Notes
Article history paragraph text
The research reported in this issue of the journal was funded by the HTA programme as project number 06/304/142. The contractual start date was in April 2007. The draft report began editorial review in April 2012 and was accepted for publication in October 2012. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen's Printer and Controller of HMSO 2013. This work was produced by Watson et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
There exists a wealth of evidence regarding the detrimental impact of hazardous alcohol consumption on the physical and mental health of the population [hazardous alcohol consumption is defined as the consumption of more than 21 standard alcohol units in any week for males and 14 for females; or half of the recommended number of standard alcohol units in any one day (10 for males, 7 for females)]. It is estimated that hazardous alcohol consumption accounts for 150,000 hospital admissions and between 15,000 and 22,000 deaths per annum in the UK. 1 In the older population (those aged ≥ 55 years), hazardous alcohol consumption is associated with a wide range of physical, psychological and social problems. 2 There is evidence of an association between increased alcohol consumption and increased risk of coronary heart disease, hypertension and haemorrhagic and ischaemic stroke, increased rates of alcohol-related liver disease and increased risk of a range of cancers. 3 Alcohol consumption is identified as one of the three main risk factors for falls,4 a major cause of morbidity and mortality in this population. The Royal College of Physicians estimates that 60% of older people admitted to hospital because of repeated falls, confusion, chest infections and heart failure have undiagnosed alcohol problems. 5 Increased alcohol consumption in older age can also contribute to the onset of dementia and other age-related cognitive deficits, Parkinson's disease and a range of psychological problems including depression and anxiety. 6 Alcohol use is implicated in one-third of all suicides in the older population. 7 It is estimated that 80% of those aged ≥ 65 years regularly take prescribed medication and that polypharmacy is common, with one-third taking at least four prescribed medications per day. 8 Alcohol is a major contraindication for many of the drugs prescribed for older people, and alcohol and medication interactions are a common phenomenon. 9 Increased alcohol consumption in older age is also associated with a range of social problems including self-neglect, poor nutrition, social isolation and hypothermia. 10
The prevalence of hazardous alcohol consumption (inclusive of harmful consumption) in those aged ≥ 55 years is generally considered to be lower than in the wider adult population. The most recent estimate derived from the Alcohol Needs Assessment Research Project11 indicates a general population prevalence of between 15% and 25% and concurs with other estimates derived from the General Household Survey. 12 There is evidence that these prevalence rates are underestimates of the true prevalence rate. 11 There is also evidence that the prevalence rate in primary care attendees is higher than in the general population. 13 Furthermore, the current Home Office Alcohol Strategy recommends that available research be used in order to understand ‘how we can best communicate the risks from alcohol, improving the public's understanding of both personal risks and societal harms. This will include whether separate advice is desirable for the maximum amount of alcohol to be drunk in one occasion and for people over 65’. 14
Recent research using data derived from the General Practice Research Database indicates that only 5% of people aged ≥ 55 years with an alcohol use disorder are identified in primary care settings. 15 Older people are less likely to seek treatment for alcohol use disorders16 and alcohol-related presentations are often atypical or masked by comorbid physical or psychiatric illness that makes alcohol-related diagnosis more difficult. 17 In 2000, 16% of the UK population was ≥ 65 years old and this is expected to increase to 21% by 2026. 8 As the average age of the population increases, the absolute number of older people consuming alcohol at hazardous levels will increase even if the prevalence rate remains stable. Opportunistic screening is a proactive screening technique that has been used with some success in a variety of health-care areas including type II diabetes18 and chlamydia infections,19 and is particularly useful in identifying conditions in populations who would not usually seek treatment.
A number of paper-based screening methods have been developed to identify hazardous alcohol consumption; these include instruments such as the Michigan Alcohol Screening Test,20 Paddington Alcohol Test,21 Fast Alcohol Screening Test,22 Single Alcohol Screening Question23 and the Alcohol Use Disorders Identification Test (AUDIT). 24 All have acceptable levels of sensitivity and specificity. The AUDIT was specifically developed for use in a primary care population and has 92% sensitivity and 92% specificity for identifying hazardous alcohol use in a UK primary care setting. 13 More specifically, in older populations (≥ 65 years) AUDIT has been demonstrated to have higher sensitivity and specificity compared with other screening tests. 25 AUDIT is a short, 10-item questionnaire that addresses frequency of alcohol consumption, alcohol-related problems and alcohol dependence symptoms. Because of the evidence of underdetection and misdiagnosis of hazardous alcohol use in older populations,16,17 the proactive application of a short universal screening method is likely to be more appropriate. There is evidence that patients are more compliant with screening protocols for alcohol use in health-care settings and that the environment provides an opportunity for a ‘teachable moment’, increasing the patient's likelihood of engaging in any intervention. 26
There is a substantial evidence base for the efficacy of brief motivational interventions, aimed at reducing alcohol consumption in primary care. Studies have demonstrated the effectiveness of brief interventions in reducing alcohol consumption in primary care populations in the UK;27 in particular, six systematic reviews focus specifically on this28–33 and all conclude that brief interventions in primary care populations are effective in reducing alcohol consumption. However, many of the studies included in these reviews exclude older patients. There are no systematic reviews or subgroup analyses specifically focusing on older patient groups. There is contradictory evidence from primary research of the efficacy of brief interventions specifically targeted at older hazardous alcohol consumers. Moore et al. 34 compared a minimal, brief advice intervention with a multifaceted intervention including physician advice and behavioural counselling in adults attending primary care centres in the USA. While reductions were observed in both groups in terms of consumption at 12 months, no significant differences were observed between the groups. Yet in a trial of brief interventions for older alcohol users in primary care in the USA, Fleming et al. 35 reported a 34% reduction in alcohol consumption and 64% reduction in those drinking at hazardous levels at 12 months, significantly better than those who received no intervention. Blow and Barry36 also reported significantly greater reduction in alcohol use in older people treated with brief interventions in primary care than in control subjects. There is also evidence from subgroup analyses of existing studies that older patients are at least as likely to benefit from brief interventions as younger patients37 and that older adults are more likely to adhere to and comply with brief intervention treatment regimes. 38
Brief interventions have been proven to be both clinically effective and cost-effective in the management of individuals with hazardous and harmful drinking in primary care settings. 27–29,39 Existing studies, however, have included few older drinkers, and this population may have different alcohol problems and, consequently, different health and social costs. The evidence of brief interventions has been criticised for failing to address a wider range of alcohol use disorders including harmful alcohol consumption40 and for failing to address more entrenched drinking behaviours.
Screening for alcohol use disorders identifies a range of needs that are likely to require a range of types and intensities of interventions. One of the primary reasons why many general practitioners (GPs) are reluctant to implement screening into routine care is because they lack the appropriate skills for dealing with the more severe cases identified. 41
Older alcohol consumers are often typified as either ‘early-onset’ drinkers, whose consumption pattern is a continuation of lifetime hazardous consumption, or ‘late-onset’ drinkers, whose excessive alcohol consumption begins in later life. ‘Late-onset’ drinkers are more likely to benefit from brief interventions than ‘early-onset’ drinkers, who often require a more intensive intervention approach. 42 One such intensive approach that has been used is motivational enhancement therapy (MET). It is relatively short (usually three 40-minute sessions delivered by a trained therapist) but is more intensive than a brief motivational intervention. Primary research has shown it to be as effective as other even more intensive interventions such as Cognitive Behavioural Therapy, Twelve-Step Facilitation Therapy and Social Behavioural Network Therapy [Matching Alcohol Treatments to Client Heterogeneity (Project MATCH)43; UK Alcohol Treatment Trial (UKATT)44].
Physiological changes that occur as part of the ageing process mean that older people are more vulnerable to the effects of alcohol and experience alcohol-related problems at lower consumption levels than younger people. Stepped care interventions offer a potentially resource-efficient means of meeting the needs of this population. Stepped care interventions provide a means of delivering more intensive interventions only to those who fail to respond to less intensive interventions, and are more in keeping with rational clinical decision-making than the blanket use of any one intervention strategy. This stepped approach has been advocated in a variety of clinical areas including depression,45,46 smoking,47 back pain48 and alcohol use. 49,50 A recent pilot study of stepped care interventions for male alcohol users in primary care indicated a potential effect size difference between stepped care and minimal intervention of 0.25 in favour of stepped care and an indication that stepped care approaches for alcohol users may be more cost-effective than minimal interventions. 51
Research objectives
-
To evaluate the clinical effectiveness and cost effectiveness of stepped care interventions for older hazardous alcohol users in primary care.
-
To screen 4170 primary care attendees aged ≥ 55 years for hazardous alcohol use using the AUDIT questionnaire.
-
To evaluate the acceptability and validity of opportunistically screening for hazardous alcohol use in older primary care attendees.
-
To estimate the prevalence of alcohol use disorders in an older primary care population.
-
To study the process of therapy as delivered by both practice nurses and trained therapists.
-
To randomise 500 hazardous alcohol users, with equal probability, to either a minimal intervention or stepped care.
-
To conduct 6- and 12-month follow-up on at least 70% of those randomised to assess alcohol consumption, alcohol-related problems, quality of life and service utilisation.
Primary hypothesis (stated as a null hypothesis)
-
Stepped care interventions for older hazardous alcohol users are no more effective at reducing alcohol consumption than a minimal intervention 12 months after randomisation.
Secondary hypotheses (stated as a null hypothesis)
-
Stepped care is no more cost-effective than minimal intervention 12 months after randomisation.
-
Stepped care will not reduce alcohol-related problems in comparison with minimal intervention 12 months after randomisation.
-
Stepped care will not increase health-related quality of life (HRQoL) compared with minimal intervention 12 months after randomisation.
Chapter 2 Methods
Trial design
The Alcohol: Evaluating Stepped care in Older Populations Study (AESOPS) was a pragmatic, multicentre, two-armed, randomised controlled, open trial with equal randomisation. Participants aged ≥ 5 years who scored ≥ 8 using the AUDIT and consented to participate were randomised (1 : 1) to receive either:
-
minimal intervention consisting of a 5-minute brief advice intervention with the practice nurse or research nurse involving feedback of the results of the screening and discussion regarding the health consequences of continued hazardous alcohol consumption; or
-
stepped care intervention consisting of three consecutive steps, in which progression between steps is dependent upon the outcome of each previous step.
The study protocol can be seen in Appendix 1.
Sample size
At the time of development, there were no previous studies of stepped care interventions for older alcohol-using adults. The closest UK pragmatic randomised controlled trials (RCTs) include that by Wallace et al. 27 and STEPWICE,52 which reported effect size differences between stepped care and minimal interventions of 0.36 and 0.27 respectively. Similar effect size differences were reported in studies from the USA. 35,53,54 There is evidence that older populations respond to brief psychosocial interventions for alcohol use as well as, or even better than, general populations. 38,55 Assuming a conservative effect size difference between stepped care and minimal intervention of the order of 0.3 would require a sample size of 175 participants in each of the two randomised groups, using power at 80% and a 5% significance level.
Our previous experience in conducting RCTs in the fields of substance use, alcohol-using populations44,52 and elderly populations indicated that, with assiduous follow-up regimes, loss to follow-up at 12 months would be in the order of 20%. Evidence also exists that older populations are more compliant with treatment regimes and follow-up protocols than younger populations. 56 Taking these factors into account, we erred on the side of caution and allowed a loss to follow-up of 30%, requiring 500 participants to be randomised (250 in each group). Previous alcohol use screening and intervention studies conducted in UK health-care settings57 suggest that 80% of those screened positive tend to be eligible and 75% of those eligible tend to consent to randomisation. This meant that the study required 834 screen-positives, of whom we predicted 500 would be eligible and consent to randomisation.
Approvals obtained
North West Research Ethics Committee approved the study on 11 April 2007.
The details of multicentre research ethics committee, local research ethics committee and Research and Development Department approvals are provided in Appendix 2.
The trial was assigned the International Standard Randomised Controlled Trial Number (ISRCTN) of ISRCTN52557360; National Research Register number N0484190633 and United Kingdom Clinical Research Network ID 3796.
Trial sites
The study was conducted in eight UK sites with 55 general practices set up to participate. These sites and practices were recruited throughout the study and represent a range of small and large practices, and urban and rural settings. The sites included were North Yorkshire and York; Hull and East Riding; Norfolk; Leeds; Fife; Kent; Tyneside; and County Durham. Details of the study sites and practices are provided in Appendix 3.
Participant eligibility
Inclusion and exclusion criteria were chosen to maintain a balance between ensuring the sample was representative of the primary care population and ensuring that the trial population was able to engage with both the interventions and follow-up.
Inclusion criteria
Patients were considered potentially eligible if they met all of the following criteria:
-
They were aged ≥ 55 years at time of screening.
-
They screened positive for hazardous alcohol use (this is inclusive of harmful and dependent alcohol use) using AUDIT criteria (i.e. scored ≥ 8).
-
They provided their contact details on the screening form.
-
They were residing in a stable place of residence.
-
They lived within commutable distance of the primary care centre.
-
They were willing to provide informed consent for randomisation, treatment and follow-up.
Exclusion criteria
Potential participants were excluded if they met any of the following criteria:
-
They had received treatment for substance use, excluding nicotine, in the previous 90 days.
-
They were already seeking help for alcohol use.
-
They had any outstanding legal issues likely to lead to imprisonment.
-
They suffered from severe mental or physical illness likely to preclude active participation in treatment or follow-up.
Recruitment into the trial
All primary care attendees aged ≥ 55 years were given the opportunity to pick up a ‘screening pack’ from the practice waiting room or from the receptionist. The screening pack contained an information sheet (Appendix 4), a copy of the AUDIT questionnaire (Appendix 5) and a freepost return envelope. The AUDIT questionnaire contained a section asking for contact details to be provided if the patient was willing to help with the research; thereby, patients had the opportunity to complete the form anonymously. This envelope could either be posted back to the University of York (allowing for completion at home if preferred) or left in a postal box within the GP practice. Returned questionnaires were entered into a secure online database that collated the responses to all 10 questions on the AUDIT questionnaire. Patients who scored ≥ 8 on the AUDIT questionnaire, and had provided their contact details, were contacted by telephone and invited to attend an appointment with the practice/research nurse, ideally within the following 7 days. At that point they had the opportunity to ask any questions. At the appointment, the study was fully explained to the patients, their eligibility to participate was ascertained and they were given an opportunity to ask any further questions. If interested and willing, they were then asked to give written informed consent.
During the study recruitment phase, a change in screening method was brought in (detailed in Chapter 3). In addition to the opportunistic screening method, all potentially eligible participants received screening packs by mail from their general practice. This revised method was implemented in some of those practices already screening opportunistically and in all new practices brought on board after the change was implemented.
Baseline assessment
After written informed consent had been obtained, the following data were collected in the baseline questionnaire (Appendix 6) prior to randomisation.
Alcohol Use Disorders Identification Test – Consumption (3-item)
This consists of the first three alcohol consumption questions from the AUDIT 10-item scale. 24
Drinking Problem Index
Alcohol-related problems were assessed using the 17-item participant-completed Drinking Problems Index (DPI). The DPI has been specifically designed and validated for use in older populations. 58
Health-related quality of life
Participants were given a baseline questionnaire to complete, comprising the Short Form Questionnaire-12 items (SF-12)59 and European Quality of Life-5 Dimensions (EQ-5D). 60
Health and social resource used
Details regarding hospital and primary health-care services use, social and care services use and contact with the police and criminal justice system were collected. The service use questionnaire covered a retrospective 6-month period.
Demographics
Details on age, sex, smoking status, main activity, living arrangements, current accommodation and education were collected.
Randomisation
Participants were randomised equally between the two trial arms: minimal intervention and stepped care intervention. Randomisation was carried out using random permuted blocks, stratified by site (North Yorkshire and York; Hull and East Riding; Norfolk; Leeds; Fife; Kent; Tyneside; or County Durham). To maintain allocation concealment, the generation of the randomisation sequence and subsequent treatment allocation were performed by an independent, secure, remote, telephone randomisation service based at the University of York. The computerised randomisation system was checked periodically during the trial following standard operating procedures. Owing to the nature of the intervention and the pragmatic aim of the evaluation, treatment allocation, once determined, was not concealed from the participant or the professional delivering the intervention.
Trial interventions
Participants were randomised to receive either:
-
minimal intervention: a 5-minute brief advice intervention with the practice nurse or research nurse involving feedback of the results of the screening and discussion regarding the health consequences of continued hazardous alcohol consumption; or
-
stepped care intervention: consisting of three consecutive steps, in which progression between steps is dependent upon the outcome of each previous step.
Intervention delivery
Originally, in some practices, the practice nurse delivered the minimal intervention and step 1 of the stepped care intervention. In other practices, a research nurse or research practitioner took on this role. Following a change to the protocol (as detailed in Chapter 3), two new sites used an alcohol health worker. For the purpose of this report, those delivering the minimal intervention and step 1 of the stepped care intervention will be referred to as the ‘practice/research nurses’.
In the majority of sites, step 2 was delivered by a different person than step 1. In four sites, the same people delivered the minimal intervention and steps 1 and 2 of the stepped care interventions. For the purpose of this report, those delivering step 2 of the stepped care intervention will be referred to as the ‘therapists’.
The set-up of the intervention delivery in each site is detailed in Appendix 7.
Training in the delivery of the minimal intervention and step 1 (behavioural change counselling)
The training was delivered by the training centre at Leeds Addiction Unit and lasted either 1 or 2 days, depending on previous experience of the staff being trained. Training for the minimal intervention involved understanding the AUDIT instrument and interpreting the score, and practice in feeding this back to participants and making recommendations for reducing consumption. It was delivered before training for the step 1 (20-minute) intervention that encompassed motivational interviewing skills, feeding back AUDIT scores in a manner that is designed to elicit concerns, and negotiating a behaviour change goal. In both cases training was supported by a written protocol. The training took the form of simulated consultation, followed by a seminar and then another simulated consultation. Each attendee had the opportunity to engage in a simulated consultation and this was recorded. As a group the practice/research nurses discussed the simulated consultations to examine and review the techniques. Prior to the staff seeing any study participants, an assessment of their competency was made using a recorded session that was rated by an independent expert. Ongoing supervision was provided throughout the study by an expert trainer from Leeds Addiction Unit.
A further training session was provided covering protocol issues and use of the study database. This session included the rationale for the study; patient eligibility; use of the online database (making and recording outcomes of appointments); recruitment procedures (including informed consent and randomisation); completion of trial documentation; conducting post-step 1 and post-step 2 assessment telephone calls; and handling of participant withdrawal.
Training in the delivery of step 2 (motivational enhancement therapy)
Motivational enhancement therapy therapists had attended specialist training at the Leeds Addiction Unit. Training was supported by a MET protocol, and follow-up supervision of video-recorded supervision was offered. Particular attention was given to understanding of the evidence base, the theoretical basis of treatment, demonstration of practice and role-play opportunities. Supervision was given in the delivery of a number of therapy sessions, and two recorded sessions were to be completed and reviewed in conjunction with a trained supervisor prior to the therapist seeing study participants. The supervision provided the main opportunity for practising skills and delivering the structure and content of the treatment. Assessment of competence was considered according to the therapist's ability to deliver MET in accordance with the designation of treatment prescribed in the treatment protocol.
A further training session was provided covering protocol issues and use of the study database. This session included the rationale for the study; patient eligibility; use of the online database (making and recording outcome of appointments); and handling of participant withdrawal.
Minimal intervention arm
The minimal intervention consisted of a 5-minute brief advice intervention with the practice/research nurse involving feedback of the results of the screening and discussion regarding the health consequences of continued hazardous alcohol consumption. The participant also received a brief self-help booklet, Safer drinking – a self help guide (Appendix 8), outlining the consequences of excessive alcohol consumption and providing information on sources of help for drinking problems locally and nationally.
Stepped care intervention arm
The stepped care intervention consisted of three consecutive steps in which progression between steps was dependent upon the outcome of each previous step.
Step 1 consisted of a 20-minute session of behavioural change counselling (BCC) delivered by the practice/research nurse. This intervention, based upon an existing evidence base of brief interventions, utilises the technique of motivational interviewing40 and aims to address the individual's motivation to change his or her drinking behaviour. The counselling was protocol guided and the practice/research nurses were trained in the delivery. Four weeks after randomisation the participant was contacted by the nurse and a short telephone assessment was made regarding the participant's alcohol consumption in the previous 4 weeks using the extended AUDIT–Consumption (3-item) (AUDIT–C). If the participant was still consuming alcohol at hazardous levels a referral was made to step 2 of the intervention.
Step 2 involved an intervention by a trained therapist in the primary care environment. The intervention, MET, was provided through three 40-minute sessions on, preferably, a weekly basis if possible. The intervention was protocol guided and addressed six basic principles of increasing motivation for change. Feedback about individual alcohol consumption included emphasis on the individual as being the agent responsible to change, advice on how to accomplish change, provision of alternative vehicles for change, maintenance of an empathetic therapeutic style and emphasis on enhancing the individual's self-efficacy. Four weeks after the final MET session, the nurse contacted the participant and a short telephone assessment was made regarding the participant's alcohol consumption in the previous 4 weeks using the extended AUDIT-C. If the participant was still consuming alcohol at hazardous levels a referral was to be made to step 3 of the intervention.
These interventions were guided by treatment protocols to specify the purpose and principles of each intervention and the structure and content of each particular treatment session.
Step 3 consisted of a referral to the local specialist alcohol services to receive specialist intervention, including, as necessary, detoxification, inpatient care, outpatient counselling, group therapy, relapse prevention treatment or medication. There was no limit on the intensity or duration of the step 3 intervention.
Participant follow-up
Appendix 9 shows a summary of the AESOPS trial.
Trial completion
Participants were deemed to have completed the trial when they had been in the trial for 12 months.
Participants were deemed to have fully withdrawn from the trial when:
-
they wished to exit the trial fully
-
their doctor or nurse withdrew them from the trial or
-
they died.
Instead of withdrawing fully from the trial, participants had the option of (1) withdrawing only from receiving trial treatment; or (2) withdrawing only from postal questionnaires.
Participants who elected to withdraw from both the trial treatment and the follow-up postal questionnaires were deemed to be full withdrawals. This ensured appropriate follow-up from the Trials Unit.
Measurement and verification of primary measure
The primary outcome measure was average drinks per day (ADD) at 12 months post randomisation, where a standard drink equates to 8 g of ethanol.
Determination of average drinks per day
This was ascertained using the self completed extended AUDIT-C. The outcome was measured at baseline and then at 6 and 12 months post randomisation.
Measurement and verification of secondary outcomes
Alcohol-related problems
Alcohol-related problems were measured at baseline, 6 and 12 months post randomisation using the 17-item DPI.
Health-related quality of life
Participants were asked to answer questions relating to their HRQoL throughout the study by completing two generic instruments (EQ-5D and SF-12). These instruments are particularly useful for comparing groups of participants while also having a broad capacity for use in economic evaluation. Their generic nature also makes them potentially responsive to side effects or unforeseen effects of treatment.
Each participant's perception of his or her general health was assessed using the acute version of the SF-1261 and the EQ-5D. 60 The SF-12 is a reliable and well-validated questionnaire,62 and has been used in UK populations, including with older people. 63 We used a layout of the SF-12 shown in previous work to yield improved response rates and quality. 64 The EQ-5D is a generic measure of health status, where health is characterised on five dimensions (mobility, self-care, ability to undertake usual activities, pain and anxiety/depression). 60 Participants were asked to describe their level of health on each dimension using one of three levels: no problems, moderate problems and severe problems. Each response locates a person in one of 245 mutually exclusive health states (the 243 states arising from the EQ-5D, plus unconscious and dead), each of which has previously been valued on the 0 (equivalent to dead) to 1 (equivalent to perfect health) ‘utility’ scale based on interviews with a sample of 3395 members of the UK public. 65 The EQ-5D has been validated in the UK and questionnaires containing both instruments were administered to participants in person at baseline and by postal questionnaire at 6 and 12 months.
Collection of resource-use data
At recruitment (baseline) and 6 and 12 months after randomisation, participants were asked to complete a questionnaire on health and social care resource use during the previous 6 months (Appendix 6). The questionnaire was designed for participant completion and was returned to the trial office using a prepaid reply envelope. Participants indicated how many times in the previous 6 months they had used health services, for example if they had seen a GP or nurse or received hospital care. In addition, they were asked about contact with the police and criminal justice system. The collection of self-reported resource-use data was continued until the patient had been in the study for 12 months or until the patient withdrew from follow-up, or fully withdrew from the study.
Quality assurance of treatment delivery
Participants were asked to provide consent to have all treatment sessions recorded. A 30% sample was randomly selected, stratified by therapist, site and treatment (minimal and stepped care). Recordings were rated by an independent rater and assessed for quality of delivery and compliance with treatment protocols. A proportion of the recordings were double rated for quality assurances and calibration.
Adverse events
There were no anticipated risks in relation to either treatment arm and there is no documented evidence of adverse events arising as a result of either minimal intervention or stepped care intervention, but a mechanism for recording these was in place in case any arose.
Non-trial participants
Patients who had returned their screening forms and entered their contact details indicating willingness to be approached, but were subsequently found not to be eligible based on their AUDIT score (i.e. they scored < 8), were sent a letter informing them of the outcome of the screening. In addition, a ‘non-participant’ questionnaire identical to the study baseline questionnaire was included and patients were asked to complete this if they so wished and return it anonymously (Appendix 6).
Clinical analyses methods
The objective of the clinical analyses was to compare the clinical effectiveness and cost-effectiveness of stepped care interventions for older hazardous alcohol users in primary care.
Outcomes
Primary
-
Average drinks per day – derived from extended AUDIT-C at 12 months. The number of times per week alcohol was consumed was calculated from question 1 of the AUDIT-C. This was multiplied by the number of standard drinks reported in question 2 and then divided by 7 to give the ADD. For example, drinking four or five times per week would give a mean number of 4.5 days. If the number of standard drinks stated was 7–9, the number of standard drinks would be taken as the midpoint, 8. The calculation would then be 4.5 × 8/7 = 5.1 drinks per day.
Secondary
-
Alcohol-related problems (DPI) at 6 and 12 months.
-
Quality of life (SF-12) at 6 and 12 months.
-
ADD (derived from extended AUDIT-C) at 6 months.
-
Extended AUDIT-C score at 6 and 12 months.
-
AUDIT-C status at 6 and 12 months (score of ≥ 5 = AUDIT-C positive).
Primary analysis
All analyses were performed on an intention-to-treat (ITT) basis using a two-sided 5% significance level. Analyses were performed in SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
The primary analysis compared minimal intervention with stepped care on the primary outcome measure, ADD, at 12 months post randomisation using a hierarchical linear model (mixed model). The mixed model was used to account for any variation due to GP practice and the allocated therapist/nurse delivering the intervention. The analysis was adjusted for baseline ADD.
The model was developed starting from the simplest model, participants nested within GP practice, treating GP as a random effect. Where the data allowed, the therapist/nurse identification was added as random effect to make a three-level hierarchical model: participant within therapist within practice. Model checking was performed by assessing residual plots to ensure all models fit the data; where necessary transformations were employed to make the model a better fit.
The effects of missing data were examined using the commands Proc Mi and Mi Analyse in SAS v9.2. The same covariates that were used in the primary analysis were included. Any baseline characteristics that predicted missingness were also included. These analyses were used as a sensitivity analysis; the reported results will be those obtained from the primary analysis.
Secondary outcomes
Average drinks per day: month 6
Average drinks per day at month 6 was analysed in the same way as the primary outcome: using a mixed model, adjusting for baseline ADD and including GP as a random effect. Model checking was performed by assessing residual plots to ensure the model fit the data; where necessary, transformations or other analysis methods were used.
Alcohol-related problems
Alcohol-related problems measured at baseline, month 6 and month 12 were assessed using the 17-item DPI. The score ranges from 0 to 17, with 17 as the most severe. This was analysed in the same way as the primary outcome: using a mixed model, adjusting for baseline DPI and including GP as a random effect.
Alcohol Use Disorders Identification Test – Consumption (3-item) score
AUDIT-C score was measured at baseline, month 6 and month 12. The score ranges from 0 to 12, with 12 as the most severe. This was analysed in the same way as the primary outcome: using a mixed model, adjusting for baseline AUDIT-C score and including GP as a random effect.
Alcohol Use Disorders Identification Test status – Consumption (3-item)
AUDIT-C status was calculated from the AUDIT-C score at baseline, month 6 and month 12. A score of ≥ 5 is classed as AUDIT-C positive and a score of < 5 as AUDIT-C negative. A positive AUDIT-C status is indicative of hazardous alcohol consumption and inclusive of both harmful and dependent consumption. This was analysed using a mixed logistic regression model, adjusting for baseline AUDIT-C score and including GP as a random effect.
Quality of life
Quality of life was measured using the SF-12 questionnaire (measured at baseline and 6 and 12 months). The scores for the physical and mental health components were analysed using a mixed model.
Process Rating Scale
The Process Rating Scale (PRS) was adapted from the validated UKATT PRS66 and contains items that were used to rate structure, content and style of the delivery of the minimal and the step 1 interventions (Appendix 10).
Hypotheses
-
Are the mean frequency and quality scores for the specific task items and practice/research nurse style items substantively different for the minimal intervention compared with the 20-minute behaviour change counselling?
-
Is the 5-minute intervention characterised by different mean frequency ratings of MET consistent practice/research nurses style items (reflective listening, empathy and open questions) and different MET inconsistent style items (closed questions and giving unsolicited advice) from the 20-minute intervention?
-
Is the session content for the two treatment groups different? Should both interventions have covered the same session content?
-
Do specialist practice/research nurses receive different mean frequency and quality scores for specific task items and style items from non-specialist practice/research nurses?
-
What level of consistency is there between the ratings of the primary and secondary raters (inter-rater reliability)?
Analysis
The mean frequency score and the mean quality score for specific task items and practice/research nurse style items for each session were calculated. Linear regression models were used to compare the scores for the 5-minute and the 20-minute interventions. The dependent variable was mean score (frequency or quality) and the independent variable was session type (5- or 20-minute session). To take account of the clustered nature of the data, a mixed model was used with practice/research nurse fitted as a random effect.
The mean frequency score of MET style items and MET inconsistent items was calculated for each session. Linear regression models were used to compare the scores for the 5-minute (minimal) and the 20-minute (step 1) interventions. The dependent variable was mean frequency score and the independent variable was session type (5- or 20-minute session). To take account of the clustered nature of the data, a mixed model was used with practice or research nurse fitted as a random effect.
Session content was assessed using a series of yes/no answers to five questions. Logistic regression models were used for each of the five questions to test for differences between the session types. Again, a hierarchical model was used, with practice/research nurse fitted as a random effect.
In order to examine differences between types of practice/research nurses, the analysis for specific task items was repeated and the type of practice/research nurse (specialist/non-specialist) was also included in addition to session type.
Inter-rater reliability of the individual frequency items of the scale was examined using the intraclass correlation coefficient (ICC) two-way mixed-effects model (case 367) to estimate the reliability of a mean of several ratings. 68 For four summary measures, the average of the two raters' summary scores was plotted against difference in their summary score69 to make pairwise comparisons between raters. This illustrates graphically whether or not the summary scores are rated consistently, how well the raters agree on average and what the limits of agreement are.
Economic analysis
Economic evaluation of health interventions is a tool used to assist decision-makers in prioritising and allocating resources in the health-care sector, by assessing the value for money (cost-effectiveness) of alternative interventions. 70
The aim of the economic evaluation was to assess the cost-effectiveness of a stepped care intervention compared with minimal intervention in the treatment of older hazardous alcohol users in primary care.
The first stage of the economic analysis was to calculate the cost of delivering the trial treatments. Opportunistic screening costs were estimated using the actual costs of screening associated with the study. The costs of delivering the minimal intervention and the first two tiers of stepped care were based on actual patient contact time from timesheets maintained by practice or research nurses and therapists. The costs of the minimal intervention and the first two tiers of the stepped care programme were based on information gathered on patient contact with the primary care and specialist services during the trial.
The costs of the trial interventions were calculated using local costs of specialist services and included an allowance for the training and supervision costs, using methods developed for the UKATT trial. 71 Utilisation of more specialist services was recorded, including the type of intervention, and costs were applied from previous research trials and a recent Department of Health-funded research project based on a range of specialist providers and intervention types. 72 The incremental cost-effectiveness of stepped care compared with the minimal intervention was assessed from both a health and a personal social services perspective following National Institute for Health and Care Excellence (NICE) guidance73 and a wider public sector resource perspective. 73 Utilisation of alcohol services outside the trial protocol, along with all other public sector services, including health, social welfare and contact with criminal justice agencies, was assessed from questionnaires administered at baseline and 6 and 12 months. Units of resource use recorded were multiplied by national sources of unit costs71,74 in order to provide generalisable results.
The economic analysis tested the hypothesis that stepped care is more cost-effective than the minimal intervention, using a cost–utility framework. Utility values were derived from the EQ-5D60 and were then used with population values and the quality-adjusted life-year (QALY) change calculated using the area under the curve method. 75 Incremental cost-effectiveness analysis combined the costs of the interventions, as detailed above, with the QALY changes, using the cost in the intervention group over and above the control, divided by the incremental QALYs in the intervention group over and above the control. The non-parametric bootstrap resampling technique was used to test the sensitivity of the calculated incremental cost-effectiveness ratios (ICERs) and cost-acceptability curves estimated to demonstrate different threshold values for a QALY. 76 This would show the probability that stepped care was the preferred treatment option at different values for the decision-maker's willingness to pay for a QALY.
The effects of the GP practice were analysed using a multilevel model programmed in MLwiN (MLwiN, Centre for Multilevel Modelling, Bristol, UK). A net benefit framework was used, estimating the benefit of treatment by multiplying QALY changes by a £30,000-per-QALY value net of treatment cost. This estimated a net benefit of treatment for each patient in the trial. The multilevel model tested the proportion of the variation in the net benefit of treatment attributable to the practice to investigate the effect of the treatment location.
Chapter 3 Protocol changes
Outcome measures
In the original protocol the intention was to ascertain a diagnosis of alcohol use disorder using the Short Form-Composite International Diagnostic Interview (SF-CIDI). It was decided to replace this instrument with the AUDIT, with a score of ≥ 16 indicative of higher-risk alcohol use and possible alcohol dependence. The AUDIT has established sensitivity and specificity for the identification of at-risk alcohol use, inclusive of higher risk and possibly dependent alcohol use, and using this instrument reduced the response burden on participants. In addition, the primary outcome measure (ADD) was to be measured using a timeline follow-back method. This method would have involved a 20- to 30-minute interview with a trained researcher at each of the outcome assessment points. We replaced this with a validated, reliable self-complete instrument: the extended AUDIT-C. Evidence exists that the timeline follow-back method acts as a brief intervention and as such may act to reduce the observed differences between allocated groups. 77 Using the extended AUDIT-C reduced this measurement effect, as well as the response burden on participants. The original protocol had outlined our plan to conduct follow-ups face to face at 6 and 12 months post randomisation. As the primary outcome measure was replaced with a self-completed instrument it was decided to replace this follow-up method with postal follow-ups at 6 and 12 months post randomisation, meaning that the participants did not have to return to the practice for any follow-up assessments.
Recruitment period
The original protocol planned to recruit 500 participants over an 18-month period with all participants being followed up for 12 months. As the study progressed, delays to practice initiations meant that the study had fallen behind schedule, with the first patient not recruited until April 2008. The original end date for recruitment was 31 April 2009. In addition, the recruitment rate was found to be under target as a result of prevalence and uptake rates being lower than anticipated. This led us to apply to the funder in November 2008 for an 18-month extension to the recruitment period. The original sample size remained unchanged. The funders elected instead to track recruitment through monthly reports until the end of January 2010, during which time it would become clearer whether or not the target was achievable within an acceptable timescale. New sites and practices were brought on board (Appendix 3 details start dates of trial sites) and, following a successful increase in recruitment, in February 2010 an extension was approved allowing recruitment to continue until the end of October 2010, with the 12-month follow-up ceasing by the end of November 2011.
Mail-out
Owing to this lower than expected recruitment in the study, a decision was made to change the screening process in only one centre in order to pilot the new method. As it stood, potentially eligible patients were identified by opportunistic screening in their GP's practice. That is, all patients aged ≥ 55 years who attended their GP's practice was able to pick up a pack containing a screening form (AUDIT) that they could choose to complete (either with contact details or anonymously) regarding how much alcohol they consume. As the reduced recruitment rate was thought to be due to a lower prevalence rate than first expected, the number of patients requiring to be screened needed to be far greater than anticipated in order to hit the study target of 500. It was felt that the number of patients screened could be increased within this one new site by mailing out forms to all patients aged ≥ 55 years in the participating GP practice, asking them to complete the AUDIT (entirely voluntarily, and it could still be completed anonymously if preferred), as opposed to distributing forms only to those screened opportunistically at the practice.
Therefore, a new site, Tyneside, was brought on board to pilot this method. In addition, two local (Tyneside) Alcohol Health Workers conducted the study in the practices and the screening form itself was redesigned to fit onto one side of A4 paper. The cover letter and screening form were accompanied by a new, coloured, z-folded patient information leaflet (Appendix 11) and a prepaid envelope allowing all completed questionnaires to be returned directly to the co-ordinating centre rather than handed in to the GP's practice.
Following the success of this method all new practices brought on board used this mail-out method. Practices already taking part were given the option to switch to the mail-out method or continue with the current opportunistic screening process.
Chapter 4 Clinical results
This chapter presents the statistical analysis of the AESOPS trial. In the first section of the chapter the clinical data are described, including tables and figures of data summaries. In the second section the statistical models fitted to the data are presented.
Trial recruitment
Eight sites participated in the study from across the UK. These were North Yorkshire and York; Hull and East Riding; Norfolk; Leeds; Fife; Kent; Tyneside; and County Durham. The number of participants recruited per site ranged from 3 to 209. Within these sites, 55 GP practices were set up, but only 53 commenced screening patients. Participants were recruited from 51 of the 53 practices and the number of participants recruited per practice ranged from 1 to 54.
Screening commenced in January 2008 and continued through to October 2010. Approximately 78,000 screening questionnaires were distributed, with over 60,000 of these by mail-out. Twenty-five practices used opportunistic screening only, 28 used mail-out only and six switched from opportunistic screening to mail-out (as detailed in Appendix 3). The majority of screening questionnaires returned were from the Tyneside and Leeds sites. The first participant was recruited in April 2008 and recruitment ceased at the end of October 2010. The participant follow-up period ended in November 2011 after which point the study ended.
In total, 21,545 completed screening forms were returned. Sixteen had insufficient information to score the AUDIT. Of the remainder, 1625 were AUDIT positive (scored ≥ 8 on the AUDIT) (1625/21,529 = 7.5%). This indicates that 7.5% of the population screened were considered hazardous or harmful drinkers, a rate much lower than anticipated (15% to 25%). The proportion of hazardous or harmful drinkers ranged from 6.6% to 10.4% across the sites (Table 1).
| Site | N; number audit positive, n (%) |
|---|---|
| York | 2360; 195 (8.3) |
| Hull | 691; 72 (10.4) |
| Norfolk | 2522; 166 (6.6) |
| Leeds | 6629; 466 (7.0) |
| Kent | 237; 20 (8.4) |
| Fife | 790; 63 (8.0) |
| Tyneside | 5877; 483 (8.2) |
| County Durham | 2423; 160 (6.6) |
| Total | 21,529; 1625 (7.5) |
In total, there were 949 patients who were AUDIT positive and provided contact details; they had an average AUDIT score of 12.10 [standard deviation (SD) 5.65], which was higher than for those who were AUDIT positive but did not provide contact details (Table 2).
| Statistic | Positive audit | ||||
|---|---|---|---|---|---|
| No | Yes | Total | |||
| Identifiable | Anonymous | Identifiable | Anonymous | ||
| n | 11,210 | 8694 | 949 | 676 | 21,529 |
| Mean (SD) | 2.21 (1.90) | 2.26 (1.88) | 12.10 (5.65) | 11.11 (4.45) | 2.95 (3.40) |
| Median (min., max.) | 2 (0, 7) | 2 (0, 7) | 10 (8, 40) | 10 (8, 40) | 2 (0, 40) |
The percentage of eligible patients (i.e. AUDIT positive with contact details provided) who were deemed fully eligible [928/1626 = 57% (i.e. 949 AUDIT positive, but 21 failed one or more of the other eligibility criteria)] and went on to be randomised was only 57% (529/928 = 57%).
Figure 1, the Consolidated Standards of Reporting Trials (CONSORT) flowchart, shows the progress of participants through the trial.
FIGURE 1.
The AESOPS CONSORT diagram.
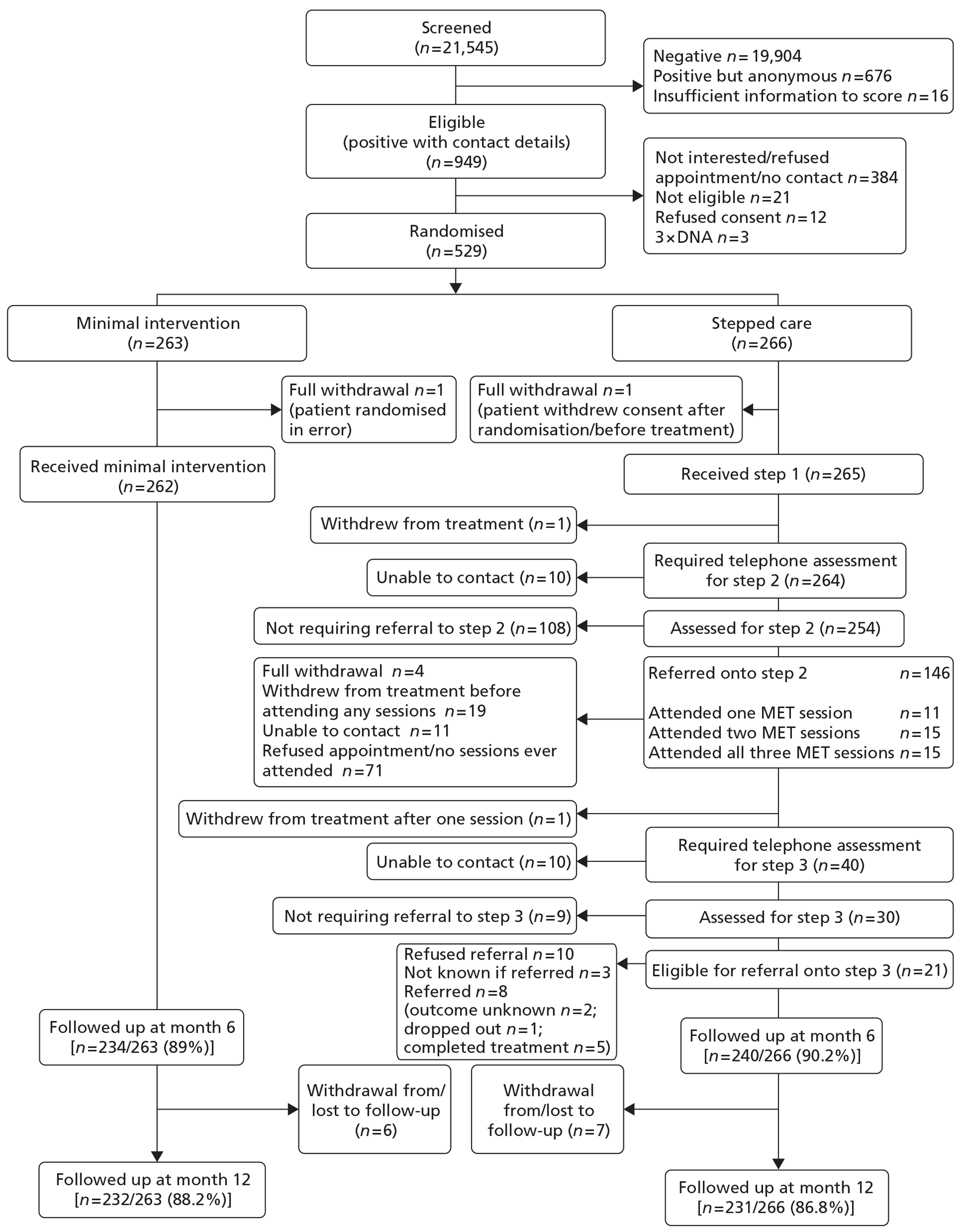
Clinical data
In total, 529 participants were randomised: 266 to stepped care and 263 to minimal intervention. A baseline questionnaire was not received from seven participants following completion: three from the stepped care group and four from the minimal group.
The majority of participants were male (n = 425; 80%) and the average age was 63 years (range 55–85 years) (Table 3). A score of ≥ 20 on the AUDIT indicates possible alcohol dependence. Overall, 7.9% of those randomised obtained a score of ≥ 20 at screening. This was higher in the minimal group (9.5%) than in the stepped care group (6.4%). This is summarised in Table 4. The average AUDIT-C score was 8.3 (SD 2.2) and ADD was 3.39 (SD 2.2). The baseline characteristics are summarised by treatment group (Tables 5 and 6).
| Characteristics | Screened (n = 21,545) | Non-participants (n = 4231) | Trial participants (n = 529) |
|---|---|---|---|
| Sex | |||
| Male (%) | 45.3 | 44.7 | 80.3 |
| Age (years) | |||
| Mean (SD) | 68 (8.6) | 67 (7.9) | 63 (5.8) |
| Median (min., max.) | 66 (55, 105) | 66 (55, 105) | 62 (55, 85) |
| AUDIT-C score | |||
| Mean (SD) | 2.6 (2.4) | 2.5 (2.0) | 8.3 (2.2) |
| Median (min., max.) | 2 (0, 12) | 2 (0, 12) | 8 (0, 12) |
| ADD | |||
| Mean (SD) | NA | 0.51 (0.76) | 3.39 (2.21) |
| Median (min., max.) | NA | 0.15 (0, 8.57) | 3 (0, 8.57) |
| Treatment arm | Screening AUDIT score ≥ 20 | ||
|---|---|---|---|
| No | Yes | Total | |
| Stepped care, n (%) | 249 (93.6) | 17 (6.4) | 266 (100) |
| Minimal, n (%) | 238 (90.5) | 25 (9.5) | 263 (100) |
| Overall, n (%) | 487 (92.1) | 42 (7.9) | 529 (100) |
| Characteristics | Stepped care (N = 266) | Minimal (N = 263) | Total (N = 529) |
|---|---|---|---|
| Sex, N | 266 | 263 | 529 |
| Male, n (%) | 220 (82.7) | 205 (77.9) | 425 (80.3) |
| Female, n (%) | 46 (17.3) | 58 (22.1) | 104 (19.7) |
| Age (years), n | 266 | 263 | 529 |
| Mean (SD) | 62.92 (5.82) | 62.74 (5.86) | 62.83 (5.83) |
| Median (min., max.) | 62 (55, 83) | 62 (55, 85) | 62.00 (55, 85) |
| Smoking status, N | 256 | 251 | 507 |
| Never smoked, n (%) | 71 (27.7) | 80 (31.9) | 151 (29.8) |
| Ex-smoker, n (%) | 141 (55.1) | 125 (49.8) | 266 (52.5) |
| Current smoker, n (%) | 44 (17.2) | 46 (18.3) | 90 (17.7) |
| Employment, N | 258 | 258 | 516 |
| In employment or self-employment, n (%) | 89 (34.5) | 93 (36.0) | 182 (35.3) |
| Retired, n (%) | 138 (53.5) | 132 (51.2) | 270 (52.3) |
| Housework, n (%) | 4 (1.5) | 3 (1.2) | 7 (1.4) |
| Student, n (%) | 1 (0.4) | 0 (0.0) | 1 (0.2) |
| Seeking work, n (%) | 9 (3.5) | 5 (1.9) | 14 (2.7) |
| Other, n (%) | 17 (6.6) | 25 (9.7) | 42 (8.1) |
| Living arrangements, N | 263 | 257 | 520 |
| Single, n (%) | 61 (23.2) | 59 (23.0) | 120 (23.1) |
| Married, n (%) | 166 (63.1) | 155 (60.3) | 321 (61.7) |
| Cohabiting, n (%) | 22 (8.4) | 17 (6.6) | 39 (7.5) |
| Widowed, n (%) | 14 (5.3) | 26 (10.1) | 40 (7.7) |
| Accommodation, N | 263 | 257 | 520 |
| Owner occupied, n (%) | 211 (80.2) | 202 (78.6) | 413 (79.4) |
| Private rented, n (%) | 14 (5.3) | 14 (5.4) | 28 (5.4) |
| Local authority/housing association, n (%) | 37 (14.1) | 40 (15.6) | 77 (14.8) |
| Temporary, n (%) | 1 (0.4) | 1 (0.4) | 2 (0.4) |
| Education continued after school, N | 262 | 258 | 520 |
| Yes, n (%) | 171 (65.3) | 158 (61.2) | 329 (63.3) |
| No, n (%) | 91 (34.7) | 100 (38.8) | 191 (36.7) |
| Degree or equivalent professional qualification, N | 261 | 256 | 517 |
| Yes, n (%) | 116 (44.4) | 100 (39.1) | 216 (41.8) |
| No, n (%) | 145 (55.6) | 156 (60.9) | 301 (58.2) |
| Outcome measure | Stepped care (N = 266) | Minimal (N = 263) | Total (N = 529) |
|---|---|---|---|
| ADD, n | 263 | 255 | 518 |
| Mean (SD) | 3.38 (2.24) | 3.41 (2.19) | 3.39 (2.21) |
| Median (min., max.) | 3 (0, 8.57) | 3 (0, 8.57) | 3 (0, 8.57) |
| DPI, n | 262 | 257 | 519 |
| Mean (SD) | 2.64 (2.90) | 3.08 (3.33) | 2.86 (3.12) |
| Median (min., max.) | 2 (0, 15) | 2 (0, 15.87) | 2 (0, 15.87) |
| SF-12 PCS, n | 260 | 256 | 516 |
| Mean (SD) | 47.67 (11.21) | 47.33 (10.99) | 47.50 (11.09) |
| Median (min., max.) | 52.00 (10.04, 70.37) | 49.81 (7.87, 67.17) | 51.02 (7.87, 70.37) |
| SF-12 MCS, n | 260 | 256 | 516 |
| Mean (SD) | 51.85 (9.51) | 50.18 (10.71) | 51.02 (10.15) |
| Median (min., max.) | 55.06 (9.13, 66.33) | 52.79 (6.98, 71.21) | 54.37 (6.98, 71.21) |
| AUDIT-C score, n | 263 | 259 | 522 |
| Mean (SD) | 8.26 (2.19) | 8.25 (2.26) | 8.26 (2.22) |
| Median (min., max.) | 8 (3, 12) | 8 (0, 12) | 8 (0, 12) |
| AUDIT-C status, N | 263 | 259 | 522 |
| Negative, n (%) | 13 (4.9) | 15 (5.8) | 28 (5.4) |
| Positive, n (%) | 250 (95.1) | 244 (94.2) | 494 (94.6) |
Comparing the trial participants with those screened, there were more males (80% compared with 45% in the screened population) and participants were slightly younger: 63 years compared with 68 years (Table 3).
Baseline data were collected on those who were willing to participate but were not eligible for the trial (non-participants) because they were AUDIT negative at screening. In order to compare the baseline characteristics of participants and non-participants, the AUDIT-C was also calculated for the screening sample. Comparing non-participants with trial participants, trial participants were younger and more likely to be male.
Analysis of clinical results
This portion of the report presents the results of the statistical models fitted to the data. It is arranged into three main sections. The first section presents the results of the modelling of the primary outcome: ADD at 12 months. The second section presents the results of the modelling of the secondary outcomes: ADD (derived from the extended AUDIT-C) at 6 months; alcohol-related problems assessed using the DPI at 6 and 12 months; extended AUDIT-C score at 6 and 12 months; and HRQoL at 6 and 12 months. The third section summarises the conclusions of the statistical analysis of the clinical outcomes.
Primary outcome
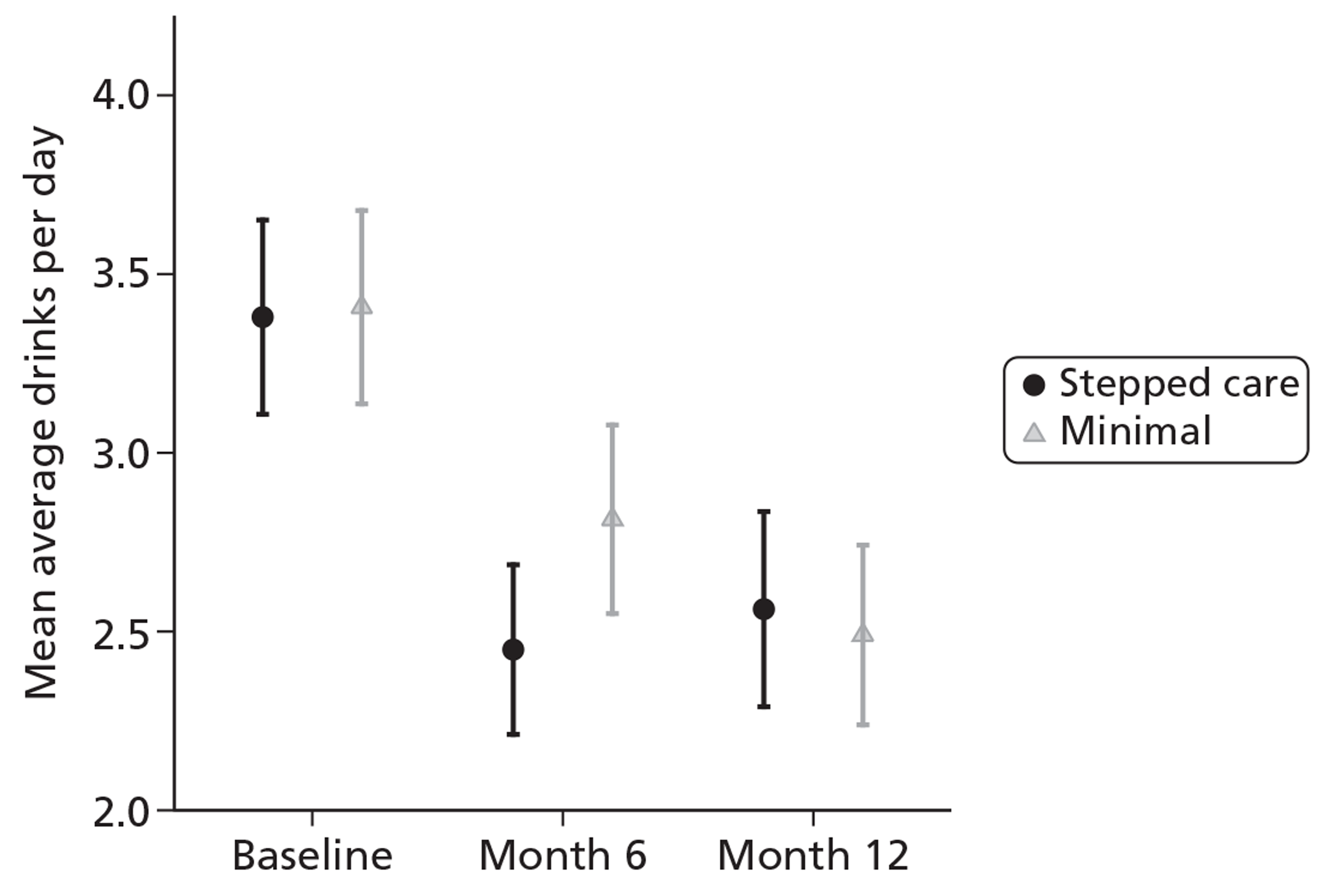
Overall, the mean ADD score in both groups decreased from baseline at both month 6 and month 12. At month 6 the stepped care group had a lower ADD but at month 12 the minimal intervention group had the lower ADD. This is summarised in Table 7. Figure 2 shows the mean ADD scores for the complete cases.
| Follow-up | Treatment arm | ||
|---|---|---|---|
| Stepped care | Minimal | Total | |
| Baseline | |||
| Valid n | 263 | 255 | 518 |
| Mean (SD) | 3.38 (2.24) | 3.41 (2.19) | 3.39 (2.21) |
| Median (min., max.) | 3.00 (0, 8.57) | 3.00 (0, 8.57) | 3.00 (0, 8.57) |
| Month 6 | |||
| Valid n | 236 | 229 | 465 |
| Mean (SD) | 2.45 (1.85) | 2.81 (2.03) | 2.63 (1.95) |
| Median (min., max.) | 1.96 (0, 8.57) | 2.25 (0, 8.57) | 2.25 (0, 8.57) |
| Month 12 | |||
| Valid n | 228 | 228 | 456 |
| Mean (SD) | 2.56 (2.09) | 2.49 (1.93) | 2.53 (2.01) |
| Median (min., max.) | 1.96 (0, 8.57) | 2.25 (0, 8.57) | 2.11 (0, 8.57) |
FIGURE 2.
Average drinks per day (complete cases).
A mixed model was used to compare ADD between the two randomised groups. The baseline value of ADD was included as a covariate and a variable was also included for treatment group; these were included as fixed effects. To account for the variation due to GP practice, this was included as a random effect in the model. Expanding the model to a three-level model to include nurse/therapist resulted in a model that failed to converge, and so the final model used was a two-level mixed model with participants nested within GP practice.
The ADD had a skewed distribution; a transformation improved the model fit. A log-transformation was used in the final model so the dependent variable was Ln(ADD_M12 + 1) (M12 = month 12).
In total, 456 participants had a response at month 12; however, in seven, ADD value at baseline was missing, and so these participants were not included in the primary analysis. The GP random effect was not significant, indicating that transformed ADD did not vary significantly between centres. There were no significant differences in ADD between the treatment groups at month 12. The stepped care group had a marginally higher ADD than the minimal intervention group but not significantly so. The results of the analysis are seen in Table 8.
| Follow-up | Stepped care estimate (SD) (n = 226) | Minimal estimate (SD)(n = 223) | Difference (95% CI) | p-value |
|---|---|---|---|---|
| Month 12 | 1.129 (0.037) | 1.104 (0.037) | 0.025 (−0.062 to 0.112) | 0.575 |
| Covariance parameter estimates | Estimate (SE) | p-value | ||
| Random GP effect | 0.013 (0.009) | 0.059 | ||
| Measurement error | 0.215 (0.015) | < 0.001 | ||
Transforming the data for the analysis was necessary because of the skewed distribution. As the transformation included the addition of 1, this meant that a back-transformation was more problematic; however, it was anticipated that this would not have a great influence on the back-transformed estimate. To verify this, an additional analysis, excluding those with an ADD of zero, was used to confirm the result. In order to summarise the results in a more meaningful way, the estimate of the difference was anti-logged. It was found that ADD at month 12 for the stepped care group was 1.025 [95% confidence interval (CI) 0.94 to 1.12] times that of the minimal group. The analysis excluding those with ADD of zero, carried out to check that the log(ADD + 1) produced a good approximation, produced very similar results, and so we concluded that these estimates were acceptable.
Missing data
The overall follow-up rate at month 12 was 87.5%, with 86.8% followed up in the stepped care group and 88.2% in the minimal intervention group. Those followed up were slightly older, more likely to be male and had a slightly lower ADD at baseline. The results are summarised in Table 9.
| Characteristics | Month 12 follow-up? | ||
|---|---|---|---|
| No | Yes | Total | |
| Stepped care, n (%) | 35 (13.2) | 231 (86.8) | 263 (100) |
| Minimal, n (%) | 31 (11.8) | 232 (88.2) | 266 (100) |
| Overall, n (%) | 66 (12.5) | 463 (87.5) | 529 (100) |
| Age (years), n | 66 | 463 | 529 |
| Mean (SD) | 61.50 (5.01) | 63.02 (5.92) | 62.83 (5.83) |
| Median (min., max.) | 61.5 (55, 75) | 62 (55, 85) | 62 (55, 85) |
| ADD, n | 62 | 456 | 518 |
| Mean (SD) | 3.82 (2.62) | 3.34 (2.15) | 3.39 (2.21) |
| Median (min., max.) | 3.27 (0.35, 8.57) | 3 (0, 8.57) | 3 (0, 8.57) |
| Sex, N | 66 | 463 | 529 |
| Male, n (%) | 50 (11.8) | 375 (88.2) | 425 (100) |
| Female, n (%) | 16 (15.4) | 88 (84.6) | 104 (100) |
The multiple imputation commands in SAS, SAS Proc MI and MI Analyse were used to perform multiple imputations in order to take into account the missing data in the analysis. Multiple imputation78 replaces each missing value with a range of possible values. Proc MI produces these imputed data sets and MI Analyse allows the results from these datasets to be combined and analysed using standard procedures. The results can be seen in Table 10. The estimates from the multiple imputation are similar to the results from the primary analysis.
| Follow-up | Primary analysis | Multiple imputation | ||
|---|---|---|---|---|
| Difference (95% CI) (n = 449) | p-value | Difference (95% CI) (n = 525) | p-value | |
| Month 12 | 0.025 (−0.062 to 0.112) | 0.575 | 0.033 (−0.065 to 0.131) | 0.470 |
Analysis of secondary outcomes
In this section, the results of the secondary analysis are presented.
Average drinks per day at month 6
The ADD at month 6 was analysed in the same way as the primary outcomes: using a mixed model, adjusting for baseline ADD and including GP as a random effect. The ADD had a skewed distribution; a transformation improved the model fit. A log transformation was used in the final model so the dependent variable was Ln(ADD_M6 + 1) (Table 11).
| Follow-up | Stepped care estimate (SD) (n = 234) | Minimal estimate (SD) (n = 230) | Difference (95% CI) | p-value |
|---|---|---|---|---|
| Month 6 | 1.119 (0.034) | 1.192 (0.034) | −0.073 (−0.156 to 0.011) | 0.088 |
At month 6, the stepped care group had a lower ADD then the minimal intervention group. This was not significant at the 5% level.
The AUDIT-C score was analysed in a similar way to the primary outcomes: using a mixed model, adjusting for baseline AUDIT-C score and including GP as a random effect. There were no significant differences in AUDIT-C score between the treatment groups at month 6 or month 12. The minimal intervention had a marginally higher AUDIT-C score at month 6, but a lower score at month 12; however, neither difference was significant (Tables 12 and 13).
| Follow-up | Intervention arm | ||
|---|---|---|---|
| Stepped care | Minimal | Total | |
| Baseline | |||
| n | 263 | 259 | 522 |
| Mean (SD) | 8.26 (2.19) | 8.25 (2.26) | 8.26 (2.22) |
| Median (min., max.) | 8 (3, 12) | 8.00 (0, 12) | 8 (0, 12) |
| Month 6 | |||
| n | 238 | 231 | 469 |
| Mean (SD) | 7.02 (2.48) | 7.38 (2.55) | 7.20 (2.52) |
| Median (min., max.) | 7 (0, 12) | 8.00 (0, 12) | 7.00 (0, 12) |
| Month 12 | |||
| n | 229 | 229 | 458 |
| Mean (SD) | 7.07 (2.48) | 6.96 (2.66) | 7.02 (2.57) |
| Median (min., max.) | 7 (0,12) | 7 (0, 12) | 7 (0, 12) |
| Follow-up | Stepped care estimates (SD) | Minimal estimates (SD) | Difference (95% CI) | p-value |
|---|---|---|---|---|
| Month 6 | 7.085 (0.159) (n = 236) | 7.373 (0.160) (n = 228) | −0.288 (−0.687 to 0.111) | 0.156 |
| Month 12 | 7.116 (0.166) (n = 227) | 6.957 (0.166) (n = 226) | 0.160 (−0.250 to 0.569) | 0.445 |
The AUDIT-C status was analysed using a hierarchical logistic regression model, adjusting from baseline status with GP practice treated as a random effect. The outcome was AUDIT-C positive. At month 6, the adjusted analysis found no significant difference in AUDIT-C status between the two treatment groups. At month 6, the stepped care group had a larger proportion of AUDIT-C positives but a smaller proportion at month 12 compared with the minimal intervention. The results can be seen in Tables 14 and 15.
| Follow-up | Stepped care | Minimal | Total |
|---|---|---|---|
| Baseline, N | 263 | 259 | 522 |
| AUDIT-C negative, n (%) | 13 (4.9) | 15 (5.8) | 28 (5.4) |
| AUDIT-C positive, n (%) | 250 (95.1) | 244 (94.2) | 494 (94.6) |
| Month 6, N | 238 | 231 | 469 |
| AUDIT-C negative, n (%) | 35 (14.7) | 26 (11.3) | 61 (13.0) |
| AUDIT-C positive, n (%) | 203 (85.3) | 205 (88.7) | 408 (87.0) |
| Month 12, N | 229 | 229 | 458 |
| AUDIT-C negative, n (%) | 35 (15.3) | 41 (17.9) | 76 (16.6) |
| AUDIT-C positive, n (%) | 194 (84.7) | 188 (82.1) | 382 (83.4) |
| Follow-up | Stepped care | Minimal | Odds ratio (95% CI) | p-value |
|---|---|---|---|---|
| Month 6 | 0.81 (0.48 to 1.37) | 0.427 | ||
| N | 236 | 228 | ||
| n (%) | 193 (82) | 186 (82) | ||
| Month 12 | 1.37 (0.76 to 2.47) | 0.289 | ||
| N | 227 | 226 | ||
| n (%) | 202 (89) | 202 (89) |
Drinking Problems Index
The DPI at month 6 and month 12 was analysed using a mixed model, adjusting for baseline DPI and including GP as a random effect. DPI scores had skewed distributions, so a log transformation was used which improved the model fit. As the DPI range included zero, one (1) was added to the total DPI score to enable logs to be calculated (Table 16 and 17).
| Follow-up | Stepped care | Minimal | Total |
|---|---|---|---|
| Baseline | |||
| n | 262 | 257 | 519 |
| Mean (SD) | 2.64 (2.90) | 3.08 (3.33) | 2.86 (3.12) |
| Median (min., max.) | 2.00 (0, 15) | 2.00 (0, 15.87) | 2.00 (0, 15.87) |
| Month 6 | |||
| n | 238 | 233 | 471 |
| Mean (SD) | 1.79 (2.60) | 2.41 (3.22) | 2.10 (2.93) |
| Median (min., max.) | 1.00 (0, 16) | 1.00 (0, 17) | 1.00 (0, 17) |
| Month 12 | |||
| n | 229 | 230 | 459 |
| Mean (SD) | 1.90 (3.03) | 2.25 (3.04) | 2.07 (3.04) |
| Median (min., max.) | 1.00 (0, 17) | 1.00 (0, 16) | 1.00 (0, 17) |
| Follow-up | Stepped care estimate (SD) | Minimal estimate (SD) | Difference (95% CI) | p-value |
|---|---|---|---|---|
| Month 6 | n = 236 | n = 229 | −0.064 (−0.173 to 0.045) | 0.247 |
| 0.799 (0.040) | 0.864 (0.040) | |||
| Month 12 | n = 227 | n = 225 | −0.018 (−0.125 to 0.088) | |
| 0.783 (0.038) | 0.802 (0.038) | 0.735 |
At month 6 and month 12, the stepped care group had a lower DPI score than the minimal intervention group. This was not significant at the 5% level.
Quality of life using the Short Form Questionnaire-12 items
The hierarchical model failed to converge so results from a simple linear regression model are presented (Tables 18 and 19).
| Component score | Stepped care | Minimal | Total |
|---|---|---|---|
| PCS | |||
| Baseline | |||
| n | 260 | 256 | 516 |
| Mean (SD) | 47.67 (11.21) | 47.33 (10.99) | 47.50 (11.09) |
| Median (min., max.) | 52 (10.04, 70.37) | 49.81 (7.87, 67.17) | 51.02 (7.87, 70.37) |
| Month 6 | |||
| n | 237 | 233 | 470 |
| Mean (SD) | 47.35 (11.33) | 47.74 (11.16) | 47.54 (11.24) |
| Median (min., max.) | 51.46 (7.02, 66.45) | 50.66 (11.38, 68.68) | 51.22 (7.02, 68.68) |
| Month 12 | |||
| n | 228 | 228 | 456 |
| Mean (SD) | 47.24 (11.87) | 47.48 (10.99) | 47.36 (11.42) |
| Median (min., max.) | 51.59 (12.69, 65.02) | 51.02 (14.37, 68.20) | 51.28 (12.69, 68.20) |
| MCS | |||
| Baseline | |||
| n | 260 | 256 | 516 |
| Mean (SD) | 51.85 (9.51) | 50.18 (10.71) | 51.02 (10.15) |
| Median (min., max.) | 55.06 (9.13, 66.33) | 52.79 (6.98, 71.21) | 54.37 (6.98, 71.21) |
| Month 6 | |||
| n | 237 | 233 | 470 |
| Mean (SD) | 51.77 (9.80) | 50.48 (10.61) | 51.13 (10.22) |
| Median (min., max.) | 54.96 (11.20, 64.40) | 54.34 (12.55, 67.52) | 54.66 (11.20, 67.52) |
| Month 12 | |||
| n | 228 | 228 | 456 |
| Mean (SD) | 51.95 (9.72) | 51.53 (9.85) | 51.74 (9.78) |
| Median (min., max.) | 55.21 (3.58, 64.74) | 54.37 (12.50, 73.02) | 54.77 (3.58, 73.02) |
| Component score | Stepped care estimate (SD) | Minimal estimate (SD) | Difference (95% CI) | p-value |
|---|---|---|---|---|
| MCS | ||||
| Month 6 | 51.214 (0.443) (n = 234) | 51.302 (0.448) (n = 228) | −0.088 (−1.329 to 1.153) | 0.889 |
| Month 12 | 51.630 (0.462) (n = 224) | 52.108 (0.463) (n = 22) | −0.478 (−0.809 to 1.766) | 0.466 |
| PCS | ||||
| Month 6 | 47.152 (0.423) (n = 234) | 47.873 (0.429) (n = 228) | −0.722 (−1.905 to 0.462) | 0.232 |
| Month 12 | 47.069 (0.489) (n = 224) | 47.707 (0.490) (n = 223) | −0.637 (−1.998 to 0.723) | 0.692 |
At month 6 and month 12, the stepped care group had a lower mental component score (MCS) than the minimal intervention group. The stepped care group also had lower physical component score (PCS) at month 6 and month 12. These differences were not significant at the 5% level.
Treatment uptake by the stepped care group
In total, 146 participants were referred to step 2; of these, 41 (28%) went on to receive step 2. The other 105 participants (72%) either declined or could not be contacted. Although those who attended step 2 had a higher average ADD than those who did not attend, it was not significantly higher (Table 20).
| Outcome | Non-attendees (n = 105) | Attendees (n = 41) | Difference (95% CI) | p-value |
|---|---|---|---|---|
| ADD score | 3.91 (2.13) | 4.24 (2.18) | 0.33 (−0.45 to 1.12) | 0.401 |
Summary
There was no evidence of a difference in ADD when comparing the stepped care group with the minimal intervention group at month 12.
There was no evidence of a difference in any of the secondary outcome measures (AUDIT score, alcohol-related problems and quality of life) at either month 6 or month 12.
Chapter 5 Economic analysis
Brief interventions have been proven to be both clinically effective and cost-effective in the management of individuals with hazardous and harmful drinking in primary care settings. 27–29,79 Cost-effectiveness for all adult drinkers for such interventions has partly been driven by reductions in health service costs of alcohol harm. The estimated wider costs of alcohol-use disorders, in terms of health care, crime, family problems and loss of productivity, was up to £258 per year in 2008. 80 Existing studies, however, have included few older drinkers, and this population may have different alcohol problems and consequently different health and social costs.
The economic analysis tests the hypothesis that a stepped care intervention is more cost-effective for older hazardous alcohol users in primary care when compared with a 5-minute brief intervention (minimal intervention).
The objectives of the economic analysis are to:
-
compare costs associated with the stepped care and minimal interventions at 6 and 12 months post randomisation
-
estimate the health benefits, measured using QALYs, from the interventions
-
assess the cost-effectiveness of the stepped care intervention compared with the minimal intervention.
Data were analysed according to the ITT principle, whereby all participants were analysed as members of their allocated group irrespective of the intervention received. Following technology appraisal guidelines used by NICE, the analysis was performed from the NHS/Personal Social Services perspective. All costs were estimated for the year 2009–10 in UK pounds (£). Follow-up was at 6 and 12 months from randomisation.
Assessment of costs
A micro-costing approach was used to compute the costs of trial interventions. The estimation of costs involved three distinct phases: identifying the relevant resource-items; measuring the use of the identified resource-items; and assigning unit costs or prices to them.
Attendees at primary care aged ≥ 55 years were screened to determine if they were eligible for the trial interventions. Opportunistic screening costs were estimated from the actual resource use associated with the screening process, which consisted of an information letter, a copy of the AUDIT questionnaire and the time input of the practice/research nurse or practitioner who contacted screen-positive patients.
The costs of the minimal intervention and the first two tiers of the stepped care programme (step 1 and step 2) were based on information gathered on patient contact with the primary care and specialist services during the trial.
Participants in the control arm received a 5-minute discussion with a practice/research nurse about the health consequences of continued hazardous alcohol consumption, and a brief self-help leaflet. Therefore, the cost of minimal intervention included practice/research nurse time and material costs of the self-help leaflet.
The costs of stepped interventions were calculated using local costs of specialist services and included an allowance for training and supervision costs. For steps 1 and 2 of the intervention, therapists were invited to participate in training sessions to provide them with skills for delivering BCC (step 1) and MET (step 2). The cost component for training included the time that trainers and therapists spent in training and supervision, plus use of space and materials. The total cost for training in each stage was allocated to the number of sessions delivered for the trial.
Step 1 of the intervention included a 20-minute session of BCC by a practice/research nurse. Step 2 consisted of three 40-minute sessions of MET on a weekly basis delivered by a therapist such as an alcohol health worker, clinical nurse manager or drug and alcohol counsellor. The actual time therapists spent delivering each intervention session was recorded and used to compute actual intervention costs by multiplying by their individual salaries.
Four weeks after each step, participants were contacted by telephone by a practice/research nurse for a reassessment of their alcohol consumption. These costs were calculated based on an average 5 minutes of practice/research nurse time and the costs of the line rental.
Step 3 of the stepped care intervention was a referral to local specialist alcohol services to receive specialist intervention. The resource use of this step was not specified in the trial and could encompass a variety of intervention approaches; interventions in this step could expand beyond the time horizon of the trial and a standard cost of £811 per patient was assumed according to the literature. 81
Data on additional utilisation of health and social care and criminal justice services outside the trial protocol were collected from questionnaires administered at baseline, 6 months and 12 months. At each time point, participants were asked about their resource use over the previous 6 months. Units of resource use recorded were then multiplied by national sources of unit costs81 in order to provide generalisable results. Table 21 presents a summary of the categories of resource use together with their unit costs.
| Resource item | Unit cost (£) | Source |
|---|---|---|
| A&E visit | 37 | Curtis81 |
| Inpatient night | 240 | Curtis81 |
| Outpatient attendance | 152 | Curtis81 |
| Day case | 637 | Curtis81 |
| Emergency ambulance | 277.80 | Curtis81 |
| Patient transport service | 57.02 | Curtis81 |
| GP (surgery) | 32 | Curtis81 |
| GP (home) | 106 | Curtis81 |
| Practice nurse (surgery) | 10 | Curtis81 |
| Practice nurse (home) | 13 | Curtis81 |
| Prescription | 8.8 | Curtis81 |
| Day centres | 36 per day | Curtis81 |
| Meals on wheels | 2.86 per meal | Oddie82 |
| Social services home care services | 92 per day | Curtis81 |
| Social worker (office) (30 minutes) | 26.50 | Curtis81 |
| Social worker (home) (60 minutes) | 53 | Curtis81 |
| Care worker (office) (30 minutes) | 25 | Curtis81 |
| Care worker (home) (60 minutes) | 50 | Curtis81 |
Assessment of outcome
The economic evaluation used QALYs as recommended by NICE as a measure of health benefit for their reference case. 73 QALYs were derived from utility scores measured by EQ-5D questionnaires at baseline and at 6-month and 12-month follow-up. The EQ-5D is a standardised instrument for use as a measure of health outcomes developed by the EuroQol Group. 60 The EQ-5D results were scored using the UK York time trade-off tariff obtained from a sample of around 3000 members of the general UK population. 83,84 Given the assumption that health status changes between measurements are smooth and gradual over time, utility scores were converted into QALYs using the area under the curve method. 85
To appropriately adjust potential imbalances and ensure comparability with the clinical analysis, multiple regression methods were applied to give the differential mean QALYs and the prediction of adjusted QALYs by controlling for baseline EQ-5D scores. 86,87
Assessment of cost-effectiveness
Incremental cost-effectiveness analysis was performed to combine the costs of the interventions with health outcomes. The mean difference in costs between the two trial groups was compared with mean difference in effectiveness to generate ICERs. 70
Here, E represents the change in effects (in this case measured QALYs), and C represents the costs of intervention, measured in monetary units, while subscripts ‘SC’ and ‘MI’ refer to stepped care and minimal intervention, respectively.
Handling uncertainty
Cost and QALY data are typically not normally distributed. Cost data are often highly right skewed because of a few cases that incur extremely high costs, while QALY data are normally left skewed because of the ceiling effect. 88–90 In this study, the non-parametric bootstrap technique was employed to explore the sensitivity of calculated ICERs. Bootstrapping is a resampling method that generates multiple replications of the statistic of interest (ICER) by sampling with replacement from the original data. 91 The bootstrap method is preferable for skewed data as it does not rely on parametric assumptions concerning the underlying distribution of data. 92,93
The results from the bootstrap resampling were used to plot cost-effectiveness planes (CEPs) and cost-effectiveness acceptability curves (CEACs) to show the decision uncertainty surrounding adoption decisions. The CEACs present the probability that stepped care is the preferred treatment option at different values for a decision-maker's willingness to pay (WTP) for a QALY. 94
Sensitivity analysis
Sensitivity analysis is an appropriate way to check on methodological uncertainty. In this study, sensitivity analysis was planned to vary assumptions about costing methods. It was found that the distributional problem that arose for cost data was mainly attributable to a few ‘extreme values’ in the distribution. Therefore, extreme values, defined as those deviating by five times the standard deviation, were excluded and the incremental cost-effectiveness analysis was repeated in the sensitivity analysis.
Results of economic analysis
A total of 21,546 primary care attendees aged ≥ 55 years were screened using the AUDIT questionnaire. A total of 529 hazardous alcohol drinkers were recruited to the trial and received either minimal or stepped care interventions.
The base-case cost-effectiveness analysis was based on 422 participants (212 in the stepped care group and 210 in the minimal intervention group) with both completed cost and outcome estimates for three different time points, i.e. baseline, 6-month and 12-month follow-up.
Costs
The breakdown of screening costs and intervention costs by allocated treatment is summarised in Table 22. The opportunistic screening costs consisted of the costs of materials provided to the 21,546 screened patients and the cost of 5 minutes of practice/research nurse time to contact screen-positive patients. The mean screening cost for every participant recruited into the trial was £5.52; this part of the cost was equal in both intervention and control groups.
| Source of cost | Intervention group | Control group |
|---|---|---|
| Opportunistic screening cost | ||
| Information letter and AUDIT questionnaire | 1.63 per participant recruited | 1.63 per participant recruited |
| Five minutes of practice/research nurse contact time with screen-positive patients | 3.89 per participant recruited | 3.89 per participant recruited |
| Minimal intervention | ||
| Five minutes of practice/research nurse time | 0.00 | 2.17 per participant |
| Self-help bookleta | 0.00 | 0.17 per participant |
| Stepped care intervention | ||
| Step 1: BCC | ||
| Training cost for practice/research nurses | 3.69 per session | 0.00 |
| Twenty-minute BCC | 8.72 (SD 0.62) per session | 0.00 |
| Self-help bookleta | 0.17 per participant | 0.00 |
| Short telephone assessment 4 weeks afterb | 2.42 per participant | 0.00 |
| Step 2: MET | ||
| Training cost for therapists | 12.71 per session | 0.00 |
| Three 40-minute sessions with trained alcohol therapist | 36.84 (SD 52.34) per participant | 0.00 |
| Short telephone assessment 4 weeks after | 2.42 per participant | 0.00 |
| Step 3: specialist alcohol services | ||
| 811 per patient | 0.00 | |
The average cost of minimal intervention was £2.34 per participant. This included £2.17 cost of practice/research nurse time and £0.17 cost of the self-help material.
For participants assigned to the stepped care group, a 20-minute BCC session together with a self-help booklet was provided for step 1 of the intervention. The intervention cost for this step was £8.89 per participant.
Step 2 consisted of three sessions of MET. Full data were available for 33 of the participants who received at least one session of therapy, averaging out at a cost of £36.84 per patient, calculated using the actual amount of therapist contact time for each patient. As part of the intervention, a practice/research nurse contacted participants and a short telephone assessment was made to reassess alcohol consumption after each step. The average reassessment cost was £2.42 per patient. The trial data show that attendance at the third stage was actually quite rare. Only five patients completed specialist interventions, with an average cost of £811 per person.
Table 23 presents the quantity of health and social care and criminal justice service utilisations at baseline and 6 months and 12 months after randomisation for both trial groups. No police and criminal justice system contacts were reported by participants.
| Resource item | Baseline | 6 months | 12 months | |||
|---|---|---|---|---|---|---|
| Control | Intervention | Control | Intervention | Control | Intervention | |
| A&E visit | 0.15 (0.58) | 0.13 (0.47) | 0.17 (0.49) | 0.17 (0.64) | 0.14 (0.61) | 0.09 (0.36) |
| Inpatient night | 0.21 (1.24) | 0.48 (3.59) | 0.20 (1.02) | 0.19 (1.00) | 0.44 (2.74) | 0.16 (0.98) |
| Outpatient attendance | 1.01 (1.86) | 1.00 (2.56) | 1.03 (2.77) | 0.97 (2.09) | 1.10 (3.47) | 0.91 (2.22) |
| Day case attendance | 0.14 (0.68) | 0.14 (0.53) | 0.17 (0.70) | 0.15 (0.58) | 0.22 (2.29) | 0.16 (0.54) |
| Use of emergency ambulance | 0.05 (0.32) | 0.06 (0.27) | 0.10 (0.42) | 0.04 (0.21) | 0.10 (0.57) | 0.02 (0.15) |
| Use of patient transport service | 0.02 (0.28) | 0.07 (0.38) | 0.02 (0.17) | 0.07 (0.59) | 0.15 (1.75) | 0.01 (0.14) |
| GP visit (surgery) | 2.45 (2.68) | 2.20 (2.19) | 2.03 (2.20) | 1.83 (1.90) | 2.31 (2.90) | 1.80 (1.98) |
| GP visit (home) | 0.01 (0.10) | 0.03 (0.26) | 0.01 (0.10) | 0.08 (0.63) | 0.06 (0.44) | 0.06 (0.45) |
| Practice nurse visit (surgery) | 1.26 (1.88) | 1.46 (2.50) | 1.17 (1.93) | 1.58 (5.35) | 1.26 (2.05) | 1.01 (1.42) |
| Practice nurse visit (home) | 0.01 (0.21) | 0.57 (8.24) | 0.07 (0.54) | 0.02 (0.17) | 0.10 (1.14) | 0.01 (0.15) |
| Prescriptions | 4.12 (5.75) | 3.58 (2.86) | 3.87 (4.04) | 4.09 (4.12) | 3.83 (3.25) | 3.60 (3.39) |
| Day centre visit | 0.52 (5.77) | 0.23 (3.30) | 0.02 (0.23) | 0.23 (3.30) | 0.20 (1.74) | 0.24 (3.30) |
| Meals on wheels | 0.03 (0.41) | 0.03 (0.41) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) |
| Social services home care services | 0.00 (0.00) | 0.08 (0.71) | 0.00 (0.00) | 0.00 (0.07) | 0.03 (0.41) | 0.01 (0.15) |
| Social worker (office) (30 minutes) | 0.06 (0.83) | 0.00 (0.07) | 0.03 (0.41) | 0.01 (0.15) | 0.02 (0.17) | 0.03 (0.30) |
| Social worker (home) (60 minutes) | 0.02 (0.28) | 0.00 (0.00) | 0.03 (0.41) | 0.01 (0.15) | 0.06 (0.83) | 0.00 (0.00) |
| Care worker (office) (30 minutes) | 0.01 (0.21) | 0.00 (0.00) | 0.02 (0.21) | 0.20 (1.83) | 0.20 (1.86) | 0.04 (0.44) |
| Care worker (home) (60 minutes) | 0.07 (0.59) | 0.01 (0.10) | 0.01 (0.14) | 0.00 (0.07) | 0.06 (0.46) | 0.06 (0.46) |
| Police and criminal justice system contacts | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) |
In addition to the costs of delivering the stepped care interventions, a unit training cost per session was added to the intervention cost to estimate a total cost for stepped care. The training costs for BCC (step 1) and MET (step 2) were £3.69 and £12.71 per session, respectively. The overall average cost of treatment for the intervention group was therefore £46.63 (SD £146) per trial participant (Table 24).
| Costs | Stepped care, £ (SD) (n = 212) | Minimal intervention, £ (SD) (n = 210) | Difference (£)a (95% CI) |
|---|---|---|---|
| Six-month resource use at baseline | 522.53 (1233.05) | 468.25 (727.41) | 54.28 (−139.67 to 248.23) |
| Six-month resource use at 6-month follow-up | 443.78 (832.70) | 467.52 (903.42) | −23.74 (−189.96 to 142.49) |
| Six-month resource use at 12-month follow-up | 410.65 (729.81) | 602.38 (2263.20) | −191.74 (−512.90 to 129.43) |
| Opportunistic screening cost | 5.52 (0.00) | 5.52 (0.00) | 0 |
| Intervention cost | 46.63 (145.88) | 2.34 (0.00) | 44.29 (24.50 to 64.08) |
| Costs at 6 monthsb | 495.53 (843.78) | 474.98 (903.42) | 20.56 (−146.75 to 187.87) |
| Costs at 6 monthsc | 488.48 (826.32) | 482.10 (826.32) | 6.38 (−164.09 to 151.33) |
| Costs at 12 monthsb | 906.18 (1369.31) | 1077.36 (2635.77) | −171.18 (−574.06 to 231.70) |
| Costs at 12 monthsc | 895.04 (2049.45) | 1088.61 (2049.47) | −193.57 (−585.06 to 197.93) |
Costs of resource use were calculated by multiplying the product of each resource-use category by its associated unit cost listed in Table 21. Total costs for each group were reported in the table by adding up resource use costs, screening costs and intervention costs (see Table 24). The results showed that resource use costs were the biggest contributor to the overall costs for both groups.
The mean total cost per participant in the stepped care group was £496 (SD £844) compared with £475 (SD £903) in the minimal intervention group at the 6-month follow-up. Using a 12-month time horizon, the mean total cost was £906 (SD £1369) and £1077 (SD £2636) in the stepped care and minimal groups, respectively.
The costs of health and social care resource use were higher in the stepped care group at baseline (difference £54; 95% CI −£140 to £248). This indicates some baseline imbalance in cost estimates, so we adjusted total costs by controlling for the imbalance in baseline resource use using the multiple regression method.
The adjusted 6-month cost for the stepped care group was £488 compared with £482 for the minimal intervention group, giving a difference of −£6.38 (95% CI −£164 to £151). At the 12-month follow-up, the adjusted mean cost was £875 in the stepped care group compared with £1089 in the minimal group (difference −£194; 95% CI −£585 to £198). The results indicated that stepped care was, on average, less costly compared with minimal intervention at 12-month follow-up, although the difference is not statistically significant.
Outcomes
Mean EQ-5D scores are reported for both groups at the baseline, 6-month and 12-month post randomisations. Figure 3 presents the change of mean EQ-5D scores over time.
FIGURE 3.
Mean EQ-5D scores at baseline, 6 months and 12 months.
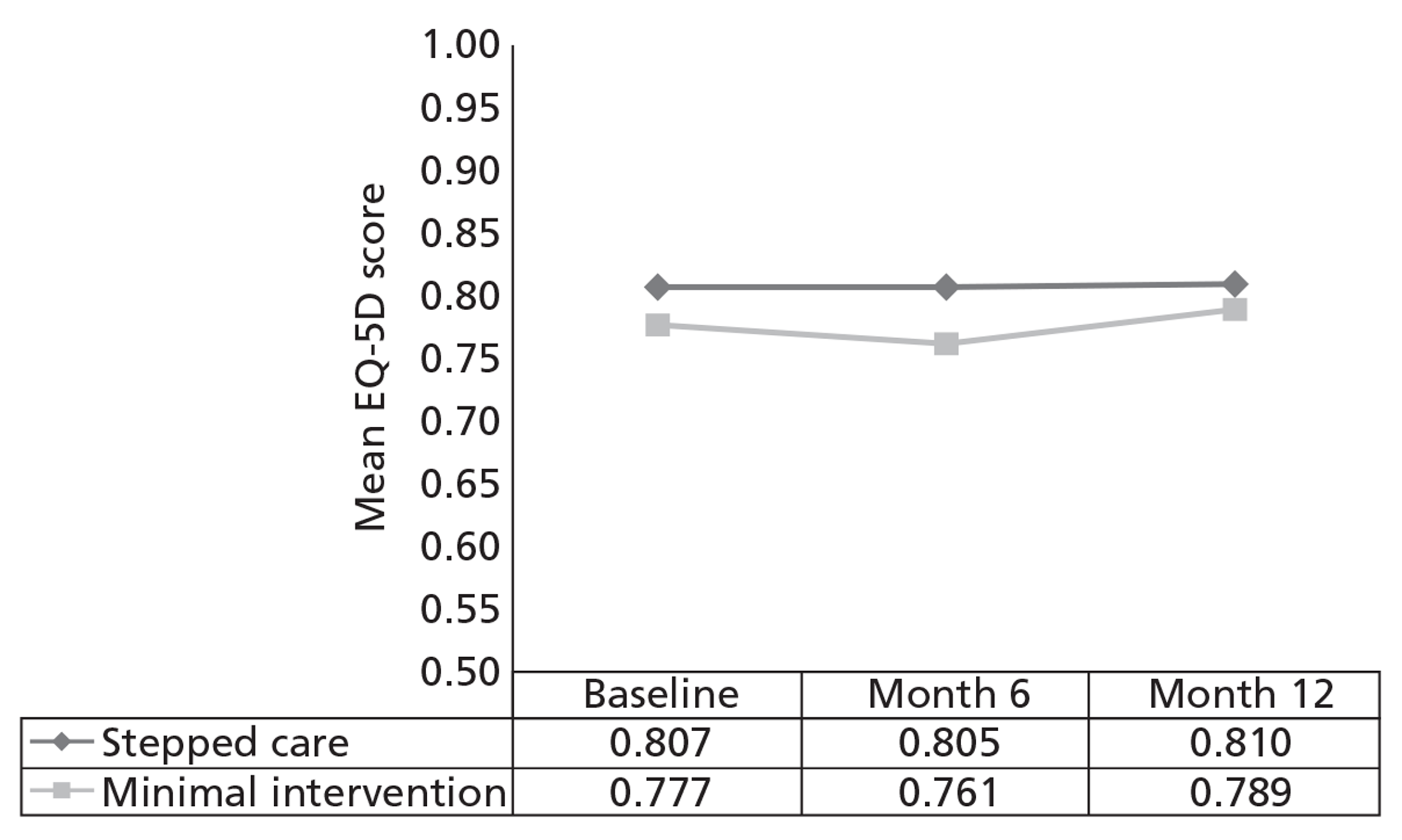
Figure 3 shows that, in both the intervention and control groups, mean EQ-5D scores at 6 months were lower than at baseline, while at the end of the 12-month follow-up, the scores increased and were higher than the baseline utilities. Changes in EQ-5D scores were transformed to estimate the QALY gains for each patient (Table 25). The mean unadjusted difference QALY gain over the 6 months from baseline was 0.4030 (SD 0.1026) in the stepped care group and 0.3843 (SD 0.1164) in the minimal intervention group. The corresponding QALY gains for the 12 months from baseline were 0.8067 (SD 0.2012) and 0.7717 (SD 0.2214), respectively.
| Outcome | Intervention group (SD) (n = 212) | Control group (SD) (n = 210) | Difference,a£ (95% CI) |
|---|---|---|---|
| Baseline EQ-5D scores | 0.8066 (0.2204) | 0.7767 (0.2507) | 0.0299 (−0.0152 to 0.0751) |
| Six-month follow-up EQ-5D scores | 0.8052 (0.2238) | 0.7606 (0.2451) | 0.0446 (−0.0003 to 0.0895) |
| Twelve-month follow-up EQ-5D scores | 0.8098 (0.2304) | 0.7891 (0.2257) | 0.0207 (−0.0229 to 0.0644) |
| QALY (6 months)b | 0.4030 (0.1026) | 0.3843 (0.1164) | 0.0186 (−0.0024 to 0.0396) |
| QALY (6 months)c | 0.3966 (0.0394) | 0.3908 (0.0394) | 0.0058 (−0.0018 to 0.0133) |
| QALY (12 months)b | 0.8067 (0.2012) | 0.7717 (0.2214) | 0.0350 (−0.0055 to 0.0755) |
| QALY (12 months)c | 0.7951 (0.1054) | 0.7834 (0.1054) | 0.0117 (−0.0084 to 0.0318) |
Similar to the cost calculation, there is imbalance occurring at the baseline in the mean EQ-5D scores in the two trial groups (Table 26). After adjusting QALYs for baseline EQ-5D, the results demonstrate that participants in the stepped care group had, on average, a slightly better quality of life than those in the minimal intervention group [difference in QALYs was 0.0058 (95% CI −0.0018 to 0.0133) at 6 months and 0.0117 (95% CI −0.0084 to 0.0318) at 12 months]. However, this difference was not statistically significant.
| Outcome | 6 months | 12 months | ||
|---|---|---|---|---|
| Stepped care (n = 212) | Minimal intervention (n = 210) | Stepped care (n = 212) | Minimal intervention (n = 210) | |
| Cost (SD) | £488 (£826) | £482 (£826) | £895 (£2049) | £1089 (£2049) |
| QALY (SD) | 0.3966 (0.0394) | 0.3908 (0.0394) | 0.7951 (0.1054) | 0.7834 (0.1054) |
| ICER (95% CI) | –£1100 per QALY (−£85,991 to £95,546) | –£7997 per QALY (−£238,341 to £172,319) | ||
Results of the cost-effectiveness analysis
Table 26 presents ICERs that combined the costs of interventions with health outcomes.
At the 6-month follow-up, both mean QALY gains and mean cost were greater in the stepped care group than in the minimal intervention group, generating an ICER of £1100 per QALY gained, while at the 12-month follow-up, minimal intervention is dominated by stepped care using the calculated average results. Stepped care participants receive more benefits (greater QALY gains) for less cost; however, none of the differences in costs and benefits between the two interventions was statistically significant. The bootstrap method was therefore employed to evaluate uncertainty surrounding cost-effectiveness estimates. Bootstrapping results were also used to generate incremental CEPs and CEACs to show uncertainty surrounding adoption decisions, shown in Figures 4 and 5.
FIGURE 4.
Cost-effectiveness plane (a), adjusted for baseline utility and costs, and cost-effectiveness acceptability curve (b), adjusted for baseline utility and costs, at 6 months (completed cases).
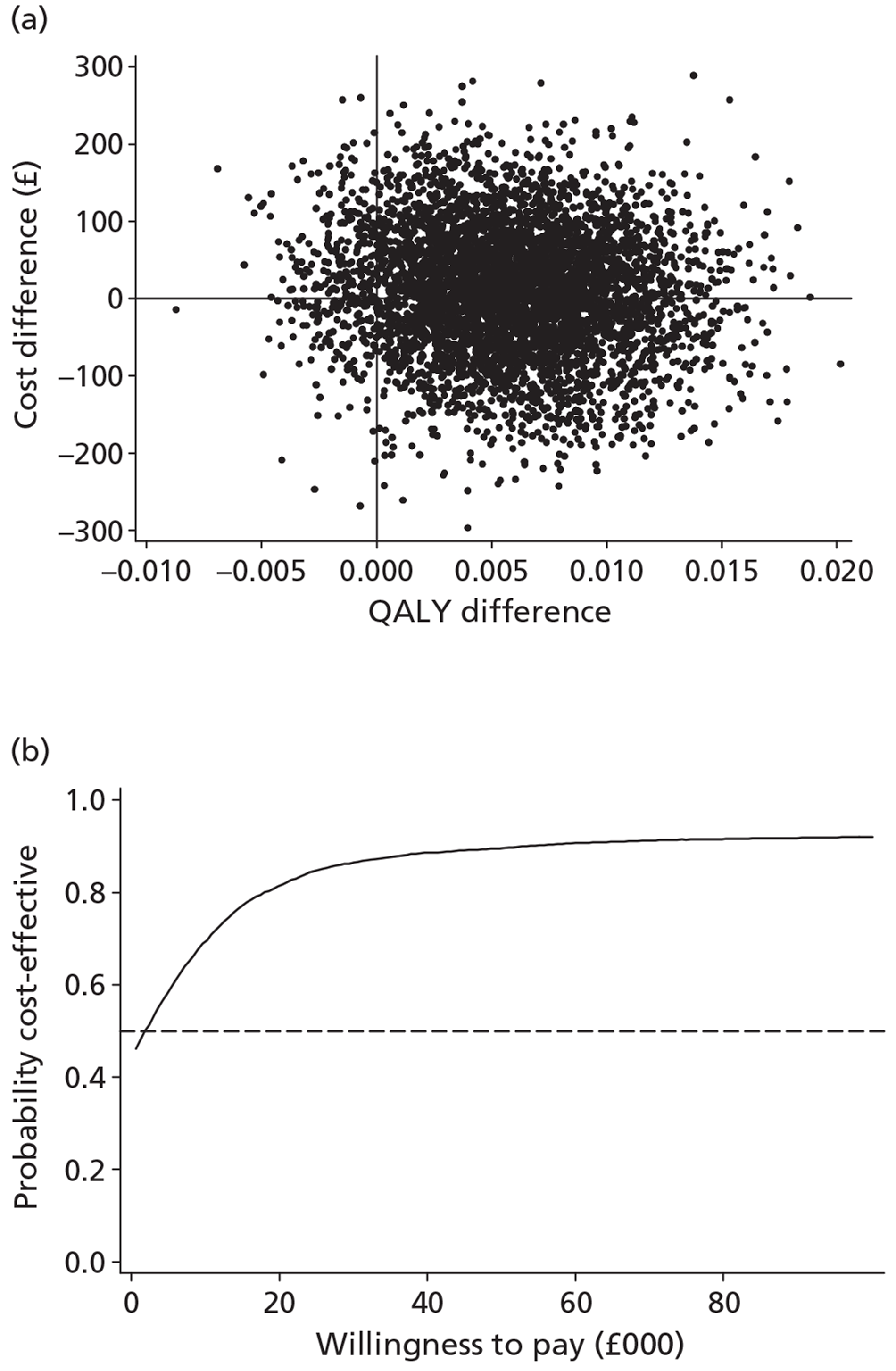
FIGURE 5.
Cost-effectiveness plane (a) and cost-effectiveness acceptability curve (b) at 12 months (completed cases).
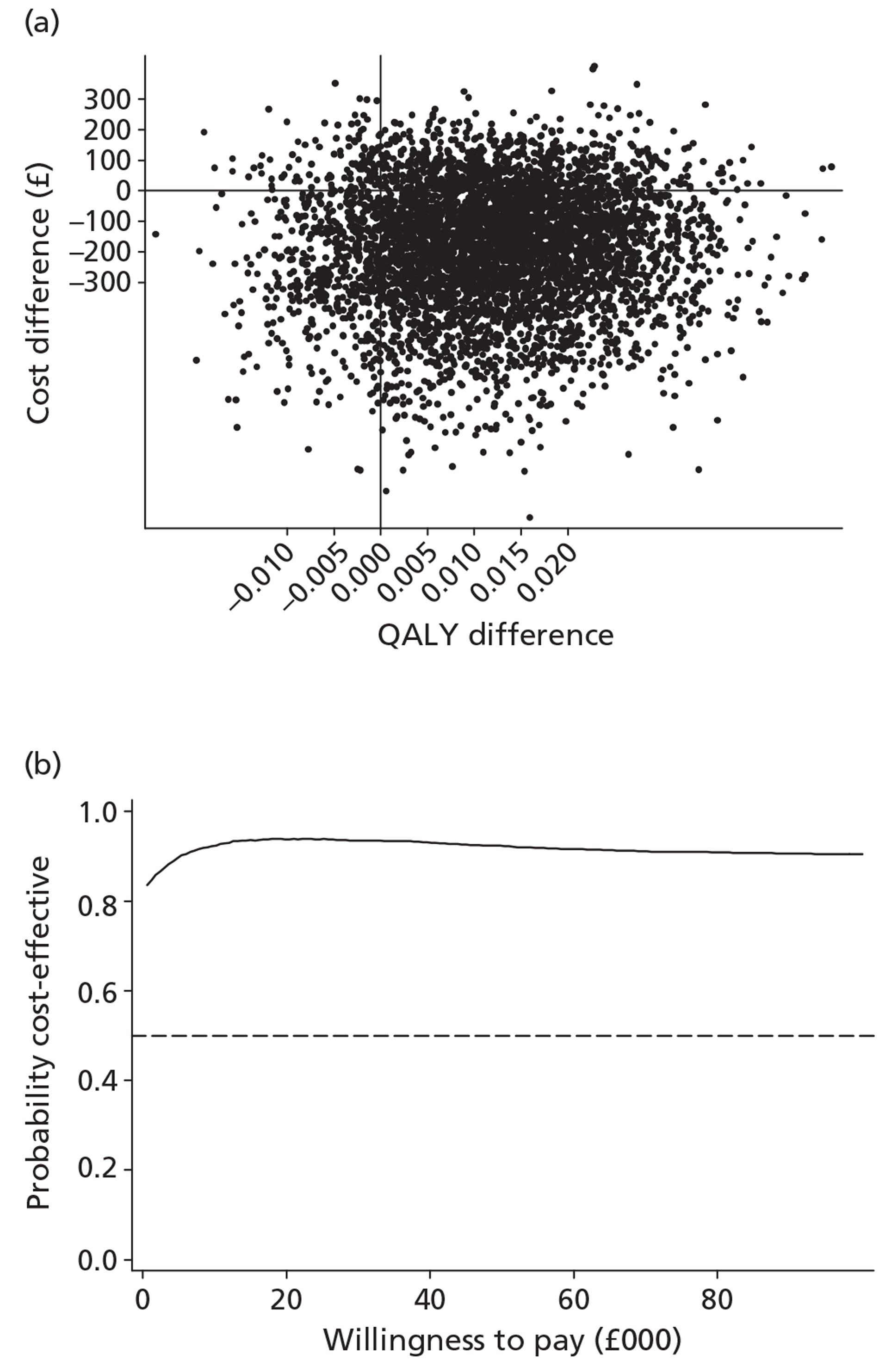
For the 6-month follow-up period, the incremental cost-effectiveness plane (Figure 4) showed over half of the plots falling into the south-east quadrant, indicating that stepped care interventions were less costly and more effective. Given WTP thresholds of £20,000–30,000 per additional QALY gained, which is the decision-making threshold used by NICE, the probability that stepped care was more cost-effective is 81.3–86.4%.
Similarly, at the 12-month follow-up (Figure 5), the majority of plots in the cost-effectiveness scatter lay in the south-east quadrant and indicated that minimal intervention was dominated by stepped care interventions. The probability of stepped care being cost-effective was between 93.5–93.84% using NICE's threshold range of £20,000–30,000 per QALY gained.
The effects of the GP practice were analysed using a multilevel model programmed in MLwiN. Net monetary benefit was calculated using a WTP threshold of £30,000 per QALY. The analysis indicated that the net monetary benefit did not significantly differ by GP practice.
Results of the sensitivity analysis
Sensitivity analysis was carried out to check on costing methods in the economic evaluation. In total, 13 participants with extreme costs were excluded in the sensitivity analysis. The changes in costs were noticeable (Figure 6). Taking the 12-month resource use as an example, the mean cost for the control group fell from £1162 to £850, and the standard deviation dropped from £2636 to £1125. With the 409 cases left, the incremental cost-effectiveness analysis was repeated. The results are summarised in Table 27.
FIGURE 6.
Twelve-month health and social care resource use before and after removing extreme cases.
| Outcome | 6 months | 12 months | ||
|---|---|---|---|---|
| Stepped care (n = 206) | Minimal intervention (n = 203) | Stepped care (n = 206) | Minimal intervention (n = 203) | |
| Cost (SD) | £430 (£530) | £368 (£530) | £827 (£1029) | £754 (£1029) |
| QALY (SD) | 0.3999 (0.0386) | 0.3926 (0.0387) | 0.8030 (0.1027) | 0.7857 (0.1027) |
| ICER (95% CI) | £8496 per QALY (−£30,395 to £73,223) | £4224 per QALY (−£37,867 to £57,965) | ||
Twelve-month health and social care resource use before and after removing extreme cases.
Taking into account the sensitivity analysis, stepped care continues to demonstrate greater mean QALY gains; however, it is now more costly. The plots on the CEPs move upwards and to the left compared with base-case results. Now the adoption of the intervention relies only on the WTP threshold. Using the NICE threshold range of £20,000–30,000 per QALY, stepped care, with an incremental cost per QALY gained of £8496 at 6 months and £4224 at 12 months, is the more cost-effective option compared with minimal intervention.
Under the new set of ICERs, and using the £20,000–30,000 per QALY gained threshold, the probability that stepped care is more cost-effective ranges between 80% and 88% at 6 months and between 87% and 90% at 12 months (Figures 7 and 8).
FIGURE 7.
Cost-effectiveness plane and cost-effectiveness acceptability curve at 6 months (sensitivity analysis).
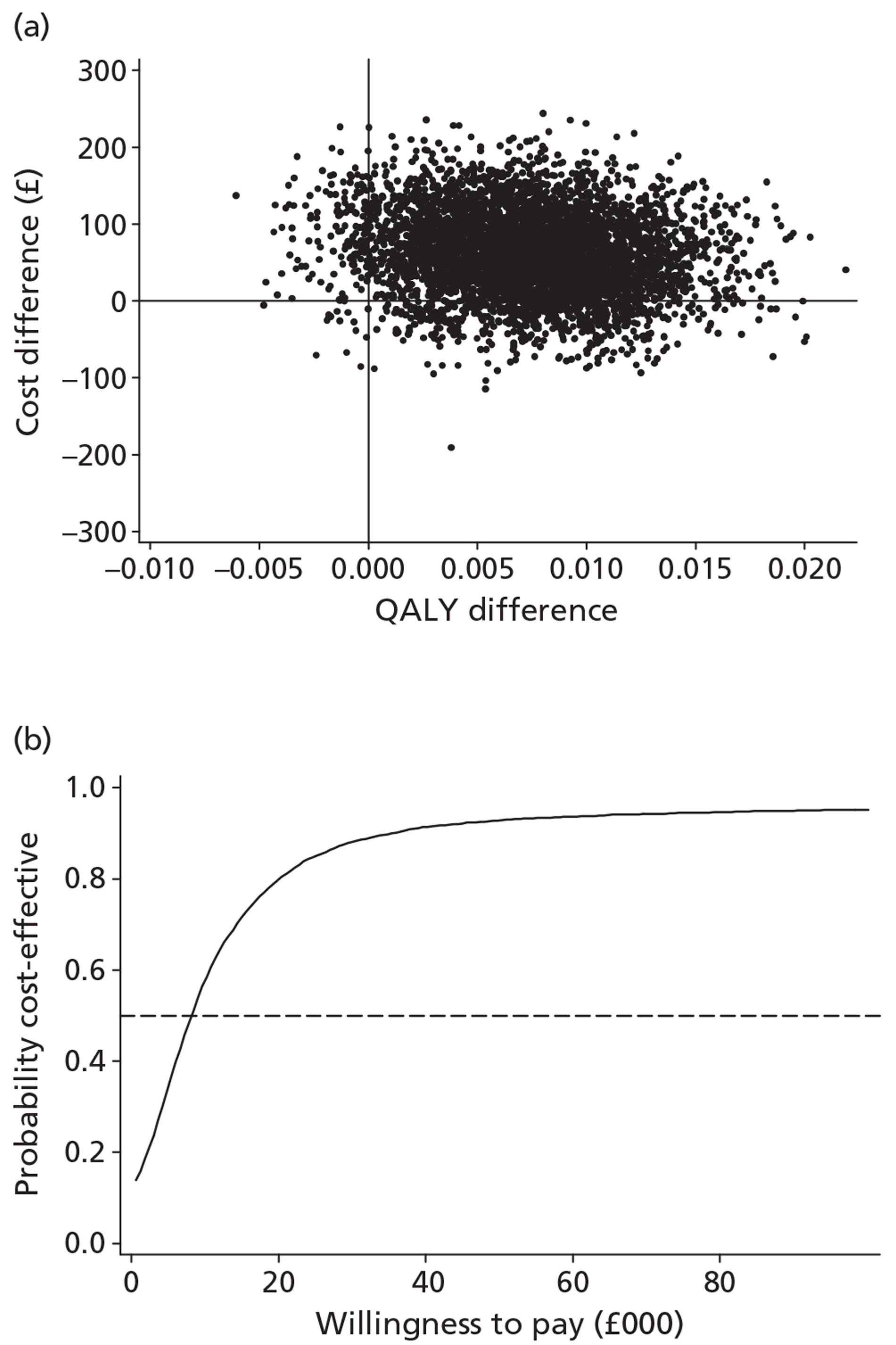
FIGURE 8.
Cost-effectiveness plane and cost-effectiveness acceptability curve at 12 months (sensitivity analysis).
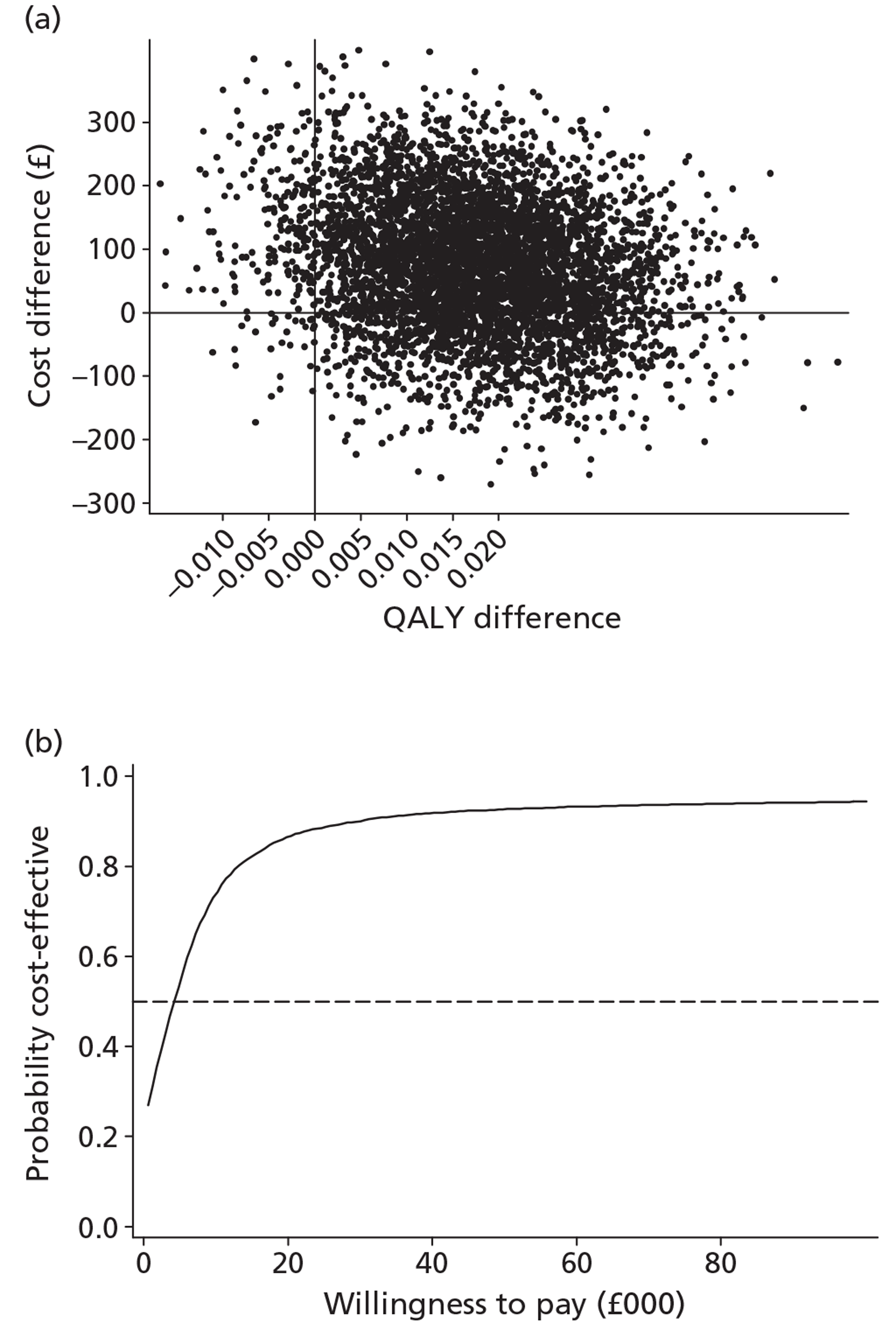
Summary
The cost-effectiveness results indicated that the costs of delivering stepped care interventions were of the order of 20 times those of the minimal intervention [£46.63 (SD £146) vs £2.34 (SD £0)]. However, the overall cost per patient, taking into account health and social care resource use, was £488 (SD £826) in the stepped care group and £482 (SD £826) in the minimal intervention group at 6 months. The mean QALY gains were slightly greater in the stepped care group than in the minimal intervention group, with a mean difference of 0.0058 (95% CI −0.0018 to 0.0133), generating an ICER of £1100 per QALY gained. At month 12, participants in the stepped care group incurred fewer costs, with a mean difference of −£194 (95% CI −£585 to £198), and had gained 0.0117 more QALYs (95% CI −0.0084 to 0.0318) compared with the control group. From an economic perspective, therefore, the minimal intervention was dominated by stepped care.
The results, based on the resampled cost-effectiveness data from the bootstrapping, showed that the probability of stepped care being cost-effective was between 81% and 86% at the 6-month follow-up, and 93.5% and 93.8% at 12 months' follow-up given the NICE decision-making threshold range of £20,000–30,000 per QALY gained. This provides decision-makers with some useful evidence that stepped care interventions are more likely to achieve better value for money than minimal interventions. However, caution is required when interpreting the results given the uncertainty surrounding the estimates.
A sensitivity analysis that excluded extreme cases altered the average costs of interventions; the ICERs were £8496 per QALY at 6 months and £4224 per QALY at 12 months. The probability that stepped care is more cost-effective ranges between 80% and 88% at 6 months and between 87% and 90% at 12 months using the £20,000–30,000 per QALY gained WTP threshold.
Chapter 6 Fidelity process rating
Treatment fidelity plays a crucial role in considering the inferences drawn from effectiveness studies. 66 It provides a means of evaluating whether or not therapists delivered the interventions as described in the session protocols and demonstrates that interventions were distinguishable from one another. 96 In other words, it reports on the internal validity of the study. It also assesses the quality of such delivery, i.e. it measures practitioner skill. This is particularly important as treatment adherence is not always related to therapist competence. 97 A therapist can adhere to a session protocol but deliver the components in a poor or unacceptable manner, such as asking questions at inappropriate times and adopting a cold and judgemental demeanour. Fidelity checks can therefore identify differences in therapist competence and enable potential treatment effects to be accurately attributed.
Methods
Development of the rating scale
The AESOPS PRS (Appendix 10) was adapted from the validated UKATT PRS66 and was designed to rate the delivery of all three trial interventions, namely the minimal intervention, the 20 minutes of BCC (step 1) and MET (step 2). Content and style items from the validated UKATT PRS, including those that rated the delivery of MET, were used as the basis for adapting the scale. These items were examined to ensure that they covered all of the treatment components specified in the session protocols. At this point an item was added to rate the number of open questions asked by the practice/research nurse.
Items described behaviours that were referred to in each of the session protocols and were therefore relevant to each intervention. Style items were largely based on a motivational interviewing approach. In order to distinguish interventions delivered in this style, two items denoting behaviours that were inconsistent with a motivational interviewing approach were included in the pilot phase. These included item 15, the extent to which the practice/research nurse provided unsolicited advice to the patient, and item 17, the number of closed questions asked by the practice/research nurse within the intervention.
The rating scale
The PRS is an 18-item scale, divided into four sections. The first section contains four items relating to overall session management. The middle two sections include eight items measuring specific tasks and five items measuring therapist style. The last section, listed as a single item, contains a session content/activity checklist.
All but three items were rated on two 5-point scales. The first scale provided a frequency rating that showed the extent to which an item was present. The second scale gave a quality rating and showed how well the practice/research nurse performed the behaviour; this scale was rated only if the item received a frequency rating. The frequency ratings ranged from 0 (‘not at all’), indicating that the item never explicitly occurred, to 4 (‘extensively’), signifying that the item was performed numerous times during the intervention. Intermittent points were labelled ‘a little’, ‘somewhat’ and ‘considerably’. On the quality scale, a rating of 0 (‘very poor’) showed that the item was performed in an unacceptable manner, and a rating of 4 (‘very well’) indicated that the therapist had demonstrated a high level of skill and expertise. Intermittent labels were ‘poor’, ‘good enough’ and ‘well’.
Global ratings were given for three of the items; two were associated with session management (‘session structure’ and ‘consistency of problem focus’) and one with therapist style (‘empathy’). The remaining items consisted of frequency counts of specific behaviours with corresponding quality ratings. Each point on the frequency scale related to a predefined number of behaviour counts. For example, a frequency rating of 2 (‘somewhat’) indicated that the item behaviour occurred either once and in some detail or three or time times but briefly. Quality scores also had corresponding definitions; for instance, 0 (‘very poor’) indicated that the practice/research nurse performed the behaviour within each item in an unacceptable manner. Where appropriate, an average quality score was given for each of the item behaviours. For example, if a practice/research nurse attempted to elicit optimism three times within the intervention and received quality scores of 2, 3 and 4, the overall quality rating given for that behaviour would be 3 (‘well’).
Two items carried a frequency rating only: item 15 (‘unsolicited advice’) and item 17 (‘closed questions’). Given that these behaviours were inconsistent with a motivational interviewing style, it followed that a rating of how well therapists performed these items was not needed. The final item, a session content/activity checklist, asked for a yes/no answer to illustrate whether or not the following content had occurred within the intervention: review AUDIT score, obtain an account of drinking, give correct advice/information, set a target, and make a drinking plan. The checklist also included a tick box question to indicate whether the recording was good or poor.
The rating manual
The rating manual was similarly adapted from that used in the UKATT study. 66 General guidelines were issued for the process of rating, such as rating practice/research nurse behaviours, distinguishing between frequency and quality scores, and avoiding sources of rater bias. Item definitions with guidelines for making higher or lower ratings were provided. These were illustrated with examples of practice/research nurse dialogue and differentiated from closely related items. Explanatory notes were included regarding the rating of session content.
Rater training and supervision
An independent rater was trained to use the adapted scale and rating manual. Supervised practice ratings were held at weekly intervals reviewing a total of 17 recordings split evenly between the trial interventions. Recordings were simultaneously rated and the scores discussed with reference to the manual and rater notes. This ensured rater consistency and prevented rater drift. Familiarity with the manual and rating scale was essential. Independent practice was carried out whereby item definitions were read each time they were scored. Recordings used during rater training were not used in the study. Regular supervision continued after training to discuss independently rated recordings. Selected recordings were rated by the independent rater and the supervisor for the purposes of calibration.
The process of rating
Following guidelines outlined in the rating manual, raters listened to the interventions and scored item behaviours. Where appropriate, frequency counts were given a corresponding quality rating. Item definitions, as specified in the manual, were referred to throughout the process in order to prevent rater drift. Raters had the option to pause the recording or consult the manual without stopping. Brief notes were made during the session to help substantiate assigned scores. These were particularly useful for discussing ratings during supervision. At the end of the session, appropriate global ratings and overall frequency and quality ratings were given. Each session was timed to ascertain duration. All scores were entered into the Statistical Package for Social Sciences (SPSS) version 19 (SPSS Inc., Chicago, IL, USA) for analysis.
Sampling
One hundred and sixty sessions of brief advice (minimal) and BCC (step 1) were selected for independent process rating (Figure 9). Only these two treatments were rated, as there were not enough MET sessions (step 2) to enable meaningful results to be obtained. The sample was stratified by site, practice/research nurse and treatment. Replacement sampling was used for eight inaudible recordings. In total, 79 sessions of brief advice and 81 sessions of BCC were rated. Nineteen per cent of these were double rated (i.e. scored by both the independent rater and supervisor): 11 sessions of brief advice and 20 of BCC.
FIGURE 9.
Flow diagram of process rating procedure.
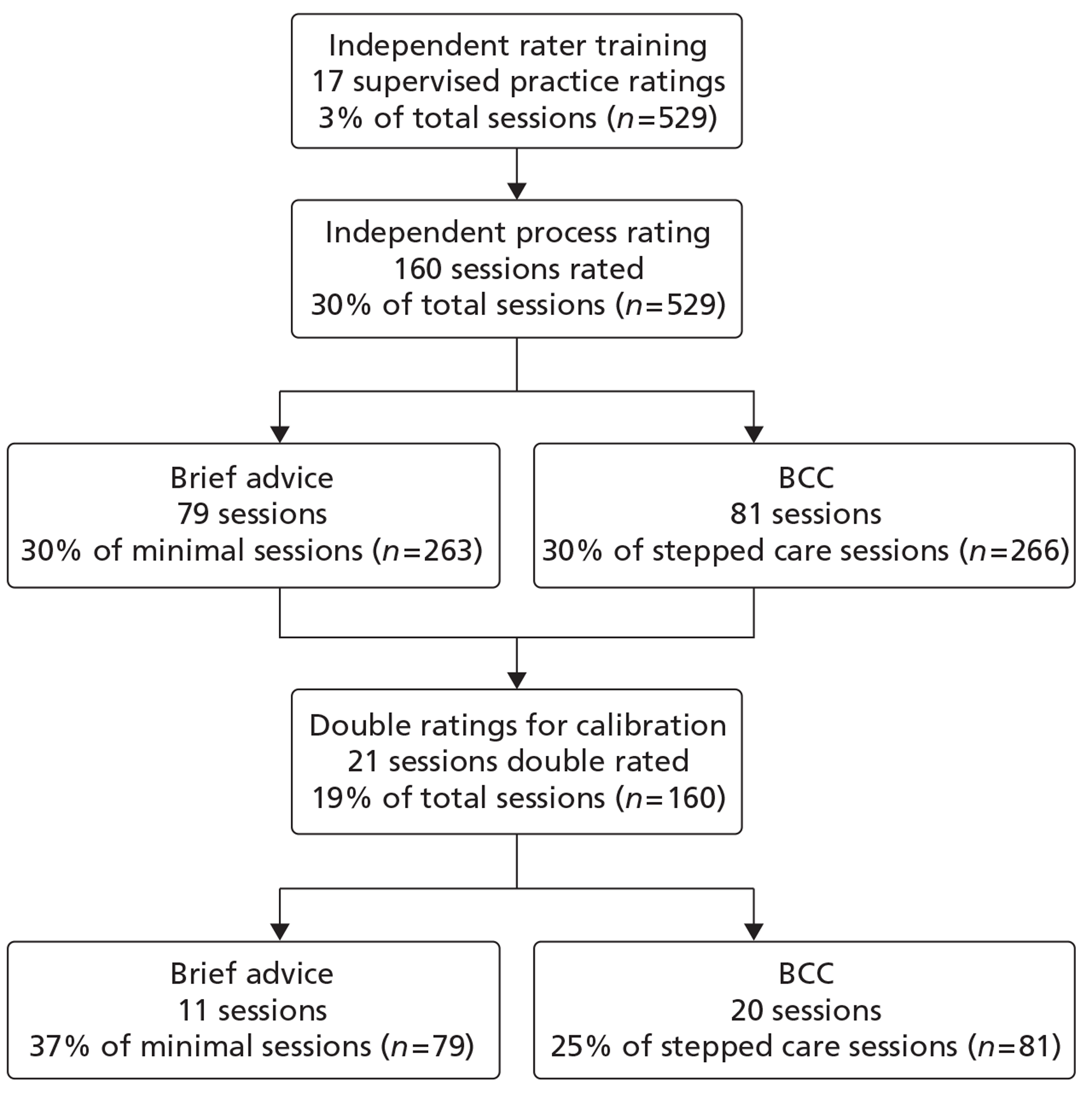
Analyses
The PRS consisted of four sections: session management, specific task, practice/research nurse style and session content. The summaries for the scores for each of the treatment sessions are displayed in Tables 28 to 32.
| Management | Intervention | ||
|---|---|---|---|
| Stepped care (n = 81) | Minimal (n = 79) | Total | |
| Maintaining structure | |||
| Frequency, mean (SD) (min., max.) | 1.9 (0.7) (0, 4) | 1.8 (0.6) (1, 3) | 1.8 (0.6) (0, 4) |
| Quality, mean (SD) (min., max.) | 1.7 (0.8) (0, 4) | 1.6 (0.6) (0, 3) | 1.7 (0.7) (0, 4) |
| Agenda setting | |||
| Frequency, mean (SD) (min., max.) | 0.9 (0.6) (0, 2) | 0.8 (0.4) (0, 2) | 0.9 (0.5) (0, 2) |
| Quality, mean (SD) (min., max.) | 1.0 (1.0) (0, 4) | 0.8 (0.8) (0, 3) | 0.9 (0.9) (0, 4) |
| Consistency of problem focus | |||
| Frequency, mean (SD) (min., max.) | 1.9 (1.1) (0, 4) | 2.0 (1.1) (0, 4) | 2.0 (1.1) (0, 4) |
| Quality, mean (SD) (min., max.) | 1.9 (1.1) (0, 4) | 2.0 (1.1) (0, 4) | 2.0 (1.1) (0, 4) |
| End of session | |||
| Frequency, mean (SD) (min., max.) | 0.2 (0.5) (0, 2) | 0.1 (0.3) (0, 2) | 0.2 (0.4) (0, 2) |
| Quality, mean (SD) (min., max.) | 0.3 (0.8) (0, 3) | 0.0 (0.2) (0, 2) | 0.2 (0.6) (0, 3) |
| Tasks | Intervention | ||
|---|---|---|---|
| Stepped care (n = 81) | Minimal (n = 79) | Total | |
| Drinking: feedback/negative consequences | |||
| Frequency, mean (SD) (min., max.) | 0.9 (0.6) (0, 3) | 0.9 (0.4) (0, 2) | 0.9 (0.5) (0, 3) |
| Quality, mean (SD) (min., max.) | 0.2 (0.5) (0, 2) | 0.2 (0.4) (0, 2) | 0.2 (0.5) (0, 2) |
| Eliciting client concerns about drinking | |||
| Frequency, mean (SD) (min., max.) | 1.6 (1.1) (0, 4) | 0.6 (0.7) (0, 3) | 1.1 (1.0) (0, 4) |
| Quality, mean (SD) (min., max.) | 0.7 (0.8) (0, 2) | 0.3 (0.6) (0, 2) | 0.5 (0.7) (0, 2) |
| Eliciting self-efficacy for change | |||
| Frequency, mean (SD) (min., max.) | 0.7 (0.8) (0, 3) | 0.1 (0.3) (0, 1) | 0.4 (0.7) (0, 3) |
| Quality, mean (SD) (min., max.) | 0.7 (0.9) (0, 3) | 0.1 (0.3) (0, 2) | 0.4 (0.8) (0, 3) |
| Commitment to drinking goal | |||
| Frequency, mean (SD) (min., max.) | 1.3 (0.9) (0, 3) | 0.7 (0.6) (0, 2) | 1.0 (0.8) (0, 3) |
| Quality, mean (SD) (min., max.) | 1.1 (0.8) (0, 3) | 0.6 (0.6) (0, 2) | 0.9 (0.8) (0, 3) |
| Ambivalence | |||
| Frequency, mean (SD) (min., max.) | 0.3 (0.5) (0, 3) | 0.0 (0.0) (0, 0) | 0.1 (0.4) (0, 3) |
| Quality, mean (SD) (min., max.) | 0.3 (0.7) (0, 3) | 0.0 (0.0) (0, 0) | 0.2 (0.5) (0, 3) |
| Creating conflict | |||
| Frequency, mean (SD) (min., max.) | 0.1 (0.4) (0, 2) | 0.0 (0.0) (0, 0) | 0.1 (0.3) (0, 2) |
| Quality, mean (SD) (min., max.) | 0.1 (0.4) (0, 2) | 0.0 (0.0) (0, 0) | 0.1 (0.3) (0, 2) |
| Eliciting commitment to change drinking | |||
| Frequency, mean (SD) (min., max.) | 1.0 (0.7) (0, 3) | 0.2 (0.4) (0, 1) | 0.6 (0.7) (0, 3) |
| Quality, mean (SD) (min., max.) | 0.8 (0.8) (0, 3) | 0.2 (0.5) (0, 3) | 0.5 (0.7) (0, 3) |
| Eliciting optimism for change | |||
| Frequency, mean (SD) (min., max.) | 1.1 (0.8) (0, 3) | 0.4 (0.7) (0, 3) | 0.7 (0.8) (0, 3) |
| Quality, mean (SD) (min., max.) | 0.8 (0.8) (0, 3) | 0.3 (0.6) (0, 2) | 0.5 (0.8) (0, 3) |
| Styles | Intervention | ||
|---|---|---|---|
| Stepped care (n = 81) | Minimal (n = 79) | Overall | |
| Frequency reflective listening, mean (SD) (min., max.) | 2.6 (0.9) (0, 4) | 1.1 (0.9) (0, 3) | 1.9 (1.2) (0, 4) |
| Quality reflective listening, mean (SD) (min., max.) | 1.7 (0.6) (0, 3) | 1.1 (0.8) (0, 3) | 1.4 (0.8) (0, 3) |
| Frequency empathy, mean (SD) (min., max.) | 1.9 (0.9) (0, 4) | 1.3 (0.9) (0, 4) | 1.6 (1.0) (0, 4) |
| Quality empathy, mean (SD) (min., max.) | 1.9 (0.9) (0, 4) | 1.4 (1.0) (0, 4) | 1.7 (1.0) (0, 4) |
| Frequency unsolicited advice, mean (SD) (min., max.) | 2.9 (1.2) (0, 5) | 3.4 (1.0) (0, 4) | 3.2 (1.1) (0, 5) |
| Frequency open questions, mean (SD) (min., max.) | 2.7 (0.8) (0, 4) | 1.0 (0.9) (0, 3) | 1.9 (1.2) (0, 4) |
| Quality open questions, mean (SD) (min., max.) | 1.9 (0.5) (0, 3) | 1.4 (0.9) (0, 3) | 1.7 (0.8) (0, 3) |
| Frequency closed questions, mean (SD) (min., max.) | 3.1 (1.0) (1, 4) | 2.1 (1.1) (1, 4) | 2.6 (1.2) (1, 4) |
| Activity | Intervention | ||
|---|---|---|---|
| Stepped care (N = 81) | Minimal (N = 79) | Total (N = 160) | |
| Review AUDIT score, n (%) | 63 (79.7) | 74 (91.4) | 137 (85.6) |
| Obtain drinking account, n (%) | 78 (98.7) | 40 (49.4) | 118 (73.8) |
| Give correct advice/information, n (%) | 76 (96.2) | 81 (100.0) | 157 (98.1) |
| Set a target, n (%) | 28 (35.4) | 11 (13.6) | 39 (24.4) |
| Make a drinking plan, n (%) | 11 (13.9) | 6 (7.4) | 17 (10.6) |
| Measures | Intervention | ||
|---|---|---|---|
| Stepped care (20 minutes) | Minimal (5 minutes) | Total | |
| Length of session (minutes and seconds) | |||
| Mean (SD) | 19:41 (05:42) | 07:10 (01:59) | 13:21 (07:34) |
| Median (min., max.) | 19:38 (03:17, 34:30) | 06:57 (03:17, 12:57) | 11:06 (03:17, 34:30) |
| Task frequency | |||
| Mean (SD) | 0.88 (0.35) | 0.36 (0.20) | 0.62 (0.39) |
| Median (min., max.) | 0.88 (0.00, 1.88) | 0.38 (0.00, 0.88) | 0.50 (0.00, 1.88) |
| Task quality | |||
| Mean (SD) | 0.59 (0.41) | 0.21 (0.22) | 0.40 (0.38) |
| Median (min., max.) | 0.50 (0.00, 1.75) | 0.13 (0.00, 1.25) | 0.25 (0.00, 1.75) |
| Style frequency | |||
| Mean (SD) | 2.64 (0.47) | 1.78 (0.53) | 2.20 (0.66) |
| Median (min., max.) | 2.80 (1.20, 3.60) | 1.80 (0.40, 3.00) | 2.20 (0.40, 3.60) |
| Style quality | |||
| Mean (SD) | 1.87 (0.55) | 1.27 (0.66) | 1.56 (0.68) |
| Median (min., max.) | 2.00 (0.33, 3.00) | 1.33 (0.00, 2.67) | 1.67 (0.00, 3.00) |
Four summary measures were calculated; these were used in the analyses in addition to the time taken to complete the sessions. The summary measures were analysed using mixed models, with practice/research nurse fitted as a random effect. There was a significant difference in time of session between the 5- and 20-minute sessions. The average time for the 5-minute session was 422.57 seconds (7 minutes) and for the 20-minute session 1174.78 seconds (20 minutes).
The 20-minute sessions had significantly higher task frequency and task quality scores. The 20-minute sessions also had significantly higher style frequency and style quality scores. The results can be seen in Table 33.
| Measures | Stepped care intervention (20 minutes), mean (SD) | Minimal intervention (5 minutes), mean (SD) | Difference (95% CI) | p-value |
|---|---|---|---|---|
| Length of session (seconds) | 1174.78 (36.53) | 422.57 (35.68) | 752.22 (674.00 to 830.44) | p < 0.001 |
| Task frequency | 0.92 (0.04) | 0.40 (0.04) | 0.52 (0.44 to 0.61) | p < 0.001 |
| Task qualitya | 0.59 (0.04) | 0.21 (0.04) | 0.38 (0.27 to 0.48) | p < 0.001 |
| Style frequencya | 2.64 (0.06) | 1.78 (0.06) | 0.87 (0.71 to 1.02) | p < 0.001 |
| Style quality | 1.82 (0.10) | 1.22 (0.10) | 0.60 (0.43 to 0.77) | p < 0.001 |
When comparing the session content, there were significant differences between the two sessions on only two measures, ‘obtaining a drinking account’ and ‘setting a target’; both of these were more likely to be performed in the 20-minute sessions. The results of all of the analyses can be seen in Table 34.
| Activity | Stepped care (N = 81) | Minimal (N = 79) | Odds ratio (95% CI) | p-value |
|---|---|---|---|---|
| Review AUDIT score, n (%) | 63 (79.7%) | 74 (91.4%) | 0.27 (0.05 to 1.42) | 0.123 |
| Obtain drinking account, n (%) | 78 (98.7%) | 40 (49.4%) | 71.7 (4.00 to 1283.6) | 0.004 |
| Give correct advice/information, n (%)a | 76 (96.2%) | 79 (100.0%) | 0.118 | |
| Set a target, n (%) | 28 (35.4%) | 11 (13.6%) | 3.41 (1.49 to 7.80) | 0.004 |
| Make a drinking plan, n (%) | 11 (13.9%) | 6 (7.4%) | 1.88 (0.81 to 4.35) | 0.140 |
The analysis of the summary measures was repeated but this time it included a variable to represent specialist practitioners. When comparing the session rating scores for specialist and non-specialist practitioners there were no significant differences found. The full results can be seen in Table 35.
| Rating | Non-specialist, mean (SD) | Specialist, mean (SD) | Difference (95% CI) | p-value |
|---|---|---|---|---|
| Task frequency | 0.69 (0.04) | 0.61 (0.05) | 0.08 (−0.07 to 0.22) | 0.260 |
| Task qualitya | 0.42 (0.04) | 0.39 (0.04) | 0.03 (−0.07 to 0.14) | 0.135 |
| Style frequency | 2.29 (0.08) | 2.18 (0.12) | 0.11 (−0.21 to 0.43) | 0.459 |
| Style quality | 1.46 (0.11) | 1.65 (0.17) | −0.18 (−0.62 to 0.26) | 0.384 |
Reliability of ratings
A sample of the recordings was rated by two raters. Inter-rater reliability of the individual frequency items of the summary scores was examined using the ICC two-way mixed-effects model (case 3). 67 For the four summary scores, the average of the two raters’ summary scores was plotted against the difference in their summary scores69 to make pairwise comparisons between raters (Figure 10).
FIGURE 10.
Bland–Altman plots.
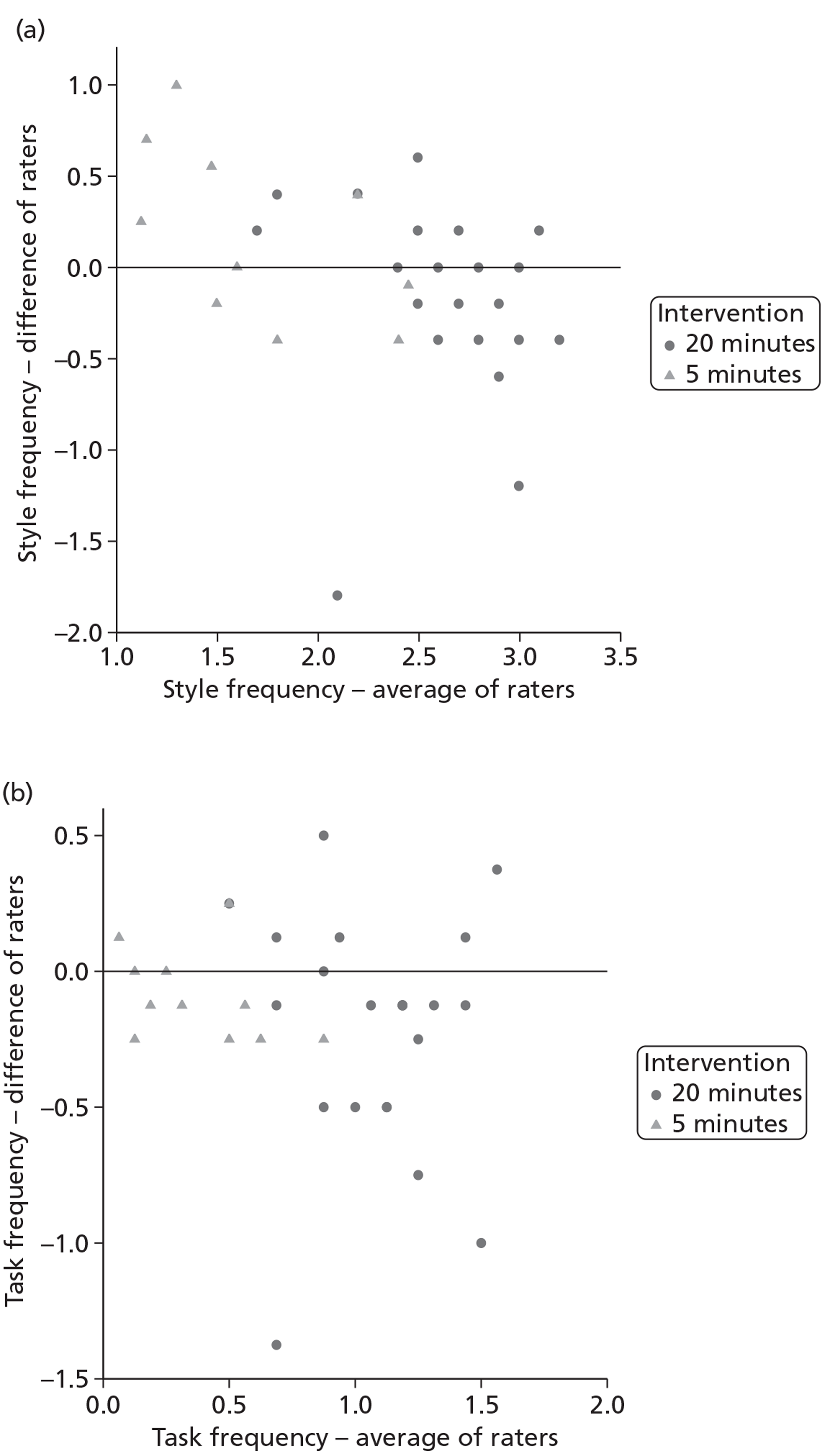
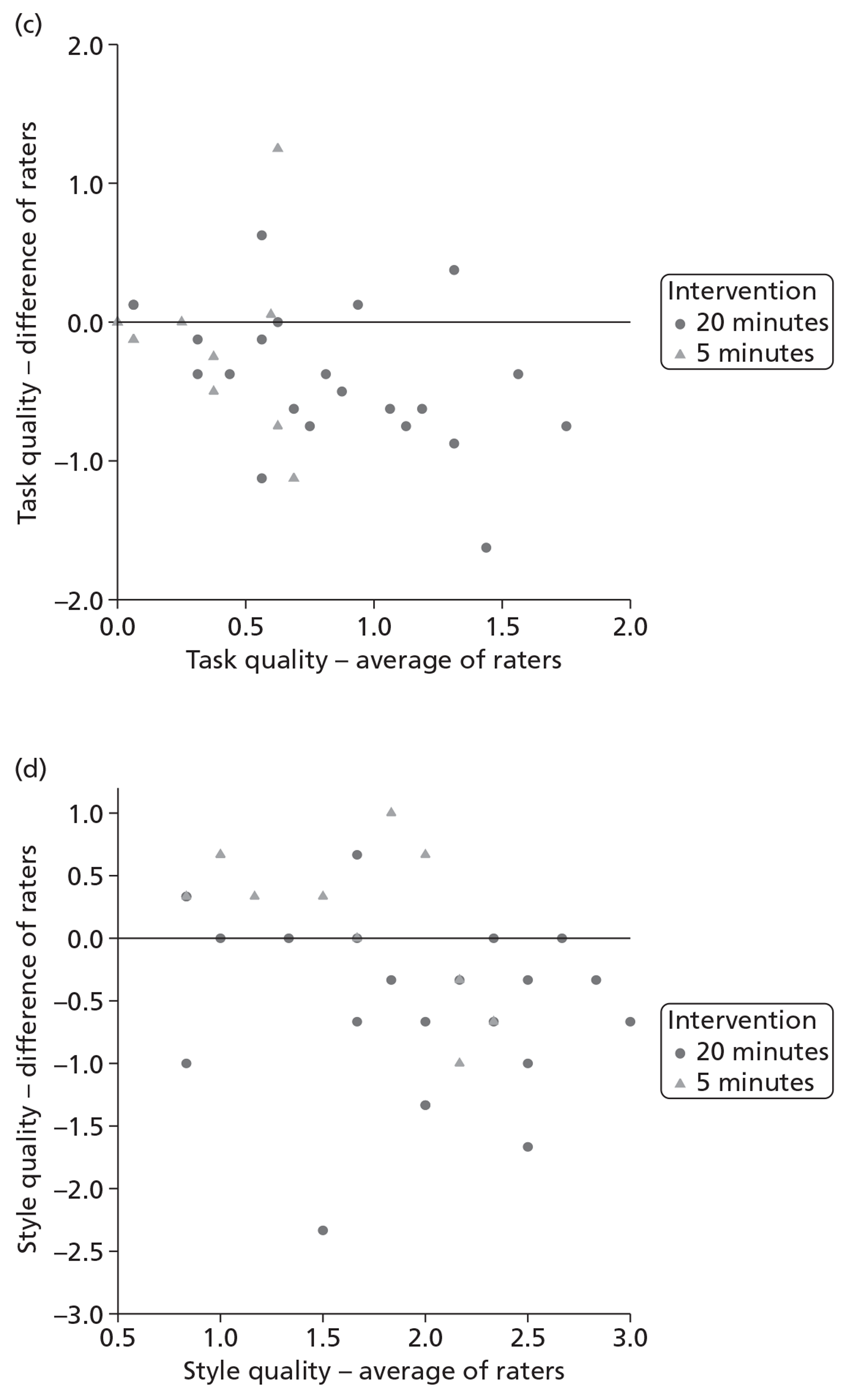
The ICCs for the summary measures ranged from 0.64 to 0.81 (Table 36), which indicates acceptable levels of agreement. 67
| Rating and raters | n | Mean rating (SD) | ICC (95% CI) |
|---|---|---|---|
| Task frequency | |||
| Rater 1 | 33 | 0.76 (0.45) | 0.815 (0.624 to 0.908) |
| Rater 2 | 33 | 0.93 (0.50) | |
| Task quality | |||
| Rater 1 | 33 | 0.51 (0.44) | 0.670 (0.332 to 0.837) |
| Rater 2 | 33 | 0.82 (0.64) | |
| Style frequency | |||
| Rater 1 | 33 | 2.30 (0.54) | 0.736 (0.465 to 0.869) |
| Rater 2 | 33 | 2.41 (0.81) | |
| Style quality | |||
| Rater 1 | 33 | 1.67 (0.60) | 0.640 (0.271 to 0.822) |
| Rater 2 | 33 | 1.95 (0.85) | |
The Bland–Altman plots for each of the summary measures compare the two raters (Figure 10). A positive difference indicates that the second rater scores higher than the first rater. A negative difference indicates than the second rater scores lower than the first.
Summary
The scale identified significant differences between the 5- and 20-minute interventions, indicating that the two types of session were distinct. Validation of the rating showed an acceptable level of agreement between the raters. There were no significant differences in the rating scores between practice/research nurses with different levels of experience (specialist vs non-specialist practitioners).
Chapter 7 Discussion
Here we report the results of a large trial of a stepped care intervention versus a minimal intervention in the management of older hazardous alcohol users in primary care.
Six published systematic reviews focus specifically upon the effectiveness of brief interventions in primary care populations28–33 although many of the studies excluded older patients. There are no systematic reviews or subgroup analyses specifically focusing on older patient groups. This trial aimed to fill that gap and was conducted and reported in accordance with international guidelines for research excellence. 98–101
One prompt to conduct this trial was research indicating the underdetection and misdiagnosis of hazardous alcohol use in older populations. 16,17 It is generally considered that the prevalence of hazardous or harmful alcohol consumption in those aged ≥ 55 years is lower than the general population. Indications are that prevalence is between 15% and 25% of the general population. 11 However, the findings of this study found the prevalence of hazardous drinking in the older population to be less than suggested by previous research. Only 7.5% of those screened were positive on the AUDIT screening questionnaire and the mean AUDIT score of those positive was 12.10 (SD 5.65).
The issues encountered in this trial highlight the fact that research can be difficult to conduct in primary care owing to a number of issues including staff time and workloads. However, the eventual successful recruitment appears to suggest that older populations are as willing and able to engage in research evaluations as the general population and, as demonstrated by the impressive questionnaire return rates, display a greater willingness to be followed up. This concurs with previous research that suggested that older populations are more compliant with follow-up protocols than younger populations. 38,56
Key findings
This study aimed to compare the effects of a minimal intervention (a 5-minute, brief advice with the practice/research nurse involving feedback of the results of the screening and discussion regarding the health consequences of continued hazardous alcohol consumption) with a stepped care intervention (progression to next step determined by reassessment of alcohol consumption after each previous step). The primary hypothesis was that a stepped care intervention reduced alcohol consumption in older hazardous alcohol users compared with a minimal intervention post randomisation. We found no evidence of a difference in the ADD after 12 months of older hazardous alcohol users when comparing the stepped care group with the minimal intervention group at month 12 [stepped care 1.129 (SD 0.037) vs minimal intervention 1.104 (SD 0.037)]. At month 6, the stepped care group had a lower ADD than the minimal interventions group, but not significantly so.
When adjusting for baseline scores and including GP as a random effect, there was no evidence of any differences in ADD at month 6, or AUDIT-C score or the DPI score at month 6 or month 12.
We investigated changes in HRQoL from baseline using the SF-12. 59 Our results showed that the stepped care group had both lower MCS and lower PCS than the minimal intervention group at months 6 and 12, although the differences were not significant at the 5% level. We cannot conclude that stepped care has any impact on HRQoL.
We evaluated whether or not stepped care was a more cost-effective treatment for the management of older alcohol users in primary care and the results revealed that, with longer follow-ups, stepped care generated greater cost savings (£194 at month 12 vs £6.38 at month 6) and greater QALY gains (0.0117 at month 12 vs 0.0058 at month 6).
The probability that stepped care was cost-effective was higher for the month 12 follow-up period (93.5–93.8%) than the month 6 follow-up (81–86%) under a conventional WTP threshold of £20,000–30,000 per QALY. This indicated that participants may benefit more from stepped care in the longer term with the reduction of alcohol-related health problems in the future. The analysis indicated that the net monetary benefit did not significantly differ by GP practice. A few ‘extreme values’ were present in the distribution (those deviating by five times the standard deviation) which were excluded, and the incremental cost effectiveness analysis was repeated in a sensitivity analysis. The sensitivity analysis altered the average costs of interventions; however, there was still over 80% certainty that stepped care is more cost-effective than minimal intervention months using the £20,000–30,000 per QALY gained threshold.
The health economic findings raise important issues when contrasted with the null clinical effectiveness findings and some further consideration of the reasons why this may occur is needed.
Firstly, in economic evaluation, an ICER combines the costs of the interventions with health outcomes. The statistic of interest is the ICER, which is estimated on the basis of four statistics from two samples: the costs of the control and intervention groups (CC and CI) and the effects of the control and intervention groups (EC and EI). This allows us to take advantage of the power to detect a difference in the joint cost-effectiveness outcome. In some cases, the power to detect a difference in this joint outcome exceeds the power to detect differences in either cost or effect alone. It is possible for a study to show no difference in clinical outcomes between interventions, but also to conclude that one intervention is more cost-effective than another, because that intervention costs less when the intervention's costs and subsequent service use are taken into account.
Secondly, the decision rule in an economic evaluation differs from traditional effect analysis. We make decisions to adopt a health technology by comparing the estimated cost-effectiveness ratio with a predefined standard or threshold value; for example, the decision-making threshold used by NICE is £20,000–30,000 per additional QALY gain. Whether or not an intervention is cost-effective varies when the decision threshold changes. Whereas in statistics an intervention is considered to be more effective if the p-value of the difference is lower than 0.05, this is not the case in an economic evaluation.
Thirdly, the traditional way of interpreting the CI may sometimes be interpreted in different ways in economic evaluation. For example, a negative ICER can arise as a result of two completely different situations. One scenario is that the intervention is more effective and less expensive. On the other side, the intervention may have a greater cost and a worse effect (cost-ineffective intervention). Therefore, we use CEACs to summarise the evidence in support of the intervention being cost-effective for all potential values of the decision rules. The CEAC presents much more information on uncertainty than it does on CIs in economic analysis, as it presents the probability that the intervention is more effective than the control at different values for a decision-maker's threshold, for example if willing to pay different values to gain one QALY.
It may be the case that the economic analysis indicates that with a far greater sample size a difference in effect of the interventions may become apparent. The sample analysed at the final outcome stage was greater than that estimated in the original sample size calculation and any small effect difference derived from a far larger sample would be unlikely to be a clinically important difference.
Consideration of possible explanations
The importance of the actual screening process itself cannot be excluded as having a possible impact on the alcohol consumption of some trial participants. Previous studies and reviews have reported possible reactivity to such assessments,77,102–105 although the exact effect is difficult to separate from the study interventions. The fact that the study involved the proactive opportunistic identification of people consuming alcohol at levels that may be detrimental to their health precluded the inclusion of a no-treatment control. Ethical considerations meant that the study compared two active interventions. The control was a minimally acceptable brief intervention and the provision of an information leaflet. The population identified using opportunistic screening exhibited lower levels of alcohol use and lower levels of alcohol-related problems than treatment-seeking populations and both groups reduced consumption over the 12 months. It may be the case that more intensive interventions in this population are no more effective than minimal interventions and similar results have been found in both systematic reviews and primary research. 28,34,106
The majority of participants engaged with both the minimal intervention and step 1 of the stepped care intervention and written comments on questionnaires were overwhelmingly positive in describing these. Of note was the fact that two-thirds of those assessed as eligible for referral to step 2 either cancelled or failed to attend. These findings are similar to other studies of stepped care for alcohol use in primary care,13 extended alcohol interventions for alcohol use in primary care106 and referrals for interventions in emergency departments. 107 It may be the case that extended multiple session interventions are not considered acceptable to a large proportion of the non-treatment-seeking population, many of whom are consuming alcohol at levels towards the lower end of the severity spectrum.
The use of the same practice/research nurse to deliver both the minimal intervention and step 1 of the stepped care intervention could have resulted in contamination due to the distinction between the interventions blurring and elements from each being found in the other. Verification of intervention fidelity not only ensures that internal validity of a study is maintained but also that external validity is enhanced. 108 This was achieved in AESOPS by each of the treatment sessions being recorded and also the fact that the PRS that was subsequently conducted did indeed identify significant differences between the 5- and 20-minute interventions, indicating that the two types of session were distinct. Therefore, in this study, having the same practice/research nurse deliver the minimal intervention and step 1 of the stepped care intervention is not considered to have affected the outcome.
In the early stages of the study, some problems had been encountered with participating practice nurses finding little time available to see study participants. As the recruitment methods changed, and an increase in potential participants identified was likely, the use of research/specialist practitioners was required (although the task of preparing and sending out the mailings did not involve the practice nurses). The possibility that these two groups (non-specialist and specialist) would be delivering the interventions differently was not proven by the PRS, where no significant differences in the rating scores between practice/research nurses with different levels of experience were found. We therefore do not consider this to have influenced the result in any way.
In the original study design we envisaged opportunistically screening patients as they attended the primary care centre and this being conducted by practice staff. In reality, difficulties in recruiting practices willing to engage in this process meant that the methods of recruitment had to be amended to include mail-out screening and the use of specialist study staff to intervene with the eligible population. This is an indication that opportunistic screening and the delivery of brief interventions embedded within primary care are not currently acceptable to primary care staff.
Comparison with previous research
Previous screening and intervention studies looking at alcohol use conducted in UK health-care settings52,57 suggested that 80% of those screened positive tend to be eligible and 75% of those eligible tend to consent to randomisation. In this study we found that only 57% of eligible patients consented to randomisation. Although still to be explored, it did not go unnoticed that a number of patients, either by written comment or by telephone, expressed the feeling that the time of the researchers would be better spent tackling binge-drinking and the perceived alcohol problems in the younger ages groups often highlighted in the media.
In addition, the prevalence of hazardous alcohol consumption, inclusive of harmful consumption and possible dependence, in those aged ≥ 55 years was estimated at 15% in the general population11 and greater, at 25%, in those attending primary care. 13 Screening results from this study found this to be only 7.5%.
The results of the clinical effectiveness aspects of the study appear to concur with recent research in the area. The population in question was an opportunistically identified population at the lower end of the alcohol use disorder spectrum. A recent systematic review of brief interventions in primary care28 found no additional benefit of more intensive versus briefer interventions. Primary research in the UK found no additional benefit of extensive brief lifestyle counselling over and above brief advice and the provision of an information leaflet for a general population identified opportunistically using AUDIT in primary care;106 in addition, a recent US study comparing minimal intervention with more intensive intervention for older alcohol users found that although alcohol use reduced in both groups there were no significant differences between the groups. 34 General population studies in primary care have established the benefits of brief interventions over and above no treatment controls28 and similar results have been reported for older people in primary care. 35,36
MEDLINE, EconLit, and NHS Economic Evaluation Database were searched for economic evaluations of alcohol treatments. Fewer than 30 full economic evaluations that compare both the costs and health consequences were found in literature. Only one trial (STEPWICE) was available that compared cost-effectiveness between stepped care and a 5-minute advice session. 51 The result of the cost-effectiveness analysis of the STEPWICE trial was very similar to AESOPS, but with a much smaller sample size (n = 112). Both studies found that stepped care was more likely to be cost-effective compared with minimal intervention using the NICE threshold range of £20,000–30,000 per QALY gained.
There are several difficulties when making direct comparisons between existing economic evaluations of alcohol treatments. Firstly, the definition of the interventions varies between studies. For example, ‘brief intervention’ was used as a common comparator in clinical trials109–112 but studies define brief intervention differently in terms of contact length, content and style. 113 Secondly, the evaluation of health consequences differs among studies. For instance, the most widely used outcome measurements were drinks per drinking day, binge-drinking episodes or heavy drinking, and percentage of days abstinent. Although some economic evaluations used QALYs as a health outcome measure following NICE's guidance71,114 none focuses on a similar population or interventions to the AESOPS study.
Strength and limitations of the study
Although we had originally estimated that we would recruit 500 participants from 15 GP practices in three sites over an 18-month period, it took twice this time and many more GP practices: 53 across eight sites. However, we did successfully recruit our target number of older hazardous alcohol users. Our finding of no evidence of a difference is not likely to be due to a lack of power.
As a result of the initial slow recruitment rate, a change in recruitment method was required, moving away from the original design of opportunistic screening in GP practice waiting rooms to the adoption of the more extensive method of mailing out forms to all patients aged ≥ 55 years on a participating GP practice register. This change, however, not only provided evidence of the limitations of using opportunistic screening at practice attendance as a recruitment method, but also allowed us to estimate a much more robust prevalence rate of hazardous alcohol consumption in this patient group, while also resulting in our recruitment target being met.
The lower than expected prevalence rate may be due to response bias, whereby those who are consuming alcohol at higher levels are less likely to respond to the AUDIT questionnaire and participate in the study. Yet participants were given an option to respond anonymously and those who did so had lower mean AUDIT scores than those who provided contact details, which may add further weight to the idea that the prevalence figure identified in the study is indicative of the true population prevalence rate. In addition, the study population was generalisable to the population of older alcohol users who were willing to be screened and to engage in an intervention to address their alcohol use.
In addition, there was a reluctance on the part of the primary care nurses to undertake these interventions, and reluctance on the part of the GPs to support their practice nurses in doing this. These issues individually, and together, made it difficult to pursue the protocol as designed without having to adapt in some way. This does, however, have important implications regarding the question of whether or not these sorts of interventions can be implemented in the primary care setting and it does not seem to be the case that practice nurses are enthusiastic about their delivery.
One limitation of this study was the low take-up by those referred to step 2 of the stepped care intervention. The precise reasons for this are unknown but a number of factors could be involved. These include possible unwillingness of participants to accept that their alcohol consumption was having a detrimental impact on their health, particularly if they consumed alcohol at lower levels of severity; unwillingness to reduce consumption any more than agreed in the initial session; or unwillingness or unavailability to attend more than one session. There may also be an issue with the time lag between identification of the problem and the intervention taking place, resulting in attendance being less likely.
Our previous experience in conducting RCTs in the fields of substance use (UKCBTMM), alcohol-using general adult populations (UKATT, STEPWICE) and elderly populations (RESPECT) indicate that, with rigorous follow-up regimes, loss to follow-up at 12 months would usually be in of the order of 20%. Taking these factors into account we had erred on the side of caution and allowed an attrition rate of 30%. In fact, in AESOPS the overall follow-up rate at month 12 was 87.5%, with 86.8% followed up in the stepped care group and 88.2% in the minimal intervention group.
In the cost-effectiveness analysis we made very conservative assumptions to ensure that the costs of the stepped care intervention were not underestimated. We did not take into account the possibility that interventions may prevent and reduce alcohol-related disease and injury, which may result in considerable cost savings, especially if these costs and expected impacts on health status were modelled over a longer time period.115 The absence of any criminal justice events within this study does suggest that the economic consequences of hazardous and harmful alcohol consumption could be different for older people, but this would need further investigation. It should also be noted that the training costs for practice nurses to deliver minimal intervention were not included in the control group. We assumed that they had already received training to deliver brief advice during their student nurse or early career training. If this part of the training cost was added into the control group, the minimal intervention would turn out to be even more costly compared with stepped care.
Generalisability of the results
The AESOPS recruited from eight sites and 53 GP practices across England and Scotland, including both urban and rural locations, in GP practices of varying sizes. With the population group within AESOPS, we are confident that these results are broadly generalisable to the population who would engage in screening and intervention for alcohol use problems in general practice.
Implications for health care
The evidence from this trial shows that there is no clinical advantage of opportunistic screening in addition to stepped care over opportunistic screening and minimal intervention in terms of the reduction in alcohol consumption at 12 months post intervention in hazardous alcohol users aged ≥ 55 years who score ≥ 8 on the AUDIT, but there is some evidence that it may be cost-effective.
Implications for research
The experience of conducting this research in primary care settings, the implication that extended interventions are not acceptable to participants or practice staff in the management of hazardous alcohol use and have no additional benefit over screening and minimal interventions, the potential to target those with more entrenched harmful or possible dependent alcohol use who are not seeking treatment and the finding that stepped care interventions appear to produce economic benefits have the following implications for future research:
-
What factors facilitate or hinder the conduct of research in primary care settings?
-
What is the effectiveness and cost-effectiveness of community-based screening and self-directed ultra-brief interventions for hazardous alcohol users compared with screening alone?
-
What is the effectiveness and cost-effectiveness of MET for non-treatment-seeking harmful and possibly dependent alcohol users delivered in primary care?
-
What are the longer-term clinical and economic impacts of stepped care interventions?
Acknowledgements
Huge thanks to the participants for taking part in this trial, as well as the practice nurses and research nurses for recruiting participants and completing the trial documentation. Thank you to the GPs, practice managers and practice administration staff. We would also like to acknowledge the Principal Investigators at each site for co-ordinating participant recruitment: York: Dr Alan Hassey; Leeds: Dr Duncan Raistrick; Hull and East Riding: Mr Tom Philips; Norfolk: Dr Daphne Rumball; Fife: Dr Alex Baldacchino; Kent: Dr John Cunningham and Dr Chris Fox; Tyneside: Dr Paul Cassidy and David Henderson; County Durham: Dr James Larcombe. We would like to thank the Primary Care Research Networks (Northern and Yorkshire; South East; East of England), Scottish Primary Care Research Network, SPHERE network (Norfolk, Great Yarmouth and Waveney), Kent and Medway NIHR Comprehensive Local Research Network, West Yorkshire Mental Health and Learning Disabilities Research Partnership and Leeds Partnership NHS Foundation Trust Research and Development Department for their help with recruiting GP practices and carrying out practice mail-outs. Finally we would like to express our gratitude to the Trial Steering Committee and Data Monitoring and Ethics Committee members for overseeing the study.
We would specifically like to thank:
Sue Allen, Lynne McAnally, Loraine Leggett, Sue Marshall, Julia Bundock, Monica Lloyd, Mary Stevenson, Polly Smith, Judith Campbell, Amanda Stevenson, Laura Stevenson, Jacqui Alland, Helen Walker, Cheryl Richardson, Sandra Simpson, June Agius, Marie Fullard, Cheryl Poole, Diane Hartley, Lisa Tindall, Ellie Gordon, Jane Boreham, Tina Taylor, Sarah Grey, Kerri Wright, Hayley Peacock, Lily Prestwood, Karon O'Flanagan, Lynne Heaney, Malcolm Bray, Kathryn Lievesley, Julie Caldwell, John Tomlinson, Jon McGowan and Linda Burr.
Collaborations and contributions of the authors
The AESOPS collaborators (current and past) are Martin Bland, Simon Coulton, Helen Crosby, Ben Cross, Veronica Dale, Colin Drummond, Christine Godfrey, Alan Hassey, Eileen Kaner, Jenny Lang, Ruth McGovern, Dorothy Newbury-Birch, Jo Orchard, Steve Parrott, Tom Phillips, Duncan Raistrick, Daphne Rumball, Gillian Tober, Valerie Wadsworth, Judith Watson and Qi Wu.
Simon Coulton was the chief investigator, chaired the Trial Management Group, edited and approved the final draft of the report.
Judith Watson was the trial manager.
Veronica Dale and Martin Bland designed the clinical analysis.
Veronica Dale conducted the clinical analyses and oversaw the process ratings analyses.
Qi Wu conducted the economic analyses.
Helen Crosby conducted the process ratings and analyses.
Gillian Tober developed the training, supervised the practice/research nurses and therapists and oversaw the process ratings.
Jenny Lang contributed to and co-ordinated recruitment to the trial in Leeds.
Dorothy Newbury-Birch and Ruth McGovern contributed to the study design and co-ordination and managed the Tyneside and County Durham recruitment to the trial.
Steve Parrott oversaw the conduct of the economic analyses.
Martin Bland oversaw the conduct of the analysis.
Colin Drummond, Christine Godfrey and Eileen Kaner contributed to the study design, conduct of the trial and the Trial Management Group.
Independent Steering Committee Members
Professor Oliver James – chairperson of Independent Steering Committee from August 2007 to end.
Professor Graham Dunn – member of Independent Steering Committee from August 2007 to end.
Professor Helen Smith – member of Independent Steering Committee from June 2009 to end.
Mrs Valerie Lipman – consumer representative and member of Independent Steering Committee from August 2007 to March 2008.
Disclaimer
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Calling Time: The Nation’s Drinking as a Major Health Issue. London: Academy of Medical Sciences; 2004.
- Coulton S. Alcohol use disorders in older people. Rev Clin Gerontol 2009;19:217-25. http://dx.doi.org/10.1017/S0959259809990244.
- Sensible Drinking. The Report of an Inter-departmental Working Group. London: Department of Health; 1995.
- Wright F, Whyley C. Accident prevention and risk taking by elderly people: The need for advice. London: Age Concern; 1994.
- Alcohol. Can the NHS afford it?. London: RCP; 2002.
- Thomas V, Rockwood K. Alcohol abuse, cognitive impairment and mortality among older people. J Am Geriatr Soc 2001;49:415-20. http://dx.doi.org/10.1046/j.1532-5415.2001.49085.x.
- Crome P, Glass I. The International Handbook of Addiction Behaviour. London: Routledge; 1991.
- Falaschetti E, Malbut K, Primatesta P, Prior G, Primatesta P. Health Survey for England HMSO. London: Her Majesty's Stationery Office; 2002.
- Moore AA, Whiteman EJ, Ward KT. Risks of combined alcohol/medication risks in older adults. Am J Geriatric Pharmacother 2007;5:64-7. http://dx.doi.org/10.1016/j.amjopharm.2007.03.006.
- Woodhouse P, Keating W, Coleshaw S. Factors associated with hypothermia in patients admitted to a group of inner city hospitals. Lancet 1987;2:1201-5.
- Drummond C, Oyefeso A, Phillips T, Cheeta S, Deluca P, Winfield H, et al. National Alcohol Research Project: The 2004 national alcohol needs assessment of England. London: Department of Health; 2005.
- General Household Survey 2007. Newport: Office for National Statistics; 2009.
- Coulton S, Drummond C, James D, Godfrey C, Bland JM, Parrott S, et al. Stepwice Research Team. Opportunistic screening for alcohol use disorders in primary care: comparative study. BMJ 2006;332:511-7. http://dx.doi.org/10.1136/bmj.38743.421574.7C.
- The Government's Alcohol Strategy. Norwich: The Stationary Office; 2012.
- Cheeta S, Drummond C, Oyefeso A, Phillips T, Deluca P, Perryman K, et al. Low identification of alcohol use disorders in general practice in England. Addiction 2008;103:766-73. http://dx.doi.org/10.1111/j.1360-0443.2008.02198.x.
- Callahan C, Tierney W. Health service use and mortality among older primary care patients with alcoholism. J Am Geriatr Soc 1995;43:1378-83.
- Reid M, Anderson P. Geriatric substance use disorders. Med Clin North Am 1997;81:999-1016. http://dx.doi.org/10.1016/S0025-7125(05)70560-5.
- Johnson SL, Tabaei BP, Herman WH. The efficacy and cost of alternative strategies for systematic screening for Type 2 diabetes in the U.S. population 45–74 years of age. Diabetes Care 2005;28:307-11. http://dx.doi.org/10.2337/diacare.28.2.307.
- Tobin C, Aggarwal R, Clarke J, Chown R, King D. Chlamydia trachomatis: opportunistic screening in primary care. Br J Gen Prac 2001;51:565-6.
- Selzer ML. The Michigan Alcoholism Screening Test (MAST): The quest for a new diagnostic instrument. Am J Psychiatry 1971;127:1653-8.
- Patton R, Crawford M, Touquet R. Hazardous drinkers in the accident and emergency department: who accepts advice?. Emerg Med J 2004;21:491-2. http://dx.doi.org/10.1136/emj.2003.011403.
- Hodgson R, Alwyn T, John B, Thom B, Smith A. The FAST Alcohol Screening Test. Alcohol Alcohol 2002;37:61-6. http://dx.doi.org/10.1093/alcalc/37.1.61.
- Canagasaby A, Vinson DC. Screening for hazardous or harmful drinking using one or two quantity-frequency questions. Alcohol Alcohol 2005;40:208-13. http://dx.doi.org/10.1093/alcalc/agh156.
- Saunders J, Aasland O, Babor T, de la Fuente J, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption–II. Addiction 1993;88:791-804. http://dx.doi.org/10.1111/j.1360-0443.1993.tb02093.x.
- Philpot M, Pearson N, Petratou V, Dayanandan R, Silverman M, Marshall J. Screening for problem drinking in older people: A comparison of CAGE and AUDIT. Age Ageing 2003;7:171-5.
- Crawford M, Patton R, Touquet R, Drummond C, Byford S, Barrett B, et al. Screening and referral for brief intervention of alcohol misusing patients in an Accident and Emergency Department: a pragmatic randomised controlled trial. Lancet 2004;364:1334-39. http://dx.doi.org/10.1016/S0140-6736(04)17190-0.
- Wallace P, Cutler S, Haines A. Randomised controlled trial of general practitioner intervention in patients with excessive alcohol consumption. BMJ 1988;297:663-8. http://dx.doi.org/10.1136/bmj.297.6649.663.
- Kaner E, Beyer F, Dickinson H, Pienaar E, Campbell F, Schlesinger C, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev 2007;2.
- Bertholet N, Daeppen J-B, Wietlisbach V, Fleming M, Burnand B. Reduction of Alcohol Consumption by Brief Alcohol Intervention in Primary Care. Arch Intern Med 2005;165:986-95. http://dx.doi.org/10.1001/archinte.165.9.986.
- Ballesteros J, Duffy JC, Querejeta I, Arino J, Gonzalez-Pinto A. Efficacy of brief interventions for hazardous drinkers in primary care: systematic review and meta-analyses. Alcohol Clin Exp Res 2004;28:608-18. http://dx.doi.org/10.1097/01.ALC.0000122106.84718.67.
- Whitlock EP, Polen MR, Green CA, Orleans T, Klein J. Behavioral counselling interventions in primary care to reduce risky/harmful alcohol use by adults: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med 2004;140:557-68.
- Poikolainen K. Effectiveness of brief interventions to reduce alcohol intake in primary health care populations: a meta analysis. Prev Med 1999;28:503-9. http://dx.doi.org/10.1006/pmed.1999.0467.
- Kahan M, Wilson L, Becker L. Effectiveness of physician-based interventions with problem drinkers: a review. Can Med Assoc J 1995;152:851-9.
- Moore A, Blow F, Hoffing M, Welgreen S, Davis J, Lin J, et al. Primary care based interventions to reduce at-risk drinking in older adults: a randomized controlled trial. Addiction 2011;106:111-20.
- Fleming M, Manwell L, Barry K, Adams W, Stauffacher E. Brief physician advice for alcohol problems in older adults. J Fam Pract 1999;48:378-84.
- Blow F, Barry K. Older patients with at risk and problem drinking patterns: New developments in brief interventions. J Geriatr Psychiatry Neurol 2000;13:115-23.
- Curtis J, Geller G, Stokes E. Characteristics, diagnosis and treatment of alcoholism in elderly patients. J Am Geriatr Soc 1989;37:310-16.
- Oslin D, Pettinati H, Volpicelli J. Alcoholism treatment adherence. Am J Geriatr Psychiatry 2002;10:740-7.
- Latimer N, Guillaume L. Prevention and early identification of alcohol use disorders in adults and young people: screening and brief interventions: cost effectiveness review. Sheffield: ScHARR Public Health Collaborating Centre; 2009.
- Rollnick S, Mason P, Butler C. Health Behaviour Change: A Guide for Practitioners. Edinburgh: Churchill Livingstone; 1999.
- Deehan A, Marshall EJ, Strang J. Tackling alcohol misuse: opportunities and obstacles in primary care. Br J Gen Pract 1998;48:1779-82.
- Menninger J. Assessment and treatment of alcoholism and substance related disorders in the elderly. Bull Menninger Clin 2002;66:166-84. http://dx.doi.org/10.1521/bumc.66.2.166.23364.
- Project MATCH Research Group Matching Alcoholism Treatments to Client Heterogeneity: Project MATCH post treatment drinking outcomes . J Stud Alcohol 1997;58:7-29.
- UKATT Research Team: Effectiveness of treatment for alcohol problems . BMJ 2005;331. http://dx.doi.org/10.1136/bmj.331.7516.541.
- Simon G, Katon W, Von Korff M, . Cost-effectiveness of a collaborative care program for primary care patients with persistent depression. Am J Psychiatry 2001;158:1638-44. http://dx.doi.org/10.1176/appi.ajp.158.10.1638.
- Scogin F, Hanson A, Welsh D. Self-administered treatment in stepped-care models of depression treatment. J Clin Psychol 2003;59:341-9. http://dx.doi.org/10.1002/jclp.10133.
- Abrams D, Orleans C, Niaura R, . Integrating individual and public health perspectives for treatment of tobacco dependence under managed health care: a combined stepped care and matching model. Ann Behav Med 1996;18:290-304. http://dx.doi.org/10.1007/BF02895291.
- Von Korff M, Moore J. Stepped care for back pain: activating approaches for primary care. Ann Intern Med 2001;134:911-17.
- Breslin F, Sobell M, Sobell L, . Problem drinkers: evaluation of a stepped-care approach. J Subst Abuse 1999;10:217-32. http://dx.doi.org/10.1016/S0899-3289(99)00008-5.
- Sobell M, Sobell L. Stepped care as a heuristic approach to the treatment of alcohol problems. J Consult Clin Psychol 2000;68:573-9. http://dx.doi.org/10.1037/0022-006X.68.4.573.
- Drummond C, Coulton S, James D, Godfrey C, Parrott S, Baxter J, et al. Effectiveness and cost-effectiveness of a stepped care intervention for alcohol use disorders in primary care: pilot study. Br J Psychiatry 2009;195:448-56. http://dx.doi.org/10.1192/bjp.bp.108.056697.
- The effectiveness and cost-effectiveness of screening and stepped care interventions for alcohol use disorders in the primary care setting. Cardiff: Welsh Office of Research & Development; 2003.
- Moyer A, Finney JW, Swearingen CE, Vergun P. Brief interventions for alcohol problems: a meta-analytic review of controlled investigations in treatment-seeking and non-treatment seeking populations. Addiction 2002;97:279-92. http://dx.doi.org/10.1046/j.1360-0443.2002.00018.x.
- Gordon A, Conigliaro J, Maisto S, McNeil M, Kraemer K, Kelley M. Comparison of consumption effects of brief interventions for hazardous drinking elderly. Subst Use Misuse 2003;38:1017-35. http://dx.doi.org/10.1081/JA-120017649.
- Lemke S, Moos R. Outcomes at 1 year and 5 years for older patients with alcohol use disorders. J Subst Abuse Treat 2003;24:43-50. http://dx.doi.org/10.1016/S0740-5472(02)00321-5.
- Atkinson R, Beresford T, Gomberg E. Alcohol and Aging. New York, NY: Oxford University Press; 1995.
- Heather N, Rollnick S, Bell A, Richmond R. Effects of brief counselling among male heavy drinkers identified on general hospital wards. Drug Alcohol Rev 1996;15:29-38. http://dx.doi.org/10.1080/09595239600185641.
- Finney J, Moos R, Brennan J. The Drinking Problems Index: An instrument to assess alcohol related problems amongst older adults. J Subst Abuse 1991;3:395-404. http://dx.doi.org/10.1016/S0899-3289(10)80021-5.
- Jenkinson C, Layte R. Development and testing of the UK SF-12 (short form health survey). J Health Serv Res Policy 1997;2:14-8.
- The EuroQol Group . EuroQol–a new facility for the measurement of health-related quality of life. The EuroQol Group. Health Policy 1990;16:199-208. http://dx.doi.org/10.1016/0168-8510(90)90421-9.
- Ware JE, Kosinski N, Keller SD. The 12-Item Short-Form Health Survey (SF-12): construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220-33. http://dx.doi.org/10.1097/00005650-199603000-00003.
- Jenkinson C, Layte R, Jenkinson D, Lawrence K, Peterson S, Paice C, et al. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies?. J Public Health Med 1997;19:179-96. http://dx.doi.org/10.1093/oxfordjournals.pubmed.a024606.
- Iglesias CP, Birks YF, Torgerson DJ. Improving the measurement of quality of life in older people: The York SF-12. QJM 2001;94:695-8. http://dx.doi.org/10.1093/qjmed/94.12.695.
- Iglesias CP, Birks YF, Torgerson DJ. Increasing response rates to postal questionnaires. Changing layout of questionnaires increases response rates. BMJ 2002;325. http://dx.doi.org/10.1136/bmj.325.7361.444.
- Kind P, Hardman G, Macran S. Centre for Health Economics discussion paper 172. York: Centre for Health Economics, University of York; 1999.
- Tober G, Clyne W, Finnegan O, Farrin A, Russell I. UKATT Research Team . Validation of a scale for rating the delivery of psycho-social sessions for alcohol dependence and misuse: The UKATT process rating scale (PRS). Alcohol Alcohol 2008;43:675-82. http://dx.doi.org/10.1093/alcalc/agn064.
- Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychol Bull 1979;86:420-28. http://dx.doi.org/10.1037/0033-2909.86.2.420.
- Fleiss J. Statistical Methods for Rates and Proportions. New York, NY: John Wiley & Sons; 1981.
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;i:307-10. http://dx.doi.org/10.1016/S0140-6736(86)90837-8.
- Drummond M, Sculpher M, Torrance G, O’Brien B, Stoddart G. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2005.
- UKATT ResearchTeam . Cost effectiveness of treatment for alcohol problems: findings of the randomised UK alcohol treatment trial (UKATT). BMJ 2005;331:544-8. http://dx.doi.org/10.1136/bmj.331.7516.544.
- Raistrick D, Tober G, Godfrey C, Parrott S, MacGregor S. Responding to Drug Misuse: Research and policy priorities in health and social care. London: Routledge; 2009.
- NICE . Guide to the Methods of Technology Appraisal 2008. www.nice.org.uk (accessed August 2012).
- Curtis L. Unit Costs of Health and Social Care 2010. Canterbury: University of Kent; 2010.
- Richardson G, Manca A. Calculation of quality adjusted life years in the published literature: a review of methodology and transparency. Health Econ 2004;13:1203-10. http://dx.doi.org/10.1002/hec.901.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87. http://dx.doi.org/10.1002/hec.635.
- McCambridge J, Kypri K. Can simply answering research questions change behavior? Systematic review and meta-analysis of alcohol brief intervention trials. PLoS One 2011;6. http://dx.doi.org/10.1371/journal.pone.0023748.
- Rubin DB. Inference and missing data. Biometrika 1976;63:581-92. http://dx.doi.org/10.1093/biomet/63.3.581.
- Latimer N, Guillaume L. Prevention and early identification of alcohol use disorders in adults and young people: screening and brief interventions: cost effectiveness review. Sheffield: ScHARR Public Health Collaborating Centre; 2009.
- The cost of alcohol harm to the NHS in England: An update to the Cabinet Office (2003) study. London: Health Improvement Analytical Team, Department of Health; 2008.
- Curtis L. Unit Costs of Health and Social Care 2010. Kent: Personal Social Services Research Unit, University of Kent; 2010.
- Oddie L. Ribble Valley Borough Council report to policy and finance committee: meals on wheels service. Clitheroe: Ribble Valley Borough Council; 2011.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. http://dx.doi.org/10.1097/00005650-199711000-00002.
- Dolan P, Gudex C, Kind P, Williams A. A social tariff for EuroQol: results from a UK general population survey. York: Centre for Health Economics; 1995.
- Richardson G, Manca A. Calculation of quality adjusted life years in the published literature: a review of methodology and transparency. Health Econ 2004;13:1203-10. http://dx.doi.org/10.1002/hec.901.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. http://dx.doi.org/10.1002/hec.944.
- Altman DG. Comparability of Randomised Groups. The Statistic 1985;34:125-36. http://dx.doi.org/10.2307/2987510.
- Willan AR, Briggs A. Statistical analysis of cost-effectiveness data. West Sussex: John Wiley & Sons Ltd; 2006.
- O’Hagan A, Stevens JW. Assessing and comparing costs: how robust are the bootstrap and methods based on asymptotic normality?. Health Econ 2003;12:33-49. http://dx.doi.org/10.1002/hec.699.
- Thompson SG, Barber JA. How should cost data in pragmatic randomised trials be analysed?. BMJ 2000;320:1197-200. http://dx.doi.org/10.1136/bmj.320.7243.1197.
- Efron B, Tibshirani R. An Introduction to the Bootstrap. New York, NY: Chapman & Hall; 1993.
- Briggs AH, Wonderling DE, Mooney CZ. Pulling cost-effectiveness analysis up by its bootstraps: a non-parametric approach to confidence interval estimation. Health Econ 1997;6:327-40.
- Glick H, Doshi J, Sonnad S, Polsky D. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2007.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87. http://dx.doi.org/10.1002/hec.635.
- Telephone advice lines for people with long term conditions: Guidance for nursing practitioners. London: Royal College of Nursing; 2006.
- Babor JP, Mercer D, Krakauer I, Calvo N. Development of an adherence/competence rating scale for individual drug counseling. Drug Alcohol Depend 1996;43:125-32. http://dx.doi.org/10.1016/S0376-8716(96)01305-1.
- Carroll KM, Nich C, Sifry R, Nuro KF, Frankforter TL, Ball SA, et al. A general system for evaluating practitioner adherence and competence in psychotherapy research in the addictions. Drug Alcohol Depend 2000;57:225-38. http://dx.doi.org/10.1016/S0376-8716(99)00049-6.
- Schulz KF, Altman DG, Moher D. CONSORT Group . CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Medicine 2010;8:18-27. http://dx.doi.org/10.1186/1741-7015-8-18.
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT Group . CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trial. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c869.
- Department of Health . Medicines for Human Use (clinical Trials) Regulations 2004. www.legislation.gov.uk/uksi/2004/1031/contents/made (accessed August 2012).
- Department of Health . Research Governance Framework for Health and Social Care 2005. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_4108962 (accessed August 2012).
- WHO Brief Intervention Study Group . A cross-national trial of brief interventions with heavy drinkers. Am J Public Health 1996;86:948-55. http://dx.doi.org/10.2105/AJPH.86.7.948.
- Fleming MF, Barry KL, Manwell LB, Johnson K, London R. Brief physician advice for problem alcohol drinkers: a randomised controlled trial in community-based primary care practices. JAMA 1997;277:1039-45. http://dx.doi.org/10.1001/jama.1997.03540370029032.
- Bien TH, Miller WR, Tonigan SJ. Brief interventions for alcohol problems: a review. Addiction 1993;88:315-36. http://dx.doi.org/10.1111/j.1360-0443.1993.tb00820.x.
- McCambridge J, Day M. Randomized controlled trial of the effects of completing the Alcohol Use Disorders Identification Test questionnaire on self-reported hazardous drinking. Addiction 2007;103:241-8. http://dx.doi.org/10.1111/j.1360-0443.2007.02080.x.
- Kaner E, Newbury-Birch D, Coulton S, Deluca P, Drummond C. Screening and brief interventions for alcohol use disorders in primary health care: a randomised controlled trial. London: Department of Health; 2011.
- Crawford MJ, Patton R, Touquet R, Drummond C, Byford S, Barrett B, et al. Screening and referral for brief intervention of alcohol-misusing patients in an emergency department: a pragmatic randomised controlled trial. Lancet 2004;364:1334-9. http://dx.doi.org/10.1016/S0140-6736(04)17190-0.
- Horner S, Rew L, Torres R. Enhancing intervention fidelity: A means of strengthening study impact. J Spec Pediatr Nurs 2005;11:80-9. http://dx.doi.org/10.1111/j.1744-6155.2006.00050.x.
- Navarro HJ, Shakeshaft A, Doran CM, Petrie DJ. The potential cost-effectiveness of general practitioner delivered brief intervention for alcohol misuse: evidence from rural Australia. Addict Behav 2011;36:1191-8. http://dx.doi.org/10.1016/j.addbeh.2011.07.023.
- Babor TF, Higgins-Biddle JC, Dauser D, Burleson JA, Zarkin GA, Bray J. Brief interventions for at-risk drinking: patient outcomes and cost-effectiveness in managed care organizations. Alcohol Alcohol 2006;41:624-31. http://dx.doi.org/10.1093/alcalc/agl078.
- Kunz FM, French MT, Bazargan-Hejazi S. Cost-effectiveness analysis of a brief intervention delivered to problem drinkers presenting at an inner-city hospital emergency department. J Stud Alcohol 2004;65:363-70.
- Kraemer KL. The cost-effectiveness and cost–benefit of screening and brief intervention for unhealthy alcohol use in medical settings. Subst Abuse 2007;28:67-7. http://dx.doi.org/10.1300/J465v28n03_07.
- Heather N. Interpreting the evidence on brief interventions for excessive drinkers: the need for caution. Alcohol Alcohol 1995;30:287-96.
- Parrott S, Godfrey C, Heather N, Clark J, Ryan T. Cost and outcome analysis of two alcohol detoxification services. Alcohol Alcohol 2006;41:84-91. http://dx.doi.org/10.1093/alcalc/agh236.
- Cobiac L, Vos T, Doran C, Wallace A. Cost-effectiveness of interventions to prevent alcohol-related disease and injury in Australia. Addiction 2009;104:1646-55. http://dx.doi.org/10.1111/j.1360-0443.2009.02708.x.
Appendix 1 Study protocol
Appendix 2 Regulatory approvals
Appendix 3 Details of study sites and practices
Appendix 4 Patient information sheet
Appendix 5 Screening questionnaire
Appendix 6 Data collection booklets
Appendix 7 Intervention delivery by site
Appendix 8 Safer drinking leaflet
Appendix 9 Study summary
Appendix 10 AESOPS Process Rating Scale
Appendix 11 Mail-out documentation
List of abbreviations
- ADD
- average drinks per day
- AESOPS
- Alcohol: Evaluating Stepped care in Older Populations Study
- AUDIT
- Alcohol Use Disorders Identification Test (10-item)
- AUDIT-C
- Alcohol Use Disorders Identification Test – Consumption (3-item)
- BCC
- behavioural change counselling
- CEAC
- cost-effectiveness acceptability curve
- CEP
- cost-effectiveness plane
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- DPI
- Drinking Problems Index
- EQ-5D
- European Quality of Life-5 Dimensions
- GP
- general practitioner
- HRQoL
- health-related quality of life
- ICC
- intraclass correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- ISRCTN
- International Standard Randomised Controlled Trial Number
- ITT
- intention to treat
- MCS
- mental component score
- MET
- motivational enhancement therapy
- NICE
- National Institute for Health and Care Excellence
- PCS
- physical component score
- PRS
- Process Rating Scale
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SD
- standard deviation
- SF-12
- Short Form Questionnaire-12 items
- UKATT
- UK Alcohol Treatment Trial
- WTP
- willingness to pay
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.