Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0108-10037. The contractual start date was in December 2009. The final report began editorial review in October 2019 and was accepted for publication in October 2020. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2021. This work was produced by Young et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2021 Queen’s Printer and Controller of HMSO
SYNOPSIS
Context and rationale for programme
Large parts of this section have been reproduced from Godfrey et al. 1 © 2013 Godfrey et al. ; licensee BioMed Central Ltd. This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Delirium
Delirium is a common and serious condition among older people, and is associated with distress for individuals, families and health-care staff;2 increased mortality; protracted lengths of hospital stay; lasting functional and cognitive decline; and increased requirement for long-term care placement. 3
Prevention of delirium
Prevention of delirium is, therefore, highly desirable; multicomponent prevention interventions that aim to attenuate modifiable delirium risk factors have consistently been shown to reduce incident delirium in hospitalised patients by about one-third in various inpatient specialties. 4–7 As a consequence of this evidence base, several national guidance documents have recommended that multicomponent delirium prevention interventions should be incorporated into routine care. 8–10
Modification of delirium risk factors typically requires a complex multicomponent system of care, comprising education and targeted interventions, directed at optimising hydration and nutrition, reducing environmental threats, increasing orientation to time and place, improving communicative practices, supporting/encouraging mobility, and improving pain and infection management. These interventions have been tested in different health systems, in diverse settings (medical, surgical, intensive care units and care homes), employing varied modes of delivery, including the Hospital Elder Life Program (HELP). 6,11–14
The Hospital Elder Life Program
The HELP is an existing, successful, standardised and manualised North American multicomponent delirium prevention system of care, which uses a skilled interdisciplinary team assisted by trained volunteers and multicomponent targeted intervention protocols. 11,12,15 The HELP was initially developed and evaluated in the USA > 15 years ago as a novel system of care to prevent delirium among medical patients admitted to hospital for unscheduled care. Effectiveness was demonstrated in a well-conducted, non-randomised, proof-of-concept, explanatory trial involving > 850 patients. 12 The effect size for delirium prevention was estimated as 40% reduction (number needed to treat = 20). Although there have been single-site randomised controlled trials and pragmatic trials of the HELP,13,16 there have not been any multisite randomised trials. Qualitative and observational studies in subsequent dissemination sites have reported factors critical for successful implementation:15,17
-
effective clinical leadership
-
ability and willingness to adapt the original HELP protocols to local hospital circumstances and constraints
-
ability to obtain longer-term resources and funding
-
senior management support.
Approximately half of the sites that express interest in the HELP do not go on to implement the programme. 17 The two most common reasons for non-adoption are lack of senior management support (53%) and perception of high start-up costs (41%). 17 Recruitment of volunteers to the programme has not emerged as a critical limiting factor, suggesting a willingness of volunteers to engage with the programme. 18
The original version of the HELP that was evaluated is referred to in the literature as the Elder Life Program and specifically focused on delirium prevention. Subsequently, the programme was widened and the scope of the intervention extended to encompass areas of good practice including protocols for discharge planning, dementia care and optimising hospital length of stay. The resulting delirium prevention system of care and additional good practice protocols became the HELP system of care. The HELP has been widely disseminated to > 200 hospitals in the USA, Canada and internationally. The HELP provides a skilled interdisciplinary team assisted by trained volunteers to implement standardised protocols targeted at six delirium risk factors: orientation, therapeutic activities, mobilisation, optimising vision and hearing, hydration, and sleep enhancement. The core interdisciplinary team facilitates system change and programme implementation, including daily support to volunteers (Table 1).
| Inclusion criteria for the HELP | |
|---|---|
|
|
| Interventions to prevent delirium | |
| Risk factor | Preventative interventiona |
| Cognitive impairment |
|
| Sleep deprivation |
|
| Immobility |
|
| Vision impairment |
|
| Hearing impairment |
|
| Dehydration |
|
| The ‘core’ interventions to prevent delirium are supplemented by a number of clinical and educational ‘program interventions’ (e.g. staff training, nurse intervention protocols, and HELP interdisciplinary rounds) | |
Although the HELP has been consistently effective for delirium prevention, not all prevention programmes have reported a reduction in delirium incidence. 19 Although some intervention components appear more significant than others, a high degree of protocol adherence facilitates success. 20
Dissemination and embedding the programme in routine care have involved local adaptation in team composition, processes of care, procedures for patient enrolment, intervention protocols and outcome tracking. 15,17,21 Although the programme has proven cost-effective for both hospitals and nursing homes in US studies,22–26 the initial start-up costs of dedicated staff time17 may hinder adoption and sustainability in some settings. 21
Delirium prevention in the NHS
A major issue faced by the NHS in England, and acknowledged by the National Institute for Health and Care Excellence (NICE),8 is the lack of a delirium prevention system of care suitable for widespread national implementation.
Fundamental to our programme of work was the modification and subsequent feasibility evaluation of the HELP, the established and successful North American multicomponent delirium prevention system of care. However, the non-critical transposing of a US health system care model to NHS hospitals, which have a different organisation of care/case mix and funding, is unlikely to be successful. A thorough review and appropriate modification of the HELP should be an initial step. At the outset of the research programme, we envisaged a new, UK-specific version (i.e. HELP-UK) suitable for general use in the NHS.
Successful implementation of a multicomponent intervention is challenging: individual change is mediated not only by the availability of evidence-based guidance but also by characteristics of the intervention and the interplay of patient, social and organisational/system factors. Our aim was to understand the ‘whole system’ within which HELP-UK would be introduced, thereby enhancing the success of implementation. Our approach drew on aspects of systems theory;27 theory-based implementation;28–30 and relationships between structure, process and outcomes, defining how a service might work in context.
Aims and objectives of the research programme
The aim of the programme was to improve delirium prevention for older people admitted to NHS acute hospitals. We sought to ameliorate the large health and social care impact of delirium among older people by undertaking linked projects to investigate the feasibility, acceptability, and potential clinical effectiveness and cost-effectiveness of a delirium prevention system of care.
The objectives were to:
-
review and adapt the HELP for use in the UK health service
-
identify strategies to support the implementation of the HELP, taking into account the potential barriers to change
-
determine the optimum methods to deliver the HELP in routine care
-
conduct a feasibility study to –
-
assess the implementation and acceptability of the adapted HELP to patients and their relatives, clinicians, support staff and volunteers
-
refine the content and delivery of the intervention
-
determine preliminary estimates of clinical effectiveness and cost-effectiveness
-
gather data to inform recruitment, appropriate outcome measure selection and sample size to design a large-scale trial.
-
The programme comprised three projects:
-
Project 1: review and adapt the HELP for use in the UK and identify candidate implementation and delivery strategies.
-
Project 2: pilot study to test implementation feasibility and acceptability of a delirium prevention intervention in terms of –
-
take-up of the intervention protocols
-
impact of the intervention on staff workload
-
impact of the intervention on patient satisfaction with care
-
acceptability to patients, carers, staff and volunteers.
-
-
Project 3: preliminary testing of the Prevention of Delirium (POD) programme system of care.
Project 1 output informed the design of the intervention, which was then tested in the preliminary pilot study (project 2). The findings of the pilot study further informed the conduct of the feasibility study (project 3) (Figure 1).
FIGURE 1.
Overview of the programme. WS, workstream.
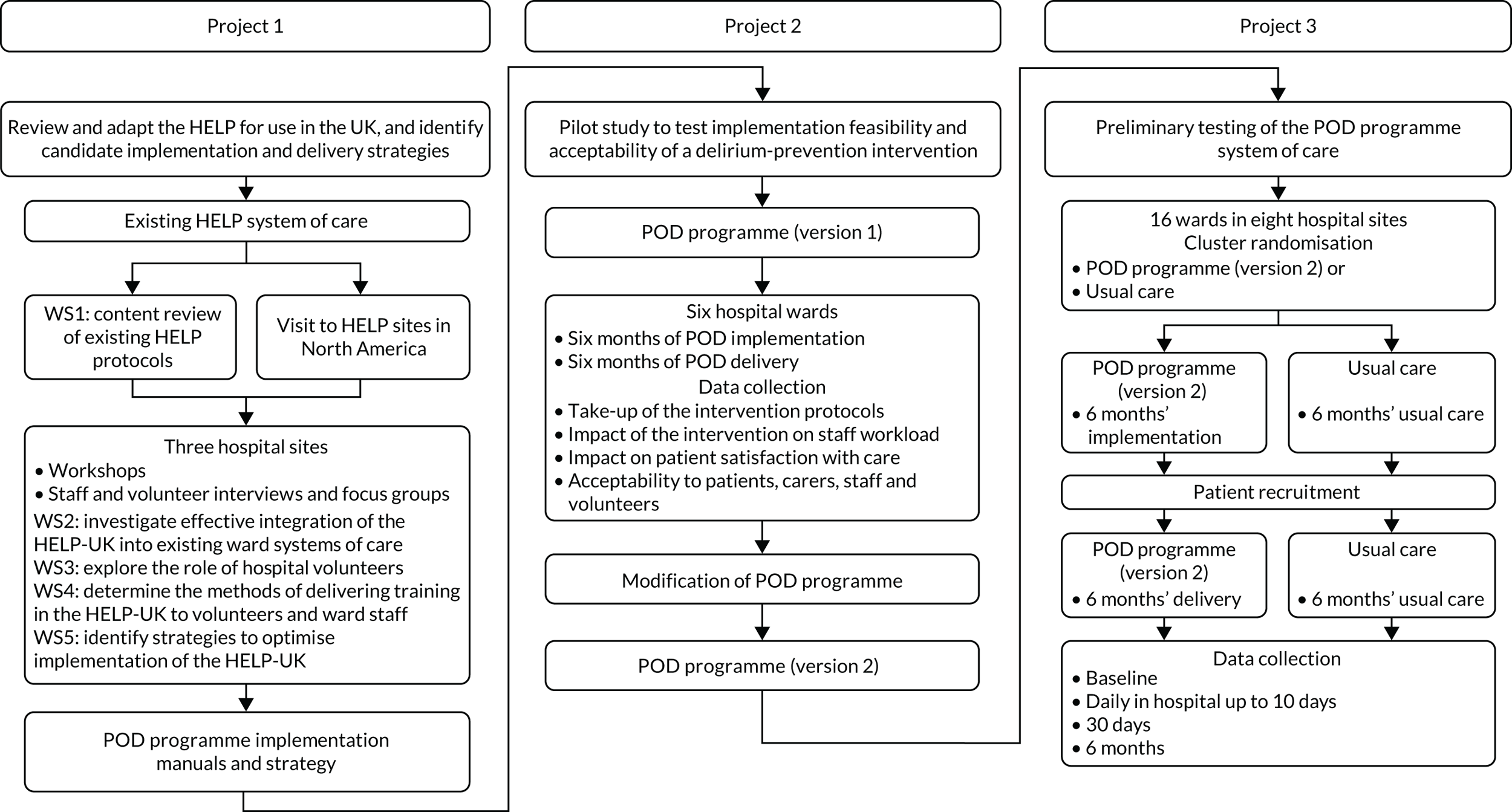
An embedded economic study assessed overall cost-effectiveness from the perspective of health and social care providers. The results of the health economic study are presented in Project 3: a multicentre, pragmatic, cluster randomised controlled feasibility study of the Prevention of Delirium programme system of care.
Project 1: review and adapt the Hospital Elder Life Program for use in the UK, and identify candidate implementation and delivery strategies
Large parts of this section have been reproduced from Godfrey et al. 1 © 2013 Godfrey et al. ; licensee BioMed Central Ltd. This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The objectives of project 1 were to:
-
review and adapt the HELP for use in the UK (HELP-UK)
-
identify strategies to support the implementation of the HELP
-
determine the optimum methods to deliver the HELP in routine care.
Project 1 comprised five workstreams (WSs):
-
WS1: content review of the existing HELP protocols.
-
WS2: investigate effective integration of the HELP-UK into existing ward systems of care.
-
WS3: explore the role of hospital volunteers.
-
WS4: determine the methods of delivering training in the HELP-UK to volunteers and ward staff.
-
WS5: identify strategies to optimise implementation of the HELP-UK.
Workstream 1 was conducted first; then the remaining four WSs were conducted concurrently.
Methods
We planned to establish HELP-UK ‘development teams’ linked to acute hospital elderly care or orthopaedic wards. HELP-UK implementation was likely to vary depending on the clinical environment in which it was introduced. By including surgical and elderly care settings, we hoped to gain insights into a range of issues related to content and implementation, reflecting these different environments. See Report Supplementary Material 1 for the research protocol.
Site recruitment and sampling
We recruited three hospital sites in the north of England to participate in WSs 2–5 (Table 2). Purposive selection included the availability of volunteers to test out the potential for them to contribute to the delirium prevention programme, as in the HELP model. Although each hospital engaged volunteers, volunteers’ degree of active involvement with patients on the wards and the maturity of the voluntary services organisation varied considerably between sites, reflecting different approaches to how hospitals deployed volunteers, which reflects the ‘real world’.
| Variable | Hospital site | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Organisation | District general hospital | Foundation trust | Foundation trust |
| Number of beds | 480 | 400 | 650 |
| Catchment | Geographically dispersed urban and rural population | Urban, ethnically diverse population | Urban and rural population |
| Catchment population | 200,000 | 350,000 | 300,000 |
| Ward | Elderly care | Elderly care |
|
| Roles of delirium prevention development team members |
|
|
|
Following meetings by the research team with relevant managers and clinical leads in the elderly care or orthopaedic units in each of the sites, agreement to participate was secured. A delirium prevention development team, which included senior and frontline staff from elderly care and other wards with potential interest/roles in the programme, was established in each hospital. Although the focus was primarily on the ward, we were also cognisant of the effect of the wider hospital environment on ward-based delirium prevention (see Table 2). In site 2, for example, participants in the workshops included staff from the medical admissions unit, and therapists in site 1 worked across wards and the medical admissions unit. We also interviewed an accident and emergency (A&E) consultant in site 1.
Workstream 1: content review of the existing Hospital Elder Life Program protocols
For WS1, we undertook a content review of the existing HELP protocols to examine their applicability to the NHS. The research team, including delirium experts (two professors of elderly care medicine, a consultant in elderly care medicine and a consultant psychiatrist, all with a research interest in delirium), reviewed the HELP protocols, implementation process and mode of delivery (manuals, training materials), alongside the then-draft NICE delirium guidelines. 8 We additionally sought the opinion of practitioners with experience in delirium management on the HELP protocols at the European Delirium Association Meeting, held in Leeds, in 2009. We also planned to ask the Cerebral Ageing and Mental Health Special Interest Group of the British Geriatrics Society to provide an external independent review of the proposed clinical protocols before presenting them to members of the delirium prevention development teams in each hospital study site.
Visits to Hospital Elder Life Program sites
Alongside the content review of the HELP protocols, and to examine delivery of the HELP in its real-life context, the research team, as part of the research plan, undertook a visit to HELP sites in the USA and Canada in the spring of 2010. We are grateful to Professor Sharon Inouye for organising this.
The Hospital Elder Life Program materials
We encountered some unforeseen difficulties with the HELP in relation to background intellectual property rights. The HELP materials were released under signed contract agreements; this restricted the extent to which we could share and discuss the detailed content with non-HELP sites. These difficulties did not compromise our programme of work. Positive solutions were arrived at through an iterative process of discussions among the central HELP team, research team, Programme Implementation Team, Programme Management Board, and empirical work and literature review. The resultant delirium prevention model drew on the HELP protocols and principles (with permission), but extended their applicability to an NHS context.
Workstreams 2–5
-
Workstream 2: investigate effective integration of the HELP-UK into existing ward systems of care.
-
Workstream 3: explore the role of hospital volunteers.
-
Workstream 4: determine the methods of delivering training in the HELP-UK to volunteers and ward staff.
-
Workstream 5: identify strategies to optimise implementation of the HELP-UK.
Workstreams 2–5 were addressed concurrently via adoption of a participatory action research approach31 with delirium development teams. Through a sequence of practitioner workshops, interviews and ward observations, we explored models of delirium prevention and delivery. This approach provided the opportunity to examine ward practice relevant to delirium and delirium prevention in the context of current clinical and experiential knowledge, to facilitate mutual learning between relevant stakeholders, and to consider strategies for implementing a delirium system of care in the light of research evidence, current practice, and the professional and organisational factors that shaped it. This iterative, dialogic and reflexive methodology, in turn, informed the conceptual framework that guided data collection and analysis.
Workstreams 2–5: conceptual framework
We employed normalisation process theory (NPT)30,32 as a sensitising lens through which to explore knowledge and ward practices on delirium and delirium prevention. NPT focuses on microsocial processes that affect implementation of a practice (or technique) in an organisation or clinical setting. Normalisation refers to the work of individuals as they engage in activities and by which ‘it becomes routinely embedded in . . . already existing, socially patterned knowledge and practices’. 30 NPT postulates four generative mechanisms that operate individually and collectively to explicate how practices (interventions) are embedded and ‘normalised’ in routine care, namely coherence, cognitive participation, collective action and reflexive monitoring (Table 3).
| Generative mechanism | Explanation |
|---|---|
| Coherence | Individually and collectively: how the work that defines and organises a practice/intervention is understood as meaningful and invested in, in respect of the knowledge, skills, behaviours and actions required to implement it |
| Cognitive participation | How the work is perceived as something worthwhile and appropriate to commit their individual time and effort (signing up) to bring about the intended outcome |
| Collective action | How work practices and the division of labour through which these are carried out are modified or adapted to implement the change/intervention |
| Reflexive monitoring | How participants individually and collectively appraise the intervention and its benefits for participants, in relation to individual and organisational goals |
Whereas NPT has been developed as a tool for examining implementation processes and to enhance understanding of the implementation ‘gap’ between research and practice, we employed it to build a picture of how delirium and delirium prevention were understood as meaningful by acute ward staff, and how the work that staff were routinely engaged in was relevant to prevention. The aim was to facilitate systematic consideration of the barriers to incorporating, and the implementation strategies necessary to incorporate, delirium prevention within existing acute service delivery. Specifically, we were interested in how the work of staff, individually and collectively, was conducted in respect of the tasks that reduced or conversely increased iatrogenic and modifiable risk factors for delirium among those who were most vulnerable. Although the value of NPT is its focus on individual and collective practices in specific settings, we were also interested in examining the wider contextual features of settings that might affect implementation. 33,34 Thus, although new practices are introduced into organisations that vary in their history, culture, learning climate and readiness for change,35,36 organisational policies and practices are located in and are shaped by national, political, economic and health policy contexts that, in combination, will affect implementation processes and outcomes. 34
Workstreams 2–5: data collection
Data collection was undertaken by members of the research team who were not connected with the clinical teams and involved multiple qualitative methods: facilitated workshops with development teams, collection of documents/records, one-to-one interviews and focus groups with multiple stakeholders, and observation of ward practices. Informed consent was obtained from participants.
Workshops
Three workshops with the three development teams, facilitated by the researchers, were conducted, as specified in the original proposal. With the consent of team members, an additional round of workshops was held to review/refine a model of prevention relevant to the NHS. Researchers and participants worked in tandem at the workshops to:
-
explore what shaped staff knowledge (or lack of awareness) of delirium and delirium prevention
-
consider barriers to and opportunities for introducing a ward-based delirium prevention programme
-
consider which risk factors should be targeted
-
assess current practice and what would need to change to implement such a programme.
We used the HELP protocols and NICE guidelines8 to frame discussions and to examine the feasibility of involving volunteers in delirium prevention. The starting points in framing the workshop discussions were, first, the HELP model (see Table 1), specifically around the feasibility of involving volunteers in delirium prevention, and then the NICE guidelines. 8 The NICE guidelines8 provided more up-to-date and UK-specific evidence about the nature and scope of interventions to prevent delirium, albeit with little focus on how to embed practice and organisational change. Each workshop lasted ≈ 2 hours. All were audio-recorded and transcribed. The average attendance (mean) across the four workshops was as follows: site 1, 10.5; site 2, 10.5; and site 3, 7.75 (see Report Supplementary Material 2).
Interviews, ward observations and collection of documents/records
Between workshops, multiple data collection methods [qualitative interviews, ward observation and collection of documents/records (Figure 2)] were employed, using NPT30,32 as a sensitising lens to explore knowledge and ward practices on delirium and delirium prevention. Specific objectives of data collection were to:
-
garner a more detailed and nuanced picture of how delirium and delirium prevention were understood by staff (knowledge of delirium/delirium prevention)
-
explore current ward routines and staff practices pertinent to the assessment of delirium risk and the delivery of a delirium prevention programme (what was the work, how did it get done and by whom)
-
examine the nature of the patient journey from A&E to the receiving ward, and the potential for identifying the risk of delirium at each point in the journey (contextual factors affecting the work)
-
consider the current usage pattern of volunteers on the wards and the opportunities for and barriers to involving them in enhancing routine care relating to delirium prevention tasks (potential for introducing, integrating and routinising new practices).
FIGURE 2.
Qualitative research and development process for WSs 2–5.
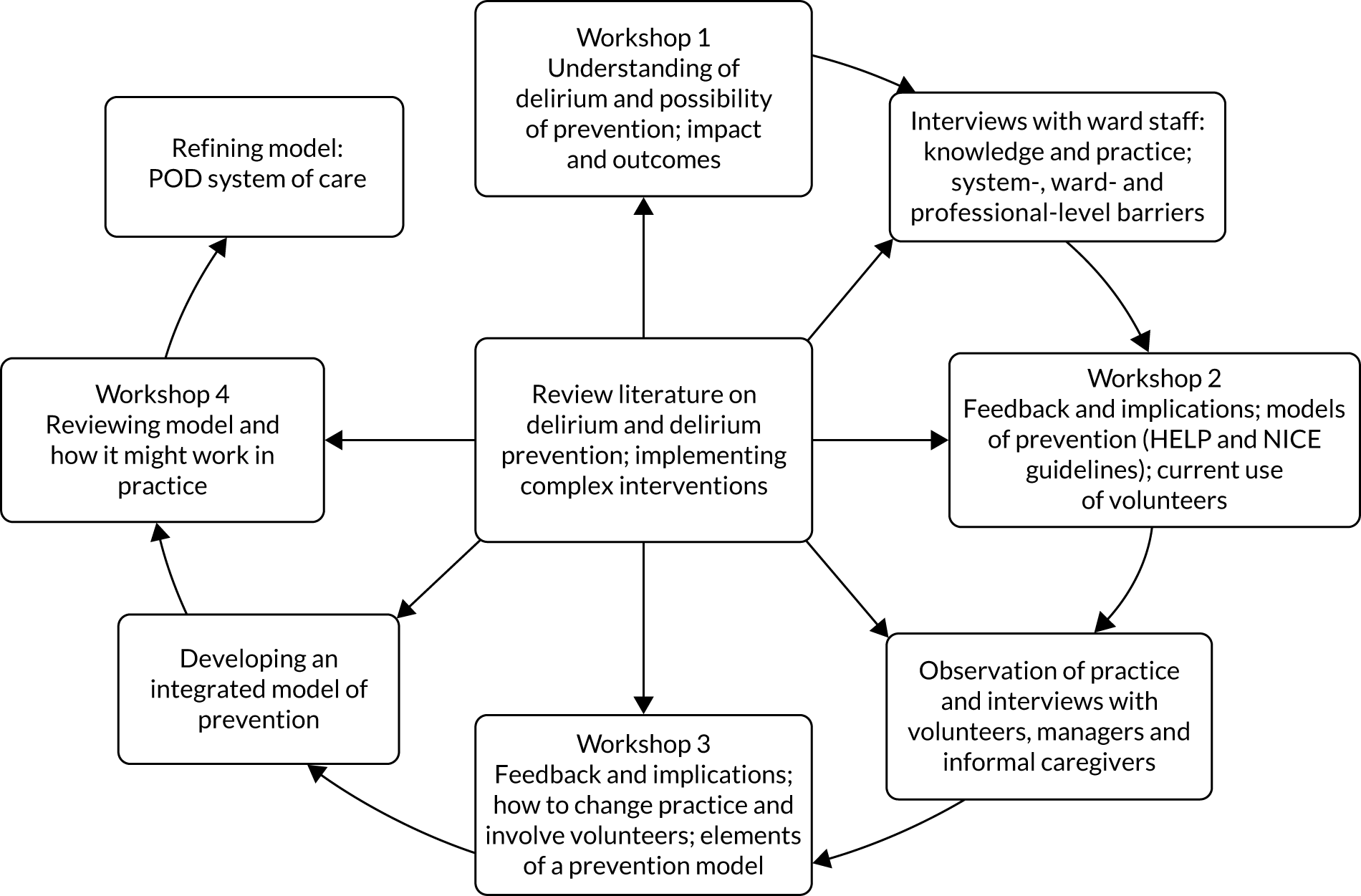
Interviews
Interviews were conducted using topic guides with purposively selected staff and other stakeholders, chosen to obtain a range of views and experience. Twenty-nine interviews (32 individuals) were carried out with clinical staff [doctors, nurses and therapists in participating elderly care and trauma orthopaedic wards, in emergency departments (A&E) and those with a specialist/managerial role in relation to older people with dementia or delirium], voluntary services managers (VSMs) and experienced volunteers whom they identified, and caregivers who had experience of caring for a relative who had developed delirium on participating wards (see Report Supplementary Material 3).
Observations
We undertook ≈ 38 hours of observation of ward practice in the three sites at different times and on different days using ethnographic methods to expand understanding of staff routines relevant to delirium prevention in the real-life, acute ward environment (see Report Supplementary Material 4). This was supplemented by the collection of relevant documents (e.g. assessment forms, care plans, ward protocols, volunteer roles).
Workstreams 2–5: analysis
Workshop proceedings and interviews were audio-recorded, transcribed and anonymised; observational notes were written up in expanded, chronological form, and all data were inputted and stored in NVivo version 9 (QSR International, Warrington, UK). Analysis and data synthesis were ongoing and iterative. Each workshop involved feedback and discussion of emerging findings and implications from the empirical data, which, in turn, generated further data collection and review of evidence on implementation strategies (see Figure 2).
We used grounded theory strategies,37 such as open and focused coding and memos, constant comparison and search for negative cases, to develop categories, their constituent properties and the relationships between them. We compared and contrasted knowledge and practices relating to delirium and delirium prevention within and across wards, the professional and organisational factors that shaped them, and the consequences for service delivery. The findings and analysis led to the development of an integrated delirium prevention programme, iteratively elaborated and refined during delirium prevention development team workshops. The programme embraced intervention protocols, an implementation process and practice tools to enhance integration of delirium prevention into routine clinical practice.
Results
Workstream 1: content review of the existing Hospital Elder Life Program protocols
The content of the HELP intervention was similar to that of the NICE guidelines,8 with the important exception that the latter included additional key risk factors (pain detection and management, infection, hypoxia and nutrition). Practitioners with experience in delirium management at the European Delirium Conference 2009 broadly agreed on the appropriateness of the content of the HELP intervention protocols (see Report Supplementary Material 5). The HELP was designed to be an integrated hospital programme delivered by a skilled interdisciplinary team (geriatricians, elder-life nurse specialists and elder-life specialists), assisted by trained volunteers. An initial review suggested that this organisation and mode of delivery might prove problematic in an NHS context because of the additional staff costs to deliver the programme: 1.25 whole-time equivalent staff for 500 at-risk patients per annum, or approximately two or three new members of staff for an average-size acute hospital trust. (After initial start-up, the additional staff might be reduced to 1.25 whole-time equivalent staff for 1000–1500 at-risk patients per annum.)
We planned to present the clinical protocols to the Cerebral Ageing and Mental Health Special Interest Group of the British Geriatrics Society for an external independent review before presenting them to members of the delirium prevention development teams in each hospital study site during the first round of workshops. However, as previously mentioned, intellectual property copyright issues with the HELP prevented us from sharing the protocols outside the research team and registered sites.
Visits to Hospital Elder Life Program sites in the USA and Canada
Members of the research team visited the active HELP sites in four hospitals in the USA and Canada. We were able to discuss and witness how the HELP system of care was organised and delivered on the ground. Although presented in the literature as a protocolised intervention, it was evident from the visits that, like most complex interventions, the content and style of the HELP varied between sites. It was also apparent that a considerable infrastructure was required to support HELP delivery, for example recruitment and training of volunteers and elder-life specialist nurses. Moreover, we observed that the ward nurses, although appreciative of the HELP system of care, seemed to have little involvement in its delivery. Following the visit, the focus on adaptation of the HELP centred on the following question:
-
Is it possible to provide an effective, integrated model of delirium prevention that minimises the need for additional staffing, but that creates a therapeutic care dynamic between ward staff, volunteers and relatives? That is, does the intervention have to be delivered by an interdisciplinary team assisted by volunteers as an addition to existing ward practice (as in the HELP model), or can it be developed as a system of care that engages staff and volunteers with relatives, as appropriate, to provide a model of enhanced care?
To explore this question, we examined the literature on implementation to identify what successfully contributes to embedding new practices/interventions in routine care and we reframed the work with development teams to consider NICE guidance8 alongside the HELP protocols.
Workstream 2: investigate effective integration of the Hospital Elder Life Program-UK into existing ward systems of care
In situating the task of developing a delirium prevention system of care, we describe how delirium and the work of delirium prevention were currently understood and accomplished by staff. We draw on NPT mechanisms to organise the findings and illustrate interpretive points from our fieldnotes and interview data. We then review the evolving model of delirium prevention developed iteratively through the empirical research and participatory process with development teams. Finally, we present the integrated delirium prevention system of care (POD system of care), including the rationale, or theory of change, underpinning it.
Knowledge and awareness of delirium
Although knowledge of delirium and interest in enhancing practice to prevent it was a key motivating factor for geriatricians’ involvement in the research, awareness of delirium was more variable among other staff. Although junior doctors might be familiar with the term ‘delirium’ and knowledge-based understanding was seen to have improved among registrars specialising in the care of older people, there was less confidence that such knowledge was routinely translated into action to prevent delirium or manage it when it occurred. For nursing and therapy staff, delirium had not featured in their professional training. Among all staff, delirium and delirium prevention were not included as part of mandatory training or in-service education programmes. This was seen to reflect the low salience attached to delirium and delirium prevention in policy and practice, such that, unlike other aspects of acute care delivery such as falls and pressure sores, there were no specific protocols relating to it in any of the sites.
Nursing, therapy and care staff generally did not use the term ‘delirium’; instead, the term ‘confusion’ or ‘acute confusion’ was more typically employed, particularly on elderly care wards:
It’s just that perhaps they don’t recognise it as delirium . . . they don’t put a label on it.
Doctor
Whatever the term used, among these staff, delirium was primarily understood in its manifestation as a problem for ward management and in the disruption it caused for other patients. Thus, awareness (unprompted) was predominantly of hyperactive delirium that resulted in difficulties for staff from problematic behaviour such as aggression, agitation, shouting and wandering. There was acknowledgement that hypoactive delirium, resulting in withdrawn, lethargic behaviour, could easily be overlooked in an acute environment. Indeed, staff awareness and understanding of the experience of delirium from the perspective of patients and caregivers was prompted through presentation of the evidence by the research team, and patients’ and caregivers’ concrete accounts of specific episodes of delirium during the workshops.
How staff perceived the nature, impact and consequences of the ‘problem’ of delirium affected how they sought to manage it. Awareness of ‘acute’ or ‘temporary’ confusion was seen to result in information-seeking from family and friends to determine whether this was of long-standing duration or of recent origin:
They might have been getting worse over a few weeks so, you really need to speak to a carer or relative; quite often we ring home care as well. We ring district nurses: ‘How are they normally? How have they been? Have you noticed any change in their condition over the last few weeks?’ . . . often the consultant . . . if we haven’t done it, will ask for us to get information from home care or whatever.
Senior nurse
Ascertaining that the change was recent and that the behaviour was atypical might precipitate a search for underlying causative factors contributory to the delirium (e.g. sepsis on elderly care wards), so as to identify solutions to address them (e.g. pain relief following surgery on orthopaedic wards). The practice consequences of identifying delirium also highlighted the process whereby delirium affects treatment and extends inpatient stay. Therapists, for example, indicated that mobilisation might need to be delayed to allow patients the chance to recover sufficiently to engage in rehabilitation:
That’s very common [delirium with sepsis], now those patients who are . . . acutely ill and we feel are in that stage, we don’t always try and do anything with them in the early days because we’re aware of the fact that, say they’d come in with a UTI [urinary tract infection] that, sometimes, just having a couple of days to recuperate means that, when we intervene, then they’ll have a much more successful outcome . . . they’re the sort of patients who we might discuss with the nursing staff and they’ll say, ‘leave it today’, you know . . .
Therapist
Some staff used the terms ‘confusion’ and ‘acute confusion’ interchangeably. This imprecision in language use denoted a lack of clarity about the distinction between acute confusion and dementia. The practice consequence was that search for a cause might not be pursued:
I think a lot of the times . . . it’s probably put down more to dementia than it is to delirium . . . when, I guess, so many people who have dementia are . . . more susceptible to having delirium . . . And then [for people with dementia], I think it’s probably more put down to: ‘they’re . . . out of their own environment, they’ve had a traumatic operation, they’re just more confused’, rather than there’s perhaps another underlying issue that’s causing it . . .
Therapist
The conflation of delirium and dementia by staff was a source of heightened anxiety and perplexity among caregivers/relatives, as the suddenness of the change and the strangeness of the behaviour of their relative was not understood by staff. Aggression and/or refusal to participate in treatment could be interpreted as ‘lack of engagement’, resulting in the patient being perceived as unsuitable for rehabilitation or berated by staff for ‘inappropriate’ behaviour. The following is an illustrative example.
Mrs Patterson’s (a pseudonym) brother-in-law was admitted to hospital ‘with a very high temperature and inflammation in his leg. I’m not quite sure what diagnosis was put on it’. He was also disabled following a stroke several years previously:
When he was admitted . . . everything went . . . during the night he just screamed for my sister . . . I went down to the hospital the following morning . . . it was obvious to me something was wrong . . . he was shouting and aggressive . . . and demanding. The nurse said to me when I went on the ward: ‘Oh, I’m glad you’re here, I want to say this in front of you [looking at the brother-in-law] that you are a very difficult man and we don’t like the way you’re speaking to us and if you continue we will refuse to nurse you’ . . . I said to her that he’s not like this usually . . . The doctor later confirmed that he had delirium.
Mrs Patterson
Generally, then, variability in how delirium was understood among different groups of staff and the lack of investment at an organisational level in respect of training and education meant that, in NPT terms, delirium identification had low coherence. Delirium diagnosis was primarily effected through use of observational cues, although how these were interpreted and acted on depended on the expertise of those making the observations. Thus, management practices following on from observations reflected the skills and interests of individual professionals, rather than a collective staff and ward response.
Delirium prevention
Given the low coherence of delirium among staff groups across sites, it is hardly surprising that delirium prevention was not perceived as meaningful. Even when senior staff had initiated action to increase awareness of delirium risk (e.g. posters displaying risk factors), this did not inform assessment and care practices: ‘it’s not in the foreground of people’s minds’ (geriatrician). Interviews and observations indicated that knowledge of delirium among individual staff did not necessarily translate into specific beliefs and behaviours (cognitive participation) and the organisation of work practices geared towards prevention (collective action).
In one elderly care ward, we observed that senior nursing staff employed the term ‘delirium’ in describing specific patients, and demonstrated awareness and knowledge of both hypoactive and hyperactive delirium, as well as sensitivity to the distress caused to patients with it. The consultant geriatrician also had a particular interest in delirium. During observation of a nursing handover meeting on this ward, it was reported that just under one-fifth of current patients were characterised as having delirium. One of the patients with delirium discussed was perceived as needing considerable assistance with eating and drinking; another was referred to as having ‘hypoactive delirium’, ‘really drowsy’, not sufficiently alert to eat and drink, incontinent and ‘on IV [intravenous] fluids’. For the former patient, it was emphasised that all staff should be alerted to ensure support at mealtimes and to encourage drinking and eating. For the latter, it was agreed that she should be moved to a bed that was more visible from the nurses’ station, although this also provoked discussion about the disorientation such a move might cause. At the same meeting, several newly admitted patients were described as having symptoms that, to the observer, might portend risk of developing delirium: an 89-year-old patient who had experienced multiple urinary tract infections and been admitted following a fall, and an 80-year-old patient with a urine infection and pneumonia who had suffered a heart attack and needed oxygen. The symptoms were presented without reference to or discussion about delirium risk or any specific preventative action to be taken. This was recognised as typical practice by the development teams. Thus, even when there was shared understanding of delirium management, this did not facilitate noting and acting on risk factors before delirium had occurred (cognitive participation in NPT).
One exception to this general gap between knowledge and practice in delirium prevention was the development and implementation of a protocol on pain management post surgery for use in hip fracture patients on the trauma orthopaedic ward. This was aimed at delirium prevention. Staff remarked on how the protocol was routinely pursued, with positive outcomes as a consequence, particularly in reducing the severity and duration of delirium episodes. The ward manager attributed success to specific features of the hip fracture patient pathway: this was direct, linear and highly protocolised, with all patients diagnosed with hip fracture fast-tracked from the A&E department to the ward to undergo surgery within 24 hours. Insertion of the protocol into the pathway was viewed as an elaboration of existing practice, rather than as a major shift in how things were done. Practice change was reinforced by the perception among nursing staff that this was a relatively simple intervention with visible positive effect in a short period of time.
By contrast, the patient journey to elderly care wards across the three hospitals was more protracted and diverse. Triage systems and initial investigation in A&E to determine a differential diagnosis and whether or not admission was warranted might be followed by further observations in a short-stay assessment facility for up to 48 hours, which could be further protracted because of a shortage of acute ward beds. The chaotic nature of A&E and short-stay assessment facility environments, compounded by the multiple potential aetiologies of delirium in these settings, was viewed as contributing to delirium risk so that the scope for preventing incident delirium on acute wards could be adversely affected by the length of the patient journey into them. Even so, delirium prevention was considered to be feasible and worthwhile in the acute ward environment, although organisational factors shaping the patient journey through the hospital also needed attention as part of a strategic approach to prevention.
Current ward routines and practices
From interviews with staff and development team discussions, the acute care ward was reported as ‘busy’, often ‘chaotic’ and challenging: a picture reinforced by research observation. Explanatory, contributory factors offered by staff included patient mix and the policy and organisational imperative to achieve rapid patient throughput. Policy and service emphasis on hospital admission of those who required specialist medical and nursing expertise that could not be provided in alternative settings meant that patients were very acutely ill. Similarly, it was expected that patients would move on from acute care once medical and functional needs were met to secure ‘safe’ discharge. Patient moves within wards across all sites were also common, reflecting various organisational contingencies.
The hectic nature of ward life had the consequence that routine practice was described by staff as being primarily directed at responding to what was immediately presented, with priority given to diagnostic, observational and interdisciplinary assessment and care-planning. This picture was reinforced from observation. Thus, particularly for nursing and auxiliary staff, ward life was organised on the basis of a structured rhythm of time-sequenced care (washing, toileting), observations, diagnostic processes and treatment, punctuated by meals and visiting times, a pattern that was prone to disruption as a result of crisis events. Alongside this daily rhythm was the management of patient flow (admissions and discharges) and associated activities (negotiating with bed managers, discharge co-ordinators, social workers, relatives and community agencies), including record-keeping.
The variability and general understanding of delirium prevention among staff meant that it was not a significant driver of ward care practice. However, although delirium preventative interventions relate primarily to features of care quality, it is pertinent to consider how relevant routine care practices were accomplished, including the barriers and contextual factors that affected them.
Nutrition, fluids and sensory aids
Although nutrition and fluid intake were viewed as components of ‘basic’ care to be undertaken by ward staff, they were primarily delivered by health-care assistants (HCAs). In each site, around one-third of patients required some direct help at mealtimes. Others might need encouragement to eat, although this provision depended on staff availability and assumed lower priority. Similarly, tasks of washing and dressing, including ensuring that patients had spectacles, dentures and hearing aids, as appropriate, were mainly undertaken by HCAs. Even so, the importance that senior nursing staff attached to care tasks affected both the value attributed to them by junior nursing staff and the extent to which they pitched in to provide assistance.
Mobilisation
Mobilisation by physiotherapy staff of patients with particular needs was limited: it appeared to occur, at most, once daily, and was intended to be augmented with support and encouragement from ward staff. Patients who merely lacked confidence in getting up and walking on their own were reliant on nurses and HCAs to provide this. Similarly, local policies on prioritising therapy for those with the potential to resume independent living meant that, for example, in one site, patients admitted from nursing homes did not receive therapy. The engagement of nurses and HCAs routinely in mobilisation work in either an enhancing or a supportive role was viewed by staff as essential to sustaining mobility among patients, most of whom were of advanced age, frail and unsteady on their feet. How consistently this was done depended on factors such as the ward physical environment and other pressures.
In one site, the confined and cluttered space of the bays was a constraint on the ability of patients to move safely, and, as the distance between bed and toilet was not more than a few steps, routine mobilisation by nurses and HCAs was limited. Only therapists walked patients for longer distances along the corridor, where the wider space enabled freer and more confident movement. In another site, by contrast, the distance between the bed and toilet was some 10–20 m. Part of the ward routine included nurses and HCAs providing direct assistance to patients and/or keeping an eye out for them as they walked from bed to toilet. It was noted over an observation period how one patient progressed from being assisted with walking to managing independently with a nurse walking behind her, and then to walking on her own. Although here the physical environment was conducive to staff encouraging mobility, this practice was facilitated and reinforced by a care ethos that placed high value on all staff, including nurses, participating in such work. This is exemplified in the following episode observed in this site, but not in others. One of the nurses was with a patient as she encouraged her to stand up from being seated. As the patient made several attempts to propel herself from a sitting to a standing position, the nurse stood by continually encouraging her by showing her how to use her arms to push and move to the edge of the chair, and praising each effort until she stood up.
Orientation and communication
Features of the ward physical environment may act as constraints to ‘good’ care practice, for example inappropriately placed clocks, lack of space or infection control policies precluding personal possessions.
There was variation between individual staff and professional groups within and between sites in the extent to which they conversed with patients in the course of their work. Therapists, for example, typically introduced themselves to patients they were working with, engaging them in general, social and orienting conversation. The pattern was more diverse among nursing and care staff. In one site, there was a buzz of chatter in the bays as nurses and HCAs conversed socially with patients as they went about their daily routines of washing, dressing, medication rounds and mealtimes. The progress of individuals was remarked on and patients were complimented on efforts at walking or dressing. In another site, interaction between staff and patients seemed primarily directed on the task in hand: ‘here are your tablets’, ‘do you have any pain?’.
The hustle and bustle of ward life, particularly from early morning to mid-afternoon as described by staff, was in marked contrast to the silence and inactivity of patients once care needs and clinical observations were completed. We could discern two parallel but distinct ward rhythms: a staff rhythm marked by frenetic movement and continuous noise – buzzers, telephones and the clatter of trolleys – and a patient rhythm distinguished by a paucity of conversation and little movement. Sustained or prolonged engagement of patients by staff was absent in all sites. Development teams remarked that this was neither feasible nor valued in the context of the priority attached to moving patients quickly through the system.
Overall, some practices pertinent to delirium prevention (assistance with meals for those who needed help with eating but not for those who required encouragement) were carried out more or less consistently for some patients across all sites. Other practices (enabling support to encourage and enhance mobility among patients lacking in confidence, and personally meaningful, as opposed to task-based, communication) were accomplished more consistently in some sites than others, depending on local policies and priorities, the physical environment in which care was delivered and the existence of a care ethos that placed high value on social engagement and care. Yet other practices (spending time with patients in one-to-one conversation or engaging patients in cognitively stimulating activities) were not routinely engaged in by staff across sites, which was seen to reflect the current acute care environment. Collective action by ward staff in practices that are preventative of delirium were contingent on local policies and priorities on patient need, staffing levels, division of labour and the care culture operating. In no site were any of these explicitly linked with delirium prevention or engaged in consistently for all patients who might exhibit delirium risk factors.
Workstream 3: exploring the role of volunteers
Discussion within development teams and interviews with VSMs, volunteers and ward staff revealed considerable variability in the size and scope of the volunteering role, supervision arrangements, training and organisation of volunteers. One hospital had had a 400-strong volunteer force since its opening some four decades previously. Here, volunteers were centrally managed under the aegis of a VSM and deputy. The post holder was responsible for recruitment, organising training, deploying volunteers to some 30 different tasks/roles and providing ongoing support to them. In another hospital, by contrast, voluntary provision was fragmented and delivered through different agencies, each focusing on discrete roles and tasks. As a consequence, there was no standardised system for recruiting, inducting, training and supporting volunteers.
Although most volunteers in all sites were primarily engaged in providing practical and orientation assistance to patients and visitors, each site had a small number of volunteers, outside the chaplaincy service, which offered one-to-one befriending with patients on the wards. These volunteers spent time conversing with patients, the purpose being to reduce isolation among patients who had few visitors. They reported variable interest in what they did among ward staff, ranging from positive reinforcement of the value attached to it to indifference and hostility. Generally, staff were seen as so busy that they were unaware of volunteer input. Sustaining volunteer involvement depended on the commitment, tenacity, skills and abilities of individual volunteers and mutual support provided to each other through informal networks. One site had developed a successful programme for trained and supervised volunteers attached to specific wards to provide assistance and encouragement to patients who needed help with meals. A similar scheme at another site had been unsuccessful, which was attributed to a lack of attention as to how to engage ward staff.
Engaging volunteers in delirium prevention tasks offered a potential resource to wards and existing direct work with patients provided the building blocks to develop it. However, the ad hoc nature of the befriending role, as typically understood by staff, and the lack of clear systems for supporting volunteers, including their purposeful integration into the work of patient care, presented obstacles to realising its potential. In NPT terms, given existing models of volunteer/ward staff engagement and practices, mechanisms for creating a common sense of purpose and value attached to the volunteer role and for establishing a division of labour that was appropriate and acceptable to both volunteers and staff were necessary to create the conditions for involving volunteers in delirium prevention.
Workstream 4: determining the methods of delivering training in the Hospital Elder Life Program-UK to volunteers and ward staff, and workstream 5: identifying strategies to optimise implementation of the Hospital Elder Life Program-UK (Prevention of Delirium system of care)
Developing a model of delirium prevention
Within development teams, and through iterative feedback of empirical findings, we pursued in-depth discussion of the content of a multicomponent delirium prevention intervention and implementation process, with particular focus at the outset on the HELP mode of delivery. With regard to the intervention, there was consensus among the development teams that the NICE components and recommendations would constitute the content, as NICE extends the HELP intervention with up-to-date evidence.
One unique aspect of the HELP mode of delivery, as described previously, is the use of trained volunteers in assisting the HELP interdisciplinary team with some of the core interventions. Development teams perceived this feature of delivery as posing major practical and conceptual difficulties, thereby challenging its feasibility in an NHS context. Conceptually, although there was considerable enthusiasm for volunteer involvement, staff considered that ward practice in respect of delirium prevention activities was central to delivering consistent, quality care, such that staff needed to be actively involved in these activities. There was understanding among some senior staff that ward care practices such as nutrition, fluid intake and mobilisation were significant not only in helping to manage delirium, but in having a preventative effect on its development. These practices were also viewed as pertinent to other areas of prevention that have been targeted for action at national and local policy levels to secure care quality improvements, such as falls and pressure sores. Engaging staff in the work of delirium prevention, then, was viewed as enhancing staff awareness of and ascribing legitimacy to work that has a wide-spectrum preventative effect, with the potential to increase patient care quality overall.
Practically, the level of resource required to emulate the HELP was viewed by all of the development teams as unachievable because they believed that substantial additional staffing would be required to deliver the intervention to all patients on a typical elderly care ward. Consequently, in collaboration with the development teams, a model of delirium prevention evolved whereby the core components of prevention were assimilated into the daily routine of staff and volunteers without the need for additional staffing. This combined a practice change in the way staff went about their daily routines in respect of the 10 core components with an enhanced role for volunteers.
However, this presented two major challenges for implementation. First, there was the paucity of knowledge and understanding of delirium prevention, particularly among nursing and care staff, whose routine practices were critical in delivering preventative interventions. Second, the enthusiasm of ward staff for involving volunteers in a more focused and direct role with patients was seen to require considerable change in the way volunteers were currently deployed.
The development of such a model, therefore, required attention to both the processes and strategies for achieving practice change and the systems and mechanisms necessary to recruit, train and support volunteers to provide an enhanced and co-ordinated role in a whole-ward intervention. Such changes, moreover, had to flow directly from a knowledge and awareness of delirium prevention as worth the investment by staff individually and collectively. This represented a shift in direction from refining the HELP for an NHS context to developing a new model of delirium prevention, namely the POD system of care.
The programme we developed (the POD system of care) was the product of the interaction of the development teams’ practice knowledge, current best evidence on delirium prevention,8 and consideration of the findings of our empirical research and recent reviews of implementation theory and research. 29,30,38 The content and implementation process documented in the resultant system of care was then further tested and refined through dialogue with the development teams.
An integrated model of delirium prevention: the Prevention of Delirium system of care
The POD system of care version 1 (PODv1) was a multicomponent intervention and implementation process organised in two manuals (Table 4). The system of care aimed to integrate delirium prevention activities into routine care.
| Section | Contents |
|---|---|
| 1. Introduction | Provides the background to the programme, the theory of change underpinning it, why it is necessary, the intended objectives and the steps that need to be in place to introduce it at ward level |
| 2. Educational materials | Comprises sets of slides, vignettes and case studies to be drawn on to raise awareness of delirium and delirium prevention and to create readiness for the introduction of the programme alongside involvement of ward staff |
| 3. Preparation for change | Sets out a detailed implementation process, mechanisms and activities for planning the work, engaging staff, executing change, and reflecting and evaluating progress and outcomes preparatory to delivery |
| 4. Implementation manual | Designed to record in detail, after completion of section 3, how each of the interventions will be implemented in routine care on the ward. This is a bespoke document, with systems and division of labour adapted to local contexts, albeit addressing common functions |
| 5. Involving volunteers | Specifies the detailed work involved in engaging volunteers alongside ward staff in implementing the integrated delirium prevention programme, one set of tasks that constitute part of section 3. It is aimed at guiding the POD action group through those issues relating to volunteers that require discussion and decisions, for example providing examples of volunteer role descriptions |
| 6. Audit and model tools | Provides a range of tools that may be helpful to draw on in implementing and reviewing the outcomes of practice change |
The Prevention of Delirium system of care interventions
The POD system of care interventions comprise actions encapsulated in protocols centring on 10 targeted clinical risk factors associated with the development of delirium among vulnerable patients. 8 The risk factors were organised hierarchically into three distinct delirium prevention ‘bundles’, according to a number of factors, including the level of expertise needed for their implementation; the bundles also provide a framework for ward staff to identify what should be done and by whom, taking into account local policies and practices:
-
Actions that might typically be carried out as part of existing medical/nursing roles (assessing and managing pain, medication management, hypoxia and infection management).
-
Actions that, depending on the level of patient need, might require skilled therapy/nursing and care input, at one end of the continuum, to, at the other end, assistance provided by volunteers with appropriate competencies (e.g. mobilisation, mealtime assistance).
-
Actions that offered scope for volunteers to enhance care practices while stimulating practice change towards providing holistic care to patients (engaging in social and stimulating activities for which volunteers can offer a unique contribution).
Prevention of Delirium system of care implementation process
The POD system of care implementation process incorporates systems and mechanisms aimed at introducing, embedding and sustaining the POD system of care interventions into routine ward care. It envisaged implementation as a process involving a number of steps, not just a single event. 38 These comprised (1) mobilisation of a staff action group, (2) staff (and volunteer) training, (3) review of current ward practice, (4) examination of delirium risk factors in relation to current ward practice, (5) implementation of delirium prevention practice and (6) the volunteer programme:
Staff action group
The first step involves the mobilisation of a staff action group with the legitimacy and authority to introduce the programme and develop a plan for change that included awareness-raising and delivering training, engaging ward staff and recruiting volunteers. The action group was to comprise relevant individuals, including ward manager, matron/senior practitioner and VSM, all central to co-ordinating and delivering the change, although others (up or down the organisational hierarchy) might be mobilised around specific objectives and tasks.
Training
With the action group in place, the second step in preparing for implementation comprised staff training based on an interactive approach to foster programme coherence. 39 Educational materials presented the theory of change underpinning the POD system of care interventions and facilitated consideration of current practice on identifying risk factors and preventative actions alongside practices to be implemented. Thus, materials placed the emphasis on staff reviewing what they actually did in respect of patients at risk of delirium and what systems needed to be in place to identify those at risk to direct attention to what was different with the POD system of care. It was envisaged that educational materials might be added to and refined depending on local need, recognising that there also existed local expertise and pre-existing work on delirium prevention in some sites.
Review of current practice
The third step in the preparatory work of implementation, and through which programme coherence was further generated, was the systematic review of current practices related to each of the delirium prevention interventions via staff observations and structured feedback to inform action-planning. Thus, using a set of suggested, adaptable audit tools, the action group was to facilitate the conduct of short periods of qualitative observation of ward practices and environment, the results of which would be discussed by the ward team. This was intended to both engage the wider ward team in understanding how the intervention departed from existing practice and secure participation in the programme of change (cognitive participation), thereby also positively affecting ward vision and culture. 34,40–42
Examination of risk factors in relation to ward practice
The fourth step in implementation planning involved the action group examining the interventions, one for each of the 10 clinical risk factors, with a structured approach to decision-making around allocating roles and responsibilities between staff and volunteers, informed by the audits and ward staff discussion.
Implementation of delirium prevention practices
The implementation process activities to insert the co-ordinated model into routine work practices comprised two sets of tasks. One set involved consideration of the appropriate role of volunteers in relation to specific delirium prevention interventions consistent with local policies. This prompted action on agreeing role descriptions, associated competencies necessary to undertake roles safely and confidently, and the appropriate training and support to do so. The other set concerned establishing and inserting into routine work practices systems and processes for the assessment and recording of clinical factors contributing to delirium risk, for communicating information and preventative tasks in respect of at-risk patients to staff and between staff and volunteers, for documenting interventions carried out by volunteers and staff, and for supervising and supporting volunteers at the ward level. These activities, which have been characterised elsewhere as the tasks of ‘planning, engaging, executing and reflecting and evaluating’,29 had the objective of enhancing ownership and commitment to the integrated model of change, thereby facilitating collective action and reflexive monitoring.
The volunteer programme
Simultaneous with system of care implementation planning at ward level was the recruitment of volunteers, the provision of training to support their involvement and a process of introducing them to ward staff to facilitate an integrated team approach to delivery.
The product of the planning for implementation was a bespoke POD system of care with the systems, processes and division of labour in place to achieve and sustain its execution, and that was adapted to local contexts. Even so, the principles underpinning POD and the steps in the change process to facilitate action on the intervention were standard. 33 We envisaged that this was not a static document, but would be subject to regular review and change based on progress, experience and documentation of actions and outcomes. 43,44
Sections 1–4 of PODv1 (see Table 4) were presented to the development teams in the third workshops; they considered the programme feasible to implement. The remaining sections were presented at the next round of workshops. Following this, we were in a position to prepare the final version of the full programme for pilot implementation in new sites in project 2, scheduled to start in June 2011. This was the main output and milestone for project 1 (see Table 4).
The project 1 sites showed considerable commitment to continuing their delirium prevention work; therefore, they each received a copy of the final system of care. Any subsequent implementation they chose to undertake was outside the auspices of the research team.
Summary
The work undertaken in project 1 focused on a central facet of complex interventions, that is developing the treatment components and the associated processes of implementation while locating them in an organisational setting (in this case, an acute hospital ward) that is itself complex and dynamic. 45
The work of delirium prevention as a meaningful set of practices posed difficult challenges for staff, as prevention necessitates a more complex understanding of a problem than understanding how to manage it. Engaging in preventative action requires knowledge at different levels: about risk factors that may predispose a patient to the problem, and the kinds of interventions or practices that have the potential to reduce modifiable risk. It also requires systems to identify those at risk, and the mobilisation of staff to carry out practices that contribute to risk reduction.
Building on the participatory method and empirical findings, PODv1 was developed as a collaborative approach to delirium prevention involving ward staff, volunteers and patients/relatives. It is distinct from the HELP in several respects. First, and in contrast to the HELP, no additional programme-specific staff are required. Rather, the programme was envisaged as becoming embedded into routine ward practices. This approach is also attractive from an intervention sustainability point of view. Second, by involving staff directly in delivering the system of care, it aimed to enhance a culture of care among staff on acute wards, recognising that communicating with patients and responding to their individual needs in a holistic manner are integral to promoting recovery and reducing adverse events. Third, by including volunteers alongside staff in providing that additional ‘bit of help’ (e.g. engaging with patients as individuals, providing cognitive and social stimulation or enhancing care through assistance with tasks such as feeding), there is the potential to increase the effectiveness of delirium prevention, with an additional positive impact on the well-being of patients and the more effective use of resources. Finally, although the POD system of care has well-described core content, it was intended to be delivered flexibly depending on pre-existing practices and local circumstances.
The research process described in project 1 led to the successful formulation of a draft delirium prevention system of care suitable for use in the NHS, which, although sharing the principles of the HELP, was substantially different from the original HELP model.
We were thus ready to embark on project 2 of the research programme: pilot-testing the novel delirium prevention system of care (POD) for feasibility and acceptability.
Project 2: pilot-testing of implementation feasibility and acceptability of the Prevention of Delirium system of care (version 1)
Parts of this section have been reproduced from Godfrey et al. 46 © 2013 Godfrey et al. ; licensee BioMed Central Ltd. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Aims and objectives
The aims and objectives of project 2 were to conduct a feasibility study to assess the implementation and acceptability of the PODv1 to patients and their relatives, clinicians, support staff and volunteers, and to refine the content and delivery of the intervention in terms of:
-
take-up of the intervention protocols
-
impact of the intervention on staff workload
-
impact on patient and carer satisfaction with care
-
acceptability to patients, carers, staff and volunteers.
Method
We undertook a before-and-after study in hospital trusts using quantitative and qualitative data collected prospectively over a 6-month baseline/implementation period and a 6-month delivery period to assess the feasibility and acceptability of the PODv1 and to refine its content and delivery.
We used a case study approach47 to collect detailed information using mixed methods. These included facilitated workshops, analysis of documentation/records, interviews and focus groups, observation, and questionnaire surveys, which provided us with data from multiple sources and from the perspectives of all potential stakeholders. This comprehensive approach to data collection was designed to facilitate identification of the adaptations required to the content, delivery, approach and context (people, systems and organisation of care) to optimise the implementation of delirium prevention.
The setting for the case study was an elderly care or orthopaedic ward in an acute hospital. The analytic lens (the case) was the work of implementing a delirium prevention system of care (i.e. PODv1) in the specific context of the care routines and practices in each ward/hospital setting. We considered three or four case studies to be practically achievable. This number would allow some cross-case comparison to take into account differences in features such as case mix, establishments and skill mix, attitudes of staff and perceived barriers to implementation.
We identified potential local sites through previous knowledge and contacts and/or interest shown in the project and recruited four elderly care wards and two orthopaedic trauma wards in four hospital trusts. In addition, a ward from project 1 independently decided to implement and deliver the POD system of care on an orthopaedic trauma ward. Although we interviewed the development lead in this site to test out the theory of change, we did not include it in the findings. Details of recruited wards are shown in Table 5.
| Descriptor | Trust | |||||
|---|---|---|---|---|---|---|
| A | B | C | D | |||
| Hospital | i | ii | iii | iv | v | |
| Number of beds | 396 | ≈ 900 | 1113 | ≈ 450 | ≈ 420 | |
| Catchment area | Town | City | City | City | Urban | |
| Catchment population | ≈ 200,000 | ≈ 500,000 | 751,480 | 213,000 | 245,000 | |
| Ward | 1 | 2 | 3 | 4 | 5 | 6 |
| Specialty | Elderly care | Orthopaedics | Elderly care | Elderly care | Elderly care | Orthopaedic/fracture neck of femur |
| Number of beds | 29 | 23 | 28 | 28 | 31 | 22 |
Engaging sites
Following recruitment, workshops were held in each case study site. Participants included patient and carer representatives, hospital managers, clinical managers and VSMs, volunteers, senior clinicians, nurses and therapists (see Report Supplementary Material 6 for participants). We explained the background to the study, the purpose and content of the delirium prevention system of care and our suggested delivery methods. Participants’ views were explored to provide an initial commentary on the practicalities of implementing PODv1, to ascertain who needed to be involved and to elicit relevant contextual knowledge (e.g. work in the hospital around delirium, key stakeholders to contact).
Data collection
For a full description, see Appendix 1.
Patient description
Anonymous ward-level patient administrative system data (sex, age on admission, length of hospital stay and discharge destination) were obtained for all admissions during the implementation and delivery phases (see Report Supplementary Material 7).
Implementation planning and delivery
We undertook qualitative interviews using topic guides with staff and volunteers; informant interviews and conversations with implementation team members; observation of ward practices, multidisciplinary team (MDT) meetings, implementation team discussions and volunteer training sessions; and collection of documents (e.g. assessment, care and discharge plans; information for patients and caregivers; and care pathways). In addition, we constructed a ward diary/events log to provide a contemporaneous account of the process of implementing and delivering PODv1; communication with teams; problems encountered; solutions arrived at; and contextual factors that affected implementation planning and delivery.
To facilitate shared learning between sites, we arranged a centrally located workshop. This had a secondary purpose: to apprise the research team with information about how sites perceived the implementation and delivery processes. Only wards 1 and 2 had, at this point, begun delivery; wards 3 and 4 were in the early stages of implementation planning and ward 5 had not started implementation planning (ward 6 had not been recruited) (see Report Supplementary Material 6 for participants). The proceedings were audio-recorded.
Take-up of the intervention
We examined the extent to which each ward instigated systems to embed PODv1 in current ward practice. This included:
-
the development and introduction of –
-
a system for identifying delirium risk
-
a daily delirium prevention plan
-
a volunteer care plan and a process for supporting volunteers
-
-
audits and observations undertaken to assess/review current practice pertinent to the 10 delirium risk factors.
Impact of the intervention on nurse workload [wards 1–4 and 6 (implementation only)]
We used a ‘dependency–acuity–quality’ method at the start of POD implementation and during POD delivery to gauge its impact on ward staff activity and modification of workload. 48,49 This involved ward-based structured observations by researchers of staff activities linked to patients’ dependency/acuity. Activities included direct care (face-to-face bedside care), indirect care (patient-related, but not face-to-face, activity), associated work (e.g. ‘hotel’-type duties) and personal time (e.g. meal breaks). To obtain a broad sample of nurses’ workloads, we undertook ward observations over 24 hours during six shifts (two early, two late and two night shifts).
Impact on patient and carer satisfaction with care (wards 1–4 and 6)
We conducted a postal patient and carer satisfaction survey at baseline and during the delivery phase using relevant questions from the Care Quality Commission’s national patient survey instrument for patients50 and, for carers, a 19-item questionnaire. 51 Both were distributed by ward staff near discharge from hospital during both study periods.
Acceptability to patients, carers, staff and volunteers (wards 1–4 and 6)
We undertook interviews with a sample of staff, volunteers, patients and carers, and organised a workshop involving implementation team members from each participating site to share and reflect on implementation experiences.
Data analysis
Interviews, focus groups and workshops were audio-recorded, fully transcribed and entered into a database (NVivo 9) for initial coding, sorting and linking. Analysis was conducted by three members of the research team using established qualitative analytic procedures: concurrent data collection and analysis, coding, memos and methods of constant comparison. 52,53 Data sets were combined to create narrative, individual case studies of implementation and delivery in context. Cross-case comparison facilitated an explanatory account of the pattern of variation, what shaped it and the consequences flowing from it.
Quantitative data were analysed using appropriate parametric and non-parametric statistical methods to give summary descriptions and investigate comparisons. Staff workload data analysis included investigation of the relationship between dependency, activity and other variables49 (see Appendix 1).
Results
Full details of implementation and delivery phases for each of the six wards can be found in Report Supplementary Material 8.
Implementation planning
Critical to the engagement of ward staff and volunteers in the POD implementation planning was involvement and direction provided by those with the authority, legitimacy and resources to make change happen, specifically the trio of ward manager, VSM and either a matron or senior nurse practitioner, to assume a proactive role in leading it. The combination of commitment and participation around a common purpose was fully achieved in wards 1, 2, 3 and 6, and failed in ward 5, as a consequence of short-staffing and the preoccupation of senior ward staff in getting through day-to-day tasks, meaning delirium was not a priority. This ward withdrew without engaging in any implementation planning work. In ward 4, an implementation team was not established: a staff member was designated by the ward manager to take implementation planning forward, including working with hospital voluntary services staff to recruit volunteers.
Features of the change management process to engage implementation team members in seeing the need for change, such as the POD system of care audits/reviews of practice, were initially a concern because of the time required to complete them. They later became valued as a lens to ‘see’ current practice, identify taken-for-granted practices that required attention and reinforce what was positive that could be built on:
The observations . . . make you ‘see’ things . . . it was good that it was me looking at my ward because it’s people I know so they . . . are comfortable with you in that situation . . . as an insider.
Staff nurse, ward 1
All five wards (1–4 and 6) conducted the audits. In ward 1, senior staff with knowledge of the ward and from different disciplines undertook audits in their area of expertise (e.g. the mental health liaison practitioner carried out the audit relating to communication and a senior therapist undertook the audit relating to mobility); in wards 3 and 4, they were carried out by the designated staff member. Important in engaging the wider ward team was that a system was established for communicating audit findings to the team and for seeking their views on the implications for practice change. In wards 1, 2, 3 and 6, this occurred through handover and staff meetings; in ward 4, findings were conveyed to senior ward staff and conveyed informally to staff ‘on the ground’.
Each ward developed its own documentation for identifying delirium risk, delirium care plans, job descriptions for volunteers and systems for communication between staff and volunteers on the work that needed to be done and how to convey it. These drew on exemplars from the PODv1 manual, but were adapted to be integrated into existing assessment and planning documentation.
Delivering training to ensure inclusion of all staff, using material in the PODv1 manual, was challenging. Creative approaches were adopted: multiple short sessions during the early morning break in ‘breakfast meetings’, a ‘delirium tree’ in the staff room with notes of risk-reducing actions hanging from the ‘branches’ and reinforcement through discussion at handovers.
In wards 1 and 2, the implementation phase was conducted as envisaged and within the timescale, driven by a matron who assumed overall responsibility for the process, with functioning implementation teams overseeing the work on each ward. In wards 3 and 4, the work of planning implementation was very protracted until a staff member was given dedicated time to pursue it. This was successful in ward 3, but was unsuccessful in ward 4 in the absence of senior ward involvement and because of ongoing staffing difficulties that, in practice, meant that the demands of nursing work made inroads into project time. In ward 6, there was a hiatus following initial implementation work with a proposed change in the ward model, and then a move to a temporary space. Once the change occurred and the move to a permanent site was completed, implementation was pursued quickly within a foreshortened timescale (< 3 months), with dedicated staff nurse time.
The flexible approach to implementation adopted in the PODv1 was based on recognition that ‘my ward is different from your ward’. In wards that fully pursued implementation, this allayed fears that the PODv1 would multiply paperwork, as staff worked at ensuring that new systems and processes were integrated into existing ones. It also fostered creativity and a problem-solving approach, and facilitated active decision-making by staff in how to make change happen, thereby contributing to staff ownership of change. Critical to engagement of the wider staff team was that ward managers acted as change ‘facilitators’, legitimating ward investment and conveying the significance of the work to the wider staff group through routine and special forums. Where this did not occur (as in ward 4), the work of implementation planning was not translated into action at the level of the ward team.
Delivery phase
Adherence to protocols
Adherence to the POD protocols in the four implementing sites was generally good: care plans for patients specifying action on each of the delirium risk factors were completed, integrated into patient notes as part of the admission process and reviewed in the MDT/ward round/handover, as necessary. Several sites developed new ways of responding to needs around communication and stimulation. For example, on ward 6, the senior occupational therapist involved occupational therapist students in engaging in therapeutic activities (reminiscence work, playing games) with patients identified as being at high risk of delirium. Occupational therapists also worked with volunteers to engage with patients based on information in the care plan on what interested them and relatives were encouraged to bring in patients’ favourite music and books. In one full implementation ward (ward 1), initial high adherence to protocols, as reflected in completion of care plans, reduced towards the end of the delivery period. Contextual factors were implicated. Early in the delivery phase, senior hospital managers conveyed their intention to close the ward as part of a strategy to reduce acute beds. The proposal was subsequently revised to merge ward 1 with an adjacent ward at the conclusion of the study, thereby effecting an overall reduction in bed capacity. However, a combination of the ward manager taking on responsibility for the two wards in the interim and uncertainty engendered among staff had the consequence that several staff moved to other posts and morale was adversely affected, which affected engagement with the research.
Changing ward practice
Similar to ward staff in the project 1 sites, nursing, therapy and care staff on all participating wards reported minimal knowledge of the significance of delirium and its adverse outcomes for patients and caregivers. In addition, there was little or no awareness that delirium might be prevented among those at risk. Education sessions, observation and structured review of current practice proved helpful and facilitated empathic connection with the experience of patients with delirium, as opposed to ‘just seeing a “problem” patient’ (sister, ward 1).
Senior ward staff in wards 1, 2, 3 and 6 and observation of practice suggested that, even prior to delivery, but during the implementation planning phase, engagement in the POD system of care had resulted in practice change:
Slowly you started noticing at handovers . . . that staff . . . wouldn’t say that Mrs X was confused, the word confused went, and people talked about whether she may have a delirium . . . it became a clinical thing, not something to be dismissed . . . and delirium wasn’t just about the person walking up and down . . . they were picking up on people who were quiet. They just started associating a sudden change in behaviour of patients with possible delirium and doing something about it.
Matron, wards 1 and 2
They also reported that, whereas before nursing and care staff might have reacted impatiently if a patient was ‘behaving badly’, now they were more patient and would spend time talking to the person, seeking to ease their confusion.
The period of implementation and delivery of the PODv1 was one of considerable organisational turbulence shaped by both national policy drivers (e.g. the imperative to achieve efficiency savings) and initiatives on improving care for people with dementia, and by local contextual factors such as ward re-organisations and staffing difficulties. This affected the engagement of the sites in both the implementation planning and delivery of the POD system of care.
Volunteers
Implementation phase
Recruitment of volunteers was completed in 6 months. Roles and ‘job descriptions’ agreed during implementation were supported with volunteer training. Even so, the volunteer component of the POD system of care was implemented to a variable extent across all the sites. The training provided was diverse, comprising specific PODv1 training in wards 1, 2 and 6; the trust’s standard volunteer training (ward 3); and provision of a leaflet (ward 4). In addition, in four wards (wards 1, 2, 4 and 6), volunteers were invited to the ward to familiarise themselves with the environment and to meet with staff prior to the start date. Dedicated training and ward introduction meetings reinforced for volunteers that they were involved in a specific programme of work as part of the ward team.
Delivery phase
The volunteer component of the PODv1 was implemented to a variable extent across all sites, being most developed in wards 1, 2, 4 and 6. The core features of the role were spending time conversing with patients; providing emotional support, reassurance and stimulation; giving practical assistance at mealtimes; and, in one ward, encouraging and supporting patients to mobilise. There were numerous examples of ways in which volunteers developed creative and person-centred approaches to communicate with very frail and cognitively impaired older people, not as patients, but as individuals with a past and present and with fears and hopes for the future, to an extent that staff were unable to offer.
The number of hours contributed monthly to each ward by volunteers was modest (mean 27 hours, range 13–56 hours). This was not a result of a lack of commitment by the volunteers – some individually contributed up to 8 hours a week – but was a consequence of the limited number of volunteers available. Except for one ward (ward 6), VSMs did not continue to recruit volunteers after the initial POD system of care implementation period.
Despite attention to introducing volunteers to ward staff prior to delivery and a dedicated POD training event for volunteers, volunteers were initially anxious and unclear as to their role and lacked confidence about how best to approach patients:
Going onto a ward where there are quite a lot of really ill people . . . you can imagine it being quite daunting. It was for me . . . I’d done loads of preparation for it and I still found it daunting so, yeah. But I think once you’ve done it once or twice you kind of know what it’s like and you know what to do . . . and staff were welcoming . . . they were all quite helpful and reassuring and they showed me what to do at the time until I got the hang of it and they knew I’d got the hang of it so, so yeah.
Young volunteer, ward 6
This early period was particularly vulnerable to volunteer attrition. Approximately half the volunteers initially recruited did not sustain their involvement over the 6 months of the PODv1 delivery. Of these, around one-third never started and most of the rest left after a few sessions either for personal reasons (illness, pressure of work) or because they found working with older people too difficult. Although volunteering was established in all four hospitals, their specific deployment directly with older people, in a dedicated system of care, was new, and the need for ongoing work and time to sustain involvement was not anticipated. The VSMs considered inadequate support at ward level to be a key factor in volunteer attrition, particularly for less confident and inexperienced volunteers. They would have liked to offer them more support, but were unable to do so because of the pressure of work. Ward staff found that supporting volunteers, at least initially, required time, effort and patience, which not all wards could easily provide. The degree of support and how it was provided varied across wards. In ward 2, for example, the nutrition assistant assumed responsibility for liaison between the ward and the volunteers; in ward 6, the ward manager took this on initially, meeting with each volunteer, assigning a staff member whom they could shadow and a more experienced volunteer to act as mentor.
Factors that appear to be important to the retention and sustainability of volunteers, and that need to be put in place by wards implementing the POD programme, are:
-
ongoing recruitment of volunteers with an expressed interest in the POD programme
-
a comprehensive POD-specific training
-
a robust support system.
Impact on staff workload
For a full description see Appendix 2.
The method for investigating nursing staff workload demonstrated small changes overall in nursing input between the implementation planning and delivery phases (direct patient care: 45% to 46%; indirect patient care 28% to 29%) (Table 6). There was a 4% increase in direct patient care by ward sister and staff nurse grades, whereas there was a 2% decrease in direct patient care in support worker grades (see Table 6). Overall, between the implementation and delivery phases, there were also small decreases in the percentage of both ‘associated work’ (i.e. non-nursing work such as hotel-type duties) (from 15% to 13%) and personal time (from 13% to 12%) undertaken by nursing staff (see Table 6). Overall, we concluded that the introduction of the PODv1 did not result in significant increases in nursing time in respect to ward routines (this did not include implementation planning work). Volunteer observations amounted to < 1% of all of the observations undertaken (see Table 6).
| Observations and activity | Staff grade | Volunteer | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Manager | Staff nurse | Support staff | ||||||||
| Phase 1 | Phase 2 | Phase 1 | Phase 2 | Phase 1 | Phase 2 | Phase 1 | Phase 2 | Phase 1a | Phase 2a | |
| Observations | ||||||||||
| n | 485 | 392 | 3338 | 2762 | 3633 | 2804 | 28 | 88 | 8257 | 6711 |
| % | 6 | 6 | 45 | 46 | 49 | 46 | 0 | 1 | 100 | 100 |
| Activity category (%) | ||||||||||
| Direct care | 21 | 25 | 42 | 46 | 48 | 46 | 82 | 63 | 45 | 46 |
| Indirect care | 46 | 45 | 38 | 36 | 18 | 21 | 0 | 13 | 28 | 29 |
| Associated care | 27 | 23 | 9 | 7 | 19 | 20 | 18 | 14 | 15 | 13 |
| Personal time | 5 | 7 | 11 | 11 | 16 | 14 | 0 | 11 | 13 | 12 |
Acceptability of the Prevention of Delirium system of care version 1
Questionnaires: patient and carer satisfaction
Patients
A total of 1360 patients were discharged during the questionnaire distribution periods; 827 questionnaires were distributed by ward staff and 134 were returned (16.2%). Return rates varied between the wards (11–38%). Satisfaction of care among patient respondents was mixed, being high for some items (e.g. choice of food, confidence and trust in doctors and nurses, enough nurses on duty to care for the patient) and low for other items (e.g. being bothered by noise at night, a number of items regarding communication) (see Appendix 3). There was a trend in a substantial number of items for less satisfaction in the delivery phase of the POD programme than in the implementation phase. This was significant for three items: item 10 – did the nurses talk in front of the patient as if they were not there?; item 12 – did a member of staff say one thing and another say something quite different?; and item 16 – did the patient find someone on the hospital staff to talk to about their worries and fears? It is possible that the focus on reviewing current practice meant that staff were more reflective on how they engaged with patients. However, the poor response rate makes it difficult to draw definitive conclusions.
Carers
Only 80 carers returned a questionnaire (of 827 questionnaires distributed). There was also a trend for less positive responses in carer questionnaires during the delivery phase than in the implementation phase across nearly all of the individual items in the questionnaire (see Appendix 3). The total score for the second subsection of the carer questionnaire was significantly lower in the delivery phase than in the implementation phase.
Sixty of the 134 patients and 51 of the 80 carers provided written comments. Across both phases of the questionnaire, the comments of the patient and carer respondents related to six common themes: staff attitude, communication, care and treatment, availability of staff, food, and environment. Overall, more patient respondents commented positively regarding staff attitude during the implementation phase than during the delivery phase. Among both patient and carer respondents, there were fewer negative comments in the delivery phase concerning staff availability than in the implementation phase. There were no other notable differences in the nature of the comments provided during each phase (see Appendix 3).
Interviews with patients and carers
For the patients and carers that were interviewed (one patient, three carers and five patient/carer dyads), it was the focus of the POD interventions that was valued: attention given to hydration and nutrition, meeting sensory needs, and help with mobilisation. Particularly valued by carers was staff spending time with their relative and getting to know what was important to them. Carers’ main criticisms related to the communication of information, such as failure of staff to actively seek or regard information about the patient, lack of involvement in discharge planning and the unavailability of nursing staff. The small sample reflects recruitment difficulties. Ethics approvals did not permit us to have direct access to patients’ personal information. It was intended that a letter inviting participation with a stamped addressed reply envelope would be given out by staff at patient discharge. This did not happen consistently. The method of recruitment was also not conducive to engaging people, many of whom were frail and living with cognitive problems.
Ward staff
Staff found some aspects of the POD system of care challenging. Of particular issue were the volume and clarity of the POD manual and the time required to implement the system of care and to support volunteers. However, staff in the majority of sites believed that the POD system of care had been beneficial and had resulted in practice change. In some wards (wards 1, 2, 3 and 6), it was considered that the ward team had developed an increased understanding and recognition of delirium and that this had enhanced practice.
Staff accepted and appreciated volunteers and considered that they brought a new and positive dimension to the ward. There were some initial concerns on the part of staff regarding the tasks that volunteers would carry out and around issues of confidentiality. In practice, they found that their fears were unfounded. Staff valued having the consistency of regular volunteers who knew what was wanted of them. The general assessment by staff of the volunteers who sustained their involvement with the ward was that they were ‘very good’ or ‘excellent’. On only one ward (ward 3) were there concerns expressed about the calibre of volunteers, specifically of some student volunteers. In ward 6, by contrast, a collaborative initiative involving sixth-form colleges was very successful in engaging volunteers interested in pursuing careers in medicine, nursing and allied professions. Although slow to establish, this was pursued beyond the POD programme, recruiting a large volunteer team of young people, which was sustained through reciprocal support of ward staff and volunteers.
Volunteers
When interviewed about their experiences, volunteers reported that they had generally found staff to be friendly and welcoming, but were not always sure that they were valued by them. Most volunteers were initially anxious about their role and lacked confidence about how best to approach patients and had sometimes felt uncertain about what they should be doing. As noted previously, the early period was particularly vulnerable to volunteer attrition. In wards where support was perceived to be inadequate, those volunteers who continued often had previous experience of volunteering or working in a care environment. Volunteers who remained reported considerable pleasure and enjoyment in their work, even if it was regarded as emotionally difficult at times.
Discussion
We undertook a before-and-after study to investigate the feasibility and acceptability of implementing and delivering the POD system of care (PODv1) developed in project 1 in six elderly care and surgical orthopaedic wards in acute hospitals. Several complementary qualitative and quantitative methods of data collection were used. The strengths of the study were the in-depth assessment of the implementation of the system of care and the range of data collection to investigate the feasibility and acceptability of the system of care, including in-depth contemporaneous recording of the process of implementation on the wards.
Feasibility of implementing the Prevention of Delirium system of care
Four of the six wards fully implemented the PODv1; one ward was a partial implementer, but primarily in relation to volunteer engagement – ward practice changes were not pursued to delivery, and one ward failed.
Critical to implementation was the combined and co-ordinated involvement of the ‘triumvirate’ of a named, individual ‘driver’ at senior level whose professional authority and vertical networks legitimated the work of POD implementation in the face of competing priorities; a ward-based ‘facilitator’, typically the ward manager, who provided support and encouragement to legitimate staff time devoted to the POD system of care and extend its reach to the wider staff team; and a VSM to recruit and support volunteers and facilitate their introduction to the ward and the POD system of care.
This combination of commitment and participation around a common purpose was fully or mainly achieved in four wards (wards 1, 2, 3 and 6); passive support of senior staff in ward 3 was compensated for by the dedicated staff member, a long-standing member of the ward team, who was highly regarded by colleagues and had a demonstrable flair for practice change. It was partially achieved in one ward (ward 4), but primarily with regard to volunteer input. It failed on one ward (ward 5). Partial implementation in ward 4, and failure to engage with the PODv1 in ward 5, posed an additional issue: namely that, in addition to leadership, implementation of what is an augmented system of care requires that there is the capacity and resources to deliver at least a basic standard of care.
These findings informed the development of four ‘readiness-for-change’ criteria, synthesised from the experience of ward implementation in project 2 to ensure recruitment of suitable sites for project 3. It should be noted that these criteria do not imply knowledge, interest or prior work on delirium prevention. Rather, they relate to the presence of contingent factors that are necessary to allow the selection of wards that are realistically going to be able to implement this complex intervention. The four criteria are:
-
commitment of the senior nurse, ward manager and VSM
-
a named person to drive implementation forward
-
dedicated time of a senior experienced nurse to lead implementation
-
adequate staffing levels.
We have mapped our wards against these criteria (Table 7). Where one or more of these criteria were absent, implementation did not occur, despite frequent contact from the research team.
| Readiness-for-change criteria | Ward (successful implementation) | |||||
|---|---|---|---|---|---|---|
| 1 (Yes) | 2 (Yes) | 3 (Yes) | 4 (Partial) | 5 (No) | 6 (Yes) | |
| Commitment | Yes | Yes | Yes | Partial | Partial | Yes |
| Named person | Yes | Yes | Yes | Yes | No | Yes |
| Dedicated time | Yes | Yes | Yes | Yes | No | Yes |
| Adequate staffing levels | Yes | Yes | Yes | No | No | Yes |
Acceptability of the Prevention of Delirium system of care intervention
Overall, the intervention was acceptable to staff, volunteers, patients and carers. In addition, and reassuringly, PODv1 delivery did not increase day-to-day nursing staff workload.
Significance of the early delivery phase
Although much attention is drawn in the implementation process and materials to engaging staff in practice change, the findings suggest that the early phase of delivery is also critical to continuance.
Volunteers
The model of delirium prevention that was adopted included a prominent role for hospital volunteers. However, most wards were not able to recruit or sustain the number of volunteers needed to have a major impact in PODv1 delivery. Therefore, a re-assessment of the role of volunteers in delirium prevention work on the ward was undertaken, preparatory to project 3.
The Prevention of Delirium programme
We engaged with site staff to elicit their comments on the PODv1 programme manuals (see Report Supplementary Material 9). Based on this feedback, and the analysis of the data collected, we modified the POD programme and manuals for use in project 3 [POD system of care version 2 (PODv2)]. Modifications included providing greater structure and clear checkpoints for the implementation phase (i.e. a built-in project management approach). This was to ensure that implementation occurred over a more restricted and manageable period (see Report Supplementary Material 10).
Summary
We concluded that the PODv1 was feasible to implement in routine care and was acceptable to staff, volunteers and patients. Some changes to the intervention were suggested by our work.
A major change was the development of the readiness-for-change criteria for the recruitment of sites in project 3. These related to requirements around leadership, commitment and resources to effect what amounted to an enhanced model of care.
Project 3: a multicentre, pragmatic, cluster randomised controlled feasibility study of the Prevention of Delirium programme system of care
Aim
The aim was to conduct a pragmatic, multicentre, cluster randomised, controlled, feasibility study to explore the potential clinical effectiveness and cost-effectiveness of the PODv2, compared with standard care, among older patients at risk of developing delirium who are admitted to hospital for emergency care.
Primary objectives
-
Estimate recruitment and follow-up rates at both patient and cluster levels.
-
Assess fidelity of the POD system of care.
-
Assess the degree of contamination at ward level due to between-ward staff movements.
-
Assess the completeness of data collection.
-
Provide a preliminary estimate of the effectiveness of the POD system of care, compared with standard care, as measured by the incidence of new-onset delirium within 10 days of recruitment (anticipated primary outcome for a definitive trial).
-
Assess the variability in the incidence of delirium within 10 days of recruitment between the hospital sites.
-
Assess fulfilment of criteria for progression to a future definitive trial.
-
Investigate differences in financial costs and benefits between the POD system of care and standard practice.
-
Estimate the sample size for a future definitive trial.
Secondary objectives
The secondary objectives were to investigate the following: differences in the severity, duration and time to first episode of delirium (including persistent delirium); falls; length of stay in hospital; in-hospital mortality; destination at discharge; participant status at 30 days; health-related quality of life and health resource use; physical and social independence; anxiety and depression; poor outcome; and safety.
Methods
Parts of the following section have been reproduced from Young et al. 54 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Parts of this section have also been reproduced from Young et al. 55 © The Author(s) 2020. Published by Oxford University Press on behalf of the British Geriatrics Society. This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/), which permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is properly cited.
Design and setting
We undertook a pragmatic, multicentre, cluster randomised, controlled feasibility study to investigate the potential effectiveness of the POD system of care, compared with standard care, in NHS hospitals in England and Wales. The study was reviewed and approved by the UK National Research Ethics Service (Research Ethics Committee reference number 13/YH/0400).
Recruitment
Hospitals and wards
We aimed to recruit 16 wards in eight NHS hospitals in England and Wales: one elderly care and one orthopaedic trauma ward in each hospital.
The inclusion criteria for ward participation were the demonstration of adequate ward nurse staffing, as assessed against national guidance,56,57 and the agreed involvement of a named ward manager, a senior nurse and a VSM (if local staff intended to use voluntary services as a component of the intervention). In addition, there was a requirement for the equivalent of 1 day per week per ward of dedicated time for 3–4 months from an experienced senior nurse to lead the implementation.
Wards were excluded if they had previously participated in the development of the POD system of care or if they intended to implement other delirium prevention initiatives during the trial.
Participants
As the POD system of care is a whole-ward intervention, all patients in the ward (regardless of eligibility and consent status) would have the potential to be exposed to the intervention. Patients were therefore recruited for completion of individual outcome assessments only.
Patients aged ≥ 65 years who were admitted to the study wards during the study period were eligible for participation.
Patients were excluded if they had prevalent delirium on admission to the ward; if discharge was planned within 48 hours of admission; if a delirium assessment had not been performed by a research assistant (RA) within 24 hours of admission (elderly care patients) or preoperatively (orthopaedic trauma patients); if consent had not been obtained with 48 hours of admission to the ward; if end-of-life care was being provided; and if they had transferred from another ward or were not under the care of the ward team. Participants were recruited for 6 months following the 6-month intervention implementation period.
Intervention
The PODv2 is a manualised, multicomponent intervention and systematic implementation process designed to secure changes in ward practice, potentially enhanced by the involvement of hospital volunteers. It comprises actions centred on 10 risk factors associated with the development of delirium: cognitive impairment and/or disorientation, dehydration and/or constipation, hypoxia, immobility or limited mobility, infection, multiple medications, pain, poor nutrition, sensory impairment, and sleep disturbance. The implementation process is supported through raising awareness and through the training of staff and volunteers in delirium prevention, including action-planning cycles of observation and audit of current practice to establish what needs to be put in place to introduce the POD system of care in a particular ward setting. These principles are embedded in the POD manual that comprises sections outlining the aims, an overview, the management of POD and the four core tasks (staff education, review of current practice, ward systems and involving volunteers), supplemented by resources including educational materials, example documents, volunteer materials and guidance.
Assessments
Primary outcome
We assessed for differences in new-onset delirium within 10 days of recruitment between patients in the intervention group (POD programme) and patients in the control group (usual care), as this is the expected primary outcome for a definitive trial. Delirium was assessed using the four-item Confusion Assessment Method (CAM)58,59 (Table 8).
| CAM item | Source of information |
|---|---|
| 1. Acute onset and fluctuating course |
|
| 2. Inattention | |
| 3. Disorganised thinking | |
| 4. Altered level of consciousness |
|
Secondary outcomes
Physical and social independence were measured by the RAs at baseline and at 3 months (postal questionnaire) using the Nottingham Extended Activities of Daily Living (NEADL) scale;62 anxiety and depression were measured by the RA using the Clinical Anxiety Scale63 and the Geriatric Depression Scale Short Form,64 respectively, at 30 days.
Research assistant Confusion Assessment Method training
A CAM training and monitoring process was developed that followed recommended practices. 65 We developed a three-stage training process. Stage 1 was central or local classroom teaching about delirium, trial research procedures and the administration of outcome measurement instruments, including the CAM. Stage 2 was specific to the CAM and involved local experiential learning consisting of (1) one-to-one practice sessions, (2) pilot interviews with patients and (3) within-site inter-rater reliability assessments. Stage 3 was a further within-site inter-rater CAM reliability performance check conducted at the local sites. 59
Data collection
Data collection was undertaken by locally based RAs who were trained in study procedures and outcome measures.
Screening data
Screening data, including demographic characteristics and admission details, were obtained by the RAs in consultation with the attending ward staff for all patients aged ≥ 65 years admitted to a study ward.
Baseline assessments
Baseline assessment by the RAs for patients providing consent comprised an initial CAM; the Charlson Comorbidity Index;66 and recording of existing hearing and or visual impairments, current medications, illness severity using the National Early Warning Score (NEWS) or equivalent,67 history of dementia and Abbreviated Mental Test Score (AMTS),60 living arrangements, and the EuroQol-5 Dimensions (EQ-5D) score. 68 Participants also completed a questionnaire relating to physical and social independence (the NEADL scale). 62
Primary outcome
The RAs performed cognitive assessments [AMTS and months of the year backwards (MotYB) test] and the CAM daily for up to 10 days post recruitment (or until discharge, if sooner) to detect the presence of new delirium. Each CAM item was assessed and recorded on a clinical research form that was dated and signed. This document was stored digitally and accessed at a later date to investigate achievement of the completeness of the CAM assessments.
Discharge assessment
At the point of discharge, the RAs recorded the date of discharge (or date of death), episodes of falls in hospital and discharge destination (living alone, living with another person, residential care home, nursing home, other).
Thirty-day assessment
At 30 days post recruitment, the RAs performed a cognitive assessment (AMTS and MotYB test) and the CAM, and asked the patient to complete a questionnaire about health-related quality of life using the EQ-5D,68 about anxiety using the Clinical Anxiety Scale,63 about depression using the Geriatric Depression Scale Short Form64 and about patient experience using selected questions from the patient-reported experience measure from the National Audit of Intermediate Care. 69
Three-month assessment
At 3 months post recruitment, postal questionnaires were used to provide information on physical and social independence (NEADL scale),62 health-related quality of life (EQ-5D),68 and health and social care resource use and living arrangements. Proxy completion of the questionnaires was permitted.
Sample size
A formal power calculation was not appropriate for this feasibility study. Assuming an average length of stay of 14 days and 25-bed wards, 50% of patients at risk of delirium, 30% of whom would provide consent (or a consultee declaration),70,71 we proposed that a recruitment target of 720 patients in 6 months was achievable.
Randomisation
The POD system of care is a ward-based intervention that aims to affect staff skills, knowledge and clinical practice. Cluster randomisation was therefore chosen to reduce between-group contamination. There remained a possibility of between-ward contamination because of staff movement. This was investigated by randomising four of the hospitals to the POD system of care or control at the hospital level, and randomising four of the hospitals at the ward level. Randomisation was stratified by ward type (elderly care medicine and orthopaedic trauma) and was a two-stage process, and was performed centrally by the statistician at the Clinical Trials Research Unit (CTRU). Sites were first randomised 1 : 1 between hospital-level allocation (both wards in the hospital received the same treatment allocation), and ward-level allocation (each ward in the hospital received a different intervention). Those sites selected for hospital-level allocation were then further randomised 1 : 1 for both of their wards to receive either the POD system of care or control. Wards in those sites selected for ward-level allocation were randomised 1 : 1 to receive either the POD system of care or control (Figure 3). 54,55
FIGURE 3.
Randomisation overview.
Implementation
Wards randomised to the intervention received the PODv2 manual. The first step was to form local implementation teams that included a study-specific ward nurse (1 day per week). An intervention overview meeting was provided by the trial co-ordinating centre (one meeting for each ward). This was followed by a 6-month implementation period to allow the intervention to be embedded in ward practice before patient recruitment to the trial occurred. Progress on implementation was monitored by regular site visits and telephone and e-mail contact, and was tracked through completion of an internal milestone checklist embedded in the POD system of care manual.
Usual-care group
Wards randomised to the usual care control group continued to deliver care as determined by local policies and practices. Any new delirium prevention measures or care processes adopted during the study period were recorded by the central trial team, following a request for information from the sites.
Blinding
The RAs administering and collecting outcome measures had no role in the intervention. It was unrealistic for RAs visiting the wards daily to conduct delirium assessments to remain blind to treatment allocation.
Assessment of intervention fidelity
We identified 21 tasks that were essential for the successful implementation and delivery of the POD system of care and grouped them in four domains based on the Conceptual Framework for Implementation method:72 (1) installation (five items; maximum score = 5), (2) delivery (12 items; maximum score = 48), (3) coverage (three items; maximum score = 16) and (4) duration of delivery (one item; maximum score = 1). 73
Data collection to inform the fidelity domains involved (1) non-participant observations, (2) extraction of standardised information from the medical and nursing records and (3) inspection of the intervention installation checklists contained in the POD system of care manual.
We used these data to populate tables of evidence for each ward relating to the four fidelity domains and their associated content items. We developed, piloted and modified a scoring system to quantify intervention fidelity and to facilitate consistency of assessor judgements. Once evidence tables had been completed, assessors were asked to provide an overall fidelity score (low compliance, ≤ 50%; medium compliance, 51–79%; high compliance, ≥ 80%) based on their judgement of the extent of completion of the essential tasks. 74
Statistical methods
All analyses and data summaries were conducted on the intention-to-treat population, defined as all participants registered, regardless of non-compliance with the protocol or withdrawal from the study. The analysis focused on descriptive statistics and confidence interval (CI) estimation, rather than on formal hypothesis testing.
Estimation of recruitment rates
We recorded the number of sites expressing an interest in participating in the trial and reasons for non-progression. To assess the feasibility of recruiting participants for a definitive trial, we calculated the number of patients screened, eligible, assessed for delirium, with prevalent delirium, with capacity to consent and for whom consent for trial participation was obtained. Baseline descriptors of the screened and recruited populations were obtained (see Characteristics of the screened and registered participants: generalisability) and compared between the groups to assess for imbalance.
Estimates of completeness of data collection and follow-up rates
The reliable calculation of delirium incidence rates requires close adherence to a predetermined delirium detection process. We therefore recorded the number of in-hospital delirium assessments and 30-day delirium assessments conducted, and calculated the missing observations. We also recorded the number and timing of participant withdrawals from follow-up data collection and the reasons for withdrawal (including deaths), and the number of participants with missing self-reported outcome questionnaires at each time point.
Assessment of between-ward intervention contamination
The number of staff moving on and off study wards within sites was collected for a sample period of 1 week. The number of participants moving wards during their hospital stay was tabulated. Incidence rates of new-onset delirium at the sites that were randomised at the hospital level were calculated and compared with those from sites that were randomised at the ward level, to assess possible between-ward contamination. Incidence rates of new-onset delirium were calculated for participants recruited within the first 3 months of sites opening to recruitment, and for participants recruited between 3 and 6 months of sites opening to recruitment to determine if the intervention delivery was sustained when more time had elapsed since initial training. Service improvements introduced on participating wards during the study period were recorded.
Estimation of a sample size for a future definitive randomised controlled trial
To inform the sample size calculation for a possible definitive trial, we calculated the incidence of new-onset delirium within 10 days of admission by ward type, by study arm and overall, together with corresponding 95% CIs. We used multilevel logistic regression that adjusted for demographic characteristics (age and sex), delirium risk factors (medications associated with delirium, e.g. benzodiazepines, opiates, H1 antihistamines),75 sensory impairment (hearing impaired, use of hearing aid or sight impaired), cognitive impairment and/or dementia, Charlson Comorbidity Index,66 NEWS67 category and ward type. In the regression model, ward type was fitted as a random effect. The number of new patients admitted per ward during the recruitment period was used to estimate cluster size. The intracluster correlation coefficient (ICC) and associated 95% CI were calculated using the covariance parameter estimate from a multilevel logistic regression without adjustment for participant or ward characteristics.
Criteria for continuation to a future definitive randomised controlled trial
A priori criteria for progression to a definitive randomised controlled trial were defined as a minimum of six of the eight wards (75%) completing the POD manual milestone checklist (to provide assurance that the POD implementation was successful) and an overall recruitment rate of at least 10% of the potential recruitment pool. The criteria did not include thresholds for projected clinical effectiveness or cost-effectiveness.
Health economics study
Model development
To estimate the cost-effectiveness of an integrated delirium prevention intervention in the context of the trial, a decision-analytic model was used. The model was developed after consultation with the research team (including clinical experts) and the parameter values were identified following targeted searches of the literature. The working report describing the model development and results is included in Appendix 4.
Cost-effectiveness study
The aim of the economic study was to establish the feasibility of conducting an economic evaluation of the POD system of care and to determine preliminary estimates of its cost-effectiveness. Specific objectives were to:
-
determine the feasibility of collecting the assessments needed (quality of life and health-care resource use) for an economic evaluation in this patient group
-
determine the number of missing data in assessments
-
determine the validity and responsiveness of quality-of-life assessments in this group
-
determine the feasibility of collecting and of using/interpreting proxy-completed assessments
-
estimate the cost of the POD intervention
-
provide estimates of the cost-effectiveness of POD, compared with usual care
-
compare these estimates with those from the earlier evaluation based on decision modelling.
Quality of life was assessed using the EQ-5D68 at baseline and at 1 and 3 months. Health-care resource use was captured using a specially designed questionnaire completed by patients (and/or proxies) at 3 months. Costs were calculated from the perspective of health services and Personal Social Services. The cost of the POD intervention was estimated to include material costs (e.g. printing of manuals), the time to deliver and receive the training and also the time to provide support during POD delivery. This information was provided by the POD research team, which kept a contemporaneous diary of visits and travel. The feasibility of data collection was determined by observing the extent of missing data.
The primary economic evaluation adopted the NICE-preferred approach of a cost–utility analysis comparing the costs and benefits of POD and usual care. 76 The analysis time horizon was 3 months, based on the trial follow-up. The main analysis result was the incremental cost-effectiveness ratio (ICER) per quality-adjusted life-year (QALY). ICERs below the range of £20,000–30,000 indicate that POD would be considered cost-effective. Non-parametric bootstrapping was employed to determine the level of sampling uncertainty. No discounting of costs or effects was conducted.
See Appendix 5 for full details of the methods.
Results
Parts of the following section have been reproduced Young et al. 55 © The Author(s) 2020. Published by Oxford University Press on behalf of the British Geriatrics Society. This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/), which permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is properly cited.
Objective 1: estimate recruitment and follow-up rates
Hospitals and wards
Twenty hospitals expressed interest. Twelve hospitals subsequently completed and returned site survey forms, and eight of these were recruited. Among the four hospitals not recruited, two withdrew (one because of poor staffing and one was unable to identify a suitable ward), one did not respond to the request for a site visit to progress towards recruitment and, for one, regulatory approvals came after the other requisite number of POD sites had gained approval. Of the 16 recruited wards, nine were elderly care medicine and seven were surgical/trauma orthopaedic. Seven of the eight hospitals registered had one elderly care ward and one orthopaedic trauma ward. The remaining hospital site had two elderly care wards registered.
None of the wards/hospitals withdrew from the study.
Patient screening and recruitment rates
Screening and recruitment took place between August 2014 and February 2015. A total of 4449 patients admitted to the 16 wards were screened for eligibility, 3274 (73.6%) of whom were considered eligible. The most common reasons for exclusion at screening of the remaining 1175 patients were as follows: 538 (45.8%; 12.1% of those screened) had a recorded diagnosis of delirium on admission, 352 (30.0%; 7.9% of those screened) had an expected duration of stay of < 48 hours, 139 (11.8%; 3.1% of those screened) were not under the care of the ward medical team and 105 (8.9%; 2.4% of those screened) were receiving end-of-life care (Figure 4).
FIGURE 4.
The Consolidated Standards of Reporting Trials flow diagram. a, Withdrawals here are from researcher questionnaires; b, withdrawals here are from postal questionnaires; one patient in the POD arm withdrew from researcher visits at 30 days, but not from postal questionnaires at 3 months.
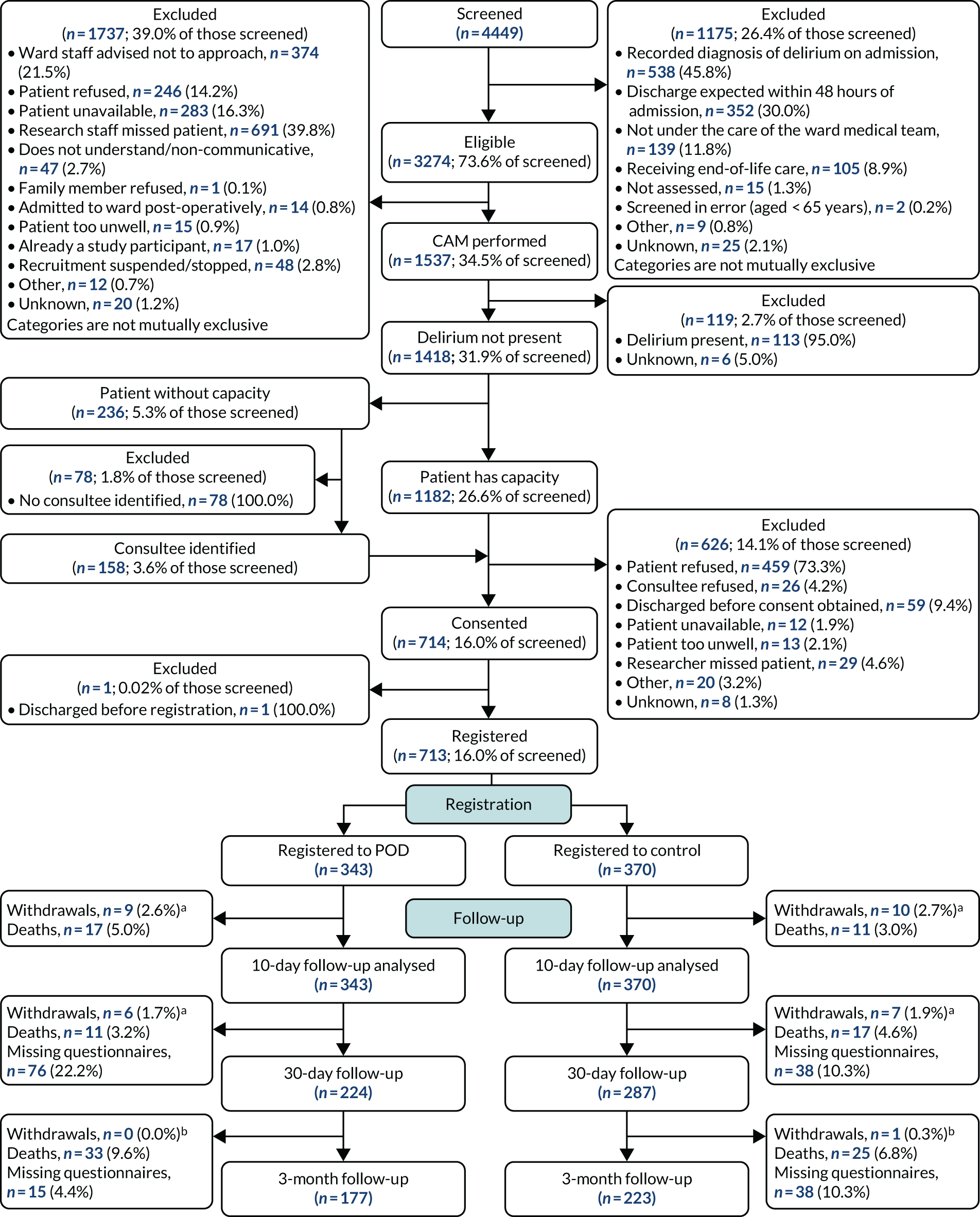
Delirium assessment was performed by the RAs on 1537 (34.5% of screened) of the 3274 eligible patients. The remaining 1737 (39.0% of screened) patients did not have a delirium assessment. The most common reasons for not performing a CAM assessment were as follows: research staff missed patient [691 (39.8% of those excluded)], ward staff advised not to approach [374 (21.5%)], patient unavailable [283 (16.3%)] and patient refused [246 (14.2%)].
Of the 1537 patients who were screened for delirium, 1418 (31.9% of screened) were assessed as not having prevalent delirium. The remaining 119 were excluded: 113 had prevalent delirium, and the reasons are unknown for six.
Of the 1418 patients assessed as not having delirium, 1340 (30.1% screened) either had capacity (n = 1182) or a consultee had been identified (n = 158); these were approached for participation. The remaining 78 patients were excluded as they were without capacity and a consultee had not been identified. Of the 1340 patients approached for participation, 626 were excluded. The most common reasons for exclusion were as follows: 459 (73.3% of those excluded) patients refused; 59 (9.4%) were discharged before consent was obtained; 29 (4.6%) were missed by the researcher; and, for 26 (4.2%), the consultee refused. Consent was obtained for 714 (16.0% of screened) patients, and 713 were registered to the study (one patient was discharged before registration): 343 were registered to the POD system of care and 370 were registered to control (see Figure 4). Figure 5 is the recruitment graph. Patient accruals to the study between the sites ranged from 65 to 105 (see Appendix 6, Table 50).
FIGURE 5.
Recruitment graph.
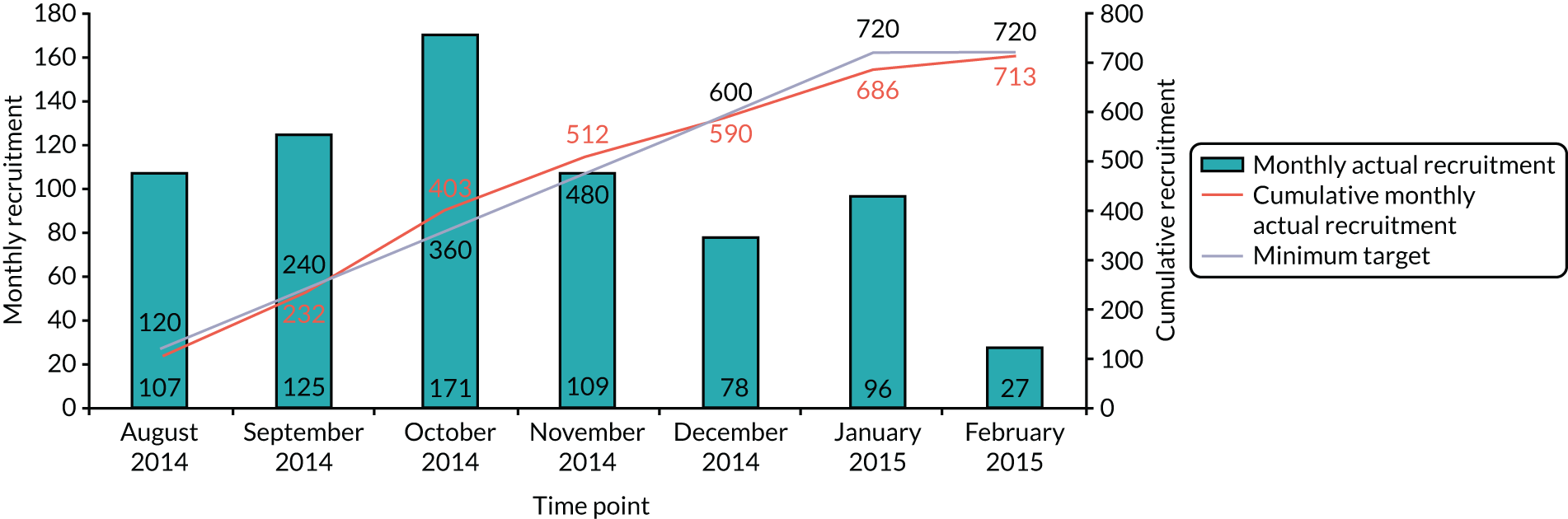
Eligibility violations
A total of 13 (1.8%) participants were identified as not fulfilling the eligibility criteria: five (1.5%) in the POD arm and eight (2.2%) in the control arm. The main criteria violated were as follows: the delirium assessment (CAM) was not performed (n = 1 in the POD arm and n = 6 in the control arm), and the participant had prevalent delirium on admission (n = 3 in POD and the POD arm and n = 2 in the control arm) (see Appendix 6, Table 51). Patients identified as breaching an eligibility criterion were included in all analyses.
Final follow-up
Thirty-three (4.6%) participants withdrew during the study period [15 (4.4%) from the POD arm and 18 (4.9%) from the control arm] (see Appendix 6, Table 52). Of these, 19 (57.6%) withdrew within 10 days of recruitment.
Characteristics of the screened and registered participants: generalisability
The characteristics of the screened and registered participants were similar with respect to age, sex and ethnicity (Table 9). In the screened and registered populations, the mean overall ages were 83.1 years [standard deviation (SD) 8.05 years] and 82.7 years (SD 7.84 years) respectively; female patients accounted, respectively, for 67.2% and 68.3% of the populations; and 89.3% and 91.7%, respectively, of the populations were of white ethnicity. Participant age and ethnicity were broadly similar across sites: the mean overall age varied between 79.0 years (SD 7.34 years) and 85.1 years (SD 7.58 years), and between 83.8% and 97.6% of participants were of white ethnicity (small differences due to more missing data for some sites). Some differences between sites were noted with respect to sex; female participants accounted for between 49.2% and 100.0% of participants (see Appendix 6, Table 53).
| Characteristic | Screened | Registered | ||||
|---|---|---|---|---|---|---|
| POD (N = 2115) | Control (N = 2334) | Total (N = 4449) | POD (N = 343) | Control (N = 370) | Total (N = 713) | |
| Age (years)a | ||||||
| Mean (SD) | 83.1 (8.15) | 83.1 (7.95) | 83.1 (8.05) | 82.5 (7.88) | 83.0 (7.81) | 82.7 (7.84) |
| Median (range) | 84 (32–109) | 84 (48–105) | 84 (32–109) | 83 (65–101) | 84 (65–99) | 83 (65–101) |
| Missing (n) | 5 | 15 | 20 | 0 | 2 | 2 |
| Sex, n (%) | ||||||
| Male | 685 (32.4) | 742 (31.8) | 1427 (32.1) | 111 (32.4) | 114 (30.8) | 225 (31.6) |
| Female | 1420 (67.1) | 1569 (67.2) | 2989 (67.2) | 231 (67.3) | 256 (69.2) | 487 (68.3) |
| Missing | 10 (0.5) | 23 (1.0) | 33 (0.7) | 1 (0.3) | 0 (0.0) | 1 (0.1) |
| Ethnicity, n (%) | ||||||
| White | 1959 (92.6) | 2012 (86.2) | 3971 (89.3) | 326 (95.0) | 328 (88.6) | 654 (91.7) |
| Mixed: white and black Caribbean | 1 (0.0) | 1 (0.0) | 2 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mixed: white and black African | 1 (0.0) | 0 (0.0) | 1 (0.0) | 1 (0.3) | 0 (0.0) | 1 (0.1) |
| Mixed: white and Asian | 1 (0.0) | 1 (0.0) | 2 (0.0) | 0 (0.0) | 1 (0.3) | 1 (0.1) |
| Other mixed background | 1 (0.0) | 1 (0.0) | 2 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Asian: Indian | 6 (0.3) | 6 (0.3) | 12 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Asian: Pakistani | 9 (0.4) | 5 (0.2) | 14 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Asian: Bangladeshi | 2 (0.1) | 1 (0.0) | 3 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other Asian background | 4 (0.2) | 1 (0.0) | 5 (0.1) | 1 (0.3) | 0 (0.0) | 1 (0.1) |
| Black: Caribbean | 7 (0.3) | 6 (0.3) | 13 (0.3) | 1 (0.3) | 1 (0.3) | 2 (0.3) |
| Chinese | 1 (0.0) | 0 (0.0) | 1 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other ethnic group | 3 (0.1) | 1 (0.0) | 4 (0.1) | 1 (0.3) | 0 (0.0) | 1 (0.1) |
| Not stated | 0 (0.0) | 2 (0.1) | 2 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 120 (5.7) | 297 (12.7) | 417 (9.4) | 13 (3.8) | 40 (10.8) | 53 (7.4) |
Baseline characteristics
The two arms were well balanced with respect to residence, hearing aid use, benzodiazepines use and comorbidities, although some imbalance between groups was evident for all other characteristics, namely reason for admission, ward type, cognitive impairment and/or dementia, highest NEWS category within 48 hours of admission, visual impairment, hearing impairment, and opiates and antihistamines prescribed (Table 10). Residence and hearing impairment were balanced across sites; however, some imbalance between sites was apparent for all other characteristics (see Appendix 6, Table 54).
| Characteristic | POD (N = 343) | Control (N = 370) | Total (N = 713) |
|---|---|---|---|
| Residence, n (%) | |||
| Home | 311 (90.7) | 339 (91.6) | 650 (91.2) |
| Nursing home | 10 (2.9) | 9 (2.4) | 19 (2.7) |
| Residential/care home | 21 (6.1) | 22 (5.9) | 43 (6.0) |
| Missinga | 1 (0.3) | 0 (0.0) | 1 (0.1) |
| Reason for admission, n (%) | |||
| Hip fracture | 71 (20.7) | 99 (26.8) | 170 (23.8) |
| Other orthopaedic condition | 60 (17.5) | 102 (27.6) | 162 (22.7) |
| Medical condition | 211 (61.5) | 169 (45.7) | 380 (53.3) |
| Missinga | 1 (0.3) | 0 (0.0) | 1 (0.1) |
| Ward type, n (%) | |||
| Elderly care | 212 (61.8) | 180 (48.6) | 392 (55.0) |
| Orthopaedic trauma/surgery | 131 (38.2) | 190 (51.4) | 321 (45.0) |
| Cognitive impairment and/or dementia, n (%) | |||
| Yes | 83 (24.2) | 67 (18.1) | 150 (21.0) |
| No | 259 (75.5) | 303 (81.9) | 562 (78.8) |
| Missing | 1 (0.3) | 0 (0.0) | 1 (0.1) |
| Highest NEWS category within 48 hours of admission,b n (%) | |||
| Low risk | 275 (80.2) | 315 (85.1) | 590 (82.7) |
| Medium risk | 54 (15.7) | 39 (10.5) | 93 (13.0) |
| High risk | 12 (3.5) | 8 (2.2) | 20 (2.8) |
| Missing | 2 (0.6) | 8 (2.2) | 10 (1.4) |
| Hearing impairment, n (%) | |||
| Yes | 120 (35.0) | 112 (30.3) | 232 (32.5) |
| No | 222 (64.7) | 258 (69.7) | 480 (67.3) |
| Missing | 1 (0.3) | 0 (0.0) | 1 (0.1) |
| Participant uses a hearing aid, n (%) | |||
| Yes | 78 (65.0) | 72 (64.3) | 150 (64.7) |
| No | 42 (35.0) | 40 (35.7) | 82 (35.3) |
| Visual impairment, n (%) | |||
| None | 43 (12.5) | 33 (8.9) | 76 (10.7) |
| Registered blind | 7 (2.0) | 6 (1.6) | 13 (1.8) |
| Partially sighted | 34 (9.9) | 29 (7.8) | 63 (8.8) |
| Wears glasses | 257 (74.9) | 301 (81.4) | 558 (78.3) |
| Missing | 2 (0.6) | 1 (0.3) | 3 (0.4) |
| Benzodiazepines prescribed, n (%) | |||
| Yes | 17 (5.0) | 15 (4.1) | 32 (4.5) |
| No | 325 (94.8) | 355 (95.9) | 680 (95.4) |
| Missing | 1 (0.3) | 0 (0.0) | 1 (0.1) |
| Opiates prescribed, n (%) | |||
| Yes | 145 (42.3) | 172 (46.5) | 317 (44.5) |
| No | 197 (57.4) | 198 (53.5) | 395 (55.4) |
| Missing | 1 (0.3) | 0 (0.0) | 1 (0.1) |
| H1 antihistamines prescribed, n (%) | |||
| Yes | 43 (12.5) | 33 (8.9) | 76 (10.7) |
| No | 299 (87.2) | 337 (91.1) | 636 (89.2) |
| Missing | 1 (0.3) | 0 (0.0) | 1 (0.1) |
| Participant comorbidities,c n (%) | |||
| Yes | 236 (68.8) | 244 (65.9) | 480 (67.3) |
| No | 106 (30.9) | 126 (34.1) | 232 (32.5) |
| Missing | 1 (0.3) | 0 (0.0) | 1 (0.1) |
| Charlson Comorbidity Indexd score | |||
| Mean (SD) | 1.7 (1.97) | 1.7 (1.88) | 1.7 (1.92) |
| Median (range) | 1 (0–12) | 1 (0–11) | 1 (0–12) |
| Missing | 1 | 0 | 1 |
Objective 2: assessment of intervention implementation
All of the eight wards allocated to the intervention group completed the milestone checklist, were deemed competent and went on to deliver the POD system of care intervention and recruit patients (Table 11). Only three wards elected to involve volunteers in the system of care. All of the sites progressed through the implementation milestones and none of the sites withdrew during either the implementation or delivery phases.
| Time taken to | Total (n = 8) |
|---|---|
| Complete staff education (weeks) | |
| Mean (SD) | 16.3 (4.10) |
| Median (range) | 17.4 (10.0–21.3) |
| Missing | 0 |
| Review current practice (weeks) | |
| Mean (SD) | 20.0 (7.88) |
| Median (range) | 17.4 (12.0–36.6) |
| Missinga | 1 |
| Implement ward system (weeks) | |
| Mean (SD) | 10.6 (7.57) |
| Median (range) | 7.9 (2.1–23.3) |
| Missing | 0 |
| Overall time taken to implement the POD system of care (weeks)b | |
| Mean (SD) | 21.4 (7.32) |
| Median (range) | 18.6 (16.4–38.4) |
| Missing | 0 |
The mean time taken to implement the POD system of care was 21.4 (SD 7.32) weeks (see Table 11), although this figure is skewed by site 8, which took 38.4 weeks, as it was temporarily located on an alternative ward owing to building works (see Appendix 6, Table 55). The median time taken to implement the POD system of care was 18.6 weeks.
Intervention fidelity
Ten health-care professionals with experience in older people’s care assessed fidelity. The mean score for each domain was as follows: installation, 4.5 points (range 3.5–5.0 points); delivery, 32.6 points (range 27.3–38.3 points); coverage, 7.9 points (range 4.2–10.1 points); and duration, 0.38 points (range 0–1.0 points). 73 Of the 10 delirium risk factors, infection, nutrition, hypoxia and pain were the most consistently addressed, and cognitive impairment, sensory impairment and multiple medications were the least consistently addressed. 73 Overall fidelity to the intervention was assessed as being high (≥ 80%) in two wards, medium (51–79%) in five wards and low (≤ 50%) in one ward.
Objective 3: assessment of between-ward intervention contamination
Staff
Contamination data were received from all sites [two sites (sites 2 and 4) provided data by telephone or e-mail only]. Site 2 stated that staff were never moved to other wards from the trauma ward and no staff were brought onto the ward. No data were provided for the elderly care ward at this site. Site 4 stated that wards did not keep any records of staff movement.
During the 1-week data collection period, there were 216 reports of staff moving into the 12 study wards that were providing data: 115 on elderly care wards and 101 on orthopaedic trauma wards, most commonly health-care assistants (51.9%) (see Appendix 6, Table 56).
Only 13 staff moves off the ward were recorded during the 1-week data collection period: four from elderly care wards and nine from orthopaedic trauma wards (see Appendix 6, Table 56).
Delirium incidence rates
The incidence rates of new-onset delirium were similar between arms at the sites randomised at the hospital level and at those randomised at the ward level (see Appendix 6, Table 57). However, incidence rates were lower when ward-level randomisation was used.
Incidence rates of new-onset delirium were similar between arms for participants recruited during the first 3 months of sites opening to recruitment and for participants recruited between 3 and 6 months of sites opening to recruitment, although incidence rates were lower during the later 3 months of recruitment (see Appendix 6, Table 58).
Patients
During their hospital stays, 135 (18.9%) participants moved wards: 58 (16.9%) in the POD group and 77 (20.8%) in the control group (see Appendix 6, Table 59). Among the sites, the percentage of participants moving wards ranged from 10.0% to 25.8% (see Appendix 6, Table 60).
Wards
No ward reported any new multicomponent delirium prevention measures during the study period. Four POD wards introduced service improvements consisting of dementia training; observations using electronic handheld devices; attempts to decrease noise levels; and identification of patients at risk of delirium with education on hearing aids, nutrition and hydration. One control ward introduced a new rounding chart.
Objective 4: completeness of data collection
Confusion Assessment Method assessments
Taking into account the length of stay and excluding the assessments not expected as a result of death, withdrawal or discharge, of an expected 5645 CAM assessments, 5065 (89.7%) were completed during the first 10 days of recruitment (Table 12).
| Number of CAMs | POD | Control | Total |
|---|---|---|---|
| Expectedb (n) | 2716 | 2929 | 5645 |
| Conducted, n (%) | 2382 (87.7) | 2683 (91.6) | 5065 (89.7) |
Non-completion rates in sites ranged from 3.5% to 14.8%. 59 The main reasons for non-completion of the CAM were participants were too ill [n = 186 (32.1%)] or participant refusal [n = 163 (28.1%)] (see Appendix 6, Table 61).
Of the 5065 CAM assessments, six (0.1%) had missing responses to CAM questions, two (0.04%) omitted the AMTS and 25 (0.5%) omitted the MotYB test (see Appendix 6, Table 62).
At 30 days, out of an expected 629 CAM assessments, 513 (81.6%) were completed (Table 13).
| Number of CAMs | POD | Control | Total |
|---|---|---|---|
| Expecteda (n) | 302 | 327 | 629 |
| Conducted, n (%) | 224 (74.2) | 289 (88.4) | 513 (81.6) |
Non-completion rates of the 30-day CAM in sites ranged from 2.8% to 30.6% (see Appendix 6, Table 63). More 30-day CAM assessments were not performed for participants in the POD group (n = 78) than for those in the control group (n = 38). Similar reasons for non-completion of the CAM were evident across the groups, although some differences between groups were noted for participant refusal (POD arm, 29.5%; control arm, 15.8%) and participants moving out of the area (POD arm, 12.8%; control arm, 21.1%) (see Appendix 6, Table 64).
Questionnaire return rates
Return rates of the questionnaire booklets to the CTRU were as follows: at baseline, 699 (98.0% of 713 registered participants); at 30 days, 511 (81.8% of 625 expected); and, at 3 months, 400 (70.5% of 567 expected) (see Appendix 6, Table 65). Participant age, sex, ethnicity and residence were broadly similar between those who did and those who did not complete the 30-day researcher questionnaire (see Appendix 6, Table 66).
The most common reasons for non-completion of the 30-day and 3-month questionnaire booklets were as follows: could not contact participant [27 (23.7%) and 92 (55.1%) for the 30-day and 3-month questionnaire booklets, respectively] and participant refused to complete them [26 (22.8%) and 31 (18.6%) for the 30-day and 3-month questionnaire booklets, respectively] (see Appendix 6, Tables 67 and 68).
Questionnaire compliance
Of the 511 30-day follow-ups, 313 (61.3%) were undertaken within ± 2 days of the due date [mean 28.9 (SD 4.93) days]. Of the 400 3-month follow-ups, 259 (64.8%) were undertaken within ± 2 weeks of the due date [mean 102.4 (SD 19.39) days].
Objective 5: estimation of effectiveness
Primary outcome
Fifty-seven (8.0%) of the 713 participants developed new-onset delirium within 10 days of recruitment: 24 (7.0%) of the 343 participants registered to wards delivering the POD system of care and 33 (8.9%) of the 370 participants registered to the control wards. New-onset delirium was slightly higher in orthopaedic trauma wards than in elderly care wards (10.0% vs. 6.4%).
Multilevel logistic regression analysis (adjusting for participant characteristics collected at registration and for ward type) was used to explore the between-group differences in delirium incidence. Although there was evidence that participants in the POD arm had lower odds of developing delirium, this result was not statistically significant (odds ratio 0.68, 95% CI 0.37 to 1.26; p-value = 0.2225) (Table 14). An unadjusted analysis confirmed this finding (odds ratio 0.77, 95% CI 0.44 to 1.33).
| Model parameter | Odds ratio (95% CI) | p-value | Unadjusted ICC (95% CI) |
|---|---|---|---|
| Randomised arm: POD vs. control | 0.68 (0.37 to 1.26) | 0.2225 | 0.0002 (–0.21 to 0.21) |
| Age (years) | 1.07 (1.02 to 1.12) | 0.0023 | |
| Sex: female vs. male | 0.94 (0.48 to 1.82) | 0.8471 | |
| Prescribed benzodiazepines vs. not | 3.50 (1.18 to 10.35) | 0.0236 | |
| Prescribed opiates vs. not | 3.53 (1.72 to 7.24) | 0.0006 | |
| Prescribed H1 antihistamines vs. not | 1.07 (0.43 to 2.66) | 0.8814 | |
| Hearing impairment vs. none | 0.97 (0.52 to 1.81) | 0.9235 | |
| Partially sighted/registered blind vs. no visual impairment | 0.35 (0.09 to 1.34) | 0.1250 | |
| Wears glasses vs. no visual impairment | 0.57 (0.23 to 1.37) | 0.2084 | |
| Orthopaedic trauma/surgery ward vs. elderly care ward | 1.27 (0.64 to 2.50) | 0.4951 | |
| EWS category: high vs. low | 1.80 (0.36 to 9.05) | 0.4768 | |
| EWS category: medium vs. low | 1.36 (0.62 to 2.96) | 0.4434 | |
| Cognitive impairment and/or dementia vs. not | 3.61 (1.88 to 6.91) | 0.0001 | |
| Charlson Comorbidity Index | 1.05 (0.90 to 1.22) | 0.5198 |
Objective 6: assessment of the variability in the incidence of delirium incidence
Delirium incidence in the eight hospital sites ranged between 4.6% and 12.9% (Table 15) (see Appendix 6, Tables 57 and 58).
| Delirium suggested? | Site, n (%) | Total (N = 713), n (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 (N = 104) | 2 (N = 82) | 3 (N = 105) | 4 (N = 90) | 5 (N = 70) | 6 (N = 65) | 7 (N = 93) | 8 (N = 104) | ||
| Yes | 5 (4.8) | 5 (6.1) | 9 (8.6) | 9 (10.0) | 9 (12.9) | 3 (4.6) | 6 (6.5) | 11 (10.6) | 57 (8.0) |
| No | 99 (95.2) | 77 (93.9) | 96 (91.4) | 81 (90.0) | 61 (87.1) | 62 (95.4) | 87 (93.5) | 93 (89.4) | 656 (92.0) |
Objective 7: criteria for continuation to the definitive randomised controlled trial
We aimed to recruit 16 wards from eight hospitals and expected that eight of these wards would successfully implement the POD intervention. To proceed to a definitive trial, it was determined that the feasibility study should show that a minimum of six wards (75%) completed the implementation milestone checklist, were deemed competent and went on to deliver the POD intervention and recruit patients. All eight (100%) wards completed the implementation milestones. The overall recruitment rate to the POD trial was 16.0% (713/4449). This exceeded the pre-stated criterion of recruitment of at least 10% of the total recruitment pool.
Objective 8: cost-effectiveness analysis
For a full description of the cost-effectiveness analysis, see Appendix 5.
Missing data
The return rate of the EQ-5D was 98.6%, 77.5% and 65.3% at baseline, 1 month and 3 months, respectively (94–98% fully completed) (see Appendix 5, Table 34). The completion rate of the resource use questionnaire was lower at 48.7%. Participants with cognitive impairment at baseline were less likely to return the questionnaire than individuals with no cognitive impairment (see Appendix 5, Table 35).
Baseline imbalance
There was some baseline imbalance between the groups, and adjustment was required. QALYs were adjusted using baseline EQ-5D, age, ward type (orthopaedic vs. general), sex and cognitive impairment status (yes vs. no).
Validity of patient outcome assessments
A significant, positive correlation existed between the EQ-5D and NEADL scale scores at 3 months (r = 0.66), indicating that they measure similar constructs in this patient group (see Appendix 5, Figure 18). The trial sample had lower EQ-5D scores at baseline than UK age-matched population norm averages (reported in Kind et al. 77) (see Appendix 5, Table 37). Patients who experienced delirium had a lower average baseline EQ-5D score than those who did not (0.09, compared with 0.26), and this difference was maintained across the different time points (1 month: 0.28, compared with 0.50; and 3 months: 0.15, compared with 0.43) (see Appendix 5, Table 38).
Validity of proxy outcomes assessments
At baseline, proxy-completed EQ-5D values were similar to self-completed (by participants) EQ-5D values, but, at 1 and 3 months, proxy-completed (or aided) EQ-5D completion underestimated quality of life (see Appendix 5, Table 39).
Costs
The POD group participants had higher average resource use for every health-care resource except general practitioner (GP) surgery visits and psychiatrist, psychologist or counsellor visits (see Appendix 5, Table 41). Participants in the POD group had, on average, 2.2 more overnight days in hospital and 1 more day in nursing/residential homes. Overall, the hospital inpatient stay appeared to be driving costs: mean costs were £4965 for the POD group and £4365 for the control group (see Appendix 5, Table 42). The average cost of the POD intervention was estimated as £10.98 per patient (see Appendix 5, Table 40).
Quality-adjusted life-years
The EQ-5D scores at baseline were slightly higher for the POD group than for the control group [mean 0.261 (SD 0.393) for the POD group vs. 0.234 (SD 0.347) for the control group]. Despite the fewer cases of delirium in the POD group, there were negligible between-group differences in QALYs, although, in all analyses, these were in favour of the control arm.
Cost-effectiveness
The trial-based ICER was dominated by standard care (see Appendix 5, Table 43). That is, the POD intervention resulted in higher costs and lower QALYs, albeit the QALY differential was negligible. The difference in cost varied from £920 in the complete-case group to £1127 for the complete-case and imputed items group. The difference in QALY varied from –0.01 in both imputation groups to –0.02 in the complete-case analysis (see Appendix 5, Table 43). NHS total cost and QALYs were replicated 10,000 times in a Monte Carlo simulation (see Appendix 5, Figures 20–22). Using a £20,000 per QALY threshold, the probability that the POD intervention was cost-effective was 0.01 (1% chance) in a simulation using adjusted QALYs and complete-case and imputed items. This chance increased to 10% when using unadjusted QALYs and complete-case data only. The findings were robust to sensitivity analyses.
The health economics model (see Model development) was updated using information from the trial. There were differences between some parameter values used in the original model and those observed in the trial: lower delirium incidence, lower delirium rate reduction and much lower utility values. As POD appeared to result in additional resource use (a difference of £419), a sensitivity analysis was run in which this was added to the POD cost; see Appendix 5, Table 47, for the updated model parameters and assumptions. The updated model showed that the POD system of care had an incremental cost and QALY of £1775 and 0.11, respectively, resulting in an ICER of £16,133, which indicated that the POD intervention was cost-effective (see Appendix 5, Table 48). The probabilistic sensitivity analyses yielded mean incremental costs and QALYs of £1774 and 0.11, respectively, and an ICER of £15,454. The cost-effectiveness acceptability curve with a ‘willingness to pay’ of £20,000 showed that POD had a 100% chance of being cost-effective (see Appendix 5, Figure 23). The results of the trial and model-based analyses were conflicted, and thus limit the confidence we can place on the economic evaluation results. It is unclear why this divergence occurred. Clearly, the model time horizon was much greater than that for the trial analysis and it is possible that the (albeit small) differential in delirium occurrence, when extrapolated over a lifetime, fully reflected the costs and benefits of the intervention, and thus explains to some degree the contrast between trial and model outcomes. The model also fixes the assumed relationship between delirium incidence and outcomes (length of stay, mortality and health-related quality of life), whereas, in the trial, we assessed the relationships directly; it could be that there was an unexpected or lower relationship between these factors than anticipated or that data quality led to bias.
There were significant issues relating to data quality (e.g. missing data and reliance on proxy reports); future research should seek to identify the optimal strategy for data collection in this population.
Objective 9: estimation of a sample size for a definitive randomised controlled trial
The unadjusted ICC was calculated as 0.0002 (95% CI –0.21 to 0.21). Assuming a significance level of 5%, a study power of 90% and a delirium incidence reduction of 30% (consistent with previous studies and our own); incorporating the observed control group incidence rate of 8.9%; allowing for 15% loss to follow-up; and using the unadjusted ICC value of 0.0002, the trial would need to recruit 5200 patients in 26 hospital clusters (200 patients per cluster). As the data to inform this calculation were obtained from the feasibility study, the estimates of delirium incidence and ICC should be treated with caution. 78 Table 16 presents a range of possible sample sizes for a future trial, with varying delirium incidence rates and ICCs (which are assumed to be low given the naturally large cluster size planned).
| Delirium incidence (%) | ICC | Total number of clusters | Total number of patients |
|---|---|---|---|
| 8.9b | 0.0002 | 26 | 5200 |
| 8.9b | 0.01 | 68 | 13,600 |
| 8.9b | 0.02 | 110 | 22,000 |
| 8.9b | 0.03 | 154 | 30,800 |
| 17.7c | 0.0002 | 14 | 2800 |
| 17.7c | 0.01 | 32 | 6400 |
| 17.7c | 0.02 | 52 | 10,400 |
| 17.7c | 0.03 | 72 | 14,400 |
Secondary objectives
Delirium
Severity of delirium episodes, duration of delirium episodes and time to first episode of delirium (including persistent delirium) were similar between the two groups (see Appendix 6, Tables 69–74).
Falls
Nineteen falls were reported in wards receiving the POD system of care and 20 falls were reported in wards receiving control, with mean falls rates of 1.6 (SD 1.00) for the intervention group and 1.3 (SD 0.39) for the control group.
Length of hospital stay
The length of stay for patients who were discharged or who died while in hospital was similar between the groups: a mean of 9.7 days (SD 7.12 days) among those patients registered to POD and a mean of 9.8 days (SD 6.91 days) among those patients registered to the control.
Deaths
A total of 104 patient deaths were reported within 3 months of recruitment (14.6% of all registered patients): 56 (16.3% of patients registered) in the POD group and 48 (13.0% of patients registered) in the control group. Of these, 28 (26.9%) deaths occurred within 10 days of patient recruitment (see Appendix 6, Table 75). The number of deaths between the sites ranged from 5 (7.7%) to 22 (21.2%).
Discharge destination
Of those patients discharged, a larger proportion of patients registered to the POD group [176/248 (71.0%)] than patients registered to the control group [194/288 (67.4%)] were discharged home (Table 17).
| Discharge location | Trial arm, n (%) | Total (N = 536), n (%) | |
|---|---|---|---|
| POD (N = 248) | Control (N = 288) | ||
| Home | 176 (71.0) | 194 (67.4) | 370 (69.0) |
| Nursing home | 9 (3.6) | 17 (5.9) | 26 (4.9) |
| Residential/care home | 32 (12.9) | 21 (7.3) | 53 (9.9) |
| Bed-based intermediate care | 28 (11.3) | 54 (18.8) | 82 (15.3) |
| Othera | 1 (0.4) | 1 (0.3) | 2 (0.4) |
| Missing | 2 (0.8) | 1 (0.3) | 3 (0.6) |
Of the 536 patients discharged, 118 (22.0%) had a change in discharge destination from independent to institutionalised accommodation: 47 out of 248 (19.0%) in the intervention arm and 71 out of 288 (24.7%) in the control arm (Table 18).
| Change in accommodation from baseline to dischargea | Trial arm, n (%) | Total (N = 536), n (%) | |
|---|---|---|---|
| POD (N = 248) | Control (N = 288) | ||
| No change: independent accommodation | 176 (71.0) | 192 (66.7) | 368 (68.7) |
| Change: institutionalised to independent accommodation | 0 (0.0) | 2 (0.7) | 2 (0.4) |
| Change: independent to institutionalised accommodation | 47 (19.0) | 71 (24.7) | 118 (22.0) |
| No change: institutionalised accommodation | 22 (8.9) | 21 (7.3) | 43 (8.0) |
| Unknown | 3 (1.2) | 2 (0.7) | 5 (0.9) |
Patient-reported outcomes
Raw (unadjusted) scores for the NEADL scale, Clinical Anxiety Scale and the Geriatric Depression Scale score showed little between-group differences, although control scores appeared very slightly higher for the NEADL at both baseline and 3 months (see Appendix 6, Tables 76–79).
Poor outcome
Poor outcome (defined as death, persistent delirium or change in accommodation at hospital discharge from home to residential care/nursing home or from residential home to nursing home) was similar: 80 out of 343 (23.3%) in the POD group and 72 out of 370 (19.5%) in the control group (see Appendix 6, Table 80).
Safety
There were no unexpected serious adverse events reported that were clearly attributable to the POD intervention.
Summary
Recruitment
We recruited the target number of 16 wards and we recruited 714 participants (99% of our target of 720) of the 4449 patients admitted to the 16 study wards, a recruitment rate of 16.0%. There was imbalance in the number of elderly care and orthopaedic trauma wards recruited and the number of participants recruited to those ward types between arms. The characteristics of the screened and registered participants were similar and showed that the populations and arms were similar with respect to age, sex and ethnicity.
Follow-up
There were few losses to follow-up [33 (4.6%) participants withdrew] and the rate of data collection was high: 89.7% of expected in-hospital CAM assessments (primary outcome) and 81.6% of the 30-day CAM assessments were undertaken as planned by the RAs.
Missing data
The return rate of the postal questionnaire booklets at 3 months was 70.5%.
Intervention implementation
All eight wards randomised to deliver the POD intervention completed the preparation for implementation and delivered the system of care. The optional volunteer element was included by only three of the eight wards. None of the sites withdrew.
Contamination
There was little evidence of contamination between the study wards, although not all wards routinely collected data on staff moves.
Health economic study
Early in the research work (project 1), and in the absence of observed data, a decision-analytic model was developed to determine the potential for the POD system of care to be cost-effective. The model made assumptions about delirium incidence, POD effectiveness, costs, survival and quality of life. It concluded, with a high degree of certainty, that POD would be cost-effective. This model was updated to include information from the trial, including POD costs, delirium rates and POD effectiveness, and the estimates of cost-effectiveness were updated. This analysis allowed us to test our previous assumptions, and also to estimate the cost-effectiveness, taking into account a longer time horizon.
The POD system of care led to fewer cases of delirium, but this did not translate to lower costs or more QALYs, regardless of the data adjustment, imputation method and Monte Carlo simulation used. Hence, POD did not appear to represent value for money in the cost–utility framework over a 3-month period. The updated decision model yielded expected costs and benefits, which were both higher for POD than for usual care. The ICER for the analysis (deterministic and probabilistic) indicated that the POD system of care was cost-effective. At a willingness-to-pay threshold of £20,000 per QALY gained, POD was cost-effective in 100% of the Monte Carlo simulations.
Discussion
Delirium is a common and serious condition in older people, and is associated with distress for individuals, families and health-care staff;2 increased mortality; protracted lengths of hospital stay; lasting functional and cognitive decline; and increased requirement for long-term care placement. 3 Prevention of delirium is, therefore, highly desirable; multicomponent prevention interventions that aim to attenuate modifiable delirium risk factors have consistently been shown to reduce incident delirium in hospitalised patients by about one-third in various inpatient specialties. 4–6 As a consequence of this evidence base, several national guidance documents have recommended that multicomponent delirium prevention interventions should be incorporated into routine care. 8–10 A major issue faced by the NHS in England, and acknowledged by NICE,8 is the lack of a delirium prevention system of care suitable for widespread national implementation.
To address this, we developed the POD system of care. 1 Our starting position was the HELP,11,12 predominantly used in the USA, for which there is evidence of effectiveness. 13 We also drew on the NICE guidelines,8 as these provided a robust summary of the international evidence base that included the need to incorporate a broader range of delirium risk factors. The National Institute for Health Research (NIHR) Programme Grant for Applied Research programme facilitated an integrated programme of work with a sequence of three studies to develop (project 1), pilot test (project 2) and then provide preliminary evidence of effectiveness and cost-effectiveness (project 3) of a multicomponent delirium prevention intervention: the POD system of care.
Project 1: review and adapt the Hospital Elder Life Program for use in the UK, and identify candidate implementation and delivery strategies
Parts of this section have been reproduced from Godfrey et al. 1 © 2013 Godfrey et al. ; licensee BioMed Central Ltd. This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
We worked with clinical teams in three acute hospital sites in the north of England. We found that delirium prevention was poorly understood by front-line ward staff. This knowledge gap has been described previously. 79–81 At the same time, multicomponent interventions aimed at reducing risk among those most vulnerable to developing delirium involved care practices that were neither consistently nor systematically carried out in routine delivery. Furthermore, it was evident that systematic and purposeful engagement in practices that contributed to reducing delirium risk, although apparently straightforward, involved a complex interplay of cultural, interdisciplinary and organisational change at ward and hospital levels. 1 At the same time, the practices that reduced delirium were those that also defined care quality in acute hospitals. 82,83 The challenge of implementation, therefore, was at the core of securing care practice change, not only to reduce delirium but also to improve care quality, particularly in respect of patients whose resilience is compromised by severe illness, cognitive impairment and frailty in advanced older age. 1
Project 1 was informative in several respects. First, in employing a theory-based approach to inform an understanding of the factors that shape routine practice around delirium and delirium prevention, it provided empirical support to inform an understanding of the behaviours and practices that need to change to implement a preventative programme. Second, it addressed a critical, albeit little-researched, area, namely how to move from existing practice to developing the strategies and skills to achieve change in complex settings. Although the implementation literature offers general insights into what works to achieve change, these also need to be rooted in the concrete contexts of specific problems in their cultural, organisational and professional environments. Third, the process of developing the POD system of care through an innovative participatory research design provided insight into aspects of constructing complex interventions that has hitherto not received much attention. Thus, the methodology that we developed has potential for generalisability beyond its specific application to delirium prevention. 1
Some problems were experienced in relation to restriction associated with the intellectual copyright protection of the HELP. This meant, for example, that the overview report that we produced at the end of WS1 (reviewing the HELP protocols) could not, as was originally intended, be shared outside the research team because of the risk of copyright infringement. Furthermore, the useful learning that the project team secured during visits to sites in the USA and Canada was also subsumed within the intellectual copyright. These difficulties reflected cultural differences between the UK and USA in approaches to research and practice change in the context of very different health-care practice environments. These difficulties did not compromise our programme of work. Solutions were arrived at through an iterative process of discussions among the research team, the Programme Implementation Team and the Programme Management Board, augmented by empirical work and literature review.
The resultant delirium prevention model draws on the HELP, but extends its applicability to an NHS context. In particular, it is broader in scope in that it encompasses actions on additional risk factors and does not require new external resources. Our ambition to develop a multicomponent intervention with potential for integration into routine care (rather than simply an additional care pathway) appeared to be realised. The PODv1 (see Table 4) emerged as a manualised, multicomponent intervention and systematic implementation process designed to secure ward practice changes consistent with a reduction in delirium. It comprised actions centred on 10 risk factors associated with the development of delirium in at-risk patients. The implementation process was supported and reinforced through the education of staff and, optionally, volunteers in delirium prevention, an action-planning cycle of observation, and an audit of current practice to establish what needs to be put in place to introduce and sustain the POD system of care. The principles underpinning the POD system of care were standardised and generalisable, but were flexible to take account of pre-existing practice and local decision-making. Fidelity plays an important independent role in the effectiveness of multicomponent delirium prevention interventions, with higher levels of fidelity resulting in lower rates of delirium incidence. 20 We investigated strategies to optimise intervention fidelity and to assess for feasibility and acceptability in a pilot study.
Project 2: pilot-testing of implementation feasibility and acceptability of the Prevention of Delirium system of care version 1
It was apparent from project 1 that the POD system of care would require new knowledge and skills; awareness and practice change; mobilisation of new resources such as the volunteers; and the volunteers’ integration into the ward team through the establishment of new relationships and ways of working.
We recruited a further six wards (four elderly care and two orthopaedic trauma) in four new NHS hospital trust sites. We initiated delirium prevention implementation teams in each site and planned to allow 6 months to introduce the PODv1 and embed new practices and procedures into routine care. Training and implementation of the PODv1 was led by the local implementation team, supported by the research team members, who served as participant observers. Data collection centred on patient and ward descriptions; the process of implementation planning and delivery; take-up of the intervention; impact of the intervention on staff workload; impact on patient satisfaction with care; and acceptability to patients, carers, staff and volunteers.
Aspects of the early installation process that engaged staff in understanding the need for ward procedure changes, such as the audit and self-observation of existing ward practice, were initially a concern to the staff because of the time involved in completing them. However, this preliminary process was favourably regarded by staff once the findings were discussed within the ward teams. The findings laid the foundations for change as they enabled the staff to identify what was happening on their ward, especially aspects of practice that required particular attention. A process for communicating the findings of the observations to staff and seeking their views on the implications for practice change was also required.
We observed differences and flexibility in the systems and mechanisms established to implement changes that were based on the recognition that ‘my ward is different from your ward.’ The variations in the delirium prevention implementation teams fostered creativity and a problem-solving approach, which contributed to staff ownership of the system of care. It also provided benefit in facilitating active decision-making by staff in how to make change happen such that new systems and processes would be integrated into existing ones, thus allaying fears that it would result in more paperwork.
Volunteers
The HELP delirium prevention system of care is predicated on a major role for trained volunteers to assist the HELP interdisciplinary team. Optimal delivery requires a rota of 21 volunteers weekly to provide input three times daily. The POD system of care, by contrast, is based on the proposition that the volunteer role is to enhance ward practice in relation to delirium prevention, particularly in respect of those tasks that appear difficult for staff to undertake consistently, for example spending time with patients and providing stimulating activities [the latter is of special relevance to patients with dementia (who have a high risk of delirium)].
Although the volunteer component of the PODv1 was implemented to some extent across the sites, the capacity was limited. The total mean number of hours contributed to each ward by volunteers monthly was modest (mean 27 hours, range 13–56 hours). This is compared with a minimum contribution of 252 hours per month for volunteers in the HELP programme (minimum based on 21 volunteers each working one 3-hour shift per week). A very large shortfall is apparent. The shortfall was not due to lack of commitment by the volunteers, indeed some contributed up to 8 hours per week, but was a consequence of the limited number of volunteers available. This was amplified by a high rate of attrition: approximately half of the volunteers initially recruited did not sustain their involvement over the 6-month delivery period. Of these, around one-third never started and, of the rest, most left after a few sessions either for personal reasons (illness, pressure of work) or because they found working with older people too difficult. The VSMs we interviewed considered inadequate support at the ward level to be a key factor in volunteer attrition, particularly for less confident and inexperienced volunteers. They would have liked to offer more support to volunteers, but were not able to do so because of the pressure of work. Ward staff found that supporting volunteers, at least initially, required time, effort and patience, which not all wards could easily provide.
‘Readiness to change’ criteria
Initial interest in the PODv1 by ward staff was not necessarily translated into commitment to and participation in the work required to implementing it. It was apparent that involvement and direction provided by those with the authority, legitimacy and resources to make the change happen was critical to the success of ward staff and volunteers in the implementation and delivery process. Specifically, the triumvirate of ward manager, VSM and either a matron or senior nurse practitioner who also assumed a proactive role in leading the change was required. We thus identified four ‘readiness to change’ criteria, which we carried forward into project 3 as ward selection criteria for the multicentre trial. These criteria did not imply knowledge, interest or prior work on delirium prevention. Rather, they related to the presence of contingent factors that seemed necessary to allow selection of wards that had a realistic opportunity to implement this complex intervention. The four criteria were as follows:
Prevention of Delirium system of care version 2
We concluded that the PODv1 was feasible to implement in routine care and was acceptable to staff, volunteers and patients. Our engagement with site staff provided comments on the PODv1 manuals. Based on this feedback, and the findings from project 2, we modified the POD manuals for use in project 3. The modifications included a more concise presentation, providing greater structure and clear check-points for the implementation phase (i.e. a prespecified project management timetable). This resulted in the second iteration of our system of care: the PODv2.
Project 3: a multicentre, pragmatic, cluster randomised controlled feasibility trial of the Prevention of Delirium system of care version 2
Parts of this section have been reproduced from Young et al. 55 © The Author(s) 2020. Published by Oxford University Press on behalf of the British Geriatrics Society. This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/), which permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is properly cited.
The multicomponent (non-pharmacological) delirium literature is dominated by small to medium-sized, predominantly single-site, randomised and non-randomised evaluation studies4–6,8,13 that are prone to several biases. 84 Ideally, future studies should be designed and conducted as multicentre and pragmatic evaluations85 to allow clinical effectiveness to be robustly evaluated. The design of such trials requires critical information such as prior estimates of effectiveness and recruitment rates. We therefore designed and conducted a multicentre, pragmatic, cluster randomised, controlled feasibility trial to obtain preliminary estimates of the effectiveness of the PODv2, and to assess recruitment and follow-up rates and fidelity to the intervention.
Recruitment and follow-up
The trial is the first successfully completed multicentre, multicomponent delirium prevention randomised controlled trial. The other similar multicentre trial involved only two hospitals, but was unable to recruit sufficient patients and had large numbers of missing data. 86 We were able to consent 714 patients from eight hospitals/16 wards over 6 months, against our target of 720 patients. We had assumed that approximately 50% of patients would be at risk of developing delirium. In fact, nearly three-quarters of the patients on these elderly care and orthopaedic trauma wards were at risk, based on the criteria published by NICE. 8 We further assumed that 30% of patients eligible for the study would be recruited. In practice, we found that the major barrier to recruitment was the inability to conduct a baseline CAM assessment to exclude prevalent delirium, largely because some patients were judged too sick by the ward staff, or because some patients were not identified for assessment within 24 hours of admission (elderly care patients) or pre operatively (orthopaedic trauma patients). Thus, the overall recruitment rate was lower than anticipated at 16%. Losses to follow-up were low: only 4.6% of patients withdrew from the trial and 14.6% of patients died within 3 months of recruitment. These are important parameter values with which to plan future similar studies involving this mixed population of elderly care and orthogeriatric patients.
Delirium incidence
Although patients in the population recruited were at high risk of delirium (elderly: mean age 82.7 years; dementia/cognitive impairment: 21%; comorbidities: 67%; hip fracture: 24%; and opiate use: 44.5%), the rate of incident (new) delirium was lower than anticipated: 8%, compared with 17.7% for a combined medical and orthogeriatric population reported in the randomised studies included in the Cochrane review (39 studies; 16,082 patients). 6 The explanation for the low delirium incidence in the trial population is unclear. It was not related to missing delirium assessments, as the RAs completed 89.7% of the expected CAM assessments during the 10 days after patient registration. The delirium incidence rates showed some variation between sites (4.6–12.9%). This suggests some variation in either individual RA delirium assessment performance or differences in local care environments that influenced the development of delirium. However, the between-site variation in delirium incidence was well within, and in no case exceeded, the pooled estimate value reported in the Cochrane review. 6
The lower than anticipated rate of delirium incidence influenced the precision of the estimate of effectiveness observed in this feasibility study. The adjusted odds ratio of 0.68 for delirium incidence for the patients randomised to the POD programme is entirely consistent with previous studies. 4–6,8 However, the 95% confidence limits were wide: 0.37 to 1.26. This finding is not surprising, as the study was not powered to provide a definitive evaluation of the POD system of care. However, a definitive cluster randomised study would need to be far larger than any previous multicomponent delirium prevention study. Assuming a significance level of 5%, a study power of 90%, a delirium incidence reduction of 30% (consistent with previous studies and our own), incorporating the control group incidence rate of 8.9% observed in this study and using the unadjusted ICC value obtained here (0.0002), the trial would need to recruit 5200 patients in 26 hospital clusters (200 patients per cluster). As the delirium incidence rate observed in this study and the ICC may be underestimates, if we incorporate the incidence of delirium observed in the Cochrane review,6 assume a larger ICC of 0.02 and keep all other assumptions constant, we would need to recruit 10,400 patients in 52 hospital clusters. This clearly represents a substantial trial, but is the only way to obtain robust evidence of clinical effectiveness to support or refute a national roll-out of the POD system of care, or a similar intervention. The findings from our feasibility trial suggest that a larger study would be achievable and provides valuable underpinning methodological information to design the study.
Primary outcome
Parts of this section have been reproduced from Green et al. 59 © The Authors. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Delirium prevention studies have universally used delirium incidence as the primary outcome. There are several methodological issues associated with this. 84 Chief among these is that:
[. . .] the detection of delirium in intervention studies is based on instruments that operationalise the Diagnostic Statistical Manual (DSM) delirium diagnostic criteria.[87] These instruments are bedside assessments that inevitably comprise some degree of interpretation by assessors. Delirium detection is therefore potentially prone to ascertainment bias caused by the relative subjectivity in interpretation of symptoms. In addition, interventions are essentially modifications to ward-based care, with the associated impracticality of effective treatment concealment. At the very least, these methodological issues imply a significant risk for an overestimate of study effect sizes in terms of delirium prevention.
Another direct consequence of using a bedside assessment is that the process is resource intensive and, therefore, expensive. Our study required 37 RAs to cover the eight hospital sites for the requisite 7 days per week. Ideally, the delirium research community needs a reliable biomarker for the condition. The development of such an objective test would lead to a step change in delirium prevention research, both pharmacological and multicomponent.
Notwithstanding these issues, we selected the CAM as our delirium assessment instrument. The CAM has been widely used in clinical practice and in research studies. 88 Administration of the CAM typically takes 5–10 minutes and is informed by brief, formal cognitive assessment. 65 Validation studies have reported high sensitivity (94–100%) and specificity (90–95%) in the hands of clinicians or researchers trained in its use. 87,89 Robust adherence to the processes described in the training manual is required to optimise diagnostic accuracy. The training process had not previously been delivered in the context of a large multicentre trial. We needed to develop a specific training programme to accommodate all 37 RAs. Using this training method, it proved possible to achieve delirium assessments for large numbers of patients with few missing data across geographically dispersed sites in multicentre studies. In the course of the feasibility trial, RAs successfully completed 5065 (89.7%) of the 5645 expected CAM assessments. The standardisation of multisite delirium assessments is an important contribution to research methodology and provides a much needed advance for the field. The recommendations based on our multisite feasibility study are contained in Table 19. 59
| Component | Requirement |
|---|---|
| Initial training and standardisation | |
| Didactic overview |
|
| Individual practice sessions |
|
| Pilot interviews with patients |
|
| Inter-rater reliability assessments (baseline standardisation) |
|
| CAM-only training |
|
| Ongoing monitoring and performance checks | |
| Coding sessions |
|
| Ongoing inter-rater reliability assessments (performance checks) |
|
| New staff training |
|
Alternative primary outcome
Embedded in our work was an investigation of a potential alternative, less resource-intensive primary outcome. It has been suggested that delirium may have a range of recovery trajectories from full recovery to continuing symptoms,90 with frailty84 or dementia90 as potential outcome mediators. The most pernicious trajectory is incomplete recovery at or shortly after discharge from hospital. These patients are at highest risk of adverse outcomes (A&E attendance, re-admission or death). 91 We hypothesised that an assessment to detect the presence of delirium symptoms at 30 days post discharge might provide a research-efficient method to identify between-group differences of clinical relevance; that is a single assessment rather than daily for 10 days during the inpatient stay. Unfortunately, owing to the lower than expected delirium incidence among the study participants, there were insufficient patients with persisting delirium symptoms (only six cases in all) to test this proposition adequately. Nonetheless, it remains an outcome of interest to investigate in future studies as it has prognostic appeal and requires a single assessment only.
Intervention fidelity
Parts of this section have been reproduced from Smith et al. 73 © The Author(s) 2020. Published by Oxford University Press on behalf of the British Geriatrics Society. This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/), which permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is properly cited.
Complex interventions such as the POD system of care are frequently implemented with lower fidelity than intended;35,92,93 an understanding of the intervention fidelity is integral to the internal validity of evaluation studies in relation to study outcome interpretation. 92 However, the methods to assess fidelity are imperfectly developed, in part because of continuing debate concerning the conceptualisation and measurement of implementation fidelity. 94 We examined the literature for appropriate frameworks to guide our fidelity assessment and identified the ‘conceptual framework for implementation’ as a potentially suitable approach. 72 This comprehensive framework includes the contribution of possible barriers to implementation and the assessment of moderating factors, including intervention complexity, facilitation strategy and participant responsiveness. However, as our randomised trial was the final part of a programme of interlinked studies, we had examined moderating factors and facilitation during the earlier projects. The intent in the current study was to investigate the extent to which the POD system of care was implemented and delivered as intended.
To our knowledge, this is the first multicentre, multicomponent delirium prevention trial to report intervention fidelity. We identified a set of 21 essential tasks as core components of the POD system of care. Accurate descriptive data are a key requirement when assessing fidelity to an intervention. 95 We used a range of methods, including non-participant observations of care delivery, case note reviews and examination of staff-completed delirium risk assessments and care plans, to obtain as complete a picture of intervention fidelity as possible. This information was then tabulated and evaluated and graded by 10 independent assessors using a standardised scoring process. Of the eight wards, two achieved an overall rating of high (≥ 80%) compliance, five achieved a rating of overall moderate (51–79%) compliance, and one was rated as low (≤ 50%) compliance. As the trial was designed as a pragmatic evaluation, the fidelity findings are likely to be generalisable to delirium prevention in routine care and provide an important context within which to interpret the outcomes of the clinical trial.
Fidelity to the individual essential tasks was variable. Of the actions relating to the 10 risk factors for delirium,8 care related to infection, poor nutrition and pain was generally the most consistently delivered, whereas multiple medications, cognitive impairment and sensory impairment received less consistent attention. Moreover, the mean overall score for intervention coverage (the patients who received the intervention compared with the at-risk patients) was low (mean score 7.9 out of a maximum of 16) and three wards were considered by all 10 assessors not to have continued the intervention for the whole of the prescribed 6 months.
Fidelity assessment has not commonly been incorporated into robust evaluations of multicomponent delirium prevention interventions. Two studies reported fidelity rates comparable to those achieved by the lower-scoring wards in our study. 70,96 In the first of these, a before-and-after study conducted on three elderly care wards in a single hospital site, the recorded fidelity to the several delirium risk factor modification protocols varied between 27% and 57%. 70 In the second, a controlled clinical trial involving patients with hip fracture, fidelity to recommendations made by the inpatient geriatric consultation team was 56.8%. 96 The former study was similar to our own in that the intervention was designed to be delivered as routine practice to all patients admitted to the ward, rather than being targeted at selected patients, as in other studies. 12,96,97
Three studies reported overall fidelity rates similar to those achieved by our higher-scoring wards. 12,97,98 In all of these studies, the intervention was implemented in a single site, compared with implementation in six hospitals in the present study. Consistent monitoring and support are probably easier to achieve in a single site and may result in increased fidelity to the intervention; our study arguably provides a more realistic, ‘real-world’ assessment of fidelity for the complex intervention of multicomponent delirium prevention. In addition, in the study that achieved a notably high rate of fidelity,12 the core intervention was delivered by an interdisciplinary team assisted by specially trained hospital volunteers as their sole remit, rather than by ward staff who may have competing calls on their time.
Conclusion
Prevention of delirium is highly desirable and multicomponent prevention interventions that aim to attenuate modifiable delirium risk factors have consistently been shown to reduce incident delirium in hospitalised patients by about one-third. NICE have recommended that multicomponent delirium prevention interventions should be incorporated into routine care. There is currently no delirium prevention system of care suitable for widespread implementation in the NHS. To address this, we developed the POD system of care. A multicentre, cluster randomised, pragmatic, feasibility study (714 participants, 16 wards, eight sites) showed that the intervention is capable of implementation and delivery in routine care. Fidelity to the intervention and preliminary estimates of clinical effectiveness and cost-effectiveness were acceptable.
Recommendation
The criteria for progression to a main trial [a minimum of six wards (75%) completing the POD manual milestone checklist and an overall recruitment rate of at least 10% of the potential recruitment pool] were fulfilled and a definitive multicentre, cluster randomised, pragmatic trial evaluation of the POD system of care should be designed and conducted in the NHS to obtain robust estimates of clinical effectiveness and cost-effectiveness.
Involvement of patients/public
We have had a long-established relationship with a group of 10–15 older, retired people in Bradford who volunteered to help and advise us with our delirium and dementia research. The group members volunteered from the much larger Bradford Older People’s Forum. The group first met in July 2007 to advise on a previous delirium research project. Since then, and following the start of the programme grant in 2009, the group met approximately every 4–6 months on 16 further occasions. The group has therefore been involved in the current programme grant since its inception, and members’ knowledge and expertise of our delirium work has expanded through their participation in this programme and related research on delirium in care homes and dementia in acute wards.
The group has been consulted on various aspects of the research, including the acceptability of delirium prevention for older people in hospital, the design and wording of the delirium prevention intervention, the design and wording of information and consent forms, and has been kept informed of the progress of the programme. Three members of the group were members of the Programme Implementation Team and, later, the Trial Management Group (project 3).
Acknowledgements
We would like to thank patients and their families who agreed to participate in the research programme. We would also like to thank the following and their colleagues for their support and participation in the research programme: Dr Paul Milnes, Airedale Hospital, Airedale NHS Foundation Trust; Dr Sion Jones, Ysbyty Gwynedd, Betsi Cadwaladr University Health Board; Dr Eric White, Bradford Royal Infirmary, Bradford Teaching Hospitals NHS Foundation Trust; Dr Gudrun Seebass, Calderdale and Huddersfield NHS Foundation Trust; Dr Jane Paisley, Harrogate District Hospital, Harrogate and District NHS Foundation Trust; Dr Julie Brache, Ipswich Hospital, East Suffolk and North Essex NHS Foundation Trust; Dr Nicola Turner, St James’ Hospital, Leeds Teaching Hospitals NHS Trust; Professor Tahir Masud, Queens Medical Centre, Nottingham University Hospitals NHS Trust; Dr Premila Fade, Poole Hospital, Poole Hospital NHS Foundation Trust; Dr Joyce Yeo, Wythenshawe Hospital, Manchester University NHS Foundation Trust; Dr David Heseltine, York Hospital, York Teaching Hospital NHS Foundation Trust; Dr Rachel Holt, Mid Yorkshire Hospitals NHS Trust; and Professor Alastair MacLullish, University of Edinburgh, for kindly providing video clips for CAM training.
We are pleased to acknowledge the help provided by our colleagues in the Academic Unit for Ageing and Stroke Research, the Clinical Trials Research Unit and the Academic Unit of Health Economics, including Dr Jenny Willson, Ms Zenia Ferreira, Dr Andrew Sutton and Ms Chantelle Browne for their contribution to the success of the programme.
This report is dedicated to the memory of Dr James George, Carlisle Royal Infirmary, who was actively engaged in delirium research throughout his career and who was a co-applicant on this research programme grant, and to the memory of Mrs Mary Godfrey, whose professional career was spent researching and improving the lives of older people with cognitive impairment, and who was a co-applicant and lead researcher on this research programme grant.
We are grateful to the members of the Programme Management Board/Trial Steering Committee, who have supported this work throughout this programme. Their input has been invaluable. The Programme Management Board/Trial Steering Committee members were as follows: Professor Finbarr Martin (chairperson), Professor Carl Thompson, Professor Deborah Sturdy, Mrs Christine Heaton, Mrs Barbara Smith, Mrs Margaret Harrison and Dr Caroline Nicholson.
We acknowledge the contribution of the HELP, LLC. Dr Sharon Inouye’s time was supported by Grants R24AG054259 (SKI), K07AG041835 (SKI) from the US National Institute on Aging.
All authors were members of the Programme Implementation Team. We would like to thank Mrs Rita Exley, Mr Ernie Lloyd and Mrs Anne Grice, who were the Consumer Group representatives on the Programme Implementation Team.
Contributions of authors
John Young (https://orcid.org/0000-0003-4085-9306) (Professor of Elderly Care Medicine) was the lead grant holder and Chief Investigator for the programme, and oversaw the design and running of the feasibility study.
John Green (https://orcid.org/0000-0003-1434-9345) (Research Programme Manager) was a co-applicant on the research programme grant and was the Programme Manager.
Mary Godfrey (https://orcid.org/0000-0002-2408-534X) (Reader in Health and Social Care) was a co-applicant on the research programme grant, and led and contributed to the set-up, design and analysis of data in projects 1 and 2. She also took a lead role in intervention development.
Jane Smith (https://orcid.org/0000-0002-6221-8844) (Senior Research Fellow) contributed to the collection and analysis of data in projects 1 and 2 and to intervention development. She took a lead role in the assessment and reporting of fidelity in the feasibility trial.
Francine Cheater (https://orcid.org/0000-0001-7392-4624) (Emeritus Professor of Public Health) was a co-applicant on the research programme grant and the lead for projects 1 and 2.
Claire Hulme (https://orcid.org/0000-0003-2077-0419) (Professor of Health Economics) was a co-applicant on the research programme grant, led the design of the health economic components of the feasibility study and was responsible for the health economic analysis, including reporting.
Michelle Collinson (https://orcid.org/0000-0003-3568-6455) (Principal Statistician) was responsible for the development of the statistical analysis plan, statistical analysis and the reporting of the feasibility study.
Suzanne Hartley (https://orcid.org/0000-0003-2346-9461) (Head of Trial Management) was responsible for the operational delivery of the feasibility study at the Clinical Trials Research Unit.
Shamaila Anwar (https://orcid.org/0000-0003-1765-7642) [Specialty Cluster Manager (Cancer, Surgery and Oral and Dental Health), NIHR Clinical Research Network] was involved in the operational delivery of the feasibility study at the Clinical Trials Research Unit.
Marie Fletcher (https://orcid.org/0000-0001-7545-1314) (Senior Trial Co-ordinator) was involved in the operational delivery of the feasibility study at the Clinical Trials Research Unit.
Gillian Santorelli (https://orcid.org/0000-0003-0427-1783) (Medical Statistician) was responsible for the development of the statistical analysis plan and statistical programming of the feasibility study.
David Meads (https://orcid.org/0000-0003-1369-2483) (Associate Professor of Health Economics) designed, supervised and conducted the economic evaluation and the reporting.
Keith Hurst (https://orcid.org/0000-0001-7364-6468) (Independent Researcher and Analyst) was a co-applicant on the research programme grant and led the staff workload study in project 2.
Najma Siddiqi (https://orcid.org/0000-0003-1794-2152) (Clinical Senior Lecturer in Psychiatry) was a co-applicant on the research programme grant and had input throughout the programme.
Dawn Brooker (https://orcid.org/0000-0001-8636-5147) (Professor of Dementia Studies) was a co-applicant on the research programme grant and had input into projects 1 and 2.
Elizabeth Teale (https://orcid.org/0000-0002-5923-3170) (Clinical Senior Lecturer in Geriatric Medicine) had input throughout the research programme and was involved in training the RAs.
Alex Brown (Director of Undergraduate Medical Education) was a co-applicant on the research programme grant and was the NHS manager.
Anne Forster (https://orcid.org/0000-0001-7466-4414) (Professor of Stroke Rehabilitation) was a co-applicant on the research programme grant and had input throughout the programme.
Amanda Farrin (https://orcid.org/0000-0002-2876-0584) (Professor of Clinical Trials and Evaluation of Complex Interventions) was a co-applicant on the research programme grant and the programme methodologist, and provided statistical oversight for the statistical analysis and reporting of the feasibility study.
Sharon Inouye (https://orcid.org/0000-0002-3663-2937) (Professor of Medicine) was a co-applicant on the research programme grant and had input throughout the programme.
Publications
Godfrey M, Smith J, Green J, Cheater F, Inouye SK, Young JB. Developing and implementing an integrated delirium prevention system of care: a theory driven, participatory research study. BMC Health Serv Res 2013;13:341. https://doi.org/10.1186/1472-6963-13-341
Young J, Cheater F, Collinson M, Fletcher M, Forster A, Godfrey M, et al. Prevention of delirium (POD) for older people in hospital: study protocol for a randomised controlled feasibility trial. Trials 2015;16:340. https://doi.org/10.1186/s13063-015-0847-2
Green JR, Smith J, Teale E, Collinson M, Avidan MS, Schmitt EM, et al. Use of the Confusion Assessment Method in multicentre delirium trials: training and standardisation. BMC Geriatr 2019;19:107. https://doi.org/10.1186/s12877-019-1129-8
Godfrey M, Green J, Smith J, Cheater F, Inouye SK, Hurst K, et al. Process of implementing and delivering the Prevention of Delirium system of care: a mixed method preliminary study. BMC Geriatrics 2020;20:1. https://doi.org/10.1186/s12877-019-1374-x
Smith J, Green J, Siddiqi N, Inouye SK, Collinson M, Farrin A, Young J. Investigation of ward fidelity to a multicomponent delirium prevention intervention during a multicentre, pragmatic, cluster randomised, controlled feasibility trial. Age Ageing 2020;49:648–55. https://doi.org/10.1093/ageing/afaa042
Young J, Green J, Farrin A, Collinson M, Hartley S, Smith J, et al. A multicentre, pragmatic, cluster randomised, controlled feasibility trial of the POD system of care. Age Ageing 2020;49:640–7. https://doi.org/10.1093/ageing/afaa044
Data-sharing statement
As a result of the methodologies involved in this study, the data generated beyond that included in the report may not be suitable for sharing; however, further information can be obtained from the corresponding author. All data requests should be submitted to the corresponding author for consideration. All data-sharing activities are subject to a data-sharing agreement after review by a subgroup of the study team, which will include data guarantor Professor Amanda Farrin.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data is used. #datasaveslives. You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health and Social Care.
References
- Godfrey M, Smith J, Green J, Cheater F, Inouye SK, Young JB. Developing and implementing an integrated delirium prevention system of care: a theory driven, participatory research study. BMC Health Serv Res 2013;13. https://doi.org/10.1186/1472-6963-13-341.
- Bélanger L, Ducharme F. Patients’ and nurses’ experiences of delirium: a review of qualitative studies. Nurs Crit Care 2011;16:303-15. https://doi.org/10.1111/j.1478-5153.2011.00454.x.
- Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA 2010;304:443-51. https://doi.org/10.1001/jama.2010.1013.
- Hshieh TT, Yue J, Oh E, Puelle M, Dowal S, Travison T, et al. Effectiveness of multicomponent nonpharmacologic delirium interventions: a meta-analysis. JAMA Intern Med 2015;175:512-20. https://doi.org/10.1001/jamainternmed.2014.7779.
- Martinez F, Tobar C, Hill N. Preventing delirium: should non-pharmacological, multicomponent interventions be used? A systematic review and meta-analysis of the literature. Age Ageing 2015;44:196-204. https://doi.org/10.1093/ageing/afu173.
- Siddiqi N, Harrison JK, Clegg A, Teale EA, Young J, Taylor J, et al. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev 2016;3. https://doi.org/10.1002/14651858.CD005563.pub3.
- Oh ES, Fong TG, Hshieh TT, Inouye SK. Delirium in older persons: advances in diagnosis and treatment. JAMA 2017;318:1161-74. https://doi.org/10.1001/jama.2017.12067.
- National Institute for Health and Care Excellence . Delirium: Diagnosis, Prevention and Management. Clinical Guideline 103 2010.
- Australian Health Ministers’ Advisory Council Health Care of Older Australians Standing Committee on behalf of the Australian Health Ministers’ Advisory Council . Delirium Care Pathways n.d. www.health.vic.gov.au/acute-agedcare (accessed November 2018).
- American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults . American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc 2015;63:142-50. https://doi.org/10.1111/jgs.13281.
- Inouye SK, Bogardus ST, Baker DI, Leo-Summers L, Cooney LM. MODELS OF GERIATRICS PRACTICE; the Hospital Elder Life Program: a model of care to prevent cognitive and functional decline in older hospitalized patients. J Am Geriatr Soc 2000;48:1697-706. https://doi.org/10.1111/j.1532-5415.2000.tb03885.x.
- Inouye SK, Bogardus ST, Charpentier PA, Leo-Summers L, Acampora D, Holford TR, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med 1999;340:669-76. https://doi.org/10.1056/NEJM199903043400901.
- Hshieh TT, Yang T, Gartaganis SL, Yue J, Inouye SK. Hospital Elder Life Program: systematic review and meta-analysis of effectiveness. Am J Geriatr Psychiatry 2018;26:1015-33. https://doi.org/10.1016/j.jagp.2018.06.007.
- Siddiqi N, Cheater F, Collinson M, Farrin A, Forster A, George D, et al. The PiTSTOP study: a feasibility cluster randomized trial of delirium prevention in care homes for older people. Age Ageing 2016;45:652-61. https://doi.org/10.1093/ageing/afw091.
- Inouye SK, Baker DI, Fugal P, Bradley EH. HELP Dissemination Project . Dissemination of the Hospital Elder Life Program: implementation, adaptation, and successes. J Am Geriatr Soc 2006;54:1492-9. https://doi.org/10.1111/j.1532-5415.2006.00869.x.
- Chen CC, Lin MT, Tien YW, Yen CJ, Huang GH, Inouye SK. Modified Hospital Elder Life Program: effects on abdominal surgery patients. J Am Coll Surg 2011;213:245-52. https://doi.org/10.1016/j.jamcollsurg.2011.05.004.
- Bradley EH, Webster TR, Schlesinger M, Baker D, Inouye SK. Patterns of diffusion of evidence-based clinical programmes: a case study of the Hospital Elder Life Program. Qual Saf Health Care 2006;15:334-8. https://doi.org/10.1136/qshc.2006.018820.
- Steunenberg B, van der Mast R, Strijbos MJ, Inouye SK, Schuurmans MJ. How trained volunteers can improve the quality of hospital care for older patients. A qualitative evaluation within the Hospital Elder Life Program (HELP). Geriatr Nurs 2016;37:458-63. https://doi.org/10.1016/j.gerinurse.2016.06.014.
- Milisen K, Foreman MD, Abraham IL, De Geest S, Godderis J, Vandermeulen E, et al. A nurse-led interdisciplinary intervention program for delirium in elderly hip-fracture patients. J Am Geriatr Soc 2001;49:523-32. https://doi.org/10.1046/j.1532-5415.2001.49109.x.
- Inouye SK, Bogardus ST, Williams CS, Leo-Summers L, Agostini JV. The role of adherence on the effectiveness of nonpharmacological interventions: evidence from the delirium prevention trial. Arch Int Med 2003;163:958-64. https://doi.org/10.1001/archinte.163.8.958.
- SteelFisher GK, Martin LA, Dowal SL, Inouye SK. Sustaining clinical programs during difficult economic times: a case series from the Hospital Elder Life Program. J Am Geriatr Soc 2011;59:1873-82. https://doi.org/10.1111/j.1532-5415.2011.03585.x.
- Rizzo JA, Bogardus ST, Leo-Summers L, Williams CS, Acampora D, Inouye SK. Multicomponent targeted intervention to prevent delirium in hospitalized older patients: what is the economic value?. Med Care 2001;39:740-52. https://doi.org/10.1097/00005650-200107000-00010.
- Leslie DL, Zhang Y, Bogardus ST, Holford TR, Leo-Summers LS, Inouye SK. Consequences of preventing delirium in hospitalized older adults on nursing home costs. J Am Geriatr Soc 2005;53:405-9. https://doi.org/10.1111/j.1532-5415.2005.53156.x.
- Rubin FH, Williams JT, Lescisin DA, Mook WJ, Hassan S, Inouye SK. Replicating the Hospital Elder Life Program in a community hospital and demonstrating effectiveness using quality improvement methodology. J Am Geriatr Soc 2006;54:969-74. https://doi.org/10.1111/j.1532-5415.2006.00744.x.
- Rubin FH, Neal K, Fenlon K, Hassan S, Inouye SK. Sustainability and scalability of the Hospital Elder Life Program at a community hospital. J Am Geriatr Soc 2011;59:359-65. https://doi.org/10.1111/j.1532-5415.2010.03243.x.
- Zaubler TS, Murphy K, Rizzuto L, Santos R, Skotzko C, Giordano J, et al. Quality improvement and cost savings with multicomponent delirium interventions: replication of the Hospital Elder Life Program in a community hospital. Psychosomatics 2013;54:219-26. https://doi.org/10.1016/j.psym.2013.01.010.
- Checkland P, Scholes J. Soft Systems Methodology in Action. Chichester: John Wiley & Sons; 1999.
- Grol RP, Bosch MC, Hulscher ME, Eccles MP, Wensing M. Planning and studying improvement in patient care: the use of theoretical perspectives. Milbank Q 2007;85:93-138. https://doi.org/10.1111/j.1468-0009.2007.00478.x.
- Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 2009;4. https://doi.org/10.1186/1748-5908-4-50.
- May C, Finch T. Implementing, embedding, and integrating practices: an outline of normalization process theory. Sociol 2009;43:535-54. https://doi.org/10.1177/0038038509103208.
- McIntyre A. Participatory Action Research. Qualitative Research Methods Series 52. London: SAGE Publications Ltd; 2008.
- May C. A rational model for assessing and evaluating complex interventions in health care. BMC Health Serv Res 2006;6. https://doi.org/10.1186/1472-6963-6-86.
- Hawe P, Shiell A, Riley T, Gold L. Methods for exploring implementation variation and local context within a cluster randomised community intervention trial. J Epidemiol Community Health 2004;58:788-93. https://doi.org/10.1136/jech.2003.014415.
- Kaplan HC, Brady PW, Dritz MC, Hooper DK, Linam WM, Froehle CM, et al. The influence of context on quality improvement success in health care: a systematic review of the literature. Milbank Q 2010;88:500-59. https://doi.org/10.1111/j.1468-0009.2010.00611.x.
- Greenhalgh T, Robert G, Macfarlane F, Bate P, Kyriakidou O. Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Q 2004;82:581-629. https://doi.org/10.1111/j.0887-378X.2004.00325.x.
- Øvretveit J, Bate P, Cleary P, Cretin S, Gustafson D, McInnes K, et al. Quality collaboratives: lessons from research. Qual Saf Health Care 2002;11:345-51. https://doi.org/10.1136/qhc.11.4.345.
- Corbin JM, Strauss A. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. Thousand Oaks, CA: SAGE Publications Inc.; 2008.
- Fixsen DL, Naoom, SF, Blase KA, Friedman RM, Wallace F. Implementation Research: A Synthesis of the Literature. Tampa, FL: University of South Florida, Louis de la Parte Florida Mental Health Institute, The National Implementation Research Network (FMHI Publication #231); 2005.
- Forsetlund L, Bjørndal A, Rashidian A, Jamtvedt G, O’Brien MA, Wolf F, et al. Continuing education meetings and workshops: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2009;2. https://doi.org/10.1002/14651858.CD003030.pub2.
- Lukas CV, Holmes SK, Cohen AB, Restuccia J, Cramer IE, Schwartz M, et al. Transformational change in healthcare systems: an organizational model. Health Care Manage Rev 2007;32:309-20. https://doi.org/10.1097/01.HMR.0000296785.29718.5d.
- Bate P, Mendel P, Robert G. Organising for Quality: The Improvement Journeys of Leading Hospitals in Europe and the United States. Oxford: Radcliffe Publishing; 2008.
- Powell AE, Rushmer RK, Davies HT. A Systematic Narrative Review of Quality Improvement Models in Health Care. Glasgow: Quality Improvement Scotland (NHS QIS); 2009.
- Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med 2006;355:2725-32. https://doi.org/10.1056/NEJMoa061115.
- Jamtvedt G, Young JM, Kristoffersen DT, O’Brien MA, Oxman AD. Audit and feedback: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2006;2. https://doi.org/10.1002/14651858.CD000259.pub2.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and Evaluating Complex Interventions: New Guidance. Medical Research Council; n.d.
- Godfrey M, Green J, Smith J, Cheater F, Inouye SK, Hurst K, et al. Process of implementing and delivering the Prevention of Delirium system of care: a mixed method preliminary study. BMC Geriatr 2020;20. https://doi.org/10.1186/s12877-019-1374-x.
- Yin R. Case Study Research: Design and Methods. Thousand Oaks, CA: SAGE Publications, Inc.; 1994.
- Hurst K. Relationships between patient dependency, nursing workload and quality. Int J Nurs Stud 2005;42:75-84. https://doi.org/10.1016/j.ijnurstu.2004.05.011.
- Hurst K. UK ward design: patient dependency, nursing workload, staffing and quality – an observational study. Int J Nurs Stud 2008;45:370-81. https://doi.org/10.1016/j.ijnurstu.2006.09.007.
- Care Quality Commission, NHS England . Adult Inpatient Survey 2009 n.d. https://nhssurveys.org/surveys/survey/02-adults-inpatients/year/2009/ (accessed 14 November 2020).
- Patterson M, Nolan M, Rick J, Brown J, Adams R, Musson G. From Metrics to Meaning: Culture Change and Quality of Acute Hospital Care for Older People. Report for the National Institute for Health Research Service Delivery and Organisation Programme 2011.
- Miles MB, Huberman AM. Qualitative Data Analysis: An Expanded Sourcebook. Thousand Oaks, CA: SAGE Publications, Inc.; 1994.
- Charmaz K. Constructing Grounded Theory: A Practical Guide Through Qualitative Analysis. London: SAGE Publications Ltd; 2006.
- Young J, Cheater F, Collinson M, Fletcher M, Forster A, Godfrey M, et al. Prevention of delirium (POD) for older people in hospital: study protocol for a randomised controlled feasibility trial. Trials 2015;16. https://doi.org/10.1186/s13063-015-0847-2.
- Young J, Green J, Farrin A, Collinson M, Hartley S, Smith J, et al. A multicentre, pragmatic, cluster randomised, controlled feasibility trial of the POD system of care. Age Ageing 2020;49:640-7. https://doi.org/10.1093/ageing/afaa044.
- Ball J. Guidance on Safe Nurse Staffing Levels in the UK n.d. www.rcn.org.uk/about-us/policy-briefings/pol-003860 (accessed November 2018).
- Hayes N, Ball J. Safe Staffing for Older People’s Wards n.d. https://my.rcn.org.uk/__data/assets/pdf_file/0009/476379/004280.pdf (accessed November 2018).
- Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. Ann Intern Med 1990;113:941-8. https://doi.org/10.7326/0003-4819-113-12-941.
- Green JR, Smith J, Teale E, Collinson M, Avidan MS, Schmitt EM, et al. Use of the Confusion Assessment Method in multicentre delirium trials: training and standardisation. BMC Geriatr 2019;19. https://doi.org/10.1186/s12877-019-1129-8.
- Hodkinson HM. Evaluation of a mental test score for assessment of mental impairment in the elderly. Age Ageing 1972;1:233-8. https://doi.org/10.1093/ageing/1.4.233.
- O’Regan NA, Ryan DJ, Boland E, Connolly W, McGlade C, Leonard M, et al. Attention! A good bedside test for delirium?. J Neurol Neurosurg Psychiatry 2014;85:1122-31. https://doi.org/10.1136/jnnp-2013-307053.
- Nouri FM, Lincoln NB. An extended ADL scale for use with stroke patients. Clin Rehabil 1987;1:301-5. https://doi.org/10.1177/026921558700100409.
- Westhuis D, Thyer BA. Development and validation of the clinical anxiety scale: a rapid assessment instrument for empirical practice. Educ Psychol Meas 1989;49:153-63. https://doi.org/10.1177/0013164489491016.
- Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol 1986;5:165-73. https://doi.org/10.1300/J018v05n01_09.
- Inouye SK. The Confusion Assessment Method (CAM): Short CAM Training Manual and Coding Guide. Boston, MA: Hospital Elder Life Program, LLC; 2014.
- Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245-51. https://doi.org/10.1016/0895-4356(94)90129-5.
- Royal College of Physicians . National Early Warning Score (NEWS): Standardising the Assessment of Acute Illness Severity in the NHS. Report of a Working Party 2012.
- EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- National Audit of Intermediate Care . Report 2013 n.d. https://static1.squarespace.com/static/58d8d0ffe4fcb5ad94cde63e/t/58f08f9fe3df28353c556761/1492160435696/naic-report-2013.pdf (accessed November 2018).
- Holt R, Young J, Heseltine D. Effectiveness of a multi-component intervention to reduce delirium incidence in elderly care wards. Age Ageing 2013;42:721-7. https://doi.org/10.1093/ageing/aft120.
- Nixon J, Nelson EA, Cranny G, Iglesias CP, Hawkins K, Cullum NA, et al. Pressure relieving support surfaces: a randomised evaluation. Health Technol Assess 2006;10. https://doi.org/10.3310/hta10220.
- Carroll C, Patterson M, Wood S, Booth A, Rick J, Balain S. A conceptual framework for implementation fidelity. Implement Sci 2007;2. https://doi.org/10.1186/1748-5908-2-40.
- Smith J, Green J, Siddiqi N, Inouye SK, Collinson M, Farrin A, et al. Investigation of ward fidelity to a multicomponent delirium prevention intervention during a multicentre, pragmatic, cluster randomised, controlled feasibility trial. Age Ageing 2020;49:648-55. https://doi.org/10.1093/ageing/afaa042.
- Borrelli B. The assessment, monitoring, and enhancement of treatment fidelity in public health clinical trials. J Public Health Dent 2011;71:52-63. https://doi.org/10.1111/j.1752-7325.2011.00233.x.
- Clegg A, Young JB. Which medications to avoid in people at risk of delirium: a systematic review. Age Ageing 2011;40:23-9. https://doi.org/10.1093/ageing/afq140.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 n.d. www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781 (accessed November 2018).
- Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. York: Centre for Health Economics, University of York; 1999.
- Eldridge SM, Costelloe CE, Kahan BC, Lancaster GA, Kerry SM. How big should the pilot study for my cluster randomised trial be?. Stat Methods Med Res 2016;25:1039-56. https://doi.org/10.1177/0962280215588242.
- Davis D, MacLullich A. Understanding barriers to delirium care: a multicentre survey of knowledge and attitudes amongst UK junior doctors. Age Ageing 2009;38:559-63. https://doi.org/10.1093/ageing/afp099.
- Teodorczuk A, Reynish E, Milisen K. Improving recognition of delirium in clinical practice: a call for action. BMC Geriatr 2012;12. https://doi.org/10.1186/1471-2318-12-55.
- Kennelly SP, Morley D, Coughlan T, Collins R, Rochford M, O’Neill D. Knowledge, skills and attitudes of doctors towards assessing cognition in older patients in the emergency department. Postgrad Med J 2013;89:137-41. https://doi.org/10.1136/postgradmedj-2012-131226.
- Rockwood K. Out of the furrow and into the fire: where do we go with delirium?. CMAJ 2002;167:763-4.
- Inouye SK, Schlesinger MJ, Lydon TJ. Delirium: a symptom of how hospital care is failing older persons and a window to improve quality of hospital care. Am J Med 1999;106:565-73. https://doi.org/10.1016/S0002-9343(99)00070-4.
- Teale E, Young J. Multicomponent delirium prevention: not as effective as NICE suggest?. Age Ageing 2015;44:915-17. https://doi.org/10.1093/ageing/afv120.
- Rowland M, Torgerson D. Understanding controlled trials: what are pragmatic trials?. BMJ 1998;316. https://doi.org/10.1136/bmj.316.7127.285.
- Heim N, van Stel HF, Ettema RG, van der Mast RC, Inouye SK, Schuurmans MJ. HELP! Problems in executing a pragmatic, randomized, stepped wedge trial on the Hospital Elder Life Program to prevent delirium in older patients. Trials 2017;18. https://doi.org/10.1186/s13063-017-1933-4.
- Wong CL, Holroyd-Leduc J, Simel DL, Straus SE. Does this patient have delirium? Value of bedside instruments. JAMA 2010;304:779-86. https://doi.org/10.1001/jama.2010.1182.
- Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc 2008;56:823-30. https://doi.org/10.1111/j.1532-5415.2008.01674.x.
- van Velthuijsen EL, Zwakhalen SM, Warnier RM, Mulder WJ, Verhey FR, Kempen GI. Psychometric properties and feasibility of instruments for the detection of delirium in older hospitalized patients: a systematic review. Int J Geriatr Psychiatry 2016;31:974-89. https://doi.org/10.1002/gps.4441.
- Cole MG, Ciampi A, Belzile E, Zhong L. Persistent delirium in older hospital patients: a systematic review of frequency and prognosis. Age Ageing 2009;38:19-26. https://doi.org/10.1093/ageing/afn253.
- Cole MG, McCusker J, Bailey R, Bonnycastle M, Fung S, Ciampi A, et al. Partial and no recovery from delirium after hospital discharge predict increased adverse events. Age Ageing 2017;46:90-5. https://doi.org/10.1093/ageing/afw153.
- Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol 2004;23:443-51. https://doi.org/10.1037/0278-6133.23.5.443.
- Durlak JA, DuPre EP. Implementation matters: a review of research on the influence of implementation on program outcomes and the factors affecting implementation. Am J Community Psychol 2008;41:327-50. https://doi.org/10.1007/s10464-008-9165-0.
- Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health 2011;38:65-76. https://doi.org/10.1007/s10488-010-0319-7.
- Mowbray CT, Holter MC, Teague GB, Bybee D. Fidelity criteria: development, measurement, and validation. Am J Eval 2003;24:315-40. https://doi.org/10.1177/109821400302400303.
- Deschodt M, Braes T, Broos P, Sermon A, Boonen S, Flamaing J, et al. Effect of an inpatient geriatric consultation team on functional outcome, mortality, institutionalization, and readmission rate in older adults with hip fracture: a controlled trial. J Am Geriatr Soc 2011;59:1299-308. https://doi.org/10.1111/j.1532-5415.2011.03488.x.
- Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc 2001;49:516-22. https://doi.org/10.1046/j.1532-5415.2001.49108.x.
- Vidán MT, Sánchez E, Alonso M, Montero B, Ortiz J, Serra JA. An intervention integrated into daily clinical practice reduces the incidence of delirium during hospitalization in elderly patients. J Am Geriatr Soc 2009;57:2029-36. https://doi.org/10.1111/j.1532-5415.2009.02485.x.
- Crow R, Gage H, Hampson S, Hart J, Kimber A, Storey L, et al. The measurement of satisfaction with healthcare: implications for practice from a systematic review of the literature. Health Technol Assess 2002;6. https://doi.org/10.3310/hta6320.
- Bergmann MA, Murphy KM, Kiely DK, Jones RN, Marcantonio ER. A model for management of delirious postacute care patients. J Am Geriatr Soc 2005;53:1817-25. https://doi.org/10.1111/j.1532-5415.2005.53519.x.
- Department of Health and Social Care . National Service Framework for Older People 2001.
- Siddiqi N, Stockdale R, Britton AM, Holmes J. Interventions for preventing delirium in hospitalised patients. Cochrane Database Syst Rev 2007;2. https://doi.org/10.1002/14651858.CD005563.pub2.
- Morandi A, Jackson JC, Ely EW. Delirium in the intensive care unit. Int Rev Psychiatry 2009;21:43-58. https://doi.org/10.1080/09540260802675296.
- Tabet N, Hudson S, Sweeney V, Sauer J, Bryant C, Macdonald A, et al. An educational intervention can prevent delirium on acute medical wards. Age Ageing 2005;34:152-6. https://doi.org/10.1093/ageing/afi031.
- Institute for Innovation and Improvement . Care Pathways for Frail Older People 2006.
- Caplan GA, Harper EL. Recruitment of volunteers to improve vitality in the elderly: the REVIVE study. Intern Med J 2007;37:95-100. https://doi.org/10.1111/j.1445-5994.2007.01265.x.
- Marcantonio ER, Flacker JM, Michaels M, Resnick NM. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc 2000;48:618-24. https://doi.org/10.1111/j.1532-5415.2000.tb04718.x.
- Lundström M, Edlund A, Karlsson S, Brännström B, Bucht G, Gustafson Y. A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. J Am Geriatr Soc 2005;53:622-8. https://doi.org/10.1111/j.1532-5415.2005.53210.x.
- Caplan GA, Coconis J, Board N, Sayers A, Woods J. Does home treatment affect delirium? A randomised controlled trial of rehabilitation of elderly and care at home or usual treatment (the REACH-OUT trial). Age Ageing 2006;35:53-60. https://doi.org/10.1093/ageing/afi206.
- Webster JR, Chew RB, Mailliard L, Moran MB. Improving clinical and cost outcomes in delirium: use of practice guidelines and a delirium care team. Ann Longterm Care 1999;7:128-34.
- O’Keeffe S, Lavan J. The prognostic significance of delirium in older hospital patients. J Am Geriatr Soc 1997;45:174-8. https://doi.org/10.1111/j.1532-5415.1997.tb04503.x.
- Bourdel-Marchasson I, Vincent S, Germain C, Salles N, Jenn J, Rasoamanarivo E, et al. Delirium symptoms and low dietary intake in older inpatients are independent predictors of institutionalization: a 1-year prospective population-based study. J Gerontol A Biol Sci Med Sci 2004;59:350-4. https://doi.org/10.1093/gerona/59.4.m350.
- Rockwood K, Cosway S, Carver D, Jarrett P, Stadnyk K, Fisk J. The risk of dementia and death after delirium. Age Ageing 1999;28:551-6. https://doi.org/10.1093/ageing/28.6.551.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2011. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
- Adamis D, Treloar A, Martin FC, Macdonald AJ. Recovery and outcome of delirium in elderly medical inpatients. Arch Gerontol Geriatr 2006;43:289-98. https://doi.org/10.1016/j.archger.2005.11.005.
- Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing 2006;35:350-64. https://doi.org/10.1093/ageing/afl005.
- NHS Digital . Hospital Episode Statistics, Admitted Patient Care – England, 2009–2010 n.d. https://digital.nhs.uk/data-and-information/publications/statistical/hospital-admitted-patient-care-activity/hospital-episode-statistics-admitted-patient-care-england-2009-10 (accessed November 2018).
- Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002;21:271-92. https://doi.org/10.1016/S0167-6296(01)00130-8.
- Duppils GS, Wikblad K. Cognitive function and health-related quality of life after delirium in connection with hip surgery. A six-month follow-up. Orthop Nurs 2004;23:195-203. https://doi.org/10.1097/00006416-200405000-00009.
- NHS Employers . Pay Circular (M&Amp;D) 1 2011 n.d. https://nhsemployers.org/-/media/Employers/Publications/Pay-circulars/Pay-Circular-_MD_-1-2011.pdf?la=en&hash=98810A32E5FC25B812F882A5F430E09C4571851B (accessed 15 February 2021).
- Netten A, Bebbington A, Darton R, Forder J, Miles K. 1996 Survey of Care Homes for Elderly People n.d. www.pssru.ac.uk/pub/dp1423∼2.pdf (accessed November 2018).
- Briggs AK, Claxton, Sculpher M. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Eeles EM, Hubbard RE, White SV, O’Mahony MS, Savva GM, Bayer AJ. Hospital use, institutionalisation and mortality associated with delirium. Age Ageing 2010;39:470-5. https://doi.org/10.1093/ageing/afq052.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
- Department of Health and Social Care . NHS Reference Costs 2013 to 2014 n.d. www.gov.uk/government/publications/nhs-reference-costs-2013-to-2014 (accessed January 2019).
Appendix 1 Data collection
We conducted a before-and-after study at each site using quantitative and qualitative data, collected prospectively at baseline and over the 6 months of intervention delivery, to determine the feasibility and acceptability of the POD programme intervention. We assumed a conservative 14-day average length of stay, a conservative 50% of patients at risk of delirium and 25 beds per ward, and estimated that approximately 150 patients per site should receive the delirium prevention system of care over 6 months.
Data collection comprised the following:
-
patient description
-
process of implementation planning and delivery
-
take-up of the intervention protocols
-
impact of the intervention on staff workload
-
impact on patient satisfaction with care
-
acceptability to patients, carers, staff and volunteers.
A summary of data collection at each site is shown in Table 20.
| Data collection | Ward | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Patient description | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ |
| Process of implementation planning and delivery | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Take-up of the intervention | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ |
| Impact of the intervention on nurse workload | ✓ | ✓ | ✓ | ✓ | ✗ | ✓a |
| Impact on patient and carer satisfaction with care | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ |
| Acceptability to patients, carers, staff and volunteers | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ |
The reasons why not all data were collected at each ward were as follows:
-
Patient description –
-
Ward 5 did not proceed to implementation and delivery of the POD system of care.
-
-
Take-up of the intervention –
-
Ward 4 did not develop paperwork for the POD programme.
-
Ward 5 did not proceed to implementation and delivery of the POD system of care.
-
Ward 6 required that all paperwork be filed in patient notes. Therefore, we did not have access to this information, although copies of some individual care plans (patient details removed) were provided.
-
-
Impact of the intervention on nurse workload –
-
Ward 5 did not proceed to implementation and delivery of the POD system of care.
-
Ward 6: the second phase of data collection was not carried out, as the nature of the ward changed substantially from surgical and orthopaedic patients (all ages) to hip fracture patients (older people). The ward was also relocated between phases, with a consequent change of physical environment.
-
-
Impact on patient and carer satisfaction with care –
-
Ward 5 did not proceed to implementation and delivery of the POD system of care.
-
-
Acceptability to patients, carers, staff and volunteers –
-
Ward 5 did not proceed to implementation and delivery of the POD system of care.
-
Patient description
We collected anonymous contextual information (age, sex, type of residence, length of hospital stay, discharge destination) to describe all of the patients admitted to the participating wards over the periods of implementation and delivery of the delirium prevention system of care. These data were obtained from the patient administration system from each participating trust.
Process of implementation planning and delivery
We developed an in-depth picture of the process of POD planning, implementation and delivery, drawing on formal interviews with staff and volunteers, observation of ward practices and routines, MDT meetings and volunteer training sessions, informal conversations with staff and the collection of relevant documents. In addition, we constructed a ward diary/events log to provide a contemporaneous account of the process of implementing and delivering the POD intervention, communication with teams, problems encountered, solutions arrived at, and contextual factors that affected implementation planning and delivery.
Take up of the intervention
The NICE delirium guidelines8 recommend that people at risk of delirium should be assessed within 24 hours of admission for 10 clinical factors that contribute to delirium. In accordance with these recommendations, when implementing the POD programme, we expected ward teams to introduce a system for the assessment and recording of delirium risk in patients admitted to the ward. Information regarding these clinical factors was needed to enable effective targeting of delirium prevention interventions.
In addition, to facilitate the consistent delivery of appropriate interventions, ward teams were asked to develop and introduce systems for planning what delirium prevention interventions were required, communicating information to staff concerning the prevention activities to be undertaken for each patient each day and recording when an activity had been completed.
An example risk assessment form and an example of a form for planning, communicating and recording delirium prevention activities (the ‘Daily Delirium Prevention Plan’) were included in the POD programme materials. Teams were able to use or modify the example documentation or they could develop their own documentation, as desired.
One of the features of the POD programme was the involvement of volunteers in the delivery of some of the delirium prevention interventions to patients at risk of delirium. To enable volunteers to work effectively and with confidence, the tasks expected of them on a day-to-day to basis needed to be set out clearly in advance. Ward teams were therefore asked to put in place a system to ensure that information about patients and appropriate interventions was available to volunteers at the start of their shift. How this was to be achieved was open and flexible, although sites could choose to use or adapt the example form provided in the POD programme if they wished.
All wards instituted one or more of these forms, with the exception of ward 4. We collected anonymised copies of completed forms from wards 1, 2 and 3 for analysis of the take-up of the intervention. We were not able to obtain the forms from ward 6 (maintained within patient case notes).
Impact of the intervention on nurse workload
The purpose of this substudy was to assess the impact of the POD intervention on staff workload.
Method
We obtained ward nurse workload data at the start of the POD implementation phase and during the delivery phase of POD implementation on participating wards to gauge the impact of POD on ward staff activity and modification of workload. We used the ‘dependency-acuity’ method, a standardised approach previously developed by a co-applicant (KH)48 and widely used in the NHS. The approach is based on previous workload planning techniques, but has the additional advantage of taking into account the dependency of patients in calculating the workload burden. This approach combines ward-based observations of the activities of staff, undertaken by non-participant observers, linked to the dependency of patients, to produce an overall assessment of the ward staff activity. To obtain a broad sample of the nurses’ workload, we undertook ward observations during the 24-hour period. We undertook the ward observations during six shifts (two early, two late and two night shifts). These observations were undertaken during the implementation phase (i.e. before POD had become established on the ward) and during the delivery phase when POD had been in use on the wards for some time.
Data collection
Data collection comprised the following:
-
A record of ward bed occupancy.
-
A measure of the dependency of patients on nursing staff to meet their needs.
As the dependency of patients affects the workload burden of staff, it was important to take account of this in the analysis. The method we adopted used a simple indicator to signify patients’ reliance on nurses to meet their needs. Patients were rated, in consultation with a senior member of the ward nursing team, on each of the categories in Table 21 on a score of 1 to 4 (lower to higher dependency).
Scores were summated to produce an overall level of dependency:
-
Independent (6–7).
-
Between independent and dependent (8–13).
-
Dependent (14–22).
-
Highly dependent (23–24).
-
Patients with a dependency level of 1 were virtually independent of nurses. Patients with a dependency level of 4, on the other hand, were dependent on nurses for most, if not all, of their needs.
-
Recording of ward nursing staff activity.
The activity and grade of each nursing member of staff and each volunteer were recorded by the non-participant observer at 10-minute intervals. Activites were chosen from a predetermined list under four headings: direct face-to-face care, indirect care, associated work and personal time.
Direct face-to-face care comprised 15 activities, including, for example, medication. Indirect care comprised five activities, including, for example, telephone conversation with a patient’s relative. Associated work comprised eight activities and included non-nursing work such as hotel-type duties. Personal time comprised four activities, for example drink breaks and unoccupied time (Table 22).
| Dependency assessment | Score |
|---|---|
| Nursing attention | |
| (a) Constant | 4 |
| (b) Every 2 hours or more often | 3 |
| (c) Every 4 hours | 2 |
| (d) Twice daily or less | 1 |
| Washing and dressing | |
| (a) Daily bed bath or open bath needing two carers | 4 |
| (b) Daily bath needing one carer | 3 |
| (c) Assistance needed to wash and dress | 2 |
| (d) Independent – relative attends to needs | 1 |
| Using the toilet | |
| (a) Incontinent or catheterised | 4 |
| (b) Help every 4 hours or more needed to use the toilet | 3 |
| (c) Needs help to use the toilet | 2 |
| (d) Independent | 1 |
| Moving | |
| (a) Immobile | 4 |
| (b) Two carers needed to help patient walk or move around | 3 |
| (c) Needs help to walk or move around | 2 |
| (d) Independent | 1 |
| Eating and drinking | |
| (a) Fed artificially (e.g. nasogastrically, intravenously) | 4 |
| (b) Depends totally on carer to eat and drink | 3 |
| (c) Needs help to eat and drink | 2 |
| (d) Independent once meal is served | 1 |
| Pressure area care | |
| (a) Necrotic areas | 4 |
| (b) High risk, needing care every 2 hours or more | 3 |
| (c) Moderate risk, needing care every 4 hours | 2 |
| (d) Low risk, needing twice daily check or less | 1 |
| Relatives | |
| (a) Relative needs constant explanation/reassurance/support/help | 4 |
| (b) Relative needs frequent help/support | 3 |
| (c) Relative needs occasional help/support | 2 |
| (d) Minimum help/support needed | 1 |
| Activity | Definition |
|---|---|
| Direct care | |
| Outpatient | Care of an outpatient on ward |
| Medical procedures | Extended-role procedure |
| Communicating with a patient | Including support/teaching/showing/explaining/assessing/observing |
| Nutrition | Help with diet and fluids, including via nasogastric/percutaneous endoscopic gastrostomy tubes and including supplements/special diets |
| Hygiene | Assist with hygiene and comfort cares and preventative pressure area care |
| Elimination | Assist/assess/record all excreted fluids/matter |
| Medication | Administer by all routes. Check, record, monitor, maintain equipment. Monitor self-medication |
| Movement | Assist in/around bed and ward, including transferring and performance of exercises |
| Vital signs | Measure, monitor, record and interpret temperature, pulse, respiration/blood pressure/saturation/blood sugar/neurological signs, weight |
| Specimens | Obtain specimens for laboratory/ward testing |
| Nursing procedure | Hand wash before and after patient care. Prepare equipment for treatments. Perform nursing procedures, for example dressings, catheterisation, enemas, pressure area care. Ensure treatments applied and maintained. Care of the deceased and their families |
| Escorting/admitting/discharging | Assist in safe transfer/discharge including plan, check identity and complete documentation. Admit or discharge to/from the ward. Discharge planning. Transfer a body to the mortuary |
| Teaching | Instruct patients |
| Assisting doctors | On ward round and during procedures/care |
| Assisting others | With patient intervention/treatments |
| Indirect care | |
| Charting | Commence/maintain nursing records |
| Reporting | Give/receive patient information (handovers, MDT meetings). Use of computer patient administration system for recording/retrieving patient information |
| Communicating with staff | Liaison with other health/social care professionals regarding specific patient requirements |
| Communicating with relatives | Support and information regarding a patient and any other issues |
| Teaching | Receive or provide professional/work-related instruction or assessment |
| Associated activities | |
| Cleaning | Organise, tidy, clean ward areas not associated with specific patient care. Empty bins and sharps bins. Dispose of soiled linen. Clean equipment and furnishings |
| Meals and drinks | Prepare for and participate in meals and drinks distribution/clearing. Change water jugs |
| Clerical | Menu lists, patient dependency records, daily bed returns, other clerical work including notes and identification bracelets, etc. Use of computer for purposes other than patient details/information |
| Communication | Administering paperwork and telephone calls (including advice line calls) |
| Errands off-ward | Deliver/collect/look for items/person, etc. |
| Supplies | Safety checks on equipment. Maintain ward supplies, restock emergency trolleys, etc. |
| Meeting/in-service training | Attend management and administrative meetings |
| Supervision/mentoring | Supervise staff, complete staff reports and appraisals. Orientate new staff members |
| Non-productive time | |
| Personal | |
| Unoccupied | |
| Breaks | |
| Other | |
Data collection was undertaken by three members of the research team (two nurses and one physiotherapist) who had previously been instructed in the method by the developer of the approach and had undertaken practice sessions in its use.
Analysis
Keith Hurst inputted the data to a Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) spreadsheet and analysed them to generate the following:
-
Bed occupancy.
This was a measure of how full the ward was, an important consideration when comparing results across time.
-
Patient dependency.
The dependency of patients affects the workload of staff. It was therefore important that this was taken into account in the analysis. We used a simple indicator to assess patients’ reliance on nurses to meet their needs (see Table 21).
-
Workload index
The workload index is a single value calculated from bed occupancy, patient dependency and direct care time. It indicates how ‘busy’ the ward care team is. Higher workload values indicate busier wards (in terms of workload).
Dependency data and staff activity data were entered into an Excel spreadsheet. Analysis was undertaken by Keith Hurst. The following analyses were undertaken: comparisons before and after implementation of the POD system of care for all wards and for each ward separately.
Impact on patient and carer satisfaction with care
Introduction
There is consistent evidence that patient satisfaction closely reflects patient–practitioner relationships, including information provision:99 both might be expected to be improved by the delirium prevention system of care. We therefore conducted a patient satisfaction survey at baseline (i.e. during the intervention implementation phase) and we repeated the survey during the intervention delivery phase.
Method
For 3 months (1 month in ward 6) during both the 6-month POD programme implementation phase and the 6-month delivery phase, we asked the ward staff to give consecutive patients about to be discharged from the participating wards an envelope containing a questionnaire, with a request asking them to complete it anonymously, with the assistance of a carer if necessary. We provided a prepaid envelope for the questionnaire’s return. The questionnaire contained questions with particular relevance to person-centred care from the Care Quality Commission’s national patient survey instrument. 50 Questions were grouped under the following subheadings:
-
the hospital and ward (four questions)
-
doctors (three questions)
-
nurses (three questions)
-
your care and treatment (10 questions)
-
leaving hospital (10 questions)
-
overall (four questions).
We also included in the envelope a questionnaire for carers about their experiences of care. We used questions taken from the NIHR Service Delivery and Organisation project From Metrics to Meaning: Culture Change and Quality of Acute Hospital Care for Older People. 51 These questions consisted of three scales:
-
Giving my relative the best – six items to assess carers’ perceptions of the level of care their relatives received.
-
Could do better – three items measuring the extent to which carers felt that their relative received negative experiences of care.
-
Feeling significant – 10 items measuring the extent to which carers felt significant and involved in their relatives’ treatment.
Patients and carers were also asked to comment in free text whether or not there was anything particularly good about the hospital care, whether or not there was anything that could be improved and whether or not they had any other comments.
We collected any undistributed quesionnaires during the implementation phase and so we were able to assess how many had been given to discharged patients by ward staff. Unfortunately, we were not able to repeat this process in the delivery phase. To be able to compare the responses in both phases of the questionnaire distribution, we estimated the number of questionnaires returned as a percentage of patient discharges during both the intervention and delivery periods.
Data analysis
We entered patient and carer responses into a database (IBM SPSS Statistics version 20.0, IBM Corporation, Armonk, NY, USA) for analysis. We tabulated the results and investigated differences between responses during the implementation phase and responses during the delivery phase for patient and carer questions using the chi-squared test, including Yates’s correction for continuity. We collapsed 2 × k tables, where > 20% of cells had an expected frequency of lower than five, to 2 × 2 tables. We used a Bonferroni correction adjustment in assessing the significance of the results because of the danger of multiple testing:
Carer satisfaction questionnaire responses were scored 5 to 1 (positive response to negative response). We totalled subsections and divided totals by the number of questions in the subsection to obtain the mean score for each subsection. We compared these mean scores for the implementation phase and the delivery phase using the Mann–Whitney U-test. We used a Bonferroni correction adjustment in assessing the significance of the results because of the danger of multiple testing:
Patient and carer comments were analysed for common themes. In each theme, comments were categorised as either positive or negative and were then compiled into reports.
Acceptability to patients, carers, staff and volunteers
Patients and carers
Patients and carers were asked in the delivery phase questionnaire if they would be willing to be contacted to be interviewed about their experiences of hospital care. Box 1 presents the topic guide for the interviews.
Introduce researcher; outline of study; confidentiality; timing
A range of questions will be asked to explore patients’ and carers’ experiences of hospital care-
Environment, for example:
-
presence of noise
-
level of privacy afforded.
-
-
Care and treatment, for example:
-
involvement in decisions about care and treatment
-
perception of quality of care.
-
-
Staff, for example:
-
availability of staff/opportunity to talk to staff
-
extent of confidence and trust in staff.
-
-
Leaving hospital, for example:
-
involvement in decisions about discharge
-
adequacy of information received about medication.
-
-
General, for example:
-
feelings about dignity and respect
-
sources of satisfaction and dissatisfaction.
-
Staff and volunteers
We interviewed a range of staff and volunteers across the sites to ascertain their thoughts on the POD programme (Table 23).
| Staff/volunteer | Trust A | Trust B | Trust C | Trust D | ||
|---|---|---|---|---|---|---|
| Ward 1 | Ward 2 | Ward 3 | Ward 4 | Ward 5 | Ward 6 | |
| Senior nurse | ✓ | ✓ | ✓ × 2 | |||
| Ward manager | ✓ | ✓ | ✓ | |||
| VSM | ✓ | ✓ | ✓ × 2 | ✓ | ||
| Seconded ‘POD’ nurse | N/A | N/A | ✓ | ✓ | N/A | ✓ |
| Ward staff | ✓ | |||||
| Occupational therapist | ✓ × 2 | |||||
| Volunteer | ✓ × 2 | ✓ × 5 | ✓ × 2 | |||
| Total (n) | 5 | 2 | 2 | 8 | 1 | 9 |
We devised topic guides for the interviews (Boxes 2 and 3).
Introduce researcher; outline of study; confidentiality; timing.
Work role detailsParticipant will be asked about their work role.
The participant’s opinion will be sought about how feasible and acceptable the delirium prevention system of care has been to introduce and deliver on the participating wardAreas for discussion will include the following:
-
Confidence in delivering the programme, for example –
-
extent to which the system of care has become routine practice
-
successful and unsuccessful delivery strategies.
-
-
Training needs and delivery, for example –
-
adequacy of training received
-
future training needs.
-
-
Team working, for example –
-
experience of working with volunteers
-
roles and relationships within the MDT.
-
-
Responses of patients and their families, for example –
-
attitude and behaviour of patients to the system of care
-
attitude and involvement of family members.
-
Welcome, introduce researchers; outline of study; confidentiality; timing.
Personal introductionsThe researcher will invite members of the group to introduce themselves in turn by saying their name and giving brief information about their role.
A range of questions will be asked about the delirium prevention system of care implementation and delivery on the ward where the volunteers workAreas for discussion will include the following:
-
Confidence in delivering the programme, for example –
-
confidence in ability to carry out interventions
-
successful and unsuccessful delivery strategies.
-
-
Training needs and delivery, for example –
-
perceptions of content and delivery of training
-
suggestions for future training.
-
-
Team-working, for example –
-
extent to which volunteers integrated into the ward team
-
communication between nursing staff and volunteers.
-
-
Responses of patients and their families, for example –
-
attitude of patients to volunteers
-
attitude and involvement of family members.
-
Appendix 2 Results and discussion of the staff workload study
Results
We observed staff activity in four wards in both the POD programme implementation phase and delivery phase. We observed one ward in the implementation phase only, as it underwent a change of specialty before the delivery phase, meaning that observations in the delivery period would not have made a valid comparison possible.
We undertook 8257 10-minute observations in the implementation phase and 6711 10-minute observations during the delivery phase: 14,968 observation in total. This equates to almost 2500 hours of staff time.
Data validity and reliability
The amount of nursing attention given to patients should be directly proportional to their dependency, with more dependent patients, on average, receiving more care (‘care ratios’). In our study, the highest-dependency patients (grade 4) received 13 times (grade 4/grade 1 = 9/0.7) and 14 times (grade 4/grade 1 = 8.4/0.6) more nursing care in the pre-POD and post-POD periods, respectively (Table 24). Any deviation from these incremental rising care times may indicate that patients’ dependency levels have been misattributed or that the non-participant observers have labelled interventions inaccurately. Either of these would be a threat to the validity and reliability of the data. Generally, the ‘care ratios’ for the wards were sound in this study, showing accuracy and consistency of data in both the pre-POD and post-POD implementation periods.
| Dependency levela | Ward 1 | Ward 2 | Ward 3 | Ward 4 | Ward 6 | All wards | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phase 1 | Phase 2 | Phase 1 | Phase 2 | Phase 1 | Phase 2 | Phase 1 | Phase 2 | Phase 1 | Phase 2 | Phase 1 | Phase 2 | |
| 1 | 0.0 | 0.0 | 1.1 | 2.5 | 2.0 | 0.0 | 0.0 | 0.0 | 0.0 | N/A | 0.7 | 0.6 |
| 2 | 5.2 | 3.1 | 2.8 | 5.7 | 2.4 | 4.5 | 3.0 | 4.1 | 3.4 | N/A | 3.2 | 4.1 |
| 3 | 3.7 | 7.2 | 7.6 | 8.7 | 6.7 | 5.9 | 4.4 | 5.9 | 8.4 | N/A | 5.2 | 6.8 |
| 4 | 10.4 | 11.4 | 15.9 | 9.5 | 4.7 | 7.2 | 7.1 | 5.7 | 7.2 | N/A | 9.0 | 8.4 |
Average ward occupancy on all wards was similar in both periods of data collection (implementation phase, 27.9 occupied beds; delivery phase, 28.2 occupied beds) (Table 25).
| Phase | Occupied beds | Percentage of patients in each dependency levela | Workload index | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| Pre POD delivery | 27.9 | 9 | 20 | 51 | 20 | 3.2 |
| Post POD delivery | 28.2 | 11 | 15 | 60 | 13 | 3.3 |
The majority of patients in both phases of the study were either dependent or highly dependent (pre POD delivery: 71% dependent or highly dependent; post POD delivery: 73% dependent or highly dependent).
The workload index for the wards was similar in the pre-POD and post-POD delivery periods (workload indices 3.2 and 3.3, respectively).
The percentages of staff at different grades observed on duty during the data collection periods were similar at both time points (see Table 6).
Ward sister or manager grade staff accounted for 6% of the observations at both time points; staff nurses accounted for 45% and 46% of the observations pre POD and post POD, respectively. Support workers accounted for almost half of the observations (49% pre POD implementation, 46% post POD implementation). Volunteers accounted for only 1% of the post-POD delivery observations (see Table 6).
There were small changes overall in direct and indirect patient care from the pre-POD to the post-POD observations (direct patient care: 45% pre POD to 46% post POD; indirect patient care: 28% pre POD to 29% post POD). There was a 4% increase in direct patient care by ward sister and staff nurse grades, whereas there was a 2% decrease in direct patient care in support worker grades (see Table 6). Overall, there were also small decreases between the implementation and delivery phases in the precentage of both associated work (from 15% to 13%) and personal time (from 13% to 12%).
Discussion
There was a modest increase in the percentage of time spent by staff in both direct and indirect care following the introduction of the POD intervention. An increase in both direct care and indirect care is unusual, as they usually demonstrate an inverse relationship; that is, if one activity increases in a ward, then the other usually falls. The reason for this is not clear.
The data support our hope that the implementation of POD would not be associated with adverse effects on nurse workload, which could have been a consequence of introducting a system of enhanced care such as the POD programme. Indeed, there was an indication that the introduction of the POD programme on the wards was associated with a small positive change overall from associated care and personal time to direct and indirect care.
An assessment of these changes to the staff workload data of the wards before and after the introduction of the POD programme to the wards is potentially vulnerable to a number of factors, including the validity and reliability of the data and the changes to bed occupancy and patient dependency. Staff activity and workload in wards are partly driven by the bed occupancy rates and patient dependency data. The data show that the pre- and post-POD data for bed occupancy and patient dependeny were similar. Therefore, comparing staff activity in pre- and post-POD periods was meaningful, that is the ward workload was not a major confounding variable. The validity and reliability of the data were also acceptable: there was consistency across the two time points. Any changes in the workload data can, therefore, reasonably be attributed to the implementation of the POD system of care.
Appendix 3 Results of the patient and carer questionnaire study
Questionnaire returns
During the POD implementation phase, 745 patients were discharged from the five participating wards and 398 questionnaires were distributed (a distribution rate of 53%) (Table 26).
| Ward | Total | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 6 | ||
| POD implementation phase | July 2011–December 2011 | July 2011–December 2011 | July 2011–April 2012 | October 2011–May 2012 | March 2012–December 2012 | |
| Distribution period | October 2011–December 2011 | October 2011–December 2011 | October 2011–December 2011 | January 2012–April 2012 | October 2012–November 2012 | |
| Patients discharged (n) | 116 | 87 | 200 | 253 | 89 | 745 |
| Questionnaires distributed (n) | 28 | 48 | 110 | 125 | 87 | 398 |
| Distribution rate (%) | 24 | 55 | 55 | 49 | 98 | 53 |
| Patient questionnaires returned (n) | 8 | 18 | 21 | 26 | 10 | 83 |
| Return rate (%) | 29 | 38 | 19 | 21 | 11 | 21 |
| Returns as % of discharges | 7 | 21 | 11 | 10 | 11 | 11 |
| Carer questionnaires returned (n) | 5 | 11 | 20 | 4 | 7 | 47 |
| POD delivery phase | January 2012–June 2012 | January 2012–June 2012 | May 2012–September 2012 | June 2012–October 2012 | January 2013–April 2013 | |
| Distribution period | April 2012–June 2012 | April 2012–June 2012 | July 2012–September 2012 | September 2012–November 2012 | March 2013–April 2013 | |
| Patients discharged (n) | 85 | 107 | 220 | 154 | 49 | 615 |
| Questionnaires distributeda (n) | 96 | 97 | 100 | 100 | 36 | 429 |
| Patient questionnaires returned (n) | 4 | 2 | 25 | 16 | 4 | 51 |
| Estimated return rate (%) | 4 | 2 | 25 | 16 | 11 | 12 |
| Returns as % of discharges | 5 | 2 | 11 | 10 | 8 | 8 |
| Carer questionnaires returned (n) | 4 | 2 | 25 | 0 | 2 | 33 |
Of the 398 questionnaires distributed in the POD implementation phase, 83 were returned (a return rate of 21%). The return rate as a percentage of patients discharged was 11%.
A total of 615 patients were discharged during the POD delivery phase from the five participating wards. Unfortunately, we do not have an exact record of how many of these patients were given a questionnaire by the ward staff; instead, the wards estimated numbers of questionnaires distributed. However, 51 patient questionnaires were returned: a return rate as a percentage of discharged patients of 8% (see Table 26). Forty-seven carer questionnaires were returned during the POD implementation phase and 33 carer questionnaires were returned during the POD delivery phase (see Table 26).
Responses to the patient questionnaire are shown in Table 27. Responses to the carer questionnaire are shown in Table 28. Numbers of positive and negative comments by patients and carers are shown in Tables 29 and 30, respectively. Patient and carer comments from the questionnaires are shown in Box 4.
| Questionnaire responses | Ward, n (%) | All wards, n (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 6 | ||||||||
| Implementation period (N = 8) | Delivery period (N = 4) | Implementation period (N = 18) | Delivery period (N = 2) | Implementation period (N = 21) | Delivery period (N = 25) | Implementation period (N = 26) | Delivery period (N = 16) | Implementation period (N = 10) | Delivery period (N = 4) | Implementation period (N = 83) | Delivery period (N = 51) | |
| The hospital and ward | ||||||||||||
| 1. Were you ever bothered by noise at night from hospital staff? | χ2 p = 1.000 | |||||||||||
| Yes | 2 (29) | 1 (33) | 2 (13) | 1 (50) | 1 (5) | 4 (18) | 6 (23) | 3 (19) | 4 (40) | 0 (0) | 15 (19) | 9 (19) |
| No | 5 (71) | 2 (67) | 14 (88) | 1 (50) | 18 (95) | 18 (82) | 20 (77) | 13 (81) | 6 (60) | 4 (100) | 63 (81) | 38 (81) |
| 2. Did you feel threatened during your stay in hospital by other patients or visitors? | χ2 p = 0.266 | |||||||||||
| Yes | 1 (14) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (9) | 2 (8) | 3 (19) | 0 (0) | 0 (0) | 3 (4) | 5 (10) |
| No | 19 (100) | 21 (91) | 17 (100) | 2 (100) | 19 (100) | 21 (91) | 24 (92) | 13 (81) | 10 (100) | 4 (100) | 76 (96) | 43 (90) |
| 3. Were you always offered a choice of food? | χ2 p = 0.215 | |||||||||||
| Yes, always | 7 (100) | 2 (67) | 14 (82) | 2 (100) | 18 (95) | 20 (87) | 23 (92) | 12 (75) | 9 (100) | 4 (100) | 71 (92) | 40 (83) |
| Yes, sometimes | 0 (0) | 1 (33) | 3 (18) | 0 (0) | 1 (5) | 3 (13) | 2 (8) | 4 (25) | 0 (0) | 0 (0) | 6 (8) | 8 (17) |
| No | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 4. Did you get enough help from staff to eat your meals? | χ2 p = 0.013a | |||||||||||
| Yes, always | 4 (67) | 0 (0) | 5 (100) | 0 (0) | 5 (56) | 5 (42) | 19 (83) | 10 (91) | 2 (50) | 1 (50) | 35 (75) | 16 (62) |
| Yes, sometimes | 2 (33) | 0 (0) | 0 (0) | 0 (0) | 4 (44) | 2 (17) | 4 (17) | 1 (9) | 1 (25) | 1 (50) | 11 (23) | 4 (15) |
| No | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 5 (42) | 0 (0) | 0 (0) | 1 (25) | 0 (0) | 1 (2) | 6 (23) |
| I did not need help to eat meals (n) | 1 | 2 | 12 | 2 | 10 | 12 | 3 | 5 | 6 | 2 | 32 | 23 |
| Doctors | ||||||||||||
| 5. When you had important questions to ask a doctor, did you get answers that you could understand? | χ2 p = 0.018 | |||||||||||
| Yes, always | 4 (57) | 1 (33) | 16 (94) | 2 (100) | 11 (65) | 8 (44) | 10 (39) | 5 (31) | 4 (50) | 2 (67) | 45 (60) | 18 (43) |
| Yes, sometimes | 3 (43) | 1 (33) | 0 (0) | 0 (0) | 5 (29) | 3 (17) | 15 (58) | 11 (69) | 4 (50) | 1 (33) | 27 (36) | 16 (38) |
| No | 0 (0) | 1 (33) | 1 (6) | 0 (0) | 1 (6) | 7 (39) | 1 (4) | 0 (0) | 0 (0) | 0 (0) | 3 (4) | 8 (19) |
| I had no need help to ask (n) | 0 | 0 | 0 | 0 | 2 | 5 | 0 | 0 | 2 | 1 | 2 | 5 |
| 6. Did you have confidence and trust in the doctors treating you? | χ2 p = 1.000a | |||||||||||
| Yes, always | 5 (71) | 1 (33) | 17 (100) | 2 (100) | 15 (83) | 16 (67) | 13 (50) | 12 (75) | 9 (90) | 4 (100) | 59 (76) | 35 (71) |
| Yes, sometimes | 2 (29) | 2 (67) | 0 (0) | 0 (0) | 3 (17) | 7 (29) | 12 (46) | 4 (25) | 1 (10) | 0 (0) | 18 (23) | 13 (27) |
| No | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (4) | 1 (4) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 1 (2) |
| 7. Did doctors talk in front of you as if you were not there? | χ2 p = 0.131 | |||||||||||
| Yes, often | 0 (0) | 1 (33) | 0 (0) | 0 (0) | 0 (0) | 5 (22) | 4 (15) | 0 (0) | 0 (0) | 0 (0) | 4 (5) | 6 (13) |
| Yes, sometimes | 2 (29) | 0 (0) | 0 (0) | 0 (0) | 3 (16) | 5 (22) | 13 (50) | 2 (13) | 1 (11) | 0 (0) | 19 (24) | 7 (15) |
| No | 5 (71) | 2 (67) | 17 (100) | 2 (100) | 16 (84) | 13 (57) | 9 (35) | 14 (88) | 8 (89) | 4 (100) | 55 (71) | 35 (73) |
| Nurses | ||||||||||||
| 8. When you had important questions to ask a nurse, did you get answers you could understand? | χ2 p = 0.026a | |||||||||||
| Yes, always | 4 (57) | 1 (50) | 15 (100) | 2 (100) | 12 (75) | 9 (45) | 14 (56) | 6 (40) | 6 (86) | 3 (100) | 51 (73) | 21 (50) |
| Yes, sometimes | 3 (43) | 0 (0) | 0 (0) | 0 (0) | 4 (25) | 7 (35) | 9 (36) | 9 (60) | 1 (14) | 0 (0) | 17 (24) | 16 (38) |
| No | 0 (0) | 1 (50) | 0 (0) | 0 (0) | 0 (0) | 4 (20) | 2 (8) | 0 (0) | 0 (0) | 0 (0) | 2 (3) | 5 (12) |
| I had no need to ask (n) | 0 | 1 | 2 | 0 | 2 | 4 | 1 | 1 | 2 | 1 | 7 | 7 |
| 9. Did you have confidence and trust in the nurses treating you? | χ2 p = 0.040a | |||||||||||
| Yes, always | 6 (86) | 2 (67) | 16 (94) | 2 (100) | 17 (90) | 11 (46) | 23 (89) | 13 (81) | 8 (80) | 4 (100) | 70 (89) | 32 (65) |
| Yes, sometimes | 1 (14) | 1 (33) | 1 (6) | 0 (0) | 2 (11) | 10 (42) | 3 (12) | 2 (12) | 2 (20) | 0 (0) | 9 (11) | 13 (27) |
| No | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (12) | 0 (0) | 1 (6) | 0 (0) | 0 (0) | 0 (0) | 4 (8) |
| 10. Did the nurses talk in front of you as if you were not there? | χ2 p = 0.001b | |||||||||||
| Yes, often | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 5 (21) | 2 (7) | 1 (6) | 0 (0) | 0 (0) | 3 (4) | 6 (12) |
| Yes, sometimes | 0 (0) | 1 (33) | 0 (0) | 0 (0) | 0 (0) | 7 (29) | 3 (12) | 3 (19) | 1 (10) | 0 (0) | 4 (5) | 11 (22) |
| No | 6 (100) | 2 (67) | 17 (100) | 2 (100) | 18 (95) | 12 (50) | 21 (81) | 12 (75) | 9 (90) | 4 (100) | 71 (91) | 32 (65) |
| 11. In your opinion, were there enough nurses on duty to care for you in hospital? | χ2 p = 0.071 | |||||||||||
| Always/nearly always enough nurses | 3 (43) | 1 (33) | 12 (67) | 2 (100) | 14 (70) | 15 (65) | 1 (4) | 8 (50) | 4 (40) | 4 (100) | 34 (42) | 30 (63) |
| Sometimes enough nurses | 3 (43) | 2 (67) | 6 (33) | 0 (0) | 4 (20) | 5 (22) | 12 (46) | 6 (38) | 6 (60) | 0 (0) | 31 (38) | 13 (27) |
| Rarely or never enough nurses | 1 (14) | 0 (0) | 0 (0) | 0 (0) | 2 (10) | 3 (13) | 13 (50) | 2 (13) | 0 (0) | 0 (0) | 16 (20) | 5 (10) |
| Your care and treatment | ||||||||||||
| 12. Sometimes in hospital, a member of staff will say one thing and another will say something quite different. Did this happen to you? | χ2 p = 0.00b | |||||||||||
| Yes, often | 1 (14) | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 6 (25) | 0 (0) | 1 (6) | 0 (0) | 0 (0) | 2 (3) | 7 (14) |
| Yes, sometimes | 2 (29) | 1 (33) | 7 (39) | 0 (0) | 4 (20) | 3 (13) | 16 (62) | 2 (13) | 4 (44) | 1 (25) | 33 (41) | 7 (14) |
| No | 4 (57) | 2 (67) | 11 (61) | 2 (100) | 15 (75) | 15 (63) | 10 (39) | 13 (81) | 5 (56) | 3 (75) | 45 (56) | 35 (71) |
| 13. Were you involved as much as you wanted to be in decisions about your care and treatment? | χ2 p = 0.004 | |||||||||||
| Yes, definitely | 3 (43) | 0 (0) | 11 (61) | 2 (100) | 10 (50) | 10 (44) | 4 (15) | 6 (38) | 8 (80) | 3 (75) | 36 (44) | 21 (44) |
| Yes, to some extent | 4 (57) | 2 (67) | 6 (33) | 0 (0) | 10 (50) | 5 (22) | 21 (81) | 10 (63) | 2 (20) | 1 (25) | 43 (53) | 18 (38) |
| No | 0 (0) | 1 (33) | 1 (6) | 0 (0) | 0 (0) | 8 (35) | 1 (4) | 0 (0) | 0 (0) | 0 (0) | 2 (3) | 9 (19) |
| 14. How much information about your condition or treatment was given to you? | χ2 p = 0.384 | |||||||||||
| Not enough | 3 (43) | 1 (33) | 1 (6) | 0 (0) | 3 (15) | 10 (44) | 5 (19) | 1 (6) | 2 (20) | 0 (0) | 14 (17) | 12 (25) |
| The right amount | 4 (57) | 2 (67) | 17 (94) | 2 (100) | 15 (75) | 12 (52) | 16 (62) | 10 (62) | 8 (80) | 4 (100) | 60 (74) | 30 (63) |
| Too much | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (10) | 1 (4) | 5 (19) | 5 (31) | 0 (0) | 0 (0) | 7 (9) | 6 (13) |
| 15. If your family or someone else close to you wanted to talk to a doctor, did they have enough opportunity to do so? | χ2 p = 0.464 | |||||||||||
| Yes, definitely | 4 (67) | 0 (0) | 6 (67) | 1 (100) | 9 (47) | 7 (35) | 3 (13) | 5 (31) | 1 (17) | 2 (68) | 23 (36) | 15 (37) |
| Yes, to some extent | 2 (33) | 1 (100) | 2 (22) | 0 (0) | 7 (37) | 7 (35) | 20 (83) | 10 (63) | 4 (67) | 1 (33) | 35 (56) | 19 (46) |
| No | 0 (0) | 0 (0) | 1 (11) | 0 (0) | 3 (16) | 6 (30) | 1 (4) | 1 (6) | 1 (17) | 0 (0) | 6 (9) | 7 (17) |
| No family or friends were involved (n) | 0 | 1 | 2 | 0 | 1 | 1 | 2 | 0 | 2 | 0 | 7 | 2 |
| Did not want or need information (n) | 0 | 1 | 6 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 7 | 4 |
| Did not want them to talk to a doctor (n) | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 2 |
| 16. Did you find someone on the hospital staff to talk to about your worries and fears? | χ2 p = 0.001b | |||||||||||
| Yes, definitely | 4 (67) | 1 (33) | 8 (89) | 2 (100) | 7 (50) | 5 (25) | 5 (20) | 8 (67) | 3 (38) | 2 (100) | 27 (44) | 18 (46) |
| Yes, to some extent | 2 (33) | 0 (0) | 1 (11) | 0 (0) | 5 (36) | 4 (20) | 18 (72) | 4 (33) | 4 (50) | 0 (0) | 30 (48) | 8 (21) |
| No | 0 (0) | 2 (67) | 0 (0) | 0 (0) | 2 (14) | 11 (55) | 2 (8) | 0 (0) | 1 (13) | 0 (0) | 5 (8) | 13 (33) |
| I had no fears or worries (n) | 0 | 0 | 0 | 0 | 5 | 3 | 1 | 4 | 2 | 2 | 17 | 9 |
| 17. Were you given enough privacy when discussing your condition or treatment? | χ2 p = 0.482a | |||||||||||
| Yes, always | 4 (57) | 1 (33) | 17 (100) | 2 (100) | 15 (75) | 12 (52) | 23 (89) | 14 (88) | 6 (60) | 4 (100) | 65 (81) | 33 (69) |
| Yes, sometimes | 3 (43) | 2 (67) | 0 (0) | 0 (0) | 4 (20) | 7 (30) | 3 (12) | 2 (12) | 2 (20) | 0 (0) | 12 (15) | 11 (23) |
| No | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 4 (17) | 0 (0) | 0 (0) | 2 (20) | 0 (0) | 3 (4) | 4 (8) |
| 18. Were you given enough privacy when being examined or treated? | χ2 p = 0.268 | |||||||||||
| Yes, always | 6 (86) | 3 (100) | 18 (100) | 2 (100) | 20 (100) | 18 (82) | 25 (96) | 16 (100) | 9 (100) | 4 (100) | 78 (98) | 43 (92) |
| Yes, sometimes | 1 (14) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (18) | 1 (4) | 0 (0) | 0 (0) | 0 (0) | 2 (3) | 4 (9) |
| No | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 19. Were you ever in any pain? | χ2 p = 0.214 | |||||||||||
| Yes | 3 (50) | 2 (67) | 13 (77) | 2 (100) | 11 (55) | 20 (91) | 8 (31) | 5 (31) | 8 (89) | 3 (75) | 43 (55) | 32 (68) |
| No | 3 (50) | 1 (33) | 4 (24) | 0 (0) | 9 (45) | 2 (9) | 18 (69) | 11 (69) | 1 (11) | 1 (25) | 35 (45) | 15 (32) |
| 20. Do you think the hospital staff did everything they could to help control your pain? | χ2 p = 0.894a | |||||||||||
| Yes, definitely | 2 (67) | 0 (0) | 12 (93) | 2 (100) | 8 (73) | 12 (57) | 4 (50) | 4 (80) | 6 (75) | 3 (100) | 32 (74) | 21 (64) |
| Yes, to some extent | 1 (33) | 2 (100) | 1 (8) | 0 (0) | 3 (27) | 8 (38) | 4 (50) | 1 (20) | 2 (25) | 0 (0) | 11 (26) | 11 (33) |
| No | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3) |
| 21. How many minutes after you used the call button did it usually take before you got the help you needed? | χ2 p = 0.776 | |||||||||||
| 0 minutes/right away | 0 (0) | 0 (0) | 2 (13) | 1 (50) | 2 (15) | 1 (6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (6) | 2 (5) |
| 1–2 minutes | 2 (33) | 0 (0) | 5 (33) | 1 (50) | 4 (31) | 4 (24) | 12 (48) | 9 (64) | 4 (67) | 2 (67) | 27 (42) | 16 (42) |
| 3–5 minutes | 2 (33) | 0 (0) | 5 (33) | 0 (0) | 5 (39) | 8 (47) | 11 (44) | 3 (21) | 2 (33) | 1 (33) | 25 (39) | 12 (32) |
| > 5 minutes | 2 (33) | 2 (100) | 3 (20) | 0 (0) | 2 (15) | 4 (24) | 2 (8) | 2 (14) | 0 (0) | 0 (0) | 9 (14) | 8 (21) |
| I never got help | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| I never used the call button (n) | 0 | 1 | 2 | 0 | 7 | 4 | 1 | 1 | 3 | 1 | 13 | 7 |
| Leaving hospital | ||||||||||||
| 22. Did you feel you were involved in decisions about your discharge from hospital? | χ2 p = 0.195 | |||||||||||
| Yes, definitely | 4 (67) | 0 (0) | 10 (63) | 2 (100) | 10 (56) | 9 (47) | 9 (35) | 10 (62) | 4 (44) | 4 (100) | 37 (49) | 25 (57) |
| Yes, to some extent | 1 (17) | 2 (67) | 5 (31) | 0 (0) | 5 (28) | 3 (16) | 16 (62) | 6 (38) | 3 (33) | 0 (0) | 30 (40) | 11 (25) |
| No | 1 (17) | 1 (33) | 1 (6) | 0 (0) | 3 (17) | 7 (37) | 1 (4) | 0 (0) | 2 (22) | 0 (0) | 8 (11) | 8 (18) |
| I did not need to be involved (n) | 0 | 0 | 2 | 0 | 3 | 4 | 0 | 0 | 1 | 0 | 6 | 4 |
| 23. Before you left hospital, were you given any written or printed information about what you should or should not do after leaving hospital? | χ2 p = 1.000 | |||||||||||
| Yes | 3 (50) | 1 (33) | 15 (94) | 2 (100) | 8 (38) | 7 (33) | 12 (46) | 12 (80) | 4 (50) | 2 (50) | 42 (55) | 24 (53) |
| No | 3 (50) | 2 (67) | 1 (6) | 0 (0) | 13 (62) | 14 (67) | 14 (54) | 3 (20) | 4 (50) | 2 (50) | 35 (46) | 21 (47) |
| 24. Did a member of staff explain the purpose of the medicines you were to take in a way you could understand? | χ2 p = 0.228 | |||||||||||
| Yes, completely | 2 (40) | 1 (33) | 17 (100) | 2 (100) | 8 (42) | 5 (26) | 7 (29) | 13 (87) | 2 (50) | 2 (67) | 36 (52) | 23 (55) |
| Yes, to some extent | 2 (40) | 1 (33) | 0 (0) | 0 (0) | 6 (32) | 6 (32) | 16 (67) | 2 (13) | 1 (25) | 1 (33) | 25 (36) | 10 (24) |
| No | 1 (20) | 1 (33) | 0 (0) | 0 (0) | 5 (26) | 8 (42) | 1 (4) | 0 (0) | 1 (25) | 0 (0) | 8 (12) | 9 (21) |
| I did not need an explanation (n) | 0 | 0 | 1 | 0 | 0 | 3 | 1 | 0 | 4 | 0 | 6 | 3 |
| I had no medicines (n) | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 2 | 1 |
| 25. Did a member of staff tell you about medication side effects to watch for when you went home? | χ2 p = 0.269 | |||||||||||
| Yes, completely | 0 (0) | 0 (0) | 5 (45) | 1 (100) | 1 (6) | 2 (10) | 6 (23) | 8 (62) | 0 (0) | 1 (100) | 12 (19) | 12 (32) |
| Yes, to some extent | 1 (20) | 0 (0) | 1 (9) | 0 (0) | 1 (6) | 4 (20) | 9 (35) | 4 (31) | 1 (25) | 0 (0) | 13 (21) | 8 (22) |
| No | 4 (80) | 2 (100) | 5 (45) | 0 (0) | 15 (88) | 14 (70) | 11 (42) | 1 (7) | 3 (75) | 0 (0) | 38 (60) | 17 (46) |
| I did not need an explanation (n) | 1 | 1 | 7 | 1 | 3 | 2 | 0 | 2 | 3 | 2 | 14 | 8 |
| 26. Were you told how to take your medication in a way you could understand? | χ2 p = 0.363 | |||||||||||
| Yes, definitely | 4 (67) | 1 (50) | 12 (92) | 2 (100) | 7 (41) | 3 (20) | 9 (36) | 10 (77) | 4 (67) | 2 (100) | 36 (54) | 18 (53) |
| Yes, to some extent | 2 (33) | 0 (0) | 0 (0) | 0 (0) | 4 (24) | 5 (33) | 15 (60) | 3 (23) | 1 (17) | 0 (0) | 22 (33) | 8 (24) |
| No | 0 (0) | 1 (50) | 1 (8) | 0 (0) | 6 (35) | 7 (47) | 1 (4) | 0 (0) | 1 (17) | 0 (0) | 9 (13) | 8 (24) |
| I did not need to be told (n) | 0 | 1 | 5 | 0 | 3 | 7 | 0 | 2 | 2 | 1 | 10 | 11 |
| 27. Were you given clear written or printed information about your medicines? | χ2 p = 0.220 | |||||||||||
| Yes, completely | 4 (67) | 1 (33) | 13 (81) | 2 (100) | 11 (58) | 5 (24) | 15 (60) | 14 (93) | 2 (33) | 3 (100) | 45 (63) | 25 (57) |
| Yes, to some extent | 1 (17) | 0 (0) | 2 (12) | 0 (0) | 4 (21) | 5 (24) | 6 (24) | 1 (7) | 2 (33) | 0 (0) | 15 (21) | 6 (14) |
| No | 1 (17) | 2 (67) | 1 (6) | 0 (0) | 4 (21) | 11 (52) | 4 (16) | 0 (0) | 2 (33) | 0 (0) | 12 (17) | 13 (30) |
| Do not know/cannot remember (n) | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 2 | 0 | 4 | 1 |
| 28. Did a member of staff tell you about any danger signals you should watch for after you went home? | χ2 p = 0.788 | |||||||||||
| Yes, completely | 0 (0) | 0 (0) | 9 (60) | 0 (0) | 2 (11) | 2 (10) | 0 (0) | 6 (43) | 1 (14) | 1 (33) | 12 (17) | 9 (23) |
| Yes, to some extent | 2 (33) | 0 (0) | 4 (27) | 0 (0) | 2 (11) | 3 (14) | 10 (42) | 8 (57) | 3 (43) | 0 (0) | 21 (30) | 11 (28) |
| No | 4 (67) | 2 (100) | 2 (13) | 0 (0) | 14 (78) | 16 (76) | 14 (58) | 0 (0) | 3 (43) | 2 (67) | 37 (53) | 20 (50) |
| It was not necessary (n) | 0 | 1 | 3 | 2 | 3 | 2 | 2 | 1 | 2 | 1 | 10 | 7 |
| 29. Did the doctors or nurses give your family or someone close to you all the information they needed to care for you? | χ2 p = 0.301 | |||||||||||
| Yes, definitely | 4 (57) | 0 (0) | 5 (38) | 1 (100) | 6 (35) | 7 (35) | 2 (8) | 7 (58) | 3 (50) | 2 (67) | 20 (29) | 17 (44) |
| Yes, to some extent | 2 (29) | 1 (33) | 3 (23) | 0 (0) | 7 (41) | 7 (35) | 16 (64) | 4 (33) | 1 (17) | 0 (0) | 29 (43) | 12 (31) |
| No | 1 (14) | 2 (67) | 5 (38) | 0 (0) | 4 (24) | 6 (30) | 7 (28) | 1 (8) | 2 (33) | 1 (33) | 19 (28) | 10 (26) |
| No family or friends were involved (n) | 0 | 0 | 1 | 0 | 2 | 2 | 1 | 0 | 3 | 0 | 7 | 2 |
| Did not want or need information (n) | 0 | 0 | 2 | 1 | 2 | 0 | 0 | 2 | 1 | 1 | 5 | 4 |
| 30. Did hospital staff tell you who to contact if you were worried about your condition or treatment after you left hospital? | χ2 p = 0.375 | |||||||||||
| Yes | 0 (0) | 1 (33) | 13 (81) | 2 (100) | 12 (63) | 5 (28) | 5 (21) | 13 (93) | 5 (63) | 3 (100) | 35 (49) | 24 (60) |
| No | 4 (100) | 2 (67) | 3 (19) | 0 (0) | 7 (37) | 13 (72) | 19 (79) | 1 (7) | 3 (38) | 0 (0) | 36 (51) | 16 (40) |
| Do not know/cannot remember (n) | 2 | 0 | 2 | 0 | 2 | 5 | 2 | 1 | 2 | 1 | 10 | 7 |
| 31. Did you receive copies of letters sent between hospital doctors and your family doctor (GP)? | χ2 p = 0.986 | |||||||||||
| Yes, I received copies | 0 (0) | 1 (33) | 8 (57) | 0 (0) | 5 (25) | 4 (21) | 22 (88) | 12 (92) | 4 (50) | 3 (75) | 39 (53) | 20 (51) |
| No, I did not receive copies | 6 (100) | 2 (67) | 6 (43) | 0 (0) | 15 (75) | 15 (79) | 3 (12) | 1 (8) | 4 (50) | 1 (25) | 34 (47) | 19 (49) |
| Not sure/do not know (n) | 0 | 0 | 3 | 2 | 1 | 4 | 1 | 2 | 2 | 0 | 7 | 8 |
| Overall | ||||||||||||
| 32. Overall, did you feel you were treated with respect and dignity while you were in hospital? | χ2 p = 0.786a | |||||||||||
| Yes, always | 17 (81) | 14 (61) | 5 (71) | 2 (67) | 18 (100) | 2 (100) | 20 (77) | 16 (100) | 10 (100) | 4 (100) | 70 (85) | 38 (79) |
| Yes, sometimes | 4 (19) | 8 (35) | 2 (29) | 1 (33) | 0 (0) | 0 (0) | 6 (23) | 0 (0) | 0 (0) | 0 (0) | 12 (15) | 9 (18) |
| No | 0 (0) | 1 (4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2) |
| 33. How would you rate how well the doctors and nurses worked together? | χ2 p = 0.050a | |||||||||||
| Excellent | 1 (14) | 1 (33) | 9 (50) | 2 (100) | 11 (52) | 5 (23) | 10 (38) | 6 (38) | 4 (40) | 3 (75) | 35 (43) | 17 (36) |
| Very good | 5 (71) | 1 (33) | 9 (50) | 0 (0) | 7 (33) | 8 (36) | 13 (50) | 10 (62) | 6 (60) | 1 (25) | 40 (49) | 20 (43) |
| Good | 1 (14) | 1 (33) | 0 (0) | 0 (0) | 1 (5) | 3 (14) | 3 (12) | 0 (0) | 0 (0) | 0 (0) | 5 (6) | 4 (9) |
| Fair | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 6 (27) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 6 (13) |
| Poor | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 0 (0) |
| 34. Overall, how would you rate the care you received? | χ2 p = 0.009 | |||||||||||
| Excellent | 2 (29) | 2 (67) | 12 (67) | 2 (100) | 11 (52) | 5 (22) | 10 (38) | 6 (38) | 3 (30) | 4 (100) | 38 (46) | 19 (40) |
| Very good | 2 (29) | 0 (0) | 5 (28) | 0 (0) | 6 (29) | 7 (30) | 12 (46) | 10 (62) | 7 (70) | 0 (0) | 32 (39) | 17 (35) |
| Good | 3 (43) | 1 (33) | 1 (6) | 0 (0) | 2 (10) | 3 (13) | 4 (15) | 0 (0) | 0 (0) | 0 (0) | 10 (12) | 4 (8) |
| Fair | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 6 (26) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 6 (13) |
| Poor | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 2 (9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 2 (4) |
| 35. During your hospital stay, were you ever asked to give your views about the quality of your care? | χ2 p = 0.636 | |||||||||||
| Yes | 0 (0) | 0 (0) | 6 (35) | 0 (0) | 5 (28) | 2 (12) | 3 (12) | 8 (50) | 2 (22) | 1 (25) | 16 (22) | 11 (28) |
| No | 5 (100) | 3 (100) | 11 (65) | 0 (0) | 13 (72) | 15 (88) | 22 (88) | 8 (50) | 7 (78) | 3 (75) | 58 (78) | 29 (73) |
| Do not know/cannot remember (n) | 1 | 0 | 1 | 2 | 3 | 6 | 1 | 0 | 1 | 0 | 7 | 8 |
| Questionnaire responses | Ward, n (%) | All wards, n (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 6 | ||||||||
| Implementation period (N = 5) | Delivery period (N = 4) | Implementation period (N = 11) | Delivery period (N = 2) | Implementation period (N = 20) | Delivery period (N = 25) | Implementation period (N = 4) | Delivery period (N = 0) | Implementation period (N = 7) | Delivery period (N = 2) | Implementation period (N = 47) | Delivery period (N = 33) | |
| Giving my relative the best | ||||||||||||
| 1. Staff took time to get to know my relative as a person | ||||||||||||
| Strongly agree | 1 (20) | 0 (0) | 4 (36) | 1 (50) | 5 (25) | 1 (4) | 1 (25) | N/A | 0 (0) | 1 (50) | 11 (23) | 3 (9) |
| Agree | 2 (40) | 3 (75) | 7 (63) | 1 (50) | 9 (45) | 10 (42) | 2 (50) | N/A | 4 (57) | 1 (50) | 24 (51) | 15 (47) |
| Neither agree nor disagree | 2 (40) | 0 (0) | 0 (0) | 0 (0) | 3 (15) | 8 (33) | 0 (0) | N/A | 3 (43) | 0 (0) | 8 (17) | 8 (25) |
| Disagree | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 2 (8) | 1 (25) | N/A | 0 (0) | 0 (0) | 2 (4) | 2 (6) |
| Strongly disagree | 0 (0) | 1 (25) | 0 (0) | 0 (0) | 2 (10) | 3 (12) | 0 (0) | N/A | 0 (0) | 0 (0) | 2 (4) | 4 (12) |
| 2. Staff always had enough time to give good-quality care | ||||||||||||
| Strongly agree | 1 (20) | 0 (0) | 4 (36) | 1 (50) | 6 (30) | 2 (8) | 2 (50) | N/A | 1 (14) | 0 (0) | 14 (30) | 3 (9) |
| Agree | 3 (60) | 2 (50) | 6 (55) | 1 (50) | 8 (40) | 10 (42) | 1 (25) | N/A | 3 (43) | 2 (100) | 21 (45) | 15 (47) |
| Neither agree nor disagree | 1 (20) | 1 (25) | 1 (9) | 0 (0) | 2 (10) | 4 (17) | 0 (0) | N/A | 2 (29) | 0 (0) | 6 (13) | 5 (16) |
| Disagree | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 6 (25) | 1 (25) | N/A | 1 (14) | 0 (0) | 3 (6) | 6 (19) |
| Strongly disagree | 0 (0) | 1 (25) | 0 (0) | 0 (0) | 3 (15) | 2 (8) | 0 (0) | N/A | 0 (0) | 0 (0) | 3 (6) | 3 (9) |
| 3. My relative always received the standard of care that I wanted | ||||||||||||
| Strongly agree | 0 (0) | 0 (0) | 4 (36) | 2 (100) | 7 (35) | 4 (17) | 2 (50) | N/A | 1 (14) | 0 (0) | 14 (30) | 6 (19) |
| Agree | 4 (80) | 2 (50) | 7 (64) | 0 (0) | 8 (40) | 9 (38) | 1 (25) | N/A | 4 (57) | 2 (100) | 24 (51) | 13 (41) |
| Neither agree nor disagree | 1 (20) | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 4 (17) | 0 (0) | N/A | 2 (29) | 0 (0) | 4 (9) | 4 (13) |
| Disagree | 0 (0) | 1 (25) | 0 (0) | 0 (0) | 3 (15) | 4 (17) | 1 (25) | N/A | 0 (0) | 0 (0) | 4 (9) | 5 (16) |
| Strongly disagree | 0 (0) | 1 (25) | 0 (0) | 0 (0) | 1 (5) | 3 (13) | 0 (0) | N/A | 0 (0) | 0 (0) | 1 (2) | 4 (13) |
| 4. Overall, the ward was a happy and welcoming place | ||||||||||||
| Strongly agree | 2 (40) | 0 (0) | 7 (64) | 2 (100) | 4 (20) | 4 (17) | 2 (50) | N/A | 1 (14) | 1 (50) | 16 (34) | 7 (22) |
| Agree | 3 (60) | 3 (75) | 4 (36) | 0 (0) | 13 (65) | 12 (50) | 2 (50) | N/A | 4 (57) | 1 (50) | 26 (55) | 16 (50) |
| Neither agree nor disagree | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (10) | 2 (8) | 0 (0) | N/A | 1 (14) | 0 (0) | 3 (6) | 2 (6) |
| Disagree | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 4 (17) | 0 (0) | N/A | 1 (14) | 0 (0) | 2 (3) | 4 (12) |
| Strongly disagree | 0 (0) | 1 (25) | 0 (0) | 0 (0) | 0 (0) | 2 (8) | 0 (0) | N/A | 0 (0) | 0 (0) | 0 (0) | 3 (9) |
| 5. Staff always seemed happy in their work | ||||||||||||
| Strongly agree | 0 (0) | 0 (0) | 2 (18) | 2 (100) | 5 (25) | 3 (13) | 0 (0) | N/A | 1 (14) | 1 (50) | 8 (17) | 6 (19) |
| Agree | 4 (80) | 3 (75) | 5 (45) | 0 (0) | 11 (55) | 10 (43) | 2 (50) | N/A | 5 (71) | 1 (50) | 27 (57) | 14 (45) |
| Neither agree nor disagree | 1 (20) | 0 (0) | 3 (27) | 0 (0) | 4 (20) | 7 (30) | 1 (25) | N/A | 1 (14) | 0 (0) | 10 (21) | 7 (23) |
| Disagree | 0 (0) | 0 (0) | 1 (9) | 0 (0) | 0 (0) | 3 (13) | 1 (25) | N/A | 0 (0) | 0 (0) | 2 (4) | 3 (10) |
| Strongly disagree | 0 (0) | 1 (25) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | N/A | 0 (0) | 0 (0) | 0 (0) | 1 (3) |
| 6. Overall, the quality of care my relative received was very good | ||||||||||||
| Strongly agree | 2 (40) | 0 (0) | 7 (64) | 2 (100) | 7 (35) | 5 (21) | 2 (50) | N/A | 1 (14) | 1 (50) | 19 (40) | 8 (25) |
| Agree | 2 (40) | 2 (50) | 3 (27) | 0 (0) | 8 (40) | 10 (42) | 1 (25) | N/A | 5 (71) | 1 (50) | 19 (40) | 13 (41) |
| Neither agree nor disagree | 1 (20) | 1 (25) | 1 (9) | 0 (0) | 1 (5) | 3 (12) | 1 (25) | N/A | 1 (14) | 0 (0) | 5 (11) | 4 (12) |
| Disagree | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (15) | 4 (17) | 0 (0) | N/A | 0 (0) | 0 (0) | 3 (6) | 4 (12) |
| Strongly disagree | 0 (0) | 1 (25) | 0 (0) | 0 (0) | 1 (5) | 3 (8) | 0 (0) | N/A | 0 (0) | 0 (0) | 1 (2) | 3 (9) |
| Mean (SD) | 4.00 (0.55) | 3.08 (1.42) | 4.32 (0.42) | N/A | 3.85 (0.91) | 3.44 (0.97) | 3.96 (1.07) | N/A | 3.79 (0.55) | 4.33 (0.24) | 3.98 (0.75) | 3.54 (1.04) |
| Median (IQR) | 4.17 (3.50–4.42) | 3.67 (1.58–4.00) | 4.33 (4.00–4.83) | N/A | 4.08 (3.08–4.50) | 3.67 (2.50–4.17) | 4.25 (2.83–4.79) | N/A | 4.00 (3.33–4.00) | 4.33 (4.17–N/A) | 4.17 (3.67–4.50) | 4.00 (2.67–4.17) |
| Mann–Whitney U-test | p = 0.077 | |||||||||||
| Could do better | ||||||||||||
| 1. Staff often spoke sharply to my relative | ||||||||||||
| Strongly disagree | 1 (20) | 0 (0) | 8 (27) | 1 (50) | 6 (30) | 2 (8) | 1 (25) | N/A | 2 (29) | 2 (100) | 18 (38) | 5 (16) |
| Disagree | 3 (60) | 1 (25) | 3 (73) | 1 (50) | 11 (55) | 10 (42) | 1 (25) | N/A | 4 (57) | 0 (0) | 22 (47) | 12 (38) |
| Neither agree nor disagree | 1 (20) | 1 (25) | 0 (0) | 0 (0) | 1 (5) | 3 (12) | 0 (0) | N/A | 1 (14) | 0 (0) | 3 (6) | 4 (12) |
| Agree | 0 (0) | 2 (50) | 0 (0) | 0 (0) | 1 (5) | 6 (25) | 2 (50) | N/A | 0 (0) | 0 (0) | 3 (6) | 8 (25) |
| Strongly agree | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 3 (12) | 0 (0) | N/A | 0 (0) | 0 (0) | 1 (2) | 3 (9) |
| 2. Staff seemed more concerned with getting the job done than caring for my relative | ||||||||||||
| Strongly disagree | 1 (20) | 0 (0) | 5 (46) | 1 (50) | 3 (15) | 0 (0) | 2 (50) | N/A | 1 (14) | 0 (0) | 12 (26) | 1 (3) |
| Disagree | 1 (20) | 2 (50) | 6 (55) | 1 (50) | 9 (45) | 7 (29) | 0 (0) | N/A | 3 (43) | 2 (100) | 19 (40) | 12 (38) |
| Neither agree nor disagree | 1 (20) | 1 (25) | 0 (0) | 0 (0) | 4 (20) | 7 (29) | 1 (25) | N/A | 3 (43) | 0 (0) | 9 (19) | 8 (25) |
| Agree | 1 (20) | 0 (0) | 0 (0) | 0 (0) | 4 (20) | 7 (29) | 1 (25) | N/A | 0 (0) | 0 (0) | 6 (13) | 7 (22) |
| Strongly agree | 1 (20) | 1 (25) | 0 (0) | 0 (0) | 0 (0) | 3 (12) | 0 (0) | N/A | 0 (0) | 0 (0) | 1 (2) | 4 (12) |
| 3. Staff did not treat my relative with dignity and respect | ||||||||||||
| Strongly disagree | 1 (20) | 1 (25) | 4 (36) | 1 (50) | 5 (26) | 2 (9) | 2 (50) | N/A | 2 (29) | 1 (50) | 14 (30) | 5 (16) |
| Disagree | 1 (20) | 2 (50) | 7 (64) | 1 (50) | 11 (58) | 11 (48) | 1 (25) | N/A | 5 (71) | 1 (50) | 25 (54) | 15 (48) |
| Neither agree nor disagree | 3 (60) | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 4 (17) | 0 (0) | N/A | 0 (0) | 0 (0) | 4 (9) | 4 (13) |
| Agree | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (11) | 2 (9) | 1 (25) | N/A | 0 (0) | 0 (0) | 3 (7) | 2 (6) |
| Strongly agree | 0 (0) | 1 (25) | 0 (0) | 0 (0) | 0 (0) | 4 (17) | 0 (0) | N/A | 0 (0) | 0 (0) | 0 (0) | 5 (16) |
| Mean (SD) | 3.53 (1.02) | 3.08 (1.26) | 4.52 (0.43) | 4.5 (0.71) | 3.86 (0.77) | 3.01 (1.06) | 3.67 (1.36) | N/A | 4.05 (0.59) | 4.50 (0.24) | 3.99 (0.81) | 3.22 (1.12) |
| Median (IQR) | 3.33 (2.67–4.50) | 3.33 (1.83–4.08) | 4.33 (4.00–5.00) | 4.50 (4.00–N/A) | 4.00 (3.33–4.33) | 3.33 (2.33–4.00) | 3.67 (2.42–4.92) | N/A | 4.00 (3.67–4.67) | 4.50 (4.33–N/A) | 4.00 (3.33–4.67) | 3.33 (2.67–4.00) |
| Mann–Whitney U-test | p = 0.003a | |||||||||||
| Feeling significant | ||||||||||||
| 1. Staff always made me feel welcome on the ward | ||||||||||||
| Strongly agree | 0 (0) | 0 (0) | 3 (27) | 2 (100) | 7 (37) | 5 (21) | 0 (0) | N/A | 1 (14) | 0 (0) | 11 (24) | 7 (22) |
| Agree | 3 (60) | 3 (75) | 7 (64) | 0 (0) | 9 (47) | 9 (38) | 3 (75) | N/A | 3 (43) | 2 (10) | 22 (54) | 14 (44) |
| Neither agree nor disagree | 2 (40) | 0 (0) | 1 (9) | 0 (0) | 1 (5) | 3 (12) | 1 (25) | N/A | 3 (43 | 0 (0) | 8 (17) | 3 (9) |
| Disagree | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (11) | 6 (25) | 0 (0) | N/A | 0 (0) | 0 (0) | 2 (4) | 6 (19) |
| Strongly disagree | 0 (0) | 1 (25) | 0 (0) | 0 (0) | 0 (0) | 1 (4) | 0 (0) | N/A | 0 (0) | 0 (0) | 0 (0) | 2 (6) |
| 2. Staff asked me for any information I might have about the wishes/needs of my relative | ||||||||||||
| Strongly agree | 0 (0) | 0 (0) | 2 (18) | 1 (100) | 4 (21) | 2 (8) | 0 (0) | N/A | 0 (0) | 0 (0) | 6 (13) | 3 (10) |
| Agree | 1 (25) | 1 (25) | 3 (27) | 0 (0) | 9 (47) | 7 (29) | 3 (75) | N/A | 1 (14) | 1 (50) | 17 (38) | 9 (29) |
| Neither agree nor disagree | 1 (25) | 0 (0) | 3 (27) | 0 (0) | 1 (5) | 4 (17) | 0 (0) | N/A | 5 (71) | 0 (0) | 10 (22) | 4 (13) |
| Disagree | 3 (16) | 8 (33) | 1 (25) | 2 (50) | 3 (16) | 8 (33) | 0 (0) | N/A | 1 (14) | 1 (50) | 8 (18) | 11 (35) |
| Strongly disagree | 2 (11) | 3 (12) | 1 (25) | 1 (25) | 2 (11) | 3 (12) | 1 (25) | N/A | 0 (0) | 0 (0) | 4 (9) | 4 (13) |
| 3. Staff provided me with enough information about the care and treatment of my relative | ||||||||||||
| Strongly agree | 0 (0) | 1 (25) | 3 (27) | 1 (100) | 7 (37) | 2 (8) | 0 (0) | N/A | 0 (0) | 0 (0) | 10 (22) | 4 (13) |
| Agree | 3 (60) | 1 (25) | 5 (45) | 0 (0) | 9 (47) | 9 (38) | 3 (75) | N/A | 3 (29) | 1 (50) | 22 (48) | 11 (35) |
| Neither agree nor disagree | 1 (20) | 0 (0) | 2 (18) | 0 (0) | 1 (5) | 4 (17) | 0 (0) | N/A | 4 (57) | 0 (0) | 8 (17) | 4 (13) |
| Disagree | 1 (20) | 1 (25) | 1 (9) | 0 (0) | 2 (11) | 7 (29) | 0 (0) | N/A | 1 (14) | 1 (50) | 5 (11) | 9 (29) |
| Strongly disagree | 0 (0) | 1 (25) | 0 (0) | 0 (0) | 0 (0) | 2 (8) | 1 (25) | N/A | 0 (0) | 0 (0) | 1 (2) | 3 (10) |
| 4. I felt fully involved in the discussions about the care and treatment of my relative | ||||||||||||
| Strongly agree | 1 (20) | 1 (25) | 3 (27) | 1 (100) | 5 (26) | 2 (8) | 0 (0) | N/A | 0 (0) | 0 (0) | 9 (20) | 4 (13) |
| Agree | 3 (60) | 1 (25) | 4 (36) | 0 (0) | 8 (42) | 5 (21) | 2 (50) | N/A | 2 (29) | 1 (50) | 19 (41) | 7 (23) |
| Neither agree nor disagree | 0 (0) | 1 (25) | 2 (18) | 0 (0) | 3 (16) | 7 (29) | 0 (0) | N/A | 3 (43) | 0 (0) | 8 (17) | 8 (26) |
| Disagree | 1 (20) | 0 (0) | 2 (18) | 0 (0) | 2 (11) | 8 (33) | 1 (25) | N/A | 2 (29) | 1 (50) | 8 (17) | 9 (29) |
| Strongly disagree | 0 (0) | 1 (25) | 0 (0) | 0 (0) | 1 (5) | 2 (8) | 1 (25) | N/A | 0 (0) | 0 (0) | 2 (4) | 3 (10) |
| 5. Staff always seemed knowledgeable about the care and treatment of my relative | ||||||||||||
| Strongly agree | 0 (0) | 0 (0) | 3 (27) | 0 (0) | 5 (26) | 3 (12) | 0 (0) | N/A | 0 (0) | 0 (0) | 8 (17) | 3 (10) |
| Agree | 5 (100) | 1 (25) | 5 (46) | 1 (100) | 7 (37) | 10 (42) | 3 (75) | N/A | 5 (71) | 2 (100) | 25 (54) | 14 (45) |
| Neither agree nor disagree | 0 (0) | 1 (25) | 3 (27) | 0 (0) | 5 (26) | 4 (17) | 0 (0) | N/A | 2 (29) | 0 (0) | 10 (22) | 5 (16) |
| Disagree | 0 (0) | 2 (50) | 0 (0) | 0 (0) | 1 (5) | 4 (17) | 0 (0) | N/A | 0 (0) | 0 (0) | 1 (2) | 6 (19) |
| Strongly disagree | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 3 (12) | 1 (25) | N/A | 0 (0) | 0 (0) | 2 (4) | 3 (10) |
| 6. Staff seemed to care about my needs as well as those of my relative | ||||||||||||
| Strongly agree | 0 (0) | 0 (0) | 1 (9) | 0 (0) | 7 (37) | 2 (8) | 0 (0) | N/A | 0 (0) | 0 (0) | 8 (17) | 2 (7) |
| Agree | 3 (60) | 0 (0) | 4 (36) | 1 (100) | 6 (32) | 8 (33) | 2 (50) | N/A | 1 (14) | 1 (50) | 16 (39) | 10 (32) |
| Neither agree nor disagree | 1 (20) | 2 (50) | 4 (36) | 0 (0) | 3 (16) | 4 (17) | 0 (0) | N/A | 5 (71) | 1 (50) | 13 (28) | 7 (23) |
| Disagree | 0 (0) | 1 (25) | 2 (18) | 0 (0) | 2 (11) | 7 (29) | 1 (25) | N/A | 1 (14) | 0 (0) | 6 (13) | 8 (26) |
| Strongly disagree | 1 (20) | 1 (25) | 0 (0) | 0 (0) | 1 (5) | 3 (12) | 1 (25) | N/A | 0 (0) | 0 (0) | 3 (7) | 4 (13) |
| 7. I could always speak to a doctor about the care of my relative | ||||||||||||
| Strongly agree | 0 (0) | 0 (0) | 2 (18) | 1 (100) | 5 (26) | 2 (8) | 0 (0) | N/A | 1 (14) | 0 (0) | 8 (18) | 3 (10) |
| Agree | 5 (100) | 1 (25) | 4 (36) | 0 (0) | 9 (47) | 7 (29) | 1 (33) | N/A | 0 (0) | 0 (0) | 19 (42) | 8 (26) |
| Neither agree nor disagree | 0 (0) | 1 (25) | 2 (18) | 0 (0) | 2 (11) | 4 (17) | 0 (0) | N/A | 3 (43) | 2 (100) | 7 (16) | 7 (23) |
| Disagree | 0 (0) | 2 (50) | 2 (18) | 0 (0) | 2 (11) | 8 (33) | 1 (33) | N/A | 3 (43) | 0 (0) | 8 (18) | 10 (32) |
| Strongly disagree | 0 (0) | 0 () | 1 (9) | 0 (0) | 1 (5) | 3 (12) | 1 (33) | N/A | 0 (0) | 0 (0) | 3 (7) | 3 (10) |
| 8. I would like to have been more involved in the care and treatment of my relative | ||||||||||||
| Strongly disagree | 0 (0) | 0 (0) | 1 (9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | N/A | 1 (14) | 0 (0) | 2 (4) | 0 (0) |
| Disagree | 1 (20) | 1 (33) | 5 (45) | 0 (0) | 7 (35) | 4 (17) | 1 (25) | N/A | 1 (14) | 1 (50) | 15 (32) | 6 (20) |
| Neither agree nor disagree | 2 (40) | 1 (33) | 4 (36) | 1 (100) | 3 (15) | 5 (21) | 1 (25) | N/A | 4 (57) | 0 (0) | 14 (30) | 7 (23) |
| Agree | 1 (20) | 1 (33) | 1 (9) | 0 (0) | 7 (35) | 10 (42) | 1 (25) | N/A | 1 (14) | 1 (50) | 11 (23) | 12 (40) |
| Strongly agree | 1 (20) | 0 (0) | 0 (0) | 0 (0) | 3 (15) | 5 (21) | 1 (25) | N/A | 0 (0) | 0 (0) | 5 (13) | 5 (17) |
| 9. Staff always listened to my views and opinions about the care of my relative | ||||||||||||
| Strongly agree | 1 (20) | 0 (0) | 1 (9) | 1 (100) | 3 (15) | 3 (12) | 1 (25) | N/A | 0 (0) | 0 (0) | 6 (13) | 4 (13) |
| Agree | 2 (40) | 1 (25) | 6 (55) | 0 (0) | 12 (60) | 4 (17) | 1 (25) | N/A | 2 (29) | 0 (0) | 23 (49) | 5 (16) |
| Neither agree nor disagree | 2 (40) | 1 (25) | 3 (27) | 0 (0) | 3 (15) | 15 (62) | 2 (50) | N/A | 4 (57) | 2 (100) | 14 (25) | 18 (58) |
| Disagree | 0 (0) | 2 (50) | 1 (9) | 0 (0) | 1 (5) | 0 (0) | 0 (0) | N/A | 1 (14) | 0 (0) | 3 (6) | 2 (7) |
| Strongly disagree | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 2 (8) | 0 (0) | N/A | 0 (0) | 0 (0) | 1 (2) | 2 (7) |
| 10. I always knew who to speak to if I had questions about the care of my relative | ||||||||||||
| Strongly agree | 0 (0) | 0 (0) | 1 (9) | 0 (0) | 5 (25) | 4 (17) | 1 (25) | N/A | 0 (0) | 0 (0) | 7 (15) | 4 (13) |
| Agree | 4 (80) | 3 (75) | 5 (45) | 1 (100) | 8 (40) | 6 (25) | 2 (50) | N/A | 4 (57) | 1 (50) | 23 (49) | 11 (35) |
| Neither agree nor disagree | 0 (0) | 1 (25) | 2 (18) | 0 (0) | 2 (10) | 6 (25) | 1 (25) | N/A | 1 (14) | 1 (50) | 6 (13) | 8 (26) |
| Disagree | 1 (20) | 0 (0) | 3 (27) | 0 (0) | 4 (20) | 5 (21) | 0 (0) | N/A | 2 (29) | 0 (0) | 10 (21) | 5 (16) |
| Strongly disagree | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 3 (12) | 0 (0) | N/A | 0 (0) | 0 (0) | 1 (2) | 3 (10) |
| Mean (SD) | 3.35 (0.70) | 2.90 (0.75) | 3.65 (0.73) | N/A | 3.68 (0.90) | 3.01 (0.94) | 2.87 (1.14) | N/A | 3.21 (0.51) | 3.30 (0.42) | 3.51 (0.81) | 3.07 (0.91) |
| Median (IQR) | 3.50 (2.63–3.93) | 3.00 (2.10–N/A) | 3.60 (3.10–4.40) | N/A | 4.00 (3.10–4.40) | 2.85 (2.33–3.90) | 3.20 (1.60–N/A) | N/A | 3.20 (2.90–3.70) | 3.30 (3.00–N/A) | 3.55 (3.10–4.08) | 3.0 (2.38–3.90) |
| Mann–Whitney U-test | p = 0.031 | |||||||||||
| Theme | Ward | All wards | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 6 | ||||||||
| Implementation period | Delivery period | Implementation period | Delivery period | Implementation period | Delivery period | Implementation period | Delivery period | Implementation period | Delivery period | Implementation period | Delivery period | |
| Questionnaires returned (n) | 8 | 4 | 18 | 2 | 21 | 25 | 26 | 16 | 10 | 4 | 83 | 51 |
| Number with comments | 4 | 4 | 12 | 1 | 11 | 12 | 3 | 3 | 8 | 2 | 38 | 22 |
| Staff attitude, n (%) | ||||||||||||
| Positive comments | 1 (25) | 1 (25) | 7 (58) | 1 (100) | 6 (54) | 2 (17) | 2 (67) | 2 (67) | 4 (50) | 0 (0) | 20 (53) | 6 (27) |
| Negative comments | 1 (25) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (25) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 3 (14) |
| Communication, n (%) | ||||||||||||
| Positive comments | 0 (0) | 0 (0) | 3 (25) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (8) | 0 (0) |
| Negative comments | 0 (0) | 0 (0) | 4 (33) | 0 (0) | 1 (9) | 2 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 5 (13) | 2 (9) |
| Care and treatment, n (%) | ||||||||||||
| Positive comments | 0 (0) | 2 (50) | 4 (33) | 1 (100) | 3 (27) | 3 (25) | 0 (0) | 1 (33) | 3 (38) | 2 (100) | 10 (26) | 9 (41) |
| Negative comments | 1 (25) | 1 (25) | 3 (25) | 0 (0) | 0 (0) | 2 (17) | 0 (0) | 0 (0) | 2 (25) | 0 (0) | 6 (16) | 3 (14) |
| Availability of staff, n (%) | ||||||||||||
| Positive comments | 0 (0) | 0 (0) | 1 (8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 0 (0) |
| Negative comments | 2 (50) | 1 (25) | 1 (8) | 0 (0) | 3 (27) | 1 (8) | 0 (0) | 2 (67) | 5 (63) | 0 (0) | 11 (29) | 4 (18) |
| Food, n (%) | ||||||||||||
| Positive comments | 1 (25) | 1 (25) | 3 (25) | 0 (0) | 2 (18) | 0 (0) | 1 (33) | 1 (33) | 0 (0) | 0 (0) | 7 (18) | 2 (9) |
| Negative comments | 1 (25) | 0 (0) | 1 (8) | 0 (0) | 0 (0) | 0 (0) | 1 (33) | 0 (0) | 2 (25) | 0 (0) | 5 (13) | 0 (0) |
| Environment, n (%) | ||||||||||||
| Positive comments | 0 (0) | 0 (0) | 1 (8) | 1 (100) | 1 (9) | 1 (8) | 1 (33) | 0 (0) | 1 (13) | 1 (50) | 4 (11) | 3 (14) |
| Negative comments | 0 (0) | 0 (0) | 3 (25) | 1 (100) | 0 (0) | 1 (8) | 0 (0) | 0 (0) | 1 (13) | 0 (0) | 4 (11) | 2 (9) |
| Theme | Ward | All wards | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 6 | ||||||||
| Implementation period | Delivery period | Implementation period | Delivery period | Implementation period | Delivery period | Implementation period | Delivery period | Implementation period | Delivery period | Implementation period | Delivery period | |
| Questionnaires returned (n) | 5 | 4 | 11 | 2 | 20 | 25 | 4 | 0 | 7 | 2 | 47 | 33 |
| Number with comments | 4 | 3 | 7 | 1 | 13 | 14 | 3 | 0 | 5 | 1 | 32 | 19 |
| Staff attitude, n (%) | ||||||||||||
| Positive comments | 0 (0) | 1 (33) | 3 (43) | 1 (100) | 2 (15) | 2 (14) | 2 (67) | – | 1 (20) | 1 (100) | 8 (25) | 5 (26) |
| Negative comments | 0 (0) | 0 (0) | 1 (14) | 0 (0) | 0 (0) | 1 (7) | 0 (0) | – | 0 (0) | 0 (0) | 1 (3) | 1 (5) |
| Communication, n (%) | ||||||||||||
| Positive comments | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 1 (8) | 0 (0) | 0 (0) | – | 1 (20) | 0 (0) | 2 (6) | 1 (5) |
| Negative comments | 0 (0) | 1 (33) | 0 (0) | 0 (0) | 2 (15) | 6 (43) | 1 (33) | – | 1 (20) | 0 (0) | 4 (12) | 7 (37) |
| Care and treatment, n (%) | ||||||||||||
| Positive comments | 1 (25) | 1 (33) | 3 (43) | 1 (100) | 2 (15) | 4 (29) | 1 (33) | – | 1 (20) | 1 (100) | 8 (25) | 7 (37) |
| Negative comments | 1 (25) | 1 (33) | 1 (14) | 0 (0) | 3 (23) | 6 (43) | 0 (0) | – | 1 (20) | 0 (0) | 6 (19) | 7 (37) |
| Availability of staff, n (%) | ||||||||||||
| Positive comments | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | – | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Negative comments | 1 (25) | 1 (33) | 0 (0) | 0 (0) | 1 (8) | 2 (14) | 1 (33) | – | 2 (40) | 0 (0) | 5 (16) | 3 (16) |
| Food, n (%) | ||||||||||||
| Positive comments | 1 (25) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | – | 0 (0) | 0 (0) | 1 (3) | 0 (0) |
| Negative comments | 1 (25) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (7) | 0 (0) | – | 0 (0) | 0 (0) | 1 (3) | 1 (5) |
| Environment, n (%) | ||||||||||||
| Positive comments | 0 (0) | 0 (0) | 1 (14) | 0 (0) | 3 (23) | 4 (29) | 1 (33) | – | 1 (20) | 0 (0) | 6 (19) | 4 (21) |
| Negative comments | 1 (25) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (7) | 0 (0) | – | 0 (0) | 0 (0) | 1 (3) | 1 (5) |
Phase 1:
The night staff were very good and helpful even more so than the day staff.
One nurse in particular was such a bully and treated us roughly while others were very helpful and caring.
Phase 2:
Very kind.
Theme: communication
Phase 1: no comments.
Phase 2: no comments.
Theme: care and treatmentPhase 1:
The hygiene of patients leaves a lot to be desired.
I have been in hospital for some time and never had a shower. When I got home my wife was horrified at the dirt between my toes and bed sores on my bottom.
Phase 2:
I felt that I was well looked after at all times.
While a patient on the ward I felt relaxed and reasonably well cared for by mostly friendly staff.
Oral hygiene (could be improved).
Theme: availability of staff
Phase 1:
The call system was not so good, we had to wait too long for attention.
More staff needed.
Phase 2:
Nurses saying they would come back with, for example, medication and not returning for anything up to an hour.
Staff were overstretched meaning bells could remain unanswered for nearly 20 minutes.
Theme: food
Phase 1:
The food was good. Small portions appreciated.
The food was not good.
Phase 2:
The food [was particularly good].
Theme: environment
Phase 1: no comments.
Phase 2: no comments.
Carer Theme: staff attitudePhase 1: no comments.
Phase 2:
Staff were kind.
Theme: communication
Phase 1: no comments.
Phase 2:
Keeping relatives/carer informed [could be improved].
Was not informed until I asked how she was. She had the D and V virus. Was never asked anything about her care.
Theme: care and treatment
Phase 1:
I was very happy with the level of care my mother received.
Night staff do not seem to be able to give the same care as day staff.
Phase 2:
The ward provided an excellent level of nursing and care.
Theme: availability of staff
Phase 1:
Buzzers not responded to quickly enough.
Phase 2: no comments.
Theme: foodPhase 1:
Good food.
Food is very important and the quality of the hospital food was not great.
Phase 2: no comments.
Theme: environmentPhase 1:
Great attention seemed to be given to cleaning, but on several occasions I had to do cleaning that had been overlooked.
Phase 2: no comments.
Ward 2 Patient Theme: staff attitudePhase 1:
The ward were very helpful and friendly.
Was very surprised that staff remembered my prior stay considering how many patients they see – very nice and personal.
Everyone treated me very well and with respect.
Auxiliary staff very friendly.
I found that the staff were both friendly and efficient.
I was treated with care and consideration and friendliness. This helped considerably as I was in pain and frightened and I am grateful to them all.
Nice to have cheery staff who work well together and care. Even cleaners, etc. very kind and friendly.
I found a friend in and a very friendly face of the trauma nurse who always managed to pop up when I was feeling anxious, always made me feel less worried as she had time to explain what and when would happen.
Phase 2:
The team work of all the staff was impressive.
Theme: communication
Phase 1:
The physio[therapy] team were helpful and listened to what I said about mobilising, etc. I felt more vulnerable at the weekend when staff tried to insist I walk further than was possible. I was upset to think that they thought I was shirking. Eventually I managed to get through to them that I had other problems than the ones evident.
Doctors explained closely what had to be done in the operation.
I was put in a side room with one other lady. She went for her operation at 1.15 p.m. and no one said hello or checked on me until about 4 p.m. I think a few smiles and hellos would have helped as I felt in isolation.
Could be improved: doctors and nurse on different shifts being informed better.
Elderly could be confused as some of the assistance or help was misled, e.g. told on admission I would be given a menu for tomorrow but was not.
Phase 2: no comments.
Theme: care and treatmentPhase 1:
The nursing care and the surgeons were excellent.
Nursing staff [were particularly good].
The help of most nurses and all the doctors [was particularly good].
[F]elt I was discharged too early and no consideration was given to my home environment and the challenges I would face.
Had no grumbles other than wish discharge process did not take all day – not exaggerating either.
Was given some equipment for nurses to use but it was never used.
Really impressed and relieved to have such good care.
Phase 2:
The care could not have been better.
Theme: availability of staff
Phase 1:
I found that there were plenty of nurses around.
Would have been improved by more nurses.
Phase 2: no comments.
Theme: foodPhase 1:
The food was excellent.
Food not always hot enough.
The food was good.
Phase 2: no comments.
Theme: environmentPhase 1:
A TV would have been helpful to pass the time.
I found the bed very difficult to move around in, also the pump noise overnight kept me awake.
On admission I requested a single room. I was informed ‘yes’, as soon as one is available. This did not happen for five days.
Pleased I got a side room – in case of pain and also enabled family to be more flexible with visits.
Phase 2:
Could be improved by: a little less noise at night.
Having my own en-suite room made a bit of difference regarding privacy.
Carer
Theme: staff attitude
Phase 1:
Even the cleaning staff were sociable.
The doctors/surgeon were caring and sympathetic to my wife’s age and condition.
My wife needed to go to the toilet but was told by a nurse it was meal time and must wait. I do not consider this to be correct for a lady of 74 years of age.
Phase 2:
Everyone cared, which makes a difference.
Theme: communication
Phase 1: no comments.
Phase 2:
A little too much pressure in asking me to look at nursing homes, when I made it clear I wanted to give my best at caring for my husband at home.
Theme: care and treatment
Phase 1:
Everything was excellent.
The care of the nursing staff was very good.
Needs to be staff to deal with dementia – as well as nursing care.
My Dad has been very happy and comfortable during his stay.
Phase 2:
Everyone knew what they were doing and did it well.
Theme: availability of staff
Phase 1: no comments.
Phase 2: no comments.
Theme: foodPhase 1: no comments.
Phase 2: no comments.
Theme: environmentPhase 1:
The ward was really modern and up to date. My wife was in a side ward which gave her privacy.
Phase 2: no comments.
Ward 3 Patient Theme: staff attitudePhase 1:
General health-care assistants attentive and supportive.
All friendly and helpful.
Caring and cheerful.
The hospital staff and nurses were wonderful.
The staff were always good and helpful.
The nursing staff were very caring and looked after myself and family.
Phase 2:
The cheerfulness of the doctors, nurses and staff on the ward. ‘BRILLIANT’.
When I left a nurse was there and wished me well.
Could be improved by: better service and attitude.
The cleaning staff unfriendly and at one point rude.
I feel that because I am older staff are inclined to treat me differently.
Theme: communication
Phase 1:
Communication between myself and my relatives. Speed of decisions regarding my conditions. Would have preferred my family to have been consulted as I am very deaf and 87 years old and at times while in hospital was delirious with infection.
Phase 2:
[Could be improved:] allowing a member of family to stay as I had problems communicating with the staff at the hospital.
Communication [could be improved].
Discharged to respite care without any involvement or notification to the family. Only knew of discharge as Dad was sat waiting for transport when we arrived to visit (having visited every day and still informed by no-one).
Theme: care and treatment
Phase 1:
The care I got from A&E to ward X and then to ward Y [POD ward], excellent.
5 star accommodation and treatment.
The nursing staff on wards X and Y [POD ward] were very caring and looked after myself and family.
Phase 2:
Overall the staff were very good apart from one night when all four patients pressed their buzzers and were ignored for eight hours. That night was torture and I asked to come home. It was Saturday night July 7th. Buzzer noise never stopped. No nurse came . . .. The nurse did say sorry but it made me feel terrible so I asked to come home.
Everything [was good].
Care on the ward [was good].
Hair washed once in four weeks. Always had to ask three or four times to be taken for bath or shower.
Very good all round.
Theme: availability of staff
Phase 1:
There could be more staff at times. It seemed as though at times that the ward was short staffed.
Never seemed to have time to talk. If I asked for something they often forgot.
Seemed short staffed at night.
Phase 2:
Not enough nurses to answer questions, they were friendly and helpful but very rushed.
Theme: food
Phase 1:
Very good food.
Food very good.
Phase 2: no comments.
Theme: environmentPhase 1:
The chance to be cared for in a single room with my own bathroom giving privacy for me and my visitors [was particularly good].
Phase 2:
Boring. Left all day sitting on chair or in bed with only visitors to talk to for 4 weeks.
Very nice and clean ward, spacious which was nice.
Ward 4
Patient
Theme: staff attitude
Phase 1:
Nurses very hard working and encouraging.
All staff top to bottom very friendly.
Phase 2:
Very friendly staff.
Care and concern from the staff.
Theme: communication
Phase 1: no comments.
Phase 2: no comments.
Theme: care and treatmentPhase 1: no comments.
Phase 2:
I think I was very well looked after by all the staff, they work very hard and in my opinion need a medal.
Theme: availability of staff
Phase 1: no comments.
Phase 2:
Could be improved by: more staff [two patients provided this comment].
Theme: food
Phase 1:
The food [could be improved].
The food was lovely.
Phase 2:
I thought the food very good and plenty, my plate was always clean!
Theme: environment
Phase 1:
Hospital ward – very clean.
Phase 2: no comments.
Carer Theme: staff attitudePhase 1:
Pleasant staff.
Very friendly staff all round. Nice experience.
Phase 2: no comments.
Theme: communicationPhase 1:
Not impressed with discharge plans – ITC package was arranged but no details passed onto family despite concerns raised about patients’ memory/capacity. Started on Oramorph [Boehringer Ingelheim, Ingelheim, Germany] but not sent home with it even though it was on discharge sheet. No details about warfarin dose. Very difficult to get updates about care and what plans were, etc., etc. Nobody seemed to know.
Phase 2: no comments.
Theme: care and treatmentPhase 1:
My wife was looked after very well.
Phase 2: no comments.
THEME: Availability of staffPhase 1:
Staff seemed, short staffed at one point, she could not talk for about 30 minutes because she was on her own.
Phase 2: no comments.
Theme: foodPhase 1: no comments.
Phase 2: no comments.
Theme: environmentPhase 1:
Clean ward.
Phase 2: no comments.
Ward 6 Patient Theme: staff attitudePhase 1:
The staff were hardworking and friendly and kind. Their patience with the elderly and dementia sufferers was wonderful.
I was very impressed with the attitude of the nursing staff – they were cheerful and patient, even with some very difficult patients.
All the nursing staff were fantastic very helpful and comforting when necessary.
On the whole the staff were good with patients in spite of being busy all the time.
Phase 2: no comments.
Theme: communicationPhase 1: no comments.
Phase 2: no comments.
Theme: care and treatmentPhase 1:
The staff nurses and sister were extremely good.
The doctors and nursing staff were very good.
I was very satisfied with the care I receive[d].
Old people were left sitting by the bed all day. I felt like they needed more stimulation, perhaps a TV room where they could talk together.
The discharge procedure is an absolute disgrace. I was asked at 8.45 p.m. to transfer to [hospital iv] and then be discharge[d] the following day. I am an 80 year old lady who lives alone.
Phase 2:
Nursing care [was particularly good].
I was well looked after for the one night I stayed in hospital after my operation.
Theme: availability of staff
Phase 1:
Number of nurses on shift [could be improved].
[Would be improved by:] more non-medical personnel to help with feeding, drinks and other non-medical tasks.
[Would be improved by:] more staff on at nights.
[Would be improved by:] more nursing staff in evening and night.
Seemed short staffed at night.
Phase 2: no comments.
Theme: foodPhase 1:
Meals [could be improved].
Nutritional value of food [could be improved].
Phase 2: no comments.
Theme: environmentPhase 1:
I was impressed with the amount of cleaning taking place on the ward. When I mentioned I had knocked over a glass of water I came back from the toilet to find not only had the floor been dealt with but my bed had been changed and fresh water put out.
The noise level was terrible, not just from patients but from ‘chirping’ beds.
Phase 2:
Clean modern surroundings.
Carer
Theme: staff attitude
Phase 1:
The nursing and ancillary staff were attentive towards me and my mum and daughter.
Phase 2:
The general attitude of the staff was very pleasant and inspired confidence.
Theme: communication
Phase 1:
The consultant who was responsible for the care of my relative expressed a wish to consult me and took time to do so after completing his operation visit. Following on to relative's discharge – I was informed by staff that my relative was on her way to her residential home – saving me a visit. The consultant was willing to see me and another close relative to discuss my mother's ongoing treatment and care.
[Could be improved by:] communication and explanation with patient/relative when there was an extended wait on a day when an operation was expected.
Phase 2: no comments.
Theme: care and treatmentPhase 1:
They made sure she was eating and drinking regularly. As she had dementia it was comforting to know she was being well cared for.
The rapid decision to discharge an 80 year old lady at 9 p.m. I find totally disgraceful.
Ideally there should be a nominated person when a patient with dementia is an inpatient. Not necessarily a nurse but someone to help with eating, drinking and generally befriend. They need not be ‘friends’ with only one patient, but it would ease the pressure on the nurses if a non-medical assistant or two were available.
Phase 2: no comments.
Theme: availability of staffPhase 1:
Number of nurses on the ward [could be improved].
The ward was understaffed. Nurses struggled at night as there was quite a few dementia patients which was quite distressful for other patients.
Phase 2:
With the staffing levels as they were, the staff did really well.
Theme: food
Phase 1: no comments.
Phase 2: no comments.
Theme: environmentPhase 1:
Hospital ward was very clean.
Phase 2: no comments.
Patient and carer comments are taken from open-text responses to questions reproduced with permission (Juliette Harrison, NHS, 2010, personal communication) from the Care Quality Commission. 50© Queen’s Printer and Controller of HMSO 2021. This work was produced by Young et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Appendix 4 Cost-effectiveness of an integrated delirium prevention intervention for elderly hospitalised patients
Jenny Willson,a David Meads,a Chantelle Browne,a Claire Hulmea and John Youngb
aAcademic Unit of Health Economics, University of Leeds, Leeds, UK
bAcademic Unit for Ageing and Stroke Research, University of Leeds, Leeds UK
Abstract
Introduction and background
Delirium is a common outcome in hospitalised older patients; with the growing concern of the burden of the UK’s ageing population on NHS budgets, it is becoming increasingly important to find cost-effective methods for both treatment and prevention. Previous work suggests that multicomponent targeted interventions (MTIs) are successful in preventing delirium. However, currently, there is no proven, transferable system of care capable of reliably addressing delirium in the UK. This study will analyse the cost-effectiveness of a MTI, which is integrated into routine care practices, in reducing the burden on the NHS of delirium among elderly hospitalised medical patients.
Method
The cost-effectiveness analysis was carried out using a state-dependent Markov model to calculate the expected lifetime costs and health benefits of a cohort of elderly hospitalised patients. In the treatment arm of the decision model, the patients receive the integrated intervention as a tool for delirium prevention; in the control arm, the patients receive usual care. Deterministic and probabilistic sensitivity analyses were used to assess the scale of the uncertainty surrounding the expected ICER. Value-of-information analysis was employed to highlight the benefits of future research in this area.
Results
The deterministic ICER of using the integrated delirium prevention intervention, as opposed to usual care, is £1057 per QALY gained. The probabilistic sensitivity analysis suggests that there is a 100% probability that the intervention will be cost-effective when the willingness to pay per QALY threshold is £30,000. At this willingness-to-pay threshold, there is no expected value of perfect information (EVPI).
Conclusion
Given the current information, we can be fairly certain that an integrated delirium prevention intervention is a cost-effective way of preventing delirium. Future research should prioritise the average lengths of stay of elderly hospitalised patients without delirium, and the utility values of health states experienced by elderly patients without delirium, in hospital and post discharge.
Keywords
Delirium; cost-effectiveness; elderly hospitalised patients.
Introduction and background
Delirium is a common disorder that affects approximately one-third of hospitalised older people and is associated with increased mortality, increased length of stay, poor functional recovery and increased costs. 100 The consequences of delirium are increasing in line with demographic changes and it is becoming increasingly important to find cost-effective methods for both treatment and prevention. 101 The aim of this analysis is to determine the cost-effectiveness of a delirium prevention intervention for hospitalised elderly patients. The focus of the analysis is on an intervention that integrates delirium prevention activities in established routine care practices.
Delirium is defined as a disturbance of consciousness and cognition with a rapid development and fluctuating course over time. 102,103 There are many causes of delirium, such as infections, surgery, metabolic problems and drugs, but people differ in their susceptibility, best described in terms of delirium risk factors. 102 The greater the number of risk factors, the greater the susceptibility to delirium. This model of delirium provides an important opportunity for prevention. Reducing the burden of delirium risk factors in individual patients might reduce the incidence of delirium. This clinical approach has been investigated in experimental studies using multicomponent interventions targeting delirium risk factors. The evidence from these studies suggests that delirium could be prevented in around 30–40% of cases. 12,97,104 This evidence was recently reviewed by NICE and a recommendation was made supporting a multicomponent intervention package in the NHS that prevents delirium by addressing 10 key risk factors: cognitive impairment/disorientation, dehydration and/or constipation, hypoxia, infection, limited mobility or immobility, pain, polypharmacy effects, poor nutrition, sensory impairment and sleep disturbance. 8 However, there is no routine, transferable care system currently available in the UK that systematically addresses these risk factors and prevents delirium in elderly hospitalised patients. 105
Several multicomponent delirium prevention interventions have been described and investigated. The HELP has been developed in North America as a standardised and manualised multicomponent delirium prevention programme and evaluated in medical patients. 12 It is delivered by additional and specifically trained hospital staff and supervised, trained hospital volunteers, and consists of detailed interventions (or ‘protocols’) that each include recommendations aimed at one of six delirium risk factors. A derivation of the HELP was investigated in Australia. 106 The intervention was delivered entirely by volunteers, and only five of the eight protocols were implemented. In a further modification, the HELP was deployed in surgical wards in Taiwan without any volunteer involvement and addressing only three delirium risk factors. 16
Other multicomponent delirium prevention interventions have been developed specifically for patients with a hip fracture. One developed in the USA involved daily structured assessments by geriatricians, leading to initiation of up to five of 10 protocol-based recommendations, such as supplemental oxygen or discontinuation of unnecessary medications. 107 A complex intervention developed in Belgium used a system of enhanced quality nursing care in which nurses were trained to identify high-risk patients and have a better understanding of delirium risk factor management. 19 A similar intervention in Sweden introduced individual delirium care plans, systematic prevention and treatment of post-operative complications, nutritional support and rehabilitation for functional retraining. 108
Finally, an intervention was developed in Australia aimed at reducing delirium by providing a ‘hospital at home’ service for geriatric rehabilitation patients. 109 This service was provided by a hospital-based multidisciplinary outreach body, comprising nurses, physiotherapists, occupational therapists and doctors.
These previous interventions are experimental, ‘proof of concept’ studies that have investigated the general approach of reducing risk factor burden in people at risk of delirium implementation, and have therefore required additional staff and other resources to supplement existing routine care. However, the risk factor approach has been referred to as ‘basic’ care, that is the sort of care that frail older people might reasonably expect to receive. 82 It is therefore reasonable to assume that delirium prevention by risk factor modification has the potential to be incorporated into routine ward care practices, rather than require additional staff. This would involve cultural and practice changes delivered by education, training and new care systems. The aim of the analysis presented here is to provide a preliminary investigation into the cost-effectiveness of such an integrated delirium prevention intervention care system and, therefore, the extent to which commissioners and providers should prioritise the approach.
An initial review of the literature was conducted to analyse previous cost-effectiveness analyses of delirium prevention interventions. Search terms included ‘cost’, ‘economic’ and ‘delirium’ and results were limited to the English language. Five economic evaluations were identified. 22,24,106,109,110 Sample sizes ranged from 37106 to 476324 patients. The perspective for analysis for all studies was either that of the health-care provider or a third-party payer, and all studies took a short-term perspective. None of the studies used a decision-analytical model in its analysis or incorporated health-state outcomes.
Three of the studies22,24,106 are economic evaluations of the HELP for delirium prevention as applied to medical patients. 12 Rizzo et al. 22 evaluated the impact of the HELP on total hospital costs, average daily costs and length of stay, and estimated the impact on the cost of specific hospital components, such as nursing costs or pharmacy costs. When the HELP delivery costs were included, there was a statistically significant hospital cost reduction for the patients at high risk of delirium, but not for patients at intermediate risk. Rubin et al. 24 evaluated a replication of the HELP and reported a 14.4% decrease in delirium rate and a reduction in length of stay of 0.3 days for patients who developed delirium, at a net saving of US$790 per patient. Caplan and Harper106 evaluated the HELP model in an Australian geriatric ward in terms of efficacy, cost-effectiveness and sustainability. Length of stay was reduced by 4.3 days for intervention patients, with a total cost saving of AU$41,820 for 16 intervention patients. 106 NICE conducted an economic evaluation of a multicomponent delirium prevention intervention for older patients admitted to medical wards. 8 The model examines the probability of an individual experiencing one of seven adverse consequences of delirium, and evaluates the expected cost and QALY outcomes associated with these adverse consequences. This health economic model indicates that the delirium prevention intervention is more effective, and less costly, than the usual care strategy.
Two health economic evaluations are available for hip fracture patients (who have a very high risk of developing delirium). Webster et al. 110 tested prospectively the impact of two different clinical practice guidelines that aimed to improve the recognition, management and outcomes in elderly hip fracture patients in the USA. 19,97,107 In the intervention phase of the study, there were 12 subjects in the control group and 29 in the intervention group; five out of 12 intervention patients and 29 out of 29 control patients developed delirium (p ≤ 0.01). The number of consultations with neurology/psychiatry departments was higher for the control group than for the intervention group (10/12 and 7/29, respectively; p ≤ 0.01) and the length of stay was longer for the control group than the intervention group (9.1 days vs. 7.4 days; p = 0.04). There were no deaths in the control group and two deaths in the intervention group; six patients from the control group and 12 from the intervention group were placed in nursing homes. A total net saving of US$57,132 was reported for 29 treatment patients. In the UK, NICE developed a health economic model to analyse the cost-effectiveness of a multicomponent delirium prevention intervention for hip fracture patients and reported that the prevention intervention was more effective and less costly than the usual care strategy. 8
Caplan et al. 109 evaluated whether or not home-based rehabilitation for frail older patients was associated with a lower incidence of delirium, lower costs and greater satisfaction than hospital rehabilitation. The costs of home rehabilitation and hospital rehabilitation (acute plus rehabilitation combined) were AU$12,185 and AU$25,042 (p = 0.0109), respectively. The overall duration of care was lower for the home rehabilitation group than for the hospital group [average 34.91 days and 40.09 days (p = 0.1889), respectively].
In summary, the published evaluations of multicomponent interventions to prevent delirium suggest that it is likely to be an effective clinical strategy to reduce the incidence of delirium in hospitals and to reduce the length of stay. Furthermore, the studies indicate that such a strategy is likely to result in cost savings, although the scale of these savings seems dependent on the intervention and clinical setting. Our literature review, and that conducted by NICE, reveals that there is a paucity of research that addresses the cost-effectiveness of delirium prevention strategies. In the main, the identified studies are simple cost evaluations. None of the empirical studies has used decision-analytical modelling to evaluate the long-term costs and outcomes of interventions or presented ICERs and accounted for uncertainty by conducting a sensitivity analysis. Moreover, the findings are based on the need for considerable additional resources to deliver the intervention, which is likely to be improbable during routine care dissemination. Thus, there is a need for more robust economic evaluations of multicomponent delirium prevention interventions in the context of routine, rather than experimental, care.
Methods
The cost-effectiveness of an integrated, routine care delirium prevention intervention was assessed using a decision-analytic framework. A state transition model (Markov model) was used to simulate the cost and effects for a hypothetical cohort of elderly hospitalised patients. Markov models describe patient progression over time through a pathway of health states, with movement between the health states being triggered by events such as hospital discharge or death. Resource use and costs are associated with each health state and the patients also accumulate QALYs in each health state. The nature of the Markov model means that it is possible to incorporate state-dependent transitions and to observe outcomes over a lifetime time horizon. The hypothetical cohort was split into a control group (who receive usual care; no specific delirium prevention measures), and a treatment group (who receive the multicomponent intervention), with separate Markov models constructed to estimate the cost-effectiveness of each strategy. The models analysed in this study were made probabilistic to demonstrate the consequences of uncertainty in the model parameters.
Model structure
The health economic model was developed as part of a UK-based research programme that is developing and investigating a new system of care that integrates delirium prevention into routine ward care in NHS hospitals. The structure of the model is a result of discussions between clinicians and health economists and relates to older people admitted for unscheduled care on medical wards. The underlying clinical pathway of the model follows the NICE guidelines for delirium diagnosis, prevention and management. 8
The model structure used in this analysis is summarised in Figure 6.
FIGURE 6.
Model structure.
The model is estimated over a lifetime time horizon using daily cycles. As Figure 6 demonstrates, the model structure remains the same for patients in both the treatment and control groups. The patients are in hospital on entering the model and they enter the Markov cohort once the decision of group allocation has been made. In the first cycle of the Markov cohort, the patients are divided into two groups: those who develop delirium and those who do not. For those who develop delirium, it is assumed that there is a period of a few delirium-free days in hospital before delirium onset. Once the onset of delirium has occurred, the patients are assigned the costs and utilities associated with delirium for the rest of their hospital stay. The model is structured this way because, even after the spell of delirium ends, a patient’s quality of life and their resource use continue to be influenced by the delirium episode because of the increased risk of adverse outcomes. 111
While patients remain in the in-hospital states, they accumulate QALYs, the values of which are based on the estimates of the quality of life of an elderly hospitalised individual and those relating to the experience of delirium. The patients also accumulate bed-day, intervention and other treatment costs. For each cycle the patient cohort spends in hospital, they have a probability of remaining in hospital, dying or being discharged from hospital.
Once discharge occurs, some patients are assumed to move to a nursing or residential home, whereas others are discharged to their own home. The patients have a probability of dying in each subsequent cycle, but will remain in the out-of-hospital states until they die. In the out-of-hospital states, the patients accumulate QALYs, the values of which will reflect the quality of life of older people post hospitalisation and in recovery from the delirium state. Costs associated with long-term nursing or residential care home use are also accumulated. The dead state is associated with zero costs and a utility value of zero.
Model population
Hypothetical cohort
The probabilistic Markov model is populated using values for a hypothetical cohort of patients. We assumed that the patients were aged ≥ 79 years, had been admitted to hospital for unscheduled care on a medical ward, and had one or more of the six key delirium risk factors (cognitive impairment, sleep deprivation, mobility impairment, vision impairment, hearing impairment and dehydration) identified by NICE. 8 The patients were assumed to be free of delirium on entering hospital, and not to have a terminal condition.
Transition probabilities
All input parameters used to populate the economic model are illustrated in Table 31. Estimates for the incidence of delirium for older people admitted to medical wards, including a systematic review, have varied between 3% and 29%. 12,111,115 The base-case estimate of the probability of delirium used a mid-range estimate of 15% from the HELP study. This estimate is well suited to our model because incident delirium was carefully confirmed and the patients were ≥ 70 years of age and had been admitted to medical wards. A sensitivity analysis was conducted using the 3–29% range of delirium incidence values reported in the systematic review. 116 The international literature, as reviewed in Introduction and background, indicates that the average reduction in delirium incidence associated with multicomponent interventions is approximately one-third. 12,116 Therefore, the base-case estimate of the probability of delirium for patients receiving the delirium prevention intervention is 10%, with an assumption that the SD is equal to the mean. 12
| Parameter | Base-case estimate | Distribution | Source | Notes/assumptions |
|---|---|---|---|---|
| Transition probabilities | ||||
| Probability of delirium in hospital | 0.15 | Beta | Inouye et al.12 | |
| Reduction in the probability of delirium in hospital with MTIs | 0.33 | Beta | Inouye et al.12 | |
| Onset of delirium | 0.26 | Beta | O’Keefe and Lavan111 | For those who will get delirium while in hospital. A beta distribution derived from the fact that 77% of patients (42/54) who developed delirium did so within 5 days of entering hospital. This is used to form a daily hazard, which has been converted to a daily probability |
| Length of stay for elderly hospitalised patients | 11 days | O’Keefe and Lavan111 | The length-of-stay figures are used to derive the daily hazard rates and probabilities of discharge from hospital | |
| Additional length of stay of a patient with delirium | 3.6 days | Rubin et al.24 | ||
| Probability of admission to care home post hospital – no delirium | 0.17 | Beta | Bourdel-Marchasson et al.112 | This is the probability that, given a patient has been discharged from hospital, their discharge location is a care home |
| Probability of admission to care home post hospital – delirium | 0.40 | Beta | Bourdel-Marchasson et al.112 | This is the probability that, given a patient has been discharged from hospital, their discharge location is a care home |
| Probability (daily) of dying in hospital – delirium | 0.0050 | Beta | O’Keeffe and Lavan111 | Using the fact that 15 out of 94 patients had died by the end of the 21-day length of stay. This is used to compute the daily hazard of death in hospital, and daily probabilities can then be computed |
| Probability (daily) of remaining in hospital – no delirium | 0.959 | Beta | Defined as 1 minus the sum of the daily probability of leaving hospital and the daily probability of dying in hospital | |
| Probability (daily) of remaining in hospital – no delirium | 0.934 | Beta | Defined as 1 minus the sum of the daily probability of leaving hospital and the daily probability of dying in hospital | |
| Probability (daily) of death for a patient with delirium discharged to home | 0.001358 | Beta | Rockwood et al.113 | Converted from a daily hazard rate, which is derived from a beta distribution, which relies on the fact that the median survival time after discharge for patients with delirium has been reported to be 510 days |
| Probability (daily) of death for elderly patient discharged to home – no delirium | 0.000618 | Beta | Rockwood et al.113 | Converted from a daily hazard rate, which is derived from a beta distribution, which relies on the fact that the median survival time after discharge for elderly, patients without delirium has been reported to be 1122 days |
| Probability (daily) of death for a patient with delirium discharged to care home | 0.001358 | Beta | Rockwood et al.113 | Same as probability of death for a patient with delirium after discharge to home. PSSRU data on survival times for elderly care home patients114 suggest that the median survival time for elderly patients in care is 493 days, which is very similar to the median survival time of 510 days used here (as reported for patients with delirium post discharge by Rockwood et al.113) |
| Probability (daily) of death for elderly patient discharged to care home – no delirium | 0.001358 | Beta | Rockwood et al.113 | Same as probability of death for a patient with delirium after discharge to home. PSSRU data on survival times for elderly care home patients suggests the median survival time for elderly patients in care is 493 days, which is very similar to the median survival time of 510 days used here (as reported for a patient with delirium post discharge by Rockwood et al.113 |
In the first cycle of the model, the patients divide into two groups using the probabilities of delirium incidence, that is some develop delirium, whereas others do not. For the patients who develop delirium, it is assumed that there is a period of delirium-free days before delirium onset. The delay in delirium onset is taken from a UK study in which 77% of patients, who had a mean age of 82 years and had been admitted to an elderly care ward, developed delirium within 5 days of admission. 111 In this study, seven out of 131 patients who did not develop delirium died while in hospital, compared with 15 out of 94 patients with delirium.
The probability that the discharge destination was a new long-term care facility was obtained from a study of patients over 75 years admitted to a medical care unit in which 40% of patients who developed delirium were discharged to new long-term care, compared with 17% who did not develop delirium. 112
The base-case estimate for the average length of hospital stay of 11 days for people aged ≥ 75 years was obtained from the Hospital Episode Statistics 2009–10 data. 117 Unfortunately, it was not possible to obtain length-of-stay information simultaneously by both admission specialty and age. The inflation factor applied to hospital length of stay due to delirium developing in hospital has been reported to range between 3.6 days22 and 116 days. 115 The base-case estimate of the additional length of stay of a patient with delirium was assumed to be 3.6 days, to generate a conservative estimate of the outcomes of the health economic model. However, the estimate of 11 days was used as a sensitivity check.
Hospital and post-admission mortality rates estimates for patients with and patients without delirium were obtained from a study that recruited patients admitted to general medical wards with a mean age of 79 years. 113 The average life expectancy for older people in long-term care, as reported by the Personal Social Services Research Unit (PSSRU),114 is very similar to the average life expectancy of patients who developed delirium in hospital. 113 Consequently, the probability of dying while in a care home for older people who developed delirium and for people who did not develop delirium was assumed to be the same as the average probability of post-discharge death for a patient who developed delirium in hospital.
Utilities
We found no studies that reported utility values in delirium. However, we identified several studies in the literature review that reported quality-of-life data, specifically the Short Form questionnaire-36 items (SF-36) or Short Form questionnaire-12 items, which can be converted to utility values. 118 Of the studies reporting quality-of-life values, only one had a reasonable sample size and included data pre and post delirium. This was a Swedish study of 115 elderly patients (mean age 83 years; 70% female) who had been admitted to hospital either with a hip fracture or for hip replacement surgery. 119 Quality-of-life assessments were conducted using the SF-36 measure at hospital admission (pre delirium) and 6 months post hospital discharge. The author of the study was contacted and agreed to provide the quality-of-life data so that they could be converted to utility values. Delirium was confirmed in 32 (28%) of the sample based on the Diagnostic and Statistical Manual of Mental Disorders, Third Edition, criteria. SF-36 quality-of-life scores were converted into Short Form questionnaire-6 Dimensions utility values using a published UK tariff based on UK general public standard gamble estimates. 118 Utility scores used in the model are summarised in Table 32.
| Parameter | Base-case estimate | Distribution | Source | Notes/assumptions |
|---|---|---|---|---|
| Utilities | ||||
| Daily QALY – delirium in hospital | 0.00163 | Beta | Duppils and Wikblad119 | This is derived from a QALY of 0.592, which comes from the below-mean QALY value for elderly hospitalised patients (0.598) minus a decrement. The decrement is the QALY change observed for patients who develop delirium between the point of admission to hospital and 6 months into the study |
| Daily QALY – in hospital and no delirium | 0.00164 | Beta | Duppils and Wikblad119 | This is derived from the mean utility value (0.598) reported for patients, on entering the study, who did not go on to develop delirium. SD was also reported |
| Daily QALY – in hospital and pre delirium onset | 0.00164 | Beta | Duppils and Wikblad119 | As daily QALY of elderly in hospital |
| Daily QALY – discharged to home and had delirium | 0.00163 | Beta | Duppils and Wikblad119 | As daily QALY of delirium in hospital |
| Daily QALY – discharged to home and never had delirium | 0.00195 | Beta | Duppils and Wikblad119 | Up until the point of 6 months since entering the model, this value is the mean daily QALY of an elderly hospitalised patient (0.00164) plus a daily QALY increment. The daily increment is derived from the QALY change between admission to hospital and 6 months into the study for the patients who do not get delirium, divided by 182 days (0.0003). From the 6-month point, this variable equals the estimated QALY for patients who did not get delirium at 6 months after entering the study (0.653). SD was also reported |
| Daily QALY – discharged to care home and had delirium | 0.00163 | Beta | Duppils and Wikblad119 | As daily QALY of delirium in hospital |
| Daily QALY – discharged to care home and never had delirium | 0.00163 | Beta | Duppils and Wikblad119 | As daily QALY of elderly in hospital |
The utility value used for older people admitted to hospital who do not have delirium is the value reported in the Swedish study119 for patients without delirium at the point of admission to hospital. This value is also used in the model for the utility of patients in hospital before they experience the onset of delirium. The utility value associated with delirium is the value reported in the Swedish study119 for patients without delirium at the point at hospital admission, plus the change in utility between admission and 6 months post discharge for patients who developed delirium. All utility values are divided by 365 to give a daily QALY value.
The utility value used for the patients without delirium discharged to home is given by the utility value they were assigned while they were in hospital, plus an increment. The increment is the change in utility between hospital admission and 6 months post discharge experienced by patients who did not develop delirium, as reported in the Swedish study. 119 The utility value assigned to patients discharged to a care home who did not have delirium is assumed to be the same as the in-hospital utility for these patients. The quality of life of patients who had delirium in hospital is assumed to remain at the same value post discharge if they are discharged to their home, or to a care home. This assumption was made because no suitable utility data were available for post-discharge elderly patients residing in long-term care. In addition, because delirium is more likely to lead to a requirement for long-term care, the assumption that discharge location does not affect utility values is a conservative one.
Costs
The cost values used in the model are described in Table 33. The costs of treating an older person in hospital with delirium or without delirium are obtained by the bed-day cost multiplied by length of stay. The bed-day cost used in the deterministic analysis is the average bed-day cost for all medical inpatients as estimated by the PSSRU. 114 In hospital, patients who develop delirium are likely to experience a greater number of complications, such as falls and pressure ulcers. 111 Although we have not explicitly taken the costs of these complications into account, the greater average length of stay of patients with delirium should capture the majority of these additional costs.
| Parameter | Base-case estimate | Distribution | Source | Notes/assumptions |
|---|---|---|---|---|
| Costs | ||||
| Cost (daily) of the MTI | 0.87 | Gamma | 2011/12 NHS Agenda for Change pay scales and NHS England Pay Circular M&D (April 2011)120 | It is assumed that each full-time equivalent additional staff will see 250 patients per year |
| Bed-day cost | 158 | Gamma | PSSRU114 | |
| Cost (daily) of stay in long-term care | 96.87 | PSSRU114 | A weighted average of the costs of a permanent stay in a private nursing home, private residential care, voluntary residential care or local authority residential care was obtained from the PSSRU. These costs include things such as community nursing, GP services and personal living expenses; personal living expenses were subtracted from the costs. The weighting was carried out using the proportion of elderly people in each of the institutions in 1996 (Netten et al.121) | |
The aim of our analysis is to assess the cost-effectiveness of a UK-based integrated delirium prevention model for elderly hospitalised medical patients. The unique feature of the intervention is that the delirium prevention activities are integrated into usual hospital care routines. In addition, it is proposed that the associated training for the clinical staff is delivered during time already allocated for this purpose. It is therefore envisaged that the integrated delirium prevention programme can be implemented at minimal or zero additional cost.
The cost associated with long-term care is a weighted average of the costs of a permanent stay in a private nursing home, private residential care, voluntary residential care or local authority residential care. The weekly cost of a permanent stay in each of these facilities was reported by the PSSRU,114 and a further study121 has reported the share of patients in each of the four care home types.
Analysis
The outcomes of the cost–utility analysis are measured by the average number of patient life-years adjusted by utility weights to produce QALYs, and cost outcomes are given as the average daily cost per patient. The overall cost-effectiveness outcome is given by the ICER, that is the ratio of the difference in costs (for treatment vs. control) divided by the difference in effects (for treatment vs. control). In the UK, the threshold ICER for an affordable treatment has been estimated to be up to £30,000. The perspective of the analysis is that of the service provider in that only direct costs to the service provider have been considered. Previous work has suggested that around 70% of long-term care residents are publicly funded;121 therefore, the effect of this was explored in the sensitivity analyses. The time horizon was the lifetime of the patient cohort and daily cycles were assumed. Half-cycle corrections and discounting at a rate of 3.5% were applied to the costs and utilities assigned to the patients in each state. The model was constructed and analysed using TreeAge Pro 2011 (TreeAge Software, Inc., Williamstown, MA, USA).
Deterministic sensitivity analysis
The base case of the model is estimated using expected values for the input parameters, and these values are subject to a degree of uncertainty. In the deterministic sensitivity analysis, estimates of the key model parameters were independently varied between upper and lower bands of plausible values.
Probabilistic sensitivity analysis
Probabilistic sensitivity analysis allows the level of overall uncertainty in the model to be assessed. To carry out the probabilistic sensitivity analysis, probability distributions were fitted to the input parameters using published SDs, or by assuming that the SD was equal to the mean when there was no available distributional information. The beta distribution was used to model the uncertainty around binomial parameters (probabilities and utilities), and the gamma distribution was used to model uncertainty around cost parameters. The analysis used 10,000 iterations by Monte Carlo simulation.
Value-of-information analysis
The decision analysis performed is based on expected cost-effectiveness given the available information. The presence of uncertainty means that there is a probability that a ‘wrong’ decision could be made and the current estimate of net benefit of a strategy may change as uncertainties surrounding model inputs are resolved. The expected cost of uncertainty, or the EVPI, is defined by the probability that a decision based on current information is wrong multiplied by the costs of making the wrong decision. The EVPI is the maximum that should be paid for further research, which will inform the decision.
The calculation of the EVPI relies on the concept of net benefit (in this case, net monetary benefit):
where λ is the cost-effectiveness threshold, ΔE is the incremental benefit, and ΔC is the incremental cost of the treatment. The EVPI is calculated by employing non-parametric methods122 using the simulated output from the Monte Carlo simulation. The net benefit of each strategy is computed at each iteration and the mean of all the net benefits is computed for each strategy, as well as the mean value of selecting the optimal strategy (greatest net benefit) at each iteration. The EVPI is the difference between the expected value of the decision made with perfect information [the expected value of choosing the alternative with the greatest net benefit at each iteration, Emax(NB)], and the expected value of the decision made on the basis of existing evidence {the value of choosing the alternative that has the greatest overall expected net benefit, max[E(NB)]}:
The population EVPI estimates are based on an annual incidence of 177,860 cases of delirium in elderly patients hospitalised as emergency cases. This estimate was calculated by multiplying the number of emergency admissions (5,177,887) as reported by the Hospital Episode Statistics in 2009/10117 by the proportion of all admissions for patients aged ≥ 75 years (0.229), giving a figure of 1,185,736.12 elderly emergency admissions. It was then assumed that 15% of these patients (177,860) would develop delirium. 12
Results
The base case of the model gives an ICER of £1057 per QALY gained associated with the integrated multicomponent delirium prevention system of care, compared with usual care. Over the lifetime of the patient cohort, comparing the multicomponent intervention with the usual care strategy, there is a marginal increase in costs of £152 and a marginal benefit of 0.14 QALYs per patient. The results from the deterministic sensitivity analysis around the base-case result are displayed in Figures 7–14.
The base case of the model assumed a reduction in the probability of incident delirium of 33%. The analysis presented in Figure 7 indicates that, even if the reduction in the probability of incident delirium was half the size of the base-case estimate, the ICER for the treatment strategy remained cost-effective, at around £1500 per QALY. Previous estimates of the probability of incident delirium for elderly hospitalised medical patients have ranged between 3% and 29%; Figure 8 demonstrates that varying the base-case estimate of the probability of delirium in the control group between 3% and 21% produces estimates of the ICER for the treatment strategy that lie between £3000 and £0 per QALY. Increasing the probability of delirium incidence to > 21% results in the treatment strategy dominating the control strategy by being cheaper and more effective.
FIGURE 7.
Impact of the reduction in the probability of delirium.
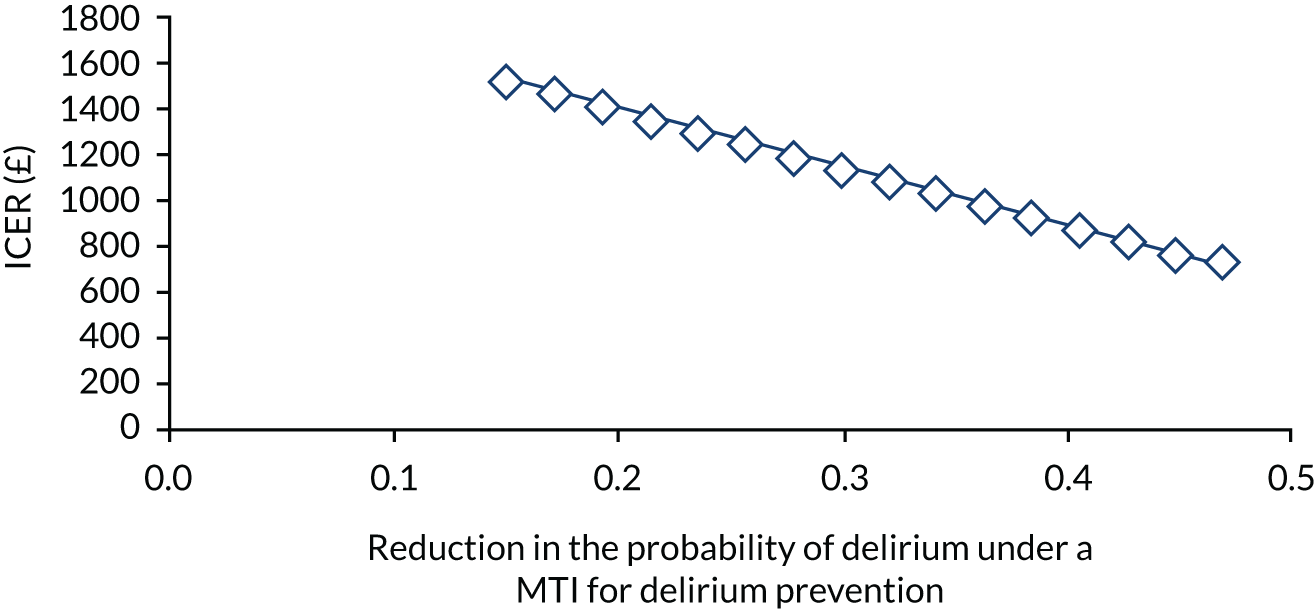
FIGURE 8.
Impact of the probability of delirium under usual care on the ICER of the control strategy.
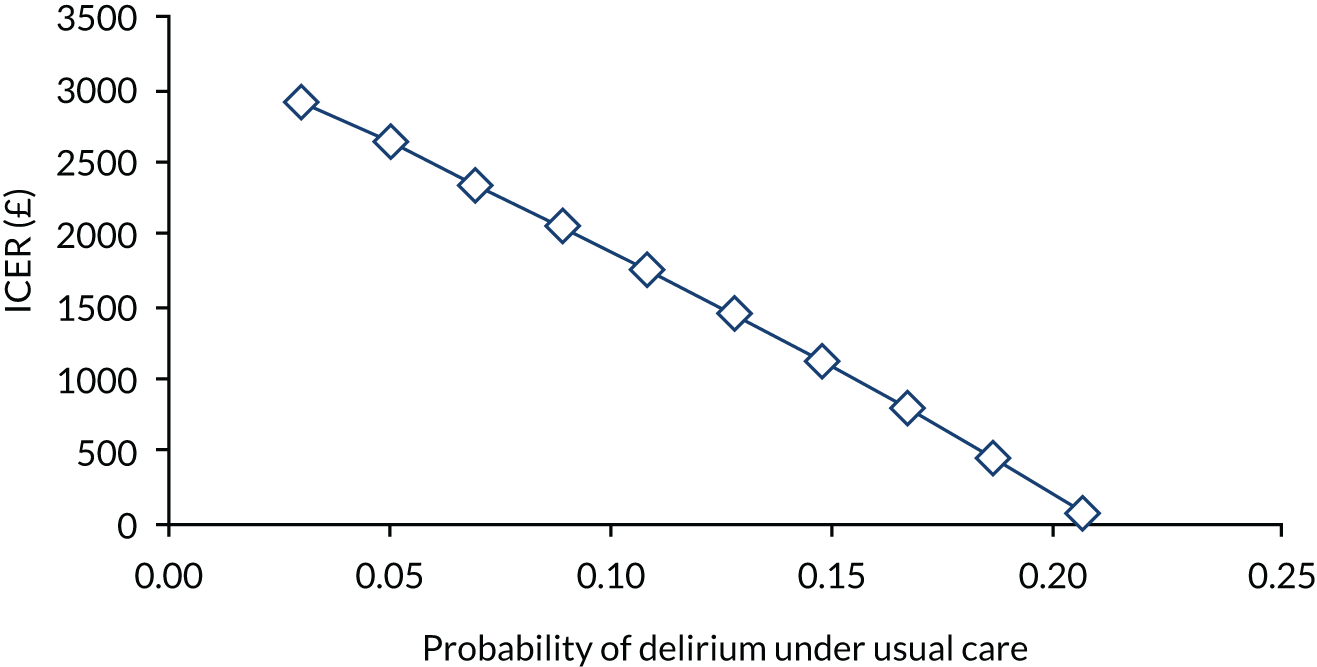
Variations in the mean length of stay of hospitalised elderly patients have a large impact on the ICER value for the treatment strategy (Figure 9). When the base-case estimate of hospital length of stay is increased by 50%, the ICER for the treatment strategy increases by > 100%, to around £2600, and, when reduced by 50%, the ICER value is around £100. However, variations in the additional length of hospital stay for patients with delirium do not have a large impact on the ICER values for the treatment strategy. The base-case estimate of the additional length of hospital stay for patients with delirium was assumed to be 3.6 days. However, previous literature has shown that this could be as high as 11 days. 123 The sensitivity analysis presented in Figure 10 indicates that, if patients with delirium stayed in hospital for 11 days longer than patients without delirium, the ICER for the treatment strategy would decrease slightly to £824 per QALY.
FIGURE 9.
Impact of the probability of the length of hospital stay for patients without delirium on the ICER of the treatment strategy.
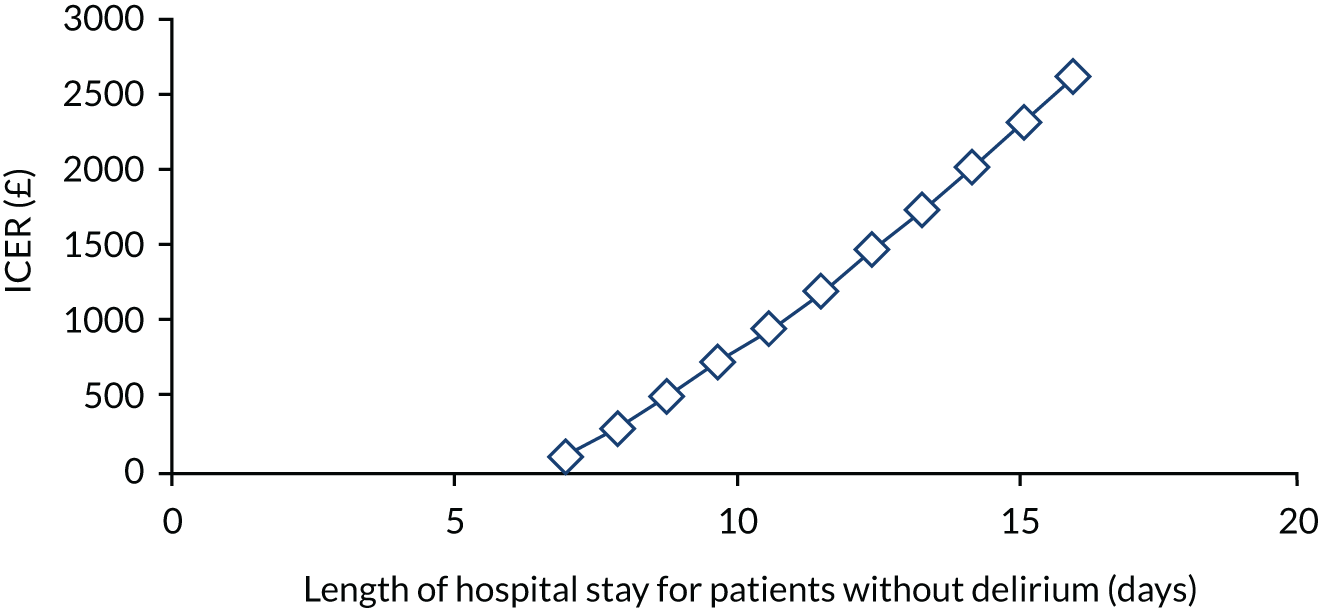
FIGURE 10.
Impact of additional hospital length of stay of patients with delirium on the ICER for the treatment strategy.
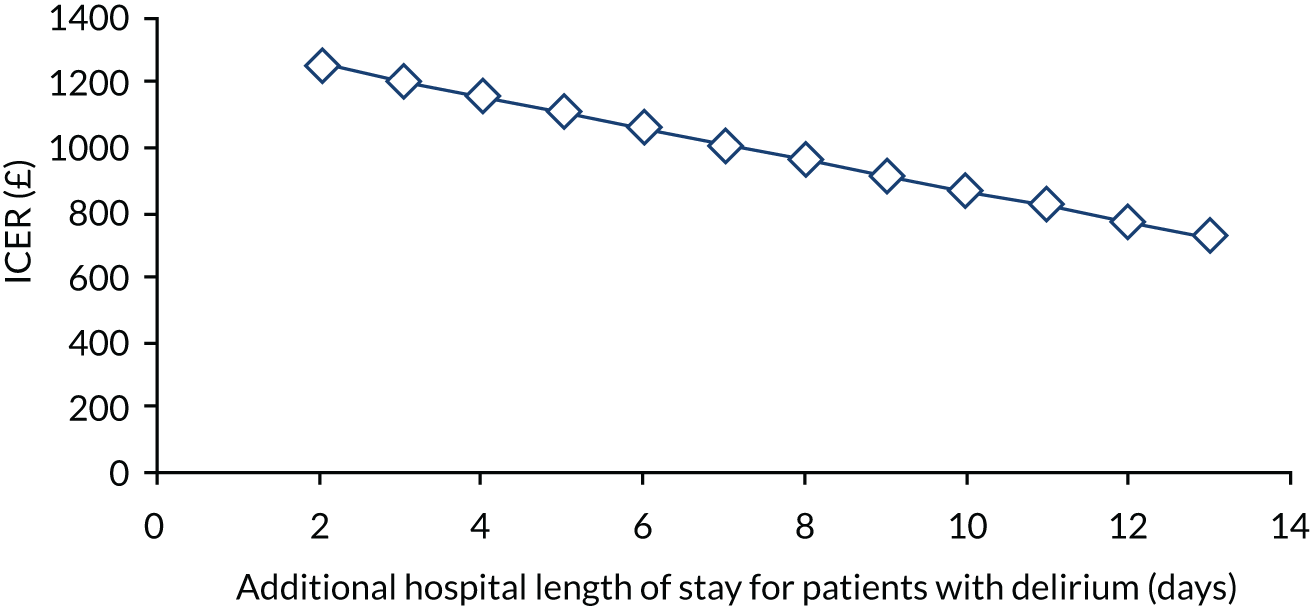
Similarly, the utility values for patients without delirium discharged home seems to have a larger impact on the potential range of the ICER than does the utility of patients with delirium discharged home (Figures 11 and 12, respectively). The base-case estimate of the daily utility of a patient without delirium discharged home is 0.0019. If this figure is reduced by 50% to 0.00095, the ICER is £2273. Increasing the daily utility of patients with delirium discharged to home by 50% increases the ICER to £1169.
FIGURE 11.
Impact of changes in the daily QALY of patients discharged to home who did not have delirium on the ICER for the treatment strategy.
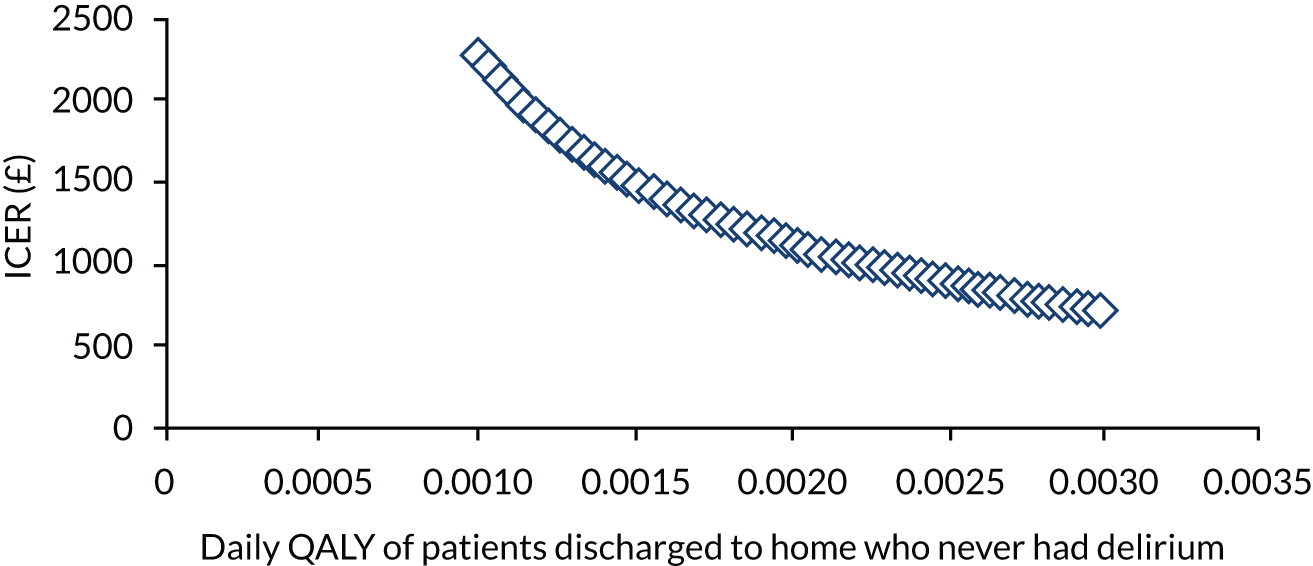
FIGURE 12.
Impact of changes in the daily QALY of patients discharged to home who had delirium on the ICER of the treatment strategy.
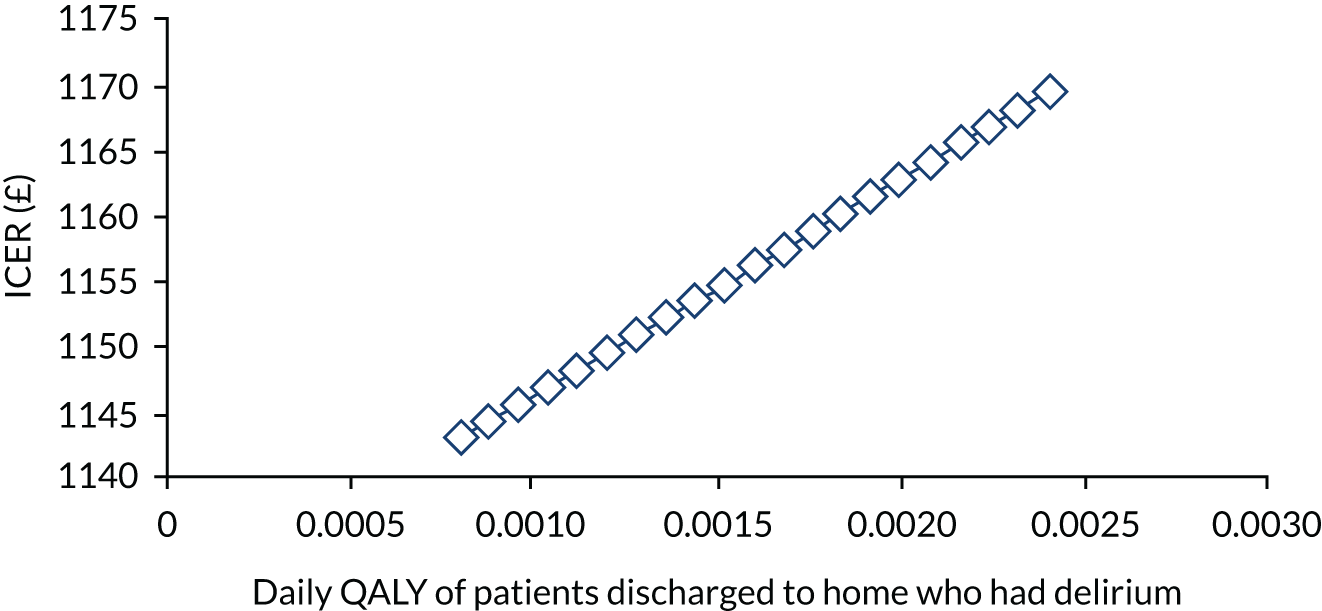
The impact of changing all other variables used in the model by 50% in either direction on the sensitivity of the ICER is demonstrated in Figures 13 and 14. A 50% decrease in the probability that a patient without delirium will die in hospital decreases the ICER for the intervention to just over £155, and a 50% increase in this parameter increases the ICER to just under £1900. This again suggests that bed-day costs are having a large impact on the ICER. Increasing the probability of discharge to a care home by 50% for patients without delirium also has a large impact on the ICER for the treatment strategy, increasing the ICER to just over £1800. However, the value of this ICER remains well under the £30,000 cost-effectiveness acceptability threshold. The ICER of the treatment strategy is not very sensitive to variation in the probability of a patient with delirium dying in hospital, or whether or not a patient is discharged home or to long-term care. Similarly, varying the utility values, the bed-day cost, the discount rate, the cost of stay in long-term care and the rate of onset of delirium by 50% in each direction had little impact on the value of the ICER.
FIGURE 13.
Sensitivity of the ICER A.
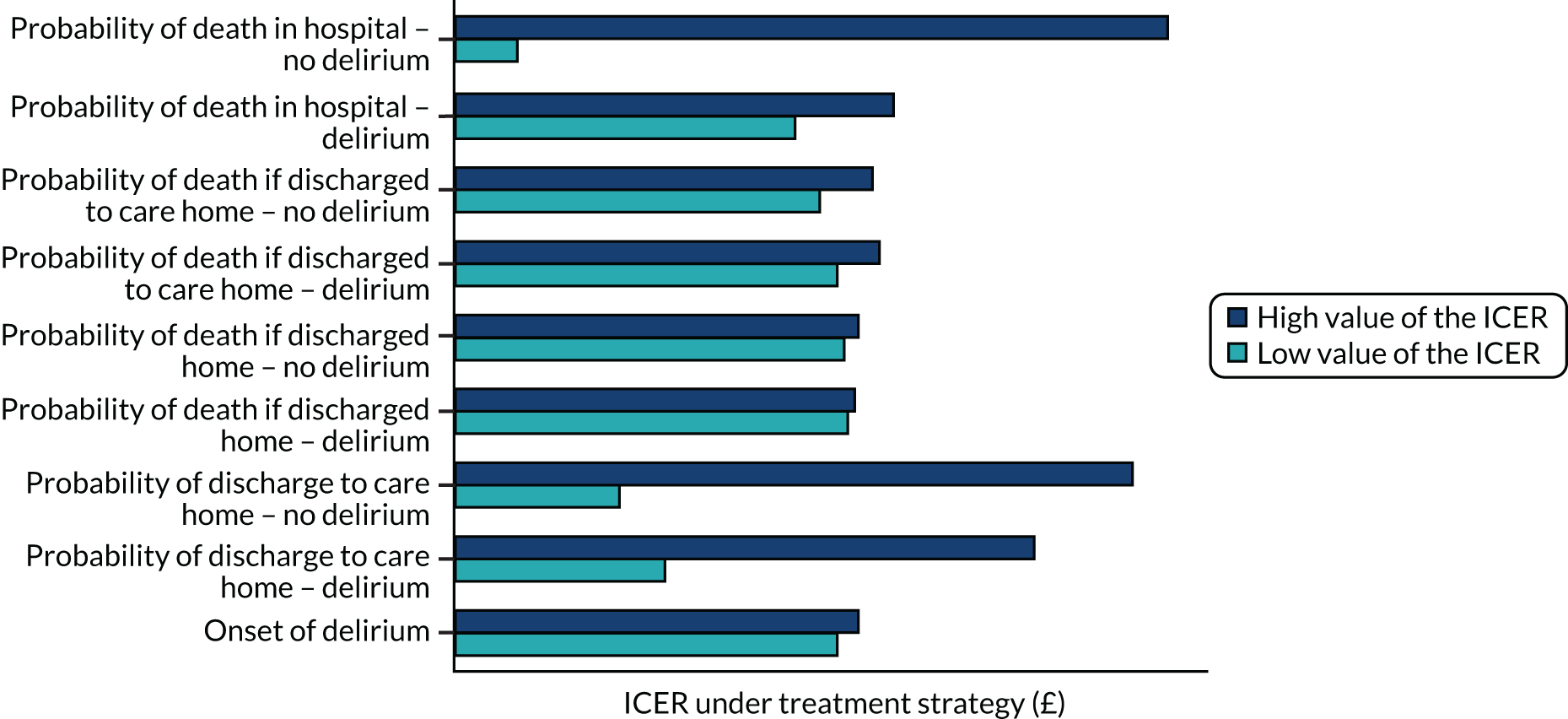
FIGURE 14.
Sensitivity of the ICER B.
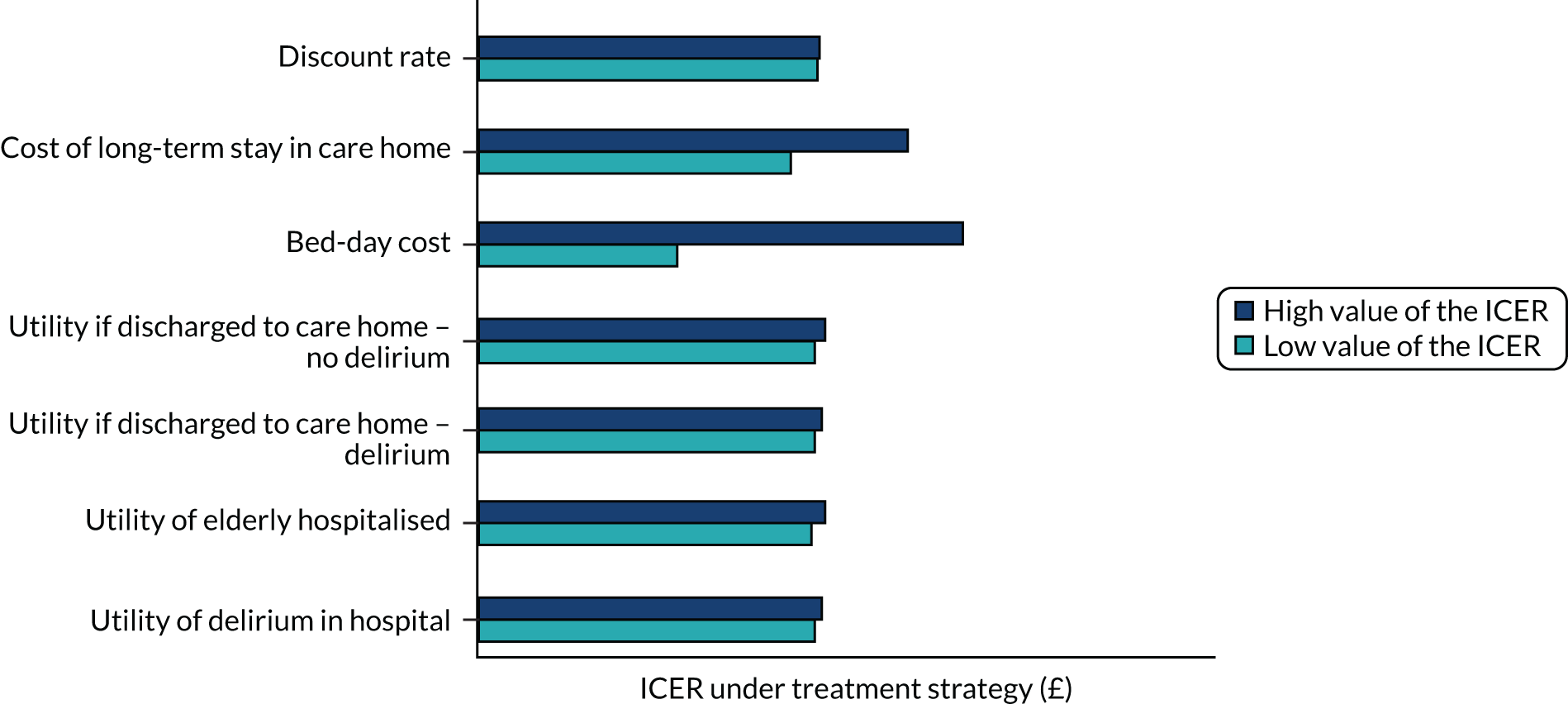
The outcomes of the probabilistic sensitivity analysis, carried out using 10,000 iterations of the Monte Carlo simulation, are displayed in Figure 15. This demonstrates that there is little uncertainty surrounding the incremental cost and effectiveness of the delirium prevention intervention, as there is relatively little variation in the outcomes of the simulation. The incremental effectiveness is likely to lie between 0.135 and 0.155 and the incremental cost is likely to lie between –£220 and £300. These results reinforce that the small magnitude of the base-case ICER is being driven by the relatively small incremental cost of the intervention and moderate incremental QALY effect of the intervention strategy, as well as the low sensitivity of the ICER illustrated in the deterministic sensitivity analysis. The small magnitudes and the lack of uncertainty in the incremental costs and effects produced by the probabilistic sensitivity analysis simulations are such that there is a 100% probability that the integrated delirium prevention intervention will be cost-effective at the £30,000 willingness-to-pay threshold. Lowering the threshold to £10,000 produces the same result (Figure 16).
FIGURE 15.
Distribution of outcomes of cost-effectiveness model after running Monte Carlo simulation.
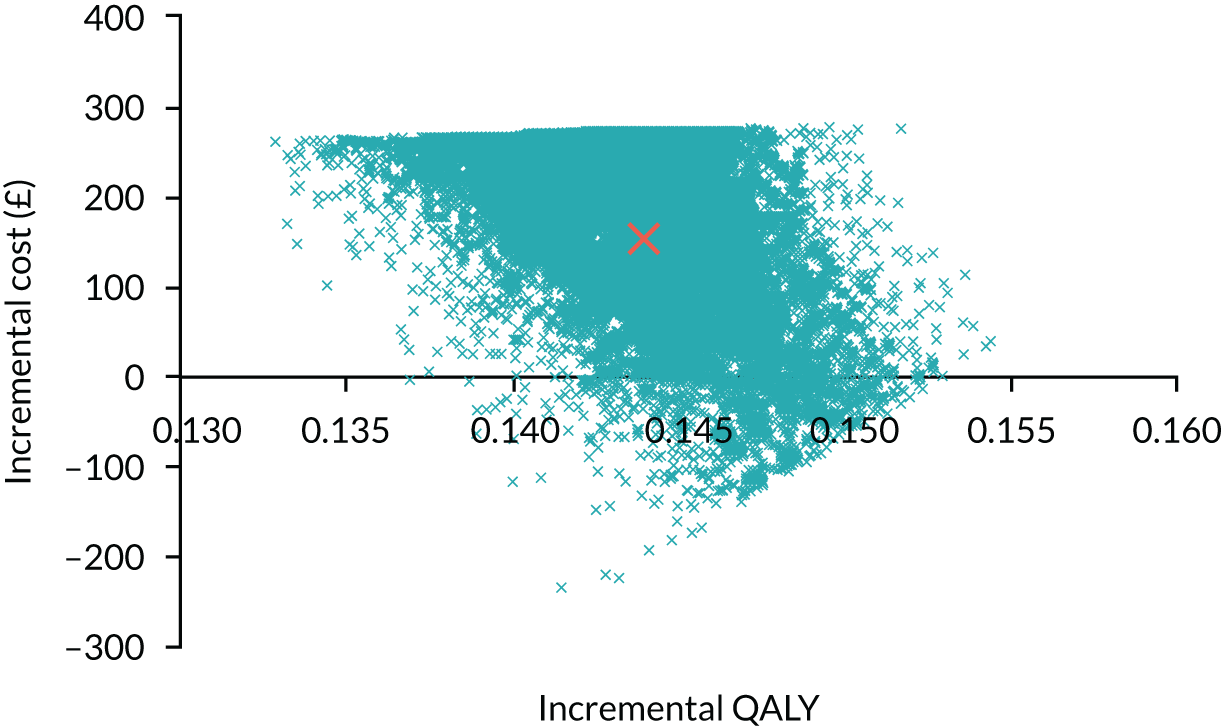
FIGURE 16.
Probability that the integrated delirium prevention intervention is cost-effective, relative to usual care.
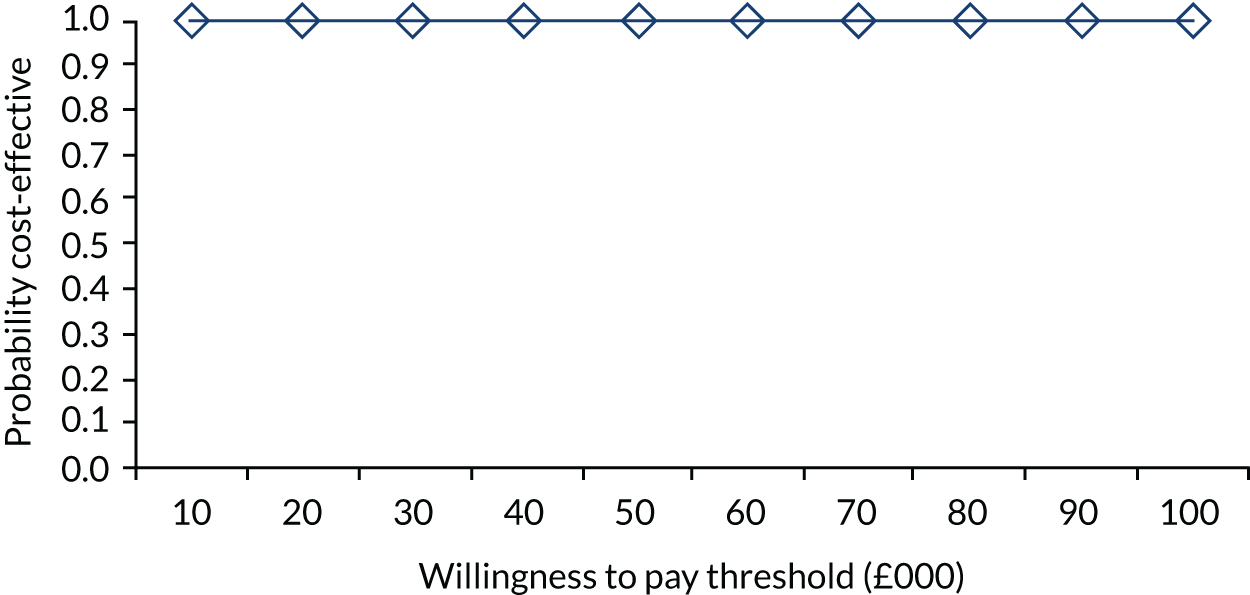
Figure 17 demonstrates the mean net benefit generated by the integrated delirium prevention intervention by willingness to pay per QALY threshold. At a willingness to pay per QALY threshold of £20,000, the mean net benefit of the intervention is just under £3000. At a threshold of £30,000, the mean net benefit is just over £4000. Figure 17 indicates that the mean net benefit increases at a constant rate with the willingness-to-pay threshold.
FIGURE 17.
Net monetary benefit of the integrated delirium prevention intervention.
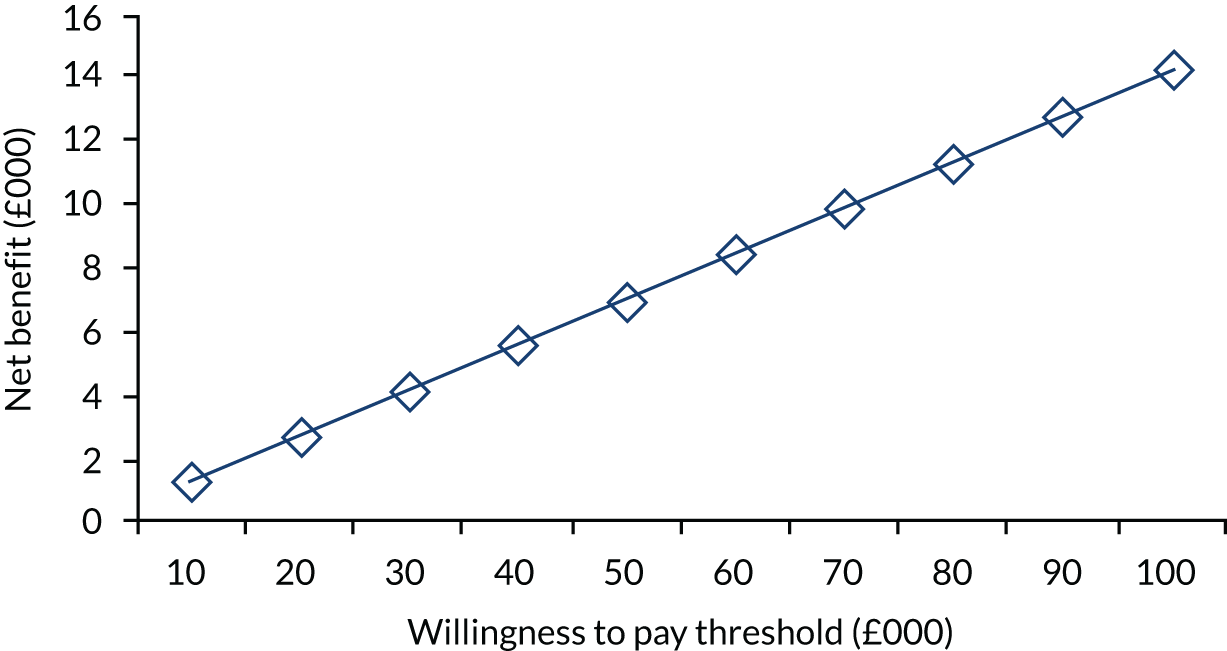
Value-of-information analysis
Because the probabilistic sensitivity simulation indicated that the intervention strategy would be cost-effective 100% of the time, in each iteration of the simulation, the intervention strategy has a greater net benefit than the control strategy. Therefore, the mean net benefit under perfect information will be the same as the mean net benefit of the intervention strategy (the overall preferred strategy with uncertain information) and the expected value of information is zero. Because the integrated delirium prevention intervention has a 100% probability of being cost-effective at any willingness to pay per QALY threshold, the EVPI will be zero for all these threshold values.
Discussion
The results demonstrate that an integrated delirium prevention intervention would be a very cost-effective way of preventing delirium in older people admitted to medical wards. At £1057, the base-case estimate for the ICER is well below the accepted £30,000 willingness-to-pay threshold. Furthermore, the probabilistic sensitivity analysis suggests that, at the £30,000 willingness to pay per QALY threshold, there is a 100% chance that the intervention strategy will be cost-effective. Thus, within the tolerances of the model input parameters, we can be nearly certain that a ward-based and integrated multicomponent intervention is a cost-effective way of preventing delirium in elderly hospitalised medical patients.
The deterministic sensitivity analysis suggests that the base-case estimate of the ICER is fairly robust to changes in most of the variables included in the model. Varying the model inputs by 50% in either direction does not produce an ICER that lies above the £30,000 willingness to pay per QALY threshold. One of the model inputs that appears to have the strongest impact on the variability of the ICER is length of hospital stay of patients without delirium. If this length of stay is increased by 50%, the ICER becomes more than double that of the base-case estimate. However, this large increase in length of stay, particularly for patients with a less complicated clinical course, is an unlikely event within an established health-care policy that is driving down lengths of hospital stay. In addition, a 50% decrease in the utility of patients discharged home who did not develop delirium in hospital produces an ICER that is around double the base-case estimate. However, because the value of the ICER is small relative to the willingness to pay per QALY threshold, even increasing the base-case estimate of length of hospital stay by 150% generates an ICER that lies well within the £30,000 willingness-to-pay threshold.
The ICER estimate result from our model is slightly smaller than that previously reported in analyses of multicomponent delirium prevention interventions. 22 This smaller ICER estimate is likely to reflect the novel, but realistic, approach to delirium prevention in routine care based on integration of the prevention risk factor protocols, rather than the use of additional staff described in the experimental studies. In addition, the ICER estimate is likely to differ from that reported by NICE. 8 In that analysis, the probability that a patient with delirium might experience one of seven adverse consequences of the condition was assessed, and expected cost and QALY outcomes were estimated. Furthermore, the difference in hospital lengths of stay of patients with and patients without delirium is not incorporated into the NICE model. 8 The current model includes delirium-related differences in length of stay and the resulting greater total bed-day costs accumulated by patients with delirium is considered to account for the range of potential adverse consequences of delirium. These adverse consequences are additionally captured in the model by assigning a lower utility value to delirious, as compared with patients without delirium. The current study also incorporates a rate of delirium onset, reflecting the fact that patients often develop delirium after being in hospital for a few days. These patients are not assigned the lower utility values associated with delirium until they actually develop delirium.
All the input parameters used in the model, other than the utility estimates, are derived from studies that have investigated delirium in general medical patients. However, the utility values used in the model are based on patients with hip fracture. If, on average, medical patients have a lower quality of life than hip fracture patients, the economic model presented in this analysis will provide a conservative estimate of the ICER for the treatment strategy. A literature search has not produced any findings that can inform how these utility weights may differ from those of medical patients. The fact that utility is likely to decrease with old age has not been incorporated into the model. Population utility values have been previously reported by age groupings,77 rather than by years of age, and therefore are not useful for use in this model. The impact of this omission on the ICER of the integrated delirium prevention strategy will depend on the extent to which utility declines with age and whether or not there is a differential in decrements between those who have and those who have not experienced delirium. However, the sensitivity analysis indicated that the cost-effectiveness of the integrated delirium prevention intervention is not particularly sensitive to changes in utility values employed in the model.
It may be argued that updated information regarding the proportion of elderly patients in each of the four types of care homes will increase the accuracy of the findings presented in this study. The information used in the model is based on 1996 estimates111 and the current proportions may be quite different. If the current composite cost of being in a care home is actually smaller than the base-case estimate used in the model, then the results may underestimate the ICER for the treatment strategy. However, the sensitivity analysis demonstrates that the ICER is relatively unresponsive to changes in the cost of a care home stay.
The model structure is constrained by a lack of available information. For instance, it is a reasonable expectation that individuals who developed delirium will be more likely to have a re-admission to hospital. A re-admission to hospital would incur costs via generating additional resource usage and would be likely to lead to a decrease in a patient’s quality of life. However, no relevant literature concerning hospital re-admission rates related to delirium could be found. If patients who develop delirium are more likely to experience a re-admission to hospital, then omitting the possibility of re-admission will provide a conservative estimate of the cost-effectiveness of the treatment strategy.
The model has also not explicitly accounted for the possibility of persistence of delirium or the possibility of post-hospital delirium recurrence. Persistence of delirium is a relatively new concept and may be present in about 25% of people at 6 months. 90 The fact that we allow for lower utilities post hospital discharge for individuals who developed delirium should at least partially capture the negative quality-of-life implications of this emerging issue. The assumption has been made that the intervention will be integrated into routine ward care without the need for additional staff. In addition, it has been assumed that any training needed will be absorbed into the regular training time schedules for hospital staff. If the programme implementation involves the need for extra staff appointments, or additional training that needs to be provided in newly scheduled time periods, these additional costs would need to be taken into account.
In terms of future research, the results are robust and suggest that the integrated delirium prevention intervention is very cost-effective. It therefore seems less important to conduct future research into the effectiveness of delirium prevention by risk factor modification if we are willing to pay £30,000, £20,000 or even £10,000 per QALY. However, the sensitivity analyses suggests that future research might usefully clarify the effects of delirium on the average length of stay of elderly hospitalised patients and the QALY values of health states experienced by patients with and patients without delirium. Moreover, the results suggest that the ICER is fairly sensitive to changes in the post-discharge utility scores for patients who did not develop delirium. The current utility values used in the model are based on hip replacement patients and, therefore, are unlikely to accurately represent the hypothetical cohort. The ability to include age-dependent utility decrements in the model would additionally improve the accuracy of the cost-effectiveness findings. The value-of-information analysis indicates the importance of increasing the accuracy in the parameters input to the model. However, further research concerning issues surrounding the accuracy of model structure and the estimates for the variables may additionally benefit the results found in this analysis. Although the omission of the probability of re-admission to hospital, the persistence of delirium and the probability of delirium recurrence from the model ensures a conservative estimate of the ICER of an integrated strategy for delirium prevention, further research into these areas will help provide a greater understanding of the overall cost and effectiveness of an integrated delirium prevention strategy for elderly hospitalised medical patients.
Appendix 5 Health economic study
Introduction
Evidence on the value for money of health-care interventions is increasingly important to decision-makers. The POD programme of research included a health economic WS whose aim was to establish the feasibility of an economic evaluation in this population and setting and to provide preliminary estimates of the cost-effectiveness of the POD intervention.
Aims and objectives
Project 3 contained an embedded economic study. The overall aim of the economic study was to establish the feasibility of conducting an economic evaluation of the POD programme and to determine preliminary estimates of its cost-effectiveness. Specific objectives were as follows:
-
determine the feasibility of collecting the assessments needed (quality of life and health-care resource use) for an economic evaluation in this patient group
-
determine the number of missing data in assessments
-
determine the validity and responsiveness of quality-of-life assessments in this group
-
determine the feasibility of collecting and of using/interpreting proxy-completed assessments
-
estimate the cost of the POD intervention
-
provide estimates of the cost-effectiveness of POD versus usual care
-
compare these estimates with those from the earlier evaluation based on decision modelling.
Methods
The data required to achieve the health economic objectives were collected in the POD feasibility trial (project 3) alongside the main trial outcomes.
Data collection
Quality of life
Quality of life was assessed using the EuroQol-5 Dimensions, three-level version (EQ-5D-3L). 68 This was collected at baseline and at 1 and 3 months. In some cases, the EQ-5D-3L was completed with the help of (or by) a proxy. At baseline and at 1 month, the EQ-5D-3L was completed face to face with a researcher or health-care professional present; at 3 months it was completed by means of a postal survey.
Health-care resource use
Health-care resource use was captured using a specially designed questionnaire, which was completed by patients (and/or proxies) at 3 months only. The questionnaire asked the respondent to record any primary care (e.g. GP visit or nurse visit) or secondary care (e.g. hospital stay) resource use in the previous 3 months. Unit costs from national sources (e.g. NHS reference costs and the PSSRU report)124 were used to cost the resource use (in Great British pounds at 2015 prices). The questionnaire was completed by means of a postal survey. We had information from the case report forms on the initial hospital stay to provide a cross-check with the patient-recalled information. In 336 cases, the total length of inpatient hospital stay reported by patients was shorter than that captured by hospital records for the initial event alone. For the main analysis, we used patient-reported stay, except when this was shorter than the hospital record, in which case we used the latter. In a sensitivity analysis, we calculated outcomes solely using patient-reported stay.
The cost of the POD intervention was also estimated. This included material costs (e.g. printing of manuals), the time to deliver and receive the training and also time to provide support during POD delivery. This information was provided by the POD research team members, who kept a contemporaneous diary of visits and travel.
Data analysis
Feasibility
The feasibility of data collection in this group was determined by establishing the number of missing data (missing questionnaires and missing items in returned questionnaires) and the validity of the assessments used.
For both the EQ-5D-3L and resource use questionnaires, counts (percentages) were produced for the number of missing questionnaires and missing items. Regression analyses were used to determine whether or not individual and clinical characteristics predicted missing data. Questionnaires with a high response rate and low numbers of missing items could be considered acceptable to patients and useful and practicable in a larger trial. Specifying what is an acceptable return and completion rate for questionnaires is difficult as it is likely to be population, time point and completion-mode specific. However, we might expect, at 3 months, the return rate to be around 60–70% and the percentage of missing data to be no more than 5–10% on each item of the completed questionnaires.
The criterion validity of the EQ-5D-3L was explored by correlating values with those from the NEADL62 and the discriminant validity was explored by calculating mean values by the groups of interest (i.e. delirium vs. no delirium). If valid in the population, we might expect the EQ-5D-3L to correlate significantly with the NEADL and to distinguish between people who did and people who did not experience delirium (with those experiencing delirium obtaining lower EQ-5D-3L values).
Missing data and baseline imbalance
In the event, a non-trivial number of missing data was observed. We adopted a number of approaches to deal with this and present the cost-effectiveness results for each. We present results based on:
-
Complete cases only (only those who completed all questionnaires and items) (n = 138).
-
Multiple imputation [multiple imputation was conducted in Stata® (StataCorp LP, College Station, TX, USA) using information on age, sex, trial arm, cognitive impairment, ward type, delirium at 10 days and existence of comorbidities] of missing item data for respondents with < 50% of the health economics questionnaire items missing (n = 314).
-
Multiple imputation (multiple imputation was conducted in Stata using information on age, sex, trial arm, cognitive impairment, ward type, delirium at 10 days and existence of comorbidities) of total NHS costs and/or EQ-5D-3L (only those who had completed at least one EQ-5D-3L had total EQ-5D-3L values imputed) values for respondents with > 50% of the health economics questionnaire responses missing or who had missed the questionnaire entirely (n = 616).
The trial data descriptives suggested that there was some baseline imbalance between trial arms; hence, adjustment was required. QALYs were adjusted using treatment arm, baseline EQ-5D-3L, age, ward type (orthopaedic vs. general), sex and cognitive impairment status (yes vs. no) as controls.
Cost-effectiveness
The primary economic evaluation adopted the NICE-preferred approach of a cost–utility analysis comparing the costs and benefits of POD with those of usual care. 8 The costs were those relating to health-care use and (for the POD arm only) those relating to the POD intervention. The benefits were measured in terms of survival, which was quality-adjusted using the EQ-5D. The analysis time horizon was 3 months based on the trial follow-up. The main analysis result was the ICER per QALY. ICERs below the range of £20,000–30,000 indicate that POD would be considered cost-effective. Non-parametric bootstrapping was employed to determine the level of sampling uncertainty. Results were presented on cost-effectiveness planes and cost-effectiveness acceptability curves.
It was stated in the economic analysis plan that a multilevel model would be used to analyse the predictors of net monetary benefit and how these vary between hospital sites and wards. An initial multilevel model analysis found sites to be insignificant predictors with extremely small coefficients. Instead, a simple linear regression was employed whereby individual variables and clinical variables, and treatment arm were entered in a regression model to predict net monetary benefit. This approach also permitted the control of baseline differences between arms. An additional cost-effectiveness analysis was conducted that presented cost per case of delirium prevented.
Costs were calculated from the perspective of the health provider and Personal Social Services. A wider cost perspective was also planned. For the trial-based cost-effectiveness analysis, no discounting of costs or effects was conducted.
Early in the research programme grant work (see Appendix 4) and in the absence of observed data, a decision-analytic model was developed to determine the potential for POD to be cost-effective. The model made assumptions about delirium prevalence, POD effectiveness and costs, survival and quality of life. It concluded with a high degree of certainty that POD would be cost-effective. This model was updated to include information from the trial, including POD costs, delirium rates and POD effectiveness, and the estimates of cost-effectiveness were updated. This analysis allowed us to test our previous assumptions, but also to estimate the cost-effectiveness, taking into account a longer time horizon.
Results
Feasibility
Missing data
Table 34 presents completion and missing rates overall and by relevant subgroups. Table 35 presents results of a regression predicting questionnaire completion. Table 36 includes missingness by questionnaire item at 3 months.
| Questionnaires | Of received questionnaires | ||||
|---|---|---|---|---|---|
| Total | Unreceived | Received | Complete EQ-5D-3L | Incomplete EQ-5D-3L | |
| Total (n) | 1939 | 362 | 1577 | 1526 | 51 |
| Time point, n (%) | |||||
| Baseline | 696 | 10 (1.4) | 686 (98.6) | 668 (97.4) | 18 (2.6) |
| 1 month | 646 | 145 (22.5) | 501 (77.5) | 490 (97.8) | 11 (2.2) |
| 3 months | 597 | 207 (34.7) | 390 (65.3) | 368 (94.4) | 22 (5.6) |
| Treatment arm, n (%) | |||||
| POD | 928 | 207 (22.3) | 721 (77.7) | 695 (96.4) | 26 (3.6) |
| Control | 1011 | 155 (15.3) | 856 (84.7) | 831 (97.1) | 25 (2.9) |
| Proxy status, n (%) | |||||
| Participant | 1339 | 1307 (97.6) | 32 (2.4) | ||
| Proxy | 93 | 85 (91.4) | 8 (8.6) | ||
| Both | 141 | 134 (95.0) | 7 (4.9) | ||
| Unknown | 4 | 0 | 4 | ||
| Delirium 10 days, n (%) | |||||
| No | 1784 | 342 (19.2) | 1442 (80.8) | 1402 (97.2) | 40 (2.8) |
| Yes | 151 | 18 (11.9) | 133 (88.1) | 122 (91.7) | 11 (8.3) |
| Unknown | 4 | 2 (50) | 2 (50) | 2 (100) | 0 (0.0) |
| Delirium 30 days, n (%) | |||||
| No | 1770 | 300 (16.9) | 1470 (83.1) | 1422 (96.7) | 48 (3.3) |
| Yes | 30 | 2 (6.7) | 28 (93.3) | 27 (96.4) | 1 (3.6) |
| Unknown | 139 | 60 (43.2) | 79 (56.8) | 77 (97.5) | 2 (2.5) |
| Age groups (years), n (%) | |||||
| 65–69 | 167 | 40 (23.9) | 127 (76.0) | 125 (98.4) | 2 (1.6) |
| 70–79 | 472 | 79 (16.7) | 393 (83.3) | 384 (97.7) | 9 (2.3) |
| 80–89 | 902 | 166 (18.4) | 736 (81.6) | 712 (96.7) | 24 (3.3) |
| ≥ 90 | 398 | 71 (17.8) | 321 (90.6) | 305 (95.0) | 16 (5) |
| Sex, n (%) | |||||
| Male | 609 | 104 (17.1) | 505 (82.9) | 486 (96.2) | 19 (3.7) |
| Female | 1330 | 258 (19.4) | 1072 (80.6) | 1040 (97.0) | 32 (3) |
| Coefficient | SE | z | P > z | Lower CI | Upper CI | |
|---|---|---|---|---|---|---|
| Age | 0.014 | 0.013 | 1.080 | 0.281 | –0.011 | 0.039 |
| Sex | 0.222 | 0.210 | 1.060 | 0.290 | –0.189 | 0.633 |
| Cognitive impairment | ||||||
| No cognitive impairment | –0.469 | 0.246 | –1.910 | 0.057 | –0.950 | 0.013 |
| Comorbidity | ||||||
| No comorbidity | 0.017 | 0.206 | 0.080 | 0.936 | –0.388 | 0.421 |
| No delirium | ||||||
| Delirium | –0.383 | 0.396 | –0.970 | 0.333 | –1.159 | 0.393 |
| Base EQ-5D-3L | –0.101 | 0.274 | –0.370 | 0.712 | –0.639 | 0.436 |
| Control/orthopaedic surgery | ||||||
| POD/orthopaedic surgery | –0.362 | 0.286 | –1.270 | 0.205 | –0.923 | 0.198 |
| Control/elderly care | 0.368 | 0.276 | 1.330 | 0.183 | –0.174 | 0.910 |
| POD/elderly care | Omitted | |||||
| Intercept | –0.840 | 1.194 | –0.700 | 0.482 | –3.181 | 1.501 |
| Variable | Missing (N = 388), n (%) |
|---|---|
| EQ-5D-3L | |
| EQ-5D-3L mobility | 8 (2.1) |
| EQ-5D-3L self-care | 4 (1.0) |
| EQ-5D-3L usual activities | 9 (2.3) |
| EQ-5D-3L pain | 8 (2.1) |
| EQ-5D-3L depression | 11 (2.8) |
| Resource use | |
| Received help with questionnaire | 12 (3.1) |
| Hospital inpatient | 53 (13.6) |
| Nursing/residential home | 44 (11.3) |
| Hospital clinic | 69 (17.7) |
| Hospital A&E | 59 (15.1) |
| GP surgery | 48 (12.3) |
| GP home | 50 (12.8) |
| Community nurse | 70 (17.9) |
| Community psychiatrist | 17 (4.4) |
| Social support | 61 (15.6) |
| Received help from family or friends | 12 (3.1) |
| Hours per week received help | 88 (22.6) |
| Time taken off work by family or friends to help | 225 (57.7) |
| Other expenses | 40 (10.3) |
| Other expense amount | 12 (3.1) |
The key findings are as follows:
-
The EQ-5D-3L return rates were 98.6%, 77.5% and 65.3% at baseline and at 1 and 3 months, respectively; on each occasion, 94–98% of these were fully complete.
-
The resource use questionnaire was in the same survey pack as the EQ-5D-3L, so return rates were the same at 3 months. However, completion rates were lower, with only 48.7% (n = 190) fully complete.
-
There did not appear to be significant differences in return rates according to treatment arm or delirium status, although a trend for higher return rates in the control arm and among those who did develop delirium was observed.
-
A regression analysis predicting survey return at 3 months found no factors that significantly explained return rate. However, individuals with cognitive impairment at baseline were less likely to return the questionnaire than individuals with no cognitive impairment, with the p-value approaching significance (see Table 35).
-
Although the returned EQ-5D-3L measures suffered from minimal missing data, some of the resource use items had high rates of missingness (see Table 36). For example, 13.6% of patients missed the question asking whether or not they had had an inpatient stay in the previous 3 months. This is important as inpatient stays represent a high proportion of total costs.
-
Twenty-three per cent of individuals did not include the number of hours per week for which they received help from friends or family. Difficulty quantifying average number of hours is common and not necessarily an indicator of poor acceptability of the question.
-
It should be noted that mode of completion was not the same across time points. At baseline and 1 month, it was face to face, whereas, at 3 months, it was via postal survey. This is likely to have contributed to the higher number of missing data at 3 months.
-
Results suggest that there was a large difference in missing resource use measure rates between the best- and worst-performing centres (results not shown).
Validity of patient outcome assessments
The scatterplot showing the correlation between baseline EQ-5D-3L and NEADL is given in Figure 18. Mean EQ-5D-3L values are provided in Table 37 and scores by delirium status are included in Table 38.
FIGURE 18.
Correlation of EQ-5D-3L and NEADL scores at 3 months.
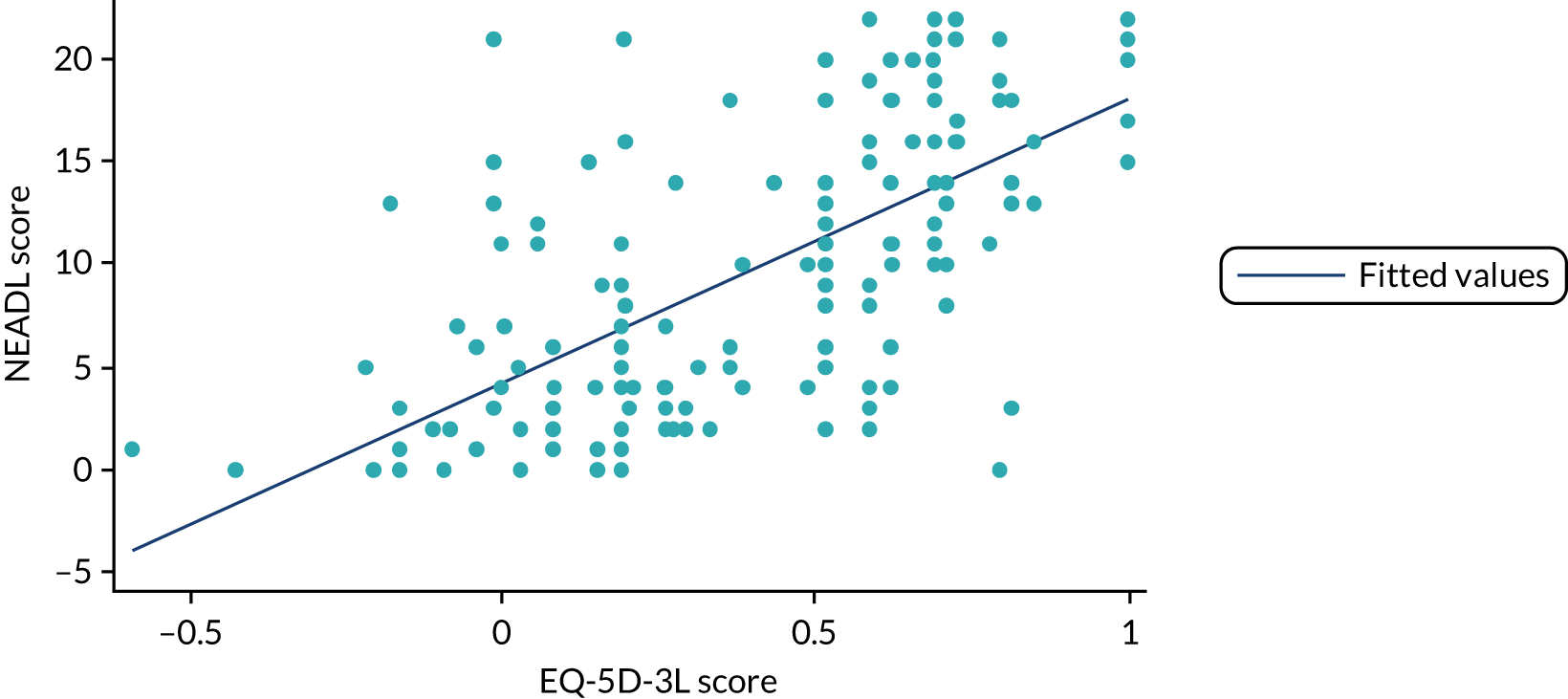
| Age group | Patients (n) | Sample mean (SD) | Population norm mean (SD) |
|---|---|---|---|
| 65 to 74 years old | 106 | 0.285 (0.389) | 0.78 (0.26) |
| Male | 45 | 0.316 (0.375) | 0.78 (0.28) |
| Female | 61 | 0.262 (0.400) | 0.78 (0.25) |
| ≥ 75 years old | 565 | 0.240 (0.367) | 0.73 (0.27) |
| Male | 168 | 0.276 (0.387) | 0.75 (0.28) |
| Female | 397 | 0.224 (0.357) | 0.71 (0.27) |
| Time point | EQ-5D-3L score | Mean (SD) dimension responsea | ||||
|---|---|---|---|---|---|---|
| Mobility | Self-care | Usual activities | Pain | Anxiety and depression | ||
| Baseline | ||||||
| No delirium (n = 544) | 0.26 (0.37) | 2.2 (0.61) | 1.9 (0.73) | 2.3 (0.72) | 1.9 (0.66) | 1.5 (0.62) |
| Delirium (n = 54) | 0.09 (0.39) | 2.6 (0.57) | 2.3 (0.76) | 2.5 (0.61) | 2.0 (0.69) | 1.6 (0.66) |
| 1 month | ||||||
| No delirium (n = 444) | 0.50 (0.32) | 1.9 (4.8) | 1.7 (0.67) | 2.0 (0.69) | 1.7 (0.60) | 1.4 (0.58) |
| Delirium (n = 53) | 0.28 (0.32) | 2.2 (0.48) | 2.3 (0.62) | 2.5 (0.59) | 1.6 (0.54) | 1.6 (0.64) |
| 3 months | ||||||
| No delirium (n = 376) | 0.43 (0.35) | 1.9 (0.50) | 1.7 (0.71) | 2.0 (0.74) | 1.8 (0.58) | 1.4 (0.59) |
| Delirium (n = 48) | 0.15 (0.29) | 2.2 (0.57) | 2.2 (0.74) | 2.5 (0.72) | 1.8 (0.54) | 1.8 (0.77) |
The key findings are as follows:
-
A significant, positive correlation existed between EQ-5D-3L and NEADL scores at 3 months (r = 0.66), indicating that they measure similar constructs in this patient group.
-
The trial sample had significantly lower EQ-5D-3L scores at baseline than the UK age-matched population norms averages (reported in Kind et al. 77) (see Table 37).
-
Patients who experienced delirium had much lower average baseline EQ-5D-3L scores than those who did not, and this difference was maintained across the three time points (see Table 38).
-
This difference appears mainly to have been driven by worse status in terms of self-care and usual activities and, to a lesser extent, mobility (see Table 38). Dimensions relating to pain and mental health were less important.
Responsiveness of patient outcome assessments
The EQ-5D-3L scores across time points are included in Table 38 and Figure 19:
-
Both those who did not have delirium and those who did at 10 days experienced a similar improvement in health status from baseline to 30 days, followed by a worsening between 30 days and 3 months (although this was more marked in the delirium group).
-
Those who had persistent delirium at 30 days did not experience an improvement in health status. It declined from baseline to 30 days and remained almost at the same level up to 3 months. However, it should be noted that the sample was small for this group.
FIGURE 19.
The EQ-5D-3L scores over time by delirium status.
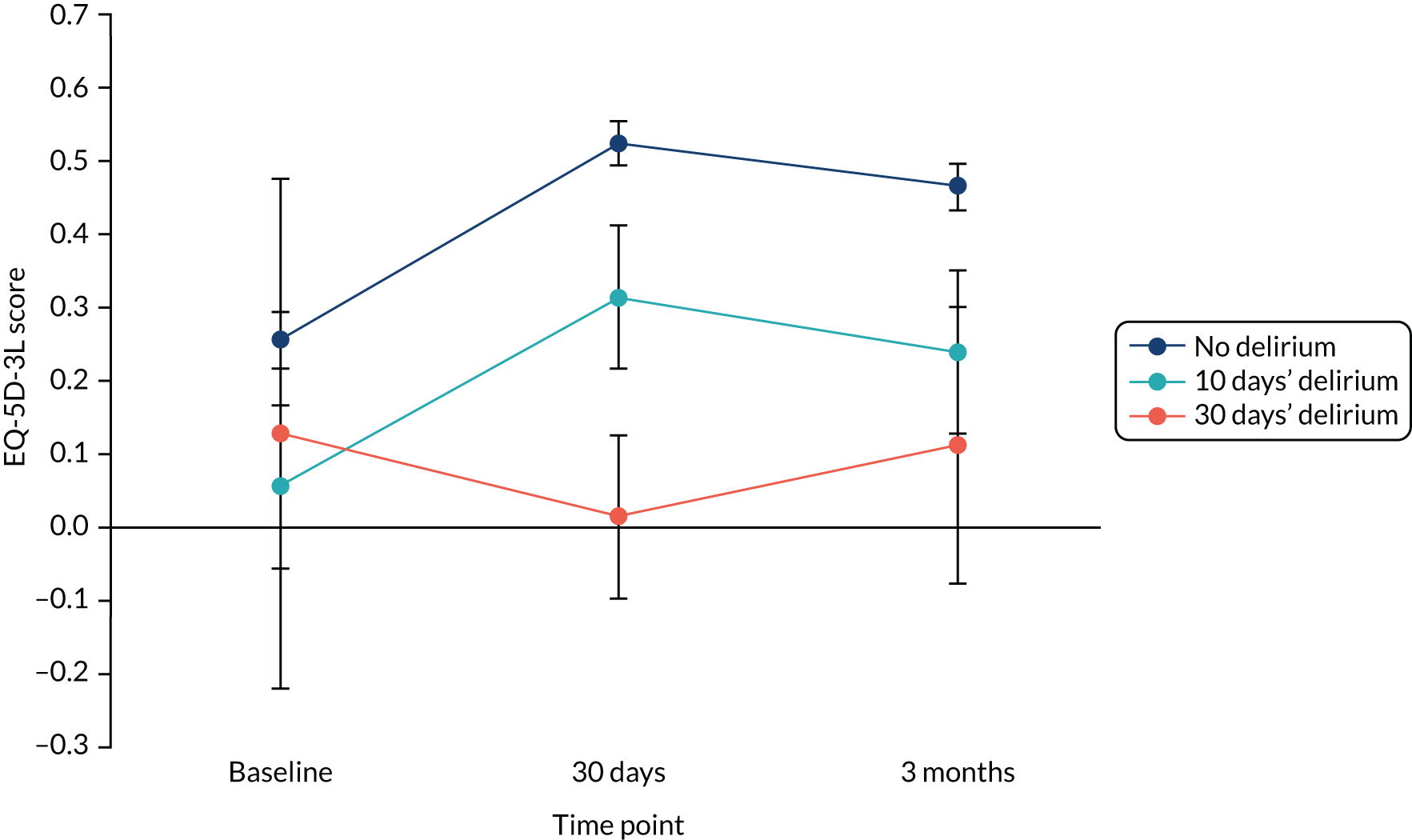
Validity of proxy outcomes assessments
The EQ-5D-3L scores according to whether or not a patient had help from a proxy are included in Table 39:
-
At baseline, proxy-completed EQ-5D-3L questionnaire values were similar to the values of those completed by participants themselves.
-
At 1 and 3 months, proxy-completed (or aided) EQ-5D-3L completion underestimated quality of life.
| Time point | EQ-5D-3L completions (n) | Mean (SD) |
|---|---|---|
| Baseline | ||
| Self-complete | 636 | 0.248 (0.373) |
| Proxy | 25 | 0.249 (0.299) |
| Both | 7 | 0.269 (0.444) |
| 1 month | ||
| Self-complete | 453 | 0.499 (0.320) |
| Proxy | 27 | 0.258 (0.280) |
| Both | 10 | 0.468 (0.258) |
| 3 months | ||
| Self-complete | 218 | 0.535 (0.311) |
| Proxy | 33 | 0.297 (0.343) |
| Both | 117 | 0.365 (0.373) |
Cost-effectiveness
Costs of Prevention of Delirium intervention
The resources used in the delivery of the POD intervention are presented in Table 40:
| Item | Unit cost (2013–14) (£) | Source |
|---|---|---|
| POD total | 39,120.37 | |
| Per patient | 10.98 | |
| POD manuals | 909.02 | The printing cost for the POD manuals was £839.02. Folders for the manuals cost approximately £70.00 |
| Introductory workshops/meetings (four times) | 3011.55 |
|
| POD facilitators | 24,302.80 | POD facilitators were local members of staff (usually at staff nurse grade), seconded for 1 day per week for 3–4 months to help POD implementation on the wards |
| POD team meetings | 6547.00 | Assumed 45-minute meetings |
| Contact support from central research team | 4350.00 |
|
-
A total of 2115 patients were screened in POD wards. However, this underestimates the number of patients to whom POD applied, as those aged < 65 years were not screened.
-
Given this, a POD cost denominator sample of 3563 was agreed to be the most suitable. This was calculated using the number of beds in POD wards multiplied by the assumed number of admissions over 6 months. The number of admissions was calculated using the number of days in 6 months divided by the average length of stay for a POD participant (10.7 days).
-
The total final cost of the POD intervention was estimated to be £39,120. This included printing of the manuals, staff time (for researcher and nurses) to attend introductory meetings, POD facilitators, POD-related team meetings and contact between researchers and the POD staff. Therefore, the per-patient cost was £10.98.
Resource use
The average resource use per completed question is presented in Table 41:
| Resource use item | Trial arm, mean (SD) | |
|---|---|---|
| POD | Control | |
| Hospital overnight stay | 18.05 (22.3); n = 144 | 15.9 (17.3); n = 193 |
| Nursing/residential home | 8.43 (22.8); n = 149 | 7.41 (21.2); n = 197 |
| Hospital clinic appointment | 1.59 (2.7); n = 144 | 1.45 (2.1); n = 177 |
| Hospital A&E department | 0.51 (1.2); n = 147 | 0.43 (0.8); n = 184 |
| GP, surgery visit | 0.84 (1.9); n = 144 | 1.02 (2.1); n = 198 |
| GP, home visit | 1.12 (1.9); n = 149 | 0.73 (1.4); n = 191 |
| District nurse or practice nurse | 8.10 (24.5); n = 137 | 5.99 (20.1); n = 183 |
| Psychiatrist, psychologist, counsellor | 0.08 (0.4); n = 159 | 0.26 (2.9); n = 214 |
| Social support (e.g. day centre, home support, social worker, support group) | 5.76 (22.8); n = 133 | 4.56 (19.7); n = 196 |
-
The POD arm participants had higher average resource use for every health-care resource except GP surgery visits and psychiatrists, psychologist or counsellor visits.
-
Participants in the POD arm had an average of 2.2 more overnight days in hospital and 1 more day in nursing/residential homes.
-
Hospital inpatient stay appears to be driving costs. The mean cost was £4965 in the POD arm and £4365 in the control arm. The mean costs per resource use item are presented in Table 42.
-
As data quality on items relating to carer time/costs was poor, and because POD no longer relied on volunteer time, it was decided not to conduct the wider-perspective cost-effectiveness analysis.
| Resource use item | Trial arm, mean cost (SD) (£) | |
|---|---|---|
| POD | Control | |
| Hospital overnight stay | 4965 (6130); n = 144 | 4365 (4755); n = 193 |
| Nursing/residential home | 665 (1798); n = 149 | 586 (1675); n = 197 |
| Hospital clinic appointment | 203 (343); n = 144 | 185 (263); n = 177 |
| Hospital A&E department | 68 (164); n = 147 | 59 (108); n = 184 |
| GP, surgery visit | 38 (89); n = 144 | 47 (95); n = 198 |
| GP, home visit | 131 (217); n = 149 | 85 (167); n = 191 |
| District nurse or practice nurse | 486 (1470); n = 137 | 359 (1206); n = 183 |
| Psychiatrist, psychologist, counsellor | 8 (35); n = 159 | 25 (273); n = 214 |
| Social support (e.g. day centre, home support, social worker, support group) | 230 (911); n = 133 | 182 (786); n = 196 |
Trial-based cost-effectiveness
The EQ-5D-3L score at baseline was slightly higher in the POD arm than in the control arm. To control for this, QALYs were adjusted using age, ward type, sex and cognitive impairment.
The key findings are as follows:
-
The ICER resulted in the POD intervention being dominated by standard care. That is, POD resulted in higher costs and lower QALYs. However, the QALY differential was negligible.
-
The difference in cost varied from £920 in the complete-case group to £1127 for complete-case and imputed items group. The difference in QALY varied from –0.01 in both imputation groups to –0.02 in the complete-case analysis. Mean cost, mean QALYs and ICER calculations are in Table 43.
-
NHS total cost and QALYs were replicated 10,000 times in a Monte Carlo simulation; the simulation is presented in Figures 20–22.
-
Using a £20,000 per QALY threshold, the probability that POD was cost-effective was 0.01 (1% chance) in a simulation using adjusted QALYs and complete-case and imputed items. This chance increased to 10% when using unadjusted QALYs and complete-case data only.
-
A sensitivity analysis was conducted using hospital inpatient length of stay from the 3-month patient questionnaire solely. The results from this analysis can be found in Table 44. The difference in cost between both arms increases to between £1148 and £1414 depending on sample group used.
-
An analysis was conducted using incremental cost and percentage of patients in each arm who experienced delirium to produce the cost per percentage of patients who avoided delirium. The cost percentage reduction in delirium ranges from £657 to £805 depending on the sample used. Detailed results are in Table 45.
| Analysis | Trial arm, mean (SD) | Difference | |
|---|---|---|---|
| POD | Control | ||
| Complete case | n = 50 | n = 88 | |
| NHS cost (£) | 5332 (6160) | 4412 (5639) | 920 |
| QALY | 0.09 (0.02) | 0.11 (0.02) | –0.02 |
| ICER | POD is dominated | ||
| Complete case and imputed items | n = 118 | n = 180 | |
| NHS cost (£) | 6173 (7614) | 5046 (5815) | 1127 |
| QALY | 0.09 (0.02) | 0.10 (0.02) | –0.01 |
| ICER | POD is dominated | ||
| Complete case, imputed items and imputed totals | n = 161 | n = 223 | |
| NHS cost (£) | 6415 (6692) | 5330 (5438) | 1085 |
| QALY | 0.09 (0.02) | 0.10 (0.02) | –0.01 |
| ICER | POD is dominated | ||
FIGURE 20.
Cost-effectiveness acceptability curve.
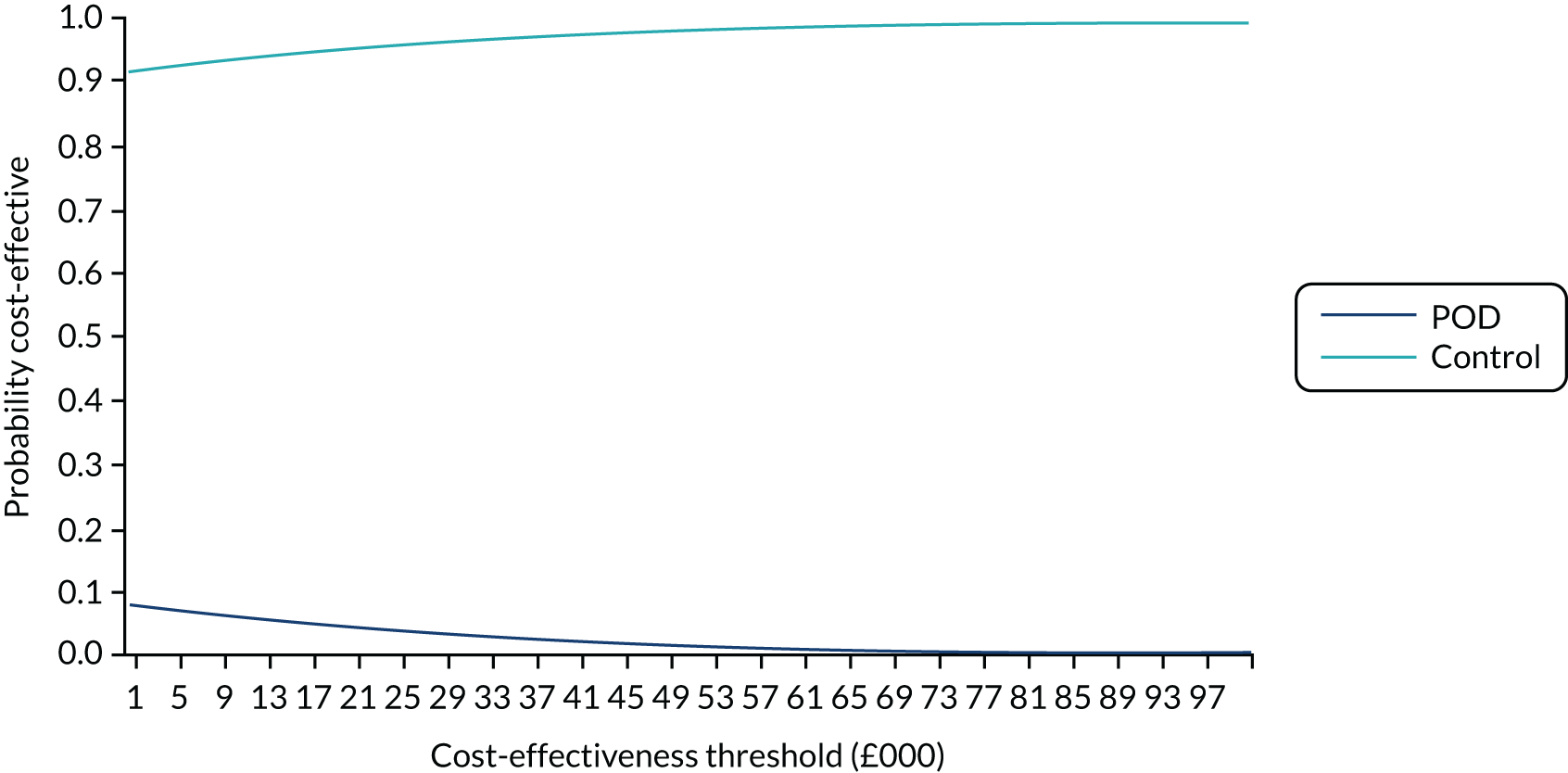
FIGURE 21.
Complete case and imputed items cost-effectiveness plane.
FIGURE 22.
Complete-case (unadjusted QALY) cost-effectiveness plane.
| Analysis | Trial arm, mean (SD) | Difference | |
|---|---|---|---|
| POD | Control | ||
| Complete case | n = 50 | n = 88 | |
| NHS cost (£) | 6707 (5585) | 5559 (5549) | 1148 |
| QALY | 0.09 (0.02) | 0.11 (0.02) | –0.02 |
| ICER | POD is dominated | ||
| Complete case and imputed items | n = 118 | n = 180 | |
| NHS cost (£) | 7562 (7223) | 6148 (5524) | 1414 |
| QALY | 0.09 (0.02) | 0.10 (0.02) | –0.01 |
| ICER | POD is dominated | ||
| Complete case, imputed items and imputed totals | n = 161 | n = 223 | |
| NHS cost (£) | 7745 (6357) | 6432 (5182) | 1313 |
| QALY | 0.09 (0.02) | 0.10 (0.02) | –0.01 |
| ICER | POD is dominated | ||
| Analysis | POD | Control | Difference |
|---|---|---|---|
| Complete case | n = 50 | n = 88 | |
| Mean NHS cost (£) | 5332 (6160) | 4412 (5639) | £920 |
| % of patients who experienced delirium | 8 | 9.4 | 1.4% |
| Cost per % of delirium avoided | £657 | ||
| Complete case and imputed items | n = 118 | n = 180 | |
| Mean NHS cost (£) | 6173 (7614) | 5046 (5815) | £1127 |
| % of patients who experienced delirium | 8 | 9.4 | 1.4% |
| Cost per % of delirium avoided | £805 | ||
| Complete case, imputed items and imputed totals | n = 161 | n = 223 | |
| Mean NHS cost (£) | 6415 (6692) | 5330 (5438) | £1085 |
| % of patients who experienced delirium | 8 | 9.4 | 1.4% |
| Cost per % of delirium avoided | £775 |
Net-benefit regression
-
Data were analysed in the net benefit regression framework. As we had employed imputation, and this may confound the model results, we opted to run the analysis on complete cases only (n = 138).
-
A multilevel model was run on the data predicting net monetary benefit with site and ward entered as levels. However, it was clear that site was not a significant factor, and the ward influence, although important, was related only to whether the ward was general or orthopaedic. For this reason, multilevel modelling was not deemed appropriate for the data and a simple linear regression was employed.
-
In the linear regression, treatment arm was not a significant predictor of net monetary benefit. However, sex and delirium status were significant predictors. Female participants experienced significantly higher benefit, whereas participants who experienced delirium had significantly lower net benefit. Results from this analysis are presented in Table 46.
| Variable | Coefficient | SD | t | P > |t| | Lower CI | Upper CI |
|---|---|---|---|---|---|---|
| Control | 1267 | 998 | 1.27 | 0.206 | –706 | 3241 |
| Female | 2362 | 999 | 2.37 | 0.019 | 387 | 4338 |
| No cognitive impairment | 1141 | 1764 | 0.65 | 0.519 | –2348 | 4631 |
| Delirium | –5969 | 2413 | –2.47 | 0.015 | –10743 | –1194 |
| No comorbidity | 217 | 986 | 0.22 | 0.826 | –1733 | 2168 |
| Orthopaedic/surgery | 620 | 964 | 0.64 | 0.521 | –1287 | 2527 |
| Intercept | –7541 | 1824 | –4.13 | 0.000 | –11149 | –3932 |
Model-based cost-effectiveness
The original model was updated using information from the trial. There were noticeable differences between some parameter values used in the 2010 modelling exercise and those observed in the feasibility trial data. For example, the model assumed a delirium incidence of 15%, versus the observed incidence of 9.4% in the trial control arm. The effectiveness of POD was also initially overestimated as it was assumed that the intervention would reduce the delirium rate by 33%. In the event, the delirium incidence between arms was 9.4% (control) and 8% (POD). There were also significant differences in quality of life. For example, the modelling assumed that hospitalised patients who went on to experience delirium had a utility of 0.598, but the trial revealed that this was much lower (0.1169). As POD appeared to result in additional resource use (a difference of £419), a sensitivity analysis was run in which this was added to the POD cost. The updated model parameters and assumptions are in Table 47.
| Parameter | Base-case estimate | Distribution | Source | Notes/assumptions |
|---|---|---|---|---|
| Transition probabilities | ||||
| Probability of delirium in hospital | 0.094 | Beta | POD feasibility trial | 34 out of 361a patients in the control arm experienced delirium |
| Reduction in the probability of delirium in hospital with MTI | 0.014 | Beta | POD feasibility trial | 8% of patients in the POD arm experienced delirium. The difference between 9.4% and 8.0% was calculated |
| Onset of delirium | 0.26 | Beta | POD feasibility trial | 93% of patients (57/61) who developed delirium did so within 10 days of entering hospital. This is used to form a daily hazard, which has been converted into a daily probability |
| Length of stay for elderly hospitalised patients | 10 days | POD feasibility trial | The length-of-stay figures are used to derive the daily hazard rates and probabilities of discharge from hospital | |
| Additional length of stay of a patient with delirium | 5 days | POD feasibility trial | ||
| Probability of admission to care home post hospital – no delirium | 0.22 | Beta | POD feasibility trial | 140 patients out of 638 patients who did not experience delirium were discharged to a care home |
| Probability of admission to care home post hospital – delirium | 0.31 | Beta | POD feasibility trial | 19 out of 61 patients who experienced delirium were discharged to a care home |
| Probability (daily) of dying in hospital – delirium | 0.00498 | Beta | O’Keefe and Lavan111 | 7 out of 131 patients had died by the end of the 11-day length of stay. This is used to compute the daily hazard of death in hospital; daily probabilities can then be computed |
| Probability (daily) of remaining in hospital – no delirium | 0.959 | N/A | Defined as 1 minus the sum of the daily probability of leaving hospital and the daily probability of dying in hospital | |
| Probability (daily) of remaining in hospital – no delirium | 0.934 | Beta | Defined as 1 minus the sum of the daily probability of leaving hospital and the daily probability of dying in hospital | |
| Probability (daily) of death for a patient with delirium discharged to home | 0.001358 | Beta | Rockwood et al.113 | Converted from a daily hazard rate that is derived from a beta distribution, which relies on the fact that the median survival time after discharge for 38 patients with delirium has been reported to be 510 days |
| Probability (daily) of death for elderly patient discharged to home – no delirium | 0.000618 | Beta | Rockwood et al.113 | Converted from a daily hazard rate that is derived from a beta distribution, which relies on the fact that the median survival time after discharge for 148 elderly patients without delirium was 1122 days |
| Probability (daily) of death for a patient with delirium discharged to care home | 0.002026 | Beta | PSSRU124 | PSSRU data on survival times for elderly care home patients suggest that the median survival time for elderly patients in care is 493 days |
| Probability (daily) of death for (non-delirious) elderly patient discharged to care home | 0.002026 | Beta | Rockwood et al.113 | PSSRU data on survival times for elderly care home patients suggest that the median survival time for elderly patients in care is 493 days |
| Probability of death in care home – no delirium | 0.002026 | Beta | PSSRU124 | As above for delirium cases |
| Probability of death in hospital – no delirium | 0.0448 | Beta | POD feasibility trial | 28 out of 638 patients die within 10 days |
| Utilities | ||||
| Utility delirium in hospital | Utility = 0.0580 | Beta | POD feasibility trial | The daily QALY of patients who experienced delirium in hospital. The QALY was divided by 365 to achieve a daily QALY value |
| Utility in care home – no delirium | Utility = 0.1169 | Beta | As delirium in hospital | |
| Utility in hospital and no delirium | 0.1169 | Beta | POD feasibility trial | This is derived from the mean utility value (0.01169) reported for patients who did not go on to develop delirium. SD was also reported |
| Utility in hospital and pre delirium onset | Utility = 0.0580 | Beta | POD feasibility trial | As daily QALY of delirium in hospital |
| Utility discharged to care home and had delirium | Utility = 0.0580 | Beta | Duppils and Wikblad119 | As daily QALY of delirium in hospital |
| Utility post discharge and had delirium | 0.2170 | Beta | POD feasibility trial | Calculated using EQ-5D-3L at 3 months for participants who experienced delirium in hospital |
| Utility – post discharge and never had delirium | 0.47768 | Beta | POD feasibility trial | Calculated using EQ-5D-3L at 3 months for participants who did not experience delirium in hospital |
| Costs | ||||
| Cost of POD | 11 | Gamma | POD feasibility trial | See Table 40 |
| Bed-day cost | 275 | Gamma | NHS reference costs 2013-14125 | National average non-elective excess bed-days |
| Cost (daily) of stay in long-term care | 96.87 | Gamma | PSSRU124 | A weighted average of the costs of a permanent stay in a private nursing home, private residential care, voluntary residential care or local authority residential care was obtained from the PSSRU. These costs include things such as community nursing, GP services and personal living expenses, personal living expenses were subtracted from the costs. The weighting was carried out using the proportion of elderly people in each of the institutions in 1996 (Netten et al.121) |
| Sensitivity analysis | ||||
| Cost of POD | 419 | Gamma | POD feasibility trial | The difference in NHS cost and hospital cost for the POD and control arms was calculated to obtain an out-of-hospital resource cost. The control cost was then subtracted from the POD cost to create a cost to reflect the extra health-care resources for the POD arm |
The key findings are as follows:
-
The lifetime time horizon cost-effectiveness results from the updated model are included in Table 48.
-
These show that POD has an incremental cost and QALY of £1775 and 0.11, respectively. This results in an ICER of £16,133, which indicates that POD is cost-effective.
-
A sensitivity analysis adding in additional resource for POD (Table 49) yields an ICER of £19,942.
-
The probabilistic sensitivity analyses yielded mean incremental costs and QALYs of £1774 and 0.11, respectively, and an ICER of £15,454. The mean incremental net monetary benefit (at λ = £20,000) was £521.90.
-
Figure 23 shows the cost-effectiveness acceptability curve and indicates (where λ =£ 20,000) that POD has a 100% chance of being cost-effective.
-
Figure 24 is the EVPI across different levels of λ. As uncertainty is low when λ = £20,000, there is a low per-person EVPI.
-
However, given the contrasting trial and model results and data quality issues, the results are, in fact, highly uncertain.
| Outcome | Control | POD | Incremental |
|---|---|---|---|
| Mean NHS cost (£) | 19,195.77 | 20,970.40 | £1774.63 |
| Mean QALY | 1.17 | 1.28 | 0.11 |
| ICER | £16,133.04 |
| Outcome | Control | POD | Incremental |
|---|---|---|---|
| Mean NHS cost (£) | 19,195.77 | 21,389.4 | £2193.63 |
| Mean QALY | 1.17 | 1.28 | 0.11 |
| ICER | £19,942.13 |
FIGURE 23.
Cost-effectiveness acceptability curve.
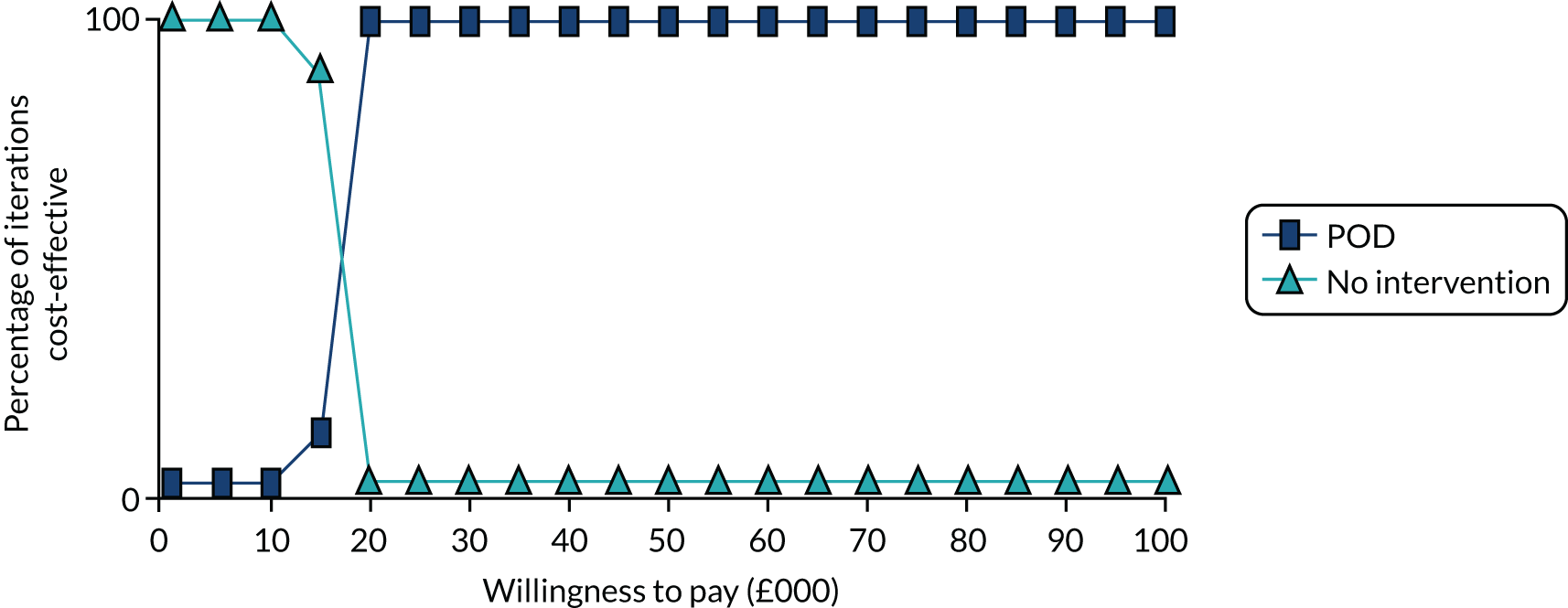
FIGURE 24.
Expected value of perfect information vs. willingness to pay.
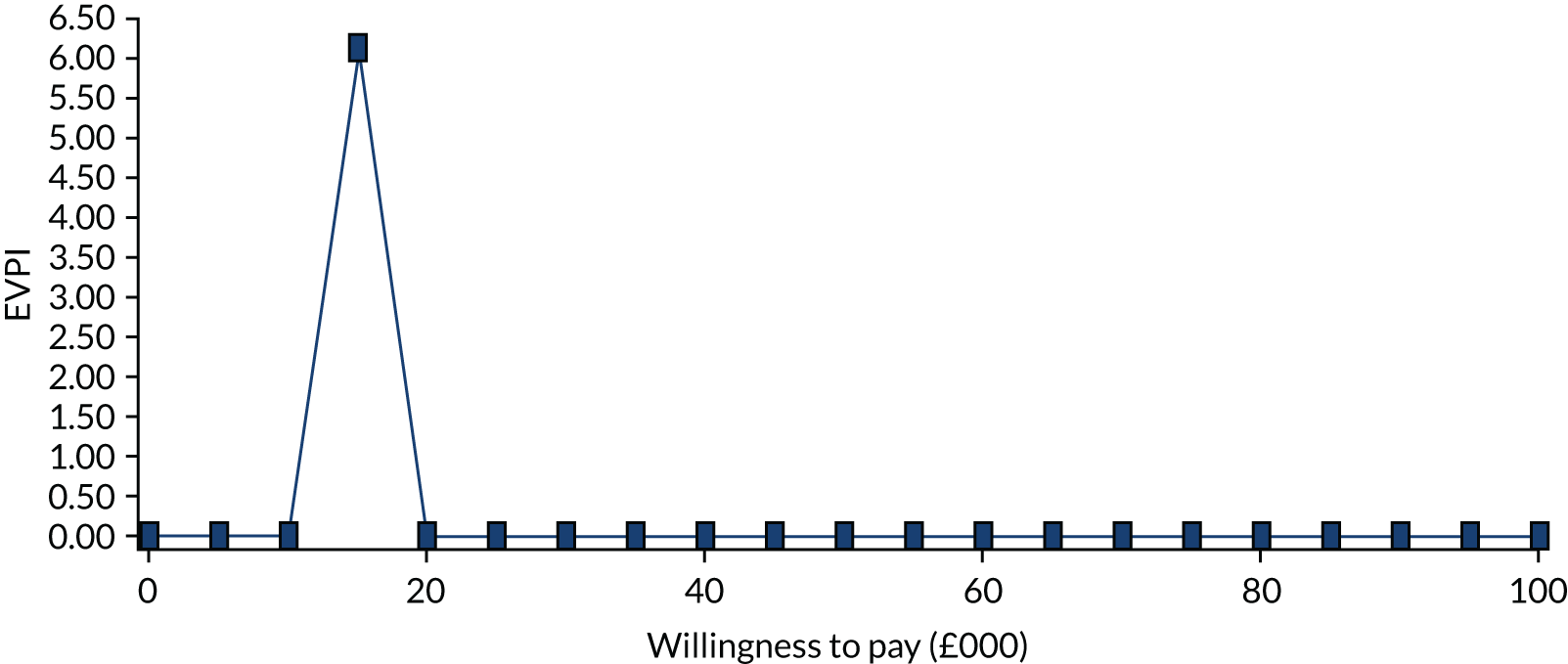
Discussion and conclusions
Feasibility: missing data
-
The return rate of questionnaires was in the range of what could be expected from an elderly group of people who had been hospitalised.
-
The completion rate of the EQ-5D-3L appeared to be acceptable.
-
The return rate did not appear to be influenced by trial arm or delirium status, which is encouraging for future studies.
-
The completion rate of the resource use measure was much lower and some items were missed by one in five people. The resource use questionnaire would benefit from further refinement to improve response rates and data quality.
-
Indeed, it is debatable whether or not self-report measures of resource use based on recall are suitable in this group. Even when items were complete, the accuracy of responses is uncertain. For example, there was a significant mismatch between self-reported hospital length of stay and that captured by hospital records (for the initial stay). Given this, it is recommended that data requests and linkage from the NHS Digital and primary care sources should be pursued in future studies (possibly alongside self-report measures).
-
Response rates typically drop off in later trial follow-ups. However, in the current study, it is also likely that the mode of completion played a part, with the 3-month measure being completed by postal survey. This was a factor in the high numbers of missing data. Consequently, greater reliance on imputation is needed, which increases uncertainty in the analysis. Because uncertainty has a cost in economic evaluations, future studies should consider the trade-off between this and research costs. On this basis, it is arguable that greater investment is warranted in data collection (i.e. face-to-face interviews) or alternative strategies are needed (e.g. routine data capture).
-
The return rates for the resource use measure also varied significantly across centres, suggesting that return rates could have been improved if best practices were followed.
Feasibility: validity and responsiveness
-
There was evidence that the EQ-5D-3L was a valid assessment in this group. It was highly correlated with the NEADL and indicated much lower health status in these patients than in age-specific general population estimates, as we might expect.
-
It was notable, however, that those patients who went on to develop delirium had poorer health status to begin with, suggesting that health status was a significant predictor of delirium onset.
-
This difference appeared to be driven by functional status, rather than mental health or pain.
-
There was evidence that the EQ-5D-3L was responsive to change in health in this group over time, as we observed an increase in status over time. However, there was a suggestion that those who develop (especially persistent) delirium do not recover to the same extent.
Feasibility: proxy completion
-
Aside from baseline assessments, there was evidence that proxy-aided completion of health status may diverge from patient reports.
-
Patient completion should be sought when possible; when not possible, a systematic approach to proxy data collection should be employed.
-
When using proxy reports, some method of calibrating these values with patient values may be needed.
Costs and effects
-
The total costs for the delivery of POD were estimated to be £39,120. Clearly, the per-patient cost depends on the number receiving the intervention and will fall over time. In this study, we defined the number in receipt as 3563, which led to a per-patient cost of £10.98.
-
We also tested other assumptions, but these had little impact on the overall total costs, as intervention costs were dwarfed by those relating to health-care resource use.
-
Health-care use appeared to be greater in the POD arm. It is unclear why this might be. It may relate to greater levels of observation or more intensive care encouraged by POD.
-
Among the group of patients who experienced delirium, 27.4% died during the trial period, compared with 6.9% of patients who did not experience delirium. In the control arm, 13% of patients died during the trial period, compared with 16.3% in the POD arm.
-
There was a slightly higher EQ-5D-3L mean value in the POD arm at baseline, which meant that adjustment was necessary. There were negligible QALY differences between arms, although, in all analyses, these were in favour of the control arm. This is despite the fact that fewer cases of delirium were detected in the POD arm. It is unclear why. This finding may be a chance occurrence or an artefact of the missing data and imputation, the influence of proxy-aided completion or the fact that lower health status predisposed patients to delirium onset.
Cost-effectiveness: trial analysis
-
The POD intervention appeared to lead to fewer cases of delirium, but this did not appear to translate to lower costs or higher QALYs, regardless of the data adjustment, imputation method and Monte Carlo simulation used.
-
In the net monetary benefit analysis, sex and experiencing delirium appeared to be significant predictors of benefit, whereas the POD intervention was not.
-
Hence, the POD intervention did not appear to represent value for money in the cost–utility framework over a 3-month period.
-
However, in the cost-effectiveness framework, the cost per percentage of delirium avoided appeared to be quite low (£657–805), although interpreting this value is difficult.
Cost-effectiveness: regression and decision modelling
-
The net benefit regression did not find treatment to be a significant predictor of net monetary benefit over the trial period. In fact, the POD intervention appeared to be associated with less monetary benefit.
-
The presence of delirium was associated with a substantial drop in net benefit (of £5969).
-
The updated decision model yielded expected costs and benefits, both of which were higher for the POD arm than for the usual care arm. The ICER for the analysis (deterministic and probabilistic) indicated that the POD intervention was, in fact, cost-effective.
-
When we are willing to pay £20,000 per QALY gained, the POD intervention was cost-effective in 100% of the Monte Carlo simulations.
-
Ordinarily, we can interpret this as meaning that, with certainty, the POD intervention would represent a cost-effective strategy and the benefit of further research (measured here as EVPI) is low.
-
However, there are a number of results that lead to doubts over the cost-effectiveness estimates, including the number of missing data and the contrast between trial- and model-based conclusions.
-
In the light of this, and all results considered, it is recommended that additional research relating to the POD intervention is conducted.
Contrasting trial and model results
The results of the trial- and model-based analyses were in conflict. It is unclear why this might have occurred, but possible explanations are as follows:
-
Different time horizons – the model has a lifetime time horizon and thus captures cost savings and benefits of delirium prevention over a much longer period.
-
The modelling assumes a robust and deterministic relationship between delirium and the outcomes of interest to the cost-effectiveness analysis (e.g. length of stay, health-related quality of life and mortality), that is that delirium avoidance has, with certainty, positive effects on these outcomes. This may perhaps give an unrealistically clean result in favour of the POD intervention in the light of positive point estimates for delirium prevention.
-
The trial analysis uses these outcome data directly and the relationship between delirium and health outcomes/costs may be weaker than presumed, or not as expected.
-
The trial data are also subject to potential bias that results from imbalance, missing data and type (potentially not at random), reliance on proxy reports and noise.
-
The incidence in delirium was small and the differential between arms was smaller still (< 2%). It is quite possible that any benefit of the POD intervention, if there was any, was subsumed by variation from the much larger proportion (< 90%) of the sample that did not experience delirium (who would potentially have used significant health-care resource unrelated to delirium).
Appendix 6 Statistical tables
| Centre | Ward identifier | Allocation | Participants recruited (n) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2014 | 2015 | Total accrual | ||||||||
| August | September | October | November | December | January | February | ||||
| Site 1 | Ward 1 | POD | 10 | 8 | 14 | 4 | 4 | 12 | – | 52 |
| Ward 2 | Control | 10 | 6 | 9 | 3 | 4 | 9 | 11 | 52 | |
| Centre accrual | 20 | 14 | 23 | 7 | 8 | 21 | 11 | 104 | ||
| Site 2 | Ward 3 | POD | 2 | 8 | 7 | 14 | 2 | 11 | – | 44 |
| Ward 4 | POD | 4 | 3 | 11 | 4 | 4 | 12 | – | 38 | |
| Centre accrual | 6 | 11 | 18 | 18 | 6 | 23 | 82 | |||
| Site 3 | Ward 5 | Control | 6 | 6 | 15 | 11 | 13 | 2 | – | 53 |
| Ward 6 | Control | 4 | 6 | 14 | 8 | 5 | 15 | – | 52 | |
| Centre accrual | 10 | 12 | 29 | 19 | 18 | 17 | 105 | |||
| Site 4 | Ward 7 | Control | 8 | 5 | 10 | 17 | 11 | 1 | – | 52 |
| Ward 8 | Control | 8 | 4 | 6 | 3 | 11 | 6 | – | 38 | |
| Centre accrual | 16 | 9 | 16 | 20 | 22 | 7 | 90 | |||
| Site 5 | Ward 9 | POD | 1 | 3 | 2 | 1 | 1 | 7 | 3 | 18 |
| Ward 10 | POD | 11 | 14 | 21 | 1 | 2 | 3 | – | 52 | |
| Centre accrual | 12 | 17 | 23 | 2 | 3 | 10 | 3 | 70 | ||
| Site 6 | Ward 11 | Control | 8 | 4 | 4 | 4 | 3 | 1 | – | 24 |
| Ward 12 | POD | 5 | 8 | 10 | 7 | 6 | 4 | 1 | 41 | |
| Centre accrual | 13 | 12 | 14 | 11 | 9 | 5 | 1 | 65 | ||
| Site 7 | Ward 13 | POD | 4 | 7 | 8 | 5 | 6 | 6 | 10 | 46 |
| Ward 14 | Control | 2 | 9 | 13 | 8 | 6 | 7 | 2 | 47 | |
| Centre accrual | 6 | 16 | 21 | 13 | 12 | 13 | 12 | 93 | ||
| Site 8 | Ward 15 | Control | 14 | 21 | 10 | 7 | – | – | – | 52 |
| Ward 16 | POD | 10 | 13 | 17 | 12 | – | – | – | 52 | |
| Centre accrual | 24 | 34 | 27 | 19 | 104 | |||||
| Total monthly accrual | ||||||||||
| POD wards | 47 | 64 | 90 | 48 | 25 | 55 | 14 | 343 | ||
| Control wards | 60 | 61 | 81 | 61 | 53 | 41 | 13 | 370 | ||
| All wards | 107 | 125 | 171 | 109 | 78 | 96 | 27 | 713 | ||
| Monthly average accrual (per ward) | 6.7 | 7.8 | 10.7 | 6.8 | 4.9 | 6.0 | 1.7 | |||
| Cumulative accrual | 107 | 232 | 403 | 512 | 590 | 686 | 713 | 713 | ||
| Eligibility violation | Participants, n (%) | ||
|---|---|---|---|
| POD (N = 343) | Control (N = 370) | Total (N = 713) | |
| Has the patient breached the eligibility criteria? | |||
| Yes | 5 (1.5) | 8 (2.2) | 13 (1.8) |
| No | 338 (98.5) | 362 (97.8) | 700 (98.2) |
| Eligibility criteria breached | |||
| Delirium assessment (CAM) was not performed | 1 (20.0) | 6 (75.0) | 7 (53.8) |
| Participant had prevalent delirium on admissiona | 3 (60.0) | 2 (25.0) | 5 (38.5) |
| Participant had a planned discharge within 48 hours | 1 (20.0) | 0 (0.0) | 1 (7.7) |
| Variable | POD (N = 343) | Control (N = 370) | Total (N = 713) |
|---|---|---|---|
| Withdrawal, n (%) | |||
| Yes | 15 (4.4) | 18 (4.9) | 33 (4.6) |
| No | 328 (95.6) | 352 (95.1) | 680 (95.4) |
| Did the patient withdraw within 10 days of providing consent?, n (%) | |||
| Yes | 9 (60.0) | 10 (55.6) | 19 (57.6) |
| No | 6 (40.0) | 8 (44.4) | 14 (42.4) |
| Time (weeks) between consent and withdrawal | |||
| Mean (SD) | 1.7 (1.52) | 1.9 (1.65) | 1.8 (1.57) |
| Median (range) | 0.7 (0.1–3.9) | 1.2 (0.1–4.7) | 0.7 (0.1–4.7) |
| Missing | 0 | 0 | 0 |
| Who requested withdrawal?, n (%) | |||
| Participant | 12 (80.0) | 16 (88.9) | 28 (84.8) |
| Family member/friend | 2 (13.3) | 1 (5.6) | 3 (9.1) |
| Missing | 1 (6.7) | 1 (5.6) | 2 (6.1) |
| Withdrawal from CAM?, n (%) | |||
| Yes | 13 (86.7) | 18 (100.0) | 31 (93.9) |
| No | 2 (13.3) | 0 (0.0) | 2 (6.1) |
| Withdrawal from researcher questionnaires?, n (%) | |||
| Yes | 15 (100.0) | 18 (100.0) | 33 (100.0) |
| Withdrawal from postal questionnaire?, n (%) | |||
| Yes | 14 (93.3) | 18 (100.0) | 32 (97.0) |
| No | 1 (6.7) | 0 (0.0) | 1 (3.0) |
| Withdrawal from data collection from notes?, n (%) | |||
| Yes | 11 (73.3) | 5 (27.8) | 16 (48.5) |
| No | 4 (26.7) | 13 (72.2) | 17 (51.5) |
| Site | Total (N = 713) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 (N = 104) | 2 (N = 82) | 3 (N = 105) | 4 (N = 90) | 5 (N = 70) | 6 (N = 65) | 7 (N = 93) | 8 (N = 104) | ||
| Age (years) | |||||||||
| Mean (SD) | 84.1 (7.32) | 81.5 (7.25) | 84.8 (7.09) | 83.0 (8.20) | 85.1 (7.58) | 79.0 (7.34) | 79.5 (8.62) | 83.6 (7.25) | 82.7 (7.84) |
| Median (range) | 85.0 (67.0–98.0) | 83.0 (66.0–98.0) | 85.0 (66.0–99.0) | 84.0 (65.0–99.0) | 86.0 (66.0–101.0) | 79.0 (65.0–95.0) | 80.0 (65.0–96.0) | 85.0 (66.0–98.0) | 83.0 (65.0–101.0) |
| (Q1, Q3) | (79.5, 90.0) | (78.0, 86.0) | (81.0, 90.0) | (77.0, 89.0) | (80.0, 91.0) | (75.0, 83.0) | (71.0, 87.0) | (79.0, 89.0) | (78.0, 89.0) |
| Missing (n) | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 2 |
| Sex, n (%) | |||||||||
| Male | 0 (0.0) | 28 (34.1) | 43 (41.0) | 33 (36.7) | 18 (25.7) | 33 (50.8) | 37 (39.8) | 33 (31.7) | 225 (31.6) |
| Female | 104 (100.0) | 54 (65.9) | 62 (59.0) | 57 (63.3) | 51 (72.9) | 32 (49.2) | 56 (60.2) | 71 (68.3) | 487 (68.3) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.1) |
| Ethnicity, n (%) | |||||||||
| White | 99 (95.2) | 80 (97.6) | 88 (83.8) | 78 (86.7) | 64 (91.4) | 59 (90.8) | 87 (93.5) | 99 (95.2) | 654 (91.7) |
| Mixed: white and black African | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.5) | 0 (0.0) | 0 (0.0) | 1 (0.1) |
| Mixed: white and Asian | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.0) | 1 (0.1) |
| Other Asian background | 0 (0.0) | 1 (1.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.1) |
| Black – Caribbean | 1 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.0) | 2 (0.3) |
| Other ethnic group | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.0) | 1 (0.1) |
| Missing | 4 (3.8) | 1 (1.2) | 17 (16.2) | 12 (13.3) | 6 (8.6) | 5 (7.7) | 6 (6.5) | 2 (1.9) | 53 (7.4) |
| Characteristic | Site, n (%) | Total, n (%) (N = 713) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 (N = 104) | 2 (N = 82) | 3 (N = 105) | 4 (N = 90) | 5 (N = 70) | 6 (N = 65) | 7 (N = 93) | 8 (N = 104) | ||
| Residence | |||||||||
| Home | 93 (89.4) | 79 (96.3) | 97 (92.4) | 83 (92.2) | 59 (84.3) | 62 (95.4) | 86 (92.5) | 91 (87.5) | 650 (91.2) |
| Nursing home | 2 (1.9) | 1 (1.2) | 1 (1.0) | 2 (2.2) | 3 (4.3) | 3 (4.6) | 4 (4.3) | 3 (2.9) | 19 (2.7) |
| Residential/care home | 9 (8.7) | 2 (2.4) | 7 (6.7) | 5 (5.6) | 7 (10.0) | 0 (0.0) | 3 (3.2) | 10 (9.6) | 43 (6.0) |
| Missinga | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.1) |
| Reason for admission | |||||||||
| Hip fracture | 0 (0.0) | 19 (23.2) | 31 (29.5) | 27 (30.0) | 42 (60.0) | 9 (13.8) | 16 (17.2) | 26 (25.0) | 170 (23.8) |
| Other orthopaedic condition | 5 (4.8) | 16 (19.5) | 25 (23.8) | 17 (18.9) | 7 (10.0) | 34 (52.3) | 32 (34.4) | 26 (25.0) | 162 (22.7) |
| Medical condition | 99 (95.2) | 47 (57.3) | 49 (46.7) | 46 (51.1) | 20 (28.6) | 22 (33.8) | 45 (48.4) | 52 (50.0) | 380 (53.3) |
| Missinga | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.1) |
| Cognitive impairment and/or dementia | |||||||||
| Yes | 13 (12.5) | 20 (24.4) | 26 (24.8) | 12 (13.3) | 31 (44.3) | 10 (15.4) | 15 (16.1) | 23 (22.1) | 150 (21.0) |
| No | 91 (87.5) | 62 (75.6) | 79 (75.2) | 78 (86.7) | 38 (54.3) | 55 (84.6) | 78 (83.9) | 81 (77.9) | 562 (78.8) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.1) |
| Highest NEWS category within 48 hours of admissionb | |||||||||
| Low | 96 (92.3) | 68 (82.9) | 89 (84.8) | 80 (88.9) | 39 (55.7) | 64 (98.5) | 58 (62.4) | 96 (92.3) | 590 (82.7) |
| Medium | 5 (4.8) | 14 (17.1) | 10 (9.5) | 5 (5.6) | 23 (32.9) | 1 (1.5) | 29 (31.2) | 6 (5.8) | 93 (13.0) |
| High | 3 (2.9) | 0 (0.0) | 2 (1.9) | 1 (1.1) | 7 (10.0) | 0 (0.0) | 5 (5.4) | 2 (1.9) | 20 (2.8) |
| Missing | 0 (0.0) | 0 (0.0) | 4 (3.8) | 4 (4.4) | 1 (1.4) | 0 (0.0) | 1 (1.1) | 0 (0.0) | 10 (1.4) |
| Hearing impairment | |||||||||
| Yes | 37 (35.6) | 27 (32.9) | 34 (32.4) | 24 (26.7) | 25 (35.7) | 19 (29.2) | 31 (33.3) | 35 (33.7) | 232 (32.5) |
| No | 67 (64.4) | 55 (67.1) | 71 (67.6) | 66 (73.3) | 44 (62.9) | 46 (70.8) | 62 (66.7) | 69 (66.3) | 480 (67.3) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.1) |
| Participant uses a hearing aid | |||||||||
| Yes | 17 (45.9) | 17 (63.0) | 19 (55.9) | 16 (66.7) | 16 (64.0) | 15 (78.9) | 19 (61.3) | 31 (88.6) | 150 (64.7) |
| No | 20 (54.1) | 10 (37.0) | 15 (44.1) | 8 (33.3) | 9 (36.0) | 4 (21.1) | 12 (38.7) | 4 (11.4) | 82 (35.3) |
| Visual impairment | |||||||||
| None | 14 (13.5) | 12 (14.6) | 8 (7.6) | 5 (5.6) | 7 (10.0) | 10 (15.4) | 7 (7.5) | 13 (12.5) | 76 (10.7) |
| Registered blind | 1 (1.0) | 2 (2.4) | 6 (5.7) | 0 (0.0) | 0 (0.0) | 1 (1.5) | 1 (1.1) | 2 (1.9) | 13 (1.8) |
| Partially sighted | 17 (16.3) | 13 (15.9) | 6 (5.7) | 4 (4.4) | 4 (5.7) | 1 (1.5) | 12 (12.9) | 6 (5.8) | 63 (8.8) |
| Wears glasses | 70 (67.3) | 55 (67.1) | 85 (81.0) | 81 (90.0) | 58 (82.9) | 53 (81.5) | 73 (78.5) | 83 (79.8) | 558 (78.3) |
| Missing | 2 (1.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (0.4) |
| Benzodiazepines prescribed | |||||||||
| Yes | 3 (2.9) | 2 (2.4) | 6 (5.7) | 6 (6.7) | 5 (7.1) | 1 (1.5) | 6 (6.5) | 3 (2.9) | 32 (4.5) |
| No | 101 (97.1) | 80 (97.6) | 99 (94.3) | 84 (93.3) | 64 (91.4) | 64 (98.5) | 87 (93.5) | 101 (97.1) | 680 (95.4) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.1) |
| Opiates prescribed | |||||||||
| Yes | 34 (32.7) | 19 (23.2) | 45 (42.9) | 45 (50.0) | 54 (77.1) | 39 (60.0) | 17 (18.3) | 64 (61.5) | 317 (44.5) |
| No | 70 (67.3) | 63 (76.8) | 60 (57.1) | 45 (50.0) | 15 (21.4) | 26 (40.0) | 76 (81.7) | 40 (38.5) | 395 (55.4) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.1) |
| H1 antihistamines prescribed | |||||||||
| Yes | 12 (11.5) | 8 (9.8) | 6 (5.7) | 13 (14.4) | 16 (22.9) | 4 (6.2) | 7 (7.5) | 10 (9.6) | 76 (10.7) |
| No | 92 (88.5) | 74 (90.2) | 99 (94.3) | 77 (85.6) | 53 (75.7) | 61 (93.8) | 86 (92.5) | 94 (90.4) | 636 (89.2) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.1) |
| Participant comorbiditiesc | |||||||||
| Yes | 80 (76.9) | 50 (61.0) | 80 (76.2) | 57 (63.3) | 46 (65.7) | 44 (67.7) | 55 (59.1) | 68 (65.4) | 480 (67.3) |
| No | 24 (23.1) | 32 (39.0) | 25 (23.8) | 33 (36.7) | 23 (32.9) | 21 (32.3) | 38 (40.9) | 36 (34.6) | 232 (32.5) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.1) |
| Charlson Comorbidity Indexd | |||||||||
| Mean (SD) | 1.9 (1.95) | 2.2 (2.73) | 2.1 (2.13) | 1.8 (1.97) | 1.5 (1.48) | 1.4 (1.48) | 1.2 (1.52) | 1.5 (1.55) | 1.7 (1.92) |
| Median (range) | 1.0 (0.0–11.0) | 1.0 (0.0–12.0) | 2.0 (0.0–11.0) | 1.0 (0.0–8.0) | 1.0 (0.0–7.0) | 1.0 (0.0–6.0) | 1.0 (0.0–7.0) | 1.0 (0.0–6.0) | 1.0 (0.0–12.0) |
| Q1, Q3 | (0.5, 3.0) | (0.0, 3.0) | (1.0, 3.0) | (0.0, 3.0) | (0.0, 2.0) | (0.0, 2.0) | (0.0, 2.0) | (0.0, 2.0) | (0.0, 3.0) |
| Missing | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Site | Ward | Ward type | Date started staff education | Date completed staff education | Time taken to complete staff education (weeks) | Date started review of current practice | Date completed review of current practice | Time taken to review current practice (weeks) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | Elderly care | 20 February 2014 | 1 May 2014 | 10.0 | 24 February 2014 | 15 June 2014 | 15.9 |
| 2 | 3 | Elderly care | 15 March 2014a | 15 July 2014a | 17.4 | 15 March 2014a | 15 July 2014a | 17.4 |
| 2 | 4 | Orthopaedic trauma | 4 March 2014 | 31 July 2014 | 21.3 | 1 March 2014 | 31 July 2014 | 21.7 |
| 5 | 9 | Elderly care | 21 May 2014 | 30 August 2014 | 14.4 | 7 May 2014 | 30 July 2014 | 12.0 |
| 5 | 10 | Orthopaedic trauma | 19 March 2014 | 31 July 2014 | 19.1 | 19 March 2014 | 31 July 2014 | 19.1 |
| 6 | 12 | Orthopaedic trauma | 15 May 2014a | 31 July 2014 | 11.0 | 15 May 2014a | – | – |
| 7 | 13 | Elderly care | 15 March 2014 | 15 July 2014 | 17.4 | 15 March 2014 | 15 July 2014 | 17.4 |
| 8 | 16 | Elderly care | 21 March 2014 | 5 August 2014 | 19.6 | 3 April 2014 | 15 December 2014a | 36.6b |
| Date started ward system implementation | Date completed ward system implementation | Time taken to implement ward system (weeks) | Date work started on POD delivery | Date work completed on POD delivery | Overall time taken to implement POD (weeks)c | |||
| 1 | 1 | Elderly care | 23 April 2014 | 10 June 2014 | 6.9 | 20 February 2014 | 15 June 2014 | 16.4 |
| 2 | 3 | Elderly care | 15 May 2014a | 15 July 2014a | 8.7 | 15 March 2014 | 15 July 2014 | 17.4 |
| 2 | 4 | Orthopaedic trauma | 11 July 2014 | 31 July 2014 | 2.9 | 1 March 2014 | 31 July 2014 | 21.7 |
| 5 | 9 | Elderly care | 11 June 2014 | 31 July 2014 | 7.1 | 7 May 2014 | 30 August 2014 | 16.4 |
| 5 | 10 | Orthopaedic trauma | 19 March 2014 | 11 July 2014 | 16.3 | 19 March 2014 | 31 July 2014 | 19.1 |
| 6 | 12 | Orthopaedic trauma | 16 July 2014 | 31 July 2014 | 2.1 | 27 March 2014 | 31 July 2014 | 18.0 |
| 7 | 13 | Elderly care | 5 March 2014 | 15 August 2014a | 23.3 | 5 March 2014 | 15 August 2014 | 23.3 |
| 8 | 16 | Elderly care | 21 March 2014 | 21 July 2014 | 17.4 | 21 March 2014 | 15 December 2014 | 38.4 |
| Variable | Moving onto the ward,a n (%) | Moving off the ward, n (%) | ||||
|---|---|---|---|---|---|---|
| Elderly care (N = 115) | Orthopaedic trauma (N = 101) | Total (N = 216) | Elderly care (N = 4) | Orthopaedic trauma (N = 9) | Total (N = 13) | |
| Staff grade | ||||||
| Health-care support worker/assistant | 66 (57.4) | 46 (45.5) | 112 (51.9) | 3 (75.0) | 2 (22.2) | 5 (38.5) |
| Senior health-care support worker/assistant | 0 (0.0) | 2 (2.0) | 2 (0.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Registered nurse | 26 (22.6) | 26 (25.7) | 52 (24.1) | 1 (25.0) | 5 (55.6) | 6 (46.2) |
| Junior sister/ward manager | 0 (0.0) | 3 (3.0) | 3 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 23 (20.0) | 24 (23.8) | 47 (21.8) | 0 (0.0) | 2 (22.2) | 2 (15.4) |
| Type of contract | ||||||
| Permanent | 33 (28.7) | 47 (46.5) | 80 (37.0) | 4 (100.0) | 9 (100.0) | 13 (100.0) |
| Agency | 16 (13.9) | 21 (20.8) | 37 (17.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Bank | 60 (52.2) | 33 (32.7) | 93 (43.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 6 (5.2) | 0 (0.0) | 6 (2.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Type of ward moved from/to | ||||||
| Elderly care | 4 (3.5) | 0 (0.0) | 4 (1.9) | 1 (25.0) | 2 (22.2) | 3 (23.1) |
| Orthopaedic trauma | 0 (0.0) | 8 (7.9) | 8 (3.7) | 0 (0.0) | 6 (66.7) | 6 (46.2) |
| Otherb | 33 (28.7) | 0 (0.0) | 33 (15.3) | 3 (75.0) | 1 (11.1) | 4 (30.8) |
| Missing | 78 (67.8) | 93 (92.1) | 171 (79.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Variable | Incidence (95% CI) | |
|---|---|---|
| POD | Control | |
| Number of CAM assessments | 2382 | 2683 |
| Randomised at hospital level | 2.5 (1.56 to 3.41) | 2.3 (1.51 to 3.08) |
| Randomised at ward level | 1.6 (0.93 to 2.31) | 1.9 (1.19 to 2.69) |
| Variable | Incidence (95% CI) | |
|---|---|---|
| POD | Control | |
| Recruited within 3 months of site opening to recruitment | 2.2 (1.51 to 2.98) | 2.6 (1.80 to 3.39) |
| Recruited between 3 and 6 months of site opening to recruitment | 1.6 (0.73 to 2.43) | 1.5 (0.79 to 2.19) |
| Number of times participants moved wards | Trial arm, n (%) | Total (N = 135), n (%) | |
|---|---|---|---|
| POD (N = 58) | Control (N = 77) | ||
| Once | 52 (89.7) | 63 (81.8) | 115 (85.2) |
| Twice | 6 (10.3) | 11 (14.3) | 17 (12.6) |
| Three times | 0 (0.0) | 2 (2.6) | 2 (1.5) |
| Four times | 0 (0.0) | 1 (1.3) | 1 (0.7) |
| Did the participant move ward? | Site, n (%) | Total (N = 713), n (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 (N = 104) | 2 (N = 82) | 3 (N = 105) | 4 (N = 90) | 5 (N = 70) | 6 (N = 65) | 7 (N = 93) | 8 (N = 104) | ||
| Yes | 23 (22.1) | 13 (15.9) | 19 (18.1) | 13 (14.4) | 7 (10.0) | 16 (24.6) | 24 (25.8) | 20 (19.2) | 135 (18.9) |
| No | 81 (77.9) | 69 (84.1) | 86 (81.9) | 77 (85.6) | 62 (88.6) | 49 (75.4) | 69 (74.2) | 84 (80.8) | 577 (80.9) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.1) |
| Variable | POD | Control | Total |
|---|---|---|---|
| Number of registered participants | 343 | 370 | 713 |
| Number (%) of participants with at least one missing daily CAM assessment | 247 (72.0) | 250 (67.6) | 497 (69.7) |
| Number (%) of participants with one missing daily CAM assessment | 154 (44.9) | 172 (46.5) | 326 (45.7) |
| Number (%) of participants with two missing daily CAM assessments | 44 (12.8) | 45 (12.2) | 89 (12.5) |
| Number (%) of participants with three or more missing daily CAM assessments | 49 (14.3) | 33 (8.9) | 82 (11.5) |
| Number of missing daily CAM assessments | 334 | 246 | 580 |
| Reason assessment not performed, n (%) | |||
| Participant too ill | 100 (29.9) | 86 (35.0) | 186 (32.1) |
| Participant refused | 92 (27.5) | 57 (23.2) | 149 (25.7.4) |
| Personal or nominated consultee refused | 3 (0.9) | 0 (0.0) | 3 (0.5) |
| Participant unavailable | 46 (13.8) | 60 (24.4) | 106 (18.3) |
| Research staff missed participant | 16 (4.8) | 13 (5.3) | 29 (5.0) |
| Ward closed | 55 (16.5) | 6 (2.4) | 61 (10.5) |
| Participant refused – delirium not suggested | 8 (2.4) | 6 (2.4) | 14 (2.4) |
| Othera | 5 (1.5) | 11 (4.5) | 16 (2.8) |
| Missing | 9 (2.7) | 7 (2.8) | 16 (2.8) |
| Variable | Trial arm, n (%) | Total (N = 5065), n (%) | |
|---|---|---|---|
| POD (N = 2382) | Control (N = 2683) | ||
| CAM assessments with missing responses | 2 (0.1) | 4 (0.1) | 6 (0.1) |
| AMTS completed? | |||
| Yes | 2381 (100.0) | 2682 (100.0) | 5063 (100.0) |
| No | 1 (0.0) | 1 (0.0) | 2 (0.0) |
| MotYB test completed? | |||
| Yes | 2368 (99.4) | 2672 (99.6) | 5040 (99.5) |
| No | 14 (0.6) | 11 (0.4) | 25 (0.5) |
| Number of 30-day CAMs | Site | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| Number expecteda | 91 | 72 | 94 | 72 | 62 | 60 | 85 | 93 | 629 |
| Number (%) conducted | 69 (75.8) | 50 (69.4) | 84 (89.4) | 70 (97.2) | 50 (80.6) | 49 (81.7) | 68 (80.0) | 73 (78.5) | 513 (81.6) |
| Reason assessment not performed | Trial arm, n (%) | Total (N = 116), n (%) | |
|---|---|---|---|
| POD (N = 78) | Control (N = 38) | ||
| Participant too ill | 14 (17.9) | 10 (26.3) | 24 (20.7) |
| Participant refused | 23 (29.5) | 6 (15.8) | 29 (25.0) |
| Personal or nominated consultee refused | 3 (3.8) | 0 (0.0) | 3 (2.6) |
| Participant unavailable | 3 (3.8) | 2 (5.3) | 5 (4.3) |
| Research staff missed participant | 3 (3.8) | 0 (0.0) | 3 (2.6) |
| Unable to contact participant | 18 (23.1) | 9 (23.7) | 27 (23.3) |
| Participant moved out of area | 10 (12.8) | 8 (21.1) | 18 (15.5) |
| Ward closed | 0 (0.0) | 1 (2.6) | 1 (0.9) |
| Participant refused – delirium not suggested | 1 (1.3) | 0 (0.0) | 1 (0.9) |
| Othera | 3 (3.8) | 2 (5.3) | 5 (4.3) |
| Variable | Baseline | 30 daysb | 3 monthsc | ||||||
|---|---|---|---|---|---|---|---|---|---|
| POD | Control | Total | POD | Control | Total | POD | Control | Total | |
| Registered participants (n) | 343 | 370 | 713 | 343 | 370 | 713 | 343 | 370 | 713 |
| Number of questionnaires expectedd | 342 | 369 | 711 | 300 | 325 | 625 | 268 | 299 | 567 |
| Booklets received, n (%) | 334 (97.7) | 365 (98.9) | 699 (98.3) | 224 (74.7) | 287 (88.3) | 511 (81.8) | 177 (66.0) | 223 (74.6) | 400 (70.5) |
| Of these, booklets received with blank questionnaires, n (%) | 0 (0.0) | 1 (0.3) | 1 (0.1) | 44 (14.7) | 11 (3.4) | 55 (8.8) | 2 (0.7) | 1 (0.3) | 3 (0.5) |
| NEADL, n (%) | 0 (0.0) | 1 (0.3) | 1 (0.1) | – | – | – | 2 (0.7) | 1 (0.3) | 3 (0.5) |
| Geriatric Depression Scale, n (%) | – | – | – | 25 (8.3) | 9 (2.8) | 34 (5.4) | – | – | – |
| Clinical Anxiety Scale, n (%) | – | – | – | 44 (14.7) | 11 (3.4) | 55 (8.8) | – | – | – |
| Booklet not returned, n (%) | 8 (2.3) | 4 (1.1) | 12 (1.7) | 76 (25.3) | 38 (11.7) | 114 (18.2) | 91 (34.0) | 76 (25.4) | 167 (29.5) |
| Characteristic | Questionnaire | Participant | Total (N = 713) | ||
|---|---|---|---|---|---|
| Returned (N = 511) | Not returned (N = 114) | Withdrew (N = 32) | Died (N = 56) | ||
| Age (years) | |||||
| Mean (SD) | 82.7 (7.76) | 81.6 (8.01) | 81.6 (8.48) | 86.1 (7.02) | 82.7 (7.84) |
| Median (range) | 83 (65–100) | 82 (66–95) | 84 (65–95) | 86 (68–101) | 83 (65–101) |
| Missing | 2 | 0 | 0 | 0 | 2 |
| Sex, n (%) | |||||
| Male | 159 (31.1) | 36 (31.6) | 9 (28.1) | 21 (37.5) | 225 (31.6) |
| Female | 352 (68.9) | 78 (68.4) | 22 (68.8) | 35 (62.5) | 487 (68.3) |
| Missing | 0 (0.0) | 0 (0.0) | 1 (3.1) | 0 (0.0) | 1 (0.1) |
| Ethnicity, n (%) | |||||
| White | 467 (91.4) | 108 (94.7) | 30 (93.8) | 49 (87.5) | 654 (91.7) |
| Mixed – white and black African | 1 (0.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.1) |
| Mixed – white and Asian | 0 (0.0) | 1 (0.9) | 0 (0.0) | 0 (0.0) | 1 (0.1) |
| Other Asian background | 1 (0.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.1) |
| Black Caribbean | 2 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.3) |
| Other ethnic group | 0 (0.0) | 1 (0.9) | 0 (0.0) | 0 (0.0) | 1 (0.1) |
| Missing | 40 (7.8) | 4 (3.5) | 2 (6.3) | 7 (12.5) | 53 (7.4) |
| Residence, n (%) | |||||
| Home | 462 (90.4) | 109 (95.6) | 31 (96.9) | 48 (85.7) | 650 (91.2) |
| Nursing home | 14 (2.7) | 2 (1.8) | 0 (0.0) | 3 (5.4) | 19 (2.7) |
| Residential/care home | 35 (6.8) | 3 (2.6) | 0 (0.0) | 5 (8.9) | 43 (6.0) |
| Missing | 0 (0.0) | 0 (0.0) | 1 (3.1) | 0 (0.0) | 1 (0.1) |
| Ward type, n (%) | |||||
| Elderly care/geriatric medicine | 259 (50.7) | 70 (61.4) | 18 (56.3) | 45 (80.4) | 392 (55.0) |
| Orthopaedic trauma/surgery | 252 (49.3) | 44 (38.6) | 14 (43.8) | 11 (19.6) | 321 (45.0) |
| Reason | Trial arm, n (%) | Total (N = 114), n (%) | |
|---|---|---|---|
| POD (N = 76) | Control (N = 38) | ||
| Could not contact | 18 (23.7) | 9 (23.7) | 27 (23.7) |
| Moved out of area | 9 (11.8) | 7 (18.4) | 16 (14.0) |
| Too unwell | 11 (14.5) | 7 (18.4) | 18 (15.8) |
| Lost at site | 4 (5.3) | 4 (10.5) | 8 (7.0) |
| Refused to complete | 22 (28.9) | 4 (10.5) | 26 (22.8) |
| Unwilling for visit | 3 (3.9) | 1 (2.6) | 4 (3.5) |
| Other | 9 (11.8) | 6 (15.8) | 15 (13.2) |
| Reason | Trial arm, n (%) | Total (N = 167), n (%) | |
|---|---|---|---|
| POD (N = 91) | Control (N = 76) | ||
| Could not contact | 48 (52.7) | 44 (57.9) | 92 (55.1) |
| Too unwell | 7 (7.7) | 5 (6.6) | 12 (7.2) |
| Refused to complete | 20 (22.0) | 11 (14.5) | 31 (18.6) |
| Unknown | 11 (12.1) | 11 (14.5) | 22 (13.2) |
| Lost in post | 2 (2.2) | 1 (1.3) | 3 (1.8) |
| Consultee/carer/relative/friend refused | 3 (3.3) | 4 (5.3) | 7 (4.2) |
| CAM severity score | POD (N = 48) | Control (N = 57) | Total (N = 105) |
|---|---|---|---|
| Mean (SD) | 3.9 (1.01) | 3.8 (0.96) | 3.8 (0.98) |
| Median (range) | 4.0 (2.7–6.0) | 3.0 (3.0–6.0) | 4.0 (2.7–6.0) |
| Missing (n) | 0 | 0 | 0 |
| Duration of delirium episode | POD (N = 24) | Control (N = 33) | Total (N = 57) |
|---|---|---|---|
| Mean (SD) | 2.3 (1.97) | 2.2 (1.85) | 2.3 (1.89) |
| Median (range) | 1.0 (1.0–8.0) | 1.0 (1.0–7.0) | 1.0 (1.0–8.0) |
| Missing (n) | 0 | 0 | 0 |
| Days between ward admission and first occurrence of delirium | POD (N = 24) | Control (N = 33) | Total (N = 57) |
|---|---|---|---|
| Mean (SD) | 4.0 (1.90) | 4.2 (2.26) | 4.1 (2.10) |
| Median (range) | 4.0 (1.0–8.0) | 4.0 (1.0–8.0) | 4.0 (1.0–8.0) |
| Missing (n) | 0 | 0 | 0 |
| Delirium suggested? | Trial arm, n (%) | Total (N = 513), n (%) | |
|---|---|---|---|
| POD (N = 224) | Control (N = 289) | ||
| Yes | 6 (2.7) | 3 (1.0) | 9 (1.8) |
| No | 218 (97.3) | 286 (99.0) | 504 (98.2) |
| CAM severity score | POD (N = 6) | Control (N = 3) | Total (N = 9) |
|---|---|---|---|
| Mean (SD) | 3.7 (0.82) | 3.3 (0.58) | 3.6 (0.73) |
| Median (range) | 3.5 (3.0–5.0) | 3.0 (3.0–4.0) | 3.0 (3.0–5.0) |
| Missing (n) | 0 | 0 | 0 |
| Did the patient have persistent delirium? | Trial arm, n (%) | Total (N = 513), n (%) | |
|---|---|---|---|
| POD (N = 224) | Control (N = 289) | ||
| Yes | 2 (0.9) | 2 (0.7) | 4 (0.8) |
| No | 222 (99.1) | 287 (99.3) | 509 (99.2) |
| Deaths | POD (N = 343) | Control (N = 370) | Total (N = 713) |
|---|---|---|---|
| Has the patient died?, n (%) | |||
| Yes | 61 (17.8) | 53 (14.3) | 114 (16.0) |
| No | 282 (82.2) | 317 (85.7) | 599 (84.0) |
| Did the patient die within 3 months of providing consent?,a n (%) | |||
| Yes | 56 (16.3) | 48 (13.0) | 104 (14.6) |
| No | 283 (82.5) | 319 (86.2) | 602 (84.4) |
| Unknown | 4 (1.2) | 3 (0.8) | 7 (1.0) |
| Did the patient die within 10 days of providing consent?, n (%) | |||
| Yes | 17 (27.9) | 11 (20.8) | 28 (24.6) |
| No | 44 (72.1) | 42 (79.2) | 86 (75.4) |
| Number of days between consent and death | |||
| Mean (SD) | 33.1 (26.91) | 36.8 (29.22) | 34.8 (27.93) |
| Median (range) | 30.0 (2.0–148.0) | 24.0 (1.0–95.0) | 27.5 (1.0–148.0) |
| Missingb (n) | 4 | 4 | 8 |
| Place of death, n (%) | |||
| Hospital | 44 (72.1) | 30 (56.6) | 74 (64.9) |
| Home | 2 (3.3) | 4 (7.5) | 6 (5.3) |
| Nursing home | 1 (1.6) | 2 (3.8) | 3 (2.6) |
| Residential care home | 1 (1.6) | 1 (1.9) | 2 (1.8) |
| Unknown | 7 (11.5) | 12 (22.6) | 19 (16.7) |
| Otherc | 2 (3.3) | 0 (0.0) | 2 (1.8) |
| Missing | 4 (6.6) | 4 (7.5) | 8 (7.0) |
| Cause of death (categorised), n (%) | |||
| Cancer | 4 (6.6) | 4 (7.5) | 8 (7.0) |
| Coronary heart disease | 4 (6.6) | 1 (1.9) | 5 (4.4) |
| Heart failure | 3 (4.9) | 4 (7.5) | 7 (6.1) |
| Pneumonia | 13 (21.3) | 4 (7.5) | 17 (14.9) |
| Sepsis | 2 (3.3) | 1 (1.9) | 3 (2.6) |
| Frailty | 5 (8.2) | 6 (11.3) | 11 (9.6) |
| Pulmonary embolism | 0 (0.0) | 1 (1.9) | 1 (0.9) |
| Unknown | 22 (36.1) | 22 (41.5) | 44 (38.6) |
| Otherd | 2 (3.3) | 2 (3.8) | 4 (3.5) |
| Missing | 6 (9.8) | 8 (15.1) | 14 (12.3) |
| Questionnaire | Baseline, mean (SD); n | 30 days, mean (SD); n | 3 months, mean (SD); n | ||||||
|---|---|---|---|---|---|---|---|---|---|
| POD | Control | Total | POD | Control | Total | POD | Control | Total | |
| Randomised (n) | 343 | 370 | 713 | ||||||
| NEADL total scorea | 36.7 (18.35); 334 | 39.7 (19.01); 364 | 38.3 (18.74); 698 | N/A | N/A | N/A | 29.5 (20.31); 173 | 33.1 (20.93); 220 | 31.5 (20.71); 393 |
| NEADL mobility score | 8.7 (6.21); 332 | 9.3 (6.43); 364 | 9.0 (6.33); 696 | N/A | N/A | N/A | 6.4 (6.31); 174 | 7.2 (6.46); 219 | 6.8 (6.40); 393 |
| NEADL kitchen score | 11.2 (4.68); 332 | 11.7 (4.70); 364 | 11.4 (4.69); 696 | N/A | N/A | N/A | 9.3 (5.54); 172 | 9.9 (5.63); 218 | 9.6 (5.59); 390 |
| NEADL domestic score | 7.7 (5.42); 334 | 8.4 (5.48); 364 | 8.1 (5.46); 698 | N/A | N/A | N/A | 5.8 (5.67); 172 | 7.0 (5.75); 217 | 6.5 (5.74); 389 |
| NEADL leisure score | 9.1 (4.80); 334 | 10.3 (4.93); 362 | 9.7 (4.90); 696 | N/A | N/A | N/A | 8.1 (5.10); 172 | 9.2 (5.22); 220 | 8.7 (5.19); 392 |
| Geriatric Depression Scale total score | N/A | N/A | N/A | 4.7 (3.49); 199 | 4.2 (3.31); 278 | 4.4 (3.39); 477 | N/A | N/A | N/A |
| Clinical Anxiety Scale total score | N/A | N/A | N/A | 16.8 (15.42); 180 | 16.9 (14.79); 276 | 16.8 (15.02); 456 | N/A | N/A | N/A |
| NEADL total and subscale scores | Baseline | 3 months | ||||
|---|---|---|---|---|---|---|
| POD (N = 334) | Control (N = 364) | Total (N = 698) | POD (N = 175) | Control (N = 222) | Total (N = 397) | |
| NEADL score | ||||||
| Mean (SD) | 36.7 (18.35) | 39.7 (19.01) | 38.3 (18.74) | 29.5 (20.31) | 33.1 (20.93) | 31.5 (20.71) |
| Median (range) | 36.0 (0.0–66.0) | 41.5 (0.0–66.0) | 39.0 (0.0–66.0) | 27.0 (0.0–66.0) | 33.8 (0.0–66.0) | 30.3 (0.0–66.0) |
| Missing (n) | 0 | 0 | 0 | 2 | 2 | 4 |
| Mobility subscale score | ||||||
| Mean (SD) | 8.7 (6.21) | 9.3 (6.43) | 9.0 (6.33) | 6.4 (6.31) | 7.2 (6.46) | 6.8 (6.40) |
| Median (range) | 8.0 (0.0–18.0) | 9.0 (0.0–18.0) | 8.7 (0.0–18.0) | 4.0 (0.0–18.0) | 5.0 (0.0–18.0) | 5.0 (0.0–18.0) |
| Missing (n) | 2 | 0 | 2 | 1 | 3 | 4 |
| Kitchen subscale score | ||||||
| Mean (SD) | 11.2 (4.68) | 11.7 (4.70) | 11.4 (4.69) | 9.3 (5.54) | 9.9 (5.63) | 9.6 (5.59) |
| Median (range) | 13.0 (0.0–15.0) | 15.0 (0.0–15.0) | 14.0 (0.0–15.0) | 11.0 (0.0–15.0) | 13.0 (0.0–15.0) | 12.0 (0.0–15.0) |
| Missing (n) | 2 | 0 | 2 | 3 | 4 | 7 |
| Domestic subscale score | ||||||
| Mean (SD) | 7.7 (5.42) | 8.4 (5.48) | 8.1 (5.46) | 5.8 (5.67) | 7.0 (5.75) | 6.5 (5.74) |
| Median (range) | 8.0 (0.0–15.0) | 9.0 (0.0–15.0) | 8.0 (0.0–15.0) | 3.0 (0.0–15.0) | 7.0 (0.0–15.0) | 6.0 (0.0–15.0) |
| Missing (n) | 0 | 0 | 0 | 3 | 5 | 8 |
| Leisure subscale score | ||||||
| Mean (SD) | 9.1 (4.80) | 10.3 (4.93) | 9.7 (4.90) | 8.1 (5.10) | 9.2 (5.22) | 8.7 (5.19) |
| Median (range) | 9.0 (0.0–18.0) | 10.0 (0.0–18.0) | 9.0 (0.0–18.0) | 8.0 (0.0–18.0) | 9.0 (0.0–18.0) | 9.0 (0.0–18.0) |
| Missing (n) | 0 | 2 | 2 | 3 | 2 | 5 |
| Geriatric Depression Scale score | POD (N = 199) | Control (N = 278) | Total (N = 477) |
|---|---|---|---|
| Mean (SD) | 4.7 (3.49) | 4.2 (3.31) | 4.4 (3.39) |
| Median (range) | 4.0 (0.0–15.0) | 3.0 (0.0–14.0) | 3.0 (0.0–15.0) |
| Categorised Geriatric Depression Scale score, n (%) | |||
| No depression | 127 (63.8) | 205 (73.7) | 332 (69.6) |
| Suggestive of depression | 56 (28.1) | 54 (19.4) | 110 (23.1) |
| Depressed | 16 (8.0) | 19 (6.8) | 35 (7.3) |
| Clinical Anxiety Scale | POD (N = 180) | Control (N = 276) | Total (N = 456) |
|---|---|---|---|
| Score | |||
| Mean (SD) | 16.8 (15.42) | 16.9 (14.79) | 16.8 (15.02) |
| Median (range) | 14.0 (0.0–100.0) | 13.1 (0.0–100.0) | 13.5 (0.0–100.0) |
| Missing (n) | 0 | 0 | 0 |
| Anxiety category, n (%) | |||
| No anxiety | 151 (83.9) | 234 (84.8) | 385 (84.4) |
| Anxiety | 29 (16.1) | 42 (15.2) | 71 (15.6) |
| Poor outcome | Trial arm, n (%) | Total (N = 713), n (%) | |
|---|---|---|---|
| POD (N = 343) | Control (N = 370) | ||
| Yes | 80 (23.3) | 72 (19.5) | 152 (21.3) |
| No | 149 (43.4) | 217 (58.6) | 366 (51.3) |
| Unknown | |||
| Without persistent delirium, still an inpatient | 41 (12.0) | 36 (9.7) | 77 (10.8) |
| Persistent delirium unknown, no change in accommodation or other discharge destination | 54 (15.7) | 31 (8.4) | 85 (11.9) |
| Persistent delirium unknown, still an inpatient | 7 (2.0) | 2 (0.5) | 9 (1.3) |
| Persistent delirium unknown, discharge destination unknown | 1 (0.3) | 0 (0.0) | 1 (0.1) |
| Participant has withdrawn | 11 (3.2) | 12 (3.2) | 23 (3.2) |
List of abbreviations
- A&E
- accident and emergency
- AMTS
- Abbreviated Mental Test Score
- CAM
- Confusion Assessment Method
- CI
- confidence interval
- CTRU
- Clinical Trials Research Unit
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EVPI
- expected value of perfect information
- GP
- general practitioner
- HCA
- health-care assistant
- HELP
- Hospital Elder Life Program
- ICC
- intracluster correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- MDT
- multidisciplinary team
- MotYB
- months of the year backwards
- MTI
- multicomponent targeted intervention
- NEADL
- Nottingham Extended Activities of Daily Living
- NEWS
- National Early Warning Score
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NPT
- normalisation process theory
- POD
- Prevention of Delirium
- PODv1
- Prevention of Delirium system of care version 1
- PODv2
- Prevention of Delirium system of care version 2
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- RA
- research assistant
- SD
- standard deviation
- SF-36
- Short Form questionnaire-36 items
- VSM
- voluntary services manager
- WS
- workstream
Notes
-
Content review of existing Hospital Elder Life Program protocols
-
Feasibility of Prevention of Delirium: leadership, planning implementation and delivery by ward
-
The Prevention of Delirium programme manuals and materials: feedback from sites
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/pgfar09040).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
