Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0610-10066. The contractual start date was in October 2012. The final report began editorial review in July 2019 and was accepted for publication in February 2021. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2021 Scott et al. This work was produced by Scott et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 Scott et al.
SYNOPSIS
The synopsis provides a narrative account of the main results from the programme. The results are reported in nine separate sections. They are preceded by this introductory section and followed by a final section that outlines key conclusions and recommendations for further research, making eleven sections in total.
The research was undertaken across three workstreams. They were characterised by their dominant research approaches. Workstream A focused on patients’ perspectives, workstream B was built around the TITRATE (Treatment Intensities and Targets in Rheumatoid Arthritis ThErapy) clinical trial and workstream C concentrated on analysing existing evidence from real-world observational studies and published clinical trials. Each of three workstreams continued throughout the duration of the programme. Labelling these workstreams A–C was for convenience and it does not imply any particular order.
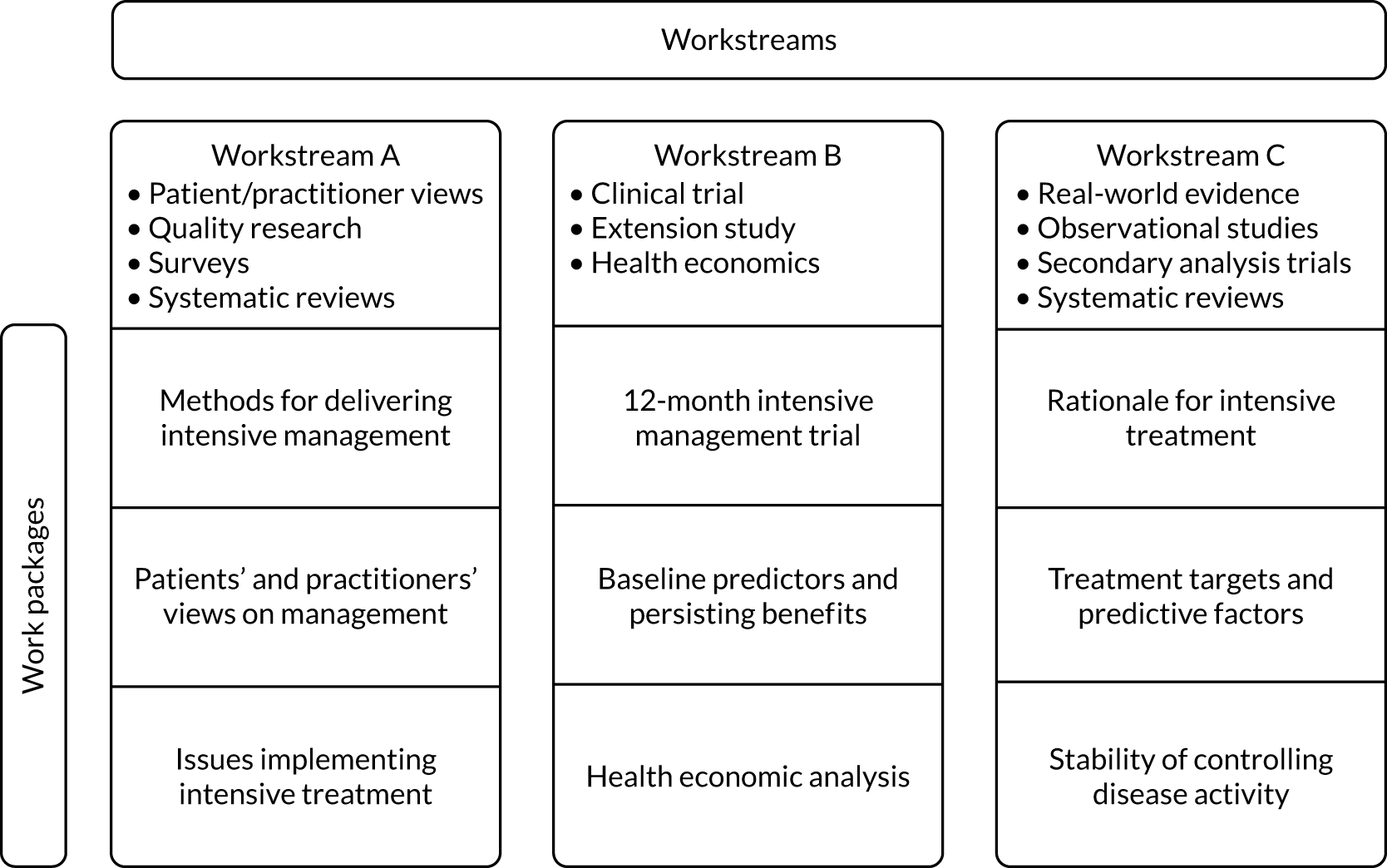
Each workstream was subdivided into three different work packages. They all comprised one large or several small studies that formed a distinct cluster. Each of these nine work packages is described in a separate section. The inter-relationship between the workstreams and work packages is outlined in Figure 1.
FIGURE 1.
Research design pathway.
The research findings could be ordered in several different ways in this synopsis and none is necessarily best. We have chosen to present them in a chronological order because we consider such an approach is most practical. Therefore, we have reported research on longstanding observational studies and completed trials first, followed by research on developing the intervention used in the TITRATE trial, then the trial results and, finally, research related to the findings in the trial. This order cuts across the different workstreams.
The nine research sections in the synopsis comprise:
-
the rationale for intensive management
-
treatment targets and predictive factors for intensive management
-
delivering intensive management
-
the TITRATE trial
-
a health economic evaluation of the TITRATE trial
-
response predictors and response persistence in the TITRATE trial
-
stability of disease control and impact on disability
-
patients’ and practitioners’ views
-
implementing intensive management.
Each of these results sections have similar substructures. These consist of aims, methods, key findings, limitations and relation to overall programme.
For most sections, detailed accounts of the patients and methods are provided in the appendices. This is because the sections are overviews of published papers and papers about to be submitted. This approach is the simplest way to make the information available to readers. However, details about patients and methods for the TITRATE trial itself, the health economic analysis of the TITRATE trial and secondary analyses of the trial are given in the main text. This is because these parts of the programme are standalone studies in which the methods and results are best considered together.
Patient and public involvement
The whole programme involved extensive patient and public involvement (PPI) and two patients are co-authors of the report. Three sections (i.e. Delivering intensive management, Patients’ and practitioners’ views and Implementing intensive management) involved extensive PPI. Patients also contributed to the trial protocol. The involvement of patients is summarised in the individual sections. The general approach is given in Patient and public involvement.
Introduction
Programme theme
The TITRATE programme studied the impact of intensive management for patients with moderately active rheumatoid arthritis (RA). Current management goals in patients with RA include minimising disease activity, decreasing physical disability and improving health-related quality of life. There is considerable evidence that, in patients with active RA, intensive management helps achieve these goals. However, many patients with RA have moderate disease activity, which falls between active disease and remission. The TITRATE programme assessed the benefits of intensively managing these patients with moderate RA disease.
Key features of rheumatoid arthritis
Overview
Rheumatoid arthritis is an immunologically driven long-term condition. Its key features are persistent synovitis of the joints, systemic inflammation and autoantibodies, such as rheumatoid factor. 1–3 RA affects 0.5–1% of adults in high-income countries, although there is considerable variation between countries and populations. 4–6 It particularly involves women and older adults. Its annual incidence is 5–50 per 100,000 people. 6–8 Uncontrolled active RA results in substantial physical disability and poor quality of life,9,10 which are often associated with loss of work and high medical and social costs. 11–13
Management
Rheumatoid arthritis is usually managed in the UK by multidisciplinary teams that comprise rheumatologists, nurses, physiotherapists, occupational therapists and other health-care professionals. The multidisciplinary teams provide education, medication, psychological support, exercise and joint protection. There is substantial variability in the nature of these teams,14 and the evidence supporting the different approaches they use also varies. 15,16 Surgical intervention may be required for end-stage joint damage. 17
Treatment focuses on the control of joint inflammation and the prevention of disease progression using disease-modifying antirheumatic drugs (DMARDs). 18 These reduce synovitis and systemic inflammation in the short term and physical disability and erosive progression in the long term. DMARDs can be categorised into several groups. We have classified them as conventional DMARDs, biologics and new orally acting Janus kinase (JAK) inhibitors. Together with short-term steroids, they are the key drug treatments for RA.
Methotrexate is the dominant conventional DMARD. 19–21 Other currently prescribed conventional DMARDs include sulfasalazine, leflunomide and hydroxychloroquine. Some patients are treated by combining two or more conventional DMARDs. Such intensive combination treatment is constrained by concerns about adverse event risks. 22,23
Biologics are used when RA is not controlled by conventional DMARDs. 24–28 They include the tumour necrosis factor inhibitors (TNFis) rituximab, abatacept and tocilizumab. All biologics are highly effective in reducing joint inflammation. They are usually combined with methotrexate or another conventional DMARD to increase efficacy and reduce the formation of blocking antibodies that could reduce their efficacy. Their use is limited by their high costs, although the advent of biosimilars is likely to reduce their costs. 29 Despite often achieving substantial reductions in disease activity, biologics are not curative. New oral drugs for RA, the JAK inhibitors, have a similar position to biologics in the treatment paradigm. 30
Steroids (glucocorticoids) are used in the short term to reduce joint inflammation. However, their long-term use is not recommended because of their side effects. 31 RA patients also receive various symptomatic treatments, including non-steroidal anti-inflammatory drugs and analgesics. 32,33 These are not usually given to control the disease process.
Clinical guidelines
There are many clinical guidelines for RA, including English guidelines from the National Institute for Health and Care Excellence (NICE), which outline its overall management. 34 High-cost treatments, such as the biologics, have specific NICE Health Technology Appraisals that recommend when they should be used. 35 The various guidelines have not resolved how best to manage patients with moderately active RA.
NHS importance
Patient load
The long-term management of RA dominates specialist rheumatology services. The 2016 national audit of early arthritis enrolled > 5000 patients, most of whom had RA. Inflammatory arthritis accounts for 40–60% of rheumatology follow-up visits. English Hospital Episode Statistics data show that there were more than 1,300,000 rheumatology outpatient follow-ups in 2017/18,38 and it is likely that a substantial proportion of these would have been for RA, potentially in the region of 300,000–600,000 outpatient follow-ups. The 2009 report from the National Audit Office estimated that 580,000 English adults had RA, with 26,000 new diagnoses each year. 39
Costs
Rheumatoid arthritis has substantial financial impacts for the NHS and the whole UK. The National Audit Office estimated that NHS costs were £560M per year and work-related disability costs were another £1800M per year. 39 Most costs are incurred by patients with high disability levels. Early intensive management targeted at remission should reduce future disability and, therefore, decrease costs. Biologic drug costs, which currently exceed £1B per year, continue to increase. 40
Need for research
The 2009 NICE guidance41 identified several unresolved questions that have been directly addressed in the TITRATE programme. Crucial issues included (1) the optimal management of patients with moderate disease activity, (2) the impact of enhancing remission rates on reducing future disability and (3) the role of self-management and the support patients need to use it.
Although more clinical guidelines have been published since 2009, including updated NICE guidance,41 and much new research has been undertaken, these questions remain important and unresolved.
Key prior research
Pathogenic heterogeneity
Rheumatoid arthritis may not be one disease. Instead, it may represent a final common pathway for several inflammatory joint diseases that vary by antibody profiles and clinical features. 42–45 Consequently, individualised management strategies are needed.
Effective treatments strategies
Both conventional DMARDs and biologics are effective treatments in active RA. However, there are uncertainties about their benefits and costs. In particular, there is debate about the relative value of combinations of conventional DMARDs compared with biologics. 46–49
Intensive management
Strategy trials have shown that combining treatments, such as conventional DMARDs, short-term steroids and, in some trials, biologics, optimise clinical outcomes. 37,50,51 Such strategy trials justify adopting treat-to-target approaches. However, there are uncertainties about the impact of intensive management on established RA patients with moderate disease activity.
Aim and objectives
Aim
The overall aim of the TITRATE programme was to assess the evidence that outcomes improve in patients with established RA who have moderate disease activity when they receive intensive management.
Objectives
There were three objectives. These were to:
-
define how to deliver intensive therapy to patients with moderate established RA
-
establish the clinical effectiveness and cost-effectiveness of intensive therapy in treatment of moderate established RA in a clinical trial
-
evaluate existing evidence supporting such intensive management in observational studies and completed trials.
Each of these objectives was studied in one of the workstreams. The first, second and third objectives were studied in workstreams A, B and C, respectively.
Rationale for intensive managements
The studies in this section place the TITRATE programme into perspective using observational studies, secondary analyses of completed trials and systematic reviews. The studies assessed changes in the outcomes and intensities of RA management in routine clinical care over the last two decades, the strength of existing evidence for the clinical effectiveness of intensive management in RA and supportive evidence for using intensive management strategies in moderate RA.
Aims
Research studies with four inter-related aims are included in this section. These aims comprised analysing temporal changes in treatment, the perspectives in RA management guidelines, the evidence base for intensive management and observational evidence supporting intensive management for moderate RA. Four parts of the research have been published. 52–55
Changes in rheumatoid arthritis management and outcomes
Rheumatoid arthritis management and outcomes continually evolve, and there is considerable evidence that RA is becoming better controlled or less severe. 56–66 We therefore examined changes in disease activity, disability and treatment intensities in observational studies in routine practice settings in recent years. We examined changes in erosive progression in a systematic review of long-term observational studies52 that measured this outcome. We had to take this approach for erosive progression because X-ray damage is not quantified in routine practice.
Clinical management guidelines
Expert guidance about managing RA also evolves. 67,68 We therefore systematically reviewed published clinical guidelines on RA management to identify current recommendations on disease assessments and intensive management (both of which are crucial for the TITRATE strategy).
Trial evidence supporting intensive management
The evidence base for intensive management of RA is also expanding. 69 We therefore undertook two systematic reviews in the area. The first systematic review focused on remissions with all intensive managements and the second systematic review assessed the clinical effectiveness and cost-effectiveness of treat-to-target strategies.
Clinical evidence for treating moderately active rheumatoid arthritis intensively
The TITRATE programme reflects two concepts. First, patients with moderate RA and persisting disease activity are likely to have substantial ongoing disability, and observational evidence supports this perspective. 17 Second, if patients with moderate disease activity subsequently achieve remission, then they will have less disability, but there is little definitive evidence for this perspective. We evaluated both assumptions by analysing an observational study and two trials. Our aim was to ensure that evidence existed in favour of treating moderate RA intensively.
Methods
Observational studies
One observational study combined four cross-sectional surveys undertaken in two adjacent specialist units from 1996 to 2014 (three surveys had previously been published70–72). The studies each enrolled 189–520 patients (see Appendix 1, Table 27, for details of these patients). Overall, 1324 patients were studied. The patients in each survey were similar: 76–80% were female, their mean age was 58–60 years and their mean disease duration was 9–10 years. No particular treatment strategy was followed.
The other observational study was a longitudinal cohort study established in 2005 at Guy’s Hospital (London, UK). It involved most of the RA patients who were managed in another specialist centre until 2015. The study enrolled 1693 patients. Seventy-five per cent of the patients were female and their mean age was 55 years and mean disease duration was 11 years at entry to study (details of these patients are given in Appendix 1, Table 28). Patients were managed intensively using a treat-to-target approach. The longitudinal observational study included 752 patients, who were followed over ≥ 3 years.
Clinical trials
The CARDERA (Combination Anti-Rheumatic Drugs in Early Rheumatoid Arthritis) trial, which lasted 24 months, enrolled 467 patients, and complete end-point data were available in 379 patients. 73 The TACIT (Tumour Necrosis Factor Inhibitors Against Combination Intensive Therapy) trial, which lasted 12 months, enrolled 208 patients, and complete end-point data were available in 179 patients. 74 Details of these patients are given in Appendix 1, Table 31.
Clinical assessments
We assessed disease activity using the Disease Activity Score for 28 joints based on the erythrocyte sedimentation rate (DAS28-ESR)75 and disability using the Health Assessment Questionnaire (HAQ). 76 Further details are given in Appendix 2.
Systematic reviews
Four systematic reviews assessed:
-
erosive progression in long-term observational studies
-
clinical guidelines for managing RA
-
intensive managements and remission
-
the clinical effectiveness and cost-effectiveness of treat-to-target strategies.
Full details of these systematic reviews are given in the supplementary online material, including PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagrams (see Report Supplementary Material 1, Figure 1), and details of the included studies (see Report Supplementary Material 1, Tables 1–5).
Statistical analyses
Data were analysed descriptively using means, standard deviations (SDs) and 95% confidence intervals (CIs) or medians and interquartile ranges (IQRs). The longitudinal observational study used mixed models to examine the changes in DAS28-ESR over time.
The systematic review of erosive damage used Larsen77 and Sharp–van der Heijde scores78 to estimate annual rates of change in a linear regression model. The systematic review of intensive management and remissions used meta-analysis with RevMan 5.3 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark) to report relative risks in random-effects models,79 using Cochrane’s chi-squared test to assess between-study heterogeneity and quantify I2 statistics. 80 The other systematic reviews were descriptive and further details are given in Appendix 3.
Key findings
Clinical studies of changes in disease activity and disability
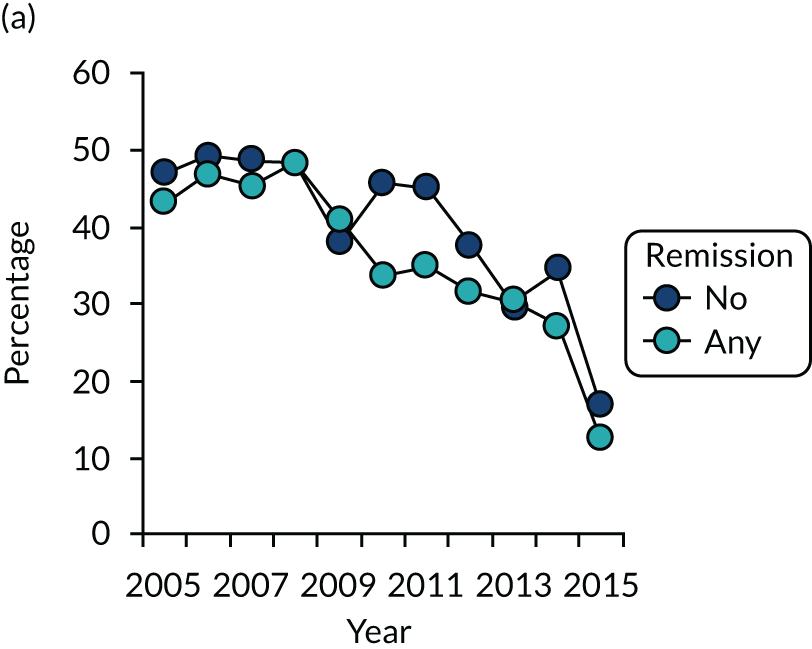
Both observational studies showed that there have been considerable reductions in disease activity levels and increases in treatment intensities over the last two decades.
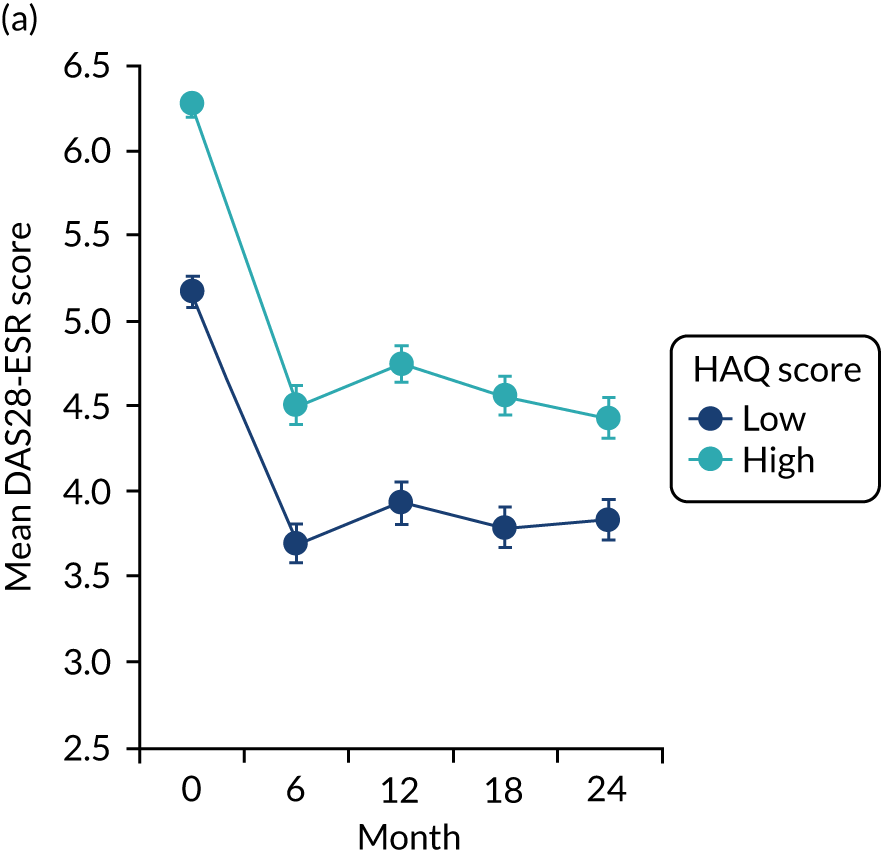
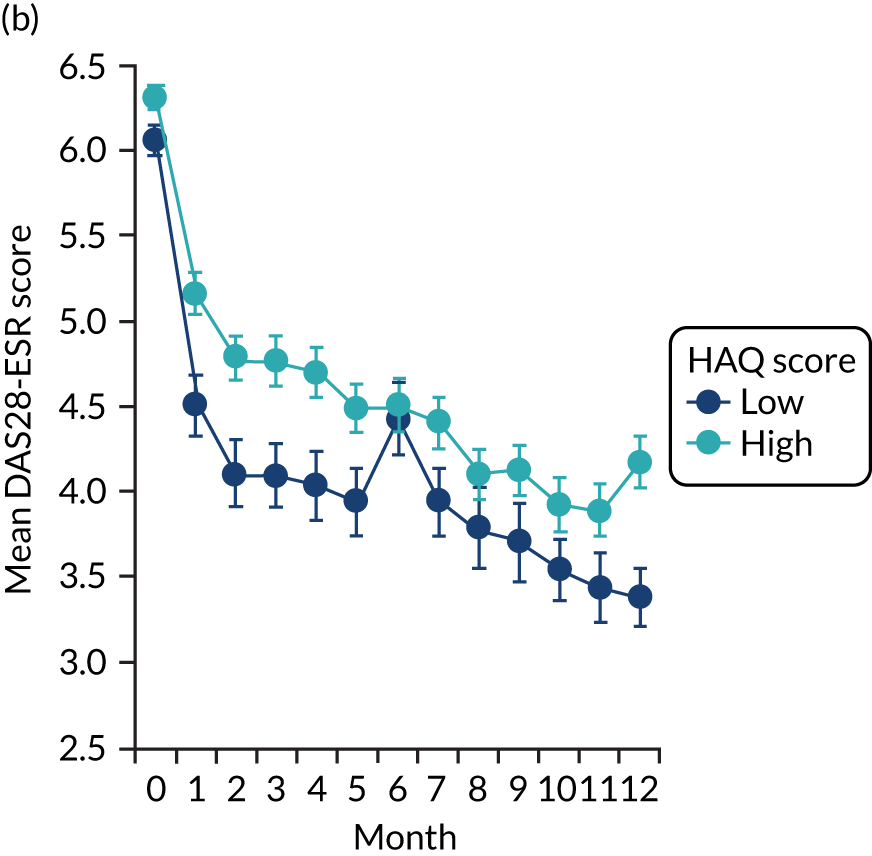
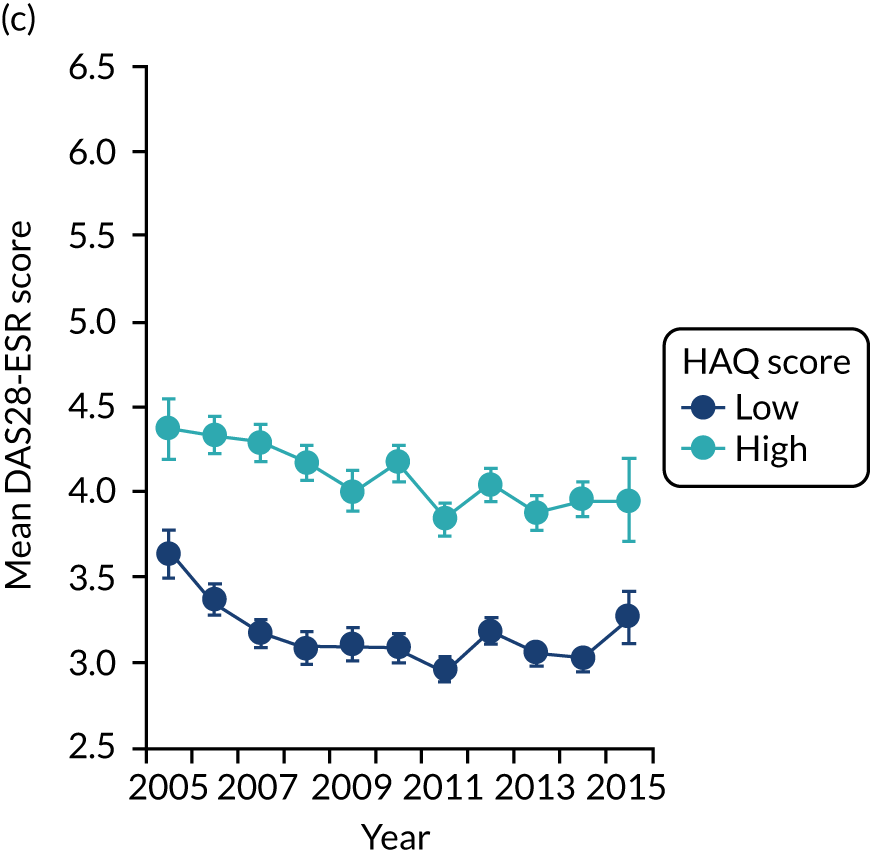
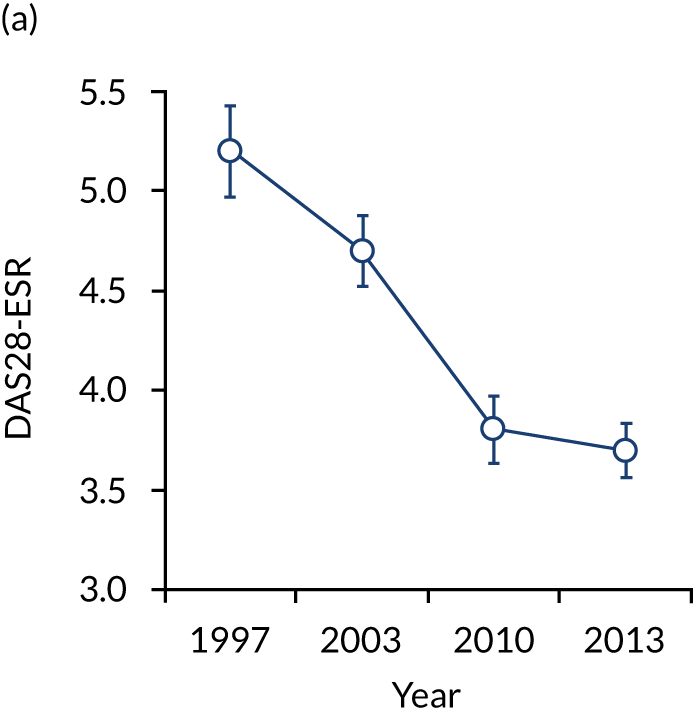
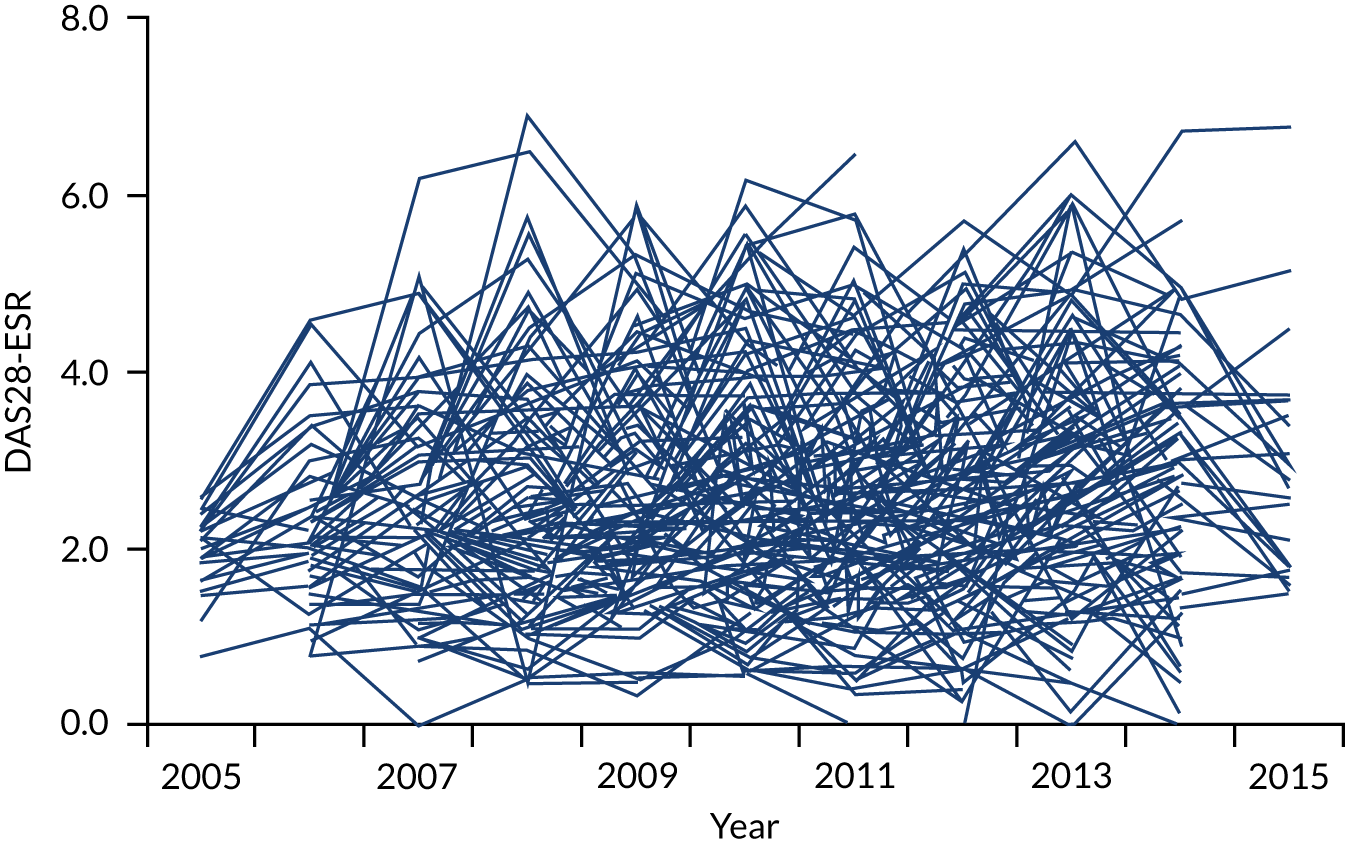
The first observational study, involving four cross-sectional surveys between 1996–7 and 2012–14, showed substantial decreases in mean DAS28-ESR scores (Figure 2). In 1996/7, mean DAS28-ESR scores were 5.2 (95% CI 5.0 to 5.4) and they decreased to 3.7 (95% CI 3.6 to 3.8) by 2012/14. DAS28-ESR remissions increased from 8% to 28%. The main treatment change was increased biologics use. None was used before 2000, but by 2012/14 biologics were used in 32% patients. Despite reductions in disease activity and increases in biologics use, disability levels were stable. In 1996/7, mean HAQ score was 1.30 (95% CI 1.08 to 1.52), and in 2012/14 it was 1.32 (95% CI 1.25 to 1.39).
FIGURE 2.
Changes in DAS28-ESR over time. (a) Cross-sectional surveys; and (b) longitudinal study.
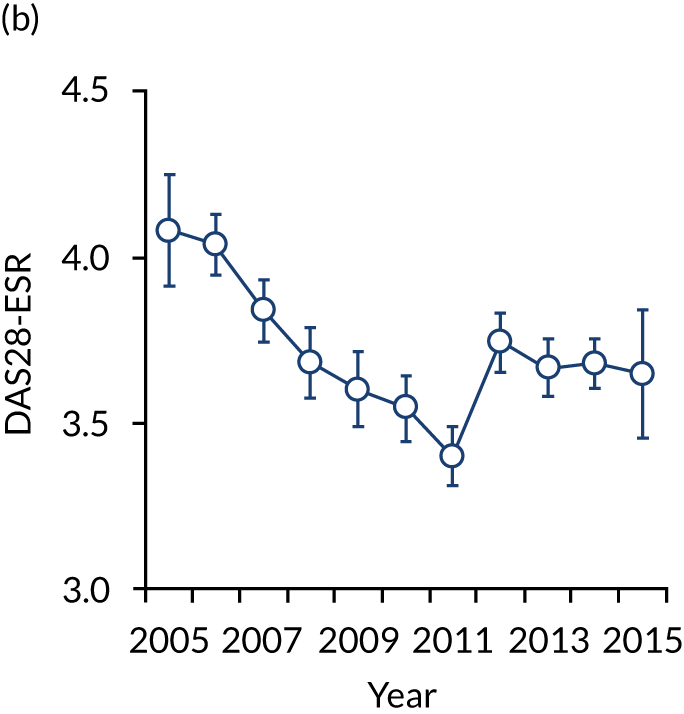
The second observational study, which comprised a longitudinal cohort study from 2005 to 2015, also showed that mean DAS28-ESR scores had decreased (see Figure 2). In 2005, the mean DAS28-ESR score was 4.1 (95% CI 3.9 to 4.3), and by 2015 it was 3.6 (95% CI 3.3 to 3.8). DAS28-ESR remissions increased from 18% to 27%. The main treatment change was increased biologics use. Biologics were prescribed for 19% of patients in 2005 and 42% of patients in 2015. The mean HAQ scores fell from 1.38 (95% CI 1.26 to 1.50) in 2005 to 1.19 (95% CI 1.04 to 1.34) in 2015.
Systematic review of changes in the progression of erosive damage
We identified 28 studies reporting RA radiological progression, and 10 studies, reported in nine papers,81–89 had sufficient data for meta-analysis. These 10 studies recruited patients from 1965 to 2000 and followed them for 5–20 years. Of 1121 patients, 73 had baseline radiological data. Five of the studies recruited from 1965 to 198981–85 and the other five studies recruited from 1990 to 2000. 85–89
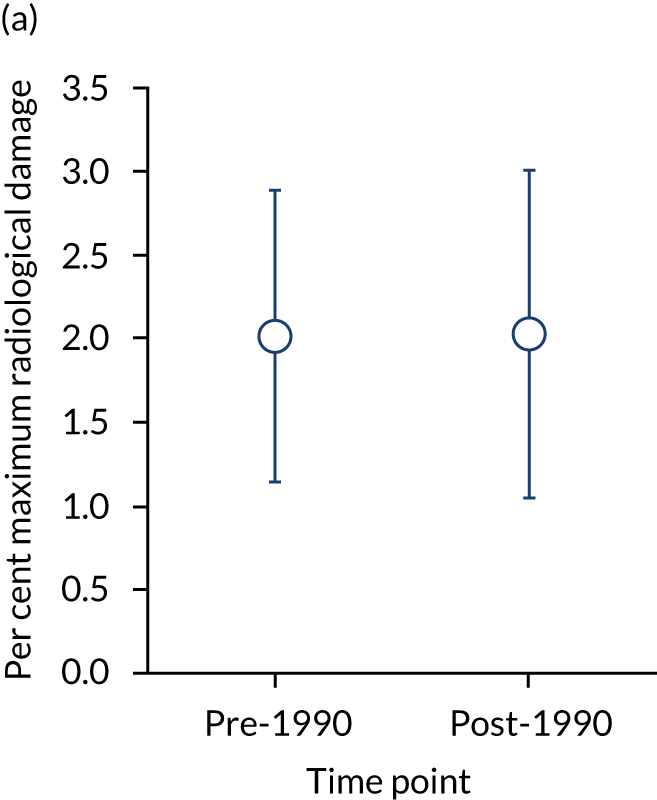
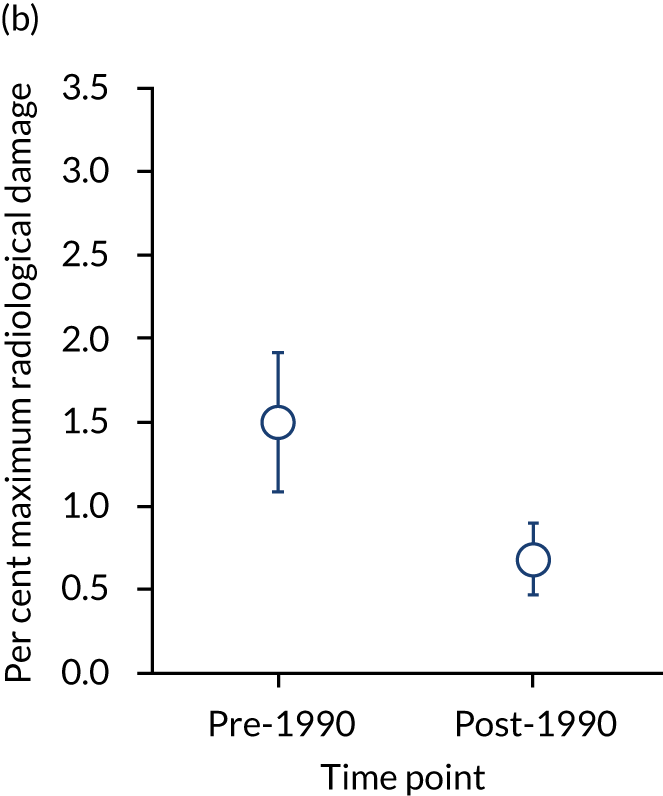
Baseline radiographic scores were similar in pre- and post-1990 studies (with a mean maximum damage of 2.01% and 2.03%, respectively). The annual rate of erosive change was higher in pre-1990 studies (mean 1.50%, 95% CI 1.08% to 1.92%) than in post-1990 studies (mean 0.68%, 95% CI 0.47% to 0.90%) and the difference was significant (p < 0.05) after adjusting for scoring methods. These changes are summarised in Figure 3.
FIGURE 3.
Initial damage and annual rate of damage in pre- and post-1990 cohorts. Means and 95% CIs are shown. (a) Initial; and (b) annual progression.
Systematic review of clinical management guidelines
We identified 22 guidelines36,90–110 (three were for early RA,99,105,107 one for established RA98 and 18 for all disease durations36,90–97,100–104,106,108–110). They were compiled by rheumatologists with variable patient involvement and contributions from nurses, allied health professionals and other experts. All guidelines dealt with drug therapies (11 guidelines covered diagnosis and 13 guidelines covered non-drug treatments).
Twenty guidelines36,90–98,100–105,107–110 recommended remission as the treatment target, and 16 guidelines36,90–97,102–104,107–110 recommended low disease activity as an alternative. Two guidelines98,106 recommended suppressing joint inflammation without defining what it implies. These recommendations are summarised in Figure 4. Remission was defined in various ways. DAS28-ESR remission was recommended in 13 guidelines,90,92–95,97,101,102,104,107–110 Simple Disease Activity Index (SDAI) in nine guidelines,36,92,95–97,102–104,108 Clinical Disease Activity Index (CDAI) in seven guidelines92,95,97,102,104,108,109 and American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) Boolean in seven guidelines. 36,92,95,97,103,104,109 Six guidelines did not recommend any specific remission criteria. 91,96,98–100,105,106
FIGURE 4.
Remission assessments and treatment targets in RA guidelines.
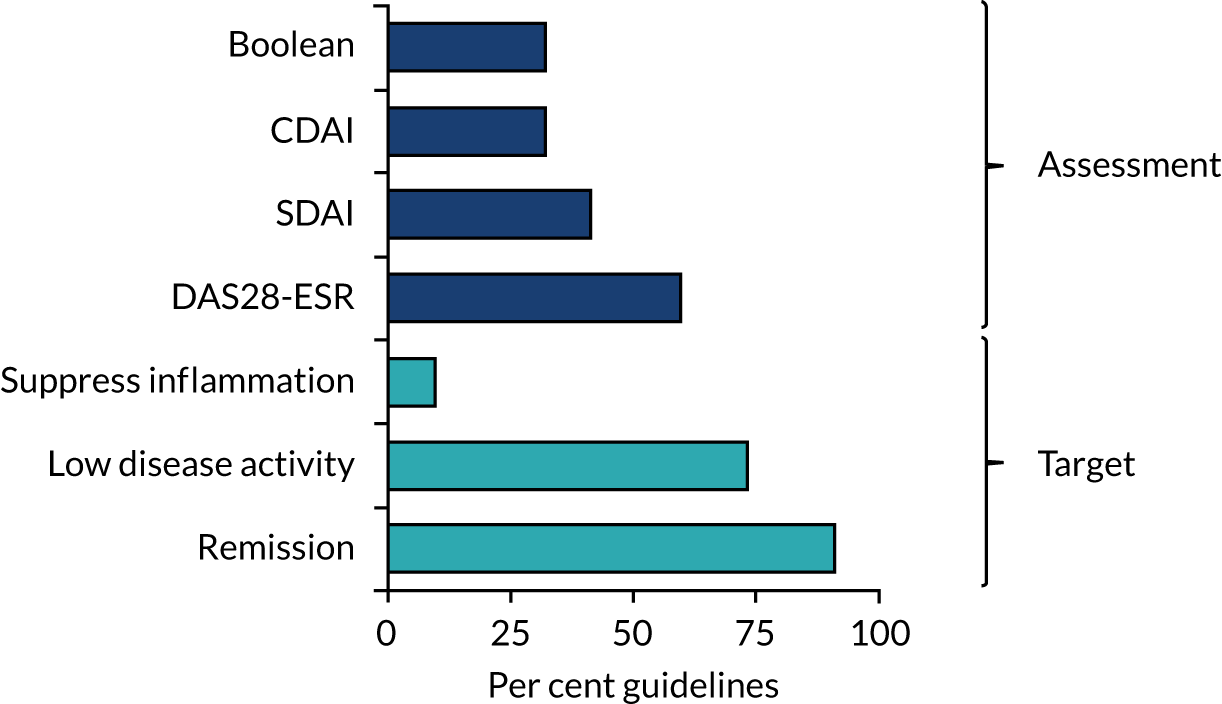
All guidelines36,90–110 recommend treating active RA. There was less unanimity about treating moderately active disease. Thirteen guidelines91,92,94–97,100,102–105,107,108 included definite recommendations about treating moderate disease, four guidelines36,90,101,109 gave implied guidance about treating moderate disease by indicating what treatment policies were needed until patients achieved remission and five guidelines93,98,99,106,110 made no recommendations.
Systematic review of trials of remissions with intensive management
We identified 53 trials reporting remissions. 74,111–162 Forty-eight trials111–132,134–139,142,143,145–162 were superiority trials in which an intensive management strategy was compared with a less intensive strategy, six trials74,128,133,140,141,144 were head-to-head trials comparing combination DMARDs with biologic treatments and one trial was in both groups. 128
In superiority studies, 3013 of 11,259 patients achieved remission with intensive management, compared with 1211 of 8493 control patients. Meta-analysis of the 53 comparisons showed a significant benefit for intensive management [risk ratio (RR) 2.23, 95% CI 1.90 to 2.61]. Intensive management increased remissions in early RA (23 comparisons; RR 1.5, 95% CI 1.38 to 1.76) and established RA (29 comparisons; RR 4.21, 95% CI 2.92 to 6.07). All intensive strategies (i.e. combination DMARDs, biologics, and JAK inhibitors) increased remissions. These effects are shown in Figure 5.
FIGURE 5.
Effectiveness in superiority and head-to-head trials assessed by random RRs. (a) Superiority trials; and (b) head-to-head trials.
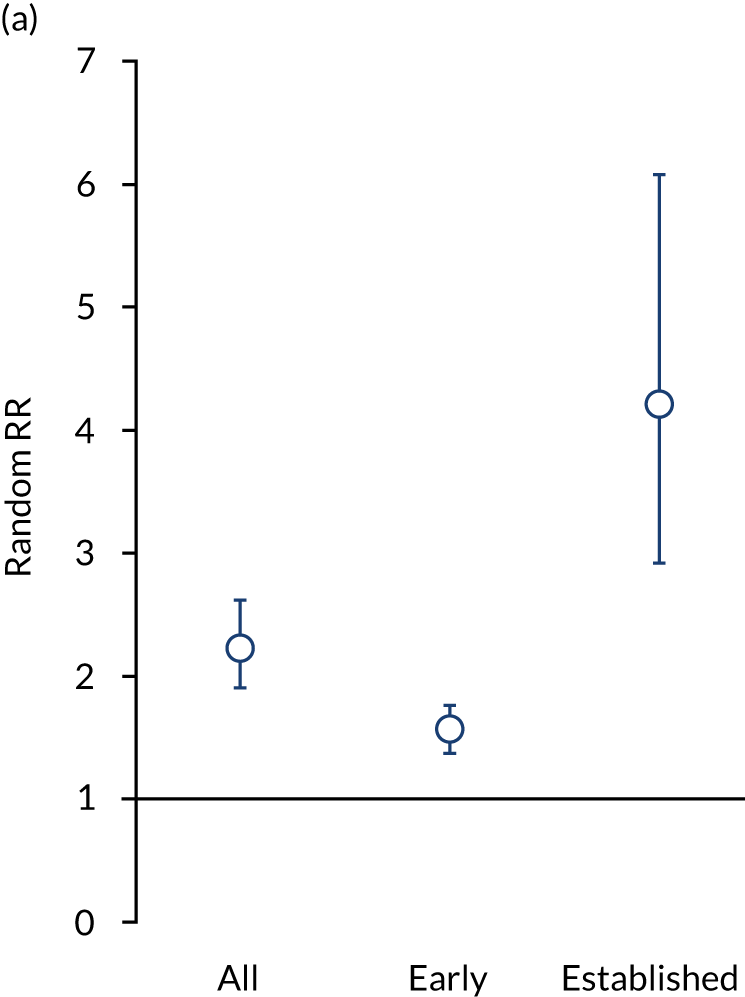
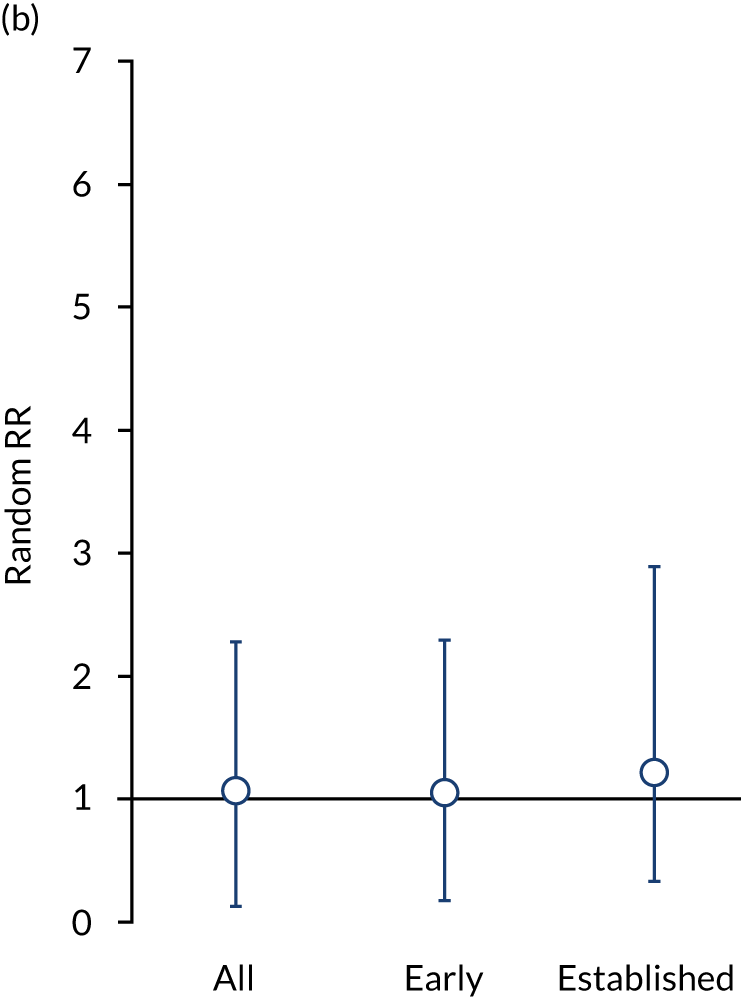
In the six head-to-head trials,74,128,133,140,141,144 317 of 787 patients achieved remission with biologics, compared with 229 of 671 patients receiving combination DMARD therapies. There was no difference between treatment strategies (RR 1.06, 95% CI 0.93 to 1.21). These effects are shown in Figure 5.
Remission frequencies differed in early and established RA. In early RA, 49% of patients had remissions with intensive management compared with 34% of control patients (RR 1.05, 95% CI 0.88 to 1.24). In established RA, 19% patients had remissions with intensive management compared with 6% of control patients (RR 1.21, 95% CI 0.88 to 1.68).
Systematic review of trials supporting using treat to target
We identified 41 papers49,113,128,129,141,148,153,155,163–195 reporting 16 relevant trials. Six trials129,158,171,179,186,190 compared treat to target with usual care, six trials49,113,128,177,183,185 compared different treatment protocols, two trials141,172 compared different treatment targets and two trials153,160 had other comparisons of conventional with intensive therapy. As the trials were too heterogeneous for meta-analysis, we undertook a narrative analysis. Details of the impact of treat-to-target strategies on remission in studies with controls receiving conventional treatment is shown in Table 1.
| Trial | Disease duration | Standard care | Intensive management | Relative risk (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Patients, n | Remission, n (%) | Treatment | Patients, n | Remission, n (%) | |||
| STREAM158 | < 1 year | Conventional | 40 | 19 (49) | Intensive | 42 | 27 (66) | 1.35 (0.88 to 2.01) |
| T-4 study186 | 1 year | Routine | 62 | 13 (21) | DAS28-ESR/MMP3 driven | 61 | 34 (56) | 2.66 (1.34 to 4.78) |
| Optimisation of adalimumab179 | Established | Routine care | 109 | 17 (16) | DAS28-ESR target | 100 | 38 (38) | 2.44 (1.44 to 4.24) |
| TICORA129 | 2 years | Routine | 55 | 9 (16) | Intensive | 55 | 36 (65) | 4.00 (2.15 to 8.02) |
| CAMERA160 | < 1 year | Conventional | 148 | 55 (37) | Intensive | 151 | 76 (50) | 1.35 (1.03 to 1.79) |
| BROSG153 | 13 years | Symptomatic | 233 | 23 (14) | Intensive | 233 | 34 (20) | 1.48 (0.87 to 2.52) |
| BeSt128 | < 1 year | Monotherapy | 126 | 36 (29) | Prednisone combination | 133 | 44 (33) | 1.16 (0.79 to 1.72) |
| Infliximab (Remicade®; Centocor Biotech Inc., Horsham, PA, USA) combination | 128 | 45 (36) | 1.23 (0.84 to 1.92) | |||||
| FIN-RACo177 | < 1 year | Single drug | 100 | 18 (18) | Combination treatment | 99 | 36 (37) | 2.02 (1.21 to 3.47) |
| U-Act-Early113 | < 1 year | Methotrexate | 108 | 48 (44) | Tocilizumab (Actemra®; Roche, Basel, Switzerland)/methotrexate | 106 | 91 (86) | 1.92 (1.56 to 2.31) |
Four of six trials comparing treat to target with usual care reported remissions and all found more remissions with intensive management. Differences were clinically and statistically significant in three trials,129,179,186 but the differences were considered meaningful in the STREAM (Strategies in Early Arthritis Management) trial. 158 Two trials171,190 comparing treat to target with usual care reported only low disease activity states. One trial171 found a significant difference and the other trial190 did not.
The six trials49,113,128,177,183,185 that compared treatment protocols all reported remissions. Two trials113,177 included conventionally treated controls and found significantly more remissions with intensive managements. Five49,128,183,185 of these trials compared different intensive management regimens and found no significant differences in remissions between regimens. One of these trials [Behandel–Strategieën (BeSt)128] included a conventionally treated group that had fewer remissions, although the difference was not significant.
Two trials141,172 compared targets in patients receiving different intensive management regimens. Both trials141,172 found no significant difference between groups. Finally, two trials153,160 that did not fit into the previous categories had conventionally treated controls and reported more remissions with intensive managements. The difference was significant in one trial,160 but not in the other. 153
Twelve trials reported harms. Deaths were reported in seven trials. 49,113,128,129,141,183,186 There were no deaths in three trials and 11 deaths in the other four trials (three deaths in two standard care arms and eight deaths in 10 intensive management arms). Serious adverse events were reported in eight trials. 49,113,128,141,158,177,185,186 Overall, 11% patients had a serious event (12% of patients receiving intensive management and 9% of patients receiving standard care).
Two studies129,153 reported cost-effectiveness. In one study,129 treat to target dominated usual care and in the other study153 step-up combination treatments were cost-effective. In 5 of the 16 studies158,172,179,183,185 included in the clinical effectiveness review, no cost-effectiveness conclusion could be reached, and in one study49 no conclusion could be drawn in the case of patients designated as low risk. In the remaining 10 studies,113,128,129,141,153,160,171,177,186,190 and among patients identified as high risk in one study,49 cost-effectiveness was inferred. In most cases, treat to target is likely to be cost-effective, except where biological treatment in early disease is used initially. No conclusions could be drawn for established RA, as there were too few studies to assess benefit.
Clinical studies of treating moderately active rheumatoid arthritis intensively
The impact of achieving remission on subsequent disease activity and disability, particularly in moderate disease patients, was studied in 752 patients in a longitudinal observational study followed for ≥ 3 years and in secondary analyses of early and established RA trials.
The frequency of moderately active disease at baseline in the 752 patients in the observational study and at 6 months in the 558 patients in the trials was substantial. It varied from 39% to 45% of patients (Table 2). In all three studies, moderate disease patients were the largest group in terms of disease activity levels. The mean end-point DAS28-ESR scores in these patients after 1–3 years’ follow-up varied between 3.5 and 4.2, and their end-point mean HAQ scores varied between 1.3 and 1.5.
| Activity status | Observational study | Established RA trial (TACIT trial74) | Early RA trial (CARDERA trial73) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n (%) | Final DAS28-ESR, mean (95% CI) | Final HAQ, mean (95% CI) | n (%) | Final DAS28-ESR, mean (95% CI) | Final HAQ, mean (95% CI) | n (%) | Final DAS28-ESR, mean (95% CI) | Final HAQ, mean (95% CI) | |
| Initial/6 months: all patients | |||||||||
| Remission | 179 (24) | 2.4 (2.3 to 2.6) | 0.7 (0.6 to 0.9) | 24 (13) | 2.6 (1.9 to 3.2) | 1.2 (0.8 to 1.5) | 74 (20) | 2.9 (2.5 to 3.2) | 0.6 (0.5 to 0.8) |
| Low | 101 (13) | 3.0 (2.8 to 3.3) | 1.1 (1.0 to 1.3) | 20 (11) | 3.3 (2.7 to 3.8) | 1.3 (1.0 to 1.7) | 37 (10) | 3.4 (3.0 to 3.8) | 0.9 (0.6 to 1.1) |
| Moderate | 322 (43) | 3.5 (3.4 to 3.7) | 1.4 (1.3 to 1.5) | 69 (39) | 3.8 (3.5 to 4.1) | 1.5 (1.3 to 1.7) | 169 (45) | 4.2 (4.0 to 4.4) | 1.3 (1.2 to 1.4) |
| High | 150 (20) | 4.1 (3.9 to 4.4) | 1.7 (1.6 to 1.8) | 66 (37) | 4.8 (4.4 to 5.2) | 1.6 (1.4 to 1.8) | 99 (26) | 5.2 (5.0 to 5.5) | 1.6 (1.5 to 1.8) |
| Subsequent | |||||||||
| Never | 167 (52) | 4.3 (4.1 to 4.4) | 1.7 (1.5 to 1.8) | 46 (67) | 4.2 (4.0 to 4.5) | 1.6 (1.4 to 1.8) | 138 (82) | 4.6 (4.4 to 4.8) | 1.4 (1.3 to 1.5) |
| Any | 155 (48) | 2.8 (2.6 to 2.9) | 1.1 (1.0 to 1.2) | 23 (33) | 2.9 (2.3 to 3.4) | 1.3 (1.0 to 1.7) | 31 (18) | 2.7 (2.2 to 3.2) | 0.7 (0.5 to 0.9) |
| Significance | < 0.001 | < 0.001 | < 0.001 | NS | < 0.001 | < 0.001 | |||
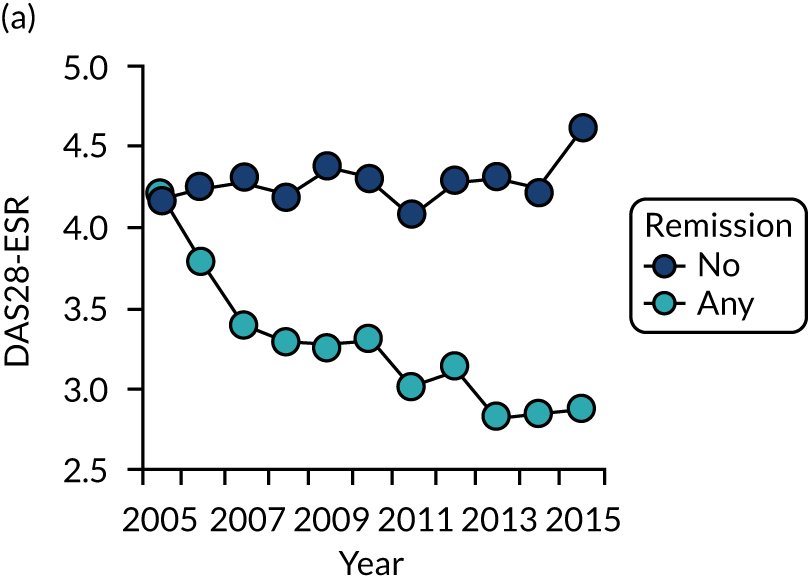
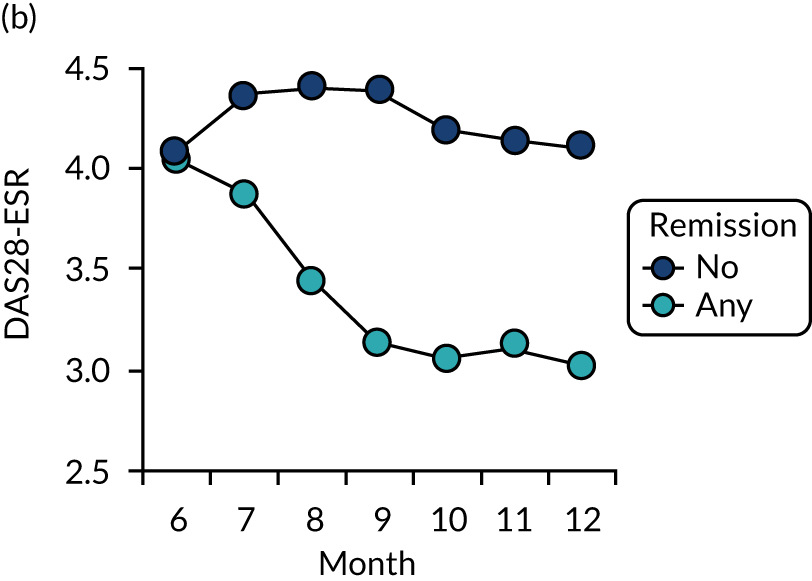
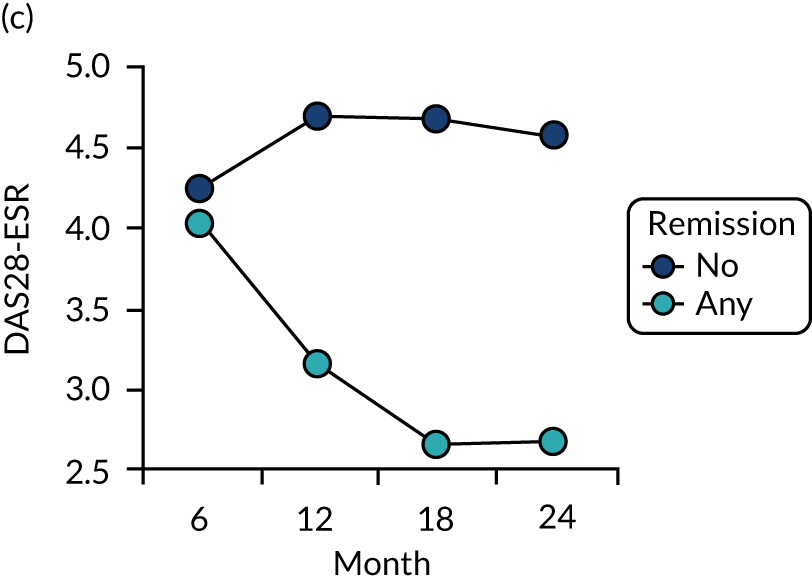
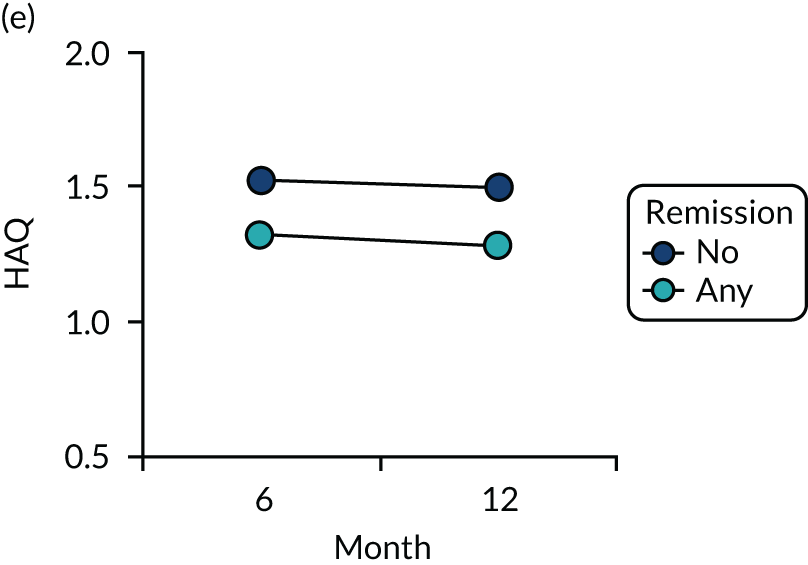
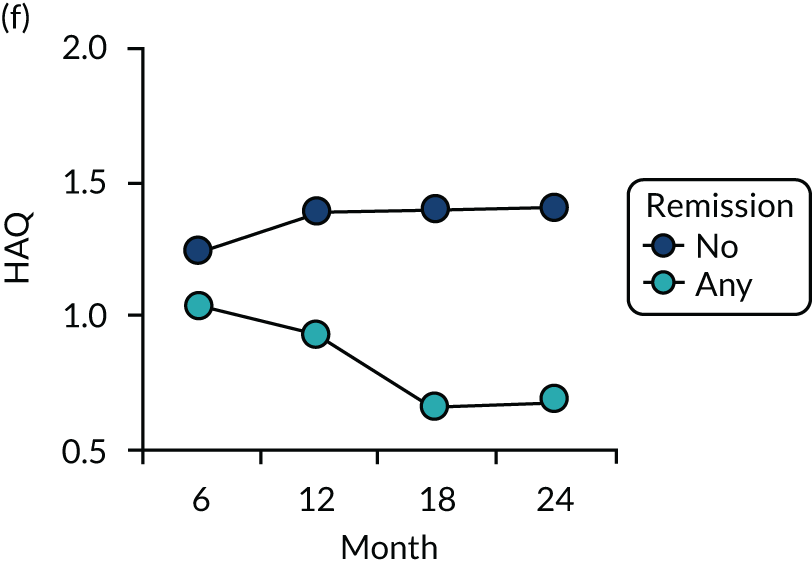
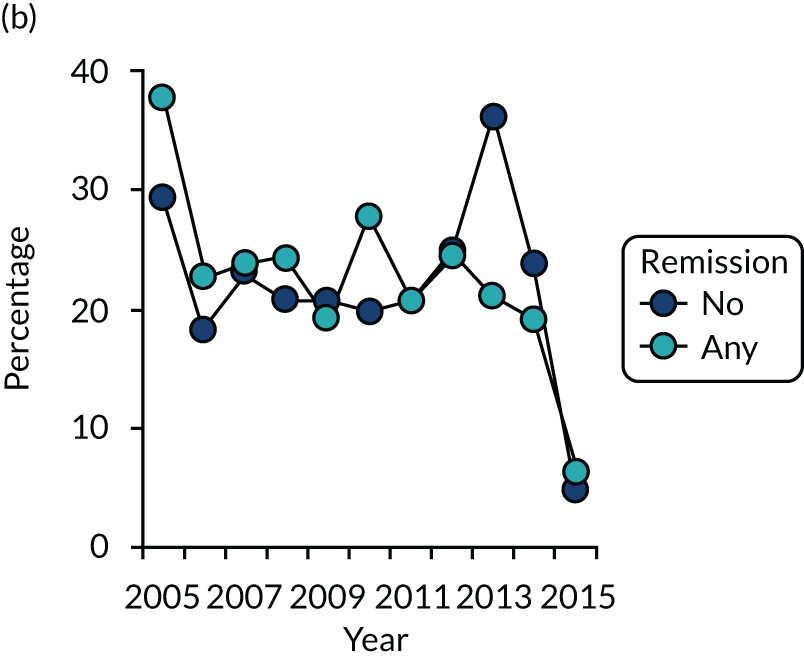
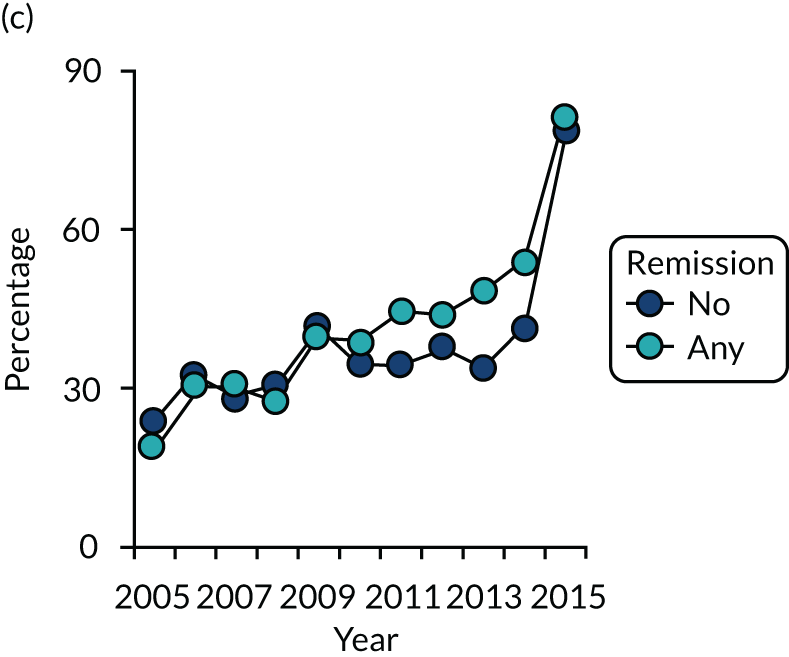
Dividing patients with initial/6-month moderate RA into those who subsequently had one or more DAS28-ESR remissions and those who did not (Figure 6) shows two things. First, those patients who achieved one or more remissions had lower subsequent mean DAS28-ESR scores than patients who did not. Second, mean HAQ scores were lower in patients who achieved one or more remissions.
Evaluating these changes in detail (see Table 2) showed that 18–48% of patients with initial/6-month moderate disease achieved one or more episodes of remission during the period of follow-up. The patients with one or more remissions had significantly lower end-point mean DAS28-ESR scores in all studies and significantly lower end-point mean HAQ scores in two studies (i.e. the observational study and the CARDERA trial73). In the other study (i.e. the TACIT trial74), end-point mean HAQ scores were lower in patients who achieved one or more remissions, but the difference was not significant. In patients with initially moderate disease, treatment intensities were comparable in patients with and without subsequent remissions (Figure 7).
FIGURE 7.
Changes in DMARD and biologic prescribing in patients with initial moderate disease activity in the observational study. Patients are divided by whether they had one or more remissions during follow-up. (a) DMARD monotherapy; (b) DMARD combination; and (c) biologics.
Limitations
The studies in this section were limited by the types of patients studied, their assessment of benefits and risks and the type of intensive management that was used.
Patients studied
It is likely that patient inclusion and follow-up strategies in observational studies have changed over time. In particular, patients with milder disease may have attended more frequently in recent years. Such changes in patient care could explain some or all of the reductions in disease activity we observed. It is also possible that the clinical phenotype of RA has evolved, with milder disease increasing in frequency. However, a recent analysis of English early RA patients since 1990 does not suggest that there have been major changes in RA clinical phenotypes. 196
Assessing benefits and risks
The assessment of remission and the duration of treatment varied in the trials of intensive management in the systematic review. This variation created unavoidable complexities when combining data from studies. When there are many studies, as occurred in the comparison of all intensive managements, combining heterogeneous data appears reasonable. However, when there are few studies, as occurred in comparisons of treat-to-target strategies, it is best avoided.
Evidence about the benefits of intensive management are almost entirely based on clinician-defined outcomes, such as changes in disease activity (e.g. remissions, reductions in disability and erosive damage). The extent to which patients consider intensive management to be beneficial is largely unknown. Patients can have different perspectives to clinicians. 197–199
One final and important issue is that we did not assess the potential of intensive management to harm patients in detail. In routine practice settings, it is particularly difficult to assess harms because they are not reported in any organised way. Nevertheless, we found no evidence of intensive management substantially increasing adverse events. Published systematic reviews of intensive management, predominantly using biological treatments, have also not found any substantial increases in adverse events with more intensive treatment regimens. 23,200,201
Treatments
There is no internationally agreed definition of what constitutes intensive management in RA. The numbers and types of treatments used, the time frames over which they are increased and the frequencies at which patients are assessed vary across studies. The absence of definite agreement makes it difficult to compare the benefits of intensive management.
Relation to overall programme
The intention in this section was to place the TITRATE programme in perspective. The various studies highlighted what is accepted and where there are continuing uncertainties.
Generally agreed areas
There is extensive evidence that intensive management in RA patients increases the frequency of remissions. One consequence has been that the use of intensive management is supported in all clinical guidelines. Another consequence has been that, over the last two decades, the intensity of treatment has increased in routine practice settings. This change has been reported in other settings. 67,202–204 Associated with the increased use of intensive managements have been reductions in overall disease activity levels and increases in the frequency of remissions. These findings have also been reported by others. 57,60 In addition, erosive progression has lessened substantially for an even longer period, and this is most likely a consequence of increased treatment intensity together with earlier diagnosis and treatment, although it remains a relevant outcome measure. This finding has also been identified in other studies and reviews. 59,205
Continuing uncertainties
There is considerable support from expert opinion in clinical guidelines for treating moderate RA intensively. We also found, in our analysis of observational data and trials, that remissions are associated with overall reductions in disease activity levels in patients with moderate disease activity. Although there is some evidence that reducing disease activity improves disability in patients with moderate RA, our results were not conclusive and further work is needed to resolve this important question.
It is also not possible to be certain that achieving remission in patients with moderate RA in our observational study and in secondary analyses of completed clinical trials was a result of the intensity of their treatment. This uncertainty can be resolved only in a prospective clinical trial that directly tests this hypothesis.
Treatment targets and predictive factors
The studies in this section evaluated RA treatment targets and simple outcome predictors using observational studies and secondary analyses of clinical trials. The studies addressed some aspects of the complex problem of what to measure when assessing RA patients.
Aims
Research studies with two overarching aims are included in this section. These aims comprised (1) defining optimal treatment targets and (2) identifying simple response predictors. They addressed the complex problem of what to measure when assessing RA patients. Optimising treatment targets and response predictors are important for interpreting the results of the TITRATE programme and implementing its findings in clinical care. Five parts of the research have been published. 206–210
Treatment targets
Targets must balance the ideal with the practical. Stringent targets may deliver optimal outcomes in some individuals, but achieve fewer overall benefits than more readily achievable targets. The TITRATE programme adopted DAS28-ESR remission as its primary target because it is the most widely used composite remission assessment. There is also extensive evidence that achieving DAS28-ESR remission optimises health-related quality of life and function and minimises radiological damage. 211–215 Sustained remission over time is particularly important because it is associated with better outcomes than remission at a single time point. 216,217 We collected components of other composite measures to compare their value as targets during intensive management.
In this section, we evaluated four aspects of treatment targets: (1) comparisons of sustained remission (persistent remission after 6 months’ treatment) and point DAS28-ESR remission and low disease activity, (2) the impact of lesser improvements in DAS28-ESR, (3) limitations of DAS28-ESR in comparison to other composite assessments and (4) associations of DAS28-ESR components with health-related quality of life.
We focused on these aspects of treatment targets, as they are important and we had access to relevant observational and trial data. We could not examine all indices, as some of the necessary data are not collected in routine care settings or our published trials. As C-reactive protein (CRP) levels and physicians’ global assessments are not usually measured in routine practice in England, we could not study the SDAI. 218
Simple baseline outcome predictors
Using baseline measures to predict clinical outcomes may help routine practice. We therefore assessed the value of simple four-point scores, HAQ scores alone and mental health status.
We selected these areas on the basis of what was practical and likely to be used in clinical practice. There was a case to assess rheumatoid factor and subtypes and other autoantibodies in predicting RA outcomes,81,219,220 but these were not used in the observational studies and trials that we could access.
Methods
Observational studies
We further studied the observational longitudinal cohort study established in 2005 at Guy’s Hospital. The study focused on 752 patients followed over ≥ 3 years. Details of these patients are given in Appendix 1, Table 28. We also studied 155 early RA patients who completed 12 months’ follow-up with clinical data at 0, 6 and 12 months in an observational study [Early Rheumatoid Arthritis Network (ERAN)]. Details of these patients are given in Appendix 1. We selected these studies because they involved patients treated in recent years. More historical studies were not used because patients received far less intensive management.
We also evaluated a compilation of single time point observational studies of outpatients with RA. 72,221–223 The patients included 747 European white RA patients, 197 black African/Caribbean British patients and 430 Arab patients seen in rheumatology clinics in Saudi Arabia. No specific treatment policies were followed in these patients. Details of these patients are given in Appendix 1, Table 29.
Clinical trials
We further evaluated the 379 patients completing the 24-month CARDERA trial73 and the 179 patients completing the TACIT trial. 74 We also evaluated patients with established RA in the OPTTIRA (Optimizing Treatment with Tumour Necrosis Factor Inhibitors In Rheumatoid Arthritis) trial. 224 The OPTTIRA trial, which lasted 6 months, enrolled 103 patients, and complete end-point data were available in 97 patients. Details of these patients are given in Appendix 1, Table 31.
Clinical assessments
We assessed disease activity using DAS28-ESR, CDAI and Routine Assessment of Patient Index Data 3 (RAPID3), disability using the HAQ, and health-related quality of life using EuroQol-5 Dimensions (EQ-5D) and the Short Form questionnaire-36 items (SF-36). 225–230
Further details are given in Appendix 2.
Statistical analyses
Data were analysed descriptively using means, SDs and 95% CIs or medians and IQRs for non-normally distributed data. Other tests included chi-squared tests, assessments of sensitivity and specificity, t-tests, regression analyses, Spearman’s correlations and multiple linear regression methods. Further details are given in Appendix 3.
Key findings
Treatment targets: DAS28-ESR and disability
The relationships between remission and low disease activity with disability and quality of life were studied in early and established RA trials and an observational cohort. In these patients, both sustained remission and sustained low disease activity were relatively uncommon. Between 5% and 9% of patients had sustained remissions and 9–16% of patients had sustained low disease activity. More patients had remission and low disease activity at single time points. Between 35% and 58% of patients had an episode of remission and between 49% and 74% of patients had an episode of low disease activity.
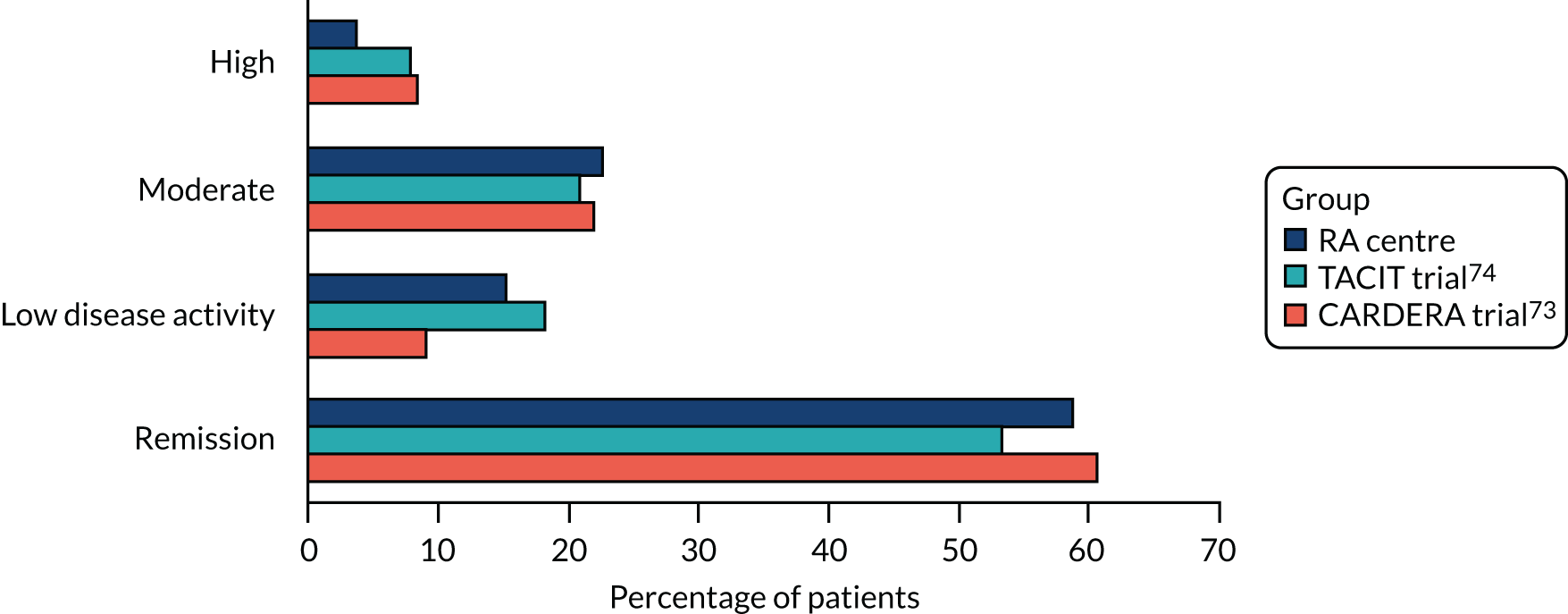
Disease Activity Score for 28 joints based on ESR scores varied substantially after patients had achieved an episode of remission. End-point DAS28-ESR scores (at 12 and 24 months in TACIT and CARDERA trials,73,74 and at final assessment in the observational study) in patients achieving an episode of remission showed that 53–61% of patients were still in remission, 9–18% of patients had low disease activity, 21–22% of patients had moderate disease activity and 4–8% of patients had high disease activity. These findings are shown in Figure 8.
FIGURE 8.
End-point DAS28-ESR category after attaining point remission.
Individual patients showed marked levels of variation in their subsequent DAS28-ESR scores following attaining remission. The extent of this within-individual variability was similar across all three cohorts. Figure 9 shows DAS28-ESR scores for all patients following attaining remission in the longitudinal observations study in patients with at least five subsequent DAS28-ESR measures.
FIGURE 9.
Within-individual variability in DAS28-ESR scores after remission for patients in the longitudinal observational study. DAS28-ESR scores for each individual patient following an episode of remission are plotted, provided there were at least five subsequent DAS28-ESR measures. Reprinted from Seminars in Arthritis and Rheumatism, Volume 49, Scott IC, Ibrahim F, Panayi G, Cope AP, Garrood T, Vincent A, et al. The frequency of remission and low disease activity in patients with rheumatoid arthritis, and their ability to identify people with low disability and normal quality of life, pp. 20–6, Copyright 2019, with permission from Elsevier. 210
Sustained and point remissions had varying impacts on end-point low disability and normal EQ-5D scores (Table 3). Sustained remissions were most specific for low disability (97–98%) and normal EQ-5D (93–97%), but lacked sensitivity (low disability: 19–29%; normal EQ-5D: 19–36%). Point remission gave a better balance between sensitivity and specificity (low disability: specificity 50–78% and sensitivity 68–89%; normal EQ-5D: specificity 42–72% and sensitivity 70–93%).
| Group | Remission/LDA status | Patients | Mean end-point DAS28 (95% CI) | End-point HAQ | End-point EQ-5D | ||||
|---|---|---|---|---|---|---|---|---|---|
| HAQ ≤ 0.5 | Specificity | Sensitivity | Normal EQ-5D | Specificity | Sensitivity | ||||
| CARDERA trial73 (n = 379) | Sustained remission | 26 (7%) | 1.80 (1.61 to 1.99) | 19/91 | 98% | 21% | 16/60 | 97% | 27% |
| Point remission | 132 (35%) | 2.81 (2.57 to 3.04) | 68/91 | 78% | 75% | 42/60 | 72% | 70% | |
| End-point remission | 80 (21%) | 1.92 (1.80 to 2.03) | 48/91 | 91% | 51% | 35/60 | 86% | 58% | |
| Sustained LDA/remission | 45 (12%) | 2.02 (1.84 to 2.19) | 33/91 | 96% | 36% | 26/60 | 94% | 33% | |
| Point LDA/remission | 187 (49%) | 3.09 (2.89 to 3.28) | 79/91 | 63% | 87% | 52/60 | 58% | 87% | |
| End-point LDA/remission | 114 (30%) | 2.20 (2.09 to 2.32) | 62/91 | 82% | 68% | 46/60 | 79% | 77% | |
| TACIT trial74 (n = 192) | Sustained remission | 10 (5%) | 1.66 (1.32 to 2.00) | 6/31 | 97% | 19% | 4/17 | 96% | 19% |
| Point remission | 80 (42%) | 2.81 (2.53 to 3.10) | 19/31 | 63% | 68% | 13/17 | 62% | 77% | |
| End-point remission | 41 (22%) | 1.92 (1.77 to 2.08) | 17/31 | 85% | 55% | 10/16 | 82% | 59% | |
| Sustained LDA/remission | 17 (9%) | 1.75 (1.50 to 2.00) | 8/31 | 94% | 26% | 6/17 | 94% | 35% | |
| Point LDA/remission | 119 (62%) | 3.18 (2.94 to 3.43) | 29/31 | 44% | 94% | 17/17 | 41% | 100% | |
| End-point LDA/remission | 66 (35%) | 2.29 (2.14 to 2.44) | 23/31 | 72% | 74% | 13/17 | 69% | 81% | |
| RA centre (n = 752) | Sustained remission | 67 (9%) | 1.56 (1.46 to 1.67) | 52/180 | 97% | 29% | 21/59 | 93% | 36% |
| Point remission | 437 (58%) | 2.83 (2.75 to 2.91) | 160/180 | 50% | 89% | 55/59 | 42% | 93% | |
| End-point remission | 167 (22%) | 1.98 (1.90 to 2.05) | 106/180 | 87% | 57% | 37/59 | 78% | 52% | |
| Sustained LDA/remission | 120 (16%) | 1.91 (1.81 to 2.01) | 73/180 | 92% | 41% | 24/59 | 86% | 41% | |
| Point LDA/remission | 560 (74%) | 3.07 (2.99 to 3.14) | 174/180 | 31% | 97% | 57/59 | 25% | 97% | |
| End-point LDA/remission | 310 (41%) | 2.41 (2.34 to 2.48) | 142/180 | 70% | 79% | 50/59 | 62% | 85% | |
Attaining sustained low disease activity was also highly specific for low disability (92–96%) and normal EQ-5D (86–94%), but lacked sensitivity (low disability: 26–41%; normal EQ-5D: 33–41%). Low disease activity at any point was highly sensitive (low disability: sensitivity 87–97%; normal EQ-5D: sensitivity 87–100%), but had only moderate specificity (low disability: specificity 31–63%; normal EQ-5D: specificity 25–58%).
Treatment targets: optimal responses in DAS28-ESR scores
An alternative way of assessing the inter-relationship between DAS28-ESR scores, disability and quality of life was examining the impact of EULAR responses in clinical trial settings. This approach was taken in another study208 that evaluated the impacts of moderate and good EULAR responses on changes in HAQ scores at the end points of early and established RA trials.
Moderate EULAR responders’ mean HAQ scores decreased by 0.39 and 0.33 in the CARDERA and TACIT trials, respectively. 73,74 In contrast, EULAR good responders had reductions of 0.88 and 0.64, respectively. In both trials, the difference between moderate and good responders exceeded the minimum clinically important difference for HAQ scores (0.22). The differences in mean reductions of 0.49 and 0.30 between moderate and good responders were significant (p < 0.01, unpaired t-test).
There were similar findings for EQ-5D scores. In moderate EULAR responders, EQ-5D scores increased by 0.18 and 0.15. In good EULAR responders, EQ-5D scores increased by 0.30 in both trials. The differences (0.12 and 0.15) between moderate and good responders also exceeded the minimum clinically important difference, which is generally considered to be 0.07, and were significant (p < 0.01, unpaired t test).
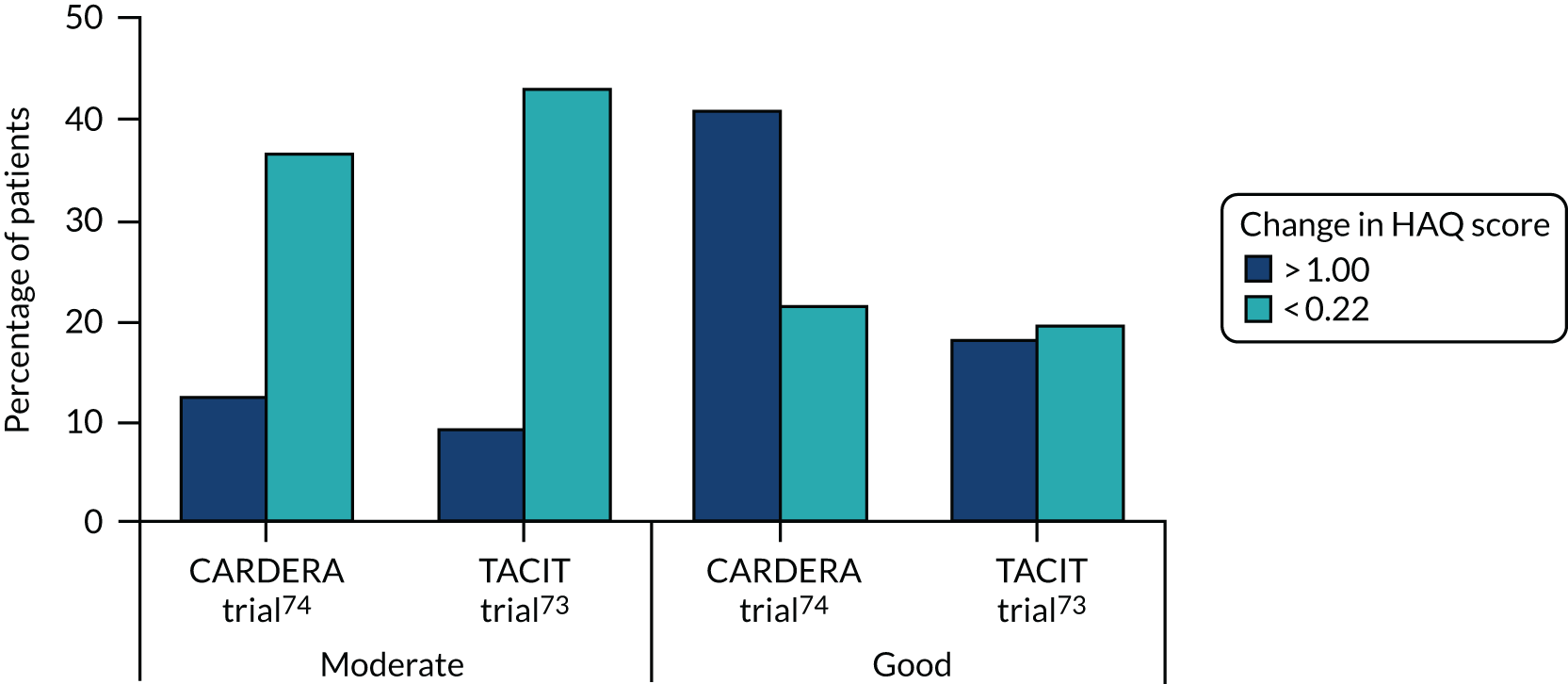
In addition, the frequencies of large and minimal improvements in disability and quality of life were assessed in these patients (Figure 10). With HAQ scores between 41% and 18%, good EULAR responders had large decreases in HAQ score (> 1.00) in early and established RA. However, only 13% and 9% of moderate EULAR responders had such reductions. By contrast, only 21% and 20% of good EULAR responders had minimal changes in HAQ score (> 0.22), compared with 37% and 43% of moderate EULAR responders.
FIGURE 10.
Changes in HAQ score in moderate and good EULAR responders. In early and established RA trials (CARDERA trial73 and TACIT trial,74 respectively). Shows per cent of patients with substantial (> 1.00) and minimal changes (< 0.22). Adapted with permission from Mian et al. 208 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
Treatment targets: DAS28-ESR and alternative assessments
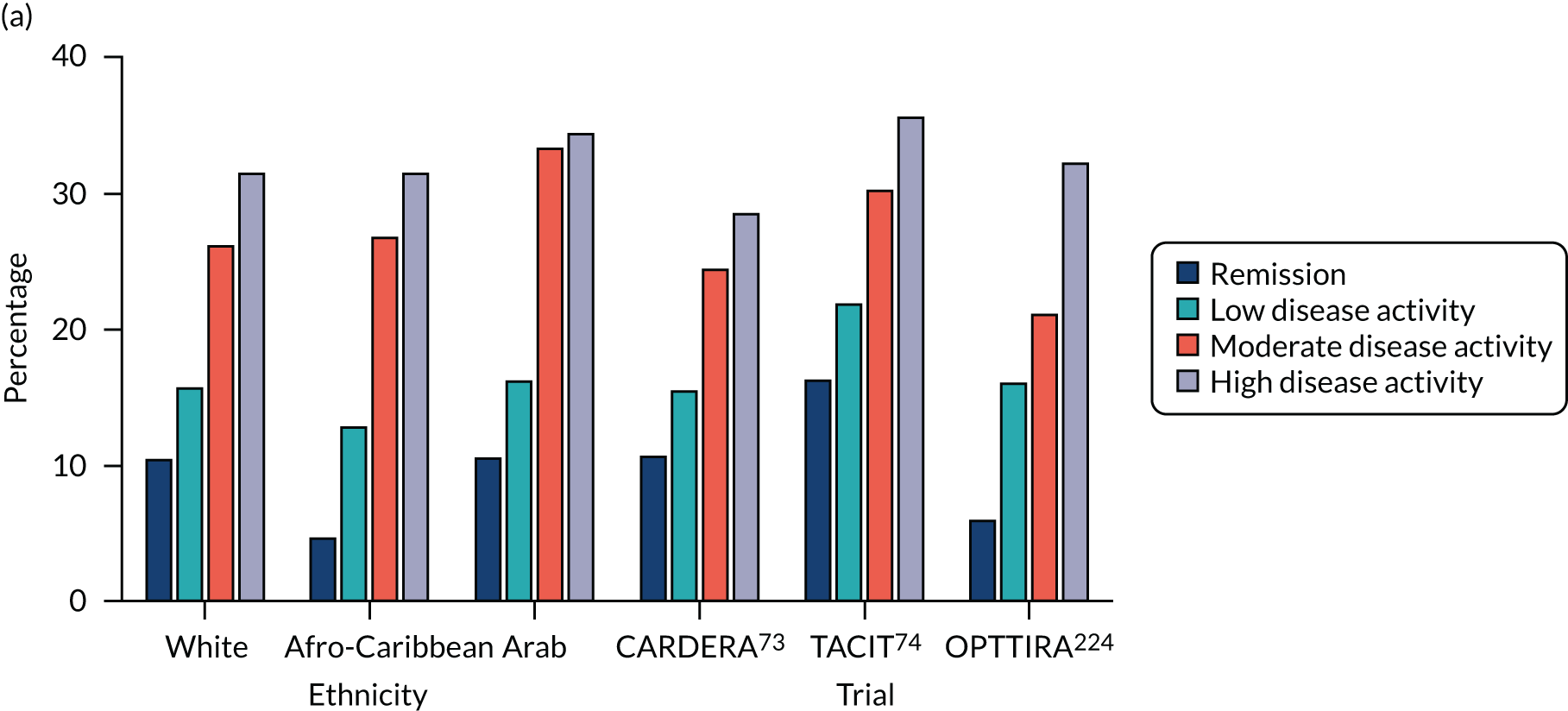
The inter-relationships between the four components of DAS28-ESR scores were assessed over the four disease activity levels: (1) remission, (2) low disease activity, (3) moderate disease activity and (4) high disease activity. Initially, these inter-relationships were assessed in an observational study of 747 European white patients. This analysis showed that ESRs contributed most to mean DAS28-ESR scores at all activity levels. However, their contribution was greatest in remission, when ESRs accounted for 70% of DAS28-ESR scores. The contribution of ESR decreased to 40% of DAS28-ESR scores in active disease. In contrast, the contributions of tender joint count to overall DAS28-ESR scores declined as DAS28-ESR fell. Swollen joint counts and patient global assessments showed small stable contributions to DAS28-ESR scores over all disease activity levels.
These findings were replicated in two further observational studies of 197 black African/Caribbean British patients223 and 430 Arab patients. 222 They were also replicated in three clinical trials (i.e. CARDERA,73 TACIT74 and OPTTIRA224) that involved 97–369 patients. Figure 11 shows the contributions of ESR and tender joint counts to DAS28-ESR disease activity levels in all six of these observational studies and trials.
FIGURE 11.
Contributions of different components to activity scores in different states of disease activity. Tender joints and ESR in DAS28 in data from six studies72–74,221–224 and components CDAI and RAPID3 in data from the CARDERA trial. 73 (a) DAS28 tender joints; (b) CDAI all components; (c) DAS28-ESR; and (d) RAPID3 all components.
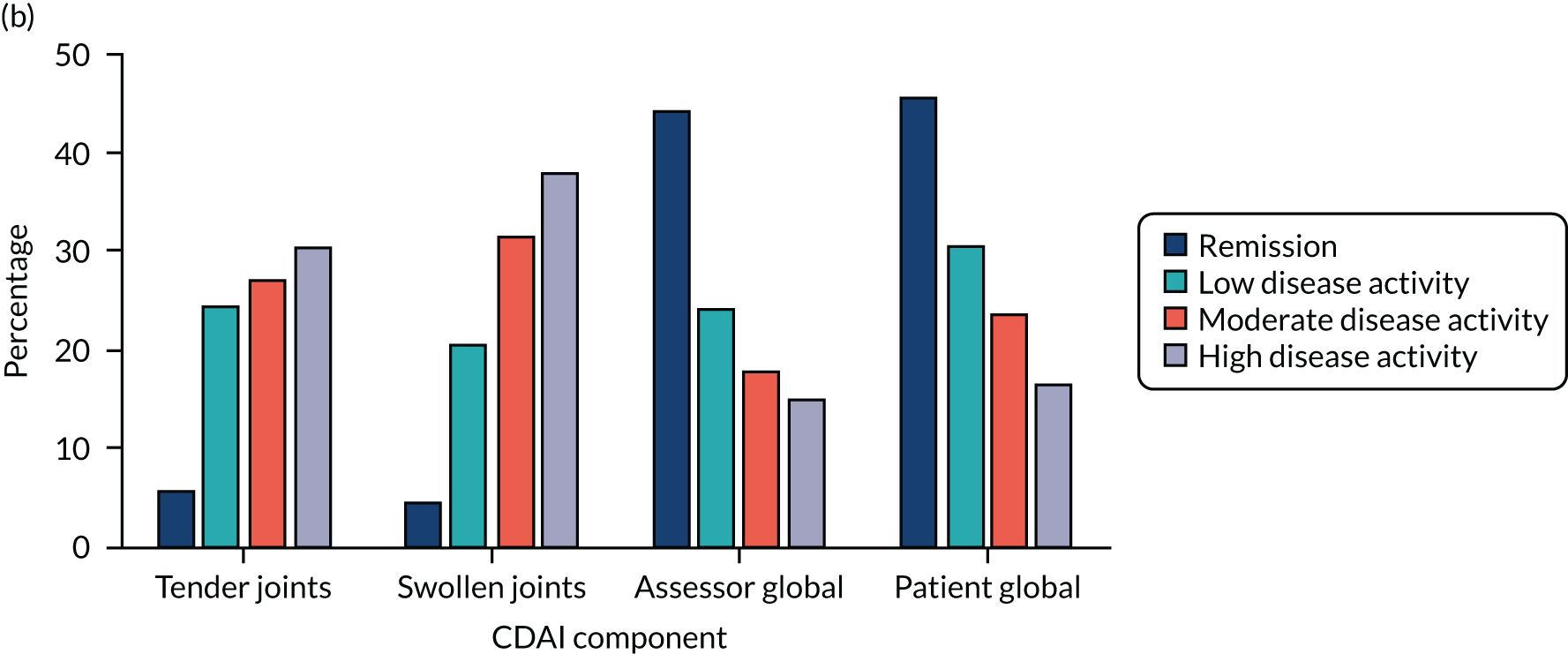
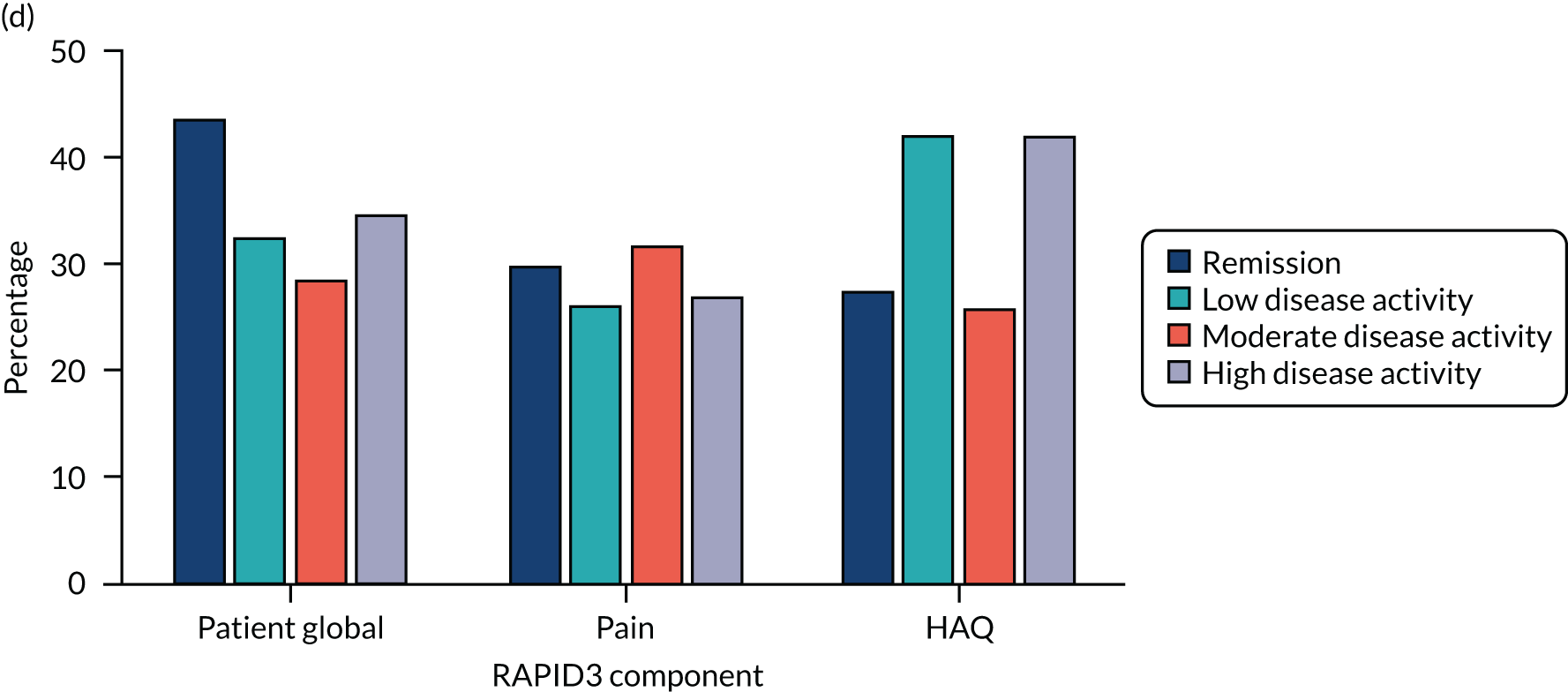
Two other composite scores – CDAI and RAPID3 – were studied in a comparative manner in one early RA trial (i.e. the CARDERA trial73). These alternative composite scores showed different patterns of variation across disease activity levels, which is also shown in Figure 11. With the CDAI, patient and assessor global assessments dominated in remission and swollen and tender joint counts dominated in active disease. With RAPID3, patient global score, pain score and HAQ score made relatively stable contributions to the overall score across all activity levels.
Treatment targets: DAS28-ESR and health-related quality of life
The association between different components of the DAS28-ESR and health-related quality of life was assessed using the SF-36. The inter-relationships were evaluated in clinical trials of 672 patients with early and established RA.
Linear regression models, which included all four DAS28-ESR components, examined the relationships to SF-36 physical component score (PCS) and mental component score (MCS). The regression models were adjusted for treatment, age, sex and disease duration. The regression models found significant correlations between patient global scores and both SF-36 summary scores in early and established RA (Table 4). Other components of DAS28-ESR had significant correlations in early RA patients, but did not have significant correlations in established RA patients.
| SF-36 | Early RA trial | Established RA trial | ||
|---|---|---|---|---|
| Standardised β (SE) | p-value | Standardised β (SE) | p-value | |
| PCS | ||||
| Swollen joint count | −0.19 (0.05) | < 0.001 | −0.06 (0.08) | 0.412 |
| Tender joint count | 0.00 (0.05) | 0.977 | −0.08 (0.09) | 0.370 |
| ESR | −0.08 (0.04) | 0.036 | 0.03 (0.07) | 0.676 |
| Patient global assessment | −0.45 (0.05) | < 0.001 | −0.36 (0.08) | < 0.001 |
| MCS | ||||
| Swollen joint count | −0.12 (0.06) | 0.029 | 0.05 (0.08) | 0.527 |
| Tender joint count | 0.15 (0.05) | 0.003 | −0.16 (0.09) | 0.080 |
| ESR | −0.12 (0.04) | 0.008 | 0.00 (0.07) | 0.974 |
| Patient global assessment | −0.43 (0.05) | < 0.001 | −0.33 (0.08) | < 0.001 |
Predictive factors: simple four-point scores
The first approach to predicting RA outcomes involved developing and testing a simple predictive score using data that are regularly collected in routine care settings. It focused on predicting persisting active RA. The score was developed in an observational study (ERAN),206 using 155 early RA patients who completed 12 months’ follow-up and had clinical data at 0, 6 and 12 months. Regression modelling identified three main predictors for persisting active disease: (1) tender joint counts, (2) HAQ scores and (3) ESR. Each of these predictors was then dichotomised (six or more tender joint counts, HAQ score of ≥ 1.0 and an ESR of ≥ 20 mm/hour) to give a four-point score. This index predicted persisting active disease (i.e. a DAS28-ESR score of > 3.2) at 6 and 12 months during follow-up in ERAN.
The value of this four-point score was then assessed in clinical trials in 558 patients with early and established RA. In the early RA trial, only 20% of patients with no predictors had persistent active disease, whereas 80% of patients with all three predictors had persistent active disease (Figure 12). This relationship was significant (p < 0.01). There was a similar relationship in the established RA trial, although this was weaker because none of the patients in the established RA trial had no initial predictive factors. In these patients, 20% of patients with one predictive factor had persistent active disease, compared with 60% of patients with all three predictors (p = 0.05).
FIGURE 12.
Simplified predictors of persistently active disease in trial patients.
Predictive factors: high baseline Health Assessment Questionnaire score as an outcome predictor
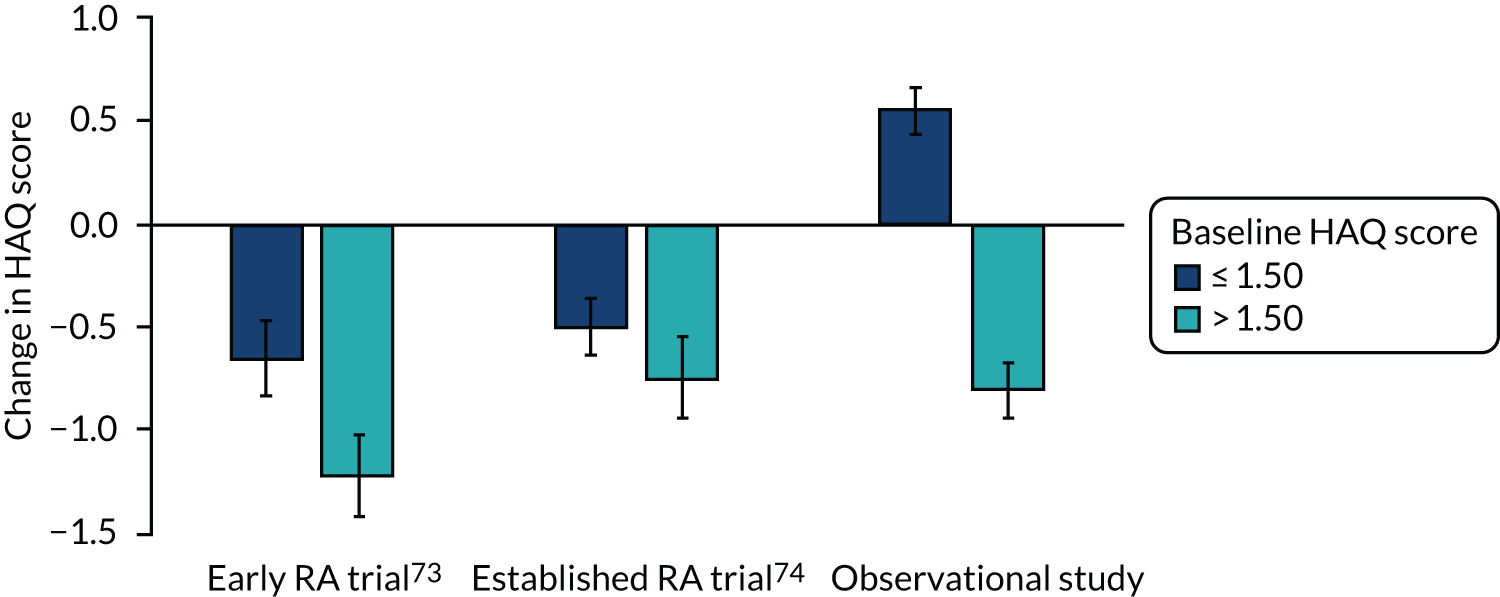
A second approach to predicting outcomes used initial HAQ scores alone. The value of baseline HAQ scores in predicting outcomes was assessed in 558 patients in early and established RA trials73,74 and 752 patients followed over ≥ 3 years in the observational study.
In both trials,73,74 patients with low baseline HAQ scores (≤ 1.50) had significantly more good EULAR responses (both p = 0.013) and significantly lower final mean DAS28-ESR scores (both p > 0.001) than patients with high baseline HAQ scores (> 1.50). These differences are shown in Figure 13. In the observational study, patients with low baseline HAQ scores had significantly lower overall mean DAS28-ESR scores (p < 0.001). In both trials, patients with high initial HAQ scores (> 1.50) had the largest end-point decreases in HAQ score if they achieved good EULAR responses. The observational study showed the same pattern.
FIGURE 13.
Initial high and low HAQ scores and changes in HAQ score in EULAR good responders. Mean and standard errors are shown.
Sequential changes in DAS28-ESR scores in both trials73,74 and the observational study showed that patients with low baseline HAQ scores had lower mean DAS28-ESR scores at all subsequent follow-up time points (Figure 14). The pattern of response was similar in all three groups, although the difference in DAS28-ESR scores attributed to baseline HAQ was larger in the early RA trial73 than in the established RA trial. 74
The relationship between baseline HAQ scores, subsequent remissions during treatment and changes in HAQ scores with treatment are shown in Table 5. The analysis shows two main things. First, patients with high baseline HAQ scores had significantly fewer remissions. Second, when remissions occurred, especially several remissions, the changes in HAQ scores were greatest in patients with high baseline HAQ.
| Study | Initial HAQ score | Remissions | HAQ score (95% CI) | Difference in HAQ score (95% CI) | |
|---|---|---|---|---|---|
| Initial | Final | ||||
| CARDERA trial73 | ≤ 1.50 | None | 1.01 (0.93 to 1.09) | 1.04 (0.92 to 1.16) | 0.03 (0.09 to 0.15) |
| One | 0.92 (0.75 to 1.08) | 0.72 (0.53 to 0.92) | –0.19 (–0.46 to 0.08) | ||
| Two or more | 0.93 (0.81 to 1.05) | 0.37 (0.24 to 0.50) | –0.56 (–0.71 to –0.40) | ||
| > 1.50 | None | 2.14 (2.08 to 2.19) | 1.04 (0.71 to 1.36) | –0.89 (–1.28 to –0.51) | |
| One | 1.93 (1.80 to 2.06) | 1.04 (0.71 to 1.36) | –0.89 (–1.28 to –0.51) | ||
| Two or more | 2.03 (1.90 to 2.16) | 0.65 (0.40 to 0.89) | –1.38 (–1.62 to –1.14) | ||
| TACIT trial74 | ≤ 1.50 | None | 1.18 (1.07 to 1.29) | 0.94 (0.74 to 1.15) | –0.24 (–0.44 to –0.03) |
| One | 0.97 (0.76 to 1.18) | 0.28 (0.01 to 0.55) | –0.69 (–1.11 to –0.27) | ||
| Two or more | 0.93 (0.73 to 1.12) | 0.53 (0.33 to 0.74) | –0.39 (–0.56 to –0.22) | ||
| > 1.50 | None | 2.25 (2.18 to 2.33) | 2.00 (1.88 to 2.12) | –0.26 (–0.35 to –0.16) | |
| One | 2.22 (2.07 to 2.38) | 1.79 (1.50 to 2.09) | –0.43 (–0.63 to –0.23) | ||
| Two or more | 2.07 (1.95 to 2.19) | 1.30 (1.07 to 1.54) | –0.77 (–1.00 to –0.54) | ||
| Observational study | ≤ 1.50 | None | 0.87 (0.78 to 0.97) | 1.29 (1.16 to 1.42) | 0.42 (0.27 to 0.56) |
| One | 0.83 (0.70 to 0.96) | 1.09 (0.92 to 1.26) | 0.25 (0.06 to 0.44) | ||
| Two or more | 0.73 (0.62 to 0.84) | 0.67 (0.51 to 0.83) | –0.06 (–0.22 to 0.09) | ||
| > 1.50 | None | 2.16 (2.10 to 2.22) | 2.11 (2.01 to 2.20) | –0.04 (–0.13 to 0.04) | |
| One | 2.09 (1.97 to 2.21) | 1.92 (1.72 to 2.13) | –0.17 (–0.40 to 0.07) | ||
| Two or more | 2.08 (1.95 to 2.22) | 1.49 (1.24 to 1.75) | –0.59 (–0.81 to –0.36) | ||
Predictive factors: anxiety and depression
The final approach to predicting outcomes assessed the impact of anxiety and depression using EQ-5D, which were related to outcomes in 379 patients in an early RA trial73 using linear regression models.
In unadjusted regression models, patients with moderate and high levels of depression and anxiety at baseline had higher HAQ and DAS28-ESR scores over time and at the trial end point. After adjusting for age, sex, disease duration, time, treatment type, baseline HAQ and DAS28-ESR scores and rheumatoid factor status, there were no longer between-group differences for HAQ score (Table 6). However, there continued to be a significant relationship between high levels of depression and anxiety at baseline and higher end-point DAS28-ESR scores.
| Model | Primary outcomes | Secondary outcomes: DAS-28 components | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HAQ | DAS-28 | SJC | ESR | PGA | TJC | |||||||||||||
| Post-treatment mean differences (SE) | Standardized mean differences | p-value | Post-treatment mean differences (SE) | Standardized mean differences | p-value | Post-treatment mean differences (SE) | Standardized mean differences | p-value | Post-treatment mean differences (SE) | Standardized mean differences | p-value | Post-treatment mean differences (SE) | Standardized mean differences | p-value | Post-treatment mean differences (SE) | Standardized mean differences | p-value | |
| Unadjusted | ||||||||||||||||||
| No depression/anxiety | ||||||||||||||||||
| Moderate depression/anxiety | 0.31 (0.07) | 0.44 | < 0.001 | 0.47 (0.15) | 0.34 | < 0.01 | 0.07 (0.08) | 0.10 | 0.33 | 0.06 (0.09) | 0.08 | 0.50 | 8.00 (2.24) | 0.37 | < 0.001 | 0.16 (0.09) | 0.18 | 0.10 |
| Extreme baseline depression/anxiety | 0.72 (0.15) | 1.01 | < 0.001 | 1.20 (0.30) | 0.86 | < 0.001 | 0.13 (0.15) | 0.19 | 0.38 | 0.18 (0.17) | 0.23 | 0.31 | 18.81 (4.56) | 0.87 | < 0.001 | 0.61 (0.18) | 0.70 | < 0.001 |
| Adjusteda | ||||||||||||||||||
| No depression/anxiety | ||||||||||||||||||
| Moderate depression/anxiety | 0.04 (0.06) | 0.06 | 0.45 | 0.10 (0.14) | 0.07 | 0.49 | −0.01 (0.08) | −0.01 | 0.95 | −0.04 (0.07) | −0.05 | 0.57 | 2.81 (2.19) | 0.13 | 0.20 | 0.05 (0.09) | 0.06 | 0.59 |
| Extreme baseline depression/anxiety | 0.21 (0.12) | 0.30 | 0.08 | 0.59 (0.29) | 0.42 | 0.04 | 0.06 (0.15) | 0.09 | 0.68 | 0.07 (0.15) | 0.09 | 0.64 | 8.72 (4.54) | 0.40 | 0.06 | 0.38 (0.17) | 0.44 | 0.02 |
At the trial end point, 80 (21%) patients had remissions (i.e. DAS-28 scores < 2.6). Patients with moderate levels of depression and anxiety at baseline had fewer clinical remissions than patients with no depression and anxiety at baseline [odds ratio (OR) 0.50, 95% CI 0.29 to 0.88; p = 0.02]. Patients with high levels of depression and anxiety symptoms at baseline also had reduced odds of reaching remission; however, this comparison was not significant (OR 0.77, 95% CI 0.25 to 2.33; p = 0.64). This is likely to reflect the small number of patients with extreme symptoms at baseline (n = 24), reducing the power to find a significant effect because of an imprecise estimate.
Limitations
Studies in this section were limited because they involved secondary analyses of previously collected data, omitted rheumatoid factor when predicting outcomes and enrolled patients using different classification criteria for RA compared with the TITRATE trial.
Secondary analyses of existing data
Most studies in this section were post hoc analyses of existing data. They did not address prespecified hypotheses. As a consequence, caution is needed interpreting their significance.
Rheumatoid factor and other autoantibodies
The prognostic studies did not consider the impact of rheumatoid factor isotypes or anti-citrullinated peptide antibodies. These are recognised response predictors that, together with smoking status, are linked to rheumatoid factor positivity. 231–234 However, as autoantibodies are measured in many different ways across centres, it is impractical to use them in current clinical practice studies. Smoking status is also not usually recorded in routine clinical practice.
Diagnostic criteria and intensive management strategies
Rheumatoid arthritis patients in all studies assessed were enrolled before the introduction of new diagnostic criteria for the classification of RA. There is evidence that these new criteria change the patients classified as having RA, particularly patients with seronegative disease. 45,235–238 The TITRATE trial uses the most recent criteria and it would have been a mistake not to do so. This change makes it difficult to relate historical findings exactly to new data.
The trials studied did not involve the same intensive management strategies used in the TITRATE programme and the impact of changing treatment in patients who did not achieve sustained remissions was not examined. These are general limitations with all trials involving intensive management strategies. 239–244
Relation to overall programme
Our findings in this section focused on the impact of different durations of remission, the effect of alternative remission assessments and the role of simple outcome predictors.
Duration of DAS28-ESR remission
We found that achieving sustained DAS28-ESR remission gave the greatest chance of minimising disability and maximising health-related quality of life; however, this was uncommon, reflecting international experience with sustained remission. 217,245–249 More patients benefited when the treatment target was to achieve DAS28-ESR remission at any time during follow-up.
End-point remission and low disease activity were both reasonable targets.
Other assessments of remission
Disease Activity Score for 28 joints based on ESR scores were dominated by the ESR at low levels and in remission. Other composite disease activity assessments, such as CDAI and RAPID3, show different patterns in their components as disease activity changes. However, there was no reason to favour one composite index over another. These findings reflect the ongoing debate about how best to use composite indices in assessing RA disease activity. 250–254 We also found that patient global assessment, a component of most composite measures, was most closely associated with patient-assessed health-related quality of life. Several other recent reports255–257 have highlighted the importance of patient global assessments in RA.
Simple outcome predictors
Poor outcomes were predicted by several simple baseline measures, including a simple four-point predictive score, initial HAQ score and the presence of anxiety and depression. The situation with the HAQ was complex, as the largest improvements with treatment were seen in patients who had high initial HAQ scores and then showed substantial clinical improvements and achieved remission. Other research has highlighted the relevance of baseline HAQ score258–261 and depression262–265 in predicting RA outcomes.
Delivering intensive management
The studies in this section helped establish how best to provide intensive management to patients and evaluated patients’ expectations and identified practical approaches for delivering care. This part of the research had considerable PPI.
Aims
Research studies with four related aims are included in this section. These aims comprised assessment of patients’ expectations, development of a patient handbook and clinician training manual, and design of supportive material, including a training course for rheumatology practitioners. There was considerable PPI in this part of the research. Four of these papers have been published. 266–269
The overall objective of workstream A was patient-led development and implementation of an experimental intensive management strategy for patients with RA with moderate disease activity. The qualitative research was pragmatic and specific in its nature, namely to examine the acceptability, development and evaluation of the intensive management intervention. A more phenomenological approach would have provided richer data, but such a perspective would have been unsuitable for the aims and objectives of the work package.
Qualitative study of patient expectations
We explored the views and expectations of patients with moderately active RA and their carers about intensive management strategies. Several previous reports have examined the views of patients with more active RA. 270–272
Patient handbook
We developed a patient handbook to support patients who received intensive management, and this reflects growing recognition of the importance of the involvement and shared decision-making of patients in their disease management. 273–277 Patients helped to identify relevant information and ensured that its content was acceptable and accessible.
Clinician training manual
We developed a training manual to support clinicians to deliver intensive management. During the development of the manual, we systematically reviewed the evidence for psychological approaches, in general, and motivational interviewing (MI) to incorporate psychological approaches to support patients receiving intensive management. Psychological interventions are likely to be beneficial as adjunctive treatments for pain, fatigue and psychological distress in RA. 278 Health-care professionals can be trained to deliver psychological interventions to support patients with common long-term disorders,279 and MI fits this niche. 280,281
Motivational interviewing was identified as a candidate psychological technique because the trial research questions focused on treat-to-target approaches. Stopping, starting and changing medications and doses is a behaviour. Therefore, the intervention required a behavioural approach to support discussions about medication, which could lead to assessment of motivation for behaviour change in routine care by specialist nurses. The clinical and research expertise of Jackie Sturt, the academic lead for the psychological intervention component, identified the potential of MI to deliver the required behavioural changes around medication changes. Researchers undertook a review of the evidence to understand whether or not MI had been used experimentally in RA and the ways in which MI had been used in long-term condition self-care behaviours in general. The wealth of evidence in many long-term conditions and the absence of evidence in RA confirmed our decision to use MI. We considered it a good fit from both the clinical and theoretical perspectives. In addition, we noted that there was no existing evidence base for its use in this population.
Other developmental activities
Two other developments did not require primary research: (1) to devise treatment plans to capture patients’ views about their treatments and (2) the development of training courses for clinicians to deliver intensive management.
Methods
Qualitative studies of patient expectations
Focus groups and one-to-one interviews were conducted with nine patients with RA and five carers from four rheumatology clinics in three London hospitals. Two non-English-speaking patients were included and were assisted by a professional translator. The groups and interviews were audio-recorded, transcribed and assessed using a framework analysis approach. 282 Details of the patients and carers are shown in Appendix 1, Table 32.
Audio-recordings were transcribed verbatim. Transcripts were analysed by the researcher (LP). A second rater (HL) appraised the emergent themes from the transcripts and consensus between both researchers was reached. The transcripts were analysed using a framework analysis approach. 283 The process of framework analysis involves a series of stages: (1) familiarisation, (2) identification of a thematic framework, (3) indexing, (4) charting and (5) mapping and interpretation.
A combined inductive–deductive approach was taken, as the study had some specific issues to explore; however, it still allowed space to discover participants’ views and concerns. The codes were based on an iterative process that incorporated both the research question and line-by-line analysis of two patient and two carer transcripts. The remaining data were indexed in a systematic way in accordance with the thematic framework. Where new codes were identified, previously indexed interview transcripts were re-read to ensure that all relevant data were coded. 283,284
Patient workshop
Handbook development was facilitated by an audio-recorded workshop that involved six patients, with another patient giving more feedback via e-mail. None had substantial prior knowledge of intensive management. The workshop transcript was analysed using thematic content analysis. 285
Systematic reviews
Two systematic reviews were undertaken, searching MEDLINE and other databases using predefined terms. The first assessed systematic reviews of psychological interventions in RA. The second assessed MI in musculoskeletal diseases. Full details of these systematic reviews are given in Report Supplementary Material 1 and 2, including a PRISMA flow diagram (see Report Supplementary Material 1, Figure 5) and details of the included studies (see Report Supplementary Material 1, Tables 6–8).
Key findings
Patients’ and carers’ views and expectations
Patients’ and carers’ views about intensive management spanned several themes and are shown in Tables 7 and 8. One theme was treatment expectations (i.e. patients want to have improved physical symptoms, reduced pain, increased mobility and greater independence). A second theme was increased medication. Patients had varying views about taking more medication, subject to the stability and benefits of their current treatment regimens. Most patients did not receive drug combinations that fully controlled their RA and they were willing to try more intensive managements, despite concerns about potential side effects. Intensive management involved more frequent clinic appointments, but these were generally acceptable to patients and carers.
| Physical Outcomes | |
|---|---|
| Reduce pain | Maybe my general pain in my body will be reduced by this treatment . . . I have got constant pain in my body(Patient 9, male, 46 years) |
| Improve mobility | Since 2006, I have to keep walking with my stick and if I don’t do that, sometimes I fall. Imagine if one day I could put it away . . . I see some people when they do the intensive management that happens to them(Patient 1, female, 52 years) |
| Stabilise RA | So as long as it (the RA) don’t get any worse . . . doesn’t spread to other parts of the body and you can contain it, then I think that’s fair enough . . . If it’s stabilised, that’s as good as it’s going to get(Carer 3, male, 71 years) |
| Reduce fatigue | Well I would like to be a bit more active, because I do get fatigue quite a lot(Patient 2, female, 62 years) |
| Increased Independence | |
| Rely less on family | . . . if you have got a wonderful family like I have got who . . . I could sit about and do nothing all day. Because they would say ‘I’ll do it, Mum you can’t do it, I’ll do it’. So a bit more independence is what I would like(Patient 4, female, 62 years) |
| Engage in more activities | It’s so frustrating; because there are things I want to do and I can’t . . . I can’t lift a kettle up if it’s too full(Patient 6, female, 64 years)I think if something could help her [patient] get that lifestyle back, where she could still do her own bits. She used to be a chef, so her not being able to cook, I think is one of the hardest things for her(Carer 1, female, 26 years) |
| Increased Medication | |
|---|---|
| Positive views | Yes, [I would try intensive management] anything that could be positive, because if it doesn’t work, it doesn’t work, we go and try another one [treatment](Patient 1, female, 52 years) |
| Negative views | Well at the moment we’re [carer and patient] doing fine . . . having had one very bad flare up about three years ago [patient], I should hate for that to happen again and I’ll be very apprehensive in changing the medication now. working, it’s balanced. Everything is nice and stable(Carer 3, male, 71 years) |
| Monthly Appointments | |
| Positive views | I wouldn’t mind . . . I would like to be able to sit down with someone, someone break down what these numbers mean from her [Mother’s] blood test . . . I just feel that if someone saw her [Mother] a bit more frequently the medication could be changed as soon as it [RA] gets worse(Carer 1, female, 26 years) |
| Negative views | How would I feel about it [attending monthly appointments]? Well, personally [hesitates] it would be a pain wouldn’t it really going up there [to the clinic] once a month? I know that sounds really ungrateful and I don’t mean that . . . what I mean is perhaps it [monthly appointments] might be a bit too much(Patient 4, female, 62 years) |
Tables 7 and 8 provide both positive and negative views, reflecting variation in participant responses. The findings identified that there was variation depending on individual circumstances.
Patients’ educational needs formed another theme. Some patients would readily take ‘whatever is prescribed’. Most wanted some information. A few preferred as much information as possible. An intervention needs to cater for all these requests. Continuity of care formed the final theme. Patients liked to see ‘their own’ rheumatologist and were concerned this specialist would ‘not know what has been happening’ when they saw different clinicians.
Development of the patient handbook: workshop for patients
Patients made several recommendations about the handbook content. They suggested that it include information on (1) the aims of intensive management, (2) its benefits above standard care and (3) the importance of patient’s active engagement with the trial.
The handbook included information about intensive management and what this would involve (e.g. monthly blood tests), including guidance on self-management (the contents of which were informed by the aims of the intervention) to help patients identify and work on key areas where they may be challenged (e.g. pain, fatigue, physical activity, medication adherence and low mood/anxiety).
Two researchers (SG and LP) collected information, for example current treatments for RA, intensive management in the TITRATE programme and self-management of life with RA. The information was gathered from evidence-based sources, including publications and current clinical guidelines, expertise from medical and allied health practitioners, and online sources from national charities National Rheumatoid Arthritis Society (NRAS) and Arthritis Research UK. The relevant information was then collated by one of the researchers (SG) into a draft handbook across nine sections. 268 Further details are given in Appendix 3.
Developing training manual: evidence for psychological support
Our systematic review of reviews on psychological support in RA identified eight relevant publications. 278,286–292 These systematic reviews all showed that psychological treatments resulted in significant improvements in functional disability, pain, fatigue, self-efficacy and coping in analyses of between 4 and 27 trials. The key findings are shown in Table 9. The effect sizes for these different interventions ranged from –0.09 for pain to 0.46 for coping.
| Outcome | Author | Measurement point | Effect size | 95% CI | Significance | Number of RCTs included in pooled result | Quality assessment |
|---|---|---|---|---|---|---|---|
| Disease activity/severity | Nyssen et al. (2016)291 | Post intervention | –0.02 | –0.37 to 0.32 | p = 0.89, NS | 3 | 10 |
| Follow-up | –0.61 | –0.96 to –0.26 | p < 0.001 | 3 | 10 | ||
| Patient global assessment | Riemsma et al. (2003)292 | Post intervention | –0.30 | –0.55 to –0.04 | p = 0.02 | 4 | 11 |
| Tender and swollen joints | Astin et al. (2002)278 | Post intervention | 0.15 | –0.09 to –0.39 | NS | 7 | 6 |
| Follow-up | 0.30 | 0.04 to –0.56 | p = 0.005 | 5 | 6 | ||
| Inflammation | Nyssen et al. (2016)291 | Post intervention | 0.10 | –0.34 to 0.53 | p = 0.67, NS | 3 | 10 |
| Functional disability | Astin et al. (2002)278 | Post intervention | 0.27 | 0.12 to –0.42 | p < 0.001 | 12 | 6 |
| Follow-up | 0.12 | –0.09 to –0.33 | NS | 7 | 6 | ||
| Riemsma et al. (2003)292 | Post intervention | –0.23 | –0.36 to –0.10 | p < 0.001 | 27 | 11 | |
| Follow-up | –0.10 | –0.23 to 0.02 | p = 0.10, NS | 18 | 11 | ||
| Knittle et al. (2010)289 | Post intervention | 0.32 | 0.13 to 0.51 | p < 0.001 | 17 | 6 | |
| Pain | Astin et al. (2002)278 | Post intervention | 0.22 | 0.07 to –0.37 | p = 0.003 | 13 | 6 |
| Follow-up | 0.06 | –0.17 to –0.29 | NS | 6 | 6 | ||
| Riemsma et al. (2003)292 | Post intervention | –0.09 | –0.19 to 0.02 | p = 0.10, NS | 26 | 11 | |
| Knittle et al. (2010)289 | Post intervention | 0.18 | 0.08 to 0.29 | p < 0.001 | 22 | 6 | |
| Fatigue | Cramp et al. (2013)287 | Post intervention | –0.24 | –0.40 to –0.07 | Significant | 13 | 11 |
| Depression | Astin et al. (2002)278 | Post intervention | 0.15 | –0.01 to –0.31 | p = 0.03 | 12 | 6 |
| Follow-up | 0.33 | –0.07 to –0.59 | p = 0.01 | 5 | 6 | ||
| Riemsma et al. (2003)292 | Post intervention | –0.14 | –0.25 to –0.04 | p = 0.009 | 13 | 11 | |
| Follow-up | 0.12 | –0.25 to 0.01 | p = 0.07, NS | 13 | 11 | ||
| Knittle et al. (2010)289 | Post intervention | 0.23 | 0.06 to 0.39 | p = 0.01 | 19 | 6 | |
| Anxiety | Knittle et al. (2010)289 | Post intervention | 0.17 | 0.02 to 0.32 | p = 0.03 | 11 | 6 |
| Self-efficacy | Astin et al. (2002)278 | Post intervention | 0.35 | 0.11 to 0.59 | p = 0.017 | 5 | 6 |
| Follow-up | 0.20 | –0.08 to –0.48 | NS | 3 | 6 | ||
| Coping | Astin et al. (2002)278 | Post intervention | 0.46 | 0.09 to 0.83 | p = 0.007 | 4 | 6 |
| Follow-up | 0.52 | –0.07 to –1.11 | p = 0.04 | 3 | 6 | ||
| Physical activity | Knittle et al. (2010)289 | Post intervention | 0.47 | 0.12 to 0.83 | p = 0.009 | 4 | 6 |
| Follow-up | 0.36 | 0.06 to 0.67 | p = 0.02 | 4 | 6 |
Developing training manual: evidence for motivational interviewing
Our systematic review identified seven relevant studies,293–299 including one systematic review,294 two clinical trials,293,298 two pilot studies297,299 and two interventional studies. 295,296
The systematic review by Chilton et al. 294 evaluated five trials of MI for the treatment of pain, fibromyalgia and osteoporosis. Although, overall, its findings were inconclusive because of the heterogeneity of the studies involved, it included considerable evidence favouring the use of MI.
The other six original research studies,293,295–299 which comprised clinical trials, pilot studies and interventional studies, also all provided some evidence in favour of using MI in these patients. Three studies293,295,299 were most relevant to the TITRATE programme. First, the trial of Ang et al. 293 showed short-term benefits on physical activity and clinical outcomes from six MI sessions. The pilot study of Ferguson et al. 299 provided some evidence that MI improved adherence with treatment. Finally, the interventional study of De Gucht295 showed that patient education that involved MI resulted in increased physical activity.
Completed patient handbook and clinician training manual
Based on these studies and associated developmental work, the handbook and training manual were finalised and used throughout the TITRATE trial.
Other developmental work
Activities that did not require primary research were the creation of a treatment plan for individual patients and devising a 2-day training course for specialist nurses and other clinicians involved in intensive management in the TITRATE trial. The course involved group-based participative experiential workshops over 2 days. It incorporated psychological and behavioural approaches to deliver supportive care together with clinical assessment and pharmacological prescribing algorithms to ensure that patients were able to receive intensive management. These were supplemented by remote one-to-one support while practitioners delivered the TITRATE intervention to their first three patients.
Limitations
The main limitations of the research in this section were doubts about the extent patients’ views should be generalised and limitations in evidence to support intensive management.
Generalisability of patients’ views
The patients’ and carers’ perspectives in the qualitative study and in the development of the handbook might not be generalisable to all patients with moderate RA. Some patients might have very different perspectives, although focus groups allow insights into the wide range of views that participants had about a specific issue, as well as how they interacted in a more ‘naturalistic’ setting. 300,301
Limitations of evidence
Although the manual and training course for clinicians delivering intensive management were evidence based, they had to reflect a range of expert opinions on management in addition to evidence-based care. There are also complex issues in the assessment of patients’ perspectives. For example, as RA predominantly affects women, the views of men with RA may be overlooked. 302
Participants were given the option of taking part in a focus group or a semistructured interview (in-person or via telephone). The reason for this was to provide choice to participants, minimise participant burden and also accommodate those experiencing RA symptoms. Focus group and semistructured interview data were analysed in the same way. This is a limitation of the study, as semistructured interviews are more suited to exploring individual experiences and focus groups more exploratory research. Telephone interviews were advantageous in this study for participants who may not have had the time or ability to take part had the interview been in person.
Relation to overall programme
Studies in this section focused on information and training needs. The studies highlighted the diversity of patients’ views and needs and showed the importance of developing a range of supportive material for patients.
Patients’ perspectives
Patients and carers had a range of views on intensive management. As they want improved physical symptoms and greater independence, most will try intensive management and accept the need for frequent appointments. However, they want sufficient information and continuity of care.
Supportive material
We developed a patient handbook and clinician training manual, which included patients’ views and the evidence about psychological interventions, and a training course for clinicians delivering intensive management. There is evidence that techniques such as MI can be used by clinicians after relatively brief training280 and that MI is relevant for patients with RA.
The TITRATE trial
This section reports the main results from the TITRATE trial, which formed the centrepiece of the programme. Previous sections have provided the rationale for the trial. These include the continuing high frequency of moderate RA, which is often associated with substantial disability, the extensive evidence that intensive management is effective and can increase remission rates, and the observational evidence that when patients with moderate RA achieve remission they have overall reductions in disease activity and disability subsequently.
Aims
The largest group of RA patients attending specialist clinics continue to have moderately active established RA and receive conventional synthetic DMARDs. These patients have potentially poor long-term outcomes. 303 The crucial unresolved question is whether or not intensive management will benefit them. The evidence in previous sections shows only one trial from the prebiologic era – the BROSG (British Rheumatoid Outcome Study Group) trial184 – that evaluated intensive management regimens in such patients. It reported only modest non-significant increases in remissions with intensive management.
The TITRATE trial bridges the gap in current evidence. It studied moderately active established RA patients receiving conventional synthetic DMARDs seen in specialist clinics. It tested the hypothesis that intensive management using drug therapy and a treatment support programme of non-drug approach given by specialist nurses resulted in higher remission rates than standard care.
In addition to optimising drug therapy, specialist nurses can provide holistic care to RA patients using other non-drug approaches. The TITRATE trial therefore also explored whether or not non-drug management by nurses can improve general symptoms such as pain and fatigue304–306 at the same time as delivering intensive management within a treat-to-target approach. The trial protocol was designed with substantial input and advice from patients and their carers. The TITRATE trial protocol and main trial findings have been published. 307,308
Methods
Design
This was an open-label 12-month pragmatic randomised multicentre, two-arm, parallel-group superiority trial.
Participants
Patients were recruited from 39 English rheumatology centres. Included patients were males and females aged > 18 years who met the 2010 RA classification criteria,309 had received at least 6 months’ treatment with conventional DMARDs, were currently receiving at least one DMARD, had moderate/intermediate disease activity (defined as a DAS28-ESR score of 3.2–5.1 with three or more swollen and/or tender joints out of 66/68 and at least one swollen joint) and who were able and willing to follow intensive management. Excluded patients included patients who had comorbidities that made intensive treatment inadvisable; in whom treatment with five or more DMARDs had failed; who had taken biologics; who had irreversible disability from extensive joint damage; who were women who were pregnant, breastfeeding or at risk of conceiving; who had recently been in another trial; or who were currently on an early RA pathway. 310
Interventions
Standard care
Clinicians followed local RA pathways for managing intermediate/moderate disease activity patients, which reflected national guidance. 304 There was no management goal, no specific treatment plan and no predefined follow-up plan.
Intensive management
Intensive management was delivered by rheumatology nurses or comparable health-care professionals trained to follow a predefined treatment support programme. Patients were reviewed monthly. The nurses (1) assessed patients’ RA and general functioning, (2) evaluated their drug treatment, (3) modified the drug treatment using a decision tool that reflected a ‘shared treatment plan’ that was planned together with patients during the first visit and (4) provided structured psychoeducation support using MI techniques.
Intensive management in the TITRATE trial spanned four strands:
-
Patients received information about their RA together with a handbook that outlined intensive treatments, possible medication side effects and ways of coping with the impact of RA on everyday life.
-
Drug treatment with conventional DMARDs and biologics was optimised following a treatment algorithm, which recommended options based on previous treatment, present treatment, contraindications, the patient’s preferences and clinical assessments.
-
Patients were given an intramuscular steroid injection if arthritis was not fully controlled.
-
Patients were provided with ‘treatment support’, with a particular focus on pain and fatigue management, physical activity, medication adherence, sleep and low mood/anxiety.
All medication given to patients was in accordance with national guidance from NICE or the national specialist society (i.e. the British Society for Rheumatology). 311
Assessments
All measures were assessed at baseline and at 6 and 12 months, except radiographs, which were taken at baseline and at 12 months. In addition, psychosocial measures (i.e. mood, anxiety, health beliefs and illness perceptions) were measured at baseline. Individual assessments were combined in composite indices [i.e. DAS28-ESR, Disease Activity Score for 28 joints based on C-reactive protein levels (DAS28-CRP), SDAI and CDAI]. A record was made of DAS28-ESR low disease activity states (i.e. a score of ≤ 3.2). Further details are given in Appendix 2.
Primary outcome measure
The primary outcome measure was DAS28-ESR remission (i.e. a DAS28-ESR score of < 2.6) at 12 months. 212,312 Alternative remission definitions consisted were a DAS28-CRP score of < 2.6, a SDAI score of ≤ 3.3, a CDAI score of ≤ 2.8 and ACR/EULAR Boolean remission at 12 months. 212,218,313,314
Secondary outcome measures
Secondary outcome measures included tender joint counts (28 and 68 joints), swollen joint counts (28 and 66 joints), ESR, CRP level, patient global assessments on 100-mm visual analogue scales (VASs), assessor global assessments on 100-mm VASs, pain and fatigue on 100-mm VASs, the HAQ,228 EuroQol-5 Dimensions, five-level version (EQ-5D-5L), score,315 plain-film radiographs of the hands and feet (scored using a modified Larsen score)316 and adverse events.
Sample size calculation
The most relevant UK trial129 compared treat to target with standard care in active early RA. Sixteen per cent of patients receiving standard care had end-point Disease Activity Score (DAS) remission. 129 We assumed that standard care in the TITRATE trial would also lead to 16% of patients achieving end-point DAS28-ESR remissions. We proposed rejecting the null hypothesis (i.e. RA patients with intermediate disease activity on DMARDs have no more remission after 12 months of intensive management) if intensive management increased remission at 12 months by ≥ 15%. Demonstrating this difference with 5% significance and 90% power meant randomising 358 patients (179 patients per group). We ended recruitment, for organisational reasons, after 3 years and when 335 patients were randomised (i.e. 94% of the planned sample size).
Randomisation
Potentially eligible patients were screened and reasons for non-entry recorded. Consenting patients were individually randomised using block randomisation with randomly varying block sizes. Stratifying by site ensured prerandomisation allocation concealment. Patients were randomised to intensive management or standard care in a 1 : 1 ratio. All staff involved in the conduct of the trial were unaware of the allocation sequence.
Blinding
The TITRATE trial was un-blinded, as patient involvement in their intensive management made blinding impossible. Independent assessors uninvolved in managing trial patients undertook clinical assessments. No specific checks were made on their knowledge of patients’ treatments. Pain, fatigue, disability and quality of life were self-assessed by patients. Radiographic reading was blinded to treatment group.
Statistical methods
Baseline characteristics were summarised by randomisation group as means and SDs and frequencies and percentages (categorical variables).
Randomised patients who received treatment were assessed on an intention-to-treat (ITT) basis. All participants had complete observations at baseline. Missing data at follow-up were imputed regardless of the reason(s) that they were missing. For subjects with missing outcomes, the baseline outcomes and other explanatory covariates (i.e. treatment group, sex, age, ethnicity, NHS region and disease duration) were used to impute the missing outcome data using predictive mean matching (PMM) with five nearest neighbours, assuming that unobserved measurements were missing at random (see Appendix 3 for detailed imputation descriptions).
A logistic regression analysis was used to analyse the primary outcome of remission at 12 months. Univariate analyses were adjusted for NHS region (design variable). Multivariable analyses were adjusted for sex, ethnicity, age, NHS region and disease duration. Alternative remission definitions were analysed similar to the primary outcome measure. Linear regression evaluated change at 12 months for the continuous primary outcome (i.e. DAS28-ESR) and secondary outcome measures.
For primary and secondary analyses that involve longitudinal measurements, linear mixed models were used to estimate the effect of treatment. Working correlation matrices were unstructured, which is not unduly complicated given that measurements were taken at three time points. Interactions between time and treatment group were also assessed in these models.
Valid/robust estimates of the precision of effects were obtained through use of the information sandwich estimator for all analyses. The estimates for primary outcome were presented as ORs with 95% CIs for the effect of intensive management. Statistical significance was determined at the 5% level using a two-sided test throughout. Serious adverse events and adverse rates in the two treatment arms were compared using comparisons of two independent proportions. Finally, complete-case analyses were also undertaken, which evaluated patients who followed the protocol and received 12 months’ treatment. All analysis was carried out using Stata® (StataCorp LP, College Station, TX, USA).
Key findings
Patients and analyses
Patients
Between August 2014 and July 2017, a total of 1405 patients were invited to participate, 459 patients were screened and 335 patients were randomised and treated (Figure 15). Of the randomised patients, 303 of 335 (90%) patients provided a primary outcome measure at 12 months, including three patients who withdrew but agreed to medical review only. Thirty-two (10%) patients were lost to follow-up.
FIGURE 15.
A CONSORT (Consolidated Standards of Reporting Trials) flow diagram for the TITRATE trial. Reproduced with permission from Scott et al. 308 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
Baseline data and numbers analysed
Demographic and disease assessments were similar in both patient groups (Table 10). The ITT analysis included all 335 randomised patients (168 patients received intensive management and 167 patients received standard care). The complete-case analysis, in which all data were present, comprised 258 patients (134 patients received intensive management and 124 patients received standard care). Additional baseline data are provided in Appendix 4, Table 38.
| Assessment | Treatment group | |
|---|---|---|
| Intensive management (N = 168) | Standard care (N = 167) | |
| Demographic | ||
| Age (years), mean (SD) | 56.4 (12.2) | 56.8 (12.0) |
| Disease duration (years), mean (SD) | 6.6 (7.0) | 5.2 (5.5) |
| Female, n (%) | 140 (83) | 130 (78) |
| Clinical assessment, mean (SD) | ||
| DAS28-ESR | 4.4 (0.5) | 4.3 (0.5) |
| DAS28-CRP | 4.5 (0.6) | 4.5 (0.6) |
| CDAI | 19.7 (6.5) | 20.4 (6.8) |
| SDAI | 20.6 (6.3) | 21.1 (6.6) |
| Tender joint counts (68 joints) | 12 (9) | 13 (9) |
| Swollen joint counts (66 joints) | 6 (5) | 5 (4) |
| ESR (mm/hour) | 18 (14) | 15 (13) |
| CRP (mg/l) | 8 (11) | 7 (8) |
| Assessor global rating (mm) | 39 (18) | 41 (18) |
| Patient global assessment (mm) | 43 (19) | 46 (21) |
| Fatigue VAS (mm) | 59 (25) | 52 (25) |
| Pain VAS (mm) | 40 (23) | 43 (23) |
| HAQ | 1.2 (0.7) | 1.2 (0.7) |
| EQ-5D-5L | 0.71 (0.16) | 0.70 (0.19) |
| Larsen score | 11 (17) | 9 (11) |
| Drug treatment, n (%) | ||
| Oral methotrexate | 59 (35) | 67 (40) |
| Subcutaneous methotrexate | 22 (13) | 19 (11) |
| Sulfasalazine | 30 (18) | 19 (11) |
| Leflunomide | 12 (7) | 11 (7) |
| Hydroxychloroquine | 29 (17) | 37 (22) |
| Azathioprine | 1 (1) | |
| Oral methotrexate/hydroxychloroquine | 7 (4) | 8 (5) |
| Oral methotrexate/sulfasalazine | 2 (1) | 1 (1) |
| Subcutaneous methotrexate/hydroxychloroquine | 3 (2) | 2 (1) |
| Subcutaneous methotrexate/sulfasalazine | 2 (1) | |
| Sulfasalazine/hydroxychloroquine | 1 (1) | 3 (2) |
Baseline treatments
All patients were taking one conventional DMARD and 15 of 168 (9%) intensive management patients and 14 of 168 (8%) standard care patients took two DMARDs. Methotrexate was the main conventional DMARD and was taken by 81 patients in the intensive management group and 86 patients in the standard care group (see Table 10).
Intensive management sessions
A total of 161 of 168 patients randomised to intensive management attended at least one session. Seven patients missed all sessions (three patients changed from intensive management to standard care after their first visit and four patients withdrew from the study and were lost to follow-up). A total of 139 of 161 (86%) patients attended at least eight (mean 11, SD 1.34) sessions. Twenty-two of 161 (14%) patients attended fewer than eight (mean 4, SD 1.94) sessions.
Intensive management treatments
A total of 140 patients started one conventional DMARD during the trial, 64 patients started a second and three patients started a third. These treatments were predominantly with non-methotrexate major DMARDs (hydroxychloroquine, n = 73; sulfasalazine, n = 55; leflunomide, n = 33). DMARD doses were increased in 69 patients and decreased in 15 patients. Biologics were given to 46 patients, and seven patients had a second biologic and two patients had a third biologic. Etanercept (as Enbrel®; Pfizer Inc., New York, NY, USA) was the main biologic and was given to 37 patients. Biologic doses were increased in two patients and reduced in two patients. Depot steroid injections were given to 72 patients (22 patients received one injection, 33 patients received two to four injections and 17 patients had five or more injections). These treatments are summarised in Table 11.
| Additional drug | Treatment group, n (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intensive management | Standard care | |||||||||
| Oral MTX (N = 68) | Subcutaneous MTX (N = 27) | SSZ (N = 31) | LEF (N = 12) | HCQ (N = 29) | Oral MTX (N = 76) | Subcutaneous MTX (N = 21) | SSZ (N = 22) | LEF (N = 11) | HCQ (N = 37) | |
| None | 13 (19) | 4 (15) | 3 (10) | 2 (7) | 20 (26) | 5 (24) | 7 (32) | 3 (27) | ||
| One DMARD | 23 (34) | 12 (44) | 12 (39) | 3 (25) | 5 (17) | 33 (43) | 12 (57) | 9 (41) | 5 (45) | 18 (49) |
| Two DMARDs | 13 (19) | 6 (22) | 11 (35) | 5 (33) | 9 (31) | 14 (18) | 1 (5) | 5 (23) | 11 (30) | |
| Enbrel | 16 (24) | 4 (15) | 4 (13) | 4 (42) | 9 (31) | 4 (5) | 1 (5) | 1 (4) | 2 (18) | 4 (11) |
| Benepali™ (Biogen Biosimilars, Maidenhead, UK) | 1 (1) | 1 (4) | 1 (3) | 1 (4) | 2 (3) | |||||
| Other TNFis | 2 (3) | 3 (10) | 3 (4) | 2 (10) | 1 (9) | 4 (11) | ||||
Standard care treatments
A total of 128 patients started one conventional DMARD during the trial, 35 patients started a second DMARD and two patients started a third DMARD. These treatments were predominantly with non-methotrexate major DMARDs (hydroxychloroquine, n = 50; sulfasalazine, n = 47; leflunomide, n = 25). DMARD doses were increased in 32 patients and decreased in nine patients. Biologics were given to 24 patients and two patients had a second biologic. Etanercept was the main biologic given to 12 patients. Biologic doses were not increased in any patient and were reduced in one patient. Depot steroid injections were given to 50 patients (28 patients received one injection, 19 patients received two to four injections and 3 patients had five or more injections). Table 11 summarises treatments during follow-up.
Primary outcome
Intensive management increased the frequency of DAS28-ESR remissions at 12 months compared with standard care. With intensive management, 32% (95% CI 25% to 40%) of patients had achieved remission, compared with 18% (95% CI 12% to 24%) of patients receiving standard care (Figure 16). Both unadjusted and adjusted logistic regression showed that these differences were significant (p < 0.01) and these differences are summarised in Table 12.
FIGURE 16.
Remission rates with intensive treatment and standard care.
| Remission classification | Unadjusted | Adjusteda | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| DAS28-ESR | 2.17 (1.28 to 3.68) | 0.004 | 2.38 (1.36 to 4.17) | 0.002 |
| DAS28-CRP | 2.44 (1.27 to 4.70) | 0.008 | 2.52 (1.28 to 4.99) | 0.008 |
| SDAI | 1.81 (0.94 to 3.47) | 0.074 | 1.90 (0.97 to 3.72) | 0.060 |
| CDAI | 1.92 (1.00 to 3.68) | 0.049 | 2.10 (1.07 to 4.09) | 0.030 |
| ACR/EULAR Boolean | 2.32 (1.04 to 5.18) | 0.040 | 2.44 (1.06 to 5.64) | 0.036 |
Other remission criteria and low disease activity at 12 months
Other types of remission
Disease Activity Score for 28 joints based on C-reactive protein levels, SDAI, CDAI and ACR/EULAR Boolean remissions at 12 months (see Table 12) also showed higher achievement of remission with intensive management (21%, 17%, 18% and 13%, respectively) than with standard care (10%, 10%, 10% and 6%, respectively). Logistic regression showed that most of these differences were significant (p < 0.05).
DAS28-ESR low disease activity
Low disease activity states were achieved by 48% (95% CI 39% to 56%) of patients receiving intensive management and 32% (95% CI 25% to 40%) of patients receiving standard care. Logistic regression showed that this difference was significant (unadjusted OR 1.94, 95% CI 1.22 to 3.10, p = 0.005; adjusted OR 2.04, 95% CI 1.25, 3.31, p = 0.004).
Clinical outcomes at 12 months
Disease activity scores and their components
The mean DAS28-ESR scores were significantly lower (p = 0.015 in unadjusted regression analyses and p = 0.001 in adjusted regression analyses) with intensive treatment (Table 13). There were also significant differences in DAS28-CRP, SDAI and CDAI scores. The mean tender and swollen joint counts and assessor and patient global scores were lower with intensive management (see Table 13). These differences were significant in unadjusted and adjusted linear regression analyses. However, mean ESR and CRP levels were unchanged during the trial, with no significant differences between treatment groups at 12 months.
| Assessment | Treatment group, mean (SE) | Linear regression | Mixed-effect model | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intensive management (n = 168) | Standard care (n = 167) | Unadjusted coefficients (95% CI) | p-value | Adjusted coefficient (95% CI) | p-value | Unadjusted coefficients (95% CI) | p-value | Adjusted coefficient (95% CI) | p-value | |
| DAS28-ESR | 3.4 (0.1) | 3.8 (0.1) | –0.6 (–0.9 to –0.3) | < 0.001 | –0.5 (–0.8 to –0.2) | 0.001 | –0.4 (–0.7 to –0.2) | 0.001 | –0.4 (–0.6 to –0.1) | 0.003 |
| Tender joints | 7.5 (0.7) | 10.8 (0.8) | –2.4 (–4.4 to –0.3) | 0.023 | –2.7 (–4.5 to –0.8) | 0.004 | –1.4 (–3.4 to 0.7) | NS | –1.7 (–3.5 to 0.2) | 0.076 |
| Swollen joints | 3.5 (0.4) | 4.9 (0.5) | –1.9 (–3.0 to –0.7) | 0.002 | –1.6 (–2.7 to –0.5) | 0.004 | –1.5 (–2.6 to –0.5) | 0.005 | –1.3 (–2.3 to –0.4) | 0.006 |
| ESR | 17 (1) | 15 (1) | –1.5 (–3.9 to 1.0) | NS | –1.1 (–3.4 to 1.1) | NS | –1.1 (–3.2 to 1.0) | NS | –0.7 (–2.7 to 1.2) | NS |
| CRP | 9 (2) | 7 (1) | 0.9 (–2.6 to 4.4) | NS | 1.5 (–1.8 to 4.7) | NS | 0.6 (–2.0 to 3.1) | NS | 1.3 (–0.9 to 3.5) | NS |
| Assessor global | 23 (2) | 31 (2) | –6 (–12 to –0.2) | 0.043 | –8 (–13 to –3) | 0.003 | –4 (–9 to 2) | NS | –5 (–10 to –1) | 0.015 |
| Patient global | 29 (2) | 41 (2) | –9 (–15 to –2) | 0.010 | –11 (–17 to –6) | < 0.001 | –6 (–12 to –1) | 0.026 | –9 (–14 to –4) | < 0.001 |
| Fatigue | 40 (2) | 50 (2) | –18 (–24 to –11) | < 0.001 | –15 (–21 to –9) | < 0.001 | –16 (–21 to –10) | < 0.001 | –13 (–18 to –8) | < 0.001 |
| Pain | 28 (2) | 37 (2) | –6.5 (–13.4 to 0.4) | 0.064 | –8.4 (–14.5 to –2.3) | 0.007 | –4 (–11 to 2) | NS | –6 (–12 to –1) | 0.015 |
| HAQ | 1.0 (0.1) | 1.1 (0.1) | –0.1 (–0.2 to 0.0) | 0.055 | –0.1 (–0.2 to 0.0) | 0.046 | –0.1 (–0.1 to 0.0) | NS | –0.1 (–0.2 to 0.0) | NS |
| EQ-5D-5L | 0.76 (0.02) | 0.72 (0.02) | 0.02 (–0.02 to 0.06) | NS | 0.03 (–0.01 to 0.07) | 0.078 | 0.02 (–0.01 to 0.05) | 0.275 | 0.02 (–0.01 to 0.05) | 0.121 |
| Larsen score | 13 (1) | 10 (1) | 0.5 (–0.1 to 1.0) | NS | 0.4 (–0.2 to 0.9) | NS | ||||
Disability and quality of life
There were only small improvements in disability assessed by the mean HAQ score and quality of life assessed by the mean EQ-5D score. The differences between treatment groups were not significant at 12 months (see Table 13).
Pain and fatigue
The mean pain and fatigue scores were significantly lower with intensive management in unadjusted and adjusted linear regression analyses (see Table 13). Clinically meaningful improvements (i.e. ≥ 10 points) in pain were achieved by 52% (95% CI 44% to 60%) of patients receiving intensive management and 42% (95% CI 34% to 49%) of patients receiving standard care. Logistic regression showed that this difference was not significant at the 5% level (adjusted OR 1.51, 95% CI 0.95 to 2.38; p = 0.080). Clinically meaningful improvements in fatigue (also 10 points of change) were achieved by 58% (95% CI 51% to 66%) of patients receiving intensive management and 35% (95% CI 28% to 42%) of patients receiving standard care. Logistic regression showed that this difference was significant (adjusted OR 2.81, 95% CI 1.76 to 4.48; p < 0.001).
Radiological scores
The mean Larsen scores increased from 11 to 13 with intensive management and from 9 to 10 with standard care, with no significant difference between groups (see Table 13).
Clinical outcomes over 6 and 12 months
Longitudinal analyses assessed changes over both 6 and 12 months using mixed-effects models (see Table 13). Unadjusted and adjusted analyses showed significant differences between groups for DAS28-ESR, swollen joint counts for 66 joints, patient global assessments, fatigue and pain. The coefficients for fatigue between treatment groups were particularly large in the unadjusted (–15.7, 95% CI –21.3 to –10.1) and adjusted (–13.1, 95% CI –18.1 to –8.1) analyses.
Complete-case analyses
Remission and changes in clinical outcomes at 12 months
The effect of intensive management on remission and clinical outcomes was similar in the complete-case analyses to the ITT analyses. DAS28-ESR remissions occurred in 43 of 134 (32%) patients receiving intensive management and 23 of 124 (19%) patients receiving standard care, with an unadjusted OR of 2.07 (95% CI 1.16 to 3.70; p = 0.014). Low disease activity on DAS28-ESR scores occurred in 61 of 134 (46%) patients receiving intensive management and 39 of 124 (31%) patients receiving standard care (OR 1.84, 95% CI 1.10 to 3.08; p = 0.020).
Remission, low disease activity, disability and quality of life with intensive management
The 134 patients who had intensive management included 43 patients with 12-month DAS28-ESR remissions, 61 patients with low disease activity and 73 patients with moderate or high disease activity. HAQ and EQ-5D scores showed only minimal changes in patients who did not achieve remissions or low disease activity states. However, in patients who achieved remissions, the 12-month change in HAQ score was –0.40 (95% CI –0.57 to –0.22) and the 12-month change in EQ-5D score was 0.13 (95% CI 0.09 to 0.17) (Table 14). There were similar improvements in patients who achieved low disease activity states. In addition, low end-point HAQ scores of < 0.5 occurred in 23 of 43 (53%) patients in DAS28-ESR remission and in 25 of 61 (41%) patients in low disease activity states compared with 13 of 73 (18%) patients with moderate or active disease activity at 12 months.
| Outcome | Time point | Remission (n = 43) | Low disease activity (n = 61) | Moderate/high disease activity (n = 73) |
|---|---|---|---|---|
| HAQ | Initial | 1.00 (0.80 to 1.21) | 1.19 (1.01 to 1.38) | 1.27 (1.13 to 1.41) |
| 12 months | 0. 60 (0.42 to 0.79) | 0.82 (0.63 to 1.01) | 1.23 (1.09 to 1.37) | |
| Change | –0.40 (–0.57 to –0.22) | –0.38 (–0.51 to –0.23) | –0.04 (–0.13 to 0.05) | |
| EQ-5D-5L | Initial | 0.74 (0.69 to 0.78) | 0.71 (0.67 to 0.75) | 0.73 (0.70 to 0.77) |
| 12 months | 0.86 (0.83 to 0.90) | 0.82 (0.78 to 0.86) | 0.72 (0.67 to 0.77) | |
| Change | 0.13 (0.09 to –0.17) | 0.11 (0.07 to 0.02) | –0.02 (–0.05 to 0.01) | |
| Fatigue | Initial | 51.8 (42.0 to 61.7) | 55.0 (47.3 to 62.1) | 62.0 (57.0 to 66.8) |
| 12 months | 24.7 (16.4 to 33.0) | 28.2 (20.9 to 35.5) | 50.6 (44.1 to 57.1) | |
| Change | –27.1 (–36.8 to –17.5) | –26.5 (–34.4 to –18.6) | –11.3 (–18.3 to –4.3) | |
| Pain | Initial | 37.6 (30.0 to 45.5) | 40.9 (34.6 to 47.2) | 40.9 (36.6 to 45.4) |
| 12 months | 9.8 (6.4 to 13.3) | 14.9 (10.3 to 19.5) | 38.6 (32.7 to 44.6) | |
| Change | –27.8 (–36.3 to –19.2) | –26.0 (–32.6 to –19.5) | –2.3 (–9.0 to 4.4) |
Remission, low disease activity, fatigue and pain with intensive management
A similar analysis of fatigue and pain showed that only the patients who achieved remission or low disease activity states had substantial improvements in fatigue and pain (see Table 14). The improvements with remission and low disease activity states were virtually identical.
Harms
Fourteen patients receiving intensive management and 11 patients receiving standard care experienced one or more serious adverse events or died (Table 15). There was no significant difference in proportion of serious adverse events between treatment groups (RR 1.27, 95% CI 0.56 to 2.92). The three patients who died comprised two in the intensive management group and one in the standard care group. None of the deaths was considered to be treatment related. Other serious adverse events spanned a range of systems and there was no indication that any of the events were treatment related.
| Category | Body system | Treatment group | |
|---|---|---|---|
| Intensive management | Standard care | ||
| Deaths | Cardiovascular | Ruptured thoracic aneurysm | |
| Neoplasia | Metastatic cancer | ||
| Respiratory | Pulmonary fibrosis | ||
| Other individual serious adverse events | Allergy | Angioedema | |
| Cardiovascular | Heart failure | Microvascular angina | |
| Myocardial infarction | Paroxysmal arrhythmia | ||
| Dyspnoea/chest tightness | |||
| Hypotension headache | |||
| Gastrointestinal | Small bowel obstruction | Diverticular disease | |
| Diverticulitis | |||
| Gallstones | |||
| Neoplasia | Breast cancer | ||
| Immunological | Tonsillitis with neutropenia | ||
| Musculoskeletal | RA flare/shoulder capsulitis | ||
| Neurological | Stroke | Sepsis | |
| Other | Pregnant | Dizziness/syncope | |
| Collapsed unknown cause | |||
| Cerebral spinal fluid leak | |||
| Respiratory | Chest infection/asthma | Exacerbation of asthma | |
| All other adverse events | Number of episodes | 114 | 151 |
| Allergies, n (%) | 1 (1) | 3 (2) | |
| Dermatological, n (%) | 8 (7) | 17 (11) | |
| Cardiovascular, n (%) | 5 (4) | 8 (5) | |
| Eyes, ear, nose and throat, n (%) | 10 (9) | 15 (10) | |
| Gastrointestinal, n (%) | 9 (8) | 27 (18) | |
| Genitourinary/renal, n (%) | 3 (3) | 10 (7) | |
| Haematological, n (%) | 5 (4) | 3 (2) | |
| Hepatic, n (%) | 6 (5) | 2 (1) | |
| Immunological, n (%) | 2 (2) | 1 (1) | |
| Musculoskeletal, n (%) | 21 (18) | 17 (11) | |
| Neoplasia, n (%) | 1 (1) | 3 (2) | |
| Neurological, n (%) | 11 (10) | 6 (4) | |
| Other, n (%) | 10 (9) | 8 (5) | |
| Psychological, n (%) | 0 (0) | 2 (1) | |
| Respiratory, n (%) | 22 (19) | 29 (19) | |
Overall, 132 patients (60 intensive management patients and 72 standard care patients) had 265 adverse events (114 in the intensive management group and 151 in the standard care group) (see Table 15). These events spanned a range of body systems. There was no evidence that intensive management increased the risk of an adverse event.
Strengths and limitations
Strengths
The TITRATE trial had two strengths. First, it was a relatively large trial, involving almost 40 different specialist centres and a range of patients. Its findings are therefore likely to be robust. Second, the predicted and the actual outcomes were very similar, showing that it delivered the expected degree of improvements based on previous studies in early RA.
Limitations
The TITRATE trial also had a number of limitations. First, it did not compare the sustainability of remission between groups;216,317 however, to assess standard care patients more often than every 6 months would mean that they were no longer receiving standard care and, therefore, invalidating them as a control group. Second, the TITRATE trial lasted only 12 months. Ideally, strategy trials would last for several years; for example, 10-year results have now been reported for the BeSt strategy trial. 175 However, undertaking such long-term trials of intensive treatment strategies has organisational and funding complexities and could not be pursued in the TITRATE trial. Third, there is uncertainty as to which outcome is preferable. 318 ACR/EULAR Boolean remissions appear ideal but are not often achieved, whereas low DAS28-ESR scores may have fewer benefits but were achieved by almost half the patients receiving intensive management. In addition, although the TITRATE trial included a range of patient-reported outcome measures, the trial was designed before newer measures, such as the Rheumatoid Arthritis Impact of Disease (RAID) score, became widely used and patients’ perspectives on their outcomes may have provided important additional information. Fourth, intensive management is not effective in all patients. The TITRATE trial does not provide any information on how best to manage patients who did not respond to intensive management. Failure to respond to different forms of intensive treatment, particularly biological therapies, is increasingly recognised as being relatively common and a source of high health-care costs. 319–321 Fifth, the use of monthly sessions was planned when the trial was designed and patients did not have any particular input into deciding if this was an optimal time for assessing their progress or if less frequent assessments would be preferable. An additional issue is that some centres may use ultrasound assessments to evaluate joint inflammation in patients with moderate disease activity, although most centres do not take this approach.
One inevitable limitation of a treatment strategy trial, like the TITRATE trial, in which patients receive a range of different interventions, is the uncertainty about the extent to which different parts of the intervention contributed to the overall benefit of intensive management. Increasing conventional DMARDs, starting biological treatments and supportive management from the specialist nurses are all likely to have contributed. However, we do not know which of these was most important or if all were needed. Such challenges are commonplace in complex interventions. 322–324
Relation to overall programme
Impact of intensive management on remission
The TITRATE trial shows that managing patients with established moderate RA who are receiving conventional DMARDs and who are being followed in specialist rheumatology clinics achieve more remission at the end of 12 months’ intensive management following a treat-to-target strategy compared with standard care. Five different remission criteria showed that intensive management was more effective than standard care. More patients also achieved low disease activity states with intensive management. Although we cannot entirely disentangle the contributions of drug therapy from support from the specialist nurses, the balance of evidence suggests that both contributed to achieving remission.
Other benefits of intensive management
The TITRATE trial also showed that when trained nurses provide holistic care in addition to adjusting drug therapy using treat-to-target approaches, it could help minimise symptoms, which is important to patients.
Safety of intensive management
There was no evidence that intensive management led to more adverse events or serious adverse events.
Extent of benefits
In the complete-case analyses, 32% of patients receiving intensive management achieved 12-month DAS28-ESR remissions and 46% of patients achieved low disease activity states. Achieving remission was associated with substantial improvements in disability and health-related quality of life. Patients receiving intensive management also had substantial improvements in fatigue and pain, and these changes were also most marked in patients achieving remission. Overall, these findings suggest that between one-third and a half of patients receiving intensive management achieved substantial benefits from this treatment approach.
Differences between strategies
Intensive management patients received more conventional DMARDs, more biologics and more steroid injections. They also had more changes in DMARD and biologic dose. However, the magnitude of these differences was relatively small. Biologic use is the most important example of the difference between strategies. With intensive management, 49 of 168 (29%) patients had 58 different biologics. With standard care, 24 of 167 (14%) patients had 26 biologics.
Health economic evaluation of the TITRATE trial
This section provides a cost-effectiveness analysis of the TITRATE trial. It assessed the economic benefits of intensive management from the perspective of English patients managed in the NHS.
Aims
This economic analysis evaluated effectiveness using quality-adjusted life-years (QALYs) during the TITRATE trial. It was conducted in line with the NICE Guide to the Methods of Technology Appraisal325 to ensure NHS relevance. The analysis used an NHS and Personal Social Services (PSS) perspective for costs in the base-case analysis. Participant variation in resource use and effectiveness were estimated separately. The economic outcome was expressed as the incremental cost per QALY gained of intensive management. As all analysis occurred at the 12-month follow-up period, no discounting of health or costs was required.
Methods
Costs
NHS resources were measured for each participant between baseline and final follow-up. This included medication costs, visits to health services and any social care and community support. Medication usage, the number of hospital visits and intensive management appointments were taken from trial records. NHS and PSS resources were self-reported by participants at 6 and 12 months, with the widely used and validated Client Service Receipt Inventory (CSRI) questionnaire. 326 It included questions related to time off work, which was used in sensitivity analysis.
Unit costs for all resources were obtained for the financial year 2018–19 from national sources. Medication use was taken from a form that collected current RA medication information over the trial. This included the duration of the medication (start date and end date of the medication), the dosage and the frequency of the medication. NHS unit costs for the medications were based on the drug tariff price reported in the British National Formulary (BNF)327 (see Appendix 5, Table 41). If the drug tariff price was not reported in the BNF, then we used the average NHS indicative price from all manufacturers of the medication. NHS and social services costs were from Unit Costs of Health and Social Care. 328
Total NHS costs and social services costs included medication, primary care, secondary care and social services contact. The unit costs are summarised in Appendix 5, Tables 41 and 42. Data on the use of secondary health-care service visits were collected by the research nurse at each centre based on forms designed to capture information on all hospital admissions and the frequency and type of intensive management sessions. Detail on the nature of the reported hospital admissions was not captured and we assumed the cost to be the cost of 1 hour with a rheumatologist. Intensive management sessions were excluded from reported hospital admissions to avoid double counting. The average costs of intensive management sessions were derived from bottom-up micro-costing based on the type of practitioner seen and the duration of the appointment (see Appendix 5, Table 44).
Primary care and social services contact were captured by the CSRI health resource questionnaire. The CSRI provides a 3-month recall and is measured at 6-month intervals. Linear interpolation was used to estimate the costs in the unobserved months. Participants were asked whether or not they had been in contact with a general practitioner (GP) (i.e. a visit to the GP, telephone call and/or GP home visit) and, if so, how many contacts they had had over the previous 3 months. This time was costed based on the average patient’s contact with a GP in the UK lasting 9.22 minutes. 328 Responses to questions on the CSRI relating to appointments with other NHS health-care workers (e.g. practice nurse, NHS physiotherapist, NHS occupational therapist) and social care workers (e.g. staff help at the participant’s home and appointments with social workers) were also recorded and staff time was costed per working hour (see Appendix 5, Table 44). Participants could also report any use of other health or social services, which was costed in a similar manner (i.e. a single working hour for staff on NHS band 5, £34) and included participants’ use of blood tests at walk-in centres, support from a mental health worker, visit to a memory clinic, installation of handrails and toilet seats, and appointments with podiatrists and orthopaedists.
Participants were asked if the NHS or social services paid for their transport to get to health-care appointments and the reported total was used for the cost of transport. The travel cost of staff to participants’ homes or to general practices for hospital-based staff was estimated as the sum of travel time and cost of transport. Travel time was assumed to be 15 minutes (i.e. one-quarter of the cost of a working hour), and we applied the NHS reimbursement rate for a 4-mile journey (i.e. 56 pence/mile, thus £2.24) for the cost of transport. Receipt of Meals on Wheels services was asked directly in the CSRI health resource questionnaire and costed for £4.40 per meal based on a 2018 national survey of the programme. 329
Indirect costs were defined as the production losses resulting from treatment when the participant was unable to return to normal activity. Information regarding participants’ recovery was collected in the CRSI questionnaire at 6 and 12 months. Individual participant costs comprised the amount of whole days or hours the individual has taken off work and the amount taken off by friends of relatives to care for them. Unit time costs were combined and costed using standard economic conventions (i.e. the human capital approach to estimating time costs) and Office for National Statistics data on UK median wages and working hours, and330 this gave a cost estimate of £92 for each day of work lost because of RA (see Appendix 5, Table 42).
The total cost to the NHS and PSS was computed by adding the estimated treatment and follow-up costs for each participant. There was no missing medication, primary care and PSS data. Missing data in the use of secondary health-care services were estimated with multiple imputation and the participant was included in the analysis. The sum of participants’ NHS, PSS and indirect costs was the societal cost of RA illness.
Health-related quality of life
The QALYs gained were estimated by applying the trapezium rule to estimate the area under the curve. QALYs were then adjusted for participants’ baseline level of utility using linear regression. Health utilities were derived from participant responses to the EQ-5D-5L at baseline and at 6 and 12 months. The EQ-5D-5L was designed in 2009 with the intention of improving the discriminative power of the instrument at levels of health near to full health, as compared with the widely used three-level version [EuroQol-5 Dimensions, three-level version (EQ-5D-3L)]. However, the position of NICE331 is that to have consistency with the current reference case analysis (which is based on EQ-5D-3L utility scores), and because of concerns raised about the validity of the EQ-5D-5L value set for England,332 the utility values should be established by mapping responses to the EQ-5D-5L descriptive system data onto the EQ-5D-3L valuation set using the mapping approach reported by Devlin et al. 333 We take this approach in the reference case. Sensitivity analysis will be undertaken with QALYs estimated using EQ-5D-5L responses and two alternative approaches for estimating EQ-5D-3L from responses to the EQ-5D-5L. 334 The mapping approaches were established based on two reference data sets of patients who completed the EQ-5D: (1) FORWARD (the National Databank for Rheumatic Diseases)334 and (2) a EuroQol Group-co-ordinated data collection study. 333,334 The EQ-5D-3L and EQ-5D-5L scores were estimated using the EQ-5D population tariffs that are based on the UK (EQ-5D-3L version) and England (EQ-5D-5L version) population responses. 333,335
Missing observations
Multiple imputation was used to create observations for missing data, which were assumed to be missing at random. Regardless of the possible reason outcomes were missing, we imputed missing values for the EQ-5D data (both EQ-5D-3L and EQ-5D-5L values) at 6 and 12 months, the number of clinical visits to the hospital and number of intensive management sessions. Missing values on the number of clinical visits to the hospital and number of intensive management sessions were dealt with in the same way as the EQ-5D. For each outcome, the method of imputation was PMM. 336 This approach imputes missing values by means of the five nearest neighbour donors, with distance based on the expected values of the missing variables. In our analysis, the expected values of missing variables were conditional on treatment group, sex, age, ethnicity, NHS region and health utility values at baseline. We then replaced each missing value with the mean value of 20 imputations created by the PMM approach (see Appendix 5, Table 43).
Economic outcomes
For each participant, we estimated total costs over the trial period and the QALYs gained. The mean differences between the two trial arms are presented with sample 95% CIs and bootstrapped bias-corrected 95% CIs. The cost and health information were combined in an estimate of incremental cost-effectiveness ratio (ICER) of intensive management compared with standard care. Non-parametric bootstrapping was used to generate CIs for the estimated mean incremental costs and effects and to summarise the uncertainty surrounding the ICERs. Uncertainty was visualised as a two-dimensional cost-effectiveness plane and as a cost-effectiveness acceptability curve, which reports the probability that the intervention is cost-effective for any given level of willingness to pay (WTP).
Sensitivity analysis
To further test the robustness of results derived from the base-case analysis, several deterministic sensitivity analyses were conducted. This included estimating an ICER with alternative approaches to establishing health utilities334 and a societal cost perspective that includes NHS and social services costs and productivity losses. 337 Such analyses examine the effect of estimated or uncertain parameters on the decision.
Subgroup analysis
To explore the sensitivity of the EQ-5D instrument to detect changes in remission, we summarise the mean QALY gain at 12 months for participants who achieved remission compared with those who did not. Remission is defined as a DAS28-ESR score of < 2.6 at 12 months.
To examine if there are subgroups of the population that respond differently to the intervention, we used regression analysis to explore associations of patient characteristics with the change in health utility score from baseline to 12 months. Logistic regression was used to examine which patient characteristics are associated with any improvement in EQ-5D-3L score at 12 months and, therefore, the dependent variable was 1 for participants who experienced any improvement at 12 months and zero otherwise. Ordinary least squares regressions explored which patient characteristics were associated with a change in the level of EQ-5D score (magnitude of the health gain) at 12 months and a separate regression explored these associations in the subgroup of patients who had achieved remission at 12 months. The regressions included as explanatory variables the region of health centre the participant receives RA treatment, sex, age, ethnicity, the duration of RA disease, intervention group and whether or not the patient moved to biologic medication during the trial. We also investigate the number of hospital visits, intensive management sessions and biologic use in participants who achieved remission compared with those who did not. The purpose of this analysis is to examine if more resources were used in patients who achieved and maintained remission, as this has implications for any future analysis that may wish to the extrapolate costs and health findings beyond the duration of this trial.
Key findings
The base-case ICER was £43,972 from an NHS and PSS cost perspective (Table 16). At £20,000 and £30,000 per QALY, which is generally considered to be the range that NICE operates in the UK for most standard health technologies, the probability that intensive management is cost-effective compared with standard care is 2% and 17%, respectively (Figure 17, see also Appendix 5, Table 45).
| Cost perspective | Treatment group (bootstrapped bias-corrected 95% CI) | Mean difference | |
|---|---|---|---|
| Intensive management (n = 168) | Standard care (n = 167) | ||
| All NHS and personal social service costs (£) | 3784 (3371 to 4246) | 2258 (1974 to 2585) | 1526 |
| Societal cost perspectivea (£) | 4697 (4076 to 5378) | 3678 (2926 to 5025) | 1019 |
| QALYs with regression adjustment for baseline EQ-5D-3L | 0.64 (0.62 to 0.66) | 0.61 (0.58 to 0.63) | 0.035; p = 0.02 |
| ICER (NHS and PSS costs) (£) | 43,972 | ||
| Sensitivity analysis | |||
| ICER (societal cost perspectivea) (£) | 29,363 | ||
| QALYs estimated using Hernández-Alava and Pudney’s334 mapping function derived from the EuroQol Group co-ordinated data set | |||
| ICER (NHS and social services costs) (£) | 47,293 | ||
| ICER (societal cost perspective) (£) | 31,580 | ||
| QALYs estimated using Hernández-Alava and Pudney’s334 mapping function derived from the FORWARD National Databank for Rheumatic Diseases | |||
| ICER (NHS and social services costs) (£) | 52,188 | ||
| ICER (societal cost perspective) (£) | 34,849 | ||
| QALYs based on EQ-5D-5L index scores | |||
| ICER (NHS and social services costs) (£) | 57,849 | ||
| ICER (societal cost perspective) (£) | 38,629 | ||
FIGURE 17.
Cost-effectiveness acceptability curve of intensive management vs. standard care from an NHS and social services cost perspective.
Figure 18 shows the empirical estimate of the joint distribution of mean incremental costs and effects for intensive management compared with standard care obtained using the results of the bootstrap replicates. The estimates indicate that intensive management is likely to be more costly to the NHS and PSS by £1526 per patient (p < 0.001), with a statistically significant (p = 0.02) increase in health benefit of 0.03 QALYs per patient over the trial period (see Table 16). A detailed summary of the between-group differences in cost components and QALY outcomes is shown in Appendix 5, Tables 47 and 48.
FIGURE 18.
Cost-effectiveness plane: intensive management vs. standard care from NHS and social services cost perspective.
Sensitivity analysis showed that the ICER fell to £29,363 when we included the value of time off work. This corresponded to a 50% probability that intensive management is cost-effective at a WTP value of £30,000 per QALY (see Appendix 5, Table 45). The ICER increases with all alternative methods for valuing health gain. The largest ICER is £57,849 when QALYs were based on EQ-5D-5L index, and this corresponded to an estimate of 7% probability that intensive management is cost-effective at a WTP value of £30,000 per QALY (see Appendix 5, Table 45).
Patients who experienced remission had an improvement in health utility of 0.151 (95% CI 0.111 to 0.191) at 12 months, compared with just 0.012 for patients without remission (Table 17). A large proportion of both groups did not respond to treatment. Sixty-four (38%) patients in the standard care group and 50 (30%) patients in the intensive management group experienced a decline in health utility at 12 months, compared with baseline, whereas there was an improvement in utility in 103 (62%) and 113 (70%) patients, respectively. We did not identify any patient characteristics that were associated with whether there was an increase in health utility at 12 months or a decline (see Appendix 5, Table 48). However, the use of biologics during the trial period was associated with an increase of 0.059 in health utility at 12 months, compared with baseline, after controlling for intervention group and patient characteristics (see Appendix 5, Table 49). This increase was slightly lower (0.056 QALYs) in patients who achieved remission at 12 months (see Appendix 5, Table 50). This suggests that greater biologics use in intensive management was a key driver of the health improvement experienced in comparison with standard care.
| EQ-5D improvement at 12 months | Mean (SD) | 95% CI | Number of observations |
|---|---|---|---|
| For patients without remissiona | |||
| Intensive management group | 0.024 (0.160) | –0.004 to 0.053 | 120 |
| Standard care group | 0.0002 (0.194) | –0.032 to 0.033 | 139 |
| Combined groups | 0.012 (0.179) | –0.010 to 0.033 | 259 |
| For patients with remissiona | |||
| Intensive management group | 0.145 (0.142) | 0.104 to 0.186 | 48 |
| Standard care group | 0.161 (0.223) | 0.075 to 0.247 | 28 |
| Combined groups | 0.151 (0.175) | 0.111 to 0.191 | 76 |
More patients achieved remission in the intensive management group (28%, n = 48) compared with the standard care group (17%, n = 28). For both groups, the use of biologics was lower in the patients who achieved remission (see Appendix 5, Table 51). Six per cent of patients (n = 10) in the intensive management group were on biologics and in remission, compared with 10% of patients (n = 16) in the standard care group. The lower percentage of patients in remission and on biologics in the intensive management group suggests that greater use of biologics is not the primary cause of the larger percentage of patients in the intensive management group who achieved remission. This leaves open other possible explanations and this must include the use of conventional DMARDs more effectively in the intensive management group. There is closer management of patients who achieved remission in the intensive management group both in comparison to patients who did not achieve remission in the intensive management group and to patients who achieved remission in the standard care group. For example, the number of hospital visits (including intensive management sessions) to the hospital for patients who achieved remission was larger in intensive management group (14.2 visits) than for the standard care group (7.4 visits) (see Appendix 5, Tables 52 and 53). In addition, within the intensive management group, the number of intensive management sessions was larger in patients with remission (11 sessions) than in patients without remission (9.1 sessions), (see Appendix 5, Table 54).
Limitations
Duration of assessment
The costs and benefits of the intervention were not extrapolated beyond the trial period in a decision-analytic model to allow a lifetime estimate of expected costs and QALYs. Such a model would require time to loss of efficacy of intensive management sessions to determine the future treatment pathway for the patient populations once a switch from intensive DMARD therapy is estimated, including biologics if patients progress to severe RA.
Duration of treatment
It is plausible that the cost per QALY gained from the intervention would improve over a longer duration of treatment. We found that a key driver of improvement in health-related quality of life was whether or not patients achieved remission, and there is evidence that patients receiving intensive management who did not achieve remission within the trial period are more likely to have done had the trial lasted longer. The number of remissions were larger in the intensive management group and patients without remission had gains in health-related quality of life (mean gain in health utility of 0.024) that were not found in the standard care group (mean gain in utility of 0.0002). In addition, if patients in the intensive management group continue to achieve and maintain remission, then the costs of intensive management treatment would be expected to decrease because we found fewer hospital visits and biologic use in intensive management patients with remission than in patients without remission. This would serve to reduce the incremental costs between groups and improve the cost-effectiveness of the intervention. Therefore, the proportion of patients who move to biologics and the time it takes for them to move to biologics is expected to be key driver of costs and possibly health benefits in an extrapolation.
Relation to overall programme
Overall health economic benefit
The ICER of intensive management compared with standard care was £43,972 and it decreased to £29,363 when a societal cost perspective was taken. Intensive management is therefore unlikely to be cost-effective at the threshold range of £20,000 to £30,000 per QALY, which is typically used by UK decision-makers when assessing short-term within-trial costs and benefits.
Longer-term perspectives
Resource constraints within the project prevented an economic evaluation beyond the 12-month follow-up period (outlined in the protocol as a potential tertiary analysis). This is needed in future research, as within-trial assessments underestimate the benefits of improved earlier treatment and potentially reduced biologic drug use over the longer term.
Response predictors and persistence in the TITRATE trial
The studies in this section evaluated different aspects of the TITRATE trial. The studies spanned predictors of responders and non-responders and the persistence of response of patients who received intensive management in the 6-month extension study.
Aims
The three research aims in this section represented separate secondary aspects of the TITRATE trial. These were (1) baseline response predictors, (2) an analysis of non-responders and (3) an evaluation of response persistence.
Baseline response predictors
We evaluated whether or not baseline response predictors would identify patients who showed limited responses to intensive management. We focused on predictors of DAS28-ESR remissions and improved fatigue scores, which were dominant clinical outcomes in the trial. Many previous studies have evaluated predictors of remission338–342 and fatigue343–348 in RA in trials and observational studies.
Analysis of non-responders
We also examined whether or not baseline factors identified patients who showed no improvement over 12 months (with decreases in DAS28-ESR of < 0.6 over 12 months). In addition, we assessed the extent to which non-responders showed some improvements in DAS28-ESR scores during treatment.
Response persistence
We undertook an 18-month follow-up assessment of patients receiving intensive management in the TITRATE trial, assessing the extent to which remissions and reductions in DAS28-ESR and fatigue were maintained after intensive management. Long-term follow-up of patients in RA intensive management trials has advantages,349 although these can be difficult to achieve.
Methods
Patients
We studied patients enrolled in the TITRATE trial. Studies of response predictors were confined to the 298 patients in whom 12-month DAS28-ESR and fatigue measures were present. Studies of response persistence were restricted to the 95 patients who were assessed at 18 months. Baseline assessments of these patients were similar from the overall group of patients recruited into the TITRATE trial. Details of these patients are shown in Appendix 4, Table 38.
Assessments
These were confined to the clinical assessments made in the TITRATE trial (see The TITRATE trial).
Statistical analyses
Data were analysed descriptively using means, SDs and 95% CIs or medians and IQRs. Predictors were assessed using logistic regression.
Key findings
Baseline predictors of remissions at 12 months
Significant predictors on unadjusted logistic regression analyses comprised male sex, baseline DAS28-ESR, HAQ scores, body mass index (BMI) and receiving intensive management. These factors remained significant in adjusted analyses (Table 18). Factors unrelated to 12-month remissions included age, disease duration, pain, fatigue, Patient Health Questionnaire-9 items (PHQ-9), Generalised Anxiety Disorder-7 (GAD-7) and scores on the Beliefs About Medicines Questionnaire (BMQ).
| Predictor | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| Coefficient (95% CI) | Significance | Coefficient (95% CI) | Significance | |
| 12-month DAS28-ESR remission | ||||
| Male sex | 1.97 (1.07 to 3.66) | p = 0.031 | 1.96 (0.99 to 3.90) | p = 0.055 |
| DAS28-ESR | 0.56 (0.34 to 0.93) | p = 0.024 | 0.58 (0.33 to 1.01) | p = 0.052 |
| HAQ | 0.53 (0.35 to 0.80) | p = 0.003 | 0.61 (0.39 to 0.95) | p = 0.027 |
| BMI | 0.91 (0.86 to 0.96) | p < 0.001 | 0.91 (0.86 to 0.96) | p = 0.001 |
| Intensive management | 2.09 (1.22 to 3.36) | p = 0.007 | 2.63 (1.46 to 4.72) | p = 0.001 |
| 12-month reductions in fatigue of ≥ 10 mm | ||||
| Fatigue | 1.03 (1.02 to 1.04) | p < 0.001 | 1.03 (1.02 to 1.04) | p < 0.001 |
| BMI | 0.96 (0.93 to 0.99) | p = 0.025 | 0.94 (0.91 to 0.98) | p = 0.004 |
| Intensive management | 2.69 (1.68 to 4.30) | p < 0.001 | 2.60 (1.58 to 4.30) | p < 0.001 |
| 12-month DAS28-ESR non-response (decreases < 0.60) | ||||
| BMI | 1.04 (1.00 to 1.08) | p = 0.028 | 1.05 (1.01 to 1.09) | p = 0.011 |
| BMQ (general) | 1.05 (1.00 to 1.11) | p = 0.042 | 1.06 (1.00 to 1.12) | p = 0.035 |
| Intensive management | 0.48 (0.30 to 0.76) | p = 0.002 | 0.48 (0.30 to 0.78) | p = 0.003 |
Combining these predictors identified a group of 40 patients who were unlikely to achieve remission irrespective of the treatment they received. The subset of patients who were obese (i.e. had a BMI of > 30 kg/m2) and had high baseline HAQ scores (i.e. > 1.50) had few remissions. Only 1 in 17 (6%) patients receiving standard care and 3 in 23 (13%) patients receiving intensive management had remissions at 12 months (chi-squared 0.6; p > 0.05).
In contrast, the 124 patients with neither of these predictors had more remissions. Fifteen of 66 (23%) patients receiving standard care and 30 of 59 (51%) patients receiving intensive management achieved remissions at 12 months (chi-squared 9.5; p = 0.002).
Baseline predictors of 12 months improved fatigue
Significant predictors on unadjusted logistic regression analyses comprised baseline fatigue, BMI and intensive management (see Table 18). Factors unrelated to 12-month improved fatigue included sex, age, disease duration, DAS28-ESR, HAQ, pain, PHQ-9, GAD-7 and the BMQ.
Non-responder at 12 months
Significant baseline predictors of non-response at 12 months on unadjusted logistic regression analyses comprised BMI, the BMQ (general) and receiving intensive management. Unrelated factors included age, disease duration, DAS28-ESR, HAQ, pain, fatigue, PHQ-9 and GAD-7. These factors remained significant in adjusted analyses (see Table 18).
Changes in DAS28-ESR during follow-up in non-responders at 12 months
One hundred and two patients who received intensive treatment did not achieve 12-month remissions. In 99 of these patients, information was available about remissions occurring during their monthly monitoring visits. Fifty-one of these patients had no remissions during these visits and 48 patients had some remissions (16 patients had a single remission, 13 patients had two remissions and 19 patients had three or more remissions), with the maximum number of remissions being eight. Leaving aside 18 patients who withdrew before 12 months and three patients for whom monthly DAS28-ESR data were not available, there were three subgroups of patients receiving active treatment: (1) 48 patients achieved remissions at 12 months, (2) 48 patients had some remissions during treatment, but these were not sustained until 12 months, and (3) 51 patients had no remissions. These patients showed differing patterns of changes in their DAS28-ESR scores. Details are shown in Appendix 4, Figure 28.
Persistence of response
The 95 patients who had received intensive management in the TITRATE trial and had attended for follow-up at 18 months were divided into two groups on the basis of achieving remission in the trial. First, 48 patients had no or only one DAS28-ESR remission during intensive management and second, 47 patients had two or more DAS28-ESR remissions in this period (median 4 remissions; range 2–10 remissions).
Analysis of DAS28-ESR remissions at 6, 12 and 18 months showed a small decline in the overall frequency of remissions at 18 months, which was least in patients achieving two or more remissions during intensive management (Figure 19). There was also a gradual return in DAS28-ESR levels towards low or moderate levels, which was least in patients achieving two or more remissions (see Figure 19). There was a more marked return in fatigue over 18 months, although this is also least in patients achieving two or more remissions. There were also marked temporal variations in frequencies of different DAS28-ESR categories. Patients with initial moderate RA showed a wide range in activity levels during intensive management (Figure 20). In addition to achieving remission and low disease activity levels, up to 20% of patients had active disease (i.e. a DAS28-ESR score of > 5.1) during follow-up.
FIGURE 19.
Disease Activity Score for 28 joints based on ESR remissions, DAS28-ESR and fatigue. Mean scores over 18 months. (a) DAS28-ESR remissions; (b) mean DAS28-ESR; and (c) mean fatigue.
FIGURE 20.
Disease activity levels over 18 months with intensive management.
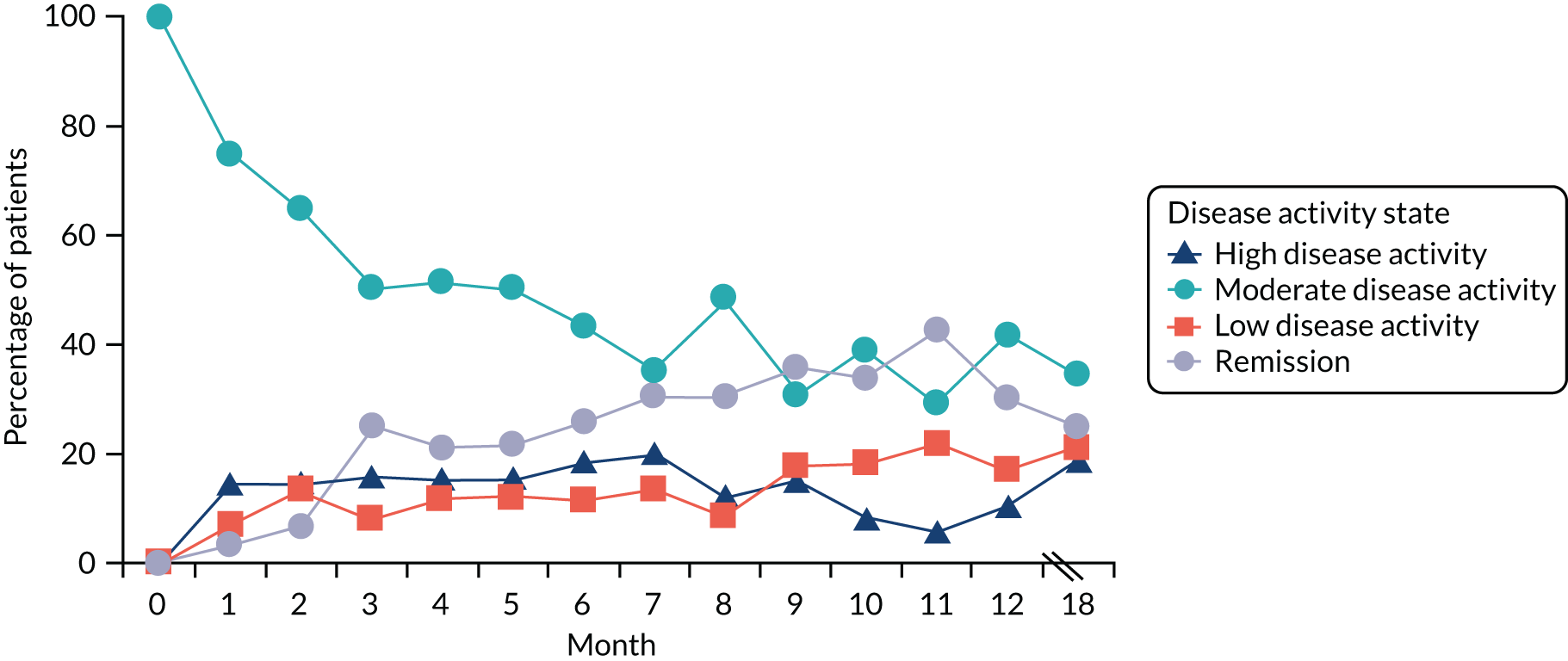
We also assessed changes in disease activity states in these patients at 12 and 18 months. This analysis showed considerable variability in the persistence of remission and low disease activity states in these patients, with many patients continuing to change between activity states. Details are shown in Appendix 4, Table 40.
Limitations
Predictive factors
Identifying predictive factors in single trials is invariably limited by uncertainty about replication. As a consequence, caution is needed and further studies need to show similar findings before any certainty can be placed on our findings. These issues have been highlighted in publications from the PROGnosis RESearch Strategy (PROGRESS) Group. 350–353
Persistence of effect
Assessing what happens to a small subset of randomised patients after a trial has ended is invariably limited by the self-selected nature of the population involved and the limited duration of follow-up. The limitations of subgroup analyses in trials are well known. 354
Relation to overall programme
Limited benefits of intensive management
Our analysis of baseline predictors suggested that some patients are unlikely to benefit from current intensive management, in particular those with a BMI > 30 kg/m2. It is likely that other approaches are needed in these patients. The negative impact of obesity has been identified with many RA patients, but may not be extend across all biologic agents. 355,356
Duration of benefits
Intensive management is unlikely to have a permanent effect in RA. Six months after stopping treatment, many patients who had benefited from intensive management were beginning to show features of returning disease activity and higher levels of fatigue. When intensive management is successful, it is likely that ongoing similar treatment is required in the longer term.
Stability of disease control and impact on disability
The research in this section focused on understanding disease course and progression and the patterns of RA-specific physical disability over time in patients receiving intensive management in ‘real-world’ clinical settings. It was intended to place treat-to-target strategies into a broader context.
Aims
The research had three inter-related aims around defining the inter-relationships between disease activity states, remissions and erosive damage. They were assessed using data from 152 patients in the REMIRA (Remissions in Rheumatoid Arthritis) study. 314,357
Transitions over time
We went beyond investigating solely the state of remission and evaluated transitions between different disease activity states defined by the DAS28-ESR.
Disability over time
We described and characterised functional disability over time (using the HAQ) with a specific focus on the impact of erosive disease at baseline and the impact of time-varying disease activity on transitions between HAQ states.
Erosive damage
We investigated erosive disease, focusing on the impact of disease activity and disability at baseline on 1-year damage progression.
Methods
Patients
The REMIRA study recruited 152 adults with RA undergoing a treat-to-target management strategy for 12 months. Inclusion criteria comprised disease duration of ≤ 10 years, receiving stable doses of conventional DMARDs or biologics for > 6 months and DAS28-ESR scores of ≤ 3.2 for 1 month or longer before recruitment. Details of these patients are shown in Appendix 1, Table 30.
Assessments
Baseline data were collected on demographics, disease duration and current treatment. Three-monthly assessments comprised DAS28-ESR and its components (i.e. 28 tender joint count, 28 swollen joint count, patient global assessment and ESR), CRP levels, HAQ, EQ-5D, Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT-F) for recording self-reported fatigue, and the Medical Outcomes Study SF-36 and its physical and mental subscales (PCS and MCS). 358–362
Posterioanterior radiographs of hands and feet at baseline and 12 months were used to define damage by radiographic erosions and damage progression by new erosions or worsening of existing erosions over 12 months.
Definition of disease states
Disease activity states were defined based on DAS28-ESR using the internationally agreed definition363 of a DAS28-ESR score of < 2.6 to indicate clinical remission, a DAS28-ESR score of 2.6–3.1 to indicate low disease activity, a DAS28-ESR score of 3.2–5.1 to indicate moderate disease activity and a DAS28-ESR score > 5.1 to indicate high disease activity.
Functional disability states were defined using the HAQ disability index categorised into four categories, (1) HAQ = 0, (2) HAQ = 0.1–0.49, (3) HAQ = 0.50–1.49 and (4) HAQ = 1.50–3.00, representing no functional disability, mild disability, moderate disability and severe disability, respectively. HAQ scores < 0.50 represent few difficulties (if any) in performing daily activities and scores > 1.50 reflect considerable difficulties or assistance required in performing daily activities. 364–367
Statistical methods
For modelling disease activity and functional disability over time, a multistate modelling approach368 based on Markov processes was adopted, as our interest lay in characterising the evolution of these disease processes as they transition between clinically meaningful disease states. In addition, multistate models naturally handle staged data where patients are under only intermittent observations. They allow the estimation of rates of transitions between the various states of disease activity or functional disability and easily incorporate the effects of covariates (both time-independent and time-dependent) on transition rates. Here, correlation among states of a patient at different assessments are directly modelled through the Markov assumption that the future evolution of the patient’s disease process depends only on his/her current disease state and not on his/her previous disease history.
For the disease activity process, we consider the three-state multistate model shown in Figure 21a, where direct transitions (forward and backwards) are allowed between adjacent states (i.e. between remission and low disease activity or between low disease activity and moderate to high disease activity). Direct transitions between remission and moderate to high disease activity are not allowed, although, as we model the disease activity process in continuous time, this simplifying assumption is not restrictive.
FIGURE 21.
Multistate diagrams for (a) disease activity states and (b) disability states.
For modelling physical functional disability, we adopt a four-state multistate model, as shown in Figure 21b, where, again, direct transitions are allowed only between adjacent states (i.e. between no disability and low disability, between low disability and moderate levels of disability, and between moderate and severe disability).
We examined the separate (univariate) and joint (multivariate) effects of select demographic and clinical variables on the transition rates in the models for both disease activity and disability. These covariates were incorporated into the models through the proportional hazards assumption. The variables considered were sex, age, ethnicity, erosive disease and treatment all at baseline, disease duration and either the HAQ score or DAS28-ESR score updated at each visit when considering either the disease activity multistate models or the disability multistate models, respectively. Damage progression was investigated through logistic regression models where only covariates measured at baseline were considered as predictors.
Key findings
Baseline features
Over 85% of the cohort had low levels of disease activity or were in clinical remission at entry. None of the patients had high baseline levels of disease activity. Approximately 50% of the cohort had low levels or no disability at baseline. Erosive disease was observed to be present in 40% of the study sample (n = 67). Most patients were receiving stable doses of methotrexate for over 6 months prior to entry. Fifty-three per cent of patients were on two or more RA medications at baseline.
Characterisation of disease activity states over time
Figure 22a displays the longitudinal profiles of disease activity states for the 150 patients with at least two visits in which DAS28-ESR scores were recorded. Of these patients, 95 had DAS28-ESR scores recorded at all five visits and 44 (46%) of those were observed to have sustained remission (i.e. observed in remission at all five visits). Table 19 summarises the numbers and types of transitions in disease activity states that were observed over the 12-month follow-up period. Seventy-two patients were not observed to have made any transitions out of their initial state. Twenty-eight patients were observed to have made one transition (16 patients were observed to have deteriorated and 12 patients to have improved). Fifty patients were observed to have made two or more transitions, with the majority (92%) of patients observed to have a fluctuating course of both deteriorations and improvements.
FIGURE 22.
Longitudinal profiles of disease activity and disability over five visits. (a) Disease activity states; and (b) disability states. NA, not applicable.
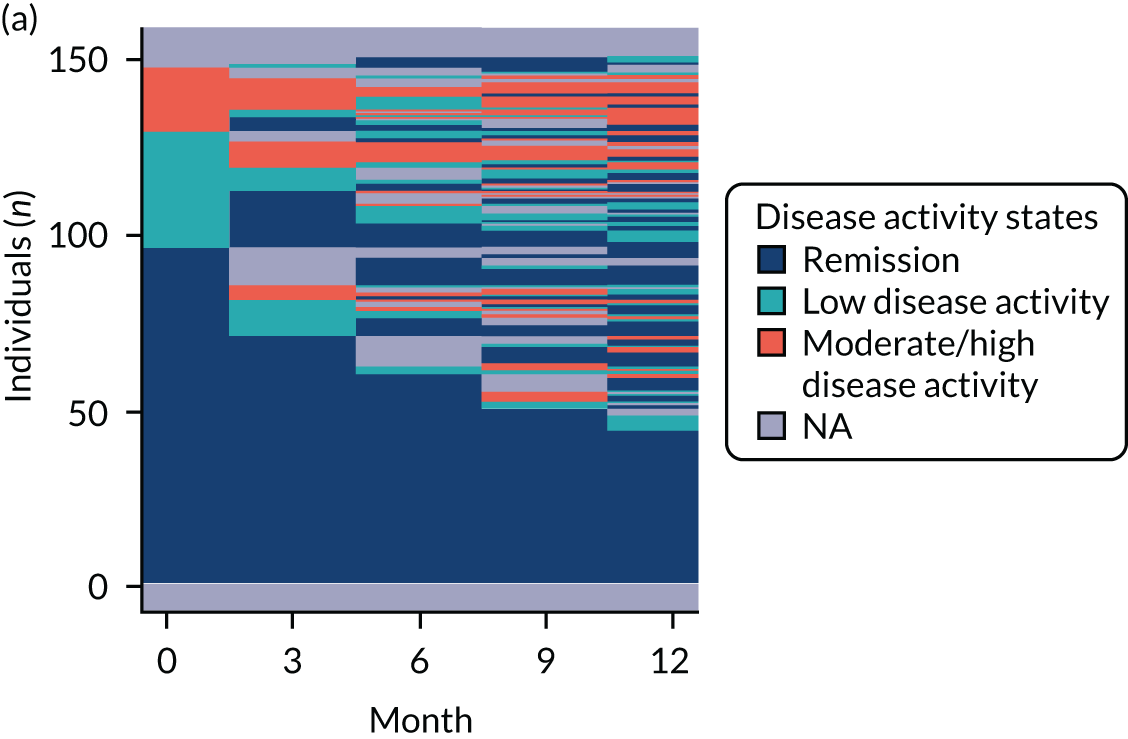
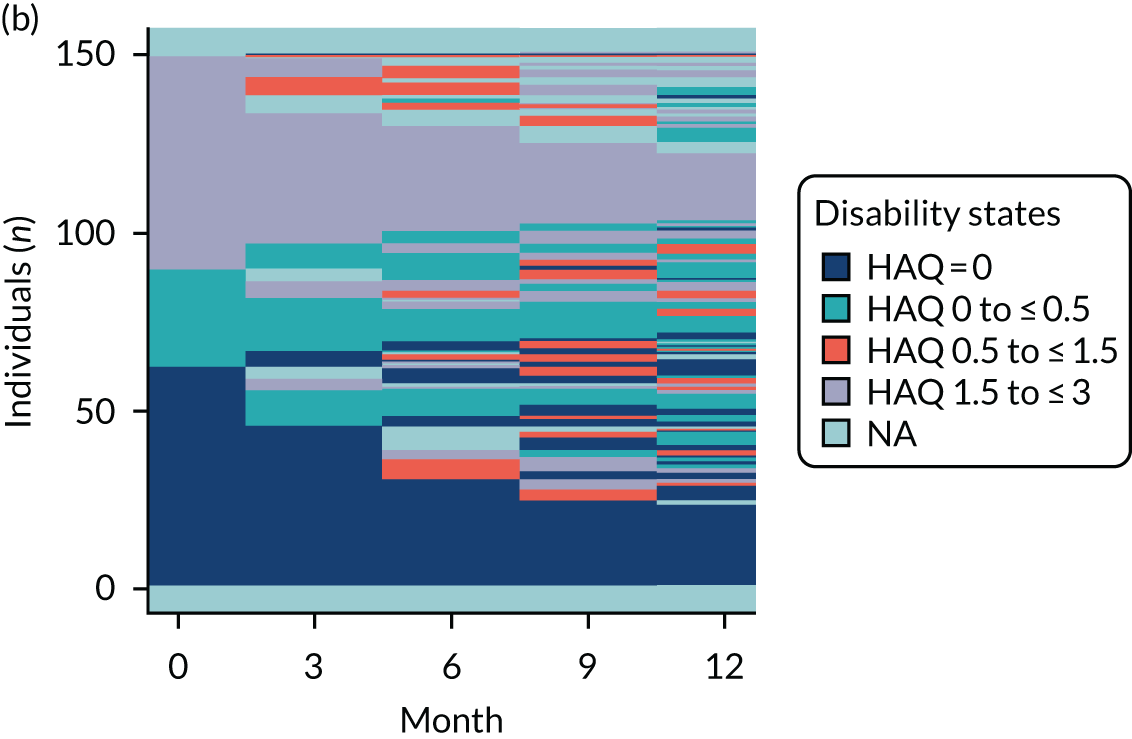
| Number of transitions | Type of transition | State at time of first observed disease activity state assessment | ||
|---|---|---|---|---|
| 1 (n = 99) | 2 (n = 33) | 3 (n = 18) | ||
| No transitions (n = 72) | 68 | 0 | 4 | |
| One transition (n = 28) | Deterioration (n = 16) | |||
| State 1 → 2 | 6 | NA | NA | |
| State 1 → 3 | 4 | NA | NA | |
| State 2 → 3 | NA | 6 | NA | |
| Improvement (n = 12) | ||||
| State 2 → 1 | NA | 7 | NA | |
| State 3 → 1 | NA | NA | 4 | |
| State 3 → 2 | NA | NA | 1 | |
| Two or more transitions (n = 50) | Steady observed deterioration (n = 4) | |||
| State 1 → 2 → 3 | 4 | NA | NA | |
| Steady observed improvement (n = 0) | ||||
| State 3 → 2 → 1 | NA | NA | 0 | |
| Fluctuating course both deterioration and improvement (n = 46) | ||||
| State 1 ↔ 2 | 10 | 9 | NA | |
| State 1 ↔ 3 | 4 | NA | 1 | |
| State 2 ↔ 3 | NA | 2 | 6 | |
| State 1 ↔ 2, 1 ↔ 3, 2 ↔ 3 | 3 | 9 | 2 | |
On fitting the three-state multistate model (see Figure 21a) with no covariates to the disease activity states, we estimated that there would be 7.28 (95% CI 5.22 to 10.16) transitions per 100 person-months from the remission state to the low disease activity state, 36.81 (95% CI 26.30 to 51.51) transitions per 100 person-months from low disease activity back to remission, and 32.21 (95% CI 20.39 to 50.86) and 24.22 (95% CI 15.26 to 38.51) transitions per 100 person-months from low disease activity to moderate/high disease activity and moderate/high back to low disease activity, respectively. Based on these transition rates, we estimate that the mean time spent in the three states of remission, low disease activity and moderate to high disease activity before exiting are 13.74 (95% CI 9.85 to 19.17) months, 1.45 (95% CI 1.08 to 1.94) months and 4.12 (95% CI 2.60 to 6.55) months, respectively. Therefore, on average, once a person enters the remission states they spend over 12 months in this state before transitioning out under a treat-to-target management strategy. In contrast, a person spends very little time continuously in either the low or moderate/high disease activity states before exiting.
The results in Table 20 show the final multivariate multistate model when covariates are explored on various transition rates. We find that males have a higher transition rate out of low disease activity to moderate/high disease activity states than females. The rate of transition to remission (from low disease activity) is 3.2 (95% CI 1.2 to 6.6) times faster for males than for females, and a 1-unit increase in HAQ increases by 2.9-fold (95% CI 1.8- to 4.7-fold) the rate of transitioning out of remission.
| Variable | Deterioration, relative risk (95% CI) | Improvement, relative risk (95% CI) | ||
|---|---|---|---|---|
| 1 → 2 | 2 → 3 | 2 → 1 | 3 → 2 | |
| Sex: male vs. female | 1 | 2.80 (1.19 to 6.62) | 3.18 (1.48 to 8.82) | 1 |
| HAQ at previous visit | 2.89 (1.79 to 4.65) | 1 | 1 | 1 |
Characterisation of functional disability over time
Figure 22b displays the longitudinal paths of disability states of the same 150 patients. The numbers of patients observed in the no disability, mild, moderate and severe disability states at first observed HAQ assessment were 64, 26, 49 and 11, respectively. Ninety-eight patients were observed to have HAQ measured at all five time points. Of these 98 patients, 23 (23.5%) remained disability free over all their visits (compared with 46.3% of patients who had five visits with DAS28-ESR measured being in sustained remission). Table 21 summarises the transition patterns in disability states that were observed over the 12-month follow-up period. Just under half of the 150 patients (n = 73) were observed to have not made any transitions out of their initial state. Twenty-five patients were observed to have made one transition (14 patients were observed to have deteriorated and 11 patients to have improved). Fifty-two patients were observed to have made two or more transitions and all but one patient were observed to have a fluctuating course of both deteriorations and improvements.
| Number of transitions | Type of transition | State at time of first observed disability assessment | |||
|---|---|---|---|---|---|
| 1 (N = 64) | 2 (N = 26) | 3 (N = 49) | 4 (N = 11) | ||
| No transitions (n = 73) | 33 | 9 | 26 | 5 | |
| One transition (n = 25) | Deterioration (n = 14) | ||||
| State 1 → 2 | 10 | NA | NA | NA | |
| State 1 → 3 | 1 | NA | NA | NA | |
| State 2 → 3 | NA | 2 | NA | NA | |
| State 3 → 4 | NA | NA | 1 | NA | |
| Improvement (n = 11) | |||||
| State 2 → 1 | NA | 4 | NA | NA | |
| State 3 → 1 | NA | NA | 1 | NA | |
| State 3 → 2 | NA | NA | 4 | NA | |
| State 4 → 3 | NA | NA | NA | 2 | |
| Two or more transitions (n = 52) | Steady observed deterioration (n = 0) | ||||
| Steady observed improvement (n = 1) | |||||
| State 3 → 2 → 1 | NA | NA | 1 | NA | |
| Fluctuating course both deterioration and improvement (n = 51) | |||||
| State 1 ↔ 2 | 15 | 4 | NA | NA | |
| State 1 ↔ 3 | 1 | NA | 0 | NA | |
| State 2 ↔ 3 | NA | 6 | 10 | NA | |
| State 3 ↔ 4 | NA | NA | 4 | 4 | |
| State 1 ↔ 2, 1 ↔ 3, 2 ↔ 3, 3 ↔ 4 | 4 | 1 | 2 | 0 | |
The results in Table 22 of fitting the final multivariate four-state multistate model provided evidence for the effects of disease duration, ethnicity and disease activity on transitions between various disability states. More precisely, we found that a 1-year increase in disease duration reduces the rates of transitioning both in and out of the no disability state by 0.8. The rate of transition to moderate disability from mild disability state increased 1.17-fold (95% CI 1.03 to 1.33) for every additional year of disease. Patients who were white had a slower rate of transitioning in and out of the no-disability state than non-white patients, and a 1-unit increase in DAS28-ESR score increased the transition rate from moderate to severe levels by around twofold (95% CI 1.24-fold to 3.92-fold). No evidence of effects of erosive disease and exposure to treatments at entry on the various transitions between disability states was found.
| Variable | Deterioration, relative risk (95% CI) | Improvement, relative risk (95% CI) | ||||
|---|---|---|---|---|---|---|
| 1 → 2 | 2 → 3 | 3 → 4 | 2 → 1 | 3 → 2 | 4 → 3 | |
| Disease duration at previous visit | 0.82 (0.71 to 0.94) | 1.17 (1.03 to 1.33) | 1 | 0.81 (0.67 to 0.97) | 1 | 1 |
| Ethnicity: white vs. rest | 0.20 (0.07 to 0.52) | 1 | 1 | 0.26 (0.09 to 0.71) | 1 | 1 |
| DAS28-ESR at previous visit | 1.30 (0.91 to 1.86) | 1 | 2.20 (1.24 to 3.92) | 1 | 1 | 1 |
Damage progression
At 1-year follow-up, 71 of the 82 (86.6%) patients observed not to have erosive disease at baseline remained damage free and four patients were observed to have progressed. For the 67 patients with baseline erosive disease, 46 (68.7%) were observed not to have progressed further and 16 were observed to have further progression. There was clear statistical evidence, as expected, that the 1-year damage progression rate was higher in those patients with baseline erosive disease than in those without (p = 0.002).
The final multivariate logistic regression model investigating baseline predictors of 1-year damage progression identified ethnicity, disease activity and erosive disease at baseline as potentially important factors (Table 23). Patients who were white were found to have higher odds of damage progression (OR 9.47, 95% CI 1.11 to 80.47; p = 0.04) than non-white patients, patients with a higher DAS28-ESR score at baseline were more likely to progress than those with lower scores (OR 2.1, 95% CI 1.04 to 2.12 for a 1-unit higher DAS28-ESR score) and RA patients with erosive disease present at baseline were also at greater risk of progression (OR 7.3, 95% 2.08 to 25.59). No statistical evidence was found for an effect of physical functional disability on damage progression (p = 0.225) after accounting for ethnicity, disease activity and erosive disease at baseline.
| Variable | OR | 95% CI | p-value |
|---|---|---|---|
| Ethnicity: white vs. rest | 9.47 | 1.11 to 80.47 | 0.040 |
| DAS28-ESR at baseline | 2.07 | 1.04 to 4.12 | 0.039 |
| Erosive at baseline: yes vs. no | 7.30 | 2.08 to 25.59 | 0.002 |
| HAQ at baseline | 0.48 | 0.14 to 1.69 | 0.225 |
Limitations
Defining remission
There is no ideal definition of clinical remission. Even sustained clinical remission may not represent an underlying state of true biological remission. Although 44 patients in the REMIRA cohort had sustained remission over the 12 months, 5 of the 44 (11%) patients had damage progression after 1 year. Clinical remission may overlook subclinical synovitis and, consequently, true remission might be better characterised molecularly using laboratory markers.
Refractory disease
We found that 18 patients in the REMIRA cohort had moderate to high disease activity states at their last visit without ever showing any clinically meaningful improvement in their disease activity. These patients may represent a refractory group of patients who do not respond well to current therapies. However, our data also suggest that, once remission is achieved, patients under a treat-to-target strategy will tend to stay in remission for > 1 year, on average, before exiting this state of clinical disease quiescence.
Relation to overall programme
Variability of remission
In the REMIRA cohort of 152 RA patients, we observed various patterns of disease activity and physical functional disability over 12 months. Forty-four patients were observed in sustained remission over all visits, whereas 23 patients were observed to be disability free at all visits and 14 patients were observed to have been both in sustained remission and disability free. Sizeable proportions of patients were observed to have a fluctuating disease course (30.7%, n = 46) and to have fluctuating levels of functional impairment (34%, n = 51). These fluctuating patterns may reflect the difficulty in controlling patients’ disease, even under a treat-to-target strategy.
Factors influencing transitions between disease states
We found that sex and levels of functional disability had an impact on the rate of transitions between disease activity states. In particular, males tended to have a higher rate of transitions out of low disease activity to moderate disease activity than females, although higher levels of disability increased the rate of transitioning out of the remission state. We also found that higher levels of disease activity increased the rate of transitioning from moderate to severe levels of disability and that ethnicity and disease duration also influenced the transition rates between disability states, in particular transitions in and out of the mild disability state.
Factors influencing erosive progression
As expected, the 1-year damage progression rate was higher in those patients with erosions at baseline. Ethnicity and disease activity at baseline were identified as possible predictors of damage progression. No evidence was found for functional disability as a predictor of 1-year damage progression in RA patients undergoing a treat-to-target strategy.
Patients’ and practitioners’ views
The research in this section evaluated patients’ and practitioners’ views on intensive management and considered additional points of relevance to patients. This part of the research had considerable PPI.
Aims
This section mainly focused on patients’ and practitioners’ views about intensive management, but it also took into account patients’ broad-based perspectives about their care. Both parts of the research have been published. 369,370
Patients’ and practitioners’ perspectives about the TITRATE trial
We explored patients’ and practitioners’ views on the feasibility and acceptability of intensive management and their experience of receiving and providing intensive management. There is considerable evidence that patients’ perspectives are important in trying to manage their long-term disorders, such as RA. 271,371–374
Broad-based perspectives
Patients discussed many aspects of their care during the TITRATE intensive management sessions, including emotional distress and treatment of pain and fatigue using self-management approaches. Practitioners provided emotional and self-management support to patients who learnt strategies to better manage symptoms. A study outside the TITRATE trial found that one other factor patients considered important was foot care; however, the TITRATE trial did not directly address this. There is substantial evidence that foot problems are particularly challenging in RA. 375–378 Two expert patients therefore led a survey of patients and clinic staff about foot care in RA.
Methods
Qualitative study to assess TITRATE trial management
Patients were recruited from the trial sites with no additional patient participant criteria to those of the trial. Fifteen patients (12 females and three males aged from 35 to 70 years) from 10 different clinics participated. Details of these patients are shown in Appendix 1, Table 33. Practitioners were those trained to deliver the TITRATE intensive management intervention who had delivered at least six sessions with the same patient. Sixteen practitioners (13 research nurses and three specialist nurses) from 13 rheumatology centres participated. Individual interviews were conducted with all patients and 13 practitioners. Eight interviews were face to face and 20 were via telephone. One focus group was held with three practitioners.
Separate semistructured topic guides were developed for patient and rheumatology practitioner participants. Initial topic guides were based on the research questions, constituent components of the intensive management intervention, and previous qualitative studies (e.g. Schoo et al. 379). The semistructured topic guides were discussed with the multidisciplinary research team and a departmental patient expert, who provided feedback on the suitability and relevance of the questions. Following the first two interviews, some small changes were made to the topic guides. These were based on how participants responded to the questions and the flow of the questions during the interviews. Further details are given in Appendix 3.
Foot health
A mixed-methods approach collected qualitative data from two focus groups of nine RA patients380 and quantitative data from 13 rheumatology team members from one site. The data were collected in a survey that had been piloted and assessed by two external clinicians and departmental patient experts living with RA for face and content validity using average congruence. 381 Further details of these patients and clinicians are given in Appendix 1.
Key findings
Patients’ and practitioners’ perspectives
Assessments covered monthly appointments, the therapeutic relationship with the practitioner, increased medication, the patient handbook and shared treatment planning (Table 24).
| Theme | Subtheme | Response |
|---|---|---|
| Monthly appointments | Monthly appointments acceptable | With working full time as well and having to go up there [clinic appointments], it was difficult, because in work I had to work my breaks to be able to make those appointments. So there was difficulty in getting there [clinic appointments], but I wanted to go |
| Access to services/consultant | We had a lady who was going on holiday and she had really bad side effects from methotrexate, so she just came in a few days before her holiday and we converted the methotrexate to subcut [subcutaneous]. A doctor saw her within an hour of her ringing on the telephone, so you wouldn’t normally get that | |
| Monthly appointments beneficial | If they decided that I needed a change in medication or increased the medication [. . .] they [practitioners] were closely monitoring it [patient’s response to medication]. It’s like fine-tuning an engine really. That’s how I equate with it [process of medication titration] | |
| Intensive management preferable to standard care | If you’ve got a clinic of seven [patients booked], you’ve got to churn them through [. . .] the nurse being a pastoral carer has gone. We’re basically following up and checking their disease activity scores and things like that now [. . .] Whilst you get that pastoral care with the TITRATE and they [patients] see it [pastoral care], they love it | |
| Therapeutic relationships | Practitioners ‘fairly’ confident using MI techniques | I think that they [the sessions] become easier as the sessions develop. I think just that first two or three [sessions] when you don’t really know each other and you’re trying to encourage that – to encourage the conversation more than just a yes or a no, it’s quite difficult |
| Patients and practitioners worked on goals together | It’s looking at the bigger picture of what else you can do. It might be that the pain has flared up because they’re [patient] sitting in a chair all day. Or is their mood affected because they’re isolated at home because they’re not able to get out and about? | |
| Importance of continuity of care | After a while once I got to know them [practitioners] we got on a lot better. I was less embarrassed I suppose is the word or maybe less reserved. I was able to talk to them [practitioners] about anything really. I suppose it’s building up trust isn’t it? | |
| Provision of helpful information | You know I’m just feeling pretty good, because my nurse helped me to understand my illness and she explained clearly how it [the medication] works, what I can expect, and that was a very good experience for me | |
| Increased medication | Improvement in RA symptoms | I’ve had arthritis for 34, 35 years [. . .] and only just recently I feel that it’s [RA] finally been controlled [. . .] I’ve got stiff swollen joints that have been damaged, they’ll never be repaired I know that, but the fact that I’m not having flare after flare after flare, which I was having that’s a great relief to me |
| Side effects of medication | At first, I found it very hard, because I was just taking medicines and medicines. I had a few little side-effects – upset stomach and feeling a bit down and drowsy. But overall when I got through the first few weeks, fine | |
| Treatment algorithm easy for practitioners to use | The rest of the intensive management stuff I’m absolutely happy with. It’s the changes in medication, where I have to rely on other people, to do prescriptions and things like that that I think held me back | |
| Patient handbook | Views on the content of the handbook | Oh yeah [. . .] it [handbook] was very informative you know, like loads of information and lots of help.A lot of it [content of the handbook] wasn’t relevant to meI do refer patients back to the information in the handbook when they’re, for example, struggling with fatigue or exercise [. . .] I’ve also given the handbook out or I’ve shown other nurses the handbook because it’s a great resourceWe’ve got those that want to read everything and then you get those that don’t want to know anything, just give me a new tablet and I’ll start it |
| Introductory use of the handbook | I can’t say I used it [handbook] often, but I did use it [. . .] I did use it to begin with more than I did at the end | |
| Shared treatment plan | Views on the shared treatment planning | It was good – it [shared treatment plan] facilitated a good getting to know you session with the guy [practitioner] that I was doing it [completing the shared treatment plan] with. It [the process of completion] was useful for me to sit down and put it [previous medication, medication preferences] in black and white. I suppose it [the process of completion] was good for him to find out where my head was atYes [. . .] I have found that [the shared treatment plan] useful but [. . .] I’ll be honest I haven’t really referred back to it very often. I don’t know if that’s just a fault of mine or whether it’s because the sessions have taken their own path |
Patient and practitioner data were analysed separately. Data were analysed using thematic analysis285 and iterative categorisation. 382 Iterative categorisation generates a clear audit trail with the data analysis, closely linked to the raw data, and involves four stages: (1) familiarisation through the reading of transcripts, (2) line-by-line coding to organise the data in preparation for analysis, (3) descriptive analysis that identifies themes and (4) interpretive analysis that explores patterns, inconsistencies and relates findings to existing knowledge.
Monthly appointments were acceptable to patients and practitioners. The benefits included (1) regular reviews of medication and (2) practitioners establishing close relationships with patients. Practitioners felt ‘fairly confident’ using MI techniques.
Therapeutic relationships with practitioners were important. Commonly reported goals included weight loss and exercise, closely followed by the management of fatigue, pain and regulation of sleep patterns. Patients described how they identified these areas with practitioners who ‘helped to organise them’ and ‘gently encouraged them’ to make changes. Practitioners’ monitoring and progress at subsequent intensive management sessions was considered helpful. Learning to pace was the most commonly reported self-management technique patients and practitioners worked on together, followed by gaining control over pain and fatigue.
Most patients said that their practitioner helped them to learn about their RA condition. Knowledge about RA and its treatments and what to expect from these is something patients found valuable. Areas mentioned specifically by patients included fatigue, pain and medication management.
Practitioners appreciated the option to offer biologics to patients with moderate RA. Most patients found that the optimised medication following monthly joint assessment helpful and side effects were generally resolved. Practitioners described the treatment algorithm as clear and easy to use. Nurses were able to seek advice from consultants about treatment changes.
The use of the patient handbook and shared treatment plans varied substantially. Practitioners described two categories of patients. Some patients would read the handbook carefully and bring the resource to the sessions and others did not show much interest in the material and did not use it.
Patients’ and clinicians’ views on foot health care for rheumatoid arthritis
A patients’ focus group highlighted the need for foot health information, the absence of regular assessment of feet in routine consultations and the importance of accessing podiatry services. The clinician survey showed that 69–85% of clinicians provided patients with foot health information (Figure 23). The feet were examined in only 47% of routine consultations. Clinicians often failed to examine feet routinely because foot examinations are not included in the DAS28-ESR score and foot examination takes additional clinic time. Although 31% of clinicians referred patients to podiatry when RA was diagnosed, none referred patients for periodic podiatry reviews. Over half of clinicians believed that patients self-reported their foot problems. Only 62% of clinicians felt fully competent examining feet.
FIGURE 23.
Information about feet provided by clinicians to RA patients. Adapted with permission from de Souza et al. 369 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
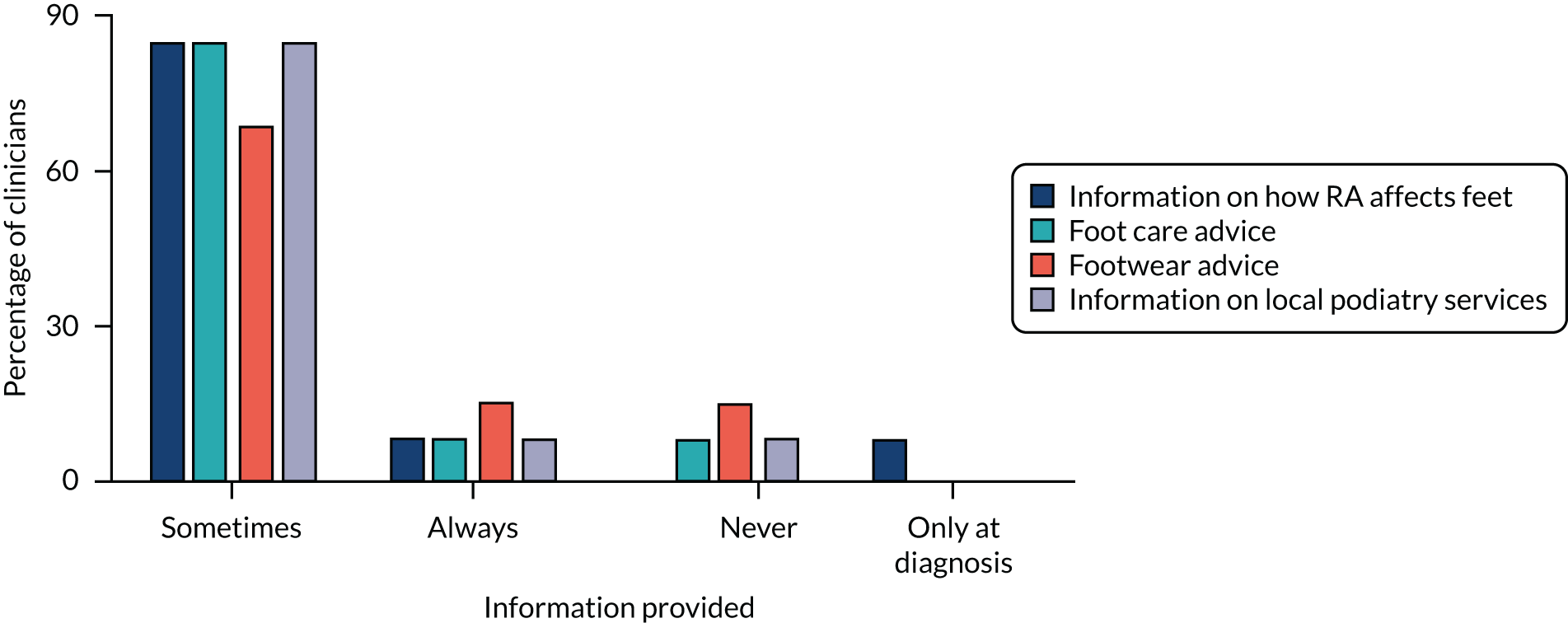
The qualitative data from the focus groups were analysed using inductive thematic analysis within a realist paradigm, whereby analysis was driven by patients’ accounts of their experiences, meaning and reality. Codes were generated by the first author and validated by a second author to negate potential patient bias. Themes and subthemes were identified by looking for recurring patterns in the data before finalisation of the themes by two authors. To enhance the validity of the findings, the simple counting method was applied, as well as providing accounts from all participants.
The survey was piloted and assessed for face and content validity using average congruence from two clinicians (external to the clinic) and our departmental patient experts (those living with RA). 381,383–385
Limitations
There were two main limitations of the studies in this section. First, there is inevitable uncertainty about the extent to which our findings are generalisable. Second, there is doubt whether or not the TITRATE trial covered all the areas that are crucial for patients.
Generalisability of patients’ and practitioners’ perspectives
Both practitioners and patients volunteered to take part in the interviews. It is possible that those who engaged more with the delivery or receipt of the intensive management intervention may have been more eager to participate. As patients in the qualitative study were largely elderly, retired and white, they were not fully representative of the RA population.
The assessment of foot health had to use a non-validated questionnaire and obtained retrospective views from clinicians because no questionnaire was available for this purpose. These methodological problems may have resulted in recall bias386 and reduced the generalisability of the findings. The perspectives of clinical staff were also restricted to clinicians practising in a single specialist unit.
What was missing from the TITRATE trial?
We do not know the extent to which the management approach in the TITRATE trial addressed all problems experienced by patients. We have highlighted the omission of foot care. Other issues that influence patients’ quality of life may have been overlooked in the management strategy.
Relation to overall programme
The most important finding is that intensive management is feasible in RA. The other relevant findings were that practitioners want to implement lifestyle modification in routine practice and that clinicians must not overlook foot care in RA.
Intensive management is feasible
We found that intensive management was acceptable to patients, whose feedback was positive. These findings reflect experience of patients in other comparable intensive treatment situations. 270,387,388 Patient participants found that increasing their medication was generally helpful. They also found that seeing the same practitioner at monthly intensive management sessions and the treatment support they provided were beneficial. Feedback from practitioners also showed that intensive management was feasible and that they could be trained to deliver it. Overall, these positive perspectives mirror the positive findings in the TITRATE trial.
Including lifestyle modification in routine care
Practitioners would like the opportunity to address lifestyle factors with patients in routine appointments. There is a strong case to examine the most practical and cost-effective way to train practitioners to adopt this approach in routine care settings. There is considerable support for such approaches in other settings. 344,389–392
Overlooking feet
Clinicians need to take into account foot symptoms when managing patients and avoid what has been termed ‘DAS blindness’369 (i.e. when consultations focus on components of the DAS28-ESR). Rheumatology clinic staff may need training in foot examination. Podiatry input should be part of the standard multidisciplinary care provided in RA, which was overlooked in the TITRATE trial, especially in view of the substantial evidence underlying the importance of foot care in RA. 393–398
Implementing intensive management
The research in this section considered issues relevant for implementing intensive management in routine clinical practice. This part of the research also had considerable PPI.
Aims
The research aims in this section span different aspects of delivering intensive management, including popular perceptions of RA and its care. We assessed the fidelity of delivering the intervention, evaluated the roles of specialist nurses and other practitioners who delivered intensive management and carried out a patient-led thematic analysis of language used about RA in UK national newspapers. One part of the research has already been published. 399
Fidelity of intervention delivery
Intervention fidelity can lead to greater confidence in results, supports research dissemination and is of particular relevance with complex interventions. 400–405 Our aim was to assess delivery of the treatment support by studying 10% of all recorded TITRATE intensive management sessions.
Nurses and other practitioners giving intensive management
The work of rheumatology nurses to deliver intensive management raises several issues. These include the published evidence for the impact of nurses in the management of RA assessed by a systematic review, the number of rheumatology nurses involved in rheumatology identified in national surveys, and the experience and background of nurses and other practitioners who delivered intensive management in the TITRATE trial.
Rheumatoid arthritis portrayal by UK national newspapers
An important source of knowledge, beliefs and attitudes about illness is the mass media. 406 Research has established the often negative and emotive language utilised by journalists to report on long-term physical and psychological illnesses. Attempts to increase the use of intensive management in RA nationally are likely to be influenced by opinions outside the immediate specialist area, including its representation in the media. 247 We therefore examined how RA is portrayed in the UK popular press.
Methods
Fidelity of intervention delivery
Intensive management consultations were audio-recorded by rheumatology practitioners with patients’ consent. A 10% sample of all recorded consultations were assessed (this comprised 126 sessions). Fidelity assessment was conducted within a team of four researchers. Establishing inter-rater reliability was undertaken between July 2015 and February 2016. Rating of the treatment support delivery commenced in March 2016 and continued for 1 year.
Systematic review of nurses contributions
We searched MEDLINE using the terms ‘nursing’ and ‘rheumatoid arthritis’. The search was limited to English publications from January 2000 to August 2018. Further details are given in Report Supplementary Material 1 and 2, including a PRISMA flow diagram (see Report Supplementary Material 1, Figure 7), the studies included (see Report Supplementary Material 1, Table 9) and study quality (see Report Supplementary Material 1, Tables 10–13).
National data about nurse numbers
Data about rheumatology nurse numbers were obtained from surveys by the National Audit Office407 and National Clinical Audits. 408
Nurses and other practitioners involved in the TITRATE trial
The TITRATE team collected data about the professional backgrounds, experience and titles of all clinicians who delivered the intensive management during the 12-month trial.
Portrayal in newspapers
The study was patient led with the support by a social scientist. The LexisNexis® [RELX (UK) Ltd, London, UK] online repository of print media was searched for articles from July 2011 to July 2016 that included RA in the headline and/or lead paragraph of 15 UK national non-specialist newspapers. Resultant articles were uploaded to NVivo (QSR International, Warrington, UK) and a realist perspective aided thematic analysis. 409 Further details are given in Report Supplementary Material 1, Tables 14 and 15.
Key findings
Fidelity of intervention delivery
Seven assessments were undertaken to assess whether or not the technique was demonstrated (Table 25). Agreement of agenda between patient and nurse, the patient talking more than 60% of the time and the use of importance and confidence rulers were evidenced in only a minority of participants’ consultations. Those techniques that were clearly more instinctive, or easier for the intensive management nurses to learn and incorporate, were providing solicited information only, demonstrating listening skills and asking open questions.
| Question | Grade | Consultations, n (%) |
|---|---|---|
| Binary questions | ||
| Discuss and agree the agenda together with the patient | 53 (42) | |
| Assess confidence/importance of changing behaviour using ruler/scale | 19 (15) | |
| Provide unsolicited advice and/or information < 50% of time | 83 (66) | |
| Provide information when solicited > 80% of the time | 92 (73) | |
| Use at least three open-ended questions | 75 (60) | |
| Allow the patient to talk > 60% of consultation | 44 (35) | |
| Summarise what the patient said on at least two occasions | 75 (60) | |
| Graded questions | ||
| Support the patient in identifying one (main) problem area | 0 | 8 (6) |
| 1 | 38 (30) | |
| 2 | 46 (37) | |
| 3 | 17 (14) | |
| 4 | 15 (12) | |
| 5 | 2 (2) | |
| Affirm the patient’s strengths, abilities or effort in any area by saying something positive or complimentary | 0 | 10 (8) |
| 1 | 33 (26) | |
| 2 | 22 (18) | |
| 3 | 47 (37) | |
| 4 | 11 (9) | |
| 5 | 3 (2) | |
| Explore reasons for and against behaviour change with the patient | 0 | 1 (1) |
| 1 | 78 (62) | |
| 2 | 13 (10) | |
| 3 | 10 (8) | |
| 4 | 6 (5) | |
| 5 | 18 (14) | |
| Help the patient identify barriers to and facilitators of behaviour change | 0 | 0 (0) |
| 1 | 78 (62) | |
| 2 | 12 (10) | |
| 3 | 8 (6) | |
| 4 | 8 (6) | |
| 5 | 20 (16) | |
| Support the patient in setting one or two goals | 0 | 0 (0) |
| 1 | 34 (27) | |
| 2 | 48 (38) | |
| 3 | 12 (10) | |
| 4 | 12 (10) | |
| 5 | 20 (16) | |
| Help the patient develop a behaviour change plan/activity diary | 0 | 0 (0) |
| 1 | 39 (31) | |
| 2 | 36 (29) | |
| 3 | 17 (14) | |
| 4 | 10 (8) | |
| 5 | 24 (19) | |
| Evoke and reinforce change talk | 0 | 1 (1) |
| 1 | 39 (31) | |
| 2 | 42 (33) | |
| 3 | 23 (18) | |
| 4 | 11 (9) | |
| 5 | 10 (8) | |
Seven questions had graded answers indicating the degree to which a technique was used. These are shown in Table 25. Between 52% and 73% of assessed consultations did not demonstrate MI skill levels beyond grade 2 on the 0–4 scale. A high level of MI technique use was observed in 5–12% of consultations. The technique that nurses demonstrated most often at moderate or high fidelity levels was affirming the patient’s strengths and abilities [in 58 consultations (46%)]. Evoking and reinforcing change talk and identifying one main problem area were observed at moderate or high fidelity skill levels in 34 (27%) and 32 (25%) consultations, respectively. Conversely, exploring reasons for and against behaviour change and helping the patient identify barriers to and facilitators of behaviour change were observed at moderate and high fidelity in only 16 (13%) consultations.
Specialist nurses and other practitioners giving intensive management
Systematic review
We identified 655 publications and included 19 papers reporting on eight trials410–417 (1974 patients), seven qualitative studies388,418–423 (242 patients) and four observational studies424–427 (1234 patients).
The main findings are summarised in Table 26. The trials were undertaken in a range of settings and used both superiority and non-inferiority designs. Overall, they provided strong evidence that patients whose follow-up was primarily managed by specialist nurses achieved similar clinical outcomes to those who were managed by doctors. Several trials showed that nurses were also able to enhance patient satisfaction and self-efficacy. The qualitative studies showed that nurses are able to increase patients’ knowledge of RA and promote self-management for patients. The observational studies, which had case–control and cohort designs, also showed that nurses provided effective and acceptable care. Overall, these studies provided strongly positive evidence in support of nurses playing key roles in the follow-up management of RA patients.
| Publication | Year | Patients (n) | Conclusion |
|---|---|---|---|
| Trials | |||
| Tijhuis et al.417 | 2002 | 210 | Nurse specialists achieve similar outcome in comparison to other approaches |
| Hill et al.411 | 2003 | 80 | Clinical outcomes similar, but patient satisfaction greater with nurse care |
| Ryan et al.416 | 2006 | 71 | Nurses improve patients’ perceived ability to cope with arthritis |
| Koksvik et al.412 | 2013 | 68 | Nurses increase satisfaction with care without loss of efficacy |
| Larsson et al.413 | 2014 | 107 | Stable patients on biologics monitored in nurse-led clinics have similar outcomes |
| Primdahl et al.415 | 2014 | 287 | Stable patients receiving biologics in nurse-led clinics have comparable clinical outcomes and enhanced self-efficacy and satisfaction |
| Ndosi et al.414 | 2014 | 181 | Nurse-led care gave similar clinical outcomes and higher general satisfaction scores |
| Dougados et al.410 | 2015 | 970 | Nurse-led programme gave short-term benefits on comorbidity management |
| Qualitative studies | |||
| Temmink et al.423 | 2000 | 128 | Patients are positive about quality and continuity of care. Some limitations in continuity of care |
| Long et al.421 | 2002 | 16 | Nurses contribute to assessment, integrating therapy and emotional support |
| Arvidsson et al.418 | 2006 | 16 | Nurses provide holistic assessments, co-ordinated care and provide insight |
| Primdahl et al.422 | 2011 | 33 | Nursing consultations are less factual and less authoritarian than medical consultations |
| Bala et al.419 | 2012 | 18 | Nurses give familial atmosphere, empathy, knowledge, accessibility and continuity |
| Larsson et al.420 | 2012 | 13 | Nurses enhance security, familiarity and participation |
| van Eijk-Hustings et al.388 | 2013 | 18 | Nurses provide education, self-management and emotional support, and help organise care |
| Observational studies | |||
| Esselens et al.424 | 2009 | 191 | Programmed care achieved better clinical outcomes and general health |
| Watts et al.427 | 2015 | 349 | Minimal differences in clinical outcomes between community and hospital follow-up |
| Solomon et al.426 | 2015 | 301 | Fewer patients seeing nurses/physician assistants had high disease activities |
| Muñoz-Fernández et al.425 | 2016 | 393 | Nurse-led clinics achieved better global assessments and less disability |
National data about nurse numbers
The 2009 National Audit Office report407 identified 377 rheumatology nurses and the 2016 Healthcare Quality Improvement Partnership report408 identified 355 nurses (in addition, there were 0.85/100,000 consultant rheumatologists and 0.64/100,000 rheumatology nurses).
Nurses and other practitioners involved in the TITRATE trial
From 2014 to 2017, TITRATE trial training was given to 100 clinicians from 39 NHS trusts (42 sites). Eighty-six participants were female, 14 were male and their mean age was 48 (range 28–72) years. The rheumatology experience of the participants varied, with a mean duration of 5 (range 0–30) years (59 participants had ≤ 4 years of experience) (Figure 24). The professional backgrounds of participants also varied: 85 participants were nurses, eight participants were other health professionals, six participants had medical backgrounds in non-consultant posts and one participant was an occupational therapist. There were marked differences in how the clinicians described their roles (Figure 25). Nurses were called rheumatology nurse, rheumatology nurse specialist, rheumatology clinical nurse specialist and rheumatology nurse practitioner. There were also considerable variations in their seniority, ranging from band 5 nurses to a modern matron.
FIGURE 24.
Rheumatology experience of TITRATE trial participants.
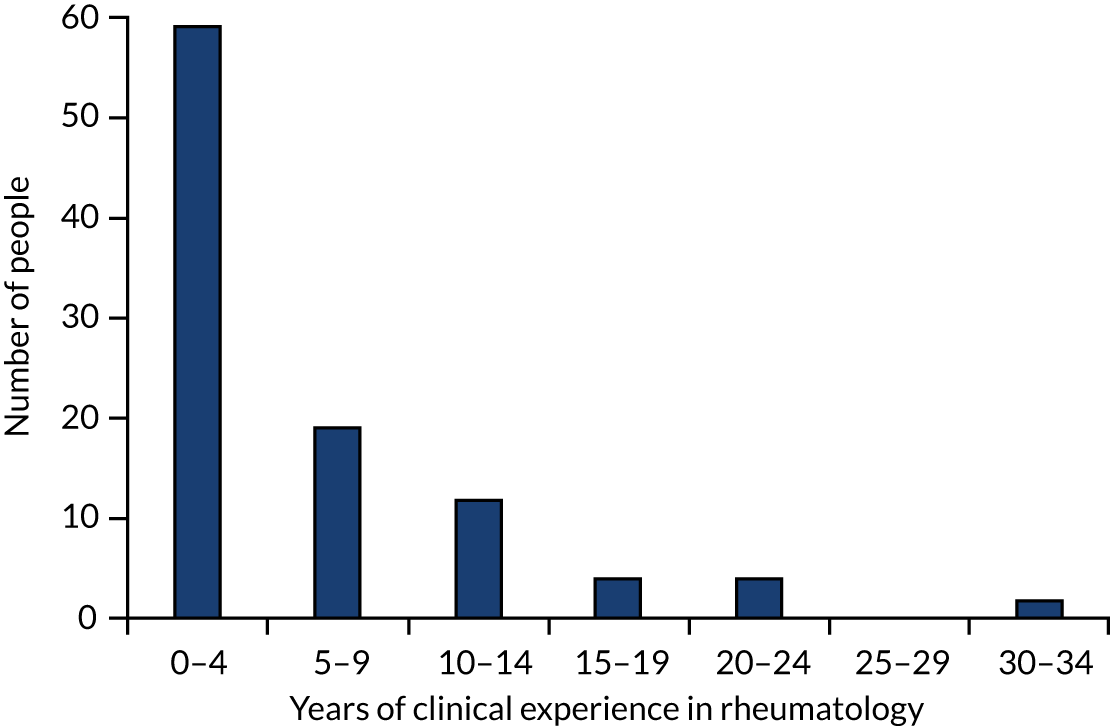
FIGURE 25.
Nurses and health-care professionals in training programme. SpN, specialist nurse; SpR, specialist registrar; ST3, year 3 of specialty training.
Portrayal by UK national newspapers
The LexisNexis search produced 413 newspaper articles, 147 of which met the inclusion criteria. Some newspapers published many articles on RA and others published few or none. Three themes were identified in the thematic analysis that conveyed how UK national newspapers used language to report on RA. These themes comprised (1) language used to describe RA, (2) language used to refer to those who live with RA and (3) language used to report on potential new treatments for RA.
In describing RA, ‘attack’ was the most cited term and was more likely to be used in the tabloid or middle market press than in the broadsheets. ‘Painful’, ‘crippling’, ‘agony’ and ‘incurable’ were also commonly used terms.
In describing people with RA, the term ‘sufferers’ had the most number of references across the entire data set of newspaper articles, and it was more likely to be found in the tabloid/middle market press than in broadsheets. ‘Patients’, ‘people’ and ‘victims’ were also commonly used terms.
The final theme, the way potential new drugs or medical technologies for RA are reported, showed that journalists often used the language of ‘hope’ to frame the discussion of potential treatments. ‘Hope’ was mainly used in the tabloid and middle market newspapers. Other terms included ‘breakthrough’, ‘ground-breaking’, ‘cure’ and even ‘miracle’ on one occasion.
Overall, newspaper articles drew on negative and emotionally laden language to convey the experience of RA. This approach was more apparent in the tabloid or middle market newspapers than in broadsheets. Neutral terms, such as ‘people’ and ‘individuals’, were used to describe those living with RA. However, they were often viewed by the popular press in a passive sense as ‘sufferers’ or ‘victims’. The media’s application of language in the context of potential new medical technologies or drugs for RA overwhelmingly stressed their positive effects. This is despite the fact that much of the scientific research highlighted in the printed press over the 5-year time frame of this study was still in development and often untested in humans.
Limitations
The research in this section of the report was more diverse as there are a broad range of issues that may influence implementation. Two main limitations need consideration: (1) the nature of intensive management and (2) the variable nature of specialist rheumatology teams.
Nature of intensive management
The treatment strategy in the TITRATE trial involved both increasing the intensity of treatment with DMARDs and biologics and supporting patients to manage their disease. Although some health-care practitioners may be most successful in increasing treatment, others may be most successful in providing supportive care. Not all practitioners may excel at all the different aspects of intensive management. This makes it challenging to judge their impact.
Variable rheumatology teams
There is extensive variation in the nature of specialist rheumatology teams across England. Some have large teams of nurses and other health-care practitioners. Others have very few staff. There is also extensive variation in the nature and experience of nurses. This variability makes it particularly difficult to assess what is available and what can be delivered locally.
Relation to overall programme
The research in this section provides some insights into some of the challenges that are likely to arise when implementing intensive management for patients using the type of approaches taken in the TITRATE trial. A key problem is the variability in expertise and training of specialist nurses and other allied health-care professionals. Many rheumatology nurses have relatively limited experience and they need more education and support. Another separate problem is the constant negative perspectives about RA in the mainstream media, which are likely to prove a disincentive for patients and clinicians.
Complexity of intervention
The fidelity assessments showed that nurses and health-care practitioners followed some but not all the approaches they were taught to use in the TITRATE trial. Training staff in the MI techniques followed in the TITRATE trial will require both time and commitment from their units.
Limited experience of specialist nurses
Most nurses delivering intensive management in the TITRATE trial had been involved in rheumatology for < 5 years. Whatever the benefits of using nurses to deliver specialist care, this level of experience of specialist nurses creates complexities in the delivery of expert care. Consultant rheumatologists, by comparison, have far more experience in the field. The challenges in training and supporting specialist nurses and other non-medical health-care professionals managing RA patients are widely understood. 428,429
Negative perspectives about rheumatoid arthritis
Media views about RA are not crucial for changing the way care is delivered. However, a persistent negative message about the disease as ‘crippling’ and patients being portrayed as ‘sufferers’ and ‘passive victims’430 is unhelpful in the promotion of intensive management approaches.
Conclusions and recommendations
Main findings
Our research covered many aspects of the intensive management of patients with moderately active RA. Several main conclusions dominate our findings about intensive management: (1) it increases remissions in active RA; (2) it is effective and safe in moderate RA; and (3) it is practical in routine care settings, although, in the short term, within-trial costs and benefits are unlikely to be cost-effective at the threshold ranges typically used by UK decision-makers (i.e. £20,000–30,000/QALY). This final section places the TITRATE trial findings into perspective. Three published reviews undertaken for the programme helped frame our conclusions. 431–433
Intensive management in active rheumatoid arthritis
There is substantial evidence from previous trials that intensive management increases remissions in patients with active RA. There is no particular reason from the perspective of efficacy to favour one type of intensive management strategy over another. However, based on custom and practice and health economic considerations, there is a case to start with combinations of conventional DMARDs. This perspective is not universally shared. There is marked international variation in conventional DMARD use,48 and some groups report low persistence rates with combinations of conventional DMARDs compared with biologic combinations. 46,47,434
Intensive management in moderately active rheumatoid arthritis
The TITRATE trial showed that intensive management increases remissions in patients with moderate RA without increasing harms. The positive findings in the main trial were supported by the associated qualitative study of patients’ and clinicians’ views.
Using intensive management in routine care
The intensive management approach used in the TITRATE trial is acceptable to patients and clinical staff and is generalisable across English rheumatology clinics. Although it can be delivered in routine care, current NHS staff are working at full capacity, and implementing it nationally would have resource implications. There are unresolved uncertainties about how best to standardise nurse-delivered intensive management strategies in routine clinical settings. In addition, the way intensive management was used in the TITRATE trial primarily reflected clinicians’ perspectives. Patients may have somewhat different views on how it should best be used.
Other important findings
Intensive management, however, does not benefit all RA patients with established moderate disease. Previous trials and the findings in the TITRATE trial indicate that it usually benefits no more than half of RA patients. Ongoing active RA has negative clinical and social impacts on patients. 435,436 There is also uncertainty about the extent to which it provides care that is considered cost-effective within current perspectives on WTP thresholds in general437–439 and how this translates to RA. 29,440,441
Remission is the key target of intensive management. Although DAS28-ESR remissions have many complexities, they appear to be a reasonable and efficient target. When patients achieve DAS28-ESR remissions for more than a single occasion, they usually continue to have reasonably well-controlled RA for some considerable time.
The routine management of RA is continually evolving. Over the last two decades patients have received more intensive management and their disease activity levels have decreased. Current management resembles many components of the approach used in the TITRATE trial, although it neither achieves the same intensity of drug treatment nor receives the same extent of supportive care from specialist nurses.
Implications for practice
Intensive management for patients with moderate rheumatoid arthritis
The positive findings from the TITRATE trial and associated research suggest that it is timely to manage some patients with moderate RA intensively. This approach is in line with the national and international guidance.
Biologics in moderate rheumatoid arthritis
The intensive management used in the TITRATE trial involved giving some patients biologics in line with current specialist guidance but outside what is currently agreed in NICE Technology Appraisal guidance for these drugs. This is an area of substantial change, as the introduction of biosimilars is currently reducing the acquisition costs of these drugs. 442–444 The TITRATE trial provides information to inform future national work in this field. It found that only a minority of patients needed these drugs over and above those meeting current guidance, which are both supportive of extending biologic use in this way. At the same time, there was no evidence in the TITRATE trial that biologics specifically contributed to the benefits of intensive management, and many patients who achieved remissions did not receive biologic drugs. Against this background, we appreciate that many elements are involved in developing national guidance and the findings from the TITRATE trial are only one factor to take into account. There is also a need to consider, in more detail, the cost-effectiveness of such an approach, as the TITRATE trial did not consider all aspects of the economic issues involved. Decisions about using relatively high-cost interventions for long-term disabling disorders like RA create complex ethics issues and it is important to take broad-based perspectives on the benefits not only to patients but also to society as a whole. Such considerations, although important, fall outside what conclusions can be reasonably be drawn by us as researchers in the field.
Regular assessments by nurses
Nurses and other health-care professionals played crucial roles in delivering intensive management in the TITRATE trial. A simplified version of the TITRATE training programme will be available for specialist units that wish to use it. A range of health-care professionals can deliver such care, and it may be possible to use telephone and digital interactions rather that needing all patients to attend clinics in person. Since our research was completed, the coronavirus pandemic changed the way clinical practice is delivered in England. Telephone and digital consultations have been widely adopted, and their impact on our findings needs careful consideration. Patient perceptions about the way their RA is assessed and managed are likely to be of particular relevance when refining how care is delivered. It may also be possible to assess patients at intervals other than monthly. Each specialist unit must decide how to deliver regular assessments by nurses and other staff based on national and international guidelines and a range of different sources of evidence, including studies such as the TITRATE trial. Not all patients require identical follow-up arrangements, and care needs to be individualised. There is also a case for undertaking further developmental work to further rationalise how intensive management is delivered. Simplifying the delivery of intensive management approaches will reduce their costs.
Recommendations for future research (in priority order)
Impact of potential predictive factors outcome
The TITRATE trial showed that patients with a high BMI were unlikely to respond to intensive management, although whether the effect was due to obesity itself or reflective of a range of underlying associations is unknown There is a growing body of evidence from observational studies that a high BMI is associated with persisting active disease and poor outcomes. 355,445–447 The reasons for these associations and the implications for management need further investigation. However, such research needs to take into account a range of genetic, educational, behavioural and environmental risk factors, rather than focusing entirely on BMI. It is also likely that social factors will be of crucial importance in predicting responses, although this was not specifically addressed in our research. Patients from socioeconomically deprived backgrounds and those from some ethnic minorities may be more likely to have poor outcomes.
Broadening the approaches to minimising disability
Achieving remission reduced disability in the TITRATE trial, but many patients continued to have considerable disability. There was also evidence that high initial disability levels reduce response to intensive management and that, although disease activity levels have declined over the last two decades, there is less evidence that disability has also reduced. These various findings suggest that disability needs to be reduced in different ways over and above minimising disease activity levels. The best way to achieve this goal needs further research. Non-drug approaches, which were not part of the TITRATE trial, may be relevant. 448–450
Part of broadening approaches to minimising disability includes reconsidering how disease activity is assessed. Although we found that intensive management reduced disease activity in moderate RA, it did not have any impact on ESR or CRP levels. It is possible that alternative blood tests may be more appropriate in patients with moderate RA, and further work may help identify which, if any, of these provide useful data when monitoring treatment responses and how they can be used in conjunction with clinical data. 451–454 It is also possible that other approaches to assessing disease activity may be more informative (e.g. using patient self-assessments in association with digital consultations).
Explore the long-term cost-effectiveness of intensive management for patients with moderate rheumatoid arthritis
The clinical findings in the TITRATE trial strongly support using intensive management approaches for patients with moderately active established RA. In contrast, the economic analysis suggested that its costs fell above the thresholds usually accepted as affordable by the NHS. However, these economic assessments were restricted to the 12-month follow-up period in the trial. They did not take into account the benefits of improved earlier treatment and potentially reduced biologic drug use over the longer term. Extrapolation of the costs and benefits of the intervention could be carried out in a decision-analytic model to produce a lifetime estimate of expected costs and QALYs. One important issue is estimating the impact of the future treatment pathway for the patient populations once a switch from intensive DMARD therapy has occurred. This will include the costs of biologics if patients progress to severe RA and the time to loss of efficacy (which could be estimated from survival analysis of the within-trial data).
Patient and public involvement
Background
This section is based on the GRIPP2 (Guidance for Reporting Involvement of Patients and the Public 2) recommendations. To appreciate the way in which patients were involved a brief background about arrangements in the host institution is likely to be helpful. In 2005, King’s College Hospital (London, UK), Department of Rheumatology, which hosted the TITRATE programme, implemented a strategy to embed PPI in research, teaching and health service delivery. This PPI strategy was led by Heidi Lempp (a TITRATE investigator).
The department established a PPI group comprising six patients and one carer. All members were provided with honorary contracts with King’s College London. One patient currently had a contract with King’s College Hospital NHS Foundation Trust (London, UK) to advise clinic staff on outpatient service delivery improvement.
Our commitment to PPI led to the setting up of patient education evenings, which have been running since 2014. These sessions take place in collaboration with clinic staff, patients and academic staff and are based on topics suggested by patients/carers. These sessions have been very successful, with several requests for their continuation. The annual patient education event takes place during the summer months (as requested by patients) and are held during early evening, starting at 5.30 p.m. The presenters are patients and clinicians from King’s College Hospital or invited external speakers. The number of attendees is gradually growing (now up to 30–40 people) and the feedback has been mostly positive (average 9/10, very good/excellent). The topics covered in 2017 included the following:
-
emotional support as part of a comprehensive rheumatology outpatient service: findings from an interview study with patients
-
adjusting to and living well with a long-term condition
-
Garment+ – redesigning clothing with patients for people
-
my home is my castle – but the drawbridge is up – accessible housing when living with long-term rheumatological conditions
-
an overview of fatigue in patients with long-term musculoskeletal conditions
-
evidence-based self-management of fatigue and emotional well-being in long-term musculoskeletal conditions.
Aims for involving patients and the public in the TITRATE programme
Patients made key contributions to many aspects of the TITRATE programme and were involved in all aspects of its design, delivery and plans for future implementation of its findings.
The three main aims for PPI were to (1) help design a care pathway for delivering intensive management in a way that patients found acceptable; (2) assess the impact of receiving intensive management and (3) consider the issues involved in implementing the findings of the programme. Patients were also encouraged to extend the work on the programme into areas they considered of particular relevant.
Methods for involving patients and the public
The local arrangements for PPI within the host department were supplemented by additional support for the TITRATE programme in two ways. First, the key patient organisation in England for people with RA – the NRAS – was involved. The chief executive of NRAS was one of the co-applicants on the TITRATE programme. During the research programme, the NRAS website provided information about the TITRATE trial, which had been approved by the Research Ethics Committee. Second, two other co-applicants in the TITRATE programme were also patients (one had no professional background in medicine and the other was both a patient and a former GP). These various arrangements enabled us to achieve a high level of involvement of patients and the public in the TITRATE programme.
One part of the TITRATE programme, workstream A, focused on developing care that is ‘best for patients’. This aspect of the research was the main focus for PPI. The workstream had three themes: (1) equipping patients for intensive treatment, (2) understanding patients’ views on intensive treatment and (3) implementing intensive treatment approaches in routine clinical care.
Outcomes from involving patients and the public
Equipping patients for intensive treatment
The patient handbook was developed with substantial PPI. First, it was supported by one focus group that involved six patients who live with RA from a single NHS hospital trust. Second, individual feedback was received from one departmental patient expert in close collaboration with two RA charities (the NRAS and Arthritis Research UK). Shared treatment plans, which are an associated part of the research, were developed with help from five patients and one carer from one hospital trust. The developmental work for the patients’ handbook and shared treatment plans has been described in one paper, which was published in 2017. 268 Two patients who were involved developing the handbook co-authored this publication.
Patients’ views on intensive treatment
Patients’ views on intensive treatment in the TITRATE programme before the trial commenced were evaluated through focus groups and one-to-one interviews with nine patients and five carers, and an additional two patients whose first language was not English. These patients and careers were from three different hospital trusts. The findings from the focus groups were published in 2016. 269 One patient co-authored this publication.
In 2017, the main focus has been an evaluation of the views of nurses and patients about their participation in the trial. Louise Prothero interviewed 13 MI practitioners from 20 different centres and a similar number of patients who participated in the active arm of the trial. The interview schedule for the patients was developed from the literature and in collaboration with one of our departmental expert patients so that the content of the questions were relevant and comprehensible to the participants. The research findings were published in 2019. 370
Input to research questions
The topic guide for the patient interviews was reviewed by a patient. Changes were made to key questions to make them more positive and understandable to patients.
Planning implementation
Four service users attended a planning meeting for implementing nurse training, together with 15 staff from national charities, health professionals and academic and clinic staff. The service users contributed substantially, based on their experiences attending nurse-led clinics. One author, a service user, agreed to co-author the report underpinning this initiative.
Foot health
A mixed-methods approach collected qualitative data from two focus groups of RA patients and quantitative data from rheumatology team members. The data were collected in a survey that had been piloted and assessed for face and content validity, using average congruence, by two external clinicians and departmental patient experts living with RA.
Rheumatoid arthritis portrayal by UK national newspapers
The mass media are an important source of knowledge, beliefs and attitudes about illness. A research team of academic staff and patients examined how RA is portrayed in the UK popular press. This was considered an important factor in influencing uptake of intensive management in routine clinical practice.
Outcomes
Supporting ongoing research
The workstream A team met 6-monthly to inform all co-applicants of the TITRATE programme about the progress of the research, including publications.
Qualitative studies
Several aspects of the research involved qualitative studies to evaluate patients’ perspectives about their management and preferred options for taking drugs and other treatments. These studies involved patients in both their designs and in their analysis. Without patient involvement, they would not have been possible.
Designing successful intensive management
The TITRATE programme centred on the trial of intensive management. There are two key challenges to overcome when asking patients with moderately active RA to participate in a treatment strategy based on intensive management. First, the treatments have to be effective. Second, patients have to be prepared to take them. The first part of the programme focused on making certain that care was given in a way that patients found acceptable in the context of treating them intensively. Patient involvement in this part of the research was crucial to the success of the programme. As intensive management was taken by patients and they achieved more remissions as a consequence, it is reasonable to conclude that this crucial aspect of patient involvement was successful.
Placing the research in context
Studies of foot health and representation of RA in newspapers were suggested by patients. They helped place the research into context relevant for implementation.
Advice on progress of research
Patients contributed to both the Trial Steering Committee and the Programme Steering Committee. This involvement has proved useful in assessing the progress of the research and in planning how to develop the programme over time. In particular, it was helpful in assessing what is reasonable for patients to be asked to do in respect of the trial and other studies, and also what questions were most relevant to patients.
Publications and dissemination
Patients were involved as co-authors of many TITRATE trial publications and conference presentations. They are also co-authoring the final report.
Discussion
We did not encounter major difficulties involving patients and the public in the TITRATE trial. This reflects the long-standing commitment of the host department to have PPI in its research portfolio. We have established a reliable and enthusiastic team of expert patients and charity involvement in workstream A of the programme. This involved keeping patient research partners informed, and in engaging them by detailed forward planning and advanced agreement in what was expected in relation to meeting deadlines for our research outputs.
Clinical research involves diverse teams. Patients were involved in some parts of the research more than others. For example, they were heavily involved in the qualitative studies but had limited roles in the systematic reviews or secondary analyses of published trials. However, this issue applies to all members of the research team, all of whom had roles that were restricted to only some aspects of the overall programme.
A particularly important issue influencing the potential future implementation of the research findings is that a substantial body of patients and key patient groups have seen all the results and have reflected and commented on them. This involvement makes it unlikely for the TITRATE trial to receive future negative criticism from patient groups that it is not relevant for them, which has occurred in some related clinical areas.
Reflections
There have been several challenges involving patients. One was recruiting patients and carers for focus groups, which has sometimes been difficult. Suitable patients must be identified by clinical teams that may not be familiar with focus group research. It can also be complex identifying times suitable for a range of patients and carers to meet, as all have other daily commitments that are unique and variable. These problems are not unique to the TITRATE trial. In particular, it is difficult to engage younger patients in work or those with family responsibilities and patients from black and ethnic communities.
A second challenge has been obtaining continuity of patient involvement in the various committees related to the TITRATE trial (e.g. the Trial Steering Committee). These meetings, which are held intermittently over several years, are relatively long and involve detailed technical discussions. Therefore, it has been difficult arranging for patients to attend all meetings, although all such meetings have involved patients. Although there are benefits from involving different patients who can provide different insights and opinions, there are advantages in having continuity of involvement. This issue, also not unique to the TITRATE trial, has no simple solution.
Acknowledgements
The TITRATE trial involved a very large number of staff who either ran the trial, served on its various committees or were involved in recruitment and patient care within the trial. The trial also involved the collaboration of a number of patients who served on committees and advised on the research programme in different ways. We are very grateful for all their help.
Trial co-sponsors
King’s College London (contact, Keith Brennan).
King’s College Hospital NHS Foundation Trust (contact, David Dawson).
TITRATE trial programme investigators
Professor David L Scott (chief investigator).
Professor Gabrielle Kingsley (deceased, replaced by Professor Frances MK Williams).
Dr Chris Deighton (retired, replaced by Dr James B Galloway).
Professor Deborah Symmons.
Ailsa Bosworth.
Professor Trudie Chalder (replaced by Professor Jackie Sturt).
Dr Claire Henderson.
Professor Vern Farewell.
Dr Brian Tom.
Dr Fowzia Ibrahim.
Professor Allan Wailoo.
Jonathan Tosh (replaced by Dr Harry Hill).
Dr Ruth Williams.
Deborah Johnson.
Dr Heidi Lempp.
Carol Simpson.
Tim Higginson.
Professor Ewan Ferlie.
Dr Nicola Gullick.
Current/previous members of the TITRATE study group
Professor David L Scott.
Dr Fowzia Ibrahim.
Dr Naomi Martin.
Richard Jenner.
Isabel Neatrour.
Dr Rhiannon R Baggott.
Dr Heidi Lempp.
Professor Jackie Sturt.
Dr Sofia Georgopoulou.
Dr Louise Prothero.
Dr Elena Nikiphorou.
Professor Frances MK Williams.
Dr James B Galloway.
King’s College London staff associated with the TITRATE trial
Dr Rachel Davis.
Holly Sandu.
Beatriz Santana Suárez.
Dr Samana Schwank.
Petra Zikatanova.
Radiograph scoring
Dr Katie Bechman.
Dr Mark Yates.
King’s College London Clinical Trials Unit
The TITRATE trial is supported by the UK Clinical Research Collaboration-registered King’s Clinical Trials Unit at King’s Health Partners, which is part funded by the National Institute for Health Research Biomedical Research Centre for Mental Health at South London and Maudsley, the NHS Foundation Trust, King’s College London and the NIHR Evaluation, Trials and Studies Coordinating Centre.
Trial Steering Committee and Programme Grant Steering Committee
Professor David G Scott (chairperson).
Professor Anisur Rahman.
Professor James Ritter.
Dr Louise Pollard.
Luke Brewer.
Jo Cumming.
Chris Ward.
Federico Muscoguiri.
Kirandeep Chana.
Quang Tan Ho.
Judith Rosheuvel.
Celia Manson.
Carrie Gibbens.
Dr Aneela Mian.
Dr Lindsay Bearne.
Joy Ellery.
Dr Nicola Gullick.
Dr Karen Walker-Bone.
Professor Anthony Woolf.
Deborah Johnson.
Ailsa Bosworth.
Professor Ewan Ferlie.
Carol Simpson.
Dr Ruth Williams.
Professor David L Scott.
Dr Fowzia Ibrahim.
Dr Naomi Martin.
Richard Jenner.
Isabel Neatrour.
Dr Rhiannon R Baggott.
Dr Heidi Lempp.
Professor Jackie Sturt.
Dr Louise Prothero.
Dr Elena Nikiphorou.
Professor Frances MK Williams.
Dr James B Galloway.
Dr Brian Tom.
Professor Allan Wailoo.
Dr Harry Hill.
Data Monitoring Committee
Dr Richard Watts (chairperson).
Dr Kimme Hyrich.
Dr Mark Lunt.
Principal investigators at clinical sites
Dr Anurag Bharadwaj, Basildon University Hospital.
Dr Chandini Rao, Blackpool Victoria Hospital.
Dr Jonathan Marks, Christchurch Hospital.
Dr Natalie Horwood, Croydon University Hospital.
Dr Owen Moore, Derriford Hospital.
Dr Martin Lee, Freeman Hospital.
Dr David Collins, Great Western Hospital.
Dr Toby Garrood, Guy’s Hospital.
Dr Claire Gorman, Homerton University Hospital.
Dr Michael Plant, James Cook University Hospital.
Dr Shabina Habibi, King George Hospital.
Dr James B Galloway, King’s College Hospital, Orpington Hospital and Queen Mary’s Hospital, Sidcup.
Dr Antigoni Grigoriou, Kingston Hospital.
Dr Kiran Putchakayala, Leighton Hospital.
Dr Elizabeth MacPhie, Minerva Health Centre.
Dr Pradip Nandi, Northampton General Hospital.
Dr David Walker, North Tyneside General Hospital.
Dr Jo Ledingham, Queen Alexandra Hospital.
Dr Vadivelu Saravanan, Queen Elizabeth Hospital, Gateshead.
Dr Abdul Khan, Queen Elizabeth The Queen Mother Hospital.
Dr Ira Pande, Queen’s Medical Centre.
Dr David Hutchinson, Royal Cornwall Hospital.
Dr Chris Deighton, Royal Derby Hospital.
Dr Richard Haigh, Royal Devon and Exeter Hospital.
Dr James Maxwell, Royal Hallamshire Hospital.
Dr Gayatri Mittal, Royal National Orthopaedics Hospital.
Dr Karen Douglas, Russells Hall Hospital.
Dr Gouri Koduri, Southend University Hospital.
Dr Nidhi Sofat, St George’s Hospital.
Dr Rod Hughes, St Peter’s Hospital.
Dr Imran Riaz, The Queen Elizabeth Hospital.
Dr Kirsten Mackay, Torbay Hospital.
Dr Coziana Ciurtin, University College London Hospital.
Dr Nicola Gullick, University Hospital.
Dr Louise Pollard, University Hospital Lewisham, Queen Elizabeth Hospital, Woolwich.
Dr Easwaradhas Gladston Chelliah, Wrightington Hospital.
Other site staff (including research nurses and co-investigators)
Karen Culfear, Basildon University Hospital.
Dr Nagui Gendi, Basildon University Hospital.
Joan Joyce, Basildon University Hospital.
Raymond Mandac, Basildon University Hospital.
Dr Anupama Nandagudi, Basildon University Hospital.
Dr Asanka Nugaliyadde, Basildon University Hospital.
Angelo Ramos, Basildon University Hospital.
Dr Shilpa Selvan, Basildon University Hospital.
Michael Villaruel, Basildon University Hospital.
Janice Booth, Blackpool Victoria Hospital.
Dr Andrew Jeffries, Blackpool Victoria Hospital.
Angela Moore, Blackpool Victoria Hospital.
Maureen Morgan, Blackpool Victoria Hospital.
Afeez Adebesin, Christchurch Hospital.
Jeanette Bennett, Christchurch Hospital.
Bengono Bessala, Christchurch Hospital.
Tanith Changuion, Christchurch Hospital.
Gail Hann, Christchurch Hospital.
Michelle Maidwell, Christchurch Hospital.
James Page, Christchurch Hospital.
Alison Pitcher, Christchurch Hospital.
Sally Sawyer, Christchurch Hospital.
Ruth Britten, Croydon University Hospital.
Dr Sarah Levy, Croydon University Hospital.
Jun Liu, Croydon University Hospital.
Gloria Nwajei, Croydon University Hospital.
Dr Cristina Tacu, Croydon University Hospital.
Esme Elloway, Derriford Hospital.
Amanda Holt, Derriford Hospital.
Dr Jonathan King, Derriford Hospital.
Sindisiwe Nyathi, Derriford Hospital.
Pauline Putt, Derriford Hospital.
Stephanie Roberts, Derriford Hospital.
Dr Lindsay Robertson, Derriford Hospital.
Helen Guy, Freeman Hospital.
Claire Humphrey, Freeman Hospital.
Panagiotis Maniatis, Freeman Hospital.
Julie Norris, Freeman Hospital.
Christine Routledge, Freeman Hospital.
Heather Russell, Freeman Hospital.
Robert Wilson, Freeman Hospital.
Natasha Chesterman, Great Western Hospital.
Suzannah Pegler, Great Western Hospital.
Dr Elizabeth Price, Great Western Hospital.
Grace Auld, Guy’s Hospital.
Cristina Blanco-Gil, Guy’s Hospital.
Estee Chan, Guy’s Hospital.
Dr Andrew Cope, Guy’s Hospital.
Vitor Goncalves, Guy’s Hospital.
Nadia Ladha, Guy’s Hospital.
Zoe McKee, Guy’s Hospital.
Maria Opena, Guy’s Hospital.
Sujith Subesinghe, Guy’s Hospital.
Susan Eisa, Homerton University Hospital.
Balinder Hans, Homerton University Hospital.
Sophia Hans, Homerton University Hospital.
Usha Mathukutty, Homerton University Hospital.
Dr Claire-Louise Murphy, Homerton University Hospital.
Animesh Patel, Homerton University Hospital.
Anna Price, Homerton University Hospital.
Dr Piero Reynolds, Homerton University Hospital.
Dolapo Sobayo, Homerton University Hospital.
Gianina Statache, Homerton University Hospital.
Dr Clare Thornton, Homerton University Hospital.
Sarah McAuliffe, James Cook University Hospital.
Dr Muddassir Shaikh, James Cook University Hospital.
Dawn Youll, James Cook University Hospital.
Andreas Georgiou, King George Hospital.
Dr Mayuri Karela, King George Hospital.
Panagiota Rousou, King George Hospital.
Dr Euthalia Roussou, King George Hospital.
Christos Tsintikidis, King George Hospital.
Elisa Visentin, King George Hospital.
Aderonke Audu, King’s College Hospital.
Carron Congreve, King’s College Hospital.
Dr Amara Ezeonyeji, King’s College Hospital.
Ella Foncel, King’s College Hospital.
Cavell Johnson, King’s College Hospital.
Margaret Ma, King’s College Hospital.
Aneela Mian, King’s College Hospital.
Hayley Noble, King’s College Hospital.
Patrizio Orru, King’s College Hospital.
Rosaria Salerno, King’s College Hospital.
Dr Laura Attipoe, Kingston Hospital.
Jennifer Crooks, Kingston Hospital.
Dr Lucy Durham, Kingston Hospital.
Tracey O’Brien, Kingston Hospital.
Duncan Bailey, Leighton Hospital.
Barbara Burnham, Leighton Hospital.
Claire Gabriel, Leighton Hospital.
Vanda Harris, Leighton Hospital.
Richard Miller, Leighton Hospital.
Lesley Ashcroft, Minerva Health Centre.
Julie Butler, Minerva Health Centre.
Joanna Cox, Minerva Health Centre.
Liana Dunn, Minerva Health Centre.
Ann Gooden, Minerva Health Centre.
Dr Ayesha Madan, Minerva Health Centre.
Dr Manoj Samaranayake, Minerva Health Centre.
Gillian Welch, Minerva Health Centre.
Kelly Wigglesworth, Minerva Health Centre.
Dr Hind Al Husain, Northampton General Hospital.
Lorraine Campey, Northampton General Hospital.
Lucy Dudgeon, Northampton General Hospital.
Alison Duncan, Northampton General Hospital.
Julie Fisher, Northampton General Hospital.
Rachael Hitchcock, Northampton General Hospital.
Ruby Hughes, Northampton General Hospital.
Dr Rachel Jeffery, Northampton General Hospital.
Andrea Kempa, Northampton General Hospital.
Jennifer Spimpolo, Northampton General Hospital.
Lynne Stockham, Northampton General Hospital.
Dr James Taylor, Northampton General Hospital.
Dr Ismaël Atchia, North Tyneside General Hospital.
Stacey Duffy, North Tyneside General Hospital.
Dr Iain Goff, North Tyneside General Hospital.
Margot Lilley, North Tyneside General Hospital.
Judith Plank, North Tyneside General Hospital.
Christina Tanney, North Tyneside General Hospital.
Robert Clark, Orpington Hospital.
Elizabeth Dragonetti, Orpington Hospital.
Anne Johnson, Orpington Hospital.
Dr Sarah Medley, Orpington Hospital.
Clemency Newman, Orpington Hospital.
Dr Dee Sreerangaiah, Orpington Hospital.
Julie Long, Queen Alexandra Hospital.
Marie White, Queen Alexandra Hospital.
Paula White, Queen Alexandra Hospital.
Julie Williams, Queen Alexandra Hospital.
Julie Dodds, Queen Elizabeth Hospital, Gateshead.
Dr Carol Heycock, Queen Elizabeth Hospital, Gateshead.
Wendy McCormick, Queen Elizabeth Hospital, Gateshead.
Susan Pugmire, Queen Elizabeth Hospital, Gateshead.
Hocine Azzoug, Queen Elizabeth Hospital, Woolwich.
Dr Gerald Coakley, Queen Elizabeth Hospital, Woolwich.
Leah Irungu, Queen Elizabeth Hospital, Woolwich.
Victoria Rodwell, Queen Elizabeth Hospital, Woolwich.
Tracey Cosier, Queen Elizabeth The Queen Mother Hospital.
Hazel Harrop, Queen Elizabeth The Queen Mother Hospital.
Tracy Hazelton, Queen Elizabeth The Queen Mother Hospital.
Eliya Nenkova, Queen Elizabeth The Queen Mother Hospital.
Anthea Potter, Queen Elizabeth The Queen Mother Hospital.
Shiny Thomas, Queen Elizabeth The Queen Mother Hospital.
Febisola Akinboyewa, Queen Mary’s Hospital, Sidcup.
Dr Nap Cheung, Queen Mary’s Hospital, Sidcup.
Dr Corina Moisoiu-Rosca, Queen Mary’s Hospital, Sidcup.
Susan Bartholomew, Queen’s Medical Centre.
Amanda Butler, Queen’s Medical Centre.
Dr Ian Gaywood, Queen’s Medical Centre.
Marie-Josèphe Pradère, Queen’s Medical Centre.
Heidi Clode, Royal Cornwall Hospital.
Anna Fouracres, Royal Cornwall Hospital.
Fiona Hammonds, Royal Cornwall Hospital.
Keely Lane, Royal Cornwall Hospital.
Andrew Pothecary, Royal Cornwall Hospital.
Gina Townley, Royal Cornwall Hospital.
Lisa Trembath, Royal Cornwall Hospital.
Leanne Welch, Royal Cornwall Hospital.
Alison Booth, Royal Derby Hospital.
Cresta Browning, Royal Devon and Exeter Hospital.
Dr Susie Earl, Royal Devon and Exeter Hospital.
Gayle Githens-Mazer, Royal Devon and Exeter Hospital.
Jane Hall, Royal Devon and Exeter Hospital.
Dr Sarah Harman, Royal Devon and Exeter Hospital.
Robert James, Royal Devon and Exeter Hospital.
Dr Ravik Mascarenhas, Royal Devon and Exeter Hospital.
Jill Moran, Royal Devon and Exeter Hospital.
Daniel Murphy, Royal Devon and Exeter Hospital.
Nicola Bennett, Royal Hallamshire Hospital.
Kay Cawthron, Royal Hallamshire Hospital.
Jane Collins, Royal Hallamshire Hospital.
Olivia Godia, Royal Hallamshire Hospital.
Joanne Kadziola, Royal Hallamshire Hospital.
Shamiso Masuka, Royal Hallamshire Hospital.
Adam Mitchell, Royal Hallamshire Hospital.
Christopher Wragg, Royal Hallamshire Hospital.
Deirdre Brooking, Royal National Orthopaedics Hospital.
Jacqueline Vinton, Royal National Orthopaedics Hospital.
Wendy Gardner, Russells Hall Hospital.
Ranjit Gidda, Russells Hall Hospital.
Stacey Jennings, Russells Hall Hospital.
Lucy Kadiki, Russells Hall Hospital.
Daljit Kaur, Russells Hall Hospital.
Dr Christos Koutsianas, Russells Hall Hospital.
Jaimini Patel, Russells Hall Hospital.
Chitra Ramful, Russells Hall Hospital.
Dr Ravinder Sandhu, Russells Hall Hospital.
Elizabeth Wells, Russells Hall Hospital.
Dr Tin Aung, Southend University Hospital.
Dr Fiona Coath, Southend University Hospital.
Prisca Gondo, Southend University Hospital.
Bernard Hadebe, Southend University Hospital.
Dr Faidra Laskou, Southend University Hospital.
Bridgett Masunda, Southend University Hospital.
Lena Assi, St George’s Hospital.
Abiola Harrison, St George’s Hospital.
Marie Buckley, St Peter’s Hospital.
Rachael Driver, St Peter’s Hospital.
Dr Susie Higgins, St Peter’s Hospital.
Margaret Walsh, St Peter’s Hospital.
Sholeh Wright, St Peter’s Hospital.
Tracy Fuller, The Queen Elizabeth Hospital.
Pauline Lingwood, The Queen Elizabeth Hospital.
Dr Bashaar Boyce, Torbay Hospital.
Christine Dixon, Torbay Hospital.
Julie Easterbrook, Torbay Hospital.
Dr Florin-Mihaela Florea, Torbay Hospital.
Diana Mill, Torbay Hospital.
Rian Penford, Torbay Hospital.
Sally-Anne Plumb, Torbay Hospital.
Lorraine Richardson, Torbay Hospital.
Alison Stokes, Torbay Hospital.
Lorraine Thornton, Torbay Hospital.
Dr Rachel Winfield, Torbay Hospital.
Elaine Wren, Torbay Hospital.
Dr Laura Attipoe, University College London Hospital.
Dr Geraint Brown, University College London Hospital.
Alice Cotton, University College London Hospital.
Jesusa Guinto, University College London Hospital.
Dr Alexis Jones, University College London Hospital.
Dr Arti Sharma, University College London Hospital.
Dr Himashi Anver, University Hospital.
Susanne Armitage, University Hospital.
Dr Siwalik Banerjee, University Hospital.
Susan Dale, University Hospital.
Dr Shirish Dubey, University Hospital.
Nigel Edwards, University Hospital.
Diane Hannon, University Hospital.
Davina Hewitt, University Hospital.
Helen Sharples, University Hospital.
Deborah Johnson, University Hospital Lewisham.
Dr Sarang Chitale, Wrightington Hospital.
Joshua Cooper, Wrightington Hospital.
Mark Gaskell, Wrightington Hospital.
Diane Heaton, Wrightington Hospital.
Pamela O’Connell, Wrightington Hospital.
Valerie Parkinson, Wrightington Hospital.
Isabelle Sykes, Wrightington Hospital.
Caroline Tierney, Wrightington Hospital.
Pfizer Inc. made Enbrel freely available for use outside NICE guidance in this study.
Contributions of authors
David L Scott (https://orcid.org/0000-0002-2198-4913) (Professor of Clinical Rheumatology) was chief investigator, led the TITRATE trial, secondary analysis of other clinical trials, observational studies and some of the systematic reviews, and drafted the final report.
Fowzia Ibrahim (https://orcid.org/0000-0002-7069-8024) (Statistician) undertook the statistical analysis of the TITRATE trial, secondary analysis of other clinical trials, observational studies and the systematic reviews, and contributed to the final report.
Harry Hill (https://orcid.org/0000-0002-0908-5595) (Research Fellow in Health Economics) undertook the health economic analysis and economic assessment of the TITRATE trial, and contributed to the final report.
Brian Tom (https://orcid.org/0000-0002-3335-9322) (Statistician) contributed the statistical analysis of the TITRATE trial, undertook statistical analysis of some of the observational studies and contributed to the final report.
Louise Prothero (https://orcid.org/0000-0002-5385-0397) (Research Assistant, Health Psychology) undertook the developmental work for intensive management and the assessment of its delivery in the TITRATE trial and contributed to the final report.
Rhiannon R Baggott (https://orcid.org/0000-0003-1228-9027) (Data Manager) organised the collection and checking of the data for the TITRATE trial and contributed to the final report.
Ailsa Bosworth (https://orcid.org/0000-0001-7634-414X) (Chief Executive, NRAS) provided patient perspectives on intensive management as both a patient and as the lead for the national patients’ organisation, and contributed to the final report.
James B Galloway (https://orcid.org/0000-0002-1230-2781) (Senior Lecturer in Rheumatology) contributed to the development of intensive management and training nurses, contributed to the analysis of the trial, the design and analysis of routine care observational studies, providing supportive information about intensive management and associated systematic reviews, and contributed to the final report.
Sofia Georgopoulou (https://orcid.org/0000-0002-8962-9985) (Research Associate, Health Psychologist) devised and delivered the work needed for delivering intensive management in the TITRATE trial, including training all specialist nurses and other health-care staff, and commented on the final report.
Naomi Martin (https://orcid.org/0000-0002-0210-7536) (Trial Co-ordinator, Clinical Psychologist) established the setup of the TITRATE trial, was responsible for integrating intensive management within the trial, oversaw the collection of trial data and commented on the final report.
Isabel Neatrour (https://orcid.org/0000-0001-5478-3053) (Trial Manager/Monitor) was responsible for monitoring the TITRATE trial across multiple English centres, ensured that the trial data were collected in an appropriate and timely manner, prepared the data for analysis and evaluation, and commented on the final report.
Elena Nikiphorou (https://orcid.org/0000-000-1-6847-3726) (Clinical Research Fellow and Consultant Rheumatologist) contributed to the analysis of the trial, the design and analysis of routine care observational studies, providing supportive information about intensive management and associated systematic reviews, and contributed to the final report.
Jackie Sturt (https://orcid.org/0000-0003-1281-1401) (Professor of Nursing) designed and oversaw the intensive management strategies used by specialist nurses in the TITRATE trial, placed the clinical findings in the trial into perspective, evaluating the delivery of care by the nurses, and contributed to the final report.
Allan Wailoo (https://orcid.org/0000-0002-9324-1617) (Professor of Health Economics) designed and oversaw the health economic analysis of the TITRATE trial, ensured that the whole programme reflected current perceptions of the economic impact of managing RA and contributed to the final report.
Frances MK Williams (https://orcid.org/0000-0002-2998-2744) (Professor of Genomic Epidemiology and Honorary Consultant Rheumatologist) contributed to the analysis of the trial and its design, contributed secondary analysis of previous trials and analysis of routine care observational studies, provided supportive information about intensive management and contributed to the final report.
Ruth Williams (https://orcid.org/0000-0002-2474-0092) (patient) played a key role in ensuring that patients’ perspectives were incorporated fully within the programme, based on her clinical background as a previous GP and the experience of living with RA over several decades, and contributed to the final report.
Heidi Lempp (https://orcid.org/0000-0003-2509-3656) (Reader in Medical Sociology) was responsible for the design, delivery and analysis of the qualitative research, oversaw the extensive PPI in the programme, enabled patients to participate within the research and contributed to the final report.
Publications
Abildtrup M, Kingsley GH, Scott DL. Calprotectin as a biomarker for rheumatoid arthritis: a systematic review. J Rheumatol 2015;42:760–70. https://doi.org/10.3899/jrheum.140628
Carpenter L, Nikiphorou E, Sharpe R, Norton S, Rennie K, Bunn F, et al. Have radiographic progression rates in early rheumatoid arthritis changed? A systematic review and meta-analysis of long-term cohorts. Rheumatology 2016;55:1053–65. https://doi.org/10.1093/rheumatology/kew004
Georgopoulou S, Prothero L, Lempp H, Galloway J, Sturt J. Motivational interviewing: relevance in the treatment of rheumatoid arthritis? Rheumatology 2016;55:1348–56. https://doi.org/10.1093/rheumatology/kev379
de Souza S, Williams R, Lempp H. Patient and clinician views on the quality of foot health care for rheumatoid arthritis outpatients: a mixed methods service evaluation. J Foot Ankle Res 2016;9:1. https://doi.org/10.1186/s13047-015-0133-2
Matcham F, Norton S, Scott DL, Steer S, Hotopf M. Symptoms of depression and anxiety predict treatment response and long-term physical health outcomes in rheumatoid arthritis: secondary analysis of a randomized controlled trial. Rheumatology 2016;55:268–78. https://doi.org/10.1093/rheumatology/kev306
Mian AN, Ibrahim F, Scott IC, Bahadur S, Filkova M, Pollard L, et al. Changing clinical patterns in rheumatoid arthritis management over two decades: sequential observational studies. BMC Musculoskelet Disord 2016;17:44. https://doi.org/10.1186/s12891-016-0897-y
Mian AN, Ibrahim F, Scott DL, Galloway J, TITRATE study group. Optimal responses in disease activity scores to treatment in rheumatoid arthritis: is a DAS28 reduction of > 1.2 sufficient? Arthritis Res Ther 2016;18:142. https://doi.org/10.1186/s13075-016-1028-8
Prothero L, Georgopoulou S, Galloway J, Williams R, Bosworth A, Lempp H. Patients’ and carers’ views and expectations about intensive management for moderate rheumatoid arthritis: a qualitative study. Psychol Health Med 2016;21:918–25. https://doi.org/10.1080/13548506.2015.1111394
Scott IC, Ibrahim F, Lewis CM, Scott DL, Strand V. Impact of intensive treatment and remission on health-related quality of life in early and established rheumatoid arthritis. RMD Open 2016;2:e000270. https://doi.org/10.1136/rmdopen-2016-000270
Gullick NJ, Mian AN, Ibrahim F, Walker D, Hassell A, Kiely PDW, et al. Predicting responses in patients with rheumatoid arthritis to disease-modifying agents using baseline clinical data. Clin Exp Rheumatol 2017;35:810–15.
Martin NH, Ibrahim F, Tom B, Galloway J, Wailoo A, Tosh J, et al. Does intensive management improve remission rates in patients with intermediate rheumatoid arthritis? (the TITRATE trial): study protocol for a randomised controlled trial. Trials 2017;18:591. https://doi.org/10.1186/s13063-017-2330-8
Prothero L, Georgopoulou S, de Souza S, Bosworth A, Bearne L, Lempp H. Patient involvement in the development of a handbook for moderate rheumatoid arthritis. Health Expect 2017;20:288–97. https://doi.org/10.1111/hex.12457
Bassett AM, de Souza S, Williams R, Lempp H. Rheumatoid arthritis portrayal by UK national newspapers 2011–2016: a service user-led thematic analysis of language used. BMC Rheumatol 2018;2:5. https://doi.org/10.1186/s41927-018-0013-z
Hughes CD, Scott DL, Ibrahim F, TITRATE Programme Investigators. Intensive therapy and remissions in rheumatoid arthritis: a systematic review. BMC Musculoskelet Disord 2018;19:389. https://doi.org/10.1186/s12891-018-2302-5
Prothero L, Barley E, Galloway J, Georgopoulou S, Sturt J. The evidence base for psychological interventions for rheumatoid arthritis: a systematic review of reviews. Int J Nurs Stud 2018;82:20–9. https://doi.org/10.1016/j.ijnurstu.2018.03.008
Gullick NJ, Ibrahim F, Scott IC, Vincent A, Cope AP, Garrood T, et al. Real world long-term impact of intensive treatment on disease activity, disability and health-related quality of life in rheumatoid arthritis. BMC Rheumatol 2019;3:6. https://doi.org/10.1186/s41927-019-0054-y
Mian A, Ibrahim F, Scott DL. A systematic review of guidelines for managing rheumatoid arthritis. BMC Rheumatol 2019;3:42. https://doi.org/10.1186/s41927-019-0090-7
Prothero L, Sturt J, de Souza S, Lempp H, TITRATE Programme Investigators. Intensive management for moderate rheumatoid arthritis: a qualitative study of patients’ and practitioners’ views. BMC Rheumatol 2019;3:12. https://doi.org/10.1186/s41927-019-0057-8
Scott IC, Ibrahim F, Panayi G, Cope AP, Garrood T, Vincent A, et al. The frequency of remission and low disease activity in patients with rheumatoid arthritis, and their ability to identify people with low disability and normal quality of life. Semin Arthritis Rheum 2019;49:20–6. https://doi.org/10.1016/j.semarthrit.2018.12.006
Carpenter L, Barnett R, Mahendran P, Nikiphorou E, Gwinnutt J, Verstappen S, et al. Secular changes in functional disability, pain, fatigue and mental well-being in early rheumatoid arthritis. A longitudinal meta-analysis. Semin Arthritis Rheum 2020;50:209–19. https://doi.org/10.1016/j.semarthrit.2019.08.006
Lempp H, Baggott R, Scott DL, Parker L, Bosworth A, Georgopoulou S, Firth J. The value, impact and role of nurses in rheumatology outpatient care: critical review of the literature. Musculoskeletal Care 2020;18:245–55. https://doi.org/10.1002/msc.1467
Scott D, Ibrahim F, Hill H, Tom B, Prothero L, Baggott RR, et al. The clinical effectiveness of intensive management in moderate established rheumatoid arthritis: the TITRATE trial. Semin Arthritis Rheum 2020;50:1182–90. https://doi.org/10.1016/j.semarthrit.2020.07.014
Presentations
Bahadur S, Mian AN, Scott I, Galloway J, Pollard L, Kingsley GH, Scott DL. Comorbidities and treatment intensity in rheumatoid arthritis. Rheumatology 2015;54:85. https://doi.org/10.1093/rheumatology/kev088.090
Georgopoulou S, Prothero L, Lempp H, Galloway J, Sturt J. Motivational interviewing: relevance in the treatment of rheumatoid arthritis? Rheumatology 2015;54:75. https://doi.org/10.1093/rheumatology/kev379
Matcham F, Mian A, Steer S, Gullick N, Scott DL, Hotopf M, et al. Psychological comorbidity and disease activity scores in rheumatoid arthritis. Rheumatology 2015;54:88. https://doi.org/10.1093/rheumatology/kev088.097
Mian A, Ibrahim F, Scott IC, Bahadur S, Filkova M, Pollard L, et al. Has the relationship between disease activity and disability in rheumatoid arthritis changed? Arthritis Rheumatol 2015;67:2765.
Mian AN, Scott DL, Kingsley GH, Ibrahim F. Impact of achieving low disease activity on general health status. Rheumatology 2015;54:24. https://doi.org/10.1093/rheumatology/kev079.006
Prothero L, Georgopoulou S, Bosworth A, Williams R, Galloway J, Lempp H. A qualitative study to explore patients' and carers' views and expectations about intensive treatment for intermediate rheumatoid arthritis. Rheumatology 2015;54:189. https://doi.org/10.1093/rheumatology/kev091.045
Gullick NJ, Ibrahim F, Mian A, Vincent A, Panayi G, Tom B, et al. Intensive treatment for rheumatoid arthritis reduces disease activity over time. Arthritis Rheumatol 2016;68:2518.
Gullick N, Ibrahim F, Mian A, Vincent A, Scott DL, Tom B, Kirkham B. Intensive treatment for rheumatoid arthritis reduces disease activity over time. Rheumatology 2016;55:i94–95. https://doi.org/10.1093/rheumatology/kew144.008
Gullick N, Ibrahim F, Scott DL, Vincent A, Tom BDM, Kirkham B. The impact of controlling disease activity in rheumatoid arthritis. Rheumatology 2016;55:i97. https://doi.org/10.1093/rheumatology/kew144.013
Hughes CD, Pollard LC, Scott DL. A systematic review of the impact of intensive therapy on remission in rheumatoid arthritis. Rheumatology 2016;55:i100. https://doi.org/10.1093/rheumatology/kew144.021
Matcham F, Scott IC, Ibrahim F, Steer S, Kingsley GH, Scott DL, Hotopf M. Disability and psychological distress in established rheumatoid arthritis: secondary analysis of a clinical trial. Rheumatology 2016;55:88. https://doi.org/10.1093/rheumatology/kew142.007
Prothero L, Barley E, Galloway J, Georgopoulou S, Sturt J. Psychological interventions for rheumatoid arthritis: a systematic review of systematic reviews. Rheumatology 2016;55:i121–2. https://doi.org/10.1093/rheumatology/kes133
Prothero L, Barley E, Galloway J, Georgopoulo S, Sturt J. Psychological interventions for rheumatoid arthritis: a systematic review of reviews. Int J Behav Med 2016;23:70. https://doi.org/10.1016/j.ijnurstu.2018.03.008
Rutherford AI, Scott DL, Subesinghe S, Ibrahim F, Galloway J. The incidence and predictors of flare in a cohort of rheumatoid arthritis patients. Rheumatology 2016;55:i73. https://doi.org/10.1093/rheumatology/kew132
Scott IC, Fowzia I, Lewis CM, Kingsley GH, Scott DL, Strand V. The impact of intensive treatment, inflammation and remission on health-related quality of life in early and established RA. Rheumatology 2016;55:i85. https://doi.org/10.1093/rheumatology/kew141.002
Scott IC, Ibrahim F, Gullick N, Kingsley G, Galloway J, Kirkham B, et al. Rationalising the treatment target in rheumatoid arthritis: defining the optimal 28-joint DAS cut-off to determine good function and normal health-related quality of life. Rheumatology 2016;55:99. https://doi.org/10.1093/rheumatology/kew144.018
Scott I, Ibrahim F, Kirkham B, Gullick N, Kingsley G, Galloway J, et al. Rationalising the treatment target in rheumatoid arthritis. Rheumatology 2016;55:i99. https://doi.org/10.1093/rheumatology/kew144.018
Scott DL. Triple therapy versus biologics: efficacy and cost-effectiveness. Rheumatology 2017;56:9. https://doi.org/10.1093/rheumatology/kex060.040
Scott IC, Ibrahim F, Safi MA, Houssien D, Scott DL. The erythrocyte sedimentation rate dominates 28-joint disease activity scores, but is not associated with post-treatment disability and health-related quality of life. Rheumatology 2017;56:14–12. https://doi.org/10.1093/rheumatology/kex062.232
Prothero L, Sturt J, de Souza S, Lempp H. Patients’ and Practitioners’ Views on Intensive Management for Moderate Rheumatoid Arthritis: A Qualitative Study. 32nd conference of the European Health Psychology Society: Health Psychology Across the Lifespan: Uniting Research, Practice and Policy, Galway, Ireland, 21–25 August 2018.
Baggott R, Scott D, Sturt J, Bosworth A, Parker L, Georgopoulou S, Lempp H. What is the value, impact and role of nurses in rheumatology outpatient care? Rheumatology 2019;58:150. https://doi.org/10.1093/rheumatology/kez108.058
Prothero L, Georgopoulou S, Lempp H, Sturt J. Fidelity Assessment of Motivational Interviewing-Based Treatment Support Delivered by Nurses. 33rd conference of the European Health Psychology Society: Individuals and Professionals: Cooperation to Health, Dubrovnik, Croatia, 3–7 September 2019.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health and Social Care.
References
- Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet 2010;376:1094-108. https://doi.org/10.1016/S0140-6736(10)60826-4.
- Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet 2016;388:2023-38. https://doi.org/10.1016/s0140-6736(16)30173-8.
- van der Woude D, van der Helm-van Mil AHM. Update on the epidemiology, risk factors, and disease outcomes of rheumatoid arthritis. Best Pract Res Clin Rheumatol 2018;32:174-87. https://doi.org/10.1016/j.berh.2018.10.005.
- Hunter TM, Boytsov NN, Zhang X, Schroeder K, Michaud K, Araujo AB. Prevalence of rheumatoid arthritis in the United States adult population in healthcare claims databases, 2004–2014. Rheumatol Int 2017;37:1551-7. https://doi.org/10.1007/s00296-017-3726-1.
- Symmons D, Turner G, Webb R, Asten P, Barrett E, Lunt M, et al. The prevalence of rheumatoid arthritis in the United Kingdom: new estimates for a new century. Rheumatology 2002;41:793-800. https://doi.org/10.1093/rheumatology/41.7.793.
- Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review. Semin Arthritis Rheum 2006;36:182-8. https://doi.org/10.1016/j.semarthrit.2006.08.006.
- Rodríguez LA, Tolosa LB, Ruigómez A, Johansson S, Wallander MA. Rheumatoid arthritis in UK primary care: incidence and prior morbidity. Scand J Rheumatol 2009;38:173-7. https://doi.org/10.1080/03009740802448825.
- Scublinsky D, Gonzalez CD. Quantifying disease in challenging conditions: incidence and prevalence of rheumatoid arthritis. J Rheumatol 2016;43:1263-4. https://doi.org/10.3899/jrheum.160522.
- Scott DL, Steer S. The course of established rheumatoid arthritis. Best Pract Res Clin Rheumatol 2007;21:943-67. https://doi.org/10.1016/j.berh.2007.05.006.
- Matcham F, Scott IC, Rayner L, Hotopf M, Kingsley GH, Norton S, et al. The impact of rheumatoid arthritis on quality-of-life assessed using the SF-36: a systematic review and meta-analysis. Semin Arthritis Rheum 2014;44:123-30. https://doi.org/10.1016/j.semarthrit.2014.05.001.
- Birnbaum H, Pike C, Kaufman R, Marynchenko M, Kidolezi Y, Cifaldi M. Societal cost of rheumatoid arthritis patients in the US. Curr Med Res Opin 2010;26:77-90. https://doi.org/10.1185/03007990903422307.
- Boonen A, Severens JL. The burden of illness of rheumatoid arthritis. Clin Rheumatol 2011;30:3-8. https://doi.org/10.1007/s10067-010-1634-9.
- Taylor PC, Moore A, Vasilescu R, Alvir J, Tarallo M. A structured literature review of the burden of illness and unmet needs in patients with rheumatoid arthritis: a current perspective. Rheumatol Int 2016;36:685-95. https://doi.org/10.1007/s00296-015-3415-x.
- Ndosi M, Ferguson R, Backhouse MR, Bearne L, Ainsworth P, Roach A, et al. National variation in the composition of rheumatology multidisciplinary teams: a cross-sectional study. Rheumatol Int 2017;37:1453-9. https://doi.org/10.1007/s00296-017-3751-0.
- Christie A, Jamtvedt G, Dahm KT, Moe RH, Haavardsholm EA, Hagen KB. Effectiveness of nonpharmacological and nonsurgical interventions for patients with rheumatoid arthritis: an overview of systematic reviews. Phys Ther 2007;87:1697-715. https://doi.org/10.2522/ptj.20070039.
- Bearne LM, Byrne AM, Segrave H, White CM. Multidisciplinary team care for people with rheumatoid arthritis: a systematic review and meta-analysis. Rheumatol Int 2016;36:311-24. https://doi.org/10.1007/s00296-015-3380-4.
- Nikiphorou E, Norton S, Young A, Carpenter L, Dixey J, Walsh DA, et al. ERAS and ERAN . Association between rheumatoid arthritis disease activity, progression of functional limitation and long-term risk of orthopaedic surgery: combined analysis of two prospective cohorts supports EULAR treat to target DAS thresholds. Ann Rheum Dis 2016;75:2080-6. https://doi.org/10.1136/annrheumdis-2015-208669.
- Burmester GR, Pope JE. Novel treatment strategies in rheumatoid arthritis. Lancet 2017;389:2338-48. https://doi.org/10.1016/S0140-6736(17)31491-5.
- Brown PM, Pratt AG, Isaacs JD. Mechanism of action of methotrexate in rheumatoid arthritis, and the search for biomarkers. Nat Rev Rheumatol 2016;12:731-42. https://doi.org/10.1038/nrrheum.2016.175.
- Cronstein BN. Low-dose methotrexate: a mainstay in the treatment of rheumatoid arthritis. Pharmacol Rev 2005;57:163-72. https://doi.org/10.1124/pr.57.2.3.
- Visser K, van der Heijde D. Optimal dosage and route of administration of methotrexate in rheumatoid arthritis: a systematic review of the literature. Ann Rheum Dis 2009;68:1094-9. https://doi.org/10.1136/ard.2008.092668.
- Donahue KE, Gartlehner G, Jonas DE, Lux LJ, Thieda P, Jonas BL, et al. Systematic review: comparative effectiveness and harms of disease-modifying medications for rheumatoid arthritis. Ann Intern Med 2008;148:124-34. https://doi.org/10.7326/0003-4819-148-2-200801150-00192.
- Hazlewood GS, Barnabe C, Tomlinson G, Marshall D, Devoe D, Bombardier C. Methotrexate monotherapy and methotrexate combination therapy with traditional and biologic disease modifying antirheumatic drugs for rheumatoid arthritis: abridged Cochrane systematic review and network meta-analysis. BMJ 2016;353. https://doi.org/10.1136/bmj.i1777.
- Alfonso-Cristancho R, Armstrong N, Arjunji R, Riemsma R, Worthy G, Ganguly R, et al. Comparative effectiveness of biologics for the management of rheumatoid arthritis: systematic review and network meta-analysis. Clin Rheumatol 2017;36:25-34. https://doi.org/10.1007/s10067-016-3435-2.
- Nam JL, Takase-Minegishi K, Ramiro S, Chatzidionysiou K, Smolen JS, van der Heijde D, et al. Efficacy of biological disease-modifying antirheumatic drugs: a systematic literature review informing the 2016 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis 2017;76:1113-36. https://doi.org/10.1136/annrheumdis-2016-210713.
- Singh JA, Christensen R, Wells GA, Suarez-Almazor ME, Buchbinder R, Lopez-Olivo MA, et al. A network meta-analysis of randomized controlled trials of biologics for rheumatoid arthritis: a Cochrane overview. CMAJ 2009;181:787-96. https://doi.org/10.1503/cmaj.091391.
- Singh JA, Hossain A, Mudano AS, Tanjong Ghogomu E, Suarez-Almazor ME, Buchbinder R, et al. Biologics or tofacitinib for people with rheumatoid arthritis naive to methotrexate: a systematic review and network meta-analysis. Cochrane Database Syst Rev 2017;5. https://doi.org/10.1002/14651858.CD012657.
- Strangfeld A, Hierse F, Kekow J, von Hinueber U, Tony HP, Dockhorn R, et al. Comparative effectiveness of tumour necrosis factor alpha inhibitors in combination with either methotrexate or leflunomide. Ann Rheum Dis 2009;68:1856-62. https://doi.org/10.1136/ard.2008.098467.
- Stevenson MD, Wailoo AJ, Tosh JC, Hernandez-Alava M, Gibson LA, Stevens JW, et al. The cost-effectiveness of sequences of biological disease-modifying antirheumatic drug treatment in England for patients with rheumatoid arthritis who can tolerate methotrexate. J Rheumatol 2017;44:973-80. https://doi.org/10.3899/jrheum.160941.
- Virtanen AT, Haikarainen T, Raivola J, Silvennoinen O. Selective JAKinibs: prospects in inflammatory and autoimmune diseases. BioDrugs 2019;33:15-32. https://doi.org/10.1007/s40259-019-00333-w.
- Palmowski Y, Buttgereit T, Dejaco C, Bijlsma JW, Matteson EL, Voshaar M, et al. ‘Official view’ on glucocorticoids in rheumatoid arthritis: a systematic review of international guidelines and consensus statements. Arthritis Care Res 2017;69:1134-41. https://doi.org/10.1002/acr.23185.
- Crofford LJ. Use of NSAIDs in treating patients with arthritis. Arthritis Res Ther 2013;15. https://doi.org/10.1186/ar4174.
- Hazlewood G, van der Heijde DM, Bombardier C. Paracetamol for the management of pain in inflammatory arthritis: a systematic literature review. J Rheumatol Suppl 2012;90:11-6. https://doi.org/10.3899/jrheum.120336.
- National Institute for Health and Care Excellence (NICE) . Rheumatoid Arthritis: The Management of Rheumatoid Arthritis in Adults 2009.
- National Institute for Health and Care Excellence (NICE) . Adalimumab, Etanercept, Infliximab, Certolizumab Pegol, Golimumab, Tocilizumab and Abatacept for Rheumatoid Arthritis Not Previously Treated With DMARDs or After Conventional DMARDs Only Have Failed 2016.
- Smolen JS, Breedveld FC, Burmester GR, Bykerk V, Dougados M, Emery P, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis 2016;75:3-15. https://doi.org/10.1136/annrheumdis-2015-207524.
- Stoffer MA, Schoels MM, Smolen JS, Aletaha D, Breedveld FC, Burmester G, et al. Evidence for treating rheumatoid arthritis to target: results of a systematic literature search update. Ann Rheum Dis 2016;75:16-22. https://doi.org/10.1136/annrheumdis-2015-207526.
- NHS Digital . Hospital Outpatient Activity, 2017–18 n.d. https://digital.nhs.uk/data-and-information/publications/statistical/hospital-outpatient-activity/2017-18 (accessed 31 March 2021).
- National Audit Office . Services for People With Rheumatoid Arthritis n.d. www.nao.org.uk/report/services-for-people-with-rheumatoid-arthritis/ (accessed 31 March 2021).
- NHS Digital . Prescribing Costs in Hospitals and the Community, England 2017 18 n.d. https://digital.nhs.uk/data-and-information/publications/statistical/prescribing-costs-in-hospitals-and-the-community/2017-18 (accessed 31 March 2021).
- National Institute for Health and Care Excellence (NICE) . Rheumatoid Arthritis in Adults: Management. n.d. www.nice.org.uk/guidance/ng100 (accessed 31 March 2021).
- Derksen VF, Ajeganova S, Trouw LA, van der Helm-van Mil AH, Hafström I, Huizinga TW, et al. Rheumatoid arthritis phenotype at presentation differs depending on the number of autoantibodies present. Ann Rheum Dis 2017;76:716-20. https://doi.org/10.1136/annrheumdis-2016-209794.
- Mankia K, D’Agostino MA, Wakefield RJ, Nam JL, Mahmood W, Grainger AJ, et al. Identification of a distinct imaging phenotype may improve the management of palindromic rheumatism. Ann Rheum Dis 2019;78:43-50. https://doi.org/10.1136/annrheumdis-2018-214175.
- Pollard LC, Kingsley GH, Choy EH, Scott DL. Fibromyalgic rheumatoid arthritis and disease assessment. Rheumatology 2010;49:924-8. https://doi.org/10.1093/rheumatology/kep458.
- van der Helm-van Mil AH, Zink A. What is rheumatoid arthritis? Considering consequences of changed classification criteria. Ann Rheum Dis 2017;76:315-17. https://doi.org/10.1136/annrheumdis-2016-209629.
- Erhardt DP, Cannon GW, Teng CC, Mikuls TR, Curtis JR, Sauer BC. Low persistence rates in rheumatoid arthritis patients treated with triple therapy are attributed to adverse drug events associated with sulfasalazine. Arthritis Care Res 2019;71:1326-35.
- Sauer BC, Teng CC, Tang D, Leng J, Curtis JR, Mikuls TR, et al. Persistence with conventional triple therapy versus a tumor necrosis factor inhibitor and methotrexate in US veterans with rheumatoid arthritis. Arthritis Care Res 2017;69:313-22. https://doi.org/10.1002/acr.22944.
- Sokka T, Envalds M, Pincus T. Treatment of rheumatoid arthritis: a global perspective on the use of antirheumatic drugs. Mod Rheumatol 2008;18:228-39. https://doi.org/10.1007/s10165-008-0056-x.
- Verschueren P, De Cock D, Corluy L, Joos R, Langenaken C, Taelman V, et al. Methotrexate in combination with other DMARDs is not superior to methotrexate alone for remission induction with moderate-to-high-dose glucocorticoid bridging in early rheumatoid arthritis after 16 weeks of treatment: the CareRA trial. Annals Rheum Dis 2015;74:27-34. https://doi.org/10.1136/annrheumdis-2014-205489.
- Emamikia S, Arkema EV, Györi N, Detert J, Chatzidionysiou K, Dougados M, et al. Induction maintenance with tumour necrosis factor-inhibitor combination therapy with discontinuation versus methotrexate monotherapy in early rheumatoid arthritis: a systematic review and meta-analysis of efficacy in randomised controlled trials. RMD Open 2016;2. https://doi.org/10.1136/rmdopen-2016-000323.
- Wailoo A, Hock ES, Stevenson M, Martyn-St James M, Rawdin A, Simpson E, et al. The clinical effectiveness and cost-effectiveness of treat-to-target strategies in rheumatoid arthritis: a systematic review and cost-effectiveness analysis. Health Technol Assess 2017;21. https://doi.org/10.3310/hta21710.
- Carpenter L, Nikiphorou E, Sharpe R, Norton S, Rennie K, Bunn F, et al. Have radiographic progression rates in early rheumatoid arthritis changed? A systematic review and meta-analysis of long-term cohorts. Rheumatology 2016;55:1053-65. https://doi.org/10.1093/rheumatology/kew004.
- Gullick NJ, Ibrahim F, Scott IC, Vincent A, Cope AP, Garrood T, et al. Real world long-term impact of intensive treatment on disease activity, disability and health-related quality of life in rheumatoid arthritis. BMC Rheumatol 2019;3. https://doi.org/10.1186/s41927-019-0054-y.
- Hughes CD, Scott DL, Ibrahim F. TITRATE Programme Investigators . Intensive therapy and remissions in rheumatoid arthritis: a systematic review. BMC Musculoskelet Disord 2018;19. https://doi.org/10.1186/s12891-018-2302-5.
- Mian AN, Ibrahim F, Scott IC, Bahadur S, Filkova M, Pollard L, et al. Changing clinical patterns in rheumatoid arthritis management over two decades: sequential observational studies. BMC Musculoskelet Disord 2016;17. https://doi.org/10.1186/s12891-016-0897-y.
- Abelson B, Sokka T, Pincus T. Declines in erythrocyte sedimentation rates in patients with rheumatoid arthritis over the second half of the 20th century. J Rheumatol 2009;36:1596-9. https://doi.org/10.3899/jrheum.081255.
- Aga AB, Lie E, Uhlig T, Olsen IC, Wierød A, Kalstad S, et al. Time trends in disease activity, response and remission rates in rheumatoid arthritis during the past decade: results from the NOR-DMARD study 2000–2010. Ann Rheum Dis 2015;74:381-8. https://doi.org/10.1136/annrheumdis-2013-204020.
- Diffin JG, Lunt M, Marshall T, Chipping JR, Symmons DP, Verstappen SM. Has the severity of rheumatoid arthritis at presentation diminished over time?. J Rheumatol 2014;41:1590-9. https://doi.org/10.3899/jrheum.131136.
- Finckh A, Choi HK, Wolfe F. Progression of radiographic joint damage in different eras: trends towards milder disease in rheumatoid arthritis are attributable to improved treatment. Ann Rheum Dis 2006;65:1192-7. https://doi.org/10.1136/ard.2005.049338.
- Kievit W, Fransen J, de Waal Malefijt MC, den Broeder AA, van Riel PL. Treatment changes and improved outcomes in RA: an overview of a large inception cohort from 1989 to 2009. Rheumatology 2013;52:1500-8. https://doi.org/10.1093/rheumatology/ket166.
- Pincus T, Sokka T, Kautiainen H. Patients seen for standard rheumatoid arthritis care have significantly better articular, radiographic, laboratory, and functional status in 2000 than in 1985. Arthritis Rheum 2005;52:1009-19. https://doi.org/10.1002/art.20941.
- Silman A, Davies P, Currey HL, Evans SJ. Is rheumatoid arthritis becoming less severe?. J Chronic Dis 1983;36:891-7. https://doi.org/10.1016/0021-9681(83)90011-5.
- Sokka T, Kautiainen H, Häkkinen A, Hannonen P. Radiographic progression is getting milder in patients with early rheumatoid arthritis. Results of 3 cohorts over 5 years. J Rheumatol 2004;31:1073-82.
- Uhlig T, Heiberg T, Mowinckel P, Kvien TK. Rheumatoid arthritis is milder in the new millennium: health status in patients with rheumatoid arthritis 1994–2004. Ann Rheum Dis 2008;67:1710-15. https://doi.org/10.1136/ard.2007.084673.
- Welsing PM, Fransen J, van Riel PL. Is the disease course of rheumatoid arthritis becoming milder? Time trends since 1985 in an inception cohort of early rheumatoid arthritis. Arthritis Rheum 2005;52:2616-24. https://doi.org/10.1002/art.21259.
- Yamanaka H, Inoue E, Singh G, Tanaka E, Nakajima A, Taniguchi A, et al. Improvement of disease activity of rheumatoid arthritis patients from 2000 to 2006 in a large observational cohort study IORRA in Japan. Mod Rheumatol 2007;17:283-9. https://doi.org/10.1007/s10165-007-0587-6.
- Judge A, Wallace G, Prieto-Alhambra D, Arden NK, Edwards CJ. Can the publication of guidelines change the management of early rheumatoid arthritis? An interrupted time series analysis from the United Kingdom. Rheumatology 2015;54:2244-8. https://doi.org/10.1093/rheumatology/kev268.
- Scott D. Guidelines for arthritis: ten years on. Clin Med 2001;1:389-91. https://doi.org/10.7861/clinmedicine.1-5-389.
- Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: a review. JAMA 2018;320:1360-72. https://doi.org/10.1001/jama.2018.13103.
- Houssien DA, McKenna SP, Scott DL. The Nottingham Health Profile as a measure of disease activity and outcome in rheumatoid arthritis. Br J Rheumatol 1997;36:69-73. https://doi.org/10.1093/rheumatology/36.1.69.
- Scott DL, Khoshaba B, Choy EH, Kingsley GH. Limited correlation between the Health Assessment Questionnaire (HAQ) and EuroQol in rheumatoid arthritis: questionable validity of deriving quality adjusted life years from HAQ. Ann Rheum Dis 2007;66:1534-7. https://doi.org/10.1136/ard.2007.073726.
- Scott IC, Ibrahim F, Johnson D, Scott DL, Kingsley GH. Current limitations in the management of cardiovascular risk in rheumatoid arthritis. Clin Exp Rheumatol 2012;30:228-32.
- Choy EH, Smith CM, Farewell V, Walker D, Hassell A, Chau L, et al. Factorial randomised controlled trial of glucocorticoids and combination disease modifying drugs in early rheumatoid arthritis. Ann Rheum Dis 2008;67:656-63. https://doi.org/10.1136/ard.2007.076299.
- Scott DL, Ibrahim F, Farewell V, O’Keeffe AG, Walker D, Kelly C, et al. Tumour necrosis factor inhibitors versus combination intensive therapy with conventional disease modifying anti-rheumatic drugs in established rheumatoid arthritis: TACIT non-inferiority randomised controlled trial. BMJ 2015;350. https://doi.org/10.1136/bmj.h1046.
- Fransen J, van Riel PL. The Disease Activity Score and the EULAR response criteria. Rheumatic Dis Clin North Am 2009;35:745-57. https://doi.org/10.1016/j.rdc.2009.10.001.
- Bruce B, Fries JF. The Stanford Health Assessment Questionnaire: dimensions and practical applications. Health Qual Life Outcomes 2003;1. https://doi.org/10.1186/1477-7525-1-20.
- Larsen A, Dale K, Eek M. Radiographic evaluation of rheumatoid arthritis and related conditions by standard reference films. Acta Radiol Diagn 1977;18:481-91. https://doi.org/10.1177/028418517701800415.
- van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol 2000;27:261-3.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. https://doi.org/10.1016/0197-2456(86)90046-2.
- Cochrane Training . Cochrane Handbook for Systematic Reviews of Interventions Version 510 [Updated March 2011] 2011. www.cochrane-handbook.org (accessed 31 March 2021).
- James D, Young A, Kulinskaya E, Knight E, Thompson W, Ollier W, et al. Early Rheumatoid Arthritis Study Group (ERAS), UK . Orthopaedic intervention in early rheumatoid arthritis. Occurrence and predictive factors in an inception cohort of 1064 patients followed for 5 years. Rheumatology 2004;43:369-76. https://doi.org/10.1093/rheumatology/keh059.
- Kuper HH, van Leeuwen MA, van Riel PL, Prevoo ML, Houtman PM, Lolkema WF, et al. Radiographic damage in large joints in early rheumatoid arthritis: relationship with radiographic damage in hands and feet, disease activity, and physical disability. Br J Rheumatol 1997;36:855-60. https://doi.org/10.1093/rheumatology/36.8.855.
- Kapetanovic MC, Lindqvist E, Algulin J, Jonsson K, Saxne T, Eberhardt K, et al. Early changes in bone mineral density measured by digital X-ray radiogrammetry predict up to 20 years radiological outcome in rheumatoid arthritis. Arthritis Res Ther 2011;13. https://doi.org/10.1186/ar3259.
- Kaarela K, Kautiainen H. Continuous progression of radiological destruction in seropositive rheumatoid arthritis. J Rheumatol 1997;24:1285-7.
- Knevel R, Kwok KY, de Rooy DP, Posthumus MD, Huizinga TW, Brouwer E, et al. Evaluating joint destruction in rheumatoid arthritis: is it necessary to radiograph both hands and feet?. Ann Rheum Dis 2013;72:345-9. https://doi.org/10.1136/annrheumdis-2012-201391.
- Bridges SL, Jr, Causey ZL, Burgos PI, Huynh BQ, Hughes LB, Danila MI, et al. Radiographic severity of rheumatoid arthritis in African Americans: results from a multicenter observational study. Arthritis Care Res 2010;62:624-31. https://doi.org/10.1002/acr.20040.
- Tanaka N, Sakahashi H, Ishii S, Sato E, Hirose K, Ishima T. Synovial membrane enhancement and bone erosion by magnetic resonance imaging for prediction of radiologic progression in patients with early rheumatoid arthritis. Rheumatol Int 2005;25:103-7. https://doi.org/10.1007/s00296-003-0404-2.
- Courvoisier N, Dougados M, Cantagrel A, Goupille P, Meyer O, Sibilia J, et al. Prognostic factors of 10-year radiographic outcome in early rheumatoid arthritis: a prospective study. Arthritis Res Ther 2008;10. https://doi.org/10.1186/ar2498.
- Viatte S, Plant D, Lunt M, Fu B, Flynn E, Parker BJ, et al. Investigation of rheumatoid arthritis genetic susceptibility markers in the early rheumatoid arthritis study further replicates the TRAF1 association with radiological damage. J Rheumatol 2013;40:144-56. https://doi.org/10.3899/jrheum.121034.
- Albrecht K, Krüger K, Wollenhaupt J, Alten R, Backhaus M, Baerwald C, et al. German guidelines for the sequential medical treatment of rheumatoid arthritis with traditional and biologic disease-modifying antirheumatic drugs. Rheumatol Int 2014;34:1-9. https://doi.org/10.1007/s00296-013-2848-3.
- Ataman S, Borman P, Evcik D, Aydog E, Ayhan F, Yildizlar D, et al. Management of rheumatoid arthritis: consensus recommendations from the Turkish League Against Rheumatism. Turk J Rheumatol 2011;26:273-94. https://doi.org/10.5606/tjr.2011.046.
- Bykerk VP, Akhavan P, Hazlewood GS, Schieir O, Dooley A, Haraoui B, et al. Canadian Rheumatology Association recommendations for pharmacological management of rheumatoid arthritis with traditional and biologic disease-modifying antirheumatic drugs. J Rheumatol 2012;39:1559-82. https://doi.org/10.3899/jrheum.110207.
- Cardiel MH. Latin American Rheumatology Associations of the Pan-American League of Associations for Rheumatology (PANLAR) . First Latin American position paper on the pharmacological treatment of rheumatoid arthritis. Rheumatology 2006;45:ii7-22. https://doi.org/10.1093/rheumatology/kei500.
- Cardiel MH, Díaz-Borjón A, Vázquez del Mercado Espinosa M, Gámez-Nava JI, Barile Fabris LA, Pacheco Tena C, et al. Update of the Mexican College of Rheumatology guidelines for the pharmacologic treatment of rheumatoid arthritis. Reumatol Clin 2014;10:227-40. https://doi.org/10.1016/j.reuma.2013.10.006.
- Gaujoux-Viala C, Gossec L, Cantagrel A, Dougados M, Fautrel B, Mariette X, et al. Recommendations of the French Society for Rheumatology for managing rheumatoid arthritis. Joint Bone Spine 2014;81:287-97. https://doi.org/10.1016/j.jbspin.2014.05.002.
- Hodkinson B, Van Duuren E, Pettipher C, Kalla A. South African recommendations for the management of rheumatoid arthritis: an algorithm for the standard of care in 2013. S Afr Med J 2013;103:576-85. https://doi.org/10.7196/samj.7047.
- Lau CS, Chia F, Harrison A, Hsieh TY, Jain R, Jung SM, et al. APLAR rheumatoid arthritis treatment recommendations. Int J Rheum Dis 2015;18:685-713. https://doi.org/10.1111/1756-185x.12754.
- Luqmani R, Hennell S, Estrach C, Basher D, Birrell F, Bosworth A, et al. British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of rheumatoid arthritis (after the first 2 years). Rheumatology 2009;48:436-9. https://doi.org/10.1093/rheumatology/ken450a.
- Luqmani R, Hennell S, Estrach C, Birrell F, Bosworth A, Davenport G, et al. British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of rheumatoid arthritis (the first two years). Rheumatology 2006;45:1167-9. https://doi.org/10.1093/rheumatology/kel215a.
- Misra R, Sharma BL, Gupta R, Pandya S, Agarwal S, Agarwal P, et al. Indian Rheumatology Association consensus statement on the management of adults with rheumatoid arthritis. Indian J Rheumatol 2008;3:S1-16. https://doi.org/10.1016/S0973-3698(10)60373-1.
- Mok CC, Tam LS, Chan TH, Lee GK, Li EK. Hong Kong Society of Rheumatology . Management of rheumatoid arthritis: consensus recommendations from the Hong Kong Society of Rheumatology. Clin Rheumatol 2011;30:303-12. https://doi.org/10.1007/s10067-010-1596-y.
- Mota LM, Cruz BA, Brenol CV, Pereira IA, Rezende-Fronza LS, Bertolo MB, et al. Guidelines for the drug treatment of rheumatoid arthritis. Rev Bras Reumatol 2013;53:158-83. https://doi.org/10.1016/S2255-5021(13)70020-8.
- Singh JA, Saag KG, Bridges SL, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2016;68:1-26. https://doi.org/10.1002/art.39480.
- Smolen JS, Landewé R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960-77. https://doi.org/10.1136/annrheumdis-2016-210715.
- Royal Australian College of General Practitioners . Clinical Guideline for the Diagnosis and Management of Early Rheumatoid Arthritis n.d. www.racgp.org.au/your-practice/guidelines/musculoskeletal/rheumatoidarthritis/ (accessed 9 August 2019).
- British Columbia . Rheumatoid Arthritis: Diagnosis, Management and Monitoring n.d. www2.gov.bc.ca/gov/content/health/practitioner-professional-resources/bc-guidelines/rheumatoid-arthritis (accessed 9 August 2019).
- Scottish Intercollegiate Guidelines Network . Management of Early Rheumatoid Arthritis n.d. www.sign.ac.uk/our-guidelines/management-of-early-rheumatoid-arthritis/ (accessed 11 April 2021).
- Baecklund E, Forsblad d’Elia H, Turesson C. Guidelines for the Pharmaceutical Management of Rheumatoid Arthritis. Swedish Society of Rheumatology n.d. http://svenskreumatologi.se/wp-content/uploads/2016/08/guidelines_for_the_pharmaceutical_management_of_rheumatoid_arthritis.pdf (accessed 31 March 2021).
- Spanish Society of Rheumatology . Update Of The Clinical Practice Guideline for the Management Of Rheumatoid Arthritis in Spain n.d. www.ser.es/wp-content/uploads/2016/01/GUIPCAR_31Marzo2012_ENG.pdf (accessed 31 March 2021).
- Royal College of Physicians . Rheumatoid Arthritis: National Clinical Guideline for Management and Treatment in Adults 2009.
- Atsumi T, Yamamoto K, Takeuchi T, Yamanaka H, Ishiguro N, Tanaka Y, et al. The first double-blind, randomised, parallel-group certolizumab pegol study in methotrexate-naive early rheumatoid arthritis patients with poor prognostic factors, C-OPERA, shows inhibition of radiographic progression. Ann Rheum Dis 2016;75:75-83. https://doi.org/10.1136/annrheumdis-2015-207511.
- Bakker MF, Jacobs JW, Welsing PM, Verstappen SM, Tekstra J, Ton E, et al. Low-dose prednisone inclusion in a methotrexate-based, tight control strategy for early rheumatoid arthritis: a randomized trial. Ann Intern Med 2012;156:329-39. https://doi.org/10.7326/0003-4819-156-5-201203060-00004.
- Bijlsma JWJ, Welsing PMJ, Woodworth TG, Middelink LM, Pethö-Schramm A, Bernasconi C, et al. Early rheumatoid arthritis treated with tocilizumab, methotrexate, or their combination (U-Act-Early): a multicentre, randomised, double-blind, double-dummy, strategy trial. Lancet 2016;388:343-55. https://doi.org/10.1016/S0140-6736(16)30363-4.
- Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, van Vollenhoven R, et al. The PREMIER study – a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum 2006;54:26-37. https://doi.org/10.1002/art.21519.
- Burmester GR, Rigby WF, van Vollenhoven RF, Kay J, Rubbert-Roth A, Kelman A, et al. Tocilizumab in early progressive rheumatoid arthritis: FUNCTION, a randomised controlled trial. Ann Rheum Dis 2016;75:1081-91. https://doi.org/10.1136/annrheumdis-2015-207628.
- Capell HA, Madhok R, Porter DR, Munro RA, McInnes IB, Hunter JA, et al. Combination therapy with sulfasalazine and methotrexate is more effective than either drug alone in patients with rheumatoid arthritis with a suboptimal response to sulfasalazine: results from the double-blind placebo-controlled MASCOT study. Ann Rheum Dis 2007;66:235-41. https://doi.org/10.1136/ard.2006.057133.
- Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum 2006;54:2793-806. https://doi.org/10.1002/art.22025.
- Detert J, Bastian H, Listing J, Weiß A, Wassenberg S, Liebhaber A, et al. Induction therapy with adalimumab plus methotrexate for 24 weeks followed by methotrexate monotherapy up to week 48 versus methotrexate therapy alone for DMARD-naive patients with early rheumatoid arthritis: HIT HARD, an investigator-initiated study. Ann Rheum Dis 2013;72:844-50. https://doi.org/10.1136/annrheumdis-2012-201612.
- Dougados M, Kissel K, Sheeran T, Tak PP, Conaghan PG, Mola EM, et al. Adding tocilizumab or switching to tocilizumab monotherapy in methotrexate inadequate responders: 24-week symptomatic and structural results of a 2-year randomised controlled strategy trial in rheumatoid arthritis (ACT-RAY). Ann Rheum Dis 2013;72:43-50. https://doi.org/10.1136/annrheumdis-2011-201282.
- Emery P, Bingham CO, Burmester GR, Bykerk VP, Furst DE, Mariette X, et al. Certolizumab pegol in combination with dose-optimised methotrexate in DMARD-naïve patients with early, active rheumatoid arthritis with poor prognostic factors: 1-year results from C-EARLY, a randomised, double-blind, placebo-controlled phase III study. Ann Rheum Dis 2017;76:96-104. https://doi.org/10.1136/annrheumdis-2015-209057.
- Emery P, Breedveld FC, Hall S, Durez P, Chang DJ, Robertson D, et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet 2008;372:375-82. https://doi.org/10.1016/S0140-6736(08)61000-4.
- Emery P, Burmester GR, Bykerk VP, Combe BG, Furst DE, Barré E, et al. Evaluating drug-free remission with abatacept in early rheumatoid arthritis: results from the phase 3b, multicentre, randomised, active-controlled AVERT study of 24 months, with a 12-month, double-blind treatment period. Ann Rheum Dis 2015;74:19-26. https://doi.org/10.1136/annrheumdis-2014-206106.
- Emery P, Deodhar A, Rigby WF, Isaacs JD, Combe B, Racewicz AJ, et al. Efficacy and safety of different doses and retreatment of rituximab: a randomised, placebo-controlled trial in patients who are biological naive with active rheumatoid arthritis and an inadequate response to methotrexate (Study Evaluating Rituximab’s Efficacy in MTX iNadequate rEsponders (SERENE)). Ann Rheum Dis 2010;69:1629-35. https://doi.org/10.1136/ard.2009.119933.
- Emery P, Fleischmann RM, Moreland LW, Hsia EC, Strusberg I, Durez P, et al. Golimumab, a human anti-tumor necrosis factor alpha monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naive patients with active rheumatoid arthritis: twenty-four-week results of a phase III, multicenter, randomized, double-blind, placebo-controlled study of golimumab before methotrexate as first-line therapy for early-onset rheumatoid arthritis. Arthritis Rheum 2009;60:2272-83. https://doi.org/10.1002/art.24638.
- Emery P, Keystone E, Tony HP, Cantagrel A, van Vollenhoven R, Sanchez A, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis 2008;67:1516-23. https://doi.org/10.1136/ard.2008.092932.
- Genovese MC, Kremer J, Zamani O, Ludivico C, Krogulec M, Xie L, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med 2016;374:1243-52. https://doi.org/10.1056/NEJMoa1507247.
- Genovese MC, McKay JD, Nasonov EL, Mysler EF, da Silva NA, Alecock E, et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum 2008;58:2968-80. https://doi.org/10.1002/art.23940.
- Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, van Zeben D, Kerstens PJ, Hazes JM, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum 2005;52:3381-90. https://doi.org/10.1002/art.21405.
- Grigor C, Capell H, Stirling A, McMahon AD, Lock P, Vallance R, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet 2004;364:263-9. https://doi.org/10.1016/S0140-6736(04)16676-2.
- Hetland ML, Stengaard-Pedersen K, Junker P, Lottenburger T, Ellingsen T, Andersen LS, et al. Combination treatment with methotrexate, cyclosporine, and intraarticular betamethasone compared with methotrexate and intraarticular betamethasone in early active rheumatoid arthritis: an investigator-initiated, multicenter, randomized, double-blind, parallel-group, placebo-controlled study. Arthritis Rheum 2006;54:1401-9. https://doi.org/10.1002/art.21796.
- Hørslev-Petersen K, Hetland ML, Junker P, Pødenphant J, Ellingsen T, Ahlquist P, et al. Adalimumab added to a treat-to-target strategy with methotrexate and intra-articular triamcinolone in early rheumatoid arthritis increased remission rates, function and quality of life. The OPERA Study: an investigator-initiated, randomised, double-blind, parallel-group, placebo-controlled trial. Ann Rheum Dis 2014;73:654-61. https://doi.org/10.1136/annrheumdis-2012-202735.
- Kavanaugh A, Fleischmann RM, Emery P, Kupper H, Redden L, Guerette B, et al. Clinical, functional and radiographic consequences of achieving stable low disease activity and remission with adalimumab plus methotrexate or methotrexate alone in early rheumatoid arthritis: 26-week results from the randomised, controlled OPTIMA study. Ann Rheum Dis 2013;72:64-71. https://doi.org/10.1136/annrheumdis-2011-201247.
- Heimans L, Wevers-de Boer KV, Visser K, Goekoop RJ, van Oosterhout M, Harbers JB, et al. A two-step treatment strategy trial in patients with early arthritis aimed at achieving remission: the IMPROVED study. Ann Rheum Dis 2014;73:1356-61. https://doi.org/10.1136/annrheumdis-2013-203243.
- Kivitz A, Olech E, Borofsky M, Zazueta BM, Navarro-Sarabia F, Radominski SC, et al. Subcutaneous tocilizumab versus placebo in combination with disease-modifying antirheumatic drugs in patients with rheumatoid arthritis. Arthritis Care Res 2014;66:1653-61. https://doi.org/10.1002/acr.22384.
- Klareskog L, van der Heijde D, de Jager JP, Gough A, Kalden J, Malaise M, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet 2004;363:675-81. https://doi.org/10.1016/S0140-6736(04)15640-7.
- Kremer J, Li ZG, Hall S, Fleischmann R, Genovese M, Martin-Mola E, et al. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med 2013;159:253-61. https://doi.org/10.7326/0003-4819-159-4-201308200-00006.
- Kremer JM, Blanco R, Brzosko M, Burgos-Vargas R, Halland AM, Vernon E, et al. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: results from the double-blind treatment phase of a randomized placebo-controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis Rheum 2011;63:609-21. https://doi.org/10.1002/art.30158.
- Kremer JM, Cohen S, Wilkinson BE, Connell CA, French JL, Gomez-Reino J, et al. A phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. Arthritis Rheum 2012;64:970-81. https://doi.org/10.1002/art.33419.
- Kremer JM, Dougados M, Emery P, Durez P, Sibilia J, Shergy W, et al. Treatment of rheumatoid arthritis with the selective costimulation modulator abatacept: twelve-month results of a phase IIb, double-blind, randomized, placebo-controlled trial. Arthritis Rheum 2005;52:2263-71. https://doi.org/10.1002/art.21201.
- Leirisalo-Repo M, Kautiainen H, Laasonen L, Korpela M, Kauppi MJ, Kaipiainen-Seppanen O, et al. Infliximab for 6 months added on combination therapy in early rheumatoid arthritis: 2-year results from an investigator-initiated, randomised, double-blind, placebo-controlled study (the NEO-RACo Study). Annals of the Rheumatic Diseases 2013;72:851-7. https://doi.org/10.1136/annrheumdis-2012-201365.
- Moreland LW, O’Dell JR, Paulus HE, Curtis JR, Bathon JM, St Clair EW, et al. A randomized comparative effectiveness study of oral triple therapy versus etanercept plus methotrexate in early aggressive rheumatoid arthritis: the treatment of Early Aggressive Rheumatoid Arthritis Trial. Arthritis Rheum 2012;64:2824-35. https://doi.org/10.1002/art.34498.
- Nam JL, Villeneuve E, Hensor EM, Wakefield RJ, Conaghan PG, Green MJ, et al. A randomised controlled trial of etanercept and methotrexate to induce remission in early inflammatory arthritis: the EMPIRE trial. Ann Rheum Dis 2014;73:1027-36. https://doi.org/10.1136/annrheumdis-2013-204882.
- Nam JL, Villeneuve EHE, Hensor EM, Conaghan PG, Keen HI, Buch MH, et al. Remission induction comparing infliximab and high-dose intravenous steroid, followed by treat-to-target: a double-blind, randomised, controlled trial in new-onset, treatment-naive, rheumatoid arthritis (the IDEA study). Ann Rheum Dis 2014;73:75-8. https://doi.org/10.1136/annrheumdis-2013-203440.
- O’Dell JR, Mikuls TR, Taylor TH, Ahluwalia V, Brophy M, Warren SR, et al. Therapies for active rheumatoid arthritis after methotrexate failure. The New Engl J Med 2013;369:307-18. https://doi.org/10.1056/NEJMoa1303006.
- Schiff M, Keiserman M, Codding C, Songcharoen S, Berman A, Nayiager S, et al. Efficacy and safety of abatacept or infliximab vs. placebo in ATTEST: a phase III, multi-centre, randomised, double-blind, placebo-controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Ann Rheum Dis 2008;67:1096-103. https://doi.org/10.1136/ard.2007.080002.
- Schipper LG, Vermeer M, Kuper HH, Hoekstra MO, Haagsma CJ, Den Broeder AA, et al. A tight control treatment strategy aiming for remission in early rheumatoid arthritis is more effective than usual care treatment in daily clinical practice: a study of two cohorts in the Dutch Rheumatoid Arthritis Monitoring registry. Ann Rheum Dis 2012;71:845-50. https://doi.org/10.1136/annrheumdis-2011-200274.
- Smolen JS, Emery P, Ferraccioli GF, Samborski W, Berenbaum F, Davies OR, et al. Certolizumab pegol in rheumatoid arthritis patients with low to moderate activity: the CERTAIN double-blind, randomised, placebo-controlled trial. Ann Rheum Dis 2015;74:843-50. https://doi.org/10.1136/annrheumdis-2013-204632.
- Smolen JS, Kay J, Doyle MK, Landewé R, Matteson EL, Wollenhaupt J, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet 2009;374:210-21. https://doi.org/10.1016/S0140-6736(09)60506-7.
- Smolen J, Landewe RB, Mease P, Brzezicki J, Mason D, Luijtens K, et al. Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A randomised controlled trial. Ann Rheum Dis 2009;68:797-804. https://doi.org/10.1136/ard.2008.101659.
- Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 2008;371:987-97. https://doi.org/10.1016/s0140-6736(08)60453-5.
- Soubrier M, Puéchal X, Sibilia J, Mariette X, Meyer O, Combe B, et al. Evaluation of two strategies (initial methotrexate monotherapy vs its combination with adalimumab) in management of early active rheumatoid arthritis: data from the GUEPARD trial. Rheumatology 2009;48:1429-34. https://doi.org/10.1093/rheumatology/kep261.
- St Clair EW, van der Heijde DM, Smolen JS, Maini RN, Bathon JM, Emery P, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum 2004;50:3432-43. https://doi.org/10.1002/art.20568.
- Symmons D, Tricker K, Harrison M, Roberts C, Davis M, Dawes P, et al. Patients with stable long-standing rheumatoid arthritis continue to deteriorate despite intensified treatment with traditional disease modifying anti-rheumatic drugs – results of the British Rheumatoid Outcome Study Group randomized controlled clinical trial. Rheumatology 2006;45:558-65. https://doi.org/10.1093/rheumatology/kei169.
- Tak PP, Rigby WF, Rubbert-Roth A, Peterfy CG, van Vollenhoven RF, Stohl W, et al. Inhibition of joint damage and improved clinical outcomes with rituximab plus methotrexate in early active rheumatoid arthritis: the IMAGE trial. Ann Rheum Dis 2011;70:39-46. https://doi.org/10.1136/ard.2010.137703.
- Takeuchi T, Yamanaka H, Ishiguro N, Miyasaka N, Mukai M, Matsubara T, et al. Adalimumab, a human anti-TNF monoclonal antibody, outcome study for the prevention of joint damage in Japanese patients with early rheumatoid arthritis: the HOPEFUL 1 study. Ann Rheum Dis 2014;73:536-43. https://doi.org/10.1136/annrheumdis-2012-202433.
- Taylor PC, Keystone EC, van der Heijde D, Weinblatt ME, Del Carmen Morales L, Reyes Gonzaga J, et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med 2017;376:652-62. https://doi.org/10.1056/NEJMoa1608345.
- van der Heijde D, Tanaka Y, Fleischmann R, Keystone E, Kremer J, Zerbini C, et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum 2013;65:559-70. https://doi.org/10.1002/art.37816.
- van Eijk IC, Nielen MM, van der Horst-Bruinsma I, Tijhuis GJ, Boers M, Dijkmans BA, et al. Aggressive therapy in patients with early arthritis results in similar outcome compared with conventional care: the STREAM randomized trial. Rheumatology 2012;51:686-94. https://doi.org/10.1093/rheumatology/ker355.
- van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, Garcia Meijide JA, Wagner S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. New Engl J Med 2012;367:508-19. https://doi.org/10.1056/NEJMoa1112072.
- Verstappen SM, Jacobs JW, van der Veen MJ, Heurkens AH, Schenk Y, ter Borg EJ, et al. Intensive treatment with methotrexate in early rheumatoid arthritis: aiming for remission. Computer Assisted Management in Early Rheumatoid Arthritis (CAMERA, an open-label strategy trial). Ann Rheum Dis 2007;66:144-39. https://doi.org/10.1136/ard.2007.071092.
- Weinblatt ME, Bingham CO, Mendelsohn AM, Kim L, Mack M, Lu J, et al. Intravenous golimumab is effective in patients with active rheumatoid arthritis despite methotrexate therapy with responses as early as week 2: results of the phase 3, randomised, multicentre, double-blind, placebo-controlled GO-FURTHER trial. Ann Rheum Dis 2013;72:381-9. https://doi.org/10.1136/annrheumdis-2012-201411.
- Westhovens R, Robles M, Ximenes AC, Nayiager S, Wollenhaupt J, Durez P, et al. Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Ann Rheum Dis 2009;68:1870-7. https://doi.org/10.1136/ard.2008.101121.
- Allaart CF, Breedveld FC, Dijkmans BA. Treatment of recent-onset rheumatoid arthritis: lessons from the BeSt study. J Rheumatol Suppl 2007;80:25-33.
- Allaart CF, Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Breedveld FC, Dijkmans BA. FARR study group . Aiming at low disease activity in rheumatoid arthritis with initial combination therapy or initial monotherapy strategies: the BeSt study. Clin Exp Rheumatol 2006;24:77-82.
- Bakker MF, Jacobs JW, Welsing PM, Vreugdenhil SA, van Booma-Frankfort C, Linn-Rasker SP, et al. Early clinical response to treatment predicts 5-year outcome in RA patients: follow-up results from the CAMERA study. Ann Rheum Dis 2011;70:1099-103. https://doi.org/10.1136/ard.2010.137943.
- De Cock D, Meyfroidt S, Joly J, Van der Elst K, Westhovens R, Verschueren P. For remission induction with glucocorticoid bridging, methotrexate monotherapy is as effective as a combination with other DMARDs, with fewer reported side effects: 4 month primary outcome of CareRA, a randomized induction strategy and treat to target trial in early rheumatoid arthritis. Arthritis Rheum 2013;65:3325-6.
- De Cock D, Westhovens R, Corluy L, Joos R, Langenaken C, Taelman V, et al. Comparison of MTX therapy with or without a moderate dose glucocorticoid bridging scheme in early rheumatoid arthritis patients lacking classical poor prognostic markers: week 16 results from the randomized multicenter CareRA trial. Ann Rheum Dis 2014;73. https://doi.org/10.1136/annrheumdis-2014-eular.2144.
- den Uyl D, ter Wee M, Boers M, Kerstens P, Voskuyl A, Nurmohamed M, et al. A non-inferiority trial of an attenuated combination strategy (‘COBRA-light’) compared to the original COBRA strategy: clinical results after 26 weeks. Ann Rheum Dis 2014;73:1071-8. https://doi.org/10.1136/annrheumdis-2012-202818.
- den Uyl D, ter Wee MM, Boers M, Voskuyl AE, Kerstens PJ, Nurmohamed MT, et al. Cobra-light therapy is clinically non-inferior to original Cobra therapy in the treatment of early rheumatoid arthritis. Ann Rheum Dis 2013;71:104-5. https://doi.org/10.1136/annrheumdis-2012-eular.1834.
- Dirven L, van den Broek M, Klarenbeek NB, Han KH, Ronday HK, Kerstens PJSM, et al. Eight year results of disease activity steered treatment in a large recent rheumatoid arthritis cohort: clinical and radiological outcomes. Arthritis Rheum 2011;63.
- Fransen J, Moens HB, Speyer I, van Riel PL. Effectiveness of systematic monitoring of rheumatoid arthritis disease activity in daily practice: a multicentre, cluster randomised controlled trial. Ann Rheum Dis 2005;64:1294-8. https://doi.org/10.1136/ard.2004.030924.
- Hodkinson B, Musenge E, Tikly M. Tight control of rheumatoid arthritis in a resource-constrained setting: a randomized controlled study comparing the clinical disease activity index and simplified disease activity index. Rheumatology 2015;54:1033-8. https://doi.org/10.1093/rheumatology/keu443.
- Klarenbeek NB, Güler-Yüksel M, van der Kooij SM, Han KH, Ronday HK, Kerstens PJ, et al. The impact of four dynamic, goal-steered treatment strategies on the 5-year outcomes of rheumatoid arthritis patients in the BeSt study. Ann Rheum Dis 2011;70:1039-46. https://doi.org/10.1136/ard.2010.141234.
- Koevoets R, Dirven L, Klarenbeek NB, van Krugten MV, Ronday HK, van der Heijde DM, et al. ‘Insights in the relationship of joint space narrowing versus erosive joint damage and physical functioning of patients with RA’. Ann Rheum Dis 2013;72:870-4. https://doi.org/10.1136/annrheumdis-2011-201191.
- Markusse IM, Akdemir G, Dirven L, Goekoop-Ruiterman YP, van Groenendael JH, Han KH, et al. Long-term outcomes of patients with recent-onset rheumatoid arthritis after 10 years of tight controlled treatment: a randomized trial. Ann Intern Med 2016;164:523-31. https://doi.org/10.7326/M15-0919.
- Markusse IM, Dirven L, van Groenendael JH, Han KH, Ronday HK, Kerstens PJSM, et al. Mortality in a large cohort of patients with early rheumatoid arthritis that were treated-to-target for 10 years. Arthritis Rheum 2014;66.
- Möttönen T, Hannonen P, Leirisalo-Repo M, Nissilä M, Kautiainen H, Korpela M, et al. Comparison of combination therapy with single-drug therapy in early rheumatoid arthritis: a randomised trial. FIN-RACo trial group. Lancet 1999;353:1568-73. https://doi.org/10.1016/S0140-6736(98)08513-4.
- Pope J, Thorne J, Haraoui B, Sampalis J. Treating to target with TNFi in active established rheumatoid arthritis results in longer drug survival than routine care with TNFi: results from the optimization of Humira RCT. Arthritis Rheum 2010;62.
- Pope JE, Haraoui B, Rampakakis E, Psaradellis E, Thorne C, Sampalis JS. Optimization of Adalimumab Trial Investigators . Treating to a target in established active rheumatoid arthritis patients receiving a tumor necrosis factor inhibitor: results from a real-world cluster-randomized adalimumab trial. Arthritis Care Res 2013;65:1401-9. https://doi.org/10.1002/acr.22010.
- Rantalaiho V, Korpela M, Hannonen P, Kautiainen H, Järvenpää S, Leirisalo-Repo M, et al. The good initial response to therapy with a combination of traditional disease-modifying antirheumatic drugs is sustained over time: the eleven-year results of the Finnish rheumatoid arthritis combination therapy trial. Arthritis Rheum 2009;60:1222-31. https://doi.org/10.1002/art.24447.
- Rantalaiho V, Korpela M, Laasonen L, Kautiainen H, Järvenpää S, Hannonen P, et al. Early combination disease-modifying antirheumatic drug therapy and tight disease control improve long-term radiologic outcome in patients with early rheumatoid arthritis: the 11-year results of the Finnish Rheumatoid Arthritis Combination Therapy Trial. Arthritis Res Ther 2010;12. https://doi.org/10.1186/ar3060.
- Rantalaiho V, Puolakka K, Korpela M, Hannonen P, Möttönen T. Long-term results of the FIN-RACo trial; treatment with a combination of traditional disease-modifying anti-rheumatic drugs is an excellent option in early rheumatoid arthritis. Clin Exp Rheumatol 2012;30:S27-31.
- Saunders SA, Capell HA, Stirling A, Vallance R, Kincaid W, McMahon AD, et al. Triple therapy in early active rheumatoid arthritis: a randomized, single-blind, controlled trial comparing step-up and parallel treatment strategies. Arthritis Rheum 2008;58:1310-17. https://doi.org/10.1002/art.23449.
- Symmons D, Tricker K, Roberts C, Davies L, Dawes P, Scott DL. The British Rheumatoid Outcome Study Group (BROSG) randomised controlled trial to compare the effectiveness and cost-effectiveness of aggressive versus symptomatic therapy in established rheumatoid arthritis. Health Technol Assess 2005;9. https://doi.org/10.3310/hta9340.
- Wee MM, den Uyl D, Boers M, Kerstens P, Nurmohamed M, van Schaardenburg D, et al. Intensive combination treatment regimens, including prednisolone, are effective in treating patients with early rheumatoid arthritis regardless of additional etanercept: 1-year results of the COBRA-light open-label, randomised, non-inferiority trial. Ann Rheum Dis 2015;74:1233-40. https://doi.org/10.1136/annrheumdis-2013-205143.
- Urata Y, Uesato R, Tanaka D, Nakamura Y, Motomura S. Treating to target matrix metalloproteinase 3 normalisation together with disease activity score below 2.6 yields better effects than each alone in rheumatoid arthritis patients: T-4 Study. Ann Rheum Dis 2012;71:534-40. https://doi.org/10.1136/annrheumdis-2011-200108.
- van den Broek M, Dirven L, Klarenbeek N, van Krugten M, Ronday H, Kerstens P, et al. Clinical and radiological outcomes of four disease activity driven treatment strategies: 8-year results of the Best Study. Ann Rheum Dis 2013;71. https://doi.org/10.1136/annrheumdis-2012-eular.1837.
- van den Broek M, Lems WF, Allaart CF. BeSt practice: the success of early-targeted treatment in rheumatoid arthritis. Clin Exp Rheumatol 2012;30:35-8.
- van der Kooij SM, de Vries-Bouwstra JK, Goekoop-Ruiterman YP, Ewals JA, Han KH, Hazes JM, et al. Patient-reported outcomes in a randomized trial comparing four different treatment strategies in recent-onset rheumatoid arthritis. Arthritis Rheum 2009;61:4-12. https://doi.org/10.1002/art.24367.
- van Hulst LT, Creemers MC, Fransen J, Li LC, Grol R, Hulscher ME, et al. How to improve DAS28 use in daily clinical practice? – a pilot study of a nurse-led intervention. Rheumatology 2010;49:741-8. https://doi.org/10.1093/rheumatology/kep407.
- Verschueren P, De Cock D, Corluy L, Joos R, Langenaken C, Taelman V, et al. Associated with a glucocorticoid bridging scheme, methotrexate is as effective alone as in combination with other DMARDs for early rheumatoid arthritis, with fewer reported side effects: 16 weeks remission induction data from the CareRA trial. Ann Rheum Dis 2014;73. https://doi.org/10.1136/annrheumdis-2014-eular.2137.
- Verschueren P, De Cock D, Corluy L, Joos R, Langenaken C, Taelman V, et al. Patients lacking classical poor prognostic markers might also benefit from a step-down glucocorticoid bridging scheme in early rheumatoid arthritis: week 16 results from the randomized multicenter CareRA trial. Arthritis Res Ther 2015;17. https://doi.org/10.1186/s13075-015-0611-8.
- Verschueren P, De Cock D, Corluy L, Joos R, Langenaken C, Taelman V, et al. Effectiveness of methotrexate with step-down glucocorticoid remission induction (COBRA Slim) versus other intensive treatment strategies for early rheumatoid arthritis in a treat-to-target approach: 1-year results of CareRA, a randomised pragmatic open-label superiority trial. Ann Rheum Dis 2017;76:511-20. https://doi.org/10.1136/annrheumdis-2016-209212.
- Verstappen SM, Bakker MF, Heurkens AH, van der Veen MJ, Kruize AA, Geurts MA, et al. Adverse events and factors associated with toxicity in patients with early rheumatoid arthritis treated with methotrexate tight control therapy: the CAMERA study. Ann Rheum Dis 2010;69:1044-8. https://doi.org/10.1136/ard.2008.106617.
- Dirven L, van den Broek M, Klarenbeek N, Van Krugten M, Van Der Lubbe P, Kerstens P. Seven year results of DAS steered treatment in the best study: clinical and radiological outcomes. Arthritis Rheum 2010;62.
- Nikiphorou E, Norton S, Carpenter L, Dixey J, Andrew Walsh D, Kiely P, et al. Early Rheumatoid Arthritis Study and the Early Rheumatoid Arthritis Network Cohorts. Arthritis Care Res 2017;69:21-7. https://doi.org/10.1002/acr.23014.
- Hewlett SA. Patients and clinicians have different perspectives on outcomes in arthritis. J Rheumatol 2003;30:877-9.
- Markenson JA, Koenig AS, Feng JY, Chaudhari S, Zack DJ, Collier D, et al. Comparison of physician and patient global assessments over time in patients with rheumatoid arthritis: a retrospective analysis from the RADIUS cohort. J Clin Rheumatol 2013;19:317-23. https://doi.org/10.1097/RHU.0b013e3182a2164f.
- Suarez-Almazor ME, Conner-Spady B, Kendall CJ, Russell AS, Skeith K. Lack of congruence in the ratings of patients’ health status by patients and their physicians. Med Decis Making 2001;21:113-21. https://doi.org/10.1177/0272989x0102100204.
- Baradat C, Degboé Y, Constantin A, Cantagrel A, Ruyssen-Witrand A. No impact of concomitant methotrexate use on serious adverse event and serious infection risk in patients with rheumatoid arthritis treated with bDMARDs: a systematic literature review and meta-analysis. RMD Open 2017;3. https://doi.org/10.1136/rmdopen-2016-000352.
- Leombruno JP, Einarson TR, Keystone EC. The safety of anti-tumour necrosis factor treatments in rheumatoid arthritis: meta and exposure-adjusted pooled analyses of serious adverse events. Ann Rheum Dis 2009;68:1136-45. https://doi.org/10.1136/ard.2008.091025.
- Edwards CJ, Arden NK, Fisher D, Saperia JC, Reading I, Van Staa TP, et al. The changing use of disease-modifying anti-rheumatic drugs in individuals with rheumatoid arthritis from the United Kingdom General Practice Research Database. Rheumatology 2005;44:1394-8. https://doi.org/10.1093/rheumatology/kei024.
- Fassmer AM, Garbe E, Schmedt N. Frequency and trends of disease-modifying antirheumatic drug (DMARD) use in Germany. Pharmacol Res Perspect 2016;4. https://doi.org/10.1002/prp2.254.
- Kim SC, Yelin E, Tonner C, Solomon DH. Changes in use of disease-modifying antirheumatic drugs for rheumatoid arthritis in the United States during 1983-2009. Arthritis Care Res 2013;65:1529-33. https://doi.org/10.1002/acr.21997.
- van der Heijde D, Landewe R. Should radiographic progression still be used as outcome in RA?. Clin Immunol 2018;186:79-81. https://doi.org/10.1016/j.clim.2017.07.022.
- Gullick NJ, Mian AN, Ibrahim F, Walker D, Hassell A, Kiely PDW, et al. Predicting responses in patients with rheumatoid arthritis to disease-modifying agents using baseline clinical data. Clin Exp Rheumatol 2017;35:810-15.
- Matcham F, Norton S, Scott DL, Steer S, Hotopf M. Symptoms of depression and anxiety predict treatment response and long-term physical health outcomes in rheumatoid arthritis: secondary analysis of a randomized controlled trial. Rheumatology 2016;55:268-78. https://doi.org/10.1093/rheumatology/kev306.
- Mian AN, Ibrahim F, Scott DL, Galloway J. TITRATE study group . Optimal responses in disease activity scores to treatment in rheumatoid arthritis: is a DAS28 reduction of >1.2 sufficient?. Arthritis Res Ther 2016;18. https://doi.org/10.1186/s13075-016-1028-8.
- Scott IC, Ibrahim F, Lewis CM, Scott DL, Strand V. Impact of intensive treatment and remission on health-related quality of life in early and established rheumatoid arthritis. RMD Open 2016;2. https://doi.org/10.1136/rmdopen-2016-000270.
- Scott IC, Ibrahim F, Panayi G, Cope AP, Garrood T, Vincent A, et al. The frequency of remission and low disease activity in patients with rheumatoid arthritis, and their ability to identify people with low disability and normal quality of life. Semin Arthritis Rheum 2019;49:20-6. https://doi.org/10.1016/j.semarthrit.2018.12.006.
- Drossaers-Bakker KW, de Buck M, van Zeben D, Zwinderman AH, Breedveld FC, Hazes JM. Long-term course and outcome of functional capacity in rheumatoid arthritis: the effect of disease activity and radiologic damage over time. Arthritis Rheum 1999;42:1854-60. https://doi.org/10.1002/1529-0131(199909)42:9<1854::AID-ANR9>3.0.CO;2-F.
- Klarenbeek NB, Koevoets R, van der Heijde DM, Gerards AH, Ten Wolde S, Kerstens PJ, et al. Association with joint damage and physical functioning of nine composite indices and the 2011 ACR/EULAR remission criteria in rheumatoid arthritis. Ann Rheum Dis 2011;70:1815-21. https://doi.org/10.1136/ard.2010.149260.
- Radner H, Smolen JS, Aletaha D. Remission in rheumatoid arthritis: benefit over low disease activity in patient-reported outcomes and costs. Arthritis Res Ther 2014;16. https://doi.org/10.1186/ar4491.
- Versteeg GA, Steunebrink LMM, Vonkeman HE, Ten Klooster PM, van der Bijl AE, van de Laar MAFJ. Long-term disease and patient-reported outcomes of a continuous treat-to-target approach in patients with early rheumatoid arthritis in daily clinical practice. Clin Rheumatol 2018;37:1189-97. https://doi.org/10.1007/s10067-017-3962-5.
- Welsing PM, van Gestel AM, Swinkels HL, Kiemeney LA, van Riel PL. The relationship between disease activity, joint destruction, and functional capacity over the course of rheumatoid arthritis. Arthritis Rheum 2001;44:2009-17. https://doi.org/10.1002/1529-0131(200109)44:9<2009::AID-ART349>3.0.CO;2-L.
- Ajeganova S, Huizinga T. Sustained remission in rheumatoid arthritis: latest evidence and clinical considerations. Ther Adv Musculoskelet Dis 2017;9:249-62. https://doi.org/10.1177/1759720X17720366.
- Einarsson JT, Geborek P, Saxne T, Kristensen LE, Kapetanovic MC. Sustained remission improves physical function in patients with established rheumatoid arthritis, and should be a treatment goal: a prospective observational cohort study from southern Sweden. J Rheumatol 2016;43:1017-23. https://doi.org/10.3899/jrheum.150995.
- Aletaha D, Ward MM, Machold KP, Nell VP, Stamm T, Smolen JS. Remission and active disease in rheumatoid arthritis: defining criteria for disease activity states. Arthritis Rheum 2005;52:2625-36. https://doi.org/10.1002/art.21235.
- Jilani AA, Mackworth-Young CG. The role of citrullinated protein antibodies in predicting erosive disease in rheumatoid arthritis: a systematic literature review and meta-analysis. Int J Rheumatol 2015;2015. https://doi.org/10.1155/2015/728610.
- Ma MH, Scott IC, Dahanayake C, Cope AP, Scott DL. Clinical and serological predictors of remission in rheumatoid arthritis are dependent on treatment regimen. J Rheumatol 2014;41:1298-303. https://doi.org/10.3899/jrheum.131401.
- Choy EH, Khoshaba B, Cooper D, MacGregor A, Scott DL. Development and validation of a patient-based disease activity score in rheumatoid arthritis that can be used in clinical trials and routine practice. Arthritis Rheum 2008;59:192-9. https://doi.org/10.1002/art.23342.
- Safi M-AA, Houssien DA, Scott DL. Disease activity and anti-cyclic citrullinated peptide (anti-CCP) antibody in Saudi RF-negative rheumatoid arthritis patients. JKAU Med Sci 2012;19:3-20. https://doi.org/10.4197/Med.19-3.1.
- Traylor M, Curtis C, Patel H, Breen G, Hyuck Lee S, Xu X, et al. Genetic and environmental risk factors for rheumatoid arthritis in a UK African ancestry population: the GENRA case–control study. Rheumatology 2017;56:1282-92. https://doi.org/10.1093/rheumatology/kex048.
- Ibrahim F, Lorente-Canovas B, Dore CJ, Bosworth A, Ma MH, Galloway JB, et al. Optimizing treatment with tumour necrosis factor inhibitors in rheumatoid arthritis-a proof of principle and exploratory trial: is dose tapering practical in good responders?. Rheumatology 2017;56:2004-14. https://doi.org/10.1093/rheumatology/kex315.
- RAND Corporation . 36-Item Short Form Survey (SF-36) n.d. www.rand.org/health-care/surveys_tools/mos/36-item-short-form.html (accessed 11 April 2021).
- EQ-5D n.d. https://euroqol.org/eq-5d-instruments/ (accessed 11 April 2021).
- Aletaha D, Smolen JS. The Simplified Disease Activity Index and Clinical Disease Activity Index to monitor patients in standard clinical care. Rheum Dis Clin North Am 2009;35:759-72. https://doi.org/10.1016/j.rdc.2009.10.006.
- Bruce B, Fries JF. The Stanford Health Assessment Questionnaire: a review of its history, issues, progress, and documentation. J Rheumatol 2003;30:167-78.
- Dougados M, Aletaha D, van Riel P. Disease activity measures for rheumatoid arthritis. Clin Exp Rheumatol 2007;25:S22-9.
- Pincus T. RAPID3, an index of only 3 patient self-report core data set measures, but not ESR, recognizes incomplete responses to methotrexate in usual care of patients with rheumatoid arthritis. Bull NYU Hosp Jt Dis 2013;71.
- Archer R, Hock E, Hamilton J, Stevens J, Essat M, Poku E, et al. Assessing prognosis and prediction of treatment response in early rheumatoid arthritis: systematic reviews. Health Technol Assess 2018;22. https://doi.org/10.3310/hta22660.
- Lundström SL, Hensvold AH, Rutishauser D, Klareskog L, Ytterberg AJ, Zubarev RA, et al. IgG Fc galactosylation predicts response to methotrexate in early rheumatoid arthritis. Arthritis Res Ther 2017;19. https://doi.org/10.1186/s13075-017-1389-7.
- Sakthiswary R, Shaharir SS, Mohd Said MS, Asrul AW, Shahril NS. IgA rheumatoid factor as a serological predictor of poor response to tumour necrosis factor α inhibitors in rheumatoid arthritis. Int J Rheum Dis 2014;17:872-7. https://doi.org/10.1111/1756-185X.12443.
- Torrente-Segarra V, Bergstra SA, Solomon-Escoto K, Da Silva J, Veale DJ, Al-Emadi S, et al. Is current smoking status and its relationship to anti-cyclic citrullinated peptide antibodies a predictor of worse response to biological therapies in rheumatoid arthritis patients?. Scand J Rheumatol 2018;47:360-3. https://doi.org/10.1080/03009742.2017.1418423.
- de Hair MJ, Lehmann KA, van de Sande MG, Maijer KI, Gerlag DM, Tak PP. The clinical picture of rheumatoid arthritis according to the 2010 American College of Rheumatology/European League Against Rheumatism criteria: is this still the same disease?. Arthritis Rheum 2012;64:389-93. https://doi.org/10.1002/art.33348.
- Nordberg LB, Lillegraven S, Aga AB, Sexton J, Olsen IC, Lie E, et al. Comparing the disease course of patients with seronegative and seropositive rheumatoid arthritis fulfilling the 2010 ACR/EULAR classification criteria in a treat-to-target setting: 2-year data from the ARCTIC trial. RMD Open 2018;4. https://doi.org/10.1136/rmdopen-2018-000752.
- Nordberg LB, Lillegraven S, Lie E, Aga AB, Olsen IC, Hammer HB, et al. Patients with seronegative RA have more inflammatory activity compared with patients with seropositive RA in an inception cohort of DMARD-naive patients classified according to the 2010 ACR/EULAR criteria. Ann Rheum Dis 2017;76:341-5. https://doi.org/10.1136/annrheumdis-2015-208873.
- Ortiz EC, Shinada S. Evolution of classification criteria for rheumatoid arthritis: how do the 2010 criteria perform?. Rheum Dis Clin North Am 2012;38:345-53. https://doi.org/10.1016/j.rdc.2012.05.004.
- Becede M, Alasti F, Gessl I, Haupt L, Kerschbaumer A, Landesmann U, et al. Risk profiling for a refractory course of rheumatoid arthritis. Semin Arthritis Rheum 2019;49:211-17. https://doi.org/10.1016/j.semarthrit.2019.02.004.
- de Thurah A, Stengaard-Pedersen K, Axelsen M, Fredberg U, Schougaard LMV, Hjollund NHI, et al. Tele-health followup strategy for tight control of disease activity in rheumatoid arthritis: results of a randomized controlled trial. Arthritis Care Res 2018;70:353-60. https://doi.org/10.1002/acr.23280.
- Harrold LR, Reed GW, John A, Barr CJ, Soe K, Magner R, et al. Cluster-randomized trial of a behavioral intervention to incorporate a treat-to-target approach to care of US patients with rheumatoid arthritis. Arthritis Care Res 2018;70:379-87. https://doi.org/10.1002/acr.23294.
- Markusse IM, Dirven L, Han KH, Ronday HK, de Sonnaville PB, Kerstens PJ, et al. Evaluating adherence to a treat-to-target protocol in recent-onset rheumatoid arthritis: reasons for compliance and hesitation. Arthritis Care Res 2016;68:446-53. https://doi.org/10.1002/acr.22681.
- Møller-Bisgaard S, Hørslev-Petersen K, Ejbjerg B, Hetland ML, Ørnbjerg LM, Glinatsi D, et al. Effect of magnetic resonance imaging vs conventional treat-to-target strategies on disease activity remission and radiographic progression in rheumatoid arthritis: the IMAGINE-RA randomized clinical trial. JAMA 2019;321:461-72. https://doi.org/10.1001/jama.2018.21362.
- Pavelka K, Akkoç N, Al-Maini M, Zerbini CAF, Karateev DE, Nasonov EL, et al. Maintenance of remission with combination etanercept-DMARD therapy versus DMARDs alone in active rheumatoid arthritis: results of an international treat-to-target study conducted in regions with limited biologic access. Rheumatol Int 2017;37:1469-79. https://doi.org/10.1007/s00296-017-3749-7.
- Einarsson JT, Willim M, Ernestam S, Saxne T, Geborek P, Kapetanovic MC. Prevalence of sustained remission in rheumatoid arthritis: impact of criteria sets and disease duration, a Nationwide Study in Sweden. Rheumatology 2019;58:227-36. https://doi.org/10.1093/rheumatology/key054.
- Hmamouchi I, Combe B, Fautrel B, Rincheval N, Lukas C. Prevalence and concordance of early and sustained remission assessed by various validated indices in the early arthritis ‘ESPOIR’ cohort. Joint Bone Spine 2014;81:409-15. https://doi.org/10.1016/j.jbspin.2014.02.007.
- Price JH, Hillman KS, Toral ME, Newell S. The public’s perceptions and misperceptions of arthritis. Arthritis Rheum 1983;26:1023-8. https://doi.org/10.1002/art.1780260812.
- Shidara K, Nakajima A, Inoue E, Hoshi D, Sugimoto N, Seto Y, et al. Continual maintenance of remission defined by the ACR/EULAR criteria in daily practice leads to better functional outcomes in patients with rheumatoid arthritis. J Rheumatol 2017;44:147-53. https://doi.org/10.3899/jrheum.160395.
- Uhlig T, Lie E, Norvang V, Lexberg Å S, Rødevand E, Krøll F, et al. Achievement of remission and low disease activity definitions in patients with rheumatoid arthritis in clinical practice: results from the NOR-DMARD study. J Rheumatol 2016;43:716-23. https://doi.org/10.3899/jrheum.151132.
- Fleischmann R, van der Heijde D, Koenig AS, Pedersen R, Szumski A, Marshall L, et al. How much does Disease Activity Score in 28 joints ESR and CRP calculations underestimate disease activity compared with the Simplified Disease Activity Index?. Ann Rheum Dis 2015;74:1132-7. https://doi.org/10.1136/annrheumdis-2013-204920.
- Kim SK, Park SH, Bae J, Son JT, Choe JY. Performance of Routine Assessment of Patient Index Data 3 (RAPID3) for assessment of rheumatoid arthritis in clinical practice: differential agreement of RAPID3 according to disease activity categories. Rheumatol Int 2014;34:1311-18. https://doi.org/10.1007/s00296-014-3042-y.
- Salaffi F, Di Carlo M, Carotti M, Sarzi-Puttini P. The subjective components of the Disease Activity Score 28-joints (DAS28) in rheumatoid arthritis patients and coexisting fibromyalgia. Rheumatol Int 2018;38:1911-18. https://doi.org/10.1007/s00296-018-4096-z.
- Schoels M, Alasti F, Smolen JS, Aletaha D. Evaluation of newly proposed remission cut-points for disease activity score in 28 joints (DAS28) in rheumatoid arthritis patients upon IL-6 pathway inhibition. Arthritis Res Ther 2017;19. https://doi.org/10.1186/s13075-017-1346-5.
- Ton E, Bakker MF, Verstappen SM, Ter Borg EJ, van Albada-Kuipers IA, Schenk Y, et al. Look beyond the disease activity score of 28 joints (DAS28): tender points influence the DAS28 in patients with rheumatoid arthritis. J Rheumatol 2012;39:22-7. https://doi.org/10.3899/jrheum.110072.
- Ferreira RJO, Carvalho PD, Ndosi M, Duarte C, Chopra A, Murphy E, et al. Impact of patient global assessment on achieving remission in patients with rheumatoid arthritis: a multinational study using the METEOR database. Arthritis Care Res 2019;71:1317-25. https://doi.org/10.1002/acr.23866.
- Ferreira RJO, Dougados M, Kirwan JR, Duarte C, de Wit M, Soubrier M, et al. Drivers of patient global assessment in patients with rheumatoid arthritis who are close to remission: an analysis of 1588 patients. Rheumatology 2017;56:1573-8. https://doi.org/10.1093/rheumatology/kex211.
- Nikiphorou E, Radner H, Chatzidionysiou K, Desthieux C, Zabalan C, van Eijk-Hustings Y, et al. Patient global assessment in measuring disease activity in rheumatoid arthritis: a review of the literature. Arthritis Res Ther 2016;18. https://doi.org/10.1186/s13075-016-1151-6.
- Fanouriakis A, Papalopoulos I, Gergianaki I, Spyrou G, Erden A, Rapsomaniki P, et al. In early arthritis patients, high HAQ at baseline and DAS28 at three months predict suboptimal outcomes at two years: a retrospective cohort study. Clin Exp Rheumatol 2018;36:806-13.
- Hyrich KL, Watson KD, Silman AJ, Symmons DP. British Society for Rheumatology Biologics Register . Predictors of response to anti-TNF-alpha therapy among patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Rheumatology 2006;45:1558-65. https://doi.org/10.1093/rheumatology/kel149.
- Lee KE, Choi SE, Xu H, Kang JH, Park DJ, Lee SS. HAQ score is an independent predictor of sustained remission in patients with rheumatoid arthritis. Rheumatol Int 2017;37:2027-34. https://doi.org/10.1007/s00296-017-3833-z.
- Nakajima A, Aoki Y, Terayama K, Sonobe M, Takahashi H, Saito M, et al. Health assessment questionnaire-disability index (HAQ-DI) score at the start of biological disease-modifying antirheumatic drug (bDMARD) therapy is associated with radiographic progression of large joint damage in patients with rheumatoid arthritis. Mod Rheumatol 2017;27:967-72. https://doi.org/10.1080/14397595.2017.1294302.
- Cook MJ, Diffin J, Scirè CA, Lunt M, MacGregor AJ, Symmons DP, et al. Predictors and outcomes of sustained, intermittent or never achieving remission in patients with recent onset inflammatory polyarthritis: results from the Norfolk Arthritis Register. Rheumatology 2016;55:1601-9. https://doi.org/10.1093/rheumatology/kew210.
- Matcham F, Ali S, Irving K, Hotopf M, Chalder T. Are depression and anxiety associated with disease activity in rheumatoid arthritis? A prospective study. BMC Musculoskelet Disord 2016;17. https://doi.org/10.1186/s12891-016-1011-1.
- Matcham F, Davies R, Hotopf M, Hyrich KL, Norton S, Steer S, et al. The relationship between depression and biologic treatment response in rheumatoid arthritis: an analysis of the British Society for Rheumatology Biologics Register. Rheumatology 2018;57:835-43. https://doi.org/10.1093/rheumatology/kex528.
- Michelsen B, Kristianslund EK, Sexton J, Hammer HB, Fagerli KM, Lie E, et al. Do depression and anxiety reduce the likelihood of remission in rheumatoid arthritis and psoriatic arthritis? Data from the prospective multicentre NOR-DMARD study. Ann Rheum Dis 2017;76:1906-10. https://doi.org/10.1136/annrheumdis-2017-211284.
- Georgopoulou S, Prothero L, Lempp H, Galloway J, Sturt J. Motivational interviewing: relevance in the treatment of rheumatoid arthritis?. Rheumatology 2016;55:1348-56. https://doi.org/10.1093/rheumatology/kev379.
- Prothero L, Barley E, Galloway J, Georgopoulou S, Sturt J. The evidence base for psychological interventions for rheumatoid arthritis: a systematic review of reviews. Int J Nurs Stud 2018;82:20-9. https://doi.org/10.1016/j.ijnurstu.2018.03.008.
- Prothero L, Georgopoulou S, de Souza S, Bosworth A, Bearne L, Lempp H. Patient involvement in the development of a handbook for moderate rheumatoid arthritis. Health Expect 2017;20:288-97. https://doi.org/10.1111/hex.12457.
- Prothero L, Georgopoulou S, Galloway J, Williams R, Bosworth A, Lempp H. Patients’ and carers’ views and expectations about intensive management for moderate rheumatoid arthritis: a qualitative study. Psychol Health Med 2016;21:918-25. https://doi.org/10.1080/13548506.2015.1111394.
- Lempp H, Hofmann D, Hatch SL, Scott DL. Patients’ views about treatment with combination therapy for rheumatoid arthritis: a comparative qualitative study. BMC Musculoskelet Disord 2012;13. https://doi.org/10.1186/1471-2474-13-200.
- Marshall NJ, Wilson G, Lapworth K, Kay LJ. Patients’ perceptions of treatment with anti-TNF therapy for rheumatoid arthritis: a qualitative study. Rheumatology 2004;43:1034-8. https://doi.org/10.1093/rheumatology/keh237.
- van Tuyl LH, Plass AM, Lems WF, Voskuyl AE, Kerstens PJ, Dijkmans BA, et al. Discordant perspectives of rheumatologists and patients on COBRA combination therapy in rheumatoid arthritis. Rheumatology 2008;47:1571-6. https://doi.org/10.1093/rheumatology/ken323.
- Bate P, Robert G. Experience-based design: from redesigning the system around the patient to co-designing services with the patient. Qual Saf Health Care 2006;15:307-10. https://doi.org/10.1136/qshc.2005.016527.
- Coulter A, Entwistle V, Gilbert D. Sharing decisions with patients: is the information good enough?. BMJ 1999;318:318-22. https://doi.org/10.1136/bmj.318.7179.318.
- Crawford MJ, Rutter D, Manley C, Weaver T, Bhui K, Fulop N, et al. Systematic review of involving patients in the planning and development of health care. BMJ 2002;325. https://doi.org/10.1136/bmj.325.7375.1263.
- Kennedy A, Robinson A, Rogers A. Incorporating patients’ views and experiences of life with IBS in the development of an evidence based self-help guidebook. Patient Educ Couns 2003;50:303-10. https://doi.org/10.1016/S0738-3991(03)00054-5.
- Nilsen ES, Myrhaug HT, Johansen M, Oliver S, Oxman AD. Methods of consumer involvement in developing healthcare policy and research, clinical practice guidelines and patient information material. Cochrane Database Syst Rev 2006;3. https://doi.org/10.1002/14651858.CD004563.pub2.
- Astin JA, Beckner W, Soeken K, Hochberg MC, Berman B. Psychological interventions for rheumatoid arthritis: a meta-analysis of randomized controlled trials. Arthritis Rheum 2002;47:291-302. https://doi.org/10.1002/art.10416.
- Alam R, Sturt J, Lall R, Winkley K. An updated meta-analysis to assess the effectiveness of psychological interventions delivered by psychological specialists and generalist clinicians on glycaemic control and on psychological status. Patient Educ Couns 2009;75:25-36. https://doi.org/10.1016/j.pec.2008.08.026.
- Miller WR, Yahne CE, Moyers TB, Martinez J, Pirritano M. A randomized trial of methods to help clinicians learn motivational interviewing. J Consult Clin Psychol 2004;72:1050-62. https://doi.org/10.1037/0022-006X.72.6.1050.
- Söderlund LL, Madson MB, Rubak S, Nilsen P. A systematic review of motivational interviewing training for general health care practitioners. Patient Educ Couns 2011;84:16-2. https://doi.org/10.1016/j.pec.2010.06.025.
- Ritchie J, Spencer L, Huberman AH, Miles MB. The Qualitative Researcher’s Companion. Thousand Oaks, CA: SAGE Publications Ltd; 2002.
- Ritchie J, Spencer L, Huberman AH, Miles MB. The Qualitative Researcher’s Companion. Thousand Oaks, CA: SAGE Publications Ltd; 2002.
- Bryman A, Burgess B. Analyzing Qualitative Data. Abingdon: Routledge; 2002.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Beltman MW, Voshaar RC, Speckens AE. Cognitive-behavioural therapy for depression in people with a somatic disease: meta-analysis of randomised controlled trials. Br J Psychiatry 2010;197:11-9. https://doi.org/10.1192/bjp.bp.109.064675.
- Cramp F, Hewlett S, Almeida C, Kirwan JR, Choy EH, Chalder T, et al. Non-pharmacological interventions for fatigue in rheumatoid arthritis. Cochrane Database Syst Rev 2013;8. https://doi.org/10.1002/14651858.CD008322.pub2.
- Dissanayake RK, Bertouch JV. Psychosocial interventions as adjunct therapy for patients with rheumatoid arthritis: a systematic review. Int J Rheum Dis 2010;13:324-34. https://doi.org/10.1111/j.1756-185X.2010.01563.x.
- Knittle K, Maes S, de Gucht V. Psychological interventions for rheumatoid arthritis: examining the role of self-regulation with a systematic review and meta-analysis of randomized controlled trials. Arthritis Care Res 2010;62:1460-72. https://doi.org/10.1002/acr.20251.
- Niedermann K, Fransen J, Knols R, Uebelhart D. Gap between short- and long-term effects of patient education in rheumatoid arthritis patients: a systematic review. Arthritis Rheum 2004;51:388-98. https://doi.org/10.1002/art.20399.
- Nyssen OP, Taylor SJ, Wong G, Steed E, Bourke L, Lord J, et al. Does therapeutic writing help people with long-term conditions? Systematic review, realist synthesis and economic considerations. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20270.
- Riemsma RP, Kirwan JR, Taal E, Rasker JJ. Patient education for adults with rheumatoid arthritis. Cochrane Database Syst Rev 2003;2. https://doi.org/10.1002/14651858.CD003688.
- Ang DC, Kaleth AS, Bigatti S, Mazzuca SA, Jensen MP, Hilligoss J, et al. Research to encourage exercise for fibromyalgia (REEF): use of motivational interviewing, outcomes from a randomized-controlled trial. Clin J Pain 2013;29:296-304. https://doi.org/10.1097/AJP.0b013e318254ac76.
- Chilton R, Pires-Yfantouda R, Wylie M. A systematic review of motivational interviewing within musculoskeletal health. Psychol Health Med 2012;17:392-407. https://doi.org/10.1080/13548506.2011.635661.
- De Gucht V. Motivational interviewing and self-regulation to increase physical activity in patients with rheumatoid arthritis. Int J Psychol 2012;47.
- Everett S, Haiduc V, Richey M, Erkan D. The short term effect of individualized nutrition counseling on nutrients and select cardiovascular risk factors in patients with systemic lupus erythematosus (SLE). Arthritis Rheum 2012;64.
- Karlsson ML, Pettersson S, Lundberg I. Smoking cessation in patients with rheumatic disease. Scand J Rheumatol 2014;43.
- Zwikker HE, van den Ende CH, van Lankveld WG, den Broeder AA, van den Hoogen FH, van de Mosselaar B, et al. Effectiveness of a group-based intervention to change medication beliefs and improve medication adherence in patients with rheumatoid arthritis: a randomized controlled trial. Patient Educ Couns 2014;94:356-61. https://doi.org/10.1016/j.pec.2013.12.002.
- Ferguson A, Ibrahim FA, Thomas V, Weinman J, Simpson C, Cope AP, et al. Improving medication adherence in rheumatoid arthritis (RA): a pilot study. Psychol Health Med 2015;20:781-9. https://doi.org/10.1080/13548506.2015.1009917.
- Green J, Thorogood N. Qualitative Methods for Health Research. London: SAGE Publications Ltd; 2009.
- Liamputtong P. Focus Group Methodology: Principle and Practice. London: SAGE Publications Ltd; 2011.
- Flurey CA, Hewlett S, Rodham K, White A, Noddings R, Kirwan J. Men, rheumatoid arthritis, psychosocial impact and self-management: a narrative review. J Health Psychol 2016;21:2168-82. https://doi.org/10.1177/1359105315572452.
- Kiely P, Walsh D, Williams R, Young A. Early Rheumatoid Arthritis Network. Outcome in rheumatoid arthritis patients with continued conventional therapy for moderate disease activity – the early RA network (ERAN). Rheumatology 2011;50:926-31. https://doi.org/10.1093/rheumatology/keq406.
- Hewlett S, Chalder T, Choy E, Cramp F, Davis B, Dures E, et al. Fatigue in rheumatoid arthritis: time for a conceptual model. Rheumatology 2011;50:1004-6. https://doi.org/10.1093/rheumatology/keq282.
- Madsen SG, Danneskiold-Samsøe B, Stockmarr A, Bartels EM. Correlations between fatigue and disease duration, disease activity, and pain in patients with rheumatoid arthritis: a systematic review. Scand J Rheumatol 2016;45:255-61. https://doi.org/10.3109/03009742.2015.1095943.
- McWilliams DF, Walsh DA. Pain mechanisms in rheumatoid arthritis. Clin Exp Rheumatol 2017;35:94-101.
- Martin NH, Ibrahim F, Tom B, Galloway J, Wailoo A, Tosh J, et al. Does intensive management improve remission rates in patients with intermediate rheumatoid arthritis? (the TITRATE trial): study protocol for a randomised controlled trial. Trials 2017;18. https://doi.org/10.1186/s13063-017-2330-8.
- Scott D, Ibrahim F, Hill H, Tom B, Prothero L, Baggott RR, et al. The clinical effectiveness of intensive management in moderate established rheumatoid arthritis: the TITRATE trial. Semin Arthritis Rheum 2020;50:1182-90. https://doi.org/10.1016/j.semarthrit.2020.07.014.
- Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580-8. https://doi.org/10.1136/ard.2010.138461.
- GOV.UK . NHS National Tariff Payment System 2016 17 n.d. www.gov.uk/government/publications/nhs-national-tariff-payment-system-201617 (accessed 31 March 2021).
- Deighton C, Hyrich K, Ding T, Ledingham J, Lunt M, Luqmani R, et al. BSR and BHPR rheumatoid arthritis guidelines on eligibility criteria for the first biological therapy. Rheumatology 2010;49:1197-9. https://doi.org/10.1093/rheumatology/keq006a.
- Felson DT, Smolen JS, Wells G, Zhang B, van Tuyl LH, Funovits J, et al. American College of Rheumatology/European League against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Ann Rheum Dis 2011;70:404-13. https://doi.org/10.1136/ard.2011.149765.
- Inoue E, Yamanaka H, Hara M, Tomatsu T, Kamatani N. Comparison of Disease Activity Score (DAS)28-erythrocyte sedimentation rate and DAS28-C-reactive protein threshold values. Ann Rheum Dis 2007;66:407-9. https://doi.org/10.1136/ard.2006.054205.
- Ma MHY, Ibrahim F, Kingsley GH, Cope A, Scott DL. Variable impacts of different remission states on health-related quality of life in rheumatoid arthritis. Clin Exp Rheumatol 2018;36:203-9.
- EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Scott DL, Houssien DA, Laasonen L. Proposed modification to Larsen’s scoring methods for hand and wrist radiographs. Br J Rheumatol 1995;34. https://doi.org/10.1093/rheumatology/34.1.56.
- Ajeganova S, van Steenbergen HW, van Nies JA, Burgers LE, Huizinga TW, van der Helm-van Mil AH. Disease-modifying antirheumatic drug-free sustained remission in rheumatoid arthritis: an increasingly achievable outcome with subsidence of disease symptoms. Ann Rheum Dis 2016;75:867-73. https://doi.org/10.1136/annrheumdis-2014-207080.
- Acebes C, Andreu JL, Balsa A, Batlle E, de Toro-Santos J, Garcia Llorente F, et al. Exploring the remission concept in rheumatoid arthritis with patients and rheumatologists: time for a new approach?. Clin Exp Rheumatol 2017;35:816-22.
- Grabner M, Boytsov NN, Huang Q, Zhang X, Yan T, Curtis JR. Costs associated with failure to respond to treatment among patients with rheumatoid arthritis initiating TNFi therapy: a retrospective claims analysis. Arthritis Res Ther 2017;19. https://doi.org/10.1186/s13075-017-1293-1.
- Harnett J, Wiederkehr D, Gerber R, Gruben D, Koenig A, Bourret J. Real-world evaluation of TNF-inhibitor utilization in rheumatoid arthritis. J Med Econ 2016;19:91-102. https://doi.org/10.3111/13696998.2015.1099538.
- Strand V, Tundia N, Song Y, Macaulay D, Fuldeore M. Economic burden of patients with inadequate response to targeted immunomodulators for rheumatoid arthritis. J Manag Care Spec Pharm 2018;24:344-52. https://doi.org/10.18553/jmcp.2018.24.4.344.
- Lysdahl KB, Mozygemba K, Burns J, Brönneke JB, Chilcott JB, Ward S, et al. Comprehensive assessment of complex technologies: integrating various aspects in health technology assessment. Int J Technol Assess Health Care 2017;33:570-6. https://doi.org/10.1017/S0266462317000678.
- Pfadenhauer LM, Gerhardus A, Mozygemba K, Lysdahl KB, Booth A, Hofmann B, et al. Making sense of complexity in context and implementation: the Context and Implementation of Complex Interventions (CICI) framework. Implement Sci 2017;12. https://doi.org/10.1186/s13012-017-0552-5.
- Shepperd S, Lewin S, Straus S, Clarke M, Eccles MP, Fitzpatrick R, et al. Can we systematically review studies that evaluate complex interventions?. PLOS Med 2009;6. https://doi.org/10.1371/journal.pmed.1000086.
- National Institute for Health and Care Excellence (NICE) . Guide to the Process of Technology Appraisal 2018.
- Chisholm D, Knapp MRJ, Knudsen HC, Amaddeo F, Gaite L, Van Wijngaarden B, et al. Client socio-demographic and service receipt inventory–European version: development of an instrument for international research: EPSILON Study 5. Br J Psychiatry 2000;177:s28-33. https://doi.org/10.1192/bjp.177.39.s28.
- Joint Formulary Committee . British National Formulary 2019.
- Curtis L. Unit Costs of Health and Social Care 2019. Canterbury: PSSRU, University of Kent; 2019.
- National Association of Care Catering . Meals on Wheels Survey 2018 n.d. http://costsectorcatering.co.uk/sites/default/files/attachment/nacc_-_meals_on_wheels_report_2018.pdf (accessed 31 March 2021).
- Office for National Statistics . Employee Earnings in the UK – 2018 2018.
- National Institute for Health and Care Excellence . NICE Position Statement on Use of the EQ-5D-5L Valuation Set for England n.d. www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/nice-technology-appraisal-guidance (accessed 25 March 2019).
- National Institute for Health and Care Excellence . Guide to the Processes of Technology Appraisal. Process and Methods [PMG19] n.d. www.nice.org.uk/process/pmg19/chapter/foreword (accessed 10 April 2021).
- Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2018;27:7-22. https://doi.org/10.1002/hec.3564.
- Hernández-Alava M, Pudney S. Econometric modelling of multiple self-reports of health states: the switch from EQ-5D-3L to EQ-5D-5L in evaluating drug therapies for rheumatoid arthritis. J Health Econ 2017;55:139-52. https://doi.org/10.1016/j.jhealeco.2017.06.013.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Di Zio M, Guarnera U. Semiparametric predictive mean matching. Adv Stat Anal 2009;93:175-86. https://doi.org/10.1007/s10182-008-0081-2.
- Jönsson B. Ten arguments for a societal perspective in the economic evaluation of medical innovations. Eur J Health Econ 2009;10:357-9. https://doi.org/10.1007/s10198-009-0173-2.
- Katchamart W, Johnson S, Lin HJ, Phumethum V, Salliot C, Bombardier C. Predictors for remission in rheumatoid arthritis patients: a systematic review. Arthritis Care Res 2010;62:1128-43. https://doi.org/10.1002/acr.20188.
- Kuusalo L, Puolakka K, Kautiainen H, Karjalainen A, Malmi T, Yli-Kerttula T, et al. Patient-reported outcomes as predictors of remission in early rheumatoid arthritis patients treated with tight control treat-to-target approach. Rheumatol Int 2017;37:825-30. https://doi.org/10.1007/s00296-017-3692-7.
- Levitsky A, Brismar K, Hafström I, Hambardzumyan K, Lourdudoss C, van Vollenhoven RF, et al. Obesity is a strong predictor of worse clinical outcomes and treatment responses in early rheumatoid arthritis: results from the SWEFOT trial. RMD Open 2017;3. https://doi.org/10.1136/rmdopen-2017-000458.
- Paulshus Sundlisæter N, Olsen IC, Aga AB, Hammer HB, Uhlig T, van der Heijde D, et al. Predictors of sustained remission in patients with early rheumatoid arthritis treated according to an aggressive treat-to-target protocol. Rheumatology 2018;57:2022-31. https://doi.org/10.1093/rheumatology/key202.
- Yu C, Jin S, Wang Y, Jiang N, Wu C, Wang Q, et al. Remission rate and predictors of remission in patients with rheumatoid arthritis under treat-to-target strategy in real-world studies: a systematic review and meta-analysis. Clin Rheumatol 2019;38:727-38. https://doi.org/10.1007/s10067-018-4340-7.
- Feldthusen C, Grimby-Ekman A, Forsblad-d’Elia H, Jacobsson L, Mannerkorpi K. Explanatory factors and predictors of fatigue in persons with rheumatoid arthritis: a longitudinal study. J Rehabil Med 2016;48:469-76. https://doi.org/10.2340/16501977-2090.
- Katz P, Margaretten M, Trupin L, Schmajuk G, Yazdany J, Yelin E. Role of sleep disturbance, depression, obesity, and physical inactivity in fatigue in rheumatoid arthritis. Arthritis Care Res 2016;68:81-90. https://doi.org/10.1002/acr.22577.
- Nikolaus S, Bode C, Taal E, van de Laar MA. Fatigue and factors related to fatigue in rheumatoid arthritis: a systematic review. Arthritis Care Res 2013;65:1128-46. https://doi.org/10.1002/acr.21949.
- Olsen CL, Lie E, Kvien TK, Zangi HA. Predictors of fatigue in rheumatoid arthritis patients in remission or in a low disease activity state. Arthritis Care Res 2016;68:1043-8. https://doi.org/10.1002/acr.22787.
- Twigg S, Hensor EMA, Freeston J, Tan AL, Emery P, Tennant A, et al. YEAR and IACON Consortia . Effect of fatigue, older age, higher body mass index, and female sex on disability in early rheumatoid arthritis in the treatment-to-target era. Arthritis Care Res 2018;70:361-8. https://doi.org/10.1002/acr.23281.
- van Hoogmoed D, Fransen J, Bleijenberg G, van Riel P. Physical and psychosocial correlates of severe fatigue in rheumatoid arthritis. Rheumatology 2010;49:1294-302. https://doi.org/10.1093/rheumatology/keq043.
- Markusse IM, Dirven L, Han KH, Ronday HK, Kerstens PJ, Lems WF, et al. Continued participation in a ten-year tight control treat-to-target study in rheumatoid arthritis: why keep patients doing their best?. Arthritis Care Res 2015;67:739-45. https://doi.org/10.1002/acr.22540.
- Hemingway H, Croft P, Perel P, Hayden JA, Abrams K, Timmis A, et al. Prognosis research strategy (PROGRESS) 1: a framework for researching clinical outcomes. BMJ 2013;346. https://doi.org/10.1136/bmj.e5595.
- Hingorani AD, Windt DA, Riley RD, Abrams K, Moons KG, Steyerberg EW, et al. Prognosis research strategy (PROGRESS) 4: stratified medicine research. BMJ 2013;346. https://doi.org/10.1136/bmj.e5793.
- Riley RD, Hayden JA, Steyerberg EW, Moons KG, Abrams K, Kyzas PA, et al. Prognosis Research Strategy (PROGRESS) 2: prognostic factor research. PLOS Med 2013;10. https://doi.org/10.1371/journal.pmed.1001380.
- Steyerberg EW, Moons KG, van der Windt DA, Hayden JA, Perel P, Schroter S, et al. Prognosis Research Strategy (PROGRESS) 3: prognostic model research. PLOS Med 2013;10. https://doi.org/10.1371/journal.pmed.1001381.
- Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine – reporting of subgroup analyses in clinical trials. N Engl J Med 2007;357:2189-94. https://doi.org/10.1056/NEJMsr077003.
- Liu Y, Hazlewood GS, Kaplan GG, Eksteen B, Barnabe C. Impact of obesity on remission and disease activity in rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res 2017;69:157-65. https://doi.org/10.1002/acr.22932.
- Shan J, Zhang J. Impact of obesity on the efficacy of different biologic agents in inflammatory diseases: a systematic review and meta-analysis. Joint Bone Spine 2019;86:173-83. https://doi.org/10.1016/j.jbspin.2018.03.007.
- Ma MHY. The REMIRA (REMission in RA) Study: Defining Low Disease Activity (LDA) States Using Clinical, Imaging and Biological Measures in Rheumatoid Arthritis. London: King’s College London; 2014.
- Cella D, Yount S, Sorensen M, Chartash E, Sengupta N, Grober J. Validation of the functional assessment of chronic illness therapy fatigue scale relative to other instrumentation in patients with rheumatoid arthritis. J Rheumatol 2005;32:811-19.
- Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum 1980;23:137-45. https://doi.org/10.1002/art.1780230202.
- Hurst NP, Kind P, Ruta D, Hunter M, Stubbings A. Measuring health-related quality of life in rheumatoid arthritis: validity, responsiveness and reliability of EuroQol (EQ-5D). Br J Rheumatol 1997;36:551-9. https://doi.org/10.1093/rheumatology/36.5.551.
- Linde L, Sørensen J, Ostergaard M, Hørslev-Petersen K, Hetland ML. Health-related quality of life: validity, reliability, and responsiveness of SF-36, 15D, EQ-5D [corrected] RAQoL, and HAQ in patients with rheumatoid arthritis. J Rheumatol 2008;35:1528-37.
- Ruta DA, Hurst NP, Kind P, Hunter M, Stubbings A. Measuring health status in British patients with rheumatoid arthritis: reliability, validity and responsiveness of the short form 36-item health survey (SF-36). Br J Rheumatol 1998;37:425-36. https://doi.org/10.1093/rheumatology/37.4.425.
- Anderson J, Caplan L, Yazdany J, Robbins ML, Neogi T, Michaud K, et al. Rheumatoid arthritis disease activity measures: American College of Rheumatology recommendations for use in clinical practice. Arthritis Care Res 2012;64:640-7. https://doi.org/10.1002/acr.21649.
- Gardiner PV, Sykes HR, Hassey GA, Walker DJ. An evaluation of the Health Assessment Questionnaire in long-term longitudinal follow-up of disability in rheumatoid arthritis. Br J Rheumatol 1993;32:724-8. https://doi.org/10.1093/rheumatology/32.8.724.
- Molenaar ET, Voskuyl AE, Dijkmans BA. Functional disability in relation to radiological damage and disease activity in patients with rheumatoid arthritis in remission. J Rheumatol 2002;29:267-70.
- Wiles N, Dunn G, Barrett E, Silman A, Symmons D. Associations between demographic and disease-related variables and disability over the first five years of inflammatory polyarthritis: a longitudinal analysis using generalized estimating equations. J Clin Epidemiol 2000;53:988-96. https://doi.org/10.1016/S0895-4356(00)00189-X.
- Wolfe F, Cathey MA. The assessment and prediction of functional disability in rheumatoid arthritis. J Rheumatol 1991;18:1298-306.
- Andersen PK, Keiding N. Multi-state models for event history analysis. Stat Method Med Res 2002;11:91-115. https://doi.org/10.1191/0962280202SM276ra.
- de Souza S, Williams R, Lempp H. Patient and clinician views on the quality of foot health care for rheumatoid arthritis outpatients: a mixed methods service evaluation. J Foot Ankle Res 2016;9. https://doi.org/10.1186/s13047-015-0133-2.
- Prothero L, Sturt J, de Souza S, Lempp H. TITRATE Programme Investigators . Intensive management for moderate rheumatoid arthritis: a qualitative study of patients’ and practitioners’ views. BMC Rheumatol 2019;3. https://doi.org/10.1186/s41927-019-0057-8.
- Dures E, Fraser I, Almeida C, Peterson A, Caesley J, Pollock J, et al. Patients’ perspectives on the psychological impact of inflammatory arthritis and meeting the associated support needs: open-ended responses in a multi-centre survey. Musculoskeletal Care 2017;15:175-85. https://doi.org/10.1002/msc.1159.
- Dures E, Hewlett S, Ambler N, Jenkins R, Clarke J, Gooberman-Hill R. Rheumatology clinicians’ experiences of brief training and implementation of skills to support patient self-management. BMC Musculoskelet Disord 2014;15. https://doi.org/10.1186/1471-2474-15-108.
- Dures E, Hewlett S, Ambler N, Jenkins R, Clarke J, Gooberman-Hill R. A qualitative study of patients’ perspectives on collaboration to support self-management in routine rheumatology consultations. BMC Musculoskelet Disord 2016;17. https://doi.org/10.1186/s12891-016-0984-0.
- Georgopoulou S, Prothero L, D’Cruz DP. Physician-patient communication in rheumatology: a systematic review. Rheumatol Int 2018;38:763-75. https://doi.org/10.1007/s00296-018-4016-2.
- Belt EA, Kaarela K, Lehto MU. Destruction and arthroplasties of the metatarsophalangeal joints in seropositive rheumatoid arthritis. A 20-year follow-up study. Scand J Rheumatol 1998;27:194-6. https://doi.org/10.1080/030097498440804.
- Otter SJ, Lucas K, Springett K, Moore A, Davies K, Cheek L, et al. Foot pain in rheumatoid arthritis prevalence, risk factors and management: an epidemiological study. Clin Rheumatol 2010;29:255-71. https://doi.org/10.1007/s10067-009-1312-y.
- van der Leeden M, Steultjens MP, Ursum J, Dahmen R, Roorda LD, Schaardenburg DV, et al. Prevalence and course of forefoot impairments and walking disability in the first eight years of rheumatoid arthritis. Arthritis Rheum 2008;59:1596-602. https://doi.org/10.1002/art.24188.
- van der Leeden M, Steultjens MP, van Schaardenburg D, Dekker J. Forefoot disease activity in rheumatoid arthritis patients in remission: results of a cohort study. Arthritis Res Ther 2010;12. https://doi.org/10.1186/ar2901.
- Schoo AM, Lawn S, Rudnik E, Litt JC. Teaching health science students foundation motivational interviewing skills: use of motivational interviewing treatment integrity and self-reflection to approach transformative learning. BMC Med Educ 2015;15. https://doi.org/10.1186/s12909-015-0512-1.
- Morgan DL. The Focus Group Guidebook. Thousand Oaks, CA: SAGE Publications Ltd; 1998.
- Popham WJ. Competency verification in the health professions via limited focus measurement. Eval Health Prof 1978;1:101-9. https://doi.org/10.1177/016327877800100308.
- Neale J. Iterative categorization (IC): a systematic technique for analysing qualitative data. Addiction 2016;111:1096-106. https://doi.org/10.1111/add.13314.
- Boyatzis R. Transforming Qualitative Information: Thematic Analysis and Code Development. Thousand Oaks, CA: SAGE Publications Ltd; 1998.
- Seale C, Seale C. The Quality of Qualitative Research. London: SAGE Publications Ltd; 1999.
- Seale C, Seale C. The Quality of Qualitative Research. London: SAGE Publications Ltd; 1999.
- Hassan E. Recall bias can be a threat to retrospective and prospective research designs. Int J Epidemiol 2006;3:339-412. https://doi.org/10.5580/2732.
- Sjö AS, Bergsten U. Patients’ experiences of frequent encounters with a rheumatology nurse – a tight control study including patients with rheumatoid arthritis. Musculoskeletal Care 2018;16:305-12. https://doi.org/10.1002/msc.1348.
- van Eijk-Hustings Y, Ammerlaan J, Voorneveld-Nieuwenhuis H, Maat B, Veldhuizen C, Repping-Wuts H. Patients’ needs and expectations with regard to rheumatology nursing care: results of multicentre focus group interviews. Ann Rheum Dis 2013;72:831-5. https://doi.org/10.1136/annrheumdis-2012-202810.
- Chehade L, Jaafar ZA, El Masri D, Zmerly H, Kreidieh D, Tannir H, et al. Lifestyle modification in rheumatoid arthritis: dietary and physical activity recommendations based on evidence. Curr Rheumatol Rev 2019;15:209-14. https://doi.org/10.2174/1573397115666190121135940.
- Fenton SAM, Veldhuijzen van Zanten JJCS, Duda JL, Metsios GS, Kitas GD. Sedentary behaviour in rheumatoid arthritis: definition, measurement and implications for health. Rheumatology 2018;57:213-26. https://doi.org/10.1093/rheumatology/kex053.
- Minnock P, Ringnér A, Bresnihan B, Veale D, FitzGerald O, McKee G. Perceptions of the cause, impact and management of persistent fatigue in patients with rheumatoid arthritis following tumour necrosing factor inhibition therapy. Musculoskeletal Care 2017;15:23-35. https://doi.org/10.1002/msc.1136.
- Thomsen T, Aadahl M, Beyer N, Hetland ML, Løppenthin K, Midtgaard J, et al. The efficacy of motivational counselling and SMS reminders on daily sitting time in patients with rheumatoid arthritis: a randomised controlled trial. Ann Rheum Dis 2017;76:1603-6. https://doi.org/10.1136/annrheumdis-2016-210953.
- Farrow SJ, Kingsley GH, Scott DL. Interventions for foot disease in rheumatoid arthritis: a systematic review. Arthritis Rheum 2005;53:593-602. https://doi.org/10.1002/art.21327.
- Graham AS, Hammond A, Williams AE. Foot health education for people with rheumatoid arthritis: the practitioner’s perspective. J Foot Ankle Res 2012;5. https://doi.org/10.1186/1757-1146-5-2.
- Hendry GJ, Turner DE, Lorgelly PK, Woodburn J. Room for improvement: patient, parent, and practitioners’ perceptions of foot problems and foot care in juvenile idiopathic arthritis. Arch Phys Med Rehabil 2012;93:2062-7. https://doi.org/10.1016/j.apmr.2012.07.007.
- Naidoo S, Anderson S, Mills J, Parsons S, Breeden S, Bevan E, et al. ‘I could cry, the amount of shoes I can’t get into’: a qualitative exploration of the factors that influence retail footwear selection in women with rheumatoid arthritis. J Foot Ankle Res 2011;4. https://doi.org/10.1186/1757-1146-4-21.
- Redmond AC, Waxman R, Helliwell PS. Provision of foot health services in rheumatology in the UK. Rheumatology 2006;45:571-6. https://doi.org/10.1093/rheumatology/kei205.
- Williams AE, Graham AS. ‘My feet: visible, but ignored. ’ A qualitative study of foot care for people with rheumatoid arthritis. Clin Rehabil 2012;26:952-9. https://doi.org/10.1177/0269215511434995.
- Bassett AM, de Souza S, Williams R, Lempp H. Rheumatoid arthritis portrayal by UK National newspapers 2011–2016: a service user-led thematic analysis of language used. BMC Rheumatol 2018;2. https://doi.org/10.1186/s41927-018-0013-z.
- Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol 2004;23:443-51. https://doi.org/10.1037/0278-6133.23.5.443.
- Mars T, Ellard D, Carnes D, Homer K, Underwood M, Taylor SJ. Fidelity in complex behaviour change interventions: a standardised approach to evaluate intervention integrity. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-003555.
- Oakley A, Strange V, Bonell C, Allen E, Stephenson J. RIPPLE Study Team . Process evaluation in randomised controlled trials of complex interventions. BMJ 2006;332:413-16. https://doi.org/10.1136/bmj.332.7538.413.
- Robb SL, Burns DS, Docherty SL, Haase JE. Ensuring treatment fidelity in a multi-site behavioral intervention study: implementing NIH Behavior Change Consortium recommendations in the SMART trial. Psycho-Oncology 2011;20:1193-201. https://doi.org/10.1002/pon.1845.
- Sturt J, Hearnshaw H, Farmer A, Dale J, Eldridge S. The Diabetes Manual trial protocol – a cluster randomized controlled trial of a self-management intervention for type 2 diabetes [ISRCTN06315411]. BMC Fam Pract 2006;7. https://doi.org/10.1186/1471-2296-7-45.
- Toomey E, Matthews J, Hurley DA. Using mixed methods to assess fidelity of delivery and its influencing factors in a complex self-management intervention for people with osteoarthritis and low back pain. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-015452.
- Bailey J, McCrossin T. Communicating diabetes in Australian print media: a change in language use between 2010 and 2014?. Aust N Z J Public Health 2016;40:493-7. https://doi.org/10.1111/1753-6405.12563.
- National Audit Office . Services For People With Rheumatoid Arthritis, 2009 2009.
- Healthcare Quality Improvement Partnership . National Clinical Audit for Rheumatoid and Early Inflammatory Arthritis First and Second Annual Reports 2015 and 2016 2016.
- Given LM. The SAGE Encyclopedia of Qualitative Research Methods. Thousand Oaks, CA: SAGE Publications Ltd; 2008.
- Dougados M, Soubrier M, Perrodeau E, Gossec L, Fayet F, Gilson M, et al. Impact of a nurse-led programme on comorbidity management and impact of a patient self-assessment of disease activity on the management of rheumatoid arthritis: results of a prospective, multicentre, randomised, controlled trial (COMEDRA). Ann Rheum Dis 2015;74:1725-33. https://doi.org/10.1136/annrheumdis-2013-204733.
- Hill J, Thorpe R, Bird H. Outcomes for patients with RA: a rheumatology nurse practitioner clinic compared to standard outpatient care. Musculoskeletal Care 2003;1:5-20. https://doi.org/10.1002/msc.35.
- Koksvik HS, Hagen KB, Rødevand E, Mowinckel P, Kvien TK, Zangi HA. Patient satisfaction with nursing consultations in a rheumatology outpatient clinic: a 21-month randomised controlled trial in patients with inflammatory arthritides. Ann Rheum Dis 2013;72:836-43. https://doi.org/10.1136/annrheumdis-2012-202296.
- Larsson I, Fridlund B, Arvidsson B, Teleman A, Bergman S. Randomized controlled trial of a nurse-led rheumatology clinic for monitoring biological therapy. J Adv Nurs 2014;70:164-75. https://doi.org/10.1111/jan.12183.
- Ndosi M, Lewis M, Hale C, Quinn H, Ryan S, Emery P, et al. The outcome and cost-effectiveness of nurse-led care in people with rheumatoid arthritis: a multicentre randomised controlled trial. Ann Rheum Dis 2014;73:1975-82. https://doi.org/10.1136/annrheumdis-2013-203403.
- Primdahl J, Sørensen J, Horn HC, Petersen R, Hørslev-Petersen K. Shared care or nursing consultations as an alternative to rheumatologist follow-up for rheumatoid arthritis outpatients with low disease activity – patient outcomes from a 2-year, randomised controlled trial. Ann Rheum Dis 2014;73:357-64. https://doi.org/10.1136/annrheumdis-2012-202695.
- Ryan S, Hassell AB, Lewis M, Farrell A. Impact of a rheumatology expert nurse on the wellbeing of patients attending a drug monitoring clinic. J Adv Nurs 2006;53:277-86. https://doi.org/10.1111/j.1365-2648.2006.03725.x.
- Tijhuis GJ, Zwinderman AH, Hazes JM, Van Den Hout WB, Breedveld FC, Vliet Vlieland TP. A randomized comparison of care provided by a clinical nurse specialist, an inpatient team, and a day patient team in rheumatoid arthritis. Arthritis Rheum 2002;47:525-31. https://doi.org/10.1002/art.10665.
- Arvidsson SB, Petersson A, Nilsson I, Andersson B, Arvidsson BI, Petersson IF, et al. A nurse-led rheumatology clinic’s impact on empowering patients with rheumatoid arthritis: a qualitative study. Nurs Health Sci 2006;8:133-9. https://doi.org/10.1111/j.1442-2018.2006.00269.x.
- Bala SV, Samuelson K, Hagell P, Svensson B, Fridlund B, Hesselgard K. The experience of care at nurse-led rheumatology clinics. Musculoskeletal Care 2012;10:202-11. https://doi.org/10.1002/msc.1021.
- Larsson I, Bergman S, Fridlund B, Arvidsson B. Patients’ experiences of a nurse-led rheumatology clinic in Sweden: a qualitative study. Nurs Health Sci 2012;14:501-7. https://doi.org/10.1111/j.1442-2018.2012.00723.x.
- Long AF, Kneafsey R, Ryan J, Berry J. The role of the nurse within the multi-professional rehabilitation team. J Adv Nurs 2002;37:70-8. https://doi.org/10.1046/j.1365-2648.2002.02059.x.
- Primdahl J, Wagner L, Hørslev-Petersen K. Being an outpatient with rheumatoid arthritis – a focus group study on patients’ self-efficacy and experiences from participation in a short course and one of three different outpatient settings. Scand J Caring Sci 2011;25:394-403. https://doi.org/10.1111/j.1471-6712.2010.00854.x.
- Temmink D, Hutten JB, Francke AL, Abu-Saad HH, van der Zee J. Quality and continuity of care in Dutch nurse clinics for people with rheumatic diseases. Int J Qual Health Care 2000;12:89-95. https://doi.org/10.1093/intqhc/12.2.89.
- Esselens G, Westhovens R, Verschueren P. Effectiveness of an integrated outpatient care programme compared with present-day standard care in early rheumatoid arthritis. Musculoskeletal Care 2009;7:1-16. https://doi.org/10.1002/msc.136.
- Muñoz-Fernández S, Aguilar MD, Rodríguez A, Almodóvar R, Cano-García L, Gracia LA, et al. Evaluation of the impact of nursing clinics in the rheumatology services. Rheumatol Int 2016;36:1309-17. https://doi.org/10.1007/s00296-016-3518-z.
- Solomon DH, Fraenkel L, Lu B, Brown E, Tsao P, Losina E, et al. Comparison of care provided in practices with nurse practitioners and physician assistants versus subspecialist physicians only: a cohort study of rheumatoid arthritis. Arthritis Care Res 2015;67:1664-70. https://doi.org/10.1002/acr.22643.
- Watts RA, Mooney J, Barton G, MacGregor AJ, Shepstone L, Irvine L, et al. The outcome and cost-effectiveness of nurse-led care in the community for people with rheumatoid arthritis: a non-randomised pragmatic study. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-007696.
- Lillie K, Ryan S, Adams J. The educational needs of nurses and allied healthcare professionals caring for people with arthritis: results from a cross-sectional survey. Musculoskeletal Care 2013;11:93-8. https://doi.org/10.1002/msc.1035.
- Vliet Vlieland TP, van den Ende CH, Alliot-Launois F, Beauvais C, Gobbo M, Iagnocco A, et al. Educational needs of health professionals working in rheumatology in Europe. RMD Open 2016;2. https://doi.org/10.1136/rmdopen-2016-000337.
- Hanson H, O’Brien N, Whybrow P, Isaacs JD, Rapley T. Drug breakthrough offers hope to arthritis sufferers: qualitative analysis of medical research in UK newspapers. Health Expect 2017;20:309-20. https://doi.org/10.1111/hex.12460.
- Abildtrup M, Kingsley GH, Scott DL. Calprotectin as a biomarker for rheumatoid arthritis: a systematic review. J Rheumatol 2015;42:760-70. https://doi.org/10.3899/jrheum.140628.
- Scott IC, Kingsley GH, Scott DL. Can we discontinue synthetic disease-modifying anti-rheumatic drugs in rheumatoid arthritis?. Clin Exp Rheumatol 2013;31:S4-8.
- Scott IC, Scott DL. Joint counts in inflammatory arthritis. Clin Exp Rheumatol 2014;32:S-7.
- Bonafede M, Johnson BH, Tang DH, Shah N, Harrison DJ, Collier DH. Etanercept-methotrexate combination therapy initiators have greater adherence and persistence than triple therapy initiators with rheumatoid arthritis. Arthritis Care Res 2015;67:1656-63. https://doi.org/10.1002/acr.22638.
- Bala SV, Samuelson K, Hagell P, Fridlund B, Forslind K, Svensson B, et al. Living with persistent rheumatoid arthritis: a BARFOT study. J Clin Nurs 2017;26:2646-56. https://doi.org/10.1111/jocn.13691.
- Kapetanovic MC, Lindqvist E, Nilsson JÅ, Geborek P, Saxne T, Eberhardt K. Development of functional impairment and disability in rheumatoid arthritis patients followed for 20 years: relation to disease activity, joint damage, and comorbidity. Arthritis Care Res 2015;67:340-8. https://doi.org/10.1002/acr.22458.
- Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19140.
- McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold: what it is and what that means. PharmacoEconomics 2008;26:733-44. https://doi.org/10.2165/00019053-200826090-00004.
- Woods B, Revill P, Sculpher M, Claxton K. Country-level cost-effectiveness thresholds: initial estimates and the need for further research. Value Health 2016;19:929-35. https://doi.org/10.1016/j.jval.2016.02.017.
- Wailoo A, Hernández Alava M, Scott IC, Ibrahim F, Scott DL. Cost-effectiveness of treatment strategies using combination disease-modifying anti-rheumatic drugs and glucocorticoids in early rheumatoid arthritis. Rheumatology 2014;53:1773-7. https://doi.org/10.1093/rheumatology/keu039.
- Wailoo AJ, Stevenson M, Tosh J, Hernández M, Stevens JW, Archer R, et al. The cost-effectiveness of biologic DMARDs in patients with severe or mild-to-severe rheumatoid arthritis after conventional DMARDs. Value Health 2014;17. https://doi.org/10.1016/j.jval.2014.08.2611.
- Braun J, Kay J. The safety of emerging biosimilar drugs for the treatment of rheumatoid arthritis. Expert Opin Drug Saf 2017;16:289-302. https://doi.org/10.1080/14740338.2017.1273899.
- Cohen S, Kay J. Biosimilars: implications for rheumatoid arthritis therapy. Curr Opin Rheumatol 2017;29:260-8. https://doi.org/10.1097/BOR.0000000000000379.
- Gulácsi L, Brodszky V, Baji P, Kim H, Kim SY, Cho YY, et al. Biosimilars for the management of rheumatoid arthritis: economic considerations. Expert Rev Clin Immunol 2015;11:43-52. https://doi.org/10.1586/1744666X.2015.1090313.
- George MD, Baker JF. the obesity epidemic and consequences for rheumatoid arthritis care. Curr Rheumatol Rep 2016;18. https://doi.org/10.1007/s11926-015-0550-z.
- Nikiphorou E, Norton S, Young A, Dixey J, Walsh D, Helliwell H, et al. Early Rheumatoid Arthritis Study and the Early Rheumatoid Arthritis Network . The association of obesity with disease activity, functional ability and quality of life in early rheumatoid arthritis: data from the Early Rheumatoid Arthritis Study/Early Rheumatoid Arthritis Network UK prospective cohorts. Rheumatology 2018;57:1194-202. https://doi.org/10.1093/rheumatology/key066.
- Poudel DR, Karmacharya P. Obesity and outcomes in rheumatoid arthritis. Semin Arthritis Rheum 2017;47. https://doi.org/10.1016/j.semarthrit.2017.03.016.
- Forestier R, André-Vert J, Guillez P, Coudeyre E, Lefevre-Colau MM, Combe B, et al. Non-drug treatment (excluding surgery) in rheumatoid arthritis: clinical practice guidelines. Joint Bone Spine 2009;76:691-8. https://doi.org/10.1016/j.jbspin.2009.01.017.
- Gossec L, Pavy S, Pham T, Constantin A, Poiraudeau S, Combe B, et al. Nonpharmacological treatments in early rheumatoid arthritis: clinical practice guidelines based on published evidence and expert opinion. Joint Bone Spine 2006;73:396-402. https://doi.org/10.1016/j.jbspin.2006.01.008.
- Vliet Vlieland TP, van den Ende CH. Nonpharmacological treatment of rheumatoid arthritis. Curr Opin Rheumatol 2011;23:259-64. https://doi.org/10.1097/BOR.0b013e32834540fb.
- Curtis JR, Chen L, Danila MI, Saag KG, Parham KL, Cush JJ. Routine use of quantitative disease activity measurements among US rheumatologists: implications for treat-to-target management strategies in rheumatoid arthritis. J Rheumatol 2018;45:40-4. https://doi.org/10.3899/jrheum.170548.
- Curtis JR, van der Helm-van Mil AH, Knevel R, Huizinga TW, Haney DJ, Shen Y, et al. Validation of a novel multibiomarker test to assess rheumatoid arthritis disease activity. Arthritis Care Res 2012;64:1794-803. https://doi.org/10.1002/acr.21767.
- Hambardzumyan K, Bolce RJ, Wallman JK, van Vollenhoven RF, Saevarsdottir S. Serum biomarkers for prediction of response to methotrexate monotherapy in early rheumatoid arthritis: results from the SWEFOT trial. J Rheumatol 2019;46:555-63. https://doi.org/10.3899/jrheum.180537.
- Norgeot B, Glicksberg BS, Trupin L, Lituiev D, Gianfrancesco M, Oskotsky B, et al. Assessment of a deep learning model based on electronic health record data to forecast clinical outcomes in patients with rheumatoid arthritis. JAMA Netw Open 2019;2. https://doi.org/10.1001/jamanetworkopen.2019.0606.
- Conaghan PG, Green MJ, Emery P. Established rheumatoid arthritis. Baillieres Best Pract Res Clin Rheumatol 1999;13:561-75. https://doi.org/10.1053/berh.1999.0046.
- Bechman K, Tweehuysen L, Garrood T, Scott DL, Cope AP, Galloway JB, et al. Flares in rheumatoid arthritis patients with low disease activity: predictability and association with worse clinical outcomes. J Rheumatol 2018;45:1515-21. https://doi.org/10.3899/jrheum.171375.
- Anderson JK, Zimmerman L, Caplan L, Michaud K. Measures of rheumatoid arthritis disease activity: Patient (PtGA) and Provider (PrGA) Global Assessment of Disease Activity, Disease Activity Score (DAS) and Disease Activity Score with 28-Joint Counts (DAS28), Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI), Patient Activity Score (PAS) and Patient Activity Score-II (PASII), Routine Assessment of Patient Index Data (RAPID), Rheumatoid Arthritis Disease Activity Index (RADAI) and Rheumatoid Arthritis Disease Activity Index-5 (RADAI-5), Chronic Arthritis Systemic Index (CASI), Patient-Based Disease Activity Score With ESR (PDAS1) and Patient-Based Disease Activity Score without ESR (PDAS2), and Mean Overall Index for Rheumatoid Arthritis (MOI-RA). Arthritis Care Res 2011;63:14-36. https://doi.org/10.1002/acr.20621.
- Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res 2011;63:240-52. https://doi.org/10.1002/acr.20543.
- Hewlett S, Hehir M, Kirwan JR. Measuring fatigue in rheumatoid arthritis: a systematic review of scales in use. Arthritis Rheum 2007;57:429-39. https://doi.org/10.1002/art.22611.
- van Riel PL, Renskers L. The Disease Activity Score (DAS) and the Disease Activity Score using 28 joint counts (DAS28) in the management of rheumatoid arthritis. Clin Exp Rheumatol 2016;34:40-4.
- Castrejon I, Ortiz AM, Garcia-Vicuna R, Lopez-Bote JP, Humbria A, Carmona L, et al. Are the C-reactive protein values and erythrocyte sedimentation rate equivalent when estimating the 28-joint disease activity score in rheumatoid arthritis?. Clin Exp Rheumatol 2008;26:769-75.
- Smolen JS, Aletaha D. Scores for all seasons: SDAI and CDAI. Clin Exp Rheumatol 2014;32:S-75.
- Castrejón I, Pincus T. Assessing remission in rheumatoid arthritis on the basis of patient reported outcomes – advantages of using RAPID3/MDHAQ in routine care. Bull Hosp Jt Dis 2014;72:136-41.
- Fransen J, Creemers MC, Van Riel PL. Remission in rheumatoid arthritis: agreement of the disease activity score (DAS28) with the ARA preliminary remission criteria. Rheumatology 2004;43:1252-5. https://doi.org/10.1093/rheumatology/keh297.
- Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res 1999;47:555-67.
- Beecham, Knapp M, Thornicroft G, Brewin C, Wing JK. Measuring Mental Health Needs. London: Gaskell; 1992.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13.
- Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092-7.
- Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health 1999;14:1-24.
- Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res 2006;60:631-7.
- Little RJ, Rubin DB. Statistical Analysis with Missing Data. Hoboken, NJ: John Wiley & Sons, Inc.; 2002.
- Scheffer J. Dealing with missing data. Res Lett Inf Math Sci 2002;3:153-60.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
Appendix 1 Baseline data in clinical studies
Studies of changes in disease activity
Data were collected at four time points from patients attending rheumatology departments at King’s College Hospital and University Hospital Lewisham (London, UK) in 1996–97 to 2013–14. All of the patients had a consultant diagnosis of RA. The first three cohorts were surveys of consecutive patients seen in routine care settings. 70–72 Data for the fourth cohort were extracted from routinely captured clinic attendance electronic patient records. The cohorts ranged from 189 to 520 patients. A total of 1324 patients were studied. The groups had similar demographic features (Table 27). The patients in these cohorts and in most of the other observational studies had established RA, which means that they had already been treated by a rheumatologist and that they did not yet have end-stage disease. The concept of established RA has been explained in detail by Conaghan et al. 455
| Characteristic | Time point | |||
|---|---|---|---|---|
| 1996–97 (N = 189) | 2001–3 (N = 310) | 2009–10 (N = 304) | 2013–14 (N = 520) | |
| Female, n (%) | 140 (74) | 237 (76) | 244 (80) | 413 (79) |
| Age (years), mean (SD) | 59 (14) | 60 (13) | 59 (15) | 58 (15) |
| Disease duration (years), median (IQR) | 8 (13) | 9 (10) | 10 (9) | 10 (9) |
Longitudinal observational study
Most RA patients attending the Guy’s and St Thomas’ NHS Trust RA Centre were included. 53 They had clinical diagnoses of RA made by experienced rheumatologists. They were seen regularly for routine care and each visit involved a clinical review and assessment of key clinical outcomes. Management followed the treat-to-target approach, with an aim of reaching DAS28-ESR remission. The patients were analysed in two ways: (1) all patients in whom data were available and (2) patients who were followed for ≥ 3 years. Details of these patient groups are shown in Table 28.
| Characteristic | All patients (N = 1693) | Patients followed for ≤ 3 years (N = 752) | Patients followed for > 3 years (N = 941) |
|---|---|---|---|
| Female, n (%) | 1262 (75) | 579 (77) | 683 (73) |
| Age (years), mean (SD) | 55 (16) | 55 (15) | 55 (14.7) |
| Disease duration (years), mean (SD) | 11 (10) | 10 (10) | 10 (9.7) |
Early Rheumatoid Arthritis Network
We studied 155 patients in the observational cohort –ERAN – who had completed 12 months’ follow-up and who had clinical data collected at 0, 6 and 12 months. These patients had mean age of 56 (SD 14) years and 101 (65%) were women. Their disease durations < 12 months.
Observational studies of limitations of DAS28-ESR
We undertook secondary analyses of three observational data sets that enrolled 1374 RA patients. The observational data sets involved 747 European white patients (enrolled in previous observational studies72,221) and 197 black African/Caribbean British patients (enrolled in one previous observational study223) who were seen in rheumatology clinics in England. A total of 430 Arab patients (enrolled in one previous observational study222) were seen in rheumatology clinics in Saudi Arabia. Details of these patients are summarised in Table 29.
| Characteristic | European (N = 747) | Black African/Caribbean British (N = 197) | Arab (N = 430) |
|---|---|---|---|
| Female, n (%) | 422 (57) | 163 (83) | 364 (85) |
| Age (years), mean (SD) | 62 (13) | 56 (15) | 46 (13) |
| Disease duration (years), mean (SD) | 12 (11) | 11 (10) | 6 (6) |
| Methotrexate, n (%) | 417 (56) | 116 (58) | 201 (47) |
| Other DMARDs, n (%) | 159 (21) | 117 (59) | 282 (66) |
| Biologics, n (%) | 45 (6) | 36 (18) | 4 (1) |
Stability of disease control in the REMIRA cohort
We studied the 152 RA patients who entered the REMIRA observational cohort study. 357,456 These patients were undergoing a treat-to-target management strategy for 12 months, had disease durations of ≤ 10 years, were receiving stable doses of conventional DMARDs or biologic DMARDs for > 6 months and had DAS28-ESR scores of ≤ 3.2 for 1 month or longer prior to entry. Details of these patients are shown in Table 30. Two-thirds of the cohort were females and three-quarters were white. The mean age at entry was 57 (SD 14) years. The mean duration of RA from diagnosis until entry was 50 (SD 32) months. Over 85% of the cohort had low levels of disease activity or were in clinical remission at entry. None of the patients had high baseline levels of disease activity. Approximately 50% of the cohort had low levels or no disability at baseline. Erosive disease was observed to be present in 44% of the study sample (67 patients), and the majority of patients were receiving stable doses of methotrexate for over 6 months prior to entry. Fifty-three per cent of patients were on two or more RA medications at baseline.
| Baseline feature | Category | Mean/number |
|---|---|---|
| Demographic | ||
| Age (years), mean (SD) | 57 (14) | |
| Disease duration (months), mean (SD) | 50 (32) | |
| Female, % | 66 | |
| Ethnicity, % | White | 76 |
| Asian | 18 | |
| Afro-Caribbean | 5 | |
| Clinical assessment | ||
| Tender joint count for 28 joints, mean (SD) | 0.6 (1.6) | |
| Swollen joint count for 28 joints, mean (SD) | 1.0 (1.8) | |
| Patient global (0- to 100-mm VAS), mean (SD) | 23 (20) | |
| ESR (mm), mean (SD) | 9.8 (8.4) | |
| CRP (mg/l), mean (SD) | 6.1 (4.0) | |
| DAS28-ESR, mean (SD) | 2.1 (0.9) | |
| DAS28-CRP, mean (SD) | 2.4 (0.6) | |
| Disease activity states, % | DAS28-ESR < 2.6 | 64 |
| DAS28-ESR = 2.6–3.2 | 22 | |
| DAS28-ESR > 32–5.1 | 12 | |
| DAS28-ESR > 5.1 | 0 | |
| Missing | 2 | |
| HAQ, mean (SD) | 0.51 (0.60) | |
| HAQ states, % | HAQ = 0 | 42 |
| HAQ 0.1–0.49 | 17 | |
| HAQ 0.5–1.49 | 33 | |
| HAQ = 1.5–3.00 | 7 | |
| Missing | 1 | |
| EQ-5D, mean (SD) | 0.75 (0.22) | |
| FACIT-F, mean (SD) | 39 (10) | |
| Fatigue (0- to 100-mm VAS), mean (SD) | 34 (23) | |
| Erosive disease, % | Yes | 44 |
| No | 54 | |
| Missing | 2 | |
| Treatments | ||
| Methotrexate | 84 | |
| Hydroxychloroquine | 39 | |
| Sulphasalazine | 26 | |
| Leflunomide | 5 | |
| Prednisolone | 2 | |
| TNFis | 16 | |
| Number of RA medications, % | One | 47 |
| Two | 37 | |
| Three | 15 | |
| Four | 1 | |
Clinical trials
CARDERA trial
The CARDERA trial73 randomised 467 patients with early active RA to receive 2 years of intensive combination treatment with methotrexate, ciclosporin and/or corticosteroids compared with methotrexate monotherapy. We evaluated the 379 completers in this trial.
TACIT trial
The TACIT trial74 randomised 205 established, active RA patients to receive 1 year of intensive DMARD therapy or TNFis. We evaluated the 146 completers in this trial.
OPTTIRA trial
The Optimizing Treatment with Tumour Necrosis Factor Inhibitors In Rheumatoid Arthritis (OPTTIRA) trial randomised 103 established RA patients receiving etanercept or adalimumab and a DMARD with DAS28 score < 3.2 for > 3 months who remained on their current biologic or had their treatment tapered over 6–12 months. 224 We evaluated the 97 patients treated in this trial.
Details of the patients in these trials are summarised in Table 31.
| Characteristic | Trial | ||
|---|---|---|---|
| CARDERA73 (N = 379) | TACIT74 (N = 192) | OPTTIRA224 (N = 97) | |
| Female, n (%) | 259 (68) | 144 (75) | 72 (74) |
| Age (years), mean (SD) | 54 (12) | 57 (12) | 57 (11) |
| Disease duration (years), mean (SD) | 0.5 (0.5) | 8 (9) | 13 (9) |
| Trial drugs | Methotrexate | Combination DMARDs | DMARDs |
| Ciclosporin | Biologics | Etanercept | |
| High-dose steroids | Adalimumab | ||
Qualitative research
Patients’ and carers’ views and expectations
Purposive sampling was used to recruit nine patients and five carers (Table 32) from four rheumatology clinics across three London hospital NHS trusts. The sample size was based on previous published qualitative studies. Patients were eligible for inclusion if they had moderate disease activity (i.e. a DAS28 score of 3.2–5.1), had received at least one DMARD for a minimum of 6 months and were currently receiving at least one DMARD. Carers were carers of eligible patients. Participants were approached by a designated member of the clinical team at each site.
| Sociodemographic feature | Patients | Carers |
|---|---|---|
| Sex | Six women and three men | Two women and three men |
| Age range (years) | 46–69 | 26–73 |
| Self-defined ethnicity | Unknown, n = 1 | British Indian, n = 1 |
| White European, n = 1 | Black British, n = 1 | |
| Turkish Cypriot, n = 1 | White British, n = 3 | |
| White British, n = 6 | ||
| Employment status | Full time, n = 1 | Full time, n = 2 |
| Not currently working, n = 5 | Retired, n = 3 | |
| Retired, n = 3 |
The patients in this and other qualitative studies had comparable ages, sexes and ethnicities to the patients in observational studies and the trial.
Patients’ and practitioners’ views of intensive treatment
This qualitative study was nested within the TITRATE trial. Patient and practitioners were recruited from the 39 trial sites. There were no additional patient participant inclusion or exclusion criteria to those of the trial. Practitioners were those trained to deliver the intensive management intervention in trial sites, provided that they had delivered at least six intensive management sessions with the same patient. During their first intensive management session, practitioners provided each patient with information about a ‘substudy’, which included an optional semistructured interview. The invitation letter requested that patients who chose to participate in the substudy should complete the consent form and return it directly to the researcher. Fifteen patients from 10 rheumatology clinics consented to participate (Table 33). Sixteen practitioners (13 research nurses and three specialist nurses) from 13 rheumatology clinics consented to participate.
| Number | Sex | Age (years) | Self-reported ethnicity | Site |
|---|---|---|---|---|
| 1 | Female | 65 | White British | A |
| 2 | Female | 64 | White British | A |
| 3 | Female | 56 | White British | A |
| 4 | Male | 70 | White British | B |
| 5 | Female | 69 | White British | B |
| 6 | Female | 54 | White | B |
| 7 | Female | 62 | White British | C |
| 8 | Male | 35 | White European | C |
| 9 | Female | 70 | White British | D |
| 10 | Female | 56 | White British | E |
| 11 | Female | 67 | White British | F |
| 12 | Male | 43 | White European | G |
| 13 | Female | 66 | White British | H |
| 14 | Female | 58 | White British | I |
| 15 | Female | 35 | White British | J |
Patients’ views on foot health care for rheumatoid arthritis
A mixed-methods approach was adopted, collecting qualitative focus group-based data from patients with RA, followed by quantitative survey-based data from clinicians.
Focus group participants were recruited by health-care staff from one tertiary rheumatology outpatient clinic in London. Inclusion criteria were adults with established RA who were able to read, speak and understand English. Collective views were gathered via two focus groups, which generated rich data about the experiences and beliefs of participants. They were facilitated by one investigator who was also a patient with established RA. An anonymous online survey allowed clinicians to provide easy completion in minimal time and a higher response rate. It comprised 11 items in six themes: (1) provision of foot health information, (2) frequency of foot examination, (3) reasons for choosing whether or not to examine feet, (4) clinician beliefs, (5) podiatry referral and (6) clinician training. All clinicians from the outpatient clinic were invited, via e-mail, by a rheumatology consultant to fill out the survey online, with one reminder sent 2 weeks later.
Appendix 2 Clinical assessments
Demographic, diagnostic and treatment information
Demographic information
Data were collected on age, sex, ethnicity and disease duration.
Diagnostic information
Evidence for the diagnosis of RA was assessed using 1987 ACR criteria and 2010 EULAR/ACR criteria or consultant-made diagnoses, depending on the study and setting.
Treatment information
Current treatments, including conventional DMARDs, biologics and steroids, were recorded, depending on the study and setting.
Disease activity assessments
Joint counts
Global assessments
Other clinical assessments
Laboratory assessments
-
ESR.
-
CRP levels.
Remission criteria
Other DAS28-ESR criteria
-
Low disease activity (i.e. DAS28-ESR score of 2.6–3.2).
-
Moderate disease activity (i.e. DAS28-ESR score of 3.2–5.1).
-
Severe or active disease (i.e. DAS28-ESR score of > 5.1).
Appendix 3 Analytic methods
Observational studies and secondary analyses of trials
Cross-sectional studies of changes in disease activity and disability
Data management and analyses used Stata. Age, disease duration, disease activity assessments and other outcomes were described using means and SDs or medians and IQRs for non-normal data. DAS28 category proportions were given as raw figures and percentages. Spearman’s correlations were used to assess relationship between the DAS28 and HAQ.
Longitudinal study of changes in disease activity and disability
Data management and analyses used Stata. Descriptive analyses used numbers of patients and percentages and mean scores with SDs or 95% CIs. We used mixed models to examine the changes in DAS28 and its components over time. We also used trend analysis to take into account repeated measures from the same patient. Subgroups were compared by chi-squared analyses or by one-way analysis of variance.
Clinical studies giving evidence for treating moderately active rheumatoid arthritis intensively
Data management and analyses used IBM SPSS Statistics (version 25; IBM Corporation, Armonk, NY, USA). Descriptive analyses used numbers of patients and percentages and mean scores with SDs or 95% CIs. Subgroups were compared by chi-squared analyses.
Treatment targets: DAS28-ESR and disability
Data management and analyses using IBM SPSS (version 23). Descriptive analyses used numbers of patients and percentages and mean scores with SDs or 95% CIs. We evaluated the sensitivity and specificity of point and sustained DAS28-ESR scores of < 2.6 and ≤ 3.2 at identifying patients with and without low HAQ scores and normal EQ-5D scores at the cohort end points.
Treatment targets: optimal responses in DAS28-ESR scores
Data management and analyses used IBM SPSS Statistics (version 22). Descriptive statistics described means, standard error and CIs. We studied patients with all data available at the trial end points. We divided patients into EULAR non-responders, moderate responders and good responders. We compared changes in HAQ and EQ-5D scores between good and moderate EULAR responders for each trial using the independent-samples t-test. We also subdivided moderate and good EULAR responders by their final DAS28 scores. In addition, we used the previous NICE criterion for remaining on treatment (i.e. a change in DAS28 score of > 1.2) to categorise patients, replicating EULAR response criteria by dividing patients into those who also achieved DAS28 low disease activity scores at the trial end point and those who did not. Finally, we assessed the numbers of patients who achieved different levels of improvement in HAQ and EQ-5D scores in both trials in relation to moderate and good EULAR responses.
Treatment targets: limitations of DAS28-ESR and alternative assessments
Data management and analyses used IBM SPSS Statistics (version 25). Disease activity assessments and other outcomes were described using means and SDs or medians and IQRs for non-normal data. We used the formula for calculating DAS28-ESR to assess the relative contributions of different components to overall scores. We took a similar approach to CDAI and RAPID3 scores. Spearman’s correlations were used to assess relationship between the quality of life and different components of DAS28-ESR and other disease activity scores.
Treatment targets: DAS28-ESR components and health-related quality of life
Analyses were performed in R (V.3.1.3; The R Foundation for Statistical Computing, Vienna, Austria). Treatment effects were evaluated using linear regression models, including the 6-month changes in each SF-36 domain and summary score as the response variable. An unadjusted model included treatment as the explanatory variable (active vs. placebo corticosteroids in the CARDERA trial73 and TNFi vs. conventional DMARD therapy in the TACIT trial74). An adjusted model included treatment, baseline SF-36 domain/summary score, age, sex and disease duration as explanatory variables. The mean SF-36 domain scores at the final time point in the CARDERA trial73 and the TACIT trial74 were plotted on spydergrams stratified by (1) DAS28-ESR activity category [i.e. remission (DAS28-ESR score of < 2.6), low disease activity (DAS28-ESR score of ≥ 2.6 to < 3.2), moderate disease activity (DAS28-ESR score of 3.2–5.1) and high disease activity (DAS28-ESR score of > 5.1)]; and (2) remission compared with non-remission according to each DAS28 component (i.e. tender joint count of ≤ 1, swollen joint count of ≤ 1, patient global assessment of disease activity on a 100-mm VAS of ≤ 10 and ESR ≤ 20 mm/hour). These component cut-off points represent the preliminary ACR/EULAR Boolean-based definition of RA remission for clinical trials. As CRP data were not available, a normal ESR level was considered indicative of acute-phase response remission.
To minimise type I error from multiple testing (four DAS28 components and eight health domains), associations between DAS28-ESR components and PCS and MCS were tested. Linear regression models used final time point PCS and MCS scores as response variables, and swollen joint count, tender joint count, ESR and patient global assessment as explanatory variables, adjusted for covariates (treatment, age, sex and disease duration). Model 1 tested each DAS28-ESR component separately. Model 2 included all DAS28-ESR components as explanatory variables. To ensure that multicollinearity between DAS28-ESR components was not an issue in model 2, variance inflation factors were calculated for each predictor. The variance inflation factor was < 2 for all explanatory variables. Standardised β-values were calculated, enabling direct comparison of effect sizes of each DAS28-ESR component on PCS and MCS. In the CARDERA trial,73 missing data had been imputed at all time points using last observation carried forward (LOCF) analysis. Missing data were imputed in 19% of patients at 24 months. An observed case analysis had excluded a significant impact of the LOCF assumption on the study end points, which included PCS and MCS scores. For consistency across studies, we imputed missing TACIT trial74 data using LOCF (undertaken at 6 months in five and 18 patients for SF-36 domain scores and DAS28-ESR components, respectively, and at 12 months in 15 and 16 patients for SF-36 domain scores and DAS28-ESR components, respectively). We undertook an additional analysis using non-imputed TACIT trial74 data to ensure that our findings were not biased by LOCF imputation.
Age- and sex-matched US normative scores were generated for CARDERA trial73 and TACIT trial74 protocol populations using data published in SF-36 manuals and updates. It was not possible to use UK age- and sex-matched norms, as these data are not publicly available, although existing studies have highlighted similarities in mean SF-36 domain scores between UK and US populations.
Predictive factors: simple four-point scores
Data management and analyses used IBM SPSS Statistics (version 17). Persistent disease activity was defined as a DAS28 score of > 3.2 at both 6- and 12-month visits. Predictors of persistent disease activity in the ERAN patients were assessed using logistic multiple regression and expressed as ORs with 95% CIs. The baseline explanatory variables considered were sex, tender joint count, swollen joint count, ESR, DAS28 and HAQ. As DAS28 was strongly associated with all other variables, it was not included in the model. Chi-squared tests compared the proportion of patients with low disease activity scores.
Predictive factors: high baseline Health Assessment Questionnaire as outcome predictor
Data management and analyses used IBM SPSS Statistics (version 25). Disease activity assessments and other outcomes were described using means and SDs or medians and IQRs for non-normal data. Groups were compared using Fisher’s exact test for categorical data and Student’s unpaired t-test for continuous data.
Predictive factors: anxiety and depression
Multilevel models were used pooling across the CARDERA trial73 2-year treatment arms. This approach was taken to account for both missing outcome data and variations between and within patients over the course of the study. The models used Stata. There were two multilevel linear models for continuous outcomes. First, unadjusted models assessed only depression/anxiety status. Second, adjusted models included age, sex, disease duration, time, baseline level of physical health, the type of treatment received and rheumatoid factor status. Further details of the methods are given in Matcham et al. 207
Qualitative research and other patient-focused activities
Patient expectations of intensive management
Semistructured topic guides were developed based on the discussions with multidisciplinary research team and three ‘patient experts’ who provided feedback on its suitability and relevance. Two separate focus groups were held for patients (n = 3) and for carers (n = 4). Semistructured interviews were also conducted with six patients and one carer, which included face-to-face and telephone interviews. Non-English-speaking patients (n = 2) were interviewed with the assistance of a translator. All audio-recorded focus groups and interviews were conducted by one researcher who was not involved in the direct care of any of the participants. On average, the focus groups lasted 1 hour and interviews 20 minutes. They took place between April and July 2014. Audio-recordings were transcribed verbatim and transcripts analysed using a framework analysis approach. A second rater (HL) appraised the emergent themes from the transcripts and consensus between both researchers was reached. To improve the validity of the data, the researcher referred back to the original transcripts throughout the analysis, including deviant accounts.
Developing patient handbook
The development of the patient handbook took place between May 2013 and April 2014 and involved five stages (Figure 26).
FIGURE 26.
Development of handbook based on the plan–do–study–act framework.
Two researchers collected information, including current treatments for RA, intensive management in the TITRATE trial and managing life with RA. The information was gathered from evidence-based sources, for example publications and current clinical guidelines, expert medical and allied health practitioners, and online from national charities (e.g. NRAS and Arthritis Research UK). It was then collated by one of the researchers into a draft handbook across nine chapters. One patient workshop was organised at an inner-city NHS foundation trust in June 2013. The workshop did not require ethics approval as its purpose was categorised as service development. The inclusion criteria comprised adults aged > 18 years with a confirmed diagnosis of RA, who were able to understand and communicate sufficiently in English to participate. All patients who agreed to take part were sent a participant information sheet describing the purpose of the workshop and its planned procedure. A week prior to the workshop, patients were sent a copy of the draft handbook. They were asked to read and consider the content and layout of the handbook and encouraged to make notes of any initial thoughts or feedback for the meeting.
The researchers who collected the information co-facilitated the workshop, which was conducted in a private room. Participants were asked to sign a written consent form before the start of the workshop. The consent form informed participants that taking part was voluntary and withdrawal from the workshop would not affect the care they receive from the outpatient clinic. It also stated that the workshop was being audio-recorded and anonymised quotations from the recording would be used in the future. The workshop began with a brief presentation and explanation about the purpose of the handbook within the context of the TITRATE trial. The draft handbook chapters were used to structure the remainder of the workshop. Taking each chapter in turn, the group was invited to provide feedback on all aspects of the draft document. There was a specific focus on the chapters about ‘intensive treatment for RA’ and ‘psychosocial support’ because these are key aspects of the intensive management intervention. The lead author took field notes during the workshop. The audio-recording was transcribed verbatim and the transcript and notes from the workshop analysed, applying thematic content analysis. This involved ‘identifying, analysing, and reporting patterns (themes) within data’. The researcher referred back to the original transcript throughout the analysis to confirm that participants’ accounts were presented accurately.
Using the audio-recording and notes written during the workshop, feedback from participants relating to the content and layout was incorporated into a revised version of the handbook. Following participants’ suggestions, selected anonymous testimonies that were expressed during the workshop were included to add context and personalise the content of the handbook. A further round of comments was then arranged for all workshop participants and an additional patient who was unable to attend the workshop. Participants were sent the revised version of the handbook via e-mail and asked to make any additional comments and send them to the researchers. A final version of the handbook was sent to all who contributed to the development for approval prior to printing.
Patients’ and practitioners’ views of intensive treatment
Data collection took place between February 2016 and September 2017. The semistructured topic guides for both groups were developed based on constituent parts of the intensive management intervention. These were discussed with the multidisciplinary research team and a patient expert who provided feedback on their suitability and relevance.
Individual interviews were conducted with patients and practitioners both face to face (n = 8) and over the telephone (n = 20). One group interview was also held with three practitioners. Interviews with patients were arranged for a date after they had completed all their intensive management sessions in case the interview influenced their views of the intervention or the trial. No pilot interviews were held; however, slight adjustments were made to the topic guides following the first two interviews with each group. The audio-recorded interviews were carried out by the lead author who was not involved in the care of any of the patients. The interviews with the practitioners were considered service evaluation and, therefore, ethics approval was not required for these.
Audio-recordings of the interviews were transcribed verbatim by an external professional transcribing agency. Both sets of transcripts were analysed using thematic analysis and iterative categorisation (supported by NVivo). Iterative categorisation generates a clear audit trial with the data analysis, closely linked to the raw data, and involves four stages: (1) familiarisation through the reading of transcripts, (2) line-by-line coding to organise the data in preparation for analysis, (3) descriptive analysis that identifies themes and (4) interpretive analysis that explores patterns, inconsistencies and relates findings to existing knowledge. To validate the data, a second experienced qualitative researcher cross-referenced the emergent themes with the lead researcher, and consensus between both researchers was reached.
Systematic reviews
Narrative descriptions
The reviews on RA guidelines, treat to target, psychological interventions (review of reviews), MI and nurse care in RA did not include any meta-analyses and only narrative descriptions of the findings were provided.
Meta-analysis of erosive progression
The means and SDs of the Larsen or Sharp–van der Heijde score were recorded at each follow-up time for each study. In cases where only a median score was obtained, the median and range were converted into a mean score and SD. To estimate annual rates of change, with standard errors, a linear regression model was conducted with follow-up year as the independent variable. Baseline scores and annual progression rates, with standard errors, were transformed into percentage maximum damage for each scoring method. These transformed scores were assessed using random-effects meta-analysis. Analysis used Stata (version 13). Further details are given in Carpenter et al. 52
To assess the strength of predictive markers, the regression coefficients and ORs with 95% CIs were collated. Unadjusted effect estimates were sought. Where these were not reported, the adjusted estimates were used. Random-effects meta-analysis was used for all models because of the likely high level of heterogeneity between studies. Analysis used Stata (version 13). Significance was assumed at a p-value < 0.05.
Meta-analysis of remissions with intensive management
Results were analysed using Review Manager 5.3 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark). The random-effects model, based on the DerSimonian and Laird method,79 was used to estimate the pooled effect sizes. This gives more equal weighting to studies of different precision in comparison with a simple inverse variance weighted approach, thereby accommodating between-study heterogeneity. For all meta-analyses, we performed Cochrane’s chi-squared test to assess between-study heterogeneity and quantified I2 statistics. 80 p-values of < 0.05 were considered significant.
Some of the randomised controlled trials had more than two treatment arms. When there were two control groups the results were combined and when there were two or more intensive treatment groups only those reporting licensed dosage regimens were included.
TITRATE trial details of imputation for primary and secondary outcome missing observations
Outcomes assessed every 6 months
Multiple imputation using chained equations with PMM using five nearest neighbours was used.
In subjects who had missing outcomes at 6 months, under the monotone assumption, baseline outcomes and explanatory covariates were used to impute the missing values at 6 months. For patients who had missing outcomes at 12 months, baseline and 6-month outcomes with explanatory covariates were used to impute the missing values. If outcome variables were missing at 6 and 12 months, then the outcome variables at 6 months was imputed first, followed by the outcomes at 12 months.
Number of cycles
The imputation was 20 cycles. At the end of the cycle, one imputed data set was created. The process was repeated to create 20 imputed data sets. The 20 data sets were combined using Rubin’s rules. 471,472 Therefore, the estimates and standard errors presented here are the combined ones.
Appendix 4 Additional details of the TITRATE trial
Complete-case/completer analyses
Baseline data and numbers analysed
This analysis evaluated patients in whom all data were present and who also followed the protocol. It evaluated 258 patients (134 patients received intensive management and 124 patients received standard care). Demographic and disease assessments were similar in both the groups (Table 34). The analysis complements the ITT analyses in The TITRATE trial.
| Assessment | Treatment group | |
|---|---|---|
| Intensive management (N = 134) | Standard care (N = 124) | |
| Age (years) | 56.5 (11.9) | 56.8 (11.8) |
| Disease duration (years) | 6.5 (7.3) | 5.1 (5.3) |
| Female | 114 (85%) | 99 (80%) |
| DAS28-ESR | 4.3 (0.5) | 4.3 (0.5) |
| DAS28-CRP | 4.5 (0.6) | 4.6 (0.6) |
| CDAI | 19.8 (6.6) | 20.6 (7.0) |
| SDAI | 20.7 (6.3) | 21.3 (6.6) |
| Tender joint counts (68 joints) | 12 (9) | 14 (9) |
| Swollen joint counts (66 joints) | 6 (5) | 6 (4) |
| ESR (mm/hour) | 18 (14) | 14 (12) |
| CRP (mg/l) | 9 (12) | 7 (7) |
| Assessor global rating (mm) | 40 (22) | 39 (18) |
| Patient global assessment (mm) | 44 (19) | 48 (22) |
| Fatigue VAS (mm) | 59 (26) | 52 (26) |
| Pain VAS (mm) | 41 (23) | 43 (23) |
| HAQ | 1.2 (0.7) | 1.2 (0.7) |
| EQ-5D-5L | 0.72 (0.16) | 0.69 (0.21) |
| Larsen score | 12 (18) | 8 (11) |
Primary outcome
Remissions based on DAS28-ESR score were more frequent with intensive management. Remissions occurred in 43 of 134 (32%) patients receiving intensive management and in 23 of 124 (19%) patients receiving standard care, with a highly significant unadjusted OR of 2.07 (95% CI 1.16 to 3.70; p = 0.014). The adjusted OR was also significantly different, as shown in Table 35.
| Remission classification | Analyses | |||
|---|---|---|---|---|
| Unadjusted | Adjusteda | |||
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| DAS28-ESR | 2.07 (1.16 to 3.70) | 0.014 | 2.39 (1.27 to 4.49) | 0.007 |
| DAS28-CRP | 2.73 (1.30 to 5.76) | 0.008 | 2.94 (1.32 to 6.53) | 0.008 |
| SDAI | 1.89 (0.93 to 3.82) | 0.077 | 1.90 (0.92 to 3.94) | 0.082 |
| CDAI | 1.97 (0.98 to 3.98) | 0.058 | 2.02 (0.98 to 4.15) | 0.056 |
| ACR/EULAR Boolean | 2.61 (1.10 to 6.20) | 0.030 | 2.65 (1.08 to 6.48) | 0.033 |
Other remission criteria and low disease activity at 12 months
Simple Disease Activity Index, DAS28-CRP, CDAI and ACR/EULAR Boolean remissions at 12 months showed higher achievement of remission with intensive management (21%, 19%, 27% and 20%, respectively) than standard care (9%, 11%, 14% and 8%, respectively) (Figure 27). Logistic regression analyses showed that most of these differences were also significant (see Table 35).
FIGURE 27.
Remissions with intensive treatment and standard care: complete-case analyses.
A total of 61 out of 134 (46%) patients receiving intensive management and 39 out of 124 (31%) patients receiving standard care had low disease activity, as measured using the DAS28-ESR (unadjusted OR 1.84, 95% CI 1.10 to 3.08; p = 0.020).
Clinical outcomes at 12 months
The mean DAS28-ESR scores were significantly lower (p = 0.002 in unadjusted and p = 0.006 in adjusted regression analyses) with intensive treatment (see Appendix 5, Table 51). The mean tender and swollen joint counts and assessor and patient global scores were lower with intensive management (Table 36). These differences were significant in unadjusted and adjusted linear regression analyses. The mean ESR and CRP levels were unchanged during the trial, with no significant differences between groups. There were only small improvements in disability assessed by mean HAQ and quality of life assessed by mean EQ-5D. Differences between groups were not significant (see Table 36). The mean pain and fatigue scores were significantly lower with intensive management in unadjusted and adjusted linear regression analyses.
| Assessment | Treatment group, mean (SD) | Linear regression | ||||
|---|---|---|---|---|---|---|
| Intensive management (n = 134) | Standard care (n = 124) | Unadjusted coefficients (95% CI) | p-value | Adjusted coefficientsa (95% CI) | p-value | |
| DAS28-ESR | 3.4 (1.5) | 3.9 (1.4) | –0.54 (–0.88 to –0.20) | 0.002 | –0.49 (–0.83 to –0.14) | 0.006 |
| Tender joint counts (68 joints) | 7.5 (8.6) | 11.1 (9.1) | –2.31 (–4.59 to –0.02) | 0.048 | –2.72 (–4.72 to –0.72) | 0.008 |
| Swollen joint counts (66 joints) | 3.6 (4.3) | 5.3 (6.1) | –1.98 (–3.31 to –0.65) | 0.004 | –1.72 (–2.93 to –0.51) | 0.006 |
| ESR (mm/hour) | 17 (18) | 15 (15) | –1.34 (–4.11 to 1.43) | 0.342 | –1.15 (–3.79 to 1.50) | 0.394 |
| CRP (mg/l) | 10 (22) | 7 (9) | 0.64 (–3.19 to 4.47) | 0.743 | 1.47 (–1.83 to 4.78) | 0.380 |
| Assessor global rating (mm) | 23 (21) | 31 (22) | –7.48 (–13.78 to –1.18) | 0.020 | –8.27 (–13.65 to –2.89) | 0.003 |
| Patient global assessment (mm) | 30 (25) | 41 (25) | –7.29 (–14.57 to –0.01) | 0.050 | –10.64 (–16.89 to –4.39) | 0.001 |
| Fatigue VAS (mm) | 40 (30) | 50 (30) | –16.84 (–24.19 to –9.50) | < 0.001 | –14.33 (–20.93 to –7.72) | < 0.001 |
| Pain VAS (mm) | 28 (25) | 38 (29) | –7.93 (–15.30 to –0.56) | 0.035 | –9.76 (–16.28 to –3.24) | 0.004 |
| HAQ | 1.05 (0.71) | 1.13 (0.77) | –0.11 (–0.23 to 0.01) | 0.083 | –0.11 (–0.22 to 0.01) | 0.078 |
| EQ-5D-5L | 0.76 (0.19) | 0.72 (0.22) | 0.02 (–0.03 to 0.06) | 0.486 | 0.03 (–0.01 to 0.07) | 0.155 |
Clinical outcomes over 6 and 12 months
Longitudinal analyses assessed changes over both 6 and 12 months using mixed-effects models (Table 37). Unadjusted and adjusted analyses showed significant differences between groups for DAS28-ESR, swollen joint counts for 66 joints and fatigue.
| Assessment | Mixed-effect model | |||
|---|---|---|---|---|
| Unadjusted coefficients (95% CI) | p-value | Adjusted coefficientsa (95% CI) | p-value | |
| DAS28-ESR | –0.24 (–0.43 to –0.05) | 0.012 | 0.22 (–0.41 to –0.03) | 0.022 |
| Tender joint counts (68 joints) | –0.66 (–2.14 to 0.82) | 0.384 | –0.94 (–2.25 to 0.37) | 0.159 |
| Swollen joint counts (66 joints) | –1.02 (–1.81 to –0.24) | 0.011 | –0.89 (–1.58 to –0.20) | 0.012 |
| ESR (mm/hour) | –0.68 (–2.23 to 0.88) | 0.392 | –0.53 (–1.96 to 0.90) | 0.464 |
| CRP (mg/l) | 0.31 (–1.63 to 2.24) | 0.756 | 1.02 (–0.59 to 2.62) | 0.215 |
| Assessor global rating (mm) | –2.95 (–6.65 to 0.76) | 0.119 | –3.59 (–6.61 to –0.57) | 0.020 |
| Patient global assessment (mm) | –3.42 (–7.52 to 0.68) | 0.102 | –5.45 (–8.92 to -1.98) | 0.002 |
| Fatigue VAS (mm) | –9.55 (–13.61 to –5.49) | < 0.001 | –8.05 (–11.71 to –4.39) | < 0.001 |
| Pain VAS (mm) | –3.37 (–7.77 to 1.02) | 0.132 | –4.74 (–8.40 to –1.08) | 0.011 |
| HAQ | –0.04 (–0.11 to 0.03) | 0.218 | –0.04 (–0.11 to 0.02) | 0.211 |
| EQ-5D-5L | 0.004 (–0.02 to 0.03) | 0.736 | 0.01 (–0.01 to 0.03) | 0.296 |
Response predictors and response persistence
Studies of response predictors evaluated the 298 patients in whom 12-month DAS28-ESR and fatigue measures were present. Studies of response persistence were restricted to the 95 patients who were assessed at 18 months. Baseline assessments of these patients are shown in Table 38. These analyses are reported in Response predictors and persistence in the TITRATE trial. Additional baseline assessments related to ethnicity, smoking and alcohol consumption, psychological assessments and BMI are shown in Table 39. Changes in disease activity states in these patients at 12 and 18 months are shown in Table 40 and Figure 28.
| Assessment | Treatment group, 12-month DAS28-ESR and fatigue scores | Intensive management with 18-month data | |||
|---|---|---|---|---|---|
| Intensive management | Standard care | All patients | Remission | ||
| None/one | Two or more | ||||
| Patients, n | 148 | 150 | 95 | 48 | 47 |
| Age (years), mean (SD) | 56.4 (12.2) | 56.9 (11.9) | 56.4 (11.2) | 57.5 (10.2) | 55.2 (12.2) |
| Disease duration (years), mean (SD) | 6.5 (7.1) | 5.1 (5.3) | 6.8 (7.2) | 7.0 (7.7) | 6.7 (6.8) |
| Female, n (%) | 122 (82) | 119 (79) | 74 (78) | 41 (85) | 33 (70) |
| DAS28-ESR, mean (SD) | 4.4 (0.5) | 4.3 (0.5) | 4.4 (0.5) | 4.5 (0.5) | 4.3 (0.6) |
| Pain, mean (SD) | 41 (22) | 43 (22) | 41 (23) | 41 (22) | 40 (24) |
| Fatigue, mean (SD) | 59 (25) | 51 (25) | 60 (25) | 64 (22) | 57 (27) |
| HAQ, mean (SD) | 1.25 (0.66) | 1.22 (0.67) | 1.30 (0.64) | 1.39 (0.60) | 1.20 (0.66) |
| Assessment | Treatment group | |
|---|---|---|
| Intensive management (N = 168) | Standard care (N = 167) | |
| Ethnicity | ||
| White | 156 | 147 |
| Black | 6 | 6 |
| Asian | 3 | 9 |
| Mixed | 1 | 4 |
| Other | 2 | 1 |
| Alcohol | ||
| Current | 120 | 118 |
| Smoking status | ||
| Current | 29 | 23 |
| Ever | 105 | 104 |
| Depression | ||
| PHQ-9 score of ≥ 10 | 60 | 59 |
| Anxiety | ||
| GAD-7 score ≥ 10 | 33 | 33 |
| BMI (kg/m2) | ||
| < 25 | 56 | 55 |
| 25–30 | 56 | 64 |
| 30–35 | 28 | 33 |
| > 35 | 28 | 14 |
| 12-month state | Per cent at 12 months | 18-month states | |||
|---|---|---|---|---|---|
| Remission | Low | Moderate | High | ||
| Remission | 30% | 11% | 8% | 9% | 3% |
| Low | 17% | 3% | 5% | 5% | 3% |
| Moderate | 42% | 11% | 9% | 15% | 8% |
| High | 11% | 1% | 0% | 4% | 5% |
FIGURE 28.
Disease Activity Score for 28 joints based on the ESR in patients receiving intensive management in the TITRATE trial by remission status. Patients divided into groups of patients who never achieved remission, patients who had one or more remissions without achieving a final 12-month remission and patients who achieved final 12-month remission. Means and standard errors shown.
Appendix 5 Additional tables for health economic evaluation
This section provides additional tables (Tables 41–54) referenced in the main economic results in Health economic evaluation of the TITRATE trial.
| Resource use item | Unit cost | Pack cost and costing assumptions | Source |
|---|---|---|---|
| Methotrexate (oral) | 5-mg dose: £0.14 | £1.73 for 24 tablets (2.5 mg) | BNF 2018327 |
| 10-mg dose: £0.95 | £47.50 for 100 tablets (10 mg) | ||
| Sulfasalazine | 500-mg dose: £0.08 | £8.43 for 112 tablets (500 mg) | BNF 2018327 |
| Leflunomide | 10-mg dose: £0.24 | £7.08 for 30 tablets (10 mg) | BNF 2018327 |
| 20-mg dose: £0.24 | £7.33 for 30 tablets (20 mg) | ||
| Hydroxychloroquine | 200-mg dose: £0.09 | £5.46 for 60 tablets (200 mg) | BNF 2018327 |
| Azathioprine | 25-mg dose: £0.06 | £1.62 for 28 tablets (25 mg) | BNF 2018327 |
| 50-mg dose: £0.04 | £2.25 for 56 tablets (50 mg) | ||
| Penicillamine | 250-mg dose: £1.59 | £88.77 for 56 tablets (250 mg) | BNF 2018327 |
| Gold injections | 20-mg dose: £4.56 | £45.55 for solution for injection (20 mg) | BNF 2018327 |
| Folic acid | 5-mg dose: £0.02 | £0.66 for 28 tablets (5 mg) | BNF 2018327 |
| Etanercept | 50-mg dose: £169.81 | £679.25 for four solutions for injection (50 mg) | BNF 2018327 |
| Adalimumab | 40-mg dose: £352.14 | £704.28 for two solutions for injections (40 mg). We used the average NHS indicative price because there was no drug tariff price | BNF 2018327 |
| Rituximab | 500-mg dose: £1266.07 | £1266.07 for one solution for injection (500 mg). We used the average NHS indicative price because there was no drug tariff price | BNF 2018327 |
| 1000-mg dose: £2532.14 | |||
| Abatacept (subcutaneous) | 125-mg dose: £302.40 | £1209.60 for four solutions for injection (125 mg). We used the average NHS indicative price because there was no drug tariff price | BNF 2018327 |
| Golimumab | 50-mg dose: £762.97 | £762.97 for one solution for injection (50 mg) | BNF 2018327 |
| 100-mg dose: £1525.94 | £1525.94 for one solution for injection (100 mg). We used the NHS indicative price because there was no drug tariff price | ||
| Tocilizumab (subcutaneous) | 162-mg dose: £228.28 | £913.12 for four solutions for injections (162 mg) | BNF 2018327 |
| Certolizumab pegol | 200-mg dose: £357.50 | £715.00 for two solutions for injections (200 mg). We used the average NHS indicative price because there was no drug tariff price | BNF 2018327 |
| 400-mg dose: £715.00 | |||
| Methylprednisolone | 1-gram dose: £17.30 | £17.30 1 g of powder | BNF 2018327 |
| 120-mg dose: £8.96 | £8.96 for one suspension for injection (120 mg) | ||
| 80-mg dose: £6.18 | £6.18 for one suspension for injection (80 mg) | ||
| 40-mg dose: £3.44 | £3.44 for one suspension for injection (40 mg) | ||
| 4-mg dose: £0.21 | £6.19 for 30 tablets (4 mg). We used the average NHS indicative price because there was no drug tariff price | ||
| Prednisone | 1-mg dose: £0.89 | £26.70 for 30 tablets (1 mg) | BNF 2018327 |
| 2-mg dose: £0.89 | £26.70 for 30 tablets (2 mg) | ||
| 5-mg dose: £0.89 | £26.70 for 30 tablets (5 mg). We used the average NHS indicative price because there was no drug tariff price | ||
| Ibuprofen | 200-mg dose: £0.04 | £0.87 for 24 tablets (200 mg) | BNF 2018327 |
| 400-mg dose: £0.06 | £1.39 for 24 tablets (400 mg) | ||
| 600-mg dose: £0.05 | £3.69 for 84 tablets (600 mg) | ||
| Diclofenac | 74-mg dose: £0.06 | £12.95 for 200 units of mouth wash (74 mg) | BNF 2018327 |
| Celecoxib | 100-mg dose: £0.04 | £2.19 for 60 tablets (100 mg) | BNF 2018327 |
| 200-mg dose: £0.06 | £1.88 for 30 tablets (200 mg) | ||
| Naproxen | 250-mg dose: £0.02 | £1.02 for 56 tablets (200 mg) | BNF 2018327 |
| 500-mg dose: £0.05 | £1.41 for 28 tablets (500 mg) | ||
| Etodolac | 600-mg dose: £0.52 | £15.50 for 30 tablets (600 mg) | BNF 2018327 |
| Etoricoxib | 30-mg dose: £0.50 | £13.99 for 28 tablets (30 mg) | BNF 2018327 |
| 60-mg dose: £0.13 | £3.58 for 28 tablets (60 mg) | ||
| 90-mg dose: £0.15 | £4.08 for 28 tablets (90 mg) | ||
| Meloxicam | 15-mg dose: £0.04 | £1.13 for 30 tablets (15 mg) | BNF 2018327 |
| Nabumetone | 500-mg dose: £0.12 | £6.90 for 56 tablets (500 mg) | BNF 2018327 |
| Tiaprofenic acid | 300-mg dose: £0.24 | £14.95 for 56 tablets (300 mg) | BNF 2018327 |
| Alendronic acid | 70-mg dose: £0.16 | £0.64 for 56 tablets (70 mg) | BNF 2018327 |
| Risedronate sodium | 35-mg dose: £0.22 | £0.88 for four tablets (35 mg) | BNF 2018327 |
| Zoledronic acid | 5-mg dose: £997.22 | £997.22 for one solution for infusion vials (5 mg) | BNF 2018327 |
| Calcium and ergocalciferol | 300-mg dose: £0.88 | £24.64 for 28 tablets (300 mg) | BNF 2018327 |
| Omeprazole | 20-mg dose: £0.24 | £0.73 for 28 tablets (20 mg) | BNF 2018327 |
| 40-mg dose: £0.98 | £6.84 for seven tablets (40 mg) | ||
| Lansoprazole | 15-mg dose: £0.03 | £0.73 for 28 tablets (15 mg) | BNF 2018327 |
| 30-mg dose: £0.04 | £1.07 for 28 tablets (30 mg) | ||
| Ranitidine | 150-mg dose: £0.02 | 0.96 for 60 tablets (150 mg) | BNF 2018327 |
| 300-mg dose: £0.03 | 0.95 for 30 tablets (300 mg) | ||
| Acetaminophen | 500-mg dose: £0.02 | £0.50 for 32 tablets (500 g) | BNF 2018327 |
| 1000-mg dose: £0.03 | |||
| Co-codamol | 8-mg dose: £0.03 | £0.76 for 100 tablets (8 mg) | BNF 2018327 |
| 15-mg dose: £0.05 | £4.59 for 100 tablets (15 mg) | ||
| 30-mg dose: £0.03 | £3.40 for 100 tablets (30 mg) | ||
| Co-dydramol | 10-mg dose: £0.02 | £0.73 for 30 tablets (10 mg) | BNF 2018327 |
| Codeine phosphate | 30-mg dose: £0.03 | £0.93 for tablets (30 mg) | BNF 2018327 |
| 60-mg dose: £0.05 | £1.45 for tablets (60 mg) | ||
| Tramadol hydrochloride | 50-mg dose: £0.08 | £4.6 for tablets (50 mg) | BNF 2018327 |
| 100-mg dose: £0.24 | £14.47 for tablets (100 mg) | ||
| Methylprednisolone acetate | 40-mg dose: £3.44 | £3.44 for one 40 mg/1 ml suspension for injection | BNF 2018327 |
| 80-mg dose: £6.18 | £6.18 for one 80 mg/1 ml suspension for injection | ||
| 125-mg dose: £4.75 | £4.75 for one powder and solvent for solution for injection vial (125 mg). We used the NHS indicative price because there was no drug tariff price | ||
| Prednisolone acetate | 2.5-mg dose: £0.03 | £0.97 for 28 tablets (5 mg) | BNF 2018327 |
| 5-mg dose: £0.03 | £0.96 for 28 tablets (2.5 mg) | ||
| 20-mg dose: £0.13 | We used the NHS indicative price because there was no drug tariff price | ||
| Triamcinolone acetonide | 10-mg dose: £0.89 | £4.47 for five suspension for injection ampoules (10 mg) | BNF 2018327 |
| 40-mg dose: £1.49 | £7.45 for five suspension for injection ampoules (40 mg) | ||
| Methotrexate (subcutaneous) | 7.5-mg dose: £13.37 | £13.37 for one solution for injection (7.5 mg) | BNF 2018327 |
| 10-mg dose: £13.77 | £13.77 for one solution for injection (10 mg) | ||
| 12.5-mg dose: £14.85 | £14.85 for one solution for injection (12.5 mg) | ||
| 15-mg dose: £14.92 | £14.92 for one solution for injection (15 mg) | ||
| 17.5-mg dose: £15.75 | £15.75 for one solution for injection (17.5 mg) | ||
| 22.5-mg dose: £16.61 | £16.61 for one solution for injection (22.5 mg) | ||
| 25-mg dose: £16.64 | £16.64 for one solution for injection (25 mg) | ||
| Abatacept (i.v.) | 125-mg dose: £302.40 | £1209.60 for four solutions for injection. We used the NHS indicative price because there was no drug tariff price | BNF 2018327 |
| Tocilizumab (i.v.) | 162-mg dose: £228.28 | £913.12 for four solutions for injection | BNF 2018327 |
| Paracetamol | 500-mg dose: £0.01 | £0.50 for 100 tablets (500 mg) | BNF 2018327 |
| 1 g dose: £0.03 | £2.50 for 100 tablets (1 g). We used the NHS indicative price for 1 g because there was no drug tariff price | ||
| Hydrocortisone | 20-mg dose: £2.76 | £82.76 for 30 tablets (20 mg) | BNF 2018327 |
| Prednisolone | 5-mg dose: £0.03 | £3.55 for 28 tablets (20 mg) | BNF 2018327 |
| 10-mg dose: £0.07 | £1.90 for 28 tablets (10 mg) | ||
| 20-mg dose: £0.13 | £0.70 for 28 tablets (5 mg) | ||
| Predisolone | 1-mg dose: £0.02 | £0.57 for 29 tablets (1 mg) | BNF 2018327 |
| 10-mg dose: £0.03 | £0.70 for 29 tablets (10 mg) | ||
| Pregabaline | 300-mg dose: £0.09 | £5.08 for 56 tablets (300 mg) | BNF 2018327 |
| Amitriptyline | 10-mg dose: £0.04 | £1.09 for 28 tablets (10 mg) | BNF 2018327 |
| 25-mg dose: £0.03 | £0.85 for 28 tablets (25 mg) | ||
| 50-mg dose: £0.10 | £2.88 for 28 tablets (50 mg) | ||
| Vitamin D | 400-unit dose: £0.10 | £8.42 for 84 capsules (400 units) | BNF 2018327 |
| 4000-unit dose: £0.08 | £6.75 for 84 capsules (4000 units) | ||
| Gabapentin | 100-mg dose: £0.02 | £2.16 for 100 capsules (100 mg) | BNF 2018327 |
| i.m. depomedrone | 120-mg dose: £8.96 | £8.96 for one suspension for injection vials (120 mg) | BNF 2018327 |
| Kenalog (Bristol Myers Squibb™, New York, NY, USA) | 40-mg dose: £1.49 | £7.45 for five suspension for injection vials (120 mg) | BNF 2018327 |
| Benepali (Biogen Biosimilars, Maidenhead, UK) | 50-mg dose: £167.88 | £671.50 for four solutions for injection (pre-filled vials). We used the average NHS indicative price for 50 mg because there was no drug tariff price | BNF 2018327 |
| Oramorph® (C.H. Boehringer Sohn AG & Co. KG, Ingelheim am Rhein, Germany) | 10-mg dose: £0.02 | £5.45 for oral solution (10 mg) | BNF 2018327 |
| Salbutamol | 100 µg: £0.02 | £3.31 for 200 units of inhalation powder (100 µg) | BNF 2018327 |
| Dihydrocodeine | 30-mg dose: £0.03 | £0.90 for 28 tablets (30 mg) | BNF 2018327 |
| Morphine sulphate SR | 10-mg dose: £0.09 | £5.20 for 60 tablets (10 mg) | BNF 2018327 |
| Mycophenolate mofetil | 500-mg dose: £0.13 | £6.53 for 50 tablets (500 mg) | BNF 2018327 |
| Rabeprazole | 10-mg dose: £0.04 | £1.24 for 28 tablets (10 mg) | BNF 2018327 |
| Cetirizine | 10-mg dose: £0.03 | £0.80 for 30 tablets (10 mg) | BNF 2018327 |
| Ibuprofen gel | 50-mg dose: £0.02 | £1.13 for 50 units of gel (50 mg) | BNF 2018327 |
| Piroxicam 0.5% gel (Accord-UK Ltd, Barnstaple, UK) | 0.5% dose: £0.03 | £1.79 for 60 units of gel (0.5%) | BNF 2018327 |
| Fenbid gel 5% (ADVANZ Pharma, London, UK) | 50-mg dose: £0.02 | £1.13 for 50 units of gel (5%). Unit cost based on ibuprofen gel | BNF 2018327 |
| Colecalciferol | 800-unit dose: £0.12 | £3.6 for 43 tablets (800 units) | BNF 2018327 |
| Baricitinib | 2-mg dose: £28.77 | £805.56 for 28 tablets (2 mg) | BNF 2018327 |
| 4-mg dose: £28.77 | £805.56 for 28 tablets (4 mg) | ||
| Fultium D3 | 400-unit dose: £0.07 | £1.85 for 28 tablets (400 units) | BNF 2018327 |
| 800-unit dose: £0.07 | £3.60 for 30 tablets (800 units) | ||
| Lidocaine | 100-mg dose: £0.44 | £4.40 for 10 100-mg ampoules for injection | BNF 2018327 |
| Buprenorphine patch | 10-mg dose: £7.89 | £31.55 for four 10 µg/hour. We used the price for transdermal patches as buprenorphine patch not listed in the BNF327 | BNF 2018327 |
| Salbutamol | 100 µg/dose: £0.02 | £3.31 for 200 100 µg/dose of dry powder | BNF 2018327 |
| Depo-medrone with lidocaine | 40-mg dose: £3.89 | £38.88 for 10 suspension for injection vials (40 mg/1 ml) | BNF 2018327 |
| Cyclizine | 50-mg dose: £0.07 | £7.45 for 100 tablets (50 mg) | BNF 2018327 |
| Pregabilin | 50-mg dose: £0.07 | £6.16 for 84 tablets (50 mg) | BNF 2018327 |
| Depo-medrone | 80-mg dose: £6.18 | £6.18 for one suspension for injection vials (80 mg/2 ml) | BNF 2018327 |
| 120-mg dose: £8.96 | £8.96 for one suspension for injection vials (120 mg/3 ml) | ||
| Arcoxia® [Organon Pharma (UK) Ltd, London, UK] | 90-mg dose: £0.15 (120 mg/3 ml) | £4.08 for 28 tablets (90 mg) | BNF 2018327 |
| Celebrex® (Upjohn UK Ltd, Sandwich, UK) | 200-mg dose: £0.06 | £1.88 for 30 capsules (200 mg) | BNF 2018327 |
| Movelat Gel (Genus Pharmaceuticals, Huddersfield, UK) | 50-mg dose: £0.02 | £1.13 for 50 gel tablets (50 mg). We used the price of ibuprofen tablets as movelat is not listed in the BNF327 | BNF 2018327 |
| Voltarol gel (GlaxoSmithKline plc, Brentford, UK) | 50-mg dose: £0.26 | £7.94 for 30 tablets (50 mg). We used the price of voltarol tablets as voltarol is not listed in BNF327 | BNF 2018327 |
| Linctus | 6.25-mg dose: £0.01 | £1.29 for 200 tablets (6.25 mg) | BNF 2018327 |
| Hydroxocobalamine | 1 mg/1 ml dose: £1.44 | £7.22 for five solutions for injection ampoules (1 mg/1 ml) | BNF 2018327 |
| Antifungal cream | 0.1% cream dose: £0.19 | £2.83 for 0.1% cream. We used the price of hydrocortisone cream | BNF 2018327 |
| Practitioner | Care sector | Unit cost (£) | Assumptions | Source |
|---|---|---|---|---|
| GP clinical visit or telephone conversation | Primary care | 37.40 | Per patient contact lasting 9.22 minutes. Unit cost includes carbon emissions (5 kgCO2e) (carbon costs < £1) direct care staff costs and qualification costs | PSSRU 2018/19 (p. 127)328 |
| GP home visit | Primary care | 100.88 | Assume 15 minutes of GP travel time per visit. Home visit lasting 10.22 minutes [PSSRU 2018/19 (p. 126)328]. Per minute of patient contact in PSSRU 2018/19 is £4 (p. 126). Unit cost includes direct care staff costs and qualification cost 328 | PSSRU 2018/19 (p. 127)328 |
| Practice nurse visit or telephone conversation | Primary care | 42 | Cost per hour, including qualifications | PSSRU 2018/19 (p. 125)328 |
| Physiotherapist home visit at participants home, general practice or elsewhere | Primary care | 45.99 | NHS band 5 (£34 per working hour). Assume a visit lasting 1 hour. In addition, 15 minutes of physiotherapist travel time per visit and a travel cost of a 4-mile return journey at the NHS reimbursement rate of 56p per mile | PSSRU 2018/19 (p. 119)328 |
| NHS occupational therapist visits at participants home, general practice or elsewhere | Primary care | 45.99 | NHS band 5 (£34 per working hour). Assume a visit lasting 1 hour. In addition, 15 minutes of physiotherapist travel time per visit and a travel cost of a 4-mile return journey at the NHS reimbursement rate of 56p per mile | PSSRU 2018/19 (p. 119)328 |
| Clinical visit to the hospital | Secondary care | 105 | A visit to the hospital was assumed to be to see a RA specialist. Cost is based on 1 hour for an associate specialist hospital (£105) | PSSRU 2018/19 (p. 161)328 |
| Meals on Wheels | PSS | 4.40 per meal | Based on the England average cost per meal | National Association of Care Catering329 |
| Home help | PSS | 29.74 | Based on the price multipliers for independent sector home care provided for social services: £22 per weekday hour. Assume a visit lasting 1 hour. In addition, 15 minutes of staff travel time per visit and a travel cost of a 4-mile return journey at the NHS reimbursement rate of 56p per mile | PSSRU 2018/19 (p. 142)328 |
| Social worker (e.g. contacted on the telephone) | PSS | £60 per hour. Unit cost includes qualification costs | PSSRU 2018/19 (p. 139)328 | |
| Participants option to self-report to any other health or social services (e.g. podiatrist) | NHS or PSS | 34.00 | NHS band 5 (£34 per working hour). Assume a 1-hour appointment | PSSRU 2018/19 (p. 119)328 |
| Transport to and from health-care appointments | NHS or PSS | Self-reported | Participant report on how much this cost them | NA |
| Absence from paid work due to RA of the participant or care from their friends or relatives | Indirect cost | 92 per day and £12.78 per hour | Median full-time weekly earnings is £460. Median per day: £92 (assuming a 5-day working week). Median hourly earnings: £12.78 | Office for National Statistics330 |
| Variable with missing data | Number of missing observations (% missing) | Mean without imputation of missing values (SD) | Mean with imputation of missing values (SD) |
|---|---|---|---|
| Health-related quality of life estimated using van Hout et al.473 mapping | |||
| Health utility score at 6 months | 24 (7.1) | 0.625 (0.212) | 0.624 (0.207) |
| Health utility score at 12 months | 29 (8.7) | 0.648 (0.210) | 0.646 (0.204) |
| Health-related quality of life estimated using EQ-5D-5L scores | |||
| Health utility score at 6 months | 24 (7.1) | 0.724 (0.203) | 0.722 (0.196) |
| Health utility score at 12 months | 29 (8.7) | 0.745 (0.201) | 0.743 (0.195) |
| Health-related quality of life estimated using Hernández-Alava and Pudney’s334 mapping based on the EuroQol Group data set | |||
| Health utility score at 6 months | 24 (7.1) | 0.610 (0.210) | 0.609 (0.205) |
| Health utility score at 12 months | 29 (8.7) | 0.637 (0.204) | 0.635 (0.199) |
| Health-related quality of life estimated using Hernández-Alava and Pudney’s334 mapping based on FORWARD: National Databank For Rheumatic Diseases | |||
| Health utility score at 6 months | 24 (7.1) | 0.618 (0.225) | 0.616 (0.220) |
| Health utility score at 12 months | 29 (8.7) | 0.647 (0.220) | 0.645 (0.217) |
| The number of clinical visits | |||
| At 6 months | 37 (11) | 2.27 (2.69) | 2.27 (2.69) |
| At 12 months | 43 (12.8) | 2.39 (3.12) | 2.39 (3.12) |
| The number of intensive management sessions | 29 (17.2) | 10.03 (2.74) | 10.03 (2.74) |
| Staff | Cost per minute (£) | Assumptions | Source |
|---|---|---|---|
| Rheumatology practitioner | 1.75 per working minute | £105 per working hour for an associate specialist hospital-based doctor | PSSRU 2018/19 (p. 161)328 |
| Matron | 2.63 per minute of patient contact | Cost per minute of patient contact is not available for band 8a (matrons). We estimated this. We find the multiplier per working hour between band 8a and band 6, which is £64 (band 8a)/£45 (band 6) = 1.42 and multiply this by the cost per minute of patient contact for a band 6 (£1.85 × 1.42 = £2.63 per minute of patient contact) | PSSRU 2018/19 (pp. 155–7)328 |
| Senior research nurse, advanced nurse practitioner or manager | 2.22 per minute of patient contact | Cost per minute of patient contact is not available for band 7 (senior nurses). We estimated this. We find the multiplier per working hour between band 8a and band 6, which is £54 (band 7)/£45 (band 6) = 1.2 and multiply this by the cost per minute of patient contact for a band 6 (£1.85 × 1.22 = £2.22 per minute of patient contact) | PSSRU 2018/19 (pp. 155–7)328 |
| Specialist nursing staff | 1.85 per minute of patient contact | £111 cost per hour of patient contact | PSSRU 2018/19 (pp. 155–7)328 |
| Nurse or research nurse | 1.5 per minute of patient contact | £90 cost per hour of patient contact | PSSRU 2018/19 (pp. 155–7)328 |
| WTP threshold value (£) | Probability (%) intensive management is cost-effective vs. standard care from an NHS and social services cost perspective | |||
|---|---|---|---|---|
| Health-related quality of life estimated using van Hout et al.473 mapping from EQ-5D-5L to EQ-5D-3L | Health-related quality of life estimated using Hernández-Alava and Pudney’s334 mapping function derived from the EuroQol Group data set | Health-related quality of life estimated using Hernández-Alava and Pudney’s334 mapping function derived from FORWARD: National Databank for Rheumatic Diseases | Health-related quality of life estimated using EQ-5D-5L index scores | |
| 10,000 | 0 | 0 | 0 | 0 |
| 15,000 | 0 | 0 | 0 | 0 |
| 20,000 | 2 | 1 | 1 | 1 |
| 25,000 | 7 | 6 | 5 | 3 |
| 30,000 | 17 | 14 | 12 | 7 |
| 35,000 | 29 | 24 | 21 | 15 |
| 40,000 | 40 | 35 | 30 | 23 |
| 45,000 | 52 | 45 | 39 | 31 |
| 50,000 | 61 | 54 | 47 | 39 |
| Cost component | Treatment group, patient mean (£) (95% CI) | Mean group difference (£) (p-value) | |
|---|---|---|---|
| Standard care | Intensive management | ||
| Intensive management sessions only | NA | 1269 (978 to 1560) | 0 |
| NHS hospital clinical visits only (includes intensive management sessions) | 628 (552 to 704) | 1761 (1454 to 2067) | 1132 (p < 0.001) |
| Biologics costs only (in patients who use biologics) | 4467 (3439 to 5496) | 3465 (2870 to 4060) | 1003 (p = 0.07) |
| Biologic costs for all patients randomised to this arm (includes many patients who do not move to biologics and, as such, have zero cost) | 642 (365 to 919) | 949 (665 to 1233) | 307 (p = 0.13) |
| NHS pharmaceutical costs only (includes biologic costs) | 1009 (726 to 1291) | 1460 (1172 to 1748) | 451 (p = 0.03) |
| All NHS and PSS costs (includes pharmaceutical costs) | 2258 (1950 to 2565) | 3784 (3345 to 4223) | 1526 (p < 0.001) |
| Societal costs (includes all NHS, PSS and productivity losses) | 3678 (2651 to 4705) | 4697 (4042 to 5352) | 1019 (p = 0.10) |
| Health-related quality of life | Treatment group, patient mean (£) (95% CI) | Mean group difference (p-value) | |
|---|---|---|---|
| Standard care | Intensive management | ||
| Health-related quality of life estimated using van Hout et al.473 mapping from EQ-5D-5L to EQ-5D-3L scores | |||
| EQ-5D-3L index score at baseline | 0.59 (0.56 to 0.62) | 0.61 (0.58 to 0.64) | 0.0160 (p = 0.42) |
| EQ-5D-3L index score at 6 months | 0.60 (0.57 to 0.64) | 0.64 (0.61 to 0.67) | 0.0375 (p = 0.10) |
| EQ-5D-3L index score at 12 months | 0.62 (0.59 to 0.65) | 0.67 (0.64 to 0.70) | 0.0478 (p = 0.03) |
| QALYs | 0.61 (0.58 to 0.63) | 0.64 (0.62 to 0.67) | 0.0264 (p = 0.07) |
| QALYs with regression adjustment for EQ-5D-3L score at baseline | 0.61 (0.58 to 0.63) | 0.64 (0.62 to 0.66) | 0.0347 (p = 0.02) |
| Alternative approaches to estimating health-related quality of life | |||
| QALYs estimated using Hernández-Alava and Pudney’s334 mapping from EQ-5D-5L to EQ-5D-3L scores derived from the EuroQol Group c data set and with regression adjustment for EQ-5D-3L score at baseline | 0.59 (0.57 to 0.62) | 0.63 (0.61 to 0.65) | 0.0323 (p = 0.03) |
| QALYs estimated using Hernández-Alava and Pudney’s334 mapping from EQ-5D-5L to EQ-5D-3L scores derived from FORWARD: National Databank for Rheumatic Diseases and with regression adjustment for EQ-5D-3L score at baseline | 0.60 (0.58 to 0.63) | 0.63 (0.61 to 0.65) | 0.0292 (p = 0.08) |
| QALYs based on EQ-5D-5L index scores with regression adjustment for EQ-5D-5L score at baseline | 0.71 (0.69 to 0.73) | 0.74 (0.72 to 0.76) | 0.0264 (p = 0.07) |
| Variable | OR | 95% CI | p-value |
|---|---|---|---|
| Region of health centre (reference category is London and South East) | |||
| South West | 0.58 | 0.32 to 1.06 | 0.08 |
| Midland | 1.44 | 0.65 to 3.18 | 0.37 |
| North East | 1.17 | 0.62 to 2.21 | 0.64 |
| Age (years) | 1.00 | 0.98 to 1.02 | 0.67 |
| Female (reference category is male) | 0.74 | 0.41 to 1.34 | 0.32 |
| Ethnicity (reference category is white) | 1.89 | 0.81 to 4.41 | 0.14 |
| Disease duration (years) | 1.00 | 0.96 to 1.04 | 0.96 |
| Intervention group (reference category is standard care group) | 1.54 | 0.96 to 2.47 | 0.07 |
| Patient moved to biologic during the trial | 1.21 | 0.67 to 2.20 | 0.52 |
| Variable | Coefficient | 95% CI | p-value |
|---|---|---|---|
| Region of health centre (reference category is London and South East) | |||
| South West | –0.030 | –0.084 to 0.023 | 0.27 |
| Midland | 0.007 | –0.060 to 0.076 | 0.82 |
| North East | 0.008 | –0.05 to 0.064 | 0.79 |
| Age (years) | 0.001 | –0.001 to 0.003 | 0.42 |
| Female (reference category is male) | –0.051 | –0.104 to 0.001 | 0.06 |
| Ethnicity (reference category is white) | 0.011 | –0.065 to 0.088 | 0.78 |
| Disease duration (years) | –0.0003 | –0.003 to 0.003 | 0.84 |
| Intervention group (reference category is standard care group) | 0.031 | –0.011 to 0.072 | 0.15 |
| Patient moved to biologic during the trial | 0.059 | 0.007 to 0.111 | 0.03 |
| Variable | Coefficient | 95% CI | p-value |
|---|---|---|---|
| Region of health centre (reference category is London and South East) | |||
| South West | –0.002 | –0.05 to 0.05 | 0.93 |
| Midland | –0.031 | –0.09 to 0.03 | 0.28 |
| North East | –0.001 | –0.05 to 0.05 | 0.98 |
| Age (years) | 0.00005 | –0.001 to 0.002 | 0.96 |
| Female (reference category is male) | –0.037 | –0.08 to 0.008 | 0.10 |
| Ethnicity (reference category is white) | –0.010 | –0.09 to 0.07 | 0.80 |
| Disease duration (years) | 0.00003 | –0.003 to 0.003 | 0.96 |
| Intervention group (reference category is standard care group) | –0.001 | –0.04 to 0.04 | 0.96 |
| Patient moved to biologic during the trial | 0.056 | 0.011 to 0.10 | 0.02 |
| On biologics | Patients who switched to biologics, n | Patients in each group who switched to biologics, % |
|---|---|---|
| For patients without remissiona | ||
| Intensive management group | 29 | 17.3 |
| Standard care group | 16 | 9.6 |
| For patients with remissiona | ||
| Intensive management group | 10 | 6 |
| Standard care group | 5 | 3 |
| Hospital visits | Mean (SD) | 95% CI | Observations, n |
|---|---|---|---|
| For patients without remissiona | |||
| Intensive management group | 14.3 (6.1) | 13.2 to 15.4 | 120 |
| Standard care group | 5.7 (4.5) | 4.9 to 6.4 | 139 |
| Combined groups | 9.7 (6.8) | 8.8 to 10.5 | 259 |
| For patients with remissiona | |||
| Intensive management group | 14.2 (3.3) | 13.3 to 15.2 | 48 |
| Standard care group | 7.4 (5.7) | 5.2 to 9.6 | 28 |
| Combined groups | 11.7 (5.4) | 10.5 to 12.9 | 76 |
| Hospital visits | Mean (SD) | 95% CI | Observations, n |
|---|---|---|---|
| For patients without remissiona | |||
| Intensive management group | 14.5 (6.2) | 13.3 to 15.6 | 107 |
| Standard care group | 5.8 (4.6) | 5.0 to 6.6 | 130 |
| Combined groups | 9.7 (6.9) | 8.8 to 10.6 | 237 |
| For patients with remissiona | |||
| Intensive management group | 14.4 (3.3) | 12.9 to 15.8 | 23 |
| Standard care group | 4.7 (4.6) | 1.7 to 7.6 | 12 |
| Combined groups | 11.0 (6.0) | 8.9 to 13.1 | 35 |
| Remissiona | Mean (SD) | 95% CI | Observations, n |
|---|---|---|---|
| Patients without remission | 9.1 (3.7) | 8.4 to 9.8 | 120 |
| Patients with remission | 10.9 (1.5) | 10.5 to 11.4 | 48 |
List of abbreviations
- ACR
- American College of Rheumatology
- BeSt
- Behandel–Strategieën
- BMI
- body mass index
- BMQ
- Beliefs About Medicines Questionnaire
- BNF
- British National Formulary
- CARDERA
- Combination Anti-Rheumatic Drugs in Early Rheumatoid Arthritis
- CDAI
- Clinical Disease Activity Index
- CI
- confidence interval
- CRP
- C-reactive protein
- CSRI
- Client Service Receipt Inventory
- DAS
- Disease Activity Score
- DAS28
- Disease Activity Score for 28 joints
- DAS28-CRP
- Disease Activity Score for 28 joints based on C-reactive protein
- DAS28-ESR
- Disease Activity Score for 28 joints based on the erythrocyte sedimentation rate
- DMARD
- disease-modifying antirheumatic drug
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- ERAN
- Early Rheumatoid Arthritis Network
- ESR
- erythrocyte sedimentation rate
- EULAR
- European League Against Rheumatism
- FACIT-F
- Functional Assessment of Chronic Illness Therapy – Fatigue
- GAD-7
- Generalised Anxiety Disorder-7
- GP
- general practitioner
- HAQ
- Health Assessment Questionnaire
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- ITT
- intention to treat
- JAK
- Janus kinase
- LOCF
- last observation carried forward
- MCS
- mental component score
- MI
- motivational interviewing
- NICE
- National Institute for Health and Care Excellence
- NRAS
- National Rheumatoid Arthritis Society
- OPTTIRA
- Optimizing Treatment with Tumour Necrosis Factor Inhibitors In Rheumatoid Arthritis
- OR
- odds ratio
- PCS
- physical component score
- PHQ-9
- Patient Health Questionnaire-9 items
- PMM
- predictive mean matching
- PPI
- patient and public involvement
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- RA
- rheumatoid arthritis
- RAPID3
- Routine Assessment of Patient Index Data 3
- REMIRA
- Remissions in Rheumatoid Arthritis
- RR
- risk ratio
- SD
- standard deviation
- SDAI
- Simple Disease Activity Index
- SF-36
- Short Form questionnaire-36 items
- TACIT
- Tumour Necrosis Factor Inhibitors Against Combination Intensive Therapy
- TITRATE
- Treatment Intensities and Targets in Rheumatoid Arthritis ThErapy
- TNFi
- tumour necrosis factor inhibitor
- VAS
- visual analogue scale
- WTP
- willingness to pay
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/pgfar09080).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.