Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-1210-12009. The contractual start date was in June 2013. The final report began editorial review in June 2019 and was accepted for publication in July 2020. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2021 Michaels et al. This work was produced by Michaels et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 Michaels et al.
SYNOPSIS
This section provides some background regarding the changes in vascular services in recent years that have arisen from technological developments and the introduction of a new specialty, which have led to the need for reconfiguration. An overview is given of the programme aims and objectives, the structure of the workstreams and the changes from the original protocol. The main components of each study are described, followed by a brief comment on patient and public involvement in the programme.
Background
In providing any clinical service, there is tension between maximising efficiency, cost-effectiveness and other desirable features of the service. Financial pressure on providers encourages efficiency, maximising activity for the minimum possible cost. Commissioners wishing to maximise cost-effective use of resources are interested in wider outcomes. However, they are limited in their ability to identify clinically meaningful outcome measures or validated proxy measures and process attributes that adequately reflect service quality and can be derived from currently available data sources. Other service attributes of importance to service users and wider society, such as equity, processes of care and dignity, are not routinely assessed but may be significantly affected by service reconfiguration. For example, when considering potential models for devolved or centralised services, the need for providers to maximise efficiency may produce adverse incentives that encourage smaller independent units to increase activity by treating cases where effective management could be better provided by a larger unit. However, the same drivers may cause centralised units to consolidate on a single site, reducing patient choice and accessibility and potentially reducing equity. It is thus vital that in planning services the key desirable attributes are identified, their relative importance is understood and there is a means available to monitor the effects of any changes.
There is currently enormous pressure for the reconfiguration of vascular services due to many conflicting requirements. Vascular surgery has separated from general surgery, progressing from a situation in the late 1990s with only a handful of centres with sufficient specialist clinicians to offer separate emergency vascular services1 to the situation when the programme was planned, when about half of vascular surgeons had no general surgical practice. 2 In July 2011 the four Departments of Health and Social Care in the UK agreed to the formation of a new specialty of vascular surgery, and a Specialist Advisory Committee was established and began specialty training year 3 in October 2013. Since then, most vascular and general services have separated completely, with < 10% of vascular services providing any general surgical cross-cover in 20153 and only 5% of vascular surgeons providing any general surgery in a 2018 vascular workforce survey. 4
These changes, and similar subspecialisation in vascular radiology, are driven partly by technological and clinical developments, such as new minimally invasive treatments for peripheral arterial disease (PAD), endovascular stent grafts for abdominal aortic aneurysms (AAAs), new treatments for varicose veins (VV) and new imaging methods, such as magnetic resonance angiography (MRA) and computerised tomography angiography (CTA).
Other drivers for service change have been the implementation of screening for AAAs (see www.gov.uk/topic/population-screening-programmes/abdominal-aortic-aneurysm; accessed 1 December 2020), scarcity of expertise in vascular interventional radiology,5 changes in training and working hours related to the European Working Time Directive and recognition of the need for sustainable out-of-hours specialist service provision. 3
Research carried out for the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme 20 years ago in a single region highlighted conflicting pressures:6
-
the need to optimise health gains by planning activity in centres with access to specialist expertise, the provision of emergency vascular and interventional radiology services and multidisciplinary working
-
workforce planning, staffing and training needs
-
improving equity of access and outcome between socioeconomic and geographic groups
-
patient preferences for attributes, such as location, travelling times, waiting times and processes of care (including the availability of newer, minimally invasive techniques)
-
links to other services, such as diabetes, general surgery, leg ulcer, cardiothoracic, stroke and renal services
-
resource availability and the capital and recurrent costs associated with reconfiguration.
Overview of the programme
The overarching aim of the research programme is to identify and draw together existing evidence regarding vascular service configuration; to develop the tools necessary to plan and assess existing services; and to predict and evaluate the results of reconfiguration. In the original application, the programme was divided into four main workstreams that are closely inter-related and include a number of substudies. Figure 1 provides an overview of the workstreams, the links between them and the relevant outputs and appendices.
FIGURE 1.
Overview of the programme, workstreams, inter-relations and outputs. CAD, carotid artery disease; EVAR, endovascular aneurysm repair; HES, Hospital Episode Statistics.
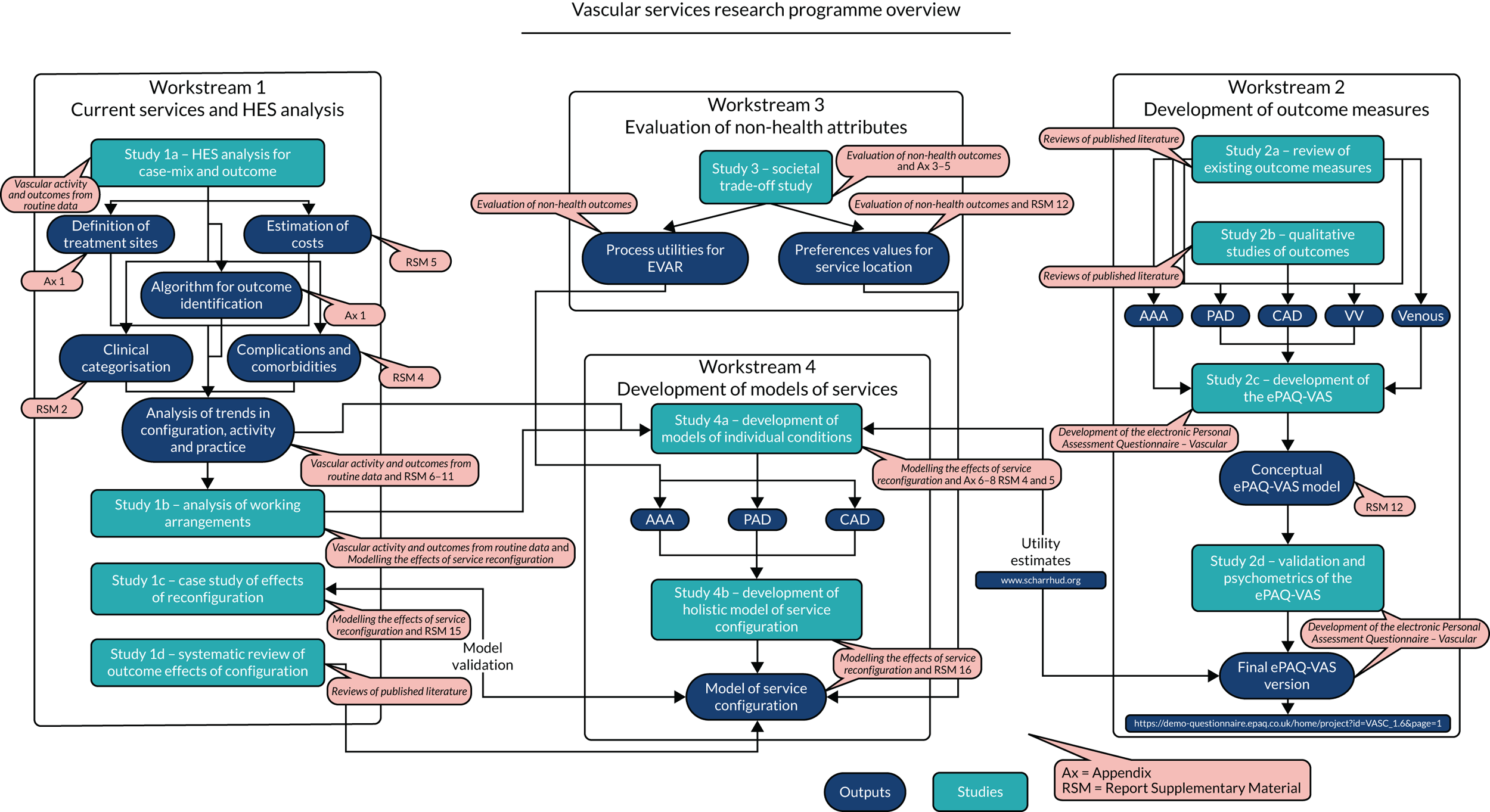
The main outputs of the programme are a new electronic Personal Assessment Questionnaire – Vascular (ePAQ-VAS) to facilitate the collection of patient-reported outcome measure (PROM) data, and a series of economic and decision analytic models that incorporate all of the current evidence to allow the characterisation of current services and prediction of the effects of any reconfiguration at either a local or a national level.
An interactive demo version of the ePAQ-VAS, a copy of a sample report and a demonstration of the management’s site are available at www.epaq.co.uk/Demo/VascularDemo (accessed 1 December 2020). The web-based interface for the organisational model can be accessed at https://modellers.sheffield.ac.uk/vascularmodel/ (accessed 1 December 2020).
To develop these, separate strands of the research have involved a thorough evaluation of existing outcome measures used in vascular services, collection of published and primary qualitative data about vascular outcomes and developing and testing the ePAQ-VAS. This has been validated as a tool to monitor clinical outcomes and is intended to be used on an individual patient basis in clinical practice, and to provide aggregate data for assessing and monitoring the effectiveness of clinical services. Further work has developed algorithms to produce consistent analysis of practice and outcomes from routinely collected hospital data, which may be used in the future to evaluate the effects of reconfiguration.
Report outline
The following is a brief overview of each of the workstreams, as originally envisaged in the application, describing the component studies and how these are incorporated into the report. The appendices provide additional details of published papers and unpublished methods and results.
Objective 1: current service arrangements
The first objective was to characterise the existing arrangements for vascular services and to establish the relationship between workload, case-mix and outcome and service configuration through an analysis of routinely collected hospital data and existing published literature. This workstream comprised four substudies. Study 1a was the analysis of Hospital Episode Statistics (HES) to identify trends and variation in activity and aspects of case-mix and outcome that can be established from routinely collected data sources. This study depended on the analysis of HES; some initial problems were encountered in obtaining the data from NHS Digital, which resulted in some delays in undertaking this aspect of the work. The task also proved to be considerably more complex than what was originally envisaged. There were a variety of reasons for this, including the lack of consistency in coding, with changes in both the coding systems and the practices over the period of the study. There were also some difficulties encountered in classifying cases with multiple and often conflicting codes and ambiguities.
Classification systems and algorithms were established that enabled consistent case-mix groups and adjustment for comorbidities allowing comparisons to be made between workload, working practices, resource use and outcomes that could be compared between centres based on service configuration and used to identify trends over time (see Vascular activity and outcomes from routine data, Appendix 1 and Report Supplementary Materials 2–11).
These classification systems and algorithms were used to populate the modelling that was carried out in workstream 4 to predict the effects of service reconfiguration on workload and outcome (see Modelling the effects of service reconfiguration). Several publications and presentations arose from this work that reported specific aspects of the trends and variations in workload, including a study of the outcomes of aortic aneurysm surgery; papers on sex differences in rates of repair of emergency AAA; papers on risk adjustment and trends in aortic aneurysm treatment; and a contribution towards another NIHR-funded project7 that examined the potential cost-effectiveness of screening women for AAA. 8–12
This proposed workstream included a survey of current working arrangements for the provision of vascular services. The intention was to carry out a survey in vascular centres in England to understand the current working arrangements. However, prior to this study commencing, the Vascular Society of Great Britain and Ireland (VSGBI) carried out a large survey of its members that covered most of the same subject matter. 3 In view of this, a decision was made that it would not be helpful to carry out a further survey because it would add little to the available information and would probably have a poor response rate from following so soon after the audit. Instead, the results of the audit were reviewed and compared with the evidence that was available from the analysis of HES to characterise working practices and identify geographical areas in which some planned reconfiguration of vascular services had taken place in recent years or was currently being planned (see Vascular activity and outcomes from routine data).
The third study that was included in workstream 1 related to an analysis of the practical effects of organisational change on activity and identifiable outcomes. This aspect of the work is included in the validation of the modelling (see Modelling the effects of service reconfiguration).
Finally, workstream 1 included a systematic review of the relationship between service configuration and clinical outcomes (see Reviews of published literature). Considerable evidence was identified in the literature and has resulted in a number of peer-reviewed publications in three separate areas: AAA repair,13 lower limb vascular surgery14 and carotid artery disease (CAD) procedures. 15
Objective 2: development of the electronic Personal Assessment Questionnaire – Vascular
The second objective of the programme was to develop, validate and implement condition-specific and generic outcome measures that could be collected in electronic form and used in clinical practice, as well as in aggregate form for the evaluation and monitoring of services. This workstream represented a considerable proportion of the total work of the programme, and included an extensive evaluation of existing outcome measures and a rigorous process for the development and implementation of a new tool for measuring outcome in vascular services. The workstream consisted of a number of related studies. The first of these was a systematic review of existing outcome measures (see Reviews of published literature). This involved an extensive set of systematic literature reviews that identified relevant outcome measures in each of the major disease areas dealt with by vascular services. The purpose of these studies was to identify any existing measures that could be incorporated into the new electronic tool and to inform its development. These reviews resulted in publications and presentations covering separate disease areas of AAA,16 PAD,17 CAD,18 VV19 and venous leg ulcers (VLUs). 20
The second planned aspects of workstream 2 were a Delphi exercise and qualitative studies to identify relevant symptoms, signs and impacts that should be included in the electronic outcome tool. In practice, this included several different aspects of the outcome tool development. Systematic reviews of qualitative evidence regarding issues that were important to patients resulted in several presentations and further publications relating to PAD,21 CAD,22 VV23 and VLUs. 24
Further primary qualitative research was carried out to identify additional themes from patients with vascular disease and a Delphi exercise was carried out with health-care professionals, both of which were used to inform the development of the new outcome measurement tool.
The third planned study in workstream 2 that was described in the original protocol related to the evaluation of existing identified measures and domains identified from the literature reviews and qualitative work to incorporate existing measures or develop new preference-based measures for the evaluation of vascular services. Careful reviewing of the existing measures found that, other than the generic EuroQol-5 Dimensions (EQ-5D), none of these was considered to be suitable to be incorporated in the new instrument. Therefore, a new preference-based measure, incorporating all of the necessary domains for the evaluation of vascular services, was developed based on the literature reviews and qualitative studies. This is described fully in Development of the electronic Personal Assessment Questionnaire – Vascular.
The final study of workstream 2 relates to the validation and evaluation of psychometric properties of the ePAQ-VAS. The final version of the ePAQ-VAS was developed following a similar process and subjected to a rigorous psychometric evaluation (see Development of the electronic Personal Assessment Questionnaire – Vascular).
Objective 3: other service attributes
The workstream that related to the third objective was designed to establish the relative value that society places on attributes other than health-related quality of life (HRQoL), such as travelling distance and treatment processes. This was carried out using a trade-off methodology, and two key areas were investigated in detail: those relating to process utility, specifically the difference between endovascular and open techniques for the repair of AAA, and the travelling distances to a local or central provider of vascular services (see Evaluation of non-health outcomes).
Objective 4: model development
The final workstream was related to the objective of developing models of service configuration. This was carried out in two parts. The initial work involved the development of three separate models that covered the clinical areas of AAA, PAD and CAD. Specific models for VV and other venous diseases were not considered necessary because the clinical consensus was that these services would not be significantly affected by reconfiguration of major arterial services.
Each of the models was developed using a conceptual framework based on clinical consensus representing the pathways of care, characterised through the analysis of HES. These were populated with data from the analysis of HES records and systematic literature reviews, and primarily used a discrete event simulation. Models were calibrated using the HES data, and the potential effects on workload and activity were validated using case studies identified from the HES statistics.
These models of individual disease areas were brought together in an interactive web-based model that enables users to identify potential reconfiguration of services within particular geographical areas and to predict the overall effects of potential service reconfiguration in terms of workload, resource use, outcomes and cost-effectiveness (see Evaluation of non-health outcomes).
Summary of changes from the proposal
Workstream 1: Hospital Episode Statistics data and service configuration
The survey of national current practice was not conducted because a survey of practice was completed at the same time by the Vascular Society.
No other changes were made to this workstream. An extension to the programme grant was approved, largely because of delays in obtaining the HES data from NHS Digital.
Workstream 2: reviewing
No changes were made to this workstream.
Workstream 2: development of the electronic Personal Assessment Questionnaire – Vascular
-
Recruitment was restricted to a single centre because of delays and complexity in gaining the necessary approvals and limitations in staff resources.
-
The results from the Delphi exercise were of limited value with regard to item reduction because of lack of agreement and a low response rate in the second round.
-
For face validity, only face-to-face interviews were carried out and no focus group was organised. This decision was taken because of the difficulty in recruiting a sufficient number of participants for a focus group in a timely manner without delaying the factor survey.
-
Rasch analysis was performed for certain sections of the ePAQ-VAS, but this was not possible for sections completed by only a small group of patients. Additional item response test analysis was performed.
Workstream 3: patient preference
Following further discussions with clinicians and modellers, it became apparent that there was a need to expand the scope of the study to look at different organisational models with follow-up locally or at the central hospital. In addition, as AAA treatment is the main driver for centralisation, it was felt that there was a need to look in more detail at preferences for endovascular and surgical treatment options per se. Finally, further developments were also required in the light of evidence of links between centralisation and outcome for certain vascular treatments. On the advice of the clinicians, VV and VLUs were dropped from the conditions to be included. This left three clinical areas to include: AAA, CAD and PAD.
The method was revised to accommodate the changes by altering some of the details of the trade-off choices, as described in Evaluation of non-health outcomes.
The initial proposal was for 200 interviews in each of the five clinical areas. With three clinical areas looking at the travel distance to hospital and one area looking at the treatment process for AAA, this initial target was revised down to 800 (i.e. four telephone surveys with 200 interviews in each).
Workstream 4: modelling
Based on clinical advice regarding the likely changes that would drive reconfiguration, the individual disease models that informed the overall model of service configuration were limited to the three clinical areas of AAA, CAD and PAD.
Patient and public involvement
The Sheffield Teaching Hospitals NHS Foundation Trust Online Public Advisory Panel provided feedback on the study materials throughout the development of the study. In the summer of 2016, the patient and public involvement (PPI) panel reviewed study documents, including the participant information sheet and invitation letter, and feedback was used to revise the wording of these documents. Moreover, members of the PPI panel participated in mock interviews using the AAA interview schedule. The overall aim was to test the language, structure and comprehension of the study materials to gather feedback and refine the survey. The feedback obtained included suggestions on the wording and design of study materials and the need to present risk (percentages) in an understandable way. All of the feedback was incorporated into the final version of the study materials. In particular, while presenting risk, a simple diagram was designed to explain what was meant by ‘chance of success’. This was included in the interview booklet and was used as a guide to decision-making during the telephone interview.
Reviews of published literature
This section reports the results of the systematic literature reviews that were carried out as part of the programme. The first section deals with the systematic reviews of published literature regarding the relationship between service configuration and outcome, which focused on three main areas of lower limb vascular disease, AAA and CAD. The subsequent sections report the results of a series of reviews to identify existing PROMs in each of the main disease areas (AAA, PAD, CAD, VV and VLU) and separate reviews to identify qualitative evidence regarding the issues of importance to patients with these conditions.
The effects of reconfiguration on practice and outcome
The relationship between the number of invasive procedures (volume) conducted by health-care institutions or individual clinicians and the outcomes, such as mortality, has been discussed and investigated in multiple conditions since the 1980s. 25,26
Although evidence of an inverse relationship between the volume of vascular procedures performed and the adverse outcomes is presented in previous systematic reviews and meta-analyses,27–37 the included data are dated. The evidence primarily represents populations in the USA38 and is, therefore, of questionable value in relation to contemporary UK practice. In the context of ongoing reconfiguration of vascular services in the UK and recent technological advances in the treatment of vascular conditions, a series of systematic reviews were conducted to evaluate the relationship between mortality and the volume of vascular procedures undertaken by individual clinicians and/or hospitals in European populations.
Methods
In general, the reviews were conducted in four stages:
-
A protocol was developed with input from clinicians, information specialists and academics and registered on PROSPERO (www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42014014850; accessed 1 December 2020) prior to the conduct of each review.
-
A single search strategy39 that combined search terms for all vascular conditions was developed and electronic searches were conducted in databases including MEDLINE, EMBASE™ (Elsevier, Amsterdam, the Netherlands), Cumulative Index to Nursing and Allied Health Literature (CINAHL) and PsycInfo® (American Psychological Association, Washington, DC, USA). Additional hand-searches and citation searches were also conducted.
-
An overview of existing systematic reviews38 was undertaken. However, no relevant current systematic review of high quality was identified that answered the research question.
-
Three systematic reviews relating to three discrete conditions, PAD, AAA and CAD, were conducted. Systematic reviews were subsequently presented at international conferences and published in peer-reviewed journals (see Appendices 2–4). 13–15
Standard systematic review methods,40 in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) recommendations,40 were followed and are detailed in the published papers. 13–15 A decision to focus on European populations was made because the organisation and delivery of ‘socialised’ health care in these countries is similar, but not identical, to the UK NHS. This is in contrast to the USA, where a market-led model is the norm.
The ideal method of identifying a causal relationship between an exposure or intervention (in this case, volume) and an outcome is to conduct a randomised controlled trial; however, the studies included in these reviews were expected to be observational rather than experimental because of the practical and ethics difficulties of randomising participants to high- or low-volume clinicians or institutions. It was, therefore, judged to be important to evaluate studies on the effects of volume in accordance with principles that would avert any understimation of the risk of bias or attribution of an inflated level of certainty to any measure of effect size. A Cochrane Risk of Bias Assessment Tool for Non-Randomised Studies of Interventions was considered to be appropriate for this reason and, therefore, was used to assess the methodological quality of the included studies. Meta-analyses, although planned, were judged to be inappropriate because of clinical and methodological heterogeneity and the risk of selection, reporting and publication bias. Narrative syntheses were, therefore, conducted, with tabulation of results reflecting the different clinical and procedural groupings presented in individual studies.
Results
Lower limb vascular disease14
Nine studies41–49 including 67,445 patients who had undergone diverse lower limb vascular surgery were eligible for inclusion in the review: three from the UK, one from the UK and Ireland, two from Sweden, two from Finland and one from Denmark. The evidence for an association between hospital/surgeon volume and mortality was contradictory, but the findings suggest that high-volume hospitals may undertake more repeated surgeries/revascularisations and limb salvage. An increase in hospital volume was associated with a decrease in post-operative amputations at 30 days and 1 year, and, similarly, an inverse relationship was identified between surgeon volume and amputation at 30 days. There were insufficient data on other variables to draw firm conclusions.
Abdominal aortic aneurysm13
Sixteen studies50–65 evaluating the volume–outcome relationship in AAA patients were included in the review: 11 studies from the UK, three from Germany, one from Norway and one from a combined UK and Swedish population. Data for 237,074 individual participants were collected from administrative databases and clinical registries incorporating a variety of clinical (elective, emergency and ruptured) and procedural (open and endoscopic repair) groups. The study quality was affected by the reliance on observational study designs. The evidence suggests that there is an inverse relationship between hospital volume and short-term mortality in AAA repair. Insufficient evidence was available to reach conclusions on the relationship between clinician volume and outcome and between hospital or clinician volume and secondary outcomes, including complications and length of hospital stay. Furthermore, clear guidance on volume thresholds for practice was not identified.
Carotid artery disease15
Eleven studies61,66–76 investigating the volume–outcome relationship met the review eligibility criteria: five from the UK, two from Sweden, one each from Germany, Finland and Italy, and a study of a combined German, Austrian and Swiss population. Data from 233,411 participants were included. Two large studies (179,736 patients) suggested that increased hospital volume was associated with reduced mortality in carotid endarterectomy (CEA), with the number needed to treat as small as 165 and for combined mortality and stroke as small as 93. The evidence was less clear for carotid artery stenting (CAS): multiple analyses in three studies did not identify convincing evidence of an association. 67,68,70,71 Limited evidence is available on the relationship between clinician volume and outcome in CAS and CEA.
Discussion
The hypothesis that individual and institutional volumes are inversely related to adverse outcomes seems plausible, and these reviews, based on the largest and highest-quality studies included, found evidence that this is the case for CEA and AAA procedures. However, volume is a convenient but potentially imprecise proxy for quality. The mechanisms by which any benefits are produced were not investigated in this review. For this reason, subsequent benefits are potentially difficult to replicate. It is questionable whether or not simply centralising vascular procedures to be conducted by high-volume clinicians in high-volume institutions will replicate the outcomes associated with a high volume of procedures.
The list of potential confounders is extensive, but includes patient age and sex, surgeon caseload, hospital volume, comorbidities, surgical variables, American Society of Anesthesiologists grade, vascular risk factors, type of hospital, day of procedure, transfer between hospitals, social deprivation, staffing levels (medical and nursing), teaching hospital status, level of research activity and ratio of high-dependency/intensive therapy unit beds to hospital beds. Any of these could be responsible for some or all of the identified volume–outcome effect, either alone or in combination with other known or unknown variables, and it is possible that interventions aimed at manipulating these variables could be effective at improving outcomes for which a wholesale reconfiguration of vascular services might not.
Existing high-volume hospitals have infrastructure and human elements that differentiate them from low-volume hospitals, and potentially from any new institution that is developed to tap into or benefit from such an assumed relationship. It could be that increased volume may be necessary, but not sufficient, to replicate the outcomes already achieved at existing high-volume hospitals. New high-volume hospitals will need to recreate the conditions prevalent in existing high-quality, high-volume hospitals if they are to achieve results comparable to the highest achieving high-volume hospitals.
The authors intended to determine the minimum procedure thresholds that doctors and institutions should practise at to achieve acceptable outcomes; however, this was complicated by the range of clinical and procedural categories that were assessed by the individual studies and it was inappropriate to make definitive recommendations on the basis of the findings.
In addition, attempts to quantify the relationship between volume and outcome do not account for the role of preference. The provision of centres that provide high enough volumes to meet minimum volume criteria might be achievable and convenient in high-density populations, such as London and other major conurbations. However, in more sparsely populated regions the provision of high-volume centres would certainly mean an additional burden of travel to access health care, which could affect patients’ decisions to access treatment. Parallel research conducted as part of this Programme Grant does consider the role of preference.
Reviews of existing patient-reported outcome measures
Methods
The aim of the reviews of PROMs was to identify primary studies reporting psychometric properties of PROMs in the relevant populations of English-speaking patients. The reviews were conducted in line with the PRISMA recommendations,40 the Oxford system77 and the Consensus-based Standards for the selection of health Measurement Instruments (COSMIN) recommendations. 78 Protocols for the reviews are available in:
-
Patient-reported Outcome Measures in Patients with Peripheral Artery Disease: Protocol for a Systematic Review 79
-
Patient Reported Outcome Measures in Patients with Abdominal Aortic Aneurysms: A Systematic Review Protocol 80
-
Systematic Review of Patient-reported Outcome Measures in Patients with Chronic Venous Insufficiency (www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42015024820; accessed 1 December 2020)
-
Patient Reported Outcome Measures in Patients with Carotid Artery Disease: A Systematic Review (www.crd.york.ac.uk/prospero/display_record.php?RecordID=23877; accessed 1 December 2020).
In summary, a two-stage search was conducted in MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations to identify potentially relevant studies. The search strategy was further translated across selected major databases. Related outputs from literature searching were presented at conferences. 39,81 Supplementary literature searches were also undertaken. Randomised studies and non-randomised studies were included if they reported the development and/or validation of generic and condition-specific PROMs in participants with AAA, CAD, PAD, VLU or VV. Owing to issues with language and cultural adaptations, PROMs administered only as original English-language questionnaires were considered eligible for inclusion. Study selection, data extraction and quality assessment were carried out independently by a minimum of two researchers. Discrepancies were checked and resolved by discussion and, if needed, by referral to a third researcher.
Study-specific criteria for assessing the methodological quality of the included studies were adapted from the COSMIN checklist, University of Oxford PROMs development criteria and other sources. 82–87 The psychometric performance of PROMs, according to population of interest, were then summarised as follows: 0, not reported; –, evidence not in favour; +/–, conflicting evidence; and +, evidence in favour. Findings were analysed and presented narratively.
Results
Details of the published systematic reviews of PROMs, with further details of the review results, are provided in Duncan et al. ,16 Poku et al. ,17 Essat et al. ,18 Aber et al. 19 and Poku et al. 20
Peripheral arterial disease17
Psychometric evaluation of six generic and seven condition-specific PROMs reported in 14 studies90–103 contributed data to the review. The most frequently reported measure was the Short Form questionnaire-36 items (SF-36) (n = 11 studies); others included the Walking Impairment Questionnaire (n = 8 studies), EQ-5D (n = 5 studies) and the Vascular Quality of Life Questionnaire (n = 3 studies). Studies included a diverse PAD population and varied in methodology, including approach to validation of PROMs. Substantial variations in the reporting of clinical presentation of PAD, management strategies and administration of instruments were noted. Evidence of superiority in the psychometric performance of a single PROM could not be established. Furthermore, no study provided evidence of a full psychometric evaluation in the patient population.
Abdominal aortic aneurysm16
Four PROMs from three studies104–107 were identified in the psychometric review of PROMs in AAA: the SF-36, the Australian Vascular Quality-of-Life Index (AUSVIQUOL), Aneurysm Dependent Quality of Life (AneurysmDQoL) and the Aneurysm Symptoms Rating Questionnaire (AneurysmSRQ). The SF-36 showed good evidence of internal consistency, construct validity and responsiveness, but did show some floor and ceiling effects in one of the studies. There was evidence to suggest low acceptability, especially in older patients. The AUSVIQUOL showed good content validity because it was developed by interviewing patients with an AAA as well as other conditions. The responsiveness and internal consistency of the AUSVIQUOL have not been assessed. The AneurysmDQoL and AneurysmSRQ are both condition-specific measures of health and HRQoL, and have comparable trend scores, but a conventional psychometric evaluation has not yet been performed. None of the identified PROMs has undergone a rigorous psychometric evaluation in the AAA population.
Carotid artery disease18
Five studies108–112 were included that reported on six PROMs: the SF-36, the EQ-5D, the Hospital Anxiety and Depression Scale (HADS), the Dizziness Handicap Inventory, the quality of life (QoL) for CAD scale and a disease-specific PROM for CAS. The rigour of the psychometric assessment of the PROMs was variable, with most attempting to assess only a single psychometric criterion. No study reported evidence on construct validity and test–retest reliability. Evidence for acceptability for the use of the SF-36, the EQ-5D and the disease-specific PROM was rated good in most studies. Only one study reported a Cronbach’s alpha score of > 0.70 as evidence of internal consistency. Overall, the psychometric evaluation of all included PROMs was rated as poor in the CAS population undergoing revascularisation.
Varicose veins88
Nine studies113–121 reported on aspects of the development and/or validation of one generic (SF-36) and three disease-specific [Aberdeen Varicose Vein Questionnaire (AVVQ), Varicose Veins Symptoms Questionnaire (VVSymQ) and Specific Quality of Life and Outcome Response – Venous] PROMs. The evidence from the included studies provided data to support the construct validity, test–retest reliability and responsiveness of the AVVQ. However, its content validity, including weighting of the AVVQ questions, was biased and based on the opinion of clinicians, and the instrument had poor acceptability. The VVSymQ displayed good responsiveness and acceptability rates. The SF-36 was considered to have satisfactory responsiveness and internal consistency.
Venous ulcers20
Ten studies122–131 with data for four generic PROMs and six condition-specific measures were identified. No generic PROM showed adequate content and criterion validity; however, the EQ-5D, Nottingham Health Profile (NHP) and the Short Form questionnaire-12 items (SF-12) had good acceptability. In general, the EQ-5D showed poor responsiveness in patients with VLUs. Most condition-specific PROMs demonstrated poor criterion and construct validity. Overall, there was some evidence of internal consistency for the Venous Leg Ulcer Quality of Life (VLU-QoL) and the Sheffield Preference-based Venous Ulcer questionnaire. Test–retest reliability was satisfactory for the Venous Leg Ulcer Self-Efficacy Tool. The NHP and VLU-QoL questionnaire seemed to be the most suitable PROMs for use by clinicians. However, a valid condition-specific PROM is still required.
Discussion
All of the systematic reviews contribute to the growing evidence of psychometric performance of PROMs in patients with vascular conditions, although a majority of the included studies (n = 41) did not report a complete psychometric evaluation of a single PROM. Widespread heterogeneity in the study methodology, patient population and treatment pathway could, in principle, limit the conclusions of the individual reviews. Therefore, these are important considerations in future research.
Reviews of qualitative evidence of vascular outcomes
Methods
The aim of the qualitative literature reviews was to examine the symptoms and quality-of-life domains that are important from the perspective of patients with PAD, AAA, CAD, VV and VLUs. Searches were conducted in CINAHL [via EBSCOhost (EBSCO Information Services, Ipswich, MA, USA)], MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations [via Ovid® (Wolters Kluwer, Alphen aan den Rijn, the Netherlands)], EMBASE (via Ovid), Psycinfo (via Ovid), Social Science Citation Index™ (Clarivate Analytics, Philadelphia, PA, USA)/Science Citation Index [via the Web of Science™ (Clarivate Analytics, Philadelphia, PA, USA) and Dissertation Abstracts (ProQuest LLC, Ann Arbor, MI, USA) and theses. To identify relevant evidence for the qualitative literature review, a search strategy was developed to include condition terms, terms for patient-reported outcomes/patient views and terms for qualitative studies. Free text and thesaurus terms, such as medical subject heading (MeSH) terms, were combined using Boolean operators. The search was based on the search strategy created for the related reviews of PROMs for each condition. 16–20 A qualitative study filter was used and combined with relevant thesaurus terms for qualitative studies. 132 Further details of the condition inclusion and exclusion criteria are listed in Table 1. The protocol for these reviews was published. 133
| Inclusion criteria | Exclusion criteria |
|---|---|
| Patient’s health, HRQoL or experience of one of the five vascular conditions listed below | Studies not in English |
| Clinicians’ views | Studies with participants aged < 16 years |
| PAD | |
| A defined population of participants with a diagnosis of PAD, also described as peripheral vascular disease, peripheral obliterate arteriopathy or peripheral arterial occlusive disease or patients with clinical evidence of any or a combination of the following, where PAD is the confirmed or stated underlying cause: rest pain, claudication, vascular spasms, ischaemic ulceration, necrosis or gangrene of the limb, amputation | Undefined population of PAD patients and patients with lower limb ulcers or amputations because of any cause other than PAD |
| AAA | |
| A defined population of participants with a diagnosis of AAA | Patients with pseudoaneurysms |
| Patients with thoracic aortic aneurysms, involving the aortic root, ascending aorta, aortic arch or descending aorta | |
| Patients with thoracoabdominal aneurysms | |
| CAD | |
| A defined population of participants with a diagnosis of CAD who need, have had or are undergoing invasive procedures. Participants undergoing treatment for stroke or TIA secondary to a diagnosis of CAD | Unspecified or mixed populations that include CAS patients. Patients with stroke or TIA not related to CAS |
| VV | |
| A defined population of participants with a diagnosis of CVI presenting with VV | An undefined population of CVI patients or patients with acute venous obstruction, such as acute deep-vein thrombosis |
| VLUs | |
| A defined population of participants with a diagnosis of CVI presenting with VLUs, oedema or skin discoloration | An undefined population of CVI patients or patients with acute venous obstruction, such as acute deep-vein thrombosis |
| Studies that include semistructured interviews, descriptions, focus groups either as standalone studies or embedded in a quantitative study. Must include both data collection and data analysis | Quantitative studies with no primary qualitative data reported |
| Published or unpublished | |
| Full-text or structured abstract with all the relevant information | Full-text or structured abstract with incomplete or unclear evidence |
Qualitative studies were included if they reported on people’s health, QoL or experience of living with one of the five vascular conditions. Studies were included if the population was people with PAD, AAA, CAD, VV or VLUs and included semistructured interviews, descriptions, focus groups as standalone studies or those embedded in a quantitative study.
Framework analysis was used for the analysis of the data for each review. 16,21,23 The PROM domains were used as the initial framework for the data analysis. The method of best fit was used to code text into domains. 134
The domains from the identified PROMs in the previous section were used for a triangulation exercise. The domains from PROMs were mapped against the themes from the qualitative review synthesis for each condition to explore which PROM items/domains captured the themes deemed to be most pertinent to patients. A triangulation approach was followed whereby the researchers evaluated whether the concepts were the same (agreement), offered similar concepts (partial agreement), were in contradiction (dissonance) or were not present (silence).
Results
Five systematic reviews of qualitative evidence were completed to understand and summarise the impact that PAD, AAA, CAD, VV and VLU have on the daily living of patients. 16,21–24 The characteristics and main findings of the studies included in these five qualitative evidence syntheses systematic reviews are summarised in Table 2.
| Condition | Number of citations | Number of included studies | Key themes |
|---|---|---|---|
| AAA | 315 | 4 | Anxiety and lack of physical symptoms |
| CAD | 964 | 4 | Symptoms, psychological and social impact, risk and service experience |
| PAD | 973 | 8 | Pain, compromised physical function, impact on social life |
| VV | 1804 | 3 | Adaptation – coping strategies employed to limit various impacts, appearance of VV |
| VLU | 1804 | 13 | Pain, odour and exudate – impact on sleep, mobility and mood |
Peripheral arterial disease21
Eight papers135–142 fulfilled the inclusion criteria and were included in the qualitative evidence synthesis. The mean age of the participants in the included studies ranged from 64 to 77 years, and the percentage of male participants was 50–79%. The included studies reported the views of 186 patients with PAD including patients with intermittent claudication (IC), chronic limb ischaemia (CLI) and amputation of lower limbs as a result of PAD. The framework analysis of the primary and secondary data in the included papers identified six main issues: symptoms, physical functioning, impact on social functioning, psychological impact, financial impact and process of care. In total, 35 themes were identified. 21
Abdominal aortic aneurysm16
Four studies were included;105,143–145 three of these four studies were conducted in Sweden in Swedish, although the publications were in English. Two of the four studies carried out semistructured interviews with patients who had an AAA identified by screening and were treated conservatively, one focused on patients who received open surgery for the AAA and the final study presented a mix of patients being treated conservatively and patients being treated with open repair (OR) or endovascular aneurysm repair (EVAR). Four overarching themes were identified from the four studies included in the qualitative synthesis: symptoms, functional outcomes, psychological outcomes and social outcomes. 16
Carotid artery disease18,23
Only four papers146–149 fulfilled the inclusion criteria and were included in the qualitative evidence synthesis: three of the included studies were from the UK and one from Sweden. The studies were published between 2002 and 2013; the age of patients who had CAD in the included studies ranged from 50 to 80 years and the percentage of male participants was 50–65%. The included studies reported the views of 62 patients with symptomatic CAS; 24 of the patients were awaiting assessment for surgery, 26 had undergone surgery and 12 were turned down for intervention and received best medical therapy. The framework analysis of the primary and secondary data of the included papers identified 18 themes. These were divided into five main domains comprising anxiety, impact on personal roles and activities, effect on independence, psychological impact, and symptoms. 22
Varicose veins23
Three independent studies150–152 that met the inclusion criteria were identified. The studies were published between 2004 and 2016 and two were conducted in the UK and the other in Sweden. Five overarching themes were identified: physical impact of VV, psychological impact, social impact, adapting to VV and reasons for seeking treatment. Within these main themes, further subthemes were identified: symptoms, symptom management, physical function, worry/anxiety, appearance, social restrictions and relationships. 88
Venous leg ulcers21
Thirteen studies reported in 16 papers153–168 met the inclusion criteria and were included in the review. The studies were published between 1995 and 2014, with eight being conducted in the UK, three in the USA, one in South Africa and one in Ireland. Following analysis of the included papers, the number of main themes was reduced from five to four: physical impact, psychological impact, social impact and treatment. Ulcer and treatment-related pain, as well as odour and exudate, appeared to have significant and direct negative effects on QoL, with additional and cumulative effects on sleep, mobility and mood.
The range of reported symptoms was broad, unique to individuals and reflected the complexity of developing instruments to measure the effects of this condition on patients’ symptoms and QoL (see Poku et al. 20).
Discussion
A total of 32 studies were included across the five reviews of existing qualitative research. The qualitative evidence from these reviews identified major themes and several subthemes within each disease area. There was some overlap between these themes. These qualitative data were used to develop the conceptual framework for the new electronic PROM developed as part of this NIHR programme grant. The evidence from the triangulation helped to identify where previous disease-specific PROMs fell short in covering issues that were deemed to be important to patients with vascular disease. Further detail about the use of these qualitative data in developing the new outcome measure is provided in Development of the electronic Personal Assessment Questionnaire – Vascular.
Vascular activity and outcomes from routine data
This section provides a descriptive summary of the various categories of inpatient activity that are related to vascular services, based on a detailed analysis of HES data. The analysis is primarily descriptive, but more detailed statistical analysis is included in the regression modelling of the key diagnostic areas that is provided in Modelling the effects of service reconfiguration.
The methods developed for classifying admissions into appropriate clinical categories of vascular activity are described, followed by the processes that were established for the identification of measures of case mix, comorbidities, complications and outcomes. Data regarding changes and variation in practice are reported, with additional detail included in Report Supplementary Materials 2–11. A further analysis considers the process of reconfiguration of services over the past 12 years and compares evidence from HES data with reports from the National Vascular Registry (NVR) and the recommendations of the NHS service specification for vascular services.
Analysis of Hospital Episode Statistics data: general methods
A major part of the first workstream of the programme was the analysis of HES data to characterise the workload of vascular services; identify trends in activity, working practices and outcomes; and relate these to service configuration. This section outlines the processes involved in this analysis, with further details given in the published papers and additional material provided in the appendices and supplementary material. The section starts with a brief description of the nature of HES and the process of obtaining the appropriate extract. Following this, there are sections relating to the process for establishing consistent and clinically relevant groupings of vascular activity, the identification of the sites at which the activity was carried out, methods for obtaining valid measures of outcome from the data, and distinguishing comorbidities and complications.
The subsequent section provides some descriptive material regarding the services that are provided, the trends in practice and configuration of services and outcomes. Further details of these analyses are provided in Appendix 1 and published papers. The detailed risk models that were derived from HES data to populate the economic models are described, along with other aspects of the modelling process, in Modelling the effects of service reconfiguration.
Data extract
The main analysis of hospital activity case-mix, resource use, working practices and outcomes was carried out based on an extract of HES data supplied by NHS Digital, and included all relevant fields in an extract of inpatient episodes covering all likely vascular procedural and diagnostic codes (see Report Supplementary Material 1). Further data extracts included linked records for all other inpatient episodes, critical care episodes and death certification records from the Office for National Statistics (ONS) for the cohort of episodes identified in the initial extract. The original data request was made in July 2013; however, because of considerable delays and changes in procedure at NHS Digital, the original data extract was not obtained until May 2015. When an extension request for the programme was made in the autumn of 2017, a further request was made for an updated HES extract to include the most recent years, but further delays resulted in the extract not being received until February 2019, leaving little time for further detailed analysis.
The initial categorisation of data, as described below, was based on the first data set and revised and updated following the receipt of additional data. However, because of the changes in coding and limited resources, a decision was made to limit the final analysis base to the 12-year period from April 2006 to March 2018, which was the most recent 12-year period that was available.
Hospital Episode Statistics data analysis
The process of analysing the HES data was largely carried out using custom-built programmes written in the R software package (The R Foundation for Statistical Computing, Vienna, Austria) (see Appendix 1). The following is a brief summary of the issues that were addressed for each of these stages of data analysis.
Cleaning of data
The HES data included a number of inconsistent, ambiguous, incomplete or duplicate records. The initial stage of ‘cleaning’ involved the removal of duplicate records or records missing critical information. Attempts were made to ensure that best use was made of the available data, for example by combining information from duplicate records if one copy was missing a piece of information, such as discharge date, that was included in another copy of the record. Thus, incomplete, overlapping or duplicate records were merged where possible to produce valid data.
The development of vascular case-mix categories
To characterise admissions, a set of case-mix categories was defined to cover the majority of vascular activity. This was an iterative process using a set of assignment rules based on procedural and diagnostic codes and other fields of the HES records, where necessary. A clinical consensus group developed an initial categorisation that included five main categories: AAA, PAD, CAD, VV and complex venous disease including VLUs. Initial categorisation was carried out and aggregate data were tabulated and considered by the clinical consensus group.
Where there were ambiguities or potential misclassification, the categories were refined using different mapping algorithms or other fields within the HES data, resulting in significant modifications of the mapping algorithms. In particular, venous ulceration was found to be largely indistinguishable from mixed arterial and venous disease or ulceration due to arterial disease, so these were combined in a single category of peripheral arterial and complex venous disease. There was also found to be a significant vascular workload outside these categories, which varied between different centres based on local practices. An additional category of ‘other vascular procedures’ was introduced to include miscellaneous upper limb, visceral vessel, arterial venous malformations and vascular access procedures.
All cases were assigned to specific case-mix groups that fell within one of five final categories: AAA, PAD, CAD, VV and ‘other’ vascular procedures. Where there were remaining ambiguities or contradictions within the data set, specific algorithms were developed for the categorisation of cases that may lead to confusion, such as where multiple conflicting codes were present or non-specific codes failed to distinguish between activities that may be relevant to vascular or other services. Decision rules were developed by the clinical consensus group for categorising such cases to relevant groups.
Examples of specific situations that required additional information for categorisation were (1) the categorisation of emergency, ruptured and elective aortic aneurysm repair; (2) the identification of amputations due to vascular disease as opposed to other causes, such as cancer or trauma; and (3) the distinction between endovascular treatments for cardiac and non-cardiac arterial disease. In addition to the categorisation based on procedural and diagnostic codes, it was necessary to develop a hierarchy of procedures to allocate cases where multiple codes relating to the same admission may result in conflicting categorisation (see Report Supplementary Material 2).
Episodes and admissions
The HES records relate to individual episodes of treatment, but a stay in hospital may generate multiple records relating to simultaneous, overlapping or sequential episodes. To provide clinically relevant categorisation and accurate estimates of length of stay, resource use and procedure-related events, all episodes were combined into continuous inpatient spells (CISs). These included all relevant information from first admission to last discharge from hospital. A ‘key’ episode was identified as the first episode that included the procedure determining the main categorisation of the hospital admission. This was merged with further information from other episodes related to that same admission, including potential indicators of comorbidity; outcome and resource use, such as secondary procedures; associated critical care episodes; and diagnostic information relating to comorbidities or complications.
Having identified relevant admissions, one or more index admission was identified for each patient using algorithms, as described in Appendix 1. For example, for AAA repair the index admission was the first one in which an aneurysm repair procedure was recorded, whereas for PAD a more complex algorithm was required. This was necessary, for example, to distinguish between amputations that were carried out as a primary procedure and amputations that might be considered an adverse outcome of a prior vascular reconstruction (see Modelling the effects of service reconfiguration).
Identification of treatment sites
To consider the effects of reconfiguration it was necessary to identify the sites at which procedures were carried out. This was found to be a complex exercise owing to the ambiguities and contradictions between the use of provider and the use of site codes within the HES record, the changes in coding, inconsistent use of particular codes and organisational mergers or other changes in providers.
Further mapping was carried out to categorise all pairs of provider and site codes based on the geographical location at which treatment took place through the identification of individual postcodes. In addition, sites and place of residence [based on lower-layer super output area (LSOA) data] were mapped to aneurysm screening areas for some of the geographical analysis (see Report Supplementary Material 3).
Identification of outcome measures
There are several potential outcome measures that can be identified from within the HES data and different outcome measures are relevant to specific case-mix categories. This section deals briefly with some of the issues in identifying outcome measures from the routine data set.
Mortality
For some high-risk procedures within vascular surgery, mortality is an important outcome measure but raises a number of issues regarding the different potential ways to measure this and the ability to correct for differences in case-mix.
The HES records include a field for discharge method, which identifies episodes that ended with the death of the patient. A single hospital admission or spell of treatment may include multiple episodes, and this is particularly true if a patient suffers a complication and is transferred to another specialty or hospital. The use of a crude assessment of mortality based on discharge method of individual episodes is likely to underestimate overall mortality. For this analysis, episodes were combined into CISs and in-hospital death was identified if any of the merged episodes included a discharge method indicating that the patient had died. An alternative for describing procedure-related mortality is to include deaths within 30 days of the procedure. Some ambiguity may be created in defining 30-day mortality where there are multiple procedures or no operative procedure during an admission. In these cases, the 30-day mortality was defined from the date of admission for unoperated cases and from the date of the first index procedure where there were multiple procedures. A patient admitted as an emergency may undergo investigative procedures followed by one or more major vascular reconstructions; in such a case, the index date would be taken as the date of the first major procedure.
Both in-hospital death and 30-day mortality were potential outcome measures and may produce different results (see Abdominal aortic aneurysm).
Re-admissions
The linkage of data allowed identification of patient pathways and re-admissions to hospital. In particular, the NHS measure of re-admission to hospital within 30 days of discharge was identified for all case-mix groups. In some cases, a further categorisation was required, as, for example, it is not uncommon for a patient to be admitted for investigation and then undergo a further subsequent planned admission for further treatment. The nature and type of re-admission were, therefore, considered on a case-by-case basis for the different categories of admission. Re-admissions for repeat procedures were identified within the full data set, and specific groups were identified that were relevant to particular diagnostic categories. Examples include re-admissions for repeat of the same procedure, further operative or endovascular procedures following treatment of peripheral arterial disease, or admissions with a stroke diagnosis following CEA. These categories are dealt with under the discussion of the results for the individual conditions.
Complications and comorbidities
Although HES records provide a rich source of diagnostic and procedural information, it can be difficult to distinguish conditions that may be pre-existing comorbidities from those that occur as complications of a treatment. A detailed piece of work was carried out to consider complications and comorbidities for different conditions based on existing published categorisation. 169 This was modified in the light of advice from the clinical consensus group and making use of evidence from linked episodes prior to the index admission to attempt to separate complications from comorbidities (see Report Supplementary Material 4).
The relevance of particular comorbidities or complications varies between the different diagnostic categories and is described in more detail below. For example, in the case of the treatment of CAD, although cerebrovascular accidents (CVAs) are an important complication of the treatment, they may also be a pre-existing condition and indication for the treatment. In the latter case, they are likely to indicate a higher-risk group of patients. By identifying those patients in whom a CVA was identified in a prior admission or the same admission as a cause for emergency admission, some separation of these groups is possible.
Cost analysis
A Healthcare Resource Group (HRG) code was assigned to each episode using the NHS Grouper algorithm and a year-specific reference cost data set was used to cost the episode based on its HRG code, financial year and other classification characteristics, such as mode of admission (elective, emergency or day case), treatment specialty, excess bed-days and other unbundled procedures. Descriptive statistics, univariate analyses and multiple regression models were used to investigate the costs and their variation. The R software (version 3.4.1) was used for all data manipulation and statistical analyses (see Modelling the effects of service reconfiguration and Report Supplementary Material 5).
Trends in case-mix, practice and outcome
Introduction
The following sections describe the overall trends in the activity carried out by vascular services over the past 12 years. Each of the following sections deals with the main trends that have been observed in the specific areas of AAA, PAD, CAD, VV and the miscellaneous ‘other’ category. For each of these categories, the individual case-mix groups are considered in terms of the demographics, the variations in practice, the introduction of new technologies and the information that is available regarding outcomes. Detailed regression analysis of the factors determining short- and long-term outcomes is provided in Appendix 6, and the relationships between hospital configuration, activity levels, practice and outcome are discussed in the next section.
Abdominal aortic aneurysm
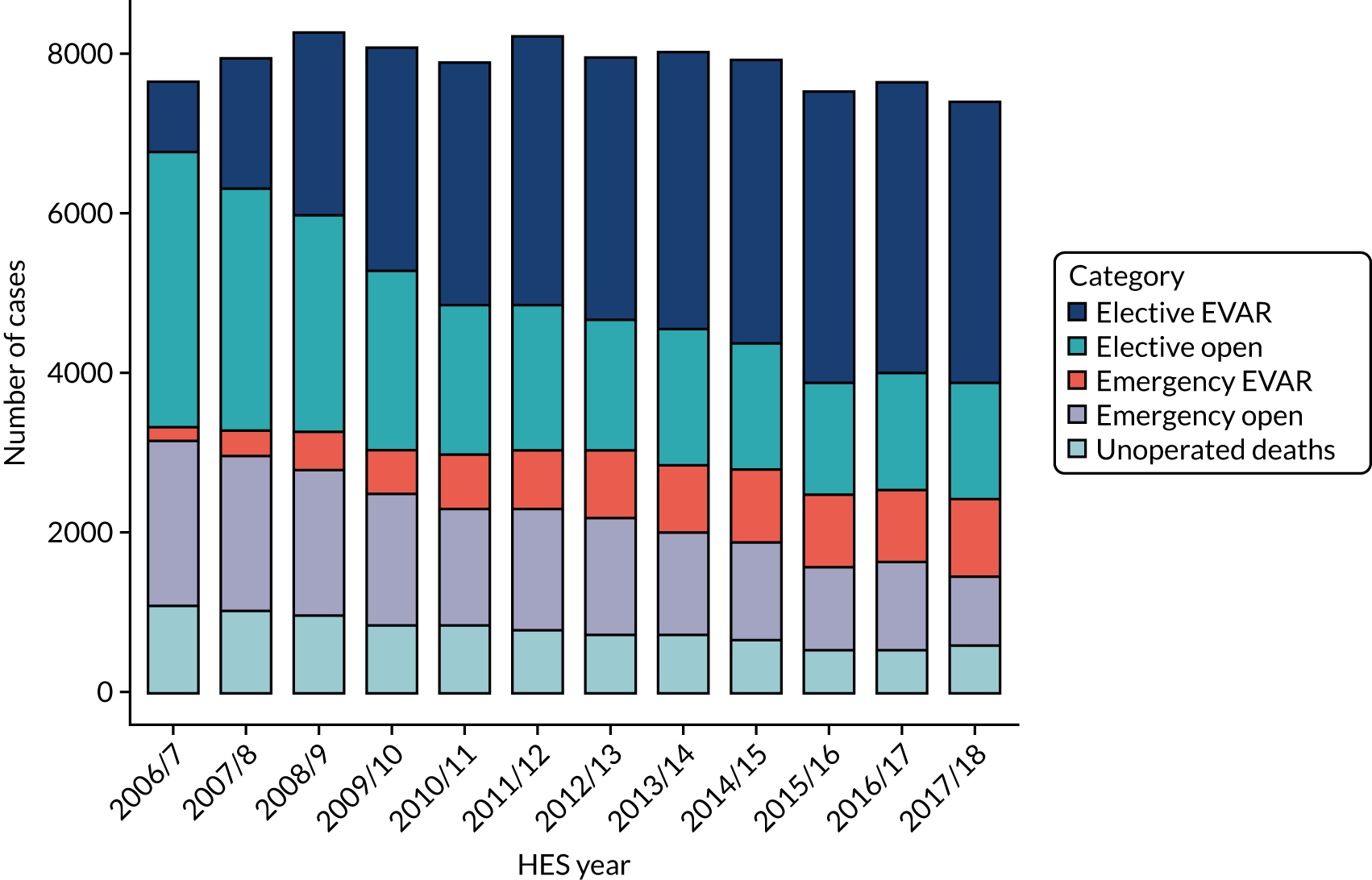
Over the past 12 years, there have been considerable changes in AAA management, with the introduction of a screening programme for men aged 65 years and the introduction of endovascular repair for elective and, increasingly, emergency aneurysm repair. The overall number of elective aneurysm repairs in England increased to a maximum of 4889 in 2011/12 and has been declining gradually since, although the proportion of endovascular repairs has risen to a steady level of approximately 70% for the most recent 3 years. Emergency procedures have declined steadily, from 3411 procedures in 2006/7 to 2302 in 2017/18, with the proportion of EVARs increasing steadily to 36.1% (Figure 2).
FIGURE 2.
Number of AAA admissions by year and category of admission.
There is considerable variation in practice with regard to the use of EVAR. The proportion of patients treated by EVAR varies between ≈ 30% and > 90% of elective cases, with similar variation for emergency admissions. Over 25% of centres do not appear to offer any emergency EVAR service (Figure 3).
FIGURE 3.
Proportion of elective AAA admissions treated by EVAR by year and volume of activity at treating centre (box plot showing median, interquartile range and 10th and 90th centiles).
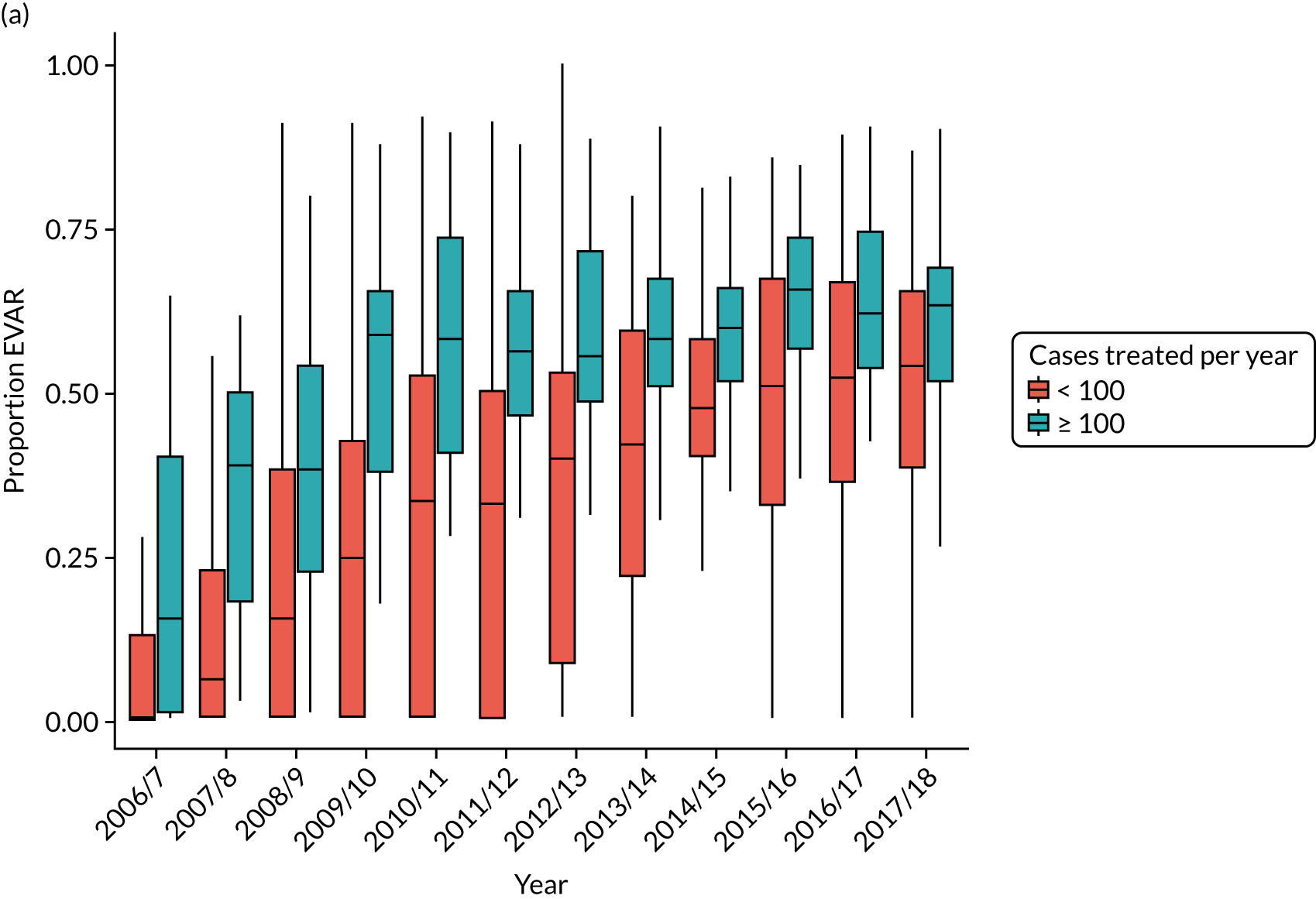
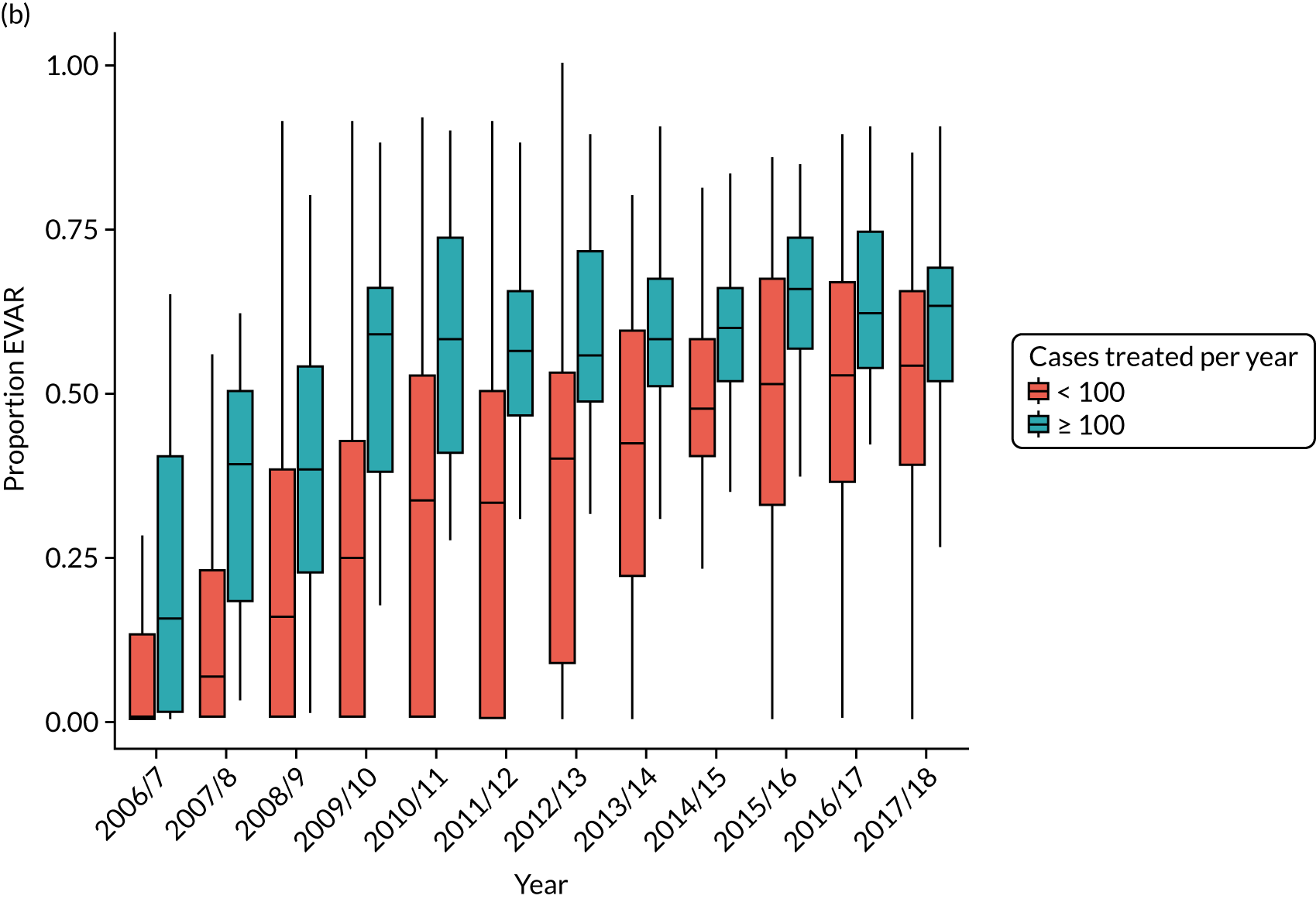
An increasing proportion of aneurysms treated by EVAR are classified as complex, being coded as juxtarenal or suprarenal aneurysms, increasing from 8.1% in 2011/12 to 18.1% in 2017/18 (see Discussion for a comparison with the estimates in the NVR report).
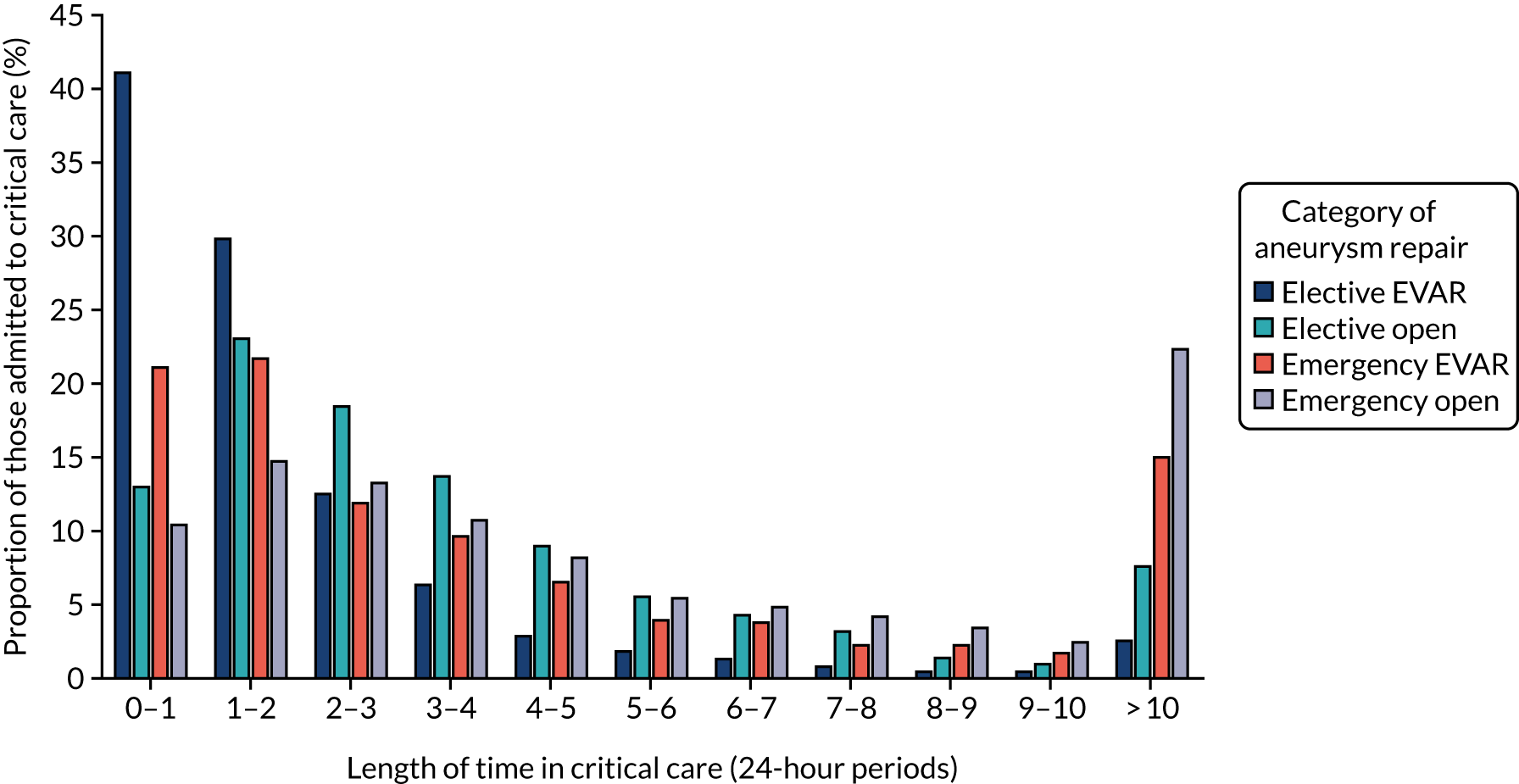
Table 3 provides summary data regarding the number and demographics, length of stay, critical care usage, re-admission rates and mortality for each of the categories of aneurysm repair, averaged over the most recent 3 years, and Figure 4 provides further details of the critical care usage for those emergency and elective AAA admissions that necessitated a critical care stay.
| Demographics | Elective EVAR | Elective open | Emergency EVAR | Emergency open | Unoperated |
|---|---|---|---|---|---|
| Number | 10,670 | 4307 | 2811 | 2989 | 1763 |
| Age (years), median (IQR) | 76.0 (70.0–81.0) | 70.0 (65.0–75.0) | 76.0 (70.0–82.0) | 75.0 (68.0–79.0) | 85.0 (79.0–89.0) |
| Age (years), mean (SD) | 75.1 (7.7) | 69.4 (9.0) | 74.0 (12.1) | 73.0 (9.9) | 83.6 (8.1) |
| Proportion male (%) | 86.8 | 87.0 | 79.7 | 82.1 | 63.1 |
| Proportion diabetic (%) | 18.2 | 13.2 | 15.1 | 11.8 | 14.3 |
| Proportion elective (%) | 100.0 | 100.0 | 0.0 | 0.0 | 1.1 |
| LOS (days), median (IQR) | 3.0 (2.0–5.0) | 8.0 (6.0–11.0) | 10.0 (5.0–19.0) | 12.0 (6.0–23.0) | 1.0 (0.0–3.0) |
| LOS (days), mean (SD) | 5.1 (10.9) | 11.6 (17.3) | 17.9 (24.9) | 20.2 (27.7) | 3.4 (8.1) |
| Wait (days), median (IQR) | 34.0 (16.0–62.0) | 29.0 (14.0–52.0) | |||
| Wait (days), mean (SD) | 48.3 (55.5) | 40.8 (41.3) | |||
| Number admitted to critical care, n (%) | 4805 (45.0) | 3828 (88.9) | 1905 (67.8) | 2563 (85.7) | 93 (5.3) |
| Hours in critical care, median (IQR) | 27.3 (22.0–52.3) | 70.1 (42.8–118.3) | 66.0 (27.8–145.0) | 98.0 (48.0–216.0) | 32.7 (11.4–88.9) |
| Hours in critical care, mean (SD) | 57.8 (147.8) | 115.8 (232.2) | 140.0 (229.7) | 195.3 (297.7) | 134.9 (389.7) |
| In-hospital death rate (%) | 1.4 | 5.1 | 13.2 | 31.9 | 100.0 |
| 30-day mortality (%) | 1.4 | 4.5 | 12.2 | 29.8 | 98.5 |
| Combined post-operative mortality (%) | 1.7 | 5.2 | 14.1 | 32.2 | 100.0 |
| Second procedure (%) | 0.3 | 0.6 | 1.7 | 1.1 | 0.0 |
| Re-admission rate of survivors within 30 days of discharge (%) | 15.1 | 11.6 | 21.8 | 17.4 |
FIGURE 4.
Length of critical stay for those emergency and elective admissions for AAA repair who were admitted to critical care.
Overall, mortality from elective AAA treatment has declined steadily, from 5.6% in 2006/7 to 2.7% in 2017/18, while mortality from emergency treatment fell from 32.2% to 22.3% over the same period. This was partly because of an increase in endovascular treatment, although there has been a reduction in mortality in all treatment categories (Table 4). The interpretation of mortality for emergency repair is confounded by the difficulty in distinguishing between emergency and ruptured cases. In addtion, the number of patients who die after being admitted with a ruptured aneurysm, but before undergoing repair, may not be included in the figures. When considering these issues, both the mortality and the turn-down rate appear to be higher for women than for men. 11
| Year | Elective admissions (%) | Emergency admissions (%) | ||||
|---|---|---|---|---|---|---|
| EVAR | Open | Overall | Ruptured | Non-ruptured | All (including unoperated) | |
| 2006/7 | 2.4 | 6.0 | 5.6 | 41.9 | 8.6 | 54.0 |
| 2007/8 | 2.9 | 6.2 | 5.4 | 39.3 | 8.0 | 51.2 |
| 2008/9 | 2.1 | 6.8 | 5.1 | 39.4 | 9.0 | 49.9 |
| 2009/10 | 2.3 | 6.1 | 4.2 | 36.7 | 9.3 | 48.2 |
| 2010/11 | 1.5 | 5.7 | 3.5 | 36.4 | 8.7 | 47.0 |
| 2011/12 | 1.8 | 6.0 | 3.6 | 37.3 | 8.3 | 45.6 |
| 2012/13 | 1.4 | 5.3 | 3.1 | 35.4 | 8.5 | 42.8 |
| 2013/14 | 1.6 | 4.8 | 3.1 | 36.2 | 9.5 | 43.2 |
| 2014/15 | 1.6 | 4.6 | 2.9 | 38.4 | 10.9 | 42.5 |
| 2015/16 | 1.2 | 5.7 | 2.7 | 38.2 | 9.4 | 40.2 |
| 2016/17 | 1.3 | 4.3 | 2.5 | 37.4 | 13.1 | 40.9 |
| 2017/18 | 1.4 | 4.4 | 2.6 | 34.9 | 9.9 | 40.1 |
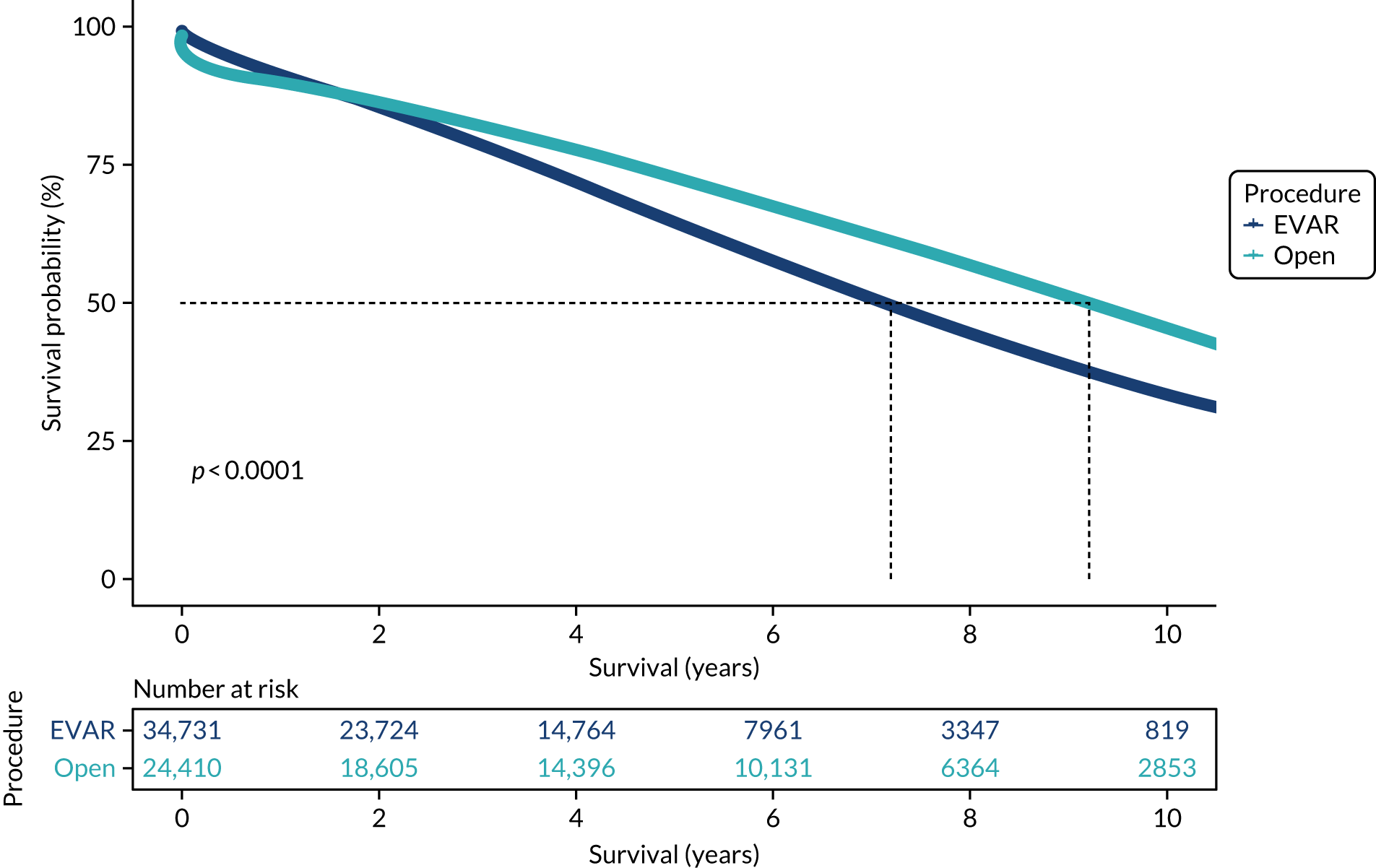
Based on linked data with repeat admission and ONS mortality data, Kaplan–Meier analysis of long-term survival was carried out for some of the key diagnostic groups. Kaplan–Meier plots for overall survival (OS) following AAA repair are provided in Figure 5, and show that OR is associated with higher initial mortality but better long-term survival. These differences persist after correction for case-mix differences (see Appendix 6 and Report Supplementary Material 6).
FIGURE 5.
Kaplan–Meier survival estimates for elective infrarenal EVAR and open AAA repair.
Peripheral arterial disease
The total number of vascular reconstructions (including all surgical and endovascular procedures) in England increased from about 25,000 in 2006/7 to a maximum of nearly 33,000 in 2013/14, decreasing to 29,000 in 2017/18. Over the same period, there was a gradual increase in the proportion that were emergency admissions, from 27.7% to 31.1%. For elective admissions, the proportion that underwent endovascular treatments remained constant at around 70%, whereas the proportion for emergency admissions increased from 41.6% to 51.7%.
The number of major amputations fell from a peak of 5851 in 2008/9 to 5522 in 2017/18, with approximately two-thirds being admitted as an emergency. Over the same period, the number of minor amputations increased from 5410 to 8280.
All procedures for PAD are more common in men but the proportion varies by category, being highest, at > 75%, for distal bypasses but just over 50% for elective minor amputations. Most patients with PAD are aged > 60 years, with those admitted as an emergency, women and those with more distal disease tending to be older.
Table 5 provides summary data regarding the demographics, length of stay, critical care usage and re-admission rates and mortality for each of the categories of PAD admission, averaged over the most recent 3 years (see Report Supplementary Material 7).
| Demographics | Investigations only | Ulcer (no procedure) | Major amputation | Minor amputation | Angioplasty (mild/moderate) | Angioplasty (severe) | Reconstruction (mild/moderate) | Reconstruction (severe) |
|---|---|---|---|---|---|---|---|---|
| Number | 78,976 | 29,211 | 16,461 | 24,035 | 37,552 | 20,217 | 10,482 | 20,202 |
| Age (years), median (IQR) | 67.0 (52.0–77.0) | 71.0 (59.0–80.0) | 66.0 (54.0–76.0) | 67.0 (56.0–77.0) | 69.0 (61.0–76.0) | 74.0 (64.0–82.0) | 69.0 (62.0–76.0) | 69.0 (58.0–77.0) |
| Age (years), mean (SD) | 63.6 (17.8) | 68.6 (15.1) | 63.8 (15.9) | 66.1 (14.5) | 67.9 (11.6) | 71.7 (13.3) | 68.4 (10.7) | 66.6 (14.9) |
| Proportion male (%) | 53.2 | 62.0 | 71.0 | 68.0 | 66.8 | 60.1 | 76.3 | 67.0 |
| Proportion diabetic (%) | 21.5 | 44.8 | 46.2 | 64.4 | 30.9 | 49.5 | 28.3 | 27.6 |
| Proportion elective (%) | 42.7 | 38.9 | 34.7 | 50.2 | 100.0 | 32.5 | 100.0 | 36.9 |
| LOS (days), median (IQR) | 0.0 (0.0–7.0) | 3.0 (0.0–12.0) | 23.0 (9.0–48.0) | 5.0 (0.0–15.0) | 0.0 (0.0–1.0) | 7.0 (1.0–17.0) | 4.0 (2.0–6.0) | 10.0 (5.0–20.0) |
| LOS (days), mean (SD) | 8.4 (27.9) | 11.3 (22.8) | 35.2 (50.7) | 13.2 (38.9) | 1.4 (26.4) | 15.0 (26.5) | 6.2 (9.7) | 17.7 (24.3) |
| Wait (days), median (IQR) | 29.0 (14.0–49.0) | 41.0 (15.0–84.0) | 23.0 (7.0–60.0) | 22.5 (8.0–55.0) | 30.0 (15.0–55.0) | 22.0 (12.0–40.0) | 34.0 (14.0–64.0) | 26.0 (11.0–55.0) |
| Wait (days), mean (SD) | 40.7 (74.3) | 61.5 (90.5) | 46.2 (66.2) | 41.8 (56.3) | 44.1 (73.7) | 31.5 (38.9) | 48.0 (52.9) | 41.8 (47.5) |
| Number admitted to critical care, n (%) | 12,077 (15.3) | 1356 (4.6) | 3177 (19.3) | 623 (2.6) | 548 (1.5) | 1649 (8.2) | 1772 (16.9) | 7336 (36.3) |
| Hours in critical care, median (IQR) | 74.0 (38.0–165.2) | 74.5 (32.9–159.3) | 69.9 (25.3–166.3) | 87.8 (33.7–206.1) | 38.8 (22.1–74.6) | 71.6 (32.5–156.9) | 29.3 (21.0–65.3) | 52.4 (24.0–114.8) |
| Hours in critical care, mean (SD) | 160.6 (314.3) | 158.6 (378.4) | 162.2 (311.2) | 197.8 (394.3) | 74.2 (133.2) | 151.8 (251.6) | 61.0 (127.3) | 115.8 (232.8) |
| In-hospital death rate (%) | 4.9 | 5.2 | 8.3 | 2.2 | 0.2 | 4.4 | 0.8 | 6.4 |
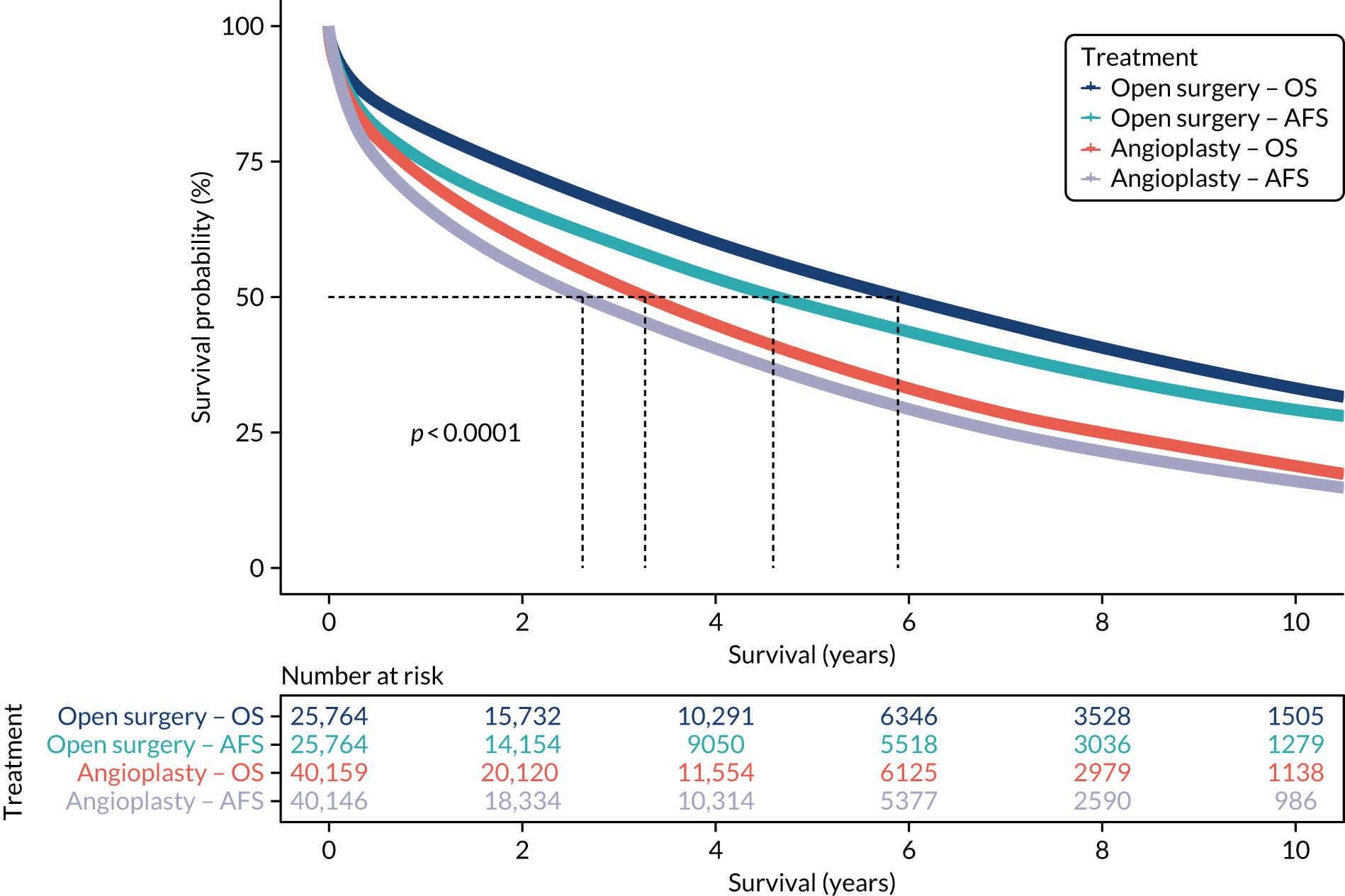
Mortality is high among patients with severe limb ischaemia (defined as emergency procedures, distal bypasses, those with a current or preceding minor or major amputation or with a diagnosis of ulcer). The median OS following angioplasty is just under 3 years and just over 6 years for open procedures, with similar differences for amputation-free survival (AFS) (Figure 6).
FIGURE 6.
Kaplan–Meier estimates for OS and AFS following open surgery and angioplasty for severe limb ischaemia. OS, overall survival.
Leg ulcers
Although the original intention was to consider those with VLUs as a separate group, it proved difficult to reliably distinguish those with an arterial and venous diagnosis. This group, therefore, includes all of those admitted with a diagnosis of leg ulcer, but no other vascular procedure including some arterial, diabetic and venous diagnostic groups (see Appendix 1).
The total number of such admissions has risen steadily, from 5184 to 7659, with about 75% being emergency admissions; the median age of such patients is 73 years, and 60% are men. Approximately 70% of the episodes relate to ulcers (with no recorded procedures) and have diagnostic codes relating to diabetes and/or arterial disease, 10% have a varicose vein diagnosis, and the remainder have no specific code to identify the cause of the ulcers.
Carotid artery disease
The number of CAD procedures increased to a maximum of 6299 in 2011/12, decreasing to 4711 in 2017/18, with the proportion of endovascular procedures increasing gradually to 10.4% in 2017/18 and the proportion of patients who were emergency admissions increasing from 14% in 2006/7, to ≈ 35%, where it has remained over the past 5 years. Approximately 66% of those patients undergoing surgery are men with an average age of 71 years; those undergoing endovascular treatment were slightly younger and including a higher proportion of women (Table 6 and Report Supplementary Material 8).
| Demographics | Endovascular | Endarterectomy |
|---|---|---|
| Number | 880 | 12,819 |
| Age (years), median (IQR) | 66.0 (56.0–74.0) | 72.0 (65.0–79.0) |
| Age (years), mean (SD) | 63.9 (13.6) | 70.9 (10.8) |
| Proportion male (%) | 59.8 | 66.4 |
| Proportion diabetic (%) | 21.0 | 25.0 |
| Proportion elective (%) | 59.0 | 66.4 |
| LOS (days), median (IQR) | 3.0 (1.0–14.0) | 3.0 (2.0–6.0) |
| LOS (days), mean (SD) | 16.5 (39.6) | 7.5 (17.6) |
| Wait (days), median (IQR) | 13.0 (5.5–34.0) | 7.0 (4.0–15.0) |
| Wait (days), mean (SD) | 26.5 (37.0) | 16.7 (41.9) |
| Number admitted to critical care, n (%) | 286 (32.5) | 5288 (41.3) |
| Hours in critical care, median (IQR) | 47.7 (22.9–137.3) | 24.0 (20.1–45.5) |
| Hours in critical care, mean (SD) | 156.1 (257.6) | 43.8 (86.4) |
| In-hospital death rate (%) | 5.9 | 1.1 |
| 30-day mortality (%) | 5.2 | 1.2 |
| Combined post-operative mortality (%) | 6.4 | 1.5 |
| Second procedure (%) | 1.5 | 0.2 |
| Re-admission within 30 days (%) | 12.5 | 10.9 |
| Proportion with prior stroke (%) | 9.2 | 17.0 |
The median survival, free of re-admission with stroke, following a CAD procedure is 8.1 years for those patients without a prior stroke and 6.2 years for those with a prior stroke (Figure 7).
FIGURE 7.
Kaplan–Meier survival estimates free of stroke re-admission following carotid treatment for those with and without a prior stroke diagnosis.
Varicose veins
The total number of admissions for treatment of VV fell substantially between 2009/10 and 2012/13, from nearly 40,000 to a low of just over 25,000 cases per year. The number of open procedures (high tie, stripping, etc.) has continued to fall and been largely replaced by the new modalities of endovenous laser treatment (EVLT) and radiofrequency ablation (RFA), with the total number of VV procedures rising again in recent years.
The demographics of the treated population has altered over the past 12 years, with an increasing proportion of men and an older population. Those treated by avulsions tend to be younger and include a higher proportion of women (Table 7).
| Demographics | RFA | EVLT | High tie, etc. | Sclerotherapy | Avulsions |
|---|---|---|---|---|---|
| Number | 35,495 | 16,831 | 16,495 | 16,947 | 5398 |
| Age (years), median (IQR) | 54.0 (42.0–67.0) | 53.0 (42.0–66.0) | 51.0 (40.0–63.0) | 57.0 (45.0–69.0) | 49.0 (39.0–60.0) |
| Age (years), mean (SD) | 54.3 (15.5) | 53.8 (15.7) | 51.6 (14.7) | 56.6 (15.5) | 50.1 (14.3) |
| Proportion male (%) | 44.4 | 43.6 | 46.0 | 37.6 | 29.3 |
| LOS (days), median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| LOS (days), mean (SD) | 0.1 (6.7) | 0.081 (3.3) | 0.79 (6.7) | 0.15 (12.9) | 0.078 (0.82) |
| Wait (days), median (IQR) | 74.0 (42.0–121.0) | 72.0 (42.0–112.0) | 69.0 (37.0–116.0) | 74.0 (41.0–120.0) | 71.0 (38.0–116.0) |
| Wait (days), mean (SD) | 90.2 (67.5) | 87.1 (66.0) | 86.9 (82.6) | 96.6 (91.3) | 88.8 (108.6) |
| Re-admission within 30 days (%) | 4.4 | 4.0 | 5.7 | 4.9 | 3.8 |
The different treatment modalities resulted in differences in re-admission rates for further VV interventions. Within 5 years of the first recorded treatment, 40% of those treated by sclerotherapy had been re-admitted, compared with ≈ 15% of those treated by open surgery, 20% treated with RFA and 27% treated with EVLT (Figure 8 and see Report Supplementary Material 9).
FIGURE 8.
Re-admission rates for VV treatments by modality of original treatment.
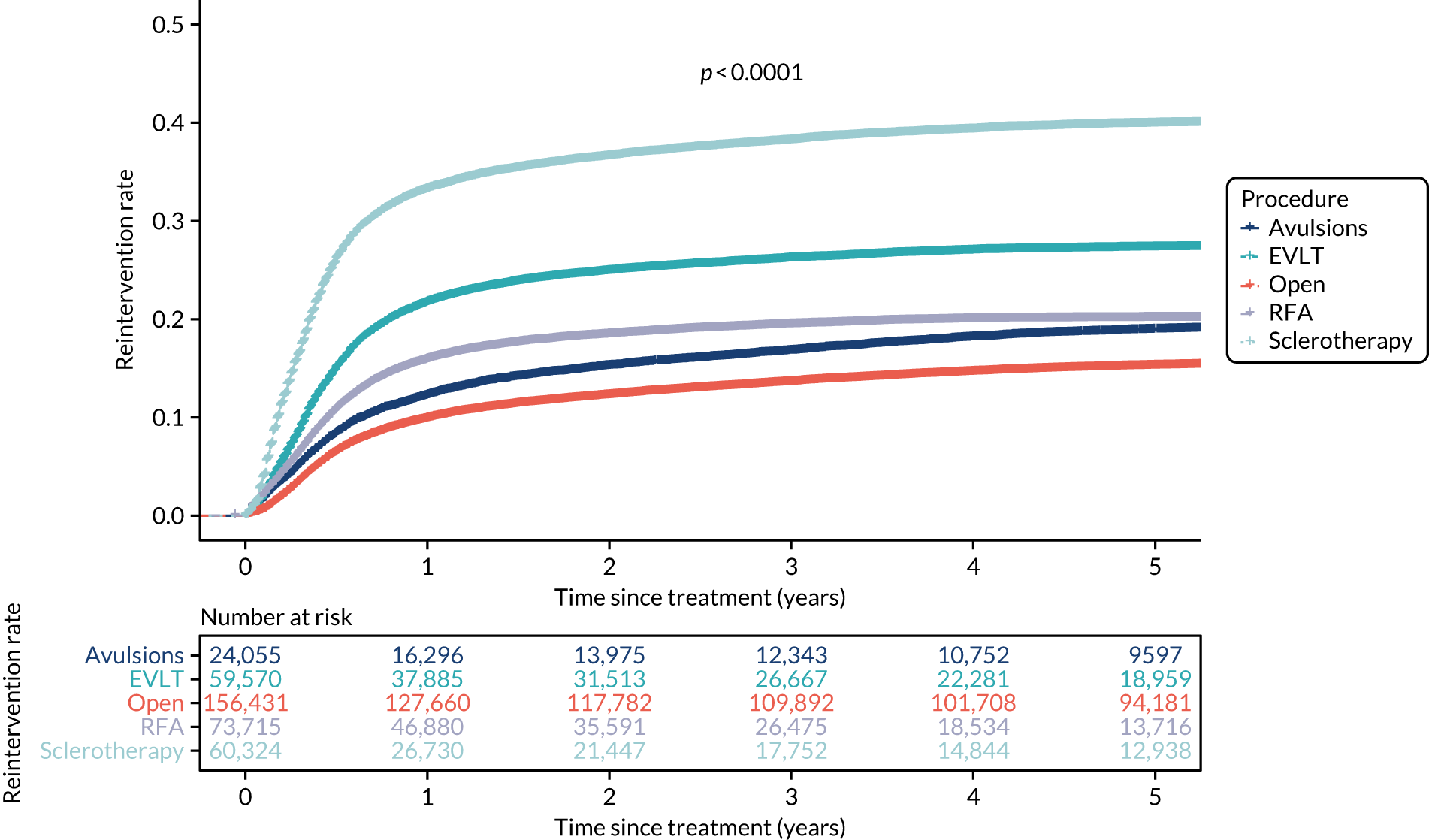
Other diagnostic groups
A number of other diagnostic groups were identified that represent less common or more specialist procedures that may, in some circumstances, be included in the vascular services workload. Less common procedures include approximately 750 upper limb procedures per year, 250 visceral vessel procedures and 300 procedures for arteriovenous malformations. An increasing proportion of these are endovascular. Vascular services may also be involved in vascular access work for people with renal failure or other diagnoses. This may represent a large workload and local arrangements vary, with an increasing proportion involving endovascular procedures (see Report Supplementary Material 10).
Discussion
Hospital Episode Statistics provides a rich source of data regarding hospital activity but the data are complex and can be difficult to interpret. There are a number of limitations in the coding systems and other pitfalls in interpreting the data that are particularly relevant to understanding the nature of vascular services. Specific issues that are important are the lack of detailed diagnostic codes for distinguishing between important subgroups of vascular patients, such as those with critical ischaemia and intermittent claudication. There are also difficulties in distinguishing between procedures or diagnoses that may be either complications of treatment or comorbidities, for example stroke can be both a complication of and an indication for carotid surgery and amputation can be a primary event or the result of a complication of failed treatment. These problems are compounded by the lack of adequate coding for laterality, which makes it impossible to distinguish between two procedures on the same leg or separate procedures on both legs.
Another difficulty in interpretation arises from the nature of the data set, which is based on episodes of treatment rather than admissions or patients; therefore, complex procedures of data linkage and identification of overlapping and repeated episodes are necessary to understand pathways of care. Where differences are identified, these may relate to real variations in clinical treatment or inconsistencies in coding practice.
Although these limitations are recognised, HES provides a very rich source of data and is the only complete database that will allow linkage of episodes throughout a patient pathway, which can also be linked to data regarding mortality.
The detailed analysis of HES data that has been carried out for this programme of research has addressed a number of the potential issues within the data through the methods developed for cleaning and linking data and modifying algorithms for identifying case-mix, comorbidities and complications, which take into account previous diagnoses and re-admissions. Although it is not possible to address all the inconsistencies in coding, some attempt can be made to produce consistent figures by identifying clinical groupings from within the data that are a proxy for certain subgroups, such as those with severe or moderate limb ischaemia and those who are admitted with a ruptured aortic aneurysm and die without vascular intervention.
Taken as a whole, the analysis of HES data demonstrates a number of trends. Overall outcomes, particularly mortality related to AAA, appear to be improving over time. Although the reduction in mortality is partly because of the move towards EVAR, there has also been mortality improvement in each of the subgroups of treatment. Overall, there is a trend towards a reducing number of AAA repairs, whereas for other diagnostic groups the overall levels appear to be relatively constant, apart from a shift towards endovascular treatment, with an older population having greater comorbidity. However, it is difficult to know whether the trend towards higher comorbidity is real or the result of an increasing depth of coding in recent years, possibly related to the introduction of HRG groupings that provide higher tariff rates for patients with additional comorbidities.
In the treatment of VV, there was a considerable reduction in the number of procedures carried out around 2011/12, but this has now gradually returned to the original rate.
There is considerable variation in practice, which is particularly notable with the adoption of new technologies. In the case of aneurysm repair, the proportion of patients treated by EVAR varies between providers from 30% to > 90%. There is also considerable variation in the proportion of AAA cases classified as complex in the use of endovascular treatments for PAD and of endovenous treatments (EVLT and RFA) for VV.
There are also marked variations in practice with regard to the use of inpatient or outpatient investigations, with some providers appearing to carry out large numbers of investigations, including duplex ultrasound as day-case admissions, whereas others appear to carry out investigations as outpatient procedures. This has considerable implications for the difference in income that may be generated from treating similar conditions.
There are a number of potential areas for further research regarding HES data, which were not within the remit of this programme. Further work is needed to identify the extent to which differences in workload and cost represent differences in coding or reflect clinical practice. There may be a potential for better classification algorithms, possibly through the use of machine-learning techniques, and linkage of HES data to other sources with richer clinical information may allow better algorithms to be developed for classification of case-mix.
The methods of classification of HES data do, however, provide a useful basis for comparison between centres and over time. This is explored further in the modelling in Modelling the effects of service reconfiguration.
Changes in working arrangements
Methods
The original protocol described a survey of working arrangements for centres undertaking the management of vascular disease. During the planning stage of this aspect of the research it became evident that the Vascular Society was carrying out an organisational audit of NHS hospital vascular services that would duplicate much of the intended survey. The results of this organisational audit were published as part of The National Vascular Registry: 2015 Annual Report10 and the audit has subsequently been repeated with details of reported configuration of vascular services included in the 2018 annual reports. 3
In view of this, a decision was made not to carry out the survey, but to carry out further work to analyse working arrangements that were evident from the HES data regarding hospital activity and to compare these with the results of the published NVR audit.
The NVR audit is commissioned by the Healthcare Quality Improvement Partnership and carried out by the VSGBI. It collects data regarding a variety of index procedures carried out by vascular specialists in the UK. These include AAA repair, peripheral arterial surgery and endovascular treatments, amputations and procedures for the treatment of CAD. Annual reports are prepared by the Vascular Society, and the 2015 and 2018 reports included the results of organisation audits of NHS vascular services that provided information regarding service configuration, the range of facilities available at each site carrying out treatment and the resources and staffing available. As part of the programme of research and the validation exercise, the analysis of HES data has been compared with some of the details contained in the NVR audit reports. This section of the report looks at the trends in vascular activity and makes a comparison in three main areas: the organisational arrangements, the activity and the measures of outcome used.
The NVR audit was based on the criteria set out in the various versions of the provision of vascular services document produced by the VSGBI and the National Service Specification for vascular services published by NHS England. 170
A detailed analysis was carried out of the trends in vascular activity. Sites were identified following the processes described in Appendix 1. Vascular activity at these sites was summarised over the 12-year period from 2006/7 to 2017/18 and sites were classified on the basis of the mix of procedures available and the levels of activity (see Report Supplementary Materials 6–11).
Sites were initially classified based on the level of AAA surgery, and further analyses considered the number of CAD admissions for this group.
There are a number of differences between the data collected by NVR and the data collected by HES, as analysed in this programme, and it is important to take these into consideration when comparing results. The NVR includes data from all four countries of the UK, whereas the HES data are restricted to England. For this reason, we used only the subset of NVR data relating to trusts in England and the corresponding data from HES. Although the NVR collects more complete clinical data, it is, a procedure-based registry without data linkage and it is, thus, impossible to distinguish between multiple procedures on the same individual and procedures on separate individuals. The analysis of HES data enabled record linkage, and thus the identification of index procedures and re-admissions, which is important when calculating mortality. The data sets may also differ because of coding errors or ambiguities in the HSE data and because some cases may be missing from the NVR.
Another issue relates to the definition of sites in NVR. These are currently classified by the three-digit provider code and providers may offer services on multiple sites. This issue becomes important, particularly when considering ‘hub and spoke’ arrangements as discussed in Organisational arrangements.
Results
Organisational arrangements
The data included in the 2018 report from the organisational survey included responses from 83 out of 89 organisations contributing to the NVR, of which 70 out of 73 were from England. Of these, 49 were hub hospitals, 11 were spoke hospitals and 10 were not part of a network. Of the 49 hub hospitals, 47 provided details of the number of spokes that they operated, which gave a total number of 131 spoke hospitals. It is not clear from the report if this number includes 11 spoke hospitals contributing to the NVR or if these are additional sites operated by the same trust or separate trusts. There are also different potential definitions of spoke hospitals, depending on the range of services that are offered, which could include outpatient clinics, day-case services, investigations, angioplasty or some inpatient surgical procedures.
For comparison, HES data identified 69 sites as carrying out elective AAA repair in 2017 (excluding those with fewer than five cases recorded). There was one additional site in which there were > 40 peripheral arterial reconstructions, a further 25 sites with > 40 angioplasties and 28 sites at which there were > 40 vascular admissions for investigations per year.
Abdominal aortic aneurysm
The trend in the number of sites offering AAA surgery is shown in Figure 9. The overall number of sites offering aneurysm repair fell from 136 to 69 between 2006/7 and 2017/18. The majority of the reduction was due to the cessation of aortic aneurysm surgery at those sites carrying out < 40 cases per year.
FIGURE 9.
Number of sites undertaking aortic surgery by total number of AAA cases per year, based on HES data.
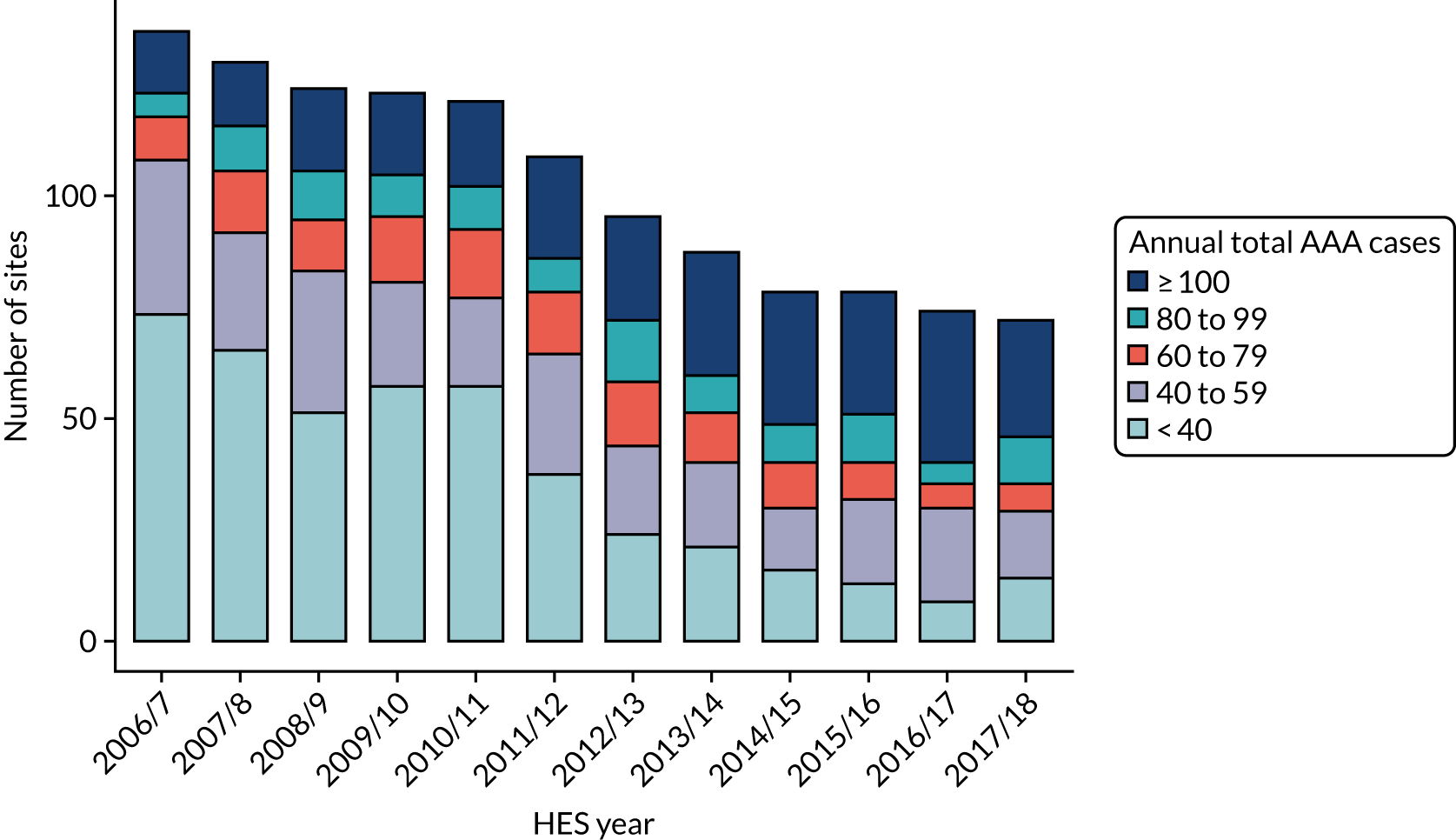
Considering the overall number of cases treated at sites with different volumes of activity, there was a reduction in the proportion of patients treated at sites with low volumes until approximately 2011. Since then, the figures have remained fairly stable, with approximately 20% of procedures carried out at sites that carry out < 60 cases per year (Figure 10).
FIGURE 10.
Proportion of AAA admissions by total number of AAA cases per year at treating site.
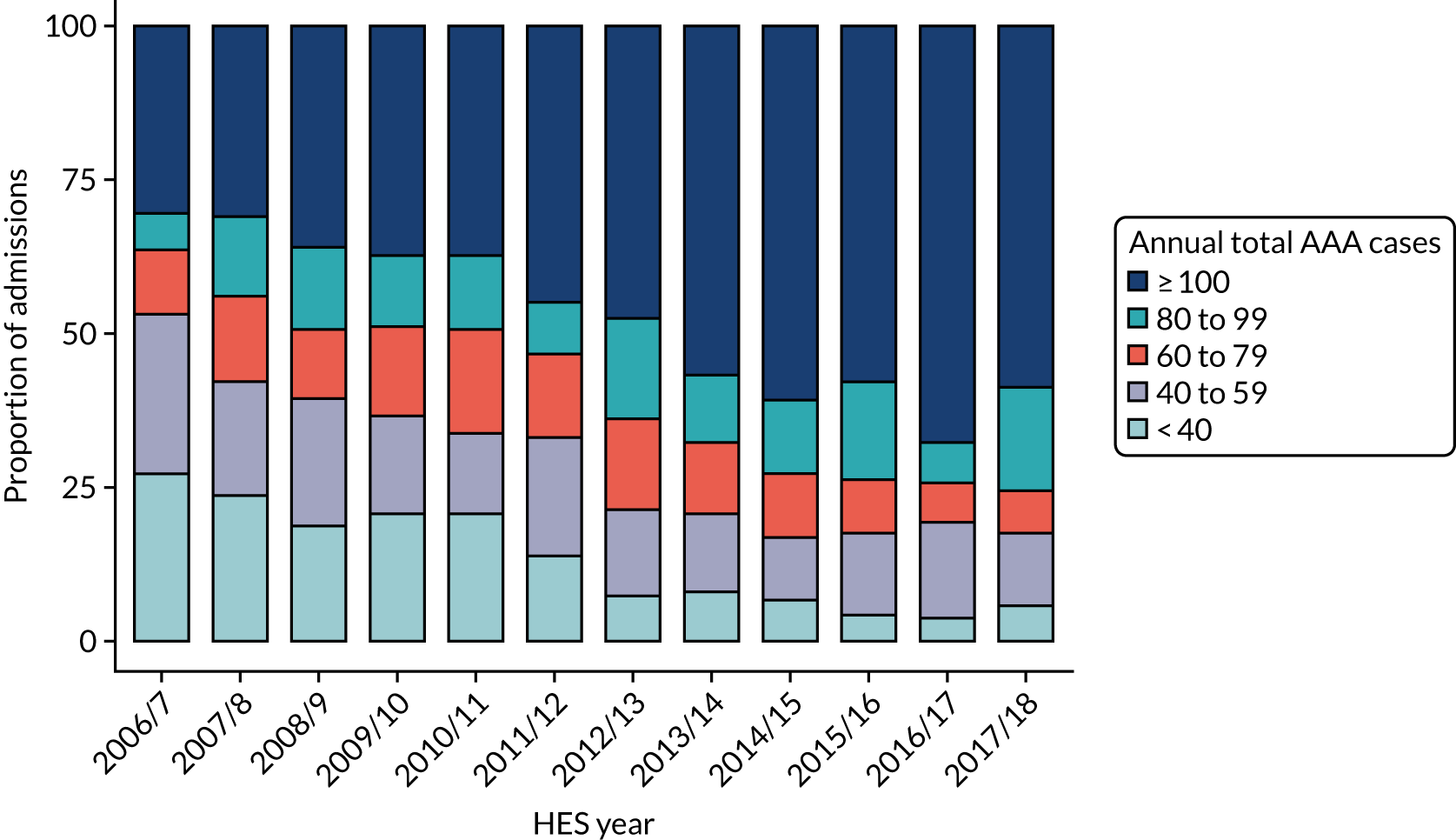
The NVR reported that the number of centres carrying out > 30 elective cases per year of infrarenal elective AAA repair had reduced to 11, compared with 58 centres carrying out more than this number. 3 For comparison, the HES data suggested that there were 12 centres in this category out of the same total of 69. This definition of activity category differs from that reported in the specification for the provision of vascular services,171 which suggests that the minimum number of cases for a designated aortic aneurysm centre should be 60 cases per year of all types of aneurysm repair, averaged over 3 years. By this criterion, the HES data suggest that 40 out of the 69 centres were compliant with current advice.
Peripheral arterial disease
Many admissions for PAD appear to have been centralised, along with the centralisation of aneurysm services, over the past 12 years (Figure 11). Those centres that have a sustained aneurysm practice undertook 96.9% of all surgical arterial reconstructions in 2017/18, compared with under 60% in 2006/7. Most other procedures have followed a similar trend, although about one-third of minor amputations are carried out at non-vascular centres.
FIGURE 11.
Trends in the proportion of categories of PAD admissions treated at sites with a current AAA practice.
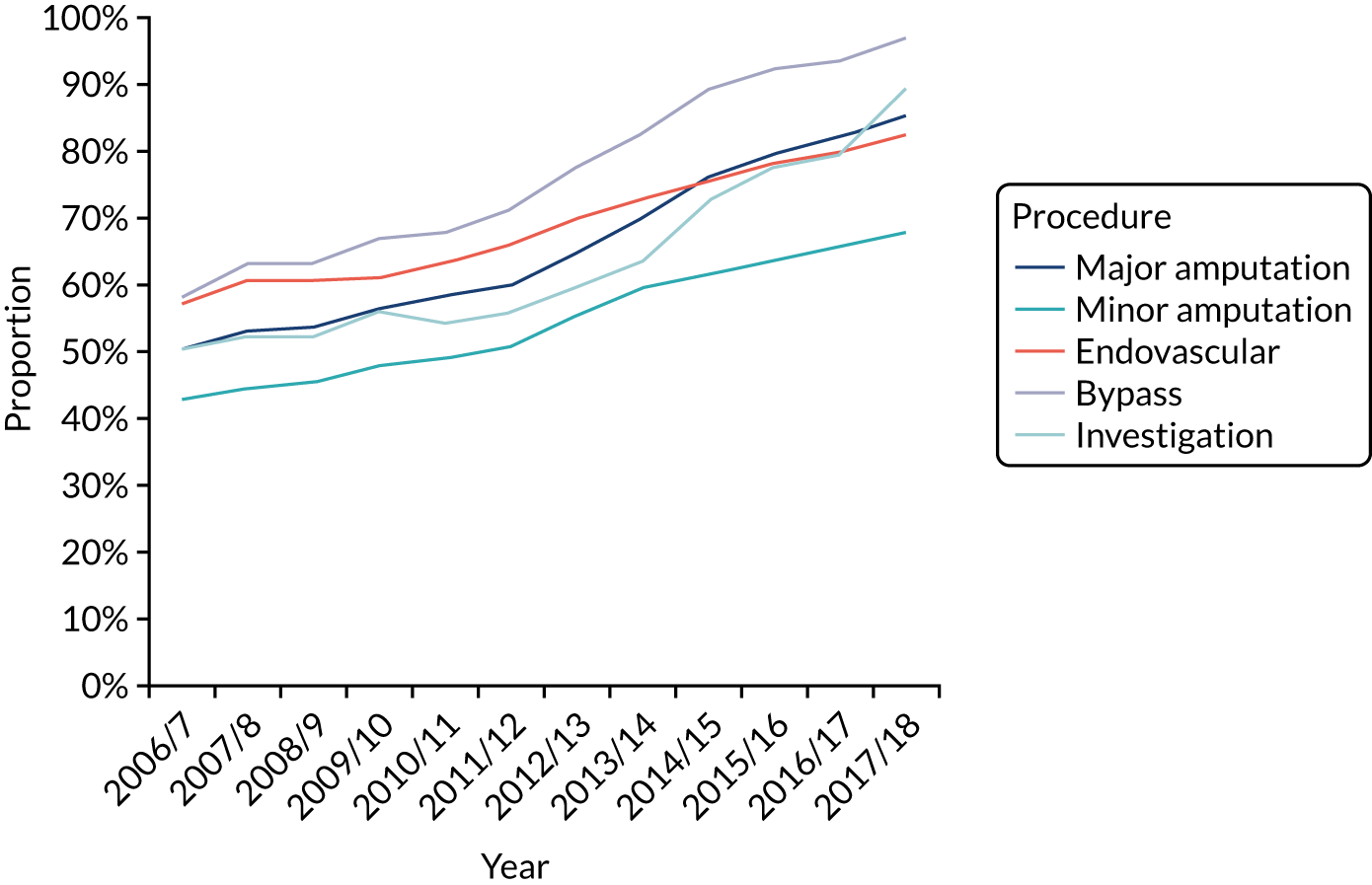
Carotid artery disease
The most recent NVR data show that 65 English trusts carry out carotid surgery, of which 23 have activity levels below the recommendation of 40 per year. In comparison, HES data analysis for the most recent year shows 74 sites, of which 26 are below the threshold. As expected, the AAA volume correlates with CAD volume, but there are a small number of sites carrying out CAD but not AAA procedures or vice versa (Figure 12).
FIGURE 12.
Number of CAD and AAA procedures for sites undertaking vascular procedures in 2017/18, compared with VSGBI recommendations. 171
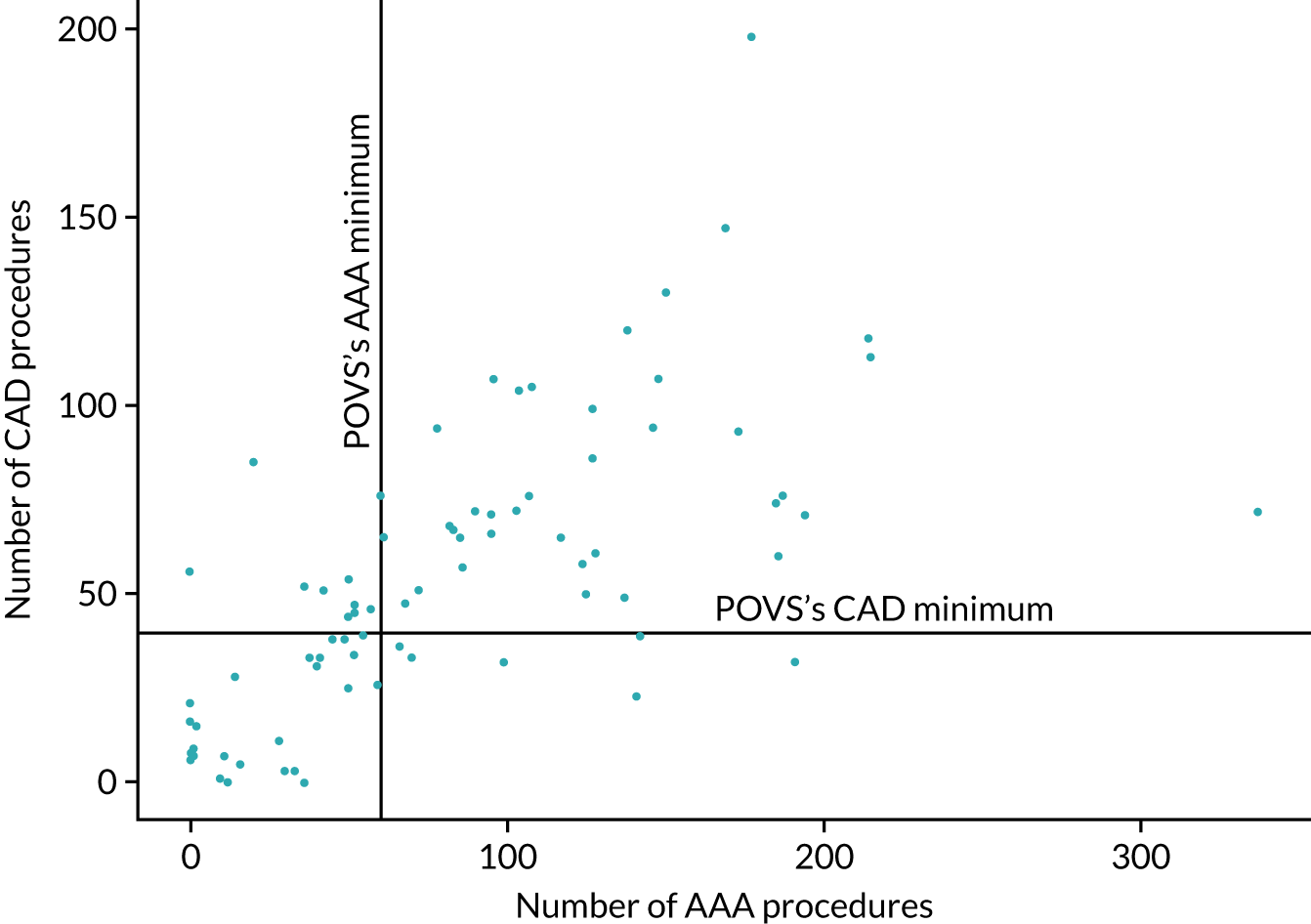
Approximately 16% of CAD procedures are carried out at sites with volumes below the recommended volume, and the postoperative mortality at these sites is significantly higher than at sites that reach the recommended volume (Table 8).
| CAD procedures | CAD annual volume | ||
|---|---|---|---|
| Under 40 | 40 and over | Overall | |
| Number of cases | 2174 | 11,525 | 13,699 |
| Mortality, % (95% CI) | 2.9 (2.2 to 3.6) | 1.6 (1.4 to 1.9) | 1.8 (1.6 to 2.1) |
Varicose veins
Although VV procedures are not included in the NVR reports, they constitute a significant workload for vascular clinicians and the reconfiguration of vascular services may affect the place or method of treatment. Between 2006/7 and 2012/13 there was a substantial reduction in the overall number of admissions related to VV procedures and an increase in the proportion treated by endovascular methods (EVLT and RFA) and at vascular centres (Figure 13). Table 9 provides details of the features of VV admissions based on the method of treatment and proportion treated at current vascular sites (those with a current AAA practice).
FIGURE 13.
Trends in the number of VV admissions treated by open and endovenous procedures at sites with and without a current AAA practice.
| Year | Non-vascular sites | Vascular sites | Proportion at vascular site (%) | ||||
|---|---|---|---|---|---|---|---|
| Number | EVLT (%) | RFA (%) | Number | EVLT (%) | RFA (%) | ||
| 2006/7 | 18,309 | 5.6 | 0.3 | 15,649 | 6.6 | 0.1 | 46.1 |
| 2007/8 | 16,670 | 11.7 | 1.2 | 17,048 | 12.1 | 1.6 | 50.6 |
| 2008/9 | 16,091 | 16.8 | 5.1 | 17,171 | 14.2 | 4.7 | 51.6 |
| 2009/10 | 15,390 | 18.2 | 14.2 | 17,146 | 18.3 | 10.7 | 52.7 |
| 2010/11 | 14,334 | 16.3 | 18.5 | 15,849 | 21.1 | 14.9 | 52.5 |
| 2011/12 | 11,586 | 15.8 | 20.8 | 13,126 | 20.9 | 20.2 | 53.1 |
| 2012/13 | 10,127 | 16.2 | 24.7 | 11,741 | 24.3 | 21.4 | 53.7 |
| 2013/14 | 10,731 | 16.0 | 31.6 | 12,614 | 25.5 | 25.1 | 54.0 |
| 2014/15 | 12,843 | 14.7 | 41.9 | 16,219 | 24.7 | 30.9 | 55.8 |
| 2015/16 | 12,047 | 15.7 | 47.0 | 17,481 | 23.7 | 33.2 | 59.2 |
| 2016/17 | 11,354 | 17.4 | 51.0 | 17,090 | 23.4 | 35.9 | 60.1 |
| 2017/18 | 10,341 | 14.5 | 55.9 | 15,578 | 21.3 | 40.6 | 60.1 |
Discussion
To understand the current arrangements for the provision of vascular services and the way that these may be affected by any future reconfiguration, a detailed analysis was carried out of the current sites at which vascular services are delivered. This was compared with data from an organisational audit carried out by VSGBI and the data on activity from the NVR. These sources of data provide rich information, but each has limitations and drawbacks. The organisational audit relies on voluntary submission of data. The NVR is a procedure-based registry that does not link to long-term re-admission and outcome data, whereas the HES data have the limitations described in the previous section relating to the coding systems and accuracy. There is, however, reasonable agreement between the sets of data in most respects.
The main findings with regard to the configuration of vascular services are that there has been a move towards centralisation of complex arterial cases, including AAA, CAD and major vascular reconstructions. As a result of the centralisation of AAA services, the number of sites at which aneurysm surgery is carried out has halved in the last 10 years. However, despite this, over the most recent 5-year period approximately 20% of aneurysm repairs are still carried out at centres with an annual volume below the 60 cases recommended in the provision of vascular services document. 171 Consideration of the distances between centres that have already amalgamated their aneurysm service and those that continue to carry out small numbers suggests that, with few exceptions, the geographical distances are not a major barrier to further centralisation.
There has been much discussion of hub-and-spoke arrangements, with various documents providing advice regarding such arrangements. 172 The organisational survey regarding such arrangements is limited in the detail that it provides. One issue in this respect is that the survey is carried out on the basis of providers, and a single provider may deliver vascular services at multiple sites. The HES data suggest that, where aneurysm services have been consolidated on a single site, many other aspects of the vascular service have followed suit, resulting in centralisation of many services rather than a hub-and-spoke arrangement. The HES data relate to inpatient activity only, so there may be hub-and-spoke arrangements with regard to outpatient clinics. However, the number of services that appear to offer day-case investigations, minor procedures or rehabilitation following amputation at satellite units appears to be limited.
Even in the case of VV surgery, which is largely carried out as a day-case procedure, there appears to be a significant degree of centralisation, with the VV activity following other major vascular services.
These results suggest that further research may be useful in identifying the drivers for and barriers to service reconfiguration and particularly to the provision of hub-and-spoke arrangements that may allow the local delivery of those aspects of patient care that can safely and appropriately be delivered at spoke sites.
Comparison between the activity and the outcomes reported from NVR and HES data suggest a number of issues that may be worthy of further investigation. The mortality estimates from NVR appear to be consistently lower than those obtained from the HES data, and there are a number of possible explanations for this. Although NVR would appear to have quite high acquisition rates, if the returns are selectively omitting cases in which there was mortality, this may result in underestimation of the mortality rate. This may occur because notes may be unavailable following deaths, so records remain incomplete, or because patients may be recorded as having survived a procedure when they have been transferred to another unit, even though they subsequently die without leaving hospital.
Another possible cause of distortion is that patients who are turned down for emergency surgery may not be recorded in the NVR. The patients identified in HES as having died with a primary diagnosis of aortic aneurysm include some such patients, although some of these underwent procedures, such as laparotomy, and, thus, should be included in the NVR data.
Another discrepancy between the HES data and the NVR relates to complex aortic aneurysm repair. Both NVR and HES suggest that there was a substantial increase in the number of EVAR procedures carried out for complex AAA, including branched and fenestrated endovascular grafts. However, the number identifiedas such in NVR is larger than the number with the relevant codes in HES data. It is not possible to distinguish between patients undergoing these complex procedures who would previously have undergone infrarenal open or endovascular procedures and patients who would not previously have been considered suitable for treatment. The distribution of such cases is uneven, with a small number of centres carrying out a higher proportion of these procedures. Considering the declining total number of AAA procedures and the absence of evidence of a large number of tertiary referrals, it seems more likely that this represents a different method of treatment of existing patients rather than a previously untreated population.
Further research may be justified to understand the appropriate place of such treatments, particularly in the light of the recent draft National Institute for Health and Care Excellence (NICE) guidance. 173
Conclusions
The detailed analysis of HES underpins the modelling that is described in Modelling the effects of service reconfiguration. The key findings in relation to service configuration are that, despite considerable centralisation of major procedures, there are still several centres that do not meet recommendations regarding activity levels and that, in most cases, this does not appear to relate to geographical limitations. Furthermore, the centralisation has been accompanied by significant changes in practice, particularly in relation to the implementation of less invasive treatments. Considerable variation in practice has been identified, some of which correlates with the degree of service centralisation. An additional finding has been the trend for centralisation of investigations and minor procedures, along with the emergency and major vascular workload.
Development of the electronic Personal Assessment Questionnaire – Vascular
In the UK, most patients with AAA, PAD, CAD, VLU and VVs are treated by the NHS’s secondary care services. Decisions on how and when to treat patients with these vascular conditions are subject to variation across the UK. Understanding the impact that the disease has on the quality of life (HRQoL) of patients can help both clinicians and patients to make treatment decisions. HRQoL is central to the individual patient’s experience of health and disease; measures of HRQoL must do more than describe a patient’s health in terms of what health professionals and society believe constitutes health. 174
One method of collecting data about HRQoL is using PROMs. PROMs are made of structured questions that ask patients about their health and HRQoL from their point of view. 175 PROMs provide information about the impact of a disease, or its associated treatment, from a patient’s perspective.
A key objective of the research programme was to identify or develop PROMs that could be collected from users of vascular services in an electronic form, to improve and monitor their treatment and to evaluate the services that they receive. This section describes an overview of the development of the ePAQ-VAS that consisted of four key stages and followed the process recommended in the US Food and Drug Administration (FDA)’s framework for PROM development. 176 A substudy was also undertaken to generate utility values of vascular patients from the EQ-5D.
Methods
Stage 1: themes generation
The various themes for the ePAQ-VAS were generated from systematic literature reviews of existing outcome measures and qualitative evidence (see Reviews of published literature).
Qualitative interviews with vascular patients
In addition, semistructured interviews were conducted with five vascular patient groups: patients with PAD, AAA, CAD, VLU and VVs. Users of vascular services attending the vascular department, Sheffield Vascular Institute (SVI) at the Sheffield Teaching Hospitals were recruited using purposive sampling techniques to ensure a range of participants at different ages, sex and stages of treatment. A clinician approached each patient and, following informed consent, a date and time were agreed for one of three researchers to visit the participant at home to carry out an interview [ethics approval: Yorkshire and Humber – Bradford Leeds Research Ethics Committee (REC) number 14/YH/1117 on 25 September 2014; an amendment to the interview schedule was approved on 18 June 2015]. The interview protocol, informed by the prior literature reviews, explored the signs, symptoms and impact that the condition had on function and the lifestyle (see Appendix 2).
Field notes were taken to aid interpretation and each interview was recorded and transcribed verbatim. Anonymised transcripts were entered into NVivo 10 (QSR International, Warrington, UK) for management and analysis using standard framework analysis techniques. 177 Between 10% and 20% of the interviews were double coded by a second researcher in NVivo 10. Inter-rater comparisons were calculated using Cohen’s kappa. Regular meetings were set up with an experienced qualitative researcher (AT) to review the frameworks and guide the analysis.
Consensus exercise with clinicians
Clinicians involved in the care of vascular patients were invited to list the key issues, the symptoms and the impact that AAA, PAD, CAD, VLU and VV had on patients suffering with these conditions. The evidence from this initial round was used to inform the qualitative evidence synthesis.
Stage 2: item generation and scale development
Items under the themes above were generated by a new PROMs Steering Committee (SCR, GJ, PP, EL and AA) to establish the initial version of the ePAQ-VAS. Face validity was assessed by separate exercises with clinicians and patients.
A different group of clinicians involved in the care of vascular patients were invited to score the relevance of items (questions) in the provisional version of the ePAQ-VAS. Participants were invited to rate the appropriateness of each question on a five-point Likert scale of ‘strongly disagree’ (0) to ‘strongly agree’ (4). This process was repeated, with members of the clinicians’ panel being presented with the aggregate findings of the previous round and again asked to score each question. This process examined the relevance of each item from the clinicians’ perspective and identified new items suggested by the clinicians. 178
Based on the work reported above, the Steering Committee employed an iterative process to incorporate evidence from the systematic reviews of qualitative studies, the qualitative study and the clinicians’ consensus study, as recommended by the FDA,176 to develop the initial item pool for the ePAQ-VAS. 179 A draft instrument was then adjusted based on further service users’ input and review by the steering committee.
Face validity of the electronic Personal Assessment Questionnaire – Vascular
Semistructured interviews were conducted with 19 patients, purposefully sampled from the five key clinical areas identified in workstream 2. A paper and/or electronic ePAQ-VAS (version 1) was presented to participants and a focused interview was conducted to investigate vascular patients’ perceptions of the whole questionnaire and individual items. Questions were asked under the headings of overall impressions, clarity, relevance and emotional response. Interviews were audio-taped, transcribed and analysed. A pragmatic approach was used for the analysis, with comments collated and presented back to the Steering Committee, which made consensus decisions on revisions to the ePAQ-VAS.
Stage 3: reducing the items, confirming the domain structure and scoring algorithms of the electronic Personal Assessment Questionnaire – Vascular
Participant recruitment
To reduce the items and confirm the conceptual framework of the ePAQ-VAS generated in stage 1, a quantitative survey was undertaken. Consecutive patients attending outpatient clinics run by SVI between June 2017 and June 2018 were invited to complete the questionnaire online before their clinic appointment using an established encrypted voucher system. Patients who had not completed the questionnaire online, and inpatients, had the option to complete the questionnaire using electronic tablet computers or computer terminals on site. The five key vascular conditions that had been identified (AAA, CAD, PAD, VLU and VV) were all represented in the sample.
Statistical analyses
The sample size calculation was based on previous studies suggesting that a ratio of at least 4–10 respondents per item would enable factor analysis and internal reliability calculations. 180,181 Because 55 items in the ePAQ-VAS contributed to eight scales, up to 550 patient completions were considered necessary.
A one-factor confirmatory factor analysis model for ordinal data182 was fitted to each of the eight scales to identify their structure and enable item reduction. Ordinal items were regressed on the domain factor by regressions estimated by a robust weighted least squares estimator with mean and variance adjustment. 183 Appropriateness of the confirmatory factor analysis model for each domain was assessed by examining the comparative fit index (CFI) and the root-mean-square error of approximation (RMSEA), where CFI of > 0.95 and RMSEA of < 0.08 were regarded as an appropriate fit. 184 Furthermore, items factor loadings (> 0.4), model residual correlations and modification indices were considered to examine local dependence within domains. 184 The magnitude of these three indices was evaluated in comparison with other items in the scale. When the modification indices (MI) were > 100 and residual correlations (RCs) were > |0.10| this was taken as the indicator of lack of fit and items were removed from the scale. 184 Following confirmatory factor analysis, redundant items were removed. Mplus version 8.2 (Muthén & Muthén, Los Angeles, CA, USA) was used for the statistical analyses.
Internal consistency reliability measurement was used to investigate the reliability of the remaining items in each domain. The Cronbach’s alpha coefficient was calculated for each domain to measure internal reliability. A Cronbach’s alpha score of ≥ 0.70 was considered acceptable; however, scores exceeding 0.92 were taken to indicate that items in the scale may be redundant. 82,185,186
Stage 3: further psychometric testing of the electronic Personal Assessment Questionnaire – Vascular
To further establish the psychometric properties of the ePAQ-VAS, the results of relevant items from a previous survey and an additional survey were used to assess test–retest reliability, construct validity and the responsiveness of the measure186 (see Report Supplementary Material 12).
Participants’ recruitment
All consecutive patients invited to outpatient clinics run by SVI from June 2018 to January 2019 were asked to participate in this study using the same voucher arrangements as for the conceptual framework analysis (CFA) study. For test–retest reliability, patients were asked to complete a second questionnaire 3–7 days later, provided that there was no change to their health status. Only patients with AAA, PAD, VLU and VV were included in this survey because CAD patients were available around the time of admission for treatment only. For responsiveness testing, patients completed the ePAQ-VAS before and 6 weeks after PAD and VV procedures. The second survey for test–retest reliability and responsiveness was administered by telephone by one of the researchers.
Statistical analyses
Scoring
Summated rating was used to score the results of the eight scales in the ePAQ-VAS and was standardised to a 0–100 scale, where 0 indicates the best HRQoL and 100 the worst HRQoL. Skipped items were allocated a score of 0; the electronic format of the instrument allows patients to skip items that are not relevant to them, so it was assumed that patients skipping any items have no problems relating to these items.
Test–retest reliability
Intraclass correlation coefficients (ICCs) were used to assess test–retest reliability. ICCs exceeding 0.7 are generally regarded to indicate reliability for population-based research and ICCs exceeding 0.9 are considered to indicate reliability for use clinically with individuals. 15,84,187
Known group validity
Known group validity was examined using hypothesis testing to examine whether or not the scales correlate well with expected clinical group differences. Correlations are considered low if r is < 0.3, moderate if r lies between 0.30 and 0.49 and high if r is > 0.5. 188 Hypotheses were stated a priori, including the postulated direction. 78,188
Responsiveness
Responsiveness was measured using standardised effect size, calculated as the change in score between post intervention and baseline divided by the standard deviation (SD) at baseline. An effect size of 0.30–0.49 is regarded as ‘small’, an effect size of 0.50–0.79 as ‘moderate’ and an effect size of ≥ 0.80 as ‘large’. 188 The standardised response mean was also calculated by measuring the mean difference between baseline and post intervention divided by the SD of the change. Similar cut-off points as for the standardised effect size above were used. Statistical analyses were performed using SPSS version 24 (IBM Corporation, Armonk, NY, USA).
Substudy EuroQol-5 Dimensions, five-level version, utility values
The purpose of this substudy was to generate utility values for use in economic evaluation models. 189 The EuroQol-5 Dimensions, five-level version (EQ-5D-5L), was incorporated into the ePAQ-VAS and participants were asked to complete an electronic version of the EQ-5D and the EQ-5D visual analogue scale. A series of clinical health states were identified by the steering group, in each of the five vascular categories, as being representative of specific vascular conditions and identifiable from the ePAQ-VAS responses. Utility values based on the EQ-5D-5L responses were calculated for each of these clinical states using the van Hout et al. 190 mapping function.
Results
Stage 1: themes generation
Overlapping themes were identified from the literature reviews for the various vascular conditions. For PAD, there were six main themes: symptoms, physical functioning, impact on social functioning, psychological impact, financial impact and process of care. For AAA, the four overarching themes identified were symptoms, functional outcomes, psychological outcomes and social outcomes. For CAD, the five main themes were anxiety, impact on personal roles and activities, effect on independence, psychological impact and symptoms. For VV, the five main themes were physical impact of VV, psychological impact, social impact, adapting to VV and reasons for seeking treatment. For VLU, the main themes were physical impact, psychological impact, social impact and treatment. 16,19,21–24
Qualitative primary study with vascular service users
Out of 111 patients invited to participate in the primary qualitative study, 56, aged 35–77 years, were interviewed to explore the impact of vascular disease on daily living: 13 patients with AAA, nine with CAD, 14 with PAD, 10 with VLU and 10 with VV. The framework analysis of the primary data identified six overarching themes relating to the impact of these five vascular conditions. These were symptoms, impact on physical function, social impact, psychological impact, financial impact and lifestyle. Identified signs, symptoms and impact of the conditions were then mapped and tabulated to see which themes were relevant to which condition and where the similarities and differences lay. 179
Pain and mobility were the most commonly reported domains by participants with PAD. The extent to which they had an impact on HRQoL was associated with the severity, age expectations and social support. Fear of the symptoms worsening and amputation were also reported. Most participants with AAA reported no physical symptoms, with a small number reporting abdominal pain or pain in their legs. Uncertainty, anxiety and fear of rupture and death appeared to have the greatest impact on HRQoL.
Patients with CAD had the widest range of signs and symptoms, reporting nine different issues. The condition had the least impact on physical and social function, although some participants reported a sense of worry and anxiety, which was most often related to fear of having a major stroke.
Most of the participants reported that VV had little impact on their overall HRQoL. Pain was reported by 8 of the 10 participants; one reported significantly diminished HRQoL owing to reduced mobility. The perceived unpleasant appearance of the VV had the greatest psychological impact and was described in detail by several of the group. Many of the participants had had their VV for very long periods of time, often just ‘putting up with it’ for numerous years before seeking help.
The impact of VLU on HRQoL differed in the group. For some there were no major issues and having a VLU was accepted as part of their current life, with the hope that it would heal eventually. For others, the impact was far more significant. Pain was reported by six of the group and in some participants was quite severe, was quite severe, leading to a significantly reduced HRQoL. Pain also had a bearing on people’s mobility and their ability, or desire, to go out and socialise. Sleep was also often disturbed because of pain. The persistence of VLU had resulted in participants suffering for long periods of time. In addition, the non-healing or recurring nature of the condition had a significant impact for many. VLU had a significant psychological impact, causing some a high degree of distress.
There were many overlapping domains between the conditions. Pain was experienced in varying degrees within and across the conditions. Many of the participants had more than one vascular condition that contributed to some of the overlap in reported symptoms. For instance, many participants with AAA reported symptoms of claudication. Comorbidities were common, particularly among patients with PAD or VLU, who described the cumulative impact that the other conditions had on their HRQoL and functioning. Overall, those patients with VVs were the youngest and reported the least impact on HRQoL. Age expectations were evident in all groups; some of the older participants reported that they expected ill health because of their age, whereas younger people often reported being much more distressed by the condition.
In total, 13 vascular clinicians reviewed the items and they scored on the relevance of the items in a two-round consensus exercise. Clinicians’ rating generally aligned with the issues identified by patients, but some additional items were added, particularly relating to specific symptoms of swallowing difficulty for CAD and abdominal pain or throbbing for AAA.
Stage 2: item generation and scale development
Based on the results above, the items of the ePAQ-VAS were arranged into four sections: generic, AAA, CAD and lower limb vascular conditions. The generic section included the EQ-5D-5L instrument that had been identified in the reviews (see Reviews of published literature) and through Steering Committee discussions as being the most appropriate generic measure. A single lower limb section was developed as common themes were identified for PAD, VLU and VVs affecting the lower limb, regardless of whether the underlying pathology was venous or arterial. An inclusive approach to development was used and a comprehensive questionnaire was produced with 168 questions.
Face validity of electronic Personal Assessment Questionnaire – Vascular
Overall, the response to the semistructured patient interviews was favourable, with participants reporting that they felt that it was fit for purpose and potentially useful. There was little consistency in items that participants found difficult. No single item was identified for which many participants had a difficulty. Issues raised included the use of abbreviations; font size and contrast between text and background; response options and scales; electronic format versus paper format; relevance to patients and clinicians; use of free-text boxes and the language and wording used; when and how to use the skip button; repetition of items and subject matter; and the possibility of emotional distress associated with questions about the possibility of deterioration or death.
In total, 59 items were eliminated owing to overlap and five items were added, as requested by clinicians, because they were relevant to clinical management. Generic items and the EQ-5D-5L questionnaire were included in the first section and all respondents answered questions about pain, altered sensation, weakness, weight/height, smoking habit, medical history and regular medication.
The subsequent three sections are condition specific and relate to CAD, AAA and lower limb vascular disease, each of which is further divided into scales. There were 55 items in eight scales in addition to the generic EQ-5D-5L instrument. The remainder of the questions did not contribute to scales but were kept because of their clinical relevance. Individual items, scales and sections and the source of the evidence for their inclusion have been detailed, along with illustration of the structure of the questionnaire following further revisions. 179
Stage 3: reducing the items, confirming the domain structure and scoring algorithms of the electronic Personal Assessment Questionnaire – Vascular
The first version of the ePAQ-VAS that was presented to patients had 114 items, of which 55 items contributed to eight factors. The other questions were either clinically relevant questions (i.e. asking about smoking, weight, etc.) or screening questions to ensure that only relevant questions are presented to the patients based on their specific vascular complaint. The ePAQ-VAS was completed by 638 vascular patients; 159 patients (24.9% of the total) completed the questionnaire online before their clinic appointment and the remainder were recruited face to face in the outpatient clinic and the vascular inpatient ward. The majority (65.2%) of patients were male. All of the patients were asked to complete relevant sections of the generic dimension. Patients were presented only with relevant disease-specific scales. In total, 49 patients completed CAD questions, 112 respondents answered the AAA dimension, 323 completed PAD-related questions and 117 and 172 patients completed the VLU and VV questions, respectively.
The CFA models were developed to test the a priori model for the eight-scale structure. 191 In the CAD section, two scales were modelled and all items in the ‘CAD-related anxiety’ section were relevant to measure the latent factor. However, two items were dropped from the ‘Impact of CAD on activities of daily living (ADL)’ domain. The first was a generic item about the impact of CAD diagnosis on enjoyment of life, which had high MI and RCs with two other items. The other asked about the impact on mood and had low factor loading. In the AAA section, only one item was deleted because of high MI and RCs with two items on the same scale, as was a similar item asking about the impact that lower limb symptoms have on the enjoyment of life. Items with a low factor loading relating to ‘cold feet’ and VLU symptoms domain were also dropped. The opinion of clinicians was sought before deleting these items.
Following item reduction, the internal consistency of each scale was examined; all scales had a Cronbach’s α coefficient of ≥ 0.70 but none exceeded 0.92. The results suggest that the items in each scale measure the same latent construct. For CFA model fit statistics see Table 10 and Aber et al. 186
| Scale | CFA initial model | CFA final model | Items deleted | ||
|---|---|---|---|---|---|
| RMSEA | CFI | RMSEA | CFI | ||
| CAD-related anxiety | 0.156 | 0.847 | 0.08 | 0.980 | None |
| Impact of CAD on ADL | ‘Impact of CAD on enjoyment of life’ and ‘low mood caused by CAD’ items were deleted. The former had high MI and RCs with items in the same domain. The latter had low factor loading | ||||
| AAA-related anxiety | 0.141 | 0.957 | 0.043 | 0.990 | None |
| Impact of AAA on ADL | ‘Impact of AAA on enjoyment of life’ had high MI and RCs with items in the same domain | ||||
| PAD symptoms | 0.127 | 0.818 | 0.077 | 0.982 | ‘Cold feet’ item was deleted because of low factor loading |
| VLU symptoms | 0.266 | 0.178 | 0.080 | 0.984 | ‘Worry about leg ulcers’ had low factor loading |
| VV symptoms | 0.187 | 0.801 | 0.078 | 0.967 | None |
Stage 4: further psychometric testing of the electronic Personal Assessment Questionnaire – Vascular
In total, 721 patients completed the ePAQ-VAS. The mean age of the participants was 63.45 years (SD 15.7 years) and 64.9% were men (n = 468). A total of 76% of patients (n = 553) completed the questionnaire in a clinical environment (clinic or ward) and the remaining patients completed the questionnaire online before their clinic. Scores were calculated for each scale (Table 11).
| Scale | Number of respondents | Mean score (out of 100) | Standard deviation |
|---|---|---|---|
| AAA-related anxiety | 121 | 23.74 | 21.84 |
| AAA impact on ADL | 121 | 17.41 | 20.82 |
| CAD-related anxiety | 50 | 44.17 | 29.61 |
| CAD impact on ADL | 50 | 32.4 | 30.29 |
| PAD symptoms | 308 | 47.08 | 26.86 |
| PAD impact on ADL | 308 | 50.28 | 30.88 |
| VLU symptoms | 122 | 34.97 | 24.37 |
| VLU impact on ADL | 122 | 55.46 | 30.91 |
| VV symptoms | 248 | 36.86 | 18.91 |
| VV impact on ADL | 248 | 28.52 | 26.69 |
Test–retest reliability
For the test–retest survey, 150 patients (60 with PAD, 39 with VLU and 51 with VVs) completed the relevant sections of a second questionnaire after 3–7 days. Test–retest results were calculated for the symptom scale and the impact that lower limb vascular disease had on ADL for patients with PAD, VLU and VVs separately. The ICC ranged from 0.59 for anxiety related to AAA to 0.94 for the impact that VLU had on ADL. 179
Known group validity
Correlations between the proposed clinical hypotheses and the CAD impact on ADL and CAD-related anxiety scores were low, ranging from –0.089 to 0.094. The correlation between size of AAA and AAA-related anxiety score was significant, and this scale captured this latent variable. There was no significant correlation between size of AAA and AAA impact on ADL. There was low correlation between AAA-related anxiety and AAA impact on ADL in preoperative patients. There was a significant correlation between rest pain and PAD symptoms and impact of PAD on ADL. The presence of an ulcer had a statistically significant correlation with the score of PAD impact on ADL.
Ulcer recurrence had a significant correlation with VLU symptom scale score. The correlation of ulcer recurrence with other scales was weak. The presence of VV with VLUs had no significant correlation with any of the scales’ correlations. The presence of VV in both legs had a significant correlation with VV symptoms only, and the presence of VV in both legs did not have strong correlations with scores of VV impact on ADL. Some of the results of known group validity are in line with proposed clinical hypotheses, for instance, ‘the larger the size of AAA, the greater the anxiety caused by the condition’ and ‘the presence of rest pain or an ulcer has a significant impact on PAD scales scores’. However, some clinical hypotheses, particularly in relation to CAD scale scores, were not in line with what was proposed. This could be because of the small size of this disease group.
Responsiveness
In total, 55 patients with VV undergoing VV surgery and 37 patients undergoing lower limb revascularisation procedures for PAD completed the ePAQ-VAS preoperatively and once more at least 6 weeks after their procedure. The effect size and standardised response mean were measured for all of the relevant scales of the ePAQ-VAS (Table 12). 179
| Scale | Number of patients | Standardised effect size | Standardised response mean |
|---|---|---|---|
| PAD symptoms | 37 | 0.69 | 0.74 |
| Impact of PAD on ADL | 37 | 0.85 | 0.69 |
| VV symptoms | 55 | 1.48 | 1.60 |
| Impact of VVs on ADL | 55 | 0.82 | 0.78 |
Substudy: EuroQol-5 Dimensions, five-level version, utility values
The average EQ-5D-5L derived utility values ranged from 0.396 for PAD patients with a major amputation to 0.886 for VV patients following treatment (Table 13).
| Health states | Number of cases | Mean utility value (SD) | Range | EQ-5D VAS score, mean |
|---|---|---|---|---|
| CAD preoperatively | 11 | 0.772 (0.207) | 0.343–1 | 77.36 |
| CAD with stroke | 10 | 0.700 (0.261) | 0.078–0.906 | 66.70 |
| AAA under surveillance | 85 | 0.711 (0.277) | –0.450–1 | 60.55 |
| AAA preoperatively | 12 | 0.778 (0.178) | 0.390–1 | 64.58 |
| AAA post repair | 3 | 0.771 (0112) | 0.691–0.850 | 76.00 |
| PAD with claudication only | 22 | 0.676 (0.215) | 0.812–1 | 66.38 |
| PAD with ulcer | 96 | 0.481 (0.315) | –0.361–1 | 55.61 |
| PAD with amputation | 39 | 0.396 (0.345) | –0.257–1 | 56.26 |
| VLU without VV | 92 | 0.473 (0.321) | –0.445–1 | 53.54 |
| VLU with VV | 22 | 0.505 (0.315) | –0.242–0.879 | 54.68 |
| Postoperative VV | 55 | 0.886 (0.181) | 0.411–1 | 75.27 |
Discussion
This section documents the steps undertaken to develop the ePAQ-VAS, a new tool to collect PROMs and other clinical information from users of vascular services. This can be used as a holistic clinical assessment tool to be completed by patients before seeing a clinician and during follow-up. The information generated can be used to help shared decision-making and to monitor the impact of intervention on the patients’ quality of life. As an electronic online measure it can potentially be completed remotely, which facilitates virtual clinics,192 and may contribute to electronic patient records or aggregated data for service evaluation. A demonstration version of the final version of the web-based form is available online at www.epaq.co.uk/Demo/VascularDemo (accessed 1 December 2020).
A major strength of the work is that the instrument has been developed through a rigorous process in line with FDA guidelines for developing PROMs. 176 Evidence from this study suggests that scales in the lower limb section of the ePAQ-VAS have good test–retest reliability, particularly the PAD and VLU scales. The results of the known group validity show that the AAA-related anxiety scale correlates with the size of AAA. Patients’ anxiety is increased and the impact that the disease has on patients’ daily living is greater for preoperative patients than for those under surveillance. The instrument shows good correlation between rest pain in PAD and increased PAD impact on ADL scores. The results of the survey also confirmed that the presence of an ulcer with PAD diagnosis increased PAD impact on ADL scores, and recurrent ulcer is associated with an increase in ulcer symptom scores. The results of the responsiveness analyses show that the following scales can detect changes following intervention: AAA-related anxiety, PAD symptoms, impact of PAD on ADL, VV symptoms scale and impact of VVs on ADL.
Another strength of the work is the inclusion of the EQ-5D-5L alongside disease-specific scales. This allows the generation of utility values for the different health states that can be used in cost-effectiveness modelling, in line with NICE’s methodology. 193 It may also facilitate further research to consider the relationship between such generic measures and the more detailed symptomatic and disease-specific description of vascular conditions provided by the new instrument.
These studies had a number of limitations. For pragmatic reasons, the development and validation were largely based on evaluation at a single site. Sample size was small for some groups of patients, because of resources and patient availability, particularly for patients with CAD and AAA. Access to some patient groups before and after treatment was difficult and the fact that some forms were completed online, some in outpatient clinics and some by telephone may have been a confounding factor. Furthermore, the sample size for responsiveness and test–retest was small for all groups, and future analysis with a larger sample size is desirable. The follow-up data for test–retest and responsiveness were collected by telephone, which may have introduced some bias. Further studies are also required to explore acceptability and completion rates, to consider the practicalities of wider implementation and to refine aspects of the process, such as reporting formats and the analysis of longitudinal and aggregate data.
Overall, the ePAQ-VAS provides a holistic data collection process that is relevant to most vascular service users and has the potential to contribute to patient-focused care and the collection of aggregate data for service evaluation.
Evaluation of non-health outcomes
Although most of the previous work on the configuration of services has been driven by evidence relating to the effect on clinical outcomes, the reconfiguration of services also affects other aspects of the service that may be important to service users. This section reports the results of trade-off studies to measure the strength of preference of a sample of the general population for aspects of the process of care. Preferences for endovascular or open surgical repair for AAA are evaluated in terms of the quality-adjusted life-year (QALY) benefit that participants would forgo for their preferred treatment. Further studies use a similar trade-off method to evaluate the strength of preferences for reduced travelling distances, in terms of the QALY benefit that would be forgone to avoid the need to travel for treatment of AAA, CAD or PAD.
Trade-off study
The reorganisation of vascular services will have an impact not only in terms of health outcomes, but also with respect to other aspects of service provision, such as travel distances and treatment processes. The aim of the proposed research was to elicit preferences from members of the public for the way in which vascular services could be organised in the NHS. Following qualitative work with vascular patients, travel distance to health facilities was identified as an extremely important factor in any reorganisation. Further work with vascular clinicians revealed a desire to elicit preferences for alternative treatment processes for AAA, as treatment of AAA is the principal driving force behind any potential reorganisation. The specific aims of the study, therefore, were to elicit preferences for (1) the treatment processes associated with EVAR and open surgical repair of AAA and (2) having to travel to specialist hospitals for treatment and follow-up for AAA, CAD and PAD. 194
Method
The method involved surveying members of the UK population, through individual telephone interviews, to elicit their preferences for treatment processes for AAA and travel distance to specialist hospitals. At the heart of the method is the notion of opportunity cost and how this relates to value. Fundamentally, something is of value to an individual only if they are willing to give up (or sacrifice) something to acquire what is being valued. Without sacrifice, there is no value. What respondents were asked to sacrifice to have their preferred treatment option or preferred hospital was small changes in the chance of treatment being successful. From their responses it was possible to quantify their strength of preference in terms of a QALY equivalent that allows those preferences to be incorporated into the cost-effectiveness models. Interview booklets for the three groups are provided in Appendices 3–5.
Participants
Participants were aged ≥ 18 years, citizens of the UK and had no previous diagnosis of a vascular condition (self-assessed). The justification for sampling members of the public rather than patients lies in the context of how the values are likely to be used, namely to inform national health-care priority-setting. In this context, NICE states a preference for QALYs to be based on general population values. The total sample size was 821 (four telephone surveys were administered, with approximately 200 participants in each).
Recruitment and consent
Participants were recruited through various NHS trusts across England. The study covered a wide geographical area including South Yorkshire, Greater London, Kent, Staffordshire, Derbyshire, Lincolnshire, Lancashire, Leicestershire, Cambridgeshire, Essex, Cumbria, North Devon, Tyne and Wear, and Northumberland. A range of approaches were used to identify potential participants. These included approaching hospital visitors on site, having recruitment stands in dining halls, using posters around the trusts with study details and recruiting staff via blanket e-mails. When approaching potential participants, efforts were made to ensure that the sample was representative of the general population with regard to age and sex. Contact details of potential participants were securely transferred to the research team, who then contacted these participants (between September 2017 and January 2018) to arrange a date and time for telephone interviews. Participants who agreed to be interviewed were sent an interview booklet approximately 1 week before the interview took place. They were advised to read the interview booklet prior to the interview to familiarise themselves with the information enclosed. Verbal consent was obtained over the telephone prior to commencing the interview and the interview was audio-recorded. The use of verbal consent was accepted and approved by the South East Coast – Brighton and Sussex Research Ethics Committee, Health Research Authority (REC number 16/LO/0943), on 20 April 2017 along with all of the study documents. An amendment to the interview documents was submitted and approved on 2 May 2017.
Results
Abdominal aortic aneurysm treatment method
A total of 209 participants completed the interview, giving a response rate of 64%. Missing data rates were low and did not exceed 2%.
When considering simple direction of preference, 167 (79.9%) participants stated that they would prefer EVAR, 40 (19.1%) participants indicated that they had a preference for open surgery and two (1.0%) participants said that they had no preference for either treatment. Factors that influenced treatment preferences for EVAR included the less invasive nature of the surgery and the quicker recovery times associated with the procedure. For open surgery, factors included having to have only one follow-up appointment and the feeling that the open procedure felt more permanent.
When participants were asked to make a sacrifice (trade-off) to have their preferred treatment, 45 and 22 respondents who had a stated a preference for EVAR and open surgery, respectively, indicated that they were not willing to sacrifice anything to support their preference and, thus, were deemed to value both treatments equally. Therefore, when strength of preference was taken into account, 122 (58.4%) participants preferred EVAR, 18 (8.6%) participants preferred open surgery and 69 (33%) participants had no preference. In the first year following treatment, those preferring EVAR were willing to give up a mean of 0.135 expected QALYs to have EVAR, whereas those preferring open surgery were willing to give up 0.033 expected QALYs to have open repair. These results indicate a clear preference for EVAR over open surgery among the sample.
Travel distance to hospital
A total of 608 participants completed the interviews (200 for AAA, 202 for CAD and 206 for PAD). Each respondent was asked to consider travelling one of four distances (5, 15, 30 or 60 miles) that were distributed equally within samples. The overall response rate was 64.4%. Missing data rates were low and did not exceed 5%.
The proportions of people willing to travel for AAA (open surgery), AAA (EVAR), CAD and PAD were 89%, 86.5%, 79.7% and 96.1%, respectively. Of these, 56.2%, 55.5%, 64.0% and 66.2% stated that they required compensation to travel for AAA (open surgery), AAA (EVAR), CAD and PAD, respectively.
Among the remaining respondents, the main reason cited for not being willing to travel was a preference for local services and/or a belief that all services should be available locally. Other reasons stated were transport difficulties with making the journey, not wanting to burden family and friends, and a concern among participants that they would feel isolated without having family and friends around them if there was no local provision.
Owing to the constraints of the study design, the maximum compensation that could be demanded by those people not willing to travel was limited to that associated with the success rate at the specialist hospital being 100%. Despite the amount of compensation required to travel by this group being unknown, to include them in the analysis they have been assigned this maximum value. This means that the value of the disutility of travelling reported below for the sample as a whole should be regarded as a minimum value.
For a typical patient aged < 65 years with one of the vascular conditions considered, the expected discounted lifetime QALYs demanded as compensation for having to travel an additional 30 miles for treatment and follow-up are as follows: 0.3541 for AAA (open surgery), 0.3869 for AAA (EVAR), 0.2041 for CAD and 0.6310 for PAD (results for the full range of distances considered are in Report Supplementary Material 13).
Taking AAA (open surgery) as an example, these results suggest that, if vascular services are reorganised so that the individual must travel an extra 30 miles for treatment and follow-up, their disutility of travelling is equal to 0.3541 QALYs. This should be netted off from any potential gain in QALYs that is expected to arise from treatment being at a specialist rather than a local hospital.
Discussion
Our results comparing EVAR with open surgery for AAA repair suggest a clear preference for EVAR in the sample. This suggests that patient preferences may be in conflict with the recent recommendation by NICE195 that EVAR should not be recommended as the first-line treatment option for most people with this condition. These findings suggest that greater consideration should be given to the value that is placed on the treatment processes of EVAR and OR. If NICE were to find a mechanism to explicitly incorporate such preferences in the decision-making process, it may increase the likelihood that recommended treatment pathways align with the preferences of the UK population. 173
Our results valuing the burden of travelling for treatment and follow-up clearly indicate that it is a potentially important component of disutility in the sample for each of the three clinical areas considered. As such, it is suggested that there is a need take this disutility into account when considering the implications of any decision to reorganise vascular services to a more centralised provision. To that end, the impact on cost-effectiveness of including the values placed on the disutility of travelling has been investigated in Modelling the effects of service reconfiguration.
Modelling the effects of service reconfiguration
The online model can be accessed at https://modellers.sheffield.ac.uk/vascularmodel/ (accessed 1 December 2020).
Summary
This section reports the evaluation of the cost and quality implications of different possible service configurations for subspecialist vascular services. A number of techniques were used, including HES data analysis, regression modelling, systematic literature review, preference elicitation, decision analysis and modelling. Costs are reported in Great British pounds at year 2017/18 prices. The NHS and Personal Social Services (PSS) perspective was adopted for the economic evaluation. Costs and outcomes are discounted at 3.5% per year. Statistical analyses and simulation were performed in R version 3.4.3.
The modelling was carried out in two parts. First, three separate models covering the clinical areas of AAA, PAD and CAD were developed. These models of individual disease areas were then brought together in an interactive web-based model that enables users to identify potential reconfiguration of services in particular geographical areas and to predict the overall effects of potential service reconfiguration in terms of workload, resource use, outcomes and cost-effectiveness.
Results at the cohort level are reported for each vascular group (AAA, CAD and PAD) separately and for the cohort as a whole. The incremental cost per QALY is reported for the combined cohort as a whole to indicate how cost-effective the new configuration is compared with the baseline configuration. The model estimates the specific results for each vascular site, before and after the reconfiguration. Scenario analysis and one-way sensitivity analysis were conducted to see how the results were affected by the model’s structure and key parameters. To assess the impact of parameter uncertainty on the model’s results, 2000 Monte Carlo simulations of the model were run in a probabilistic sensitivity analysis (PSA). Each PSA simulation ran the model with a sample of inputs for stochastic parameters.
General overview
The conceptual modelling framework was based on clinical consensus representing the pathways of care, characterised through the analysis of HES. The aim of the model is to predict the changes in workloads, distance from patients’ homes to hospitals, hospital costs and outcomes when vascular services are reconfigured (i.e. merging small centres into larger centres). Our model was a patient-level simulation. We chose a patient-level modelling approach to better utilise patient-level data from HES and to accommodate the complexity of the decision problem, capturing both patient-level information and hospital-level information. Our simulation used discrete time-to-event rather than time cycles in modelling. This reduced unnecessary computer time in running the model. System dynamics was not suitable for our modelling problem. The microsimulation models were populated with data from regression analysis of HES records, systematic literature reviews and preference information.
Service reconfiguration
Options
The model tested two different service configurations: devolved and centralised services. Devolved services are typically hospitals with a general surgical service that also offer vascular services and tend to be associated with a low volume of vascular procedures, whereas centralised services have sufficient subspecialist staff to provide a full range of emergency and elective services and are associated with a higher volume of procedures.
Definition of volume
‘All AAA repairs’ per year was used as the definition of volume. This is to ensure comparability with the criteria used by NHS England170 and the VSGBI provision of vascular services document. 171 The annual volume for each specific year was used, rather than average annual volume across the years, so that year-on-year changes could be considered.
Effects of reconfiguration
Service reconfiguration could affect clinical outcomes (e.g. mortality, length of stay, complication rate, re-admissions and survival) and costs. Moreover, patient satisfaction may be affected if they are sent to a service that is further from home than their usual vascular centre.
The HES data were used to estimate the relationship between volume and outcomes/costs, after adjusting for the patient case-mix factors. The short-term outcomes included mortality, length of stay, complication rate and re-admissions. The relationship between volume and long-term survival was also estimated. Regression equations of the hospital admission costs as a function of volume were also estimated from the HES data analysis (see Report Supplementary Material 5).
Conceptual model
The model aims to predict the changes in workloads, distance from patients’ homes to hospitals, hospital costs and outcomes when vascular services are reconfigured (i.e. merging small centres into larger centres). The model structure is similar across the three main vascular groups: AAA, CAD and PAD (Figure 14).
FIGURE 14.
The model structure. TIA, transient ischaemic attack.
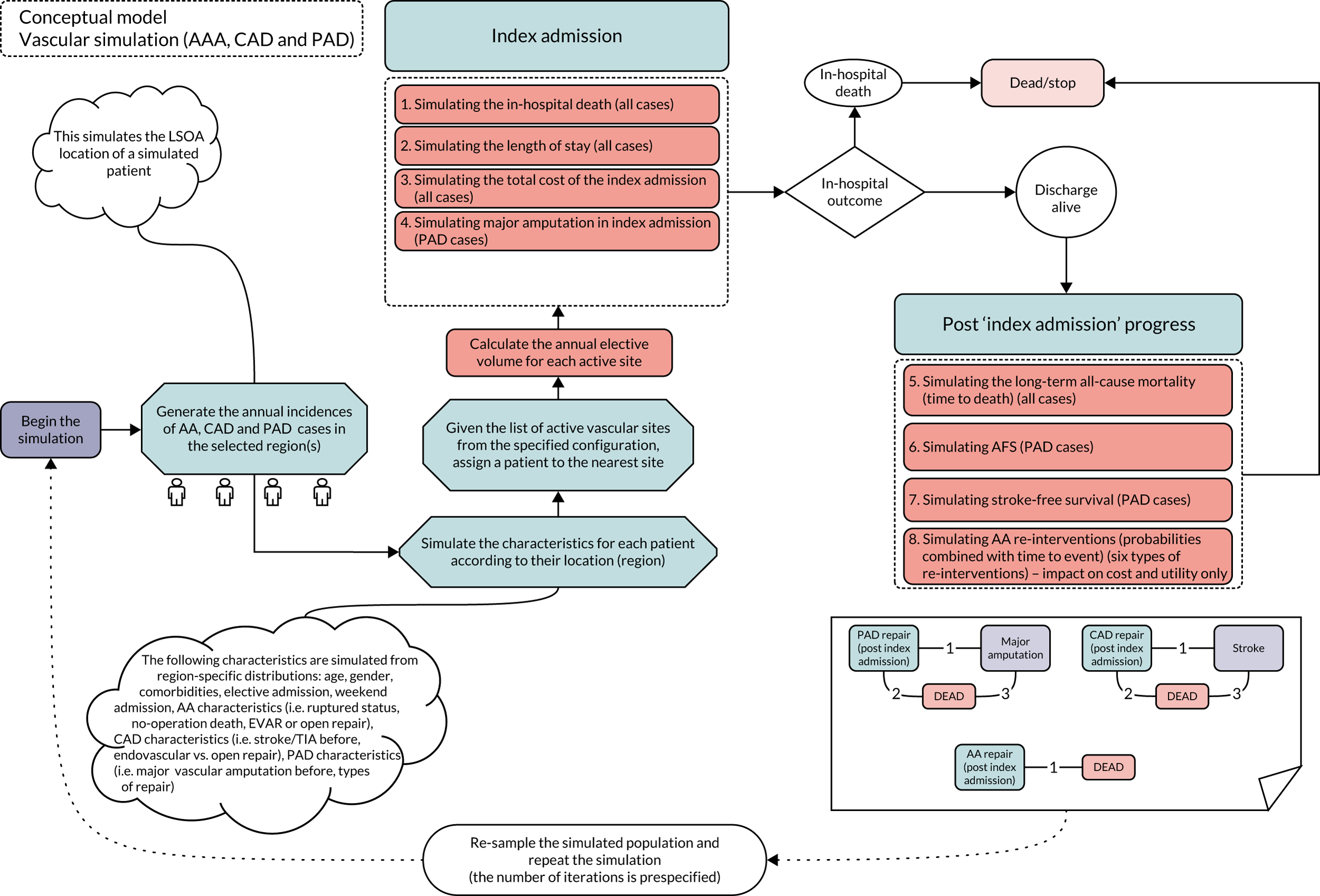
Service configuration and patient cohort
The baseline configuration of services and the new configuration (reconfiguration) can be specified for specific region(s). The aim of the evaluation is to compare the costs and outcomes between these two options.
Given the options of service configuration, the simulation starts by simulating the patient cohort for the region(s). First, it creates a sample of patient LSOA locations for AAA, CAD and PAD annual cases. Second, other patient characteristics in each vascular group are simulated based on descriptive information from HES data.
Patients are assumed to go to the nearest vascular centre (straight-line distance) and the annual AAA volume (counting all AAA repairs) of a vascular centre is calculated accordingly for a service configuration. It is assumed that in the base case the treatment option (e.g. EVAR vs. OR) is not affected by the configuration (same treatment is given before and after the reconfiguration). The base case also assumed that reconfiguration does not affect the proportion of AAA no operation deaths.
Index admission
The following outcomes were modelled for the index admission of vascular repairs: in-hospital death, length of stay, hospital costs of the admission and major amputation in the index admission (with PAD cases only). They were simulated using regression models that were developed from HES data. The list of covariates considered in the regression is given in Appendix 6 and details of these regression models are given (see Report Supplementary Material 14).
Given the patient characteristics and the volume of procedures carried out in the vascular centre where they receive the repair, probabilities of in-hospital death from the index admissions are predicted using logistic regression models developed from HES data. This is then simulated to determine whether a patient lives or dies in the index admission. Length of stay is then estimated by generalised linear regression models. This gives the duration of the patient stay in the index admission. The costs of index admissions are also estimated by regression models. For PAD cases, they also have their probabilities of having a major amputation in the index admission predicted by regression models.
Post index admission
Patients who survive and are discharged from the index admission move to the post-index admission progress/state, where their long-term survival and progression are predicted using survival models developed from HES data. OS is estimated using time-to-death (TTD) equations for each patient given their characteristics and centre volume. Patients also experience complications related to their index repairs. For AAA, the complication included various re-intervention re-admissions. For CAD, the complication was stroke-related re-admission. For PAD, the complication was major amputation re-admission.
Costs/quality-adjusted life-years
The following areas of costs are captured for the patients: costs of index admission, costs of re-interventions/complications and long-term costs. Total costs of the index admission are predicted for each patient by the regression models developed from HES data. The predicted costs include all resource use during the index admission (covering main procedures, critical care and complications in the index admission). Costs of re-admissions with major amputation were also estimated from HES data analysis. Other long-term costs are estimated from targeted systematic literature reviews; typically, these are presented as annual health-care costs that are combined with the OS to estimate the overall costs.
The following utility values were captured for the patients: utility of index admission and long-term utility with and without complications. A targeted systematic literature review was conducted to identify sources of utility values for various health states of vascular patients. Where possible, and where evidence allowed, utility values were estimated separately for different age groups, sex and treatments (e.g. EVAR vs. OR).
Modelling
Methods
The HES data between 2006/07 and 2017/18 were analysed to provide the main inputs for the simulation. First, it is the information needed to create the simulated patient cohort and vascular centres in England. Second, HES analyses provide regression models that are used in the simulation to predict the hospital costs and outcomes after vascular repairs. Data for utilities and other hospital costs (that are not available from HES data) were searched and extracted from the literature (see Appendix 7).
All costs are reported in Great British pounds at year 2017/18 prices. Costs and outcomes are discounted at 3.5% per year. All statistical analyses and simulations were performed in R. The simulation has an interactive user interface and it can be accessed online at https://modellers.sheffield.ac.uk/vascularmodel/ (accessed 1 December 2020).
Verification and validation
The model’s conceptual structure was validated by the clinical advisory panel. The design process went through several stages starting from the natural disease to the patterns identified from HES data. The clinical advisory panel were involved throughout the process to come up with the final design of the conceptual model.
The model codes were verified internally throughout the model implementation. Simulated patients were checked to make sure that they behaved logically as expected (i.e. their characteristics were changing and they followed expected pathways). The model outputs at different levels (individual, vascular centres, disease groups and whole cohort) were checked against various input combinations to see if they agree with the input data and the conceptual model. Intermediate results were also checked and compared for model consistency.
The regression models that were used for predicting costs and outcomes were developed through several stages and each stage involved an assessment by the clinical advisory panel to agree the final set of regression models to be used in the cost-effectiveness analysis (see Report Supplementary Material 14).
The model data were also compared with raw HES data to establish face validity. The outputs for comparison include the characteristics of the cohort, the allocation of patients to vascular centres before and after the reconfiguration, and various outcomes after vascular repairs, such as in-hospital mortality, length of stay, costs of index admission and long-term survival. A case study of past reconfigurations in the Yorkshire and the Humber region was conducted to validate the main assumptions in the model (see Report Supplementary Material 15).
Abdominal aortic aneurysm model
Methods
Patient cohort
Each patient is assigned a start age, mode of admission (elective vs. non-elective), weekend or weekday admission, comorbidities and index of multiple deprivation score. A proportion of AAA patients are allocated to no-operation deaths (i.e. patients died in hospital with a main diagnosis of AAA and no previous AAA repair). Among those with AAA repairs, a proportion are allocated to complex/suprarenal repairs and the rest are allocated to infrarenal repairs. They are also assigned a ruptured status and a type of repair (EVAR vs. OR). That is, AAA patients are classified into AAA no-operation deaths, infrarenal repairs (EVAR vs. OR) and suprarenal repairs (EVAR vs. OR).
Index admission
The following outcomes were modelled for the index admission of vascular repairs: in-hospital death, length of stay and hospital costs of the admission. The base case assumes that the proportion of AAA no-operation deaths does not change with the reconfiguration.
Post index admission
The main outcome of post index admission progress for AAA cases is OS. Survival models that predict TTD for a specific patient given their characteristics and the centre volume were fitted using HES data for AAA repairs.
Patients with AAA can also experience re-interventions/complications related to their index repairs. The modelling of re-interventions here is to account for their effects on the utilities (HRQoL) and costs of AAA patients only. Six types of re-interventions/complications were modelled based on a recent conceptual model developed by NICE196 and discussions with clinicians: life-threatening re-intervention, serious re-intervention, hernia, lysis of adhesion, bowel resection and other laparotomy interventions (see Appendix 8).
Costs
The costs (and utilities) were sourced from recent good-quality studies that were UK based and had sufficient detail on both costs and utilities. For the long-term costs of AAA, the values were sourced from the HTA report197 of cost-effectiveness analysis based on the EVAR-1 and EVAR-2 trials, which report costs in 2014/15 values. The cost of surveillance was reported as £227, which inflated to 2017/18 using the health services index198 is £234. EVAR patients have one surveillance visit (outpatient attendance and ultrasound) every year and OR patients have one visit every 5 years, which results in £234 and £47 per year for EVAR and OR patients, respectively. The cost of re-intervention was £8670 in 2014/15 values, which inflated to year 2017/18 is £8932. For PSA, because of the lack of data, mean values ± 10% were used.
Utilities
Without the impacts of the surgery and re-interventions, all patients are assumed to have a normal HRQoL, which are taken from a study in 1999199 that reports the UK general population utilities differentiated by age and sex.
Patient disutilities after AAA repair were sourced from the HTA report of cost-effectiveness analysis based on the EVAR-1 and EVAR-2 trials197 and are summarised below in Table 14. The utility value in the first 6 months is lower relative to baseline, with EVAR patients doing better than OR patients, and the utility reverts to baseline after 6 months from the initial operation. However, there is a disutility for 6 months after a re-intervention and in the 6 months prior to death.
| Disutility | Mean | Standard error |
|---|---|---|
| OR 0–6 months disutility | –0.0802 | 0.0119 |
| Difference EVAR vs. OR 0–3 months | 0.0599 | 0.0166 |
| Difference EVAR vs. OR 3–6 months | 0 | – |
| Disutility of re-intervention (for 6 months) | –0.0604 | 0.0258 |
| Disutility before death (for 6 months) | –0.149 | 0.0166 |
Peripheral arterial disease model
Methods
Patient cohort
Each patient is assigned a start age, mode of admission (elective vs. non-elective), weekend or weekday admission, comorbidities and index of multiple deprivation score. A proportion of them are assigned to have a major amputation before the admission with the PAD repair and a proportion of them are also assigned to have a leg ulcer. PAD patients are also assigned a type of PAD repair.
Index admission
The following outcomes were modelled for the index admission of vascular repairs: in-hospital death, length of stay and hospital costs of the admission. The probability of having a major amputation in the index admission is also predicted by regression models.
Post index admission
The main outcomes of post-index admission progress for PAD cases are OS and AFS. Survival models were used for the simulation of PAD post-index admission progress: a model of OS and a model of AFS (defined as death or major amputation).
Costs and utilities
The long-term costs and utilities of PAD were sourced from the HTA report of cost-effectiveness analysis of enhancements to angioplasty for PAD;200 and the data are presented below in Table 15. Costs were inflated to 2017/18 values, and for PSA mean values ± 10% were used. The distributions used for the utilities in the PSA are similar to those in the original HTA report. 6
| Health state | Utility | Monthly costs (£) |
|---|---|---|
| IC | 0.7, normal(0.7, 0.23/280) | 115 |
| CLI | 0.35, normal(0.35, 0.23/280) | 361 |
| Amputation | 0.48, normal(0.48, 0.23/280) | 2200 |
The cost of a hospital admission for major amputation was estimated from the HES data between 2008/9 and 2017/18. First, the cost of a major amputation hospital admission was summarised by financial years. There is a steady increase in the mean cost of a major amputation admission from £14,331 in 2008/9 to £22,135 in 2017/18. The cost data chosen for estimating individual patients’ costs of major amputation admission in the model are those for 2017/18, because this was the most recent year for which data are available. Different distributions were fitted and compared on this cost data using the ‘fitdistrplus’ package in R, and the best distribution was a beta distribution with the following parameters: shape1 (alpha) = 1.024011 and shape2 (beta) = 3.400307. This distribution was used to assign individual patient costs of major amputation admission. Its variance and covariance matrix (Table 16) was used in PSA.
| shape1 | shape2 | |
|---|---|---|
| shape1 | 0.000686 | 0.001975 |
| shape2 | 0.001975 | 0.010405 |
Carotid artery disease model
Methods
Patient cohort
Each patient is assigned a start age, mode of admission (elective vs. non-elective), weekend or weekday admission, comorbidities and index of multiple deprivation score. The patients are classified according to different types of CAD repairs (endovascular vs. OR).
Index admission
The following outcomes were modelled for the index admission of vascular repairs: in-hospital death, length of stay and hospital costs of the admission.
Post index admission
The main outcomes of post index admission progress for CAD cases are OS and stroke-free survival. Survival models were used for the simulation of CAD post-index admission progress: a model of OS and a model of stroke-free survival (defined as death or major stroke re-admissions).
Costs
The long-term costs of CAD were sourced from the HTA report of cost-effectiveness analysis based on the International Carotid Stenting Study, which reported the costs in 2013/14 values. 201 The 5-year follow-up costs were reported as £2204 and £2563 for endarterectomy and stenting, respectively. These costs inflated to year 2017/18 are £2292 and £2665, respectively, which results in annual costs of £458 and £533, respectively. For PSA, the mean values ± 10% were used. Costs for stroke were also modelled separately for endarterectomy and stenting patients. The costs for stroke patients treated by endarterectomy were £7570 in the first year and £1770 in subsequent years. For patients treated by stenting, the costs were £6020 in the first year and £1400 in subsequent years.
Utilities
The baseline utilities after CAD repair were also sourced from the same HTA report,201 which reports the mean utility per patient as 0.766 for both patients treated with endarterectomy and those treated by stenting. For PSA, the mean values ± 10% were used. The utility decrement for stroke was estimated as 0.243 based on the meta-analysis of stroke utilities by Tengs et al. ,202 and a beta distribution with an alpha of 64 and a beta of 200 was used for PSA based on the NICE clinical guidelines for PAD. 203 This was used in the model as a stroke multiplicative factor of 0.757 (i.e. 1 – 0.243) and combined with the baseline utility of 0.766 resulted in a utility of 0.58 for patients who had stroke in the CAD model.
Combined model of service configuration
The three patient-level simulation models for the vascular groups AAA, CAD and PAD were combined to develop an overall model. This model predicts the changes in workloads, distance from patients’ homes to hospitals, hospital costs and outcomes when vascular services are reconfigured (i.e. merging small centres to form larger centres) taking the three main vascular groups into account. The simulation has an interactive user interface (UI) and is deployed as a web-based application that can be accessed online at https://modellers.sheffield.ac.uk/vascularmodel/ (accessed 1 December 2020).
The user interface (UI) allows the users to specify a particular service configuration (a list of available vascular centres) that they want to be modelled and evaluated. It also allows the users to specify the type of analysis (deterministic or probabilistic sensitivity analysis), number of sample iterations and changes in inputs, such as one-off cost of the reconfiguration, probability of switching from OR to EVAR and per cent increase (or decrease) in no-operation deaths owing to the reconfiguration. Figure 15 illustrates these features of the UI.
FIGURE 15.
Example of the specification component of the UI.
The decision problem
The NHS England service specification for vascular services170 and the VSGBI171 currently recommend an AAA volume threshold of 60 cases per year. 171 This was used to define the decision problem at the national level for service configuration: is it cost-effective to reconfigure the current landscape of vascular services in England to achieve this threshold? The baseline option is then the current configuration of services in 2017/18 (the latest year of the data) and the intervention option is a new configuration of services, so that the minimum AAA volume of each vascular centre is 60.
To identify the current configuration of services in 2017/18, the total number of AAA repairs was summarised for each vascular site in the data. Sites carrying out fewer than five AA repairs per annum were considered non-vascular and were excluded. To identify the new configuration of services that satisfies the AAA volume threshold of 60 cases per year for all vascular sites, an iterative process is adopted. First, the vascular site with the minimum volume from the current configuration was identified. Second, this site was excluded from the current configuration and its patients re-allocated to their nearest available sites. Third, the AAA volume for each site was recalculated and the revised values became the current configuration (i.e. excluding the site with minimum volume). Step 1 was repeated until a configuration with no site having a volume fewer than 60 cases per year was identified.
This method identified a baseline configuration of services that included 72 active vascular sites in England in 2017/18 and a new configuration that met a minimum volume threshold of 60 AAA cases per year at all sites. In this new configuration major vascular surgery was transferred from 23 vascular centres with low volume to those with higher volumes; the final arrangement included only 49 active centres. Figure 16 illustrates the AAA volume distribution in the baseline configuration and in the new configuration and Table 17 describes the changes in each region.
FIGURE 16.
Abdominal aortic aneurysm volume distribution at baseline and the new configuration.
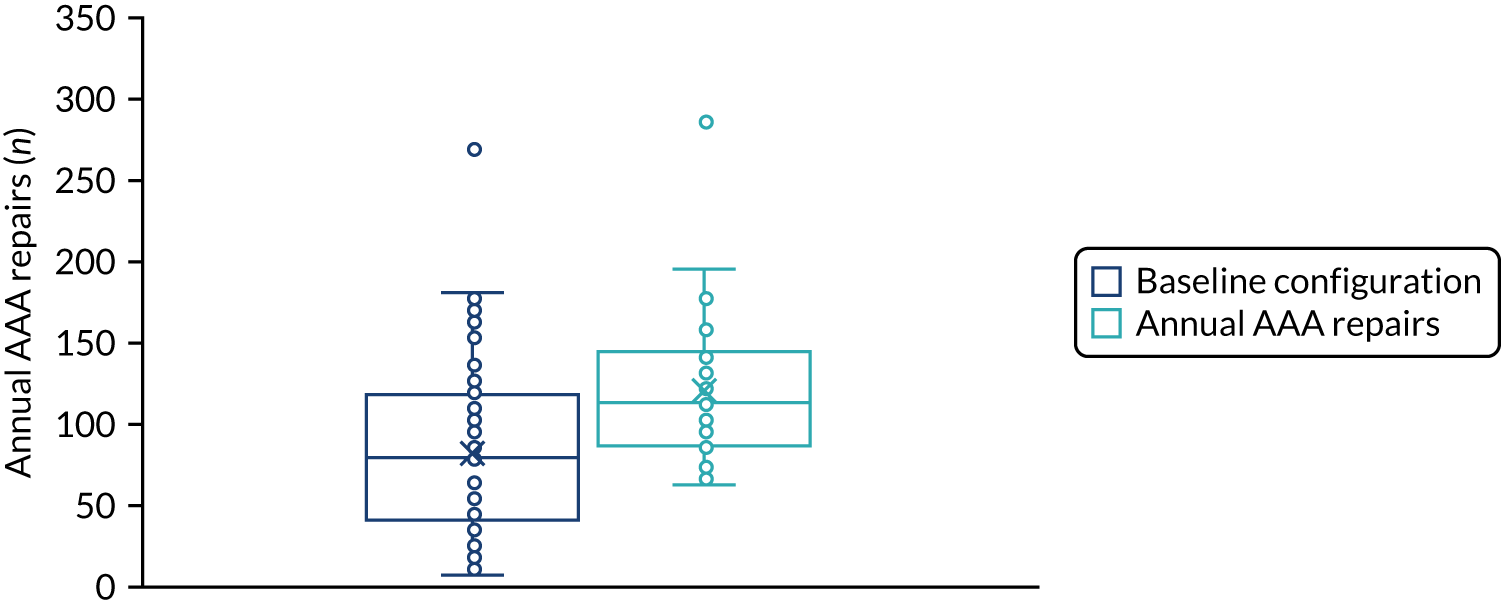
| Region | Active centres | ||
|---|---|---|---|
| Baseline | New | Change | |
| East Midlands | 6 | 4 | –2 |
| East of England | 11 | 7 | –4 |
| London | 11 | 5 | –6 |
| North East | 4 | 3 | –1 |
| North West | 10 | 7 | –3 |
| South East | 7 | 6 | –1 |
| South West | 9 | 6 | –3 |
| West Midlands | 7 | 6 | –1 |
| Yorkshire and the Humber | 7 | 5 | –2 |
| Total | 72 | 49 | –23 |
Base-case deterministic results
Table 18 shows the results of the deterministic model for AAA patients. The reconfiguration reduced in-hospital death (–0.75%), but it had a negative impact on the long-term survival of those who survived the index repairs, which lead to a decrease in the total number of QALYs (–0.056 reduction in discounted QALYs gained per patient). This is consistent with what was observed in the HES data. The reconfiguration increased the costs of index admission (£706 per patient) and the total lifetime costs (including other costs) (£728 per patient).
| Results | Baseline | New configuration | Incremental |
|---|---|---|---|
| Number of patients | 7393 | 7393 | 0 |
| AAA volume per site | 92 | 135 | 43 |
| Mean age (years) | 74.4 | 74.4 | 0.0 |
| Male (%) | 82 | 82 | 0 |
| Length of stay (days) | 10.33 | 10.35 | 0.02 |
| Hospital death (%) | 20.49 | 19.74 | –0.75 |
| Life-years (undiscounted) | 12.524 | 12.330 | –0.194 |
| QALYs (undiscounted) | 9.770 | 9.621 | –0.149 |
| Life-years (discounted) | 9.095 | 9.020 | –0.075 |
| QALYs (discounted) | 7.068 | 7.011 | –0.056 |
| Costs of index admission (£) | 17,021 | 17,727 | 706 |
| Total costs (undiscounted) (£) | 19,069 | 19,787 | 718 |
| Total costs (discounted) (£) | 18,614 | 19,343 | 728 |
Table 19 shows the results of the deterministic model for CAD patients. The reconfiguration reduced in-hospital death (–0.1%) and had a positive impact on long-term survival and stroke-related re-admission-free survival of those who survived the index repairs, leading to an increase in the total number of QALYs (0.078 additional discounted QALYs gained per patient) and an increase in the number of stroke-free days (98 days per patient). The reconfiguration increased the costs of index admission (£103 per patient) but saved resources on other longer-term costs (because there were fewer stroke-related re-admissions). Overall, this led to an increase in the total costs (£79 undiscounted and £101 discounted per patient).
| Results | Baseline | New configuration | Incremental |
|---|---|---|---|
| Number of patients | 5024 | 5024 | 0 |
| Mean age (years) | 70.5 | 70.5 | 0.0 |
| Male (%) | 66 | 66 | 0 |
| Length of stay (days) | 5.1 | 5.0 | –0.1 |
| Hospital death (%) | 1.04 | 0.94 | –0.11 |
| Stroke-free days | 7274 | 7372 | 98 |
| Life-years (undiscounted) | 20.480 | 20.696 | 0.216 |
| QALYs (undiscounted) | 15.585 | 15.760 | 0.175 |
| Life-years (discounted) | 13.299 | 13.395 | 0.096 |
| QALYs (discounted) | 10.139 | 10.217 | 0.078 |
| Costs of index admission (£) | 8991 | 9094 | 103 |
| Total costs (undiscounted) (£) | 19,448 | 19,527 | 79 |
| Total costs (discounted) (£) | 15,507 | 15,607 | 101 |
Table 20 shows the results of the deterministic model for PAD patients. The reconfiguration reduced in-hospital death (–0.12%) and had a positive impact on long-term survival and AFS of those who survived the index repair, leading to an increase in the total number of QALYs (0.035 additional discounted QALYs gained per patient) and an increase in the number of amputation-free days (60 days). The reconfiguration increased the costs of the index admission (£832 per patient) but saved resources on other longer-term costs (because there were fewer amputation-related re-admissions). This led to a reduction in the total costs (–£916 undiscounted and –£15 discounted per patient).
| Results | Baseline | New configuration | Incremental |
|---|---|---|---|
| Number of patients | 22,295 | 22,295 | 0 |
| Mean age (years) | 69 | 69 | 0 |
| Male (%) | 64 | 64 | 0 |
| Length of stay (days) | 8.7 | 9.1 | 0.4 |
| Hospital death (%) | 3.64 | 3.52 | –0.12 |
| Major amputation in index (%) | 2.53 | 2.48 | –0.04 |
| Amputation-free days | 5854.4 | 5914.8 | 60.4 |
| Life-years (undiscounted) | 17.277 | 17.380 | 0.103 |
| QALYs (undiscounted) | 9.861 | 9.939 | 0.078 |
| Life-years (discounted) | 11.256 | 11.306 | 0.050 |
| QALYs (discounted) | 6.369 | 6.404 | 0.035 |
| Costs of index admission (£) | 9436 | 10,268 | 832 |
| Total costs (undiscounted) (£) | 93,398 | 92,482 | –916 |
| Total costs (discounted) (£) | 58,782 | 58,767 | –15 |
Table 21 shows the results of the deterministic model when the whole combined cohort of AAA, PAD and CAD patients was considered. The reconfiguration reduced in-hospital death (–0.25%) and had a positive impact on long-term survival of those who survived the index admission, leading to an increase in the total number of QALYs (0.022 additional discounted QALYs gained per patient). The reconfiguration increased the costs of index admission (£700 per patient) but saved resources on other longer-term costs. This led to a reduction in the total costs when discounting was not included (–£424 per patient) but an increase in the total costs when discounting was included (£160 per patient).
| Results | Baseline | New configuration | Incremental |
|---|---|---|---|
| Number of patients | 34,712 | 34,712 | 0 |
| Mean age (years) | 70 | 70 | 0 |
| Male (%) | 68 | 68 | 0 |
| Length of stay (days) | 8.5 | 8.8 | 0.3 |
| Hospital death (%) | 6.85 | 6.60 | –0.25 |
| Travel distance (miles) | 8.5 | 10.3 | 1.8 |
| Life-years (undiscounted) | 16.728 | 16.784 | 0.056 |
| QALYs (undiscounted) | 10.670 | 10.714 | 0.044 |
| Life-years (discounted) | 11.092 | 11.122 | 0.030 |
| QALYs (discounted) | 7.0631 | 7.0851 | 0.0219 |
| Costs of index admission (£) | 10,987 | 11,687 | 700 |
| Total costs (undiscounted) (£) | 66,864 | 66,440 | –424 |
| Total costs (discounted) (£) | 43,964 | 44,124 | 160 |
| Incremental costs per QALYs (£) | 7312.90 |
The new configuration was associated with an incremental cost per QALY of £7313. This is well below the NICE cost-effectiveness threshold of £20,000 per QALY (λ). The net monetary benefit (NMB) of the reconfiguration of vascular services was £278 per patient at the £20,000 per QALY threshold (NMB = λ × incremental QALYs – incremental costs). Given the population size (34,712 patients), the population NMB was £9,644,639.
Deterministic sensitivity analysis results
Table 22 shows the results from the scenario analysis (see Methods).
| Scenario | AAA | CAD | PAD | All | ICER | ||||
|---|---|---|---|---|---|---|---|---|---|
| Incremental cost (£) | Incremental QALYs | Incremental cost (£) | Incremental QALYs | Incremental cost (£) | Incremental QALYs | Incremental cost (£) | Incremental QALYs | ||
| 0 | 728 | –0.056 | 101 | 0.078 | –15 | 0.035 | 160 | 0.022 | 7313 |
| 1 | 722 | –0.071 | 101 | 0.078 | –15 | 0.035 | 159 | 0.019 | 8448 |
| 2 | 625 | –0.120 | 96 | 0.075 | –14 | 0.034 | 138 | 0.007 | 19,161 |
| 3 | 2125 | 0.685 | 147 | 0.110 | 13 | 0.050 | 482 | 0.194 | 2488 |
| 4 | 731 | –0.027 | 106 | 0.050 | 248 | 0.023 | 330 | 0.017 | 19,891 |
| 5 | 724 | –0.053 | 101 | 0.075 | 95 | 0.034 | 230 | 0.021 | 10,779 |
| 6 | 733 | –0.060 | 100 | 0.081 | –124 | 0.037 | 91 | 0.023 | 4014 |
| 7 | 721 | 0.059 | 111 | 0.011 | 875 | 0.003 | 732 | 0.016 | 45,177 |
Figure 17 shows the results from the one-way sensitivity analysis (see Methods).
FIGURE 17.
Results from the one-way sensitivity analysis. LCI, lower 95% CI; OWSA, one-way sensitivity analysis; UCI, upper 95% CI.
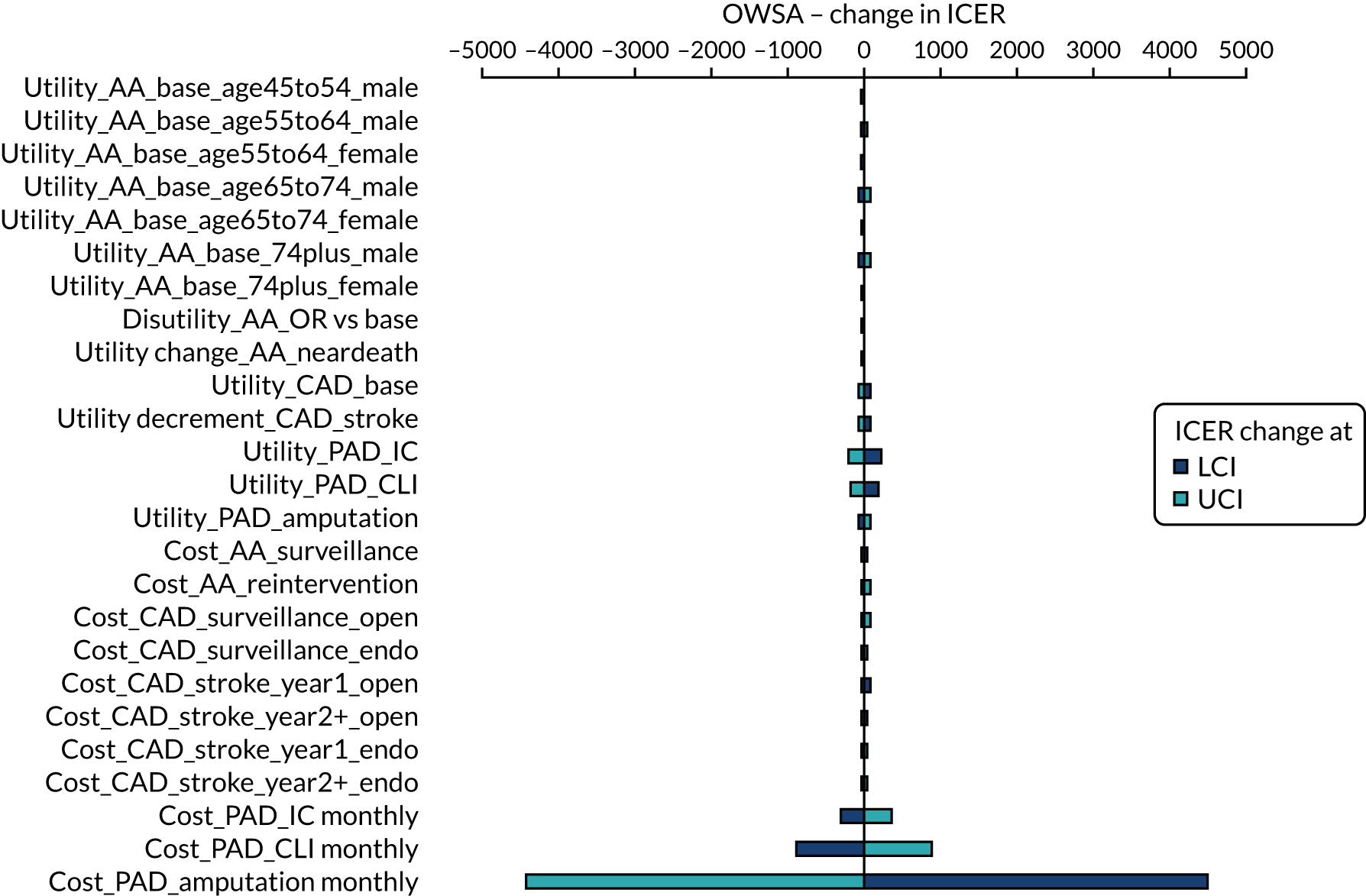
The parameters with the utilities and monthly long-term costs of PAD cases were the most influential on the results, although none of them was sufficient to increase the incremental cost-effectiveness ratio (ICER) to above £20,000 per QALY.
Probabilistic sensitivity analysis results
Figure 18 shows the PSA results for incremental QALYs and incremental cost of the vascular service reconfiguration on a cost-effectiveness plane.
FIGURE 18.
The PSA results on a cost-effectiveness plane.
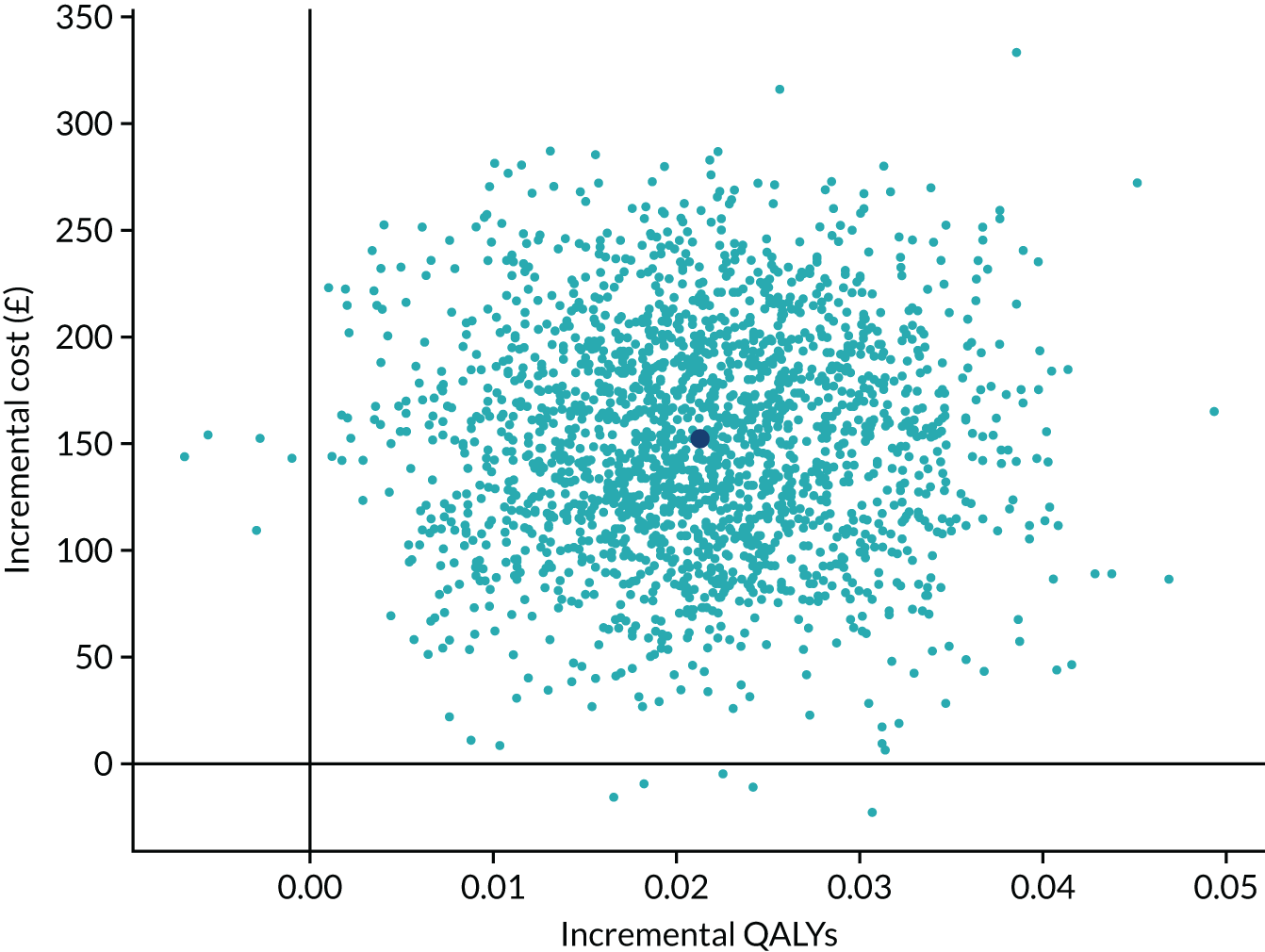
The values of the ICER of the service reconfiguration in PSA crossed into two different quadrants on the cost-effectiveness plane: 0.25% on the south-east (SE) quadrant (dominant – less expensive and more effective) and 0.25% on the north-west (NW) quadrant (dominated – more expensive and less effective). The probabilistic mean ICER was £7169 per QALY (compared with £7313 per QALY in the deterministic results).
Figure 19 shows the cost-effectiveness acceptability curve together with the cost-effectiveness acceptability frontier. At the threshold of £20,000 per QALY, the probability of the service reconfiguration being cost-effective was 96%.
FIGURE 19.
Cost-effectiveness acceptability curve and frontier.
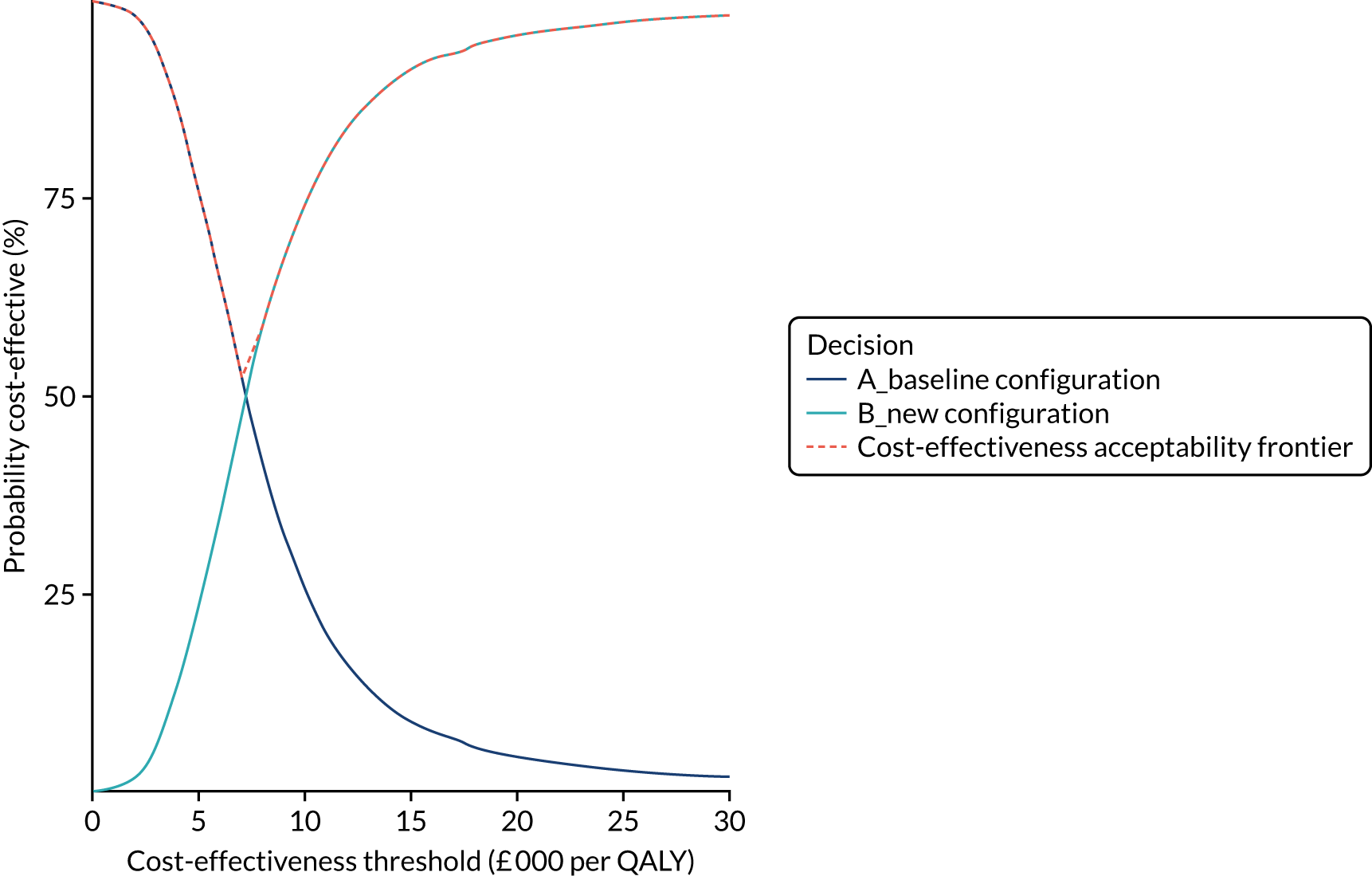
Figure 20 presents the change in individual expected value of perfect information across different values of the cost-effectiveness threshold.
FIGURE 20.
Individual patient-expected value of perfect information.
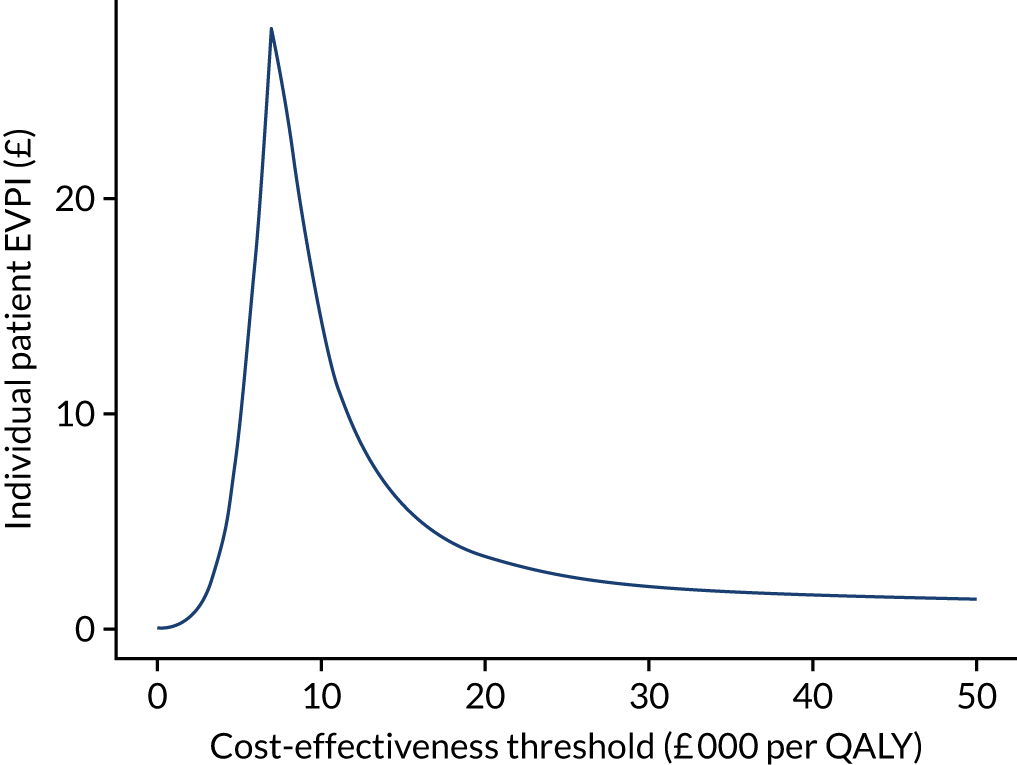
The data analysed in Vascular activity and outcomes from routine data suggests that further improvements in outcome may be achieved by increasing the threshold of activity for AAA to 100 cases per year, rather than the 60 cases considered in this analysis. A further exploratory analysis was, therefore, carried out to consider the implications of a higher threshold (see Report Supplementary Material 16).
Discussion and conclusion
This study is the first model-based economic evaluation study evaluating the cost-effectiveness of a national strategy to reconfigure the vascular services in England. The decision problem concerns the merging of vascular centres with a low annual AAA repair volume and centres with higher volume so that all active vascular centres would have an annual AAA repair volume of at least 60 cases, as recommended by NHS England170 and VSGBI. 171 Using HES data between 2008/9 and 2017/18, the current baseline configuration of services in 2017/18 (72 active vascular centres) was identified. A re-allocation algorithm identified the new service configuration that satisfied the volume threshold; this reconfiguration required transfer of major vascular surgery from 23 vascular centres with low volume to the remaining 49 active centres in the new configuration. The two configurations (baseline and new) were assessed by a patient-level cost-effectiveness simulation that simulated the pathways, costs and outcomes of three patient groups: patients with AAA, CAD and PAD.
The deterministic results suggest that this reconfiguration of vascular services is cost-effective based on the NICE cost-effectiveness threshold of £20,000 per QALY. The incremental cost per QALY (ICER) for the reconfiguration was £7313. This was translated to a NMB of the reconfiguration of vascular services of £278 per patient and £9,644,639 for the whole population at the £20,000 per QALY threshold.
However, the cost-effectiveness results were sensitive to the following assumptions in the model: (1) the assumption about the impact that reconfiguration would have on the proportion of AAA no-operation deaths; (2) the discount rate used in the model; and (3) the assumption about the volume effect on long-term outcomes (i.e. survival) of survivors of the index admissions (see Deterministic sensitivity analysis results). One-way sensitivity analysis on the model parameters on utilities and other costs informed by the literature shows that, varying these parameters, one at a time between their lower confidence interval (CI) value and upper CI value did not cause the cost-effectiveness to exceed £20,000 per QALY. The PSA estimated the mean ICER at £7169 per QALY.
The main strength of the study is that it was based on a patient-level cost-effectiveness simulation that utilised 12 years of HES data between 2006/7 and 2017/18. The richness of information from HES data enabled us to develop a simulation describing all main vascular activities (AAA, PAD and CAD) at three levels: (1) the individual patient level; (2) the hospital level, at which patients are treated; and (3) the regional level, at which patients live. The model was verified and validated with the actual observations in HES data and expert opinions from vascular clinicians. The model’s results were tested extensively in scenario analyses and one-way sensitivity analysis, and the uncertainty with the model’s parameters was quantified in a PSA.
However, there are limitations with the study. First, there are challenges with processing and analysing HES data that include defining the outcome measures and predictors; identifying comorbidities; and various aspects concerning the statistical methods for model development (modelling framework, model fitting, assessment of model performance, recalibration, handling competing risks in survival models, estimating hospital resources used, etc.). 204
Facing the challenges raised by data quality, changes in coding across the years and past service reconfigurations required subjective choice and judgement in the analytical decisions. Such challenges were addressed throughout the modelling process in group discussions combining the clinical and clinical coding, statistical and modelling expertise of the group.
The results of the analyses on the relationship between volume and in-hospital outcomes for AAA patients generally agreed with previous volume–outcome studies;52 however, the results on the relationship between volume and long-term outcomes for those AAA patients who survived and were discharged from the index admissions did not agree with a previous study. 52 Patients discharged from centres with higher AAA volumes had worse long-term survival (even after case-mix adjustment), whereas Holt et al. 54 found the reverse relationship. It should be noted that Holt et al. 54 used HES data between 1 April 2000 and 31 March 2005 (before the specific procedure codes for EVAR were introduced in HES), whereas this analysis used HES data between 1 April 2006 and 31 March 2018 and several aspects of the methods attempted to address the limitations of the methods of Holt et al. 54 In this respect, it is of note that the HES data showed an association between higher volume and higher rates of EVAR, and the recent modelling for the NICE guideline196 on AAA suggested that EVAR is dominated by OR, being both more costly and less effective in the long term. This could potentially explain the apparently poorer long-term outcomes associated with higher-volume sites.
Second, our approach in HES data analysis was relatively simplistic and pragmatic owing to time and resource constraints. Not all possible alternative approaches were tested in developing the risk predictive models, such as machine-learning methods or more advanced multistate survival analysis.
The outpatient data set and the accident and emergency data set in the HES analyses were not used, owing to time and resource constraints. These data sets could improve our estimates of resources used and costs of vascular repairs and, thus, would improve our predictive models in the cost-effectiveness simulation.
Third, data for utilities and other long-term costs used in the model were sought from a rapid systematic review of the literature. Values for these parameters came mainly from three UK HTA reports for AAA, PAD and CAD. It could be argued that a more comprehensive review and data extraction could have been carried out to improve the estimates for these parameters. Nevertheless, the impact of changing these values between their lower and upper CI levels was tested in a one-way sensitivity analysis and it was found that doing so did not significantly change the cost-effectiveness results. Furthermore, the uncertainty with the model’s parameters was assessed in our PSA.
Fourth, our model is based on a fundamental assumption that the associations identified in the regression models reflect direct or indirect causal relationships. The model does not account for many complex influential factors, such as capacity constraints, linked hospital services, the experience of individual vascular surgeons and local factors (e.g. transport links) that may influence real-world choice and the undoubted complex disruptions associated with organisational change. Furthermore, the method of sequentially redistributing workload from the lowest-volume site may not achieve an ideal or optimal configuration, as there may be advantages in transferring services from several sites to one central location. Another issue is the overall selection of procedures carried out at different sites. For example, with appropriate collaboration many minor procedures, investigations and rehabilitation services may be provided at sites different from those carrying out major vascular procedures.
This point may be particularly relevant in the light of the findings of the preference work described in Evaluation of non-health outcomes, which suggests that the strength of preference for local services, when considered in terms of the QALYs that would be traded is high when compared with the average per-patient benefit of the effects of reconfiguration.
The overall economic benefit of reconfiguration is small and uncertain and, furthermore, is not consistent across the three vascular conditions considered: AAA, CAD and PAD. The primary economic benefit from reconfiguration arises from improved CAD outcomes and improved costs and outcomes associated with amputations in PAD. There appears to be a complex trade-off between short- and long-term outcomes in EVAR and OR in AAA that may indicate a potential for further economic gains from reconfiguration that is worthy of further research.
Another limitation of the study is that the modelling was confined to an analysis of the effects on the inpatient aspects of the three main diagnostic areas of AAA, CAD and PAD. Although these represent the majority of workload that may be expected to transfer with the reconfiguration of vascular services, vascular specialists provide services, such as VV surgery, outpatient clinics, joint diabetic foot services and, in some areas, vascular access. It is not necessary that such services are co-located with major vascular inpatient services, and there exist various arrangements through which some of these may be provided in ‘non-vascular’ centres or community settings. However, the evidence in Vascular activity and outcomes from routine data suggests that centralisation of vascular services may be accompanied by changes in practice and relocation of some services. As the arrangements for such services may vary depending on geography and local facilities, application of the model to a specific local reconfiguration would require separate consideration of the intended working arrangements in these respects.
The model provides estimates of the emergency and elective workload, bed requirements and resource use that will support the planning of reconfiguration. However, specific recommendations regarding workforce, bed numbers and facilities in any planned reconfiguration would also require additional local adaptation to account for intended arrangements regarding any ‘non-mandatory’ changes in configuration, such as the location of investigative, minor procedure, rehabilitation and outpatient provision.
Programme outcomes and conclusions
Overview of programme outcomes
The vascular research programme has resulted in the development of two major products: an electronic web-based tool for the collection of PROMs and other clinical data (the ePAQ-VAS), and a tool for the computer simulation of potential service reconfiguration, which can be used to estimate the effects on workload, resource use, outcomes and cost-effectiveness of potential service reconfiguration. In the process of developing these, there have been a number of other outputs from the programme, including publications and analyses. These provided evidence regarding a variety of outcome measures of relevance to vascular services, estimates of utility associated with vascular-related health states, the details of patient preferences for aspects of service provision and the development of methods for the analysis of workload and outcome from routine data.
The electronic Personal Assessment Questionnaire – Vascular
The ePAQ-VAS is a web-based tool for the collection of PROMs and clinical information of relevance to patients using vascular services. It can be completed by patients from home using a web interface, with secure access being provided through a personalised voucher code that can be distributed with an appointment letter or by other means. It may also be completed by patients at the time of outpatient appointments using a tablet or computer workstation. The form is divided into a number of sections relating to generic information and specific sections relating to aneurysm, leg symptoms or CAD. Relevant sections may be completed or skipped depending on the nature of the condition that is being addressed. The form is designed to collect data of clinical relevance but also to produce outcome estimates including the generic EQ-5D and specific domains related to clinical conditions. These can be used to monitor individual patient progress or for overall service evaluation. The reliability and validity of the tool have been established through extensive psychometric evaluation. A demonstration version of the final version of the form is available online at www.epaq.co.uk/Demo/VascularDemo (accessed 1 December 2020).
The vascular services simulation model
A computerised simulation model has been developed with a web-based interface that allows the modelling of potential reconfiguration of the sites providing vascular services in England. The web-based interface allows users to select specific regions and combinations of hospital sites and allows the selection of various parameters for sensitivity analysis. By selecting different sites for a comparison, the effects of reconfiguration of services can be modelled, providing estimates of the overall cost-effectiveness of a reconfiguration along with calculated estimates of the workload in the different categories and resource use required.
Other outcomes
In addition to these two main products, the programme has generated evidence and research outputs from a number of other aspects of the studies. Several papers describe extensive reviews of the evidence relating to outcome measures for different aspects of vascular disease,17–24 the available evidence relating to patient preferences,194 and to the relationship between service configuration and outcomes. 11,13–15
The societal preference studies provide estimates of the strength of preference for EVAR compared with OR and for the strength of preference relating to travelling distance for access to vascular services.
The algorithms developed for the analysis of HES data provide a basis for consistent evaluation of case-mix and outcomes from this complex routine data set.
In addition to the main web-based model, the modelling has provided a detailed analysis of the relationship between activity outcomes and resource use.
Utility values for health states related to conditions dealt with by vascular services have been obtained from two sources: a detailed literature review to support the modelling and direct measurement in a patient sample based on EQ-5D-5L values measured by the ePAQ-VAS. The details of these have been added to the School of Health and Related Research Health Utilities Database (ScHARRHUD) (see www.scharrhud.org/; accessed 1 December 2020), where they are publicly available.
Patient and public involvement
A non-disease-specific PPI group was engaged for the relevant work on the NIHR Vascular Programme Grant.
The PPI group, recruited through the Sheffield Clinical Research Group was used on a number of occasions, for example to review documentation that was required for ethics approval [e.g. participant information sheets and invitation letters (see Appendix 10)].
There were two main periods of interaction with the PPI group, as detailed below:
-
In summer 2016 when the group reviewed study documents [participant information sheet and invitation letter (see Appendix 10)] and participated in mock interviews using the AAA interview schedule (this was relevant to workstream 3).
-
In spring 2017 when the group again participated in mock interviews (this was relevant to workstream 3).
The feedback from the mock interviews was used to revise the interview schedule and interview booklets to ensure that they were designed and delivered in line with the PPI panel recommendations regarding accessibility for all readers and participants.
Appendix 9 outlines a record of the changes that were recommended by the PPI panel to ensure maximum efficacy of the documentation shown in Appendix 10.
Details of the patient and public involvement panel
The PPI panel is a lay advisory panel that is not focused on a specific disease. Further information about the PPI panel is available at www.sheffieldclinicalresearch.org/for-patients-public/how-to-get-involved/ (accessed 1 December 2020).
Publications
The PPI group has been acknowledged in the publications resulting from this aspect of the programme of work. The PPI group lead was made aware of any decisions to include the PPI group in publication acknowledgements to ensure correct reference.
Successes and limitations
Successes
Overall, this has been a very successful programme. A good team was developed and the number of high-quality peer-reviewed publications exceeded expectations. In many areas the work was ground-breaking and required considerable methodological development, alongside more established methods. Some of these are likely to be relevant to further research in other fields. Particular examples of such methodological developments are the processes used for staged identification of appropriate literature relating to outcomes, the methods used to identify and classify vascular activity and outcomes from HES, the development of methods to evaluate process utilities, and the methods used to develop a simulation model of vascular service configuration from HES data.
The research has already had significant impact on NHS services in several areas. The analyses of HES data were used to contribute to another NIHR-funded research project that was commissioned to examine the potential expansion of aneurysm screening to women (the SWAN study). 7
Some initial work has already been carried out in conjunction with the National Aortic Aneurysm Screening Programme (NAASP) to develop a modified version of the ePAQ-VAS to collect additional HRQoL data alongside the screening programme. This has led to the commissioning of further work to pilot and roll out the electronic questionnaire.
The research group also registered as a stakeholder in the recent consultation on the NICE guideline205 on AAA and contributed to the consultation.
Limitations
The most significant problem encountered during the programme was the provision of access to HES data. The original supply of the data extract required for the analysis took nearly 2 years and was partly responsible for significant delays to the programme. When an extension to the programme was granted, a further application was made to update the HES extract with the most recent data. Once again, severe delays were encountered, resulting in a number of complaints to NHS Digital, and the final data extract was received only in February 2019, allowing only a few months for a complex analysis. This limited the ability of the research programme to fully explore the data and realise the full potential from this aspect of the research. A preliminary analysis of the data is included in Vascular activity and outcomes from routine data. A ‘no-cost’ extension to the use of data has been agreed with NHS Digital (in response to a series of formal complaints), so there is potential for further analysis and publications if sufficient resources and personnel can be identified.
In addition to this, the analysis of HES data proved to be far more complex than was originally envisaged. This was largely related to the complexity of the data structures, changes in coding and inconsistencies in the data that required numerous iterations of analysis to provide suitable algorithms for case-mix classification purposes.
There were recruitment challenges for the study, for example in workstream 3 recruitment was slow and difficult. Often, potential participants were called three to five times before a time to do the interview could be arranged, and various people would commit and then not answer on the day. This made it difficult to recruit a sample that was representative of the general population (i.e. having a 50 : 50 sex split). Trusts were asked to provide a more balanced sample and at one point to specifically recruit male participants in an effort to rectify this. The trusts commented that it was generally more difficult to recruit male participants, as they were less willing to participate and less widely available.
Implications for research
The programme has resulted in the development of two main tools that should be beneficial in the planning and evaluation of vascular services; however, there is considerable potential for further work to be carried out on these. The ePAQ-VAS has been developed to a stage at which it is in a useable format and has undergone extensive psychometric evaluation, showing good validity and reliability. The next stage in its development is the wider application in clinical practice with its evaluation both as a clinical tool for monitoring outcomes and as a potential method for collection of data for the evaluation of services. For this purpose, the usability and nature of report formats requires some further development and the tool requires evaluation in different clinical settings and geographical regions.
The web-based model of service provision has considerable potential for further development both in evaluating its usefulness in the real-world situation of regions undergoing a reorganisation of vascular services and in considering its potential applicability to other and more widespread clinical services.
In addition to further work on the tools that have been established by the programme, there are a number of areas that have been highlighted as worthy of further studies:
-
Studies of the drivers of and barriers to organisational change within vascular services.
It is clear from the data that have been analysed that many centres have managed to amalgamate services for major vascular surgery and radiology on a single site. However, there are a number of other areas in which the geographical distribution of population and services would appear to differ little from those where there has been successful reorganisation and yet there remain services that fail to meet the published recommendations for service configuration. It is suggested that detailed qualitative studies with the various stakeholders in such areas may aid an understanding of the barriers to such reconfiguration.
-
Further development of vascular registries and data linkage.
The current procedure-based NVR is an invaluable source of information regarding practice and outcome. However, it is limited by being a procedure-based registry that does not track subsequent procedures and outcomes, collect data relating to selection processes or collect data regarding patients who are turned down for elective or emergency surgery. There is potential for linkage between data sources including HES, ONS statistics and data from the NAASP. This has the potential to provide a more complete picture of practice and outcomes in vascular services.
-
The measurement and application of process utilities.
The studies carried out in this programme suggest that society places a significant value on aspects of the service other than HRQoL outcomes. This includes aspects of the process of care, such as the invasive nature of an intervention and the location of services. This is a new area of research and further investigation is warranted to further develop methods of measurement of process utilities and to investigate the ways in which these may be incorporated into cost-effectiveness analyses.
-
Exploration of variations in practice and coding.
The data analysed for this programme demonstrate considerable variation in practice, resource use and cost between different units. It is unclear from the data the extent to which these represent local variations in the way that patients are managed, geographical differences in case-mix or differences in coding practice. All of these may have significant implications for the cost-effectiveness of the service provided.
Implications for practice and policy
This programme of research has confirmed the potential clinical effectiveness and cost-effectiveness of consolidating services for major vascular surgery on single sites with a higher volume of activity. Considering the existing recommendation for a minimum of 60 aortic aneurysm repairs per year, averaged over a 3-year period,3,171,172 approximately 20% of aneurysm repairs in England continue to be carried out in centres that do not meet this criterion. Data analysis suggests that extending this to a higher threshold of approximately 100 cases per year may have further advantages with regard to clinical outcomes.
Examination of the geographical arrangements suggests that the distances involved, with a few exceptions, are unlikely to be a barrier to such reconfiguration and consideration could be given to how such changes may be achieved.
In considering service reconfiguration, there are a number of other issues that need to be taken into consideration. Patient preferences for local services and the need to maintain links to other specialties would suggest that some form of hub-and-spoke arrangement may allow some services to be provided more locally, where it is safe to do so. Providing services, such as investigations, minor procedures, day-case surgery and amputation rehabilitation, at a spoke site through an integrated hub-and-spoke service would maintain a regular presence of vascular clinicians and health-care professionals and promote linkages with other services. The evidence from the data analysed suggest that there is a trend towards moving such procedures into the central site, rather than maintaining hub-and-spoke arrangements. However, there would appear to be a potential for greater integration of services with outreach or in-reach arrangements that could minimise the movement of resources, pressure on facilities and workforce implications associated with centralisation of major operating. The potential for such distributed services would depend on existing local staffing and facilities, and could be usefully explored through further modelling in any further planned reorganisation of services.
In this respect, there is also a case for considering how preferences for aspects of the service that are not included in current cost-effectiveness calculations may be more formally incorporated in policy-making processes.
The evaluation of PROMs suggests that most of the existing measures that apply to vascular services are poorly validated and not widely implemented, hence the development of the new ePAQ-VAS instrument. There is a need for more widely applicable tools for the collection of such data in vascular patients both for clinical management and for the evaluation of services. It is suggested that the implementation of an appropriate outcome measure, such as the ePAQ-VAS, would be a potentially beneficial development.
There is considerable variation in practice with regard to the introduction of new technology and in coding practices. It is suggested that there is a need for standardisation of procedures for the introduction of new technology, better monitoring of compliance with guidelines and audit of sites that appear to be outliers, with regard to practice or coding, to address such variability.
Acknowledgements
The authors would like to thank the many people who have supported the research programme.
All of the patients and members of the public who participated in the development and piloting of the ePAQ-VAS and the preference elicitation study and those who contributed to the programme through the Sheffield Teaching Hospitals NHS Foundation Trust Online Public Advisory Panel.
Kath Wilson for providing excellent administrative support throughout the programme.
Cliff Shearman, chairperson of the Independent Steering Committee, and the other members of the committee: Paul Roderick, Duncan Ettles, Keith Abrams and Peter Maufe.
The many health-care professionals who have contributed to the various consensus groups, clinical advisory groups and supported the recruitment of patients.
Stephen Walters and John Brazier for providing statistical and methodological advice. The research team also acknowledges the support of the NIHR Clinical Research Network.
Contributions of authors
Jonathan Michaels (https://orcid.org/0000-0002-3422-7102) is the principal investigator and made a substantial contribution to the conception, design, analysis, interpretation and writing up of all aspects of the study.
Emma Wilson (https://orcid.org/0000-0002-4695-2184) was the programme manager throughout the full 6 years of the programme. She was involved with and had oversight of the design, development and implementation of all of the workstreams in the programme. She was involved in the day-to-day research (governance approvals, data collection and data analysis) for workstreams 2 and 3.
Ravi Maheswaran (https://orcid.org/0000-0002-3899-4421) was co-lead for the workstream relating to analysis of HES to identify trends and variation in activity and aspects of case-mix and outcome that can be established from routinely collected data sources. He made a substantial contribution to the conception, design, data acquisition, analysis, interpretation and writing up of the study, particularly in relation to epidemiological aspects.
Stephen Radley (https://orcid.org/0000-0002-3980-7712) reviewed the manuscript, including the electronic web-based questionnaire (ePAQ-VAS) materials and updating the website and associated links. He was responsible for the design and support of web-based questionnaire and associated platform technology.
Georgina Jones (https://orcid.org/0000-0002-5267-1776) was lead for workstream 2. She made a substantial contribution to the conception, design, data acquisition, analysis, interpretation and writing up of the study, particularly in relation to the stages of work that underpinned the development and psychometric testing of the ePAQ-VAS.
Thai-Son Tong (https://orcid.org/0000-0002-9307-6850) was the lead for the workstream relating to the development of the cost-effectiveness model and the analysis of HES data supporting the model development. He was also the main data analyst to manage and process HES data to support other workstreams. He made a substantial contribution to the conception, design, data acquisition, analysis, interpretation and writing up of the study, particularly in relation to HES analysis and modelling studies.
Eva Kaltenthaler (https://orcid.org/0000-0002-6185-8321) contributed to the conception, design, analysis and interpretation of the study, particularly in relation to the systematic reviews of PROMs and volume–outcome relationships.
Ahmed Aber (https://orcid.org/0000-0002-5707-2430) made a significant contribution to the conception, design, data acquisition, analysis, interpretation and writing up of the study, particularly in relation to the systematic reviews of valid PROMs, qualitative systematic reviews, development of the ePAQ-VAS and psychometric analysis of the ePAQ-VAS. He made substantial contribution to HES data analysis and the design of health states to measure the process utilities associated with vascular services reconfiguration.
Andrew Booth (https://orcid.org/0000-0003-4808-3880) made a significant contribution to the conception, design and write up of the qualitative evidence synthesis, providing methodological advice on the use of framework synthesis and advising on the triangulation of concepts from the literature with those from the PROMs.
Helen Buckley Woods (https://orcid.org/0000-0002-0653-9803) made a significant contribution to the project as information specialist, particularly in the systematic reviews of PROMs and volume–outcome relationships, and the qualitative literature review of patients’ perspectives.
James Chilcott (https://orcid.org/0000-0003-1231-7817) contributed to the conception, design, data acquisition, analysis, interpretation and writing up of the study, particularly in relation to the health economic modelling workstream.
Rosie Duncan (https://orcid.org/0000-0003-2712-4743) made a significant contribution to the methods of the qualitative reviews, the data extraction from the systematic reviews and the design and collection of the qualitative interviews that informed the development of the PROMs.
Munira Essat (https://orcid.org/0000-0003-2397-402X) made a significant contribution to the conception, design, data acquisition, analysis, interpretation and writing up of the study, particularly in relation to the systematic reviews of PROMs and volume–outcome relationships and in the identification of economic model parameters.
Edward Goka (https://orcid.org/0000-0002-6754-3312) contributed to the conception, design and writing up (data collection, analysis and interpretation of findings) of the manuscript on relationship between volume and outcomes in lower limb. He also contributed to data extraction, and critically reviewing and revising intellectual content of the manuscripts for the systematic reviews on abdominal aneurysm and carotid artery, and approved the final versions of all the three reviews and the final report.
Aoife Howard (https://orcid.org/0000-0002-1589-5586) made a significant contribution to the conception, design, data collection, analysis, interpretation and writing up of the study, particularly in relation to the large-scale national telephone survey of public preferences for non-health outcomes related to vascular services. Aoife also contributed to systematic reviews of valid PROMs and the impact of vascular conditions on quality of life.
Anju Keetharuth (https://orcid.org/0000-0001-8889-6806) contributed to data collection and analysis of and interpretation of psychometric properties of PROMs in patients with AAA and PAD.
Elizabeth Lumley (https://orcid.org/0000-0002-8962-7568) made a significant contribution to the conception, design, data acquisition, analysis, interpretation and writing up of the study, particularly in relation to the qualitative quality of life study, qualitative systematic reviews and development of the ePAQ-VAS.
Shah Nawaz (https://orcid.org/0000-0002-7282-5816) made a significant contribution to the conception, design, data acquisition, analysis, interpretation and writing up of the study. He was also significantly involved across the workstreams in relation to the ePAQ-VAS, HES and PPI.
Suzy Paisley (https://orcid.org/0000-0002-1739-6852) contributed to the design of systematic reviews including scope definition, literature search design and study selection processes.
Simon Palfreyman (https://orcid.org/0000-0002-5927-1951) contributed to the conception, design, data acquisition and analysis of the study, particularly in relation to PROMs. He was also significantly involved across the workstreams in relation to the ePAQ-VAS, systematic reviews, HES and PPI.
Edith Poku (https://orcid.org/0000-0001-6549-5081) made a substantial contribution to the conception, design, data acquisition, analysis, interpretation and writing up of the study, particularly in relation to the systematic reviews of PROMs and volume–outcome relationships and in the identification of economic model parameters.
Patrick Phillips (https://orcid.org/0000-0003-0241-1527) made a significant contribution to the conception, design, data acquisition, analysis, interpretation and writing up of the study, particularly in relation to the systematic reviews of volume–outcome relationships and PROMs, the development of the ePAQ-VAS and in the evaluation of non-health outcomes.
Gill Rooney (https://orcid.org/0000-0002-8388-9444) made a significant contribution to data collection, analysis and writing up of the final report, in particular with relation to the evaluation of public preferences for non-health outcomes related to vascular services. She also assisted in preparing and co-ordinating the final report for submission.
Praveen Thokala (https://orcid.org/0000-0003-4122-2366) contributed to the conception, design, analysis, interpretation and writing up of the HES analysis and modelling studies.
Steven Thomas (https://orcid.org/0000-0001-5855-2842) made a significant contribution to the conception, design, data acquisition, analysis, interpretation and writing up of the study. He was also significantly involved across the workstreams in relation to the ePAQ-VAS, HES and economic model parameters.
Angela Tod (https://orcid.org/0000-0001-6336-3747) contributed to the conception, design, data acquisition, analysis, interpretation and writing up of the qualitative components of the research programme, especially in the initial 3 years.
Nyantara Wickramasekera (https://orcid.org/0000-0002-6552-5153) made a significant contribution to the conception, design, data collection, analysis, interpretation and writing up of the study, particularly in relation to the large-scale national telephone survey of public preferences for non-health outcomes related to vascular services.
Phil Shackley (https://orcid.org/0000-0002-1862-0596) made a substantial contribution to the conception, design, data acquisition, analysis, interpretation and writing up of the study. He led the workstream relating to the elicitation of public preferences for treatments for abdominal aortic aneurysms and the disutility of having to travel for treatment, and conceived the methodology and study design used in the workstream.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review. Data from Hospital Episode Statistics can be requested through NHS Digital (https://digital.nhs.uk/data-and-information).
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data are vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it is important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health and Social Care.
References
- Wolfe JH. The future of vascular services: the need for a strategy. BMJ 1997;315:695-6. https://doi.org/10.1136/bmj.315.7110.695.
- The Vascular Society of Great Britain and Ireland . The Provision of Services for Patients With Vascular Disease 2012 2012. www.vascularsociety.org.uk/_userfiles/pages/files/Document%20Library/Provision-of-Services-for-Patients-with-Vascular-Disease.pdf (accessed 13 May 2019).
- The Vascular Society of Great Britain and Ireland . The National Vascular Registry: 2018 Annual Report 2018. www.vsqip.org.uk/content/uploads/2018/12/2018-NVR-Annual-Report.pdf (accessed 13 May 2019).
- The Vascular Society of Great Britain and Ireland . Vascular Surgery United Kingdom Workforce Survey 2018 2018. www.vascularsociety.org.uk/_userfiles/pages/files/Document%20Library/Vascular%20Surgery%20Workforce%20Survey%20%202018%20V4_0.pdf (accessed 13 May 2019).
- The Royal College of Radiologists . Clinical Radiology; UK Workforce Census 2017 Report 2017. www.rcr.ac.uk/system/files/publication/field_publication_files/bfcr185_cr_census_2017.pdf (accessed 13 May 2019).
- Michaels J, Brazier J, Palfreyman S, Shackley P, Slack R. Cost and outcome implications of the organisation of vascular services. Health Technol Assess 2000;4. https://doi.org/10.3310/hta4110.
- Thompson SG, Bown MJ, Glover MJ, Jones E, Masconi KL, Michaels JA, et al. Screening women aged 65 years or over for abdominal aortic aneurysm: a modelling study and health economic evaluation. Health Technol Assess 2018;22. https://doi.org/10.3310/hta22430.
- Sweeting MJ, Masconi KL, Jones E, Ulug P, Glover MJ, Michaels JA, et al. Analysis of clinical benefit, harms, and cost-effectiveness of screening women for abdominal aortic aneurysm. Lancet 2018;392:487-95. https://doi.org/10.1016/S0140-6736(18)31222-4.
- The Vascular Society of Great Britain and Ireland . The Provision of Emergency Vascular Services 2001. www.vascularsociety.org.uk/_userfiles/pages/files/Document%20Library/VSGBI-Emergency_3.pdf (accessed 1 December 2020).
- The Vascular Society of Great Britain and Ireland . The National Vascular Registry: 2015 Annual Report 2015. www.vascularsociety.org.uk/_userfiles/pages/files/Resources/NVR-2015-Annual-Report.pdf (accessed 13 May 2019).
- Aber A, Tong TS, Chilcott J, Thokala P, Maheswaran R, Thomas SM, et al. Sex differences in national rates of repair of emergency abdominal aortic aneurysm. Br J Surg 2019;106:82-9. https://doi.org/10.1002/bjs.11006.
- Sidloff DA, Saratzis A, Sweeting MJ, Michaels J, Powell JT, Thompson SG, et al. Sex differences in mortality after abdominal aortic aneurysm repair in the UK. Br J Surg 2017;104:1656-64. https://doi.org/10.1002/bjs.10600.
- Phillips P, Poku E, Essat M, Woods HB, Goka EA, Kaltenthaler EC, et al. Procedure volume and the association with short-term mortality following abdominal aortic aneurysm repair in European populations: a systematic review. Eur J Vasc Endovasc Surg 2017;53:77-88. https://doi.org/10.1016/j.ejvs.2016.10.007.
- Goka EA, Phillips P, Poku E, Essat M, Woods HB, Walters SJ, et al. The relationship between hospital or surgeon volume and outcomes in lower limb vascular surgery in the United Kingdom and Europe. Ann Vasc Surg 2017;45:271-86. https://doi.org/10.1016/j.avsg.2017.04.031.
- Phillips P, Poku E, Essat M, Woods HB, Goka EA, Kaltenthaler EC, et al. Systematic review of carotid artery procedures and the volume–outcome relationship in Europe. Br J Surg 2017;104:1273-83. https://doi.org/10.1002/bjs.10593.
- Duncan R, Essat M, Jones G, Booth A, Buckley Woods H, Poku E, et al. Systematic review and qualitative evidence synthesis of patient-reported outcome measures for abdominal aortic aneurysm. Br J Surg 2017;104:317-27. https://doi.org/10.1002/bjs.10407.
- Poku E, Duncan R, Keetharuth A, Essat M, Phillips P, Woods HB, et al. Patient-reported outcome measures in patients with peripheral arterial disease: a systematic review of psychometric properties. Health Qual Life Outcomes 2016;14. https://doi.org/10.1186/s12955-016-0563-y.
- Essat M, Aber A, Phillips P, Poku E, Woods HB, Howard A, et al. Patient-reported outcome measures in carotid artery revascularization: systematic review and psychometric analysis. Ann Vasc Surg 2018;50:275-83. https://doi.org/10.1016/j.avsg.2017.12.008.
- Aber A, Poku E, Phillips P, Essat M, Buckley Woods H, Palfreyman S, et al. Systematic review of patient-reported outcome measures in patients with varicose veins. Br J Surg 2017;104:1424-32. https://doi.org/10.1002/bjs.10639.
- Poku E, Aber A, Phillips P, Essat M, Buckley Woods H, Palfreyman S, et al. Systematic review assessing the measurement properties of patient-reported outcomes for venous leg ulcers. BJS Open 2017;1:138-47. https://doi.org/10.1002/bjs5.25.
- Aber A, Lumley E, Phillips P, Woods HB, Jones G, Michaels J. Themes that determine quality of life in patients with peripheral arterial disease: a systematic review. Patient 2018;11:489-502. https://doi.org/10.1007/s40271-018-0307-7.
- Aber A, Howard A, Woods HB, Jones G, Michaels J. Impact of carotid artery stenosis on quality of life: a systematic review. Patient 2019;12:213-22. https://doi.org/10.1007/s40271-018-0337-1.
- Lumley E, Phillips P, Aber A, Buckley-Woods H, Jones GL, Michaels JA. Experiences of living with varicose veins: a systematic review of qualitative research. J Clin Nurs 2019;28:1085-99. https://doi.org/10.1111/jocn.14720.
- Phillips P, Lumley E, Duncan R, Aber A, Woods HB, Jones GL, et al. A systematic review of qualitative research; experiences of living with venous leg ulcers. J Adv Nurs 2018;74:550-63. https://doi.org/10.1111/jan.13465.
- Hughes RG, Garnick DW, Luft HS, McPhee SJ, Hunt SS. Hospital volume and patient outcomes. The case of hip fracture patients. Med Care 1988;26:1057-67. https://doi.org/10.1097/00005650-198811000-00004.
- Luft HS, Hunt SS, Maerki SC. The volume–outcome relationship: practice-makes-perfect or selective-referral patterns?. Health Serv Res 1987;22:157-82.
- Awopetu AI, Moxey P, Hinchliffe RJ, Jones KG, Thompson MM, Holt PJE. Systematic review and meta-analysis of the relationship between hospital volume and outcome for lower limb arterial surgery. Br J Surg 2010;97:797-803. https://doi.org/10.1002/bjs.7089.
- Gandjour A, Bannenberg A, Lauterbach KW. Threshold volumes associated with higher survival in health care: a systematic review. Med Care 2003;41:1129-41. https://doi.org/10.1097/01.MLR.0000088301.06323.CA.
- Henebiens M, van den Broek TAA, Vahl AC, Koelemay MJW. Relation between hospital volume and outcome of elective surgery for abdominal aortic aneurysm: a systematic review. Eur J Vasc Endovasc Surg 2007;33:285-92. https://doi.org/10.1016/j.ejvs.2006.10.010.
- Holt PJE, Poloniecki JD, Loftus IM, Thompson MM. Meta-analysis and systematic review of the relationship between hospital volume and outcome following carotid endarterectomy. Eur J Vasc Endovasc Surg 2007;33:645-51. https://doi.org/10.1016/j.ejvs.2007.01.014.
- Holt PJE, Poloniecki JD, Gerrard D, Loftus IM, Thompson MM. Meta-analysis and systematic review of the relationship between volume and outcome in abdominal aortic aneurysm surgery. Br J Surg 2007;94:395-403. https://doi.org/10.1002/bjs.5710.
- Hoornweg LL, Storm-Versloot MN, Ubbink DT, Koelemay MJW, Legemate DA, Balm R. Meta analysis on mortality of ruptured abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2008;35:558-70. https://doi.org/10.1016/j.ejvs.2007.11.019.
- Killeen SD, Andrews EJ, Redmond HP, Fulton GJ. Provider volume and outcomes for abdominal aortic aneurysm repair, carotid endarterectomy, and lower extremity revascularization procedures. J Vasc Surg 2007;45:615-26. https://doi.org/10.1016/j.jvs.2006.11.019.
- Marlow NE, Barraclough B, Collier NA, Dickinson IC, Fawcett J, Graham JC, et al. Effect of hospital and surgeon volume on patient outcomes following treatment of abdominal aortic aneurysms: a systematic review. Eur J Vasc Endovasc Surg 2010;40:572-9. https://doi.org/10.1016/j.ejvs.2010.07.001.
- Wilt TJ, Lederle FA, MacDonald R, Jonk YC, Rector TS, Kane RL. Comparison of endovascular and open surgical repairs for abdominal aortic aneurysm. Evid Rep Technol Assess 2006;144:1-113.
- Shackley P, Slack R, Booth A, Michaels J. Is there a positive volume–outcome relationship in peripheral vascular surgery? Results of a systematic review. Eur J Vasc Endovasc Surg 2000;20:326-35. https://doi.org/10.1053/ejvs.2000.
- Young EL, Holt PJE, Poloniecki JD, Loftus IM, Thompson MM. Meta-analysis and systematic review of the relationship between surgeon annual caseload and mortality for elective open abdominal aortic aneurysm repairs. J Vasc Surg 2007;46:1287-94. https://doi.org/10.1016/j.jvs.2007.06.038.
- Phillips P, Poku E, Essat M, Buckley Woods H, Goka EA, Kaltenthaler E, et al. Volume–Outcome Relationships in Peripheral Vascular Surgery: An Overview of Reviews n.d. https://scharr.dept.shef.ac.uk/vascular-research/wp-content/uploads/sites/9/2016/03/HTAi-2016-Tokyo-PP1.pptx (accessed 11 January 2021).
- Buckley Woods H, Phillips P, Poku E, Essat M, Edward G, Kaltenthaler E, et al. Searching for Evidence for Systematic Reviews of Volume Outcome Relationship in Peripheral Vascular Surgery n.d. https://scharr.dept.shef.ac.uk/vascular-research/wp-content/uploads/sites/9/2016/03/HTAi-2016-Tokyo-HBW.pptx (accessed 11 January 2021).
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339. https://doi.org/10.1136/bmj.b2700.
- Berridge DC, Scott DJ, Beard JD, Hands L. Trials and tribulations of vascular surgical benchmarking. Br J Surg 1998;85:508-10. https://doi.org/10.1046/j.1365-2168.1998.00655.x.
- Moxey PW, Hofman D, Hinchliffe RJ. Volume–outcome relationships in lower extremity arterial bypass surgery. Ann Surg 2012;256:1102-7. https://doi.org/10.1097/SLA.0b013e31825f01d1.
- Elfstrom J, Troeng T, Stubberod A. Adjusting outcome measurements for case-mix in a vascular surgical register – is it possible and desirable?. Eur J Vasc Endovasc Surg 1996;12. https://doi.org/10.1016/S1078-5884(96)80015-3.
- Kantonen I, Lepantalo M, Luther M, Salenius P, Ylönen K. Factors affecting the results of surgery for chronic critical leg ischemiaea–nationwide survey. Finnvasc Study Group. J Vasc Surg 1998;27:940-7. https://doi.org/10.1016/S0741-5214(98)70276-9.
- The Vascular Society of Great Britain and Ireland. Critical limb ischaemia: management and outcome . Report of a national survey. The Vascular Surgical Society of Great Britain and Ireland. Eur J Vasc Endovasc Surg 1995;10:108-13. https://doi.org/10.1016/S1078-5884(05)80206-0.
- Goode SD, Keltie K, Burn J, Patrick H, Cleveland TJ, Campbell B, et al. Effect of procedure volume on outcomes after iliac artery angioplasty and stenting. Br J Surg 2013;100:1189-96. https://doi.org/10.1002/bjs.9199.
- Troeng T, Janzon L, Bergqvist D. Adverse outcome in surgery for chronic leg ischaemiaerisk factors and risk prediction when using different statistical methods. Eur J Vasc Surg 1992;6:628-35. https://doi.org/10.1016/S0950-821X(05)80840-0.
- Bredahl K, Jensen LP, Schroeder TV, Sillesen H, Nielsen H, Eiberg JP. Mortality and complications after aortic bifurcated bypass procedures for chronic aortoiliac occlusive disease. J Vasc Surg 2015;62:75-82. https://doi.org/10.1016/j.jvs.2015.02.025.
- Biancari F, Kantonen I, Albäck A, Mätzke S, Luther M, Lepäntalo, et al. Limits of infrapopliteal bypass surgery for critical leg ischemia: when not to reconstruct. World J Surg 2000;24:727-33. https://doi.org/10.1007/s002689910117.
- Hentschker CRM, Mennicken R. The volume outcome relationship and minimum volume standards – empirical evidence for Germany. Health Econ 2015;24:644-58. https://doi.org/10.1002/hec.3051.
- Trenner M, Haller B, Sollner H, Storck M, Umscheid T, Niedermeier H, et al. Twelve years of the quality assurance registry abdominal aortic aneurysm of the German Vascular Society (DGG): Part 3: Predictors of the perioperative outcome with focus on annual caseload. Gefasschirurgie 2015;20:32-44. https://doi.org/10.1007/s00772-014-1401-3.
- Holt PJE, Poloniecki JD, Loftus IM, Michaels JA, Thompson MM. Epidemiological study of the relationship between volume and outcome after abdominal aortic aneurysm surgery in the UK from 2000 to 2005. Br J Surg 2007;94:441-48. https://doi.org/10.1002/bjs.5725.
- Holt PJE, Poloniecki JD, Khalid U, Hinchliffe RJ, Loftus IM, Thompson MM. Effect of endovascular aneurysm repair on the volume–outcome relationship in aneurysm repair. Circ Cardiovasc Qual Outcomes 2009;2:624-32. https://doi.org/10.1161/CIRCOUTCOMES.109.848465.
- Holt PJE, Karthikesalingam A, Hofman D, Poloniecki JD, Hinchliffe RJ, Loftus IM, et al. Provider volume and long-term outcome after elective abdominal aortic aneurysm repair. Br J Surg 2012;99:666-72. https://doi.org/10.1002/bjs.8696.
- Hafez H. National Vascular Database analysis: the relationship between AAA repair volume and outcome. Br J Surg 2012;99.
- Eckstein HH, Bruckner T, Heider P, Wolf O, Hanke M, Niedermeier HP, et al. The relationship between volume and outcome following elective open repair of abdominal aortic aneurysms (AAA) in 131 German hospitals. Eur J Vasc Endovasc Surg 2007;34:260-6. https://doi.org/10.1016/j.ejvs.2007.05.006.
- Holt PJE, Karthikesalingam A, Poloniecki JD, Hinchliffe RJ, Loftus IM, Thompson MM. Propensity scored analysis of outcomes after ruptured abdominal aortic aneurysm. Br J Surg 2010;97:496-503. https://doi.org/10.1002/bjs.6911.
- Powell JT, Surgery V. Observations from the IMPROVE trial concerning the clinical care of patients with ruptured abdominal aortic aneurysm. Br J Surg 2014;101:216-24. https://doi.org/10.1002/bjs.9410.
- Karthikesalingam A, Holt PJ, Vidal-Diez A, Ozdemir BA, Poloniecki JD, Hinchliffe RJ, et al. Mortality from ruptured abdominal aortic aneurysms: clinical lessons from a comparison of outcomes in England and the USA. Lancet 2014;383:963-9. https://doi.org/10.1016/S0140-6736(14)60109-4.
- Ozdemir BA, Karthikesalingam A, Sinha S, Poloniecki JD, Vidal-Diez A, Hinchliffe RJ, et al. Association of hospital structures with mortality from ruptured abdominal aortic aneurysm. Br J Surg 2015;102:516-24. https://doi.org/10.1002/bjs.9759.
- Sidloff DA, Gokani VJ, Stather PW, Choke E, Bown MJ, Sayers RD. National Vascular Registry Report on surgical outcomes and implications for vascular centres. Br J Surg 2014;101:637-42. https://doi.org/10.1002/bjs.9462.
- Haug ES, Romundstad P, Saether OD, Jørgenvåg R, Myhre HO. Quality of data reported on abdominal aortic aneurysm repair – a comparison between a national vascular and a national administrative registry. Eur J Vasc Endovasc Surg 2005;29:571-8. https://doi.org/10.1016/j.ejvs.2005.02.002.
- Jibawi A, Hanafy M, Guy A. Is there a minimum caseload that achieves acceptable operative mortality in abdominal aortic aneurysm operations?. Eur J Vasc Endovasc Surg 2006;32:273-6. https://doi.org/10.1016/j.ejvs.2006.03.013.
- Karthikesalingam A, Wanhainen A, Holt PJ, Vidal-Diez A, Brownrigg JR, Shpitser I, et al. Comparison of long-term mortality after ruptured abdominal aortic aneurysm in England and Sweden. Br J Surg 2016;103:199-206. https://doi.org/10.1002/bjs.10049.
- Karthikesalingam A, Holt PJ, Vidal-Diez A, Bahia SS, Patterson BO, Hinchliffe RJ, et al. The impact of endovascular aneurysm repair on mortality for elective abdominal aortic aneurysm repair in England and the United States. J Vasc Surg 2016;64:321-7. https://doi.org/10.1016/j.jvs.2016.01.057.
- Holt PJE, Poloniecki JD, Loftus IM, Thompson MM. The relationship between hospital case volume and outcome from carotid endartectomy in England from 2000 to 2005. Eur J Vasc Endovasc Surg 2007;34:646-54. https://doi.org/10.1016/j.ejvs.2007.07.021.
- Kantonen I, Lepäntalo M, Salenius JP, Mätzke S, Luther M, Ylönen K. Influence of surgical experience on the results of carotid surgery. The Finnvasc Study Group. Eur J Vasc Endovasc Surg 1998;15:155-60. https://doi.org/10.1016/S1078-5884(98)80137-8.
- Kragsterman B, Logason K, Ahari A, Troëng T, Parsson H, Bergqvist D. Risk factors for complications after carotid endarterectomy – a population-based study. Eur J Vasc Endovasc Surg 2004;28:98-103. https://doi.org/10.1016/j.ejvs.2004.03.016.
- Kuehnl A, Tsantilas P, Knappich C, Schmid S, König T, Breitkreuz T, et al. Significant association of annual hospital volume with the risk of in hospital stroke or death following carotid endarterectomy but likely not after carotid stenting: secondary data analysis of the statutory German carotid quality assurance database. Circ Cardiovasc Interv 2016;9. https://doi.org/10.1161/CIRCINTERVENTIONS.116.004171.
- Kuhan G, Gardiner ED, Abidia AF, Chetter IC, Renwick PM, Johnson BF, et al. Risk modelling study for carotid endarterectomy. Br J Surg 2001;88:1590-4. https://doi.org/10.1046/j.0007-1323.2001.01938.x.
- Kuhan G, Marshall EC, Abidia AF, Chetter IC, McCollum PT. A Bayesian hierarchical approach to comparative audit for carotid surgery. Eur J Vasc Endovasc Surg 2002;24:505-10. https://doi.org/10.1053/ejvs.2002.1763.
- Lindström D, Jonsson M, Formgren J, Delle M, Rosfors S, Gillgren P. Outcome after 7 years of carotid artery stenting and endarterectomy in Sweden – single centre and national results. Eur J Vasc Endovasc Surg 2012;43. https://doi.org/10.1016/j.ejvs.2012.01.024.
- Loftus IM, Paraskevas KI, Johal A, Waton S, Heikkila K, Naylor AR, et al. Editor’s choice – delays to surgery and procedural risks following carotid endarterectomy in the UK National Vascular Registry. Eur J Vasc Endovasc Surg 2016;52:438-43. https://doi.org/10.1016/j.ejvs.2016.05.031.
- McCollum PT, Da Silva A, Ridler BD, De Cossart L. Audit Committee . Carotid endarterectomy in the UK and Ireland: audit of 30-day outcome. Eur J Vasc Endovasc Surg 1997;14:386-91. https://doi.org/10.1016/S1078-5884(97)80289-4.
- Parlani G, De Rango P, Verzini F, Cieri E, Simonte G, Cao P. RR1. Safety of carotid stenting (CAS) is based on the center experience more than on the individual performance. J Vasc Surg 2012;55:85S-86S. https://doi.org/10.1016/j.jvs.2012.03.210.
- Theiss W, Hermanek P, Mathias K, Brückmann H, Dembski J, Hoffmann FJ, et al. Predictors of death and stroke after carotid angioplasty and stenting: a subgroup analysis of the pro-CAS data. Stroke 2008;39:2325-30. https://doi.org/10.1161/STROKEAHA.108.514356.
- Terwee CB, Mokkink LB, Knol DL, Ostelo RW, Bouter LM, de Vet HC. Rating the methodological quality in systematic reviews of studies on measurement properties: a scoring system for the COSMIN checklist. Qual Life Res 2012;21:651-7. https://doi.org/10.1007/s11136-011-9960-1.
- Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, et al. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Qual Life Res 2010;19:539-49. https://doi.org/10.1007/s11136-010-9606-8.
- Poku E, Essat M, Duncan R, Phillips P, Woods H, Palfreyman S, et al. Patient-Reported Outcome Measures in Patients With Peripheral Artery Disease: Protocol for a Systematic Review 2016.
- Essat M, Poku E, Duncan R, Phillips P, Woods H, Palfreyman S, et al. Patient Reported Outcome Measures in Patients With Abdominal Aortic Aneurysms: A Systematic Review Protocol 2016.
- Essat M, Buckley Woods H, Poku E, Kaltenthaler E, Michaels J. PRM210 - methods used to identify patient-reported outcome measures in vascular diseases. Value Health 2018;21. https://doi.org/10.1016/j.jval.2018.09.2328.
- Lamping DL, Schroter S, Marquis P, Marrel A, Duprat-Lomon I, Sagnier PP. The community-acquired pneumonia symptom questionnaire: a new, patient-based outcome measure to evaluate symptoms in patients with community-acquired pneumonia. Chest 2002;122:920-9. https://doi.org/10.1378/chest.122.3.920.
- Lamping DL, Schroter S, Kurz X, Kahn SR, Abenhaim L. Evaluation of outcomes in chronic venous disorders of the leg: development of a scientifically rigorous, patient-reported measure of symptoms and quality of life. J Vasc Surg 2003;37:410-19. https://doi.org/10.1067/mva.2003.152.
- Fitzpatrick R, Davey C, Buxton MJ, Jones DR. Evaluating patient-based outcome measures for use in clinical trials. Health Technol Assess 1998;2. https://doi.org/10.3310/hta2140.
- Guyatt GH, Deyo RA, Charlson M, Levine MN, Mitchell A. Responsiveness and validity in health status measurement: a clarification. J Clin Epidemiol 1989;42:403-8. https://doi.org/10.1016/0895-4356(89)90128-5.
- Morris C, Janssens A, Allard A, Thompson CJ, Shilling V, Tomlinson R, et al. Informing the NHS Outcomes Framework: evaluating meaningful health outcomes for children with neurodisability using multiple methods including systematic review, qualitative research, Delphi survey and consensus meeting. Health Serv Deliv Res 2014;2. https://doi.org/10.3310/hsdr02150.
- Terwee CB, Bot SD, de Boer MR, van der Windt DA, Knol DL, Dekker J, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol 2007;60:34-42. https://doi.org/10.1016/j.jclinepi.2006.03.012.
- Aber A, Poku E, Phillips P, Essat M, Buckley Woods H, Palfreyman S. Systematic review of patient-reported outcome measures in patients with varicose veins. J Vasc Surg: Venous Lymphat Disord 2018;6. https://doi.org/10.1016/j.jvsv.2017.10.007.
- Essat M, Aber A, Phillips P, Poku E, Wood HB, Howard A, et al. OP27 patient-reported outcome measures in carotid artery revascularization. Int J Tech Assess Health Care 2017;33:12-3. https://doi.org/10.1017/S0266462317001271.
- Smith MJ, Borchard KL, Hinton E, Scott AR. The Australian Vascular Quality of Life Index (AUSVIQUOL): an improved clinical quality of life tool for peripheral vascular disease. Eur J Vasc Endovasc Surg 2007;34:199-205. https://doi.org/10.1016/j.ejvs.2007.02.005.
- Chetter IC, Spark JI, Dolan P, Scott DJ, Kester RC. Quality of life analysis in patients with lower limb ischaemia: suggestions for European standardisation. Eur J Vasc Endovasc Surg 1997;13:597-604. https://doi.org/10.1016/S1078-5884(97)80070-6.
- Chong PFS, Garratt AM, Golledge J, Greenhalgh RM, Davies AH. The intermittent claudication questionnaire: a patient-assessed condition-specific health outcome measure. J Vasc Surg 2002;36:764-71. https://doi.org/10.1016/S0741-5214(02)00131-3.
- Gulati S, Coughlin PA, Hatfield J, Chetter IC. Quality of life in patients with lower limb ischemia; revised suggestions for analysis. J Vasc Surg 2009;49:122-6. https://doi.org/10.1016/j.jvs.2008.08.011.
- Mehta T, Venkata SA, Chetter I, McCollum P. Assessing the validity and responsiveness of disease-specific quality of life instruments in intermittent claudication. Eur J Vasc Endovasc Surg 2006;31:46-52. https://doi.org/10.1016/j.ejvs.2005.08.028.
- Morgan MB, Crayford T, Murrin B, Fraser SC. Developing the Vascular Quality of Life Questionnaire: a new disease-specific quality of life measure for use in lower limb ischemia. J Vasc Surg 2001;33:679-87. https://doi.org/10.1067/mva.2001.112326.
- Tew G, Copeland R, Le Faucheur A, Gernigon M, Nawaz S, Abraham P. Feasibility and validity of self-reported walking capacity in patients with intermittent claudication. J Vasc Surg 2013;57:1227-34. https://doi.org/10.1016/j.jvs.2012.02.073.
- Mazari FAK, Carradice D, Rahman MN, Khan JA, Mockford K, Mehta T, et al. An analysis of relationship between quality of life indices and clinical improvement following intervention in patients with intermittent claudication due to femoropopliteal disease. J Vasc Surg 2010;52:77-84. https://doi.org/10.1016/j.jvs.2010.01.085.
- Coyne KS, Margolis MK, Gilchrist KA, Grandy SP, Hiatt WR, Ratchford A, et al. Evaluating effects of method of administration on Walking Impairment Questionnaire. J Vasc Surg 2003;38:296-304. https://doi.org/10.1016/S0741-5214(03)00312-4.
- Izquierdo-Porrera AM, Gardner AW, Bradham DD, Montgomery PS, Sorkin JD, Powell CC, et al. Relationship between objective measures of peripheral arterial disease severity to self-reported quality of life in older adults with intermittent claudication. J Vasc Surg 2005;41:625-30. https://doi.org/10.1016/j.jvs.2005.01.012.
- McDermott MM, Liu K, Guralnik JM, Martin GJ, Criqui MH, Greenland P. Measurement of walking endurance and walking velocity with questionnaire: validation of the walking impairment questionnaire in men and women with peripheral arterial disease. J Vasc Surg 1998;28:1072-81. https://doi.org/10.1016/S0741-5214(98)70034-5.
- Regensteiner JG, Steiner JF, Panzer RJ, Hiatt WR. Evaluation of walking impairment by questionnaire in patients with peripheral arterial disease. Eur J Vasc Endovasc Surg 1990;2:142-52.
- Spertus J, Jones P, Poler S, Rocha-Singh K. The peripheral artery questionnaire: a new disease-specific health status measure for patients with peripheral arterial disease. Am Heart J 2004;147:301-8. https://doi.org/10.1016/j.ahj.2003.08.001.
- Treat-Jacobson D, Lindquist RA, Witt DR, Kirk LN, Schorr EN, Bronas UG, et al. The PADQOL: development and validation of a PAD-specific quality of life questionnaire. Vasc Med 2012;17:405-15. https://doi.org/10.1177/1358863X12466708.
- Peach G, Romaine J, Holt PJE, Thompson MM, Bradley C, Hinchliffe RJ. Quality of life, symptoms and treatment satisfaction in patients with aortic aneurysm using new abdominal aortic aneurysm-specific patient-reported outcome measures. Br J Surg 2016;103:1012-19. https://doi.org/10.1002/bjs.10182.
- Peach G, Romaine J, Wilson A, Holt PJ, Thompson MM, Hinchliffe RJ, et al. Design of new patient-reported outcome measures to assess quality of life, symptoms and treatment satisfaction in patients with abdominal aortic aneurysm. Br J Surg 2016;103:1003-11. https://doi.org/10.1002/bjs.10181.
- Borchard KLa, Hewitt PM, Wotherspoon S, Scott AR. Australian Vascular Quality of Life Index (Ausviquol): a pilot study of a disease-specific quality of life measure. ANZ J Surg 2006;76:208-13. https://doi.org/10.1111/j.1445-2197.2006.03697.x.
- Mangione CM, Goldman L, Orav EJ, Marcantonio ER, Pedan A, Ludwig LE, et al. Health-related quality of life after elective surgery. J Gen Int Med 1997;12:686-97. https://doi.org/10.1046/j.1525-1497.1997.07142.x.
- Stolker JM, Mahoney EM, Safley DM, Pomposelli FB, Yadav JS, Cohen DJ. Health-related quality of life following carotid stenting versus endarterectomy: results from the SAPPHIRE (Stenting and Angioplasty with Protection in Patients at HIgh Risk for Endarterectomy) trial. JACC Cardiovasc Interv 2010;3:515-23. https://doi.org/10.1016/j.jcin.2010.02.009.
- Attigah N, Kutter J. Assessment of patients’ satisfaction in carotid surgery under local anaesthesia by psychometrical testing – a prospective cohort study. Eur J Vas Endovasc Surg 2011;41:76-82. https://doi.org/10.1016/j.ejvs.2010.08.020.
- Cohen DJS. Health-related quality of life after carotid stenting versus carotid endarterectomy: Results from CREST (Carotid Revascularization Endarterectomy versus Stenting Trial). J Am Col Cardiol 2011;58:1557-65. https://doi.org/10.1016/j.jacc.2011.05.054.
- Hsu LC, Chang FC, Teng MMH, Chern CM, Wong WJ. Impact of carotid stenting in dizzy patients with carotid stenosis. J Chin Med Assoc 2014;77:403-8. https://doi.org/10.1016/j.jcma.2014.05.005.
- Ivanova P, Kikule I, Zvirgzdins V, Krievins D. Quality of life assessment for asymptomatic high-grade carotid stenosis patients before and after carotid endarterectomy. Gaz Med Ital Arch Sci Med 2015;174:33-42.
- Garratt AM, Macdonald LM, Ruta DA, Russell IT, Buckingham JK, Krukowski ZH. Towards measurement of outcome for patients with varicose veins. Qual Health Care 1993;2:5-10. https://doi.org/10.1136/qshc.2.1.5.
- Garratt AM, Ruta DA, Abdalla MI, Buckingham JK, Russell IT. The SF36 health survey questionnaire: an outcome measure suitable for routine use within the NHS?. BMJ 1993;306:1440-4. https://doi.org/10.1136/bmj.306.6890.1440.
- Garratt AM, Ruta DA, Abdalla MI, Russell IT. SF 36 health survey questionnaire: II. Responsiveness to changes in health status in four common clinical conditions. Qual Health Care 1994;3:186-92. https://doi.org/10.1136/qshc.3.4.186.
- Garratt AM, Ruta DA, Abdalla MI, Russell IT. Responsiveness of the SF-36 and a condition-specific measure of health for patients with varicose veins. Qual Life Res 1996;5:223-4. https://doi.org/10.1007/BF00434744.
- Lattimer CR, Kalodiki E, Azzam M, Geroulakos G. The Aberdeen varicose vein questionnaire may be the preferred method of rationing patients for varicose vein surgery. Angiology 2014;65:205-9. https://doi.org/10.1177/0003319712474953.
- Lattimer CR, Kalodiki E, Azzam M, Geroulakos G. Responsiveness of individual questions from the venous clinical severity score and the Aberdeen varicose vein questionnaire. Phlebology 2014;29:43-51. https://doi.org/10.1258/phleb.2012.012080.
- Paty J, Turner-Bowker DM, Elash CA, Wright D. The VVSymQ® instrument: use of a new patient-reported outcome measure for assessment of varicose vein symptoms. Phlebology 2016;31:481-8. https://doi.org/10.1177/0268355515595193.
- Shepherd AC, Gohel MS, Lim CS, Davies AH. A study to compare disease-specific quality of life with clinical anatomical and hemodynamic assessments in patients with varicose veins. J Vasc Surg 2011;53:374-82. https://doi.org/10.1016/j.jvs.2010.09.022.
- Wright DD, Paty J, Turner-Bowker DM, Bradbury A. Psychometric evaluation of a new patient-reported outcome (PRO) symptom diary for varicose veins: VVSymQ® instrument. Patient 2016;9:335-48. https://doi.org/10.1007/s40271-015-0159-3.
- Bland JM, Dumville JC, Ashby RL, Gabe R, Stubbs N, Adderley U, et al. Validation of the VEINES-QOL quality of life instrument in venous leg ulcers: repeatability and validity study embedded in a randomised clinical trial. BMC Cardiovasc Disord 2015;15. https://doi.org/10.1186/s12872-015-0080-7.
- Brown A, Kendall S, Flanagan M, Cottee M. Encouraging patients to self-care – the preliminary development and validation of the VeLUSET©, a self-efficacy tool for venous leg ulcer patients, aged 60 years and over. Int Wound J 2014;11:326-34. https://doi.org/10.1111/iwj.12199.
- Franks PJ, Moffatt CJ. Health related quality of life in patients with venous ulceration: use of the Nottingham health profile. Qual Life Res 2001;10:693-700. https://doi.org/10.1023/A:1013825924765.
- Hareendran A, Doll H, Wild DJ, Moffatt CJ, Musgrove E, Wheatley C, et al. The venous leg ulcer quality of life (VLU-QoL) questionnaire: development and psychometric validation. Wound Repair Regen 2007;15:465-73. https://doi.org/10.1111/j.1524-475X.2007.00253.x.
- Iglesias CP, Birks Y, Nelson EA, Scanlon E, Cullum NA. Quality of life of people with venous leg ulcers: a comparison of the discriminative and responsive characteristics of two generic and a disease specific instruments. Qual Life Res 2005;14:1705-18. https://doi.org/10.1007/s11136-005-2751-9.
- Jull A, Parag V, Walker N, Rodgers A. Responsiveness of generic and disease-specific health-related quality of life instruments to venous ulcer healing. Wound Repair Regen 2010;18:26-30. https://doi.org/10.1111/j.1524-475X.2009.00556.x.
- Palfreyman S. Assessing the impact of venous ulceration on quality of life. Nurs Times 2008;104:34-7.
- Palfreyman S, Michaels J, Brazier J. Development of a tool to examine the effect of venous ulcers on patients’ quality of life. Nurs Stand 2007;21:57-8. https://doi.org/10.7748/ns2007.07.21.45.57.c4585.
- Smith JJ, Guest MG, Greenhalgh RM, Davies AH. Measuring the quality of life in patients with venous ulcers. J Vasc Surg 2000;31:642-9. https://doi.org/10.1067/mva.2000.104103.
- Walters SJ, Morrell CJ, Dixon S. Measuring health-related quality of life in patients with venous leg ulcers. Qual Life Res 1999;8:327-36. https://doi.org/10.1023/A:1008992006845.
- Grant MJ. How does your searching grow? A survey of search preferences and the use of optimal search strategies in the identification of qualitative research. Health Info Libr J 2004;21:21-32. https://doi.org/10.1111/j.1471-1842.2004.00483.x.
- Duncan R, Booth A, Woods HB, Munira E, Phillips P, Poku E, et al. Understanding the Experience and Impact of Living with a Vascular Condition from the Patients Perspective: A Qualitative Evidence Synthesis Protocol. Sheffield: Health Economics and Decision Science, School of Health and Related Research (ScHARR), University of Sheffield; 2016.
- Carroll C, Booth A, Cooper K. A worked example of ‘best fit’ framework synthesis: a systematic review of views concerning the taking of some potential chemopreventive agents. BMC Med Res Methodol 2011;11. https://doi.org/10.1186/1471-2288-11-29.
- Gibson JM, Kenrick M. Pain and powerlessness: the experience of living with peripheral vascular disease. J Adv Nurs 1998;27:737-45. https://doi.org/10.1046/j.1365-2648.1998.00599.x.
- Cunningham M, Swanson V, Pappas E, O’Carroll R, Holdsworth RJ. Illness beliefs and walking behaviour after revascularisation for intermittent claudication: a qualitative study. J Cardiopulm Rehabil Prev 2014;34:195-201. https://doi.org/10.1097/HCR.0000000000000046.
- Wann-Hansson C, Hallberg IR, Klevsgard R, Andersson E. Patients’ experiences of living with peripheral arterial disease awaiting intervention: a qualitative study. Int J Nurs Stud 2005;42:851-62.
- Wann-Hansson C, Hallberg IR, Klevsgard R, Andersson E. The long-term experience of living with peripheral arterial disease and the recovery following revascularisation: a qualitative study. Int J Nurs Stud 2008;45:552-61. https://doi.org/10.1016/j.ijnurstu.2006.11.006.
- Egberg L, Andreassen S, Mattiasson AC. Experiences of living with intermittent claudication. J Vasc Nurs 2012;30:5-10. https://doi.org/10.1016/j.jvn.2011.11.001.
- Treat-Jacobson D, Halverson SL, Ratchford A, Regensteiner JG, Lindquist R, Hirsch AT. A patient-derived perspective of health-related quality of life with peripheral arterial disease. J Nurs Scholarsh 2002;34:55-60. https://doi.org/10.1111/j.1547-5069.2002.00055.x.
- Schorr EN, Peden-McAlpine C, Treat-Jacobson D, Lindquist R. Characterization of the peripheral artery disease symptom experience. Geriatr Nurs 2015;36:293-300. https://doi.org/10.1016/j.gerinurse.2015.03.004.
- Suckow BD, Goodney PP, Nolan BW, Veeraswamy RK, Gallagher P, Cronenwett JL, et al. Domains that determine quality of life in vascular amputees. Ann Vasc Surg 2015;29:722-30. https://doi.org/10.1016/j.avsg.2014.12.005.
- Brännström M, Björck M, Strandberg G, Wanhainen A. Patients’ experiences of being informed about having an abdominal aortic aneurysm – a follow-up case study five years after screening. J Vasc Nurs 2009;27:70-4. https://doi.org/10.1016/j.jvn.2009.04.001.
- Letterstål A, Olofsson P, Forsberg C. Risk attitude and preferences in person’s hypothetically facing open repair of abdominal aortic aneurysm. J Vasc Nurs 2012;30:112-17. https://doi.org/10.1016/j.jvn.2012.04.004.
- Pettersson M, Bergbom I. To be under control. J Cardiovasc Nurs 2013;28:387-95. https://doi.org/10.1097/JCN.0b013e31824bd965.
- Gibson J. Use of qualitative research to analyze patient and clinician decision making in carotid endarterectomy. J Vasc Nurs 2002;20:60-5. https://doi.org/10.1067/mvn.2002.125224.
- Hallin A, Bergqvist D, Fugl-Meyer K, Holmberg L. Areas of concern, quality of life and life satisfaction in patients with peripheral vascular disease. Eur J Vasc Endovasc Surg 2002;24:255-63. https://doi.org/10.1053/ejvs.2002.1647.
- Gibson J, Watkins C. People’s experiences of the impact of transient ischaemic attack and its consequences: qualitative study. J Adv Nurs 2012;68:1707-15. https://doi.org/10.1111/j.1365-2648.2011.05849.x.
- Gibson J, Watkins C. The use of formal and informal knowledge sources in patients’ treatment decisions in secondary stroke prevention: qualitative study. Health Expect 2013;16:e13-23. https://doi.org/10.1111/j.1369-7625.2011.00724.x.
- Franz A, Wann-Hansson C. Patients’ experiences of living with varicose veins and management of the disease in daily life. J Clin Nurs 2015;25:733-41. https://doi.org/10.1111/jocn.13023.
- Hudson BF, Ogden J, Whiteley MS. A thematic analysis of experiences of varicose veins and minimally invasive surgery under local anaesthesia. J Clin Nurs 2015;24:1502-12. https://doi.org/10.1111/jocn.12719.
- Palfreyman SJ, Drewery-Carter K, Rigby K, Michaels JA, Tod AM. Varicose veins: a qualitative study to explore expectations and reasons for seeking treatment. J Clin Nurs 2004;13:332-40. https://doi.org/10.1046/j.1365-2702.2003.00840.x.
- Brown A. Chronic leg ulcers, part 1: do they affect a patients social life?. Br J Nurs 2005;14:894-8. https://doi.org/10.12968/bjon.2005.14.17.19751.
- Brown A. Chronic leg ulcers, part 2: do they affect a patients social life?. Br J Nurs 2005;14:986-1000. https://doi.org/10.12968/bjon.2005.14.18.19888.
- Byrne O, Kelly M. Living with a chronic leg ulcer. J Comm Nurs 2010;24:46-54.
- Charles H. The impact of leg ulcers on patients’ quality of life. Prof Nurs 1995;10.
- Chase SK, Melloni M, Savage A. A forever healing: the lived experience of venous ulcer disease. J Vasc Nurs 1997;15:73-8. https://doi.org/10.1016/S1062-0303(97)90004-2.
- Douglas V. Living with a chronic leg ulcer: an insight into patients’ experiences and feelings. J Wound Care 2001;10:355-60. https://doi.org/10.12968/jowc.2001.10.9.26318.
- Flaherty E. The views of patients living with healed venous leg ulcers. Nurs Stand 2005;19:78-89. https://doi.org/10.7748/ns.19.45.78.s56.
- Hareendran A, Bradbury A, Budd J, Geroulakos G, Hobbs R, Kenkre J, et al. Measuring the impact of venous leg ulcers on quality of life. J Wound Care 2005;14:53-7. https://doi.org/10.12968/jowc.2005.14.2.26732.
- Hopkins A. Disrupted lives: Investigating coping strategies for non-healing leg ulcers. Br J Nurs 2004;13:556-63. https://doi.org/10.12968/bjon.2004.13.9.12972.
- Jones JE. The Prevalence and Experience of Emotional Distress in Patients With Chronic Venous Ulceration 2007.
- Jones JE, Robinson J, Barr W, Carlisle C. Impact of exudate and odour from chronic venous leg ulceration. Nurs Stand 2008;22:53-61. https://doi.org/10.7748/ns2008.07.22.45.53.c6592.
- Krasner D. Painful venous ulcers: themes and stories about living with the pain and suffering. J Wound Ostomy Continence Nurs 1998;25:158-68. https://doi.org/10.1097/00152192-199805000-00008.
- Krasner D. Painful venous ulcers: themes and stories about their impact on quality of life. Ostomy Wound Manage 1998;44:38-42.
- Palfreyman S, Michaels J, Brazier J. Development of a tool to examine the effect of venous ulcers on patients’ quality of life. Nurs Stand 2007;21:57-69. https://doi.org/10.7748/ns.21.45.57.s55.
- Theron B. Quality of Life of Adults With Venous Leg Ulcers 2008.
- Wellborn J, Moceri JT. The lived experiences of persons with chronic venous insufficiency and lower extremity ulcers. J Wound Ostomy Continence Nurs 2014;41:122-6. https://doi.org/10.1097/WON.0000000000000010.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. https://doi.org/10.1016/0021-9681(87)90171-8.
- NHS England . Specialised Vascular Services (Adults): Service Specification 2017. www.england.nhs.uk/wp-content/uploads/2017/06/specialised-vascular-services-service-specification-adults.pdf (accessed 13 May 2019).
- The Vascular Society of Great Britain and Ireland . The Provision of Services for Patients With Vascular Disease 2018 2018. www.vascularsociety.org.uk/_userfiles/pages/files/Document%20Library/VS%202018%20Final.pdf (accessed 13 May 2019).
- The Vascular Society of Great Britain and Ireland . Vascular Reconfiguration Top Tips 2018. www.vascularsociety.org.uk/_userfiles/pages/files/Document%20Library/Vascular%20Reconfiguration%20Top%20Tips%202018.pdf (accessed 13 May 2019).
- National Institute for Health and Care Excellence . Abdominal Aortic Aneurysm: Diagnosis and Management 2018. www.nice.org.uk/guidance/gid-cgwave0769/documents/html-content (accessed 20 May 2019).
- Carr AJ, Higginson IJ. Are quality of life measures patient centred?. BMJ 2001;322:1357-60. https://doi.org/10.1136/bmj.322.7298.1357.
- Wu AW, Snyder C. Getting ready for patient-reported outcomes measures (PROMs) in clinical practice. Healthc Pap 2011;11:48-53. https://doi.org/10.12927/hcpap.2012.22705.
- US Food and Drug Administration . Guidance for Industry – Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims 2009.
- Ritchie J, Spencer L, Huberman AM, Miles MB. The Qualitative Researcher’s Companion. Thousand Oaks, CA: SAGE Publications, Inc.; 2002.
- Hsu C-C, Sandford BA. The Delphi technique: making sense of consensus. PARE 2007.
- Aber A, Phillips P, Lumley E, Radley S, Thomas SM, Nawaz S, et al. Mixed methods study to develop the content validity and the conceptual framework of the electronic patient reported outcome measure for vascular conditions. BMJ Open 2020;10. https://doi.org/10.1136/bmjopen-2019-034154.
- McDowell I, Newell C. Measuring Health: A Guide to Rating Scales and Questionnaires. Oxford University Press; 1996.
- Kline P. A Psychometrics Primer. London: Free Association; 2000.
- Muthén B. A general structural equation model with dichotomous, ordered categorical, and continuous latent variable indicators. Psychometrika 1984;49:115-32. https://doi.org/10.1007/BF02294210.
- Beauducel A, Herzberg PY. On the performance of maximum likelihood versus means and variance adjusted weighted least squares estimation in CFA. Struct Equ Model: Multi J 2006;13:186-203. https://doi.org/10.1207/s15328007sem1302_2.
- Schreiber JB, Nora A, Stage FK, Barlow EA, King J. Reporting structural equation modeling and confirmatory factor analysis results: a review. J Educ Res 2006;99:323-38. https://doi.org/10.3200/JOER.99.6.323-338.
- Brazier J, Deverill M. A checklist for judging preference-based measures of health related quality of life: learning from psychometrics. Health Econ 1999;8:41-5. https://doi.org/10.1002/(SICI)1099-1050(199902)8:1<41::AID-HEC395>3.0.CO;2-#.
- Aber A, Phillips P, Hughes J, Keetharuth AD, Rooney G, Radley S, et al. Electronic personal assessment questionnaire for vascular conditions (ePAQ-VAS): development and validity. Br J Surg 2020;107:1004-12. https://doi.org/10.1002/bjs.11531.
- Streiner DL, Norman GR, Cairney J. Health Measurement Scales: A Practical Guide to Their Development and Use. Oxford: Oxford University Press; 2015.
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Mahwah, NJ: Lawrence Erlbaum Associates; 1988.
- Rees A, Paisley S, Brazier J, Cantrell A, Poku E, Williams K. Development of the Scharr HUD (Health Utilities Database). Value Health 2013;16. https://doi.org/10.1016/j.jval.2013.08.1585.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- King-Kallimanis BL, Oort FJ, Nolte S, Schwartz CE, Sprangers MA. Using structural equation modeling to detect response shift in performance and health-related quality of life scores of multiple sclerosis patients. Qual Life Res 2011;20:1527-40. https://doi.org/10.1007/s11136-010-9844-9.
- Jones G, Brennan V, Jacques R, Wood H, Dixon S, Radley S. Evaluating the impact of a ‘virtual clinic’ on patient experience, personal and provider costs of care in urinary incontinence: a randomised controlled trial. PLOS ONE 2018;13. https://doi.org/10.1371/journal.pone.0189174.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013.
- Wickramasekera N, Howard A, Philips P, Rooney G, Hughes J, Wilson E, et al. Strength of public preferences for endovascular or open aortic aneurysm repair. Br J Surg 2019;106:1775-83. https://doi.org/10.1002/bjs.11265.
- National Institute for Health and Care Excellence (NICE) . Abdominal Aortic Aneurysm: Diagnosis and Management 2020. www.nice.org.uk/guidance/ng156 (accessed 8 December 2020).
- National Institute for Health and Care Excellence (NICE) . Abdominal Aortic Aneurysm: Diagnosis and Management [NG156]. Health Economics Appendix 2020. www.nice.org.uk/guidance/ng156/evidence/y-health-economics-appendix-pdf-255167681407 (accessed 8 December 2020).
- Patel R, Powell JT, Sweeting MJ, Epstein DM, Barrett JK, Greenhalgh RM. The UK EndoVascular Aneurysm Repair (EVAR) randomised controlled trials: long-term follow-up and cost-effectiveness analysis. Health Technol Assess 2018;22. https://doi.org/10.3310/hta22050.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2019. Canterbury: Personal Social Services Research Unit, University of Kent; 2019.
- Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. York: University of York, Centre for Health Economics; 1999.
- Simpson EL, Kearns B, Stevenson MD, Cantrell AJ, Littlewood C, Michaels JA. Enhancements to angioplasty for peripheral arterial occlusive disease: systematic review, cost-effectiveness assessment and expected value of information analysis. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18100.
- Featherstone RL, Dobson J, Ederle J, Doig D, Bonati LH, Morris S, et al. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): a randomised controlled trial with cost-effectiveness analysis. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20200.
- Tengs TO, Lin TH. A meta-analysis of quality-of-life estimates for stroke. PharmacoEconomics 2003;21:191-200. https://doi.org/10.2165/00019053-200321030-00004.
- National Clinical Guideline Centre . National Institute for Health and Clinical Excellence: Guidance. Lower Limb Peripheral Arterial Disease: Diagnosis and Management 2012.
- Bottle R, Gaudoin R, Goudie R, Jones S, Aylin P. Can valid and practical risk-prediction or casemix adjustment models, including adjustment for comorbidity, be generated from English hospital administrative data (Hospital Episode Statistics)? A national observational study. Health Serv Deliv Res 2014;2. https://doi.org/10.3310/hsdr02400.
- National Institute for Health and Care Excellence (NICE) . Abdominal Aortic Aneurysm: Diagnosis and Management 2020.
- NHS Digital . Hospital Episode Statistics Data Dictionary 2020. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics/hospital-episode-statistics-data-dictionary (accessed 1 December 2020).
- NHS Digital . The NHS Digital Classifications Browser n.d. https://classbrowser.nhs.uk/ (accessed 15 March 2021).
- Aber A, Tong T, Chilcott J, Maheswaran R, Thomas SM, Nawaz S, et al. Outcomes of aortic aneurysm surgery in England: a nationwide cohort study using hospital admissions data from 2002 to 2015. BMC Health Serv Res 2019;19. https://doi.org/10.1186/s12913-019-4755-0.
Appendix 1 Vascular activity and outcomes from routine data
Analysis of Hospital Episode Statistics data: general methods
A major part of the first workstream of the programme was the analysis of HES data, as described in summary in Vascular activity and outcomes from routine data, and in more detail in Report Supplementary Materials 1–11, to characterise the workload of vascular services, to identify trends in activity, working practices and outcomes and to relate these to service configuration. This appendix describes the processes involved in this analysis.
Data extract
Hospital Episode Statistics comprises several databases containing the details of all admissions, accident and emergency attendances and outpatient appointments at NHS hospitals in England. It is now managed by NHS Digital and contains extensive information, as described in the HES data dictionary. 206 Data from HES are available through a data access request service that enables bespoke extracts to be obtained from NHS Digital. For the purpose of this programme, a data extract that included all of the major vascular procedures and diagnostic areas was requested in July 2013. The basic structure of the requested information included the available data regarding patient characteristics, geographical information regarding place of residence and treatment, diagnostic and procedural information, and details of the dates and destination for admission and discharge for each episode fitting a set of criteria that were likely to pick up the majority of vascular-related conditions and procedures. Having identified all potentially relevant episodes, data linkage using a pseudo-anonymised identifier was used to obtain all further episodes for the same cohort of patients, details of critical care episodes for those patients and linked data from the ONS obtained from death certification that provided the date and cause of death (see Report Supplementary Material 1).
Owing to numerous iterations and changes in procedure at NHS Digital, there were considerable delays in obtaining the data, which were eventually provided in May 2015. When a request for an extension to the programme was made in the autumn of 2017, a further request was made to update the HES extract; however, because of further delays in changes in procedure, the final extract was not received until February 2019, which left little time for further analysis.
Although the original data extract contained information from 1999 and the updated data extend to March 2018, a decision was made to limit the final analysis to the 12-year period from 2006 to 2018: the most recent 12-year period that was available. This decision was made because, owing to changes in coding, geographical data and provider arrangements, it was felt that it would be difficult to draw clear comparisons with data prior to 2006.
Overview of process
The analysis of HES data was an extensive and time-consuming process because of the very large data sets and complexity of the information. The majority of analyses were carried out through custom programmes written in the R software environment for statistical computing. The processing consisted of a number of stages. Initially, the data were ‘cleaned’ to remove records missing critical information or containing inconsistent data and to identify and remove duplicate records.
The second stage was to identify and characterise individual hospital admissions. The structure of the inpatient records is based on episodes of treatment [finished consultant episodes (FCEs)] that differ from definitions of ‘spells’ or ‘admission’, in that multiple episodes may occur within the same period of admission to hospital for treatment. To produce consistent data, the decision was made to consider admissions in terms of CISs that include the full duration of a patient’s hospital journey from the date of first admission to hospital to the last discharge, in which episodes were overlapping or sequential without a break even if there was a transfer between specialties or hospitals during that period.
To categorise the admission, a series of case-mix categories were defined with the help of a clinical consensus group to classify admissions into clinically meaningful groups. The case-mix groups were ordered in a hierarchy to determine the category of those cases for which there were multiple procedural or diagnostic codes that may assign the case to different clinical groupings. Assignment rules were also developed to identify an index admission to categorise patients with multiple admissions and characterise pathways of care, and to prevent double-counting of patients who may fall into several categories at different times.
Having merged episodes into single records for admissions relating to the specific case-mix groups, further work was carried out to identify the site of treatment to link data with critical care and ONS mortality data. These were used to develop measures of outcome to classify those comorbidities and complications that were identifiable from the data and to carry out an analysis of costs for specific procedures and pathways of care.
Further details of the various process involved are provided in the following sections.
Clinical consensus group
A group of clinicians involved with the management of vascular disease assisted in the classification of HES data. The exact composition of the group varied over the duration of the programme, but included vascular surgeons, physicians, vascular interventional radiologists and clinical nurse specialists. Their input was an essential part of the process in identifying the appropriate categorisation of clinical activity. As can be seen from the detailed descriptions, there are a number of issues where ambiguities or inconsistencies in the coding led to uncertainties in the categorisation of patient groups. Where such difficulties arose, the clinical group considered the various options for categorisation and determined additional information that may be of assistance in determining the optimum categorisation. For example, where there was doubt about whether or not certain procedures were related to vascular disease, the consensus group suggested potential tabulations of data, based on other fields in the data set (e.g. specialty, diagnostic codes or data in linked admissions), that may help to clarify the most appropriate categorisation. This was an iterative process in which further analyses were suggested and data tabulated for further discussion by the clinical consensus group.
Examples of areas for which clinical input was essential in determining appropriate categorisation are the various methods for categorising the treatment of emergency or ruptured AAAs and for distinguishing between amputations for vascular and amputations for other conditions. These specific issues in these cases are discussed in more detail below and in Report Supplementary Materials 2 and 4.
Definition of case-mix groups
To facilitate the analysis, and in keeping with the proposed modelling, the analysis plan involved the development of specific categories of vascular activity for which pathways could be described. In each of these categories, activity was divided into clinically meaningful case-mix groups. This was carried out through an iterative process in which a group of clinicians and expert advisers provided some textual description of appropriate groups and categories. These were then translated into algorithms for identification based on the Office of Population Censuses and Surveys (OPCS)’s and diagnostic codes. Some initial identification using the categorisation was carried out, and there were several iterations where records were sampled both from those included in the various groupings and from those that would be excluded. These were reviewed and, where it became apparent that the categorisation was ambiguous or unsatisfactory, further attempts were made to refine the categorisation by including decision rules based on combinations of diagnostic and procedural codes or other fields that are available from the HES records.
The initial decision was to divide the workload into five case-mix categories:
-
AAA
-
PAD
-
CAD
-
VV
-
venous ulceration.
During the subsequent work, it became evident that the diagnostic codes for venous ulceration were not sufficiently sensitive to differentiate between chronic venous disease and leg or foot ulceration related to PAD. As a result, the PAD and venous ulcer categories were combined to make a single category of peripheral arterial and complex venous disease.
In addition to this, there was found to be a significant vascular workload that was not directly related to these categories, which included conditions for which there were differences in practice between centres and some more specialist procedures. These were treated as an additional category of ‘other vascular procedures’. This category included procedures on the visceral vessels, vascular access procedures, upper limb vascular procedures and arteriovenous malformations.
Initially, consideration was given to using existing coding systems for categorisation; the various versions of the HRG classification were considered but suffered from a number of drawbacks. There have been several iterations of these, with a changing categorisation over the years, and, although it was possible to allocate using the most recent grouper, when this was attempted the expert advisory group felt that the categorisation was not ideal for the purpose of identifying differences in practice that may be significantly related to the service configuration. The main rationale for this was that certain areas lacked the granularity to distinguish what were felt to be important clinical distinctions. Therefore, it was agreed that the categorisation procedure would be undertaken from scratch based on the OPCS and International Classification of Diseases (ICD) codes. 207
As a first step, all OPCS codes relating to vascular surgical procedures (largely L codes) and those relating to amputations (X codes) were considered individually and classified according to whether or not they were likely to be within the remit of vascular services. Those codes that were felt to be non-specific and may or may not relate to vascular services were identified separately. The OPCS codes were assigned to the vascular groups as detailed in the tables in Report Supplementary Material 2. (This gives the final categorisation following the iterative process described below.)
For each of the assigned groups, records were then sampled in two ways. Initially, random cases were drawn from the categories and a clinician reviewed all of the HES fields including age, sex, specialty and the text relating to OPCS, HRG and ICD coding, to establish whether or not they felt that the cases were being correctly categorised.
The second set of checks were carried out by cross-tabulating various aspects of the identified groups, looking at them by the most common primary diagnoses, the main specialty and treatment specialty and any other fields that were felt to be relevant in specific cases.
This process resulted in refinement of a number of the categories and groupings. The following sections describe the specific issues that were encountered and the decisions that were made regarding categorisation for each of the case-mix categories.
Abdominal aortic aneurysm
There were several issues encountered in identifying AAA surgery.
Emergency versus elective admission or procedure
In the HES data record, the OPCS data include separate codes for emergency and elective aortic aneurysm repair (e.g. L18X vs. L19X). There are also different codes for ruptured and non-ruptured aneurysm (e.g. I713 vs. I714) and the codes regarding the admission method distinguished between elective and emergency admission to hospital.
Cross-tabulating the method of admission against either the diagnostic or the OPCS codes suggested that there was considerable inconsistency in the way that the coding was carried out. Following discussions with clinical advisors, it was decided that the best way to handle this was to divide admissions into elective and emergency admissions, irrespective of the categorisation of the procedure or diagnosis. Among the emergency admissions, two categories were separately identified that would be a proxy for ruptured and non-ruptured urgent cases based on a combination of the ICD codes and the delay between admission and first operation.
On inspecting cross-tabulation of these data, it became evident that the combination of using the code for ruptured aneurysm with a delay between admission and surgery of < 3 days, and a code for a non-ruptured aneurysm with surgery on the day of admission resulted in discrimination between two groups of emergency aortic aneurysm, with a considerable difference in mortality. This was felt to reflect a best attempt at a meaningful separation between the clinically distinct categories of ruptured and non-ruptured urgent aneurysm repair.
Aortoiliac and aortobifemoral bypass
The OPCS procedure codes have separate codes for aneurysm repair and aortic bypass (e.g. L19X vs. L21X). The procedure carried out for an aortic aneurysm may sometimes be described as an aortic bypass, and examination of HES records showed that the codes often coexisted. Even where there was no code in the OPCS fields for an aortic aneurysm procedure, a significant number of cases in which there were aortic bypasses included a diagnostic code representing AAA. The aortic bypass procedures were, therefore, amalgamated and, where the ICD codes included a field containing a code for AAA, the procedures were categorised as infrarenal aneurysm repair, whereas those without such an ICD code were categorised as aortic bypass for PAD.
Endovascular aneurysm repair
New OPCS codes for EVAR (stent graft) were introduced in 2005. Prior to this, it is not possible to reliably separate EVAR from open surgical repair. After the introduction of separate codes for endovascular repair, it was found that many cases had records including OPCS codes for both non-specific aneurysm repair and endovascular repair. In some cases, this may be a reflection of multiple procedures within the same admission; however, this is quite an unusual event and is considered separately in relation to identifying cases with multiple operations in the same admission. In cases where the coding represents multiple codes being used for the same procedure the clinical advice, supported by considering differences in mortality rates and hospital stay, suggested that the majority of these were instances of a single endovascular procedure with multiple procedural codes. For this reason, those cases where there were both codes in the record were classified as endovascular repairs.
Juxtarenal and suprarenal aneurysms
Historically, aneurysms above the renal artery were dealt with in cardiothoracic units or a few specialist vascular units; however, with the increasing use of fenestrated and branched endovascular stent grafts, several vascular units are now undertaking significant numbers of these procedures. Examination of the records suggested that the majority of cases classified with codes representing these procedures continue to be carried out at specialist cardiothoracic units. However, a proportion represent juxtarenal aneurysms that fall into the workload of vascular services. In the absence of any better way to categorise these, they were classified on the basis of treatment specialty or, where this was not available, on main specialty, including vascular and general surgery and interventional radiology within the vascular workload. All other cases (most of which were cardiothoracic or cardiology specialties) were excluded.
Aneurysm-related deaths
A proportion of patients admitted as an emergency with a ruptured AAA die either before or during surgery. It felt important to try and capture these, as the selection of patients for intervention may potentially represent a difference owing to different organisational arrangements. We examined the records of a sample of patients who died in hospital and whose diagnostic codes included AAAs. This revealed that there were a significant number of cases in which a procedure, such as laparotomy, a diagnostic procedure or insertion of a venous line, was recorded in the procedure codes or in which there was a death on the day of admission without a procedure. However, there were also other cases for which aneurysm was included as a secondary diagnosis and the main reason for admission appeared to be other than the aneurysm, particularly cardiac or respiratory diagnoses. A vascular group was, therefore, defined in which the treatment and/or main specialty was vascular or general surgery, the primary diagnosis field included the code for AAA and the discharge method identified a death in hospital.
Summary
The considerations above gave rise to a final category of AAA-related admissions with the procedures being grouped as suprarenal and juxtarenal endovascular repair (complex endovascular); suprarenal and juxtarenal OR (complex open); infrarenal endovascular repair; infrarenal OR (to include aortic bypasses with a diagnosis of aneurysm); and patients with a diagnosis of an aneurysm who died without an aortic procedure being recorded.
Peripheral arterial disease
Procedures that were included in the peripheral arterial category covered a wide range of endovascular and open procedures and amputations carried out for lower limb vascular disease. The procedures were divided into 12 categories: aortoiliac bypass, extra-anatomical bypass, femoroproximal and femorodistal bypass, femoral endarterectomy, major and minor amputation, iliac, femoral and unspecified angioplasty, embolectomy, and investigations. Some of these categories required some further refinement, particularly where codes were non-specific.
Aortoiliac bypass
As referred to above, some aortoiliac bypasses were carried out in patients with diagnostic codes for aneurysm, and these were, therefore, classified above with the infrarenal aneurysm repairs. The remainder were categorised as PAD procedures; however, there was an additional group that included non-specific codes for operations on the aorta, such as unspecified bypasses of a segment of aorta (L219) and endarterectomy of aorta NEC (L252). Where the codes were non-specific, further investigation showed that the diagnostic and specialty codes suggested that some of these were carried out for cardiothoracic diagnoses and the cases were, therefore, limited to those with a treatment specialty or main specialty of general or vascular surgery.
Endovascular treatment
The OPCS codes have been modified in recent years to include a number of different endovascular procedures, including transluminal placement of stent, transluminal angioplasty, other transluminal procedures and separate codes for placement of different numbers of bare metallic or drug-eluting stents. Many of these codes do not specify a site; however, there are additional codes for transluminal procedures on the femoral or iliac arteries, but not for procedures distal to the knee. The transluminal procedures were, therefore, divided into three categories of iliac angioplasty, femoral angioplasty and unspecified angioplasty to include all transluminal therapeutic procedures. The unspecified group were found to contain a large number of procedures that were carried out under cardiac or cardiothoracic specialties, and these were, therefore, further filtered on the basis of the treatment or main specialty codes.
Amputation
The majority of lower limb amputations in the UK are carried out for vascular and diabetic disease and, therefore, fall largely within the remit of vascular services. There are, however, some that are carried out for malignancy, trauma or orthopaedic problems. The initial intention was, therefore, to limit amputations on the basis of specialty; however, it became apparent that in some places there is significant crossover, with orthopaedic surgeons carrying out some amputations on patients with diagnoses of diabetes or vascular disease, whereas in other areas vascular or general surgeons would be involved in amputations resulting from non-vascular causes. The amputations were, therefore, selected on the basis of excluding a set of diagnostic codes for trauma and malignancy, leaving a less specific group that could be explored further in relation to vascular treatment pathways.
Carotid disease
Carotid treatments were divided into endovascular treatments (codes L311–L319 and L767) and open procedures that included endarterectomy, carotid body operations and carotid bypass procedures.
Varicose veins
There have been recent changes in the treatments available for VV. Up until 2005/6, the only procedures for which OPCS codes were available were injection sclerotherapy and open surgical procedures. Since 2006/7, additional codes have been introduced for radiofrequency ablation and laser ablation. Where available, the category of VV has been split into five separate groups for open surgery, including high ligation and surgery on the truncal veins, phlebectomy/avulsions, laser treatment, radiofrequency ablation and sclerotherapy.
Venous ulceration was originally intended to be defined as a separate category based on ICD codes; however, sampling of records demonstrated that the codes for leg ulcers were non-specific and sometimes associated with other codes and procedures that suggested complex venous disease or mixed arterial disease. It was, therefore, considered that these would be best analysed as part of the PAD pathway.
Other procedures
There are a large number of other procedures that may potentially fall under the remit of vascular services. Although these include a large number of codes, many of them are not specific to vascular services and the overall numbers that appear to have been admitted under the care of vascular services are relatively small compared with the main diagnostic groups. However, examination of this showed that some were procedures that may represent complications of other treatments for vascular disease, they also contain a large number of therapeutic endovascular procedures, many of which may be carried out by a vascular interventional radiologist, as a service to other specialties and may therefore represent an important group in respect to potential reconfiguration of services. These were, therefore, identified and grouped for subsequent analysis; these resulted in a number of different groups that may require separate consideration.
Arteriovenous malformations
There are separate codes for open and endovascular treatment of arteriovenous malformations; however, these do not specify the treatment site. Although there is the facility for identifying sites within the ICD codes, examination of the records showed that these were used inconsistently and many arteriovenous malformations are treated by other specialties. These were, therefore, divided into two groups for endovascular and open treatments but restricted to those cases in which the treatment and/or main specialty was general surgery, vascular surgery or interventional radiology.
Upper limb procedures
Again, these were divided into open and endovascular procedures on the basis of the OPCS codes.
Vascular access procedures
There is considerable variation in practice as to the specialties that undertake vascular access procedures, particularly those required for cancer treatments and for renal access. In some places these are carried out by vascular surgeons and there is an increasing use of endovascular placement, often with the involvement of interventional radiologists. It was, therefore, decided to include these procedures in the analysis and, for the purpose of categorisation, they were divided into four groups for renal access and other forms of access, each of which were subdivided into endovascular and open procedures.
Visceral and renal artery procedures
These are relatively uncommon procedures and, therefore, renal and visceral procedures were grouped together but subdivided into endovascular and open procedures.
There are a range of other procedures that may contribute to the workload of vascular services; those that are open procedures largely relate to unspecified procedures on arteries and veins, which were found to be unusual on their own, but often combined with another procedure. These were classified as ‘other open vascular procedures’. There is also a wide range of miscellaneous endovascular procedures including therapeutic occlusion of vessels, procedures relating to thrombolysis, embolisation, embolectomy and insertion of filters. Some of these may represent a significant workload for vascular radiology services and they were, therefore, identified as a separate group for further evaluation.
Hierarchy of procedures
To ensure consistency across the entire data set, the first 12 fields containing procedure codes were checked for relevant OPCS codes that could be assigned to one of the above categories. Because each episode contains multiple procedure fields and each admission may have multiple episodes, a process was developed to flag whether or not a specific episode and a specific admission contained codes that would result in assignment to each of the groups. A hierarchy was then developed on the basis of consultation with clinicians to assign those cases that contained codes relating to multiple case-mix groups to the most appropriate primary grouping (see Report Supplementary Material 2).
In general, the rationale was that specific categories were preferred to non-specific ones (e.g. if there were codes for femoral angioplasty and non-specific codes relating to angioplasty, the case would be assigned as a femoral angioplasty). There were some specific instances that required value judgements that were made in consultation with the clinical expert advisers:
-
Cases with both aneurysm procedures and peripheral arterial procedures were categorised as aneurysm. The rationale for this was that when arterial procedures and aneurysm-related procedures are carried out in the same admission, it is often the case that the arterial procedure either is an aspect of the aneurysm treatment (e.g. iliac angioplasty or embolisation related to endovascular aneurysm repair) or might relate to a complication of the aneurysm treatment, such as distal embolisation, bypass or limb loss following aneurysm surgery.
-
In the aneurysm group, those with both endovascular and open procedures were assigned to endovascular procedures. The basis of this was examination of records, which suggested that codes for aneurysm repair are sometimes used in conjunction with endovascular procedures and, where an OR and an endovascular procedure occur separately in the same admission, it was felt most likely that the OR represented a failure of an intended endovascular procedure. This latter point will be investigated further when looking at multiple procedures within the same admission.
-
In the VV category, episodes often contained multiple codes for procedures and the clinical advice was that certain surgical procedures or sclerotherapy may be carried out in conjunction with laser or radiofrequency treatment for truncal veins. Cases where there were codes for the newer procedures were, therefore, assigned to these, with other open procedures taking precedence over avulsions or sclerotherapy.
-
Where groups within the ‘other’ category were present alongside codes for aneurysm, PAD or carotid treatments the case was assigned to one of the last two categories.
-
Although cases were assigned to a single group and category to allow the development of pathways, the flags for each of the individual groups were retained to allow subsequent consideration of patterns of activity in which there were multiple procedures or multiple categories of treatment.
Identification of treatment sites
One of the main aspects of the analysis of HES data was to characterise the relationship between hospital volume and aspects of practice, resource use and outcome to model the effects of changes in service configuration. To do this, it is necessary to identify the sites at which procedures are carried out. The HES data set contains two fields that identify the provider site. The first is ‘procode’, which should contain the provider code for the NHS provider under which the episode is categorised, and the second is ‘sitetret’, which should identify the site at which the treatment takes place. Examination of these fields showed that there is considerable variation in the way in which the codes are used, and this is compounded by changes in organisational structures, as a result of which the relationship between individual sites and NHS providers may have changed over the years.
In theory, all NHS acute trusts should be represented by a three-digit alphanumeric code beginning with the letter R. NHS trust sites are identified by a five-digit alphanumeric in which the first three digits are those of the provider trust and the last two digits identify the specific site. There is, however, considerable inconsistency in the way that these codes are used. In some cases, the full five-digit site appears in the provider code, whereas in others there is a three-digit code or three digits followed by non-specific digits, such as 00 or XX. In some cases, the ‘sitetret’ field contains just two digits representing the site; in others it contains a full five digits. There are some instances of the initials of the hospital being used rather than the published code and some hospitals have multiple site codes representing different wards or units within the hospital.
Expert opinion was that it was important to identify the sites in which procedures were carried out rather than to rely on provider codes alone, as there have been many changes in provider trusts and the merger of several hospitals into a large single provider for administrative processes may or may not be accompanied by changes in the sites at which vascular services are delivered. An exercise was, therefore, carried out to try to identify hospital sites in a consistent way, so that changes in practice with regard to the sites at which procedures are carried out could be comparable across the years of the study. It was felt that this was particularly important for major vascular procedures and, therefore, an attempt was made to identify specifically all sites where elective AAA repair were carried out during the period of study.
To achieve this, all combinations of provider and site code were identified and all combinations of codes were examined individually. Where the provider and site codes did not correspond and map to a consistent specific site, both codes were examined individually to categorise them wherever possible. In many cases, it was possible to identify the site in this way. A further exercise was carried out to identify multiple sites that represented different units or wards within the same hospital and to reclassify sites where the coding had changed owing to organisational codes and to map these onto the current provider sites.
In this way, a look-up table was produced of all sites at which aortic aneurysm repair had been carried out. These were identified by a site identifier (ID) consisting of the current three-digit provider code plus a fourth digit that represented the individual site. Where a provider had only a single site carrying out aortic surgery or where there was insufficient coding to identify the site beyond the three-digit provider code this was designated XYZ0 (where XYZ is the three-digit provider code), and where a specific site was identified these would be numbered XYZ1, XYZ2, etc. In this way, a look-up table was generated that allowed the concatenated provider and site codes to be used to look-up the specific four-digit site identifier. In addition, as some sites had changed name and some hospital moves had taken place, further identification and amalgamation of sites was carried out by matching site postcodes.
Since the introduction of the NAASP, aneurysm services in England have been grouped into 43 screening areas and, based on digitised mapping of these areas, all resident LSOAs and provider sites were classified to a specific screening area for consideration of geographical variations in configuration and practice (see Report Supplementary Material 3).
Identification of admissions
The HES provides records at the level of consultant episode. Where patients are transferred between consultants or units within a single admission, this may generate multiple episodes within the same stay in hospital. Each episode may have duplicate or unique information regarding procedures, diagnoses or other fields within the HES record. To develop patient pathways for specific treatments and conditions it is necessary to combine the episode-level data into admissions. In the majority of cases, a single episode is present in an admission so that the episode start and end dates are the same as the admission and discharge dates; however, where there were multiple episodes it is necessary to aggregate the relevant information across multiple episodes to produce an admission-level set of data.
A significant proportion of episodes do not contain a discharge date, so the first stage in this procedure was to identify admissions as being all unique combinations of admission and anonymised HES ID for the same patient. In some cases, this results in admissions with multiple discharge dates. This may occur if there is a day-case admission followed by re-admission or transfer to another hospital on the same day, or if one or more of the episodes contains an incorrect discharge date.
In developing the admission-level data set, all admissions on the same date for an individual patient were combined while retaining the episode-level data to identify transfers between sites, re-admissions and multiple procedures within the same admission. To create an overall admission record, the discharge dates were aggregated to identify the last discharge date, taken as the end of the full admission.
Examination of the data showed that there are a number of anomalies within the records. For example, there may be multiple admissions that appear to have the same discharge date for the same patient. This may occur if, for example, a patient is discharged and then re-admitted and discharged or dies on the same day. It may also be due to a data entry error. Because the former circumstance in particular is relevant to the outcome of treatment, these episodes were combined into the same admission record by creating a minimum admission date for all admissions with the same end date.
Further evaluation of the data showed that there are numerous anomalous situations in which there appear to be concurrent or overlapping admissions. Theoretically, if the rules for characterising admissions and discharges are followed, there should not be overlapping episodes, or episodes where admission and discharge dates are within another admission. However, the HES data show that this is not uncommon, and examination of records where this occurred suggested different circumstances where it seems to happen. One is commonly associated with admissions for investigations or dialysis, where it would appear that patients may remain in the HES data as apparently being an inpatient in one hospital, while having a separate admission for dialysis or an investigation, often as a day case. The other situation is where there are apparently discrepancies in the recorded admission or discharge date for what appear to be episodes within the same admission.
To allow further analysis of these situations, all overlapping and coincident admissions were combined into a single record based on a combined maximum discharge date and minimum admission date, with those in which there were multiple sites or conflicting dates being flagged for further consideration.
Identification of outcome measures
There are a number of potential outcome measures that can be identified from within HES data and different outcome measures are particularly relevant to particular case-mix categories. This section deals briefly with some of the issues in identifying outcome measures from the routine data set, but further details are provided in Report Supplementary Material 4 and in published papers. 7,11,208
Mortality
For some high-risk procedures within vascular surgery, mortality is an important outcome measure but raises a number of issues regarding the different potential ways to measure this and the ability to correct for differences in case-mix.
The HES records include a field for discharge method that identifies episodes that ended with the death of the patient. In the past, this was commonly used as a method to identify mortality. As discussed above, a single hospital admission or spell of treatment may include multiple episodes, which is particularly true if a patient suffers a complication and is transferred to another specialty or hospital. In view of this, the use of a crude assessment of mortality based on discharge method of individual episodes is likely to underestimate overall mortality. For the current analysis, episodes were combined into continuous impatient spells and in-hospital death was identified if any of the merged episodes included a discharge method indicating that the patient had died. An alternative that is commonly used for describing procedure-related mortality is to include deaths within 30 days of a procedure. This was available through linkage of the episode data to ONS mortality data. Some ambiguity may be created in defining 30-day mortality where there are multiple procedures or no operative procedure during an admission. In these cases, the 30-day mortality was defined as 30 days from the date of admission for unoperated cases and as 30 days from the date of the first index procedure where there were multiple procedures in the same admission. For example, a patient admitted as an emergency may undergo investigative procedures followed by one or more major vascular reconstructions, and in such a case the index date would be taken as the date of the first major procedure.
Both in-hospital death and 30-day mortality were used as potential outcome measures and produced different results, an issue that is discussed further in the results relating to aortic aneurysm repair in Vascular activity and outcomes from routine data.
Re-admissions
The linkage of data allowed identification of patient pathways and re-admissions to hospital; in particular, the NHS measure of re-admission to hospital within 30 days of discharge was identified for all case-mix groups. In some cases, a further categorisation was required, because, for example, it is not uncommon for a patient to be admitted for investigation and then undergo a further subsequent planned admission for further treatment. The nature and type of re-admission was, therefore, looked at on a case-by-case basis for the different categories of admission. Re-admissions for repeat procedures was identified within the full data set and specific groups were identified that were relevant to particular diagnostic categories. Examples include re-admissions for repeat of the same procedure, further operative or endovascular procedures following treatment of PAD, and admissions with a stroke diagnosis following carotid endarterectomy. These categories are dealt with under the discussion of the results for the individual conditions.
Complications and comorbidities
Although HES records provide a rich source of diagnostic and procedural information, it can be difficult to distinguish some conditions that may be pre-existing comorbidities from those that occur as complications of a treatment. A detailed piece of work was carried out to consider complications and comorbidities for different conditions based on existing published categorisation (see Report Supplementary Material 4), but modifying this in the light of advice from the clinical consensus group and making use of evidence from linked episodes prior to the index admission to attempt to separate complications from comorbidities. Further details of this process are described in Report Supplementary Material 4.
The relevance of different comorbidities or complications varies between the different diagnostic categories, and is described in more detail below. For example, in the case of the treatment of CAD, although CVAs are an important complication of the treatment, they may also be a pre-existing condition and indication for the treatment, in which case they are likely to indicate a higher-risk group of patients. By identifying those patients in whom a CVA was identified in a prior admission or the same admission as a cause for emergency admission, some separation of these groups is possible.
Appendix 2 Qualitative interview topic guide
Qualitative interviews topic guide patient number XXXXXXXXXXXXXXXXXX
| Welcome and introduction | Confirm consent – complete form |
| Confirm confidentiality | |
| OK to tape | |
| Can stop any time | |
| History | Could you tell me a little bit about why you are attending the vascular clinic? |
| Symptoms | |
| Diagnosis | |
| Understanding about the disease? | |
| Quality of life | Tell me about your experiences of living with your condition |
| Overall, how would you say your condition affects your quality of life? | |
| Physical symptoms | How does your disease affect you physically? |
| Pain (triggers and relievers, intensity, location) | |
| Mobility (walking distance, stairs) | |
| Sleep/fatigue (getting to sleep, waking up, feeling tired) | |
| Physical functioning | What impact does your condition have on your day-to-day functioning? |
| Ability to get out and about | |
| Daily activities (shopping, self-care, house-hold chores) | |
| Sex life | |
| Exercise | |
| Limited in type of activity you would like to do | |
| What would you say is the most significant impact on your daily life? | |
| Emotional impact | How does this impact on how you feel? |
| Well-being | |
| Mood (emotional, upset, angry, scared) | |
| Health concerns (present, future) | |
| Limitations | |
| Social impact | How does your condition impact on your ability to get out and about and see people? |
| Friends | |
| Family | |
| Work (economic impact, time off work, relationship with colleagues) | |
| Hobbies | |
| Impact on others (support needs) | |
| Self-care | What lifestyle advice have you been given – e.g. smoking cessation, exercise, weight management, etc.? |
| Process of care | Thinking about your experiences at the hospital – how do you feel about the care you have received? |
| Was it a prompt referral? | |
| How are the staff? | |
| Is the clinic easy to get to? | |
| Treatment? | |
| What has gone well/not so well? | |
| What are your views on how long you had to wait in clinic to see medical staff/specialists? | |
| Is there anything you feel could have been improved? | |
| What are your views on the time you had to wait for your procedure following your referral? Would it have made much difference to you if the waiting time had been shorter? | |
| What about follow-up appointments? Were these at the hospital or at your home? | |
| Did you have far to travel for your procedure? | |
| How big a problem would it have been if you’d have had to travel further for your procedure and/or follow-up appointments? | |
| Did you see the same people at each follow-up appointment? If yes, is that something you valued? If no, would you have preferred to see the same people? | |
| Did you have to stay in hospital following your procedure? How long? Would you have preferred a longer or shorter stay? | |
| Apart from the outcome of the procedure itself, what was the most important thing to you about the process of care that you’ve undergone? | |
| Which aspect of care would you most like to see improved? | |
| Closing | Is there anything else you feel would be useful for other people with your condition to improve their care? |
Appendix 3 Abdominal aortic aneurysm trade-off interview booklet
Appendix 4 Carotid artery disease trade-off interview booklet
Appendix 5 Peripheral arterial disease trade-off interview booklet
Appendix 6 List of covariates in volume–outcome regression models
| Covariate | Name | inhosd_AAA | inhosd_CAD | inhosd_PAD | surv_AAA | surv_CAD | surv_PAD | los_AAA | lso_CAD | los_PAD | cost_AAA | cost_CAD | cost_PAD | amp_PAD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| startage | Age (years) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| male | Male (vs. female) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| evar | EVAR (vs. OR) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| imd04 | Index of Multiple Deprivation (2004) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| elective | Admitted as elective | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| weekend | Admitted at weekend (Saturday or Sunday) | em_rup | em | em | ✗ | ✗ | ✗ | em_rup | em | em | em_rup | em | em | em |
| urban | Living in urban area | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| distml | Distance from home to hospital | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| hesyear200708 | hesyear200708 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ✗ | ✗ | ✗ | 1 |
| hesyear200809 | hesyear200809 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| hesyear200910 | hesyear200910 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| hesyear201011 | hesyear201011 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| hesyear201112 | hesyear201112 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| hesyear201213 | hesyear201213 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| hesyear201314 | hesyear201314 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| hesyear201415 | hesyear201415 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| hesyear201516 | hesyear201516 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| hesyear201617 | hesyear201617 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| hesyear201718 | hesyear201718 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| co1 | Coronary artery disease | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| co2 | Heart failure | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| co3 | Cerebrovascular disease | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| co4 | COPD | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| co5 | Peptic ulcer disease | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| co6 | Mild liver disease | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| co7 | Diabetes | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| co8 | Paraplegia and hemiplegia | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| co9 | Renal disease | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| co10 | Cancer | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| co11 | Moderate or severe liver disease | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| co12 | Smoking | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| co13 | Obesity | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| co14 | Primary hypertension | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| co15 | Dyslipidaemia | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| vol | Total annual volume | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| ruptured | AAA ruptured | 1 | ✗ | ✗ | 1 | ✗ | ✗ | 1 | ✗ | ✗ | 1 | ✗ | ✗ | ✗ |
| cad_b4 | Having either TIA, stroke or I652 (occlusion and stenosis of the carotid artery) before index admission | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| amaj_c | Major amputation in index admission | ✗ | ✗ | 1 | ✗ | ✗ | 1 | ✗ | ✗ | 1 | ✗ | ✗ | 1 | ✗ |
| by_subEb | Embolectomy subgroup (ref: aibip_PAD) | ✗ | ✗ | 1 | ✗ | ✗ | 1 | ✗ | ✗ | 1 | ✗ | ✗ | 1 | 1 |
| by_subFdisby | Femorodistal subgroup (ref: aibip_PAD) | ✗ | ✗ | 1 | ✗ | ✗ | 1 | ✗ | ✗ | 1 | ✗ | ✗ | 1 | 1 |
| by_subFmoby | Femoroproximal subgroup (ref: aibip_PAD) | ✗ | ✗ | 1 | ✗ | ✗ | 1 | ✗ | ✗ | 1 | ✗ | ✗ | 1 | 1 |
| death_c | Died in hospital | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | 1 | 1 | 1 | 1 | 1 | 1 | ✗ |
| vulcer_c | Leg ulcer in index admission | ✗ | ✗ | 1 | ✗ | ✗ | 1 | ✗ | ✗ | 1 | ✗ | ✗ | 1 | 1 |
| los_findex | Length of stay in index admission | ✗ | ✗ | ✗ | 1 | 1 | 1 | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| stroke_inad | Stroke in index admission | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| amaj_b4 | Major amputation before index admission | ✗ | ✗ | 1 | ✗ | ✗ | 1 | ✗ | ✗ | 1 | ✗ | ✗ | 1 | 1 |
Appendix 7 List of model parameters informed by literature searches
| vasgroup | group | sub1 | sub2 | Mean | SD |
|---|---|---|---|---|---|
| AA | General | Under 25 | Male | 0.94 | 0.12 |
| AA | General | Under 25 | Female | 0.94 | 0.12 |
| AA | General | 25 to 34 | Male | 0.93 | 0.16 |
| AA | General | 25 to 34 | Female | 0.93 | 0.15 |
| AA | General | 35 to 44 | Male | 0.91 | 0.17 |
| AA | General | 35 to 44 | Female | 0.91 | 0.15 |
| AA | General | 45 to 54 | Male | 0.84 | 0.27 |
| AA | General | 45 to 54 | Female | 0.85 | 0.23 |
| AA | General | 55 to 64 | Male | 0.78 | 0.28 |
| AA | General | 55 to 64 | Female | 0.81 | 0.26 |
| AA | General | 65 to 74 | Male | 0.78 | 0.28 |
| AA | General | 65 to 74 | Female | 0.78 | 0.25 |
| AA | General | 75 + | Male | 0.75 | 0.28 |
| AA | General | 75 + | Female | 0.71 | 0.27 |
| AA | Repair | OSRvsbase | month0to3 | –0.0802 | 0.30 |
| AA | Repair | OSRvsbase | month3to6 | –0.0802 | 0.30 |
| AA | Repair | evarvsOSR | month0to3 | 0.0599 | 0.42 |
| AA | Reintervention | vsbase | upto6month | –0.0604 | 0.65 |
| AA | Neardeath | vsbase | before6month | –0.149 | 0.42 |
| CAD | Base | 0.766 | 0.25 | ||
| CAD | Stroke_decrement | vsperfect | Mean | 0.243 | |
| CAD | Stroke_decrement | vsperfect | Alpha | 64 | |
| CAD | Stroke_decrement | vsperfect | Beta | 200 | |
| PAD | Base | IC | 0.7 | 0.23 | |
| PAD | Base | CLI | 0.35 | 0.23 | |
| PAD | Amputation | 0.48 | 0.23 | ||
| AA | Surveillance_cost | 234 | |||
| AA | Reintervention_cost | 8932 | 893.20 | ||
| CAD | Surveillance_cost | Open | 458 | 45.8 | |
| CAD | Surveillance_cost | Endo | 533 | 53.3 | |
| CAD | Stroke_cost | Open | Year1 | 7570 | 757 |
| CAD | Stroke_cost | Open | Year2onward | 1770 | 177 |
| CAD | Stroke_cost | Endo | Year1 | 6020 | 602 |
| CAD | Stroke_cost | Endo | Year2onward | 1400 | 140 |
| PAD | IC_cost | monthly | 115 | 11.5 | |
| PAD | CLI_cost | monthly | 361 | 36.1 | |
| PAD | Amputation_folcost | monthly | 2200 | 220 |
Appendix 8 Reinterventions following abdominal aortic aneurysm treatment
| Reintervention category | Severity | OPCS codes |
|---|---|---|
| Conversion to OR | Life-threatening reinterventions (classed by EVAR 1 group) | Any AAA OPCS code after primary repair |
| Reintervention for graft infection – open | Divide these into open interventions vs. EVAR re-intervention | |
| Reintervention for graft infection – endovascular | ||
| Reoperation of OR – open | ||
| Known aneurysmal extension above or below original graft – OR | ||
| Known aneurysmal extension above or below original graft – endovascular | ||
| Replacement stent graft | ||
| Fenestrated EVAR | ||
| Axillo – bifemoral bypass graft | Life-threatening reinterventions (classed by EVAR 1 group) | PAD (extra-anatomical) only |
| Staple or ligation (EVAR) | Serious reintervention (classed by EVAR 1 group) | L472 Percutaneous transluminal embolisation of visceral branch of abdominal aorta NEC |
| Embolisation (of endoleak) | L713 Percutaneous transluminal embolisation of artery | |
| Sclerosis of endoleaks | L702 Open embolisation of artery NEC | |
| Femoral–femoral crossover graft | All PAD procedure except extanat | |
| Distal limb procedure/revascularisation – open | ||
| Distal limb procedure/revascularisation – endovascular | ||
| Amputation | ||
| Reintervention for thrombosis of graft limb – endovascular | ||
| False femoral aneurysm – open | ||
| Re-intervention for thrombosis of graft limb – open procedure | ||
| Incisional hernia – open repair | T251, T252, T253, T258, T259, T261, T262, T263, T264, T268, T269 | |
| Laparotomy | Mentioned in reintervention | Y502 Laparotomy approach |
| T301 Reopening of abdomen and re-exploration of intra-abdominal operation site and surgical arrest of postoperative bleeding | ||
| T302 Reopening of abdomen and re-exploration of intra-abdominal operation site NEC | ||
| T303 Reopening of abdomen NEC | ||
| T304 Opening of abdomen and exploration of groin | ||
| T308 Other specified opening of abdomen | ||
| T309 Unspecified opening of abdomen | ||
| Lysis of adhesions | Mentioned in reintervention | T412 Division of band of peritoneum |
| T413 Freeing of adhesions of peritoneum | ||
| T414 Open removal of foreign body from peritoneum | ||
| T415 Freeing of extensive adhesions of peritoneum | ||
| T418 Other specified other open operations on peritoneum | ||
| T419 Unspecified other open operations on peritoneum | ||
| Bowel resection | Mentioned in reintervention | H041–H139 |
Appendix 9 Feedback from the Patient Advisory Panel
Online Patient Advisory Panel: reviewer form
Please complete this form electronically and return to xxxxxxxxxxxx by the agreed deadline.
We recommend that you read all the information we send you first, before completing the review.
Please have a common sense practical view by ensuring that your comments are meaningful, considered and informed by your experiences, and where appropriate challenging.
If you have any questions whilst reviewing this information please get in touch with xxx by email or call xxxxxxxxxxx.
Section 1: comments on the invite letter
Is the invite letter clearly written and easy to understand? (if you feel there are elements of the invite letter that could be improved, please elaborate on this in your answer).
Reviewer 1 – Yes.
Reviewer 2 – I think that the letter is written in a clearly and easy to understand way mostly, and the layout is also clear and easy to understand. The part of the letter that mentions types of vascular problems that you can have could be taken out. This is because there would be very few people that would know what they were and even less understands them. As the participants of the projects are healthy therefore don’t suffer from any vascular problems, they probably won’t know what the conditions are, so the names of some of the problems would just confuse them when they read it.
Reviewer 3 – Is the invite letter clearly written and easy to understand? (if you feel there are elements of the invite letter that could be improved, please elaborate on this in your answer) Yes it is clearly written and understandable. It could be improved by defining the terms: vascular, services and vascular insufficiency, and also by using less medical terminology i.e. talking about blood circulation problems rather than vascular conditions.
Reviewer 4 – The letter is fairly clearly written, although terms are used that might not be understood and I wasn’t entirely clear about the purpose of the research from the letter.
It isn’t clear what a ‘healthy’ adult is – whether other conditions are excluded or just vascular conditions.
Also, it would be better to have the explanation of what ‘vascular problems’ are right at the start as the term is used in the title and first paragraph without explanation.
What are vascular ‘services’? in-patient/out-patient and/or community services?
I was confused as to how ‘public’ views on ‘vascular’ services could be explored.
Many people won’t know what ‘aortic aneurysm’ or ‘venous insufficency’ mean and there’s no explanation of ‘health-care treatments’ and ‘interventions’ ‘patient-focused’ may not be a familiar term to everyone.
Reviewer 5 – Reasonably clear however a description of what ‘Vascular’ services are, is needed. I feel the word vascular itself is too technical.
Also most of paragraph 3 needs to come before paragraph 2.
Using the phrase general public is condescending ‘public’ is fine.
Reviewer 6 – The letter is brief and to the point. I did however keep rereading to try to understand why you want the opinion of people who do not need to access this service.
Reviewer 7 – I think you should make it clear at the very beginning that this is an interview study, not a clinical trial or experiment.
Reviewer 8 – It is a well written, clear, logical and just the right length. This is difficult as not sure how it could be improved. It’s the correct length and gets right to the point without any unnecesarry information. I would perhaps want to maybe know a bit more information on how this study would impact the health service.
Reviewer 9 – The letter is very clearly written in plain English and easy to understand.
Reviewer 10 – It is clear, but I suggest the actual details of the participant’s contribution – the short interview possibly face to face-could be set out in the first paragraph just to engage them more.
Reviewer 11 – I am very happy that the invite letter is clearly written and easy to understand. It clearly states that the research is aimed at improving patient-focused care: I feel this is important when embarking upon a research study.
Reviewer 12 – It is, but where are you getting their name from? I think that needs including as you are hinting at the fact you know something about them as you know they don’t have a vascular condition. I think people need to assured that their contribution can make a difference and explain the rationale for interviewing this group. A lot of people will think they cannot comment on a condition they have no experience of and a service they have never used. I certainly fall into this category.
Having read the invite letter, do you think it is a project that you would consider taking part in?
Reviewer 1 – Yes.
Reviewer 2 – I would consider it, because it doesn’t sound like it would inconvenience me too much, as the interview is mostly likely to take place over telephone, and would only take around 20–30 minutes.
Reviewer 3 – Yes. It appears to be a worthwhile project in which to become involved.
Reviewer 4 – Not without more explanation – and I was put off by the technical nature of both documents and the hypothetical scenario methodology.
Reviewer 5 – Yes.
Reviewer 6 – I see no reason not to take part, it’s a painless half an hour of my time; I would like to know why you’re seeking the opinions of the general public, people unaffected by the service, to help shape the service. Why should I be interested?
Reviewer 7 – Yes.
Reviewer 8 – I would be interested in taking part to see how the services are being changed and from the letter it would suggest that re-structuring of services are taking place, and the researchers and clinicians are asking the public what they want and what they would benefit from.
Reviewer 9 – It is a project I would take part in, although there is no direct benefit to myself, it may help others.
Reviewer 10 – Yes, if I were ‘healthy’!
Reviewer 11 – Yes, I would be keen to take part in this project.
Reviewer 12 – Not currently, I would want the information I have asked for above.
Section 2: comments on the participant information sheet
Does the document clearly describe and explain what will be involved for a patient who wants to participate in the research?
Reviewer 1 – “Participant interviews will most likely take place over the phone, but some interviews will be in person”: Better said in Invite Letter: “This will most likely be a telephone interview, but in some cases could be in person, either at a clinic or hospital”. Why one or the other, and why not knowing in advance?
Reviewer 2 – Yes, apart from the part that mentions about some of the interviews being in person at clinic or in hospital. Is there a reason why some people would be interviewed in person, or is that an option in case some people can’t do the interview by phone? This would need clarifying, as it may put some people off, as they would find it an inconvenience for them if they would have to go in in person.
Reviewer 3 – Yes I believe so. The use of the term “patient” here is inappropriate I think as it is addressed to “healthy volunteers”.
Reviewer 4 – No – I don’t think so. It still uses medical terms and I found the interview structure unclear.
Reviewer 5 – Not really I found it very confusing is it about quality of services or how far I would travel for the service. Also, as a patient, would I be interviewed over the phone or face to face – why the difference and who decides? Finally what is described in the structure doesn’t seem like an interview to me more like a ‘virtual or imaginary game’ and this does need more clarification.
Reviewer 6 – Very clear, easy to read. Describes the process well.
Reviewer 7 – Yes I think it’s very clear.
Reviewer 8 – This document very clearly starts with something I look out for. ‘No obligation to complete this study’ and ‘contact details can be found at the end’. It then follows into what is expected and a general example of how the interview will be conducted.
‘The disadvantages and risks involved are minimal’. – I’m not sure about this? How can a phone call be risky? This is too general, you need to outline the risk clearly or if no, which I suspect, say “there are no risks involved with the interview”.
Reviewer 9 – The document is very thorough and clear on what would be involved for the patient. There is no harm involved in the study and data storage and use was explain clearly and thoroughly.
Reviewer 10 – yes.
Reviewer 11 – The participant information sheet is very clearly written, and explains everything in good detail. I feel it also sets out the aims of the project very clearly.
Reviewer 12 – It is, but I think it could have more information in it as described for the invitation letter above. A description of the documents they will be sent would be useful, as it sounds offputting and bit much like hard work for no benefit. It would be useful to give some examples of where this type of research has been successful before.
Would you say that the document is written in a language that patients would understand, if not, what parts need changing?
Reviewer 1 – Definition of “Trade-off techniques”.
Reviewer 2 – It is mainly written in a language that the patients would understand, apart from one part. The paragraph that talks about the purpose of the study includes a list of vascular problems near the end, “peripheral arterial disease, aortic aneurysm, carotid arterial disease, varicose veins and venous insufficiency”. The inclusion of this is confusing and not really that necessary for the patient information sheet as they won’t understand what they’re reading.
Reviewer 3 – As in my response to Section 1 I would like to see the terms vascular, services, insufficiency, explained in more everyday language so as to be more readily understood by lay persons.
Reviewer 4 – The ‘purpose’ paragraph is unclear – if this is aimed at the public who don’t have vascular conditions they are unlikely to understand the medical terms used. Also – it still doesn’t explain how public views can be related to services they don’t access. Same points as above re letter – use of ‘healthy’ ‘patient-focused’, etc. I found the interview structure unclear – would I be asked entirely hypothetical questions? And how much explanation would there be of the health states and treatments?
Reviewer 5 – I think a lot of the language is quite technical such as ‘vascular health-care treatments’ and ‘rank them in order of how good or bad’ and ‘trade-off technique’ So they need descriptions in short and simple sentences. Also what do you mean by good or bad? This is very vague does it mean ‘is this painful or uncomfortable or embarrassing?’ I found a lot of the language quite patronising.
Reviewer 6 – Seems straightforward and easy to understand, clearly laid out. Being picky, I don’t like the use of ‘so therefore’ in the paragraph about disadvantages and risks. It’s clumsy and unnecessary to use both words.
Reviewer 7 – Yes but I think it might interest people more if you clarified what ‘vascular services’ comprises. Is it surgery, medicine and radiology? You might also consider encouraging potential subjects by telling them how their responses will make a difference.
Reviewer 8 – A very clear and logical information sheet. I cannot see anything that would need altering other than the disadvantages and risks involved section.
Reviewer 9 – Perhaps define ‘vascular’ slightly sooner. The document is understandable and clear. It is lengthy; however, I believe all the information is necessary.
Reviewer 10 – yes.
Reviewer 11 – I’m happy that it’s all written in a manner that’s easy to understand.
Reviewer 12 – The language is fine.
If you were given this document in clinic, would you have any questions for the researchers after reading this document?
Reviewer 1 – No.
Reviewer 2 – It says that they would be sent documents to prepare for the interview. Would they be long and time consuming to read, as this may not be possible for people who are very busy and wouldn’t have that much time to read long documents, therefore maybe making it an inconvenience and put them off.
Reviewer 3 – Yes as stated above.
Reviewer 4 – How can questions relating to hypothetical conditions and situations help to inform provision of a specific set of services for specific conditions? Wouldn’t it be better to ask those patients who already use them? Where are the locked cabinets for storing transcripts kept?
Reviewer 5 – I would be very worried about confidentiality I don’t find what has been written is reassuring in any way whatsoever. You need to be very specific about coding procedures and how all identifiers are removed. You need to state how researchers are monitored and what their access procedures are. Most patients are happy to give a researcher plenty of information about themselves just as long as they, the patient, is not identifiable.
Reviewer 6 – I still don’t understand why you want healthy volunteers with no interest in the service, I know what you want, and how it is carried out, and that it is part of a much larger study, but why would my opinion be valuable?
Reviewer 7 – I think the main question would be around the arrangements for the interview. Can it be done at the weekend or in the evening? If the subject has to travel are you facilitating this by paying their expenses? If I was sitting in a clinic waiting area reading this I’d like to know if the interview could be done during that visit to the hospital. This would benefit you because as they leave hospital they’ll forget all about the request to be involved.
Reviewer 8 – Is there a need for this study to decide where certain vascular services will be situated and structured in Sheffield because there will be some remodeling and moving? What do you expect to understand from the trade-off technique? How do my details, such as occupation, help the study? Why are you using healthy volunteers and not people with vascular disease states?
Reviewer 9 – I might wish for the researchers to elaborate on the meaning of ‘trade-off’ technique.
Reviewer 10 – yes, I would want to know more about ‘the broad structure of the interviews will be as follows:’ as I would be intrigued!
Reviewer 11 – If I have any other health conditions (i.e. not vascular), would this affect my eligibility to take part in the research? How many participants do you hope to interview? Do you have any links to the ‘larger research study’ you mention on the first page? Are there any factors that determine whether a phone interview or a face-to-face interview is conducted? Would I be able to read the transcript of my interview (after it’s been written up), such that I can confirm that it gives a true representative view of my interview?
Reviewer 12 – Why me? How can I possibly comment on something I know nothing about?
Section 3: do you have any other comments you would like to feedback to the researcher?
Reviewer 1 – None.
Reviewer 2 – None.
Reviewer 3 – None.
Reviewer 4 – None.
Reviewer 5 – You have decided that distance to the vascular service as a key factor, I would suggest most patients would consider local transport links and parking issues at a site are more important than distance
Reviewer 6 – None.
Reviewer 7 – None.
Reviewer 8 – None.
Reviewer 9 – The information looks thorough and clear.
Reviewer 10 – If you are going to tease the participant with the last section, either be more specific with what it entails, or leave it out altogether until the day. If you have any questions that you would like to ask the researcher regarding their project, please include them here.
Reviewer 11 – I think this is a really important project, especially given the aim is to assess current vascular treatment provision, and suggest improvements. I’d be very keen to be involved in the study. I wish you every success.
Reviewer 12 – None.
Appendix 10 Participant information sheet and invitation letter
The design, development and commissioning of patient focused vascular services: public preferences for vascular treatment
Participant information sheet for volunteers from the general public: telephone interviews
We would like to invite you to take part in a research project, which is looking at different ways in which services for vascular disease could be provided in the UK. The reason for inviting you is that we are interested in finding out the views of the general public.
Before deciding if you would like to take part, it is important that you understand why the research is being done, and what taking part would involve for you. Please take the time to read through the following information carefully, and feel free to discuss it with any family or friends if you wish. If you have any questions or would like any more information before deciding, please contact the research team whose details are included at the end of this leaflet.
Please note that taking part is entirely voluntary and you do not have to do so.
What are vascular services?
Vascular services are health-care treatments for people who have vascular disease (problems with their blood circulation system). Examples of vascular disease include varicose veins, venous leg ulcers, peripheral arterial disease, carotid arterial disease (which can lead to strokes) and aortic aneurysms. To take part in the research study you do not need to have any medical knowledge or know anything about vascular disease.
What is the purpose of the study?
We are interested in finding out what members of the general public think about the way vascular services are organised. We are particularly interested in your views about how far people have to travel to be treated for vascular disease. This research study is part of a large 5-year research study, funded by the Department of Health, to evaluate vascular services in the UK. The results of the study will be used to make recommendations about how vascular services could be better organised to produce the most benefit for patients.
Why have I been invited to take part?
You have been asked to take part in this study because you are a member of the general public and do not suffer from a vascular condition.
What will happen to me if I take part?
If you agree to take part, we will arrange for you to have a one-time only interview with a member of the research team. This will most likely be a telephone interview, but interviews could be arranged in person, at either the clinic or hospital, if preferred. The interview will be one-to-one, with just you and the researcher. One of the research team members will contact you to arrange the interview at a time that is convenient for you. You will also be sent some documents through the post for you to look at during the interview.
The broad structure of the interviews will be as follows:
-
You will be provided with some descriptions of symptoms that are experienced by people with different vascular conditions, and asked to compare the conditions in terms of how good or bad you think the conditions are. For instance you could be asked to compare the symptoms of a person who has had their leg amputated with those of a person who has not had an amputation, but who has moderate amounts of pain in their leg.
-
You will then be asked to imagine that you are experiencing some of the vascular conditions and to value them using a technique that is commonly used in this kind of research. For instance you could be asked to decide whether you would prefer to continue living with some pain or to have a treatment to reduce the pain but which has some risk attached to it. We will explore how your choices might change for different levels of risk.
-
You will then be given a description of a typical treatment process for a vascular condition and asked about how far you would be prepared to travel to be treated if you had that condition.
-
Finally, you will be asked to answer some general questions about yourself, such as where you live and your occupation. You will also be given the opportunity to make any general comments about the interview and your experience of it.
The researcher will carefully talk you through each stage of the interview and will clearly explain all of the information presented to you during the process. The documents posted to you before the interview will help with this. The interview will be recorded. This will avoid the need for the researcher to take lots of notes and will help them concentrate on what you are saying. Your responses will be entered into a database but the information will be made anonymous so you will not be recognised. This means any names or information that make you identifiable will be removed. The researcher will check that the recording and the noted responses are the same, and will then erase the recording. The interviews will last approximately 20–30 minutes.
Do I have to take part?
It is entirely your decision whether or not to take part, and you are under no obligation to do so. If you do decide to take part you will be asked to give us your consent over the phone before going ahead with the interview. If the interview takes place in person, you will be asked to sign a consent form. You will be given a copy of the participant information sheet and consent form to keep. If you decide to take part, you are still free to withdraw at any time, and you do not have to give a reason.
What are the possible disadvantages and risks of taking part?
The disadvantages and risks involved are very small. The interview may take up to 20–30 minutes to complete, so may be a little time consuming.
What are the possible benefits of taking part?
The study will not help you directly. However, the information we get from this study will help improve the treatment and care provision to people with vascular disease.
What if there is a problem?
There is no obvious reason why any problems should arise from you taking part, or that you may be harmed as a result of participating in the research.
If you have any complaint about the way you have been dealt with during the study, the normal NHS complaints procedures are available to you. In the first instance you should contact the trust’s patient services team by telephone XXXX.
Will my taking part in the study be kept confidential?
The recordings and written records of your interview will be kept safely and confidentially. They will be stored in locked cabinets and on password-protected computers. Only the researchers will have access to them. Any information that we use will be anonymised so that you will not be able to be identified. All information that is collected about you during the course of the research will be kept strictly confidential.
What will happen to the results of the research study?
The results of the study will be used to make recommendations about how vascular services could be better organised to produce the most benefit for patients, and written up in reports and other publications. If you wish, you can provide us with your email or address and we will send you a copy of the final report of the study.
Who is organising and funding the research?
A team led by Professor Jonathan Michaels, funded by an NIHR Programme Grant, to examine the provision of vascular services. The research is a collaboration between the University of Sheffield, Sheffield Teaching Hospitals and the NHS Sheffield Clinical Commissioning Group.
The project lead for this part of the study is Dr Phil Shackley.
Who has reviewed the study?
All research in the NHS is looked at by an independent group of people, called a Research Ethics Committee, to protect your interests. This study has been reviewed by South East Coast – Brighton and Sussex Research Ethics Committee.
Contacts for further information
Aoife Howard, Research Associate XXXX.
List of abbreviations
- AAA
- abdominal aortic aneurysm
- ADL
- activities of daily living
- AFS
- amputation-free survival
- AneurysmDQoL
- Aneurysm Dependent Quality of Life
- AneurysmSRQ
- Aneurysm Symptoms Rating Questionnaire
- AUSVIQUOL
- Australian Vascular Quality-of-Life Index
- AVVQ
- Aberdeen Varicose Vein Questionnaire
- CAD
- carotid artery disease
- CAS
- carotid artery stenting
- CEA
- carotid endarterectomy
- CFI
- comparative fit index
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CIS
- continuous inpatient spell
- CLI
- chronic limb ischaemia
- COSMIN
- Consensus-based Standards for the selection of health Measurement Instruments
- CVA
- cerebrovascular accident
- ePAQ-VAS
- electronic Personal Assessment Questionnaire – Vascular
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- EVAR
- endovascular aneurysm repair
- EVLT
- endovenous laser treatment
- FDA
- Food and Drug Administration
- HES
- Hospital Episode Statistics
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- IC
- intermittent claudication
- ICC
- intraclass correlation coefficient
- ICD
- International Classification of Diseases
- ICER
- incremental cost-effectiveness ratio
- ID
- identifier
- LSOA
- lower-layer super output area
- MI
- modification indices
- NAASP
- National Aortic Aneurysm Screening Programme
- NHP
- Nottingham Health Profile
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMB
- net monetary benefit
- NVR
- National Vascular Registry
- ONS
- Office for National Statistics
- OPCS
- Office of Population Censuses and Surveys
- OR
- open repair
- OS
- overall survival
- PAD
- peripheral arterial disease
- PPI
- patient and public involvement
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- PROM
- patient-reported outcome measure
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RC
- residual correlation
- REC
- Research Ethics Committee
- RFA
- radiofrequency ablation
- RMSEA
- root-mean-square error of approximation
- SD
- standard deviation
- SF-12
- Short Form questionnaire-12 items
- SF-36
- Short Form questionnaire-36 items
- SVI
- Sheffield Vascular Institute
- TTD
- time to death
- UI
- user interface
- VLU
- venous leg ulcer
- VLU-QoL
- Venous Leg Ulcer Quality of Life
- VSGBI
- Vascular Society of Great Britain and Ireland
- VV
- varicose veins
- VVSymQ
- Varicose Veins Symptoms Questionnaire
Notes
-
Geographical mapping of LSOA, to districts and screening areas
-
Detailed summary tables of data regarding other diagnostic groups
-
Draft paper on the trade-off study regarding travelling distance
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/pgfar09050).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
