Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 13/142/04. The contractual start date was in April 2016. The draft report began editorial review in January 2022 and was accepted for publication in May 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Lois et al. This work was produced by Lois et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Lois et al.
Chapter 1 Introduction
Background
People with diabetes are at risk of experiencing permanent sight loss because of complications of diabetic retinopathy, including diabetic macular oedema (DMO), proliferative diabetic retinopathy (PDR), and macular ischaemia. 1
In DMO (Figure 1) fluid, and at times blood and lipid (fat), leaks from the retinal blood vessels, damaged by the chronically high glucose environment and its consequences, and builds up in the centre of the retina, the macula, the area responsible for central vision. As a result, the macular function is compromised, and the person’s sight is reduced.
FIGURE 1.
Infrared and optical coherence tomography (OCT) images of patients with and without diabetic macular oedema (DMO). (a) Infrared image and (b) optical coherence tomography (OCT) scan of the macula (left eye) of a patient with DMO. Fluid is observed as areas or reduced reflectance (arrows) on the OCT scan. For comparison, (c) infrared image and (d) OCT scan of the macula (right eye) of another patient in whom DMO cleared following treatment. The normal depression at the centre of the macula (fovea) is present (arrowhead).
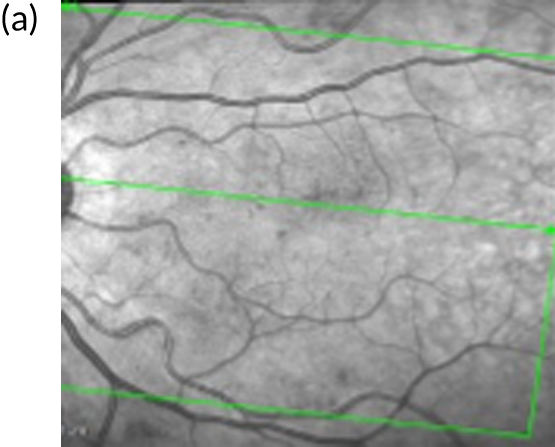
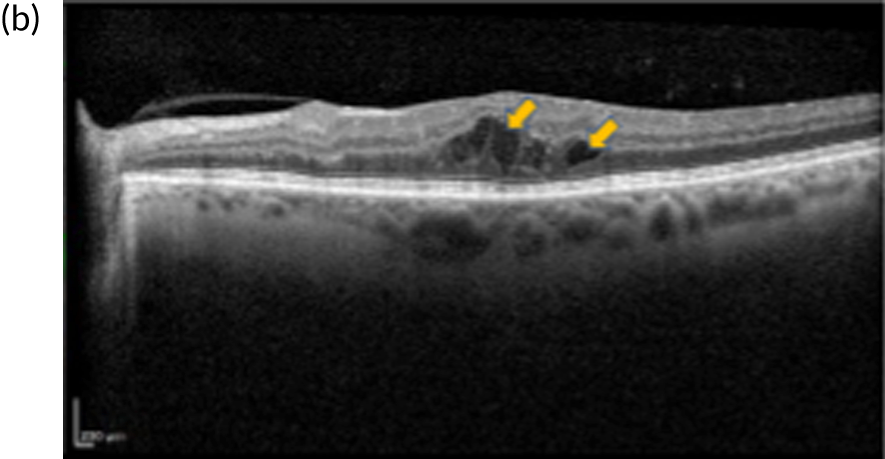
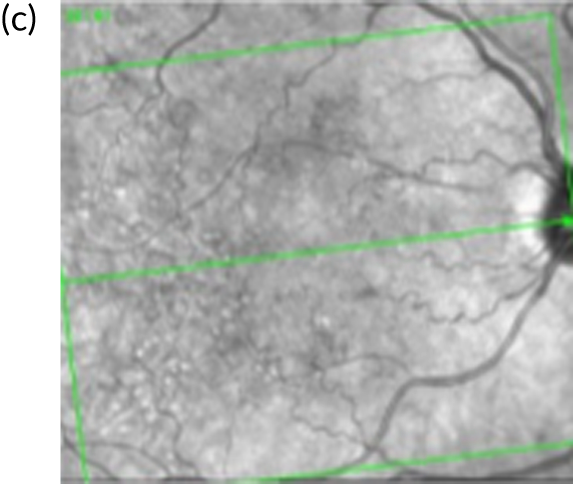
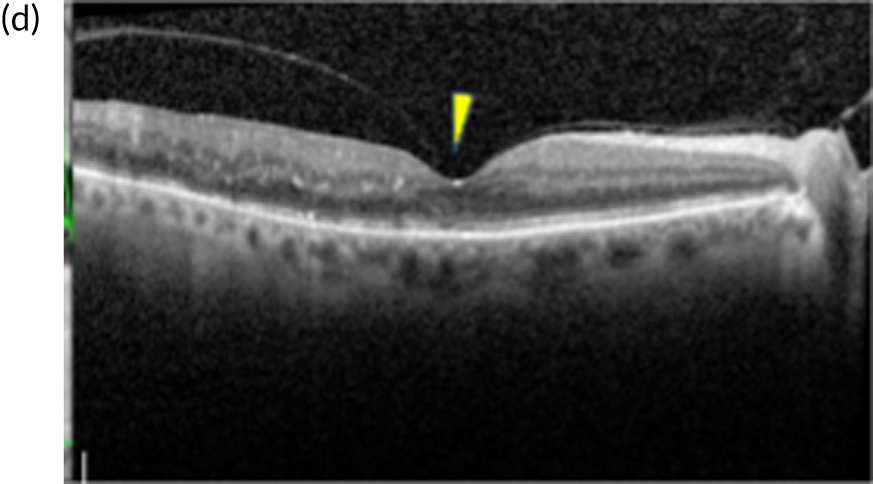
Currently, depending on the amount of fluid present [this can be measured using a diagnostic technology called optical coherence tomography (OCT) (Figures 1b and 1d)], different treatment options are recommended for patients with DMO. If the amount of fluid is considerable [i.e. people with a central retinal subfield thickness (CRT), as measured with OCT, of ≥ 400 µm], the National Institute for Health and Care Excellence (NICE) recommends treatment with intravitreal eye injections of drugs; this is known as anti-vascular endothelial growth factor (anti-VEGF) therapy. 2,3 Anti-VEGF therapy is required monthly during the first year of treatment for most patients and at less frequent intervals thereafter, until the fluid clears. If the amount of fluid in DMO is less severe (a CRT of < 400 µm on OCT) macular laser treatment is recommended by NICE as the treatment of choice as, for this group, macular laser is as clinically effective as anti-VEGF therapy but less costly. 1,2,4 Macular laser is applied in a single session with topical anaesthetic drops; injections of local anaesthetic are not required as it causes no discomfort/pain to patients. The topical anaesthesia is used as to apply the laser, a viewing contact lens needs to be placed on the surface of the eye, so an appropriately magnified and sharp view of the macula is obtained to perform the treatment. Laser sessions may need to be repeated every 3–4 months until the fluid clears.
The first randomised clinical trial (RCT) that showed a benefit of laser treatment for people with diabetes and macular oedema was the Early Treatment Diabetic Retinopathy Study (ETDRS). 5 The ETDRS demonstrated that macular laser reduced the risk of visual loss (≥ 3 ETDRS line loss) by 50% at 3 years in patients with what was defined as clinically significant diabetic macular oedema (CSMO). 5 The definition of CSMO was based on the presence of the following characteristics on clinical examination (slit-lamp biomicroscopy): (1) thickening of the retina at or within 500 µm from the centre of the macula, (2) hard exudation at or within 500 µm from the centre of the macula provided that it was associated with adjacent retinal thickening or (3) thickening of the retina of the size of the optic nerve head or larger within one disc diameter from the centre of the macula. In the ETDRS only 3% of patients had improvement in vision of ≥ 15 letters, but 85% of eyes included in the study had excellent vision at baseline (Snellen equivalent ≥ 20/40) which may have accounted for the limited visual improvement observed. More recent RCTs on laser treatment for centre-involving DMO showed laser can indeed improve vision, with improvements of ≥ 10 ETDRS letters observed in 32% of eyes at 2 years and in 44% of eyes at 3 years. 6,7
It is important to note that centre-involving DMO may not necessarily mean CSMO. In regards to this, trials conducted comparing anti-VEGF therapy with macular laser included people with centre-involving DMO. Centre-involving DMO is defined based on OCT findings. The natural history of centre-involving DMO diagnosed by OCT is less clear than that of CSMO.
Macular laser is also used in patients with DMO who do not fully respond to anti-VEGF therapy. In a randomised trial comparing different anti-VEGF drugs to treat centre-involving DMO, 41–64% of eyes receiving anti-VEGF therapy required macular laser by 2 years following treatment initiation to control the disease. 8
In the ETDRS study,5 laser treatment was undertaken using a continuous wave laser. This laser [referred to as ‘threshold’ laser, and here, throughout this report, as standard threshold macular laser (SL)] produces a visible burn in the retina and is considered the ‘standard’ manner in which to perform macular laser. The laser energy when using this SL is predominantly absorbed by one of the layers of the retina, the retinal pigment epithelium (RPE), and converted into heat. Although the mechanism of action of macular laser is not completely understood, it is believed that it has its effect, at least partly, by acting on still viable RPE cells around the area of the burn. Given that heat spreads by conduction, there could be damage to the retinal layers overlying the RPE, including photoreceptor cells (i.e. the cones and rods which are the visual cells of the retina). SL can ‘burn’ the retina and, if applied to the centre of the fovea (the centre of the macula, which is the area of the retina that provides maximal central vision) could cause marked central sight loss. Therefore, this form of laser requires considerable expertise by the clinician administering it as the fovea may not be easily identifiable when thickened by DMO. Ideally, as advised by the ETDRS,5 a fundus fluorescein angiogram (FFA) would be performed to identify areas of leakage that should be aimed at by the laser treatment. However, clinicians may opt to use SL to treat areas of macular thickening as observed on OCT. 6,7 Side effects of SL, besides the potential burning of the fovea as explained above, could include paracentral scotomas (areas of loss of sight around the centre) which can potentially affect driving ability, colour vision deficits and formation of scarring over or under the retina (i.e. epiretinal membrane/subretinal fibrosis, respectively). If strong laser is applied close to the centre of the macula, the size of the ‘burn’ (i.e. the area where retinal cells are lost) could expand over time and, even if initially the centre is not affected, central loss of vision could occur over time.
Macular laser treatment can also be delivered using what is called ‘subthreshold’ micropulse laser (SML). In SML, a series of repetitive, very short pulses of laser are delivered, with each pulse of active ‘on’ laser separated by a long ‘off’ time. This ‘off’ time allows for cooling of the retina, preventing the development of a ‘burn’ and, thus, leaving the RPE and overlying neurosensory retina, including photoreceptors, intact. It is believed SML acts by directly stimulating the RPE. As there is no destruction of the retina, this treatment could be applied to larger areas of the retina (not only those with ‘leaking’ blood vessels or thickened by DMO) in a standardised fashion and repeated as many times as needed.
Although the lack of a ‘burn’ in the retina would appear to be of clear advantage, the absence of clinically objective changes in the retina following SML has led to some clinicians/researchers being sceptical of SML having equally beneficial effects as SL. Earlier studies, however, including small RCTs, have shown comparable or superior results using SML. Lavinsky et al. 9 showed superiority regarding improvement in vision and reduction in CRT at 12 months following high-density SML in a three-arm trial that included 123 participants with CSMO (n = 42 and n = 39 randomised to high-density and low-density SML, respectively; and n = 42 to SL). Similarly, in a smaller RCT which included 62 eyes from 50 patients with centre-involving CSMO (32 eyes received SML and 30 eyes received SL) Vujosevic et al. 10 found no statistically significant differences in vision or CRT between laser groups, but a statistically significant increased retinal sensitivity (i.e. better retinal function) on microperimetry testing following SML. Other trials by Figueira et al. (n = 53 patients with CSMO according to the ETDRS definition),11 Venkatesh et al. 12 (n = 33 patients with CSMO), Kumar et al. 13 (n = 20 patients with CSMO according to ETDRS criteria) and Laursen et al. 14 (n = 16 patients with CSMO), with follow-up of 18 weeks and 8, 12 and 5 months, respectively, found no statistically significant differences in vision and CRT between both types of laser. A more recent RCT by Xie et al. 15 which included 84 patients (99 eyes) receiving standard argon laser (n = 49 eyes) or SML (n = 50 eyes) also found comparable results between laser groups.
In a systematic review and meta-analysis by Chen et al. ,16 which included all but one (Kumar et al. 13) of the RCTs mentioned above, and based on data from 398 eyes (203 eyes in the SML group and 195 in the SL group), SML was found to be statistically significantly superior to SL in terms of mean change of best-corrected visual acuity (BCVA) at 3, 9 and 12 months following treatment, with no statistically significant difference in change in CRT. The largest difference in BCVA between laser arms was observed at 12 months (0.1 log-MAR) favouring SML, but this seemed to be due to the effect of one trial (Lavinsky et al. 9), and to the high-density arm of this trial; the difference would not be considered, in any case, clinically important (0.1 log-MAR, equivalent to 5 ETDRS letters).
Qaio et al. 17 subsequently published another systematic review comparing SML laser with SL, which included seven RCTs, all six included in Chen et al. ’s review16 and, in addition, the study by Kumar et al. ,13 with a total of 467 eyes from 379 participants. The primary outcomes were change in BCVA and CRT. Secondary outcomes included contrast sensitivity and retinal damage; neither quality of life nor cost-effectiveness of the treatments were evaluated. Meta-analysis found no statistically significant differences in outcomes at any time point (longest follow-up was 12 months). There was comparable preservation of contrast sensitivity with SML with less retinal damage. The review authors concluded that SML was as good as SL but caused less retinal damage.
The results of the review by Qiao et al. 17 differ somewhat from those of Chen et al. 16 owing to the choice of arm and outcome from the RCT conducted by Lavinsky et al. 9 The review by Qiao et al. 17 reported mean BCVA at 12 months with three trials, Figueira et al.,11 Lavinsky et al.,9 and Vujosevic et al.,10 whereas the review by Chen et al. 16 reported change from baseline with only two trials at 12 months, Lavinsky et al. 9 and Vujosevic et al. 10 The Lavinsky et al. 9 RCT had two SML arms, one with normal and another with high-density SML treatment, and reported better results with the latter. The Chen et al. 16 meta-analysis used only the high-density arm whereas the Qiao et al. 17 meta-analysis used only the normal density arm. Their different conclusions were due to the Lavinsky et al. trial,9 deemed to give a significant result in Chen et al. 16 (based on change from baseline) but not in Qiao et al. 17 (based on mean BCVA at 12 months).
A Bayesian network meta-analysis by Wu et al. 18 found no significant difference in mean change in BCVA and CRT between SL and SML. A 2018 Cochrane systematic review19 and meta-analysis by Jorge et al. concluded SML may be as effective as SL, but the GRADE (Grading of Recommendations Assessment, Development and Evaluation) assessment was of low certainty.
All conducted meta-analyses were limited by the inherent limitations of the available RCTs, including the short follow-up (longest follow-up of 12 months). Furthermore, it is unclear what the proportion of participants with a CRT of < 400 µm was in the RCTs included, which based on NICE guidelines would be the participants most likely to respond to macular laser. 1,2,4
Recently (and thus not included in any of the systematic reviews mentioned above) a small (68 participants, 34 in each group), very short-term (4 months) RCT by Fazel et al. 20 compared SML with SL in people with CSMO and with a CRT of less than 450 µm. Changes in CRT were statistically significantly higher in the SML group than the SL group. Changes in macular volume and visual acuity were only significant in the SML group but not in the SL group.
In summary, published data suggest that SML is comparable, and potentially even superior, to SL, but stronger evidence is required. None of the trials conducted comparing SML and SL included patient-reported outcome measures (PROMs) or the cost-effectiveness of the treatments. Furthermore, outcomes were measured, at the longest, at 12 months. Given the lack of destruction of retinal tissue, SML can be given to larger areas of the macula and repeated as needed, potentially indefinitely. SML may allow for a more standardised delivery of treatment (e.g. using set grids that cover the entire macula), which could, in turn, minimise variability in the quality of treatment delivered, with successful delivery being less dependent on the surgeon’s skills. The lack of deleterious effects when used over the fovea21 makes SML very safe (i.e. sight loss as a result of an inadvertent foveal burn is obviated), potentially facilitating training on its application to junior ophthalmologists and ophthalmic-allied non-medical staff. This would be highly advantageous considering the major problems with capacity currently faced in ophthalmic clinics throughout the world.
On this basis, the DIAMONDS (DIAbetic Macular Oedema aNd Diode Subthreshold micropulse laser) trial was designed as a non-inferiority trial of the efficacy of SML when compared with SL, although the trial was also powered to test equivalence and superiority (if this were to exist). We chose central vision as the primary outcome, as this is very important to people with diabetes and DMO, and set the non-inferiority margin at 5 ETDRS letters (equivalence margin as ± 5 ETDRS letters), as visual changes of this size should not be clinically relevant to patients and could even be due to test/re-test variability.
Chapter 2 Clinical trial methods
Parts of this chapter are adapted or reproduced from Lois et al. 22 and Costa et al. 23 These are Open Access articles distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original texts.
Aims
The DIAMONDS trial aimed to evaluate the clinical effectiveness and cost-effectiveness of SML when compared with SL for the treatment of patients with centre-involving DMO with a CRT on spectral domain optical coherence tomography (SD-OCT) of < 400 µm.
Primary objective
The primary objective of the trial was to determine whether or not SML is non-inferior (or equivalent) to SL at improving or preserving vision 24 months after treatment in patients with centre-involving DMO with a CRT of < 400 µm. The non-inferiority margin was set at 5 ETDRS letters (and the equivalence margin at ± 5 ETDRS letters), as a difference of this size is not considered to be clinically relevant and could even be due to test/re-test variability. 2,3,8,24–26
Secondary objectives
The secondary objectives of the trial were to determine whether or not SML is superior to SL at improving or preserving binocular vision and visual field; reducing or clearing DMO; allowing treated patients to achieve driving standards; and improving their health- and vision-related quality of life 24 months after treatment. The relative cost-effectiveness of SML when compared with SL was also evaluated, as well as the side effects of these treatments, the number of laser treatments required and the need for additional treatments (other than laser).
Trial design
The protocol for the DIAMONDS trial was published in February 2019. 22 The DIAMONDS trial was a pragmatic, multicentre, allocation-concealed, non-inferiority, randomised, double-masked (participants and outcome assessors), prospective clinical trial set within specialist hospital eye services (HES) (n = 16) in the UK.
Patient eligibility and recruitment
Potential participants were identified at each of the participating sites through electronic databases, through referrals to HES or while in the clinic. Patients identified through electronic databases or referrals were approached by telephone or invitation letter. Verbal and written information about the study was given to potential participants. Informed consent was obtained from patients willing to take part in the trial and they were subsequently recruited. Patients identified while in the clinic were verbally informed about the study and given a patient information leaflet. They were given time to think about their participation and ask questions. If they wished to be enrolled on the same day, they were recruited into the trial following informed consent. If they wanted more time to think about their potential participation, a further visit was organised and, if they were willing to participate at this visit, they were recruited.
Inclusion criteria
Inclusion criteria for the trial were centre-involving DMO, as determined by slit-lamp biomicroscopy and SD-OCT, in one or both eyes, with either (1) a CRT of > 300 µm but < 400 µm in the central subfield (central 1 mm) owing to DMO as determined by SD-OCT, or (2) a CRT of < 300 µm provided that intraretinal and/or subretinal fluid was present in the central subfield (central 1 mm) owing to DMO.
The following conditions also had to be met:
-
visual acuity of > 24 Early Treatment Diabetic Retinopathy Study (ETDRS) letters (Snellen equivalent > 20/320)
-
amenable to laser treatment, as judged by the treating ophthalmologist
-
aged ≥ 18 years.
Exclusion criteria
A patient’s eyes were not eligible for the study if their macular oedema was owing to causes other than DMO or if their eyes met the following criteria:
-
ineligible for macular laser, as judged by the treating ophthalmologist
-
DMO with a CRT of ≥ 400 µm
-
active PDR requiring treatment
-
received intravitreal anti-VEGF therapy within the previous 2 months
-
received macular laser treatment within the previous 12 months
-
received intravitreal injection of steroids
-
cataract surgery within the previous 6 weeks
-
panretinal photocoagulation (PRP) within the previous 3 months.
In addition, patients who were otherwise eligible were not included in the study if they met the following criteria:
-
on pioglitazone (Actos®, Takeda UK Ltd.), and the drug could not be stopped 3 months before joining the trial and for its entire duration (because this drug could be responsible for the presence of macular oedema)
-
chronic renal failure requiring dialysis or kidney transplant
-
any other condition that in the opinion of the investigator would preclude participation in the study (such as unstable medical status or severe disease that would make it difficult for the patient to complete the study)
-
very poor glycaemic control that required their starting intensive therapy within the previous 3 months
-
using an investigational drug.
If both eyes were eligible, both eyes would receive the same type of laser but one was designated as the ‘study eye’. This was the eye with the best BCVA at randomisation or, if vision was the same in both eyes, the eye with the lesser CRT.
If the fellow eye was not eligible, baseline data and information on whether or not participants developed DMO or PDR in this eye during the study and about treatments administered to it were recorded in the patient’s case report form (CRF) at months 12 and 24 to determine any possible effects of these events on outcomes.
The DIAMONDS trial participants were similar to those enrolled in the original ETDRS5 in that, like those enrolled in ETDRS, they had mild to moderate non-proliferative diabetic retinopathy and had a visual acuity of 20/200 or better. As mentioned above, ETDRS was the first trial demonstrating the clinical effectiveness of macular laser for the treatment of DMO. However, unlike in ETDRS, DIAMONDS trial participants did not necessarily have to have CSMO to be enrolled, as per ETDRS definition, and they could have only centre-involving DMO as determined using SD-OCT, a technology not available at the time ETDRS was conducted. This follows standard clinical practice.
Ethics approval and consent
The protocol for the DIAMONDS trial22 was approved by the Office for Research Ethics Committees Northern Ireland (ORECNI) (15/NI/0197). A Clinical Trial Authorisation (CTA) was obtained from the Medicines and Healthcare products Regulatory Agency (MHRA) (32485/0029/001–0001). The trial was registered with the European Union Drug Regulating Authorities Clinical Trials (EudraCT) database (2015-001940-12), in the International Standard Randomised Controlled Trial Number (ISRCTN) register (ISRCTN16962255), and at clinicaltrials.gov (NCT03690050).
Informed consent
Informed consent to participate in the study was sought from potentially eligible participants by the ophthalmologist or a designee at each participating site. The treating ophthalmologist also obtained informed consent before carrying out the allocated laser procedure.
Participant withdrawal
Participants could withdraw from the study at any time without prejudice. In the event of consent withdrawal, patients were to be asked for their permission to use the data already collected. If this permission was declined, then any collected data on that patient would not be used in the trial analysis. Withdrawal of consent was recorded on the patient’s CRF.
Interventions
Patients were randomised to one of two groups: (1) 577 nm SML or (2) SL [e.g. argon, frequency-doubled neodymium-doped yttrium aluminium garnet (Nd:YAG) 532 nm laser]. Application of the allocated laser was in accordance with the processes described in Micropulse laser and Standard laser. Information on laser type, parameters used and time spent applying the treatment was recorded in the patient’s CRF. In participants with both eyes eligible and included in the trial, both study eye and fellow eye received the same type of laser (i.e. the type randomly allocated).
Micropulse laser
Subthreshold micropulse laser was delivered using a 577 nm optically pumped diode laser (IQ 577™ laser system; IRIDEX Corporation, Mountain View, CA, USA). The IRIDEX Corporation laser system was used as IRIDEX was, at the time the trial was designed and initiated, the only manufacturer that produced a 577 nm wavelength laser that could have potential benefits when compared with other available wavelengths, including lack of absorption by the macular pigment, very good absorption by melanin and good penetration through lens opacities. SML was applied confluently to the macular area, using three 7 × 7 spot grids above and below the fovea (500 µm from its centre) and one 7x7 spot grid at each side (temporal and nasal) of the fovea (500 µm from its centre). In addition, treatment was also applied to areas of thickening located outside this central area. Firstly, a threshold was set by titrating the power of the laser upwards, starting from 50 mW, in 10 mW increments, in an area where oedema was present, around > 2 disc diameters from the foveal centre (if possible), and until a barely visible tissue reaction was seen. If a reaction was evident with 50 mW, the power was not increased. Then, the laser was switched to the micropulse mode with the power of the laser set at x4 the threshold identified (e.g. if a barely visible reaction was seen at 50 mW, then micropulse laser was applied with a 200mW power). SML was then undertaken using a spot size of 200 µm, duty cycle of 5%, and ‘on’ duration of 200 ms (composed of multiple 0.1 ms of ‘on’ pulses, with 1.9 ms of ‘off’ time in between ‘on’ pulses). A standard operating procedure (SOP) for SML was prepared for the trial (see Report Supplementary Material 1).
Standard laser
For patients allocated to SL, the laser was applied to areas of thickened retina, macular non-perfusion (away from and non-contiguous with the perifoveal capillaries) and leaking microaneurysms, in accordance with the ETDRS and the Royal College of Ophthalmologists guidelines. 5,27 FFA and SD-OCT were used to identify areas of non-perfusion and leakage (FFA) and thickening (SD-OCT) before treatment, at the discretion of the treating ophthalmologist. Treatment was applied to obtain a mild grey–white burn evident beneath leaking microaneurysms and in other areas of leakage or non-perfusion not affecting the perifoveal capillaries based on FFA, if FFA had been obtained, or to cover areas of thickening if treatment was given based on OCT findings, or both. The treatment was intended to spare the central 500 µm and the area within 500 µm from the optic nerve head. A standard operating procedure (SOP) for SL was prepared and used in the trial (see Report Supplementary Material 2).
The SL treatment in the DIAMONDS trial was performed using a modified ETDRS technique. In the ETDRS, argon laser was used, whereas in the DIAMONDS trial other types of lasers were allowed, given that argon laser is no longer widely available. The technique and parameters used for SL treatment in the DIAMONDS trial is representative of the technique used in other macular laser trials6,7 and that used in standard clinical practice.
Retreatment
Where necessary, retreatments were carried out with the same laser allocated by randomisation. When retreating, treatment of areas within 300–500 µm from the centre of the fovea was allowed. Details of retreatments were recorded in the patient’s CRF.
Rescue treatment
Where necessary, rescue treatment, with anti-VEGF therapy or steroids, as appropriate based on judgement by the treating ophthalmologist, was allowed in both treatment groups if the CRT increased to ≥ 400 µm at any point during the patient’s follow-up or if a loss of ≥ 10 ETDRS letters occurred in relation to DMO. The type and date of any rescue treatment was recorded in the patient’s CRF.
Participating sites and experience
Sixteen NHS HES sites participated in the recruitment, management and follow-up of DIAMONDS trial participants. All treating ophthalmologists had extensive experience in diabetic retinopathy and DMO as well as in delivering laser treatment for DMO. The participating sites were: Royal Victoria Hospital, Belfast Health & Social Care Trust; Bristol Eye Hospital, University Hospitals Bristol NHS Foundation Trust; Frimley Park Hospital NHS Foundation Trust; Hinchingbrooke Hospital, North West Anglia NHS Trust; King’s College Hospital NHS Foundation Trust; Manchester Royal Eye Hospital, Central Manchester University Hospitals NHS Foundation Trust; National Institute for Health and Care Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust; Newcastle Eye Centre, Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust; John Radcliffe Hospital, Oxford University Hospitals NHS Foundation Trust; Sheffield Teaching Hospitals NHS Foundation Trust; Sunderland Eye Infirmary, South Tyneside and Sunderland NHS Foundation Trust; Bradford Royal Infirmary, Bradford Teaching Hospitals NHS Trust; James Cook University Hospital, South Tees Hospitals NHS Foundation Trust; Hull and East Yorkshire Hospital, Hull and East Yorkshire NHS Trust; Stoke Mandeville Hospital, Buckinghamshire NHS Trust; and Hillingdon Hospitals NHS Foundation Trust.
Randomisation and masking
Randomisation
Following informed consent, eligible participants were randomised 1:1 to receive either SML or SL using a minimisation algorithm within the automated randomisation system Sealed Envelope (Sealed Envelope Ltd, London, UK; URL: www.sealedenvelope.com), with the allocation concealed to the ophthalmologist randomising the patient until the patient had joined the trial. The local ophthalmologist used this automated system to ensure post-randomisation masking of allocation to the outcome assessors. Although most patients received their allocated therapy at the baseline visit, it was acceptable for it to be performed at a later visit within two weeks of the baseline visit. If there was a longer interval between the baseline visit and the laser treatment, eligibility was re-confirmed before treatment.
The randomisation system used minimisation to balance allocation of patients across intervention groups for the following prognostic factors: centre, distance BCVA at presentation [≥ 69 ETDRS letters (Snellen equivalent of ≥ 20/40; log-MAR ≥ 0.3); 24–68 ETDRS letters (Snellen equivalent ≤ 20/50; log-MAR 0.4–1.2)] and previous administration of anti-VEGF therapy or macular laser in the study eye. A random element was used in the minimisation to provide a probability of 0.85 for assigning to the treatment group that minimised imbalance.
Masking
The DIAMONDS trial was a pragmatic RCT so that its results would be applicable immediately in an NHS setting once the trial was completed. For this reason, ophthalmologists undertaking laser treatments for DMO at each of the participating centres delivered the treatment for the trial and, thus, were not masked to the laser used. However, participants and outcome assessors (e.g. optometrists measuring visual function; photographers, technicians and nurses obtaining OCT images; and ophthalmic technicians obtaining visual fields) were all masked to treatment allocation. Patients were not informed before, during or after the laser treatment about which laser was used and remained masked until the trial ended. Similarly, investigators obtaining outcome measures had access to the CRF booklet only (and not to the notes of the patients) and this booklet did not contain information about the type of laser the patient had been allocated to or received (this information was held in a locked cabinet/locked room by the treating ophthalmologists).
Patient assessments
Patients were assessed during the study according to the schedule of assessments shown in Table 1.
| Trial procedures and assessments | Baselinea | Months post randomisationa | |||||
|---|---|---|---|---|---|---|---|
| 4 | 8 | 12 | 16 | 20 | 24 | ||
| Informed consent | ✓ | ||||||
| Medical history | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Blood test: HbA1cb | ✓ | ||||||
| BCVA in study eye and fellow eye | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Binocular distance vision | ✓ | ✓ | ✓ | ||||
| Humphrey 10–2 visual field in study eye | ✓ | ✓ | ✓ | ||||
| Esterman binocular visual field | ✓ | ✓ | ✓ | ||||
| SD-OCT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| NEI-VFQ-25 | ✓ | ✓ | ✓ | ||||
| EQ-5D-5L | ✓ | ✓ | ✓ | ||||
| VisQoL | ✓ | ✓ | ✓ | ||||
| Randomisation | ✓ | ||||||
| Subthreshold micropulse laser/standard lasera,c | ✓ d | ||||||
| Adverse events | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
Participants’ BCVA was measured in both eyes using ETDRS visual acuity charts at 4 m at baseline and at months 4, 8, 12, 16, 20 and 24. BCVA was obtained following refraction at baseline and at 12 and 24 months by optometrists masked to treatment allocation. At all other visits, BCVA could be obtained by other masked staff using the most recently obtained refraction. Binocular BCVA was obtained to give an indication of the person’s vision ‘in real life’, using both eyes (i.e. with both eyes opened simultaneously). It was obtained by masked optometrists using the ETDRS visual acuity charts at 4 m at baseline and at 12 and 24 months. A refraction protocol was followed by the optometrists to obtain BCVA. ETDRS visual acuity scores were recorded for study and fellow eyes in the patient’s CRF at each study visit.
Testing of the 10–2 Humphrey visual field was performed in the study eye (and the fellow eye if this was included in the trial) by a visual field technician masked to the allocated treatment at baseline at 12 and 24 months. An Esterman binocular visual field (to determine the patient’s ability to fulfil driving standards) was obtained at the same time points. Visual fields eligible for analysis had to achieve pre-defined reliability criteria (false positives < 15%). If the visual fields were not reliable, they were repeated. The mean deviation (MD) value for the 10–2 Humphrey visual fields and the number of points seen and missed for the Esterman binocular visual fields were recorded in the patient’s CRF.
Participants’ CRT, as determined using SD-OCT, was obtained in both eyes at baseline and at months 4, 8, 12, 16, 20 and 24. SD-OCT was obtained by technicians, photographers or nurses, as per standard clinical practice at each of the participating centres, who were masked to the treatment allocation. The measure of thickness at the central 1 mm (i.e. CRT) was recorded in the patient’s CRF and used for analysis. Total and maximal macular volume were also recorded in the CRF. Presence or absence of intraretinal or subretinal fluid was determined in a masked fashion at the 24-month follow-up visit by masked readers at the Central Administrative Research Facility (CARF) at Queen’s University Belfast. Images sent to CARF were anonymised. The Heidelberg Spectralis SD-OCT (Heidelberg Engineering GmbH, Heidelberg, Germany) was used to obtain the CRT measurements for each participant at baseline and at each follow-up visit, unless for any reason, this was not possible.
Two vision-related quality of life tools, the National Eye Institute Visual Function Questionnaire – 25 (NEI-VFQ-25) and the Vision and Quality of Life Index (VisQol), were used in the DIAMONDS trial, in addition to a generic preference-based health-related quality of life measure to generate utility data [the EuroQol-5 Dimensions, five-level version (EQ-5D-5L)]. Questionnaires were self-completed by patients at baseline and at 12 and 24 months. Baseline questionnaires were completed before the first session of laser treatment.
Participants were followed up at 4-month intervals following laser treatment for a total of seven visits. Additional visits (interim visits) took place, if required. To maximise retention, the DIAMONDS trial was designed as a pragmatic trial, with the 4-monthly visits being akin to those in usual, routine care. In most visits, with the exception of those at baseline and at 12 and 24 months, the tests were the same as those done in routine care (i.e. a measure of visual acuity and SD-OCT scans). Consent was also obtained from the participants to allow for an evaluation of longer-term patient outcomes (at 5 years), which would be subject to a future funding application.
Outcomes
The primary outcome was the difference between treatment groups in the mean change in BCVA in the study eye from baseline to month 24.
The secondary outcomes comprised the following:
-
mean change in binocular BCVA from baseline to month 24
-
mean change in CRT in the study eye, as determined by SD-OCT, from baseline to month 24
-
mean change in the MD of the Humphrey 10–2 visual field in the study eye from baseline to month 24
-
change in the percentage of people meeting driving standards from baseline to month 24
-
mean change in EQ-5D-5L, NEI-VFQ-25 and VisQoL scores from baseline to month 24
-
cost per quality-adjusted life-year (QALY) gained
-
adverse effects
-
number of laser treatments carried out
-
additional treatments required (other than laser).
Data collection and management
Case report forms were used to collect data for each participant in the DIAMONDS trial. On-site monitoring visits during the trial checked the accuracy of entries on CRFs against the source documents and adherence to the protocol and procedures, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use – Good Clinical Practice (ICH-GCP) guidelines, and regulatory requirements. Monitoring visits were undertaken by a monitor from the Northern Ireland Clinical Trials Unit (NICTU). To ensure accurate, complete and reliable data were collected, the chief investigator and the NICTU provided training to site staff through investigator meetings and site initiation visits.
Data quality
The chief investigator and the NICTU provided training to unit staff on trial processes and procedures including CRF completion and data collection. Monitoring during the trial included adherence to the protocol, trial-specific procedures and good clinical practice. Within the NICTU, the clinical data management process was governed by SOPs, which ensured standardisation and adherence to International Conference of Harmonisation Good Clinical Practice guidelines and regulatory requirements.
Data quality control checks were carried out by a data manager following the NICTU SOP. Data validation was implemented and discrepancy reports were generated following data entry to identify discrepancies such as out-of-range values, inconsistencies or protocol deviations based on data validation checks. Changes to data were recorded and fully auditable. Data errors were documented and corrective actions implemented.
A Data Monitoring and Ethics Committee (DMEC) and a Trial Steering Committee (TSC) were convened for the DIAMONDS trial, the former to carry out reviews of the accumulating data at regular intervals during the study, to ensure the safety of participants and the latter to monitor the progress of the trial, among other tasks.
Adverse events
The safety of the treatment was assessed at each visit by noting any complications during or after laser treatment, including self-reported visual disturbances, and an ETDRS visual acuity loss of ≥ 10 letters and ≥ 15 letters occurring from visit to visit. Patients were asked specifically about reduced colour vision, presence of paracentral scotomas or distortion (‘waviness’ of straight lines) at each visit and responses were recorded in the appropriate CRF. Although serious adverse events (SAEs) related to the study procedures were unlikely to occur as a result of any of the study procedures (see below), all SAEs were recorded on the patient’s CRF and the sponsor and the Research Ethics Committee were informed of these. The DMEC was also provided with information on all SAEs on a routine basis.
As the DIAMONDS trial did not investigate medicinal products, adverse event reporting followed the Health Research Authority guidelines on safety reporting in non-Clinical Trials of Investigational Medicinal Products (non-CTIMP) studies. Adverse events (AEs) and SAEs were recorded on the patient’s CRF and the information was updated with the date and time of resolution or confirmation that the event was due to the participant’s illness when this information became available.
An AE was defined as any untoward medical occurrence in a participant in a research study, including occurrences which were not necessarily caused by or related to the study. AEs relating to pre-existing underlying diseases were not recorded in the DIAMONDS trial.
A SAE was defined as an untoward occurrence that met one or more of the following criteria:
-
resulted in death
-
was life-threatening
-
required hospitalisation or prolongation of existing hospitalisation
-
resulted in persistent or significant disability or incapacity
-
consisted of a congenital anomaly or birth defect
-
was otherwise considered medically significant by the investigator.
Hospitalisation was defined as an inpatient admission regardless of length of stay, even if the hospitalisation was a precautionary measure for continued observation. Hospitalisations for a pre-existing condition, including elective procedures, did not constitute a SAE.
Anticipated adverse events due to laser treatment
The following were listed in the protocol22 as potential AEs in the DIAMONDS trial:
-
foveal burn
-
central/paracentral scotomas
-
epiretinal membrane formation
-
choroidal neovascularisation related to the laser
-
self-reported reduced colour vision
-
self-reported metamorphopsia.
Statistical methods for effectiveness analyses
Pre-trial power calculation
The DIAMONDS trial was powered to demonstrate not only non-inferiority but also equivalence of SML compared with SL with respect to the primary outcome (i.e. mean change in BCVA in the study eye between baseline and month 24). This was because, based on the existing knowledge when the trial was designed, it was possible that no differences could be found in the primary outcome but that differences could exist in other important secondary outcomes, such as PROMs. The DIAMONDS trial was also sufficiently powered to determine superiority of one laser over the other, if this were to exist.
Based on a mean of 0.08 log-MAR [standard deviation (SD) 0.23 log-MAR] for BCVA change from baseline for the standard care laser9 and a permitted maximum difference of 0.1 log-MAR (± 5 ETDRS letters) between groups, we estimated that the DIAMONDS trial would require 113 randomised participants per group, at 90% power and 0.05 level of significance. Allowing for up to a 15% dropout rate during the 24 months of follow-up, as observed in other randomised trials on DMO with outcomes determined at 24 months,28,29 the recruitment target was set at 266 patients.
A permitted maximal difference of 5 ETDRS letters between groups was chosen as the non-inferiority margin (± 5 ETDRS letters for equivalence) because a difference of this size or less is not considered clinically relevant or meaningful to patients. 2,3,8,24–26
In addition, 24-month data for 113 participants per group would also be sufficient to detect a mean difference between lasers of 37.7 µm in CRT (based on a SD of 86.810 µm) and of 6.55 in NEI-VFQ-25 scores (based on a SD of 15.1 score as per Tranos et al. 30). These are important secondary outcomes and such differences in CRT and NEI-VFQ-25 scores have been shown to be clinically relevant. 31,32
Analysis principles
The DIAMONDS trial was designed with sufficient power to detect not only non-inferiority but also equivalence of SML when compared with SL and the primary statistical analysis was per protocol (PP), but an intention-to treat (ITT) analysis was also undertaken. ITT is recommended for superiority trials but, for non-inferiority or equivalence trials, a PP analysis is preferred because ITT increases the risk of a type I error for such trials. The main analyses were as pre-specified in the protocol, but some additional post hoc analyses were also undertaken (these are detailed in Post hoc analyses).
The difference between lasers for change in BCVA [with 95% confidence intervals (CIs)] from baseline to month 24 (primary end point) was compared with the permitted maximum difference of 5 ETDRS letters (0.1 log-MAR). SML would be deemed non-inferior to SL if the lower limit of the 95% CI of the treatment difference was above this non-inferiority margin. If the 95% CI of the treatment difference was wholly within both the upper and lower margins of the permitted maximum difference (± 5 ETDRS letters), then SML would be deemed to be equivalent to SL.
Change in BCVA from baseline to month 24 was compared between the two intervention groups using an independent two-sample t-test with a secondary analysis using an analysis of covariance (ANCOVA) model adjusted for baseline BCVA score, baseline CRT and minimisation factors/covariates comprising centre; distance BCVA at baseline of ≥ 69 ETDRS letters (Snellen equivalent of ≥ 20/40; log-MAR ≥ 0.3) or 24–68 ETDRS letters (Snellen equivalent ≤ 20/50; log-MAR 0.4–1.2); previous use of anti-VEGF therapies in the study eye; and previous use of macular laser in the study eye.
The primary analysis was based on data from the study eye only. When performing a secondary analysis on the subset of participants with both eyes eligible and treated, study eye was included as a random effect within the mixed model. The principal analysis was based on available case data with no imputation of missing values. Intention-to-treat analyses were used for all secondary outcomes because the aim was to assess superiority for these outcomes.
Side effects of laser treatment and use of additional treatments (e.g. steroids or anti-VEGF therapy) were analysed using logistic regression models with adjustment for the minimisation covariates. Analyses of secondary measures of visual function and anatomical outcomes (MD of the 10–2 visual field test, CRT and macular volume) and number of treatments required were undertaken using linear regression models adjusted for baseline BCVA score and minimisation variables. Analysis of ‘driving ability’ (i.e. meeting standards for driving) was undertaken using a logistic regression model adjusted for baseline BCVA and the minimisation variables. The number of AEs, adverse reactions (ARs), SAEs, serious adverse reactions (SARs) and suspected unexpected serious adverse reactions (SUSARs), and the number and percentage of participants experiencing these events are reported. The chi-square test (or Fisher’s exact test if appropriate) and proportion test were used to check if incidences of AEs differed between intervention groups. Relative risks with 95% CIs are reported. Baseline characteristics, follow-up measurements and safety data are presented using appropriate descriptive summary measures depending on the scale of measurement and distribution.
Statistical diagnostic methods were used to check for violations of the model assumptions.
Statistical significance was based on two-sided tests, with p < 0.05 taken as the criterion for statistical significance and with no adjustment for multiple testing.
Sensitivity analyses
Sensitivity analyses were undertaken to assess the impact of missing data by imputing extreme values (lowest and highest) and last observation carried forward; the impact of including patients who were not treatment naive (i.e. excluding those who have had previous laser treatment for DMO in the study eye or previous anti-VEGF therapy for DMO or PDR in the study eye); and the impact of using month-24 data that were collected outside of ± 14 days of the due date.
Subgroup analyses
We conducted pre-specified subgroup analyses of the primary outcome based on clinical rationale. These subgroups were centre; distance BCVA at baseline of ≥ 69 ETDRS letters (Snellen equivalent of ≥ 20/40; log-MAR ≥ 0.3) or 24–68 ETDRS letters (Snellen equivalent ≤ 20/50; log-MAR 0.4–1.2); previous administration of anti-VEGF therapy; and previous use of macular laser in the study eye. These analyses were carried out by including the corresponding interaction term in the regression model and 99% CI.
We also conducted analyses to identify whether or not any group of participants was at high risk of poor outcomes. High-risk participants were defined as participants with a baseline glycated haemoglobin type A1c (HbA1c) value of ≥ 53 mmol/mol (≥ 7%). These were analysed in exploratory subgroup analyses.
Primary analysis
The primary analysis was performed on the mean change in BCVA in the study eye from baseline to month 24 and comprised the following: mean change in BCVA by intervention group (with SDs); difference in means with 95% CI; p-value from independent two-sample t-test; and secondary analysis using ANCOVA adjusted for baseline BCVA and minimisation variables. The non inferiority (and equivalence) margin was compared against the 95% CI for both PP and ITT analyses.
Secondary analyses
The secondary analyses, and how they were reported, were as follows:
-
Mean change in binocular BCVA from baseline to month 24 – mean (SD) by intervention group, difference in means with 95% CI and p-value from linear regression adjusted for baseline BCVA and minimisation variables
-
mean change in CRT as determined by SD-OCT, from baseline to month 24 – mean (SD) by intervention group, difference in means with 95% CI and p-value from linear regression adjusted for baseline BCVA and minimisation variables
-
mean change in the MD of the Humphrey 10–2 visual field from baseline to month 24 – mean with SD by intervention group, difference in means with 95% CI and p-value from linear regression adjusted for baseline BCVA and minimisation variables
-
change in the percentage of participants meeting driving standards from baseline to month 24 – number and percentage of participants meeting driving standards by intervention group, odds ratio with 95% CI and p-value from logistic regression adjusted for baseline BCVA and minimisation variables
-
number of participants experiencing side effects from baseline to month 24 – number and percentage of participants by intervention group, odds ratio with 95% CI and p-value from logistic regression adjusted for minimisation variables
-
number of laser treatments used in the study eye from baseline to month 24 – mean with SD by intervention group, difference in means with 95% CI and p-value from linear regression adjusted for baseline BCVA and minimisation variables
-
number of participants receiving at least one additional treatment (other than laser) from baseline to month 24 – number and percentage of participants by intervention group, odds ratio with 95% CI and p-value from logistic regression adjusted for minimisation variables.
Additional analyses specified in the statistical analysis but not in the published protocol
Additional analyses specified in the statistical analysis but not in the published protocol, and how they were reported, were as follows:
-
mean change in BCVA in the study eye from baseline to month 12 – mean with SD by intervention group, difference in means with 95% CI and p-value from ANCOVA adjusted for baseline BCVA and minimisation variables
-
mean change in binocular BCVA from baseline to month 12 – mean with SD by intervention group, difference in means with 95% CI and p-value from linear regression adjusted for baseline BCVA and minimisation variables
-
mean change in CRT, as determined by SD-OCT, from baseline to month 12 – mean with SD by intervention group, difference in means with 95% CI and p-value from linear regression adjusted for baseline BCVA and minimisation variables
-
mean change in the MD of the Humphrey 10–2 visual field from baseline to month 12 – mean with SD by intervention group, difference in means with 95% CI and p-value from linear regression adjusted for baseline BCVA and minimisation variables
-
change in the percentage of people meeting driving standards from baseline to month 12 – number and percentage of participants meeting driving standards by intervention group, odds ratio with 95% CI and p-value from logistic regression adjusted for baseline BCVA and minimisation variables
-
number of participants experiencing side effects from baseline to month 12 – number and percentage of participants by intervention group, odds ratio with 95% CI and p-value from logistic regression adjusted for minimisation variables
-
number of laser treatments used in study eye from baseline to month 12 – mean with SD by intervention group, difference in means with 95% CI and p-value from linear regression adjusted for baseline BCVA and minimisation variables
-
number of participants with at least one additional treatment (other than laser) from baseline to month 12 – number and percentage of participants by intervention group, odds ratio with 95% CI and p-value from logistic regression adjusted for minimisation variables
-
number of steroid injections as additional treatments from baseline to month 12 and from baseline to month 24 – mean with SD by intervention group, difference in means with 95% CI and p-value from linear regression adjusted for minimisation variables
-
number of participants with at least one steroid injection from baseline to month 12 and from baseline to month 24 – number and percentage of participants by intervention group, odds ratio with 95% CI and p-value from logistic regression adjusted for minimisation variables
-
number of anti-VEGF treatments as additional treatments from baseline to month 12 and from baseline to month 24 – mean with SD by intervention group, difference in means with 95% CI and p-value from linear regression adjusted for minimisation variables; and as number and percentage of participants by category (≤ 4, 5–10 and > 10)
-
number of participants with at least one anti-VEGF treatment as additional treatment from baseline to month 12 and from baseline to month 24 – number and percentage of participants by intervention group, odds ratio with 95% CI and p-value from logistic regression adjusted for minimisation variables
-
number of participants receiving rescue treatments from baseline to month 12 and from baseline to month 24 – number and percentage of participants by intervention group, odds ratio with 95% CI and p-value from logistic regression adjusted for minimisation variables
-
number of participants satisfying rescue criteria from baseline to month 12 and from baseline to month 24 – number and percentage of participants by intervention group, odds ratio with 95% CI and p-value from logistic regression adjusted for adjusted for baseline BCVA and minimisation variables
-
number of participants experiencing a loss of 10 or more ETDRS letters between baseline and month 24 – number and percentage of participants by intervention group, odds ratio with 95% CI and p-value from logistic regression adjusted for baseline BCVA and minimisation variables.
Post hoc analyses
Post hoc analyses, and how they were reported, were as follows:
-
mean change in macular volume from baseline to month 24: mean with SD by intervention group, difference in means with 95% CI and p-value from linear regression adjusted for baseline BCVA and minimisation variables
-
number of participants experiencing a loss of 5 or more ETDRS letters from baseline to month 24
-
number of participants experiencing an increase in BCVA or a BCVA loss not greater than 5 ETDRS letters from the baseline value to month 24
-
number of participants satisfying rescue criteria at least once at any time from baseline to month 24
-
number of participants satisfying rescue criteria at least once and receiving rescue treatment at any time from baseline to month 24
-
number of participants satisfying rescue criteria at least once and not receiving rescue treatment at any time from baseline to month 24
-
number of participants who did not satisfy rescue criteria at all from baseline to month 24
-
number of participants who did not satisfy rescue criteria at all but received rescue treatment from baseline to month 24.
Health economics methods
Overview
The main objective of the health economics evaluation was to conduct a short-term (baseline to 2 years’ follow-up) within-trial analysis comparing the cost-effectiveness of SML with SL in patients with DMO fulfilling the DIAMONDS trial eligibility criteria. To achieve this, a systematic comparison of the costs of resource inputs used by participants in the two treatment groups and the consequences associated with the interventions was conducted. The primary analysis adopted an NHS and Personal Social Services (PSS) perspective. The economic evaluation took the form of a cost–utility analysis, expressed in terms of incremental cost per QALY gained. Costs and outcomes beyond the first year of follow-up were discounted at 3.5% in line with the NICE reference case. 33
For the health economics analysis, we adopted an ITT approach as reported in the health economics analysis plan. ITT requires that study participants are analysed according to their treatment assignment regardless of actual treatment received. This is the approach preferred by NICE for cost-effectiveness analyses as stated in their methods guide. 33 We report the results for the per-protocol analysis in a sensitivity analysis.
Measuring resource use and costs
Data were collected on resource use and costs associated with delivery of laser treatment (direct intervention costs) and broader health service resource use during the 24 months of follow-up. All costs were expressed in Great British pounds and valued in 2019–20 prices. Where appropriate, costs were inflated to 2019–20 prices using the NHS Cost Inflation Index. 34
Direct intervention costs
Direct intervention costs were costs associated with the delivery of laser treatment. These included staff costs and equipment costs (i.e. costs of laser machines) (see Appendix 1, Table 22 and Table 23). Unit costs for staff were obtained from the Personal Social Services Research Unit (PSSRU) Unit Costs of Health and Social Care 2020 compendium34 and were multiplied by the time it took to perform specified procedures for each participant. The time it took to undertake various procedures was recorded on the DIAMONDS trial CRFs and comprised: (1) the time it took imaging technicians to obtain FFA and SD-OCT scans to guide laser treatment and (2) the time taken by consultants and associate specialists to perform the laser procedure, as well as the time invested in counselling the patient. Costs of laser machines were obtained directly from manufacturers. An annual equivalent cost of equipment was obtained by annuitising the capital costs of the item over its useful life span, applying a discount rate of 3.5% per annum. We derived a per-patient cost of equipment (including annual maintenance costs) by assuming that the machine will be used to perform laser procedures on 3000 patients per year (Professor Noemi Lois, Queen’s University Belfast, 2021, personal communication).
Measuring and valuing resource use
Resource use data were captured on trial CRFs at scheduled clinic visits over the 24-month follow-up period, at 4, 8, 12, 16, 20 and 24 months. On-site monitoring visits during the trial ensured the accuracy of entries in the CRF. The CRFs captured details related to the eye condition of inpatient and day case admissions, outpatient attendances, other tests or investigations, medication use including anti-VEGF therapy/steroids or other rescue treatments, and laser retreatments.
Resource inputs were valued by attaching unit costs derived from national compendia in accordance with NICE’s Guide to the Methods of Technology Appraisal. 33 The key databases for deriving unit cost data included the Department of Health and Social Care’s Reference Costs 2018–19 schedules,35 the PSSRU’s Unit Costs of Health and Social Care 2019 compendium34 and volume 80 of the British National Formulary. 36 Appendix 1, Table 22 gives a summary of the unit cost values and data sources for broader resource use categories identified within the follow-up questionnaires.
The following assumptions were made in costing outpatient attendances. Where an outpatient attendance was reported but no procedure undertaken, the average unit cost of an outpatient ophthalmology visit was used; this varied between £80 and £101 per consultation depending on whether the consultation was ‘non-consultant’ or ‘consultant-led’. We used data captured on trial CRFs documenting the grade of professional that attended to the patient to select the most appropriate unit costs. For example, if a consultant attended to the patient, then the consultant-led unit cost was applied. Where a procedure(s) was undertaken as part of the visit, the relevant Healthcare Resource Group (HRG) code was derived using the HRG4 + Reference Costs Grouper Software (NHS Digital, Leeds, UK). High-cost drugs (specifically anti-VEGF therapy) were separately costed as these are considered an unbundled HRG. We also costed steroid rescue treatment. Costing of laser retreatments followed the same approach as costing for the index (first-session) laser procedure.
Summary statistics were generated for resource use variables by treatment allocation and assessment point. Mean resource use and cost values were compared between groups using two-sample t-tests. Differences between groups, along with 95% CIs, were estimated using non-parametric bootstrap estimates (10,000 replications).
Health outcomes
The primary outcome of the within-trial economic evaluation is the QALY, as recommended in the NICE reference case. 33 The QALY is a measure that combines quantity and quality of life lived into a single metric, with one QALY equating to 1 year of full health. QALY estimates are generated from combining length (survival) and health-related quality of life (HRQoL) data from participants for the period covering the trial time horizon through an area under the curve (AUC) approach using a linear extrapolation. 37 Since AUC estimates are predicted to correlate with baseline scores (and thus potential baseline imbalances), AUC estimates were adjusted for baseline scores within regression analyses. HRQoL was converted into health-state utilities indexed at 0 and 1, where 0 represents death and 1 represents full health. Patients who died during the study were subsequently scored 0 at later scheduled follow-up visits for both cost and HRQoL scores and were included as observed data.
To calculate QALYs, it is imperative to obtain health state values for participants within the trial. The HRQoL of trial participants was assessed at baseline and at 12 and 24 months’ post randomisation using the EQ-5D-5L instrument. The EQ-5D-5L consists of the descriptive system and the visual analogue scale. The descriptive system includes five questions addressing mobility, self-care, usual activities, pain/discomfort and anxiety/depression, with each dimension assessed at five levels from no problems to extreme problems. Responses to the EQ-5D-5L instrument were converted into health utility scores using the EQ-5D-5L Crosswalk Index Value Calculator currently recommended by NICE, which maps the EQ-5D-5L descriptive system data onto the EuroQol-5 Dimensions, three-level version (EQ-5D-3L) valuation set. 38 A detailed description of the mapping methodology is provided in the study by van Hout et al. 38
Health-related quality of life was also assessed by determining the vision-related quality of life using two vision specific measures: the NEI-VFQ-25 and the VisQoL. 39–41 The NEI-VFQ-25 is a vision-specific patient-reported quality of life tool. This validated questionnaire has been used widely to evaluate visual outcomes in patients with eye diseases, including diabetic retinopathy. In addition to eliciting information about general health and vision, it specifically addresses difficulty with near vision, distance vision, driving and the effect of light conditions on vision, providing a comprehensive evaluation of vision-related quality of life. The NEI-VFQ-25 scoring is done in two-stages: (1) each item is scored on a scale of 0 (lowest) to 100 (highest), where a higher score represents better functioning; and (2) items within each subscale are averaged together (11 subscales in total for the NEI-VFQ-25). To obtain the combined score for the questionnaire, the average of the subscales (excluding the general health rating question) is undertaken. Averaging across the subscales scores, rather than individual items, gives equal weight to each subscale.
The VisQol questionnaire is shorter than the NEI-VFQ-25 with only six attributes: physical well-being, independence, social well-being, self-actualisation, planning and organisation. However, it has not been widely validated. The utilities for VisQoL were developed using a time trade-off exercise in people who were visually impaired including patients with age-related macular degeneration, diabetic retinopathy and glaucoma. 41
The health utility values and QALYs accrued over the 24-month follow-up period were summarised by treatment group and assessment point and presented as means and associated standard errors (SEs). Between-group differences were compared using the two-sample t-test, in a similar way to the descriptive analyses of resource inputs and costs.
Handling of missing data
Multiple imputation by chained equations was used to predict missing health status (utility) scores and costs based on the assumption that data were missing at random (MAR). The MAR assumption was tested through a series of logistic regression analyses comparing participants’ characteristics for those with and without missing end-point data. Imputation was achieved using predictive mean matching, which has the advantage of preserving non–linear relationships and correlations between variables within the data. 42 Twenty imputed data sets were generated and used to inform the base-case analyses. Parameter estimates were pooled across the 20 imputed data sets using Rubin’s rules to account for between- and within-imputation components of variance terms associated with parameter estimates. 43 Imputed and observed values were compared to establish that imputation did not introduce bias into subsequent estimation.
Cost-effectiveness analysis
The base-case cost-effectiveness analysis was performed using an ITT approach. Mean incremental costs and QALYs were estimated using seemingly unrelated regression methods that account for the correlation between costs and outcomes. The seemingly unrelated regression adjusted for the following covariates: baseline utilities, baseline body mass index (BMI), baseline BCVA, patient-reported previous use of anti-VEGF therapy at baseline and previous use of macular laser. The joint distributions of costs and outcomes were generated using non-parametric bootstrap methods and used to populate the cost-effectiveness plane. The incremental cost-effectiveness ratio (ICER) for SML compared with SL was calculated by dividing the between-group difference in adjusted mean total costs by the between-group difference in adjusted mean QALYs. Mean ICER values were compared against cost-effectiveness threshold values ranging between £15,000 and £30,000 per QALY gained, in line with NICE guidance. 33 The cost-effectiveness thresholds provide an indication of society’s willingness to pay for an additional QALY; lower ICER values than the threshold could be considered cost-effective for use in the UK NHS. The incremental net monetary benefit (NMB) of switching from SL to SML was calculated for cost-effectiveness thresholds ranging from £15,000 to £30,000 per QALY gained. NMB is calculated as the net benefit of an intervention (expressed in monetary terms) considering all costs associated with the intervention. A positive incremental NMB, suggests that, on average, SML is cost-effective compared with SL at the given cost-effectiveness threshold. In that case, the cost to derive the benefit is less than the maximum amount that the decision-maker would be willing to pay for this benefit. 44
Trial management
The chief investigator had overall responsibility for the conduct of the DIAMONDS trial. The chief investigator and NICTU undertook trial management including clinical trial applications (Ethics and Research Governance), site initiation and training, monitoring, analysis and reporting. The trial co-ordinator was responsible on a day-to-day basis for overseeing and co-ordinating the work of the multi-disciplinary trial team and was the main contact between the trial team and other parties. Before the DIAMONDS trial started, site training ensured that all relevant essential documents and trial supplies were in place and that site staff were fully aware of the study protocol and procedures. The following trial committees were established.
Trial Management Group
The Trial Management Group (TMG) was chaired by the chief investigator and included representation from the NICTU and other investigators or collaborators involved in the study. The TMG had responsibility for the day-to-day operational management of the DIAMONDS trial and met monthly throughout the trial to discuss and monitor its progress.
Trial Steering Committee
The conduct of DIAMONDS was overseen by a TSC, on behalf of the sponsor and funder. The TSC comprised an independent chair, additional independent members (including a patient representative) and members of the trial team (including the chief investigator). TSC meetings were arranged so that they coincided with meetings of the DMEC, to allow members in both committees to discuss issues and recommendations raised by the DMEC.
Data Monitoring and Ethics Committee
The DMEC was responsible for safeguarding the interests of participants in the DIAMONDS trial. The DMEC monitored the main outcome measures including safety and efficacy. The DMEC comprised two clinicians and a statistician who were independent of the trial.
DIAMONDS patient and public involvement group
At the very early stages of the DIAMONDS trial conception, a DIAMONDS patient and public involvement (PPI) group was established. The DIAMONDS PPI group contributed to the trial design, including the selection of outcomes, preparation of patient-related materials for the trial, recruitment strategies, interpretation of trial results and preparation of the Plain English summary.
Sponsor
The Belfast Health and Social Care Trust (BHSCT) was the sponsor for the DIAMONDS trial.
Reporting
The reporting of the DIAMONDS trial follows the Consolidated Standards of Reporting Trials (CONSORT) guidelines45 and guidelines on reporting for equivalence and non-inferiority trials. 46
Changes in trial methodology since trial conception
There were no changes made in the design, outcomes or conductance of the DIAMONDS trial after trial commencement.
Chapter 3 Clinical effectiveness
Participating sites and characteristics of DIAMONDS trial participants
Table 2 lists the 16 participating sites, the date when they were opened to recruitment and the total number of participants screened and recruited at each site.
| Site code | Site name | Date site opened | Total screened | Total recruited |
|---|---|---|---|---|
| 01 | Belfast Health and Social Care Trust | 18 January 2017 | 44 | 38 |
| 02 | Bristol Eye Hospital | 19 April 2017 | 23 | 19 |
| 03 | Frimley Park Hospital | 28 February 2017 | 49 | 42 |
| 04 | Hinchingbrooke Hospital | 20 July 2017 | 12 | 12 |
| 05 | London King’s College Hospital | 03 April 2017 | 32 | 29 |
| 06 | Manchester Royal Eye Hospital | 22 March 2017 | 25 | 12 |
| 07 | Moorfields Eye Hospital | 22 February 2017 | 27 | 20 |
| 08 | Newcastle Eye Centre | 12 April 2017 | 16 | 15 |
| 09 | John Radcliffe Hospital (Oxford) | 04 April 2017 | 12 | 6 |
| 10 | Sheffield Royal Hallamshire Hospital | 28 February 2017 | 14 | 10 |
| 11 | Sunderland Eye Infirmary | 24 February 2017 | 30 | 21 |
| 12 | Bradford Hospital | 18 October 2017 | 12 | 10 |
| 13 | James Cook Hospital (South Tees) | 13 October 2017 | 20 | 16 |
| 14 | Hull & East Yorkshire Hospitals NHS Trust | 17 August 2018 | 11 | 8 |
| 15 | Stoke Mandeville Hospital | 05 September 2018 | 2 | 1 |
| 16 | Hillingdon Hospital | 14 September 2018 | 7 | 7 |
Participants
Participant flow
The CONSORT flow diagram in Figure 2 details the flow of patients through the DIAMONDS trial.
FIGURE 2.
Participant flow in the DIAMONDS trial. a, Total number of reasons can be greater than number of patients excluded as patients can have multiple exclusion reasons; b, n = 1 patient withdrew permission for their data to be used and will not be included in any analysis going forward. Reproduced with permission from Lois et al. 47 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure above includes minor additions and formatting changes to the original figure.
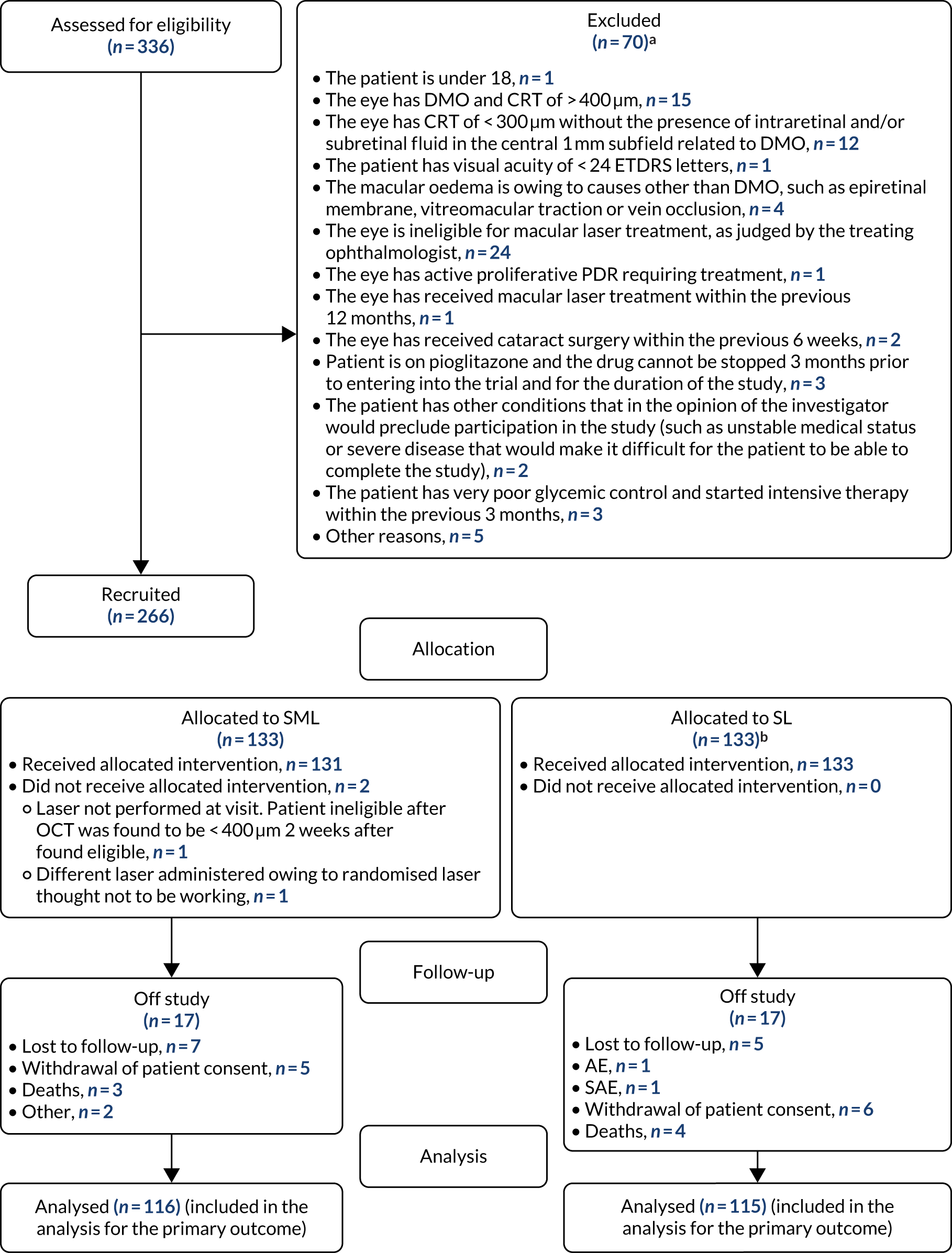
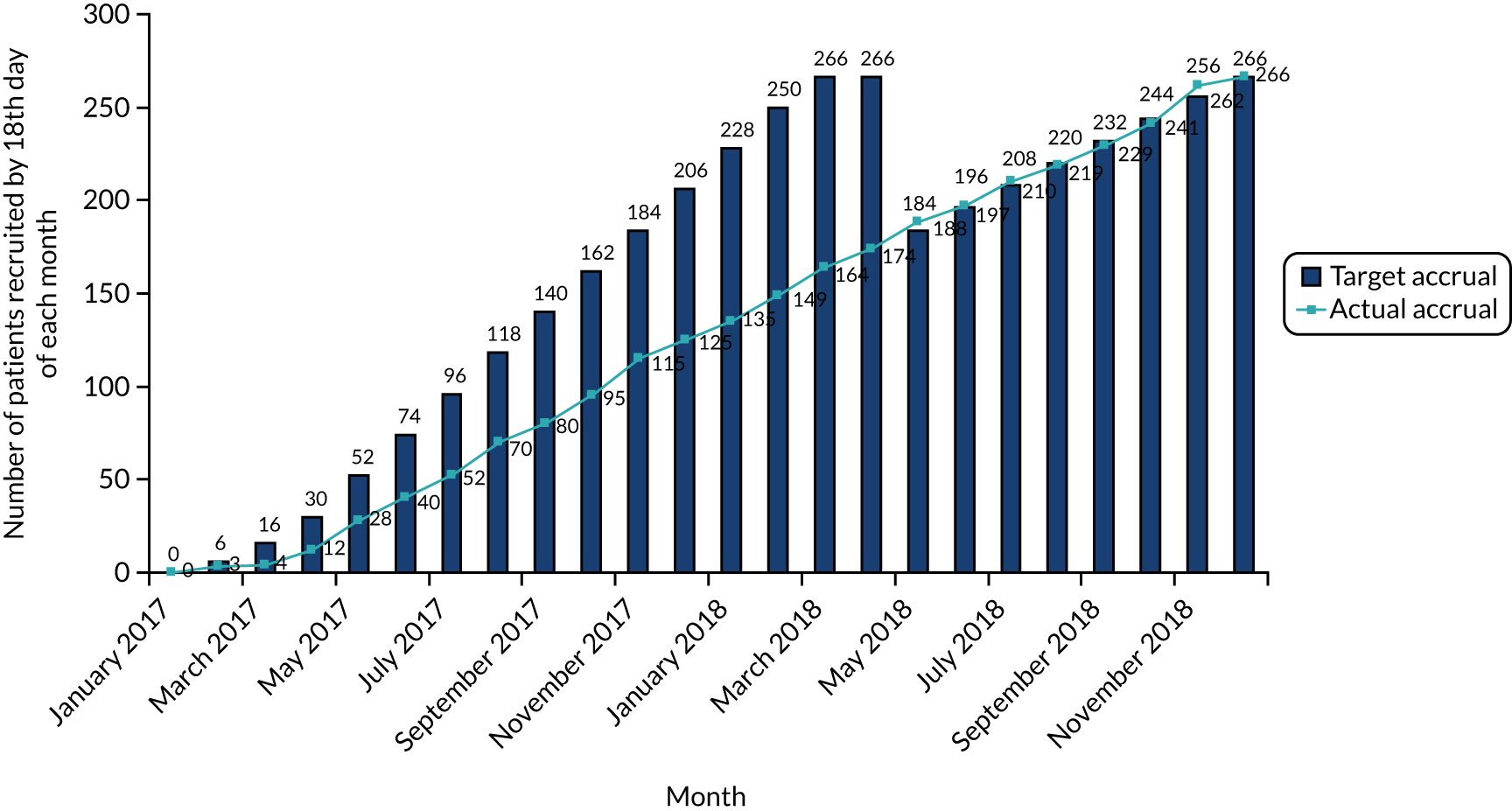
Patient recruitment took place between 18 January 2017 and 20 November 2018. A total of 336 participants were assessed for eligibility and 266 (79%) of those assessed as eligible agreed to join the trial and were randomised (intervention, n = 133; control, n = 133). One participant withdrew consent for their data to be used and was excluded from all analyses. The first month 24 follow-up visit was conducted on 25 January 2019 and the final month 24 follow-up visit was conducted on 22 December 2020. Recruitment was initially due to be completed by April 2018 but, at that time, the recruitment of participants had not been completed. An extension was subsequently approved to extend this timeline until December 2018; recruitment was then completed. The cumulative patient recruitment against the anticipated pre-trial sample size is shown in Figure 3.
FIGURE 3.
Patient recruitment.
Patient baseline characteristics
Patient baseline characteristics were broadly similar across intervention groups (Table 3).
| Baseline characteristics at trial entry | Treatment group | Total, N = 265 (100.0%)a | |
|---|---|---|---|
| SML, N = 133 (50.2%) | SL, N = 132 (49.8%) | ||
| Sex, n (%) | |||
| Male | 91 (68.4) | 95 (72.0) | 186 (70.2) |
| Female | 42 (31.6) | 37 (28.0) | 79 (29.8) |
| Age (years), mean (SD) | 61.9 (10.1) | 62.6 (10.4) | 62.2 (10.3) |
| Ethnicity, n (%) | |||
| White | 105 (79.0) | 100 (75.8) | 205 (77.4) |
| Asian | 15 (11.3) | 15 (11.4) | 30 (11.3) |
| Black (African) | 12 (9.0) | 14 (10.6) | 26 (9.8) |
| Other | 1 (0.8) | 2 (1.5) | 3 (1.1) |
| Middle East | 0 (0.0) | 1 (0.8) | 1 (0.4) |
| Black (African American) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hispanic | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Diabetes | |||
| Type 1, n (%) | 20 (15.0) | 18 (13.6) | 38 (14.3) |
| Duration of type 1 (years), mean (SD) | 28.8 (12.9) | 23.6 (9.0) | 26.4 (11.4) |
| Type 2, n (%) | 113 (85.0) | 113 (85.6) | 226 (85.3) |
| Duration of type 2 (years), mean (SD) | 16.0 (8.4) [n = 112] | 15.3 (6.7) [n = 112] | 15.7 (7.6) [n = 224] |
| Other, n (%) | 0 (0.0) | 1 (0.8) | 1 (0.4) |
| Duration of other (years), mean (SD) | – | 14.3 (–) | 14.3 (–) |
| Smoking status | |||
| Current smoker, n (%) | 10 (7.5) | 7 (5.3) | 17 (6.4) |
| Number of years smoked (current smoker), mean (SD) | 27.9 (15.1) | 38.7 (7.9) | 32.4 (13.5) |
| Past smoker, n (%) | 50 (37.6) | 46 (34.9) | 96 (36.2) |
| Number of years smoked (past smoker), mean (SD) | 16.5 (12.8) [n = 47] | 19.1 (13.2) [n = 46] | 17.8 (13.0) [n = 93] |
| Never smoked, n (%) | 73 (54.9) | 79 (59.9) | 152 (57.4) |
| DMO diagnosis (study eye) | |||
| Present, n (%) | 133 (100.0) | 132 (100.0) | 265 (100.0) |
| Mean (SD) duration of diagnosis (years) | 3.0 (5.8) | 2.1 (2.6) | 2.5 (4.5) |
| Absent, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| DMO diagnosis (non-study eye) | |||
| Present, n (%) | 93 (69.9) | 82 (62.1) | 175 (66.0) |
| Duration of diagnosis (years), mean (SD) | 4.1 (6.7) | 2.9 (3.2) | 3.5 (5.3) |
| Absent, n (%) | 40 (30.1) | 50 (37.9) | 90 (34.0) |
| Previous DMO laser treatment (study eye) | |||
| Yes, n (%) | 32 (24.1) | 32 (24.2) | 64 (24.2) |
| No, n (%) | 101 (75.9) | 100 (75.8) | 201 (75.9) |
| Number of previous DMO laser sessions, median (IQR) | 1 (1–2) [n = 30] | 1 (1–2) | 1 (1–2) [n = 62] |
| Time since last DMO laser session (years), mean (SD) | 4.7 (6.6) [n = 30] | 3.7 (2.1) | 4.2 (4.8) [n = 62] |
| Previous DMO laser treatment (non-study eye) | |||
| Yes, n (%) | 39 (29.3) | 33 (25.0) | 72 (27.2) |
| No, n (%) | 94 (70.7) | 99 (75.0) | 193 (72.8) |
| Number of previous DMO laser sessions, median (IQR) | 1 (1–2) [n = 37] | 1 (1–2) | 1 (1–2) [n = 70] |
| Time since last DMO laser session (years), mean (SD) | 5.3 (6.7) [n = 37] | 4.4 (3.1) [n = 32] | 4.9 (5.3) [n = 69] |
| Previous anti-VEGF therapies (study eye), n (%) | |||
| Bevacizumab (Avastin®, Roche): yes | 1 (0.8) | 1 (0.8) | 2 (0.8) |
| Bevacizumab: no | 132 (99.3) | 131 (99.2) | 263 (99.3) |
| Ranibizumab (Lucentis®, Roche): yes | 14 (10.5) | 11 (8.3) | 25 (9.4) |
| Ranibizumab: no | 119 (89.5) | 121 (91.7) | 240 (90.6) |
| Aflibercept (Eylea®, Bayer): yes | 9 (6.8) | 5 (3.8) | 14 (5.3) |
| Aflibercept: no | 124 (93.2) | 127 (96.2) | 251 (94.7) |
| Pegaptanib (Macugen®, Pfizer Inc.): yes | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Pegaptanib: no | 133 (100.0) | 132 (100.0) | 265 (100.0) |
| Any previous anti-VEGF therapies in study eye | 17 (12.8) | 14 (10.6) | 31 (11.7) |
| Previous anti-VEGF therapies (non-study eye), n (%) | |||
| Bevacizumab: yes | 3 (2.3) | 2 (1.5) | 5 (1.9) |
| Bevacizumab: no | 129 (97.7) | 130 (98.5) | 259 (98.1)c |
| Ranibizumab: yes | 22 (16.7) | 12 (9.1) | 34 (12.9) |
| Ranibizumab: no | 110 (83.3) | 120 (90.9) | 230 (87.1)c |
| Aflibercept: yes | 11 (8.3) | 7 (5.3) | 18 (6.8) |
| Aflibercept: no | 122 (91.7) | 125 (94.7) | 247 (93.2) |
| Pegaptanib: yes | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Pegaptanib: no | 133 (100.0) | 132 (100.0) | 265 (100.0) |
| Any previous anti-VEGF therapies in non-study eye | 28 (21.1) | 19 (14.4) | 47 (17.7) |
| Anti-VEGF treatments during trial (study eye) | |||
| Number of anti-VEGF injections, median (IQR) | 7 (5–10) [n = 1] | 8.5 (4–13) [n = 14] | 8 (4–12) [n = 31] |
| Time since last anti-VEGF injection (years), mean (SD) | 0.9 (0.9) [n = 17] | 0.9 (1.0) [n = 14] | 0.9 (0.9) [n = 31] |
| Anti-VEGF treatments during trial (study eye) | |||
| Number of anti-VEGF injections, median (IQR) | 7 (5–9.5) [n = 28] | 8 (5–13) [n = 19] | 7 (5–11) [n = 47] |
| Time since last anti-VEGF injection (years), mean (SD) | 1.5 (2.0) [n = 28] | 0.6 (1.1) [n = 19] | 1.1 (1.7) [n = 47] |
| History of PDR (study eye), n (%) | |||
| Yes | 17 (12.8) | 6 (4.6) | 23 (8.7) |
| No | 116 (87.2) | 126 (95.5) | 242 (91.3) |
| History of PDR (non-study eye), n (%) | |||
| Yes | 17 (12.8) | 9 (6.8) | 26 (9.8) |
| No | 116 (87.2) | 123 (93.2) | 239 (90.2) |
| Previous PRP (study eye) | |||
| Yes, n (%) | 17 (12.8) | 6 (4.6) | 23 (8.7) |
| No, n (%) | 116 (87.2) | 126 (95.5) | 242 (91.3) |
| Time since last PRP session (years), mean (SD) | 6.3 (11.7) [n = 16] | 1.7 (0.9) [n = 4] | 5.4 (10.6) [n = 20] |
| Previous PRP (non-study eye) | |||
| Yes, n (%) | 17 (12.8) | 7 (5.3) | 24 (9.1) |
| No, n (%) | 116 (87.2) | 125 (94.7) | 241 (90.9) |
| Time since last PRP session (years), mean (SD) | 6.2 (11.3) [n = 16] | 1.2 (1.0) [n = 5] | 5.0 (10.0) [n = 21] |
| Lens status (study eye), n (%) | |||
| Phakic: yes | 106 (79.7) | 116 (87.9) | 222 (83.8) |
| Phakic: no | 27 (20.3) | 16 (12.1) | 43 (16.2) |
| Pseudophakic: yes | 27 (20.3) | 16 (12.1) | 43 (16.2) |
| Pseudophakic: no | 106 (79.7) | 116 (87.9) | 222 (83.8) |
| Aphakic: yes | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Aphakic: no | 133 (100.0) | 132 (100.0) | 265 (100.0) |
| Lens status (non-study eye), n (%) | |||
| Phakic: yes | 104 (78.2) | 119 (90.2) | 223 (84.2) |
| Phakic: no | 29 (21.8) | 13 (9.9) | 42 (15.9) |
| Pseudophakic: yes | 29 (21.8) | 13 (9.9) | 42 (15.9) |
| Pseudophakic: no | 104 (78.2) | 119 (90.2) | 223 (84.2) |
| Aphakic: yes | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Aphakic: no | 133 (100.0) | 132 (100.0) | 265 (100.0) |
| Weight (kg), mean (SD) | 92.3 (19.2) [n = 129] | 90.6 (19.1) [n = 129] | 91.4 (19.1) [n = 258] |
| Height (cm), mean (SD) | 170.9 (9.8) [n = 130] | 170.2 (10.2) [n = 130] | 170.6 (10.0) [n = 260] |
| BMI | |||
| Overall (kg/m2), mean (SD) | 31.8 (7.5) [n = 129] | 31.2 (6.2) [n = 129] | 31.5 (6.9) [n = 258] |
| BMI < 18.5 (underweight), n (%) | 0 (0.0) | 1 (0.8) | 1 (0.4) |
| BMI 18.5–24.9 (healthy weight), n (%) | 14 (10.5) | 17 (12.9) | 31 (11.7) |
| BMI 25–29.9 (overweight), n (%) | 47 (35.3) | 48 (36.4) | 95 (35.9) |
| BMI 30–39.9 (obese), n (%) | 50 (37.6) | 52 (39.4) | 102 (38.5) |
| BMI ≥ 40 (morbidly obese), n (%) | 22 (16.5) | 14 (10.6) | 36 (13.6) |
| HbA1c mmol/mol, mean (SD) | 69.8 (17.8) [n = 130] | 69.1 (18.9) | 69.5 (18.4) [n = 262] |
| HbA1c (%), mean (SD)b | 8.5 (3.8) | 8.5 (3.9) | 8.5 (3.8) |
| BCVA | |||
| Study eye (ETDRS letters), mean (SD) | 80.2 (8.2) | 80.1 (8.7) | 80.2 (8.4) |
| ≥ 69 ETDRS letters, n (%) | 121 (91.0) | 122 (92.4) | 243 (91.7) |
| 24–68 ETDRS letters, n (%) | 12 (9.0) | 10 (7.6) | 22 (8.3) |
| Non-study eye (ETDRS letters), mean (SD) | 79.5 (11.0) | 78.9 (14.4) | 79.2 (12.8) |
| CRT (µm), mean (SD) | |||
| Study eye | 326.0 (38.7) | 332.6 (35.6) | 329.2 (37.3) |
| Non-study eye | 303.7 (55.8) | 307.6 (46.3) [n = 130] | 305.6 (51.3) [n = 263] |
| Study eye selection, n (%) | |||
| One eye eligible | 125 (94.0) | 119 (90.2) | 244 (92.1) |
| Both eyes eligible | 8 (6.0) | 13 (9.8) | 21 (7.9) |
| Patient meets UK driving standards, n (%) | |||
| Yes | 126 (96.2) | 129 (97.7) | 255 (97.0) |
| No | 5 (3.8) | 3 (2.3) | 8 (3.0)d |
| Treatment naive (study eye), n (%) | |||
| Yes | 92 (69.2) | 94 (71.2) | 186 (70.2) |
| No | 41 (30.8) | 38 (28.8) | 79 (29.8) |
| Treatment naive (non-study eye), n (%) | |||
| Yes | 81 (60.9) | 87 (65.9) | 168 (63.4) |
| No | 52 (39.1) | 45 (34.1) | 97 (36.6) |
With respect to the trial inclusion criteria, all participants had DMO present in the study eye with an overall mean duration of diagnosis of 2.5 years (SD 4.5 years). In addition, 24.2% (n = 64) of participants had received previous macular laser treatment prior to joining the trial, with a mean number of laser sessions of 1 [median 1, interquartile range (IQR) 1–2] and a mean length of time since the last laser session of 4.2 years (SD 4.8 years). The mean CRT was 329.2 µm (SD 37.3 µm) and the mean BCVA was 80.2 ETDRS letters (SD 8.4 ETDRS letters).
The randomisation minimisation factors were as follows:
-
site – sites participating in the DIAMONDS trial are shown in Table 2
-
distance BCVA at baseline – 91.7% (n = 243) of participants had ≥ 69 ETDRS letters and 8.3% (n = 22) of participants had 24–68 ETDRS letters at baseline
-
previous use of anti-VEGF therapy in the study eye – 11.7% (n = 31) of participants reported previous use of anti-VEGF therapies in the study eye, with a mean number of injections of 8 (median 8, IQR 4–12), and a mean length of time since last injection of 0.9 years (SD 0.9 years)
-
previous use of macular laser in the study eye – 24.2% (n = 64) of participants reported previous use of macular laser in the study eye.
The majority of participants were male (70.2%, n = 186) and sex distribution was similar between treatment groups. The overall mean age was 62.2 years (SD 10.3 years). A total of 85% (n = 226) of participants had type 2 diabetes with a mean duration of diabetes since diagnosis of 15.7 years (SD 7.6 years); type 1 diabetes was noted in 14.3% (n = 38) of participants, with a mean duration of 26.4 years (SD 11.4 years) [in one (0.4%) participant ‘other’ type of diabetes was noted in the CRF with a duration of 14.3 years). Most participants were overweight, obese, or morbidly obese (88%), with a mean HbA1c value of 69.5 mmol/mol (SD 18.4 mmol/mol) or 8.5% (SD 3.8%).
Table 3 presents the mean and SD or median and IQR for continuous data, and the number and percentage for categorical data for the baseline information in the treatment groups.
Treatment after trial entry
Participants were followed up at 4-month intervals (baseline, and at months 4, 8, 12, 16, 20 and 24) for a total of seven visits. Table 4 summarises protocol deviations reported by treatment group for both, number of events and number of patients. There were a total of 83 protocol deviations (4%) reported relating to missed visits out of the total possible number of visits (1855) which affected 58 participants, balanced across both groups [SML, n = 31 (23%) and SL, n = 41 (21%)].
| Category | SML group (N = 133) | SL group (N = 132) | ||
|---|---|---|---|---|
| Number of events (n) | Number of patients, n (%) | Number of events (n) | Number of patients, n (%) | |
| Classification: major | ||||
| Did not meet eligibility criteria for rescue treatment | 7 | 7 (5.3) | 10 | 10 (7.6) |
| Eligibility | 2 | 2 (1.5) | 1 | 1 (0.8) |
| Study intervention not administered as per protocol | 2 | 2 (1.5) | 0 | 0 (0.0) |
| Classification: minor | ||||
| HbA1c outside baseline assessment window | 13 | 12 (9.0) | 7 | 6 (4.5) |
| Incorrect version of PIS/CF used: patient reconsented | 4 | 4 (3.0) | 3 | 3 (2.3) |
| Late CRF submission | 4 | 4 (3.0) | 3 | 3 (2.3) |
| Late SAE reporting | 3 | 3 (2.3) | 1 | 1 (0.8) |
| Missed visit | 42 | 31 (23.3) | 41 | 27 (20.5) |
| Missing data | 59 | 47 (35.3) | 63 | 43 (32.6) |
| Other: image | 0 | 0 (0.0) | 10 | 3 (2.3) |
| Randomisation error due to incorrect data entry by site | 26 | 26 (19.5) | 24 | 24 (18.2) |
| Site staff training and delegation | 3 | 3 (2.3) | 7 | 7 (5.3) |
| Study intervention not administered as per protocol | 4 | 4 (3.0) | 0 | 0 (0.0) |
| Treatment allocation recorded in medical notes | 8 | 8 (6.0) | 7 | 7 (5.3) |
| Unmasking of treatment allocation | 1 | 1 (0.8) | 0 | 0 (0.0) |
| Visit outside visit window | 77 | 58 (43.6) | 80 | 60 (45.5) |
Overall, there were 35 participants not available for the main primary outcome analyses: 12 participants were lost to follow up, 11 participants withdrew consent to continue in the trial but gave permission for their data to be used, 1 was lost to follow-up because of an adverse event, 1 was lost to follow-up because of a serious adverse event, 7 patients died and 2 were lost for ‘other’ reasons. One participant withdrew completely from the trial, also withdrawing permission for any of their data to be used.
Of the 265 participants randomised and included in the analysis, 50% (n = 133/265) of participants were randomised to receive SML and 50% (n = 132/265) were randomised to receive SL. The majority received treatment as allocated, with 0.8% (n = 2) of participants not receiving allocated treatment at baseline: one participant was found to be ineligible and did not receive any laser and another received SL because the local team considered the SML not to be working appropriately.
Treatment after trial entry for the study eye is shown in Table 5 (and for the fellow eye in Appendix 1, Table 24).
| Treatment after trial entry (study eye) | Treatment group | |
|---|---|---|
| SML (N = 133) | SL (N = 132) | |
| Spot size (µm), n (%) | ||
| 50 | 0 (0.0) | 3 (2.3) |
| 60 | 0 (0.0) | 8 (6.1) |
| 80 | 0 (0.0) | 2 (1.5) |
| 100 | 0 (0.0) | 88 (66.7) |
| 160 | 0 (0.0) | 2 (1.5) |
| 200 | 131c (100.0) | 29 (22.0) |
| Duration (ms), n (%)a | ||
| 10 | 0 (0.0) | 50 (37.9) |
| 20 | 0 (0.0) | 60 (45.5) |
| 50 | 0 (0.0) | 1 (0.8) |
| 70 | 0 (0.0) | 1 (0.8) |
| 100 | 0 (0.0) | 20 (15.2) |
| 200 | 131c (100.0) | 0 (0.0) |
| Laser power (mW) (micropulse power for DSML), mean (SD) | 256.1 (63.3) [n = 128] | 120.7 (37.8) |
| Number of spots, mean (SD) | 356.9 (215.0) [n = 128] | 66.4 (53.9) |
| Number of treatments, mean (SD) | 2.4 (1.7) | 1.9 (1.2) |
| Number of treatments, n (%) | ||
| 1 | 53 (39.9) | 66 (50.0) |
| 2 | 37 (27.8) | 37 (28.0) |
| 3 | 17 (12.8) | 17 (12.9) |
| 4 | 9 (6.8) | 4 (3.0) |
| 5 | 4 (3.0) | 6 (4.6) |
| 6 | 9 (6.8) | 2 (1.5) |
| 7 | 4 (3.0) | 0 (0.0) |
| Withdrawal of consent (per patient), n | ||
| Refused use of data already collected | 0 | 1 |
| Refused permission for clinical data to be reviewed | 0 | 1 |
| Did not receive allocated treatment (which includes those who received no treatment and those who received the treatment of other group), n (%) | 2 (1.5) | 0 (0.0) |
| Received treatment of other group, n (%)b | 1 (0.8) | 0 (0.0) |
A total of 512 protocol deviations were recorded, 255 (49.8%) in the SML group and 257 (50.2%) in the SL group (Table 4); 22 of these were classified as major by the chief investigator and NICTU, 11 (50.0%) in the SML group and 11 (50.0%) in the SL group. The two major deviations classified as ‘study intervention not administered as per protocol’ related to one participant who was randomised to SML but received SL in error at the month 4 visit, and one participant who was randomised to SML but received SL at the baseline visit. The three major deviations classified as “eligibility” related to one participant who met the inclusion criteria and was randomised but when rechecked during the baseline visit was found to have OCT > 400 µm and so laser was not performed; one participant who was randomised but later identified as being treated with pioglitazone; and one participant who was mistakenly randomised with a baseline OCT of 401 µm. The remaining 17 major protocol deviations related to rescue treatment, that is the patient did not meet eligibility criteria for rescue treatment.
The 50 minor deviations classified as ‘randomisation error due to incorrect data entry by site’ related to an issue that was identified with data entry into the Sealed Envelope electronic randomisation system following a reconciliation with the clinical trial database on MACRO version 4.9.1 (Ennov UK, Eaton Socon, UK). It was found that Sealed Envelope contained some data entry errors relating to the minimisation criteria, and it was not possible to amend the data on the Sealed Envelope system. Protocol deviations were recorded on the clinical trial database for all patients affected. These deviations were classed as minor as they did not affect the randomisation process itself. The four minor deviations classified as ‘study intervention not administered as per protocol’ related to one patient who received SML administered with lower power than what was required at one of the laser sessions and three patients who received treatment only on certain areas of the macula, rather than the full grid as advised in the pertinent SOP.
Primary outcome: mean change in best-corrected visual acuity in the study eye at 24 months after treatment
The primary analysis was PP and included all participants who satisfied the PP criteria and had BCVA data at baseline and month 24. Of the 265 participants randomised (excluding the single patient that fully withdrew consent for their data to be used), primary outcome data were available for 87% (n = 231; SML, n = 116 and SL, n = 115).
The primary outcome was the mean change in BCVA in the study eye from baseline to month 24. The difference between lasers in change in BCVA (with a 95% CI) from baseline to month 24 (primary endpoint) was compared with the permitted maximum difference of 5 ETDRS letters (0.1 log-MAR) in the non-inferiority analysis. The mean change in BCVA in the study eye from baseline to month 24 was –2.43 ETDRS letters (SD 8.2 ETDRS letters) in the SML group and –0.45 ETDRS letters (SD 6.72 ETDRS letters) in the SL group. The difference in the mean change in BCVA in the study eye from baseline to month 24 was –1.98 ETDRS letters (95% CI –3.9 to –0.0 ETDRS letters; p = 0.046), which although statistically significant was not clinically relevant. Therefore, SML was deemed to be non-inferior to SL because the lower limit of the 95% confidence interval of the treatment difference (–3.9 ETDRS letters) was above the non-inferiority margin (–5.0 ETDRS letters). Furthermore, SML was also deemed equivalent to SL as the 95% CI (–3.9 to –0.0 ETDRS letters) was wholly within both the upper and lower margins of the permitted maximum difference (–5.0 to 5.0 ETDRS letters). An ITT analysis was also undertaken, which supported the findings from the PP analysis. Table 6 displays the analysis results for the primary outcome for both the PP and ITT analyses.
| Analysis | Mean change in BCVA in the study eye from baseline to month 24 (ETDRS letters), mean (SD) | Difference in ETDRS letters (95% CI) | p-valueb | |
|---|---|---|---|---|
| SML group (n = 116a) | SL group (n = 115a) | |||
| PP analysis | –2.43 (8.20) [n = 115] | –0.45 (6.72) | –1.98 (–3.9 to –0.0) | 0.046 |
| ITT analysis | –2.41 (8.16) | –0.45 (6.72) | –1.96 (–3.9 to –0.0) | 0.047 |
Figure 4 shows graphically the non-inferiority margin of –5 ETDRS letters; the shaded area represents the equivalence zone. It illustrates that the 95% CIs from both the PP and ITT analyses lay wholly within the equivalence zone and were also above the non-inferiority margin of –5 ETDRS letters.
FIGURE 4.
Primary outcome (observed values). Dashed line represents the non-inferiority margin and shaded area represents the equivalence zone. PP difference is –1.98 ETDRS letters (95% CI –3.93 to –0.035 ETDRS letters); ITT difference is –1.96 ETDRS letters (95% CI –3.90 to –0.022 ETDRS letters).
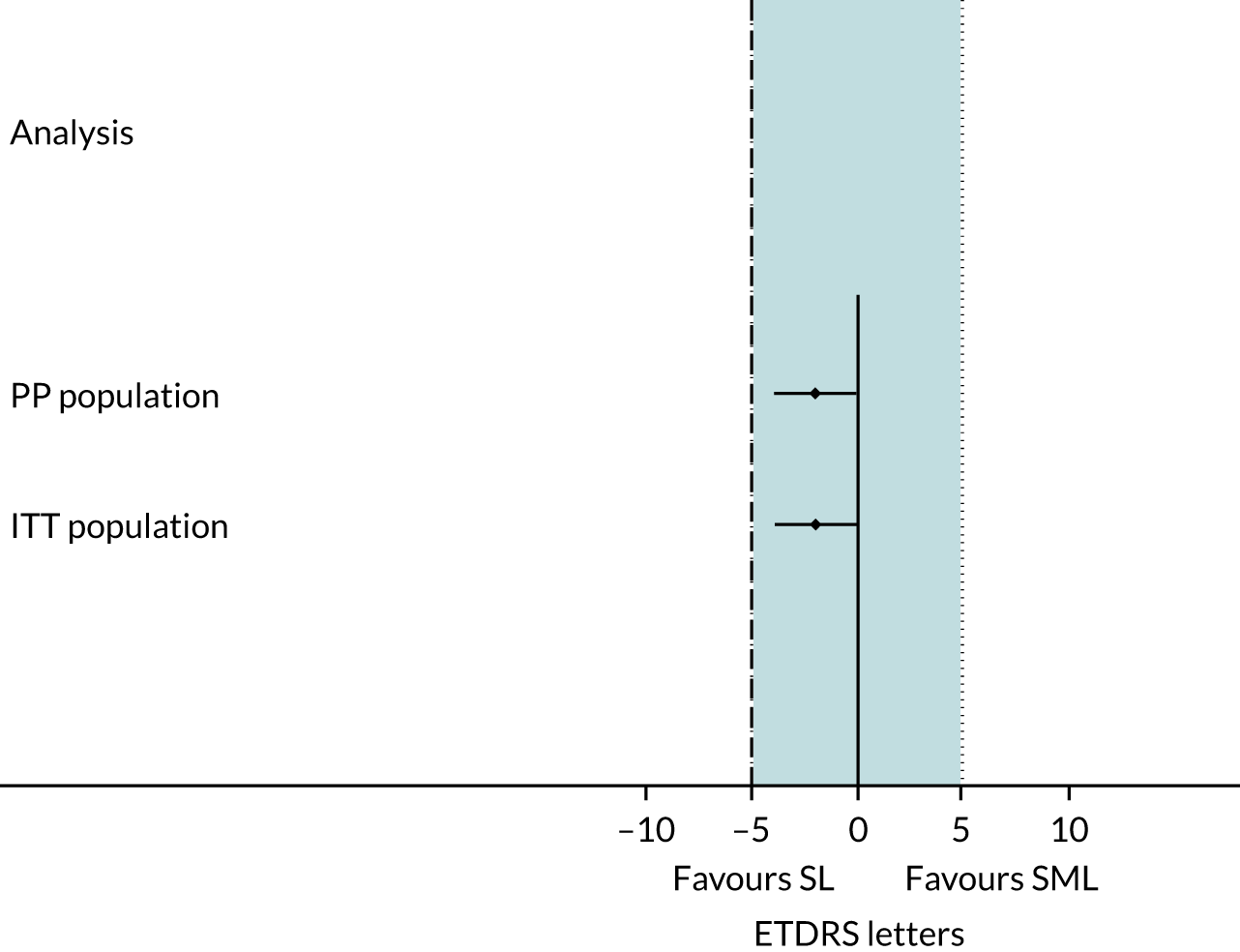
In accordance with the statistical analysis plan, the primary outcome was also adjusted for baseline BCVA score, baseline CRT, and previous cataract surgery in the study eye prior to enrolment in the trial as well as the minimisation factors on both the PP and the ITT populations. These results support the findings from the unadjusted analyses in Table 6. The adjusted mean change in BCVA in the study eye from baseline to month 24 was –2.36 ETDRS letters (SE 0.67 ETDRS letters) in the SML group and –0.53 ETDRS letters (SE 0.67 ETDRS letters) in the SL group. The difference of –1.84 ETDRS letters (95% CI –3.72 to 0.047 ETDRS letters; p = 0.056) was found to be neither statistically significant nor clinically important.
Table 7 displays the analysis results for the primary outcome, for both PP and ITT analyses, after adjusting for baseline BCVA score, baseline CRT, cataract surgery in the study eye prior to enrolment in the trial, and the following minimisation factors/covariates: centre, distance BCVA at baseline, previous use of anti-VEGF therapies and previous use of macular laser in the study eye.
| Adjusted analysis | Mean change in BCVA in the study eye from baseline to month 24 (ETDRS letters), mean (SE) | Difference in ETDRS letters (95% CI)b | p-value | |
|---|---|---|---|---|
| SML group (n = 116a) | SL group (n = 115a) | |||
| PP analysis | –2.36 (0.67) [n = 115] | –0.53 (0.67) | –1.84 (–3.7 to 0.0) | 0.056 |
| ITT analysis | –2.34 (0.66) | –0.53 (0.66) | –1.81 (–3.7 to 0.1) | 0.058 |
A secondary analysis was performed on the subset of participants with both eyes included in the trial (Table 8), including study eye as a random effect within the mixed model. The mean change in BCVA in both eyes from baseline to month 24 was –2.34 ETDRS letters (SE 8.10 ETDRS letters) in the SML group and –0.52 ETDRS letters (SE 6.81 ETDRS letters) in the SL group. The difference of –1.82 ETDRS letters (95% CI –2.42to –1.23 ETDRS letters; p = < 0.001) was statistically significant but is not considered clinically important.
| Primary outcome | Mean change in BCVA in both eyesa from baseline to month 24 (ETDRS letters), mean (SD) | Difference in ETDRS letters (95% CI) | p-value | |
|---|---|---|---|---|
| SML group (n = 119b) | SL group (n = 123b) | |||
| PP analysis | –2.34 (8.10) | –0.52 (6.81) | –1.82 (–2.42 to –1.23) | < 0.001 |
Secondary outcomes
Secondary outcomes reported in this section are mean change from baseline to month 24 in binocular BCVA, CRT, MD of the Humphrey 10–2 visual field in the study eye, percentage of people meeting driving standards; adverse effects, number of laser treatments carried out and additional treatments. EQ-5D-5L, NEI-VFQ-25, and VisQoL scores and cost per QALY gained are reported in Chapter 4.
Results for the secondary outcomes are presented in Table 9. There was no statistically significant difference in the following secondary outcomes: mean change in binocular BCVA (mean difference 0.32 ETDRS letters, 95% CI –0.99 to 1.64 ETDRS letters; p = 0.63), CRT (mean difference –0.64 µm, 95% CI –14.25 µm to 12.98 µm; p = 0.93), MD of the 10–2 Humphrey visual field (0.39 dB, 95% CI –0.23 dB to 1.02 dB; p = 0.21), percentage meeting driving standards (percentage point difference 1.6%, 95% CI –25.3% to 28.5%; p = 0.91), side effects [risk ratio (RR) 0.28, 95% CI 0.06, 1.34; p = 0.11] and additional treatments (percentage point difference –2.8%, 95% CI –13.1% to 7.5%; p = 0.59). The number of laser treatments performed was higher in the SML group (mean difference 0.48 treatments, 95% CI 0.18 to 0.79 treatments; p = 0.002). On closer inspection of the data, this difference appeared to be driven by a small number of participants requiring a larger number of laser treatments in the SML group. Specifically, 13 participants required six or seven sessions in the SML group compared with two participants in the SL group who needed this number of sessions.
| Secondary outcome | SML group | SL group | Difference (95% CI)a | p-value |
|---|---|---|---|---|
| Mean change in binocular BCVA from baseline to month 24 (ETDRS letters), mean (SE)b | –1.36 (0.47) [n = 115] | –1.68 (0.47) [n = 115] | 0.32 (–0.99 to 1.64) | 0.63 |
| Mean change in CRT in the study eye, as determined by SD-OCT from baseline to month 24, mean (SE)b | –17.45 (4.84) [n = 115] | –16.81 (4.84) [n = 115] | –0.64 (–14.25 to 12.98) | 0.93 |
| Mean change in the MD of the Humphrey 10–2 visual field in the study eye from baseline to month 24 (dB), mean (SE)b | –0.47 (0.22) [n = 91] | –0.87 (0.22) [n = 95] | 0.39 (–0.23 to 1.02) | 0.21 |
| Number of patients meeting driving standards at month 24, n (%)c | 104 (95.4) [n = 108] | 106 (97.3) [n = 109] | OR: 0.84 (0.14 to 5.27) | 0.86 |
|
Percentage point difference: 1.6 (–25.3 to 28.5) |
0.91 | |||
| Number of patients experiencing side effects from baseline to month 24, n (%)d | 2 (1.5) [n = 133] | 7 (5.3) [n = 132] | OR: 0.27 (0.056 to 1.34) | 0.11 |
| RR: 0.28 (0.060 to 1.34) | 0.11 | |||
| Number of laser treatments used from baseline to month 24 in study eye, mean (SE)b | 2.37 (0.11) [n = 133] | 1.89 (0.11) [n = 132] | 0.48 (0.18 to 0.79) | 0.002 |
| Number of patients with at least one additional treatment (other than laser) from baseline to month 24 (anti-VEGF therapies or steroids i.e. rescue treatments), n (%)d | 24 (18.1) [n = 133] | 28 (21.2) [n = 132] | OR: 0.78 (0.42 to 1.45) | 0.44 |
|
Percentage point difference: –2.8 (–13.1 to 7.5) |
0.59 |
Adverse events
A total of 70 SAEs were reported, affecting 46 (17%) participants. Participants randomised to the SML group had 0.8 times the risk of a SAE of those randomised to the SL group (RR 0.8, 95% CI 0.5 to 1.4; p = 0.50).
There were no SAEs reported that were deemed to be related to study treatment (i.e. SARs).
A total of 418 AEs were reported, affecting 157 (59%) participants. Participants randomised to the SML group had 0.9 times the risk of an AE of those randomised to the SL group (RR 0.9, 95% CI 0.8 to 1.1; p = 0.48).
Appendix 1, Table 25 displays safety by treatment group, overall and by the reported System Organ Class. There were no statistically significant differences identified between treatment groups.
There were nine AEs reported that were deemed to be related to study treatments [i.e. adverse reactions (ARs)], affecting six (2%) of participants (Table 10). Participants randomised to SML had 0.5 times the risk of an AR of those who randomised to the SL group (RR 0.5, 95% CI 0.1 to 2.7; p = 0.45)
| AEs | SML group (n = 133) | SL group (n = 132) | RR (for number of patients) (95% CI) | p-value | ||
|---|---|---|---|---|---|---|
| Number of events (n) | Number of patients, n (%) | Number of events (n) | Number of patients, n (%) | |||
| Foveal burn | 0 | 0 (0.0) | 0 | 0 (0.0) | – | – |
| Central/paracentral scotomas | 2 | 2 (1.5) | 3 | 3 (2.3) | –0.01 (–0.04 to 0.03) | 0.68 |
| Eye disorders, other, self-reported paracentral scotomas, study eye | 0 | 0 | 2 | 2 | – | – |
| Eye disorders, other, self-reported paracentral scotomas, non-study eye | 1 | 1 | 0 | 0 | – | – |
| Eye disorders, other, self-reported paracentral scotomas, both eyes | 1 | 1 | 1 | 1 | – | – |
| Epiretinal membrane formation | 0 | 0 (0.0) | 3 | 3 (2.3) | – | 0.12 |
| Eye disorders, other, epiretinal membrane, study eye | 0 | 0 | 2 | 2 | – | – |
| Eye disorders, other, epiretinal membrane, non-study eye | 0 | 0 | 1 | 1 | – | – |
| Choroidal neovascularisation | 0 | 0 (0.0) | 1a | 1 (0.7) | – | 0.50 |
| Self-reported reduced colour vision | 2 | 2 (1.5) | 0 | 0 (0.0) | – | 0.50 |
| Self-reported metamorphopsia | 3 | 2 (1.5) | 2 | 2 (1.5) | 1.0 (0.1 to 6.9) | 1.00 |
A total of 161 unanticipated eye-related AEs occurring in each of the laser groups were reported, affecting 38% of participants (n = 102) as detailed in Table 11.
| AEs | SML group (n = 133) | SL group (n = 132) | RR (for number of patients) (95% CI) | p-value | ||
|---|---|---|---|---|---|---|
| Number of events (n) | Number of patients, n (%) | Number of events (n) | Number of patients, n (%) | |||
| Blurred vision | 2 | 2 (1.5) | 2 | 2 (1.5) | 1.0 (0.1 to 6.9) | 1.00 |
| Eye disorders, keratitis, study eye | 2 | 2 (1.5) | 1 | 1 (0.8) | 2.0 (0.2 to 21.6) | 1.00 |
| Eye disorders, other, blocked meibomian gland | 0 | 0 (0.0) | 1 | 1 (0.8) | – | 0.50 |
| Eye disorders, other, both eyes, reduced colour vision | 1 | 1 (0.8) | 0 | 0 (0.0) | – | 1.00 |
| Eye disorders, other, cataract surgery | 6 | 6 (4.5) | 2 | 1 (0.8) | 3.0 (0.6 to 14.5) | 0.28 |
| Eye disorders, other, central retinal artery occlusion | 1 | 1 (0.8) | 0 | 0 (0.0) | – | 1.00 |
| Eye disorders, other, conjunctivitis | 1 | 1 (0.8) | 1 | 1 (0.8) | 1.0 (0.06 to 15.7) | 1.00 |
| Eye disorders, other, corneal abrasion | 1 | 1 (0.8) | 2 | 1 (0.8) | 1.0 (0.06 to 15.7) | 1.00 |
| Eye disorders, other, corneal oedema | 1 | 1 (0.8) | 0 | 0 (0.0) | – | 1.00 |
| Eye disorders, other, decreased central vision | 0 | 0 (0.0) | 1 | 1 (0.8) | – | 0.50 |
| Eye disorders, other, entropion, study eye | 2 | 2 (1.5) | 0 | 0 (0.0) | – | 0.50 |
| Eye disorders, other, foreign body left eye | 0 | 0 (0.0) | 1 | 1 (0.8) | – | 0.50 |
| Eye disorders, other, left epiphora | 0 | 0 (0.0) | 2 | 1 (0.8) | – | 0.50 |
| Eye disorders, other, left eye – itching and running | 1 | 1 (0.8) | 0 | 0 (0.0) | – | 1.00 |
| Eye disorders, other, neovascularisation in the iris | 1 | 1 (0.8) | 0 | 0 (0.0) | – | 1.00 |
| Eye disorders, other, ophthalmic cobweb effect right eye | 0 | 0 (0.0) | 1 | 1 (0.8) | – | 0.50 |
| Eye disorders, other, parafoveal intraretinal haemorrhage | 3 | 3 (2.3) | 0 | 0 (0.0) | – | 0.25 |
| Eye disorders, other, proliferative diabetic retinopathy, study eye | 1 | 1 (0.8) | 1 | 1 (0.8) | 1.0 (0.06 to 15.7) | 1.00 |
| Eye disorders, other, raised IOP | 0 | 0 (0.0) | 1 | 1 (0.8) | – | 0.50 |
| Eye disorders, other, reduced left superior field of vision | 0 | 0 (0.0) | 1 | 1 (0.8) | – | 0.50 |
| Eye disorders, other, smoke effect in right eye | 1 | 1 (0.8) | 0 | 0 (0.0) | – | 1.00 |
| Eye disorders, other, subconjunctival haemorrhage | 0 | 0 (0.0) | 1 | 1 (0.8) | – | 0.50 |
| Eye disorders, other, vitreous subhyaloid haemorrhage | 0 | 0 (0.0) | 1 | 1 (0.8) | – | 0.50 |
| Eye disorders, other, vitreous syneresis | 0 | 0 (0.0) | 1 | 1 (0.8) | – | 0.50 |
| Eye disorders, other, worsening of macula oedema | 1 | 1 (0.8) | 0 | 0 (0.0) | – | 1.00 |
| Eye pain | 1 | 1 (0.8) | 0 | 0 (0.0) | – | 1.00 |
| Floaters | 1 | 1 (0.8) | 2 | 2 (1.5) | 0.5 (0.05 to 5.4) | 0.62 |
| Glaucoma | 1 | 1 (0.8) | 2 | 1 (0.8) | 1.0 (0.06 to 15.7) | 1.00 |
| Other, reduced vision, non-study eye, consecutive visits | 5 | 5 (3.8) | 5 | 5 (3.8) | 1.0 (0.3 to 3.3) | 1.00 |
| Other, reduced vision, study eye, consecutive visits | 47 | 37 (27.8) | 37 | 29 (22.0) | 1.3 (0.8 to 1.9) | 0.32 |
| Other, reduced vision, study eye, since last visit | 2 | 2 (1.5) | 2 | 2 (1.5) | 1.0 (0.1 to 6.9) | 1.00 |
| Retinal detachment | 1 | 1 (0.8) | 0 | 0 (0.0) | – | 1.00 |
| Retinal tear | 0 | 0 (0.0) | 1 | 1 (0.8) | – | 0.50 |
| Vision decreased | 0 | 0 (0.0) | 1 | 1 (0.8) | – | 0.50 |
| Vitreous haemorrhage | 7 | 5 (3.8) | 1 | 1 (0.8) | 5.0 (0.6 to 41.9) | 0.21 |
| Watery eyes, non-study eye | 1 | 1 (0.8) | 0 | 0 (0.0) | – | 1.00 |
The primary outcome was analysed within pre-specified subgroups for which there was a clinical rationale (centre, distance BCVA at baseline, previous use of anti-VEGF therapies and macular laser in the study eye) by including the corresponding interaction term in the regression model and using 99% CIs. Analyses were carried out to identify whether or not any groups of participants [e.g. those with higher HbA1c or those having had previous cataract surgery (i.e. those with pseudophakic eyes)] were at high risk of poorer outcomes (Table 12). There was a statistically significant interaction for the centre subgroup analysis (p = 0.013), but this result was unreliable because of wide variability in the number of participants recruited at each centre. No other statistically significant interactions were identified.
| Subgroup | Mean change in BCVA in the study eye from baseline to month 24 (ETDRS letters), mean (SD) | Difference in ETDRS letters (99% CI) | Interaction termb | |
|---|---|---|---|---|
| SML group (n = 115a) | SL group (n = 115a) | |||
| Centre | ||||
| Site 01 | 0.74 (7.36) [n = 19] | –1.61 (6.16) [n = 18] | 2.35 (–3.98 to 8.68) | 0.013 |
| Site 02 | –1.38 (4.66) [n = 8] | 0.00 (6.27) [n = 10] | –1.38 (–10.50 to 7.75) | |
| Site 03 | –0.32 (5.74) [n = 19] | –2.32 (6.00) [n = 19] | 2.00 (–4.24 to 8.24) | |
| Site 04 | –10.00 (16.96) [n = 5] | 0.67 (3.78) [n = 6] | –10.67 (–22.32 to 0.99) | |
| Site 05 | –3.42 (5.37) [n = 12] | 3.64 (9.25) [n = 14] | –7.06 (–14.63 to 0.51) | |
| Site 06 | –4.20 (6.22) [n = 5] | –1.80 (5.50) [n = 5] | –2.40 (–14.57 to 9.77) | |
| Site 07 | –4.00 (4.60) [n = 6] | –3.71 (3.55) [n = 7] | –0.29 (–10.99 to 10.42) | |
| Site 08 | –3.50 (8.90) [n = 8] | 2.67 (5.68) [n = 6] | –6.17 (–16.56 to 4.23) | |
| Site 09 | –12.00 (–) [n = 1] | 0.25 (6.08) [n = 4] | –12.25 (–33.76 to 9.26) | |
| Site 10 | –3.00 (7.96) [n = 4] | –3.00 (3.46) [n = 3] | 0.00 (–14.70 to 14.70) | |
| Site 11 | –2.00 (7.00) [n = 9] | –0.64 (6.41) [n = 11] | –1.36 (–10.01 to 7.29) | |
| Site 12 | –0.67 (10.07) [n = 3] | –14.00 (9.90) [n = 2] | 13.33 (–4.23 to 30.90) | |
| Site 13 | –6.38 (15.96) [n = 8] | 4.33 (6.74) [n = 6] | –10.71 (–21.10 to –0.32) | |
| Site 14 | –1.75 (8.66) [n = 4] | 6.00 (–) [n = 1] | –7.75 (–29.26 to 13.76) | |
| Site 15 | [n = 0] | –4.00 (–) [n = 1] | – | |
| Site 16 | –2.75 (1.71) [n = 4] | 0.00 (5.66) [n = 2] | –2.75 (–19.42 to 13.92) | |
| Distance BCVA at baseline | ||||
| ≥ 69 ETDRS letters (Snellen equivalent of ≥ 20/40; log-MAR ≥ 0.3) | –2.89 (7.92) [n = 106] | –0.95 (5.83) [n = 107] | –1.93 (–4.54 to 0.68) | 0.70 |
| 24–68 ETDRS letters (Snellen equivalent ≤ 20/50; log-MAR 0.4–1.2) | 2.89 (10.01) [n = 9] | 6.25 (12.88) [n = 8] | –3.36 (–12.61 to 5.89) | |
| Previous use of anti-VEGF therapies in the study eye | ||||
| Yes | –4.56 (6.85) [n = 16] | –1.25 (4.85) [n = 12] | –3.31 (–10.75 to 4.13) | 0.60 |
| No | –2.09 (8.37) [n = 99] | –0.36 (6.92) [n = 103] | –1.73 (–4.47 to 1.01) | |
| Previous use of macular laser treatment in the study eye | ||||
| Yes | –2.36 (7.38) [n = 28] | –0.58 (4.65) [n = 26] | –1.78 (–7.11 to 3.55) | 0.91 |
| No | –2.46 (8.48) [n = 87] | –0.42 (7.24) [n = 89] | –2.04 (–4.99 to 0.90) | |
| High-risk baseline HbA1c value of ≥ 53 mmol/molc | ||||
| Yes | –2.88 (8.60) [n = 92] | –0.74 (5.97) [n = 94] | –2.14 (–5.01 to 0.73) | 0.75 |
| No | –0.45 (6.54) [n = 20] | 0.86 (9.47) [n = 21] | –1.31 (–7.42 to 4.81) | |
Sensitivity analyses
Sensitivity analyses were undertaken to assess the impact of missing data by imputing extreme values (lowest and highest) and last observation carried forward; the impact of including patients who were not treatment naive (i.e. excluding those who had had previous laser treatment for DMO in the study eye or previous anti-VEGF therapy for DMO or PDR in the study eye); the impact of including patients who had received cataract surgery in the study eye prior to entering into the trial (i.e. those with pseudophakic eyes); and the impact of using month-24 data collected outside ± 14 days of the due date (Table 13). These results support the findings of the unadjusted analyses in Table 6, and the lower limit of the 95% CIs for each of the treatment differences in these sensitivity analyses lie above the non-inferiority margin (–5.0 ETDRS letters) and within the equivalence margins (± 5.0 ETDRS letters).
| Sensitivity analysis | Mean change in BCVA in the study eye from baseline to month 24 (ETDRS letters), mean (SD) | Difference in ETDRS letters (95% CI) | p-valueb | |
|---|---|---|---|---|
| SML group (n = 131a) | SL group (n = 132) | |||
| Highest value imputed | –1.62 (8.14) [n = 131] | 0.02 (6.57) [n = 132] | –1.64 (–3.44 to 0.15) | 0.073 |
| Lowest value imputed | –2.77 (7.98) [n = 131] | –0.58 (6.33) [n = 132] | –2.19 (–3.94 to –0.44) | 0.014 |
| Last observation carried forward | –2.14 (8.28) [n = 131] | –0.16 (6.55) [n = 132] | –1.98 (–3.79 to –0.17) | 0.033 |
| No previous laser treatment for DMO in study eye | –2.46 (8.48) [n = 87] | –0.42 (7.24) [n = 89] | –2.04 (–4.39 to 0.30) | 0.087 |
| No previous anti-VEGF therapy for DMO in study eye | –2.09 (8.37) [n = 99] | –0.36 (6.92) [n = 103] | –1.73 (–3.86 to 0.40) | 0.11 |
| No cataract surgery in the study eye | –3.21 (8.24) [n = 92] | –0.36 (6.80) [n = 101] | –2.85 (–4.99 to –0.71) | 0.009 |
| Excluding participants with month 24 follow-up outside ± 14 days of the due date | –2.00 (7.67) [n = 40] | –1.07 (8.52) [n = 46] | –0.93 (–4.43 to 2.56) | 0.60 |
Chapter 4 Health economics analysis: results
Overview of the health economics analysis
The protocol for the DIAMONDS trial22 envisaged that economic modelling might be required if visual outcomes differed between treatment groups. However, the protocol was designed to minimise any visual loss. As per standard clinical practice, laser treatment was repeated following the initial session at the discretion of the treating ophthalmologists if it was felt to be required. Similarly, as per standard clinical care, rescue treatment with anti-VEGF therapy was allowed if rescue criteria were met. Intravitreal steroid injections could also be used if needed, at the discretion of the treating ophthalmologist.
Subthreshold micropulse laser was found to be not only non-inferior but indeed equivalent to SL. Thus, modelling was not required. This chapter provides the results from the within-trial cost-effectiveness analysis comparing SML with SL. The chapter presents (1) missing data by treatment group, (2) resource use and economic costs for different health resource categories, (3) health outcomes (EQ-5D-5L utility, NEI-VFQ-25 and VisQoL scores) and (4) cost-effectiveness results of the base-case and sensitivity analyses.
Results of the health economics analysis
Missing data
Table 14 shows the degree of missing health economics data by treatment group and follow-up time point. The missing data pattern was non-monotonic as individuals with missing data at one follow-up time point could have subsequent data entries. For example, there are more missing EQ-5D-5L data at 12 months than at 24 months. Overall, the percentage of missing data was low, ranging between 2% and 21% across all variables.
| Health economic variable by time-pointa | Number of missing values, n (%) | Total number of missing values, n (%) | |
|---|---|---|---|
| SML group | SL group | ||
| EQ-5D-5L utility scores | |||
| Baseline | 5 (3.76) | 6 (4.54) | 11 (4.15) |
| 12 months | 24 (18.05) | 16 (12.12) | 40 (15.09) |
| 24 months | 20 (15.04) | 16 (12.12) | 36 (13.58) |
| Costs of resource use: first laser procedureb | 5 (3.76) | 0 (0.0) | 5 (1.89) |
| Laser retreatment costs | |||
| Baseline to 4 months | 10 (7.52) | 10 (7.58) | 20 (7.55) |
| 4–8 months | 9 (6.77) | 14 (10.61) | 23 (8.68) |
| 8–12 months | 19 (14.29) | 16 (12.12) | 35 (13.21) |
| 12–16 months | 27 (20.3) | 21 (15.91) | 48 (18.11) |
| 16–20 months | 29 (21.8) | 26 (19.7) | 55 (20.75) |
| 20–24 months | 28 (21.05) | 25 (18.94) | 53 (20.00) |
| Anti-VEGF therapy costs | |||
| Baseline to 4 months | 7 (5.26) | 6 (4.55) | 13 (4.91) |
| 4–8 months | 7 (5.26) | 14 (10.61) | 21 (7.93) |
| 8–12 months | 12 (9.02) | 13 (9.85) | 25 (9.43) |
| 12–16 months | 15 (11.28) | 13 (9.85) | 28 (10.57) |
| 16–20 months | 22 (16.54) | 16 (12.12) | 38 (14.34) |
| 20–24 months | 14 (10.53) | 13 (9.85) | 27 (10.19) |
| Hospital outpatient services costs | |||
| Baseline to 4 months | 7 (5.26) | 6 (4.55) | 13 (4.91) |
| 4–8 months | 7 (5.26) | 14 (10.61) | 21 (7.93) |
| 8–12 months | 13 (9.77) | 13 (9.85) | 26 (9.81) |
| 12–16 months | 15 (11.28) | 13 (9.85) | 28 (10.57) |
| 16–20 months | 22 (16.54) | 16 (12.12) | 38 (14.34) |
| 20–24 months | 14 (10.53) | 14 (10.61) | 28 (10.57) |
Economic costs and health-care resource use
Table 15 summarises the NHS costs associated with resource use among complete cases (i.e. cases with complete cost data) by cost category and follow-up period. We present the costs of the first laser procedure separately from that of the repeated laser procedures. Nonetheless, the total costs of laser therapy for each patient includes costs of the first laser procedure plus any subsequent laser retreatments they had (as captured in the trial CRF). The mean direct intervention cost for the first laser procedure was £47.11 (SE £2.65) for the SML group compared with £41.16 (SE £2.30) for the SL group; the mean difference of £5.95 was not statistically significant at the 5% level (95% CI: –1.00 to 12.90; p = 0.09). Equipment costs constituted a small proportion of the total costs as we assumed that the machine will be used to perform laser procedures on 3000 patients per year (see Appendix 1, Table 23). Further details on variables used to calculate laser treatment costs, for example times to complete laser treatments, are provided in Appendix 1, Table 26 and in Appendix 2, Figure 9.
| Parameter | Cost, mean (SE) | Mean difference in costs (bootstrap 95% CI) | |
|---|---|---|---|
| SML group (N = 64) | SL group (N = 76) | ||
| Index laser procedure | 47.11 (2.65) | 41.16 (2.30) | 5.95 (–1.00 to 12.91) |
| Laser retreatments | 64.73 (8.34) | 45.04 (6.71) | 19.68 (–1.50 to 40.87) |
| Outpatient care | 170.70 (42.83) | 163.33 (53.36) | 7.37 (–127.94 to 142.69) |
| Anti-VEGF costs | 615.29 (176.68) | 876.12 (223.81) | –260.84 (–824.77 to 303.09) |
| Total NHS and PSS costsa | 897.83 (189.24) | 1125.66 (250.09) | –227.83 (–848.02 to 392.35) |
The mean total NHS and PSS costs were lower in the SML group than the SL group (£897.83 vs. £1125.66) between baseline and 24 months post randomisation; the mean difference of £227.83 was not statistically significant at the 5% level. Table 16 presents more granular economic cost data by time period. The table also captures information on the number of participants who accessed a service over specified time periods.
The total costs in Tables 15 and 16 differ because one uses only data from participants with compete cost data at all time points whereas the other refers to participants with complete cost data at each of the specified time points. Outpatient visit costs are higher in those with complete attendance, as would be expected. The main difference is in anti-VEGF therapy rescue costs, which are much higher in participants with missing data.
| Parameter by scheduled follow-up visit | SML group | SL group | Mean difference in costs (£) (bootstrap 95% CI) | ||||
|---|---|---|---|---|---|---|---|
| N a | n (%) | Cost (£), mean (SE)b | N a | n (%) | Cost (£), mean (SE)b | ||
| Index laser procedure | 128 | – | 45.57 (1.70) | 132 | – | 42.29 (1.69) | 3.28 (–1.40 to 7.96) |
| Laser retreatments (at scheduled visits) | |||||||
| 4 months | 123 | 60 (48.8) | 14.81 (1.68) | 122 | 38 (31.1) | 9.68 (1.56) | 5.13 (0.61 to 9.65) |
| 8 months | 124 | 34 (27.4) | 8.71 (1.46) | 118 | 28 (23.7) | 8.28 (1.45) | 2.05 (–3.62 to 4.49) |
| 12 months | 115 | 29 (25.4) | 8.25 (1.49) | 117 | 15 (12.9) | 4.55 (1.21) | 3.70 (–0.08 to 7.47) |
| 16 months | 108 | 23 (21.7) | 6.65 (1.47) | 113 | 15 (13.5) | 5.53 (1.54) | 1.12 (–5.28 to 2.97) |
| 20 months | 107 | 20 (19.2) | 5.78 (1.31) | 109 | 14 (13.2) | 4.64 (1.39) | 1.14 (–2.61 to 4.90) |
| 24 months | 108 | 12 (11.4) | 3.50 (1.03) | 111 | 4 (3.7) | 1.30 (0.65) | 2.20 (–0.20 to 4.60) |
| Intermediate/interim sessions | 132 | 5 (3.8) | 1.61 (0.73) | 130 | 3 (2.3) | 1.32 (0.88) | 0.29 (–1.96 to 2.52) |
| 4 to 24 monthsc | 70 | 183 | 62.09 (7.71) | 77 | 117 | 42.61 (6.64) | 17.48 (–2.53 to 37.50) |
| Outpatient visits | |||||||
| Baseline to 4 months | 126 | 5 (4) | 8.46 (5.11) | 126 | 11 (8.7) | 12.73 (5.50) | –4.27 (–18.94 to 10.40) |
| 4–8 months | 126 | 8 (6.3) | 10.75 (4.35) | 118 | 13 (11) | 19.24 (7.03) | –8.49 (–24.89 to 7.90) |
| 8–12 months | 120 | 20 (16.7) | 29.58 (9.36) | 119 | 12 (10.1) | 16.80 (6.04) | 12.79 (–9.27 to 34.84) |
| 12–16 months | 118 | 23 (19.5) | 30.62 (10.17) | 119 | 23 (19.3) | 33.30 (16.04) | –2.68 (–40.17 to 34.80) |
| 16–20 months | 111 | 19 (17.1) | 30.03 (8.03) | 116 | 15 (12.9) | 20.43 (6.77) | 9.60 (–12.36 to 31.56) |
| 20–24 months | 119 | 12 (10.1) | 17.71 (7.11) | 118 | 23 (19.5) | 30.10 (9.32) | –12.39 (–35.46 to 10.68) |
| Baseline to 24 months | 91 | 87 | 142 (31.94) | 101 | 97 | 150.89 (41.54) | –8.89 (–113.91 to 96.12) |
| Rescue treatments: anti-VEGF injections | |||||||
| Baseline to 4 months | 126 | 4 | 19.61 (15.72) | 126 | 5 | 24.13 (19.61) | –4.52 –(56.94 to 35.83) |
| 4–8 months | 126 | 13 | 48.25 (24.83) | 118 | 26 | 138.59 (48.85) | –90.33 (–183.29 to 21.44) |
| 8–12 months | 121 | 20 | 89.77 (29.33) | 119 | 27 | 137.84 (42.86) | –48.07 (–153.30 to 63.47) |
| 12–16 months | 118 | 32 | 141.21 (51.96) | 119 | 40 | 196.89 (50.92) | –55.69 (–214.99 to 97.67) |
| 16–20 months | 111 | 20 | 103.00 (36.71) | 116 | 27 | 137.14 (36.71) | –34.14 (–139.06 to 84.48) |
| 20–24 months | 119 | 21 | 104.91 (37.98) | 119 | 42 | 211.47 (73.34) | –106.56 (–266.76 to 54.07) |
| Baseline to 24 months | 92 | 37d | 447.50 (112.87) | 101 | 65d | 809.24 (184.92) | –361.74 (–813.66 to 117.36) |
Economic costs and resource use summary
-
The mean number of laser treatments was 2.4 in the SML group and 1.9 in the SL group, difference 0.48 (p = 0.002). Of these treatments, 80% and 86%, respectively, were given in the first 12 months (Table 9).
-
The proportion of patients requiring rescue treatment (almost all with anti-VEGF therapy – only one patient had a steroid injection) in the study eye was 18% in the SML group and 21% in the SL group, and the difference was not statistically significant (Table 9). About half of the patients receiving anti-VEGF therapies did so in the first 12 months (9.8% in the SML group and 12.9% in the SL group) (see Appendix 1, Table 27).
-
The proportion of participants that met the criteria for rescue treatment at least once was 33% for the SML group and 31% for the SL group, therefore of those who at any one time point met the criteria, only 54% and 68%, respectively, were treated (see Appendix 1, Table 27). Some of those not treated had only temporary increases of a few micrometres in CRT that resolved without treatment.
-
The mean number of anti-VEGF treatments in the SL group was skewed by five patients who received more than 10 treatments. None of the participants in the SML group required 10 or more injections.
-
Laser treatment costs were based on the time from the patient entering the laser room to the time of their leaving the room, as recorded in the CRF.
Health outcomes
The EQ-5D-5L utility scores showed no statistically significant differences between the two laser groups (Table 17). The EuroQol-5 Dimensions (EQ-5D) visual analogue scale (VAS) scores followed a similar pattern across time periods for the two treatment groups (Table 18).
| Time point | SML group (N = 133) | SL group (N = 132) | Between-group difference (95% CI) | p-value | |||
|---|---|---|---|---|---|---|---|
| n | EQ-5D-5L utility score, unadjusted mean (SD) | n | EQ-5D-5L utility score, unadjusted mean (SD) | Unadjusted | Adjusteda | ||
| Baseline | 128 | 0.757 (0.272) | 126 | 0.772 (0.231) | –0.0148 (–0.079 to 0.050) | –0.017 (–0.080 to 0.048) | 0.615 |
| 12 months | 109 | 0.798 (0.237) | 116 | 0.770 (0.251) | 0.0281 (–0.040 to 0.096) | –0.001 (–0.067 to 0.065) | 0.969 |
| 24 months | 113 | 0.747 (0.284) | 116 | 0.759 (0.285) | –0.0117 (–0.079 to 0.056) | –0.011 (–0.077 to 0.054) | 0.737 |
| Time point | Treatment group | Mean (SD) | Median | Quartile 1 (Q1) | Quartile 3 (Q3) | IQR |
|---|---|---|---|---|---|---|
| Baseline | SML | 74.74 (19.65) | 80 | 65 | 90 | 25 |
| SL | 75.20 (18.61) | 80 | 70 | 90 | 20 | |
| 12 months | SML | 76.53 (18.29) | 80 | 70 | 90 | 20 |
| SL | 73.86 (18.79) | 80 | 68 | 90 | 22 | |
| 24 months | SML | 72.27 (20.42) | 75 | 60 | 90 | 30 |
| SL | 75.13 (17.27) | 80 | 65 | 90 | 25 |
National Eye Institute Visual Functioning Questionnaire – 25 and Vision and Quality of Life Index outcomes
The VisQoL analysis showed no statistically significant differences in utility scores between the two treatment groups for any of the VisQoL dimensions at any follow-up time point (see Appendix 1, Table 28). Similar results were observed for the NEI-VFQ-25 subscales (Figures 5 and 6, and Appendix 1, Table 29). Overall baseline (pre laser treatment) utility scores from analysis of all six VisQoL dimensions and NEI-VFQ-25 subscales are detailed in Appendix 1, Tables 28 and 29 and in Report Supplementary Materials 3 and 4. The tables show that there are no statistically significant differences in utility scores for the VisQoL or the NEI-VFQ-25 subscales between baseline and 24 months.
FIGURE 5.
The baseline NEI-VFQ-25 subscale and composite scores in participants treated with SML vs. SL.
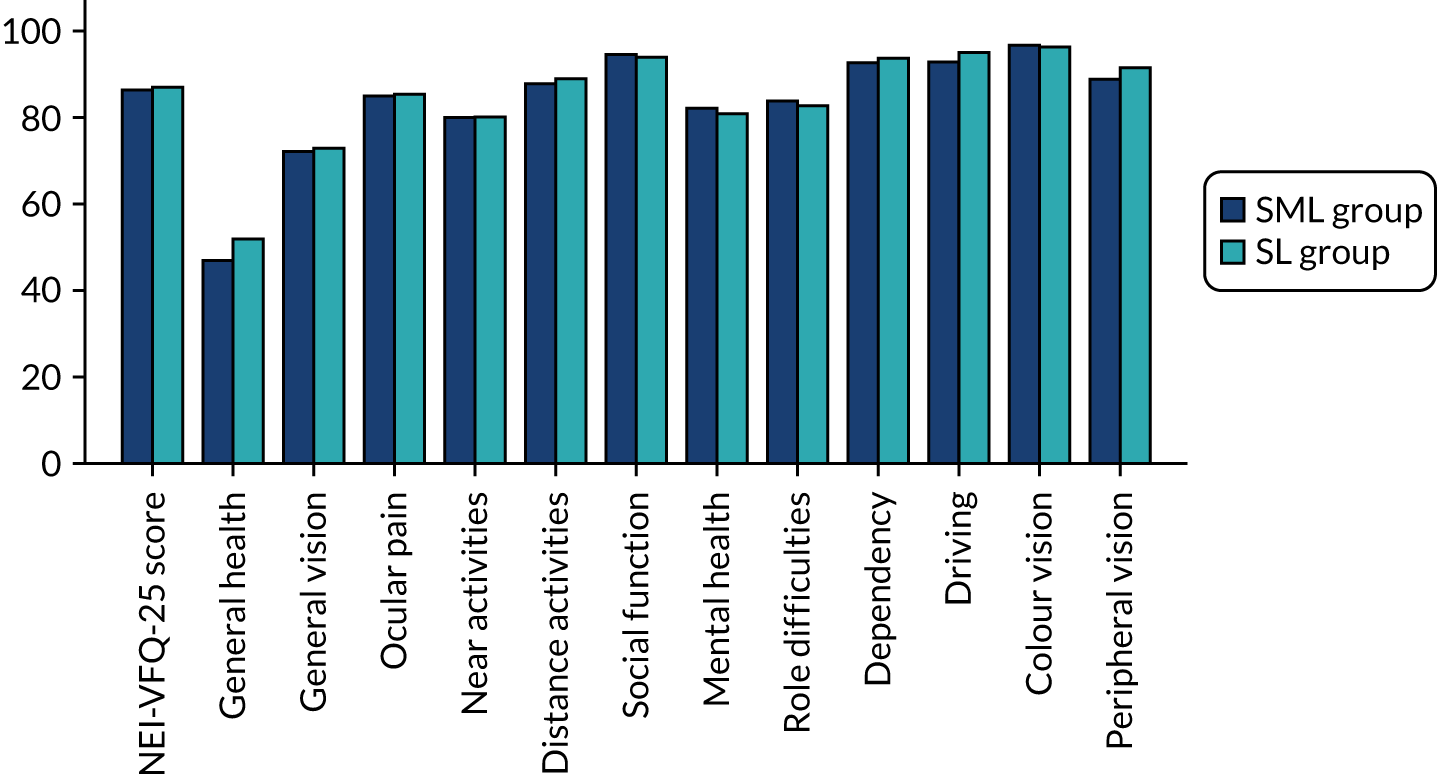
FIGURE 6.
The NEI-VFQ-25 subscale and composite scores in participants treated with SML vs. SL at 24 months.
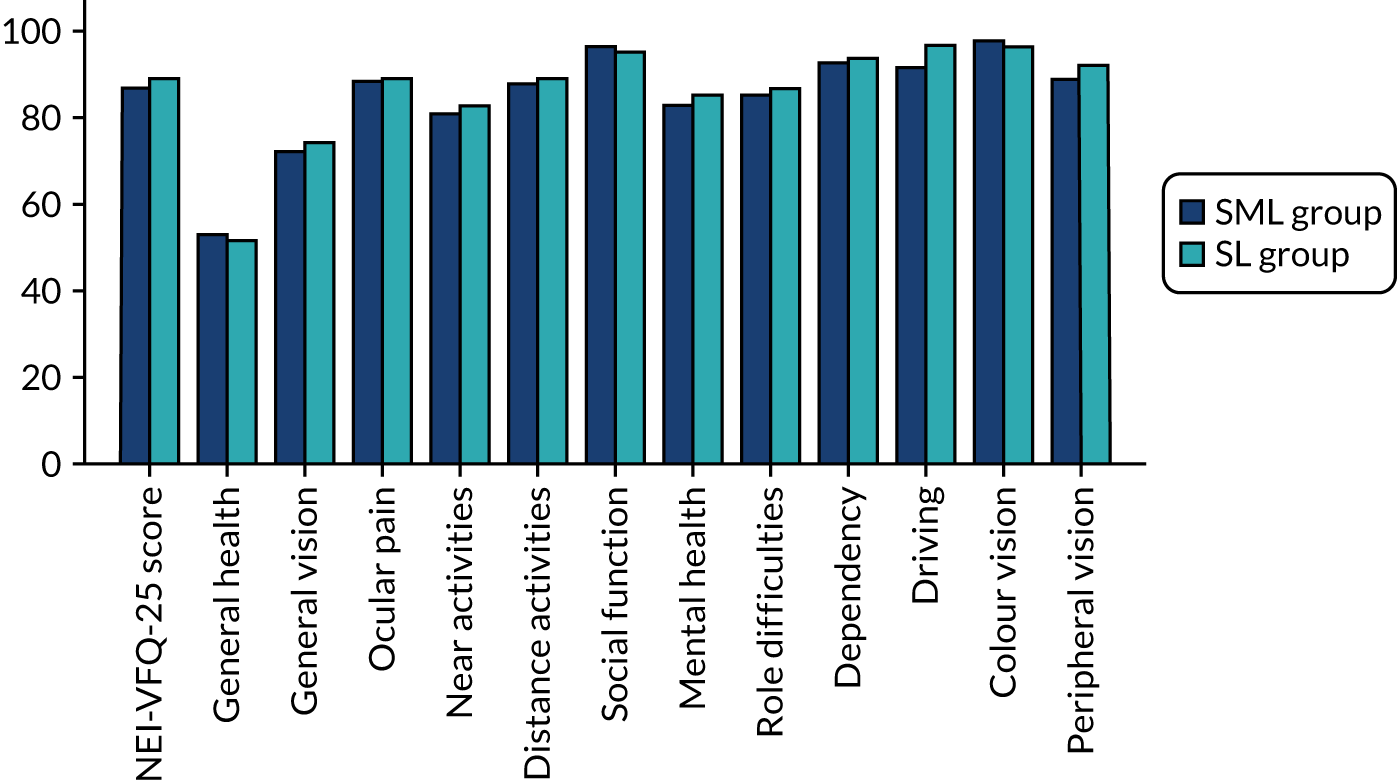
Cost-effectiveness analysis
The cost-effectiveness results are presented in Table 19 with the SL group as the referent and the SML group as the comparator (i.e. SML minus SL) for the estimation of ICER values. The time horizon is the 24-month follow-up period of the trial. The joint distributions of costs and outcomes for the base-case analysis are represented in Figures 7 and 8.
| Scenario | SML vs. SL | ICER | Probability of cost-effectiveness | NMB (£) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Incremental cost (£), mean (95% CI) | Incremental QALYs, mean (95% CI) | At £15,000/QALY | At £20,000/QALY | At £30,000/QALY | At £15,000/QALY | At £20,000/QALY | At £30,000/QALY | ||
| Base-case analysis | |||||||||
| ITT approach: imputed attributable costs and QALYs, covariate adjusteda | –365 (–822 to 93) | 0.008 (–0.059 to 0.075) | SML dominant | 0.80 | 0.763 | 0.716 | 479 (–652 to 1610) | 520 (–925 to 1965) | 600 (–1495 to 2695) |
| Sensitivity analyses | |||||||||
| PP approach: imputed attributable costs and QALYs, covariate adjusteda | –374 (–830 to 83) | 0.0077 (–0.056 to 0.072) | SML dominant | 0.811 | 0.773 | 0.724 | 483 (–606 to 1572) | 521 (–868 to 1910) | 598 (–1413 to 2609) |
| 25% reduction in anti-VEGF costs | –327 (–729 to 75) | 0.008 (–0.059 to 0.075) | SML dominant | 0.789 | 0.751 | 0.706 | 422 (–676 to 1520) | 463 (–954 to 1880) | 543 (–1529 to 2615) |
| 25% increase in anti-VEGF costs | –402 (–922 to 118) | 0.008 (–0.059 to 0.075) | SML dominant | 0.811 | 0.774 | 0.726 | 536 (–634 to 1706) | 577 (–902 to 2056) | 657 (–1464 to 2778) |
FIGURE 7.
Cost-effectiveness scatterplot with 95% confidence ellipses at 24 months for the base case. Imputed, additionally controlled for baseline utilities and minimisation variables (previous use of anti-VEGF therapy and previous laser treatment).
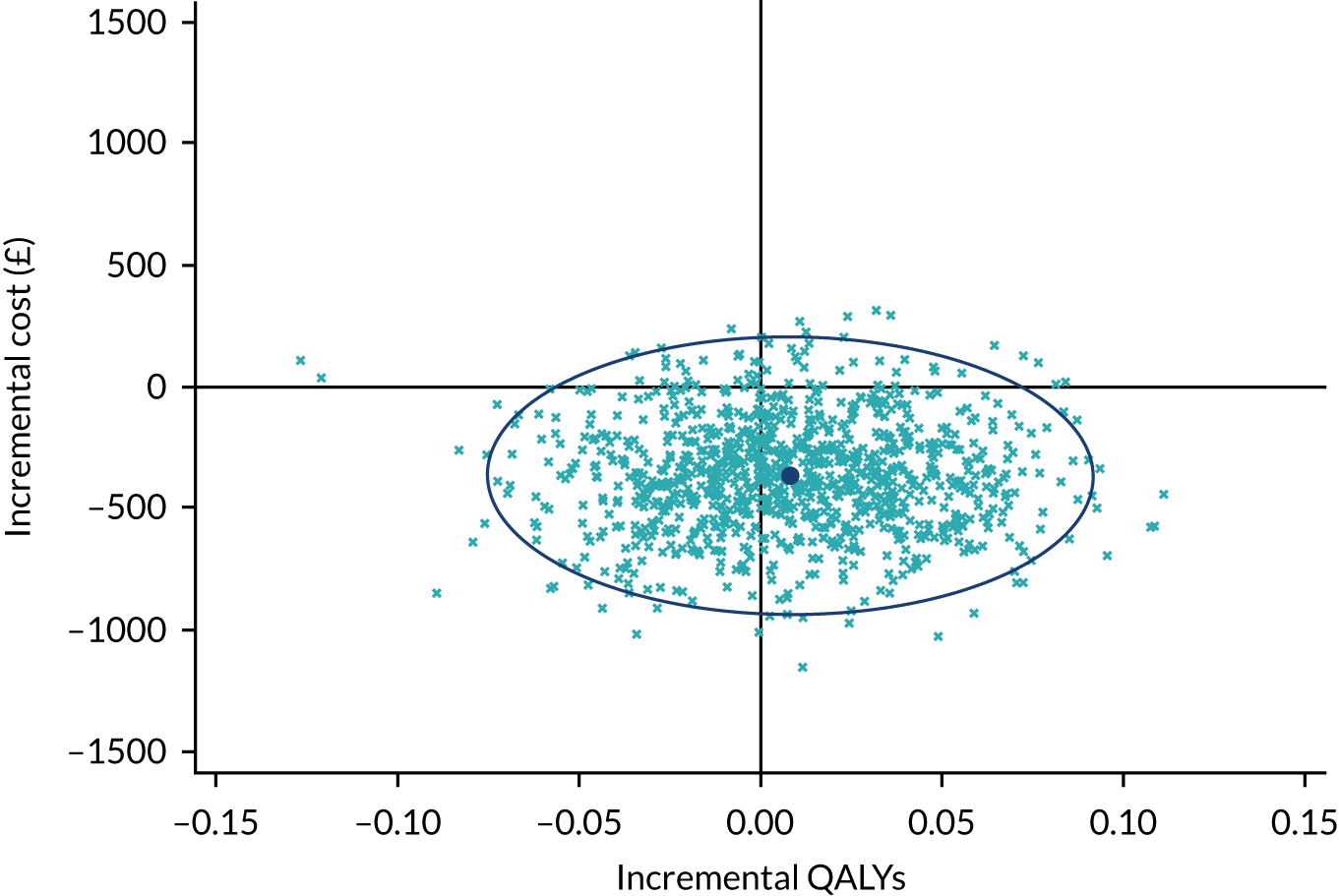
FIGURE 8.
Cost-effectiveness acceptability curves at 24 months for base case. Imputed, additionally controlled for baseline utilities and baseline minimisation variables.
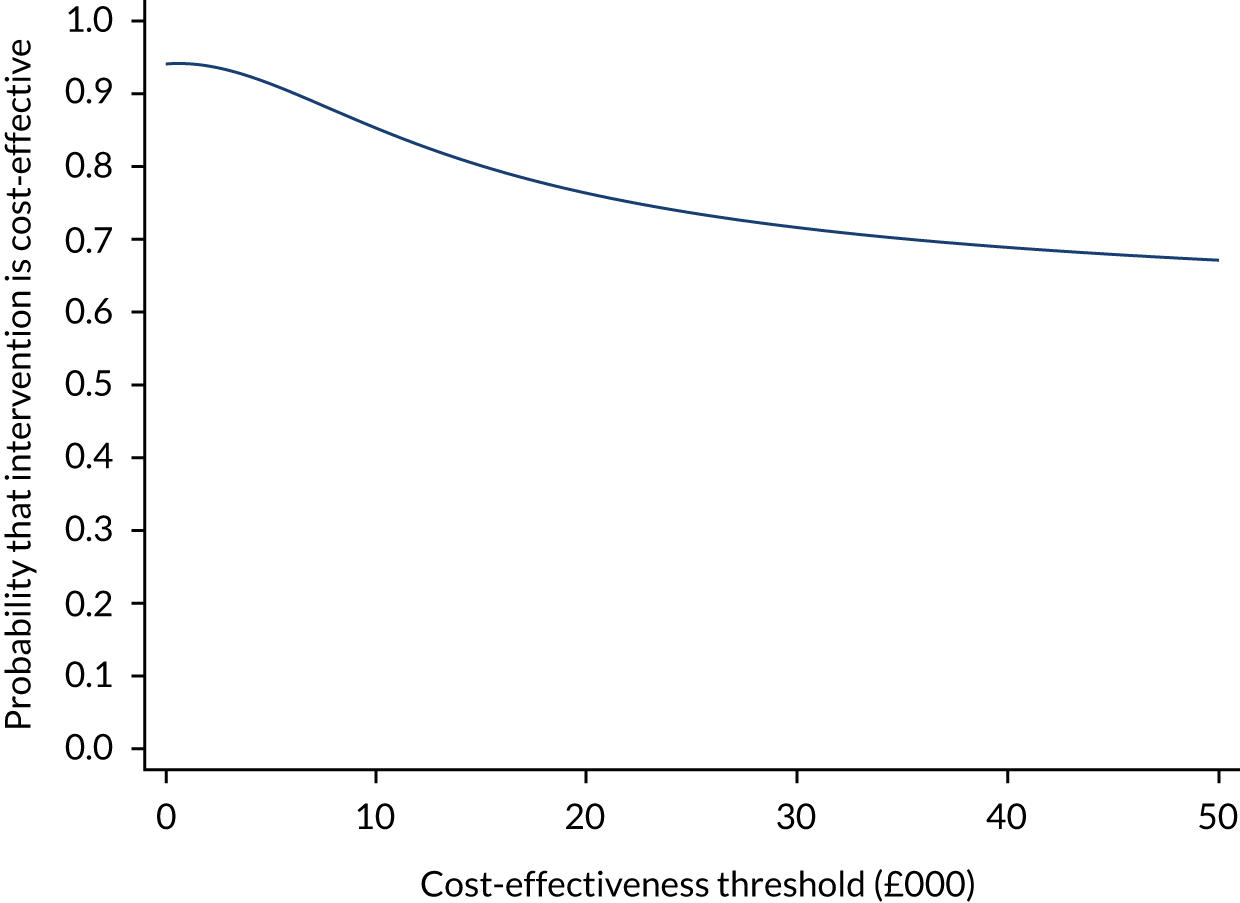
Base-case analysis
Over the 24-month follow-up period, participants in the SML group, compared with the SL group, experienced a non-statistically significant increase of 0.008 QALYs (circa 3 days of good quality of life) (95% CI –0.059 to 0.075 QALYs). In addition, the mean NHS and PSS costs were lower in the SML group compared with the SL group (mean cost difference –£365, 95% CI –£822 to £93). However, it should be noted that the CI for the cost difference is wide and ranges from cost saving to cost increasing. The ICER for the base-case analysis indicates that SML dominates, as average costs for this intervention were lower and average benefits were marginally higher than those for SL.
Assuming cost-effectiveness thresholds of £15,000 per QALY, £20,000 per QALY and £30,000 per QALY, the probability that SML was cost-effective compared with SL ranged from 71% to 80%, and the NMB associated with SML was positive (Table 19). However, the QALY difference of 0.008 was not significant. The results of the sensitivity analyses follow a similar pattern to the base-case analysis.
Discussion
Total costs were higher in the SL group, but the difference seemed to be driven by a higher number of anti-VEGF rescue injections, largely owing to five patients in the SL group having 10 or more injections. The CI around total costs were wide and overlapped, so findings should be interpreted with caution. There was no statistically significant difference in EQ-5D-5L scores and no clinically relevant difference on visual outcomes; SML and SL were found to be clinically equivalent.
Chapter 5 Discussion
Statement of principal findings
The DIAMONDS trial was a pragmatic, allocation-concealed, multicentre, randomised, non-inferiority clinical trial (but powered to detect equivalence and superiority, if it were to exist) in which outcome assessors and participants were masked to treatment allocation. The DIAMONDS trial recruited in full, for a total of 266 participants with 133 participants allocated to each laser group; one participant in the SL group withdrew consent for their data to be used and thus 265 participants were included in the analysis. Despite the COVID-19 pandemic, which occurred during the second year of follow-up for most participants, primary outcome data (measured at 24 months from trial initiation) was available in the majority of participants: 116 (87%) and 115 (86%) in the SML group and SL group, respectively. Participants were predominantly white males with uncontrolled (mean HbA1c of 8.5%) type 2 diabetes and were mostly overweight, obese, or morbidly obese. Participants had a mean age of 62 years, a mean duration of DMO diagnosis of 2.5 years, a mean BCVA of 80 letters (6/7.5 Snellen Equivalent) and a mean CRT of 329.2 µm on SD-OCT.
Best-corrected visual acuity was selected as the primary outcome in the DIAMONDS trial. Central vision is very important to people with diabetes as it is used in many tasks including recognising faces and reading (many people with diabetes need to read medication labels and instructions specifying, for example, the amount of insulin needed for their treatment). The non-inferiority margin of 5 ETDRS letters (equivalence margin of± 5 ETDRS letters) was chosen as changes within this range are not considered to be clinically relevant and could be the result of test/re-test variability. The DIAMONDS trial found SML to be equivalent to SL, with an adjusted mean change in BCVA in the study eye from baseline to month 24 of –2.36 ETDRS letters (SE 0.67 ETDRS letters) in the SML group compared with –0.53 ETDRS letters (SE 0.67 ETDRS letters) in the SL group (mean difference –1.84 ETDRS letters, 95% CI –3.72 to 0.05 ETDRS letters; p = 0.056).
There were no statistically significant differences in the pre-defined secondary outcomes with the exception of the number of laser treatments performed, which was slightly higher (less than one further session) in the SML group. This finding appeared to be driven, however, by a small number of participants (n = 13) that required a larger number of laser treatments in the SML group. Most participants (approximately 80% and 90% of participants in SML group and SL group, respectively) required 1–3 laser sessions throughout the 2-year period (see Appendix 1, Table 30).
A similar number of participants in each laser treatment group (approximately one-third) met eligibility criteria to receive rescue treatment at any time during the 2-year period of the study. Fewer participants, however, actually received rescue treatment [24 (18%) in the SML group and 28 (21%) in the SL group; odds ratio 0.78, 95% CI 0.42 to 1.45; p = 0.44 and percentage point difference –2.8, 95% CI –13.1 to 7.5; p = 0.59]; all received anti-VEGF therapy and one, in the SL group, received intravitreal steroids in addition. Most participants maintained good vision throughout the trial, with only a small proportion [25 (9%) participants: 17 (13%) in the SML group and 8 (6%) in the SL group] experienced a drop of 10 ETDRS letters (which would be considered a clinically relevant change) from baseline to month 24 (see Appendix 1, Tables 31–33). Similarly, most participants (over 95% for each type of laser) met driving standards at the 24-month trial visit. Meeting driving standards was identified at the time of trial conception by the DIAMONDS PPI group and in consultation with patients with diabetic retinopathy and DMO to be very important to people with diabetes; as a result this was one of the secondary outcomes investigated. The DIAMONDS PPI group contributed not only to the design of the trial, elaboration of participant-related materials and planning of recruitment strategies, but also to the interpretation and dissemination of findings.
Most participants maintained good HRQoL and vision-related quality of life throughout the trial period. The total cost of the treatment, including the laser (first session and subsequent laser sessions), any additional treatments required and follow-up for the 2 years was £897.83 and £1125.66 for the SML group and SL group, respectively.
The DIAMONDS trial was designed as a pragmatic trial. 49 On its conception, we followed the PRECIS-2 (PRagmatic Explanatory Continuum Indicator Summary 2) tool50 to ensure as much as possible that trial results would be generalisable and reproducible in clinical practice if implemented in the NHS.
Published randomised trials and systematic reviews comparing subthreshold micropulse laser with standard laser
We carried out a literature review at the time the DIAMONDS trial was designed. In addition, we ran auto-alerts during the duration of the trial, up to January 2021, to identify studies that could be pertinent to the DIAMONDS trial. We then extended the searches up to December 2021. We found six systematic reviews comparing SML with SL published since 2016: Chen et al. ,16 Qiao et al. ,17 Wu et al. ,18 Jorge et al. ,19 Scholz et al. 51 and Blindbaek et al. 2018. 52 These were quality assessed using the National Institutes of Health (NIH) checklist (see Appendix 1, Table 34). 53 Based on this, four of these reviews (Chen et al. ,16 Jorge et al. ,19 Qiao et al. 17 and Wu et al. 18) were judged to be of good or high quality.
Chen et al. 16 found statistically significantly better visual acuity (0.1 log-MAR) with SML laser treatment at 12 months follow-up only,16 but this was due to one trial by Lavinsky et al. 9 and the high-density arm of that trial. The review concluded that SML laser is an effective therapy for treating DMO, but that compared with SL, the changes were of limited clinical significance.
Qiao et al. 17 included seven RCTs; six of these seven trials were included in the review by Chen et al. 16 mentioned above. Meta-analysis found no statistically significant differences in BCVA or central macular thickness at any time point. The results of meta-analyses for contrast sensitivity were mixed, with some trials favouring SL and others favouring SML.
The results in the Qiao et al. 17 review differ somewhat from those of Chen et al. 16 Qiao et al. 17 reported mean BCVA at 12 months with three trials, Figueira et al. ,11 Lavinsky et al. 9 and Vujosevic et al. ,10 whereas Chen et al. reported change from baseline in only two trials, Lavinsky et al. 9 and Vujosevic et al. 10 The Chen et al. review16 used only the high-density arm of Lavinsky et al.,9 whereas Qiao et al. 17 used only the normal density arm of this trial. Their different conclusions between these two reviews appear to be due to the results of Lavinsky et al. ,9 deemed to give a significant result in Chen et al. 16 (based on change in BCVA from baseline) but not in Qiao et al. 17 (based on mean BCVA at 12 months).
The review by Wu et al. 18 included a Bayesian network meta-analysis of RCTs and quasi-randomised trials comparing any two treatments of interest, including SML or SL photocoagulation as monotherapy or SL combined with anti-VEGF therapy. The review included 18 studies: 15 reported BCVA and 16 reported CRT and were included in the network meta-analysis, and 11 studies compared SL alone with laser plus ranibizumab. Of seven trials comparing SL with SML, four (Casson et al. ,54 Laursen et al. ,14 Venkatesh et al. 12 and Xie et al. 15) had only six months’ follow-up. The remaining three were those by Figueira et al. ,11 Lavinsky et al. 9 and Vujosevic et al. 10 Wu et al. 18 found no statistically significant difference in visual acuity improvement between monotherapy laser photocoagulation with SL and SML. 18
Scholz et al. 51 reviewed the effects of SML for the treatment of macular disorders including central serous chorioretinopathy, DMO and retinal vein occlusion. Although a comprehensive search was undertaken, the review did not specify eligibility criteria or any other review methods and, thus, was subsequently rated as being of low quality. Eleven prospective studies in DMO were included, although the review stated that a larger number of studies were identified. The review calculated change scores for CRT and BCVA but the methods of these calculations were not reported. The conclusions made by the authors, who stated that included studies demonstrated efficacy of SML, should, therefore, be taken with caution.
A review by Blindbaek et al. 52 of the potential role of focal/grid laser photocoagulation in DMO treatment was not rated as high quality. They noted that few high-quality studies have compared SML with SL therapy and that results were mixed. Quality of life was not discussed.
One of the problems with all available meta-analyses was that only three trials were analysed, each with a follow-up period of 12 months; none had a longer follow-up period. The mean CRTs in the trials by Lavinsky et al. 9 and Vujosevic et al. 10 were approximately 370 µm, so many patients would have had a CRT of > 400 µm, unlike those participants included in the DIAMONDS trial. None of the reviews reported effects on quality of life or costs.
Fazel et al. 20 conducted a single centre trial that randomised 68 eyes of 68 patients to SML or SL. Inclusion criteria included a CRT of 300–449 µm. With SL there was no statistically significant change in BCVA whereas a significant change was observed with SML, with an improvement of 0.07 Log-MAR by 4 months. CRT was reduced by 13 µm following SML and by 5 µm following SL. The small number of patients included in each treatment group and the short follow-up period (maximum 4 months) limits greatly the usefulness of the results presented. There was no power calculation for the trial shown. Owing to its year of publication, this trial was not included in the reviews by Chen et al. ,16 Qiao et al. 17 and Wu et al. 18
A review by Cooper et al. 55 aimed to synthesise the evidence on the psychological, social and everyday visual impact of diabetic retinopathy and DMO from the patient perspective. Eighty-five studies with a range of study designs were included; 23 of these studies assessed quality of life. However, the studies appeared to focus on diabetic retinopathy; quality of life in DMO was not discussed. Visual functioning and psychological wellbeing measures were reported in other studies, including some in DMO; however, limited numerical data were reported and no data on DMO were summarised.
Assessment for meta-analysis
We considered adding the DIAMONDS 12-month results into a meta-analysis with the three previous RCTs with a 12-month follow-up. However, there were important differences in baseline characteristics such as CRT, previous treatments and BCVA, as well as in laser regimens used (Tables 20 and 21). The trial by Lavinsky et al. 9 used two SML regimens, standard and high dose, and only the high-dose regimen showed any advantage. 9 In the DIAMONDS trial the response to laser did not seem to be affected by previous treatments, so that may not prevent combining the studies in a meta-analysis. However, the high baseline mean CRTs in the trials by Lavinsky et al. 9 and Vujosevic et al. 10 suggest that these trials included many participants with CRTs well over 400 µm, which would be an impediment to amalgamating them in a meta-analysis with the DIAMONDS data. The baseline BCVA in Lavinsky et al. 9 was much lower than that of other studies, so there would be potentially more scope for participants in this trial to gain vision. None of the previous RCTs comparing SL with SML used the 577 nm SML that was used in the DIAMONDS trial. It may be that all SML may have similar effects independently of the wavelength used, but we do not yet know if this is the case. For example, a study undertaken by Vujosevic et al. 56 randomised 53 eyes from 53 patients to 810 nm infrared SML or 577 nm yellow SML and found no difference in effectiveness or safety.
| Trial characteristic | DIAMONDS | Figueira et al. 200911 | Lavinsky et al. 20119 | Vujosevic et al. 201010 | |||||
|---|---|---|---|---|---|---|---|---|---|
| SML (N = 133) | SL (N = 132) | SML (N = 44a) | SL (N = 40a) | SML (HD) (N = 42) | SML (ND) (N = 39) | SL (N = 42) | SML (N = 32a) | SL (N = 30a) | |
| Baseline characteristics | |||||||||
| BCVA | |||||||||
| ETDRS letters | 80.2 (8.2) | 80.1 (8.7) | 78.4 (8.1) | 78.0 (7.8) | 40b | 50b | 45b | 75b | 75b |
| log-MAR | 0.1b | 0.1b | 0.1–0.2b | 0.1–0.2b | 0.90 (0.30–1.30) | 0.70 (0.40–1.30) | 0.80 (0.30–1.30) | 0.21 (0.30) | 0.29 (0.3) |
| CRT (µm) | 326.0 (38.7) | 332.6 (35.6) | 248.9 (58.7) | 255.0 (61.9) | 371 (297–879) | 379 (279–619) | 370 (269–710) | 358.3 (93.7) | 378.4 (94.5) |
| HbA1c (%) | 8.5 (3.8) | 8.5 (3.9) | 9.0 (1.6) | 9.1 (1.6) | 8.2 (0.6) | 8.0 (0.6) | 7.9 (0.6) | 8.9 (0.5) | 8.8 (0.4) |
| Previous treatments, n (%) | |||||||||
| Laser | 32 (24.1) | 32 (24.2) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| anti-VEGF | 17 (12.8) | 14 (10.6) | NR | NR | 0 | 0 | 0 | 0 | 0 |
| Other aspects | |||||||||
| Laser re-treatments allowed? | Yes, 2.4 in SML group and 1.9 in SL group | Yes, after 4 months from baseline treatment. No data provided | Yes, more frequent in high dose arm | Yes, mean number of treatments 2 in both arms | |||||
| Laser details | DIAMONDS | Figueira et al. 200911 | Lavinsky et al. 2011 (normal)9 | Lavinsky et al. 2011 (high dose)9 | Vujosevic et al. 201010 |
|---|---|---|---|---|---|
| SML equipment | Iridex IQ 577 system | Iridex Oculite SLx (IRIDEX Corporation, Mountain View, CA, USA) | Opto FastPulse (Opto Electronics, San Paolo, Brazil) | Iridex Oculite SLx | |
| Type of laser | 577 nm diode laser | 810 nm | 810 nm | 810 nm | |
| Number of spots | Three 7 × 7 spot grids above and below the fovea (at 500 µm from its centre) and one 7 × 7 spot grid at each side (temporal and nasal at 500 µm from the foveal centre), plus laser to areas of thickening outside these, if present | Not reported | Grid treatment above, below and nasally around centre of macula. Same grid as mETDRS protocol. 500–3000 µm from centre of macula. No coagulation of microaneurysms | Grid treatment above, below and nasally around centre of macula. Confluent invisible burns, no targeting or avoidances of MAs | Number according to extent of CSMO, up to 250–300 µm from centre of fovea |
| Size of spots (microns) | 200 | First burn 125 spot size | 125 | 125 | 125 |
| Power | Start at 50 mW titrating up in 10 mW increments until barely visible reaction seen | Increased until visible burn seen, then changed to micropulse mode and power doubled | Power titrated upwards until white burn seen, using continuous mode, then switched to micropulse mode | 750 mW | |
| Duty cycle (%) | 5 | 15 | 15 | 5 | |
We therefore concluded that owing to the heterogeneity among the trials, a meta-analysis was not appropriate.
Other recent studies
One retrospective study by Mansouri et al. 57 compared the effects of SML in patients with DMO and a CRT of ≤ 400 µm with those with a CRT of > 400 µm. The study included 63 eyes from 58 patients. Patients could have repeated laser treatment if DMO had not improved at 3 months, and they could also have rescue anti-VEGF therapy with bevacizumab if, by 6 months, DMO remained. The group with a CRT of ≤ 400 µm achieved an average reduction of 55 µm in CRT and a 0.2 log-MAR gain in visual acuity at 12 months and none required rescue anti-VEGF therapy with bevacizumab. The group with a CRT of > 400 µm did not improve, despite 19 of the 30 patients receiving repeated laser treatment, and all required rescue anti-VEGF treatment with bevacizumab between 6 and 12 months.
Another retrospective study by Kim et al. ,58 using ‘selective retina therapy’ with the Q-switched neodymium-doped yttrium lithium fluoride laser system, also found that eyes with a CRT of < 400 µm (n = 35) experienced a statistically significant improvement in BCVA and reduction in CRT at 6 months following treatment, whereas no statistically significant change in either of these outcomes was observed in eyes with a CRT of ≥ 400 µm (n = 37). These studies provide further support for the decision made by NICE1,2,4 to advise treatment with macular laser for patients with DMO where the CRT is < 400 µm.
A prospective study by Citirik59 included 80 eyes from 40 patients who had previously undergone ranibizumab treatment for DMO and, subsequently, had recurrent DMO and received SML treatment. Patients were grouped as having a CRT between 251 µm and 300 µm (Group 1; n = 20 eyes), between 301 µm and 400 µm (Group 2; n = 18 eyes) and > 400 µm (Group 3). There was also a group of 20 eyes with a CRT of 250–300 µm in patients who did not accept additional treatment (i.e. laser) and were used as controls (Group 4). At 2 months’ follow-up, eyes in Group 1 experienced a 37.43 µm reduction in CRT (p = 0.03) whereas eyes in groups 2, 3, and 4 experienced no statistically significant change (p = 0.47, p = 0.58 and p = 0.32, respectively). Similarly, the mean BCVA in Group 1 increased from Log-MAR 0.52 to Log-MAR 0.38 (p = 0.01), whereas there was no improvement in BCVA in groups 2, 3, and 4 (p = 0.74, p = 0.88 and p = 0.46, respectively). The results of this study, however, are limited by the very small number of participants in each group and the very short follow-up period and, thus, should be interpreted cautiously.
Adverse events
If SML has a similar effectiveness to SL, as most trials including the DIAMONDS trial suggest, the next question is whether or not it has significantly fewer adverse effects, and whether or not these are clinically meaningful.
In the DIAMONDS trial both lasers proved to be very safe, with only a very small number of participants (the highest proportion of participants for any of the following events being ≈ 2%) experiencing AEs potentially related to the laser treatment, including central/paracentral scotomas, epiretinal membrane, choroidal neovascularisation, and self-reported reduced colour vision and metamorphopsia. No statistically significant differences in the occurrence of AEs were found between laser groups. These potential AEs owing to laser treatment were identified a priori before the trial commenced; patients were questioned at each of the follow-up visits about their occurrence and ophthalmologists also evaluated participants to determine whether or not any of these had happened. None of these reported AEs, however, was regarded by the investigators as being definitively caused by the laser treatment.
In the RCT by Vujosevic et al. ,10 it was found that contrast sensitivity measured by microperimetry increased after SML, but decreased after SL. Qiao et al. 17 included in their meta-analysis three trials (Kumar et al. 13 and Venkatesh et al. 12 at 6 months, and Figueira et al. 11 at 3 months) that had included contrast sensitivity and found no difference overall between SML and SL. There was considerable heterogeneity among trials.
Chhablani et al. 60 randomised 30 eyes from 20 patients with non-centre-involving DMO to two SML strategies (5% and 15% duty cycle) or to SL. Patients had microperimetry, CRT and BCVA measured at baseline and at 6 and 12 weeks post treatment. Retinal sensitivity increased in the SML group but decreased in the SL group.
The majority of previously published studies9,10,13 reported a lack of visible retinal changes after SML, whereas laser scars are most often present after SL. Figueira et al. 11 reported scars in 59% of eyes after SL versus 14% after SML after a follow-up of 12 months.
None of the above studies assessed patient-reported outcomes, with the exception of the DIAMONDS trial. The DIAMONDS trial showed no differences in health-related or vision-related quality of life, suggesting that differences in retinal sensitivity and the presence of retinal scars may not be perceived by and be relevant to patients or, alternatively, that the quality of life tools used are unable to detect them, if they were to exist.
Clinical implications
For many years, macular laser was the mainstay therapy to treat DMO, with the ETDRS demonstrating in 1985 that it reduced the risk of visual loss (loss of ≥ 3 ETDRS lines = loss of 15 ETDRS letters) by 50% at 3 years in people with CSMO. 5 As only a small (≈ 3%) proportion of participants in the ETDRS experienced an improvement in vision of 15 ETDRS letters, it has been usually stated that macular laser does not improve vision. The great majority of eyes in the ETDRS (1903/2243; 85%) had excellent vision, of 20/40 or better at baseline,5 which may explain the limited improvement in vision observed in this large trial. Indeed, a Diabetic Retinopathy Clinical Research Network trial showed that 32% and 44% of patients with centre-involving DMO experienced a 10-letter visual acuity improvement at 2 and 3 years, respectively, following macular laser. 6,7 Thus, macular laser can improve vision in people with centre-involving DMO. However, with the advent of anti-VEGF therapies the benefits of macular laser have been ignored by many, as shown in the published European Society of Retina Specialists (EURETINA) guidelines. 61 The DIAMONDS trial shows that for patients with DMO fulfilling the DIAMONDS inclusion criteria, and as advised by NICE based on best available evidence, macular laser has still an important place in the management of people with centre-involving DMO. 2,3 In addition, as highlighted by Bakri et al. in the American Society of Retinal Specialists guidelines,62 macular laser also remains the treatment of choice in patients with non-centre-involving DMO. Furthermore, it has been shown in a publicly funded RCT that 41–64% of people receiving anti-VEGF therapies (bevacizumab, ranibizumab or aflibercept) required macular laser to control DMO during the 2-year period following initiation of therapy. 8 Thus, macular laser is still needed even in people treated with anti-VEGF therapy.
A retrospective study by Lai et al. 63 which included 164 eyes from 164 patients with centre-involving DMO and a CRT of > 300 µm treated with either SML (86 eyes) or intravitreal aflibercept monotherapy (78 eyes) found that although at the 6-month follow-up a higher percentage of eyes in the aflibercept group experienced at least a 5-letter visual acuity improvement when compared with those in the SML group (56% vs. 38%; p = 0.044), this was no longer the case at 12 months (45% vs. 49%; p = 0.584) or at 24 months (49% vs. 57%; p = 0.227). Similarly, although at the 6-month visit the aflibercept group had a higher percentage of eyes with an improvement of at least 10% in CRT than the SML group (73% vs. 49%; p = 0.005), this was no longer the case at the 12-month (73% vs. 70%; p = 0.995) or 24-month visits (85% vs. 84%; p = 0.872).
A publicly funded RCT by the Diabetic Retinopathy Clinical Research Network compared observation, standard macular laser and anti-VEGF therapy (aflibercept) in patients with centre-involving DMO with good vision, with the primary outcome being a loss of ≥ 5 ETDRS letters from baseline to 2 years. 64 A total of 625 of the 702 participants (89%) completed the 2-year visit. Participants had normal vision [mean ETDRS letter score 85 ETDRS letters (6/6)], and had better glycaemic control (median HbA1c 7.6) and less severe DMO [mean CRT ≈ 300 µm (306 µm, 314 µm and 314 µm in aflibercept, laser and observation groups, respectively)] than those included in DIAMONDS. The percentage of eyes with a loss of ≥ 5-ETDRS letters at 2 years was 16% in the aflibercept arm, 17% in the macular laser arm and 19% in the observation arm, with no statistically significant differences among groups. Similarly, there were no statistically significant differences in change in CRT from baseline to 2 years among treatment groups. The median number of aflibercept injections over 2 years in the aflibercept group was eight (interquartile range 6–11) and macular laser was additionally required in 6% of eyes in this group. A small proportion of participants (9%, 7% and 7% in the aflibercept, macular laser and observation arms, respectively) experienced a loss of 10 or more ETDRS letters from baseline to month 24. The authors concluded that observation without treatment, unless visual acuity worsens, was a reasonable strategy for eyes with centre-involving DMO. Given that DIAMONDS trial participants had poor diabetes control (mean HbA1c 8.5%), reduced vision [mean ETDRS score of 80 letters (6/7.5 Snellen equivalent)] and more severe DMO (mean CRT 329.2 µm), observation is unlikely to have been the right approach for the management of their DMO.
The DIAMONDS trial showed that SML had comparable efficacy and cost to SL, suggesting that either treatment could be equally offered to patients with centre-involving DMO with a CRT of < 400 µm that was suitable for macular laser. Given that SML has been shown to preserve photoreceptor cells (visual cells),21,56,65 RPE and neurosensory retina,10,21 and produce no burn or objective damage, it should be easier and safer to teach to, for example, junior ophthalmologists and non-medical staff, as a foveal burn would be avoided. The possibility of having trained non-medical staff contributing to the management of people living with complications of diabetic retinopathy could help the NHS to cope with the high and ever-increasing demand for care in HES. Specialist nurses and trained hospital optometrists are already providing intravitreal injections for people with DMO in the NHS. 66 A new pathway involving allied health care professionals has been recently shown to have good sensitivity and acceptable specificity, as well as to be cost saving, for the surveillance of people with previously treated complications of diabetic retinopathy, including DMO. 47,67–69 Thus, the possibility of expanding the role of allied non-medical staff to deliver macular laser treatment using SML seems to be reasonable and worth pursuing.
Strengths and limitations
The DIAMONDS trial was a robustly designed and appropriately powered multicentre RCT. It was powered to detect not only non-inferiority of SML when compared with SL, but also equivalence and superiority if these were to exist. It was powered to detect differences not only in the primary outcome but also in important secondary outcomes (CRT and vision-related quality of life). It was estimated at the trial conception that 113 participants in each laser arm were required to have completed primary outcome data at month 24 for the trial to answer the research questions formulated; at trial completion (24-month follow-up) a higher number of participants (116 and 115 in the SML and SL groups, respectively) were available. The trial was designed as a pragmatic trial so that its results could be reproduced in routine clinical practice. Unlike many RCTs evaluating treatments for DMO, in which the primary outcome was measured at 1 year, the DIAMONDS trial set the primary outcome at 2 years, as it is possible that benefits of treatments may wane overtime or, as shown in some laser trials, improve over time. Similarly, unlike many RCTs evaluating treatments for DMO, the DIAMONDS trial included patient-reported outcomes and, importantly, incorporated a clinical outcome that was suggested by people with diabetes and DMO, namely meeting driving standards. This highlights the importance of PPI in the design of research studies, including RCTs. Furthermore, and unlike most trials on treatments for DMO, it incorporated a prospective within-trial health economic evaluation to compare the cost-effectiveness of the alternative treatments investigated. Limitations include the fact that the great majority of participants enrolled had poorly controlled diabetes and, thus, it is possible that better outcomes in both treatment groups could be achieved in people with adequately controlled diabetes.
Implications for health care
The results of the DIAMONDS trial show that ≈ 80% of patients with centre-involving DMO and a CRT of < 400 µm can be managed successfully with macular laser alone and that either SML or SL can be used given that both laser treatments are equivalent. Only ≈ 20% of people in the DIAMONDS trial required additional anti-VEGF treatment. Despite this, one European group61 has recommended anti-VEGF therapy over laser treatment for people with DMO. However, because of the cost of these drugs and the frequency of patient visits required when anti-VEGF therapies are used, implementation of this anti-VEGF strategy for all patients with DMO would be much more expensive for, and would increase demand for care within, health-care systems. Moreover, it is likely that macular laser may be more convenient for patients, given that it requires fewer visits to the clinic for monitoring/repeating treatment when needed and is less painful.
Recommendations for research
The DIAMONDS trial showed that laser treatment remains an effective treatment strategy for patients with centre-involving DMO with a CRT of < 400 µm as determined by SD-OCT. A study determining which patient’s baseline characteristics, besides CRT, are associated with a higher likelihood of an adequate functional (visual acuity) and anatomical (clearance of DMO) treatment response to macular laser would be recommended, so that a more individualised treatment strategy could be offered to patients (i.e. those less likely to respond to macular laser could be offered alternative therapies).
A RCT comparing anti-VEGF monotherapy with anti-VEGF plus laser therapy once the CRT has decreased to less than 400 µm following anti-VEGF treatment would be of interest, as it may reduce the number of anti-VEGF injections required and may improve functional and anatomical outcomes more effectively than either treatment alone. Such a study has not been undertaken. In RCTs in which macular laser was combined with anti-VEGF therapy, it was not stipulated in the protocol that macular laser should be performed only once CRT had decreased to < 400 µm following anti-VEGF injections.
A study evaluating the feasibility of allied non-medical staff delivering SML to patients with DMO would be of benefit as this strategy, if safe and acceptable to patients, could increase capacity in the NHS.
Patient and public involvement
The DIAMONDS PPI group had a substantial input in the trial. As stated in Chapter 2, DIAMONDS patient and public involvement group, the PPI group contributed to the trial design, including the selection of outcomes, preparation of patient related materials for the trial and recruitment strategies. One outcome (meeting driving standards) came directly from this group (i.e. it had not been anticipated by the trial investigators) as it was identified as being a very important outcome for people living with diabetes and DMO. The PPI group participated in the interpretation of trial results and voiced the importance of the overall finding of the trial (i.e. the fact that SML, which does not cause any detectable damage in the retina, is as effective as SL in the treatment of people with centre-involving DMO suitable for macular laser and with a CRT of < 400 µm) and the subsequent importance of future implementation of trial data into clinical practice. The PPI group is currently taking part in the dissemination of the trial results.
Conclusions
Subthreshold micropulse laser was found to be equivalent to SL in clinical benefits, safety and associated costs and, thus, either laser can be used for the treatment of people with centre-involving DMO suitable for macular laser and with a CRT of < 400 µm.
Acknowledgements
The authors would like to specially thank all DIAMONDS trial participants. We thank the NIHR Health Technology Assessment programme for providing funding for the trial. We also thank the UK Ophthalmology Clinical Research Network, and Maurice O’Kane, Julie Silvestri, Jonathan Jackson, Paul Biagioni of the Northern Ireland Ophthalmology Clinical Research Network, as well as Alison Murphy and Lynn Murphy for their support to this trial. We thank Pamela Royle for conducting the literature searches. We thank Danielle Logan (Supporting Statistician, NICTU) for her assistance with the statistical analyses. We would like to thank IRIDEX for providing a free loan for the laser equipment for the trial and William, Moore, Joan Stauffer, George Marcellino (in memoriam), Franz Weingartner, Gareth Hymas, Nick Fitrzyk and Suzanne Kelly, for their support. IRIDEX had no role in the design, data analysis or interpretation of the DIAMONDS trial data or in the writing of this report.
The authors would also like to thank independent TSC members Alistair Laidlaw, Ian Pearce, Tom Rush, and Andrew Elders, and DMEC members Graeme MacLennan, Baljean Dhillon and Tom Williamson.
Ethics approval for the DIAMONDS trial was obtained on 17 August 2016 from the Office for Research Ethics Committees Northern Ireland (ORECNI) (15/NI/0197) prior to the initiation of the trial. A Clinical Trial Authorisation (CTA) was also obtained from the Medicines and Healthcare products Regulatory Agency (MHRA) (32485/0029/001–0001). Informed consent to participate in the study was obtained from all participants. The DIAMONDS trial was conducted in accordance with the World Medical Association Declaration of Helsinki. 70
Patient and public involvement
We would like to thank the DIAMONDS PPI Group and especially Martin Adams for providing a patient and public perspective on the design, findings and interpretation of the results of this trial. We thank the members of the ‘Diabetes Family’ Facebook (Meta Platforms, Inc., Menlo Park, CA, USA) group, who provided very important feedback at the time of trial conception. This included the suggestion of adding ‘meeting driving standards’ as an outcome in the trial, after having recognised this to be a very important outcome for people living with diabetes.
DIAMONDS study group
Noemi Lois, Queen’s University and Royal Victoria Hospital, BHSCT; Evie Gardner, NICTU; Norman Waugh, Warwick University; Augusto Azuara-Blanco, Queen’s University and Royal Victoria Hospital, BHSCT; Hema Mistry, Warwick University; Danny McAuley, Queen’s University and Royal Victoria Hospital, BHSCT; Mike Clarke, Queens University; Tariq M Aslam, Manchester Royal Eye Hospital, Central Manchester University Hospitals NHS Foundation Trust; Clare Bailey, Bristol Eye Hospital, University Hospitals Bristol NHS Foundation Trust; Victor Chong, Royal Free Hospital NHS Foundation Trust, London; Sobha Sivaprasad, Moorfields Eye Hospital NHS Foundation Trust; David H Steel, Sunderland Eye Infirmary, City Hospitals Sunderland NHS Foundation Trust; James Stephen Talks, Newcastle Eye Centre, Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust; Catherine Adams, NICTU; Christina Campbell, NICTU; Matthew Mills, NICTU; Paul Doherty, NICTU; Aby Joseph, NICTU; Nachiketa Acharya, Sheffield Teaching Hospitals NHS Foundation Trust; Louise Downey, Hull and East Yorkshire Hospital, Hull and East Yorkshire NHS Trust; Haralabos Eleftheriadis, King’s College Hospital NHS Foundation Trust; Samia Fatum, John Radcliffe Hospital, Oxford University Hospitals NHS Foundation Trust; Sheena George, Hillingdon Hospitals NHS Foundation Trust; Faruque Ghanchi, Bradford Teaching Hospitals NHS Trust; Markus Groppe, Stoke Mandeville Hospital, Buckinghamshire NHS Trust; Robin Hamilton, Moorfields Eye Hospital NHS Foundation Trust; Geeta Menon, Frimley Park Hospital NHS Foundation Trust; Ahmed Saad, James Cook University Hospital, South Tees Hospitals NHS Foundation Trust; and Marianne Shiew; Hinchingbrooke Hospital North West Anglia NHS Trust.
Research teams at participating sites
The DIAMONDS trial was a collaborative effort across many sites. We thank all staff at the participating sites for making a valuable contribution to the trial, both in delivering the intervention and in measuring patient outcomes. We are also grateful to those staff at the NICTU who worked on the early phases of the trial, including Catherine Adams, Fiona McCourt, Evie Gardner, Mairead North and Matthew Mills.
Royal Victoria Hospital, Belfast Health and Social Care Trust
Nuala-Jane Lavery, Rebecca Denham, Louise Scullion, Vittorio Silvestri, Lesley Doyle, Graham Young, Kate Graham, Jonathan Jackson, Jonathan Keenan, Georgina Sterret, Deirdre Burns, Claire Maguire and Emma McConnell.
Bristol Eye Hospital, University Hospitals Bristol NHS Foundation Trust
Geraldine Salahi-Ali, Poppy Lavender and Mona-Lisa Bora.
Frimley Park Hospital NHS Foundation Trust
Clare Denford, Lynda Francis, Manju Chandran (Consultant Ophthalmic Surgeon and Sub Investigator) and Abigail Raguro (Research Nurse).
Hinchingbrooke Hospital, North West Anglia NHS Trust
Debra Jones and Linda Molloy.
King’s College Hospital NHS Foundation Trust
Noimot Timson.
Manchester Royal Eye Hospital, Central Manchester University Hospitals NHS Foundation Trust
Stephanie Clarke, Rasia Platt and Sony-Rose Augustine.
Moorfields Eye Hospital NHS Foundation Trust
Samantha Gibson, Taniya Chowdhury, Supeetha David, Tabassum Master, Simona Esposti, Elaina Reid, Tran Dang, Maria Gemenetzi and Filipa Rodriguez.
Newcastle Eye Centre, Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust
Patricia Day and Violet Andrew.
John Radcliffe Hospital, Oxford University Hospitals NHS Foundation Trust
Janette Savage.
Sheffield Teaching Hospitals NHS Foundation Trust
Helen Pokora.
Sunderland Eye Infirmary, South Tyneside and Sunderland NHS Foundation Trust
Maged Habib, Lauren Gardner and Steve Dodds.
Bradford Royal Infirmary, Bradford Teaching Hospitals NHS Trust
Nicola Hawes, Sarah Moss, Hayley Barker, Zeid Madanat and Farahat Butt.
James Cook University Hospital, South Tees Hospitals NHS Foundation Trust
Catherine Williamson and Adele Szekeres.
Hull and East Yorkshire Hospital, Hull and East Yorkshire NHS Trust
Angie Atkinson and Julia Twigger.
Stoke Mandeville Hospital, Buckinghamshire NHS Trust
Judith Abrams.
Hillingdon Hospitals NHS Foundation Trust
Rahila Naureen.
Trial Management Group
The following were members of the TMG for some or all of the duration of the study: Professor Noemi Lois (chief investigator, chair); Professor Augusto Azuara-Blanco, Professor Mike Clarke, Professor Danny McAuley, Mr Paul Doherty, Ms Catherine Adams and Ms Fiona McCourt (trial managers); Mr Matthew Mills and Ms Pauline McElhill (clinical trial co-ordinators); Mr Aby Joseph and Ms Omowumi Odusina (data managers); Mr Mark Wilson (database developer); Ms Christina Campbell, Ms Evie Gardner and Ms Mairead North (statisticians); Professor Norman Waugh (health economist), Dr Hema Mistry and Dr Mandy Maredza (health economists); Ms Nicola Duff and Ms Lesley-Anne Dougan (clinical trial administrators); and Ms Emma McAuley and Mr Ryan Craig (clinical research monitors).
Trial Steering Committee
Mr Alistair Laidlaw (clinician, chair), Mr Andrew Elders (statistician), Mr Ian Pearce (clinician) and Mr Tom Rush (PPI representative).
Data Monitoring and Ethics Committee
Mr Graeme MacLennan (statistician, chair), Professor Baljean Dhillon (clinician) and Professor Tom Williamson (clinician).
Sponsor
BHSCT.
Contributions of authors
Noemi Lois (https://orcid.org/0000-0003-2666-2937) (Clinical Professor of Ophthalmology) is the Chief Investigator for the DIAMONDS trial; led the team of co-applicants, researchers and trial supporting staff; conceived and designed the trial with input from co-applicants; and contributed to the identification, recruitment, examination and treatment of all participants enrolled at the Belfast site, as well as to data collection, analysis and interpretation of the trial findings. She led the drafting of this report, revised it critically for important intellectual content and approved its final draft for submission.
Christina Campbell (https://orcid.org/0000-0002-8446-523X) (Trial Statistician) performed the statistical analyses, assisted with the interpretation of data, contributed to the drafting of the report and critically reviewed it.
Norman Waugh (https://orcid.org/0000-0003-0934-4961) (Professor of Public Health and Health Technology Assessment) and Hema Mistry (https://orcid.org/0000-0002-5023-1160) (Health Economist) contributed to the design of the health economics plan and provided oversight for all aspects of the economic evaluation including its design, conduct, analysis and reporting.
Augusto Azuara-Blanco (https://orcid.org/0000-0002-4805-9322) (Clinical Professor of Ophthalmology) contributed to the design of this trial, to the interpretation of the data and to the critical review of this report.
Mandy Maredza (https://orcid.org/0000-0002-2030-3338) (Health Economist) undertook the economic evaluation, supported by Norman Waugh and Hema Mistry.
Danny McAuley (https://orcid.org/0000-0002-3283-1947) (Clinical Professor and Consultant in Intensive Care Medicine and former Director of the Northern Ireland Clinical Trials Unit) contributed to the design of this trial, the interpretation of the data and the critical review of this report.
Nachiketa Acharya (https://orcid.org/0000-0002-0794-689X) (Consultant Ophthalmologist) contributed to the critical review of this report, as well as to the recruitment and evaluation of participants enrolled and followed up in this trial.
Tariq M Aslam (https://orcid.org/0000-0002-9739-7280) (Consultant Ophthalmologist) contributed to the design of this trial, the interpretation of the data and the critical review of this report, as well as to the recruitment and evaluation of participants enrolled and followed up in this trial.
Clare Bailey (https://orcid.org/0000-0003-2141-4512) (Consultant Ophthalmologist) contributed to the design of this trial, the interpretation of the data and the critical review of this report, as well as to the recruitment and evaluation of participants enrolled and followed up in this trial.
Victor Chong (https://orcid.org/0000-0002-7693-522X) (Consultant Ophthalmologist) contributed to the design of this trial, the interpretation of the data and the critical review of this report, as well as to the recruitment and evaluation of participants enrolled and followed up in this trial.
Louise Downey (https://orcid.org/0000-0001-5944-3437) (Consultant Ophthalmologist) contributed to the critical review of this report, as well as to the recruitment and evaluation of participants enrolled and followed up in this trial.
Haralabos Eleftheriadis (https://orcid.org/0000-0002-9980-4070) (Consultant Ophthalmologist) contributed to the critical review of this report, as well as to the recruitment and evaluation of participants enrolled and followed up in this trial.
Samia Fatum (https://orcid.org/0000-0002-5760-4038) contributed to the critical review of this report, as well as to the recruitment and evaluation of participants enrolled and followed up in this trial.
Sheena George (https://orcid.org/0000-0002-9953-1834) (Consultant Ophthalmologist) contributed to the critical review of this report, as well as to the recruitment and evaluation of participants enrolled and followed up in this trial.
Faruque Ghanchi (https://orcid.org/0000-0002-4448-8162) (Consultant Ophthalmologist) contributed to the critical review of this report, as well as to the recruitment and evaluation of participants enrolled and followed up in this trial.
Markus Groppe (https://orcid.org/0000-0003-1963-3484) (Consultant Ophthalmologist) contributed to the critical review of this report, as well as to the recruitment and evaluation of participants enrolled and followed up in this trial.
Robin Hamilton (https://orcid.org/0000-0003-2861-8761) (Consultant Ophthalmologist) contributed to the critical review of this report, as well as to the recruitment and evaluation of participants enrolled and followed up in this trial.
Geeta Menon (https://orcid.org/0000-0001-7532-6564) (Consultant Ophthalmologist) contributed to the critical review of this report, as well as to the recruitment and evaluation of participants enrolled and followed up in this trial.
Ahmed Saad (https://orcid.org/0000-0002-5159-8001) (Consultant Ophthalmologist) contributed to the critical review of this report, as well as to the recruitment and evaluation of participants enrolled and followed up in this trial.
Sobha Sivaprasad (https://orcid.org/0000-0001-8952-0659) (Consultant Ophthalmologist) contributed to the design of this trial, the interpretation of the data and the critical review of this report, as well as to the recruitment and evaluation of participants enrolled and followed up in this trial.
Marianne Shiew (https://orcid.org/0000-0003-4774-3576) (Consultant Ophthalmologist) contributed to the critical review of this report, as well as to the recruitment and evaluation of participants enrolled and followed up in this trial.
David H Steel (https://orcid.org/0000-0001-8734-3089) (Consultant Ophthalmologist) contributed to the design of this trial, the interpretation of the data and the critical review of this report, as well as to the recruitment and evaluation of participants enrolled and followed up in this trial.
James Stephen Talks (https://orcid.org/0000-0001-6126-6476) (Consultant Ophthalmologist) contributed to the design of this trial, the interpretation of the data and the critical review of this report, as well as to the recruitment and evaluation of participants enrolled and followed up in this trial.
Paul Doherty (https://orcid.org/0000-0002-3263-7052) (Trial Manager) provided training and support to sites, co-ordinated and managed the trial, was involved in the management of the study and critically reviewed the manuscript.
Clíona McDowell (https://orcid.org/0000-0002-7644-7197) (Trial Statistician) contributed to the statistical analyses, assisted with the interpretation of data, contributed to the drafting of the statistical sections of the report and critically reviewed it.
Mike Clarke (https://orcid.org/0000-0002-2926-7257) (Professor of Research Methodology at the MRC Methodology Hub and Director of the Northern Ireland Clinical Trials Unit) was involved in the conception and design of this trial, contributed to the interpretation of data and drafting of this report, and revised it critically for important intellectual content.
Publications
Lois N, Gardner E, Waugh N, Azuara-Blanco A, Mistry H, McAuley D, et al. Diabetic macular oedema and diode subthreshold micropulse laser (DIAMONDS): study protocol for a randomised controlled trial. Trials 2019;20:122.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data or trial materials may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Stitt AW, Curtis TM, Chen M, Medina RJ, McKay GJ, Jenkins A, et al. The progress in understanding and treatment of diabetic retinopathy. Prog Retin Eye Res 2016;51:156-86. https://doi.org/10.1016/j.preteyeres.2015.08.001.
- National Institute for Health and Care Excellence (NICE) . Ranibizumab for Treating Diabetic Macular Oedema 2013. www.nice.org.uk/guidance/ta274 (accessed 7 January 2022).
- National Institute for Health and Care Excellence (NICE) . Aflibercept for Treating Diabetic Macular Oedema 2015. www.nice.org.uk/guidance/ta346 (accessed 7 January 2022).
- National Institute for Health and Care Excellence (NICE) . Faricimab for Treating Diabetic Macular Oedema 2022. www.nice.org.uk/guidance/TA799 (accessed 17 November 2022).
- Early Treatment Diabetic Retinopathy Study Research Group . Photocoagulation for diabetic macular edema: early treatment diabetic retinopathy report number 1. Arch Ophthalmol 1985;103:1796-806. https://doi.org/10.1001/archopht.1985.01050120030015.
- Aiello LP, Edwards AR, Beck RW, Bressler NM, Davis MD, Ferris F, et al. Factors associated with improvement and worsening of visual acuity 2 years after focal/grid photocoagulation for diabetic macular edema. Ophthalmology 2010;117:946-53. https://doi.org/10.1016/j.ophtha.2009.10.002.
- Beck RW, Edwards AR, Aiello LP, Bressler NM, Ferris F, Glassman AR, et al. Three-year follow-up of a randomized trial comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmol 2009;127:245-51. https://doi.org/10.1001/archophthalmol.2008.610.
- Wells JA, Glassman AR, Ayala AR, Jampol LM, Bressler NM, Bressler SB, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology 2016;123:1351-9. https://doi.org/10.1016/j.ophtha.2016.02.022.
- Lavinsky D, Cardillo JA, Melo LA, Dare A, Farah ME, Belfort R. Randomized clinical trial evaluating mETDRS versus normal or high-density micropulse photocoagulation for diabetic macular edema. Invest Ophthalmol Vis Sci 2011;52:4314-23. https://doi.org/10.1167/iovs.10-6828.
- Vujosevic S, Bottega E, Casciano M, Pilotto E, Convento E, Midena E. Microperimetry and fundus autofluorescence in diabetic macular edema: subthreshold micropulse diode laser versus modified early treatment diabetic retinopathy study laser photocoagulation. Retina 2010;30:908-16. https://doi.org/10.1097/IAE.0b013e3181c96986.
- Figueira J, Khan J, Nunes S, Sivaprasad S, Rosa A, de Abreu JF, et al. Prospective randomised controlled trial comparing sub-threshold micropulse diode laser photocoagulation and conventional green laser for clinically significant diabetic macular oedema. Br J Ophthalmol 2009;93:1341-4. https://doi.org/10.1136/bjo.2008.146712.
- Venkatesh P, Ramanjulu R, Azad R, Vohra R, Garg S. Subthreshold micropulse diode laser and double frequency neodymium: YAG laser in treatment of diabetic macular edema: a prospective, randomized study using multifocal electroretinography. Photomed Laser Surg 2011;29:727-33. https://doi.org/10.1089/pho.2010.2830.
- Kumar V, Ghosh B, Mehta DK, Goel N. Functional outcome of subthreshold versus threshold diode laser photocoagulation in diabetic macular oedema. Eye 2010;24:1459-65. https://doi.org/10.1038/eye.2010.53.
- Laursen ML, Moeller F, Sander B, Sjoelie AK. Subthreshold micropulse diode laser treatment in diabetic macular oedema. Br J Ophthalmol 2004;88:1173-9. https://doi.org/10.1136/bjo.2003.040949.
- Xie TY, Guo QQ, Wang Y, Wang Q, Chen XY. Randomized, controlled clinical trial comparison of SDM laser versus argon ion laser in diabetic macular edema. Int Eye Sci 2013;13:2370-2.
- Chen G, Tzekov R, Li W, Jiang F, Mao S, Tong Y. Subthreshold micropulse diode laser versus conventional laser photocoagulation for diabetic macular edema: a meta-analysis of randomized controlled trials. Retina 2016;36:2059-65. https://doi.org/10.1097/IAE.0000000000001053.
- Qiao G, Guo HK, Dai Y, Wang XL, Meng QL, Li H, et al. Sub-threshold micro-pulse diode laser treatment in diabetic macular edema: a meta-analysis of randomized controlled trials. Int J Ophthalmol 2016;9:1020-7. https://doi.org/10.18240/ijo.2016.07.15.
- Wu Y, Ai P, Ai Z, Xu G. Subthreshold diode micropulse laser versus conventional laser photocoagulation monotherapy or combined with anti-VEGF therapy for diabetic macular edema: a bayesian network meta-analysis. Biomed Pharmacother 2018;97:293-9. https://doi.org/10.1016/j.biopha.2017.10.078.
- Jorge EC, Jorge EN, Botelho M, Farat JG, Virgili G, El Dib R. Monotherapy laser photocoagulation for diabetic macular oedema. Cochrane Database Syst Rev 2018;10. https://doi.org/10.1002/14651858.CD010859.pub2.
- Fazel F, Bagheri M, Golabchi K, Jahanbani Ardakani H. Comparison of subthreshold diode laser micropulse therapy versus conventional photocoagulation laser therapy as primary treatment of diabetic macular edema. J Curr Ophthalmol 2016;28:206-11. https://doi.org/10.1016/j.joco.2016.08.007.
- Luttrull JK, Sinclair SH. Safety of transfoveal subthreshold diode micropulse laser for fovea-involving diabetic macular edema in eyes with good visual acuity. Retina 2014;34:2010-20. https://doi.org/10.1097/IAE.0000000000000177.
- Lois N, Gardner E, Waugh N, Azuara-Blanco A, Mistry H, McAuley D, et al. Diabetic macular oedema and diode subthreshold micropulse laser (DIAMONDS): study protocol for a randomised controlled trial. Trials 2019;20. https://doi.org/10.1186/s13063-019-3199-5.
- Costa ML, Achten J, Wagland S, Marian IR, Maredza M, Schlüssel MM, et al. Plaster cast versus functional bracing for Achilles tendon rupture: the UKSTAR RCT. Health Technol Assess 2020;24. https://doi.org/10.3310/hta24080.
- Mosely J. Visual Function Endpoints: The Regulatory Perspective. London: European Medicines Agency; 2011.
- Diabetic Retinopathy Clinical Research Network . Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med 2015;372:1193-203. https://doi.org/10.1056/NEJMoa1414264.
- Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. CATT Research Group . Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011;364:1897-908. https://doi.org/10.1056/NEJMoa1102673.
- The Royal College of Ophthalmologists . Diabetic Retinopathy Guidelines n.d. www.rcophth.ac.uk/wp-content/uploads/2014/12/2013-SCI-301-FINAL-DR-GUIDELINES-DEC-2012-updated-July-2013.pdf (accessed 7 January 2022).
- Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 2012;119:789-801. https://doi.org/10.1016/j.ophtha.2011.12.039.
- Gillies MC, Sutter FK, Simpson JM, Larsson J, Ali H, Zhu M. Intravitreal triamcinolone for refractory diabetic macular edema: two-year results of a double-masked, placebo-controlled, randomized clinical trial. Ophthalmology 2006;113:1533-8. https://doi.org/10.1016/j.ophtha.2006.02.065.
- Tranos PG, Topouzis F, Stangos NT, Dimitrakos S, Economidis P, Harris M, et al. Effect of laser photocoagulation treatment for diabetic macular oedema on patient’s vision-related quality of life. Curr Eye Res 2004;29:41-9. https://doi.org/10.1080/02713680490513191.
- Fiore T, Androudi S, Iaccheri B, Lupidi M, Giansanti F, Fabrizio G, et al. Repeatability and reproducibility of retinal thickness measurements in diabetic patients with spectral domain optical coherence tomography. Curr Eye Res 2013;38:674-9. https://doi.org/10.3109/02713683.2013.781191.
- Lloyd AJ, Loftus J, Turner M, Lai G, Pleil A. Psychometric validation of the Visual Function Questionnaire-25 in patients with diabetic macular edema. Health Qual Life Outcomes 2013;11. https://doi.org/10.1186/1477-7525-11-10.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013. www.nice.org.uk/process/pmg9 (accessed 6 September 2022).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2020. Canterbury: PSSRU, University of Kent; 2020.
- Department of Health and Social Care . NHS Reference Costs 2018 to 2019 n.d. https://improvement.nhs.uk/resources/reference-costs/ (accessed 21 May 2018).
- Joint Formulary Committee . British National Formulary 2022.
- Glick HA, Doshi JA, Sonnad SS. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press Oxford; 2014.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD. National Eye Institute Visual Function Questionnaire Field Test Investigators . Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol 2001;119:1050-8. https://doi.org/10.1001/archopht.119.7.1050.
- Misajon R, Hawthorne G, Richardson J, Barton J, Peacock S, Iezzi A, et al. Vision and quality of life: the development of a utility measure. Invest Ophthalmol Vis Sci 2005;46:4007-15. https://doi.org/10.1167/iovs.04-1389.
- Peacock S, Misajon R, Iezzi A, Richardson J, Hawthorne G, Keeffe J. Vision and quality of life: development of methods for the VisQoL vision-related utility instrument. Ophthalmic Epidemiol 2008;15:218-23. https://doi.org/10.1080/09286580801979417.
- Morris TP, White IR, Royston P. Tuning multiple imputation by predictive mean matching and local residual draws. BMC Med Res Methodol 2014;14. https://doi.org/10.1186/1471-2288-14-75.
- Little RJ, Rubin DB. Statistical Analysis with Missing Data. Hoboken, NJ: John Wiley & Sons; 2019.
- York Health Economics Consortium . Net Monetary Benefit. 2016. https://yhec.co.uk/glossary/net-monetary-benefit/ (accessed 7 January 2022).
- Piaggio G, Elbourne DR, Pocock SJ, Evans SJ, Altman DG. CONSORT Group . Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA 2012;308:2594-604. https://doi.org/10.1001/jama.2012.87802.
- Le Henanff A, Giraudeau B, Baron G, Ravaud P. Quality of reporting of noninferiority and equivalence randomized trials. JAMA 2006;295:1147-51. https://doi.org/10.1001/jama.295.10.1147.
- Lois N, Cook JA, Wang A, Aldington S, Mistry H, Maredza M, et al. Evaluation of a new model of care for people with complications of diabetic retinopathy: the EMERALD study. Ophthalmology 2021;128:561-73. https://doi.org/10.1016/j.ophtha.2020.10.030.
- DiaSys . HbA1c Calculator n.d. www.hba1cnet.com/hba1c-calculator/ (accessed 19 September 2022).
- Ford I, Norrie J. Pragmatic trials. N Engl J Med 2016;375:454-63. https://doi.org/10.1056/NEJMra1510059.
- Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ 2015;350. https://doi.org/10.1136/bmj.h2147.
- Scholz P, Altay L, Fauser S. A review of subthreshold micropulse laser for treatment of macular disorders. Adv Ther 2017;34:1528-55. https://doi.org/10.1007/s12325-017-0559-y.
- Blindbæk SL, Peto T, Grauslund J. How do we evaluate the role of focal/grid photocoagulation in the treatment of diabetic macular edema?. Acta Ophthalmol 2019;97:339-46. https://doi.org/10.1111/aos.13997.
- National Heart, Lung, and Blood Institute . Study Quality Assessment Tools n.d. www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed 7 January 2022).
- Casson RJ, Raymond G, Newland HS, Gilhotra JS, Gray TL. Pilot randomized trial of a nanopulse retinal laser versus conventional photocoagulation for the treatment of diabetic macular oedema. Clin Exp Ophthalmol 2012;40:604-10. https://doi.org/10.1111/j.1442-9071.2012.02756.x.
- Cooper OAE, Taylor DJ, Crabb DP, Sim DA, McBain H. Psychological, social and everyday visual impact of diabetic macular oedema and diabetic retinopathy: a systematic review. Diabet Med 2020;37:924-33. https://doi.org/10.1111/dme.14125.
- Vujosevic S, Martini F, Longhin E, Convento E, Cavarzeran F, Midena E. Subthreshold micropulse yellow laser versus subthreshold micropulse infrared laser in center-involving diabetic macular edema: morphologic and functional safety. Retina 2015;35:1594-603. https://doi.org/10.1097/IAE.0000000000000521.
- Mansouri A, Sampat KM, Malik KJ, Steiner JN, Glaser BM. Medscape. Efficacy of subthreshold micropulse laser in the treatment of diabetic macular edema is influenced by pre-treatment central foveal thickness. Eye 2014;28:1418-24. https://doi.org/10.1038/eye.2014.264.
- Kim M, Park YG, Jeon SH, Choi SY, Roh YJ. The efficacy of selective retina therapy for diabetic macular edema based on pretreatment central foveal thickness. Lasers Med Sci 2020;35:1781-90. https://doi.org/10.1007/s10103-020-02984-6.
- Citirik M. The impact of central foveal thickness on the efficacy of subthreshold micropulse yellow laser photocoagulation in diabetic macular edema. Lasers Med Sci 2019;34:907-12. https://doi.org/10.1007/s10103-018-2672-9.
- Chhablani J, Alshareef R, Kim DT, Narayanan R, Goud A, Mathai A. Comparison of different settings for yellow subthreshold laser treatment in diabetic macular edema. BMC Ophthalmol 2018;18. https://doi.org/10.1186/s12886-018-0841-z.
- Schmidt-Erfurth U, Garcia-Arumi J, Bandello F, Berg K, Chakravarthy U, Gerendas BS, et al. Guidelines for the management of diabetic macular edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica 2017;237:185-222. https://doi.org/10.1159/000458539.
- Bakri SJ, Wolfe JD, Regillo CD, Flynn HW, Wykoff CC. Evidence-based guidelines for management of diabetic macular edema. J VitreoRetin Dis 2019;3:145-52. https://doi.org/10.1177/2474126419834711.
- Lai FHP, Chan RPS, Lai ACH, Tsang S, Woo TTY, Lam RF, et al. Comparison of two-year treatment outcomes between subthreshold micropulse (577 nm) laser and aflibercept for diabetic macular edema. Jpn J Ophthalmol 2021;65:680-8. https://doi.org/10.1007/s10384-021-00846-4.
- Baker CW, Glassman AR, Beaulieu WT, Antoszyk AN, Browning DJ, Chalam KV, et al. Effect of initial management with aflibercept vs laser photocoagulation vs observation on vision loss among patients with diabetic macular edema involving the center of the macula and good visual acuity: a randomized clinical trial. JAMA 2019;321:1880-94. https://doi.org/10.1001/jama.2019.5790.
- Wells-Gray EM, Doble N, Ohr MP, Choi SS. Structural integrity of individual cone photoreceptors after short-wavelength subthreshold micropulse laser therapy for diabetic macular edema. Ophthalmic Surg Lasers Imaging Retina 2018;49:946-54. https://doi.org/10.3928/23258160-20181203-07.
- Gallagher MJ. Introduction of a nurse-led intravitreal injection service in ophthalmology. Br J Nurs 2017;26:800-3. https://doi.org/10.12968/bjon.2017.26.14.800.
- Lois N, Cook J, Aldington S, Waugh N, Mistry H, Sones W, et al. Effectiveness of Multimodal imaging for the Evaluation of Retinal oedema And new vesseLs in Diabetic retinopathy (EMERALD). BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2018-027795.
- Lois N, Cook J, Wang A, Aldington S, Mistry H, Maredza M, et al. Multimodal imaging interpreted by graders to detect re-activation of diabetic eye disease in previously treated patients: the EMERALD diagnostic accuracy study. Health Technol Assess 2021;25. https://doi.org/10.3310/hta25320.
- Maredza M, Mistry H, Lois N, Aldington S, Waugh N. EMERALD Study Group . Surveillance of people with previously successfully treated diabetic macular oedema and proliferative diabetic retinopathy by trained ophthalmic graders: cost analysis from the EMERALD study. Br J Ophthalmol 2021;106:1549-54. https://doi.org/10.1136/bjophthalmol-2021-318816.
- World Medical Association . Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191-4. https://doi.org/10.1001/jama.2013.281053.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2019. Canterbury: PSSRU, University of Kent; 2019.
- NHS England . 2019/20/National/Cost/Collection/Data/Publication n.d. www.england.nhs.uk/publication/2019-20-national-cost-collection-data-publication/ (accessed 22 November 2022).
Appendix 1 Additional tables
| Resource Item | Unit cost (£, 2020) | Measurement unit | Source |
|---|---|---|---|
| Staff Costs | |||
| Consultant | 114 | Per working hour | PSSRU 2020,34 page 158 |
| Associate specialist/staff grade | 117 | Per working hour | PSSRU 2020,34 page 158 |
| Retina fellow | 50 | Per working hour | PSSRU 2019,71 page 158 |
| Ophthalmic photographer/imaging technician | 52a | Per working hour | PSSRU 2020,34 page 151 |
| Anti-VEGF costs | |||
| Ranibizumab | 569 | Per dose | NHS Reference Costs 2019–2072 |
| Aflibercept | 634 | Per dose | NHS Reference Costs 2019–2072 |
| Bevacizumab | 277 | Per dose | NHS Reference Costs 2019–2072 |
| Laser type | Current cost (£)a | Lifespan (years) | Annual throughput (n)b | Total annual discounted costs (£) | Cost per patient (£) |
|---|---|---|---|---|---|
| Complete scanning Laser Module TxCell/Haag Streit Fit/IQ577 nm with Micropulse®c |
79,800: purchase price 6990: total cost for a 5-year preventative maintenance contract (with the first 2 years being warranty) plus VAT |
14 | 3000 | 8860 | 2.95 |
| Nidek GYC-1000 laser (including installation)d |
14,090: purchase price 3653: maintenance costs over 5 years |
7 | 3000 | 3113 | 1.04 |
| Pascal lasere | 51,522: purchase price (excluding optional extras) | 14 | 3000 | 6266 | 2.09 |
| 1548: annual discounted maintenance costs. Assumed similar maintenance costs as DSML, i.e. £6,990 over 5 years | |||||
| Argon laser (new design high quality ophthalmic laser) |
14,000: purchase price 809: annual discounted maintenance costs. Assumed similar maintenance costs as DSML, i.e. £6,990 over 5 years |
7 | 3000 | 3010 | 1.03 |
| Treatment after trial entry (fellow eye on the study) | Treatment group | |
|---|---|---|
| SML (N = 8) | SL (N = 13) | |
| Treatment given, n (%) | ||
| Yes | 4 (50.0) | 9 (69.2) |
| No | 4 (50.0) | 4 (30.8) |
| Spot size (µm), n (%) | ||
| 100 | 0 (0.0) | 5 (55.6) |
| 200 | 4 (100.0) | 4 (44.4) |
| Duration (ms), n (%)a | ||
| 10 | 0 (0.0) | 1 (11.1) |
| 20 | 0 (0.0) | 4 (44.4) |
| 100 | 0 (0.0) | 4 (44.4) |
| 200 | 4 (100.0) | 0 (0.0) |
| Laser power (mW) (micropulse power for DSML), mean (SD) | 260.0 (69.3) [n = 4] | 116.7 (37.4) [n = 9] |
| Number of spots, mean (SD) | 479.3 (140.9) [n = 4] | 34.4 (18.3) [n = 9] |
| Number of treatments, n (%) | ||
| Mean (SD) | 4.5 (2.6) [n = 4] | 2.3 (1.7) [n = 9] |
| 1 | 1 (25.0) | 5 (55.6) |
| 3 | 0 (0.0) | 1 (11.1) |
| 4 | 1 (25.0) | 2 (22.2) |
| 5 | 0 (0.0) | 1 (11.1) |
| 6 | 1 (25.0) | 0 (0.0) |
| 7 | 1 (25.0) | 0 (0.0) |
| SAEs and AEs | SML group (N = 133) | SL group (N = 132) | RR (for number of patients) (95% CI) | p-value | ||
|---|---|---|---|---|---|---|
| Events (n) | Patients, n (%) | Events (n) | Patients, n (%) | |||
| Total SAEs | 35 | 21 (15.8) | 35 | 25 (18.9) | 0.8 (0.5 to 1.4) | 0.50 |
| Related to study treatment | 0 | 0 (0.0) | 0 | 0 (0.0) | – | – |
| Related to study treatment and unexpected | 0 | 0 (0.0) | 0 | 0 (0.0) | – | – |
| Total AEs | 212 | 76 (57.1) | 206 | 81 (61.4) | 0.9 (0.8 to 1.1) | 0.48 |
| Related to study treatment | 5 | 2 (1.5) | 5 | 4 (3.0) | 0.5 (0.1 to 2.7) | 0.45 |
| SAEs | ||||||
| Blood and lymphatic system disorders | 1 | 1 (0.8) | 0 | 0 (0.0) | – | 1.00 |
| Cardiac disorders | 8 | 5 (3.8) | 4 | 4 (3.0) | 1.2 (0.3 to 4.5) | 1.00 |
| Eye disorders | 1 | 1 (0.8) | 1 | 1 (0.75) | 1.0 (0.1 to 15.7) | 1.00 |
| Gastrointestinal disorders | 3 | 3 (2.3) | 3 | 3 (2.3) | 1.0 (0.2 to 4.8) | 1.00 |
| General disorders and administration | 1 | 1 (0.8) | 0 | 0 (0.0) | – | 1.00 |
| Hepatobiliary disorders | 1 | 1 (0.8) | 1 | 1 (0.75) | 1.0 (0.1 to 15.7) | 1.00 |
| Infections and infestations | 5 | 4 (3.0) | 8 | 4 (3.0) | 1.0 (0.3 to 3.9) | 1.00 |
| Injury, poisoning and procedural complications | 1 | 1 (0.8) | 3 | 3 (2.3) | 0.3 (0.0 to 3.1) | 0.37 |
| Investigations | 1 | 1 (0.8) | 0 | 0 (0.0) | – | 1.00 |
| Musculoskeletal and connective tissue | 0 | 0 (0.0) | 1 | 1 (0.75) | – | 0.50 |
| Neoplasms benign and malignant | 1 | 1 (0.8) | 5 | 5 (3.8) | 0.2 (0.0 to 1.7) | 0.12 |
| Nervous system disorders | 1 | 1 (0.8) | 2 | 2 (1.5) | 0.5 (0.1 to 5.4) | 0.62 |
| Renal and urinary disorders | 1 | 1 (0.8) | 2 | 2 (1.5) | 0.5 (0.1 to 5.4) | 0.62 |
| Respiratory, thoracic and mediastinal | 7 | 2 (1.5) | 3 | 2 (1.5) | 1.0 (0.1 to 6.9) | 1.00 |
| Surgical and medical procedures | 1 | 1 (0.8) | 1 | 1 (0.75) | 1.0 (0.1 to 15.7) | 1.00 |
| Vascular disorders | 2 | 2 (1.5) | 1 | 1 (0.75) | 2.0 (0.2 to 21.6) | 1.00 |
| AEs | ||||||
| Blood and lymphatic system disorders | 2 | 2 (1.5) | 4 | 4 (3.0) | 0.5 (0.1 to 2.7) | 0.45 |
| Cardiac disorders | 8 | 5 (3.8) | 7 | 7 (5.3) | 0.7 (0.2 to 2.2) | 0.55 |
| Ear and labyrinth disorders | 1 | 1 (0.8) | 1 | 1 (0.75) | 1.0 (0.1 to 15.7) | 1.00 |
| Eye disorders | 97 | 57 (42.9) | 81 | 50 (37.8) | 1.1 (0.8 to 1.5) | 0.41 |
| Gastrointestinal disorders | 14 | 9 (6.8) | 10 | 8 (6.1) | 1.1 (0.4 to 2.8) | 0.81 |
| General disorders and administration | 2 | 2 (1.5) | 2 | 2 (1.5) | 1.0 (0.1 to 6.9) | 1.00 |
| Hepatobiliary disorders | 1 | 1 (0.8) | 1 | 1 (0.75) | 1.0 (0.1 to 15.7) | 1.00 |
| Infections and infestations | 37 | 20 (15.0) | 27 | 17 (12.9) | 1.2 (0.6 to 2.1) | 0.61 |
| Injury, poisoning and procedural complications | 5 | 4 (3.0) | 8 | 8 (6.1) | 0.5 (0.2 to 1.6) | 0.25 |
| Investigations | 1 | 1 (0.8) | 0 | 0 (0.0) | – | 1.00 |
| Metabolism and nutrition disorders | 1 | 1 (0.8) | 1 | 1 (0.75) | 1.0 (0.1 to 15.7) | 1.00 |
| Musculoskeletal and connective tissue | 5 | 5 (3.8) | 10 | 10 (7.6) | 0.5 (0.2 to 1.4) | 0.18 |
| Neoplasms benign and malignant | 4 | 4 (3.0) | 15 | 10 (7.6) | 0.4 (0.1 to 1.2) | 0.11 |
| Nervous system disorders | 9 | 6 (4.5) | 8 | 7 (5.3) | 0.85 (0.3 to 2.5) | 0.77 |
| Psychiatric disorders | 1 | 1 (0.8) | 1 | 1 (0.75) | 1.0 (0.1 to 15.7) | 1.00 |
| Renal and urinary disorders | 3 | 3 (2.3) | 6 | 5 (3.8) | 0.6 (0.2 to 2.4) | 0.50 |
| Respiratory, thoracic and mediastinal | 9 | 3 (2.3) | 8 | 7 (5.3) | 0.4 (0.1 to 1.6) | 0.22 |
| Skin and subcutaneous tissue disorders | 2 | 2 (1.5) | 7 | 7 (5.3) | 0.3 (0.1 to 1.3) | 0.10 |
| Surgical and medical procedures | 6 | 5 (3.8) | 1 | 1 (0.75) | 5.0 (0.6 to 41.9) | 0.21 |
| Vascular disorders | 4 | 4 (3.0) | 8 | 7 (5.3) | 0.6 (0.2 to 1.9) | 0.38 |
| ARs | ||||||
| Eye disorders | 4 | 4 (3.0) | 5 | 5 (3.8) | 0.8 (0.2 to 2.9) | 0.75 |
| Surgical and medical procedures | 1 | 1 (0.8) | 0 | 0 (0.0) | – | 1.00 |
| Laser type | Mean (SD) | Median | Minimum | Maximum |
|---|---|---|---|---|
| SML | 19.44 (9.80) | 19 | 7 | 100 |
| SL | 17.77 (7.33) | 17 | 3 | 45 |
| Total | 18.58 (8.65) | 17 | 3 | 100 |
| Outcome | SML group | SL group | Difference (95% CI)a | p-value |
|---|---|---|---|---|
| Change in BCVA in the study eye from baseline to month 12, mean (SE)b | –1.02 (0.56) [n = 119] | –0.41 (0.56) [n = 118] | –0.61 (–2.19 to 0.98) | 0.45 |
| Change in binocular BCVA from baseline to month 12, mean (SE)c | –0.82 (0.44) [n = 119] | –1.21 (0.44) [n = 118] | 0.39 (–0.85 to 1.64) | 0.53 |
| Change in CRT in the study eye, as determined by SD-OCT, from baseline to month 12, mean (SE)c | –5.58 (4.37) [n = 120] | –17.71 (4.41) [n = 118] | 12.13 (–0.25 to 24.50) | 0.055 |
| Change in the MD of the Humphrey 10–2 visual field in the study eye from baseline to month 12, mean (SE)c | –0.33 (0.26) [n = 112] | –0.65 (0.27) [n = 105] | 0.32 (–0.43 to 1.07) | 0.40 |
| People meeting driving standards at month 12, n (%)d | 111 (95.7) [n = 116] | 113 (95.0) [n = 119] | OR: 1.16 (0.30 to 4.45) | 0.83 |
| Percentage point difference: 1.4 (–23.6 to 26.4) | 0.91 | |||
| Patients experiencing side effects from baseline to month 12, n (%)e | 2 (1.5) | 6 (4.6) | OR: 0.32 (0.064 to 1.62) | 0.17 |
| RR: 0.33 (0.068 to 1.61) | 0.17 | |||
| Laser treatments in study eye needed from baseline to month 12, mean (SE)c | 1.92 (0.074) [n = 127] | 1.64 (0.074) [n = 129] | 0.28 (0.070 to 0.49) | 0.009 |
| Participants receiving additional treatments other than laser in study eye (at least one anti-VEGF treatment or steroids, i.e. rescue treatments) from baseline to month 12, n (%)e | 13 (9.8) | 17 (12.9) | OR: 0.69 (0.32 to 1.51) | 0.36 |
| RR: 0.75 (0.38 to 1.46) | 0.39 | |||
| Percentage point difference: –6.0 (–14.5 to 2.5) | 0.17 | |||
| Patients with at least one steroid injection in study eye (as additional treatment) from baseline to month 12, n (%)e | 0 (0.0) | 0 (0.0) | ||
| Patients with at least one steroid injection in study eye (as additional treatment) from baseline to month 24, n (%)e | 0 (0.0) | 1 (0.8) | ||
| Steroid injections in study eye (as additional treatment) from baseline to month 12, n (%)f | 0 (0.0) | 0 (0.0) | ||
| Steroid injections in study eye (as additional treatment) from baseline to month 24, n (%)f | 0 (0.0) | 1 (0.8) | ||
| Patients receiving at least one anti-VEGF treatment (as additional treatment) from baseline to month 12, n (%)e | 13 (9.8) | 17 (12.9) | OR: 0.69 (0.32 to 1.51) | 0.36 |
| RR: 0.75 (0.38 to 1.46) | 0.39 | |||
| Percentage point difference: –6.0 (–14.5 to 2.5) | 0.17 | |||
| Patients receiving at least one anti-VEGF treatment (as additional treatment) from baseline to month 24, n (%)e | 24 (18.1) [n = 133] | 28 (21.2) [n = 132] | OR: 0.78 (0.42 to 1.45) | 0.44 |
| Percentage point difference: –2.8 (–13.1 to 7.5) | 0.59 | |||
| Anti-VEGF treatments (as additional treatment) from baseline to month 12, mean (SE)f | 0.26 (0.10) [n = 133] | 0.46 (0.10) [n = 132] | –0.20 (–0.48 to 0.086) | 0.17 |
| Anti-VEGF treatments (as additional treatment) from baseline to month 24, mean (SE)f | 0.80 (0.23) [n = 133] | 1.30 (0.23) [n = 132] | –0.50 (–1.14 to 0.14) | 0.13 |
| Anti-VEGF treatments (as additional treatment) from baseline to month 12, n (%)fg | ||||
| 1–2 | 7 (53.9) [n = 13] | 6 (35.3) [n = 17] | ||
| 3–4 | 3 (23.1) [n = 13] | 7 (41.2) [n = 17] | ||
| 5–10 | 3 (23.1) [n = 13] | 4 (23.5) [n = 17] | ||
| > 10 | 0 (0.0) [n = 13] | 0 (0.0) [n = 17] | ||
| Anti-VEGF treatments (as additional treatment) from baseline to month 24, n (%)fg | ||||
| 1–2 | 4 (16.7) [n = 24] | 7 (25.0) [n = 28] | ||
| 3–4 | 10 (41.7) [n = 24] | 7 (25.0) [n = 28] | ||
| 5–10 | 10 (41.7) [n = 24] | 9 (32.1) [n = 28] | ||
| > 10 | 0 (0.0) [n = 24] | 5 (17.9) [n = 28] | ||
| Participants satisfying rescue criteria at least once in study eye from baseline to month 12, n (%)d | 32 (24.1) [n = 133] | 32 (24.2) [n = 132] | OR: 0.97 (0.55 to 1.71) | 0.91 |
| RR: 0.95 (0.62 to 1.45 | 0.81 | |||
| Percentage point difference: 0.02 (–11.7 to 11.7) | 1.00 | |||
| Participants satisfying rescue criteria at least once in study eye from baseline to month 24, n (%)d | 44 (33.1) [n = 133] | 41 (31.1) [n = 132] | OR: 1.08 (0.64 to 1.81) | 0.78 |
| RR: 1.01 (0.71 to 1.44) | 0.94 | |||
| Percentage point difference: 2.48 (–11.0 to 16.0) | 0.72 | |||
| Participants experiencing a loss of 10 or more ETDRS letters from baseline to month 24, n (%)d | 17 (14.7) [n = 116] | 8 (7.0) [n = 115] | OR: 2.28 (0.93 to 5.57) | 0.071 |
| RR: 2.03 (0.91 to 4.51) | 0.083 | |||
| VisQol dimension | Time point | SML group (N = 133) | SL group (N = 132) | Between-group difference (95% CI) | p-value | ||
|---|---|---|---|---|---|---|---|
| n | Unadjusted mean (SD) | n | Unadjusted mean (SD) | ||||
| Injure (likely to injure self) | Baseline | 130 | 0.953 (0.140) | 116 | 0.967 (0.093) | –0.023 (–0.007 to 0.053) | 0.141 |
| 12 months | 130 | 0.975 (0.082) | 109 | 0.950 (0.177) | –0.018 (–0.05 to 0.014) | 0.272 | |
| 24 months | 113 | 0.985 (0.047) | 112 | 0.955 (0.159) | 0.004 (–0.029 to 0.037) | 0.804 | |
| Cope (coping with life demands) | Baseline | 130 | 0.942 (0.109) | 116 | 0.956 (0.119) | 0.001 (–0.025 to 0.028) | 0.915 |
| 12 months | 130 | 0.943 (0.105) | 109 | 0.957 (0.121) | –0.007 (–0.036 to 0.021) | 0.614 | |
| 24 months | 112 | 0.963 (0.099) | 111 | 0.956 (0.159) | 0 (–0.030 to 0.028) | 0.963 | |
| Friendships (ability to have friendships) | Baseline | 130 | 0.915 (0.147) | 116 | 0.940 (0.143) | 0.003 (–0.031 to 0.037) | 0.879 |
| 12 months | 130 | 0.918 (0.141) | 109 | 0.944 (0.143) | –0.008 (–0.045 to 0.028) | 0.650 | |
| 24 months | 130 | 0.918 (0.127) | 111 | 0.939 (0.136) | –0.005 (–0.042 to 0.032) | 0.805 | |
| Assistance (organising assistance) | Baseline | 131 | 0.970 (0.093) | 116 | 0.983 (0.080) | –0.001 (–0.023 to 0.02) | 0.904 |
| 12 months | 129 | 0.969 (0.117) | 110 | 0.979 (0.086) | –0.007 (–0.030 to 0.015) | 0.528 | |
| 24 months | 112 | 0.991 (0.043) | 113 | 0.977 (0.138) | –0.002 (–0.0248 to 0.021) | 0.880 | |
| Roles (difficult to fulfil roles) | Baseline | 131 | 0.927 (0.181) | 116 | 0.961 (0.117) | 0.025 (–0.007 to 0.057) | 0.119 |
| 12 months | 129 | 0.953 (0.121) | 110 | 0.966 (0.083) | –0.005 (–0.039 to 0.029) | 0.769 | |
| 24 months | 113 | 0.966 (0.113) | 113 | 0.953 (0.138) | –0.012 (–0.047 to 0.022) | 0.478 | |
| Confidence (confidence to join activities) | Baseline | 131 | 0.936 (0.158) | 116 | 0.970 (0.075) | 0.015 (–0.012 to 0.041) | 0.269 |
| 12 months | 129 | 0.950 (0.122) | 110 | 0.963 (0.076) | –0.005 (–0.033 to 0.023) | 0.710 | |
| 24 months | 113 | 0.975 (0.046) | 112 | 0.954 (0.121) | –0.009 (–0.037 to 0.020) | 0.546 | |
| Time point | Variable | Observations, n | Mean (SD) | Minimum | Maximum |
|---|---|---|---|---|---|
| SML group | |||||
| Baseline | NEI-VFQ-25 composite score | 131 | 86.38 (13.83) | 30.88 | 100 |
| General health | 131 | 46.95 (25.39) | 0.00 | 100 | |
| General vision | 130 | 72.15 (13.75) | 40.00 | 100 | |
| Ocular pain | 131 | 85.02 (20.65) | 0.00 | 100 | |
| Near activities | 130 | 80.00 (19.84) | 8.33 | 100 | |
| Distance activities | 130 | 87.82 (16.09) | 16.67 | 100 | |
| Vision social function | 130 | 94.62 (13.56) | 37.50 | 100 | |
| Vision mental health | 131 | 82.16 (19.95) | 18.75 | 100 | |
| Vision role difficulties | 130 | 83.85 (23.11) | 0.00 | 100 | |
| Vision dependency | 131 | 92.68 (19.28) | 8.33 | 100 | |
| Driving | 92 | 92.84 (12.08) | 33.33 | 100 | |
| Colour vision | 129 | 96.71 (11.85) | 50.00 | 100 | |
| Peripheral vision | 130 | 88.85 (18.95) | 25.00 | 100 | |
| 12 months | NEI-VFQ-25 composite score | 113 | 89.61 (9.99) | 45.92 | 100 |
| General health | 113 | 50.00 (23.62) | 0.00 | 100 | |
| General vision | 112 | 74.11 (13.05) | 40.00 | 100 | |
| Ocular pain | 113 | 89.82 (17.40) | 12.50 | 100 | |
| Near activities | 113 | 83.67 (19.30) | 8.33 | 100 | |
| Distance activities | 112 | 90.29 (15.06) | 33.33 | 100 | |
| Vision social function | 113 | 96.68 (8.35) | 50.00 | 100 | |
| Vision mental health | 113 | 86.06 (15.38) | 18.75 | 100 | |
| Vision role difficulties | 112 | 86.27 (20.95) | 0.00 | 100 | |
| Vision dependency | 113 | 96.31 (12.35) | 25.00 | 100 | |
| Driving | 77 | 94.53 (9.57) | 50.00 | 100 | |
| Colour vision | 113 | 98.45 (8.37) | 25.00 | 100 | |
| Peripheral vision | 112 | 93.08 (14.32) | 50.00 | 100 | |
| 24 months | NEI-VFQ-25 composite score | 114 | 87.19 (14.08) | 22.65 | 100 |
| General health | 114 | 52.85 (29.33) | 0.00 | 100 | |
| General vision | 114 | 72.63 (15.57) | 20.00 | 100 | |
| Ocular pain | 114 | 88.38 (16.61) | 25.00 | 100 | |
| Near activities | 114 | 81.18 (21.55) | 0.00 | 100 | |
| Distance activities | 114 | 87.35 (17.57) | 12.50 | 100 | |
| Vision social function | 113 | 95.24 (12.87) | 25.00 | 100 | |
| Vision mental health | 113 | 83.13 (20.22) | 0.00 | 100 | |
| Vision role difficulties | 113 | 85.07 (24.31) | 0.00 | 100 | |
| Vision dependency | 111 | 93.09 (19.72) | 0.00 | 100 | |
| Driving | 81 | 91.82 (13.57) | 16.67 | 100 | |
| Colour vision | 110 | 97.73 (9.90) | 50.00 | 100 | |
| Peripheral vision | 113 | 88.50 (20.60) | 25.00 | 100 | |
| SL group | |||||
| Baseline | NEI-VFQ-25 composite score | 130 | 87.00 (12.73) | 44.63 | 100 |
| General health | 130 | 51.92 (25.22) | 0.00 | 100 | |
| General vision | 130 | 72.92 (15.52) | 40.00 | 100 | |
| Ocular pain | 130 | 85.38 (17.82) | 25.00 | 100 | |
| Near activities | 130 | 80.16 (19.33) | 25.00 | 100 | |
| Distance activities | 130 | 89.01 (14.17) | 41.67 | 100 | |
| Vision social function | 130 | 93.94 (13.68) | 25.00 | 100 | |
| Vision mental health | 130 | 80.87 (20.62) | 6.25 | 100 | |
| Vision role difficulties | 129 | 82.75 (26.48) | 0.00 | 100 | |
| Vision dependency | 130 | 93.72 (14.57) | 33.33 | 100 | |
| Driving | 91 | 95.05 (9.38) | 50.00 | 100 | |
| Colour vision | 130 | 96.35 (11.69) | 25.00 | 100 | |
| Peripheral vision | 130 | 91.54 (16.63) | 25.00 | 100 | |
| 12 months | NEI-VFQ-25 composite score | 117 | 88.47 (13.78) | 29.21 | 100 |
| General health | 116 | 49.78 (25.00) | 0.00 | 100 | |
| General vision | 116 | 75.17 (14.59) | 40.00 | 100 | |
| Ocular pain | 117 | 88.25 (15.25) | 37.50 | 100 | |
| Near activities | 117 | 83.62 (19.29) | 8.33 | 100 | |
| Distance activities | 117 | 88.89 (17.02) | 25.00 | 100 | |
| Vision social function | 116 | 94.29 (14.22) | 25.00 | 100 | |
| Vision mental health | 117 | 86.38 (18.99) | 6.25 | 100 | |
| Vision role difficulties | 117 | 85.26 (24.27) | 0.00 | 100 | |
| Vision dependency | 116 | 92.74 (19.37) | 0.00 | 100 | |
| Driving | 81 | 93.26 (14.65) | 33.33 | 100 | |
| Colour vision | 115 | 98.48 (6.86) | 50.00 | 100 | |
| Peripheral vision | 115 | 91.96 (16.07) | 25.00 | 100 | |
| 24 months | NEI-VFQ-25 composite score | 115 | 88.80 (13.78) | 29.08 | 100 |
| General health | 115 | 51.96 (24.59) | 0.00 | 100 | |
| General vision | 114 | 74.39 (14.94) | 20.00 | 100 | |
| Ocular pain | 115 | 89.35 (15.55) | 37.50 | 100 | |
| Near activities | 115 | 82.79 (20.03) | 16.67 | 100 | |
| Distance activities | 115 | 89.53 (17.41) | 16.67 | 100 | |
| Vision social function | 115 | 94.57 (13.05) | 12.50 | 100 | |
| Vision mental health | 115 | 85.43 (21.26) | 0.00 | 100 | |
| Vision role difficulties | 114 | 86.73 (21.76) | 0.00 | 100 | |
| Vision dependency | 114 | 94.01 (19.03) | 0.00 | 100 | |
| Driving | 78 | 96.42 (7.35) | 58.33 | 100 | |
| Colour vision | 115 | 96.52 (11.89) | 25.00 | 100 | |
| Peripheral vision | 115 | 91.96 (16.74) | 25.00 | 100 | |
| Laser retreatments since trial entry, n | SML (N = 133), n (%) | SL (N = 132), n (%) | Total (N = 265), n (%) |
|---|---|---|---|
| 0 | 53 (39.8) | 66 (50.0) | 119 (44.9) |
| 1 | 37 (27.8) | 37 (28.0) | 74 (27.9) |
| 2 | 17 (12.8) | 17 (12.9) | 34 (12.8) |
| 3 | 9 (6.8) | 4 (3.0) | 13 (4.9) |
| 4 | 4 (3.0) | 6 (4.5) | 10 (3.8) |
| 5 | 9 (6.8) | 2 (1.5) | 11 (4.2) |
| 6 | 4 (3.0) | 0 (0.0) | 4 (1.5) |
| Outcome | SML group (N = 116), n (%) | SL group (N = 115), n (%) | Difference (95% CI) | p-value |
|---|---|---|---|---|
| Participants experiencing a loss of ≥ 5 ETDRS letters from baseline to month 24a | 40 (34.5) | 25 (21.7) | OR 1.86 (1.03 to 3.38) | 0.041 |
| RR 1.53 (1.00 to 2.34) | 0.052 | |||
| Participants experiencing a change of ± 5 ETDRS letters from the baseline valuea | 80 (69.0) | 93 (80.9) | OR 0.53 (0.28 to 0.98) | 0.041 |
| RR 0.85 (0.73 to 0.98) | 0.028 | |||
| Percentage point difference –11.0 (–33.7 to 10.9) | 0.32 |
| Outcome | SML group (N = 17), n (%) | SL group (N = 8), n (%) |
|---|---|---|
| Participants satisfying rescue criteria at least once from baseline to month 24 | 10 (58.8) | 6 (75.0) |
| Participants satisfying rescue criteria at least once and receiving rescue treatment from baseline to month 24 | 4 (23.5) | 3 (37.5) |
| Participants satisfying rescue criteria at least once and not receiving rescue treatment from baseline to month 24 | 6 (35.3) | 3 (37.5) |
| Participants who did not satisfy rescue criteria at all from baseline to month 24 | 7 (41.2) | 2 (25.0) |
| Participants who did not satisfy rescue criteria at all but received rescue treatment from baseline to month 24 | 2 (11.8) | 0 (0.0) |
| Outcome | SML group (N = 115) | SL group (N = 115) | Difference (95% CI) | p-value |
|---|---|---|---|---|
| Change in macular volume (mm3) from baseline to month 24, mean (SE)a | –0.23 (0.06) | –0.24 (0.06) | 0.007 (–0.16 to 0.18) | 0.94 |
| Review | Focused questiona | Eligibility criteriab | Searchesc | Dual reviewd | Validitye | Study detailsf | Publication biasg | Heterogeneityh |
|---|---|---|---|---|---|---|---|---|
| Blindbæk et al. 201952 | Yes | No | No | No | No | Partial | No | N/A |
| Chen et al. 201616 | Yes | Yes | Yes | Partial | No | Yes | No | Yes |
| Jorge et al. 201819 | Yes | Yes | Yes | Yes | Yes | Yes | N/A | N/A |
| Qiao et al. 201617 | Yes | Yes | Yes | Yes | Yes | Partial | Yes | Yes |
| Scholz et al. 201751 | Yes | No | Yes | No | Yes | Yes | No | No |
| Wu et al. 201818 | Yes | Yes | Yes | Yes | Yes | Partial | No | Yes |
Appendix 2 Additional figures
FIGURE 9.
Boxplot showing the distribution in time taken to complete the first laser procedure.
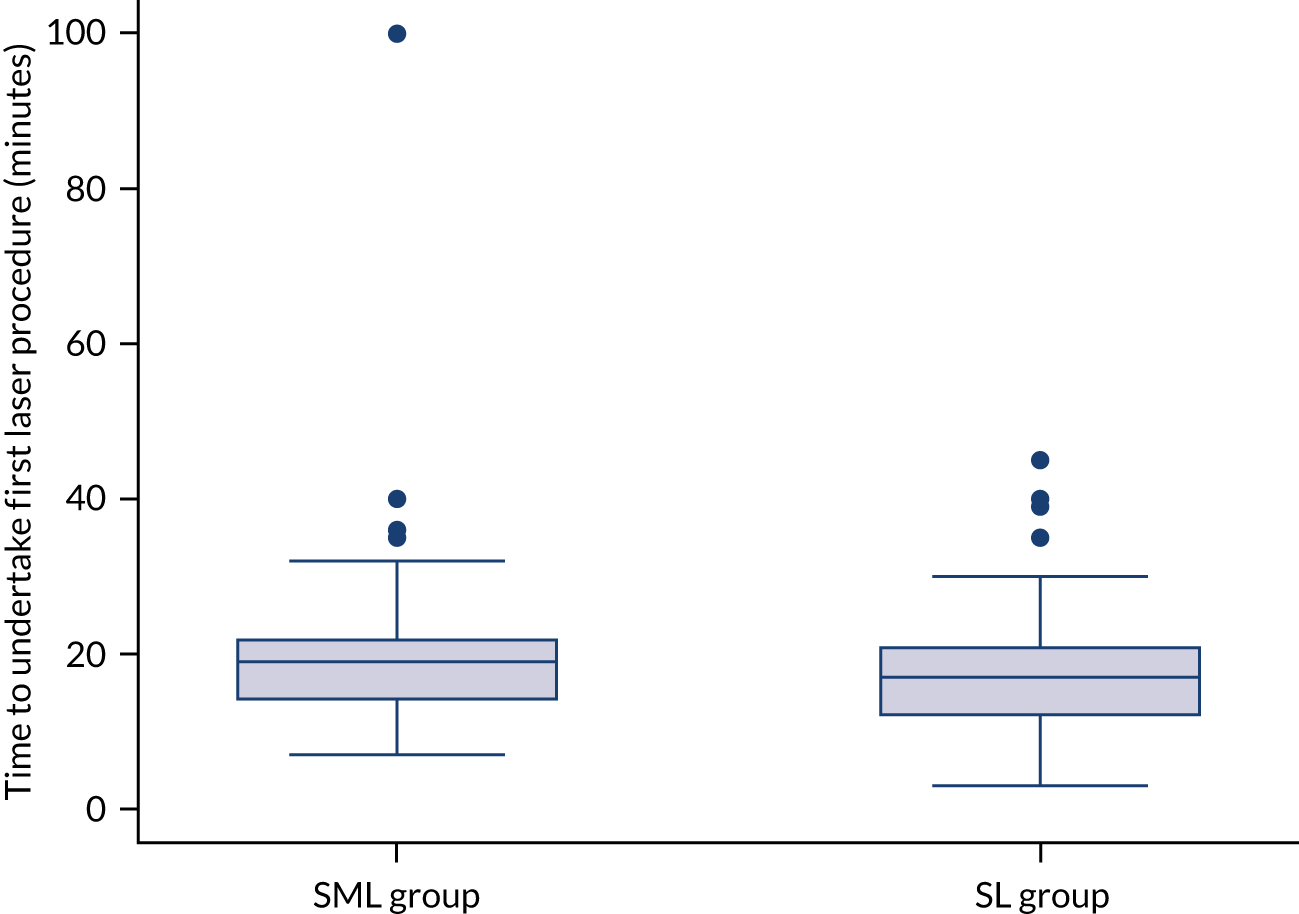
List of abbreviations
- AE
- adverse event
- ANCOVA
- analysis of covariance
- anti-VEGF
- anti-vascular endothelial growth factor
- AR
- adverse reaction
- AUC
- area under the curve
- BCVA
- best-corrected visual acuity
- BHSCT
- Belfast Health and Social Care Trust
- BMI
- body mass index
- CARF
- Central Angiographic Resource Facility
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- CRT
- central retinal subfield thickness
- CSMO
- clinically significant diabetic macular oedema
- DIAMONDS
- DIAbetic Macular Oedema aNd Diode Subthreshold micropulse laser
- DMEC
- Data Monitoring and Ethics Committee
- DMO
- diabetic macular oedema
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- EQ-5D VAS
- EuroQol-5 Dimensions visual analogue scale
- ETDRS
- Early Treatment Diabetic Retinopathy Study
- FFA
- fundus fluorescein angiography
- HbA1c
- glycated haemoglobin type A1c
- HES
- hospital eye services
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- ITT
- intention to treat
- MAR
- missing at random
- MD
- mean deviation
- NEI-VFQ-25
- National Eye Institute Visual Functioning Questionnaire – 25
- NICE
- National Institute for Health and Care Excellence
- NICTU
- Northern Ireland Clinical Trials Unit
- NIHR
- National Institute for Health and Care Research
- NMB
- net monetary benefit
- OCT
- optical coherence tomography
- PDR
- proliferative diabetic retinopathy
- PP
- per protocol
- PPI
- patient and public involvement
- PROM
- Patient-reported outcome measure
- PRP
- panretinal photocoagulation
- PSS
- Personal Social Services
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RPE
- retinal pigment epithelium
- RR
- risk ratio
- SAE
- serious adverse event
- SAR
- serious adverse reaction
- SD
- standard deviation
- SD-OCT
- spectral domain optical coherence tomography
- SE
- standard error
- SL
- standard threshold macular laser
- SML
- subthreshold micropulse laser
- SOP
- standard operational procedure
- SUSAR
- suspected unexpected serious adverse reaction
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- VAS
- visual analogue scale
- VisQoL
- Vision and Quality of Life Index
Notes
-
Supplementary Figure 1: Standard Operating Procedure (SOP) (treatment guideline) for subthreshold micropulse laser (SML)
-
Supplementary Figure 2: Standard Operating Procedure (SOP) (treatment guideline) for standard threshold laser
-
Supplementary Table 1: VisQoL dimension values ‘disvalues’, by treatment group per follow-up time period
-
Supplementary Table 2: VisQoL dimension utility scores by treatment group per follow-up time period
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/SZKI2484).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
