal, which will be discussed in a subsequent section. The findings of this study for the outcome of AMI are reported in Table 17.
No AMIs occurred in the 34 patients receiving combination acenocoumarol and aspirin compared with 5 out of 265 (1.9%) AMIs in those receiving acenocoumarol alone. 54 The rate of AMIs was lower in all three combination therapy arms than in the arm with adjusted-dose acenocoumarol alone. No AMIs occurred in those receiving acenocoumarol plus triflusal 600 mg or acenocoumarol plus aspirin 100 mg, and one patient receiving acenocoumarol plus triflusal 300 mg experienced an AMI [1/120 (0.8%)] compared with 5 out of 265 (1.9%) patients receiving adjusted-dose warfarin alone.
Flaker et al. 69 also reported non-randomised comparisons for ximelagatran (36 mg) plus aspirin (100 mg) with ximelagatran (36 mg) alone, over a mean follow-up period of 16.5 months. A slightly higher rate of AMIs was observed in patients on combined therapy than in those on ximelagatran alone [10/531 (1.9%) vs 40/3120 (1.3%), respectively]. However, it is to be noted that aspirin use was indicated in patients with previous CAD in the original SPORTIF studies. 65,68
No studies were identified that reported randomised comparisons for AMI outcome comparing ximelagatran in combination with aspirin with ximelagatran alone.
The NASPEAF study39 compared adjusted-dose acenocoumarol (INR 1.4–2.4) plus triflusal (600 mg) with adjusted-dose acenocoumarol alone (INR 2.0–3.0) in high-risk patients during a median follow-up of 2.95 years, and combination adjusted-dose acenocoumarol (INR 1.2–2.0) plus triflusal (600 mg) with adjusted-dose acenocoumarol alone (INR 2.0–3.0) in intermediate-risk patients during a median follow-up of 2.6 years. This study specified outcomes for non-fatal AMIs. No non-fatal AMIs occurred in the NASPEAF study. 39
Bover et al. 54 reported non-randomised AMI outcome data comparing adjusted-dose acenocoumarol (INR 1.9–2.5) plus triflusal in two different regimes (600 mg or 300 mg) or aspirin (100 mg) with adjusted-dose acenocoumarol (INR 2.0–3.0) alone, over a mean follow-up period of 4.92 years.
The rate of AMI was lower in all three combination therapy arms than for adjusted-dose acenocoumarol alone. No AMIs occurred in those receiving acenocoumarol plus triflusal 600 mg or acenocoumarol plus aspirin 100 mg, and one patient receiving acenocoumarol plus triflusal 300 mg experienced an AMI [1/120 (0.8%)] compared with 5 out of 265 (1.9%) patients receiving adjusted-dose acenocoumarol alone.
The combination of adjusted-dose acenocoumarol (target INR 1.9–2.5) with either triflusal 600 mg or triflusal 300 mg or aspirin (100 mg) demonstrated fewer events of AMI [1/155 (0%), 1/120 (0.8%) and 0/34 (0%), respectively] than acenocoumarol given alone in adjusted dose alone with target INR of 2.0–3.0 [5/265 (1.9%)].
The differences in AMI outcomes reported in the included studies may reflect the methodological differences between these studies discussed in detail above (see Between-study differences). In addition, only two studies,42,69 one randomised42 and one non-randomised69 provided a specific definition for AMI, whereas three others39,43,54 (two randomised39,43 and one non-randomised54) did not. Both the AFASAK II42 and SPORTIF III and V69 studies used the same standard definition of AMI. Four studies42,43,54,69 (two randomised42,43 and two non-randomised54,69) reported all AMIs, whereas one randomised study39 reported only non-fatal AMI events. Of note here for the non-randomised comparisons54,69 is the potential confounding of the addition of APT to ACT at physicians' discretion, which may have resulted in patients at risk of an AMI being given APT, which may account for variation in the reported event rates.
Very few AMIs were reported. Although the rate of AMI was numerically lower with combined ACT plus APT compared with ACT alone in four42,43,54,69 (two randomised42,43 and two non-randomised54,69) of five43 studies reporting this outcome, there was no evidence of a significant benefit of combination therapy in the reduction of AMIs. However, in the non-randomised comparisons the addition of APT is confounded by indication. 54,69
Outcome 6: in-stent thrombosis
No studies were identified that reported in-stent thrombosis outcome data comparing ACT plus APT with anticoagulant alone in an AF population.
Outcome 7: vascular death
Four studies, reported in seven articles39,41–45,47 yielded outcome data for vascular death. Of these, all four studies reported randomised comparisons39,41–43 (Table 18) supported by three subgroup analyses. 44,45,47 No non-randomised comparisons reported vascular death as an outcome.
| Author, year | Stroke risk, follow-up | ACT + APT, n | No. of events/total participants in ACT + APT arm (%) | ACT (alone or ACT + placebo), n | No. of events/total participants in ACT arm (%) | RR (95% CI) |
|---|---|---|---|---|---|---|
| aPérez-Gómez et al., 2004, RCT – NASPEAF39 | High risk,b 2.95 years | Adjusted-dose acenocoumarol (INR 1.4-2.4) + trifusal (600 mg), n = 223 | Vascular all: 6/223 (2.7) | Adjusted-dose acenocoumarol (INR 2.0–3.0), n = 247 | Vascular all: 17/247 (6.9) | 0.39 (0.16 to 0.97) |
| • Vascular (bleed): 1/223 (0.4) | • Vascular (bleed): 0/247 (0) | 3.32 (0.14 to 81.12) | ||||
| • Vascular (SE): 0/223 (0) | • Vascular (SE): 2/247 (0.8) | 0.22 (0.01 to 4.59) | ||||
| • Vascular (stroke): 4/223 (1.8) | • Vascular (stroke): 6/247 (2.4) | 0.74 (0.21 to 2.58) | ||||
| • Vascular (AMI): 0/223 (0) | • Vascular (AMI): 1/247 (0.4) | 0.37 (0.02 to 9.01) | ||||
| • Vascular (HF): 0/223 (0) | • Vascular (HF): 2/247 (0.8) | 0.22 (0.01 to 4.59) | ||||
| • Vascular (HF-avascular): 0/223 (0) | • Vascular (HF- avascular): 2/247 (0.8) | 0.22 (0.01 to 4.59) | ||||
| • Vascular (sudden): 1/223 (0.4) | • Vascular (sudden): 4/247 (1.6) | 0.28 (0.03 to 2.46) | ||||
| Intermediate risk,c 2.6 years | Adjusted-dose acenocoumarol (INR 1.25-2.0) + trifusal (600 mg), n = 222 | Vascular all: 2/222 (0.9) | Adjusted-dose acenocoumarol (INR 2.0–3.0), n = 232 | Vascular all: 11/232 (4.7) | 0.19 (0.04 to 0.85) | |
| • Vascular (bleed): 1/222 (0.5) | • Vascular (bleed): 0/232 (0) | 3.13 (0.13 to 76.54) | ||||
| • Vascular (SE): 0/222 (0) | • Vascular (SE): 0/232 (0) | Not estimable | ||||
| • Vascular (stroke): 0/222 (0) | • Vascular (stroke): 3/232 (1.3) | 0.15 (0.01 to 2.87) | ||||
| • Vascular (AMI): 0/222 (0) | • Vascular (AMI): 0/232 (0) | Not estimable | ||||
| • Vascular (HF): 0/222 (0) | • Vascular (HF): 3/232 (1.3) | 0.15 (0.01 to 2.87) | ||||
| • Vascular (HF- avascular): 0/222 (0) | • Vascular (HF- avascular): 1/232 (0.4) | 0.35 (0.01 to 8.50) | ||||
| • Vascular (sudden): 1/222 (0.5) | • Vascular (sudden): 4/232 (1.7) | 0.26 (0.03 to 2.32) | ||||
| Lechat et al., 2001 RCT –FFAACS41 | High risk,d 0.84 years | Adjusted-dose fluindione (INR 2.0–2.6) + aspirin (100 mg), n = 76 | Vascular: 3/76 (3.9) | Adjusted-dose fluindione (INR 2.0–2.6) + placebo, n = 81 | Vascular: 2/81 (2.5) | 1.60 (0.27 to 9.31) |
| eGullov et al., 1998 RCT -AFASAK II42 | Risk NR, 3.5 years | Fixed-dose warfarin (1.25 mg) + aspirin (300 mg), n = 171 | Vascular: 3/171 (1.8) | Adjusted-dose warfarin (INR 2.0–3.0), n = 170 | Vascular: 5/170 (2.9) | 0.60 (0.14 to 2.46) |
| Fixed-dose warfarin (1.25 mg), n = 167 | Vascular: 2/167 (1.2) | 1.46 (0.25 to 8.66) | ||||
| SPAF investigators, 1996 RCT – SPAF III43 | High risk,f 1.1 years | Adjusted-dose warfarin (INR 1.2–1.5) + aspirin (325 mg), n = 521 | Vascular: 27/521 (5.2) | Adjusted-dose warfarin (INR 2.0–3.0), n = 523 | Vascular: 27/523 (5.2) | 1.00 (0.60 to 1.69) |
Vascular death was defined as sudden or any other death occurring within 30 days after a vascular event or progressive HF in the NASPEAF study. 39 The FFAACS study41 reported vascular death as one due to any of the following reasons: ischaemic or haemorrhagic stroke (Rankin score between 4 and 5 followed by death), an AMI, sudden, fatal SE, fatal haemorrhage, arterial aneurysm rupture, gangrene secondary to severe ischaemia and/or pulmonary embolism.41 Vascular death was not defined separately in the AFASAK II study42 or the SPAF III study. 43 The definitions were considered broadly similar for the purposes of this review.
Three randomised comparisons41–43 compared a VKA plus aspirin with a VKA alone. The AFASAK II42 and SPAF III43 studies both compared warfarin plus aspirin with warfarin alone, although the warfarin and aspirin regimes differed between the studies. The FFAACS41 study compared fluindione plus aspirin with fluindione alone.
The AFASAK II42 study compared fixed-dose warfarin (1.25 mg daily) plus aspirin (300 mg daily) with adjusted-dose warfarin (INR 2.0–3.0) or fixed-dose warfarin (1.25 mg daily). The risk profile of the patients enrolled in this study was not specified. The rates of vascular death were low and there were no significant differences in the rate of vascular death between the treatment groups during the median 3.5 years of follow-up: combination therapy compared with adjusted-dose warfarin (INR 2.0–3.0) alone [3/171 (1.8%) vs 5/170 (2.9%), respectively, RR 0.60 (95% CI 0.14 to 2.46)] and compared with fixed-dose (1.25 mg) warfarin alone [3/171 (1.8%) vs 2/167 (1.2%), respectively, RR 1.46 (95% CI 0.25 to 8.66)]. 42
The SPAF III43 study compared adjusted-dose warfarin (INR 1.2–1.5) plus aspirin (325 mg daily) with adjusted-dose warfarin (INR 2.0–3.0) alone in high-risk patients with AF. The rate of vascular death was the same in both the combination therapy and warfarin-alone arms [27/521 (5.2%) vs 27/523 (5.2%), respectively, RR 1.00 (95% CI 0.6 to 1.69)] during the mean 1.1-year follow-up period. 43
The FFAACS41 study compared adjusted-dose fluindione (INR 2.0–2.6) plus aspirin 100 mg with adjusted-dose fluindione (INR 2.0–2.6) alone. The number of vascular deaths in both groups was small and the difference was not significant [3/76 (3.9%) vs 2/81 (2.5%), respectively, RR 1.60 (95% CI 0.27 to 9.31)] during a mean 0.84-year follow-up.
The rate of vascular death differed between the studies. Among those patients receiving combination therapy, the rate of vascular death was highest in the SPAF III43 study: 27 out of 521 patients (5.2%) compared with 3 out of 171 patients (1.8%) in the AFASAK II42 study and 3 out of 76 patients (3.9%) in the FFAACS study. 41 Among those receiving anticoagulation alone, again the rate of vascular death was highest in the SPAF III study,43 27 out of 523 (5.2%) patients, with rates of 1.2% (2/167) and 2.9% (5/170) among those fixed- and adjusted-dose warfarin in the AFASAK II study, respectively, and 2.5% (2/81) in those patients receiving fluindione in the FFAACSs. 41 The NASPEAF39 randomised comparison compared adjusted-dose acenocoumarol (INR 1.4–2.4) in combination with triflusal 600 mg with adjusted-dose acenocoumarol (INR 2.0–3.0) alone in high-risk patients and adjusted-dose acenocoumarol (INR 1.2–2.0) and triflusal 600 mg in combination compared with adjusted-dose acenocoumarol (INR 2.0–3.0) alone in intermediate-risk patients.
Fewer vascular deaths occurred in patients receiving combination therapy than in those receiving acenocoumarol alone in both the high-risk [6/223 (2.7%) vs 17/247 (6.9%), respectively] and intermediate-risk [2/222 (0.9%) vs 11/232 (4.7%), respectively] groups, but these differences were not significant: RR 0.39 (95% CI 0.16 to 0.97) and RR 0.19 (95% CI 0.04 to 0.85), respectively.
No studies reported non-randomised comparisons of ACT plus APT compared with ACT alone for the outcome of vascular death.
The differences in vascular mortality reported in the included studies may reflect the methodological differences between these studies discussed in detail above (see Between-study differences). Of the four randomised studies,39,41–43 only two provided a specific definition of vascular death,39,41 which may reflect the variation in vascular mortality reported between the included studies.
Secondary outcomes
Outcome 8: all-cause mortality
Ten articles39,41–43,47,50,54,58,68,69 yielded outcome data for all-cause mortality. Of these, four studies in five articles39,41–43,47 reported randomised comparisons. The remaining five articles50,54,58,68,69 reported non-randomised comparisons, of which three were primary studies,54,54,58 and two were secondary analyses of the SPORTIF III and SPORTIF V studies by White et al. 68 and Flaker et al. 69
Of the studies that reported non-randomised comparisons, those by Lopes et al. ,50 Stenestrand et al. 58 and White et al. 68 are not mentioned further in this section because two of these50,68 did not furnish details of number of patients (denominator) in either therapy group and one did not report the number of events. 58 The reasons for non-inclusion of their data have been reported in Appendix 7. The characteristics of these studies have been reported previously (see Table 6).
All-cause mortality was frequently classified as death from non-vascular, indeterminant, unknown or sudden causes. A precise definition of these groupings or subclassifications of mortality was not always supplied in the study reports and/or the definitions may vary between studies for the same subclassification.
The findings of the included studies for each of these composites and/or subclassifications of all-cause mortality are detailed in Tables 19 and 20.
| Author, year | Stroke risk, follow-up | ACT + APT, n | No. of events/total participants in ACT + APT arm (%) | ACT (alone or ACT + placebo), n | No. of events/total participants in ACT arm (%) | RR (95% CI) |
|---|---|---|---|---|---|---|
| Pérez-Gómez et al., 2004, RCT – NASPEAF39 | High risk,a 2.95 years | Adjusted-dose acenocoumarol (INR 1.4–2.4) + triflusal (600 mg), n = 223 | Total:b 12/223 (5.4) | Adjusted-dose acenocoumarol (INR 2.0–3.0), n = 247 | Total:b 23/247 (9.3) | 0.58 (0.29 to 1.13) |
| Non-vascular: 6/223 (2.7) | Non-vascular: 6/247 (2.4) | 1.11 (0.36 to 3.38) | ||||
| Intermediate risk,c 2.6 years | Adjusted-dose acenocoumarol (INR 1.25–2.0) + triflusal (600 mg), n = 222 | Total:b 6/222 (2.7) | Adjusted-dose acenocoumarol (INR 2.0–3.0), n = 232 | Total:b 20/232 (8.6) | 0.31 (0.13 to 0.77) | |
| Non-vascular: 4/222 (1.8) | Non-vascular: 9/232 (3.9) | 0.46 (0.15 to 1.49) | ||||
| Lechat et al., 2001 RCT – FFAACS41 | High risk,d 0.84 years | Adjusted-dose fluindione (INR 2.0–2.6) + aspirin (100 mg), n = 76 | All cause: 3/76 (3.9) | Adjusted-dose fluindione (INR 2.0–2.6) + placebo, n = 81 | All cause: 3/81 (3.7) | 1.07 (0.22 to 5.12) |
| eGullov et al., 1998 RCT – AFASAK II42 | Risk NR, 3.5 years | Fixed-dose warfarin (1.25 mg) + aspirin (300 mg), n = 171 | Total:f 9/171 (5.3) | Adjusted-dose warfarin (INR 2.0–3.0), n = 170 | Total:f 6/167 (3.6) | 1.46 (0.53 to 4.03) |
| Non-vascular: 1/171 (0.6) | Non-vascular: 0/167 (0) | 2.93 (0.12 to 71.42) | ||||
| Unknown cause: 2/171 (1.2) | Unknown cause: 2/167 (1.2) | 0.98 (0.14 to 6.85) | ||||
| Fixed-dose warfarin (1.25 mg), n = 167 | Total:f 17/170 (10.0) | 0.53 (0.24 to 1.15) | ||||
| Non-vascular: 2/170 (1.2) | 0.50 (0.05 to 5.43) | |||||
| Unknown cause: 3/170 (1.8) | 0.66 (0.11 to 3.92) | |||||
| SPAF investigators, 1996 RCT – SPAF43 | High risk,f 1.1 years | Adjusted-dose warfarin (INR 1.2–1.5) + aspirin (325 mg), n = 521 | Total:f 42/521 (8.1) | Adjusted-dose warfarin (INR 2.0–3.0), n = 523 | Total:f 35/523 (6.7) | 1.20 (0.78 to 1.86) |
| Non-vascular: 12/521 (2.3) | Non-vascular: 8/523 (1.5) | 1.51 (0.62 to 3.65) | ||||
| Indeterminant: 3/521 (0.6) | Indeterminant: 0/523 (0) | 7.00 (0.36 to 135.18) |
| Author, year | Stroke risk, follow-up | ACT + APT, n | No. of events/total participants in ACT + APT arm (%) | ACT (alone or ACT + placebo), n | No. of events/total participants in ACT arm (%) |
|---|---|---|---|---|---|
| Bover et al., 200954 | Risk NR, 4.92 years | Adjusted-dose acenocoumarol (INR 1.9–2.5) + triflusal (600 mg), n = 155 | Non-cardiac: 6/155 (3.9) | Adjusted-dose acenocoumarol (INR 2.0–3.0), n = 265 | – |
| Sudden: 4/155 (2.6) | |||||
| Adjusted-dose acenocoumarol (INR 1.9–2.5) + triflusal (300 mg), n = 120 | Non-cardiac: 3/120 (2.5) | Non-cardiac: 3/265 (1.1) | |||
| Sudden: 0/120 (0) | Sudden: 3/265 (1.1) | ||||
| Adjusted-dose acenocoumarol (INR 1.9–2.5) + aspirin (100 mg), n = 34 | Non-cardiac: 1/34 (2.9) | ||||
| Sudden: 1/34 (2.9) | |||||
| Flaker et al., 200669 | High risk,a 16.5 months | Adjusted-dose warfarin (INR 2.0–3.0) + aspirin (≤ 100 mg), n = 481 | All: 17/481 (3.5) | Adjusted-dose warfarin (INR 2.0–3.0), n = 3172 | All: 112/3172 (3.5) |
| Ximelagatran (36 mg) + aspirin (≤ 100 mg), n = 531 | All: 3/531 (0.6) | Ximelagatran (36 mg), n = 3120 | All: 95/3120 (3.0) |
All-cause mortality
Two randomised comparisons (AFASAK II42 and SPAF III43) and one non-randomised comparison69 compared the combination of warfarin plus aspirin with warfarin alone.
The AFASAK II42 randomised comparison compared combined fixed-dose warfarin (1.25 mg) plus aspirin (300 mg daily) with either adjusted-dose warfarin (target INR 2.0–3.0) alone or with fixed-dose warfarin (1.25 mg daily) alone. The risk profile of the patients enrolled in this study was not specified. The rate of all-cause mortality was lower among those patients receiving combined therapy than in those receiving fixed-dose warfarin [9/171 (5.3%) vs 17/170 (10%), respectively, RR 0.53 (95% CI 0.24 to 1.15)] and higher than those patients receiving adjusted-dose warfarin [9/171 (5.3%) vs 6/167 (3.6%), respectively, RR 1.46 (95% CI 0.53 to 4.03)] over a mean follow-up period of 3.5 years, although these differences were not significant. 42 The SPAF III study43 compared adjusted-dose warfarin (INR 1.2–1.5) plus aspirin (325 mg) with adjusted-dose warfarin (target INR 2.0–3.0) alone in high-risk patients with AF. The study43 demonstrated similar rates of all-cause mortality for patients treated with adjusted-dose warfarin (INR1.2–1.5) in combination with aspirin (325 mg) compared with those treated with adjusted-dose warfarin (INR 2.0–3.0) alone [42/521 (8.1%) vs 35/523 (6.7%), respectively, RR 1.20 (95% CI 0.78 to 1.86)] over a mean follow-up period of 1.1 years.
There were small differences in the rate of all-cause mortality in these two RCTs. 42,43 In the combination therapy arm of the SPAF III study43 the mortality rate was slightly higher at 8.1% (42/521) than 5.3% (9/171) in the combined therapy arm in the AFASAK II study. 42 Among those patients receiving adjusted-dose warfarin alone, the rate of all-cause mortality was also higher in the SPAF III43 study [35/523 (6.7%)] than in the AFASAK II42 study [6/167 (3.6%)], but lower than those receiving fixed-dose warfarin alone in the AFASAK II study42 [35/523 (6.7%) vs 17/170 (10%), respectively].
The pooled analysis of the SPORTIF studies by Flaker et al. 69 compared adjusted-dose warfarin (INR 2.0–3.0) plus aspirin (100 mg) with adjusted-dose warfarin (INR 2.0–3.0) alone, over a mean follow-up period of 16.5 months. The rate of all-cause mortality was the same in patients receiving combined therapy or warfarin alone [17/481 (3.5%) vs 112/3172 (3.5%), respectively].
The mortality rate was much higher in the combined therapy arm of the AFASAK II42 and SPAF III43 studies than in the combined therapy arm in the SPORTIF III and V69 studies [9/171 (5.3%), 42/521 (8.1%) and 17/481 (3.5%), respectively]. The mortality rate was also much higher in patients receiving adjusted-dose warfarin alone in the SPAF III43 study [35/523(6.7%)] and fixed-dose warfarin alone in the AFASAK II42 study [17/170 (10%)], but similar among those patients receiving adjusted-dose warfarin alone in the AFASAK II42 and SPORTIF III and V studies [6/167 (3.6%) and 112/3172 (3.5%), respectively].
One study (NASPEAF39) reported randomised comparisons on all-cause mortality comparing adjusted-dose acenocoumarol (INR 1.4–2.4) plus triflusal (600 mg) with acenocoumarol alone (INR 2.0–3.0) in high-risk patients, and the combination of adjusted-dose acenocoumarol (INR 1.2–2.0) plus triflusal (600 mg) with acenocoumarol alone (INR 2.0–3.0) in intermediate-risk patients. The findings of this study are presented in Table 19.
The study demonstrated lower rates of all-cause mortality with combined therapy than acenocoumarol alone in the high-risk group [12/223 (5.4%) vs 23/247 (9.3%), respectively; RR 0.58 (95% CI 0.29 to 1.13)] over a median 2.95 year follow-up period, as well as in the intermediate-risk group [6/222 (2.7%) vs 20/232(8.6%), respectively; RR 0.31 (95% CI 0.13 to 0.77), over a median follow-up of 2.6 years, although these differences were not significant.
There was no non-randomised evidence available for all-cause mortality for this comparison.
The FFAACS41 study demonstrated very similar rates of all-cause mortality for patients treated with adjusted-dose fluindione (INR 2.0–2.6) in combination with aspirin (100 mg) to those with adjusted-dose fluindione (INR 2.0–2.6) plus placebo [3/76 (3.9%) vs 3/81 (3.7%), respectively; RR 1.07 (95% CI 0.22 to 5.12], over a mean follow-up period of 0.84 years.
There was no non-randomised evidence available for all-cause mortality for this comparison.
The pooled analysis of SPORTIF trials by Flaker et al. 69 reported non-randomised comparisons for all-cause mortality comparing ximelagatran (36 mg) plus aspirin (100 mg) with ximelagatran (36 mg) alone, over a mean follow-up period of 16.5 months. Fewer deaths were observed in patients on combined therapy than in those on ximelagatran alone [3/531 (0.6%) vs 95/3120 (3.0%), respectively]. However, it is to be noted that aspirin use was based on clinical need and thus the comparison may be confounded by indication. 65,68
There was no randomised evidence available for all-cause mortality for this comparison.
Mortality due to non-vascular causes
The AFASAK II42 and SPAF III43 studies reported randomised comparisons for mortality due to non-vascular causes comparing combinations of different regimes of warfarin plus aspirin to warfarin alone. There were no non-randomised comparisons identified for this outcome.
The AFASAK II42 RCT demonstrated similar rates of mortality due to non-vascular causes in patients receiving the combination of fixed-dose warfarin (1.25 mg) and aspirin (300 mg) compared with those receiving fixed-dose warfarin (1.25 mg) alone [1/171 (0.6%) vs 2/170 (1.2%), respectively); RR 0.50 (95% CI 0.05 to 5.43)] over a mean follow-up period of 3.5 years. No non-vascular deaths occurred in patients receiving adjusted-dose warfarin (INR 2.0–3.0) alone [1/171 (0.6%) vs 0/167 (0%), respectively); RR 2.93 (95% CI 0.12 to 71.42)]. The stroke risk of this population was not specified. 42
The SPAF III43 study demonstrated similar rates of mortality due to non-vascular causes in high-risk patients treated with adjusted-dose warfarin (INR 1.2–1.5) plus aspirin (325 mg) compared with those treated with adjusted-dose warfarin (INR 2.0–3.0) alone [12/521 (2.3%) vs 8/523(1.5%), respectively); RR 1.51 (95% CI 0.62 to 3.65)] over a mean follow-up period of 1.1 years. 43
There were very few non-vascular deaths in these two RCTs. 42,43 In the combination therapy arms, the event rate was higher in the SPAF III43 study at 2.3% (12/521) compared with 0.6% (1/171) in the AFASAK II study. 42 Rates of non-vascular mortality were similar in those receiving adjusted-dose warfarin alone in the SPAF III43 study [8/523 (1.5%)] and fixed-dose warfarin in the AFASAK II study [2/170 (1.2%)]. No non-vascular deaths occurred in the AFASAK II42 study among patients receiving adjusted-dose warfarin. The differences might reflect the methodological heterogeneity between studies as explained previously (see Between-study differences).
The NASPEAF39 study reported randomised comparisons on non-vascular cause mortality comparing adjusted-dose acenocoumarol (INR 1.4–2.4) plus triflusal (600 mg) with acenocoumarol alone (INR 2.0–3.0) in high-risk patients, and the combination of adjusted-dose acenocoumarol (INR 1.2–2.0) plus triflusal (600 mg) with acenocoumarol alone (INR 2.0–3.0) in intermediate-risk patients. The findings of this study are presented in Table 19.
The study demonstrated similar rates of non-vascular death when combined therapy was compared with acenocoumarol alone in the high-risk group [6/223 (2.7%) vs 6/247(2.4%), respectively; RR 1.11 (95% CI 0.36 to 3.38)] and lower but non-significant non-vascular mortality rates in the intermediate-risk group on combined therapy compared with those on acenocoumarol alone [4/222 (1.8%) vs 9/232 (0.48%), respectively; RR 0.46 (95% CI 0.15 to 10.49].
There were no non-randomised comparisons identified for this outcome.
Mortality due to indeterminant or unknown cause
The AFASAK II42 and SPAF III43 studies reported randomised comparisons for mortality due to unknown causes comparing combinations of different regimes of warfarin plus aspirin with warfarin alone. There were no non-randomised comparisons identified for this outcome.
The AFASAK II study42 demonstrated similar rates of mortality from unknown causes across all arms (see Table 19). The event rate was 1.2% in patients receiving combination therapy (2/171) and those receiving adjusted-dose warfarin alone [2/167); RR 0.98 (95% CI 0.14 to 6.85)] and similar in those receiving fixed-dose warfarin alone [3/170 (1.8%); RR 0.66 (95% CI 0.11 to 3.92)] over a mean follow-up period of 3.5 years. The stroke risk of this population was not specified. 42
The SPAF III43 study demonstrated a higher but statistically non-significant rate of mortality owing to indeterminant causes in high-risk patients treated with adjusted-dose warfarin (INR 1.2–1.5) plus aspirin (325 mg) than in those treated with adjusted-dose warfarin (INR 2.0–3.0) alone [3/521 (0.6%) vs 0/523 (0%), respectively; RR 7.00 (95% CI 0.36 to 135.18)] over a mean follow-up period of 1.1 years. 43
The rates of indeterminate mortality were slightly lower in the SPAF III study43 than in the AFASAK II study42 in both the combined therapy group as well as those receiving warfarin alone, despite the methodological differences between these two randomised comparisons. 42,43
Other definitions
Bover et al. 54 reported non-randomised comparisons for non-cardiac and sudden mortality comparing adjusted-dose acenocoumarol (INR 1.9–2.5) plus three different antiplatelet regimes (triflusal 600 mg, triflusal 300 mg or aspirin 100 mg) with adjusted-dose acenocoumarol (INR 2.0–3.0) alone, over a mean follow-up period of 4.92 years. A specific definition for either outcome was not specified. There were no randomised comparisons identified for this outcome.
More non-cardiac deaths were observed in patients receiving any of the combined therapy regimes (triflusal 600 mg, triflusal 300 mg or aspirin 100 mg) [6/155 (3.9%, 3/120 (2.5%) and 1/34 (2.9%), respectively] than those receiving adjusted-dose acenocoumarol alone [3/265 (1.1%)]. 54
The study reported a higher proportion of sudden deaths in patients receiving a combination of either acenocoumarol plus triflusal 600 mg or acenocoumarol plus aspirin 100 mg than with acenocoumarol alone [4/155 (2.6%), 1/34 (2.9%) vs 3/265 (1.1%), respectively] and a lower rate in those receiving combined acenocoumarol plus triflusal 300 mg than in those receiving acenocoumarol alone [0/120 (0%) vs 3/265 (1.1%), respectively].
There is no evidence from one non-randomised study for the benefit of combination ACT and APT over ACT alone in the reduction of either non-cardiac or sudden death. 54
The differences in all-cause mortality reported in the included studies may reflect the methodological differences between these studies discussed above (see Between-study differences). In addition, although all-cause mortality was frequently classified as death from non-vascular, indeterminant, unknown or sudden causes, a precise definition of these groupings or subclassifications of mortality was not always supplied in the study reports and/or the definitions may vary between studies for the same subclassification, which may account for some variation in the reported event rates.
Overall summary for mortality (excluding vascular death)
Five studies (three randomised39,41,42 and two non-randomised54,69) demonstrated that there is no evidence that combination therapy with ACT plus APT significantly reduces the risk of all-cause39,41,42,54,69, non-vascular,39,42 or non-cardiac54 mortality, mortality from unknown causes,42,43 and sudden death54 compared with ACT alone.
Outcome 9: bleeding
Twenty-seven articles yielded outcome data for bleeding. 39–45,47,51,53,54,56,57,59–65,72,73 Five of these studies in eight articles reported randomised comparisons. 39–45,47 The remaining 19 articles reported non-randomised comparisons of which 14 were primary studies51,53,54,56,57,59–65,72,73 and five were secondary analyses of the SPORTIF III and SPORTIF V studies. 66–70 Of those that reported non-randomised comparisons, data from four articles are reported in this section,54,63,69,73 as the others do not report any further relevant data. These other studies are reported in Appendix 7 except for the study by Akins et al. ,67 which has been reported elsewhere in the results section of the report; however, for the outcome of bleeding it does not report the number of bleeding events by therapy group.
Bleeding events were reported either on their own or in conjunction with other events such as embolism and mortality. In those studies that reported bleeding alone, bleeding was classified as major, minor or non-severe, and intracranial. A precise definition of these subclassifications was not always supplied in the study reports and/or the definitions may vary between studies for the same subclassification. The findings of the included studies for each of these subclassifications of bleeding are detailed in Tables 21 and 22.
| Author, year, study name | Stroke risk, follow-up | ACT + APT, n | No. of events/total participants in ACT + APT arm (%) | ACT (alone or ACT + placebo), n | No. of events/total participants in ACT arm (%) | RR (95% CI) |
|---|---|---|---|---|---|---|
| aPérez-Gómez et al., 2004, RCT – NASPEAF 39 | High risk,b 2.95 years | Adjusted-dose acenocoumarol (INR 1.4–2.4) + triflusal (600 mg), n = 223 | ICH: 2/223 (0.9) | Adjusted-dose acenocoumarol (INR 2.0–3.0), n = 247 | ICH: 5/247 (2.0) | 0.44 (0.09 to 2.26) |
| Severec:12/223 (5.4) | Severe:c13/247 (5.3) | 1.02 (0.47 to 2.19) | ||||
| Severe – other:d 2/223 (0.9) | Severe – other:d5/247 (2.0) | 0.44 (0.09 to 2.26) | ||||
| Non-severe: 20/223 (8.9) | Non-severe: 18/247 (7.3) | 1.23 (0.67 to 2.27) | ||||
| Intermediate risk,e 2.6 years | Adjusted-dose acenocoumarol (INR 1.25–2.0) + triflusal (600 mg), n = 222 | ICH: 1/222 (0.5) | Adjusted-dose acenocoumarol (INR 2.0–3.0), n = 232 | ICH: 4/232 (1.7) | 0.48 (0.05 to 4.21) | |
| Severe:c 5/222 (2.3) | Severe:c 10/232 (4.3) | 0.52 (0.18 to 1.50) | ||||
| Severe – other:d 1/222 (0.5) | Severe – other:d 5/232 (2.2) | 0.21 (0.02 to 1.77) | ||||
| Non-severe: 16/222 (7.2) | Non-severe: 15/232 (6.5) | 1.11 (0.56 to 2.20) | ||||
| Lidell et al., 200340 | Risk NR, 22 days | Adjusted-dose warfarin (INR 2.0–3.0) + clopidogrel (75 mg), n = 20 | Minor: 0/20 (0) | Adjusted-dose warfarin (INR 2.0–3.0) + placebo, n = 23 | Minor: 5/23 (21.8) | 0.10 (0.01,1.77) |
| Lechat et al., 2001 RCT – FFAACS41 | High risk,f 0.84 years | Adjusted-dose fluindione (INR 2.0–2.6) + aspirin (100 mg), n = 76 | Severe: 3/76 (3.9) | Adjusted-dose fluindione (INR 2.0–2.6) + placebo, n = 81 | Severe:1/81 (1.2) | 3.19 (0.34 to 30.07) |
| Non-severe: 10/76 (13.2) | Non-severe: 1/81 (1.2) | 10.66 (1.39 to 81.28) | ||||
| All: 13/76 (17.1) | All: 2/81 (2.5) | 6.93 (1.62 to 29.69) | ||||
| gGullov et al., 1998 RCT – AFASAK II42 | Risk NR, 3.5 years | Fixed-dose warfarin (1.25 mg) + aspirin (300 mg), n = 171 | ICH: 0/171 (0) | Adjusted-dose warfarin (INR 2.0–3.0), n = 170 | ICH: 2/170 (1.2) | 0.19 (0.01 to 4.11) |
| Major:h 4/170 (2.4) | 0.25 (0.03 to 2.20) | |||||
| Major:h 1/171 (0.6) | Minor:42/170 (24.7) | 0.66 (0.43 to 1.02) | ||||
| Fixed-dose warfarin (1.25 mg), n = 167 | ICH: 1/167 (0.6) | 0.33 (0.13 to 7.94) | ||||
| Minor: 28/171 (16.4) | Major:h 3/167 (1.8) | 0.33 (0.03 to 3.09) | ||||
| Minor: 21/167 (12.6) | 1.30 (0.77 to 2.19) | |||||
| SPAF investigators, 1996 RCT – SPAF III43 | High risk,i 1.1 years | Adjusted-dose warfarin (INR 1.2–1.5) + aspirin (325 mg), n = 521 | ICH: 5/521 (0.9) | Adjusted-dose warfarin (INR 2.0–3.0), n = 523 | ICH: 3/523 (0.6) | 1.67 (0.40 to 6.96) |
| Major:h 13/521 (2.5) | Major:h 12/523 (2.3) | 1.08 (0.50 to 2.36) | ||||
| Minor: 6/521 (1.2) | Minor: 4/523 (0.8) | 1.5 (0.43 to 5.30) |
| Author, year, study name | Stroke risk, follow-up | ACT + APT, n | No. of events/total participants in ACT + APT arm (%) | ACT (alone or ACT + placebo), n | No. of events/total participants in ACT arm (%) |
|---|---|---|---|---|---|
| aHansen et al., 201063 | Risk NR, 3.3 years | Warfarin + aspirin, n = 18,345 | All: 1209/18,345 (6.6) | Warfarin, n = 50,919 | All: 3642/50,919 (7.2) |
| Warfarin + clopidogrel, n = 1430 | All: 69/1430 (4.8) | ||||
| Warfarin + aspirin + clopidogrel, n = 1261 | All: 64/1261 (5.1) | ||||
| bBover et al., 200954 | Risk NR, 4.92 years | Adjusted-dose acenocoumarol (INR 1.9–2.5) + triflusal (600 mg), n = 155 | Severe:c10/155 (6.5) | Adjusted-dose acenocoumarol (INR 2.0–3.0), n = 265 | Severe:a 32/265 (12.1) |
| Fatal: 0/155 (0) | Fatal: 7/265 (2.6) | ||||
| GI: 8/155 (5.2) | GI: 6/265 (2.3) | ||||
| Adjusted-dose acenocoumarol (INR 1.9–2.5) + triflusal (300 mg), n = 120 | Severe:c6/120 (5.0) | ||||
| Fatal: 1/120 (0.8) | |||||
| GI: 5/120 (4.2) | |||||
| Adjusted-dose acenocoumarol (INR 1.9–2.5) + aspirin (100 mg), n = 34 | Severe:c7/34 (20.6) | Dabigatran (50 mg), n = 59 | |||
| Fatal: 2/34 (5.9) | |||||
| GI: 0/34 (0) | |||||
| Ezekowitz et al., 2007, RCT – PETRO73 | ≥ 1 stroke risk criteria,d22 weeks | Dabigatran (50 mg) + aspirin (81 mg), n = 21 | Major: 0/21 (0) | Major: 0/59 (0) | |
| Clinical relevant + major: 1/21 (4.8) | Clinical relevant + major: 0/59 (0) | ||||
| All:e 2/21 (9.5) | All:e 2/59 (3.4) | ||||
| Dabigatran (50 mg) + aspirin (325 mg), n = 27 | Major: 0/27 (0) | ||||
| Clinical relevant + major: 1/27 (3.7) | |||||
| All:e 3/27 (11.1) | |||||
| Dabigatran (150 mg) + aspirin (81 mg), n = 36 | Major: 0/36 (0) | Dabigatran (150 mg), n = 100 | Major: 0/100 (0) | ||
| Clinical relevant + major: 2/36 (5.6) | Clinical relevant + major: 9/100 (9.0) | ||||
| All:e 8/36 (22.2) | All:e 15/100 (15.0) | ||||
| Dabigatran (150 mg) + aspirin (325 mg), n = 33 | Major: 0/33 (0) | ||||
| Clinical relevant + major: 2/33 (6.1) | |||||
| All:e 7/33 (21.2) | |||||
| Dabigatran (300 mg) + aspirin (81 mg), n = 34 | Major: 1/34 (2.9) | Dabigatran (300 mg), n = 105 | Major: 0/105 (0) | ||
| Clinical relevant + major: 5/34 (14.7) | Clinical relevant + major: 6/105 (5.7) | ||||
| All:e 11/34 (32.4) | All:e 14/105 (13.3) | ||||
| Dabigatran (300 mg) + aspirin (325 mg), n = 30 | Major: 3/30 (10.0) | ||||
| Clinical relevant + major: 6/30 (20.0) | |||||
| All:e 14/30 (46.7) | |||||
| f Flaker et al., 200669 | High risk,g 16.5 months | Adjusted-dose warfarin (INR 2.0–3.0) + aspirin (≤ 100 mg), n = 481 | Major:h 25/481 (5.2) | Adjusted-dose warfarin (INR 2.0–3.0), n = 3172 | Major:h 100/3172 (3.2) |
| Major/minor: 251/481 (52.2) | Major/minor: 1199/3172 (37.8) | ||||
| Ximelagatran (36 mg b.i.d.) + aspirin (≤ 100 mg), n = 531 | Major:h 2/531 (0.4) | Ximelagatran (36 mg b.i.d.), n = 3120 | Major:h 78/3120 (2.5) | ||
| Major/minor: 202/531 (38.0) | Major/minor: 1013/3120 (32.5) |
All bleeding outcomes
Three studies36,41,73 reported all bleeding outcomes, one randomised41 and two non-randomised36,73 comparisons.
One randomised comparison41 in high-risk patients compared adjusted-dose fluindione (INR 2.0–2.6) plus aspirin 100 mg with adjusted-dose fluindione (INR 2.0–2.6) plus placebo. There were significantly more bleeding events in patients receiving combined therapy than in those on fluindione plus placebo [13/76 (17.1%) vs 2/81 (2.5%), respectively; RR 6.93 (95% CI 1.62 to 29.69] during the mean 0.84-year follow-up.
One non-randomised comparison (PETRO73) compared combinations of different doses of dabigatran (50, 150 and 300 mg) plus different regimes of aspirin (81 and 325 mg) with dabigatran alone (50 mg, 150 mg, 300 mg). Higher proportions of bleeding were found in patients receiving combination therapy at all doses of dabigatran plus aspirin than in those receiving dabigatran alone (see Table 22). A higher proportion of bleeding events were observed in patients on the combination therapy of dabigatran 50 mg plus either aspirin 81 mg or 325 mg [2/21 (9.5%) and 3/27 (11.1%), respectively] than in those receiving dabigatran 50 mg alone [2/59 (3.4%)]. A higher proportion of events were observed in patients on the combined therapy of dabigatran 150 mg plus either aspirin 81 or 325 mg [8/36 (22.2%), 7/33 (21.2%), respectively] than in those receiving dabigatran 150 mg alone [15/100 (15%)]. A higher proportion of patients on the combined therapy of dabigatran 300 mg plus either aspirin 81 or 325 mg [11/34 (32.4%), 14/30 (46.7%), respectively] suffered a bleeding event than in those receiving dabigatran 300 mg alone [14/105 (13.3%)] during a mean follow-up period of 22 weeks. Randomised comparisons for dabigatran plus an antiplatelet agent compared with dabigatran alone were not identified.
Hansen et al. 63 reported registry data comparing warfarin (INR target not stated) in combination with either aspirin (dose not stated) or clopidogrel (dose not stated) or both clopidogrel and aspirin (dose not stated), with warfarin alone (dose not stated). The rate of bleeding was similar among patients receiving warfarin plus aspirin or warfarin alone [1209/18,345 (6.6%) vs 3642/50,919 (7.2%], respectively), although the rate of bleeding was slightly lower in patients receiving either warfarin plus clopidogrel (69/1430 (4.8%)] or triple therapy [64/1261 (5.1%)] than in those receiving warfarin alone [3642/50,919 (7.2%)]. However, the use of an antiplatelet agent is confounded by indication and given that bleeding is a contraindication to ATT-only patients felt to be at low risk of bleeding may have been given combination therapy in this non-randomised comparison.
There is conflicting evidence regarding the effect of combination ACT plus APT compared with ACT alone on the risk of all bleeding. Two studies (one randomised41 and one non-randomised73) demonstrated higher rates of overall bleeding with some combination therapy (fluindione plus aspirin41 and dabigatran plus aspirin73) over ACT alone, whereas one other non-randomised study63 found similar levels of bleeding with combination therapy (warfarin plus aspirin or clopidogrel) compared with ACT alone. 54
Major (or severe) haemorrhage
Four randomised comparisons39–43 and three non-randomised comparisons54,69,73 reported data on major (or severe) haemorrhage.
The AFASAK II42 study defined major haemorrhage as fatal, life-threatening, or potentially life-threatening, requiring surgical treatment or blood transfusion. All life-threatening bleeds were confirmed from hospital records. The SPAF III43 study defined major haemorrhage according to the Landfeld criteria, i.e. overt bleeding that was fatal, life-threatening, potentially life-threatening, or acute or subacute leading to reoperation or moderate or severe blood loss. 93 The NASPEAF39 study defined severe haemorrhage as requiring hospital admission, blood transfusion, or surgery. The FFAACS study defined severe haemorrhage as needing treatment (including transfusion) or hospitalisation. 41 These definitions are broadly comparable and are considered equivalent for the purposes of this review.
The AFASAK II42 and SPAF III43 studies reported randomised comparisons for major haemorrhage comparing different regimes of combined warfarin plus aspirin with warfarin alone. The findings of these studies are reported in Table 21.
The AFASAK II42 study reported very low event rates with a non-significant difference in rates of major bleeding between combined fixed-dose warfarin (1.25 mg daily) plus aspirin (300 mg daily) and adjusted-dose warfarin (INR 2.0–3.0) alone [1/171 (0.6%) vs 4/170 (2.4%), respectively, RR 0.25, 95% CI 0.03 to 2.20] or fixed-dose warfarin (1.25 mg daily) alone [1/171 (0.6%) vs 3/167 (1.8%), respectively, RR 0.33, 95% CI 0.03 to 3.09] during the mean 3.5 years of follow-up. The risk profile of the patients enrolled in this study42 was not specified.
The SPAF III43 study reported very similar rates of major bleeding in patients on either adjusted-dose warfarin (INR 1.2–1.5) plus aspirin (325 mg daily) or adjusted-dose warfarin (INR 2.0–3.0) alone in high-risk patients with AF [13/521 (2.5%) vs 12/523 (2.3%), respectively, RR 1.08, 95% CI 0.5 to 2.36] during the mean 1.1-year follow-up period. 43
Flaker et al. 69 reported non-randomised data on a pooled analysis of the SPORTIF III and V studies comparing combined adjusted-dose warfarin (INR 2.0–3.0) plus aspirin (100 mg) with adjusted-dose warfarin alone (INR 2.0–3.0) (Table 22). Higher rates of major bleeding were reported in the combined therapy group than in the warfarin alone group [25/481 (5.2%) vs 100/3172 (3.2%), respectively] during the mean 16.5-month follow-up.
There were small differences in the event rates of major bleeding in the two RCTs. In the combination therapy arms of the randomised comparisons, the rate of major bleeding was higher in the SPAF III43 study than in the AFASAK II42 study [13/521 (2.5%) vs 1/171 (0.6%), respectively], and much lower than the rate of major bleeding with combination warfarin and aspirin therapy in the SPORTIF III and V69 studies [25/481 (5.2%)]. However, rates were similar in those receiving warfarin alone in the SPAF III study,43 12 out of 523 patients (2.3%) to those on either adjusted-dose warfarin (INR 2.0–3.0) alone or those receiving fixed-dose warfarin (1.25 mg) alone [4/170 (2.4%) and 3/167 (1.8%), respectively] in the AFASAK II study,42 and adjusted-dose warfarin alone in SPORTIF III and V69 studies [100/3172 (3.2%)]. Of note is the fact that the SPAF III43 and AFASAK II42 studies included intracerebral haemorrhage events in their definitions of major bleeding; however, the SPORTIF studies69 include both ICH as well as fatal bleed in the total rate of major haemorrhage. This might also explain the differences in the event rates between these studies in addition to the methodological heterogeneity discussed in detail above (see Between-study differences).
The NASPEAF trial39 reported very similar major bleeding event rates in patients on combined adjusted-dose acenocoumarol (INR 1.4–2.4) plus triflusal (600 mg) and those on adjusted-dose acenocoumarol (INR 2.0–3.0) alone in high-risk patients during the median 2.6-year follow-up [12/223 (5.4%) vs 13/247 (5.3%), respectively, RR 1.02 95% CI 0.47 to 2.19]. The rate of major bleeding was lower, but not significantly so, among intermediate-risk patients receiving combined adjusted-dose acenocoumarol (INR 1.2–2.0) and triflusal (600 mg) than in those receiving adjusted-dose acenocoumarol (INR 2.0–3.0) alone during a median 2.9-year follow-up [5/222 (2.3%) vs 10/232 (4.3%), respectively, RR 0.52, 95% CI 0.18 to 1.50]. 39
Bover et al. 54 reported data comparing combined adjusted-dose acenocoumarol (INR 1.9–2.5) in combination with three antiplatelet regimes (triflusal 600 mg and 300 mg, aspirin 100 mg) to adjusted-dose acenocoumarol (INR 2.0–3.0) alone. Higher rates of bleeding were observed in patients on combined acenocoumarol plus aspirin [7/34 (20.6%)] and lower rates in those on combined acenocoumarol plus triflusal 600 mg [10/155 (6.5%)] or combined acenocoumarol plus triflusal 300 mg [6/120 (5.0%)] than in those on acenocoumarol alone [35/265 (12.1%)] during the mean 4.92 years of follow-up. 54 However, the population in this study was derived from a cohort of another RCT (see Between-study differences).
The FFAACS study41 reported higher, but not significantly different, rates of major bleeding with combined adjusted-dose fluindione (INR 2.0–2.6) plus aspirin (100 mg) than with adjusted-dose fluindione (INR 2.0–2.6) plus placebo in high-risk patients during the mean 0.84-year follow-up [3/76 (3.9%) vs 1/81 (1.2%), respectively, RR 3.19, 95% CI 0.34 to 30.07]. 41
There was no non-randomised evidence for this comparison identified for major bleeding.
Flaker et al. 69 reported data on a pooled analysis of the SPORTIF III and V studies comparing combined ximelagatran (36 mg twice daily) and aspirin (≤ 100 mg) with ximelagatran (36 mg twice daily) alone. Lower rates of major haemorrhage were reported in patients on combination therapy than in those on ximelagatran alone [2/531 (0.4%) vs 78/3120 (2.5%), respectively] during the 16.5-month follow-up.
The PETRO study73 reported no major bleeding events in patients on dabigatran 50 mg or 150 mg (in combination with aspirin or given alone). However, a higher proportion of patients on combined therapy of dabigatran 300 mg plus either aspirin 81 mg or 325 mg [1/34 (2.9%), 3/30 (10%) respectively] suffered a major bleeding event than those on dabigatran 300 mg alone [0/105 (0%)] during a mean follow-up period of 22 weeks.
There is conflicting evidence regarding the effect of combination ACT plus APT compared with ACT alone on the risk of major bleeding. Four randomised studies reported relatively low event rates and demonstrated no significant increase in the risk of major bleeding with combination therapy compared with ACT alone. 39,41–43 Three non-randomised studies reported inconsistent data, with two demonstrating higher rates of major bleeding with some combination therapy (VKAs plus aspirin)54,69 over ACT alone, and lower bleeding rates with other combined therapy (VKA plus triflusal54 or ximelagatran plus aspirin69), whereas the other study reported an increased risk of major bleeding only with the highest dose of ACT plus APT compared with ACT alone. 73
Intracranial haemorrhage
Three randomised comparisons39,42,43 and no non-randomised comparisons reported data on ICH. None of the studies included a definition of ICH.
The AFASAK II42 and SPAF III43 studies reported randomised comparisons for ICH comparing different regimes of combined warfarin plus aspirin to warfarin alone. The findings of these studies are reported in Table 21.
The AFASAK II42 study reported very low event rates with a non-significant difference in rates of intracranial bleeding between combined fixed-dose warfarin (1.25 mg daily) plus aspirin (300 mg daily) and adjusted-dose warfarin (INR 2.0–3.0) alone [0/171 (0%) vs 2/170 (1.2%), respectively, RR 0.19, 95% CI 0.01 to 4.11] or fixed-dose warfarin (1.25 mg daily) alone [0/171, (0%) vs 1/167 (0.6%), respectively, RR 0.33, 95% CI 0.13 to 7.94] during the median 3.5 years of follow-up. The risk profile of the patients enrolled in this study was not specified. 42
The SPAF III43 study reported very similar rates of ICH in patients on either adjusted-dose warfarin (INR 1.2–1.5) plus aspirin (325 mg daily) or adjusted-dose warfarin (INR 2.0–3.0) alone in high-risk patients with AF [5/521 (0.9%) vs 3/523 (0.6%), respectively, RR 1.67, 95% CI 0.4 to 6.96] during the mean 1.1-year follow-up period. 43
The rate of ICH was very low and similar in both of these RCTs. In the combined therapy arm, the rate of ICH was 0.9% (5/521) in the SPAF III43 study compared with 0% in the AFASAK II study. 42 Rates of ICH were similar in those receiving either fixed- or adjusted-dose warfarin in the AFASAK II42 study [2/170 (1.2%) and 1/167 (0.6%), respectively] and adjusted-dose warfarin in the SPAF III study [3/523 (0.6%)]. The difference in the rates may be explained by methodological heterogeneity between the included studies (see Between-study differences).
The NASPEAF trial39 reported low event rates with non-significant differences in rates of ICH between combined adjusted-dose acenocoumarol (INR 1.4–2.4) plus triflusal (600 mg), and adjusted-dose acenocoumarol (INR 2.0–3.0) alone [2/223 (0.9%) vs 5/247 (2.0%), respectively, RR 0.44, 95% CI 0.09 to 2.26] in high-risk patients during the median 2.6-year follow-up, or combined adjusted-dose acenocoumarol (INR 1.2–2.0) plus triflusal (600 mg) compared with adjusted-dose acenocoumarol (INR 2.0–3.0) alone [1/222 (0.5%) vs 4/232 (1.7%), respectively, RR 0.48, 95% CI 0.05 to 4.21] in intermediate-risk patients during a median 2.9-year follow-up. 39
Minor (or non-severe) bleeding
Five randomised comparisons39–43 and no non-randomised comparisons reported data on minor or non-severe bleeding. Definitions for minor bleeding were not clearly specified in these studies.
The AFASAK II42 and SPAF III43 studies reported randomised comparisons for minor bleeding comparing different regimes of combined warfarin plus aspirin with warfarin alone. The findings of these studies are reported in Table 21.
The AFASAK II42 study reported a non-significant difference in rates of minor bleeding when combined fixed-dose warfarin (1.25 mg daily) plus aspirin (300 mg daily) was compared with either adjusted-dose warfarin (INR 2.0–3.0) alone [28/171 (16.4%) vs 42/170 (24.7%), respectively, RR 0.66, 95% CI 0.43 to 1.02] or fixed-dose warfarin (1.25 mg daily) alone [28/171 (16.4%) vs 21/167 (12.6%), respectively, RR 1.30, 95% CI 0.77 to 2.19] during the median 3.5 years of follow-up. The risk profile of the patients enrolled in this study42 was not specified.
The SPAF III43 study also reported similar rates of minor haemorrhage in patients on either adjusted-dose warfarin (INR 1.2–1.5) plus aspirin (325 mg daily) or adjusted-dose warfarin (INR 2.0–3.0) alone in high-risk patients with AF [6/521 (1.2%) vs 4/523 (0.8%), respectively, RR 1.5 95% CI 0.43 to 5.30] during the mean 1.1-year follow-up period. 43
The rates of minor bleeding were much higher in the AFASAK II42 study than in the SPAF III43 study for both the combination therapy [28/171 (16.4%) vs 6/521 (1.2%), respectively] and warfarin-alone arms [adjusted-dose warfarin alone 42/170 (24.7%) vs 4/523 (0.8%), respectively] and 21/167 (12.6%) for fixed-dose warfarin alone in the AFASAK II42 study arms.
There was no non-randomised evidence reported for this comparison/outcome combination.
Lidell et al. 40 reported a non-significant difference in rates of minor bleeding between patients on either combined adjusted-dose warfarin (INR 2.0–3.0) plus clopidogrel (75 mg), or adjusted-dose warfarin (2.0–3.0) plus placebo [0/20 (0%) vs 5/23 (21.8%), respectively, RR 0.10, 95% CI 0.01 to 1.17] during the mean follow-up of 22 days.
There was no non-randomised evidence for this comparison identified for minor bleeding.
The NASPEAF study39 reported non-significant differences in rates of non-severe haemorrhage between combined adjusted-dose acenocoumarol (INR 1.4–2.4) plus triflusal (600 mg) and adjusted-dose acenocoumarol (INR 2.0–3.0) alone in high-risk patients during the median 2.6-year follow-up [20/223 (8.9%) vs 18/247 (7.3%), respectively, RR 1.23, 95% CI 0.67 to 2.27] or combined adjusted-dose acenocoumarol (INR 1.2–2.0) plus triflusal (600 mg) compared with adjusted-dose acenocoumarol (INR 2.0–3.0) alone [16/222 (7.2%) vs 15/232 (6.5%), respectively, RR 1.11, 95% CI 0.56 to 2.20] in intermediate-risk patients during a median follow-up of 2.9 years.
There was no non-randomised evidence for this comparison identified for minor bleeding.
The FFAACS trial41 reported a significant difference in rates of non-severe bleeding, with more events in patients on combined adjusted-dose fluindione (INR 2.0–2.6) plus aspirin (100 mg), than in those on adjusted-dose fluindione (INR 2.0–2.6) plus placebo in high-risk patients during the mean 0.84-year follow-up [10/76 (13.2%) vs 1/81 (1.2%), respectively, RR 10.66, 95% CI 1.39 to 81.28].
There was no non-randomised evidence for this comparison identified for minor bleeding.
The differences in bleeding outcomes reported in the included studies may reflect the methodological differences between these studies, which are discussed in detail above (Between-study differences). Various definitions of major bleeding were used across included studies (although these were considered broadly comparable for the purposes of this review), and subclassifications of bleeding varied between studies and were not always clearly defined. In addition, the likelihood of bleeding is reduced when the INR is < 3.0 and, therefore, studies using INR targets < 3.039,42,43,54 in either the intervention and/or comparator arms may have resulted in few bleeding events. Furthermore, only four studies39,40,43,54 (three randomised39,40,43 and one non-randomised54) reported TTR for ACT plus APT and ACT alone. TTR is associated with the incidence of bleeding events; when TTR is better (≥ 70%) the likelihood of adverse bleeding events is significantly reduced. 94 Therefore, differences in the TTR may help to explain differences in the bleeding event rates reported. Moreover, in the combined therapy group in the non-randomised studies, those patients with a high risk of bleeding may not have received additional APT and, therefore, potential confounding by indication may also account for differences in the bleeding rates reported.
Four randomised studies39,40,42,43 demonstrated no significant increased risk in minor or non-severe bleeding with combination therapy compared with anticoagulation alone, whereas another small randomised study41 reported a significant increase in the risk of minor/non-severe bleeding with combined therapy.
Outcome 10: patient quality of life
Of the included studies, no study was identified that reported quality-of-life outcome for the comparisons of interest.
Outcome 11: major adverse events (all-cause mortality, non-fatal myocardial infarction and stroke) and other composite outcomes
No study was identified that reported major adverse events comprising all-cause mortality, non-fatal MI and stroke. Six articles39,41,43 –45,54 reported other composite events, which included combined end points consisting of two or more previously reported outcomes. Three studies (in five articles39,41,43 –45) reported randomised comparisons, and one study54 reported non-randomised comparisons for various composite end points. The findings of these studies are reported in Table 23 and 24, respectively.
| Author, year | Stroke risk, follow-up | ACT + APT, n | No. of events/participants in ACT + APT arm (%) | ACT (alone or ACT + placebo), n | No. of events/total participants in ACT arm (%) | RR (95% CI) |
|---|---|---|---|---|---|---|
| aPérez-Gómez et al., 2004, RCT – NASPEAF39 | High risk,b 2.95 years | Adjusted-dose acenocoumarol (INR 1.4–2.4) + triflusal (600 mg), n = 223 | Severe bleeding, non-fatal stroke, TIA, SE and vascular death: 22/223 (9.9) | Adjusted-dose acenocoumarol (INR 2.0–3.0), n = 247 | Severe bleeding, non-fatal stroke, TIA, SE and vascular death: 34/247 (13.8) | 0.72 (0.43 to 1.19) |
| Embolism, stroke, AMI and vascular death: 13/223 (5.8) | Embolism, stroke, AMI and vascular death: 25/247 (10.1) | 0.58 (0.30 to 1.10) | ||||
| Non-fatal stroke, TIA, SE, and vascular death: 14/223 (6.3) | Non-fatal stroke, TIA, SE, and vascular death: 29/247 (11.7) | 0.53 (0.29 to 0.99) | ||||
| Intermediate risk,c2.6 years | Adjusted-dose acenocoumarol (INR 1.25–2.0) + triflusal (600 mg), n = 222 | Severe bleeding, non-fatal stroke, TIA, SE and vascular death: 8/222 (3.6) | Adjusted-dose acenocoumarol (INR 2.0–3.0), n = 232 | Severe bleeding, non-fatal stroke, TIA, SE and vascular death: 21/232 (9.1) | 0.40 (0.18 to 0.88) | |
| Embolism, stroke, AMI and vascular death: 4/222 (1.8) | Embolism, stroke, AMI and vascular death: 8/232 (3.4) | 0.52 (0.16 to 1.71) | ||||
| Non-fatal stroke, TIA, SE, and vascular death: 5/222 (2.3) | Non-fatal stroke, TIA, SE, and vascular death: 15/232 (16.5) | 0.35 (0.13 to 0.94) | ||||
| Lechat et al., 2001, RCT – FFAACS41 | High risk,d 0.82 years | Adjusted-dose fluindione (INR 2.0–2.6) + aspirin (100 mg), n = 76 | SE, death: 5/76 (6.6) | Adjusted-dose fluindione (INR 2.0–2.6) + placebo, n = 81 | SE and death: 2/81 (2.5) | 2.66 (0.53 to 13.33) |
| SPAF investigators, 1996 RCT – SPAF III43 | High risk,e 1.1 years | Adjusted-dose warfarin (INR 1.2–1.5) + aspirin (325 mg), n = 521 | Stroke, SE, vascular death: 66/521 (12.7) | Adjusted-dose warfarin (INR 2.0–3.0), n = 523 | Stroke, SE and vascular death: 37/523 (7.1) | 1.79 (1.22 to 2.63) |
| Author, year | Stroke risk, follow-up | ACT + APT, n | No. of events/total participants in ACT + APT group (%) | ACT (alone), n | No. of events/total participants in ACT group (%) |
|---|---|---|---|---|---|
| Bover et al., 200954 | Stroke risk NR, 4.92 years | Adjusted-dose acenocoumarol (INR 1.9–2.5) + triflusal (600 mg), n = 155 | Ischaemic events (all): 4/155 (2.6) | Adjusted-dose acenocoumarol (INR 2.0–3.0), n = 265 | |
| Stroke, a systemic/coronary ischaemic events, AMI and mortality: 9/155 (5.8) | |||||
| Adjusted-dose acenocoumarol (INR 1.9–2.5) + triflusal (300 mg), n = 120 | Ischaemic events (all): 11/120 (9.2) | Ischaemic events – all: 22/265 (8.3) | |||
| Stroke,a systemic/coronary ischaemic events, AMI and mortality: 12/120 (10) | Stroke,a systemic/coronary ischaemic events, AMI and mortality: 37/265 (13.9) | ||||
| Adjusted-dose acenocoumarol (INR 1.9–2.5) + aspirin (100 mg), n = 34 | Ischaemic events (all): 0/34 (0) | ||||
| Stroke,a systemic/coronary ischaemic events, AMI and mortality: 3/34 (8.8) |
Severe bleeding, non-fatal stroke, transient ischaemic attack, systemic embolism and vascular death
The NASPEAF study reported a randomised comparison on the composite outcome of severe bleeding, non-fatal stroke, TIA, SE and vascular death comparing adjusted-dose acenocoumarol (INR 1.4–2.4) plus triflusal (600 mg) with acenocoumarol alone (INR 2.0–3.0) in high-risk patients, and the combination of adjusted-dose acenocoumarol (INR 1.2–2.0) plus triflusal (600 mg) with acenocoumarol alone (INR 2.0–3.0) in intermediate-risk patients. A lower but statistically non-significant rate of the composite end point occurred in the combined therapy group than in those receiving anticoagulant alone in the high-risk patients [22/223 (9.9%) vs 34/247 (13.8%), respectively, RR 0.72 (95% CI 0.43 to 1.19)] during a median follow-up of 2.95 years. A similar trend was observed in the intermediate-risk group, for which the combination therapy arm demonstrated a lower composite event rate than the acenocoumarol-alone arm [8/222 (3.6%) vs 21/232 (9.1%) respectively, RR 0.40 (95% CI 0.18 to 0.88)] after a median follow-up of 2.6 years (see Table 23).
No other study was identified that evaluated this composite outcome.
Embolism, stroke, acute myocardial infarction and vascular death
The NASPEAF study39 reported a randomised comparison on the composite outcome of embolism, stroke, AMI and vascular death.
A lower but statistically non-significant rate of the composite end point was observed in patients receiving combined therapy than in those on anticoagulant alone, in both the high-risk patients [13/223 (5.8%) vs 25/247 (10.1%), respectively, RR 0.58 (95% CI 0.30 to 1.10)] during a median follow-up of 2.95 years, as well as the intermediate-risk patients [4/222 (1.8%) vs 8/232 (3.4%), respectively, RR 0.52 (95% CI 0.16 to 1.71)] after a median follow-up of 2.6 years (see Table 23). No other study reporting a composite end point of embolism, stroke, AMI and vascular death was identified.
Non-fatal stroke, transient ischaemic attack, systemic embolism and vascular death
The NASPEAF study39 reported a lower rate of non-fatal stroke, TIA, SE and vascular death as a composite end point in patients receiving combined therapy than in those on anticoagulant alone, in both the high-risk patients [14/223 (6.3%) vs 29/247 (11.7%), respectively, RR 0.53 (95% CI 0.29, 0.99)] during a median follow-up of 2.95 years, as well as the intermediate-risk patients [5/222 (2.3%) vs 15/232 (16.5%), respectively, RR 0.35 (95% CI 0.13 to 0.94)] after a median follow-up of 2.6 years (see Table 23).
No other study reporting a composite end point of embolism, stroke, AMI and vascular death was identified.
Systemic embolism and death
The FFAACS41 study reported randomised data comparing adjusted-dose fluindione (INR 2.0–2.6) plus aspirin 100 mg to adjusted-dose fluindione (INR 2.0–2.6) alone. Although not significantly different, composite events of SE and death were reported among patients receiving combination therapy compared with patients receiving fluindione alone [5/76 (6.6%) vs 2/81 (2.5%), respectively, RR 2.66 (95% CI 0.53 to 13.33)] during a mean 0.84-year follow-up (see Table 23).
No other study reporting the composite end point of SE and death was identified.
Stroke, systemic embolism and vascular death
The SPAF III study43 reported a randomised comparison for rates of the composite outcome of stroke, SE and vascular death comparing adjusted-dose warfarin (INR 1.2–1.5) plus aspirin (325 mg) with adjusted-dose warfarin (target INR 2.0–3.0) alone in high-risk patients with AF. A significantly higher incidence of the composite end point was observed in the combined therapy arm than in those receiving warfarin alone [66/521 (12.7%) vs 37/523 (7.1%), respectively, RR 1.79 (95% CI 1.22 to 2.63)] over a mean follow-up period of 1.1 years. 43
No other studies reporting data on this composite end point were identified.
Ischaemic events (all)
Bover et al. 54 reported non-randomised data on the composite outcome of all ischaemic events comparing adjusted-dose acenocoumarol (INR 1.9–2.5) plus three different regimes of APT (triflusal 600 mg or 300 mg, aspirin 100 mg) with adjusted-dose acenocoumarol alone (INR 2.0–3.0) over a mean follow-up period of 4.92 years (see Table 24).
A combination of adjusted-dose acenocoumarol (target INR 1.9–2.5) with triflusal 600 mg or aspirin 100 mg demonstrated fewer ischaemic events [4/155 (2.6%) and 0/34 (0%), respectively] than acenocoumarol alone [22/265 (8.3%)]. However, patients receiving acenocoumarol plus triflusal 300 mg demonstrated more ischaemic events than those on acenocoumarol alone [11/120 (9.2%) vs 22/265 (8.3%), respectively] (see Table 23).
There were no randomised comparisons identified that reported a composite end point of all ischaemic events.
Stroke, systemic/coronary ischaemic events, acute myocardial infarction and mortality
Bover et al. 54 reported lower rates of the composite end point of stroke, systemic/coronary ischaemic events, AMI and mortality in patients on combined therapy of acenocoumarol with either triflusal 600 mg, triflusal 300 mg or aspirin 100 mg [9/155 (5.8%), 12/120 (10%) and 3/34 (8.8%), respectively] than those on acenocoumarol alone [37/265 (13.9%)] over a mean follow-up period of 4.92 years. Of note is the fact that this study consisted of the majority of patients enrolled from another RCT (see Between-study differences).
There were no randomised comparisons identified that reported the composite end point of stroke, systemic/coronary ischaemic events, AMI and mortality.
The differences in major adverse event outcomes reported in the included studies may reflect the methodological differences between these studies discussed in detail above (Between-study differences). Different combinations of major adverse events were examined in composite events in each of the included studies and, therefore, it is not possible to compare across studies.
Although lower major adverse event rates were observed in three studies39,41,54 (two randomised39,41 and one non-randomised54) with combination therapy for the composite end points of severe bleeding, non-fatal stroke, TIA, SE and vascular death,39 non-fatal stroke, TIA, SE and vascular death,39 embolism, stroke, AMI and vascular death,39 SE and death,41 and stroke, systemic/coronary ischaemic events, AMI and mortality,54 and all ischaemic events54 than anticoagulation alone, the reduction was not significantly different between the ACT and APT vs ACT alone in the two randomised studies. 39,41 Combination therapy conferred a significantly increased risk of the composite end point of stroke, SE and vascular death compared with ACT alone in one randomised study. 43
Outcome 12: revascularisation procedures
No studies were identified that reported the outcome of revascularisation procedures comparing combined anticoagulant plus APT with ACT alone.
Outcome 13: percentage time in therapeutic international normalised ratio range
Four studies39,40,42,54 reported in four articles provided outcome data on percentage time in therapeutic INR range (TTR) for ACT in both the intervention (combined anticoagulation plus APT) and comparator (ACT-alone) arms. Of these, three studies39,40,42 reported randomised comparisons and one study54 reported non-randomised comparisons. The characteristics of these studies have been reported previously in Tables 4 and 6, respectively, and the findings of these studies are reported in Tables 25 and 26, respectively.
| Author, year | Stroke risk, follow-up, no. of centresa | ACT + APT, n | TTR [% (SD)] in ACT + APT arm | ACT (alone or ACT + placebo), n | TTR [% (SD)] in ACT-alone arm |
|---|---|---|---|---|---|
| Pérez-Gómez et al., 2004, RCT – NASPEAF39 | High risk,b 2.95 years, NR | Adjusted-dose acenocoumarol (INR 1.4–2.4) + triflusal (600 mg), n = 223 | 73 (22) | Adjusted-dose acenocoumarol (INR 2.0–3.0), n = 247 | 67 (22) |
| Intermediate risk,c 2.6 years, NR | Adjusted-dose acenocoumarol (INR 1.25–2.0) + triflusal (600 mg), n = 222 | 66 (25) | Adjusted-dose acenocoumarol (INR 2.0–3.0), n = 232 | 65 (22) | |
| Lidell et al.40 | Stroke risk NR, 22 days, 1 | Adjusted-dose warfarin (INR 2.0–3.0) + clopidogrel (75 mg), n = 20 | 100 | Adjusted-dose warfarin (INR 2.0–3.0), n = 23 | 100 |
| SPAF investigators, 1996 RCT – SPAF43 | High risk,d 1.1 years, multiplee | Adjusted-dose warfarin (INR 1.2–1.5) + aspirin (325 mg), n = 521 | 54 | Adjusted-dose warfarin (INR 2.0–3.0), n = 170 | 61 |
| Author, year | Stroke risk, follow-up, no. of centresa | ACT + APT, n | TTR % in ACT + APT arm | ACT (alone), n | TTR % in ACT-alone arm |
|---|---|---|---|---|---|
| Bover et al., 200954 | Risk NR. 4.92 years, 2 | Adjusted-dose acenocoumarol (INR 1.9–2.5) + triflusal (600 mg), n = 155 | 54.2 | Adjusted-dose acenocoumarol (INR 2.0–3.0), n = 265 | 62 |
| Adjusted-dose acenocoumarol (INR 1.9–2.5) + triflusal (300 mg), n = 121 | 59.1 | ||||
| Adjusted-dose acenocoumarol (INR 1.9–2.5) + aspirin (100 mg), n = 34 | 53 |
Lidell et al. 40 and the SPAF III study43 reported TTR for warfarin plus clopidogrel40 or warfarin plus aspirin43 and warfarin alone. 40,43 In the study by Lidell et al. ,40 TTR was reported to be 100% in both therapy arms,40 whereas the SPAF III43 study reported TTR to be 54% in the combined therapy arm and 61% in the warfarin-alone arm. 43 It should be noted that the SPAF III study43 consisted of a longer follow-up period of a mean of 1.1 years, whereas Lidell et al. 40 followed up only 43 patients over a mean follow-up period of 22 days. Furthermore, the SPAF III43 study used multiple centres utilising testing reagents with multiple sensitivities, whereas Lidell et al. 40 report a central assessment laboratory for all samples.
The NASPEAF study39 reported TTR for acenocoumarol plus triflusal and acenocoumarol alone in high- and intermediate-risk groups. A TTR of 73% was reported in patients receiving combination therapy and 67% in those receiving acenocoumarol alone in the high-risk category. TTR was similar in both therapy arms in the intermediate-risk group (66% in combination therapy arm and 65% in acenocoumarol alone).
The non-randomised comparison by Bover et al. 54 reported a lower TTR in the patients receiving combination acenocoumarol plus triflusal 600 mg (54.2%) and those receiving combination acenocoumarol plus aspirin 100 mg (53%) than in those receiving adjusted-dose acenocoumarol alone (62%). TTR was similar in patients receiving combination acenocoumarol plus triflusal 300 mg to those receiving acenocoumarol alone (59.1% vs 62%, respectively).
The TTR varied markedly between the studies. The study by Lidell et al. 40 achieved 100% TTR in both treatment groups, probably as a result of the small sample size and the relatively short follow-up period. In the combined therapy arms of the other two randomised comparisons, TTR was higher in NASPEAF39 in both the high- and intermediate-risk groups than in the SPAF III43 study (73% and 66% vs 54%, respectively). TTR was lower in all three combined therapy arms of the non-randomised comparison54 than in the combined therapy arms in two of the RCTs,39,40 which may be a reflection of the tighter INR control undertaken in RCTs than in non-RCTs settings but similar to TTR in the SPAF III study. 43 TTR was similar in the anticoagulation-alone arms of NASPEAF39 (67% and 65% in high- and intermediate-risk patients, respectively), the SPAF III43 study (61%) and Bover et al. 54 (62%).
Of the four studies39,40,43,54 that reported percentage TTR, TTR was higher in those receiving combination ACT plus APT in one randomised study,39 the same (100% TTR) in another randomised study40, and lower in two other studies43,54 (one randomised43 and one non-randomised54) than in those receiving ACT alone. INR control, evidenced by TTR, may have impacted on the event rates for each of the outcomes reported.
Summary of results according to interventions and comparator
Vitamin K antagonist plus antiplatelet therapy compared with vitamin K antagonist alone
Warfarin, acenocoumarol and fluindione were the VKAs investigated in the included studies. A summary of their findings according to the intervention and comparator are detailed as follows, and Forrest plots (without summary estimates) are available in Appendix 8.
Warfarin plus aspirin compared with warfarin alone
This comparison was investigated in five articles. 42,43,63,68,69 Of these, two studies reported randomised comparisons42,43 and the remaining three were non-randomised comparisons. 63,68,69Table 27 presents the outcomes of these studies. Warfarin and aspirin dosage differed across the studies, along with significant population heterogeneity.
| Name of study | Population, design | Intervention: ACT + APT | Comparator: ACT only | Outcomes: event % in ACT + APT vs event % in ACT alone, RR (95% CI) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study design, stroke risk, follow-up | Dose of ACT, dose of APT (n) | Dose (n) | Stroke | TIA | SE | Stroke + SE | AMI | Vascular mortality | All-cause mortality | Bleeding (any) | TTR | Composite events | |
| Warfarin + aspirin vs warfarin alone | |||||||||||||
| Gullov et al., 1998 (RCT – AFASAK II42) | Rand, risk NR, 3.5 years | Fixed dose 1.25 mg, 300 mg (171) | Adjusted dose INR 2.0–3.0, (170) | All: 6.4 vs 5.9, 1.09 (0.48 to 2.51) | 1.2 vs 0.6, 1.99 (0.18 to 21.72) | All: 0.6 vs 1.2, 0.50 (0.05 to 5.43) | 7.0 vs 7.1, 0.99 (0.46 to 2.15) | 0 vs 2.4, 0.11 (0.01 to 2.04) | 1.8 vs 2.9, 0.60 (0.14 to 2.46) | Total: 5.3 vs 3.6, 1.46 (0.53 to 4.03) | ICH: 0 vs 1.2, 0.19 (0.01 to 4.11) | N/A | NR |
| Non-infarct: 1.8 vs 1.8, 0.99 (0.20 to 4.86) | Fatal: 0.6 vs 0, 2.98 (0.12 to 72.70) | Non-vascular: 0.6 vs 0, 2.93 (0.12 to 71.42) | Major: 0.6 vs 2.4, 0.25 (0.03 to 2.20) | ||||||||||
| Minor: 2.3 vs 0, 8.95 (0.49 to 164.92) | Unknown cause: 1.2 vs 1.2, 0.98 (0.14 to 6.85) | Minor: 16.4 vs 24.7, 0.66 (0.43 to 1.02) | |||||||||||
| Disabling: 2.3 vs 1.8, 1.33 (0.30 to 5.83) | |||||||||||||
| Fatal: 0 vs 0 to not estimable | |||||||||||||
| Haemorrhagic: 0 vs 0.6, 0.33 (0.01 to 8.08) | |||||||||||||
| Ischaemic: 4.7 vs 1.8, 2.65 (0.72 to 9.82) | |||||||||||||
| Non-disabling: 1.8 vs 2.4, 0.75 (0.17 to 3.28) | |||||||||||||
| – | Fixed dose 1.25 mg, (167) | All: 6.4 vs 7.8, 0.83 (0.38 to 1.79) | 1.2 vs 2.4, 0.49 (0.09 to 2.63) | All: 0.6 vs 0.6, 0.98 (0.06 to 15.49) | 7.0 vs 8.4, 0.84 (0.40 to 1.76) | 0 vs 3.6, 0.08 (0.00 to 1.32) | 1.8 vs 1.2, 1.46 (0.25 to 8.66) | Total: 5.3 vs 10.0, 0.53 (0.24 to 1.15) | ICH: 0 vs 0.6, 0.33 (0.13 to 7.94) | N/A | NR | ||
| Non-infarct: 1.8 vs 3.6, 0.49 (0.12 to 1.92) | Fatal: 0.6 vs 0.6, 0.98 (0.06 to 15.49) | Non-vascular: 0.6 vs 1.2, 0.50 (0.05 to 5.43) | Major: 0.6 vs.1.8, 0.33 (0.03 to 3.09) | ||||||||||
| Minor: 2.3 vs 1.8, 1.30 (0.30 to 5.73) | Unknown cause: 1.2 vs 1.8, 0.66 (0.11 to 3.92) | Minor: 16.4 vs.12.6, 1.30 (0.77 to 2.19) | |||||||||||
| Fatal: 0 vs 1.2 to 0.20 (0.01 to 4.04) | |||||||||||||
| Haemorrhagic: 0 vs 0, not estimable | |||||||||||||
| Ischaemic: 4.7 vs 2.9, 1.56 (0.52 to 4.68) | |||||||||||||
| Non-disabling: 1.8 vs 2.4, 0.73 (0.17 to 3.22) | |||||||||||||
| SPAF investigators, 1996 (RCT – SPAF III)43 | Randomised, high risk,a NR, 3.5 years | Adjusted dose INR 1.2–1.5, 325 mg (521) | Adjusted dose INR 2.0–3.0 (523) | Disabling: 5.9 vs 1.9, 2.83 (1.44 to 5.57) | 4.4 vs 2.9, 1.54 (0.81 to 2.92) | 0.2 vs 0, 3.01 (0.12 to 73.75) | 8.4 vs 2.1, 4.02 (2.10 to 7.69) | 1.9 vs 1.0, 2.01 (0.69 to 5.83) | 5.2 vs 5.2, 1.00 (0.60 to 1.69) | Total: 8.1 vs 6.7, 1.20 (0.78 to 1.86) | ICH: 0.9 vs 0.6, 1.67 (0.40 to 6.96) | 54 vs 61 | Stroke, SE, vascular death: 12.7 vs 7.1, 1.79 (1.22 to 2.63) |
| Ischaemic: 8.3 vs 2.1, 3.92 (2.05 to 7.52) | Non-vascular: 2.3 vs 1.5, 1.51 (0.62 to 3.65) | Major: 2.5 vs 2.3, 1.08 (0.50 to 2.36) | |||||||||||
| Ischaemic (fatal): 0.9 vs 0.2, 5.02 (0.59 to 42.81) | Indeterminant: 0.6 vs 0, 7.00 (0.36 to 135.18) | Minor: 1.2 vs 0.8, 1.5 (0.43 to 5.30) | |||||||||||
| Hansen et al., 201063 | Non-randomised, risk NR, 3.3 years | NR, NR (18,345) | NR (50,919) | NR | NR | NR | NR | NR | NR | NR | All: 6.6 vs 7.2 | NR | |
| Flaker et al., 200669 | Non-randomised, high risk,b 16.5 months | Adjusted dose INR 2.0–3.0, ≤ 100 mg (481) | Adjusted dose INR 2.0–3.0 (3172) | All: 2.3 vs 2.1 | NR | NR | 2.3 vs 2.2 | 0.8 vs 1.5 | NR | 3.5 vs 3.5 | Major: 5.2 vs 3.2 | NR | |
| Akins et al., 200767 | Non-randomised, high riskb, 16.5 months | Adjusted dose INR 2.0–3.0, ≤ 100 mg (156) | Adjusted dose INR 2.0–3.0 (567) | NR | NR | NR | Major/minor: 52.2 vs 37.8 | ||||||
| 6.9 vs 4.1 | NR | NR | NR | NR | NR | ||||||||
| Warfarin + clopidogrel vs warfarin alone | |||||||||||||
| Lidell et al., 2003 40 | Randomised, risk NR, 22 days | Adjusted dose INR 2.0–3.0, 75 mg (20) | Adjusted dose INR 2.0–3.0 (23) | NR | NR | NR | NR | NR | NR | Minor: 0 vs 21.8, 0.10 (0.01 to 1.77) | 100 vs 100 | ||
| Hansen et al., 2010 63 | Non-randomised, risk NR, 3.3 years | NR, NR (1430) | NR (50,919) | NR | NR | NR | NR | NR | NR | All: 4.8 vs 7.2 | NR | ||
| Warfarin + aspirin + clopidogrel vs warfarin alone | |||||||||||||
| Hansen et al., 201063 | Non-randomised, risk NR, 3.3 years | NR, NR (1261) | NR (50,919) | NR | NR | NR | NR | NR | All: 5.1 vs 7.2 | NR | |||
| Acenocoumarol + aspirin vs acenocoumarol alone | |||||||||||||
| Bover et al., 200954 | Non-randomised, risk NR, 4.92 years | Adjusted dose INR 1.9–2.5, 100 mg (34) | Adjusted dose INR 2.0–3.0 (265) | All: 2.9 vs 5.7 | NR | 0 vs 2.6 | NR | 0 vs 1.9 | NR | Non-cardiac: 2.9 vs 1.1 | Severe: 20.6 vs 12.1 | 53 vs 62 | Ischaemic events (all): 0 vs 8.3 |
| Haemorrhagic: 2.9 vs 1.9 | Sudden: 2.9 vs 1.1 | Fatal: 5.9 vs 2.6 | Stroke, systemic/coronary ischaemic events, AMI and mortality: 8.8 vs 13.9 | ||||||||||
| Lethal: 2.9 vs 1.5 | GI: 0 vs 2.3 | ||||||||||||
| Non-GI: 20.6 vs 9.8 | |||||||||||||
| Acenocoumarol + triflusal vs acenocoumarol alone | |||||||||||||
| Pérez-Gómez et al., 2004, (RCT – NASPEAF39) | Randomised, high risk,c 2.95 years | Adjusted dose INR 1.4–2.4, 600 mg (223) | Adjusted dose INR 2.0–3.0 (247) | Non-fatal: 2.7 vs 2.4, 1.11 (0.36 to 3.38) | 0.9 vs 1.2, 0.74 (0.12 to 4.38) | Non-fatal: 0 vs 1.2, 0.16 (0.01 to 3.05) | Stroked/any embolism: 5.4 vs 8.1, 0.66 (0.33 to 1.33) | 0 vs 0, not estimable | 2.7 vs 6.9, 0.39 (0.16 to 0.97) | Total: 5.4 vs 9.3, 0.58 (0.29 to 1.13) | ICH: 0.9 vs 2.0, 0.44 (0.09 to 2.26) | 73 vs 67 | Severe bleeding, non-fatal stroke, TIA, SE and vascular death: 9.9 vs 13.8, 0.72 (0.43 to 1.19) |
| Stroked/fatal embolism: 1.8 vs 3.2, 0.55 (0.17 to 1.81) | Non-vascular: 2.7 vs 2.4, 1.11 (0.36 to 3.38) | Severe: 5.4 vs 5.3, 1.02 (0.47 to 2.19) | Embolism, stroke, AMI and vascular death: 5.8 vs.10.1, 0.58 (0.30 to 1.10) | ||||||||||
| Severe – other: 0.9 vs 2.0, 0.44 (0.09 to 2.26) | Non-fatal stroke, TIA, SE, and vascular death: 6.3 vs 11.7, 0.53 (0.29 to 0.99) | ||||||||||||
| Non-severe: 8.9 vs 7.3, 1.23 (0.67 to 2.27) | |||||||||||||
| GI: 3.6 vs 1.2, 2.95 (0.79 to 10.99) | |||||||||||||
| Randomised, intermediate risk,e 2.6 years | Adjusted dose INR 1.25–2.0, 600 mg (222) | Adjusted dose INR 2.0–3.0 (232) | Non-fatal: 1.4 vs 1.3, 1.05 (0.21 to 5.12) | 0 vs 0, not estimable | Non-fatal: 0 vs 0.4, 0.35 (0.01 to 8.50) | Stroked/any embolism: 1.4 vs 3.0, 0.45 (0.12 to 1.71) | 0 vs 0, not estimable | 0.9 vs 4.7, 0.19 (0.04 to 0.85) | Total: 2.7 vs 8.6, 0.31 (0.13 to 0.77) | ICH: 0.5 vs 1.7, 0.48 (0.05 to 4.21) Severe:d 2.3 vs 4.3, 0.52 (0.18 to 1.50) | 66 vs 65 | Severe bleeding, non-fatal stroke, TIA, SE and vascular death: 3.6 vs 9.1 to 0.40 (0.18 to 0.88) | |
| Stroked/fatal embolism: 0 vs 1.3, 0.15 (0.01 to 2.87) | Non-vascular: 1.8 vs 3.9, 0.46 (0.15 to 1.49) | Severe – other: 0.5 vs 2.2, 0.21 (0.02 to 1.77) | Embolism, stroke, AMI and vascular death: 1.8 vs 3.4, 0.52 (0.16 to 1.71) | ||||||||||
| Non-severe: 7.2 vs 6.5, 1.11 (0.56 to 2.20) | Non-fatal stroke, TIA, SE, and vascular death: 2.3 vs 16.5, 0.35 (0.13 to 0.94) | ||||||||||||
| GI: 1.4 vs 0.43, 3.13 (0.33 to 29.91) | |||||||||||||
| Bover et al., 200954 | Non-randomised, risk NR, 4.92 years | Adjusted dose INR 1.9–2.5, 600 mg (155) | Adjusted dose INR 2.0–3.0 (265) | All: 3.2 vs 5.7 | NR | 0 vs 2.6 | NR | 0 vs 1.9 | NR | Non-cardiac: 3.9 vs 1.1 | Severe: 6.5 vs 12.1 | 54.2 vs 62 | Ischaemic events (all): 2.6 vs 8.3 |
| Haemorrhagic: 0.6 vs 1.9 | Sudden: 2.6 vs 1.1 | Fatal: 0 vs 2.6 | Stroke, systemic/coronary ischaemic events, AMI and mortality: 5.8 vs 13.9 | ||||||||||
| Lethal: 1.3 vs 1.5 | GI: 5.2 vs 2.3 | ||||||||||||
| Adjusted dose INR 1.9–2.5, 300 mg (120) | Adjusted dose INR 2.0–3.0 (265) | All: 6.7 vs 5.7 | NR | 1.7 vs 2.6 | Non-GI: 1.3 vs 9.8 | ||||||||
| Haemorrhagic: 0 vs 1.9 | NR | 0.8 vs 1.9 | NR | Non-cardiac: 2.5 vs 1.1 | Severe: 5.0 vs 12.1 | 59.1 vs 62 | Ischaemic events (all): 9.2 vs 8.3 | ||||||
| Lethal: 2.5 vs 1.5 | Sudden: 0 vs 1.1 | Fatal: 0.8 vs 2.6 | Stroke, systemic/coronary ischaemic events, AMI and mortality: 10.0 vs 13.9 | ||||||||||
| GI: 4.2 vs 2.3 | |||||||||||||
| Non-GI: 0.8 vs 9.8 | |||||||||||||
| Dabigatran + aspirin vs dabigatran alone | |||||||||||||
| Ezekowitz et al., 2007 (RCT – PETRO)73 | Non-randomised, ≥ stroke risk criteria,f 12 weeks | 300 mg,d 81 mg (34) | 300 mgd (105) | NR | NR | 0 vs 0 | NR | NR | NR | NR | Major: 2.9 vs 0 Clinical relevant + major: 14.7 vs 5.7 | N/A | |
| All: 32.4 vs 13.3 | |||||||||||||
| 300 mg,d 325 mg (30) | NR | NR | 0 vs 0 | NR | NR | NR | NR | Major: 10.0 vs 0 Clinical relevant + major: 20.0 vs 5.7 | N/A | ||||
| All: 46.7 vs 13.3 | |||||||||||||
| 150 mg,d 81 mg (36) | 150 mgd (100) | NR | NR | 0 vs 0 | NR | NR | NR | NR | Major: 0 vs 0 clinical relevant + major: 5.6 vs 9.0 | N/A | |||
| All: 22.2 vs 15.0 | |||||||||||||
| 150 mg,d 325 mg (33) | NR | NR | 0 vs 0 | NR | NR | NR | NR | Major: 0 vs 0 clinical relevant + major: 6.1 vs 9.0 | N/A | ||||
| All: 21.2 vs 15.0 | |||||||||||||
| 50 mg,d 81 mg (21) | 50 mgd (59) | NR | NR | 4.8 vs 1.7 | NR | NR | NR | NR | Major: 0 vs 0 clinical relevant + major: 4.8 vs 0 | N/A | |||
| All: 9.5 vs 3.4 | |||||||||||||
| 50 mg,d 325 mg (27) | NR | NR | 0 vs 1.7 | NR | NR | NR | NR | Major: 0 vs 0 clinical relevant + major: 3.7 vs 0 | N/A | ||||
| All: 11.1 vs 3.4 | |||||||||||||
| Fluindione + aspirin vs fluindione alone | |||||||||||||
| Lechat et al., 2001 (RCT – FFAACS41) | Randomised, high risk,g 0.82 years | Adjusted dose INR 2.0–2.6, 100 mg (76) | Adjusted dose INR 2.0–2.6 (81) | NR | NR | TE: 2.6 vs 1.2, 2.13 (0.20 to 23.03) | NR | NR | 3.9 vs 2.5, 1.60 (0.27 to 9.31) | 3.9 vs 3.7, 1.07 (0.22 to 5.12) | Severe: 3.9 vs 1.2, 3.19 (0.34 to 30.07) | NR | SE, death: 6.6 vs 2.5, 2.66 (0.53 to 13.33) |
| Non-severe: 13.2 vs 1.2, 10.66 (1.39 to 81.28) | |||||||||||||
| All: 17.1 vs 2.5, 6.93 (1.62 to 29.69) | |||||||||||||
| Ximelagatran + aspirin vs ximelagatran alone | |||||||||||||
| Flaker et al., 200669 | Non-randomised, high risk,b 16.5 months | 36 mg,d 100 mg (531) | 36 mgd (3120) | All: 2.1 vs 1.6 | NR | NR | 2.3 vs 1.9 | 1.9 vs 1.3 | NR | 0.6 vs 3.0 | Major: 0.4 vs 2.5 | N/A | |
| Akins et al., 200767 | Non-randomised, high risk,h 16.5 months | 36 mg,d 100 mg (157) | 36 mgd (629) | NR | NR | NR | Major/minor: 38.0 vs 32.5 | ||||||
| 7.0 vs 3.5 | NR | NR | NR | NR | N/A | ||||||||
In both RCTs, AFASAK II42 and SPAF III,43 event rates for all categories of stroke were low and similar in patients on combined warfarin [fixed dose42 or adjusted dose (INR 1.2–1.5)43] plus aspirin (30042 or 325 mg43) to those on warfarin [fixed dose42 or adjusted dose (INR 1.2–1.5)42,43] alone, except for ischaemic strokes, for which both studies rates were higher with combination therapy than ACT alone, but not significantly so.
Of the non-randomised comparisons, only Flaker et al. 69 reported outcome data for stroke comparing adjusted-dose warfarin (INR 2.0–3.0) plus ≤ 100 mg aspirin with adjusted-dose warfarin (INR 2.0–3.0) alone, indicating a similar rate of strokes across the two arms.
Differences in the rates of TIA and SE outcomes between the study arms was not significantly different in both the AFASAK II42 and SPAF III studies. 43 No non-randomised study was identified that reported TIA and SE outcome for warfarin plus aspirin compared with warfarin alone.
The rate of the combined end point of stroke and SE was similar across the study arms in the AFASAK II study,42 whereas the SPAF III43 study reported higher rates in patients on combined warfarin plus aspirin than in those with adjusted-dose warfarin (INR 2.0–3.0) alone. The non-randomised comparison by Flaker et al. 69 demonstrated similar rates across the study arms. A subgroup of patients from this cohort with a history of previous embolism was analysed by Akins et al. ,68 demonstrating a higher proportion of patients in the combined therapy arm suffering the end point of stroke or SE than in those on warfarin alone.
The SPAF III43 and AFASAK II42 RCTs did not demonstrate a significant difference in the event rates of AMI between combination therapy and warfarin alone. Flaker et al. ,69 in their non-randomised comparison also demonstrated similar events of AMI in patients on combined therapy compared with those on warfarin alone.
Similar rates of vascular mortality were observed across the study arms in the two RCTs. 42,43 No non-randomised comparisons were identified that reported vascular mortality comparing combined warfarin plus aspirin with warfarin alone.
The AFASAK II42 and SPAF III43 studies demonstrated no significant difference in the rates of all-cause mortality in the combined therapy arms compared with adjusted-dose warfarin (INR 2.0–3.0) alone. Flaker et al. 69 reported a similar proportion of all-cause mortality across arms in a non-randomised comparison.
Similar rates of haemorrhage (intracranial, major and minor) were reported in the combined therapy group compared with warfarin alone in both the AFASAK II42 and SPAF III43 studies. Of the non-randomised comparisons, Hansen et al. 63 reported a smaller proportion of patients suffering a haemorrhagic event in patients on combined therapy than in those on warfarin alone, in a large non-randomised cohort of patients with AF (n = 118,606), followed up over a period of 3.3 years. The study by Flaker et al. ,69 however, demonstrated a higher proportion of patients experiencing haemorrhage in the combined therapy group than in those on warfarin alone over a period of 16.5 months.
Significantly higher rates of the composite end point of stroke, SE and vascular death were reported in patients on combined warfarin plus aspirin than in those on warfarin alone in the SPAF III study. 43 No other study reported outcomes for this comparison.
The SPAF III43 RCT reported TTRs that were within the therapeutic range (in this case between INR 1.5–2.5) for patients on combined therapy for 54% of the time and those on warfarin alone were reported to be within therapeutic range (INR 2.0–3.0) for 61% of the time.
Of the studies that reported randomised comparisons, the AFASAK II study42 was prematurely terminated when results of the SPAF-III trial42 were published, demonstrating the superiority of adjusted-dose warfarin (INR 2.0–3.0) alone, over the combination of adjusted-dose warfarin (INR 1.2–2.5) and aspirin 325 mg, in preventing stroke or SE. 42 Both of these comparisons used different open-label warfarin regimes in the combination and comparator arm, and different doses of aspirin (300 mg AFASAK II42 and 325 mg SPAF III43), and had varying lengths of follow-up (mean 3.5 years in the AFASAK II42 study and mean 1.1 years in the SPAF III43 study). The SPAF III43 study did not consider diabetes mellitus a stroke risk factor, which could have introduced patients at lower risk of stroke into the study, whereas the AFASAK II42 study did not specify stroke risk. Of the non-randomised studies, aspirin was administered at the physician's discretion. 63,69 One study was conducted on hospitalised patients in whom the dosage of warfarin and aspirin was not reported. 63 These factors make it potentially difficult to infer a clear effect of combined therapy on vascular events in a high-risk AF population.
Warfarin plus clopidogrel compared with warfarin alone
This comparison was investigated in two studies, of which one was a randomised comparison40 (the other reported a non-randomised comparison63). Table 27 presents the outcomes of these studies. Of note is the dearth of studies conducted on a group of patients with AF at a specified high risk of stroke randomised to combined therapy of adjusted-dose warfarin (INR of 2.0–3.0) plus clopidogrel and adjusted-dose warfarin (INR 2.0–3.0) alone.
Data were available only for rates of haemorrhage in these two studies. Lidell et al. 40 reported very low event rates for minor haemorrhage in a randomised comparison of a small, predominantly male, sample size (n = 43), followed up over a very short period of time (22 days). Furthermore, Hansen et al. 63 reported a higher proportion of patients suffering from haemorrhage in the warfarin group than in the combined therapy group in a large sample size (n = 118,606) of hospitalised patients followed up over a period of 3.3 years. Clopidogrel was administered according to physician's discretion in this study. Furthermore, the dosage of both warfarin or clopidogrel was unknown in this study. 63 Therefore, from the available evidence, it is difficult to determine the effect of combined therapy on vascular events.
Warfarin plus aspirin plus clopidogrel (triple therapy) compared with warfarin alone
One non-randomised study63 investigated this comparison. 63
Data were available only for rates of haemorrhage for this comparison. Hansen et al. 63 reported a higher proportion of patients suffering from haemorrhage in the warfarin-only group than in the triple therapy group. Although the study was conducted on a large sample size (n = 118,606) over a mean of 3.3 years of follow-up, the dosage of warfarin, aspirin or clopidogrel was not reported. Furthermore, APT was administered at physician's discretion. The evidence is, therefore, insufficient to determine the benefit of combined therapy over warfarin alone for vascular events.
Fluindione plus aspirin compared with fluindione alone
This comparison was investigated in one randomised study41 comparing fluindione (INR 2.0–2.6) plus aspirin (100 mg) with fluindione (INR 2.0–2.6) plus placebo in high-risk patients with AF over a mean follow-up period of 0.84 years. Non-randomised evidence was not identified for this comparison.
The study41 reported very low event rates of SE, vascular death, all-cause mortality, and the composite end point of non-fatal SE and vascular death, with non-significant differences between combined therapy and fluindione plus placebo. However, a significantly higher rate of haemorrhage was observed in patients on combination therapy than in those on fluindione plus placebo. The study was conducted on a small sample size (n = 157) over a mean follow-up period of 0.84 years on a high-risk AF population, 85% of whom were anticoagulant experienced at entry. Of note is the low event rate and premature termination of the trial because of a low enrolment rate. All of these factors render it difficult to meaningfully evaluate the benefit of combination therapy over anticoagulant alone for this combination.
Acenocoumarol plus aspirin compared with acenocoumarol alone
This comparison was investigated in one non-randomised comparison by Bover et al. ,54 comparing adjusted-dose acenocoumarol targeting an INR range of 1.9–2.5 plus aspirin 100 mg with adjusted-dose acenocoumarol (INR 2.0–3.0) alone. The study54 also compared the combination of acenocoumarol plus different regimes of triflusal (300 and 600 mg) with acenocoumarol alone. These comparisons have been reported in previous sections. Many of the patients in the study had been participants in the NASPEAF RCT;39 however, it was difficult to identify which patients these were, what – if any –subsequent treatment they received and, thus, their influence on the findings of this non-randomised comparison. 54
The study54 reported a very small number of outcome events, with fewer events of strokes (total), SE and AMI in the combined therapy group than in the acenocoumarol-alone group. The study54 also reported the composite end points of ischaemic events, stroke, AMI and mortality with no significant differences in events in patients on combined therapy compared with those on acenocoumarol alone. However, patients on combination therapy demonstrated more non-cardiac and sudden deaths, along with a greater prevalence of severe, fatal, and non-GI bleeding than those on acenocoumarol alone.
Of note is, the considerably greater prevalence of stroke risk factors in the patients on acenocoumarol plus aspirin (embolism or age > 75 years, males, HF, diabetes mellitus, dyslipidaemia, coronary disease, smokers) than in those on acenocoumarol alone. 54 There were very few patients in the combined therapy group (n = 34) compared with those on acenocoumarol alone (n = 265). Therefore, it is difficult to conclude the benefit of combined therapy over acenocoumarol alone.
Acenocoumarol plus triflusal compared with acenocoumarol alone
This comparison was investigated in two studies: one reporting a randomised comparison39 and one a non-randomised comparison. 54 No study was identified with a clearly specified group of patients with AF, at a high-risk of stroke, randomised to combination therapy of adjusted-dose acenocoumarol targeting an INR of 2.0–3.0 plus triflusal and adjusted-dose acenocoumarol (INR 2.0–3.0) alone.
Acenocoumarol and triflusal dosage differed between the studies. The NASPEAF study39 compared adjusted-dose acenocoumarol in different regimes (INR 1.4–2.4 and INR 1.25–2.0) plus triflusal 600 mg, with adjusted-dose acenocoumarol (INR 2.0–3.0) alone in patients at a high risk and intermediate risk of stroke. Bover et al. 54 compared adjusted-dose acenocoumarol (INR 1.9–2.5) combined with different regimes of triflusal (600 mg, 300 mg) with adjusted-dose acenocoumarol (INR 2.0–3.0) alone, wherein most patients consisted of previously randomised patients in the NASPEAF RCT. 39 As mentioned previously it was difficult to identify the specific distribution of these patients. 54 It is also important to note that patients on combined therapy had more stroke risk factors than patients on acenocoumarol alone (combination with triflusal 600 mg; greater percentage of patients with previous embolism and dyslipidaemia; combination therapy with triflusal 300 mg consisted of more patients with previous embolism or age > 75 years, diabetes mellitus, dyslipidaemia).
The NASPEAF RCT39 reported no difference in rates of non-fatal stroke between combination ACT plus APT and ACT alone in either a high- or intermediate-risk population. 39 Bover et al. 54 reported a higher proportion of patients on acenocoumarol alone suffering a stroke than in those on combination therapy with acenocoumarol plus triflusal 600 mg, whereas a similar number of events were reported in patients on combined acenocoumarol and triflusal 300 mg than in those receiving acenocoumarol alone (see Table 27).
Similar rates of TIA were observed in both treatment arms in the high- and intermediate-risk population in the NASPEAF RCT. 39 No non-randomised evidence was identified reporting TIA for this comparison.
Very few events of non-fatal SE were observed in the NASPEAF study. 39 The non-randomised comparison study by Bover et al. 54 demonstrated fewer events of SE in patients on combined therapy (with either triflusal 600 mg or 300 mg) than in those on acenocoumarol alone.
Rates of the combined end point of stroke and SE were similar across the arms in both the high-risk group as well as the intermediate-risk group in the NASPEAF study. 39 Non-randomised comparisons were not identified for this end point.
No AMI events were reported in the NASPEAF study. 39 However, Bover et al. 54 demonstrated slightly fewer AMI events in patients on combined therapy (with either triflusal 600 mg or 300 mg) than in those on acenocoumarol alone. 54
The NASPEAF study39 demonstrated significantly lower rates of vascular mortality in patients on combined acenocoumarol plus triflusal 600 mg than in those on acenocoumarol alone in both the high- and intermediate-risk groups.
A non-significant lower rate of all-cause mortality was reported in the high-risk group in the NASPEAF study39 for the combination therapy. This difference was more pronounced in intermediate-risk patients and reached statistical significance. A lower rate of all-cause mortality was reported in patients on combined therapy than in those on acenocoumarol alone in the intermediate-risk group. 39 Furthermore, Bover et al. ,54 in their non-randomised comparison, reported a higher proportion of non-cardiac deaths in patients on combined therapy (acenocoumarol plus either triflusal 600 mg or triflusal 300 mg) than in those on acenocoumarol alone.
No significant differences in the rates of intracranial, severe, non-severe or gastrointestinal (GI) haemorrhage were reported in the randomised NASPEAF study39 comparing the combination of acenocoumarol plus triflusal with acenocoumarol alone in high- and intermediate-risk patients. 39 Bover et al. 54 reported a smaller proportion of patients suffering a severe, fatal or a non-GI haemorrhage in the combined therapy group(s) (acenocoumarol plus either triflusal 600 mg or triflusal 300 mg) than in the acenocoumarol-alone group. However, more patients in the combination therapy group(s) demonstrated GI bleeding than those on acenocoumarol alone.
The rate of the combined end points of non-fatal stroke, TIA, SE and vascular death was significantly lower in patients on combined acenocoumarol plus additional triflusal 600 mg than in those on acenocoumarol alone in both high- and intermediate-risk groups. 39 A similar trend was observed for the combined end point of severe bleeding, non-fatal stroke, TIA, SE and vascular death in the intermediate-risk group in the NASPEAF study. 39 Furthermore, Bover et al. 54 reported fewer events of composite end points (ischaemic events, and stroke, systemic events, AMI and mortality) in the combined therapy group(s) than in the acenocoumarol-alone group.
The NASPEAF study39 reported slightly better TTR of acenocoumarol in the combination therapy arm than in the acenocoumarol-alone arm in the high-risk group. However, in a non-randomised comparison, Bover et al. 54 reported slightly better TTR in patients on acenocoumarol alone than in those on combination therapy of acenocoumarol plus either triflusal 300 mg or 600 mg.
Overall, there seem to be fewer negative events in the combined therapy arms, with statistically significant differences in the mortality rates and composite end points, than in the acenocoumarol-alone arms in either the high- or the intermediate-risk groups in the randomised NASPEAF study. 39 However, there seems to be no statistically significant difference in rate of haemorrhage between the two arms in either risk group. 39 A similar trend was demonstrated in the non-randomised comparison by Bover et al. ,54 with fewer patients suffering stroke, SE, bleeding and composite end points in the combined therapy group than in the acenocoumarol-alone group.
Other anticoagulants
Dabigatran and ximelagatran do not belong to the VKA class of anticoagulant and, therefore, have been dealt with separately in the sections below.
Dabigatran plus aspirin compared with dabigatran alone
This comparison was investigated in the PETRO73 study comparing different regimes of dabigatran (50/150/300 mg twice daily) plus aspirin 81 mg or 325 mg doses with adjusted-dose dabigatran (50/150/300 mg twice daily) alone.
This PETRO study73 reported none or very small number of systemic embolic events in each therapy group. A higher proportion of patients on combined dabigatran (300/150/50 mg twice daily) plus aspirin (81 or 325 mg) experienced a haemorrhagic event than in those on dabigatran (300/150/50 mg twice daily) alone.
The PETRO study73 was conducted on a sample of 502 antithrombotic-experienced patients with AF (82% males) at a high risk of stroke over a follow-up period of 12 weeks. 73 However, after entry of about half of the patients, the requirement for patients to have a history of CAD was removed to facilitate inclusion, which could have allowed inclusion of lower-risk patients as well. The numerical distribution of patients in each group (dabigatran and dabigatran plus aspirin) was uneven, and it was not clear if aspirin was administered at random or conditionally. Therefore, the benefit or harm of combined therapy over anticoagulant alone is not clear for this comparison.
Ximelagatran plus aspirin compared with ximelagatran alone
This comparison was investigated in the pooled analyses of the SPORTIF trials (SPORTIF III64 and SPORTIF V65) by Flaker et al. ,69 and Akins et al. ,67 comparing ximelagatran (36 mg twice daily) plus aspirin (100 mg), with ximelagatran (36 mg twice daily) alone. The study by Flaker et al. 69 demonstrated that a higher proportion of patients on combined ximelagatran plus aspirin suffered a stroke, AMI, haemorrhage, or combined end point of stroke and SE than those on ximelagatran alone. Akins et al. 67 conducted an analysis on a high-risk subgroup (those with history of embolism) for the same cohort, and demonstrated a similar trend for the combined end point of stroke and SE (Table 27). Furthermore, Flaker et al. 69 demonstrated fewer major bleeding events and lower all-cause mortality in the combination arm than in those on ximelagatran alone. The SPORTIF trials64,65 were conducted on patients with AF with at least one risk factor for stroke over a mean follow-up of 16.5 months. Aspirin was indicated for patients with CAD, and there were significant baseline differences between patients administered combined therapy and those on anticoagulant alone. 69 There was no randomised evidence identified for this comparison. Therefore, the benefit of combination therapy over ximelagatran alone is difficult to evaluate from the available evidence.
Chapter 4 Discussion
Statement of principal findings
The purpose of this review was to assess the clinical effectiveness of adding APT to ACT compared with ACT alone in reducing vascular events in patients with AF at a high risk of TEs resulting from atrial fibrillation.
Clinical effectiveness
A total of five studies39 – 43 that reported randomised comparisons, and 1850 – 65,72,73 that reported non-randomised comparisons, were included in this assessment.
Overall, there were few stroke events reported with conflicting evidence regarding the benefit of ACT plus APT over ACT alone in the reduction of all stroke events, with two studies (one randomised42 and one non-randomised69) reporting no differences, whereas another non-randomised study54 reports equivocal data, demonstrating fewer strokes with two combination regimes of ACT plus APT over ACT alone. 54
Studies that differentiated between types of strokes did not report significant differences in the rates between patients on ACT plus APT and those on ACT alone. Two randomised studies42,43 and one non-randomised study54 found no significant reduction in the risk of fatal stroke with ACT plus APT over ACT alone. Furthermore, combination therapy did not decrease the risk of non-fatal stroke compared with anticoagulation alone in another randomised study. 39 Of the few events reported in one randomised42 and one non-randomised54 study, there was no evidence of an increased risk of haemorrhagic stroke with combination ACT plus APT over ACT alone. There is conflicting evidence regarding the benefit of combination therapy in the reduction of ischaemic stroke, with one randomised study42 demonstrating no significant difference, whereas another randomised study suggests a significantly increased risk of ischaemic stroke with combination therapy. 43 There is also conflicting evidence regarding the benefit of combination ACT plus APT compared with ACT alone in the reduction of disabling stroke, with one randomised study42 demonstrating no significant difference, whereas another randomised study43 suggests a significantly increased risk of disabling stroke with combination therapy. However, given the methodological heterogeneity and study quality issues, it is difficult to comment on a clear benefit of one therapeutic regime over another.
No significant benefit of combination therapy over anticoagulation alone was observed to reduce the risk of TIAs. 39,42,43
The majority of included studies do not provide significant evidence of any benefit for combination therapy over ACT alone in the reduction of the combined end point of stroke and SE from two randomised39,42 and two non-randomised67,69 studies, apart from one RCT43 that suggests a significant increased risk of the combined end point of stroke and SE with the combination of ACT and APT compared with ACT alone.
There is also no evidence that combination ACT plus APT is associated with a significant reduction in systemic embolic events compared with ACT alone in the included studies.
There is no clear evidence of a significant benefit of combination therapy in the reduction of AMI despite numerically lower rates of the event with combined ACT plus APT than with ACT alone. 42,43,54,69
The available evidence does not indicate a clear benefit of combination therapy in reducing the risk of vascular death compared with ACT alone. 39,41 – 43 In a similar way, six studies39,41–43,54,69 demonstrated that combination therapy with ACT and APT did not confer a significant reduction in all-cause, non-vascular or mortality from unknown causes, over ACT alone.
Combination therapy was observed to significantly increase the risk of bleeding compared with ACT alone in two studies41,73 (one randomised41 and one non-randomised73), whereas one large non-randomised study63 reported similar levels of bleeding with combination therapy, including triple therapy, compared with anticoagulation alone. 63 There is conflicting evidence regarding the effect of combination ACT plus APT compared with ACT alone on the risk of major bleeding with no randomised evidence reporting a significant increase in the risk with combination therapy compared with ACT alone. 39,41–43 Furthermore, the non-randomised studies reported inconsistent data, with two demonstrating higher rates of major bleeding with some combination therapy (VKAs plus aspirin)54,69 over ACT alone, and lower bleeding rates with other combined therapy (VKA plus triflusal54 or ximelagatran plus aspirin69), whereas the other study73 reported an increased risk of major bleeding only with the highest dose of ACT plus APT compared with ACT alone. The rate of ICH reported in three randomised studies was very low and there was no evidence of a significantly increased risk of ICH with combination therapy over ACT alone. 39,42,43
No significant increased risk in minor or non-severe bleeding was observed with combination therapy compared with anticoagulation alone,39,40,42,43 whereas another small randomised study41 reported a significant increase in the risk of minor/non-severe bleeding with combined therapy.
Although lower major adverse event rates were observed in three studies39,41,54 (two randomised39,41 and one non-randomised54) with combination therapy for the composite end points of severe bleeding, non-fatal stroke, TIA, SE and vascular death,39 non-fatal stroke, TIA, SE, and vascular death,39 embolism, stroke, AMI, and vascular death,39 SE and death,41 and stroke, systemic/coronary ischaemic events, AMI and mortality,54 and all ischaemic events54 than with anticoagulation alone, the difference between ACT plus APT and ACT alone was not significantly different in the two randomised studies. 39,41 Combination therapy conferred a significantly increased risk of the composite end point of stroke, SE and vascular death, compared with ACT alone, in one randomised study. 43
Not all the randomised studies were of good quality. The mean duration of the studies varied from as low as 22 days40 to 3.5 years,42 with a sample size ranging from 43 patients40 to 1209,39 and compared an antiplatelet agent (aspirin, clopidogrel, triflusal) added to an anticoagulant agent (warfarin, acenocoumarol, fluindione) with anticoagulant alone (or ACT plus placebo). Most studies furnished clear information on the randomisation design and method; however, the majority undertook therapies in an open-label fashion. 39,42,43 No study reported a robust, randomised comparison in a high-risk AF population (with a specified CHADS2 score of ≥ 2) between combined therapy of ACT targeting a standard therapeutic INR target of 2.0–3.0 plus additional APT, and ACT alone (target INR 2.0–3.0). Only one study41 compared fluindione (target INR 2.0–2.6) plus additional aspirin with fluindione plus placebo (target INR 2.0–2.6) in a high-risk AF population. With a mean follow-up of 0.84 years and premature termination of the trial because of slow recruitment, the study results were less than adequate to be generalisable. Other studies investigated different doses of anticoagulant plus antiplatelet to anticoagulant alone in patients at variable (or unspecified) stroke risks.
The quality of those studies that reported non-randomised comparisons was generally poor. The sample size in these studies varied from 228 patients59 to 118,606,63 with follow-up periods of between 8 weeks62 and 7.2 years. 52,61 Most studies were retrospective in nature, with patient data identified from a register of records, and with no or unclear information on blinding of assessors. APT was used at physicians' discretion in most studies, clearly indicated for cardiovascular diseases in a few, or for specific reasons which were not reported in others. The time of antiplatelet administration also varied across the studies or was not clearly specified.
Quality assessment of included studies was undertaken for this review. However, given the issues around heterogeneity between included studies, it was felt that extensive reporting of quality had little meaning in the context of this review. Therefore, in the results section, only summary tables of quality are provided (see Tables 5 and 7, and Appendix 6).
Methodology and issues
Several issues regarding methodological and clinical heterogeneity were encountered during the course of the review. A few are outlined in the following sections.
Population
The review aimed to assess the clinical benefit of combined therapy with ACT plus APT over ACT alone on vascular events in a population at high risk of stroke, with high risk determined either by history of AMI with PCI with or without stent, or having a CHADS2 score of ≥ 2. However, not all studies identified such a population.
Risk of stroke
None of the included studies reported data for a high-risk population with a CHADS2 score of ≥ 2. Those studies that evaluated stroke risk according to CHADS2 score failed to report the outcomes for each CHADS2 score category separately (high, moderate and low risk, respectively). 50,72
Of the five studies that reported randomised comparisons, three39,41,43 specified a high-risk AF population. However, the definition of high risk varied across the studies. None of the included studies specified diabetes mellitus as one of their stroke risk assessment criteria (diabetes mellitus being one of the risk score criteria of the CHADS2 scheme). Of those that reported non-randomised comparisons, the majority did not specify the stroke risk of the sample, whereas the definitions of high risk varied across the studies in those that specified stroke risk. 50,55,58,64,65,72,73
Study setting
Almost all non-randomised studies were conducted in hospital patients. This could have included more frail patients with multiple comorbidities that might place them at a higher risk for events and, therefore, would make the results from such studies less generalisable to a wider population. 51
Of the studies reporting non-randomised data, six were based on reviews of hospital records. 56–58,60,61,63 Of these, one was a large study63 on 118,606 patients over a mean follow-up of 3.3 years. It is important to note that such studies are at high risk of selection bias with less information on ethnicity and dosage and prone to poor documentation. 95 Results from these studies, therefore, need to be considered with caution.
Valvular diseases
Studies of patients with valvular diseases were included in the review. Those studies that included patients with valve replacements or mechanical heart valves were, however, excluded, despite the fact that this population is considered to be at high risk of stroke, because of different clinical target of anticoagulant INR range. If a study did not specify that subjects with valvular replacements were excluded, it was not excluded. For this reason, the studies by NASPEAF,39 SPAF III43 and Hansen et al. 63 were included in the review.
Intervention and comparator
The review was aimed at investigating combined ACT plus APT in comparison with ACT alone (plus placebo), with the dose of ACT adjusted to target the recommended INR range of 2.0–3.0 in both study arms.
Types of therapies
The type of both ACT and APT varied across the studies. Of the included randomised studies, three40,42,43 reported data for warfarin therapy in different regimes in combination with different APT (aspirin, triflusal, clopidogrel). Of the remaining two RCTs, the FFAACS41 study reported data for fluindione (ACT) and the NASPEAF study39 assessed acenocoumarol (ACT) in combination with triflusal (APT). Both fluindione and triflusal are not known to be widely used in Europe and the UK. There was no further evidence available on these technologies.
Of those reporting non-randomised data, 14 studies reported data on warfarin in various regimes combined with an APT. Bover et al. 54 reported data for acenocoumarol plus triflusal, an APT that is not known to be widely used in the UK or Europe. This study54 included a majority of patients enrolled in the NASPEAF trial. 39 The PETRO study73 reported non-randomised data for dabigatran plus aspirin compared with dabigatran alone. No further evidence was available for this comparison. Ximelagatran was investigated in the two SPORTIF studies64,65 and their six66–71 supporting post hoc analyses. The AMADEUS study72 did not specify the specific ACT (idraparinux or VKA) used in the comparison of ACT plus aspirin compared with ACT alone, whereas another study failed to identify the ACT. 58 Three studies did not report the name of the APT in the study. 52,59,60
Dosage
Only two40,41 of the five included randomised studies investigated ACT with the recommended target INR range of 2.0–3.0 in both study arms. Both studies were conducted on a small sample size (n = 43,40n = 15741) over a short period of follow-up. One did not specify either the stroke risk or the sample size calculations for the study. 40 The FFAACS study41 was terminated early because of slow recruitment, which might have resulted in an overestimation of therapeutic efficacy. 96 Most studies reporting non-randomised comparisons reported the dosage of both therapies. Most studies reporting data for patients on warfarin specified the target INR of 2.0–3.0 in both study arms. 51,53,57,59,61,62,64,65 However, data from many of these did not add further information to the RCT data. The reasons for non-inclusion of data from these studies have been reported in Appendix 7.
Previous antithrombotic therapy
Of the randomised studies, two41,43 of the included studies consisted of an anticoagulant-experienced population. Two other included RCTs39,40 did not report this information and one42 specified an anticoagulant-naive population. The majority of non-randomised studies also reported a population with a history of antithrombotic medication,53–55,58,59,61,63,69,72,73 whereas others did not report this information. Such a population group might have potential implications of lower event rates because of patients' tolerance to an ACT in comparison with those who have no prior experience of ATT.
Outcomes
The review aimed to assess the benefit of combined therapy over ACT alone on vascular events in a high-risk AF population.
The primary outcome measures assessed were stroke, TIA, SE, the composite end point of SE and stroke, MI, vascular death along with secondary outcomes of all-cause mortality, bleeding events and composite end point consisting of various primary outcomes. Composite end points of stroke and SE were not specified in the review protocol; however, it was considered clinically relevant and reported in a considerable number of included studies and, therefore, was agreed to be reported in the review. The review protocol also specified in-stent thrombosis, revascularisation procedures and quality-of-life outcome measures; however, none of the included studies was found to report these events.
Outcome definitions
The review protocol specified definitions for each of the outcomes, which were broadly comparable with those specified in individual included studies. However, many studies failed to provide precise definitions of the outcomes.
Stroke, symbolic embolism, composite of stroke/systemic embolism, transient ischaemic attack, acute myocardial infarction and composite events
The majority of the included studies did not report a significant difference in event rates between the patients on combined therapy compared with those on ACT alone. Of the studies reporting randomised comparisons, one43 reported a statistically significant higher risk of events in the combined therapy arm compared with ACT alone for the number of stroke events, composite of SE or stroke, and composite of stroke, SE and vascular deaths. However, the study compared warfarin [with a lower than recommended target INR range (1.2–1.5)] in combination with aspirin 300 mg with warfarin alone, targeting an INR of 2.0–3.0 in high-risk patients with AF in an open-label RCT with no blinding. 43 The population risk criteria in this study did not include diabetes mellitus, contrary to the current established stroke risk schemes such as CHADS2. 27 Furthermore, the NASPEAF RCT39 reported fewer events of composite of non-fatal stroke, TIA, SE and vascular death in combined therapy arm than in patients on acenocoumarol alone in both intermediate- and high-risk patients. 39 The INR range of acenocoumarol was below the recommended target of 2.0–3.0 in this study and established stroke risk assessment schemes were not used.
Risk of these events varied across the studies that reported non-randomised comparisons with low event rates and confounding of results by indication of APT.
Mortality: all cause and vascular
Most studies did not report a significant difference in mortality rates between the two therapy groups. Of the studies reporting randomised comparisons, only one study39 reported a significantly lower rate of vascular death in patients on combined acenocoumarol plus triflusal than in those with acenocoumarol alone in either high- or low-risk patients. However, the low event rates in the study warrant cautious interpretation. 39 Non-randomised evidence for mortality was not free from bias, as evident from the previous sections. Therefore, it is difficult to deduce the benefits of combined ACT plus APT compared with ACT alone on mortality from the evidence available.
Bleeding
Significant differences in bleeding rates were not reported in the majority of the included studies. Only one RCT41 reported significantly higher rates of bleeding in the combined therapy arm than with fluindione plus placebo. 41 However, the trial was prematurely terminated because of a small sample size with slow recruitment of patients (n = 157), resulting in a low study power to detect a meaningful effect of combined therapy on embolic events. Non-randomised evidence from one study69 reported a larger number of bleeding events in the combined therapy group of warfarin plus aspirin than with warfarin alone. However, these groups were not evenly distributed and indication of aspirin for patients with CAD confounded the results.
Strengths and limitations
Strengths of the assessment
-
Studies included in the assessment consisted of both randomised and non-randomised comparisons in an attempt to investigate all the available evidence around the subject.
-
A comprehensive search strategy was undertaken encompassing all relevant databases.
-
Robust review methodology was used.
Limitations of the assessment
-
It was originally intended that an IPD analysis would be undertaken to specifically address the effect of APT added to ACT (compared with ACT alone) on various outcomes (including time to first vascular event/first major haemorrhage or clinically relevant bleeding/death and time within therapeutic INR range) (see Appendix 1 and Chapter 4, Changes to protocol). Predefined subgroup analyses were to be developed to possibly include stent type; warfarin-naive versus warfarin-experienced subjects; short- and long-term outcomes; patients with diabetes mellitus; and CHADS2 score ≥ 2 and < 2. However, it became clear from the range of included studies that the methodological heterogeneity between studies, the clinical heterogeneity within and between studies, and the relatively small number of events was against such analyses being able to appreciably add to the findings of the review. To aid explanation, we draw the reader's attention to Table 27. This table summarises the key features and findings of the included studies grouped by similar intervention and comparator. Examining the section of the table containing the five studies that investigated combination ACT plus APT compared with ACT alone reveals that the intervention and comparator regimens were heterogeneous for both elements and, furthermore, the study designs were a mix of randomised and non-randomised comparisons. As with aggregate patient data meta-analysis, clinical and methodological study homogeneity are still overriding considerations prior to undertaking IPD meta-analyses and, thus, it was not an option to pool data across all studies in this case. Only two of the studies67,69 had similar intervention/comparator characteristics and these were the same non-randomised comparison where aspirin was added to warfarin therapy based on clinical indication. Thus, as mentioned previously in this report, the treatment comparison in these studies was confounded by indication and IPD meta-analyses would therefore also be confounded. Where IPD analysis might have been beneficial is in possibly revealing data on outcomes previously unreported for a given study. In the current example, the greatest potential for this was with the two non-randomised comparisons with similar intervention/comparisons or for the outcome of TTR for all warfarin/aspirin studies. However, the utility of this was limited given the aforementioned limitation of combining data across studies. Similar issues also affected the value of IPD analyses for other intervention/comparator combinations (see Table 27). Thus, although the benefits of an IPD approach are well recognised97 in the current report, the approach offered limited advantage. These issues were discussed with the NIHR and with their agreement it was decided not to undertake the planned the IPD analysis. As such, some aspirational aspects of the current work could not be achieved.
-
Individual participant data analysis could not be undertaken for various reasons. Included studies reported low event rates, with methodological heterogeneity and ambiguity along with the fact that it was very difficult to identify studies with similar study designs, population characteristics, intervention and comparator therapies, and outcome measures. There is paucity of directly relevant randomised evidence to undertake a meta-analysis for the merits of one technology over another.
-
The evidence was such that three stages of study selection were required, with one of these stages being unforeseen. With hindsight, this process might have been more efficiently achieved.
-
Although the review initially aimed to identify high-risk patients, none of the included studies specified a high-risk group as per the established stroke risk assessment criteria. Studies were also included if stroke risk was not specified. This might have introduced studies with patients at a lower risk of stroke.
-
It was intended to include only those studies that reported data for patients on combined ACT plus APT with a target INR range for ACT of between 2.0 and 3.0. However, this criterion could have been too restrictive; therefore, those studies in which no INR range was specified were also included, as it could not be ruled out that the appropriate INR was utilised.
Ongoing studies
Given the advent of novel oral anticoagulants, the direct thrombin inhibitors (e.g. dabigatran) and factor Xa inhibitors (e.g. apixaban, rivaroxaban and endoxaban), members of the steering committee are aware of planned post hoc non-randomised comparisons, between ACT plus APT and ACT alone, for the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY),21 the Apixaban for Reduction In STroke and Other ThromboemboLic Events in Atrial Fibrillation (ARISTOTLE)22 and the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET-AF). 23 Two members from the steering committee are also the co-authors of the non-randomised comparison between ACT plus APT compared with ACT only in the post hoc analysis of the AMADEUS study,72 which was published after the search strategy of the current review. Therefore, it is not included in this assessment.
Implications for future research
It is clear from the results of this systematic review that there are not sufficient data from the five randomised comparisons and 18 non-randomised comparisons to conclude whether or not there are high-risk patients with AF who would benefit from a reduction in vascular events with combined therapy of anticoagulation and APT compared with ACT alone.
Given the paucity of data, and the clinical and methodological heterogeneity encompassed in the studies from which the data comes, an individual participant data analysis is unlikely to prove beneficial. Likewise, it is recommended that a cost-effectiveness analysis at this point would be premature.
Given the absence of ongoing trials addressing the benefit of anticoagulation plus APT compared with anticoagulation alone in patients with AF at high-risk of TEs, it is recommended that a definitive prospective RCT needs to be undertaken. Any future trial would need to consider the following issues:
-
The population would need to be clearly defined. This would mean taking into account the different risk stratification scores, which currently exist in order to allow clinicians and policy-makers to interpret any findings within their specific health economy. Any future study should consider including a population at high risk of atherosclerotic coronary artery and other vascular events (following ACS ± stenting) and those patients at high risk of AF-mediated TEs.
-
The intervention would need to be clearly defined. There are currently data available from studies utilising different classes of drugs with ongoing post hoc analyses becoming available for new classes of both anticoagulant and antiplatelet agents. Any future study would need to address these potential class effects. From the UK context, at the time of writing, any future trial should compare adjusted-dose warfarin (INR 2.0–3.0) plus aspirin (75–325 mg) with adjusted-dose warfarin (INR 2.0–3.0). However, given the emergence of newer anticoagulation agents (dabigatran, rivaroxaban and apixaban) this prioritisation may need to be revisited in the future to reflect current best clinical practice.
-
Any future study should include a health economic analysis.
-
The comparator group would need to receive the same ACT as the intervention group; thus, if the anticoagulant under investigation was a VKA, then the comparator group should have the same INR target as the intervention group. Similarly, achieved INRs in terms of therapeutic time in range should be reported for both groups.
-
All outcomes would need to be clearly defined in order to allow clinicians and policy-makers to interpret any findings within their specific health economy.
-
All outcomes would need to be independently validated in line with international definitions.
-
Analysis of outcomes would need to be undertaken in line with contemporary methods of assessing net clinical benefit.
-
Duration of follow-up needs to be sufficient to allow (1) confidence that the findings would reflect real world utilisation of the technologies and (2) a reasonable number of events. This will obviously be dependent on sample size, but should be at least 1 year.
Conclusion
This systematic review identified five randomised and 18 non-randomised studies that compared treatment with anticoagulation and APT with treatment with ACT alone in patients with AF. These studies were generally of poor quality, utilised different anticoagulant and APTs, investigated different populations of patients in terms of risk, had different follow-up periods and used different outcome measures, with various definitions of these outcomes.
The data from these studies are not sufficient to conclude whether or not there are patients with AF in whom the addition of an antiplatelet agent to an anticoagulant is warranted in terms of benefit from reduction of vascular events compared with an increased risk of bleeding.
It is recommended that a definitive prospective RCT is undertaken, preferably in a population at high risk of atherosclerotic coronary artery and other vascular events in addition to being at high risk of AF-mediated TEs, utilising interventions and comparators that include current and emerging ACT and APT strategies, which also takes into account the findings of this review.
Acknowledgements
The authors wish to thank Joanna Hine for the administrative support and all of those who contributed to the translation of the foreign-language papers. In addition, the authors would like to thank the members of the steering committee (Matt Fay, Carl Heneghan, Sue Jowett, Lalit Kalra, Eve Knight, Gregory Lip, Jonathan Mant, Ellen Murray, Peter Rose and Rod Taylor) for their advice and support.
Contribution of authors
Deirdre A Lane, joint principal investigator, contributed to the development of the protocol, study selection, data extraction and quality assessment, writing of the report and provided clinical input.
Smriti Raichand contributed to the development of the protocol, study selection, data extraction and quality assessment, and writing of the report.
David Moore contributed to the development of the protocol, provided methodological input, and contributed to study selection and writing of the report.
Martin Connock contributed to the development of the protocol and study selection (abstract screening).
Anne Fry-Smith, devised the search strategy and carried out the searches.
David Fitzmaurice, joint principal investigator, contributed to the development of the protocol, study selection, report writing and provided clinical input.
All authors provided input to the development of the review report, commented on various drafts of the chapters and contributed to their editing.
Disclaimer
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation – Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the European Society of Cardiology Committee for practice guidelines (Writing committee to revise the 2001 guidelines for the management of patients with atrial fibrillation). Circulation 2006;114:700-52.
- Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2369-429. http://dx.doi.org/10.1093/eurheartj/ehq278.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983-8. http://dx.doi.org/10.1161/01.STR.22.8.983.
- Wolf PA, Mitchell JB, Baker CS, Kannel WB, D’Agostino RB. Impact of atrial fibrillation on mortality, stroke, and medical costs. Arch Intern Med 1998;158:229-34. http://dx.doi.org/10.1001/archinte.158.3.229.
- Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998;98:946-52. http://dx.doi.org/10.1161/01.CIR.98.10.946.
- Dorian P, Jung W, Newman D, Paquette M, Wood K, Ayers GM, et al. The impairment of health-related quality of life in patients with intermittent atrial fibrillation: implications for the assessment of investigational therapy. J Am Coll Cardiol 2000;36:1303-9. http://dx.doi.org/10.1016/S0735-1097(00)00886-X.
- Thrall G, Lane D, Carroll D, Lip GYH. Quality of life in patients with atrial fibrillation: a systematic review. Am J Med 2006;119. http://dx.doi.org/10.1016/j.amjmed.2005.10.057.
- Heeringa J, van der Kuip DA, Hofman A, Kors JA, van HG, Stricker BH, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J 2006;27:949-53. http://dx.doi.org/10.1093/eurheartj/ehi825.
- Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation 2004;110:1042-6. http://dx.doi.org/10.1161/01.CIR.0000140263.20897.42.
- Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006;114:119-25. http://dx.doi.org/10.1161/CIRCULATIONAHA.105.595140.
- Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001;285:2370-5. http://dx.doi.org/10.1001/jama.285.18.2370.
- Hobbs FD, Fitzmaurice DA, Mant J, Murray E, Jowett S, Bryan S, et al. A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study. Health Technol Assess 2005;9.
- Jorgensen HS, Nakayama H, Reith J, Raaschou HO, Olsen TS. Acute stroke with atrial fibrillation. The Copenhagen Stroke Study. Stroke 1996;27:1765-9. http://dx.doi.org/10.1161/01.STR.27.10.1765.
- Steger C, Pratter A, Martinek-Bregel M, Avanzini M, Valentin A, Slany J, et al. Stroke patients with atrial fibrillation have a worse prognosis than patients without: data from the Austrian Stroke registry. Eur Heart J 2004;25:1734-40. http://dx.doi.org/10.1016/j.ehj.2004.06.030.
- Lin HJ, Wolf PA, Kelly-Hayes M, Beiser AS, Kase CS, Benjamin EJ, et al. Stroke severity in atrial fibrillation. The Framingham Study. Stroke 1996;27:1760-4. http://dx.doi.org/10.1161/01.STR.27.10.1760.
- Wolfe CDA, Crichton SL, Heuschmann PU, McKevitt CJ, Toschke AM, Grieve AP, et al. Estimates of outcomes up to ten years after stroke: analysis from the prospective South London Stroke Register. PLoS Med 2011;8. http://dx.doi.org/10.1371/journal.pmed.1001033.
- Estimating the direct costs of atrial fibrillation to the NHS in the constituent countries of the UK and at SHA level in England, 2008. London: OHE; 2009.
- Stewart S, Murphy NF, Walker A, McGuire A, McMurray JJ. Cost of an emerging epidemic: an economic analysis of atrial fibrillation in the UK. Heart 2004;90:286-92. http://dx.doi.org/10.1136/hrt.2002.008748.
- Stroke Risk in Atrial Fibrillation Working Group . Independent predictors of stroke in patients with atrial fibrillation: a systematic review. Neurology 2007;69:546-54. http://dx.doi.org/10.1212/01.wnl.0000267275.68538.8d.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Int Med 2007;146:857-67.
- Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139-51. http://dx.doi.org/10.1056/NEJMoa0905561.
- Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981-92. http://dx.doi.org/10.1056/NEJMoa1107039.
- Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883-91. http://dx.doi.org/10.1056/NEJMoa1009638.
- London: Royal College of Physicians; 2006.
- Gage BF, van WC, Pearce L, Hart RG, Koudstaal PJ, Boode BS, et al. Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation 2004;110:2287-92. http://dx.doi.org/10.1161/01.CIR.0000145172.55640.93.
- Atrial Fibrillation Investigators . Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Int Med 1994;154:1449-57. http://dx.doi.org/10.1001/archinte.1994.00420130036007.
- Hart RG, Pearce LA, McBride R, Rothbart RM, Asinger RW. Factors associated with ischemic stroke during aspirin therapy in atrial fibrillation: analysis of 2012 participants in the SPAF I-III clinical trials. The Stroke Prevention in Atrial Fibrillation (SPAF) Investigators. Stroke 1999;30:1223-9. http://dx.doi.org/10.1161/01.STR.30.6.1223.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001;285:2864-70. http://dx.doi.org/10.1001/jama.285.22.2864.
- Wang TJ, Massaro JM, Levy D, Vasan RS, Wolf PA, D’Agostino RB, et al. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: the Framingham Heart Study. JAMA 2003;290:1049-56. http://dx.doi.org/10.1001/jama.290.8.1049.
- Becker RC, Meade TW, Berger PB, Ezekowitz M, O’Connor CM, Vorchheimer DA, et al. The primary and secondary prevention of coronary artery disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edn). Chest 2008;133:776-814.
- Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation. Chest 2010;137:263-72. http://dx.doi.org/10.1378/chest.09-1584.
- May AE, Geisler T, Gawaz M. Individualized antithrombotic therapy in high risk patients after coronary stenting. A double-edged sword between thrombosis and bleeding. Thromb Haemost 2008;99:487-93.
- Singer DE, Albers GW, Dalen JE, Fang MC, Go AS, Halperin JL, et al. Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edn). Chest 2008;133:546-92.
- Rubboli A, Halperin JL. Pro: ‘Antithrombotic therapy with warfarin, aspirin and clopidogrel is the recommended regime in anticoagulated patients who present with an acute coronary syndrome and/or undergo percutaneous coronary interventions’. Thromb Haemost 2008;100:752-3.
- Lip GY, Huber K, Andreotti F, Arnesen H, Airaksinen KJ, Cuisset T, et al. Management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous coronary intervention/stenting. Thromb Haemost 2010;103:13-28. http://dx.doi.org/10.1160/TH09-08-0580.
- Rubboli A, Halperin JL, Airaksinen KE, Buerke M, Eeckhout E, Freedman SB, et al. Antithrombotic therapy in patients treated with oral anticoagulation undergoing coronary artery stenting. An expert consensus document with focus on atrial fibrillation. Ann Med 2008;40:428-36. http://dx.doi.org/10.1080/07853890802089786.
- Higgins JPT, Green Se. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 (updated March 2011). Copenhagen: The Cochrane Collaboration; 2011.
- Khan KS, ter Riet G, Popay J, Nixon J, Kleijnen J. Conducting the review, Phase 5: Study quality and assessment. Centre for Reviews and Dissemination. Systematic reviews: CRD's guidance for undertaking reviews in health care. York: University of York; 2001.
- Pérez-Gómez F, Alegría E, Berjón J, Iriarte JA, Zumalde J, Salvador A, et al. Comparative effects of antiplatelet, anticoagulant, or combined therapy in patients with valvular and nonvalvular atrial fibrillation: a randomized multicenter study. J Am Coll Cardiol 2004;44:1557-66. http://dx.doi.org/10.1016/j.jacc.2004.05.084.
- Lidell C, Svedberg LE, Lindell P, Bandh S, Job B, Wallentin L. Clopidogrel and warfarin: absence of interaction in patients receiving long-term anticoagulant therapy for non-valvular atrial fibrillation. Thromb Haemost 2003;89:842-6.
- Lechat P, Lardoux H, Mallet A, Sanchez P, Derumeaux G, Lecompte T, et al. Anticoagulant (fluindione)-aspirin combination in patients with high-risk atrial fibrillation. A randomized trial (Fluindione, Fibrillation Auriculaire, Aspirin et Contraste Spontane; FFAACS). Cerebrovasc Dis 2001;12:245-52. http://dx.doi.org/10.1159/000047711.
- Gullov AL, Koefoed BG, Petersen P, Pedersen TS, Andersen ED, Godtfredsen J, et al. Fixed minidose warfarin and aspirin alone and in combination vs adjusted-dose warfarin for stroke prevention in atrial fibrillation: Second Copenhagen Atrial Fibrillation, Aspirin, and Anticoagulation Study. Arch Int Med 1998;158:1513-21. http://dx.doi.org/10.1001/archinte.158.14.1513.
- Stroke Prevention in Atrial Fibrillation Investigators . Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: Stroke Prevention in Atrial Fibrillation III randomised clinical trial. Lancet 1996;348:633-8. http://dx.doi.org/10.1016/S0140-6736(96)03487-3.
- Pérez-Gómez F, Iriarte JA, Zumalde J, Berjón J, Salvador A, Alegría E, et al. Antithrombotic therapy in elderly patients with atrial fibrillation: effects and bleeding complications: a stratified analysis of the NASPEAF randomized trial. Eur Heart J 2007;28:996-1003. http://dx.doi.org/10.1093/eurheartj/ehl364.
- Pérez-Gómez F, Salvador A, Zumalde J, Iriarte JA, Berjón J, Alegría E, et al. Effect of antithrombotic therapy in patients with mitral stenosis and atrial fibrillation: a sub-analysis of NASPEAF randomized trial. Eur Heart J 2006;27:960-7. http://dx.doi.org/10.1093/eurheartj/ehi667.
- Pérez-Gómez F, Fernandez C. Antiplatelet and anticoagulant therapy in patients with atrial fibrillation. Cardiol Rev 2006;23:44-8.
- Gullov AL, Koefoed BG, Petersen P. Bleeding during warfarin and aspirin therapy in patients with atrial fibrillation: the AFASAK 2 study. Atrial Fibrillation Aspirin and Anticoagulation. Arch Int Med 1999;159:1322-8. http://dx.doi.org/10.1001/archinte.159.12.1322.
- Hart RG, Pearce LA, Miller VT, Anderson DC, Rothrock JF, Albers GW, et al. Cardioembolic vs. noncardioembolic strokes in atrial fibrillation: frequency and effect of antithrombotic agents in the stroke prevention in atrial fibrillation studies. Cerebrovasc Dis 2000;10:39-43. http://dx.doi.org/10.1159/000016023.
- Blackshear JL, Zabalgoitia M, Pennock G, Fenster P, Strauss R, Halperin J, et al. Warfarin safety and efficacy in patients with thoracic aortic plaque and atrial fibrillation. Am J Cardiol 1999;83:453-5. http://dx.doi.org/10.1016/S0002-9149(98)00886-8.
- Lopes RD, Elliott LE, White HD, Hochman JS, Van de WF, Ardissino D, et al. Antithrombotic therapy and outcomes of patients with atrial fibrillation following primary percutaneous coronary intervention: results from the APEX-AMI trial. Eur Heart J 2009;30:2019-28. http://dx.doi.org/10.1093/eurheartj/ehp213.
- Abdelhafiz AH, Wheeldon NM. Risk factors for bleeding during anticoagulation of atrial fibrillation in older and younger patients in clinical practice. Am J Geriatr Pharmacother 2008;6:1-11. http://dx.doi.org/10.1016/j.amjopharm.2008.03.005.
- Toda G, Akiyama K, Sakuragawa K, Iliev II, Hayano M, Yano K. Thromboembolic complication in atrial fibrillation in a long-term follow-up: the relationship with underlying disease, type of atrial fibrillation, and antithrombotic therapy. Japanese Circ J 1998;62:255-60. http://dx.doi.org/10.1253/jcj.62.255.
- Albers GW, Yim JM, Belew KM, Bittar N, Hattemer CR, Phillips BG, et al. Status of antithrombotic therapy for patients with atrial fibrillation in university hospitals. Arch Int Med 1996;156:2311-16. http://dx.doi.org/10.1001/archinte.1996.00440190053006.
- Bover R, Perez-Gomez F, Maluenda MP, Asenjo S, Perez-Saldana R, Igea A, et al. Long-term follow-up of atrial fibrillation patients in the NASPEAF study. Prospective evaluation of different antiplatelet treatments. Rev Esp Cardiokl 2009;62:992-1000. http://dx.doi.org/10.1016/S1885-5857(09)73265-7.
- Hart RG, Pearce LA, Rothbart RM, McAnulty JH, Asinger RW, Halperin JL. Stroke with intermittent atrial fibrillation: incidence and predictors during aspirin therapy. Stroke Prevention in Atrial Fibrillation Investigators. J Am Coll Cardiol 2000;35:183-7. http://dx.doi.org/10.1016/S0735-1097(99)00489-1.
- Suzuki S, Yamashita T, Kato T, Fujino T, Sagara K, Sawada H, et al. Incidence of major bleeding complication of warfarin therapy in Japanese patients with atrial fibrillation. Circ J 2007;71:761-5. http://dx.doi.org/10.1253/circj.71.761.
- Burton C, Isles C, Norrie J, Hanson R, Grubb E. The safety and adequacy of antithrombotic therapy for atrial fibrillation: a regional cohort study. Br J Gen Pract 2006;56:697-702.
- Stenestrand U, Lindback J, Wallentin L, RIKS-HIA R. Anticoagulation therapy in atrial fibrillation in combination with acute myocardial infarction influences long-term outcome: a prospective cohort study from the Register of Information and Knowledge About Swedish Heart Intensive Care Admissions (RIKS-HIA). Circulation 2005;112:3225-31. http://dx.doi.org/10.1161/CIRCULATIONAHA.105.552984.
- Johnson CE, Lim WK, Workman BS. People aged over 75 in atrial fibrillation on warfarin: the rate of major hemorrhage and stroke in more than 500 patient-years of follow-up. J Am Geriatr Soc 2005;53:655-9. http://dx.doi.org/10.1111/j.1532-5415.2005.53215.x.
- Shireman TI, Howard PA, Kresowik TF, Ellerbeck EF. Combined anticoagulant-antiplatelet use and major bleeding events in elderly atrial fibrillation patients. Stroke 2004;35:2362-7. http://dx.doi.org/10.1161/01.STR.0000141933.75462.c2.
- Blich M, Gross B. Thromboembolic prophylaxis in nonrheumatic atrial fibrillation: utilization patterns, efficacy, and complications in a long-term follow-up of community patients. Int J Cardiol 2004;96:89-95. http://dx.doi.org/10.1016/j.ijcard.2003.06.014.
- Klein AL, Murray RD, Grimm RA, Li J, Person-Hansen C, Jasper SE, et al. Bleeding complications in patients with atrial fibrillation undergoing cardioversion randomized to transesophageal echocardiographically guided and conventional anticoagulation therapies. Am J Cardiol 2003;92:161-5. http://dx.doi.org/10.1016/S0002-9149(03)00531-9.
- Hansen ML, Sorensen R, Clausen MT, Fog-Petersen ML, Raunso J, Gadsboll N, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Int Med 2010;170:1433-41. http://dx.doi.org/10.1001/archinternmed.2010.271.
- Olsson SB. Executive Steering Committee of the SPORTIF III Investigators . Stroke prevention with the oral direct thrombin inhibitor ximelagatran compared with warfarin in patients with non-valvular atrial fibrillation (SPORTIF III): randomised controlled trial. Lancet 2003;362:1691-8. http://dx.doi.org/10.1016/S0140-6736(03)14841-6.
- Albers GW, Diener HC, Frison L, Grind M, Nevinson M, Partridge S, et al. Ximelagatran vs warfarin for stroke prevention in patients with nonvalvular atrial fibrillation: a randomized trial. JAMA 2005;293:690-8. http://dx.doi.org/10.1001/jama.293.6.690.
- Teitelbaum JS, von KR, Gjesdal K, Kristinsson A, Gahn G, Albers GW, et al. Effect of ximelagatran and warfarin on stroke subtypes in atrial fibrillation. Can J Neurol Sci 2008;35:160-5.
- Akins PT, Feldman HA, Zoble RG, Newman D, Spitzer SG, Diener HC, et al. Secondary stroke prevention with ximelagatran versus warfarin in patients with atrial fibrillation: pooled analysis of SPORTIF III and V clinical trials. Stroke 2007;38:874-80. http://dx.doi.org/10.1161/01.STR.0000258004.64840.0b.
- White HD, Gruber M, Feyzi J, Kaatz S, Tse HF, Husted S, et al. Comparison of outcomes among patients randomized to warfarin therapy according to anticoagulant control: results from SPORTIF III and V. Arch Int Med 2007;167:239-45. http://dx.doi.org/10.1001/archinte.167.3.239.
- Flaker GC, Gruber M, Connolly SJ, Goldman S, Chaparro S, Vahanian A, et al. Risks and benefits of combining aspirin with anticoagulant therapy in patients with atrial fibrillation: an exploratory analysis of stroke prevention using an oral thrombin inhibitor in atrial fibrillation (SPORTIF) trials. Am Heart J 2006;152:967-73. http://dx.doi.org/10.1016/j.ahj.2006.06.024.
- Douketis JD, Arneklev K, Goldhaber SZ, Spandorfer J, Halperin F, Horrow J. Comparison of bleeding in patients with nonvalvular atrial fibrillation treated with ximelagatran or warfarin: assessment of incidence, case-fatality rate, time course and sites of bleeding, and risk factors for bleeding. Arch Int Med 2006;166:853-9. http://dx.doi.org/10.1001/archinte.166.8.853.
- Halperin JL. Ximelagatran: oral direct thrombin inhibition as anticoagulant therapy in atrial fibrillation. J Am Coll Cardiol 2005;45:1-9. http://dx.doi.org/10.1016/j.jacc.2004.09.049.
- Bousser MG, Bouthier J, Buller HR, Cohen AT, Crijns H, Davidson BL, et al. Comparison of idraparinux with vitamin K antagonists for prevention of thromboembolism in patients with atrial fibrillation: a randomised, open-label, non-inferiority trial. Lancet 2008;371:315-21. http://dx.doi.org/10.1016/S0140-6736(08)60168-3.
- Ezekowitz MD, Reilly PA, Nehmiz G, Simmers TA, Nagarakanti R, Parcham-Azad K, et al. Dabigatran with or without concomitant aspirin compared with warfarin alone in patients with nonvalvular atrial fibrillation (PETRO Study). Am J Cardiol 2007;100:1419-26. http://dx.doi.org/10.1016/j.amjcard.2007.06.034.
- Andersen LV, Vestergaard P, Deichgraeber P, Lindholt JS, Mortensen LS, Frost L. Warfarin for the prevention of systemic embolism in patients with non-valvular atrial fibrillation: a meta-analysis. Heart 2008;94:1607-13. http://dx.doi.org/10.1136/hrt.2007.135657.
- Garwood CL, Corbett TL. Use of anticoagulation in elderly patients with atrial fibrillation who are at risk for falls. Ann Pharmacother 2008;42:523-32. http://dx.doi.org/10.1345/aph.1K498.
- Hughes M, Lip GY. Guideline Development Group for the NICE national clinical guideline for management of atrial fibrillation in primary and secondary care . Risk factors for anticoagulation-related bleeding complications in patients with atrial fibrillation: a systematic review. QJM 2007;100:599-607. http://dx.doi.org/10.1093/qjmed/hcm076.
- Dentali F, Douketis JD, Lim W, Crowther M. Combined aspirin-oral anticoagulant therapy compared with oral anticoagulant therapy alone among patients at risk for cardiovascular disease: a meta-analysis of randomized trials. Arch Int Med 2007;167:117-24. http://dx.doi.org/10.1001/archinte.167.2.117.
- Cooper NJ, Sutton AJ, Lu G, Khunti K. Mixed comparison of stroke prevention treatments in individuals with nonrheumatic atrial fibrillation. Arch Int Med 2006;166:1269-75. http://dx.doi.org/10.1001/archinte.166.12.1269.
- Lip GY, Edwards SJ. Stroke prevention with aspirin, warfarin and ximelagatran in patients with non-valvular atrial fibrillation: a systematic review and meta-analysis. Thrombosis Research 2006;118:321-33. http://dx.doi.org/10.1016/j.thromres.2005.08.007.
- Larson RJ, Fisher ES. Should aspirin be continued in patients started on warfarin?. J Gen Int Med 2004;19:879-86. http://dx.doi.org/10.1111/j.1525-1497.2004.30419.x.
- Lip GY, Rothwell P, Sudlow C. Stroke prevention. Clin Evid 2004;12:253-84.
- McNamara RL, Tamariz LJ, Segal JB, Bass EB. Management of atrial fibrillation: review of the evidence for the role of pharmacologic therapy, electrical cardioversion, and echocardiography. Ann Int Med 2003;139:1018-33.
- Perret-Guillaume C, Wahl DG. Low-dose warfarin in atrial fibrillation leads to more thromboembolic events without reducing major bleeding when compared to adjusted-dose: a meta-analysis. Thromb Haemost 2004;91:394-402.
- Sanchez-Pena P, Lechat P. Anticoagulant treatment in non-valvular atrial fibrillation. Méd Thér 2002;8:277-84.
- Segal JB, McNamara RL, Miller MR, Kim N, Goodman SN, Powe NR, et al. Prevention of thromboembolism in atrial fibrillation. A meta-analysis of trials of anticoagulants and antiplatelet drugs. J Gen Int Med 2000;15:56-67. http://dx.doi.org/10.1046/j.1525-1497.2000.04329.x.
- Aronow WS. Management of the older person with atrial fibrillation. J Am Geriatr Soc 1999;47:740-8.
- Ezekowitz MD, Levine JA. Preventing stroke in patients with atrial fibrillation. JAMA 1999;281:1830-5. http://dx.doi.org/10.1001/jama.281.19.1830.
- Fera MS, Giovannini E. Predictors of thromboembolic risk and role of antithrombolic prophylaxis in nonrheumatic atrial fibrillation. G Ital Cardiol 1999;29:193-21.
- Loewen P, Sunderji R, Gin K. The efficacy and safety of combination warfarin and ASA therapy: a systematic review of the literature and update of guidelines. Can J Cardiol 1998;14:717-26.
- Howard PA, Duncan PW. Primary stroke prevention in nonvalvular atrial fibrillation: implementing the clinical trial findings. Ann Pharmacother 1997;31:1187-96.
- Poli D, Antonucci E, Marcucci R, Mannini L, Falciani M, Abbate R, et al. The quality of anticoagulation on functional outcome and mortality for TIA/stroke in atrial fibrillation patients. Int J Cardiol 2009;132:109-13. http://dx.doi.org/10.1016/j.ijcard.2007.10.041.
- Connolly SJ, Pogue J, Eikelboom J, Flaker G, Commerford P, Franzosi MG, et al. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation 2008;118:2029-37. http://dx.doi.org/10.1161/CIRCULATIONAHA.107.750000.
- Landefeld CS, Anderson PA, Goodnough LT, Moir TW, Hom DL, Rosenblatt MW, et al. The bleeding severity index: Validation and comparison to other methods for classifying bleeding complications of medical therapy. J Clin Epidemiol 1989;42:711-18. http://dx.doi.org/10.1016/0895-4356(89)90066-8.
- Wan Y, Heneghan C, Perera R, Roberts N, Hollowell J, Glasziou P, et al. Anticoagulation control and prediction of adverse events in patients with atrial fibrillation: a systematic review. Circ Cardiovas Qual Outcomes 2008;1:84-91. http://dx.doi.org/10.1161/CIRCOUTCOMES.108.796185.
- Le-Kim L, Kerr D, Kelly A-M. Are patients on warfarin with high INR treated according to published guidelines?. Int J Hematol 2009;5.
- Singer DE, Hughes RA, Gress DR, Sheehan MA, Oertel LB, Ward MS, et al. The effect of low-dose warfarin on the risk of stroke in patients with nonrheumatic atrial fibrillation. N Engl J Med 1990;323:1505-11. http://dx.doi.org/10.1056/NEJM199011293232201.
- Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ 2010.
Appendix 1 Final protocol
Appendix 2 Literature search strategies
Appendix 3 Publications not available after contacting authors
Publications not available after contacting authors (PDF download)
Appendix 4 List of excluded studies
Appendix 5 Summary of the systematic reviews and meta-analyses included in the review
Summary of the systematic reviews and meta-analyses included in the review (PDF download)
Appendix 6 Quality assessment of randomised comparisons using the Cochrane Collaboration risk-of-bias tools
Quality assessment of randomised comparisons using the Cochrane Collaboration risk-of-bias tools (PDF download)
Appendix 7 Studies with data not included in the review and reasons
Studies with data not included in the review and reasons (PDF download)
Appendix 8 Forest plots (without summary estimates) for all outcomes by intervention and comparator
Forest plots (without summary estimates) for all outcomes by intervention and comparator (PDF download)
Glossary
- Acute coronary syndrome
- Acute coronary artery disease, including unstable angina and non-ST-segment elevation myocardial infarction and ST-segment elevation myocardial infarction.
- Antiplatelet agent
- Type of anticlotting agent that works by inhibiting blood platelets. Antiplatelet drugs include clopidogrel, dipyridamole and aspirin.
- Aspirin
- A salicylate drug inhibitor of platelet aggregation.
- Cerebrovascular
- Pertaining to the blood vessels of the brain.
- Clopidogrel
- A thienopyridine – an inhibitor of platelet aggregation.
- Coronary arteries
- The arteries that supply the heart muscle with blood.
- Coronary artery disease
- Gradual blockage of the coronary arteries, usually by atherosclerosis.
- Coronary heart disease
- Narrowing or blockage of the coronary arteries of the heart by atheroma; often leads to angina, coronary thrombosis or heart attack, heart failure and/or sudden death.
- Dipyridamole
- Inhibitor of platelet aggregation, also available in combination with aspirin.
- Electrocardiogram
- A recording of the electrical signals from the heart.
- Haemorrhagic stroke
- Death of brain cells because of bleeding in the brain.
- Heterogeneity
- Variability among studies, which could be clinical, methodological or statistical.
- Infarction
- Death of tissue following interruption of the blood supply.
- Intention-to-treat analysis
- A method of data analysis in which all patients are analysed in the group to which they were assigned at randomisation, regardless of any variation to this.
- International normalised ratio
- A measure for reporting the results of blood coagulation (clotting) tests for individuals on vitamin K antagonists.
- Ischaemia
- A low oxygen state, usually due to obstruction of the arterial blood supply or inadequate blood flow leading to hypoxia in the tissue.
- Ischaemic stroke
- Death of brain cells caused by blockage in a cerebral blood vessel.
- Meta-analysis
- A quantitative method for synthesising data by combining similar outcomes of many similar studies.
- Myocardial infarction
- Damage to the heart muscle caused by obstruction of circulation to a region of the heart. Also called a heart attack.
- Non-ST-segment elevation myocardial infarction
- A myocardial infarction that is not associated with elevation of the ST segment on an electrocardiogram.
- Occlusive vascular event
- An event caused by the blockage of an artery, due to myocardial infarction, unstable angina, ischaemic stroke, transient ischaemic attack or peripheral arterial disease.
- Peripheral arterial disease
- A condition in which the arteries that carry blood to the arms or legs become narrowed or clogged, slowing or stopping the flow of blood. Also known as peripheral vascular disease.
- Plaque
- Atheromatous plaque is a swelling on the inner surface of an artery produced by lipid deposition.
- Relative risk
- The proportion of people experiencing the event of interest among those exposed to the relevant (risk) factor (e.g. drug) divided by the proportion of people experiencing the event of interest among those not exposed to the risk factor.
- ST-segment elevation myocardial infarction
- A myocardial infarction associated with elevation of the ST segment on the electrocardiogram.
- Stroke
- The sudden death of brain cells because of a lack of oxygen when blood flow to the brain is impaired by a blockage or rupture of an artery to the brain, causing neurological dysfunction.
- Thrombus
- An aggregation of blood factors, primarily platelets and fibrin with entrapment of cellular elements; frequently causes vascular obstruction at the point of its formation.
- Transient ischaemic attack
- A brain disorder caused by temporary disturbance of blood supply to an area of the brain, resulting in a sudden, brief (< 24 hours, usually < 1 hour) decrease in brain function.
- Unstable angina
- Angina pectoris (chest pain) in which the cardiac pain has changed in pattern or occurs at rest.
- Vascular disease
- Any disease of the circulatory system.
List of abbreviations
- ACC
- American College of Cardiology
- ACS
- acute coronary syndrome
- ACT
- anticoagulant therapy
- AF
- atrial fibrilliation
- AFASAK II
- Second Copenhagen Atrial Fibrillation, Aspirin and Anticoagulation Study
- AHA
- American Heart Association
- AMI
- acute myocardial infarction
- APT
- antiplatelet therapy
- ATT
- antithrombotic therapy
- CAD
- coronary artery disease
- CHADS2
- Congestive heart failure, Hypertension, Age ≥ 75 years, Diabetes mellitus, and prior Stroke or TIA or thromboembolism
- CI
- confdence interval
- CRD
- Centre for Reviews and Dissemination
- ESC
- European Society of Cardiology
- FFAACS
- Fluindione, Fibrillation Auriculaire, Aspirin et Contraste Spontané study
- GI
- gastrointestinal
- HF
- heart failure
- HTA
- Health Technology Assessment
- ICH
- intracranial haemorrhage
- INR
- international normalised ratio
- IPD
- individual participant data
- ITT
- intention to treat
- LV
- left ventricle/ventricular
- LVEF
- left ventricular ejection fraction
- MI
- myocardial infarction
- NASPEAF
- NAtional Study for Prevention of Embolism in Atrial Fibrillation
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- OAC
- oral anticoagulant
- ODTI
- oral direct thrombin inhibitors
- PCI
- percutaneous coronary intervention
- PETRO
- dabigatran with or without concomitant aspirin compared with warfarin alone in patients with non-valvular atrial fibrillation study
- RCT
- randomised controlled trial
- RR
- relative risk
- SE
- systemic embolism
- SPAF III
- Stroke Prevention in Atrial Fibrillation III study
- SPORTIF
- Stroke Prevention using an ORal Thrombin Inhibitor in atrial Fibrillation study
- TE
- thromboembolism/thromboembolic event
- TIA
- transient ischaemic attack
- TTR
- time in therapeutic range
- VKA
- vitamin K antagonist
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.