Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number NIHR127519. The protocol was agreed in November 2018. The assessment report began editorial review in May 2019 and was accepted for publication in November 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Martine Harris has received point-of-care creatinine devices and consumables for use in research studies from Nova Biomedical (Runcorn, UK), Abbott Laboratories (Chicago, IL, USA) and Radiometer Ltd (Crawley, UK). She has co-authored academic papers in this area from 2016 to present and contributed (from August 2017 to January 2018) as an expert commentator for the National Institute for Health and Care Excellence’s Medtech innovation briefing number 136 (MIB136) entitled ‘Point-of-care creatinine tests before contrast-enhanced imaging’. James Altunkaya is funded via a National Institute for Health Research Research Methods Fellowship. Sofia Dias has received Medical Research Council funding.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Corbett et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Description of the health problem
The use of computed tomography (CT) imaging has transformed the way the body can be visualised to detect disease and inform treatment decisions across a range of diseases. This is illustrated by the increase in the number of CT scans performed in hospitals in England, from just over 1 million in 1996–7 to almost 5 million in 2012–13. 1 In clinical situations in which the use of contrast is deemed beneficial before CT imaging is performed, an iodine-based (iodinated) contrast agent is normally given to patients to enhance image quality and diagnostic performance. Different types of agent are available, with the dose varying depending on the type of scan or procedure required. However, intravenously administered contrast agents are thought to occasionally cause kidney damage or acute kidney injury (AKI), particularly in patients with existing kidney disease. Historically, high-osmolar contrast agents were used for radiological examinations, but these agents were considered to pose a significant risk of contrast-induced AKI and other adverse events. The term contrast-induced AKI (CI-AKI) or contrast-induced nephropathy (CIN) describes an AKI occurring within a few days of receiving a contrast agent that cannot be attributed to other causes. However, the development of safer contrast media (low-osmolar agents and iso-osmolar agents) and their widespread adoption in clinical practice means that it is now difficult to ascribe contrast as the cause of an AKI. Much of the research literature on the risks of CI-AKI is limited, being based on single-group cohorts, but the inclusion of adequate control populations in more recent studies has generated results that question the risk of AKI from contrast agents. This had led to the current debate about whether or not low-osmolar and iso-osmolar contrast agents pose any meaningful risk of AKI. 2–5 In the light of this uncertainty, the term post-contrast AKI (PC-AKI) is now increasingly used to describe such events. Definitions of AKI vary, but often include absolute increases in baseline levels of serum creatinine (SCr) ≥ 0.5 mg/dl or relative increases of 25–50%. 6
Although many possible clinical risk factors for PC-AKI have been suggested and studied, most risk factors relate to chronic kidney disease (CKI) or AKI more broadly, rather than specifically to PC-AKI. Renal dysfunction appears to be the most important risk factor for PC-AKI. A creatinine blood test is used to identify patients at risk; elevated creatinine levels indicate likely kidney dysfunction. In clinical practice, creatinine blood test results are often used to calculate eGFRs (estimated glomerular filtration rates). eGFRs are considered a better measure of kidney function than creatinine alone; eGFR is calculated using details on age, sex, race and creatinine level. Several different methods exist to calculate eGFR in adults, with the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation7 and the MDRD (Modification of Diet in Renal Disease) equation8 frequently used in the NHS. eGFR results are used to evaluate patient risk of PC-AKI before a contrast agent is administered so that any risk from contrast agents can be minimised or removed. Patients with abnormal eGFR results may need prophylactic intravenous hydration (IVH) to reduce the risk of AKI or alternative imaging strategies may be used that do not require the use of a contrast agent.
The risk of PC-AKI can be quickly assessed in most hospital patients awaiting a CT scan or procedure. All inpatients should have a recent eGFR or creatinine measurement available as part of other hospital tests, as should many outpatients. However, some outpatients do not have a recent result available when their CT appointment is due. Although a blood sample could be taken and sent to the hospital laboratory, results typically become available only more than 1 hour after the blood is taken. Moreover, some radiology services offer extended-day and 7-day services, which may not be in line with laboratory provision. Kidney function will therefore be unknown in these patients at the time of their appointment, so their risk of PC-AKI will be more difficult to evaluate. Consequently, rather than patients being subject to an uncertain risk of PC-AKI, their CT appointment may be rescheduled or performed without a contrast agent. The former can result in patient stress and a lost appointment slot for the radiology department, whereas the latter will result in less accurate CT images. Sometimes contrast may be administered in patients thought to be at a low risk of AKI based on other clinical information. Point-of-care (POC) measurement devices allow rapid blood sampling and measurement of eGFRs, enabling PC-AKI risk to be assessed and, if the risk is low, the CT appointment to go ahead as planned.
Current service provision and care pathway
A 2015 review of the quality of available clinical practice guidance documents on different aspects of PC-AKI, and of their recommendations, found variation in how PC-AKI was defined, how patients at risk should be identified, and found limited consensus on the use of interventions for preventing PC-AKI. 9 In light of the significant number of recent and ongoing studies in these areas of research, it is important that any clinical guidance is kept up to date.
Guidelines published in 2018 on the use of contrast media include the European Society of Urogenital Radiology (ESUR) guidelines on PC-AKI,10 The Royal Australian and New Zealand College of Radiologists (RANZCR) Iodinated Contrast Media Guideline11 and the ACR (American College of Radiology) Manual on Contrast Media. 6 The ESUR guidelines recommend measurement of eGFR before administration of an intravascular iodinated contrast agent in either all patients or patients who have a history of renal disease (i.e. patients with an eGFR of < 60 ml/minute/1.73 m2), kidney surgery, proteinuria, hypertension, hyperuricaemia or diabetes mellitus. Two guidelines recommend using the CKD-EPI equation to calculate eGFR. 10,11
Broadly, there is a consensus across all three guidelines about how to identify patients who may be at risk of PC-AKI, with agreement that there is very little evidence that iodinated contrast material is an independent risk factor for AKI in patients with an eGFR ≥ 30 ml/minute/1.73 m2. An eGFR threshold of < 30 ml/minute/1.73 m2 is therefore often used to identify patients at risk of PC-AKI. Nevertheless, the RANZCR guideline notes that intravascular iodinated contrast agents should be given to any patient regardless of renal function status if the perceived diagnostic benefit to the patient, in the opinion of the radiologist and the referrer, justifies this administration. 11 Similarly, the ACR guideline advises that any threshold put into practice must be weighed on an individual patient level with the benefits of administering contrast material. 6
In patients identified as being at a higher risk of developing PC-AKI, pre- and post-procedural 0.9% intravenous saline is recommended in the RANZCR guidelines as the first-line preventative strategy to mitigate the risk. 11 The ESUR guidelines recommend that in high-risk patients (with an eGFR < 30 ml/minute/1.73 m2 or known/suspected acute renal failure) clinicians should:
-
consider an alternative imaging method not using iodine-based contrast media
-
use intravenous saline (3–4 hours before and 4–6 hours after contrast) or sodium bicarbonate (1 hour before contrast agent administration)
-
individualise preventative hydration in patients with severe congestive heart failure or patients with end-stage renal failure (i.e. patient with an eGFR < 15 ml/minute/1.73 m2).
The ESUR guidelines also recommend measurement of eGFRs 48 hours after contrast agent administration, patient monitoring for at least 30 days and eGFR measurement at regular intervals if, at 48 hours, PC-AKI is diagnosed.
In terms of clinical practice adopted across NHS radiology departments, two surveys conducted in 2015 identified inconsistent or poor compliance with guidance, with the wide variation in practice being thought to reflect inconsistencies in published guidance. 12,13 One of the surveys reported that most (of the responding) NHS CT departments required renal function to be assessed via a blood test for all patients, although in some departments only patients at high risk of PC-AKI were assessed. 12 It is thought that risk-stratifying questionnaires may be a more efficient way to identify patients at high risk of PC-AKI,14 with blood test results needed only for high-risk patients, although conclusive evidence on this approach is still needed. One of the NHS surveys asked about the eGFR or creatinine threshold levels at which contrast agents were contraindicated. Although the most frequently used threshold was an eGFR of < 30 ml/minute/1.73 m2 (used in 45% of NHS trusts), overall there was notable variation, with 19 different thresholds identified, each leading to different prophylactic treatment strategies. 12
Variation across the NHS also exists in the way creatinine is measured in laboratories. 15 The Jaffe (alkaline picrate) method is a colorimetric assay that can be affected by interfering substances (such as ketones and bilirubin) and so is prone to overestimate creatinine. Alternatively, enzymatic laboratory methods can be used, which are more accurate (because they are less prone to interference), but are also more expensive. In order to reduce error and maximise the comparability of creatinine measurements between laboratories, methods should be calibrated against isotope dilution mass spectrometry (IDMS). Similarly, there is variation in the way eGFR is calculated across the NHS. 15 Although the CKD-EPI equation is recommended in recent guidelines, the MDRD equation is also commonly used, even though it is more prone to underestimate eGFR in some patients. 16
Regardless of which particular group of patients has their renal function assessed, previous blood test results are not always available prior to CT appointments, which can result in cancellations and re-bookings. The use of POC devices presents a possible solution to this problem by providing eGFR measurements in time frames short enough to avoid cancellation of CT appointments. POC testing could be done on all patients with missing results or just on those patients identified as being at high risk of PC-AKI using a questionnaire. Alternatively, some radiology departments avoid this problem by adopting a ‘no blood test result – no booking’ policy, whereas others mitigate it by making efforts to chase up missing blood results. 12
Description of the technologies under assessment
Several POC devices are being assessed, based on their ability to output results as eGFRs: StatSensor® (Nova Biomedical, Runcorn, UK), i-STAT Alinity (Abbott Point of Care, Inc., Princeton, NJ, USA), ABL90 FLEX PLUS and ABL800 FLEX (Radiometer Ltd, Crawley, UK), epoc® (Siemens Healthineers AG, Erlangen, Germany) and Piccolo Xpress® (Abaxis, Inc., Union City, CA, USA) and DRI-CHEM NX 500 (Fujifilm Corporation, Tokyo, Japan).
Point-of-care creatinine devices are either handheld, portable or tabletop and require only very small blood samples (usually obtained via finger prick). Some devices use test cartridges and others test strips. Levels of creatinine are measured using enzymatic methods either as one of several analytes or as a single measurement. Although POC devices provide results quickly, their results may not be as accurate as those derived from laboratory reference test analyses.
Currently, only around 10% of NHS CT departments use POC devices to get a blood test result for patients attending without a recent result. 12 For POC devices to be adopted more widely in outpatient settings, assurances will be needed about their accuracy in providing reliable estimates of eGFR at the POC, when compared with estimates derived from laboratory reference test analyses. Another area of concern lies in whether or not POC devices can store and transmit results to hospital databases to ensure patient records are as up to date and complete as possible.
Chapter 2 Aims and objectives
Overall aims and objectives of assessment
The purpose of this assessment was to assess the clinical effectiveness and cost-effectiveness of POC creatinine tests to assess kidney function, for people who need contrast-enhanced CT imaging in a non-emergency situation and who do not have a recent SCr measurement. To achieve this, the following objectives were proposed.
Clinical effectiveness
-
To perform a systematic review of studies that compare the results of POC creatinine tests with laboratory-based tests to assess kidney function in a non-emergency setting.
-
To perform a systematic review of the clinical impacts and implementation of POC creatinine tests to assess kidney function before CT imaging. This will include assessment of the associated mortality and morbidity, patient-centred outcomes, adverse events, acceptability to clinicians and patients, and compliance.
Cost-effectiveness
-
To perform a systematic review of published cost-effectiveness studies of the use of POC creatinine tests in a secondary care setting to assess kidney function before contrast-enhanced imaging.
-
To develop a decision model to estimate the cost-effectiveness of the use of POC creatinine tests to assess kidney function before contrast-enhanced imaging. The relevant population is people who need contrast-enhanced imaging in a non-emergency situation and who do not have a recent SCr measurement.
-
The objective of the decision model will link the diagnostic accuracy of POC creatinine tests to short-term costs and consequences (e.g. the impact on cancelled or delayed appointments, use and volume of contrast media and associated risks, such as PC-AKI). Short-term risks of PC-AKI will be linked to potential longer-term costs and consequences (e.g. CKD, end-stage renal disease and death) using the best-available evidence. Depending on the robustness of the evidence, additional exploratory analyses using assumptions and expert opinion may be also undertaken.
-
The feasibility of extending the decision model to include other clinical outcomes that could be affected by any changes in the imaging decision based on the POC tests will also be assessed. These outcomes could include (i) any anxiety associated with having a delayed or cancelled CT scan and (ii) morbidity and mortality implications of performing unenhanced scans, or using lower doses of contrast agent. However, given that these outcomes will differ depending on the specific population and the underlying reason for imaging, it is envisaged that any extension of this nature will need to be constrained to a specific population/reason for the scan. The practicalities and value of developing a specific ‘exemplar’ application (with potentially limited generalisability) will be considered versus using a simpler and more generic approach (e.g. using threshold analysis to determine the magnitude of any impact necessary to result in a different decision based on conventional cost-effectiveness decision rules).
-
The cost-effectiveness of the alternative POC tests will be expressed in terms of incremental cost per quality-adjusted life-year (QALY) and/or net health (or monetary) benefits.
Chapter 3 Assessment of clinical effectiveness
Literature searches
Comprehensive searches of the literature were conducted to identify studies relating to POC devices for measuring creatinine levels in the blood.
The search strategy was developed in MEDLINE (via Ovid) by an information specialist with input from the review team. The strategy comprised a set of terms for POC tests combined with terms for either creatinine or eGFR. Text word searches in the title and abstracts of records and relevant subject headings were included in the strategy. No date or language limits were applied and the searches were not restricted by study design. The MEDLINE strategy was adapted for use in all other resources searched.
The searches were carried out in November 2018. The following databases were searched: MEDLINE (including Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE), Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Database of Systematic Reviews (CDSR), Cumulative Index to Nursing and Allied Health Literature (CINAHL) Plus, Database of Abstracts of Reviews of Effects (DARE), EMBASE, Health Management Information Consortium (HMIC), Health Technology Assessment (HTA) Database, PubMed and the Science Citation Index.
In addition, the following resources were searched for ongoing, unpublished or grey literature: ClinicalTrials.gov, Conference Proceedings Citation Index: Science, EU Clinical Trials Register, Open Access Theses and Dissertations, ProQuest Dissertations & Theses Global™ (ProQuest, Ann Arbor, MI, USA), PROSPERO, the World Health Organization (WHO)’s International Clinical Trials Registry Platform portal and manufacturers’ websites. References submitted by the manufacturers to the National Institute for Health and Care Excellence (NICE) were also checked. The websites of manufacturers of POC creatinine devices were checked and the reference lists of relevant reviews and included studies were scanned.
Search results were imported into EndNote x8 [Clarivate Analytics (formerly Thomson Reuters), Philadelphia, PA, USA] and deduplicated. Full search strategies can be found in Appendix 1.
Separate searches were also made to identify evidence to inform estimation of the risk of an AKI following a contrast-enhanced CT scan (see Pragmatic reviews of further evidence to inform the economic model).
Selection criteria
Two reviewers independently screened all titles and abstracts. Full papers of any titles and abstracts deemed potentially eligible were obtained where possible, and the relevance of each study assessed independently by two reviewers according to the criteria below. Any disagreements were resolved by consensus. Conference abstracts were included provided that they reported sufficient data to assess eligibility.
The following eligibility criteria were used to identify relevant studies.
Participants
To maximise the number of data on test accuracy, the eligible population for test accuracy studies was any adult patient group receiving POC creatinine testing compared with laboratory testing in a non-emergency/intensive care setting.
For studies reporting clinical or implementation outcomes, only studies of adult patients receiving POC tests before CT imaging in a non-emergency, outpatient setting were included.
Interventions
For test accuracy studies, details of the POC devices eligible for the review are presented in Table 1. This list is broader than those reported in the NICE scope and in the study protocol, which were restricted to devices that reported eGFRs. This was done to maximise the available evidence base because early on during the screening process it became evident that many studies were of devices that did not calculate eGFR (i.e. creatinine was measured), with eGFR being calculated manually by the study investigators. These studies were included where it was thought (following clinical and technical advice) that the model in question was sufficiently similar to the most recent version of the device (all the most recent models have the facility to present eGFR results). New versions of a device may sometimes incorporate software improvements (to allow eGFR outputs), a different interface or improved functionality, rather than changes in the way creatinine is analysed. For example, the recently released i-STAT Alinity was ‘built on the proven technology of the i-STAT System’,17 and hence studies were included that used an ‘i-STAT’ device.
| Manufacturer and devices | Device format | Parameters measured | Sample volume | Analysis time | eGFR equation used |
|---|---|---|---|---|---|
| Nova Biomedical StatSensor | Handheld | Creatinine only | 1.2 µl | 30 seconds | MDRD, CKD-EPI, Cockcroft–Gault, Schwartz and Counahan–Barratt |
|
Related models: StatSensor-i, StatSensor Xpress-i All models allow offset adjustment of results to correct for measurement bias; StatSensor and StatSensor-i also allow slope adjustment |
|||||
|
Abbott Point of Care i-STAT Alinity |
Handheld | Multiple parameters | 65 µl | 2 minutes | MDRD and CKD-EPI |
| Related models: i-STAT1, many studies simply state ‘i-STAT’ | |||||
|
Radiometer Ltd ABL90 FLEX PLUS |
Portable | 19 parameters | 65 µl | 35 seconds | CKD-EPI, MDRD and Schwartz |
| ABL800 FLEX | Tabletop | 18 parameters | 125–250 µl | 1 minute | CKD-EPI and MDRD |
|
Related models: ABL827 and ABL837 All models allow offset and slope adjustment of results to correct for measurement bias |
|||||
| Siemens Healthineers AG epoc | Handheld | 11 parameters on one test card | 92 µl | < 1 minute | CKD-EPI, MDRD and Schwartz |
|
Abaxis, Inc. Piccolo Xpress |
Tabletop | Multiple parameters | 100 µl | < 14 minutes | MDRD |
|
Fujifilm Corporation DRI-CHEM NX 500 |
Tabletop | Multiple parameters | 10 µl | 5 minutes | Expected |
All the eligible devices measure whole-blood creatinine using an enzymatic method. The devices are either handheld, tabletop or portable and need very small volumes of blood. Creatinine levels may be analysed either as one component of a panel of parameters or as a single measurement via a test card or specific cartridge.
Reference standard
-
Non-urgent (results available after 1 hour) laboratory-based SCr measurement:
-
Jaffe method
-
enzymatic method.
-
-
Urgent (results available within an hour) laboratory-based SCr measurement:
-
Jaffe method
-
enzymatic method.
-
-
No testing, clinical judgement alone.
Outcomes
The eligible intermediate outcome measures were:
-
diagnostic accuracy of POC creatinine devices compared with laboratory-based creatinine devices
-
correlation between POC creatinine devices and laboratory-based creatinine devices
-
test failure rates
-
number of delayed or cancelled and rescheduled scans
-
volume of intravenous contrast material used
-
number of unenhanced scans
-
number of hospital admissions
-
hospital length of stay.
All relevant outcome definitions and cut-off points were extracted.
In addition, the following clinical outcomes were eligible:
-
AKI (either PC-AKI or CI-AKI)
-
fall in baseline eGFR or rise of baseline creatinine
-
temporary renal replacement therapy
-
new-onset CKD (stage 3 or worse)
-
end-stage renal disease with the need for permanent renal replacement therapy
-
health-related quality of life (HRQoL)
-
mortality.
Eligible outcomes related to the implementation of the interventions of interest and related practical issues included:
-
acceptability of POC devices (to clinicians and patients)
-
patient satisfaction
-
training requirements
-
uptake and compliance.
Study designs
Diagnostic accuracy and correlation studies
Studies in which the POC test and laboratory reference test were performed independently on the same patients were eligible.
Clinical effectiveness/implementation
Any experimental or observational study that compared POC tests with laboratory testing and that reported relevant clinical outcomes as listed in Outcomes were eligible. Studies with a single-group design were also eligible. Relevant publications reporting issues that were related to the implementation of, or practical advice relating to, POC creatinine test technologies (experimental or observational studies or reviews) were also included.
Case reports and studies focusing only on technical aspects of POC creatinine test technologies (such as technical descriptions of the testing process or specifications of machinery) were excluded.
Data extraction
Data on study characteristics and results were extracted by one reviewer using a standardised data extraction form and independently checked by a second reviewer (MC and AL). Discrepancies were resolved by discussion, with involvement of a third reviewer (SD) where necessary. Data from relevant studies with multiple publications were extracted and reported as a single study, quoting the most recent or most complete publication. Given the large number of included studies, the checking of reference lists of included studies, to identify further studies, was not systematically undertaken. Where appropriate, study authors and manufacturers were contacted to seek more detailed or missing diagnostic or clinical data. If data on mean measurement bias were reported without 95% limits of agreement [or confidence intervals (CIs)] these were estimated if a standard deviation and sample size was reported using the Bland and Altman formula. 18
The type of diagnostic accuracy data and synthesis required for this assessment are different from the typical diagnostic accuracy study in which a device might be tested for its ability to detect a dichotomous (yes/no) risk of PC-AKI. As the definition of PC-AKI risk has changed over time, sensitivity and specificity data at a given threshold are not relevant as both the laboratory reference test and POC device thresholds for defining risk have changed. Therefore, reported sensitivity and specificity will refer to different diagnoses of risk. In addition, this assessment aimed to describe the accuracy of the POC devices in correctly classifying individuals according to their PC-AKI risk categories determined by different levels of eGFR as given in Table 2. These thresholds were chosen because they reflect both the thresholds used in guidelines – which have varied over time – and the thresholds used in defining CKD. 19,20
| Category | eGFR (ml/minute/1.73 m2) |
|---|---|
| 1 | 0–29 |
| 2 | 30–44 |
| 3 | 45–59 |
| 4 | ≥ 60 |
Therefore, the probability that individuals are correctly classified into the four risk categories in Table 2 was estimated and the probabilities that they are incorrectly classified into one of the other categories were estimated.
Therefore, data were primarily extracted on the number of individuals in each of the cells in a four-by-four table, defined by the categories in Table 2. A data extraction template is presented in Appendix 2, Table 38. Where data were reported as a combination of these categories (e.g. number of individuals with an eGFR of < 60 ml/minute/1.73 m2), these were also extracted.
Critical appraisal
The quality of the diagnostic accuracy studies was assessed using the QUADAS-2 (quality assessment of diagnostic accuracy studies 2) tool, modified to incorporate review-specific issues. QUADAS-2 evaluates both risk of bias and concerns about study applicability to the review question. The Cochrane risk-of-bias tool was used to evaluate randomised controlled trials (RCTs) identified in the pragmatic reviews. The quality of other studies included in the review was not assessed formally, as these studies did not directly inform the quantitative synthesis or parameters informing the economic analyses. Quality assessments were performed by one reviewer (AL) and independently checked by a second reviewer (MC). Disagreements were resolved through consensus and, where necessary, by consulting a third reviewer (SD).
Methods of data synthesis
Synthesis of diagnostic accuracy data
For each device, estimates of the probabilities that individuals are classified by the POC device as having an eGFR in one of the four categories in Table 2 given their true eGFR is in one of those categories were required. These probabilities relate to the sensitivity and specificity of each device, which were used to populate the economic model in Diagnostic accuracy of point-of-care creatinine tests. Individuals are categorised as being at risk of PC-AKI if their eGFR is < 30 ml/minute/1.73 m2 (i.e. category 1 in Table 2). Therefore, the probability that each POC device correctly classifies individuals in this category will reflect their sensitivity to detecting individuals at risk. To calculate the specificity of each POC device it is necessary to know the underlying distribution of patients across the different eGFR categories (see Diagnostic accuracy of point-of-care creatinine tests for details).
Separate syntheses were carried out for POC devices for which two or more studies reported data on individuals classified into the different categories by laboratory reference test and POC device. Devices with sufficient data were StatSensor (including StatSensor, StatSensor-i and StatSensor Xpress-i), i-STAT (including i-STAT and i-STAT1) and ABL (including ABL827 and ABL800 FLEX); hence, three separate analyses were carried out, pooling the data on three devices (i.e. StatSensor, i-STAT and ABL), assuming that the different specifications of each device does not differ in their diagnostic characteristics.
For each study i reporting data on all cells of Table 38 in Appendix 2 the number of individuals classified by a POC device as belonging to eGFR category k = 1, . . . ., 4, given true eGFR category (as determined by the laboratory reference test) j = 1, . . . ., 4, rijk were assumed to follow a multinomial distribution, which is a generalisation of the binomial distribution to more than two categories:
with nij defining the number of individuals with true eGFR in category j in study i, and pjk defining the probabilities of being classified by a POC device in eGFR category k, when the true category is j (j, k = 1, . . . ., 4), which were assumed common to all studies.
The model was estimated in a Bayesian framework using Markov chain Monte Carlo in OpenBUGS (version 3.2.3; OpenBUGS Foundation, Imperial College London, London, UK),21,22 in which the probabilities were given a non-informative Dirichlet prior distribution:
The Dirichlet distribution is an extension of the beta distribution to multiple dimensions and ensures that the estimated probabilities always add to one. 21,23 Setting all the parameters equal to one, as in Equation 2, assigns equal density a priori to any vector of probabilities that sums to one.
Studies reporting only on collapsed categories were assumed to provide information on a function of the probabilities pjk. This function varied depending on which categories were collapsed, with relationships determined using partitioning properties of conditional probabilities. Estimation of the probability that an individual in an included study (as opposed to the underlying population of interest for this assessment – see Diagnostic accuracy of point-of-care creatinine tests) has true eGFR in category j, T[j] was also required. For details see Appendix 3, Model for the probability that an individual has a true estimated glomerular filtration rate in each category.
As the posterior distributions of the probabilities are bounded at zero and one, they are expected to be highly skewed. Therefore, results are reported as posterior medians with 95% credible intervals (CrIs) and plotted as density strips. In Diagnostic accuracy of point-of-care creatinine tests, the mean probability estimates, calculated from 1000 simulated values from the posterior distribution obtained by thinning the 30,000 posterior values generated in each analysis of the evidence synthesis, were used to derive specificity and sensitivity. Density strips are horizontal rectangles that can represent an entire probability distribution in one dimension: the rectangle is darkest at the point of highest probability density, then shaded with darkness proportional to the density, gradually fading to white at points of zero density. 24 The width of the rectangle itself has no meaning, and is used only to distinguish between distributions arising from different analyses. Standard lines representing point and interval estimates tend to give the impression that the data equally support all points in the interval, whereas density strips give a better description of the uncertainty in a probability distribution, particularly for non-symmetric distributions.
Each model was run until convergence was satisfactory and then the results were based on a further sample of iterations from two separate chains. Convergence was assessed by inspecting history and Brooks–Gelman–Rubin plots. 25,26
Data from different studies were pooled under the assumption that they estimate common probabilities, given a true eGFR category (i.e. using a fixed-effects model). Extension to a model allowing for between-study heterogeneity in probabilities was considered, but as a result of the small number of studies reporting data on all categories and the small number of individuals in some categories (including several zeros), this was not deemed feasible. The OpenBUGS code and data used are given in Appendix 4.
Clinical effectiveness results
Quantity and quality of research available
Figure 1 presents the study selection process in a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram. The literature searches identified a total of 3350 unique records. After title and abstract screening, 171 references were retrieved and 54 unique studies were included in the review. Of these, 12 studies reported diagnostic accuracy data (expressed as, or allowing calculation of, sensitivity and specificity) for eGFRs,27–38 seven reported diagnostic accuracy data for only SCr,39–45 and 50 studies presented data on correlation and/or measurement bias between a POC device and a laboratory reference test. 14,27,28,30–76 Six studies reported data on workflow or clinical outcomes. 29,59,62,77–79
FIGURE 1.
Study identification process: PRISMA flow diagram.
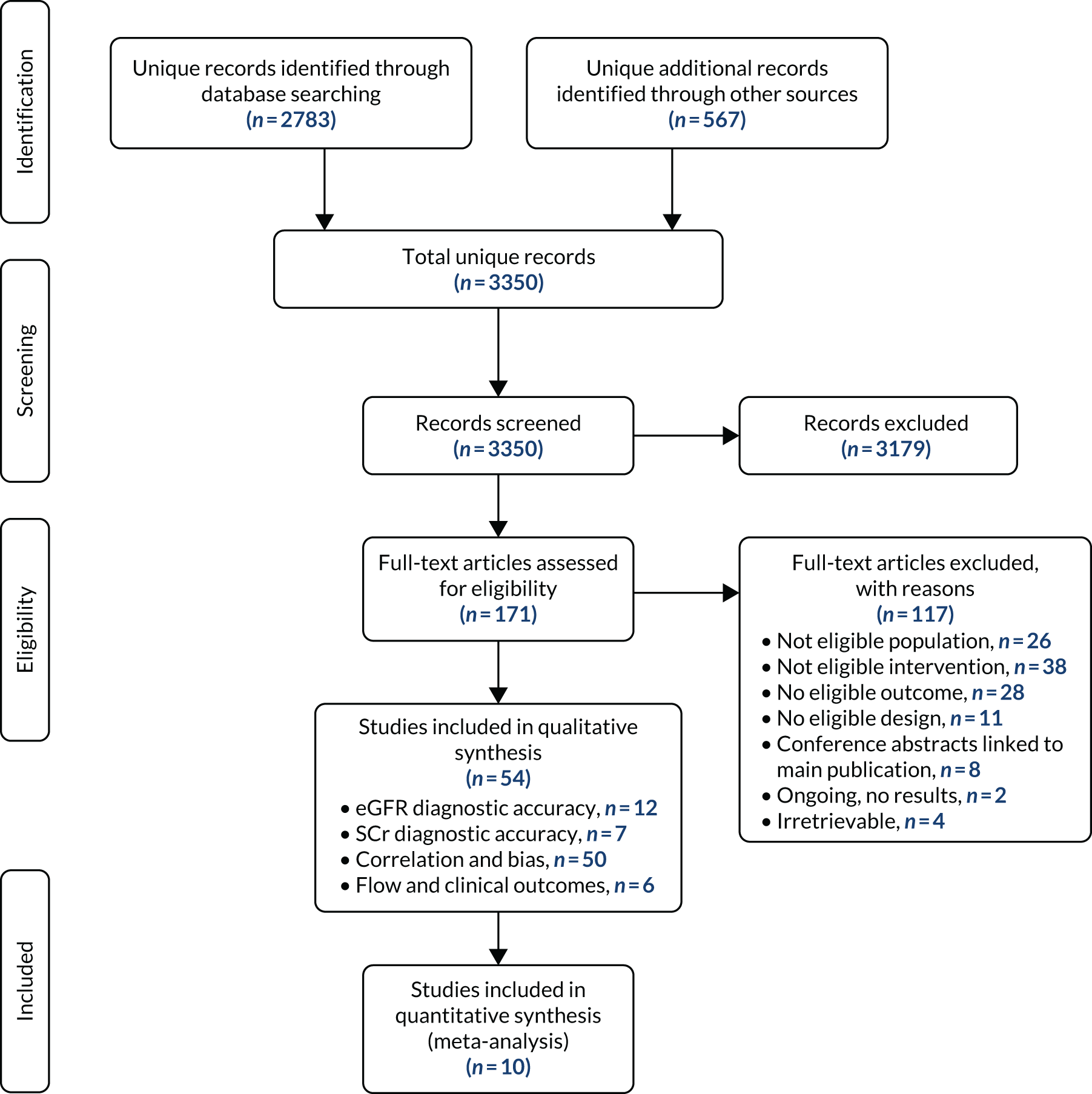
All studies that reported data on diagnostic accuracy of either eGFR or SCr also reported correlation/measurement bias results, except one. 29 Three of the studies that reported data on workflow or clinical outcomes also reported data on diagnostic accuracy or correlation/bias. 29,59,62
Risk-of-bias assessment
Table 3 summarises the results of the QUADAS-2 assessment, split by POC device. Full results, including all signalling questions, are reported in Appendix 5.
| Study (author and year of publication) | Assessment | ||||||
|---|---|---|---|---|---|---|---|
| Risk of bias | Concerns about applicability | ||||||
| Patient selection | POC and laboratory reference tests | Flow and timing | Population | Thresholds | Test | ||
| POC | Laboratory reference | ||||||
| Radiometer studies | |||||||
| Botz et al., 201327 | ? | + | + | ? | + | + | + |
| Korpi-Steiner et al., 200931 | + | + | + | + | – | – | + |
| Snaith et al., 201837 | + | + | + | + | + | + | + |
| i-STAT studies | |||||||
| aBotz et al., 201327 | ? | + | + | ? | + | + | + |
| Korpi-Steiner et al., 200931 | + | – | + | + | – | – | + |
| Nichols et al., 200733 | + | + | + | + | – | + | + |
| aObrador et al., 201234 | ? | – | + | – | – | – | + |
| aShephard et al., 200835 | ? | – | ? | ? | – | – | + |
| Snaith et al., 201837 | + | + | + | + | + | + | + |
| Snaith et al., 201938 | + | + | + | + | + | + | + |
| StatSensor studies | |||||||
| Dorward et al., 201828 | + | + | + | – | – | + | + |
| Houben et al., 201729 | + | ? | + | – | + | + | + |
| Inoue et al., 201730 | + | + | + | + | – | – | + |
| Korpi-Steiner et al., 200931 | + | – | + | + | – | – | + |
| Krige, 201732 | + | – | + | – | + | + | + |
| Shephard et al., 201036 | ? | – | + | – | – | + | + |
| Snaith et al., 201837 | + | + | + | + | + | + | + |
Six studies were rated as being at low risk across all risk-of-bias domains, including two studies of ABL800,31,37 three studies of i-STAT33,37,38 and three studies of StatSensor. 28,30,37 Among the six studies27,29,34–36 with at least one domain rated as being at unclear or high risk of bias, three used correction factors after comparing initial POC results with laboratory reference test results from the same samples, including two studies of i-STAT34,35 and one StatSensor study. 36 Correction factors can be entered into StatSensor devices to correct for measurement bias (see Table 1). However, in these studies the correction was applied to align POC test results with the reference standard results using the same samples. Therefore, adjusted analyses reported in these studies may overestimate the accuracy of the POC devices. None of the ABL studies reported using its offset correction functionalities. Four studies27,34–36 (including three conference abstracts27,34,35) reported insufficient information to assess bias related to patient selection. Other risk-of-bias issues included the use of different MDRD equations between the index test and the reference standard,31 and the use of a Jaffe method for the laboratory reference test (vs. an enzymatic method for the POC test). 32
Only two studies had low applicability concerns across all domains, including one study of ABL800, i-STAT and StatSensor,37 and one study of i-STAT. 38 The most common applicability concern was the use of eGFR threshold. Three studies of i-STAT,31,33,35 three of StatSensor28,31,36 and one ABL800 study31 used an eGFR cut-off point of 60 ml/minute/1.73 m2 or above (see Background). Several studies included disease-specific populations, including two StatSensor studies28,36 and two i-STAT studies;29,34 therefore, their applicability to a broader population of outpatients referred to CT without a recent eGFR may be limited. One study used a non-standard CKD staging34 and one study30 used a country-specific Japanese equation to calculate eGFR, which limits their applicability to the review question.
Overall, two studies were rated as being at low risk of bias and had low applicability concerns across all domains assessed, including one that evaluated ABL800, i-STAT and StatSensor,37 and one of i-STAT only. 38
Some studies are presented in several lines as they compare multiple devices (e.g. the 2018 publication by Snaith et al. 37).
Studies reporting bias or correlation outcomes
Fifty studies reported bias or correlation outcomes. 14,27,28,30–76 Eighteen studies were available only as conference abstracts (Table 4). Where reported, sample sizes ranged from 10 to 3087 patients. Four studies were set in the UK37,38,43,53 and 11 studies were reported as being conducted in a radiology or CT setting14,27,30,31,38,40,41,46,59,62,74
| Study (author and year of publication) | Population (N) and country | POC device(s) | Laboratory reference | Results (for creatinine unless stated) and notes | |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aumatell et al., 201046 |
24 undergoing CT scans Australia |
StatSensor | VITROS® (version 5; Ortho Clinical Diagnostics, Raritan, NJ, USA) |
r2 values for three different StatSensor devices were 0.9886, 0.9866 and 0.9935 (mean 0.990) The B–A plot indicated underestimation of creatinine using StatSensor (a small negative bias), but no further bias results were reported |
|||||||||||||||||||||||||||||||||||||||||||||
| Azzouz et al., 201414 |
1467 outpatients with renal dysfunction before MRI or CT Denmark |
StatSensor | NR | This study evaluated a structured questionnaire and reported an r2 = 0.9 when comparing laboratory reference with StatSensor | |||||||||||||||||||||||||||||||||||||||||||||
| Bahar et al., 201647 |
244 oncology outpatients split into three cohorts corresponding to three different periods USA |
i-STAT | Jaffe (Beckman Coulter DxC 800, Beckman Coulter, Inc., Pasadena, CA, USA) |
|
|||||||||||||||||||||||||||||||||||||||||||||
| Baier et al., 200348 |
15 organ donors USA |
i-STAT | NR | r2 = 0.95 | |||||||||||||||||||||||||||||||||||||||||||||
| aBender et al., 201249 |
54 patients prescribed carboplatin chemotherapy and zoledronic acid; and 56% of patients were female USA |
i-STAT | Enzymatic (VITROS 5600, Ortho Clinical Diagnostics) |
The study was designed to determine if whole blood and SCr measurements were interchangeable when calculating dosages for carboplatin and zoledronic acid For the CG eGFR results i-STAT had an average negative bias of –19.25 mg/dl, whereas the MDRD eGFR and CKD-EPI eGFR results had positive biases of 115.2 mg/dl and 28.0 mg/dl, respectively |
|||||||||||||||||||||||||||||||||||||||||||||
| aBetman et al. 201550 |
Not reported USA |
i-STAT and epoc | Olympus platform (no other details) |
Patient serum samples with known creatinine levels were pooled to create three standards: normal, high and very high range creatinine. Serial dilutions of hydroxycarbamide were added to aliquots of each standard i-STAT: a typical dose of hydroxycarbamide could result in a creatinine level with a positive bias of 6.15 mg/dl. i-STAT SCr measurements showed a dose–response relationship, with the concentration of hydroxycarbamide, but epoc did not |
|||||||||||||||||||||||||||||||||||||||||||||
| aBobilewicz, 200851 |
70 potential organ donors, post-extensive surgery Poland |
ABL 800 | Enzymatic (Cobas INTEGRA® 800, Roche Holding AG, Basel, Switzerland) | r2 = 0.997 | |||||||||||||||||||||||||||||||||||||||||||||
| aBotz et al., 201327 |
2042 patients at risk of renal disease prior to radiological examinations; and 43% of patients were female USA |
ABL827 and i-STAT1 (sample type NR) | Enzymatic, (Cobas C-501, Roche Holding AG) |
Mean bias for i-STAT was + 0.03 mg/dl (SD 0.13 mg/dl, 95% LoA estimated by EAG as –0.22 to 0.28) Mean bias for ABL827 was –0.06 mg/dl (SD 0.13 mg/dl, 95% LoA estimated by the EAG as –0.31 to 0.19 mg/dl) |
|||||||||||||||||||||||||||||||||||||||||||||
| Cao et al., 201752 |
10 patients USA |
epoc | VITROS 5600 (Ortho Clinical Diagnostics) |
r2 = 0.9313 Mean bias: −0.025 mg/dl (−3.4%) |
|||||||||||||||||||||||||||||||||||||||||||||
| aCory et al., 201853 |
15 pregnant women and non-pregnant control patients UK |
StatSensor Xpress | Enzymatic (type NR) |
r2 = 0.95 r2 = 0.96 (pregnant population subgroup, n = 11) The median difference with the reference test was 12 µmol/l |
|||||||||||||||||||||||||||||||||||||||||||||
| Dimeski et al., 201354 |
40 laboratory staff and renal outpatients Australia |
i-STAT | Jaffe (Beckman Coulter DxC 800) | Results presented by method of blood sampling:
|
|||||||||||||||||||||||||||||||||||||||||||||
| bDohnal et al., 200855 |
NRc Czech Republic |
Piccolo Xpress | VITROS 950 and Konelab 60 (Thermo Fisher Scientific, Waltham, MA, USA) | Statistically significant bias (8%; p < 0.05) | |||||||||||||||||||||||||||||||||||||||||||||
| dDorward et al., 201828 |
187 HIV-positive patients from a POC RCT; median age 31 years; 62% female; and mean creatinine concentration of 69.0 µmol/l South Africa |
StatSensor Xpress-I (capillary) | Enzymatic (Dimension® EXL™ 200 IDMS, Siemens AG, Munich, Germany) | Mean POC bias was 10.4 µmol/l (95% LoA –17.6 to 38.3 µmol/l); r2 = 0.58 | |||||||||||||||||||||||||||||||||||||||||||||
| Gault et al., 200156 |
149 randomly selected samples, with a mean creatinine concentration of 220 µmol/l Canada |
i-STAT | Jaffe (Beckman Coulter Synchron CX7, Beckman Coulter, Inc., Pasadena, CA, USA) | r2 = 0.99; mean bias 10.9%; mean difference 20.1 µmol/l (SD 30.3 µmol/l); 95% LoA estimated by the EAG as –39.3 to 79.5 µmol/l | |||||||||||||||||||||||||||||||||||||||||||||
| aGeorgievskaya et al., 201157 |
33 oncology patients Country NR |
i-STAT | Enzymatic (Dimension Vista® System, Siemens AG, Munich, Germany) | r2 = 0.926; mean bias –0.02 mg/dl | |||||||||||||||||||||||||||||||||||||||||||||
| Griffin et al., 201839 | Two studies of field workers:
|
StatSensor Xpress | Jaffe | Creatinine overestimated before adjustment:
|
|||||||||||||||||||||||||||||||||||||||||||||
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |||||||||||||||||||||||||||||||||||||||||||||
| Haneder et al., 201241 |
401 referred for CT scan at two centres; mean age was 62 years (SD 14 years); and 63% male Germany |
StatSensor (two devices: A and B) | Jaffe (Dimension RXL, Siemens AG; Olympus AU2700, Beckman Coulter, Inc.) |
Centre 1:Centre 2:Creatinine was underestimated by StatSensor before adjustment Centre 1 (n = 201): |
|||||||||||||||||||||||||||||||||||||||||||||
| Inoue et al., 201730 |
123 (with unadjusted results), scheduled for CT; mean eGFR 75.3 ml/minute/1.73 m2 (SD 21.4 ml/minute/1.73 m2); mean creatinine 0.8 mg/dl (SD 0.29 mg/dl) Japan |
StatSensor-i (capillary) | Enzymatic (BioMajesty™ BM2250, Jeol Ltd, Tokyo, Japan) |
r2 for eGFR = 0.80; r2 for creatinine = 0.88. Mean bias not reported B–A plots indicated a positive bias (overestimation) with StatSensor for creatinine and a negative bias for eGFR |
|||||||||||||||||||||||||||||||||||||||||||||
| aJanetto et al., 200658 |
85 heparinised samples USA |
ABL800 FLEX | Jaffe (Olympus AU5431, Beckman Coulter, Inc.) | r2 = 0.996; mean bias –0.22 mg/dl | |||||||||||||||||||||||||||||||||||||||||||||
| Korpi-Steiner et al., 200931 |
266 excess samples taken before CT procedures; mean age 68 years; and 39% female USA |
ABL800 FLEX, i-STAT StatSensor (with slope and intercept offset option) Heparinised venous samples |
Enzymatic, (Cobas INTEGRA 400, Roche Holding AG) | Mean bias:
|
|||||||||||||||||||||||||||||||||||||||||||||
| Kosack et al., 201542 |
60 patients and laboratory workers The Netherlands |
StatSensor Xpress | VITROS 5,1FS (Ortho Clinical Diagnostics) | r2 = 0.97
|
|||||||||||||||||||||||||||||||||||||||||||||
| fKrige, 201732 |
103 mixed-ancestry South Africans; mean age 52 years; and 69% female South Africa |
StatSensor (capillary) | Jaffe (AU5800 Clinical Chemistry Analyzer, Beckman Coulter, Inc.) | Mean bias not reported, but the B–A plot of creatinine showed a negative bias | |||||||||||||||||||||||||||||||||||||||||||||
| Lee-Lewandrowski et al., 201259 |
3087 referred for contrast-enhanced scan (CT or MRI) without a recent eGFR USA |
i-STAT | Jaffe (Cobas C501, Roche Holding AG) |
r2 = 0.99 for creatinine B–A plot: i-STAT values were slightly lower for SCr values > 2 mg/dl, whereas a t-test showed no difference for values < 2 mg/dl |
|||||||||||||||||||||||||||||||||||||||||||||
| f,gLehtonen, 201360 |
n = 63 samples Finland |
i-STAT | Modular EVO | Mean bias: 8.8% (NS) | |||||||||||||||||||||||||||||||||||||||||||||
| aMahlow et al., 201661 |
540 samples; oncology outpatients presenting for chemotherapy infusion USA |
i-STAT | Enzymatic (COBAS 8000, Roche Holding AG) |
Small but consistent positive bias: i-STAT SCr values were on average higher than the laboratory analyser by 0.11 mg/dl (SD 0.04 mg/dl, 95% LoA estimated by EAG as 0.03 to 0.19 mg/dl) r2 = 0.926 eGFR was underestimated by 4–12% depending on gender and absolute creatinine value |
|||||||||||||||||||||||||||||||||||||||||||||
| aMcGough et al., 201843 |
33 dialysis patients UK |
StatSensor | Jaffe (Cobas 8000, Roche Holding AG) | Mean bias was –0.15 mg/dl (–3.4%) | |||||||||||||||||||||||||||||||||||||||||||||
| Minnings et al., 201544 |
100 patients from a health centre or hospital setting; 70% female; and median SCr concentration of 0.72 mg/dl Nicaragua |
StatSensor Xpress | Jaffe (Roche Cobas INTEGRA 400, Roche Holding AG) | Median bias was 0.32 mg/dl | |||||||||||||||||||||||||||||||||||||||||||||
| Morita et al., 201162 |
113 patients scheduled for CT or MRI without a recent eGFR measurement Japan |
StatSensor | Enzymatic (7700 Clinical Analyzer, Hitachi High-Technologies America, Inc., Tokyo, Japan) |
For creatinine: mean bias = −0.10 mg/dl (95% LoA −0.43 to 0.22 mg/dl); r2 = 0.74. For eGFR: mean bias = 11 ml/minute/1.73 m2 (95% LOA –22.4 to 44.4 ml/minute/1.73 m2); r2 = 0.74 |
|||||||||||||||||||||||||||||||||||||||||||||
| Murata et al., 201863,80 |
60 residual samples USA |
Piccolo Xpress | VITROS 5600 (Ortho Clinical Diagnostics) |
r2 = 0.93 B–A plot indicted a negative bias |
|||||||||||||||||||||||||||||||||||||||||||||
| dNaugler et al., 201464 |
Discarded samples Canada |
i-STAT | Enzymatic (Cobas 6000, Roche Holding AG) |
eGFR: mean bias of −2.18 ml/minute/1.73 m2 B–A plot indicated better agreement for lower eGFR values than for higher values (i.e. > 60 ml/minute/1.73 m2) |
|||||||||||||||||||||||||||||||||||||||||||||
| Nichols et al., 200733 |
50 chemotherapy patients USA |
i-STAT (venous) | Enzymatic (Roche Holding AG) and Jaffe | Positive bias for i-STAT compared with Jaffe (mean difference 14.1 µmol/l, 95% CI 11.5 to 16.8 µmol/l; r2 = 0.997) and with enzymatic (mean difference 19.4 µmol/l, 95% CI 16.8 to 22.1 µmol/l; r2 = 0.998) | |||||||||||||||||||||||||||||||||||||||||||||
| aObrador et al., 201234 |
257 diabetic patients; mean age, 57 years; 62% women; and mean creatinine concentration of 0.8 mg/dl (SD 0.4 mg/dl) Mexico |
i-STAT (capillary) | NR (Olympus AU5400 High Volume Chemistry Immuno Analyzer, Olympus Corporation of the Americas, Center Valley, PA, USA) |
r2 = 0.93 (capillary) r2 = 0.90 (venous) |
|||||||||||||||||||||||||||||||||||||||||||||
| Park et al., 200965 |
60 samples (20 low, 20 medium and 20 high levels of SCr) The Republic of Korea (published in Korean) |
Piccolo Xpress | TBA 200-FR (Toshiba Co., Tokyo, Japan) | r2 = 0.9978; mean bias –0.2 mg/dl (SD 0.2 mg/dl, 95% LoA estimated by the EAG as –0.59 to 0.19 mg/dl) | |||||||||||||||||||||||||||||||||||||||||||||
| aRensburg et al., 201445 |
Number NR South Africa |
StatSensor | Jaffe (ADVIA®, Siemens Healthineers) | r2 = 0.987 | |||||||||||||||||||||||||||||||||||||||||||||
| aSchnabl et al., 200866 | 40 samples, a broad range of concentrations of SCr | Piccolo Xpress | NR (ARCHITECT c8000, Abbott, Abbott Park, IL, USA) | Average positive bias for SCr: 14%: ‘good correlation’ (r2 = NR, but ≥ 0.88) | |||||||||||||||||||||||||||||||||||||||||||||
| Schnabl et al., 201067 |
191 patients, which included 97 pre-dialysis and 57 post-dialysis patients Canada |
StatSensor | Jaffe (ARCHITECT c8000) |
r2 = 0.9328 overall; r2 = 08312 for pre-dialysis patients; r2 = 0.9347 for post-dialysis patients Few bias data were reported: a negative bias was seen at high creatinine concentrations, especially in pre-dialysis patients in which the bias was –30% |
|||||||||||||||||||||||||||||||||||||||||||||
| aShephard et al., 200835 |
101 venous blood samples Australia |
i-STAT (venous) | Enzymatic (NR) | The i-STAT displayed a positive bias relative to the IDMS-aligned laboratory method (mean % bias of 5.6% overall, 10.4% for samples < 150 µmol/l and 4.5% for samples > 150 µmol/l). This bias was eliminated by applying a correction formula and IDMS alignment | |||||||||||||||||||||||||||||||||||||||||||||
| Shephard et al., 201036 |
100; 63 renal/dialysis patients attending clinic, 37 healthy patients; and 52% female Australia |
StatSensor (capillary) | Enzymatic (Creatinine Plus assay, Roche Holding AG) | Better concordance in patients with higher SCr levels for both StatSensor devices pre and post calibration. There was greater bias for both StatSensor devices pre calibration, that is, before-and-after correction of a mean positive bias of 5.6% and alignment to the IDMS reference methodRecalibration time pointr2Mean bias (µmol/l) (95% CI)Pre recalibrationLow levels of SCr (i.e. < 150 µmol/l)StatSensor 10.83–7.3 (–11.0 to –3.6)StatSensor 20.84–6.7 (–10.3 to –3.1)AllStatSensor 10.97–47.3 (–63.6 to –31.1)StatSensor 20.97–46.5 (–63.6 to –29.3)Post recalibrationLow levels of SCr (i.e. < 150 µmol/l)StatSensor 10.834.2 (–0.2 to 8.7)StatSensor 20.845.0 (0.8 to 9.3)AllStatSensor 10.97–4.3 (–14.5 to 5.9)StatSensor 20.97–5.5 (–16.4 to 5.3) | Recalibration time point | r 2 | Mean bias (µmol/l) (95% CI) | Pre recalibration | Low levels of SCr (i.e. < 150 µmol/l) | StatSensor 1 | 0.83 | –7.3 (–11.0 to –3.6) | StatSensor 2 | 0.84 | –6.7 (–10.3 to –3.1) | All | StatSensor 1 | 0.97 | –47.3 (–63.6 to –31.1) | StatSensor 2 | 0.97 | –46.5 (–63.6 to –29.3) | Post recalibration | Low levels of SCr (i.e. < 150 µmol/l) | StatSensor 1 | 0.83 | 4.2 (–0.2 to 8.7) | StatSensor 2 | 0.84 | 5.0 (0.8 to 9.3) | All | StatSensor 1 | 0.97 | –4.3 (–14.5 to 5.9) | StatSensor 2 | 0.97 | –5.5 (–16.4 to 5.3) | ||||||||||||
| Recalibration time point | r 2 | Mean bias (µmol/l) (95% CI) | |||||||||||||||||||||||||||||||||||||||||||||||
| Pre recalibration | |||||||||||||||||||||||||||||||||||||||||||||||||
| Low levels of SCr (i.e. < 150 µmol/l) | |||||||||||||||||||||||||||||||||||||||||||||||||
| StatSensor 1 | 0.83 | –7.3 (–11.0 to –3.6) | |||||||||||||||||||||||||||||||||||||||||||||||
| StatSensor 2 | 0.84 | –6.7 (–10.3 to –3.1) | |||||||||||||||||||||||||||||||||||||||||||||||
| All | |||||||||||||||||||||||||||||||||||||||||||||||||
| StatSensor 1 | 0.97 | –47.3 (–63.6 to –31.1) | |||||||||||||||||||||||||||||||||||||||||||||||
| StatSensor 2 | 0.97 | –46.5 (–63.6 to –29.3) | |||||||||||||||||||||||||||||||||||||||||||||||
| Post recalibration | |||||||||||||||||||||||||||||||||||||||||||||||||
| Low levels of SCr (i.e. < 150 µmol/l) | |||||||||||||||||||||||||||||||||||||||||||||||||
| StatSensor 1 | 0.83 | 4.2 (–0.2 to 8.7) | |||||||||||||||||||||||||||||||||||||||||||||||
| StatSensor 2 | 0.84 | 5.0 (0.8 to 9.3) | |||||||||||||||||||||||||||||||||||||||||||||||
| All | |||||||||||||||||||||||||||||||||||||||||||||||||
| StatSensor 1 | 0.97 | –4.3 (–14.5 to 5.9) | |||||||||||||||||||||||||||||||||||||||||||||||
| StatSensor 2 | 0.97 | –5.5 (–16.4 to 5.3) | |||||||||||||||||||||||||||||||||||||||||||||||
| Skurup et al., 200868 |
104 samples Denmark |
ABL837 | Enzymatic (Cobas INTEGRA, Roche Holding AG) |
r2 = 0.999 B–A plot indicated a very small positive bias that appeared to decrease as levels of creatinine increased |
|||||||||||||||||||||||||||||||||||||||||||||
| Snaith et al., 201837 |
300 phlebotomy outpatients attending for routine blood tests; mean age 60 years; 47% female; and mean creatinine concentration of 92 µmol/l UK |
ABL800 FLEX, StatSensor (capillary) and i-STAT (venous) |
Enzymatic (Cobas 8000, Roche Holding AG) | ABL800 FLEX had the strongest agreement with laboratory-measured SCr concentrations (r2 = 0.991; mean bias = −0.86 µmol/l, 95% LoA −9.6 to 7.9 µmol/l) followed by i-STAT (r2 = 0.985; mean bias = 3.88 µmol/l, 95% LoA −8.8 to 16.6 µmol/l) and StatSensor (r2 = 0.891; mean bias = 3.56 µmol/l, 95% LoA −27.7 to 34.8 µmol/l) | |||||||||||||||||||||||||||||||||||||||||||||
| Snaith et al., 201938 |
300 adult outpatients attending for a contrast-enhanced CT scan, mean age 65 years, 48% female UK |
i-STAT (venous) | Enzymatic (Cobas 8000, Roche Holding AG) | Mean bias –0.21 (units not reported), 95% LoA –13.94 to 13.51; r2 = 0.948 | |||||||||||||||||||||||||||||||||||||||||||||
| Srihong et al., 201269 |
40 random blood samples from the central laboratory Thailand |
StatSensor | Jaffe (Beckman Coulter DxC 800) | r2 = 0.984 | |||||||||||||||||||||||||||||||||||||||||||||
| aStojkovic et al., 201770 |
56 participants; 48% female; and mean age around 53 years Serbia |
StatSensor | Enzymatic (Cobas, Roche Holding AG) |
B–A plot showed a mean eGFR bias of –2 ± 10 ml/minute/1.73 m2 CKD-EPI equation used for eGFR |
|||||||||||||||||||||||||||||||||||||||||||||
| aStraseski et al., 200971 |
50 inpatients; and median creatinine concentration of 1.30 mg/dl USA |
StatSensor (‘EZ CHEM’) | Enzymatic (Roche Holding AG, Hitachi Modular) | Mean bias reported only for subgroups. 0.69 mg/dl for the 14 samples (10 patients) with discordant results (differed by > 0.5 mg/dl between the two methods). A control group (n = 10) that was age, gender and race matched to the patients with discordant results had a mean bias of 0.14 mg/dl | |||||||||||||||||||||||||||||||||||||||||||||
| aStraseski et al., 201072 |
150 inpatients USA |
StatSensor (‘EZ CHEM’) | Enzymatic (Roche Holding AG, Hitachi Modular) and IDMS |
r2 = 0.791 when compared with IDMS method Higher discordance in patients with elevated creatinine values (> 2.0 mg/dl). Compared with the enzymatic method, 34 (23%) samples differed by > 0.5 mg/dl. Of these samples, 23 (68%) had enzymatic creatinine results > 2.0 mg/dl. Correlation with enzymatic method was not reported |
|||||||||||||||||||||||||||||||||||||||||||||
| Straseski et al., 201173 |
119 intensive care and oncology inpatients; 45% female; and mean age 59 years USA |
StatSensor | Enzymatic (Roche Holding AG, Hitachi Modular) and IDMS |
When compared with the enzymatic method there was increased discordance for results at higher creatinine concentrations r2 = 0.88 B–A plot suggested a negative bias. 22 patients had creatinine concentration results that differed by ≥ 0.50 mg/dl. 19 of the 22 patients had eGFR values < 30 ml/minute/1.73 m2 |
|||||||||||||||||||||||||||||||||||||||||||||
| aTreves and Boehre, 201174 |
NR; radiology setting France |
StatSensor | LX20 (Beckman-Coulter) and RXL (Siemens) | r2 = 0.908 | |||||||||||||||||||||||||||||||||||||||||||||
| Too et al., 201575 |
52 ‘leftover’ blood samples Singapore |
StatSensor | NR | Positive bias of 11.3% (95% LoA –24.3% to 47.0%) | |||||||||||||||||||||||||||||||||||||||||||||
| van Lint et al., 201576 |
138 kidney transplant outpatients The Netherlands |
StatSensor Xpress-i | Enzymatic (Modular P800, Roche Holding AG) | Mean bias = –12.38 µmol/l (95% LoA –58.8 to 34.1 µmol/l) | |||||||||||||||||||||||||||||||||||||||||||||
Studies of StatSensor devices
Twenty-six studies reported measurement bias or correlation results for a StatSensor POC device14,28,30–32,36,37,39–46,53,62,67,69–76 Eight studies were available only as a conference abstract. 40,43,45,53,70–72,74 A large majority of studies were of the StatSensor or StatSensor-i model, with six studies being of the StatSensor Xpress (or Xpress-I) model. 28,39,42,44,53,76 Sample sizes ranged from 15 to 1467 patients. Most studies reported measurement bias results based on levels of creatinine, with only three studies reporting results based on eGFR. 30,62,70 Among the studies that either explicitly reported mean measurement bias results or for which an indication of mean bias could be derived from Bland–Altman plots, there appeared to be no clear trend in terms of the direction of bias, with nearly as many studies reporting positive bias (in StatSensor creatinine measurements) as reporting negative bias. Only two studies reported results following offset correction to adjust for bias. 39,41
Enzymatic laboratory reference methods are far more specific for measuring creatinine than Jaffe laboratory methods. The latter methods are prone to overestimate creatinine (especially at low concentrations) as picric acid reacts with other metabolites or drugs. Results from studies that use enzymatic laboratory methods are therefore preferable to those using Jaffe methods. Of the 10 studies28,30,31,36,37,53,62,70,73,76 that used an enzymatic laboratory reference, five reported a positive measurement bias in creatinine levels when using StatSensor28,30,37,53,70 and five reported a negative bias. 31,36,62,73,76 However, some bias results were reported only as percentage changes. The results of those enzymatic reference standard studies that reported mean biases in mg/dl or µmol/l (including the often wide limits of agreement) indicated that many StatSensor creatinine measurements are likely to be inaccurate enough to have a clinically significant impact on subsequent eGFR calculations. This impact was evident in studies that reported bias results based on eGFRs; for example, Morita et al. 62 reported a mean eGFR bias of 11 ml/minute/1.73 m2 (95% limits of agreement –22.4 to 44.4 ml/minute/1.73 m2). Even studies that did not report significant mean bias reported the presence of important bias in measures of variance around the mean; for example, in the study by Snaith et al. 37 the mean bias was very small at 3.56 µmol/l (0.04 mg/dl), but the 95% limits of agreement were −27.7 µmol/l (–0.31 mg/dl) to 34.8 µmol/l (0.39 mg/dl). Several studies did not report a measure-of-bias variance. Five studies indicated that bias tended to increase at higher creatinine concentrations. 39,42,67,72,73
Most of the studies that reported data on how well StatSensor results correlate with laboratory results (r2) found high levels of correlation. However, these data have limited relevance to this assessment because good correlation of results does not necessarily mean there is good agreement between the two methods of measurement.
Studies of i-STAT devices
Eighteen studies reported measurement bias or correlation results for an i-STAT POC device. 27,31,33–35,37,38,47–50,54,56,57,59–61,64 Seven were available only as conference abstracts. 27,34,35,49,50,57,61 Sample sizes ranged from 15 to 3087 patients. Most studies reported bias results based on levels of creatinine; two studies reported results based on eGFRs. 61,64 Most studies reported using enzymatic laboratory methods; two studies used Jaffe methods. 54,56 One study focused on bias following the addition of serial dilutions of hydroxycarbamide. 50 Eight studies indicated that there were positive biases in creatinine values derived from i-STAT devices when compared with laboratory results,31,33,35,56,60,61,64 whereas two studies showed a negative bias. 38,47 In four other studies the bias was very small, being close to zero. 27,37,54,57 Many of the biases appeared large enough to have a clinically significant impact on subsequent eGFR calculations. The two studies61,64 that examined the effect on eGFR reported a underestimation by 4–12%,61 depending on gender and absolute creatinine value, and a mean bias of –2.2 ml/minute/1.73 m2. 64 Limits of agreement (where available) were mostly narrow, indicating that the biases were quite consistent and predictable.
Studies of ABL series devices
Six studies reported measurement bias or correlation results relating to an ABL device,27,31,37,51,58,68 although three studies were available only as conference abstracts. 27,51,58 Four studies were of the ABL800 device,31,37,51,58 one was of the ABL82727 and one was of the ABL837. 68 Sample sizes ranged from 7051 to 2042. 27 All studies used an enzymatic laboratory reference method except one. 58 All bias data related to levels of creatinine. Very small negative mean biases from ABL devices were reported in two studies,27,31 with both estimates having narrow 95% limits of agreement. One study reported a mean bias that was close to zero37 but with 95% limits of agreement that were notably broader than the two aforementioned studies. 27,31 One study58 reported a substantial negative bias (i.e. of –0.22 mg/dl) without an accompanying measure of variance.
Studies of Piccolo Xpress devices
Four studies reported measurement bias or correlation data for the Piccolo Xpress device. 55,63,65,66 One study was reported in Czech,55 so only minimal data could be extracted, and one study was available only as a conference abstract. 66 It was unclear whether enzymatic or Jaffe laboratory reference methods were used in all four studies. 55,63,65,66 All the studies were small (n ≤ 60), although this information could not be extracted for the study published in Czech. 55 Two studies reported bias data only as percentages, with both studies reporting positive biases (of 8%55 and 14%66), one study did not report an numerical estimate of bias (but did present a Bland–Altman plot),63 and one study65 reported a negative bias of – 0.2 mg/dl (95% limits of agreement estimated as –0.25 to –0.15 mg/dl).
Studies of epoc devices
One study reported measurement bias and correlation data for an epoc device. 52 This study found that epoc device measurements resulted in a small negative mean bias (i.e. of − 0.025 mg/dl). The other epoc study – available only as a conference abstract – investigated whether or not hydroxycarbamide caused interference in creatinine measurements using i-STAT and epoc devices, and whether or not the interference resulted in bias. 50 No interference was found for the epoc device.
Studies that compared different types of device
Three of the studies listed in Table 4 directly compared different types of POC device. 27,31,37 The Snaith et al. 37 and Korpi-Steiner et al. 31 studies both compared StatSensor, i-STAT and ABL800 FLEX devices. Both studies found that the ABL800 FLEX had the strongest agreement with laboratory-measured SCr, followed by i-STAT and then StatSensor. The study available only as a conference abstract compared an ABL827 device with an i-STAT, concluding that creatinine results from both devices correlated well with laboratory-measured SCr. 27
Summary
Overall, results from the StatSensor studies illustrate wide variation in the size and direction of measurement bias that can be encountered when using this device. It may be relevant for users to be aware of the availability of the offset functionality to correct for any bias observed with an individual StatSensor device. Only two StatSensor studies reported using an offset adjustment for measurement bias. This raises the possibility that issues such as lack of awareness or difficulties in implementing the adjustment function to align the POC test to local laboratory methods could be relevant in clinical practice. The tendency for measurement bias to increase at higher creatinine levels (as seen in some studies) is also a concern, as this has important implications for the care decisions made about sicker patients. Although potentially important measurement bias was identified in some studies of i-STAT and ABL devices, in most of these studies the concordance of results was generally better than was found in most of the StatSensor studies. Few studies were available on the epoc and Piccolo Xpress devices; the limited data and reporting in these studies, coupled with their small sample sizes, made it difficult to draw conclusions about creatinine measurement biases.
Although the concordance and measurement bias results reported in these studies suggest that there may be important limitations to using POC devices to measure creatinine, it is more important to consider the impact of any measurement bias on results categorised according to clinically important thresholds that may be used for clinical decision-making. Studies that report such data are presented in Studies reporting diagnostic accuracy results using estimated glomerular filtration rate thresholds.
Studies reporting diagnostic accuracy results based on creatinine thresholds
Seven studies reported diagnostic accuracy data relating to creatinine thresholds (Table 5), with four being reported as published papers39,41,42,44 and three as conference abstracts. 40,43,45 Where reported, sample sizes ranged from 33 to 401 patients. Population details were limited with one study (appearing to be) set in the UK43 and one reported as being of patients due to receive CT scans. 41 All the studies were of StatSensor POC devices. Six studies used a Jaffe method39–41,43–45 for the laboratory reference standard and in one study this was unclear. 42
| Study (author and year of publication) | Population (N) and country | POC device(s) | Laboratory reference | Results and notes |
|---|---|---|---|---|
| Griffin et al., 201839 | Two studies of field workers:
|
StatSensor Xpress | Jaffe | Adjusted results with unadjusted results in brackets:
|
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Haneder et al., 201241 |
401 patients referred for a CT scan at two centres; mean age was 62 years (SD 14 years); and 63% male Germany |
StatSensor (two devices: A and B) | Jaffe (Dimension RXL, Siemens AG; Olympus AU2700, Beckman Coulter, Inc.) | Centre 1 at a cut-off point of 1.2 mg/dl:
|
| Kosack et al., 201542 |
60 patients and laboratory workers The Netherlands |
StatSensor Xpress | VITROS 5,1FS (Ortho Clinical Diagnostics) | At a cut-off point of ≥ 115 µmol/l (1.3 mg/dl):
|
| aMcGough et al., 201843 |
33 dialysis patients UK |
StatSensor | Jaffe (Cobas 8000, Roche Holding AG) | At a cut-off point of 1.5 mg/dl both sensitivity and specificity were 100% |
| Minnings et al., 201544 |
100 patients from a health centre or hospital setting; 70% female; and a median SCr concentration of 0.72 mg/dl Nicaragua |
StatSensor Xpress | Jaffe (Roche Cobas INTEGRA 400, Roche Holding AG) | At a cut-off point of 1.1 mg/dl:
|
| aRensburg et al., 201445 |
Number NR South Africa |
StatSensor | Jaffe (ADVIA®, Siemens Healthineers) | At a cut-off point of 130 µmol/l (1.5 mg/dl):
|
The creatinine thresholds used in the studies (to calculate sensitivity and specificity) ranged from 1.1 mg/dl to 1.5 mg/dl (i.e. 97 µmol/l to 133 µmol/l). As eGFR (rather than creatinine alone) is used to estimate kidney function in clinical practice, diagnostic accuracy results based on creatinine thresholds are not as clinically relevant or useful than those based on eGFR thresholds. Moreover, all these (creatinine) studies are of the StatSensor POC device, which allows users to implement offset adjustment of biased results. Two of the seven studies explicitly reported results that incorporated an offset adjustment. 39,41 The other five studies did not report using offset adjustment. 40,42–45 Notwithstanding these limitations, most studies reported unadjusted sensitivities that were higher than specificities, indicating that StatSensor tended to overestimate creatinine levels compared with laboratory Jaffe results. The exceptions were the study by Haneder et al. ,41 which reported much lower (unadjusted) sensitivities than specificities in the two devices tested, and the small UK study which reported both a sensitivity and specificity of 100%. 43 Although most studies indicated overestimation of creatinine by StatSensor, the Haneder et al. study41 illustrated that some StatSensor devices may underestimate creatinine. This variation in over- or underestimation was also seen across the studies that reported results for creatinine level bias (see Studies reporting bias or correlation outcomes).
The results of the Griffin et al. 39 and Haneder et al. studies41 indicate that, even after offset adjustment of creatinine results, StatSensor can produce false-negative (FN) and false-positive (FP) results. This has the potential to result in unnecessary prophylactic treatment or scans without contrast (i.e. FP) or to unnecessarily expose high-risk patients to contrast (i.e. FN). The laboratory reference standards used in these studies also limits their value, as the adjustments may themselves be inaccurate, being based on Jaffe methods rather than more accurate enzymatic methods.
Studies reporting diagnostic accuracy results using estimated glomerular filtration rate thresholds
Table 6 summarises the characteristics of the 12 studies that reported diagnostic accuracy data of eGFR measurements with POC creatinine test devices.
| Study (author and year of publication) | Population (N) and country | POC device(s) (sample type) | Laboratory reference | eGFR equation | Results and notes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aBotz et al., 201327 |
2042 patients at risk of renal disease prior to radiological examinations; 43% of patients were female USA |
ABL827 and i-STAT1 (sample type NR) | Enzymatic, (Cobas C-501, Roche Holding AG) | MDRD |
Contingency table: ABL827 and i-STAT accuracy at eGFR 30 and 60 ml/minute/1.73 m2 cut-off points Source: publicationDevice, number of tests (n)ABL827i-STATeGFR < 30 ml/minute/1.73 m2≥ 30 ml/minute/1.73 m2eGFR < 30 ml/minute/1.73 m2eGFR ≥ 30 ml/minute/1.73 m2Laboratory eGFR < 30 ml/minute/1.73 m2263122 eGFR ≥ 30 ml/minute/1.73 m2NRNRNRNReGFR < 60 ml/minute/1.73 m2eGFR ≥ 60 ml/minute/1.73 m2eGFR < 60 ml/minute/1.73 m2eGFR ≥ 60 ml/minute/1.73 m2 eGFR < 60 ml/minute/1.73 m2520183NRNR eGFR ≥ 60 ml/minute/1.73 m2242517NRNRNotesSensitivity and specificity for i-STAT at < 60 and ≥ 60 ml/minute/1.73 m2 were both 93%. n = 3244 for ABL827 and n = 2042 for i-STAT (for patients with same-day measurements). |
Source: publication | Device, number of tests (n) | ABL827 | i-STAT | eGFR < 30 ml/minute/1.73 m2 | ≥ 30 ml/minute/1.73 m2 | eGFR < 30 ml/minute/1.73 m2 | eGFR ≥ 30 ml/minute/1.73 m2 | Laboratory | eGFR < 30 ml/minute/1.73 m2 | 26 | 3 | 12 | 2 | eGFR ≥ 30 ml/minute/1.73 m2 | NR | NR | NR | NR | eGFR < 60 ml/minute/1.73 m2 | eGFR ≥ 60 ml/minute/1.73 m2 | eGFR < 60 ml/minute/1.73 m2 | eGFR ≥ 60 ml/minute/1.73 m2 | eGFR < 60 ml/minute/1.73 m2 | 520 | 183 | NR | NR | eGFR ≥ 60 ml/minute/1.73 m2 | 24 | 2517 | NR | NR | Notes | Sensitivity and specificity for i-STAT at < 60 and ≥ 60 ml/minute/1.73 m2 were both 93%. | n = 3244 for ABL827 and n = 2042 for i-STAT (for patients with same-day measurements). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Source: publication | Device, number of tests (n) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ABL827 | i-STAT | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR < 30 ml/minute/1.73 m2 | ≥ 30 ml/minute/1.73 m2 | eGFR < 30 ml/minute/1.73 m2 | eGFR ≥ 30 ml/minute/1.73 m2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Laboratory | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR < 30 ml/minute/1.73 m2 | 26 | 3 | 12 | 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR ≥ 30 ml/minute/1.73 m2 | NR | NR | NR | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR < 60 ml/minute/1.73 m2 | eGFR ≥ 60 ml/minute/1.73 m2 | eGFR < 60 ml/minute/1.73 m2 | eGFR ≥ 60 ml/minute/1.73 m2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR < 60 ml/minute/1.73 m2 | 520 | 183 | NR | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR ≥ 60 ml/minute/1.73 m2 | 24 | 2517 | NR | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Notes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sensitivity and specificity for i-STAT at < 60 and ≥ 60 ml/minute/1.73 m2 were both 93%. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| n = 3244 for ABL827 and n = 2042 for i-STAT (for patients with same-day measurements). | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| bDorward et al., 201828 |
187 HIV-positive patients from a POC RCT; median age 31 years; 62% female; and a mean creatinine concentration of 69.0 µmol/l South Africa |
StatSensor Xpress-I (capillary) | Enzymatic (Dimension® EXL 200 IDMS, Siemens AG) |
Modified MDRD (without race) |
At an eGFR < 90 ml/minute/1.73 m2 threshold, sensitivity was 87.1% (95% CI 76.2% to 94.3%), specificity was 52% (95% CI 42.9% to 61.0%). One patient had a laboratory-measured eGFR of < 60 ml/minute/1.73 m2; this was correctly identified by StatSensor At a creatinine threshold of > 106 µmol/l (1.2 mg/dl), sensitivity was 100% and specificity 95.1% (95% CI 90.9% to 97.7%) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Houben et al., 201729 |
351 women due for contrast-enhanced spectral mammography The Netherlands |
StatSensor CREAT (capillary) | Enzymatic (Cobas 8000, Roche) | MDRD |
Contingency table: StatSensor accuracy at eGFR 30 and 60 ml/minute/1.73 m2 cut-off points Source: publicationDevice, number of tests (n)StatSensoreGFR < 30 ml/minute/1.73 m2eGFR 30–44 ml/minute/1.73 m2eGFR 45–59 ml/minute/1.73 m2eGFR ≥ 60 ml/minute/1.73 m2Laboratory eGFR < 30 ml/minute/1.73 m20000 eGFR 30–44 ml/minute/1.73 m20012 eGFR ≥ 60 ml/minute/1.73 m200348NotesSeven patients had an eGFR < 60 ml/minute/1.73 m2, necessitating additional preparation prior to contrast delivery. The POC device failed to categorise six of these seven patients (86%), leading to unwanted contrast administration. Two patients (including one of the three patients with an eGFR of 45 ml/minute/1.73 m2) subsequently developed CIN after 2–5 days, which was normalised after 30 days. |
Source: publication | Device, number of tests (n) | StatSensor | eGFR < 30 ml/minute/1.73 m2 | eGFR 30–44 ml/minute/1.73 m2 | eGFR 45–59 ml/minute/1.73 m2 | eGFR ≥ 60 ml/minute/1.73 m2 | Laboratory | eGFR < 30 ml/minute/1.73 m2 | 0 | 0 | 0 | 0 | eGFR 30–44 ml/minute/1.73 m2 | 0 | 0 | 1 | 2 | eGFR ≥ 60 ml/minute/1.73 m2 | 0 | 0 | 348 | Notes | Seven patients had an eGFR < 60 ml/minute/1.73 m2, necessitating additional preparation prior to contrast delivery. The POC device failed to categorise six of these seven patients (86%), leading to unwanted contrast administration. Two patients (including one of the three patients with an eGFR of 45 ml/minute/1.73 m2) subsequently developed CIN after 2–5 days, which was normalised after 30 days. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Source: publication | Device, number of tests (n) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| StatSensor | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR < 30 ml/minute/1.73 m2 | eGFR 30–44 ml/minute/1.73 m2 | eGFR 45–59 ml/minute/1.73 m2 | eGFR ≥ 60 ml/minute/1.73 m2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Laboratory | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR < 30 ml/minute/1.73 m2 | 0 | 0 | 0 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR 30–44 ml/minute/1.73 m2 | 0 | 0 | 1 | 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR ≥ 60 ml/minute/1.73 m2 | 0 | 0 | 348 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Notes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Seven patients had an eGFR < 60 ml/minute/1.73 m2, necessitating additional preparation prior to contrast delivery. The POC device failed to categorise six of these seven patients (86%), leading to unwanted contrast administration. Two patients (including one of the three patients with an eGFR of 45 ml/minute/1.73 m2) subsequently developed CIN after 2–5 days, which was normalised after 30 days. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Inoue et al., 201730 |
123 patients (with unadjusted results), scheduled for CT; mean eGFR 75.3 ml/minute/1.73 m2 (SD 21.4 ml/minute/1.73 m2); mean creatinine 0.8 mg/dl (SD 0.29 mg/dl) Japan |
StatSensor-i (capillary) | Enzymatic (BioMajesty™ BM2250, Jeol Ltd) |
Modified MDRD (Japanese CKD patients) |
Contingency table: StatSensor accuracy at eGFR < 30, 30–44 and ≥ 45 ml/minute/1.73 m2 cut-off points (unadjusted results) Source: publication table and plotsDevice, number of tests (n)eGFR < 30 ml/minute/1.73 m2eGFR 30–44 ml/minute/1.73 m2eGFR ≥ 45 ml/minute/1.73 m2Laboratory eGFR < 30 ml/minute/1.73 m2400 eGFR 30–44 ml/minute/1.73 m2170 eGFR ≥ 45 ml/minute/1.73 m211199 Adjustment was performed by applying offset correction on the basis of the slope and intercept of internal sample Plots presented after correction suggested that eGFR laboratory measurements were unexpectedly affected by this adjustment; therefore, only unadjusted results were extracted |
Source: publication table and plots | Device, number of tests (n) | eGFR < 30 ml/minute/1.73 m2 | eGFR 30–44 ml/minute/1.73 m2 | eGFR ≥ 45 ml/minute/1.73 m2 | Laboratory | eGFR < 30 ml/minute/1.73 m2 | 4 | 0 | 0 | eGFR 30–44 ml/minute/1.73 m2 | 1 | 7 | 0 | eGFR ≥ 45 ml/minute/1.73 m2 | 1 | 11 | 99 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Source: publication table and plots | Device, number of tests (n) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR < 30 ml/minute/1.73 m2 | eGFR 30–44 ml/minute/1.73 m2 | eGFR ≥ 45 ml/minute/1.73 m2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Laboratory | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR < 30 ml/minute/1.73 m2 | 4 | 0 | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR 30–44 ml/minute/1.73 m2 | 1 | 7 | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR ≥ 45 ml/minute/1.73 m2 | 1 | 11 | 99 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Korpi-Steiner et al., 200931 |
266 excess samples taken before CT procedures; mean age 68 years; and 39% female USA |
ABL800 FLEX, i-STAT, StatSensor (with slope and intercept offset option) Heparinised venous samples |
Enzymatic, (Cobas INTEGRA 400, Roche Holding AG) | INTEGRA 400 and ABL800 used adjusted MDRD (IDMS traceable). i-STAT and StatSensor used conventional MDRD |
Contingency table: ABL800 and i-STAT accuracy at eGFR 60 ml/minute/1.73 m2 cut-off points Source: publicationDevice, number of tests (n)ABL800i-STATeGFR < 60 ml/minute/1.73 m2eGFR ≥ 60 ml/minute/1.73 m2eGFR < 60 ml/minute/1.73 m2eGFR ≥ 60 ml/minute/1.73 m2Laboratory eGFR < 60 ml/minute/1.73 m25513662 eGFR ≥ 60 ml/minute/1.73 m2619232166 Contingency table: StatSensor accuracy at eGFR 60 ml/minute/1.73 m2 cut-off points, with and without correction offset Source: publicationDevice, number of tests (n)StatSensorStatSensor offseteGFR < 60 ml/minute/1.73 m2eGFR ≥ 60 ml/minute/1.73 m2eGFR < 60 ml/minute/1.73 m2eGFR ≥ 60 ml/minute/1.73 m2Laboratory eGFR < 60 ml/minute/1.73 m211574028 eGFR ≥ 60 ml/minute/1.73 m2019824174 An offset of 0.28 mg/dl was applied that maximised overall concordance between the POC test and the laboratory reference in this data set |
Source: publication | Device, number of tests (n) | ABL800 | i-STAT | eGFR < 60 ml/minute/1.73 m2 | eGFR ≥ 60 ml/minute/1.73 m2 | eGFR < 60 ml/minute/1.73 m2 | eGFR ≥ 60 ml/minute/1.73 m2 | Laboratory | eGFR < 60 ml/minute/1.73 m2 | 55 | 13 | 66 | 2 | eGFR ≥ 60 ml/minute/1.73 m2 | 6 | 192 | 32 | 166 | Source: publication | Device, number of tests (n) | StatSensor | StatSensor offset | eGFR < 60 ml/minute/1.73 m2 | eGFR ≥ 60 ml/minute/1.73 m2 | eGFR < 60 ml/minute/1.73 m2 | eGFR ≥ 60 ml/minute/1.73 m2 | Laboratory | eGFR < 60 ml/minute/1.73 m2 | 11 | 57 | 40 | 28 | eGFR ≥ 60 ml/minute/1.73 m2 | 0 | 198 | 24 | 174 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Source: publication | Device, number of tests (n) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ABL800 | i-STAT | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR < 60 ml/minute/1.73 m2 | eGFR ≥ 60 ml/minute/1.73 m2 | eGFR < 60 ml/minute/1.73 m2 | eGFR ≥ 60 ml/minute/1.73 m2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Laboratory | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR < 60 ml/minute/1.73 m2 | 55 | 13 | 66 | 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR ≥ 60 ml/minute/1.73 m2 | 6 | 192 | 32 | 166 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Source: publication | Device, number of tests (n) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| StatSensor | StatSensor offset | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR < 60 ml/minute/1.73 m2 | eGFR ≥ 60 ml/minute/1.73 m2 | eGFR < 60 ml/minute/1.73 m2 | eGFR ≥ 60 ml/minute/1.73 m2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Laboratory | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR < 60 ml/minute/1.73 m2 | 11 | 57 | 40 | 28 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR ≥ 60 ml/minute/1.73 m2 | 0 | 198 | 24 | 174 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| cKrige, 201732 |
103 mixed-ancestry South Africans; mean age 52 years; and 69% female South Africa |
StatSensor (capillary) | Jaffe (AU5800 Clinical Chemistry Analyzer, Beckman Coulter, Inc.) | MDRD (SI units) |
Contingency table: StatSensor-i accuracy at eGFR < 30, 30–44, 45–59 and ≥ 60 ml/minute/1.73 m2 cut-off points Source: individual patient data in thesisDevice, number of tests (n)eGFR < 30 ml/minute/1.73 m2eGFR 30–44 ml/minute/1.73 m2eGFR 45–59 ml/minute/1.73 m2eGFR ≥ 60 ml/minute/1.73 m2Laboratory eGFR < 30 ml/minute/1.73 m21000 eGFR 30–44 ml/minute/1.73 m20000 eGFR 45–59 ml/minute/1.73 m20011 eGFR ≥ 60 ml/minute/1.73 m2000100NotesThe three low eGFR values were:• POC, 22 ml/minute/1.73 m2; laboratory, 1 ml/minute/1.73 m2• POC, 48 ml/minute/1.73 m2; laboratory, 49 ml/minute/1.73 m2• POC, > 90 ml/minute/1.73 m2; laboratory, 56 ml/minute/1.73 m2 |
Source: individual patient data in thesis | Device, number of tests (n) | eGFR < 30 ml/minute/1.73 m2 | eGFR 30–44 ml/minute/1.73 m2 | eGFR 45–59 ml/minute/1.73 m2 | eGFR ≥ 60 ml/minute/1.73 m2 | Laboratory | eGFR < 30 ml/minute/1.73 m2 | 1 | 0 | 0 | 0 | eGFR 30–44 ml/minute/1.73 m2 | 0 | 0 | 0 | 0 | eGFR 45–59 ml/minute/1.73 m2 | 0 | 0 | 1 | 1 | eGFR ≥ 60 ml/minute/1.73 m2 | 0 | 0 | 0 | 100 | Notes | The three low eGFR values were: | • POC, 22 ml/minute/1.73 m2; laboratory, 1 ml/minute/1.73 m2 | • POC, 48 ml/minute/1.73 m2; laboratory, 49 ml/minute/1.73 m2 | • POC, > 90 ml/minute/1.73 m2; laboratory, 56 ml/minute/1.73 m2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Source: individual patient data in thesis | Device, number of tests (n) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR < 30 ml/minute/1.73 m2 | eGFR 30–44 ml/minute/1.73 m2 | eGFR 45–59 ml/minute/1.73 m2 | eGFR ≥ 60 ml/minute/1.73 m2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Laboratory | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR < 30 ml/minute/1.73 m2 | 1 | 0 | 0 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR 30–44 ml/minute/1.73 m2 | 0 | 0 | 0 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR 45–59 ml/minute/1.73 m2 | 0 | 0 | 1 | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR ≥ 60 ml/minute/1.73 m2 | 0 | 0 | 0 | 100 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Notes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The three low eGFR values were: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| • POC, 22 ml/minute/1.73 m2; laboratory, 1 ml/minute/1.73 m2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| • POC, 48 ml/minute/1.73 m2; laboratory, 49 ml/minute/1.73 m2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| • POC, > 90 ml/minute/1.73 m2; laboratory, 56 ml/minute/1.73 m2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Nichols et al., 200733 |
50 chemotherapy patients USA |
i-STAT (venous) | Enzymatic (Roche Holding AG) and Jaffe | Cockcroft–Gault and MDRD |
Diagnostic accuracy of i-STAT against two laboratory reference methods and two eGFR equations at an eGFR < 60 ml/minute/1.73 m2 cut-off point Source: publicationSensitivity (%)Specificity (%)MDRD Jaffe10087.2CG Jaffe10059.2MDRD enzymatic10085CG enzymatic10072.5CG, Cockcroft–Gault. |
Source: publication | Sensitivity (%) | Specificity (%) | MDRD Jaffe | 100 | 87.2 | CG Jaffe | 100 | 59.2 | MDRD enzymatic | 100 | 85 | CG enzymatic | 100 | 72.5 | CG, Cockcroft–Gault. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Source: publication | Sensitivity (%) | Specificity (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MDRD Jaffe | 100 | 87.2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CG Jaffe | 100 | 59.2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MDRD enzymatic | 100 | 85 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CG enzymatic | 100 | 72.5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CG, Cockcroft–Gault. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| aObrador et al., 201234 |
257 diabetic patients; mean age, 57 years; 62% women; and mean creatinine concentration of 0.8 mg/dl (SD 0.4 mg/dl) Mexico |
i-STAT (capillary) | NR (Olympus AU5400 High Volume Chemistry Immuno Analyzer, Olympus Corporation of the Americas) | CKD-EPI |
Contingency table: i-STAT accuracy by CKD stage (stages 0–4) Source: table in abstractDevice, number (n)IDMS SCr – laboratory referenceCKD stage01234i-STAT SCr CKD stage 01540000 1053500 2041330 3103152 400004Total1555721186 Simple linear regression was used to estimate a correction factor to align i-STAT SCr to IDMS-SCr. Following this correction, no patient was incorrectly classified as not having CKD by i-STAT (capillary sample) (100% sensitivity). One patient was incorrectly classified as having CKD (99.4% specificity) |
Source: table in abstract | Device, number (n) | IDMS SCr – laboratory reference | CKD stage | 0 | 1 | 2 | 3 | 4 | i-STAT SCr | CKD stage | 0 | 154 | 0 | 0 | 0 | 0 | 1 | 0 | 53 | 5 | 0 | 0 | 2 | 0 | 4 | 13 | 3 | 0 | 3 | 1 | 0 | 3 | 15 | 2 | 4 | 0 | 0 | 0 | 0 | 4 | Total | 155 | 57 | 21 | 18 | 6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Source: table in abstract | Device, number (n) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IDMS SCr – laboratory reference | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CKD stage | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 0 | 1 | 2 | 3 | 4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| i-STAT SCr | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CKD stage | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 0 | 154 | 0 | 0 | 0 | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 | 0 | 53 | 5 | 0 | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | 0 | 4 | 13 | 3 | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | 1 | 0 | 3 | 15 | 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | 0 | 0 | 0 | 0 | 4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | 155 | 57 | 21 | 18 | 6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| aShephard et al., 200835 |
101 venous blood samples Australia |
i-STAT (venous) | Enzymatic (IDMS aligned) (device NR) | NR | The i-STAT had a positive measurement bias relative to the IDMS-aligned laboratory method (mean bias of 5.6% overall, 10.4% for samples < 150 mmol/l and 4.5% for samples > 150 mmol/l). This bias was corrected and an IDMS alignment performed using a correction formula based on the regression equation between the i-STAT and laboratory methods:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shephard et al., 201036 |
100; 63 renal/dialysis patients attending clinic and 37 healthy patients; and 52% female Australia |
StatSensor (capillary) | Enzymatic (Creatinine Plus assay, Roche Holding AG) | MDRD |
Diagnostic accuracy of two StatSensor devices at an eGFR 60 ml/minute/1.73 m2 cut-off point before and after recalibration Source: publicationSensitivity (%)Specificity (%)Pre-laboratory recalibrationStatSensor 186.8100StatSensor 282.4100Post-laboratory recalibrationStatSensor 196.278.7StatSensor 292.278.7 After correction of a mean positive bias of 5.6% and alignment to the IDMS reference method Contingency table: StatSensor accuracy at an eGFR 60 ml/minute/1.73 m2 cut-off point before and after recalibration Source: publication in paperDevice, number of tests (n)StatSensor 1Pre recalibrationPost recalibration*LaboratoryeGFR < 60 ml/minute/1.73 m2eGFR ≥ 60 ml/minute/1.73 m2eGFR < 60 ml/minute/1.73 m2eGFR ≥ 60 ml/minute/1.73 m2 eGFR < 60 ml/minute/1.73 m2467512 eGFR ≥ 60 ml/minute/1.73 m20461037 After correction of a mean positive bias of 5.6% and alignment to the IDMS reference method Contingency table: StatSensor accuracy at eGFR < 30, 30–59 and ≥ 60 ml/minute/1.73 m2 cut-off points before and after recalibration Source: study figureDevice, number of tests (n)StatSensor 1Pre recalibrationPost recalibrationeGFR < 30 ml/minute/1.73 m2eGFR 30–59 ml/minute/1.73 m2eGFR ≥ 60 ml/minute/1.73 m2eGFR < 30 ml/minute/1.73 m2eGFR 30–59 ml/minute/1.73 m2eGFR ≥ 60 ml/minute/1.73 m2Laboratory eGFR < 30 ml/minute/1.73 m226613210 eGFR 30–59 ml/minute/1.73 m201461172 eGFR ≥ 60 ml/minute/1.73 m2004701037 |
Source: publication | Sensitivity (%) | Specificity (%) | Pre-laboratory recalibration | StatSensor 1 | 86.8 | 100 | StatSensor 2 | 82.4 | 100 | Post-laboratory recalibration | StatSensor 1 | 96.2 | 78.7 | StatSensor 2 | 92.2 | 78.7 | Source: publication in paper | Device, number of tests (n) | StatSensor 1 | Pre recalibration | Post recalibration* | Laboratory | eGFR < 60 ml/minute/1.73 m2 | eGFR ≥ 60 ml/minute/1.73 m2 | eGFR < 60 ml/minute/1.73 m2 | eGFR ≥ 60 ml/minute/1.73 m2 | eGFR < 60 ml/minute/1.73 m2 | 46 | 7 | 51 | 2 | eGFR ≥ 60 ml/minute/1.73 m2 | 0 | 46 | 10 | 37 | Source: study figure | Device, number of tests (n) | StatSensor 1 | Pre recalibration | Post recalibration | eGFR < 30 ml/minute/1.73 m2 | eGFR 30–59 ml/minute/1.73 m2 | eGFR ≥ 60 ml/minute/1.73 m2 | eGFR < 30 ml/minute/1.73 m2 | eGFR 30–59 ml/minute/1.73 m2 | eGFR ≥ 60 ml/minute/1.73 m2 | Laboratory | eGFR < 30 ml/minute/1.73 m2 | 26 | 6 | 1 | 32 | 1 | 0 | eGFR 30–59 ml/minute/1.73 m2 | 0 | 14 | 6 | 1 | 17 | 2 | eGFR ≥ 60 ml/minute/1.73 m2 | 0 | 0 | 47 | 0 | 10 | 37 | ||||||||||||||||||||||||||||||||||||||||||||
| Source: publication | Sensitivity (%) | Specificity (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pre-laboratory recalibration | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| StatSensor 1 | 86.8 | 100 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| StatSensor 2 | 82.4 | 100 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Post-laboratory recalibration | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| StatSensor 1 | 96.2 | 78.7 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| StatSensor 2 | 92.2 | 78.7 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Source: publication in paper | Device, number of tests (n) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| StatSensor 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pre recalibration | Post recalibration* | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Laboratory | eGFR < 60 ml/minute/1.73 m2 | eGFR ≥ 60 ml/minute/1.73 m2 | eGFR < 60 ml/minute/1.73 m2 | eGFR ≥ 60 ml/minute/1.73 m2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR < 60 ml/minute/1.73 m2 | 46 | 7 | 51 | 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR ≥ 60 ml/minute/1.73 m2 | 0 | 46 | 10 | 37 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Source: study figure | Device, number of tests (n) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| StatSensor 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pre recalibration | Post recalibration | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR < 30 ml/minute/1.73 m2 | eGFR 30–59 ml/minute/1.73 m2 | eGFR ≥ 60 ml/minute/1.73 m2 | eGFR < 30 ml/minute/1.73 m2 | eGFR 30–59 ml/minute/1.73 m2 | eGFR ≥ 60 ml/minute/1.73 m2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Laboratory | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR < 30 ml/minute/1.73 m2 | 26 | 6 | 1 | 32 | 1 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR 30–59 ml/minute/1.73 m2 | 0 | 14 | 6 | 1 | 17 | 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR ≥ 60 ml/minute/1.73 m2 | 0 | 0 | 47 | 0 | 10 | 37 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Snaith et al., 201837 |
300 phlebotomy outpatients attending for routine blood tests; mean age 60 years; 47% female; and mean creatinine concentration of 92 µmol/l UK |
ABL800 FLEX, StatSensor (capillary) and i-STAT (venous) |
Enzymatic (Cobas 8000, Roche Holding AG) | CKD-EPI (and MDRD for comparison) |
After correction of a mean positive bias of 5.6% and alignment to IDMS reference method No further accuracy results were reported for StatSensor 2 Contingency table: i-STAT accuracy at eGFR < 30, 30–44, 45–59 and ≥ 60 ml/minute/1.73 m2 cut-off points Source: correspondence with authorDevice, number of tests (n)i-STATeGFR < 30 ml/minute/1.73 m2eGFR 30–44 ml/minute/1.73 m2eGFR 45–59 ml/minute/1.73 m2eGFR ≥ 60 ml/minute/1.73 m2Laboratory eGFR < 30 ml/minute/1.73 m212000 eGFR 30–44 ml/minute/1.73 m232500 eGFR 45–59 ml/minute/1.73 m205291 eGFR ≥ 60 ml/minute/1.73 m20114210 Contingency table: ABL800 accuracy at eGFR < 30, 30–44, 45–59 and ≥ 60 ml/minute/1.73 m2 cut-off points Source: correspondence with authorDevice, number of tests (n)ABL800eGFR < 30 ml/minute/1.73 m2eGFR 30–44 ml/minute/1.73 m2eGFR 45–59 ml/minute/1.73 m2eGFR ≥ 60 ml/minute/1.73 m2Laboratory eGFR < 30 ml/minute/1.73 m212000 eGFR 30–44 ml/minute/1.73 m202440 eGFR 45–59 ml/minute/1.73 m202312 eGFR ≥ 60 ml/minute/1.73 m2001224 Contingency table: StatSensor accuracy at eGFR < 30, 30–44, 45–59 and ≥ 60 ml/minute/1.73 m2 cut-off points Source: correspondence with authorDevice, number of tests (n)StatSensoreGFR < 30 ml/minute/1.73 m2eGFR 30–44 ml/minute/1.73 m2eGFR 45–59 ml/minute/1.73 m2eGFR ≥ 60 ml/minute/1.73 m2Laboratory eGFR < 30 ml/minute/1.73 m28400 eGFR 30–44 ml/minute/1.73 m231780 eGFR 45–59 ml/minute/1.73 m2010178 eGFR ≥ 60 ml/minute/1.73 m20133191 Test failures occurred four times with StatSensor and once with ABL800 FLEX (none for i-STAT). All 5-second tests were successful |
Source: correspondence with author | Device, number of tests (n) | i-STAT | eGFR < 30 ml/minute/1.73 m2 | eGFR 30–44 ml/minute/1.73 m2 | eGFR 45–59 ml/minute/1.73 m2 | eGFR ≥ 60 ml/minute/1.73 m2 | Laboratory | eGFR < 30 ml/minute/1.73 m2 | 12 | 0 | 0 | 0 | eGFR 30–44 ml/minute/1.73 m2 | 3 | 25 | 0 | 0 | eGFR 45–59 ml/minute/1.73 m2 | 0 | 5 | 29 | 1 | eGFR ≥ 60 ml/minute/1.73 m2 | 0 | 1 | 14 | 210 | Source: correspondence with author | Device, number of tests (n) | ABL800 | eGFR < 30 ml/minute/1.73 m2 | eGFR 30–44 ml/minute/1.73 m2 | eGFR 45–59 ml/minute/1.73 m2 | eGFR ≥ 60 ml/minute/1.73 m2 | Laboratory | eGFR < 30 ml/minute/1.73 m2 | 12 | 0 | 0 | 0 | eGFR 30–44 ml/minute/1.73 m2 | 0 | 24 | 4 | 0 | eGFR 45–59 ml/minute/1.73 m2 | 0 | 2 | 31 | 2 | eGFR ≥ 60 ml/minute/1.73 m2 | 0 | 0 | 1 | 224 | Source: correspondence with author | Device, number of tests (n) | StatSensor | eGFR < 30 ml/minute/1.73 m2 | eGFR 30–44 ml/minute/1.73 m2 | eGFR 45–59 ml/minute/1.73 m2 | eGFR ≥ 60 ml/minute/1.73 m2 | Laboratory | eGFR < 30 ml/minute/1.73 m2 | 8 | 4 | 0 | 0 | eGFR 30–44 ml/minute/1.73 m2 | 3 | 17 | 8 | 0 | eGFR 45–59 ml/minute/1.73 m2 | 0 | 10 | 17 | 8 | eGFR ≥ 60 ml/minute/1.73 m2 | 0 | 1 | 33 | 191 | ||||||||||||||||||||||||||||||
| Source: correspondence with author | Device, number of tests (n) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| i-STAT | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR < 30 ml/minute/1.73 m2 | eGFR 30–44 ml/minute/1.73 m2 | eGFR 45–59 ml/minute/1.73 m2 | eGFR ≥ 60 ml/minute/1.73 m2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Laboratory | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR < 30 ml/minute/1.73 m2 | 12 | 0 | 0 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR 30–44 ml/minute/1.73 m2 | 3 | 25 | 0 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR 45–59 ml/minute/1.73 m2 | 0 | 5 | 29 | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR ≥ 60 ml/minute/1.73 m2 | 0 | 1 | 14 | 210 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Source: correspondence with author | Device, number of tests (n) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ABL800 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR < 30 ml/minute/1.73 m2 | eGFR 30–44 ml/minute/1.73 m2 | eGFR 45–59 ml/minute/1.73 m2 | eGFR ≥ 60 ml/minute/1.73 m2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Laboratory | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR < 30 ml/minute/1.73 m2 | 12 | 0 | 0 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR 30–44 ml/minute/1.73 m2 | 0 | 24 | 4 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR 45–59 ml/minute/1.73 m2 | 0 | 2 | 31 | 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR ≥ 60 ml/minute/1.73 m2 | 0 | 0 | 1 | 224 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Source: correspondence with author | Device, number of tests (n) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| StatSensor | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR < 30 ml/minute/1.73 m2 | eGFR 30–44 ml/minute/1.73 m2 | eGFR 45–59 ml/minute/1.73 m2 | eGFR ≥ 60 ml/minute/1.73 m2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Laboratory | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR < 30 ml/minute/1.73 m2 | 8 | 4 | 0 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR 30–44 ml/minute/1.73 m2 | 3 | 17 | 8 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR 45–59 ml/minute/1.73 m2 | 0 | 10 | 17 | 8 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR ≥ 60 ml/minute/1.73 m2 | 0 | 1 | 33 | 191 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Snaith et al., 201938 |
300 adult outpatients attending for a contrast-enhanced CT scan, mean age 65 years, 48% female UK |
i-STAT (venous) | Enzymatic (Cobas 8000, Roche Holding AG) | CKD-EPI |
Contingency table: i-STAT accuracy at eGFR < 30, 30–44, 45–59 and ≥ 60 ml/minute/1.73 m2 cut-off points Source: correspondence with authorDevice, number of tests (n)i-STATeGFR < 30 ml/minute/1.73 m230–44 eGFR 30–44 ml/minute/1.73 m2eGFR 45–59 ml/minute/1.73 m2eGFR ≥ 60 ml/minute/1.73 m2Laboratory eGFR < 30 ml/minute/1.73 m20000 30–44 eGFR 30–44 ml/minute/1.73 m21940 eGFR 45–59 ml/minute/1.73 m202357 eGFR ≥ 60 ml/minute/1.73 m2017234 Six POC test failures were recorded |
Source: correspondence with author | Device, number of tests (n) | i-STAT | eGFR < 30 ml/minute/1.73 m2 | 30–44 eGFR 30–44 ml/minute/1.73 m2 | eGFR 45–59 ml/minute/1.73 m2 | eGFR ≥ 60 ml/minute/1.73 m2 | Laboratory | eGFR < 30 ml/minute/1.73 m2 | 0 | 0 | 0 | 0 | 30–44 eGFR 30–44 ml/minute/1.73 m2 | 1 | 9 | 4 | 0 | eGFR 45–59 ml/minute/1.73 m2 | 0 | 2 | 35 | 7 | eGFR ≥ 60 ml/minute/1.73 m2 | 0 | 1 | 7 | 234 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Source: correspondence with author | Device, number of tests (n) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| i-STAT | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR < 30 ml/minute/1.73 m2 | 30–44 eGFR 30–44 ml/minute/1.73 m2 | eGFR 45–59 ml/minute/1.73 m2 | eGFR ≥ 60 ml/minute/1.73 m2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Laboratory | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR < 30 ml/minute/1.73 m2 | 0 | 0 | 0 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 30–44 eGFR 30–44 ml/minute/1.73 m2 | 1 | 9 | 4 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR 45–59 ml/minute/1.73 m2 | 0 | 2 | 35 | 7 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| eGFR ≥ 60 ml/minute/1.73 m2 | 0 | 1 | 7 | 234 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
All included studies were observational. The sample size ranged from 50 to 2042 participants. Two studies included outpatients referred for a contrast-enhanced CT scan. 30,38 Two studies included patients undergoing a radiological examination, but did not specify what proportion were outpatients. 27,31 Four studies included disease-specific populations, including people with CKD,36 cancer,33 diabetes mellitus34 or infected with human immunodeficiency virus (HIV). 28 One study focused on women referred for contrast-enhanced spectral mammography. 29 Other studies included phlebotomy outpatients37 and mixed-ancestry South African patients. 32
Three studies were conducted in the USA. 27,31,33 Two studies each were conducted in the UK,37,38 Australia35,36 and South Africa. 28,32 A single study was conducted in the following countries: the Netherlands,29 Japan30 and Mexico. 34 Three studies were reported only as a conference abstract. 27,34,35
Seven studies evaluated i-STAT27,31,33–35,37,38 and seven studies evaluated a StatSensor device. 28–32,36,37 Three studies included Radiometer Ltd’s POC device, including ABL80031,37 and ABL827. 27 Two studies evaluated three POC devices (ABL, i-STAT and StatSensor)31,37 and one study evaluated two devices (ABL and i-STAT). 27 There were no studies of other eligible POC tests, such as ABL90 FLEX PLUS, DRI-CHEM NX 500, epoc and Piccolo Xpress.
All sample types used with StatSensor were capillary,28–30,32,36,37 except in one study (which used a venous sample). 31 Conversely, most i-STAT devices used venous samples31,33,35,37,38 except in one study (which used a capillary sample). 34 Another i-STAT study did not specify the sample type used. 27 None of the studies compared the accuracy of a single device using two different sample types.
Three StatSensor30,31,36 and two i-STAT studies34,35 reported using an offset correction to estimate concordance between the POC test and laboratory reference test derived from the study sample. Adjusted and unadjusted results were reported in all three StatSensor studies, but only adjusted results were presented by the two i-STAT studies.
The laboratory reference method was Jaffe in two studies32,33 and not reported in one study. 34 All other studies used an enzymatic method. Equations used to calculate eGFR varied across the studies, and only three studies used CKD-EPI. 34,37,38
Individual study results, including contingency tables, are presented in Table 6. Eight studies reported sufficient data to calculate accuracy at an eGFR threshold of 30 ml/minute/1.73 m2. 27,29,30,32,33,36–38 Four studies only reported results using higher eGFR thresholds: two studies used an eGFR cut-off point of 60 ml/minute/1.73 m2;31,35 one study used an eGFR threshold of 90 ml/minute/1.73 m2 (although some limited data on an eGFR threshold of 60 ml/minute/1.73 m2 were extractable);28 and one study only reported eGFR results according to a non-standard CKD classification (stages 0–4). 34 Two studies were conference abstracts and did not provide sufficient data to be included in the synthesis. 34,35 Both studies evaluated i-STAT and reported accuracy results following an offset correction.
Shephard et al. 35 compared the accuracy of i-STAT against an enzymatic method using 101 venous blood samples. After correction of a mean positive bias of 5.6% and alignment to the IDMS reference method, i-STAT had 96% sensitivity and 96% specificity for an eGFR threshold of 60 ml/minute/1.73 m2 compared with the laboratory reference test.
Obrador et al. 34 evaluated the accuracy of i-STAT in 257 diabetic patients. Concordance with the laboratory reference test was evaluated according to a CKD classification ranging from 0 to 4, with 0 indicating no CKD. No further details were provided on the CKD classification; therefore, it is not clear how these results compare to the standard Kidney Disease Improving Global Outcomes (KDIGO) classification, as presented in Table 2. The study used a simple linear regression to estimate a correction factor to align i-STAT SCr to IDMS SCr. After this correction, the Obrador et al. 3 study found that all patients with CKD (stages 1–4) were correctly classified by the POC test (100% sensitivity) and all but one were correctly classified as CKD free (99.4% specificity).
Available data for quantitative synthesis
Studies of StatSensor devices
Data from the seven studies28–32,36,37 included in the analysis for StatSensor devices are given in Appendix 2 (see Table 42). One study28 provided limited data on only one individual with an eGFR of < 60 ml/minute/1.73 m2 who was correctly classified by StatSensor Xpress-i, but no other data on individuals in other eGFR categories. For StatSensor-I, one study30 reported data on collapsed categories of eGFR and another reported data on all eGFR categories. 32 The StatSensor device was compared in four studies,29,31,36,37 one of which37 reported data on all eGFR categories in Table 2.
Two studies31,36 of StatSensor devices included a user-specified adjustment (see Table 1) to correct for systematic measurement bias. A third study30 reported data using an alternative adjustment that cannot be applied directly to the device. A possible scenario for use of this device in clinical practice is to identify whether or not there is a systematic bias in device performance and then incorporate an adjustment into the device, to correct subsequent samples. To assess the performance of StatSensor under this scenario, an additional ‘adjusted data’ analysis was carried out, in which the reported adjusted data from Korpi-Steiner et al. 31 and Shephard et al. 36 were used. However, Inoue et al. 30 was removed, as bias was identified but the correction was not one that could be implemented in practice.
Studies of i-STAT devices
Data from the five studies27,31,33,37,38 included in the analysis for i-STAT devices are given in Appendix 2 (see Table 40). All studies presented results for the i-STAT device, except for Botz et al. ,27 which provided limited data on individuals with an eGFR of < 30 ml/minute/1.73 m2 and their classification using i-STAT1. Two studies37,38 reported data on all eGFR categories, although Snaith et al. 38 did not observe any individuals with an eGFR of < 30 ml/minute/1.73 m2.
Studies of ABL series devices
Data from the three studies27,31,37 included in the analysis for ABL (Radiometer Ltd) devices are given in Appendix 2 (see Table 41). Two types of device were compared: ABL800 FLEX31,37 and ABL827. 27 Only one study provided data on all eGFR categories. 37
Studies calculating estimated glomerular filtration rate using Chronic Kidney Disease Epidemiology Collaboration
All studies used the MDRD equation to calculate eGFR except for two, which used CKD-EPI. 37,38 The first of these studies included StatSensor, i-STAT and ABL800 FLEX devices37 and the second study included only the i-STAT device. 38 In addition, these two studies37,38 were the only ones rated as being at low risk of bias and with applicability concerns (see Table 3). An additional analysis using only the data in these two studies was carried out to check for any differences in classification accuracy. Although only one study included a StatSensor or ABL device, in order to properly quantify the uncertainty in the probabilities, the model described in Synthesis of diagnostic accuracy data (see Equations 1 and 2) was still used.
Results: assessment of diagnostic accuracy
Convergence was achieved for all synthesis models at (or before) 5000 iterations. A further 30,000 iterations on two chains were run; therefore, all results are based on 60,000 post-convergence iterations.
Probability of belonging to each category
The probabilities that an individual belongs to each eGFR category in Table 2 were calculated from the number of individuals in each category reported by all included studies (i.e. regardless of the device being evaluated, one study reporting results on two sets of patients27). The probabilities reported in each study are given in Table 7 (raw data in Tables 39–41). The pooled probabilities of belonging to each of the four categories of interest, T[j], j = 1,2,3,4, used in the main synthesis model are given in Table 8.
| Laboratory eGFR category (ml/minute/1.73 m2) | Study (author and year of publication), probability | ||
|---|---|---|---|
| Snaith et al., 201837 | Snaith et al., 201938 | Krige 201732 | |
| < 30 | 0.040 | 0.000 | 0.010 |
| 30–44 | 0.093 | 0.047 | 0.000 |
| 45–59 | 0.117 | 0.147 | 0.019 |
| ≥ 60 | 0.750 | 0.807 | 0.971 |
| Inoue et al., 201730 | Houben et al., 201729 | ||
| < 30 | 0.033 | 0.000 | |
| 30–44 | 0.065 | 0.009 | |
| ≥ 45 | 0.902 | 0.991 | |
| Shephard et al., 201036 | Botz et al., 2013 (ABL)27 | ||
| < 30 | 0.330 | 0.009 | |
| 30–59 | 0.200 | 0.208 | |
| ≥ 60 | 0.470 | 0.783 | |
| Botz et al., 2013 (i-STAT)27 | |||
| < 30 | 0.007 | ||
| ≥ 30 | 0.993 | ||
| Korpi-Steiner et al., 200931 | Dorward et al., 201828 | Nichols et al., 200733 | |
| < 60 | 0.256 | 0.005 | 0.184 |
| ≥ 60 | 0.744 | 0.995 | 0.816 |
| Probability | Estimated probability | |||
|---|---|---|---|---|
| All data | Shephard et al., 201036 removed | |||
| Median | 95% CrI | Median | 95% CrI | |
| T[1] | 0.014 | 0.011 to 0.017 | 0.009 | 0.007 to 0.012 |
| T[2] | 0.051 | 0.039 to 0.064 | 0.051 | 0.039 to 0.064 |
| T[3] | 0.143 | 0.127 to 0.159 | 0.143 | 0.127 to 0.159 |
| T[4] | 0.792 | 0.780 to 0.803 | 0.797 | 0.785 to 0.808 |
Most studies included a few individuals in category 1 (i.e. an eGFR of < 30 ml/minute/1.73 m2) and more individuals in higher eGFR categories. However, Shephard et al. 36 included a majority of renal patients and, therefore, individuals had a higher probability of being in category 1, than those in other included studies (33% compared with 0–4%). Excluding Shephard et al. 36 reduced the pooled probability of patients being in category 1, T[1], slightly but hardly impacted the other probabilities (Table 8). A sensitivity analysis was conducted to assess how this affected the estimation of the main probabilities of interest (see Sensitivity analysis for true probability calculations).
Probability of classification by point-of-care device, given a laboratory-defined category
The pooled probabilities of being classified by a POC device in category k, given the laboratory classification j, pjk = p[j,k], with j,k = 1,2,3,4, are given in Table 9 and plotted as density strips in Figure 2.
| Probability | Device, pooled probabilitity | |||||
|---|---|---|---|---|---|---|
| StatSensor | i-STAT | ABL | ||||
| Median | 95% CrI | Median | 95% CrI | Median | 95% CrI | |
| p[1,1] | 0.74 | 0.61 to 0.85 | 0.85 | 0.69 to 0.94 | 0.87 | 0.75 to 0.95 |
| p[1,2] | 0.18 | 0.08 to 0.30 | 0.04 | 0.00 to 0.18 | 0.03 | 0.00 to 0.14 |
| p[1,3] | 0.03 | 0.00 to 0.12 | 0.04 | 0.00 to 0.18 | 0.03 | 0.00 to 0.14 |
| p[1,4] | 0.04 | 0.01 to 0.11 | 0.04 | 0.00 to 0.16 | 0.04 | 0.00 to 0.15 |
| p[2,1] | 0.09 | 0.03 to 0.19 | 0.10 | 0.04 to 0.21 | 0.02 | 0.00 to 0.11 |
| p[2,2] | 0.57 | 0.42 to 0.71 | 0.77 | 0.64 to 0.87 | 0.78 | 0.61 to 0.90 |
| p[2,3] | 0.22 | 0.12 to 0.36 | 0.10 | 0.04 to 0.21 | 0.15 | 0.05 to 0.29 |
| p[2,4] | 0.10 | 0.03 to 0.24 | 0.01 | 0.00 to 0.06 | 0.03 | 0.00 to 0.15 |
| p[3,1] | 0.01 | 0.00 to 0.03 | 0.01 | 0.00 to 0.05 | 0.02 | 0.00 to 0.08 |
| p[3,2] | 0.14 | 0.09 to 0.20 | 0.10 | 0.04 to 0.17 | 0.06 | 0.01 to 0.16 |
| p[3,3] | 0.25 | 0.16 to 0.34 | 0.81 | 0.72 to 0.88 | 0.74 | 0.62 to 0.85 |
| p[3,4] | 0.60 | 0.51 to 0.69 | 0.08 | 0.04 to 0.13 | 0.17 | 0.09 to 0.26 |
| p[4,1] | 0.00 | 0.00 to 0.01 | 0.00 | 0.00 to 0.01 | 0.00 | 0.00 to 0.01 |
| p[4,2] | 0.00 | 0.00 to 0.01 | 0.01 | 0.00 to 0.02 | 0.00 | 0.00 to 0.01 |
| p[4,3] | 0.06 | 0.04 to 0.08 | 0.08 | 0.06 to 0.10 | 0.01 | 0.00 to 0.01 |
| p[4,4] | 0.94 | 0.91 to 0.95 | 0.91 | 0.89 to 0.93 | 0.99 | 0.98 to 0.99 |
FIGURE 2.
Density strips for classification probabilities for each device with vertical lines defining the median and 95% CrI. (a) StatSensor; (b) i-Stat; and (c) ABL. p[j,k], probabilities of being classified in category k, given a laboratory classification j. Categories are described in Table 2.
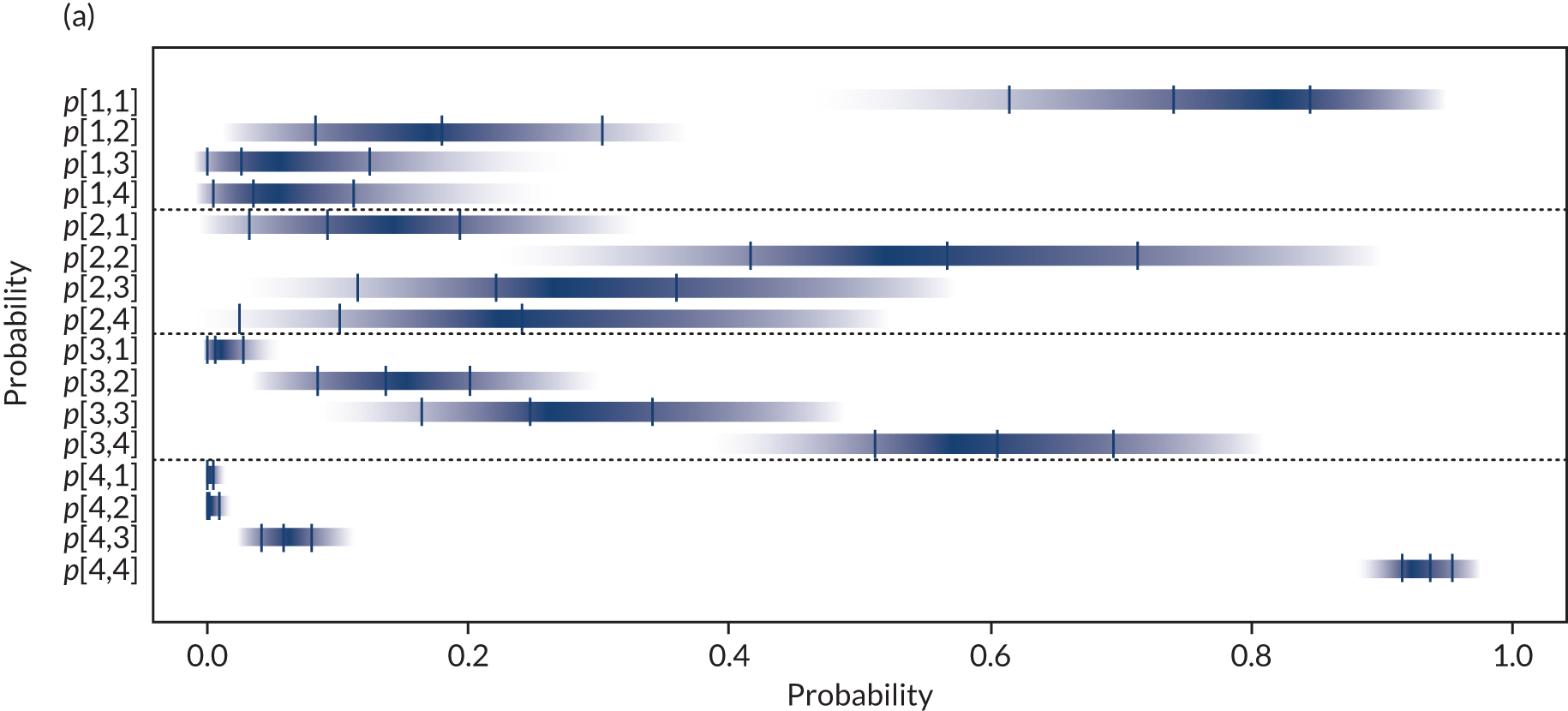
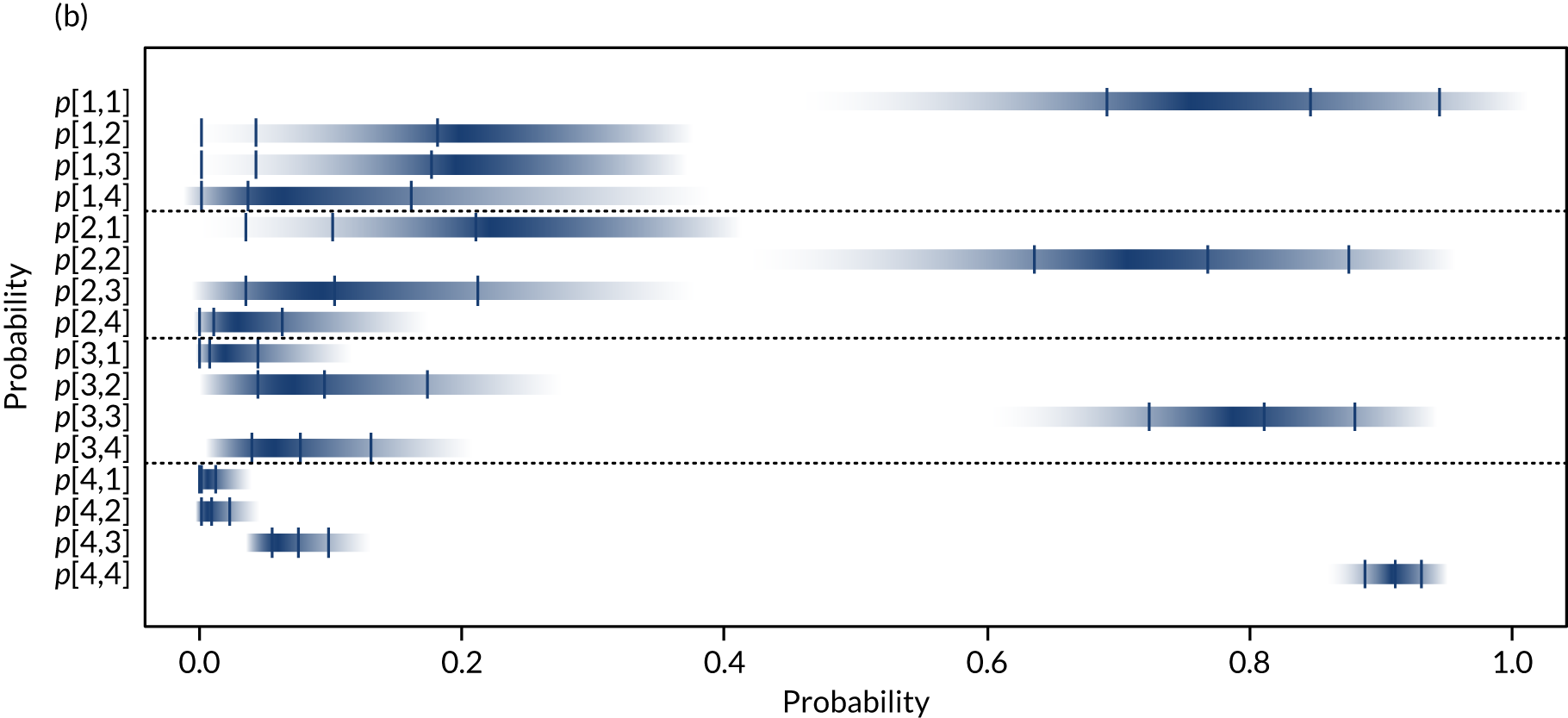
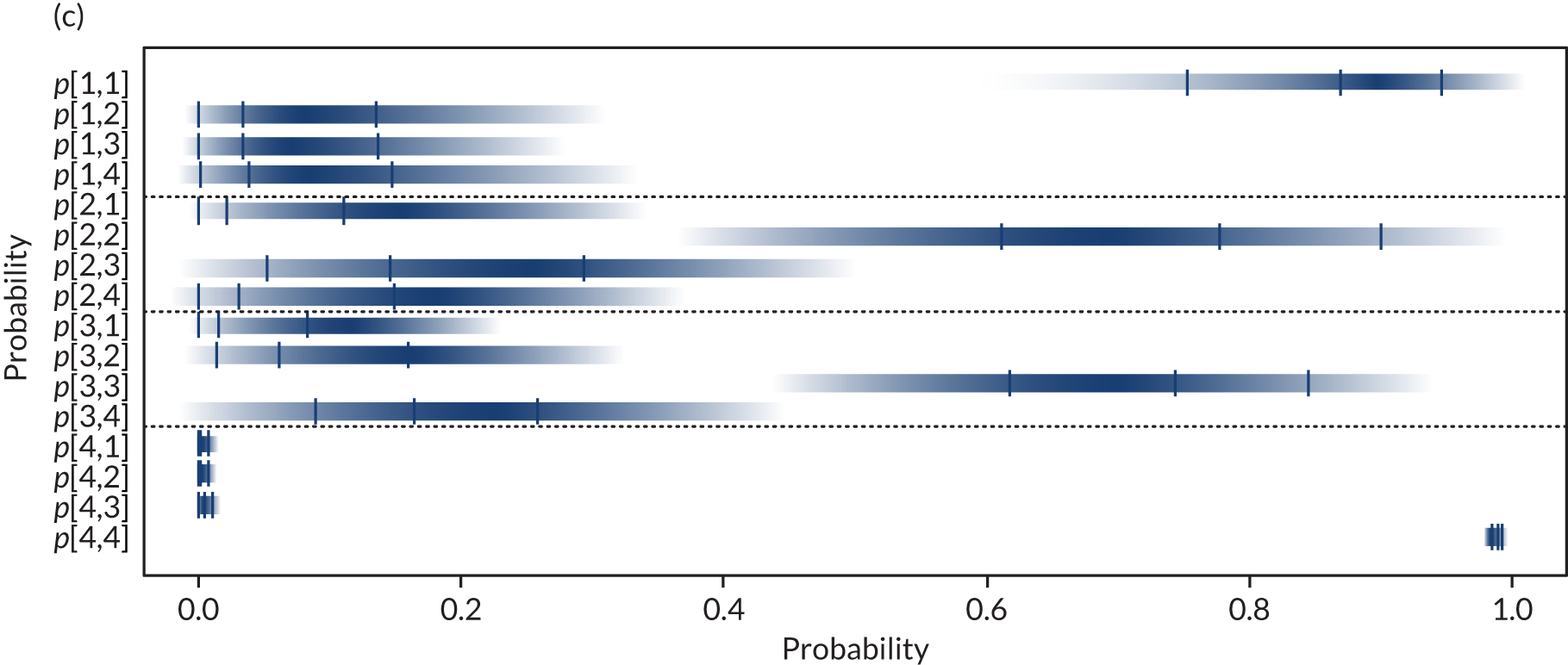
The i-STAT and ABL devices have higher median probabilities of correct classification in each of the three lowest categories (i.e. p[1,1], p[2,2], p[3,3]) than the StatSensor, with StatSensor appearing particularly poor at correctly classifying individuals in category 3 (i.e. individuals with an eGFR of 45–59 ml/minute/1.73 m2). However, there is considerable uncertainty in these probabilities for all devices.
The median probabilities of being correctly classified as being at risk of PC-AKI (i.e. defined as an eGFR of < 30 ml/minute/1.73 m2, sensitivity) using i-STAT or ABL devices are similar (85% and 87%, respectively), whereas for StatSensor devices this median probability is lower (74%). The median probability of being incorrectly classified as being at risk of PC-AKI by the POC device for individuals with an eGFR of 30–44 ml/minute/1.73 m2 ranges from 2% for ABL devices to 9–10% for StatSensor and i-STAT devices; however, there is some uncertainty around these values. The probabilities of being incorrectly classified as at risk reduce considerably for individuals with an eGFR ≥ 45 ml/minute/1.73 m2.
Additional analyses
Two (non-prespecified) additional analyses were conducted: one using adjusted data for StatSensor devices and a second using data only from studies using the CKD-EPI equation to calculate eGFR (see Available data for quantitative synthesis).
StatSensor-adjusted data analysis
Adjusted data reported by Korpi-Steiner et al. 31 and Shephard et al. 36 are given in Table 42 (in Appendix 2). The pooled probabilities for StatSensor obtained using these adjusted data and removing Inoue et al. 30 data are given in Table 10. Figure 3 presents density strips for the probabilities obtained for StatSensor in the main analysis (dark blue, wide), and using the adjusted data (light blue, narrow).
| Probability | Pooled probability | |
|---|---|---|
| Median | 95% CrI | |
| p[1,1] | 0.84 | 0.73 to 0.93 |
| p[1,2] | 0.11 | 0.04 to 0.22 |
| p[1,3] | 0.02 | 0.00 to 0.08 |
| p[1,4] | 0.01 | 0.00 to 0.08 |
| p[2,1] | 0.11 | 0.04 to 0.22 |
| p[2,2] | 0.51 | 0.35 to 0.67 |
| p[2,3] | 0.28 | 0.15 to 0.44 |
| p[2,4] | 0.09 | 0.02 to 0.22 |
| p[3,1] | 0.01 | 0.00 to 0.04 |
| p[3,2] | 0.12 | 0.06 to 0.20 |
| p[3,3] | 0.49 | 0.37 to 0.60 |
| p[3,4] | 0.38 | 0.28 to 0.49 |
| p[4,1] | 0.00 | 0.00 to 0.01 |
| p[4,2] | 0.00 | 0.00 to 0.01 |
| p[4,3] | 0.12 | 0.09 to 0.14 |
| p[4,4] | 0.88 | 0.85 to 0.90 |
There is good overlap of the 95% CrIs for classifications of individuals with true eGFRs in the first two categories, although the adjusted analysis gives a higher probability that individuals are correctly classified as being at risk of PC-AKI (sensitivity) (p[1,1] median is 84%, in Table 10, compared with 74% in the unadjusted analysis, in Table 9).
However, there is conflict between results from the adjusted data analysis and the main analysis for categories 3 and 4, particularly for estimated probabilities p[3,3], p[3,4], p[4,3] and p[4,4]. The main analysis suggests a lower probability of correctly classifying individuals in category 3, but a higher probability of correctly classifying individuals in category 4, than in the adjusted data analysis. In addition, the main analysis suggests that individuals in category 3 have a lower probability of being classified as belonging to this category than to category 4, whereas this is not the case in the adjusted analysis.
Including only studies using Chronic Kidney Disease Epidemiology Collaboration equation
The pooled probabilities of being classified by POC device in category k, given a laboratory classification in category j, pjk = p[j,k], with j,k = 1,2,3,4, for StatSensor and ABL800 FLEX estimated from data from the only study that used the CKD-EPI equation,37 and for i-STAT using data from the two studies37,38 that used the CKD-EPI equation, are presented in Table 11.
| Probability | Device, pooled probabilitity | |||||
|---|---|---|---|---|---|---|
| StatSensor | i-STAT | ABL800 FLEX | ||||
| Median | 95% CrI | Median | 95% CrI | Median | 95% CrI | |
| p[1,1] | 0.56 | 0.32 to 0.79 | 0.83 | 0.60 to 0.96 | 0.83 | 0.60 to 0.96 |
| p[1,2] | 0.31 | 0.12 to 0.55 | 0.05 | 0.00 to 0.22 | 0.05 | 0.00 to 0.22 |
| p[1,3] | 0.05 | 0.00 to 0.22 | 0.05 | 0.00 to 0.22 | 0.04 | 0.00 to 0.22 |
| p[1,4] | 0.05 | 0.00 to 0.22 | 0.05 | 0.00 to 0.22 | 0.05 | 0.00 to 0.22 |
| p[2,1] | 0.12 | 0.04 to 0.26 | 0.10 | 0.04 to 0.21 | 0.02 | 0.00 to 0.11 |
| p[2,2] | 0.56 | 0.39 to 0.73 | 0.76 | 0.63 to 0.87 | 0.79 | 0.63 to 0.90 |
| p[2,3] | 0.28 | 0.14 to 0.45 | 0.10 | 0.04 to 0.21 | 0.15 | 0.05 to 0.30 |
| p[2,4] | 0.02 | 0.00 to 0.11 | 0.02 | 0.00 to 0.08 | 0.02 | 0.00 to 0.11 |
| p[3,1] | 0.02 | 0.00 to 0.09 | 0.01 | 0.00 to 0.04 | 0.02 | 0.00 to 0.09 |
| p[3,2] | 0.28 | 0.15 to 0.43 | 0.09 | 0.04 to 0.17 | 0.07 | 0.02 to 0.18 |
| p[3,3] | 0.46 | 0.31 to 0.62 | 0.79 | 0.69 to 0.86 | 0.83 | 0.69 to 0.92 |
| p[3,4] | 0.23 | 0.11 to 0.37 | 0.11 | 0.05 to 0.18 | 0.07 | 0.02 to 0.18 |
| p[4,1] | 0.00 | 0.00 to 0.02 | 0.00 | 0.00 to 0.01 | 0.00 | 0.00 to 0.02 |
| p[4,2] | 0.01 | 0.00 to 0.02 | 0.01 | 0.00 to 0.02 | 0.00 | 0.00 to 0.02 |
| p[4,3] | 0.15 | 0.11 to 0.20 | 0.05 | 0.03 to 0.07 | 0.01 | 0.00 to 0.02 |
| p[4,4] | 0.84 | 0.79 to 0.88 | 0.95 | 0.92 to 0.96 | 0.98 | 0.96 to 1.00 |
StatSensor results
Figure 3 presents density strips for the probabilities obtained for StatSensor using only the CKD-EPI data (orange, narrow). These results broadly agree with the adjusted data analysis (light blue, narrow), although uncertainty in the probabilities for an eGFR < 30 ml/minute/1.73 m2 is larger in the CKD-EPI analysis as only one study37 is used with only a few individuals in this category.
FIGURE 3.
StatSensor: density strips for classification probabilities for the main analysis (dark blue, wide), the adjusted data analysis (light blue, narrow) and the analysis including only CKD-EPI data (orange, narrow). Vertical lines define the medians and 95% CrIs. p[j,k] is the probability of being classified by the POC device in eGFR category k, given laboratory classification in category j. Categories are described in Table 2.
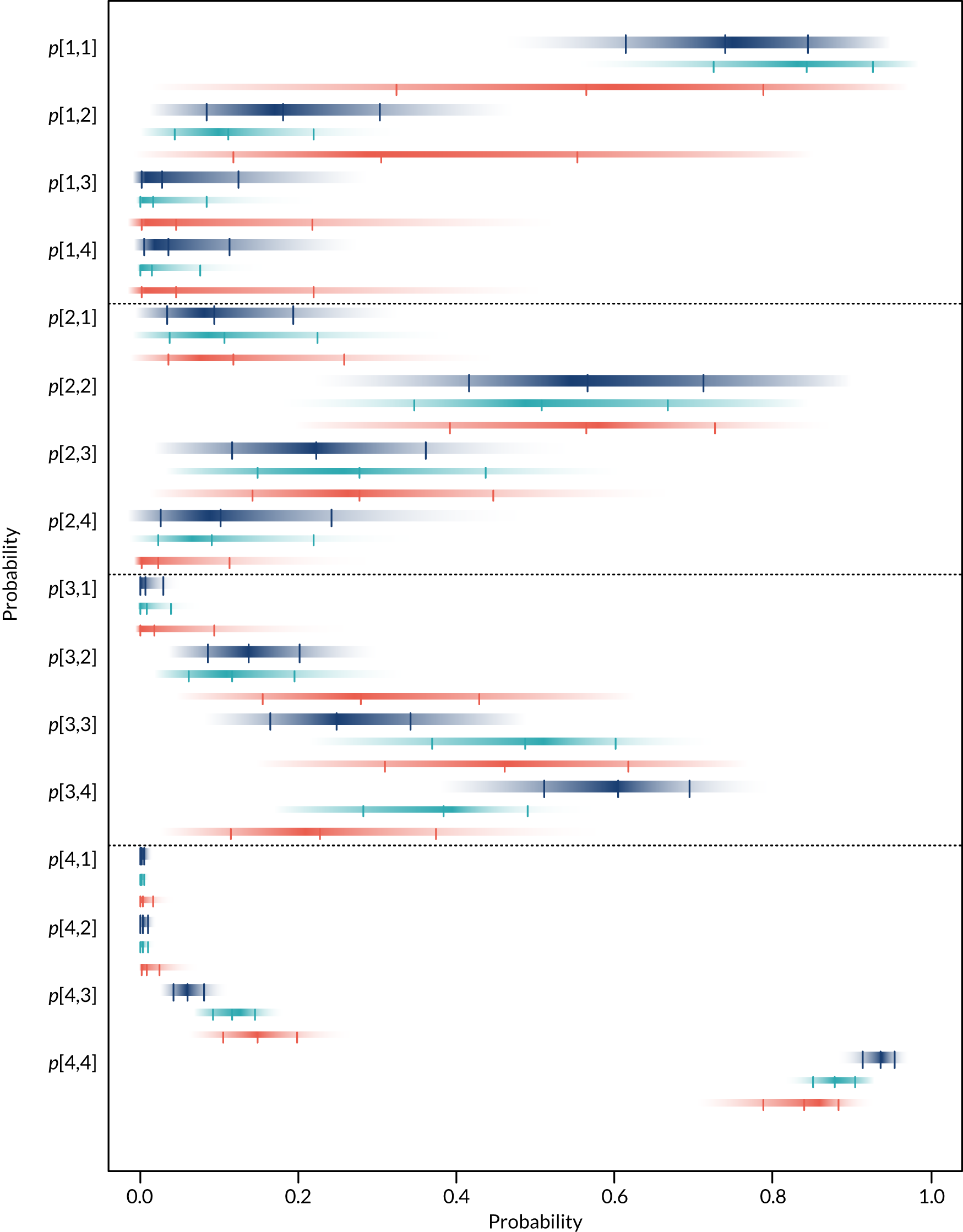
i-STAT results
Figure 4 presents density strips for the probabilities obtained for i-STAT in the main analysis (dark blue, wide) and the analysis using only the CKD-EPI data37,38 (orange, narrow). There is good overlap of all density strips, with the main analysis producing slightly more precise results.
FIGURE 4.
i-STAT: density strips for classification probabilities for the main analysis (dark blue, wide) and the sensitivity analysis including only CKD-EPI data (orange, narrow). Vertical lines define the medians and 95% CrIs. p[j,k] is the probability of being classified by the POC device in eGFR category k, given laboratory classification in category j. Categories are described in Table 2.
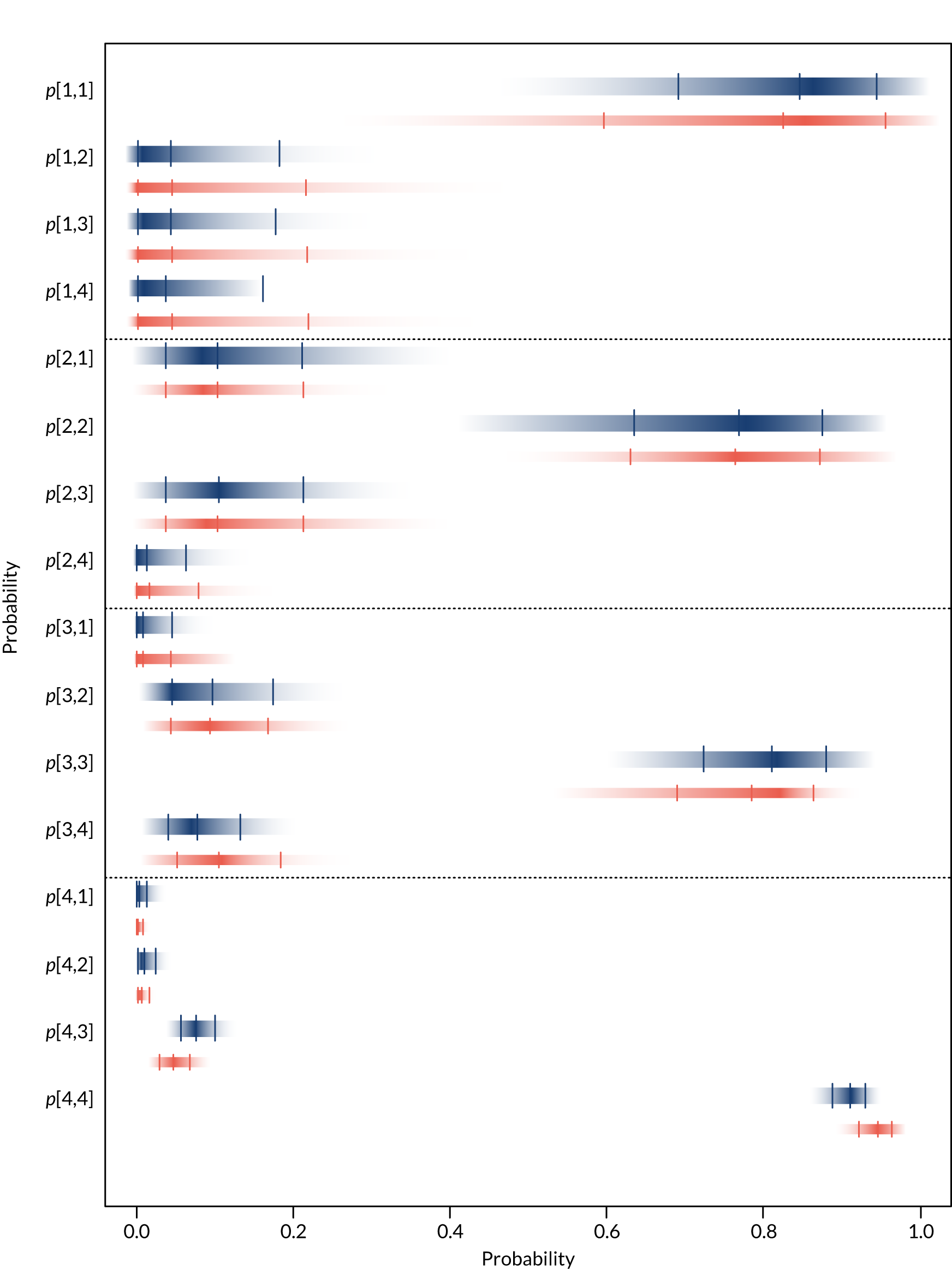
ABL results
Figure 5 presents density strips for the probabilities obtained for ABL devices in the main analysis (dark blue, wide) and the analysis using only the CKD-EPI data37 (orange, narrow). There is good overlap of all density strips, with the main analysis producing slightly more precise results, particularly for the probabilities of being correctly classified as at risk of PC-AKI (eGFR < 30 ml/minute/1.73 m2).
FIGURE 5.
ABL800 FLEX: density strips for classification probabilities for the main analysis (dark blue, wide) and the sensitivity analysis including only data from Snaith et al. 78 (orange, narrow). Vertical lines define the medians and 95% CrIs. p[j,k] is the probability of being classified by the POC device in eGFR category k, given laboratory classification in category j. Categories are described in Table 2.
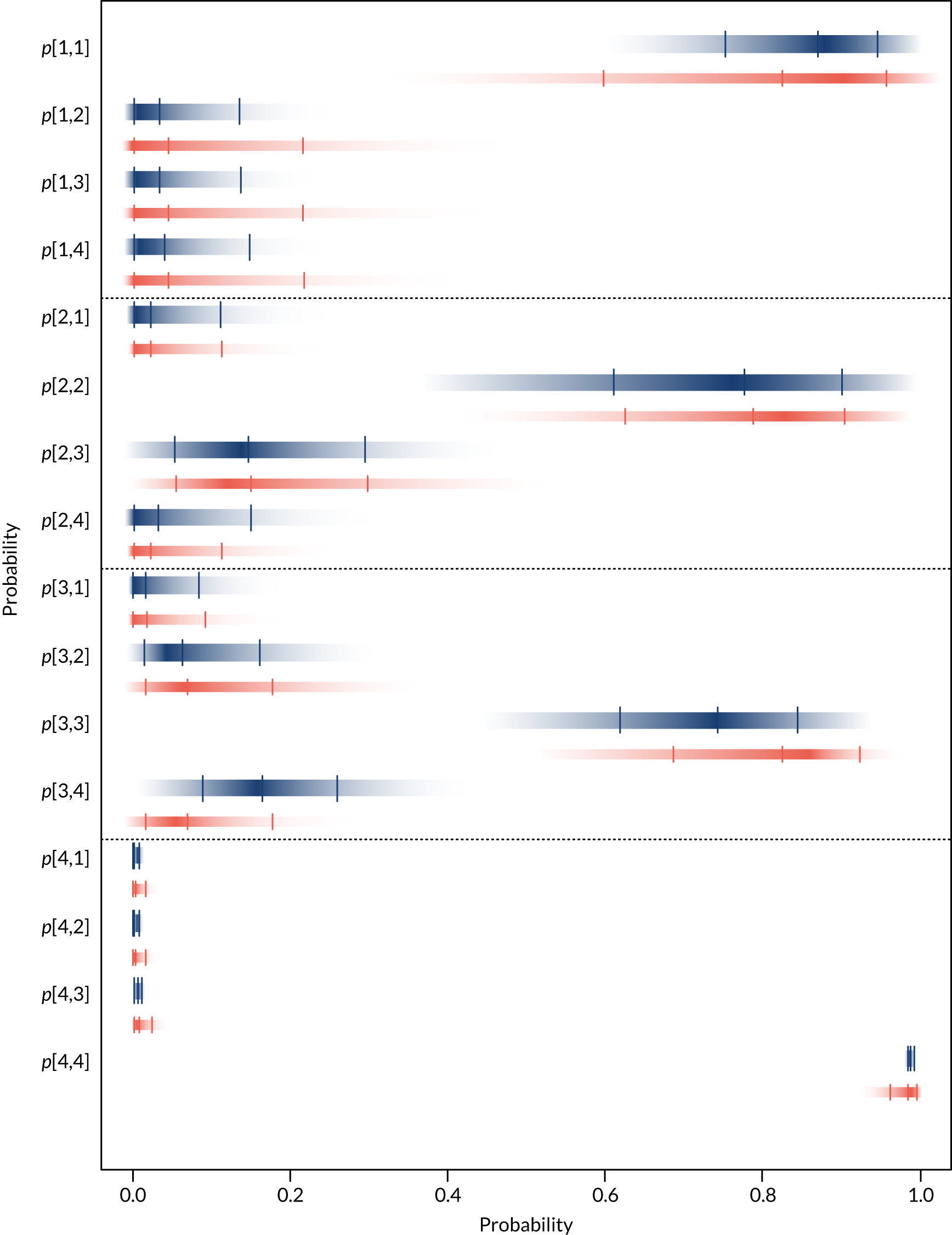
Sensitivity analysis for true probability calculations
To assess the impact of using different values of T[j] (see Table 8) in the model for the probabilities of interest, pjk, a sensitivity analysis was conducted for each device with Shephard et al. 36 removed from the calculation of the T[j] (but retained in the StatSensor synthesis of pj). The resulting probabilities are reported in Table 12 and are very similar to those reported in the main analysis (see Table 9).
| Probability | Device, pooled probability | |||||
|---|---|---|---|---|---|---|
| StatSensor | i-STAT | ABL | ||||
| Median | 95% CrI | Median | 95% CrI | Median | 95% CrI | |
| p[1,1] | 0.74 | 0.61 to 0.85 | 0.84 | 0.69 to 0.94 | 0.87 | 0.75 to 0.95 |
| p[1,2] | 0.18 | 0.08 to 0.30 | 0.04 | 0.00 to 0.18 | 0.03 | 0.00 to 0.14 |
| p[1,3] | 0.03 | 0.00 to 0.13 | 0.04 | 0.00 to 0.18 | 0.03 | 0.00 to 0.14 |
| p[1,4] | 0.03 | 0.00 to 0.11 | 0.04 | 0.00 to 0.17 | 0.04 | 0.00 to 0.15 |
| p[2,1] | 0.09 | 0.03 to 0.19 | 0.10 | 0.04 to 0.21 | 0.02 | 0.00 to 0.11 |
| p[2,2] | 0.57 | 0.41 to 0.71 | 0.77 | 0.64 to 0.87 | 0.78 | 0.61 to 0.90 |
| p[2,3] | 0.22 | 0.12 to 0.36 | 0.10 | 0.04 to 0.21 | 0.15 | 0.05 to 0.30 |
| p[2,4] | 0.10 | 0.03 to 0.24 | 0.01 | 0.00 to 0.06 | 0.03 | 0.00 to 0.15 |
| p[3,1] | 0.01 | 0.00 to 0.03 | 0.01 | 0.00 to 0.04 | 0.02 | 0.00 to 0.08 |
| p[3,2] | 0.14 | 0.09 to 0.20 | 0.10 | 0.05 to 0.17 | 0.06 | 0.02 to 0.16 |
| p[3,3] | 0.25 | 0.16 to 0.34 | 0.81 | 0.72 to 0.88 | 0.74 | 0.62 to 0.84 |
| p[3,4] | 0.60 | 0.51 to 0.69 | 0.08 | 0.04 to 0.13 | 0.16 | 0.09 to 0.26 |
| p[4,1] | 0.00 | 0.00 to 0.01 | 0.00 | 0.00 to 0.01 | 0.00 | 0.00 to 0.01 |
| p[4,2] | 0.00 | 0.00 to 0.01 | 0.01 | 0.00 to 0.02 | 0.00 | 0.00 to 0.01 |
| p[4,3] | 0.06 | 0.04 to 0.08 | 0.08 | 0.06 to 0.10 | 0.01 | 0.00 to 0.01 |
| p[4,4] | 0.94 | 0.91 to 0.95 | 0.91 | 0.89 to 0.93 | 0.99 | 0.98 to 0.99 |
Summary
Data on the classification of individuals according to their PC-AKI risk by POC devices compared with a laboratory reference test were pooled to estimate the probabilities that individuals are correctly or incorrectly classified into one of the four eGFR categories used to determine PC-AKI risk. Results suggest that i-STAT and ABL devices are better than StatSensor devices at correctly categorising individuals, particularly for the lower eGFR categories: StatSensor was less accurate at correctly classifying patients with true eGFRs < 30 ml/minute/1.73 m2 (i.e. lower sensitivity).
The StatSensor device can incorporate an adjustment to better align results with those of the reference laboratory test. An additional analysis using adjusted data improved this device’s classification of individuals with low eGFRs, although there were still larger probabilities of misclassification at higher eGFR values than for the other devices.
Analyses that included studies that only measured eGFR with the CKD-EPI equation showed that the results were consistent and robust for i-STAT and ABL, whereas results for StatSensor showed some differences. Overall, results suggest that i-STAT and ABL devices show better agreement with the reference laboratory test in the classification of individuals’ eGFR, particularly for the lower categories, which are of greatest clinical importance.
Studies reporting clinical, workflow or implementation outcomes
Six studies reported clinical, workflow or implementation outcomes relating to POC devices (Table 13). 29,59,62,77–79 One study was available only as a conference abstract. 79 Patient sample sizes ranged from 113 to 3087 and one study was a survey of staff at 68 NHS trust sites. 78 Any POC device was eligible to be included in this section of the review: three studies used StatSensor,29,62,79 one used an i-STAT device59 and one used a Reflotron® Plus (Roche Holding AG, Basel, Switzerland) POC device (and a screening questionnaire). 77
| Study (author and year of publication) | Population (N) and country | Device(s) | eGFR equation | Results and notes |
|---|---|---|---|---|
| Houben et al., 201729 |
351 women due for contrast-enhanced spectral mammography The Netherlands |
POC device(s) | MDRD | Seven patients had an eGFR < 60 ml/minute/1.73 m2, necessitating additional preparation prior to contrast delivery. The POC device failed to categorise six of these seven patients (86%), leading to unwanted contrast administration. Two patients (including one of the three patients with an eGFR of 45 ml/minute/1.73 m2) subsequently developed CIN after 2–5 days, which was normalised after 30 days |
| Ledermann et al., 201077 |
796 of 1766 patients scheduled for contrast-enhanced CT with at least 1 ESUR risk factor for renal insufficiency; 55% female; and mean age 61 years Switzerland |
Reflotron plus and screening questionnaire | MDRD (Levey modified) | The diagnostic procedure was adapted in 132 patients (16.6%): 85 (10.7%) had a contrast dose reduction, 40 (5.0%) had CT without contrast, three (0.38%) had MRI scanning and four (0.5%) had scintigraphy |
| Lee-Lewandrowski et al., 201259 |
3087 patients were referred for contrast-enhanced scan (CT or MRI) without a recent eGFR USA |
i-STAT | MDRD | 1-month audit: 285 (74%) of 384 patients referred for CT/MRI had a normal eGFR and could undergo a scan with contrast. Of the 99 patients (26%) with an abnormal eGFR (< 60 ml/minute/1.73 m2), 73 (74%) received a scan with contrast and 26 (26.3%) without contrast |
| Morita et al., 201162 |
113 patients scheduled for CT or MRI without a recent eGFR measurement Japan |
StatSensor | Modified Japanese Society of Nephrology–Chronic Kidney Disease Initiatives |
Seven patients with an eGFR of 30–50 ml/minute/1.73 m2 underwent i.v. hydration No symptoms of PC-AKI were observed [the median follow-up period from the examination day was 94 days (range 2–248 days)] Test failures in 10 patients (8.8%), of which six were due to ‘flow errors’, although measurements were successfully made at the second attempt |
| Snaith et al., 201678 |
Survey of NHS trusts sites; and 68 out of 174 responded (39%) UK |
NA | NA | 26 sites had considered using POC technology. Six sites indicated POC tests would be carried out if a result was unavailable. POC was in regular use at a further two sites and was currently being evaluated at another six. The remaining 12 sites had rejected POC technology as an adjunct, mostly for cost reasons. Other reasons for rejected POC technology included a lack of support from pathology, reliability and accuracy of the equipment and incompatibility with pathology measures. Three sites also raised concerns that the immediacy of a POC result could lead to a reduction in imaging capacity (e.g. lost slot) |
| aStahr et al., 201079 |
360 patients in a PET/CT unit Denmark |
StatSensor | NR | Before-and-after (introduction of StatSensor) comparison of scans performed with and without i.v. contrast:
|
In Lee-Lewandrowski et al. ’s59 US study, an average of 5.3% of patients presented for a CT or magnetic resonance imaging (MRI) requiring a contrast agent, but without a recent creatinine or eGFR result. A 1-month audit of these patients (n = 384) found that the i-STAT POC device identified 74% of patients as having normal results (defined as an eGFR ≥ 60 ml/minute/1.73 m2), with the CT/MRI study proceeding as planned. Of the patients with an abnormal eGFR (i.e. an eGFR of < 60 ml/minute/1.73 m2), 74% of scans were performed with contrast and 26% without contrast. The authors commented that the decision to use contrast agents in patients with abnormal eGFRs considered the type of study being performed (vascular vs. non-vascular) and an assessment of the overall risk/benefit of administering or not administering a contrast agent. Houben et al. 29 also used an eGFR threshold of < 60 ml/minute/1.73 m2 for identifying abnormal results, with StatSensor failing to identify six of the seven patients with abnormal results as measured in the laboratory reference test. This resulted in unwanted contrast agent administration. Two patients subsequently developed PC-AKI after 2–5 days, which was normalised after 30 days.
Ledermann et al. 77 studied 1766 patients referred for contrast-enhanced CT at a private Swiss radiology facility. Only 3.5% of patients had external SCr values on their referral forms (as was requested). A Reflotron POC device was used on patients who had risk factors for PC-AKI (identified using a questionnaire). No fixed eGFR threshold on which to base decisions was adopted; although 116 the 796 patients with a risk factor had a POC-measured eGFR of < 60 ml/minute/1.73 m2, the diagnostic procedure was modified in 132 patients. The most frequently adopted changes in the management of these 132 patients was a reduction in contrast agent volume (in 64% of patients) and CT scanning performed without a contrast agent (30%). Morita et al. 62 studied the effect of using a StatSensor device on 113 Japanese patients awaiting CT or MRI examinations who did not have a recent eGFR. 62 Twenty-one patients had an eGFR of < 60 ml/minute/1.73 m2. The seven patients who had an eGFR of 30–50 ml/minute/1.73 m2 underwent IVH with 500 ml of saline.
Snaith et al. 78 considered implementation issues in a survey that examined adherence of UK hospitals to guidance on the use of gadolinium-based contrast agents in MRI; the risk of nephrogenic systemic fibrosis is elevated in patients with impaired renal function. Six out of 68 sites indicated that POC creatinine testing would be carried out where recent blood test results were unavailable. Twelve sites had rejected using a POC device as an adjunct, mostly for cost reasons.
Stahr et al. ’s79 study reported the proportion of scans involving intravenous contrast agent before and after the introduction of a StatSensor device. However, the results are limited by the study design used, the small sample size and the details reported (it was available only as a conference abstract).
Together, the results of these studies illustrate variation in practice both in terms of the proportions of patients who do not have a recent eGFR result and in the management decisions taken when a POC device indicates an ‘abnormal’ eGFR. However, many of these studies were undertaken several years ago, so the value of their results is somewhat limited because the eGFR thresholds for defining an abnormal result have decreased over time.
Pragmatic reviews of further evidence to inform the economic model
Evidence of the risk of acute kidney injury from contrast agents
Patients who need contrast-based imaging sometimes have other risk factors for AKI that make it difficult to ascribe a causative role to contrast agents. Determining the true incidence of CI-AKI from the published literature can be difficult as many studies do not include a control group of patients not receiving contrast agents. Such studies will probably also include kidney injuries unrelated to contrast agents. Another important issue when considering the risk of kidney injury following administration of contrast agents is the outcomes being evaluated. AKI is typically defined as a specific change (relative or absolute) in SCr levels, which makes it a surrogate outcome. The clinical significance of surrogate events can be questionable as they sometimes resolve spontaneously without the patient being aware of their existence. Wherever possible, the identification of the risk of real clinical outcomes – such as mortality or the need for dialysis – is more important and useful to patients, clinicians and researchers alike.
These issues seem particularly important in patients with high SCr levels. In a retrospective study81 of 32,161 patients who had not received iodinated contrast material, researchers analysed SCr levels over 5 consecutive days. The study found that, during the 5-day period, more than two-fifths of patients showed a change in level (up or down) of at least 0.4 mg/dl, with higher initial creatinine values being associated with a higher frequency of a given absolute change. 81 These results are important given that some commonly used definitions of AKI cover absolute increases in SCr of ≥ 0.3 to 0.5 mg/dl. 6 Similarly, a retrospective study in a more relevant population (11,588 patients undergoing CT investigations either with or without contrast agents) found that the incidence of AKI increased with increasing baseline creatinine concentration in both contrast and no-contrast groups, concluding that much of the creatinine elevation was attributable to background fluctuation, underlying disease or treatment. 82 Finally, a prospective study of 716 CT or MRI outpatients found that eGFR values varied independently of whether or not patients received a contrast agent. When comparing pre-imaging values with those 3 days after, 45% of CT patients had a change > ± 10 ml/minute/1.73 m2 in the contrast group (n = 237), compared with 59% in the smaller control group (n = 97). 83
It is anticipated that a large number of studies would report on the risk of kidney injury after contrast agent administration; therefore, initially it was sought to identify any recent reviews on the subject. A search of MEDLINE was undertaken for reviews reporting data on the risk of AKI in CT patients. The search was run to identify papers published from 2012 to present; the start year was chosen pragmatically to keep the review manageable and to restrict it to the more up-to-date evidence (literature search strategy details are presented in Appendix 1). From the 291 titles and abstracts retrieved, five potentially relevant reviews were identified. However, the results from three reviews had limited applicability to the outpatient population considered in this assessment, as they were of kidney transplant patients,84 critically ill patients,85 and a mixture of emergency, ICU and inpatients. 86 In the two remaining reviews, the quality of included studies was limited because the studies lacked non-contrast agent control groups. 87,88
Therefore, we focused on the most recent of the five reviews identified (i.e. Aycock et al. 86), which was also the largest study in terms of patient numbers and the broadest in terms of populations. The study reported that, compared with non-contrast CT, intravenous contrast-enhanced CT was not significantly associated with AKI [odds ratio (OR) 0.94, 95% CI 0.83 to 1.07], need for renal replacement therapy (OR 0.83, 95% CI 0.59 to 1.16) or all-cause mortality (OR 1.0, 95% CI 0.73 to 1.36). Although all the studies in the Aycock et al. 86 review had control groups, many studies were small and most did not attempt to match groups on factors associated with outcomes. Therefore, the largest studies were identified with matched control groups included in this review: retrospective studies by McDonald et al. (n = 21,346)89 and Davenport et al. (n = 20,242). 90 The McDonald et al. 89 study looked at AKI, mortality and the need for renal replacement therapy, reporting similar results to the pooled results reported in the Aycock et al. 86 review (described above). The Davenport et al. 90 study reported results by subgroups based on SCr thresholds, concluding that iodinated contrast material is a nephrotoxic risk factor for AKI, but not in patients with a stable SCr levels < 1.5 mg/dl.
In outpatient clinical practice it is eGFR, not creatinine alone, that is used to estimate kidney function (and make decisions on whether or not to use contrast agents), so studies that quantify the risk of AKI in populations subgrouped by baseline eGFR thresholds are more relevant to this assessment. Citation searching using Google Scholar (Google Inc., Mountain View, CA, USA), together with reference lists searches, identified large propensity score-matched studies by the same research groups that reported results risk stratified by eGFR thresholds. 91,92 The characteristics and results of these two studies are presented in Table 14.
| Study details | Study (author and year of publication) | |
|---|---|---|
| McDonald et al., 201492 | Davenport et al., 201391 | |
| Population | Around 90% inpatients, 10% outpatients | All inpatients |
| Sample size | 12,508 (CT examinations between 2000 and 2010) | 17,652 (CT examinations between 2000 and 2010) |
| eGFR method | MDRD | Not reported |
| AKI definition | Increase of ≥ 0.5 mg/dl SCr, 24–72 hours after CT | Increase of ≥ 0.3 mg/dl of SCr or a SCr increase 1.5-fold above baseline within 48 hours (AKIN criteria) |
| Propensity score-matching methods | Generated separately for each eGFR subgroup using logistic regression derived from 13 clinical variables. Nearest-neighbour one-to-one matching (with calliper) without replacement | Generated for the whole group using logistic regression derived from 13 clinical variables |
| eGFR thresholds and results: number of AKIs | eGFR:
|
eGFR:
|
| AKI incidencea | eGFR:
|
eGFR:
|
Propensity score-matching attempts to account for the selection bias inherent in non-randomised studies by accounting for patient characteristics that are associated with the development of AKI and other clinical outcomes, and which can affect decisions on whether or not to use a contrast agent. Matched propensity score analyses matches patients based on risk factors, which predict both whether or not a contrast-enhanced scan is given and the outcome, by calculating a propensity score that reflects the likelihood that a patient is offered a contrast-enhanced scan, if the risk factors are present. The choice of covariates used to calculate the propensity score is crucial: all covariates believed to be related to both the decision to use contrast agent and the outcome should be measured and included. Propensity score analyses can only adjust for known and measured covariates, as opposed to randomised studies, in which both known and unknown confounders tend to be balanced across groups, thus the possibility of residual confounding cannot be completely ruled out. Inclusion of covariates that are related to contrast assignment but not outcome may reduce efficiency of the method, although this is not a serious limitation in large data sets. 93 In addition, the choice of matching method can affect the amount of residual bias. 94
Although the eGFR thresholds to define subgroups mostly differ, the studies’ results are concordant for the risk of AKI in patients with an eGFR ≥ 45 ml/minute/1.73 m2, with contrast agent not being associated with increased risk. The results differ most notably for the eGFR < 30 ml/minute/1.73 m2 subgroups, with the McDonald et al. 92 study reporting no increased risk and the Davenport et al. 91 study reporting a statistically significant increase in risk in patients receiving contrast agent (see Table 14). Although the Davenport et al. study91 has the largest overall sample size, it has far fewer patients in the eGFR < 30 ml/minute/1.73 m2 subgroup (i.e. 116 vs. 1486), which is reflected in its very wide CIs for the estimated ORs.
Another factor that may have contributed to the eGFR < 30 ml/minute/1.73 m2 subgroup results being different is the difference in AKI definitions. Davenport et al. 91 used a lower absolute SCr increase of 0.3 mg/dl, compared with the 0.5 mg/dl increase used by McDonald et al. 92 Given the (previously discussed) natural fluctuation in SCr levels, the use of a lower threshold is likely to detect more AKI events in patients with higher baseline SCrs. These events may be less likely to be clinically significant (in terms of their impact on real clinical outcomes) than AKIs defined using larger increases in SCr. This ‘noise’ of excess events may hamper interpretation of the Davenport et al. 91 study results, given the very small denominators in the eGFR < 30 ml/minute/1.73 m2 subgroups. The difference in propensity score adjustment methods and matching may also contribute to the differences in results. McDonald et al. 92 derived the propensity scores separately for each eGFR subgroup, which will better account for the different clinical characteristics expected in patients with a lower eGFR score and lead to better matching. In contrast, Davenport et al. 91 derived propensity scores for the whole cohort, with mixed eGFR scores, which may explain why differences between two covariates (whether or not CT was performed in the intensive care unit and whether or not the patient had type 1 diabetes mellitus) remained statistically significant after matching.
The Davenport et al. 91 study required that patients have both a baseline and an index pre-CT creatinine measurement – around 16,000 patients were excluded as a result of unstable kidney function. McDonald et al. 92 did not require a baseline creatinine measurement, although the study excluded patients with pre-existing dialysis requirements and patients with acute renal failure in the preceding 14 days. These different criteria may explain the differing numbers across the two studies of patients with an eGFR < 30 ml/minute/1.73 m2. The small numbers mean that the Davenport et al. 91 study eGFR < 30 ml/minute/1.73 m2 results may be prone to chance effects. This can be investigated by calculating the fragility index of the eGFR < 30 ml/minute/1.73 m2 subgroup result. The fragility index is the minimum number of patients whose status would have to change from a non-event to an event in order to turn a statistically significant result to a non-significant result: the smaller the fragility index, the more ‘fragile’ the result. 95 The fragility index is calculated using a Fisher’s exact test, although other methods, such as a chi-squared test, are often used in studies. The p-value from a Fisher’s exact test can be discrepant from a chi-squared test, especially for small studies. In cases in which a Fisher’s exact test produces a non-significant p-value (i.e. without converting a patient from a non-event to an event), the fragility index is reported as zero, indicating a lack of robustness of the result. For the Davenport et al. 91 study, for an eGFR < 30 ml/minute/1.73 m2 result based on the published summary patient data, the fragility index is zero (i.e. the result is not statistically significant using Fisher’s exact test). However, as mentioned previously, following propensity matching the Davenport et al. 91 study OR was adjusted, and the fragility index for the statistically significant OR of 2.96 cannot be calculated from the data available.
If it was assumed that the Davenport et al. 91 study eGFR < 30 ml/minute/1.73 m2 subgroup result was robust, then the ‘number need to harm’ is six, that is, for every six inpatients with an eGFR < 30 ml/minute/1.73 m2 who receive contrast, one inpatient will have an AKI caused by the contrast agent. However, it should be remembered that this result is for a surrogate outcome – it is unclear to what extent increases of 0.3 mg/dl in the SCr level of patients with a baseline eGFR < 30 ml/minute/1.73 m2 translate into real clinical outcomes, such as mortality or the need for dialysis. The McDonald et al. 92 study identified in the in the Aycock et al. 86 systematic review reported data on real clinical outcomes – the results suggested that there was no association between the use of contrast agents and need for dialysis, or death, for all eGFR subgroup analyses (eGFR subgroups were based on stages of chronic renal failure). 89 Although the number of clinical events in this study were quite small, particularly for the dialysis outcome. Moreover, if there is a risk of CI-AKI associated with an eGFR < 30 ml/minute/1.73 m2, it is likely to be lower in the outpatient population of interest in this assessment, given that inpatients are more likely to have other AKI risk factors (including acute illness and exposure to nephrotoxic treatments). Nevertheless, uncertainty about the level of risk remains, primarily because of the unmeasured clinical characteristics, which could not contribute to the propensity scores – most notably the level of prophylactic measures (e.g. IVH) used in the contrast groups and the prevalence of potentially nephrotoxic medication use at time of scanning.
The citation and reference searching identified three further publications of interest on the risk of AKI from contrast agents. 96–98 The first was a review of propensity score-matching studies on AKI after contrast,96 which lists several studies by McDonald et al. 92 and Davenport et al. 91 research groups. This review96 also cited a large study (n = 17,934) by a different research group that reported results by baseline eGFR subgroups. 97 The study setting was an emergency department – different from the inpatients studied by McDonald et al. 92 and Davenport et al. 91 – with comparisons made between contrast-enhanced CT, unenhanced CT and no-CT groups. The results were similar to those reported by McDonald et al. ,92 with rates of AKI being similar among all groups, including the eGFR 15–30 ml/minute/1.73 m2 subgroups.
The review of propensity score-matching studies96 also cited a further study by McDonald et al. 98 that reported the effect of contrast agents on dialysis and mortality, reported by baseline eGFR subgroups. The study was of 5758 inpatients, emergency patients and outpatients who had a CT scan either with or without contrast agents. Contrast agents were not associated with higher rates of dialysis or mortality for any subgroup comparisons, including the CKD stages 4–5 subgroup (i.e. patients with an eGFR < 30 ml/minute/1.73 m2), although the last results are limited by the small number of patients in the contrast group (90, falling to 76 after propensity score matching).
Summary
Although debate about the risk of AKI from contrast agents is ongoing,2–4 evidence from large propensity score-matching studies of inpatients is consistent in suggesting that there is no association between the use of contrast agents and the risk of AKI in patients with an eGFR ≥ 45 ml/minute/1.73 m2. In patients with an eGFR < 45 ml/minute/1.73 m2, there is some uncertainty about whether or not contrast agents are associated with a small risk, although the most robust evidence available suggests that there is no association in inpatients. If a risk does exist, it would be expected to be lower in outpatients than in inpatients.
Evidence on prophylactic interventions for post-contrast acute kidney injury
Pragmatic searches of MEDLINE and recent guidelines were conducted to identify evidence on the clinical effectiveness and safety of standard prophylaxis intravenous saline hydration for preventing PC-AKI in high-risk patients. Recent systematic reviews (from 2012 onwards) of RCTs comparing IVH with oral hydration, placebo or no treatment for preventing PC-AKI in patients with chronic renal failure (defined as an eGFR < 60 ml per min/1.73 m2) undergoing radiological procedures requiring low-osmolality contrast media were included. Risk of bias was assessed using the Cochrane risk-of-bias tool. 99
Review of reviews
Three recent systematic reviews with meta-analysis were identified. 100–102 Characteristics and results of the reviews are summarised in Table 15.
| Review details | Study (author and year of publication) | ||
|---|---|---|---|
| Ahmed et al., 2018102 | Agarwal et al., 2015101 | Hiremath et al., 2013100 | |
| Number of studies; number of participants | 197; 42,273 | 5; 447 | 6; 513 |
| Search date | Up to April 2017 | Up to April 2015 | Up to November 2011 |
| Population |
|
|
|
| Interventions: number of studies; number of participants | 44 types including:
|
|
|
| Contrast media type: % studies |
|
|
|
| Synthesis method |
|
|
|
| Outcomes | CI-AKI: ≥ 25% relative increase or ≥ 0.5 mg/dl increase from baseline creatinine 1–5 days post contrast exposure | CIN (multiple definitions) > 44.2 µmol/l (> 0.5 mg/dl) absolute increase or > 25% relative increase in SCr level, within 48–72 hours of contrast exposure | CIN (multiple definitions) > 44.2 µmol/l (0.5 mg/dl) absolute increase or > 26.4 mmol/l (0.3 mg/dl), or > 25% relative increase in SCr level, within 48–72 hours of contrast exposure |
| Main findings | Top ranked interventions were:
|
PC-AKI incidence:RR of 0.97 (95% CI 0.36 to 2.94; I2 = 48%) Subgroup of three studies with CKD patients: RR 1.73 (95% CI 0.69 to 4.33) |
PC-AKI incidence:
|
| Conclusions | Some options (particularly allopurinol, PGE1 and oxygen) deserve to be tested in larger RCTs | Oral hydration is at least as effective as i.v. hydration with saline to prevent PC-AKI | Oral hydration may be as effective as i.v. hydration for the prevention of PC-AKI |
All three reviews100–102 included RCTs evaluating prophylactic treatments to prevent PC-AKI in patients undergoing contrast-enhanced procedures. Two meta-analyses evaluated the relative efficacy of intravenous and oral hydration in head-to-head comparisons and one network meta-analysis evaluated 44 different prophylactic interventions. Most of the evidence focused on patients undergoing cardiac procedures. Overall, all three reviews found no significant difference between intravenous and oral hydration to prevent PC-AKI. None of the reviews reported data on mortality, dialysis outcomes or complications of IVH.
Ahmed et al. 102 conducted a large systematic review and network meta-analysis comparing the efficacy of 44 therapies for the prevention of PC-AKI in patients undergoing a contrast-enhanced procedure. The review included 197 RCTs (including 42,273 participants). Nearly three-quarters of the patients included underwent coronary angiography and 8% underwent a CT procedure. Half of included patients had reduced kidney function, which was defined as either an eGFR < 60 ml/minute/1.73 m2 or a SCr level of > 1.3 mg/dl (114 mmol/l). The number of patients with an eGFR < 45 ml/minute/1.73 m2 was not reported. The most common interventions were N-acetylcysteine (68 studies, 6095 participants), IVH (41 studies, 5136 participants), NaHCO3 (sodium bicarbonate) (32 studies, 3393 participants) and statins (14 studies, 3040 participants). Oral hydration was also evaluated (five studies; 254 participants). The most common comparators were placebo (70 studies, 7044 participants) and control/no treatment (88 studies, 9120 participants). Over half of the studies (55.5%) reported using low-osmolar contrast agents. Most studies were in cardiac patients; coronary angiography was the contrast-dependent procedure in 72.5% of studies. The primary outcome of the review was PC-AKI (referred to as CI-AKI in the review), defined as ≥ 25% relative increase or ≥ 0.5 mg/dl increase from baseline creatinine level 1–5 days post contrast agent exposure. Overall, the review found that the best-ranked interventions were allopurinol, prostaglandin E1 and oxygen, although these results are based on a few trials with a small number of participants. There was no significant difference in the ORs of PC-AKI between IVH or oral hydration compared with placebo (IVH vs. placebo: OR 0.91, 95% CI 0.60 to 1.34 in all studies; OR 0.97, 95% CI 0.52 to 1.9 in studies with low eGFRs/high baseline renal profile; oral hydration vs. placebo: OR 1.09, 95% CI 0.41 to 2.75), and there was no significant difference between intravenous and oral hydration (OR 0.83, 95% CI 0.35 to 1.95). Compared with control/no treatment, there was a statistically significant difference favouring IVH (OR 0.71, 95% CI 0.52 to 0.99), but not oral hydration (OR 1.09, 95% CI 0.41 to 2.75). Overall heterogeneity was 0.55 (95% CrI 0.41 to 0.69, using a vague prior distribution) and 0.50 (95% CrI 0.37 to 0.64, using an informative prior distribution), which is moderate to large on the log-OR scale. Although Ahmed et al. 102 state that consistency was assessed using an inconsistency plot, reported results are insufficient to conclude whether or not it was present.
Agarwal et al. 101 reported on a meta-analysis of five RCTs (447 participants) comparing oral and IVH for the prevention of CIN (thereafter PC-AKI) in patients receiving low-osmolar contrast agents. All five RCTs were also included in Ahmed et al. 102 Two-thirds of included participants had CKD (not defined), and all except one study only included patients undergoing cardiac procedures. There was no significant difference in the incidence of PC-AKI between IVH (7.7%) and oral hydration (8.2%) [risk ratio (RR) 0.97, 95% CI 0.36 to 2.94; I2 = 48%]. A subgroup analysis of CKD patients (not defined) found no statistically significant difference between treatment arms (RR 1.73, 95% CI 0.69 to 4.33; I2 = 0%). The review concluded that oral hydration is at least as effective as IVH to prevent PC-AKI.
Hiremath et al. 100 included six RCTs (513 participants) that compared the relative efficacy of oral hydration and IVH. Four of these trials were also included in Agarwal et al. ,101 and all were included in Ahmed et al. 102 All except one study (i.e. Dussol et al. 103) focused exclusively on patients undergoing cardiac procedures. There was no significant difference in the incidence of PC-AKI between IVH (8.1%) and oral hydration (9.6%) (OR 1.19, 95% CI 0.46 to 3.10; I2 = 57%).
Randomised controlled trial evidence
As most of the review evidence focused on patients undergoing cardiac procedures, the applicability of the review findings may be limited for the population of outpatients scheduled for contrast-enhanced CT scanning without a recent eGFR measurement who may be at a higher risk of PC-AKI. Therefore, references of studies included in the reviews were checked for RCTs comparing oral hydration or IVH versus no treatment for preventing post-contrast AKI in outpatients with chronic renal failure (i.e. an eGFR < 60 ml/minute/1.73 m2) undergoing non-cardiac radiological procedures requiring non-ionic, low-osmolality contrast agents.
Two trials met this study’s inclusion criteria: A MAastricht Contrast-Induced Nephropathy Guideline (AMACING)104,105 and Dussol et al. 103 The characteristics and results of both trials are reported in Table 16. Risk-of-bias assessment is summarised in Table 48 (in Appendix 5). AMACING104 was designed as an non-inferiority trial and was therefore not sufficiently powered to detect a significant difference between treatments. Dussol et al. 103 was significantly smaller (with approximately one-quarter of the participants being assigned to IVH or to the control) and did not report allocation concealment methods; therefore, a risk of bias cannot be excluded. 103 Both trials could not blind study participants and study personnel, although this is unlikely to significantly affect the assessment of PC-AKI.
| Study (trial acronym/author and year of publication) | Characteristics | Results | |||||
|---|---|---|---|---|---|---|---|
| Design | Selection criteria | Population characteristics | Interventions | Mean volume of contrast agent (SD)a | PC-AKI definition | ||
| AMACING104,105 |
Randomised, parallel-group, open-label, non-inferiority trial The Netherlands N = 660 |
|
|
i.v. hydration 0.9% NaClb or no i.v. hydration |
|
Increase in SCr levels by > 25% or 44 µmol/l within 2–6 days post contrast agent | PC-AKI incidence (2–6 days’ follow-up):
|
| Dussol et al., 2006103 |
Randomised, parallel-group, four-arm, open-label trial France N = 330 |
|
|
i.v. hydration 0.9% NaCl or oral hydration |
|
Increase in SCr levels ≥ 0.5 mg/dl (44 µmol/l) above baseline at 48 hours post contrast agent |
PC-AKI incidence (48 hours of follow-up):No significant differences in between-arm differences at 24 hours’ follow-up (results NR in study) Dialysis, fluid overload, significant increase in BP (48 hours’ follow-up): none in either trial arm Other adverse events (48 hours’ follow-up):Further results for theophylline and furosemide arms were reported |
The AMACING104 study was a single-centre, randomised, parallel-group, open-label, Phase 3, non-inferiority trial of no prophylaxis compared with guideline-recommended prophylaxis in preventing what the authors termed CIN (thereafter PC-AKI). In addition, the trial was to explore the effect on long-term post-contrast agent exposure adverse outcomes. A total of 660 adults with an eGFR between 30 and 59 ml/minute/1.73 m2 undergoing an elective procedure requiring an iodinated contrast agent were randomised to standard intravenous prophylactic hydration or no prophylaxis. PC-AKI was measured at 2–6 days post contrast agent exposure. The trial found no significant difference in the incidence of PC-AKI between intravenous prophylaxis (2.7%) and no treatment (2.6%) at follow-up (RR 1.04, 95% CI 0.39 to 2.73). No haemodialysis or related deaths occurred within 35 days. Eighteen (5.5%) patients in the intravenous prophylaxis group experienced IVH treatment-related adverse events. At 1 year following contrast agent exposure, there was no significant difference in the proportion of patients requiring dialysis between intravenous prophylaxis and the control group (0.6% incidence in both groups; RR 1.01, 95% CI 0.14 to 7.14), and no difference in mortality [IVH 9.8% vs. control 10.8%; hazard ratio (HR) 1.12, 95% CI 0.70 to 1.80].
The study by Dussol et al. 103 was a single-centre, randomised, parallel-group, open-label trial comparing the efficacy of oral saline hydration with that of intravenous saline hydration, with or without theophylline or furosemide, for preventing PC-AKI. Patients undergoing radiological procedures with a non-ionic, low-osmolality contrast agent with an eGFR ranging between 15 and 60 ml/minute/1.73 m2 were randomised to one of four groups: oral hydration, standard IVH, IVH with theophylline and IVH with furosemide. The proportion of patients with an eGFR < 30 ml/minute/1.73 m2 was not reported. The study found no significant difference in the incidence of PC-AKI between intravenous prophylaxis (6.6%) and oral hydration (5.2%) (RR 1.27, 95% CI 0.35 to 4.54) at 48 hours post contrast agent exposure. There were no significant adverse events in either study arm.
Overall, both trials found that oral hydration was not inferior to IVH for preventing AKI in patients with an eGFR < 60 ml/minute/1.73 m2. There was mixed evidence on the safety of IVH: one trial (AMACING104) suggested that IVH was associated with treatment-related complications, and another found no adverse events. 103
Non-randomised evidence
Owing to the lack of RCT-based evidence in patients with an eGFR < 30 ml/minute/1.73 m2, further pragmatic MEDLINE searches were conducted to identify relevant non-randomised evidence. One retrospective cohort study was found. 106
Nijssen et al. 106 included patients referred for an elective procedure who received intravascular iodinated contrast material administration with an eGFR < 30 ml/minute/1.73 m2 and who were excluded from the AMACING trial. 104 Outcomes included CIN (as referred to in the trial, thereafter PC-AKI) (2–6 days’ follow-up), dialysis and mortality within 35 days post contrast agent exposure, and complications of prophylactic IVH. The characteristic and results of Nijssen et al. 106 are reported in Table 43.
Of the 155 patients with an eGFR < 30 ml/minute/1.73 m2 who received contrast material, 119 (76.8%) received 0.9% intravenous sodium chloride (i.e. standard IVH), 12 (7.8%) received 1.4% NaHCO3 hydration and 24 (15.5%) received no prophylaxis. Reasons for deviation from standard prophylaxis are reported in Table 43. Data on 2- to 6-day SCr measurements were available for only 59 (50%) of the standard prophylaxis patients. Data on other clinical outcomes were available for 99–100% of standard prophylaxis patients. The incidence of clinical outcomes were reported separately for patients with an eGFR < 30 ml/minute/1.73 m2 receiving standard prophylaxis, NaHCO3 hydration and no prophylaxis. PC-AKI occurred in 8 out of 59 (13.6%) patients with standard prophylaxis, in 1 out of 12 (8.3%) NaHCO3-hydrated patients, and in 1 out of 18 (5.6%) no-prophylaxis patients. Dialysis within 35 days of contrast agent exposure occurred in 1 out of 118 (0.85%) standard prophylaxis patients, in 1 out of 12 (8.3%) NaHCO3-hydrated patients and in none of the 23 patients receiving no prophylaxis. Death within 35 days of post-contrast agent exposure occurred in 11 out of 119 (9.2%) of standard prophylaxis patients. There were no deaths in patients receiving NaHCO3 or no prophylaxis.
Results of patients with an eGFR < 30 ml/minute/1.73 m2 who received standard prophylaxis were analysed against the IVH arm of the AMACING trial104 in unadjusted or unmatched comparisons. Compared with the AMACING trial104 active-arm participants, the incidence of PC-AKI was significantly higher in patients with an eGFR < 30 ml/minute/1.73 m2 (13.6% vs. 2.7%; p = 0.0019). Death within 35 days of contrast agent exposure was also higher in the cohort arm (9.2% vs. 0.0%; p < 0.0001). There was no difference in the incidence of complications of prophylactic IVH (5.9% vs. 5.5%; p = 0.8529) and 35-day dialysis (0.9% vs. 0.0%; p = 0.2646) between the two trial groups.
Results from Nijssen et al. 106 may not be reliable as a result of the lack of randomisation, the lack of matching and adjusted comparison, and the significant rate of missing PC-AKI data in higher-risk patients undergoing standard hydration.
Summary of prophylaxis evidence
This study found three recent systematic reviews and meta-analyses evaluating prophylactic treatments to prevent PC-AKI in patients undergoing contrast-enhanced procedures. The reviews were consistent in showing no evidence of a difference in effectiveness between intravenous and oral hydration to prevent PC-AKI. However, relevant pooled estimates from meta-analyses had wide CIs and there was evidence of heterogeneity; therefore, the true effect (or lack of effect) of IVH compared with oral hydration to prevent PC-AKI remains uncertain. None of the reviews reported on mortality, dialysis or complications from IVH. Most evidence from systematic reviews focused on patients undergoing cardiac procedures, and incidence of PC-AKI was significantly higher than that reported in outpatient populations scheduled for contrast-enhanced CT scanning without a recent eGFR measurement; therefore, the applicability of much of the evidence to this study’s population of interest is uncertain.
The evidence in patients at higher risk of PC-AKI who are referred for a non-emergency scan with contrast media is more limited. Two RCTs of non-cardiac outpatients with CKD (i.e. an eGFR < 60 ml/minute/1.73 m2) were identified, and both found no evidence that intravenous prophylaxis reduced the incidence of post-contrast agent AKI compared with no IVH. This is consistent with the broader evidence from the systematic reviews that were identified, which primarily included cardiac patients. This study found only limited non-RCT evidence for patients with an eGFR < 30 ml/minute/1.73 m2. There was mixed evidence on the safety of IVH in non-cardiac outpatients with CKD (eGFR < 60 ml/minute/1.73 m2): one trial suggested that IVH was associated with treatment-related complications and another found no adverse events.
Overall, there is no evidence to suggest that IVH is more effective than oral hydration or placebo in preventing PC-AKI, renal replacement therapy (RRT) or reducing mortality. Evidence on complications of IVH is inconclusive. The certainty of the evidence on the efficacy of IVH is limited by the lack of precision in intermediate outcome estimates, lack of hard clinical outcomes and broader issues surrounding the existence of PC-AKI in patients with CKD.
Evidence of practice variation in renal function assessment
Two quite recent studies that have evaluated how renal function assessment practice varies in the UK were identified by reference list searching and citation searching. A survey undertaken in 2015 by Cope et al. 13 assessed compliance with UK 2013 guidelines107,108 for the prevention, recognition and management of CI-AKI. All UK acute NHS providers with a clinical radiology audit lead registered with the Royal College of Radiologists (RCR) were invited to complete a questionnaire. In order to demonstrate guidance compliance in daily practice, audit data on 40 consecutive stable outpatients who had undergone CT with intravenous iodine-based contrast agents were also requested from each NHS provider.
Eighty-nine of the 172 (52%) health service providers responded to the questionnaire and 91 out of 212 (43%) hospitals provided audit data. In general, the paper by Cope et al. 13 noted wide variation in clinical practice and poor compliance with guidelines. Although kidney function test results within 3 months of the scan were available for 86% of outpatients, eGFR results (as recommended in the guidelines) were available for only 66%. Responsibility for checking baseline kidney function was taken by the radiology department in 49% of departments; in 51%, the responsibility was either devolved to the referring clinician or was not clearly defined. Only 30% of radiology departments had a policy for management of patients who developed PC-AKI or had locally agreed arrangements in place for the care of patients when repeat blood tests demonstrate PC-AKI. The requirement for intravenous volume expansion for high-risk patients prior to the scan was met by 64% of departments.
Audit data were available for 3590 fit outpatients. Analyses were reported for a subgroup of 513 patients with a baseline eGFR < 60 ml/minute/1.73 m2; 288 (56%) had pre- and post-contrast kidney function tests – no change was seen in the median SCr level 2 days post contrast. The incidence of clinically significant (requiring treatment or resulting in death) PC-AKI was zero in 3590 outpatients.
Harris et al. 12 also undertook a UK survey in 2015, requesting data from CT managers in 174 NHS trusts to identify screening practices prior to outpatient contrast-enhanced CT. The response rate (47%) was similar to that reported in Cope et al. ’s13 survey. The RCR guideline109 was most frequently used, although 20% of responders did not cite the use of a specific guideline. Most responding sites (75/82, 92%) required renal function to be assessed via a blood test; most sites did this for all patients, although 20% of sites assessed only ‘high-risk’ patients. Variation in how blood tests were organised was found, with most radiology departments sharing the responsibility with the referring clinician. Most radiology departments removed or minimised the risk of patients attending radiology without a recent kidney function result by checking blood results either before booking appointments (56%) or when appointments were made (16%), with blood tests booked if needed. Just over one-quarter of radiology departments (28%) indicated that results are reviewed on the day of the scan (or the night before).
Variation was also evident in the eGFR or SCr thresholds at which contrast was deemed to be contraindicated; 19 different threshold levels were identified, each leading to different prophylactic strategies. The most frequently used threshold was an eGFR of < 30 ml/minute/1.73 m2, which was used in 35 of the 77 (45%) NHS trusts. Blood test results were not checked by 7 out of 82 (9%) sites – sites indicated that it was the referrer’s responsibility. For patients attending without a recent blood result, 45% send the patient away to have a blood test and scan either on the same day (if possible) or on a different day, and 11% of sites use POC devices to get a quick blood test result. Most of the remaining sites said that they would seek advice from a consultant radiologist. Data on practice variation in obtaining follow-up (post-contrast) blood tests were also reported. The authors concluded that the wide variation in practice is a reflection of inconsistencies in published guidance and that an evidence-based consensus on risk thresholds was needed.
Chapter 4 Assessment of existing economic evidence on point-of-care testing
This chapter provides an overview of existing cost-effectiveness evidence on the use of POC creatinine tests in an outpatient non-emergency secondary care setting to assess kidney function before contrast-enhanced CT imaging. The relevant population includes adult patients who do not have a recent eGFR measurement. Eligible studies were systematically identified and the main findings narratively summarised and tabulated for comparison. Other sources of evidence, with more qualitative consideration of the potential implications of introducing POC testing in the context of the current decision problem, were also reviewed. These sources of evidence included:
-
one existing Medtech innovation briefing (MIB) on POC devices for creatinine testing
-
a report produced by the King’s Technology Evaluation Centre (KiTEC; King’s College London, London, UK) to support the External Assessment Group (EAG)’s report.
The findings from the reviews helped inform the development of a new decision-analytic model reported in Chapter 5, Independent economic assessment.
Methodology of the cost-effectiveness review
Searches
The literature search reported in Chapter 3, Assessment of clinical effectiveness, Searches, was also used to identify studies reporting on the cost-effectiveness of POC creatinine testing in an outpatient non-emergency setting before contrast-enhanced CT imaging.
Selection process
A broad range of studies were considered in the review, including economic evaluations conducted alongside trials, modelling studies and analyses of administrative databases. Only full economic evaluations that compared two or more options and considered both costs and consequences (i.e. cost-minimisation, cost-effectiveness, cost–utility and cost–benefit analyses) were included in the review. The inclusion criteria also defined the relevant population as non-emergency outpatients scheduled to receive intravenous contrast-enhanced CT imaging.
The selection of relevant studies was performed in two stages:
-
Titles and abstracts identified by the search strategy were examined and screened for possible inclusion.
-
Full texts of the potentially relevant studies were obtained and screened for inclusion.
Two researchers (AD and JA) independently screened the titles and abstracts of all reports identified by the bibliographic searches and full-text papers were subsequently obtained for assessment and screened by at least two researchers. Any disagreement was resolved by consensus.
Confidential information
This report contains reference to confidential information provided as part of the NICE appraisal process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
Results
A total of 3628 records were identified by the initial search of economic databases. Three studies were identified as potentially relevant from their titles and/or abstracts. The full-text articles of these records were assessed for eligibility; however, none was found to meet the inclusion criteria. Figure 6 presents a flow diagram of the selection process. Table 49 in Appendix 6 lists excluded studies alongside reasons for exclusion.
FIGURE 6.
Assessment of cost-effectiveness: summary of study selection and exclusion.
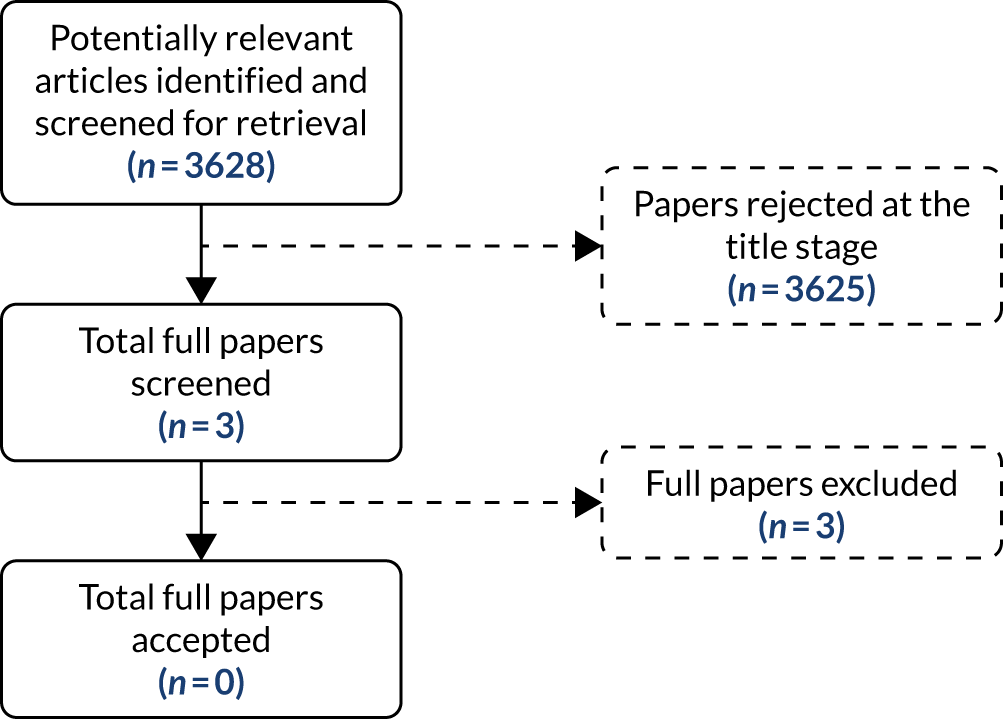
Although no published studies were identified from the systematic review, one unpublished economic study was identified, which was considered potentially relevant (Professor Beverley Snaith, Mid Yorkshire Hospitals NHS Trust, 2019, personal communication). Following discussion with the lead author, a draft version of the manuscript was provided. This draft was provided by the lead author in academic in confidence.
Review of Shinkins et al.
Overview
(Confidential information has been removed.)
FIGURE 7.
Confidential information has been removed.


Relevance of findings
(Confidential information has been removed.)
Overview of other sources of evidence
Although no other studies were identified that met the review inclusion criteria, several additional sources of evidence were identified that provided a more qualitative consideration of the potential implications of introducing POC testing in an outpatient non-emergency secondary care setting to assess kidney function before contrast-enhanced CT imaging. These additional sources of evidence are briefly summarised below.
Resourcing implications identified in the Medtech innovation briefing
The MIB (specifically MIB13615) identifies POC testing technologies as an alternative to laboratory-based testing in those patients who present for contrast CT scanning without a recent eGFR measurement. In the absence of a recent creatinine measurement, these patients may otherwise have their imaging cancelled or rescheduled – given national guidelines for a recent eGFR to be available before imaging. 108,110 If the scan is not cancelled, the authors of MIB suggest that the patient would either undergo non-contrast-enhanced scanning or continue with contrast scanning as planned, thus putting the patient at risk of kidney injury.
The authors therefore identify a key benefit of POC devices to be reducing the incidence of cancelled CT scans as a result of the expectation of a reduced patient waiting time for eGFR measurements for those patients who present without a recent eGFR measurement. MIB specialist commentators note the administrative cost of cancelling or rescheduling scans, and the impact of cancellations on overall scanning capacity. The other benefit is more accurately identifying the subset of patients without a recent eGFR measurement who should not proceed with a contrast CT scan because of their elevated risk of kidney disease (i.e. those patients with an eGFR < 30 ml/minute/1.73 m2). These patients are most likely to suffer adverse effects of contrast-induced kidney injury and, thus, should not generally proceed to contrast CT scanning unless appropriate prophylaxis is provided, their contrast dose is reviewed or they are in urgent need of diagnostic information provided only by contrast-enhanced imaging.
The MIB authors note that POC devices are expected to deliver eGFR results from a whole-blood sample in ≤ 9 minutes, compared with laboratory testing, which can take between 60 minutes and 24 hours. The specialist clinical group that was consulted note that this reduction in waiting time would reduce the need for additional appointments, delayed appointments and increase patient throughput. The MIB authors note that POC devices would be most useful in assessing kidney function in the subgroup of the overall patient population at highest risk of kidney disease, including those patients with diabetes mellitus, people taking metformin and older people.
The MIB authors note that POC testing would increase upfront costs compared with standard laboratory-based testing. The unit cost of a laboratory test for blood/serum/plasma creatinine was £1.29 at 2015/16 prices (i.e. reference cost DAPS04111). The MIB authors note that the unit cost per POC test for the devices that they consider vary between £0.17 and £4.75. The authors also note the significant upfront capital costs of POC devices. On a practical front, the authors note the potential requirement for staff training and compliance and quality assurance policies, as well as an increase in storage space for POC consumables; however, the MIB authors also note that all of these requirements would be unlikely to be a significant change. The MIB authors also note that additional resources may be required for participation in external quality assurance schemes, with specialist commentators also suggesting potential costs for the integration of recording POC results with the existing hospital reporting system. The specialist group of clinical advisors held divergent opinions on whether or not POC testing would replace central laboratory testing or supplement it.
The MIB authors note some economic benefits of early diagnosis of CKD through the use of POC testing as opposed to waiting for GP testing; however, the authors note that these savings would be minimal. The authors also cite a US-based study112 that showed a reduction in waiting times for eGFR results from an average of 1 hour 54 minutes to 5 minutes following the introduction of radiology POC testing. This US study also suggested that the volume of contrast material used was also reduced for 26.4% of patients (33/125 patients). Although not directly reported in the MIB, this study suggests that rapid testing will enable radiology departments to reduce costs by reducing the number of full-time-equivalent administrative positions needed for checking laboratory results prior to testing. In addition, the rapid testing will also reduce technician overtime as a result of the reduced need to accommodate delayed examination times due to waiting for laboratory results.
Implications for the care pathway identified in the King’s Technology Evaluation Centre’s report
As part of the report produced by KiTEC to support the EAG’s report,113 clinical experts were also interviewed regarding their views on the implications of introducing POC creatinine testing within the current CT imaging pathway. The KiTEC report noted that all the clinical experts that were interviewed expressed concerns regarding the use of these devices in their departments. The report highlighted two main reasons for these concerns. First, the clinical experts highlighted that referring clinicians would rely even more on the radiology department to check patients’ eGFR. As a result of this behavioural change, the clinical experts thought that this would result in an increase in the number of patients referred for a CT appointment without a recent eGFR measurement. Second, the clinicians noted that this would increase the responsibility and resourcing required by the radiology department not only to action upon a low eGFR but also to explain to the attending patient that their result was abnormal and may require further investigations and changes in management.
Discussion of existing cost-effectiveness evidence and relevance to current decision problem
(Confidential information has been removed.)
To address the issues and uncertainties identified in the review and, in particular, to inform the cost-effectiveness of POC creatinine testing for the specific decision population under consideration, a new independent decision model was developed.
Chapter 5 Independent economic assessment
Overview
Chapter 4 identified several issues and uncertainties arising from previously published studies. A number of important limitations were also identified in relation to the current decision problem, specifically:
-
Only one cost–consequence analysis was identified and no studies have formally assessed the cost-effectiveness of POC testing in the decision context considered in this appraisal.
-
The lack of any study that has attempted to formally compare different POC testing devices.
-
The absence of any study that has attempted to quantify the benefits and risks associated with incorporating POC testing within the current CT imaging pathway.
For these reasons, it has been necessary to develop a de novo decision model.
Contribution of the model
The purpose of the decision model is to assess the cost-effectiveness of POC testing to assess kidney function in people who need contrast-enhanced CT imaging in a non-emergency situation and who do not have a recent eGFR measurement. The model provides a quantitative framework to link the diagnostic accuracy of POC creatinine tests to short-term costs and consequences (e.g. the impact on cancelled or delayed appointments, use of contrast media with and without IVH and associated risks such as PC-AKI) and final health outcomes (e.g. end-stage renal disease and death) expressed in terms of QALYs. This linkage is necessary in order to provide decision makers with an indication of the health gain achieved by POC tests, relative to their additional cost, in units that permit comparison with other uses of health service resources.
The purpose of the POC and existing laboratory-based tests (urgent and non-urgent) is to inform subsequent scanning decisions, specifically the use of contrast material, prophylactic hydration or the use of alternative imaging modalities. The model characterises the impact of the alternative tests (POC vs. laboratory based) based on the person’s estimated eGFR and the subsequent decisions according to specific eGFR thresholds. These decisions will affect the use of contrast, prophylaxis and alternative imaging modalities. For example, the volume of contrast will depend on whether or not a decision is made to proceed with CT imaging using contrast material or to proceed with an unenhanced CT scan or even to an alternative imaging modality. These decisions, and the subsequent use of contrast material and prophylactic hydration, also need to be linked to any possible impact on the risks of PC-AKI and to final health outcomes, including morbidity and mortality.
The use of POC testing within the current CT pathway has implications to the health system that relate to the following main components:
-
System level and resourcing: the use of POC testing may reduce system inefficiencies related to ensuring that a recent laboratory-based eGFR measure is available prior to the CT appointment. Although significant efforts are often made to ensure that a recent eGFR measure is available prior to the scheduled CT appointment, a proportion of individuals may present on the day of the scan without a recent eGFR measurement. As a result, these individuals may be sent for blood tests in the hospital laboratory, which means that the planned CT scan appointment may need to be delayed or rescheduled.
-
Diagnostic (in)accuracy: POC tests (used with or without additional risk questionnaires) inevitably introduce some level of misclassification compared with laboratory testing, in that some of the individuals may be misclassified as having a high risk of PC-AKI (i.e. FP) and others, who are truly high risk, may be misclassified as low risk (i.e. FN). As a consequence of misclassification, these individuals may not receive the appropriate clinical management strategies, leading to potential morbidity and even mortality implications.
-
Risk of PC-AKI: equally, POC devices may help to identify individuals at high risk of PC-AKI, particularly those patients presenting at their appointment without a recent eGFR measurement and for whom a decision to proceed to contrast-enhanced CT scanning is made based on clinical judgement alone. By providing a timely eGFR measurement, more individuals at a higher risk of PC-AKI may be identified, allowing more appropriate management strategies to be followed. That is, preventative strategies can be put in place, including the use of oral hydration or IVH or identifying individuals for whom the use of contrast media can be avoided, without significantly compromising accuracy by performing an unenhanced CT scan or changing diagnostic modality.
The modelling proposed in this study is designed to address these three components and to be able to determine the overall value of POC testing inferred from each of the possible risks and benefits. The following sections outline the decision problem and the structure of the model. In addition, the sections also provide an overview of the key assumptions and data sources used to populate the model.
Model structure
Overview
The model evaluates the cost and health outcomes of a cohort of outpatients presenting for a non-emergency contrast-enhanced CT scan without a recent eGFR measurement. The model is populated using the results from the quantitative synthesis of the diagnostic accuracy of POC testing as described in Chapter 3, Results: assessment of diagnostic accuracy. Other relevant parameters were informed by a series of additional reviews described throughout this section. These parameters are used to provide a link between the diagnostic accuracy of a given testing strategy, the impact on subsequent treatment decisions and the ultimate effect on health outcomes and costs.
Costs are presented from the perspective of the NHS and Personal Social Services (PSS) and are reported in Great British pounds at a 2018 price base. Outcomes are expressed in terms of QALYs. Outcomes beyond the first year are discounted at a rate of 3.5% per annum.
The model uses a decision tree cohort approach to estimate, based on best available data, the costs and health outcomes of the relevant testing and treatment strategies. The model structure captures:
-
individuals’ true eGFR status (with the cohort dichotomised based on a cut-off point of 30 ml/minute/1.73 m2)
-
how these individuals are subsequently classified by different testing strategies (with classification dichotomised on the same eGFR cut-off point of 30 ml/minute/1.73 m2 and probabilities conditional on true eGFR status)
-
any actions taken to mediate PC-AKI risk in patients identified (correctly or incorrectly) as below the eGFR cut-off point
-
the subsequent risk of PC-AKI (conditional on eGFR status and any actions taken to mediate PC-AKI risk)
-
the risk of renal replacement therapy (conditional on whether or not a patient experienced PC-AKI).
Costs and QALYs are linked to the use of screening tests, mediating actions taken and the use of RRT.
A simplified model schematic is shown in Figure 8. Patients are defined as true positives (TPs), FPs, true negatives (TNs) and FNs according to their overall classification across each testing strategy and not in relation to individual tests in the sequence. Testing approaches may combine up to three testing elements to identify patients. The elements of testing considered were:
-
screening on the basis of a risk factor questionnaire
-
testing with a POC device
-
testing with a laboratory test (urgent or non-urgent).
Patients identified as negative by the testing approach will receive no alternative management and undergo contrast-enhanced CT. Patients identified as positive will receive mediating action, which in the base case is assumed to be the use of IVH prior to undergoing contrast-enhanced CT. Following their scan, patients may experience a PC-AKI and may subsequently undergo RRT.
FIGURE 8.
Decision tree general schematics.
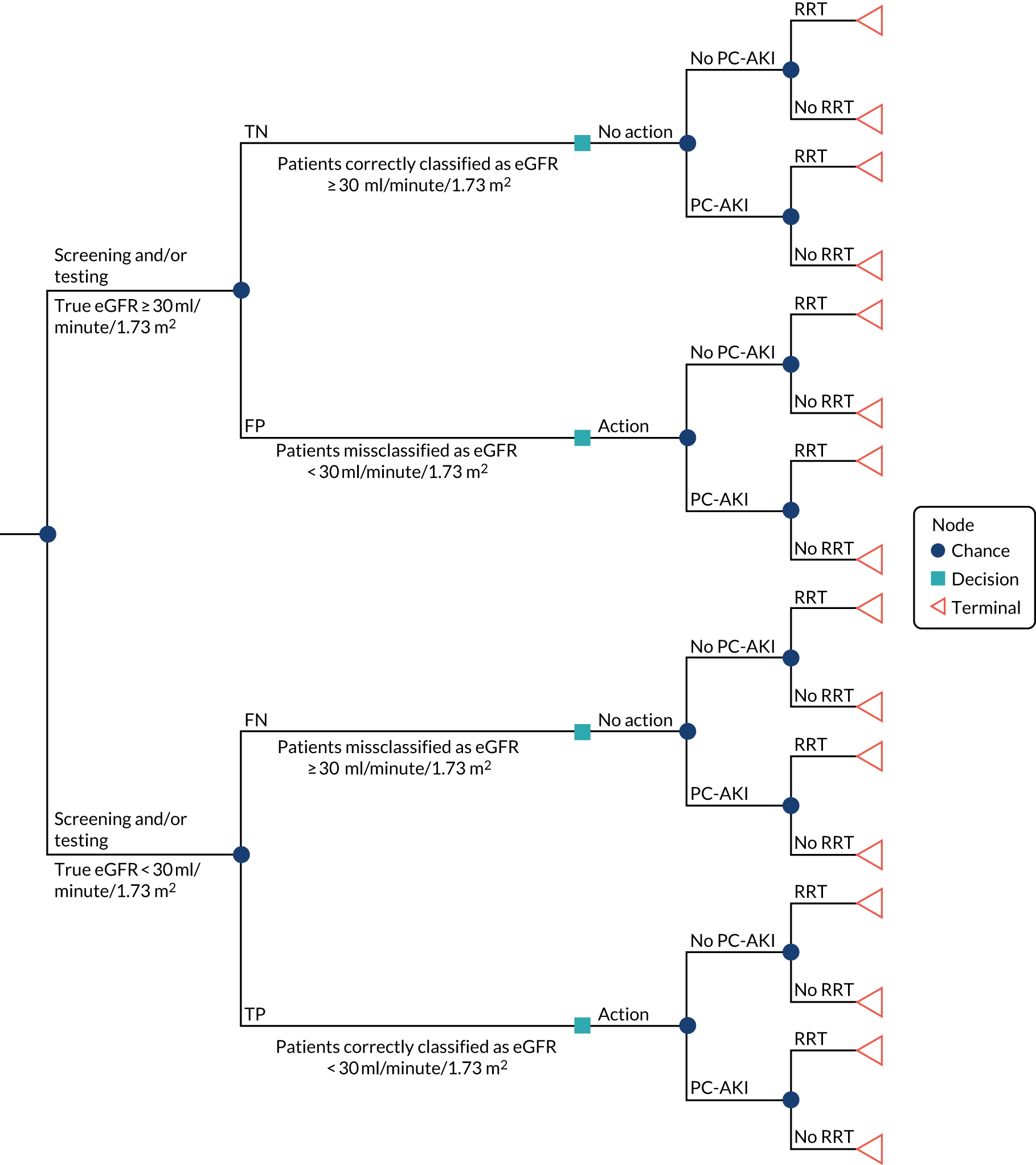
A key assumption in the base-case analysis is that all individuals will eventually proceed to contrast-enhanced CT. Hence, the only difference between the alternative testing strategies that were evaluated concerns the costs and potential health impact of delayed or rescheduled CT, whether or not any mediating action is taken to reduce the risk of PC-AKI (i.e. use of IVH) and the consequences of PC-AKI. The base-case analysis does not attempt to include other clinical outcomes that could be affected by changes to the imaging decision itself. These outcomes could include anxiety associated with delayed or cancelled scanning, and morbidity and mortality implications of performing unenhanced scanning or using an alternative imaging modality. This simplification was considered necessary given the limited data available and the challenges of characterising the heterogeneity in the overall population and the underlying reason for imaging and linking this to individualised clinical decision-making and associated outcomes.
The challenges of linking different decisions regarding the use of contrast media in imaging to patient outcomes were also highlighted in KiTEC’s report. 113 Clinical experts interviewed in the KiTEC report stated that it is difficult to quantify the impact of decisions regarding the use of contrast media on patient outcomes as the benefits of using intravenous contrast vary depending on the underlying population and scanning indication. The use of intravenous contrast was considered by the clinical experts to be well-established practice, but none was aware of any landmark study that could be used to quantify the benefits compared with alternative imaging decisions.
Although the base-case analysis imposes boundaries around the specific clinical outcomes assessed because of practical considerations and data gaps, a series of additional scenario analyses were undertaken to explore the robustness of the base-case analysis to alternative assumptions concerning the potential impact of alternative imaging decisions on costs and outcomes. These scenarios considered the potential costs as well as any anxiety effects associated with delayed CT scan or a use of an alternative imaging modality. The full set of scenarios are discussed in more detail in later sections.
The model evaluates the cost-effectiveness of 14 alternative testing strategies to identify and manage patients with an eGFR < 30 ml/minute/1.73 m2. The likelihood of an individual being classified as positive (i.e. with an eGFR < 30 ml/minute/1.73 m2) or negative (i.e. with an eGFR ≥ 30 ml/minute/1.73 m2) is estimated for each strategy based on an individual’s true eGFR status and the diagnostic accuracy (sensitivities and specificities) of the different elements of testing that constitute the overall testing strategy. Where a strategy involves multiple tests, an individual will progress from one test to the next if the first test classifies them as positive, which in the case of risk factor screening will involve them being classified as at risk or, in the case of a POC device, of having an eGFR < 30 ml/minute/1.73 m2. An individual will be identified as positive (either TP or FP) if the final test in the strategy classifies them as having an eGFR < 30 ml/minute/1.73 m2.
The risk of PC-AKI is conditioned on an individual’s true eGFR value, with higher risk assumed in patients with an eGFR < 30 ml/minute/1.73 m2. This risk is assumed to be modifiable by providing either prophylactic measures prior to the provision of contrast agent or changing the imaging modality. Individuals who test negative are managed with their planned contrast-enhanced CT scan, whereas those individuals who test positive are managed to reduce their risk of PC-AKI. The model assumes that the risk of PC-AKI is modifiable only for patients who have a true eGFR measure < 30 ml/minute/1.73 m2. Therefore, individuals who are misclassified as positive (i.e. FP) will incur the costs of the actions taken to reduce their perceived PC-AKI risk, but do not derive any health benefit in terms of a reduction in PC-AKI risk and subsequent clinical events. Individuals who are misclassified as negative (i.e. FN) will not incur the cost of these mediating actions, but will fail to realise the health benefits of receiving an action that would reduce their risk of PC-AKI. In the base case, the mediating action is assumed to be IVH prior to a full-contrast CT scan. In a scenario analysis, individuals were considered to receive a range of possible mediating actions, with a proportion of patients receiving IVH prior to a full-contrast CT scan, a proportion receiving an unenhanced CT scan and a proportion receiving a MRI scan.
Based on evidence from a series of reviews, all individuals are assumed to be at risk of requiring temporary RRT within 6 months of imaging and this risk is assumed to be conditional solely on experiencing a PC-AKI. Based on this evidence, it is also assumed that PC-AKI has no impact on mortality, and that there are no differences between strategies in terms of patients’ costs and HRQoL after 6 months post imaging.
The model considers the costs of testing patients according to the combination of testing components in each strategy. In the base case, undertaking a laboratory test was assumed to always cause a delay and cancellation of the initial CT scan, with consequent loss of the imaging time slot and associated costs. Scenario analyses explored the robustness of the results to alternative assumptions, including that a proportion of the laboratory tests would be urgent and would not result in a delay unless a positive test result was obtained requiring mediating action. Risk factor screening and POC testing would cause the delay and cancellation of the initial CT scan only if they are the final testing component in that strategy and the final result was positive resulting in mediating action being taken. For individuals who undergo mediating actions (i.e. IVH in the base case), the cost of the action taken and any associated adverse events were captured. PC-AKI events are assumed to impose no costs, although PC-AKI events do alter the risk of a patient requiring RRT, which was costed.
Outcomes of patients are captured in QALYs over their remaining lifetime. All patients in the model are assumed to have the same life expectancy and HRQoL as the age- and sex-adjusted general population, with HRQoL decrements applied to those patients who require RRT for a duration of 3 months. No further HRQoL impacts are assumed in the base-case analysis. A scenario analysis considered a HRQoL decrement as a result of anxiety caused by any delay of the CT scan or use of an alternative imaging modality.
Further details of the main structural and input assumptions and the sources of evidence considered for each are discussed in detail in later sections of the report.
Strategies
The strategies included in the model represent the potential pathways that are either part of current clinical practice or represent ways in which POC testing could be integrated into clinical practice. These can be grouped into six types of strategy, according to the testing approach followed:
-
laboratory testing only
-
risk factor screening combined with POC testing
-
risk factor screening combined with laboratory testing
-
risk factor screening combined with POC testing and laboratory testing
-
POC testing only
-
POC testing combined with laboratory testing.
A strategy of ‘no testing and manage all with contrast-enhanced CT’ was not included in the base-case analysis. Although this represents a potentially feasible strategy, it was not deemed to be clinically appropriate given the consistent recommendations reported across clinical guidelines recommending the use of some form of screening or testing to identify individuals at risk of PC-AKI. However, for completeness and to aid the overall interpretation of the results, this strategy was included in a separate scenario. Similarly, a strategy of risk factor screening alone was initially considered but then excluded, as this strategy was not deemed to be clinically feasible as a result of the high rate of FPs that would require IVH and the limited capacity to provide this.
Laboratory testing consists of performing a blood test on all individuals presenting without a recent eGFR measurement prior to imaging. Although the NICE scope distinguished between urgent and non-urgent laboratory tests, no evidence was subsequently identified concerning differences in test performance or unit costs. However, access to urgent laboratory testing has important implications for the timing of clinical decisions and the impact on scanning decisions (i.e. whether or not the scan can be rescheduled within the same day or requires the scan to be rebooked for a separate day). Inevitably, there exists significant heterogeneity across NHS sites in terms of provision and access to urgent laboratory testing. In the base-case analysis, it was assumed that laboratory testing would require the CT scan to be rescheduled on a separate day (i.e. only non-urgent testing). A series of scenarios were also undertaken that assumed that a proportion of patients (i.e. 25%, 50%, 75% and 100%) would receive urgent laboratory testing, allowing their CT scan to be rescheduled for the same day and hence avoiding the full opportunity cost of a lost CT scan appointment.
Individuals who test negative with laboratory testing are assumed to be managed with the planned contrast-enhanced CT scan. Those individuals who test positive receive mediating action to reduce their PC-AKI risk, with management consisting of IVH followed by contrast-enhanced CT in the base-case analysis.
Figure 9 provides a schematic of the model structure for the laboratory testing strategy.
FIGURE 9.
Model structure: laboratory testing. p1, probability of an eGFR < 30 ml/minute/1.73 m2; p2, probability of AKI conditional on an eGFR ≥ 30 ml/minute/1.73 m2 and contrast-enhanced CT scan; and p3, probability of AKI conditional on an eGFR < 30 ml/minute/1.73 m2 and contrast-enhanced CT scan with prophylactic IVH.
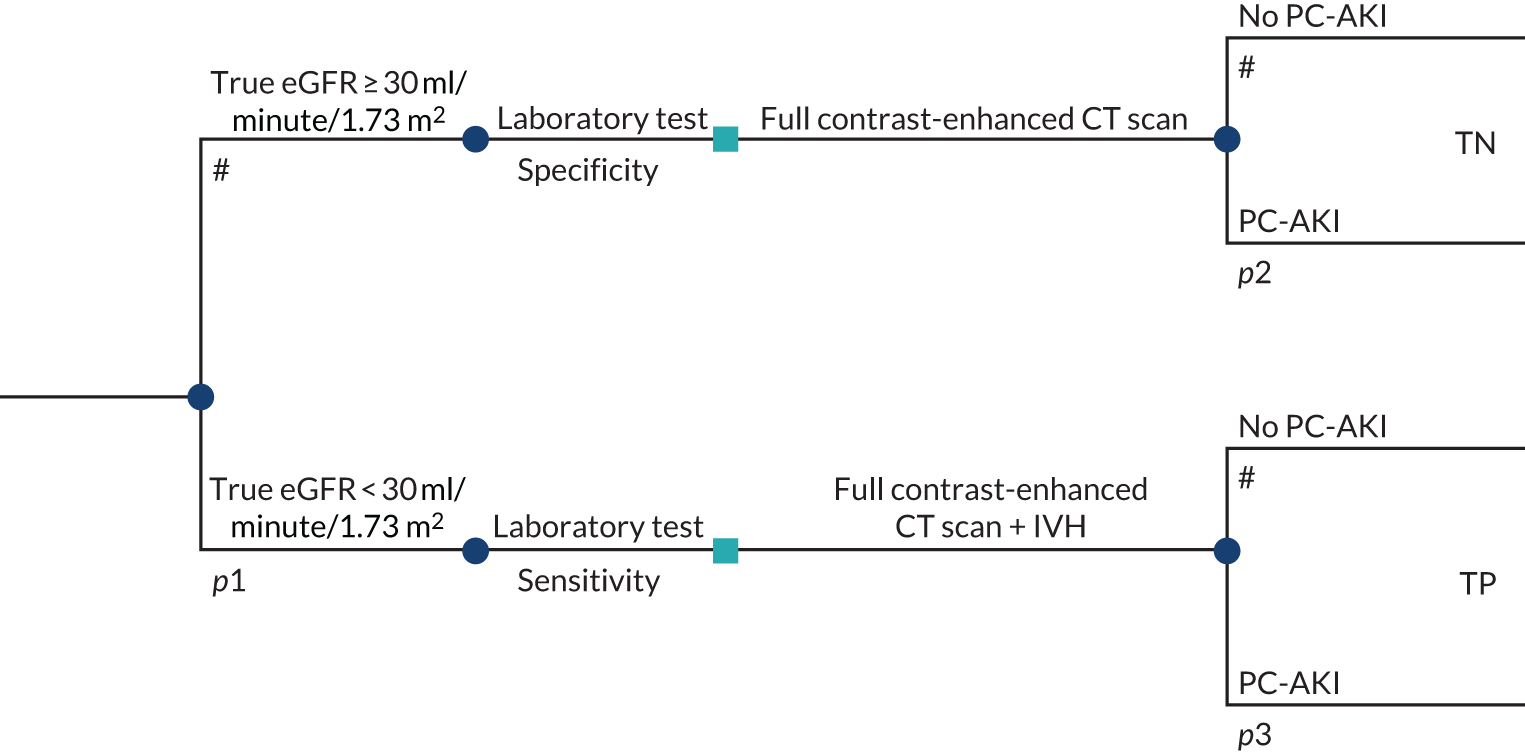
Risk factor screening combined with POC testing consists of screening individuals with a risk factor questionnaire followed by a POC test for individuals identified with at least one risk factor (risk factor positive). Individuals who screen risk factor negative or test negative with the POC test are assumed to proceed with the planned contrast-enhanced CT scan. Individuals who screen positive and have an eGFR measurement of < 30 ml/minute/1.73 m2 with the POC device receive IVH to reduce their PC-AKI risk.
Figure 10 provides a schematic of the model structure for the risk factor screening combined with POC testing strategy.
FIGURE 10.
Model structure: risk factor screening combined with POC testing. p1, probability of an eGFR < 30 ml/minute/1.73 m2; p2, probability of AKI conditional on an eGFR ≥ 30 ml/minute/1.73 m2 and contrast-enhanced CT scan; p3, probability of AKI conditional on an eGFR ≥ 30 ml/minute/1.73 m2 and contrast-enhanced CT scan with prophylactic IVH; p4, probability of AKI conditional on an eGFR < 30 ml/minute/1.73 m2 and contrast-enhanced CT scan; and p5, probability of AKI conditional on an eGFR < 30 ml/minute/1.73 m2 and contrast-enhanced CT scan with prophylactic IVH. RF, risk factor.
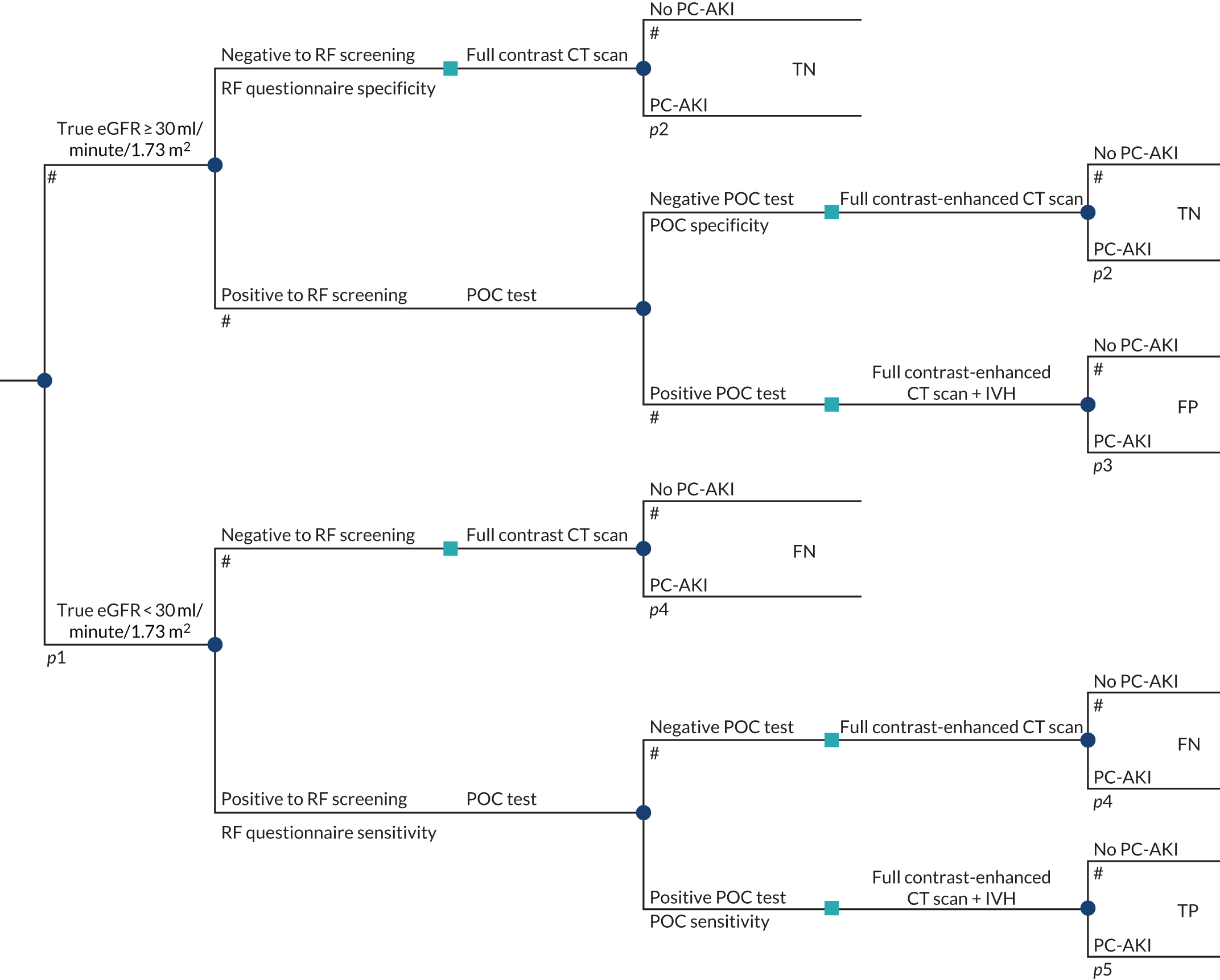
Risk factor screening combined with laboratory testing consists of screening individuals with a risk factor questionnaire followed by a laboratory test for those individuals who screen positive for at least one risk factor. Individuals who have no risk factors, and those who test negative on the laboratory test, receive contrast-enhanced CT scanning. Individuals who screen and test positive receive additional management to reduce their risk of PC-AKI.
Figure 11 provides a schematic of the model structure for the risk factor screening combined with laboratory testing strategy.
FIGURE 11.
Model structure: risk factor screening combined with laboratory testing. p1, probability of an eGFR < 30 ml/minute/1.73 m2; p2, probability of AKI conditional on an eGFR ≥ 30 ml/minute/1.73 m2 and contrast-enhanced CT scan; p3, probability of AKI conditional on an eGFR < 30 ml/minute/1.73 m2 and contrast-enhanced CT scan; and p4, probability of AKI conditional on an eGFR < 30 ml/minute/1.73 m2 and contrast-enhanced CT scan with prophylactic IVH. RF, risk factor.
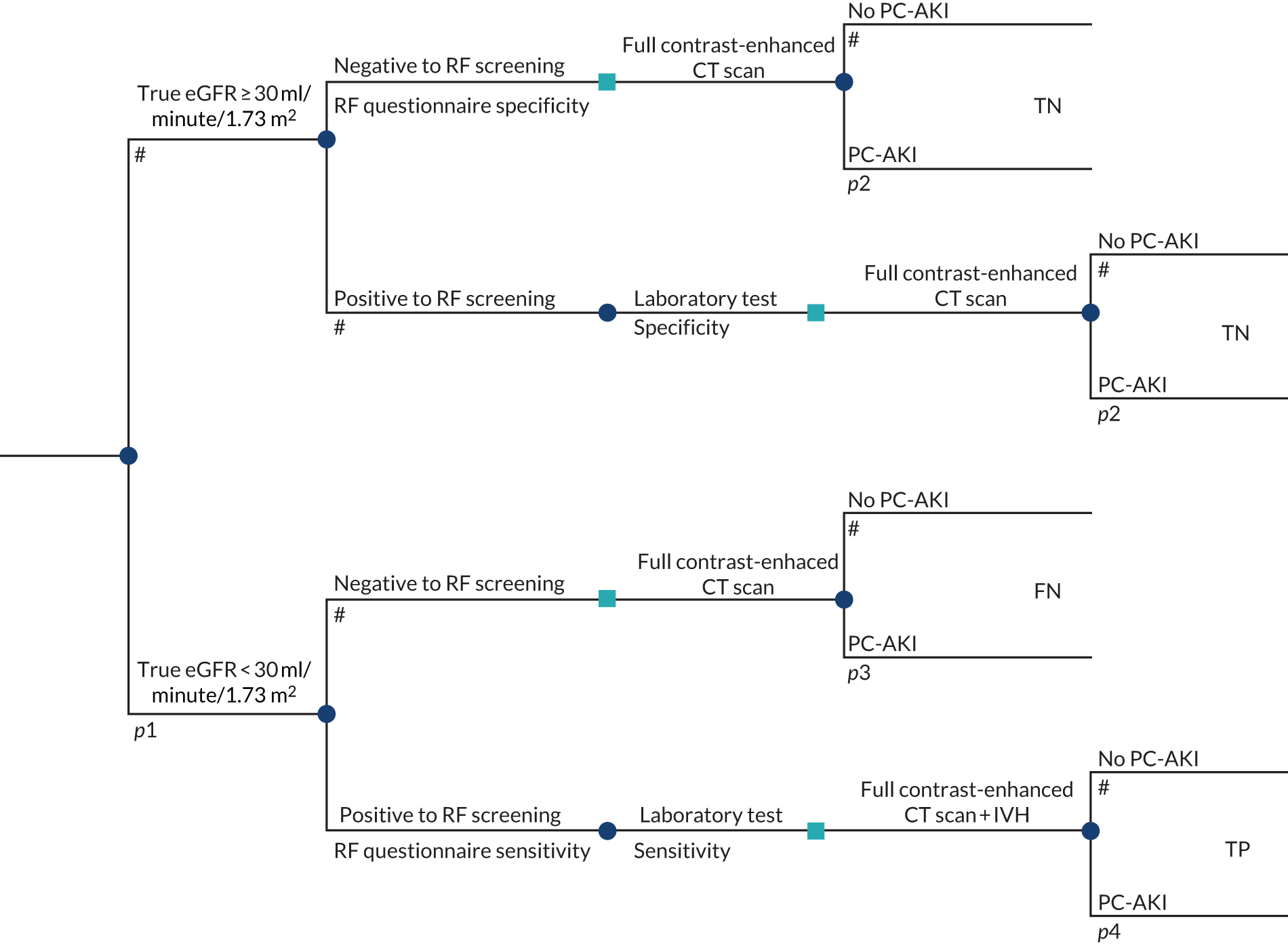
Risk factor screening combined with POC and laboratory testing comprises a three-step testing sequence that involves screening all individuals for risk factors, testing with POC devices those with at least one risk factor, and providing individuals who screen and test positive (with POC devices) with a confirmatory laboratory test. All individuals that have a negative result at any point in the testing sequence are managed with a contrast-enhanced CT scan. Individuals who test positive at all three steps of the testing sequence receive management to reduce their risk of PC-AKI.
Figure 12 provides a schematic of the model structure for the risk factor screening combined with POC and laboratory testing strategy.
FIGURE 12.
Model structure: risk factor screening combined with POC and laboratory testing. p1, probability of an eGFR < 30 ml/minute/1.73 m2; p2, probability of AKI conditional on an eGFR ≥ 30 ml/minute/1.73 m2 and contrast-enhanced CT scan; p3, probability of AKI conditional on an eGFR < 30 ml/minute/1.73 m2 and contrast-enhanced CT scan; and p4, probability of AKI conditional on an eGFR < 30 ml/minute/1.73 m2 and contrast-enhanced CT scan with prophylactic IVH. RF, risk factor.
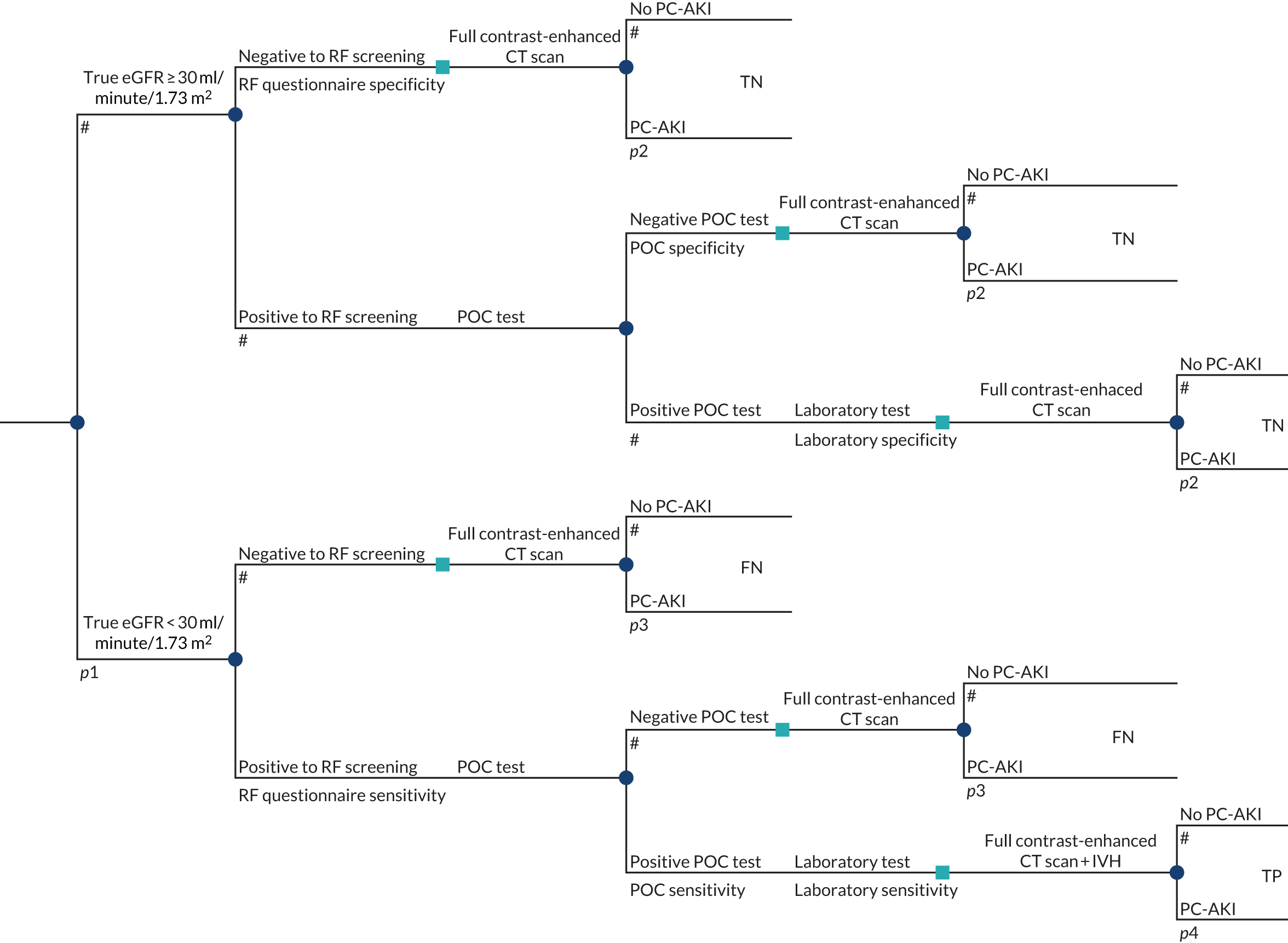
Point-of-care testing consists of testing all individuals with a POC device, with those individuals testing negative managed with a contrast-enhanced CT scan and those individuals testing positive receiving mediating action to reduce their risk of PC-AKI.
Figure 13 provides a schematic of the model structure for the POC testing strategy.
FIGURE 13.
Model structure: POC testing. p1, probability of an eGFR < 30 ml/minute/1.73 m2; p2, probability of AKI conditional on an eGFR ≥ 30 ml/minute/1.73 m2 and contrast-enhanced CT scan; p3, probability of AKI conditional on an eGFR ≥ 30 ml/minute/1.73 m2 and contrast-enhanced CT scan with prophylactic IVH; p4, probability of AKI conditional on an eGFR < 30 ml/minute/1.73 m2 and contrast-enhanced CT scan; and p5, probability of AKI conditional on an eGFR < 30 ml/minute/1.73 m2 and contrast-enhanced CT scan with prophylactic IVH.
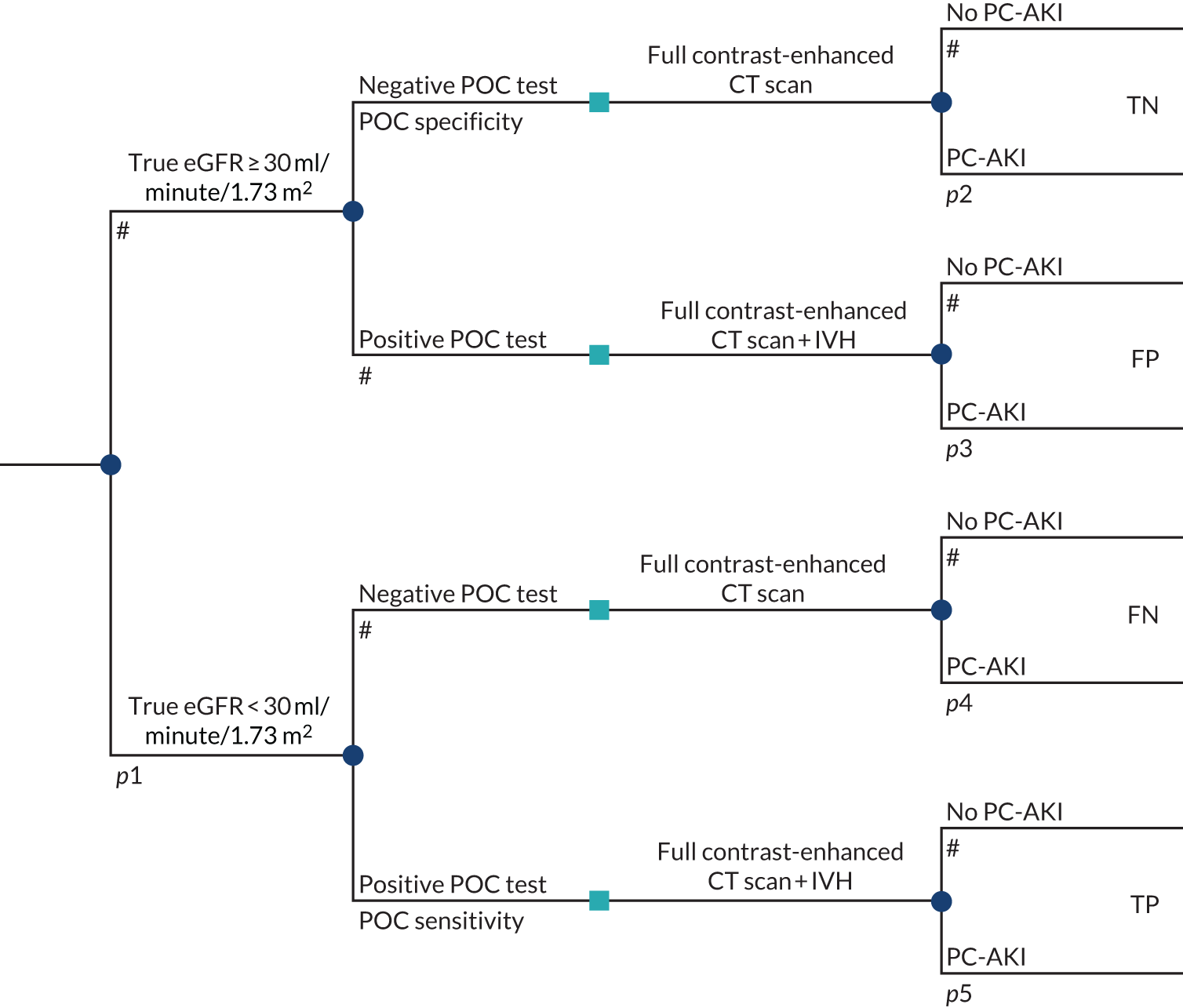
The last strategy type combines POC testing with laboratory testing. Individuals who test positive on the POC test receive a confirmatory laboratory test. Those testing negative on either test receive a contrast-enhanced CT scan, and those testing positive on both sequences receive mediating action to reduce their risk of PC-AKI.
Figure 14 provides a schematic of the model structure for the POC testing combined with laboratory testing strategy.
FIGURE 14.
Model structure: POC testing combined with laboratory testing. p1, probability of an eGFR < 30 ml/minute/1.73 m2; p2, probability of AKI conditional on an eGFR ≥ 30 ml/minute/1.73 m2 and contrast-enhanced CT scan; p3, probability of AKI conditional on an eGFR < 30 ml/minute/1.73 m2 and contrast-enhanced CT scan; and p4, probability of AKI conditional on an eGFR < 30 ml/minute/1.73 m2 and contrast-enhanced CT scan with prophylactic IVH.
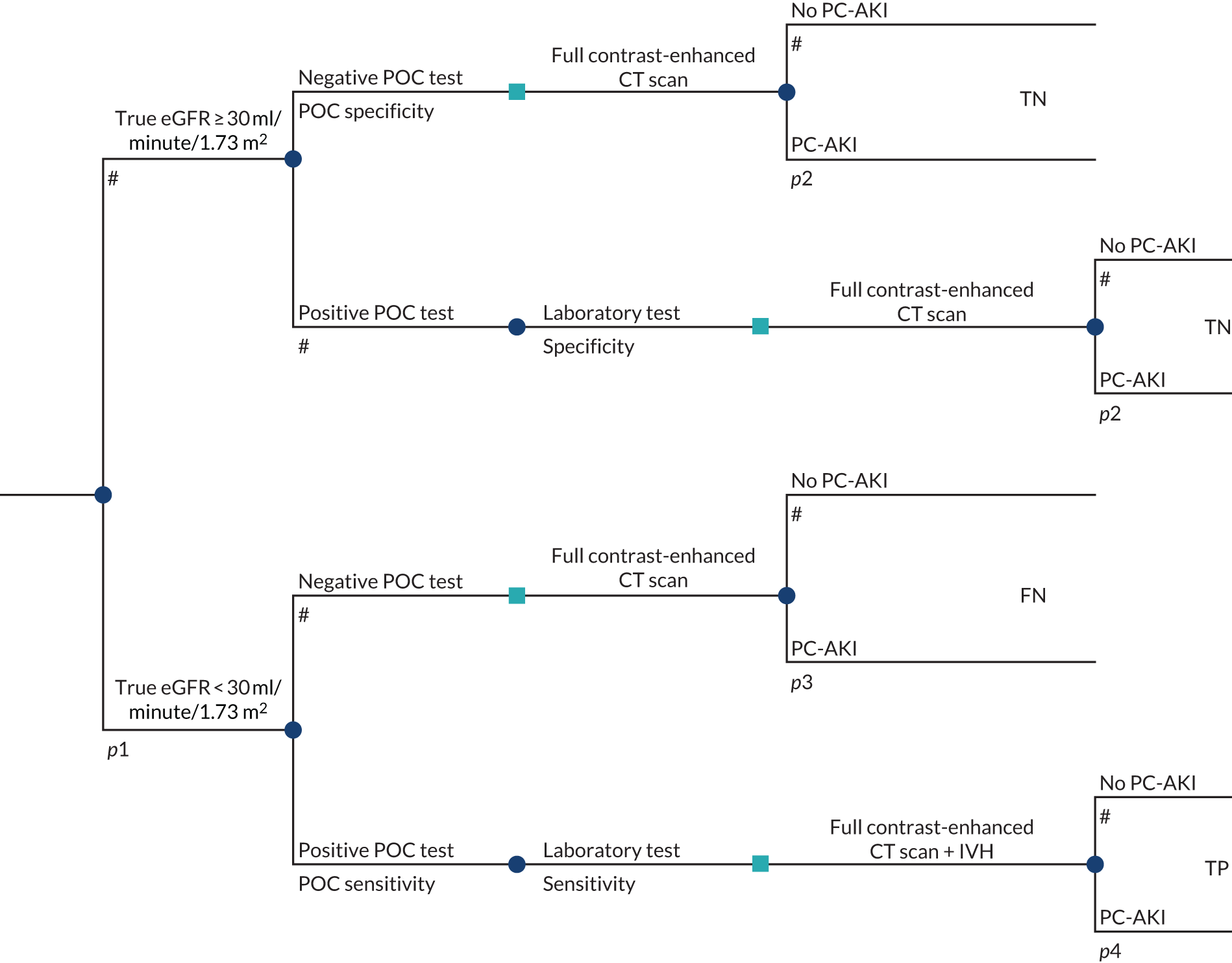
For each type of strategy that includes POC testing, the model considers separate strategies for each of the POC devices. The POC devices considered in the cost-effectiveness analysis are restricted to those that reported diagnostic accuracy data using eGFR thresholds reported in the quantitative synthesis (see Chapter 3, Results: assessment of diagnostic accuracy). The three devices considered in the model are i-STAT Alinity, ABL800 FLEX and StatSensor. In line with the clinical effectiveness review, the different models of i-STAT, ABL 800 series and StatSensor are assumed to be equivalent in terms of diagnostic accuracy data within brand, whereas the costs are derived for the models that are commercially available in the UK, according to the manufacturer.
Although different types of laboratory-based SCr tests are used in clinical practice to derive eGFR values, it is assumed that these values are all equivalent in terms of diagnostic accuracy and costs. The laboratory test is assumed to have perfect diagnostic accuracy (i.e. 100% sensitivity and specificity) and, therefore, laboratory-measured eGFR is assumed equivalent to a ‘true’ eGFR value.
Clinical guidelines recommend that only individuals considered at high risk of PC-AKI have their eGFR measured prior to undergoing contrast-enhanced CT. 6,10–12 However, these guidelines do not recommend the use of any particular screening tool, and there is a lack of consistency across this literature regarding the specific criteria that would allow the identification of high-risk individuals. Therefore, screening in the model was assumed to be conducted with a generic risk factor questionnaire.
Laboratory testing requires time for the test to be processed, which means that some individuals may not be able to undergo CT on the same day. In the base case it was assumed that all individuals undergoing a laboratory test would have their CT scan cancelled. However, a scenario analysis allowed for a proportion of patients to receive a rapid laboratory test, and those patients who test negative are assumed not to have their CT scan cancelled.
Risk factor screening and POC testing are assumed to be conducted within the original CT scan time slot and, therefore, do not introduce any further delays (and associated costs). However, if individuals are identified as requiring alternative management to mitigate the PC-AKI risk, it may also be unfeasible to conduct this within the same day for which their original CT scan was planned. The base case assumes that all patients who require a laboratory test or test positive at the last step of the testing sequence will incur the costs of delay. The proportions requiring delay are varied in scenario analyses.
The model considers three alternative management options for patients who are identified as having an eGFR < 30 ml/minute/1.73 m2 by any of the testing approaches described above. These management approaches are:
-
IVH followed by contrast-enhanced CT scan
-
unenhanced CT scan
-
unenhanced MRI scan.
It is assumed that all approaches are equivalent in terms of diagnostic accuracy of the imaging modality, but differ in terms of cost and effect on the risk of PC-AKI. As previously stated, all patients in the base-case analysis identified as being at high risk of PC-AKI are assumed to be managed with prophylactic IVH and proceed with a full-contrast dose CT scan. It is assumed that adverse events from IVH are associated only with costs and not with any HRQoL loss. Separate scenarios are presented assuming alternative management approaches.
Table 17 summarises the 14 strategies evaluated in the base-case cost-effectiveness analysis.
| Strategy number | Testing | Management | |
|---|---|---|---|
| Label | Description | ||
| 1 | Lab | Test all with a laboratory test |
Test negative:a contrast-enhanced CT scan Test positive:b IVH + contrast-enhanced CT scan |
| 2 | RF + i-STAT | Screen with RF questionnaire. Patients who screen positive are tested with i-STAT | |
| 3 | RF + ABL800 FLEX | Screen with RF questionnaire. Patients who screen positive are tested with ABL800 Flex | |
| 4 | RF + StatSensor | Screen with RF questionnaire. Patients who screen positive are tested with StatSensor | |
| 5 | RF + Lab | Screen with RF questionnaire. Patients who screen positive are also tested with a laboratory test | |
| 6 | RF + i-STAT + Lab | Screen with RF questionnaire. Patients who screen positive are tested with i-STAT. Patients who test positive with POC testing are also tested with a laboratory test | |
| 7 | RF + ABL800 FLEX + Lab | Screen with RF questionnaire. Patients who screen positive are tested with ABL800 Flex. Patients who test positive with POC testing are tested with a laboratory test | |
| 8 | RF + StatSensor + Lab | Screen with RF questionnaire. Patients who screen positive are tested with StatSensor. Patients who test positive with POC testing are tested with a laboratory test | |
| 9 | i-STAT | Test with i-STAT. Patients who test positive with POC testing are tested with a laboratory test | |
| 10 | ABL800 FLEX | Test with ABL800 Flex. Patients who test positive with POC testing are tested with a laboratory test | |
| 11 | StatSensor | Test with StatSensor. Patients who test positive with POC testing are tested with a laboratory test | |
| 12 | i-STAT + Lab | Test with i-STAT. Patients who test positive with POC testing are tested with a laboratory test | |
| 13 | ABL800 FLEX + Lab | Test with ABL800 Flex. Patients who test positive with POC testing are tested with a laboratory test | |
| 14 | StatSensor + Lab | Test with StatSensor. Patients who test positive with POC testing are tested with a laboratory test | |
Model input parameters
Population characteristics
The cost-effectiveness of the alternative strategies will be dependent on the characteristics of the patient population being considered, including the distribution of eGFR and the number of patients who are likely to present without a recent eGFR measurement. The population considered here is non-emergency adult outpatients presenting for intravenous contrast-enhanced CT scanning without an available eGFR measurement at the radiology department.
Distribution of estimated glomerular filtration rate
No published studies were identified in non-emergency adult outpatients presenting for intravenous contrast-enhanced CT scanning without an available eGFR measurement that presented sufficient information to determine the underlying distribution of eGFR. Therefore, additional evidence was sought from the clinical adviser to the EAG (Martine Harris; Dr Martine Harris, Mid Yorkshire Hospitals NHS Trust, 2019, personal communication). Dr Harris provided 1 month’s routine outpatient audit data across three sites from the Mid Yorkshire Hospitals NHS Trust. Data were grouped by bins of eGFR width of 10 ml/minute/1.73 m2 (with an eGFR < 30 ml/minute/1.73 m2 and > 90 ml/minute/1.73 m2 treated as individuals bins) and were available for 816 outpatients, of whom 104 attended radiology without a recent eGFR measure.
Table 54 (see Appendix 8) presents the distribution in the overall sample of 816 outpatients and in the subgroup of patients who attended radiology without a recent eGFR measure. Only one patient in the overall sample (i.e. ‘all outpatients’) had an eGFR < 30 ml/minute/1.73 m2 (0.12%), whereas no patients in the subgroup who attended without a prior eGFR had a measure < 30 ml/minute/1.73 m2. The overall sample and the subgroup without a prior eGFR measurement appear to be broadly comparable, with similar proportions falling into each eGFR bin.
The data provided by Dr Harris (personal communication) were further disaggregated by the reason for referral for CT (suspected cancer, urgent and routine referrals). Table 55 (see Appendix 8) presents the eGFR distribution by reason for referral in the overall sample and in the subgroup of patients who attended radiology without a recent eGFR measure. The reasons for referral appear to differ between the overall sample and the subgroup without a prior eGFR measurement, with the majority of those patients without a prior eGFR measurement being referred routinely (74%), whereas only one-third of the overall sample were referred routinely. Given the additional stratification and, therefore, smaller numbers, the percentages within each eGFR bin appear more variable across reason for referral within the subgroup without prior eGFR measurement. In the overall sample, the percentages across the eGFR bins for each reason for referral appear broadly comparable.
Evidence at less disaggregated eGFR levels (i.e. bands of < 30, 30–60 and ≥ 60 ml/minute/1.73 m2) was also available from two published studies38,114 and a separate report by KiTEC,113 which was commissioned to support this appraisal. The KiTEC report113 provided evidence on the eGFR distribution from a 2-week audit of outpatient radiology patients at Guy’s and St Thomas’ NHS Foundation Trust (GSTT; London, UK).
Table 18 summarises the evidence from these studies compared with the data provided by Dr Harris (personal communication). Both of the Harris populations (i.e. all outpatients and the subgroup without a prior eGFR measurement) appear broadly similar to the populations from the two published studies, although the population in Moos et al. 114 appears slightly less severe, with a higher percentage of patients with eGFR scores > 60 ml/minute/1.73 m2. The audit of outpatient radiology patients at GSTT reports a more severe population, with 15.86% of patients reported to have an eGFR < 30 ml/minute/1.73 m2. The reason for this marked difference was not clear based on the evidence provided in the KiTEC report;113 however, it highlights that the underlying eGFR distribution may vary considerably across different NHS sites.
| eGFR category (ml/minute/1.73 m2) | Study (first author and year), % of patients | ||||
|---|---|---|---|---|---|
| Harris,a 2019 | Moos et al., 2014114 | Snaith et al., 201938 | KiTEC, 2019113 | ||
| All outpatients | Patients without a prior eGFR measurement | ||||
| < 30 | 0.12% | 0.00% | 0.32% | 0.00% | 15.86% |
| 30–60 | 22.18% | 22.12% | 9.84% | 19.33% | 25.17% |
| > 60 | 77.70% | 77.88% | 89.84% | 80.67% | 58.97% |
| Total | 816 | 104 | 925 | 300 | 580 |
Given the granularity with regard to the narrower eGFR bins of the Harris data (personal communication) and comparability with the two published studies,38,114 the Harris data were used to inform the distribution of eGFR of patients in the base-case analysis. In addition, given the similarity in overall eGFR distribution in the overall sample and the subgroup without a prior eGFR measurement, the eGFR distribution in the larger overall sample is used in the base-case analysis. Separate scenario analyses were undertaken using the eGFR distribution from the subgroup with missing eGFRs at presentation and the alternative eGFR distribution provided in the KiTEC report113 from GSTT.
Parametric distributions were fitted to estimate the probability a patient falls into four eGFR categories. These categories represent the eGFR bands reported in the clinical effectiveness review and synthesis (i.e. < 30, 30–45, 45–60 and ≥ 60 ml/minute/1.73 m2). Fitting distributions to the full set of data points resulted in a poor visual fit at the lower levels of eGFRs; therefore, the distribution was fitted only up to an eGFR of 60 ml/minute/1.73 m2, with the probability of being above or below 60 ml/minute/1.73 m2 estimated separately. The log-normal distribution was considered to provide the best visual fit. The resulting probabilities are shown in Table 19. For the overall sample, the fitted log-normal distribution predicted a probability of 0.62% of a patient having an eGFR < 30 ml/minute/1.73 m2.
| eGFR category (ml/minute/1.73 m2) | Probability of eGFR in category | |
|---|---|---|
| All patients (n = 816) | Patients with missing eGFR (n = 104) | |
| < 30 | 0.62% | 0.27% |
| 30–45 | 6.28% | 5.1% |
| 45–60 | 15.45% | 16.44% |
| > 60 | 77.67% | 78.18% |
Number of patients without a recent estimated glomerular filtration rate measurement
The number of patients who present for a contrast-enhanced CT scan without a recent eGFR measurement will determine the size of the population to which POC testing may be offered in the NHS. Based on surveys of NHS services12,13 and discussions with clinical advisers, the behaviour of practices regarding the absence of eGFR measurements is likely to be heterogeneous.
The type of practice behaviour most commonly seen in the NHS can be characterised as follows:
-
CT scans are not allowed to be booked until a recent eGFR measurement can be reported in the referral request; this implies that no individuals arrive for a CT scan without a recent eGFR measurement.
-
CT scans are allowed to be booked without a record of a recent eGFR measurement, but efforts are made by the radiology department to obtain a recent measurement prior to the scan appointment (i.e. by checking electronic records, requesting a blood test from the referrer or directly instigating a laboratory test).
-
CT scans are allowed to be booked without a record of a recent eGFR measurement, but no further checks are implemented by the radiology department prior to the CT scan appointment.
The first type of practice behaviour means that individuals will not present without a recent eGFR measurement and, hence, implies no role for POC creatinine testing; therefore, this type of practice behaviour is not explicitly considered in the model.
Practices that allow booking of a contrast-enhanced CT scan without a confirmed recent eGFR measure differ in terms of the processes and protocols followed regarding how eGFR measurements missing at the time of booking are obtained prior to the scan appointment. Thus, practice behaviour will determine the proportion of patients without a recent eGFR at the point of CT scan. This also has implications for patient throughput and the costs of POC tests. It may also affect the underlying eGFR distribution of patients without a recent eGFR measure.
A formal assessment of the cost-effectiveness of different types of practice behaviour was considered beyond the scope of this appraisal. Instead, a series of assumptions were made concerning the proportion of patients likely to attend without a recent eGFR measurement. Scenario analysis was undertaken to explore the impact of using alternative assumptions and throughput estimates.
Table 20 summarises the evidence identified that reported on the proportion of patients in an outpatient setting presenting with and without recent eGFR values at the different stages at which eGFR measurements are checked.
| Availability of eGFR measurements | Study (first author and year) | |||||
|---|---|---|---|---|---|---|
| Cope et al., 201713 | Snaith et al., 201938 | Harris,a 2019 – all outpatients data | KiTEC, 2019113 | |||
| Clinical experts | GSTT data | |||||
| Audit 2015 | January 2019 | |||||
| % eGFR measurements available (n/N) at referral/vettingb | NR | 54.0 (162/300) | 43.9 (358/816) | NR | 53.5 (77/144) | 47.7 (580/1215) |
| % eGFR measurements that were provided after booking by referrer or from other records | NR | NR | 43.4 (354/816) | NR | 26.4 (38/144) | NR |
| % eGFR measurements missing (n/N) with test instigated by the radiology department | NR | 12.3 (37/300) | 12.7 (104/816) | NR | NR | NR |
| % eGFR measurements missing (n/N) at CT scan | 34 (1220/3584) | 1.33 (4/300) | 1.1 (9/816) | Small but non-zero | 16.7 (24/144) | NR |
The report by Cope et al. 13 provides the largest source of UK evidence. However, the results from this audit are aggregated for all responding practices and, thus, the heterogeneity of practice behaviour cannot be characterised. Therefore, the percentage of patients with missing eGFR values (34%) will include all types of practice behaviour.
Another source of data on outpatients was the sample of 1-month CT attendance data retrospectively collected for the three radiology sites of the Mid Yorkshire Hospitals NHS Trust (Dr Martine Harris, personal communication) [also used by Shinkins and colleagues (Dr Bethany Shinkins, University of Leeds, 2019, personal communication)], which was also used to inform the eGFR distribution in the model. These data may be more reflective of what would be observed in a practice similar to practice type 2, in which patients are actively chased for an eGFR measurement up until the scan. When POC testing is not available, the radiology department would try to obtain a laboratory result up until the day of the scan, and 1.1% of patients would still present on the day without a valid eGFR measurement. However, if POC creatinine testing was an option, it was assumed that the radiology department would be unlikely to directly instigate any laboratory tests and the proportion of patients presenting to the CT scan without an eGFR measurement would be closer to 12.7%. The results from Snaith et al. 38 appear broadly consistent with this.
The KiTEC report presents results from three sources of data:
-
interviews with clinical experts
-
an internal audit data conducted at GSTT
-
a raw data extraction of patients records for outpatients referred for a CT scan at GSTT over 2 weeks in January 2019.
The clinical experts provided only qualitative data that cannot be used in the model. According to the audit data, a fairly high proportion of patients will present to a CT scan without a recent eGFR measurement (i.e. 16.7%). The GSTT raw data included information only on patients at the point of referral, so the proportion of patients with missing eGFR values at the point of scan is unknown. The only data available are the proportion of patients with a valid eGFR measure at the point of referral (47.7%), which is lower than in Snaith et al. 38 (54.0%), but higher than in the Mid Yorkshire Hospitals NHS Trust data (43.4%).
Of the sources identified, the estimates from Cope et al. 13 were considered to be the most representative of the ‘average’ practice behaviour in a UK setting. Therefore, the base-case analysis assumes that 34% of patients have missing eGFR values at the point of CT scan. Scenario analyses were also undertaken to explore the impact of heterogeneity and implications for the throughput assumptions informing the costs of POC testing.
Subgroups
The NICE scope identified two subgroups: (1) people with known existing kidney disease and (2) people at different levels of risk of PC-AKI. In the absence of diagnostic accuracy data specific to these separate subgroups or data reporting the underlying eGFR distributions, a formal assessment of cost-effectiveness in these subgroups was not possible. Although the alternative testing strategies included in the model consider the use of POC testing in different subgroups (i.e. POC testing in all individuals or restricted to only those individuals identified at high risk of PC-AKI), diagnostic accuracy is assumed to be the same for each device regardless of where POC testing is used within the overall patient pathway.
Diagnostic accuracy
Diagnostic accuracy of point-of-care creatinine tests
The model is parameterised using the diagnostic accuracy data from the quantitative synthesis presented in Chapter 3, Results: assessment of diagnostic accuracy. The diagnostic accuracy of the POC devices in Chapter 3, Results: assessment of diagnostic accuracy, are presented in terms of the probability a patient is classified in a given eGFR category (i.e. < 30, 30–44, 45–59 and ≥ 60 ml/minute/1.73 m2) by a POC device conditional on their true eGFR category. However, the economic model considers only a single cut-off point of an eGFR of < 30 ml/minute/1.73 m2 for informing alternative management decisions. In addition, evidence reported in later sections suggests sufficient similarity in risks of PC-AKI and effects of mediating actions on PC-AKI across the range of eGFR in individuals with an eGFR ≥ 30 ml/minute/1.73 m2. 115 Hence, the model structure was further simplified by dichotomising the overall population into those with an eGFR < 30 ml/minute/1.73 m2 and those with an eGFR ≥ 30 ml/minute/1.73 m2. The true eGFR value is assumed to correspond to the laboratory measurement regardless of the method used, although there are variations in diagnostic accuracy across the different laboratory methods. This is a necessary simplifying assumption.
Dichotomising the population based on a single eGFR threshold (i.e. an eGFR < 30 and an eGFR ≥ 30 ml/minute/1.73 m2) means that the sensitivity and specificity of the POC devices for this threshold need to be derived from the probabilities reported for each eGFR category (i.e. < 30, 30–44, 45–59 and ≥ 60 ml/minute/1.73 m2) in Chapter 3, Results: assessment of diagnostic accuracy. The sensitivity of the tests can be taken directly from the results of the quantitative synthesis as the probability that an individual with an eGFR < 30 ml/minute/1.73 m2 is correctly categorised as eGFR < 30 ml/minute/1.73 m2 (p[1,1]). However, by simplifying the model and combining the patients with a true eGFR of > 30 ml/minute/1.73 m2 into one group, it was necessary to combine information on the distribution of population eGFR with the probability of being classified as an eGFR < 30 ml/minute/1.73 m2 for a given true eGFR category (p[i,1] for i [2,3,4]) to estimate the specificity of the POC devices.
The specificity is estimated as the weighted average of the probabilities of being classified as eGFR < 30 ml/minute/1.73 m2 for the eGFR categories (30–44, 45–59 and > 60 ml/minute/1.73 m2) with the weights based on the proportions of patients falling into the eGFR categories. Specificity was estimated using the following equation for each device:
in which p[i,1] is the probability that a patient with true eGFR category i is classified as an eGFR < 30 ml/minute/1.73 m2 and Weighti represents the proportion of the patient population with an eGFR > 30 ml/minute/1.73 m2 who fall into true eGFR category i.
Given that specificity is based on not only the diagnostic accuracy evidence from Chapter 3, Results: assessment of diagnostic accuracy, but also the distribution of population eGFR, it should be noted that when this distribution is altered the specificity of the device will also change.
The base-case analysis estimates are informed by the main analysis reported from the quantitative synthesis. Additional scenario analyses were undertaken using results based on the sensitivity analysis reported in Chapter 3, Results: assessment of diagnostic accuracy, and included:
-
a StatSensor-adjusted data analysis
-
an analysis with studies using the CKD-EPI equation to calculate eGFR.
Table 21 reports POC creatinine diagnostic accuracy estimates applied in the base-case and the scenario analyses. Mean p[i,j] estimates were calculated from 1000 simulated values from the posterior distribution obtained by thinning the 30,000 posterior values generated in each analysis of the evidence synthesis, and used to derive specificity and sensitivity. Means were preferred to medians to ensure that the expected costs and health outcomes predicted by the model reflect the average patient. The model sampled from the p[i,j]-simulated values to derive specificity and sensitivity in the probabilistic sensitivity analysis.
| Analysis | Device | Diagnostic accuracy evidence synthesis | |||||
|---|---|---|---|---|---|---|---|
| i-STAT | ABL800 FLEX | StatSensor | |||||
| Sensitivity | Specificity | Sensitivity | Specificity | Sensitivity | Specificity | ||
| Base case | 84.1% | 98.9% | 86.1% | 99.2% | 73.9% | 99.1% | Base-case (main) analysis |
| Scenario | 84.1% | 98.9% | 86.1% | 99.2% | 84.1% | 99.0% | StatSensor-adjusted data analysis |
| 81.7% | 98.9% | 81.4% | 99.1% | 56.4% | 98.4% | Analysis with the CKD-EPI equation studies only | |
Diagnostic accuracy of risk screening questionnaires
Clinical guidelines recommend risk factor screening for patients without prior eGFR measurements presenting for contrast-enhanced CT scans to avoid unnecessary blood testing. 6,10,11,62 However, these guidelines do not recommend the use of any particular screening tool, and there is a lack of consistency across this literature regarding the specific criteria to identify high-risk patients. 12 Furthermore, survey data of UK radiology departments suggest that different guidelines are followed in clinical practice, resulting in heterogeneity of clinical practice behaviour to prevent PC-AKI.
Another issue relates to the evidence context in which the guidelines and risk factor questionnaires were developed. The eGFR cut-off point at which PC-AKI risk is considered to increase to clinically relevant values has altered over time, and patients are now considered to be at high risk of PC-AKI only at eGFR values < 30 ml/minute/1.73 m2. Therefore, it is unclear if existing screening tools would accurately identify patients at risk of PC-AKI under the currently used diagnostic criterion, especially in patient populations in which the average eGFR is expected to be high, as is the case for non-emergency CT scan outpatients.
Studies identified through reference list searching and citation searching, conducted as part of the pragmatic reviews described in Pragmatic reviews of further evidence to inform the economic model, were examined to identify diagnostic accuracy evidence for risk factor questionnaires. Four studies14,75,114,116 that examined the diagnostic accuracy of risk factor screening questionnaires in an outpatient setting were identified as potentially relevant. In addition, unpublished risk factor screening diagnostic accuracy data were obtained from the 2019 Snaith et al. 38 study (Professor Beverley Snaith, personal communication).
Table 56 (in Appendix 8) summarises the risk factors included in each of the questionnaires. The studies examined 12 different questionnaires used to identify individuals at increased risk of PC-AKI. None of the questionnaires included exactly the same risk factors, but all questionnaires considered previous renal disease and diabetes mellitus as risk factors.
Three of the studies14,38,75 compared the diagnostic accuracy of risk factor screening questionnaires against POC devices, whereas three had a laboratory test as a reference test. 38,114,116 Only three studies14,38,75 included exclusively outpatients and all included patients presenting for a contrast-enhanced CT scan. Data for the relevant eGFR cut-off point (i.e. eGFR < 30 ml/minute/1.73 m2) were reported for three of the studies. 14,38,75
Diagnostic accuracy estimates at different eGFR thresholds are reported, alongside study characteristics, for studies using laboratory and POC test as a reference test in Table 57 (in Appendix 8) and Table 22, respectively.
| Questionnaire (first author and year) | Reference test | eGFR equation | Population | eGFR category (ml/minute/1.73 m2) | |||||
|---|---|---|---|---|---|---|---|---|---|
| < 30 | < 45 | < 60 | |||||||
| Sensitivity | Specificity | Sensitivity | Specificity | Sensitivity | Specificity | ||||
| Azzouz et al., 201414 | StatSensor | CKD-EPI | Outpatients scheduled for a CT scan with and without contrast and MRI | 88.2% | 45.2% | 85.4% | 47.1% | – | – |
| Too et al., 201575 | |||||||||
| Original | StatSensor | CKD-EPI | Outpatients without recent measurement scheduled for contrast-enhanced CT scan | 100.0% | 65.2% | 92.9% | 65.3% | 65.9% | 65.8% |
| Modified | – | – | 85.7% | 86.0% | 43.2% | 86.3% | |||
| Snaith et al., 201938 | |||||||||
| Originala | i-STAT | CKD-EPI | Outpatients attending for a contrast-enhanced CT scan | 100.0% | 47.8% | 69.2% | 48.4% | 62.7% | 50.2% |
| Modifieda | 100.0% | 67.6% | 38.5% | 67.6% | 35.6% | 68.0% | |||
| RANZCR RF | 0.0% | 82.9% | 23.1% | 83.3% | 25.4% | 85.1% | |||
Although diagnostic accuracy data comparing risk factor questionnaires to a gold standard reference test would have been preferable to inform the model, no studies reported these data for the diagnostic cut-off point of interest (i.e. eGFR < 30 ml/minute/1.73 m2). However, the data reported for the eGFR < 45 and < 60 ml/minute/1.73 m2 cut-off points in the studies against a laboratory reference suggest that the sensitivity of the questionnaires is high; sensitivity becomes 100% for the majority of most questionnaires as there is a move from a higher to a lower eGFR cut-off point. The only questionnaires that do not have a sensitivity of 100% at an eGFR < 45 ml/minute/1.73 m2 are those applied in the Snaith et al. study38 (Professor Beverley Snaith, personal communication).
The diagnostic accuracy data from studies comparing risk factor questionnaires to POC devices also suggest that high sensitivity tends to be 100% at the lower eGFR cut-off point (i.e. < 30 ml/minute/1.73 m2). The questionnaire based on The RANZCR guidelines11 is the exception with a sensitivity of 0%, but it is also worth noting that only one patient in Snaith et al. 38 had an eGFR < 30 ml/minute/1.73 m2 and, thus, results are very uncertain. Specificity at an eGFR < 30 ml/minute/1.73 m2 varies between 45.2% and 82.9%. The questionnaire with the lowest overall diagnostic accuracy was that examined by the Azzouz et al. study. 14
In the base-case analysis, the diagnostic accuracy estimates for risk factor screening data were derived from the study by Too et al. 75 This study reported a sensitivity of 100%, which is consistent with the data reported for the studies that used laboratory test as a reference (albeit at higher diagnostic cut-off points). As uncertainty about the diagnostic performance of screening tools remains, a scenario analysis was conducted with data from the Azzouz et al. questionnaire. 14
Table 23 summarises the risk factor screening diagnostic accuracy estimates applied in the model. Beta distributions were fitted to the sensitivity and specificity data to generate random distributions of these parameters in the probabilistic sensitivity analysis.
Risks of post-contrast acute kidney injury
Clinical guidelines have highlighted that individuals with an eGFR < 30 ml/minute/1.73 m2 are potentially at an increased risk of PC-AKI following contrast-enhanced CT and that actions should be taken to mitigate that risk, such as considering an alternative imaging method not using iodine-based contrast media or by providing IVH prophylaxis prior to undertaking contrast-enhanced CT. 6,10,11 Whether or not there is an elevated risk in those individuals with an eGFR between 30 and 45 ml/minute/1.73 m2 undergoing contrast-enhanced CT remains unclear. 6,11
For the purposes of modelling the impact of identifying patients with low eGFRs, it is important to establish the risk of PC-AKI conditional on eGFR and any actions taken to mitigate the risk (e.g. providing IVH). This section considers the evidence for the risk of PC-AKI conditional on eGFRs in individuals receiving contrast-enhanced CT, the effect of IVH on that risk and the effect of removing intravenous contrast on that risk.
Risk of post-contrast acute kidney injury conditional on estimated glomerular filtration rate
Most evidence on the risk of PC-AKI following contrast-enhanced CT comes from inpatient settings in which patients’ creatinine levels are routinely monitored following a scan. However, these patients are not considered representative of the outpatient population considered in this appraisal as these patients are likely to have greater comorbidities and associated risk factors for PCI-AKI. Therefore, further evidence was sought to estimate the risk of PC-AKI conditional on eGFR in a non-emergency outpatient setting.
Eight studies containing PC-AKI evidence in outpatients were identified through reference list searching and citation searching conducted as part of the pragmatic reviews described in Chapter 3, Pragmatic reviews of further evidence to inform the economic model. Three of the eight studies identified104,115,117 had a high percentage of patients with complete follow-up data for all patients, rather than only for patients considered at risk at baseline. Park et al. 115 was considered the most relevant study to identify baseline risk in the population because it contained data from 8 years of follow-up, including patients across eGFR subgroups considered, and used contemporary PC-AKI definitions of an absolute increase in levels of SCr of 0.5 ml/minute or 25% from baseline levels. The study by Park et al. 115 also reported data on the consequences of PC-AKI in terms of mortality and need for RRT, which are discussed in later sections.
Park et al. 115 examined the risk of PC-AKI in 1666 patients with an eGFR < 60 ml/minute/1.73 m2 undergoing contrast-enhanced CT after receiving prophylactic IVH and presented the PC-AKI rate for different eGFR categories (i.e. < 30, 30–44 and 45–60 ml/minute/1.73 m2). These rates are presented in Table 24. Patients with an eGFR < 30 ml/minute/1.73 m2 had a PC-AKI rate of 10.80%, and this decreased to 2.39% in patients with an eGFR between 45 and 60 ml/minute/1.73 m2.
| eGFR (ml/minute/1.73 m2) | Number of patients | Number of PC-AKI events | PC-AKI rate |
|---|---|---|---|
| < 30 | 250 | 27 | 10.80% |
| 30–44 | 579 | 14 | 2.42% |
| 45–60 | 837 | 20 | 2.39% |
| All patients | 1666 | 61 | 3.66% |
Several other outpatient studies were identified that presented the risks of PC-AKI conditional on an eGFR in patients with an eGFR < 60 ml/minute/1.73 m2. The results from these other studies are presented in Table 58 (in Appendix 8). The results from these studies are broadly comparable with those from Park et al. ,115 with the PC-AKI rate in the 30–60 ml/minute/1.73 m2 eGFR group and ranging from 1.3% to 2.6% and from 10.8% to 12.07% in the < 30 ml/minute/1.73 m2 eGFR group.
Given the size of the patient population and its comparability with the other identified outpatient studies, the estimates from Park et al. 115 were used to inform the model. Given the similarity in AKI risk in the eGFR 30–44 ml/minute/1.73 m2 and the eGFR 45–60 ml/minute/1.73 m2 groups, these eGFR categories were pooled, resulting in separate PC-AKI risks applied in the model for an eGFR < 30 and ≥ 30 ml/minute/1.73 m2.
As all patients in the Park et al. 115 study received IVH, additional evidence was also sought to inform the PC-AKI rate in individuals who would be incorrectly misclassified and, hence, would not receive IVH.
Effect of prophylactic intravenous hydration on post-contrast acute kidney injury risk
To account for the effect of prophylactic IVH on the risk of PC-AKI following contrast-enhanced CT imaging, evidence from meta-analyses and other randomised and non-randomised studies was examined. Full details of study sources considered are provided in Chapter 3, Evidence on prophylactic interventions for post-contrast acute kidney injury.
Three meta-analyses100–102 examined the effectiveness of contrast-associated AKI prevention methods, the largest and most recent of which was used to parameterise the model. 102 The study by Ahmed et al. 102 considered the impact of prophylactic IVH in patients with an eGFR < 60 ml/minute/1.73 m2 and found for the comparison against placebo an OR of 0.97 (95% CI 0.52 to 1.9). However, data from the AMACING study104 indicated that there was no effect of IVH on PC-AKI in patients with an eGFR between 30 and 60 ml/minute/1.73 m2. Therefore, for the base case, it was assumed that the prophylactic IVH OR of 0.97 (95% CI 0.52 to 1.9) would be applied to patients with an eGFR < 30 ml/minute/1.73 m2, but that there would be no effect on risk in patients with an eGFR ≥ 30 ml/minute/1.73 m2. A scenario analysis was undertaken using the lower bound of the OR (i.e. 0.52), implying a greater protective effect of IVH compared with the base-case analysis.
Effect of contrast on post-contrast acute kidney injury risk
A review of propensity-matched evidence, identified from the recent Aycock et al. 86 meta-analysis, was conducted to identify studies providing evidence on the effect of contrast agents on PC-AKI stratified by eGFR. Three studies91,92,97 provided evidence on contrast-enhanced CT against unenhanced scans by eGFR category. Table 25 summarises the evidence from these three studies, two of which are reported in detail in Chapter 3, Evidence of the risk of acute kidney injury from contrast agents.
| Study (author and year of publication) | Outcome of interest | Type | OR (95% CI) |
|---|---|---|---|
| Hinson et al., 201797 | AKI and an eGFR 15–29 ml/minute/1.73 m2 | 0.3 mg/dl or 50% above baseline | 0.96 (0.86 to 1.08) |
| Davenport et al., 201390 | AKI and an eGFR < 30 ml/minute/1.73 m2 | 0.3 mg/dl or 50% above baseline | 2.96 (1.22 to 7.17) |
| McDonald et al., 201489 | AKI and an eGFR < 30 ml/minute/1.73 m2 | 0.5 mg/dl above baseline | 0.97 (0.72 to 1.30) |
Hinson et al. 97 was a large propensity-matched study identified through citation searching of the Aycock et al. 86 study. The study by Hinson et al. 97 was excluded from the clinical effectiveness review of evidence of PC-AKI because it was set in an emergency department. However, given the conflicting findings reported by Davenport et al. 90 and McDonald et al. ,89 the additional evidence reported by Hinson et al. 97 was considered relevant and the results from all three studies were pooled to inform the model inputs.
A fixed-effects meta-analysis of these three studies suggested no effect of contrast agents on PC-AKI risk (OR 0.98, 95% CI 0.88 to 1.08). Hence, it was assumed in the base case that there was no effect of contrast agents on the risk of PC-AKI.
Risks of post-contrast acute kidney injury conditional on estimated glomerular filtration rate, prophylactic intravenous hydration and use of contrast agents
For the cost-effectiveness model, the risk of PC-AKI, conditional on eGFR and with and without the use of prophylactic IVH and/or contrast agents was required.
The evidence on PC-AKI conditional on eGFR from the Park et al. 115 study was combined with evidence on the impact of IVH from Ahmed et al. 102 to estimate the probability of a PC-AKI in patients with an eGFR < 30 ml/minute/1.73 m2 and ≥ 30 ml/minute/1.73 m2 who did not receive IVH (with the values for those receiving prophylactic IVH taken directly from Park et al. 115). It was assumed that patients with an eGFR ≥ 60 ml/minute/1.73 m2 had the same risk as those in the eGFR 30–60 ml/minute/1.73 m2 group. Based on the meta-analysis reported in Effect of contrast on post-contrast acute kidney injury risk, it was assumed that there was no impact of contrast agents on the risk of PC-AKI in the base-case analysis.
Table 26 summarises the PC-AKI risks used in the cost-effectiveness model.
| eGFR (ml/minute/1.73 m2) | Risk of PC-AKI | ||
|---|---|---|---|
| Contrast-enhanced CT scan | Unenhanced CT scan | ||
| With IVH | Without IVH | ||
| < 30 | 10.80% | 11.1% | 11.1% |
| ≥ 30 | 2.40% | 2.40% | 2.40% |
The parameters in Table 26 were set up probabilistically in the model by fitting beta distributions to the probabilities of PC-AKI with IVH (for both an eGFR ≥ 30 ml/minute/1.73 m2 and an eGFR < 30 ml/minute/1.73 m2) from Park et al. 115 and a log-normal distribution to the OR of PC-AKI for IVH versus placebo from Ahmed et al. 102
Acute kidney injury consequences and overall mortality
A separate review of published models focusing on the management and consequences of AKI was conducted to further inform the model structure, parameter inputs and assumptions. Further details of the review are reported in Appendix 9.
Based on the review’s findings, the main consequences of PC-AKI include potential mortality risks and the need for RRT. The literature reviewed to inform the risks of PC-AKI in the model was examined for evidence on mortality and risk of RRT conditional on PC-AKI. Park et al. 115 was considered the most relevant to characterise the consequences of PC-AKI in outpatients presenting for CT scan, as the publication reports risks of mortality and initiation of RRT over time by PC-AKI status.
Park et al. 115 present Kaplan–Meier curves by PC-AKI status (PC-AKI vs. no PC-AKI) for time from CT scan until event for (1) death and (2) initiation of RRT (renal survival). Two analyses are presented for each outcome: before and after propensity score matching. The study by Park et al. 115 also reports HRs comparing PC-AKI with no PC-AKI for the full study sample and subgroups by eGFR category (i.e. < 30 vs. ≥ 30 ml/minute/1.73 m2) and timing of events (within 6 months vs. after 6 months of contrast-enhanced CT scan), which are reported in Table 59 (in Appendix 8).
The published Kaplan–Meier curves suggest no difference in terms of mortality for patients who had PC-AKI compared with those who did not, as the curves are largely overlapping for the two groups of patients. This is further supported by the mortality HRs comparing PC-AKI with no PC-AKI, which are consistently non-statistically significant across all analyses; therefore, mortality in the model is assumed to be the same for all patients regardless of PC-AKI status. Mortality was incorporated in the model by applying the costs and QALYs to the PC-AKI pay-offs in the model to the proportion of patients alive at 6 months in Park et al. 115 (i.e. 94.5%). This proportion is assumed to be the same for patients with and without PC-AKI. As baseline mortality risks were not reported by eGFR category, mortality was also assumed to be independent of eGFR levels.
A significant effect of PC-AKI was identified on the probability of RRT initiation. Statistically significant HRs for RRT initiation for the full follow-up period and when events occurring only within 6 months of a CT scan are considered (see Table 59 in Appendix 8). The effect of PC-AKI on the probability of RRT initiation does not appear to be statistically significant in the analysis excluding patients with events after the first 6 months, suggesting that any impact of PC-AKI on the rates of RRT initiation occurs within 6 months of contrast-enhanced CT scanning.
The baseline probability of RRT initiation in the model (i.e. 0.014) is derived from the probability of not having started RRT at 6 months, which is derived from the Kaplan–Meier figure reported for the group who did not experience PC-AKI. The HR for the within-6-months subgroup (i.e. 8.61) is applied to the baseline risk of RRT initiation to estimate the probability of RRT initiation for individuals who experience a PC-AKI event (i.e. 0.111). The HR for RRT initiation for PC-AKI compared with no PC-AKI was set up probabilistically in the model by fitting a log-normal distribution to the data reported in Park et al. 115
Mortality and health-related quality of life
Quality-adjusted life-years were estimated based on estimated mortality and HRQoL. QALYs were discounted at an annual rate of 3.5%. Mortality over 6 months was estimated from a study of post-CT scan patients,115 with mortality post 6 months based on the general population (age and sex adjusted). HRQoL was based on the general population (age and sex adjusted) with utility decrements applied for adverse outcomes, namely undergoing RRT or anxiety resulting from delayed scans. In the base case, RRT is considered the only source of disutility. A scenario analysis also considers the disutility associated with anxiety from delayed scans.
The proportion of patients expected to be alive 6 months post CT scan was derived from Park et al. 115 (i.e. 94.5%) and was estimated as a weighted average of the proportion of patients alive in this study at 6 months post contrast-enhanced CT scan by PC-AKI status (PC-AKI and no PC-AKI). A beta distribution was fitted to the proportion of patients alive at 6 months to derive probabilistic estimates for this parameter. UK life tables were sourced from the Office for National Statistics118 for mortality post 6 months.
Age- and sex-specific general population HRQoL was derived using the equation proposed by Ara and Brazier,119 and applied to the proportion of patients expected to be alive each year (from start age in the model until 100 years old).
Renal replacement therapy was assumed to consist of haemodialysis, based on the study by Kim et al. 117 reporting an earlier data cut-off value of Park et al. 115 The disutility associated with RRT was sourced from a meta-analysis and a metaregression of utilities in CKD patients120 that was identified on the reference list of one of the studies (i.e. Hall et al. 121) examined in the context of the AKI models systematic review. The estimate of –0.11 represents the disutility from dialysis. A gamma distribution was fitted to the utility estimate in the model to generate random draws of the parameter for the probabilistic sensitivity analysis. The disutility is applied for 3 months in the model based on NICE’s Clinical Guideline 169. 108,110 Disutility from anxiety was calculated by assuming that patients would incur the disutility from a EuroQol-5 Dimensions, three-level version (EQ-5D-3L), questionnaire score change from level 1 to level 3 (i.e. –0.236) in the depression/anxiety domain for 2 weeks. The 2-week duration of anxiety was assumed to be the maximum time that patients would have to wait before they could have a CT scan after cancellation of the originally planned scan.
Table 27 details the disutility estimates applied in the model alongside the respective sources and assumptions.
| Adverse outcomes | Utility value (95% CI) | Source | Assumptions |
|---|---|---|---|
| RRT | –0.11 (–0.15 to –0.08) | Wyld et al., 2012120 | 3 months’ duration |
| Anxiety | –0.236 (NA) | EQ-5D-3L score decrement change from level 1 to 3 on the depression/anxiety domain122 | 2 weeks’ duration |
The model does not consider the impact from the delay of the planned CT scan on patient outcomes as a result of any change in their underlying condition during the waiting period. Given the heterogeneity in reasons for referral for a CT scan in the relevant population, and the lack of data sources to characterise the potential impact of delay on HRQoL and disease progression across a wide range of conditions, it was considered unfeasible to include this element in the model. No disutility from PC-AKI was considered, as clinical opinion suggests that the majority of PC-AKI events are asymptomatic.
The potential disutility from adverse events associated with IVH was also not included in the model. The AMACING trial, which compared the cost-effectiveness of IVH to prevent PC-AKI in patients with an eGFR between 30 and 60 ml/minute/1.73 m2 with no IVH, found a small difference in excess hospitalisation days due to adverse events from IVH between treatment arms (i.e. 0.06 days). 104 Therefore, it was considered that any adverse events from IVH would have a short duration and have a very limited impact on HRQoL.
Resource use and costs
Point-of-care device costs
Six manufacturers of a total of seven devices (one manufacturer producing two of the devices) provided evidence on the device costs. These costs included the capital costs per device, consumables per test, quality control consumable costs and annual maintenance costs. The cost of training was not included in the test cost estimates because of a lack of data to inform these parameters. Resource use estimates provided included the time to conduct a test, the time to conduct a quality control procedure and the frequency of quality control procedures required. Information was also provided on the expected lifespan of each device.
Table 28 below details the capital cost per device. For the three devices considered in Chapter 5, the price per device ranged from £4995 to £37,495. The higher capital cost of the Radiometer Ltd ABL800 FLEX reflects that this device is a benchtop unit that allows the user to measure a full panel of up to 18 STAT parameters on the same blood sample. This contrasts with the handheld, single-use design provided by i-STAT Alinity and StatSensor devices.
| Device (manufacturer; device) | Capital cost (per device) | VAT status |
|---|---|---|
| Devices included in the model | ||
| Abbott; i-STAT Alinity | £6500 | Excluding |
| Nova Biomedical; StatSensor | £4995 | Uncertain |
| Radiometer Ltd; ABL800 FLEX | £37,495 | Excluding |
| Other devices | ||
| Abaxis, Inc.; Piccolo Xpress | £11,000 | Excluding |
| Fujifilm Corporation; DRI-CHEM NX 500 | £8500 | Excluding |
| Radiometer Ltd; ABL90 FLEX PLUS | £14,995 | Excluding |
| Siemens Healthineers AG; epoc | £6240 | Excluding |
In terms of the lifespan of the devices, only two manufacturers provided a lifespan estimate. Radiometer Ltd stated that the maximum lifespan of devices would be 7–10 years, whereas Fujifilm Corporation considered the maximum lifespan of its device as 6 years. Other manufacturers noted that it is difficult to assess lifespan of devices, as it will be conditional on the way the devices are used.
Capital costs were annuitised in the model over the expected lifetime of the devices. Given the difficulties in obtaining robust lifetime estimates across the devices, the model assumed a common lifetime estimate of 7 years for all of the devices considered, to estimate the expected annual capital cost of the device.
Table 60 (in Appendix 8) details the consumables cost per test for each device, as well as the expected time taken for the test to report results. For the three devices considered in the model (see Table 28), the cost of consumables per test ranged from £2.88 to £4.75, and the time the devices took to report results varied from 30 seconds to 2 minutes.
Table 61 (in Appendix 8) details the costs of a quality control check required for each device (including, where necessary, multiple levels), as well as the frequency of quality control checks recommended by the manufacturer. The cost per quality control is presented in two ways: the first way includes the total cost of quality control materials for a complete quality control test (this is based on the splitting of quality control materials from larger vials as required); and the second way also includes the cost of any test-based consumables required for the quality control procedure (see Table 60 in Appendix 8).
For the three POC devices included in the cost-effectiveness model (see Table 28), the cost per quality control check excluding test-based consumables ranged from £0.20 to £5.01 and when test-based consumables were also included from £4.15 to £6.80. For two of the POC devices considered, quality control needs to be conducted each day, whereas for the other test it must be conducted every week or every 25 tests, whichever is more frequent.
Table 62 (in Appendix 8) details the annual maintenance costs for each device. The cost for the devices considered in the model ranges from £850 per annum to £4685 per annum.
To estimate the cost per POC test it is necessary to combine this information on costs with expected throughput. Throughput affects the amount of capital cost, the annual maintenance cost and the quality control cost attributed per test conducted (with test consumable costs not being affected by throughput).
Estimates of throughput were based on the data provided by Dr Harris (Dr Martine Harris, personal communication) based on 1 month’s routine outpatient audit data across three sites from the Mid Yorkshire Hospitals NHS Trust. Over a 1-month period, 816 individuals were scanned across three separate sites (272 per site per month). Combining this estimate with the percentage of patients who are assumed to present at their scan appointment without a recent eGFR measurement (34% in the base-case analysis), results in an estimated monthly throughput of 92.6 patients (i.e. 1111 per annum) for the POC devices. If a risk factor questionnaire is used to screen individuals prior to a POC test, fewer individuals will undergo a POC test, resulting in lower throughput and higher costs per POC test. In such cases, throughput for the POC device will be conditional on the accuracy of the risk factor screening and the distribution of eGFRs in the population. In the base case, risk factor screening prior to a POC test results in a POC throughput of 32.6 patients per month. Alternative throughput assumptions were considered in separate scenario analyses.
Table 63 (in Appendix 8) presents the total device cost per POC test based on the expected monthly throughput of 92.6 patients undergoing a POC test assumed in the base-case analysis. For the three devices included in the model, the total device cost per test ranged from £6.71 to £14.07. It should be noted that these costs do not include any consumables for collecting or transferring blood to the POC device, nor are any additional costs included for storage of consumables (e.g. additional refrigerator capacity).
Point-of-care testing will also involve the use of staff time to conduct the tests, including taking blood samples, using the device and conducting quality control checks. Details of the staff time required for each device for pretesting, time to use the device and for quality controls are provided in Table 64 (in Appendix 8).
It was assumed that an additional 3 minutes of staff time would be required for pretesting (i.e. collecting blood), which is assumed to be taken after the patient is cannulated in preparation for the administration of contrast. The time for using the device was based on manufacturers’ estimates of the time it takes the device to report results, with the assumption that the staff member would not conduct any other activities while the device was analysing the sample. For quality control testing, it was assumed that preparation of quality control material would take 1.5 minutes for each device (based on one manufacturer’s reported time) and that conducting the quality control test would take the same time as the device takes to analyse a sample. Where the quality control checking was automatic (two devices), no staff costs were assumed.
Table 64 (in Appendix 8) also reports the estimated total staff cost per test conducted and per quality control procedure conducted (all assumed to be conducted by a band 3 clinical support worker). The staff cost for each test for the three devices considered ranged from £1.66 to £2.14 and the staff cost for conducting the quality control check ranged from £0.00 to £1.46. As with the device-related quality control costs, quality control staff costs need to be attributed per test conducted based on expected throughput. The final column in Table 64 shows the estimated total staff cost per test conducted (including the allocated quality control staff cost). For the three devices considered in the model, this estimated total staff cost ranged from £1.66 to £2.14 based on a monthly throughput of 92.6 patients (1111 per annum). It should be noted that no staff time has been considered for training.
Other costs
Testing costs
The previous section considered costs associated with the POC devices, including staff costs for conducting tests and quality control costs. Other costs considered in the model in the testing stage include risk factor screening, laboratory testing and a phlebotomist’s time. Risk factor screening was assumed to take 2 minutes and 40 seconds by a clinical support worker,77 whereas taking a blood sample was assumed to take (confidential information has been removed) of a phlebotomist’s time (Dr Bethany Shinkins, personal communication). These costs were combined with published national unit costs to estimate the cost per test. 123 The cost of laboratory testing was taken from the National Schedule of Reference Costs – Year 2017–18 – NHS Trust and NHS Foundation Trusts. 124
Table 29 details the unit costs for each of these costs and the cost per POC test (inclusive of capital, consumable, quality control and staff costs) based on the base-case throughput assumptions of 92.6 patients receiving a POC test without risk factor screening and 32.6 patients with risk factor screening.
| Cost category | Resource use | Units | Source | Unit cost | Source/assumptions | Cost |
|---|---|---|---|---|---|---|
| RF screening | Clinical support worker | 2.67 minutes | Ledermann et al., 201077 | £25.00/hour |
Curtis and Burns,123 2017 Assumed the equivalent to a hospital nurse (band 3) |
£1.11 |
| Laboratory test | Laboratory worker | One test | – | £1.11/test |
National Schedule of Reference Costs – Year 2017–18 – NHS Trust and NHS Foundation Trusts 124 Reference cost DAPS04,111 directly accessed clinical biochemistry |
|
| Phlebotomist | Confidential information has been removed | Dr Bethany Shinkins, personal communication | Confidential information has been removed | Confidential information has been removed | ||
| Total cost of a laboratory test | £3.31 | |||||
| POC tests | i-STAT – without RF screening | One test | See Point-of-care device costs | £8.85/test | See Point-of-care device costs | £8.85 |
| ABL800 FLEX – without RF screening | One test | £15.73/test | £15.73 | |||
| StatSensor – without RF screening | One test | £8.52/test | £8.52 | |||
| i-STAT – with RF screening | One test | £11.96/test | £11.96 | |||
| ABL800 FLEX – with RF screening | One test | £36.36/test | £36.36 | |||
| StatSensor – with RF screening | One test | £14.25/test | £14.25 | |||
Table 30 reports the testing costs for each stage of all of the strategies, as well as the total identification costs if a patient undergoes all of the screening and test steps for that strategy. Risk factor screening costs £1.11, whereas POC test costs vary from £8.52 to £15.73 when used without risk factor screening and from £11.96 to £36.36 when used with risk factor screening. A laboratory test costs £3.31.
| Strategy | Costs | |||
|---|---|---|---|---|
| Risk factor screening | POC testa | Laboratory test | Total testing (excluding additional phlebotomist cost for a positive POC test) | |
| 1. Lab | – | – | £3.31 | £3.31 |
| 2. RF + i-STAT | £1.11 | £11.96 | – | £13.07 |
| 3. RF + ABL800 FLEX | £1.11 | £36.36 | – | £37.47 |
| 4. RF + StatSensor | £1.11 | £14.25 | – | £15.36 |
| 5. RF + Lab | £1.11 | – | £3.31 | £4.42 |
| 6. RF + i-STAT + Lab | £1.11 | £11.96 | £3.31 | £16.38 |
| 7. RF + ABL800 FLEX + Lab | £1.11 | £36.36 | £3.31 | £40.78 |
| 8. RF + StatSensor + Lab | £1.11 | £14.25 | £3.31 | £18.67 |
| 9. i-STAT | – | £8.85 | – | £8.85 |
| 10. ABL800 FLEX | – | £15.74 | – | £15.74 |
| 11. StatSensor | – | £8.52 | – | £8.52 |
| 12. i-STAT + Lab | – | £8.85 | £3.31 | £12.16 |
| 13. ABL800 FLEX + Lab | – | £15.74 | £3.31 | £19.05 |
| 14. StatSensor + Lab | – | £8.52 | £3.31 | £11.83 |
For POC test costs, there is an additional £2.50 cost for setting up the cannula if the contrast-enhanced CT scan is cancelled because of a positive POC test result. This was based on the assumption that 6 minutes of a clinical support worker’s time is needed to set up the cannula for the admission of intravenous contrast agents for the CT scan, which is done prior to the taking of blood for the POC test (which was assumed to take an additional 3 minutes of the clinical support worker’s time). This cost is captured in the contrast-enhanced CT Healthcare Resource Group (HRG) and so is already reflected in the cost applied if the patient goes on to receive a contrast-enhanced CT scan (described in Management and imaging costs). However, if the CT scan is cancelled, the cost of an unenhanced CT scan HRG is used to reflect the cost of a cancelled test, which would not include the cost of the initial cannulisation. Therefore, the additional cost of 6 minutes of a clinical support worker’s time is added. For laboratory testing, whether or not cannulisation is done subsequently to a POC test, it is assumed that an additional 6 minutes of a phlebotomist’s time is required, and the cost of £3.31 for the phlebotomist (£2.20) and laboratory work (£1.11) is always applied.
Management and imaging costs
In addition to the identification costs, there are also the costs associated with patient management and the imaging conducted. Management costs include cancellation and rebooking of appointments, follow-up appointments with nephrologists for those patients categorised as having an eGFR < 30 ml/minute/1.73 m2, IVH for patients before undergoing full-contrast CT scans and costs associated with adverse events from IVH. Imaging considered includes contrast-enhanced CT, unenhanced CT and MRI.
Table 31 summarises the costs used for patient management and imaging. Costs were estimated based on resource use estimates and assumptions and combined with national reference costs. 123,124 If a scan is cancelled, the cost of an unenhanced CT scan (£87.92) is applied to reflect the cost of the cancelled scan. It is assumed that it takes (confidential information has been removed) of a staff member’s time to rebook a CT scan and/or book IVH, costing (confidential information has been removed) (Dr Bethany Shinkins, personal communication). If a patient is identified as having an eGFR < 30 ml/minute/1.73 m2, it is assumed that they will have a follow-up appointment with a nephrologist to discuss their CKD, costing £186.49.
| Cost category | Resource use | Units | Source | Unit cost | Source/assumptions | Cost |
|---|---|---|---|---|---|---|
| Imaging | CT scan – contrast enhanced | One scan | – | £111.65 per scan | NHS Reference Costs 2017/18;124 activity-weighted average of HRG currency codes RD21A, RD24Z, RD25Z for outpatients and direct access undergoing CT scanning with contrast | £111.65 |
| CT scan – unenhanced | One scan | – | £87.92 per scan | NHS Reference Costs 2017/18;124 activity-weighted average of HRG currency codes RD20A, RD23Z, RD25Z for outpatients and direct access undergoing CT scanning without contrast | £87.92 | |
| MRI | One scan | – | £151.98 per scan | NHS Reference Costs 2017/18;124 activity-weighted average of HRG currency code RD04Z for outpatients and direct access undergoing MRI without contrast | £170.53 | |
| Cancellations | Rebooking CT scan and/or hydration | Confidential information has been removed | Dr Bethany Shinkins, personal communication | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Cancellation | One scan | Assumption | £87.92/scan | Same as an unenhanced CT scan | £87.92 | |
| Follow-up | Nephrologist | One visit | Assumption | £186.49 | NHS Reference Costs 2017/18;124 all outpatient, consultant led, Nephrology | £186.49 |
| i.v. hydration | Admission | 1 day | – | £340.89 per day | NHS Reference Costs 2017/18;124 weighted average of HRG KC05K-N, fluid or electrolyte disorders, without interventions | £340.89 |
| AEs from i.v. hydration | Hospitalisation | 0.06 | Nijssen et al., 2017104 | £431.00 per night | NHS Reference Costs 2017/18;124 elective inpatient excess bed-days (across all codes) | |
| Specialist inpatient consultation | 0.04 | Nijssen et al., 2017104 | £143.44 per visit | NHS Reference Costs 2017/18;124 average across HRGs of outpatient consultant-led appointments | ||
| In-hospital diagnostics | 0.02 | Nijssen et al., 2017104 | £58.36 per test | NHS Reference Costs 2017/18;124 activity-weighted average of HRG currency code AA33C. Total HRG activity excluding excess bed-days | ||
| Total cost of AEs from i.v. hydration per patient | £32.76 | |||||
Patients who require IVH are assumed to be admitted as a day case at a cost of £340.89. IVH is also associated with adverse events including hospitalisation, specialist inpatient consultation and in-hospital diagnostics. The probability of these adverse events occurring was taken from Nijssen et al. 104 and the costs of each from NHS reference costs, resulting in an expected cost of adverse events per patient undergoing IVH of £32.76. To reflect the variation in the number of areas being scanned and whether the scans were costed as outpatients or direct access, weighted averages of HRG codes were used to estimate the cost of each type of scan (i.e. unenhanced CT, contrast-enhanced CT and MRI), with the weight reflecting the total number of each type of HRG in the NHS. The costs of imaging were £87.92 for an unenhanced CT scan, £111.65 for a contrast-enhanced CT scan and £151.98 for a MRI scan.
Costs associated with outcomes
The model considers the occurrence of PC-AKI and RRT. Clinical opinion suggests that the majority of PC-AKI events in the study population are asymptomatic and, therefore, unlikely to require the use of health-care resources. Hence, only the costs associated with RRT are included in the model.
The cost of RRT is applied to patients who underwent RRT in the model. Table 65 (in Appendix 8) summarises the costs of RRT. As highlighted in Mortality and health-related quality of life, RRT was assumed to consist of haemodialysis and have a duration of 3 months. The number of haemodialysis sessions per week was sourced from NICE’s clinical guideline number 169,108,110 and unit costs taken from NHS reference costs. 124 The total cost of RRT applied in the model was £9758.
Analytic methods
Overview
The decision-analytic model is evaluated deterministically and probabilistically for the base-case analysis using 1000 Monte Carlo simulations to reflect the joint uncertainty across all of the inputs according to the probability distributions assigned to each input. The parameters set up probabilistically in the model are POC devices diagnostic accuracy data; risk factor questionnaire diagnostic accuracy data; risks of PC-AKI; the HR for the initiation of RRT; the proportion of patients alive at 6 month post contrast; and disutility from RRT.
Following conventional decision rules for cost-effectiveness, the mean costs and QALYs for the various strategies are presented and cost-effectiveness compared by estimating the incremental cost-effectiveness ratios (ICERs), as appropriate.
A limitation of conventional ICER decision rules is that the interpretation of negative and positive ICERs is ambiguous without reference to the cost-effectiveness plane. In contrast to conventional ICER decision rules, the net benefit approach provides an unambiguous decision rule. Net benefits can be expressed on the effect scale [i.e. net health benefits (NHBs)] or the cost scale [i.e. net monetary benefits (NMBs)] and are estimated by rearranging the elements of the conventional ICER equation, where:
In contrast to conventional ICER decision rules, the net benefit approach provides an unambiguous decision rule. For a given cost-effectiveness threshold, the strategy with the highest net benefit is the same strategy that would be considered cost-effective when comparing ICERs against the threshold. A further advantage of using the net benefit framework in the current appraisal is that it may provide a more useful way to summarise results when there are very small differences in QALYs between strategies. In this situation ICERs can be highly sensitive to very small changes in the denominator (i.e. QALY differences).
Uncertainty regarding the appropriate source of data, the appropriate assumptions or model structure and other scenarios are explored using a series of deterministic scenario analysis, as described further in Scenario analyses.
Base-case analysis
The parameters and main assumptions used within the base-case economic model, and their characteristics, are summarised in Table 66 (in Appendix 8).
Scenario analyses
To investigate the impact of several key parameter and structural assumptions, a series of deterministic scenario analyses were undertaken. These scenarios are summarised in Table 32.
| Number | Scenario name | Element of uncertainty | Description |
|---|---|---|---|
| 1 | StatsSensor-adjusted analysis | Diagnostic accuracy – additional analyses | Data for StatSensor based on adjusted data analysis (see Additional analyses) |
| 2 | CKD-EPI equation studies | Diagnostic accuracy – additional analyses | Quantitative synthesis based only on studies calculating eGFR using CKD-EPI equation (see Additional analyses) |
| 3 | Alternative risk factor questionnaire | Diagnostic accuracy – quantitative synthesis | Diagnostic accuracy of risk factor screening questionnaires informed by data on an alternative questionnaire (from the Azzouz et al. study14) |
| 4 | eGFR distribution – Harris subgroup | eGFR distribution | Distribution of eGFRs based on the subgroup of individuals without a prior eGFR measurement at referral (the Mid Yorkshire Hospitals NHS Trust) |
| 5 | eGFR distribution – GSTT audit | eGFR distribution | Distribution of eGFRs based on a raw data extraction of patient records for outpatients referred to a CT scan at the GSTT over 2 weeks in January 2019 |
| 6.1 | Throughput | Throughput estimates |
Throughput estimates adjusted for alternative assumptions concerning the proportion of individuals attending a scan appointment without a recent eGFR measurement 12.7% (compared with 34% in base-case analysis) based on data from the Mid Yorkshire Hospitals NHS Trust |
| 6.2 | Throughput | Throughput estimates | Throughput estimates 50% lower than base case |
| 6.3 | Throughput | Throughput estimates | Throughput estimates 50% higher than base case |
| 7.1 | Proportion of cancelled CT scans (0%) | Opportunity cost of delayed/rescheduled CT scan | 0% of CT scans are cancelled as a result of requiring a laboratory test (i.e. all laboratory testing assumed to be urgent) |
| 7.2 | Proportion of cancelled CT scans (25%) | Opportunity cost of delayed/rescheduled CT scan | 25% of CT scans are cancelled as a result of requiring a laboratory test (i.e. 75% of laboratory testing assumed to be urgent and 25% non-urgent) |
| 7.3 | Proportion of cancelled CT scans (50%) | Opportunity cost of delayed/rescheduled CT scan | 50% of CT scans are cancelled as a result of requiring a laboratory test (i.e. 50% of laboratory testing assumed to be urgent and 50% non-urgent) |
| 7.4 | Proportion of cancelled CT scans (75%) | Opportunity cost of delayed/rescheduled CT scan | 75% of CT scans are cancelled as a result of requiring a laboratory test (i.e. 25% of laboratory testing assumed to be urgent and 75% non-urgent) |
| 8 | Anxiety from delay | HRQoL impact of scan delay | Disutility from anxiety is included for patients who have their CT scan delayed |
| 9 | Effect of i.v. hydration (PC-AKI risk) | Effect of i.v. hydration on PC-AKI risk (an eGFR < 30 ml/minute/1.73 m) | The effect of i.v. hydration in reducing the risk of PC-AKI was increased using the lower bound of the treatment effect reported by Ahmed et al.102 (OR 0.52 vs. 0.97 applied in the base-case analysis) |
| 10.1 | Management approach for test positives | Management approach assumed for patients who test positive to POC/laboratory tests |
50% receive i.v. hydration followed by a contrast-enhanced CT scan 50% receive unenhanced CT scan |
| 10.2 | Management approach for test positives | Management approach assumed for patients who test positive to POC/laboratory tests |
One-third receive i.v. hydration followed by a contrast-enhanced CT scan One-third receive an unenhanced CT scan One-third receive a MRI |
| 11.1 | No testing – i.v. contrast media for all | Exclusion of no-testing strategy in the base case | All patients assumed to be given i.v. contrast with no additional testing |
| 11.2 | No testing – i.v. contrast media for all | Exclusion of no-testing strategy in the base case and more optimistic assumption concerning the effect of i.v. hydration is reducing PC-AKI risk (an eGFR < 30 ml/minute/1.73 m) | Combination of scenarios 9 and 11.1 |
Model validation
The model was developed by one researcher (AD) and the programming was checked by a second researcher (MS). A separate version of the model was independently programmed by a third researcher (SW), who successfully replicated the base-case results.
Results of the independent economic assessment
Base case
Deterministic and probabilistic results expressed in NMB and NHB at a cost-effectiveness threshold of £20,000 per QALY are presented in Tables 33 and 34, respectively. Strategy ranking from the highest (1) to the lowest (14) average net benefit is presented in both tables. Incremental net benefit was calculated for each strategy compared with laboratory testing (‘Lab’). Results for the upper bound of the cost-effectiveness threshold recommended by NICE, that is, £30,000 per additional QALY, are not presented, with the exception of probabilities, which are presented for the range of cost-effectiveness thresholds. Results were consistent across the range of cost-effectiveness thresholds considered, and for both deterministic and probabilistic analyses.
| Identification | Management | Total | At £20,000 per QALY | NB rank | |||||
|---|---|---|---|---|---|---|---|---|---|
| Costs | QALYs | NHB (QALYs) | NMB | INHB (QALYs) | INMB | ||||
| 1 | Lab | £363.26 | 9.991371010 | 9.97321 | £199,464.16 | 0.00000 | £0.00 | 14 | |
| 2 | RF + i-STAT | £278.02 | 9.991371002 | 9.97747 | £199,549.40 | 0.00426 | £85.25 | 4 | |
| 3 | RF + ABL800 FLEX | £285.87 | 9.991371003 | 9.97708 | £199,541.55 | 0.00387 | £77.39 | 9 | |
| 4 | RF + StatSensor | £277.84 | 9.991370997 | 9.97748 | £199,549.58 | 0.00427 | £85.42 | 3 | |
| 5 | RF + Lab | £304.06 | 9.991371010 | 9.97617 | £199,523.36 | 0.00296 | £59.20 | 13 | |
| 6 | RF + i-STAT + Lab | £275.84 | 9.991371002 | 9.97758 | £199,551.58 | 0.00437 | £87.42 | 1 | |
| 7 | RF + ABL800 FLEX + Lab | £284.39 | 9.991371003 | 9.97715 | £199,543.03 | 0.00394 | £78.87 | 8 | |
| 8 | RF + StatSensor + Lab | £276.15 | 9.991370997 | 9.97756 | £199,551.27 | 0.00436 | £87.11 | 2 | |
| 9 | i-STAT | £286.35 | 9.991371002 | 9.97705 | £199,541.07 | 0.00385 | £76.91 | 10 | |
| 10 | ABL800 FLEX | £290.99 | 9.991371003 | 9.97682 | £199,536.43 | 0.00361 | £72.28 | 12 | |
| 11 | StatSensor | £283.96 | 9.991370997 | 9.97717 | £199,543.46 | 0.00396 | £79.30 | 7 | |
| 12 | i-STAT + Lab | £280.08 | 9.991371002 | 9.97737 | £199,547.34 | 0.00416 | £83.18 | 6 | |
| 13 | ABL800 FLEX + Lab | £286.70 | 9.991371003 | 9.97704 | £199,540.72 | 0.00383 | £76.56 | 11 | |
| 14 | StatSensor + Lab | £279.09 | 9.991370997 | 9.97742 | £199,548.33 | 0.00421 | £84.17 | 5 | |
| Identification | Management | Total | At £20,000 per QALY | NB rank | Probability cost-effective at | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Costs | QALYs | NHB (QALYs) | NMB | INHB (QALYs) | INMB | £20,000 per QALY | £30,000 per QALY | ||||
| 1 | Lab | £367.12 | 9.993255191 | 9.97490 | £199,497.99 | 0.00000 | £0.00 | 14 | 0.0% | 0.0% | |
| 2 | RF + i-STAT | £281.87 | 9.993255167 | 9.97916 | £199,583.23 | 0.00426 | £85.24 | 4 | 0.0% | 0.0% | |
| 3 | RF + ABL800 FLEX | £289.72 | 9.993255171 | 9.97877 | £199,575.39 | 0.00387 | £77.40 | 9 | 0.0% | 0.0% | |
| 4 | RF + StatSensor | £281.70 | 9.993255154 | 9.97917 | £199,583.40 | 0.00427 | £85.42 | 3 | 0.0% | 0.0% | |
| 5 | RF + Lab | £307.94 | 9.993255191 | 9.97786 | £199,557.17 | 0.00296 | £59.18 | 13 | 0.0% | 0.0% | |
| 6 | RF + i-STAT + Lab | £279.70 | 9.993255167 | 9.97927 | £199,585.40 | 0.00437 | £87.42 | 1 | 79.3% | 79.3% | |
| 7 | RF + ABL800 FLEX + Lab | £288.24 | 9.993255171 | 9.97884 | £199,576.87 | 0.00394 | £78.88 | 8 | 0.0% | 0.0% | |
| 8 | RF + StatSensor + Lab | £280.01 | 9.993255154 | 9.97925 | £199,585.09 | 0.00436 | £87.10 | 2 | 20.7% | 20.7% | |
| 9 | i-STAT | £290.20 | 9.993255167 | 9.97875 | £199,574.90 | 0.00385 | £76.91 | 10 | 0.0% | 0.0% | |
| 10 | ABL800 FLEX | £294.83 | 9.993255171 | 9.97851 | £199,570.27 | 0.00361 | £72.28 | 12 | 0.0% | 0.0% | |
| 11 | StatSensor | £287.82 | 9.993255154 | 9.97886 | £199,577.29 | 0.00396 | £79.30 | 7 | 0.0% | 0.0% | |
| 12 | i-STAT + Lab | £283.93 | 9.993255167 | 9.97906 | £199,581.17 | 0.00416 | £83.19 | 6 | 0.0% | 0.0% | |
| 13 | ABL800 FLEX + Lab | £290.55 | 9.993255171 | 9.97873 | £199,574.55 | 0.00383 | £76.57 | 11 | 0.0% | 0.0% | |
| 14 | StatSensor + Lab | £282.95 | 9.993255154 | 9.97911 | £199,582.15 | 0.00421 | £84.17 | 5 | 0.1% | 0.1% | |
The strategy with highest incremental net benefit is strategy 6, that is, ‘RF + i-STAT + Lab’, with an incremental NMB of £87.42 (Table 33) compared with ‘Lab’. Strategy 6 is also the strategy with the highest probability of being the most cost-effective (Table 34; 79.3% for cost-effectiveness thresholds of £20,000 and £30,000 per additional QALY). The strategy ‘RF + i-STAT + Lab’ is also the least costly of all strategies under comparison, with expected total costs of £275.84, but generates fewer QALYs than the majority of other strategies.
Table 77 (in Appendix 10) shows the results of the fully incremental ICER analysis. The ICER of strategy 5, RF + Lab, compared with strategy 6, ‘RF + i-STAT + Lab’, is £3.61M per additional QALY and, therefore, suggests that strategy 6 is the most cost-effective strategy at conventional cost-effectiveness threshold ranges. As highlighted in Analytical methods, Overview, the fully incremental ICERs appear particularly sensitive to the small effect differences between strategies, limiting their interpretability. Given the small effect differences, and challenges of interpreting the ICER results, fully incremental ICER results are presented only for the base case, with all other results expressed in terms of net benefits.
In general, strategies that combine risk factor screening with POC and laboratory testing result in higher net benefit than other types of strategies involving a POC testing component, as the strategies that combine risk factor screening with POC and laboratory testing have a high positive predictive value (PPV) (Table 35) at a lower average total cost (Table 36). Strategies combining risk factor screening with POC testing and laboratory testing all have a PPV of 1, meaning that all patients identified as positive are TPs. This avoids unnecessary management of FPs with IVH, which imposes costs associated with cancelling and rebooking CT scans (for those patients identified as being TN at only the laboratory testing stage), delivery of IVH, treatment of IVH adverse events and patient follow-up. The appropriate management of patients with a true eGFR > 30 ml/minute/1.73 m2 appears to be a key driver of cost-effectiveness, with the appropriate management of patients with a true eGFR < 30 ml/minute/1.73 m2 being less important given their low prevalence. The next highest ranking strategies are those that combine risk factor screening with POC testing but which do not use confirmatory laboratory testing. These strategies have lower overall specificity and result in more FPs than risk factor screening combined with POC and confirmatory laboratory testing, with increased costs from unnecessary management of patients misclassified as positive (cancelling and rebooking CT scans, delivery of IVH, treatment of IVH adverse events and patient follow-up).
| Identification | Management | Diagnostic accuracy | Probability of | |||||
|---|---|---|---|---|---|---|---|---|
| FPa | FNa | Test positivea | PPV | PC-AKI | RRT | |||
| 1 | Lab | 0.0000 | 0.0000 | 0.0062 | 1.000 | 0.024529 | 0.0158936 | |
| 2 | RF+ i-STAT | 0.0039 | 0.0010 | 0.0091 | 0.569 | 0.024532 | 0.0158939 | |
| 3 | RF + ABL800 FLEX | 0.0027 | 0.0009 | 0.0080 | 0.664 | 0.024532 | 0.0158938 | |
| 4 | RF + StatSensor | 0.0031 | 0.0016 | 0.0076 | 0.599 | 0.024534 | 0.0158941 | |
| 5 | RF + Lab | 0.0000 | 0.0000 | 0.0062 | 1.000 | 0.024529 | 0.0158936 | |
| 6 | RF + i-STAT + Lab | 0.0000 | 0.0010 | 0.0052 | 1.000 | 0.024532 | 0.0158939 | |
| 7 | RF + ABL800 FLEX + Lab | 0.0000 | 0.0009 | 0.0053 | 1.000 | 0.024532 | 0.0158938 | |
| 8 | RF + StatSensor + Lab | 0.0000 | 0.0016 | 0.0046 | 1.000 | 0.024534 | 0.0158941 | |
| 9 | i-STAT | 0.0113 | 0.0010 | 0.0165 | 0.315 | 0.024532 | 0.0158939 | |
| 10 | ABL800 FLEX | 0.0077 | 0.0009 | 0.0130 | 0.407 | 0.024532 | 0.0158938 | |
| 11 | StatSensor | 0.0088 | 0.0016 | 0.0133 | 0.342 | 0.024534 | 0.0158941 | |
| 12 | i-STAT + Lab | 0.0000 | 0.0010 | 0.0052 | 1.000 | 0.024532 | 0.0158939 | |
| 13 | ABL800 FLEX + Lab | 0.0000 | 0.0009 | 0.0053 | 1.000 | 0.024532 | 0.0158938 | |
| 14 | StatSensor + Lab | 0.0000 | 0.0016 | 0.0046 | 1.000 | 0.024534 | 0.0158941 | |
| Identification | Management | Probability of | Costs | Total costs | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Incurring a delay | Unnecessary IVH | Testing | Cancellation and rebooking | Follow-up | IVH and AEs | CT scan | Post contrast | ||||
| 1 | Lab |
Test negativea – contrast-enhanced CT scan |
1.0000 | 0.0000 | £3.31 | £89.75 | £1.15 | £2.30 | £111.65 | £155.09 | £363.26 |
| 2 | RF + i-STAT | 0.0091 | 0.0039 | £5.34 | £0.82 | £1.70 | £3.41 | £111.65 | £155.10 | £278.02 | |
| 3 | RF + ABL800 FLEX | 0.0080 | 0.0027 | £13.92 | £0.72 | £1.49 | £2.99 | £111.65 | £155.10 | £285.87 | |
| 4 | RF + StatSensor | 0.0076 | 0.0031 | £6.14 | £0.68 | £1.42 | £2.84 | £111.65 | £155.10 | £277.84 | |
| 5 | RF + Lab | 0.3519 | 0.0000 | £2.28 | £31.58 | £1.15 | £2.30 | £111.65 | £155.09 | £304.06 | |
| 6 | RF + i-STAT + Lab | 0.0091 | 0.0000 | £5.37 | £0.82 | £0.97 | £1.94 | £111.65 | £155.10 | £275.84 | |
| 7 | RF + ABL800 FLEX + Lab | 0.0080 | 0.0000 | £13.95 | £0.72 | £0.99 | £1.98 | £111.65 | £155.10 | £284.39 | |
| 8 | RF + StatSensor + Lab | 0.0076 | 0.0000 | £6.17 | £0.68 | £0.85 | £1.70 | £111.65 | £155.10 | £276.15 | |
| 9 | i-STAT | 0.0165 | 0.0113 | £8.89 | £1.48 | £3.07 | £6.16 | £111.65 | £155.10 | £286.35 | |
| 10 | ABL800 FLEX | 0.0130 | 0.0077 | £15.77 | £1.17 | £2.43 | £4.87 | £111.65 | £155.10 | £290.99 | |
| 11 | StatSensor | 0.0133 | 0.0088 | £8.55 | £1.20 | £2.49 | £4.98 | £111.65 | £155.10 | £283.96 | |
| 12 | i-STAT + Lab | 0.0165 | 0.0000 | £8.94 | £1.48 | £0.97 | £1.94 | £111.65 | £155.10 | £280.08 | |
| 13 | ABL800 FLEX + Lab | 0.0130 | 0.0000 | £15.81 | £1.17 | £0.99 | £1.98 | £111.65 | £155.10 | £286.70 | |
| 14 | StatSensor + Lab | 0.0046 | 0.0000 | £8.59 | £1.20 | £0.85 | £1.70 | £111.65 | £155.10 | £279.09 | |
Strategies with POC testing and laboratory testing have a lower average net benefit than risk factor screening combined with POC testing strategies, despite not misclassifying patients as FPs (with associated costs of management), because of the higher costs of testing arising when all patients receive POC testing.
The strategies where POC testing is used in isolation are the lowest ranking among strategies involving POC testing, because they misclassify more patients as FPs than any other strategies and all patients incur the cost of POC testing.
Although the highest ranking strategy at £20,000 per additional QALY is strategy 6, ‘RF + i-STAT + Lab’, it is worth noting that the corresponding strategy with StatSensor, strategy 8, has only a marginally smaller average incremental net benefit (i.e. £87.11 compared with £87.42 for strategy 6). i-STAT and StatSensor are both handheld devices with similar diagnostic accuracy, with StatSensor having a slightly higher specificity (99.1% vs. 98.9%) and lower sensitivity (81.7% vs. 84.1%). The cost per test appears higher for StatSensor (£14.25) than for i-STAT (£11.96) when these tests are preceded by risk factor screening, but similar when POC testing is the first step of the testing sequence (£8.52 and £8.85 for StatSensor and i-STAT, respectively), because of the impact of different throughput assumptions. In all other types of strategies involving POC testing (i.e. risk factor screening combined with POC testing, POC testing with laboratory testing and POC testing only), the strategies with StatSensor have a higher net benefit than corresponding ones with i-STAT. This highlights the importance of specificity in the model given the high costs associated with FPs.
Strategies including testing with ABL800 FLEX (i.e. strategies 3, 7, 10 and 13) have a consistently lower net benefit than corresponding strategies with i-STAT and StatSensor, as a result of the higher costs of testing with this device. The ABL800 FLEX is a benchtop device with much higher capital costs than the handheld devices (see Resource use and costs, Point-of-care device costs). The cost per ABL800 FLEX test is, therefore, considerably higher than that of i-STAT and StatSensor, especially at lower patient throughputs (e.g. when strategies including risk factor screening determine that fewer patients receive POC tests). Although ABL800 FLEX is the best-performing device in terms of diagnostic accuracy, any net benefit gains from avoided misclassification are offset by the higher cost of the device.
The strategies that yield the higher QALY gains, that is, strategies 1, ‘Lab’, and 5, ‘RF + Lab’, are those that avoid misclassification of patients resulting in no FPs or FNs. These are also the strategies with the lowest average net benefit because the small QALY benefits from the appropriate management of patients are offset by the highest costs of cancellation and rebooking (especially for strategy 1) and of managing patients who test positive.
The base-case cost-effectiveness results appear to be largely driven by the balance between the costs of testing and the costs associated with mismanagement of FPs. The reduction of PC-AKI risk, and thus the probability of RRT (see Table 35), do not appear to be major drivers in the model. Owing to the low prevalence of patients who have a true eGFR < 30 ml/minute/1.73 m2, the low risk of PC-AKI in the model population and lack of evidence of impact of IVH in reducing this risk, the expected risk of PC-AKI is similar across strategies. Consequently, the QALY gains (see Table 33) and the costs resulting from RRT (see Table 36) are also similar across all strategies. The QALY gains of appropriately managing patients who have a true eGFR < 30 ml/minute/1.73 m2 are small (i.e. the QALY difference between TP and FN is only 0.0000079237), whereas costs of managing patients who test positive are high. The low prevalence of patients who have a true eGFR < 30 ml/minute/1.73 m2 combined with other factors means that specificity appears a more important cost-effectiveness driver than sensitivity, as avoiding FPs translates into considerably higher net benefit gains than mismanaging FNs.
The deterministic results for the scenario analyses are presented in Appendix 11 (see Tables 78 and 93). Table 37 summarises the ranking of each strategy in terms of net benefit at £20,000 per additional QALY for the base-case and scenario analyses. Figure 15 shows strategy ranks from the highest (top line) to the lowest (bottom line) net benefit across scenario analyses. The strategies are labelled with their corresponding number within the circles.
| Strategy number | Base case | Scenario | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6.1 | 6.2 | 6.3 | 7.1 | 7.2 | 7.3 | 7.4 | 8 | 9 | 10.1 | 10.2 | ||
| 6 | 1 | 1 | 2 | 2 | 1 | 5 | 1 | 1 | 2 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 8 | 2 | 2 | 1 | 1 | 2 | 1 | 3 | 2 | 1 | 4 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 4 | 3 | 4 | 4 | 4 | 4 | 2 | 5 | 4 | 3 | 5 | 3 | 3 | 3 | 3 | 3 | 4 | 4 |
| 2 | 4 | 3 | 3 | 6 | 3 | 6 | 2 | 3 | 4 | 6 | 4 | 4 | 4 | 4 | 4 | 3 | 3 |
| 14 | 5 | 5 | 5 | 3 | 5 | 3 | 6 | 6 | 5 | 7 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| 12 | 6 | 6 | 6 | 5 | 6 | 7 | 4 | 5 | 6 | 8 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| 11 | 7 | 8 | 11 | 7 | 7 | 4 | 8 | 7 | 9 | 9 | 8 | 7 | 7 | 8 | 7 | 7 | 7 |
| 7 | 8 | 7 | 7 | 8 | 8 | 8 | 9 | 9 | 7 | 10 | 9 | 8 | 8 | 7 | 8 | 9 | 9 |
| 3 | 9 | 9 | 9 | 11 | 10 | 10 | 11 | 10 | 8 | 12 | 11 | 9 | 9 | 9 | 9 | 10 | 10 |
| 9 | 10 | 10 | 8 | 9 | 9 | 9 | 7 | 8 | 11 | 11 | 10 | 10 | 10 | 11 | 10 | 8 | 8 |
| 13 | 11 | 11 | 10 | 10 | 11 | 11 | 12 | 11 | 10 | 13 | 12 | 11 | 11 | 10 | 11 | 11 | 11 |
| 10 | 12 | 12 | 12 | 12 | 12 | 12 | 13 | 12 | 12 | 14 | 13 | 13 | 12 | 12 | 12 | 12 | 12 |
| 5 | 13 | 13 | 13 | 13 | 13 | 13 | 10 | 13 | 13 | 1 | 7 | 12 | 13 | 13 | 13 | 13 | 13 |
| 1 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 2 | 14 | 14 | 14 | 14 | 14 | 14 | 14 |
FIGURE 15.
Summary of net benefit ranking across scenario analysis. Scenario 1, StatSensor-adjusted analysis; scenario 2, CKD-EPI equation studies; scenario 3, alternative risk factor questionnaire; scenario 4, eGFR distribution – Harris subgroup without prior eGFR; scenario 5, eGFR distribution – GSTT audit data population; scenario 6.1, throughput – 12.7% without a prior eGFR; scenario 6.2, throughput – 50% lower than base case; scenario 6.3; throughput – 50% higher than base case; scenario 7.1, proportion of cancelled CT scans (0%); scenario 7.2, proportion of cancelled CT scans (25%); scenario 7.3, proportion of cancelled CT scans (50%); scenario 7.4, proportion of cancelled CT scans (75%); scenario 8, anxiety from delay; scenario 9, effect of IVH (PC-AKI risk); scenario 10.1, management approach for test positives (50% IVH + contrast CT scan and 50% no contrast CT scan); and scenario 10.2, management approach for test positives (one-third IVH + contrast CT scan, one-third no contrast CT scan and one-third MRI). BC, base case.
The results suggest that strategy 6, ‘RF + i-STAT + Lab’, has the highest net benefit across the majority of scenarios. However, this finding appears sensitive to alternative assumptions in terms of diagnostic accuracy (scenarios 2 and 3), eGFR distribution (scenario 5), throughput estimates (scenario 6.3) and opportunity costs of delayed/rescheduled scan (scenario 7.1). Despite some changes in rankings, differences in net benefits between strategies, and particularly between i-STAT and StatSensor, appear extremely small. The clinical and economic importance of the differences between individual devices and different types of strategies may be limited.
When the diagnostic accuracy of POC devices is sourced solely from studies using the CKD-EPI equation to calculate eGFRs (scenario 2), there is a switch in the net benefit rank between strategy 6 (RF + i-STAT + Lab) and 8 (RF + StatSensor + Lab). When this source of data is used, the sensitivity of all POC devices decreases compared with the base case, with StatSensor having the greatest decrease in sensitivity compared with the base case (56.4% vs. 73.9%). This results in an increase in the proportion of FNs for strategy 8, ‘RF + StatSensor + Lab’, with a consequent decrease in costs from managing positive patients. The decrease in costs is sufficient to offset the higher costs of testing for strategy 8, ‘RF + StatSensor + Lab’, compared with strategy 6, ‘RF + i-STAT + Lab’, and, under this scenario, the strategy becomes the cost-effective alternative.
In scenario 3, it is assumed that risk factor screening is performed with a questionnaire with worse diagnostic accuracy. Compared with the base-case analysis, the sensitivity of the questionnaire is reduced from 100% to 88.2%, whereas specificity is reduced from 65.2% to 45.2%. The lower specificity of the questionnaire results in an increase in throughput for POC testing for strategies where POC testing is preceded by risk factor screening, with a consequent reduction in the costs of POC testing. The cost per test of StatSensor (with risk factor screening) reduces proportionately more than with i-STAT and, despite remaining the more costly of the two tests (£11.06 vs. £10.23, respectively), this small difference in the cost of testing is now offset by the lower costs of managing patients identified as positive by StatSensor. Therefore, strategy 8, ‘RF + StatSensor + Lab’, switches with strategy 6, ‘RF + i-STAT + Lab’, as the cost-effective alternative for scenario 3. Strategy 14, ‘StatSensor + Lab’, also has a higher net benefit than both strategy 2, ‘RF + STAT’, and strategy 4, ‘RF + StatSensor’. This higher net benefit is due to an increase in the costs of testing in the strategies including risk factor screening, given that the lower specificity of the questionnaire results in more patients being tested with POC (even if the cost per POC test reduces).
Scenario 5 assumes that the underlying distribution of eGFR values in the relevant population matches that of the GSTT audit population. This population is characterised by having a higher proportion of patients with an eGFR < 30 ml/minute/1.73 m2 than the base case (15.9% vs. 0.6%). When the proportion of patients with a true eGFR < 30 ml/minute/1.73 m2 is higher, there will be more patients testing positive and thus receiving more intensive patient management. There will also be more patients who can benefit from management to reduce PC-AKI (as risk will be overall higher), but the benefit of being managed with IVH remains small. The proportion of patients who test positive (and incur more costs for a small benefit) will be higher for strategies with lower specificity and higher sensitivity. In this scenario, the strategy with the highest net benefit is strategy 8, ‘RF + StatSensor + Lab’, followed by strategy 4, ‘RF + StatSensor’, and then strategy 14, ‘StatSensor + Lab’. As StatSensor is the POC device with lowest sensitivity, strategies including this device will result in proportionally fewer positive POC tests with lower costs from delays and, where POC is not followed by laboratory testing, lower costs from managing patients who test positive across the testing strategy. The increase in the proportion of patients with a true eGFR < 30 ml/minute/1.73 m2 also results in a reduction in the cost per test for all POC devices when combined with risk factor screening, but proportionally more for StatSensor than for i-STAT. The cost-effectiveness of strategies including POC testing with StatSensor is more favourable than that of strategies with other devices when the proportion of patients with a true eGFR < 30 ml/minute/1.73 m2 increases to 15.9% despite its lower sensitivity.
Higher levels of throughput (i.e. scenario 6.3) result in a switch in the net benefit rank between strategies 6 and 8, with strategy 8, ‘RF + StatSensor + Lab’ generating higher net benefit. Higher throughput reduces the cost per POC test for all devices. The cost per test of StatSensor is more sensitive (as a result of the costs of quality control) to changes in throughput than i-STAT, and reduces proportionately more compared with base case than the cost per i-STAT test. Therefore, strategy 8, ‘RF + StatSensor + Lab’, becomes less costly than strategy 6, ‘RF + i-STAT + Lab’, and becomes the cost-effective strategy in scenario 6.3.
Scenarios 7.1–7.4 explore uncertainty in the proportion of patients who can have their laboratory test and/or IVH performed urgently and, therefore, without incurring the opportunity costs of a delayed CT scan. The results of the base-case analysis are robust to all alternative assumptions tested under this scenario except when it is assumed that all patients are urgent cases and none incurs the opportunity costs of a delayed CT scan (i.e. scenario 7.1). If there were no delays to CT scanning from laboratory testing and/or IVH, strategy 5, ‘RF + Lab’, would become the strategy with the highest net benefit, followed by strategy 1, ‘Lab’. The two strategies are equivalent in terms of QALY gains (as risk factor screening is assumed to be 100% sensitive), but risk factor screening allows the reduction in the overall costs of testing as only patients who are risk factor positive receive the laboratory test. Under scenario 7.1, these strategies become the least costly across all other strategies, because all other costs of managing test-positive patients are incurred only by TPs (the strategies do not allow for misclassification) and the costs of testing are lower than for the other strategies.
Scenarios 8–10.2 explored alternative assumptions concerning the impact of anxiety as a result of delay (scenario 8), the effect of IVH (scenario 9) and the costs of alternative imaging decisions (scenarios 10.1 and 10.2). Although there were some minor changes in rankings across these scenarios, strategies 6 (RF + i-STAT + Lab) and 8 (RF+StatSensor + Lab) remained the highest ranked strategies across all these scenarios.
As detailed in Strategies, a strategy of ‘no testing and manage all with contrast-enhanced CT’ was not included in the base-case analysis, as this strategy was not deemed to be clinically appropriate given the consistent recommendations reported across clinical guidelines recommending the use of some form of screening or testing to identify individuals at risk of PC-AKI. However, for completeness, and to aid the overall interpretation of the results, two additional scenarios were included (i.e. scenarios 11.1 and 11.2). Scenario 11.1 (see Table 94 in Appendix 11) replicated the base-case analysis, but included an additional ‘no-testing’ strategy. Scenario 11.2 (see Table 95 in Appendix 11) included the additional ‘no-testing’ strategy and also altered the assumptions concerning the effectiveness of IVH in reducing the risk of PC-AKI.
In both scenarios, that is 11.1 and 11.2, the ‘no testing and manage all with contrast-enhanced CT’ was associated with the highest net benefit.
Discussion of the independent economic assessment
The purpose of the decision model was to assess the cost-effectiveness of POC testing to assess kidney function, for people who need contrast-enhanced CT imaging in a non-emergency situation and who do not have a recent eGFR measurement. The decision model considered the potential benefits to, and possible risks of, using a range of alternative POC testing approaches within the current CT pathway.
A potential limitation of the model is the assumption made in the base-case analysis that all individuals will eventually proceed to a contrast-enhanced CT scan. This simplification was considered necessary given the limited data available, the challenges of characterising the heterogeneity in the overall population and the underlying reason for imaging and linking this to individualised clinical decision-making and associated outcomes. In a real-world setting, the decision between alternative imaging modality will depend on the balance between each patient’s risk of PC-AKI and the impact on diagnostic accuracy of choosing a different imaging modality (which depends on the underlying condition). However, an extensive series of scenario analyses were undertaken to explore the potential impact of alternative assumptions. Uncertainties remain in terms of other clinical outcomes that could be affected by alternative imaging decisions (e.g. potential health loss from using a suboptimal imaging modality to inform treatment) or by delaying imaging (e.g. a change in underlying condition while waiting for a rescheduled scan).
The simplifying assumption on the opportunity cost of cancelling and rescheduling a CT scan may also not hold across NHS trusts, as this depends on whether or not the loss of the CT scan slot can be avoided. For example, some NHS trusts may be able to obtain laboratory tests and deliver any required risk-mitigating actions within the same day or fill the cancelled slots with scans for non-elective patients. Although there is uncertainty on the proportion of CT scans that would be cancelled and rescheduled, the results of the base-case analysis were robust to the range of alternative assumptions tested under scenario analysis on this parameter, except when it is assumed that no patient incurs the opportunity costs of a delayed CT scan.
The evidence on POC diagnostic accuracy is sparse; these estimates are informed by studies with small cohorts of patients and with few patients with eGFRs < 30 ml/minute/1.73 m2. Although the small number of patients below this diagnostic threshold is reflective of the expected distribution of eGFRs in an outpatient population, it introduces uncertainty on the estimates of POC diagnostic accuracy and, therefore, on the estimates of cost-effectiveness. Moreover, comparative evidence was available for only three devices, which precluded the inclusion in the analysis of strategies of other commercially available POC devices. The distribution of eGFR values in the model population was also informed by audit data from a single NHS trust, which had only one individual with an eGFR < 30 ml/minute/1.73 m2. Scenario analysis using audit data from another NHS trust with a higher prevalence of eGFR < 30 ml/minute/1.73 m2 did not, however, change the type of optimal testing strategy.
Another potential limitation of the analysis is that it excludes the costs of implementing the use of POC devices in the NHS, namely costs associated with staff training and laboratory governance. No evidence was identified to inform these parameters, but training costs per patient are anticipated to be low compared with the other elements of cost already included in the costs per POC test. The magnitude of laboratory governance costs will vary across NHS trusts, as it will depend on whether or not POC testing is already in use in radiology departments and if the trust has suitable IT connectivity. However, the costs of implementation and laboratory governance would have to be substantial (i.e. in excess of £80,000 per annum) to change the conclusions of the analysis. Furthermore, if POC devices were available in radiology departments they might be used to measure eGFRs outside the bounds of this particular decision problem, which could potentially reduce the costs per test by increasing throughput.
The evidence on the clinical outcomes of the relevant population is also affected by data sparsity. The rates of PC-AKI conditional on eGFR values, risk of RRT and mortality subsequent to PC-AKI in outpatients undergoing contrast-enhanced CT scan were informed by a single study. Furthermore, the rates required additional assumptions on the links between PC-AKI and subsequent patient outcomes (e.g. the assumption that the risk of RRT is independent of underlying eGFR value and depends on only PC-AKI status). No evidence on the effect of contrast agents on risk of PC-AKI in an outpatient population was identified (see Evidence of the risk of acute kidney injury from contrast agents), and pooled evidence from three large propensity score-matched studies in inpatient populations suggested no effect of contrast on PC-AKI risk (see Effect of contrast agents on post-contrast acute kidney injury). Given that one of the studies included in the meta-analysis suggested a detrimental effect of contrast on PC-AKI risk91 and the ongoing scientific debate on whether or not contrast agents modify the risk of PC-AKI, this remains an area of uncertainty. However, findings were robust to scenario analysis assuming an increased risk of PC-AKI from contrast agents.
The prophylactic effect of IVH on the risk of PC-AKI across different eGFR categories is also an area of uncertainty that potentially limits the findings of this study. Although the majority of evidence identified suggests that there is no effect of IVH on the risk of PC-AKI for patients with an eGFR ≥ 30 ml/minute/1.73 m2, there is a lack of randomised evidence in patients with an eGFR < 30 ml/minute/1.73 m2 (see Evidence on prophylactic interventions for post-contrast acute kidney injury). In the absence of relevant evidence for patients with an eGFR < 30 ml/minute/1.73 m2, an assumption on the effect of IVH on the risk of PC-AKI for these patients was required in the model (i.e. a small statistically non-significant effect of 0.97 from IVH). Despite this limitation, the results were robust to scenario analysis increasing the prophylactic effect of IVH for patients with an eGFR < 30 ml/minute/1.73 m2.
The finding that a scenario including a ‘no testing and manage all with contrast-enhanced CT’ strategy had the highest net benefit of all the strategies suggests that additional testing costs required to obtain either a laboratory assessment or a POC test result may not provide sufficient improvement in patient outcomes to warrant routine testing. Such a strategy is, however, unlikely to be considered clinically acceptable. These findings also need to be considered alongside the limitations of the model assumptions and the uncertainties that remain regarding the effect of contrast media on the risk of PC-AKI, and the benefits of appropriate prophylactic management to reduce the risk of PC-AKI.
Conclusions of the cost-effectiveness section
The base-case cost-effectiveness results showed that the testing strategy with highest net benefit (i.e. the strategy that appears to be cost-effective) was a three-step testing sequence that involves initially screening all individuals for risk factors, testing with a POC device those individuals identified with at least one risk factor and including a final confirmatory laboratory test for individuals who also test positive with a POC device. Within this testing approach type, the specific POC device with the highest net benefit was i-STAT; however, differences in the net benefit between the i-STAT and StatSensor devices were very small. These findings appeared robust to a wide range of scenario analyses. Despite some changes in rankings, differences in net benefits between many of the individual strategies remained extremely small.
Differences in the cost and diagnostic specificity of the individual testing strategies appeared more important drivers than diagnostic sensitivity. The reduction of PC-AKI risk and associated consequences were not major drivers in the model as a result of the low risk of PC-AKI estimated for this population, the lack of evidence suggesting an increased risk of PC-AKI associated with the use of contrast media and the lack of evidence of impact of IVH in reducing the risk of PC-AKI.
Chapter 6 Discussion
Statement of principal findings
Most of the 54 studies that were eligible for inclusion in the systematic review reported only measurement bias or correlation outcomes and so were of limited relevance to the economic modelling part of the assessment. Correlation results data are limited because results that might appear impressive (i.e. correlation coefficients close to 1) can sometimes hide imperfect agreement between methods. Of the studies reporting data on creatinine/eGFR measurement bias, results from the StatSensor studies demonstrated wide variation in both the size and direction of bias. It is therefore important that StatSensor users are aware of the availability of the offset facility to correct for any measurement bias observed, as this did not appear to have been done in most StatSensor studies. It is also preferable that any bias corrections should be informed by data from enzymatic laboratory reference methods, rather than Jaffe methods, which are well known to be less accurate than enzymatic methods for measuring levels of creatinine (unless they are IDMS aligned). Although potentially important measurement bias was also identified in some studies of the i-STAT and ABL devices, in most of these studies the concordance of results was generally better than was found in most of the StatSensor studies. No eligible studies were available on the DRI-CHEM NX 500 device and few studies were available on the epoc and Piccolo Xpress devices; the limited data and reporting in these studies, coupled with their small sample sizes, made it difficult to draw conclusions about creatinine measurement biases.
All seven studies that reported diagnostic accuracy results based on creatinine thresholds were of the StatSensor device. However, these studies were of limited value to this assessment because only two of the seven studies explicitly reported results that incorporated an offset adjustment (both of which were based on Jaffe laboratory methods) and diagnostic accuracy results based on creatinine thresholds are not as clinically relevant as results based on eGFR thresholds.
Twelve studies reported eGFR diagnostic accuracy data, but these covered only three types of device: StatSensor, i-STAT and ABL devices. Although half of these studies were assessed as having results with a low risk of bias, there were some concerns about the applicability of results to the outpatient CT setting in all but two studies. Results of the eGFR data synthesis show better sensitivity to detect risk of PC-AKI for i-STAT and ABL devices than for StatSensor device. In addition, i-STAT and ABL devices also have higher probabilities of correctly classifying individuals in the same eGFR categories as the reference laboratory than StatSensor devices. This is particularly marked for the lower categories that are of greatest clinical importance. Additional analyses carried out using adjusted StatSensor data and including studies that used only the CKD-EPI equation confirmed these findings.
A three-step testing sequence that involves combining a risk factor questionnaire, POC testing and confirmatory laboratory testing would potentially reduce unnecessary delays or rescheduling of CT scans. In the light of existing evidence, this testing approach appears more cost-effective than the current approach, which involves obtaining a recent laboratory-based measurement prior to administering contrast media.
Strengths and limitations of the assessment
The systematic review was performed using transparent, reproducible and robust methods. Our comprehensive literature searches sought to identify all relevant published and unpublished studies, which minimised the possibility of publication or language biases affecting the review results. Similarly, key review processes were performed in duplicate, which minimised the possibility of any reviewer errors and biases. This study also successfully obtained previously unpublished data from two important studies of diagnostic accuracy based on eGFR thresholds. Study quality was evaluated in studies reporting eGFR diagnostic accuracy data using a modified version of the QUADAS-2 tool. Appropriate synthesis methods were used to evaluate the accuracy of the devices and provide the inputs needed to the economic evaluation in the form of probabilities of correct classification by the POC device into the same eGFR range as the reference laboratory. Uncertainty in the data was taken into account, although it was not possible to fully account for between-study differences in results.
A further strength of this review was the broadness of its scope: in addition to studies reporting diagnostic accuracy data, the review sought studies reporting measurement bias and clinical or workflow outcomes.
The de novo decision model is the first formal evaluation of the potential clinical benefits, risks and costs of incorporating POC testing to assess kidney function for people who need contrast-enhanced CT imaging in a non-emergency outpatient setting and who present without a recent eGFR measurement. The main strength of the decision model is the linkage between the diagnostic accuracy of a given strategy, the impact on subsequent treatment decisions and the ultimate effect on health outcomes and costs.
Some diagnostic accuracy studies were limited by small sample sizes, and most studies had few patients with eGFR values of < 30 ml/minute/1.73 m2. Although this is reflective of outpatient populations, it limits the data available for analyses based on the most important eGFR threshold of < 30 ml/minute/1.73 m2 and it contributes to the uncertainty around diagnostic accuracy estimates. Few studies directly compared different POC creatinine devices and eGFR diagnostic accuracy data were not available for the ABL90 FLEX PLUS, DRI-CHEM NX 500, epoc and Piccolo Xpress POC devices. Available data on the underlying distribution of eGFR values in the relevant population were also sparse and suggested that few radiology outpatients have eGFR values of < 30 ml/minute/1.73 m2. This may, however, vary across NHS trusts and is likely to depend on local-level organisation characteristics (e.g. whether the hospital is a specialist centre and/or has renal services on site). Scenario analysis suggests, however, that the findings on the optimal type of strategy are robust to alternative assumptions on the distribution of eGFRs.
Another potential limitation of this assessment is the assumption made in the base-case analysis that all individuals will eventually proceed to a contrast-enhanced CT scan. This simplification was considered necessary given the limited data available, the challenges of characterising the heterogeneity in the overall population and the underlying reason for imaging and linking this to individualised clinical decision-making and associated outcomes. However, an extensive series of scenario analyses were undertaken to explore the potential impact of alternative assumptions.
The assumption that all cancelled and rescheduled CT scans will result in the loss of the CT slot (i.e. incur the cost of one CT scan) may also not reflect clinical practice across all NHS trusts. Although this could limit the generalisability of the results, the cost-effectiveness results were mostly robust to the range of alternative assumptions tested under scenario analysis on this parameter. The only exception was the scenario assuming that no patient incurs the opportunity costs of a delayed CT scan.
The cost-effectiveness analysis did not include the costs of implementing the use of POC devices in the NHS. Data were not available to fully quantify these costs, and the costs are likely to vary widely across NHS trusts. Nevertheless, the addition of these costs is unlikely to change the findings of the cost-effectiveness analysis.
The linkage of diagnostic accuracy data to clinical outcomes in the model relied on sparse data and on a number of assumptions regarding the risk of PC-AKI conditional on eGFR values, and the link between PC-AKI and subsequent patient outcomes. The effect of contrast media on the risk of PC-AKI and the effect of intravenous prophylaxis in reducing the risk of PC-AKI are areas of uncertainty. However, findings were robust to scenario analysis assuming an increased risk of PC-AKI from contrast agents, so resolving this uncertainty is unlikely to change the results of the cost-effectiveness analysis.
Uncertainties
There were few studies that reported data on the impact of POC devices in CT departments on the use (or rates of non-use) of contrast agents for diagnostic procedures, nor were there few data on the use of prophylactic treatments or workflow outcomes, such as cancelled appointments. No data were available on studies of POC device on clinical outcomes, such as need for renal replacement therapy or hospital admissions. The impact of POC devices on these important outcomes is therefore uncertain.
The model relied on a number of assumptions to establish a link between diagnostic accuracy data and clinical outcomes given the data limitations. The following remain areas of uncertainty:
-
diagnostic accuracy of POC devices in patients with an eGFR < 30 ml/minute/1.73 m2
-
underlying distribution of eGFR
-
proportion of CT scan slots lost as a result of cancelled and rescheduled CT scans
-
impact on clinical outcomes from alternative imaging decisions and delays to imaging
-
link between PC-AKI and subsequent patient outcomes
-
effect of contrast media on risk of PC-AKI by category of eGFR
-
effect of IVH on risk of PC-AKI by category of eGFR.
Among these areas of uncertainty, the proportion of CT scan slots lost is the most likely to affect the results of the cost-effectiveness analysis, but only if all loss of CT scan slots can be avoided.
The finding that a scenario including a ‘no testing and manage all with contrast-enhanced CT’ strategy had the highest net benefit of all the strategies suggests that additional testing costs required to obtain either a laboratory assessment or a POC test result may not provide sufficient improvements in patient outcomes to warrant routine testing. Such a strategy is, however, unlikely to be considered clinically acceptable. Furthermore, the health benefits from providing prophylactic management to patients with eGFRs are small given the proportion of patients with an eGFR < 30 ml/minute/1.73 m2, the assumption that contrast media do not increase the risk of PC-AKI and the modest effect of prophylactic IVH in reducing PC-AKI. Thus, these findings also need to be considered alongside the limitations of the model assumptions and the uncertainties that remain regarding the effect of contrast media on the risk of PC-AKI, and the benefits of appropriate prophylactic management to reduce the risk of PC-AKI.
Chapter 7 Conclusions
Results from this systematic review of POC creatinine devices showed that i-STAT and ABL800/827 devices are more accurate than StatSensor devices at correctly detecting individuals with an eGFR < 30 ml/minute/1.73 m2 (better sensitivity). The synthesis also indicated that i-STAT and ABL devices have higher probabilities than StatSensor devices of correctly classifying individuals in the same eGFR categories as the reference laboratory. Additional analyses carried out using adjusted StatSensor data and including only studies that used the CKD-EPI equation confirmed these findings.
A pragmatic review identified evidence from large studies of inpatients that suggests there is no association between contrast agents and the risk of AKI in patients with an eGFR ≥ 45 ml/minute/1.73 m2, although uncertainty exists about whether or not contrast agents are associated with a small risk in patients with an eGFR < 45 ml/minute/1.73 m2. There was no evidence to suggest that IVH is more effective than oral hydration for preventing PC-AKI or RRT or reducing mortality. In the light of existing evidence, a three-step testing sequence, consisting of initially screening all individuals for risk factors, testing with a POC device those individuals identified with at least one risk factor and including a final confirmatory laboratory test for individuals who also test positive with a POC device, appears to be cost-effective. Within this testing approach, the i-STAT device had the highest net benefit; however, differences in the net benefit between the i-STAT and StatSensor devices were very small.
Implications for health care
The findings suggest that the use of POC devices, compared with current practice, may reduce costs to the health system arising from unnecessary delays in CT scanning appointments for the majority of individuals. Any savings also need to be considered against the potential risks arising from misclassification. However, although the use of POC devices results in a marginal reduction in outcomes compared with a strategy of obtaining a laboratory measurement for all individuals, the loss in outcomes appears more than offset by the estimated cost savings. These findings need to be considered alongside the uncertainties and limitations of the analysis described in Chapter 6.
Suggested research priorities
Research is needed to provide more accurate and precise estimates of the distribution of eGFR results in CT outpatient settings, particularly with respect to patients with an eGFR of < 30 ml/minute/1.73 m2.
Further studies are needed on the diagnostic accuracy and impact on workflow of different risk-stratifying questionnaires for identifying CT outpatients attending without a recent eGFR who are at high risk of PC-AKI. Uncertainty exists regarding questionnaire accuracy using the (currently) most frequently used diagnostic threshold of eGFR < 30 ml/minute/1.73 m2 and also regarding which are the optimal criteria to be included in the questionnaires.
Evidence on the diagnostic accuracy of the Piccolo, ABL90 FLEX PLUS, DRI-CHEM NX 500 and epoc devices in outpatient CT settings is needed, as there are currently no available studies. Although it would be useful to have further studies comparing the diagnostic accuracy of different POC devices in CT outpatient settings at an eGFR threshold of < 30 ml/minute/1.73 m2, feasibility issues make it difficult to recommend such studies, given the scarcity of CT outpatients without a recent laboratory eGFR result who have an eGFR < 30 ml/minute/1.73 m2. Broadening a study population to inpatients could solve the issue of patient numbers, but nevertheless be problematic to undertake as such patients would already have a recent laboratory eGFR result and so the use of POC devices would not be warranted. If such a study was undertaken, a key limitation would be the uncertainty about the applicability of its results to CT outpatients. Nearly all i-STAT studies included in the review used whole-blood samples, whereas nearly all studies of StatSensor used capillary samples. It is not clear whether or not the observed differences in diagnostic accuracy between the two devices may be explained by the use of different blood samples. Therefore, a study comparing the pros and cons (including accuracy, convenience and cost) of using capillary samples versus whole-blood samples in POC devices may be relevant. Debate exists about how best to resolve the issue of the risks of contrast media, with some suggesting a need for a randomised study to fully determine the contribution of intravenous contrast media to the development AKI. 97 Others have documented that prospective studies in patients with an eGFR < 30 ml/minute/1.73 m2 have been attempted but had to be terminated early; further clarification on the risk from contrast agents could be gained from studies of specific patient subgroups that did not receive intravenous prophylaxis (e.g. CT angiography), irrespective of renal function. 96
Acknowledgements
We would like to thank Professor Beverly Snaith (Clinical Professor of Radiography, University of Bradford, Bradford, UK) for sharing data from recent studies and Ms Tracey Eastwood (Pinderfields Hospital, The Mid Yorkshire Hospitals NHS Trust, Wakefield, UK) for providing further technical details on POC devices.
We also thank the following specialist committee members appointed for this topic who provided advice during the assessment: Dr Anne Dawnay (Consultant Clinical Scientist in Clinical Biochemistry, Barts Health NHS Trust, The Royal London Hospital, London, UK), Dr Mark Devonald (Consultant Nephrologist, Nottingham University Hospitals NHS Trust, Nottingham, UK), Dr Andrew Lewington (Consultant Kidney Specialist, Leeds Teaching Hospitals, Leeds, UK), Professor Beverly Snaith (Clinical Professor of Radiography, University of Bradford, Bradford, UK) and Ms Annette Thomas (Consultant Clinical Scientist, Cardiff and Vale University Health Board, University Hospital of Wales, Cardiff, UK).
Contributions of authors
Mark Corbett (https://orcid.org/0000-0002-5937-1493) contributed to writing the protocol, conducted study selection, data extraction, validity assessment, interpretation of evidence and co-wrote the clinical sections of the report.
Ana Duarte (https://orcid.org/0000-0002-0528-4773) contributed to the writing of the protocol and the cost-effectiveness section, developed the economic model and performed the economic analysis.
Alexis Llewellyn (https://orcid.org/0000-0003-4569-5136) conducted study selection, data extraction, validity assessment and co-wrote the clinical sections of the report.
James Altunkaya (https://orcid.org/0000-0002-8293-3466) contributed to the writing of the cost-effectiveness section.
Melissa Harden (https://orcid.org/0000-0003-2338-6869) devised the search strategy, carried out the literature searches and wrote the search sections of the report.
Martine Harris (https://orcid.org/0000-0003-1924-3718) provided clinical advice during the project and previously unpublished data, and commented on drafts of the report.
Simon Walker (https://orcid.org/0000-0002-5750-3691) contributed to the writing of the cost-effectiveness section, assisted with the economic analysis and validated the economic model.
Stephen Palmer (https://orcid.org/0000-0002-7268-2560) contributed to the writing of the protocol and the cost-effectiveness section, commented on drafts of the report and contributed to all aspects of the project.
Sofia Dias (https://orcid.org/0000-0002-2172-0221) contributed to writing the protocol, developed the data synthesis model, contributed to data extraction and interpretation of evidence, and co-wrote the clinical sections of the report.
Marta Soares (https://orcid.org/0000-0003-1579-8513) contributed to the writing of the cost-effectiveness section and development of the economic model, contributed to model validation, and had overall responsibility for the cost-effectiveness section of the report.
Data-sharing statement
The data used in the analyses of this report are predominantly drawn from published and publicly available sources, as cited throughout the report. Summaries of the non-confidential data and of the models used are available on request from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Committee on Medical Aspects of Radiation in the Environment (COMARE) . Sixteenth Report. Patient Radiation Dose Issues Resulting from the Use of CT in the UK 2014.
- Ehrmann S, Aronson D, Hinson JS. Contrast-associated acute kidney injury is a myth: yes. Intensive Care Med 2018;44:104-6. https://doi.org/10.1007/s00134-017-4950-6.
- Weisbord SD, du Cheryon D. Contrast-associated acute kidney injury is a myth: No. Intensive Care Med 2018;44:107-9. https://doi.org/10.1007/s00134-017-5015-6.
- Kashani K, Levin A, Schetz M. Contrast-associated acute kidney injury is a myth: we are not sure. Intensive Care Med 2018;44:110-14. https://doi.org/10.1007/s00134-017-4970-2.
- McDonald RJ, McDonald JS, Newhouse JH, Davenport MS. Controversies in contrast material-induced acute kidney injury: closing in on the truth?. Radiology 2015;277:627-32. https://doi.org/10.1148/radiol.2015151486.
- ACR Committee on Drugs and Contrast Media . ACR Manual on Contrast Media: Version 10.3 2018 2018.
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604-12. https://doi.org/10.7326/0003-4819-150-9-200905050-00006.
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461-70. https://doi.org/10.7326/0003-4819-130-6-199903160-00002.
- Vanommeslaeghe F, De Mulder E, Van de Bruaene C, Van de Bruaene L, Lameire N, Van Biesen W. Selecting a strategy for prevention of contrast-induced nephropathy in clinical practice: an evaluation of different clinical practice guidelines using the AGREE tool. Nephrol Dial Transplant 2015;30:1300-6. https://doi.org/10.1093/ndt/gfv220.
- European Society of Urogenital Radiology (ESUR) . ESUR Guidelines on Contrast Agents v10.0 2018. https://www.esur-cm.org/ (accessed 28 February 2019).
- The Royal Australian and New Zealand College of Radiologists . Iodinated Contrast Media Guideline V2.3 2018.
- Harris MA, Snaith B, Clarke R. Strategies for assessing renal function prior to outpatient contrast-enhanced CT: a UK survey. Br J Radiol 2016;89. https://doi.org/10.1259/bjr.20160077.
- Cope LH, Drinkwater KJ, Howlett DC. RCR audit of compliance with UK guidelines for the prevention and detection of acute kidney injury in adult patients undergoing iodinated contrast media injections for CT. Clin Radiol 2017;72:1047-52. https://doi.org/10.1016/j.crad.2017.07.002.
- Azzouz M, Romsing J, Thomsen HS. Can a structured questionnaire identify patients with reduced renal function?. Eur Radiol 2014;24:780-4. https://doi.org/10.1007/s00330-013-3065-x.
- Gbinigie O, Thompson M, Price CP, Heneghan C, Plüddemann A. Point-of-Care Creatinine Testing for the Detection and Monitoring of Chronic Kidney Disease. Oxford: NIHR Diagnostic Evidence Co-operative Oxford; 2014.
- McFadden EC, Hirst JA, Verbakel JY, McLellan JH, Hobbs FDR, Stevens RJ, et al. Systematic review and metaanalysis comparing the bias and accuracy of the modification of diet in renal disease and chronic kidney disease epidemiology collaboration equations in community-based populations. Clin Chem 2018;64:475-85. https://doi.org/10.1373/clinchem.2017.276683.
- Abbott . I-STAT Alinity 2019. www.pointofcare.abbott/int/en/offerings/istat-alinity (accessed 20 March 2019).
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307-10. https://doi.org/10.1016/S0140-6736(86)90837-8.
- Kidney Disease: Improving Global Outcomes (KDIGO) . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:1-150.
- National Institute for Health and Care Excellence (NICE) . Chronic Kidney Disease in Adults: Assessment and Management. Clinical Guideline [CG182] 2014. www.nice.org.uk/guidance/cg182/chapter/1-Recommendations (accessed 21 May 2019).
- Lunn D, Jackson C, Best N, Thomas A, Spiegelhalter D. The BUGS Book. Boca Raton, FL: CRC Press; 2013.
- Lunn D, Spiegelhalter D, Thomas A, Best N. The BUGS project: evolution, critique and future directions. Stat Med 2009;28:3049-67. https://doi.org/10.1002/sim.3680.
- Gelman A, Carlin JB, Stern HS, Dunson DB, Vehtari A, Rubin DB. Bayesian Data Analysis. London: Chapman & Hall; 2014.
- Jackson CH. Displaying uncertainty with shading. Am Stat 2008;62:340-7. https://doi.org/10.1198/000313008X370843.
- Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat 1998;7:434-55. https://doi.org/10.1080/10618600.1998.10474787.
- Gelman A, Rubin DB. Inference from iterative simulation using multiple sequences. Stat Sci 1992;7:457-72. https://doi.org/10.1214/ss/1177011136.
- Botz C, Sorenson L, Karon B. Comparison of whole blood and serum creatinine and estimated glomerular filtration rate for screening of at-risk patients prior to radiographic procedures. Clin Chem 2013;1.
- Dorward J, Yende-Zuma N, Samsunder N, Karim QA, Drain PK, Garrett N. Clinic-based evaluation of a point-of-care creatinine assay to screen for renal impairment among HIV-positive patients receiving tenofovir disoproxil fumarate. J Acquir Immune Defic Syndr 2018;77:e36-e39. https://doi.org/10.1097/QAI.0000000000001613.
- Houben IPL, van Berlo CJLY, Bekers O, Nijssen EC, Lobbes MBI, Wildberger JE. Assessing the risk of contrast-induced nephropathy using a finger stick analysis in recalls from breast screening: the CINFIBS Explorative Study. Contrast Media Mol Imaging 2017;2017. https://doi.org/10.1155/2017/5670384.
- Inoue A, Nitta N, Ohta S, Imoto K, Yamasaki M, Ikeda M, et al. StatSensor-i point-of-care creatinine analyzer may identify patients at high-risk of contrast-induced nephropathy. Exp Ther Med 2017;13:3503-8. https://doi.org/10.3892/etm.2017.4389.
- Korpi-Steiner NL, Williamson EE, Karon BS. Comparison of three whole blood creatinine methods for estimation of glomerular filtration rate before radiographic contrast administration. Am J Clin Pathol 2009;132:920-6. https://doi.org/10.1309/AJCPTE5FEY0VCGOZ.
- Krige T. Screening for Chronic Kidney Disease (CKD) in a High Risk Population Using a Point of Care Instrument for Creatinine Measurement: a Community Based Study (The Bellville South Africa Study. Stellenbosch: Stellenbosch University; 2017.
- Nichols JH, Bartholomew C, Bonzagi A, Garb JL, Jin L. Evaluation of the IRMA TRUpoint and i-STAT creatinine assays. Clin Chim Acta 2007;377:201-5. https://doi.org/10.1016/j.cca.2006.09.024.
- Obrador G, Olvera N, De La Pe DO, Gutieez V, Villa A. Validation of serum creatinine (SCR) measurement with the point-of-care testing (POCT) device i-STAT in a Mexican government sponsored-chronic kidney disease (CKD) screening program. Nephrol Dial Transplant 2012;27.
- Shephard M, Corso O, Mathew T. Creatinine can be measured at the point-of-care. Nephrology 2008;13:A101-A.
- Shephard M, Peake M, Corso O, Shephard A, Mazzachi B, Spaeth B, et al. Assessment of the Nova StatSensor whole blood point-of-care creatinine analyzer for the measurement of kidney function in screening for chronic kidney disease. Clin Chem Lab Med 2010;48:1113-19. https://doi.org/10.1515/CCLM.2010.238.
- Snaith B, Harris MA, Shinkins B, Jordaan M, Messenger M, Lewington A. Point-of-care creatinine testing for kidney function measurement prior to contrast-enhanced diagnostic imaging: evaluation of the performance of three systems for clinical utility. Clin Chem Lab Med 2018;56:1269-76. https://doi.org/10.1515/cclm-2018-0128.
- Snaith B, Harris MA, Shinkins B, Messenger M, Lewington A, Jordaan M, et al. Point of care creatinine testing in diagnostic imaging: a feasibility study within the outpatient computed tomography setting. Eur J Radiol 2019;112:82-7. https://doi.org/10.1016/j.ejrad.2019.01.007.
- Griffin BR, Butler-Dawson J, Dally M, Krisher L, Cruz A, Weitzenkamp D, et al. Unadjusted point of care creatinine results overestimate acute kidney injury incidence during field testing in Guatemala. PLOS ONE 2018;13. https://doi.org/10.1371/journal.pone.0204614.
- Hamza M. Evaluation of the StatSensor Whole Blood Creatinine Monitoring System for Screening Patients in the Radiology Department n.d.
- Haneder S, Gutfleisch A, Meier C, Brade J, Hannak D, Schoenberg SO, et al. Evaluation of a handheld creatinine measurement device for real-time determination of serum creatinine in radiology departments. World J Radiol 2012;4:328-34. https://doi.org/10.4329/wjr.v4.i7.328.
- Kosack CS, de Kieviet W, Bayrak K, Milovic A, Page AL. Evaluation of the Nova StatSensor® Xpress™ Creatinine point-of-care handheld analyzer. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0122433.
- McGough L, Malic A, Beukinga I, Blairon L. Management of the Prevention of Kidney Injury in Patients at Risk of Renal Failure by StatSensor Blood Creatinine Monitoring System: Evaluation of Method n.d.
- Minnings K, Kerns E, Fiore M, Fiore M, Parekh RS, DuBois J, et al. Chronic kidney disease prevalence in Rivas, Nicaragua: use of a field device for creatinine measurement. Clin Biochem 2015;48:456-8. https://doi.org/10.1016/j.clinbiochem.2015.01.005.
- Rensburg M, Hudson C, Erasmus R. Evaluation and performance of the nova StatSensor creatinine point-of-care monitoring system. Clin Chem Lab Med 2014;1.
- Aumatell A, Sharpe D, Reed W. Validation of the StatSensor creatinine meter for testing blood before contrast computed tomography studies. Point Care 2010;9:25-31. https://doi.org/10.1097/POC.0b013e3181d2d8a5.
- Bahar B, DeChristopher PJ, Holmes EW, Kahn SE. Investigation of falsely decreased creatinine results observed from the Abbott I-STAT point-of-care device in use for testing specimens from ambulatory oncology patients. Point Care 2016;15:72-7. https://doi.org/10.1097/POC.0000000000000093.
- Baier KA, Markham LE, Flaigle SP, Nelson PW, Shield CF, Muruve NA, et al. Point-of-care testing in an organ procurement organization donor management setting. Clin Transplant 2003;17:48-51. https://doi.org/10.1034/j.1399-0012.17.s9.9.x.
- Bender LM, Gearhart M, Faso A, Hammett-Stabler C. Comparison of whole blood creatinine measurement to serum creatinine measurements in renal dose calculations. Clin Chem 2012;58:A165-A6.
- Betman SA, Hod EA, Kratz A. Hydroxyurea interference in point-of-care creatinine and glucose measurements. Am J Clin Pathol 2015;1. https://doi.org/10.1093/ajcp/143.suppl1.031.
- Bobilewicz D. Comparison of Creatinine Concentration in Serum, Plasma and Whole Blood on Different Routinely Used Analysers 2008.
- Cao J, Edwards R, Chairez J, Devaraj S. Validation of capillary blood analysis and capillary testing mode on the epoc point of care system. Pract Lab Med 2017;9:24-7. https://doi.org/10.1016/j.plabm.2017.07.003.
- Cory R, Clark K, Snowball O, Bramham K. Evaluation of the StatSensor Xpress for the point of care determination of capillary creatinine concentration in a pregnant population. Pregnancy Hypertens 2018;13. https://doi.org/10.1016/j.preghy.2018.08.410.
- Dimeski G, Tilley V, Jones BW, Brown NN. Which point-of-care creatinine analyser for radiology: direct comparison of the i-Stat and StatStrip creatinine methods with different sample types. Ann Clin Biochem 2013;50:47-52. https://doi.org/10.1258/acb.2012.012081.
- Dohnal L, Kloudova M, Cechak P. Comparison of Some Characteristics in Results of Four Analytical Systems for 12 Basic Biochemical Analytes. Klini Biochemi Metabol 2008;16:33-9.
- Gault MH, Seymour ME, Howell WE. Evaluation of i-STAT creatinine assay. Nephron 2001;88:178-82. https://doi.org/10.1159/000045982.
- Georgievskaya ZA, Creger RA, Schmotzer CL. Under-estimation of plasma creatinine using iSTAT point-of-care analyzer: impact on carboplatin dosing in an academic cancer center. Clin Chem 2011;1.
- Janetto P, Shi RZ, Scheider C, Breitenfeld D. Comparison of Whole Blood Versus Plasma Creatinine Measurements on the ABL800 FLEX and Olympus AU5431 Analyzer (Modified Jaffe Method) 2006.
- Lee-Lewandrowski E, Chang C, Gregory K, Lewandrowski K. Evaluation of rapid point-of-care creatinine testing in the radiology service of a large academic medical center: impact on clinical operations and patient disposition. Clin Chim Acta 2012;413:88-92. https://doi.org/10.1016/j.cca.2011.05.006.
- Lehtonen A. Sodium, Potassium and Creatinine Result Level Comparison Between Point-of-Care Analyser and Core Laboratory Automatic Analyser. Tampere: Tampere University of Applied Sciences; 2013.
- Mahlow J, Reineks E. Accuracy of i-STAT point-of-care creatinine measurements in outpatient chemotherapy patients: a direct comparison to laboratory based methods. Clin Chem 2016;62.
- Morita S, Suzuki K, Masukawa A, Ueno E. Assessing renal function with a rapid, handy, point-of-care whole blood creatinine meter before using contrast materials. Jpn J Radiol 2011;29:187-93. https://doi.org/10.1007/s11604-010-0536-8.
- Murata K, Glaser L, Nardiello M, Ramanathan LV, Carlow DC. Data from the analytical performance of the Abaxis Piccolo Xpress point of care analyzer in whole blood, serum, and plasma. Data Brief 2018;16:81-9. https://doi.org/10.1016/j.dib.2017.11.006.
- Naugler C, Redman L, Sadzradeh H. Comparison of estimated glomerular filtration rates using creatinine values generated by iSTAT and Cobas 6000. Clin Chim Acta 2014;429:79-80. https://doi.org/10.1016/j.cca.2013.11.033.
- Park H, Ko DH, Kim JQ, Song SH. Performance evaluation of the Piccolo Xpress point-of-care chemistry analyzer. Korean J Lab Med 2009;29:430-8. https://doi.org/10.3343/kjlm.2009.29.5.430.
- Schnabl KL, Tarek M, Cursio C, Yip PM. Evaluation of the Piccolo Xpress chemistry analyzer for point of care testing of plasma liver function markers, electrolytes and metabolites. Clin Biochem 2008;41:1284-5. https://doi.org/10.1016/j.clinbiochem.2008.08.058.
- Schnabl KL, Bagherpoor S, Diker P, Cursio C, Dubois J, Yip PM. Evaluation of the analytical performance of the Nova StatSensor creatinine meter and reagent strip technology for whole blood testing. Clin Biochem 2010;43:1026-9. https://doi.org/10.1016/j.clinbiochem.2010.04.055.
- Skurup A, Kristensen T, Wennecke G. National Kidney Disease Education Program Laboratory Working Group . New creatinine sensor for point-of-care testing of creatinine meets the National Kidney Disease Education Program guidelines. Clin Chem Lab Med 2008;46:3-8. https://doi.org/10.1515/CCLM.2008.004.
- Srihong C, Pangsapa K, Chuaboonmee K, Kotipan Y, Charuruks N. Evaluation of the analytical performance of the nova StatSensor creatinine meter for blood testing. J Med Assoc Thai 2012;95:1225-31.
- Stojkovic V, Delanaye P, Schleck M, Le Goff C, Cavalier E. Estimated glomerular filtration rate (GFR) using a point of care (POC) measure of creatinine in patients with iohexol determinate GFR. Clin Chem Lab Med 2017;55.
- Straseski J, Phelan L, Clarke W. Creatinine levels in patients with renal disease: discordance between POC meter and automated enzymatic methods. Clin Chem 2009;55:A102-A.
- Straseski JA, Clarke W, Molinaro R. Investigating possible causes of discrepant creatinine results: discordance in patients with renal insufficiency. Am J Clin Pathol 2010;134.
- Straseski JA, Lyon ME, Clarke W, Dubois JA, Phelan LA, Lyon AW. Investigating interferences of a whole-blood point-of-care creatinine analyzer: comparison to plasma enzymatic and definitive creatinine methods in an acute-care setting. Clin Chem 2011;57:1566-73. https://doi.org/10.1373/clinchem.2011.165480.
- Treves C, Boehre JL. Controlled trial between measurement of glomerular filtration rate on venous blood (automated laboratory) and capillary blood (bedside system). Clin Chem Lab Med 2011;1.
- Too CW, Ng WY, Tan CC, Mahmood MI, Tay KH. Screening for impaired renal function in outpatients before iodinated contrast injection: comparing the Choyke questionnaire with a rapid point-of-care-test. Eur J Radiol 2015;84:1227-31. https://doi.org/10.1016/j.ejrad.2015.04.001.
- van Lint CL, van der Boog PJ, Romijn FP, Schenk PW, van Dijk S, Rovekamp TJ, et al. Application of a point of care creatinine device for trend monitoring in kidney transplant patients: fit for purpose?. Clini Chem Lab Med 2015;53:1547-56. https://doi.org/10.1515/cclm-2014-0932.
- Ledermann HP, Mengiardi B, Schmid A, Froehlich JM. Screening for renal insufficiency following ESUR (European Society of Urogenital Radiology) guidelines with on-site creatinine measurements in an outpatient setting. Eur Radiol 2010;20:1926-33. https://doi.org/10.1007/s00330-010-1754-2.
- Snaith B, Harris MA, Clarke R. Screening prior to gadolinium based contrast agent administration: a UK survey of guideline implementation and adherence. Radiography 2016;22:S24-S27. https://doi.org/10.1016/j.radi.2016.07.006.
- Stahr K, Lindell E, Ahnlide J, Law I. The impact of Nova StatSensor creatinine point-of-care monitoring system on the number of i.v. contrast-enhanced CT scanning procedures performed in a clinical PET/CT unit. Eur J Nucl Med Mol Imag 2010;2:S496-S7.
- Murata K, Glaser L, Nardiello M, Richardson S, Ramanathan LV, Carlow DC. Analytical performance of the Abaxis Piccolo Xpress® point of care analyzer in whole blood, serum, and plasma. Clin Biochem 2015;48:1344-6. https://doi.org/10.1016/j.clinbiochem.2015.08.002.
- Newhouse JH, Kho D, Rao QA, Starren J. Frequency of serum creatinine changes in the absence of iodinated contrast material: implications for studies of contrast nephrotoxicity. AJR Am J Roentgenol 2008;191:376-82. https://doi.org/10.2214/AJR.07.3280.
- Bruce RJ, Djamali A, Shinki K, Michel SJ, Fine JP, Pozniak MA. Background fluctuation of kidney function versus contrast-induced nephrotoxicity. AJR Am J Roentgenol 2009;192:711-18. https://doi.org/10.2214/AJR.08.1413.
- Azzouz M, Rømsing J, Thomsen HS. Fluctuations in eGFR in relation to unenhanced and enhanced MRI and CT outpatients. Eur J Radiol 2014;83:886-92. https://doi.org/10.1016/j.ejrad.2014.02.014.
- Cheungpasitporn W, Thongprayoon C, Mao MA, Mao SA, D’Costa MR, Kittanamongkolchai W, et al. Contrast-induced acute kidney injury in kidney transplant recipients: a systematic review and meta-analysis. World J Transplant 2017;7:81-7. https://doi.org/10.5500/wjt.v7.i1.81.
- Ehrmann S, Quartin A, Hobbs BP, Robert-Edan V, Cely C, Bell C, et al. Contrast-associated acute kidney injury in the critically ill: systematic review and Bayesian meta-analysis. Intensive Care Med 2017;43:785-94. https://doi.org/10.1007/s00134-017-4700-9.
- Aycock RD, Westafer LM, Boxen JL, Majlesi N, Schoenfeld EM, Bannuru RR. Acute kidney injury after computed tomography: a meta-analysis. Ann Emerg Med 2018;71:44-53. https://doi.org/10.1016/j.annemergmed.2017.06.041.
- Kooiman J, Pasha SM, Zondag W, Sijpkens YW, van der Molen AJ, Huisman MV, et al. Meta-analysis: serum creatinine changes following contrast enhanced CT imaging. Eur J Radiol 2012;81:2554-61. https://doi.org/10.1016/j.ejrad.2011.11.020.
- Moos SI, van Vemde DN, Stoker J, Bipat S. Contrast induced nephropathy in patients undergoing intravenous (IV) contrast enhanced computed tomography (CECT) and the relationship with risk factors: a meta-analysis. Eur J Radiol 2013;82:e387-99. https://doi.org/10.1016/j.ejrad.2013.04.029.
- McDonald RJ, McDonald JS, Carter RE, Hartman RP, Katzberg RW, Kallmes DF, et al. Intravenous contrast material exposure is not an independent risk factor for dialysis or mortality. Radiology 2014;273:714-25. https://doi.org/10.1148/radiol.14132418.
- Davenport MS, Khalatbari S, Dillman JR, Cohan RH, Caoili EM, Ellis JH. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material. Radiology 2013;267:94-105. https://doi.org/10.1148/radiol.12121394.
- Davenport MS, Khalatbari S, Cohan RH, Dillman JR, Myles JD, Ellis JH. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: risk stratification by using estimated glomerular filtration rate. Radiology 2013;268:719-28. https://doi.org/10.1148/radiol.13122276.
- McDonald JS, McDonald RJ, Carter RE, Katzberg RW, Kallmes DF, Williamson EE. Risk of intravenous contrast material-mediated acute kidney injury: a propensity score-matched study stratified by baseline-estimated glomerular filtration rate. Radiology 2014;271:65-73. https://doi.org/10.1148/radiol.13130775.
- Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med 1997;127:757-63. https://doi.org/10.7326/0003-4819-127-8_Part_2-199710151-00064.
- Rosenbaum P, Rubin D. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat 1985;39:33-8. https://doi.org/10.1080/00031305.1985.10479383.
- Walsh M, Srinathan SK, McAuley DF, Mrkobrada M, Levine O, Ribic C, et al. The statistical significance of randomized controlled trial results is frequently fragile: a case for a Fragility Index. J Clin Epidemiol 2014;67:622-8. https://doi.org/10.1016/j.jclinepi.2013.10.019.
- Dekkers IA, van der Molen AJ. Propensity score matching as a substitute for randomized controlled trials on acute kidney injury after contrast media administration: a systematic review. AJR Am J Roentgenol 2018;211:822-6. https://doi.org/10.2214/AJR.17.19499.
- Hinson JS, Ehmann MR, Fine DM, Fishman EK, Toerper MF, Rothman RE, et al. Risk of acute kidney injury after intravenous contrast media administration. Ann Emerg Med 2017;69:577-86. https://doi.org/10.1016/j.annemergmed.2016.11.021.
- McDonald JS, McDonald RJ, Williamson EE, Kallmes DF. Is intravenous administration of iodixanol associated with increased risk of acute kidney injury, dialysis, or mortality? A propensity score-adjusted study. Radiology 2017;285:414-24. https://doi.org/10.1148/radiol.2017161573.
- Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011. The Cochrane Collaboration; 2011.
- Hiremath S, Akbari A, Shabana W, Fergusson DA, Knoll GA. Prevention of contrast-induced acute kidney injury: is simple oral hydration similar to intravenous? A systematic review of the evidence. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0060009.
- Agarwal SK, Mohareb S, Patel A, Yacoub R, DiNicolantonio JJ, Konstantinidis I, et al. Systematic oral hydration with water is similar to parenteral hydration for prevention of contrast-induced nephropathy: an updated meta-analysis of randomised clinical data. Open Heart 2015;2. https://doi.org/10.1136/openhrt-2015-000317.
- Ahmed K, McVeigh T, Cerneviciute R, Mohamed S, Tubassam M, Karim M, et al. Effectiveness of contrast-associated acute kidney injury prevention methods; a systematic review and network meta-analysis. BMC Nephrol 2018;19. https://doi.org/10.1186/s12882-018-1113-0.
- Dussol B, Morange S, Loundoun A, Auquier P, Berland Y. A randomized trial of saline hydration to prevent contrast nephropathy in chronic renal failure patients. Nephrol Dial Transplant 2006;21:2120-6. https://doi.org/10.1093/ndt/gfl133.
- Nijssen EC, Rennenberg RJ, Nelemans PJ, Essers BA, Janssen MM, Vermeeren MA, et al. Prophylactic hydration to protect renal function from intravascular iodinated contrast material in patients at high risk of contrast-induced nephropathy (AMACING): a prospective, randomised, phase 3, controlled, open-label, non-inferiority trial. Lancet 2017;389:1312-22. https://doi.org/10.1016/S0140-6736(17)30057-0.
- Nijssen EC, Nelemans PJ, Rennenberg RJ, van Ommen V, Wildberger JE. Prophylactic intravenous hydration to protect renal function from intravascular iodinated contrast material (AMACING): long-term results of a prospective, randomised, controlled trial. EClinicalMedicine 2018;4–5:109-16. https://doi.org/10.1016/j.eclinm.2018.10.007.
- Nijssen EC, Nelemans PJ, Rennenberg RJ, van Ommen V, Wildberger JE. Evaluation of safety guidelines on the use of iodinated contrast material: conundrum continued. Invest Radiol 2018;53:616-22. https://doi.org/10.1097/RLI.0000000000000479.
- The Renal Association, British Cardiovascular Intervention Society and the Royal College of Radiologists . Prevention of Contrast Induced Acute Kidney Injury (CI-AKI) in Adult Patients 2013. www.rcr.ac.uk/publications.aspx?PageID=310%26PublicationID=391 (accessed 31 January 2019).
- National Institute for Health and Care Excellence (NICE) . Acute Kidney Injury: Prevention, Detection and Management of Acute Kidney Injury up to the Point of Renal Replacement Therapy. Clinical Guidelines, CG169 2013. https://guidance.nice.org.uk/cg169 (accessed 31 January 2019).
- Royal College of Radiologists . Standards for Intravascular Contrast Administration to Adult Patients 2015.
- National Clinical Guideline Centre . Acute kidney injury: prevention, detection and management up to the point of renal replacement therapy. Royal Coll Physicians 2013;8.
- Department of Health and Social Care . National Schedule of Reference Costs 2015–2016 2016. www.gov.uk/government/publications/nhs-reference-costs-2015-to-2016 (accessed 15 April 2019).
- Nowacek A, McClintock D. Point-of-care creatinine/estimated glomerular filtration rate testing within the radiology department of an academic medical center improves workflow and reduces costs. Am J Clin Pathol 2015;1. https://doi.org/10.1093/ajcp/143.suppl1.045.
- Chalkidou A, Erskine J. RX205 Point-of-care Creatinine Tests. London: KiTEC – King’s Technology Evaluation Centre; 2019.
- Moos SI, Stoker J, Nagan G, de Weijert RS, van Vemde DN, Bipat S. Prediction of presence of kidney disease in a general patient population undergoing intravenous iodinated contrast enhanced computed tomography. Eur Radiol 2014;24:1266-75. https://doi.org/10.1007/s00330-014-3149-2.
- Park S, Kim MH, Kang E, Park S, Jo HA, Lee H, et al. Contrast-induced nephropathy after computed tomography in stable CKD patients with proper prophylaxis: 8-year experience of outpatient prophylaxis program. Medicine 2016;95. https://doi.org/10.1097/MD.0000000000003560.
- Schreuder SM, Stoker J, Bipat S. Prediction of presence of kidney disease in patients undergoing intravenous iodinated contrast enhanced computed tomography: a validation study. Eur Radiol 2017;27:1613-21. https://doi.org/10.1007/s00330-016-4478-0.
- Kim SM, Cha RH, Lee JP, Kim DK, Oh KH, Joo KW, et al. Incidence and outcomes of contrast-induced nephropathy after computed tomography in patients with CKD: a quality improvement report. Am J Kidney Dis 2010;55:1018-25. https://doi.org/10.1053/j.ajkd.2009.10.057.
- Office for National Statistics . National Life Tables: England 2018. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesenglandreferencetables (accessed 7 February 2020).
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18. https://doi.org/10.1111/j.1524-4733.2010.00700.x.
- Wyld M, Morton RL, Hayen A, Howard K, Webster AC. A systematic review and meta-analysis of utility-based quality of life in chronic kidney disease treatments. PLOS Med 2012;9. https://doi.org/10.1371/journal.pmed.1001307.
- Hall PS, Mitchell ED, Smith AF, Cairns DA, Messenger M, Hutchinson M, et al. The future for diagnostic tests of acute kidney injury in critical care: evidence synthesis, care pathway analysis and research prioritisation. Health Technol Assess 2018;22. https://doi.org/10.3310/hta22320.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: Personal Social Services Research Unit, University of Kent; 2017.
- Department of Health and Social Care . National Schedule of Reference Costs – Year 2017–18 – NHS Trust and NHS Foundation Trusts 2018. https://improvement.nhs.uk/resources/reference-costs/ (accessed 15 April 2019).
- Duffy S, de Kock S, Misso K, Noake C, Ross J, Stirk L. Supplementary searches of PubMed to improve currency of MEDLINE and MEDLINE In-Process searches via Ovid. J Med Libr Assoc 2016;104:309-12. https://doi.org/10.3163/1536-5050.104.4.011.
- Adams DA, Buus-Frank M. Point-of-care technology: the i-STAT system for bedside blood analysis. J Pediatr Nurs 1995;10:194-8. https://doi.org/10.1016/S0882-5963(05)80084-3.
- Canadian Agency for Drugs and Technologies in Health . Point of Care Testing for Human Chorionic Gonadotropin, Creatinine, and Blood Urea Nitrogen: Cost-Effectiveness 2013. https://www.crd.york.ac.uk/CRDWeb/ShowRecord.asp?ID=32014000136 (accessed 21 May 2019).
- Canadian Agency for Drugs and Technologies in Health (CADTH) . CADTH Database Search Filters 2016. www.cadth.ca/resources/finding-evidence/strings-attached-cadths-database-search-filters (accessed 13 January 2019).
- Chicaíza-Becerra LA, García-Molina M, Gamboa Ó. Cost-effectiveness of iso- versus low-osmolality contrast media in outpatients with high risk of contrast medium-induced nephropathy. Biomedica 2012;32:182-8. https://doi.org/10.1590/S0120-41572012000300005.
- Iannazzo S, Vandekerckhove S, De Francesco M, Nayak A, Ronco C, Morana G, et al. Economic evaluation of intravenous iodinated contrast media in Italy. Int J Technol Assess Health Care 2014;30:69-77. https://doi.org/10.1017/S0266462313000706.
- De Smedt DM, Elseviers MM, Lins RL, Annemans L. Economic evaluation of different treatment modalities in acute kidney injury. Nephrol Dial Transplant 2012;27:4095-101. https://doi.org/10.1093/ndt/gfs410.
- Ethgen O, Schneider AG, Bagshaw SM, Bellomo R, Kellum JA. Economics of dialysis dependence following renal replacement therapy for critically ill acute kidney injury patients. Nephrol Dial Transplant 2015;30:54-61. https://doi.org/10.1093/ndt/gfu314.
- Kerr M, Bedford M, Matthews B, O’Donoghue D. The economic impact of acute kidney injury in England. Nephrol Dial Transplant 2014;29:1362-8. https://doi.org/10.1093/ndt/gfu016.
- Petrovic S, Bogavac-Stanojevic N, Lakic D, Peco-Antic A, Vulicevic I, . Cost-effectiveness analysis of acute kidney injury biomarkers in pediatric cardiac surgery. Biochemia Medica 2015;25:262-71. https://doi.org/10.11613/BM.2015.027.
- James MT, Ghali WA, Knudtson ML, Ravani P, Tonelli M, Faris P, et al. Associations between acute kidney injury and cardiovascular and renal outcomes after coronary angiography. Circulation 2011;123:409-16. https://doi.org/10.1161/CIRCULATIONAHA.110.970160.
- Mueller C, Buerkle G, Buettner HJ, Petersen J, Perruchoud AP, Eriksson U, et al. Prevention of contrast media-associated nephropathy: randomized comparison of 2 hydration regimens in 1620 patients undergoing coronary angioplasty. Arch Intern Med 2002;162:329-36. https://doi.org/10.1001/archinte.162.3.329.
- Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009;360:961-72. https://doi.org/10.1056/NEJMoa0804626.
- Eriksen BO, Ingebretsen OC. The progression of chronic kidney disease: a 10-year population-based study of the effects of gender and age. Kidney Int 2006;69:375-82. https://doi.org/10.1038/sj.ki.5000058.
- Tajima R, Kondo M, Kai H, Saito C, Okada M, Takahashi H, et al. Measurement of health-related quality of life in patients with chronic kidney disease in Japan with EuroQol (EQ-5D). Clin Exp Nephrol 2010;14:340-8. https://doi.org/10.1007/s10157-010-0304-1.
- Kind P, Dolan P, Gudex C, Williams A. Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ 1998;316:736-41. https://doi.org/10.1136/bmj.316.7133.736.
- Sullivan PW, Slejko JF, Sculpher MJ, Ghushchyan V. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Making 2011;31:800-4. https://doi.org/10.1177/0272989X11401031.
- Department of Health and Social Care . National Schedule of Reference Costs 2011–2012 2012. www.gov.uk/government/publications/nhs-reference-costs-financial-year-2011-to-2012 (accessed 15 April 2019).
- Curtis L. Unit Costs of Health and Social Care 2012. Canterbury: Personal Social Services Research Unit, University of Kent; 2012.
- Joint Formulary Committee . British National Formulary (BNF) 2013.
- National Institute for Health and Care Excellence (NICE) . Anaemia Management in People With Chronic Kidney Disease Clinical Guidelines, CG114 2011. www.nice.org.uk/guidance/cg114 (accessed 21 April 2020).
- Solomon R. The role of osmolality in the incidence of contrast-induced nephropathy: a systematic review of angiographic contrast media in high risk patients. Kidney Int 2005;68:2256-63. https://doi.org/10.1111/j.1523-1755.2005.00684.x.
- Nguyen SA, Suranyi P, Ravenel JG, Randall PK, Romano PB, Strom KA, et al. Iso-osmolality versus low-osmolality iodinated contrast medium at intravenous contrast-enhanced CT: effect on kidney function. Radiology 2008;248.1:97-105. https://doi.org/10.1148/radiol.2481071484.
- Solomon R, DuMouchel W. Contrast media and nephropathy: findings from systematic analysis and Food and Drug Administration reports of adverse effects. Invest Radiol 2006;41.8:651-60. https://doi.org/10.1097/01.rli.0000229742.54589.7b.
- From AM, Bartholmai BJ, Williams AW, Cha SS, McDonald FS. Mortality associated with nephropathy after radiographic contrast exposure. Mayo Clin Proc 2008;83:1095-100. https://doi.org/10.4065/83.10.1095.
- Klarenbach SW, Pannu N, Tonelli MA, Manns BJ. Cost-effectiveness of hemofiltration to prevent contrast nephropathy in patients with chronic kidney disease. Crit Care Med 2006;34:1044-51. https://doi.org/10.1097/01.CCM.0000206287.22318.C3.
- Aguirre Caicedo M. Nefropatía por medios de contraste. Acta Med Colomb 2007;32:68-79.
Appendix 1 Literature search strategies
Database search strategies
MEDLINE (Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE®)
URL: via Ovid – https://ovidsp.ovid.com/.
Date range searched: 1946 to 5 November 2018.
Date searched: 6 November 2018.
Records retrieved: 935.
Search strategy
-
Point-of-Care Systems/ (11,059)
-
Point-of-Care Testing/ (999)
-
point-of-care.ti,ab,kf. (15,874)
-
(POC or POCT).ti,ab,kf. (4593)
-
(rapid$ adj3 (test$ or determin$ or assess$ or analys$ or analyz$ or identif$ or measur$ or screen$)).ti,ab. (72,301)
-
((bedside$ or bed-side$) adj3 (test$ or determin$ or assess$ or analys$ or analyz$ or identif$ or measur$ or screen$)).ti,ab. (3654)
-
((on-site or onsite) adj3 (test$ or determin$ or assess$ or analys$ or analyz$ or identif$ or measur$ or screen$)).ti,ab. (2472)
-
(near adj4 patient$ adj4 test$).ti,ab. (429)
-
(near adj4 patient$ adj4 determin$).ti,ab. (18)
-
(near adj4 patient$ adj4 assess$).ti,ab. (40)
-
(near adj4 patient$ adj4 analys$).ti,ab. (52)
-
(near adj4 patient$ adj4 analyz$).ti,ab. (21)
-
(near adj4 patient$ adj4 identif$).ti,ab. (38)
-
(near adj4 patient$ adj4 measur$).ti,ab. (88)
-
(near adj4 patient$ adj4 screen$).ti,ab. (15)
-
or/1-15 (98,921)
-
Creatinine/ (53,591)
-
creatinin$ .ti,ab,kf. (103,420)
-
serumcreatinin$ .ti,ab,kf. (4)
-
SCr.ti,ab,kf. (6111)
-
or/17-20 (127,272)
-
16 and 21 (584)
-
Kidney Function Tests/ (24,304)
-
Glomerular Filtration Rate/ (40,393)
-
((kidney$ or renal) adj3 (function$ or dysfunction$)).ti,ab. (122,372)
-
glomerul$ filtration rate$ .ti,ab,kf. (39,656)
-
glomerulofiltration rate$ .ti,ab,kf. (6)
-
GFR.ti,ab,kf. (17,926)
-
eGFR.ti,ab,kf. (49,812)
-
or/23-29 (208,018)
-
16 and 30 (531)
-
22 or 31 (933)
-
Computers, Handheld/ (3272)
-
((handheld or hand held) adj2 (device$ or analyser$ or analyzer$)).ti,ab. (1598)
-
((desktop or desk top) adj2 (device$ or analyser$ or analyzer$)).ti,ab. (74)
-
((table top or tabletop or bench top or benchtop) adj2 (device$ or analyser$ or analyzer$)).ti,ab. (145)
-
((portab$ or transportab$) adj2 (device$ or analyser$ or analyzer$)).ti,ab. (3217)
-
(near patient$ adj2 (device$ or analyser$ or analyzer$)).ti,ab. (28)
-
or/33-38 (8033)
-
21 or 30 (290,065)
-
39 and 40 (50)
-
32 or 41 (966)
-
(i-STAT or iSTAT).ti,ab,kf. (486)
-
40 and 43 (23)
-
(StatSensor or Stat Sensor).ti,ab,kf. (16)
-
ABL90 FLEX PLUS.ti,ab,kf. (0)
-
(ABL800 FLEX or ABL800FLEX or ABL 800 FLEX).ti,ab,kf. (25)
-
Dri-chem NX500.ti,ab,kf. (0)
-
epoc Blood Analysis.ti,ab,kf. (3)
-
Piccolo Xpress.ti,ab,kf. (7)
-
or/44-50 (69)
-
42 or 51 (1003)
-
exp animals/not humans/ (4,511,292)
-
54 52 not 53 (935).
Key
/ = indexing term [medical subject heading (MeSH) heading].
exp = exploded indexing term (MeSH heading).
$ = truncation.
ti,ab = terms in either title or abstract fields.
kf = author keywords field.
adj3 = terms within three words of each other (any order).
Cochrane Central Register of Controlled Trials (CENTRAL)
URL: via Wiley Online Library – https://onlinelibrary.wiley.com/.
Date range searched: issue 10 of 12, October 2018.
Date searched: November 2018.
Records retrieved: 107.
The strategy below was used to search both CENTRAL and CDSR.
Search strategy
-
#1 MeSH descriptor: [Point-of-Care Systems] this term only (387)
-
#2 MeSH descriptor: [Point-of-Care Testing] this term only (46)
-
#3 point-of-care:ti,ab,kw (1465)
-
#4 (POC or POCT):ti,ab,kw (1329)
-
#5 (rapid* near/3 (test* or determin* or assess* or analys* or analyz* or identif* or measur* or screen*)):ti,ab,kw (2811)
-
#6 ((bedside* or bed-side*) near/3 (test* or determin* or assess* or analys* or analyz* or identif* or measur* or screen*)):ti,ab,kw (330)
-
#7 ((on-site or onsite) near/3 (test* or determin* or assess* or analys* or analyz* or identif* or measur* or screen*)):ti,ab,kw (179)
-
#8 (“near” near/4 patient* near/4 test*):ti,ab,kw (46)
-
#9 (“near” near/4 patient* near/4 determin*):ti,ab,kw (3)
-
#10 (“near” near/4 patient* near/4 assess*):ti,ab,kw (9)
-
#11 (“near” near/4 patient* near/4 analys*):ti,ab,kw (1)
-
#12 (“near” near/4 patient* near/4 analyz*):ti,ab,kw (1)
-
#13 (“near” near/4 patient* near/4 identif*):ti,ab,kw (4)
#14 (“near” near/4 patient* near/4 measur*):ti,ab,kw (8)
-
#15 (“near” near/4 patient* near/4 screen*):ti,ab,kw (1)
-
#16 {OR #1-#15} (5677)
-
#17 MeSH descriptor: [Creatinine] this term only (3779)
-
#18 creatinin*:ti,ab,kw (17,537)
-
#19 serumcreatinin*:ti,ab,kw (34)
-
#20 SCr:ti,ab,kw (890)
-
#21 122-#20-#20-#20-#20 (17,896)
-
#22 #16 AND #21 (61)
-
#23 MeSH descriptor: [Kidney Function Tests] this term only (1162)
-
#24 MeSH descriptor: [Glomerular Filtration Rate] this term only (2488)
-
#25 ((kidney* or renal) near/3 (function* or dysfunction*)):ti,ab,kw (14,814)
-
#26 glomerul* next filtration next rate*:ti,ab,kw (7103)
-
#27 glomerulofiltration next rate*:ti,ab,kw (0)
-
#28 GFR:ti,ab,kw (4858)
-
#29 eGFR:ti,ab,kw (4823)
-
#30 {OR #23-#29} (21,219)
-
#31 #16 AND #30 (65)
-
#32 #22 OR #31 (103)
-
#33 MeSH descriptor: [Computers, Handheld] this term only (230)
-
#34 ((handheld or “hand held”) near/2 (device* or analyser* or analyzer*)):ti,ab,kw (227)
-
#35 ((desktop or “desk top”) near/2 (device* or analyser* or analyzer*)):ti,ab,kw (6)
-
#36 ((“table top” or tabletop or “bench top” or benchtop) near/2 (device* or analyser* or analyzer*)):ti,ab,kw (5)
-
#37 ((portab* or transportab*) near/2 (device* or analyser* or analyzer*)):ti,ab,kw (330)
-
#38 ((“near patient” or “near patients”) near/2 (device* or analyser* or analyzer*)):ti,ab,kw (1)
-
#39 {OR #33-#38} (775)
-
#40 #21 OR #30 (32,349)
-
#41 #39 AND #40 (9)
-
#42 #32 OR #41 (112)
-
#43 (i-STAT or iSTAT):ti,ab,kw (20)
-
#44 (StatSensor or Stat-Sensor):ti,ab,kw (0)
-
#45 “ABL90 FLEX PLUS”:ti,ab,kw (0)
-
#46 (ABL800 FLEX or ABL800FLEX or ABL 800 FLEX):ti,ab,kw (4)
-
#47 Dri-chem NX500:ti,ab,kw (0)
-
#48 “epoc Blood Analysis”:ti,ab,kw (0)
-
#49 Piccolo Xpress:ti,ab,kw (0)
-
#50 123-#49-#49-#49-#49 (22)
-
#51 #42 OR #50 (133)
-
#52 #42 or #50 in Cochrane Reviews (26)
-
#53 #42 or #50 in Trials (107)
Key
MeSH descriptor = indexing term (MeSH heading).
* = truncation.
ti,ab,kw = terms in either title or abstract or keyword fields.
near/3 = terms within three words of each other (any order).
next = terms are next to each other.
Cochrane Database of Systematic Reviews (CDSR)
URL: via Wiley Online Library – https://onlinelibrary.wiley.com/.
Date range searched: issue 11 of 12, November 2018.
Date searched: 6 November 2018.
Records retrieved: 26.
See Cochrane Central Register of Controlled Trials (CENTRAL) for search strategy used.
Cumulative Index to Nursing and Allied Health Literature (CINAHL Plus)
URL: via EBSCOhost – www.ebscohost.com/.
Date range searched: inception to 5 November 2018.
Date searched: 6 November 2018.
Records retrieved: 398.
Search strategy
-
S1 MH “Point-of-Care Testing” OR MH “Clinical Information Systems” (8790)
-
S2 TI point-of-care OR AB point-of-care (5832)
-
S3 TI (POC or POCT) OR AB (POC or POCT) (1220)
-
S4 TI ( rapid* N3 (test* or determin* or assess* or analys* or analyz* or identif* or measur* or screen*) ) OR AB (rapid* N3 (test* or determin* or assess* or analys* or analyz* or identif* or measur* or screen*) ) (8379)
-
S5 TI ( (bedside* or bed-side*) N3 (test* or determin* or assess* or analys* or analyz* or identif* or measur* or screen*)) OR AB ( (bedside* or bed-side*) N3 (test* or determin* or assess* or analys* or analyz* or identif* or measur* or screen*) ) (1641)
-
S6 TI ( (on-site or onsite) N3 (test* or determin* or assess* or analys* or analyz* or identif* or measur* or screen*) ) OR AB ( (on-site or onsite) N3 (test* or determin* or assess* or analys* or analyz* or identif* or measur* or screen*) ) (10,344)
-
S7 TI (near N4 patient* N4 test*) OR AB (near N4 patient* N4 test*) (152)
-
S8 TI (near N4 patient* N4 determin*) OR AB (near N4 patient* N4 determin*) (11)
-
S9 TI (near N4 patient* N4 assess*) OR AB (near N4 patient* N4 assess*) (23)
-
S10 TI (near N4 patient* N4 analys*) OR AB (near N4 patient* N4 analys*) (23)
-
S11 TI (near N4 patient* N4 analyz*) OR AB (near N4 patient* N4 analyz*) (5)
-
S12 TI (near N4 patient* N4 identif*) OR AB (near N4 patient* N4 identif*) (24)
-
S13 TI (near N4 patient* N4 measur*) OR AB (near N4 patient* N4 measur*) (36)
-
S14 TI (near N4 patient* N4 screen*) OR AB (near N4 patient* N4 screen*) 4)
-
S15 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 (31,354)
-
S16 MH “Creatinine” (8128)
-
S17 TI creatinin* OR AB creatinin* (13,520)
-
S18 TI serumcreatinin* OR AB serumcreatinin* (2)
-
S19 TI SCr OR AB SCr (737)
-
S20 S16 OR S17 OR S18 OR S19 (17,758)
-
S21 S15 AND S20 (186)
-
S22 MH “Kidney Function Tests” (2679)
-
S23 MH “Glomerular Filtration Rate” (8043)
-
S24 TI ( (kidney* or renal) N3 (function* or dysfunction*) ) OR AB ( (kidney* or renal) N3 (function* or dysfunction*) ) (16,250)
-
S25 TI glomerul* N1 filtration N1 rate* OR AB glomerul* N1 filtration N1 rate* (6789)
-
S26 TI glomerulofiltration N1 rate* OR AB glomerulofiltration N1 rate* (2)
-
S27 TI GFR OR AB GFR (2398)
-
S28 TI eGFR OR AB eGFR (8593)
-
S29 S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 (30,731)
-
S30 S15 AND S29 (160)
-
S31 S21 OR S30 (289)
-
S32 MH “Computers, Hand-Held” (3826)
-
S33 MH “Portable Equipment” (1004)
-
S34 TI ( (handheld or “hand held”) N2 (device* or analyser* or analyzer*) ) OR AB ( (handheld or “hand held”) N2 (device* or analyser* or analyzer*) ) (629)
-
S35 TI ( (desktop or “desk top”) N2 (device* or analyser* or analyzer*) ) OR AB ( (desktop or “desk top”) N2 (device* or analyzer* or analyzer*) ) (36)
-
S36 TI ( (“table top” or tabletop or “bench top” or benchtop) N2 (device* or analyser* or analyzer*) ) OR AB ( (“table top” or tabletop or “bench top” or benchtop) N2 (device* or analyser* or analyzer*) ) (36)
-
S37 TI ( (portab* or transportab*) N2 (device* or analyser* or analyzer*) ) OR AB ( (portab* or transportab*) N2 (device* or analyser* or analyzer*) ) (870)
-
S38 TI ( ((“near patient” or “near patients”) N2 (device* or analyser* or analyzer*)) ) OR AB ( ((“near patient” or “near patients”) N2 (device* or analyser* or analyzer*)) ) (6)
-
S39 S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 (6102)
-
S40 S20 OR S29 (41,597)
-
S41 S39 AND S40 (11)
-
S42 S31 OR S41 (296)
-
S43 TI ( i-STAT or iSTAT ) OR AB ( i-STAT or iSTAT ) (92)
-
S44 TI ( StatSensor or Stat-Sensor ) OR AB ( StatSensor or Stat-Sensor ) (3)
-
S45 TI ABL90 FLEX PLUS OR AB ABL90 FLEX PLUS (0)
-
S46 TI ( (ABL800 FLEX or ABL800FLEX or ABL 800 FLEX) ) OR AB ( (ABL800 FLEX or ABL800FLEX or ABL 800 FLEX) ) (7)
-
S47 TI Dri-chem NX500 OR AB Dri-chem NX500 (0)
-
S48 TI epoc Blood Analysis OR AB epoc Blood Analysis (6)
-
S49 TI Piccolo Xpress OR AB Piccolo Xpress (2)
-
S50 S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 (108)
-
S51 S42 OR S50 (398)
Key
MH = indexing term (CINAHL heading).
* = truncation.
TI = terms in the title.
AB = terms in the abstract.
N3 = terms within three words of each other (any order).
Database of Abstracts of Reviews of Effects (DARE)
URL: via www.crd.york.ac.uk/CRDWeb/.
Date range searched: from inception to 31 March 2015.
Date searched: 6 November 2018.
Records retrieved: four.
Search strategy
The search strategy below was used to search all three of the Centre for Reviews and Dissemination (CRD) databases: DARE, the HTA database and NHS Economic Evaluations Database (NHS EED). As the term near is a stop word in the CRD databases it cannot be used as a search term. Therefore, lines 8–15 and line 38 of the MEDLINE strategy were omitted from the search of the CRD databases.
-
MeSH DESCRIPTOR Point-of-Care Systems (157)
-
MeSH DESCRIPTOR Point-of-Care Testing (1)
-
(point-of-care) (224)
-
(POC or POCT) (23)
-
(rapid* NEAR3 (test* or determin* or assess* or analys* or analyz* or identif* or measur* or screen*)) (370)
-
((test* or determin* or assess* or analys* or analyz* or identif* or measur* or screen*) NEAR3 rapid*) (128)
-
((bedside* or bed-side*) NEAR3 (test* or determin* or assess* or analys* or analyz* or identif* or measur* or screen*)) (27)
-
((test* or determin* or assess* or analys* or analyz* or identif* or measur* or screen*) NEAR3 (bedside* or bed-side*)) (14)
-
((on-site or onsite) NEAR3 (test* or determin* or assess* or analys* or analyz* or identif* or measur* or screen*)) (11)
-
((test* or determin* or assess* or analys* or analyz* or identif* or measur* or screen*) NEAR3 (on-site or onsite)) (6)
-
#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 (645)
-
MeSH DESCRIPTOR Creatinine (114)
-
(creatinin*) (499)
-
(serumcreatinin*) (0)
-
(SCr) (17)
-
#12 OR #13 OR #14 OR #15 503)
-
#11 AND #16 (7)
-
MeSH DESCRIPTOR Kidney Function Tests (53)
-
MeSH DESCRIPTOR Glomerular Filtration Rate (92)
-
((kidney* or renal) NEAR3 (function* or dysfunction*)) OR ((function* or dysfunction*) NEAR3 (kidney* or renal) ) (541)
-
(glomerul* filtration rate*) (176)
-
(glomerulofiltration rate*) (0)
-
(GFR) OR (eGFR) (194)
-
#18 OR #19 OR #20 OR #21 OR #22 OR #23 (784)
-
#11 AND #24 (2)
-
#17 OR #25 (9)
-
MeSH DESCRIPTOR Computers, Handheld (13)
-
((handheld or hand held) NEAR2 (device* or analyser* )or analyzer*)) OR ((device* or analyser* or analyzer*) NEAR2 (handheld or hand held)) (19)
-
((desktop or desk top) NEAR2 (device* or analyser* or analyzer*)) OR ((device* or analyser* or analyzer*) NEAR2 (desktop or desk top)) (2)
-
((table top or tabletop or bench top or benchtop) NEAR2 (device* or analyser* or analyzer*)) OR ((device* or analyser* or analyzer*) NEAR2 (table top or tabletop or bench top or benchtop)) (0)
-
((portab* or transportab*) NEAR2 (device* or analyser* or analyzer*)) OR ((device* or analyser* or analyzer*) NEAR2 (portab* or transportab*)) (29)
-
#27 OR #28 OR #29 OR #30 OR #31 (59)
-
#16 OR #24 (1095)
-
#32 AND #33 (1)
-
#26 OR #34 (10)
-
(i-STAT) OR (iSTAT) (3)
-
(StatSensor) OR (Stat Sensor) (0)
-
(ABL90 FLEX PLUS) (0)
-
(ABL800 FLEX or ABL800FLEX or ABL 800 FLEX) (0)
-
(Dri-chem NX500) (0)
-
(epoc Blood Analysis) (0)
-
(Piccolo Xpress) (0)
-
#35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 (13).
Key
MeSH DESCRIPTOR = indexing term (MeSH heading).
* = truncation.
NEAR3 = terms within three words of each other (order specified).
EconLit
URL: via Ovid – https://ovidsp.ovid.com/.
Date range searched: 1886 to 1 November 2018.
Date searched: 6 November 2018.
Records retrieved: 0.
Search strategy
-
point-of-care.mp. (9)
-
(POC or POCT).mp. (14)
-
(rapid$ adj3 (test$ or determin$ or assess$ or analys$ or analyz$ or identif$ or measur$ or screen$)).mp. (319)
-
((bedside$ or bed-side$) adj3 (test$ or determin$ or assess$ or analys$ or analyz$ or identif$ or measur$ or screen$)).mp. (1)
-
((on-site or onsite) adj3 (test$ or determin$ or assess$ or analys$ or analyz$ or identif$ or measur$ or screen$)).mp. (28)
-
(near adj4 patient$ adj4 test$).mp. (2)
-
(near adj4 patient$ adj4 determin$).mp. (0)
-
(near adj4 patient$ adj4 assess$).mp. (0)
-
(near adj4 patient$ adj4 analys$).mp. (0)
-
10 (near adj4 patient$ adj4 analyz$).mp. (0)
-
11 (near adj4 patient$ adj4 identif$).mp. (0)
-
12 (near adj4 patient$ adj4 measur$).mp. (0)
-
13 (near adj4 patient$ adj4 screen$).mp. (0)
-
14 or/1-13 (369)
-
15 creatinin$ .mp. (8)
-
16 serumcreatinin$ .mp. (0)
-
17 SCr.mp. (53)
-
18 or/15–17 (61)
-
19 14 and 18 (0)
-
20 ((kidney$ or renal) adj3 (function$ or dysfunction$)).mp. (7)
-
21 glomerul$ filtration rate$ .mp. (1)
-
22 glomerulofiltration rate$ .mp. (0)
-
23 GFR.mp. (6)
-
24 eGFR.mp. (1)
-
25 or/20-24 (15)
-
26 14 and 25 (0)
-
27 19 or 26 (0)
-
28 ((handheld or hand held) adj2 (device$ or analyser$ or analyzer$)).mp. (16)
-
29 ((desktop or desk top) adj2 (device$ or analyser$ or analyzer$)).mp. (2)
-
30 ((table top or tabletop or bench top or benchtop) adj2 (device$ or analyser$ or analyzer$)).mp. (0)
-
31 ((portab$ or transportab$) adj2 (device$ or analyser$ or analyzer$)).mp. (8)
-
32 (near patient$ adj2 (device$ or analyser$ or analyzer$)).mp. (1)
-
33 or/28-32 (25)
-
34 18 or 25 (74)
-
35 33 and 34 (0)
-
36 27 or 35 (0)
-
37 (i-STAT or iSTAT).mp. (180)
-
38 34 and 37 (0)
-
39 (StatSensor or Stat Sensor).mp. (0)
-
40 ABL90 FLEX PLUS.mp. (0)
-
41 (ABL800 FLEX or ABL800FLEX or ABL 800 FLEX).mp. (0)
-
42 Dri-chem NX500.mp. (0)
-
43 epoc Blood Analysis.mp. (0)
-
44 Piccolo Xpress.mp. (0)
-
45 38 or 39 or 40 or 41 or 42 or 43 or 44 (0)
-
46 36 or 45 (0).
Key
$ = truncation.
mp = terms in either title, abstract, or heading word fields.
adj3 = terms within three words of each other (any order).
EMBASE
URL: via Ovid – https://ovidsp.ovid.com/.
Date range searched: 1974 to 5 November 2018.
Date searched: 6 November 2018.
Records retrieved: 1967.
Search strategy
-
“point of care testing”/ (106,79)
-
rapid test/ (3395)
-
point-of-care.ti,ab,kw. (22,688)
-
(POC or POCT).ti,ab,kw. (7243)
-
(rapid$ adj3 (test$ or determin$ or assess$ or analys$ or analyz$ or identif$ or measur$ or screen$)).ti,ab. (88,530)
-
((bedside$ or bed-side$) adj3 (test$ or determin$ or assess$ or analys$ or analyz$ or identif$ or measur$ or screen$)).ti,ab. (5676)
-
((on-site or onsite) adj3 (test$ or determin$ or assess$ or analys$ or analyz$ or identif$ or measur$ or screen$)).ti,ab. (3323)
-
(near adj4 patient adj4 test$).ti,ab. (596)
-
(near adj4 patient$ adj4 determin$).ti,ab. (33)
-
(near adj4 patient$ adj4 assess$).ti,ab. (68)
-
(near adj4 patient$ adj4 analys$).ti,ab. (74)
-
(near adj4 patient$ adj4 analyz$).ti,ab. (27)
-
(near adj4 patient$ adj4 identif$).ti,ab. (70)
-
(near adj4 patient$ adj4 measur$).ti,ab. (125)
-
(near adj4 patient$ adj4 screen$).ti,ab. (22)
-
or/1-15 (124,452)
-
creatinine/ (156,366)
-
creatinine blood level/(97,275)
-
creatinin$ .ti,ab,kw. (164,758)
-
serumcreatinin$ .ti,ab,kw. (161)
-
SCr.ti,ab,kw. (10,539)
-
or/17-21 (240,268)
-
16 and 22 (1184)
-
kidney function test/ (10,451)
-
exp glomerulus filtration rate/(84,857)
-
((kidney$ or renal) adj3 (function$ or dysfunction$)).ti,ab. (179,335)
-
glomerul$ filtration rate$ .ti,ab,kw. (55,656)
-
glomerulofiltration rate$ .ti,ab,kw. (10)
-
GFR.ti,ab,kw. (33,036)
-
eGFR.ti,ab,kw. (93375)
-
or/24-30 (315,007)
-
16 and 31 (988)
-
23 or 32 (1837)
-
portable equipment/ (2209)
-
((handheld or hand held) adj2 (device$ or analyser$ or analyzer$)).ti,ab. (2365)
-
((desktop or desk top) adj2 (device$ or analyser$ or analyzer$)).ti,ab. (98)
-
((table top or tabletop or bench top or benchtop) adj2 (device$ or analyser$ or analyzer$)).ti,ab. (220)
-
((portab$ or transportab$) adj2 (device$ or analyser$ or analyzer$)).ti,ab. (4155)
-
(near patient$ adj2 (device$ or analyser$ or analyzer$)).ti,ab. (45)
-
or/34-39 (8570)
-
22 or 31 (476,117)
-
40 and 41 (98)
-
or 42 (1905)
-
(i-STAT or iSTAT).ti,ab,kw,dv. (923)
-
44 and 41 (79)
-
(StatSensor or Stat Sensor).ti,ab,kw,dv. (37)
-
ABL90 FLEX PLUS.ti,ab,kw,dv. (3)
-
(ABL800 FLEX or ABL800FLEX or ABL 800 FLEX).ti,ab,kw,dv. (106)
-
Dri-chem NX500.ti,ab,kw,dv. (0)
-
epoc Blood Analysis.ti,ab,kw,dv. (8)
-
Piccolo Xpress.ti,ab,kw,dv. (34)
-
or/45-51 (256)
-
43 or 52 (2077)
-
(animal/ or animal experiment/ or animal model/ or animal tissue/ or nonhuman/) not exp human/ (5,588,968)
-
53 not 54 (1967)
Key
/ = indexing term (Emtree heading).
exp = exploded indexing term (Emtree heading).
$ = truncation.
ti,ab = terms in either title or abstract fields.
kw = terms in the author keywords field.
dv = terms in the device trade name field.
adj3 = terms within three words of each other (any order).
Health Management Information Consortium (HMIC)
URL: via Ovid – https://ovidsp.ovid.com/.
Date range searched: 1979 to July 2018.
Date searched: 6 November 2018.
Records retrieved: five.
Search strategy
-
near patient tests/ (26)
-
point-of-care.mp. (225)
-
(POC or POCT).mp. (45)
-
(rapid$ adj3 (test$ or determin$ or assess$ or analys$ or analyz$ or identif$ or measur$ or screen$)).mp. (280)
-
((bedside$ or bed-side$) adj3 (test$ or determin$ or assess$ or analys$ or analyz$ or identif$ or measur$ or screen$)).mp. (23)
-
((on-site or onsite) adj3 (test$ or determin$ or assess$ or analys$ or analyz$ or identif$ or measur$ or screen$)).mp. (32)
-
(near adj4 patient$ adj4 test$).mp. (63)
-
(near adj4 patient$ adj4 determin$).mp. (0)
-
(near adj4 patient$ adj4 assess$).mp. (3)
-
(near adj4 patient$ adj4 analys$).mp. (3)
-
(near adj4 patient$ adj4 analyz$).mp. (0)
-
(near adj4 patient$ adj4 identif$).mp. (2)
-
(near adj4 patient$ adj4 measur$).mp. (0)
-
(near adj4 patient$ adj4 screen$).mp. (1)
-
or/1-14 (605)
-
creatinine/ (3)
-
creatinin$ .mp. (116)
-
serumcreatinin$ .mp. (0)
-
SCr.mp. (22)
-
16 or 17 or 18 or 19 (138)
-
15 and 20 (3)
-
((kidney$ or renal) adj3 (function$ or dysfunction$)).mp. (139)
-
glomerul$ filtration rate$ .mp. (60)
-
glomerulofiltration rate$ .mp. (0)
-
GFR.mp. (17)
-
eGFR.mp. (37)
-
22 or 23 or 24 or 25 or 26 (187)
-
15 and 27 (0)
-
portable equipment/ (74)
-
exp Portability/ (16)
-
((handheld or hand held) adj2 (device$ or analyser$ or analyzer$)).mp. (16)
-
((desktop or desk top) adj2 (device$ or analyser$ or analyzer$)).mp. (5)
-
((table top or tabletop or bench top or benchtop) adj2 (device$ or analyser$ or analyzer$)).mp. (0)
-
((portab$ or transportab$) adj2 (device$ or analyser$ or analyzer$)).mp. (19)
-
(near patient$ adj2 (device$ or analyser$ or analyzer$)).mp. (0)
-
29 or 30 or 31 or 32 or 33 or 34 or 35 (123)
-
20 or 27 (286)
-
36 and 37 (0)
-
21 or 28 or 38 (3)
-
(i-STAT or iSTAT).mp. (2)
-
(StatSensor or Stat Sensor).mp. (0)
-
ABL90 FLEX PLUS.mp. (0)
-
(ABL800 FLEX or ABL800FLEX or ABL 800 FLEX).mp. (0)
-
Dri-chem NX500.mp. (0)
-
epoc Blood Analysis.mp. (0)
-
Piccolo Xpress.mp. (0)
-
40 or 41 or 42 or 43 or 44 or 45 or 46 (2)
-
39 or 47 (5)
Key
/ = subject heading search.
$ = truncation.
mp = terms in either title, abstract, heading word or other title fields.
adj3 = terms within three words of each other (any order).
Health Technology Assessment (HTA) database
URL: via https://www.crd.york.ac.uk/CRDWeb/.
Date range searched: from inception to 31 March 2018.
Date searched: 6 November 2018.
Records retrieved: five.
See Database of Abstracts of Reviews of Effects (DARE) for search strategy used.
NHS Economic Evaluations Database (NHS EED)
URL: via www.crd.york.ac.uk/CRDWeb/.
Date range searched: from inception to 31 March 2015.
Date searched: 6 November 2018.
Records retrieved: four.
See Database of Abstracts of Reviews of Effects (DARE) for search strategy used.
PubMed
URL: www.ncbi.nlm.nih.gov/pubmed/.
Date searched: 5 November 2018.
Records retrieved: 499.
Search strategy
Search ((((((((((((((“Creatinine”[Mesh:NoExp]) OR creatinin*[Title/Abstract]) OR serumcreatinin*[Title/Abstract]) OR SCr[Title/Abstract])) OR (((((((“Kidney Function Tests”[Mesh:NoExp]) OR “Glomerular Filtration Rate”[Mesh:NoExp]) OR (((kidney*[Title/Abstract] OR renal[Title/Abstract])) AND (function*[Title/Abstract] OR dysfunction*[Title/Abstract]))) OR glomerul* filtration rate*[Title/Abstract]) OR glomerulofiltration rate*[Title/Abstract]) OR GFR[Title/Abstract]) OR eGFR[Title/Abstract]))) AND ((((((((“Point-of-Care Systems”[Mesh]) OR “Point-of-Care Testing”[Mesh:NoExp]) OR point-of-care[Title/Abstract]) OR ((POC[Title/Abstract] OR POCT[Title/Abstract]))) OR ((rapid*[Title/Abstract]) AND (test[Title/Abstract] OR tests[Title/Abstract] OR testing[Title/Abstract] OR testings[Title/Abstract] OR tested[Title/Abstract] OR determin*[Title/Abstract] OR assess*[Title/Abstract] OR analys*[Title/Abstract] OR analyz*[Title/Abstract] OR identif*[Title/Abstract] OR measur*[Title/Abstract] OR screen*[Title/Abstract]))) OR (((bedside*[Title/Abstract] OR bed-side*[Title/Abstract])) AND (test[Title/Abstract] OR tests[Title/Abstract] OR testing[Title/Abstract] OR testings[Title/Abstract] OR tested[Title/Abstract] OR determin*[Title/Abstract] OR assess*[Title/Abstract] OR analys*[Title/Abstract] OR analyz*[Title/Abstract] OR identif*[Title/Abstract] OR measur*[Title/Abstract] OR screen*[Title/Abstract]))) OR (((on-site[Title/Abstract] OR onsite[Title/Abstract])) AND (test[Title/Abstract] OR tests[Title/Abstract] OR testing[Title/Abstract] OR testings[Title/Abstract] OR tested[Title/Abstract] OR determin*[Title/Abstract] OR assess*[Title/Abstract] OR analys*[Title/Abstract] OR analyz*[Title/Abstract] OR identif*[Title/Abstract] OR measur*[Title/Abstract] OR screen*[Title/Abstract]))) OR near patient*[Title/Abstract]))) OR ((((((((“Creatinine”[Mesh:NoExp]) OR creatinin*[Title/Abstract]) OR serumcreatinin*[Title/Abstract]) OR SCr[Title/Abstract])) OR (((((((“Kidney Function Tests”[Mesh:NoExp]) OR “Glomerular Filtration Rate”[Mesh:NoExp]) OR (((kidney*[Title/Abstract] OR renal[Title/Abstract])) AND (function*[Title/Abstract] OR dysfunction*[Title/Abstract]))) OR glomerul* filtration rate*[Title/Abstract]) OR glomerulofiltration rate*[Title/Abstract]) OR GFR[Title/Abstract]) OR eGFR[Title/Abstract]))) AND (((((“Computers, Handheld”[Mesh:NoExp]) OR (((handheld[Title/Abstract] OR hand-held[Title/Abstract])) AND (device*[Title/Abstract] OR analyser*[Title/Abstract] OR analyzer*[Title/Abstract]))) OR (((desktop[Title/Abstract] OR desk-top[Title/Abstract])) AND (device*[Title/Abstract] OR analyser*[Title/Abstract] OR analyzer*[Title/Abstract]))) OR (((table-top[Title/Abstract] OR tabletop[Title/Abstract] OR bench-top[Title/Abstract] OR benchtop[Title/Abstract])) AND (device*[Title/Abstract] OR analyser*[Title/Abstract] OR analyzer*[Title/Abstract]))) OR (((portab*[Title/Abstract] OR transportab*[Title/Abstract])) AND (device*[Title/Abstract] OR analyser*[Title/Abstract] OR analyzer*[Title/Abstract]))))) OR (((((((((((i-STAT[Title/Abstract] OR iSTAT[Title/Abstract]))) AND ((((((“Creatinine”[Mesh:NoExp]) OR creatinin*[Title/Abstract]) OR serumcreatinin*[Title/Abstract]) OR SCr[Title/Abstract])) OR (((((((“Kidney Function Tests”[Mesh:NoExp]) OR “Glomerular Filtration Rate”[Mesh:NoExp]) OR (((kidney*[Title/Abstract] OR renal[Title/Abstract])) AND (function*[Title/Abstract] OR dysfunction*[Title/Abstract]))) OR glomerul* filtration rate*[Title/Abstract]) OR glomerulofiltration rate*[Title/Abstract]) OR GFR[Title/Abstract]) OR eGFR[Title/Abstract])))) OR ((StatSensor[Title/Abstract] OR Stat-Sensor))) OR ABL90 FLEX PLUS[Title/Abstract]) OR ((ABL800 FLEX[Title/Abstract] OR ABL800FLEX[Title/Abstract] OR ABL 800 FLEX[Title/Abstract]))) OR Dri-chem NX500[Title/Abstract]) OR epoc Blood Analysis[Title/Abstract]) OR Piccolo Xpress[Title/Abstract]))) NOT ((animals[mh] NOT humans[mh])))) AND ((pubstatusaheadofprint OR publisher[sb] OR pubmednotmedline[sb])).
The above search strategy incorporates the following search line to limit to studies found in PubMed but not available in Ovid MEDLINE:
(pubstatusaheadofprint OR publisher[sb] OR pubmednotmedline[sb])
See Duffy et al. 125
Key
[Mesh] = exploded indexing term (MeSH heading).
[Mesh:noexp] = indexing term (MeSH heading) not exploded.
* = truncation.
[Title/Abstract]) = terms in either title or abstract fields.
Science Citation Index
URL: via Web of Science [Clarivate Analytics (formerly Thomson Reuters), Philadelphia, PA, USA] – https://clarivate.com/.
Date range searched: 1900 to 5 November 2018.
Date searched: 6 November 2018.
Records retrieved: 1011.
Search strategy
# 34 #32 not #33 (1011)
Indexes=SCI-EXPANDED Timespan=1900-2018
-
# 33 TI=(animal or animals or rat or rats or mouse or mice or rodent or rodents or porcine or murine or sheep or lamb or lambs or ewe or ewes or pig or pigs or piglet or piglets or sow or sows or minipig or minipigs or rabbit or rabbits or cat or cats or kitten or kittens or dog or dogs or puppy or puppies or monkey or monkeys or horse or horses or foal or foals or equine or calf or calves or cattle or heifer or heifers or hamster or hamsters or chicken or chickens or livestock or alpaca* or llama*) (2,864,727)
-
# 32 #31 OR #30 OR #28 OR #20 OR # (161,053)
-
# 31 TS=(StatSensor or Stat-Sensor or ABL90 FLEX PLUS or ABL800 FLEX or ABL800FLEX or ABL 800 FLEX or Dri-chem NX500 or epoc Blood Analysis or Piccolo Xpress) (75)
-
# 30 #29 AND #27 (26)
-
# 29 TS=(i-STAT or iSTAT) (455)
-
# 28 #27 AND #26 (56)
-
# 27 #19 OR #15 (255,088)
-
# 26 #25 OR #24 OR #23 OR #22 OR #21 (10,534)
-
# 25 TS=(near-patient* NEAR/2 (device* or analyser* or analyzer*)) (38)
-
# 24 TS=((portab* or transportab*) NEAR/2 (device* or analyser* or analyzer*)) (7004)
-
# 23 TS=((table-top or tabletop or bench-top or benchtop) NEAR/2 (device* or analyser* or analyzer*)) (281)
-
# 22 TS=((desktop or desk-top) NEAR/2 (device* or analyser* or analyzer*)) (201)
-
# 21 TS=((handheld or hand-held) NEAR/2 (device* or analyser* or analyzer*)) (3280)
-
# 20 #19 AND #14 (562)
-
# 19 #18 OR #17 (190,586)
-
# 18 TS=((glomerul* NEAR/1 filtration NEAR/1 rate*) OR (glomerulofiltration NEAR/1 rate*) OR GFR OR eGFR) (93,612)
-
# 17 TS=((kidney* or renal) NEAR/3 (function* or dysfunction*)) (118,800)
-
# 16 #15 AND #14 (550)
-
# 15 TS=(creatinin* or serumcreatinin* or SCr) (99,211)
-
# 14 #13 OR #12 OR #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 (137,790)
-
# 13 TS=(“near” NEAR/4 patient* NEAR/4 screen*) (22)
-
# 12 TS=(“near” NEAR/4 patient* NEAR/4 measur*) (110)
-
# 11 TS=(“near” NEAR/4 patient* NEAR/4 identif*) (53)
-
# 10 TS=(“near” NEAR/4 patient* NEAR/4 analyz*) (20)
-
# 9 TS=(“near” NEAR/4 patient* NEAR/4 analys*) (65)
-
# 8 TS=(“near” NEAR/4 patient* NEAR/4 assess*) (67)
-
# 7 TS=(“near” NEAR/4 patient* NEAR/4 determin*) (32)
-
# 6 TS=(“near” NEAR/4 patient* NEAR/4 test*) (500)
-
# 5 TS=((“on-site” or “onsite”) NEAR/3 (test* or determin* or assess* or analys* or analyz* or identif* or measur* or screen*)) (5961)
-
# 4 TS=((bedside* or bed-side*) NEAR/3 (test* or determin* or assess* or analys* or analyz* or identif* or measur* or screen*)) (3668)
-
# 3 TS=(rapid* NEAR/3 (test* or determin* or assess* or analys* or analyz* or identif* or measur* or screen*)) (109,855)
-
# 2 TS=(POC or POCT) (7275)
-
# 1 TS=(point-of-care) (16,121)
Key
TS = topic tag; searches terms in title, abstract, author keywords and keywords plus fields.
TI = search in title field.
* = truncation.
“ ” = phrase search.
NEAR/3 = terms within three words of each other (any order).
Ongoing, unpublished or grey literature search strategies
ClinicalTrials.gov
URL: https://clinicaltrials.gov/.
Date searched: 8 November 2018.
Records retrieved: 103.
Search strategy
Twenty-six studies found for:
(creatinine OR serumcreatinine OR SCr) AND (point-of-care OR near patient)
Twenty-six studies found for:
(kidney function OR renal function OR kidney dysfunction OR renal dysfunction) AND (point-of-care OR near patient)
Eight studies found for:
(glomerular filtration rate OR GFR OR eGFR) AND (point-of-care OR near patient)
Forty-three studies found for:
istat OR i-stat OR StatSensor OR Stat-Sensor OR ABL90 FLEX PLUS OR ABL800 FLEX OR ABL800FLEX OR ABL 800 FLEX OR Dri-chem NX500 OR epoc Blood Analysis OR Piccolo Xpress
Conference Proceedings Citation Index: Science
URL: via Web of Science, Clarivate Analytics – https://clarivate.com/.
Date range searched: 1990 to 5 November 2018.
Date searched: 6 November 2018.
Records retrieved: 78.
Search strategy
# 34 #32 not #33 (78)
Indexes=CPCI-S Timespan=1900-2018
-
# 33 TI=(animal or animals or rat or rats or mouse or mice or rodent or rodents or porcine or murine or sheep or lamb or lambs or ewe or ewes or pig or pigs or piglet or piglets or sow or sows or minipig or minipigs or rabbit or rabbits or cat or cats or kitten or kittens or dog or dogs or puppy or puppies or monkey or monkeys or horse or horses or foal or foals or equine or calf or calves or cattle or heifer or heifers or hamster or hamsters or chicken or chickens or livestock or alpaca* or llama*) (258,819)
-
# 32 #31 OR #30 OR #28 OR #20 OR #16 (80)
-
# 31 TS = (StatSensor or Stat-Sensor or ABL90 FLEX PLUS or ABL800 FLEX or ABL800FLEX or ABL 800 FLEX or Dri-chem NX500 or epoc Blood Analysis or Piccolo Xpress) (6)
-
# 30 #29 AND #27 (3)
-
# 29 TS=(i-STAT or iSTAT) (73)
-
# 28 #27 AND #26 (4)
-
# 27 #19 OR #15 (28,719)
-
# 26 #25 OR #24 OR #23 OR #22 OR #21 (8738)
-
# 25 TS=(near-patient* NEAR/2 (device* or analyser* or analyzer*)) (3)
-
# 24 TS=((portab* or transportab*) NEAR/2 (device* or analyser* or analyzer*)) (5017)
-
# 23 TS=((table-top or tabletop or bench-top or benchtop) NEAR/2 (device* or analyser* or analyzer*)) (114)
-
# 22 TS=((desktop or desk-top) NEAR/2 (device* or analyser* or analyzer*)) (308)
-
# 21 TS=((handheld or hand-held) NEAR/2 (device* or analyser* or analyzer*)) (3501)
-
# 20 #19 AND #14 (32)
-
# 19 #18 OR #17 (21,751)
-
# 18 TS=((glomerul* NEAR/1 filtration NEAR/1 rate*) OR (glomerulofiltration NEAR/1 rate*) OR GFR OR eGFR) (9710)
-
# 17 TS=((kidney* or renal) NEAR/3 (function* or dysfunction*)) (13,364)
-
# 16 #15 AND #14 (53)
-
# 15 TS=(creatinin* or serumcreatinin* or SCr) (9631)
-
# 14 #13 OR #12 OR #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 (20,101)
-
# 13 TS=(“near” NEAR/4 patient* NEAR/4 screen*) (5)
-
# 12 TS=(“near” NEAR/4 patient* NEAR/4 measur*) (16)
-
# 11 TS=(“near” NEAR/4 patient* NEAR/4 identif*) (8)
-
# 10 TS=(“near” NEAR/4 patient* NEAR/4 analyz*) (5)
-
# 9 TS=(“near” NEAR/4 patient* NEAR/4 analys*) (6)
-
# 8 TS=(“near” NEAR/4 patient* NEAR/4 assess*) (8)
-
# 7 TS=(“near” NEAR/4 patient* NEAR/4 determin*) (3)
-
# 6 TS=(“near” NEAR/4 patient* NEAR/4 test*) (42)
-
# 5 TS=((“on-site” or “onsite”) NEAR/3 (test* or determin* or assess* or analys* or analyz* or identif* or measur* or screen*)) (2391)
-
# 4 TS=((bedside* or bed-side*) NEAR/3 (test* or determin* or assess* or analys* or analyz* or identif* or measur* or screen*)) (356)
-
# 3 TS=(rapid* NEAR/3 (test* or determin* or assess* or analys* or analyz* or identif* or measur* or screen*)) (13,933)
-
# 2 TS=(POC or POCT) (1280)
-
# 1 TS=(point-of-care) (2689)
Key
TS = topic tag; searches terms in title, abstract, author keywords and keywords plus fields.
TI = search in title field.
* = truncation.
“ ” = phrase search.
NEAR/3 = terms within three words of each other (any order).
EU Clinical Trials Register
URL: www.clinicaltrialsregister.eu/ctr-search/search.
Date searched: 7 November 2018.
Records retrieved: 15.
Search strategy
1. Four results found for:
(creatinine OR serumcreatinine) AND (“point of care” OR point-of-care OR “near patient”)
2. Two results found for:
(“kidney function” OR “renal function” OR “kidney dysfunction” OR “renal dysfunction”) AND (“point of care” OR point-of-care OR “near patient”)
3. Three results found for:
(“glomerular filtration rate” OR “glomerulofiltration rate” OR GFR OR eGFR) AND (“point of care” OR point-of-care OR “near patient”)
4. Six results found for:
istat OR i-stat OR “i stat” OR StatSensor OR Stat-Sensor OR “Stat Sensor” OR “ABL90 FLEX PLUS”
5. No results found for:
“ABL800 FLEX” OR ABL800FLEX OR “ABL 800 FLEX” OR “Dri-chem NX500”
6. No results found for:
“epoc Blood Analysis” OR “Piccolo Xpress”.
Open Access Theses and Dissertations
URL: https://oatd.org/.
Date searched: 8 November 2018.
Records retrieved: 36.
Search strategy
-
(creatinine OR serumcreatinine OR SCr) AND (“point of care”) (15)
-
(creatinine OR serumcreatinine OR SCr) AND (“near patient”) (0)
-
(“kidney function” OR “renal function” OR “kidney dysfunction” OR “renal dysfunction”) AND (“point of care” OR “near patient”) (11)
-
(“glomerular filtration rate” OR GFR OR eGFR) AND (“point of care” OR “near patient”) (2)
-
(istat OR “i-stat”) AND (creatinine OR serumcreatinine OR SCr OR “glomerular filtration rate” OR GFR OR eGFR) (2)
-
StatSensor OR “Stat-Sensor” OR “ABL90 Flex Plus” OR “ABL800 FLEX” OR “ABL800FLEX” OR “ABL 800 FLEX” OR “Dri-chem NX500” OR “epoc Blood analysis” OR “Piccolo Xpress”(6)
ProQuest Dissertations & Theses Global A&I
URL: via ProQuest – www.proquest.com/.
Date searched: 6 November 2018.
Records retrieved: 68.
Search strategy
1. (TI,AB,IF(point-of-care) OR TI,AB,IF(POC OR POCT)) AND (TI,AB,IF(creatinin* OR serumcreatinin* OR SCr) OR TI,AB,IF((kidney* OR renal) NEAR/3 (function* OR dysfunction*)) OR TI,AB,IF(glomerul* filtration rate*) OR TI,AB,IF(glomerulofiltration rate*) OR TI,AB,IF(GFR OR eGFR))
Fifteen results.
2. (TI,AB,IF(rapid* NEAR/3 (test* OR determin* OR assess* OR analys* OR analyz* OR identif* OR measur* OR screen*)) OR TI,AB,IF((bedside* OR bed-side*) NEAR/3 (test* OR determin* OR assess* OR analys* OR analyz* OR identif* OR measur* OR screen*))) AND (TI,AB,IF(creatinin* OR serumcreatinin* OR SCr) OR TI,AB,IF((kidney* OR renal) NEAR/3 (function* OR dysfunction*)) OR TI,AB,IF(glomerul* filtration rate*) OR TI,AB,IF(glomerulofiltration rate*) OR TI,AB,IF(GFR OR eGFR))
Twenty-nine results.
3. TI,AB,IF((on-site OR onsite) NEAR/3 (test* OR determin* OR assess* OR analys* OR analyz* OR identif* OR measur* OR screen*)) AND (TI,AB,IF(creatinin* OR serumcreatinin* OR SCr) OR TI,AB,IF((kidney* OR renal) NEAR/3 (function* OR dysfunction*)) OR TI,AB,IF(glomerul* filtration rate*) OR TI,AB,IF(glomerulofiltration rate*) OR TI,AB,IF(GFR OR eGFR))
Three results.
4. (TI,AB,IF(“near” NEAR/4 patient* NEAR/4 test*) OR TI,AB,IF(“near” NEAR/4 patient* NEAR/4 determin*) OR TI,AB,IF(“near” NEAR/4 patient* NEAR/4 assess*) OR TI,AB,IF(“near” NEAR/4 patient* NEAR/4 analys*) OR TI,AB,IF(“near” NEAR/4 patient* NEAR/4 analyz*) OR TI,AB,IF(“near” NEAR/4 patient* NEAR/4 identif*) OR TI,AB,IF(“near” NEAR/4 patient* NEAR/4 measur*) OR TI,AB,IF(“near” NEAR/4 patient* NEAR/4 screen*)) AND (TI,AB,IF(creatinin* OR serumcreatinin* OR SCr) OR TI,AB,IF((kidney* OR renal) NEAR/3 (function* OR dysfunction*)) OR TI,AB,IF(glomerul* filtration rate*) OR TI,AB,IF(glomerulofiltration rate*) OR TI,AB,IF(GFR OR eGFR))
Three results.
5. ((TI,AB,IF(creatinin* OR serumcreatinin* OR SCr) OR TI,AB,IF((kidney* OR renal) NEAR/3 (function* OR dysfunction*)) OR TI,AB,IF(glomerul* filtration rate*) OR TI,AB,IF(glomerulofiltration rate*) OR TI,AB,IF(GFR OR eGFR)) AND (TI,AB,IF((handheld OR hand-held) NEAR/2 (device* OR analyser* OR analyzer*)) OR TI,AB,IF((desktop OR desk-top) NEAR/2 (device* OR analyser* OR analyzer*)) OR TI,AB,IF((table-top OR tabletop OR bench-top OR benchtop) NEAR/2 (device* OR analyser* OR analyzer*)) OR TI,AB,IF((portab* OR transportab*) NEAR/2 (device* OR analyser* OR analyzer*)) OR TI,AB,IF((“near patient” OR “near patients”) NEAR/2 (device* OR analyser* OR analyzer*)))) OR TI,AB,IF(i-STAT OR iSTAT OR StatSensor OR Stat-Sensor OR ABL90 FLEX PLUS OR ABL800 FLEX OR ABL800FLEX OR ABL 800 FLEX OR Dri-chem NX500 OR epoc Blood Analysis OR Piccolo Xpress)
Eighteen results.
PROSPERO
URL: www.crd.york.ac.uk/PROSPERO/.
Searched on: 6 November 2018.
Records retrieved: 13.
Search strategy
-
#1 MeSH DESCRIPTOR Point-of-Care Systems (41)
-
#2 MeSH DESCRIPTOR Point-of-Care Testing (14)
-
#3 point-of-care (171)
-
#4 POC or POCT (51)
-
#5 rapid* adj3 (test* or determin* or assess* or analys* or analyz* or identif* or measur* or screen*) (210)
-
#6 (test* or determin* or assess* or analys* or analyz* or identif* or measur* or screen*) adj3 rapid* (88)
-
#7 ((bedside* or bed-side*) adj3 (test* or determin* or assess* or analys* or analyz* or identif* or measur* or screen*)) (32)
-
#8 (test* or determin* or assess* or analys* or analyz* or identif* or measur* or screen*) adj3 ((bedside* or bed-side*)) (15)
-
#9 ((on-site or onsite) adj3 (test* or determin* or assess* or analys* or analyz* or identif* or measur* or screen*)) (8)
-
#10 “near” adj4 patient* adj4 test* (5)
-
#11 “near” adj4 patient* adj4 determin* (0)
-
#12 “near” adj4 patient* adj4 assess* (0)
-
#13 “near” adj4 patient* adj4 analys* (0)
-
#14 “near” adj4 patient* adj4 analyz* (0)
-
#15 “near” adj4 patient* adj4 identif* (0)
-
#16 “near” adj4 patient* adj4 measur* (0)
-
#17 “near” adj4 patient* adj4 screen* (0)
-
#18 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 (432)
-
#19 MeSH DESCRIPTOR Creatinine (12)
-
#20 creatinin* (452)
-
#21 serumcreatinin* (0)
-
#22 SCr (54)
-
#23 #19 OR #20 OR #21 OR #22 (480)
-
#24 #18 AND #23 (5)
-
#25 MeSH DESCRIPTOR Kidney Function Tests (4)
-
#26 MeSH DESCRIPTOR Glomerular Filtration Rate (10)
-
#27 ((kidney* or renal) adj3 (function* or dysfunction*)) (536)
-
#28 glomerul* filtration rate* (192)
-
#29 glomerulofiltration rate* (0)
-
#30 GFR (167)
-
#31 eGFR (246)
-
#32 #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 (786)
-
#33 #32 AND #18 (7)
-
#34 #24 OR #33 (12)
-
#35 MeSH DESCRIPTOR Computers, Handheld (8)
-
#36 ((handheld or hand held) NEAR2 (device* or analyser* or analyzer*)) (40)
-
#37 ((device* or analyser* or analyzer*) NEAR2 (handheld or hand held)) (3)
-
#38 ((handheld or hand held) adj2 (device* or analyser* or analyzer*)) (40)
-
#39 ((device* or analyser* or analyzer*) adj2 (handheld or hand held)) (3)
-
#40 ((desktop or desk top) adj2 (device* or analyser* or analyzer*)) (0)
-
#41 ((device* or analyser* or analyzer*) adj2 (desktop or desk top)) (2)
-
#42 ((table top or tabletop or bench top or benchtop) adj2 (device* or analyser* or analyzer*)) (1)
-
#43 ((device* or analyser* or analyzer*) adj2 (table top or tabletop or bench top or benchtop)) (0)
-
#44 ((portab* or transportab*) adj2 (device* or analyser* or analyzer*)) (40)
-
#45 ((device* or analyser* or analyzer*) adj2 (portab* or transportab*)) (3)
-
#46 ((device* or analyser* or analyzer*) adj2 (“near” patient*)) (0)
-
#47 ((“near” patient*) adj2 (device* or analyser* or analyzer*)) (0)
-
#48 #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 (82)
-
#49 ((function* or dysfunction*) adj3 (kidney* or renal)) (107)
-
#50 #32 OR #49 (808)
-
#51 #18 AND #50 (7)
-
#52 #50 OR #23 (1002)
-
#53 #52 AND #48 (1)
-
#54 #24 OR #51 OR #53 (13)
-
#55 i-STAT or iSTAT (1)
-
#56 StatSensor or Stat Sensor (0)
-
#57 ABL90 FLEX PLUS (0)
-
#58 ABL800 FLEX or ABL800FLEX or ABL 800 FLEX (0)
-
#59 Dri-chem NX500 (0)
-
#60 epoc Blood Analysis (0)
-
#61 Piccolo Xpress (0)
-
#62 #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 (13)
Key
MeSH DESCRIPTOR = indexing term (MeSH heading).
* = truncation.
adj3 = terms within three words of each other (order specified).
World Health Organization’s International Clinical Trials Registry Platform
URL: www.who.int/ictrp/search/en/.
Date searched: 8 November 2018.
Records retrieved: 28.
Search strategy
1. Six records for six trials found for:
creatinine AND point of care
2. No results were found for:
creatinine AND near patient
3. No results were found for:
serumcreatinine AND point of care
4. No results were found for:
serumcreatinine AND near patient
5. No results were found for:
SCr AND point of care
6. No results were found for:
SCr AND near patient
7. Two records for two trials found for:
kidney function AND point of care
8. One trial found for:
kidney function AND near patient
9. Two records for two trials found for:
renal function AND point of care
10. One trial found for:
renal function AND near patient
11. No results were found for:
kidney dysfunction AND point of care
12. No results were found for:
kidney dysfunction AND near patient
13. No results were found for:
glomerular filtration rate AND point of care
14. No results were found for:
glomerular filtration rate AND near patient
15. No results were found for:
glomerulofiltration rate AND point of care
16. No results were found for:
glomerulofiltration rate AND near patient
17. No results were found for:
GFR AND point of care
18. No results were found for:
GFR AND near patient
19. No results were found for:
eGFR AND point of care
20. No results were found for:
eGFR AND near patient
21. Seventeen records for 16 trials found for:
istat OR i-stat OR StatSensor OR Stat-Sensor OR ABL90 FLEX PLUS OR ABL800 FLEX OR ABL800FLEX OR ABL 800 FLEX OR Dri-chem NX500 OR epoc Blood Analysis OR Piccolo Xpress
Search for review evidence on the risk of acute kidney injury from contrast agents following computed tomography scans
Ovid MEDLINE® ALL
URL: https://ovidsp.ovid.com.
Date range searched: 1946 to 27 November 2018.
Date searched: 28 November 2018.
Records retrieved: 291.
Search strategy
-
exp Acute Kidney Injury/ (42,013)
-
(acute adj2 (renal or kidney) adj2 (fail$ or injur$ or insufficien$)).ti,ab. (41,624)
-
((acute or renal or kidney) adj2 tubular necrosis).ti,ab. (3611)
-
or/1-3 (60,170)
-
tomography, x-ray computed/ or colonography, computed tomographic/ or four-dimensional computed tomography/ or positron emission tomography computed tomography/ or single photon emission computed tomography computed tomography/or tomography, spiral computed/ or multidetector computed tomography/ (374,663)
-
((compute$ adj2 tomograph$) or tomodensitometry or cine-CT).ti,ab. (268,668)
-
((CT or CAT) adj2 (scan$ or imag$)).ti,ab. (118,123)
-
((CT or CAT) adj2 contrast).ti,ab. (8124)
-
(cross-sectional adj2 (scan$ or imag$)).ti,ab. (6368)
-
((emission or positron or proton) adj2 tomograph$).ti,ab. (68,380)
-
(PET or PET-CT$ or PET?CT$ or CT-PET$ or CT?PET$).ti,ab. (85,352)
-
(SPECT or SPECT-CT$ or SPECT?CT$ or CT-SPECT$ or CT?SPECT$).ti,ab. (26,355)
-
(SPET or SPET-CT$ or SPET?CT$ or CT-SPET$ or CT?SPET$).ti,ab. (1327)
-
or/5-13 (624,723)
-
exp Administration, Intravenous/ (137,931)
-
(intravenous or IV).ti,ab. (609,985)
-
15 or 16 (670,695)
-
4 and 14 and 17 (223)
-
(contrast induced adj (AKI or acute kidney injury or nephropathy or tubular necrosis)).ti,ab. (2295)
-
(radiocontrast induced adj (AKI or acute kidney injury or nephropathy or tubular necrosis)).ti,ab. (115)
-
((postcontrast or post-contrast) adj (AKI or acute kidney injury or nephropathy or tubular necrosis)).ti,ab. (22)
-
((contrast or radiocontrast) adj nephropathy).ti,ab. (376)
-
(CI-AKI or CIAKI or PC-AKI).ti,ab. (403)
-
or/19–23 (2730)
-
25 14 and 24 (326)
-
18 or 25 (488)
-
exp animals/ not humans/ (4,519,266)
-
not 27 (480)
-
limit 28 to yr=“2012 -Current” (291)
Appendix 2 Data extraction
| Laboratory reference result – eGFR (ml/minute/1.73 m2) | POC device result – eGFR (ml/minute/1.73 m2) | |||
|---|---|---|---|---|
| 0–29 | 30–44 | 45–59 | ≥ 60 | |
| 0–29 | ||||
| 30–44 | ||||
| 45–59 | ||||
| ≥ 60 | ||||
| eGFR (ml/minute/1.73 m2) | Study (author and year of publication) | eGFR (ml/minute/1.73 m2) | Study (author and year of publication) | eGFR (ml/minute/1.73 m2) | Study (author and year of publication) | eGFR (ml/minute/1.73 m2) | Study (author and year of publication) | eGFR (ml/minute/1.73 m2) | Study (author and year of publication) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lab | POC | Snaith et al., 201837 | Krige, 201732 | Lab | POC | Houben et al., 201729 | Lab | POC | Inoue et al., 201730 | Lab | POC | Shephard et al., 201036 | Lab | POC | Korpi-Steiner et al., 200931 | Dorward et al., 201828 |
| < 30 | < 30 | 8 | 1 | < 30 | < 30 | 0 | < 30 | < 30 | 4 | < 30 | < 30 | 26 | < 60 | < 60 | 11 | 1 |
| 30–44 | 4 | 0 | 30–44 | 0 | 30–44 | 0 | 30–59 | 6 | ≥ 60 | 57 | 0 | |||||
| 45–59 | 0 | 0 | 45–59 | 0 | ≥ 45 | 0 | > 60 | 1 | ||||||||
| ≥ 60 | 0 | 0 | ≥ 60 | 0 | ||||||||||||
| Number of tests | 12 | 1 | Number of tests | 0 | Number of tests | 4 | Number of tests | 33 | Number of tests | 68 | 1 | |||||
| 30–44 | < 30 | 3 | 0 | 30–44 | < 30 | 0 | 30–44 | < 30 | 1 | 30–59 | < 30 | 0 | ≥ 60 | < 60 | 0 | NA |
| 30–44 | 17 | 0 | 30–44 | 0 | 30–44 | 7 | 30–59 | 14 | ≥ 60 | 198 | NA | |||||
| 45–59 | 8 | 0 | 45–59 | 1 | ≥ 45 | 0 | > 60 | 6 | ||||||||
| ≥ 60 | 0 | 0 | ≥ 60 | 2 | ||||||||||||
| Number of tests | 28 | 0 | Number of tests | 3 | Number of tests | 8 | Number of tests | 20 | Number of tests | 198 | 186 | |||||
| 45–59 | < 30 | 0 | 0 | ≥ 45 | < 30 | 0 | ≥ 45 | < 30 | 1 | ≥ 60 | < 30 | 0 | ||||
| 30–44 | 10 | 0 | 30–44 | 0 | 30–44 | 11 | 30–59 | 0 | ||||||||
| 45–59 | 17 | 1 | ≥ 45 | 348 | ≥ 45 | 99 | > 60 | 47 | ||||||||
| ≥ 60 | 8 | 1 | ||||||||||||||
| Number of tests | 35 | 2 | Number of tests | 348 | Number of tests | 111 | Number of tests | 47 | ||||||||
| ≥ 60 | < 30 | 0 | 0 | |||||||||||||
| 30–44 | 1 | 0 | ||||||||||||||
| 45–59 | 33 | 0 | ||||||||||||||
| ≥ 60 | 191 | 100 | ||||||||||||||
| Number of tests | 225 | 100 | ||||||||||||||
| eGFR (ml/minute/1.73 m2) | Study (author and year of publication) | eGFR (ml/minute/1.73 m2) | Study (author and year of publication) | eGFR (ml/minute/1.73 m2) | Study (author and year of publication) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lab | POC | Snaith et al., 201837 | Snaith et al., 201938 | Lab | POC | aBotz et al., 201327 | Lab | POC | Korpi-Steiner et al., 200931 | Nichols et al., 200733 |
| < 30 | < 30 | 12 | 0 | < 30 | < 30 | 12 | < 60 | < 60 | 66 | 9 |
| 30–44 | 0 | 0 | ≥ 30 | 2 | ≥ 60 | 2 | 0 | |||
| 45–59 | 0 | 0 | ||||||||
| ≥ 60 | 0 | 0 | ||||||||
| Number of tests | 12 | 0 | Number of tests | 14 | Number of tests | 68 | 9 | |||
| 30–44 | < 30 | 3 | 1 | ≥ 30 | < 30 | NA | ≥ 60 | < 60 | 32 | 6 |
| 30–44 | 25 | 9 | ≥ 30 | NA | ≥ 60 | 166 | 34 | |||
| 45–59 | 0 | 4 | ||||||||
| ≥ 60 | 0 | 0 | ||||||||
| Number of tests | 28 | 14 | Number of tests | 2028 | Number of tests | 198 | 40 | |||
| 45–59 | < 30 | 0 | 0 | |||||||
| 30–44 | 5 | 2 | ||||||||
| 45–59 | 29 | 35 | ||||||||
| ≥ 60 | 1 | 7 | ||||||||
| Number of tests | 35 | 44 | ||||||||
| ≥ 60 | < 30 | 0 | 0 | |||||||
| 30–44 | 1 | 1 | ||||||||
| 45–59 | 14 | 7 | ||||||||
| ≥ 60 | 210 | 234 | ||||||||
| Number of tests | 225 | 242 | ||||||||
| eGFR (ml/minute/1.73 m2) | Study (author and year of publication) | eGFR (ml/minute/1.73 m2) | Study (author and year of publication) | eGFR (ml/minute/1.73 m2) | Study (author and year of publication) | |||
|---|---|---|---|---|---|---|---|---|
| Lab | POC | Snaith et al., 201837 | Lab | POC | Botz et al., 201327 | Lab | POC | Korpi-Steiner et al., 200931 |
| < 30 | < 30 | 12 | < 30 | < 30 | 26 | < 60 | < 60 | 55 |
| 30–44 | 0 | ≥ 30 | 3 | ≥ 60 | 13 | |||
| 45–59 | 0 | |||||||
| ≥ 60 | 0 | |||||||
| Number of tests | 12 | Number of tests | 29 | Number of tests | 68 | |||
| 30–44 | < 30 | 0 | 30–59 | < 30 | NA | ≥ 60 | < 60 | 6 |
| 30–44 | 24 | ≥ 30 | NA | ≥ 60 | 192 | |||
| 45–59 | 4 | |||||||
| ≥ 60 | 0 | |||||||
| Number of tests | 28 | Number of tests | 674 | Number of tests | 198 | |||
| 45–59 | < 30 | 0 | ≥ 60 | 0–60 | 24 | |||
| 30–44 | 2 | ≥ 60 | 2517 | |||||
| 45–59 | 31 | |||||||
| ≥ 60 | 2 | |||||||
| Number of tests | 35 | Number of tests | 2541 | |||||
| ≥ 60 | < 30 | 0 | ||||||
| 30–44 | 0 | |||||||
| 45–59 | 1 | |||||||
| ≥ 60 | 224 | |||||||
| Number of tests | 225 | |||||||
| eGFR (ml/minute/1.73 m2) | Study (author and year of publication) | eGFR (ml/minute/1.73 m2) | Study (author and year of publication) | ||
|---|---|---|---|---|---|
| True | POC | Shephard et al., 201036 (StatSensor – adjusted) | True | POC | Korpi-Steiner et al., 200931 (StatSensor – with offset) |
| < 30 | < 30 | 32 | < 60 | < 60 | 40 |
| 30–59 | 1 | ≥ 60 | 28 | ||
| ≥ 60 | 0 | ||||
| Number of tests | 33 | Number of tests | 68 | ||
| 30–59 | < 30 | 1 | ≥ 60 | < 60 | 24 |
| 30–59 | 17 | ≥ 60 | 174 | ||
| ≥ 60 | 2 | ||||
| Number of tests | 20 | Number of tests | 198 | ||
| ≥ 60 | < 30 | 0 | |||
| 30–59 | 10 | ||||
| ≥ 60 | 37 | ||||
| Number of tests | 47 | ||||
| Study (author and year of publication) | Characteristics | Results | |||||
|---|---|---|---|---|---|---|---|
| Design | Selection criteria | Population characteristicsa | Contrast volume | Intervention | PC-AKI definition | ||
| Nijssen et al., 2018106 |
Retrospective cohort Uncontrolled comparison with patients with an eGFR 30–59 ml/minute/1.73 m2 from the AMACING trial |
eGFR < 30 ml/minute/1.73 m2 referred for an elective procedure with intravascular iodinated contrast material administration and excluded from the AMACING trial Exclusion criteria: |
|
81 ml (45 ml) | Increase in SCr levels by > 25% or 44 µmol/l within 2–6 days post contrast |
PC-AKI
|
|
Appendix 3 Modelling collapsed category data
Studies reporting on only collapsed categories were assumed to provide information on a function of the probabilities of interest. This function varied depending on which categories were collapsed, with relationships determined using conditional partitioning of probabilities:
in which C is the true reported category, which is collapsed over (i.e. contains) categories A1 and A2 from Table 2, and B is the category estimated by the POC device. Note also that as A1 and A2 are contained in C, Equation 6 can be simplified to:
For each value of B, A1 and A2, Pr(B | A1) and Pr(B | A2) can be expressed in terms of the probabilities of interest, Pjk.
In addition, it was also necessary to calculate Pr(A1 | C) and Pr(A2 | C), which are the conditional probabilities of an individual belonging to true eGFR categories A1 and A2, given that they belong to the joint category C. The probability that an individual included in a study in the synthesis has true eGFR in category j, Tj, was estimated separately and used to calculate the conditional true probabilities as:
The number of individuals classified by a POC device as belonging to the collapsed eGFR category, given their true collapsed eGFR category (determined by the laboratory test), was also assumed to follow a multinomial distribution, with dimensions depending on the number of categories reported and the probabilities written in terms of Pjk, using Equation 7. For an example, see Appendix 3, Example likelihood and probability calculations for collapsed data. Equations 7 and 8 can also be extended to collapsing over more than two consecutive categories, when necessary.
Model for the probability that an individual has a true estimated glomerular filtration rate in each category
All included studies were used to estimate the probability that an individual has true eGFR (as measured by the laboratory) in each of the four categories of interest (see Table 2).
Let yij be the number of individuals in study i with true eGFR in category j, which is assumed to follow a multinomial distribution:
with Ni defining the total number of individuals in study i, and Tj the probabilities that an individual has true eGFR in category j.
The model was estimated in a Bayesian framework using Markov chain Monte Carlo in OpenBUGS (version 3.2.3),21,22 in which the probabilities were given a non-informative Dirichlet prior distribution with equal probabilities in each category:
Studies reporting on collapsed categories contributed to the corresponding sum of probabilities Tj. The number of individuals in the collapsed categories were assumed to follow a multinomial distribution with an appropriate number of dimensions and probabilities written as functions of Tj.
Example likelihood and probability calculations for collapsed data
Shephard et al. 36 reported the number of patients classified as having eGFRs of < 30, ≥ 30 and 30–59 ml/minute/1.73 m2 by the laboratory and StatSensor POC device (see Table 39).
The number of individuals classified by the POC device as belonging to each eGFR category, given true eGFR category j=1, 2, 3, rj1*, rj2*, rj3*, were assumed to follow a multinomial distribution:
with nj defining the number of individuals with true eGFR in category j in this study. The probabilities pjk* were written as a function of the probabilities of interest pjk, according to Equation 7 by writing:
Thus, letting B = POC eGFR < 30 ml/minute/1.73 m2, it can be written as:
Letting B = POC eGFR < 30–59 ml/minute/1.73 m2 and noting that:
It can be written:
and letting B = POC eGFR ≥ 60 ml/minute/1.73 m2, it can be written as:
thus linking all the probabilities with data available with the probabilities of interest.
Appendix 4 OpenBUGS code for analyses
StatSensor main analysis (includes calculation of probability of true estimated glomerular filtration rate in each category)
OpenBUGS data
i-STAT main analysis (includes calculation of probability of true estimated glomerular filtration rate in each category)
OpenBUGS data
ABL main analysis (includes calculation of probability of true estimated glomerular filtration rate in each category)
OpenBUGS data
Appendix 5 Quality assessment details
QUADAS-2: risk of bias – patient selection
Selection question 1: was a consecutive or random sample of patients enrolled?
Selection question 2: did the study avoid inappropriate exclusions?
Risk of bias: could the selection of patients have introduced bias?
Answers to the above questions for the patient selection domain are presented in Table 44.
| Study (author and year of publication) | Description | Selection question | Risk of bias | Notes | |
|---|---|---|---|---|---|
| 1 | 2 | ||||
| aBotz et al., 201327 | 2042 patients at risk of renal disease prior to radiological examinations; 43% female; USAWe retrospectively obtained all i-STAT1 and Radiometer Ltd 827 whole blood creatinine results performed on the same day of service as a serum creatinine for the period January 1-December 31, 2011 | UC | UC | UC |
Retrospective selection of patients with both POC and refence standard It is not clear how the patients were classified as at risk of renal disease Conference abstract |
| Dorward et al., 201828 |
187 HIV-positive patients who recently initiated first-line ART, median age 31 years (IQR 27–38 years); 62% female; South Africa Prospectively recruited trial arm population |
Yes | No | Low |
Excluded one patient with an eGFR < 30 ml/minute/1.73 m2, who was clinically unstable Unlikely to introduce significant bias |
| Houben et al., 201729 |
351 women due for contrast-enhanced spectral mammography; the Netherlands Women eligible for contrast-enhanced spectral mammography between December 2014 and June 2016 The women ‘were asked to voluntarily participate in this observational study’ |
UC | Yes | Low |
Not explicitly stated if consecutively recruited, but appears likely No inappropriate exclusions |
| Inoue et al., 201730 |
233 consecutive outpatients scheduled for contrast-enhanced CT studies Of the 233 patients, 123 patient samples were evaluated prior to adjustment and the other 110 following adjustment |
Yes | Yes | low |
Consecutive No inappropriate exclusions |
| Korpi-Steiner et al., 200931 | Sample selection was not consecutive because staff were available only during selected hours to perform creatinine testing. Institutional protocol requires creatinine/eGFR measurement for patients older than 70 years, patients with a history of diabetes mellitus, and patients with a history of renal disease or renal transplantation | No | UC | Low |
Reasons provided for non-consecutive recruitment are acceptable and unlikely to introduce bias There was no evidence of inappropriate exclusion |
| Krige, 201732 | 103 mixed-ancestry healthy South Africans; mean age 52 years; 69% female | Yes | UC | Low | Random sampling |
| Nichols et al., 200733 | 50 consecutive patients requiring creatinine levels prior to chemotherapy administration; 52% male; 6% black African | Yes | Yes | Low |
Consecutive No inappropriate exclusions reported |
| aObrador et al., 201234 | 257 diabetic patients; mean age 56.9 years (SD 12.5 years); 62% women | UC | UC | UC |
Insufficient information Conference abstract |
| aShephard et al., 200835 |
101 venous blood samples No other information |
UC | UC | UC |
Insufficient information Conference abstract |
| Shephard et al., 201036 | 100 patients (63 renal/dialysis patients attending clinic, 37 healthy); 52% female | UC | UC | UC |
No information suggesting recruitment was consecutive or random 67% dialysis patients and 33% healthy volunteers |
| Snaith et al., 201837 | Over a 6-week period in September and October 2016, consecutive adult outpatients (≥ 18 years) attending a UK hospital phlebotomy department for routine Urea and Electrolytes (U&E) blood tests were approached. No upper age limit was adopted, but pregnant individuals and those unable to consent were excluded300 attending for routine blood tests (phlebotomy outpatients); mean age 60 years; 47% female; mean creatinine concentration 92 µmol/l | Yes | Yes | Low |
Consecutive No inappropriate exclusions reported, although 61 consenting patients were excluded because target sample size of 300 had been reached |
| Snaith et al., 201938 | CT outpatients without recent (i.e. within 3 months) eGFROver an eight-week period between February and April 2017 consecutive adult outpatients (≥ 18 years) attending for a contrast-enhanced CT scan were approached | Yes | Yes | Low |
Consecutive No inappropriate exclusions reported |
QUADAS-2: risk of bias – index test and reference standard
Selection question 1: is the reference standard likely to measure eGFR/creatinine accurately enough?
Selection question 2: was the same method used to calculate eGFR/creatinine for both index test and reference standard?
Risk of bias: could the conduct or interpretation of the index test or reference standard have introduced bias?
Answers to the above questions for the index test and reference standard domain are presented in Table 45.
| Study (author and year of publication) | Description | Selection question | Risk of bias | Notes | |
|---|---|---|---|---|---|
| 1 | 2 | ||||
| aBotz et al., 201327 |
i-STAT1 and Radiometer Ltd 827 whole-blood creatinine Roche Cobas Enzymatic C-501 analyzer MDRD formula |
Yes | UC | Low |
Conference abstract No information suggesting the method used to calculate eGFR/creatinine for both index test and reference standard were different |
| Dorward et al., 201828 |
Calibrated StatSensor Xpress-I using finger-prick capillary whole blood Dimension EXL 200 Enzymatic StatSensor Xpress-i, ‘factory calibrated’ setting was used, so (it appears that) the authors did not add an offset to the device, even though the device has that functionality Only non–offset results are reported |
Yes | UC | Low | No information suggesting that the method used to calculate eGFR/creatinine for both index test and reference standard were different |
| Houben et al., 201729 |
StatSensor used according to manual instructions Enzymatic reference standard StatSensor CREAT, no mention of offset or adjustments and only raw results are reported |
Yes | UC | UC | Unclear if MDRD equation used for POC and the laboratory reference are the same (factor 186 for POC vs. 175 for laboratory reference?) |
| Inoue et al., 201730 |
Adjusted and unadjusted plots and table of results show that the laboratory eGFR measurements also change, which is not supposed to happen (it should only adjust device values). The reported adjusted results from this study may not represent NHS practice. In addition, therefore, only the unadjusted results were used for the synthesis Uses StatSensor-i and included an adjustment (‘adjustment by applying offset correction on the basis of the slope and intercept of internal sample’) |
Yes | Yes | Low | Low as assessment applies only to unadjusted accuracy estimates |
| Korpi-Steiner et al., 200931 |
Different MDRD equations used for laboratory reference and i-STAT and StatSensor For laboratory reference and ABL800: standard MDRD calibrated to IDMS traceability: eGFR (ml/minute) = 175 × Cr−1.154 × Age−0.203 (× 0.742 if female) (× 1.212 if African American) For i-STAT and StatSensor: MDRD equation originally validated with conventional creatinine calibrations: eGFR (ml/minute) = 186 × Cr−1.154 × Age−0.203 (× 0.742 if female) (× 1.212 if African American) Results with offset (0.28 mg/dl) and no offset were reported |
Yes | No |
Low (ABL800) High (i-STAT and StatSensor) |
Different MDRD equations used for laboratory reference and i-STAT and StatSensor |
| Krige, 201732 |
Capillary sample for POC Siemens ADVIA 1800, which used an IDMS-standardised kinetic Jaffe assay method StatSensor: no offset used |
Yes | No | High | Jaffe method for reference laboratory (vs. enzymatic for POC test) |
| Nichols et al., 200733 |
MDRD formula Jaffe and enzymatic used Note that this assessment focuses on only the MDRD enzymatic laboratory reference, which was used for the pooled analyses |
Yes | Yes | Low |
No significant concerns MDRD used for both POC and laboratory reference |
| aObrador et al., 201234 |
Simple linear regression was used to estimate a correction factor to align i-STAT SCr to IDMS-SCr CKD staging was not standard (0–4) It is unclear what eGFR values correspond to each CKD stage Diagnostic accuracy results were reported only post correction |
Yes | UC | High |
Simple linear regression was used to estimate a correction factor to align i-STAT SCr to IDMS-SCr Diagnostic accuracy results were reported only post correction |
| aShephard et al., 200835 |
The i-STAT had a positive bias relative to the IDMS-aligned laboratory method (mean % bias of 5.6% overall, 10.4% for samples < 150 mmol/l and 4.5% for samples > 150 mmol/l) This bias was eliminated, and an IDMS alignment performed, by applying a correction formula Accuracy estimates were reported only post correction and alignment Reference standard used was enzymatic, with no further details reported |
UC | UC | High |
The i-STAT had a positive bias relative to the laboratory method Mean % bias of 5.6% overall, 10.4% for samples < 150 mmol/l and 4.5% for samples > 150 mmol/l Correction and alignment were performed Accuracy estimates were reported only post correction and alignment The reference laboratory test used was enzymatic, with no further details reported |
| Shephard et al., 201036 |
MDRD An eGFR cut-off point of 60 ml/minute/1.73 m2 Two devices were tested: Nova 1 and Nova 2 2 × 2 table available only for Nova 1 Tests were performed before and after calibration Two MDRD equations were used: the factory factor was 186, and the factor used post calibration was 175 (standard) For POC, 186 and 175 factors were both used to calculate sensitivity/specificity estimates before calibration; post calibration, only 175 factors were used Laboratory reference MDRD equation used factor 175 before and after calibration Plasma samples were used only for the laboratory reference On calibration:Results pre and post correction are reported |
Yes | No (pre-adjustment) | High |
High risk after calibration and adjustment as the offset adjustment was performed against the laboratory reference using the same samples For pre-calibration results it appears that the eGFR MDRD equation was used with factor 186 (vs. factor 175 for the laboratory test) Plasma was used for the laboratory reference test |
| Snaith et al., 201837 | CKD-EPI was used for POC tests and laboratory reference for the main analysis No offset adjustments done for any of the devices Laboratory reference method: enzymatic (Cobas 8000, Roche)Clarification from Dr Martine Harris (personal communication): Samples were taken based on how they would be clinical practice. Both the ABL800 and i-STAT were used with venous samples only, the StatSensor was the only device where a capillary sample was used |
Yes | Yes | Low |
CKD-EPI used for POC tests and laboratory reference for the main analysis Enzymatic reference standard |
| Snaith et al., 201938 |
I-STAT and enzymatic (Cobas 8000, Roche) CKD-EPI used for both |
Yes | Yes | Low | No significant concerns |
QUADAS-2: risk of bias – flow and timing
Selection question 1: did all patients receive both the index test and reference standard?
Selection question 2: were all patients included in the analysis?
Selection question 3: did all patients receive the same reference standard?
Selection question 4: was there an acceptable time gap between taking the index test blood and the reference standard blood samples?
Risk of bias: could the patient flow have introduced bias?
Answers to the above questions for the flow and timing domain are presented in Table 46.
| Study (author and year of publication) | Description | Selection question | Risk of bias | Notes | |||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||||
| aBotz et al., 201327 |
Retrospective Analysed all i-STAT1 whole-blood creatinine results performed on the same day (not clear how long in-between) of service as a SCr within 1 year Radiometer 827 results did not appear to be all on the same day |
Yes | Yes | Yes | UC | Low | See Description |
| Dorward et al., 201828 | Eight reference samples were excluded as a result of a laboratory strike or because they were processed 48 hours after sampling | No | No | Yes | Yes | Low | Exclusions are unlikely to have significantly biased the results |
| Houben et al., 201729 |
14 excluded ‘due to the inability to withdraw venous blood through the vacuum system used’ Blood drawn for laboratory measurement within 15 minutes of the POC test |
No | No | Yes | Yes | Low | Exclusions are unlikely to have introduced bias |
| Inoue et al., 201730 |
Reported as consecutive though retrospective All blood samples taken in the radiology suite prior to CT Time gap unknown, but unlikely to be significant |
Yes | Yes | Yes | UC | Low | Unlikely |
| Korpi-Steiner et al., 200931 | Excess samples of lithium heparin whole blood were removed after sample mixing to run on the i-STAT, StatSensor, and Radiometer methods (in that order). This was followed by centrifugation of the sample for 2 minutes at 4500 g for the analysis of plasma creatinine on the INTEGRA 400 | Yes | Yes | Yes | Yes | Low | Retrospective, but no significant concerns about flow |
| Krige, 201732 |
Both capillary and venous blood samples were collected at the same time Time gap between analysis of sample types was not reported |
Yes | Yes | Yes | Yes | Low | Considered low, though gap between analysis of sample types was not reported |
| Nichols et al., 200733 | All blood analyses were completed within 2 h of specimen collection. One specimen had too little sample to allow duplicate testing . . . and was excluded from the analysisAll samples were collected over 3 days | Yes | Yes | Yes | Yes | Low | See Description |
| aObrador et al., 201234 |
No description Conference abstract |
Yes | Yes | Yes | UC | Low |
Insufficient information Conference abstract |
| aShephard et al., 200835 |
No description Conference abstract |
UC | UC | UC | UC | UC |
Insufficient information Conference abstract |
| Shephard et al., 201036 |
Predialysis results from one patient were omitted from graphs and statistical calculations because of very inconsistent results Collection of POC and laboratory reference samples at the same time, but time gap between POC and laboratory reference analysis is unclear |
Yes | No | Yes | Yes | Low | See Description |
| Snaith et al., 201837 | Where there was incomplete data, i.e. results not available across all methods, the participants were excluded from the sampleAfter venous blood was collected:Capillary blood sampling was performed from the fingertip of each participant by two research radiographers as would be the case in routine practice. The skin was pierced with a spring-loaded lancet and the sample collected directly onto the analysis strip avoiding squeezing of the finger or milking of bloodContacted author – time gap between samples was within 10 minutes | Yes | Yes | Yes | Yes | Low | See Description |
| Snaith et al., 201938 |
If the POC test result identified a decline in kidney function from its baseline result, this prompted a requirement to wait for laboratory confirmation before CT Contacted author – time gap between samples was within 10 minutes Only four samples excluded: |
Yes | Yes | Yes | Yes | Low | See Description |
QUADAS-2: applicability concerns
Applicability concerns 1: are there concerns that the included patients do not match the review question?
Applicability concerns 2: are there concerns that the eGFR/creatinine thresholds used do not match the review question?
Applicability concerns 3: are there concerns that the index test, its conduct or interpretation differ from the review question?
Applicability concerns 4: are there concerns that the reference standard, its conduct or interpretation differ from the review question?
Answers to the above questions for applicability concerns are presented in Table 47.
| Study (author and year of publication) | Description | Applicability concerns | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| aBotz et al., 201327 |
Not clear how participants were classified as at risk of renal disease Not clear participants were outpatients Conference abstract eGFR thresholds: 30 and 60 ml/minute/1.73 m2 Whole-blood samples used for POC |
UC | Low | Low | Low |
| Dorward et al., 201828 |
HIV-positive population, younger and a higher proportion of women than the average outpatient population Only one patient had an eGFR < 60 ml/minute/1.73 m2 eGFR threshold: 90 Finger-prick whole-blood sample used for POC |
High | High | Low | Low |
| Houben et al., 201729 |
Only women referred for contrast-enhanced spectral mammography were recruited Data on all relevant thresholds were extractable |
High | Low | Low | Low |
| Inoue et al., 201730 |
The pre-adjustment study included 123 consecutive outpatients (74 males, 49 females, mean age 66.7 ± 12.5 years) who underwent contrast-enhanced CT between September 2011 and February 2012 SCr levels of the patients had not been assessed in the month preceding hospital admittance In the post-adjustment study, 110 consecutive outpatients (62 males, 48 females, mean age 70.1 ± 12.7 years) who underwent contrast-enhanced CT at Kohka Public Hospital between June and November 2012, were included < 30, 30–45 and > 45 ml/minute/1.73 m2 thresholds extractable, but equation used to calculate eGFR is not standard (Japanese Society of Nephrology-Chronic Kidney Disease Initiatives) Uses StatSensor-i and included an adjustment (‘adjustment by applying offset correction on the basis of the slope and intercept of internal sample’) Adjusted and unadjusted plots and table of results show the laboratory eGFR measurements also change, which is not supposed to happen (it should adjust only the device values). So the reported adjusted results from this study may not represent NHS practice. Therefore, only unadjusted results were assessed and used in the meta-analysis |
Low | High | High | Low |
| Korpi-Steiner et al., 200931 |
Patients referred for CT without a recent eGFR/SCr measurement considered at risk < 60 ml/minute/1.73 m2 threshold only Excess lithium heparinised whole-blood samples used |
Low | High | High | Low |
| Krige, 201732 |
103 mixed-ancestry healthy outpatients attending nephrology clinic; South Africans; mean age 52 years; 69% female Jaffe method used IPD reported allowed derivation of < 30 ml/minute/1.73 m2 cut-off point data |
High | Low | Low | Low |
| Nichols et al., 200733 |
Only chemotherapy patients, but no significant reasons to believe that they depart from the main population of interest Only eGFR < 60 ml/minute/1.73 m2 cut-off point assessed |
Low | High | Low | Low |
| aObrador et al., 201234 |
Only diabetics; 62% women Simple linear regression was used to estimate a correction factor to align i-STAT SCr to IDMS-SCr Diagnostic accuracy results were reported only post correction CKD staging was not standard (0–4) It was unclear what eGFR values corresponded to each CKD stage |
High | High | High | Low |
| aShephard et al., 200835 |
Insufficient information (conference abstract) Results reported only for eGFRs of 60 ml/minute/1.73 m2 Accuracy estimates were calculated only post correction and alignment |
UC | High | High | Low |
| Shephard et al., 201036 |
67% were dialysis patients; 33% were healthy volunteers Only an eGFR < 60 ml/minute/1.73 m2 cut-off point was assessed Low applicability concerns for post-calibration method (uses standard MDRD factor, as per laboratory reference) Study mentions StatSensor (not clear which model), but used an adjustment to correct for bias Results pre and post correction are reported A similar adjustment could in theory be implemented in the StatSensor Xpress-I, so potentially used on the NHS |
High | High | Low | Low |
| Snaith et al., 201837 |
Phlebotomy outpatients Characteristics may differ from outpatients scheduled for CT without recent SCr measurement, but deemed unlikely to significantly affect applicability All relevant eGFR cut-off points reported Study states device as only the StatSensor (unclear which model) and did not use any offset Only the raw results are available |
Low | Low | Low | Low |
| Snaith et al., 201938 |
CT outpatients without recent (within 3 months) eGFR No significant concerns Venous samples were used for POC testing |
Low | Low | Low | Low |
| Study [author (trial acronym) and year of publication] | Random sequence allocation (selection bias) | Allocation concealment (selection bias) | Blinding of participants and personnel (performance bias) | Blinding of outcome assessment (detection bias) | Incomplete outcome data (attrition bias) | Selective reporting (reporting bias) |
|---|---|---|---|---|---|---|
| Nijssen et al., (AMACING) 2017 and 2018104,105 | + | + | – | + | + | + |
| Dussol et al., 2006103 | + | ? | – | + | + | ? |
Appendix 6 Systematic review of cost-effectiveness studies
Table 49 lists the studies excluded from the review alongside reasons for exclusion.
| Study (author and year of publication) | Reason for rejection |
|---|---|
| Adams et al., 1995126 |
Study does not include any comparators No health outcomes were considered |
| Canadian Agency for Drugs and Technologies in Health, 2013127 | Not a cost-effectiveness analysis |
| Lee-Lewandrowski et al., 201259 | Cost analysis, no health outcomes were considered |
Appendix 7 Review of Shinkins et al. (unpublished data)
Appendix 8 Model inputs
| eGFR (ml/minute/1.73 m2) | All outpatients, n (% of total) | Patients without a prior eGFR measurement, n (% of total) |
|---|---|---|
| < 30 | 1 (0.12) | 0 (0) |
| 30–40 | 31 (3.8) | 4 (3.85) |
| 41–50 | 59 (7.23) | 5 (4.81) |
| 51–60 | 91 (11.15) | 14 (13.46) |
| 61–70 | 141 (17.28) | 29 (27.88) |
| 71–80 | 154 (18.87) | 24 (23.08) |
| 81–90 | 150 (18.38) | 16 (15.38) |
| > 90 | 189 (23.16) | 12 (11.54) |
| Total | 816 | 104 |
| eGFR ( ml/minute/1.73 m2) | All outpatients, n (% of total) | Patients without a prior eGFR measurement, n (% of total) | ||||
|---|---|---|---|---|---|---|
| Reason for referral | Reason for referral | |||||
| Suspected cancer | Urgent | Routine | Suspected cancer | Urgent | Routine | |
| < 30 | 0 (0.0) | 0 (0.0) | 1 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 30–40 | 21 (5.4) | 4 (2.6) | 6 (2.2) | 0 (0.0) | 1 (6.7) | 3 (3.9) |
| 41–50 | 26 (6.7) | 15 (9.6) | 18 (6.7) | 0 (0.0) | 0 (0) | 5 (6.5) |
| 51–60 | 47 (12.0) | 18 (11.5) | 26 (9.6) | 2 (16.7) | 1 (6.7) | 11 (14.3) |
| 61–70 | 59 (15.1) | 31 (19.9) | 51 (18.9) | 3 (25.0) | 6 (40.0) | 20 (26.0) |
| 71–80 | 70 (17.9) | 29 (18.6) | 55 (20.4) | 3 (25.0) | 4 (26.7) | 17 (22.1) |
| 81–90 | 71 (18.2) | 27 (17.3) | 52 (19.3) | 3 (25.0) | 1 (6.7) | 12 (15.6) |
| > 90 | 96 (24.6) | 32 (20.5) | 61 (22.6) | 1 (8.3) | 2 (13.3) | 9 (11.7) |
| Total | 390 (48) | 156 (19) | 270 (33) | 12 (12) | 15 (14) | 77 (74) |
Risk factor screening questionnaires
| Risk factors | Study (author and year of publication) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Azzouz et al., 201414 | Too et al., 201575 | Snaith et al., 201938 | Schreuder et al., 2017116 | Moos et al., 2014114 | |||||||
| Original | Modified | Original/modified | RANZCR RF | Model A | Model B | Model 1 | Model 2 | Model 3 | Model 4 | ||
| Renal disease | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Renal surgery | ✗ | ✗ | |||||||||
| Hypertension | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||||
| Gout | ✗ | ✗ | |||||||||
| Diabetes mellitus and/or metformin | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Proteinuria | ✗ | ||||||||||
| Recent/current illness | ✗ | ||||||||||
| Cardiovascular disease | ✗ | ✗ | |||||||||
| Age (years) | |||||||||||
| > 75 | ✗ | ✗ | |||||||||
| > 60 | ✗ | ✗ | |||||||||
| Congestive heart failure | ✗ | ✗ | ✗ | ||||||||
| Anaemia | ✗ | ||||||||||
| Use of diuretics | ✗ | ||||||||||
| Malignancy | ✗ | ||||||||||
| Multiple myeloma | ✗ | ||||||||||
| Waldenström’s macroglobulinaemia | ✗ | ||||||||||
| Questionnaire | Reference test | eGFR equation | Population | eGFR (ml/minute/1.73 m2) | |||
|---|---|---|---|---|---|---|---|
| < 45 | < 60 | ||||||
| Sensitivity | Specificity | Sensitivity | Specificity | ||||
| Schreuder et al., 2017116 | |||||||
| Model A | Laboratory | MDRD | Non ICU and non-emergency patients scheduled to i.v. contrast-enhanced CT | 100.0% | 46.3% | 88.0% | 58.7% |
| Model B | 100.0% | 58.7% | 76.1% | 61.5% | |||
| Moos et al., 2014114 | |||||||
| Model 1 | Laboratory | MDRD | Non-ICU and non-emergency patients scheduled to i.v. contrast-enhanced CT | 100.0% | 18.8% | 96.4% | 20.1% |
| Model 2 | 100.0% | 26.1% | 96.4% | 28.1% | |||
| Model 3 | 100.0% | 38.8% | 89.3% | 41.1% | |||
| Model 4 | 100.0% | 57.6% | 76.8% | 60.0% | |||
| Snaith et al., 201938 | |||||||
| Originala | Laboratory | CKD-EPI | Outpatients attending for a contrast-enhanced CT | 71.4% | 48.6% | 65.5% | 50.8% |
| Modifieda | 38.5% | 67.6% | 35.6% | 68.0% | |||
| RANZCR RF | 35.7% | 83.9% | 25.9% | 85.1% | |||
| eGFR (ml/minute/1.73 m2) | Study (author and year of publication), % of total | |||
|---|---|---|---|---|
| Park et al., 2016115 | Nijssen et al., 2017104 | Nijssen et al., 2018106 | Kim et al., 2010117 | |
| < 30 | 10.80% | N/A | 11.24% | 12.07% |
| 30–60 | 2.40% | 2.65% | N/A | 1.30% |
| Number of patients (eGFR < 30, 30–60 ml/minute/1.73 m2) | 1666 (250, 1416) | 603 (N/A, 603) | 89 (89, N/A) | 520 (58, 462) |
| Secondary outcome | Propensity matching | |||
|---|---|---|---|---|
| Before | After | |||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Death | 1.05 (0.58 to 1.91) | 0.86 | 0.90 (0.46 to 1.76) | 0.75 |
| Within 6 months | 0.80 (0.31 to 2.07) | 0.64 | 0.81 (0.29 to 2.31) | 0.70 |
| After 6 months | 1.15 (0.53 to 2.49) | 0.72 | 0.99 (0.41 to 2.40) | 0.98 |
| eGFR | ||||
| ≥ 30 ml/minute/1.73 m2 | 1.20 (0.53 to 2.72) | 0.66 | 0.93 (0.35 to 2.51) | 0.89 |
| < 30 ml/minute/1.73 m2 | 0.87 (0.35 to 2.15) | 0.76 | 0.79 (0.29 to 2.13) | 0.64 |
| Initiation of RRT | 2.75 (1.52 to 4.98) | 0.001 | 3.05 (1.43 to 6.47) | 0.003 |
| Within 6 months | 4.54 (1.93 to 10.71) | 0.001 | 8.61 (2.28 to 32.61) | 0.002 |
| After 6 months | 1.73 (0.62 to 4.81) | 0.30 | 1.15 (0.34 to 3.86) | 0.83 |
| eGFR | ||||
| ≥ 30 ml/minute/1.73 m2 | 4.47 (1.33 to 15.07) | 0.02 | 5.23 (0.57 to 47.64) | 0.14 |
| < 30 ml/minute/1.73 m2 | 2.58 (1.34 to 4.97) | 0.004 | 2.65 (1.15 to 6.15) | 0.02 |
| Device | Testing material costed | Per test | |
|---|---|---|---|
| Cost | Time (minutes) | ||
| Devices included in the model | |||
|
Abbott Point of Care i-STAT Alinity |
Creatinine cartridge | £4.75 | 2 |
|
Nova Biomedical StatSensor |
Creatinine test strip | £3.95 | 0.5 |
|
Radiometer Ltd ABL800 FLEX |
Per-test proportion of all testing materials | £2.88 | 1 |
| Other devices | |||
|
Abaxis, Inc. Piccolo Xpress |
Kidney check rotor | £12.00 | 12 |
|
Fujifilm Corporation DRI-CHEM NX 500 |
DRI-CHEM creatinine slide; Fujifilm plasma filter | £3.73 | 1 |
|
Radiometer Ltd ABL90 FLEX PLUS |
Per-test proportion of all testing materials | £2.71 | 1 |
| Siemens Healthineers AG epoc | Test cartridge | £5.75 | 1 |
| Device | Cost per quality control check | Time to prepare QC materials (minutes) | Frequency of quality control | |
|---|---|---|---|---|
| Excluding test-based consumables | Including test-based consumables | |||
| Devices included in model | ||||
|
Abbott Point of Care i-STAT Alinity |
£2.05 | £6.80 | 45 minutes to bring to ambient temperature, 1–2 minutes to prepare materials | Every week/every 25 tests |
|
Nova Biomedical StatSensor |
£0.20 | £4.15 | Not known | Every 24 hours |
|
Radiometer Ltd ABL800 FLEX |
£5.01 | £5.01 | Automatic – no time to prepare materials | Every 24 hours |
| Other devices | ||||
|
Abaxis, Inc. Piccolo Xpress |
£19.20 | £31.20 | 30 minutes to bring to ambient temperature | Every 30 days/every 10 tests |
|
Fujifilm Corporation DRI-CHEM NX 500 |
£11.97 | £15.70 | 30 minutes to bring to ambient temperature, 30 minutes to mix | Every 30 days |
|
Radiometer Ltd ABL90 FLEX PLUS |
£3.76 | £3.76 | Automatic – no time to prepare materials | Every 24 hours |
| Siemens Healthineers AG epoc | £28 | £33.75 | 60 minutes to bring to ambient temperature | Every 50 tests |
| Device | Annual maintenance cost | Guarantee period |
|---|---|---|
| Devices included in model | ||
|
Abbott Point of Care i-STAT Alinity |
£850 | 1 year |
|
Nova Biomedical StatSensor |
£850 | 1 year |
|
Radiometer Ltd ABL800 FLEX |
£4685 | 1 year |
| Other devices | ||
|
Abaxis, Inc. Piccolo Xpress |
£1675 | 1 year |
|
Fujifilm Corporation DRI-CHEM NX 500 |
£750 | 1 year |
|
Radiometer Ltd ABL90 FLEX PLUS |
£1315 | 1 year |
| Siemens Healthineers AG epoc | £816 | 1 year |
| Device | Capital cost | Annual servicing | Consumables | Quality control materials (including test consumables) | Total device cost per test (based on a throughput of 92.6 patients per month) |
|---|---|---|---|---|---|
| Devices included in model | |||||
|
Abbott Point of Care i-STAT Alinity |
£0.92 | £0.77 | £4.75 | £0.27 | £6.71 |
|
Nova Biomedical StatSensor |
£0.71 | £0.77 | £3.95 | £1.36 | £6.79 |
|
Radiometer Ltd ABL800 FLEX |
£5.33 | £4.22 | £2.88 | £1.65 | £14.07 |
| Other devices | |||||
|
Abaxis, Inc. Piccolo Xpress |
£1.56 | £1.51 | £12.00 | £3.12 | £18.19 |
|
Fujifilm Corporation DRI-CHEM NX 500 |
£1.21 | £0.68 | £3.73 | £0.17 | £5.78 |
|
Radiometer Ltd ABL90 FLEX PLUS |
£2.13 | £1.18 | £2.71 | £1.24 | £7.27 |
| Siemens Healthineers AG epoc | £0.89 | £0.73 | £5.75 | £1.58 | £8.95 |
| Device | Pre-testing staff time (minutes) | Time to use the device to analyse a sample (minutes) | Staff cost per test conducted | Time for quality control (minutes) | Total quality control staff cost | Total staff cost per test conducted (including quality control)a |
|---|---|---|---|---|---|---|
| Devices included in model | ||||||
|
Abbott Point of Care i-STAT Alinity |
3 | 2 | £2.08 | 3.5 | £1.46 | £2.14 |
|
Nova Biomedical StatSensor |
3 | 0.5 | £1.46 | 2 | £0.83 | £1.73 |
|
Radiometer Ltd ABL800 FLEX |
3 | 1 | £1.66 | 0 | £0.00 | £1.66 |
| Other devices | ||||||
|
Abaxis, Inc. Piccolo Xpress |
3 | 12 | £6.25 | 13.5 | £5.63 | £6.81 |
|
Fujifilm Corporation DRI-CHEM NX 500 |
3 | 1 | £1.67 | 2.5 | £1.04 | £1.68 |
|
Radiometer Ltd ABL90 FLEX PLUS |
3 | 1 | £1.49 | 0 | £0.00 | £1.49 |
| Siemens Healthineers AG epoc | 3 | 1 | £1.67 | 2.5 | £1.04 | £1.71 |
| Cost category | Resource use | Units | Source | Parameter, unit cost | Source/assumptions | Cost |
|---|---|---|---|---|---|---|
| RRT | Haemodialysis sessions | Thrice-weekly for 3 months | NICE CG169108 | £271.06 per session |
NHS Reference Costs 2017/18 111 HRG currency code LE01 A, haemodialysis for acute kidney injury, ≥ 19 years111 |
£9758 |
| Parameter | Value | Source | Probabilistic model setup |
|---|---|---|---|
| Population characteristics | |||
| Probability of eGFR (ml/minute/1.73 m2) | < 30:a 0.006 | Gamma distribution fitted to the Mid Yorkshire Hospitals NHS Trust data | NA |
| 30–44:a 0.063 | |||
| 45–59:a 0.154 | |||
| ≥ 60:a 0.777 | |||
| Age and male proportion | 65 years, 51.7% | Snaith et al., 201938 | NA |
| % missing an eGFR | 34% | Cope et al., 201713 | NA |
| Patients per site | 272 monthly | Harris all outpatient data | NA |
| Diagnostic accuracy | |||
| Laboratory test |
Sensitivity: 100% Specificity: 100% |
Assumption | NA |
| i-STAT |
Sensitivity: 84.1% Specificity: 98.9% |
Evidence synthesis of POC diagnostic accuracy – main analysis | Model draws from 1000 simulated values from the meta-analysis posterior distribution |
| ABL |
Sensitivity: 86.1% Specificity: 99.2% |
||
| StatSensor |
Sensitivity: 73.9% Specificity: 99.1% |
||
| Risk factor questionnaire |
Sensitivity: 100% Specificity: 65.2% |
Too et al., 201575 |
Independent beta distributions fitted to the diagnostic accuracy 2 × 2 tables Sensitivity: α = 10.01; β = 0.01 (continuity correction of 0.01) Specificity: α = 470; β = 881 |
| Probability of AKI with contrast conditional on an | |||
| eGFR | |||
| < 30 ml/minute/1.73 m2 and no i.v. hydration | 11.1% |
Park et al. , 2016115 Ahmed et al. , 2018102 |
Log-normal distribution fitted to an OR of PC-AKI with i.v. hydration [14] = 0.97; ln(SE) = 0.33 |
| < 30 ml/minute/1.73 m2 and i.v. hydration | 10.8% | Park et al., 2016115 | Beta distribution: α = 27; β = 223 |
| ≥ 30 ml/minute/1.73 m2 with no i.v. hydration | 2.4% | Assumption | Beta distribution: α = 34; β = 1385 |
| ≥ 30 ml/minute/1.73 m2 with i.v. hydration | 2.4% | Park et al., 2016115 | Beta distribution: α = 34; β = 1385 |
| Probability of RRT (no PC-AKI) | 1.4% | Park et al., 2016115 | Beta distribution: α = 22; β = 1583 |
| Probability of RRT (PC-AKI) | 11.1% | Park et al., 2016115 | Log-normal distribution fitted to a HR of RRT given PC-AKI = 8.61; ln(SE) = 0.679 |
| Proportion of patients alive at 6 months post imaging | 94.5% | Park et al., 2016115 | Independent beta distributions fitted to proportion of alive patients:
|
| HRQoL-adjusted life expectancy | 9.80 QALYs | Calculated from ONS mortality data,118 and Ara and Brazier’s119 general population utility equation | NA |
| QALY loss from RRT | –0.0275 | Wyld et al.120 and assuming 3 months of RRT | Gamma distribution fitted to utility decrement from RRT = 0.11; SE = 0.02 |
| QALY loss from anxiety due to delays | 0 | Assumption | NA |
| Costs | |||
| Laboratory test | £3.31 | NHS Reference Costs 2017/18 124 | NA |
| Risk factor screening | £1.11 | Ledermann et al., 2010;77 and NHS Reference Costs 2017/18124 | NA |
| i-STAT without RF screening | £8.85 | See Point-of-care device costs | NA |
| ABL800 FLEX without RF screening | £15.73 | See Point-of-care device costs | NA |
| StatSensor without RF screening | £8.52 | See Point-of-care device costs | NA |
| i-STAT with RF screening | £11.96 | See Point-of-care device costs | NA |
| ABL800 FLEX with RF screening | £36.36 | See Point-of-care device costs | NA |
| StatSensor with RF screening | £14.25 | See Point-of-care device costs | NA |
| Contrast-enhanced CT scan | £111.65 | NHS Reference Costs 2017/18 124 | NA |
| CT scan rebooking | Confidential information has been removed | Shinkins et al. (in submission)b | NA |
| CT scan cancellation | £87.92 | NHS Reference Costs 2017/18,124 assumed to be the cost of an unenhanced CT scan | NA |
| i.v. hydration | £340.89 | NHS Reference Costs 2017/18 124 | NA |
| Adverse events from i.v. hydration | £32.76 | Nijssen et al., 2017;104 and NHS Reference Costs 2017/18124 | NA |
| Follow-up if test was positivea | £186.49 | NHS Reference Costs 2017/18 124 | NA |
| RRT | £9758 | NHS Reference Costs 2017/18124 and assuming thrice weekly sessions for 3 months | NA |
| Mediating action if positivea | |||
| i.v. hydration and contrast-enhanced CT scan | 100% of patients | Assumption | NA |
| Unenhanced CT scan | 0% of patients | Assumption | NA |
| MRI | 0% of patients | Assumption | NA |
| Proportion of rebooked and cancelled scans if test was positivea | 100% | Assumption | NA |
Appendix 9 Supplementary cost-effectiveness review
Given that the initial scoping searches conducted while drafting the study protocol indicate that the existing cost-effectiveness literature addressing the relevant decision problem is likely to be limited, one targeted search was also conducted to identify further evidence. The aim of this search was to identify cost-effectiveness studies evaluating the treatment and management of AKI. The additional review should mitigate some of the potential limitations of the existing cost-effectiveness literature, as one of the key conceptual issues concerns the nature of the linked evidence modelling required to estimate the occurrence of PC-AKI and its associated consequences (e.g. CKD, end-stage renal disease and death).
Methods
Searches
Searches were undertaken to identify cost-effectiveness studies evaluating the treatment and management of AKI. A search strategy was developed in MEDLINE (via Ovid) consisting of terms for AKI combined with a search strategy developed by the Canadian Agency for Drugs and Technologies in Health (CADTH) to limit retrieval to cost-effectiveness studies. 128 The search was limited to studies published from 2012 onwards in any language. The MEDLINE strategy was adapted for use in all other databases searched.
The following databases were searched in January 2019: MEDLINE ALL (including Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE), EconLit, EMBASE, NHS EED, Research Papers in Economics (RePEc) and the Science Citation Index.
Database
Ovid MEDLINE(R) ALL.
Date range searched: 1946 to 11 January 2019.
Date searched: 14 January 2019.
Records retrieved: 2972 retrieved, of which 2157 remained after deduplication.
Search strategy
-
exp Acute Kidney Injury/ (42,239)
-
(acute adj2 (renal or kidney$ or nephr$) adj2 (fail$ or injur$ or insufficien$)).ti,ab. (42,016)
-
((acute or renal or kidney$ or nephr$) adj2 tubular necrosis).ti,ab. (3660)
-
or/1-3 (60,629)
-
(contrast adj3 (kidney$ or renal or nephr$) adj3 (injur$ or fail$ or insufficien$ or tubular necrosis)).ti,ab. (1049)
-
(contrast adj3 (AKI or nephropath$ or nephrotoxic$)).ti,ab. (2895)
-
((radiocontrast or radio-contrast) adj3 (kidney$ or renal or nephr$) adj3 (injur$ or fail$ or insufficien$ or tubular necrosis)).ti,ab. (69)
-
((radiocontrast or radio-contrast) adj3 (AKI or nephropath$ or nephrotoxic$)).ti,ab. (299)
-
((postcontrast or post-contrast) adj3 (kidney$ or renal or nephr$) adj3 (injur$ or fail$ or insufficien$ or tubular necrosis)).ti,ab. (19)
-
((postcontrast or post-contrast) adj3 (AKI or nephropath$ or nephrotoxic$)).ti,ab. (13)
-
(CI-AKI or CIAKI or PC-AKI or PCAKI).ti,ab. (406)
-
or/5-11 (3991)
-
4 or 12 (62,842)
-
economics/ (26,988)
-
exp “costs and cost analysis”/ (221,067)
-
economics, dental/ (1901)
-
exp “economics, hospital”/ (23,279)
-
economics, medical/ (8991)
-
economics, nursing/ (3986)
-
economics, pharmaceutical/ (2833)
-
exp “Fees and Charges”/ (29,548)
-
exp Budgets/ (13,436)
-
budget*.ti,ab,kf. (26,878)
-
(economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* or pharmaco-economic* or expenditure or expenditures or expense or expenses or financial or finance or finances or financed).ti,kf. (207,844)
-
(economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* or pharmaco-economic* or expenditure or expenditures or expense or expenses or financial or finance or finances or financed).ab. /freq=2 (255,303)
-
(cost* adj2 (effective* or utilit* or benefit* or minimi* or analy* or outcome or outcomes)).ab,kf. (142,936)
-
(value adj2 (money or monetary)).ti,ab,kf. (2107)
-
exp models, economic/ (13,754)
-
economic model*.ab,kf. (2928)
-
markov chains/ (13,149)
-
markov.ti,ab,kf. (19,884)
-
monte carlo method/ (26,253)
-
monte carlo.ti,ab,kf. (44,643)
-
exp Decision Theory/ (11,296)
-
(decision* adj2 (tree* or analy* or model*)).ti,ab,kf. (20,435)
-
or/14-35 (664,050)
-
13 and 36 (769)
-
exp animals/ not humans/ (4,535,562)
-
37 not 38 (763)
-
limit 39 to yr=“2012 -Current” (425).
Study selection
Studies using decision models to evaluate the cost-effectiveness of AKI management and published from 2012 until 2019 were considered for inclusion. Only full economic evaluations that compared two or more options and considered both costs and consequences (i.e. cost-minimisation, cost-effectiveness, cost–utility and cost–benefit analyses) were considered.
Two researchers (AD and JA) independently screened the titles and abstracts of all reports identified by the bibliographic searches, and full-text papers were subsequently obtained for assessment and screened by at least two researchers. Disagreements were resolved by consensus.
Results
The initial search of economic databases identified a total of 2972 records, of which 2157 remained after deduplication. Eight titles108,110,121,129–134 were identified as potentially relevant based on their titles and/or abstracts. The full-text articles of these records were assessed for eligibility. Four studies108,110,121,129,130 were found to meet the selection criteria and were included in the review. These studies were not subject to a formal assessment, but were used to assist in the overall development of the new analytical model. Table 67 shows the results of the searches, and Table 68 lists excluded studies alongside reasons for exclusion. The studies identified in the review are summarised in Table 69.
| Database | Number of records | |
|---|---|---|
| Retrieved before deduplication | After deduplication | |
| MEDLINE Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) | 425 | 420 |
| EMBASE (via Ovid) | 1649 | 1242 |
| EconLit (via Ovid) | 3 | 2 |
| NHS EED (via CRD databases) | 6 | 0 |
| Science Citation Index (via Clarivate Analytics) | 877 | 486 |
| RePEc | 12 | 7 |
| Total in EndNote | 2972 | 2157 |
| Study (author and year of publication) | Reason for rejection |
|---|---|
| De Smedt et al., 2012131 | Area under the curve model |
| Ethgen et al., 2015132 | Does not compare patients with and patients without AKI |
| Kerr et al., 2014133 | Not a comparison of alternative interventions |
| Petrovic et al., 2015134 | Uses the RIFLE criteria to define AKI and had a paediatric population |
| Study (author, year of publication and country) | Interventions | Patient population | Time horizon | Model type | Health states | Key results |
|---|---|---|---|---|---|---|
| Chicaíza-Becerra et al., 2012,129 Colombia | Iso- and low-osmolality contrast media | Outpatients at high risk of CI-AKI | Lifetime | Decision tree |
|
Other alternatives dominated by iopamidol and iodixanol Iodixanol vs. iopamidol = US$ 14,660/LYG |
| CG169,108,110 UK | Prophylactic hydration to prevent CI-AKI | Patients at high risk of CI-AKI | Lifetime | Markov model | CKD stages 3–4, CKD stage 5, CI-AKI, death |
NAC + 0.9% sodium chloride – NMB = £47,957 Sodium bicarbonate – NMB = £47,585 At a threshold of £20,000 per additional QALY |
| Hall et al., 2018,121 UK | NEPHROCHECK® (bioMérieux, Marcy-l’Étoile, France), cystatin C in urine, plasma and serum; and NGAL in urine, plasma and serum | ICU patients | Lifetime | Decision tree + two-period decision model | Decision tree:
|
Cystatin C (urine and plasma) and NGAL (urine and plasma) dominated by cystatin C (serum) ICERs for cystatin C (serum), NGAL (serum) and NEPHROCHECK were £11,476, £25,492 and £12,855,101 per additional QALY, respectively |
| Iannazzo et al., 2014,130 Italy | Iodixanol vs. low-osmolar contrast media | Patients with i.v. contrast media CT | Lifetime | Markov model | AKI free, AKI, myocardial infarction and death | Iodixanol dominated low-osmolar contrast media |
Two studies quantify the impact of the interventions under comparison on costs and outcomes by modelling CKD progression after AKI108,110,121 with a Markov model structure. One study130 also uses a model Markov structure to compare cost-effectiveness between alternative contrast media, but does not characterise CKD progression and considers only progression to myocardial infarction. One study129 follows a more simplified structure, whereby a decision tree structure is used to quantify the pay-offs in terms of costs and outcomes of dialysis and death.
The models from the National Clinical Guideline Centre108,110 and Chicaíza-Becerra et al. 129 were considered the most relevant examples of how costs and outcomes associated with AKI can be quantified, as they represent the two extremes of model structure complexity in the context of CI-AKI. The studies were examined with the aim of identifying important structural assumptions and parameter estimates, and highlighting key areas of uncertainty. These studies are summarised and highlight the elements potentially relevant to inform the conceptualisation and development of the new decision model.
Review of Clinical Guideline number 169
The National Clinical Guideline Centre developed a Markov model to assess the cost-effectiveness of prophylactic hydration strategies for the prevention of CI- AKI in patients at stage 3–4 CKD (with and without diabetes) who need a CT scan. 108,110 The analysis followed the perspective of the NHS and PSS. Costs were expressed as Great British pounds (2011/12) and health outcomes as QALYs. Costs and outcomes are discounted at an annual rate of 3.5%.
Model structure
The model considers a lifetime horizon and 3-month cycles. The structure of the model is depicted elsewhere. 110
The model is composed of four mutually exclusive health states: stage 3–4 CKD, stage 5 CKD, PC-AKI (CI-AKI in the original text) and death. Patients enter the model through the stage 3–4 CKD state and undergo a CT scan, and can then transition to PC-AKI, remain on the initial state or transition to stage 5 CKD. Patients who transition to PC-AKI will remain on that state for one cycle only (i.e. 3 months), and either return to stage 3–4 CKD or progress to stage 5 CKD. After the first cycle, a continuous risk of PC-AKI from repeated scans is assumed for patients in the stage 3–4 CKD state. Patients in stage 5 CKD can only remain in the state or die. The model assumes no regression from stage 5 CKD to less severe CKD states, and no further PC-AKI after transition to stage 5 CKD. Patients can transition to death from any other state in the model.
Baseline transition probabilities and treatment effects
The population consists of patients with known stage 3–4 CKD (average age 70 years) presenting for a CT scan in an unspecified setting. The data sources used to inform baseline transition probabilities to the PC-AKI state, treatment effects from prophylactic hydration and PC-AKI mortality were drawn mostly from studies in cardiovascular patients receiving contrast agents. The severity of AKI was assumed to affect mortality rates only for the PC-AKI state. AKI stage-specific mortality rates were obtained from a large observational study135 in coronary angiography patients. The rates were weighted by the relative proportion of patients at each AKI severity stage following the cardiovascular intervention in the same study to estimate the overall probability of death following PC-AKI. The baseline risk of PC-AKI was informed by the incidence of AKI in the renal insufficiency subgroup prophylactically intravenously hydrated with the 0.9% sodium chloride treatment from a trial comparing two hydration strategies in patients undergoing coronary angiography. 136 The probability of a repeat scan was derived from the probability of repeat PCI in a trial of patients with coronary artery disease,137 and applied to the baseline risk of PC-AKI to calculate the risk of PC-AKI from the second cycle in the model onwards.
The age-dependent probability of disease progression from stage 3–4 to stage 5 CKD was derived from a retrospective longitudinal study of stage 3 CKD patients. 138 The probability of death on stage 3–4 CKD was estimated by applying age- and sex-dependent standardised mortality rates (SMRs) to UK general population life tables, and converting the annual rates to 3-month probabilities. The model implicitly assumed the same rate of progression to stage 5 CKD and mortality for both stage 3 and stage 4 CKD patients, despite the latter having more severe renal function impairment.
Mortality on stage 5 CKD was estimated by applying age- and sex-dependent SMRs from a prospective cohort study in an end-stage renal disease population to UK general population life tables.
Baseline treatment properties in the model are summarised in Table 70, along with the sources of evidence.
| Transition | Probability | Source |
|---|---|---|
| Stage 3–4 to PC-AKI (first cycle) | 0.0217 | Mueller et al., 2002136 |
| Stage 3–4 (second cycle and subsequent cycles) | 0.0007 |
Mueller et al. , 2002136 Serruys et al. , 2009137 |
| PC-AKI stage 1 to stage 5 CKD | 0.015 |
James et al. , 2011135 Applied to 83% of PC-AKI patients |
| PC-AKI stage 2–3 to stage 5 CKD | 0.109 |
James et al. , 2011135 Applied to 17% of PC-AKI patients |
| PC-AKI to stage 5 CKD | 0.031 | Calculated |
| CKD stage 3–4 to CKD stage 5 (mean age dependent) | 0.001 | Eriksen and Ingebretsen, 2006138 |
| PC-AKI stage 1 to death | 0.136 |
James et al. , 2011135 Applied to 83% of PC-AKI patients |
| PC-AKI stage 2–3 to death | 0.378 |
James et al. , 2011135 Applied to 17% of PC-AKI patients |
| PC-AKI to death | 0.182 | Calculated |
The treatment effect of each alternative prophylactic IVH strategy was estimated as a relative risk of PC-AKI using a mix of direct and indirect comparisons, and applied to the baseline risk of PC-AKI for the reference hydration strategy (i.e. 0.9% sodium chloride). Figure 16 illustrates the treatment effects (i.e. RRs) estimated in comparison with 0.9% sodium chloride. Adverse events from prophylaxis were not considered in the model.
FIGURE 16.
Comparisons of relative treatment effects available from the meta-analysis of trials (CG169). CG, clinical guideline; NAC, N-acetylcysteine; SB, sodium bicarbonate; SC, sodium chloride.
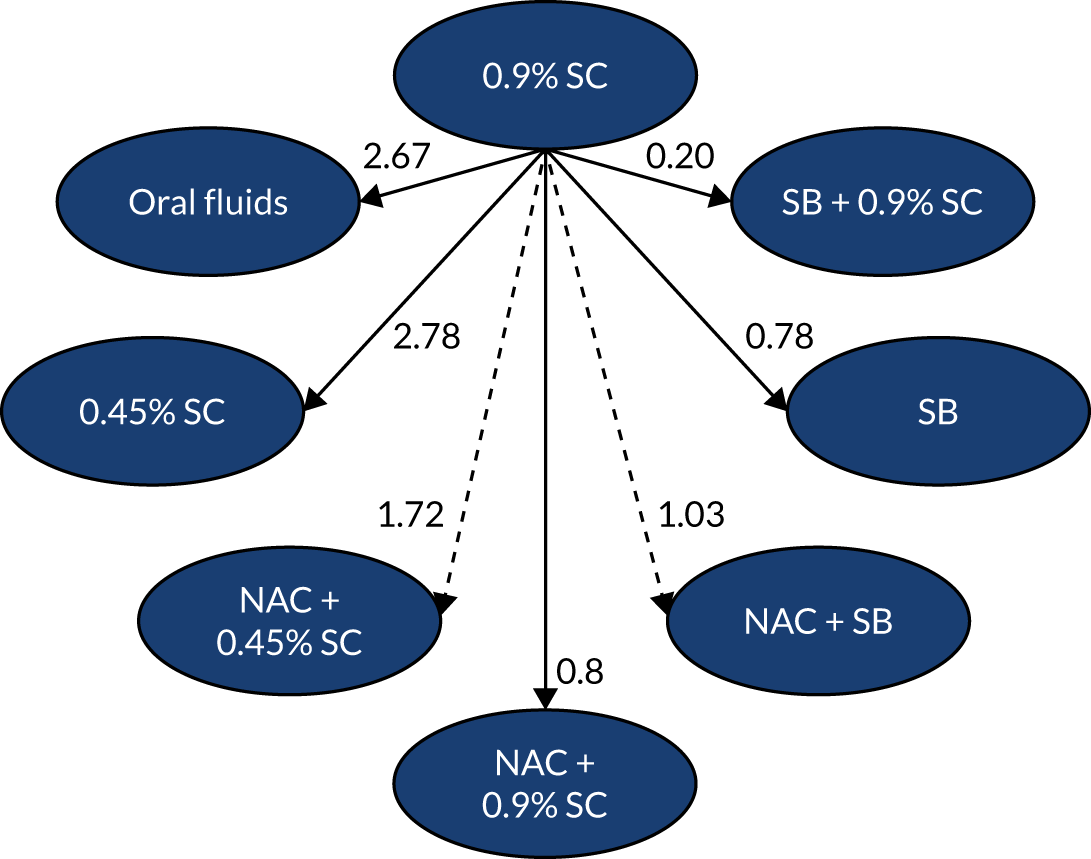
Health-related quality of life
Health state utility was informed by a literature review conducted by the authors. Estimates from a Japanese139 study reporting EQ-5D utility scores by CKD stage (i.e. 1–5) were applied to the UK general population utility estimate for the 65–74 years age bracket140 to yield health state utility estimates. The PC-AKI health state was estimated by multiplying the utility estimate for renal failure, from a UK-based catalogue of EQ-5D index scores,141 by the same general UK population utility estimate used to adjust the CKD states’ estimates.
Resource use and costs
The resource use and costs included in the model were the ones associated with the acquisition and administration of the hydration strategies, and health state costs.
The acquisition unit costs for the hydration strategies were sourced from published national sources, manufacturers’ price lists, and personal communications with the Commercial Medicines Unit of the UK Department of Health and Social Care. The resource use associated with infusion (and dose) was based on the hydration regimes that constituted each strategy, rather than the regimes on the trials informing treatment effectiveness. It was assumed that only infusions lasting > 8 hours would require hospitalisation. No administration costs were included for hydration strategies administered over a shorter period. The unit cost for infusions requiring hospitalisation was that of a coronary angiography excess bed-day from the National Schedule of Reference Costs 2011–2012. 142
Health state unit costs were sourced from national published sources: National Schedule of Reference Costs 2011–2012,142 Unit Costs of Health and Social Care 2012,143 the British National Formulary (BNF) 62144 and other NICE guidance. Resource use was based on assumptions informed by expert opinion. Tables 71–74 summarise health states resource use and costs.
| Category | Resource use | Source/details | Unit cost | Source | Cost per cycle |
|---|---|---|---|---|---|
| PC-AKI | 1 | Event in the model | £2013 | Weighted average of AKI-related HRG codes (LA07C-G) from the National Schedule of Reference Costs 2011–2012142 | £2013 |
The PC-AKI health state costs were estimated by pooling the average costs of all AKI-related HRG codes in the NHS reference costs weighted by their respective activity. The cost per cycle was £2013.
Costs in stage 3–4 CKD include specialist appointments, eGFR measurements, anaemia management with epoetin alpha and diuretics. The cost per cycle on this state was £176.
| Category | Resource use | Source/details | Unit cost | Source | Cost per cycle |
|---|---|---|---|---|---|
| Nephrologist appointment | 1 | Per cycle, assumption | £157 | National Schedule of Reference Costs 2011–2012 142 | £157 |
| eGFR measurement | |||||
| Biochemistry | 1 | Per cycle, assumption | £1.26 | National Schedule of Reference Costs 2011–2012 142 | £1.26 |
| Phlebotomist | 5 minutes | Per cycle, assumption | £3.42 | Unit Costs of Health and Social Care 2012, 2012143 | £3.42 |
| Drugs | |||||
| Diuretics | 40 mg/day | Assumed that 26% of patients were in stage 4 and, of these patients, around 60% would be treated with furosemide | £0.26 | BNF144 | £4 |
| Epoetin alpha | 1788 units/week | Applied to 9% of those patients who are assumed to require treatment for anaemia. The dose and proportion of patients were informed by previous NICE guidance | £0.0051 | BNF144 | £11 |
Patients in stage 5 CKD will incur costs associated with either RRT or conservative management (management without RRT). It was assumed that 90% of patients received RRT and 10% received conservative management. For patients on RRT the costs in stage 5 CKD differed for cycle 1 (£7252) and for cycle 2 onwards (£6284 per cycle), with higher resource use intensity in cycle 1 as a result of the need to perform access procedures for RRT before starting treatment. Access procedures are then assumed to be required once every 1–5 years. Patients on conservative management for CKD stage 5 also incur the costs of diuretic drugs and additional check-ups. The cost per cycle of conservative management was £642. Considering all patients (RRT and conservative management), the cost on the first cycle was £6585 and £5512 per subsequent cycle.
| Cycle | Category | Resource use | Source/details | Unit cost | Source | Cost per cycle |
|---|---|---|---|---|---|---|
| First cycle | ||||||
| Nephrologist appointment | 2 | Per cycle, assumption | £157 | National Schedule of Reference Costs 2011–2012 142 | £374 | |
| eGFR measurement | 12 | See Table 72 | £4.67 | See Table 72 | £56 | |
| Epoetin alpha | 1.788 units/week | Applied to 33% of patients who are assumed to require treatment for anaemia. The dose and proportion of patients informed by previous NICE guidance | £0.01 | BNF144 | £39 | |
| Access procedure | 1 | Assumption | £1323 | Pooled average of HRG codes for RRT access procedures from the National Schedule of Reference Costs 2011–2012142 | £1323 | |
| RRT |
3 haemodialysis sessions/week 7 peritoneal dialysis sessions/week |
Assumption Distribution of patients on peritoneal dialysis and haemodialysis (21% and 79% of patients on RRT, respectively) and frequency of sessions were informed by the Renal Registry report |
£157.76 haemodialysis £54.70 peritoneal dialysis |
Activity-weighted average of HRG codes for RRT procedures from the National Schedule of Reference Costs 2011–2012142 | £5460 | |
| After first cycle | ||||||
| Nephrologist appointment | 2 | Per cycle, assumption | £157 | National Schedule of Reference Costs 2011–2012 142 | £314 | |
| eGFR measurement | 12 | See Table 72 | £4.67 | See Table 72 | £56 | |
| Epoetin α | 1.788 units/week | Applied to 33% of patients who are assumed to require treatment for anaemia. The dose and proportion of patients informed by NICE CG114145 | £0.01 | BNF62144 | £39 | |
| Access procedure | 0.15 | Assumption | £1323 | Pooled average of HRG codes for RRT access procedures from the National Schedule of Reference Costs 2011–2012142 | £199 | |
| RRT | 3-weekly haemodialysis sessions or 7-weekly peritoneal dialysis sessions |
Assumption Distribution of patients on peritoneal dialysis and haemodialysis (21% and 79% of patients on RRT, respectively) and frequency of sessions were informed by the Renal Registry report |
£157.76 for haemodialysis £54.70 for peritoneal dialysis | Activity-weighted average of HRG codes for RRT procedures from the National Schedule of Reference Costs 2011–2012142 | £5460 | |
| Category | Resource use | Source/details | Unit cost | Source | Cost per cycle |
|---|---|---|---|---|---|
| Nephrologist appointment | 2 | Per cycle, assumption | £157 | National Schedule of Reference Costs 2011–2012 142 | £374 |
| Specialist nurse | |||||
| Telephone call | 12 | Per cycle, assumption | £5.30 | Unit Costs of Health and Social Care 2012 143 | £64 |
| Home visit | 3 | Per cycle, assumption | £22.08 | Unit Costs of Health and Social Care 2012 143 | £66 |
| eGFR measurement | 12 | See Table 72 | £4.67 | See Table 72 | £56 |
| Drugs | |||||
| Epoetin alpha | 1.788 units/week | Applied to 33% of patients who are assumed to require treatment for anaemia. The dose and the proportion of patients were informed by NICE CG114145 | £0.01 | BNF144 | £39 |
| Diuretics | 80 mg/day | Assumed that 90% of patients would be treated with furosemide | £0.26 | BNF144 | £43 |
Uncertainty
Joint parameter uncertainty was considered in the model by performing probabilistic sensitivity analysis. Probabilistic distributions were attributed to most parameters in the model, and random draws of these distributions were sampled over 1000 model simulations to yield probabilistic cost-effectiveness estimates.
The authors conducted an extensive number of scenario analyses testing assumptions around resource use associated with IVH, costs of PC-AKI, age in the model, baseline risk of PC-AKI, probability of repeat scans, treatment effect of hydration, health state utilities and discount rates.
Findings
Under base-case assumptions, the cost-effective strategy to prevent PC-AKI in patients with stage 3–4 CKD undergoing CT was considered to be IVH with 0.9% sodium chloride in addition to N-acetylcysteine (NAC), with a NMB of £47,957 at a threshold of £20,000 per additional QALY. This strategy also had the highest probability of cost-effectiveness (43%) at the same cost-effectiveness threshold. Sodium bicarbonate with 0.9% sodium chloride was the most effective strategy, generating 0.006 additional QALYs on average compared with 0.9% sodium chloride in addition to NAC. However, the additional QALYs did not offset the incremental costs when comparing these two strategies (£370).
The results were robust to the majority of the scenario analysis undertaken. The key drivers of the model were identified as the cost of admission for the IVH regimens requiring it and the treatment effectiveness estimates.
When it was assumed that all patients were inpatients and no additional costs of hospital admission were considered for IVH strategies administered over periods longer than 8 hours, the cost-effective strategy became sodium bicarbonate with 0.9% sodium chloride, with a NMB of £47,738 and 90% probability of cost-effectiveness at £20,000 per QALY gained. When it was assumed that strategies containing either 0.9% sodium chloride or sodium bicarbonate patients required a hospital admission, sodium bicarbonate with 0.9% sodium chloride was also the cost-effective strategy with a NMB of £47,304 and 70% probability of cost-effectiveness at £20,000 per QALY gained.
Applying the treatment effect for NAC plus sodium bicarbonate versus 0.9% sodium chloride estimated from an alternative indirect link in the treatment effectiveness meta-analysis (RR = 0.63 instead of 1.03), the cost-effective strategy was NAC plus sodium bicarbonate with a NMB of £47,670 and 48% probability of cost-effectiveness at £20,000 per QALY gained.
Limitations of the model in the context of our study
The model structure does not consider patients with normal kidney function and at earlier stages of renal disease (i.e. CKD stages 1 and 2), as these were not part of the study population. Therefore, the model would require substantial structural adaptations to include these patients.
The parameter estimates informed by evidence generated in the context of PCI and coronary angiography are unlikely to be directly generalisable to the study population, as the underlying risk of CI-AKI, severity of AKI and associated mortality are likely to be much higher for patients who:
-
undergo intra-arterial contrast administration
-
have more cardiovascular-related comorbidities that are also risk factors for AKI (e.g. diabetes mellitus) than would be expected to be observed in an outpatient population referred for intravenous contrast-enhanced CT.
Review of the Chicaíza-Becerra et al.129 publication
The authors used a decision tree model to evaluate the cost-effectiveness of iso- and low-osmolality contrast media for outpatients at high risk of PC-AKI from the perspective of the Colombian NHS. Costs were expressed as US dollars, 2009 price year, and health outcomes as life-years gained. The base-case results are presented for undiscounted costs and outcomes, as well as applying a 3% annual rate on both.
Model structure
The decision tree considers a lifetime horizon and is illustrated in Chicaíza-Becerra et al. 129
All patients undergo a procedure (not described) that requires administration of one of four possible contrast media alternatives: iohexol, iodixanol, iopamidol or other low-osmolality contrast media. The structure of the decision tree is the same for each of the four contrast agent options. The first chance node divides patients according to their probability of having PC-AKI (CIN in the original paper) after contrast administration. Patients who do not have PC-AKI can die or survive. Patients without PC-AKI may have to undergo dialysis or not. All patients who suffer a PC-AKI event have a PC-AKI-specific mortality risk at the last chance node. Surviving patients have the full life expectancy of the Colombian general population (i.e. 74 years).
Probabilities and treatment effects
The study population is described as outpatients at high risk of PC-AKI; however, the authors do not define what constitutes high risk in this context. The patients’ average age in the model is 63 years. Table 75 summarises the probability estimates in the model and sources of evidence.
| Probability | Point estimate | Source |
|---|---|---|
| Probability of PC-AKI | ||
| Iohexol | 0.21 |
Solomon, 2005146 Nguyen et al. , 2008147 Solomon and DuMouchel, 2006148 |
| Iodixanol | 0.09 | |
| Iodixanol | 0.1 | |
| Other low-osmolality media | 0.18 | |
| Mortality PC-AKI | 0.16 | From et al., 2008149 |
| Mortality no AKI | 0.05 | |
| Probability of dialysis (if PC-AKI) | 0.36 |
Klarenbach et al. , 2006150 Aguirre Caicedo, 2007151 |
| Probability of hospitalisation on a CCU | 0.29 | |
The authors do not state if dialysis is transient or permanent, or the period of time considered to estimate the probability of dialysis. As the risk of death is not conditional on dialysis, dialysis is likely to be transient. Furthermore, only 6 days of hospitalisation were considered for patients who initiate dialysis (see Chicaíza-Becerra et al. 129). The time period considered for the estimation of the probabilities of death is not described.
Health-related quality of life
Health-related quality of life was not considered in the model because of the lack of health utility estimates specific to Colombia at the time of the study. Effectiveness was measured in life-years gained, and the average life expectancy of the Colombian population was assumed for patients who survived in the model.
Resource use and costs
The study included the following elements of resource use and costs: direct costs related to contrast media, and the treatment of associated renal complications. The cost of prophylactic IVH was not included, as the same costs would apply to every strategy under comparison. The unit costs for contrast media were market prices, and the unit cost of health-care use for handling complications was taken from the Colombian national tariff set for medicines and health procedures. Table 76 summarises the resource use in the model. Unit costs were not extracted as it was not clear whether or not the costs reported in the study were unit costs.
| Category | Resource use | Source/details |
|---|---|---|
| Contrast media | ||
| Iopamidol | 17.5 ml |
Assumes 5 ml of contrast agent for each kilogram of a patient’s body weight divided by SCr level The average weight in the model is assumed to be 70 kg and the average SCr level 2 mg/dl |
| Iohexol | ||
| Other low osmolality | ||
| Iodixanol | ||
| Days of hospitalisation | ||
| Without nephropathy | 2 |
Klarenbach et al. , 2006150 Aguirre Caicedo, 2007151 |
| With nephropathy and no dialysis | 4 | |
| With nephropathy and dialysis | 6 | |
| Dialysis | 1 | |
| Placement of temporary venous catheter | 1 | Not clear to whom these costs apply in the model |
| Creatinine, BUN, electrolyte and blood gas analyses | 1 |
Uncertainty
The model considered joint parameter uncertainty by performing probabilistic sensitivity analysis. Probabilistic distributions were attributed to most parameters in the model, but no further details are provided on the probabilistic sensitivity analysis. The authors conducted univariate deterministic sensitivity analysis by varying most parameters within a range of values. The rationale for each range of values was not presented.
Findings
The iohexol and other low-osmolality contrast media were dominated by iopamidol and iodixanol in the base-case analysis. At a cost-effectiveness threshold of US$5356 per additional QALY (the Colombian threshold value), iopamidol was identified as the cost-effective option for the analyses applying a 0% and 3% annual discount rate on both costs and outcomes. Iopamidol was also the strategy most likely to be cost-effective at a willingness to pay ranging between US$0 and US$11,740.
The results were sensitive to variation in the risk of PC-AKI for iopamidol (if it became higher than 0.11, iodixanol would dominate all strategies), and to the costs of the contrast media. Iopamidol became less cost-effective when the price per 50-ml vial was higher than US$51 (base case US$26.6), whereas iodixanol became cost-effective when the price for a 50-ml vial was lower than US$28 (base case US$52.7).
Limitations of the model in the context of the study
Although the model structure is flexible enough to consider the full population of non-emergency outpatients presenting for a CT scan, the evidence sources informing the model are mostly informed by studies in patients at a higher risk of PC-AKI (and subsequent events). Furthermore, the assumptions on time frame for the occurrence of short-term events (i.e. death and dialysis) and for the duration of adverse outcomes (dialysis) are not explicitly stated. Finally, the model does not consider HRQoL.
Conclusion
The structure of the model described in Chicaíza-Becerra et al. 129 links PC-AKI to the relevant outcomes in terms of costs and HRQoL, and can easily be adapted to address the decision problem in our study. Although the model developed for CG169108,110 also allows us to establish this link, the additional evidence that is required to parameterise this more complex model is not available for the population of interest. The increased complexity of the CG169 model was necessary to capture the impact of PC-AKI in a specific population with pre-existing grade 3–4 CKD disease, but is less relevant in the context of this study. Furthermore, the model structure in CG169 does not consider patients with normal kidney function and at earlier stages of renal disease (i.e. CKD stage 1 or 2), and would, therefore, require substantial structural adaptation to reflect the population in our decision problem.
Appendix 10 Base-case analysis results
| Identification | Management | Total | Incremental | ICER (per QALY) | |||
|---|---|---|---|---|---|---|---|
| Costs | QALYs | Costs | QALYs | ||||
| 6 | RF + i-STAT + Lab | £275.84 | 9.99137100231 | – | – | – | |
| 8 | RF + StatSensor + Lab | £276.15 | 9.99137099733 | £0.31 | –0.000000005 | Dominated | |
| 4 | RF + StatSensor | £277.84 | 9.99137099733 | £1.99 | –0.000000005 | Dominated | |
| 2 | RF+ i-STAT | £278.02 | 9.99137100231 | £2.17 | 0.00000000000 | Dominated | |
| 14 | StatSensor + Lab | £279.09 | 9.99137099733 | £3.25 | –0.000000005 | Dominated | |
| 12 | i-STAT + Lab | £280.08 | 9.99137100231 | £4.23 | 0.00000000000 | Dominated | |
| 11 | StatSensor | £283.96 | 9.99137099733 | £8.12 | –0.00000000499 | Dominated | |
| 7 | RF + ABL800 FLEX + Lab | £284.39 | 9.99137100330 | £8.55 | 0.00000000099 | Extendedly dominated | |
| 3 | RF + ABL800 FLEX | £285.87 | 9.99137100330 | £10.03 | 0.00000000099 | Dominated | |
| 9 | i-STAT | £286.35 | 9.99137100231 | £10.51 | 0.00000000000 | Dominated | |
| 13 | ABL800 FLEX + Lab | £286.70 | 9.99137100330 | £10.86 | 0.00000000099 | Dominated | |
| 10 | ABL800 FLEX | £290.99 | 9.99137100330 | £15.14 | 0.00000000099 | Dominated | |
| 5 | RF + Lab | £304.06 | 9.99137101011 | £28.22 | 0.00000000779 | £3,620,669,780 | |
| 1 | Lab | £363.26 | 9.99137101011 | £87.42 | 0.00000000779 | Dominated | |
Appendix 11 Scenario analyses results
| Identification | Management | Total | At £20,000 per QALY | NB rank | |||||
|---|---|---|---|---|---|---|---|---|---|
| Costs | QALYs | NHB (QALYs) | NMB | INHB (QALYs) | INMB | ||||
| 1 | Lab | £363.26 | 9.991371010 | 9.97321 | £199,464.16 | 0.00000 | £0.00 | 14 | |
| 2 | RF + i-STAT | £278.02 | 9.991371002 | 9.97747 | £199,549.40 | 0.00426 | £85.25 | 3 | |
| 3 | RF + ABL800 FLEX | £285.87 | 9.991371003 | 9.97708 | £199,541.55 | 0.00387 | £77.39 | 9 | |
| 4 | RF + StatSensor | £278.51 | 9.991371002 | 9.97745 | £199,548.91 | 0.00424 | £84.75 | 4 | |
| 5 | RF + Lab | £304.06 | 9.991371010 | 9.97617 | £199,523.36 | 0.00296 | £59.20 | 13 | |
| 6 | RF + i-STAT + Lab | £275.84 | 9.991371002 | 9.97758 | £199,551.58 | 0.00437 | £87.42 | 1 | |
| 7 | RF + ABL800 FLEX + Lab | £284.39 | 9.991371003 | 9.97715 | £199,543.03 | 0.00394 | £78.87 | 7 | |
| 8 | RF + StatSensor + Lab | £276.61 | 9.991371002 | 9.97754 | £199,550.81 | 0.00433 | £86.66 | 2 | |
| 9 | i-STAT | £286.35 | 9.991371002 | 9.97705 | £199,541.07 | 0.00385 | £76.91 | 10 | |
| 10 | ABL800 FLEX | £290.99 | 9.991371003 | 9.97682 | £199,536.43 | 0.00361 | £72.28 | 12 | |
| 11 | StatSensor | £285.13 | 9.991371002 | 9.97711 | £199,542.29 | 0.00391 | £78.13 | 8 | |
| 12 | i-STAT + Lab | £280.08 | 9.991371002 | 9.97737 | £199,547.34 | 0.00416 | £83.18 | 6 | |
| 13 | ABL800 FLEX + Lab | £286.70 | 9.991371003 | 9.97704 | £199,540.72 | 0.00383 | £76.56 | 11 | |
| 14 | StatSensor + Lab | £279.62 | 9.991371002 | 9.97739 | £199,547.80 | 0.00418 | £83.65 | 5 | |
| Identification | Management | Total | At £20,000 per QALY | NB rank | |||||
|---|---|---|---|---|---|---|---|---|---|
| Costs | NMB | NHB (QALYs) | NMB | INHB (QALYs) | INMB | ||||
| 1 | Lab | £363.26 | 9.991371010 | 9.97321 | £199,464.16 | 0.00000 | £0.00 | 14 | |
| 2 | RF + i-STAT | £277.73 | 9.991371001 | 9.97748 | £199,549.69 | 0.00428 | £85.54 | 3 | |
| 3 | RF + ABL800 FLEX | £286.05 | 9.991371001 | 9.97707 | £199,541.37 | 0.00386 | £77.21 | 9 | |
| 4 | RF + StatSensor | £278.67 | 9.991370989 | 9.97744 | £199,548.75 | 0.00423 | £84.60 | 4 | |
| 5 | RF + Lab | £304.06 | 9.991371010 | 9.97617 | £199,523.36 | 0.00296 | £59.20 | 13 | |
| 6 | RF + i-STAT + Lab | £275.72 | 9.991371001 | 9.97758 | £199,551.70 | 0.00438 | £87.54 | 2 | |
| 7 | RF + ABL800 FLEX + Lab | £284.26 | 9.991371001 | 9.97716 | £199,543.16 | 0.00395 | £79.01 | 7 | |
| 8 | RF + StatSensor + Lab | £275.68 | 9.991370989 | 9.97759 | £199,551.74 | 0.00438 | £87.59 | 1 | |
| 9 | i-STAT | £285.70 | 9.991371001 | 9.97709 | £199,541.72 | 0.00388 | £77.57 | 8 | |
| 10 | ABL800 FLEX | £291.85 | 9.991371001 | 9.97678 | £199,535.57 | 0.00357 | £71.41 | 12 | |
| 11 | StatSensor | £287.65 | 9.991370989 | 9.97699 | £199,539.77 | 0.00378 | £75.61 | 11 | |
| 12 | i-STAT + Lab | £279.90 | 9.991371001 | 9.97738 | £199,547.52 | 0.00417 | £83.36 | 6 | |
| 13 | ABL800 FLEX + Lab | £286.67 | 9.991371001 | 9.97704 | £199,540.75 | 0.00383 | £76.59 | 10 | |
| 14 | StatSensor + Lab | £279.03 | 9.991370989 | 9.97742 | £199,548.39 | 0.00421 | £84.23 | 5 | |
| Identification | Management | Total | At £20,000 per QALY | NB rank | |||||
|---|---|---|---|---|---|---|---|---|---|
| Costs | QALYs | NHB (QALYs) | NMB | INHB (QALYs) | INMB | ||||
| 1 | Lab | £363.26 | 9.991371010 | 9.97321 | £199,464.16 | 0.00000 | £0.00 | 14 | |
| 2 | RF+ i-STAT | £280.52 | 9.991370997 | 9.97734 | £199,546.90 | 0.00414 | £82.74 | 6 | |
| 3 | RF + ABL800 FLEX | £287.38 | 9.991370998 | 9.97700 | £199,540.04 | 0.00379 | £75.89 | 11 | |
| 4 | RF + StatSensor | £279.71 | 9.991370993 | 9.97739 | £199,547.71 | 0.00418 | £83.55 | 4 | |
| 5 | RF + Lab | £322.14 | 9.991371004 | 9.97526 | £199,505.28 | 0.00206 | £41.12 | 13 | |
| 6 | RF + i-STAT + Lab | £277.09 | 9.991370997 | 9.97752 | £199,550.33 | 0.00431 | £86.18 | 2 | |
| 7 | RF + ABL800 FLEX + Lab | £285.03 | 9.991370998 | 9.97712 | £199,542.39 | 0.00391 | £78.23 | 8 | |
| 8 | RF + StatSensor + Lab | £277.05 | 9.991370993 | 9.97752 | £199,550.37 | 0.00431 | £86.22 | 1 | |
| 9 | i-STAT | £286.35 | 9.991371002 | 9.97705 | £199,541.07 | 0.00385 | £76.91 | 9 | |
| 10 | ABL800 FLEX | £290.99 | 9.991371003 | 9.97682 | £199,536.43 | 0.00361 | £72.28 | 12 | |
| 11 | StatSensor | £283.96 | 9.991370997 | 9.97717 | £199,543.46 | 0.00396 | £79.30 | 7 | |
| 12 | i-STAT + Lab | £280.08 | 9.991371002 | 9.97737 | £199,547.34 | 0.00416 | £83.18 | 5 | |
| 13 | ABL800 FLEX + Lab | £286.70 | 9.991371003 | 9.97704 | £199,540.72 | 0.00383 | £76.56 | 10 | |
| 14 | StatSensor + Lab | £279.09 | 9.991370997 | 9.97742 | £199,548.33 | 0.00421 | £84.17 | 3 | |
| Identification | Management | Total | At £20,000 per QALY | NB rank | |||||
|---|---|---|---|---|---|---|---|---|---|
| Costs | QALYs | NHB (QALYs) | NMB | INHB (QALYs) | INMB | ||||
| 1 | Lab | £361.06 | 9.991371782 | 9.97332 | £199,466.38 | 0.00000 | £0.00 | 14 | |
| 2 | RF+ i-STAT | £275.59 | 9.991371778 | 9.97759 | £199,551.85 | 0.00427 | £85.47 | 3 | |
| 3 | RF + ABL800 FLEX | £283.62 | 9.991371779 | 9.97719 | £199,543.81 | 0.00387 | £77.44 | 10 | |
| 4 | RF + StatSensor | £275.65 | 9.991371776 | 9.97759 | £199,551.78 | 0.00427 | £85.41 | 4 | |
| 5 | RF + Lab | £301.65 | 9.991371782 | 9.97629 | £199,525.78 | 0.00297 | £59.41 | 13 | |
| 6 | RF + i-STAT + Lab | £273.62 | 9.991371778 | 9.97769 | £199,553.82 | 0.00437 | £87.44 | 1 | |
| 7 | RF + ABL800 FLEX + Lab | £282.16 | 9.991371779 | 9.97726 | £199,545.28 | 0.00395 | £78.90 | 8 | |
| 8 | RF + StatSensor + Lab | £274.16 | 9.991371776 | 9.97766 | £199,553.27 | 0.00434 | £86.90 | 2 | |
| 9 | i-STAT | £283.47 | 9.991371778 | 9.97720 | £199,543.96 | 0.00388 | £77.59 | 9 | |
| 10 | ABL800 FLEX | £288.69 | 9.991371779 | 9.97694 | £199,538.74 | 0.00362 | £72.37 | 12 | |
| 11 | StatSensor | £281.34 | 9.991371776 | 9.97730 | £199,546.10 | 0.00399 | £79.72 | 7 | |
| 12 | i-STAT + Lab | £277.80 | 9.991371778 | 9.97748 | £199,549.63 | 0.00416 | £83.26 | 6 | |
| 13 | ABL800 FLEX + Lab | £284.47 | 9.991371779 | 9.97715 | £199,542.96 | 0.00383 | £76.59 | 11 | |
| 14 | StatSensor + Lab | £277.05 | 9.991371776 | 9.97752 | £199,550.39 | 0.00420 | £84.01 | 5 | |
| Identification | Management | Total | At £20,000 per QALY | NB rank | |||||
|---|---|---|---|---|---|---|---|---|---|
| Costs | QALYs | NHB (QALYs) | NMB | INHB (QALYs) | INMB | ||||
| 1 | Lab | £460.78 | 9.991336844 | 9.96830 | £199,365.95 | 0.00000 | £0.00 | 14 | |
| 2 | RF+ i-STAT | £374.98 | 9.991336644 | 9.97259 | £199,451.76 | 0.00429 | £85.80 | 6 | |
| 3 | RF + ABL800 FLEX | £384.19 | 9.991336669 | 9.97213 | £199,442.54 | 0.00383 | £76.59 | 10 | |
| 4 | RF + StatSensor | £364.55 | 9.991336515 | 9.97311 | £199,462.18 | 0.00481 | £96.23 | 2 | |
| 5 | RF + Lab | £410.83 | 9.991336844 | 9.97080 | £199,415.90 | 0.00250 | £49.95 | 13 | |
| 6 | RF + i-STAT + Lab | £372.81 | 9.991336644 | 9.97270 | £199,453.93 | 0.00440 | £87.97 | 5 | |
| 7 | RF + ABL800 FLEX + Lab | £383.05 | 9.991336669 | 9.97218 | £199,443.69 | 0.00389 | £77.73 | 8 | |
| 8 | RF + StatSensor + Lab | £362.79 | 9.991336515 | 9.97320 | £199,463.94 | 0.00490 | £97.98 | 1 | |
| 9 | i-STAT | £383.53 | 9.991336644 | 9.97216 | £199,443.20 | 0.00386 | £77.25 | 9 | |
| 10 | ABL800 FLEX | £389.08 | 9.991336669 | 9.97188 | £199,437.66 | 0.00359 | £71.70 | 12 | |
| 11 | StatSensor | £371.11 | 9.991336515 | 9.97278 | £199,455.62 | 0.00448 | £89.67 | 4 | |
| 12 | i-STAT + Lab | £376.46 | 9.991336644 | 9.97251 | £199,450.27 | 0.00422 | £84.32 | 7 | |
| 13 | ABL800 FLEX + Lab | £384.94 | 9.991336669 | 9.97209 | £199,441.79 | 0.00379 | £75.84 | 11 | |
| 14 | StatSensor + Lab | £365.34 | 9.991336515 | 9.97307 | £199,461.39 | 0.00477 | £95.44 | 3 | |
| Identification | Management | Total | At £20,000 per QALY | NB rank | |||||
|---|---|---|---|---|---|---|---|---|---|
| Costs | QALYs | NHB (QALYs) | NMB | INHB (QALYs) | INMB | ||||
| 1 | Lab | £361.06 | 9.991371782 | 9.97332 | £199,466.38 | 0.00000 | £0.00 | 14 | |
| 2 | RF+ i-STAT | £278.41 | 9.991371778 | 9.97745 | £199,549.02 | 0.00413 | £82.65 | 2 | |
| 3 | RF + ABL800 FLEX | £302.33 | 9.991371779 | 9.97626 | £199,525.11 | 0.00294 | £58.73 | 11 | |
| 4 | RF + StatSensor | £280.85 | 9.991371776 | 9.97733 | £199,546.58 | 0.00401 | £80.21 | 5 | |
| 5 | RF + Lab | £301.65 | 9.991371782 | 9.97629 | £199,525.78 | 0.00297 | £59.41 | 10 | |
| 6 | RF + i-STAT + Lab | £276.44 | 9.991371778 | 9.97755 | £199,550.99 | 0.00423 | £84.62 | 1 | |
| 7 | RF + ABL800 FLEX + Lab | £300.87 | 9.991371779 | 9.97633 | £199,526.57 | 0.00301 | £60.19 | 9 | |
| 8 | RF + StatSensor + Lab | £279.36 | 9.991371776 | 9.97740 | £199,548.07 | 0.00408 | £81.70 | 3 | |
| 9 | i-STAT | £286.30 | 9.991371778 | 9.97706 | £199,541.14 | 0.00374 | £74.76 | 7 | |
| 10 | ABL800 FLEX | £307.40 | 9.991371779 | 9.97600 | £199,520.04 | 0.00268 | £53.66 | 13 | |
| 11 | StatSensor | £286.54 | 9.991371776 | 9.97704 | £199,540.90 | 0.00373 | £74.52 | 8 | |
| 12 | i-STAT + Lab | £280.62 | 9.991371778 | 9.97734 | £199,546.81 | 0.00402 | £80.44 | 4 | |
| 13 | ABL800 FLEX + Lab | £303.18 | 9.991371779 | 9.97621 | £199,524.25 | 0.00289 | £57.88 | 12 | |
| 14 | StatSensor + Lab | £282.25 | 9.991371776 | 9.97726 | £199,545.19 | 0.00394 | £78.81 | 6 | |
| Identification | Management | Total | At £20,000 per QALY | NB rank | |||||
|---|---|---|---|---|---|---|---|---|---|
| Costs | QALYs | NHB (QALYs) | NMB | INHB (QALYs) | INMB | ||||
| 1 | Lab | £363.26 | 9.991371010 | 9.97321 | £199,464.16 | 0.00000 | £0.00 | 14 | |
| 2 | RF+ i-STAT | £279.71 | 9.991371002 | 9.97739 | £199,547.71 | 0.00418 | £83.56 | 3 | |
| 3 | RF + ABL800 FLEX | £297.07 | 9.991371003 | 9.97652 | £199,530.35 | 0.00331 | £66.20 | 10 | |
| 4 | RF + StatSensor | £280.95 | 9.991370997 | 9.97732 | £199,546.47 | 0.00412 | £82.31 | 4 | |
| 5 | RF + Lab | £304.06 | 9.991371010 | 9.97617 | £199,523.36 | 0.00296 | £59.20 | 13 | |
| 6 | RF + i-STAT + Lab | £277.53 | 9.991371002 | 9.97749 | £199,549.89 | 0.00429 | £85.73 | 1 | |
| 7 | RF + ABL800 FLEX + Lab | £295.59 | 9.991371003 | 9.97659 | £199,531.83 | 0.00338 | £67.67 | 9 | |
| 8 | RF + StatSensor + Lab | £279.27 | 9.991370997 | 9.97741 | £199,548.15 | 0.00420 | £84.00 | 2 | |
| 9 | i-STAT | £288.04 | 9.991371002 | 9.97697 | £199,539.38 | 0.00376 | £75.22 | 8 | |
| 10 | ABL800 FLEX | £302.18 | 9.991371003 | 9.97626 | £199,525.24 | 0.00305 | £61.08 | 12 | |
| 11 | StatSensor | £287.07 | 9.991370997 | 9.97702 | £199,540.35 | 0.00381 | £76.19 | 7 | |
| 12 | i-STAT + Lab | £281.77 | 9.991371002 | 9.97728 | £199,545.65 | 0.00407 | £81.49 | 5 | |
| 13 | ABL800 FLEX + Lab | £297.90 | 9.991371003 | 9.97648 | £199,529.52 | 0.00327 | £65.36 | 11 | |
| 14 | StatSensor + Lab | £282.21 | 9.991370997 | 9.97726 | £199,545.21 | 0.00405 | £81.06 | 6 | |
| Identification | Management | Total | At £20,000 per QALY | NB rank | |||||
|---|---|---|---|---|---|---|---|---|---|
| Costs | QALYs | NHB (QALYs) | NMB | INHB (QALYs) | INMB | ||||
| 1 | Lab | £363.26 | 9.991371010 | 9.97321 | £199,464.16 | 0.00000 | £0.00 | 14 | |
| 2 | RF+ i-STAT | £277.45 | 9.991371002 | 9.97750 | £199,549.97 | 0.00429 | £85.81 | 4 | |
| 3 | RF + ABL800 FLEX | £282.14 | 9.991371003 | 9.97726 | £199,545.28 | 0.00406 | £81.12 | 8 | |
| 4 | RF + StatSensor | £276.80 | 9.991370997 | 9.97753 | £199,550.62 | 0.00432 | £86.46 | 3 | |
| 5 | RF + Lab | £304.06 | 9.991371010 | 9.97617 | £199,523.36 | 0.00296 | £59.20 | 13 | |
| 6 | RF + i-STAT + Lab | £275.28 | 9.991371002 | 9.97761 | £199,552.14 | 0.00440 | £87.98 | 2 | |
| 7 | RF + ABL800 FLEX + Lab | £280.66 | 9.991371003 | 9.97734 | £199,546.76 | 0.00413 | £82.60 | 7 | |
| 8 | RF + StatSensor + Lab | £275.12 | 9.991370997 | 9.97762 | £199,552.30 | 0.00441 | £88.14 | 1 | |
| 9 | i-STAT | £285.79 | 9.991371002 | 9.97708 | £199,541.63 | 0.00387 | £77.47 | 11 | |
| 10 | ABL800 FLEX | £287.25 | 9.991371003 | 9.97701 | £199,540.17 | 0.00380 | £76.01 | 12 | |
| 11 | StatSensor | £282.93 | 9.991370997 | 9.97722 | £199,544.49 | 0.00402 | £80.34 | 9 | |
| 12 | i-STAT + Lab | £279.52 | 9.991371002 | 9.97740 | £199,547.90 | 0.00419 | £83.75 | 6 | |
| 13 | ABL800 FLEX + Lab | £282.97 | 9.991371003 | 9.97722 | £199,544.45 | 0.00401 | £80.29 | 10 | |
| 14 | StatSensor + Lab | £278.06 | 9.991370997 | 9.97747 | £199,549.36 | 0.00426 | £85.21 | 5 | |
| Identification | Management | Total | At £20,000 per QALY | NB rank | |||||
|---|---|---|---|---|---|---|---|---|---|
| Costs | QALYs | NHB (QALYs) | NMB | INHB (QALYs) | INMB | ||||
| 1 | Lab | £273.51 | 9.991371010 | 9.97770 | £199,553.91 | 0.00000 | £0.00 | 2 | |
| 2 | RF+ i-STAT | £277.20 | 9.991371002 | 9.97751 | £199,550.22 | –0.00018 | –£3.69 | 6 | |
| 3 | RF + ABL800 FLEX | £285.15 | 9.991371003 | 9.97711 | £199,542.27 | –0.00058 | –£11.64 | 12 | |
| 4 | RF + StatSensor | £277.16 | 9.991370997 | 9.97751 | £199,550.26 | –0.00018 | –£3.64 | 5 | |
| 5 | RF + Lab | £272.48 | 9.991371010 | 9.97775 | £199,554.94 | 0.00005 | £1.03 | 1 | |
| 6 | RF + i-STAT + Lab | £275.03 | 9.991371002 | 9.97762 | £199,552.39 | –0.00008 | –£1.51 | 3 | |
| 7 | RF + ABL800 FLEX + Lab | £283.67 | 9.991371003 | 9.97719 | £199,543.75 | –0.00051 | –£10.16 | 10 | |
| 8 | RF + StatSensor + Lab | £275.47 | 9.991370997 | 9.97760 | £199,551.95 | –0.00010 | –£1.96 | 4 | |
| 9 | i-STAT | £284.87 | 9.991371002 | 9.97713 | £199,542.55 | –0.00057 | –£11.36 | 11 | |
| 10 | ABL800 FLEX | £289.82 | 9.991371003 | 9.97688 | £199,537.60 | –0.00082 | –£16.30 | 14 | |
| 11 | StatSensor | £282.77 | 9.991370997 | 9.97723 | £199,544.65 | –0.00046 | –£9.25 | 9 | |
| 12 | i-STAT + Lab | £278.60 | 9.991371002 | 9.97744 | £199,548.82 | –0.00025 | –£5.09 | 8 | |
| 13 | ABL800 FLEX + Lab | £285.54 | 9.991371003 | 9.97709 | £199,541.88 | –0.00060 | –£12.02 | 13 | |
| 14 | StatSensor + Lab | £277.90 | 9.991370997 | 9.97748 | £199,549.52 | –0.00022 | –£4.39 | 7 | |
| Identification | Management | Total | At £20,000 per QALY | NB rank | |||||
|---|---|---|---|---|---|---|---|---|---|
| Costs | QALYs | NHB (QALYs) | NMB | INHB (QALYs) | INMB | ||||
| 1 | Lab | £295.95 | 9.991371010 | 9.97657 | £199,531.47 | 0.00000 | £0.00 | 14 | |
| 2 | RF+ i-STAT | £277.40 | 9.991371002 | 9.97750 | £199,550.02 | 0.00093 | £18.55 | 4 | |
| 3 | RF + ABL800 FLEX | £285.33 | 9.991371003 | 9.97710 | £199,542.09 | 0.00053 | £10.62 | 11 | |
| 4 | RF + StatSensor | £277.33 | 9.991370997 | 9.97750 | £199,550.09 | 0.00093 | £18.62 | 3 | |
| 5 | RF + Lab | £280.37 | 9.991371010 | 9.97735 | £199,547.05 | 0.00078 | £15.58 | 7 | |
| 6 | RF + i-STAT + Lab | £275.23 | 9.991371002 | 9.97761 | £199,552.19 | 0.00104 | £20.72 | 1 | |
| 7 | RF + ABL800 FLEX + Lab | £283.85 | 9.991371003 | 9.97718 | £199,543.57 | 0.00060 | £12.10 | 9 | |
| 8 | RF + StatSensor + Lab | £275.64 | 9.991370997 | 9.97759 | £199,551.78 | 0.00102 | £20.31 | 2 | |
| 9 | i-STAT | £285.24 | 9.991371002 | 9.97711 | £199,542.18 | 0.00054 | £10.71 | 10 | |
| 10 | ABL800 FLEX | £290.11 | 9.991371003 | 9.97687 | £199,537.31 | 0.00029 | £5.84 | 13 | |
| 11 | StatSensor | £283.07 | 9.991370997 | 9.97722 | £199,544.35 | 0.00064 | £12.88 | 8 | |
| 12 | i-STAT + Lab | £278.97 | 9.991371002 | 9.97742 | £199,548.45 | 0.00085 | £16.98 | 6 | |
| 13 | ABL800 FLEX + Lab | £285.83 | 9.991371003 | 9.97708 | £199,541.59 | 0.00051 | £10.12 | 12 | |
| 14 | StatSensor + Lab | £278.20 | 9.991370997 | 9.97746 | £199,549.22 | 0.00089 | £17.75 | 5 | |
| Identification | Management | Total | At £20,000 per QALY | NB rank | |||||
|---|---|---|---|---|---|---|---|---|---|
| Costs | QALYs | NHB (QALYs) | NMB | INHB (QALYs) | INMB | ||||
| 1 | Lab | £318.39 | 9.991371010 | 9.97545 | £199,509.03 | 0.00000 | £0.00 | 14 | |
| 2 | RF+ i-STAT | £277.61 | 9.991371002 | 9.97749 | £199,549.81 | 0.00204 | £40.78 | 4 | |
| 3 | RF + ABL800 FLEX | £285.51 | 9.991371003 | 9.97710 | £199,541.91 | 0.00164 | £32.88 | 9 | |
| 4 | RF + StatSensor | £277.50 | 9.991370997 | 9.97750 | £199,549.92 | 0.00204 | £40.89 | 3 | |
| 5 | RF + Lab | £288.27 | 9.991371010 | 9.97696 | £199,539.15 | 0.00151 | £30.12 | 12 | |
| 6 | RF + i-STAT + Lab | £275.44 | 9.991371002 | 9.97760 | £199,551.98 | 0.00215 | £42.95 | 1 | |
| 7 | RF + ABL800 FLEX + Lab | £284.03 | 9.991371003 | 9.97717 | £199,543.39 | 0.00172 | £34.35 | 8 | |
| 8 | RF + StatSensor + Lab | £275.81 | 9.991370997 | 9.97758 | £199,551.61 | 0.00213 | £42.57 | 2 | |
| 9 | i-STAT | £285.61 | 9.991371002 | 9.97709 | £199,541.81 | 0.00164 | £32.77 | 10 | |
| 10 | ABL800 FLEX | £290.40 | 9.991371003 | 9.97685 | £199,537.02 | 0.00140 | £27.99 | 13 | |
| 11 | StatSensor | £283.36 | 9.991370997 | 9.97720 | £199,544.06 | 0.00175 | £35.02 | 7 | |
| 12 | i-STAT + Lab | £279.34 | 9.991371002 | 9.97740 | £199,548.08 | 0.00195 | £39.05 | 6 | |
| 13 | ABL800 FLEX + Lab | £286.12 | 9.991371003 | 9.97706 | £199,541.30 | 0.00161 | £32.27 | 11 | |
| 14 | StatSensor + Lab | £278.50 | 9.991370997 | 9.97745 | £199,548.92 | 0.00199 | £39.89 | 5 | |
| Identification | Management | Total | At £20,000 per QALY | NB rank | |||||
|---|---|---|---|---|---|---|---|---|---|
| Costs | QALYs | NHB (QALYs) | NMB | INHB (QALYs) | INMB | ||||
| 1 | Lab | £340.83 | 9.991371010 | 9.97433 | £199,486.60 | 0.00000 | £0.00 | 14 | |
| 2 | RF+ i-STAT | £277.81 | 9.991371002 | 9.97748 | £199,549.61 | 0.00315 | £63.01 | 4 | |
| 3 | RF + ABL800 FLEX | £285.69 | 9.991371003 | 9.97709 | £199,541.73 | 0.00276 | £55.13 | 9 | |
| 4 | RF + StatSensor | £277.67 | 9.991370997 | 9.97749 | £199,549.75 | 0.00316 | £63.16 | 3 | |
| 5 | RF + Lab | £296.17 | 9.991371010 | 9.97656 | £199,531.25 | 0.00223 | £44.66 | 13 | |
| 6 | RF + i-STAT + Lab | £275.64 | 9.991371002 | 9.97759 | £199,551.78 | 0.00326 | £65.18 | 1 | |
| 7 | RF + ABL800 FLEX + Lab | £284.21 | 9.991371003 | 9.97716 | £199,543.21 | 0.00283 | £56.61 | 8 | |
| 8 | RF + StatSensor + Lab | £275.98 | 9.991370997 | 9.97757 | £199,551.44 | 0.00324 | £64.84 | 2 | |
| 9 | i-STAT | £285.98 | 9.991371002 | 9.97707 | £199,541.44 | 0.00274 | £54.84 | 10 | |
| 10 | ABL800 FLEX | £290.69 | 9.991371003 | 9.97684 | £199,536.73 | 0.00251 | £50.13 | 12 | |
| 11 | StatSensor | £283.66 | 9.991370997 | 9.97719 | £199,543.76 | 0.00286 | £57.16 | 7 | |
| 12 | i-STAT + Lab | £279.71 | 9.991371002 | 9.97739 | £199,547.71 | 0.00306 | £61.12 | 6 | |
| 13 | ABL800 FLEX + Lab | £286.41 | 9.991371003 | 9.97705 | £199,541.01 | 0.00272 | £54.41 | 11 | |
| 14 | StatSensor + Lab | £278.79 | 9.991370997 | 9.97743 | £199,548.63 | 0.00310 | £62.03 | 5 | |
| Identification | Management | Total | At £20,000 per QALY | NB rank | |||||
|---|---|---|---|---|---|---|---|---|---|
| Costs | QALYs | NHB (QALYs) | NMB | INHB (QALYs) | INMB | ||||
| 1 | Lab | £363.26 | 9.982325151 | 9.96416 | £199,283.24 | 0.00000 | £0.00 | 14 | |
| 2 | RF+ i-STAT | £278.02 | 9.991288549 | 9.97739 | £199,547.75 | 0.01323 | £264.51 | 4 | |
| 3 | RF + ABL800 FLEX | £285.87 | 9.991298687 | 9.97701 | £199,540.10 | 0.01284 | £256.86 | 9 | |
| 4 | RF + StatSensor | £277.84 | 9.991302192 | 9.97741 | £199,548.20 | 0.01325 | £264.96 | 3 | |
| 5 | RF + Lab | £304.06 | 9.988187660 | 9.97298 | £199,459.69 | 0.00882 | £176.45 | 13 | |
| 6 | RF + i-STAT + Lab | £275.84 | 9.991288549 | 9.97750 | £199,549.93 | 0.01333 | £266.69 | 1 | |
| 7 | RF + ABL800 FLEX + Lab | £284.39 | 9.991298687 | 9.97708 | £199,541.58 | 0.01292 | £258.34 | 7 | |
| 8 | RF + StatSensor + Lab | £276.15 | 9.991302192 | 9.97749 | £199,549.89 | 0.01333 | £266.65 | 2 | |
| 9 | i-STAT | £286.35 | 9.991221895 | 9.97690 | £199,538.08 | 0.01274 | £254.84 | 11 | |
| 10 | ABL800 FLEX | £290.99 | 9.991253148 | 9.97670 | £199,534.08 | 0.01254 | £250.84 | 12 | |
| 11 | StatSensor | £283.96 | 9.991250450 | 9.97705 | £199,541.05 | 0.01289 | £257.81 | 8 | |
| 12 | i-STAT + Lab | £280.08 | 9.991221895 | 9.97722 | £199,544.36 | 0.01306 | £261.12 | 6 | |
| 13 | ABL800 FLEX + Lab | £286.70 | 9.991253148 | 9.97692 | £199,538.36 | 0.01276 | £255.12 | 10 | |
| 14 | StatSensor + Lab | £279.09 | 9.991250450 | 9.97730 | £199,545.92 | 0.01313 | £262.67 | 5 | |
| Strategy | Identification | Management | Total | At £20,000 per QALY | |||||
|---|---|---|---|---|---|---|---|---|---|
| Costs | QALYs | NHB (QALYs) | NMB | INHB (QALYs) | INMB | ||||
| 1 | Lab | £363.26 | 9.991371010 | 9.97321 | £199,464.16 | 0.00000 | £0.00 | 14 | |
| 2 | RF+ i-STAT | £278.09 | 9.991370798 | 9.97747 | £199,549.33 | 0.00426 | £85.17 | 4 | |
| 3 | RF + ABL800 FLEX | £285.93 | 9.991370825 | 9.97707 | £199,541.48 | 0.00387 | £77.33 | 9 | |
| 4 | RF + StatSensor | £277.96 | 9.991370662 | 9.97747 | £199,549.46 | 0.00426 | £85.30 | 3 | |
| 5 | RF + Lab | £304.06 | 9.991371010 | 9.97617 | £199,523.36 | 0.00296 | £59.20 | 13 | |
| 6 | RF + i-STAT + Lab | £275.92 | 9.991370798 | 9.97757 | £199,551.50 | 0.00437 | £87.34 | 1 | |
| 7 | RF + ABL800 FLEX + Lab | £284.46 | 9.991370825 | 9.97715 | £199,542.96 | 0.00394 | £78.80 | 8 | |
| 8 | RF + StatSensor + Lab | £276.27 | 9.991370662 | 9.97756 | £199,551.14 | 0.00435 | £86.98 | 2 | |
| 9 | i-STAT | £286.43 | 9.991370798 | 9.97705 | £199,540.99 | 0.00384 | £76.83 | 10 | |
| 10 | ABL800 FLEX | £291.05 | 9.991370825 | 9.97682 | £199,536.37 | 0.00361 | £72.21 | 12 | |
| 11 | StatSensor | £284.08 | 9.991370662 | 9.97717 | £199,543.33 | 0.00396 | £79.17 | 7 | |
| 12 | i-STAT + Lab | £280.15 | 9.991370798 | 9.97736 | £199,547.26 | 0.00416 | £83.11 | 6 | |
| 13 | ABL800 FLEX + Lab | £286.77 | 9.991370825 | 9.97703 | £199,540.65 | 0.00382 | £76.49 | 11 | |
| 14 | StatSensor + Lab | £279.21 | 9.991370662 | 9.97741 | £199,548.20 | 0.00420 | £84.04 | 5 | |
| Identification | Management | Total | At £20,000 per QALY | NB rank | |||||
|---|---|---|---|---|---|---|---|---|---|
| Costs | QALYs | NHB (QALYs) | NMB | INHB (QALYs) | INMB | ||||
| 1 | Lab | £362.04 | 9.991370986 | 9.97327 | £199,465.38 | 0.00000 | £0.00 | 14 | |
| 2 | RF+ i-STAT | £276.20 | 9.991370982 | 9.97756 | £199,551.22 | 0.00429 | £85.84 | 3 | |
| 3 | RF + ABL800 FLEX | £284.28 | 9.991370982 | 9.97716 | £199,543.14 | 0.00389 | £77.76 | 10 | |
| 4 | RF + StatSensor | £276.33 | 9.991370979 | 9.97755 | £199,551.09 | 0.00429 | £85.71 | 4 | |
| 5 | RF + Lab | £302.84 | 9.991370986 | 9.97623 | £199,524.58 | 0.00296 | £59.20 | 13 | |
| 6 | RF + i-STAT + Lab | £274.82 | 9.991370982 | 9.97763 | £199,552.60 | 0.00436 | £87.22 | 1 | |
| 7 | RF + ABL800 FLEX + Lab | £283.34 | 9.991370982 | 9.97720 | £199,544.08 | 0.00394 | £78.70 | 9 | |
| 8 | RF + StatSensor + Lab | £275.25 | 9.991370979 | 9.97761 | £199,552.17 | 0.00434 | £86.79 | 2 | |
| 9 | i-STAT | £283.07 | 9.991370982 | 9.97722 | £199,544.35 | 0.00395 | £78.97 | 8 | |
| 10 | ABL800 FLEX | £288.39 | 9.991370982 | 9.97695 | £199,539.03 | 0.00368 | £73.65 | 12 | |
| 11 | StatSensor | £281.31 | 9.991370979 | 9.97731 | £199,546.11 | 0.00404 | £80.73 | 7 | |
| 12 | i-STAT + Lab | £279.05 | 9.991370982 | 9.97742 | £199,548.37 | 0.00415 | £82.99 | 6 | |
| 13 | ABL800 FLEX + Lab | £285.65 | 9.991370982 | 9.97709 | £199,541.77 | 0.00382 | £76.39 | 11 | |
| 14 | StatSensor + Lab | £278.19 | 9.991370979 | 9.97746 | £199,549.23 | 0.00419 | £83.85 | 5 | |
| Identification | Management | Total | At £20,000 per QALY | NB rank | |||||
|---|---|---|---|---|---|---|---|---|---|
| Costs | QALYs | NHB (QALYs) | NMB | INHB (QALYs) | INMB | ||||
| 1 | Lab | £361.77 | 9.991370978 | 9.97328 | £199,465.65 | 0.00000 | £0.00 | 14 | |
| 2 | RF+ i-STAT | £275.80 | 9.991370975 | 9.97758 | £199,551.62 | 0.00430 | £85.97 | 3 | |
| 3 | RF + ABL800 FLEX | £283.93 | 9.991370975 | 9.97717 | £199,543.49 | 0.00389 | £77.84 | 10 | |
| 4 | RF + StatSensor | £275.99 | 9.991370973 | 9.97757 | £199,551.43 | 0.00429 | £85.78 | 4 | |
| 5 | RF + Lab | £302.57 | 9.991370978 | 9.97624 | £199,524.85 | 0.00296 | £59.20 | 13 | |
| 6 | RF + i-STAT + Lab | £274.59 | 9.991370975 | 9.97764 | £199,552.83 | 0.00436 | £87.18 | 1 | |
| 7 | RF + ABL800 FLEX + Lab | £283.11 | 9.991370975 | 9.97722 | £199,544.31 | 0.00393 | £78.66 | 9 | |
| 8 | RF + StatSensor + Lab | £275.05 | 9.991370973 | 9.97762 | £199,552.37 | 0.00434 | £86.72 | 2 | |
| 9 | i-STAT | £282.34 | 9.991370975 | 9.97725 | £199,545.08 | 0.00397 | £79.43 | 8 | |
| 10 | ABL800 FLEX | £287.81 | 9.991370975 | 9.97698 | £199,539.60 | 0.00370 | £73.95 | 12 | |
| 11 | StatSensor | £280.72 | 9.991370973 | 9.97734 | £199,546.70 | 0.00405 | £81.05 | 7 | |
| 12 | i-STAT + Lab | £278.82 | 9.991370975 | 9.97743 | £199,548.60 | 0.00415 | £82.95 | 6 | |
| 13 | ABL800 FLEX + Lab | £285.42 | 9.991370975 | 9.97710 | £199,542.00 | 0.00382 | £76.35 | 11 | |
| 14 | StatSensor + Lab | £277.99 | 9.991370973 | 9.97747 | £199,549.43 | 0.00419 | £83.78 | 5 | |
| Identification | Management | Total | At £20,000 per QALY | NB rank | |||||
|---|---|---|---|---|---|---|---|---|---|
| Costs | QALYs | NHB (QALYs) | NMB | INHB (QALYs) | INMB | ||||
| 1 | Lab | £363.26 | 9.991371010 | 9.97321 | £199,464.16 | 0.00000 | £0.00 | 15 | |
| 2 | RF+ i-STAT | £278.02 | 9.991371002 | 9.97747 | £199,549.40 | 0.00426 | £85.25 | 5 | |
| 3 | RF + ABL800 FLEX | £285.87 | 9.991371003 | 9.97708 | £199,541.55 | 0.00387 | £77.39 | 10 | |
| 4 | RF + StatSensor | £277.84 | 9.991370997 | 9.97748 | £199,549.58 | 0.00427 | £85.42 | 4 | |
| 5 | RF + Lab | £304.06 | 9.991371010 | 9.97617 | £199,523.36 | 0.00296 | £59.20 | 14 | |
| 6 | RF + i-STAT + Lab | £275.84 | 9.991371002 | 9.97758 | £199,551.58 | 0.00437 | £87.42 | 2 | |
| 7 | RF + ABL800 FLEX + Lab | £284.39 | 9.991371003 | 9.97715 | £199,543.03 | 0.00394 | £78.87 | 9 | |
| 8 | RF + StatSensor + Lab | £276.15 | 9.991370997 | 9.97756 | £199,551.27 | 0.00436 | £87.11 | 3 | |
| 9 | i-STAT | £286.35 | 9.991371002 | 9.97705 | £199,541.07 | 0.00385 | £76.91 | 11 | |
| 10 | ABL800 FLEX | £290.99 | 9.991371003 | 9.97682 | £199,536.43 | 0.00361 | £72.28 | 13 | |
| 11 | StatSensor | £283.96 | 9.991370997 | 9.97717 | £199,543.46 | 0.00396 | £79.30 | 8 | |
| 12 | i-STAT + Lab | £280.08 | 9.991371002 | 9.97737 | £199,547.34 | 0.00416 | £83.18 | 7 | |
| 13 | ABL800 FLEX + Lab | £286.70 | 9.991371003 | 9.97704 | £199,540.72 | 0.00383 | £76.56 | 12 | |
| 14 | StatSensor + Lab | £279.09 | 9.991370997 | 9.97742 | £199,548.33 | 0.00421 | £84.17 | 6 | |
| 15 | No testing | Contrast-enhanced CT | £266.77 | 9.991370961 | 9.97803 | £199,560.65 | 0.00482 | £96.50 | 1 |
| Identification | Management | Total | At £20,000 per QALY | NB rank | |||||
|---|---|---|---|---|---|---|---|---|---|
| Costs | QALYs | NHB (QALYs) | NMB | INHB (QALYs) | INMB | ||||
| 1 | Lab | £363.26 | 9.991371010 | 9.97321 | £199,464.16 | 0.00000 | £0.00 | 15 | |
| 2 | RF+ i-STAT | £278.09 | 9.991370798 | 9.97747 | £199,549.33 | 0.00426 | £85.17 | 5 | |
| 3 | RF + ABL800 FLEX | £285.93 | 9.991370825 | 9.97707 | £199,541.48 | 0.00387 | £77.33 | 10 | |
| 4 | RF + StatSensor | £277.96 | 9.991370662 | 9.97747 | £199,549.46 | 0.00426 | £85.30 | 4 | |
| 5 | RF + Lab | £304.06 | 9.991371010 | 9.97617 | £199,523.36 | 0.00296 | £59.20 | 14 | |
| 6 | RF + i-STAT + Lab | £275.92 | 9.991370798 | 9.97757 | £199,551.50 | 0.00437 | £87.34 | 2 | |
| 7 | RF + ABL800 FLEX + Lab | £284.46 | 9.991370825 | 9.97715 | £199,542.96 | 0.00394 | £78.80 | 9 | |
| 8 | RF + StatSensor + Lab | £276.27 | 9.991370662 | 9.97756 | £199,551.14 | 0.00435 | £86.98 | 3 | |
| 9 | i-STAT | £286.43 | 9.991370798 | 9.97705 | £199,540.99 | 0.00384 | £76.83 | 11 | |
| 10 | ABL800 FLEX | £291.05 | 9.991370825 | 9.97682 | £199,536.37 | 0.00361 | £72.21 | 13 | |
| 11 | StatSensor | £284.08 | 9.991370662 | 9.97717 | £199,543.33 | 0.00396 | £79.17 | 8 | |
| 12 | i-STAT + Lab | £280.15 | 9.991370798 | 9.97736 | £199,547.26 | 0.00416 | £83.11 | 7 | |
| 13 | ABL800 FLEX + Lab | £286.77 | 9.991370825 | 9.97703 | £199,540.65 | 0.00382 | £76.49 | 12 | |
| 14 | StatSensor + Lab | £279.21 | 9.991370662 | 9.97741 | £199,548.20 | 0.00420 | £84.04 | 6 | |
| 15 | No testing | Contrast-enhanced CT | £267.22 | 9.991369679 | 9.97801 | £199,560.17 | 0.00480 | £96.02 | 1 |
List of abbreviations
- ACR
- American College of Radiology
- AKI
- acute kidney injury
- AMACING
- A MAastricht Contrast-Induced Nephropathy Guideline
- CDSR
- Cochrane Database of Systematic Reviews
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CG
- clinical guideline
- CI
- confidence interval
- CI-AKI
- contrast-induced acute kidney injury
- CIN
- contrast-induced nephropathy
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CKD
- chronic kidney disease
- CKD-EPI
- Chronic Kidney Disease Epidemiology Collaboration
- CRD
- Centre for Reviews and Dissemination
- CrI
- credible interval
- CT
- computed tomography
- EAG
- External Assessment Group
- eGFR
- estimated glomerular filtration rate
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- ESUR
- European Society of Urogenital Radiology
- FN
- false negative
- FP
- false positive
- GSTT
- Guy’s and St Thomas’ NHS Foundation Trust
- HIV
- human immunodeficiency virus
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IDMS
- isotope dilution mass spectrometry
- IVH
- intravenous hydration
- KiTEC
- King’s Technology Evaluation Centre
- MDRD
- Modification of Diet in Renal Disease
- MeSH
- medical subject heading
- MIB
- Medtech innovation briefing
- MRI
- magnetic resonance imaging
- NAC
- N-acetylcysteine
- NHB
- net health benefit
- NHS EED
- NHS Economic Evaluations Database
- NICE
- National Institute for Health and Care Excellence
- NMB
- net monetary benefit
- OR
- odds ratio
- PC-AKI
- post-contrast acute kidney injury
- POC
- point of care
- PPV
- positive predictive value
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- QUADAS
- quality assessment of diagnostic accuracy studies
- RANZCR
- The Royal Australian and New Zealand College of Radiologists
- RCR
- Royal College of Radiologists
- RCT
- randomised controlled trial
- RePEc
- Research Papers in Economics
- RR
- risk ratio
- RRT
- renal replacement therapy
- SCr
- serum creatinine
- SMR
- standardised mortality rate
- TN
- true negative
- TP
- true positive
This monograph is based on the Technology Assessment Report produced for NICE. The full report contained a considerable number of data that were deemed confidential. The full report was used by the Appraisal Committee at NICE in their deliberations. The full report with each piece of confidential data removed and replaced by the statement ‘confidential information (or data) removed’ is available on the NICE website: www.nice.org.uk.
The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.
