Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 14/167/01. The contractual start date was in January 2016. The draft report began editorial review in May 2019 and was accepted for publication in December 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Athimalaipet V Ramanan has received speaker fees/honoraria/consulting fees from Abbvie Inc. (North Chicago, IL, USA), Union Chimique Belge (Brussels, Belgium), Eli Lilly and Company (Indianapolis, IN, USA), Novartis (Basel, Switzerland), Roche Holding AG (Basel, Switzerland) and Bristol-Myers Squibb (New York, NY, USA). Paula R Williamson reports grants from the National Institute for Health Research (NIHR) Health Technology Assessment programme outside the submitted work and involvement with a clinical trials unit funded by NIHR.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Jones et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
In the UK, juvenile idiopathic arthritis (JIA) is the most common inflammatory disorder in childhood, affecting 10 : 100,000 children and young people (CYP) each year, with a population prevalence of around 1 : 1000. 1 JIA is a set of related disorders, all including chronic arthritis, with or without other, extra-articular, features, and with no other associated diagnoses, such as infection or other autoimmune multisystem disorders. It is difficult to assess arthritis in JIA, as active arthritis causes several symptoms or clinical signs. Pain in the joint is not always present in active JIA and can also be caused by many non-inflammatory conditions. Signs of arthritis can include joint swelling, tenderness, warmth and restriction of movement, and can be seen in varying degrees and combinations. However, some joints, such as spinal joints and the hips, are enclosed in bone or deeply hidden from direct touch, making it impossible to feel tenderness and swelling in these joints. Similarly, restriction of movement can be due to both acute inflammation and later joint damage.
The natural untreated outcomes of JIA are serious and disabling, but with modern treatment regimens, including biologic drugs, the prognosis has improved dramatically. However, there is no sustainably effective treatment or cure and some patients, and some individual joints, are still relatively resistant to treatment with available drugs. In addition, the ability to withdraw treatment fully without subsequent flare is possible in only about one-third of cases. 2,3
For these reasons, arthritis activity is measured using a combination of clinical variables known as a core outcome set (COS). These are variously composed of the Physician’s Global Assessment of Disease Activity (PGA), the number of active joints (both swollen and/or tender), the number of joints with limited movement, physical functional ability [measured with the validated Childhood Health Assessment Questionnaire (CHAQ)], self- and parent-reported disease activity [measured by the Patient/Parent Assessment of Global Assessment (Pa/PtGA)] and the erythrocyte sedimentation rate (ESR). These can be combined into the American College of Rheumatology Paediatric (ACR Pedi) responses4 or the Juvenile Arthritis Disease Activity Score (JADAS). 5 The ACR Pedi responses define relative symptom improvement and include the ACR Pedi 30%, the ACR Pedi 50%, the ACR Pedi 70% and the ACR Pedi 90%. To achieve the ACR Pedi 30%, the patient must exhibit improvement from baseline of at least 30% in three of any six variables in the COS, with no more than one of the remaining variables worsening by > 30%. ACR Pedi 50%, ACR Pedi 70% and ACR Pedi 90% improvement criteria are defined in the same way, but require improvements of 50%, 70% and 90%, respectively. These improvement measures have been used in clinical trials, but the considerable complexity of calculating improvement makes use of these measures difficult in real-time clinical practice. 6
Activity can also be measured by the JADAS using 71 joints, 27 joints or a maximum score of the first 10 active joints. The score is the summation of the active joint count, the PGA and the Pa/PtGA, with the ESR. 5 More recently, the clinical Juvenile Arthritis Disease Activity Score (cJADAS), without ESR, has been shown to be accurate and is simpler to score, and can be assigned in clinic at each visit. 6
The Childhood Arthritis Prospective Study (CAPS) (www.caps-childhoodarthritisprospectivestudy.co.uk; accessed 5 June 2020) is the largest incident cohort study of JIA worldwide, studying the management and outcome of JIA, and has delivered one publication on the health economics of treating arthritis7 among many clinical publications from the cohort. This health economic study found that the costs of clinician time are partly a reflection of the unpredictable nature of the inflammatory response and the clinical flares of arthritis, and the need for urgent reviews and treatment intensification.
Corticosteroids (CSs) have been used in the treatment of JIA since the 1950s. 8 It is well known that CSs can transform disease activity in JIA and that the majority of JIA patients receive CSs during their care. 9 However, the widespread clinical practice of using high-dose CSs for a limited period, to downgrade the inflammatory response with the aim of inducing initial remission, is not evidence based. CSs have been used in the treatment of JIA both at initial presentation and at subsequent disease flares, and they are also sometimes used to maintain long-term disease remission. 7 The intention of clinicians is generally to spare the long-term use of CSs, although actual practice differs, as demonstrated by the CAPS disease cohort data,7 and, in reality, CSs are still a very common treatment in JIA.
Minimising the perpetuation of chronic disease and the prevention of long-term damage from inflammatory presentations and disease flares in JIA are associated with treatment inductions, which cause early and complete remission. 10 Although disease-modifying anti-rheumatic drugs (DMARDs), especially methotrexate (MTX), are well established in the treatment of JIA, they are slow to act if used alone. This can leave the inflammatory process essentially unchecked for the 6–12 weeks11 that are required for MTX to work, with associated morbidity and amplification of the inflammatory response from under treatment. Biologic drugs may be highly effective in inducing remission in early disease, but there are no guidelines that include immediate use of these agents at diagnosis or as intermittent-pulsed treatments to control flares. This is because of a combination of the cost of the biologic drugs and the possible long-term, as yet unknown, safety issues. The reasonable reserving of biologic drugs to second-line treatment is well established in JIA. In adult rheumatoid arthritis (RA), treatment with additional disease-modifying agents, or increased doses of existing drugs added at frequent clinic review until remission is established, is known as treating to target. However, all such regimens include the use of CSs. 12–15 Increasingly, UK and international centres treating JIA are also adopting this treat-to target approach. 16
Routes of corticosteroid administration
Corticosteroids are currently administered by four routes: (1) orally, (2) by intravenous (IV) infusion, (3) by intramuscular (IM) injection or (4) by intra-articular injection; however, the only informative evidence base of effectiveness and efficacy in JIA is for intra-articular CS injections (IACIs). However, the above routes, either alone or in combination, are widely used on the basis that the initial systemic CS treatments suppress the severity of the inflammatory response and reduce the number of active joints that will eventually require IACIs. Many patients receive CSs by more than one route in a single flare, but there is no robust evidence base to guide the decision as to which route or dose to use.
In theory, and in clinical practice, there is no reason to use different routes of administration of CSs in new or flaring patients or in patients with different JIA disease subtypes. The fact that IACIs are widely used is, in part, because the oligoarticular subtype of JIA, in which four or fewer joints are involved, is the most common subtype. The more localised nature of the arthritis lends itself to IACIs, and it is thought that localised treatment may be associated with fewer systemic CS side effects and be more effective in suppressing local inflammation. However, there have been no comparative trials to test this hypothesis. In addition, disease flares, irrespective of subtype, may also be limited to a few joints at any time, making IACIs an attractive initial treatment in this case also.
The CAPS17 treatment data is invaluable for its real-world prospective information on the use of CS in JIA. Six UK centres recruited a total of 1477 new patients with JIA, of whom 759 were followed up for 3 years. Among this group, 340 (45%) received oral, IM or IV CSs. However, very few patients were treated with IM injections (n = 8) compared with oral CSs (n = 265), IV CSs (n = 191) or IACIs (n = 603) (noting that some patients received treatment by more than one route) (Professor Wendy Thomson, Deputy Director Arthritis Research UK Centre for Genetics and Genomics, University of Manchester, 2017, personal communication).
From discussions with physicians in specialty groups and international study groups we know that routes and doses of CSs in clinical practice are based on physician preference. For example, individual physicians may administer high-dose methylprednisolone IV infusions for 1–3 consecutive days on 1 or 2 consecutive weeks. Patients may then be changed to oral CSs, or IACIs may be used to treat individual joints that remain active. By contrast, in adult RA, treatment flares are often treated with IM injections of CSs. 18 In paediatric practice, some centres use the IM route only infrequently, but the reasons for this are not clear. Clinicians who use this route anecdotally describe good treatment responses and excellent patient acceptability, but the extent of such practice is currently not known. It is possible that the IM route is considered too painful for use in children, but this has not been formally studied. On the other hand, the IM route could provide better long-term remission, and is potentially the cheapest route with the lowest CS adverse event burden, but this has not been studied in JIA. Given these uncertainties, this study addresses the acceptability of including the IM injection modality in a final trial protocol.
We believe that it is important to understand why and how clinicians and patients together choose to use or, even more importantly, to reject different modalities of CS treatment in JIA. Given that there is no firm evidence base for CS use, that some CS delivery methods may be more costly or difficult to use than others and that CS doses vary greatly, it appears that the factors influencing choice may be linked to individual practice and experience, as well as to the availability of treatment facilities. However, there are pros and cons in terms of delivery experience, as well as risks and benefits for each type of treatment, and without an evidence base on efficacy and duration of effect of each modality it is not possible to make this a fully informed decision. Even if simple non-inferiority of one route of CS administration were demonstrated, other factors, such as acceptability, speed of delivery, cost of treatment and duration of effect, could justify the choice of an alternative route.
Intra-articular corticosteroid injections are also frequently used to control individual joint disease in patients with active JIA, with single or multiple injections being administered in a single treatment session, but the extent to which CS administered by this method is functioning as a de facto CS depot, affecting distant joints as well as acting directly on the injected joints, is not known. The IM injection depot route is considered to work in exactly this way. In some patients, multiple IACIs are administered repeatedly without the use of additional DMARDs. Upwards of 20 joints are injected at one time in some JIA patients. 19 IACIs are sometimes administered with conscious sedation (inhaled nitrous oxide), but multiple injections need to administered in theatre under general anaesthetic (GA), often with radiographic or ultrasound guidance. In some centres with poor availability of theatre space, this can lead to a long waiting list for treatment.
It is not known whether the best route to achieve long-term remission is the direct intra-articular route, which can be used in any inflamed joint, or IV infusion of a larger ‘pulse’ dose (rapidly effective but possibly with a shorter duration of action). Oral administration of a moderate dose may result in a smoother CS profile, but it is not known if the response is complete or if this route results in more long-term side effects (as the cumulative dose is nearly always higher). On the other hand, the high-dose IV infusion can produce immediate systemic side effects that may be more severe than those associated with lower-dose oral CSs. IM injection is intended to provide a slow-release depot of CS. IM injection, in contrast to IACI does not necessitate direct joint needling and as a result does not increase the volume/pressure of the joint cavity or lead to subcutaneous fat atrophy. In this study we elicited the views of patients and parents on these different delivery routes and investigated real-world data on the choice of the CS-dosing regimen used in different delivery routes.
It is not known if some subtypes of JIA respond better to some routes of administration than to others, or if the duration of action of CSs, in terms of suppression of inflammation, differs between routes. There is no evidence regarding the relative side effect profile associated with each route, considering the balance of the harmful effects of inflammation on the health of the joint and the well-being of the patients. This is especially pertinent given the evidence of the impact of JIA on general well-being. 20
Standardisation of JIA treatment could reduce the time taken to achieve disease control, which would in turn reduce the time to induction of remission. As is to be expected given the lack of an evidence base, JIA treatment rates and the choice of CS treatment regimen vary greatly. Consensus guidelines for the treatment of JIA are being produced by the European Union (EU)-funded Single Hub and Access point for paediatric Rheumatology in Europe (SHARE)21 but do not include the CS regimens to be used. The variability in CS regimens causes confusion and complicates analyses of outcome data from other studies, such as the biologic drug long-term safety registry studies (for example Kearsley-Fleet et al. ). 22
To our knowledge, there have been no studies of patient preference in the choice of CS administration route. There have not been any head-to-head studies of different routes of CS administration in induction regimens to assess non-inferiority in terms of efficacy, patient acceptability, pharmacokinetic (PK)/pharmacodynamic (PD) variables, overall CS burden and the frequency of CS-related side effects. In this study we asked patients, their families and health-care professionals (HCPs) about their experiences of treatment with the various modalities and their views on a possible drug trial.
Undertaking a definitive randomised controlled trial (RCT) is inherently costly. Therefore, undertaking a definitive study in an area where national practice is so varied, where stakeholder acceptability is unknown and without an agreed primary outcome poses a significant and unnecessary risk. Designing a trial to explore different routes of CS administration in patients with JIA is complicated by a number of factors, including differences in JIA subtype, differences in joints affected, whether initially or during flares, differences in disease severity, the wide variety of treatment choices and uncertainty regarding the willingness of units or patients to embark on a trial of well-established treatments. This study provides much needed information that will help to guide the design of any future such trial.
The Health Technology Assessment (HTA) Programme Commissioning Board concluded that a well-run feasibility study of CS induction in JIA is needed to justify a definitive trial. For example, the number of patients in each group may prove to be too small to justify a full trial and the number of treatment combinations and JIA subtypes must be clearly rationalised, something that we achieved using a consensus process.
To our knowledge, there have been no previous studies of patient preference in the choice of CS administration routes. Nor to our knowledge have there been any head-to-head studies of different routes of CS administration in induction regimens to assess non-inferiority in terms of efficacy, patient acceptability, PK/PD variables, overall CS burden and the frequency of CS-related side effects. In this study we asked patients, their families and HCPs about their experiences of treatment with the various modalities and their views on a possible drug trial.
Compliance with Health Technology Assessment commissioned brief
The HTA commissioned brief arose from the important clinical question ‘What is the best initial treatment to induce remission of JIA and which treatment should be used to manage significant disease flares?’.
A literature search and horizon scan for this application found only four interventional studies of CSs in JIA, all relating to IACI, only two of which were RCTs. 23,24 We identified no interventional studies and only 13 observational studies of other forms of CS treatment, with two prospective studies23,24 examining the current management of JIA, including oral CSs. Damage in JIA is the result of joint erosions that lead to cartilage loss and bony eburnation with resultant pain, functional disability and an increased need for early joint replacement. 25 Disorders of local bone growth and restriction of overall growth in height are frequent in inadequately controlled disease. 26,27 CSs would be used as part of most treatment to target or treatment regimens with tight control of inflammation in adults who have RA; however, in a relatively recent evidence summary it was concluded that there is a ‘near complete lack of published evidence’ for the use of systemic glucocorticoids in JIA. 28 In addition, Dueckers et al. 29 state that ‘there are no controlled trials and no standardised therapeutic regimens for the use of systemic glucocorticoids’. It is well known and widely reported that CSs are used frequently to induce remission of JIA. 30 Most clinical trials of therapeutic agents in JIA,, such as biologic drugs, have attempted to control for CS effect by controlling the allowed changes in CS dosing. However, no trials have directly compared the different CS induction regimens while controlling for other DMARDs and/or biologic agents.
Both patient/family treatment acceptability and physician decision-making processes play a large part in the choice of route of CS administration in JIA. A RCT comparing the different routes of CS administration is unlikely to succeed unless the reasons behind treatment decisions are understood and patients are willing to be randomised to different administration routes. There is a paucity of robust data to aid the decision as to which of the most commonly used CS regimens should be chosen as the comparator arm.
Safety, clinical and cost-effectiveness of corticosteroids in juvenile idiopathic arthritis
High-dose CSs and CSs given for protracted periods result in significant adverse drug reactions (ADRs), including:
-
reduction in growth in height
-
weight gain
-
facial puffiness
-
striae
-
acne
-
behavioural issues
-
sleep alteration
-
immunosuppression
-
increased blood pressure
-
hirsutism
-
propensity to diabetes
-
cardiovascular complications
-
osteoporosis.
Subcutaneous fat atrophy occurs in approximately 8% of individuals who receive CSs by intra-articular injection,31 but rates of ADRs associated with other routes of CS administration are not known. It is essential to optimise the CS dosage to maximise the benefit while minimising cumulative dose-related ADRs. However, the brief of this feasibility study did not encompass the collection of ADR rates; therefore, this is an issue to be discussed before carrying out a full trial. However, we know from consumer groups in the UK, including the National Institute for Health Research (NIHR) Clinical Studies Group (CSG) in paediatric rheumatology, that the issue of CS side effects is one of the top research priorities of CYP with JIA, as well as their parents and carers (personal communication). There are currently still no validated methods for scoring the severity of CS-related ADRs in inflammatory disease, and this is a factor that must be included in a future full trial proposal. There has been no systematic data collection of ADRs associated with different routes of treatment; however, this is an important part of the risk–benefit ratio needed to make clinical choices about treatment.
Corticosteroids have a significant effect on halting the radiological progression of rheumatoid arthritis. There are still large differences in doses, health-care costs and patient burden between the different CS treatment regimens across the UK. There are no studies involving head-to-head comparisons of CS treatment while controlling for other treatment modalities, such as DMARDs or biologic agents, although CSs are frequent concurrent medications in clinical trials in JIA.
Available evidence for the effectiveness of corticosteroids in juvenile idiopathic arthritis
-
A Cochrane review included 15 RCTs (1414 patients receiving CSs in the first 2 years of treatment). 32 A small RCT in 22 patients with systemic-onset JIA found that IV infusion of methylprednisolone in combination with low-dose oral prednisolone produced a better response than with oral prednisolone alone. 33
-
Data from a study of the treatment of JIA with IACI demonstrated that, when administered by intra-articular injection, triamcinolone hexacetonide (TH) was superior to triamcinolone acetonide (TA), with a longer duration of action and a lower relapse rate. 34
-
A British Society of Paediatric and Adolescent Rheumatology (BSPAR)-led audit of CS use in 2006 received data from three out of 12 tertiary paediatric rheumatology referral centres that were approached and two out of seven district general hospitals with paediatric rheumatology clinics that were approached. The results showed that, among 86 patients with all JIA subtypes receiving CSs in the previous 2 years, 68 (79%) were treated with IACIs and nine (10%) received oral CSs alone. Only one patient (1%) received IV CSs and two (2%) received only IM CSs, with the remaining 25 patients (29%) receiving CSs by a combination of delivery routes. In all 39 treatment episodes of IV infusion of methylprednisolone the doses used were uniform. Three patients (3%) received different doses and types of IM CSs. However, with such a low unit response rate (26%) to this audit, the results are not generalisable. The low response rate to the audit also highlights difficulties with busy units supporting clinical studies, something that this feasibility study sought to address.
It is possible that the long-term concurrent use of DMARDs or very expensive biologic drugs could be reduced or avoided in some patients by using instead repeated short courses of systemic CSs or multiple and repeated IACIs, but this has not been studied. The advent of DMARD and biologic treatment has led to an impression of a reduced role for CSs in JIA, but available databases such as CAPS show that CSs are still commonly used in JIA. The annual cost of the average biologic drug was > £10,000 in 2010,35 although the costs of most biologics used in JIA are now significantly lower owing to the availability of biosimilars. However, if even a few patients were prevented from requiring biologic treatment by satisfactory suppression of inflammation from timely CS doses with or without cheaper DMARDs, then the health-economic effect of evidence-based CS use could be marked.
Patient and public engagement/involvement
Patient and public involvement (PPI) has been an integral component of this study since conception. Preliminary PPI commenced prior to the study application, when PPI contributors ranked the study question as a key priority for young people and their families as part of the NIHR Clinical Research Network (NIHR CRN): Children/Versus Arthritis (Chesterfield, UK) Paediatric Rheumatology CSG problem/population, intervention/indicator, comparison, outcome (PICO) prioritisation exercise. In addition, young person and parent-led prioritisation exercises revealed that any CS treatment, the uncertainty of treatment scheduling/outcomes and treatment side effects were all major concerns for young people with JIA and their parents/carers.
During the study outline application process, discussions regarding the planning of the study were undertaken with the study co-applicant (SS), who is a young adult living with childhood-onset JIA and who subsequently helped to lead PPI in the overall study. The study co-applicant (SS) was involved in the submission of the outline application, and in the response to reviewers and the submission of the study application, which was subsequently approved and funded. SS was also involved in the management and oversight of the study, as a member of the study management group (SMG), while an external PPI contributor (CT), who is also a young person living with childhood-onset JIA, was identified to sit on the study steering committee (SSC). An external PPI co-ordinator (HB) was identified as an individual to support the study co-applicant in undertaking PPI activities as part of the study. PPI contributors were also provided with continuous professional development certificates after their attendance at the consensus meetings, to document their contributions.
The chief investigator (EMB) and study co-ordinator (GN) acted as mentors and liaison individuals for PPI contributors, although a positive working relationship was established between PPI contributors and other academic/clinical members of the study team, enabling an open, honest and transparent working relationship that was built on equality and respect to follow. Enabling PPI contributors to feel valued as part of a research team is integral to successful partnerships built on these qualities. PPI contributors were embedded within existing networks, such as the CSG, and so were able to liaise between external PPI contributors with an interest in the study.
Our PPI contributors provided valuable input into the design and conduct of the study across the different phases, including the literature review, qualitative study, consensus process and feasibility study. They were also involved in the submission process by seeking ethics approval for the study and by providing specific comments on topics relevant to young people and parents/carers taking part in the study. Specific activities that were related to the study design and conduct included the design of advertisement leaflets for young people, parents/carers and HCPs; reviewing participant information sheets; reviewing assent/consent forms; and the identification and refinement of potential outcome measures for inclusion, including outcome descriptions. PPI contributors also facilitated recruitment of participants to the study, as well as facilitating the identification of PPI members to take part in the consensus process.
Our PPI contributors were heavily involved in the consensus process, including consensus meetings that were held in July 2017 and December 2018. Simon R Stones and Heather Baguley co-delivered several of the presentations and sessions at both meetings and acted as named individuals for other PPI contributors in attendance. PPI contributors were also encouraged and supported by academic/clinical members of the study team to document the consensus meeting process and to contribute to the preparation of reports and dissemination of findings from the meeting(s).
In addition, PPI contributors were involved in advising on data analysis, for example in discussions about emergent themes from the literature review and the qualitative phase of the study. They were also heavily involved in the preparation and dissemination of study findings, including the co-authorship of conference abstracts and manuscripts, and the subsequent preparation and delivery of findings, such as conference posters. Simon R Stones also led the writing of conference abstracts and a manuscript about the first consensus meeting, seeking input from academic/clinical members of the team. PPI contributors also suggested conferences that would be suitable for presentation of the study findings and led on the translation of scientific findings into accessible plain language summaries, available to those who took part in the study, as well as to young people and parents/carers more broadly. This included the dissemination of findings on social media and among peer networks.
Finally, PPI contributors were involved in the writing of this report. This section was prepared by Simon R Stones and Chapter 6 was co-authored by Simon R Stones. Simon R Stones also reviewed all chapters of the report during the review process by the entire study team.
Important outputs of this feasibility study
Many RCTs find recruitment difficult if clinical teams are not involved in the development of study protocols and, therefore, are not committed to the study through the ownership of the study questions and the need for evidence. The design of this feasibility study was planned to maximise HCP ‘buy-in’ to a final RCT by adapting the protocol and outcome measure choice following the literature review, extensive surveys, qualitative interviews and structured survey and stakeholder consensus process of opinions and refining agreements in areas of difference. 36
A head-to-head RCT of different CS regimens is the eventual goal. However, because of the difficulties in achieving such a study, a detailed feasibility study was required to discover whether or not such a RCT is acceptable and achievable, and whether or not the results will be meaningful.
Irrespective of whether not the findings of this feasibility study suggest that a future full trial is possible, this study also generated significant outputs of value and impact to the wider national and international research community and to NIHR, in terms of, for example, the joint consumer and HCP choice of primary outcome, the treatment preferences and a wider UK paediatric rheumatology unit engagement with research by the active participation with the research question and protocol development.
Aims and objectives
We identified specific study aims with objective research questions underpinning each aim. There is currently a lack of consensus as to which regimen of CS induction should be used in children with various disease subtypes and severities of JIA.
Overall aim
The overall aim was to establish the feasibility and acceptability of conducting a RCT to evaluate the safety and efficacy of different CS regimens. We proposed a mixed-methods study with engagement of stakeholders [HCPs, patients and parents (PPI)] fundamental to each phase of the process.
The study delivered a review of published literature on CS outcomes in JIA; a prospective study of the actual number of JIA patients treated with CS (including doses and routes of administration); HCP- and PPI-informed (where possible) choice of control, intervention and outcome for a proposed RCT; and a prospective evaluation of change in chosen primary outcome over 12 weeks. Willingness to randomise (HCP) and willingness to participate (patients and parents) were estimated.
The overall feasibility study comprised several phases:
-
A review of the literature identifying candidate outcomes to be evaluated as a consensus primary outcome for a RCT.
-
An exploratory phase providing quantitative and qualitative information on current practice informing potential regimens to be tested –
-
A national e-survey of HCPs’ and UK paediatric rheumatology units’ current practice in CSs use to be used to define the treatment arms of a RCT and to identify the most common treatment to be used as the control arm of a future RCT. The survey asked about factors influencing choice of regimen, for example individual and unit treatment protocols, and experiences of response; outcomes measured and recording of ADRs, and factors influencing HCP choice of route of administration.
-
Qualitative interviews with patients and parents with a specific focus on acceptability of a full RCT of CS regimens, to allow in-depth discussion of views and experience of factors influencing individual treatment choice (including administration route) and willingness to be randomised.
-
-
A national survey and consensus process refining the primary outcome measure of induction of response for a proposed RCT.
-
A consensus stakeholder meeting – finalising key parameters, including inclusion/exclusion criteria, primary outcome, minimally important clinical difference and treatment arms.
-
An observational prospective feasibility study – identifying patients nationally with agreed eligibility criteria receiving current/proposed treatment arms with observational data on consensus primary outcome collected at baseline and after CS treatment and at 12 weeks, to inform estimate of sample size for a future RCT.
-
An overall feasibility study report – providing summary of key parameters to enable decision to be made by the HTA Commissioning Board on feasibility of a future RCT.
Research aim 1
The first aim was to establish the current practice for CS use in treating JIA in the UK. In particular, we aimed to obtain a snapshot of the numbers of patients with JIA of different degrees of severity who were attending hospital and requiring CS treatment, as well as the unit HCPs’ capacity to deliver a RCT.
Objective research questions (RQs):
-
RQ 1 – what types, routes and doses of CSs are used and in which JIA patients?
-
RQ 2 – what clinical criteria are used for commencing CSs and choosing the route of CS administration?
-
RQ 3 – what are the key issues/concerns with regard to capacity and capability in the conduct of a future RCT?
-
RQ 4 – how many potentially eligible CYP with JIA of different degrees of severity attending hospital in the UK require CS treatment and could be randomised in a comparative treatment study?
To fulfil the commissioning brief, this report will characterise current practice and inform an estimate of eligible patients for a future RCT.
Research aim 2
The second research aim was to determine the control, intervention and patient group(s) for a future RCT and to establish HCPs’ willingness to randomise and the likely consent rate.
Objective RQs:
-
RQ 5 – what characteristics would HCPs and parents/patients want to see included in a future RCT of CSs in JIA? Which patients should be included/excluded? What would be the most appropriate control group in a future trial? How would active disease or a disease flare be defined?
-
RQ 6 – how willing would patients/parents be to consent to be randomised in a future clinical trial and how willing would HCPs be to randomise?
-
RQ 7 – how would patients’/parents’ preference for mode of CS delivery influence their willingness to participate in a future RCT?
To fulfil the commissioning brief, this report will identify the clinician- and patient-directed control and intervention for a RCT and inform randomisation and consent rates in a RCT.
Research aim 3
The third research aim was to choose the primary outcome for use in a future clinical trial of CSs in JIA.
Objective RQs:
-
RQ 8 – what primary outcome is important to HCPs?
-
RQ 9 – what primary outcome is important to parents/patients?
-
RQ 10 – what would a minimally important clinical difference be for any potential primary outcome?
To fulfil the commissioning brief, this report will identify the primary outcome.
Research aim 4
The fourth research aim was to conduct a prospective observational study of newly diagnosed patients with JIA who fulfil the proposed inclusion/exclusion criteria and who naturalistically receive the proposed control or treatment arms, and to observe change and variance in the proposed consensus primary outcome over a 12-week period to inform the precision of the sample size calculation.
To fulfil the commissioning brief, this report will inform the sample size estimate for the RCT and characterise further the estimate of eligible patients for the RCT.
Research aim 5
The fifth research aim was to develop a report for the HTA programme on the feasibility for a definitive study defining the design, control and intervention arms, with recommendations to the inclusion and exclusion criteria, primary outcome, sample size based on the primary outcome and subtypes of JIA to be included.
Research methodology
This mixed-methods study included:
-
A national structured survey on current treatment practice in JIA was carried out among stakeholders, including HCPs (paediatric rheumatologists, paediatricians with a specialist interest in rheumatology, adolescent rheumatologists with expertise in JIA and specialist nurses in paediatric rheumatology), combined with the results from the unit screening log. The survey asked about –
-
Current practice including criteria for starting CSs, the proportion of patients with JIA receiving CSs, the timing of reviews, dosing criteria with any systemic CS reduction regimens, as well as the number receiving more than one CS modality.
-
Capability including the proportion of Good Clinical Practice-trained nursing/medical staff, out-of-hours consultant/research nurse and clinical nurse specialist cover, number of available day-case facilities for in-hospital CS delivery by the IM injection, IV infusion and IACI routes (e.g. occupancy, staffing ratios).
-
Acceptability, in broad terms, of a RCT on the use of each of the four CS delivery methods in different JIA subtypes and patient age groups. This component also assessed the barriers perceived by HCPs (identified using an online survey) to accepting a treatment regimen as a trial arm when this is not part of the current treatment choice for the team.
-
-
Determining the choice of primary outcome and CS treatment regimen for the future clinical trial in JIA through:
-
Review of the literature, review of the latest revision of the European Medicines Agency guideline on JIA trial design, review of the outcomes of the SHARE conclusions.
-
Stakeholder consultation through –
-
– A survey and stakeholder consensus process, including parents, patients and HCPs, to achieve consensus on the primary outcome, inclusion/exclusion criteria and treatment modalities.
-
– A stakeholder meeting [using formal consensus techniques including the Grading of Recommendations Assessment, Development and Evaluation (GRADE) scoring] to present and discuss the findings from the structured survey on the parameters of the proposed trial. A joint HCP and consumer/PPI consensus meeting to determine the primary outcome measure, acceptability of the CS routes and the treatment decisions around choice of CS induction regimen and aspects of the feasibility trial design including type and timing of intervention and barriers to recruitment.
-
-
A qualitative study of patient/parent views and experiences of the use of CSs and of a future trial involving randomisation between delivery routes. Patients (≥ 8 years) and parents were sampled from the co-applicant centres where one or more of the delivery routes were used.
-
-
An observational prospective feasibility study was conducted. This collected primary outcome and treatment data on newly diagnosed, or flaring, JIA patients receiving CS treatments. The study focused on the JIA disease subtype, the doses and routes given and on data relevant to the primary outcome at baseline and at 6 weeks and 12 weeks post commencement of the CS therapy, with assessment of a priori definitions of remission, from changes in primary outcome measure over the 12-week study period. The number of newly treated or flaring patients with CSs in each JIA subtype was determined to allow for reliable power calculations to be made for a potential future randomised trial.
-
Preparation of a project report has included the basic consideration of whether or not a definitive trial is truly feasible based on defining the appropriate eligibility, sample size, primary outcome and choice of CS interventions and the route for the control arm, based on 1–3 above. In addition, the willingness of HCPs to consider enrolling patients into such a trial, and for patients to be enrolled, was a central factor in defining whether or not a formal trial could succeed. Unit buy-in and clinician enthusiasm for such a study was also explored in combination with practical questions such as study set-up times and times for site regulatory approvals at participating NHS trusts.
Chapter 2 Literature review on the use of corticosteroids in juvenile idiopathic arthritis
Introduction
The ultimate goals in managing JIA are to prevent or control joint damage, to prevent loss of function and to decrease pain. JIA is a chronic disease that is characterised by periods of remission and flare. Treatment is aimed at inducing rapid remission while minimising toxicity from medications, in the hope of inducing a permanent remission.
The standardised outcome measures used during the management of patients with JIA are of importance for both clinical care and research. To date, to our knowledge there have been no controlled trials on the use of intra-articular (IA) CSs versus systemic agents in children with JIA. To our knowledge, there are also no controlled trials on the dosage and route of systemic CSs (oral vs. IV). Outcome measures used in clinical trials in JIA to date have used the ACR Pedi responses,4 but these are hard to calculate in clinical practice.
Aim and objectives
Our aim was to undertake a literature review on the use of CSs in children with JIA. Our objectives were to identify whether or not there is a standardised use of CSs published in the literature and also to identify the outcome measures used in these JIA studies. This activity was a crucial part of the method to determine an agreed outcome measure(s) for the planned prospective feasibility study SIRJIA (Study of the Induction of Remission in Juvenile Idiopathic Arthritis).
For the purpose of this review, we included the articles published in English from 2000 to May 2016. We searched in the databases CINAHL, the Cochrane Library, EMBASE, MEDLINE, the Turning Research into Practice (TRIP) database, International Clinical Trials Registry, UK NIHR CRN study Portfolio and the Australian clinical trials register.
Methods
We carried out a literature search in the above databases for articles published from 2000 until May 2016 in English, using the following PICO format:
-
patient, population – CYP with JIA
-
intervention – treatment with CSs
-
comparison – not applicable
-
outcome – remission, reduction in disease activity, JIA core outcomes and JADAS.
We also gathered information from the referenced articles with the intention of obtaining as much evidence as possible from the literature regarding the use of CSs in JIA, and the outcome measures used.
A further literature search in MEDLINE and EMBASE was carried out by the clinical librarian to include on any additional studies published from 2016 to 2018. As the literature review was not systematic, Preferred Reporting Items for Systematic Reviews (PRISMA) or its extensions do not necessarily apply.
Results
The combined electronic searches identified 272 records from eight databases, of which 25 records were removed after accounting for duplicates, leaving 247 records for further consideration. The titles and abstracts of the remaining records were screened for eligibility using the PICO screening tool, and 101 full-text papers were found to be potentially eligible for inclusion. The abstract and methodology sections of these articles were further analysed, and subsequently 54 articles contributed to the results outlined in this paper. In addition, references from the original articles, including expert opinions and review articles, were also included in the results despite being published prior to the study period (systematic reviews, n = 17; evidence summaries, n = 13; research articles, n = 103; case reports, n = 2; surveys, n = 2; expert overviews, n = 18).
The flow chart in Figure 1 illustrates the study selection process.
FIGURE 1.
Flow diagram of the literature review.
Synthesis of evidence
Intra-articular corticosteroids
The use of IACIs for the treatment of inflammatory arthritis in adults was initially reported by Hollander et al. in 1951. 37 The use of IACIs in children was first noted anecdotally in 1979,38 and the first prospective evaluation of IACIs in the management of chronic arthritis in children was reported by Allen et al. in 1986. 39 In that prospective study, 53 knees were injected in 40 children with chronic arthritis and responses were evaluated at 6, 12 and 24 months for good clinical response, relapse and time to relapse. All knees responded well to treatment, with 36.7% relapse. IACIs appeared to be a safe and effective treatment option for the management of JIA, particularly the management of oligoarticular JIA. 40,41 IACIs are an effective way to control disease activity and to induce resolution of synovitis, decrease the presence of joint and limb deformities, improve function, provide pain relief and serve as an adjunct to the longer-term, first-line DMARD therapy, MTX. Various technical aspects, different formulations of CSs, duration of disease at treatment, concomitant use of other medications and disease severity might impact the efficacy of IACIs, although conclusive data that elucidate these factors are scarce. 42
A systematic review to evaluate the advantages of administering CSs during arthrocentesis for temporomandibular disorders, including JIA, found no significant differences in the clinical outcomes observed between the CS injection group and the control groups (which were injected with normal saline, physiological salt water or Ringer’s lactate). 43
Ravelli et al. 44 conducted a multicentre, prospective, randomised, open-label trial that compared the effects of IACIs with IACIs plus oral MTX (15 mg/m2, maximum 20 mg). The CS used was TH (for shoulder, elbow, wrist, knee and tibiotalar joints) or methylprednisolone acetate (for subtalar and tarsal joints). The trial reported that concomitant administration of oral MTX did not augment the effect of IACI therapy.
Comparative effects of different corticosteroids in intra-articular injections
A double-blinded study of adults with rheumatoid arthritis of the knee found that intra-articular injection of TH resulted in significantly greater initial and more lasting improvement in joint inflammation than prednisolone t-butyl acetate or methylprednisolone acetate. 45 In the first double-blinded comparison of CS preparations reported in children, TH was found to be superior to betamethasone in reducing knee swelling and stiffness in children with oligoarticular JIA. 46 Honkanen et al. 47 compared the effect of IACIs in the knee in 45 children with JIA who were administered 1.5 mg/kg methylprednisolone acetate and 34 children injected with 0.7 mg/kg TH, and found a significantly higher remission rate (p < 0.005) in the TH group. Zulian et al. 48 found TH to be superior to TA, even when the TA dose was doubled from 2 mg/kg to a maximum of 80 mg. The joints in patients injected with TA relapsed first in 53.8% of the joints injected, compared with only six (15.4%) of the joints injected with TH. Eberhard et al. ,49 who carried out a retrospective study of 227 joints, reported a longer time to relapse with TH than with TA, with the mean time to relapse being 10.14 ± 0.49 months and 7.75 ± 0.49 months in the TH group and the TA group, respectively. The updated literature search identified a report of TA use in recent practice in India owing to the easier availability of the drug. 50
The duration of response to IACI is dependent on the actual CS used, with less soluble preparations providing a longer duration of response. The greater efficacy of TH in IACI can probably be explained by its PD properties: it is less soluble and absorbed more slowly than TA; thus synovial levels are maintained for longer and systemic corticoid levels are reduced. 51 As a result of this study, TH has been recognised among paediatric rheumatologists as the medication of choice for IA administration of CYP with JIA.
Dosage regimen for intra-articular corticosteroids
The dosage regimen for TH currently used by the majority of UK paediatric rheumatologists is 1 mg/kg for large joints (i.e. knees, hips and shoulders) and 0.5 mg/kg for medium-sized joints (i.e. ankles, wrists and elbows). 52 There is more variability in the smaller joints in the hands, where methylprednisolone acetate is recommended. 52 In addition, TH is recommended for the hands and feet at 1–2 mg per joint for metacarpophalangeals/metatarsophalangeals and 0.6–1 mg per joint for proximal interphalangeals. 52
Paediatric rheumatologists frequently administer multiple IACIs in a single episode of treatment of JIA, while simultaneously initiating therapy with a DMARD and/or biologic/biosimilar agents. This strategy, the so-called ‘bridge’ effect, is regarded as an alternative to systemic CSs. The aim is to induce prompt remission of synovitis, that is to achieve a quick control of inflammatory symptoms, while awaiting the full therapeutic effect of a DMARD or biologic/biosimilar medication. 53
Use of systemic corticosteroids
Systemic CSs, that is CSs administered orally or by IV infusion or IM injection, are used in the treatment of children with acute severe arthritis, both around the time of presentation and during disease flare. Systemic CSs have been used mainly for the treatment of children with polyarticular or systemic JIA. However, to date, there are no standardised protocols or guidelines and no RCTs comparing the effect of various forms and dosages of systemic CSs to treat children with systemic JIA. It is important to note that the choice of CS in systemic JIA is likely to be influenced by the tendency for this type of JIA to be complicated by potentially life-threatening macrophage activation syndrome.
Michels54 published the results of a survey conducted among paediatric rheumatologists from North America, Israel, Australia and Europe. The survey, utilising a standardised questionnaire, elicited rheumatologists’ personal definitions of low-dose, long-term CS treatment of JIA. The results obtained from 99 respondents revealed that paediatric rheumatologists’ definitions of low-dose, long-term CS therapy varies within a wide range. The dosage that was still considered low was 0.26 ± 0.14 mg/kg/day prednisolone (minimum–maximum, 0.04–0.5 mg/kg/day). Reported dosages were higher in northern Europe (0.29 ± 0.12 mg/kg/day; n = 9), western Europe (0.42 ± 0.14 mg/kg/day; n = 7), southern Europe (0.30 ± 0.14 mg/kg/day; n = 9), eastern Europe (0.25 ± 0.14 mg/kg/day; n = 6) and North America (0.33 ± 0.17 mg/kg/day; n = 16) than in central Europe (0.19 ± 0.09 mg/kg/day; n = 43).
The ReACCh-Out study55 was a multicentre, prospective, inception cohort study of CYP with JIA who were diagnosed within 12 months before enrolment. Prospective data were collected at enrolment, 6-monthly for 2 years and then annually. Initial data published in 2010 showed that oral prednisolone was used most often in CYP with systemic JIA and rheumatoid factor (RF)-positive polyarticular JIA. 23 The probability of instituting systemic CSs within 6 months of diagnosis was 79% and 61% in systemic JIA and RF-positive polyarticular JIA, respectively. 55 The literature includes a case report of an adolescent with systemic JIA that responded to oral CSs56 and a report of CS-dependent refractory systemic JIA. 57 The reports do not include the details of all the dosing regimens used.
The Childhood Arthritis and Rheumatology Research Alliance (CARRA) developed standardised consensus treatment plans (CTPs) for systemic JIA with the goal of comparing their effectiveness (in achieving clinically inactive disease) using data collected for the CARRA registry. 58 The physicians chose the actual CTPs for each of their patients with systemic JIA and an electronic survey was sent to voting members of CARRA regarding CTP choice for CYP with new-onset systemic JIA in whom treatment with non-steroidal anti-inflammatory drug (NSAID) monotherapy had failed. Respondents were asked to select one or more of the following CTPs for each clinical case scenario: (a) systemic CSs only, (b) MTX (with or without CSs), (c) anti-interleukin 1 (IL-1) therapy (with or without CSs) or (d) anti-IL-6 therapy (with or without CSs). Respondents could choose more than one CTP if factors such as insurance limitations or family preference might affect treatment. The results showed significant variability in systemic JIA treatment approaches, no clear standard of care among CARRA members58 and widespread use of MTX and CSs. MTX use increased with more arthritis features. However, MTX and CSs are frequently used, regardless of presenting disease features. Overall, concurrent CS use was indicated by the majority of respondents across all CTPs.
In a prospective cohort study (TREAT) of 85 children with polyarticular JIA, patients were randomised blindly to MTX, etanercept or rapidly tapered prednisolone and assessed for clinically inactive disease (CID) over 1 year of treatment. 59 Patients starting on methylprednisolone acetate achieved CID earlier, and had more study days in CID, than those starting MTX, but the differences were not significantly different.
Uveitis is the one of the most severe complications of JIA. There are studies60,61 reporting the use of systemic CSs for severe uveitis associated with JIA, and these were included in this literature review, as uveitis may be an indication for CSs in JIA in the absence of any specific joint indication. It was felt that evidence for the use of systemic CSs to treat uveitis could usefully supplement the evidence regarding response to different modalities of treatment as either arthritis or uveitis could be the indication for CS treatment at different times and convergence of treatment plans could be helpful. However, the evidence base of use of systemic CSs in uveitis is also limited. Marvillet et al. 60 conducted a retrospective analysis of records of 70 children with JIA. Severe ocular involvement necessitated systemic CS therapy in 29 (42.0%) patients. 60 Among immunomodulating agents, MTX and cyclosporine were used in 41 patients and tumour necrosis factor α antagonists were used in 15 patients. Twelve patients (17.4%) achieved complete resolution of uveitis, whereas 14 (20.3%) experienced a relapsing course and in 23 (33.3%) uveitis became chronic, with relapses as soon as the treatment was decreased and 21 (30.4%) had a severe course. Nouair et al. 61 reported use of CSs in 41% of patients in a retrospective analysis of children with non-infectious uveitis who were followed between 2004 and 2013.
With regard to IV infusion of CSs in JIA, the literature search revealed little of note apart from a single case report of a patient with systemic JIA57 in whom a methylprednisolone succinate pulse of 1 g for 2 days was followed by a maintenance dose of prednisolone of 1.3 mg/kg/day.
The literature review revealed a lack of standardised dosing regimens for either induction or maintenance therapy and no evidence of tapering doses of CSs.
In the STIVEA trial,62 adult patients with very early inflammatory polyarthritis (4–10 weeks’ duration) were randomised to receive three IM injections of 80 mg of either methylprednisolone acetate or placebo, administered at weekly intervals. 62 Assessments were carried out monthly for the first 6 months after the first injection, with a final 6-month review at 12 months after the first injection. Patients in the placebo group (76%) were more likely to need DMARDs during the first 6 months of the trial than patients in the CS group (61%) [adjusted odds ratio (OR) = 2.11, 95% confidence interval (CI) 1.16 to 3.85; p = 0.015). Disease activity did not differ between the two groups at 12 months. After 12 months, arthritis had resolved without the need for DMARDs in 9.9% (11/111) of patients in the placebo group and in 19.8% (22/111) of patients in the CS-treated group (adjusted OR = 0.42, 95% CI 0.18 to 0.99; p = 0.048). We found no RCTs of the use of IM CSs in children with JIA.
Corticosteroid side effects
There is a paucity of studies in the literature to guide practitioners in counselling CYP about the anticipated weight changes and side effects following CS initiation in JIA.
In a prospective study63 that examined CS-related changes in body mass index among CYP with rheumatic diseases, the median starting CS dosage was 1.3 mg/kg/day prednisone equivalents. However, the study participants included those with other rheumatological diagnoses and only 38% of the study participants had a diagnosis of JIA.
Cleary et al. 40 reported that it is likely that multiple IACIs (10 or more joints, including large joints) result in sufficient systemic absorption of CS to produce a Cushingoid appearance. The clinical features of Cushing’s syndrome can include a moon-shaped face, centripetal adiposity, supraclavicular fat pad accumulation, bruising, striae and proximal myopathy, but adverse effects can also be subclinical or overlap with those of other medical conditions. There is an increased risk of developing diabetes or hypertension and a predisposition to opportunistic infections. 57 Huppertz and Pfüller64 reported transient suppression of cortisol release detected by a low morning peak value of salivary cortisol. No adverse events were recorded secondary to this transient adrenal suppression.
There is a case report of severe acneiform rashes in two adolescents with JIA following bilateral knee IACI of TH. 65
Valta et al. 66 reported a prospective study evaluating bone health and growth in 62 CYP with JIA. No correlation was found between the bone mineral density and the disease characteristics or cumulative CS dose. 66 This study showed low prevalence of osteoporosis and normal growth in CYP with JIA. However, the study population was small and this question warrants larger controlled trials.
Outcome measures used in juvenile idiopathic arthritis studies
We analysed outcome measures used in studies that were identified by our literature review. The most widely used primary outcome measures that were identified were the number of active and restricted joints, the number of flares, medications, ESR, JIA core outcome variables, CHAQ, visual analogue scale (VAS), Pa/PtGA and PGA and clinical remission (based on clinical examination, ACR Pedi 30 or ACR Pedi 70).
Esbjörnsson and colleagues measured gait dynamics using three-dimensional gait analysis and foot-related disability using the Juvenile Arthritis Foot disability Index in their study of the effect of IACIs on foot and walking function. 67 In the BSPAR etanercept study,68 JADAS, ACR, Juvenile Arthritis Disease Activity Score, 71-joint count (JADAS-71), ACR Pedi 90 and minimal disease activity at 1 year were measured.
There is limited literature regarding the use of outcome measures in systemic JIA specifically. The main outcomes used are clinical inactivity with no arthritis, no fever, rash, serositis and generalised lymphadenopathy. In studies specifically of temporomandibular joint arthritis, jaw pain or dysfunction, maximal incisal opening distance and magnetic resonance imaging-detected inflammation have been used as outcome measures. The Disease Activity Score-28 was used in one study. Other measures identified include duration of morning stiffness, knee joint circumference and knee joint flexion in degrees.
There was no evidence of any PPI involvement in the choice of outcome measures used in any of these studies.
Limitations
Our review was a scoping review of literature on the use of CSs rather than a systematic analysis. Articles in languages other than English were not included in the study.
Discussion
There is good evidence for the use of IACIs in CYP with JIA, leading to grade A recommendations for use. There is reasonable evidence supporting the efficacy of systemic CSs in CYP with systemic JIA, polyarticular JIA and JIA-associated uveitis; however, the optimal mode of administration remains unclear. In children with polyarticular JIA, IACIs are more often used as a bridging therapy while waiting for the disease to show a complete response to DMARD agents and biologic therapy.
No standardised dosing regimen for either induction or maintenance therapy, or any defined tapering dose of CS, is available in the literature. It appears that there is widespread variation in the use of CSs among different clinical settings and centres. There is very little evidence regarding CS treatment regimens and, in particular, there is no good evidence of the relative efficacy and tolerability of oral and IV modes of CS administration.
It is of note that this review is limited to published clinical trials, as would be expected when attempting to establish evidence-based practice. However, one cannot ignore the fact that, historically, in the field of clinical medicine, reasons for favouring certain methods of treatment over others may be educated by individual experiences. These experiences contribute to herd opinions among HCPs. Patient preferences may change with time, and knowledge of the pathogenesis and evolution of a chronic disease may lead to a different treatment focus at different times. A good example of this is macrophage activation syndrome, which is now diagnosed much more frequently in the systemic JIA subset because awareness has increased. CS treatments are now more intensive in an attempt to prevent the high mortality rate associated with this disorder.
There is a clear and pressing need for future studies comparing the efficacy, toxicity and tolerability of different CS treatment regimens (multiple IACIs, oral and systemic routes of administration). The length of follow-up of a future RCT needs to consider both short-term efficacy and longer-term outcomes, such as future flare rate, as demonstrated by several of the reviewed studies.
Chapter 3 Families’ views on a proposed randomised controlled trial of corticosteroid induction regimen in juvenile idiopathic arthritis: qualitative study
Some excerpts in this chapter are adapted from Sherratt et al. 69 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Introduction
The number of RCTs being initiated for JIA is increasing. 70 Recruitment of patients is crucial to the success of such trials; poor recruitment can inflate costs,71 result in underpowered trials and lead to potentially effective interventions being abandoned or their introduction delayed. 72 Approximately 45% of multicentre trials in the UK do not achieve their original recruitment target, which often results in costly recruitment extensions. 73
Patient recruitment can be especially challenging in paediatric studies and in relatively uncommon conditions, such as JIA. 74,75 Compared with trials in adult rheumatology, informed consent can be more complex in paediatric studies owing to the need to involve both child and parents in enrolment decisions. 76 In addition, some treatments may be poorly tolerated by younger children,77 leading to high drop-out rates. Innovative trial methodology and involving families in the design of JIA trials have the potential to speed up the trajectory towards making evidence-based treatments available to young patients. 78,79
Increasingly, researchers are also using qualitative methods to explore the feasibility of trials from the perspective of patients and to proactively identify and address problems that may undermine a future trial. 80,81 Early-stage feasibility studies without a randomised component, such as the one we report here, are a distinct subtype of feasibility study. These are often used to resolve fundamental questions, such as which treatments to compare or uncertainties about the ‘in principle’ acceptability of a trial to patients and recruiters before committing resources to a pilot or full randomised trial.
Although studies involving CYP are advocated in the literature,82,83 we are aware of only one early-stage feasibility study84 that qualitatively accessed the perspectives of both parents and CYP to inform the design and conduct of a trial. This recent study focused on the experiences of CYP with acute osteomyelitis or septic arthritis, whereas the current study explores the perspectives of CYP with JIA. As JIA is a long-term condition, CYP with JIA may find trial participation especially challenging as a result of the high level of burden of their condition,82 which often requires patients to attend for frequent blood tests and reviews, or as a result of their previous treatment experiences. This study aims to shed light on such issues.
This chapter explores what an early-phase qualitative feasibility study that involves interviews with both parents and CYP can tell us about the viability of a proposed JIA trial to investigate CS induction therapy regimens.
Aims and objectives
The overarching project had several aims and objectives, as detailed elsewhere. In particular, the qualitative study aimed to address aspects of research aims 2 and 3:
-
research aim 2 – to determine the control, intervention and patient group(s) for a future RCT and to establish HCPs’ willingness to randomise and the likely consent rate
-
research aim 3 – to choose the primary outcome for use in a future clinical trial of CSs in JIA.
Specifically, the aims of the qualitative study (see Appendix 1, Figure 4) included:
-
establishing the characteristics that parents and patients would want to see included in a future RCT on CSs in JIA
-
exploring how willing patients and parents are to consent to be randomised in a future RCT
-
exploring whether or not patients’ and parents’ preferences for mode of CS delivery influence their willingness to participate in a future RCT
-
establishing which treatment outcomes are most important to parents and patients with JIA to inform the selection of a primary outcome for use in a future RCT.
Methods
Qualitative research methods
We adopted a qualitative approach involving semistructured interviews. Qualitative research provides in-depth insights into participants’ experiences and perspectives. Such studies are typically characterised by smaller sample sizes but many rich data. 83 More specifically, qualitative research has been used to identify and address key uncertainties in planning and designing proposed trials. 80 Semistructured interviews are well suited to exploring the patients’ perceptions of complex and sensitive issues,85 as they enable families to raise matters of importance to them and enable interviewers to inform families about trials and to clarify any misunderstandings.
A Research Ethics Committee (REC) in the north-east of England (the North East – Newcastle and North Tyneside 2) approved this study (16/NE/0047).
Participant selection
Clinicians from four paediatric and adolescent rheumatology centres in the UK approached the families of CYP who met the eligibility criteria (Box 1), briefly described the study and requested verbal permission for a researcher to contact them. CYP were required to have a confirmed diagnosis of JIA in line with current guidance. 86,87 Frances C Sherratt and Louise Roper, both experienced in qualitative research in health settings, conducted the interviews after seeking informed consent from parents and assent or consent from CYP. Interviews took place from August 2016 to March 2017 and were audio-recorded and transcribed. The research team monitored sampling characteristics to ensure that the sample was inclusive of patients of varying ages, time since diagnosis/flare and CS delivery route experience. Sampling for interviews ceased when data saturation was reached, that is when further interviews were no longer contributing new information. 88
-
CYP were aged ≤ 16 years.
-
CYP had received a clinical diagnosis of JIA in line with current practice. 86,87
-
CYP had recent experience (≤ 12 months) of at least one out of four CS regimens: (1) oral prednisolone (tablets), (2) IV methylprednisolone, (3) IACIs (triamcinolone hexacatonide) or (4) IM depot methylprednisolone.
Interview protocol
Semistructured, topic-guided interviews with CYP and parents covered the topics identified in Box 2 (see Report Supplementary Material 1 for an example of the topic guide for parent interviews). These interviews were conversational to allow participants to raise and reflect on matters of concern to them. Although we interviewed all parents regardless of their child’s age, only children who were aged ≥ 8 years were interviewed. We developed separate topic guides for parents, children (8–12 years) and young people (13–16 years) and adapted these throughout the study, guided by the ongoing analysis. We also developed stimulus materials to facilitate the interviews, including a video, a flow chart that illustrated the proposed trial and prompt cards that detailed potential benefits and side effects of the four CS delivery routes (see Report Supplementary Material 2). Consultant paediatric rheumatologists advised on the development of the prompt cards. These cards listed key points about the delivery routes that the rheumatologists perceived as important. The cards were designed to facilitate discussion in the interviews, rather than to provide an exhaustive list of benefits and side effects of the delivery routes.
In brief, the proposed trial was described to participants as comparing four CS delivery routes (hereon referred to as treatments): (1) oral prednisolone (referred to herein as tablets), (2) intravenous methylprednisolone (IV), (3) triamcinolone hexacatonide intra-articular CS injection(s) (IACIs) and (4) IM depot methylprednisolone. These four treatments are widely used in clinical practice to treat JIA, although the only informative evidence base of effectiveness and efficacy is for IACIs. 40,89 Referring to the prompt cards, the interviewer described the four delivery routes to families, including the process of delivery (e.g. duration) and the potential pros and cons. Although a trial could help to establish which of the treatments is most effective in treating JIA, the feasibility of such a trial is uncertain.
-
Patient’s initial symptoms
-
Receiving a diagnosis and initial treatments offered
-
Decision-making and experiences of initial treatments
-
CS delivery routes
-
Experiences of delivery routes (good and bad points)
-
Perceptions of delivery routes (good and bad points)
-
Delivery route preferences based on experiences and perceptions
-
Experienced/anticipated treatment outcomes
-
Description of proposed RCT
-
Understanding of RCT
-
Acceptability of randomisation
-
Willingness to participate
-
Other factors that might influence willingness to participate
-
Providing further information on pros and cons of delivery routes
-
Treatment acceptability
Qualitative analysis
We drew on the framework method,83 an approach to the thematic analysis of qualitative of data,90 and used NVivo 10 (QSR International, Warrington, UK) to assist with data indexing and coding. Frances C Sherratt, Louise Roper and Bridget Young initially read a selection of transcripts and discussed the developing analysis. Frances C Sherratt and Louise Roper double-coded approximately 10% of transcripts, discussing divergences and their resolution, leading to the development of a preliminary coding framework. The remaining transcripts were coded by either Frances C Sherratt or Louise Roper, and further data analysis meetings were organised involving the research team (BY, LR, and FS) throughout the course of analysis to discuss convergences and divergences, identify quotes for report writing and agree when data saturation had been reached.
In quantitative research, numbers contribute to the persuasive ‘power’ of the findings, whereas in qualitative research, the words of participants are essential to enable the reader to judge whether or not the research team’s interpretations of the data are grounded in the experience of the participants. 91 The research team discussed and selected participant quotes for this report to illuminate and substantiate the researchers’ synopsis of the themes identified through systematic data analysis. 92,93 A draft report of the analysis that contained extensive data extracts was circulated to the wider study team. This enabled investigator triangulation helping to ‘test’ and refine the analysis. Participants were sent a summary of the study findings.
Results
Participants
All 26 eligible families, who were identified by clinicians, agreed to researcher contact. Of these, nine (35%) could not subsequently be contacted and two (8%) declined trial participation. Twenty-eight participants from 15 families completed or attempted an interview (58% response rate), comprising nine patients and 19 parents (Table 1). In one of the 28 interviews, we were unable to sufficiently engage the interest of one of the patients (C8_8–10y) in discussing the concepts that we wished to explore. As no meaningful data were obtained, this patient’s interview was not transcribed or analysed, although their parents’ interview was. Another patient’s interview (C11_8–10y) was unusually short (6 minutes) because the family had limited time available, but the patient’s mother was also interviewed and both interviews were analysed. Excluding the outlier interview (C11_8–10y), interviews lasted from 23 to 76 minutes (median 43 minutes, interquartile range 35–54 minutes).
| Family number | Parent interviewed | CYP age (years) | CYPs’ JIA status (reported by clinician) | Treatments experienced by the child/young persona | |||
|---|---|---|---|---|---|---|---|
| Oral tablets | IV infusion | IM injection | IACI | ||||
| 1b | Mother and father | 11–13 | Flare | ✓ | ✓ | ✓ | ✓ |
| 2 | Mother | 5–7 | Flare | ✓ | ✓ | ||
| 3b | Mother | 14–16 | Flare | ✓ | ✓ | ✓ | |
| 4c | Mother and father | 1–4, 5–7 | Diagnosis, flare | ✓ | ✓ | ||
| 5 | Mother | 1–4 | Diagnosis | ✓ | |||
| 6 | Mother | 5–7 | Diagnosis | ✓ | ✓ | ||
| 7 | Mother and father | 1–4 | Diagnosis | ✓ | |||
| 8b | Mother and father | 8–10 | Flare | ✓ | ✓ | ||
| 9b | Mother | 11–13 | Diagnosis | ✓ | ✓ | ||
| 10b | Mother | 14–16 | Flare | ✓ | ✓ | ✓ | ✓ |
| 11b | Mother | 8–10 | Flare | ✓ | ✓ | ||
| 12b | Mother | 11–13 | Diagnosis | ✓ | ✓ | ||
| 13b | Mother | 14–16 | Flare | ✓ | ✓ | ||
| 14b | Mother | 14–16 | Flare | ✓ | ✓ | ||
| 15 | Father | 14–16 | Flare | ✓ | ✓ | ||
Participants were interviewed in their homes (n = 9), in a private setting in the paediatric rheumatology clinic (n = 4), in the parent’s workplace (n = 1) or by telephone (n = 1). Of the nine CYP, two were interviewed jointly with one parent and seven were interviewed separately. Of 19 parents, 14 were interviewed separately and five were interviewed with their child present, including the two noted above and three parents whose children were ≤ 7 years and ineligible for interview.
As shown in Table 1, CYP ranged in age from 1 to 16 years and most were female (9/16, 56%). One family had two CYP with JIA. All but two families (13/15 families) had experienced at least two treatments, and most CYP had established JIA with a recent disease flare (10/16 CYP). IACI was the treatment most commonly experienced (14/16 CYP). Although the inclusion criteria stated that patients needed to have had CSs within the past 12 months (see Box 1), all families reported that patients were treated within the past 6 months, and almost one-third of families (4/15 families) were actively receiving at least one CS treatment at the time of interview. We obtained families’ postcodes wherever possible and used the Indices of Multiple Deprivation (IMD), a measure of socioeconomic status, to inform sampling and ensure that there was representation from all socioeconomic groups: 8 out of 13 (62%) families lived in areas of highest deprivation (IMD quintiles 4–5), 2 out of 13 (15%) lived in areas of moderate deprivation (IMD quintile 3) and 3 out of 13 (23%) lived in areas of lowest deprivation (IMD quintile 1 or 2). 94
In the quotes in the following sections, each participant is indicated (Mo = mother, Fa = father or C = child) with a family number (see Table 1 for family number reference). Patients’ age categories are provided (e.g. 14–16y). Ellipses (. . .) indicate omitted text and square brackets indicate explanatory text.
Experiences and perceptions of corticosteroid delivery routes
Intra-articular corticosteroid injection
Use and effect
Thirteen families had first-hand experience of IACIs and several parents reported that this delivery route had been effective in treating their child’s JIA:
[Since treatment] . . . [patient] wants to be off, she wants, she will never sit still, which is how she was anyway before the diagnosis.
Fa7_1–4y
Several parents reported that the effect of IACIs was usually apparent with 48 hours, but that they continued to see symptoms improve weeks later. However, some families who had experienced a number of disease flares described IACIs as initially effective but less effective in their most recent experiences:
. . . this last time with [patient] . . . was strange, wasn’t it . . . she was fine for a week and then it seemed to come back again. We hadn’t had that.
Mo4_1–4, 5–7y
Benefits and drawbacks
Participants tended to view IACIs as a more intensive and concentrated treatment, as it ‘gets straight to the joint’ (C9_11–13y). Therefore, families tended to view IACIs as more suitable for patients with fewer affected joints:
. . . it’s powerful for a few joints but not when you’ve got more than a few affected.
Mo10_14–16y
Intra-articular corticosteroid injections were also viewed by some as a more invasive procedure and, for this reason, some parents believed it was best suited for disease that was towards the more severe end of the spectrum (established by pain or swelling). This led some parents to vary in their treatment preferences over time, depending on the nature and perceived severity of a specific flare:
It depends on how bad [child] is, if his joints are really swollen and a lot of inflammation on, then yeah, joint injections all the way. You know, if he’s flared up a little then maybe tablets.
Mo3_14–16y
Parents and CYP described several drawbacks of IACIs, including the need for the patient to undergo general anaesthesia during administration of the treatment, the need for the patient to be nil-by-mouth in the hours before treatment and the pain that the patient can experience after treatment. Some parents reported that the process of anaesthesia was stressful for them and their child, irrespective of the child’s age.
Parents believed that their children were anxious because of a fear of dying during the procedure, a fear of injection, feeling out of control and the smell and taste of the anaesthetic mask. One parent explained:
She was scared she was gonna die and not wake up. She didn’t actually say it but you know, that, that was her worry . . . at that age they wanna be in control of the situation and she’s just not got any control.
Mo10_14–16y
However, interviewed children rarely described their experience of anaesthesia as distressing, and one mother even described her child as:
. . . excited by it all . . . the building was exciting, even getting up to the car park he found exciting . . . he was just fascinated with it all.
Mo6_5–7y
This contrasted with how this mother felt about taking her child to hospital for IACI:
. . . but I just, you know, just taking your child to hospital’s scary.
Mo6_5–7y
Another mother of a younger child described her first experience of IACI as:
Horrendous . . . one of the worst days we’ve had to go through as a parent really . . . take our little girl and sort of put her under anaesthetic, and we weren’t going to be there with her for that period of time . . . because she is little as well . . . I’m not saying it would be any easier if she was 7 or 8 or whatever, but at least . . . she would understand that we are going to be back, we’ll see you in a bit.
Mo7_1–4y
As noted above, children receiving GA had to be nil-by-mouth for several hours before the procedure. Parents, particularly those with younger children, commented that this was a source of distress for children and a considerable inconvenience for the wider family:
It’s not so nice because, you know, you need to starve for a whole day and you are 8, you can’t drink water even so you can do nothing and, and you’re in hospital.
Mo8_8–10y
However, again, CYP did not comment on this.
Some families also described how their child had experienced increased pain in the injection site immediately after the IACI but that this was short-lived. However, two families described the pain as more intense than the pain associated with the child’s JIA:
It hurts a lot afterwards, like it does get worse before it gets better, but only for like a few days like it gets worse . . . they did swell up and like it felt like your hand was broken . . . I couldn’t have gone into school and like done all the writing and things.
C10_14–16y
I don’t think anything was really painful before, whereas this time his wrist was so painful.
Mo3_14–16y
Other negative aspects of IACI that families commented on, but less frequently, included children feeling tired after the procedure, the need to take time off school for the procedure and bruising or dimpling at the injection site.
Oral prednisolone (tablets)
Use and effect
Ten families had first-hand experience of CS tablets; however, few reported tablets to be effective in reducing symptoms long term. Tablets were often solely prescribed for less severe JIA or prescribed alongside another CS delivery route for more severe disease. Families who reported tablets to be effective described a reduction in symptoms within 48 hours:
They gave us her steroid tablets and it went down then. The steroid tablets, it went down overnight, didn’t it?
Mo4_1–4, 5–7y
The effect of the tablets was often reported as short term, with a return of symptoms once the course was reduced or completed:
They were okay the tablets but they were really short term . . . they would only work while she was taking them, after that the flare-up would come back.
Mo1_11–13y
I don’t think we saw any effect with the tablets so I think that’s why we progressed to other things.
Mo10_14–16y
Nevertheless, in contrast to IACIs, some parents viewed the tablets as more suited to patients with several affected joints, founded on the belief that oral medicines travel throughout the body and, therefore, would reach multiple joints:
The tablet’s going right the way round his bloodstream so it’s leaving a bit of steroid everywhere it goes.
Mo6_5–7y
Tablets were also commonly viewed as a less intensive delivery route and, perhaps linked to this, as less effective. One parent explained:
If he’s flared up a little then maybe tablets . . . it depends on how bad [my child] actually is, and which method to go down.
Mo3_14–16y
One parent who had no experience of using tablets said:
We’ve just had a real intense treatment to get him to where he needed to be before they put him on the immune suppressants . . . so I think tablets probably wouldn’t be as effective because by the time the tablets started working it would be quite progressed.
Mo9_11–13y
This comment also indicates the mother’s concern that CS tablets, which patients could be allocated to as part of a future trial, could replace or interfere with current treatment regimens that are deemed to be effective.
Benefits and drawbacks
Regardless of whether or not they had first-hand experience of tablets, families listed several benefits and drawbacks of tablets compared with other delivery routes. Families of children who had milder forms of JIA or fewer affected joints viewed tablets as more convenient than other treatments; for example, some described opting for tablets because of upcoming holidays or because they wanted to avoid their child being hospitalised:
I think [clinician] would have done the joint injections sooner um but we were due to go on holiday, so that’s why we tried the oral steroids first, ’cause he could continue taking those while we were on holiday.
Mo6_5–7y
Some parents without first-hand experience of tablets anticipated that they would be difficult to administer to younger children; however, those with experience of dissolvable tablets described the process as easy:
. . . it was eight little tablets just dissolved into juice, um so they were quite easy to administer.
Mo6_5–7y
Furthermore, children who had previously been treated with MTX were reluctant to take tablets in general, as they associated tablets with vomiting:
I didn’t like them at all, they made me feel sick even thinking about it, so I think it was just a mental thing.
C1_11–13y
One parent, whose child had a pronounced fear of injections, commented that their child preferred tablets over any other delivery route:
Tablets – your favourite. No needles are needed.
Mo8_8–10y
Families reported side effects that they associated with tablets, including an increase in their child’s appetite and changes in their mood and behaviour. Most parents viewed increases in appetite and associated weight gain negatively:
. . . he was just eating anything he could find, so he gained a lot of weight.
Mo6_5–7y
Bad, they give you a really big appetite . . . I was on it for about 3 months and I put about 2 or 3 stone.
C3_14–16y
Some parents described a change in their child’s mood and behaviour that was associated with taking tablets:
Off your head, weren’t you? . . . Crazy! . . . Loads of energy.
Mo2_5–7y
In particular, one parent felt that the tablets exacerbated her child’s attention deficit hyperactivity disorder (ADHD):
His behaviour changed in the summer . . . when he was on the oral steroids . . . his temper was quite . . . it was quicker than normal, um and his aggression would last longer and, you know, with his ADHD you can calm him down . . . whilst he was on these oral steroids it was quite a task.
Mo2_5–7y
Intravenous injection
Use and effect
Seven families had first-hand experience of IV infusions, but patients from two of these families had not yet completed IV treatment at the time of interview, so they were unable to assess the effect. Some families reported reduced symptoms following IV infusion, whereas others reported a limited effect; however, patients often received IV infusion concurrently with another type of CS treatment, making it difficult for them to know which delivery route to attribute the effect to. For example, when the interviewer asked a child who was receiving two treatments concurrently about which treatment she preferred she explained:
I do not know because like I feel like the IV and the tablets go along with each other in a way because I’m on both at the minute . . . It would depend what works the best for me.
C12_11–13y
Benefits and drawbacks
Regardless of whether or not they had first-hand experience of IV infusion, most parents and children believed that IV infusion was fast-acting, treated the whole body and may, therefore, be particularly suitable for children with several affected joints. One parent explained:
I’d rather she went on to, you know, IV and that treats her whole body then, rather than the joint injection will just treat a specific area.
Mo1_11–13y
The belief that IV infusion affects the whole body was typically regarded positively, as families associated this with treatment efficacy. However, one parent indicated that IV infusion could be more harmful than localised treatment:
. . . the IV one would affect the whole body, so I don’t know whether that would be a good thing or not . . . if that would cause more damage for steroids in your whole system rather than just a targeted area.
Mo2_5–7y
Some families without experience of IV infusion suggested that it might be less time-consuming than other delivery routes:
I’d rather just go with, have it IV . . . a better way . . . rather than keep dragging it on.
Mo5_1–4y
Conversely, others commented that IV infusion necessitated at least 1 day in hospital, which they viewed as an inconvenience. One child identified that a drawback of IV infusion was that it entailed missing school; however, no parents identified this as a problem:
. . . it took a long time, so I had to miss quite a lot of school, and I had to catch up on everything, so it means I’d have to write a lot.
C1_11–13y
Several parents who did not have experience of IV infusion also queried whether or not the treatment would be suitable for younger children, given the challenges of occupying or distracting them during the procedure:
I don’t think she would bode well with, having something on her all the time either, with her age. Maybe if somebody were a bit older.
Mo7_1–4y
However, we were unable to interview any families of CYP in the 1–3 years and 4–7 years categories who had first-hand experience of IV infusion. Some parents without experience of IV infusion viewed the prospect of this treatment as quite frightening, but did not explicitly comment that this would deter them from allowing their child to have the treatment. One further parent, whose child had a fear of injections, explained that both she and her child would refuse this treatment:
No chance for any cannulas . . . and we wouldn’t even agree for it.
Mo8_8–10y
One mother reported that IV infusion had increased her child’s appetite and led him to gain weight, which her son had found upsetting:
Because he’d put a lot of weight on in the space of a couple of weeks he was really depressed at that time, very depressed.
Mo9_11–13y
When interviewers presented parents and CYP with a list of potential pros and cons for each of the four delivery routes, parents’ comments on the drawbacks associated with IV infusion (such as ‘funny taste’ and ‘red, puffy cheeks’) were particularly prominent (i.e. these side effects were mentioned more often and with a serious tone) relative to the drawbacks presented for other treatments.
Intramuscular injection
Use and effect
Intramuscular injection was the least common CS delivery route, with only three families reporting first-hand experience of this treatment, the youngest child being 11 years old. One patient had received IM CSs only few days before the interview, so was unable to comment on the longevity of its effect. The other two patients had received IM CSs many months before the interview and reported the treatment to be effective for at least 6 months in reducing their symptoms:
The muscle injection is definitely the best one for me.
C1_11–13y
Yeah, and then that worked for a little bit but it just didn’t last . . . [it lasted about] 6 months.
C14_14–16y
One of the patients who received CSs by IM injection was treated concurrently with CSs delivered by another route.
Benefits and drawbacks
One of the CYP who had first-hand experience of IM injection described the procedure and its immediate aftermath as painful:
. . . the nurse was a bit of a rush at giving it, she just ran at me, but she didn’t tell me . . . it hurt for a while afterwards as well . . . maybe an hour and a half at most.
C10_14–16y
However, when asked if this might deter her from having the treatment again, the patient answered:
No . . . if I thought it worked then I’d probably have it again. It doesn’t really matter about if it hurt . . . if it makes me better in the long term.
C10_14–16y
Families with no experience of IM injection tended to view it favourably, particularly compared with the other treatments. Indeed, several parents described IM injection as straightforward, quick and, therefore, preferable to other treatments such as tablets, as no course of medication was required, and to IACI, as no anaesthetic was required. This was appealing to parents:
. . . it’s so quick, she’s just getting one shot and then that’s it, there’s no drama of morning times, trying to get the tablets down her and she’s crying ’cause she doesn’t want to take them.
Mo2_5–7y
However, as with IV infusion, some parents with no experience of IM injection viewed it as an appropriate treatment for patients with several affected joints:
. . . if it was just one or two joints I would probably go with the joint injection, but if it was like flaring up in different places then I’d probably go with the muscle or the IV.
Mo9_11–13y
This is somewhat surprising as injection is often problematic for children.
Nevertheless, parents without experience of the IM injection treatment felt that it would be acceptable because their child was accustomed to injections:
In [child’s] case she’s used to needles and all that, you know, she has a lot of them . . . that sort of thing doesn’t bother her.
Mo1_11–13y
Similarly, a parent whose child had a fear of needles commented that the prospect of an injection without anaesthetic was daunting, yet she anticipated that her child would be more likely to tolerate IM injection than IV infusion:
No chance for that IV injection.
Mo8_8–10y
And is it the same for the muscle injection as well?
Interviewer
Muscle injection is just, is just anyway just a few seconds so it’s like with those injections which he had now, so he will cope with it eventually but no chance for, um, any cannulas or stuff like this, no, never.
Mo8_8–10y
The children who had first-hand experience of IM injection were of secondary school age and it is unclear if this route is used in, or considered suitable for, younger children. It is notable that one child with no experience of IM injection commented that the injection ‘might hurt a bit more’ (C9_11–13y) and that this prospect could deter other children from having it.
In response to the list of treatment pros and cons that were presented, some parents commented on the fact that IM injection involves a lower dose of CSs than the other routes. One parent went on to question whether or not IM injection would be as effective as the other routes:
That looks alright . . . but do you get the same benefit out of it? . . . I mean it’s saying that you get a lower dose than your joint injections.
Mo3_14–16y
Another parent questioned how IM injection would be effective in treating an affected joint, as it is the muscle and not the joint that is injected, whereas another suggested that the procedure might be frightening for a parent, as it is undertaken in hospital.
Views on the proposed future trial
Willingness to be randomised in a future trial
Families recognised the need for JIA trials and some expressed enthusiasm for the proposed trial:
I would [participate] ’cause I’ve always been up for like helping with research.
Mo2_5–7y
It is a very good topic to actually do a research on . . . it’s one that’s needed because there are many ways of [delivering] steroids.
C3_14–16y
Children and young people often suggested that participation in the proposed trial could be of benefit to either themselves or others:
I’d try it [the trial], yeah . . . It seems fine . . . If it’s going to help, it’s going to help.
C14_14–16y
How CYP and parents perceived randomisation influenced their views of the trial. For the most part, both parties appeared to grasp the rationale for randomisation as a means to avoid bias:
I do think it will be best if it’s chosen by a computer because some patients might be biased.
C3_14–16y
However, at times some parents struggled to reconcile randomisation with a need to trust in clinicians’ expertise:
I’d go with just whatever’s the best one and that’s why, that’s why I’m trusting [the doctors] to get her better . . . I wouldn’t be comfortable with the computer randomly selecting what would be the best course of treatment for her.
Fa15_14–16y
Other parents struggled to reconcile randomisation with their need as parents to have confidence in the care being provided for their child:
We were told the steroid injections would help her, so that sort of put confidence in us. But for someone to say we’re not quite sure which one the best one is, so we’re going to do it randomly, I think that would put a lot of sort of discomfort in us.
Mo7_1–4y
In contrast, only one young person was opposed to participating in the proposed trial, rejecting randomisation in favour of a clinician-informed treatment:
I would have said no because . . . the doctor needs to tell you what’s best for you and not a computer because a computer doesn’t know that.
C12_11–13y
The comments of other CYP indicated that they were open to participating, and some expressed a degree of nonchalance about their treatment being randomised as part of research participation:
I’d be like ‘It’s been happening for this long, I don’t care any more, you can do whatever, do anything with my body, it’s alright!’.
C13_14–16y
This distinction between parents and CYP was also reflected in the following joint interview, during which C13 was more permissive towards the research than his mother:
[At diagnosis] I haven’t experienced any of them [treatments] so far so it wouldn’t hurt to try any of them . . .
C13_14–16y
I think I might have been a little bit more concerned ’cause I think I would want, at the beginning when we didn’t know a lot about it, I think I would have been thinking, ‘oh no, I need to have the consultant say what’s best for him’.
Mo13_14–16y
This permissive attitude among CYP may have been founded in part on feeling confident that clinicians would always prioritise their care over the trial:
I’d probably take part in it just to like help the research and, ‘cause I think if something didn’t work on me I know that . . . they’d just put me on something else like in the end.
C9_11–13y
Beliefs about treatments and willingness to be randomised
Past treatment experiences
Families’ experience of different treatments over numerous disease flares since diagnosis heavily influenced their views on the proposed trial and their willingness to participate in the planned trial. Reflecting on a treatment that ‘had worked’, one mother indicated that she would like her child to receive the same treatment if they experienced a future flare-up:
When you’re first diagnosed you will try any of the medication, but now we know what works for [my child] I would want to stick with that.
Mo14_14–16y
Conversely, on realising that joining the trial could mean that her child received a treatment that had previously ‘not worked’, another mother questioned the logic of offering that same treatment to her child in the context of a trial:
If it’s not worked this time, what makes them think it’s going to work the second time?
Mo6_5–7y
Hence, parents struggled to see the logic behind randomising a child to a treatment that they had previously experienced as ineffective, or the logic behind their child being randomised to a ‘new’ treatment when they had experienced, first hand, their child’s symptoms being relieved by another treatment. This was a highly important issue for parents and one that they would want clinicians to address, should such a trial be offered to them.
Differing treatment preferences within families
Older children and their parents frequently differed in their treatment preferences, with both parties often noting drawbacks of treatments that the other had not identified:
I’d rather [have IV].
C12_11–13y
You’d rather have [IV] than just have a needle in your bum? I think I’d rather have the needle in my bum than be sat here.
Mo12_11–13y
Wouldn’t [IM injection] hurt? That would hurt more surely?
C12_11–13y
. . . well you’ve had to come here for 3 days [for IV].
Mo12_11–13y
It doesn’t bother me. It’s like an hour on this and then it’s done.
C12_11–13y
. . . to me, [IM] would be a lot less painful going in your butt ’cause you’ve got that little meatiness, do you know, something to put it in.
Mo13_14–16y
Any muscle anywhere else I probably would have been fine. Like you could just stick it in my thigh or something that’s okay but I don’t want to have to get the moon out for you.
C13_14–16y
As noted above, some CYP were receiving CSs at the time that they were interviewed. Despite being unable to assess the effect of the current treatment in reducing symptoms at that time point, one young person explained that he preferred his current treatment over other treatments, even ones he had not previously experienced:
I’d say [I prefer] this one [IV] . . . because my experience right now . . . I feel quite, quite fine on this. Probably [I would secondly choose] tablets since I haven’t experienced those.
C13_14–16y
This implies that treatment side effects weighed heavily in this young person’s treatment preferences.
Factors to consider in designing a proposed trial
As well as the views of CYP and parents described above, other important factors that should be considered in designing a future trial include patient age, patient’s number of joints affected, the time since diagnosis, whether or not the patient is receiving multiple treatments, the clinicians’ treatment preferences and trial inconvenience.
Patient age and the number of affected joints
Although families did not view any one of the four delivery routes as unsuitable to be evaluated in the proposed trial overall, they did perceive certain treatments as more or less acceptable to certain CYP, particularly because of patient age and the number of affected joints. Families held these views even when they did not have direct experience of the treatment concerned.
Parents believed that treatment suitability depended on a child’s age; for example, a mother anticipated that her 8-year-old would not tolerate IV infusion:
He’s not going to want to stand there for hours.
Mo11_8–10y
However, this mother thought that her child might tolerate IV infusion when he was older:
I would imagine if he got lots and lots of flare-ups, and he is older and he understands it, I can see the logic behind that.
Mo11_8–10y
Although a child’s age seemed important to parents in making decisions about treatments and, therefore, in designing the trial, CYP did not comment on this.
Both parents and CYP also believed that treatments varied in their suitability by the number of affected joints that a patient has; for example, one child suggested that IV infusion is more suitable when more than one joint is affected:
If you have a flare and it doesn’t just affect one joint, then [IV], it’d kind of help you more.
C9_11–13y
A mother of a child with localised JIA echoed the view that treatments varied in their suitability for different JIA subtypes and questioned whether, owing to its systemic nature, IV infusion would be harmful to her child:
The IV one would affect the whole body, so I don’t know whether that would be a good thing or not. I’ve never experienced that, we’ve only ever had the joint injections.
Mo2_5–7y
Therefore, parents and some children struggled to see the logic of a trial in which they might receive a treatment that, based on their knowledge of JIA and how treatments worked, was ill-suited to their personal experience of JIA.
Time since diagnosis
Families’ accounts indicated that time since diagnosis is a further factor to consider in designing the proposed trial. Most participants were more comfortable with the idea of participating in the trial if they were invited at initial diagnosis, when the treatments would be new to families:
[We would participate] at the beginning when he had it, probably it will be different but now, no way . . . we had such a big, big problems with his injection.
Mo8_8–10y
Nevertheless, there were some exceptions. Several parents recalled the emotional upheaval of learning that their child had a life-changing condition and their strong desire for certainty at that time. One mother explained that if she was asked to enter her child into a trial in the aftermath of diagnosis ‘I’d probably say “not yet” ’ (Mo9_11–13y).
Children and young people did not view trial recruitment at diagnosis to be problematic, again pointing to divergence in the views between CYP and parents. Overall, parents often described their children as resilient and CYP themselves rarely described the emotional upheaval of receiving a diagnosis, but they did reflect on how the diagnosis had affected their parents:
What was it like being told about having arthritis?
Interviewer
I don’t know, I think I took it a bit better than my mum, ’cause mums are mums, so they get a bit worried.
C9_11-13y
Receiving multiple treatments
Children and young people with multiple affected joints often received more than one CS either simultaneously or in short succession, which made it difficult for them and their parents to differentiate the effect of an individual treatment:
I don’t know [which is best] because like I feel like the IV and the tablets go along with each other in a way because I’m on both at the minute.
C12_11–13y
Most families also had experience of non-CS treatments for JIA, such as other immunosuppressive medication, hydrotherapy or physiotherapy, and some were concerned that the use of multiple treatments might cloud interpretation of the proposed trial’s findings:
The challenge is that they don’t have this treatment independently of other things, do they? It’s always combined with something else or it has been in our experience. So, it’ll be steroids plus something else.
Mo10_14–16y
Some parents also queried whether or not they could continue with existing treatments if their child were to participate in a trial of CSs:
I don’t know, would they stop the MTX or would they just carry it on?
Mo4_1-4, 5–7y
These illustrate further issues that clinicians would need to address when inviting families to take part in JIA trials.
Families’ accounts of clinicians’ treatment preferences
Both parents and CYP referred to the trusting relationships that they had with their clinician, and how they relied on the clinician to accurately inform them about treatments and provide them with optimal care:
The doctors have been so good . . . I’ve got to trust what they are telling me and what they think is best.
Fa15_14–16y
Who do you think would be the best person to give you more information about the research study?
Interviewer
Probably doctors and that so you know it’s true, in case like random people just say this works.
C1_11–13y
Families spoke about treatment preferences that their clinician had voiced when making recommendations about treatment during routine consultations, and indicated that the preferences of clinicians also depended on a child’s age, medical history and JIA subtype and/or number of affected joints:
And age thing as well, and how long they’ve had [JIA]. Because [the doctor] tends to do it with their age doesn’t she?
Mo1_11–13y
Not surprisingly, participants also indicated that the treatment preferences of clinicians influenced their own perceptions of treatment efficacy and suitability:
Probably the joint injections [would be best] ‘cause [the doctor] said they work better ‘cause it’s straight into the joint.
C9_11–13y
Inconvenient site visits
Some parents questioned or remarked on the number of visits to the clinic that the proposed trial would entail, noting that it was already challenging to attend clinic appointments because of work or school commitments. Parents thereby implied that their willingness to participate would hinge on the convenience or otherwise of the trial:
Would that entail, mean more hospital visits though, to do this trial? ’Cause obviously with work we’d be struggling, it depends how often that would be.
Mo2_5–7y
Some CYP acknowledged these factors but did not suggest that this would be a barrier to participation:
Mam doesn’t really mind going to the hospital and that, because obviously she does it because it helps me.
C14_14–16y
Important treatment outcomes for families
Parents and CYP who had a long-term diagnosis of JIA often spoke about ‘controlling’ JIA, rather than ‘curing’ it:
That’s all we know and it’s just basically . . . controlling it really, as opposed to curing it.
Mo11_8–10y
[Medication is] keeping it under control at the minute.
Fa15_14–16y
For many parents and CYP, a reduction in symptom severity, as opposed to complete eradication, seemed to define treatment success:
To compare what it was before, he’s had a huge improvement.
Fa8_8–10y
It would stop any more swelling happening . . . reduce some . . . or control it at least.
Mo9_11–13y
Parents were especially cautious of a returning flare and often spoke about the short-lived or temporary nature of treatment effects:
At the moment he’s doing . . . now for today he’s fine.
Mo8_8–10y
You think it’s under control and, and then it comes back again . . . she has had moments where she has been flare-free . . . but then it’s just like a rollercoaster . . . finding out that it’s back.
Mo2_5–7y
Children and young people tended to be more optimistic about treatment outcomes:
Getting into remission hopefully, ’cause I was close with the last one, I was off it for . . . it must have been about 2 weeks and then a fail, it was another fail.
C13_14–16y
Some CYP described previous experiences of complete symptom eradication for months and even years, whereas others spoke of getting back to ‘normal’:
It just goes back to normal, like you feel like normal.
C14_14–16y
Completely flipped over, a different child . . . it was back to normal . . .
Fa1_11–13y
Parents and CYP described treatment outcomes that were of greatest importance to them. Reduction in pain was one of the most prominent outcomes discussed:
. . . it’s mostly just, pain. I’m not feeling it any more, I feel fine, I feel healthy . . .
C13_14–16y
I felt proper awake from the first dose and I felt like no pain, I was in no pain whereas as soon as I was like off them, my pain’s just the same.
C12_11–13y
Parents and CYP also described how stiffness and swelling was an important outcome:
When the swelling goes down, because the swelling does go down after like 24 hours after having it.
C14_14–16y
Parents and CYP also described fatigue and mood as being important treatment outcomes. Parents and older patients explained that treatment had made them more tired:
This time I just feel the same level before, like I’m just really tired still and everything ’cause with my arthritis I get more tired but then with all the drugs and everything . . . it’s harder to sleep and I can wake up.
C12_11–13y
Children and young people also explained that their energy levels had increased and often attributed this to their CS treatment:
My mum said that I was all hyper and I was running around because I had so much energy.
C1_11–13y
I was very like uplifted I guess you could say. Like I felt more active, I felt like I could do anything pretty much. I felt amazing, like outstanding . . . it changed my mood quite a bit I must say.
C13_14–16y
Parents also described the impact of CSs on energy and mood [see Oral prednisolone (tablets)].
Mobility was also an important outcome, especially for parents of younger children, as babies and toddlers were less able to communicate pain:
By sort of afternoon/evening time (after treatment) she was better . . . so she was more likely to crawl, more likely to sort of walk a few steps holding your hand.
Mo7_1–4y
One parent described a previous course of CSs that her child had received when they were younger and the guilt that she had felt as a parent for misinterpreting her child’s symptoms:
She wouldn’t have been mobile at all [if she’d not had the treatment]. She was struggling getting down the stairs, and even in the mornings I used to say how good she was for not getting out the bed, then I realised the reason why, she couldn’t get out the bed . . . I did feel dead guilty about that.
Mo2_5–7y
Older CYP also tended to discuss weight gain and Cushing’s syndrome as important treatment outcomes:
They give you a really big appetite and I’ve got a few pictures on my phone in a minute that I’ll show you where I was on it for about 3 months and I put about 2 or 3 stone.
C3_14–16y
One child experienced puffiness in his cheeks and was bullied as a result of this:
Once they started to do like the steroids, ’cause I went puffy and stuff, and then people would say stuff . . . It used to like affect me like emotionally.
C9_11–13y
Finally, some parents and CYP described how the symptoms of JIA had resulted in poor school attendance and prevented their children from participating in sports or social activities:
She’s been off school for the last, definitely the last week solidly and then, but for the last year she’s had quite a lot of time off. So she flags up on school reports as being a low attender.
Mo10_14–16y
[CS] made me a bit like able to do stuff like more normal, like I would normally do like go to my cousin’s and play with her.
C9_11–13y
Discussion
To our knowledge, this is the first pre-trial qualitative feasibility study that has involved both parents and CYP with a long-term condition. Studies involving CYP are advocated in the literature,95,96 but we are aware of only one other pre-trial qualitative feasibility study84 that included parental and CYP input, which explored families’ views on the feasibility of a trial of treatments for acute osteomyelitis or septic arthritis. 84 The current study recruited CYP with a long-term condition, for whom trial participation can be especially challenging. 82 Although much research has focused on deficits in patients’ understanding of trials,72 we found that parents and CYP were able to engage with the logic of the proposed trial and identify potential flaws in its design, despite the scenario being hypothetical. 97,98 This suggests that pre-trial qualitative feasibility studies with CYP and their parents can inform and potentially optimise trial design71,72 by drawing on the in-depth knowledge and insight that families acquire in coping with a long-term condition.
In contrast to previous research,99 we found that families’ willingness to participate in a proposed trial and their treatment preferences frequently differed and, although CYP were more permissive towards the trial than their parents, they sometimes identified concerns that parents did not raise. This also echoes a previous pre-trial feasibility study that found that patients with acute osteomyelitis or septic arthritis often downplayed the impact of the illness or focused on different issues to their parents. 84 Previous research has found that parents often adopt an executive or managerial role in their child’s treatment and care,100 working to identify, anticipate and meet their child’s needs. 101 Based on this previous work and findings from the current study, we propose that CYP’s permissiveness reflects their reliance on their parents to protect them from harm and co-ordinate their care. CYP also trusted clinicians with their care and, unlike parents, they did not identify trial inconveniences (e.g. travel), which may have also contributed to their permissive orientation. Current guidance encourages recruiters to support families in sharing decisions regarding research. 29 Given that our study and others84 show that CYP can provide important input to trial design, the viewpoints of both parents and CYP are likely to be valuable in informing and improving future paediatric clinical trials and clinical practice. The findings have helped us to develop recommendations for a proposed CS induction regimen trial (Box 3). Several of these recommendations will be useful in developing future trials in JIA more broadly and in avoiding common recruitment pitfalls.
-
The views of parents and children are important in informing trial design and conduct.
-
Although families may not deem one treatment being unsuitable overall, triallists need to establish whether or not all treatments are suitable for all patients with JIA. If this is not possible, amendments to the eligibility criteria or a trial comparing fewer treatments will be needed.
-
Further qualitative work should explore clinicians’ views on a future CS induction regimen trial in JIA to establish clinical equipoise and support for the trial.
-
Triallists should consider methods of making a future trial more accessible to parents and children, such as combining trial follow-up assessments with clinic appointments.
Although families did not deem any single treatment as unsuitable to evaluate in the proposed trial, they believed that treatments needed to be tailored to CYP depending on factors such as age and the number of affected joints. Previous experience of treatments was an important influence on such beliefs. When families have treatment preferences that are experience based (e.g. trials evaluating established treatments) rather than anticipated (e.g. trials of treatments that are new to families), recruitment and randomisation is likely to be especially challenging,102–105 particularly as CYP with JIA receive treatments on repeated occasions. 89 Clinicians who are recruiting to trials that are investigating treatments that families have directly experienced will need to be prepared to respond to probing questions from families. For example, as one parent asked when contemplating the possibility of her child being randomised to a treatment that she had previously experienced to be ineffective:
. . . if it’s not worked this time, what makes them think it’s going to work the second time?
Mo6_5–7y
Triallists should also consider which treatment options can be evaluated in a trial and will be tolerated by CYP with JIA. Some trials may need to adapt the eligibility criteria or the treatments that are included to ensure that the trial is acceptable to families. It is also currently unclear how decision-making about trial participation is managed within families when treatment preferences conflict; further research would be beneficial.
Families described how clinicians had shaped their treatment preferences via previous discussions about the suitability of different treatments, based on factors such as a child’s age and the number of joints affected. Such discussions are part of good care in routine clinical practice, but equally, when invited to consider participation in a trial, families cannot be expected to put aside knowledge that they have previously gleaned from clinicians. Although clinician and patient treatment preferences are often regarded as separate entities, our findings indicate that in long-term conditions the two can be intertwined and that the influence of previous clinician–family discussions about treatments needs to be considered when communicating with families about trials. For example, when recruiting to any future RCT, clinicians will need to respond to families’ concerns about using a treatment that clinicians may have previously told them was unsuitable. Indeed, clinicians and their treatment preferences can often be a considerable barrier to patient participation in paediatric trials. 106,107 Further qualitative work exploring clinicians’ views of future JIA trials would help to address such potential difficulties.
Similar to previous research,108 we found that trial inconvenience, such as additional appointments, will also be a likely recruitment barrier for parents. Triallists will need to explore options to reduce trial burden, such as completing follow-up appointments by telephone or Skype™ (Microsoft Corporation, Redmond, WA, USA) and arranging appointments at participants’ convenience (e.g. out of school hours).
Accessing parents’ and CYP’s unique perspectives and experiences of JIA also enabled us to identify the treatment preferences and outcomes of greatest importance to families with JIA. These insights can be used as part of a wider process to inform the selection of outcomes in a future trial, as well as everyday clinical practice. Furthermore, qualitative research with parents and patients is recommended to inform the development of a COS. 109 Although a COS was developed for use in JIA trials in 1997,4 the set did not involve parent and patient input and a recent special interest group highlighted the need to update the set to consider parent- and patient-centred outcomes. 110 The findings could help inform such an update.
Strengths and limitations
We interviewed socioeconomically diverse families from across England, including males and females and CYP of different ages who had varied treatment experiences. Although we included data from only one patient aged 8–10 years in the analysis, the parents we interviewed had children aged 1–16 years. In accessing the perspectives of CYP, our approach is consistent with current guidance on including the voices of children in research. 95 Furthermore, the competence of chronically ill children in health-related matters can often exceed their chronological age. 111
We did not interview families with a recent JIA diagnosis who did not yet have first-hand experience of CSs. We acknowledge that the views of such families may differ markedly from those in our sample. However, interviewing families at diagnosis, but before CS treatment, would probably have been challenging, particularly given the difficulties families experience at this time111 as the families in this study reported.
This study’s sample size is typical and appropriate for a qualitative study given that the inferences drawn are not about prevalence or statistical distribution. 83 Rather, our inferences concern the nature of families’ perspectives and the value of these in informing the design of JIA trials. Similar to deliberative engagement methods,112 we used stimulus materials (see Report Supplementary Material 2) and discursive interviews to enable families to raise matters of importance to them, and for us to inform families about trials and clarify any key misunderstandings.
Conclusion
This pre-trial qualitative feasibility study indicates that families could engage with the logic of a proposed JIA trial and provide valuable input into trial design before further investment of resources. We identified potential barriers to recruitment for a CS induction regimen trial in JIA, divergent views between parents and CYP, and areas for further exploration such as clinician treatment preferences. This study highlights the importance of including families in pre-trial feasibility work to illuminate barriers to recruitment and inform strategies to improve informed consent and recruitment.
Chapter 4 Assessment of current UK practice
Introduction
This chapter will look at the feasibility of conducting the proposed trial of CSs in the induction of remission in new or flaring JIA cases, by exploring the opinions of clinicians about their current practice. This is to ascertain the acceptability of a proposed trial and to identify potential barriers to participation in a trial103 and how these could be addressed. A prospective screening log exercise was also conducted to allow collection of data on JIA patients who were treated in real time with CSs to ascertain the numbers of potential patients who are treated in the UK in a given period.
Aims and objectives
The national survey and screening log exercise addressed specific aspects of the research aims: research aims 1 (establishing current practice) and 2 (acceptability of treatment arms). These results fed into research aim 3 (structured survey and discussion group with stakeholder consensus), research aim 4 (feasibility study to test study design) and research aim 5 (consensus meeting and final protocol choice).
The specific questions that the national survey and screening log exercise aimed to address were:
-
RQ 1 – what types, routes and doses of CS are used, and in which patients?
-
RQ 2 – what clinical criteria are used for commencing CS treatment and choosing the route of administration?
-
RQ 3 – what are the key issues/concerns with regard to capacity and capability in the conduct of a future RCT?
-
RQ 4 – how many potentially eligible CYP attend hospital in the UK with varying severities of JIA requiring CS treatment who could be randomised in a comparative treatment study?
-
RQ 5 – what characteristics would HCPs and parents/patients want to see included in a future RCT of CSs in JIA? Which patients should be included/excluded? What would be the most appropriate control agent in a future trial? How would active disease or a disease flare be defined?
-
RQ 6 – how willing would patients/parents be to consent to be randomised in a future clinical trial and how willing would be HCPs be to randomise to it?
Methods
The project plan consisted of two work streams that were developed to meet its aims and objectives. First, the project plan required the development of a questionnaire survey with which to conduct a sample survey of clinical practice and opinions on the use of CSs to treat JIA, and also of opinions on the proposed RCT. Second, a prospective screening exercise was conducted of JIA patients who were treated with CSs with a timed screening log completed by each participating centre. These two work streams were carried out independently and participants were not aware of the results of either.
The survey was sent to a wide group of HCPs using BSPAR members, the BSPAR nurses and the Barbara Ansell National Network for Adolescent Rheumatology (BANNAR) mailing lists.
National opinion survey of clinical practice
The national survey of clinical practice among HCPs (paediatric rheumatologists, paediatricians with a specialist interest in rheumatology, adolescent rheumatologists with expertise in JIA and specialist nurses in paediatric rheumatology) who are involved in the care of CYP with JIA was devised by the research team. The aim was to include both open and closed questions, as well as comment boxes. Topic guides for the survey were developed by the SMG to explore acceptability of the proposed trial and the feasibility of the trial design, and to identify potential barriers to participation in a trial and how these could be addressed.
A draft survey was circulated to the SMG to review ease of use, comprehension and interpretation; the survey was then refined based on the comments/suggestions received. The survey was pilot tested on a group of paediatricians and underwent final modification.
The survey was produced using SurveyMonkey® (Palo Alto, CA, USA) (URL: www.surveymonkey.co.uk). A hyperlink to the opinion survey was embedded in an e-mail sent on 21 October 2016 to selected paediatric rheumatology HCPs (n = 162) inviting them to participate. As the initial response rate was poor (19 out of 162 participants; 12%), the SMG agreed that survey link should be re-sent to try and improve the completion rate. Invitees were sent a follow-up e-mail and telephone reminders, as required. Following the final reminder, a further 2 weeks was allowed to complete the survey, after which no further responses could be submitted.
Advice was sought from the National Research Ethics Service on the requirement for ethics approval for completion of the structured survey. As the structured survey was seeking opinion only, ethics approval was not required. Consent for participation in the online structured survey was implied by submission of a response.
Screening exercise
Paediatric rheumatologists at tertiary and secondary sites were invited to provide screening log data on JIA patients who were treated with CSs. These sites were chosen to reflect different types of hospital-based services (secondary/tertiary centre) in different regions. Completion of screening logs enabled the identification of all patients who were treated with CSs who met the inclusion criteria in the study period. No sample size was determined prior to data collection, as this was dependent on admission/attendance rates.
Data on the number of CYP with different subtypes and severity of disease (in terms of the numbers of active joints) and disease duration pre-treatment, as well as duration of flare pre-treatment, were collated from purposively chosen sites through prospective screening logs. These were co-investigator sites and they were also contracted by the study to be collaborator sites for the final feasibility part of the study.
Sites were asked to record all patients who had newly diagnosed or flaring JIA who attended their hospital as inpatients, day cases or outpatients over a 6-week period. The HCPs at each site were asked to complete information on date of treatment/treatment decision, child age, JIA diagnosis, whether the child was newly diagnosed or flaring, whether or not they were prescribed CSs, the route of administration and if any other concomitant medications commenced, including biologic agents. Flaring patients whose flare was treated without CSs, for example by starting on biologics instead, were also included in the screening log for completeness and to allow us assess how often flares are treated without CSs. Sites were asked to screen patients for 6 weeks or until 20 patients were screened.
Following the protocol for similar studies conducted by this team, it was confirmed by the study sponsor (Alder Hey Children’s NHS Foundation Trust) that the screening log element did not require Health Research Authority (HRA) approval.
Steps were taken to ensure that data were anonymised to adhere to the Data Protection Act and Caldicott principles. Data were collected and stored in locked cupboards, in locked offices or on password-protected computers, in accordance with local university and hospital research governance policies. Centres were free to choose any route of CS administration, as per existing clinical decision-making.
Results
Results are reported below for both the national opinion survey of practice and the national screening logs, as both asked similar questions but in different ways and without reference to the results of the other method. This enabled researchers to assess if the hypothetical survey results were mirrored in the real-time screening log.
Baseline characteristics
National opinion survey of clinical practice
A total of 39 (24%) responses were received from 162 HCPs: 22 (56%) from NHS consultants, five (13%) from grid trainees, eight (21%) from clinical nurse specialists and four (10%) from ‘other’. Of the NHS consultants (n = 22), 17 (77%) were clinical NHS consultant paediatric rheumatologists and five (23%) were mixed consultants; 21 (95%) were paediatric rheumatologists and one (5%) was an adult rheumatologist with a special interest in paediatric rheumatology. This represented responses from the majority of the consultant paediatric rheumatologists, grid trainees and specialist paediatric rheumatology nurses in the UK and, therefore, was a representative and useful response rate from the paediatric rheumatology specialist community. The non-responders were a mixture of adult rheumatologists and HCPs who were members of BSPAR at the time.
Screening logs
In total, 297 participants from 13 centres were screened in just less than 6 months. The first participant was screened on 2 September 2016 and the last participant was screened on 28 December 2017 (Table 2). Five centres (Royal Hospital for Sick Children, Edinburgh; Royal Hospital for Children, Glasgow; Royal Aberdeen Children’s Hospital, Aberdeen; Birmingham Children’s Hospital, Birmingham; and Royal Manchester Children’s Hospital, Manchester) were unable to take part in the screening log exercise. Although REC and HRA approvals were not required for this particular activity, some NHS research and development (R&D) departments enforced local permission processes, which caused delays and led to the deadline for completing the screening log exercise being missed.
| Site name | Date of first screening | Date of last screening | Number of screenings |
|---|---|---|---|
| Addenbrookes Children’s Hospital | 4 October 2016 | 15 November 2016 | 20 |
| Alder Hey Children’s NHS Foundation Trust | 13 October 2016 | 24 July 2017 | 40 |
| Bristol Royal Hospital for Children | 28 November 2016 | 28 December 2017 | 20 |
| Great North Children’s Hospital | 2 November 2016 | 24 November 2016 | 20 |
| Great Ormond Street Hospital for Sick Children | 16 January 2017 | 27 February 2017 | 42 |
| New Cross Hospital | 2 September 2016 | 24 November 2016 | 12 |
| Norfolk and Norwich University Hospitals NHS Foundation Trust | 1 December 2016 | 12 January 2017 | 12 |
| Nottingham Children’s Hospital | 9 January 2017 | 3 February 2017 | 20 |
| Royal Belfast Hospital for Sick Children | 25 October 2016 | 22 December 2016 | 35 |
| Royal Stoke University Hospital | 3 October 2016 | 5 December 2016 | 13 |
| Southampton General Hospital | 1 November 2016 | 9 December 2016 | 16 |
| St James’s University Hospital | 3 October 2016 | 10 November 2016 | 27 |
| The Children’s Hospital Sheffield | 24 October 2016 | 2 December 2016 | 20 |
| Total number of screenings | 297 |
Participants ranged in age between 1.17 and 17.83 years (mean age approximately 9 years). In total, 107 (36%) participants were diagnosed as having oligoarthritis and 96 (32%) were diagnosed with RF-negative polyarthritis. A total of 97 (33%) participants were newly diagnosed and 262 (88%) participants were prescribed CSs at their visit; 35 (12%) were managed with non-steroid treatments. Table 3 shows the baseline characteristics of patients from the screening exercise.
| Characteristic | Participants (N = 297) |
|---|---|
| Agea (years), mean ± SD | 9.08 ± 4.91 |
| Type of JIA, n (%) | |
| Oligoarthritis | 107 (36) |
| RF-positive polyarthritis | 15 (5) |
| RF-negative polyarthritis | 96 (32) |
| Enthesitis-related arthritis | 18 (6) |
| Psoriatic arthritis | 13 (4) |
| Systemic-onset JIA | 13 (4) |
| Extended oligoarthritis | 33 (11) |
| Diagnosis not recorded | 4 (1) |
| Diagnosis type, n (%) | |
| New diagnosis | 97 (33) |
| Flaring | 200 (67) |
| CSs prescribed, n (%) | |
| Yes | 262 (88) |
| No | 35 (12) |
Research question 1a: what types and routes of corticosteroids are used?
The majority of clinicians who were surveyed (n = 17, 47%) said that they would treat new patients and flaring patients with the same CS remission induction regimen.
The first-line treatment for a new diagnosis with extended oligoarthritis, RF-negative and RF-positive polyarthritis, enthesitis-related arthritis and psoriatic arthritis was mainly DMARDs and IA CSs. IA CSs alone was the first-line choice in oligoarthritis, whereas for systemic-onset JIA the first-line choice was DMARDs plus IV infusion of methylprednisolone plus oral CSs (Table 4). A summary of the first-line treatments that clinicians would use in patients with a new diagnosis of the different subtypes of active JIA is given in Table 4 and a summary of all the possible treatments that HCPs could use is given in Table 5.
| JIA subtype | Treatment, n (%) (N = 39) | No response (n) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NSAIDs alone | DMARDs alone | IA CSs | Oral CSs alone | DMARDs plus IV infusion of methylprednisolone | DMARDs plus IV infusion of methylprednisolone plus oral CSs | DMARDs plus IV infusion of methylprednisolone plus IA CSs | DMARDs plus IA CSs | DMARDs plus IM injection of CSs | ||
| Enthesitis-related arthritis | 2 (5) | 3 (8) | 1 (3) | 1 (3) | 4 (11) | 5 (13) | 2 (5) | 20 (53) | 0 (0) | 1 |
| Extended oligoarthritis | 1 (3) | 1 (3) | 4 (11) | 1 (3) | 0 (0) | 3 (8) | 2 (5) | 25 (68) | 0 (0) | 2 |
| Oligoarthritis | 4 (10) | 0 (0) | 34 (87) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 0 |
| RF-negative polyarthritis | 0 (0) | 2 (5) | 1 (3) | 0 (0) | 1 (3) | 9 (24) | 9 (24) | 15 (41) | 0 (0) | 2 |
| RF-positive polyarthritis | 0 (0) | 2 (5) | 1 (3) | 0 (0) | 1 (3) | 11 (30) | 7 (19) | 15 (41) | 0 (0) | 2 |
| Psoriatic arthritis | 0 (0) | 2 (5) | 3 (8) | 0 (0) | 2 (5) | 4 (11) | 4 (11) | 23 (61) | 0 (0) | 1 |
| Systemic-onset JIA | 0 (0) | 1 (3) | 0 (0) | 2 (5) | 3 (8) | 29 (76) | 3 (8) | 0 (0) | 0 (0) | 1 |
| JIA subtype | Treatment, n (%) (N = 39) | No response (n) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NSAIDs alone | DMARDs alone | IA CSs | DMARDs plus IV infusion of methylprednisolone | DMARDs plus IV infusion of methylprednisolone plus oral CSs | DMARDs plus IV infusion of methylprednisolone plus IA CSs | DMARDs plus IM injection of CSs | DMARDs plus IA CSs | Oral CSs alone | ||
| Enthesitis-related arthritis | 14 (36) | 14 (36) | 26 (67) | 20 (51) | 22 (56) | 24 (62) | 12 (31) | 33 (85) | 5 (13) | 0 |
| Extended oligoarthritis | 4 (10) | 12 (31) | 26 (67) | 22 (56) | 23 (59) | 24 (62) | 12 (31) | 37 (95) | 5 (13) | 0 |
| Oligoarthritis | 20 (51) | 8 (21) | 38 (97) | 3 (8) | 2 (5) | 2 (5) | 6 (15) | 22 (56) | 5 (13) | 0 |
| RF-negative polyarthritis | 0 (0) | 10 (26) | 21 (54) | 26 (67) | 30 (77) | 26 (67) | 16 (41) | 32 (82) | 4 (10) | 0 |
| RF-positive polyarthritis | 0 (0) | 8 (21) | 19 (49) | 27 (69) | 30 (77) | 27 (69) | 15 (38) | 33 (85) | 4 (10) | 0 |
| Psoriatic arthritis | 8 (21) | 14 (37) | 27 (71) | 21 (55) | 23 (61) | 19 (50) | 12 (32) | 36 (95) | 6 (16) | 1 |
| Systemic-onset JIA | 5 (13) | 3 (8) | 10 (26) | 27 (69) | 36 (92) | 20 (51) | 6 (15) | 15 (38) | 12 (31) | 0 |
For all subtypes of JIA (except systemic-onset JIA) the most common treatments for patients with a disease flare were IA CSs or a combination of DMARDs and IA CSs. In systemic-onset JIA, the most common treatment for a new flare was DMARDs plus IV infusion of methylprednisolone plus oral CSs or oral CSs alone (Table 6).
| JIA subtype | Treatment, n (%) (N = 39) | No response(n) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NSAIDs alone | DMARDs alone | IA CSs | DMARDs plus IV infusion of methylprednisolone | DMARDs plus IV infusion of methylprednisolone plus oral CSs | DMARDs plus IV infusion of methylprednisolone plus IA CSs | DMARDs plus IM injection of CSs | DMARDs plus IA CSs | Oral CSs alone | ||
| Enthesitis-related arthritis | 5 (24) | 7 (33) | 14 (67) | 10 (48) | 12 (57) | 11 (52) | 7 (33) | 17 (81) | 12 (57) | 18 |
| Extended oligoarthritis | 3 (14) | 5 (24) | 15 (71) | 10 (48) | 7 (33) | 9 (43) | 7 (33) | 17 (81) | 9 (43) | 18 |
| Oligoarthritis | 7 (33) | 4 (19) | 19 (90) | 5 (24) | 2 (10) | 3 (14) | 3 (14) | 14 (67) | 5 (24) | 18 |
| RF-negative polyarthritis | 3 (14) | 6 (29) | 15 (71) | 11 (52) | 14 (67) | 11 (52) | 8 (38) | 17 (81) | 14 (67) | 18 |
| RF-positive polyarthritis | 2 (10) | 5 (24) | 15 (71) | 13 (62) | 13 (62) | 11 (52) | 8 (38) | 17 (81) | 15 (71) | 18 |
| Psoriatic arthritis | 4 (19) | 7 (33) | 14 (67) | 11 (52) | 10 (48) | 10 (48) | 7 (33) | 16 (76) | 9 (43) | 18 |
| Systemic-onset JIA | 2 (10) | 6 (29) | 7 (33) | 13 (62) | 19 (90) | 10 (48) | 2 (10) | 8 (38) | 15 (71) | 18 |
The two treatments that were the least commonly prescribed by HCPs were IM CSs alone and high-dose methylprednisolone IV infusion followed by IACIs, with the main reason for this being that these were not the preferred choice (Table 7).
| Treatment | Is this treatment prescribed, and if not why? n (%) (N = 39) | No response | ||||||
|---|---|---|---|---|---|---|---|---|
| Yes | Treatment cost | Lack of access | Lack of resource | Not my preferred choice | Too painful | Increased side effects | ||
| Oral CSs (soluble) | 30 (83) | 2 (6) | 4 (11) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 |
| Oral CSs (enteric coating) | 31 (84) | 4 (11) | 1 (3) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 2 |
| High-dose methylprednisolone IV infusion | 33 (92) | 0 (0) | 1 (3) | 0 (0) | 2 (6) | 0 (0) | 0 (0) | 3 |
| High-dose methylprednisolone IV infusion followed by oral CSs | 35 (95) | 1 (3) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 2 |
| High-dose methylprednisolone IV infusion followed by IACIs | 27 (75) | 0 (0) | 0 (0) | 0 (0) | 9 (25) | 0 (0) | 0 (0) | 3 |
| IM injection of CSs | 22 (59) | 0 (0) | 0 (0) | 0 (0) | 11 (30) | 4 (11) | 0 (0) | 2 |
| IACIs | 36 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 |
The majority of HCPs (n = 23, 74% of the 31 respondents) reported that, in their experience, IM injections were generally well tolerated. However, eight respondents (26% of respondents) felt that they were not well tolerated and eight did not give a response. Twenty-six (72%) respondents provided the age for the youngest patient to whom they had administered IM injections, 10 (28%) respondents said they rarely or never used IM injections and two HCPs did not respond. Among HCPs who had experience of administering IM injections, the mean and median age of patients at the time of treatment was 11.5 years and 12.5 years, respectively.
Health-care professionals were asked about the duration of effectiveness of IM injections; the majority of HCPs (n = 16, 67% out of 39 respondents) reported that IM injections were effective for between 1 and 2 months, with only one HCP (4%) stating that they felt it would be effective for 5 months. There were no responses from 12 participants, and it was not clear if this was because they believed IM injections to be ineffective or because they did not use them. In total, 30 HCPs stated that they did not have a preference for IM injection over other methods of CS administration. Most HCPs were of the opinion that IM are no more likely than oral or IV CSs or IACIs to induce remission (Table 8).
| IM injections considered better than | Response | Number of responses, n (% of respondents) (N = 39) |
|---|---|---|
| Oral CSs | Yes | 8 (27%) |
| No | 22 (73%) | |
| No response | 9 | |
| IV CSs | Yes | 2 (7%) |
| No | 28 (93%) | |
| No response | 9 | |
| IACIs | Yes | 4 (13%) |
| No | 26 (87%) | |
| No response | 9 |
Data from the screening log exercise showed that in all subtypes of JIA the most commonly used route of CS administration was intra-articular injection. There was evidence of the use of IM injections in two patients with a diagnosis of polyarthritis (RF negative) and two with extended oligoarthritis (Table 9). The most commonly used CS in all subtypes of JIA was TH, with prednisolone also commonly used (except in oligoarthritis) (Table 10).
| JIA subtype | Treatment, n (%) (N = 297) | |||||
|---|---|---|---|---|---|---|
| IM | IV | Oral | IACI | CS not given | Route of CS missing | |
| Oligoarthritis (n = 107) | 0 (0) | 1 (1) | 2 (2) | 104 (97) | 6 (5.6) | 0 (0) |
| Polyarthritis (RF positive) (n = 15) | 0 (0) | 2 (13) | 3 (20) | 10 (67) | 2 (13) | 0 (0) |
| Polyarthritis (RF negative) (n = 96) | 2 (2) | 20 (21) | 29 (30) | 47 (49) | 13 (14) | 0 (0) |
| Enthesitis-related arthritis (n = 18) | 0 (0) | 2 (11) | 7 (39) | 11 (61) | 3 (17) | 0 (0) |
| Psoriatic arthritis (n = 13) | 0 (0) | 1 (8) | 2 (15) | 10 (77) | 2 (15) | 0 (0) |
| Systemic-onset JIA (n = 13) | 0 (0) | 3 (23) | 4 (31) | 9 (69) | 2 (15) | 0 (0) |
| Extended oligoarthritis (n = 33) | 2 (6) | 3 (9) | 9 (27) | 18 (55) | 7 (21) | 0 (0) |
| No diagnosis recorded (n = 4) | 0 (0) | 0 (0) | 2 (50) | 2 (50) | 0 (0) | 0 (0) |
| Total | 4 | 32 | 58 | 211 | 35 | 0 |
| JIA subtype | Type of CS, n (%) (N = 297) | |||||||
|---|---|---|---|---|---|---|---|---|
| Depo-Medronea | IV methylprednisoloneb | THc | Triamcinolone hexacetonide | Prednisoloned | Kenaloge | Triamcinolone acetonidee | No CS given | |
| Oligoarthritis (n = 107) | 2 (2) | 1 (1) | 99 (93) | 2 (2) | 2 (2) | 0 (0) | 1 (1) | 6 (6) |
| Polyarthritis (RF positive) (n = 15) | 0 (0) | 2 (13) | 10 (67) | 0 (0) | 3 (20) | 0 (0) | 0 (0) | 2 (13) |
| Polyarthritis (RF negative) (n = 96) | 2 (2) | 20 (21) | 45 (47) | 0 (0) | 30 (31) | 1 (1) | 1 (1) | 13 (14) |
| Enthesitis-related JIA (n = 18) | 1 (6) | 2 (11) | 9 (50) | 1 (6) | 7 (39) | 0 (0) | 0 (0) | 3 (17) |
| Psoriatic arthritis (n = 13) | 0 (0) | 1 (8) | 10 (77) | 0 (0) | 2 (15) | 0 (0) | 0 (0) | 2 (15) |
| Systemic-onset arthritis (n = 13) | 2 (15) | 3 (23) | 7 (54) | 0 (0) | 4 (31) | 0 (0) | 0 (0) | 2 (15) |
| Extended oligoarthritis (n = 33) | 2 (6) | 3 (9) | 15 (45) | 2 (6) | 9 (27) | 0 (0) | 1 (3) | 7 (21) |
| No diagnosis recorded (n = 4) | 0 (0) | 0 (0) | 2 (50) | 0 (0) | 4 (50) | 0 (0) | 0 (0) | 0 (0) |
Research question 1b: what doses of corticosteroids are used in the survey?
As part of the detailed questioning regarding corticosteroids dosing, units were asked about their frequency of dose review and their weaning processes. The majority of HCPs reported that they would review the CS treatment regimen of patients who were being treated with IM injections every 6 weeks, IV infusions weekly, oral CSs every 4 weeks and IACIs every 12 weeks (Table 11).
| Treatment | Routine review, n (%) (N = 39) | No response | ||||
|---|---|---|---|---|---|---|
| Weekly | Every 4 weeks | Every 6 weeks | Every 12 weeks | > 12 weeks | ||
| IM injection | 3 (12) | 6 (23) | 9 (35) | 6 (23) | 2 (8) | 13 |
| IV infusion | 17 (47) | 10 (28) | 8 (22) | 0 (0) | 1 (3) | 3 |
| Oral CSs | 4 (12) | 17 (50) | 11 (33) | 2 (9) | 0 (0) | 5 |
| IACI | 2 (6) | 3 (9) | 12 (35) | 15 (44) | 2 (6) | 5 |
Health-care professionals were asked how often they would usually wean down oral CSs. Thirty (83%) of the 36 HCPs who responded stated that weaning would follow an individualised regimen depending on the severity of the patient’s disease, and six (17%) stated that they would use a standard unit regimen (three HCPs did not respond).
When asked about the maximum number of IACIs that HCPs would administer at any one time, answers were very mixed, with the number varying from one to ‘no maximum’; several HCPs responded that the number of injections would depend on the individual patient. The majority of HCPs (n = 19, 53%) stated that (for IACIs only) they would give fewer than five injections at one time, and 15 (42%) HCPs stated they would give 5–10 injections. The survey found that the highest frequency range of IACIs that a HCP would give in one administration was 16–20, which was reported by one (3%) HCP. When asked on how many occasions in a year that they would administer IACIs in an individual patient, 13 (36%) HCPs stated that they would do this on three occasions and 16 stated that they would do this on either two or three or three or four occasions.
Research question 2: what clinical criteria are used for commencing corticosteroids and choosing the route of administration?
The key criteria that HCPs (35 answered the question, with four missing values) stated that they used for commencing CSs and for choosing the route of administration were rapid induction of remission (n = 31, 89%), high disease activity (n = 31, 89%), severity of systemic JIA (n = 30, 86%) and level of inflammation (n = 28, 80%). Other common criteria included JIA subtype, the desire to target specific joints and the level of disability. When asked to rank the reasons for choosing the route of administration of CSs, the number 1 determinant was disease severity, followed by disease subtype. Those determinants judged to be less important included fear of needles and social factors.
Research question 3: what are the key issues/concerns with regard to capacity and capability in the conduct of a future randomised controlled trial?
When asked about the proportion of clinical staff who were responsible for conducting clinical research, the vast majority of HCPs (n = 31, 89%) stated that between 80% and 100% were good clinical practice (GCP) trained and, similarly, 29 HCPs (81%) stated that between 80% and 100% of their nursing staff who conducted clinical research were GCP trained.
When asked about out-of-hours medical and nursing cover that was available within the research team, 24 (69%) HCPs stated that there was a consultant available, eight HCPs (24%) stated that there was a research nurse available and eight HCPs (24%) stated that a clinical nurse specialist was available.
Day-case facilities to enable same-day IM CS treatment were commonly available, with 20 (59%) HCPs stating that this was the case, 12 (35%) stating that treatment was available within 1 week and only one HCP stating that no such facility was available. The majority of HCPs (n = 28, 78%) stated that facilities were available to provide IV CS treatment within 1 week, whereas availability of facilities for IACI treatment meant that this treatment was most commonly carried out within 2–4 weeks (n = 15, 43%).
Research question 4: how many potentially eligible children and young people attending hospital in the UK with varying severities of juvenile idiopathic arthritis requiring corticosteroid treatment could be randomised in a comparative treatment study?
A total of 297 patients were identified during the screening exercise. Further information on the number of potential eligible patients is provided and further discussed in Chapter 6.
Extrapolation of results from this screening exercise requires two assumptions to be made:
-
when extrapolating the screening period to a full year effect, there are no seasonal differences
-
a means of estimating the population coverage of the responding centres is available so that results can be extrapolated to the UK total population (i.e. a surrogate for the centres we did not approach/did not respond).
Research question 5: what characteristics would health-care professionals and parents/patients want to see included in a future randomised controlled trial on corticosteroids in juvenile idiopathic arthritis? Which patients should be included/excluded? What would be the most appropriate control in a future trial? How would active disease or a disease flare be defined?
Health-care professionals’ perceptions of the available evidence to support the use of any particular type or route of CS over any other was that it was generally poor (n = 27, 77%), with only two respondents stating that they felt that the available evidence was good or excellent. When asked about the mode of administration of CSs that they felt was best tolerated by patients, HCPs felt that IACIs followed by IV infusions were the best tolerated and IM injections and oral CSs not being used as an adjunct to DMARDs were the least tolerated.
When asked about their thoughts on whether or not each subtype of JIA required a different CS research/protocol, the response from HCPs was equivocal: 13 (36%) thought that ‘yes’ they should, eight (22%) replied ‘no’ and 12 (33%) were ‘not sure’. Table 12 shows the responses given by HCPs when asked which mode of CS treatment is most appropriate in each JIA subtype.
| JIA subtype | Treatment, n (%) (N = 39) | ||||||
|---|---|---|---|---|---|---|---|
| Oral | IV infusion | IM injection | IACI | I do not think CSs as an adjunct to DMARDs are necessary in this subtype | All routes equally effective | No response | |
| Enthesitis-related arthritis | 4 (11) | 8 (23) | 2 (6) | 13 (37) | 1 (3) | 7 (20) | 4 |
| Extended oligoarthritis | 2 (6) | 2 (6) | 1 (3) | 24 (71) | 1 (3) | 4 (12) | 5 |
| Oligoarthritis | 1 (3) | 0 (0) | 0 (0) | 34 (97) | 0 (0) | 0 (0) | 4 |
| RF-negative polyarthritis | 3 (8) | 13 (36) | 1 (3) | 11 (31) | 0 (0) | 8 (22) | 3 |
| RF-positive polyarthritis | 2 (6) | 15 (42) | 2 (6) | 9 (25) | 0 (0) | 8 (22) | 3 |
| Psoriatic arthritis | 5 (14) | 6 (17) | 0 (0) | 17 (47) | 0 (0) | 8 (22) | 3 |
| Systemic-onset JIA | 6 (17) | 29 (81) | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 3 |
Research question 6: how willing would patients/parents be to consent to be randomised in a future clinical trial and how willing would health-care professionals be to randomise?
When asked whether or not they would be willing to randomise patients to the various modes of administration of CSs, responses from HCPs were generally positive and 15 (47%) HCPs replied that they would be happy to randomise patients to any of the four delivery methods (Table 13).
| Treatment | Response | Number of responses, n (%) (N = 39) |
|---|---|---|
| Oral | Yes | 20 (69) |
| No | 2 (7) | |
| Not sure | 7 (24) | |
| No response | 10 | |
| IV infusion | Yes | 20 (69) |
| No | 2 (7) | |
| Not sure | 7 (24) | |
| No response | 10 | |
| IM injection | Yes | 15 (52) |
| No | 7 (24) | |
| Not sure | 7 (24) | |
| No response | 10 | |
| IACI | Yes | 21 (72) |
| No | 1 (3) | |
| Not sure | 7 (24) | |
| No response | 10 | |
| To any of the four delivery methods | Yes | 15 (47) |
| No | 6 (19) | |
| Not sure | 11 (34) | |
| No response | 7 |
Discussion
Key findings
The results from the national survey of clinical practice showed that practices in the management of new patients with JIA and those that are flaring vary. Data captured from the survey showed that the first-line treatment for a new diagnosis in several subtypes of JIA is mainly DMARDs plus IA CSs, with IA CSs alone being the first-line treatment in oligoarthritis and DMARDs plus IV infusion of methylprednisolone plus oral CSs required in systemic JIA.
For polyarticular-course JIA (polyarticular RF positive or RF negative, extended oligoarticular, enthesitis-related arthritis and psoriatic arthritis) the most commonly used first-line treatment is DMARDs plus IA CSs.
Data from the screening log exercise confirmed HCPs’ direct reports and showed that in all subtypes of JIA the most commonly used route of CSs is intra-articular injection. However, there was also evidence of the use of IM injections.
The majority of HCPs who completed this survey indicated that they would be prepared to consider entering patients into a trial that randomised to the various modes of administration of CSs, and approximately half of the HCPs replied that they would be happy to randomise patients to any of the four delivery methods.
Strengths of study
The major strength of the national survey of clinical practice was that it allowed HCPs (paediatric rheumatologists, paediatricians with a specialist interest in rheumatology, adolescent rheumatologists with expertise in JIA and specialist nurses in paediatric rheumatology) who are involved in the care of CYP with JIA to describe their clinical experiences in the management of patients. It also gave them an opportunity to voice their opinions about the acceptability of a future RCT.
The screening log exercise allowed further investigation into the management of patients over a 6-week period and allowed examination of the number of patients who may be potentially available for a future trial in JIA in the UK.
Limitations of study
If the results of a feasibility study are to be incorporated into the planning and design of a future RCT, the RCT should be carried out relatively quickly before the results of the feasibility study are incorporated into clinical practice. Time constraints in waiting for responses from HCPs to the national survey of clinical practice may have led to a more limited response; however, we believe that the responses received were representative of national practice, particularly as the paediatric rheumatologists who completed the survey constituted a high proportion of the total number of these professionals in the UK.
There were four centres that were unable to take part in the screening log exercise because the locally imposed R&D department permissions were not in place; this meant that we could not include data from these centres in the results. Although REC and HRA approvals were not required for this particular activity, some R&D departments enforced local permission processes that were not commensurate with the timing of the screening log exercise. This highlights the difficulties that could be faced when trying to open these centres in a future trial and will inform site selection.
Chapter 5 Choosing a patient-important primary outcome measure
Introduction
Outcomes should be chosen at the beginning of a RCT, and be prespecified and clearly defined in the trial protocol. They are used to compare either the beneficial or the harmful effects between the different interventions under investigation. 113 The primary outcome should give the most patient-important outcome perspective and give convincing evidence directly related to the objective of the trial. 114 Secondary outcomes can help to support the objectives of the trial and to evaluate additional effects of the interventions of interest. The choice of outcomes as primary or secondary is essential in the design of a RCT. In choosing a primary outcome, minimisation of bias is one of the most important factors. If used to influence policy and practice, the outcomes need to be relevant to CYP and their families as well as to HCPs and others making decisions about health care. 115
Given a previous lack of consensus as to which regimen of CS induction should be used in CYP with various disease subtypes and severities of JIA, a RCT of different CS regimens is needed. However, in view of the multiple subtypes of JIA, with flares affecting different joints and systems at different times, the difficulties in achieving such a study are such that a detailed feasibility study was essential to discover whether or not a RCT is acceptable, achievable and will be meaningful to CYP, families and HCPs. Many RCTs find recruitment difficult if CYP, their families and HCPs are not involved in the development of study questions and study protocols. Involving people in the design of RCTs can improve the quality of research and the quality of future care. 116 These principles inspired the concept for this process, whereby a consensus approach was used to decide which outcomes should be measured during the proposed study of CS regimens for CYP with JIA and which should be considered the primary outcome.
This chapter will look at which outcomes are of most importance to both HCPs and parents, by exploring their views in a face-to-face consensus meeting and also by exploring the views of a wider audience by conducting a national survey.
Aims and objectives
The face-to-face meeting and the survey addressed specific aspects of research aim 3 (structured survey and discussion group with stakeholder consensus). The specific questions that the face-to-face meeting and survey aimed to address were:
-
RQ 8 – what primary outcome is important to HCPs?
-
RQ 9 – what primary outcome is important to parents/patients?
-
RQ 5 – which patients should be included/excluded? What would be the most appropriate control in a future trial?
The aim of the face-to-face meeting was to achieve consensus among CYP, their families and HCPs about the primary outcome measure and desired inclusion/exclusion criteria for a prospective feasibility study of CS regimens in CYP with JIA. This unique approach to selecting the primary outcome measure and inclusion/exclusion criteria in a feasibility study of this nature reflects the importance of ensuring that research addresses the needs and concerns of those living with and caring for people with JIA.
The aim of the survey was to investigate the choice of primary outcome by exploring the views of a wider group of CYP, their families and HCPs.
Methods
A modified nominal group technique (NGT) was used to achieve consensus on the most appropriate primary outcome measure to be included in a prospective feasibility study, in addition to the desired inclusion/exclusion criteria of a future study. The NGT was selected as a systematic and robust group process that would permit social interaction and discussions, while limiting dominance by vocal members and normative pressure for conformity, through the use of individual, private voting opportunities, to achieve democratic, consensus agreement between multiple stakeholders with different experiences. 117–119 Individual, hand-held ResponseCard keypads (Turning Technologies, Youngstown, OH, USA)120 were used for voting. Votes were anonymised, with only the stakeholder group (either CYP/families or HCPs) identifiable to the research team.
Following on from the consensus meeting, a survey was conducted to gain further information to help choose a primary outcome for the future RCT. The survey was sent to a wider group of HCPs using BSPAR members, the BSPAR nurses and the BANNAR mailing lists. CYP and their families were identified through dissemination of the opportunity to get involved on social media platforms and by electronic mailing to patient/parent and charitable organisations, co-ordinated by the study’s PPI representative (SS) (see Report Supplementary Material 3).
Definition of consensus
The outcome of individual votes was classified as ‘consensus in’, ‘consensus out’ or ‘no consensus’ in accordance with the following criteria based on the use of a 9-point Likert scale (from 1, not important, to 9, very important):121
-
consensus in – > 70% of participants scoring 7 to 9 and < 15% of participants scoring 1 to 3
-
consensus out – > 70% of participants scoring 1 to 3 and < 15% of participants scoring 7 to 9
-
no consensus – anything else that does not meet the ‘consensus in’ or ‘consensus out’ criteria.
Participants involved in consensus meeting
The NGT is designed to be a small-group technique, recommended for groups of approximately 10 participants. 117,119 We advertised the consensus meeting on social media relevant to CYP and their families, and also recruited through charity/peer networks and by contacting participants of the previous SIRJIA qualitative study. 36 Those who expressed an interest in attending the consensus meeting were asked to provide contact details. HCPs were informed of the consensus meeting through the professional networks previously described. The research team then shared full details of the consensus meeting, including the date, time, location and the expenses and child care policy with CYP and their families. Participants’ time and contributions were recognised, in line with INVOLVE guidance on budgeting for public involvement in research. 122 Child-care services were provided in a child-friendly environment at the Museum of Liverpool, to enable parents/carers with younger children to attend.
Procedure of consensus meeting
The NGT is a structured process that follows a prescribed set of problem-solving stages to facilitate the decision-making process. The process is described in Figure 2, with each of the stages of the NGT discussed below. The ultimate outcome of the process is the aggregation of preferences from multiple stakeholders to give an overall response to the preferred primary outcome measure for inclusion in a prospective feasibility study. 119
FIGURE 2.
Itemised process of the SIRJIA consensus meeting using a modified NGT.
The selection of the most appropriate inclusion/exclusion criteria followed the process of identifying the preferred primary outcome measure. A group of non-voting observers and facilitators ensured that the process was not dominated by any one individual with strong views, while encouraging those with less confidence to voice their opinions.
Stage 1: identification of candidate primary outcome measures
Prior to the SIRJIA consensus meeting,36 multi-stakeholder discussions facilitated the compilation of a list of 10 candidate primary outcome measures for potential inclusion in a prospective feasibility study. This built on work that was conducted by an interdisciplinary team in the UK to develop a minimal core data set for CYP with JIA. 123 A group of patient research partners representing patient organisations [Versus Arthritis and the Scottish Network for Arthritis in Children (SNAC) (Edinburgh, UK)], Your Rheum (Manchester, UK), the paediatric rheumatology CSG and the patient research partner on the SIRJIA SMG reviewed the draft outline and scoring mechanism of the candidate primary outcome measures (Table 14) prior to its inclusion in the SIRJIA consensus meeting. 36
| Outcome measure | Outcome measure description |
|---|---|
| Patient or Parent/Carer Global Assessment of Wellbeing4 | A 10-point VAS score self-reported by CYP and/or their families, describing how JIA has affected the child in the past week, considering all of the ways the condition has affected the child |
| Physician Global Assessment of Disease Activity4 | A 10-point VAS score self-reported by a qualified HCP, assessing overall JIA disease activity in CYP. Judgement is made by interpreting the physical examination, reported symptoms and test results |
| Morning stiffness in minutes | A self-reported numerical estimate of the number of minutes of morning stiffness experienced by CYP with JIA |
| Number of joints with active arthritis (swollen and tender joints), as stated by the physician4 | Defined as the number of swollen and tender joints that a HCP identifies when examining CYP with JIA |
| Number of joints with active arthritis (swollen and tender joints), as stated by the patient/parenta | Defined as the number of swollen and tender joints that CYP with JIA and/or families self-report to HCPs |
| Number of joints with active arthritis (swollen only), as stated by the physician | Defined as the number of swollen joints that a HCP identifies when examining CYP with JIA |
| Number of joints with active arthritis (swollen only), as stated by the patient/parentsa | Defined as the number of swollen joints that CYP with JIA and/or families self-report to HCPs |
| JADAS-715 | This is a composite outcome measure using four variables measured in the clinical setting:
|
| Wallace criteria for CID124 | This is a composite outcome measure using a combination of factors to decide whether or not CYP have inactive disease (no active arthritis or uveitis). For inactive disease, CYP should have:
|
| JIA Core Set4 | This is a composite outcome measure consisting of:
|
Stage 2: introductory discussions in a face-to-face group setting
Participants attended the 1-day SIRJIA consensus meeting in Liverpool, UK, in July 2017. The meeting was chaired by one of the SIRJIA HCP co-applicants and was formally opened by two patient research partners, one of whom sits on the SIRJIA SMG as a co-applicant. The patient research partners welcomed everyone to the meeting and initiated an icebreaker activity to enable the participants to get to know each other. Participants were then briefed about the process and agenda of the consensus meeting (Table 15). Participants were provided with handouts detailing each of the primary outcome measures for consideration for reference throughout the meeting. There was a PowerPoint® (Microsoft Corporation, Redmond, WA, USA) presentation about the 10 outcomes to be decided between; however, the first voting exercise was a preliminary vote, to assess choices by participants with no or limited prior knowledge.
| Time | Outline |
|---|---|
| 09:30 | Welcome, introduction and icebreaker activity |
| 09:50 | Stage 2: outline of the SIRJIA consensus meeting and the SIRJIA research overview |
| 10:20 | Stage 3: first voting exercise of primary outcome measures and presentation of qualitative study. Participants are shown the results before they break into groups |
| 11:00 | Stage 4: group-based discussion (HCPs alongside CYP with their families) of the results of the first voting exercise, data collection frequencies and the inclusion/exclusion criteria for the prospective feasibility study |
| Stage 5: facilitators of the two groups then feedback to the wider group about the discussions that were held, before the second voting exercise | |
| 12:00 | Lunch and networking |
| 13:00 | Stage 6: the third voting exercise is conducted |
| 14:00 | Break and collation of the results |
| 14:30 | Stage 7: presentation of the final vote results, highlighting the majority consensus and the selected primary outcome measure |
| Stage 8: voting of the inclusion/exclusion criteria for the prospective feasibility study | |
| 15:30 | Summary, next steps and closure of the meeting |
Stage 3: first voting exercise of primary outcome measures
Each of the 10 outcome measures was described to participants using a PowerPoint presentation, and participants were encouraged to ask questions to enable them to understand each of the outcome measures. Voting occurred anonymously and independently using an electronic voting system that counted votes in real time. This prevented participants being influenced by others’ votes. Voting was intercalated with the explanations of the candidate outcome measures, with participants voting for an outcome measure immediately after its explanation on screen. This vote was recorded as the first vote. The votes were counted and verified by two observers with statistical expertise (AJ and TM).
The first vote was preliminary in nature, as previously described, to ease participants into the process in the light of no or limited prior knowledge. The subsequent discussions were then useful in clarifying the 10 outcome measures that had previously been selected for potential inclusion in a feasibility study, helping to eliminate misunderstanding, particularly among non-HCPs. 119 To add additional context to the SIRJIA consensus meeting, participants were given a short presentation that highlighted the results from the preceding qualitative study that explored families’ views on outcomes of importance to them in receiving CS treatment for JIA,125 as part of the larger SIRJIA study. 36
Stage 4: group-based discussion (health-care professionals alongside children and young people with families) of the results of the first voting exercise and the inclusion/exclusion criteria for the prospective feasibility study
Prior to conducting the second vote, the participants were split into two separate groups for 1 hour: one group for CYP and their families and the other group for HCPs. Discussions were carried out on the choice of primary outcome measures, the suggested time intervals for the collection of outcome data and decisions regarding the inclusion and exclusion criteria for the prospective feasibility study. These subgroups were each led by one patient research partner (HB or SS) and one researcher (FS or BY). The purpose of these subgroup-based discussions was to enable all participants to have the opportunity, in a more relaxed and familiar environment, to voice their thoughts and concerns in the light of the first (preliminary) vote, discussions and qualitative study presentation; thus, these subgroups enabled participants to voice concerns that could then be anonymously presented back to the larger group. Indeed, when the groups convened, one selected individual from each of the subgroups presented their summarised discussions back to the wider group, which prompted discussion among participants.
Stage 5: second voting exercise and discussion
The second vote was then conducted using the same technique as the first vote in stage 3, with discussion as necessary. The votes were counted and verified by two observers (AJ and TM).
Stage 6: third voting exercise
Following the presentation of the first and second votes, and another brief explanation of the final candidate outcome measures, a third and final vote was conducted. The votes were counted and verified by two observers (AJ and TM).
Stage 7: selection of the primary outcome measure
The majority vote choice was considered as the primary outcome for the prospective feasibility study. The results were then reported back to all of the participants.
Stage 8: voting of the inclusion/exclusion criteria for the prospective feasibility study and time for assessing the primary outcome measure
Discussions took place regarding the time intervals for the collection of SIRJIA feasibility study36 outcome data and for measuring the primary outcome. This naturally fed into a discussion that was led by the chairperson (MWB) on the inclusion/exclusion criteria for the prospective feasibility study. Facilitators emphasised that in the prospective feasibility study, details and reasons for exclusion would be noted on the screening log. It was suggested that the following groups be considered ineligible for and be excluded from the feasibility study:
-
CYP with arthritis as part of another health condition, such as a connective tissue disease (e.g. lupus)
-
CYP with JIA and haemophagocytic lymphohistiocytosis complicating their JIA, where current standard of care will be used as their treatment
-
CYP with JIA and a severe infection complicating their JIA at the time of disease flare.
A vote to choose eligible patients for a prospective feasibility study was conducted, with participants voting ‘yes’ or ‘no’ to the questions below:
-
Should CYP with oligoarticular JIA (four or fewer joints involved) be included in the study?
-
Should CYP with polyarticular JIA (five or more joints involved) be included in the study?
-
Should CYP with systemic JIA be included in the study?
-
Should only CYP aged ≤ 16 years be allowed to take part in the study?
The meeting was closed by the chief investigator (EMB), who thanked all of the participants for attending and for their active contributions to shaping the future direction of this research.
Results
Participants
Fifteen individuals participated in the SIRJIA consensus meeting [two CYP, one sibling, six parents/carers and six HCPs (three clinical nurse specialists and three doctors)]. Parents/carers included those with children aged ≤ 5 years and children aged 5–11 years and parents/carers of young people aged 11–18 years. CYP and their families represented a spread of geographical locations across the UK, including Northern Ireland, Scotland and England. HCPs were spread across England, with invited participants from the devolved nations being unable to attend.
The majority of families and CYP with JIA in attendance were diagnosed with systemic JIA, as reported by parents/carers. There was an unplanned and unexpected preponderance of the systemic subtype and this was a result of many factors, including availability on the day, and the relationship with teams from around the country that recommended participants to the study. The fact that systemic patients had more long-term interactions with their medical teams probably increased their willingness to volunteer to help. It is also likely that they had had more experience with all of the various types of CSs as part of their treatments. However, it was clear from the discussions that this group of CYP and their families were well aware of the other subtypes of arthritis. They themselves had also experienced flares involving only a few joints without systemic features and the quality of their discussion was consciously inclusive of other subtypes. The choice of the JADAS-71 and the cJADAS was made despite the fact that these measures do not include systemic features at present (although it is likely that there will be a validated systemic JADAS in the near future). We take this to illustrate that, regardless of their disease subtype, the majority of the CYP and their families who participated were able to choose the composite score that was of the widest application at the time of the consensus meeting.
To our knowledge, this was first time when using JIA consensus methods that CYP and their families outnumbered HCPs (by 9 to 6). Despite this being a consensus process on a complex medical topic, the level of understanding was such that all concerned were confident in the outcome of the process.
In addition, 10 non-voting observers and facilitators with academic, clinical and lay experience were present. These comprised two consultants in paediatric rheumatology (MWB and FMcE), two researchers (FS and BY), two public involvement champions (HB and SS), one statistician (AJ), two trial management colleagues (TM and GN) and the chief investigator (EMB), who assumed a spectator role, to eliminate bias as much as possible, but was available to answer questions. This was important so that queries about the design of the feasibility study could be addressed appropriately.
Identifying a primary outcome measure to include in a prospective feasibility study
In the first vote, JADAS and Physician Global Assessment Score were ‘consensus in’ and there was ‘no consensus’ for all other outcomes. During subgroup discussions with HCPs and CYP and their families, the need for a composite score was emphasised. Participants highlighted that a composite score would be more inclusive of all subtypes of JIA, having discussed within subgroups the subjective nature of other individual outcome measures in their own right. Of the three candidate composite outcome measures, the JADAS did not capture data about fever, which was relevant for those with systemic JIA. However, during subsequent discussions with all of the participants, JADAS was regarded well by most participants as a good composite score of current disease activity that would include systemic features. CYP and family participants preferred the term ‘active disease’ to ‘joint damage’ in the JADAS, considering this a more positive and optimistic phrase to use when assessing disease activity. CYP and their families reaffirmed that outcome measures should include the perspectives of CYP and their families, for example by including CYP- and family-reported measures, as well as those reported by HCPs. However, HCP participants pointed out a potential drawback of the JADAS, in that some centres no longer measure ESR routinely and, therefore, the JADAS would need to include measurement of C-reactive protein (CRP); alternatively, the cJADAS could be used.
Participants went on to discuss the Wallace criteria for CID124 and the JIA Core Set4 in more detail; however, participants felt that this may be too stringent an outcome for acute flares. This was developed to assess outcome at 6 months; however, even if the timescale for this measure was shortened, the need for truly inactive disease was felt to be unrealistic as a primary outcome. The JIA Core Set assesses a limited number of joints and includes the CHAQ, which HCP participants professed to dislike because, although it produces a good validated score, they believe that it is often used inconsistently across the UK in clinical practice (although this opinion was largely based on anecdotal experience). CYP and their families also expressed their dislike of the CHAQ, stating that the measure is no longer relevant to 21st-century CYP with JIA (e.g. it does not assess the use of technology and asks questions regarding the ability of CYP with JIA to complete household jobs, which parents/carers joked about given that their children would be unlikely to do such jobs even when they were well).
In addition, participants highlighted that the study should assess the wider effect of CSs on CYP’s physical and psychosocial health, and not just the isolated effects of CSs on JIA. The need for families to provide input here was discussed on multiple occasions, and it was felt that a parental or carer assessment of pain alone using a VAS was inadequate and, instead, parental/carer assessment of global disease activity was merited. In addition, CYP and their families described how it is very difficult to report aspects such as pain and stiffness, given that these symptoms often wax and wane. Measures such as morning stiffness in minutes do not account for the temporal fluidity of symptoms nor for the difficulty of retrospective recollection that those with fatigue and cognitive difficulties often experience. The tone of the conversation also changed direction during the HCP subgroup discussion as the topic of side effects was considered. Participants raised the issue of including both effectiveness and the experience of CSs as primary outcome measures and debated if there would come a point during a study of CS treatment when side effect burden would take priority over the effectiveness of CSs, and vice versa. Participants concluded that patient experience was integral to any trial of therapy among CYP with JIA. It was foreseen that experience would be captured within a composite outcome measure that included a patient-reported/parent- or carer-reported assessment of well-being. However, HCP participants emphasised that experience could be factored as a secondary measure in its own right given that experience could impact on the outcomes observed. This was reflected in the CYP and families subgroup discussion, in which participants felt that many CS-induced side effects would not be captured with the outcomes discussed, including fatigue, a Cushingoid appearance and behaviour changes. In addition, CYP and their families reported that CYP can develop an aversion to the colour yellow, which they negatively associate with MTX and sharps bins, and, therefore, suggested that any future study documentation should avoid this colour.
When the whole group reconvened, participants raised a number of important issues that they felt were not necessarily captured by some of the existing outcome measures, for example the effect of fatigue and pain on the lives of CYP, as well as the impact of treatment on psychosocial health and quality of life. Similarly, CS side effects were not captured by the candidate primary outcome measures. Families also expressed the potential difficulty of encouraging their child to take treatment at times. In summary, participants suggested that some outcomes, including side effects and compliance, could become secondary outcome measures in a prospective feasibility study.
In the second vote, ‘morning stiffness in minutes’ was ‘consensus out’, whereas JADAS and the JIA Core Set were ‘consensus in’ (Table 16). This led to a discussion about the validation of outcome measures, including the shortlisted composite measures. It was explained to participants that all of the proposed measures had previously been validated, although the single-item outcomes are often used as secondary outcomes in trials. This helped to clarify the purpose of the primary outcome measure prior to the third vote. In the third vote, two outcome measures were shortlisted through the ‘consensus in’ approach: JADAS and the JIA Core Set. The results of the third vote clearly identified JADAS as the primary outcome measure of choice among all participants (100%).
| Outcome | Score | Subgroup, n (%) | Overall, n (%) (N = 15) | |
|---|---|---|---|---|
| HCP (n = 6) | CYP and their family (n = 9) | |||
| Patient or Parent/Carer Global Assessment of Wellbeing4 | 1–3 | 2 (33) | 5 (56) | 7 (47) |
| 4–6 | 4 (67) | 2 (22) | 6 (40) | |
| 7–9 | 0 (0) | 2 (22) | 2 (13) | |
| Physician Global Assessment of Disease Activity4 | 1–3 | 2 (33) | 1 (11) | 3 (20) |
| 4–6 | 2 (33) | 5 (56) | 7 (47) | |
| 7–9 | 2 (33) | 3 (33) | 5 (33) | |
| Morning stiffness in minutes | 1–3 | 4 (67) | 8 (89) | 12 (80) |
| 4–6 | 2 (33) | 1 (11) | 3 (20) | |
| 7–9 | 0 (0) | 0 (0) | 0 (0) | |
| Number of joints with active arthritis (swollen and tender joints), as stated by the physician4 | 1–3 | 3 (50) | 1 (11) | 4 (27) |
| 4–6 | 3 (50) | 4 (44) | 7 (47) | |
| 7–9 | 0 (0) | 4 (44) | 4 (27) | |
| Number of joints with active arthritis (swollen and tender joints), as stated by the patient/parent | 1–3 | 5 (83) | 0 (0) | 5 (33) |
| 4–6 | 1 (17) | 5 (56) | 6 (40) | |
| 7–9 | 0 (0) | 4 (44) | 4 (27) | |
| Number of joints with active arthritis (swollen only), as stated by the physician | 1–3 | 4 (67) | 3 (33) | 7 (47) |
| 4–6 | 2 (33) | 4 (44) | 6 (40) | |
| 7–9 | 0 (0) | 2 (22) | 2 (13) | |
| Number of joints with active arthritis (swollen only), as stated by the patient/parents | 1–3 | 4 (67) | 3 (33) | 7 (47) |
| 4–6 | 2 (33) | 4 (44) | 6 (40) | |
| 7–9 | 0 (0) | 2 (22) | 2 (13) | |
| JADAS-715 | 1–3 | 0 (0) | 0 (0) | 0 (0) |
| 4–6 | 0 (0) | 3 (33) | 3 (20) | |
| 7–9 | 6 (100) | 6 (67) | 12 (80) | |
| Wallace criteria for CID124 | 1–3 | 1 (17) | 0 (0) | 1 (7) |
| 4–6 | 4 (67) | 3 (33) | 7 (47) | |
| 7–9 | 1 (17) | 6 (67) | 7 (47) | |
| JIA Core Set4 | 1–3 | 1 (17) | 1 (11) | 2 (13) |
| 4–6 | 0 (0) | 1 (11) | 1 (7) | |
| 7–9 | 5 (83) | 7 (78) | 12 (80) | |
Time intervals for the collection of outcome data
Participants went on to discuss the time at which outcome measures should be reported, prioritising age and JIA subtype, long-term disease activity and side effects as the most influential factors in determining a schedule for data collection. Informal consensus among participants confirmed that outcomes need to be measured sooner than 3 months post CS delivery, given that existing long-term treatments used as part of standard of care would influence the outcomes recorded. Some participants felt that the first time point should be at around 6 weeks post CS delivery, given the acute action of CSs and the fact that the effect of some single-injection delivery routes would decrease with time. In principle, there was general agreement that a 6-week measurement of primary outcome was appropriate. Participants implied that secondary outcomes should be collected more frequently between standard collection points post CS delivery, to generate a better picture of the short- and long-term effect of CS treatment. A variety of time points were suggested: CYP and their families suggested biweekly, weekly and fortnightly, whereas HCPs suggested every 48 hours initially, with longer intervals over time. Families pointed out that their children can experience rapid changes, within 1 week, and felt that this would not be appropriately captured at 6 weeks or 3 months.
Regardless of the time interval chosen, participants emphasised that the process needs to be as easy as possible and suggested that real-time digital capture mechanisms are the most appropriate method of data collection. Examples included short message service (SMS) text messaging, a mobile phone or tablet application, or a secure website. It was recognised that the introduction of technological methods of data collection could require additional effort for all stakeholders involved, for example the governance challenges of implementing electronic data collection from CYP, as well as the requirement for CYP and their families to self-report data during their existing daily lives. In addition, participants agreed that the prospective feasibility study would last for 3 months, given the rapid onset of action of CSs.
Agreement of the inclusion and exclusion criteria for the prospective feasibility study
The majority of HCPs and CYP and their families (93%) voted for the inclusion of all disease subtypes of JIA (including oligoarticular, polyarticular and systemic JIA). Broken down, 93% of participants voted to include CYP with oligoarticular JIA, 100% voted to include CYP with polyarticular JIA and 87% of participants voted to include CYP with systemic JIA. Participants raised some concerns during this voting process, given that some non-HCP participants felt that this was a difficult question to answer, particularly if their knowledge of disease subsets was limited or restricted to a certain JIA subtype. Participants queried whether or not CYP who had severe systemic JIA would realistically be eligible to participate in the study, which CYP and parent/carer participants suspected not, because IACI or oral CS delivery would not be suitable given the urgent need for rapid treatment action. A level of severity could be defined specifically in the exclusion criteria for the trial. A solution to this would be to split the trial into JIA subtypes. CYP and their families queried how systemic features such as rashes would be captured. It would be important that HCPs treating CYP with JIA in the future study identify subtype-specific features, as well as the overall outcome measures of response to CSs.
A small majority of participants (53%) were not in favour of setting an inclusion criterion of age ≤ 16 years in the feasibility trial. Participants felt that the age limit should be extended beyond 16 years, given that the study could be of use to those with childhood-onset JIA who have active disease in adulthood. In addition, this age cut-off point was seen to be discriminatory to older adolescents with JIA, who would be denied access to a trial that could directly benefit them. Discussions then took place about the proposed reason for the age cut-off point that had been specified in the original HTA Commissioning Board brief. This could include the existing complexity of the study, the increasing complexity of collecting measurements with increasing age and the need to agree on a sensible cut-off point that currently corresponds to the definition of JIA as a disease with onset before the age of 16 years. However, the facilitators agreed to communicate this concern to the relevant funding body during the analysis of the overall SIRJIA study. 36 This proposal resonated with participants, as they felt that their voices in research issues, such as defining inclusion/exclusion criteria by age, should be heard and actioned.
Survey findings
There were a total of 45 responses from HCPs: 30 (66%) NHS consultants, six (13%) clinical nurse specialists, five (11%) grid trainees, three (7%) clinical research fellows and one (2%) academic; there was a total of 16 responses from CYP and/or their families.
The results for each of the outcomes can be seen in Table 17. Overall, three outcomes had an overall score in the category of 7–9 of > 70%. These were:
-
number of joints with active arthritis (swollen only), as stated by the physician
-
JADAS-71
-
Wallace criteria for CID. 124
| Outcome | Score | Subgroup, n (%) | Overall, n (%) | |
|---|---|---|---|---|
| HCP | CYP and their family | |||
| Patient or Parent/Carer Global Assessment of Wellbeing | 1–3 | 3 (7) | 0 (0) | 3 (5) |
| 4–6 | 22 (49) | 8 (50) | 30 (50) | |
| 7–9 | 20 (44) | 7 (44) | 27 (45) | |
| Physician Global Assessment of Disease Activity | 1–3 | 5 (11) | 1 (6) | 6 (10) |
| 4–6 | 9 (20) | 10 (63) | 19 (31) | |
| 7–9 | 31 (69) | 5 (31) | 36 (59) | |
| Morning stiffness in minutes | 1–3 | 12 (27) | 6 (38) | 18 (30) |
| 4–6 | 22 (49) | 5 (31) | 27 (44) | |
| 7–9 | 11 (24) | 5 (31) | 16 (26) | |
| Number of joints with active arthritis (swollen and tender joints), as stated by the physician | 1–3 | 3 (7) | 2 (12.5) | 5 (8) |
| 4–6 | 8 (18) | 7 (44) | 15 (25) | |
| 7–9 | 34 (76) | 7 (44) | 41 (67) | |
| Number of joints with active arthritis (swollen only), as stated by the physician | 1–3 | 2 (4) | 1 (6) | 3 (5) |
| 4–6 | 5 (11) | 6 (38) | 11 (18) | |
| 7–9 | 38 (84) | 8 (50) | 46 (77) | |
| JADAS-715 | 1–3 | 2 (4) | 0 (0) | 2 (3) |
| 4–6 | 8 (18) | 4 (25) | 12 (20) | |
| 7–9 | 35 (78) | 11 (69) | 46 (77) | |
| Wallace criteria for CID124 | 1–3 | 3 (7) | 0 (0) | 3 (5) |
| 4–6 | 8 (18) | 6 (38) | 14 (23) | |
| 7–9 | 34 (76) | 10 (63) | 44 (72) | |
| JIA Core Set4 | 1–3 | 1 (2) | 1 (6) | 2 (3) |
| 4–6 | 15 (33) | 2 (13) | 17 (28) | |
| 7–9 | 29 (64) | 12 (75) | 41 (68) | |
The survey results revealed three outcomes that were classified as ‘consensus in’: number of joints with active arthritis (swollen only), as stated by the physician, and JADAS. No outcomes were classified as ‘consensus out’.
Participants were asked to choose one of the proposed outcomes as the ‘very best primary outcome’ for a CS study in JIA (Table 18). The majority (53%) of HCPs reported that they would prefer the primary outcome of choice to be JADAS, with 20% stating that they thought the Wallace criteria for CID124 should be the primary outcome. There was a two-way tie among CYP and their families with regard to what they thought that the primary outcome for a future trial should be. Equal numbers (n = 5, 31%) chose the JADAS and the number of joints with active arthritis (swollen and tender joints), as stated by the physician.
| Outcome | Subgroup, n (%) | Overall (N = 61), n (%) | |
|---|---|---|---|
| HCP (N = 45) | CYP and their family (N = 16) | ||
| Patient or Parent/Carer Global Assessment of Wellbeing | 1 (2) | 0 (0) | 1 (2) |
| Physician Global Assessment of Disease Activity | 2 (4) | 0 (0) | 2 (3) |
| Morning stiffness in minutes | 0 (0) | 1 (6) | 1 (2) |
| Number of joints with active arthritis (swollen and tender joints), as stated by the physician | 0 (0) | 5 (31) | 5 (8) |
| Number of joints with active arthritis (swollen only), as stated by the physician | 3 (7) | 0 (0) | 3 (5) |
| JADAS5 | 24 (53) | 5 (31) | 29 (48) |
| Wallace criteria for CID124 | 9 (20) | 3 (19) | 12 (20) |
| JIA Core Set4 | 6 (13) | 2 (13) | 8 (13) |
Discussion
This consensus process demonstrates the unique insights that can be generated by working with a group of stakeholders, including CYP with JIA, their families and HCPs. The fact that CYP and their families outnumbered HCPs by 9 to 6 demonstrates their willingness to discuss and express opinion about complex medical scenarios,126 to define inclusion/exclusion criteria and to identify what CYP and their families find acceptable in terms of taking part in research. 127,128 By engaging and involving key stakeholders in the SIRJIA from the outset, CYP, families and HCPs have truly informed and shaped the direction of a possible future RCT to deliver research findings that are both meaningful and relevant to CYP with JIA and their families. 129
By using NGT to develop consensus among a variety of stakeholders, we found that the preferred primary outcome measure for inclusion in a prospective feasibility study was a composite measure. 130 The choice of a composite measure became increasingly clear between votes, once participants had familiarised themselves with the candidate outcome measures. The option to have secondary outcomes in any future study in addition to the primary outcome measure was clarified. This indicates that discussions and re-ranking did not significantly change participants’ views on the need for a composite primary outcome measure to paint a broader picture of the health of CYP within the study. However, there was an example in which the specific components of the shortlisted composite measures were scrutinised and deemed ‘consensus out’, which may reflect the impact of the detailed discussions that took place about the relevance of specific composite measure components to CYP currently living with JIA. Such discussions, and the consensus that followed, may not have happened if CYP and their families were not involved in the consensus process, given that it was CYP and their families who felt that the JIA Core Set and Wallace criteria for CID were less appropriate to them than the JADAS.
Furthermore, CYP and their families favoured the idea of recruiting CYP with JIA who were aged ≥ 16 years; however, such outcome measures are not necessarily suitable or validated in these populations, which prompted careful planning at this stage of study development to ensure that the selected outcome measure was age appropriate. Questions were also raised as to whether or not the Juvenile Arthritis Disease Activity Score based on C-reactive protein (JADAS-CRP) and the clinical JADAS were validated in trials, recognising that some centres no longer routinely test the ESR. Indeed, the JADAS-CRP has been validated131,132 and would be a realistic and practical alternative. The clinical or three-part JADAS has also been validated. 133
Participants raised a number of valid and important points about outcomes that were not necessarily captured by the candidate outcome measures, including the composite measures. These revolved around symptoms such as fatigue and pain, the side effects of CS treatment and the physical and psychosocial impact of treatment on the lives of CYP and their families. It was agreed that these are important outcomes to report and that they would be considered as possible secondary outcomes in a prospective feasibility study. However, HCPs highlighted the difficulties in capturing the long-term side effects of CS treatment, but recognised the need for the community to know about the short- and long-term side effects. CS side effect scores are in the process of being developed, but none is yet validated for clinical use CYP and their families supported this notion and confirmed that existing information was scarce. Furthermore, CYP and their families asked whether or not certain outcomes could be added or combined to existing composite measures, for example whether or not fever could be added to the JADAS. Discussions were held about why this could not be achieved, because the JADAS was already being validated in its current form. However, participants were reassured that fever in CYP with systemic JIA would be measured as a secondary outcome.
Interestingly, participants suggested that secondary outcome measures could be self-reported by CYP and/or their families through real-time remote monitoring using digital technology;134,135 this could be achieved through SMS text message, mobile phone application or a secure website. Participants indicated that this would appeal to CYP and their families given that it would potentially reduce the frequency of clinical study visits. However, participants stated that such collection methods should not be labelled as a ‘diary’ as this can be counterintuitive for participation and engagement.
A visible difference in engagement was seen among participants when they were actively involved in voting and discussing the primary outcome measures and inclusion/exclusion criteria, compared with discrete points in the agenda when didactic presentations were given to participants. Although it is sometimes necessary to provide content in such a way to inform participants of relevant concepts before their participation in activities, this observation acts as a reminder to encourage the co-design of appropriate, informative and participatory activities that share knowledge with participants while conserving their engagement. 136
This consensus process confirms the essential role of CYP and their families in co-designing research,137 which is evident by the important and critical comments that were communicated during the process. CYP and their families also expressed an explicit desire to be involved in preparing information about trials that would be given to CYP and their families. This requires careful attention, from the information content to the overall design of such information and recognising the sensitivities that need to be addressed, such as the use of appropriate aesthetics and language. 138 Adherence to treatment is a concern and issue for many CYP. Hence, any clinical trial involving treatment requires careful and rigorous development with meaningful input from CYP and their families from the outset, to mitigate against challenges in the delivery of the clinical trial itself. 125
The survey that was conducted after the consensus meeting was completed by 45 HCPs and 16 CYP and their families. This allowed a wider group of individuals to provide their opinion in the choice of outcomes that should be used in any future trial. Both the HCPs and the CYP and their families chose the JADAS as the primary outcome measure in future trials, with CYP and their families also choosing the number of joints with active arthritis (swollen and tender joints), as stated by the physician, as a joint favourite.
The JADAS outcome was voted as ‘consensus in’ during both the consensus meeting and the survey; inclusion of the JIA COS, although voted as ‘consensus in’ during the meeting, did not achieve consensus in the survey. The number of joints with active arthritis (swollen and tender joints), as stated by the physician, was also voted as ‘consensus in’ during the survey and there was ‘no consensus’ from the meeting. Morning stiffness was voted as ‘consensus out’ during the meeting and ‘no consensus’ from the results of the survey.
Strengths and limitations
A key strength of this study is the way in which CYP with JIA and their families have been involved in co-designing the study with HCPs and researchers. Post-consensus meeting feedback was overwhelmingly positive, with participants expressing their satisfaction in the process and in the opportunity to discuss and shape the SIRJIA. 36 For future iterations of such workshops, participants suggested that a guided pathway could be provided about treatment options for CYP with JIA that contextualised where CSs fit into the average treatment regimen. In addition, an information document that distinguished the different subtypes of JIA would have been helpful, as participants felt ill-informed when selecting which subsets of JIA should be included or excluded. Significant preparation is required to equip participants with the appropriate knowledge and support to make informed decisions.
When using the NGT to achieve consensus, some individuals may feel that the process is inflexible, given that the shortlisted primary outcome measures were prespecified prior to the consensus meeting. Although the NGT has been recommended for groups of no more than 10 participants,139 in our consensus process the larger number of 15 participants (including CYPs, their families and HCPs) was needed for fuller representation of all stakeholders.
It has been suggested that larger groups should be split into two or more smaller groups and the consensus votes pooled together;139 however, we did not want to separate CYP, their families and HCPs during voting to achieve a true partnership in voting. Previous research has questioned the merit of having mixed groups of participants in developing consensus using the NGT. 139,140 However, we have challenged this and now emphasise the importance of using a mixed-group approach to understand and integrate the views of CYP, their families and HCPs. The integration of views from different participants was aided by an experienced facilitator, with the support of impartial observers. Private anonymous voting using remote controllers also helped to reduce social pressure to conform. However, despite best efforts to empower and engage all of the participants, there remains the possibility that CYP and their families may have felt less confident and able to voice their opinions in the presence of HCPs, particularly during discussions. Likewise, HCPs may have felt in a similar position, reluctant to risk offending or upsetting any CYP and their families. We took practical steps to avoid such situations, including facilitation by an independent chairperson and patient research partners, in addition to the appointment of spokespeople within subgroups to feed back to the wider group.
Conducting the survey of opinions after the consensus had both strengths and limitations. The major strength was that a far greater number of CYP, their families and HCPs were able to take part and provide their opinion on what is a very important decision in the planning of a RCT. However, unlike at the consensus meeting, there was no facility to discuss the actual detail of the outcomes, detail that attendees on the day of the consensus meeting felt was hugely beneficial to their understanding.
Conclusion
In this consensus process, we have demonstrated the feasibility of involving multiple stakeholders, including CYP and their families, in synthesising complex concepts to agree, by consensus, the design components of clinical research, using the NGT. The primary outcome measures for inclusion in a prospective feasibility study of CS regimens in CYP with JIA were co-prioritised by all key stakeholders, with CYP, their families and HCPs sharing the role in the ultimate selection of the JADAS as an appropriate composite outcome measure by consensus agreement. We recommend that all key stakeholders, including CYP, families and HCPs, be involved in designing clinical research that will address the needs and preferences of CYP with JIA and their families, while ensuring the design of such research is methodologically and clinically appropriate.
Chapter 6 Observational prospective feasibility study
Aims and objectives
We aimed to conduct an observational prospective feasibility study in centres across the UK. This prospective feasibility study included newly diagnosed patients with JIA who fulfilled the proposed inclusion/exclusion criteria and naturalistically received treatments that would become the proposed control or treatment arms; the most common treatment would be considered to be the control treatment arm. This would address research aim 4 (to conduct a prospective observational study of newly diagnosed patients with JIA who fulfil the proposed inclusion/exclusion criteria and who naturalistically receive the proposed control or treatment arms, and to observe change and variance in the primary outcome over a 12-week period to inform the precision of the sample size calculation).
The main objectives were to:
-
collect further information on the number of eligible patients for a future trial
-
identify suitable sites and collaborators
-
determine the potential sample size for any future trial
-
collect information on the outcome measures chosen in the first consensus process (both primary and secondary) to be used in a future trial.
Methods
Study design
This was an observational prospective feasibility study in participants who had received a new diagnosis of JIA, or those participants who had flaring disease requiring induction of remission.
Sites were asked to treat participants as per routine clinical care, with the CS route and doses chosen to be in keeping with their prior clinician decision.
Eligibility criteria
Children and adolescents aged < 16 years who had a new diagnosis of JIA or flaring disease requiring induction of remission that needed CS treatment were eligible for registration.
Participants who had arthritis as part of another disorder, such as connective tissue disease, or who had haemophagocytic lymphohistiocytosis or a severe infection complicating their JIA at the time of disease flare, were not eligible to take part in the study.
Ethics approval
The trial protocol was not initiated until it had received the favourable opinion of the main REC (16/NE/0047) on 21 September 2017. Subsequent to this, it was reviewed at the R&D offices at participating sites.
The trial and any subsequent amendments were reviewed and approved by North East – Newcastle & North Tyneside 3 REC (16/NE/0047).
Recruitment
The study was carried out in 15 UK centres. Participants were identified through paediatric rheumatology outpatient clinics.
Informed consent
This study recruited CYP aged < 16 years. Informed consent procedures reflected the legal and ethics requirements to obtain valid informed consent for this population. Prior written informed consent was required for all trial participants.
Information was provided to potential participants and their families verbally and in writing. All of the participants had the opportunity to discuss the project with the responsible investigator at the site and/or a designated member of the research team. Discussions were supported with detailed written and ethically approved patient information sheets and consent forms that were provided to CYP and their families.
All CYP and their families were given the opportunity to ask any questions that they had, to discuss the study with their surrogates and time to consider the information prior to agreeing to participate.
Registration
Participants were registered using a web-based system once all of the eligibility criteria had been confirmed.
Outcome measures
Data were collected at three time points during the course of the study: baseline, 6 weeks and 12 weeks.
The following outcomes were measured:
-
JADAS.
This is composed of four components:
-
physician global assessment of disease activity (measured on a 0–10 point scale)
-
parent/patient global assessment of well-being (measured on a 0–10 point scale)
-
active joint count in 71 joints
-
ESR.
The cJADAS is composed of all of the above components but does not include the ESR or the CRP. The JADAS and cJADAS are calculated as a simple sum of scores from the components detailed above, which give global scores of 0–101 and 0–91 for the JADAS-71 and cJADAS-71, respectively.
-
functional ability (CHAQ)
-
Physician Global Assessment of Disease Activity
-
number of active joints
-
ACR Pedi JIA core set – ACR Pedi 30, ACR Pedi 50, ACR Pedi 70, ACR Pedi 90 and ACR Pedi 100 levels.
The JIA core set criteria assessed at each study visit are:
-
physician global assessment of disease activity
-
parent/patient assessment of overall well-being
-
functional ability (CHAQ)
-
number of joints with active arthritis
-
number of joints with limited range of movement
-
ESR.
The ACR Pedi 30, 50, 70, 90 and 100 levels are defined as 30%, 50%, 70%, 90% and 100% improvement, respectively, in a minimum of three variables in the core set with worsening of one variable by no more than 30% as defined in the ACR Pedi criteria.
-
patient/parent global assessment of disease activity
-
Wallace criteria for CID. 124
Statistical analysis
The mean, SD, minimum, median, maximum, range and IQR for the change from baseline to 6 and 12 weeks (and each time point) for each of the continuous outcomes (JADAS-71, cJADAS-71, Physician Global Assessment of Disease activity, patient/parent global assessment, CHAQ and the number of active joints) will be presented overall and by mode of treatment received (IM injection, oral, IV infusion, intra-articular injection and two or more CS routes).
For each ACR Pedi level, the number of patients meeting the eligibility criteria will be presented overall and by mode of treatment received (IM injection, oral, IV infusion, intra-articular injection and two or more CS routes) from baseline to 6 weeks and from baseline to 12 weeks. The number and percentage of patients who have inactive disease at baseline and at 6 and 12 weeks will be presented overall and by mode of treatment received (IM injection, oral CS, IV infusion, intra-articular injection and two or more CS routes).
All available data were analysed. The proportions of missing data are presented, but owing to the nature of this study missing data were not imputed.
Results
Participant recruitment
The first participant was registered to the SIRJIA study on 29 June 2018 and the last patient was registered on 21 September 2018. The last study visit took place on 11 January 2019.
Twelve out of the 15 sites registered at least one participant and two sites registered 10 or more participants to the study. The flow of participants through the study is presented in Figure 3.
FIGURE 3.
Number of patients screened and registered in the SIRJIA.
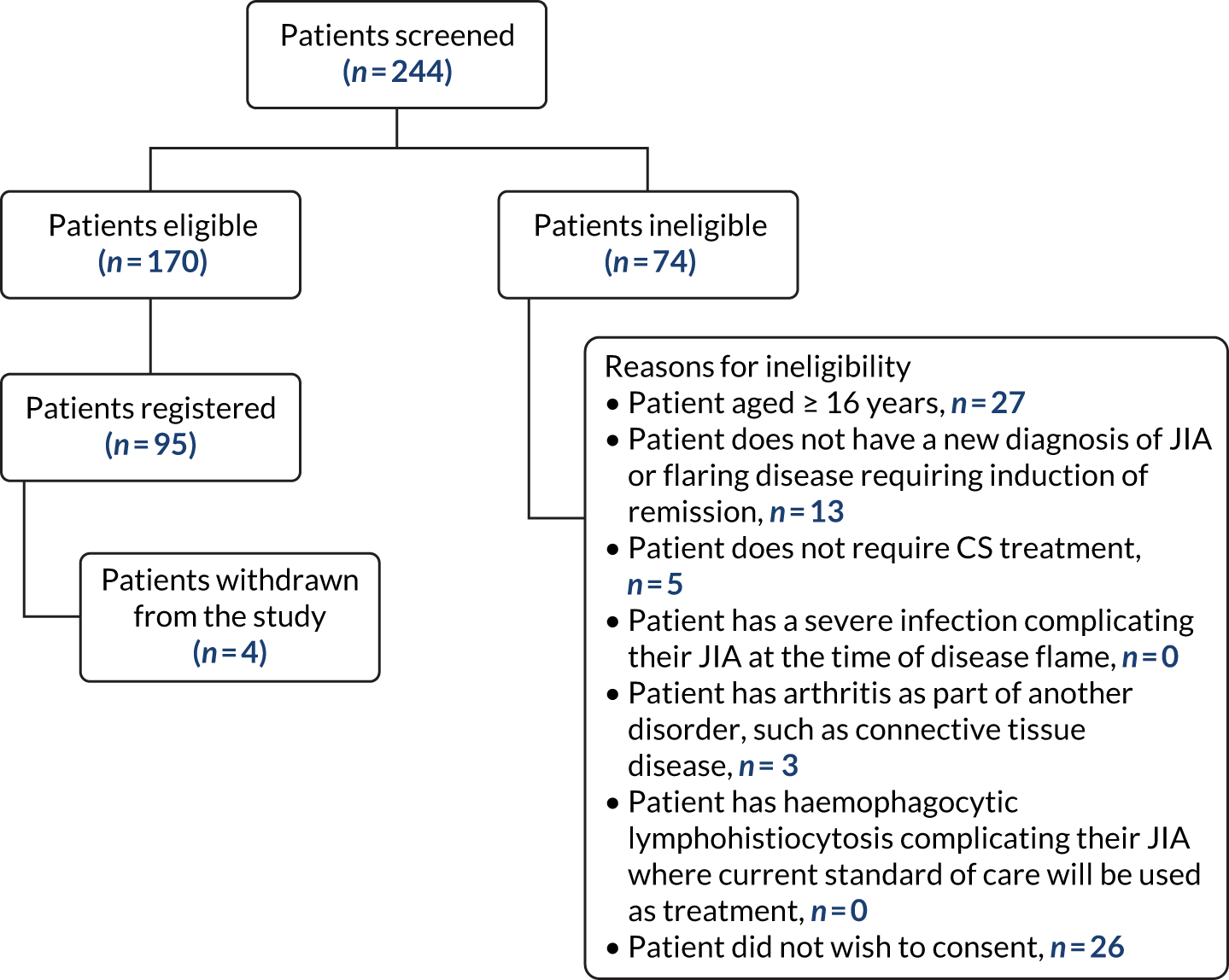
A total of 244 patients were assessed for eligibility for the study.
The number of participants enrolled overall by site is given in Table 19. The main reasons for ineligibility included that the patient was aged ≥ 16 years (n = 27; 36%), the patient did not have a new diagnosis of JIA or flaring disease requiring induction of remission (n = 13; 17%) or the patient did not wish to give consent (n = 26; 35%).
There were 170 patients who were eligible to participate in the study and 95 were registered.
| Site | Number of patients screened | Number of patients eligible | Number of patients registered | Date of first registration | Date of last registration |
|---|---|---|---|---|---|
| Cambridge University Hospitals NHS Foundation Trust | 8 | 8 | 7 | 11 July 2018 | 29 August 2018 |
| The Royal Wolverhampton NHS Trust | 4 | 4 | 4 | 29 June 2018 | 26 July 2018 |
| Norfolk and Norwich University Hospitals NHS Foundation Trust | 17 | 14 | 7 | 12 July 2018 | 20 September 2018 |
| Leeds Teaching Hospitals NHS Trust | 24 | 11 | 11 | 20 July 2018 | 21 September 2018 |
| University Hospital Southampton NHS Foundation Trust | 53 | 44 | 5 | 17 July 2018 | 6 August 2018 |
| Birmingham Women’s and Children’s NHS Foundation Trust | 12 | 9 | 8 | 2 August 2018 | 14 September 2018 |
| University Hospitals of North Midlands NHS Trust | 12 | 9 | 6 | 3 July 2018 | 21 August 2018 |
| University Hospital Southampton NHS Foundation Trust | 7 | 7 | 6 | 30 July 2018 | 20 August 2018 |
| Sheffield Children’s NHS Foundation Trust | 5 | 5 | 5 | 20 July 2018 | 24 August 2018 |
| Great Ormond Street Hospital For Children NHS Foundation Trust | 21 | 7 | 4 | 10 August 2018 | 20 September 2018 |
| Royal Aberdeen Children’s Hospital | 6 | 6 | 6 | 27 July 2018 | 20 September 2018 |
| Royal Belfast Hospital for Sick Children | 30 | 11 | 11 | 20 July 2018 | 30 August 2018 |
| The Great North Children’s Hospital | 5 | 4 | 4 | 19 September 2018 | 19 September 2018 |
| Nottingham University Hospitals NHS Trust | 35 | 26 | 6 | 20 July 2018 | 21 September 2018 |
| University Hospital Bristol NHS Foundation Trust | 5 | 5 | 5 | 6 July 2018 | 8 August 2018 |
| Total | 244 | 170 | 95 |
Baseline characteristics
The demographic baseline data of the 95 participants who were registered across all centres are shown in Table 20. A total of 69 (72.6%) patients were female and the mean age of participants 9.7 years. The vast majority were described as white British (n = 84, 88.4%). Twenty-eight (29.5%) patients had recently received a new diagnosis of JIA and 67 (70.5%) had had a flare of their JIA.
| Baseline characteristics | Number of patients (N = 95), n (%) |
|---|---|
| Gender | |
| Female | 69 (72.6) |
| Male | 26 (27.4) |
| Ethnicity | |
| White British | 84 (88.4) |
| Any other ethnic group | 7 (7.4) |
| Black/black British | 2 (2.1) |
| Asian/Asian British | 1 (1.1) |
| Unobtainable | 1 (1.1) |
| New diagnosis of JIA | |
| No | 67 (70.5) |
| Yes | 28 (29.5) |
| Flare of JIA | |
| No | 27 (28.4) |
| Yes | 67 (70.5) |
| Unobtainable | 1 (1.1) |
| International League Against Rheumatology subtype | |
| Systemic-onset JIA | 5 (5.3) |
| Oligoarthritis persistent | 34 (35.8) |
| Oligoarthritis extended | 20 (21.1) |
| RF-negative polyarthritis | 25 (26.3) |
| RF-positive polyarthritis | 3 (3.2) |
| Psoriatic JIA | 4 (4.2) |
| Enthesis-related arthritis | 3 (3.2) |
| Undifferentiated arthritis | 1 (1.1) |
| ESR | |
| Abnormal | 31 (32.6) |
| Normal | 32 (33.7) |
| Not done | 31 (32.6) |
| Unobtainable | 1 (1.1) |
| CRP | |
| Abnormal | 18 (18.9) |
| Normal | 44 (46.3) |
| Not done | 32 (33.7) |
| Unobtainable | 1 (1.1) |
| Does the participant experience stiffness in the morning? | |
| Yes | 75 (78.9) |
| No | 17 (17.9) |
| Unobtainable | 3 (3.2) |
| Duration of morning stiffness (minutes)a | |
| Mean (SD) | 50.9 (49.9) |
| Age (years) | |
| Mean (SD) | 9.7 (4.6) |
| Height (cm)a | |
| Mean (SD) | 139.2 (24.1) |
| Weight (kg) | |
| Mean (SD) | 38 (17.9) |
There were no patients who had plasma viscosity measured at baseline. Five (5.3%) patients had active uveitis. Three patients (3.2%) had fever, rash, serositis, splenomegaly or generalised lymphadenopathy that was attributable to their JIA.
A total of 37 (38.9%) clinicians considered other CS routes prior to making a final decision on the treatment route chosen. In addition, patient preference (n = 33, 34.7%), unit protocol/local practice (n = 73, 76.8%) or another factor (n = 28, 29.5%) influenced the decision made by clinicians.
Treatment
In total, 55 (57.9%) participants were treated with IACIs alone, 16 (16.8%) patients were treated with oral CSs alone and two were treated with IV infusion CSs alone. During the data collection period there were no participants who received IM injection CSs. A total of 22 patients (23.2%) received CSs by a combination of routes, with the majority of patients receiving IACIs and oral CSs (n = 9, 9.5%) or IV infusion and oral CSs (n = 8, 8.4%) (Table 21).
| Route of CS treatment | Number of patients, n (%) |
|---|---|
| IACI | 55 (57.9) |
| Two or more routes | 22 (23.2) |
| IACI and oral CS | 9 (9.5) |
| IV infusion and oral CS | 8 (8.4) |
| IACI and IV infusion | 3 (3.2) |
| IACI and IM injection | 1 (1.1) |
| IACI, IV infusion and oral CS | 1 (1.1) |
| Oral CS | 16 (16.8) |
| IV infusion | 2 (2.1) |
| IM injection | 0 (0) |
JADAS-71 results
The results for JADAS-71 are presented by route and overall in Table 22 for 6 weeks and in Table 23 for 12 weeks.
| Outcome | Route of administration CS | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IA | IV infusion | Oral | Two or more routes | Overall | |||||||||||
| Baseline | Week 6 | Change | Baseline | Week 6 | Change | Baseline | Week 6 | Change | Baseline | Week 6 | Change | Baseline | Week 6 | Change | |
| JADAS-71 | |||||||||||||||
| Mean (SD) | 8.8 (4.0) | 6 (6.5) | 4.3 (4) | 29.7 (–) | 17.2 (–) | – | 14.8 (9.8) | 5.9 (3.6) | 6.7 (9.1) | 19.6 (15.7) | 12.2 (8.6) | 8.1 (13.7) | 12.5 (10.1) | 8.4 (7.4) | 5.9 (8.7) |
| Median (IQR) | 9.8 (5.6) | 4.7 (6.5) | 3.9 (5.4) | 29.7 (0) | 17.2 (0) | – | 14.7 (11.2) | 7.5 (7.2) | 10.6 (16.9) | 17.3 (11.4) | 14.0 (17.3) | 2.6 (12.0) | 10.8 (9.1) | 7.4 (6.9) | 3.4 (8.7) |
| Missing | n = 35 missing = 20 | n = 14 missing = 41 | n = 13 missing = 42 | n = 1 missing = 1 | n = 1 missing = 1 | n = 0 missing = 2 | n = 9 missing = 7 | n = 7 missing = 9 | n = 3 missing = 13 | n = 13 missing = 9 | n = 11 missing = 11 | n = 8 missing = 14 | n = 58 missing = 37 | n = 33 missing = 62 | n = 24 missing = 71 |
| cJADAS-71 | |||||||||||||||
| Mean (SD) | 8.3 (4.3) | 4.6 (4.9) | 4.0 (4.1) | 24.3 (7.6) | 23.6 (11.0) | 0.7 (3.4) | 13.4 (8.9) | 6.6 (5.7) | 6.7 (6.7) | 15.4 (12.2) | 8.3 (7.1) | 7.5 (10.6) | 11.1 (8.3) | 6.5 (6.6) | 5.3 (6.9) |
| Median (IQR) | 8.4 (6.8) | 4.1 (5.8) | 3.7 (5.3) | 24.3 (10.8) | 23.6 (15.6) | 0.7 (4.8) | 10.4 (11.5) | 5.7 (5.5) | 4.1 (9.0) | 12.8 (13.9) | 4.8 (10.6) | 4.1 (11.9) | 9.6 (7.8) | 4.8 (6.7) | 3.8 (6.0) |
| Missing | n = 53 missing = 2 | n = 36 missing = 19 | n = 36 missing = 19 | n = 2 missing = 0 | n = 2 missing = 0 | n = 2 missing = 0 | n = 16 missing = 0 | n = 15 missing = 1 | n = 15 missing = 1 | n = 19 missing = 3 | n = 17 missing = 5 | n = 17 missing = 5 | n = 90 missing = 5 | n = 70 missing = 25 | n = 70 missing = 25 |
| Physician Global Assessment of Disease activity | |||||||||||||||
| Mean (SD) | 2.5 (1.6) | 0.9 (1.2) | 1.5 (1.5) | 5.3 (0.6) | 5.2 (1.5) | 0.1 (0.9) | 3.5 (2.1) | 1 (1.2) | 2.4 (2.3) | 4.6 (2.2) | 2.5 (2.2) | 2.3 (2.7) | 3.1 (2) | 1.4 (1.8) | 1.8 (2) |
| Median (IQR) | 2.1 (1.8) | 0.5 (1.0) | 1.4 (2.0) | 5.3 (0.8) | 5.2 (2.1) | 0.1 (1.3) | 2.9 (3.4) | 0.3 (2.1) | 1.9 (2.5) | 4.7 (2.9) | 2.1 (4.0) | 2.1 (3.3) | 2.6 (3.2) | 0.6 (2.0) | 1.5 (2.3) |
| Missing | n = 54 missing = 1 | n = 40 missing = 15 | n = 40 missing = 15 | n = 2 missing = 0 | n = 2 missing = 0 | n = 2 missing = 0 | n = 16 missing = 0 | n = 15 missing = 1 | n = 15 missing = 1 | n = 19 missing = 3 | n = 18 missing = 4 | n = 17 missing = 5 | n = 91 missing = 4 | n = 75 missing = 20 | n = 74 missing = 21 |
| Patient/parent global assessment | |||||||||||||||
| Mean (SD) | 3.5 (2.7) | 3.0 (2.7) | 0.7 (2.2) | 6.0 (2.6) | 3.4 (2.6) | 2.6 (0.0) | 5.2 (2.9) | 3.5 (2.8) | 1.6 (2.3) | 4.0 (2.9) | 3.3 (2.8) | 0.4 (2.1) | 3.9 (2.9) | 3.2 (2.7) | 0.8 (2.2) |
| Median (IQR) | 2.8 (4.2) | 2.1 (4.6) | 0.5 (2.1) | 6.0 (3.7) | 3.4 (3.7) | 2.6 (0.0) | 5.0 (4.4) | 2.9 (3.9) | 0.6 (2.8) | 2.7 (4.5) | 2.7 (4.0) | 0.5 (2.0) | 3.4 (4.6) | 2.7 (4.2) | 0.5 (2.3) |
| Missing | n = 53 missing = 2 | n = 37 missing = 18 | n = 37 missing = 18 | n = 2 missing = 0 | n = 2 missing = 0 | n = 2 missing = 0 | n = 16 missing = 0 | n = 15 missing = 1 | n = 15 missing = 1 | n = 22 missing = 0 | n = 17 missing = 5 | n = 17 missing = 5 | n = 93 missing = 2 | n = 71 missing = 24 | n = 71 missing = 24 |
| CHAQ | |||||||||||||||
| Mean (SD) | 0.8 (0.7) | 0.5 (0.6) | –0.3 (0.4) | 1.4 (0.2) | 1.4 (0.5) | 0 (0.7) | 1.1 (0.9) | 0.9 (0.9) | –0.2 (0.5) | 0.9 (0.7) | 0.8 (0.6) | –0.1 (0.4) | 0.9 (0.7) | 0.7 (0.7) | –0.2 (0.5) |
| Median (IQR) | 0.6 (1.0) | 0.3 (0.9) | –0.3 (0.6) | 1.4 (0.3) | 1.4 (0.8) | 0 (1.0) | 1.1 (1.3) | 0.6 (1.6) | –0.1 (0.4) | 0.8 (0.9) | 0.7 (0.6) | –0.1 (0.4) | 0.8 (1.0) | 0.5 (1.0) | –0.1 (0.5) |
| Missing | n = 53 missing = 2 | n = 39 missing = 16 | n = 39 missing = 16 | n = 2 missing = 0 | n = 2 missing = 0 | n = 2 missing = 0 | n = 16 missing = 0 | n. = 15 missing = 1 | n = 15 missing = 1 | n = 22 missing = 0 | n = 18 missing = 4 | n = 18 missing = 4 | n = 93 missing = 2 | n = 74 missing = 21 | n = 74 missing = 21 |
| Number of active joints | |||||||||||||||
| Mean (SD) | 2.8 (2.8) | 0.7 (2) | 1.9 (2.6) | 13 (5.7) | 15 (9.9) | –2 (4.2) | 4.8 (5.5) | 2.1 (3.5) | 2.8 (4) | 6.8 (.1) | 2.5 (2.7) | 4.6 (8.4) | 4.2 (5.7) | 1.8 (3.6) | 2.6 (5) |
| Median (IQR) | 2 (3) | 0 (1) | 1 (2) | 13 (8) | 15 (14) | –2 (6) | 3 (4.5) | 0 (3) | 2 (4) | 4 (7) | 2 (4) | 3 (7) | 2 (4) | 0 (2) | 1 (2) |
| Missing | n = 55 missing = 0 | n = 41 missing = 14 | n = 41 missing = 14 | n = 2 missing = 0 | n = 2 missing = 0 | n = 2 missing = 0 | n = 16 missing = 0 | n = 15 missing = 1 | n = 15 missing = 1 | n = 22 missing = 0 | n = 19 missing = 3 | n = 19 missing = 3 | n = 95 missing = 0 | n = 77 missing = 18 | n = 77 missing = 18 |
| Outcome | Route of administration of CS | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IA | IV | Oral | Two or more routes | Overall | |||||||||||
| Baseline | Week 12 | Change | Baseline | Week 12 | Change | Baseline | Week 12 | Change | Baseline | Week 12 | Change | Baseline | Week 12 | Change | |
| JADAS-71 | |||||||||||||||
| Mean (SD) | 8.8 (4.0) | 5 (6) | 4.7 (4.8) | 29.7 (–) | 13.1 (–) | – | 14.8 (9.8) | 9.3 (4.2) | 1.3 (7.5) | 19.6 (15.7) | 8.7 (5.3) | 9.2 (9) | 12.5 (10.1) | 7.3 (5.6) | 5.4 (7) |
| Median (IQR) | 9.8 (5.6) | 2.6 (9.4) | 3.3 (9.1) | 29.7 (0) | 13.1 (0) | – | 14.7 (11.2) | 9.8 (6.7) | –0.3 (10.5) | 17.3 (11.4) | 8.9 (7.4) | 9.5 (14.8) | 10.8 (9.1) | 6.8 (8.9) | 3.3 (11.1) |
| Missing | n = 35 missing = 20 | n = 15 missing = 37 | n = 10 missing = 42 | n = 1 missing = 1 | n = 1 missing = 1 | n = 0 missing = 2 | n = 9 missing = 7 | n = 8missing = 8 | n = 4 missing = 12 | n = 13 missing = 9 | n = 9 missing = 12 | n = 6 missing = 15 | n = 58 missing = 37 | n = 33 missing = 58 | n = 20 missing = 71 |
| cJADAS-71 | |||||||||||||||
| Mean (SD) | 8.3 (4.3) | 4.4 (4.4) | 3.9 (4.5) | 24.3 (7.6) | 12.1 (–) | 6.8 (–) | 13.4 (8.9) | 7.8 (4.7) | 6.5 (9.9) | 15.4 (12.2) | 7.1 (5.3) | 8.1 (12.9) | 11.1 (8.3) | 5.8 (4.9) | 5.4 (8.2) |
| Median (IQR) | 8.4 (6.8) | 3.1 (4.9) | 2.8 (6.6) | 24.3 (10.8) | 12.1 (0) | 6.8 (0) | 10.4 (11.5) | 7 (7.7) | 4.5 (8.6) | 12.8 (13.9) | 6.8 (7.1) | 6.3 (12.0) | 9.6 (7.8) | 4.7 (7.1) | 4.7 (8.3) |
| Missing | n = 53 missing = 2 | n = 41 missing = 11 | n = 40 missing = 12 | n = 2 missing = 0 | n = 1 missing = 1 | n = 1 missing = 1 | n = 16 missing = 0 | n = 13 missing = 3 | n = 13 missing = 3 | n = 19 missing = 3 | n = 17 missing = 4 | n = 16 missing = 5 | n = 90 missing = 5 | n = 72 missing = 19 | n = 70 missing = 21 |
| Physician Global Assessment of Disease activity | |||||||||||||||
| Mean (SD) | 2.5 (1.6) | 0.7 (1.1) | 1.7 (1.8) | 5.3 (0.6) | 3.5 (–) | 2.2 (–) | 3.5 (2.1) | 2 (2.1) | 1.5 (3.2) | 4.6 (2.2) | 2.4 (1.8) | 2 (3.2) | 3.1 (2) | 1.4 (1.7) | 1.8 (2) |
| Median (IQR) | 2.1 (1.8) | 0.2 (1.3) | 1.3 (1.8) | 5.3 (0.8) | 3.5 (0) | 2.2 (0) | 2.9 (3.4) | 1.1 (3.8) | 1.4 (4.6) | 4.7 (2.9) | 2.3 (2) | 2.6 (4.4) | 2.6 (3.2) | 0.8 (2.2) | 1.4 (2.6) |
| Missing | n = 54 missing = 1 | n = 46 missing = 6 | n = 45 missing = 7 | n = 2 missing = 0 | n = 1 missing = 1 | n = 1 missing = 1 | n = 16 missing = 0 | n = 16 missing = 0 | n = 16 missing = 0 | n = 19 missing = 3 | n = 20 missing = 1 | n = 18 missing = 3 | n = 91 missing = 4 | n = 83 missing = 8 | n = 80 missing = 11 |
| Patient/parent global assessment | |||||||||||||||
| Mean (SD) | 3.4 (2.7) | 2.8 (2.8) | 0.6 (2.9) | 6.0 (2.6) | 0.5 (–) | 3.7 | 5.2 (2.9) | 3.4 (2.4) | 1.8 (2.7) | 4.0 (2.9) | 3.1 (2.7) | 0.4 (2.9) | 3.9 (2.9) | 2.9 (2.7) | 0.8 (2.9) |
| Median (IQR) | 2.8 (4.2) | 2.3 (4.7) | 0.4 (3.1) | 5.9 (3.7) | 0.5 (0) | 3.7 (0) | 5.0 (4.4) | 3.9 (2.8) | 1.7 (3.1) | 2.7 (4.5) | 2.6 (3.8) | 0.2 (3.3) | 3.4 (4.6) | 2.4 (4.5) | 0.3 (3.3) |
| Missing | n = 53 missing = 2 | n = 41 missing = 11 | n = 40 missing = 12 | n = 2 missing = 0 | n = 1 missing = 1 | n = 1 missing = 1 | n = 16 missing = 0 | n = 13 missing = 3 | n = 13 missing = 3 | n = 22 missing = 0 | n = 18 missing = 3 | n = 18 missing = 3 | n = 93 missing = 2 | n = 73 missing = 18 | n = 72 missing = 19 |
| CHAQ | |||||||||||||||
| Mean (SD) | 0.8 (0.7) | 0.5 (0.7) | –0.2 (0.7) | 1.4 (0.2) | 0.5 (–) | –1 (–) | 1.1 (0.9) | 0.7 (0.7) | –0.5 (0.5) | 0.9 (0.7) | 0.6 (0.5) | –0.1 (0.6) | 0.9 (0.7) | 0.6 (0.6) | –0.3 (0.6) |
| Median (IQR) | 0.6 (1.0) | 0.3 (0.5) | –0.2 (0.5) | 1.4 (0.3) | 0.5 (0) | –1 (0) | 1.1 (1.3) | 0.6 (0.9) | –0.3 (0.8) | 0.8 (0.9) | 0.6 (0.8) | –0.1 (0.6) | 0.8 (1.0) | 0.4 (0.8) | –0.3 (0.6) |
| Missing | n = 53 missing = 2 | n = 42 missing = 10 | n = 40 missing = 12 | n = 2 missing = 0 | n = 1 missing = 0 | n = 1 missing = 1 | n = 16 missing = 0 | n = 15 missing = 1 | n = 15 missing = 1 | n = 22 missing = 0 | n = 18 missing = 3 | n = 18 missing = 3 | n = 93 missing = 2 | n = 76 missing = 15 | n = 74 missing = 17 |
| Number of active joints | |||||||||||||||
| Mean (SD) | 2.8 (2.8) | 0.8 (1.3) | 2 (3) | 13 (5.7) | 8 (–) | 1 (–) | 4.8 (5.5) | 1.8 (2.7) | 2.9 (5.5) | 6.8 (.1) | 2 (2.1) | 4.8 (8.8) | 4.2 (5.7) | 1.3 (2) | 2.8 (5.5) |
| Median (IQR) | 2 (3) | 0 (1) | 1 (1.5) | 13 (8) | 8 (0) | 1 (0) | 3 (4.5) | 0 (3) | 2 (4) | 4 (7) | 1 (4) | 3 (7) | 2 (4) | 0 (2) | 1 (2) |
| Missing | n = 55 missing = 0 | n = 48 missing = 4 | n = 48 missing = 4 | n = 2 missing = 0 | n = 1 missing = 1 | n = 1 missing = 1 | n = 16 missing = 0 | n = 16 missing = 0 | n = 16 missing = 0 | n = 22 missing = 0 | n = 21 missing = 0 | n = 21 missing = 0 | n = 95 missing = 0 | n = 86 missing = 5 | n = 86 missing = 5 |
Overall, the mean [standard deviation (SD)] JADAS-71 score at baseline was 12.5 (10.1) and at 6 weeks had fallen by 5.9 (8.7), and by 12 weeks slightly more, by 5.4 (7). The proportion of missing data for the JADAS-71 was considerable (75%) both at 6 weeks and at 12 weeks. The main reason for missing data for the JADAS-71 at these time points was because these patients were not clinically unwell and, therefore, there was no need to carry out a blood test. The JADAS-71 requires information on all four components and, therefore, owing to missing ESR values, this could not be calculated.
The cJADAS-71 had fewer missing data at 6 weeks (26%) and at 12 weeks (22%) than the JADAS-71. The mean change in overall score at both of these time points was slightly lower in the cJADAS-71 than in the JADAS-71; the mean change for the cJADAS was 5.3 at 6 weeks and 5.4 at 12 weeks (see Table 23).
Physician global assessment and patient/parent assessment of global assessment
The overall mean Physician Global Assessment score at baseline was 3.1 and the mean change from baseline was 1.8 at both 6 and 12 weeks. The number of missing data was higher at 6 weeks (22%) than at 12 weeks (12%) (see Tables 22 and 23) because some units were unable to achieve an extra 6-week follow-up in all patients.
The overall mean Patient/Parent Assessment of Global Assessment at baseline was 3.9 and the mean change from baseline to 6 and 12 weeks was 0.9 and 0.8, respectively. Similar to the Physician Global Assessment, the number of missing data was higher at 6 weeks (25%) than at 12 weeks (20%) (see Tables 22 and 23).
Childhood Health Assessment Questionnaire
The mean CHAQ score at baseline was 0.9 and was slightly lower at 6 and 12 weeks (–0.2 and –0.3, respectively). The number of missing data at 6 weeks was higher than at 12 weeks (see Tables 22 and 23).
Number of active joints
The overall mean number of active joints at baseline was 4.2, and this reduced to 1.8 at 6 weeks and 1.3 at 12 weeks. The number of missing data at 6 weeks was again higher than at 12 weeks (see Tables 22 and 23).
Number of patients with inactive disease
There were no patients who had inactive disease at baseline. The definition of inactive disease using the Wallace criteria124 meant that all criteria must be met at any given time point for the patient to be classified as having inactive disease. At 6 weeks there were only three (3.3%) patients who had inactive disease, and at 12 weeks there were four patients (4.4%) (Table 24).
| Disease status | Baseline, n (%) | 6 weeks, n (%) | 12 weeks, n (%) |
|---|---|---|---|
| IA | |||
| Active disease | 55 (100) | 46 (83.6) | 38 (73.1) |
| Inactive disease | 0 (0) | 1 (1.8) | 2 (3.8) |
| Missing | 0 (0) | 8 (14.5) | 12 (23.1) |
| IV infusion | |||
| Active disease | 2 (100) | 2 (100) | 2 (100) |
| Inactive disease | 0 (0) | 0 (0) | 0 (0) |
| Missing | 0 (0) | 0 (0) | 0 (0) |
| Oral | |||
| Active disease | 16 (100) | 14 (87.5) | 14 (87.5) |
| Inactive disease | 0 (0) | 0 (0) | 2 (12.5) |
| Missing | 0 (0) | 2 (12.5) | 0 (0) |
| Two or more routes | |||
| Active disease | 22 (100) | 20 (90.9) | 19 (90.5) |
| Inactive disease | 0 (0) | 2 (9.1) | 0 (0) |
| Missing | 0 (0) | 0 (0) | 2 (9.5) |
| Overall | |||
| Active disease | 95 (100) | 82 (86.3) | 73 (80.2) |
| Inactive disease | 0 (0) | 3 (3.2) | 4 (4.4) |
| Missing | 0 (0) | 10 (10.5) | 14 (15.4) |
There were missing data for 10 (10.5%) patients at 6 weeks and 14 (15.4%) patients at 12 weeks.
American College of Rheumatology Scores
The results of the number of patients achieving ACR Pedi 30, ACR Pedi 50, ACR Pedi 70, ACR Pedi 90 and ACR Pedi 100 at 6 weeks and 12 weeks are given in Tables 25 and 26, respectively.
| ACR Pedi | CS route, n (%) | Overall | |||
|---|---|---|---|---|---|
| IA | IV infusion | Oral | Two or more routes | ||
| ACR Pedi 30 | |||||
| Yes | 17 (30.9) | 0 (0.0) | 9 (56.3) | 8 (36.4) | 34 (35.8) |
| No | 19 (34.5) | 2 (100) | 5 (31.3) | 9 (40.9) | 35 (36.8) |
| Missing | 19 (34.5) | 0 (0.0) | 2 (12.5) | 5 (22.7) | 26 (27.4) |
| ACR Pedi 50 | |||||
| Yes | 16 (29.1) | 0 (0.0) | 9 (56.3) | 7 (31.8) | 32 (33.7) |
| No | 20 (36.4) | 2 (100) | 5 (31.3) | 10 (45.5) | 37 (38.9) |
| Missing | 19 (34.5) | 0 (0.0) | 2 (12.5) | 5 (22.7) | 26 (27.4) |
| ACR Pedi 70 | |||||
| Yes | 12 (21.8) | 0 (0.0) | 8 (50) | 4 (18.2) | 24 (25.3) |
| No | 24 (43.6) | 2 (100) | 6 (37.5) | 13 (59.1) | 45 (47.4) |
| Missing | 19 (34.5) | 0 (0.0) | 2 (12.5) | 5 (22.7) | 26 (27.4) |
| ACR Pedi 90 | |||||
| Yes | 7 (12.7) | 0 (0.0) | 4 (25) | 4 (18.2) | 15 (15.8) |
| No | 29 (52.7) | 2 (100) | 10 (62.5) | 13 (59.1) | 54 (56.8) |
| Missing | 19 (34.5) | 0 (0.0) | 2 (12.5) | 5 (22.7) | 26 (27.4) |
| ACR Pedi 100 | |||||
| Yes | 6 (10.9) | 0 (0.0) | 3 (18.8) | 2 (9.1) | 11 (11.6) |
| No | 30 (54.5) | 2 (100) | 11 (68.8) | 15 (68.2) | 58 (61.1) |
| Missing | 19 (34.5) | 0 (0.0) | 2 (12.5) | 5 (22.7) | 26 (27.4) |
| ACR Pedi | CS route, n (%) | Overall | |||
|---|---|---|---|---|---|
| IA | IV | Oral | Two or more routes | ||
| ACR Pedi 30 | |||||
| Yes | 18 (34.6) | 1 (50) | 9 (56.3) | 7 (33.3) | 35 (38.5) |
| No | 21 (40.4) | 0 (0.0) | 5 (31.3) | 12 (57.1) | 38 (41.8) |
| Missing | 13 (25) | 1 (50) | 2 (12.5) | 2 (9.5) | 18 (19.8) |
| ACR Pedi 50 | |||||
| Yes | 17 (32.7) | 0 (0.0) | 6 (37.5) | 6 (28.6) | 29 (31.9) |
| No | 22 (42.3) | 1 (50) | 8 (50) | 13 (61.9) | 44 (48.4) |
| Missing | 13 (25) | 1 (50) | 2 (12.5) | 2 (9.5) | 18 (19.8) |
| ACR Pedi 70 | |||||
| Yes | 14 (26.9) | 0 (0.0) | 4 (25) | 4 (19) | 22 (24.2) |
| No | 25 (48.1) | 1 (50) | 10 (62.5) | 15 (71.4) | 51 (56) |
| Missing | 13 (25) | 1 (50) | 2 (12.5) | 2 (9.5) | 18 (19.8) |
| ACR Pedi 90 | |||||
| Yes | 10 (19.2) | 0 (0.0) | 4 (25) | 3 (14.3) | 17 (18.7) |
| No | 29 (55.8) | 1 (50) | 10 (62.5) | 16 (76.2) | 56 (61.5) |
| Missing | 13 (25) | 1 (50) | 2 (12.5) | 2 (9.5) | 18 (19.8) |
| ACR Pedi 100 | |||||
| Yes | 9 (17.3) | 0 (0.0) | 2 (12.5) | 3 (14.3) | 14 (15.4) |
| No | 30 (57.7) | 1 (50) | 12 (75) | 16 (76.2) | 59 (64.8) |
| Missing | 13 (25) | 1 (50) | 2 (12.5) | 2 (9.5) | 18 (19.8) |
Twenty-six (27.4%) patients could not be included in the analysis at 6 weeks and 18 (19.8%) patients could not be included at 12 weeks.
Overall, 34 (35.8%) patients achieved ACR Pedi 30, 32 (33.7%) patients achieved ACR Pedi 50, 24 (25.3%) patients achieved ACR Pedi 70, 15 (15.8%) patients achieved ACR Pedi 90 and 11 (11.6%) patients achieved ACR Pedi 100 at 6 weeks. There were similar findings at 12 weeks for each of the outcomes.
Discussion
The findings from this study show that there is an eligible population of patients who are potentially willing to take part in a future RCT. Almost 70% of patients who took part in this study were experiencing a flare of their JIA that necessitated CSs. The two main routes of CS that were given across the 15 sites that took part in this study were intra-articular injection and oral; the combinations of intra-articular and oral CSs and of IV and oral CSs were also common. Only two patients in the study received CSs by IV infusion alone and no patients in any of the sites received only IM injection of CSs.
The fact that a large number of data for the proposed primary outcome (JADAS-71) were missing showed that it would not be feasible to use this as a primary outcome at 6 weeks. There were two main reasons for the high proportion of missing data. The first was that several sites that took part in this study did not routinely bring patients back for a 6-week visit; participation in any future RCT would require sites to ensure that they could adhere to this protocol. The second was that not all patients needed a blood test when they attended for their visit, meaning that ESR was not measured and the JADAS-71 could not be completed. Previous work133 has shown that the cJADAS can be calculated by collecting the core outcome variables of the JADAS, but excluding the ESR. It was shown that the amended score (omitting the ESR) correlates well with the JADAS and, therefore, could be used instead. 133
In our study, there were still missing data for the cJADAS (i.e. at least one of active joint counts, PGA score and, most often, the Pa/PtGA score was not available for analysis). The proportion of missing data was similar to that reported in the CAPS and to the rate of data collected in real time in clinic. Online data entry at the time of clinical assessment could reduce the number of missing data, and the CAPTURE JIA study141 is trialling an online platform for these scores that may be available for use in a future study.
The data collected during this study were used in the power calculations that are given in Chapter 8.
The collection of data should be made as simple as possible for patients and their families, and innovative solutions (such as touchscreen technologies) should be considered when designing large trials to try to minimise the number of missing data. Careful monitoring of the outcomes of interest and of the components of the composite outcomes should be considered prior to the commencement of any large trial.
Chapter 7 Study management
Introduction
When setting up a feasibility study one can encounter unexpected challenges. The challenges that the SIRJIA faced included delays in approval, in achieving engagement from sites and in making changes to the study design, and these delays had financial implications. Having SMG members who were already experienced in trial management enabled us to find solutions to these problems, to exceed our recruitment target and to engage with PPI representatives and consumers, which is imperative to achieving the aims of any study.
Study approval process
Initially, the study encountered a number of unfortunate delays due to the newly released HRA process. There was subsequent uncertainty among sites with regards to the new process and the documents that were required for approval. The HRA process delay inevitably had an impact on the SIRJIA timelines and the scheduled qualitative interviews, which were due to start in March 2016 but did not commence until October 2016 (7 months later). Table 27 shows the timelines for approval of the documents for the SIRJIA protocol. The detailed description of amendments is provided in Table 28.
| Application | Approval date |
|---|---|
| Initial ethics submission | 25 January 2016 |
| Ethics approval by proportionate review provisional opinion | 8 February 2016 |
| Final ethics approval | 18 March 2016 |
| HRA submission pre-approval | 10 June 2016 |
| HRA approval | 10 August 2016 |
| Amendment number | Details of amendment | Date submitted to multicentre REC | Date of REC letters/correspondence | Date REC approved |
|---|---|---|---|---|
| Original application | Protocol version 1.0, dated 15 January 2015 | 15 January 2016 | 21 September 2017 | 21 September 2017 |
| Number 1: non-substantial | Protocol version 2.0:
|
5 September 2016 | 3 October 2016 | 3 October 2016 |
| Number 1: substantial |
|
14 June 2017 | 14 July 2017 | 14 July 2017 |
| Number 2: non-substantial | Change of principal investigator for Birmingham Children’s Hospital | 20 September 2017 | 21 September 2017 | 21 September 2017 |
| Number 2: substantial |
|
21 February 2018 | 6 March 2018 | 6 March 2018 |
| Number 3: substantial | Poster to advertise survey for PPI consumers through Simon Stones (PPI representative) Facebook (Facebook, Inc., Menlo Park, CA, USA) page | 4 September 2018 | 20 September 2018 | 20 September 2018 |
| Number 4: substantial |
|
8 November2018 | 10 December 2018 | 8 December 2018 |
These delays had a compound effect resulting in the study seeking an initial 15 months costed extension.
Changes to study design
The original protocol study design was to collect anonymised routine clinical data; therefore, informed consent was not required from patients. However, during the set-up phase, it became apparent that staff from 10 out of the 15 centres who were initially trained felt that consent was necessary for the centre to take part in the study. This was because of the change to the study visit schedule to include an additional proposed study visit at 6 weeks. The reason for adding the 6-week visit was that clinicians on the SMG felt, on reflection, that the duration of the action of CSs administered by intra-articular or IM injection, was such that the effect might have worn off by 12 weeks, the time point originally chosen for assessment of the primary outcome. This would then blunt the primary outcome findings and disadvantage the shorter-acting CS regimens.
This, therefore, required a further submission to the REC of the amended protocol (version 3.0, 30 May 2017) and consent forms (version 1.0, 23 May 2017), among other documents.
Delphi process changed to discussion and consensus meeting
The Delphi method is a decision-making process based on responses to multiple rounds of questionnaires. The anonymous responses are aggregated and shared with the respondents after each round, which can run over a number of months. Considering that we had already lost 9 months of study time, the SMG and SSC made the decision to replace the Delphi method with e-surveys (see Chapter 5) and discussion and consensus meetings to facilitate a quicker response in deciding the feasibility study primary outcome and agreeing on the aspects of a future full RCT.
Study oversight committees
The oversight members for the SMG and SSC were identified and agreed, as indicated in the oversight members list in Appendix 2.
Study management group meetings were scheduled on a monthly basis, whereas SSC meetings were scheduled annually. There was no Data Safety Monitoring Committee, as the study was not collecting safety data. The SSC took an active role in advising and on decision-making. Study documents, such as protocols, detailed programmes for meetings, journal articles and promotion materials, were circulated to committee members for review.
The day-to-day running of the study was co-ordinated by the Liverpool Clinical Trials Centre (LCTC), University of Liverpool (see Appendix 3).
Study management group
The SMG meetings did not take place monthly as planned owing to difficulties in finding convenient times that suited everyone, the clinicians in particular. The fact that the study co-ordinator worked part-time made it difficult to find days that suited both her and the rest of the SMG members, especially clinicians. To ensure the smooth running of the study, other communication methods (such as e-mail and telephone calls) were used to agree study issues. The chief investigator held teleconferences with key people (clinicians) to discuss specific study issues when it was not possible to convene a meeting of everyone. The SMG meetings facilitated important dialogues to drive forward study activities.
Study Steering Committee
The first SSC meeting was held on 19 July 2016 and meetings were held annually thereafter; the final SSC meeting took place on 13 March 2019 to present the final study results. The meetings comprised one face-to-face meeting, two teleconference meetings and one mixed face-to-face and teleconference meeting (this being the last one). The SSC members were instrumental in advising on study issues such as the methodology, extensions and future RCT designs, and on the conduct of study activities, such as e-surveys and discussion and consensus meetings. Nicholas Webb resigned from his role owing to relocation to Basel, Switzerland, and was replaced by Indi Banarjee.
Patient and public involvement
The PPI representatives (SS and HB) were instrumental in the design of study documents, protocol amendments, dissemination of study e-surveys, choosing the feasibility study primary outcome and agreeing the aspects of the future protocol. The study benefited from the unique and integrated involvement of patients and families in the protocol design. The PPI representatives helped to identify participants for discussion and consensus meetings, as well as through advertising the e-surveys. The detailed role played by PPI representatives in the study is explicitly spelt out in Chapter 5. The contacts who assisted in identifying PPI representatives included Simon R Stones (PPI representative/co-applicant and SMG member), Katharine Cresswell [Young people's Opinions Underpinning Rheumatology Research (YOURR) group co-ordinator], Catherine Wright (Northern Ireland Versus Arthritis Young People and Family Manager) and Sharon Douglas (Board Member of SNAC Group and liaison to the BSPAR parent group).
The PPI co-applicant Simon R Stones was proactively involved in the study, from the pre-application phase to the dissemination of the findings and preparation of this report. He helped to promote the study at various conferences, including the British Society for Rheumatology/BSPAR conference at Royal Manchester Children’s Hospital in Manchester (28–30 November 2016). His other roles and links allowed him to advocate for the voice of the Paediatric Rheumatology CSG Consumers, the BSPAR parent group, the various JIA charities in the UK and the YOUR RHEUM young person advisory group, all at a UK level. He is also involved in advocacy at a European level and was able to link this UK work to an international context. He is involved in the European League Against Rheumatism Standing Committee of People with Arthritis/Rheumatism in Europe and was a founding member of its youth group, Young People with Arthritis/Rheumatism in Europe.
The study logo and other materials were designed with PPI support. We had PPI representatives’ support with the development of the BSPAR poster, BSPAR leaflet and the interview guides for the qualitative work. The patient-facing documents were reviewed by PPI representatives, including Heather Bagley (LCTC PPI Co-ordinator) and other PPI representatives, before submission to the REC.
Sites and site issues
We initially identified seven sites to participate in qualitative interviews, but unfortunately, owing to delays with the local R&D procedures to give approvals after the initiation of the new HRA process, only four sites participated.
None of the Scottish sites was able to participate in the completion of the early screening log exercise, as their R&D process meant that they had to have complete study approval in place prior to this activity, regardless of the fact that the data were anonymised routine clinical data. Their non-participation meant that the screening data were not representative of the national experience; however, for the actual feasibility study, one Scottish site and one site from Northern Ireland were involved in consented recruitment. In addition, clinicians and PPI consumers from the two devolved nations participated in other study activities, such as e-surveys and discussion and consensus meetings. We were also able to identify a further two sites elsewhere in the UK to replace the sites that had dropped out.
The SMG had agreed that to maximise the number of participants recruited during the 3-month recruitment period the study co-ordinator would work towards obtaining approval from all sites that were identified, with the intention that all sites would open to recruitment on the same day. However, this did not happen for various local reasons, such as team capacity and internal approval processes; we opened all sites between 20 June 2018 and 31 August 2018.
Data management
Common issues arising from case report form completion
The definition of flare and re-flare in clinical practice is routinely decided by the clinician. However, given that the SIRJIA did not include a consensus on how to define flare, this caused confusion at sites. For example, some patients who were potentially recruited for an ‘ongoing flare’ but required a treatment change should have been captured as re-flare. There is need in a future RCT for the 6-week visit to be added as an extra visit, as many sites do not have this visit as part of routine treatment owing to clinic capacity issues.
Conclusion
Management failures that are common in research are inadequate research skills, exaggerated project projections, ineffective communication among stakeholders and inappropriate projections and planning. 2–4 Study management is less burdensome when the team works consistently towards a common goal that achieves the study aims. The effective study management processes made possible the attainment of positive outcomes: feasibility study recruitment (96 participants recruited of 50 targeted), reaching consensus on choosing the primary outcome and agreeing aspects of the future protocols for RCTs.
Chapter 8 Final consensus meeting
Introduction
As part of the overall SIRJIA study objectives, there was a need for a final discussion and consensus meeting to agree details of a final protocol to be recommended to the HTA Commissioning Board. The final consensus meeting was designed to present and review the results of the whole feasibility study, covering all aspects of the study brief through to reviewing the study results. Subsequent discussions with consensus voting were planned to agree on a final trial protocol or protocols. A complex set of questions to be answered prior to the final choice was prepared. These covered aspects of future trial design, such as capability, capacity, randomisation, type and timing of intervention, definition of usual standard of care to be the comparator arm, treatment threshold criteria with inclusion and exclusion criteria, primary and secondary outcomes, minimally clinically important differences in the primary outcome, secondary outcomes, randomisation, blinding and dosing details. Methods were discussed to address any potential barriers to participation in a larger study, as well as the acceptability of a future randomised trial to CYP, their families and HCPs.
Aims and objectives
The aim of the final discussion and consensus meeting was to discuss and choose the future RCT protocol, or protocols, and to achieve consensus among CYP, their families and HCPs.
Methods
Eight possible treatment protocols were suggested and developed by the research team and were agreed by the SMG. These were circulated to the study principal investigators and HCPs who would be attending the final consensus meeting so that they could vote on them prior to the face-to-face meeting.
A modified NGT was used to achieve consensus on the most appropriate prospective randomised study protocol, as well as the desired inclusion and exclusion criteria in a future study. This methodology has been described in Chapter 5. Individual, hand-held, interactive response keypads were used for voting. Votes were anonymised, with only the stakeholder group identifiable to the research team (either CYP, their families or HCPs).
This meeting was multidisciplinary and included both HCPs and PPI partners, including CYP and their families. The HCPs who had experience of treating JIA and associated PPI consumers were identified through consumer groups such as SNAC, Arthritis Care, the YOURR Group, the British Society for Rheumatology/BSPAR and BANNAR. However, as aspects of the final protocol were highly specialised and required medical knowledge, there were fewer PPI partners than HCPs and they did not vote on all protocol aspects with HCPs on this occasion. The PPI partners were involved in parallel discussions on the details of the study and voted on details of the study that were within their experience and expertise.
Where appropriate, we used a consensus approach to finalise any remaining areas where there was a lack of agreement on the final trial design to be recommended. The formal stakeholder consensus and voting meeting was held after the discussion meeting process, using the NGT to agree aspects of the future RCT protocol(s). The consensus criteria were agreed prior to the meeting.
The definition of consensus was specified prior to round 1; any outcome voted for by > 60% of participants was considered to have achieved consensus. If no consensus was met, further discussion would take place and then the group voted again. This method of achieving consensus by stakeholders was adopted, as it allows exploration of a wide range of viewpoints among a diverse group of people who have relevant experience.
This meeting was also used to feed back the summary results of the SIRJIA study to both HCPs and PPI representatives. A schedule of the meeting is presented in Table 29.
| Time | Activity |
|---|---|
| 09:00–09:30 | Breakfast and coffee |
| 09:30–10:45 | PPI consumers and HCPs together |
| 10:00–11:00 |
|
| 10:45–11:00 | Break |
| 11:00–12:30 |
Future protocol design Overview on the results from e-survey and final protocol(s) choicePPI consumers and HCPs in separate groups |
| 12:30–13:30 | Lunch |
| 13:30–14:00 |
|
| 14:00–14:20 | Break. Ratification of results based on the percentage of votes cast by participants in favour of each criterion |
| 14:20–14:45 |
|
| 14:45–15:45 |
|
| 15:30–15:45 | Drinks available before leaving |
| 15:45–16:00 |
|
Meeting report
After an explanation of clinical trial research, attendees discussed in pairs what research meant to them and were asked to draw an annotated picture of the results of their discussion, as an ice-breaker, to feed back to the meeting. A generalised welcome was followed by an overview of the day and an explanation of the process of discussion and voting to achieve consensus on details of the final two protocols remaining after the HCP online voting. The results of other stages of the SIRJIA study36 that would be pertinent to the meeting were then presented.
Qualitative research presentation
The qualitative team presented their findings that described CYP’s and their parents’ views on various aspects of the routes of CS treatments and on their willingness to be recruited to a future RCT. They explained that their research had included people with a mix of experiences of taking CSs and of perceptions of other drugs used to treat JIA and that a key finding was that randomisation would be more difficult for CYP who had experienced CS treatments before and who favoured one route over another.
In response to a question about the favoured route of CS administration among CYP, the presenters responded that the numbers of individuals studied with experience of each route were too small to give a clear answer. There was agreement among attendees that a full trial would be needed to answer this question.
Results presentation on the assessment of UK practice
The results of previous study activities, including the screening log exercise, the survey of practice and the feasibility study were presented, and covered the numbers of individuals with each disease subtype and who received treatment by each route and the number of patients with active JIA currently receiving CS treatment.
When asked if the research had found a favoured CS route among CYP, the presenters pointed out that pre-trial qualitative studies are designed not to answer such questions, but, rather to provide in-depth information about patients’ views of a future RCT, so that these can be taken into account in the design and conduct of such a trial. There was agreement among attendees that a full trial would be needed to answer this question.
The groups were asked to discuss the minimum age at which potential study participants should be treated with each route of CS administration. It was felt that, although specific safety issues may be relevant to patients aged < 4 years or aged < 2 years, the trial should not specify a lower age limit for participation.
Attendees also raised and discussed what the maximum age for inclusion should be. The original HTA Commissioning Board remit had not allowed this feasibility study to include patients aged > 16 years. However, the meeting felt strongly and voted unanimously that this age cut-off point disadvantaged older adolescents and young adults with JIA who are not studied separately in adult trials. It was voted that the issue should be raised again with the HTA Commissioning Board and it was recommended that young people, at least up to the age of 18 years, be allowed to participate in a future trial.
Protocol design session
The group discussed the two protocols for the consensus voting. It was decided that each of these protocols should be worked up in detail, with votes on particular aspects of each, to allow future investigators to have a choice of two valid options. The HCP group agreed that if, in the case of protocol 8, IM injection showed no benefit after 3 months this arm could be dropped and information used in the intention-to-treat analysis. It was also recommended that the trial would continue with two arms.
Results
The eight original protocols are outlined in Appendix 4 and are as follows:
-
Protocol 1 – IACI versus IM injection. The control arm is IACI and the treatment arm is a single IM dose of methylprednisolone (Depo-Medrone) to a maximum dose of 150 mg.
-
Protocol 2 – IACI versus IV infusion of methylprednisolone, completely excluding patients with oligoarticular JIA.
-
Protocol 3 – IACI versus oral prednisolone, completely excluding patients with oligoarticular JIA.
-
Protocol 4 – oral prednisolone versus IV infusion of methylprednisolone completely, excluding patients with oligoarticular JIA.
-
Protocol 5 – oral prednisolone versus IM injection of Depo-Medrone, including patients with oligoarticular JIA flares.
-
Protocol 6 – IM injection of Depo-Medrone versus IV infusion of methylprednisolone, including patients with oligo-articular IA flares.
-
Protocol 7 – a staged CS protocol approach in which entry is through failed IACI and is, therefore, open to patients with all subtypes of JIA. Inclusion: patients with any JIA subtype in whom IACI has failed are randomised to one of IV infusion, IM injection or oral prednisolone.
-
Protocol 8 – an adaptive design in which all routes of CS delivery are compared with IACI, and the least effective route is removed from further study at an interim analysis. This protocol has IACI as the control group. Patients with first presentation of oligoarticular JIA are excluded, but those experiencing subsequent flares of oligoarticular JIA can be included.
Survey
Voting on protocol choice was carried out before the final consensus meeting by 110 HCPs, including all of the site principal investigators and all of the meeting participants. The respondents were asked to choose the most important head-to-head study protocol from protocols 1–6 and then one of the more complex protocols, either protocol 7 or protocol 8.
Although only 17 replies were received in the timeframe, we know that the respondents were mostly consultant paediatric rheumatologists and, therefore, the reply was highly representative of those actually making treatment recommendations for CYP with JIA. The results were very clear in prioritising protocol 2 (8/17, 47%) and protocol 8 (11/16, 69%). Thus, the planned consensus meeting format was modified from that outlined in Methods. The SMG decided to work on the two chosen protocols and to use consensus voting to decide on detailed aspects of the protocol, such as inclusion and exclusion criteria.
The final consensus meeting was attended by 10 HCPs, most of whom were site principal investigators for the SIRJIA study but not on the SMG, and eight PPI representatives. The discussion sessions were led by three SMG members and an independent PPI facilitator, and the whole meeting, as well as the voting, was overseen by an independent chairperson with no previous knowledge of the SIRJIA study. The CI was present only to present results from the previous study activities and to answer questions as they arose, but did not express any opinions with regard to details of the final protocol choices.
Protocol design session
Important issues were raised by the discussion sessions. The full notes of the PPI consumers and the HCPs discussion groups are given in Appendix 5.
The group discussed the two protocols for the consensus voting. It was agreed by the HCP group in protocol 8 that if, after 3 months of the trial, any arm of the study (e.g. the IM injection arm) showed no benefit, this arm could be dropped and any information could be used in the intention-to-treat analysis. It was also recommended that the trial would then continue with the other arms. This is part of the adaptive design.
Results of the consensus meeting voting
The HCPs and PPI representatives discussed very similar issues; however, these were voted on only by the HCPs in view of the number of clinical experience-linked issues. The HCP meeting focused on the technical aspects of the medications and the PPI representatives’ meeting focused more on the experiential and inclusivity aspects of the protocol.
Protocol 2
Protocol 2: initial protocol preconsensus discussion and voting
Treatment arms
The two arms were IACI (control arm) and IV infusion of methylprednisolone (treatment arm), completely excluding patients with oligoarticular JIA.
Initial inclusion criteria
-
Patients with polyarticular JIA or a polyarticular joint course.
-
Patients with systemic-onset juvenile idiopathic arthritis (SOJIA), psoriatic arthritis and enthesitis-related arthritis are included in line with the conditions stated in the exclusion criteria.
-
Patients with extended oligoarticular JIA.
Initial exclusion criteria:
-
Patients with oligoarticular JIA.
-
Patients with active systemic JIA with systemic features or a serum ferritin level above 1000 ng/ml.
-
Patients with active infection.
-
Patients with active uveitis as the main indication for CS treatment.
Initial control arm
The most commonly used route of CS administration is IACI; therefore, this route will be used as the control arm of the study. A maximum of 10 joints will be treated with a maximum of 40 mg of TH per joint (with more detailed dosing per joint for the final study).
Initial treatment arm
It was decided that the initial treatment arm would be IV infusion of methylprednisolone at a dose of 20–30 mg/kg to a maximum of 1 g given on 3 consecutive days with gastric protection.
Protocol 2 voting questions and the results of health-care professional voting
-
Do you agree that patients with polyarticular JIA or a polyarticular joint course should be included?
Response: yes (100%).
-
Do you agree that patients with SOJIA features with active arthritis but without systemic features should be included?
Response: yes (100%).
-
Do you agree that patients with psoriatic arthritis and enthesitis-related arthritis should be included?
Response: yes (78%) and no (22%).
-
Do you agree that patients with extended polyarticular JIA should be included?
Response: yes (100%).
-
Do you agree that all patients with oligoarticular JIA should be excluded?
Response: yes (22%) and no (78%).
-
Do you agree that all patients with oligoarticular JIA in a second flare should be included as long as they have more than two active joints?
Response: yes (78%) and no (22%).
-
Do you agree that all patients with oligoarticular JIA in a second flare should be included as long as they have more than three active joints?
Response: yes (100%).
-
Do you agree that all patients with active systemic JIA will be excluded?
Response: yes (89%) and no (11%).
-
The most commonly used route of administration of CSs in the initial treatment of JIA is IACI and this route will, therefore, be used as the control arm of the study. Do you agree?
Response: yes (89%) and no (11%).
-
A maximum of 10 joints will be treated to a maximum dose of 40 mg of TH per joint. Do you agree with this?
Response: yes (56%) and no (44%).
-
The dose of IV infusion of methylprednisolone will be 20–30 mg/kg to a maximum of 1 g given on 3 consecutive days with gastric protection. Do you agree with this?
Response: yes (67%) and no (33%).
-
Should there be a maximum number of joints as part of the treatment regimen?
Response: yes (22%) and no (78%).
Protocol 2 with changes after voting
Treatment arms
The two arms were IACI (control arm) and IV infusion of methylprednisolone (treatment arm), including in patients with oligoarticular JIA.
Final inclusion criteria
-
Patients with polyarticular JIA or a polyarticular joint course.
-
Patients with SOJIA, psoriatic arthritis and enthesitis-related arthritis are included in line with the conditions stated in the exclusion criteria.
-
Extended oligoarticular JIA.
-
Patients with oligoarticular JIA in a second flare should be included as long as they have more than two active joints.
Final exclusion criteria
-
Patients with active systemic JIA with systemic features or a serum ferritin level above 1000 ng/ml.
-
Patients with active infection.
-
Patients with active uveitis as the main indication for CS treatment.
Final voted control arm
The most commonly used route of administration of CSs is IACI and this route will, therefore, be used as the control arm of the study. Dosing will be to a maximum of 40 mg of TH per joint (with more detailed dosing per joint for the final study). There will be no maximum number of joints injected and this will instead be left to the discretion of the physician.
Final voted treatment arm
Based on the final vote, the treatment arm will receive IV infusion of methylprednisolone at a dose of 20–30 mg/kg to a maximum of 1 g given on 3 consecutive days with gastric protection.
Protocol 8
Initial protocol preconsensus discussion and voting
An adaptive design in which all routes of CS delivery are compared with IACI as the control arm, and the least effective route is removed from further study at an interim analysis.
Initial control arm treatments
Control
The most commonly used CS route was IACI and this route will, therefore, be used as the control arm of the study. Dosing will be up to a maximum of 10 joints with a maximum of 40 mg of TH per joint (with more detailed dosing per joint for the final study).
Detailed treatment arms
The participants will be randomised to one of the following: IV infusion, oral prednisolone or IM injection.
First initial inclusion criteria
Does the patient have oligoarticular JIA with active joints in the first flare? Is the patient able to have IACI in a timely manner (within 2 weeks)? If yes, recruit to study and treat with IACI.
Second initial inclusion criteria
Treat flares of oligoarticular JIA in the same way as polyarticular disease, including patients with:
-
systemic-onset JIA without systemic features
-
enthesitis-related arthritis or psoriatic arthritis
-
extended oligoarticular JIA.
Initial exclusion criteria
-
Patients with active systemic JIA with systemic features or a serum ferritin level above 1000 ng/ml.
-
Patients with active infection.
-
Patients with active uveitis as the main indication for CS treatment.
Protocol 8 voting questions and results
-
Do you agree that this protocol can apply to patients with all subtypes of JIA, including patients with persistent oligoarticular JIA experiencing a second or subsequent flare?
Response: yes (67%) and no (33%).
-
Should this protocol include patients with additional active enthesitis?
Response: yes (78%) and no (22%).
-
Should this protocol include patients with additional active tenosynovitis as well as arthritis?
Response: yes (100%).
-
Should this protocol exclude patients with active systemic JIA features?
Response: yes (89%) and no (11%).
-
Should this protocol exclude patients with active macrophage activation syndrome?
Response: yes (78%) and no (22%).
-
Should this protocol include patients with active arthritis even if they have severe psoriasis?
Response: yes (11%) and no (89%).
-
Should the age limits for inclusion be the same for all modalities of treatment?
Response: yes (89%) and no (11%).
-
Should only patients above the age of 6 years be included in this protocol?
Response: no (100%).
-
Should recruitment be limited to patients above the age of 10 years in this protocol?
Response: no (100%).
-
Should recruitment be limited to patients above the age of 2 years in this protocol?
Response: yes (22%) and no (78%).
-
Should there be a lower age limit in this protocol?
Response: yes (11%) and no (89%).
-
Do you agree that patients should have at least three active joints at the time of randomisation to treatment?
Response: yes (75%) and no (25%).
-
Should any joints that fail to respond fully to a randomised treatment within the first 6 weeks be treated with additional intra-articular injections of CSs of the physician’s choice?
Response: yes (33%) and no (67%).
-
Should the maximum dose of Depo-Medrone IM injection be 120 mg?
Response: yes (100%).
-
Should the dose of IV infusion of methylprednisolone be 30 mg/kg up to a maximum of 1 g?
Response: yes (88%) and no (13%).
-
Should the dose of methylprednisolone be administered by IV infusion once daily for 3 days?
Response: yes (75%) and no (25%).
-
Should the dose of methylprednisolone be administered by a single IV infusion only?
Response: yes (25%) and no (75%).
-
Which oral CS induction regimen would you prefer?
Response:
-
A – 2 mg/kg to a maximum of 60 mg given for 1 week, reducing to 25% of the original dose over 5 weeks.
-
B – 1 mg/kg to a maximum of 40 mg for 2 weeks, reducing to 38% of the original dose over 5 weeks.
-
C – 1 mg/kg to a maximum of 40 mg for 1 week, reducing to 38% of the original dose over 3 weeks.
-
-
Which oral CS induction regimen would you prefer?
Response:
-
A – 1 mg/kg to a maximum of 40 mg for 2 weeks, reducing to 50% of the original dose over 5 weeks.
-
B – 1 mg/kg to a maximum of 40 mg for 1 week reducing to 50% of the original dose over 3 weeks.
-
-
Should the oral prednisolone dose be 1 mg/kg to a maximum of 40 mg for 1 week, with a 5-week weaning regimen?
Response: yes (100%).
-
Should this protocol include patients with additional active tenosynovitis as well arthritis?
Response: yes (100%).
Protocol 8 final voted protocol
An adaptive design in which all routes of CS delivery are compared with IACI as the control arm, and the least effective route is removed from further study at an interim analysis.
Final voted control arm
The most commonly used CS route was IACI and this route will, therefore, be used as the control arm of the future RCT. Dosing will be to a maximum of 40 mg of TH per joint (with more detailed dosing per joint for the final study). There will be no maximum number of joints injected; instead this will be left to the discretion of the physician.
Detailed treatment arms
-
Oral prednisolone 1 mg/kg to a maximum of 40 mg for 1 week, with a 5-week weaning regimen.
-
IV infusion methylprednisolone at a dose of 30 mg/kg to a maximum dose of 1 g given for 3 consecutive days.
-
Depo-Medrone IM injection up to a maximum dose of 120 mg.
Voted inclusion criteria
-
All age groups were included, with a recommendation to extend the cut-off point up to the participants’ 19th birthday.
-
Patients should have at least three active joints at the time of randomisation to treatment.
-
Patients with persistent oligoarticular JIA experiencing a second or subsequent flare.
-
Does the patient have a polyarticular-course JIA, including –
-
systemic-onset JIA without systemic features
-
enthesitis-related arthritis including with additional active enthesitis
-
psoriatic arthritis
-
extended oligoarticular JIA
-
arthritis as well as additional active tenosynovitis?
-
Voted exclusion criteria
-
Patients with active systemic JIA with systemic features or a serum ferritin level above 1000 ng/ml.
-
Patients with active infection.
-
Patients with active uveitis as the main indication for CS treatment.
-
Patients with severe psoriasis even if they have active arthritis.
-
Patients with active macrophage activation syndrome.
Patients with any joints that fail to respond fully to a randomised treatment within the first 6 weeks should not be treated with additional IA CSs unless the physician decides to remove the patient from the study.
Additional combined considerations from the discussion groups
See Appendix 5 for the full meeting notes.
Additional considerations from the discussion groups are as follows:
-
It was agreed that the safety, tolerability and cost-effectiveness of all routes should be recorded as secondary outcomes with special attention to any occurrences of avascular necrosis.
-
It was suggested that patients with polyarticular disease with neck involvement should not be randomised.
-
Patients with active enthesitis only without active arthritis should be excluded.
-
Patients with active tenosynovitis only without active arthritis should be excluded.
-
Patients with active temporomandibular joint inflammation should not be excluded.
-
Analysis of associated concomitant medications that are DMARDs will be necessary and the recommendation is that these should be kept unchanged before randomisation and for 6 weeks after CS treatment.
-
The timescale for flare needs to be clarified and it was queried if this exclusion criterion was actually required.
Trial design
In this section, different sample size estimates are proposed for protocol 2 and protocol 8. The sample size estimates below pool data from all of the various subgroups of JIA that were included in the study and patients with various degrees of disease activity.
Design proposal for protocol 2 and sample size calculations
In this protocol, the aim of the trial is to compare IV infusion with the control treatment of IACI. The sample size calculations are based on the primary outcome of change in cJADAS from baseline to 6 weeks. The mean baseline cJADAS in the IACI arm was 8.3 and the change from baseline to 6 weeks was 4.0 (SD 4.1).
A sample size of 41 participants in each group would have 90% power to detect a difference in means of 3 (the difference between the IACI group mean difference, µ1, of 4 and an IV infusion mean, µ2, of 7) assuming that the common standard deviation is 4.1 using a two-group t-test with a 0.05 two-sided significance level. This does not take into account any inflation for missing data.
Sample sizes (for each group) for difference in means of 2, 1 and 0.5 are 90, 355 and 1414 participants, respectively.
Design proposals for protocol 8 and sample size calculations
In this protocol, the aim of the trial is to compare the control treatment (IACI) with oral prednisolone, IM injection or IV infusion. A fixed sample size will be considered as well as an adaptive design. This design will allow arms that are not performing as well as the control arm to be stopped early.
Sample size calculations are presented that allow for up to three analyses (up to two interim analyses and one final analysis). At each of the planned analyses, comparative test statistics, Zi (i = 1, 2, 3), for each of the three arms are computed and then compared with the IV infusion arm. If at any of the interim analyses the test statistic for any of the three groups lies below zero, then that arm will dropped and not studied any further. Should all of the test statistics lie below zero, then the entire study should be stopped with the conclusion that none of the three treatment options is better than IACI.
If any test statistic lies above an upper cut-off value, then the study should be stopped and that treatment should be deemed superior to IACI (control). If the test statistic lies between zero and the upper cut-off value, then the trial should continue and further patients should be randomised to each of the remaining intervention arms and the control arm. At the final analysis, the remaining test statistics are compared with a critical value; should any of these test statistics exceed this value, then that particular treatment would be said to be superior to IACI.
The assumptions below have been made:142,143
-
The outcome is normally distributed with known variance.
-
Equal numbers of participants are randomised to each of the four arms.
-
O’Brien and Fleming144 boundaries are used to determine the stopping boundaries.
-
The minimum important difference between the experimental arm and the control arm is denoted δ.
-
The probability that any of the three experimental arms is better than IACI is α (one-sided).
-
The probability that any of the three experimental arms is better than the control arm when it is better by δ and all others are worse by at least δ0 is 1 – β.
Sample size
The primary outcome was chosen during the consensus process and was the change from baseline to 6 weeks for the cJADAS between the three treatment arms and the IACI control arm. The estimate of variability for the change from baseline to 6 weeks in the cJADAS is taken from the feasibility study and is estimated to be σ = 4.3.
The sample size estimates that are provided in Table 30 all use an estimate of the variability to be σ = 4.3, an ‘uninteresting effect’ δ0 = 4.0 and δ to be 3.5, 3, 2 and 1, respectively.
| Number of analyses | Stopping bounds | Total (N) | E (N/H0) | E (N/H1) |
|---|---|---|---|---|
| δ = 4.5, δ0 = 4.0, α = 0.05, 1 – β = 0.9 | ||||
| 1 | 2.06 | 1340 | 1340 | 1340 |
| 2 | (2.93, 2.073) | 2680 | 1340 | 1340 |
| 3 | (3.61, 2.56, 2.09) | 4020 | 1340 | 1340 |
| δ = 5, δ0 = 4.0, α = 0.05, 1 – β = 0.9 | ||||
| 1 | 2.06 | 336 | 336 | 336 |
| 2 | (2.93, 2.07) | 672 | 336 | 336 |
| 3 | (3.61, 2.56, 2.09) | 1008 | 337 | 336 |
| δ = 6, δ0 = 4.0, α = 0.05, 1 – β = 0.9 | ||||
| 1 | 2.06 | 88 | 88 | 88 |
| 2 | (2.93, 2.07) | 168 | 99 | 86 |
| 3 | (3.61, 2.56, 2.09) | 192 | 105 | 82 |
| δ = 7, δ0 = 4.0, α = 0.05, 1 – β = 0.9 | ||||
| 1 | 2.06 | 44 | 44 | 44 |
| 2 | (2.93, 2.07) | 56 | 48 | 38 |
| 3 | (3.61, 2.56, 2.09) | 60 | 46 | 38 |
The columns headed E (N/H0) and E (N/H1) show, respectively, the expected sample size when none of the three experimental arms is better than IACI and when exactly one of the three is better than IACI.
It can be seen from Table 30 that in the case of a fixed sample size design comparing three ‘experimental’ arms with the IACI control arm (with an interesting difference of 7 and an expected 6-week cJADAS of 1, assuming a baseline score of 8.1), a change from baseline to 6 weeks requires a total of 44 patients (11 per arm) when a one-sided family-wise error of 5% and a power of 90% is required. For any of the three experimental arms, a corresponding test statistic > 2.06 can conclude superiority over IACI.
A two-stage design comprising an interim analysis and one final analysis would require, at most, 56 patients for the same error rates. At the interim analysis, which would be conducted half-way through the recruitment of patients, a test statistic > 2.93 would lead to a claim to superiority of that arm and the trial could be stopped. If the interim analysis revealed a test statistic below zero (which would indicate that that arm is no better than IACI), no further participants should be allocated to that arm. If none of the three arms were found to be superior to the IACI arm at the interim analysis, then a further seven patients should be randomised to each arm. At the final analysis, if any of the test statistics were > 2.07, then that treatment could be considered superior to IACI.
For a trial arm to be dropped or the trial to be stopped early would require 28 patients (if at least one treatment is clearly superior to IACI or all treatments are dropped), 42 patients (if only one experimental arm is progressed to the final analysis), 49 patients (if two arms are progressed) or 56 patients (if all arms continue). The expected (average) sample size of the design is 48 patients if no treatment is better than IACI and 38 patients if exactly one treatment is better than IACI.
The results for the design with two interim analyses and one final analysis, and for the designs with other ‘interesting’ effects can be interpreted in the same manner.
Discussion
The most common treatment throughout the SIRJIA study was IACIs and this will, therefore, be recommended as the ‘treatment as usual’ or control arm as defined by the HTA Commissioning Board brief. Therefore, in both of the protocols discussed in the final discussion and consensus meeting, IACI was defined and agreed as the control treatment. There was subsequent detailed discussion about the practicalities of IACI as the control treatment. Factors raised were the minimum and maximum number of joints to be treated in the control arm. There was agreement in the HCP discussion that no maximum number of joints should be cited but that this decision should be left to physician discretion. However, PPI participants felt that 10 or more joints injected at one time would be too much.
The fact that multiple IACIs may need to be carried out under GA and the limited availability of theatre facilities to carry out joint injection were suggested as possible sources of increased risk in the control arm. The acceptability of randomising patients to a treatment arm necessitating GA was also raised, but as such treatment is routine and well established, and general anaesthesia brief and light, there was general agreement that this should not preclude proceeding with IACI as the control treatment. There was a concern that the capacity to carry out treatment under GA within 2 weeks would be tricky even for large units. However, treatment delay resulting from limited injection list space in theatre could be mitigated by measuring baseline disease activity on the day of treatment (or within 1 week of treatment) with the primary outcome planned to be measured at 6 weeks after the actual date of treatment. In addition, many units have access to Entonox sedation, and several joints can be injected at once with this modality of sedation. This did not cause any major issues in the feasibility study, apart from raising the practical need for 1 week’s leeway in the baseline measure to allow for the fact that research staff may not be present on the day of the IACI.
It is recommended that psychological support should be available for patients with needle phobias, in view of the fact that three of the treatment arms (IACI, IV infusion and IM injection) in protocol 8 include needles. However, this is part of routine clinical care in JIA, which commonly involves diagnostic and monitoring blood tests, and most patients do actually receive IACIs at some point during their care.
Parents and families throughout the SIRJIA study raised the relevant concern that CYP with JIA who had already experienced a treatment that had not worked fully might be reluctant to enter a study in which randomisation might lead to the same treatment again. However, the variability in response to a treatment in different flares is known by clinicians and is something that would be dealt with during recruitment. It is recommended that this factor be specifically detailed on the future trial patient information leaflets (PILs).
There was unanimous agreement that the age range for recruitment to all treatment arms should have no lower limit, with this being left to physician and family discretion. The PPI representatives were concerned about the specific issues relating to very young children in trials, with regard to, for example, IV infusion cannulation, IM injections or general anaesthesia; these are factors that will be considered on balance in individual patient recruitment. However, there was a clear agreement that the upper age limit should be extended at least to the 18th year, that is to those aged ≤ 19 years, and that consideration should be given to the inclusion of young adults up to the age of 21 years. The general view was that this age group is currently disadvantaged in being excluded from JIA treatment trials. It was not felt to be correct that young adults with JIA could expect to be included in trials of rheumatoid arthritis in adulthood only, under the assumption that their disease has changed, which is usually not the case.
There was discussion among HCPs about whether or not the dosing of CSs between treatment arms should aim for equivalence of CS; this was not generally supported as the study aim is to evaluate currently favoured treatment regimens. The dosing of IACI would need to be confirmed for each joint, but with a maximum of 40 mg of TH for large joints. There is no total cumulative maximum dose per treatment session used in clinical practice. The dosing of CSs administered by IM injection in small children would need to be clarified by the trial team at the time of a future trial protocol preparation, because there are currently no published per-kg dosing recommendations. The IV infusion dosing was voted to be 30 mg/kg to a maximum of 1 g administered on 3 consecutive days, with gastric protection cover. The durations/rates of the IV infusions will need to be specified and could be 4 hours on the first day and over 1 hour on the subsequent 2 days. The dilution of methylprednisolone and the amount of normal saline for the dilution must be specified. The oral dosing of preference was voted on in the meeting and the favoured regimen was oral prednisolone at a dose of 1 mg/kg for 1 week, with a 5-week weaning period.
It was argued that the side effects and adverse events of the treatments are so important in the burden of the disease and its treatment that they must be included as secondary outcomes. This is particularly true of CS-induced side effects, despite the fact that there are no validated scoring systems for CS-induced adverse events. Special note was made of the need to record any cases of avascular necrosis.
Similarly, cost-effectiveness should be included as a secondary outcome. If the results of a trial show very little difference in the effectiveness of different regimens, then perhaps the cheapest treatment should be used in future.
There was general discussion as to whether or not the study should aim for equivalence in treatment effects or for superiority of effect. Both outcomes would be useful in their own right, with equivalence empowering CYP and their families to decide with their clinician which route is most appropriate for them. On the other hand, if one route were revealed to be clearly superior in terms of efficacy this would constitute an important evidence base for treatment.
The feasibility study did not include a definition of JIA flare or specify a timescale for flare, leaving this to physician discretion and intention to treat. HCPs were clear that flare should be defined and a timescale specified in the inclusion criteria, but these issues could not be dealt with in the limited time available at the consensus meeting. We are not aware of any drug trials in JIA that have quantified duration of flare, but this will be added to the post study work list.
The cJADAS was still supported as the primary outcome measure of choice. However, a clinically meaningful difference in cJADAS has not been previously studied, nor was the clinician experience of the measure enough to set the required change for a future trial. The results from the feasibility data and power calculations will inform this decision. However, the fact that JIA is now treated more aggressively means that the starting point for treatment is generally much lower for many patients and, therefore, the starting point for the outcome measure is lower. For example, defining a clinically meaningful difference as a change in cJADAS of > 10 would mean that the majority of patients would be unable to achieve this, by definition, as their activity score at baseline is likely to be much lower than 10 to start with.
There was agreement that the primary outcome should be measured at 6 weeks, as earlier measurement would mask a slower response in some but later measurement would risk the development of new flares and similarly mask the treatment response. In addition, the 12-week outcome visit was not challenged; however, thought will need to be given in a future RCT as to the benefits of a 6-, 9- or 12-month follow-up to allow subsequent flare rates to be compared between treatment groups as an important secondary outcome.
Preparation of study materials for a final trial would be helped by adapting those developed for this SIRJIA study. 36 This should include the graphics and study explanation flow sheets from the qualitative study. The consent forms and PILs could readily be adapted. The PPI group recommended that videos would be a useful way to present information to both parents and children; animations or real-life videos were both viewed as acceptable. The videos should show what would be involved in taking part in the trial and what would happen at each stage. In the case of an animation, the characters would need to be realistic. Parents thought that some information that could be included on a trial website, such as the risks of all treatments, would be too much for children but useful for parents. One parent mentioned resources that had been developed by (or with) SNAC, where the videos related to ‘what and why’ and were seen to be potentially useful.
For patients to have the access to know about clinical trials that may be relevant to them, it is important to have ongoing schemes in place to publicise research. For example, no one had heard of the NIHR ‘OK to Ask’ and the ‘I am Research’ campaigns. It was suggested that these schemes need to be run repeatedly in centres. Parents talked about a scarcity of information about what research was available to take part in. They usually heard about things through their consultant, although one parent, as a result of attending the meeting, heard about another research study of interest.
Chapter 9 Conclusions
Summary of main findings
-
The literature review showed that although there is sufficient evidence to support the use of IACI injections in CYP with JIA, evidence for the use of other methods of administration of CSs and standardised dosing regimens is still lacking.
-
Although the qualitative study with CYP and their families did not identify one treatment as unsuitable overall, it showed the importance of taking account of CYP and parent treatment preferences in the design and conduct of any future RCT. Such a RCT would need to ensure that all of the treatments that are being investigated are suitable for all patients with JIA and, if this is not the case, eligibility criteria should be amended.
-
A future trial should be as accessible as possible to CYP and their families, for example by combining trial follow-up assessments with clinic appointments.
-
The national survey of clinical practice showed that there is no standard treatment for CYP with a new diagnosis of JIA or for those who are undergoing a flare.
-
Health-care professionals who took part in the national survey indicated that they would be willing to randomise CYP into a future trial.
-
A consensus process involving CYP, their families and HCPs chose the JADAS-71 as an appropriate primary outcome.
-
There were 95 CYP who were registered into the feasibility study. Missing data were highlighted as an issue for the JADAS-71, but the cJADAS-71 could be used in its place. Future trials should ensure that adequate monitoring is in place to ensure that any issues with regard to missing data are highlighted as early as possible.
-
Two trial protocols were considered by HCPs as the most appropriate to answer key questions regarding the most effective method of administrating CSs.
Overall conclusions
This mixed-methods study has confirmed the lack of a published evidence base for the CS regimen of choice for use in new or flaring JIA.
We have shown that this is an important question for CYP with JIA, their families and HCPs alike, as CSs are a long-established part of treatment in JIA, but decisions as to the CS route and dose are typically devolved to clinicians based on their opinion and experience, coupled with patient choice.
We have demonstrated excellent agreement and ‘buy-in’ to a multicentre study and have developed two different possible study protocols that have been worked up in a truly open and consensus-derived manner.
The unit-based screening log numbers, which revealed 250 patients screened by 12 centres over 6 weeks, coupled with double the anticipated recruitment to the feasibility study (with 95 patients recruited from 15 centres), show that there are enough CYP with JIA currently receiving courses of CSs.
The involvement of CYP, their families and HCPs led to a consensus in the choice of a composite primary outcome in the form of the cJADAS. Two trial protocols were developed and any future trial should consider the use of the treatment regimens and designs that have been discussed.
It is important that the results of this research are disseminated as widely as possible; the current outputs from this study are presented in Appendix 6.
Acknowledgements
The authors would like to acknowledge all of the children, young people, family members and health-care professionals who have contributed to many aspects of the SIRJIA study both as participants in the clinical study section and as contributors to the consensus meetings.
The authors would like to thank Katharine Cresswell, Sharon Douglas (member of BSPAR Parent Group and co-founder of SNAC) and Catherine Wright for facilitating the invitation of CYP and their families to become involved in this consensus process, and Dr Madeline Rooney for her contribution throughout the course of the study.
We are very grateful for the attendance at the final discussion and consensus meeting that was held on 3 December 2018 by the following researchers: Professor Robert Moots (chairperson), Dr Alice Leahy, Dr Eslam Al-abadi, Dr Valentina Leone, Dr Clarissa Pilkington, Dr Gulshan Malik, Dr Kate Armon, Dr Jonathan Packham, Dr Clare Pain and Dr Liza McCann.
We thank all of the members of the SSC (see Appendix 2) and all of those from the LCTC who contributed to the study activities at various times of the study (see Appendix 3).
This study was sponsored by Alder Hey Children’s NHS Foundation Trust.
Contributions of authors
Ashley P Jones (https://orcid.org/0000-0001-5253-730X) (Statistical Lead) led the statistical team throughout the study, contributed to the overall study design, the design of specific substudies and protocol development. He contributed to the final report (drafting and reviewer) and was a member of the SMG and SSC.
Dannii Clayton (https://orcid.org/0000-0003-3535-3466) (Trial Statistician) was responsible for the analysis of the quantitative data. She contributed to the final report (drafting and reviewing) and was a member of the SMG.
Gloria Nkhoma (https://orcid.org/0000-0002-7771-2626) (Trial Co-ordinator) contributed to protocol development, all aspects of governance and study delivery and preparation of progress reports. She contributed to the final report (drafting and reviewer) and was a member of the SMG.
Frances C Sherratt (https://orcid.org/0000-0003-4147-9305) (Research Associate) is a researcher on the SIRJIA qualitative study and contributed to data collection, analysis and report writing. She also provided support at both study consensus meetings. She contributed to the final report (drafting and reviewer) and was a member of the SMG.
Matthew Peak (https://orcid.org/0000-0003-1909-3211) (Co-applicant) contributed to overall study design, the design of specific substudies and protocol development. He contributed to the final report (drafting and reviewer) and was a member of the SMG and SSC.
Simon R Stones (https://orcid.org/0000-0002-5943-1310) (PPI Representative/Patient Research Partner/Co-applicant) contributed to protocol development, championed the involvement of young people and their families throughout the study and gave guidance to aspects of governance and study delivery. He contributed to the final report (drafting and reviewing) and was a member of the SMG.
Louise Roper (https://orcid.org/0000-0002-2918-7628) (Research Associate) is a researcher on the SIRJIA qualitative study and contributed to data collection, analysis and report writing. She also provided support at the first study consensus meeting. She contributed to the final report (drafting and reviewer) and was a member of the SMG.
Bridget Young (https://orcid.org/0000-0001-6041-9901) (Co-applicant) was a supervisor for the qualitative study, was a member of the SMG and contributed to the final report (drafting and reviewer).
Flora McErlane (https://orcid.org/0000-0002-8434-1771) (Co-applicant) was a member of the SMG, contributed to outcome measure discussions and to the final report (reviewer).
Tracy Moitt (https://orcid.org/0000-0002-5579-996X) (Senior Trials Manager) contributed to protocol development, gave guidance and support on all aspects of governance and study delivery and supported the preparation of progress reports. She contributed to the final report (drafting and reviewer) and was a member of the SMG.
Athimalaipet V Ramanan (https://orcid.org/0000-0001-8663-2401) (Co-applicant) was a member of the SMG and reviewed the final report.
Helen E Foster (https://orcid.org/0000-0001-8824-2546) (Co-applicant) was a member of the SMG, contributed to the review of survey questions and substudy development.
Paula R Williamson (https://orcid.org/0000-0001-9802-6636) (Co-applicant) contributed to the overall study design and reviewed the final report.
Samundeeswari Deepak (https://orcid.org/0000-0003-3222-1441) (Consultant Paediatric Rheumatologist) was a member of the SMG, contributed to the literature review on the use of CSs and writing Chapter 2 of the report, and participated in the discussions during the teleconferences and in the final consensus meeting.
Michael W Beresford (https://orcid.org/0000-0002-5400-9911) (Co-applicant) contributed to overall study design and protocol development. He chaired the first consensus meeting on outcome measures and reviewed the final report.
Eileen M Baildam (https://orcid.org/0000-0001-8463-6388) (Chief Investigator) developed the initial study design and contributed to protocol development, was an applicant on the grant, was the chairperson of the SMG, has overseen all aspects of the study and contributed to all parts of the final report.
Publication
Sherratt FC, Roper L, Stones SR, McErlane F, Peak M, Beresford MW, et al. Protective parents and permissive children: what qualitative interviews with parents and children can tell us about the feasibility of juvenile idiopathic arthritis trials. Pediatr Rheumatol 2018;16:76.
Data-sharing statement
Requests for data can be made through the corresponding author. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data is used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Symmons DP, Jones M, Osborne J, Sills J, Southwood TR, Woo P. Pediatric rheumatology in the United Kingdom: data from the British Pediatric Rheumatology Group National Diagnostic Register. J Rheumatol 1996;23:1975-80.
- Baszis K, Garbutt J, Toib D, Mao J, King A, White A, et al. Clinical outcomes after withdrawal of anti-tumor necrosis factor α therapy in patients with juvenile idiopathic arthritis: a twelve-year experience. Arthritis Rheum 2011;63:3163-8. https://doi.org/10.1002/art.30502.
- Chang CY, Meyer RM, Reiff AO. Impact of medication withdrawal method on flare-free survival in patients with juvenile idiopathic arthritis on combination therapy. Arthritis Care Res 2015;67:658-66. https://doi.org/10.1002/acr.22477.
- Giannini EH, Ruperto N, Ravelli A, Lovell DJ, Felson DT, Martini A. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum 1997;40:1202-9. https://doi.org/10.1002/1529-0131(199707)40:7<1202::AID-ART3>3.0.CO;2-R.
- Consolaro A, Ruperto N, Bazso A, Pistorio A, Magni-Manzoni S, Filocamo G, et al. Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Rheum 2009;61:658-66. https://doi.org/10.1002/art.24516.
- McErlane F, Beresford MW, Baildam EM, Thomson W, Hyrich KL. Recent developments in disease activity indices and outcome measures for juvenile idiopathic arthritis. Rheumatology 2013;52:1941-51. https://doi.org/10.1093/rheumatology/ket150.
- Thornton J, Lunt M, Ashcroft DM, Baildam E, Foster H, Davidson J, et al. Costing juvenile idiopathic arthritis: examining patient-based costs during the first year after diagnosis. Rheumatology 2008;47:985-90. https://doi.org/10.1093/rheumatology/ken039.
- Boland EW. The effects of cortisone and adrenocorticotropic hormone (ACTH) on certain rheumatic diseases. Calif Med 1950;72:405-14.
- Davies R, Carrasco R, Foster HE, Baildam EM, Chieng SEA, Davidson JE, et al. Treatment prescribing patterns in patients with juvenile idiopathic arthritis (JIA): analysis from the UK Childhood Arthritis Prospective Study (CAPS). Semin Arthritis Rheum 2016;46:190-5. https://doi.org/10.1016/j.semarthrit.2016.06.001.
- Wallace CA, Ringold S, Bohnsack J, Spalding SJ, Brunner HI, Milojevic D, et al. Extension study of participants from the trial of early aggressive therapy in juvenile idiopathic arthritis. J Rheumatol 2014;41:2459-65. https://doi.org/10.3899/jrheum.140347.
- Petty RE, Laxer RM, Lindsley CB, Wedderburn LR. Textbook of Pediatric Rheumatology 7th edition. Amsterdam: Elsevier; 2016.
- Grigor C, Capell H, Stirling A, McMahon AD, Lock P, Vallance R, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet 2004;364:263-9. https://doi.org/10.1016/S0140-6736(04)16676-2.
- Hetland ML, Stengaard-Pedersen K, Junker P, Østergaard M, Ejbjerg BJ, Jacobsen S, et al. Radiographic progression and remission rates in early rheumatoid arthritis – MRI bone oedema and anti-CCP predicted radiographic progression in the 5-year extension of the double-blind randomised CIMESTRA trial. Ann Rheum Dis 2010;69:1789-95. https://doi.org/10.1136/ard.2009.125534.
- Smolen JS, Aletaha D, Bijlsma JW, Breedveld FC, Boumpas D, Burmester G, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis 2010;69:631-7. https://doi.org/10.1136/ard.2009.123919.
- Rantalaiho V, Kautiainen H, Korpela M, Puolakka K, Blåfield H, Ilva K, et al. Physicians’ adherence to tight control treatment strategy and combination DMARD therapy are additively important for reaching remission and maintaining working ability in early rheumatoid arthritis: a subanalysis of the FIN-RACo trial. Ann Rheum Dis 2014;73:788-90. https://doi.org/10.1136/annrheumdis-2013-204271.
- Hissink Muller P, Brinkman DMC, Schonenberg-Meinema D, van den Bosch WB, Koopman-Keemink Y, Brederije ICJ, et al. Treat to target (drug-free) inactive disease in DMARD-naive juvenile idiopathic arthritis: 24-month clinical outcomes of a three-armed randomised trial. Ann Rheum Dis 2019;78:51-9. https://doi.org/10.1136/annrheumdis-2018-213902.
- Hyrich KL, Lal SD, Foster HE, Thornton J, Adib N, Baildam E, et al. Disease activity and disability in children with juvenile idiopathic arthritis one year following presentation to paediatric rheumatology. Results from the Childhood Arthritis Prospective Study. Rheumatology 2010;49:116-22. https://doi.org/10.1093/rheumatology/kep352.
- Choy EH, Kingsley GH, Khoshaba B, Pipitone N, Scott DL. Intramuscular Methylprednisolone Study Group. A two year randomised controlled trial of intramuscular depot steroids in patients with established rheumatoid arthritis who have shown an incomplete response to disease modifying antirheumatic drugs. Ann Rheum Dis 2005;64:1288-93. https://doi.org/10.1136/ard.2004.030908.
- Papadopoulou C, Kostik M, Gonzalez-Fernandez MI, Bohm M, Nieto-Gonzalez JC, Pistorio A, et al. Delineating the role of multiple intraarticular corticosteroid injections in the management of juvenile idiopathic arthritis in the biologic era. Arthritis Care Res 2013;65:1112-20. https://doi.org/10.1002/acr.21947.
- Tong A, Jones J, Craig JC, Singh-Grewal D. Children’s experiences of living with juvenile idiopathic arthritis: a thematic synthesis of qualitative studies. Arthritis Care Res 2012;64:1392-404. https://doi.org/10.1002/acr.21695.
- Kuemmerle-Deschner JB, Hansmann S, Wulffraat NM, Vastert SJ, Hens K, Anton J, et al. Recommendations for collaborative paediatric research including biobanking in Europe: a Single Hub and Access point for paediatric Rheumatology in Europe (SHARE) initiative. Ann Rheum Dis 2018;77:319-27.
- Kearsley-Fleet L, Davies R, Baildam E, Beresford MW, Foster HE, Southwood TR, et al. Factors associated with choice of biologic among children with Juvenile Idiopathic Arthritis: results from two UK paediatric biologic registers. Rheumatology 2016;55:1556-65. https://doi.org/10.1093/rheumatology/kev429.
- Oen K, Duffy CM, Tse SM, Ramsey S, Ellsworth J, Chédeville G, et al. Early outcomes and improvement of patients with juvenile idiopathic arthritis enrolled in a Canadian multicenter inception cohort. Arthritis Care Res 2010;62:527-36. https://doi.org/10.1002/acr.20044.
- Shen CC, Yao TC, Yeh KW, Huang JL. Association of disease activity and anti-rheumatic treatment in juvenile idiopathic arthritis with serum lipid profiles: a prospective study. Semin Arthritis Rheum 2013;42:590-6. https://doi.org/10.1016/j.semarthrit.2012.10.002.
- Gäre BA, Fasth A. The natural history of juvenile chronic arthritis: a population based cohort study. I. Onset and disease process. J Rheumatol 1995;22:295-307.
- Weiss JE, Ilowite NT. Juvenile idiopathic arthritis. Rheum Dis Clin North Am 2007;33:441-70.
- Cannizzaro E, Schroeder S, Müller LM, Kellenberger CJ, Saurenmann RK. Temporomandibular joint involvement in children with juvenile idiopathic arthritis. J Rheumatol 2011;38:510-15. https://doi.org/10.3899/jrheum.100325.
- Beukelman T, Patkar NM, Saag KG, Tolleson-Rinehart S, Cron RQ, DeWitt EM, et al. 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res 2011;63:465-82. https://doi.org/10.1002/acr.20460.
- Dueckers G, Guellac N, Arbogast M, Dannecker G, Foeldvari I, Frosch M, et al. Evidence and consensus based GKJR guidelines for the treatment of juvenile idiopathic arthritis. Clin Immunol 2012;142:176-93. https://doi.org/10.1016/j.clim.2011.10.003.
- Hashkes PJ, Laxer RM. Medical treatment of juvenile idiopathic arthritis. JAMA 2005;294:1671-84. https://doi.org/10.1001/jama.294.13.1671.
- Job-Deslandre C, Menkes CJ. Complications of intra-articular injections of triamcinolone hexacetonide in chronic arthritis in children. Clin Exp Rheumatol 1990;8:413-16.
- Kirwan JR, Bijlsma JW, Boers M, Shea BJ. Effects of glucocorticoids on radiological progression in rheumatoid arthritis. Cochrane Database Syst Rev 2007;1. https://doi.org/10.1002/14651858.CD006356.
- Picco P, Gattorno M, Buoncompagni A, Pistoia V, Borrone C. 6-methylprednisolone ‘mini-pulses’: a new modality of glucocorticoid treatment in systemic onset juvenile chronic arthritis. Scand J Rheumatol 1996;25:24-7. https://doi.org/10.3109/03009749609082663.
- Zulian F, Martini G, Gobber D, Plebani M, Zacchello F, Manners P. Triamcinolone acetonide and hexacetonide intra-articular treatment of symmetrical joints in juvenile idiopathic arthritis: a double-blind trial. Rheumatology 2004;43:1288-91. https://doi.org/10.1093/rheumatology/keh313.
- National Institute for Health and Care Excellence (NICE) . Adalimumab, Etanercept, Infliximab, Rituximab and Abatacept for the Treatment of Rheumatoid Arthritis After the Failure of a TNF Inhibitor 2010.
- National Institute for Health Research . High Flow Humidified Oxygen As an Early Intervention in Children With Acute Severe Asthma. A Feasibility Study (HiFlo ASA) n.d. www.fundingawards.nihr.ac.uk/award/PB-PG-1217-20024 (accessed 15 November 2019).
- Hollander JL, Brown EM, Jessar RA, Brown CY. Hydrocortisone and cortisone injected into arthritic joints; comparative effects of and use of hydrocortisone as a local antiarthritic agent. J Am Med Assoc 1951;147:1629-35. https://doi.org/10.1001/jama.1951.03670340019005.
- Bernstein BT, Thompson DM, Singsen BH, Miller JJ. Juvenile Rheumatoid Arthritis. Littleton, MA: PSG Publishing Co; 1979.
- Allen RC, Gross KR, Laxer RM, Malleson PN, Beauchamp RD, Petty RE. Intraarticular triamcinolone hexacetonide in the management of chronic arthritis in children. Arthritis Rheum 1986;29:997-1001. https://doi.org/10.1002/art.1780290808.
- Cleary AG, Murphy HD, Davidson JE. Intra-articular corticosteroid injections in juvenile idiopathic arthritis. Arch Dis Child 2003;88:192-6. https://doi.org/10.1136/adc.88.3.192.
- Cunha AL, Miotto E, Silva VB, Osaku FM, Niemxeski LB, Furtado RN, et al. Intra-articular injection in patients with juvenile idiopathic arthritis: factors associated with a good response. Rev Bras Reumatol Engl Ed 2016;56:490-6. https://doi.org/10.1016/j.rbr.2015.08.010.
- Gotte AC. Intra-articular corticosteroids in the treatment of juvenile idiopathic arthritis: safety, efficacy, and features affecting outcome. A comprehensive review of the literature. Open Access Rheumatol 2009;1:37-49. https://doi.org/10.2147/OARRR.S5103.
- Davoudi A, Khaki H, Mohammadi I, Daneshmand M, Tamizifar A, Bigdelou M, et al. Is arthrocentesis of temporomandibular joint with corticosteroids beneficial? A systematic review. Med Oral Patol Oral Cir Bucal 2018;23:e367-75. https://doi.org/10.4317/medoral.21925.
- Ravelli A, Davì S, Bracciolini G, Pistorio A, Consolaro A, van Dijkhuizen EHP, et al. Intra-articular corticosteroids versus intra-articular corticosteroids plus methotrexate in oligoarticular juvenile idiopathic arthritis: a multicentre, prospective, randomised, open-label trial. Lancet 2017;389:909-16. https://doi.org/10.1016/S0140-6736(17)30065-X.
- Bird HA, Ring EF, Bacon PA. A thermographic and clinical comparison of three intra-articular steroid preparations in rheumatoid arthritis. Ann Rheum Dis 1979;38:36-9. https://doi.org/10.1136/ard.38.1.36.
- Balogh Z, Ruzsonyi E. Triamcinolone hexacetonide versus betamethasone. A double-blind comparative study of the long-term effects of intra-articular steroids in patients with juvenile chronic arthritis. Scand J Rheumatol Suppl 1987;67:80-2. https://doi.org/10.3109/03009748809105305.
- Honkanen VE, Rautonen JK, Pelkonen PM. Intra-articular glucocorticoids in early juvenile chronic arthritis. Acta Paediatr 1993;82:1072-4. https://doi.org/10.1111/j.1651-2227.1993.tb12815.x.
- Zulian F, Martini G, Gobber D, Agosto C, Gigante C, Zacchello F. Comparison of intra-articular triamcinolone hexacetonide and triamcinolone acetonide in oligoarticular juvenile idiopathic arthritis. Rheumatology 2003;42:1254-9. https://doi.org/10.1093/rheumatology/keg358.
- Eberhard BA, Sison MC, Gottlieb BS, Ilowite NT. Comparison of the intraarticular effectiveness of triamcinolone hexacetonide and triamcinolone acetonide in treatment of juvenile rheumatoid arthritis. J Rheumatol 2004;31:2507-12.
- Bagri NK, Tripathy SK. Intra-articular corticosteroid administration. Indian Pediatr 2018;55. https://doi.org/10.1007/s13312-018-1310-8.
- Derendorf H, Möllmann H, Grüner A, Haack D, Gyselby G. Pharmacokinetics and pharmacodynamics of glucocorticoid suspensions after intra-articular administration. Clin Pharmacol Ther 1986;39:313-17. https://doi.org/10.1038/clpt.1986.45.
- Southwood TR. ABC of rheumatology. Arthritis in children. BMJ 1995;310:728-32. https://doi.org/10.1136/bmj.310.6981.728.
- Ravelli A, Lattanzi B, Consolaro A, Martini A. Glucocorticoids in paediatric rheumatology. Clin Exp Rheumatol 2011;29:148-52.
- Michels H. What is low-dose corticosteroid therapy in juvenile idiopathic arthritis? A worldwide, questionnaire-based survey. Z Rheumatol 2000;59:127-30. https://doi.org/10.1007/PL00022855.
- Guzman J, Oen K, Tucker LB, Huber AM, Shiff N, Boire G, et al. The outcomes of juvenile idiopathic arthritis in children managed with contemporary treatments: results from the ReACCh-Out cohort. Ann Rheum Dis 2015;74:1854-60. https://doi.org/10.1136/annrheumdis-2014-205372.
- Lord M, Allaoua M, Ratib O. Positron emission tomography findings in systemic juvenile idiopathic arthritis. Rheumatology 2011;50. https://doi.org/10.1093/rheumatology/ker136.
- Mellos A, Mauro A, Granato C, Gicchino MF, Perrone L, Olivieri AN. Refractory systemic juvenile idiopathic arthritis complicated by coxarthrosis. Pediatric Rheumatol 2014;12. https://doi.org/10.1186/1546-0096-12-S1-P232.
- Weiss JE, DeWitt EMM, Beukelman T, Schanbrg LE, Schneider R, Kimura Y. Choice of Systemic JIA Treatment Among Childhood Arthritis and Rheumatology Research Alliance (CARRA) Rheumatologists n.d.
- Wallace CA, Giannini EH, Spalding SJ, Hashkes PJ, O’Neil KM, Zeft AS, et al. Clinically inactive disease in a cohort of children with new-onset polyarticular juvenile idiopathic arthritis treated with early aggressive therapy: time to achievement, total duration, and predictors. J Rheumatol 2014;41:1163-70. https://doi.org/10.3899/jrheum.131503.
- Marvillet I, Terrada C, Quartier P, Quoc EB, Bodaghi B, Prieur AM. Ocular threat in juvenile idiopathic arthritis. Joint Bone Spine 2009;76:383-8. https://doi.org/10.1016/j.jbspin.2008.10.015.
- Nouar D, Hoarau C, Uettwiller F, Le Lez ML. Non-infectious pediatric uveitis in a single french tertiary center. Pediatric Rheumatol 2014;12. https://doi.org/10.1186/1546-0096-12-S1-P233.
- Verstappen SM, McCoy MJ, Roberts C, Dale NE, Hassell AB, Symmons DP. STIVEA investigators . Beneficial effects of a 3-week course of intramuscular glucocorticoid injections in patients with very early inflammatory polyarthritis: results of the STIVEA trial. Ann Rheum Dis 2010;69:503-9. https://doi.org/10.1136/ard.2009.119149.
- Shiff NJ, Brant R, Guzman J, Cabral DA, Huber AM, Miettunen P, et al. Glucocorticoid-related changes in body mass index among children and adolescents with rheumatic diseases. Arthritis Care Res 2013;65:113-21. https://doi.org/10.1002/acr.21785.
- Huppertz HI, Pfüller H. Transient suppression of endogenous cortisol production after intraarticular steroid therapy for chronic arthritis in children. J Rheumatol 1997;24:1833-7.
- Goldzweig O, Carrasco R, Hashkes PJ. Systemic adverse events following intraarticular corticosteroid injections for the treatment of juvenile idiopathic arthritis: two patients with dermatologic adverse events and review of the literature. Semin Arthritis Rheum 2013;43:71-6. https://doi.org/10.1016/j.semarthrit.2012.12.006.
- Valta H, Lahdenne P, Jalanko H, Aalto K, Mäkitie O. Bone health and growth in glucocorticoid-treated patients with juvenile idiopathic arthritis. J Rheumatol 2007;34:831-6.
- Esbjörnsson AC, Iversen MD, André M, Hagelberg S, Schwartz MH, Broström EW. Effect of intraarticular corticosteroid foot injections on walking function in children with juvenile idiopathic arthritis. Arthritis Care Res 2015;67:1693-701. https://doi.org/10.1002/acr.22624.
- Kearsley-Fleet L, Davies R, Lunt M, Southwood TR, Hyrich KL. Factors associated with improvement in disease activity following initiation of etanercept in children and young people with Juvenile Idiopathic Arthritis: results from the British Society for Paediatric and Adolescent Rheumatology Etanercept Cohort Study. Rheumatology 2016;55:840-7. https://doi.org/10.1093/rheumatology/kev434.
- Sherratt FC, Roper L, Stones SR, McErlane F, Peak M, Beresford MW, et al. Protective parents and permissive children: what qualitative interviews with parents and children can tell us about the feasibility of juvenile idiopathic arthritis trials. Pediatr Rheumatol Online J 2018;16. https://doi.org/10.1186/s12969-018-0293-2.
- Ruperto N, Giannini EH, Pistorio A, Brunner HI, Martini A, Lovell DJ. Is it time to move to active comparator trials in juvenile idiopathic arthritis?: a review of current study designs. Arthritis Rheum 2010;62:3131-9. https://doi.org/10.1002/art.27670.
- Al-Shahi Salman R, Beller E, Kagan J, Hemminki E, Phillips RS, Savulescu J, et al. Increasing value and reducing waste in biomedical research regulation and management. Lancet 2014;383:176-85. https://doi.org/10.1016/S0140-6736(13)62297-7.
- Fletcher B, Gheorghe A, Moore D, Wilson S, Damery S. Improving the recruitment activity of clinicians in randomised controlled trials: a systematic review. BMJ Open 2012;2. https://doi.org/10.1136/bmjopen-2011-000496.
- Sully BG, Julious SA, Nicholl J. A reinvestigation of recruitment to randomised, controlled, multicenter trials: a review of trials funded by two UK funding agencies. Trials 2013;14. https://doi.org/10.1186/1745-6215-14-166.
- Tishler CL, Reiss NS. Pediatric drug-trial recruitment: enticement without coercion. Pediatrics 2011;127:949-54. https://doi.org/10.1542/peds.2010-2585.
- Berard RA, Laxer RM. Learning the hard way: clinical trials in juvenile idiopathic arthritis. Ann Rheum Dis 2018;77:1-2. https://doi.org/10.1136/annrheumdis-2017-211108.
- Caldwell PH, Murphy SB, Butow PN, Craig JC. Clinical trials in children. Lancet 2004;364:803-11. https://doi.org/10.1016/S0140-6736(04)16942-0.
- Smyth RL, Weindling AM. Research in children: ethical and scientific aspects. Lancet 1999;354:21-4. https://doi.org/10.1016/S0140-6736(99)90253-2.
- Balevic SJ, Becker ML, Cohen-Wolkowiez M, Schanberg LE. Clinical trial design in juvenile idiopathic arthritis. Paediatr Drugs 2017;19:379-89. https://doi.org/10.1007/s40272-017-0244-2.
- Douglas SL. A consumer perspective on embedding research in paediatric rheumatology. Rheumatology 2014;53:1915-16. https://doi.org/10.1093/rheumatology/ket461.
- O’Cathain A, Hoddinott P, Lewin S, Thomas KJ, Young B, Adamson J, et al. Maximising the impact of qualitative research in feasibility studies for randomised controlled trials: guidance for researchers. Pilot Feasibility Stud 2015;1. https://doi.org/10.1186/s40814-015-0026-y.
- O’Cathain A, Thomas KJ, Drabble SJ, Rudolph A, Goode J, Hewison J. Maximising the value of combining qualitative research and randomised controlled trials in health research: the QUAlitative Research in Trials (QUART) study – a mixed methods study. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18380.
- Beadle-Brown J, Ryan S, Windle K, Holder J, Turnpenny A, Smith N, et al. Engagement of People with Long Term Conditions in Health and Social Care Research: Barriers and Facilitators to Capturing the Views of Seldom-Heard Populations. Canterbury: Quality and Outcomes of Person-Centred Care Policy Research Unit, University of Kent; 2012.
- Richie J, Lewis J. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: SAGE Publications Ltd; 2003.
- Lees A, Payler J, Ballinger C, Lawrence P, Faust SN, Meads G. Positioning children’s voice in clinical trials research: a new model for planning, collaboration, and reflection. Qual Health Res 2017;27:2162-76. https://doi.org/10.1177/1049732317726760.
- Barriball KL, While A. Collecting data using a semi-structured interview: a discussion paper. J Adv Nurs 1994;19:328-35. https://doi.org/10.1111/j.1365-2648.1994.tb01088.x.
- Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 2004;31:390-2.
- Petty RE, Southwood TR, Baum J, Bhettay E, Glass DN, Manners P, et al. Revision of the proposed classification criteria for juvenile idiopathic arthritis: Durban, 1997. J Rheumatol 1998;25:1991-4.
- Ness LR. Are we there yet? Data saturation in qualitative research. Qualitative Report 2015;20:1408-16.
- Marti P, Molinari L, Bolt IB, Seger R, Saurenmann RK. Factors influencing the efficacy of intra-articular steroid injections in patients with juvenile idiopathic arthritis. Eur J Pediatr 2008;167:425-30. https://doi.org/10.1007/s00431-007-0525-9.
- Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-117.
- Morrow SL. Quality and trustworthiness in qualitative research in counseling psychology. J Couns Psychol 2005;52. https://doi.org/10.1037/0022-0167.52.2.250.
- O’Brien BC, Harris IB, Beckman TJ, Reed DA, Cook DA. Standards for reporting qualitative research: a synthesis of recommendations. Acad Med 2014;89:1245-51. https://doi.org/10.1097/ACM.0000000000000388.
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007;19:349-57. https://doi.org/10.1093/intqhc/mzm042.
- Ministry of Housing, Communities and Local Government . English Indices of Deprivation 2015: Research Report 2015.
- Nuffield Council on Bioethics . Children and Clinical Research: Ethical Issues 2015.
- Shaw C, Brady LM, Davey C. Guidelines for Research with Children and Young People. London: National Children’s Bureau Research Centre; 2011.
- Boland L, McIsaac DI, Lawson ML. Barriers to and facilitators of implementing shared decision making and decision support in a paediatric hospital: a descriptive study. Paediatr Child Health 2016;21:e17-21. https://doi.org/10.1093/pch/21.3.e17.
- Zwaanswijk M, Tates K, van Dulmen S, Hoogerbrugge PM, Kamps WA, Beishuizen A, et al. Communicating with child patients in pediatric oncology consultations: a vignette study on child patients’, parents’, and survivors’ communication preferences. Psycho-Oncology 2011;20:269-77. https://doi.org/10.1002/pon.1721.
- Madden L, Shilling V, Woolfall K, Sowden E, Smyth RL, Williamson PR, et al. Questioning assent: how are children’s views included as families make decisions about clinical trials?. Child Care Health Dev 2016;42:900-8. https://doi.org/10.1111/cch.12347.
- Young B, Dixon-Woods M, Windridge KC, Heney D. Managing communication with young people who have a potentially life threatening chronic illness: qualitative study of patients and parents. BMJ 2003;326. https://doi.org/10.1136/bmj.326.7384.305.
- Williams C. Alert assistants in managing chronic illness: the case of mothers and teenage sons. Sociol Health Illn 2000;22:254-72. https://doi.org/10.1111/1467-9566.00202.
- Mills EJ, Seely D, Rachlis B, Griffith L, Wu P, Wilson K, et al. Barriers to participation in clinical trials of cancer: a meta-analysis and systematic review of patient-reported factors. Lancet Oncol 2006;7:141-8. https://doi.org/10.1016/S1470-2045(06)70576-9.
- Ross S, Grant A, Counsell C, Gillespie W, Russell I, Prescott R. Barriers to participation in randomised controlled trials: a systematic review. J Clin Epidemiol 1999;52:1143-56. https://doi.org/10.1016/S0895-4356(99)00141-9.
- King M, Nazareth I, Lampe F, Bower P, Chandler M, Morou M, et al. Impact of participant and physician intervention preferences on randomized trials: a systematic review. JAMA 2005;293:1089-99. https://doi.org/10.1001/jama.293.9.1089.
- Elliott D, Husbands S, Hamdy FC, Holmberg L, Donovan JL. Understanding and improving recruitment to randomised controlled trials: qualitative research approaches. Eur Urol 2017;72:789-98. https://doi.org/10.1016/j.eururo.2017.04.036.
- Shilling V, Williamson PR, Hickey H, Sowden E, Beresford MW, Smyth RL, et al. Communication about children’s clinical trials as observed and experienced: qualitative study of parents and practitioners. PLOS ONE 2011;6. https://doi.org/10.1371/journal.pone.0021604.
- Caldwell PH, Butow PN, Craig JC. Pediatricians’ attitudes toward randomized controlled trials involving children. J Pediatr 2002;141:798-803. https://doi.org/10.1067/mpd.2002.129173.
- Prescott RJ, Counsell CE, Gillespie WJ, Grant AM, Russell IT, Kiauka S, et al. Factors that limit the quality, number and progress of randomised controlled trials. Health Technol Assess 1999;3. https://doi.org/10.3310/hta3200.
- Williamson PR, Altman DG, Bagley H, Barnes KL, Blazeby JM, Brookes ST, et al. The COMET Handbook: version 1.0. Trials 2017;18. https://doi.org/10.1186/s13063-017-1978-4.
- Morgan EM, Riebschleger MP, Horonjeff J, Consolaro A, Munro JE, Thornhill S, et al. Evidence for updating the core domain set of outcome measures for juvenile idiopathic arthritis: report from a special interest group at OMERACT 2016. J Rheumatol 2017;44:1884-8. https://doi.org/10.3899/jrheum.161389.
- Alderson P. Competent children? Minors’ consent to health care treatment and research. Soc Sci Med 2007;65:2272-83. https://doi.org/10.1016/j.socscimed.2007.08.005.
- Morain SR, Whicher DM, Kass NE, Faden RR. Deliberative engagement methods for patient-centered outcomes research. Patient 2017;10:545-52. https://doi.org/10.1007/s40271-017-0238-8.
- Akobeng AK. Understanding randomised controlled trials. Arch Dis Child 2005;90:840-4. https://doi.org/10.1136/adc.2004.058222.
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) . Guideline: Statistical Principles for Clinical Trials 1998.
- Williamson PR, Altman DG, Blazeby JM, Clarke M, Devane D, Gargon E, et al. Developing core outcome sets for clinical trials: issues to consider. Trials 2012;13. https://doi.org/10.1186/1745-6215-13-132.
- Fergusson D, Monfaredi Z, Pussegoda K, Garritty C, Lyddiatt A, Shea B, et al. The prevalence of patient engagement in published trials: a systematic review. Res Involv Engagem 2018;4. https://doi.org/10.1186/s40900-018-0099-x.
- Jones J, Hunter D. Consensus methods for medical and health services research. BMJ 1995;311:376-80. https://doi.org/10.1136/bmj.311.7001.376.
- Allen J, Dyas J, Jones M. Building consensus in health care: a guide to using the nominal group technique. Br J Community Nurs 2004;9:110-14. https://doi.org/10.12968/bjcn.2004.9.3.12432.
- Delp P, Thesen A, Motiwalla J, Seshardi N. Systems Tools for Project Planning. Bloomington, IN: International Development Institute, University of Indiana; 1977.
- Products: ResponseCard RF Accessibility n.d. www.turningtechnologies.co.uk/response-options (accessed 15 November 2019).
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2 . Framing the question and deciding on important outcomes. J Clin Epidemiol 2011;64:395-400. https://doi.org/10.1016/j.jclinepi.2010.09.012.
- Cartwright J, Kabir T, Simons L. Budgeting for Involvement. London: INVOLVE; 2013.
- McErlane F, Foster HE, Armitt G, Bailey K, Cobb J, Davidson JE, et al. Development of a national audit tool for juvenile idiopathic arthritis: a BSPAR project funded by the Health Care Quality Improvement Partnership. Rheumatology 2018;57:140-51. https://doi.org/10.1093/rheumatology/kex322.
- Wallace CA, Ruperto N, Giannini E. Childhood Arthritis and Rheumatology Research Alliance. Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J Rheumatol 2004;31:2290-4.
- Sherratt F, Roper L, Baildam E, Peak M, McErlane F, Stones S, et al. Family Perspectives on the Feasibility of a Corticosteroid Induction Regimen Randomised Controlled Trial in Juvenile Idiopathic Arthritis: Results of a Qualitative Study n.d.
- Gorst SL, Gargon E, Clarke M, Blazeby JM, Altman DG, Williamson PR. Choosing important health outcomes for comparative effectiveness research: an updated review and user survey. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0146444.
- Gal T, Duramy B. International Perspectives and Empirical Findings on Child Participation: From Social Exclusion to Child-Inclusive Policies. Oxford: Oxford University Press; 2015.
- Roberts H, Christensen P, James A. Research with Children. London: Routledge; 2017.
- Liabo K, Boddy K, Burchmore H, Cockcroft E, Britten N. Clarifying the roles of patients in research. BMJ 2018;361. https://doi.org/10.1136/bmj.k1463.
- Freemantle N, Calvert MJ. Interpreting composite outcomes in trials. BMJ 2010;341. https://doi.org/10.1136/bmj.c3529.
- Consolaro A, Giancane G, Schiappapietra B, Davì S, Calandra S, Lanni S, et al. Clinical outcome measures in juvenile idiopathic arthritis. Pediatr Rheumatol Online J 2016;14. https://doi.org/10.1186/s12969-016-0085-5.
- Nordal EB, Zak M, Aalto K, Berntson L, Fasth A, Herlin T, et al. Validity and predictive ability of the juvenile arthritis disease activity score based on CRP versus ESR in a Nordic population-based setting. Ann Rheum Dis 2012;71:1122-7. https://doi.org/10.1136/annrheumdis-2011-200237.
- McErlane F, Beresford MW, Baildam EM, Chieng SE, Davidson JE, Foster HE, et al. Validity of a three-variable Juvenile Arthritis Disease Activity Score in children with new-onset juvenile idiopathic arthritis. Ann Rheum Dis 2013;72:1983-8. https://doi.org/10.1136/annrheumdis-2012-202031.
- Yardley L, Spring BJ, Riper H, Morrison LG, Crane DH, Curtis K, et al. Understanding and promoting effective engagement with digital behavior change interventions. Am J Prev Med 2016;51:833-42. https://doi.org/10.1016/j.amepre.2016.06.015.
- Sommer C, Zuccolin D, Arnera V, Schmitz N, Adolfsson P, Colombo N, et al. Building clinical trials around patients: evaluation and comparison of decentralized and conventional site models in patients with low back pain. Contemp Clin Trials Commun 2018;11:120-6. https://doi.org/10.1016/j.conctc.2018.06.008.
- Bergold J, Thomas S. Participatory research methods: a methodological approach in motion. Historical Social Research Historische Sozialforschung 2012;13:191-222.
- Curran JA, Bishop A, Chorney J, MacEachern L, Mackay R. Partnering with parents to advance child health research. Healthc Manage Forum 2018;31:45-50. https://doi.org/10.1177/0840470417744568.
- Sheridan R, Martin-Kerry J, Watt I, Higgins S, Stones SR, Taylor DH, et al. User testing digital, multimedia information to inform children, adolescents and their parents about healthcare trials. J Child Health Care 2019;23:468-82. https://doi.org/10.1177/1367493518807325.
- Cantrill JA, Sibbald B, Buetow S. The Delphi and nominal group techniques in health services research. Int J Pharm Pract 1996;4:67-74. https://doi.org/10.1111/j.2042-7174.1996.tb00844.x.
- Wainwright D, Boichat C, McCracken LM. Using the nominal group technique to engage people with chronic pain in health service development. Int J Health Plann Manage 2014;29:52-69. https://doi.org/10.1002/hpm.2163.
- McErlane F, Armitt G, Cobb J, Bailey K, Cleary G, Douglas S, et al. CAPTURE-JIA: a consensus-derived core dataset to improve clinical care for children and young people with juvenile idiopathic arthritis. Rheumatology 2020;59:137-45. https://doi.org/10.1093/rheumatology/kez214.
- Jaki T, Magirr D. Considerations on covariates and endpoints in multi-arm multi-stage clinical trials selecting all promising treatments. Stat Med 2013;32:1150-63. https://doi.org/10.1002/sim.5669.
- Magirr D, Jaki T, Whitehead J. A generalized Dunnett test for multi-arm multi-stage clinical studies with treatment selection. Biometrika 2012;99:494-501. https://doi.org/10.1093/biomet/ass002.
- O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics 1979;35:549-56. https://doi.org/10.2307/2530245.
Appendix 1 The SIRJIA mixed-methods design
FIGURE 4.
The SIRJIA mixed-methods design.
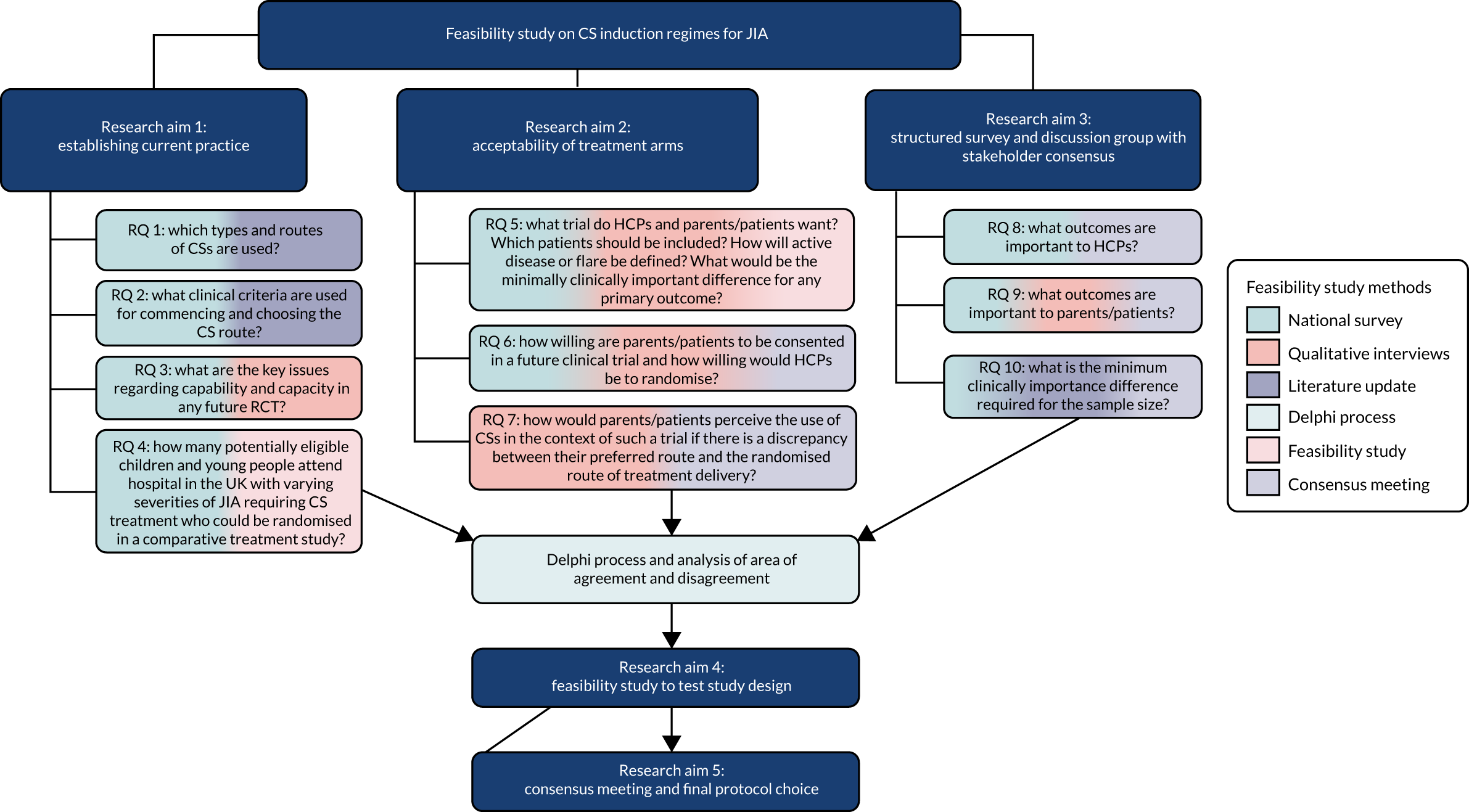
Appendix 2 Study Oversight Committees
Study Steering Committee
Independent members
Professor Danielle van der Windt (chairperson), Research, Institute for Primary Care and Health Sciences, Keele University, UK.
Ms Catriona Graham, Statistician, Wellcome Trust Clinical Research Facility, Western General Hospital, Crewe Road South, Edinburgh, UK.
Miss Carrie Thompson, Public Member.
Professor Nicholas Webb, Translational Medicine Discovery Director, Renal and Transplantation at Novartis Institutes for BioMedical Research, Switzerland (Professor Webb resigned from the committee on 3 July 2018 owing to a relocation).
Dr Indi Banarjee, Consultant Paediatric Endocrinologist, Manchester Royal Children’s Hospital, Manchester, UK.
Study management group
Dr Eileen Baildam, Chief Investigator, Consultant Paediatric Rheumatologist, Alder Hey Children’s Foundation NHS Trust, Liverpool, UK.
Professor Michael Beresford, Brough Chairperson, Professor of Child Health, University of Liverpool, NIHR Clinical Research Network: Specialty Cluster Lead for Children, Reproductive & Childbirth, Musculoskeletal, Ophthalmology and Haematology, Honorary Consultant Paediatric Rheumatologist, Director NIHR Alder Hey Clinical Research Facility, Director UK Experimental Arthritis Treatment Centre for Children.
Miss Dannii Clayton, Trial Statistician, LCTC, University of Liverpool, Liverpool, UK.
Ms Lucy Cooper, Sponsor Contracts, Alder Hey Children’s Foundation NHS Trust, Liverpool, UK.
Dr Sam Deepak, Locum Consultant Paediatric Rheumatologist, Queen’s Hospital, Nottingham, UK.
Dr Helen Foster, Professor Paediatric Rheumatology, Newcastle University Medicine Malaysia (NUMed), Malaysia.
Dr Ashley Jones, Acting Head of Statistics, LCTC, University of Liverpool, Liverpool, UK.
Dr Flora McErlane, Consultant Paediatric Rheumatologist, Great North Children’s Hospital, Newcastle Hospitals NHS Foundation Trust and Institute of Cellular Medicine, Newcastle University, Newcastle, UK.
Mrs Tracy Moitt, Senior Trial Manager, LCTC, University of Liverpool, Liverpool, UK.
Ms Gloria Nkhoma, Trial Co-ordinator, LCTC, University of Liverpool, Liverpool, UK.
Professor Matthew Peak, Director of Research Alder Hey Children’s NHS Trust, Clinical Lead NIHR CRN: Children North West Coast, Lead Liverpool Paediatric Medicine Research Unit.
Professor Athimalaipet Ramanan, Consultant Paediatric Rheumatologist, Bristol Royal Hospital for Children & Royal National Hospital for Rheumatic Disease, UK.
Dr Madeline Rooney Consultant Paediatric Rheumatologist, Belfast Children’s Hospital, Belfast, Northern Ireland.
Dr Fran Sherratt, Research Associate, Institute of Population Health Sciences, University of Liverpool, Liverpool, UK.
Dr Louise Roper, Research Associate, Institute of Population Health Sciences, University of Liverpool, Liverpool, UK.
Mr Simon Stones, Patient Advocate and Consultant, Collaboro Consulting, Bolton, UK, and Postgraduate Researcher, School of Healthcare, University of Leeds, Leeds, UK.
Professor Bridget Young, Professor of Psychology, Institute of Population Health Sciences, University of Liverpool, Liverpool, UK.
Appendix 3 Study management team
All study management was conducted through the LCTC at the University of Liverpool.
Liverpool Clinical Trials Centre director
Professor Paula Williamson.
Senior statistician
Dr Ashley Jones.
Supervising study manager
Mrs Tracy Moitt.
Senior data manager
Mrs Clare Jackson.
Study manager
Mrs Gloria Nkhoma.
Statisticians, data manager and information systems and administration
Dr Duncan Appelbe: information systems.
Miss Dannii Clayton: study statistician.
Mr Kieran Crabtree: trial co-ordinator assistant.
Mrs Michelle Girvan: data manager.
Mrs Janet Harrison: clinical data management systems team leader.
Mrs Linda Kane: quality control information systems.
Mr Keith Kennedy: information systems bespoke developer.
Dr Laura Sutton: statistician.
Mr Meirion Thomas: information systems.
Mrs Dianne Wheatley: database developer.
Appendix 4 Eight possible protocols for voting on prior to final consensus meeting
Appendix 5 Detailed discussion notes from final consensus meeting
Appendix 6 Output from the study
Journal articles
Sherratt FC, Roper L, Stones SR, McErlane F, Peak M, Beresford MW, et al. Protective parents and permissive children: what qualitative interviews with parents and children can tell us about the feasibility of juvenile idiopathic arthritis trials. Pediatr Rheumatol Online J 2018;16:76.
Conference proceedings
Baildam EM, Jones A, Clayton D, Nkhoma G, Peak M, Ramanan A, et al. A national survey of clinical practice of corticosteroid use in newly diagnosed or flaring cases of juvenile idiopathic arthritis (JIA) across the UK. Annals of the Rheumatic Diseases 2020; in press.
Stones SR, Bagley H, Jones A, McErlane F, Moitt T, Nkhoma G, et al. Identifying the primary outcome measure and protocol components for a prospective feasibility study of corticosteroid regimens for children and young people with juvenile idiopathic arthritis using consensus methods with young people, families and professionals. Annals of the Rheumatic Diseases 2020; in press.
Stones SR, Bagley H, Sherratt FC, Roper L, Baildam EM. Co-designing a comparative randomised controlled clinical trial of corticosteroid regimens with children, young people and parents living with juvenile idiopathic arthritis. Annals of the Rheumatic Diseases 2020; in press.
Roper L, Sherratt F, Baildam EM, McErlane F, Beresford MW, Foster H, Young B. Children’s and parents’ perspectives on outcomes for JIA trials comparing different corticosteroid delivery routes: a qualitative study. Pediatric Rheumatology 2018;16(Suppl. 2):P250.
Deepak S, Baildam E, McErlane F. Literature review on the use of corticosteroids in Juvenile idiopathic arthritis (JIA). Rheumatology 2017;56(Suppl. 7):kex390.051.
Deepak S, Baildam E, McErlane F. The use of corticosteroids in children with juvenile idiopathic arthritis: a literature review as part of the SIRJIA study. Rheumatology 2017;56(Suppl. 2):kex062.299.
Deepak S, Baildam E, McErlane F and the HTA 14/167/01 SIRJIA Trial Management Group. A literature review of outcome measures used in juvenile idiopathic arthritis trials- as part of the steroid induction of remission in JIA (SIRJIA) study. Pediatric Rheumatology 2017;15(Suppl. 2):P230.
Sherratt FC, Roper L, Baildam EM, Beresford MW, Stones SR, Ramanan A, et al. Families’ views on the feasibility of a corticosteroid trial in JIA: a qualitative study. Pediatric Rheumatology 2017;15(Suppl. 2):P248.
Sherratt F, Roper L, Baildam E, Peak M, McErlane F, Stones SR, Young B. Family perspectives on the feasibility of a corticosteroid induction regimen randomised controlled trial in juvenile idiopathic arthritis: results of a qualitative study. Trials 2017;18(Suppl. 1):P384.
Presentations
Roper, L. Children’s and Parents’ Perspectives on Outcomes for JIA Trials Comparing Different Corticosteroid Delivery Routes: A Qualitative Study. Proceedings of the 25th Paediatric Rheumatology European Society Congress, 5–8 September 2018, Lisbon, Portugal.
Baildam, E. Literature Review of Outcome Measures Used in JIA Trials – As Part of the SIRJIA Study. Proceedings of the 24th Paediatric Rheumatology European Society Congress, 14–17 September 2017, Athens, Greece, and Proceedings of the PReS Young Investigators Meeting, 13–14 September 2017, Athens, Greece.
Baildam, E. The Use of Corticosteroids in Children with JIA: A Literature Review as part of the SIRJIA Study. Proceedings of the British Society for Rheumatology Conference, 25–27 April 2017, Birmingham, UK and Proceedings of the BSPAR Conference, 28–30 November 2016, Manchester, UK.
Sherratt, F. Family Perspectives on the Feasibility of a Corticosteroid Induction Regimen Trial in Juvenile Idiopathic Arthritis. Proceedings of the 4th International Clinical Trials Methodology Conference (ICTMC), 7–10 May 2017, Liverpool, UK.
Sherratt, F. Families’ Views on the Feasibility of a Corticosteroid Trial in JIA: A Qualitative Study. Proceedings of the 24th Paediatric Rheumatology European Society Congress, 14–17 September 2017, Athens, Greece.
Sherratt, F. Families’ Perspectives on the Feasibility of a Corticosteroid Induction Regimen Randomised Controlled Trial in Juvenile Idiopathic Arthritis: Results from Qualitative Interviews. Poster presented at the North West Rheumatology Club Meeting, 2017, Manchester, UK.
List of abbreviations
- ACR Pedi
- American College of Rheumatology Paediatric
- ADHD
- attention deficit hyperactivity disorder
- ADR
- adverse drug reaction
- BANNAR
- Barbara Ansell National Network for Adolescent Rheumatology
- BSPAR
- British Society of Paediatric and Adolescent Rheumatology
- CAPS
- Childhood Arthritis Prospective Study
- CARRA
- Childhood Arthritis and Rheumatology Research Alliance
- CHAQ
- Childhood Health Assessment Questionnaire
- CI
- confidence interval
- CID
- clinically inactive disease
- cJADAS
- clinical Juvenile Arthritis Disease Activity Score
- cJADAS-71
- clinical Juvenile Arthritis Disease Activity Score, 71-joint count
- COS
- core outcome set
- CRP
- C-reactive protein
- CS
- corticosteroid
- CSG
- Clinical Studies Group
- CTP
- consensus treatment plan
- CYP
- children and young people
- DMARD
- disease modifying anti-rheumatic drug
- ESR
- erythrocyte sedimentation rate
- GA
- general anaesthetic
- GCP
- good clinical practice
- HCP
- health-care professional
- HRA
- Health Research Authority
- HTA
- Health Technology Assessment
- IA
- intra-articular
- IACI
- intra-articular corticosteroid injection
- IL
- interleukin
- IM
- intramuscular
- IMD
- Indices of Multiple Deprivation
- IV
- intravenous
- JADAS
- Juvenile Arthritis Disease Activity Score
- JADAS-71
- Juvenile Arthritis Disease Activity Score, 71-joint count
- JADAS-CRP
- Juvenile Arthritis Disease Activity Score based on C-reactive protein
- JIA
- juvenile idiopathic arthritis
- LCTC
- Liverpool Clinical Trials Centre
- MTX
- methotrexate
- NGT
- nominal group technique
- NIHR
- National Institute for Health Research
- NIHR CRN
- National Institute for Health Research Clinical Research Network
- NSAID
- non-steroidal anti-inflammatory drug
- OR
- odds ratio
- Pa/PtGA
- Parent/Patient Global Assessment of Disease Activity
- PD
- pharmacodynamic
- PGA
- Physician’s Global Assessment of Disease Activity
- PICO
- problem/population, intervention/indicator, comparison, outcome
- PIL
- patient information leaflet
- PK
- pharmacokinetic
- PPI
- patient and public involvement
- R&D
- research and development
- RA
- rheumatoid arthritis
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RF
- rheumatoid factor
- RQ
- research question
- SD
- standard deviation
- SHARE
- Single Hub and Access point for paediatric Rheumatology in Europe
- SIRJIA
- Study of the Induction of Remission in Juvenile Idiopathic Arthritis
- SMG
- study management group
- SMS
- short message service
- SNAC
- Scottish Network for Arthritis in Children
- SOJIA
- systemic onset juvenile idiopathic arthritis
- SSC
- study steering committee
- TA
- triamcinolone acetonide
- TH
- triamcinolone hexacetonide
- VAS
- visual analogue score
- YOURR
- Young people's Opinions Underpinning Rheumatology Research
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/hta24360).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
