Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 16/150/01. The contractual start date was in April 2018. The draft report began editorial review in December 2020 and was accepted for publication in June 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Šumilo et al. This work was produced by Šumilo et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Šumilo et al.
Chapter 1 Background, aims and objectives
Background and rationale
In the UK, a caesarean section (CS) is a common surgical procedure, with around one-third of babies being delivered this way. 1–4 The risk of maternal post-partum infections is considerably higher following CS than following vaginal delivery (VD). 5 National Institute for Health and Care Excellence (NICE) Guideline 192 on caesarean birth6 recommends offering women undergoing CS prophylactic antibiotics that work against the organisms causing wound infections, endometritis and urinary tract infections to reduce the risk of post-partum infections.
Before 2011, this NICE clinical guideline recommended giving intravenous prophylactic antibiotics after cord clamping to prevent the baby from being exposed to the maternal antibiotics. In 2011, the recommendation was changed to offering antibiotics to women before skin incision for CS. 7 This change in recommendation was based on evidence that earlier administration reduces the risk of maternal infections, although most of these infections are mild and tend to respond well to treatment. 8 A Cochrane review9 on the timing of intravenous prophylactic antibiotics for preventing post-partum infectious morbidity in women undergoing cs delivery included evidence from 10 randomised controlled trials (including a total of 5041 women). The review showed a significant reduction in the risk of combined post-partum infections [43%, 95% confidence interval (CI) 28% to 55%], wound infection (41%, 95% CI 19% to 56%) and endometritis (46%, 95% CI 21% to 64%) in women receiving antibiotics prior to cord clamping, compared with administering antibiotics after cord clamping. 9
It is known, however, that these high-dose broad-spectrum pre-operative antibiotics cross the placenta, with concentrations detectable within 20–30 minutes, resulting in babies being exposed to the antibiotics at the time of birth. 9,10 Although the Cochrane review found no evidence of neonatal adverse effects,9 intrapartum antibiotics have been shown to significantly disrupt the intestinal microbiota of babies. 11 During birth, the gut is colonised by microbes12 and the mode of delivery is known to significantly affect the composition of intestinal microbiota both in the neonatal period and later in infancy. 13 These microbiota appear to be key for intestinal immune tissue to differentiate resident commensal bacteria from potential pathogens. 14 In addition, there is a growing body of evidence that intestinal microbe composition in infants plays a key role in the development of their immune system, including regulation of response to different antigens and inflammation, and disruptions to intestinal microbiota are associated with susceptibility to asthma, allergies and other immune-related diseases later in childhood. 15–22
A recent systematic review14 explored the association between intestinal microbiota and the development of asthma, eczema and allergic sensitisation to food allergens in newborns and children. The review found evidence that reduced bacterial diversity and a greater or reduced abundance of particular bacteria was associated with the development of these health conditions. There is also an association between reduced intestinal bacterial diversity in the first months of life and development of allergic rhinitis. 23
Some evidence also suggests that gut microbiota may have an influence on the development of autoimmune diseases. For example, gut microbiota have been linked to the development of type 1 diabetes,24 coeliac disease25 and systemic connective tissue disorders. 26,27 The evidence in this area is acknowledged to be limited. It is, however, hypothesised that the mechanisms driving autoimmune disease development are similar to those involved in the development of allergic diseases. 28
There is limited evidence that explores the link between the gut microbiome and infectious and inflammatory disease and other immune system-related conditions. Intestinal microbiota are likely to be involved in the development of necrotising enterocolitis and inflammatory bowel disease. 29 It has also been hypothesised that childhood acute lymphoblastic leukaemia development could have an immune component driven by microbial exposures in early life. 30
One study has shown an increased risk of cerebral palsy in children born to mothers who were given antibiotics in spontaneous pre-term labour. 31 There is also a small body of research evidence that suggests associations between differences in gut microbiota and other neurodevelopmental conditions, but there is a consensus that further longitudinal assessment of the role of the gut microbiome in neurodevelopmental disorders is required. 32,33
In the broader area of child health, there is a small amount of evidence looking at gut microbiota and symptoms, such as colic, and general health measures, such as expected weight gain. Infants affected by colic have been reported to have different levels of intestinal bacterial diversity from unaffected infants. 34 Some types of gut bacteria appear to be associated with different growth patterns in babies. 35
As antibiotic exposure around the time of birth leads to different gut microbiota patterns in newborns and different microbiota patterns are associated with longer-term health conditions, the change in the timing of when mothers receive prophylactic antibiotics presents a natural experiment. Babies born to mothers who were provided antibiotics after cord clamping are exposed to no antibiotics. Those born to mothers given pre-operative antibiotics are exposed to high-dose broad-spectrum antibiotics that are likely to affect their gut microbiota.
In this study, we aimed to compare the outcomes for babies born by CS who were and who were not exposed to antibiotics given to the mother at the time of birth. The primary outcomes of interest in this study were asthma and eczema. These are the two most frequent allergy-related, doctor-diagnosed conditions in childhood and are associated with changes in gut microbiota composition in infants. 18,20 They are commonly seen in primary care and are well recorded. 36,37 With a prevalence of 3% in children aged 0–5 years, asthma is a relatively common health condition in childhood, with the largest numbers of emergency hospital admissions for any long-term condition in young children in the UK. 38,39 Although deaths due to asthma in children are rare, they do occur, accounting for 1–2% of all asthma deaths. 38,40
Eczema is a very common condition in childhood, with a prevalence of 18% of ‘ever diagnosed eczema’ in children aged 0–5 years reported in the UK. 41 Eczema usually develops in very early childhood and can affect a child’s sleep, their social and emotional development, and quality of life. It incurs significant societal costs because of lost school days, lost working days for parents and costs to the NHS, including prescription of topical corticosteroids. 42
As the evidence regarding the role of microbiota in the development of diseases is evolving, we also assessed whether or not in-utero exposure to broad-spectrum antibiotics immediately prior to birth increases the risk of a range of other immune system-related health conditions in the first 5 years of life in children born by CS.
Aims and objectives
Overall aim
The overall aim of the study was to examine the impact, if any, that the change in the national recommendation from advising administering prophylactic antibiotics post-cord clamping to administering antibiotics before skin incision has had on the risk of allergic and other related health outcomes in children delivered by CS.
Primary objectives
The main objective was to investigate if prophylactic antibiotic exposure of the fetus immediately before birth (intervention) compared with no antibiotic exposure (comparator) increases the risk of (1) asthma and (2) eczema (outcomes) in children delivered by CS (population).
Secondary objectives
-
To investigate the impact of exposure to pre-incision antibiotics in children delivered by CS on (1) other allergic and allergy-related health conditions, (2) autoimmune conditions, (3) infections and inflammation, (4) other immune system-related health conditions, (5) neurodevelopmental health conditions and (6) less specific child health measures (i.e. colic and failure to thrive).
-
To investigate the impact of exposure to pre-incision antibiotics in children delivered by CS on health service use (defined as overall primary care consultation frequency and hospital admissions).
-
To investigate if the effects of reducing post-partum infections in mothers, as reported in randomised controlled trials conducted outside the UK,9 can be replicated in the UK using routine health-care records.
Chapter 2 Methods
Study design and setting
The primary objective and first two secondary objectives were addressed by undertaking a controlled interrupted time series study. 43 It comprised a cohort of children born in the UK between 2006 and 2018 and their mothers, whose health-care records were included on a primary care database [i.e. The Health Improvement Network (THIN) or the Clinical Practice Research Datalink (CPRD)] or a secondary care database [Hospital Episode Statistics (HES)].
The interrupted time series component of the study compared rates of diagnosis of asthma, eczema and other immune system-related diseases over the study period in children delivered by CS between time periods when the mother of each child was likely to receive antibiotics before skin incision and periods when it was likely that antibiotics would be administered after cord clamping.
Validity in this comparison of incidence rates is mostly threatened by temporal changes in the diagnosis and recording of health conditions and different exposures that can have an impact on the number of cases identified in routine data, rather than from case mix confounders, as indications for CS and CS incidence have changed little over the study period. As an example, patterns of asthma diagnosis have changed over time, which can, in part, be attributed to the revisions in the national asthma management guidance44 and the potentially conflicting compliance and prevalence issues faced in meeting asthma-specific Quality and Outcomes Framework (QOF) indicators introduced in 2004. 45
Conducting an analysis dependent on adjustment for confounding factors was unlikely to succeed in controlling for these changes, because (1) it is uncertain what all the drivers of all these changes have been, (2) it is uncertain whether or not any covariates exist that accurately describe these changes without substantial missing data and (3) there are challenges in specification of a functional form for the relationship between these covariates and outcome. Instead, to account for such temporal changes, we included children born by VD as a control group, as these children would have been subject to all the same temporal changes as children delivered by CS, but would not have routinely received prophylactic antibiotics. Figure 1 shows that, although the national uptake of pre-incision antibiotic policy has increased over the study period, incidence rates of asthma decreased equally in children born by CS and those born by VD.
FIGURE 1.
Illustration of the need to use VDs as the comparator group because of changing temporal patterns in outcome events unrelated to routine exposure to pre-incision antibiotics, using asthma as an example. (Rates after 2014 are further reduced because of < 5 years of follow-up and much lower incidence of asthma in very young children.)
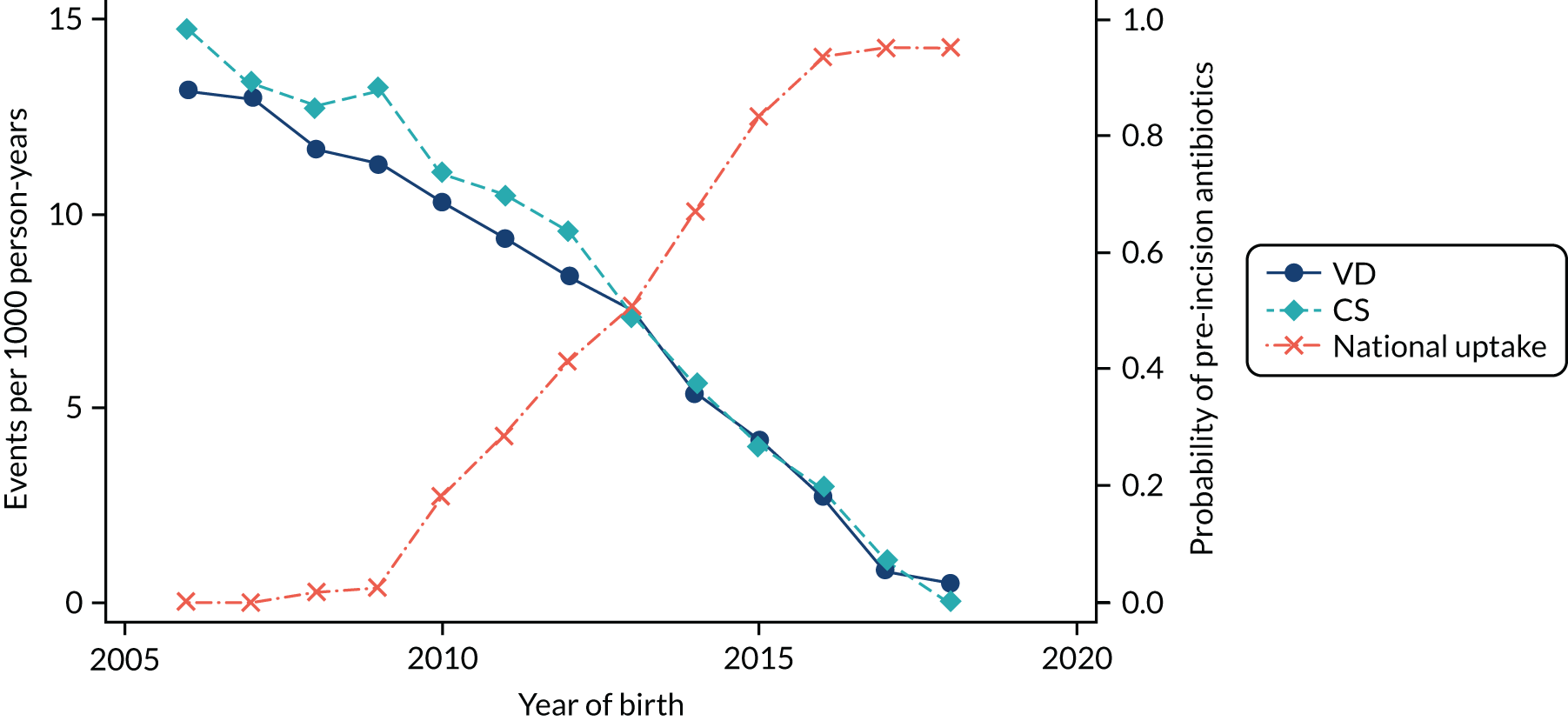
Our model first computed the ratio of the incidence of outcomes in those receiving CS compared with VD in each year and age group. The incidence of some outcomes, including asthma, is known to differ by delivery mode. 46–48 It then compared these ratios between periods when mothers of children delivered by CS would have received antibiotics before skin incision and periods when mothers of children delivered by CS would have received antibiotics after cord clamping. This ‘ratio-of-ratios’ is an indicator of how the incidence of disease has increased (if > 1) with pre-incision antibiotics, stayed the same (if = 1) or decreased (if < 1), while adjusting for temporal changes through the matching of outcomes in children born vaginally. Subject to the assumption that the model of the difference between CS and VD rates is transferable across the time periods, differences between the observed and counterfactual CS event rates were interpreted as likely to be caused by the change in practice.
In the primary analysis, for primary care data, national policy uptake rates in the year of delivery were used as a basis for estimating a probability that each mother had received pre-incisional antibiotics and, therefore, the analysis actually used this probability as the explanatory variable, rather than a 0–1 variable. For secondary care data, we used each hospital’s response indicating the year of local policy implementation. This was obtained from our national survey of maternity care providers, which provided a 0–1 variable.
To address the final secondary objective, we compared the incidence of post-partum maternal infectious morbidity post intervention with pre-intervention rates to investigate if the effects of reduced morbidity that are shown in randomised controlled trials outside the UK9 that form the basis for the NICE guideline change7 can be replicated in the UK using routine health-care data. This analysis has been carried out for two reasons: (1) to measure the assumed impact using ‘real-world data’ to enable us to compare the benefits of pre-incisional antibiotics on maternal outcomes against any potential harms for the child and (2) to assure us that any lack of effect of the different prophylactic antibiotic policies was not due to the quality of the routine data. We calculated the crude estimates and, because of the observed overall changes in the incidence of different maternal post-partum infections recorded in routine data over time, we also undertook controlled analysis adjusting for delivery mode.
Population and eligibility criteria
To be eligible for inclusion in the study, records of all live-born children needed to meet the following criteria:
-
The year of birth was between 2006 and 2018.
-
It was possible to link the baby and their mother’s health-care record in primary care (THIN or CPRD) databases or a secondary care (HES) database.
-
The delivery mode (CS or VD) could be determined based on recording in primary (THIN or CPRD) and/or secondary care (HES).
If children had missing delivery information, they were excluded. In the case of multiple births (such as twins), one of the children was included randomly to ensure independence of observations. 49
The duration of follow-up of children born between 2006 and 2014 was until the age of 5 years. The follow-up of children born in 2015, 2016, 2017 and 2018 was limited to 4, 3, 2 and 1 years, respectively.
For primary care data, the exposure, outcomes and covariates were defined using the Read clinical code classification system. In HES, International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) was used for clinical diagnoses and the Office of Population Censuses and Surveys Classification of Surgical Operations and Procedures, 4th revision (OPCS-4) for procedures. The code lists are available online at URL: https://github.com/NIHR-Pre-incision-Antibiotics/ClinicalCodes (accessed 8 July 2021).
The process for Read code selection was as follows:
-
Stage 1 – a list of key words and synonyms for each variable of interest was compiled.
-
Stage 2 – the terms identified in stage 1 were used to search the description column of the Read code dictionary/database to identify the main chapter and appropriate level 3 stem code in the Read code dictionary.
-
Stage 3 – stem codes were then used to identify other related Read codes, up to level 7, associated with the main stem codes. These Read codes were then selected and stored.
-
Stage 4 – where available, for each outcome of interest, online Read code repositories and supplementary information from published articles that had used a similar set of Read codes were identified. These were then compared with our selected set of Read codes and further useful codes were added at this point.
-
Stage 5 – with reference to the inclusion and exclusion criteria, the team identifying and selecting Read codes allocated weights to each Read code as follows: (1) definite (the team were sure that the Read code met the study inclusion criteria), (2) probable (the team felt that these codes could meet the inclusion criteria but needed further discussion), and (3) excluded (the study team felt that these codes definitely did not meet the inclusion criteria).
-
Stage 6 – consensus was sought through consultation with study team researchers and public contributors, general practitioners (GPs) or specialty field experts on the final list of codes to be used, which were utilised for electronic data extraction.
Selection of ICD-10 and OPCS-4 codes to obtain admitted patient care data was generally straightforward. The validity of each coding algorithm was checked through triangulation of a number of sources. First, recently published studies based on routinely collected data were screened to check for consensus in the research community as to how to code specific reasons for admission. Second, checks of resources for clinical coders (the NHS coding clinic and the Terminology and Classifications Service in NHS Digital) were carried out. Third, technical appendices of outputs produced by the NHS and the Office for National Statistics were checked to see what coding definitions were used in reporting by public bodies. Last, to check for the specificity of coding (i.e. the extent to which fourth digits were commonly used or, if coding tended to default to ‘other’ or ‘not elsewhere classified’) individual analyses of English admitted patient care data were undertaken.
The delivery mode is recorded well in hospital records. 2 We used OPCS-4 procedure codes, which are available for 98% of births in HES,50 to derive the mode of delivery and ICD-10 codes for the remaining deliveries. Our estimates showed that the delivery mode is also accurately recorded in primary care using Read codes (98% verified against hospital records). The recording, however, was incomplete (the delivery mode was known for 50% and 69% of children in THIN and CPRD data sets, respectively, with the higher proportion in CPRD due to the supplied data set being restricted to the records in the CPRD pregnancy register). For THIN and CPRD records not linked to HES, we used the Read codes to identify the mode of delivery, which increased the number of children in the sample.
Study outcomes
The primary outcomes in this study were the incidence of (1) asthma and (2) eczema. Asthma and eczema outcomes in the THIN–CPRD data set were defined using lists of relevant Read codes, which were created following the steps described above (see Population and eligibility criteria). Furthermore, Read code lists for both asthma and eczema have been previously validated in primary care. 51,52 Therefore, existing validated lists were used as the basis for the outcome definition in this study and further codes were added following consultation with the wider research team, which included a GP and an allergy and immunology consultant. In line with the paper on the validation of eczema coding,51 in addition to a Read code for eczema diagnosis, at least two prescriptions on separate dates for any eczema-related treatment were also required to reflect the chronic nature of the condition. In the HES data set, asthma and eczema were defined if a ICD-10 code for asthma or eczema was used as the main primary diagnosis for the hospital admission.
The main analysis for these outcomes was carried out separately in the primary care data set and the secondary care (HES) data set (the latter analysis including only those hospitals for which the year of antibiotic prescribing policy change was known).
We also explored the relationship between antibiotic exposure and asthma and eczema severity in the primary care data set. Moderate/severe asthma was defined as having a Read code for asthma diagnosis and a prescription code for a corticosteroid inhaler and either a leukotriene receptor antagonist or a systemic steroid. As the severity of asthma is not reliably coded, a prescription for a leukotriene receptor antagonist or a systemic steroid is indicative of asthma that is uncontrolled on inhaled corticosteroids (i.e. moderate or severe asthma). Moderate/severe eczema was defined as having a Read code for eczema diagnosis and at least two prescriptions on separate dates, with at least one prescription for treating moderate or severe eczema (i.e. moderate, potent or very potent steroid, topical calcineurin, systemic therapy or phototherapy), as described in the NICE guideline for eczema. 53
Secondary outcomes are listed in Table 1. We excluded immune deficiencies from the outcome list, as Read and ICD-10 codes did not allow us to differentiate between congenital and acquired immunodeficiencies. We also did not include pelvic abscess as a separate outcome in the analysis of secondary care data, as there was no specific ICD-10 code for this condition.
| Outcome | Corresponding secondary objective | Data set analysed | |
|---|---|---|---|
| Primary care | Secondary carea | ||
| Health conditions and symptoms in children | To investigate the impact of exposure to pre-incision antibiotics in children delivered by CS | ||
| Other allergic and allergy-related conditions | To investigate the impact of exposure to pre-incision antibiotics in children delivered by CS on other allergic and allergy-related health conditions | ||
| Food allergy/intolerance | ✓ | ||
| Allergic rhinitis and conjunctivitis | ✓ | ||
| One or more allergy-related disease (e.g. asthma, eczema, food allergy/intolerance, allergic rhinitis and conjunctivitis) | ✓ | ||
| Penicillin allergyb | ✓ | ||
| Anaphylaxisb | ✓ | ✓ | |
| High risk of anaphylactic reaction (prescribing of automatic injection devices containing adrenaline)b | ✓ | ||
| Autoimmune diseases | To investigate the impact of exposure to pre-incision antibiotics in children delivered by CS on autoimmune conditions | ||
| Type 1 diabetesb | ✓ | ✓ | |
| Coeliac diseaseb | ✓ | ✓ | |
| Juvenile idiopathic arthritisb | ✓ | ✓ | |
| Scleroderma/systemic sclerosisb | ✓ | ✓ | |
| Inflammatory myopathiesb | ✓ | ✓ | |
| Systemic lupus erythematosusb | ✓ | ✓ | |
| Autoimmune (idiopathic) thrombocytopenic purpurab | ✓ | ✓ | |
| Juvenile pernicious (megaloblastic) anaemiab | ✓ | ✓ | |
| Childhood vitiligob | ✓ | ||
| Infections and inflammation | To investigate the impact of exposure to pre-incision antibiotics in children delivered by CS on infections and inflammation | ||
| Neonatal sepsis (early and late onset) | ✓ | ||
| Other sepsisb | ✓ | ||
| Wheeze | ✓ | ||
| Upper respiratory tract infectionsb | ✓ | ||
| Lower respiratory tract infectionsb | ✓ | ✓ | |
| Bronchiolitisb | ✓ | ✓ | |
| Gastroenteritisb | ✓ | ✓ | |
| Inflammatory bowel disease | ✓ | ✓ | |
| Urinary tract infectionsb | ✓ | ✓ | |
| Antibiotic prescribingb | ✓ | ||
| Other immune system-related conditions | To investigate the impact of exposure to pre-incision antibiotics in children delivered by CS on other immune system-related health conditions | ||
| Necrotising enterocolitis | ✓ | ||
| Leukaemiab | ✓ | ✓ | |
| Neurodevelopmental conditions | To investigate the impact of exposure to pre-incision antibiotics in children delivered by CS on neurodevelopmental health conditions | ||
| Cerebral palsy | ✓ | ||
| Autism spectrum disorderb | ✓ | ||
| ADHDb | ✓ | ||
| Less specific measures of child health | To investigate the impact of exposure to pre-incision antibiotics in children delivered by CS on less-specific child health measures | ||
| Colicb | ✓ | ||
| Failure to thriveb | ✓ | ||
| Health-care utilisation in children | To investigate the impact of exposure to pre-incision antibiotics in children delivered by CS on utilisation of health services (defined as overall primary care consultation frequency and hospital admissions) | ||
| Primary care consultationsb | ✓ | ||
| Hospital admissionsb | ✓ | ||
| Maternal outcomes (up to 6 weeks post partum) | To investigate if the effects of reducing post-partum infections in mothers, as reported in randomised controlled trials conducted outside the UK, can be replicated in the UK using routine health-care records | ||
| Composite infectious morbidity (wound infection, endometritis/endomyometritis, pelvic abscess,c maternal sepsis, maternal deathd) | ✓ | ✓ | |
| Endometritis/endomyometritis | ✓ | ✓ | |
| Wound infection | ✓ | ✓ | |
| Urinary tract infection/cystitis/pyelonephritis | ✓ | ✓ | |
| Sepsis | ✓ | ✓ | |
| Pelvic abscess | ✓ | ||
| Maternal deathb,e | ✓ | ||
| Antibiotic prescribingb | ✓ | ||
| Length of hospital stayb | ✓ | ||
Covariates
To assess the validity of the model we used in controlling for temporal confounders, we explored changes in the case mix covariates to identify any differential changes over time in relation to the mode of birth. To identify relevant covariates, a literature review of reviews was conducted on the risk factors for the primary study outcomes (i.e. asthma and eczema) and postnatal maternal infections. MEDLINE and Google Scholar (Google Inc., Mountain View, CA, USA) were searched for articles written in English that reported the effect of any risk factor for the conditions mentioned. Identified covariates included maternal demographic characteristics,54 behaviour-related factors,54–58 pregnancy- and labour-related factors,54,57 antibiotic prescribing during pregnancy,56,59,60 maternal long-term allergy-related conditions,61–64 deprivation,54,55,58,65,66 child demographic and birth characteristics,54,55,58,59,62,64–67 breastfeeding,55,67 child health conditions55,61–65,67 and antibiotic prescribing in childhood55,60,67 (Table 2).
| Covariate | Data set analysed | ||
|---|---|---|---|
| Primary care | Secondary care | National maternity surveys | |
| Maternal characteristic | |||
| Age at childbirth | ✓ | ✓ | |
| Ethnicity | ✓ | ✓ | |
| Smoking status | ✓ | ||
| BMI before pregnancy | ✓ | ||
| Parity | ✓ | ||
| Premature rupture of membranes | ✓ | ||
| Post-partum haemorrhage | ✓ | ||
| Manual placental removal/retained products of conception | ✓ | ||
| Antibiotic prescribing during pregnancy | ✓ | ||
| Asthma | ✓ | ||
| Eczema | ✓ | ||
| Allergic rhinitis and conjunctivitis | ✓ | ||
| Household characteristic | |||
| Deprivation score | ✓ | ✓ | |
| Child characteristic | |||
| Sex | ✓ | ✓ | |
| Ethnicity | ✓ | ✓ | |
| Gestational age | ✓ | ||
| Birthweight | ✓ | ||
| Agea | ✓ | ✓ | |
| Breastfeeding (first few days and 3 months) | ✓ | ||
| Asthmab | ✓ | ✓ | |
| Eczemab | ✓ | ✓ | |
| Allergic rhinitis and conjunctivitisb | ✓ | ||
| Food allergy/intoleranceb | ✓ | ||
| Upper respiratory tract infectionsb | ✓ | ||
| Lower respiratory tract infectionsb | ✓ | ✓ | |
| Bronchiolitisb | ✓ | ✓ | |
| Antibiotic prescribingb | ✓ | ||
Data sources and mother–baby linkage
Electronic primary and secondary health-care records
THIN and CPRD
The Health Improvement Network and CPRD GOLD are two UK-wide primary care databases of anonymised patient records from general practices that use the same computer software (Vision, Cegedim Rx Ltd and In Practice Systems Ltd, London, UK), together covering over 10% of the UK population. 68 In addition, a significant proportion of patients (about 30% in THIN and 60% in CPRD) have linked secondary care (HES) data. Both THIN and CPRD GOLD databases are broadly generalisable to the UK population in terms of patient demographics and health condition prevalence. 69,70 The University of Birmingham (Birmingham, UK) holds a sublicence for the full THIN database. The CPRD GOLD data set was acquired from the CPRD centre of the Medicines and Healthcare products Regulatory Agency (MHRA) for use in this specific study.
Identification and removal of duplicate practices
The Health Improvement Network and CPRD GOLD databases have some overlap at practice level, with 37% and 46% unique practices, respectively, reported previously. 68 Our sample size calculations showed that both primary care databases were required to provide sufficient power to answer the research questions. We adapted a previously published algorithm to identify practices common to both CPRD GOLD and THIN. 71 Although the databases do not use the same identifiers for patients or practices, the overlapping practices can be identified and removed reliably using practice-related information, patient registration, demographic and medical record information. 68,71
We extracted practice- and patient-level data to identify common/duplicate practices in THIN and CPRD. The following practice-level data were extracted: (1) the number of males and females born between 1905 and 1910, between 1910 and between 1915 and 1915 and 1920; (2) the number of males and females newly diagnosed with type 2 diabetes in the year 2010; and (3) the total number of patients who received at least one drug prescription in the year 2010. In total, this amounted to nine practice-level variables that we extracted from the THIN database and the same variables were provided to us on request from CPRD GOLD.
For patient-level data, we examined aggregated information from a sample of patients. To extract our patient-level variables, we calculated an eligible time period where both THIN and corresponding CPRD GOLD practices were contributing data to their respective data set. The eligible period was defined as the time between the ‘latest’ and ‘earliest’ set of dates for each database as follows: latest of (1) THIN Vision date, (2) THIN acceptable mortality reporting date, (3) THIN computerisation date or (4) CPRD GOLD ‘up to standard’ date (a quality indicator); and the earliest of (1) THIN collection date or (2) CPRD collection date. Defining the eligible time period was important, as the patient-level data we extracted for deduplication can vary outside this period. For example, there might be patients who were registered with THIN earlier, but were transferred out before the practice started contributing data to CPRD GOLD. If such patient-level data were extracted from THIN, then we would not be able to confirm their characteristics in CPRD GOLD, as their data were never present in it. Alternatively, we might have found similar patient-level characteristics by chance in candidate practices that are not duplicates.
For each THIN and CPRD GOLD practice pair, we used CPRD GOLD data and randomly selected 10 patients who were registered in the practice within the eligible period. We extracted their registration date, year and month of birth, sex, family identification number, first three consultation types, consultation dates and duration of consultations for matching. We created two new scores, (1) ‘patient point’ and (2) ‘consultation point’, and set both to zero. We looked in the corresponding THIN practice to find patients with the same information. If a patient was found in THIN with the same registration data, sex and date of birth information, then the patient point was incremented by 1 and, for the same patient, we incremented consultation points by 1 for each consultation information that was matched as equal in both databases.
After considering patient- and practice-level scores, we found 450 practices contributing to both THIN and CPRD. Among the duplicate practices, we retained the practice (from THIN or CPRD GOLD) that contributed the highest number of babies to the study.
Linkage of mothers and babies
Information on mothers and their babies can be linked in CPRD GOLD and THIN using the family identification number, delivery codes, mother’s registered or estimated date of delivery, child’s birth month, gestational age at delivery and, where available, the sex of the baby, with reported identification of the vast majority of mother–child pairs. 72,73
Clinical Practice Research Datalink GOLD data held by the CPRD centre of the MHRA include a mother–baby link and, because of CPRD data minimisation requirements, we were given access to already linked mother–baby CPRD data. CPRD has linked mothers and children using several methods, including delivery-related information, information indicating pregnancy and pre- and postnatal information, which is explained in detail elsewhere. 74
To link mothers and babies in the THIN–HES and THIN primary care database, for each mother and for each delivery record she had within the study period, we looked in the associated database record to see if there was a baby with the same family number as that of the mother and linked them to the mother if the following conditions were true:
-
The baby’s year and month of birth were the same as the mother’s year and month of delivery.
-
The baby was registered with the practice within a year of birth.
In addition, after linkage, if the sex of the newborn was provided in the mother’s HES delivery record, then we cross-checked it with that in the primary care data to ensure accuracy. In addition, where a mother had more than one baby matching (e.g. twins), we randomly chose one baby.
We excluded delivery records and baby records that did not meet our matching criteria.
Defining index and exit date for patients in THIN and CPRD
For each of the records containing delivery information in HES, we checked if patient data existed in the associated THIN/CPRD practice records. If found, for each mother–baby pair, we set the index date as the date of delivery, defined as the OPCS-4 date or the hospital episode end date if the OPCS-4 date was missing. For mother and baby pairs for whom HES data did not exist, we assigned the date of birth of the baby as the index date for the baby and the date of delivery for the mother. These two dates can differ because only the month of birth is given in the baby’s records. At the same time, delivery date may reflect the date the information was entered in primary care records, as opposed to the true delivery date. We also defined the patient start date separately for the mother and baby as the latest of the date the quality of data was verified, study start date (1 January 2006) or registration date with the practice. For the mother, the day when they reached age 13 years was also included in determining the patient start date. We also defined the patient end date separately for the mother and baby, to determine the end of maximum follow-up for each patient, as the earliest of the death date, the date the patient left the general practice, 31 December 2018 or the last practice data collection date. If the patient was a mother, we added 6 weeks to the date of delivery in the list when calculating the patient end date, as maternal outcomes were limited to the post-partum period. If the patient was a baby, we included their date of birth plus 5 years in the list when calculating the patient end date. Once we defined these three key dates (i.e. the index date, patient start date and patient end date), we removed any patients whose start date was after their end date (e.g. error in recording of dates), whose index date was not between the start and end dates (e.g. if the HES data had information after the patient left the practice) or whose age at index date was not between 13 and 50 years (mother only).
HES data covering the whole of England
A mother–baby linked database was created using anonymised HES data collected for all NHS hospital admissions in England75 to allow the investigation of more severe outcomes of interest resulting in hospital admissions.
We requested HES data from NHS Digital on all delivery events occurring in English hospitals from financial years 2005/6 to 2017/18. We also asked for any admissions data relating to patients under the age of 5 years with the same encrypted HES identifier as a baby delivered in any of these delivery events and, for the mothers who underwent a delivery episode, any admissions either 1 month before delivery or 6 weeks after the start of the delivery episode. The application process was protracted. We completed the application in August 2018, but received data for the financial years 2005/6 to 2016/17 in April 2019. A data update for 2017/18 and 2018/19 was received in March 2020, but because of the project time restrictions, we were able to add only the latest admission data for the mother–baby pairs that we had already linked in the database using records for 2005/6 to 2016/17.
There is no shared identifier in the UK to link maternal and baby records in HES. We adapted a strategy that had already been validated by another recent study76 using deterministic and probabilistic linkage algorithms, allowing linkage of up to 98% of baby and mother secondary care records in England and still remaining nationally representative for the main birth characteristics (e.g. gestational age, birthweight, sex and maternal age). The processing workflow is outlined below.
We identified all of the delivery episodes and the spells to which they belonged. Episodes in which maternity care is delivered have a specific set of fields that contain critical data about the delivery pathway, as well as the usual data set of diagnostic and procedure fields that occur in any other kind of hospital admission episode. These are called the ‘maternity tail’ and include variables such as the weight of the baby, the maternal age, the pregnancy term in weeks and other clinical details. There is not an automatic linking field between babies and mothers in the anonymised data available in HES and so a simple linkage allowing details of a birth to be linked to future admissions involving the child is not possible. However, a baby born in hospital is technically admitted to the hospital and has an admission episode, even if they require no clinical treatment. This episode should contain all of the same data as the ‘maternity tail’ in the mother’s record. In practice, there are often a number of missing data items in the mother’s record and, more often, in the baby’s record. It is possible, however, to create an algorithm that evaluates the likelihood of a particular baby’s episode being linked to a particular mother’s birth event. For example, if all of the maternity fields match, if the baby’s admission date is the same day as the delivery and if the registered GP, the hospital and the postal district prefix of the postcode of residence are the same, then the chance that this delivery did not relate to this baby would be very low. If most of the data matched, but there were some missing items and no data items that contradicted each other, then the chances of mismatch would also be very small. If there was only one likely baby match, we could assume a deterministic linkage. The chances of an erroneous match would increase a little with each missing pair of data items, and it is possible that a situation could occur in which more than one baby could be matched to the birth episode could occur. In these cases, we had to apply probabilistic matching based on a matching threshold.
The first major data manipulation task was identifying all of the delivery episodes that we were going to match. This is not a straightforward task, as episodes that contain data relating to deliveries are sometimes not specifically flagged as a delivery episode. In addition, some episodes that are clearly flagged as delivery episodes do not contain all of the data needed to obtain a match. This is because there can be a number of episodes in a spell in hospital that include a delivery and, despite coding and clerking standards, some data that belong in one episode can be spread across more than one. We identified any episode that met the criteria used by Harron et al. ,76 namely that the episode should contain a code indicating the delivery of a live infant, a procedure code for a delivery procedure or have two or more ‘maternity tail’ fields that are completed with a valid (i.e. not null or system missing) value. When these episodes had been identified, we used the unique spell identifier to extract all episodes in each spell containing a delivery episode.
Cleaning was carried out to remove duplicate records, episode durations with implausible date ranges and births where there was an implausible interval between two or more birth events. This happens rarely and is usually because a mother is misidentified in the data set and is given the same NHS identifier (ID) as someone else. If both these women had given birth within, for example, 6 weeks of each other, then it appears that one woman has given birth twice, with an implausible duration between the two deliveries. In these cases, records for both women were censored.
Mother–baby linkage
The Harron et al. 76 methodology was based on a complex synthetic data set where iterations of data removal were undertaken in key mother and baby variables and probabilities of mismatches were calculated given that different variable or combinations of variable were missing on one side or the other of the match. Through this process, a series of probabilities for different matching scenarios were calculated, as well as a series of weights to determine the likelihood of a valid match, given that a specific variable was matched or not matched. The process was based on 1 year of data for 2012/13. Data recording in HES was improving year on year in the period of our study. It is likely that the data years before 2012/13 would have had more missingness than the Harron et al. 76 data and the years afterwards. As Harron et al. ’s76 weights were calculated roughly in the middle of our period of study, we felt that they could be applied reliably to our data. We decided that rather than replicating the original synthetic data set creation, which would have been outside the resource capabilities of this study as originally designed, we would repeat Harron et al. ’s76 matching methods, but use the matching weights published in their paper. 76
During key stages in the work, we had clarification from Harron et al. 76 on some of the aspects of their method that were not covered in detail in the publication. The linkage was carried out in two stages. Where data could be linked using a simple rules-based approach following the original study, we followed the original authors in referring to this as deterministic linkage. Any delivery events that could not be linked deterministically were linked probabilistically.
Deterministic (rules-based) linkage
Mother and baby records were treated as matching if records matched on general practice, maternal age, birthweight, gestation, birth order and sex. The matching could allow for missing data, but not for any contradictory data on either side of the match. If three or more of the variables matched exactly, and no variables were mismatched, the match was assumed to be valid.
Probabilistic linkage
Any delivery records that were unmatched deterministically were compared with baby records on 21 separate variables. Each one had a positive match weight where there was a match and a negative weight where there was a mismatch. These were applied to a matching matrix and the weights summed to derive a total weight for each candidate pair. The weights were determined from the synthetic data analysis produced by Harron et al. 76 and were available in their published paper. Their team also used the same threshold (a match score of 20) to decide whether or not the match was probable.
Linkage to survey data
The final stage involved matching national survey data on hospital prophylactic antibiotic administration policies (see National survey data for more details on the survey) to births. This involved identifying the NHS organisation code for each site about which the respondent was answering. This was carried out by cross-checking all of the hospital names with the NHS Coding and Classification Service records. In addition, each site had to be checked to see whether or not its site code had changed during the period of study; this happens when the trust responsible for the site changes. This also involved manual searches on a database of successor and predecessor organisations held by the NHS Coding and Classification Service. Not all deliveries were matched because not all sites replied to the questionnaire. There were some cases where individual units did not collect their site codes with sufficient specificity. This can happen when one trust manages a large number of smaller sites. There is a highly specific site code capable of attributing activity to an individual site; however, some organisations do not use the code appropriately, choosing to apply a more generic code with a trust prefix but no suffix (i.e. the part of the codes that identifies the site specifically). We linked only those questionnaire data where a valid and site-specific code was captured in HES and was the same as a site code attributed to a hospital that had responded to the survey.
National survey data
National survey of hospital prophylactic antibiotic administration policies
Information about which prophylactic antibiotics are given and the timing of their administration is not available in HES. Therefore, we obtained the year when the policy to administer pre-incision antibiotics was introduced in each hospital from a survey of maternity care providers.
A national survey of the clinical directors for UK maternity units was completed to investigate (1) to what extent the recommendation in the NICE Clinical Guideline 1327 regarding the timing of prophylactic antibiotic administration for CS has been adopted across the country, (2) when the updated national guidance was implemented and (3) the types of pre-incision antibiotics used in practice.
The survey was developed using a secure, web-based software platform REDCap (Research Electronic Data Capture) designed to support data capture for research studies (version 7, Vanderbilt University, Nashville, TN, USA)77 and a link to the survey was sent to all clinical directors of maternity units in 2017, with follow-up of non-responders undertaken by e-mail, telephone and at national professional conferences. During the follow-up, an option to complete the survey in Microsoft Word (Microsoft Corporation, Redmond, WA, USA) was also offered. A copy of the survey questionnaire is included in Report Supplementary Material 1. If the respondents did not specify the exact year of the policy change, but had entered a free-text comment saying, for example, a year before which the policy was changed (e.g. ‘before 2009’) then this year was used in the analysis as the year of the policy change.
National maternity care surveys
Information on breastfeeding in health-care data is incomplete and we, therefore, investigated the trends in this case mix variable by the mode of delivery using data from the national maternity surveys, with the survey methodology described in detail elsewhere. 78,79 Briefly, the Care Quality Commission undertook nationwide surveys of women who had a live birth and received care from NHS trusts in 2007, 2010, 2013, 2015 and 2017 in England, asking about their experiences during labour and birth, including the type of delivery and newborn feeding in the first few days after birth. 79 Care Quality Commission survey data were available from the UK Data Service. 80 National surveys were also carried out in 2006, 2010 and 2014 by the National Perinatal Epidemiology Unit (Oxford, UK) using a random sample from live birth registrations in England. The questionnaires were posted to women at 3 months after delivery and asked questions on antenatal and postnatal care and health, including questions on the mode of delivery and infant feeding at the time of the survey (e.g. breast milk only, both breast milk and formula milk, and formula milk only). 78
Estimated sample size and statistical power
THIN–CPRD database sample size estimates
To estimate the number of CSs and VDs in primary care over our study period, we first calculated the completeness of recording in THIN database using THIN data linked to secondary care records in 2010. This was extrapolated over all years in our study (2006–18), taking into consideration the changes in the numbers of births nationally over time. We assumed that the overall size of the THIN and CPRD databases, the proportions with linked secondary care data and the reported overlap between THIN and CPRD databases remained constant over the study period. We also assumed that 86% of mothers and babies could be linked. The number of births we expected to have data on over the study period was 777,389 (206,615 children born by CS and 570,774 children born by VD).
Our estimates of asthma incidence in 0- to 5-year-old children in the UK using the THIN database matched the previously published figures. 38 In 2014, the incidence ranged from 0.8 and 6.5 per 1000 person-years in those aged 0–1 and 1–2 years, respectively, to 12.1 and 11.4 in those aged 3–4 and 4–5 years, respectively. Based on our estimates of the incidence of asthma in each age group in each year from 2006 onwards, and assuming a 5% annual loss of follow-up due to patients changing their general practice, Table 3 shows the estimated number of children available for follow-up depending on their year of birth, and the number of new asthma cases in each of the study years by the mode of delivery.
| Birth yeara | Person-years of follow-up by year of life | Asthma cases by year of life | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |
| Children born by CS | ||||||||||
| 2006 | 14,448 | 13,725 | 13,038 | 12,386 | 11,766 | 70 | 214 | 230 | 254 | 183 |
| 2007 | 15,136 | 14,379 | 13,660 | 12,977 | 12,328 | 56 | 196 | 220 | 236 | 166 |
| 2008 | 15,308 | 14,542 | 13,814 | 13,123 | 12,466 | 38 | 169 | 201 | 207 | 140 |
| 2009 | 15,394 | 14,624 | 13,892 | 13,197 | 12,537 | 35 | 153 | 179 | 194 | 147 |
| 2010 | 15,781 | 14,991 | 14,241 | 13,528 | 12,851 | 31 | 140 | 160 | 184 | 157 |
| 2011 | 15,824 | 15,032 | 14,280 | 13,566 | 12,887 | 28 | 126 | 166 | 193 | 159 |
| 2012 | 16,254 | 15,441 | 14,668 | 13,934 | 13,237 | 26 | 116 | 175 | 206 | 166 |
| 2013 | 16,082 | 15,277 | 14,513 | 13,787 | 13,097 | 19 | 107 | 154 | 185 | 157 |
| 2014 | 15,996 | 15,196 | 14,436 | 13,714 | 13,028 | 12 | 99 | 134 | 166 | 149 |
| 2015 | 16,598 | 15,768 | 14,979 | 14,230 | 8 | 95 | 121 | 156 | ||
| 2016 | 16,598 | 15,768 | 14,979 | 5 | 88 | 106 | ||||
| 2017 | 16,598 | 15,768 | 3 | 82 | ||||||
| 2018 | 16,598 | 3 | ||||||||
| Children born vaginally | ||||||||||
| 2006 | 43,172 | 41,013 | 38,962 | 37,013 | 35,162 | 209 | 640 | 688 | 759 | 548 |
| 2007 | 44,462 | 42,238 | 40,126 | 38,119 | 36,213 | 163 | 576 | 646 | 692 | 486 |
| 2008 | 44,892 | 42,647 | 40,514 | 38,488 | 36,563 | 112 | 497 | 590 | 608 | 412 |
| 2009 | 44,720 | 42,484 | 40,359 | 38,341 | 36,423 | 101 | 445 | 520 | 564 | 427 |
| 2010 | 45,744 | 43,456 | 41,283 | 39,218 | 37,257 | 91 | 405 | 463 | 534 | 454 |
| 2011 | 45,580 | 43,301 | 41,135 | 39,078 | 37,124 | 82 | 364 | 477 | 555 | 459 |
| 2012 | 45,408 | 43,137 | 40,980 | 38,931 | 36,984 | 72 | 324 | 490 | 575 | 463 |
| 2013 | 43,344 | 41,176 | 39,117 | 37,161 | 35,302 | 51 | 288 | 415 | 500 | 423 |
| 2014 | 42,484 | 40,359 | 38,341 | 36,423 | 34,601 | 32 | 262 | 355 | 442 | 396 |
| 2015 | 42,742 | 40,604 | 38,573 | 36,644 | 21 | 245 | 312 | 402 | ||
| 2016 | 42,742 | 40,604 | 38,573 | 14 | 227 | 273 | ||||
| 2017 | 42,742 | 40,604 | 9 | 211 | ||||||
| 2018 | 42,742 | 9 | ||||||||
HES database sample size estimates
Using the HES secondary care database available for England and assuming 95% linkage between mothers and babies (the linkage estimate varied between 91% in the earlier years and 98% in 2012/13),76 we estimated that we would obtain data from 8,043,600 children with a known mode of delivery across 2006–18 (2,070,500 children born by CS and 5,973,100 born by VD).
Annually, there are around 10,000 hospital admissions with asthma as the primary diagnosis in children aged 0–4 years. 81 Assuming a re-admission rate during the follow-up of 50% (our estimates using HES indicate that, in the later years of the cohort, the proportion of children re-admitted was as low as 29–39%), we calculated the numbers of children who were admitted to hospital for the first time with asthma as the primary diagnosis in each year of follow-up (Table 4).
| Birth yeara | Person-years of follow-up by year of life | Asthma cases by year of life | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |
| Children born by CS | ||||||||||
| 2006 | 145,200 | 145,178 | 144,838 | 144,499 | 144,160 | 22 | 343 | 342 | 341 | 340 |
| 2007 | 151,800 | 151,777 | 151,422 | 151,067 | 150,713 | 23 | 358 | 357 | 357 | 356 |
| 2008 | 152,400 | 152,377 | 152,021 | 151,664 | 151,308 | 23 | 360 | 359 | 358 | 357 |
| 2009 | 153,700 | 153,677 | 153,317 | 152,958 | 152,599 | 23 | 363 | 362 | 361 | 360 |
| 2010 | 157,400 | 157,376 | 157,008 | 156,640 | 156,272 | 24 | 371 | 371 | 370 | 369 |
| 2011 | 158,800 | 158,776 | 158,405 | 158,033 | 157,662 | 24 | 375 | 374 | 373 | 372 |
| 2012 | 162,700 | 162,676 | 162,295 | 161,914 | 161,534 | 24 | 384 | 383 | 382 | 381 |
| 2013 | 160,700 | 160,681 | 160,321 | 159,961 | 159,602 | 19 | 360 | 359 | 358 | 358 |
| 2014 | 160,200 | 160,187 | 159,846 | 159,505 | 159,164 | 13 | 340 | 339 | 338 | 337 |
| 2015 | 166,900 | 166,887 | 166,531 | 166,176 | 13 | 354 | 353 | 352 | ||
| 2016 | 166,900 | 166,887 | 166,531 | 13 | 354 | 353 | ||||
| 2017 | 166,900 | 166,887 | 13 | 354 | ||||||
| 2018 | 166,900 | 13 | ||||||||
| Children born by VD | ||||||||||
| 2006 | 452,400 | 452,364 | 451,400 | 450,437 | 449,475 | 68 | 1059 | 1056 | 1054 | 1052 |
| 2007 | 465,400 | 465,363 | 464,371 | 463,380 | 462,391 | 70 | 1089 | 1087 | 1084 | 1082 |
| 2008 | 467,500 | 467,463 | 466,467 | 465,471 | 464,478 | 70 | 1094 | 1092 | 1089 | 1087 |
| 2009 | 466,000 | 465,963 | 464,970 | 463,978 | 462,987 | 70 | 1090 | 1088 | 1086 | 1083 |
| 2010 | 477,300 | 477,262 | 476,245 | 475,229 | 474,214 | 72 | 1117 | 1114 | 1112 | 1110 |
| 2011 | 476,600 | 476,600 | 476,600 | 476,600 | 476,600 | 71 | 1115 | 1115 | 1115 | 1115 |
| 2012 | 474,900 | 474,900 | 474,900 | 474,900 | 474,900 | 71 | 1111 | 1111 | 1111 | 1111 |
| 2013 | 453,700 | 453,700 | 453,700 | 453,700 | 453,700 | 54 | 1012 | 1012 | 1012 | 1012 |
| 2014 | 444,500 | 444,500 | 444,056 | 443,167 | 441,834 | 36 | 947 | 946 | 944 | 941 |
| 2015 | 448,700 | 448,664 | 447,708 | 446,753 | 36 | 956 | 954 | 952 | ||
| 2016 | 448,700 | 448,664 | 447,708 | 36 | 956 | 954 | ||||
| 2017 | 448,700 | 448,664 | 36 | 956 | ||||||
| 2018 | 448,700 | 36 | ||||||||
Estimates of statistical power
To estimate statistical power, and to assess the impact of misclassification of antibiotic exposure on estimates of increase in risk with pre-incision prophylactic antibiotics for CS, we simulated the study (1) based on our estimates using the THIN database regarding the number of children for whom maternal data could be linked and the asthma diagnosis rates in each year group between 0 and 5 years, and (2) using HES data based on our estimates of children with linked maternal data and rates of children newly hospitalised for asthma based on HES statistics.
In each simulation, we created a data set for the whole study, with 13 birth cohorts from 2006 to 2018 and follow-up included across the first 5 years of life (curtailed at the end of 2018). Each birth was classified by the delivery mode and, for those children born by CS, whether or not prophylactic antibiotics were administered prior to skin incision (generated randomly using a binomial random number generator using an underlying probability identified in the national survey of hospital prophylactic antibiotic administration policies during that year of birth). 49
We randomly simulated outcome events according to the year-age event rates using a binomial random number generator, with increased rates in all those born by CS and further increases in those who had pre-incision antibiotics (Table 5). A ratio of incidence ratios of 1.20 was used for increased risk of asthma with CS. 47 Further increases (ratio of incidence ratios from 1.10 to 1.20, increasing in steps of 0.02) were used for the increase with pre-incision antibiotics rather than antibiotics after cord clamping.
| Variable | THIN–CPRD database, n | HES database, n |
|---|---|---|
| CS births | 206,615 | 2,070,500 |
| Post-cord clamping antibiotics | 111,508 | 1,115,670 |
| Pre-incision antibiotics | 95,107 | 954,830 |
| VD births | 570,774 | 5,973,100 |
| Total births | 777,389 | 8,043,600 |
| CS person-years of follow-up | 792,265 | 8,661,832 |
| Post-cord clamping antibiotics | 501,401 | 5,524,890 |
| Pre-incision antibiotics | 290,864 | 3,136,942 |
| VD person-years of follow-up | 2,215,405 | 25,339,526 |
| Total person-years of follow-up | 3,007,670 | 34,001,358 |
| New events in children born by CS | 7173 | 15,333 |
| Post-cord clamping antibiotics | 5324 | 10,454 |
| Pre-incision antibiotics | 1849 | 4880 |
| New events in children born by VD | 20,378 | 44,906 |
| Total events | 27,551 | 60,240 |
| Average event rate per 1000 person-years | 9.2 | 1.8 |
Simulations were repeated 1000 times and statistical power estimated by noting the proportion of simulations for which the lower limit of the 95% CI for the variable indicating if antibiotics were administered prior to skin incision was greater than a ratio of incidence ratios of 1. We also assessed attenuation bias created by misclassification.
The model that was fitted to analyse the simulation data included a trend term for the probability of receiving antibiotics before skin incision, where the value for 2006–9 = 0, 2010 = 0.2, 2011 = 0.4, 2012 = 0.6, 2013 = 0.8 and 2014–18 = 1 (with a zero value for those who were born by VD). Although in the analysis we used values obtained from the national survey on hospital prophylactic antibiotic administration policies, as the simulations used the national survey to allocate women to a treatment, we would have overestimated power if we had used exactly the same function in the analysis model.
For outcomes in primary care, we estimated that we would have 80% power of detecting a ratio of incidence ratios of 1.16 and > 90% power of detecting ratio of incidence ratios of 1.18, being able to estimate them with a maximum of 15% underestimation from misclassification.
For outcomes recorded in secondary care, in the full HES data set, we estimated that we would have > 80% power to detect a ratio of incidence ratios of 1.10 and 90% power to detect a ratio of incidence ratios of 1.12, with similar underestimation rates due to misclassification.
This relates to detecting an increase of seven additional new cases of asthma per 1000 children aged 0–4 years or an increase of 4% (1400/35,000) of all new asthma cases in 0- to 4-year-olds annually in the UK. This also relates to detecting an increase of one additional new case of asthma requiring hospital admission per 1000 children aged 0–4 years or an increase of 3% (200/7000) of all newly hospitalised asthma cases in 0- to 4-year-olds annually.
We also estimated that this study would be adequately powered to detect differences in some of the other outcomes of interest, as their incidence is higher than asthma incidence in children, including:
Statistical methods and analyses
The statistical analysis methods for this project can be broken down into the methods used to analyse the primary care data set (THIN–CPRD) and the methods used to analyse the secondary care data set (HES). We describe these methods separately for each data set.
Primary care data (THIN–CPRD)
Summarising data in primary care
The primary outcomes (of asthma and eczema) are summarised in the THIN–CPRD data set using the number of children diagnosed with asthma or eczema and the length of time at risk. Each child is classified as either diagnosed with the outcome or not, using the methods of diagnosing asthma and eczema described earlier (see Study outcomes). For each outcome, the length of time at risk is calculated as the time from birth to the earliest of the child’s fifth birthday, the date the child is lost to follow-up (e.g. the child moves practice or the practice is not included in THIN–CPRD), the study end date (31 December 2018) or the date at which the child is diagnosed with the outcome. We present the rate of the outcome per 1000 person-years, which is calculated as the ratio of the number of events and the exposure time multiplied by 1000. We do not present results stratified by prophylactic antibiotic status, as this was not possible (see Analysis methods used).
All dichotomous secondary outcomes are summarised in the same way as the primary outcomes. Secondary outcomes that are of a count form, such as the number of primary care consultations in the first 12 months, are summarised using the total number of events and the length of time at risk. The length of time at risk is calculated as the time from birth to the earliest of the date the child is lost to follow-up or the date at which the outcome-specific length of time has passed (e.g. 12 months). The rate of the outcome is presented per 1000 person-years.
Maternal outcomes are summarised using the total number of events and the percentage of total births to which this corresponds. All maternal outcomes in primary care were dichotomous. For the composite maternal infectious morbidity, we categorised mothers as having either ‘none’ or ‘one or more’ of the individual composite outcomes (i.e. wound infection, endometritis/endomyometritis, sepsis and pelvic abscess) recorded in primary care data. Results are presented stratified by delivery mode (VD or CS).
Analysis methods used
To analyse the primary outcomes (asthma and eczema), we calculated for each child’s year of life whether or not a diagnosis of the outcome was made and the exposure time for that particular year of life. For example, a child diagnosed with asthma aged 18 months would have zero events and 12 months’ exposure during their first year of life, and one event and 6 months’ exposure during their second year of life. This allowed us to explore cases by year of birth and year of life. To estimate the impact of antibiotic timing (pre-incision or post-cord clamping prophylactic antibiotics), we fitted a Poisson regression model to the data, with an offset for exposure time and robust standard errors to allow for misspecification. The model included terms for year of birth and age (year of life), and their interaction. Two further terms were also included in the model: (1) mode of birth (CS or VD’) to allow for differential risk of the outcome in those receiving CS compared with VD’ and (2) antibiotic timing’ to allow for differential risk in those receiving pre-incision rather than post-cord clamping prophylactic antibiotics. For all CS births, as the exact timing of antibiotic provision was not known, we used the probability of antibiotic use before skin incision derived from the national survey. This coefficient provides an estimate of the effect of the policy change in antibiotic timing, adjusting for misclassification. As the policy change was applicable for CS births only, all VD births were classified as pre-policy change. We present the incidence rate ratio (IRR) and the 95% CI for the antibiotic timing variable, comparing the rate of events in those given prophylactic antibiotics pre incision with the rate of events in those given prophylactic antibiotics post cord clamping.
All dichotomous secondary outcomes are analysed in the same manner as the primary outcomes. For outcomes with a small number of events (< 200), we present the summary statistics only. For count outcomes, a Poisson model without robust standard errors was fitted to the data. All other terms in the model were the same as those used for the primary outcomes.
Sensitivity analyses
To assess the robustness of the results, we conducted several sensitivity analyses. Sensitivity analyses were performed for the two co-primary outcomes (asthma and eczema). Some secondary outcomes were included in the sensitivity analyses to check the robustness of the primary analysis, particularly where results suggested a significant association with the timing of antibiotic provision and the association was unexpected.
In the primary care data set, we conducted the following sensitivity analyses:
-
outcome definition
-
definition of timing of the prophylactic antibiotic policy change
-
data recording quality
-
random effect.
The rationale for these sensitivity analyses and the statistical methods used for each of them are described in more detail below.
Outcome definition
The main outcome definition for asthma in primary care was based on relevant Read codes and the main definition of eczema was based on relevant Read codes in combination with two eczema-related prescriptions on separate dates, as described earlier. We considered the impact of prophylactic antibiotic timing on severity of asthma and eczema based on prescribing of medications indicative of moderate or severe asthma and eczema. In addition, we also considered several alternative definitions of asthma and eczema, including:
-
asthma diagnosed in children aged 3–5 years (as it is more difficult to distinguish asthma from other more common diagnoses, such as bronchiolitis, in very young children)
-
asthma definition based on Read codes used in the QOF
-
eczema diagnosis based on Read codes in children aged 1–5 years and at least two prescriptions within 90 days before or 365 days after the eczema Read code record
-
eczema diagnosis based on Read codes only.
Definition of timing of the prophylactic antibiotic policy change
For the primary analysis, we used a probability that the prophylactic antibiotics were given pre incision using data from the national survey. To test the sensitivity of this method, we restricted the data set to the births from 2006 to 2010 and from 2013 to 2018. This was carried out to compare births in the years before the change in the NICE guidelines (i.e. 2006–10) with the years in which > 50% of hospitals had introduced the policy (i.e. 2013–18). Children born by CS were dichotomised as having prophylactic antibiotics post cord clamping (children born 2006–10) or prophylactic antibiotics pre incision (children born 2013–18). A Poisson model was fitted to the data, with an offset for exposure time and robust standard errors. The model included terms for year of birth, year of life, their interaction, delivery mode (CS or VD) and antibiotic timing (pre incision or post cord clamping).
Data recording quality
To evaluate the impact of data recording quality, we restricted the data set to THIN–CPRD records linked to secondary care records. It was expected that these records would be the most accurate source for mode of delivery. A Poisson model was fitted to the data in the same manner as the primary analysis.
Random effect
Our primary analysis assumes that all observations are independent. As the outcomes are based on Read codes that have been entered onto an electronic medical health system at the general practice, it is possible that observations from children within the same general practice are correlated in some way (both because of access to the same general practice and also because they are likely to share socioeconomic similarities). To explore the possibility of non-independence of observations, we explored whether or not the results were robust to the inclusion of a random effect for general practice. This allows observations within a general practice to be correlated. To this end, a mixed-effects Poisson model was fitted to the data, with an offset for exposure time and robust standard errors and a random effect for general practice. Fixed effects were included for year of birth, year of life, their interaction, mode of birth (CS or VD) and antibiotic timing (i.e. probability of pre-incision prophylactic antibiotics).
Subgroup analyses
Children born by elective CS are likely to be exposed to antibiotics in utero for longer than children born by emergency CS. To assess whether or not the impact of the antibiotic policy change is different in elective CS and emergency CS, we categorised each birth as being elective CS, emergency CS or VD. A Poisson model was fitted to the data, with an offset for exposure time and robust standard errors. Fixed effects were included for year of birth and year of life, and their interaction. There were also terms for mode of delivery (i.e. emergency CS, elective CS or VD) and antibiotic timing (i.e. probability of pre-incision prophylactic antibiotics), and an interaction between them. We report the IRR for antibiotic timing in the emergency CS and elective CS groups, and the p-value for the interaction between them. This p-value indicates the strength of evidence of a differential effect of antibiotic timing based on CS type.
Secondary care data (HES)
Summarising data in secondary care
The primary outcomes (asthma and eczema) are summarised in the HES database using the number of children admitted to hospital at least once and the length of time at risk. For each outcome, an admission is indicated by ICD-10 codes, as described earlier (see Study outcomes). Children are categorised as having ‘no admission’ or as having ‘one or more admissions’. The length of time at risk is calculated as the time from birth to the earliest of the child’s fifth birthday or the study end date of 31 March 2019. We present the rate of the outcome per 1000 person-years, which is calculated as the ratio of the number of events and the exposure time multiplied by 1000.
Most dichotomous secondary outcomes are summarised in the same way, where we present the number of children admitted at least once, the total follow-up time and the outcome rate per 1000 person-years. For secondary outcomes with short exposure time (such as neonatal sepsis), we present the outcome rate per 1000 births.
Dichotomous maternal outcomes are summarised in the HES data set using the number of events and the percentage of total events to which this corresponds. One continuous maternal outcome (i.e. hospital length of stay) was not normally distributed and so the median and interquartile range are presented. All maternal outcomes are summarised stratified by delivery mode (VD or CS).
Analysis methods used
To analyse the primary outcomes (asthma and eczema), for each child we calculated whether or not they had been admitted to hospital admission for the primary reason of asthma or eczema and their exposure time. Owing to data limitations, we did not have the date of admission and so could not look at cases by year of life. We did, however, examine cases by year of birth. To estimate the impact of antibiotic timing (pre-incision or post-cord clamping prophylactic antibiotics), a Poisson model was fitted to the data, with an offset for exposure time and robust standard errors to allow for misspecification. The model included terms for year of birth, mode of birth (CS or VD) and antibiotic timing. Data were not available on the antibiotic timing (pre incision or post cord clamping) for individual births, but data on the hospital policy for each year were known. It was assumed that prophylactic antibiotics for each CS were administered in accordance with the hospital’s policy for that particular year. Antibiotic timing was categorised as before policy change, year of policy change and after policy change. As the policy change was applicable to CS births only, all VD births were classified as ‘before policy change’. The comparison between children born after the policy change and those born before the policy provides an estimate of the effect of the policy change in antibiotic timing. This comparison did not include those children born in the year of policy change, as their exposure to prophylactic antibiotics is not known. We present the IRR and 95% CI for the antibiotic timing variable, comparing the rate of events in those given prophylactic antibiotics pre incision with the rate of events in those given prophylactic antibiotics post cord clamping.
All dichotomous secondary outcomes are analysed in the same manner as the primary outcomes. For outcomes with a small number of events (< 200), we present the summary statistics only. For continuous outcomes (i.e. maternal length of stay), we fitted a linear regression model, with fixed effects for year of birth, mode of birth (CS or VD) and antibiotic timing. We present the difference in mean length of stay between those children born by CS with pre-incision prophylactic antibiotics and those children born by CS with post-cord clamping prophylactic antibiotics.
Sensitivity analyses
To assess the robustness of the results, we conducted several sensitivity analyses. Sensitivity analyses were performed for the two co-primary outcomes (asthma and eczema). Some secondary outcomes were included in the sensitivity analyses to check for the robustness of the primary analysis.
In the secondary care data set (HES), we conducted the following sensitivity analyses:
-
definition of timing of the prophylactic antibiotic policy change
-
random effect
-
exploratory discordant sibling analysis.
Definition of timing of the prophylactic antibiotic policy change
The primary analysis of the secondary care data set is limited to hospitals that responded to the national survey, as we required information on whether or not the antibiotic policy was changed and the year in which the change was made. As not all hospitals responded to the survey, we wanted to explore the sensitivity of the results to the timing of antibiotic policy change. To this end, we estimated the probability of pre-incision prophylactic antibiotics based on calendar year. These probabilities were calculated using responses to the national survey from England hospitals, as HES includes data from hospitals in England only. A Poisson model was fitted to the data, with an offset for exposure time and robust standard errors. Fixed effects were included for year of birth, mode of birth (CS or VD) and antibiotic timing (probability of pre-incision prophylactic antibiotics).
Random effect
Our primary analysis assumes that observations are independent. However, it is possible that observations from children born from the same hospital are correlated in some way, perhaps because of sociodemographic and health-care access similarities. To explore the possibility of non-independence of observations, we explored whether or not the results were robust to the inclusion of a random effect for hospital. This allows observations from children born within the same hospital to be correlated. To this end, a mixed-effects Poisson model was fitted to the data, with an offset for exposure time and robust standard errors, and a random effect for hospital. Fixed effects were included for year of birth, mode of birth (CS or VD) and antibiotic timing (pre incision or post cord clamping).
Discordant sibling analysis
In an attempt to control further for family-related genetic and environmental factors, we employed a discordant sibling analysis. For this, we created a subset of data in which women gave birth at least twice using the same mode of birth (VD or CS) during the study, including before and after the change in antibiotic policy. A Poisson model was fitted to the data in the same manner as the primary analysis of the secondary care data.
Subgroup analyses
In the secondary care data set, we performed two subgroup analyses: (1) CS type analysis (i.e. elective or emergency) and (2) antibiotic regiment analysis (i.e. cefuroxime only, cefuroxime and metronidazole, and co-amoxiclav only).
Caesarean section type
To assess whether or not the impact of the antibiotic policy change differs between elective CS and emergency CS, we categorised each birth as being elective CS, emergency CS or VD. A Poisson model was fitted to the data, with an offset for exposure time and robust standard errors. Fixed effects were included for mode of delivery (emergency CS, elective CS or VD) and antibiotic timing (using probability of antibiotic policy change), and an interaction between them. Adjustments were included for year of birth. We report the IRR for antibiotic timing in the emergency and elective CS groups, and the p-value for the interaction between them.
Antibiotic regimen
Different antibiotic regimens may have a differential effect on longer-term child health. Based on the national survey, the most common antibiotics used were co-amoxiclav alone, cefuroxime alone, and cefuroxime and metronidazole. We restricted the analysis to hospitals that used the same antibiotics before and after the policy change. A Poisson model was fitted to the data, with an offset for exposure time and robust standard errors. Fixed effects were included for year of birth, mode of delivery (CS of VD) and antibiotic timing (pre incision or post cord clamping). We fitted a model separately for each antibiotic type (co-amoxiclav alone, cefuroxime alone, cefuroxime and metronidazole). We report the IRR for antibiotic timing separately for each antibiotic type. This exploratory subgroup analysis was undertaken for primary outcomes (asthma and eczema) and necrotising enterocolitis because of the previously reported increased risk of this condition in babies born to mothers who were exposed to co-amoxiclav. 83
Analysis was carried out using Stata® MP version 16 (StataCorp LP, College Station, TX, USA).
Research Ethics Committee approval
The Ethics Review Committee of the University of Birmingham provided the ethics approval required for this study (reference number ERN_17-1675).
The NHS South-East Multi-Centre Research Ethics Committee approved the use of the THIN database, subject to independent scientific review to help ensure appropriate analysis and interpretation of the data. 84 The Independent Scientific Ethical Advisory Committee – Scientific Review Committee Panel of the data provider IQVIA (Durham, NC, USA) approved the use of the THIN and HES-linked data in this study (reference number 18THIN047).
The CPRD data set has ethics approval from a National Research Ethics Service Committee for observational research using anonymised data. 85 For this study, the use of CPRD has been approved by the Independent Scientific Advisory Committee for MHRA Database Research (reference number 18_181AR2).
The use of the HES database is exempt from NHS Research Ethics Committee approval because it involves the analysis of an existing data set of non-identifiable data. The standard NHS Digital data approval process was followed to obtain approval for the use of HES data. 86 The Health Research Authority confirmed that Health Research Authority approval was not required for this research, as it involved linking anonymised patient data from established databases for this study only.
Changes to the protocol
There were no changes to the protocol.
Patient and public involvement
Aims
There were two main aims of patient and public involvement (PPI) in this project. First, we involved women to ensure that we included outcomes that are important for parents and children. We explored with parents and parents-to-be which health conditions, particularly allergy- and immune system-related conditions and maternal infections after birth, are most important to them and, therefore, which health conditions the study should ideally investigate (assuming that information for these conditions is well recorded in routine medical practice). Second, our public involvement activities helped ensure that key messages from the research about the timing of prophylactic antibiotic administration in CS were communicated to relevant audiences in ways that were clear and accessible. We report our PPI here using the format recommended by the Guidance for Reporting Involvement of Patients and the Public Short Form (GRIPP2-SF). 87
Methods
The chief investigator (DS) provided leadership for all public involvement in the project and was the first point of contact for public contributors and researchers on all matters relating to public involvement. Two women (RH and MS) were recruited to be involved in the study. Ruth Hewston is a volunteer with the National Childbirth Trust (London, UK) and has experience of involvement in a range of maternity projects. Magdalena Skrybant had a CS in 2013 and has an interest in maternity research. Both Magdalena Skrybant and Ruth Hewston were invited to attend all Project Management Group meetings. Materials were sent in advance of meetings and Ruth Hewston and Magdalena Skrybant could contact Dana Šumilo at any point to discuss the project. Both Magdalena Skrybant and Ruth Hewston were invited to contribute to all reports and they are acknowledged as co-authors to publications. Public contributors were also offered an honorarium for their involvement.
We also recruited an independent public contributor to our Project Steering Group (MC). MC was recruited through the Royal College of Obstetricians and Gynaecologists (RCOG) (London, UK) Women’s Voices Involvement Panel. Maria Clark was invited to attend all meetings, received paperwork in advance and could contact Dana Šumilo to discuss the study.
To gain wider public perspectives, public involvement sessions took place as part of the ‘Yoga for Bump’ sessions, which were facilitated by National Institute for Health and Care Research (NIHR) Collaborations for Leadership in Applied Health Research and Care and funded through a Wellcome Trust (London, UK) Engagement Fund. These sessions were free for women to attend and included an informal chat with a midwife, a research engagement session and then a pregnancy yoga session. The sessions were held in June 2018 in two different community locations in the West Midlands (Hall Green and Perry Barr) and at two different times of the day, which helped maximise the diversity of the contributors. Eight mothers or mothers-to-be took part in the sessions. They came from a variety of backgrounds, and included women from ethnic minority communities, an often under-represented group in research. The researcher (DS) and a PPI advisor (MS) of the project explained the background of this research and the research questions. This was followed by the discussion, in relation to this study, of which health conditions are most important to women and how the findings of the study can be shared.
We also obtained perspectives from women from the RCOG Women’s Voices Involvement Panel who responded to the wider clinician and public consultation survey in July 2018. A link to the online survey was also sent to the British Intrapartum Care Society (London, UK), the British Maternal & Fetal Medicine Society (London, UK) and the British Association of Perinatal Medicine (Bristol, UK). In the survey, respondents were asked to select up to three conditions from each group of health conditions that we were interested in for inclusion as study outcomes (the survey questionnaire is included in Report Supplementary Material 2). Twenty-nine respondents (22 clinicians and seven parents) completed the survey.
Both Magdalena Skrybant and Ruth Hewston contributed to the writing of this report, including Patient and public involvement and the Plain English summary.
Results
Patient and public involvement, including the public involvement sessions and the wider survey, helped us to confirm that we should look at a wide range of outcomes, including neurodevelopmental conditions. From the proposed outcome list in the online survey, there were no outcomes that were not selected by the respondents, apart from septic thrombophlebitis, which we excluded from the final outcome list. Our public involvement sessions also confirmed that we should consider the severity of outcomes.
Patient and public involvement also helped us to identify other information mothers-to-be wanted explained in relation to the study, including how antibiotics work, what antibiotics are recommended, the difference between intrapartum antibiotics and antibiotics during pregnancy, and what impact antibiotics may have depending on when they are provided. PPI also confirmed the importance of balancing the risks and benefits to the mother and baby when communicating the study findings.
Discussion and conclusions
Patient and public involvement helped us to confirm the finding from our initial PPI while developing this research proposal that a broad range of outcomes should be investigated. PPI engagement sessions informed the development of the wider consultation through the online survey and the final outcome list. PPI also confirmed the importance of capturing and interpreting the data, taking into account the severity of health conditions, which was addressed in this study by assessing the health conditions in both primary care and hospital data. Furthermore, PPI strengthened the importance of comparing the impact on children with the impact on mothers and helped us to better understand what relevant information women need to know when communicating the results of the study.
Reflections
The study benefited from having two very experienced public contributors as full members of the Project Management Group since the inception of this research and also a very experienced independent public contributor as a member of the Project Steering Group.
Wider PPI activities were useful in confirming that the outcomes we were planning to investigate were relevant to parents and parents-to-be and in identifying additional outcomes of interest. Public involvement sessions were also useful in identifying what contextual information needs to be provided when communicating the findings to the wider public. The 30-minute duration of the public involvement sessions was sufficient for addressing the main objectives of this PPI. Given the complexity of the research topic and uncertainty around the potential impact the antibiotics may have, it was initially difficult for women to comment on the outcomes that were important to them. It was very helpful to have an experienced PPI advisor facilitating public involvement sessions, maximising the benefit gained from the sessions.
Chapter 3 Results
THIN–HES-linked and THIN primary care data sets descriptions
During the study period, 219,390 deliveries were identified in the THIN–HES-linked data set. We excluded 32,808 delivery records, as they did not have a baby with the same family number as the mother. We then matched a baby for each of the remaining 186,582 deliveries. We excluded a further 72,807 records, either because they were linked to more than one potential mother or because the baby was not registered within the first year from birth. This left us with a total of 113,775 valid deliveries linked to a baby identified in the THIN–HES-linked data set.
In the THIN primary care data set, we looked for incident deliveries (VD or CS) from 1 January 2006 to 31 December 2018 in women aged 13–50 years at the time of delivery who were already registered with the practice. We found a total of 441,540 delivery records in the database, of which 54,939 were excluded, as there was no baby born in the same month and year of delivery with the same family number as the mother. We further excluded 135,318 records of babies who were either linked to more than one potential mother or not registered within a year from birth. This left a total of 251,283 mother–baby pairs identified using Read codes in the THIN primary care database. Finally, we extracted information from records of the babies who had ‘born by vaginal delivery’ or ‘born by caesarean section’ Read codes from the THIN primary care database and found 46,593 records. All of these babies, however, were already present in our linked primary care data set derived from the delivery codes noted in the mother’s medical record and, therefore, did not add any new babies that could be linked to a mother.
Combined, there were a total of 365,058 mother–baby pairs from THIN–HES-linked and THIN primary care data sets. A total of 57,317 of these pairs were identified in both the THIN primary care and THIN–HES-linked databases (i.e. duplicates) and, therefore, we removed those records from the THIN primary care database. This left us with a final total of 307,741 unique mother–baby pairs from the THIN primary care and THIN–HES-linked databases (Figure 2).
FIGURE 2.
Study population selection, including mother–baby data linkage in THIN data sets.
CPRD–HES-linked and CPRD primary care data sets descriptions
The CPRD mother–child-linked database contained 646,967 mother–baby pairs in total. When examining CPRD data, our main goal was to identify linked mothers and babies who had a recorded mode of delivery (VD/CS).
We used the same criteria to define patient start date and patient end date for the CPRD database as we did for THIN. There were 444,823 mother–baby pairs in the CPRD–HES-linked data set. To retain only the valid mother–baby pairs in the CPRD–HES-linked data, we excluded (1) 11,172 records because information on babies was not present in the primary care practice data and (2) 171,269 records because either the mother or baby did not meet the study inclusion criteria (78,229 babies were excluded because they were not registered within 1 year from date of birth, 61,553 mothers were excluded because their delivery date was not in between the patient start date and the patient end date or their age at delivery did not match study inclusion criteria, and 31,487 mother–baby pairs were excluded because both the mother and the baby did not meet the study inclusion criteria). This left us with 262,382 mother–baby pairs that had an OPCS-4 or ICD-10 delivery code identifying the mode of delivery and met our study inclusion criteria (as explained in Chapter 2).
In the CPRD primary care data set, there were 452,353 mother–baby pairs, from which we excluded 121,598 records as they did not meet the study inclusion criteria, leaving us with 330,755 mother–baby pairs with a recorded mode of delivery in the CPRD primary care database.
In total, we had 593,137 mother–baby pairs from both CPRD data sets, of which we excluded 183,230 duplicates (valid mother–baby pairs found independently in both the CPRD primary care and CPRD–HES data sets), removing them from the CPRD primary care data set. This left us with 409,907 valid mother–baby pairs across the two CPRD data sets (Figure 3).
FIGURE 3.
Study population selection in CPRD data sets.
Final THIN–CPRD data set description
Across all data sets, we identified 717,648 valid deliveries. We then excluded 201,703 (28.1%) duplicates (163,408 from THIN data sets and 38,295 from the CPRD data sets). This resulted in a final total of 515,945 mother–baby pairs for inclusion in our analysis [144,333 (28.0%) from THIN data sets combined and 371,612 (72.0%) from CPRD data sets combined] (Figure 4).
FIGURE 4.
Flow diagram of the final THIN–CPRD data set.
Out of the total of 515,945 births, 144,861 (28.1%) were births by CS and the remaining 371,084 (71.9%) were by VD. The number of actual linked mother–baby pairs is smaller than the estimate in our sample size justification. This was mainly because we used THIN data from the year 2010 to derive our estimates and, since 2010, many practices have left the Vision system and in turn the number of practices contributing to THIN and CPRD GOLD has substantially reduced over the years, resulting in decreasing numbers of deliveries in the THIN–CPRD data set in the more recent years (Figure 5). At the time of producing our sample size estimates, we overlooked this phenomenon and did not account for it and, hence, ended up with an overestimate of the number of deliveries in this combined data set.
FIGURE 5.
The number of births by delivery mode and year in the final THIN–CPRD data set.
Overall, 57.2% (295,079/515,945) of the births in the final THIN–CPRD data set were from THIN–HES- or CPRD–HES-linked data sets (Figure 6). The proportion of births from HES-linked data sets decreased over the years, with the majority of births from 2015 coming from primary care databases, and HES-linked data were not available after the beginning of 2018. As the recording of delivery mode is less complete in primary care, the decrease of births from HES-linked data sets explains the larger proportions of CS births in the later years of the study in the final THIN–CPRD data set. Over one-quarter [26.0% (76,819/295,079)] of births identified in HES-linked data sets were delivered by CS.
FIGURE 6.
The number of births by delivery mode and year from the THIN–CPRD data set, with linked HES data used for sensitivity analysis.
HES data set description
The derivation of the HES analysis set covering the whole of England, which was produced from the matching of delivery episodes and baby records, is summarised in Figure 7.
FIGURE 7.
Flow diagram for derivation of the mother–baby-linked HES data set covering the whole of England and linkage to the national survey of the hospital prophylactic antibiotic administration policies.
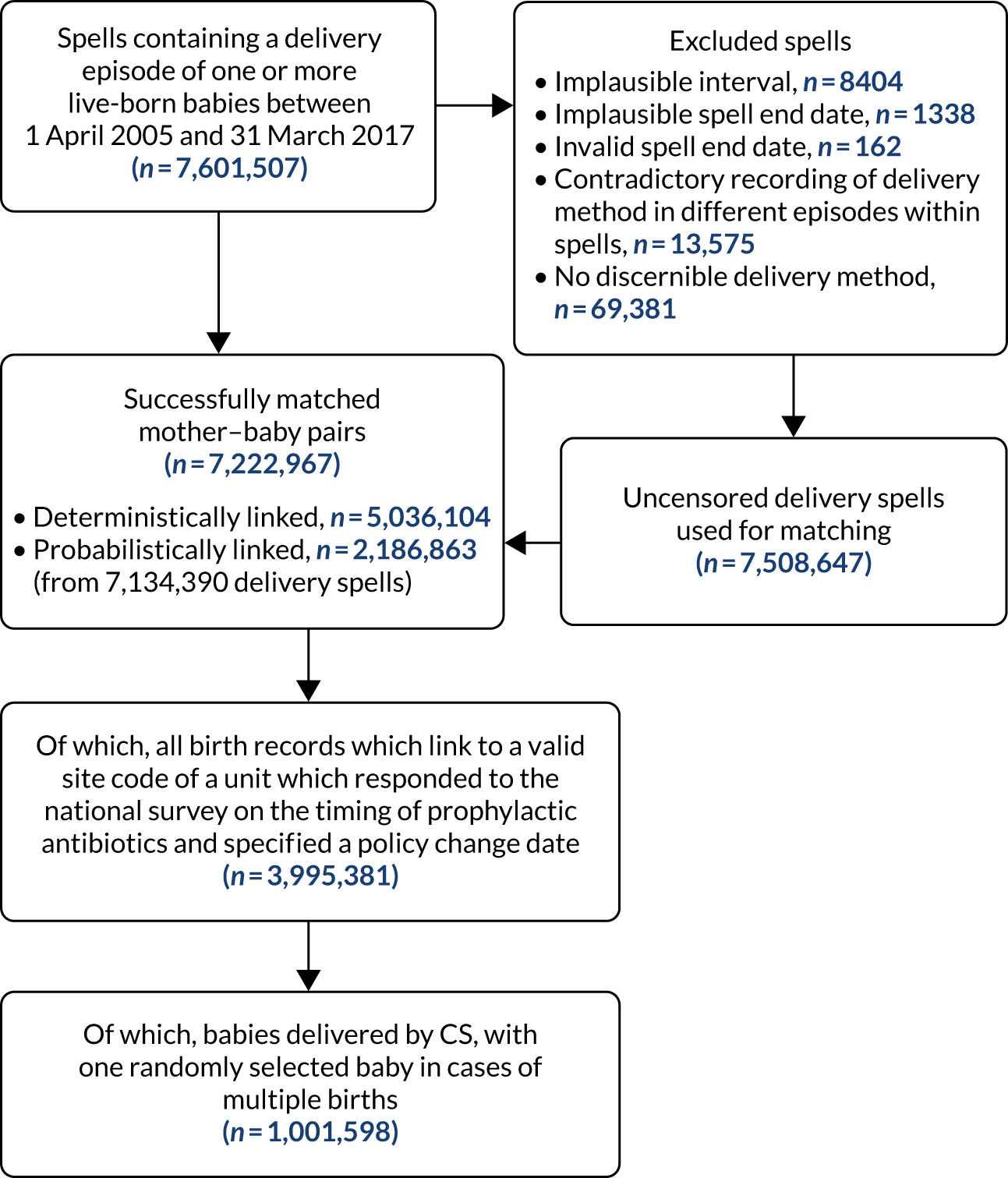
After randomly selecting one baby in cases of multiple births from 7,222,967 successfully matched mother–baby pairs, the mother-baby-linked HES data set covering the whole of England included 7,147,884 mother–baby pairs, of which 25.4% (1,813,799/7,147,884) were CS births. The data set linked to the responses to the national survey of hospital prophylactic antibiotic administration policies contained records of 3,945,351 mother–baby pairs (with one baby in the data set in cases of multiple births), 25.4% (n = 1,001,598) of which were CS births. Figure 8 shows the absolute numbers of CS and VD births over time in the full HES data set and the HES data set linked to the survey data. The sharp drop in the number in the final year is because it includes births only until the end of March 2017.
FIGURE 8.
The number of births by delivery mode and year in (a) the HES data set covering the whole of England; and (b) the HES data set linked to the data from the national survey of hospital prophylactic antibiotic administration policies used in the main analysis.
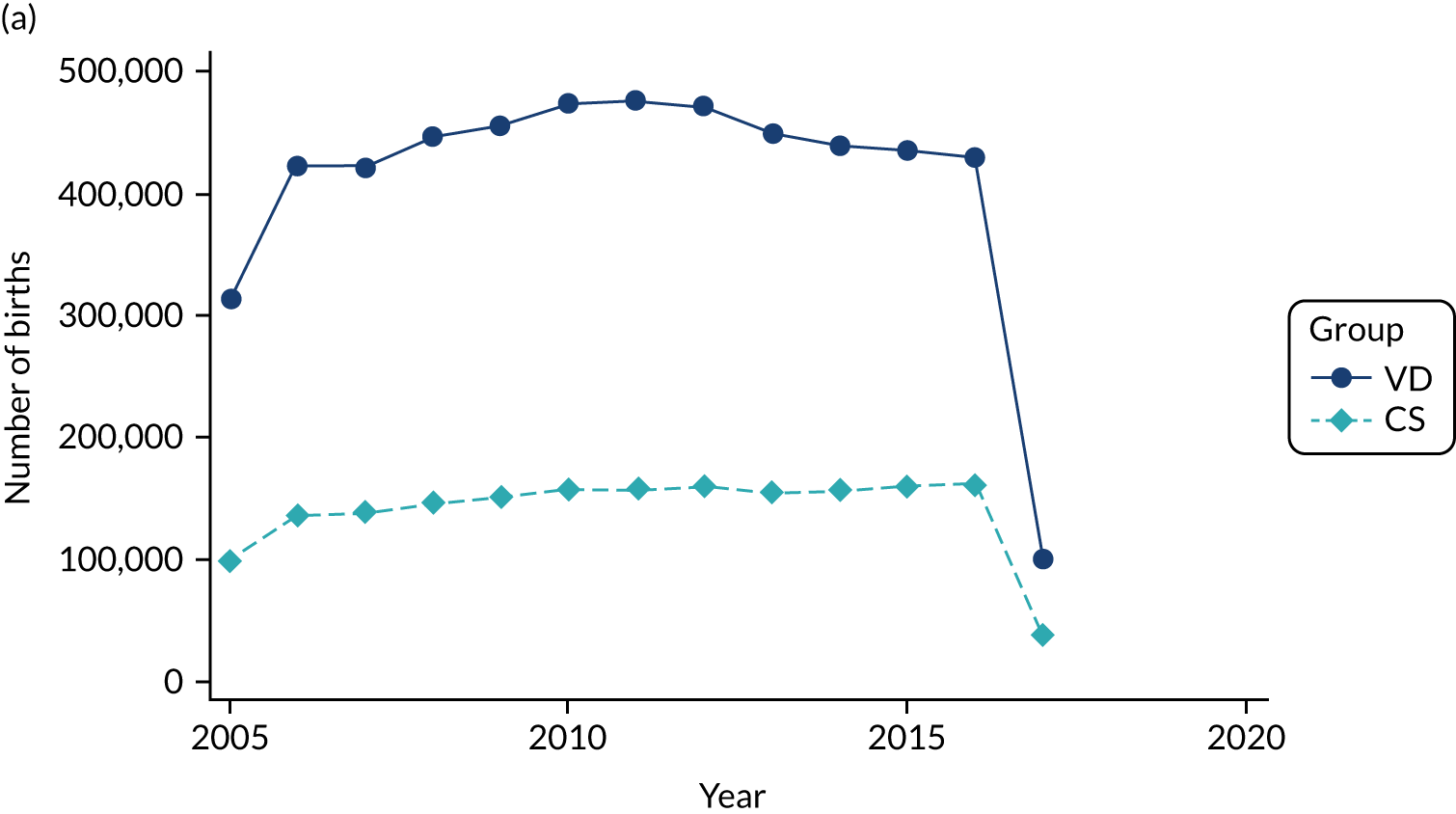
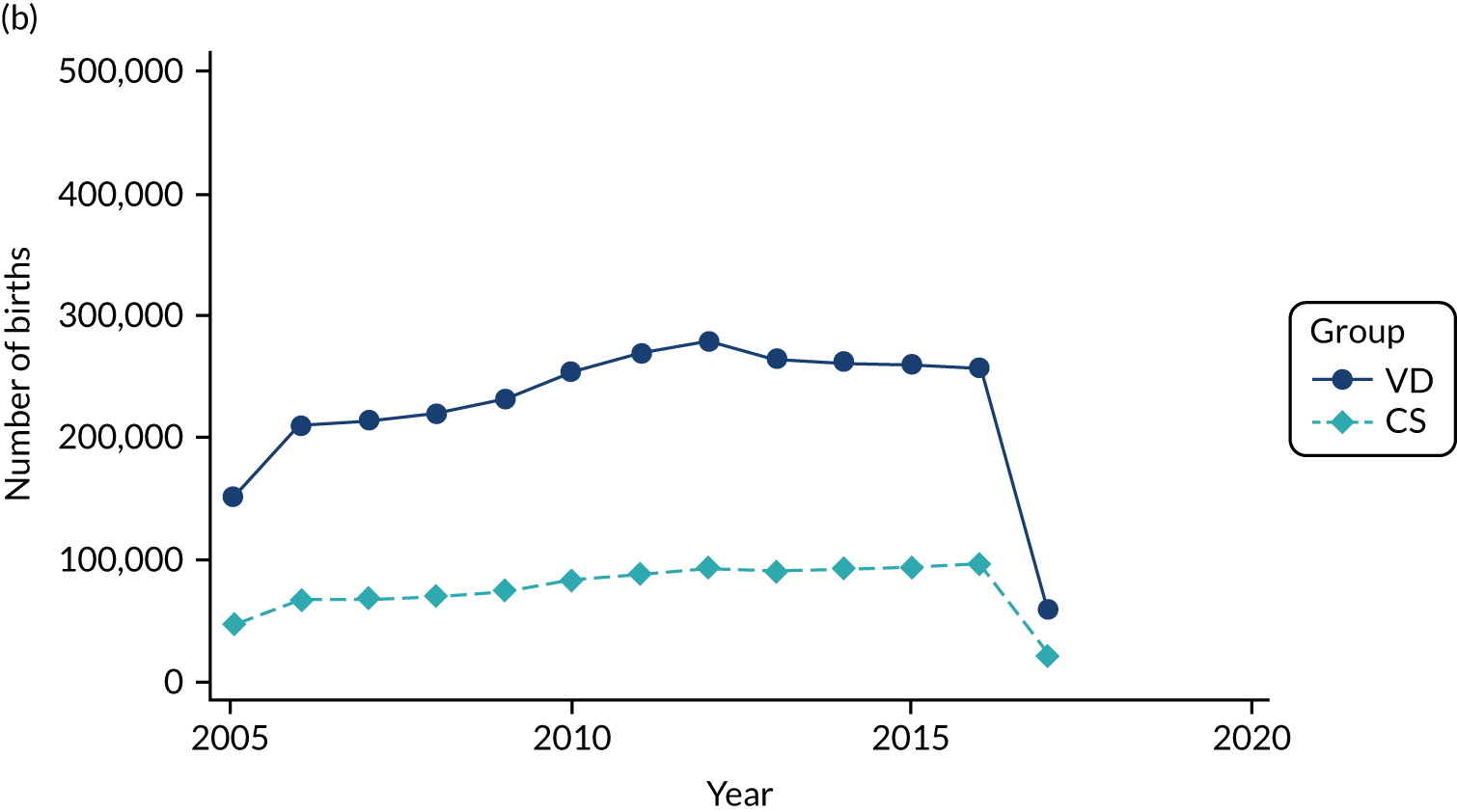
Results of the national survey of hospital prophylactic antibiotic administration policies
An overall survey response rate of 75.7% (143/189) was achieved.
The vast majority of hospitals [95.8% (137/143)] reported that their unit’s policy was to provide antibiotic prophylaxis before skin incision and five (or fewer) hospitals had this policy for either pre-labour or in-labour CS only.
Of the hospitals with a pre-incision antibiotic policy, 86.9% (119/137) indicated in which year they had implemented this policy change. The increase in the proportion of hospitals with a pre-incision antibiotics policy was gradual (Figure 9) and there was relatively little variation between different countries in the UK and regions within England (Appendix 1). No hospitals reported that they had implemented a pre-incision antibiotic policy before 2008. By 2013, over half of all hospitals had a policy to administer antibiotic prophylaxis before skin incision.
FIGURE 9.
Cumulative percentage of hospitals over time with policies to administer antibiotic prophylaxis before skin incision for women undergoing CS in the whole of the UK and England separately.
All of the hospitals that responded to the question about the type of prophylactic antibiotics currently administered used broad-spectrum antibiotics. The most common regimens were co-amoxiclav (40.6%, 54/133), cefuroxime (29.3%, 39/133) and cefuroxime together with metronidazole (26.3%, 35/133).
Of the 125 hospitals that had changed the practice to pre-incision antibiotics and responded to whether or not they used the same antibiotics before the policy change, 72.0% (90/125) had not changed their antibiotic regimen. The vast majority (≥ 95%) of those hospitals that had changed antibiotics after implementing pre-incision antibiotic policy had previously used co-amoxiclav.
Clindamycin was the most commonly used prophylactic antibiotic for women with a penicillin allergy (71.4%, 95/133), either administered on its own (53.4%, 71/133) or used along with gentamicin (12.8%, 17/133). Other regimens included gentamicin and teicoplanin and/or metronidazole in 12.8% (17/133) of hospitals. Metronidazole was used on its own or along with other antibiotics (such as cefuroxime or cefaclor) in 7.5% (10/133) of hospitals and teicoplanin alone was administered in 6.0% (8/133) of hospitals. Of the 123 hospitals that had changed their practice to pre-incision antibiotics and responded to whether or not they had used the same antibiotics before the policy change for women with penicillin allergy, 91.1% (112/123) used the same antibiotic regimen.
Eight hospitals with pre-incision antibiotic policies had conducted audits from 2014 onwards regarding the proportion of women given prophylactic antibiotics before skin incision, and reported that 70–100% of women had received pre-incision antibiotics.
Results of covariate analysis
Covariate analysis graphs are included in Appendix 2. There were no marked differential changes over time between the CS and VD groups in terms of maternal, household and child characteristics.
Primary outcome results
Main analysis
Outcomes recorded in primary care
Table 6 shows the numbers of children with a first-time diagnosis of asthma and eczema, the total years of follow-up and the overall rate per 1000 person-years (note that these rates are not directly comparable to the rates in the total 0- to 4-year-old population, as rates differ by age and the maximum age to which the children born in 2015, 2016, 2017 and 2018 were followed up to was 4 years, 3 years, 2 years and 1 year, respectively). As the rates of outcomes are compared according to the probability that each mother had received pre-incision antibiotics based on the year of delivery and the national uptake of the pre-incision antibiotic policy for CS in that year, it is not possible to present this information in a table by exposure group. Therefore, we have plotted the rates of outcomes by delivery mode, year of birth and age in Appendix 3.
| Outcome | Total number (follow-up time in person-years) | Overall rate per 1000 person-years | Pre-incision vs. post-clamping antibioticsa (based on national policy uptake, %) | ||
|---|---|---|---|---|---|
| IRR | 95% CI | p-value | |||
| Asthma | 16,540 (1,670,173) | 9.90 | 0.91 | 0.78 to 1.05 | 0.18 |
| Eczema | 102,888 (1,430,708) | 71.91 | 0.98 | 0.94 to 1.03 | 0.46 |
There was a 9% decrease in diagnosis of asthma in children exposed to pre-incision antibiotics compared with those with no such exposure (IRR 0.91, 95% CI 0.78 to 1.05). There was a 2% decrease in diagnosis of eczema in children with pre-incisional antibiotics compared with those with no such exposure (IRR 0.98, 95% CI 0.94 to 1.03). Together, these show that there was not enough evidence of an association between timing of prophylactic antibiotics administration and asthma and eczema diagnoses. The minimum increase in risk we estimated that we would be able to detect during our power calculations was not within the width of the CIs.
As part of the analysis model, a term was included to allow for delivery mode (CS or VD). For each study outcome, we have calculated an IRR comparing CS with VD to ascertain the difference in risk based on delivery mode (see Appendix 4).
Outcomes recorded in secondary care
Table 7 shows the numbers of children with a first-time hospital admission for asthma and eczema, the total years of follow-up (calculated assuming a follow-up of 5 years for children born before 2015 and follow-up of 4 years, 3 years, 2 years and 1 year for the children born in 2015, 2016, 2017 and 2018, respectively) and the overall rate per 1000 person-years. Pre-incision antibiotics were not associated with statistically significant increases in the risk of a childhood asthma or eczema resulting in hospital admission.
| Outcome | Total number (follow-up time in person-years) | Overall rate per 1000 person-years | Pre-incision vs. post-clamping antibioticsa (based on the year of policy change in each hospital) | ||
|---|---|---|---|---|---|
| IRR | 95% CI | p-value | |||
| Asthma | 30,274 (18,137,690) | 1.67 | 1.05 | 0.99 to 1.11 | 0.12 |
| Eczema | 1154 (18,137,690) | 0.06 | 0.96 | 0.71 to 1.29 | 0.77 |
There was a 5% increase in first-time hospital admissions for asthma in children exposed to pre-incision antibiotics compared with those with no such exposure (IRR 1.05, 95% CI 0.99 to 1.11). There was a 4% decrease in first-time hospital admissions for eczema in children exposed to pre-incision antibiotics compared with those with no such exposure (IRR 0.96, 95% CI 0.71 to 1.29). Together, these findings show that there was not enough evidence of an association between timing of prophylactic antibiotics administration and asthma and eczema resulting in hospital admission, although, owing to a small number of hospital admissions, the CIs for eczema were very wide.
Sensitivity analyses
The results of sensitivity analyses for asthma diagnosis in primary care (Table 8) were fairly similar to the results of the main analysis, with generally slightly lower IRRs than in the main analysis, including for moderate and severe asthma, based on prescribing information. We performed several additional sensitivity analyses for asthma outcome definition and these IRRs were also similar to the IRRs reported in the main analysis.
| Outcome | Total number (follow-up time in person-years) | Overall rate per 1000 person-years | Pre-incision vs. post-clamping antibioticsa | |
|---|---|---|---|---|
| IRR | 95% CI | |||
| Sensitivity to population changes | ||||
| Asthma (2006–10 vs. 2013–18)b,c | 13,488 (1,339,371) | 10.07 | 0.91 | 0.82 to 1.00 |
| Asthma (HES-linked primary care data set)b,d | 9494 (933,557) | 10.17 | 0.89 | 0.72 to 1.10 |
| Sensitivity to model changes | ||||
| Asthma (random effect for general practice)b | 16,540 (1,670,173) | 9.90 | 0.89 | 0.77 to 1.03 |
| Sensitivity to outcome definition | ||||
| Asthma (age ≥ 3 years)b | 12,125 (1,687,601) | 7.18 | 0.86 | 0.70 to 1.05 |
| Asthma (QOF Read codes)e | 12,512 (1,677,394) | 7.46 | 0.94 | 0.79 to 1.11 |
| Moderate/severe asthmab,f | 13,895 (1,676,660) | 8.29 | 0.84 | 0.72 to 0.99 |
| Moderate/severe asthmae,f | 10,987 (1,681,617) | 6.53 | 0.89 | 0.74 to 1.07 |
The sensitivity analysis of HES data for more severe asthma resulting in hospital admission produced very similar results to the main analysis, with no indication of significantly increased or reduced risk with introduction of pre-incision antibiotics (Table 9).
| Outcome | Total number (follow-up time in person-years) | Overall rate per 1000 person-years | Pre-incision vs. post-clamping antibioticsa | |
|---|---|---|---|---|
| IRR | 95% CI | |||
| Asthma [based on national policy uptake (%) in England]b | 56,748 (33,094,684) | 1.71 | 1.04 | 0.97 to 1.11 |
| Asthma (random effect for hospital) | 30,274 (18,137,690) | 1.67 | 1.03 | 0.97 to 1.10 |
| Asthma (discordant sibling analysis) | 3756 (2,183,199) | 1.72 | 0.97 | 0.83 to 1.13 |
The results of sensitivity analyses for eczema were similar to the main results, suggesting no significant impact of pre-incision antibiotics on the risk of eczema, including for moderate and severe eczema, defined based on prescribing information (Table 10) and admission to hospital (Table 11).
| Outcome | Total number (follow-up time in person-years) | Overall rate per 1000 person-years | Pre-incision vs. post-clamping antibioticsa | |
|---|---|---|---|---|
| IRR | 95% CI | |||
| Sensitivity to population changes | ||||
| Eczema (2006–10 vs. 2013–18)b,c | 81,704 (1,149,270) | 71.10 | 0.99 | 0.96 to 1.02 |
| Eczema (HES-linked primary care data set)b,d | 61,328 (793,746) | 77.26 | 0.96 | 0.90 to 1.02 |
| Sensitivity to model changes | ||||
| Eczema (random effect for general practice)b | 102,888 (1,430,708) | 71.91 | 0.99 | 0.95 to 1.04 |
| Sensitivity to outcome definition | ||||
| Eczema (age ≥ 1 year)b,e | 46,663 (1,597,254) | 29.21 | 1.02 | 0.94 to 1.10 |
| Eczemaf | 121,938 (1,367,826) | 89.15 | 0.98 | 0.94 to 1.02 |
| Moderate/severe eczemab,g | 44,380 (1,565,679) | 28.35 | 0.96 | 0.90 to 1.04 |
| Outcome | Total number (follow-up time in person-years) | Overall rate per 1000 person-years | Pre-incision vs. post-clamping antibioticsa | |
|---|---|---|---|---|
| IRR | 95% CI | |||
| Eczema [based on national policy uptake (%) in England]b | 2004 (33,094,684) | 0.06 | 0.78 | 0.58 to 1.06 |
| Eczema (random effect for hospital) | 1154 (18,137,690) | 0.06 | 0.95 | 0.69 to 1.31 |
| Eczema (discordant sibling analysis)c | 124 (2,183,199) | 0.06 | ||
A Poisson model has the potential for overdispersion, although we included robust standard errors to allow for the misspecification of the variance. A comparison of the results from the Poisson model and the results from a negative binomial model (with the same covariates) showed very similar results.
Secondary outcome results
Outcomes in children
Outcomes recorded in primary care
We found no evidence of a significant risk difference for any of the other allergic, allergy-related, autoimmune diseases, infections and less specific measures of child health (i.e. colic and failure to thrive) recorded in primary care in relation to the timing of prophylactic antibiotic administration (Table 12). The absolute numbers of children with autoimmune and neurodevelopmental diseases were small in this data set, resulting in a high level of uncertainty around the estimates. Given that multiple tests were undertaken and many of the included outcomes were exploratory because of a poor evidence base for an association between microbiota, early antibiotic exposure and these outcomes, a higher degree of caution is required when considering these results where the strength of the association is not high. Some of the autoimmune conditions and immune system-related diseases were extremely rare in children aged < 5 years [i.e. juvenile idiopathic arthritis, systemic sclerosis and scleroderma, inflammatory myopathies, systemic lupus erythematosus, autoimmune (idiopathic) thrombocytopenic purpura, juvenile pernicious (megaloblastic) anaemia, inflammatory bowel disease and leukaemia], preventing any meaningful calculation of risk.
| Outcome | Total number (follow-up time in person-years) | Overall rate per 1000 person-years | Pre-incision vs. post-clamping antibioticsa (based on national policy uptake, %) | ||
|---|---|---|---|---|---|
| IRR | 95% CI | p-value | |||
| Other allergic and allergy-related conditions | |||||
| Food allergy/intolerance | 8609 (1,680,729) | 5.12 | 1.02 | 0.89 to 1.17 | 0.76 |
| Allergic rhinitis and conjunctivitis | 10,511 (1,680,973) | 6.25 | 0.98 | 0.83 to 1.16 | 0.81 |
| More than one allergy-related disease | 20,146 (1,634,317) | 12.33 | 0.99 | 0.88 to 1.12 | 0.85 |
| Penicillin allergyb | 1020 (1,698,282) | 0.60 | 0.68 | 0.42 to 1.08 | 0.10 |
| Anaphylaxisb | 253 (1,700,221) | 0.15 | 1.07 | 0.43 to 2.63 | 0.89 |
| High risk of anaphylactic reactionb | 2365 (1,696,513) | 1.39 | 0.81 | 0.60 to 1.09 | 0.17 |
| Autoimmune diseases | |||||
| Type 1 diabetesb | 251 (1,700,377) | 0.15 | 0.57 | 0.20 to 1.67 | 0.30 |
| Coeliac diseaseb | 285 (1,700,259) | 0.17 | 0.54 | 0.21 to 1.39 | 0.20 |
| Juvenile idiopathic arthritisb,c | 115 (1,700,555) | 0.07 | |||
| Scleroderma/systemic sclerosisb,c | ≤ 5 (1,700,760) | 0.00 | |||
| Inflammatory myopathiesb,c | 16 (1,700,750) | 0.01 | |||
| Systemic lupus erythematosusb,c | ≤ 5 (1,700,754) | 0.00 | |||
| Autoimmune (idiopathic) thrombocytopenic purpurab,c | 164 (1,700,430) | 0.10 | |||
| Juvenile pernicious (megaloblastic) anaemiab,c | 9 (1,700,741) | 0.01 | |||
| Childhood vitiligob | 206 (1,700,390) | 0.12 | 1.13 | 0.36 to 3.52 | 0.84 |
| Infections and inflammation | |||||
| Wheeze | 49,079 (1,576,600) | 31.13 | 1.00 | 0.93 to 1.06 | 0.87 |
| Upper respiratory tract infectionsb | 314,783 (823,002) | 382.48 | 0.99 | 0.97 to 1.02 | 0.47 |
| Lower respiratory tract infections (excluding bronchiolitis)b | 81,784 (1,495,270) | 54.70 | 1.04 | 0.99 to 1.10 | 0.14 |
| Bronchiolitisb | 33,133 (1,604,809) | 20.65 | 1.05 | 0.98 to 1.12 | 0.20 |
| Gastroenteritisb | 56,403 (1,550,710) | 36.37 | 1.03 | 0.97 to 1.10 | 0.37 |
| Inflammatory bowel diseasec | 10 (1,700,743) | 0.01 | |||
| Urinary tract infectionb | 24,416 (1,655,549) | 14.75 | 1.00 | 0.90 to 1.11 | 0.99 |
| Antibiotic prescribingb | 336,893 (783,562) | 429.95 | 1.03 | 1.00 to 1.06 | 0.02 |
| Other immune system-related conditions | |||||
| Leukaemiab,c | 116 (1,700,571) | 0.07 | |||
| Neurodevelopmental conditions | |||||
| Cerebral palsy | 375 (1,699,874) | 0.22 | 1.65 | 0.79 to 3.45 | 0.18 |
| Autism spectrum disorderb | 2021 (1,698,425) | 1.19 | 1.14 | 0.77 to 1.67 | 0.52 |
| ADHDb | 436 (1,700,183) | 0.26 | 2.53 | 1.05 to 6.12 | 0.04 |
| Less-specific measures of child health | |||||
| Colicb | 20,949 (1,633,464) | 12.82 | 1.06 | 0.96 to 1.16 | 0.26 |
| Failure to thriveb | 4018 (1,688,880) | 2.38 | 1.08 | 0.87 to 1.34 | 0.47 |
| Health-care utilisation in children | |||||
| Consultations recorded in primary care (in the first 12 months)b | 6,688,462 (513,518) | 13,024.80 | 1.02 | 1.01 to 1.02 | < 0.001 |
We observed a very small (3%) increase in the likelihood of being prescribed antibiotics in early childhood (IRR 1.03, 95% CI 1.00 to 1.06), which was statistically significant because of the overall very high rates of antibiotic prescribing in young children. Similarly, there was a very small (2%) relative increase in the total number of consultations for any reason recorded in primary care (IRR 1.02, 95% CI 1.01 to 1.02).
Outcomes recorded in secondary care
We did not find evidence of a significant increase in the relative risk associated with pre-incision antibiotics for any of the following more severe secondary outcomes of interest resulting in a hospital admission [i.e. anaphylaxis, type 1 diabetes, juvenile idiopathic arthritis, autoimmune (idiopathic) thrombocytopenic purpura, lower respiratory tract infections and bronchiolitis, urinary tract infection, necrotising enterocolitis and leukaemia] (Table 13). The numbers of children with a diagnosis of coeliac disease, systemic sclerosis and scleroderma, inflammatory myopathies, systemic lupus erythematosus, juvenile pernicious (megaloblastic) anaemia and inflammatory bowel disease were very small in the study population and we could not estimate IRRs for these outcomes in secondary care. There was a very small (2%) increase in the risk of being admitted to hospital for gastroenteritis (IRR 1.02, 95% CI 1.00 to 1.05) and a very small (1%) relative increase in the risk of having a hospital admission for any reason (IRR 1.01, 95% CI 1.01 to 1.02). These outcomes were exploratory and, although the differences in risk were statistically significant, they may not be clinically significant.
| Outcome | Total number (follow-up time in person-years) | Overall rate per 1000 person-years | Pre-incision vs. post-clamping antibioticsa (based on the year of policy change in each hospital) | ||
|---|---|---|---|---|---|
| IRR | 95% CI | p-value | |||
| Other allergic and allergy-related conditions | |||||
| Anaphylaxisb | 1665 (18,137,690) | 0.09 | 1.01 | 0.82 to 1.25 | 0.91 |
| Autoimmune diseases | |||||
| Type 1 diabetesb | 2445 (18,137,690) | 0.13 | 0.99 | 0.82 to 1.20 | 0.94 |
| Coeliac diseaseb,c | 178 (18,137,690) | 0.01 | |||
| Juvenile idiopathic arthritisb | 297 (18,137,690) | 0.02 | 0.97 | 0.54 to 1.74 | 0.92 |
| Scleroderma/systemic sclerosisb,c | ≤ 5 (18,137,690) | 0.00 | |||
| Inflammatory myopathiesb,c | 26 (18,137,690) | 0.00 | |||
| Systemic lupus erythematosusb,c | ≤ 5 (18,137,690) | 0.00 | |||
| Autoimmune (idiopathic) thrombocytopenic purpurab | 1506 (18,137,690) | 0.08 | 1.11 | 0.88 to 1.39 | 0.37 |
| Juvenile pernicious (megaloblastic) anaemiab,c | ≤ 5 (18,137,690) | 0.00 | |||
| Infections and inflammation | |||||
| Early-onset neonatal sepsis (within 3 days of birth)d | 3336 (3,945,351) | 0.85 | 0.75 | 0.65 to 0.87 | < 0.001 |
| Late-onset neonatal sepsis (between 4 and 28 days after birth)d | 7226 (3,945,351) | 1.83 | 0.88 | 0.80 to 0.97 | 0.008 |
| Other sepsis (from 29 days after birth)b | 15,182 (17,835,240) | 0.85 | 0.98 | 0.91 to 1.04 | 0.48 |
| Lower respiratory tract infections (excluding bronchiolitis)b | 128,399 (18,137,690) | 7.08 | 0.99 | 0.96 to 1.01 | 0.19 |
| Bronchiolitisb | 153,411 (18,137,690) | 8.46 | 1.01 | 0.98 to 1.03 | 0.64 |
| Gastroenteritisb | 145,293 (18,137,690) | 8.01 | 1.02 | 1.00 to 1.05 | < 0.001 |
| Inflammatory bowel diseasec | 87 (18,137,690) | 0.00 | |||
| Urinary tract infectionb | 41,676 (18,137,690) | 2.30 | 1.00 | 0.95 to 1.04 | 0.86 |
| Other immune system-related conditions | |||||
| Necrotising enterocolitisd | 1178 (3,945,351) | 0.30 | 1.16 | 0.95 to 1.42 | 0.16 |
| Leukaemiab | 1094 (18,137,690) | 0.06 | 0.97 | 0.74 to 1.29 | 0.86 |
| Health-care utilisation in children | |||||
| Any hospital admissionb | 1,459,776 (18,137,690) | 80.48 | 1.01 | 1.01 to 1.02 | < 0.001 |
Having a pre-incision antibiotics policy was associated with a 25% relative reduction in early-onset neonatal sepsis (IRR 0.75, 95% CI 0.65 to 0.87) and a 12% reduction in late-onset sepsis (IRR 0.88, 95% CI 0.80 to 0.97), although the overall recorded incidence of neonatal sepsis has increased over the study period both in children born by CS and in those born by VD (see Appendix 3).
Maternal outcomes
Table 14 shows the overall numbers and percentages of women who developed maternal infections during the study period by delivery mode, the uncontrolled risk estimates comparing pre-incision antibiotics with antibiotics administered after cord clamping and the estimates adjusting for rates in the VD comparator group, as there were changes in the recorded incidence of different maternal post-partum infection indicators in routine data over the time (see Appendix 3).
| Outcome | Delivery mode, n (%)a | Pre-incision vs. post-clamping antibiotics (based on national policy uptake, %) | ||||||
|---|---|---|---|---|---|---|---|---|
| CS | VD | IRRb | 95% CI | p-value | IRRc | 95% CI | p-value | |
| Composite infectious morbidityd | 12,466 (8.61) | 5303 (1.43) | 0.67 | 0.56 to 0.79 | < 0.001 | 0.70 | 0.63 to 0.77 | < 0.001 |
| Endometritis/endomyometritis | 617 (0.43) | 1269 (0.34) | 0.63 | 0.26 to 1.53 | 0.31 | 0.98 | 0.72 to 1.33 | 0.88 |
| Wound infection | 11,717 (8.09) | 3800 (1.02) | 0.64 | 0.53 to 0.77 | < 0.001 | 0.62 | 0.55 to 0.69 | < 0.001 |
| Urinary tract infection/cystitis/pyelonephritis | 2551 (1.76) | 4935 (1.33) | 0.64 | 0.43 to 0.97 | 0.04 | 1.01 | 0.87 to 1.17 | 0.93 |
| Sepsis | 212 (0.15) | 254 (0.07) | 11.55 | 3.66 to 34.41 | < 0.001 | 1.40 | 0.85 to 2.31 | 0.19 |
| Pelvic abscesse | ≤ 5 (0.00) | 7 (0.00) | ||||||
| Antibiotic prescribing | 39,000 (26.92) | 59,138 (15.94) | 0.94 | 0.85 to 1.03 | 0.17 | 0.89 | 0.85 to 0.92 | < 0.001 |
We found a significant negative association between giving antibiotics before skin incision and the composite outcome of maternal infectious morbidity, with a relative reduction in infectious morbidity of 33% in the uncontrolled analysis in primary care (IRR 0.67, 95% CI 0.56 to 0.79) and 30% in the analysis controlling for infection rates in the VD group (IRR 0.70, 95% CI 0.63 to 0.77). The overall rates of maternal sepsis recorded in routine health-care records increased substantially over the study period (see Appendix 3). The observed reduction in the risk of the maternal infectious morbidity outcome was principally due to the marked decrease in wound infections in the CS group recorded in primary care over the study period, and differences in endometritis and maternal sepsis in the adjusted analyses were not statistically significant. In the controlled analysis, there was also no evidence of a significantly reduced risk of urinary tract infections. Pre-incision antibiotic policy was associated with a 6% (IRR 0.94, 95% CI 0.85 to 1.03) and an 11% reduction (IRR 0.89, 95% CI 0.85 to 0.92) in women being prescribed antibiotics in the post-natal period in primary care in the uncontrolled analysis and analysis adjusted for delivery mode, respectively.
Table 15 shows results for maternal infectious morbidity recorded in hospital data. Diagnoses were recorded after delivery, during the birth episode or as a new hospital admission in the post-partum period and, therefore, the incidence (apart for maternal sepsis) is lower than that in the primary care records.
| Outcome | Delivery mode | Pre-incision vs. post-clamping antibiotics (based on the year of policy change in each hospital) | ||||||
|---|---|---|---|---|---|---|---|---|
| CS | VD | IRRa | 95% CI | p-value | IRRb | 95% CI | p-value | |
| Composite infectious morbidity, n (%)c,d | 18,934 (1.89) | 9112 (0.31) | 0.98 | 0.94 to 1.02 | 0.31 | 0.84 | 0.81 to 0.87 | < 0.001 |
| Endometritis/endomyometritis, n (%)c,e | 41 (0.00) | 40 (0.00) | ||||||
| Wound infection, n (%)c | 16,185 (1.62) | 6701 (0.23) | 0.94 | 0.90 to 0.98 | 0.006 | 0.84 | 0.81 to 0.87 | < 0.001 |
| Urinary tract infection/cystitis/pyelonephritis, n (%)c | 3148 (0.31) | 5526 (0.19) | 1.14 | 1.03 to 1.26 | 0.01 | 1.04 | 0.96 to 1.14 | 0.32 |
| Sepsis, n (%)c | 2878 (0.29) | 2389 (0.08) | 1.22 | 1.09 to 1.36 | < 0.001 | 1.15 | 1.05 to 1.26 | 0.003 |
| Maternal deaths, n (%)c,e,f | ≤ 5 (0.00) | ≤ 5 (0.00) | ||||||
| Length of stay in days, median (IQR) | 3 (2–4) | 1 (1–2) | –0.004g | –0.02 to 0.01 | 0.66 | –0.23g | –0.24 to –0.21 | < 0.001 |
The relative risk for the composite maternal infectious morbidity outcome was significantly reduced in the analysis adjusted for delivery mode only, showing a 16% reduction associated with pre-incision antibiotic policy (IRR 0.84, 95% CI 0.81 to 0.87). For individual outcomes, the risk of wound infection recorded in secondary care was reduced by 16% in the adjusted analysis (IRR 0.84, 95% CI 0.81 to 0.87); however, the risk of maternal sepsis was still increased, even after adjusting for increasing rates of maternal sepsis in women who delivered vaginally in the same locations where CSs were performed. After adjusting for delivery mode, there was no significant association between urinary tract infections and giving antibiotics before skin incision. The length of hospital stay was slightly reduced in the analysis adjusted for delivery mode.
Additional sensitivity analyses
The effect sizes in the sensitivity analyses for all childhood outcomes with unexpected statistically significant findings in primary care were reduced, compared with the main analysis (Table 16). Sensitivity analyses suggest a high degree of imprecision in the estimate of the risk of attention deficit hyperactivity disorder (ADHD) in relation to exposure to pre-incision antibiotics. Given the poor existing evidence base on whether or not antibiotics or microbiota changes are clearly associated with ADHD, these results may be spurious.
| Outcome | Total n (follow-up time in person-years) | Overall rate per 1000 person-years | Pre-incision vs. post-clamping antibioticsa | |
|---|---|---|---|---|
| IRR | 95% CI | |||
| Antibiotic prescribing | ||||
| 2006–10 vs. 2013–18b | 268,872 (629,840) | 426.89 | 1.01 | 1.00 to 1.03 |
| HES-linked primary care data only | 192,224 (443,140) | 433.78 | 1.02 | 0.98 to 1.06 |
| Random effect for general practice | 336,893 (783,562) | 429.95 | 1.03 | 1.00 to 1.05 |
| ADHDc | ||||
| 2006–10 vs. 2013–18b | 344 (1,364,177) | 0.25 | 1.24 | 0.66 to 2.34 |
| HES-linked primary care data only | 237 (950,702) | 0.25 | 2.42 | 0.61 to 9.58 |
| Consultations recorded in primary care (12 months)c | ||||
| 2006–10 vs. 2013–18b | 5,319,832 (414,034) | 12,848.79 | 1.01 | 1.01 to 1.02 |
| HES-linked primary care data only | 3,850,551 (295,070) | 13,049.62 | 1.02 | 1.01 to 1.03 |
Overall, the results of the sensitivity analyses in HES data were similar to the results of the main analysis. They confirm the reduced risk for early-onset neonatal sepsis; however, there was more uncertainty around the estimates for late-onset sepsis. The associations between pre-incision antibiotics and hospital admission for gastroenteritis, as well as admission to hospital for any reason, remained statistically significant, although the size of the increased risk remains very small (Table 17).
| Outcome | Total n (follow-up time in person-years) | Overall rate per 1000 person-years | Pre-incision vs. post-clamping antibioticsa | |
|---|---|---|---|---|
| IRR | 95% CI | |||
| Early-onset neonatal sepsisc | ||||
| Based on national policy uptake (%) in Englandb | 12,629 (7,147,884) | 1.77 | 0.76 | 0.68 to 0.85 |
| Random effect for hospital | 7226 (3,945,351) | 1.83 | 0.86 | 0.77 to 0.97 |
| Late-onset neonatal sepsisc | ||||
| Based on national policy uptake (%) in Englandb | 6075 (7,147,884) | 0.85 | 0.68 | 0.58 to 0.81 |
| Random effect for hospital | 3336 (3,945,351) | 0.85 | 0.82 | 0.57 to 1.17 |
| Gastroenteritisd | ||||
| Based on national policy uptake (%) in Englandb | 249,521 (33,094,684) | 7.54 | 1.08 | 1.05 to 1.11 |
| Any hospital admissiond | ||||
| Based on national policy uptake (%) in Englandb | 2,572,391 (33,094,684) | 77.73 | 1.02 | 1.01 to 1.02 |
Results of subgroup analyses
Type of caesarean section
The results of subgroup analyses comparing the effect of pre-incision antibiotics with post-cord clamping antibiotics in emergency and elective CS on asthma and eczema, adjusted for rates of VD, were similar to the main results (Tables 18 and 19). Although uncertainty was larger around the estimates, the results do not suggest an increased risk associated with a change from post-cord clamping to pre-incision antibiotics in either elective or emergency CS.
| Outcome | Pre-incision vs. post-cord clamping antibioticsa (based on national policy uptake, %) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Emergency CS | Elective CS | p-value interaction | |||||||||
| Total n (follow-up time in person-years) | Overall rate per 1000 person-years | IRR | 95% CI | p-value | Total n (follow-up time in person-years) | Overall rate per 1000 person-years | IRR | 95% CI | p-value | ||
| Asthma | 2256 (210,139) | 10.74 | 1.05 | 0.86 to 1.27 | 0.65 | 1786 (179,873) | 9.93 | 0.86 | 0.69 to 1.07 | 0.16 | 0.15 |
| Eczema | 14,564 (176,401) | 82.56 | 1.03 | 0.97 to 1.09 | 0.38 | 10,428 (155,825) | 66.92 | 0.98 | 0.92 to 1.05 | 0.58 | 0.28 |
| Outcome | Pre-incision vs. post-cord clamping antibioticsa (based on the year of policy change in each hospital) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Emergency CS | Elective CS | p-value interaction | |||||||||
| Total n (follow-up time in person-years) | Overall rate per 1000 person-years | IRR | 95% CI | p-value | Total n (follow-up time in person-years) | Overall rate per 1000 person-years | IRR | 95% CI | p-value | ||
| Asthma | 4984 (2,675,442) | 1.86 | 1.05 | 0.98 to 1.13 | 0.17 | 3104 (1,897,257) | 1.64 | 1.04 | 0.96 to 1.14 | 0.34 | 0.90 |
| Eczema | 175 (2,675,442) | 0.07 | 0.87 | 0.60 to 1.26 | 0.47 | 106 (1,897,257) | 0.06 | 1.15 | 0.73 to 1.80 | 0.55 | 0.34 |
Antibiotic regimen
Table 20 shows results of analyses restricted to hospitals that were administering the same antibiotic regimen before and after policy change regarding the timing of prophylactic antibiotics. No particular antibiotic regimen was associated with a higher risk of being admitted to hospital because of asthma. Hospital admissions due to eczema were very rare and more evidence would be required to confirm if there may be a clinically significant decrease in the risk of eczema associated with pre-incision administration of co-amoxiclav. Overall, there was no evidence that a particular antibiotic regimen was associated with a different risk of necrotising enterocolitis when comparing pre-incision and post-clamping antibiotics.
| Outcome | Total n (follow-up time in person-years) | Overall rate per 1000 person-years | Pre-incision vs. post-clamping antibioticsa (based on the year of policy change in each hospital) | ||
|---|---|---|---|---|---|
| IRR | 95% CI | p-value | |||
| Asthma | |||||
| Cefuroxime alone | 5697 (3,555,311) | 1.60 | 0.88 | 0.77 to 1.01 | 0.08 |
| Cefuroxime and metronidazole | 5236 (2,951,897) | 1.77 | 1.04 | 0.91 to 1.19 | 0.59 |
| Co-amoxiclav alone | 10,477 (6,013,240) | 1.74 | 1.08 | 0.98 to 1.19 | 0.13 |
| Eczema | |||||
| Cefuroxime alone | 253 (3,555,311) | 0.07 | 1.36 | 0.75 to 2.46 | 0.30 |
| Cefuroxime and metronidazoleb | 171 (2,951,897) | 0.06 | |||
| Co-amoxiclav alone | 447 (6,013,240) | 0.07 | 0.55 | 0.32 to 0.97 | 0.04 |
| Necrotising enterocolitisc | |||||
| Cefuroxime alone | 213 (771,945) | 0.28 | 1.38 | 0.83 to 2.28 | 0.22 |
| Cefuroxime and metronidazole | 232 (639,624) | 0.36 | 1.22 | 0.76 to 1.95 | 0.41 |
| Co-amoxiclav alone | 347 (1,310,208) | 0.26 | 1.32 | 0.92 to 1.89 | 0.13 |
Chapter 4 Discussion and implications
This large population-wide study of routine primary and secondary health-care records did not find evidence of an increased risk of asthma and eczema (including severe asthma and eczema, and other allergic and allergy-related conditions) in children aged < 5 years in relation to the change in the guidelines recommending administration of antibiotics before skin incision in CSs.
There is also no convincing evidence that pre-incision antibiotics are associated with significant risk of development of autoimmune and other conditions investigated in this study, including infections, necrotising enterocolitis, leukaemia, neurodevelopmental conditions, colic and failure to thrive. Most of these outcomes in our study were purely exploratory, as there was insufficient evidence regarding the role of microbiota in the development of these conditions. Many of these health conditions are rare in young children, resulting in large uncertainty around the estimates. Our study was not sufficiently powered to detect clinically important differences in the rarer outcomes.
We found a statistically significant reduction in neonatal sepsis similar to the relative reduction in the point estimate found in a systematic review of randomised controlled trials, although the review’s results were not statistically significant. 89 The results confirm previous findings89 that antibiotics before skin incision do not increase the risk of neonatal sepsis, but the observed relative reduction in the risk associated with pre-incision antibiotics in babies born by CS, which was estimated by controlling for the risk of sepsis in babies born by VD, should be interpreted with caution in this study. The overall incidence of neonatal sepsis diagnoses recorded in routine health-care data has increased over the years. Clinically diagnosed neonatal sepsis often has no organism isolated from blood culture. 90 The diagnosis of neonatal sepsis in our study is likely to be culture-confirmed sepsis, in which case the receipt of antibiotics immediately prior to birth may result in an increase in the likelihood of being culture negative, even if the baby develops signs of neonatal sepsis (i.e. it may be an artefact of the antibiotics rather than an actual decrease in the relative risk of disease).
The very small (ranging from 1% to 3%) relative increases in the number of consultations recorded in primary care, the increased likelihood of children exposed to pre-incision antibiotics being prescribed antibiotics in early childhood, the increased likelihood of hospital admission for gastroenteritis and ever being admitted to hospital for any reason that where observed in this study are all common events and, therefore, produce statistically significant differences. Residual confounding and bias may explain these differences; however, even if they are real, they are very small and unlikely to be clinically important.
In this research, we obtained risk estimates for > 50 outcomes across primary and secondary care data sets; however, because of multiple comparisons, some of the statistically significant differences we observed may have occurred by chance. Some of the health conditions, such as ADHD, may show symptoms in early childhood, but are most commonly diagnosed later in life, with the incidence being the highest in school-aged children. 91 Therefore, the numbers of children born by CS with a new diagnosis of ADHD in primary care were very small in each year during our study, resulting in large variations in the incidence between the years. More evidence would be required to confirm or refute this finding before any conclusion of an association can be made. Previous research has shown conflicting results regarding exposure to antibiotics in early life and ADHD. For example, recent population-based Canadian and Danish studies92,93 did not observe a significant association between antibiotic exposure in the first years of life and ADHD, particularly in sibling-controlled analysis that adjusted for familial confounding factors. However, a large Finnish study94 suggested an association between exposure to antibiotics in early life and ADHD that may not be fully explained by familial confounding.
Our study did not identify a significant association between necrotising enterocolitis and pre-incision antibiotics for CS, including co-amoxiclav. There was some degree of uncertainty around the estimates, and they do not exclude the possibility of an increased risk. There was a slight increase in the overall incidence of necrotising enterocolitis over the study period, as recorded in the routine secondary care records. In this study, we assessed the overall association only and did not examine the risk in any specific subgroups of pregnancies and babies previously reported to be at higher risk of necrotising enterocolitis. 83,95
The relative reduction in the composite outcome of maternal infectious morbidity in the post-partum period associated with pre-incision antibiotics in CS that we observed in this study is comparable to the reduced risks previously reported in randomised controlled trials. 9,89 We found an overall increase in maternal sepsis recorded in health-care records, which may be due to an increase in diagnosis, prevention and treatment of sepsis over the study period. The relative increase in the CS group may, therefore, be an artefact, as any operation increases the suspicion of sepsis, leading to an increased rate of diagnosis. Evidence from randomised controlled trials shows that prophylactic antibiotics significantly reduce serious infectious complications compared with no antibiotic prophylaxis for CS. 96
In this study, we investigated the impact of the timing of prophylactic antibiotics in CS deliveries. Babies born by CS have a different composition of gut microbiota after birth and are more likely to be colonised by opportunistic pathogenic bacteria than babies born by VD. 13 Therefore, the impact of antibiotic prophylaxis around the time of birth in babies born by VD may be different.
The strengths of this study include using data from large population-based mother–baby linked data sets and examining the impact of pre-incision antibiotic policy on a wide range of allergy, immune system and other related outcomes. It also assessed the impact on the severity of outcomes by examining the risk of health conditions recorded in primary care and health conditions resulting in a hospital admission. The study employed a strong design, using VD as a comparison group to control for any temporal changes (e.g. changes in diagnoses and recording in medical records) over time, which could not be achieved by simple adjustment for confounding factors. We also undertook a number of sensitivity analyses to assess the robustness of our findings, including the impact of missing data, and the results were similar to those of the main analyses.
The study also has several limitations that need to be taken into account when interpreting the findings. This was an observational study that used routinely collected health-care data, and validated case definitions for different health conditions in electronic medical records, where such exist, have mostly been derived from populations that include older age groups. 51,52,97 Making diagnoses for some conditions in early childhood can be difficult and rely on symptoms (e.g. asthma objective tests can be performed in children aged ≥ 5 years only). 44,98 Some health conditions we investigated in this study were very rare in young children and our study was not sufficiently powered to detect clinically significant differences in risks for these outcomes. The timing of prophylactic antibiotic administration was not recorded in routine health-care records and, therefore, we were not able to ascertain exposure to pre-incision antibiotics at an individual level. It is also possible that there is residual confounding that was not fully accounted for by using VD as a comparator group. Finally, the results may not be generalisable to settings where different prophylactic antibiotic regimens are used.
Implications for practice and research recommendations
The overall findings from this study provide further evidence for the currently prevailing practice in the UK of giving prophylactic antibiotics before skin incision to help reduce the risk of maternal infections in the post-partum period.
Some of the secondary health outcomes we investigated in this study were exploratory because of a lack of or inconclusive evidence regarding an association between newborn microbiota, early exposure to antibiotics and children’s development. Many of the health conditions were also very rare in young children, resulting in large uncertainty around the estimates. We suggest undertaking a further study using routinely collected health-care data in other countries where there was no change in the timing of prophylactic antibiotic administration to assess if there have also been changes in the risk over time of developing these health conditions, particularly ADHD and gastroenteritis, irrespective of the exposure to prophylactic antibiotics around the time of birth. The current study includes health-care records until 2018, but it could also be repeated in the future to capture outcomes of interest in children aged > 5 years.
Other research is ongoing in the UK using the Born in Bradford birth cohort, allowing the ascertainment of antibiotic exposure at an individual level. The original Born in Bradford cohort included 13,776 children, and the majority of these children were born before the recommendation to give antibiotics pre incision. 99 This may limit the ability of this study to assess the risk for most of the outcomes of interest investigated in this research.
Acknowledgements
We would like to thank our independent Steering Group members (Elizabeth Draper, Alison Wright, Maria Clark and Daniel Prieto-Alhambra) for providing expert advice and oversight of the study.
We would also like to thank Anuradhaa Subramanian for helping with the literature review, Max Feltham, the Birmingham Surgical Trials Consortium (Birmingham, UK) and Mary Perrins for their help with the survey on prophylactic antibiotic policies; Fiona Alderdice and Jane Henderson for providing aggregate data on breastfeeding from the National Perinatal Epidemiology Unit’s Maternity Surveys; Rachel Rowe for her advice regarding information on free-standing midwifery units; and Katie Harron for her advice on mother–baby linkage in the HES data set.
We also thank all of the women who were involved in our PPI engagement sessions, and the members of the RCOG’s Women’s Voices Involvement Panel and clinicians who completed the survey.
Dana Šumilo is affiliated to the National Institute for Health and Care Research Health Protection Research Unit (NIHR HPRU) in Gastrointestinal Infections at University of Liverpool in partnership with the UK Health Security Agency (UKHSA), in collaboration with University of Warwick. Dana Šumilo is based at the University of Warwick. The views expressed are those of the author(s) and not necessarily those of NIHR, the Department of Health and Social Care or UKHSA.
This study is based, in part, on data from the CPRD, obtained under licence from the UK NHRA, and THIN, obtained under licence from IQVIA. IQVIA Medical Research Data incorporates data from THIN, a Cegedim database. Reference made to THIN is intended to be descriptive of the data asset licensed by IQVIA. This work used de-identified data provided by patients as a part of their routine care.
Contributions of authors
Dana Šumilo (https://orcid.org/0000-0001-6732-2459) (Consultant and Honorary Senior Research Fellow, Public Health and Epidemiology) contributed to the study design, analysis and interpretation of the results and led the writing of the report.
Krishnarajah Nirantharakumar (https://orcid.org/0000-0002-6816-1279) (Professor, UK Research and Innovation Clinical Fellow and Honorary Consultant, Public Health and Health Informatics) led the preparation of the THIN–CPRD data set and contributed to the analysis, interpretation of the results and writing of the final report.
Brian H Willis (https://orcid.org/0000-0002-0821-8624) (Medical Research Council Clinician Scientist and General Practitioner) contributed to the study design, development of study outcome definitions, interpretation of the results and writing of the final report.
Gavin Rudge (https://orcid.org/0000-0002-9857-6547) (Research Fellow, Health Informatics) was responsible for data linkage and preparation of the HES data set, and contributed to the development of study outcome definitions, analysis, interpretation of the results and writing of the final report.
James Martin (https://orcid.org/0000-0002-6949-4200) (Lecturer, Statistics) was responsible for statistical analysis and contributed to the interpretation of the results and writing of the final report.
Krishna Gokhale (https://orcid.org/0000-0002-2167-9572) (Research Fellow, Computer Science) was responsible for data linkage and preparation of the THIN–CPRD data set and contributed to the analysis, interpretation of the results and writing of the final report.
Rasiah Thayakaran (https://orcid.org/0000-0002-4422-4095) (Research Fellow, Statistics) was responsible for preparation of the THIN–CPRD data set and contributed to the analysis, interpretation of the results and writing of the final report.
Nicola J Adderley (https://orcid.org/0000-0003-0543-3254) (Lecturer, Health Informatics and Epidemiology) contributed to data acquisition, interpretation of the results and writing of the final report.
Joht Singh Chandan (https://orcid.org/0000-0002-9561-5141) (Academic Clinical Lecturer, Public Health) contributed to data acquisition, preparation of the THIN–CPRD data set, development of study outcome definitions and writing of the final report.
Kelvin Okoth (https://orcid.org/0000-0002-2745-4083) (Research Fellow, Epidemiology) contributed to the development of study outcome definitions and preparation of the THIN–CPRD data set.
Isobel M Harris (https://orcid.org/0000-0001-8125-3832) (Research Fellow, Systematic Reviews) contributed to the literature review for this study, and the analysis and writing of the final report.
Ruth Hewston (https://orcid.org/0000-0002-2035-184X) (Patient and Public Contributor) advised on all PPI aspects of the project, including defining study outcomes, wider PPI engagement, interpretation of the results, development of lay messages and writing of the final report.
Magdalena Skrybant (https://orcid.org/0000-0001-7119-2482) (Patient and Public Contributor) advised on all PPI aspects of the project, including defining study outcomes, wider PPI engagement, interpretation of the results, development of lay messages and writing of the final report.
Jonathan J Deeks (https://orcid.org/0000-0002-8850-1971) (Professor, Biostatistics) developed statistical methodology and oversaw the analysis, and contributed to the study design, interpretation of the results and writing of the report.
Peter Brocklehurst (https://orcid.org/0000-0002-9950-6751) (Professor, Women’s Health and Perinatal Epidemiology) contributed to the study design, development of study outcome definitions, interpretation of the results and writing of the report.
Publications
Šumilo D, Nirantharakumar K, Willis BH, Rudge G, Martin J, Gokhale K, et al. Long term impact of giving antibiotics before skin incision versus after cord clamping on children born by caesarean section: protocol for a longitudinal study based on UK electronic health records. BMJ Open 2019;9:e033013.
Šumilo D, Willis BH, Rudge GM, Martin J, Gokhale K, Thayakaran R, et al. Long term impact of prophylactic antibiotic use before incision versus after cord clamping on children born by caesarean section: longitudinal study of UK electronic health records. BMJ 2022;377:e069704.
Data-sharing statement
All data queries and requests should be submitted to the corresponding author for consideration in the first instance. Please note that data for this study were derived from THIN and CPRD GOLD primary care and linked data obtained under licence from IQVIA World Publications Ltd and UK MHRA. A secondary care database was derived from HES data obtained under licence from the Health and Social Care Information Centre (NHS Digital). Data for similar cohorts can be requested from IQVIA World Publications Ltd, the UK MHRA and NHS Digital, subject to protocol approval and licence agreements.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Public Health Scotland . Births in Scottish Hospitals n.d. www.isdscotland.org/Health-Topics/Maternity-and-Births/Births/ (accessed 27 January 2020).
- NHS Digital . NHS Maternity Statistics n.d. https://digital.nhs.uk/data-and-information/publications/statistical/nhs-maternity-statistics (accessed 27 January 2020).
- Public Health Agency of Northern Ireland . Children’s Health in Northern Ireland n.d. www.publichealth.hscni.net/directorates/operations/statistics (accessed 27 January 2020).
- Thomas C. Maternity and Birth Statistics: 2020 2021. https://gov.wales/maternity-and-birth-statistics-2020-html (accessed 27 January 2020).
- Leth RA, Møller JK, Thomsen RW, Uldbjerg N, Nørgaard M. Risk of selected postpartum infections after cesarean section compared with vaginal birth: a five-year cohort study of 32,468 women. Acta Obstet Gynecol Scand 2009;88:976-83. https://doi.org/10.1080/00016340903147405.
- National Institute for Health and Care Excellence (NICE) . Caesarean Birth 2021.
- National Institute for Health and Care Excellence (NICE) . Caesarean Section. NICE Clinical Guideline CG132 2011.
- Bailey SR, Field N, Townsend CL, Rodger AJ, Brocklehurst P. Antibiotic prophylaxis for women undergoing caesarean section and infant health. BJOG 2016;123:875-6. https://doi.org/10.1111/1471-0528.13701.
- Mackeen AD, Packard RE, Ota E, Berghella V, Baxter JK. Timing of intravenous prophylactic antibiotics for preventing postpartum infectious morbidity in women undergoing cesarean delivery. Cochrane Database Syst Rev 2014;12. https://doi.org/10.1002/14651858.CD009516.pub2.
- Sutton AL, Acosta EP, Larson KB, Kerstner-Wood CD, Tita AT, Biggio JR. Perinatal pharmacokinetics of azithromycin for cesarean prophylaxis. Am J Obstet Gynecol 2015;212:812.e1-6. https://doi.org/10.1016/j.ajog.2015.01.015.
- Azad MB, Konya T, Persaud RR, Guttman DS, Chari RS, Field CJ, et al. Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. BJOG 2016;123:983-93. https://doi.org/10.1111/1471-0528.13601.
- Korpela K, de Vos WM. Early life colonization of the human gut: microbes matter everywhere. Curr Opin Microbiol 2018;44:70-8. https://doi.org/10.1016/j.mib.2018.06.003.
- Shao Y, Forster SC, Tsaliki E, Vervier K, Strang A, Simpson N, et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 2019;574:117-21. https://doi.org/10.1038/s41586-019-1560-1.
- Zimmermann P, Messina N, Mohn WW, Finlay BB, Curtis N. Association between the intestinal microbiota and allergic sensitization, eczema, and asthma: a systematic review. J Allergy Clin Immunol 2019;143:467-85. https://doi.org/10.1016/j.jaci.2018.09.025.
- Lawley TD, Walker AW. Intestinal colonization resistance. Immunology 2013;138:1-11. https://doi.org/10.1111/j.1365-2567.2012.03616.x.
- Murgas Torrazza R, Neu J. The developing intestinal microbiome and its relationship to health and disease in the neonate. J Perinatol 2011;31:29-34. https://doi.org/10.1038/jp.2010.172.
- Honda K, Littman DR. The microbiome in infectious disease and inflammation. Annu Rev Immunol 2012;30:759-95. https://doi.org/10.1146/annurev-immunol-020711-074937.
- Penders J, Thijs C, van den Brandt PA, Kummeling I, Snijders B, Stelma F, et al. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study. Gut 2007;56:661-7. https://doi.org/10.1136/gut.2006.100164.
- Tamburini S, Shen N, Wu HC, Clemente JC. The microbiome in early life: implications for health outcomes. Nat Med 2016;22:713-22. https://doi.org/10.1038/nm.4142.
- Bisgaard H, Bønnelykke K, Stokholm J. Immune-mediated diseases and microbial exposure in early life. Clin Exp Allergy 2014;44:475-81. https://doi.org/10.1111/cea.12291.
- Neu J, Rushing J. Cesarean versus vaginal delivery: long-term infant outcomes and the hygiene hypothesis. Clin Perinatol 2011;38:321-31. https://doi.org/10.1016/j.clp.2011.03.008.
- Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol 2015;21:8787-803. https://doi.org/10.3748/wjg.v21.i29.8787.
- Bisgaard H, Li N, Bonnelykke K, Chawes BL, Skov T, Paludan-Müller G, et al. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J Allergy Clin Immunol 2011;128:646-52.e1. https://doi.org/10.1016/j.jaci.2011.04.060.
- Vatanen T, Franzosa EA, Schwager R, Tripathi S, Arthur TD, Vehik K, et al. The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature 2018;562:589-94. https://doi.org/10.1038/s41586-018-0620-2.
- Verdu EF, Galipeau HJ, Jabri B. Novel players in coeliac disease pathogenesis: role of the gut microbiota. Nat Rev Gastroenterol Hepatol 2015;12:497-506. https://doi.org/10.1038/nrgastro.2015.90.
- Rosser EC, Mauri C. A clinical update on the significance of the gut microbiota in systemic autoimmunity. J Autoimmun 2016;74:85-93. https://doi.org/10.1016/j.jaut.2016.06.009.
- Volkmann ER, Hoffmann-Vold AM, Chang YL, Jacobs JP, Tillisch K, Mayer EA, et al. Systemic sclerosis is associated with specific alterations in gastrointestinal microbiota in two independent cohorts. BMJ Open Gastroenterol 2017;4. https://doi.org/10.1136/bmjgast-2017-000134.
- Vangay P, Ward T, Gerber JS, Knights D. Antibiotics, pediatric dysbiosis, and disease. Cell Host Microbe 2015;17:553-64. https://doi.org/10.1016/j.chom.2015.04.006.
- Arrieta MC, Stiemsma LT, Amenyogbe N, Brown EM, Finlay B. The intestinal microbiome in early life: health and disease. Front Immunol 2014;5. https://doi.org/10.3389/fimmu.2014.00427.
- Greaves M. A causal mechanism for childhood acute lymphoblastic leukaemia. Nat Rev Cancer 2018;18:471-84. https://doi.org/10.1038/s41568-018-0015-6.
- Kenyon S, Pike K, Jones DR, Brocklehurst P, Marlow N, Salt A, et al. Childhood outcomes after prescription of antibiotics to pregnant women with spontaneous preterm labour: 7-year follow-up of the ORACLE II trial. Lancet 2008;372:1319-27. https://doi.org/10.1016/S0140-6736(08)61203-9.
- Kelly JR, Minuto C, Cryan JF, Clarke G, Dinan TG. Cross talk: the microbiota and neurodevelopmental disorders. Front Neurosci 2017;11. https://doi.org/10.3389/fnins.2017.00490.
- Prehn-Kristensen A, Zimmermann A, Tittmann L, Lieb W, Schreiber S, Baving L, et al. Reduced microbiome alpha diversity in young patients with ADHD. PLOS ONE 2018;13. https://doi.org/10.1371/journal.pone.0200728.
- Dubois NE, Gregory KE. Characterizing the intestinal microbiome in infantile colic: findings based on an integrative review of the literature. Biol Res Nurs 2016;18:307-15. https://doi.org/10.1177/1099800415620840.
- White RA, Bjørnholt JV, Baird DD, Midtvedt T, Harris JR, Pagano M, et al. Novel developmental analyses identify longitudinal patterns of early gut microbiota that affect infant growth. PLOS Comput Biol 2013;9. https://doi.org/10.1371/journal.pcbi.1003042.
- Pols DH, Wartna JB, Moed H, van Alphen EI, Bohnen AM, Bindels PJ. Atopic dermatitis, asthma and allergic rhinitis in general practice and the open population: a systematic review. Scand J Prim Health Care 2016;34:143-50. https://doi.org/10.3109/02813432.2016.1160629.
- Punekar YS, Sheikh A. Establishing the incidence and prevalence of clinician-diagnosed allergic conditions in children and adolescents using routinely collected data from general practices. Clin Exp Allergy 2009;39:1209-16. https://doi.org/10.1111/j.1365-2222.2009.03248.x.
- British Lung Foundation . Asthma Statistics n.d. https://statistics.blf.org.uk/asthma (accessed 27 January 2020).
- NHS England . Childhood Asthma n.d. www.england.nhs.uk/ourwork/ltc-op-eolc/ltc-eolc/si-areas/childhood-asthma/ (accessed 27 January 2020).
- Office for National Statistics (ONS) . Death Registration Summary Statistics, England and Wales, 2015 2015.
- Simpson CR, Newton J, Hippisley-Cox J, Sheikh A. Trends in the epidemiology and prescribing of medication for eczema in England. J R Soc Med 2009;102:108-17. https://doi.org/10.1258/jrsm.2009.080211.
- Scottish Intercollegiate Guidelines Network (SIGN) . Management of Atopic Eczema in Primary Care. A National Clinical Guideline 125 2011.
- Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol 2017;46:348-55. https://doi.org/10.1093/ije/dyw098.
- British Thoracic Society . British Guideline on the Management of Asthma 2019. www.sign.ac.uk/media/1773/sign158-updated.pdf (accessed 22 November 2020).
- NHS Digital . Quality and Outcomes Framework (QOF), Enhanced Services and Core Contract Extraction Specifications (Business Rules) n.d. http://content.digital.nhs.uk/qof (accessed 27 January 2020).
- Black M, Bhattacharya S, Philip S, Norman JE, McLernon DJ. Planned cesarean delivery at term and adverse outcomes in childhood health. JAMA 2015;314:2271-9. https://doi.org/10.1001/jama.2015.16176.
- Thavagnanam S, Fleming J, Bromley A, Shields MD, Cardwell CR. A meta-analysis of the association between caesarean section and childhood asthma. Clin Exp Allergy 2008;38:629-33. https://doi.org/10.1111/j.1365-2222.2007.02780.x.
- Sandall J, Tribe RM, Avery L, Mola G, Visser GH, Homer CS, et al. Short-term and long-term effects of caesarean section on the health of women and children. Lancet 2018;392:1349-57. https://doi.org/10.1016/S0140-6736(18)31930-5.
- Šumilo D, Nirantharakumar K, Willis BH, Rudge G, Martin J, Gokhale K, et al. Long-term impact of giving antibiotics before skin incision versus after cord clamping on children born by caesarean section: protocol for a longitudinal study based on UK electronic health records. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2019-033013.
- Martin P, Cortina-Borja M, Newburn M, Harper G, Gibson R, Dodwell M, et al. Timing of singleton births by onset of labour and mode of birth in NHS maternity units in England, 2005–2014: a study of linked birth registration, birth notification, and hospital episode data. PLOS ONE 2018;13. https://doi.org/10.1371/journal.pone.0198183.
- Abuabara K, Magyari AM, Hoffstad O, Jabbar-Lopez ZK, Smeeth L, Williams HC, et al. Development and validation of an algorithm to accurately identify atopic eczema patients in primary care electronic health records from the UK. J Invest Dermatol 2017;137:1655-62. https://doi.org/10.1016/j.jid.2017.03.029.
- Nissen F, Quint JK, Wilkinson S, Mullerova H, Smeeth L, Douglas IJ. Validation of asthma recording in electronic health records: a systematic review. Clin Epidemiol 2017;9:643-56. https://doi.org/10.2147/CLEP.S143718.
- National Institute for Health and Care Excellence . Atopic Eczema in Under 12s: Diagnosis and Management. 2007. www.nice.org.uk/guidance/cg57 (accessed 11 February 2020).
- Galvão A, Braga AC, Gonçalves DR, Guimarães JM, Braga J. Sepsis during pregnancy or the postpartum period. J Obstet Gynaecol 2016;36:735-43. https://doi.org/10.3109/01443615.2016.1148679.
- Beasley R, Semprini A, Mitchell EA. Risk factors for asthma: is prevention possible?. Lancet 2015;386:1075-85. https://doi.org/10.1016/S0140-6736(15)00156-7.
- Kantor R, Kim A, Thyssen JP, Silverberg JI. Association of atopic dermatitis with smoking: a systematic review and meta-analysis. J Am Acad Dermatol 2016;75:1119-25.e1. https://doi.org/10.1016/j.jaad.2016.07.017.
- Lakhan P, Doherty J, Jones M, Clements A. A systematic review of maternal intrinsic risk factors associated with surgical site infection following caesarean sections. Healthc 2010;15:35-41. https://doi.org/10.1071/hi10001.
- Oland AA, Booster GD, Bender BG. Psychological and lifestyle risk factors for asthma exacerbations and morbidity in children. World Allergy Organ J 2017;10. https://doi.org/10.1186/s40413-017-0169-9.
- Castro-Rodriguez JA, Forno E, Rodriguez-Martinez CE, Celedon JC. Risk and protective factors for childhood asthma: what is the evidence?. J Allergy Clin Immunol Pract 2016;4:1111-22. https://doi.org/10.1016/j.jaip.2016.05.003.
- Tsakok T, McKeever TM, Yeo L, Flohr C. Does early life exposure to antibiotics increase the risk of eczema? A systematic review. Br J Dermatol 2013;169:983-91. https://doi.org/10.1111/bjd.12476.
- Fortson E, Feldman S, Strowd L. Management of Atopic Dermatitis: Methods and Challenges. New York, NY: Springer Publishing; 2017.
- Bao Y, Chen Z, Liu E, Xiang L, Zhao D, Hong J. Risk factors in preschool children for predicting asthma during the preschool age and the early school age: a systematic review and meta-analysis. Curr Allergy Asthma Rep 2017;17. https://doi.org/10.1007/s11882-017-0753-7.
- Federico MJ, Hoch HE, Anderson WC, Spahn JD, Szefler SJ. Asthma management for children: risk identification and prevention. Adv Pediatr 2016;63:103-26. https://doi.org/10.1016/j.yapd.2016.04.010.
- Milligan KL, Matsui E, Sharma H. Asthma in urban children: epidemiology, environmental risk factors, and the public health domain. Curr Allergy Asthma Rep 2016;16. https://doi.org/10.1007/s11882-016-0609-6.
- Hatfield SJ, Rogers NK, Lloyd-Lavery A, Grindlay D, Barnett R, Thomas KS. What’s new in atopic eczema? An analysis of systematic reviews published in 2014. Part 1. Epidemiology, risk factors and outcomes. Clin Exp Dermatol 2016;41:843-6. https://doi.org/10.1111/ced.12977.
- Pyun BY. Natural history and risk factors of atopic dermatitis in children. Allergy Asthma Immunol Res 2015;7:101-5. https://doi.org/10.4168/aair.2015.7.2.101.
- Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab 2015;66:8-16. https://doi.org/10.1159/000370220.
- Petersen I, McCrea RL, Sammon CJ, Osborn DP, Evans SJ, Cowen PJ, et al. Risks and benefits of psychotropic medication in pregnancy: cohort studies based on UK electronic primary care health records. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20230.
- Blak BT, Thompson M, Dattani H, Bourke A. Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care 2011;19:251-5. https://doi.org/10.14236/jhi.v19i4.820.
- Williams T, van Staa T, Puri S, Eaton S. Recent advances in the utility and use of the General Practice Research Database as an example of a UK Primary Care Data resource. Ther Adv Drug Saf 2012;3:89-9. https://doi.org/10.1177/2042098611435911.
- Cai B, Xu W, Bortnichak E, Watson DJ. An algorithm to identify medical practices common to both the General Practice Research Database and The Health Improvement Network database. Pharmacoepidemiol Drug Saf 2012;21:770-4. https://doi.org/10.1002/pds.3277.
- Cea-Soriano L, García Rodríguez LA, Fernández Cantero O, Hernández-Díaz S. Challenges of using primary care electronic medical records in the UK to study medications in pregnancy. Pharmacoepidemiol Drug Saf 2013;22:977-85. https://doi.org/10.1002/pds.3472.
- Charlton R, Snowball J, Sammon C, de Vries C. The Clinical Practice Research Datalink for drug safety in pregnancy research: an overview. Therapie 2014;69:83-9. https://doi.org/10.2515/therapie/2014007.
- Williams R. CPRD Mother Baby Link Documentation. London: Medicines and Healthcare products Regulatory Agency; 2017.
- NHS Digital . Hospital Episode Statistics (HES) n.d. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics (accessed 27 September 2021).
- Harron K, Gilbert R, Cromwell D, van der Meulen J. Linking data for mothers and babies in de-identified electronic health data. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0164667.
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377-81. https://doi.org/10.1016/j.jbi.2008.08.010.
- National Perinatal Epidemiology Unit . National Maternity Surveys n.d. www.npeu.ox.ac.uk/maternity-surveys (accessed 27 January 2020).
- Care Quality Commission . NHS Patient Surveys n.d. https://nhssurveys.org/surveys/survey/04-maternity/ (accessed 27 January 2020).
- Care Quality Commission, Picker Institute Europe . Maternity Services Survey, 2010 2011. https://doi.org/10.5255/UKDA-SN-6745-1 (accessed 15 November 2020).
- NHS Digital . Hospital Episode Statistics for England. Admitted Patient Care Statistics; 2014–15 2015.
- Public Health Wales . Caesarean-Section Surgical Site Infection Surveillance. Annual Report: 2014 2015.
- Kenyon SL, Taylor DJ, Tarnow-Mordi W. ORACLE Collaborative Group . Broad-spectrum antibiotics for preterm, prelabour rupture of fetal membranes: the ORACLE I randomised trial. Lancet 2001;357:979-88. https://doi.org/10.1016/S0140-6736(00)04233-1.
- NHS Health Research Authority . The Health Improvement Network (THIN) Database n.d. www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/the-health-improvement-network-thin-database/ (accessed 27 January 2020).
- Clinical Practice Research Datalink . Safeguarding Patient Data n.d. www.cprd.com/safeguarding-patient-data (accessed 27 January 2020).
- NHS Digital . Obtaining Data from NHS Digital from Health Research – A Guide for Researchers 2017. https://mrc.ukri.org/documents/pdf/obtaining-nhs-digital-data-guidance-v300317/ (accessed 27 September 2021).
- Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ 2017;358. https://doi.org/10.1136/bmj.j3453.
- Šumilo D, Willis BH, Rudge GM, Martin J, Gokhale K, Thayakaran R, et al. Long term impact of prophylactic antibiotic use before incision versus after cord clamping on children born by caesarean section: longitudinal study of UK electronic health records. BMJ 2022;377. https://doi.org/10.1136/bmj-2021-069704.
- Bollig C, Nothacker M, Lehane C, Motschall E, Lang B, Meerpohl JJ, et al. Prophylactic antibiotics before cord clamping in cesarean delivery: a systematic review. Acta Obstet Gynecol Scand 2018;97:521-35. https://doi.org/10.1111/aogs.13276.
- Klingenberg C, Kornelisse RF, Buonocore G, Maier RF, Stocker M. Culture-negative early-onset neonatal sepsis – at the crossroad between efficient sepsis care and antimicrobial stewardship. Front Pediatr 2018;6. https://doi.org/10.3389/fped.2018.00285.
- Holden SE, Jenkins-Jones S, Poole CD, Morgan CL, Coghill D, Currie CJ. The prevalence and incidence, resource use and financial costs of treating people with attention deficit/hyperactivity disorder (ADHD) in the United Kingdom (1998 to 2010). Child Adolesc Psychiatry Ment Health 2013;7. https://doi.org/10.1186/1753-2000-7-34.
- Hamad AF, Alessi-Severini S, Mahmud SM, Brownell M, Kuo IF. Antibiotic exposure in the first year of life and the risk of attention-deficit/hyperactivity disorder: a population-based cohort study. Am J Epidemiol 2019;188:1923-31. https://doi.org/10.1093/aje/kwz178.
- Axelsson PB, Clausen TD, Petersen AH, Hageman I, Pinborg A, Kessing LV, et al. Investigating the effects of cesarean delivery and antibiotic use in early childhood on risk of later attention deficit hyperactivity disorder. J Child Psychol Psychiatry 2019;60:151-9. https://doi.org/10.1111/jcpp.12961.
- Lavebratt C, Yang LL, Giacobini M, Forsell Y, Schalling M, Partonen T, et al. Early exposure to antibiotic drugs and risk for psychiatric disorders: a population-based study. Transl Psychiatry 2019;9. https://doi.org/10.1038/s41398-019-0653-9.
- Kenyon SL, Taylor DJ, Tarnow-Mordi W. ORACLE Collaborative Group . Broad-spectrum antibiotics for spontaneous preterm labour: the ORACLE II randomised trial. Lancet 2001;357:989-94. https://doi.org/10.1016/S0140-6736(00)04234-3.
- Smaill FM, Grivell RM. Antibiotic prophylaxis versus no prophylaxis for preventing infection after cesarean section. Cochrane Database Syst Rev 2014;10. https://doi.org/10.1002/14651858.CD007482.pub3.
- McBrien KA, Souri S, Symonds NE, Rouhi A, Lethebe BC, Williamson TS, et al. Identification of validated case definitions for medical conditions used in primary care electronic medical record databases: a systematic review. J Am Med Inform Assoc 2018;25:1567-78. https://doi.org/10.1093/jamia/ocy094.
- National Institute for Health and Care Excellence (NICE) . Asthma: Diagnosis, Monitoring and Chronic Asthma Management 2017. www.nice.org.uk/guidance/ng80 (accessed 22 November 2020).
- Pembrey L, Petherick E, Bird P, Wright J, Pearce N, Oddie S. In-Utero Antibiotic Exposure in Babies Born by Caesarean Section and Subsequent Risk of Allergic Disease and Asthma in the Born in Bradford Birth Cohort 2018. www.journalslibrary.nihr.ac.uk/programmes/hta/1615006/#/ (accessed 22 November 2020).
Appendix 1 Uptake of pre-incision antibiotics policy for caesarean section across England and the UK
FIGURE 10.
Cumulative percentage of hospitals with policies to administer antibiotic prophylaxis before skin incision for women undergoing CS over time and in different countries in the UK.
FIGURE 11.
Cumulative percentage of hospitals with policies to administer antibiotic prophylaxis before skin incision for women undergoing CS over time and in different regions in England.
Appendix 2 Covariate graphs
Maternal and household characteristics
FIGURE 12.
Proportion of delivery mode in the THIN–CPRD data set over time and across different maternal age groups at childbirth: (a) < 20 years; (b) 20–29 years; (c) 30–39 years; and (d) ≥ 40 years.
FIGURE 13.
Proportion of delivery mode in the HES data set over time and by maternal age groups at childbirth: (a) < 20 years; (b) 20–29 years; (c) 30–39 years; and (d) ≥ 40 years.
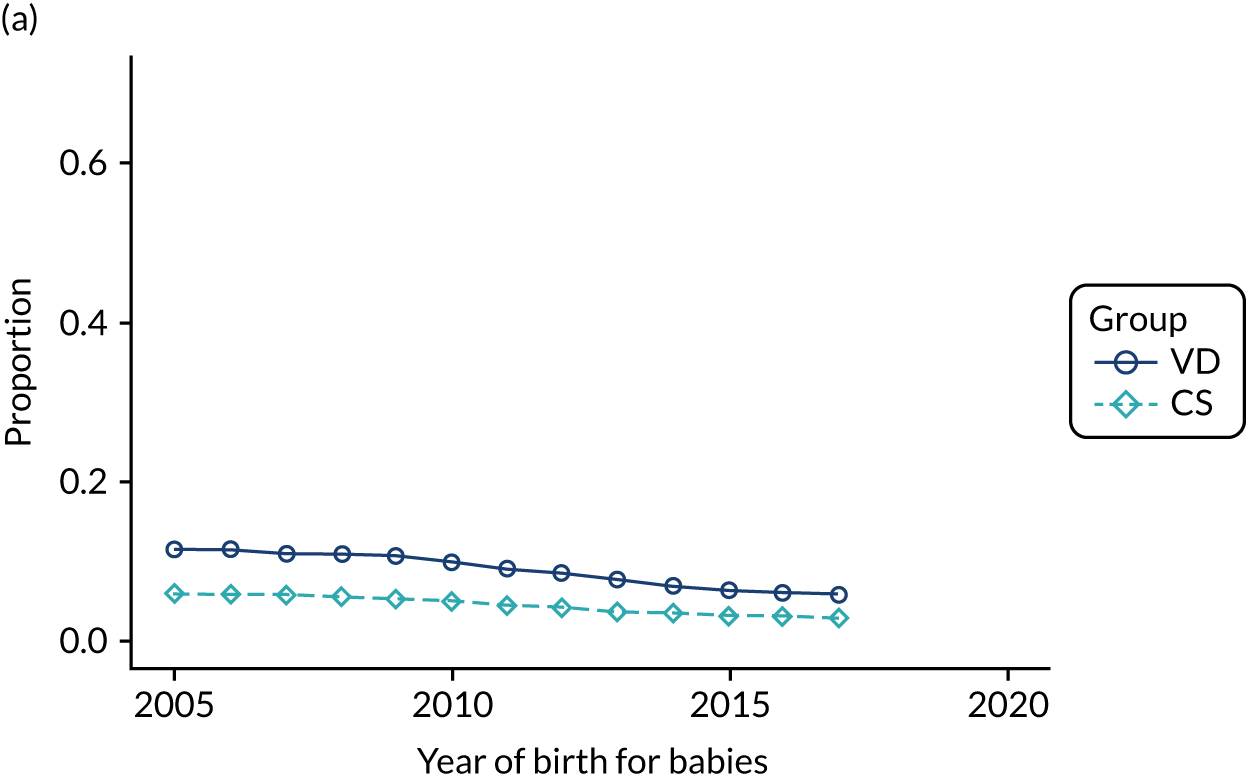
FIGURE 14.
Proportion of delivery mode in the THIN–CPRD data set over time and by maternal ethnicity: (a) black; (b) missing; (c) mixed; (d) other; (e) South Asian; and (f) white.
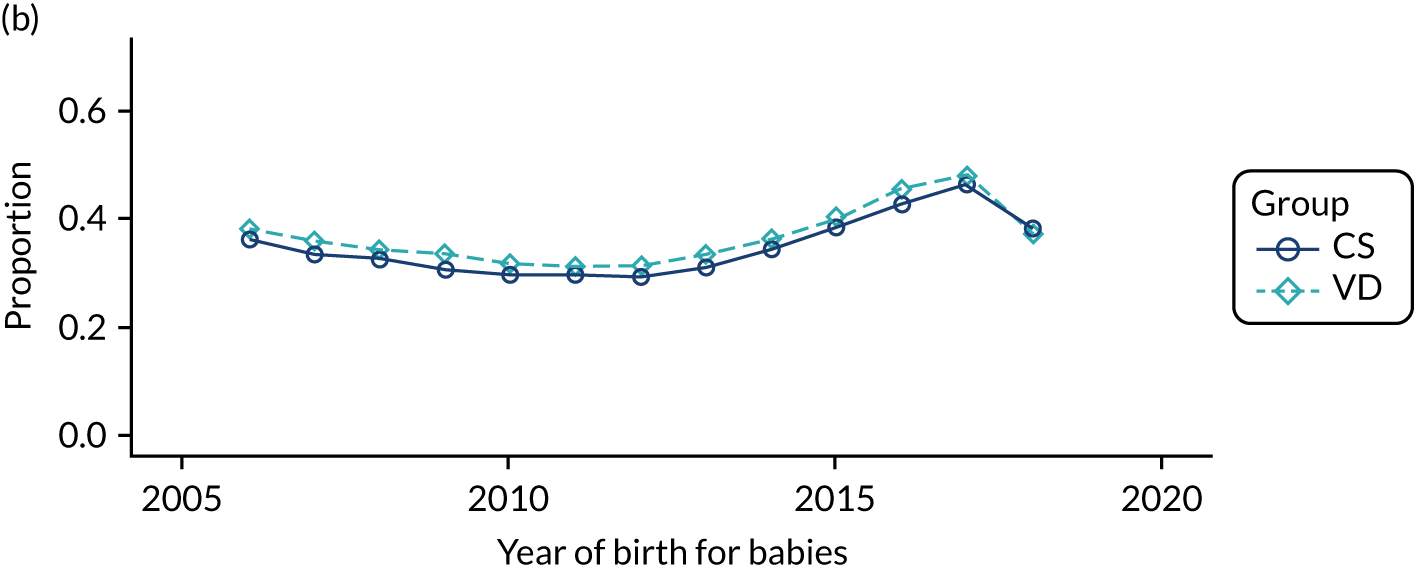
FIGURE 15.
Proportion of delivery mode in the HES data set over time and by maternal ethnicity: (a) black; (b) missing; (c) mixed; (d) other; (e) South Asian; and (f) white.
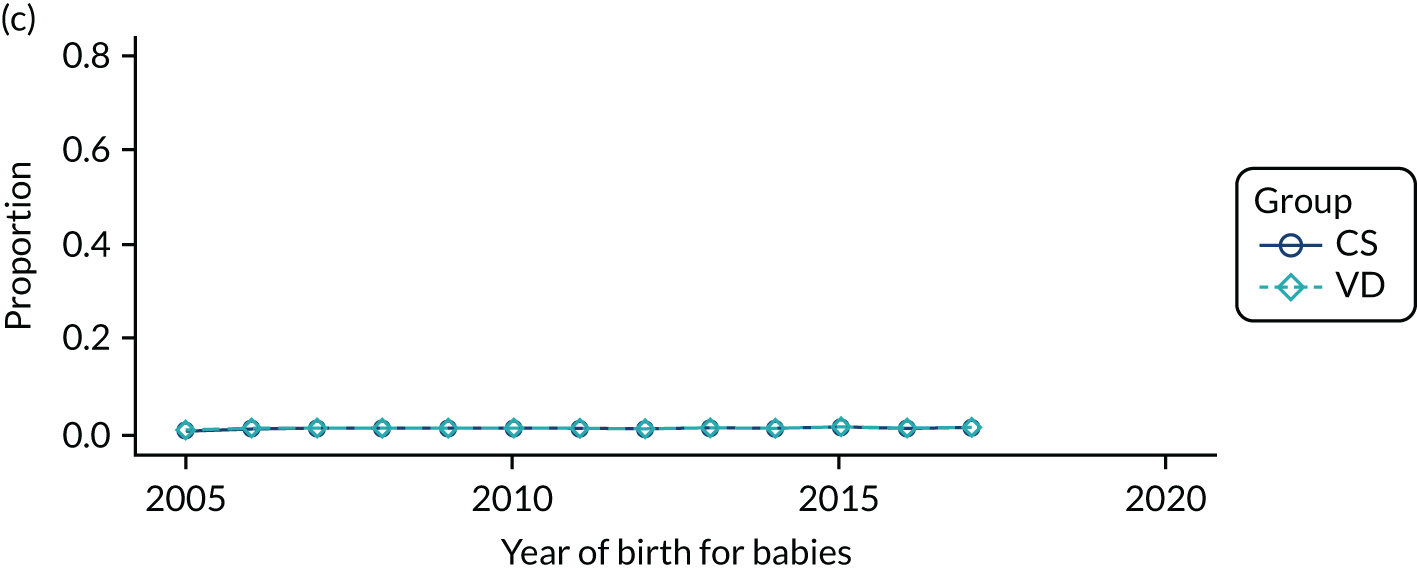
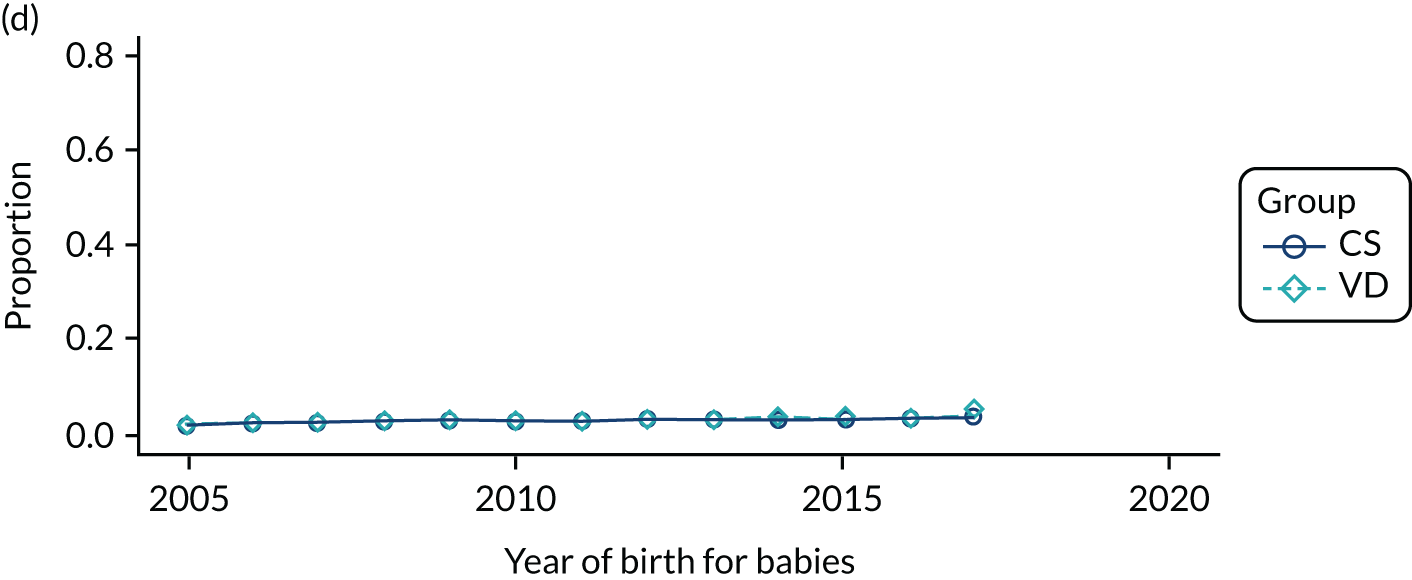
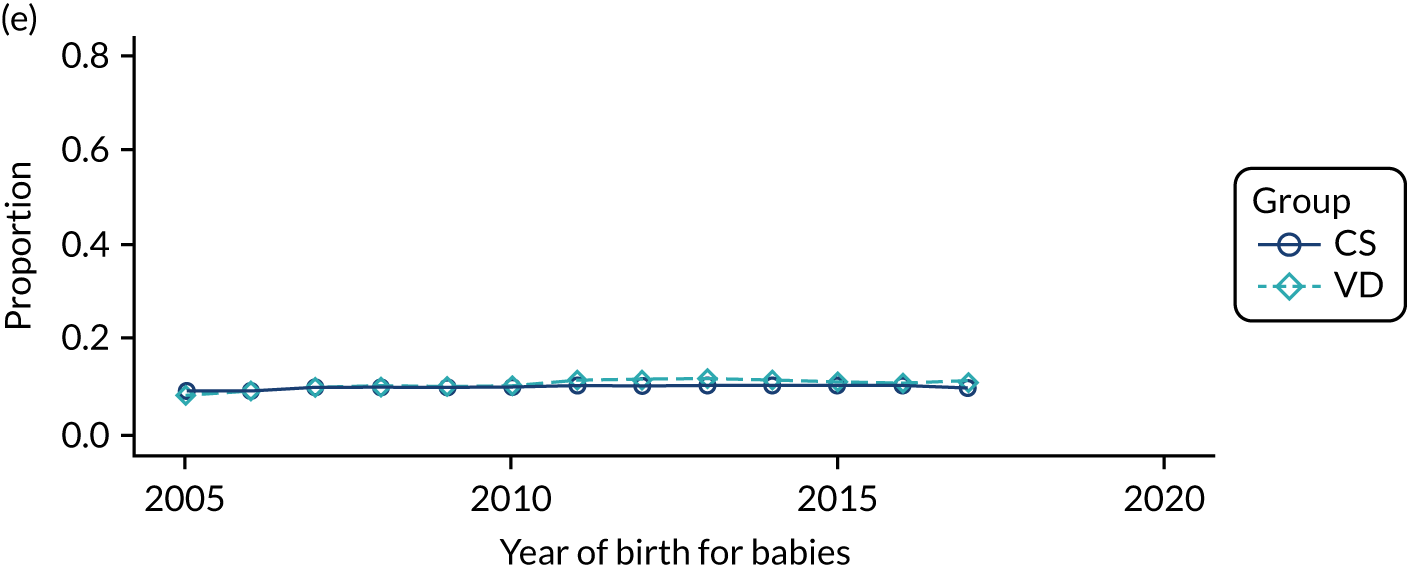
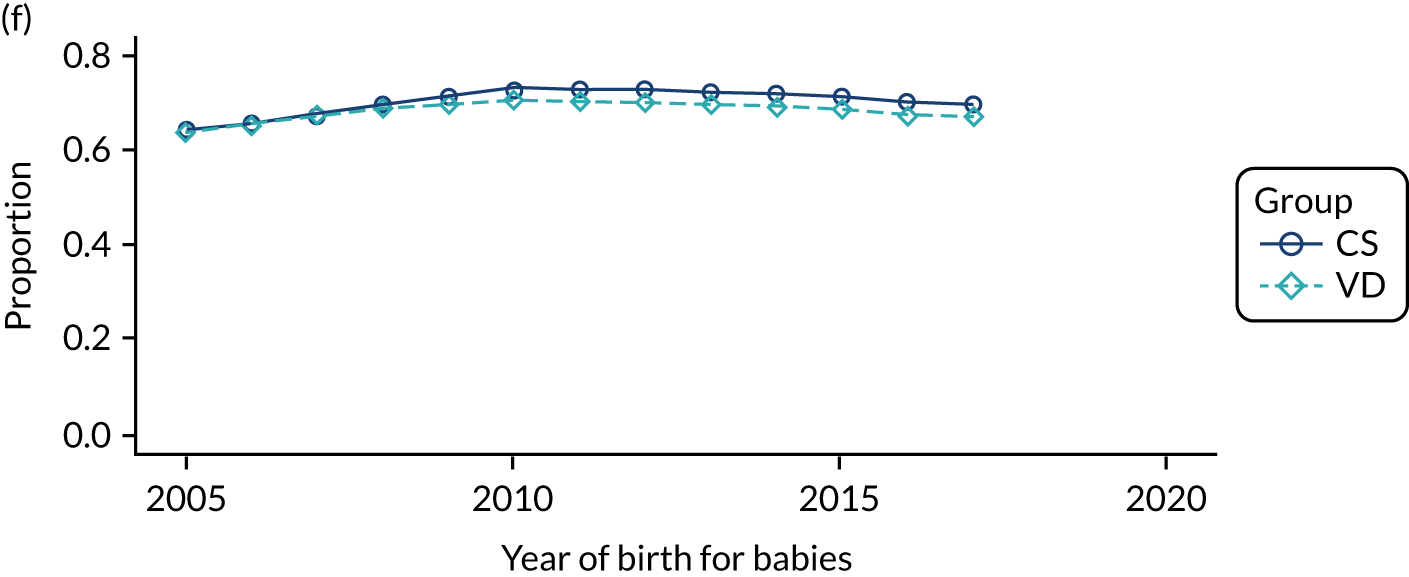
FIGURE 16.
Proportion of delivery mode in the THIN–CPRD data set over time and by maternal smoking status before delivery: (a) discontinued; (b) missing; (c) no; and (d) yes.
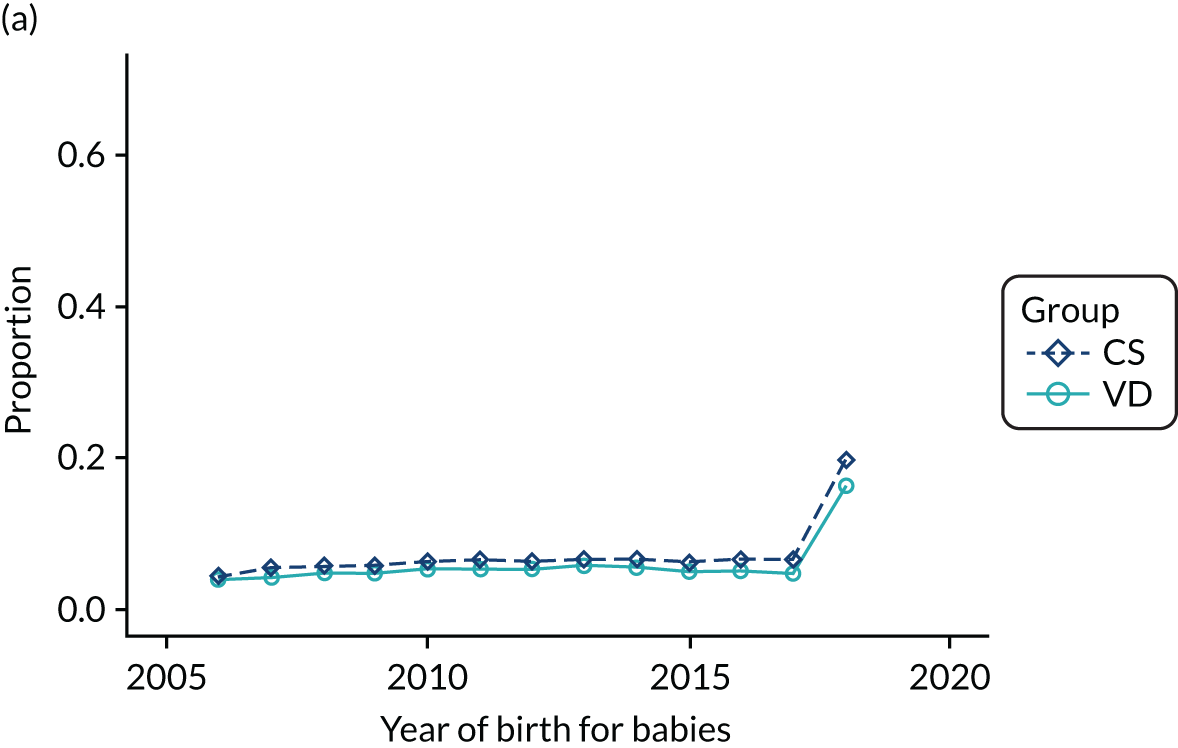
FIGURE 17.
Proportion of delivery mode in the THIN–CPRD data set over time and by different maternal body mass index groups (latest measurement was 9 months before delivery): (a) 0–18.5 kg/m2; (b) 18.5–25 kg/m2; (c) 25–30 kg/m2; (d) 30–34 kg/m2; (e) ≥ 35 kg/m2; and (f) missing.
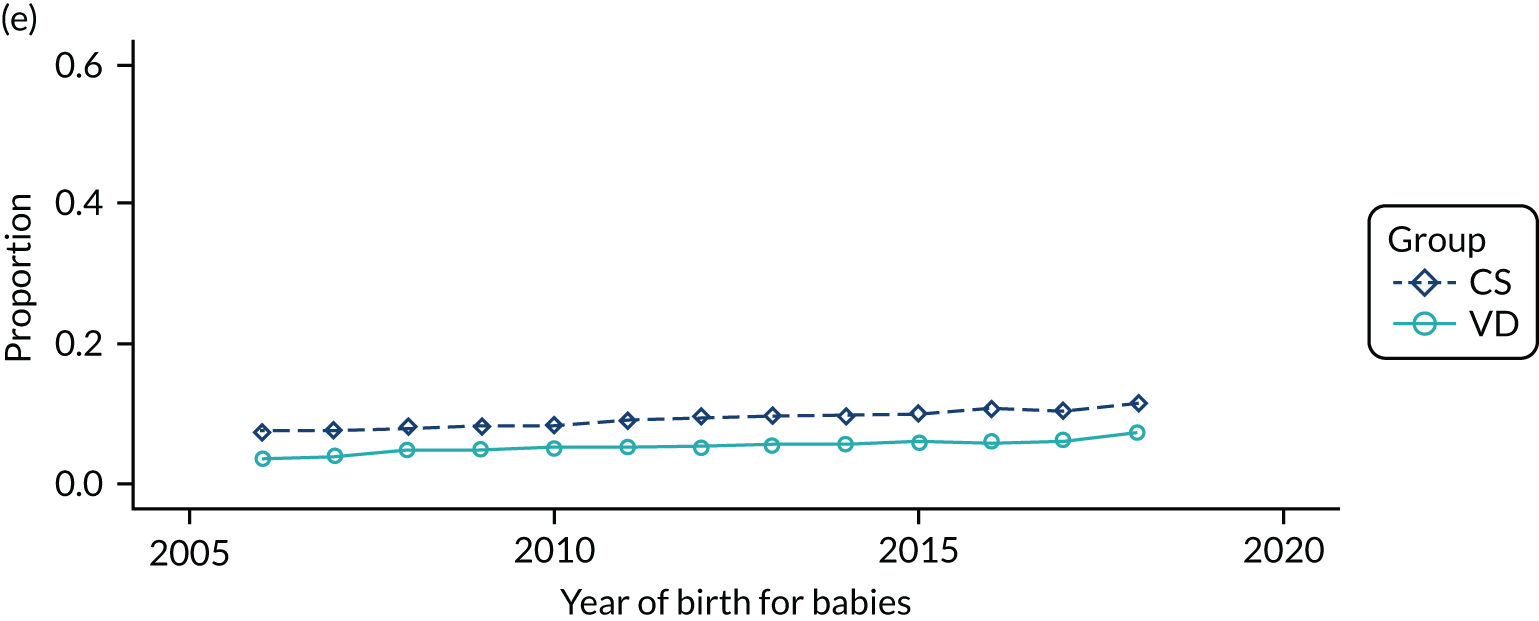
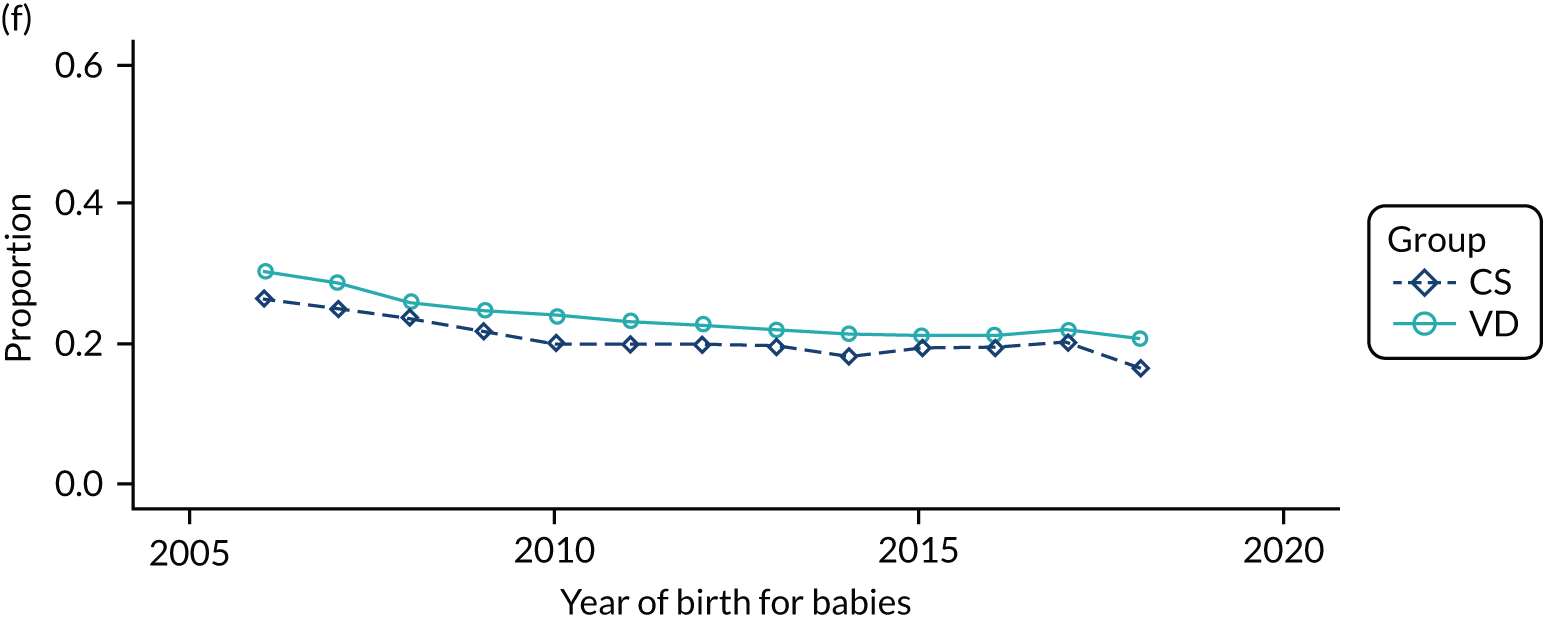
FIGURE 18.
Proportion of delivery mode in the HES data set over time and by parity: (a) 0; (b) 1; (c) ≥ 2; and (d) missing.
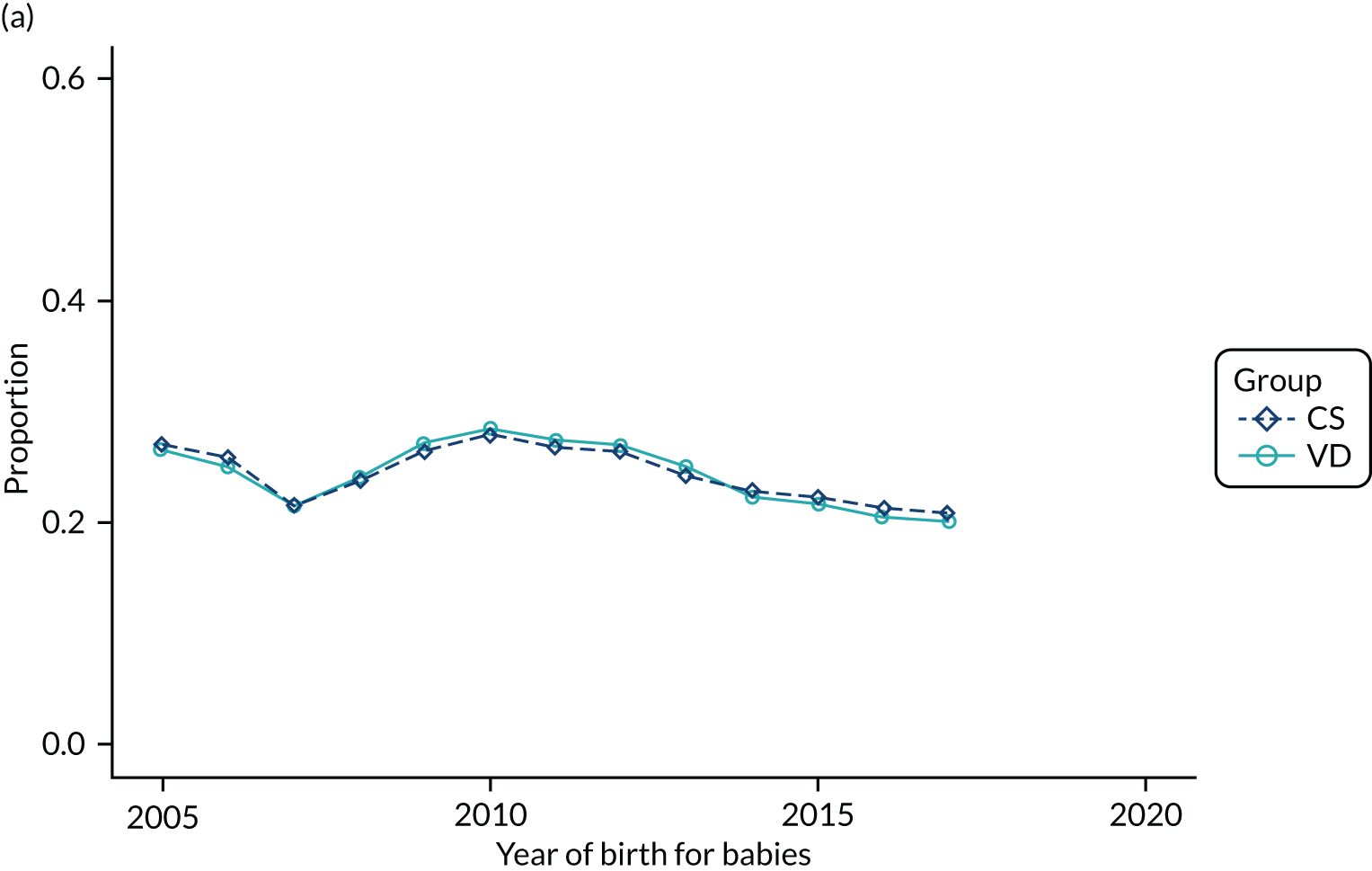
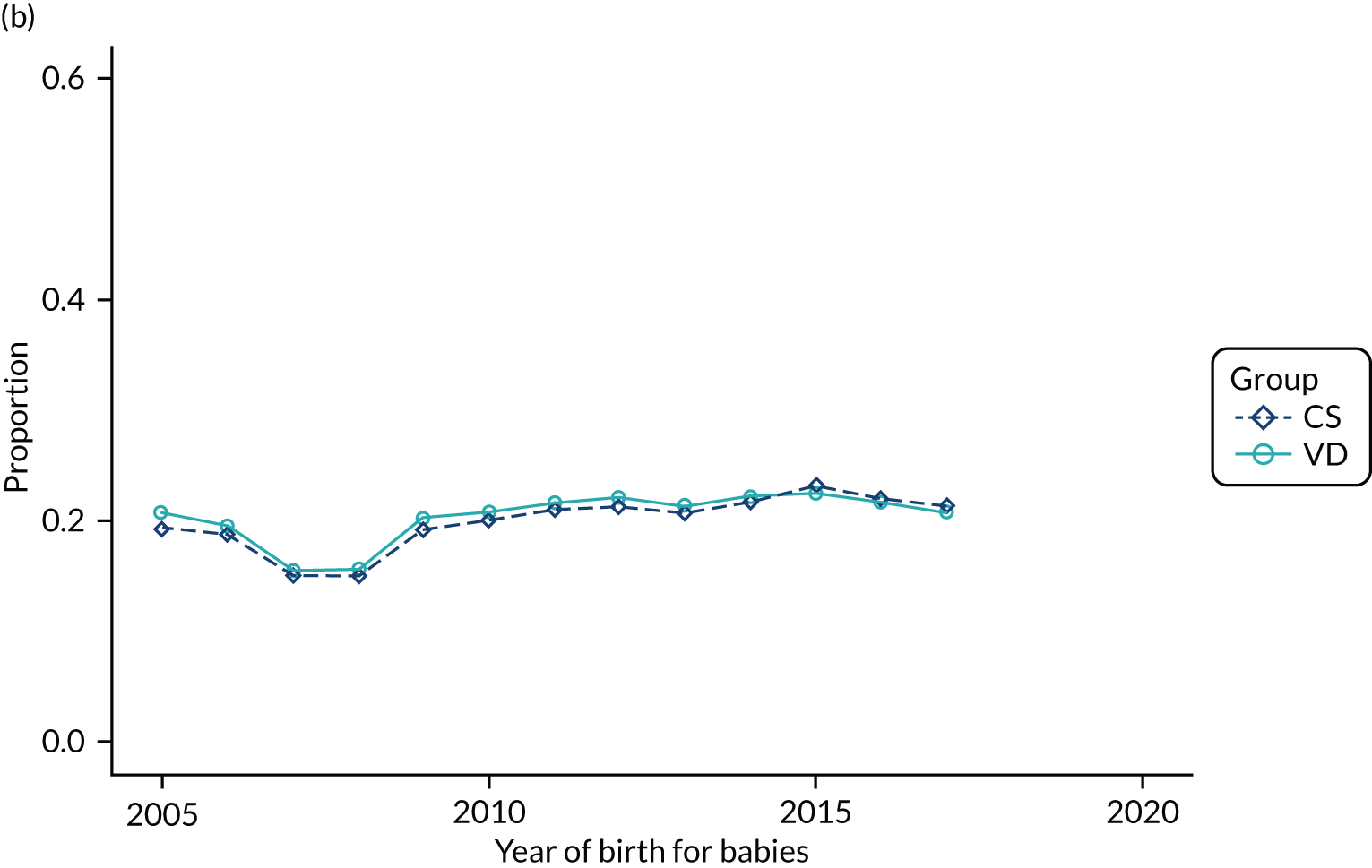
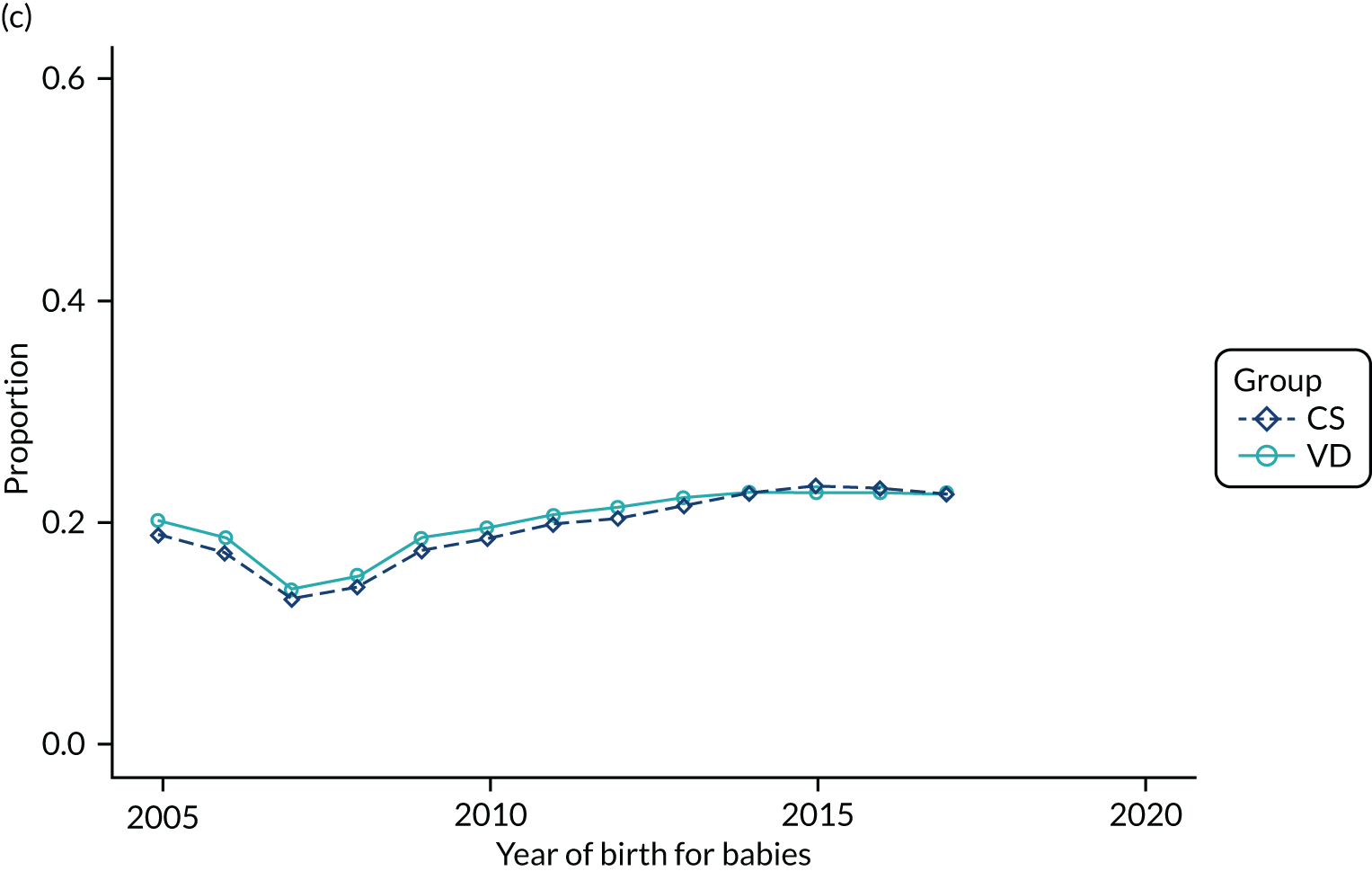
FIGURE 19.
Proportion of delivery mode in the HES data set with premature rupture of membranes over time.
FIGURE 20.
Proportion of delivery mode in the HES data set with post-partum haemorrhage over time.
FIGURE 21.
Proportion of delivery mode in the HES data set with manual placental removal/retained products of conception over time.
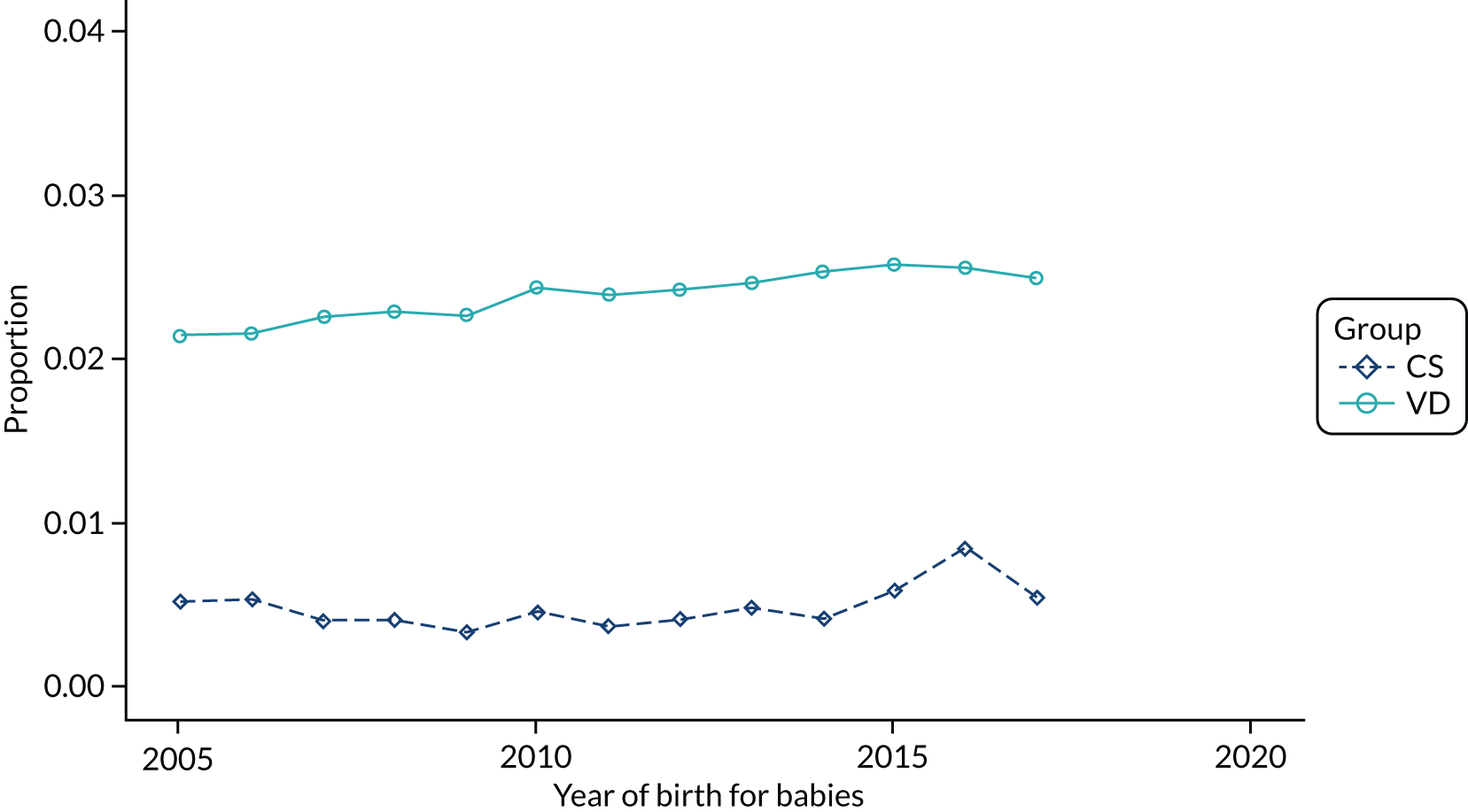
FIGURE 22.
Proportion of women by delivery mode in the THIN–CPRD data set who were prescribed antibiotics during pregnancy over time.
FIGURE 23.
Proportion of women by delivery mode in the THIN–CPRD data set over time and with a record of (a) asthma; (b) eczema; and (c) allergic rhinitis and conjunctivitis.
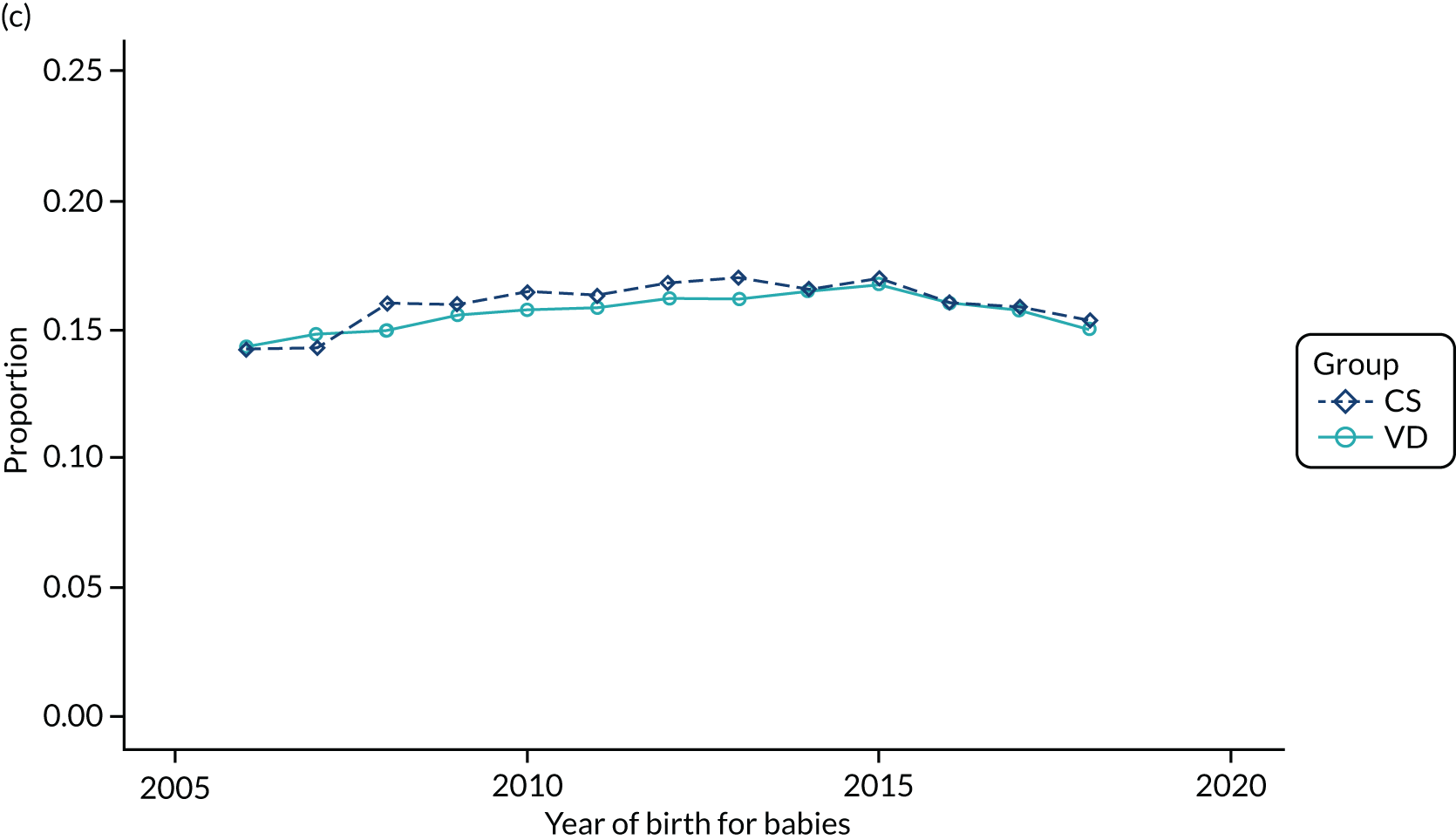
FIGURE 24.
Proportion of delivery mode in the THIN–CPRD data set over time and living in different areas of deprivation (as measured by Townsend score, with 1 being most affluent and 5 being most deprived): (a) Townsend score of 1; (b) Townsend score of 2; (c) Townsend score of 3; (d) Townsend score of 4; (e) Townsend score of 5; and (f) missing.
FIGURE 25.
Proportion of delivery mode in the HES data set over time and living in different areas of deprivation (as measured by Index of Multiple Deprivation 2015 score, with the lowest score being most affluent and the highest score being most deprived): (a) 0–0.2; (b) 0.2–0.4; (c) 0.4–0.6; (d) 0.6–0.8; (e) 0.8–1; and (f) missing.
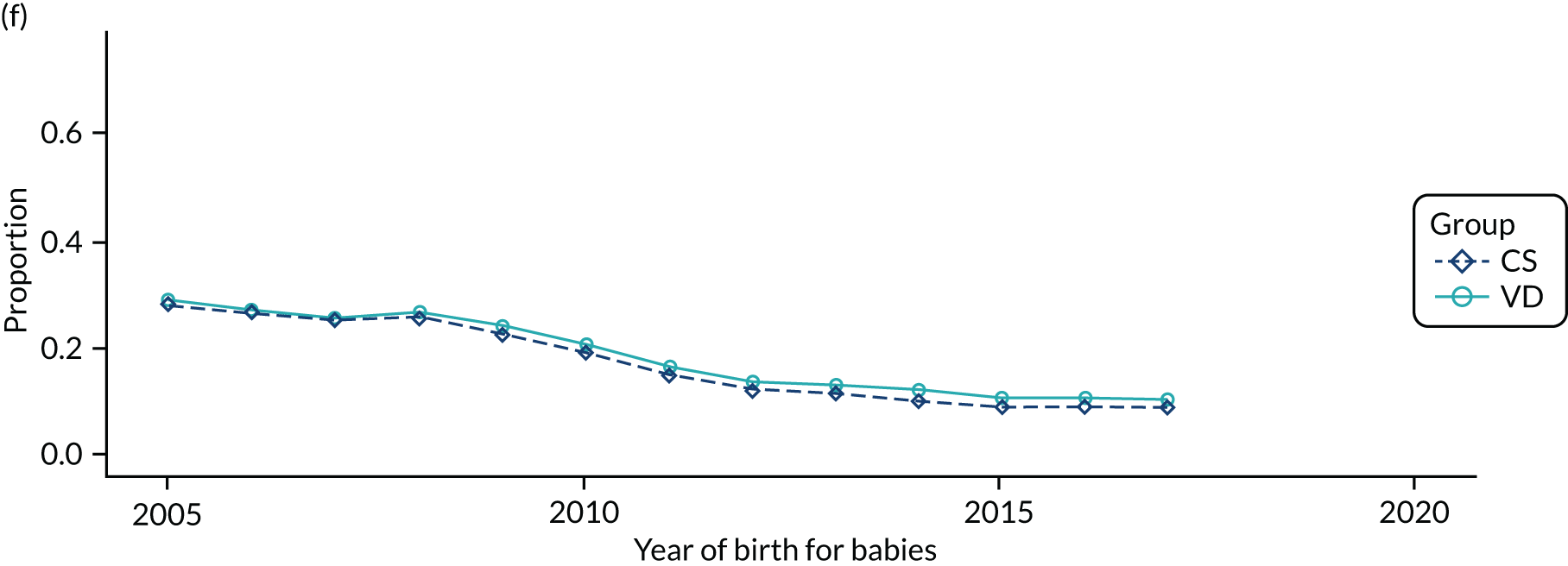
Child characteristics
FIGURE 26.
Proportion of boys in CS and VD births over time in (a) the THIN–CPRD data set and (b) the HES data set.
FIGURE 27.
Proportion of delivery mode in the THIN–CPRD data set over time and by child’s ethnicity: (a) black; (b) missing; (c) mixed; (d) other; (e) South Asian; and (f) white.
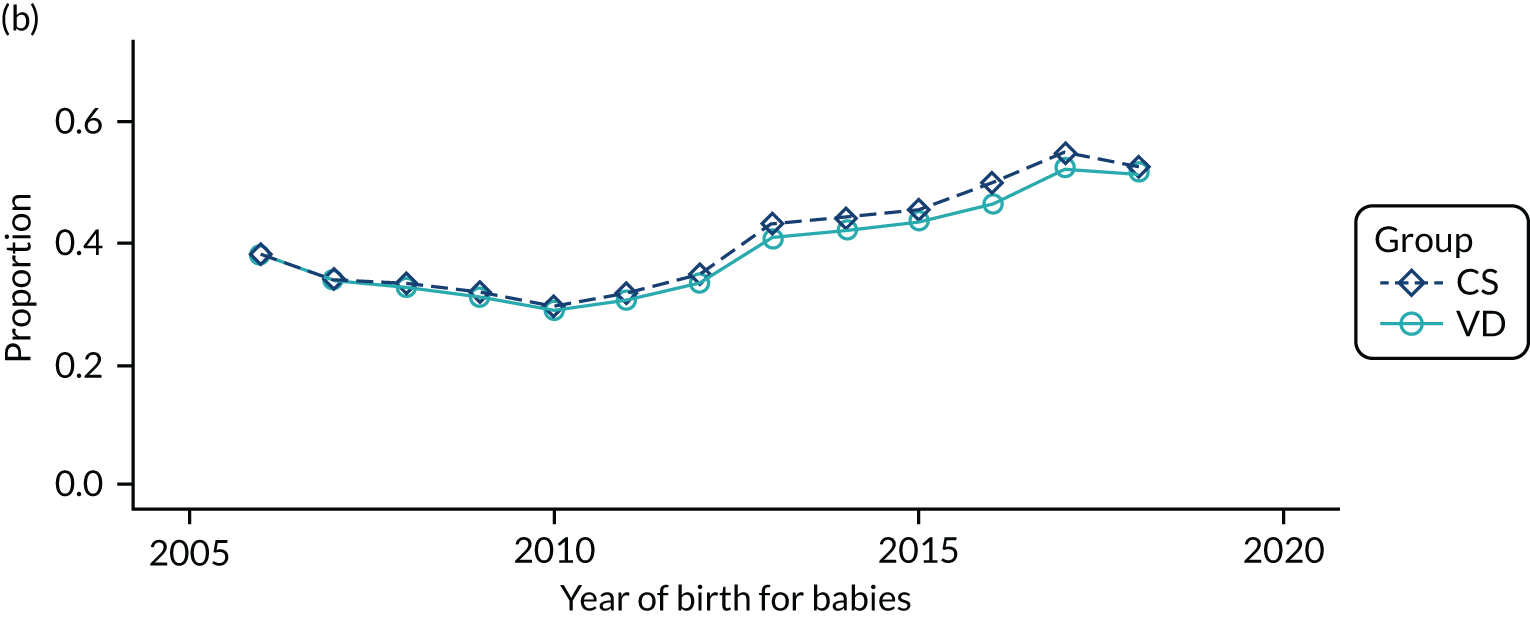
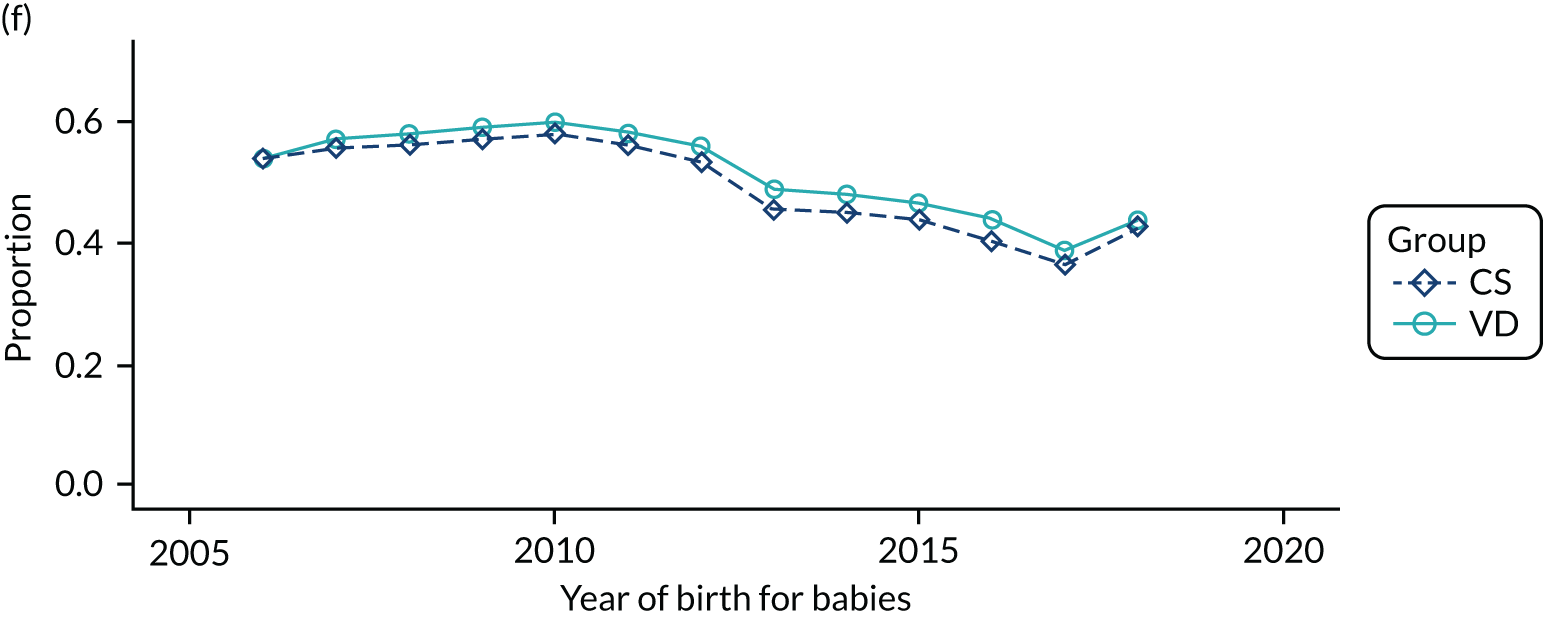
FIGURE 28.
Proportion of delivery mode in the HES data set over time and by child’s ethnicity: (a) black; (b) missing; (c) mixed; (d) other; (e) South Asian; and (f) white.
FIGURE 29.
Proportion of delivery mode in the HES data set over time and by gestational age: (a) < 32 weeks; (b) 32–36 weeks; (c) ≥ 37 weeks; and (d) missing.
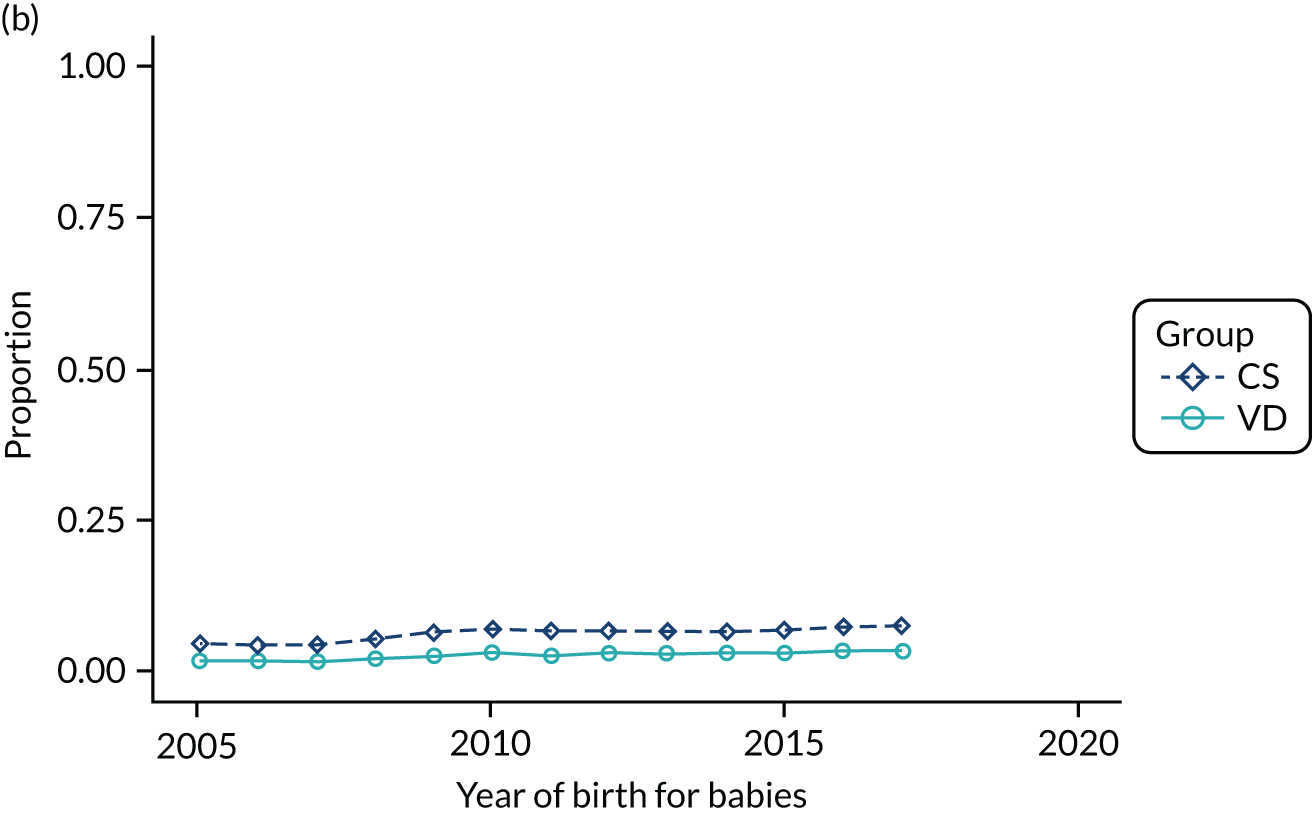
FIGURE 30.
Proportion of delivery mode in the HES data set over time and by the child’s birthweight: (a) < 2500 g; (b) 2500–3999 g; (c) ≥ 4000 g; and (d) missing.
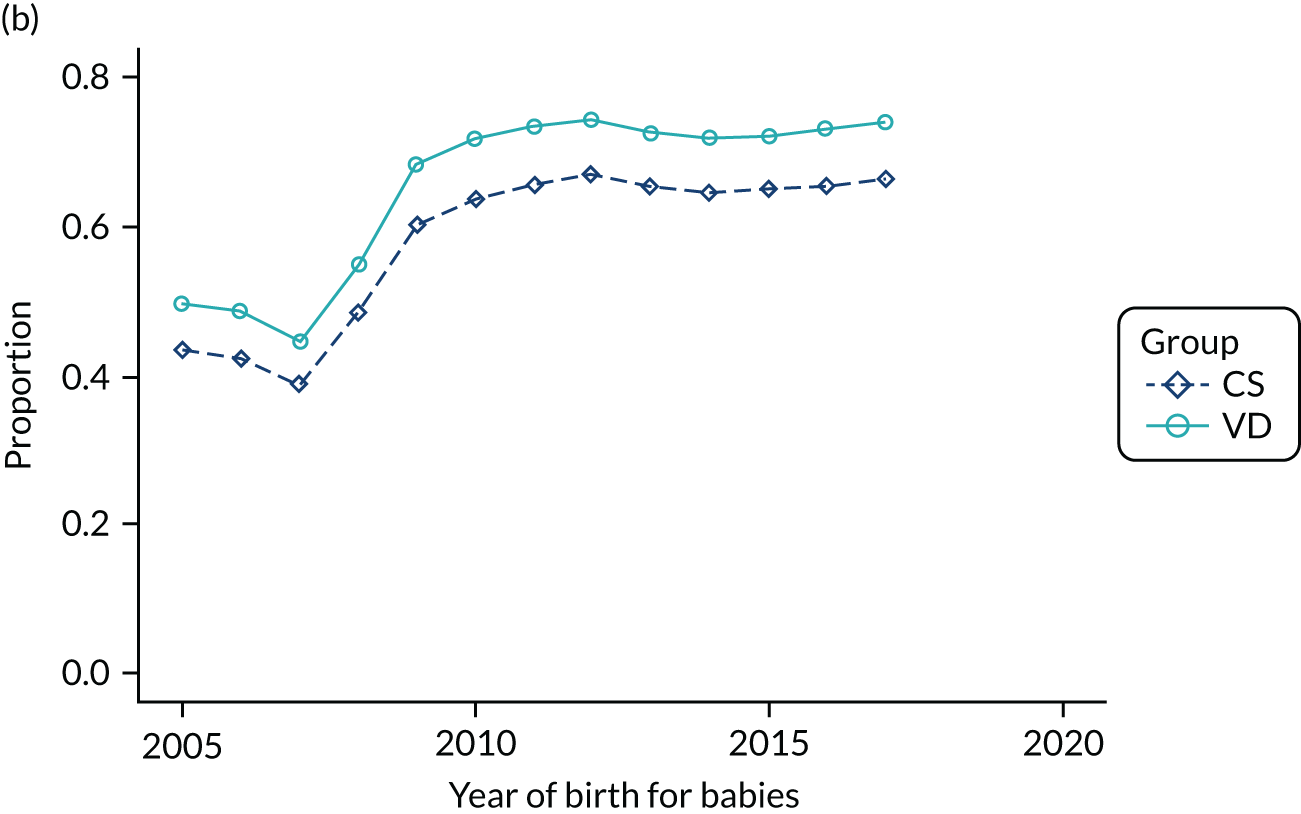
FIGURE 31.
Proportion of women feeding their babies with breast milk only in the few days after birth by delivery mode (data source: Care Quality Commission’s Maternity Surveys in 2007, 2010, 2013 and 2017). 79
FIGURE 32.
Proportion of women feeding their babies with breast milk only at 3 months of age by delivery mode (data source: National Perinatal Epidemiology Unit maternity surveys in 2006, 2010 and 201478).
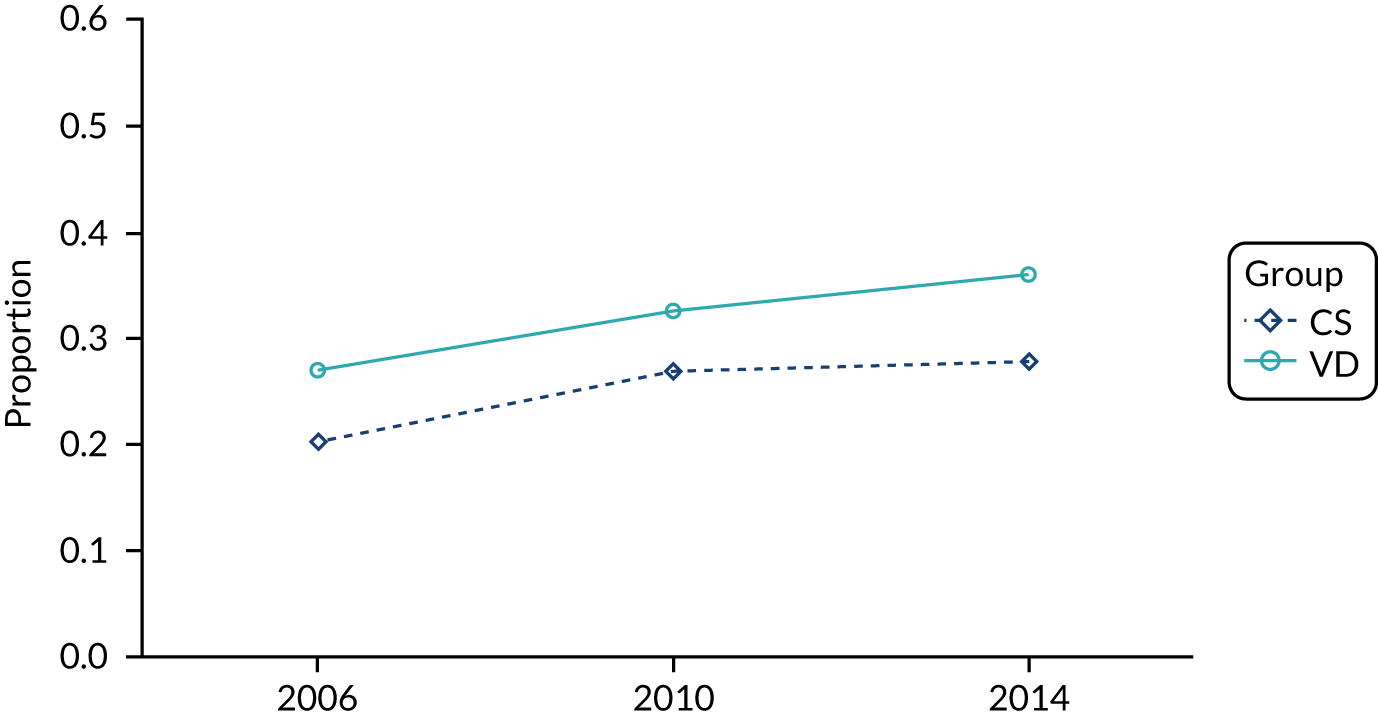
Appendix 3 Rates of outcomes of interest in the study population over time and by delivery mode
In this appendix, we present graphs displaying the rates of different outcomes in the study population over time. In each graph, we present the rates separately for delivery mode (CS or VD). In the primary care data set, individual graphs are presented for each year of life. No CIs have been included on these graphs; however, to aid the interpretation of the precision of these rates, we provide some example CIs for the rate of the outcome (per 1000 person-years) for a series of event rates based on the numbers of events and follow-up time found in primary care data (Table 21) and secondary care data (Table 22).
| Number of events | Follow-up time (person-years) | Rate per 1000 person-years | CI for rate per 1000 person-years |
|---|---|---|---|
| 2 | 11,868 | 0.17 | 0.02 to 0.61 |
| 71 | 11,850 | 5.99 | 4.68 to 7.56 |
| 1189 | 11,329 | 104.95 | 99.07 to 111.09 |
| 5583 | 9254 | 603.34 | 587.61 to 619.37 |
| Number of events | Follow-up time (person-years) | Rate per 1000 person-years | CI for rate per 1000 person-years |
|---|---|---|---|
| 235 | 764,195 | 0.31 | 0.27 to 0.35 |
| 1865 | 764,195 | 2.44 | 2.33 to 2.55 |
| 4864 | 149,522 | 32.53 | 31.62 to 33.46 |
| 53,595 | 764,195 | 70.1 | 69.54 to 70.72 |
Outcomes in the THIN–CPRD data set
FIGURE 33.
Incidence rate of asthma per 1000 person-years in children born by CS and VD by age and year of birth in the THIN–CPRD data set. Age (a) 0 years; (b) 1 year; (c) 2 years; (d) 3 years; and (e) 4 years.
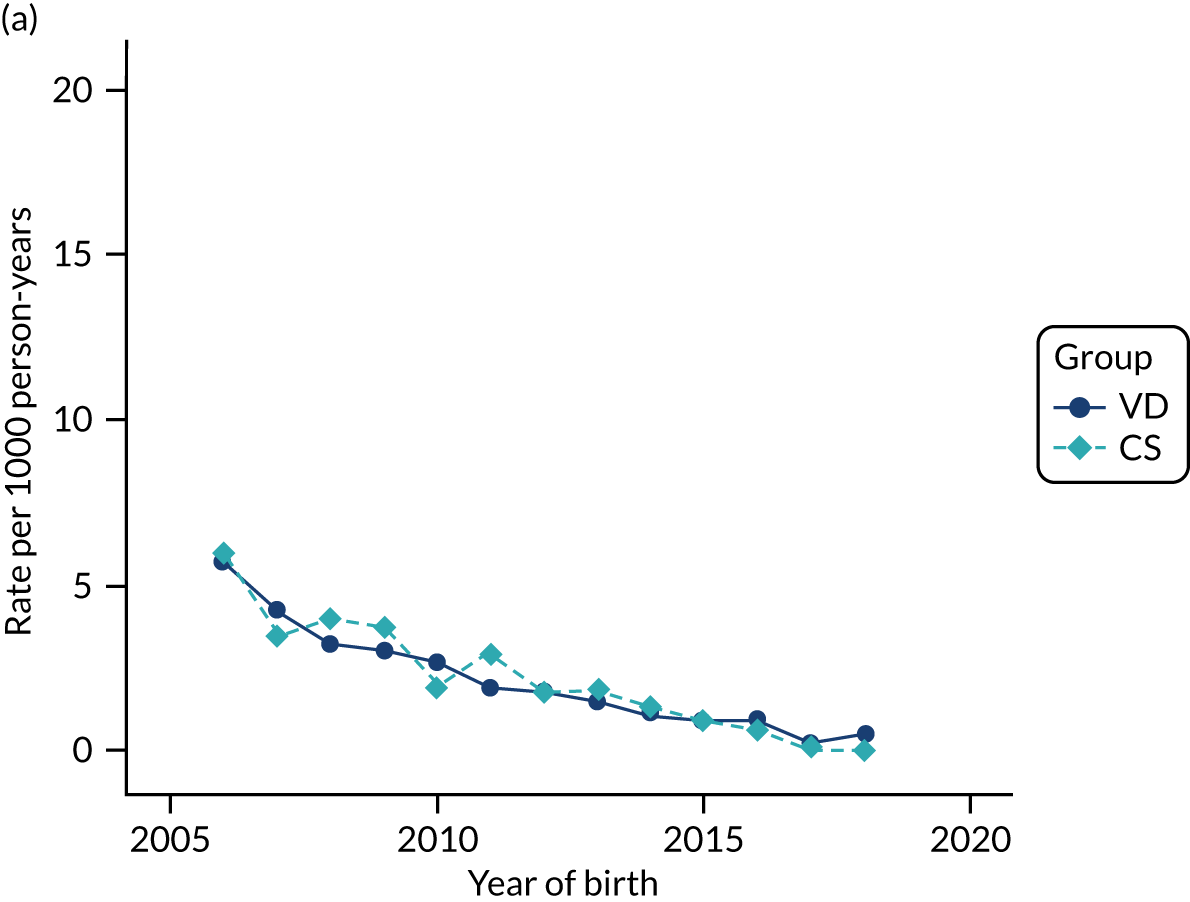
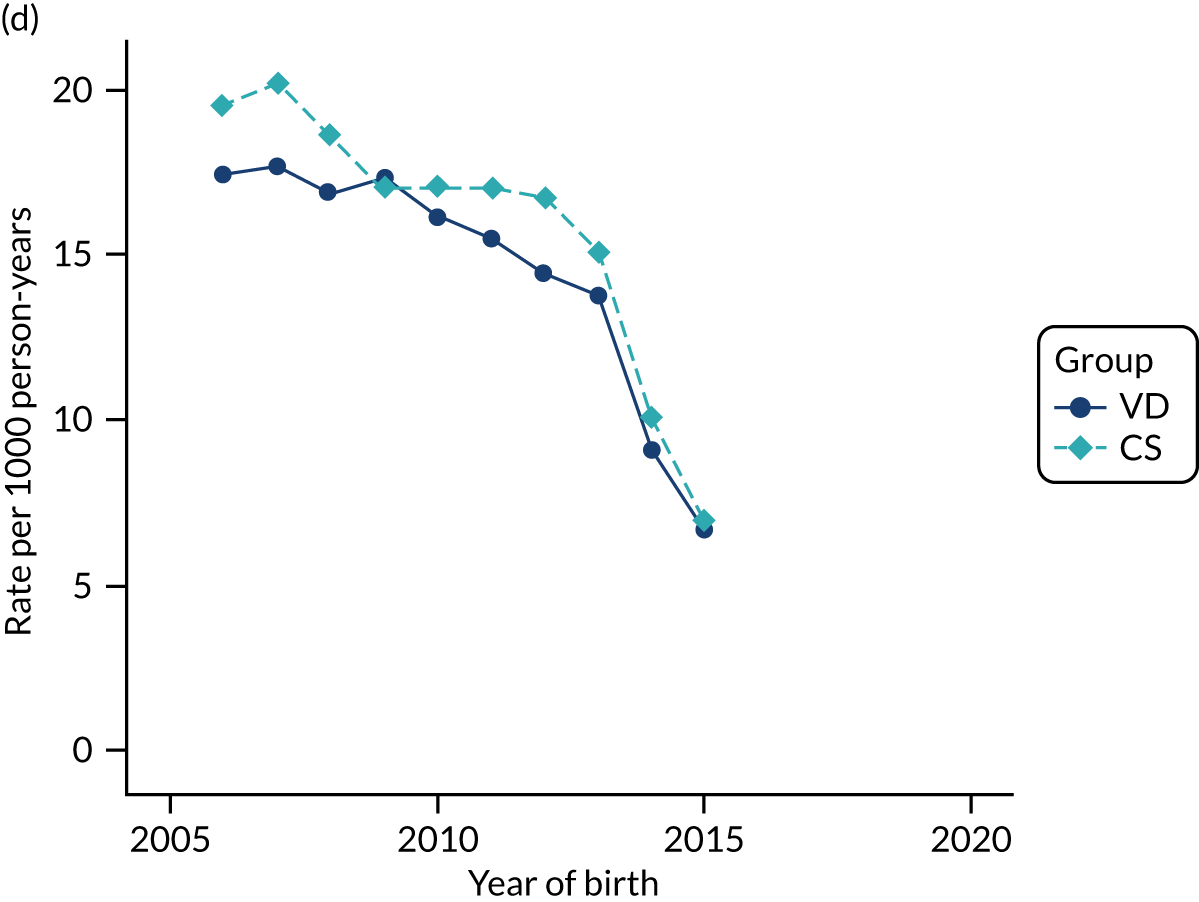
FIGURE 34.
Incidence rate of eczema per 1000 person-years in children born by CS and VD by age and year of birth in the THIN–CPRD data set. Age (a) 0 years; (b) 1 year; (c) 2 years; (d) 3 years; and (e) 4 years.
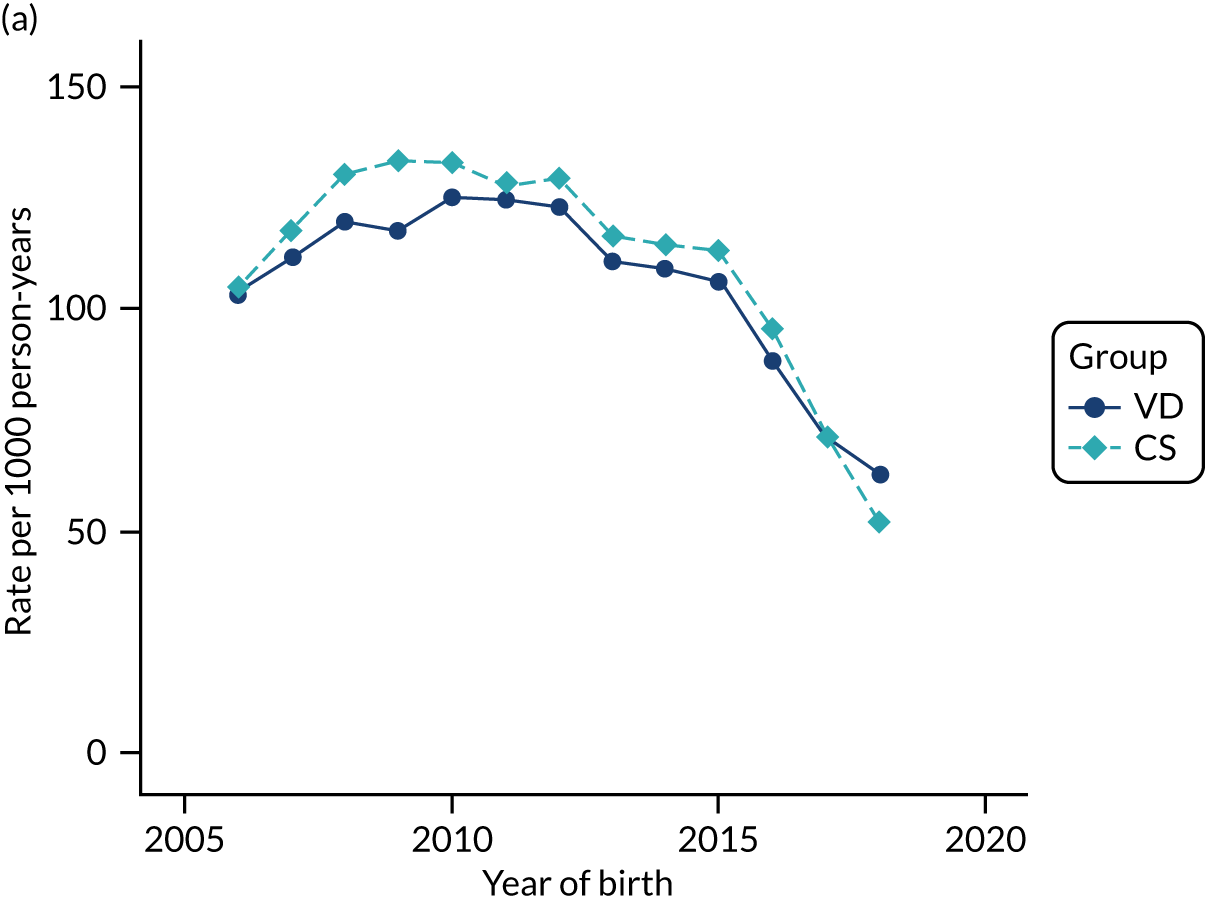
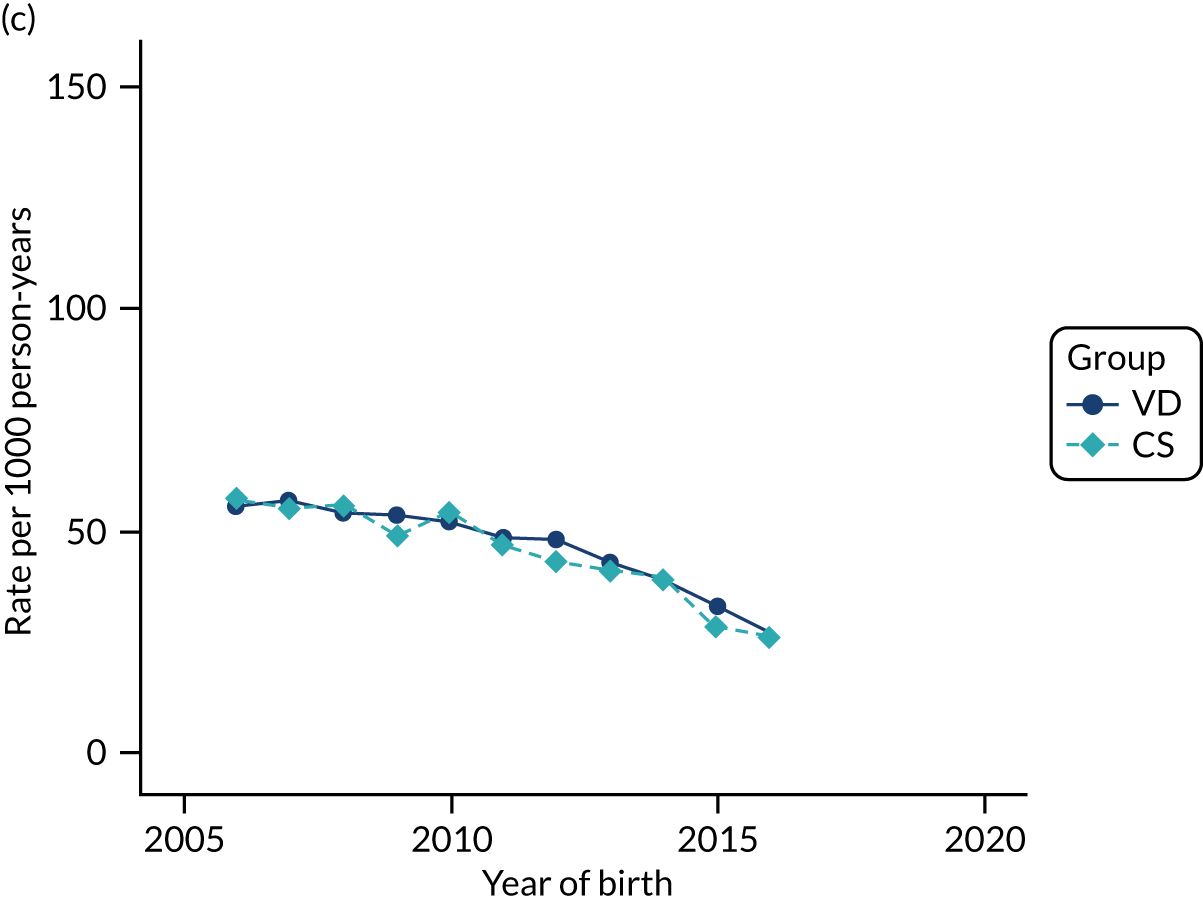
FIGURE 35.
Incidence rate of allergic rhinitis and conjunctivitis per 1000 person-years in children born by CS and VD by age and year of birth in the THIN–CPRD data set. Age (a) 0 years; (b) 1 year; (c) 2 years; (d) 3 years; and (e) 4 years.
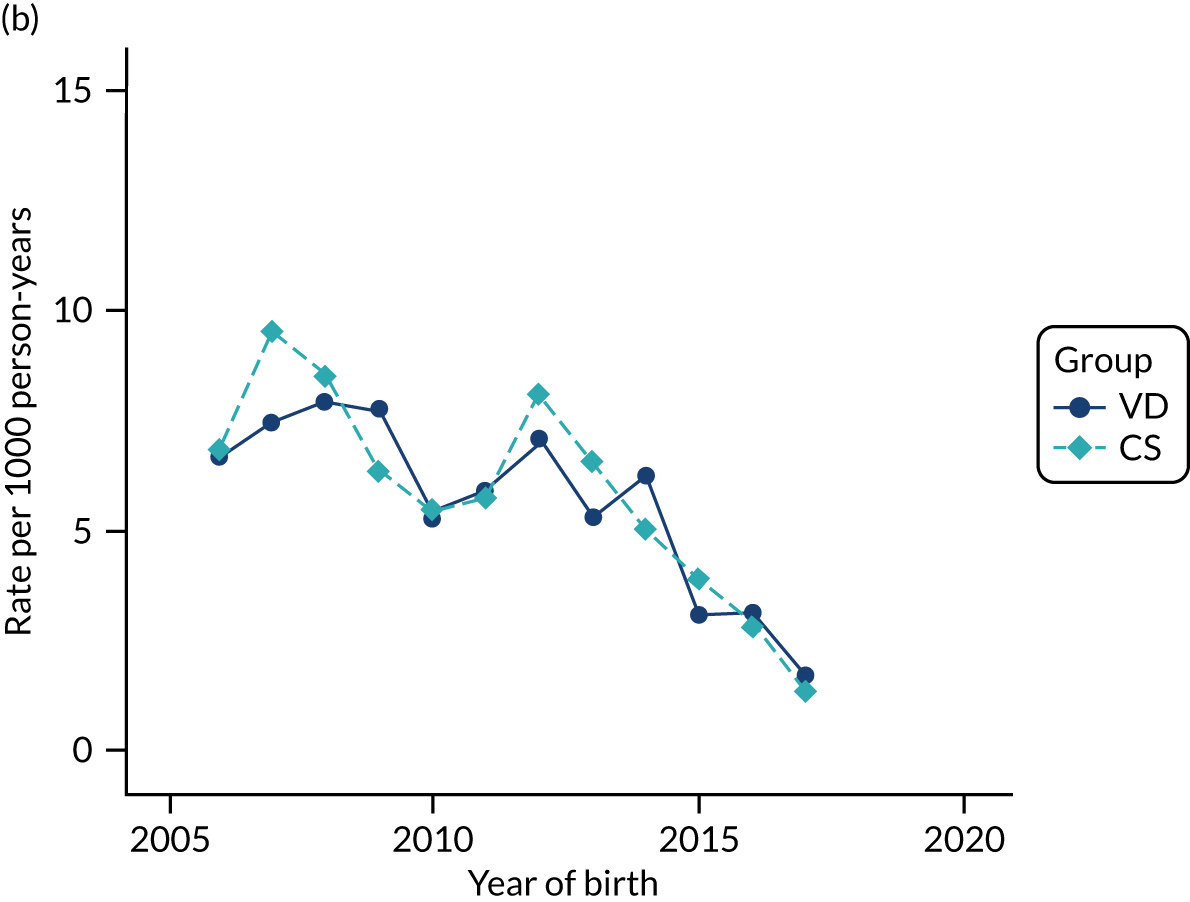
FIGURE 36.
Incidence rate of food allergy and intolerance per 1000 person-years in children born by CS and VD by age and year of birth in the THIN–CPRD data set. Age (a) 0 years; (b) 1 year; (c) 2 years; (d) 3 years; and (e) 4 years.
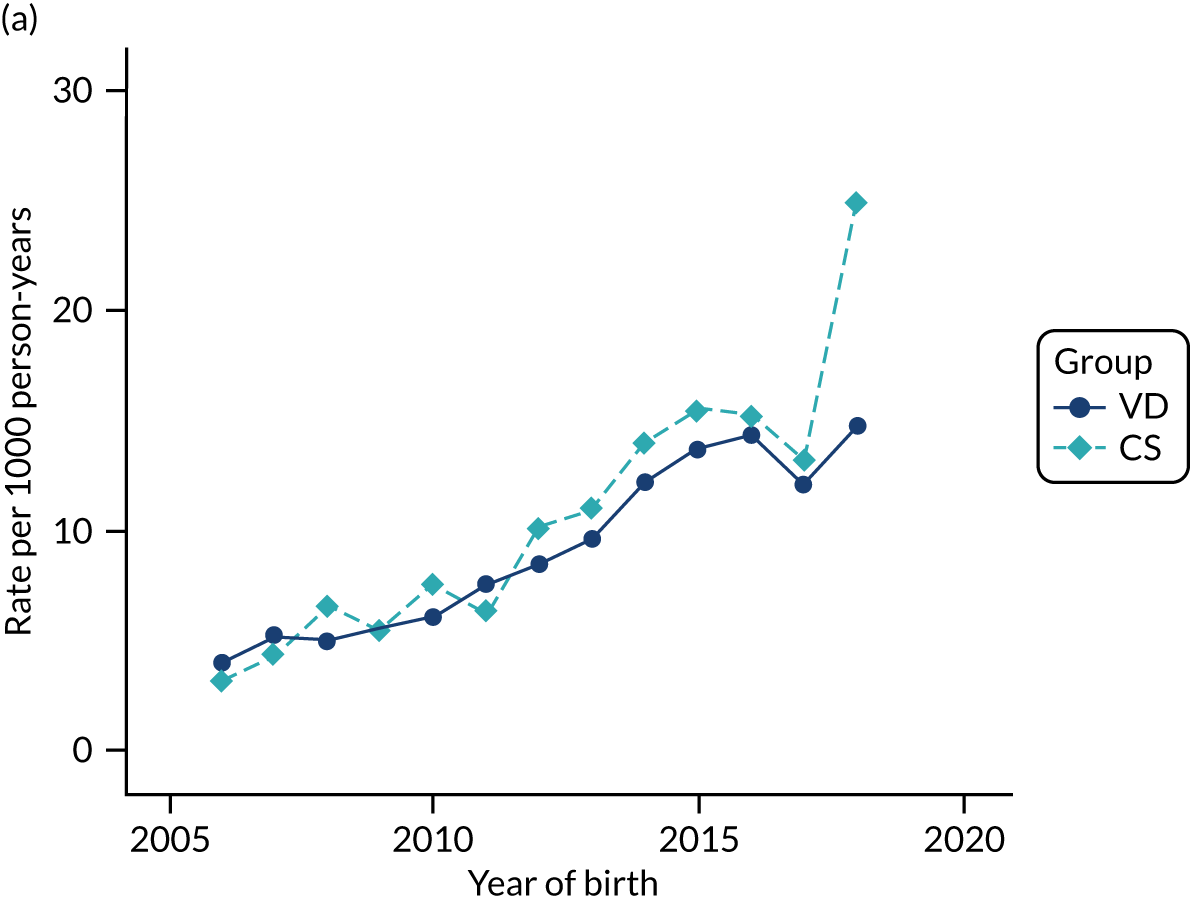
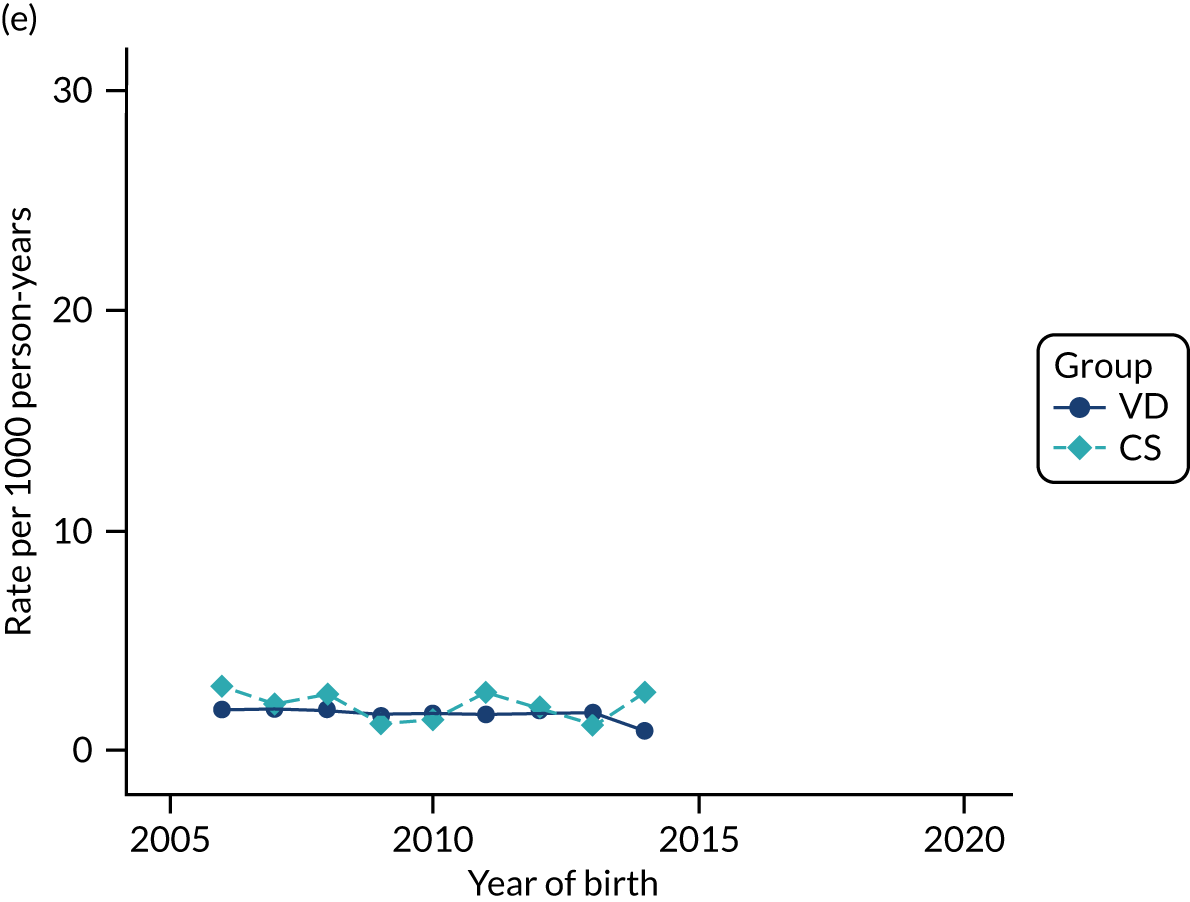
FIGURE 37.
Incidence rate of having two or more allergy-related conditions (e.g. asthma, eczema, food allergy/intolerance, allergic rhinitis and conjunctivitis) per 1000 person-years in children born by CS and VD by age and year of birth in the THIN–CPRD data set. Age (a) 0 years; (b) 1 year; (c) 2 years; (d) 3 years; and (e) 4 years.
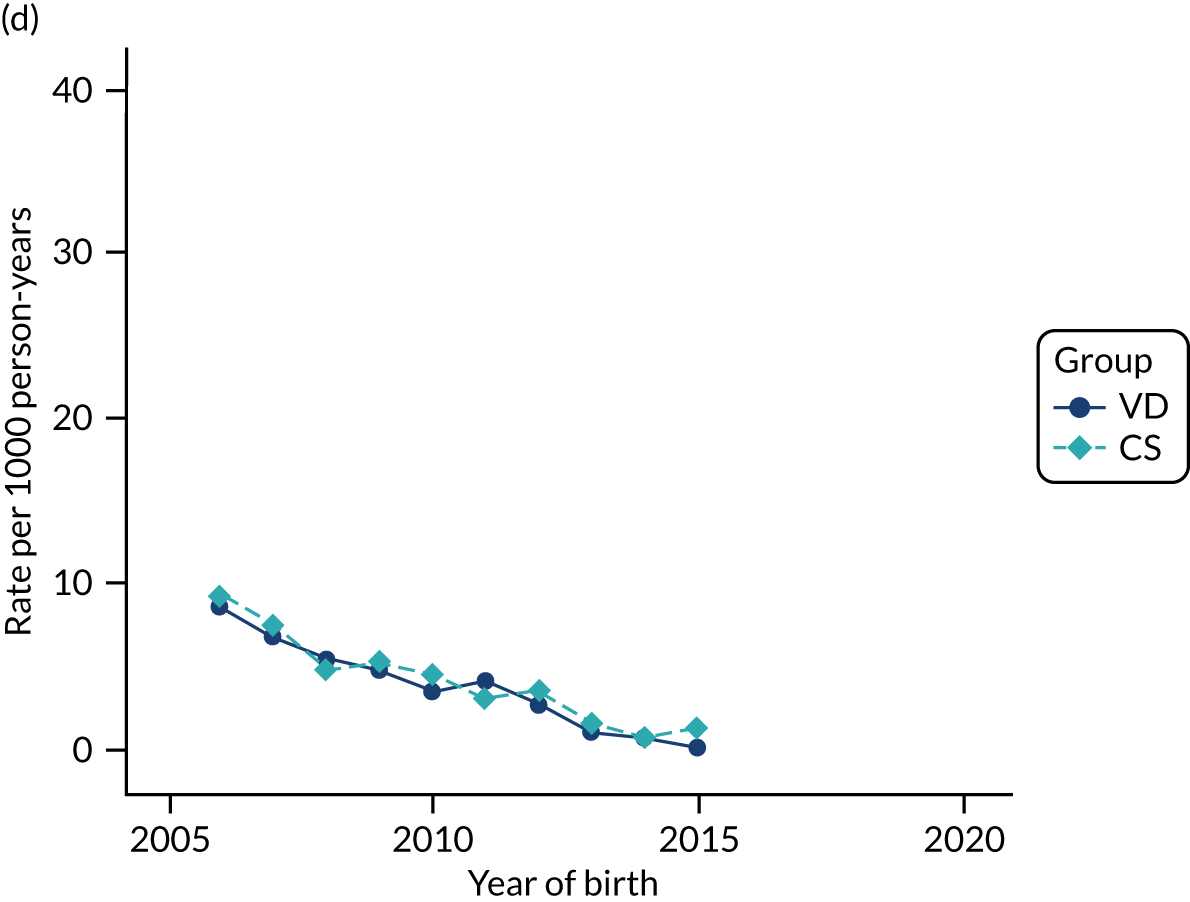
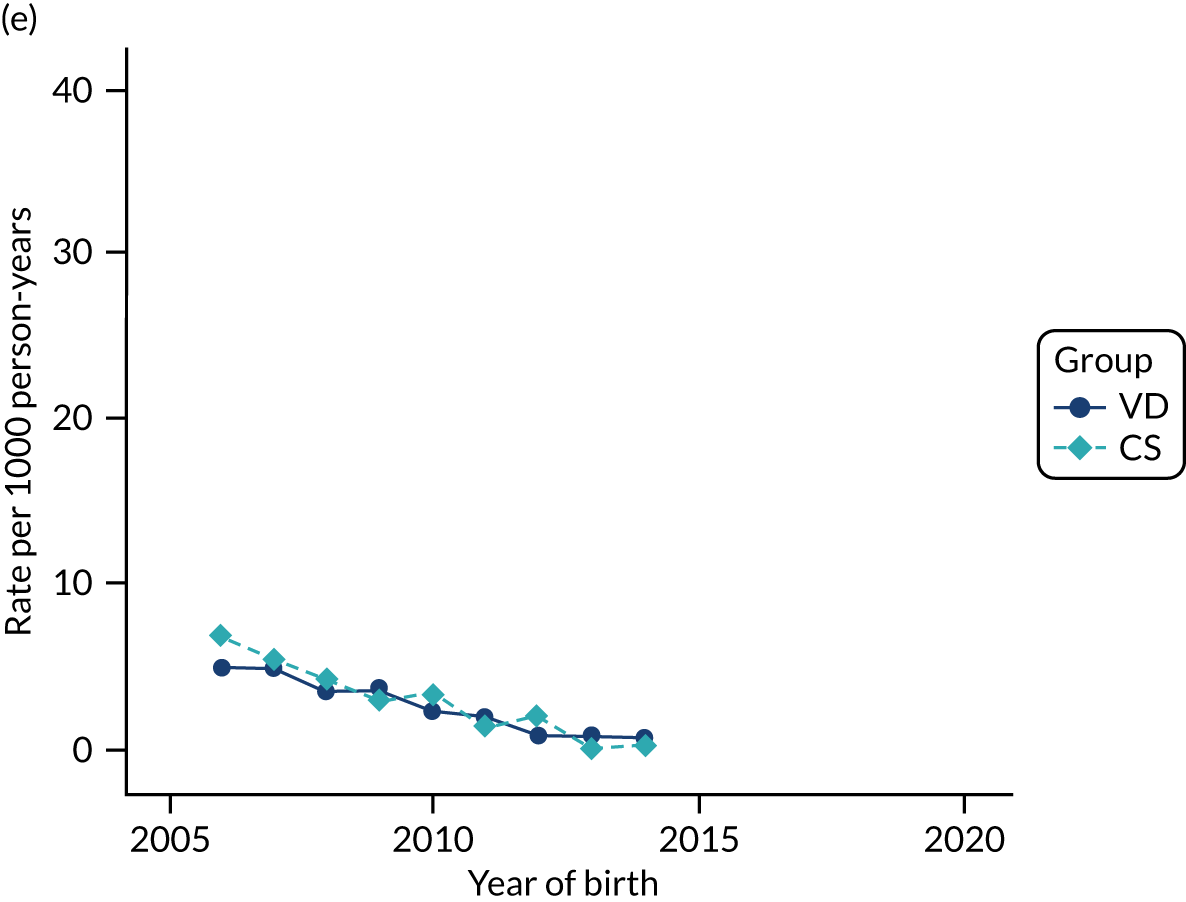
FIGURE 38.
Incidence rate of penicillin allergy per 1000 person-years in children born by CS and VD by age and year of birth in the THIN–CPRD data set. Age (a) 0 years; (b) 1 year; (c) 2 years; (d) 3 years; and (e) 4 years.
FIGURE 39.
Incidence rate of anaphylaxis per 1000 person-years in children born by CS and VD by age and year of birth in the THIN–CPRD data set. Age (a) 0 years; (b) 1 year; (c) 2 years; (d) 3 years; and (e) 4 years.
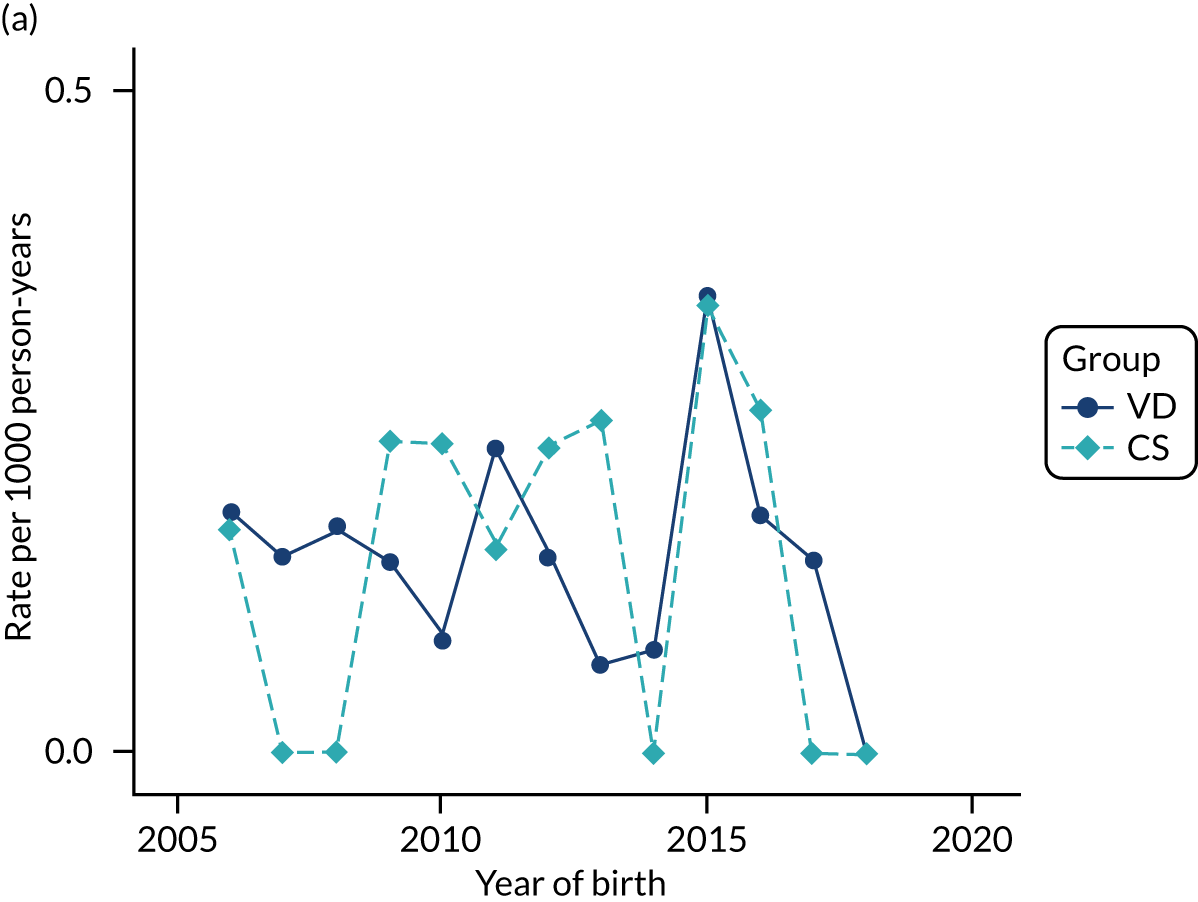
FIGURE 40.
Incidence rate of high risk of anaphylactic reaction (prescribing of automatic injection devices containing adrenaline) per 1000 person-years in children born by CS and VD by age and year of birth in the THIN–CPRD data set. Age (a) 0 years; (b) 1 year; (c) 2 years; (d) 3 years; and (e) 4 years.
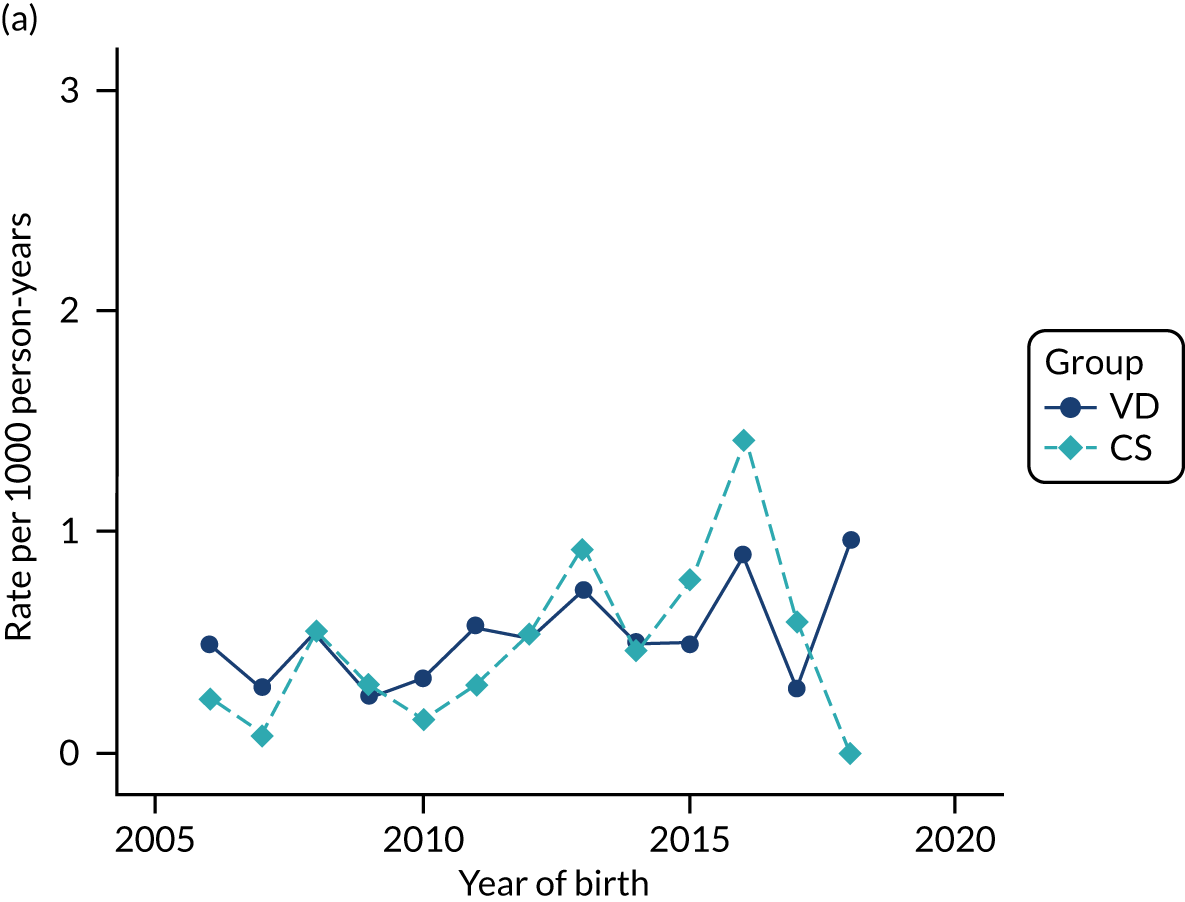
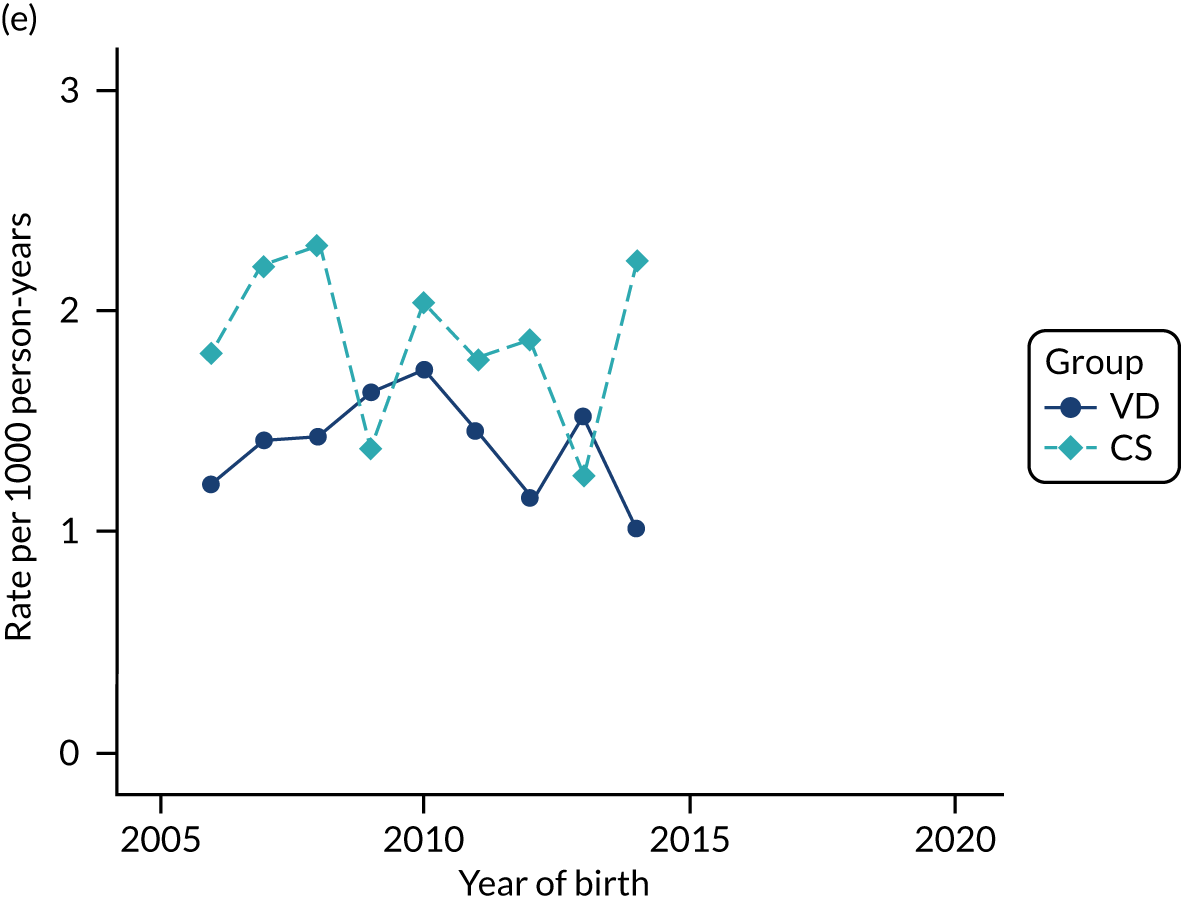
FIGURE 41.
Incidence rate of type 1 diabetes per 1000 person-years in children born by CS and VD by age and year of birth in the THIN–CPRD data set. Age (a) 0 years; (b) 1 year; (c) 2 years; (d) 3 years; and (e) 4 years.
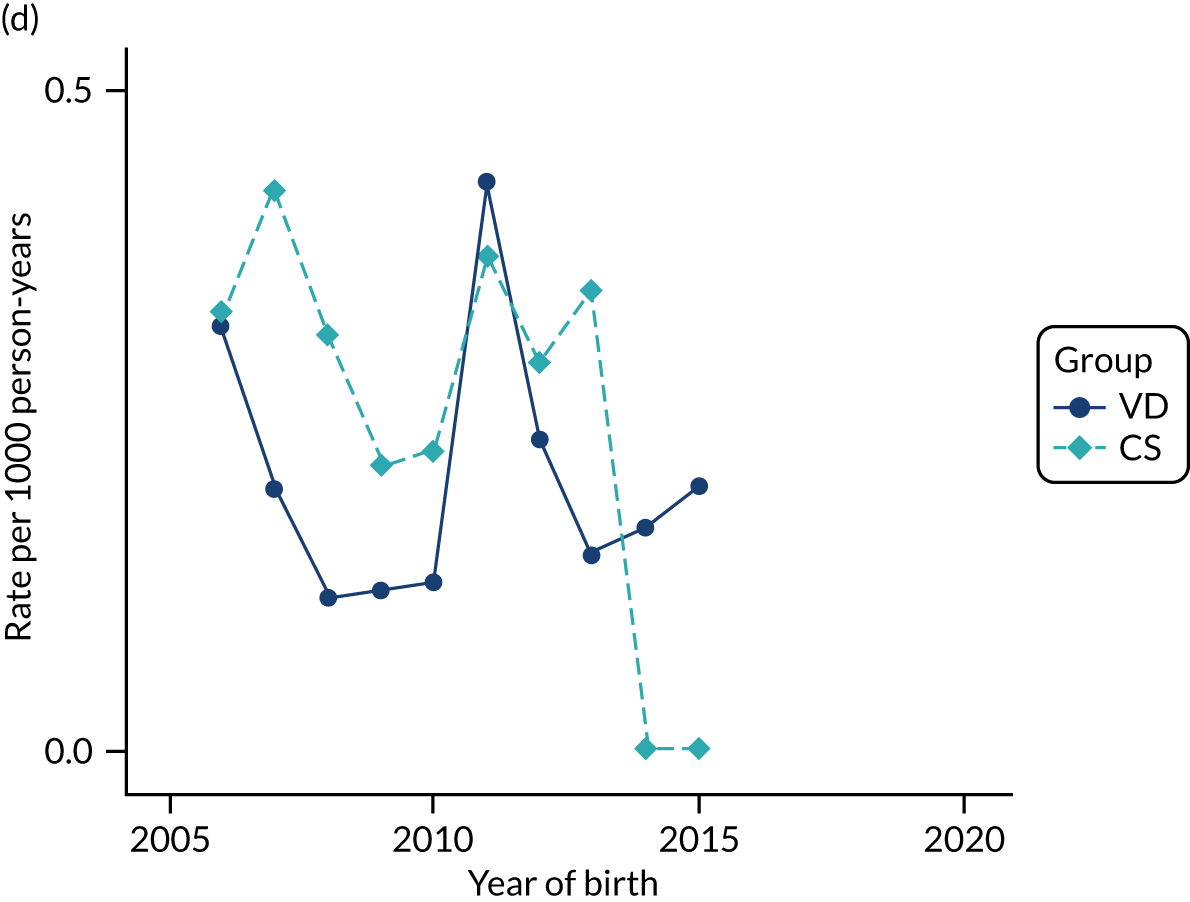
FIGURE 42.
Incidence rate of coeliac disease per 1000 person-years in children born by CS and VD by age and year of birth in the THIN–CPRD data set. Age (a) 0 years; (b) 1 year; (c) 2 years; (d) 3 years; and (e) 4 years.
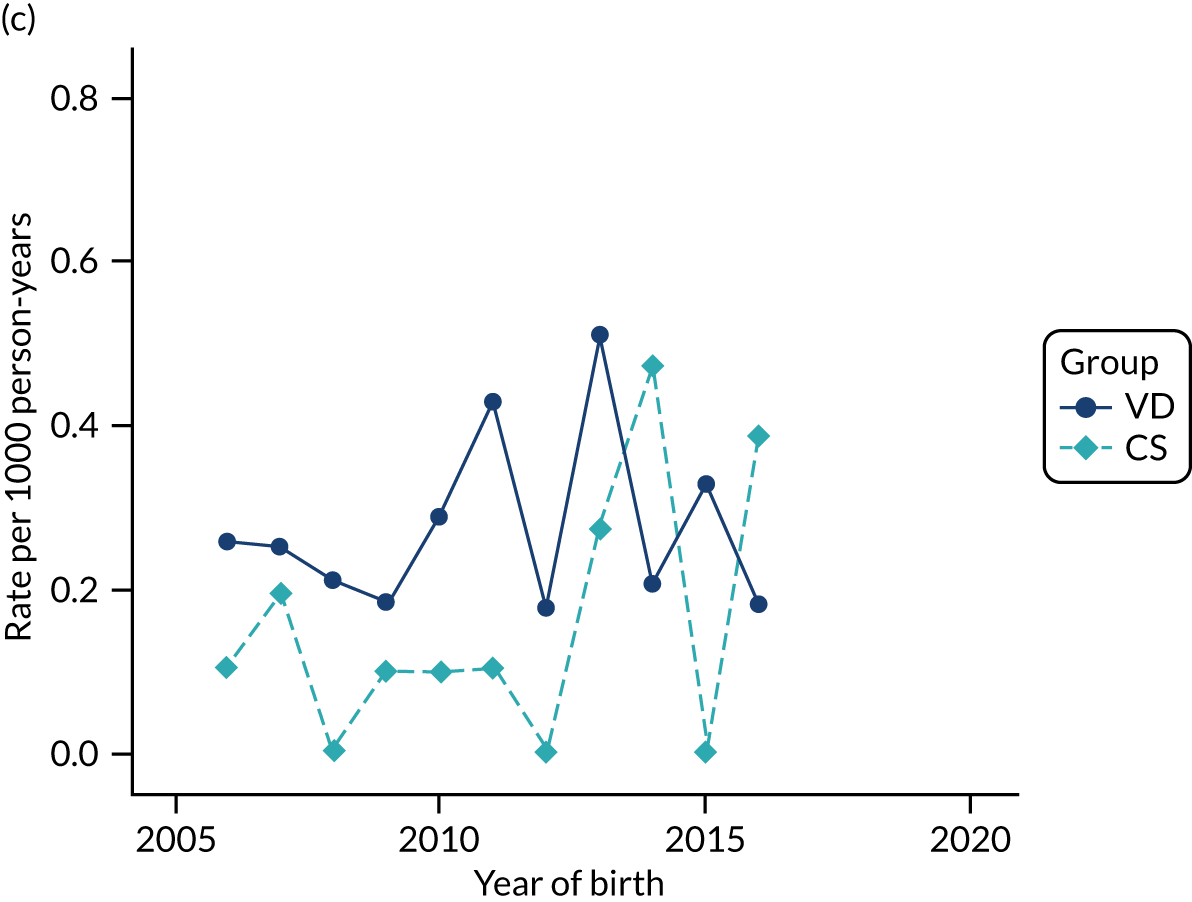
FIGURE 43.
Incidence rate of childhood vitiligo per 1000 person-years in children born by CS and VD by age and year of birth in the THIN–CPRD data set. Age (a) 0 years; (b) 1 year; (c) 2 years; (d) 3 years; and (e) 4 years.
FIGURE 44.
Incidence rate of wheeze per 1000 person-years in children born by CS and VD by age and year of birth in the THIN–CPRD data set. Age (a) 0 years; (b) 1 year; (c) 2 years; (d) 3 years; and (e) 4 years.
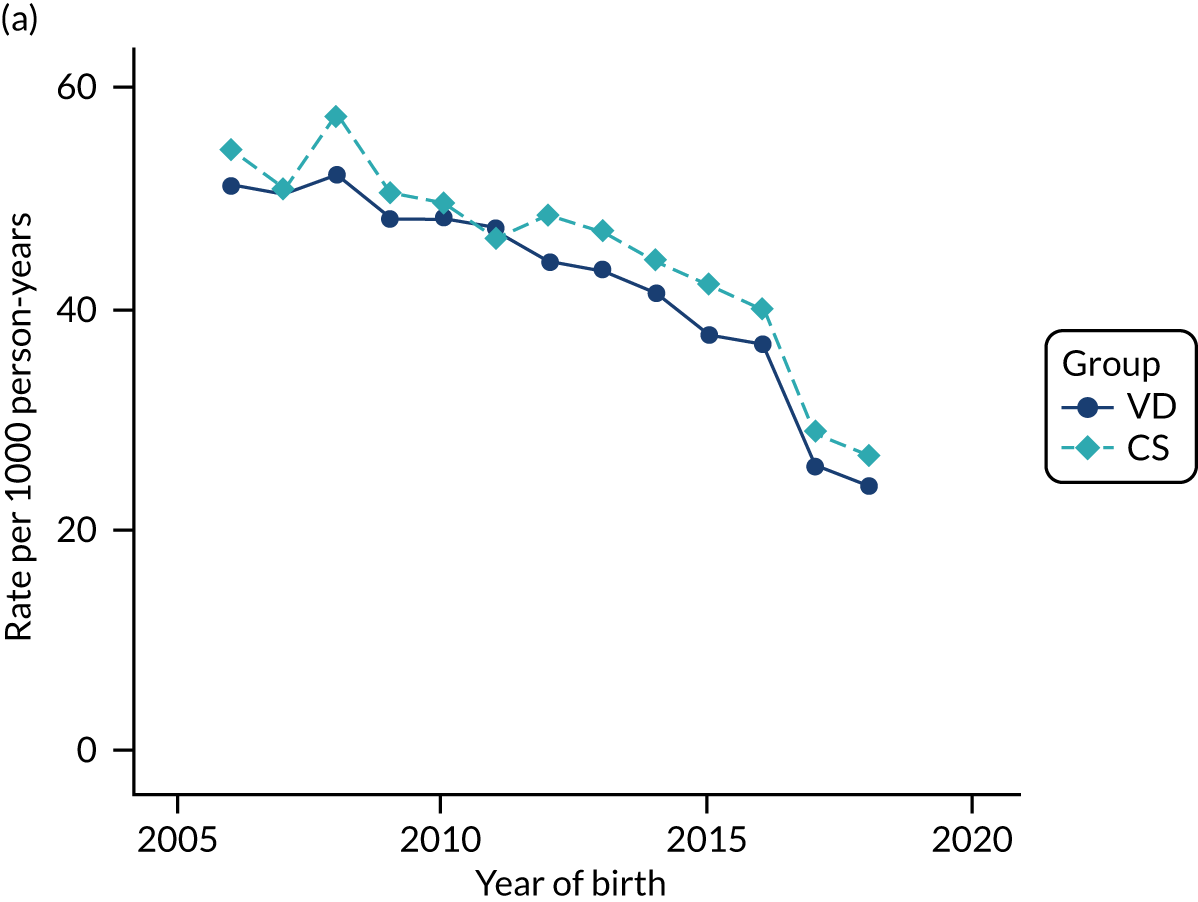
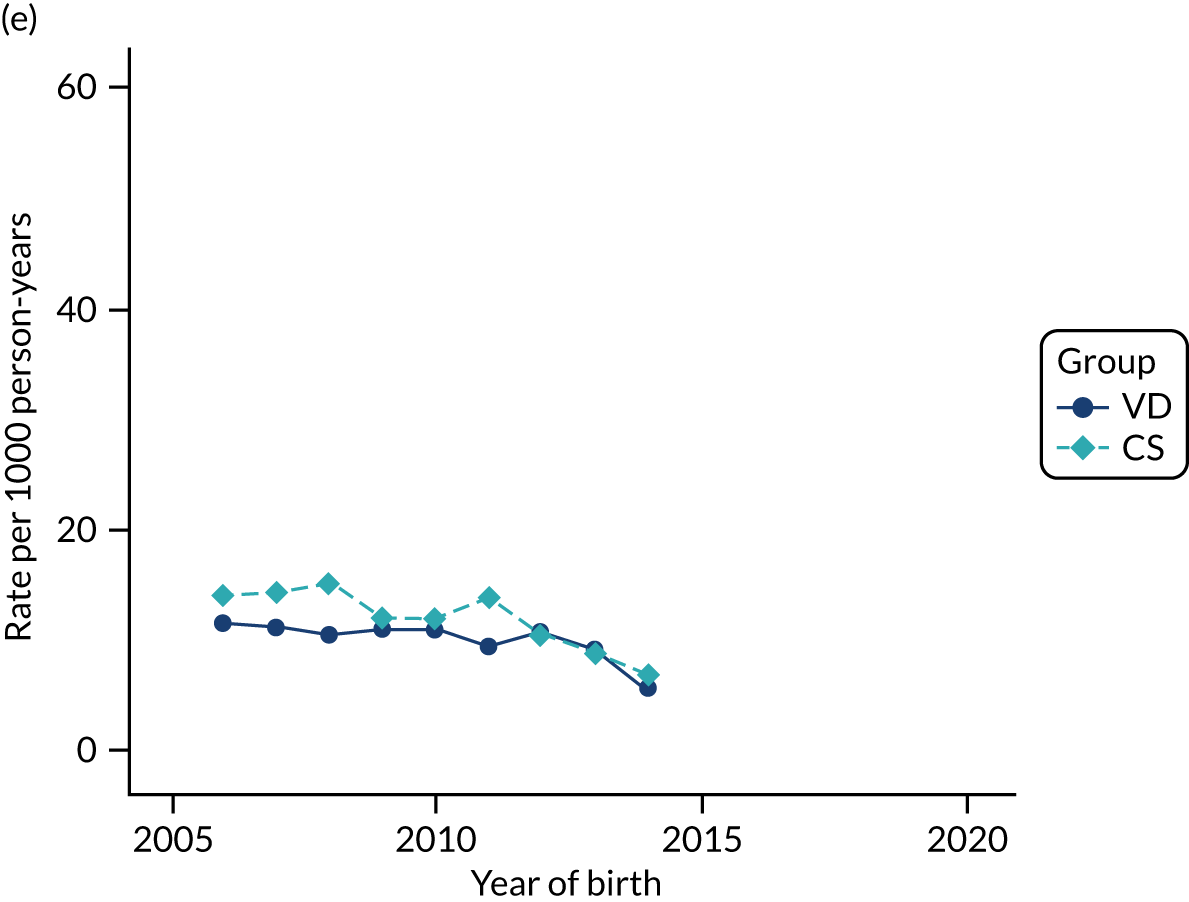
FIGURE 45.
Incidence rate of upper respiratory tract infections per 1000 person-years in children born by CS and VD by age and year of birth in the THIN–CPRD data set. Age (a) 0 years; (b) 1 year; (c) 2 years; (d) 3 years; and (e) 4 years.
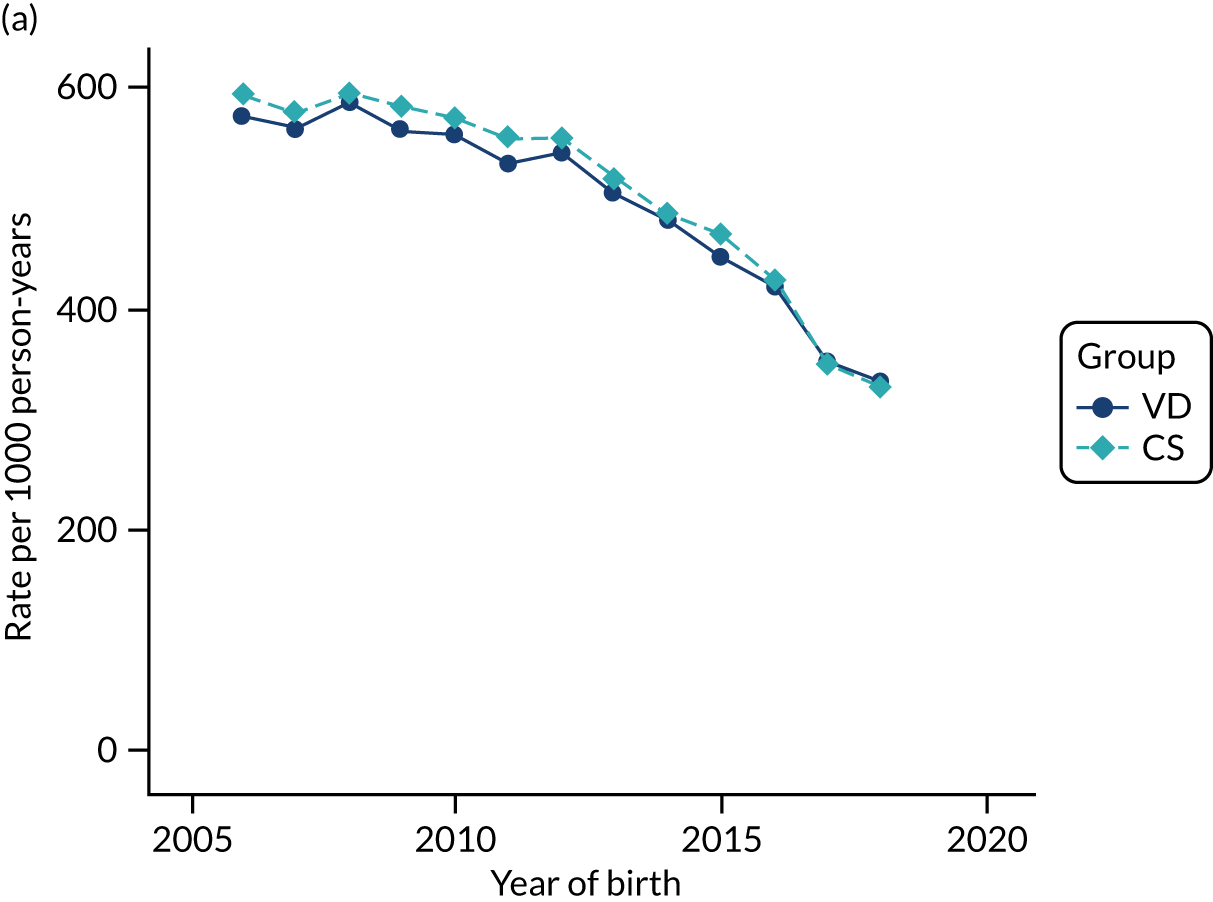
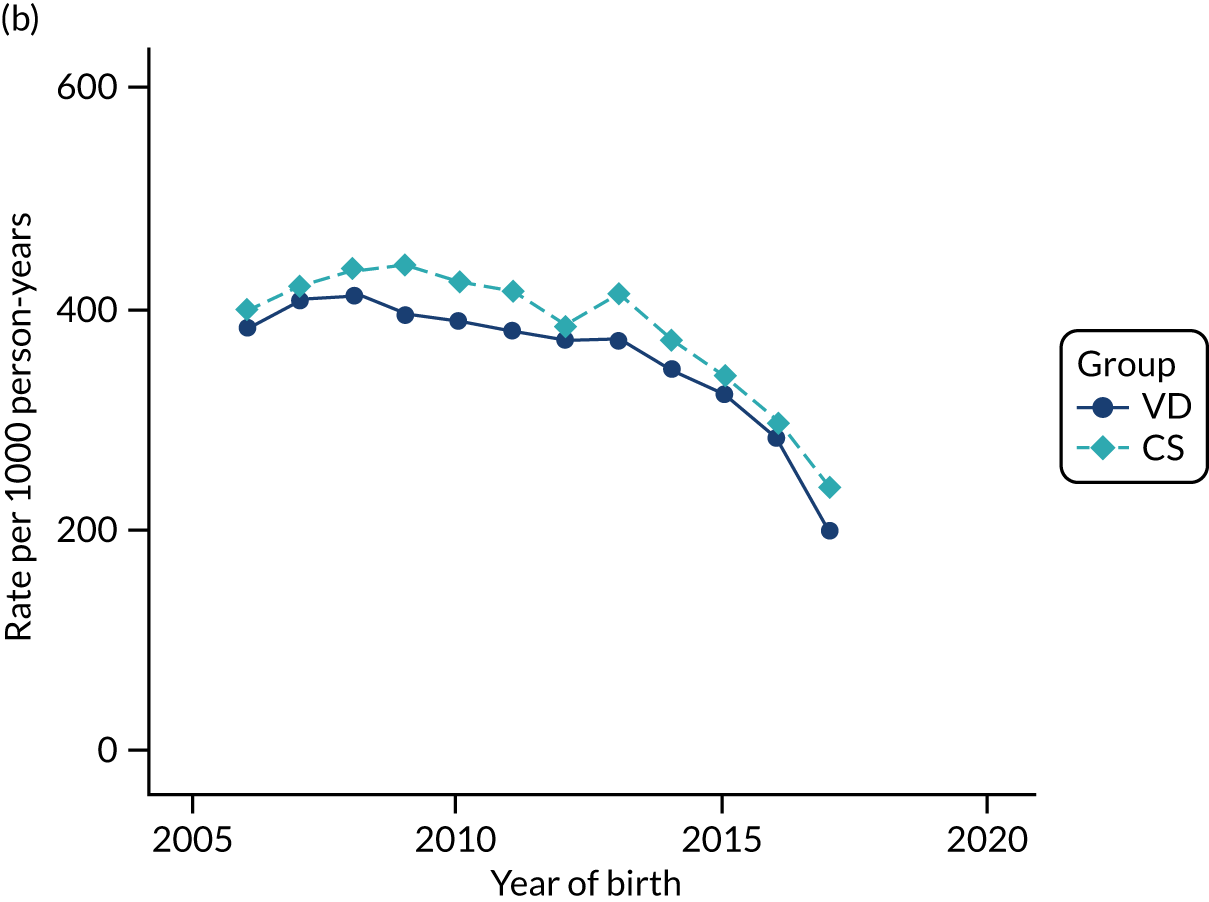
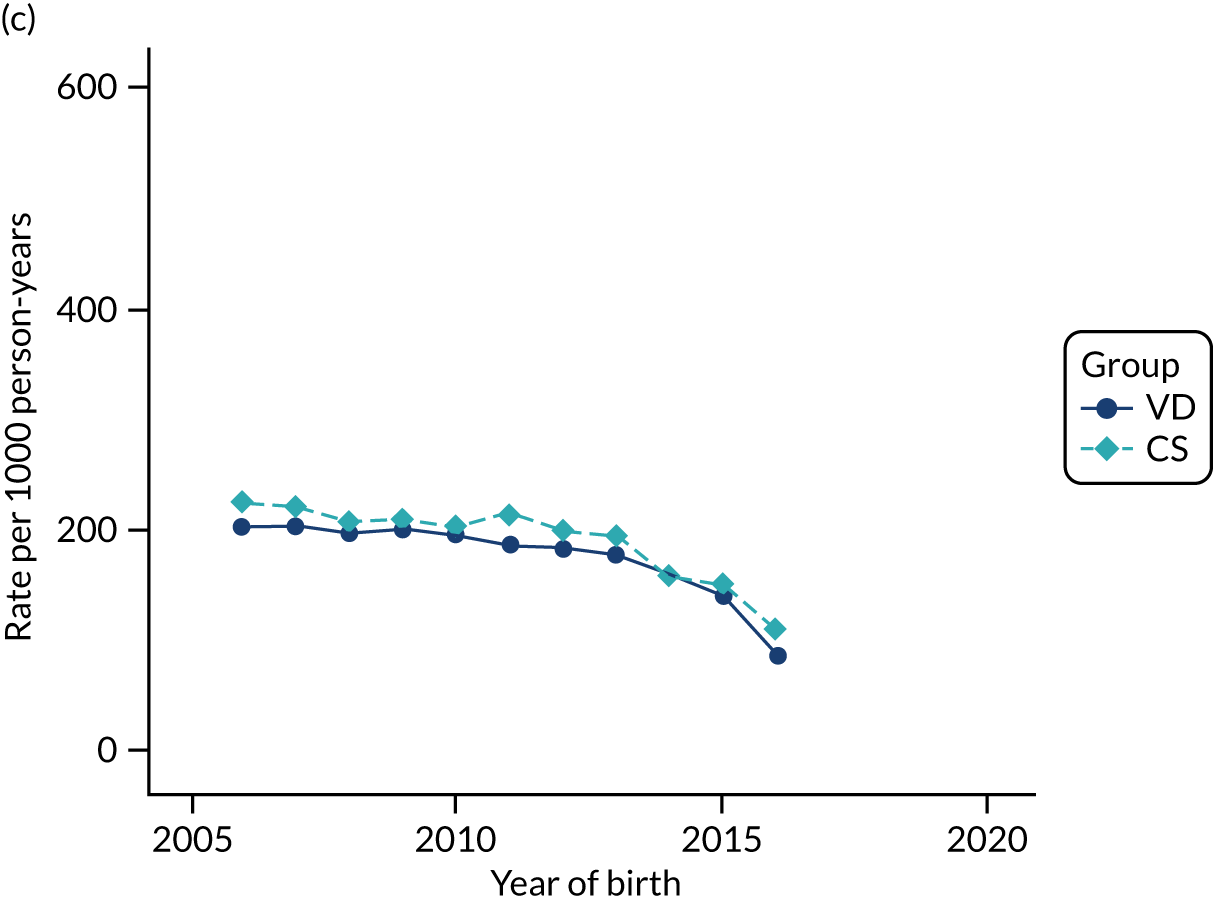
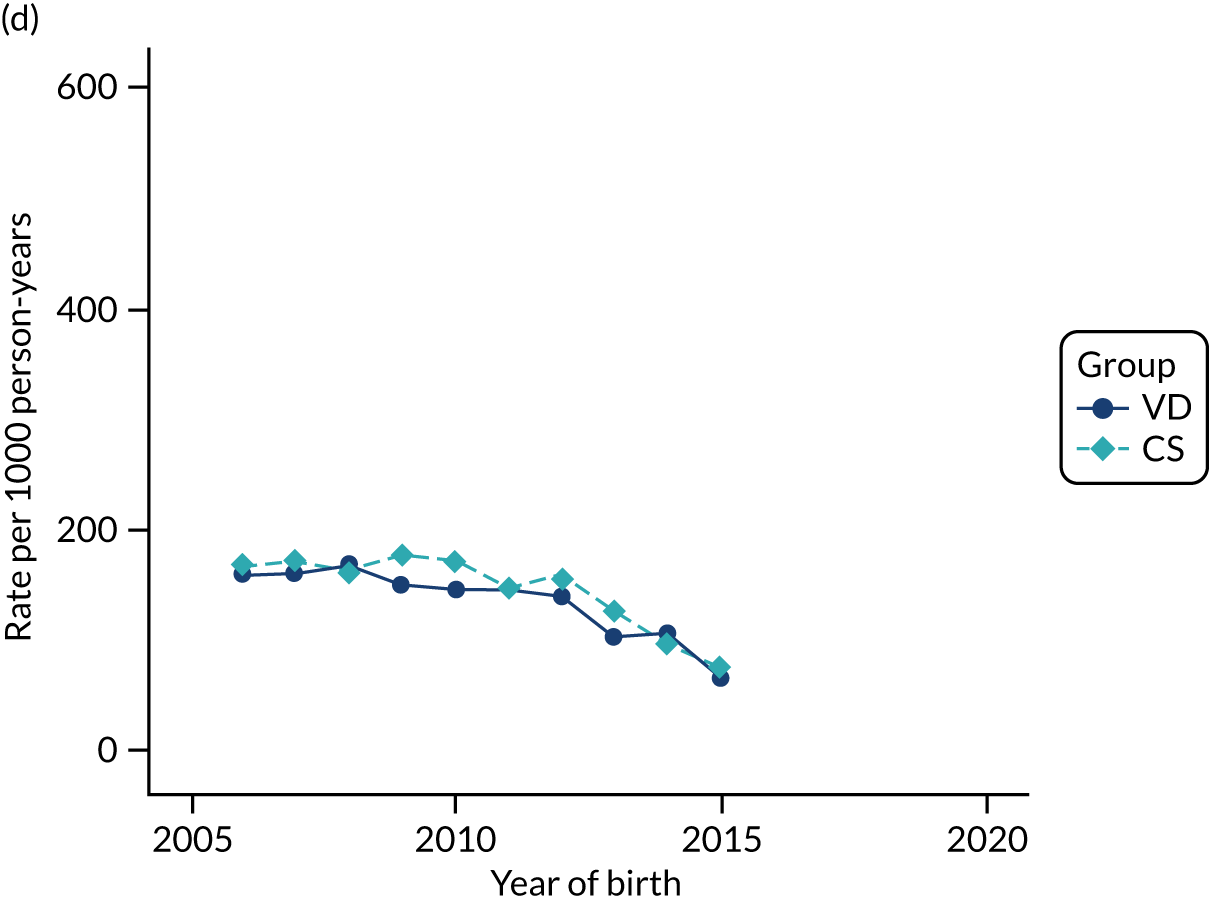
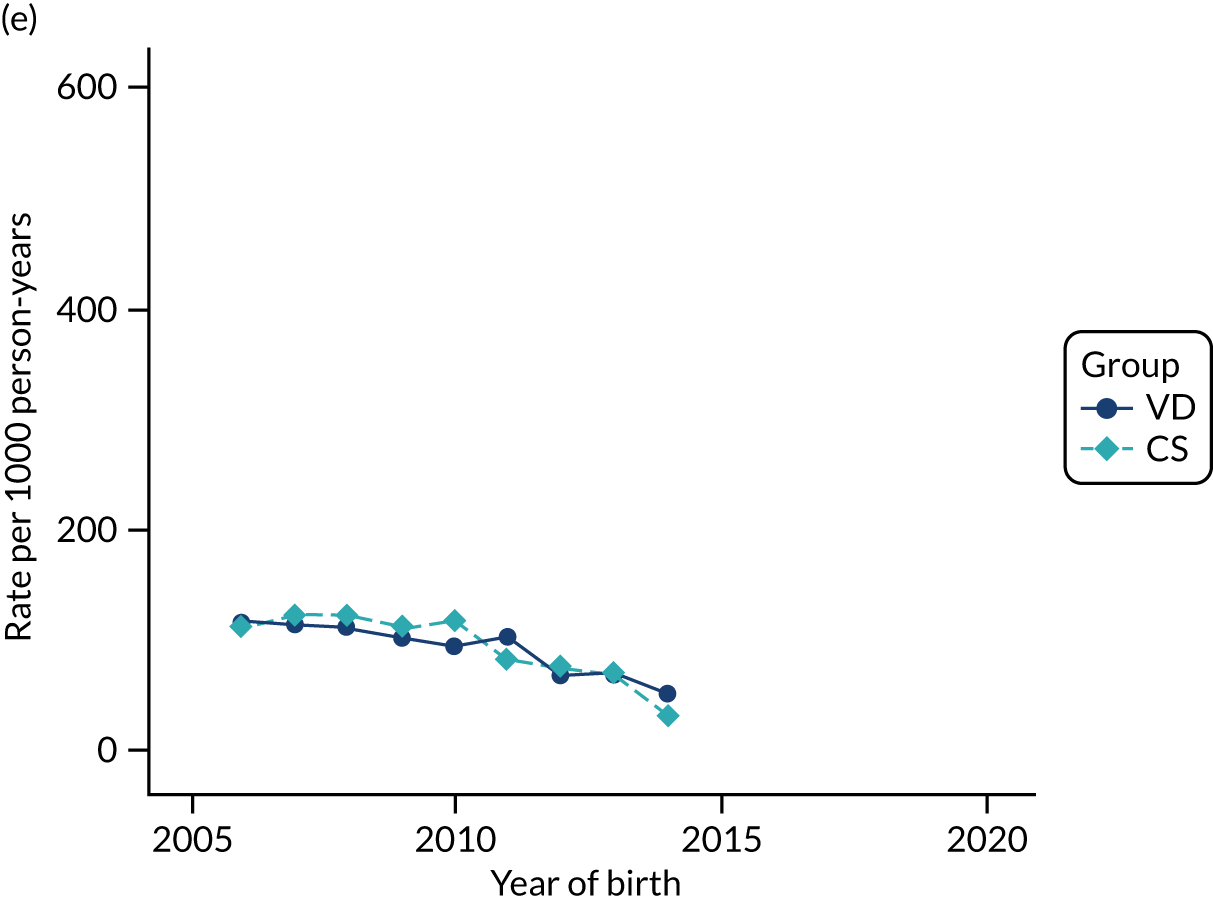
FIGURE 46.
Incidence rate of lower respiratory tract infections per 1000 person-years in children born by CS and VD by age and year of birth in the THIN–CPRD data set. Age (a) 0 years; (b) 1 year; (c) 2 years; (d) 3 years; and (e) 4 years.
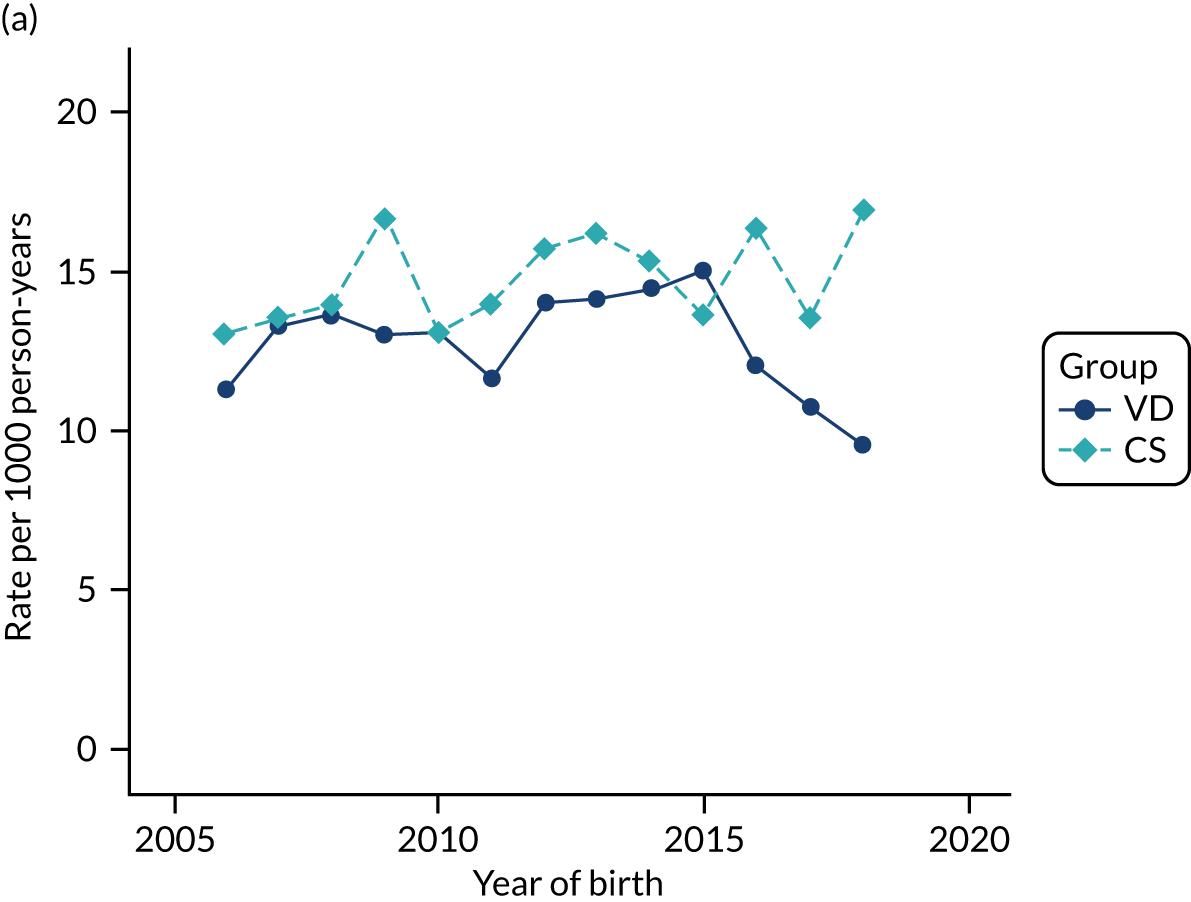
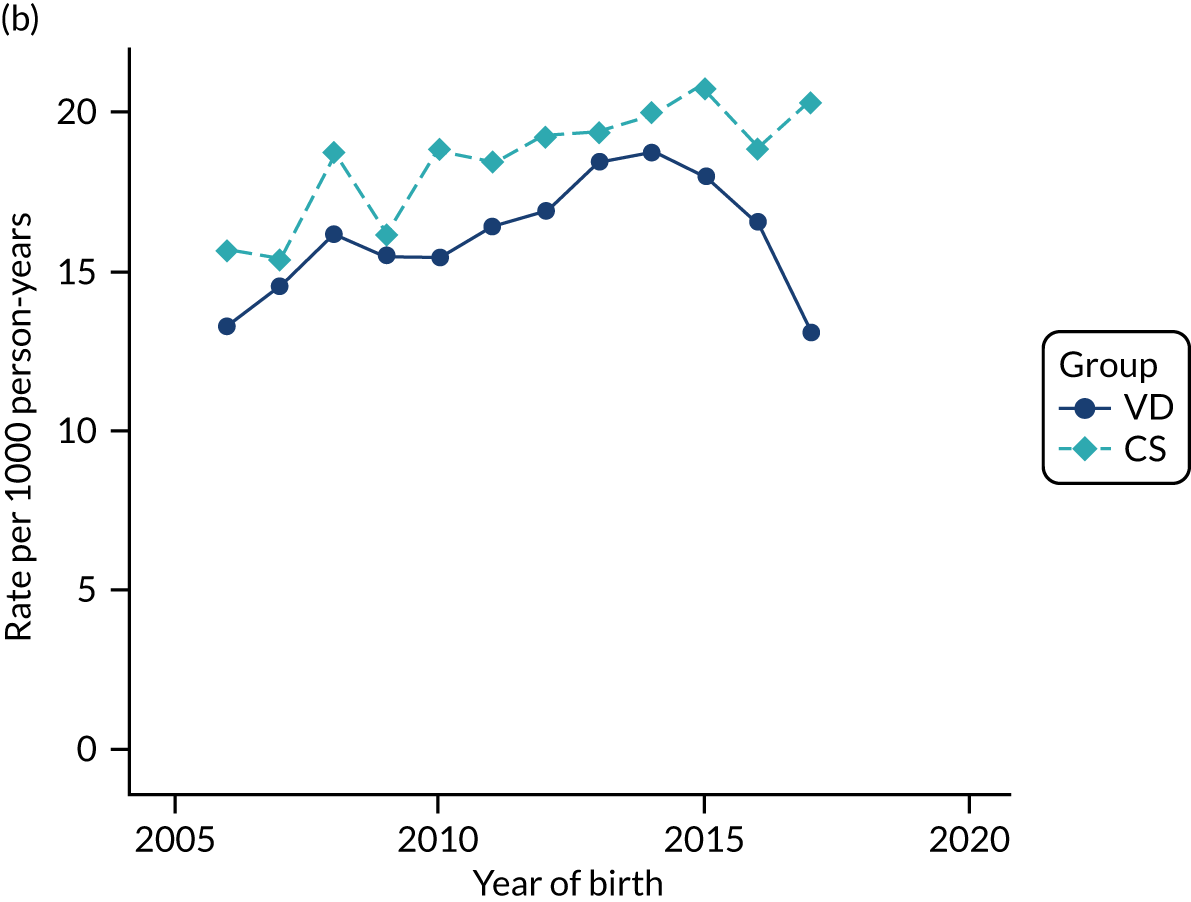
FIGURE 47.
Incidence rate of bronchiolitis per 1000 person-years in children born by CS and VD by age and year of birth in the THIN–CPRD data set. Age (a) 0 years; (b) 1 year; (c) 2 years; (d) 3 years; and (e) 4 years.
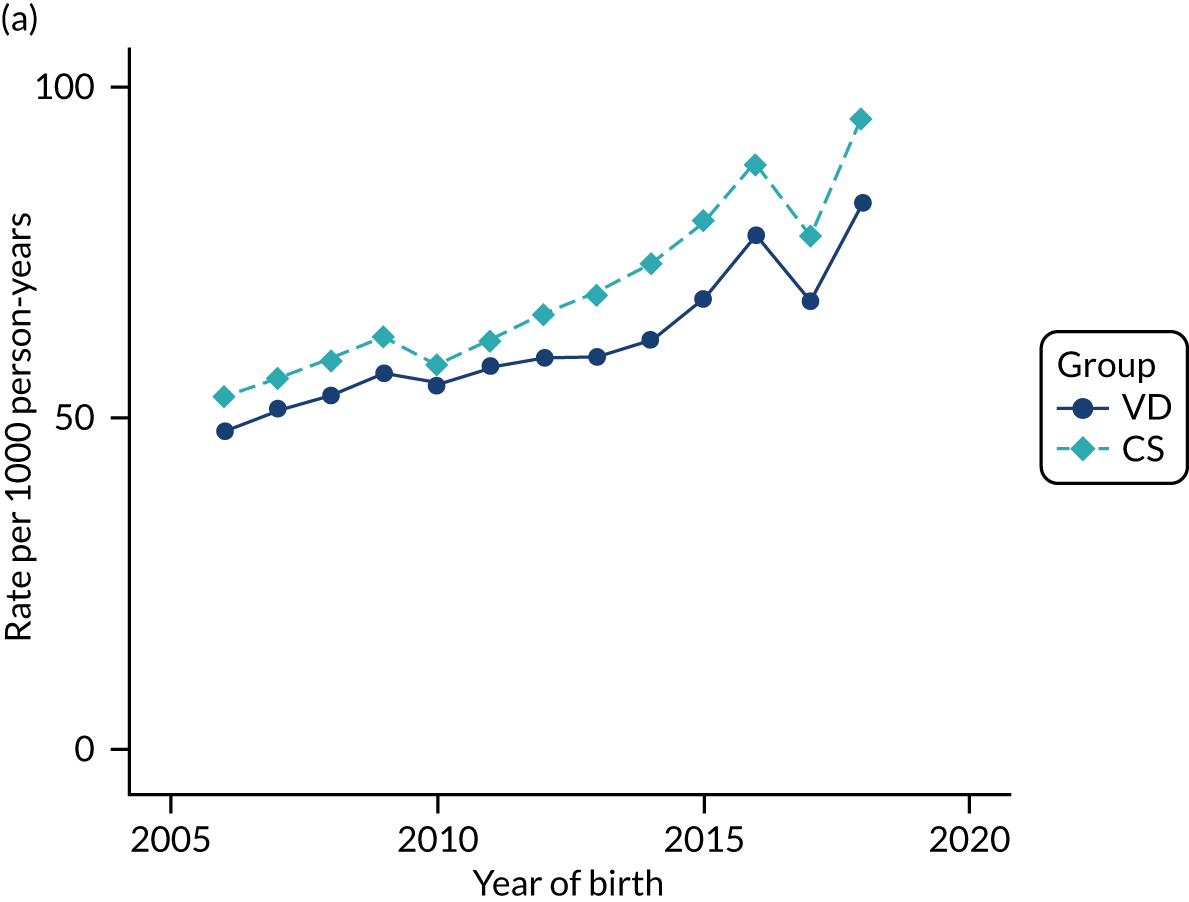
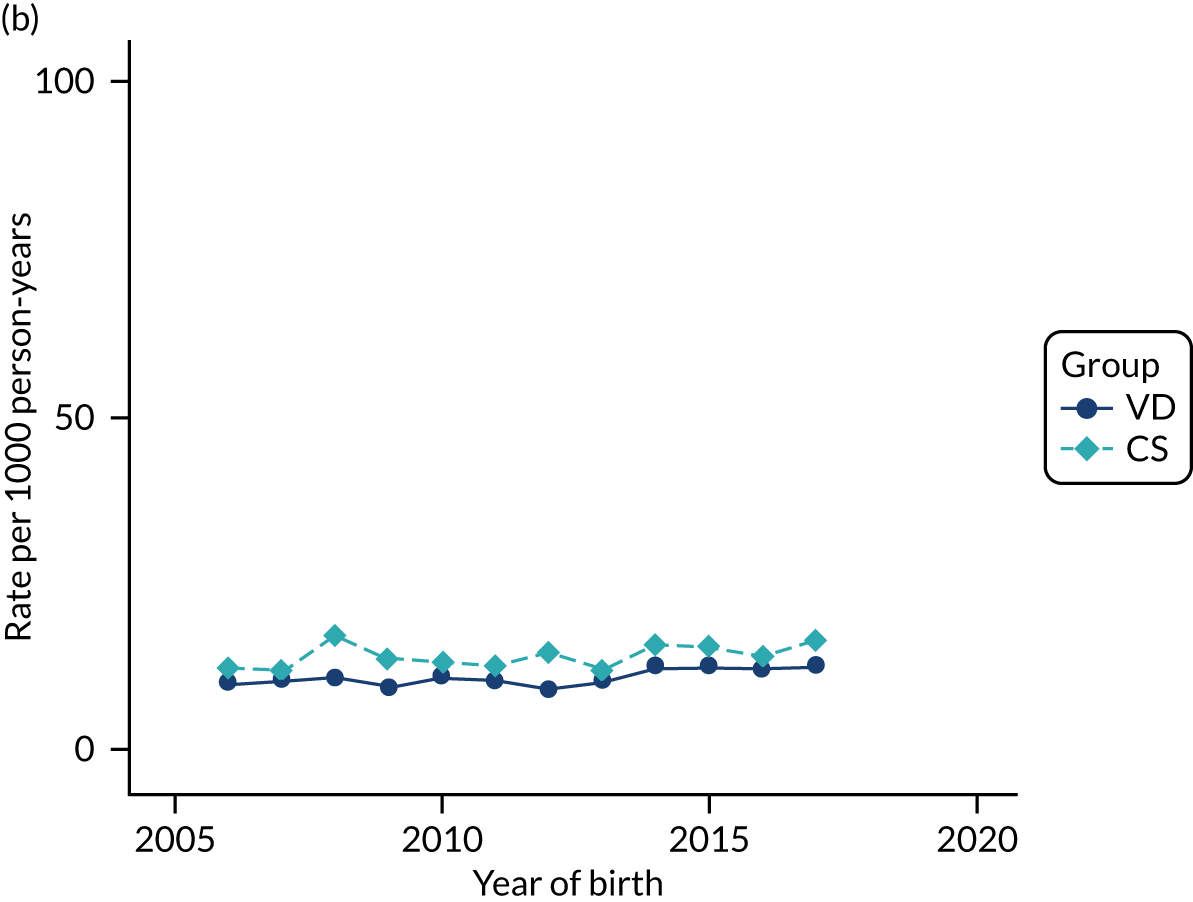
FIGURE 48.
Incidence rate of gastroenteritis per 1000 person-years in children born by CS and VD by age and year of birth in the THIN–CPRD data set. Age (a) 0 years; (b) 1 year; (c) 2 years; (d) 3 years; and (e) 4 years.
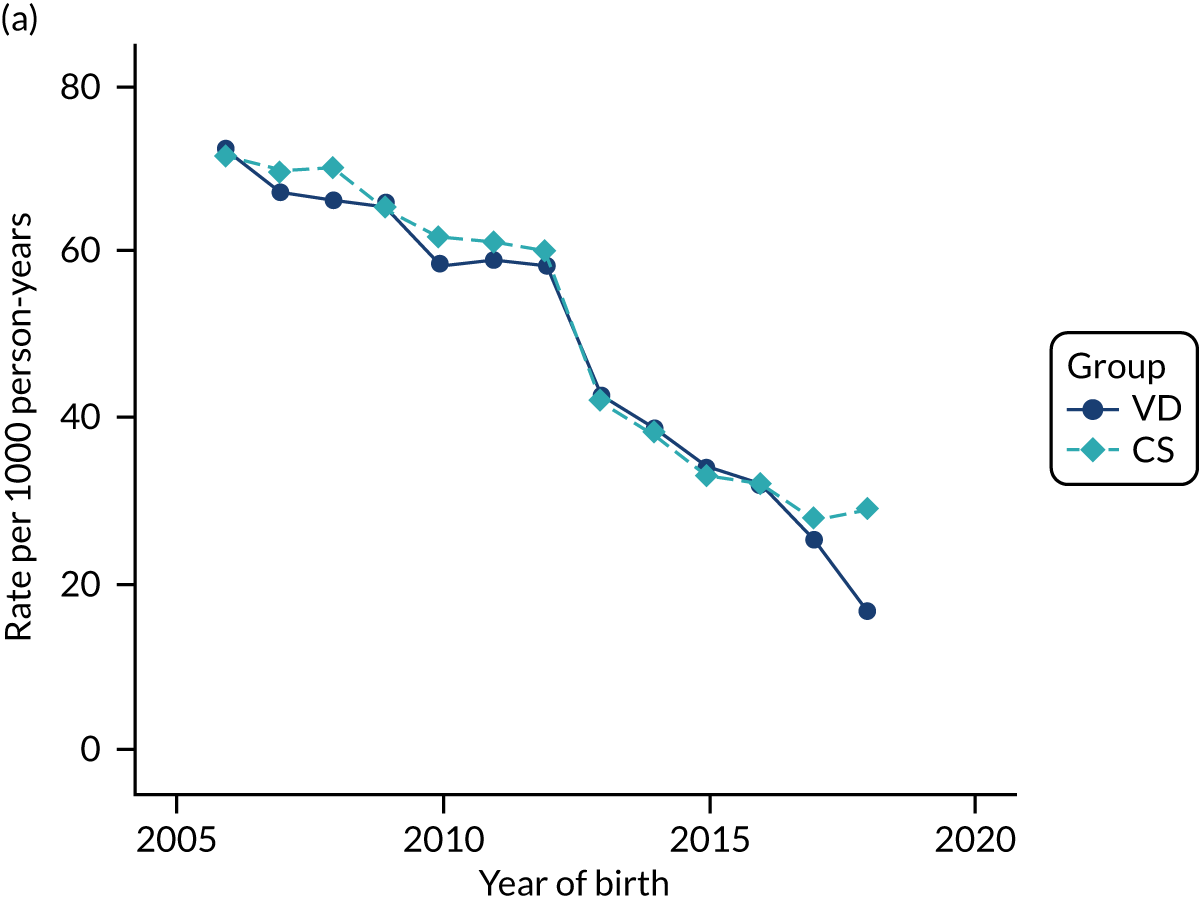
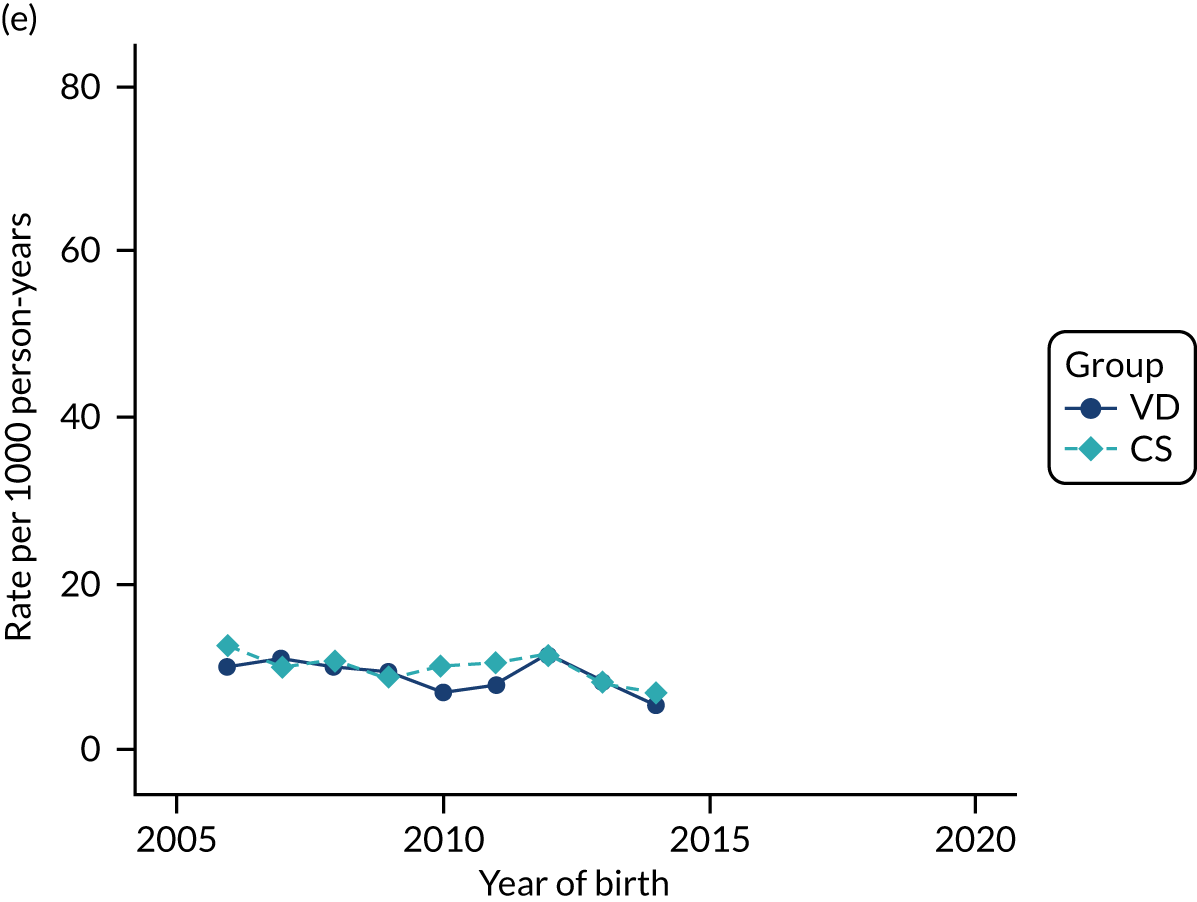
FIGURE 49.
Incidence rate of urinary tract infection per 1000 person-years in children born by CS and VD by age and year of birth in the THIN–CPRD data set. Age (a) 0 years; (b) 1 year; (c) 2 years; (d) 3 years; and (e) 4 years.
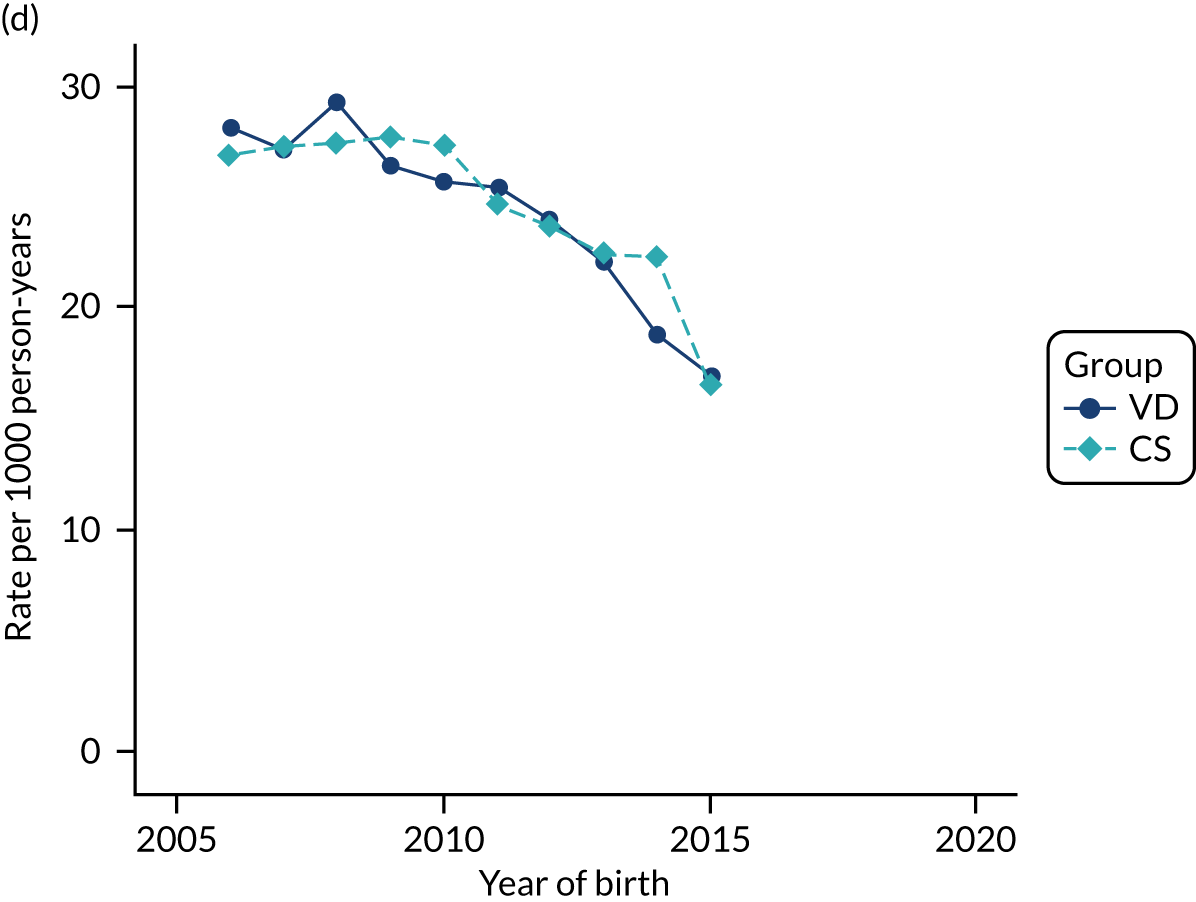
FIGURE 50.
Incidence rate of first-time antibiotic prescription per 1000 person-years in children born by CS and VD by age and year of birth in the THIN–CPRD data set. Age (a) 0 years; (b) 1 year; (c) 2 years; (d) 3 years; and (e) 4 years.
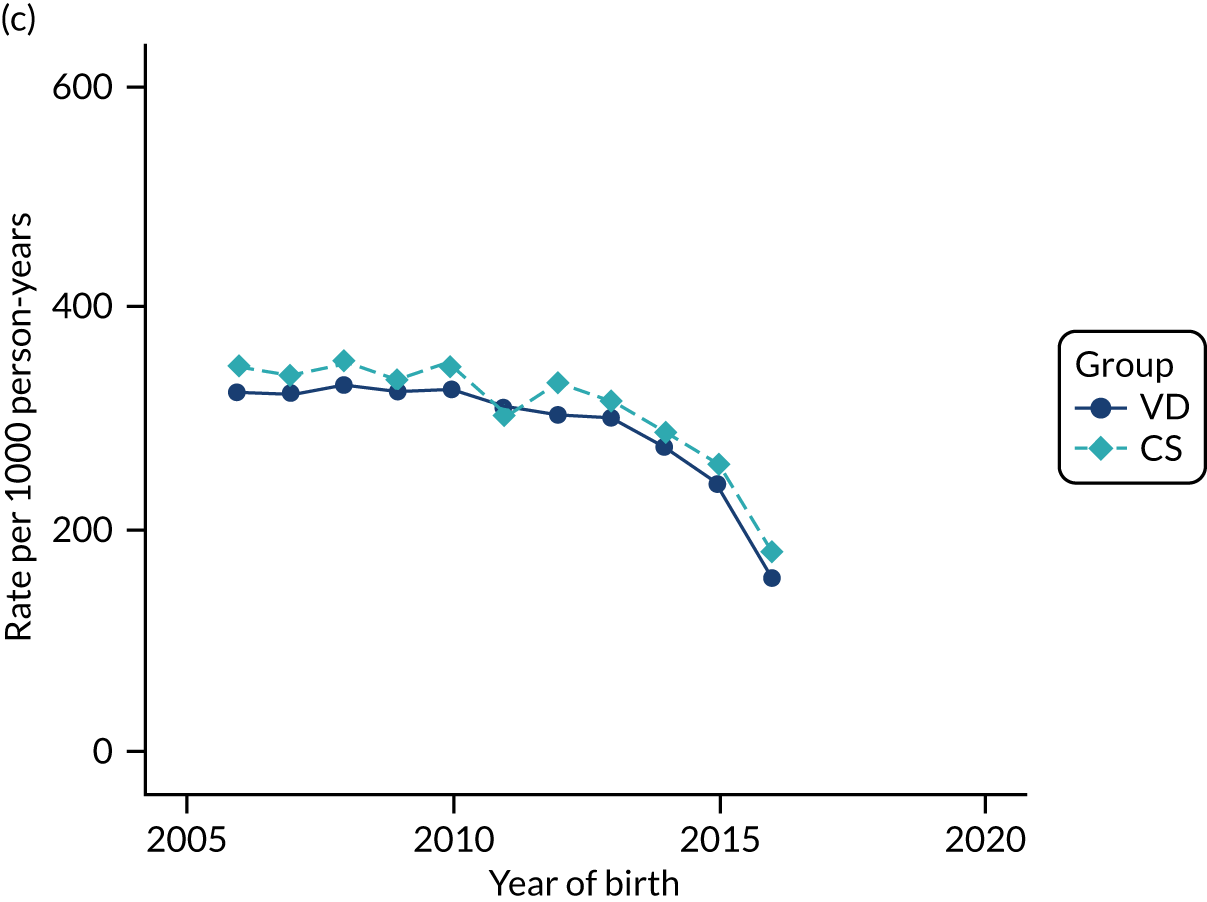
FIGURE 51.
Incidence rate of cerebral palsy per 1000 person-years in children born by CS and VD by age and year of birth in the THIN–CPRD data set. Age (a) 0 years; (b) 1 year; (c) 2 years; (d) 3 years; and (e) 4 years.
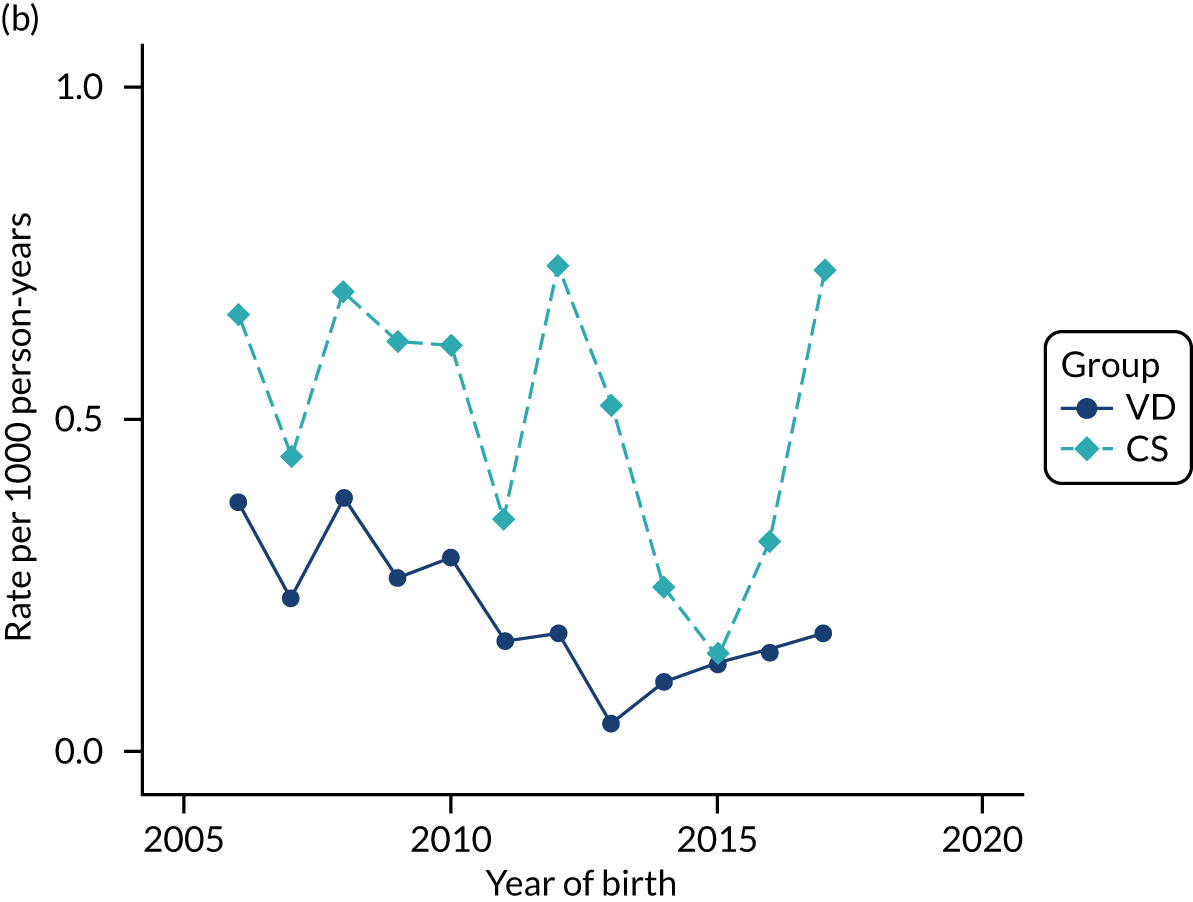
FIGURE 52.
Incidence rate of autism spectrum disorder per 1000 person-years in children born by CS and VD by age and year of birth in the THIN–CPRD data set. Age (a) 0 years; (b) 1 year; (c) 2 years; (d) 3 years; and (e) 4 years.
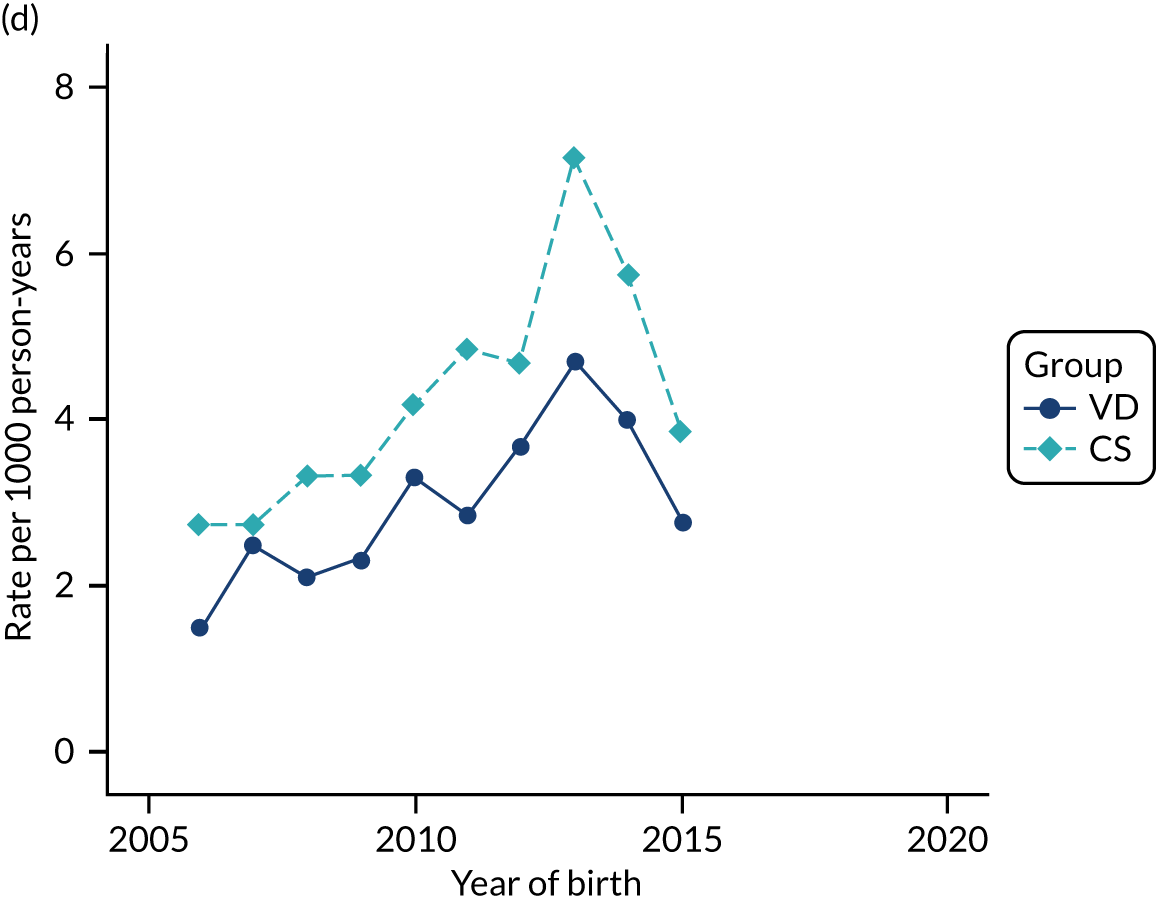
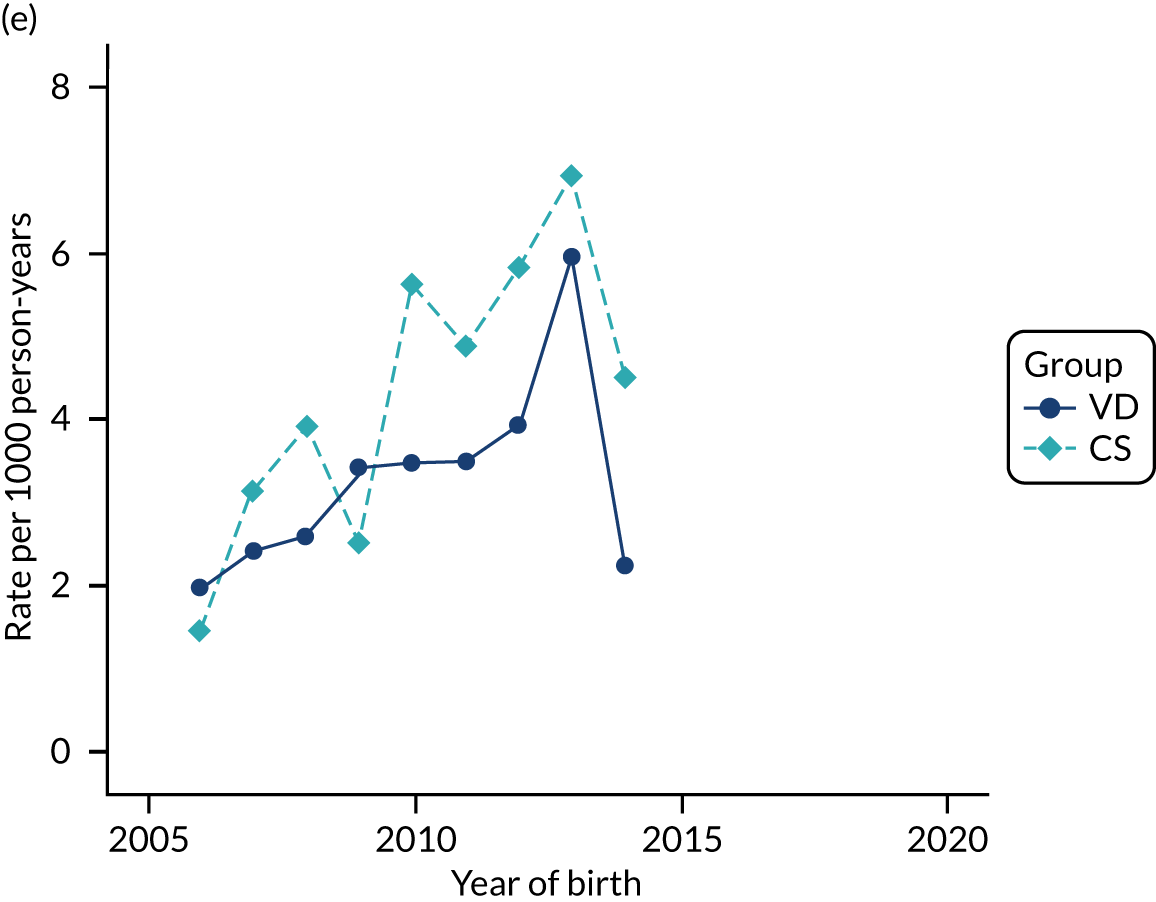
FIGURE 53.
Incidence rate of ADHD per 1000 person-years in children born by CS and VD by age and year of birth in the THIN–CPRD data set. Age (a) 0 years; (b) 1 year; (c) 2 years; (d) 3 years; and (e) 4 years.
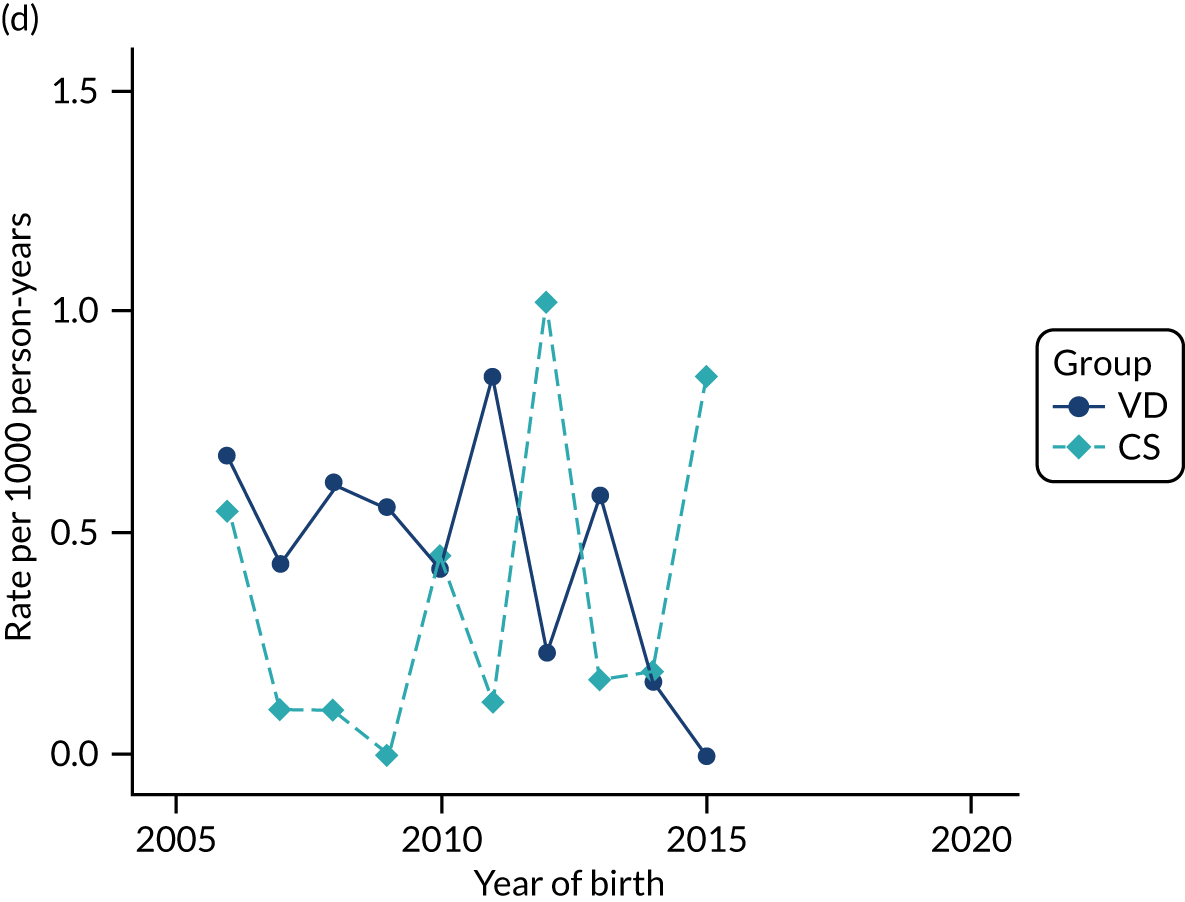
FIGURE 54.
Incidence rate of colic per 1000 person-years in children born by CS and VD by age and year of birth in the THIN–CPRD data set. Age (a) 0 years; (b) 1 year; (c) 2 years; (d) 3 years; and (e) 4 years.
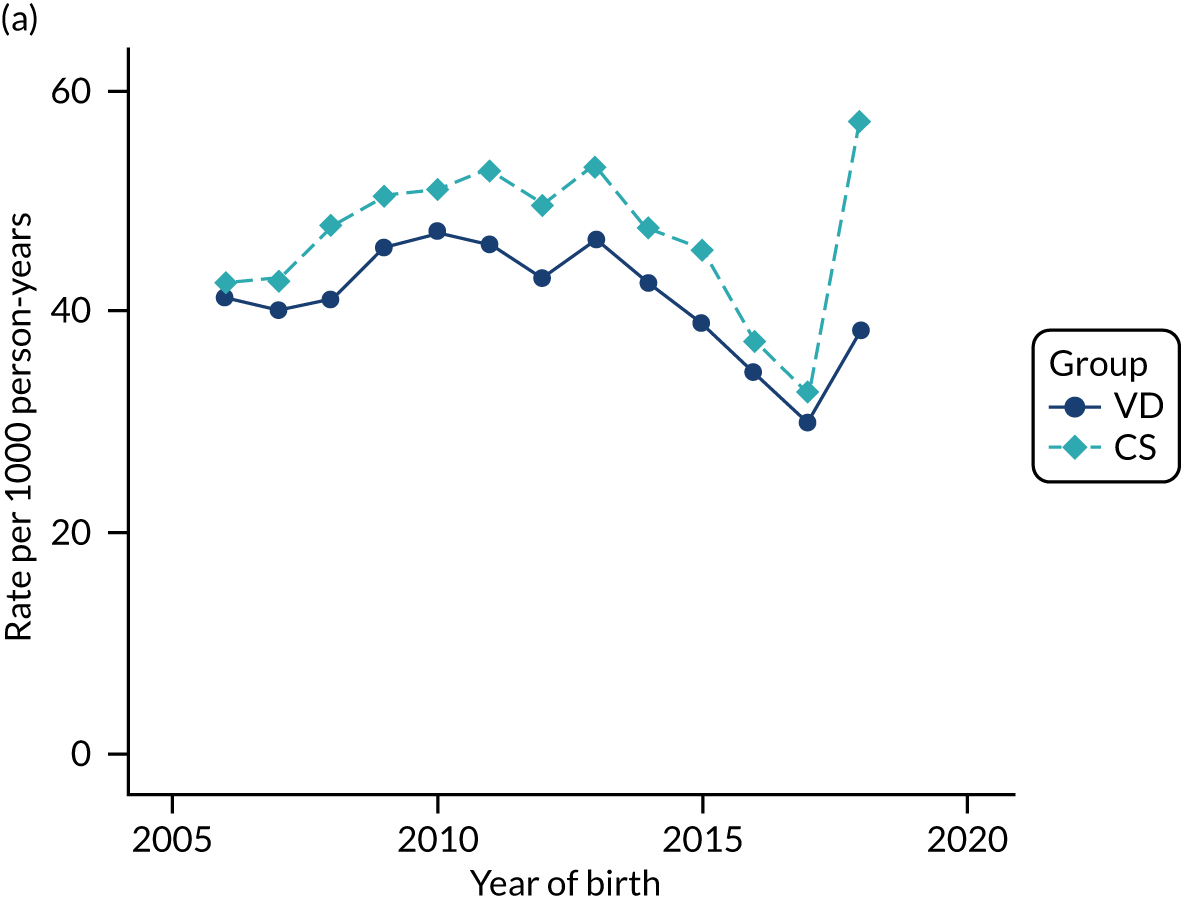
FIGURE 55.
Incidence rate of failure to thrive per 1000 person-years in children born by CS and VD by age and year of birth in the THIN–CPRD data set. Age (a) 0 years; (b) 1 year; (c) 2 years; (d) 3 years; and (e) 4 years.
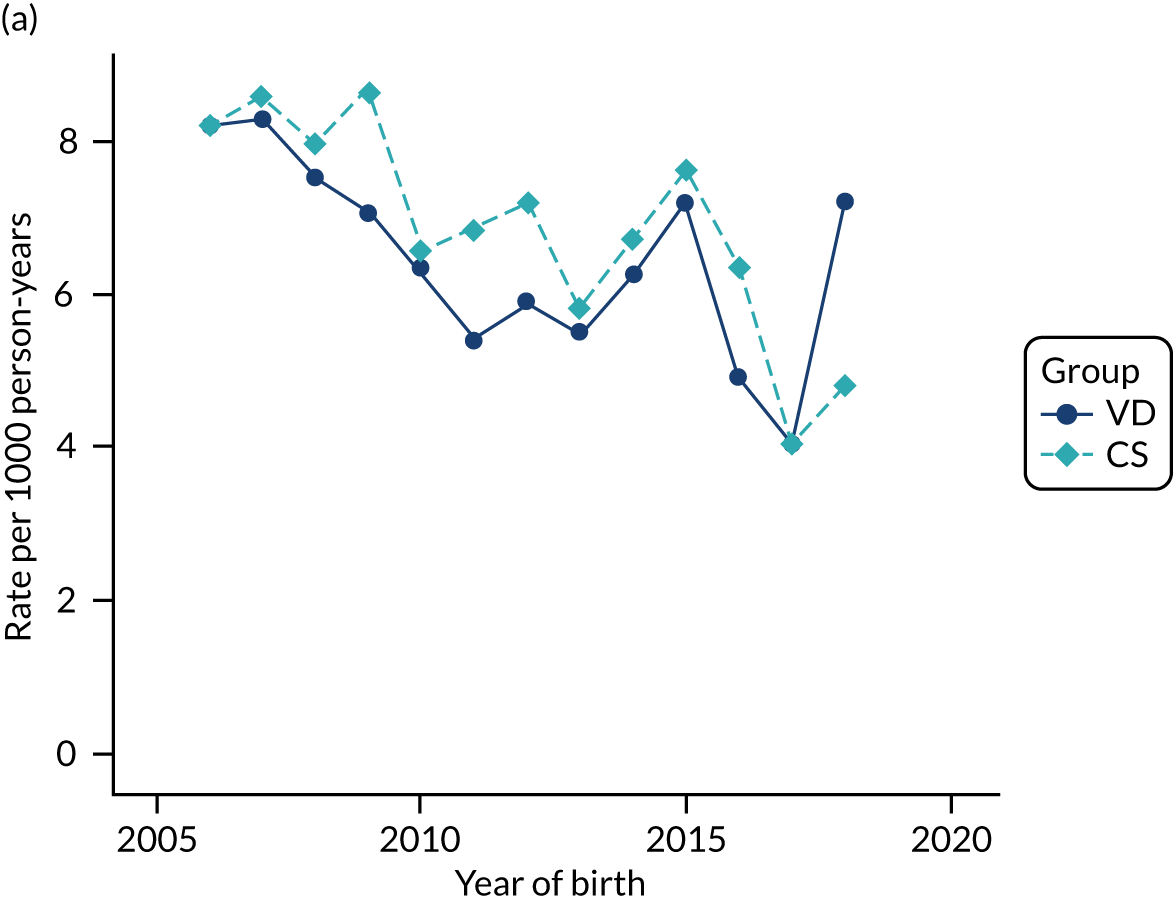
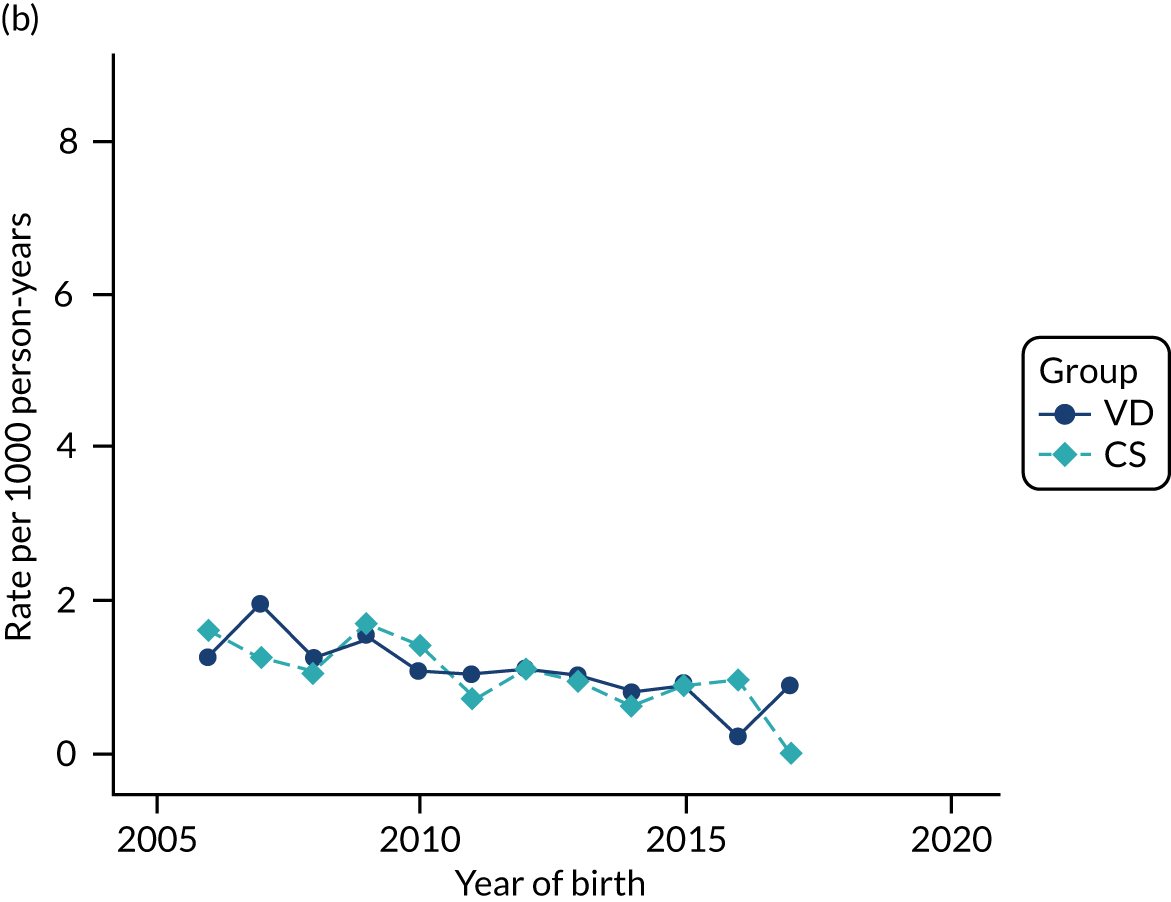
FIGURE 56.
Incidence rate of primary care consultations in the first 12 months of life per 1000 person-years in children born by CS and VD by age and year of birth in the THIN–CPRD data set.
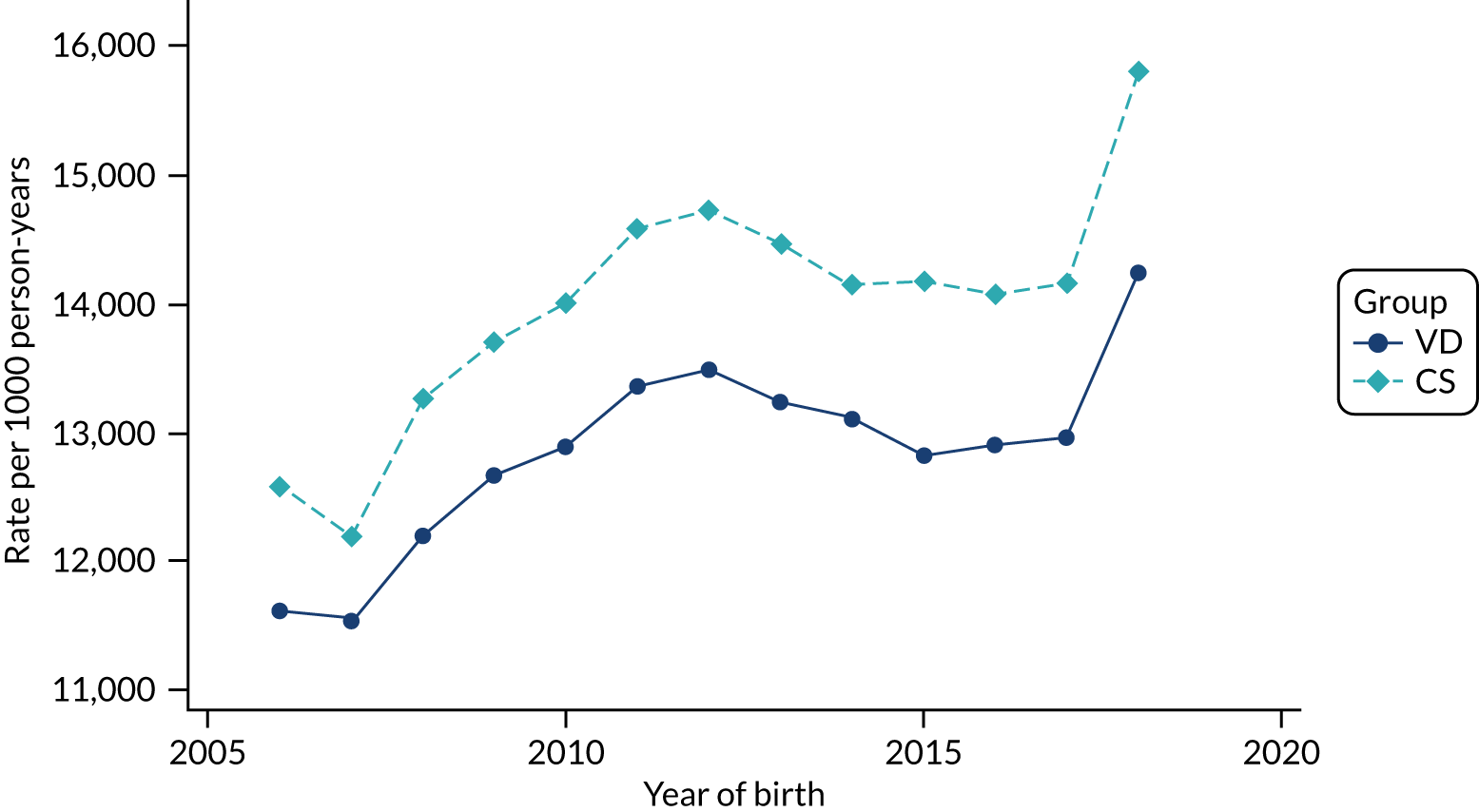
FIGURE 57.
Incidence of maternal composite infectious morbidity (e.g. wound infection, endometritis/endomyometritis, pelvic abscess and maternal sepsis) per 1000 births by CS and VD by year of delivery in the THIN–CPRD data set.
FIGURE 58.
Incidence of wound infection per 1000 births by CS and VD by year of delivery in the THIN–CPRD data set.
FIGURE 59.
Incidence of endometritis/endomyometritis per 1000 births by CS and VD by year of delivery in the THIN–CPRD data set.
FIGURE 60.
Incidence rate of maternal sepsis per 1000 births by CS and VD by year of delivery in the THIN–CPRD data set.
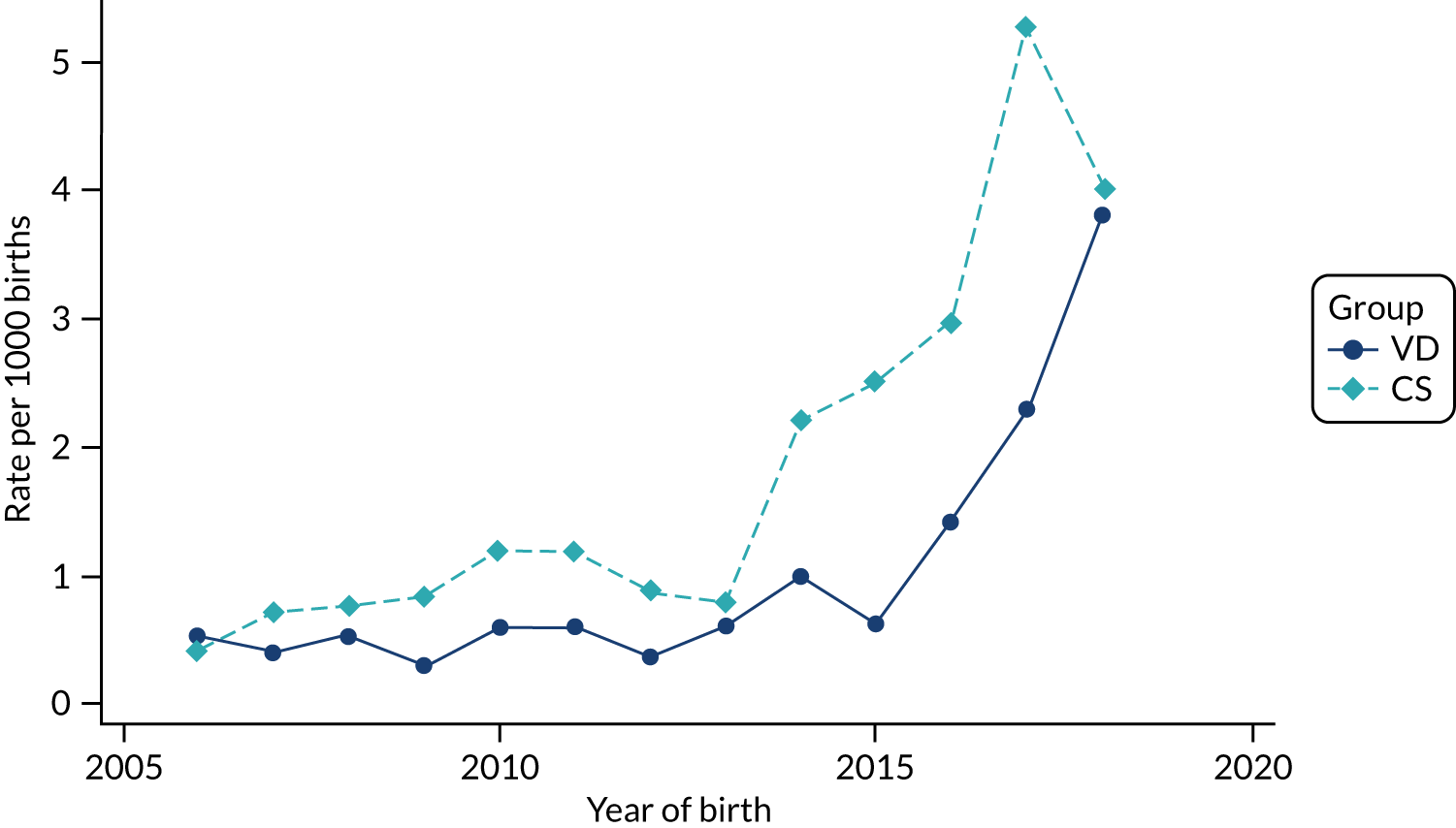
FIGURE 61.
Incidence rate of maternal urinary tract infection per 1000 births by CS and VD by year of delivery in the THIN–CPRD data set.
FIGURE 62.
Incidence of antibiotic prescribing in the post partum period per 1000 births by CS and VD by year of delivery in the THIN–CPRD data set.
Outcomes in the HES data set
As HES captures all hospital admissions in England, all children were assumed to be followed up until age 5 years if they were born before 2015. For the birth years of 2015, 2016 and 2017, children were followed up to age 4, 3 and 2 years, respectively, and, therefore, for these years the incidence rate reflects the incidence of the outcome of interest in these age groups.
FIGURE 63.
Incidence rate of asthma per 1000 person-years in children born by CS and VD by year of birth in the HES data set.
FIGURE 64.
Incidence rate of eczema per 1000 person-years in children born by CS and VD by year of birth in the HES data set.
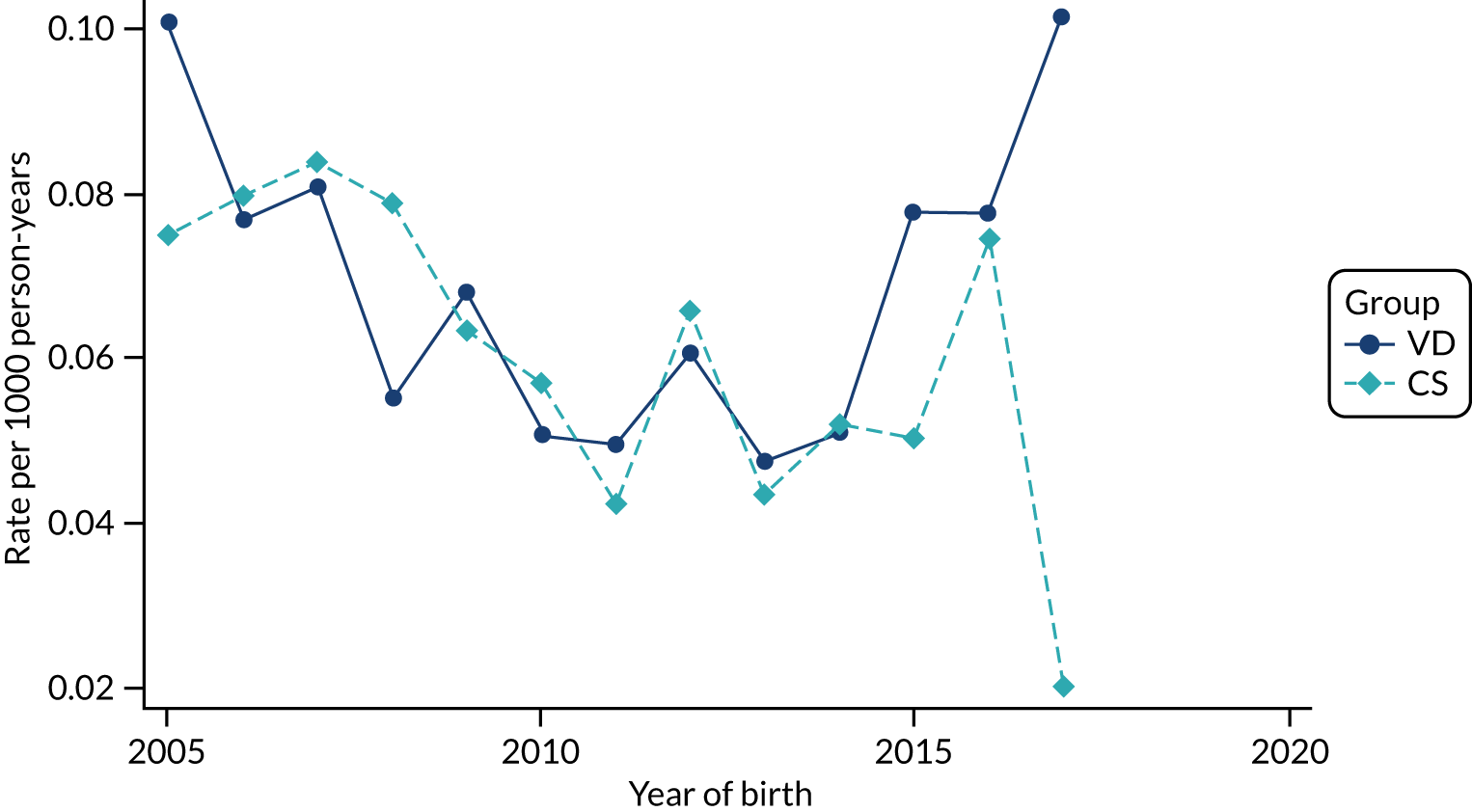
FIGURE 65.
Incidence rate of anaphylaxis per 1000 person-years in children born by CS and VD by year of birth in the HES data set.
FIGURE 66.
Incidence rate of type 1 diabetes per 1000 person-years in children born by CS and VD by year of birth in the HES data set.
FIGURE 67.
Incidence rate of juvenile idiopathic arthritis per 1000 person-years in children born by CS and VD by year of birth in the HES data set.
FIGURE 68.
Incidence rate of autoimmune (idiopathic) thrombocytopenic purpura per 1000 person-years in children born by CS and VD by year of birth in the HES data set.
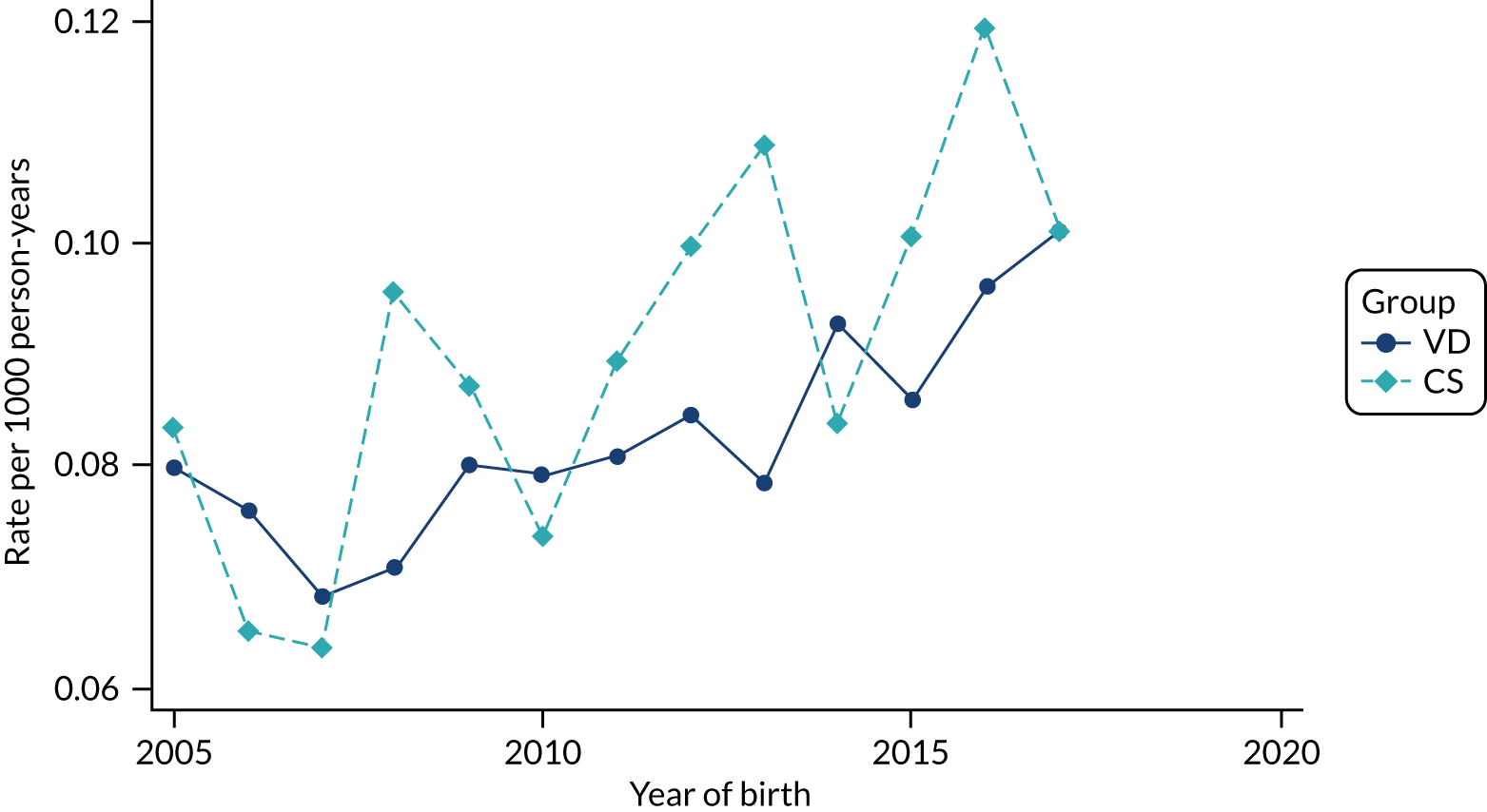
FIGURE 69.
Incidence of late-onset neonatal sepsis per 1000 births in children born by CS and VD by year of birth in the HES data set.
FIGURE 70.
Incidence of early-onset neonatal sepsis per 1000 births in children born by CS and VD by year of birth in the HES data set.
FIGURE 71.
Incidence rate of other sepsis in children (developed after the neonatal period of 28 days after birth) per 1000 person-years in children born by CS and VD by year of birth in the HES data set.
FIGURE 72.
Incidence rate of lower respiratory tract infections per 1000 person-years in children born by CS and VD by year of birth in the HES data set.
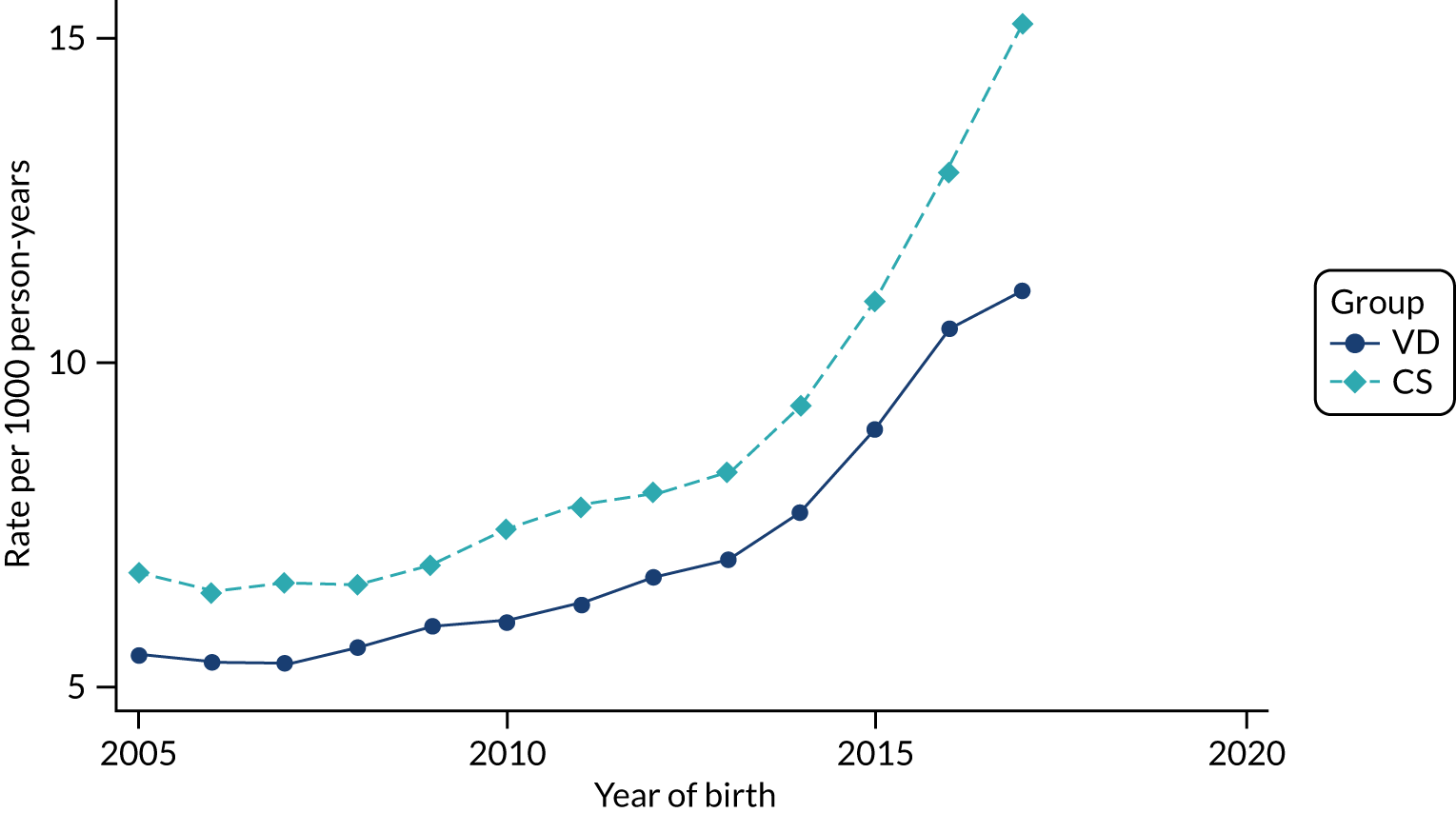
FIGURE 73.
Incidence rate of bronchiolitis per 1000 person-years in children born by CS and VD by year of birth in the HES data set.
FIGURE 74.
Incidence rate of gastroenteritis per 1000 person-years in children born by CS and VD by year of birth in the HES data set.
FIGURE 75.
Incidence rate of urinary tract infections per 1000 person-years in children born by CS and VD by year of birth in the HES data set.
FIGURE 76.
Incidence of necrotising enterocolitis per 1000 births in children born by CS and VD by year of birth in the HES data set.
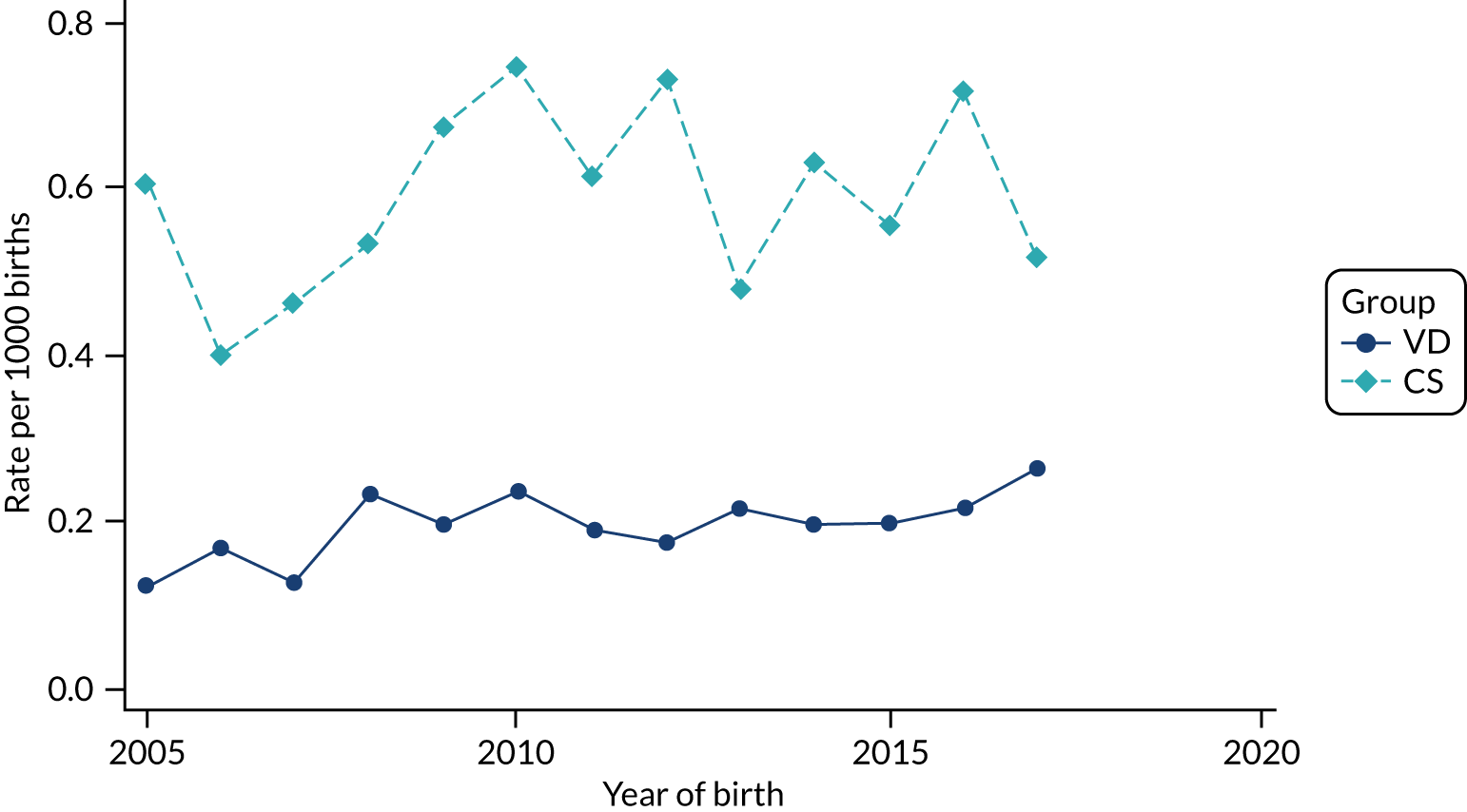
FIGURE 77.
Incidence rate of leukaemia per 1000 person-years in children born by CS and VD by year of birth in the HES data set.
FIGURE 78.
Incidence rate of first-time hospital admission per 1000 person-years in children born by CS and VD by year of birth in HES data set.
FIGURE 79.
Incidence of maternal composite infectious morbidity (e.g. wound infection, endometritis/endomyometritis and maternal sepsis) per 1000 births by CS and VD by year of delivery in HES data set.
FIGURE 80.
Incidence of wound infection per 1000 births by CS and VD by year of delivery in HES data set.
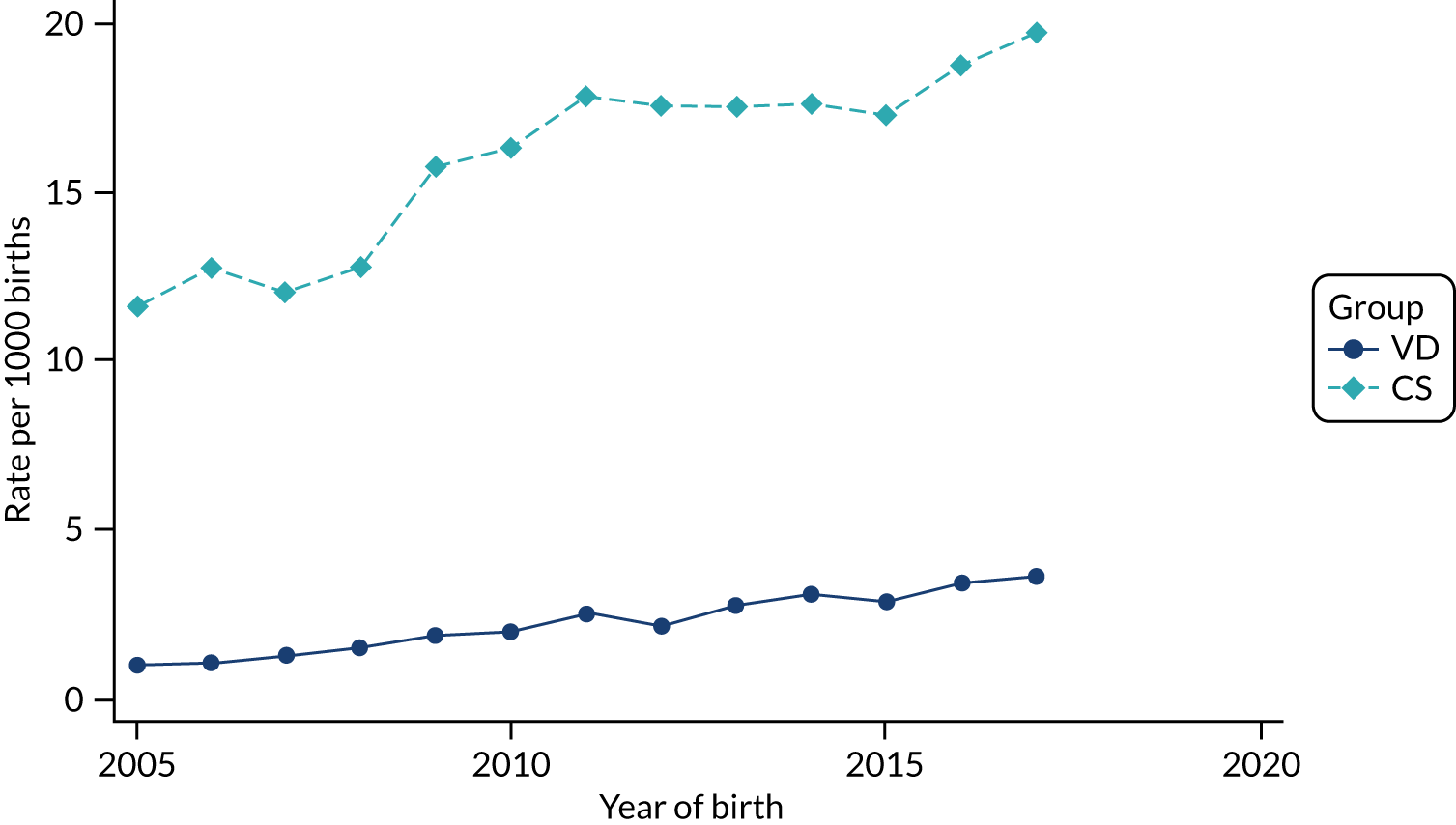
FIGURE 81.
Incidence of maternal sepsis per 1000 births by CS and VD by year of delivery in HES data set.
FIGURE 82.
Incidence of maternal urinary tract infection per 1000 births by CS and VD by year of delivery in HES data set.
Appendix 4 Risk of different study outcomes by delivery mode
Primary outcomes
Outcomes in children recorded in primary care
| Outcome | CS vs. VDa | ||
|---|---|---|---|
| IRR | 95% CI | p-value | |
| Asthma | 1.10 | 1.06 to 1.15 | < 0.001 |
| Eczema | 1.04 | 1.02 to 1.05 | < 0.001 |
Outcomes in children recorded in secondary care
| Outcome | CS vs. VDa | ||
|---|---|---|---|
| IRR | 95% CI | p-value | |
| Asthma | 1.09 | 1.05 to 1.12 | < 0.001 |
| Eczema | 0.95 | 0.81 to 1.12 | 0.56 |
Secondary outcomes
Outcomes in children recorded in primary care
| Outcome | CS vs. VDa | ||
|---|---|---|---|
| IRR | 95% CI | p-value | |
| Other allergic and allergy-related conditions | |||
| Food allergy/intolerance | 1.11 | 1.03 to 1.18 | 0.004 |
| Allergic rhinitis and conjunctivitis | 1.07 | 1.02 to 1.13 | 0.01 |
| One or more allergy-related disease | 1.10 | 1.06 to 1.15 | < 0.001 |
| Penicillin allergyb | 1.12 | 0.93 to 1.34 | 0.22 |
| Anaphylaxisb | 0.87 | 0.59 to 1.29 | 0.49 |
| High risk of anaphylactic reactionb | 1.21 | 1.07 to 1.36 | 0.002 |
| Autoimmune diseases | |||
| Type 1 diabetesb | 1.12 | 0.78 to 1.60 | 0.55 |
| Coeliac diseaseb | 1.24 | 0.88 to 1.75 | 0.22 |
| Childhood vitiligob | 0.97 | 0.65 to 1.46 | 0.89 |
| Infections and inflammation | |||
| Wheeze | 1.10 | 1.07 to 1.13 | < 0.001 |
| Upper respiratory tract infectionsb | 1.04 | 1.03 to 1.05 | < 0.001 |
| Lower respiratory tract infections (excluding bronchiolitis)b | 1.07 | 1.05 to 1.10 | < 0.001 |
| Bronchiolitisb | 1.12 | 1.08 to 1.16 | < 0.001 |
| Gastroenteritisb | 1.04 | 1.02 to 1.07 | < 0.001 |
| Urinary tract infectionb | 1.04 | 1.00 to 1.08 | 0.03 |
| Antibiotic prescribingb | 1.05 | 1.04 to 1.06 | < 0.001 |
| Other immune system-related conditions | |||
| Leukaemiab | 1.13 | 0.67 to 1.92 | 0.65 |
| Neurodevelopmental conditions | |||
| Cerebral palsy | 2.27 | 1.74 to 2.95 | < 0.001 |
| Autism spectrum disorderb | 1.26 | 1.10 to 1.44 | 0.001 |
| ADHDb | 0.79 | 0.60 to 1.05 | 0.10 |
| Less-specific measures of child health | |||
| Colicb | 1.10 | 1.05 to 1.15 | < 0.001 |
| Failure to thriveb | 1.06 | 0.96 to 1.16 | 0.24 |
| Health-care utilisation in children | |||
| Consultations recorded in primary care (12 months)b | 1.08 | 1.077 to 1.082 | < 0.001 |
Outcomes in children recorded in secondary care
| Outcome | CS vs. VDa | ||
|---|---|---|---|
| IRR | 95% CI | p-value | |
| Other allergic and allergy-related conditions | |||
| Anaphylaxisb | 1.15 | 1.004 to 1.32 | 0.04 |
| Autoimmune diseases | |||
| Type 1 diabetesb | 1.13 | 1.02 to 1.26 | 0.02 |
| Coeliac diseaseb | 0.95 | 0.62 to 1.46 | 0.82 |
| Juvenile idiopathic arthritisb | 1.02 | 0.75 to 1.38 | 0.91 |
| Autoimmune (idiopathic) thrombocytopenic purpurab | 1.08 | 0.94 to 1.24 | 0.28 |
| Infections and inflammation | |||
| Early-onset neonatal sepsis | 1.21 | 1.13 to 1.29 | < 0.001 |
| Late-onset neonatal sepsis | 1.31 | 1.19 to 1.44 | < 0.001 |
| Other sepsisb | 1.23 | 1.17 to 1.29 | < 0.001 |
| Lower respiratory tract infections (excluding bronchiolitis)b | 1.22 | 1.20 to 1.24 | < 0.001 |
| Bronchiolitisb | 1.19 | 1.17 to 1.21 | < 0.001 |
| Gastroenteritisb | 0.70 | 0.37 to 1.33 | 0.28 |
| Urinary tract infectionb | 1.07 | 1.04 to 1.10 | < 0.001 |
| Other immune system-related conditions | |||
| Necrotising enterocolitis | 2.95 | 2.57 to 3.38 | < 0.001 |
| Leukaemiab | 1.09 | 0.93 to 1.28 | 0.31 |
| Health-care utilisation in children | |||
| Any hospital admissionb | 1.07 | 1.06 to 1.07 | < 0.001 |
Maternal outcomes
| Outcome | Group, n (%) | CS vs. VDa | |||
|---|---|---|---|---|---|
| CS | VD | IRR | 95% CI | p-value | |
| Composite infectious morbidity | 12,466 (8.61) | 5303 (1.43) | 6.82 | 6.52 to 7.14 | < 0.001 |
| Endometritis/endomyometritis | 617 (0.43) | 1269 (0.34) | 1.27 | 1.11 to 1.45 | < 0.001 |
| Wound infection | 11,717 (8.09) | 3800 (1.02) | 9.72 | 9.22 to 10.24 | < 0.001 |
| Urinary tract infection/cystitis/pyelonephritis | 2551 (1.76) | 4935 (1.33) | 1.34 | 1.25 to 1.43 | < 0.001 |
| Sepsis | 212 (0.15) | 254 (0.07) | 1.67 | 1.20 to 2.32 | 0.002 |
| Pelvic abscessb | ≤ 5 (0.00) | 7 (0.00) | |||
| Antibiotic prescribing | 39,000 (26.92) | 59,138 (15.94) | 1.88 | 1.85 to 1.92 | < 0.001 |
| Outcome | CS | VD | CS vs. VDa | ||
|---|---|---|---|---|---|
| IRR | 95% CI | p-value | |||
| Composite infectious morbidity, n (%)b | 18,934 (1.9) | 9112 (0.3) | 6.49 | 6.31 to 6.68 | < 0.001 |
| Endometritis/endomyometritis, n (%)c | 41 (0.004) | 40 (0.001) | |||
| Wound infection, n (%) | 16,185 (1.62) | 6701 (0.23) | 7.56 | 7.32 to 7.81 | < 0.001 |
| Urinary tract infection/cystitis/pyelonephritis, n (%) | 3148 (0.31) | 5526 (0.19) | 1.62 | 1.53 to 1.71 | < 0.001 |
| Sepsis, n (%) | 2878 (0.3) | 2389 (0.1) | 3.08 | 2.84 to 3.34 | < 0.001 |
| Maternal deaths, n (%)c | ≤ 5 (0.00) | ≤ 5 (0.00) | |||
| Length of stay in days, median (IQR) | 3 (2 to 4) | 1 (1 to 2) | 1.75d | 1.74 to 1.75 | < 0.001 |
| Outcome | CS vs. VDa | ||
|---|---|---|---|
| IRR | 95% CI | p-value | |
| Asthma | |||
| Cefuroxime alone | 1.09 | 1.02 to 1.16 | 0.02 |
| Cefuroxime plus metronidazole | 1.05 | 0.98 to 1.13 | 0.19 |
| Co-amoxiclav alone | 1.11 | 1.06 to 1.17 | < 0.001 |
| Eczema | |||
| Cefuroxime alone | 0.72 | 0.49 to 1.05 | 0.09 |
| Cefuroxime plus metronidazole | 0.94 | 0.59 to 1.49 | 0.78 |
| Co-amoxiclav alone | 1.15 | 0.91 to 1.46 | 0.25 |
| Necrotising enterocolitis | |||
| Cefuroxime alone | 1.99 | 1.25 to 3.15 | 0.003 |
| Cefuroxime plus metronidazole | 2.50 | 1.61 to 3.90 | < 0.001 |
| Co-amoxiclav alone | 3.57 | 2.45 to 5.21 | < 0.001 |
Subgroup analyses
| Primary outcome | CS vs. VDa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Emergency CS | Elective CS | |||||||||
| n (follow-up time in person-years) | Rate per 1000 person-years | IRR | 95% CI | p-value | n (follow-up time in person-years) | Rate per 1000 person-years | IRR | 95% CI | p-value | |
| Asthma | 2256 (210,139) | 10.74 | 1.11 | 1.05 to 1.17 | < 0.001 | 1786 (179,873) | 9.93 | 1.06 | 1.00 to 1.13 | 0.06 |
| Eczema | 14,564 (176,401) | 82.56 | 1.13 | 1.11 to 1.16 | < 0.001 | 10,428 (155,825) | 66.92 | 0.95 | 0.93 to 0.99 | < 0.001 |
| Primary outcome | CS vs. VDa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Emergency CS | Elective CS | |||||||||
| n (follow-up time in person-years) | Rate per 1000 person-years | IRR | 95% CI | p-value | n (follow-up time in person-years) | Rate per 1000 person-years | IRR | 95% CI | p-value | |
| Asthma | 4984 (2,675,442) | 1.86 | 1.13 | 1.09 to 1.17 | < 0.001 | 3104 (1,897,257) | 1.64 | 1.02 | 0.97 to 1.06 | 0.45 |
| Eczema | 175 (2,675,442) | 0.07 | 1.06 | 0.88 to 1.28 | 0.55 | 106 (1,897,257) | 0.06 | 0.79 | 0.61 to 1.02 | 0.07 |
List of abbreviations
- ADHD
- attention deficit hyperactivity disorder
- CI
- confidence interval
- CPRD
- Clinical Practice Research Datalink
- CS
- caesarean section
- GP
- general practitioner
- HES
- Hospital Episode Statistics
- ICD-10
- International Statistical Classification of Diseases and Related Health Problems, Tenth Revision
- IRR
- incidence rate ratio
- MHRA
- Medicines and Healthcare products Regulatory Agency
- NICE
- National Institute for Health and Care Excellence
- OPCS-4
- Office of Population Censuses and Surveys Classification of Surgical Operations and Procedures, 4th revision
- PPI
- patient and public involvement
- QOF
- Quality and Outcomes Framework
- RCOG
- Royal College of Obstetricians and Gynaecologists
- THIN
- The Health Improvement Network
- VD
- vaginal delivery
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/ZYZC8514).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
