Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 11/67/01. The contractual start date was in March 2013. The draft report began editorial review in April 2020 and was accepted for publication in December 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2021 Wu et al. This work was produced by Wu et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 Wu et al.
Chapter 1 Introduction
Background
Cancer requiring chemotherapy is common. The National Cancer Registration and Analysis Service reported 169,000 patients receiving chemotherapy for years 2013 and 2014. 1 Although the frequency of administrations is not stated, based on data from 2009 it is likely to be approximately 500,000 chemotherapy deliveries per annum. When chemotherapy has to be administered intravenously, it can be given either through a peripheral cannula or short catheter (midline) into an arm vein or through a dedicated venous access device. These include a centrally inserted tunnelled catheters or Hickman-type devices (Hickman), peripherally inserted central catheters (PICCs) and centrally inserted totally implantable venous access device (PORTs). When the duration of the chemotherapy regime is over several months, these devices have the advantage that they all deliver the drug into a large high-flow central vein (superior vena cava). This avoids local problems from the irritant nature of many chemotherapeutic drugs, which can damage and rapidly occlude small peripheral arm veins and ulcerate the skin should they extravasate.
In 2012, the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme released two commissioning calls to address the clinical effectiveness and cost-effectiveness of venous access devices for central delivery of chemotherapy:2 specifically, comparisons between (1) subcutaneously tunnelled central lines (i.e. Hickman) and PICCs, and (2) subcutaneously tunnelled central lines and implantable venous access PORTs. The following year, the Cancer And Venous Access (CAVA) trial was funded to address not only the two original research questions, but also the additional comparison of PICCs with PORTs.
The current decision-making processes behind device choice remain poorly understood. At the time of commissioning, Hickman were the most commonly used device in clinical practice. 3 Despite the lack of strong evidence on the relative effectiveness and safety of these devices, the use of PICCs has increased over the past decade as an alternative to the Hickman and PORTs. The reasons for this include perceived lower cost, alleged greater safety, reduced waiting times and ease of insertion (often by nursing teams) in that they do not require an operating theatre or expensive fluoroscopic imaging.
The existing evidence on the devices is heterogeneous and insufficient to inform decision-making. A systematic review evaluated the risk of infectious and non-infectious complications associated with the use of Hickman compared with PORTs. 4 Although the evidence base was heterogeneous, the overall evidence showed that Hickman were associated with greater risk complications than those of PORTs. A more recent systematic review evaluated the complications and costs of PICCs compared with those of PORTs. 5 Based on data from 15 cohort studies, the study showed an increased risk of complications, including thrombosis, occlusion, infection, malposition and accidental removal, with PICCs.
All three devices are currently used in the UK and are produced by several manufacturers. Currently, there is no prescriptive protocol as to how, where or by whom these devices should be placed. In general, clinical practice adheres to the evidence-based practice in infection control (epic) guidelines6 and the guidance from the National Institute for Health and Care Excellence (NICE) (clinical guideline). 7 These guidelines were both updated during the lifetime of this trial.
Aim and objectives
The aim of the CAVA trial was to evaluate the clinical effectiveness and cost-effectiveness of venous access devices for the central delivery of chemotherapy.
The objectives were to determine:
-
whether or not PICCs are non-inferior to Hickman with regard to complication rates
-
whether or not PORTs are superior to Hickman with regard to complication rates
-
whether or not PORTs are superior to PICCs with regard to complication rates
-
the cost-effectiveness of Hickman, PICCs and PORTs
-
the acceptability of Hickman, PICCs and PORTs to patients and clinical staff.
Chapter 2 Methods
Study design
The CAVA trial was an open-label, multicentre, randomised controlled trial (RCT) to determine the clinical effectiveness and cost-effectiveness of three routinely used central venous access devices (CVADs): centrally inserted tunnelled catheter (Hickman), PICCs and centrally inserted totally implantable venous access device (PORTs). Alongside the trial, pre- and post-trial qualitative research was also carried out to examine the acceptability of these devices to patients and clinical staff.
The trial was registered on 26 March 2013 as ISRCTN44504648 and the trial protocol was published in The Lancet. 8
Settings and locations
Initially, six recruiting sites were set up. However, during the course of the trial, a further 12 sites were included.
Original sites
-
Beatson West of Scotland Cancer Centre (BWoSCC), Glasgow (lead centre).
-
Queen Elizabeth Hospital Birmingham, Birmingham.
-
Christie Hospital, Manchester.
-
Freeman Hospital, Newcastle upon Tyne.
-
Guy’s Hospital, London.
-
St James’s University Hospital, Leeds.
Additional sites
-
University Hospital of North Durham, Durham.
-
Darlington Memorial Hospital, Darlington.
-
Northampton General Hospital, Northampton.
-
Weston Park Hospital, Sheffield.
-
Charing Cross Hospital, London.
-
Royal United Hospital, Bath.
-
Forth Valley Royal Hospital, Larbert.
-
Cumberland Infirmary, Carlisle.
-
Kent and Canterbury Hospital, Canterbury.
-
Royal Cornwall Hospital, Treliske.
-
Broomfield Hospital, Chelmsford.
-
Sunderland Royal Hospital, Sunderland.
-
Western General Hospital, Edinburgh.
Participants
All adult patients who were expected to receive chemotherapy over a long period of time to treat malignancy were eligible for enrolment if they met the following inclusion criteria:
-
aged ≥ 18 years
-
receiving or to receive anticancer intravenous therapy
-
duration of anticancer intravenous therapy of ≥ 12 weeks
-
intended duration of continuous device placement of ≥ 12 weeks with no temporary removal for surgery
-
clinical team uncertain as to which device is optimal for this indication
-
solid or haematological malignancy
-
suitable upper extremity vein for all the access devices to which the patient may be randomised
-
able to provide written informed consent.
Patients were excluded from the trial according to the following exclusion criteria:
-
life or treatment expectancy of < 3 months
-
previous venous access device removed as a result of complications within the last 2 weeks
-
patient has any evidence of active infection
-
requirement for high volume of flow rate (apheresis line)
-
requirement for catheter to be placed in a non-upper extremity vein.
Randomisation
The trial had four randomisation options for each eligible participant:
-
Hickman versus PICCs versus PORTs
-
PICCs versus Hickman
-
PORTs versus Hickman
-
PORTs versus PICCs.
This was to maximise recruitment by facilitating recruitment to the study in cases in which one device was not feasible to consider. For instance, a patient may have a strong dislike for one particular device, a patient may be considered to be unsuitable for a particular device or a particular device may be unavailable at the recruiting site. Clinicians could choose from any of the four randomisations depending on the individual patient and the practice at their individual site. Treatment allocations were obtained by contacting the Cancer Research UK (CRUK) Clinical Trials Unit (CTU), Glasgow. The three-way randomisation was initially set up with a 2 : 2 : 1 (Hickman–PICCs–PORT) ratio to over-recruit to the arms involved in the non-inferiority comparison, which required more patients. The numbers of patients assigned to each treatment arm in the three-way and two-way randomisations were monitored, with the plan to make adjustments to the three-way randomisation ratio as appropriate to ensure the required balance of arms; however, no adjustments were required. Randomisations were performed using a minimisation algorithm incorporating a random component. The stratification factors used in the minimisation were:
-
centre
-
body mass index (BMI) – < 20 kg/m2, 20 to < 30 kg/m2, 30 to < 40 kg/m2, ≥ 40 kg/m2
-
device history – patients with no prior devices fitted, patients having previously had at least one device fitted > 3 months prior to the study, patients having had devices fitted within 3 months of the study
-
type of disease – haematological malignancies, solid tumours
-
planned treatment mode – inpatient, outpatient.
Interventions
Subcutaneously tunnelled central catheter (Hickman)
Introduced in 1979, subcutaneously tunnelled central catheters are commonly known as Hickman and consist of a thin plastic tube that is inserted into a central vein in the neck or upper chest region. It is ‘tunnelled’ under the skin for a few centimetres before exiting and has a Dacron™ cuff (DuPont de Nemours, Inc., Wilmington, DE, USA) to improve stability and minimise the risk of infection. Ultrasound is used to target the vein. These catheters are inserted by a variety of specialists (nurse practitioners, interventional radiologists, anaesthetists and surgeons) but, currently, nursing experience in placement is limited. Maintenance involves regular dressing change and weekly line flushing. The cost of a Hickman lies between those of PICCs and PORTs. Removal requires simple dissection to free the Dacron cuff.
Peripherally inserted central catheters
Introduced in 1975, a PICC line is a thin plastic tube that is inserted into a peripheral vein in the upper arm. These catheters are commonly inserted by nurse practitioners, but also by interventional radiologists. Ultrasound is used to target the vein. Maintenance involves regular dressing change and weekly line flushing. It is the cheapest device to purchase and the simplest device to place. When it is no longer required, removal of the line is straightforward.
Implantable chest wall PORTs
Introduced in 1981, a chest wall port is a small, coin-sized device with a silicone membrane that is buried just under the skin in a subcutaneous pocket. It connects to a thin plastic tube similar to the other two devices. The entire device is completely implanted with nothing exiting the skin. Ultrasound is used to target the vein. The PORT has to be punctured via the skin with a special needle each time it is used. PORTs are inserted by a variety of specialists (nurse practitioners, interventional radiologists, anaesthetists and surgeons) but, currently, nursing experience in placement is limited. Given that PORTs are totally implanted, there is no dressing requirement and flushing is needed only monthly. PORTs are the most expensive of the three devices and the most complicated to insert and remove, requiring a minor surgical procedure.
Outcomes measures
Complication and quality-of-life data were reported monthly until device removal or withdrawal up to a period of 12 months.
Primary outcome
The primary outcome was complication rate, a composite of the inability to aspirate blood, infection associated with the device (suspected, confirmed or exit site), venous thrombosis, pulmonary embolism related to the device, mechanical failure (line fracture, line separation from chest wall port, exposure of line cuff, exposure of chest wall PORT or breakdown of wound, chest wall port flip, line fallen out or line migration requiring intervention) and other complications.
Secondary outcome
The secondary outcome measures were:
-
Incidence of individual complications – inability to aspirate blood from the device, venous thrombosis related to the device, pulmonary embolism related to the device, laboratory-confirmed bloodstream infection, suspected catheter-related bloodstream infection, exit site infection, mechanical failure and other complications.
-
Complications per catheter-week – defined as the number of complications divided by the number of weeks that the device was in place.
-
Time to first complication – defined as the time from randomisation to the first documented complication. Patients not experiencing a complication were censored at their device removal date or last available date (last chemotherapy date, last status assessment date or date of death) if the device was still in place at the end of the study.
-
Duration of chemotherapy treatment interruptions – overall and for each individual complication.
-
EuroQoL-5 Dimensions, three-level version (EQ-5D-3L) – a validated, generic, health-related quality-of-life (HRQoL) measure comprising five dimensions (i.e. mobility, self-care, usual activities, pain/discomfort and anxiety/depression). A value of 1 indicates perfect health, whereas a value of –0.594 indicates the worst health state (worse than dead). The visual analogue score for general health is reported on a scale of 0–100 (0 being the worst imaginable health state and 100 being the best).
-
European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire C30 (QLQ-C30) – comprising scores for the five functional scales (i.e. physical, role, emotional, cognitive and social), nine symptom scales (i.e. fatigue, nausea and vomiting, pain, dyspnoea, insomnia, appetite loss, constipation, diarrhoea and financial difficulties) and a global health status score, all on a scale of 0–100 (a high score represents a higher response level).
-
Venous Access Device Questionnaire (see Report Supplementary Material 1) – comprising 16 different questions (relating to continuing to drive, getting in and out of a car, using public transport, going shopping, eating, hygiene, sleeping, mobility, usual work, exercise, hobbies, feeling of self-consciousness). These items are assessed on a scale of 1–4 (not at all, a little, quite a bit, very much; as piloted in the Glasgow feasibility study). 3
Sample size
The sample size was based on the three hypotheses of interest.
Hypothesis 1: peripherally inserted central catheters are non-inferior to Hickman in terms of complication rate
Based on the assumption that the Hickman complication rate is 55%, PICCs would be considered non-inferior if their complication rate is no more than 10% higher (i.e. 65%). To rule out this difference with 80% power, and a one-sided significance level of 2.5% required 778 patients in total using a 1 : 1 randomisation.
Hypothesis 2: PORTs have a lower complication rate and are more cost-effective than Hickman
The minimum requirement here was to demonstrate that PORTs have a lower complication rate than Hickman. Based on the assumption that the Hickman complication rate is 55%, we aimed to detect at least a 15% reduction with PORTs. To detect this reduction with 95% power, and a two-sided significance level of 5% required 550 patients in total using a 1 : 1 randomisation.
Hypothesis 3: PORTs have a lower complication rate and are more cost-effective than peripherally inserted central catheters
The minimum requirement here was to demonstrate that PORTs have a lower complication rate than PICCs. Based on the assumption that the PICCs complication rate is 55%, we aimed to detect at least a 15% reduction with PORTs. To detect this reduction with 80% power, and a two-sided significance level of 5% required 341 patients in total using a 1 : 1 randomisation.
Statistical analysis
The analysis was performed separately for the three pairwise comparisons of interest. All analyses were based on the intention-to-treat (ITT) population, which was defined as all randomised patients, and study arms were based on the device that patients were assigned to at randomisation. Per-protocol sensitivity analyses were undertaken for the primary analysis of each comparison excluding patients who were not fitted with the device assigned at randomisation.
The primary outcome was complication rate. This was analysed using logistic regression, including terms for treatment arm, randomisation stratification factors and whether the data came from the relevant two-way or three-way randomisation (Figure 1). The incidence of venous thrombosis was compared using the same logistic regression approach as the primary analysis. The total durations of treatment interruptions were summarised and compared using Mann–Whitney U-tests for each complication and overall. The binary stratification factors of treatment mode and type of disease were excluded because of the small numbers of patients in one category across all comparisons (≤ 13% and ≤ 10%, respectively). BMI, device history and site were re-parameterised for the same reason. BMI was dichotomised into < 30 and ≥ 30 mg/kg2, device history was categorised as yes or no, and site retained the six sites with the highest recruitment (i.e. BWoSCC, Freeman Hospital, St James’s University Hospital, University Hospital of North Durham, Christie Hospital and Charing Cross Hospital) and combined the smaller sites into one ‘Other’ site.
FIGURE 1.
Four randomisations contributing to three comparisons.
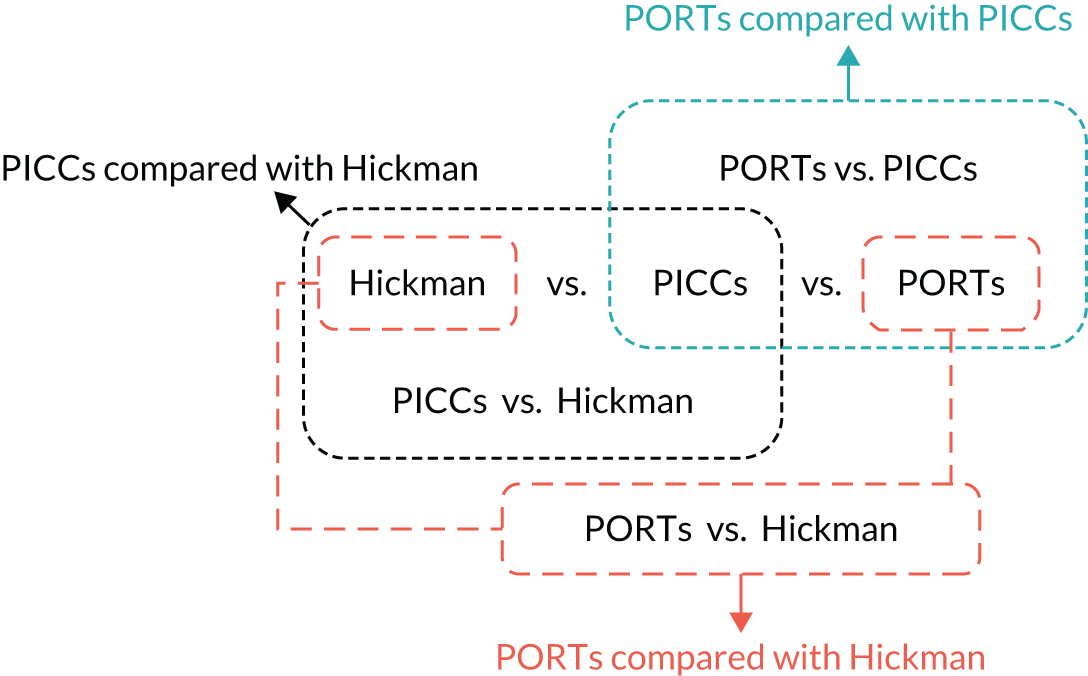
For the PICCs versus Hickman comparison only, to judge non-inferiority in terms of an odds ratio (OR), the allowable 10% limit on the increase in complication rate (from 55% to 65%), as used in the sample size calculations, was converted to an OR. This 10% increase limit corresponds to an OR (PICCs/Hickman) limit of 1.519.
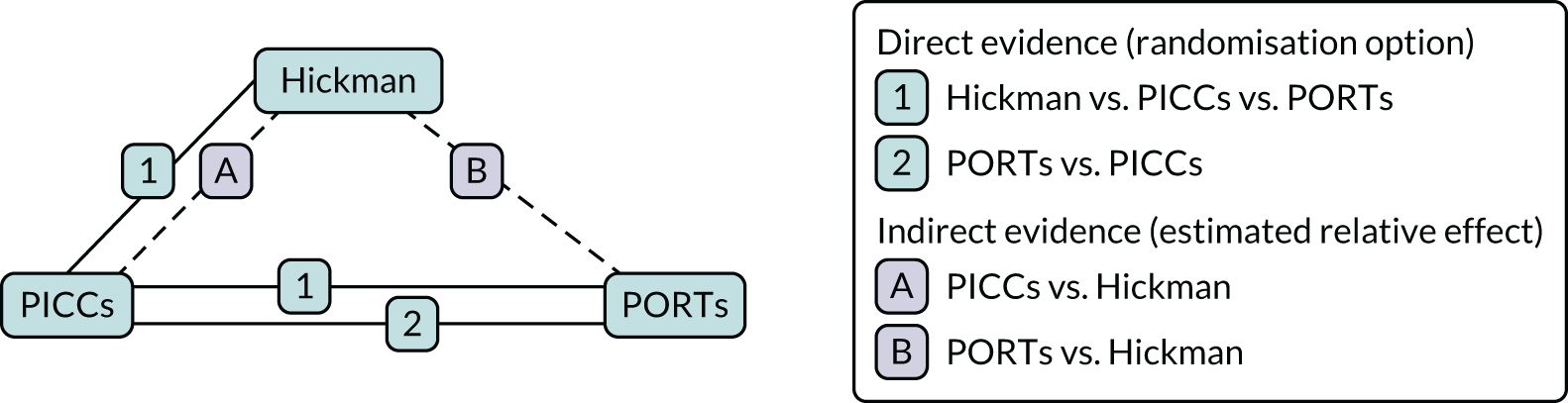
In addition, a network meta-analysis (NMA) of the four randomisation options was carried out. 9 The relative effects of each device compared with their comparator were estimated using both direct and indirect evidence, thereby generating a more precise estimate of the relative treatment effects. The direct evidence is based on the head-to-head randomisation options for each of the three comparisons. The indirect evidence was obtained omitting patients from the three-way randomisation and the final effect was estimated by augmenting the direct estimate with the indirect estimates using a fixed-effect estimates approach. The Q-statistic was used as a measure of heterogeneity between the direct and the indirect estimates to measure consistency to ensure that these could be appropriately combined for analysis. An illustration using the PORTs versus PICCs comparison is presented in Figure 2.
FIGURE 2.
Direct and indirect evidence for estimating the effects of PORTs vs. PICCs.
Multiple imputation was applied to missing EuroQoL-5 Dimensions (EQ-5D) data10 prior to calculating the index values; five imputed data sets were created. For each patient, the area under the curve (AUC) was estimated. 11 The AUCs described by the EQ-5D index score (both the original data and the imputed data) were standardised by the time spent in the study (from randomisation to device removal, withdrawal, death or a maximum of 12 months if the patient was still alive with the device in place). These standardised AUCs were adjusted by having the baseline value (value reported prior to the device being fitted) subtracted from them. These adjusted standardised AUC scores were compared between the arms; the five imputed data sets were analysed separately using the Mann–Whitney U-test before their results were combined to provide a single p-value for the difference between arms. The same approach was taken for the EQ-5D visual analogue scale for health. The resultant p-values for the individual scores were adjusted for multiple comparisons using the false discovery rate approach [calculated using the p. adjust function (FDR option) of the stats library in R (The R Foundation for Statistical Computing, Vienna, Austria); URL: www.r-project.org]. 12
The EORTC QLQ-C30 data were imputed and analysed as with the EQ-5D data, with the exception that the multiple imputation techniques were applied after the data had been scored in accordance with standard EORTC conventions. Unadjusted and adjusted p-values were obtained for the differences between arms for the five functional scales of the EORTC QLQ-C30 (i.e. physical, role, emotional, cognitive and social), nine symptom scales (i.e. fatigue, nausea and vomiting, pain, dyspnoea, insomnia, appetite loss, constipation, diarrhoea and financial difficulties) and the global health status score.
The worst responses for each question from the Venous Access Device Questionnaire were summarised and compared across arms via Mann–Whitney U-tests. The resultant p-values for the individual questions were adjusted for multiple comparisons using the false discovery rate approach, as with the EQ-5D data.
Changes to the study protocol
Substantial amendment 1 added the inclusion criterion of intended duration of continuous device placement of at least 12 weeks, with no temporary removal for surgery. This was in response to a site query regarding patients who have chemotherapy ahead of initial neo adjuvant surgery (if the line was expected to remain in situ during surgery then the patient was eligible and if it was expected to be removed the patient was ineligible).
Substantial amendment 2 amended the exclusion criterion for previous venous access device removal owing to complication from 3 months to 2 weeks. This was to maximise recruitment, as the 3-month exclusion was a barrier to recruitment. In addition, this amendment also added the exclusion criterion of any evidence of active infection to clarify the requirement that patients must not have an overt infection prior to study entry.
Substantial amendment 3 amended the sample size to 1300 patients following the results of the internal pilot study, which took place during the first 18 months of the recruitment period. The independent Trial Steering Committee (TSC) formally assessed the study progress against the following criteria:
-
At least 35% of the target recruitment for that time point met (individually for each of the three two-way comparisons).
-
If this milestone was not met for a particular comparison, the TSC would have considered stopping recruitment to that comparison, but this was not the case at the end of the pilot period.
Other amendments
-
In the protocol, the definition of the primary end point was a composite of infection associated with the device (suspected or confirmed) and/or mechanical failure only. This was amended as more complications were specified as the study developed. It was an error that the protocol was not updated accordingly.
-
The protocol incorrectly stated that the power for the PICCs versus Hickman comparison was intended to be 90%; this should have been corrected to state 80%.
-
An analysis was planned based on complication event rate data13 to estimate the relative effect of the devices on infections (laboratory-confirmed, suspected catheter related and exit site infections) versus non-infections (all other complications combined) to allow an assessment of the similarity of these effects over time via a likelihood ratio test. The test was to be conducted by comparing the likelihood of a standard joint frailty model [modelling complication (no distinction of type) and device removal rates simultaneously] with the likelihood of a multivariate joint frailty model [modelling the two complication types (infection and non-infection) and device removal rates simultaneously]. Unfortunately, no software could be found to apply the multivariate joint frailty model successfully to the CAVA trial data and the analysis was abandoned.
Economic evaluation
In line with the aim of the overall trial, to address the questions of the original NIHR commissioning brief, the objectives of the economic evaluation were to determine the cost-effectiveness of:
-
PICCs compared with Hickman
-
PORTs compared with Hickman
-
PORTs compared with PICCs.
An economic evaluation was undertaken from the perspective of the UK NHS. A within-trial analysis was conducted over the time horizon of 1 year (i.e. from randomisation to the end of CAVA trial follow-up period) to evaluate the three comparisons. Costs and outcomes were not discounted.
Resource use and costs
All health-care resource use data were collected by a research nurse involved in the delivery of care to patients in the CAVA trial. These include:
-
Procedure details relating to device insertion – staffing team (nurse, radiographer, anaesthesiologist, radiologist, doctor and surgeon), setting (theatre, treatment room, radiology department and bedside), type of anaesthesia and type of imaging. These components formed the basis of the intervention costs.
-
Device removal – device removal for any reason during the trial period.
-
Health-care resource use during follow-up period – number of admissions and length of stay, and outpatient visits. These were recorded monthly during the trial and formed the basis of the non-intervention costs.
Unit costs were based on national sources (Table 1). Where costs were not routinely available, resource use and unit costs were estimated through consultation with clinical experts. All unit costs were presented in Great British pounds for the price year 2017/18.
The total cost per patient was calculated by attaching relevant unit costs to the resource use data. In addition to the cost of the device, the cost associated with device insertion was calculated. This included the cost of the primary operator and any additional staff required for insertion, setting, imaging, anaesthesia and use of prophylactic antibiotics. Unit costs associated with staff and setting were applied to the time taken for device insertion based on data recorded within the trial. Similarly, unit costs for imaging, anaesthesia and the use of prophylactic antibiotics were applied to resource use data from the trial. The cost of device removal took into account staff time required to perform the procedure. The time required to remove each device was obtained from two separate clinical experts. Unplanned follow-up costs, including inpatient stays in hospital and outpatient visits recorded following device insertion (which were not part of the patient’s ongoing chemotherapy care), were collected. For the purposes of the analysis, device insertion costs (the device itself and device insertion costs) and unplanned follow-up costs (inpatient stay, outpatient visits, device removal and replacement costs) were also estimated alongside total costs.
| Resource | Unit | Unit cost (£) | Source |
|---|---|---|---|
| Device cost | |||
| Hickman | Per device | 165 | Manufacturer (Vygon SA, Écouen, France) |
| PICC | Per device | 120 | Manufacturer (Vygon) |
| PORT | Per device | 340 | Manufacturer (Vygon) |
| Staff | |||
| Nurse | Per working hour | 55 | PSSRU 201914 |
| Radiographer | Per working hour | 109 | PSSRU 201914 |
| Anaesthesiologist | Per working hour | 109 | PSSRU 201914 |
| Radiologist | Per working hour | 109 | PSSRU 201914 |
| Doctor | Per working hour | 109 | PSSRU 201914 |
| Setting | |||
| Theatre | Per procedure | 571 | Walker and Todd15 |
| Treatment room | Per procedure | 286 | Expert clinical opinion |
| Radiology department | Per procedure | 571 | Walker and Todd15 |
| Beside | Per procedure | 14 | Expert clinical opinion |
| Imaging | |||
| Ultrasound | Per procedure | 168 | NHS Reference Costs (2017/18)16 |
| Fluoroscopy | Per procedure | 33 | NHS Reference Costs (2017/18)16 |
| Sherlock tracking | Per procedure | 60 | Taxbro et al. 202017 |
| X-ray | Per procedure | 11 | NHS Reference Costs (2017/18)16 |
| Anaesthesia | |||
| Local only | Per procedure | 11 | Calvert et al.18 |
| General anaesthesia | Per procedure | 35 | Calvert et al.18 |
| Other | |||
| Prophylactic antibiotics | Per procedure | 1.18 | Taxbro et al. 202017 |
| Device removal | |||
| Hickman | Per procedure | 483 | Expert clinical opiniona |
| PICC | Per procedure | 14 | Expert clinical opinionb |
| PORT | Per procedure | 501 | Expert clinical opinionc |
| Device replacement | |||
| Hickman | 1052 | Estimated from the CAVA trial data | |
| PICC | 484 | Estimated from the CAVA trial data | |
| PORT | 1135 | Estimated from the CAVA trial data | |
| Complications | |||
| Inpatient stay | Per day | 969 | ISD Scotland19 |
| Outpatient visit | Per visit | 136 | NHS Reference Costs (2017/18)16 |
Health-related quality of life
In accordance with NICE guidance on estimating cost-effectiveness,20 health outcomes were expressed as quality-adjusted life-years (QALYs) gained. Data on HRQoL were collected using the EQ-5D-3L at baseline, and monthly thereafter until device removal, death or the end of follow-up at 12 months. The EQ-5D-3L assesses five health domains (i.e. mobility, self-care, usual activities, pain/discomfort and anxiety/depression) according to three levels (i.e. no problems, some problems and extreme difficulty). Responses to EQ-5D-3L were converted into a single global measure of health utility for each time point. 21 For patients who had the device removed within the trial period, we did not collect data subsequent to device removal. Therefore, we estimated the quality-adjusted time spent on device per patient as a proxy for QALY gained. A baseline-adjusted AUC approach was used to estimate the QALYs gained in the trial, while adjusting for the life-years gained by the HRQoL experienced over the study period. 22
Missing data
Resource use data comprised data on inpatient stays and outpatient visits during the follow-up period. It was assumed that where a patient had no record of outpatient visits or inpatient admissions during the follow-up period, none had taken place. Therefore, we included resource use data only where they were reported and did not impute for missing resource use data.
Multiple imputation by chained equations was used to impute missing EQ-5D health–utility values. 23 For patients with missing values, health–utility values from all other available time points were used to predict missing values,24 while adjusting for the trial stratification factors (age, sex, BMI, device history and treatment arm).
Cost-effectiveness analysis
The cost-effectiveness analysis was based on the imputed ITT patient population. The mean total costs and QALYs were estimated by fitting generalised linear models to the data and adjusting for age, sex, BMI, device history, treatment arm and baseline EQ-5D (QALY estimates only). The appropriate family for the generalised linear model was selected based on the results of the modified Park’s test. The final cost model was based on the log-link and gamma family; the final QALY model was based on the identity link and Gauss family. Based on the estimation of the final statistical model, the total cost and QALY difference between arms were based on the marginal prediction. All cost and QALY calculations were conducted in Stata® 14 (StataCorp LP, College Station, TX, USA).
Non-parametric bootstrapping was used to explore uncertainty in our estimates of mean cost and QALYs and to describe how this uncertainty affects the model outcomes. A 1000-iteration bootstrap was undertaken to estimate the 95% confidence intervals (CIs) for mean cost and QALYs for each device. The resultant distribution of mean costs and QALYs was then presented graphically on the cost-effectiveness plane.
Where appropriate, cost-effectiveness was expressed as incremental cost-effectiveness ratios (ICERs), calculated as the difference between devices in mean total costs divided by mean total QALYs. In addition, cost-effectiveness was also expressed as net monetary benefit (NMB) based on a willingness-to-pay threshold of £20,000. The NMB is a measure of the health benefit, expressed in monetary terms:
where E = effectiveness, WTP = willingness-to-pay threshold and C = cost.
When comparing two devices, a positive incremental NMB indicates that the device of interest is cost-effective compared with the alternative. Cost-effectiveness acceptability curves were used to present the uncertainty in the decision regarding the most cost-effective option over a variety of monetary willingness-to-pay thresholds.
Sensitivity and scenario analyses
Several sensitivity and scenario analyses were undertaken to test the robustness of the cost-effectiveness estimates. These included:
-
Exploring the results at 3 and 6 months. There have been suggestions from the literature and from clinical practice that device-related complications typically occur within the first 6 months of device placement. The time to first complication was also investigated in the CAVA trial. A sensitivity analysis was conducted by restricting the analysis to 3 and 6 months of follow-up.
-
Exploring the results of patients with solid tumours only. The CAVA trial population included patients with solid tumours and haematological malignancies. There have been suggestions from the literature and from clinical practice that these patients are managed differently. This has also been reflected by the small number of patients with haematological malignancies being recruited into the trial. A sensitivity analysis was carried out by restricting the analysis to patients with solid tumours only.
-
Exploring a nurse-led model of care. There are variations across clinical practice in how care is provided for patients requiring venous access devices for chemotherapy, specifically with regard to device implantation, removal and replacement of PORTs. Where Hickman or PORTs devices are required, these are typically inserted in a theatre or radiology department by a radiologist. The associated resource requirements are greater than those for a PICC device. Furthermore, waiting times for theatre space and radiologist availability may also increase the time taken to start a patient on treatment. Personal communication with clinicians currently involved in the routine insertion of PORTs suggested that this procedure can be performed by a nurse-led service. A sensitivity analysis was carried out to examine this scenario. For such a nurse-led service, we assumed that three nurses would be required and that the procedure was undertaken in a treatment room (instead of in an ‘air handled’ room). We also included the cost of regular device maintenance required for Hickman and PICCs, delivered by a nurse in the community, which is currently standard practice but was not captured within the clinical trial. This involved a 30-minute visit per week from a district nurse.
Pre-trial qualitative study
The pre-trial qualitative study was carried out to explore patient and staff views about participation in a RCT, with the aim of maximising recruitment to the CAVA trial.
Sampling and recruitment
Following ethics approval of the study protocol, patients were approached in chemotherapy clinics and day care units and were invited to participate in the study. A patient information leaflet was sent out by post or handed out to interested patients. The research team followed up the initial contact with a telephone call to discuss any queries and to recruit participants. Initial plans to hold one focus group of 10 patients were modified when recruitment proved more difficult than anticipated. Barriers to recruitment included ill health, treatment schedules, travel issues, work commitments and a reluctance to join a group discussion. Therefore, following consultation with the CAVA project management group, the decision was taken to carry out three separate focus groups. A convenience sample of nine patients receiving treatment for cancer at the lead centre (BWoSCC, Glasgow) took part in three separate focus groups, each with three participants. Four male and five female patients participated. Participants’ ages ranged from 48 to 66 years. All participants had solid tumours, including two with metastatic disease. Three participants had experience of an implanted PORT, three participants had experience of a PICC line and two participants had experience of a tunnelled cuffed central catheter. One participant had experience of both a PICC and a tunnelled cuffed central catheter. Four participants had previously participated in a RCT.
Clinical staff were also invited to be interviewed because it was anticipated that there would be variations in attitudes regarding equipoise across specialties and roles, and differences in local practice, which may act as facilitators of and barriers to randomisation. One-to-one, semistructured interviews were conducted with 23 clinical staff (five nurse managers, four research nurses, four oncologists, six interventional radiologists, three haematologists and one anaesthetist) from six different centres (BWoSCC, Glasgow; Queen Elizabeth Hospital Birmingham, Birmingham; St James’s University Hospital, Leeds; Christie Hospital, Manchester; Freeman Hospital, Newcastle upon Tyne; University Hospital of North Durham, Durham). The research team made initial contact with the relevant individuals via the local principal investigator at each site and clinical staff information sheets were e-mailed to potential participants.
Procedure
Focus groups with patients took place in a private room at the lead centre and were facilitated by one of the authors (MS), who has received considerable training in qualitative research methodology and has a master of laws (medical law and health-care ethics), as well as a background in nursing. The moderator, who had no prior relationship with the participants, was accompanied by an assistant, but no one else was present during data collection. Signed consent to participation and to audio-recording of the discussion was obtained. A focus group guide was used (see Report Supplementary Material 2), which encompassed questions on participants’ attitudes, experiences and preferences relating to the three devices, and on participants’ understanding of the study design and willingness to participate in a randomised trial of the three devices. Learning aids included laminated photographs of the devices in situ, copies of the patient information sheet (PIS) for the trial and a demonstration of the three devices. Focus groups lasted approximately 1 hour and were digitally recorded.
The same qualitative researcher visited each centre to interview staff. Written consent to participation and to audio-recording of the interview was obtained prior to participation. A semistructured interview guide was used (see Report Supplementary Material 3) that aimed to elicit information on perceived barriers to and facilitators of recruitment, as well as the attitudes to the three devices and to RCTs. Feedback was obtained on trial materials including PISs and consent forms. All interviews were digitally recorded.
Analysis
All recordings, along with field notes taken during data collection, were transcribed verbatim, anonymised and thematically analysed. 25 The QSR NVivo10 (QSR International, Warrington, UK) software program was used to facilitate data analysis. First, initial codes were identified, based on careful reading and re-reading of the data by two members of the research team independently. Second, these codes were sorted into potential themes. Finally, the themes were refined through repeated investigation of both similar and anomalous examples.
Post-trial qualitative study
The post-trial qualitative study sought to examine patient and staff acceptability of the three devices, as well as experiences of trial participation.
Sampling and recruitment
Forty-two patients enrolled in the CAVA trial participated in eight focus groups. Participants were purposively sampled from the CAVA trial’s six largest recruitment centres: BWoSCC, Glasgow; St James’s Hospital, Leeds; Christie Hospital, Manchester; Freeman Hospital, Newcastle upon Tyne; University Hospital of North Durham, Durham; and Hammersmith Hospital, London. Three further participants who had agreed to participate were too unwell to attend, one each at Leeds, Durham and London. To include a range of perspectives and experiences, participants at each site were chosen for maximum variation in terms of age, sex, cancer diagnosis and device allocation, as well as positive and negative clinical experiences with CVADs. Eligible participants were initially contacted by local trial nurses with whom they had prior contact and who provided information sheets in person or by mail.
Originally, six focus groups (one at each centre) comprising participants with a mix of all three CAVA trial devices was planned. This plan was amended for two reasons. First, one site (Christie Hospital) had ceased recruitment at the time of focus group planning and only participants with PORT devices remained on the trial. Second, it was felt that, although mixed-device groups would be well suited to comparisons between devices, single-device groups could offer greater insight into attitudes and experiences of each device. The design was amended to include four large mixed-device groups (4–11 participants) and four smaller single-device groups (two PORTs only, one Hickman only, one PICC only; two or three participants). The two additional groups were sampled at the trial’s Glasgow site, which had higher recruitment rates than the other sites.
Clinical staff (nurses, oncologists, radiologists and anaesthetists) from each of the trial’s centres were contacted via e-mail by the research team, provided with copies of the information sheet and invited to be interviewed. The decision was made to also invite trial staff from non-clinical research backgrounds to take part, as it was felt that these staff could offer useful insights, particularly regarding the organisational and administrative aspects of the trial. Twenty-six one-to-one semistructured interviews with clinical staff from 13 centres were carried out.
Procedure
Focus groups took place on site at six CAVA trial centres in quiet meeting rooms and were moderated by one of the authors (CR), a female psychologist (PhD; Doctor of Philosophy) and an experienced qualitative researcher who had no prior relationship with the participants. A trial nurse attended part of one focus group (Leeds) to address patient queries; otherwise no other persons were present. Prior to each session, details about data collection, analysis and use were discussed, and written informed consent was obtained. The moderator started by explaining her own background and role in the trial. She then reminded participants about the purpose of the broader trial and current focus group, using A4 cards depicting each device type and reiterating current clinical equipoise. A focus group guide was used to ensure that all relevant topics would be addressed (see Report Supplementary Material 2). Topics included CAVA trial participation and day-to-day experiences relating to their device. To create a communication situation resembling a naturally occurring interaction, interference with the discussion was kept to a minimum. Focus group discussions lasted approximately 1 hour and were audio-recorded with participants’ permission. To assist with transcription and analysis, relevant field notes were compiled after each focus group.
Interviews with staff took place in person or by telephone by the same researcher. Face-to-face interviews took place in interviewees’ offices or in quiet rooms on site. As above, a semistructured interview guide was used to facilitate data collection (see Report Supplementary Material 3). Topics covered included the interviewee’s knowledge, experiences and opinions regarding the use of CVADs in the context of anticancer treatment, as well as their experience of participating in the CAVA trial. Interviews lasted, on average, 30 minutes and were audio-recorded using a digital voice recorder.
Analysis
All recordings, along with field notes taken during data collection, were transcribed verbatim, anonymised and uploaded to the QSR NVivo 10 qualitative software program. Data were analysed using thematic analysis, ‘a method for identifying, analysing and reporting patterns (themes) within data’. 25 Transcripts were read and re-read to ensure familiarity. A coding framework was developed based on patterns and repeated topics identified in the data. Data were coded, and coded chunks of data were grouped into initial themes. These processes were conducted by a single researcher in the first instance and reviewed by two further researchers at different stages. Data were then re-read and the appropriateness of themes interrogated. Particular attention was paid to similarities and differences across device types, and to discrepancies between developed themes and the data. As a final step, the specifics of each theme were refined, and clear definitions for each theme were formulated.
Chapter 3 Pre-trial qualitative results
The difficulties associated with recruitment, especially in multicentre RCTs, have been well documented. 26,27 Qualitative research methods have been shown to be successful in increasing rates of randomisation in RCTs, especially when undertaken at the feasibility stage of a trial or fully integrated into the design of the RCT. 28–30 The CAVA pre-trial qualitative study was informed by the Glasgow feasibility study and incorporated into the design of the Phase III study from conception. 31 The primary goal of the study was to explore patient and staff views about participation in the CAVA trial, with the aim of maximising recruitment.
Focus groups with patients
Four main themes were identified from the analysis of the focus group discussions with the patients: (1) taking part in RCTs, (2) views on randomisation, (3) study documentation and (4) familiarity with the devices. Each is discussed in turn below.
Taking part in randomised controlled trials
All participants were in favour of patients being invited to participate in clinical research. Factors influencing this were both self-benefiting and altruistic, while including an appreciation of the serious nature of cancer, a desire to help future patients and an understanding that progress and current practice is based on the results of previous clinical trials, together with a hope of being randomised to a device not currently available outside the study. As one of the participants said:
Well I think it is, because had I not taken part in the first clinical trial, then I wouldn’t be still here. It’s as simple as that. I think that clinical trials are absolutely essential.
Female, Hickman, prior experience of a RCT
Similarly, another patient with no previous experience of RCTs said:
I think for future care as well, em, the experiences of patients now helps to form better care for people coming along, you know, kind of after us if you like.
Female, PICC, no prior experience of a RCT
Views on randomisation
All patients expressed an understanding of the process and reasons for randomisation. The majority either had participated in or would be prepared to participate in a RCT, acknowledging that this is an informed decision. Yet, despite professing to understand the concept of RCTs, participants’ comments indicated a degree of confusion over common trial terms (e.g. ‘trial’ was equated with ‘experiment’ and ‘treatment arm’ with ‘chemotherapy regime’). One patient considered that there should be a degree of patient choice in device allocation, assuming that this would not affect the numbers required to be allocated to each device:
I think it’s an informed choice, you know, If you are prepared to go ahead with the trial, it is made very clear to you, you know, it is randomised, you’ve got a chance of having, you know, one of the three and if you’re happy with that then, you know, yeh, go ahead.
Female, PICC, no prior experience of a RCT
Other factors influencing this decision were the severity of illness and whether or not participation would have an impact on other issues, such as treatment schedules:
If I chose the Hickman or the PORT, it would delay my next chemotherapy by a week. And my last chemotherapy finished on my birthday and I was determined to get to that last one on that day.
Female, PICC, no prior experience of a RCT
Certain patients, however, stated that they would not want to take part in a randomised trial of the three devices, as they wished to retain an element of choice in relation to line type:
I can understand why you want to do it, ehm . . . but eh, I know that given the choice I wouldn’t want to be randomly selected for a Hickman line or one that is implanted . . .
Male, PICC, no prior experience of a RCT
Study documentation
Comments on the language used in the PIS varied between ‘straightforward’ and ‘jargon free’ and, by contrast, ‘patronising’. Similarly, views on the length of the PIS also varied. Although several participants were not concerned about the length of the information sheet, considering that this would be what was expected, other views were that it was too long and would be overwhelming for patients. One participant, in particular, felt strongly that the design and layout of the PIS could be significantly improved. He considered that, initially, patients should be provided with a summary sheet, that is, the ‘technical specification’. The opportunity to find out further information should then be in the form of frequently asked questions. This could also be supplemented by links to a website (that could be accessed while attending for treatment). The longer document could be retained for reference.
Familiarity with the devices
Patients were not neutral in their stance towards the three devices. Some had received more than one device over the course of their treatment and this experience had coloured their judgement. For instance, a patient who previously had a PICC mentioned:
Well, probably not the PICC, I wouldn’t want that . . . That looped inside you.
Male, Hickman, prior experience of a RCT
Others were influenced by family members’ and friends’ experiences of the devices:
My wife had the Hickman line and eh, she . . . got a lot of infections, she eventually at one stage got septicaemia. She was on holiday at the time and had to be airlifted off one of the islands so that was a bit of a trauma.
Male, PICC, no prior experience of a RCT
As in every area of medical care, the level of patients’ knowledge and understanding of the subject varied widely. Many had conducted their own internet research on the three devices before deciding whether or not to enter the study. However, other patients admitted to being reluctant to know too much about the disease or treatment, preferring information to be filtered or summarised by health-care professionals.
Clinical staff interviews
Analysis of staff interviews identified five main themes: (1) getting staff on board, (2) clinical staff perceptions and preferences, (3) logistics of device insertion, (4) lack of experience and training, and (5) attitudes towards trial materials.
Getting staff on board
It was clear that the CAVA trial would necessitate a relatively complex patient pathway crossing several different specialties, including oncology, haematology and radiology, as well as having implications for primary care staff. The need to have all staff informed, involved and committed to the study was a recurrent theme with both nursing and medical staff across all specialties and centres; this was acknowledged to be a priority and essential for effective recruitment and was frequently described as getting colleagues ‘on board’:
I have no concern, I mean I am absolutely confident that the staff, the nursing in particular, will be very supportive, but we need everybody to be absolutely onboard.
Oncologist, Glasgow
The CAVA champion was perceived to be instrumental in informing, advising and motivating staff at each centre, providing a point of contact for clinical and nursing staff and raising awareness of the study. Several initiatives were reported to be under way across centres to ensure that staff were kept up to date with the progress of study set-up, including sending out slides and flyers to advise of the study and holding meetings to introduce the study to relevant colleagues and visiting key clinicians and departments to raise awareness. The qualitative study, particularly the interviewing of clinical staff, also appeared to play a significant part in raising awareness of the study and stimulating discussion and debate.
The heavy workload typical of all departments treating patients with cancer, the number of RCTs carried out in cancer centres and the adverse impact that this can have on trial recruitment was not an unexpected finding and the need to ensure that staff are fully informed and motivated with regard to the study was reiterated. In addition, it became apparent that the oncology and haematology departments were much more familiar with drug trials than with device trials, raising the risk of a device trial being overlooked. The concept of ‘trial fatigue’ and the potential for a non-drug trial to be less visible was a valuable prompt to raise considerations of adequate notification of the study, particularly to those carrying out ongoing care, ensuring complete data collection and complications reporting. Initiatives to raise visibility of the study included colour coding patient-held diaries and case record forms, along with adding proformas to the front of case notes in clinics:
We are very busy, that can be the over-riding sort of factor in saying: ‘OK, fine, I’ll just get on with the standard work rather than spend another half an hour or an hour explaining a trial that the patient may or may not take up’.
Oncologist, Glasgow
Clinical staff perceptions and preferences
A significant finding of the pre-trial qualitative study was a lack of equipoise regarding the three devices for particular patient groups. Haematologists in all but one centre expressed concern regarding the suitability of PORTs in their patient subgroup. In addition to issues of staff inexperience with the device, concern was expressed over the risks posed by the more invasive nature of the procedure to insert and remove the device in thrombocytopenic and neutropenic patients. Consequently, in some centres there was a lack of engagement by haematologists in the study (Manchester and Durham). In Leeds, haematologists intended to randomise between PICCs and Hickman-type devices only, whereas in Glasgow concern was also expressed regarding the use of PICCs, which were considered to present difficulties in transfusing blood products and other supportive therapies required for acute leukaemic and transplant participants:
We are actually not familiar with the PORT in adult haematology or oncology really . . . It would be a major practical problem to be inserting it into our haematology patients in terms of the insertion itself, there is a possibility that the bleeding risk and the infection risk could create more problems for my type of patients, because these are acute leukaemia patients . . .
Haematologist, Leeds
Newcastle was the only centre where the haematologists were completely on board, willing to randomise between all three devices and to introduce PORTs for chemotherapy delivery in haematology patients.
With the exception of the issues discussed above, most clinicians and nursing staff interviewed seemed to be proactive in the conduct of RCTs and ostensibly at ease with introducing the concepts of equipoise and randomisation to cancer patients:
This is our, if you like, ‘bread and butter’. We are involved, myself and my colleagues . . . are involved in trials in general; Phase III trials, and randomisation is the core part of it. So, we do discuss randomisation with patients on a regular basis and I don’t see this as a complex trial at all, I think it is quite straightforward.
Oncologist, Glasgow
Logistics of device insertion
The logistics of the line insertion appointments was also a recurrent theme across clinical staff interviews. Several participants thought that the element of randomisation between devices could create an additional level of complexity in the process of commencing a patient on chemotherapy. This was particularly relevant in oncology and haematology, where the time from referral for line insertion to treatment can be short and any perceived potential delays to chemotherapy delivery may impact negatively on patient recruitment. Services at each centre varied significantly, and there was acknowledgement that there might be a scarcity of slots for PORT insertion in some centres. In others, PORT insertion for chemotherapy was in addition to existing workload:
I think, obviously you know kind of access to interventional radiology might be the decider here because if there is a waiting list to put a PORT in, what have you, then your treatment targets have to be met either for cancer waiting time target or from a clinical need target type of thing.
Oncologist, Newcastle
The introduction of PORTs for chemotherapy delivery also created a requirement for PORT removal appointments, either because of complications or because removal was requested by patients at the end of treatment. In many centres, this was in addition to current workload and, therefore, raised the issue of accommodating this requirement. Responses to this varied across centres; in one, this was not considered an appropriate use of interventional theatre time and arrangements were made for a surgical referral for PORT removal when necessary, whereas, in others, interventional radiologists were prepared to undertake line removal for study patients as required.
Lack of experience and training
Experience of PORTs varied widely between centres. In several centres, PORTs were already in use for chemotherapy delivery; however, in others experience was limited and the need for additional training was apparent. Staff interviews in Glasgow highlighted a lack of confidence and experience with PORTs as a major area of concern. Despite a programme of training, the learning curve generated by the new device resulted in additional referrals to interventional radiology for assistance with maintenance issues and additional hospital visits by patients for procedures normally carried out by the primary care team. The qualitative interviews did highlight, however, the nursing staff’s enthusiasm and commitment to developing the necessary skills:
So that’s a big buzz, eh, you know when we problem solve in a successful way. And I know that once we get used to PORTs, we’ll love them.
Oncology nurse, Glasgow
Attitudes towards trial materials
Clinical staff in all centres raised a few minor issues with trial materials, specifically the PIS. It was felt that illustrations were not as clear as they could be and that the inclusion of necklaces was distracting and did not demonstrate best practice. Some inconsistencies with local practice were raised, specifically the reference to pumps that were not universally used across all centres. It was felt that greater clarity was required in two areas: (1) that the radiation dose was less than that of a radiograph and (2) that monthly questionnaires did not involve a further hospital visit. Finally, two clinicians raised the point that the references to randomisation by ‘computer’ and ‘tossing a coin’ were considered not to be concepts that patients feel comfortable with in relation to decision-making. A summary of these points was forwarded to the project manager and the Trial Management Group for further discussion at a forthcoming Trial Management Group meeting, and the PIS was modified as appropriate.
Discussion
This qualitative study was one of a few fully integrated, qualitative studies in large multicentre RCTs. This integration has allowed the analysis, feedback and interventions to take place prior to the study opening to recruitment. Our findings showed that patients had a vested interest in the device used for the delivery of their chemotherapy and demonstrated a willingness to be involved in research on CVADs. The focus groups also raised awareness of the need for clarity around the concept of the RCT and, in particular, the process of randomisation. In addition, we found that clear information and education on the three devices was required to ensure fully informed consent. The main themes identified from the pre-trial clinical staff interviews, namely logistics and complexity of service delivery, the need for education and training, and, finally, the lack of equipoise, had the potential to present significant barriers to recruitment. Consequently, the remit of the funded role of the CAVA champion (i.e. a dedicated member of the study team at each centre) was developed to encompass not only recruitment and randomisation, but also co-ordination and facilitation of device insertion appointments, communication and liaison across specialties, and education and dissemination of knowledge (see Report Supplementary Material 4). Centres with a champion recruited well and the ability to move a champion from a centre with high recruitment to support one that was performing less well proved invaluable. In response to the need for training, The Implanted PORT Training Day, a 1-day workshop on PORT insertion and maintenance using cadavers, was devised by the chief investigator and has been held annually for clinicians, nurses and industry representatives. Furthermore, effective training models and manikins, sourced through industry, have been shared between centres. Additional meetings between the chief investigator and the haematologists were undertaken to address issues around equipoise, leading to some emerging support from haematologists for the use of PORTs as the study progressed. The above findings and measures were presented to the CAVA trial principal investigators and champions at a pre-recruitment meeting, at the Christie Hospital in Manchester, to maximise dissemination and with the aim of having a positive impact on recruitment.
Chapter 4 Recruitment
Target recruitment
Overall, 1061 participants were recruited from 18 UK sites. At each site, clinicians could choose any of the four randomisations depending on the individual patient and the practice at their individual site: (1) Hickman versus PICCs versus PORTs; (2) PICCs versus Hickman; (3) PORTs versus Hickman; and (4) PORTs versus PICCs. Recruitment commenced on 8 November 2013 and was originally planned to take place over a period of 3 years. The initial target sample size was a total of 1500 patients (Table 2).
| Sample size assumption | Comparison | ||
|---|---|---|---|
| PICCs vs. Hickman | PORTs vs. Hickman | PORTs vs. PICCs | |
| Expected difference in complication rate | 10% (70% vs. 60%) | 20% (40% vs. 60%) | 20% (40% vs. 60%) |
| Power | 90% | 90% | 90% |
| Significance level | 2.5% | 5% | 5% |
| Target number of patients to recruit | 1000 | 250 | 250 |
An assessment on recruitment was prespecified at 18 months and was carried out in April 2015. If any of the comparisons had < 35% of the target sample size projected for this period, that comparison could be discontinued. The recruitment target was met for all of the comparisons; however, it was noted that the recruitment rate was below the predicted rate for the PICCs versus Hickman comparison and for the PORTs versus PICCs comparison. There were concerns that the target recruitment numbers could not be achieved within the remaining recruitment period. Furthermore, the results for the Glasgow feasibility study (PORTs vs. Hickman) became available at this time. The sample sizes and recruitment plan were re-estimated on the basis of these results and the observed recruitment (Table 3). This was approved by the Data Monitoring Committee (DMC) in November 2015. Recruitment to randomisation to comparisons involving PICCs was challenging, with below-expected recruitment rates. In January 2017, a variation in contract was granted by the NIHR HTA programme to allow an additional 15 months of recruitment (until the end of February 2018).
| Sample size assumption | Comparison | ||
|---|---|---|---|
| PICCs vs. Hickman | PORTs vs. Hickman | PORTs vs. PICCs | |
| Expected difference in complication rate | 10% (65% vs. 55%) | 15% (40% vs. 55%) | 15% (40% vs. 55%) |
| Power | 80% | 95% | 80% |
| Significance level | 2.5% | 5% | 5% |
| Target number of patients to recruit | 778 | 550 | 341 |
Recruitment by randomisation option
With the exception of one randomisation option, all randomisation options were open to recruitment until the end of the recruitment period. The randomisation option of PORTs versus Hickman was closed at the end of November 2015 given that it was clear that the required number of patients for the comparison would be achieved by the end of the study via the three-way comparison. The numbers of patients contributing to each of the comparisons of interest is shown in Table 4. Subsequently, these patients formed the population base for the three comparisons. The Hickman versus PORTs comparison had the highest recruitment; 42% of the recruited patients contributed to this comparison.
| Arm | Arm, n (%) | Total, n (%) | ||
|---|---|---|---|---|
| PORTs | PICCs | Hickman | ||
| Randomisation option | ||||
| PORTs vs. PICCs vs. Hickman | 53 (20.0) | 106 (40.0) | 106 (40.0) | 265 (100.0) |
| PICCs vs. Hickman | 0 (0.0) | 106 (50.0) | 106 (50.0) | 212 (100.0) |
| PORTs vs. Hickman | 200 (50.4) | 0 (0.0) | 197 (49.6) | 397 (100.0) |
| PORTs vs. PICCs | 94 (50.3) | 93 (49.7) | 0 (0.0) | 187 (100.0) |
| Total | 347 (32.7) | 305 (28.7) | 409 (38.5) | 1061 (100.0) |
| Comparison | ||||
| PICCs vs. Hickman | 0 (0.0) | 212 (50.0) | 212 (50.0) | 424 (100.0) |
| PORTs vs. Hickman | 253 (45.5) | 0 (0.0) | 303 (54.5) | 556 (100.0) |
| PORTs vs. PICCs | 147 (42.5) | 199 (57.5) | 0 (0.0) | 346 (100.0) |
| Total | 400 (30.2) | 411 (31.0) | 515 (38.8) | 1326 (100.0) |
Hickman versus peripherally inserted central catheters comparison: recruitment
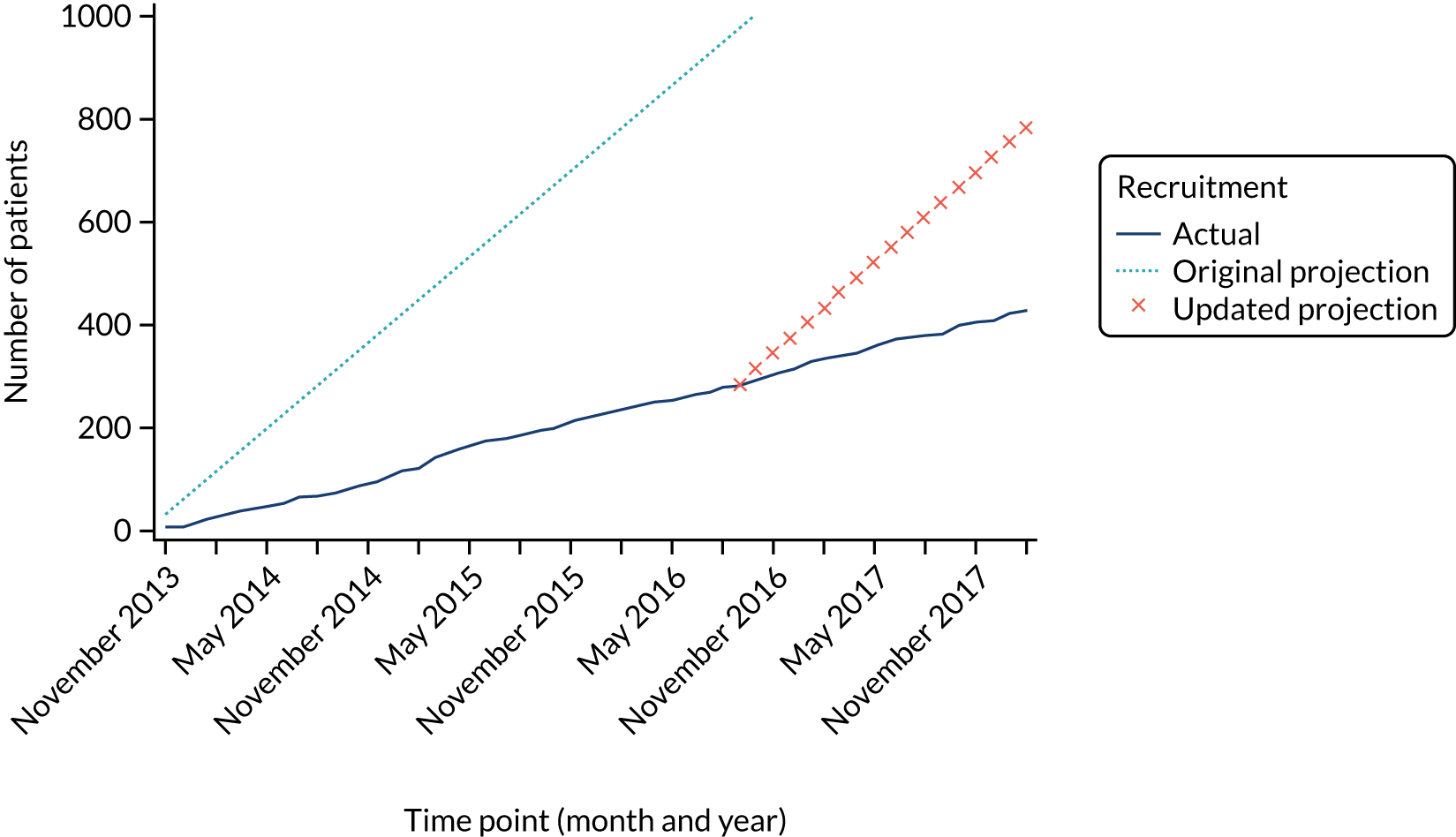
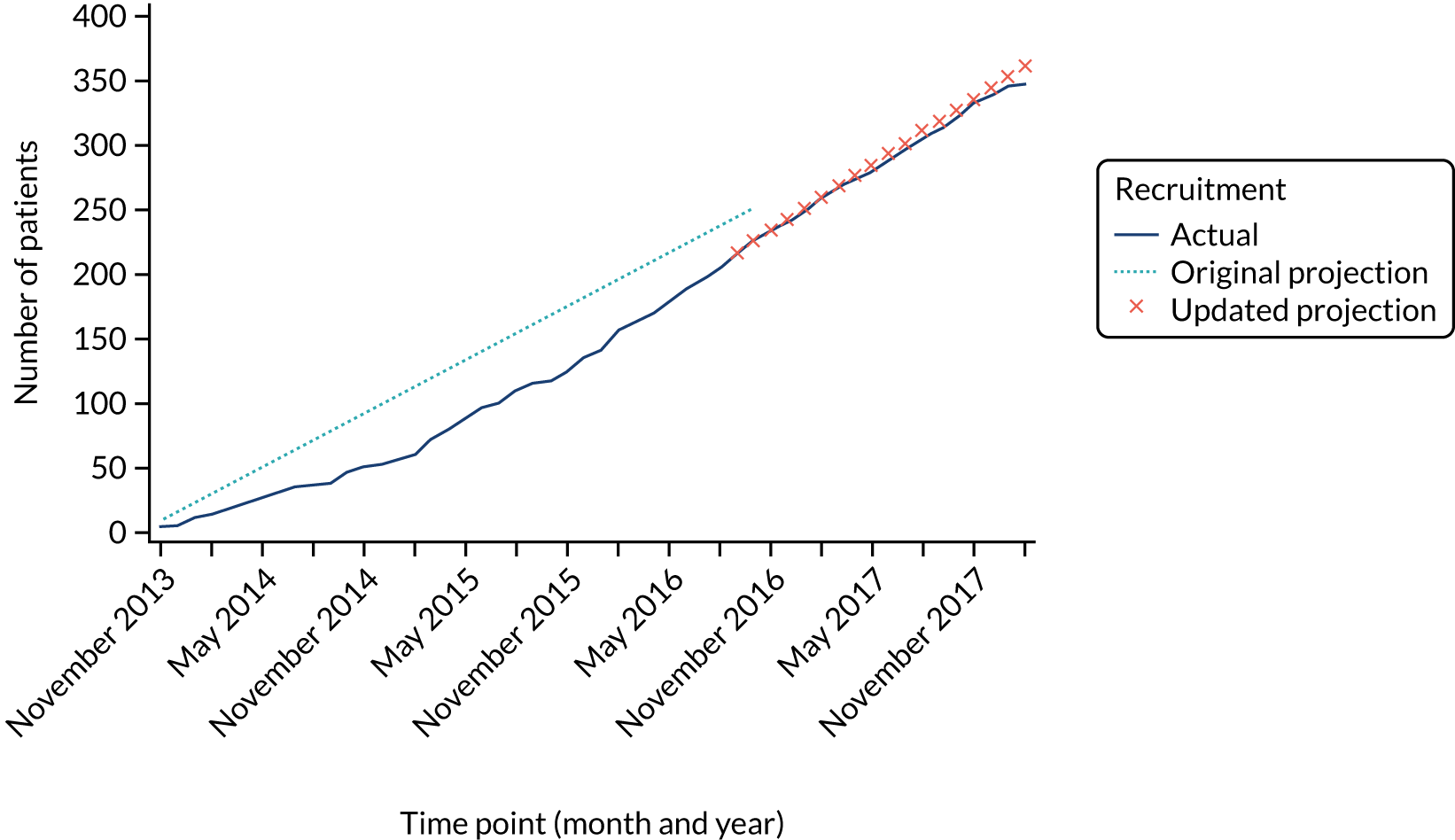
Recruitment to the Hickman versus PICCs comparison was challenging and was consistently below the target recruitment rate. The recruitment target was revised in April 2015, but overall recruitment remained below the target (Figure 3). In total, 424 patients from 15 sites contribute to the PICCs versus Hickman comparison (see Report Supplementary Material 5). This is notably less than the required 778 patients to ensure 80% power for our non-inferiority comparison. The resultant power of this comparison is 54%.
FIGURE 3.
Recruitment projections for the PICCs vs. Hickman comparison.
PORTs versus Hickman comparison: recruitment
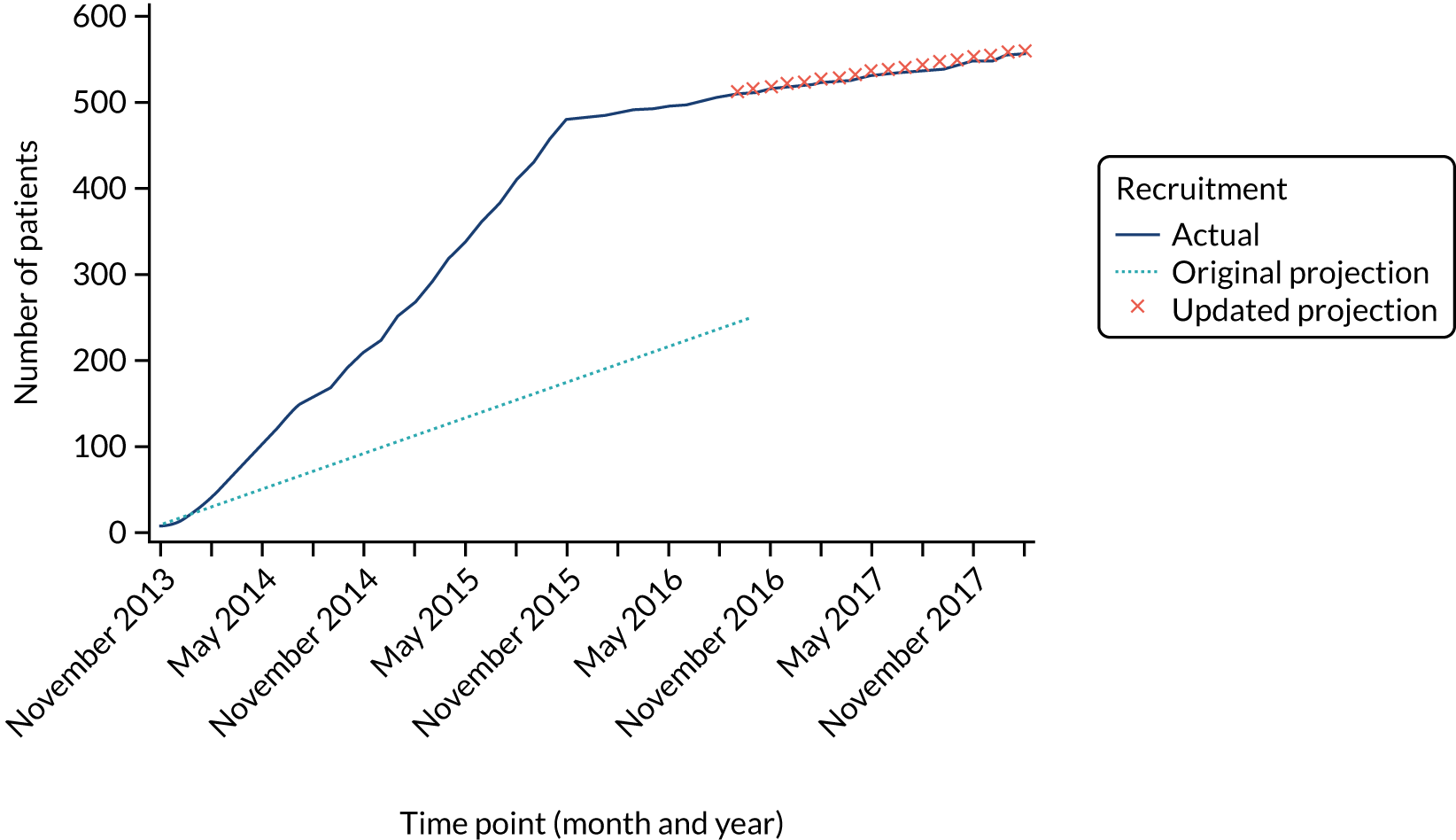
Recruitment for the PORTs versus Hickman comparison was above the target recruitment rate (Figure 4). As a consequence, the two-way randomisation option for this comparison closed at the end of November 2015. However, patients continued to accrue to this comparison via the three-way randomisation until recruitment ended in February 2018. A target of 550 patients was required and a total of 556 patients were recruited from 10 sites (see Report Supplementary Material 5), which results in a final power for this comparison of 95%.
FIGURE 4.
Recruitment projections for the PORTs vs. Hickman comparison.
PORTs versus peripherally inserted central catheters comparison: recruitment
The initial recruitment rate for the PORTs versus PICCs comparison was lower than the expected rate. The recruitment rate was reassessed in April 2015 and a revised target was produced. Subsequently, the trial recruited to target for this comparison (Figure 5). A total of 341 patients were required from 11 sites for the PORTs versus PICCs comparison to ensure 80% power for the final comparison (see Report Supplementary Material 5).
FIGURE 5.
Recruitment projections for the PORTs vs. PICCs comparison.
Discussion
The estimation of target recruitment to the CAVA trial was complex. This was primarily because of the necessity of offering four randomisation options to accommodate the clinical services that recruiting sites can offer, as not all sites were able to deliver all three devices. Our recruitment target was 1500 patients. However, at the pre-planned interim assessment, it was clear that recruiting for the PICCs versus Hickman comparison was extremely challenging, and, to some extent, this was also the case for recruitment to the PORTs versus PICCs comparison. By contrast, we were able to over-recruit to the PORTs versus Hickman comparison. This is primarily as a result of the Hickman-type device no longer being the preferred device during this period and PICCs becoming the dominant strategy in clinical practice. Following the interim assessment, we were able to recruit to the revised target recruitment for the PORTs versus Hickman and PORTs versus PICCs comparisons. However, we were not able to do so for the PICCs versus Hickman comparison. As a result, the final power of this comparison was substantially reduced from 80% to 54%. We did not feel it appropriate to attempt to ‘artificially’ boost the power by modifying the significance level or the type of comparison from non-inferiority. We were able to increase the power (to 64%) of this comparison by borrowing strength from indirect estimates of the difference between the PICCs and Hickman-type devices from the other comparisons using a NMA approach. Despite this being less than the intended power, this is still the largest number of patients ever randomised into a trial comparing these devices.
Chapter 5 Results for the peripherally inserted central catheters versus Hickman comparison
Study participants
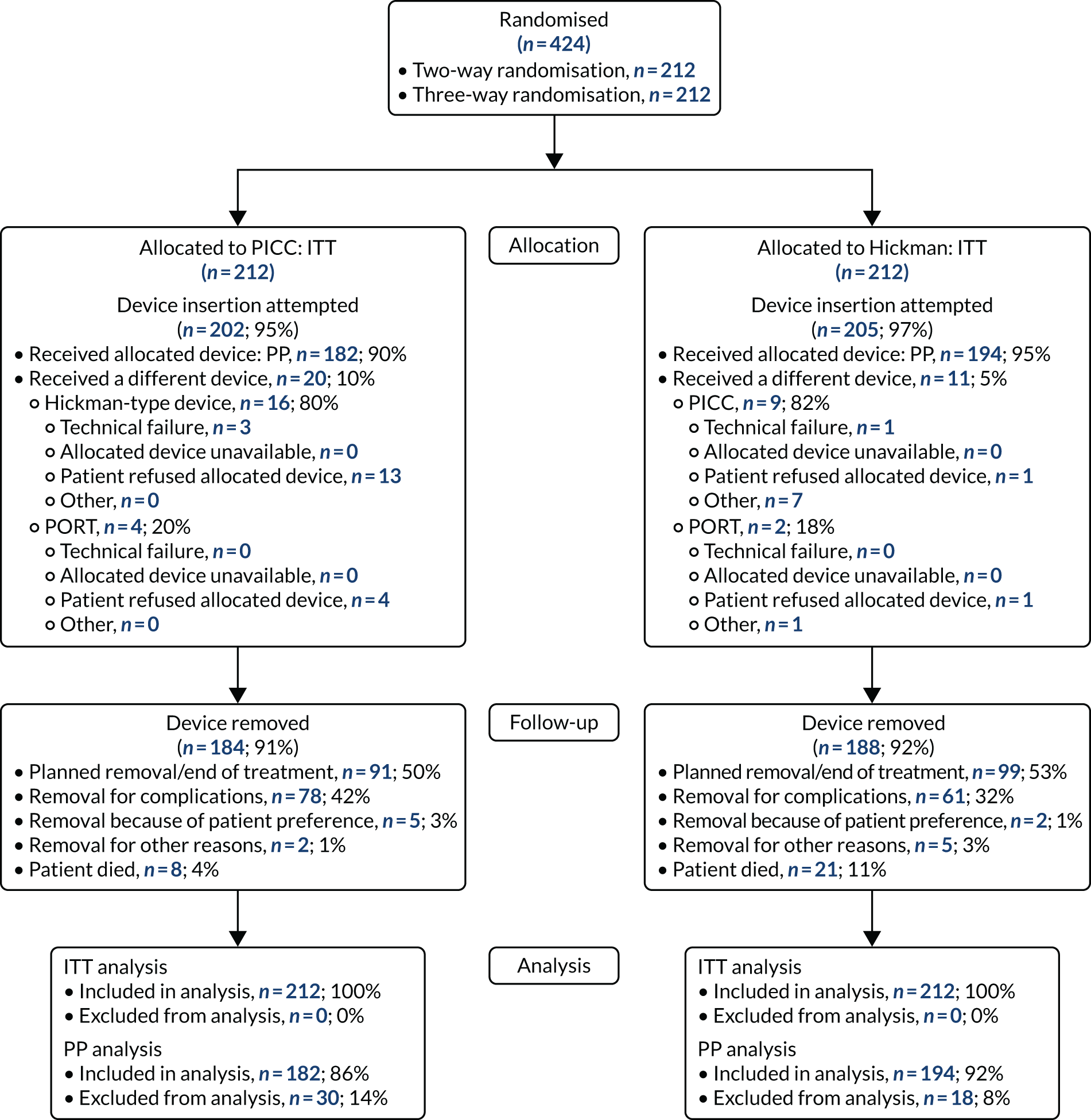
Study participants were recruited from both two-way (Hickman vs. PICCs) and three-way (Hickman vs. PICCs vs. PORTs) randomisations. In total, 424 participants were included in the PICCs versus Hickman comparison (Figure 6). The two-way and the three-way randomisations both contributed equal numbers of participants to each arm. Of all of the patients who entered the trial, 212 were randomised to receive PICCs and 212 were randomised to receive Hickman-type devices. All patients were included in the ITT analysis. Device insertion was attempted with 95% (n = 202) and 97% (n = 205) of patients randomised to PICCs and Hickman, respectively. Of these patients, 10% (n = 20) in the PICCs arm and 5% (n = 11) in the Hickman arm received a different device from the one to which they were randomised. The per-protocol population consisted only of patients who received the device to which they were randomised: 194 patients in the Hickman arm and 182 patients in the PICCs arm. All patients were followed up monthly until device removal, death or to the end of the 12-month follow-up.
FIGURE 6.
The PICCs vs. Hickman comparison CONSORT flow diagram. PP, per protocol.
Baseline characteristics
The baseline characteristics were well balanced between the two arms (Table 5). There was no difference between the two arms with respect to age, sex, BMI and ethnicity. The mean age of participants was 61 ± 12 years. Over half of the participants (53%) were male. The majority of patients were white (98%) and had a BMI between 20 and 30 mg/kg2 (68%).
The majority (87%) of patients were solid tumour patients with metastatic disease; there was no difference between the two arms in respect of solid tumour and haematological malignancy. Among those with solid tumours, 61% of the patients had colorectal primary tumours, which included a greater proportion of the Hickman arm than the PICCs arm. The proportion of patients with pancreatic cancer was greater in the PICCs arm than in the Hickman arm (15% vs. 8%, respectively). Among those with haematological malignancies, the proportions varied across acute myeloid leukaemia, high-grade non-Hodgkin’s lymphoma and Hodgkin’s disease. Across the two arms, the majority of the participants had planned outpatient treatments (92%) and did not have any prior venous access device (85%). The mean EQ-5D-3L health–utility score was 0.7 ± 0.2; there were no differences across the two arms. Similar patterns were observed with the mean EQ-5D health state score and the mean QLQ-C30 quality-of-life score.
| Characteristic | Arm | Total, n (%) | |
|---|---|---|---|
| PICCs | Hickman | ||
| Mean age (years) (SD, range) | 62 (11, 19–85) | 61 (12, 20–87) | 61 (12, 19–87) |
| Sex, n (%) | |||
| Female | 102 (48.1) | 96 (45.3) | 198 (46.7) |
| Male | 110 (51.9) | 116 (54.7) | 226 (53.3) |
| BMI (mg/kg2),a n (%) | |||
| < 20 | 10 (4.7) | 12 (5.7) | 22 (5.2) |
| 20 to < 30 | 145 (68.4) | 145 (68.4) | 290 (68.4) |
| 30 to < 40 | 51 (24.1) | 49 (23.1) | 100 (23.6) |
| ≥ 40 | 6 (2.8) | 6 (2.8) | 12 (2.8) |
| Ethnic origin, n (%) | |||
| White | 204 (96.2) | 210 (99.1) | 414 (97.6) |
| Asian | 3 (1.4) | 1 (0.5) | 4 (0.9) |
| Afro-Caribbean | 1 (0.5) | 1 (0.5) | 2 (0.5) |
| Missing | 4 (1.9) | 0 (0.0) | 4 (0.9) |
| Type of disease,a n (%) | |||
| Solid tumour | 185 (87.3) | 184 (86.8) | 369 (87.0) |
| Colorectal | 104 (56.2) | 120 (65.2) | 224 (60.7) |
| Breast | 21 (11.4) | 21 (11.4) | 42 (11.4) |
| Pancreatic | 27 (14.6) | 15 (8.2) | 42 (11.4) |
| Other (two missing in PICCs) | 31 (16.8) | 28 (15.2) | 59 (16.0) |
| Haematological malignancy, n (%) | 27 (12.7) | 28 (13.2) | 55 (13.0) |
| Acute myeloid leukaemia | 7 (25.9) | 11 (39.3) | 18 (32.7) |
| High-grade non-Hodgkin’s lymphoma | 5 (18.5) | 8 (28.6) | 13 (23.6) |
| Hodgkin’s disease | 4 (14.8) | 3 (10.7) | 7 (12.7) |
| Other (one missing in PICCs) | 10 (37.0) | 6 (21.4) | 16 (29.1) |
| Metastatic disease (solid tumour patients only), n (%) | |||
| Yes | 114 (61.6) | 108 (58.7) | 222 (60.2) |
| No | 68 (36.8) | 76 (41.3) | 144 (39.0) |
| Missing | 3 (1.6) | 0 (0.0) | 3 (0.8) |
| Patients being administered 5-fluorouracil | 137 (64.6) | 143 (67.5) | 280 (66.0) |
| Planned treatment mode,a n (%) | |||
| Inpatient | 17 (8.0) | 19 (9.0) | 36 (8.5) |
| Outpatient | 195 (92.0) | 193 (91.0) | 388 (91.5) |
| Device historya | |||
| No prior device, n (%) | 181 (85.4) | 180 (84.9) | 361 (85.1) |
| ≥ 1 previous device inserted > 3 months before study entry, n (%) | 26 (12.3) | 26 (12.3) | 52 (12.3) |
| ≥ 1 previous device inserted ≤ 3 months before study entry, n (%) | 5 (2.4) | 6 (2.8) | 11 (2.6) |
| Baseline quality-of-life scores, mean (SD, range) | |||
| EQ-5D index value | 0.7 (0.3, –0.3 to 1.0) | 0.8 (0.2, –0.2 to 1.0) | 0.7 (0.2, –0.3 to 1.0) |
| EQ-5D health state score | 70.6 (20.7, 10.0 to 100.0) | 70.3 (18.6, 10.0 to 100.0) | 70.4 (19.6, 10.0 to 100.0) |
| QLQ-C30 global health status | 65.3 (22.6, 0.0 to 100.0) | 68.0 (21.1, 0.0 to 100.0) | 66.7 (21.9, 0.0 to 100.0) |
Device details
The majority (74%) of PICCs came from two manufacturers [Vygon and CR Bard Inc. (Franklin Lakes, NJ, USA)], whereas Hickman-type devices were primarily from one manufacturer (Vygon) (see Appendix 1). In both arms, the majority of patients received single-lumen devices (86% in the PICCs arm and 73% in the Hickman arm). Antimicrobial coating was present in devices received by 11% of the patients in the PICCs arm and 3% of patients in the Hickman arm. Overall, 54% of PICCs were computerised tomography (CT) pump compatible, compared with 17% of Hickman-type devices. Valves were present in 36% of PICCs and 14% of Hickman-type devices.
Overall, the majority of the patients had their device fitted within 1 week of randomisation: median 7 days [interquartile range (IQR) 4–13 days] for patients in the PICCs arm and 6 days (IQR 3–11 days) for patients in the Hickman arm. The majority of PICCs were inserted by nurses (67%) in either a treatment room or a radiology department. Only 13% were placed by radiologists. In 76% of device fittings, the basilic or cephalic vein proximal to the elbow was used. Hickman-type devices were inserted by a variety of primary operators (radiologist 46%, nurse 23% and anaesthetist 20%) working in different environments using mainly the jugular vein (85%). Perioperative antibiotics were given to only 2% of patients in both arms. The majority of device insertions were completed within 30 minutes (55% PICCs vs. 72% Hickman), but, overall, the length of time for device insertion was longer with PICCs.
Complications and device removal
Approximately half of the patients experienced one or more complications: 52% of patients in the PICCs arm and 49% of patients in the Hickman arm. Of those patients who experienced complications, the majority had only one complication (Table 6). To conclude non-inferiority of PICCs at a significance level of 2.5%, the upper end of the 95% CI for the OR had to be < 1.519, which it was not for the primary analysis (OR 1.15, 95% CI 0.78 to 1.71) or for the NMA (OR 1.10, 95% CI 0.78 to 1.55) (detailed outputs are presented in Report Supplementary Material 6). Regardless of device allocation, complications tend to occur in the first 15 weeks of the follow-up period. The mean time to first complication was 25 ± 1.3 weeks in the Hickman arm, compared with 24 ± 1.5 weeks in the PICCs arm (Figure 7); the median time to first complication was 26 weeks (IQR 8 weeks–not estimable) in the Hickman arm, compared with 17 weeks (IQR 6–48 weeks) in the PICCs arm.
However, PICCs were in place for a shorter duration (median 113 days, 95% CI 106 to 123 days) than Hickman-type devices (median 158 days, 95% CI 140 to 175 days). The mean number of complications per catheter-week was 0.12 ± 0.02 in the PICCs arm, compared with 0.07 ± 0.01 in the Hickman arm. The proportion of patients reporting complications classed as Society of Interventional Radiology (SIR) classification C or above was 25% in the PICCs arm and 50% in the Hickman arm.
| Complication and device removal | Arm | |||
|---|---|---|---|---|
| PICCs | Hickman | |||
| Patients | Complications | Patients | Complications | |
| Number of complications, n (%) | ||||
| 0 | 102 (48.1) | 109 (51.4) | ||
| 1 | 72 (34.0) | 63 (29.7) | ||
| 2 | 25 (11.8) | 25 (11.8) | ||
| ≥ 3 | 13 (6.1) | 15 (7.1) | ||
| Total number of patients | 212 | 212 | ||
| Complication type | ||||
| Inability to aspirate blood, n (%) | 45 (21.2) | 66 (38.2) | 33 (15.6) | 43 (25.3) |
| Venous thrombosis, n (%) | 13 (6.1) | 14 (8.1) | 10 (4.7) | 10 (5.9) |
| Pulmonary embolism, n (%) | 6 (2.8) | 6 (3.5) | 4 (1.9) | 4 (2.4) |
| Infection, n (%) | 23 (10.8) | 27 (15.6) | 63 (29.7) | 78 (45.9) |
| Laboratory-confirmed bloodstream infection | 10 (4.7) | 11 (6.4) | 41 (19.3) | 43 (25.3) |
| Suspected catheter-related bloodstream infection | 10 (4.7) | 12 (6.9) | 18 (8.5) | 23 (13.5) |
| Exit site infection | 4 (1.9) | 4 (2.3) | 19 (9.0) | 22 (12.9) |
| Mechanical failure, n (%) | 31 (14.6) | 31 (17.9) | 7 (3.3) | 7 (4.1) |
| Other, n (%) | 23 (10.8) | 29 (16.8) | 16 (7.5) | 18 (10.6) |
| Total number of complications | 173 | 170 | ||
| Severe complications according to SIR classifications (% of total complications) | 25 | 50 | ||
| Rate of complications per patient | 0.82 | 0.80 | ||
| Mean number of complications per catheter-week ± SE | 0.12 ± 0.02 | 0.07 ± 0.01 | ||
| Planned removal/end of treatment, n (%) | 91 (49.5) | 99 (52.7) | ||
| Removal owing to complications, n (%) | 78 (42.4) | 61 (32.4) | ||
| Removal owing to patient preference, n (%) | 5 (2.7) | 2 (1.1) | ||
| Removal owing to other reasons, n (%) | 2 (1.1) | 5 (2.7) | ||
| Patient died, n (%) | 8 (4.3) | 21 (11.2) | ||
| Total devices removed, (n) | 184 | 188 | ||
Approximately half of the device removals in each arm were planned. Over one-third of the devices were removed because of a complication, a slightly higher proportion in the PICCs arm (n = 78, 42%) than in the Hickman arm (n = 61, 32%). Overall, eight deaths were recorded in the PICCs arm and 21 in the Hickman arm. Chemotherapy interruption owing to complications was recorded in 9% (n = 18) of patients in the PICCs arm and 13% (n = 27) of patients in the Hickman arm. The mean number of days of chemotherapy interruption owing to complications was smaller in the PICCs arm (0.91 ± 3.99 days) than in the Hickman arm (2.24 ± 8.73 days).
FIGURE 7.
Time to first complication for the PICCs vs. Hickman comparison.
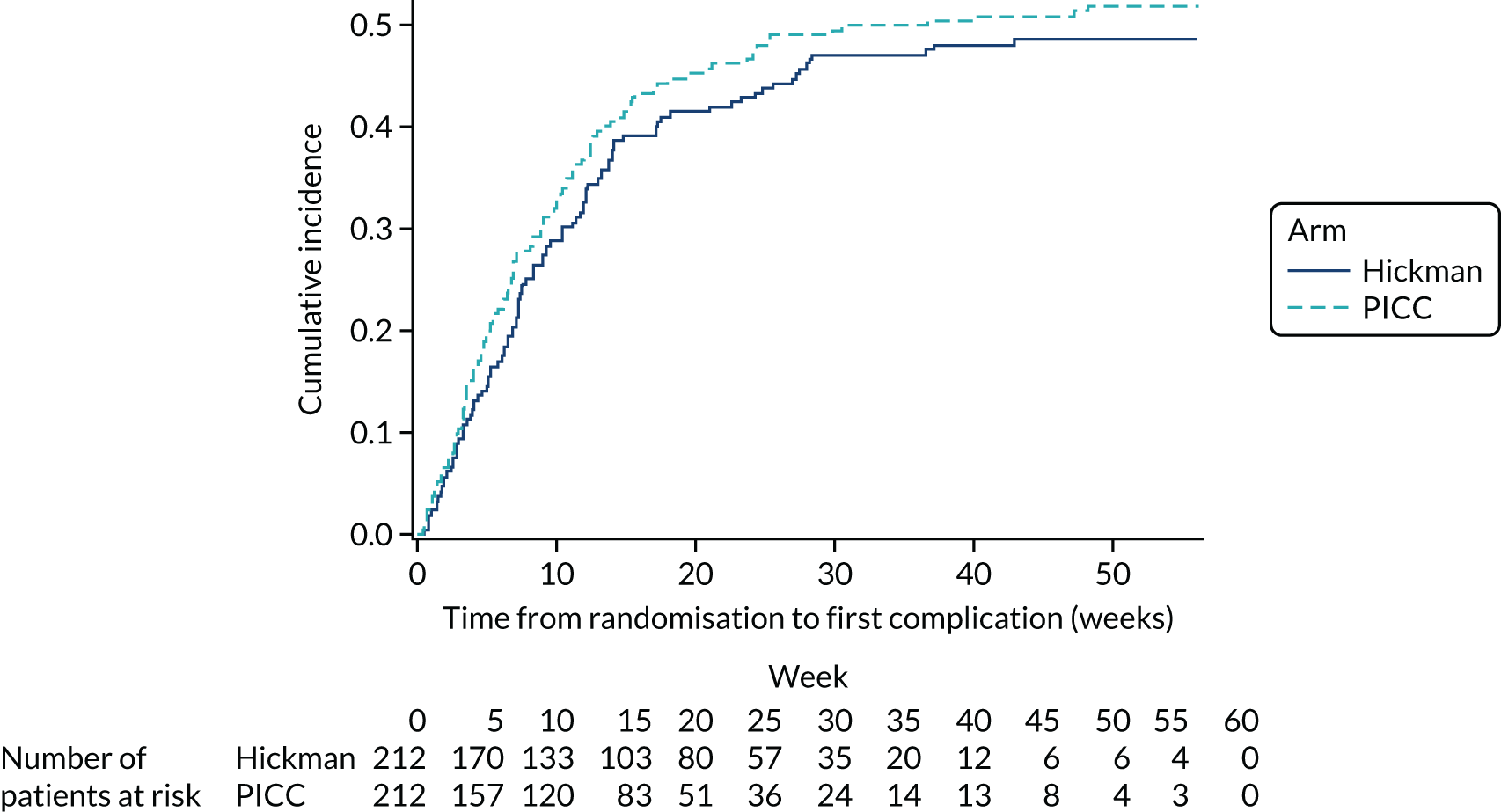
Specific complications and device removal
Inability to aspirate blood
The proportion of patients who experienced one or more occurrences of an inability to aspirate blood was larger in the PICCs arm (n = 45; 21%) than in the Hickman arm (n = 33; 16%). A simple flush was commonly performed in approximately one-third of all ‘actions undertaken’ to manage the complications. Other procedures included thrombolytic locks (21% of procedures undertaken) and infusion, further imaging and manipulation of the device in situ. Only one patient in each arm had a complication classified as SIR category C or above. As a consequence of an inability to aspirate blood, 36% (n = 16) of the patients with this complication in the PICCs arm had their device removed, compared with 12% of these patients (n = 4) in the Hickman arm. Chemotherapy was prematurely stopped in three patients (7%) in the PICCs arm.
Laboratory-confirmed infection
Laboratory-confirmed bloodstream infection was recorded in 5% (n = 10) of patients in the PICCs arm, compared with 19% (n = 41) in the Hickman arm. In both arms, these infections were primarily identified by culture of recognised pathogens on blood samples and were treated with antibiotics. Five patients in the PICCs arm and 34 patients in the Hickman arm had a complication classified as SIR category C or above; one death was recorded in the Hickman arm. As a consequence of laboratory-confirmed bloodstream infection, 60% (n = 6) of the patients with this complication in the PICCs arm had their device removed, compared with 66% (n = 27) of those patients in the Hickman arm. Chemotherapy was prematurely stopped in six of the Hickman arm patients.
Suspected catheter-related bloodstream infection
Suspected catheter-related bloodstream infection was recorded in 5% (n = 10) of patients in the PICCs arm, compared with 9% (n = 18) of patients in the Hickman arm. In both arms, these infections primarily presented as fever and were treated with antibiotics. Eight patients in the PICCs arm, compared with 14 patients in the Hickman arm, had a complication classified as SIR category C or above. As a consequence of suspected catheter-related bloodstream infection, 20% (n = 2) of the patients with this complication in the PICCs arm had their device removed, compared with 61% (n = 11) of those patients in the Hickman arm. Chemotherapy was prematurely stopped in two of the PICCs arm patients.
Exit site infection
Exit site infection was recorded in 2% (n = 4) of patients in the PICCs arm, compared with 9% (n = 19) of patients in the Hickman arm. All infections were confirmed and antibiotics were given in the majority of cases. One patient in the PICCs arm and four patients in the Hickman arm, had a complication classified as SIR category C or above. As a consequence of exit site infection, no patients in the PICCs arm and three patients in the Hickman arm had their device removed. Only one patient in the Hickman arm had their chemotherapy prematurely stopped.
The mean time to first infection (any infection) was similar in both arms: 33 ± 1.2 weeks in the Hickman arm and 33 ± 8 weeks in the PICCs arm. The median time to first infection (any infection) cannot be estimated because less than half of the patients experienced an event.
Venous thromboembolism and pulmonary embolism
Venous thrombosis was uncommon: 6% (n = 13) of patients in the PICCs arm and 5% (n = 10) of patients in the Hickman arm experienced venous thrombosis, of whom one patient in the PICCs arm reported two instances of venous thrombosis. Non-inferiority of PICCs in terms of venous thrombosis could not be concluded at a significance level of 2.5% for the logistic regression analysis (OR 1.30, 95% CI 0.55 to 3.08). The most frequent site for venous thrombosis in the PICCs arm was upper extremity veins (71%), whereas in the Hickman arm it was the internal jugular (40%) and subclavian (40%) veins. Patients were primarily treated with anticoagulants. Six patients in the PICCs arm and three patients in the Hickman arm had a complication classified as SIR category C or above. As a consequence, six patients in the PICCs arm and five patients in the Hickman arm had their device removed. Chemotherapy was prematurely stopped in no patients in the PICCS arm and in two patients in the Hickman arm.
Pulmonary embolism (PE) was rare, occurring in 3% (n = 6) of patients in the PICCs arm and 2% (n = 4) of patients in the Hickman arm. All patients received anticoagulants. As a consequence, none of the PICCs arm patients and one of the Hickman arm patients had their device removed. Chemotherapy was prematurely stopped in two of the PICCs arm patients and one of the Hickman arm patients.
Mechanical failure and other complications
Mechanical failure was reported in 15% (n = 31) of patients in the PICCs arm, compared with 3% (n = 7) of patients in the Hickman arm. Of these patients, six in the PICCs arm and three in the Hickman arm reported line fracture, 10 patients in the PICCs arm and two in the Hickman arm reported ‘device fallen out’, and 15 patients in the PICCs arm and two patients in the Hickman arm reported line migration that required intervention. Four patients in the PICCs arm and two patients in the Hickman arm had a complication classified as SIR category C or above. As a consequence, 28 patients in the PICCs arm and seven in the Hickman arm had their device removed. Chemotherapy was prematurely stopped in one patient in the Hickman arm.
Other complications were reported for 11% (n = 23) of patients in the PICCs arm, compared with 8% (n = 16) of patients in the Hickman arm. Two patients in the PICCs arm and three patients in the Hickman arm had a complication classified as SIR category C or above. As a consequence, 12 patients in the PICCs arm and five patients in the Hickman arm had their device removed. Chemotherapy was prematurely stopped in one Hickman patient.
Quality of life
Quality-of-life data were collected monthly, until device removal or withdrawal, for up to 12 months. However, the proportion of missing data increased substantially with follow-up time (see Appendix 2 and Report Supplementary Material 7).
Health-related quality of life
EuroQoL-5 Dimensions index values were calculated by patient and time point according to standard conventions (a value of 1 indicates no problems on any of the five dimensions and a value of –0.594 indicates maximum problems on all five dimensions). Health state scores were reported on a scale of 0–100, where 0 is the worst imaginable health state and 100 the best. Overall, there was a trend of small improvement over time in both the index values and the health state score (Figure 8). During the first 6 months, the study arms were similar. However, from around 7 months onwards, both the index values and the health state scores tended to be marginally higher and less variable in the Hickman arm than in the PICCs arm (see Figure 8). For each patient, the AUC described by the EQ-5D index values and health state scores were standardised by the time spent in the study, leading to values on the same scale as the original data. The standardised AUCs were then adjusted by subtracting the baseline value. The mean adjusted standardised AUC for the original EQ-5D index value data was –0.009 ± 0.01 for the PICCs arm and –0.007 ± 0.01 for the Hickman arm. The pooled estimate over the imputed data resulted in mean values of –0.095 ± 0.43 for the PICCs arm and –0.129 ± 0.03 for the Hickman arm. These scores were compared using the Mann–Whitney U-test, and a two-sided p-value was found not to be statistically significant at the 5% level. The mean adjusted standardised AUC for the original EQ-5D health state score data was –13.93 ± 2.42 for the PICCs arm and –8.79 ± 1.99 for the Hickman arm. The pooled estimate over the imputed data resulted in mean values of –8.57 ± 1.99 for the PICCs arm and –7.71 ± 1.73 for the Hickman arm. The difference in scores was not statistically significant at the 5% level (see Appendix 3).
FIGURE 8.
The PICCs vs. Hickman comparison for (a) EuroQoL-5 Dimensions index value and (b) health states. The error bars represent ± 1 standard error.
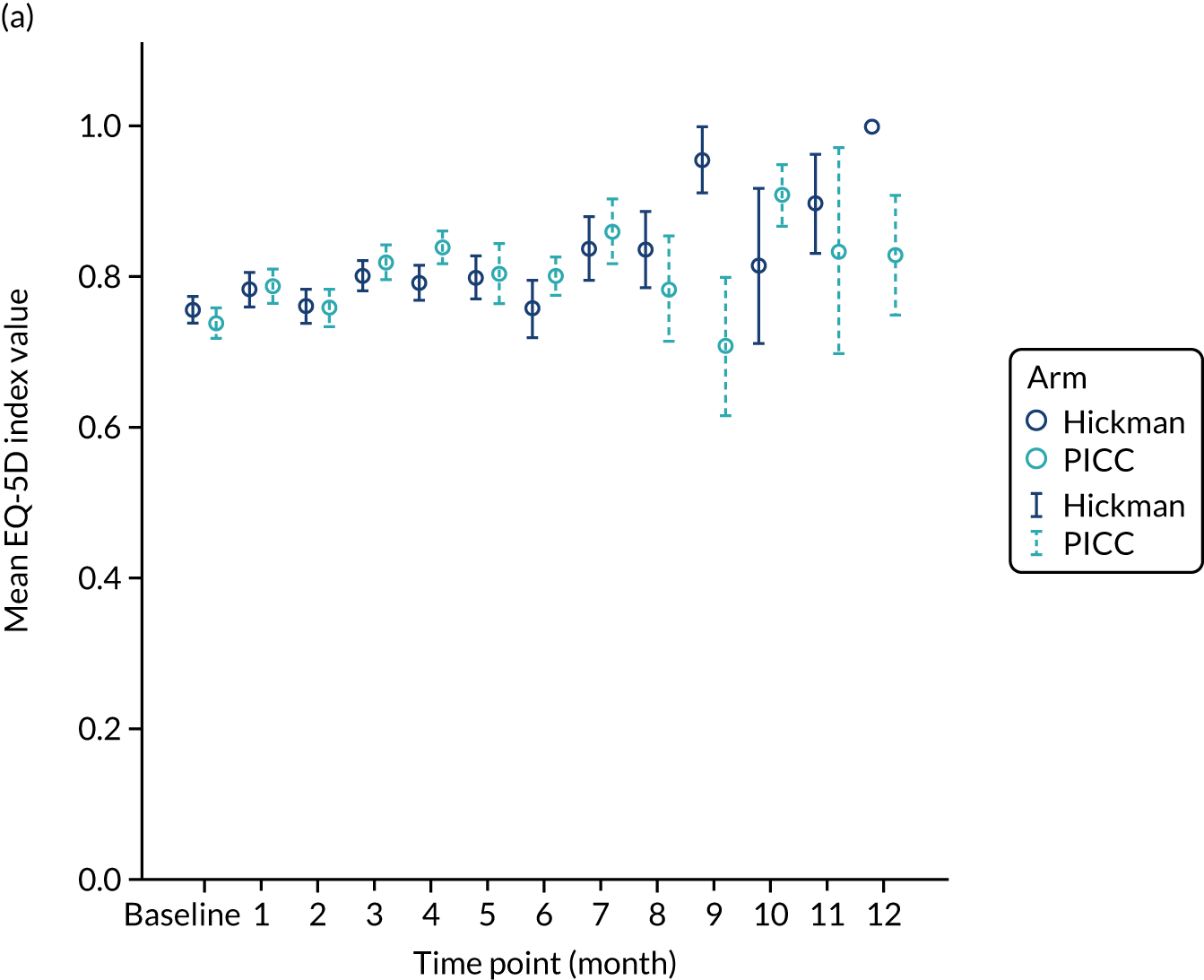
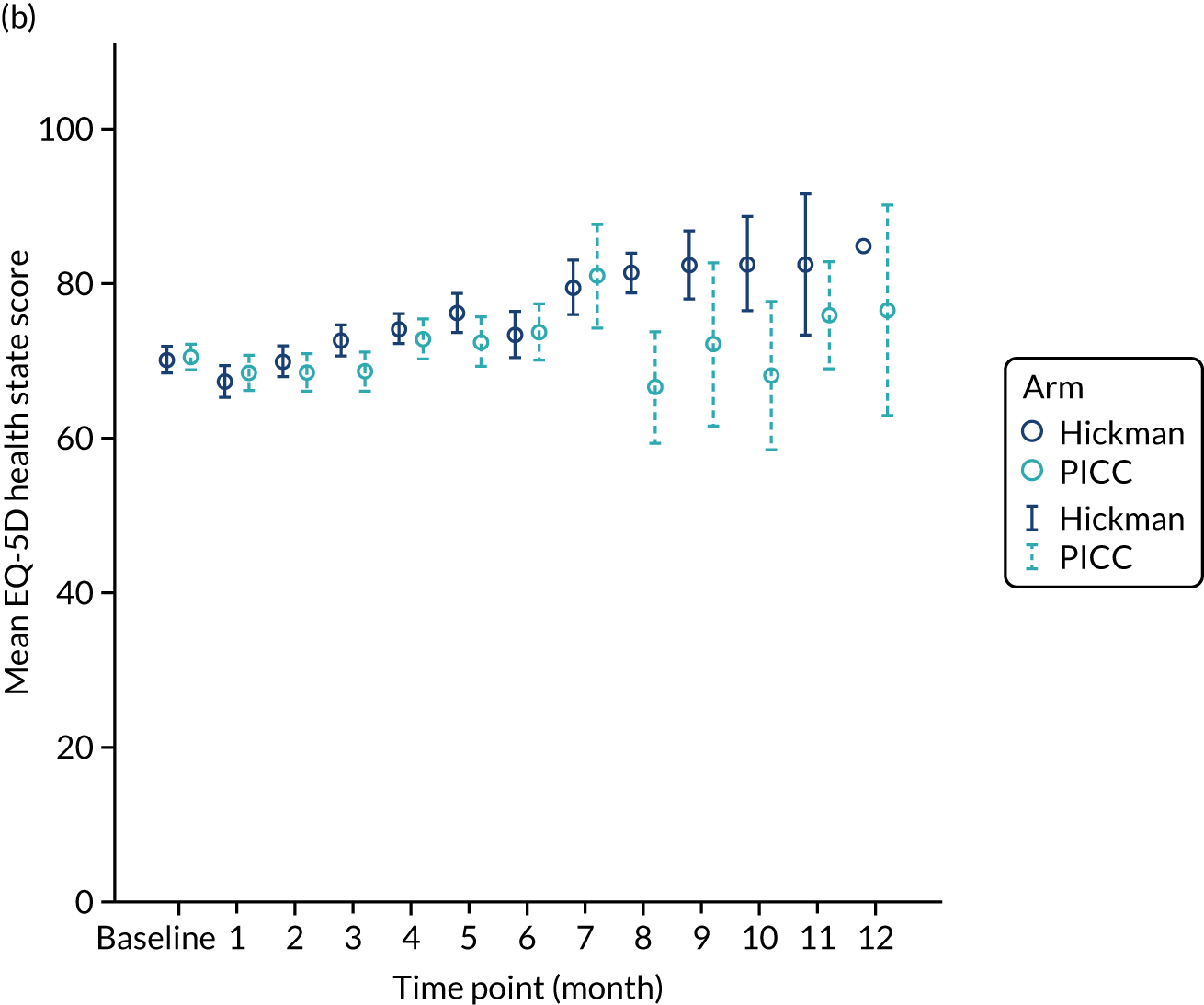
Quality of life: Quality of Life Questionnaire C30
The QLQ-C30 scale scores were calculated in accordance with standard EORTC conventions, resulting in values between 0 and 100; a high score represents a higher response level. During the first 6 months, the study arms were similar (Figure 9); however, from month 8, there was a tendency for apparently more markedly different patterns between the arms. The results were consistent at the 5% significance level for both the unadjusted and the adjusted approaches for all of the scales; there were no statistically significant differences between arms (see Appendix 4).
FIGURE 9.
The EORTC QLQ-C30 scores for the PICCs vs. Hickman comparison.
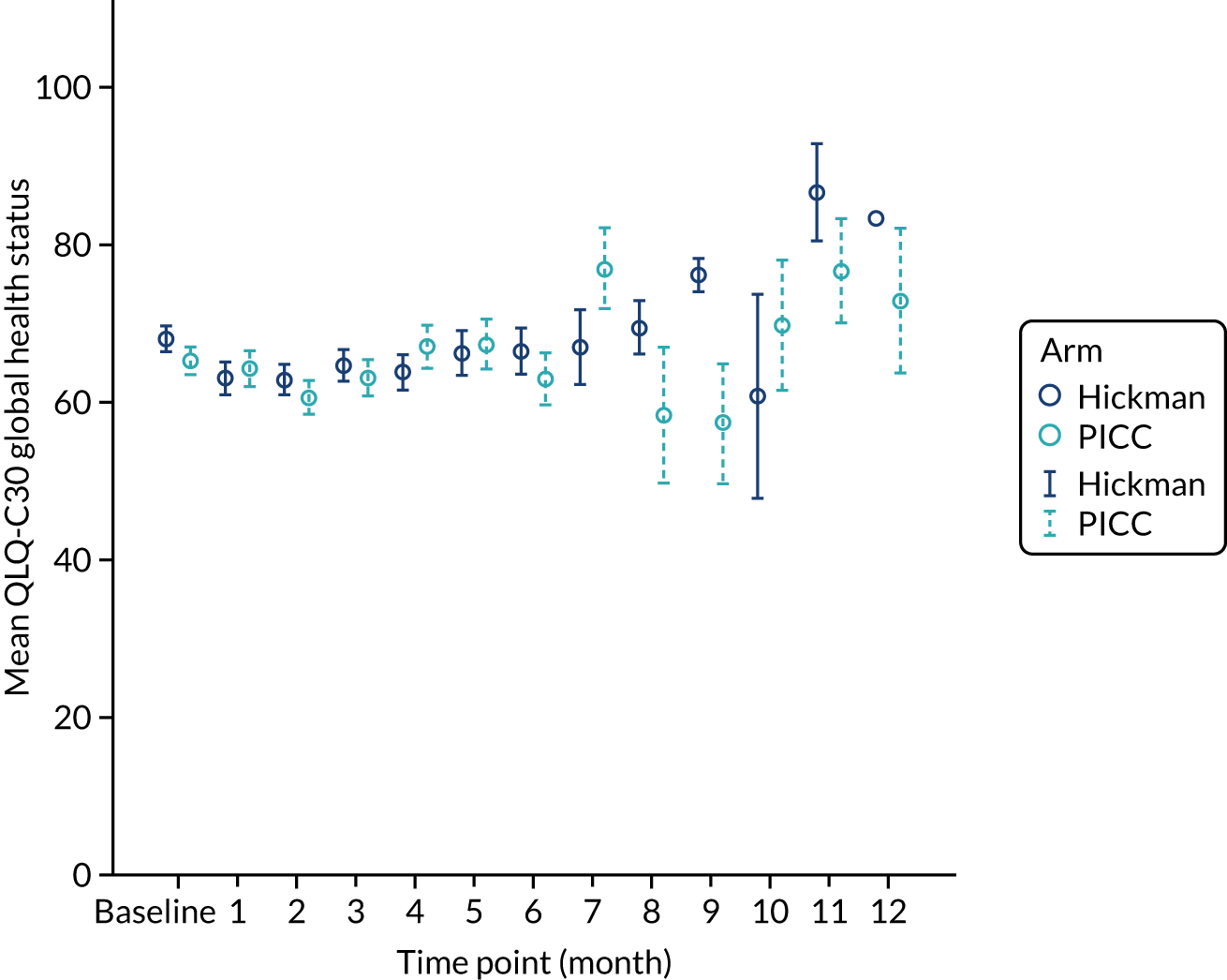
Venous access device-related quality of life
The worst score reported during the study for each question was established and compared across the two arms for the 403 ITT patients with a device fitted (excluding those patients with a different device fitted because of technical insertion failure) (Figure 10). There were statistically significant differences via the unadjusted p-values at the 5% level for hygiene and hobbies; however, these were not statistically significant when adjusted for the other questions. Both of these results appear to favour the Hickman over the PICC (see Appendix 5).
FIGURE 10.
Device-specific questionnaire for the PICCs vs. Hickman comparison.
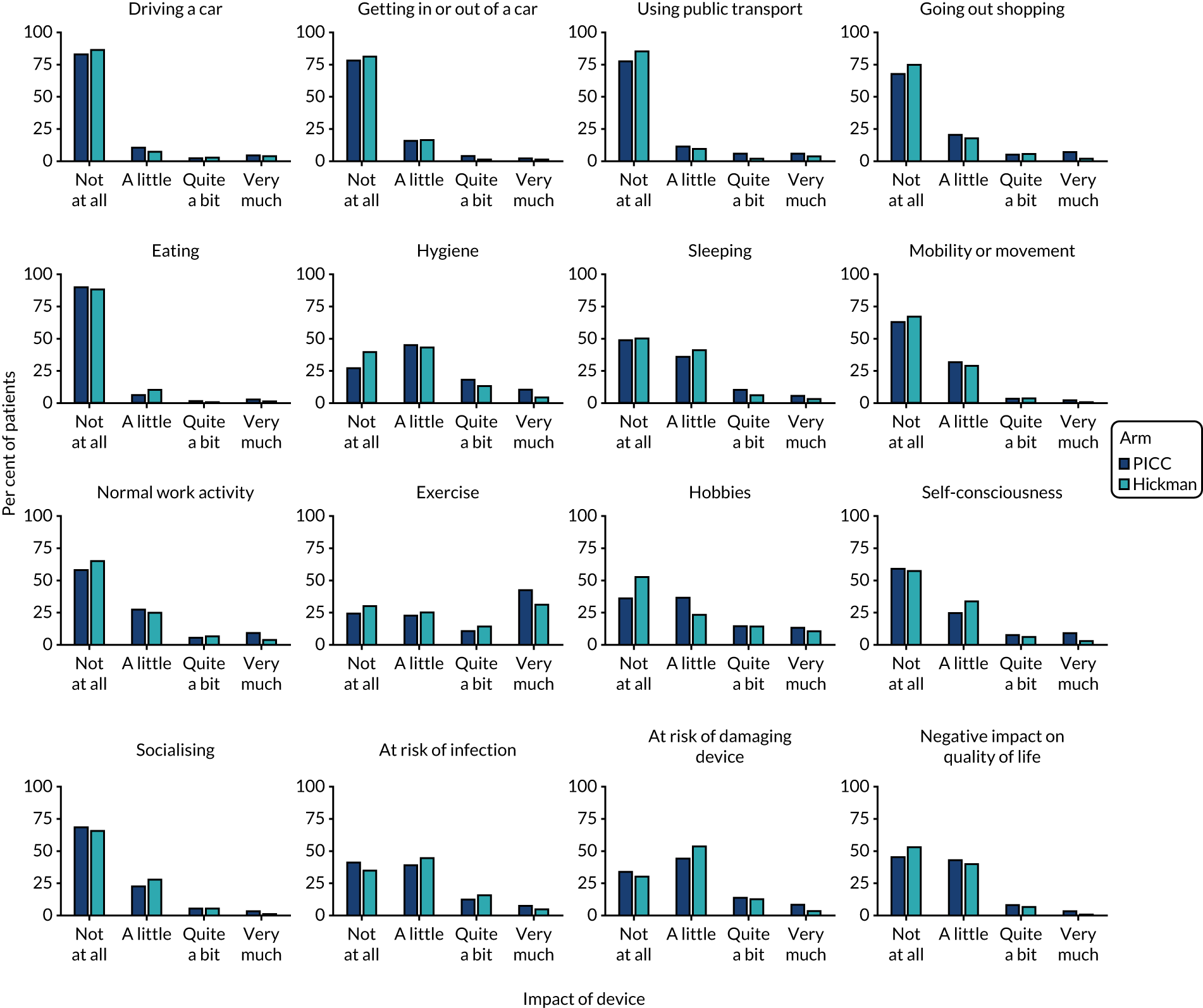
Discussion
The objective of this component of the CAVA trial was to determine whether or not PICCs were non-inferior to Hickman with regard to complication rates. We observed similar overall complication rates across the two arms (52% with PICCs and 49% with Hickman). However, we were unable to confirm non-inferiority (OR 1.15, 95% CI 0.78 to 1.71 based on primary analysis); this was probably due to the comparison being underpowered. We did not present the results of the per-protocol sensitivity analysis as the conclusions drawn were the same as those of the ITT analysis.
Overall, PICCs were in situ for a shorter duration than Hickman-type devices (difference in median of 25 days). When this was taken into account, PICCs were found to be associated with a higher rate of complications per catheter-week (0.12 ± 0.02) than Hickman-type devices (0.07 ± 0.01). Device removal as a result of complications was common in both arms (42% PICCs and 32% Hickman). This may in part be because of the ease of removal of both devices. PICCs were associated with higher rates of an inability to aspirate blood (21% PICCs vs. 16% Hickman) and mechanical failure (11% PICCs vs. 8% Hickman) than Hickman. By contrast, Hickman-type devices were associated with higher rates of all types of infections than PICCs (11.3% PICCs vs. 37% Hickman). Furthermore, we found the highest infection rates in the haematological cancers: 30% in the PICCs arm and 57% in the Hickman arm (data not shown). Although these cancers made up only 13% of the ITT population, this could suggest that PICCs may be a better choice than Hickman from a perspective of averting infection complications. However, a large-scale study is needed to confirm this hypothesis. Similar rates of venous thrombosis, pulmonary embolism and other complications were reported. Venous thrombosis was uncommon (6% in the PICCs arm compared with 5% in the Hickman arm). This is in contrast to a large systematic review of > 29,000 patients that showed a 2.5-fold increased risk of a venous thrombosis with a PICC compared with a centrally inserted catheter. 32 In the CAVA trial, we imaged only patients with symptomatic venous thrombosis and there was no attempt to detect incidental thrombosis, which is thought to be fairly common; this may, in part, explain the discrepancies.
We found no significant difference in the quality of life, as measured by the EQ-5D or the EORTC QLQ-C30. It is likely that these instruments are not sensitive to the devices but are dominated by the underlying malignancy and its management. The device-specific quality-of-life instrument did show a significant benefit in favour of Hickman for 2 of the 16 questions, hygiene and hobbies, but this significance was lost when adjusted for multiple testing.
One yellow card report was submitted to the Medicines and Healthcare products Regulatory Agency for a Hickman patient who had possible endocarditis related to thrombus at the distal end of the tunnelled central venous line. This was considered to be a serious related and unexpected event, which was treated with antibiotics and line removal.
At present, there is very limited evidence in the literature comparing these two devices, and no RCTs have been performed in a cancer setting. There is only one small RCT (n = 102) comparing these two devices in the administration of total parenteral nutrition. 33 The primary outcome, a complication necessitating device removal, was higher in the PICCs arm (56%) than in the Hickman arm (33%). Venous thrombosis was more common with a PICC, although infection rates were similar.
The key limitation of this comparison was the low statistical power owing to under-recruitment. There was heterogeneity in the choice and management of these devices across centres. The choice of device inserted and the management of the devices was left to each centre (e.g. catheters with single or multiple lumens of different diameters and construction; antibiotic coated or not; different external anchorage devices for anchorage; different flushing regimens; dressings; cleaning strategy). However, we understood that most centres followed evidence-based practice in infection control (epic) guidelines at that time. 6
In conclusion, the CAVA trial has shown that the complication rates when using PICCs are similar to those when using Hickman. Infection was the dominant complication in the Hickman arm, and mechanical complications were the dominant complications in the PICCs arm. Although infection is usually regarded as one of the most important complications afflicting intravenous catheters, mechanical problems are also important and can cause delay in the administration of chemotherapy and may mandate replacement of the device. However, we were unable to draw firm conclusions on whether or not PICCs are non-inferior to Hickman.
Chapter 6 Results for the PORTs versus Hickman comparison
Study participants
Study participants were recruited from both two-way (Hickman vs. PORT) and three-way (Hickman vs. PICC vs. PORT) randomisations; the two-way randomisation contributed 71% of the participants. In total, 556 participants were included in the PORTs versus Hickman comparison (Figure 11). Of all of the patients who entered the trial, 253 were randomised to receive PORTs and 303 were randomised to receive Hickman. All participants were included in the ITT analysis. Device insertion was attempted with 97% (n = 245) and 93% (n = 283) of all patients randomised to PORTs and Hickman, respectively. The per-protocol population consisted only of participants who received the device to which they were randomised: n = 239 in the PORTs arm and n = 277 in the Hickman arm. All patients were followed up monthly until device removal, death or to the end of the 12-month follow-up.
FIGURE 11.
The PORTs vs. Hickman comparison CONSORT flow diagram. PP, per protocol.
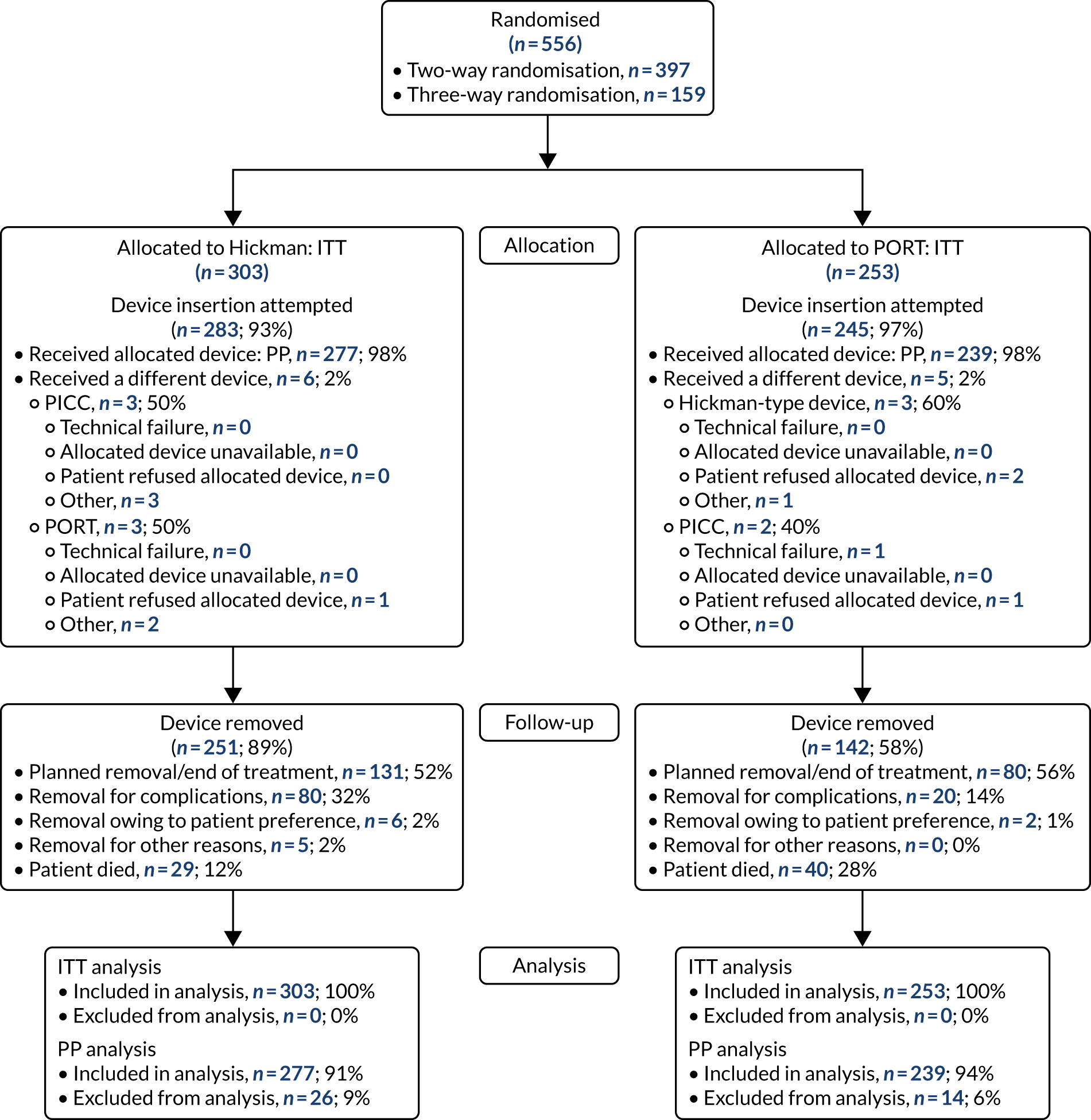
Baseline characteristics
The baseline characteristics were well balanced between the two arms (Table 7). There was no difference between the two arms in respect of age, sex, BMI and ethnicity. The mean age of participants was 59 years [standard deviation (SD) 13 years]. Over half of the participants (53%) were males. The majority of patients were white (97%) and had a BMI of between 20 kg/m2 and 30 kg/m2 (68%).
The majority (93%) of patients were solid tumour patients with metastatic disease; there was no difference between the two arms in respect of solid tumour and haematological malignancy. Among those with solid tumours, the majority of participants had colorectal primary tumours (59%). Among those participants with haematological malignancies, a larger proportion of those in the Hickman arm had acute myeloid leukaemia (57%) than in the PORTs arm (28%), whereas the proportions with high-grade non-Hodgkin’s lymphoma and Hodgkin’s disease were higher in the PORTs arm (both 22%) than in the Hickman arm (both 13%). However, the numbers were very small. Across the two arms, the majority of the participants had planned outpatient treatments (91%) and did not have any prior venous access device fitted (79%). The mean EQ-5D-3L health–utility score was 0.7 (SD 0.3); there were no differences across the two arms. Similar patterns were observed with the mean EQ-5D health state score and the mean QLQ-C30 quality-of-life score.
| Characteristic | Arm | Total | |
|---|---|---|---|
| PORTs | Hickman | ||
| Mean age (years) (SD, range) | 59 (13, 19–86) | 60 (13, 20–87) | 59 (13, 19–87) |
| Sex, n (%) | |||
| Female | 112 (44.3) | 151 (49.8) | 263 (47.3) |
| Male | 141 (55.7) | 152 (50.2) | 293 (52.7) |
| BMI (kg/m2),a n (%) | |||
| < 20 | 13 (5.1) | 16 (5.3) | 29 (5.2) |
| 20 to < 30 | 171 (67.6) | 209 (69.0) | 380 (68.3) |
| 30 to < 40 | 61 (24.1) | 70 (23.1) | 131 (23.6) |
| ≥ 40 | 8 (3.2) | 8 (2.6) | 16 (2.9) |
| Ethnic origin, n (%) | |||
| White | 246 (97.2) | 293 (96.7) | 539 (96.9) |
| Asian | 4 (1.6) | 1 (0.3) | 5 (0.9) |
| South-East Asian | 1 (0.4) | 0 (0.0) | 1 (0.2) |
| Afro-Caribbean | 2 (0.8) | 3 (1.0) | 5 (0.9) |
| Other | 0 (0.0) | 1 (0.3) | 1 (0.2) |
| Missing | 0 (0.0) | 5 (1.7) | 5 (0.9) |
| Type of disease,a n (%) | |||
| Solid tumour | 235 (92.9) | 280 (92.4) | 515 (92.6) |
| Colorectal | 138 (58.7) | 168 (60.0) | 306 (59.4) |
| Breast | 27 (11.5) | 42 (15.0) | 69 (13.4) |
| Pancreatic | 16 (6.8) | 18 (6.4) | 34 (6.6) |
| Other (four missing in Hickman arm) | 54 (23.0) | 48 (17.1) | 102 (19.8) |
| Haematological malignancy | 18 (7.1) | 23 (7.6) | 41 (7.4) |
| Acute myeloid leukaemia | 5 (27.8) | 13 (56.5) | 18 (43.9) |
| High-grade non-Hodgkin’s lymphoma | 4 (22.2) | 3 (13.0) | 7 (17.1) |
| Hodgkin’s disease | 4 (22.2) | 3 (13.0) | 7 (17.1) |
| Other (one missing in Hickman arm) | 5 (27.8) | 3 (13.0) | 8 (19.5) |
| Metastatic disease (solid tumour patients only), n (%) | |||
| Yes | 156 (66.4) | 191 (68.2) | 347 (67.4) |
| No | 79 (33.6) | 85 (30.4) | 164 (31.8) |
| Missing | 0 (0.0) | 4 (1.4) | 4 (0.8) |
| Patients being administered 5-fluorouracil | 179 (70.8) | 198 (65.3) | 377 (67.8) |
| Planned treatment mode,a n (%) | |||
| Inpatient | 25 (9.9) | 26 (8.6) | 51 (9.2) |
| Outpatient | 228 (90.1) | 277 (91.4) | 505 (90.8) |
| Device history,a n (%) | |||
| No prior device | 198 (78.3) | 239 (78.9) | 437 (78.6) |
| ≥ 1 previous device inserted > 3 months before study entry | 46 (18.2) | 53 (17.5) | 99 (17.8) |
| ≥ 1 previous device inserted ≤ 3 months before study entry | 9 (3.6) | 11 (3.6) | 20 (3.6) |
| Baseline quality-of-life scores, mean (SD, range) | |||
| EQ-5D index value | 0.7 (0.3, –0.1 to 1.0) | 0.7 (0.3, –0.3 to 1.0) | 0.7 (0.3, –0.3 to 1.0) |
| EQ-5D health state score | 71.0 (21.0, 0.0 to 100.0) | 69.4 (19.8, 0.0 to 100.0) | 70.2 (20.3, 0.0 to 100.0) |
| QLQ-C30 global health status | 66.0 (21.9, 0.0 to 100.0) | 64.2 (22.1, 0.0 to 100.0) | 65.0 (22.0, 0.0 to 100.0) |
Device details
The PORTs came from a variety of companies, whereas the majority of Hickman-type devices (93%) were from one manufacturer (Vygon) (see Appendix 5). The majority of Hickman-type devices (84%) were of silicone construction, whereas half of the PORTs were silicone and the other half polyurethane constructions. In both arms, the majority of devices had one lumen (PORTs 94% and Hickman 85%). Antimicrobial coating was not used in any PORTs and was used in only 1% of Hickman-type devices. Overall, 89% of PORTs were CT pump compatible, compared with 14% of Hickman-type devices. Valves were present on 1% of PORTs and on 4% of Hickman-type devices.
The majority of the patients in both arms had their device fitted within 1 week of randomisation: median 6 days (IQR 4–12 days) for the PORTs arm and 6 days (IQR 3–10 days) for the Hickman arm. All devices were placed using local anaesthesia; a minority of patients also had conscious sedation. PORTs were inserted by a variety of primary operators (radiologist 59%, nurse 24% and anaesthetist 11%) working mainly in the radiology department (70%) using the jugular vein (90%). Similarly, Hickman-type devices were inserted by a variety of primary operators (radiologist 48%, nurse 35% and anaesthetist 13%) working in different environments using mainly the jugular vein (88%). Perioperative antibiotics were given to 14% of patients in the PORTs arm compared with 1% of patients in the Hickman arm. The majority of PORTs insertions took between 30 and 60 minutes (77%), whereas Hickman insertions took < 30 minutes (80%).
Complications and device removal
The proportion of patients experiencing one or more complications was considerably lower in the PORTs arm than in the Hickman arm (i.e. 29% vs. 43%) (Table 8). This difference was found to be statistically significant in both the primary comparison (OR 0.54, 95% CI 0.37 to 0.77) and the NMA (OR 0.59, 95% CI 0.42 to 0.82). (Detailed outputs are presented in Report Supplementary Material 6.) Regardless of device allocation, complications tend to occur in the first 3 months of the follow-up period (Figure 12). The mean time to first complication was 41 ± 1.4 weeks in the patients of the PORTs arm, compared with 27 ± 1.1 weeks in the Hickman arm. The median time to first complication was not estimable in the patients of the PORTs arm, whereas the median time to first complication was 39 weeks (IQR 9 weeks–not estimable) in the patients of the Hickman arm.
However, PORTs were in place for much longer (median 367 days, 95% CI 324 to 393 days) than Hickman-type devices (median 165 days, 95% CI 149 to 177 days). The mean number of complications per catheter-week was 0.02 ± 0.00 in the PORTs arm, compared with 0.06 ± 0.01 in the Hickman arm. Overall, major complications (as defined by SIR classifications) were observed in 47% of patients in the Hickman arm and 45% of patients in the PORTs arm.
| Complication and device removal | Arm | |||
|---|---|---|---|---|
| PORTs | Hickman | |||
| Patients | Complications | Patients | Complications | |
| Number of complications, n (%) | ||||
| 0 | 180 (71.1) | 172 (56.8) | ||
| 1 | 44 (17.4) | 87 (28.7) | ||
| 2 | 15 (5.9) | 31 (10.2) | ||
| ≥ 3 | 14 (5.5) | 13 (4.3) | ||
| Total number of patients | 253 | 303 | ||
| Complication type | ||||
| Inability to aspirate blood, n (%) | 38 (15.0) | 63 (47.7) | 42 (13.9) | 60 (30.0) |
| Venous thrombosis, n (%) | 3 (1.2) | 3 (2.3) | 7 (2.3) | 7 (3.5) |
| Pulmonary embolism, n (%) | 3 (1.2) | 3 (2.3) | 4 (1.3) | 4 (2.0) |
| Infection, n (%) | 36 (14.2) | 47 (35.6) | 77 (25.4) | 102 (51.0) |
| Laboratory-confirmed bloodstream infection | 14 (5.5) | 16 (12.1) | 49 (16.2) | 54 (27.0) |
| Suspected catheter-related bloodstream infection | 19 (7.5) | 21 (15.9) | 15 (5.0) | 16 (8.0) |
| Exit site infection | 10 (4.0) | 10 (7.6) | 26 (8.6) | 32 (16.0) |
| Mechanical failure | 2 (0.8) | 2 (1.5) | 9 (3.0) | 9 (4.5) |
| Other | 14 (5.5) | 14 (10.6) | 17 (5.6) | 18 (9.0) |
| Total number of complications (n) | 132 | 200 | ||
| Severe complications according to SIR classifications (% of total complications) | 34 | 26 | ||
| Rate of complications per patient | 0.52 | 0.66 | ||
| Mean number of complications per catheter-week ± SE | 0.02 ± 0.00 | 0.06 ± 0.01 | ||
| Planned removal/end of treatment, n (%) | 80 (56.3) | 131 (52.2) | ||
| Removal owing to complications, n (%) | 20 (14.1) | 80 (31.9) | ||
| Removal owing to patient preference, n (%) | 2 (1.4) | 6 (2.4) | ||
| Removal owing to other reasons, n (%) | 0 (0.0) | 5 (2.0) | ||
| Patient died, n (%) | 40 (28.2) | 29 (11.6) | ||
| Total devices removed (n) | 142 | 251 | ||
Over half of the device removals in each arm were planned. Around one-quarter of the devices were removed because of a complication, a lower proportion in the PORTs arm (15%) than in the Hickman arm (32%). Overall, 40 deaths were recorded in the PORTs arm and 29 deaths were recorded in the Hickman arm. Chemotherapy interruption owing to complications was recorded in 7% (n = 17) of PORTs patients and 11% (n = 32) of Hickman patients. The mean total number of days of chemotherapy interruption owing to any complication was smaller in the PORTs arm (0.58 ± 2.82 days) than in the Hickman arm (1.5 ± 5.58 days).
FIGURE 12.
Time to first complication for the PORTs vs. Hickman comparison.
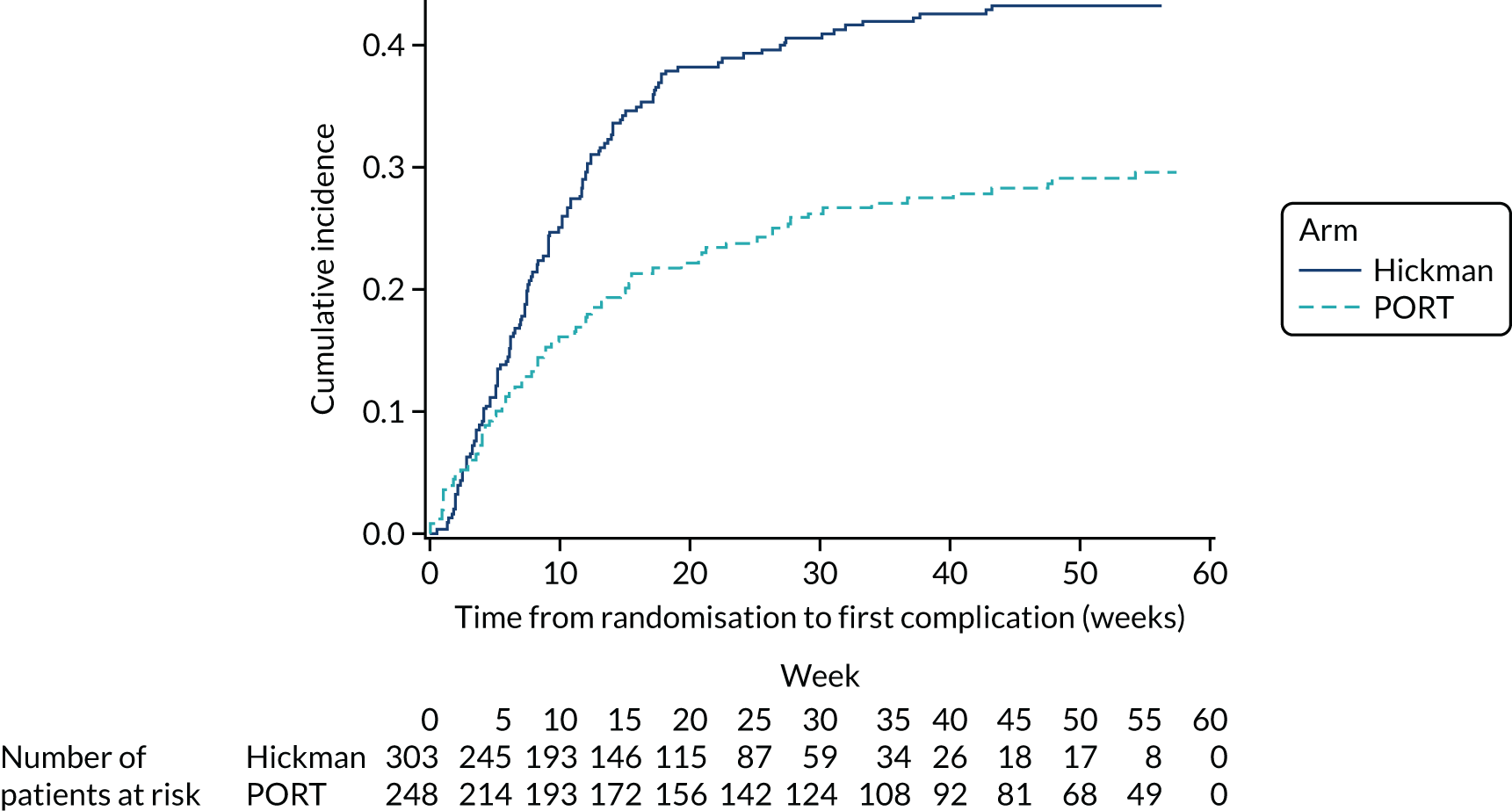
Specific complications and device removal
Inability to aspirate blood
Similar proportions of patients in the PORTs arm (n = 38; 15%) and in the Hickman arm (n = 42; 14%) experienced one or more occurrence of an inability to aspirate blood; this accounts for 63 complications in the PORTs arm and 60 complications in the Hickman arm. A simple flush was commonly performed to manage the complications. Other procedures included thrombolytic locks and infusion, further imaging and manipulation of the device in situ. Six patients in the PORTs arm and five patients in the Hickman arm had a complication classified as SIR category C or above. As a consequence of an inability to aspirate blood, 5% (n = 2) of the PORTs patients with this complication and 29% (n = 12) of the Hickman patients had their device removed.
Laboratory-confirmed infection
Laboratory-confirmed bloodstream infection was recorded in 6% (n = 14) of patients in the PORTs arm, compared with 16% (n = 49) of patients in the Hickman arm. In both arms, these infections were primarily identified by culture of recognised pathogens on blood samples and were treated with antibiotics. Twelve patients in the PORTs arm and 39 patients in the Hickman arm had a complication classified as SIR category C or above. As a consequence of laboratory-confirmed bloodstream infection, 50% (n = 7) of the patients with this complication in the PORTs arm had their device removed compared with 76% (n = 37) in the Hickman arm. Chemotherapy was prematurely stopped in two of the PICCs arm patients and seven of the Hickman arm patients.
Suspected catheter-related bloodstream infection
Suspected catheter-related bloodstream infection was recorded in 8% (n = 19) of patients in the PORTs arm, compared with 5% (n = 15) of patients in the Hickman arm. In both arms, these primarily presented as fever and were treated with antibiotics. Fourteen patients in the PORTs arm compared with nine patients in the Hickman arm had a complication classified as SIR category C or above. As a consequence of suspected catheter-related bloodstream infection, 37% (n = 7) of the patients with this complication in the PORTs arm had their device removed compared with 60% (n = 9) in the Hickman arm. One patient in each of the two arms had chemotherapy prematurely stopped.
Exit site infection
Exit site infection was recorded in 4% (n = 10) of patients in the PORTs arm, compared with 9% (n = 26) of patients in the Hickman arm. All infections were confirmed and antibiotics were given in the majority of cases. Three patients in the PORTs arm and nine patients in the Hickman arm had a complication classified as SIR category C or above. As a consequence of exit site infection, no patients in the PORTs arm and six patients in the Hickman arm had their device removed. One patient in the PORTs arm and two in the Hickman arm had their chemotherapy prematurely stopped.
The mean time to first infection (any infection) was 42 ± 0.9 weeks for patients in the PORTs arm, compared with 37 ± 1.2 weeks for patients in the Hickman arm. The median time to first infection (any infection) could not be estimated because less than half of the patients experienced an event.
Venous thromboembolism
Venous thrombosis was uncommon: 1% (n = 3) of patients in the PORTs arm and 2% (n = 7) of patients in the Hickman arm. No statistically significant difference was detected using logistic regression. Patients were primarily treated with anticoagulants. Thromboses occurred in three patients in the PORTs arm, occurring in the superior vena cava, upper extremity and internal jugular veins. In the seven patients in the Hickman arm in whom thromboses occurred, the majority of cases occurred in the internal jugular or subclavian vein. One patient in the PORTs arm and two patients in the Hickman arm had a complication classified as SIR category C or above. As a consequence, one patient in the PORTs arm and three patients in the Hickman arm had their device removed. No patients had their chemotherapy prematurely stopped.
Pulmonary embolism was rare and occurred in 1% (n = 3) of patients in the PORTs arm and 1% (n = 4) of patients in the Hickman arm. All but one patient received anticoagulants. None of the patients had their device removed as a consequence of PE. Chemotherapy was prematurely stopped in one of the Hickman patients.
Mechanical failure and other complications
Mechanical failure was reported in 1% (n = 2) of patients in the PORTs arm, compared with 3% (n = 9) of patients in the Hickman arm. Both patients in the PORTs arm reported exposure of the PORT through the wound. In the Hickman arm, two patients reported line fracture, one reported exposure to fixation cuff, two reported ‘device fallen out’ and four reported line migration that required intervention. Two patients in the Hickman arm had a complication classified as SIR category C or above. As a consequence, all patients in the Hickman arm who had mechanical failure had their device removed. Chemotherapy was prematurely stopped in one patient in the Hickman arm.
Other complications were reported in 6% of patients in the PORTs arm (n = 14) and in 6% of patients in the Hickman arm (n = 17). Two patients in the PORTs arm and one patient in the Hickman arm had a complication classified as SIR category C or above. As a consequence, five patients in each arm had their device removed. Chemotherapy was prematurely stopped in two PORTs patients.
Quality of life
Quality-of-life data were collected monthly, until device removal or withdrawal, up to a period of 12 months. However, the proportion of missing data increased substantially with the follow-up time (see Appendix 6 and Report Supplementary Material 7).
Health-related quality of life
Overall, there was little or no change in HRQoL over time. During the first 6 months, the study arms were similar. However, from month 7 onwards the mean index values and health state scores tended to be marginally higher and less variable for patients in the PORTs arm; however, at almost all time points, the standard error bars overlap (Figure 13). The mean adjusted standardised AUC for the original EQ-5D index values was –0.003 ± 0.02 in the PORTs arm and –0.010 ± 0.01 in the Hickman arm. The pooled estimate over the imputed data resulting in mean values was –0.157 ± 0.03 for the PORTs arm and –0.139 ± 0.02 for the Hickman arm. These scores were not statistically significantly different at the 5% level. The mean adjusted standardised AUC for the original EQ-5D health state score data were –9.72 ± 1.47 for patients in the PORTs arm and –10.90 ± 1.67 for patients in the Hickman arm. The pooled estimate over the imputed data resulting in mean values were –11.39 ± 1.73 for the PORTs arm and –8.74 ± 1.53 for the Hickman arm. The difference in these scores was not statistically significant at the 5% level (see Appendix 8).
FIGURE 13.
The PORTs vs. Hickman comparison for (a) EQ-5D index value; and (b) health state.
Quality of life: Quality of Life Questionnaire C30
The mean QLQ-C30 global health status was very similar across the two arms at all time points, with the exception of month 11 (Figure 14). The analyses of the AUC data over the duration of device insertion showed no statistically significant difference between the devices for this scale. There were statistically significant differences at the 5% significance level in terms of cognitive functioning (unadjusted p = 0.040 in favour of Hickman) and appetite loss (unadjusted p = 0.007 in favour of PORTs), as assessed by the QLQ-C30; however, after adjusting for multiple comparisons, these differences were no longer of statistical significance (see Appendix 7).
FIGURE 14.
The EORTC QLQ-C30 scores for the PORTs vs. Hickman comparison. The error bars represent ± 1 standard error.
Venous access device-related quality of life
The worst score reported during the study for each question was established and compared across the two arms for the 545 ITT patients with a device fitted (excluding those patients with a different device fitted owing to technical insertion failure) (Figure 15). There were statistically significant differences via the unadjusted p-values at the 5% level for 11 of the 16 questions. These remained statistically significant when adjusted for the other analyses. Hence, both of these results appear to favour PORTs over Hickman (see Appendix 8).
FIGURE 15.
Device-specific questionnaire for the PORTs vs. Hickman comparison.
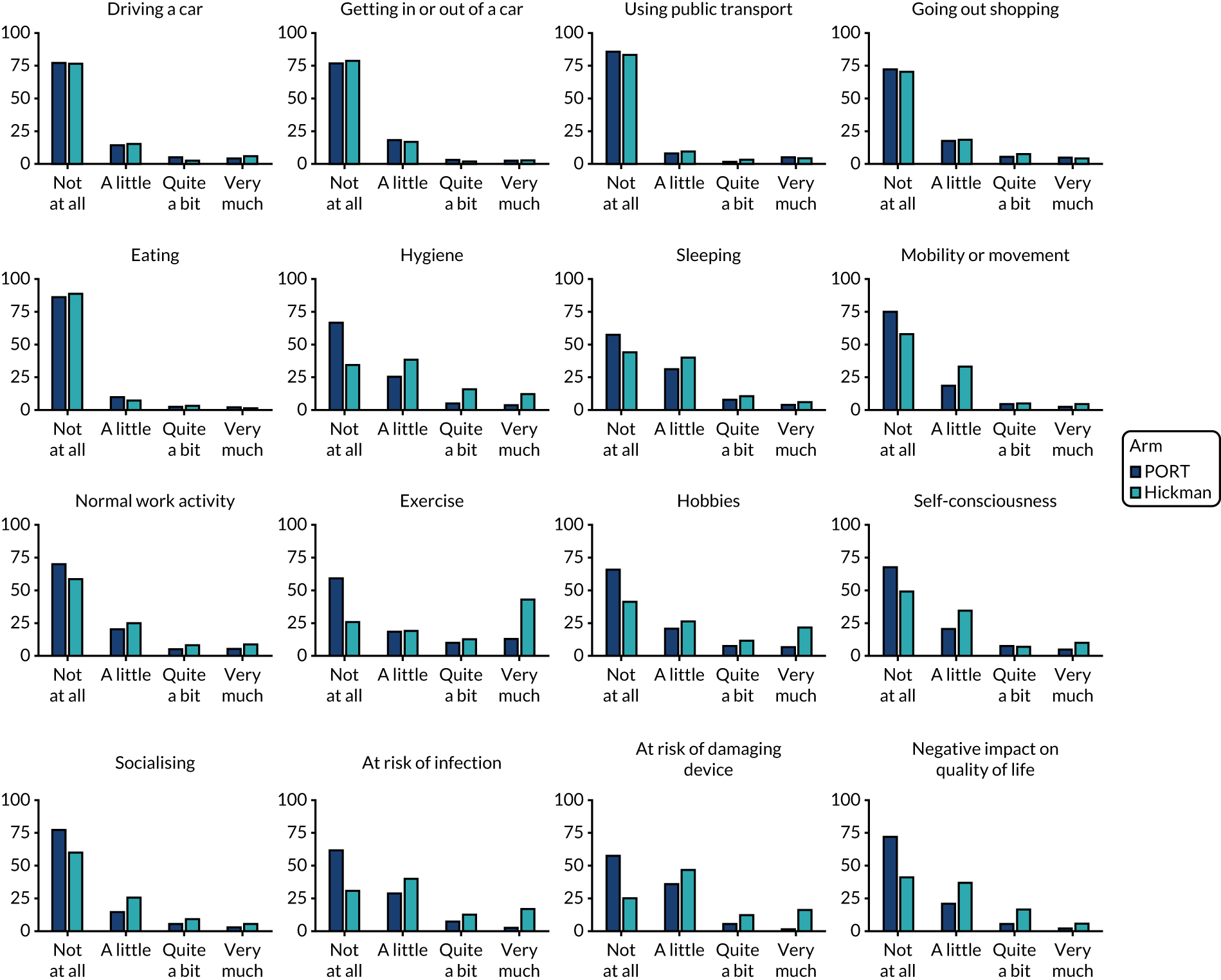
Discussion
The objective of this component of the CAVA trial was to determine whether or not PORTs were superior to Hickman with regard to their complication rate. We observed a statistically significant reduction in overall complications associated with PORTs when compared with Hickman (OR 0.54, 95% CI 0.37 to 0.77). We did not present the results of the per-protocol sensitivity analysis because the conclusions drawn were the same as those from the ITT analysis.
Overall, PORTs were in situ for a substantially longer period than Hickman-type devices (difference in median of 202 days). When this was taken into account, PORTs were found to be associated with 0.02 ± 0.00 complications per catheter-week, compared with 0.06 ± 0.01 complications per catheter-week in the Hickman arm. Device removal as a result of complications was far less frequent in the PORTs arm (14%) than in the Hickman arm (32%). This may be a reflection on the reluctance to remove PORTs and the desire to rescue the device, where possible. Both devices reported similar rates of the inability to aspirate blood (15% PORTs vs. 14% Hickman) and other complications (5% PORTs vs. 6% Hickman). PORTs were associated with substantially lower rates of laboratory-confirmed bloodstream infection (5% PORTs vs. 16% Hickman), exit site infection (4% PORTs vs. 9% Hickman) and mechanical failure (0.8% PORTs vs. 3% Hickman) than Hickman. However, rates of suspected catheter-related bloodstream infection was slightly higher in the patients of the PORTs arm (8%) than in the Hickman arm (5%). Similar to the PICCs–Hickman comparison, we found the highest infection rates in patients with haematological cancers: 61% in both the PORTs arm and the Hickman arm (data not shown). Although these cancers made up only 7% of the ITT population, this questions the role of these two devices for patients with haematological malignancies. There may be patient groups for whom PORTs are not a practical choice (e.g. for the administration of large volumes of blood products or when they are used continuously for days and weeks at a time, when needle access becomes difficult and may increase the risk of local infection). This was cited as one of the reasons for the original reluctance by most haematology centres to randomise patients to the PORTs comparisons.
Venous thrombosis was rare, being reported in 1% of patients in the PORTs arm and in 2% of patients in the Hickman arm. The low incidence of venous thrombosis is consistent with a pilot RCT (n = 100), the results of which were published in 2016, in which the incidence of venous thrombosis was 4% in the PORTs arm and 1% in the Hickman and PORT arms. 3 However, as discussed in Chapter 5, the low incidence may, in part, be because of imaging symptomatic patients only.
We found no significant difference in the quality of life, as measured by the EQ-5D or the EORTC QLQ-C30. As discussed in the Chapter 5, it is likely that these instruments are not sensitive to the devices but dominated by the underlying malignancy and its management. By contrast, the device-specific quality-of-life instrument did show a significant benefit in favour of PORTs for 11 of the 16 questions.
A systematic review published in 2014 revealed the poor evidence base in comparing these groups. 4 Although it appeared that infections were more common in the Hickman arm (pooled OR approximately two-fold), this failed to reach statistical significance and the risk of complications varied widely between studies. 4 Existing 2015 guidelines from the USA state that there is no clear preference between a PORT and a Hickman. 34 Furthermore, the European Society for Medical Oncology and the American Society of Clinical Oncology make no specific device recommendation. 35,36 Similarly, the Association of Anaesthetists of Great Britain and Ireland states that either device is suitable when a central catheter is required for long-term access. 37
The CAVA trial was carried out in 18 centres across the UK using the existing facilities of those centres. This comparison of Hickman-type devices and PORTs was the quickest to recruit and recruitment closed early as a result. In total, 556 patients were included in this comparison and the analysis was adequately powered. Therefore, the generalisability of the results should be high both in oncology and in other patients.
The key limitation of this comparison relates to the heterogeneity in the choice and management of these devices across centres. However, we understood that most centres followed epic guidelines at that time. 6 As PORTS were not in routine use in most of the centres, there was an inevitable learning curve. This may have resulted in a higher complication rate, particularly in the ongoing nursing care for the device (e.g. difficulties or lack of confidence and experience with needle access to PORTs). Furthermore, all PORTs were centrally placed; therefore, we cannot comment on any attempted comparison with peripheral arm PORTs.
In conclusion, the CAVA trial has shown that PORTs approximately halved the overall risk of complication when compared with Hickman-type devices. This was primarily driven by the impact on laboratory-confirmed bloodstream infections and exit site infections. Of these two complications, infection is regarded as more clinically significant than the inability to aspirate blood.
Chapter 7 Results for the peripherally inserted central catheters versus PORTs comparison
Study participants
Study participants were recruited from both two-way (PORTs vs. PICCs) and three-way (Hickman vs. PICCs vs. PORTs) randomisations; the two-way randomisation contributed 54% of the participants. In total, 346 participants were included in the PORTs versus PICCs comparison (Figure 16). Of all of the patients who entered the trial, 147 were randomised to receive PORTs and 199 were randomised to receive PICCs. All participants were included in the ITT analysis. Device insertion was attempted with 97% (n = 143) of participants randomised to PORTs and 94% (n = 187) of participants randomised to PICCs. The per-protocol population consisted only of participants who received the device to which they were randomised: n = 131 in the PORTs arm and n = 159 in the PICCs arm. All patients were followed up monthly until device removal, death or to the end of the 12-month follow-up.
FIGURE 16.
The PORTs vs. PICCs comparison CONSORT flow diagram.
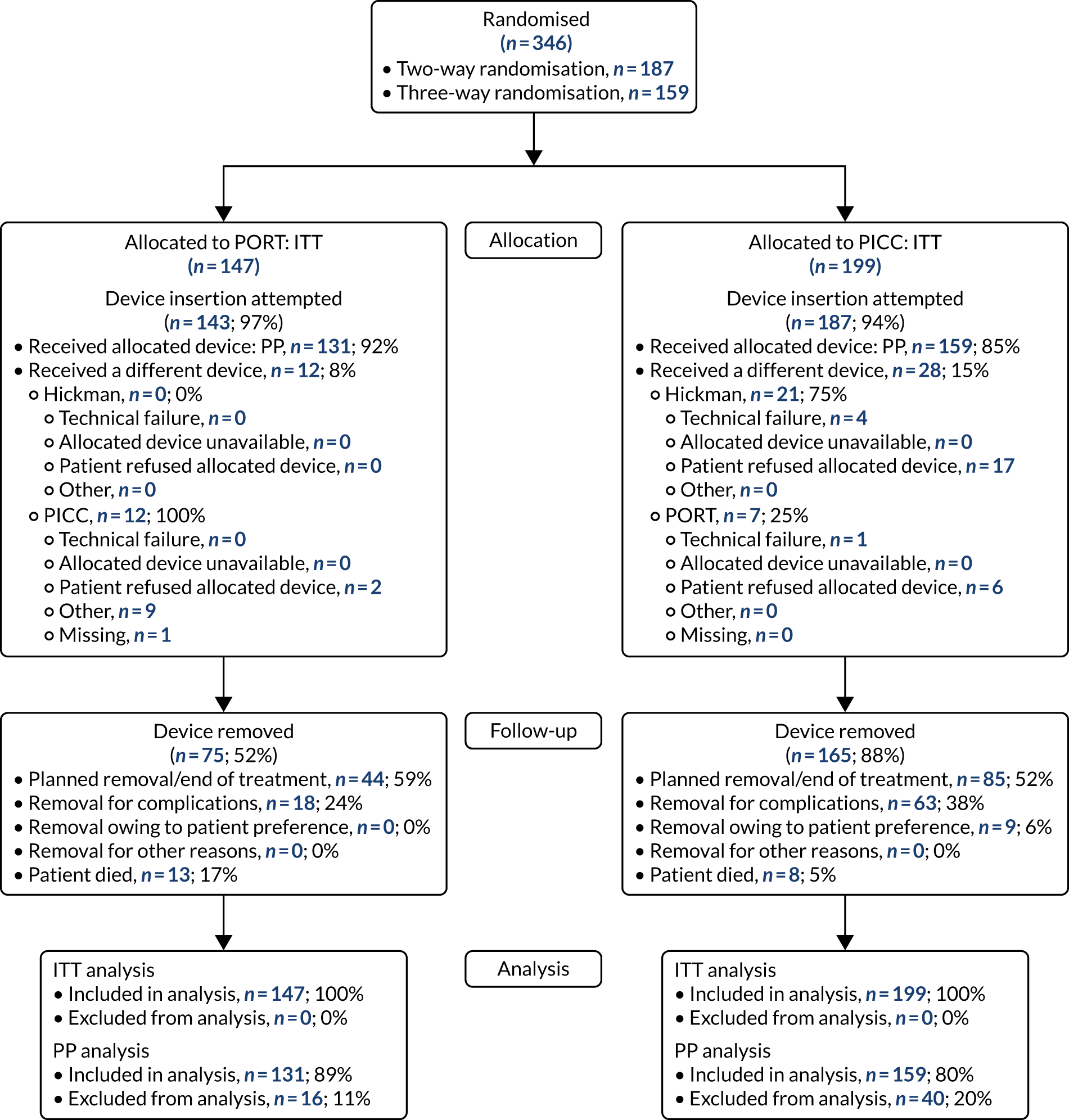
Baseline characteristics
The baseline characteristics were well balanced between the two arms (Table 9). There was no difference between the two arms in respect of age, sex, BMI and ethnicity. The mean age of participants was 61 ± 13 years. Over half of the participants (54%) were females. The majority of patients were white (92%) and had a BMI of between 20 kg/m2 and 30 kg/m2 (69%).
| Characteristic | Arm | Total | |
|---|---|---|---|
| PORT | PICCs | ||
| Mean age in years (SD, range) | 61 (12, 28–86) | 61 (13, 19–84) | 61 (13, 19–86) |
| Sex, n (%) | |||
| Female | 81 (55.1) | 107 (53.8) | 188 (54.3) |
| Male | 66 (44.9) | 92 (46.2) | 158 (45.7) |
| BMI (mg/kg2),a n (%) | |||
| < 20 | 9 (6.1) | 8 (4.0) | 17 (4.9) |
| 20 to < 30 | 98 (66.7) | 139 (69.8) | 237 (68.5) |
| 30 to < 40 | 36 (24.5) | 47 (23.6) | 83 (24.0) |
| ≥ 40 | 4 (2.7) | 5 (2.5) | 9 (2.6) |
| Ethnic origin, n (%) | |||
| White | 137 (93.2) | 182 (91.5) | 319 (92.2) |
| Asian | 3 (2.0) | 5 (2.5) | 8 (2.3) |
| South-East Asian | 3 (2.0) | 0 (0.0) | 3 (0.9) |
| Afro-Caribbean | 3 (2.0) | 6 (3.0) | 9 (2.6) |
| Other | 1 (0.7) | 4 (2.0) | 5 (1.4) |
| Missing | 0 (0.0) | 2 (1.0) | 2 (0.6) |
| Type of disease,a n (%) | |||
| Solid tumour | 142 (96.6) | 190 (95.5) | 332 (96.0) |
| Colorectal | 65 (45.8) | 89 (46.8) | 154 (46.4) |
| Breast | 22 (15.5) | 27 (14.2) | 49 (14.81) |
| Pancreatic | 12 (8.5) | 25 (13.2) | 37 (11.1) |
| Other (one missing on PICC) | 43 (30.3) | 48 (25.3) | 91 (27.4) |
| Haematological malignancy | 5 (3.4) | 9 (4.5) | 14 (4.0) |
| Acute myeloid leukaemia | 2 (40.0) | 1 (11.1) | 3 (21.4) |
| High-grade non-Hodgkin’s lymphoma | 0 (0.0) | 4 (44.4) | 4 (28.6) |
| Hodgkin’s disease | 0 (0.0) | 1 (11.1) | 1 (7.1) |
| Other (one missing on PICC) | 3 (60.0) | 2 (22.2) | 5 (35.7) |
| Metastatic disease (solid tumour patients only), n (%) | |||
| Yes | 93 (65.5) | 123 (64.7) | 216 (65.1) |
| No | 48 (33.8) | 65 (34.2) | 113 (34.0) |
| Missing | 1 (0.7) | 2 (1.1) | 3 (0.9) |
| Patients being administered 5-fluorouracil | 91 (61.9) | 122 (61.3) | 213 (61.6) |
| Planned treatment mode,a n (%) | |||
| Inpatient | 5 (3.4) | 6 (3.0) | 11 (3.2) |
| Outpatient | 142 (96.6) | 193 (97.0) | 335 (96.8) |
| Device historya | |||
| No prior device | 123 (83.7) | 168 (84.4) | 291 (84.1) |
| ≥ 1 previous device inserted > 3 months before study entry | 21 (14.3) | 27 (13.6) | 48 (13.9) |
| ≥ 1 previous device inserted ≤ 3 months before study entry | 3 (2.0) | 4 (2.0) | 7 (2.0) |
| Baseline quality-of-life scores, mean (SD, range) | |||
| EQ-5D index value | 0.8 (0.2, 0.0–1.0) | 0.8 (0.2, 0.0–1.0) | 0.8 (0.2, 0.0–1.0) |
| EQ-5D health state score | 74.3 (17.5, 30.0–100.0) | 73.6 (19.6, 20.0–100.0) | 73.9 (18.6, 20.0–100.0) |
| QLQ-C30 global health status | 67.8 (19.9, 0.0–100.0) | 69.8 (20.6, 0.0–100.0) | 68.9 (20.3, 0.0–100.0) |
The majority (96%) were solid tumour patients with metastatic disease; there was no difference between the two arms in respect of solid tumour and haematological malignancy. Among those with solid tumours, the majority of participants had colorectal primary tumours (46%). Among those with haematological malignancies, a greater proportion of the patients in the PORTs arm had acute myeloid leukaemia (40%) than in the PICCs arm (11%), whereas more patients in the PICCs arm had high-grade non-Hodgkin’s lymphoma (44%) than in the PORTs arm (0%). However, the numbers were very small. Across the two arms, the majority of the participants had planned outpatient treatments (97%) and did not have any prior venous access device (84%). The mean EQ-5D-3L health–utility score was 0.8 ± 0.2; there were no difference across the two arms. Similar patterns were observed with the mean EQ-5D health state score and the mean QLQ-C30 quality-of-life score.
Device details
The PORTs came from various companies, but the majority of PICCs (59%) were from one manufacturer (Vygon) (see Appendix 11). Approximately half of the PORTs were silicone and the other half polyurethane, whereas the majority of PICCs (87%) were of polyurethane construction. The majority of devices had one lumen (PORTs 97% and PICCs 88%). Antimicrobial coatings were used in only 2% of PORTs and 10% of PICCs. Overall, 84% of PORTs were CT pump compatible, compared with only 39% of PICCs. Valves were present on 4% of PORTs and 28% of PICCs.
The majority of the patients in the PICC arm had their device fitted within 1 week of randomisation, median 7 days (IQR 5–12 days), whereas the median in the PORTs arm was 8 days (IQR 4–16 days). With the exception of five PORT patients who had general anaesthesia, all devices were placed using local anaesthesia. A minority of PORT patients also had conscious sedation.
The majority of PORTs were inserted by radiologists (78%) in the radiology department. PICCs were inserted predominantly by nurses (73%) in a treatment room, in a room in the radiology department or at the bedside using mainly the peripheral arm vein above the elbow (80%). The jugular vein was the main vein used for PORTs insertion (90%). Perioperative antibiotics were given to 18% of PORTs patients and 2% of PICCs patients. The majority of PORT insertions took between 30 and 60 minutes (70%), whereas the majority of PICC insertions took < 30 minutes (52%).
Complications and device removal
The proportion of patients experiencing one or more complications was considerably lower in the PORTs arm than in the PICCs arm (32% vs. 47%) (Table 10). This difference was found to be statistically significantly different in the primary comparison (OR 0.52, 95% CI 0.33 to 0.83) and the NMA (OR 0.50, 95% CI 0.34 to 0.73). (Detailed outputs are presented in Report Supplementary Material 6.) Regardless of device allocation, complications tended to occur in the first 15 weeks of the follow-up period (Figure 17). The mean time to first complication was 35 ± 1.6 weeks in the PORTs arm, compared with 23 ± 1.3 weeks in the PICCs arm. The median time to first complication was not estimable in the PORTs arm and was 21 weeks (IQR 7 weeks–not estimable) in the PICCs arm.
However, PORTs were in place for longer (median 393 days, 95% CI 324 to 393 days) than PICCs (median 119 days, 95% CI 109 to 130 days). The mean number of complications per catheter-week was 0.05 ± 0.02 in the PORTs arm, compared with 0.13 ± 0.2 in the PICCs arm. Overall, major complications (as defined by SIR classifications) were observed in 34% of the PORTs arm and 26% of the PICCs arm.
| Complication and device removal | Arm | |||
|---|---|---|---|---|
| PORTs | PICCs | |||
| Patients | Complications | Patients | Complications | |
| Number of complications, n (%) | ||||
| 0 | 100 (68.0) | 106 (53.3) | ||
| 1 | 28 (19.0) | 61 (30.7) | ||
| 2 | 10 (6.8) | 23 (11.6) | ||
| ≥ 3 | 9 (6.1) | 9 (4.5) | ||
| Total number of patients (n) | 147 | 199 | ||
| Complication type, n (%) | ||||
| Inability to aspirate blood | 23 (15.6) | 33 (38.8) | 37 (18.6) | 55 (39.6) |
| Venous thrombosis | 3 (2.0) | 3 (3.5) | 22 (11.1) | 24 (17.3) |
| Pulmonary embolism | 3 (2.0) | 3 (3.5) | 1 (0.5) | 1 (0.7) |
| Infection, n (%) | 18 (12.2) | 24 (28.2) | 16 (8.0) | 16 (11.5) |
| Laboratory-confirmed bloodstream infection | 8 (5.4) | 9 (10.6) | 7 (3.5) | 7 (5.0) |
| Suspected catheter-related bloodstream infection | 8 (5.4) | 11 (12.9) | 5 (2.5) | 5 (3.6) |
| Exit site infection | 4 (2.7) | 4 (4.7) | 4 (2.0) | 4 (2.9) |
| Mechanical failure, n (%) | 4 (2.7) | 4 (4.7) | 21 (10.6) | 21 (15.1) |
| Other, n (%) | 16 (10.9) | 18 (21.2) | 19 (9.5) | 22 (15.8) |
| Total number of complications (n) | 85 | 139 | ||
| Severe complications according to SIR classifications (% of total complications) | 34 | 26 | ||
| Rate of complications per patient | 0.58 | 0.70 | ||
| Mean number of complications per catheter-week ± SE | 0.05 ± 0.02 | 0.13 ± 0.02 | ||
| Planned removal/end of treatment, n (%) | 44 (58.7) | 85 (51.5) | ||
| Removal due to complications, n (%) | 18 (24.0) | 63 (38.2) | ||
| Removal due to patient preference, n (%) | 0 (0.0) | 9 (5.5) | ||
| Removal due to other reasons, n (%) | 0 (0.0) | 0 (0.0) | ||
| Patient died, n (%) | 13 (17.3) | 8 (4.8) | ||
| Total devices removed (n) | 75 | 165 | ||
Over half of the device removals in each arm were planned. Over one-third of the devices were removed because of a complication, with a lower proportion in the PORTs arm (24%, n = 18) than in the PICCs arm (38%, n = 63). The mean total number of days of chemotherapy interruption owing to any complication was larger in the PORTs arm (1.0 ± 4.09 days) than in the PICCs arm (0.6 ± 3.27 days).
FIGURE 17.
Time to first complication for the PORTs vs. PICCs comparison.
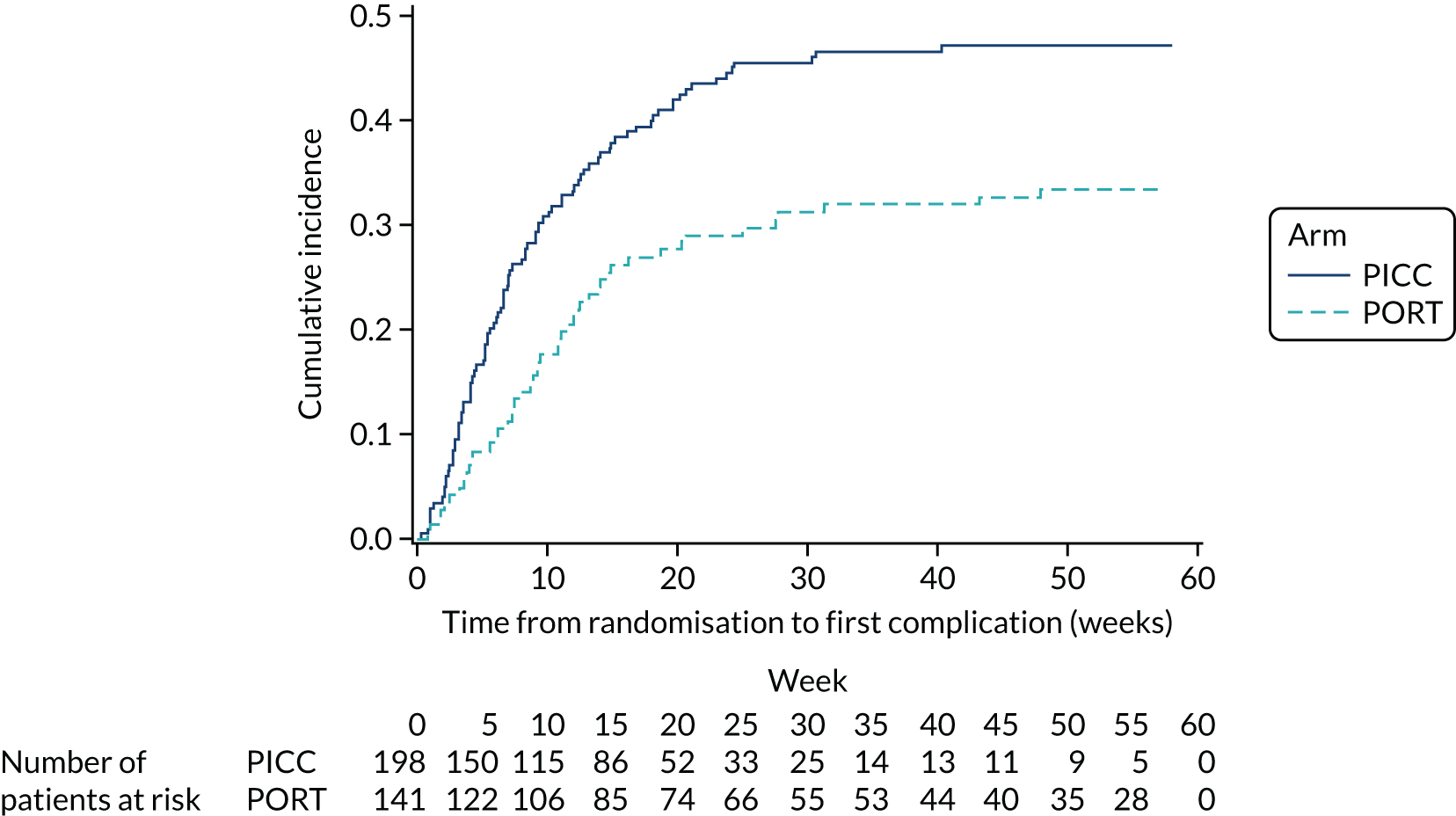
Specific complications and device removal
Inability to aspirate blood
Similar proportions of patients in the PORTs arm (n = 23; 16%) and the PICCs arm (n = 37; 19%) experienced one or more occurrence of the inability to aspirate blood; this accounts for 33 complications in the PORTs arm and 55 complications in the PICCs arm. A simple flush was commonly performed to manage the complications. Other procedures included thrombolytic locks and infusion, further imaging and manipulation of the device in situ. Two patients in the PICCs arm had a complication classified as SIR category C or above. As a consequence of an inability to aspirate blood, 4% (n = 1) of PORTs patients with this complication and 27% (n = 10) of PICCs patients had their device removed. Three PICCs patients had their chemotherapy stopped prematurely.
Laboratory-confirmed infection
Laboratory-confirmed bloodstream infection was recorded in 5% (n = 8) of patients in the PORTs arm, compared with 4% (n = 7) of patients in the PICCs arm. In both arms, these infections were primarily identified by culture of recognised pathogens on blood samples and were treated with antibiotics. Six patients in the PORTs arm and four patients in the PICCs arm had a complication classified as SIR category C or above. As a consequence of laboratory-confirmed bloodstream infection, 63% (n = 5) of the patients with this complication in the PORTs arm had their device removed, compared with 86% (n = 6) of those patients in the PICCs arm. Chemotherapy was prematurely stopped in one patient in the PORTs arm and two patients in the PICCs arm.
Suspected catheter-related bloodstream infection
Suspected catheter-related bloodstream infection was recorded in 5% (n = 8) of patients in the PORTs arm, compared with 3% (n = 5) of patients in the PICCs arm. In both arms, these infections primarily presented as fever, and all patients were given antibiotics. Six patients in the PORTs arm, compared with five patients in the PICCs arm, had a complication classified as SIR category C or above. As a consequence of suspected catheter-related bloodstream infection, 50% (n = 4) of the patients with this complication in the PORTs arm had their device removed, compared with 60% (n = 3) of those patients in the PICCs arm. One patient in the PICCs arm had chemotherapy prematurely stopped.
Exit site infection
Exit site infection was recorded in 3% (n = 4) of patients in the PORTs arm, compared with 2% (n = 4) of patients in the PICCs arm. All infections were confirmed and antibiotics were given in the majority of cases. Two patients in the PICCs arm had a complication classified as SIR category C or above. As a consequence of exit site infection, no patients in the PORTs arm and three patients in the PICCs arm had their device removed. No patients had their chemotherapy prematurely stopped.
The mean time to first infection (any infection) was 43 ± 01.2 weeks in the PORTs arm, compared with 44 ± 1.1 weeks in the PICCs arm. The median times to first infection (any infection) could not be estimated because less than half of the patients experienced an event.
Venous thrombosis and pulmonary embolism
Venous thrombosis was recorded in 2% (n = 3) of patients in the PORTs arm and 11% (n = 22) of patients in the Hickman arm. This difference was found to be statistically significantly different at the 5% significance level (OR 0.14, 95% CI 0.04 to 0.50). Patients were primarily treated with anticoagulants. Two patients in the PORTs arm and nine patients in the PICCs arm had a complication classified as SIR category C or above. As a consequence, one patient in the PORTs arm and nine patients in the PICCs arm had their device removed. One patient in the PORTs arm had their chemotherapy prematurely stopped.
Pulmonary embolism was rare and occurred in 2% (n = 3) of patients in the PORTs arm and 1% (n = 1) of patients in the PICCs arm. All patients received anticoagulants. No patients with PE had their device removed or their chemotherapy prematurely stopped.
Mechanical failure and other complications
Mechanical failure was reported in 3% (n = 4) of patients in the PORTs arm, compared with 11% (n = 21) of patients in the PICCs arm. Of these patients, five in the PICCs arm reported line fracture, one in the PORTs arm reported exposure of the PORT through their wound, one in the PORTs arm and eight in the PICCs arm reported ‘device fallen out’, and two in the PORTs arm and eight in the PICCs arm reported line migration that required intervention. Two patients in the PICCs arm had a complication classified as SIR category C or above. As a consequence, one patient in the PORTs arm and 19 in the PICCs arm who had mechanical failure had their device removed. No patient had their chemotherapy prematurely stopped.
Other complications were reported in 11% (n = 16) of patients in the PORTs arm and 10% (n = 19) in the PICCs arm. Three patients in the PORTs arm and two in the PICCs arm had a complication classified as SIR category C or above. As a consequence, six patients in the PORTs arm and 12 patients in the PICCs arm had their device removed. No patient had their chemotherapy prematurely stopped.
Quality of life
Quality-of-life data were collected monthly, until device removal or withdrawal, up to a period of 12 months. However, the proportion of missing data increased substantially with the follow-up time (see Appendix 12 and Report Supplementary Material 7).
Health-related quality of life
Overall, there was little or no change in HRQoL over time. During the first 6 months, the study arms were similar. Following that, however, the mean index scores look to be marginally higher in the PICCs arm (Figure 18). The mean adjusted standardised AUCs for the original EQ-5D index values were –0.031 ± 0.01 for the PORTs arm and –0.017 ± 0.01 for the PICCs arm. The pooled estimate over the imputed data resulting in mean values were –0.216 ± 0.04 for the PORTs arm and –0.113 ± 0.04 for the PICCs arm. These scores were not statistically significantly different at the 5% level. The mean adjusted standardised AUCs for the original EQ-5D health state score data were –17.09 ± 2.84 for the PORTs arm and –19.08 ± 2.84 for the PICCs arm. The pooled estimate over the imputed data resulting in mean values were –14.51 ± 1.99 for the PORTs arm and –9.78 ± 2.21 for the PICCs arm. The difference in these scores was not statistically significant at the 5% level (see Appendix 11).
FIGURE 18.
The PORTs vs. PICCs comparison for (a) EQ-5D index value and (b) health state. The error bars represent ± 1 standard error.
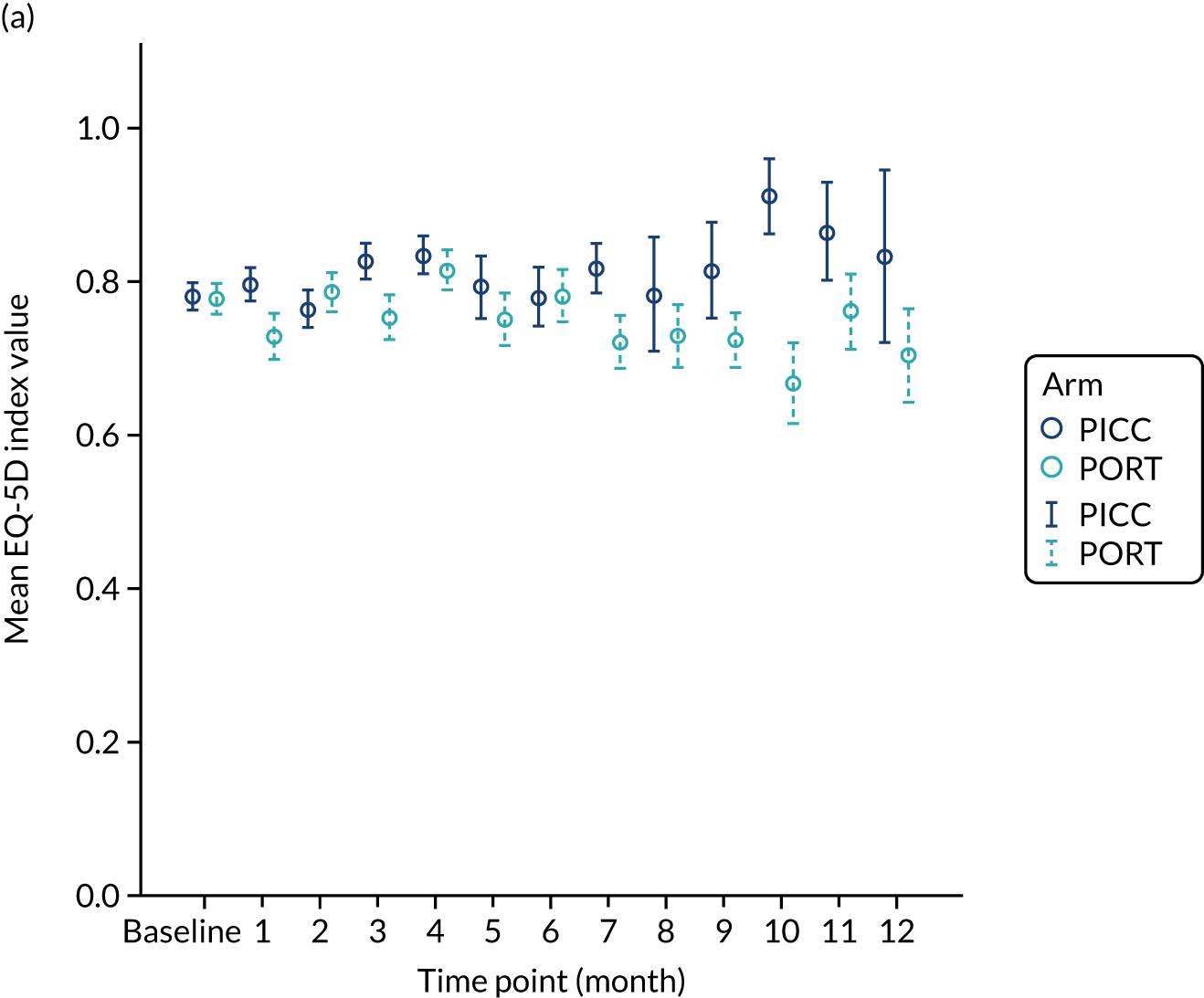
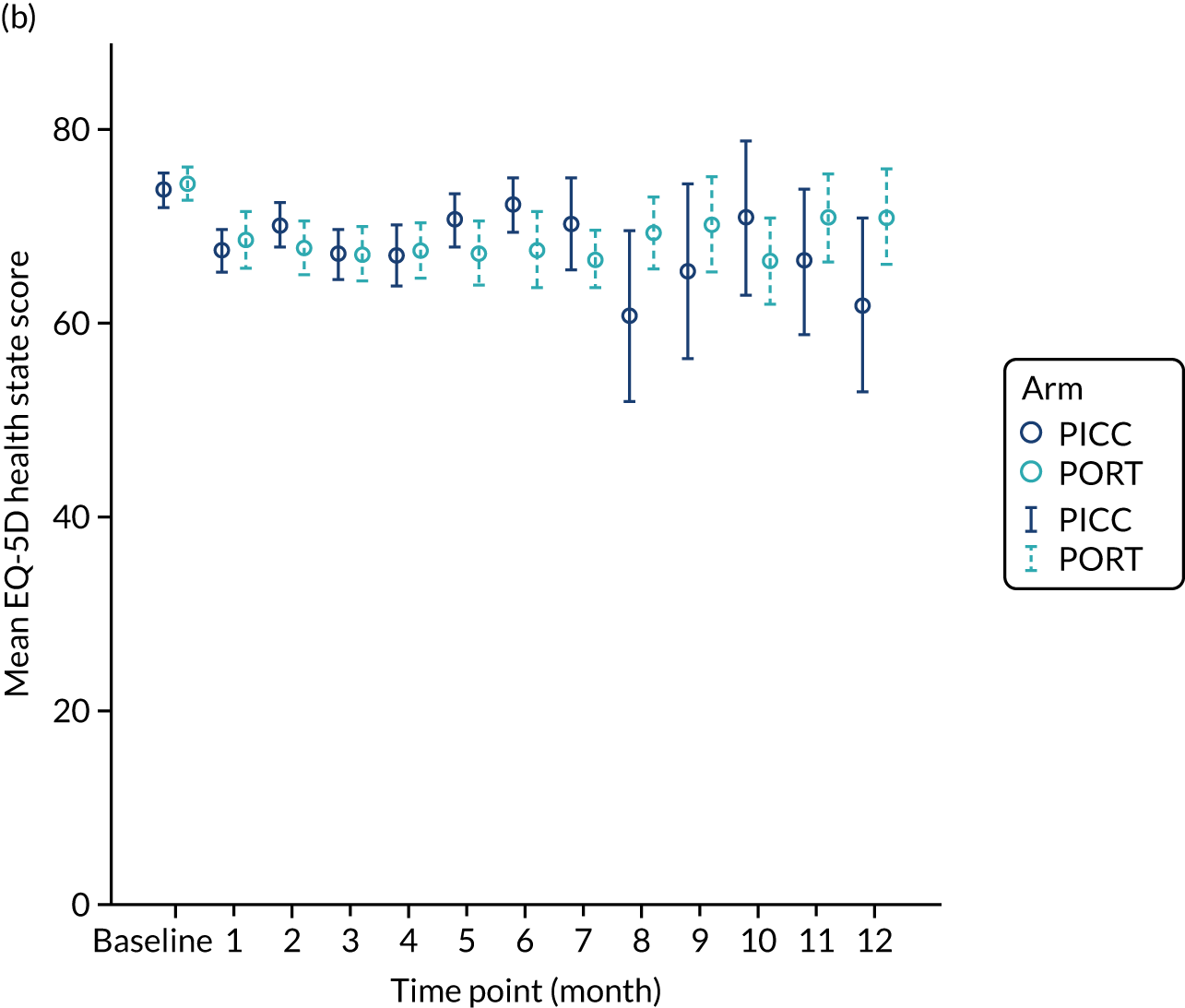
Quality of life: Quality of Life Questionnaire C30
The mean QLQ-C30 global health status was very similar across the two arms at all time points, with the exception of months 7 and 10 (Figure 19). The analysis of the AUC data over the duration of device insertion showed no statistically significant difference between the devices for this scale. There was a statistically significant difference at the 5% significance level in terms of constipation (unadjusted p = 0.029 in favour of PICCs), as assessed by the QLQ-C30; however, after adjusting for multiple comparisons, this statistical significance was lost (see Appendix 11).
FIGURE 19.
The EORTC QLQ-C30 scores. The error bars represent ± 1 standard error.
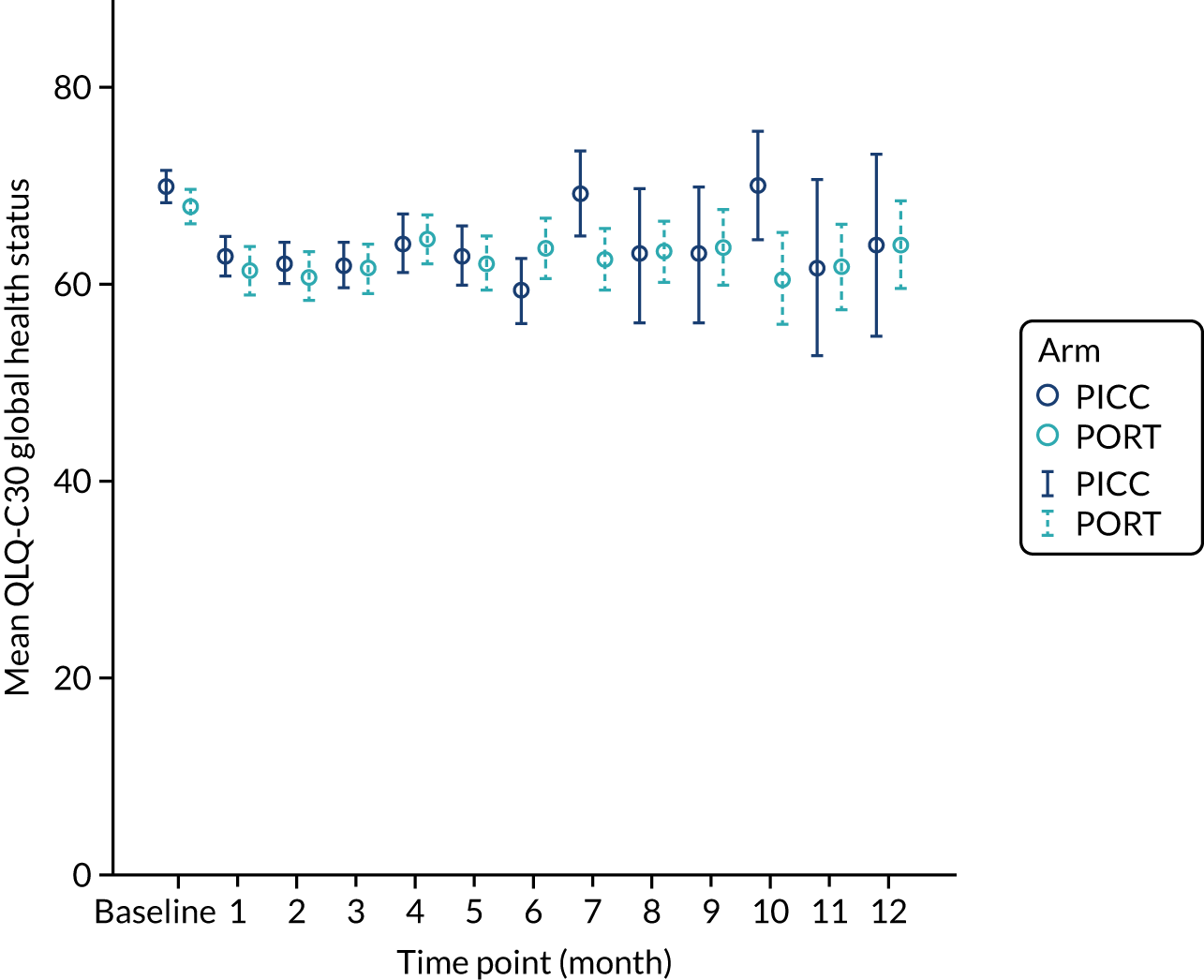
Venous access device-related quality of life
The worst score reported during the study for each question was established and compared across the two arms for the 325 ITT patients who had a device fitted (excluding those patients who had a different device fitted owing to technical insertion failure) (Figure 20). There were statistically significant differences favouring PORTs over PICCs at the 5% significance level for 8 of the 16 questions relating to hygiene, exercise, hobbies, self-consciousness, socialising, at risk of damaging device, at risk of infection and impact on quality of life. The adjusted p-values were all < 0.001, with the exception of self-consciousness (p = 0.016), socialising (p = 0.009) and risk of infection (p = 0.003) (see Appendix 12).
FIGURE 20.
Device-specific questionnaire for the PORTs vs. PICCs comparison.
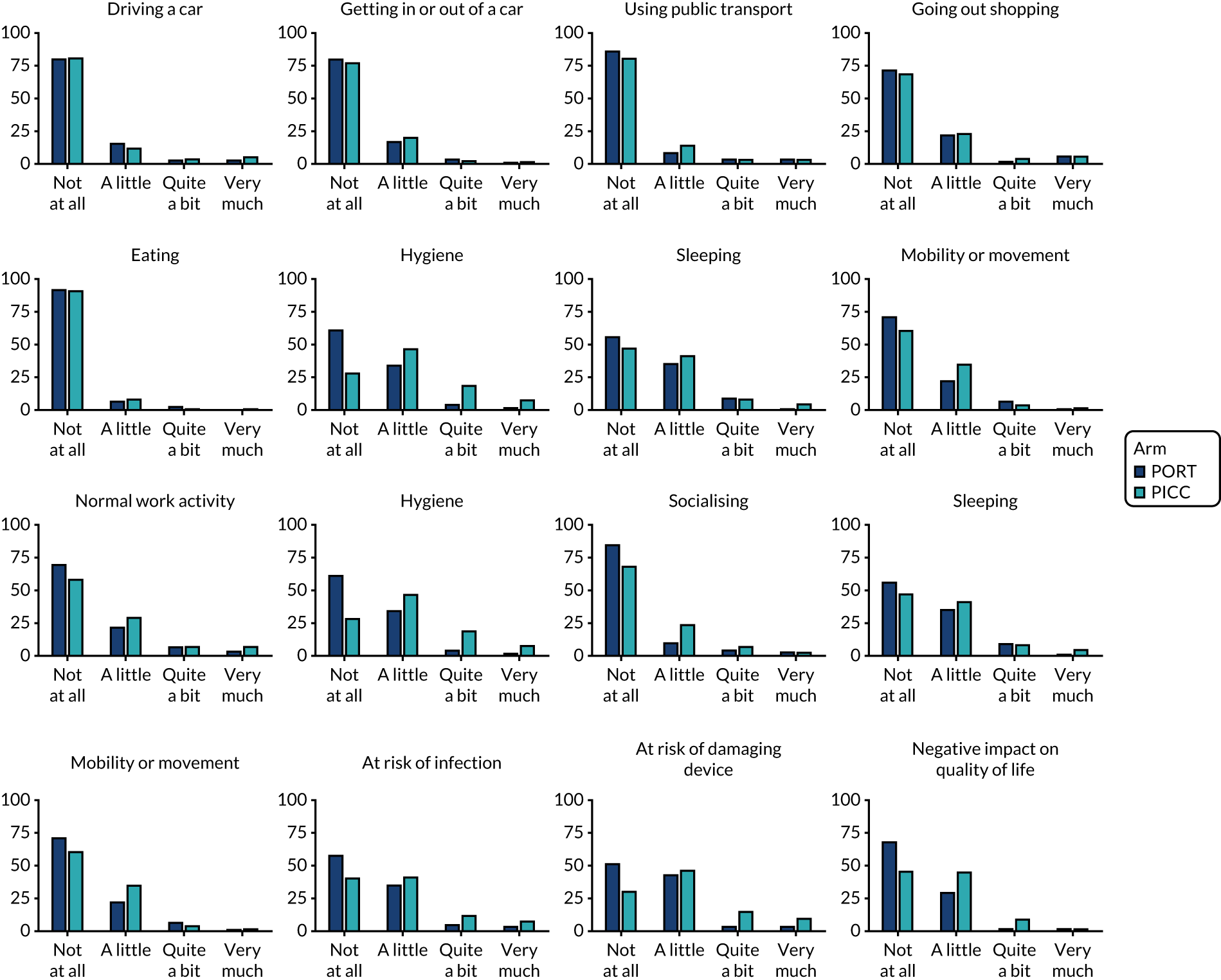
Discussion
The objective of this component of the CAVA trial was to determine whether or not PORTs were superior to PICCs with regard to their complications rate. We observed a statistically significant reduction of overall complications associated with PORTs when compared with PICCs (OR 0.52, 95% CI 0.33 to 0.83). We did not present the results of the per-protocol sensitivity analysis because the conclusions drawn were the same as those from the ITT analysis.
Overall, PORTs were in situ for a substantially longer period than PICCs (difference in median of 274 days). When this was taken into account, PORTs were found to be associated with 0.05 ± 0.02 complications per catheter-week, compared with 0.13 ± 0.02 complications per catheter-week in the PICCs arm. Device removal as a result of complications was less frequent in the PORTs arm (24%) than in the PICCs arm (38%). As discussed in the previous chapters, this may be a reflection of the ease of PICCs removal, and on the reluctance to remove PORTs and the desire to rescue the device, where possible. The PORTs arm was associated with a lower rate of an inability to aspirate blood (16%) than in the PICCs arm (19%). Although infection rates (any type) were reported in a greater proportion of PORTs patients than PICCs patients (12% vs. 8%, respectively), the mean number of infections per catheter-week was similar when device time in situ was taken into account (0.02 in both arms; data not shown). Venous thrombosis was reported in 2% of the PORTs arm but in 11% of the PICCs arm; this difference in complication rates was statistically significant. Mechanical failure was reported in 3% of the PORTs arm, compared with 11% of the PICCs arm.
We found no significant difference in quality of life as measured by the EQ-5D or the EORTC QLQ-C30. As discussed in the previous chapters, it is likely that these instruments are not sensitive to the devices but dominated by the underlying malignancy and its management. By contrast, the device-specific quality-of-life instrument did show a significant benefit in favour of PORTs for 8 out of the 16 questions.
Our findings are consistent with those from two recent RCTs, which also showed a significantly higher complication rate with PICCs than with PORTs. 38,39 One RCT was conducted in patients with solid cancers in Sweden; PICCs were shown to be associated with a 2.7-fold increase in complications (composite of catheter-related adverse events requiring intervention, thrombotic, occlusive, infections and mechanical). 38 The other RCT was conducted in women with early breast cancers in France. 39 This trial reported a two-fold increased risk of catheter-related severe adverse events (grade ≥ III, or delay in chemotherapy administration or device removal) in the PICCs arm than in the PORTs arm.
The infection rate in our trial was higher than in either of these trials. This is mostly explained by our inclusion of ‘suspected catheter-related bloodstream infections’. We did this to capture patients who did not fulfil the strict definition of catheter-related bloodstream infection but were treated on the assumption that they have infection clinically. Similar to the PICCs–Hickman and the PORTs–Hickman comparisons, we found the highest infection rates in the haematological cancers PICCs (33%) and PORTs (80%). Although these cancers made up only 4% of the ITT population, this questions the role of a PORT for these malignancies.
In both trials, venous thrombosis was the most frequent complication and the authors concluded that it is becoming increasingly recognised as a risk factor when choosing to use a PICC. 38,39 The venous thrombosis is likely to be because of the presence of the catheter in the narrower calibre arm veins rather than in a central catheter placement (i.e. catheter to vein diameter ratio). Furthermore, a large systematic review of > 29,000 patients showed a 2.5-fold increased risk of a venous thrombosis with PICCs than with centrally inserted catheters. 32
The CAVA trial was carried out in 18 centres across the UK using the existing facilities of those centres. This comparison was the quickest to recruit to and recruitment closed early as a result. In total, 346 patients were included in this comparison and the analysis was adequately powered. Therefore, the generalisability of the results should be high in both oncology patients and other patients.
The key limitation of this comparison is similar to that of the PORTs–Hickman comparison. This relates to the heterogeneity in the choice and management of these devices across centres. However, we understood that most centres followed epic guidelines at that time. 6 As PORTs were not in routine use in most of the centres, there was an inevitable learning curve. Furthermore, similar to the two recent RCTs, all PORTs in the CAVA trial were centrally placed; therefore, we cannot comment on any attempted comparison with peripheral arm PORTs.
In conclusion, the CAVA trial has shown that the use of PORTs approximately halved the risk of complication when compared with the use of PICCs. This questions the current situation in the UK, where the use of PICCs is the dominant strategy.
Chapter 8 Economic evaluation
The objective of the economic evaluation was to determine the cost-effectiveness of the device comparisons over a 12-month period: (1) PICCs compared with Hickman, (2) PORTs compared with Hickman and (3) PORTs compared with PICCs. Details of the methods are reported in Chapter 2.
Resource use
Device insertion
The resource required for device insertion varied across the three devices. The insertion of Hickman-type devices was most commonly led by radiologists in a radiology department (46–48%). Nurse-led and anaesthesiologist-led insertions in a procedure or treatment room accounted for > 20% of the patients who had a device fitted (Table 11). The insertions of PICCs were most commonly nurse led and performed in a procedure or treatment room (67–73%); radiologist-led insertions were less frequent (11–13%). Similar to Hickman insertions, PORTs insertions were primarily radiologist led in a radiology department. Nurse-led PORTs insertions varied substantially (2–24%). All devices were inserted under local anaesthetic, with the exception of five PORTs patients who received general anaesthetic. The use of prophylactic antibiotics was uncommon and non-antimicrobial dressings were the most commonly applied across all three devices.
Device insertions for the majority of cases required an additional nurse to be present during the procedure (86–95% among Hickman patients and 81–97% among PORTs patients) (Table 12); however, this was less frequent for PICCs (65–81% of patients). Similarly, the use of an additional radiographer was common for the insertion of Hickman-type devices and PORTs but was less common, for PICCs. During the procedure for each of the devices, it was not common to have an additional anaesthesiologist, radiologist, doctor or surgeon present. The majority of patients received ultrasound imaging (Table 13). Fluoroscopy was far more frequently used in patients receiving Hickman-type devices and PORTs. Approximately one-quarter of patients receiving a PICC required the use of fluoroscopy. The use of chest X-rays was more common with PICCs than with Hickman-type devices and PORTs.
| Primary operator and setting | Arm, n (%) | |||||
|---|---|---|---|---|---|---|
| Hickman vs. PICC | Hickman vs. PORT | PICC vs. PORT | ||||
| Hickman | PICC | Hickman | PORT | PICC | PORT | |
| Primary operator | ||||||
| Nurse | 47 (22.7) | 128 (67.4) | 97 (34.6) | 59 (24.3) | 125 (73.1) | 3 (2.2) |
| Radiographer | 15 (7.2) | 10 (5.3) | 10 (3.6) | 5 (2.1) | 7 (4.1) | 3 (2.2) |
| Anaesthesiologist | 42 (20.3) | 7 (3.7) | 36 (12.9) | 27 (11.1) | 5 (2.9) | 14 (10.2) |
| Radiologist | 96 (46.4) | 24 (12.6) | 133 (47.5) | 144 (59.3) | 18 (10.5) | 107 (78.1) |
| Doctor | 4 (1.9) | 1 (0.5) | 4 (1.4) | 7 (2.9) | 1 (0.6) | 5 (3.6) |
| Surgeon | 1 (0.5) | 3 (1.6) | 0 (0) | 0 (0) | 0 (0) | 5 (3.6) |
| Other | 1 (0.5) | 16 (8.4) | 0 (0) | 0 (0) | 13 (7.6) | 0 (0) |
| Missing | 1 (0.5) | 1 (0.5) | 0 (0) | 1 (0.4) | 2 (1.2) | 0 (0) |
| Total | 207 (100) | 190 (100) | 280 (100) | 243 (100) | 171 (100) | 137 (100) |
| Setting | ||||||
| Theatre | 61 (29.5) | 11 (5.8) | 50 (17.9) | 57 (23.5) | 5 (2.9) | 28 (20.4) |
| Procedure/treatment room | 39 (18.8) | 103 (54.2) | 86 (30.7) | 9 (3.7) | 61 (35.7) | 1 (0.7) |
| Radiology department | 103 (49.8) | 50 (26.3) | 140 (50) | 171 (70.4) | 42 (24.6) | 106 (77.4) |
| Bedside | 0 (0) | 12 (6.3) | 0 (0) | 0 (0) | 44 (25.7) | 0 (0) |
| Missing | 0 (0) | 0 (0) | 4 (1.4) | 5 (2.1) | 17 (9.9) | 2 (1.5) |
| Other | 4 (1.9) | 14 (7.4) | 0 (0) | 1 (0.4) | 2 (1.2) | 0 (0) |
| Total | 207 (100) | 190 (100) | 280 (100) | 243 (100) | 171 (100) | 137 (100) |
| Type of anaesthesia | ||||||
| Local only | 180 (87) | 188 (98.9) | 268 (95.7) | 216 (88.9) | 168 (98.2) | 115 (83.9) |
| Local and conscious sedation | 26 (12.6) | 1 (0.5) | 12 (4.3) | 27 (11.1) | 1 (0.6) | 17 (12.4) |
| General anaesthesia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 5 (3.6) |
| Missing | 1 (0.5) | 1 (0.5) | 0 (0) | 0 (0) | 2 (1.2) | 0 (0) |
| Total | 207 (100) | 190 (100) | 280 (100) | 243 (100) | 171 (100) | 137 (100) |
| Prophylactic antibiotics given | ||||||
| Yes | 4 (1.9) | 3 (1.6) | 2 (0.7) | 34 (14) | 3 (1.8) | 24 (17.5) |
| No | 199 (96.1) | 179 (94.2) | 272 (97.1) | 200 (82.3) | 160 (93.6) | 109 (79.6) |
| Missing | 4 (1.9) | 8 (4.2) | 6 (2.1) | 9 (3.7) | 8 (4.7) | 4 (2.9) |
| Total | 207 (100) | 191 (100) | 280 (100) | 243 (100) | 171 (10) | 137 (10) |
| Type of dressing applied | ||||||
| Non-antimicrobial | 144 (69.9) | 159 (83.7) | 221 (78.9) | 226 (93) | 140 (81.9) | 121 (88.3) |
| Antimicrobial | 60 (29) | 29 (15.3) | 58 (20.7) | 10 (4.1) | 25 (14.6) | 12 (8.8) |
| Missing | 3 (1.4) | 2 (1.1) | 1 (0.4) | 7 (2.9) | 6 (3.5) | 4 (2.9) |
| Total | 207 (100) | 190 (100) | 280 (100) | 243 (100) | 171 (100) | 137 (100) |
| Additional staff | Arm, n (%) | |||||
|---|---|---|---|---|---|---|
| Hickman vs. PICC | Hickman vs. PORT | PICC vs. PORT | ||||
| Hickman | PICC | Hickman | PORT | PICC | PORT | |
| Nurse | ||||||
| Present | 197 (95.2) | 124 (65.3) | 240 (85.7) | 197 (81.1) | 101 (59.1) | 133 (97.1) |
| Not present | 10 (4.8) | 66 (34.7) | 40 (14.3) | 46 (18.9) | 70 (40.9) | 4 (2.9) |
| Total | 207 (100) | 190 (100) | 280 (100) | 243 (100) | 171 (100) | 137 (100) |
| Radiographer | ||||||
| Present | 140 (67.6) | 41 (21.6) | 179 (63.9) | 200 (82.3) | 33 (19.3) | 117 (85.4) |
| Not present | 67 (32.4) | 149 (78.4) | 101 (36.1) | 43 (17.7) | 138 (80.7) | 20 (14.6) |
| Total | 207 (100) | 190 (100) | 280 (100) | 243 (100) | 171 (100) | 137 (100) |
| Anaesthesiologist | ||||||
| Present | 7 (3.4) | 2 (1.1) | 4 (1.4) | 1 (0.4) | 0 (0) | 6 (4.4) |
| Not present | 200 (96.6) | 188 (98.9) | 276 (98.6) | 242 (99.6) | 171 (100) | 131 (95.6) |
| Total | 207 (100) | 190 (100) | 280 (100) | 243 (100) | 171 (100) | 137 (100) |
| Radiologist | ||||||
| Present | 13 (6.3) | 4 (2.1) | 9 (3.2) | 19 (7.8) | 2 (1.2) | 11 (8) |
| Not present | 194 (93.7) | 186 (97.9) | 271 (96.8) | 224 (92.2) | 169 (98.8) | 126 (92) |
| Total | 207 (100) | 190 (100) | 280 (100) | 243 (100) | 171 (100) | 137 (100) |
| Doctor | ||||||
| Present | 4 (1.9) | 1 (0.5) | 8 (2.9) | 8 (3.3) | 2 (1.2) | 5 (3.6) |
| Not present | 203 (98.1) | 189 (99.5) | 272 (97.1) | 235 (96.7) | 169 (98.8) | 132 (96.4) |
| Total | 207 (100) | 190 (100) | 280 (100) | 243 (100) | 171 (100) | 137 (100) |
| Other | ||||||
| Present | 33 (15.9) | 55 (28.9) | 31 (11.1) | 49 (20.2) | 59 (34.5) | 12 (8.8) |
| Not present | 174 (84.1) | 135 (71.1) | 249 (88.9) | 194 (79.8) | 112 (65.5) | 125 (91.2) |
| Total | 207 (100) | 190 (100) | 280 (100) | 243 (100) | 171 (100) | 137 (100) |
| Imaging | Arm, n (%) | |||||
|---|---|---|---|---|---|---|
| Hickman vs. PICC | Hickman vs. PORT | PICC vs. PORT | ||||
| Hickman | PICC | Hickman | PORT | PICC | PORT | |
| Any imaging? | ||||||
| Yes | 1 (0.5) | 4 (2.1) | 2 (0.7) | 0 (0) | 5 (2.9) | 0 (0) |
| No | 206 (99.5) | 186 (97.9) | 278 (99.3) | 243 (100) | 166 (97.1) | 137 (100) |
| Total | 207 (100) | 190 (100) | 280 (100) | 243 (100) | 171 (100) | 137 (100) |
| Ultrasound | ||||||
| Yes | 191 (92.3) | 150 (78.9) | 256 (91.4) | 212 (87.2) | 144 (84.2) | 130 (94.9) |
| No | 16 (7.7) | 40 (21.1) | 24 (8.6) | 31 (12.8) | 27 (15.8) | 7 (5.1) |
| Total | 207 (100) | 190 (100) | 280 (100) | 243 (100) | 171 (100) | 137 (100) |
| Fluoroscopy | ||||||
| Yes | 158 (76.3) | 51 (26.8) | 193 (68.9) | 233 (95.9) | 40 (23.4) | 128 (93.4) |
| No | 49 (23.7) | 139 (73.2) | 87 (31.1) | 10 (4.1) | 131 (76.6) | 9 (6.6) |
| Total | 207 (100) | 190 (100) | 280 (100) | 243 (100) | 171 (100) | 137 (100) |
| Sherlock tracking | ||||||
| Yes | 0 (0) | 14 (7.4) | 0 (0) | 0 (0) | 1 (0.6) | 0 (0) |
| No | 207 (100) | 176 (92.6) | 280 (100) | 243 (100) | 170 (99.4) | 137 (100) |
| Total | 207 (100) | 190 (100) | 280 (100) | 243 (100) | 171 (100) | 137 (100) |
| Chest X-ray | ||||||
| Yes | 3.9 (18.8) | 57 (30) | 61 (21.8) | 45 (18.5) | 65 (38) | 17 (12.4) |
| No | 168 (81.2) | 133 (70) | 219 (78.2) | 198 (81.5) | 106 (62) | 120 (87.6) |
| Total | 207 (100) | 190 (100) | 280 (100) | 243 (100) | 171 (100) | 137 (100) |
| Other | ||||||
| Yes | 1 (0.5) | 16 (8.4) | 0 (0) | 8 (3.3) | 11 (6.4) | 1 (0.7) |
| No | 206 (99.5) | 174 (91.6) | 280 (100) | 235 (96.7) | 160 (93.6) | 136 (99.3) |
| Total | 207 (100) | 190 (100) | 280 (100) | 243 (100) | 171 (100) | 137 (100) |
Unplanned outpatient visits
In the PICCs versus Hickman comparison, the proportion of patients reporting unplanned outpatient visits was similar when comparing PICCs (28%) with Hickman (28%) during the follow-up period (Table 14). The mean and median number of outpatient visits were similar across the two arms. In the PORTs versus Hickman comparison, 13% of the PORTs patients reported outpatient visits, compared with 27% of Hickman patients. The mean number of visits in the PORTs arm was approximately half that of the Hickman arm. In the PORTs versus PICCs comparison, 17% of PORTs patients reported outpatient visits compared with 28% of PICCs patients. The mean number of outpatient visits was substantially smaller in the PORTs arm (0.250 ± 0.63) than in the PICCs arm (0.476 ± 1.19).
| Arm | n | Outpatient visits | |||
|---|---|---|---|---|---|
| Patients with outpatient visits, n (%) | Mean number (SD) | Median number (IQR) | Minimum, maximum number | ||
| Hickman vs. PICCs comparison | |||||
| Hickman | 212 | 58 (28) | 0.512 (1.03) | 0 (0–1) | 0, 8 |
| PICCs | 212 | 58 (28) | 0.500 (1.22) | 0 (0–1) | 0, 13 |
| PORTs vs. Hickman comparison | |||||
| Hickman | 303 | 77 (27) | 0.544 (1.23) | 0 (0–1) | 0, 9 |
| PORTs | 252 | 32 (13) | 0.230 (0.75) | 0 (0–0) | 0, 7 |
| PORTs vs. PICCs comparison | |||||
| PICCs | 199 | 52 (28) | 0.476 (1.19) | 0 (0–1) | 0, 13 |
| PORTs | 147 | 24 (16) | 0.250 (0.63) | 0 (0–1) | 0, 3 |
Unplanned inpatient admissions
In the PICCs versus Hickman comparison, 14% of the PICCs patients reported unplanned inpatient admissions during the follow-up period, compared with 20% of the Hickman patients (Table 15). The mean length of stay for these patients was shorter in the PICCs arm (1.198 ± 5.05 days) than in the Hickman arm (2.279 ± 6.92 days). In the PORTs versus Hickman comparison, 12% of PORTs patients and 18% of Hickman patients reported unplanned inpatient admissions. The mean length of stay was shorter in the PORTs arm (1.05 ± 4.23 days) than in the Hickman arm (1.50 ± 4.59 days). In the PORTs versus PICCs comparison, a similar proportion of patients reported unplanned inpatient admission in both arms (12% of PORTs and 11% of PICCs). Patients in the PORTs arm were associated with a longer length of stay (1.45 ± 6.37 days) than patients in the PICCs arm (0.50 ± 2.02 days). Regardless of the device inserted, few patients reported extended hospital stay. The maximum reported length of stay ranged from 19 days in the PICCs arm (when compared with the PORTs arm) to 56 days in both the PICCs arm and the Hickman arm (when compared with each other).
| Arm | n | Inpatient admissions | |||
|---|---|---|---|---|---|
| Patients with inpatient admissions, n (%) | Mean length of stay in days (SD) | Median length of stay in days (IQR) | Minimum, maximum length of stay | ||
| Hickman vs. PICCs comparison | |||||
| Hickman | 212 | 43 (20) | 2.28 (6.92) | 0 (0–12) | 0, 56 |
| PICCs | 212 | 30 (14) | 1.20 (5.05) | 0 (0–7) | 0, 56 |
| PORTs vs. Hickman comparison | |||||
| Hickman | 303 | 53 (18) | 1.50 (4.59) | 0 (0–9) | 0, 56 |
| PORTs | 252 | 29 (12) | 1.05 (4.23) | 0 (0–7) | 0, 36 |
| PORTs vs. PICCs comparison | |||||
| PICCs | 199 | 22 (11) | 0.50 (2.02) | 0 (0–3) | 0, 19 |
| PORTs | 147 | 18 (12) | 1.45 (6.37) | 0 (0–7) | 0, 51 |
Costs
The mean costs associated with device insertion, unplanned follow-up (outpatient and inpatient visits) and total costs are presented in Table 16. The mean device insertion costs were approximately £1100 for Hickman, £500 for PICCs and £1400 for PORTs. These costs were consistent across the three comparisons. In the PICCs versus Hickman comparison, regardless of the type of device inserted, the mean unplanned follow-up costs constituted approximately 70% of total costs. In the PORTs versus Hickman comparison, mean unplanned follow-up costs constituted 43% of total costs in the PORTs arm and 59% of total costs in the Hickman arm. In the PORTs versus PICCs comparison, mean unplanned follow-up costs constituted 51% of total costs in the PORTs arm and 53% of total costs in the PICCs arm. As highlighted in previous chapters, PORTs are in situ for substantially longer periods than both Hickman-type devices and PICCs. As a consequence, patients in the PORTs arm have the potential to accumulate a higher level of resource use and costs. When catheter dwell times were taken into account, the difference in costs per catheter-week were –£126 (95% CI –£279 to £28) when comparing PICCs with Hickman-type devices, –£45 (95% CI –£744 to £655) when comparing PORTs with Hickman-type devices, and –£41 (95% CI –£227 to £147) when comparing PORTs with PICCs.
| Arm | n | Cost (£) | ||||||
|---|---|---|---|---|---|---|---|---|
| Device insertion (95% CI) | Per cent of total | Outpatients (95% CI) | Inpatients (95% CI) | Total unplanned follow-up (95% CI)a | Per cent of total | Total (95% CI) | ||
| PICCs vs. Hickman comparison | ||||||||
| Hickman | 212 | 1106 (1064 to 1148) | 33 | 70 (48 to 91) | 2226 (1110 to 3342) | 2295 (1197 to 3394) | 67 | 3401 (2224 to 4429) |
| PICC | 212 | 504 (484 to 523) | 29 | 67 (46 to 88) | 1160 (574 to 1746) | 1228 (636 to 1819) | 71 | 1732 (1126 to 2555) |
| PORTs vs. Hickman comparison | ||||||||
| Hickman | 303 | 1064 (1035 to 1092) | 41 | 74 (50 to 98) | 1451 (851 to 2052) | 1526 (917 to 2134) | 59 | 2590 (2023 to 3012) |
| PORT | 252 | 1397 (1355 to 1438) | 57 | 31 (20 to 42) | 1012 (560 to 1465) | 1044 (595 to 1514) | 43 | 2441 (1887 to 2906) |
| PORTs vs. PICCs comparison | ||||||||
| PICC | 199 | 495 (473 to 515) | 48 | 65 (41 to 88) | 482 (192 to 772) | 546 (240 to 853) | 53 | 1044 (725 to 1031) |
| PORT | 147 | 1388 (1319 to 1456) | 49 | 34 (20 to 48) | 1416 (440 to 2392) | 1450 (521 to 2379) | 51 | 2882 (1789 to 3734) |
Health-related quality-of-life data, based on the EQ-5D questionnaire, were collected monthly for patients with a device in situ; however, a large proportion of these patients did not complete their monthly EQ-5D questionnaire (Figure 21). These distributions of complete data, missing data and device removal/death were similar between the two arms over time in the Hickman versus PICCs comparison. Complete data were available for only 12% of all patients in this comparison. The number of missing data was consistently large in both arms (41–58% of patients with a device in situ did not complete the EQ-5D questionnaires in the first 6 months; this increased to 64–93% in the final 6 months).
As highlighted in previous chapters, PORTs were in situ for a longer period than Hickman-type devices and PICCs. Consequently, the proportion of complete data was higher for PORTs than for the comparator devices. However, the number of missing data was consistently large across all arms. In the PORTs versus Hickman comparison, 35–61% of PORTs patients with a device in situ did not complete the EQ-5D questionnaires following the baseline assessment, compared with 40–67% of Hickman patients. Complete data were available for only 9% of all patients in this comparison. In the PORTs versus PICCs comparison, the distributions of complete data, missing data and device removal/death were similar between the two arms over time. In patients with a device in situ, 40–67% of the PORTs patients and 43–75% of the PICCs patients did not complete EQ-5D questionnaires. Complete data were available for only 10% of all patients in this comparison.
FIGURE 21.
The EQ-5D data: relative proportions of patients with complete data, missing data and device removed or death. (a) Hickman vs. PICCs: Hickman arm; (b) Hickman vs. PICCs: PICCs arm; (c) PORTs vs. Hickman: PORTs arm; (d) PORTs vs. Hickman: Hickman arm; (e) PORTs vs. PICCs: PORTs arm; and (f) PORTs vs. PICCs: PICCs arm.
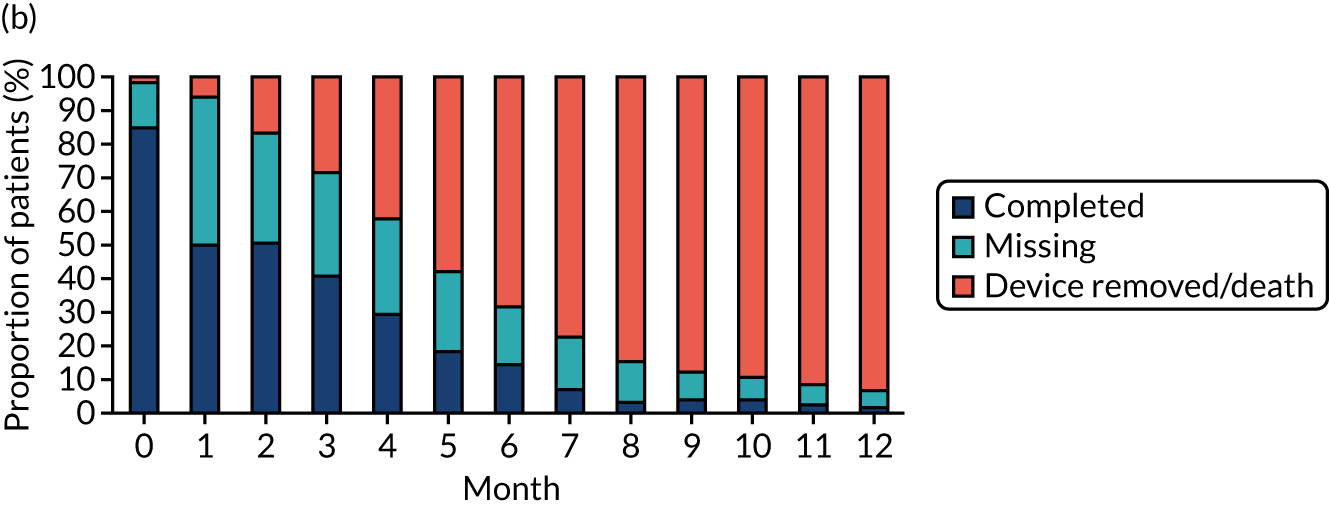
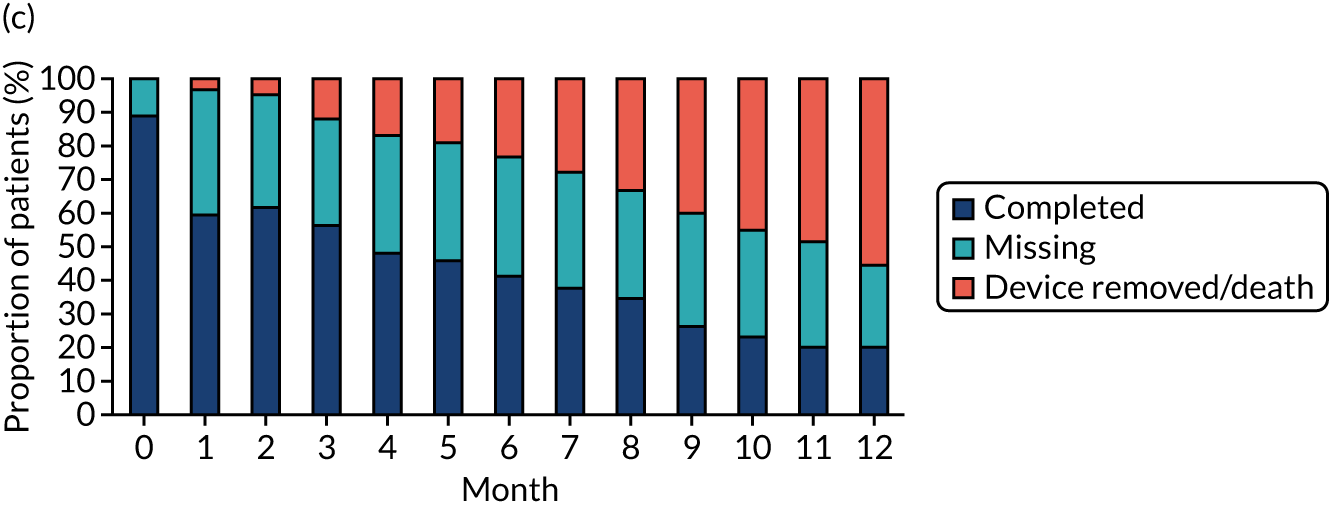
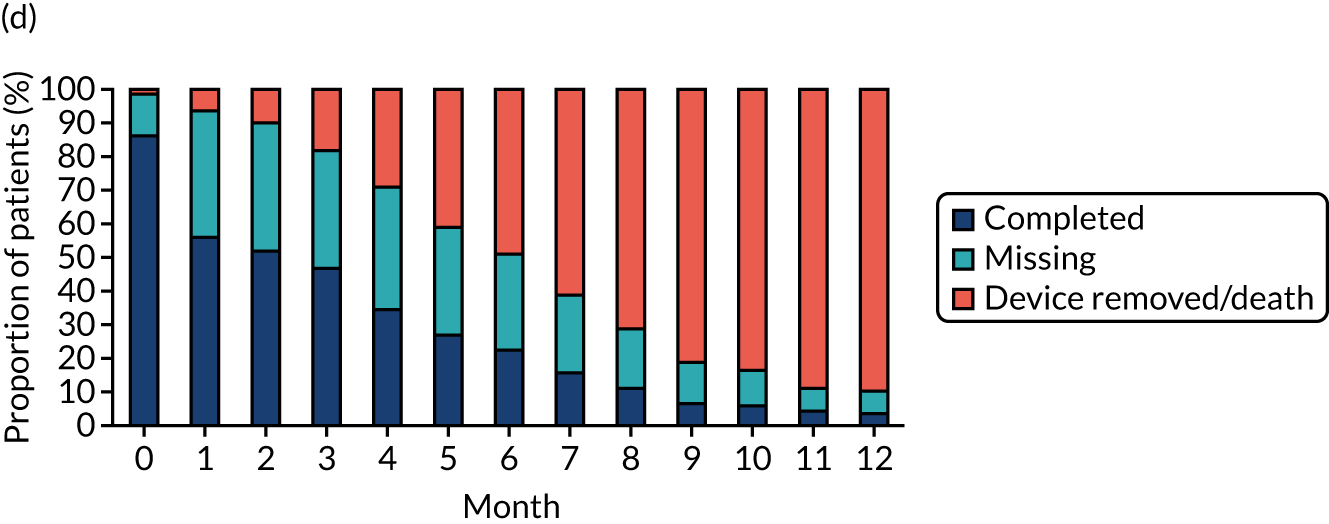
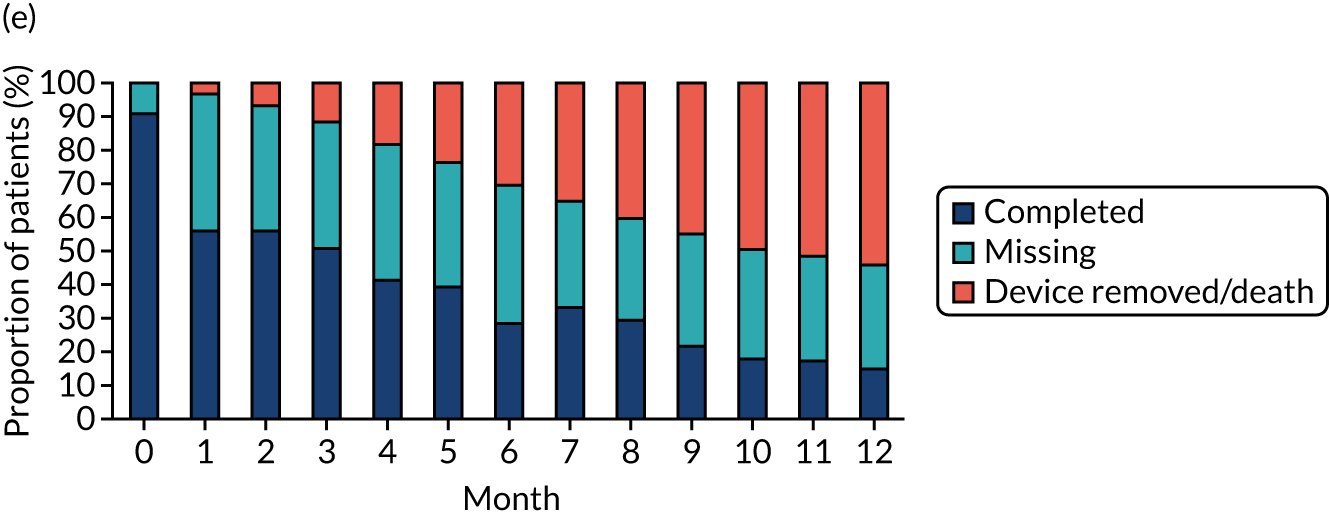
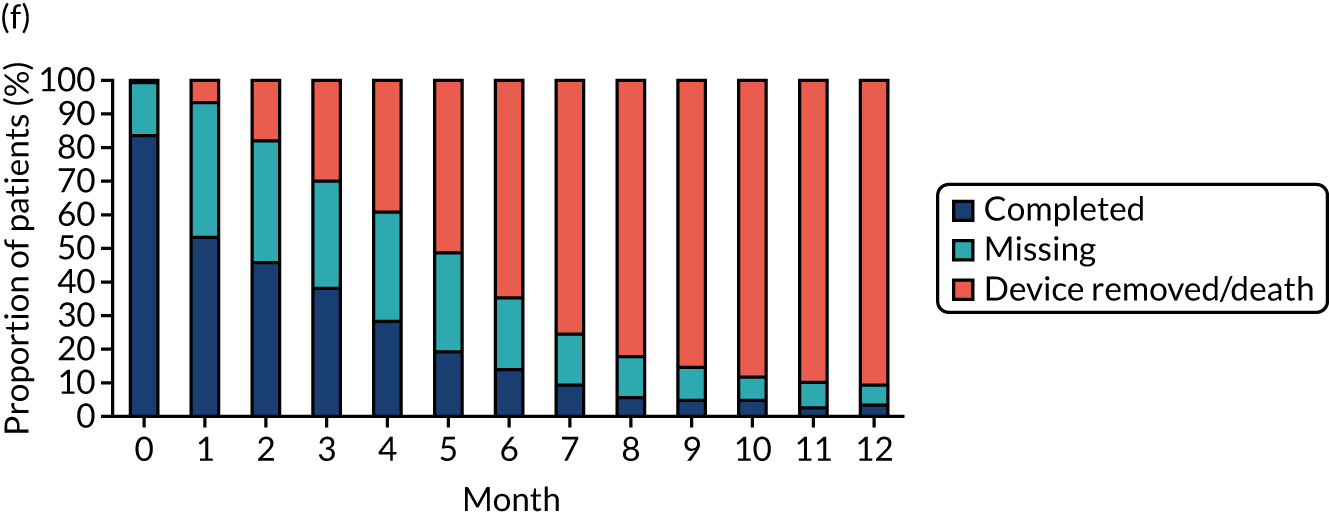
The proportion of patients who reported any problems with the EQ-5D domains at baseline, 3 months, 6 and 12 months are presented in Figure 22. In the PICCs versus Hickman comparison, approximately 50% of patients reported any problems with usual activities, and pain and discomfort. A smaller proportion of patients (30–40%) reported any problems with mobility, and anxiety or depression. Approximately 10% of patients reported any problems with self-care. This is consistent across the two devices. At 3 months, there was an improvement with pain or discomfort, and anxiety or depression. However, at 6 months, there was a general deterioration across all of the domains. At 12 months, data were available from only very few patients in the Hickman arm, of whom approximately 50% and 75% reported any problems with mobility and usual activities, respectively.
In the PORTs versus Hickman comparison, the trends at baseline and 3 months were consistent with those observed in the PICCs versus Hickman comparison. However, the improvement at 3 months was maintained at 6 months. Similar to the other comparison, 12-month data were only available from very few patients. The data showed a much greater proportion of patients in the Hickman arm than in the PORTs arm reported any problems with mobility; however, this needs to be interpreted with caution.
In the PORTs versus PICCs comparison, similar proportions of patients reported any problems with pain and discomfort, and anxiety or depression to that of the other two comparisons. However, the proportion of patients who reported any problems with the other three domains were lower than in the other two comparisons. At 3 months, a greater proportion of PORTs patients than PICCs patients reported any problems with mobility, usual activities, pain and discomfort, and anxiety or depression. However, this was no longer the case at 6 months, with very little differences between the two devices. Similar to other comparisons, 12-month data were available for only a few patients. The data showed much greater proportions of PORTs patients than PICCs patients reporting any problems with the five domains, but this needs to be interpreted with caution.
For the EQ-5D index values, see Figures 8, 13 and 18. Overall, there is little or no change in HRQoL over time across all devices and comparisons.
FIGURE 22.
Proportion of respondents with any problems with each of the EQ-5D domains.
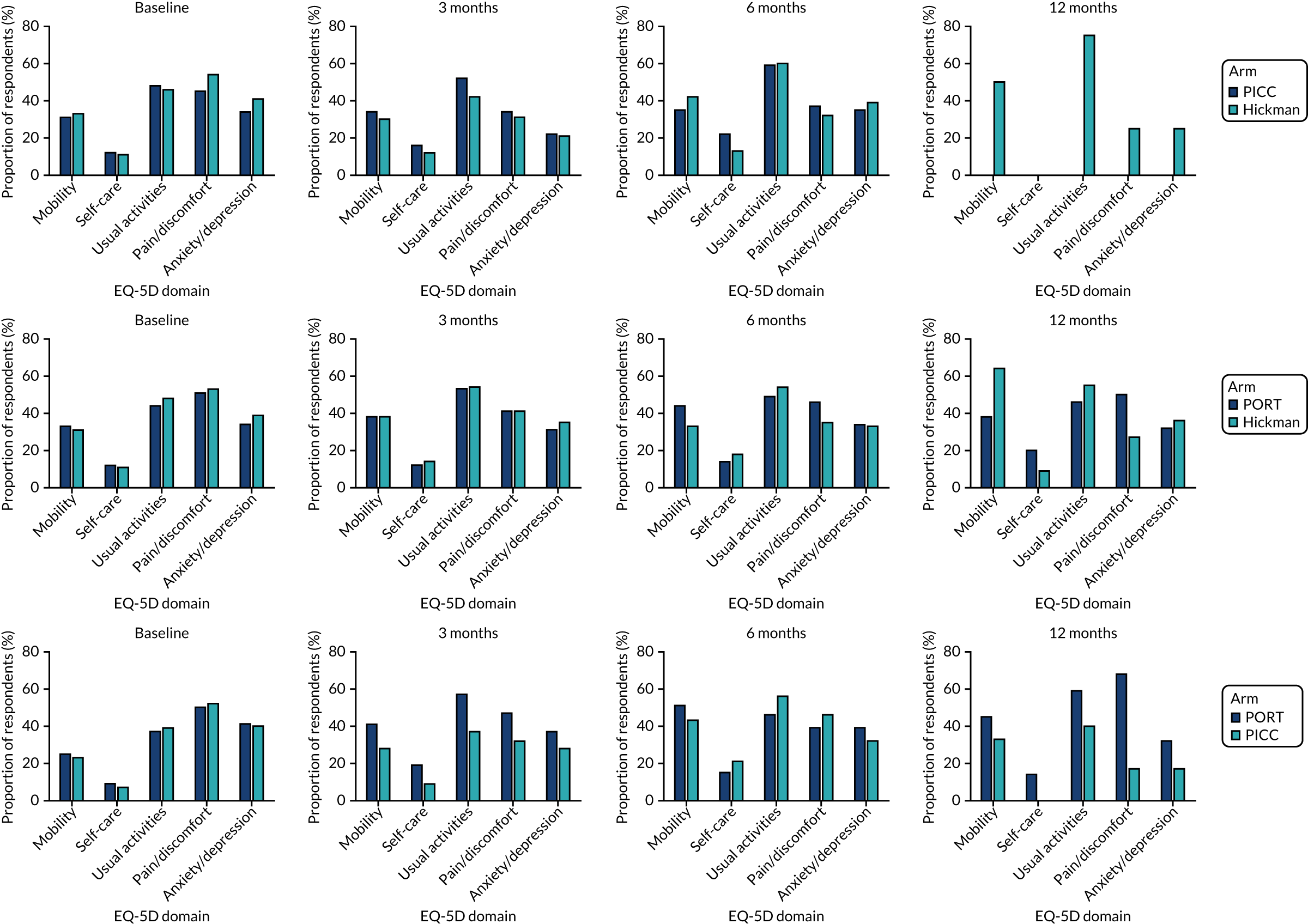
Cost-effectiveness results (base case)
Hickman versus peripherally inserted central catheters comparison
There was a total of 424 patients included in the PICCs (n = 212) versus Hickman (n = 212) comparison, who reported 110 and 103 complications, respectively (Table 17). The predicted mean total cost, which accounted for the skewed distribution and stratification factors in the trial, was £1708 in the PICCs arm, compared with £3262 in the Hickman arm. This lower cost (–£1553) in the PICCs arm was associated with lower mean QALY gained (–0.009 QALYs; equates to a loss of 3 days of perfect health over a 12-month period) and a higher complications rate (52% in the PICCs arm and 49% in the Hickman arm). However, the differences in QALYs and in complication rates were not statistically significant. Compared with Hickman-type devices, PICCs were associated with an ICER of £172,556 saved per QALY lost and, based on a cost-effectiveness ceiling ratio of £20,000, a NMB of £1373.
| Results | Arm | Difference | |
|---|---|---|---|
| PICCs | Hickman | ||
| N | 212 | 212 | |
| Complications (n) | 110 | 103 | 7 |
| Mean total costs (£) (95% CI) | 1708 (1153 to 2262) | 3262 (2227 to 4296) | –1553 (–2639 to –468) |
| Mean QALYs gained (95% CI) | 0.755 (0.739 to 0.771) | 0.763 (0.747 to 0.779) | –0.009 (–0.031 to 0.014) |
A visual illustration of the uncertainty surrounding the mean cost and QALY gain associated with each device over 1000 iterations of bootstrap analysis plotted on the cost-effectiveness plane is presented in Figure 23. The x-axis represents the incremental QALY gain, and the y-axis represents the incremental cost, associated with the use of PICCs compared with Hickman-type devices. The majority of the costs and QALY estimates lie in the south-west quadrant of the cost-effectiveness plane. Despite some uncertainty relating to the magnitude of the difference in costs between the two devices, the overall finding is that PICCs were associated with lower costs and a decrement in QALY gains when compared with Hickman. Irrespective of the willingness-to-pay threshold, the probability of the Hickman-type device being cost-effective compared with PICCs is low (see Figure 23).
FIGURE 23.
The PICCs vs. Hickman (a) cost-effectiveness plane and (b) acceptability curve.
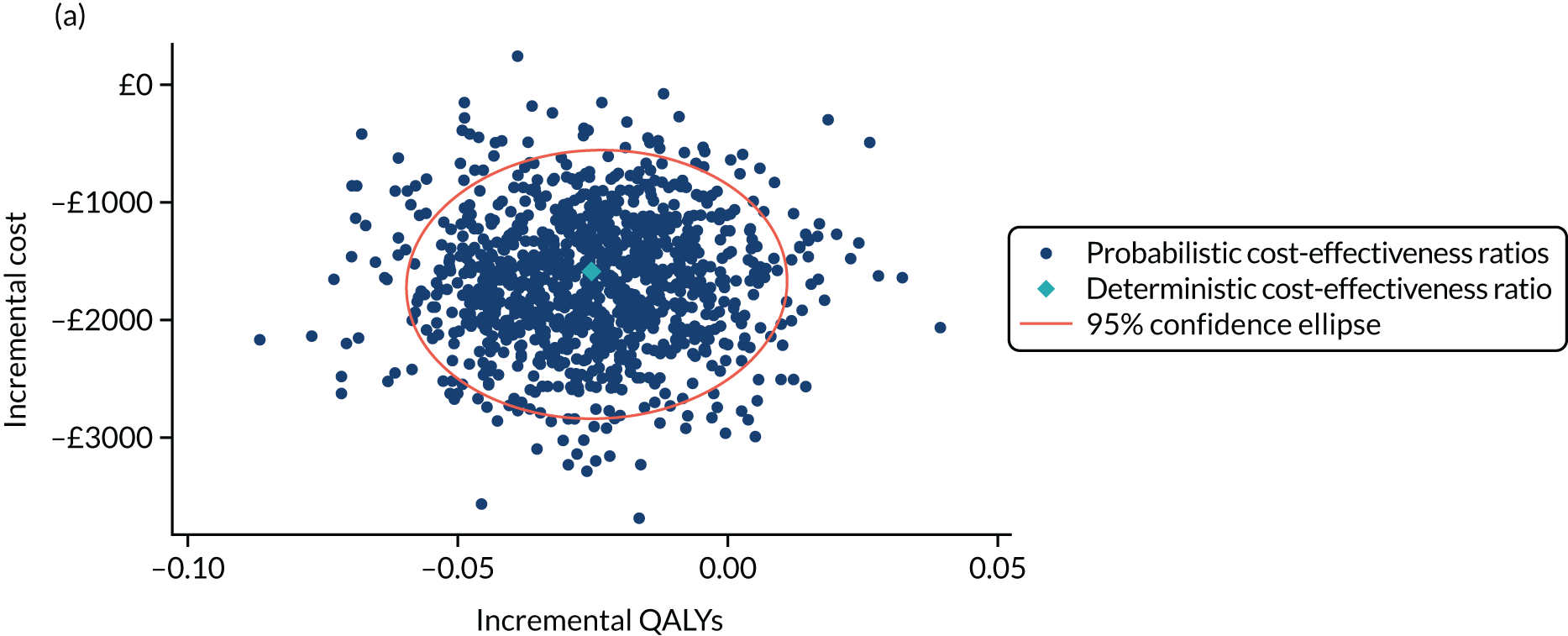
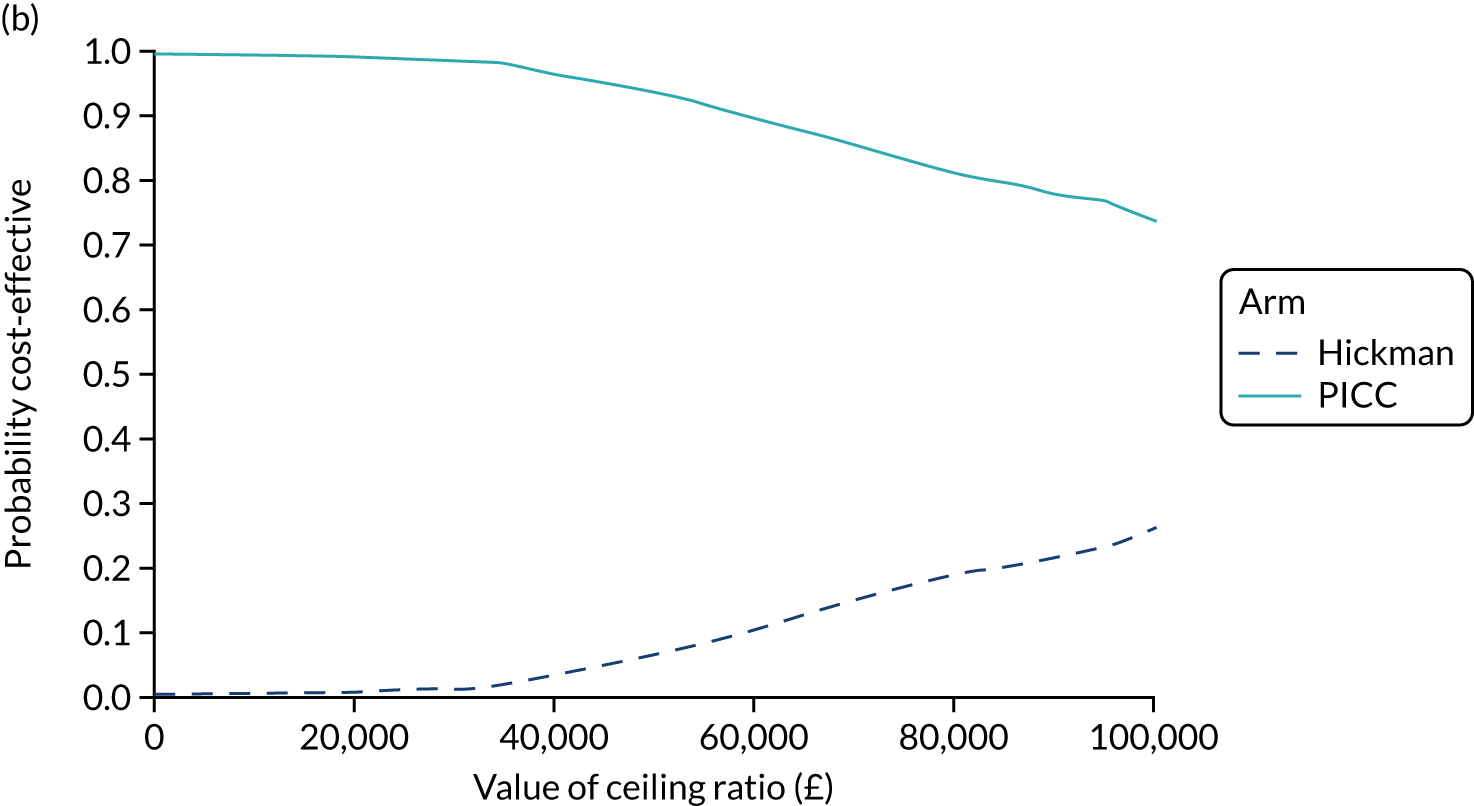
PORTs versus Hickman comparison
There was a total of 556 patients included in the PORTs (n = 253) versus Hickman (n = 303) comparison, who reported 73 and 131 complications, respectively (Table 18). The predicted mean total cost was £2436 in the PORTs arm, compared with £2481 in the Hickman arm. There was a very small difference in mean QALYs gained (0.004) favouring PORTs. Furthermore, the PORTs arm was associated with a substantially lower complication rate (29%) than the Hickman arm (43%). In this scenario, where the cost of PORTs was lower than that of Hickman with additional gain in QALYs, PORTs dominate Hickman. Similarly, PORTs dominate Hickman from the perspective of costs and complications averted. Compared with Hickman, PORTs were associated with an ICER of –£11,250 per QALY gained (note that a negative ICER in this case owing to PORTs being dominant) and, based on a cost-effectiveness ceiling ratio of £20,000, a NMB of £125.
| Results | Arm | Difference | |
|---|---|---|---|
| PORTs | Hickman | ||
| N | 253 | 303 | |
| Complications (n) | 73 | 131 | –58 |
| Mean total costs (£) (95% CI) | 2436 (1927 to 2946) | 2481 (2007 to 2954) | –45 (–744 to 655) |
| Mean QALYs gained (95% CI) | 0.746 (0.728 to 0.763) | 0.742 (0.726 to 0.759) | 0.004 (–0.020 to 0.027) |
A visual illustration of the uncertainty surrounding the mean cost and QALY gain associated with each device over 1000 iterations of bootstrap analysis plotted on the cost-effectiveness plane is presented in Figure 24. The majority of the simulated mean costs and mean QALYs were centred around the origin; there is little difference in costs and QALYs between the two arms.
FIGURE 24.
The PORTs vs. Hickman cost-effectiveness plane.
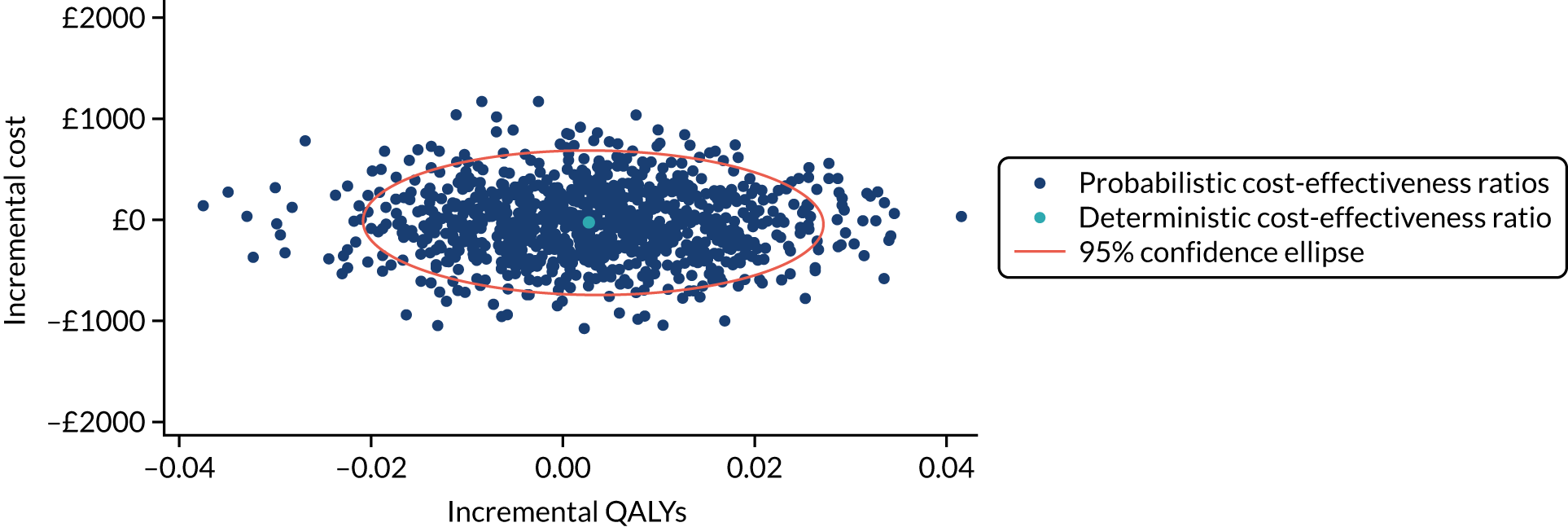
PORTs versus peripherally inserted central catheters comparison
There was a total of 346 patients included in the PORTs (n = 147) versus PICCs (n = 199) comparison, who reported 47 and 93 complications, respectively (Table 19). The predicted mean total cost was £2706 in the PORTs arm, compared with £1041 in the PICCs arm. There was a difference in mean QALYs gained between the two arms (0.741 in the PORTs arm and 0.759 in the PICCs arm; –0.018 equates to a loss of 6.5 days of perfect health over a 12-month period); this was not statistically significant. However, the PORTs arm was associated with a substantially lower complications rate (32%) than the PICCs arm (47%). In this scenario, PORTs were dominated by PICCs (the cost of PORTs was greater and the QALYs were lower). Compared with PICCs, PORTs were associated with an ICER of –£56 per QALY gained (note that a negative ICER in this case owing to PORTs being dominated) and, based on a cost-effectiveness ceiling ratio of £20,000, a NMB of –£2025. However, PORTs were associated with a 15% reduction in complication rates. The incremental cost per complication averted (based on the number of complications averted per 100 patients) was £104.
| Results | Arm | Difference | |
|---|---|---|---|
| PORTs | PICCs | ||
| N | 147 | 199 | |
| Complications (n) | 47 | 93 | –46 |
| Mean total costs (£) (95% CI) | 2706 (1899 to 3513) | 1041 (764 to 1316) | 1665 (766 to 2564) |
| Mean QALYs gained (95% CI) | 0.741 (0.723 to 0.759) | 0.759 (0.744 to 0.775) | –0.018 (–0.042 to 0.006) |
A visual illustration of the uncertainty surrounding the mean cost and QALY gain associated with each device over 1000 iterations of bootstrap analysis plotted on the cost-effectiveness plane is presented in Figure 25. The majority of the simulated mean costs and mean QALYs were centred around the origin; there is little difference in costs and QALYs between the two arms. Despite some uncertainty related to the magnitude of the difference in costs between the two devices, the overall finding is that PORTs were associated with greater costs and a small decrement in QALY gains than PICCs.
FIGURE 25.
The PORTs vs. PICCs cost-effectiveness plane.
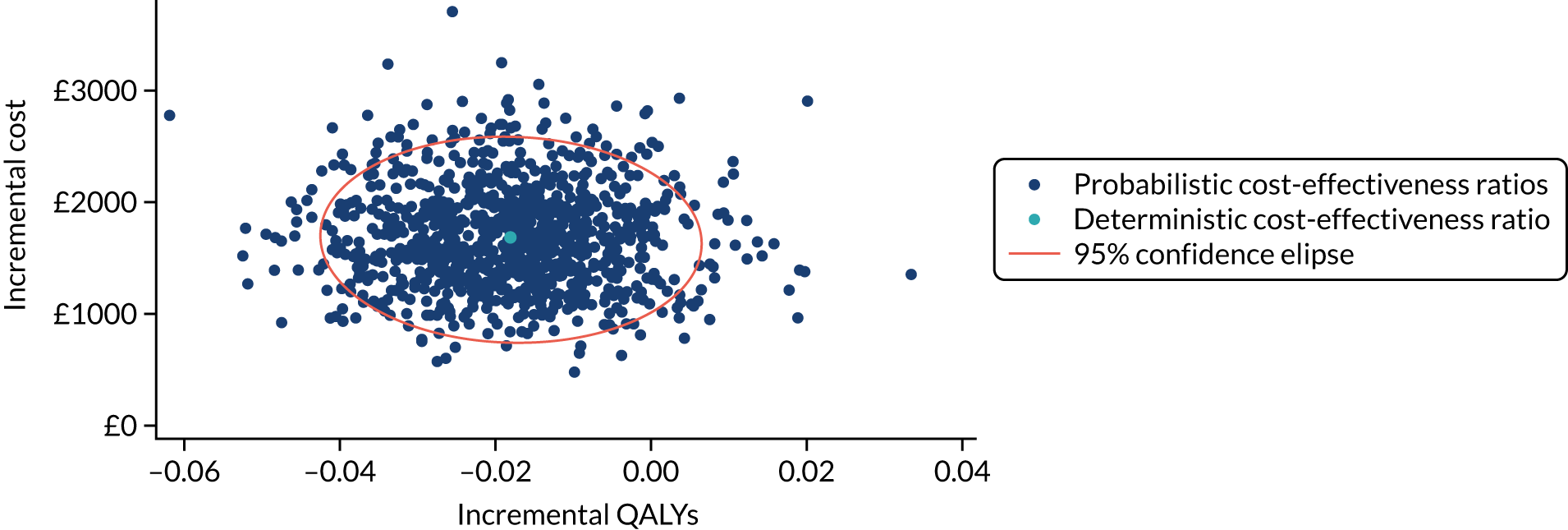
Sensitivity and scenario analysis
Time horizon of 3 and 6 months
The majority of the complications reported in the CAVA trial occurred within the first 6 months of the follow-up period. In addition, the data for the EQ-5D were also more complete in the first 6 months than the final 6 months of the follow-up. Therefore, the sensitivity analysis was conducted by restricting the data to 3 and 6 months of follow-up. In the PICCs versus Hickman comparison, at 3 months, PICCs were associated with lower costs (–£1077) but also with a small decrement in QALYs gained (–0.020); similar findings were observed at 6 months (Table 20). These results were in line with the base case. In the PORTs versus Hickman comparison, at 3 months, PORTs were associated with lower costs (–£135) but also with a small decrement in QALYs gained (–0.009; equivalent to 3 days of perfect health); similar findings were observed at 6 months. This small QALY decrement was not observed with the base case, which showed an incremental QALY gain of 0.004 at 12 months. In the PORTs versus PICCs comparison at 3 months, PORTs were associated with greater costs (£1039) but with a small decrement in QALYS gained (–0.010; equivalent to 3.5 days of perfect health); similar findings were observed at 6 months. This is in line with the results of the base case.
| Time point | Arm | Difference | |
|---|---|---|---|
| PICCs | Hickman | ||
| Base case (n) | 212 | 212 | |
| Mean total costs (£) (95% CI) | 1708 (1153 to 2262) | 3262 (2227 to 4296) | –1553 (–2639 to –468) |
| Mean QALYs gained (95% CI) | 0.755 (0.739 to 0.771) | 0.763 (0.747 to 0.779) | –0.009 (–0.031 to 0.014) |
| 3-month follow-up (n) | 174 | 152 | |
| Mean total costs (£) (95% CI) | 998 (714 to 1282) | 2076 (1479 to 2673) | –1077 (–1755 to –400) |
| Mean QALYs gained (95% CI) | 0.751 (0.733 to 0.768) | 0.752 (0.735 to 0.769) | –0.002 (–0.026 to 0.022) |
| 6-month follow-up (n) | 104 | 67 | |
| Mean total costs (£) (95% CI) | 1520 (997 to 2.043) | 2675 (1743 to 3606) | –1155 (–2097 to –214) |
| Mean QALYs gained (95% CI) | 0.754 (0.737 to 0.770) | 0.757 (0.741 to 0.774) | –0.003 (–0.026 to 0.019) |
| Time point | Arm | Difference | |
| PORTs | Hickman | ||
| Base case (n) | 253 | 303 | |
| Mean total costs (£) (95% CI) | 2436 (1927 to 2946) | 2481 (2007 to 2954) | –45 (–744 to 655) |
| Mean QALYs gained (95% CI) | 0.746 (0.728 to 0.763) | 0.742 (0.726 to 0.759) | 0.004 (–0.020 to 0.027) |
| 3-month follow-up (n) | 222 | 245 | |
| Mean total costs (£) (95% CI) | 1693 (1282 to 2104) | 1829 (1419 to 2239) | –135 (–713 to 441) |
| Mean QALYs gained (95% CI) | 0.739 (0.721 to 0.757) | 0.748 (0.731 to 0.764) | –0.009 (–0.033 to 0.016) |
| 6-month follow-up (n) | 192 | 153 | |
| Mean total costs (£) (95% CI) | 1971 (1475 to 2467) | 2159 (1657 to 2660) | –187 (–8741,829 to 500) |
| Mean QALYs gained (95% CI) | 0.736 (0.717 to 0.754) | 0.749 (0.732 to 0.767 | –0.014 (–0.039 to 0.012) |
| Time point | Arm | Difference | |
| PORTs | PICCs | ||
| Base case (n) | 147 | 199 | |
| Mean total costs (£) (95% CI) | 2706 (1899 to 3513) | 1041 (764 to 1316) | 1665 (766 to 2564) |
| Mean QALYs gained (95% CI) | 0.741 (0.723 to 0.759) | 0.759 (0.744 to 0.775) | –0.018 (–0.042 to 0.006) |
| 3-month follow-up (n) | 129 | 138 | |
| Mean total costs (£) (95% CI) | 1975 (1251 to 2699) | 936 (639 to 1232) | 1039 (239 to 1839) |
| Mean QALYs gained (95% CI) | 0.758 (0.740 to 0.777) | 0.769 (0.753 to 0.785) | –0.010 (–0.034 to 0.013) |
| 6-month follow-up (n) | 101 | 70 | |
| Mean total costs (£) (95% CI) | 2133 (1354 to 2912) | 981 (671 to 1291) | 1152 (281 to 2023) |
| Mean QALYs gained (95% CI) | 0.755 (0.737 to 0.773) | 0.764 (0.748 to 0.780 | –0.009 (–0.034 to 0.015) |
Solid tumours only
A sensitivity analysis was performed on patients with solid tumours only (Table 21). The main impact seen in this analysis is the reduction in mean total costs, primarily driven by the mean unplanned follow-up associated with PORTs. The mean unplanned follow-up costs associated with PORTs reduced from £2436 in the base case to £2096 when compared with Hickman; similarly, a reduction from £2706 (base case) to £2286 was observed when compared with PICCs. In the PICCs versus Hickman comparison, the results were consistent with the base case. In the PORTs versus Hickman comparison, PORTs were associated with lower costs (–£59) and a small decrement in QALYs gained (–0.001; equivalent to less than 1 day of perfect health) than Hickman. In the PORTs versus PICCs comparison, the results were consistent with the base case.
| Results | Arm | Difference | |
|---|---|---|---|
| PICCs | Hickman | ||
| Base case (n) | 212 | 212 | |
| Mean total costs (£) (95% CI) | 1708 (1153 to 2262) | 3262 (2227 to 4296) | –1553 (–2639 to –468) |
| Mean QALYs gained (95% CI) | 0.755 (0.739 to 0.771) | 0.763 (0.747 to 0.779) | –0.009 (–0.031 to 0.014) |
| Solid tumours only (n) | 109 | 204 | |
| Mean total costs (£) (95% CI) | 1439 (995 to 1883) | 2767 (1939 to 3594) | –1327 (–2099 to –555) |
| Mean QALYs gained (95% CI) | 0.767 (0.750 to 0.784) | 0.778 (0.761 to 0.795) | –0.011 (–0.035 to 0.014) |
| Results | Arm | Difference | |
| PORTs | Hickman | ||
| Base case (n) | 253 | 303 | |
| Mean total costs (£) (95% CI) | 2436 (1927 to 2946) | 2481 (2007 to 2954) | –45 (–744 to 655) |
| Mean QALYs gained (95% CI) | 0.746 (0.728 to 0.763) | 0.742 (0.726 to 0.759) | 0.004 (–0.020 to 0.027) |
| Solid tumours only (n) | 235 | 280 | |
| Mean total costs (£) (95% CI) | 2096 (1710 to 2481) | 2154 (1791 to 2518) | –59 (–587 to 469) |
| Mean QALYs gained (95% CI) | 0.742 (0.724 to 0.760) | 0.743 (0.727 to 0.759) | –0.001 (–0.023 to 0.023) |
| Results | Arm | Difference | |
| PORTs | PICCs | ||
| Base case (n) | 147 | 199 | |
| Mean total costs (£) (95% CI) | 2706 (1899 to 3513) | 1041 (764 to 1316) | 1665 (766 to 2564) |
| Mean QALYs gained (95% CI) | 0.741 (0.723 to 0.759) | 0.759 (0.744 to 0.775) | –0.018 (–0.042 to 0.006) |
| Solid tumours only (n) | 142 | 190 | |
| Mean total costs (£) (95% CI) | 2286 (1641 to 2932) | 977 (724 to 1229) | 1310 (662 to 1957) |
| Mean QALYs gained (95% CI) | 0.745 (0.727 to 0.763) | 0.763 (0.748 to 0.779) | –0.018 (–0.042 to 0.006) |
Nurse-led service of care
The health-care resource use required for PORTs insertion varied across the sites within the CAVA trial (Table 22). A scenario analysis based on a nurse-led service of care was conducted. Under this scenario, it was assumed that the insertion of PORTs will be led by a senior nurse, with the support of two others. Overall, this reduced the cost of PORTs insertion and subsequently the total costs. When compared with Hickman, PORTs are no longer associated with greater costs (–£297). At lower costs and when there is no difference in QALYs, PORTs become the dominant option. When compared with PICCs, the incremental cost of PORTs was less than that of the base case (£1194 vs. £1665); however, PICCs remained the dominant strategy (lower costs and greater QALYs gained).
| Results | Arm | Difference | |
|---|---|---|---|
| PORTs (n = 253) | Hickman (n = 303) | ||
| Base case | |||
| Mean total costs (£) (95% CI) | 2436 (1927 to 2946) | 2481 (2007 to 2954) | –45 (–744 to 655) |
| Mean QALYs gained (95% CI) | 0.746 (0.728 to 0.763) | 0.742 (0.726 to 0.759) | 0.004 (–0.020 to 0.027) |
| Nurse-led PORTs insertion | |||
| Mean total costs (£) (95% CI) | 1781 (1280 to 2282) | 2078 (1537 to 2620) | –297 (–1043 to 449) |
| Mean QALYs gained (95% CI) | 0.746 (0.728 to 0.763) | 0.742 (0.726 to 0.759) | 0.004 (–0.020 to 0.027) |
| Results | Arm | Difference | |
| PORTs (n = 147) | PICCs (n = 199) | ||
| Base case | |||
| Mean total costs (£) (95% CI) | 2706 (1899 to 3513) | 1041 (764 to 1316) | 1665 (766 to 2564) |
| Mean QALYs gained (95% CI) | 0.741 (0.723 to 0.759) | 0.759 (0.744 to 0.775) | –0.018 (–0.042 to 0.006) |
| Nurse-led PORTs insertion | |||
| Mean total costs (£) (95% CI) | 2132 (1239 to 3024) | 938 (589 to 1288) | 1194 (163 to 2225) |
| Mean QALYs gained (95% CI) | 0.741 (0.723 to 0.759) | 0.759 (0.744 to 0.775) | –0.018 (–0.042 to 0.006) |
Discussion
We have estimated the costs and QALYs associated with the use of three venous access devices commonly used for the delivery of long-term chemotherapy: Hickman, PICCs and PORTs. Overall, our results showed that PORTs were associated with a similar mean total cost as Hickman, but with a greater total cost than PICCs. However, across all three devices, there was little difference in mean QALYs.
Our base case showed that, compared with Hickman, PICCs were associated with a substantially lower cost (–£1553) and a decrement in QALYs gained (–0.009); this was accompanied by a higher complications rate (3%) for PICCs. Under such circumstances, PICCs could be considered as a cost-effective alternative to Hickman if we were willing to accept health loss at a given level of cost savings. The current NICE threshold for cost-effectiveness of willingness to pay of £20,000 per QALY gained could be interpreted as a minimum saving of £20,000 required to forgo one QALY. In the PICCs versus Hickman scenario, a cost saving of £1553 would result in the loss of 0.009 QALYs, equivalent to a saving of £172,556 for each QALY forgone (which far exceeds the £20,000 savings required). On balance, we would consider PICCs to be a cost-effective alternative to Hickman.
However, there is much uncertainty associated with these findings, primarily as a result of this comparison being underpowered within the CAVA trial. If the trial had had sufficient power to show PICCs to be non-inferior to Hickman, in accordance with the trial hypothesis, there would be less uncertainty associated with these findings.
PORTs were associated with a small incremental cost when compared with Hickman (£45), but with a significantly greater cost than PICCs (£1665). However, PORTs were associated with an increment in QALYs gained (0.004) when compared with Hickman and a decrement in QALYs gained (–0.018; difference was not statistically significant) when compared with PICCs. This was alongside a lower complications rate than with Hickman (14%) and with PICCs (15%). Based on the framework of a cost–utility analysis, under such circumstances, PORTs dominate Hickman and are considered cost-effective; however, PORTs would not be considered cost-effective when compared with PICCs. However, if we were to consider infections averted as the health outcome in a cost-effectiveness analysis, PORTs were associated with an incremental cost of £1.36 per complication averted when compared with Hickman and £104 per complication averted when compared with PICCs. PORTs were in situ for a significantly longer period than both PICCs and Hickman. Accounting for device dwelling time would result in a lower cost per catheter-week for PORTs than for Hickman (–£47, 95% CI –£166 to £73), and for PICCs (–£41, 95% CI –£227 to £147).
Despite lower complication rates in the PORTs arm than in the Hickman arm and the PICCs arm, the mean follow-up costs were higher in the PORTs arm than the PICCs arm. This was primarily driven by the longer length of stay in the follow-up period. This may be owing to differing approaches to managing complications across the devices. It may be the case that the threshold for removing a device in the case of a complication is greater for PORTs, than for other devices, especially when compared with PICCs. Unlike PICCs, which can be readily removed, there may be a reluctance to remove PORTs when patients experience complications. For instance, in the case of infections, it is likely that PICCs can be readily removed and replaced while the patient receives a course of oral antibiotics. However, in the case of PORTs, it is likely that patients would be admitted for intravenous antibiotics to treat the infection while the device is kept in situ.
Based on data from EQ-5D collected during the trial, we estimated little difference in mean QALYs across the three devices. However, there are major limitations to our EQ-5D data, such as follow-up being restricted to patients with the device in situ only and the large number of missing data from these patients. There is also some concern that EQ-5D may not be sensitive to changes in quality of life associated with the venous access devices. The CAVA trial comprised cancer patients who were expected to receive long-term chemotherapy. It is possible, therefore, that the impact of any preference for venous access device is dominated by the cancer diagnosis and the burden of chemotherapy treatment. Furthermore, contrary to the EQ-5D findings, assessment of changes in quality of life based on the Venous Access Device Questionnaire revealed significant improvement in several domains when comparing PORTs with Hickman and with PICCs. This may not have been adequately captured by the EQ-5D and the subsequent QALY estimates. The economic evaluation alongside the CAVA trial has adhered to good practice set out in the NICE guidance. 20 However, given the potential limitations of the EQ-5D in this context, the NICE-preferred cost–utility framework may not be appropriate for decision-making.
In the CAVA trial, complications data were not explicitly linked to resource use. For this reason, it was not possible to attribute specific complications to specific outpatient visits and inpatient admissions. Hence, resource use recorded during the overall follow-up period is an imperfect proxy for complication costs in our analysis. We also performed sensitivity analysis to test the robustness of our results. The results from the sensitivity and scenario analysis were consistent with the base case. The majority of the complications occurred within the first 6 months of the follow-up period, and the majority of the costs had been incurred at that stage. The results from the 3-month and the 6-month follow-ups were consistent with the base case. In the sensitivity analysis of patients with solid tumours only, PORTs were associated with lower costs and a small QALY gain when compared with Hickman; in this scenario, PORTs were considered cost-effective. PORTs were associated with greater cost of insertion than both Hickman and PICCs. An alternative service delivery model for PORTs has the potential to reduce this cost. We explored a nurse-led service, but there may also be a role for manufacturers to play here, in terms of reducing the cost of PORTs. The cava trial has shown that currently PORTs are not routinely delivered through a nurse-led model. If such a service delivery model were to be implemented, a programme of education and training would be required to prepare clinical staff for the routine delivery of PORTs in this patient population.
There are few studies that have formally evaluated the cost-effectiveness of venous access devices. One study published in 2007 evaluated the complication rates and costs associated with the use of Hickman compared with PORTs in patients with solid tumours. 40 The study found that, despite the greater initial cost (purchase and implantation) of PORTs, the lower rate of complications led to an overall cost saving from the use of PORTs (£1483) compared with the use of Hickman-type devices (£1512). Our sensitivity analysis on patients with solid tumours only came to similar conclusions. In 2016, our team conducted a pilot study comparing PORTs with Hickman-type devices. 3 We reported that PORTs were associated with a lower cost, a lower rate of complications and a smaller number of QALYs gained than Hickman-type devices. However, this was a very small study based on a single centre. Only the cost of the devices and not the costs associated with insertion of the devices was taken into account. Since then, one study has evaluated complication rates, costs and quality of life associated with PORTs compared with PICCs. 41 Based on data from 70 patients with solid tumours, the results showed a greater initial cost of device insertion associated with PORTs, but a lower cost relating to complications with PICCs. This resulted in no difference in overall total mean cost between devices. Similar to the CAVA trial, this study also collected quality-of-life data using a non-validated study-specific central venous line questionnaire, which covered functional status, sleep and hygiene disturbance for 36 patients. No difference in quality of life was reported at the end of the trial (6 months). However, patients commented that the questionnaire did not ask about several aspects of quality of life that were affected by the choice of device (e.g. showering, bathing and swimming), and that the study found there was a preference for PORTs among those patients who had previously received a PICC line. A more recent study evaluating complication rates and cost associated with the use of PICCs compared with PORTs found that, despite the initial higher costs associated with PORTs insertion, few complications among those receiving PORTs led to a higher total cost per patient associated with the use of PICCs (£825) than with the use of PORTs (£662). 17 Although this study did not formally collect validated quality-of-life data, it was noted that patients in the trial reported more insertion-related discomfort in the PORTs arm, although there was a smaller impact on activities of daily life.
Chapter 9 Post-trial qualitative results
Despite recent growth in patient-centred approaches, the views of patients are often not factored into device selection, and patient support can focus on the clinical aspects of devices to the exclusion of patients’ day-to-day experiences. 42 In part, this reflects poor understanding of these views and experiences. Staff attitudes and experiences, and the ways in which these may affect device selection and management, are similarly poorly understood. The post-trial qualitative study sought to examine patient and staff acceptability of the three CAVA trial devices, as well as the experiences of trial participation. 43
Focus groups with patients
The characteristics of the 42 participants are presented in Table 23.
| Characteristic | Participants |
|---|---|
| Age (years) | |
| Mean | 61.7 |
| SD | 8.6 |
| Range | 45–79 |
| Sex (n) | |
| Male | 22 |
| Female | 20 |
| Type of cancer (n) | |
| Colorectal | 24 |
| Breast | 5 |
| Ovarian | 4 |
| Pancreatic | 3 |
| Endometrioid | 2 |
| Lung | 1 |
| Oesophageal | 1 |
| Prostate | 1 |
| Acute myeloid leukaemia | 1 |
| CVAD use (n) | |
| Hickman type | 11 |
| PICC | 15 |
| PORT | 16 |
| CVADs prior to CAVA trial participation (n) | |
| None | 30 |
| At least one, inserted > 3 months prior to study entry | 11 |
| At least one, inserted ≤ 3 months prior to study entry | 1 |
| Weeks elapsed since consenting to participate in the trial | |
| Mean | 40.9 |
| SD | 34.2 |
| Range | 3–137 |
Four main themes were identified from the analysis of the focus group data: (1) acceptability of CVADs, (2) living with a CVAD, (3) patterns of preference and (4) experiences of trial participation. In general, attitudes towards all three devices appeared to be positive, with patients viewing their CVAD as part of their treatment and recovery. Participants with PICCs and Hickman-type devices tended to compare their device favourably with peripheral cannulation. By comparison, participants with PORTs consistently compared their device with PICCs and Hickman-type devices, emphasising the perceived superiority of PORTs. PORTs were perceived to offer unique psychological benefits, including a greater sense of freedom and less intrusion in the context of personal relationships. Participation in the trial was motivated by prosocial intentions and potential access to otherwise unavailable devices and, for most participants, was viewed as a source of new knowledge and additional support.
Acceptability of central venous access devices
Most participants expressed satisfaction with their device and, in general, devices were regarded as having less of an impact than other aspects of participants’ journeys, including the side effects of chemotherapy, surgical interventions and the effects of their illness more broadly. Participants with experience of chemotherapy via peripheral cannulation said that their device facilitated treatment, making administration less painful and easier (for themselves and staff). Participants tended to frame satisfaction in terms of the role of their device in their treatment and in their overall journey. They saw their device as part of their treatment and, hence, part of the reason that they were getting better or were ‘still here’: ‘It’s part of my treatment so it’s part of my life, it’s there’ (female, PICC). In addition, when expressing satisfaction with their device, many participants referred to having had a problem-free experience with their device. That being said, our sample included people who had experienced various difficulties and complications with each of the devices. We found that even these patients remained satisfied with their overall experience. Complications, including partial or minor malfunctions, did not appear to affect acceptability or satisfaction, as long as the device remained functional.
Living with a central venous access device
Notwithstanding acceptance, living with a CVAD presented distinct challenges, which necessitated meaningful adjustments and adaptations. Although the experience of individual participants differed substantially, we provide an inclusive account of the wide range of experiences described, highlighting points of difference between devices.
Practical challenges
Participants with PICCs and those with Hickman-type devices described comparable challenges and responses, which centred on keeping their external line clean, secure and comfortable. At a minimum, most reported needing to change the ways in which they bathed or slept. To keep their line dry, participants described sourcing and using various waterproof covers or innovating their own solutions using household items [e.g. ‘I put cling-film on it’ (male, PICC)]. Some described adopting a conscientious approach to choosing clothing that could both conceal and accommodate their devices: ‘I certainly think about what I’m going to wear’ (female, Hickman). Here, too, they exhibited resourcefulness; they described improvising protective covers and finding ways of securing lines with underclothing or sterile dressings [e.g. ‘I had an old pair of tights I used to cut them and I had different colours and that worked fine’ (female, PICC)]. These difficulties were heightened when portable systemic anticancer therapy infusion pumps were attached to devices for treatment, preventing participants from securing or covering their line in the usual way:
Quite often I slept downstairs on the couch because I was worried about my partner who does [big arm movements] you know in his sleep and I thought it’s better with these wires going from here and up through your jammies, so I just slept downstairs, so that’s what I did on those nights [when pump was attached].
Female, PICC
For participants with PORTs, experiences varied more markedly depending on whether or not a portable systemic anti-cancer treatment pump was attached to their device. With pumps in place, participants with PORTs experienced the same challenges described by those with PICCs and Hickman-type devices. Most of the time, without a pump attached, participants experienced few concerns regarding everyday activities, although some reported feeling cautious about their device, especially early on and during sleep. In general, participants with PORTs claimed that based on their observations or direct experiences (some had experienced different devices prior to the CAVA trial), PORTs were more discreet, more secure, less disruptive of hobbies and activities, and easier to live with and maintain than PICCs and Hickman-type devices:
It’s just ease of use and the fact that it’s not in usage 24/7 [24 hours a day, 7 days a week] for months on end like the Hickman lines size of a packet of fags hanging on your chest, or the cannula sticking out your arm constantly that you have to protect and worry about.
Male, PORT
Gaps in knowledge
Across all three devices, the adjustments and adaptations described by participants were often associated with moments of uncertainty regarding proper care of their device or moments when their device complicated ordinary activities:
Well, it’s like the showering. ‘Cause you don’t know until you’re home and you think ‘Oh, right. How am I gonna get around this now?’. So, you suss it out for yourself.
Female, PORT
Where participants were unable to find a solution to a particular problem, they sometimes gave up and disregarded care advice:
I abandoned trying to keep it dry and I was just very naughty and I would shower regardless.
Female, PICC
Some of those who found that their device was affected by seatbelts reported wearing their belt in unconventional ways and were uncertain if what they were doing was safe or legal:
There is a question about eh, you know, is it safe? Is it legal?
Male, PORT
Participants with PORTs demonstrated a unique knowledge gap; many were unclear as to how long their device would remain in place and what this might mean for ongoing care and support.
Clinical care
Participants spoke at length about interactions with clinical staff, particularly those who they encountered outside oncology departments: mainly community nurses (district nurses), but also staff at local clinics and GP surgeries and other hospital departments. These discussions centred on a lack of staff experience and knowledge regarding CVADs. Although this applied to all devices, it was especially pervasive in the case of PORTs:
But they [district nurses] would NOT touch the port. They wanted nothing to do with it whatsoever.
Male, PORT: prior experience of PICC
Insufficient staff training resulted in several issues for participants, including avoidance of the device by clinical staff and inexpert handling of the device, as well as formal and informal participant involvement in staff training. Participants reported feeling disappointment or frustration when clinical staff were unwilling or unable to use their device for purposes such as drawing blood or administering contrast materials for CT scans. This typically caused delays, missed appointments and other inconveniences. It also meant additional needlesticks or the use of peripheral cannulation, which participants had hoped to avoid.
Emotional and psychological impact
The practical benefits associated with the lack of external lines with PORTs (i.e. less visible and easier maintenance) meant that they appeared to be less psychologically burdensome. In particular, participants with PORTs repeatedly stressed that it was easy for them to ‘forget’ about their device for days or weeks at a time. Participants described feeling ‘free’ between rounds of treatment:
This thing [PORT] is kind of a plug and play approach. Plug it in, introduce the chemicals, take it out, chuck it away and you are free, it’s nothing, it’s as if it wasn’t there.
Male, PORT
Although some participants with PICCs and Hickman-type devices also described being able to completely forget about their device, the majority were reminded of it several times daily, mostly when bathing and dressing. One participant compared his current PORT with his previous PICC in this regard, explaining that he had been aware of his PICC ‘24/7’ and had come to resent his treatment because he conflated treatment and mode of delivery (i.e. PICC). By contrast, he could forget about his PORT entirely and no longer resented treatment. In fact, he found the lack of external line so beneficial that he described the experience of having his portable pump disconnected in the following terms:
As soon as that [portable pump] came off, bang, that was it. You almost felt alive again. Because it just detaches everything. It all disappears. And mentally as well as physically you can begin to forget it because you don’t really know it’s there. And I think that’s a huge difference. It sort of lifts you back up again.
Male, PORT: prior experience of PICC
Participants also discussed the effects of CVADs on relationships and intimacy, and found the PORT to be beneficial in important ways. External lines, but not PORTs, caused worry in the context of close physical contact. One participant with a Hickman-type device explained that it had affected her relationship with her husband because its external lines made her feel unattractive. Another participant explained that her external line had got in the way when she and her husband needed to comfort each other. She talked about the importance of moments of physical comfort in the context of cancer:
But even, you know, my husband just putting his arm across me to give me a cuddle at night-time and we were denied that because he was so worried that he touched it and I was equally worried that it would be caught up as well. And it was when you need it most, some comfort, you were denied that comfort because of this line hanging out.
Female, Hickman: prior experience of PORT
Participants with different devices expressed different sets of emotions when discussing their decision to show their device to others or to conceal it. For instance, participants with PICCs and Hickman-type devices expressed discomfort regarding the appearance of their device and how it would make others feel to see it:
I don’t like seeing a tube going into my chest, so I don’t imagine other people want to see it either.
Female, Hickman
In our sample, this sentiment was not echoed by participants with PORTs. Among these participants, some talked about showing their device to others in terms of sharing a sense of awe or fascination with their device. Some even expressed humour; three participants with PORTs (currently or previously) mentioned nicknames (based on the idea of a button, e.g. ‘the magic button’) that they and others (friends, family) used for their device.
Patterns of preference
Among our sample, participants tended to prefer their own device and most participants stated that, if they had to choose a CVAD they would choose the one that they currently had. Noticeable differences between devices were evident with regard to how satisfaction and preferences were formulated and expressed. For instance, we observed differences in the comparators participants used when discussing device satisfaction. Participants with PICCs and Hickman-type devices tended to compare their devices (favourably) with peripheral cannulation. Participants in a Hickman-only focus group compared Hickman and PICCs and preferred the former, which they felt was less obtrusive. Unless prompted, participants with either of these devices tended not to make comparisons with PORTs. When prompted, there was ambivalence. Some were unsure about the idea of a needlestick for device access or, among those with PICCs, a device in their chest. Some claimed to lack sufficient knowledge about PORTs to compare. Others, although happy with their current device, professed an interest in PORTs. By comparison, participants with PORTs consistently compared them with PICCs and Hickman-type devices and explicitly regarded PORTs as superior CVADs:
The whole three [CVADs] were an option and I was hoping that it would be the PORT just because you didn’t see anything.
Female, Hickman
Furthermore, participants with PICCs and Hickman-type devices tended to express satisfaction with their device in personal terms. In the following extract, when ‘participant 1’ was asked if he would recommend PICCs to others he was reluctant, stressing the personal nature of his preference. This was not the case with ‘participant 2’, who had a PORT device:
Participant 1: Well I wouldn’t suggest, I wouldn’t give anybody advice on it because it’s up to them what that they do, it’s everybody’s personal choice what they like, em, but I would just say, well I find this a lot easier you know, so that’s . . . [to participant 2] obviously you’ve had a PORT . . .
Male, PICC
Participant 2: I would recommend the PORT.
Female, PORT
As illustrated here, participants who preferred PORTs were more forthright in their preferences, which tended to be based on the characteristics of the device itself, rather than familiarity or personal factors. This forthrightness was noted with interest by one participant with a PICC:
Loving the fact that the PORT owners are really enthusiastic, there is this sense of like evangelical ‘yeah this is great’. Whereas with the PICC line we are all indifferent, different experiences, it’s not as clear cut, whereas you guys are.
Male, PICC
Participants who could directly compare PORTs with other devices were unequivocal in their preference for PORTs and a positive regard for PORTs held even among those who had experienced painful placement procedures and device complications:
So, it [PORT] hasn’t worked for me, but if it had worked I would think it was the best one.
Female, PORT
Reflecting on the provision of PORTs by the NHS, participants advocated for greater access to PORTs, arguing that the benefits justify the additional costs:
And I feel quite strongly the very small difference between the Hickman-line costs and PORT costs and the overall benefits to people who are going, suffering enough anyway, their life should be made easier. And it’s a small difference, you know. And as I say I have had the benefit of trying these different things, and I find just, there’s no comparison.
Female, Hickman: prior experience of PORT
Experiences of trial participation
Most participants intimated a prosocial motivation for taking part in the CAVA trial. They were motivated to help other patients who came later to ‘give something back’ to the hospital or health system or to advance cancer treatment and, as one participant put it, help to ‘end this horrible disease’. Part of this motivation was a result of attaching a high value to research. One participant described a culture of research at the centre he attended and reported pre-emptively asking if there were any trials that he could take part in. For others, personal or family connections to the NHS or to health research reinforced motivation:
I signed up for all the trials when I got diagnosed, so owt [‘anything’] I could do to help the research.
Male, PORT
Participants’ decisions to take part in the trial also depended on their opinions regarding the CVADs under investigation and their feelings regarding randomisation. When invited to take part, many participants reported a preference for one device (most often PORTs), either a long-standing or a newly formed preference based on the information that they received about the three devices. Indeed, for some patients, being informed about the trial prompted them to do their own research into different vascular access options. These participants tended to form strong preferences. Others did not form opinions of their own but reported observing preferences among staff and inferring the superiority of that device (again, usually PORTs). PORTs were not routinely offered or available to NHS patients at several trial centres. This was a key incentive for many. Some of these patients reported that the randomisation process felt like taking part in a lottery; they felt lucky or unlucky, depending on the outcome:
I actually wanted a PORT and I was told the only way I could have one in Glasgow was by paying for it. Or by joining the trial. So, that’s why I joined the trial and I was thinking about paying for a PORT, but because I ended up having weekly chemo [chemotherapy] I didn’t mind having the PICC line, because it meant I was in here every week to have bloods, so it would get re-dressed then.
Male, PICC
Some participants professed not to prefer any device. These participants tended to feel that they did not know much about any of the devices, that all were equally unknown and there was nothing to be lost by leaving device selection to chance. Some were well informed but unable to choose; opting for randomisation was a way of solving their dilemma. Finally, some participants reported having been confused about the nature of randomisation when first informed about the trial, but stated that this had been addressed before participation.
For the vast majority of participants, the trial appeared to be a source of information and new knowledge about vascular access options and/or an opportunity to gain access to otherwise unavailable devices. For many, it also represented a source of additional support. For instance, one participant who was nervous about having a line said that the trial nurse took the time to explain the device and procedure to her, which was very helpful. A few patients, however, reported confusion or conflation of the trial with treatment as usual. Two patients, for example, referred to their PORT devices as ‘the CAVA’ or ‘the CAVA PORT’. Furthermore, one participant who had a PORT device and lived in an area in which PORTs were available only as part of the trial thought that the care and maintenance that she received for her PORT was also only part of the trial:
This is what I’m not clear of on the study, as to when the study comes to an end, what happens to us all going around with PORTs?
Female, PORT
Following device placement, further contact with the trial varied. Participants received and completed regular questionnaires (i.e. self-report measures of quality of life). Sometimes, questionnaires were brought to them in person by CAVA trial staff, who sat and went through the questionnaire with them. For some participants, this was an appreciated extra point of contact with a member of clinical staff who had a good understanding of their devices: an opportunity to ask questions or just chat about their experience.
Interviews with staff
The analysis of interview data identified three main themes: (1) acceptability of CVADs, (2) experiences of trial participation and (3) post-trial expectations. In general, staff regarded all three devices as acceptable vascular access options; each device was associated with a distinct set of perceived advantages and disadvantages. Although the CAVA trial was regarded positively by participants, a number of difficulties relating to participation were reported, focusing mainly on issues around recruitment. Regardless of the outcomes that they expected or hoped for, interviewees were clear that all three devices had a place within anticancer treatment and that none should be confined to the shelf, unless it was found to pose an unacceptable risk.
Acceptability of central venous access devices
All devices were regarded as acceptable by participants, and no device was considered inherently superior. Interviewees felt that all three had a place and purpose in the context of anticancer treatment. It was also noted that direct comparisons between different CVADs were not always reasonable, as several independent factors meant that certain devices cannot/should not be used under certain circumstances, such as a specific treatment regimen, patient vasculature and other patient factors (e.g. support and access to transport):
I think we’ve learned, eh, more through routine clinical practice than the trial, that they all have risks and complications and none of them are perfect. You can’t say oh, this one’s head and shoulders above. And that’s why the trial is so important, because I honestly do not know which is best.
Oncologist
Although interviewees consistently acknowledged a lack of empirical evidence comparing the PICC, Hickman and PORT devices, they were willing to express personal perspectives. These tended to be couched as subjective opinions, based on personal observations, experience or common sense. Perceived advantages and disadvantages primarily concerned device safety, including complications (e.g. thrombosis, haematoma, movement/displacement of device and clot), infections and the durability of the device. Additional points of comparison included ease of use and maintenance, compatibility with local services, ease of insertion and costs.
Perceived advantages and disadvantages of the three central venous access devices
Each device was associated with a unique set of pros and cons. PORTs were seen as having a lower risk of infection and complications, but were often not well supported by local services and entailed an intrusive and burdensome insertion procedure. PICC lines were regarded as more prone to negative outcomes (i.e. infections and complications) and as more labour-intensive to maintain, but were understood to be inexpensive, and quick and easy to insert and remove. There was less consensus regarding Hickman-type devices. Some interviewees saw them as equally prone to complications as PICCs, whereas others saw them as safer and more reliable. One participant commented that Hickman-type devices offered no unique advantages over PICCs or PORTs, but carried the disadvantages of both. The main advantage associated with Hickman-type devices was the greater range of functionality, including higher gauge and multiple lumens that allow for delivery of additional products (e.g. blood products, stem cell and bone marrow transfusion):
We started off obviously when the trial opened, which was a couple of years ago, probably a bit more now, with three-way randomisation between a PICC, a PORT and a Hickman em, and the Hickman-PORT randomisation closed and actually that was the one, personally, I think was the less interesting because I think if you’re going to have a long line into your chest, then the PORT has obvious advantages over a Hickman, but a Hickman doesn’t have obvious advantages over a PICC from my point of view, well not huge advantages anyway.
Oncologist
The importance of patient experience
All participants were fundamentally concerned with the experience of patients and emphasised the importance of being able to live normally when you have cancer. Although interviewees tended to describe perceived clinical and practical advantages and disadvantages in an even-handed way, they were more likely to describe the superiority of one device over the others when considering a patient’s point of view. In general, PORTs were seen as preferable from a patient perspective.
Participants did acknowledge certain disadvantages of PORTs, including a more invasive insertion procedure (potentially making them unsuitable for short-term treatment) and a lack of training among local staff, along with various inconveniences that come with that. However, they also cited many benefits, all reflecting topics discussed by focus group participants, demonstrating good insight into patients’ experiences. Interviewees recognised both practical and psychological/emotional benefits of PORTs. They described PORTs as less visible (hence, less of a reminder of illness), less intrusive into everyday life (not interfering with swimming, bathing or caring for children), easier to manage (monthly as opposed to weekly flushing) and less subject to worries about infection than with Hickman and PICCs:
A lot of people complain about being able to shower, wash their hair properly and everything like that, with the Hickman line, the PICC line in, because you’ve got an open line, external to the body so you can catch it, you can lie on it . . . If they sleep with no top on, or even women, it’s just, you know, you’ve got to strap it up in some way. So, it’s fairly intrusive in your life I would imagine, that’s how it looks and, whereas the PORT, because it’s under the skin, em, you can shower, you can swim if you wanted to, it’s less intrusive. I know it can be still visible and some people had a little bit of a problem about the visibility but overall . . . It’s far better in my opinion.
Non-clinical researcher
Interviewees reported seeing a general preference for PORTs among patients. Those involved with trial randomisation processes described participants’ elation or disappointment when they were not randomly selected to have a PORT. Preferences for PORTs, however, were not universal. Some interviewees regarded PORTs as less acceptable than other devices owing to a significantly more invasive insertion procedure. Some commented that patients prefer not to have a device in their chest, emphasising the advantage of PICCs in this respect. Overall, however, although participants stressed that PICCs were quick and easy to place and remove, external lines were seen as difficult and unpleasant for patients, both practically and psychologically; patients use terms such as ‘hanging out of chest’ to stress this point.
Experiences of trial participation
The CAVA trial was regarded positively by participants, who saw it as necessary research, addressing important questions. The trial itself was seen as straightforward to run, and participant information materials were seen as well designed and helpful. At some centres, taking part had increased or even initiated the use of devices that were not commonly used prior to the CAVA trial, typically PORTs. This was attributed to an increased awareness of these devices as an option for patients and an increased capacity to place and manage PORTs:
And we had free PORTs to start with . . . I think, we ended up with about six or eight free PORTs, which was great, and they just have this kind of get off the ground and allow people to see them.
Non-clinical researcher
The CAVA trial was also seen as an extra source of support for patients. Trial staff became an extra point of contact for patients with queries about their device. Contact necessitated by the trial (e.g. administering quality-of-life questionnaires) presented patients with an opportunity that they might not otherwise have had, namely to chat about concerns. A few participants also suggested that the very fact that the trial was taking place would raise awareness of the general dearth of evidence relating to CVADs, and hopefully inspire further work:
Sadly, like all these trials, it will probably raise as many questions as it answers, but I think it will, it will put the sort of problem on the map and it will, even if it doesn’t give all the answers we’d like, I think it will start to focus people’s attention as to the fact that there is no evidence on this and will encourage others to sort of build on that. So, I think it will sort of get a momentum because you’ll need, you’ll need a whole series of trials, you know, like is it better to put lines on the right or the left, you know, are big lines better than small lines, and all this stuff.
Anaesthetist
Barriers to participant recruitment
Although positively regarded overall, interviewees also described a number of difficulties with the trial, mostly related to participant recruitment. At most centres, potentially eligible patients were identified in the first instance by consultant oncologists, who tended not to be directly involved with the CAVA trial. Interviewees highlighted several reasons why eligible patients might not be referred to the trial team. Sometimes, this was because of a lack of awareness on the part of those responsible for identifying eligible patients. Interviewees reported that consultant oncologists and others in this position were prone to forgetting about the trial. Sometimes, failure to refer was attributed to a lack of enthusiasm or disapproval regarding the trial; for instance, consultants sometimes disliked certain devices and were reluctant to suggest trial participation to patients. Some did not perceive any value in taking part in the trial, either because they regarded existing evidence as sufficient or because they perceived CVADs to be an unimportant factor in the context of anticancer treatment:
The problem that we’ve encountered is that we have, we’ve got some clinicians here who don’t care. They don’t care what sort of device goes in as long as the patient gets their chemo [chemotherapy], but then we have other clinicians who have very strong ideas about what device they think and it’s just based on their own experience just, as you know, there’s not good evidence to show that one device is better than another, you know? So, em, so our biggest challenge has been getting those clinicians on board.
Radiologist
Interviews showed that some consultants were not against any device in principle but were concerned that the trial in general or that PORT or Hickman placement in particular would entail delays in device placement and the onset of treatment. Concerns regarding delays of this kind were not unfounded. In many trial centres, appointments for PORT or Hickman insertion were relatively infrequent owing to the unavailability of placement staff, surgical theatre slots, day beds and other related needs. Where commencing treatment was seen as urgent, patients tended not to be included in the trial:
If there’s a real urgency that they want the line in, then you probably wouldn’t randomise a patient. I have had a couple of consultants say ‘no, I’m not waiting for that, I need this line in next week and I need them to start chemo [chemotherapy]’.
Nurse (research)
Interviewees cited additional local factors that impeded recruitment. Some explained that there was a large amount of research taking place locally and that certain other trials took priority or that patients were overwhelmed with invitations to participate:
So, we’ve got, we’ve had some of these patients being approached for four trials, but our clinicians will tend to feel that the ones that could involve drugs that could affect the outcome of their health are pretty important.
Nurse (research)
Other centres reported small patient numbers, which limited recruitment potential, as well as staffing problems, limited resources and heavy workloads, which undermined staff capacity to recruit:
I think for a while we had the challenge with staffing levels, we went through a period of time where there were no staff because I cover quite a lot of treatment trials and priority shifted to looking after the patients who were on the treatment trial, so there wasn’t as much time for me to perhaps screen.
Nurse (research)
Factors facilitating participant recruitment
One factor cited by interviewees as bolstering recruitment was patient motivation. Like focus group participants, interviewees described two motivating factors, namely prosocial intentions and potential access to otherwise unavailable devices. Moreover, participants talked extensively about the importance of ensuring that clinicians who had initial contact with potential participants were reminded of the trial. Interviewees described keeping constant contact with these clinicians or maintaining a visible presence in key clinics to sustain awareness of the trial. For instance, one trial nurse talked about going in person to see other departments rather than relying on e-mail or telephone. Another lamented that she did not have enough time to ‘hang around in clinics’; she thought that she would have recruited more participants had she been able to do so.
Interviewees described working to allay the concerns of sceptical clinicians or consultants, and to engage others who were not interested. They talked about winning consultants over and forging connections. Often, allaying concerns meant taking active measures to avoid or minimise delays. To ensure timely device placement, staff responsible for recruitment worked out ways of navigating and negotiating complicated systems. When scheduling insertion, they had either to wait until randomisation had taken place and the allocated device was known (which meant securing last-minute appointments) or to book a potentially inappropriate appointment and amend if necessary. Some promoted support for the trial by taking responsibility for all vascular access appointments for trial patients, both unburdening clinicians and making the case that this aspect of patient care is best left to a specialist vascular access service:
And really the way that we’ve tried to do that is just to say we’ll take the whole venous access thing away from you, so you just have to say this patient needs venous access and then we will sort everything else out. So, you know, we’ll sort out all their bloods, you know, all their swabs for all of the device, we’ll get it put in, em, and that’s it. So, but we had to make it, em, to try and get patients from those clinicians, if you see what I mean, we had to make it easy for them.
Nurse (vascular access)
Both options required a level of flexibility and amenability on the part of staff and departments not directly involved with the trial. Trial staff, therefore, relied on good working relationships with these individuals and departments. Good communication was regarded as essential; establishing and keeping open lines of communication facilitated co-ordination. Staff also commented on the importance of being known and trusted by relevant others. Experience in the sense of long service at a particular centre was of benefit to some of the most successful trial staff. From experience, they had a good understanding of how local services operated, what options were available and feasible, and where to look for support or apply pressure. Experienced, well-known staff were also able to leverage their expertise and ask for favours:
I think 54 consultants work in the hospital, I know a large percentage of them, they trust me, they totally trust me, [. . .] I can get favours. I don’t consider them favours, I consider them people doing their job, but they know me and I hope I have done a professional job following through and I follow through with as many patients as I can. I’m always on the case, I’m always speaking to patients when I’m on duty if I can, so I am, I made myself part of the fabric, I’ve made myself a real go to person, the consultants get in touch with me on a daily basis and I never deny them. And will say to me ‘that’s not your job’, but I’ll say to him ‘that’s why we’ve been so successful because I go that extra mile’.
Nurse (research)
When speaking about successful recruitment, interviewees often referenced the positivity and energy of the local trial team and other clinical staff who came into contact with the trial. They used terms such as committed, engaged, enthusiastic, motivated, passionate and ‘on board’. Motivation was underpinned by positive opinions of research in general or of the trial itself, as well as a commitment to patient care. Although not required to do so, interviewees also described following patients through their journey on the CAVA trial, maintaining contact and offering support:
So from the moment they are identified I really have to get a move on because I have to contact the radiology team to let them know this is a potential trial patient, they get them booked in [inaudible] and put down that they may be eligible for the trial so they could potentially receive one of three devices. With the PICC lines it’s a walk in, walk out the policy is so they could potentially receive it . . . With the Hickman and the PORT they would require day medicine so they would require a bed and he would also require bloods done before as well as to check coagulation so that’s another reason why it has to be quick and then as soon as I’ve spoken with the patient I’ll issue them with the information and give them some time to consider it and ask any questions and then if they’re happy to I would try and get them within 24 hours to get the consent process underway and get them randomised.
Nurse (research)
Post-trial expectations
Interviewees believed that results in three domains would be important for informing practice: safety, cost and patient outcomes. Regarding safety, staff were interested in evidence relating to infection rates, device durability and complications, including device malfunction, movement, blood clots and thrombosis. Some wished for evidence regarding the effects of quality of care (handling, staff experience and proper placement) on these factors. Discussions of cost-effectiveness mainly centred on one issue: interviewees suspected that the belief that PICCs are the most cost-effective of the three CVADs (and PORTs the least) was not correct and were curious to see if this suspicion would be borne out by the evidence. Participants were also interested in patient-reported outcomes, with many expecting that PORTs would have the least negative impact on quality of life.
Almost all participants expressed a willingness to respond to new evidence. Most wished that, whatever the outcome of the CAVA trial, there would be an appropriate response broadly and/or locally. Some interviewees hoped that results would find one device to be consistently superior across domains, which would greatly simplify device selection. Others held that this would not be likely or, in fact, desirable:
And eh, I wouldn’t like to see a situation whereby the end result is that this device is better than the other, because there are too many factors involved and I don’t believe you can say that.
Nurse (vascular access)
Among those who expected a ‘winner’, most believed that PORTs would be identified as superior, certainly safer and preferred by patients. These interviewees hoped that PORTs would become a more widely available option for patients. Indeed, most interviewees emphasised the importance that all three devices be made equally available in future, which, in practice, often meant increasing the availability of PORTs. In any case, it was hoped that the trial findings would be used to support evidence-based decisions and recommendations both in individual cases and in terms of service provision.
Finally, in broader terms regarding the future of central venous access in chemotherapy, most participants felt that early intervention was important (i.e. avoidance of cannulation), emphasising the experience of patients or the importance of preserving patients’ veins for purposes beyond anticancer treatment. In general, our analysis found that, regardless of the outcomes that they had expected or hoped for, interviewees were clear that all three devices had a place within anticancer treatment and that none should be confined to the shelf, unless it was found to pose an unacceptable risk.
Discussion
To the best of our knowledge, this is the first qualitative study concomitantly exploring patient and staff experiences of the three most routinely used CVADs for the delivery of intravenous systemic anti-cancer therapy. Our results resonate with prior findings suggesting that all three CVADs are generally well accepted by patients,44–48 but also reveal a notable consistency in patient and staff attitudes. Although all three devices presented challenges for staff and patients, all three were regarded as acceptable and preferable to peripheral cannulation for the purposes of systemic anticancer therapy. Across our sample, both patients and staff expressed a generalised preference for PORT devices. For staff, this preference was based to some extent on perceptions (which, staff acknowledged, currently lack sufficient empirical evidence) that PORTs were safer than other devices. Staff preferences were based even more on understanding patients’ experiences. Therefore, although PORTs were acknowledged to be more challenging from a clinical and management perspective, staff favoured them because they were seen as better for patients. Indeed, staff were very well attuned to patients’ experiences and cited the same practical conveniences of PORTs, as well as the emotional and psychological benefits of a less conspicuous or obtrusive device that patients themselves described. Many of the disadvantages of PORTs related to limitations of local services and expertise relating to PORTs rather than characteristics of the device itself. Nonetheless, preferences for PORTs were general, but not universal. Some staff and some patients did not prefer PORTs, and none envisaged a scenario in which any options are withdrawn. Indeed, the ideal scenario supported by the evidence within our sample is one in which all three devices are, where clinically appropriate, available as options, and where device selection is a collaborative process between patients and clinicians, informed by robust empirical evidence.
Chapter 10 Discussion
The CAVA trial recruited 1061 participants and, to our knowledge, is both the largest randomised control trial ever conducted comparing three devices commonly used for the long-term administration of chemotherapy and the only study incorporating both quantitative and qualitative data and a full health economic evaluation from a UK NHS perspective.
The first comparison (non-inferiority of PICCs in relation to Hickman) showed no difference in complication rates but was underpowered to conclude on non-inferiority. This was partly because of a marked reduction in the use of Hickman-type devices during the course of the trial, coupled with a large expansion of nurse-led PICC services across UK oncology sites. Approximately half of all patients in both arms reported at least one complication. Interestingly, the dominant complication in the Hickman arm was infection, whereas in the PICCs arm it was the inability to aspirate blood. The presence of a cuff with Hickman-type devices reduces the risk of mechanical issues, such as accidental removal; however, it challenges the perception that the cuff is also an impediment to infection. The risk of venous thrombosis and PE was low and similar in both arms. There were no significant differences in quality of life based on the EQ-5D, the EORTC QLQ-30 and the device-specific questionnaire. PICCs were associated with substantially lower costs (–£1553) and a small but non-statistically significant decrement in QALYs (–0.009). Furthermore, the results remained consistent when accounting for device dwell time; PICCs were associated with a lower cost per catheter-week than Hickman (–£126). PICCs could be considered as a cost-effective alternative to Hickman if we were willing to accept health forgone at a given level of cost savings (e.g. a saving of ≥ £20,000 per additional QALY forgone).
The second comparison (superiority of PORTs compared with Hickman) recruited well ahead of target and the direct randomisation to this comparison closed early. The risk of overall complications associated with PORTs was approximately half that of Hickman (OR 0.54, 95% CI 0.37 to 0.77). This difference was largely explained by a reduction in infective complications. The most frequent complication in the PORTs arm was an inability to aspirate blood, whereas in the Hickman arm it was infection. Venous thrombosis was uncommon but twice as frequent with a Hickman-type device. PE was rare and similar in both arms. There were no significant differences in quality of life based on the EQ-5D and the EORTC QLQ-30. However, the device-specific questionnaire showed most aspects of quality of life (11 out of 16 questions) to be significantly better with a PORT and none to be better with a Hickman-type device. The total cost associated with PORTs was less than that associated with Hickman; however, this difference was small (–£45). This incremental cost was associated with a small gain in QALYs (0.004). In this scenario, PORTs dominate Hickman. In addition, PORTs were associated with reduced complication rates, resulting in an incremental cost of £1.36 per complication averted. Furthermore, the results remained consistent when accounting for device dwell time: PORTs were associated with a lower cost per catheter-week than Hickman (–£47). We, therefore, conclude that PORTs are the preferred option in this comparison, especially when considering patients with solid tumours.
The third comparison (superiority of PORTs compared with PICCs) showed that the risk of overall complication associated with PORTs was approximately half that of PICCs (OR 0.52, 95% CI 0.33 to 0.83). This difference was largely explained by a reduction in mechanical and thrombotic complications. The most common complication in both arms was an inability to aspirate blood. Interestingly, we found the infective complications to be almost double with PORTs than with PICCs. This is difficult to understand, but has been reported by others. 38 It could be explained by a learning curve phenomenon in the aftercare of PORTs in centres in which PORTs were recently introduced. Venous thrombosis was much more frequent with a PICC, and this has been reported by several other groups. 32,39 This may be related to the fact that PICCs are placed in a much smaller calibre arm vein than other devices, which are placed in a central vein puncture, thereby avoiding the arm completely. PE was again rare in both arms but more common in the PICCs arm. There were no significant differences in quality of life based on the EQ-5D and the EORTC QLQ-30. However, the device-specific questionnaire showed most aspects of quality of life (8 out of 16 questions) to be significantly better with a PORT and none to be significantly better with a PICC. However, compared with PICCs, PORTs were associated with an incremental cost of £1665. Although this incremental cost was associated with a decrement in QALYs (–0.018; loss of 6.5 days), it was also associated with a reduction in complication rate. The incremental cost per complication averted was £104. Based on a cost–utility analysis approach, the cost-effectiveness threshold for adoption is £20,000 per QALY gained. In this context, PORTs are dominated by PICCs and would not be considered to be a cost-effective alternative. When accounting for device dwell time, PORTs were associated with a lower cost per catheter-week than PICCs (–£41). However, given the limitations of the EQ-5D and the significant reduction in the risk of complications, the incremental cost per complication averted ought to be taken into consideration. Depending on the decision-maker’s willingness to pay to avert a complication, PORTs may be suitable for adoption in clinical practice.
The CAVA trial was a challenging study to conduct. This was mainly because of a changing service delivery model in which nurse-led teams expanded the PICCs option. The PICCs could often be inserted more timeously than either Hickman or, more particularly, PORTs, for which medical input was often required. The qualitative research confirmed this to be a barrier to randomisation, together with a lack of equipoise in some staff groups; however, the presence of a trial champion on site at six centres helped to reduce these biases. The CAVA trial was a pragmatic trial encompassing both solid and haematological cancers and involved a mix of different staff groups across the 18 centres placing the devices. Therefore, the trial was fairly heterogeneous from these perspectives. In addition, there appeared to be differences in the way that complications were handled clinically across the devices; for example, PICCs, the easiest type of device to place, are, not surprisingly, also the easiest to remove. PORTs, by contrast, are more complicated to remove and require medical or surgical input. It is likely that this affected how a complication was managed and may have biased costs in favour of PICCs. In addition, the dwell time of PORTs was longer than that of the other two devices, again potentially biasing the complication rate against PORTs.
The majority of patients in the CAVA trial had metastatic disease, reflecting the population requiring a central venous catheter (CVC) for chemotherapy. Treatment breaks, for example in metastatic colon cancer, are becoming more common, and it is more likely that a PICC, and to a lesser extent a Hickman, would be removed than a PORT, which, being more complex to place and remove, would be left in situ. We did not collect data on subsequent devices following device removal. This could lead to an underestimation of costs for PICCs and Hickman, because we did not capture subsequent device and reinsertion costs, and could lead to an underestimation of complication rates associated with subsequent devices.
The two recent RCTs comparing PICCs with PORTs have either taken all eligible patients, as in the CAVA38 trial, or selected patients requiring adjuvant chemotherapy for one tumour type (breast). 39 We consider that our approach makes the findings more generalisable for all patients receiving chemotherapy who require a CVC. We attempted to determine whether or not a PE was related to the device; however, that proved very difficult clinically and we are of the view that the source of most pulmonary emboli was unknown.
We found neither the EQ-5D nor the EORTC QLQ-C30 instruments to be sensitive to any of the device-related complications, possibly because of the burden of the underlying disease. The device-specific quality-of-life questionnaire that we designed during our feasibility study asked specifically about how the device affected quality of life. 3 This is in contrast to the EQ-5D and EORTC QLQ-C30, which do not address the device as a specific factor; therefore, they are more likely to be influenced by the underlying health state (i.e. cancer). The device-specific tool did show significant differences in favour of PORTs. This was also supported by the post-trial qualitative research, which indicated that, although patients felt that all three devices were acceptable and preferable to peripheral cannulation, PORTs had some advantages. Unfortunately, the quality-of-life benefits revealed by the device-specific quality-of-life questionnaire could not be quantified and incorporated into the economic analysis. Overall, the HRQoL questionnaires completion rate was low and fell markedly with time. This could be partly explained by the need for monthly returns as well as patients’ deteriorating health owing to their underlying condition. In the CAVA trial, patients were asked to complete and return the questionnaires by post if not attending routine follow-up clinics. The free-text reasons for not completing the questionnaires were provided in approximately two-thirds of cases. However, the majority (approximately 80%) of these were reported as ‘not completed/returned’ or ‘patient not contacted/contactable’, which does not provide much additional information. Unfortunately, quality-of-life data cannot be queried or chased, and capturing these data remains challenging. It is possible that those experiencing the worst health states were less likely to return the questionnaires, thus overestimating the perceived scores. The analyses of EQ-5D and the EORTC QLQ-C30 were based on multiple imputation. The analysis of the unimputed data drew the same conclusions as the analyses of imputed data for both the EQ-5D and the EORTC QLQ-C30. We did not perform complete-case analyses, as in all three comparisons < 13% of patients had complete data and this subset of patients was not considered to be representative of the patient population. These are likely to have been patients in better health and, for this reason, the results of the analyses may have been biased.
In determining the value of these devices, decision-makers need to trade off all of the potential clinical consequences and patient benefits against all relevant costs. Although we have expressed cost-effectiveness as incremental costs per QALY gained to be judged against the £20,000 willingness-to-pay threshold, this may not have captured all of the potential clinical and patient benefits associated with these devices. A cost–consequence approach to inform a multicriteria decision rule may be of more benefit in this context.
Conclusions
On balance, taking into account the complication rates, quality-of-life outcomes and total costs associated with the devices, we were unable to draw firm conclusions between the choice of PICCs and the choice of Hickman. The risk of a complication from a PORT is approximately half that of either a PICC or a Hickman. PORTs are associated with greater health benefits than Hickman; this is evident from the significant reduction in complication rates associated with PORTs. The use of PORTs in patients with solid tumours is considered to be cost-effective. However, the use of PORTs, compared with PICCs, is not likely to be cost-effective from an incremental cost per QALY gained perspective. However, if we take into account the health benefits associated with reduced complications, and if the service delivery model was changed to one in which all three devices are placed by nurse-led teams, there is the potential to change this model in favour of PORTs.
Implications for practice
Decision-makers need to trade off overall complication rates, infection rates, HRQoL impacts, patient preference and costs when selecting a device for patients. An agreed protocol on the choice, placement and maintenance of these devices is needed.
Patient and public involvement
It proved difficult to get significant engagement from the public, despite protracted efforts. We were unable to recruit patients or members of the public onto any of the groups that worked on the protocol or subsequent running of the trial. The pre-trial qualitative research, however, proved very useful in understanding patients’ opinions on both the device and the concept of a randomised trial. We were able to modify the PIS and improve the delivery of the service in several centres in response to these comments. Furthermore, the CAVA trial was endorsed by the CRUK CTU In-house Trial Advisory Board at the time of trial opening and was reviewed annually by the CRUK CTU Steering Committee. Both the advisory board and the committee have patient and public involvement members.
Recommendations for research
Although the CAVA trial has enriched our understanding of these three devices, further challenges remain and provide a rich area for future research. One of the biggest challenges is to understand and modify the service delivery of these devices so that patients and providers can be reassured that all three can be delivered timeously and as cost-effectively as possible. For example, most Hickman and almost all PORTs are placed by medical staff; this introduces barriers regarding cost and delivery time. Several centres have challenged this model and use nurse-led teams to place all three devices. More research is required to understand the barriers to this and how to disseminate good practice to the other centres in the UK. Clearly, a nurse-led system has much to commend it, with reduced costs, increased efficiency and releasing medical staff for their more conventional roles.
We had so few patients with haematological malignancies in the CAVA trial that no firm conclusions could be drawn. This remains an area for further research. Furthermore, we looked at these devices in the context of cancer only; however, they are also used for other purposes, such as antibiotics treatment, total parenteral nutrition and renal dialysis. More evidence is needed in these scenarios. Technical advances continue, which include coating and flushing the catheters with antimicrobial agents. It is not yet clear what if any advantage these additions may make. There is little or no information available to help providers choose between devices offered by different manufacturers. Although there may be little difference, it is also an area worthy of further research.
In the CAVA trial, we were able to evaluate the consequence of the initial device only. The impact of subsequent devices and their associated impact on complications, HRQoL and costs need to be evaluated.
Acknowledgements
The authors wish to thank the patients who participated in the CAVA trial, as well as the principal investigators research staff and nursing staff at each participating site for facilitating the recruitment, treatment and follow-up of trial participants.
The authors wish to thank Jane Murray (patient representative) for leading the writing of the Plain English summary.
The authors also wish to thank the independent members of the TSC and DMC:
-
Rob Jones (Professor of Clinical Cancer Research, Clinical Trials) contributed clinical expertise and protocol development.
-
Rebecca Shaw (Lecturer in Social Science for Public Health, Health Economics) designed the qualitative components of the CAVA trial.
-
Linda Kelly (Lecturer in Advanced Clinical Practice, Vascular Access) contributed clinical expertise in vascular access.
-
Andrew Willis (Consultant Diagnostic and Interventional Radiologist, Radiology) contributed clinical expertise in interventional radiology and was a local principal investigator.
-
Steve Hill (Procedure Team Manager, Vascular Access) contributed clinical expertise in vascular access and was a local principal investigator.
-
Sumaira Macdonald (Consultant Vascular/Interventional Radiologist, Radiology) contributed clinical expertise in interventional radiology and was a local principal investigator.
-
Hannah Hesselgreaves (Associate Professor, Qualitative Research) led the qualitative components of the CAVA trial following the departure of Dr Rebecca Shaw from academia.
Trial Steering Committee
Alison Birtle (chairperson), Gareth Griffiths, Annette Rauf, Steve Thomas, Andrew Johnston, Lisa Dougherty, Jonathan Moss, James Paul, Olivia Wu, Elaine McCartney and Judith Dixon-Hughes.
Data Monitoring Committee
Simon Crabb (chairperson), Fergus Daly and Graeme Houston.
Investigator group
-
Beatson West of Scotland Cancer Centre, Glasgow – Jonathan Moss and Donna Mellon.
-
Queen Elizabeth Hospital Birmingham, Birmingham – Andrew Willis and Catherine Prest.
-
Christie Hospital, Manchester – Steve Hill and Jo Allen.
-
Freeman Hospital, Newcastle upon Tyne – Tobias Menne and Sonia Poolan.
-
Guy’s Hospital, London – Sharon Whelan and Laura Green.
-
St James’ University Hospital, Leeds – Andrew Bodenham, Anne Crossley and Alison Judge.
-
University Hospital of North Durham, Durham (including Darlington Memorial Hospital, Darlington) – Stuart Marsden, Andrea Kay and Susan Wadd.
-
Northampton General Hospital – Roshan Agarwal and Andrea Kempa.
-
Weston Park Hospital, Sheffield – Josh Wright and Rachel Clarke.
-
Charing Cross Hospital, London – Alison Graham and Shaun O’Gara.
-
Royal United Hospital, Bath – Emma De Winton and Samantha Curtis.
-
Forth Valley Royal Hospital, Larbert – Nik Arestis and Anne Todd.
-
Cumberland Infirmary, Carlisle – Jonathan Nicoll.
-
Kent and Canterbury Hospital, Canterbury – Matthew Jones and Bonny Appleby.
-
Royal Cornwall Hospital, Treliske – Andrew Edwards and Kerry Atkinson.
-
Broomfield Hospital, Chelmsford – Gopalakrishnan Srinivasan and Liz Dawson.
-
Sunderland Royal Infirmary, Sunderland – Mark Carpenter, Paula Newton and Hayley Anderson.
-
Western General Hospital, Edinburgh – Peter Hall and Heather McVicars.
Contributions of authors
Olivia Wu (https://orcid.org/0000-0002-0570-6016) (Lindsay Chair of Health Economics) contributed to the conception and design of the trial, led the health economics component, contributed to the interpretation of the clinical and health economics results, and led the writing/editing of the report.
Elaine McCartney (https://orcid.org/0000-0002-0260-4161) (Statistician) contributed to the conception and design of the trial, conducted the statistical analyses, contributed to the interpretation of the results and the writing/editing of the report.
Robert Heggie (https://orcid.org/0000-0001-7396-4773) (Health Economist) conducted the economic evaluation and contributed to the writing/editing of the report.
Evi Germeni (https://orcid.org/0000-0001-5576-8816) (Lecturer in Qualitative Methods for Health Research) led the qualitative components of the trial and contributed to the writing/editing of the report.
James Paul (https://orcid.org/0000-0001-7367-5816) (Head of Biostatistics) contributed to the conception and design of the trial, conducted the statistical analyses, the interpretation of the results and contributed to the drafting of the report.
Eileen Soulis (https://orcid.org/0000-0001-9022-9295) (Project Manager 2018–present) was responsible for the day-to-day management of the trial and contributed to the writing/editing of the report.
Susan Dillon (https://orcid.org/0000-0002-6676-1393) (Clinical Trial Co-ordinator) was responsible for data collection and contributed to the review of the report.
Caoimhe Ryan (https://orcid.org/0000-0001-7279-563X) (Postdoctoral Research Associate) conducted the post-trial qualitative study and reviewed the report.
Moira Sim (https://orcid.org/0000-0003-1294-356X) (Qualitative Researcher) conducted the pre-trial qualitative study and review of the report.
Judith Dixon-Hughes (https://orcid.org/0000-0002-5596-4400) (Project Manager 2012–18) was responsible for the day-to-day management of the trial and contributed to the writing of the report.
Roshan Agarwal (https://orcid.org/0000-0002-3626-2008) (Consultant Medical Oncologist) contributed to the recruitment of participants and review of the report.
Andrew Bodenham (https://orcid.org/0000-0002-4726-4527) (Consultant in Anaesthesia and Intensive Care Medicine) contributed to the conception and design of the trial, recruitment of participants and review of the report.
Tobias Menne (https://orcid.org/0000-0002-3289-2344) (Consultant Haematologist) contributed to the recruitment of participants and review of the report.
Brian Jones (https://orcid.org/0000-0002-9439-2353) (Consultant Medical Microbiologist) contributed to the conception and design of the trial and review of the report.
Jonathan Moss (https://orcid.org/0000-0002-4589-9174) (Professor of Interventional Radiology) contributed to the conception and design of the trial, recruitment of participants, conduct of the trial, interpretation of the results and the writing/editing of the report.
Other information
Sponsor: NHS Greater Glasgow and Clyde.
Trials Unit: Cancer Research UK Glasgow Clinical Trials Unit (CRUK Glasgow CTU). Partner of Cancer Clinical Trials Unit Scotland (CaCTUS).
Protocol: version 4, 19 February 2016; URL: www.crukctuglasgow.org/media/CAVA/MI102.pdf.
PORTs were provided free of charge by several manufacturers [Cook Medical LLC (Bloomington, IN, USA), Perouse Medical Inc. (Ivry-le-Temple, Oise, France) and AngioDynamics, Inc. (Latham, NY, USA)] to centres where there were funding issues for these devices. None of these companies had any role in data collection, handling or processing, or the writing of this report.
Publication
Moss JG, Wu O, Bodenham AR, Agarwal R, Menne TF, Jones BL, et al. Central venous access devices for the delivery of systemic anticancer therapy (CAVA): a randomised controlled trial [published online ahead of print June 20 2021]. Lancet 2021.
Data-sharing statement
The CAVA trial investigators are committed to furthering cancer research by sharing de-identified individual patient data from the CAVA trial with others in the field who wish to use the data for high-quality research. We are happy to consider proposals from researchers and will share individual patient data to the maximum extent, subject to individual study constraints relating to:
-
ethics approval and informed consent
-
contractual and legal obligations
-
publication timelines (data will not normally be shared prior to the publication of the primary results).
In addition, all proposals will be reviewed for their scientific merit by the lead CAVA trial investigators and study chief investigator. Only data relevant to the objectives of a particular proposal will be provided. An independent review process will be undertaken in cases of disagreement between the applicant and the CTU/chief investigator.
If you wish to have an initial discussion about accessing data from CTU studies please contact:
Caroline Kelly
Head of Biostatistics
Cancer Research UK Clinical Trials Unit, Glasgow
Level 0
Beatson West of Scotland Cancer Centre
1053 Great Western Road
Glasgow
G12 0YN
E-mail: caroline.kelly@glasgow.ac.uk
Tel: 0141 301 7188.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data is used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- National Cancer Registration and Analysis Service . Cancer Reports n.d. http://ncin.org.uk/publications/reports/ (accessed 21 April 2021).
- National Institute for Health Research . Funding and Awards: Research Award n.d. https://fundingawards.nihr.ac.uk/award/11/67/01 (accessed 21 April 2021).
- Wu O, Boyd K, Paul J, McCartney E, Ritchie M, Mellon D, et al. Hickman catheter and implantable port devices for the delivery of chemotherapy: a phase II randomised controlled trial and economic evaluation. Br J Cancer 2016;114:979-85. https://doi.org/10.1038/bjc.2016.76.
- Kulkarni S, Wu O, Kasthuri R, Moss JG. Centrally inserted external catheters and totally implantable ports for the delivery of chemotherapy: a systematic review and meta-analysis of device-related complications. Cardiovasc Intervent Radiol 2014;37:990-1008. https://doi.org/10.1007/s00270-013-0771-3.
- Pu YL, Li ZS, Zhi XX, Shi YA, Meng AF, Cheng F, et al. Complications and costs of peripherally inserted central venous catheters compared with implantable port catheters for cancer patients: a meta-analysis. Cancer Nurs 2020;43:445-67. https://doi.org/10.1097/NCC.0000000000000742.
- Pratt RJ, Pellowe C, Loveday H, Robinson N, Smith GW, Barrett S, et al. The epic project: developing national evidence-based guidelines for preventing healthcare associated infections: foreword. J Hosp Infect 2001;47:S3-S4. https://doi.org/10.1053/jhin.2000.0886.
- National Institute for Health and Care Excellence (NICE) . Healthcare-Associated Infections: Prevention and Control in Primary and Community Care n.d. www.nice.org.uk/guidance/cg139 (accessed 21 April 2021).
- Moss J, Willis A, Bodenham A, Hill S, Menne T, Cox J. Protocol 13PRT 7956: Cancer And Venous Access (CAVA): A Randomised Controlled Trial With Associated Qualitative Research of Venous Access Devices for the Delivery of Long-Term Chemotherapy (ISCRTN No 44504648). n.d. https://thelancet.com/protocol-reviews/13PRT-7956 (accessed 21 April 2021).
- Hoaglin DC, Hawkins N, Jansen JP, Scott DA, Itzler R, Cappelleri JC, et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on indirect treatment comparisons good research practices: part 2. Value Health 2011;14:429-37. https://doi.org/10.1016/j.jval.2011.01.011.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons; 1987.
- Qian W, Parmar MK, Sambrook RJ, Fayers PM, Girling DJ, Stephens RJ. Analysis of messy longitudinal data from a randomized clinical trial. MRC Lung Cancer Working Party. Stat Med 2000;19:2657-74. https://doi.org/10.1002/1097-0258(20001015)19:19<2657::AID-SIM557>3.0.CO;2-3.
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 1995;57:289-300. https://doi.org/10.1111/j.2517-6161.1995.tb02031.x.
- Zhu L, Sun J, Tong X, Srivastava DK. Regression analysis of multivariate recurrent event data with a dependent terminal event. Lifetime Data Anal 2010;16:478-90. https://doi.org/10.1007/s10985-010-9158-9.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2019. Canterbury: Personal Social Services Research Unit, University of Kent; 2019.
- Walker G, Todd A. Nurse-led PICC insertion: is it cost effective?. Br J Nurs 2013;22:S9-S15. https://doi.org/10.12968/bjon.2013.22.Sup19.S9.
- NHS Improvement . Reference Costs 2017–2018: Highlights, Analysis and Introduction to the Data 2018.
- Taxbro K, Hammarskjöld F, Juhlin D, Hagman H, Bernfort L, Berg S. Cost analysis comparison between peripherally inserted central catheters and implanted chest ports in patients with cancer – a health economic evaluation of the PICCPORT trial. Acta Anaesthesiol Scand 2020;64:385-93. https://doi.org/10.1111/aas.13505.
- Calvert N, Hind D, McWilliams R, Davidson A, Beverley CA, Thomas SM. Ultrasound for central venous cannulation: economic evaluation of cost-effectiveness. Anaesthesia 2004;59:1116-20. https://doi.org/10.1111/j.1365-2044.2004.03906.x.
- Public Health Scotland . Data and Intelligence. Acute Medical: Specialty Costs n.d. https://isdscotland.org/Health-Topics/Finance/Costs/Detailed-Tables/Speciality-Costs/Acute-Medical.asp (accessed 21 April 2021).
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Ramsey S, Willke R, Briggs A, Brown R, Buxton M, Chawla A, et al. Good research practices for cost-effectiveness analysis alongside clinical trials: the ISPOR RCT-CEA Task Force report. Value Health 2005;8:521-33. https://doi.org/10.1111/j.1524-4733.2005.00045.x.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Watson JM, Torgerson DJ. Increasing recruitment to randomised trials: a review of randomised controlled trials. BMC Med Res Methodol 2006;6. https://doi.org/10.1186/1471-2288-6-34.
- McDonald AM, Knight RC, Campbell MK, Entwistle VA, Grant AM, Cook JA, et al. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials 2006;7. https://doi.org/10.1186/1745-6215-7-9.
- Donovan J, Mills N, Smith M, Brindle L, Jacoby A, Peters T, et al. Quality improvement report: improving design and conduct of randomised trials by embedding them in qualitative research: ProtecT (prostate testing for cancer and treatment) study. Commentary: presenting unbiased information to patients can be difficult. BMJ 2002;325:766-70. https://doi.org/10.1136/bmj.325.7367.766.
- Hamilton DW, de Salis I, Donovan JL, Birchall M. The recruitment of patients to trials in head and neck cancer: a qualitative study of the EaStER trial of treatments for early laryngeal cancer. Eur Arch Otorhinolaryngol 2013;270:2333-7. https://doi.org/10.1007/s00405-013-2349-8.
- Paramasivan S, Huddart R, Hall E, Lewis R, Birtle A, Donovan JL. Key issues in recruitment to randomised controlled trials with very different interventions: a qualitative investigation of recruitment to the SPARE trial (CRUK/07/011). Trials 2011;12. https://doi.org/10.1186/1745-6215-12-78.
- Ritchie M, Kelly LJ, Moss J, Paul J, Shaw R. Exploring attitudes towards a randomised controlled trial of venous access devices – a nested pre-trial qualitative study. J Vasc Access 2015;16:407-12. https://doi.org/10.5301/jva.5000447.
- Chopra V, Anand S, Hickner A, Buist M, Rogers MA, Saint S, et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet 2013;382:311-25. https://doi.org/10.1016/S0140-6736(13)60592-9.
- Cowl CT, Weinstock JV, Al-Jurf A, Ephgrave K, Murray JA, Dillon K. Complications and cost associated with parenteral nutrition delivered to hospitalized patients through either subclavian or peripherally-inserted central catheters. Clin Nutr 2000;19:237-43. https://doi.org/10.1054/clnu.2000.0103.
- Chopra V, Flanders SA, Saint S, Woller SC, O’Grady NP, Safdar N, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med 2015;163:S1-40. https://doi.org/10.7326/M15-0744.
- Schiffer CA, Mangu PB, Wade JC, Camp-Sorrell D, Cope DG, El-Rayes BF, et al. Central venous catheter care for the patient with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2013;31:1357-70. https://doi.org/10.1200/JCO.2012.45.5733.
- Sousa B, Furlanetto J, Hutka M, Gouveia P, Wuerstlein R, Mariz JM, et al. Central venous access in oncology: ESMO Clinical Practice Guidelines. Ann Oncol 2015;26:v152-68. https://doi.org/10.1093/annonc/mdv296.
- Bodenham A, Babu S, Bennett J, Binks R, Fee P, Fox B, et al. Association of Anaesthetists of Great Britain and Ireland: safe vascular access 2016. Anaesthesia 2016;71:573-85. https://doi.org/10.1111/anae.13360.
- Taxbro K, Hammarskjöld F, Thelin B, Lewin F, Hagman H, Hanberger H, et al. Clinical impact of peripherally inserted central catheters vs. implanted port catheters in patients with cancer: an open-label, randomised, two-centre trial. Br J Anaesth 2019;122:734-41. https://doi.org/10.1016/j.bja.2019.01.038.
- Clatot F, Fontanilles M, Lefebvre L, Lequesne J, Veyret C, Alexandru C, et al. Randomised phase II trial evaluating the safety of peripherally inserted catheters versus implanted port catheters during adjuvant chemotherapy in patients with early breast cancer. Eur J Cancer 2020;126:116-24. https://doi.org/10.1016/j.ejca.2019.11.022.
- Ng F, Mastoroudes H, Paul E, Davies N, Tibballs J, Hochhauser D, et al. A comparison of Hickman line- and Port-a-Cath-associated complications in patients with solid tumours undergoing chemotherapy. Clin Oncol (R Coll Radiol) 2007;19:551-6. https://doi.org/10.1016/j.clon.2007.04.003.
- Patel GS, Jain K, Kumar R, Strickland AH, Pellegrini L, Slavotinek J, et al. Comparison of peripherally inserted central venous catheters (PICC) versus subcutaneously implanted port-chamber catheters by complication and cost for patients receiving chemotherapy for non-haematological malignancies. Support Care Cancer 2014;22:121-8. https://doi.org/10.1007/s00520-013-1941-1.
- Corriere MA, Avise JA, Peterson LA, Stafford JM, Easterling D, Boone DS, et al. Exploring patient involvement in decision making for vascular procedures. J Vasc Surg 2015;62:1032-9.e. https://doi.org/10.1016/j.jvs.2015.04.443.
- Ryan C, Hesselgreaves H, Wu O, Moss J, Paul J, Dixon-Hughes J, et al. Patient acceptability of three different central venous access devices for the delivery of systemic anticancer therapy: a qualitative study. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2018-026077.
- Harrold K, Martin A, Scarlett C. Proactive PICC placement: evaluating the patient experience. Br J Nurs 2016;25:S4-1. https://doi.org/10.12968/bjon.2016.25.8.S4.
- Kreis H, Loehberg CR, Lux MP, Ackermann S, Lang W, Beckmann MW, et al. Patients’ attitudes to totally implantable venous access port systems for gynecological or breast malignancies. Eur J Surg Oncol 2007;33:39-43. https://doi.org/10.1016/j.ejso.2006.08.003.
- Parás-Bravo P, Paz-Zulueta M, Santibañez M, Fernández-de-Las-Peñas C, Herrero-Montes M, Caso-Álvarez V, et al. Living with a peripherally inserted central catheter: the perspective of cancer outpatients-a qualitative study. Support Care Cancer 2018;26:441-9. https://doi.org/10.1007/s00520-017-3815-4.
- Sharp R, Grech C, Fielder A, Mikocka-Walus A, Cummings M, Esterman A. The patient experience of a peripherally inserted central catheter (PICC): a qualitative descriptive study. Contemp Nurse 2014;48:26-35. https://doi.org/10.5172/conu.2014.48.1.26.
- Alpenberg S, Rosengren K, Joelsson G. Feeling confident in using PICC lines: patients experiences of living with a PICC line during chemotherapy treatment. Home Health Care Manag Pract 2015;27:119-25. https://doi.org/10.1177/1084822314566300.
Appendix 1 Peripherally inserted central catheter versus Hickman: device details
| Device details | Device, n (%) | Total, n (%) | |
|---|---|---|---|
| PICC | Hickman | ||
| Manufacturer | |||
| CR Bard Inc. | 57 (30.0) | 39 (18.8) | 96 (24.2) |
| Cook Medical LLC (Bloomington, IN, USA) | 27 (14.2) | 8 (3.9) | 35 (8.8) |
| Vygon | 84 (44.2) | 158 (76.3) | 242 (61.0) |
| Med CMP (Harleysville, PA, USA) | 2 (1.1) | 0 (0.0) | 2 (0.5) |
| AngioDynamics, Inc. (Latham, NY, USA) | 9 (4.7) | 0 (0.0) | 9 (2.3) |
| Other | 11 (5.8) | 2 (1.0) | 13 (3.3) |
| Catheter material | |||
| Silicone | 26 (13.7) | 149 (72.0) | 175 (44.1) |
| Polyurethane | 160 (84.2) | 54 (26.1) | 214 (53.9) |
| Other | 1 (0.5) | 2 (1.0) | 3 (0.8) |
| Missing | 3 (1.6) | 2 (1.0) | 5 (1.3) |
| Number of lumens | |||
| 1 | 164 (86.3) | 150 (72.5) | 314 (79.1) |
| 2 | 24 (12.6) | 57 (27.5) | 81 (20.4) |
| Missing | 2 (1.1) | 0 (0.0) | 2 (0.5) |
| Antimicrobial coating | |||
| Yes | 21 (11.1) | 7 (3.4) | 28 (7.1) |
| No | 167 (87.9) | 196 (94.7) | 363 (91.4) |
| Missing | 2 (1.1) | 4 (1.9) | 6 (1.5) |
| CT pump compatible | |||
| Yes | 103 (54.2) | 36 (17.4) | 139 (35.0) |
| No | 85 (44.7) | 167 (80.7) | 252 (63.5) |
| Missing | 2 (1.1) | 4 (1.9) | 6 (1.5) |
| Valve | |||
| Yes | 68 (35.8) | 28 (13.5) | 96 (24.2) |
| No | 121 (63.7) | 173 (83.6) | 294 (74.1) |
| Missing | 1 (0.5) | 6 (2.9) | 7 (1.8) |
Appendix 2 Peripherally inserted central catheter versus Hickman: quality-of-life compliance
| Month | Arm | |||||
|---|---|---|---|---|---|---|
| PICC | Hickman | |||||
| Present (n) | Expected (n) | Compliance (%) | Present (n) | Expected (n) | Compliance (%) | |
| 0 | 196 | 209 | 93.8 | 203 | 212 | 95.8 |
| 1 | 131 | 199 | 65.8 | 144 | 201 | 71.6 |
| 2 | 122 | 177 | 68.9 | 127 | 189 | 67.2 |
| 3 | 94 | 152 | 61.8 | 111 | 175 | 63.4 |
| 4 | 69 | 123 | 56.1 | 97 | 153 | 63.4 |
| 5 | 44 | 90 | 48.9 | 66 | 126 | 52.4 |
| 6 | 29 | 67 | 43.3 | 49 | 104 | 47.1 |
| 7 | 18 | 48 | 37.5 | 29 | 80 | 36.3 |
| 8 | 11 | 32 | 34.4 | 18 | 58 | 31.0 |
| 9 | 11 | 26 | 42.3 | 8 | 40 | 20.0 |
| 10 | 7 | 22 | 31.8 | 8 | 32 | 25.0 |
| 11 | 5 | 17 | 29.4 | 5 | 18 | 27.8 |
| 12 | 4 | 14 | 28.6 | 2 | 15 | 13.3 |
| Total | 741 | 1176 | 63.0 | 867 | 1403 | 61.8 |
Appendix 3 Peripherally inserted central catheter versus Hickman: EuroQoL-5 Dimensions and Quality of Life Questionnaire C30 analysis results
| Questionnaire | Original AUC data, mean (SE) | Pooled AUC imputed data, mean (SE) | Two-sided p-value | |||
|---|---|---|---|---|---|---|
| PICC | Hickman | PICC | Hickman | Unadjusted | Adjusted | |
| EQ-5D | ||||||
| Index value | –0.009 (0.01) | –0.007 (0.01) | –0.095 (0.04) | –0.129 (0.03) | 0.239 | 0.478 |
| Health state score | –13.93 (2.42) | –8.79 (1.85) | –8.57 (1.99) | –7.71 (1.73) | 0.891 | 0.891 |
| QLQ-C30: global health status | ||||||
| Global health status | –5.09 (1.32) | –5.82 (1.34) | –5.19 (1.37) | –4.76 (1.62) | 0.858 | 0.968 |
| Functional scales | ||||||
| Physical functioning | –4.16 (1.02) | –5.85 (1.10) | –5.91 (1.22) | –5.99 (1.19) | 0.968 | 0.968 |
| Role functioning | –7.16 (1.95) | –7.57 (1.92) | –7.81 (1.84) | –6.07 (2.05) | 0.482 | 0.968 |
| Emotional functioning | 0.32 (1.18) | 0.48 (1.12) | 0.66 (1.39) | 1.74 (1.34) | 0.490 | 0.968 |
| Cognitive functioning | –4.27 (1. 11) | –4.54 (0.88) | –5.36 (1.27) | –4.44 (1.04) | 0.607 | 0.968 |
| Social functioning | –4.30 (1.86) | –5.54 (1.65) | –4.49 (1.81) | –4.20 (1.71) | 0.943 | 0.968 |
| Symptom scales | ||||||
| Fatigue | 7.46 (1.59) | 8.39 (1.41) | 6.79 (1.63) | 6.58 (1.80) | 0.714 | 0.968 |
| Nausea and vomiting | 1.31 (1.24) | 3.40 (1.07) | 2.42 (1.38) | 1.23 (1.52) | 0.478 | 0.968 |
| Pain | –1.47 (1.64) | –2.47 (1.45) | –1.31 (1.69) | –2.95 (1.48) | 0.666 | 0.968 |
| Dyspnoea | 1.99 (1.48) | 2.68 (1.40) | 2.84 (1.67) | 2.36 (1.96) | 0.893 | 0.968 |
| Insomnia | 1.97 (2.01) | 1.57 (1.87) | 0.36 (1.89) | –0.64 (2.13) | 0.846 | 0.968 |
| Appetite loss | –0.31 (1.91) | 3.04 (179) | –0.82 (1.99) | 0.71 (1.88) | 0.822 | 0.968 |
| Constipation | 0.10 (1.59) | 0.79 (1.91) | –1.25 (1.77) | 0.32 (1.77) | 0.285 | 0.968 |
| Diarrhoea | 7.87 (1.70) | 7.82 (1.68) | 7.68 (1.92) | 6.55 (1.72) | 0.739 | 0.968 |
| Financial difficulties | 0.17 (1.70) | 2.14 (1. 16) | –1.22 (1.54) | 1.46 (1.83) | 0.342 | 0.968 |
Appendix 4 Peripherally inserted central catheter versus Hickman: Venous Access Device Questionnaire analysis results
| Activity | p-value | |
|---|---|---|
| Unadjusted | Adjusted | |
| Driving a car | 0.446 | 0.595 |
| Getting in or out of a car | 0.432 | 0.595 |
| Using public transport | 0.073 | 0.292 |
| Going out shopping | 0.121 | 0.323 |
| Eating | 0.681 | 0.798 |
| Hygiene | 0.004 | 0.064 |
| Sleeping | 0.437 | 0.595 |
| Mobility or movement | 0.400 | 0.595 |
| Normal work activity | 0.159 | 0.363 |
| Exercise | 0.071 | 0.292 |
| Hobbies | 0.020 | 0.160 |
| Self-consciousness | 0.736 | 0.798 |
| Socialising | 0.748 | 0.798 |
| At risk of infection | 0.441 | 0.595 |
| At risk of damaging device | 0.800 | 0.800 |
| Negative impact on quality of life | 0.108 | 0.323 |
Appendix 5 PORT versus Hickman: device details
| Device details | Device, n (%) | Total, n (%) | |
|---|---|---|---|
| PORT | Hickman | ||
| Manufacturer | |||
| CR Bard Inc. | 3 (1.2) | 18 (6.4) | 21 (4.0) |
| Cook Medical LLC | 47 (19.3) | 1 (0.4) | 48 (9.2) |
| Vygon | 2 (0.8) | 260 (92.9) | 262 (50.1) |
| Med CMP | 36 (14.8) | 0 (0.0) | 36 (6.9) |
| Smith Medical, Inc. (Minneapolis, MN, USA) | 22 (9.1) | 0 (0.0) | 22 (4.2) |
| AngioDynamics, Inc. | 15 (6.2) | 0 (0.0) | 15 (2.9) |
| Other | 116 (47.7) | 1 (0.4) | 117 (22.4) |
| Missing | 2 (0.8) | 0 (0.0) | 2 (0.4) |
| Catheter material | |||
| Silicone | 119 (49.0) | 236 (84.3) | 355 (67.9) |
| Polyurethane | 118 (48.6) | 41 (14.6) | 159 (30.4) |
| Other | 0 (0.0) | 1 (0.4) | 1 (0.2) |
| Missing | 6 (2.5) | 2 (0.7) | 8 (1.5) |
| Number of lumens | |||
| 1 | 229 (94.2) | 237 (84.6) | 466 (89.1) |
| 2 | 10 (4.1) | 43 (15.4) | 53 (10.1) |
| Missing | 4 (1.6) | 0 (0.0) | 4 (0.8) |
| Antimicrobial coating | |||
| Yes | 0 (0.0) | 2 (0.7) | 2 (0.4) |
| No | 238 (97.9) | 275 (98.2) | 513 (98.1) |
| Missing | 5 (2.1) | 3 (1.1) | 8 (1.5) |
| CT pump compatible | |||
| Yes | 217 (89.3) | 40 (14.3) | 257 (49.1) |
| No | 22 (9.1) | 237 (84.6) | 259 (49.5) |
| Missing | 4 (1.6) | 3 (1.1) | 7 (1.3) |
| Valve | |||
| Yes | 2 (0.8) | 11 (3.9) | 13 (2.5) |
| No | 238 (97.9) | 265 (94.6) | 503 (96.2) |
| Missing | 3 (1.2) | 4 (1.4) | 7 (1.3) |
Appendix 6 PORT versus Hickman: quality-of-life compliance
| Month | Arm | |||||
|---|---|---|---|---|---|---|
| PORT | Hickman | |||||
| Present (n) | Expected (n) | Compliance (%) | Present (n) | Expected (n) | Compliance (%) | |
| 0 | 243 | 253 | 96.0 | 284 | 297 | 95.6 |
| 1 | 185 | 243 | 76.1 | 191 | 281 | 68.0 |
| 2 | 168 | 238 | 70.6 | 183 | 270 | 67.8 |
| 3 | 161 | 222 | 72.5 | 156 | 245 | 63.7 |
| 4 | 142 | 209 | 67.9 | 116 | 214 | 54.2 |
| 5 | 126 | 203 | 62.1 | 90 | 177 | 50.8 |
| 6 | 116 | 192 | 60.4 | 72 | 153 | 47.1 |
| 7 | 100 | 182 | 54.9 | 55 | 117 | 47.0 |
| 8 | 89 | 166 | 53.6 | 33 | 86 | 38.4 |
| 9 | 72 | 152 | 47.4 | 21 | 55 | 38.2 |
| 10 | 63 | 137 | 46.0 | 18 | 49 | 36.7 |
| 11 | 55 | 130 | 42.3 | 12 | 34 | 35.3 |
| 12 | 56 | 114 | 49.1 | 11 | 30 | 36.7 |
| Total | 1576 | 2441 | 64.6 | 1242 | 2008 | 61.9 |
Appendix 7 PORT versus Hickman: EuroQoL-5 Dimensions and Quality of Life Questionnaire C30 analysis results
| Questionnaire | Original AUC data, mean (SE) | Pooled imputed AUC data, mean (SE) | Two-sided p-value | |||
|---|---|---|---|---|---|---|
| PORT | Hickman | PORT | Hickman | Unadjusted | Adjusted | |
| EQ-5D | ||||||
| Index value | –0.003 (0.02) | –0.010 (0.01) | –0.157 (0.03) | –0.139 (0.03) | 0.363 | 0.363 |
| Health state score | –9.72 (1.47) | –10.90 (1.67) | –11.39 (1.73) | –8.74 (1.53) | 0.129 | 0.258 |
| QLQ-C30: global health status | ||||||
| Global health status | –4.68 (1.27) | –4.18 (1.18) | –4.74 (1.46) | –3.86 (1.16) | 0.691 | 0.942 |
| Functional scales | ||||||
| Physical functioning | –5.27 (1.02) | –4.88 (1.02) | –5.73 (1.06) | –5.33 (0.97) | 0.793 | 0.965 |
| Role functioning | –5.80 (1.72) | –7.04 (1.52) | –5.94 (2.01) | –7.01 (1.94) | 0.965 | 0.965 |
| Emotional functioning | 0.95 (1.20) | 0.88 (0.95) | 1.45 (1.46) | 1.71 (0.96) | 0.941 | 0.965 |
| Cognitive functioning | –4.64 (1.08) | –2.31 (0.98) | –5.35 (1.06) | –2.61 (1.02) | 0.040 | 0.300 |
| Social functioning | –3.28 (1.63) | –2.48 (1.44) | –2.82 (1.72) | –1.41 (1.55) | 0.454 | 0.851 |
| Symptom scales | ||||||
| Fatigue | 7.39 (1.55) | 6.23 (1.21) | 7.79 (1.55) | 5.86 (1.36) | 0.212 | 0.636 |
| Nausea and vomiting | 5.99 (1.21) | 2.19 (1.17) | 4.89 (1.19) | 0.57 (1.17) | 0.081 | 0.405 |
| Pain | –1.93 (1.69) | –3.28 (1.27) | –1.77 (1.67) | –4.45 (1.50) | 0.108 | 0.405 |
| Dyspnoea | 3.50 (1.50) | 4.35 (1.29) | 3.21 (1.70) | 4.39 (1.51) | 0.662 | 0.942 |
| Insomnia | –1.94 (2.08) | 0.58 (1.54) | –2.65 (1.97) | –0.30 (2.00) | 0.392 | 0.840 |
| Appetite loss | 5.20 (1.84) | –1.04 (1.62) | 4.98 (1.88) | –1.99 (1.74) | 0.007 | 0.105 |
| Constipation | 3.18 (1.62) | 1.00 (1.60) | 2.80 (1.66) | –0.75 (1.65) | 0.297 | 0.743 |
| Diarrhoea | 4.74 (1.54) | 5.82 (1.43) | 5.08 (1.40) | 4.98 (2.00) | 0.949 | 0.965 |
| Financial difficulties | –0.79 (1.45) | 0.74 (1.11) | –0.66 (1.73) | 0.58 (1.46) | 0.665 | 0.942 |
Appendix 8 PORT versus Hickman: Venous Access Device Questionnaire analysis results
| Activity | p-value | |
|---|---|---|
| Unadjusted | Adjusted | |
| Driving a car | 0.923 | 0.923 |
| Getting in or out of a car | 0.606 | 0.693 |
| Using public transport | 0.524 | 0.645 |
| Going out shopping | 0.676 | 0.721 |
| Eating | 0.452 | 0.603 |
| Hygiene | < 0.001 | < 0.001 |
| Sleeping | 0.007 | 0.011 |
| Mobility or movement | < 0.001 | < 0.001 |
| Normal work activity | 0.011 | 0.016 |
| Exercise | < 0.001 | < 0.001 |
| Hobbies | < 0.001 | < 0.001 |
| Self-consciousness | < 0.001 | < 0.001 |
| Socialising | < 0.001 | < 0.001 |
| At risk of infection | < 0.001 | < 0.001 |
| At risk of damaging device | < 0.001 | < 0.001 |
| Negative impact on quality of life | < 0.001 | < 0.001 |
Appendix 9 PORT versus peripherally inserted central catheter: device details
| Device details | Device, n (%) | Total, n (%) | |
|---|---|---|---|
| PORT | PICC | ||
| Manufacturer | |||
| CR Bard Inc. | 7 (5.1) | 26 (15.2) | 33 (10.7) |
| Cook Medical LLC | 26 (19.0) | 21 (12.3) | 47 (15.3) |
| Vygon | 2 (1.5) | 101 (59.1) | 103 (33.4) |
| Med CMP | 10 (7.3) | 0 (0.0) | 10 (3.2) |
| Smith Medical, Inc. | 26 (19.0) | 0 (0.0) | 26 (8.4) |
| AngioDynamics, Inc. | 12 (8.8) | 11 (6.4) | 23 (7.5) |
| Other | 54 (39.4) | 11 (6.4) | 65 (21.1) |
| Missing | 0 (0.0) | 1 (0.6) | 1 (0.3) |
| Catheter material | |||
| Silicone | 62 (45.3) | 18 (10.5) | 80 (26.0) |
| Polyurethane | 69 (50.4) | 148 (86.5) | 217 (70.5) |
| Other | 1 (0.7) | 1 (0.6) | 2 (0.6) |
| Missing | 5 (3.6) | 4 (2.3) | 9 (2.9) |
| Number of lumens | |||
| 1 | 133 (97.1) | 150 (87.7) | 283 (91.9) |
| 2 | 3 (2.2) | 16 (9.4) | 19 (6.2) |
| Missing | 1 (0.7) | 5 (2.9) | 6 (1.9) |
| Antimicrobial coating | |||
| Yes | 2 (1.5) | 17 (9.9) | 19 (6.2) |
| No | 131 (95.6) | 149 (87.1) | 280 (90.9) |
| Missing | 4 (2.9) | 5 (2.9) | 9 (2.9) |
| CT pump compatible | |||
| Yes | 115 (83.9) | 67 (39.2) | 182 (59.1) |
| No | 18 (13.1) | 99 (57.9) | 117 (38.0) |
| Missing | 4 (2.9) | 5 (2.9) | 9 (2.9) |
| Valve | |||
| Yes | 6 (4.4) | 47 (27.5) | 53 (17.2) |
| No | 128 (93.4) | 120 (70.2) | 248 (80.5) |
| Missing | 3 (2.2) | 4 (2.3) | 7 (2.3) |
Appendix 10 PORT versus peripherally inserted central catheter: quality-of-life compliance
| Month | Arm | |||||
|---|---|---|---|---|---|---|
| PORT | PICC | |||||
| Present (n) | Expected (n) | Compliance (%) | Present (n) | Expected (n) | Compliance (%) | |
| 0 | 142 | 147 | 96.6 | 189 | 197 | 95.9 |
| 1 | 98 | 141 | 69.5 | 126 | 185 | 68.1 |
| 2 | 96 | 135 | 71.1 | 100 | 162 | 61.7 |
| 3 | 83 | 129 | 64.3 | 82 | 138 | 59.4 |
| 4 | 65 | 119 | 54.6 | 62 | 120 | 51.7 |
| 5 | 64 | 111 | 57.7 | 39 | 97 | 40.2 |
| 6 | 46 | 101 | 45.5 | 26 | 70 | 37.1 |
| 7 | 52 | 95 | 54.7 | 19 | 49 | 38.8 |
| 8 | 46 | 87 | 52.9 | 13 | 35 | 37.1 |
| 9 | 39 | 80 | 48.8 | 12 | 29 | 41.4 |
| 10 | 27 | 74 | 36.5 | 11 | 23 | 47.8 |
| 11 | 28 | 70 | 40.0 | 4 | 20 | 20.0 |
| 12 | 26 | 67 | 38.8 | 6 | 19 | 31.6 |
| Total | 812 | 1356 | 59.9 | 689 | 1144 | 60.2 |
Appendix 11 PORT versus peripherally inserted central catheter: EQ-5D and Quality of Life Questionnaire C30 analysis results
| Questionnaire | Original AUC data | Pooled imputed AUC data | Two-sided p-value | |||
|---|---|---|---|---|---|---|
| PORT | PICC | PORT | PICC | |||
| Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | Unadjusted | Adjusted | |
| EQ-5D | ||||||
| Index value | –0.031 (0.01) | –0.017 (0.01) | –0.216 (0.04) | –0.113 (0.04) | 0.003 | 0.006 |
| Health state score | –17.09 (2.84) | –19.08 (2.84) | –14.51 (1.99) | –9.78 (2.21) | 0.164 | 0.410 |
| QLQ-C30: global health status | ||||||
| Global health status | –4.23 (1.34) | –7.68 (1.20) | –5.71 (1.52) | –7.55 (1.70) | 0.490 | 0.725 |
| Functional scales | ||||||
| Physical functioning | –5.52 (1.06) | –5.92 (1.06) | –7.21 (1.45) | –6.70 (1.23) | 0.738 | 0.852 |
| Role functioning | –6.14 (1.91) | –10.52 (1.83) | –7.85 (2.33) | –10.62 (2.09) | 0.532 | 0.725 |
| Emotional functioning | –0.50 (1.13) | –0.10 (1.20) | –0.28 (1.38) | –0.36 (1.22) | 0.710 | 0.852 |
| Cognitive functioning | –4.22 (1.18) | –3.49 (1.18) | –6.31 (1.30) | –4.64 (1.41) | 0.481 | 0.725 |
| Social functioning | –4.33 (1.84) | –6.02 (1.78) | –6.27 (2.08) | –6.28 (2.07) | 0.942 | 0.942 |
| Symptom scales | ||||||
| Fatigue | 9.60 (1.61) | 8.44 (1.39) | 11.49 (1.64) | 9.10 (2.00) | 0.306 | 0.725 |
| Nausea and vomiting | 5.18 (1.60) | 2.46 (1.32) | 6.11 (1.53) | 2.97 (1.43) | 0.160 | 0.513 |
| Pain | –0.43 (1.57) | 1.28 (1.64) | 0.28 (1.69) | 1.90 (1.67) | 0.532 | 0.725 |
| Dyspnoea | 2.51 (1.77) | 4.95 (1.33) | 3.50 (1.84) | 5.43 (1.53) | 0.901 | 0.942 |
| Insomnia | 0.03 (2.27) | 3.72 (1.99) | –0.71 (2.33) | 3.79 (2.13) | 0.150 | 0.513 |
| Appetite loss | 3.17 (2.30) | 0.96 (1.79) | 3.85 (2.60) | 0.30 (2.83) | 0.376 | 0.725 |
| Constipation | 2.08 (1.96) | –4.17 (1.87) | 2.49 (2.05) | –3.42 (1.82) | 0.029 | 0.435 |
| Diarrhoea | 2.14 (2.28) | 5.99 (1.69) | 2.89 (2.23) | 7.22 (1.66) | 0.171 | 0.513 |
| Financial difficulties | 5.08 (1.68) | 1.17 (1.46) | 4.67 (1.69) | –0.06 (1.65) | 0.123 | 0.513 |
Appendix 12 PORT versus peripherally inserted central catheter: Venous Access Device Questionnaire analysis results
| Activity | p-value | |
|---|---|---|
| Unadjusted | Adjusted | |
| Driving a car | 0.968 | 0.968 |
| Getting in or out of a car | 0.593 | 0.678 |
| Using public transport | 0.287 | 0.383 |
| Going out shopping | 0.581 | 0.678 |
| Eating | 0.848 | 0.905 |
| Hygiene | < 0.001 | < 0.001 |
| Sleeping | 0.136 | 0.198 |
| Mobility or movement | 0.118 | 0.189 |
| Normal work activity | 0.059 | 0.105 |
| Exercise | < 0.001 | < 0.001 |
| Hobbies | < 0.001 | < 0.001 |
| Self-consciousness | 0.008 | 0.016 |
| Socialising | 0.004 | 0.009 |
| At risk of infection | 0.001 | 0.003 |
| At risk of damaging device | < 0.001 | < 0.001 |
| Negative impact on quality of life | < 0.001 | < 0.001 |
Glossary
- Hickman
- Centrally inserted, Hickman-type, cuffed tunnelled venous access device.
- PICC
- Peripherally inserted central venous access device.
- PORT
- Centrally inserted totally implantable venous access device.
List of abbreviations
- 24/7
- 24 hours a day, 7 days a week
- AUC
- area under the curve
- BMI
- body mass index
- BWoSCC
- Beatson West of Scotland Cancer Centre
- CAVA
- Cancer And Venous Access
- CI
- confidence interval
- CRUK
- Cancer Research UK
- CT
- computerised tomography
- CTU
- Clinical Trials Unit
- CVAD
- central venous access device
- CVC
- central venous catheter
- DMC
- Data Monitoring Committee
- EORTC
- European Organisation for Research and Treatment of Cancer
- epic
- evidence-based practice in infection control
- EQ-5D
- EuroQoL-5 Dimensions
- EQ-5D-3L
- EuroQoL-5 Dimensions, three-level version
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- ITT
- intention to treat
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMA
- network meta-analysis
- NMB
- net monetary benefit
- OR
- odds ratio
- PE
- pulmonary embolism
- PICC
- peripherally inserted central catheter
- PIS
- patient information sheet
- QALY
- quality-adjusted life-year
- QLQ-C30
- Quality of Life Questionnaire C30
- RCT
- randomised controlled trial
- SACT
- systemic anticancer therapy
- SD
- standard deviation
- SIR
- Society of Interventional Radiology
- TSC
- Trial Steering Committee
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/hta25470).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
